UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended December 31 , 2022
OR
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For transition period from to
Commission File Number 001-39311
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |||||||
(647 ) 812-2417
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||||||||||||
The | ||||||||||||||
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
1
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | ||||||||
| ☒ | Smaller reporting company | ||||||||||
| Emerging growth company | |||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
Indicate by check mark whether the Registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☐ No ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to Section 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act): Yes ☐ No ☐
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the common shares on the NASDAQ Global Select Market on June 30, 2022, was $504.0 million.
As of March 22, 2023, there were approximately 105,682,677 shares of the registrant's common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
2
TABLE OF CONTENTS
| Page | |||||
3
References throughout this document to POINT or the Company include POINT Biopharma Global Inc., and its consolidated subsidiaries. In accordance with the Securities and Exchange Commission’s “Plain English” guidelines, this Annual Report on Form 10-K (this "Form 10-K") has been written in the first person. In this document, the words “we”, “our”, “ours” and “us” refer only to POINT Biopharma Global Inc. and its consolidated subsidiaries and not any other person.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward-looking statements” which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions (including the negative of any of the foregoing) may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. The forward-looking statements in this Form 10-K are based on current expectations and beliefs concerning future developments and their potential effects. These forward-looking statements are made only as of the date of this Form 10-K. There can be no assurance that future developments affecting us will be those that we have anticipated. By their nature, forward-looking statements in this Form 10-K may or may not occur in the future. Forward-looking statements involve known and unknown risks and uncertainties that may cause our actual results in future periods to differ materially from those projected or contemplated in the forward-looking statements as a result of factors including, but not limited to, the following:
•the success, cost and timing of our product development activities and clinical trials, our plans for clinical development of our product candidates and the initiation and completion of any other clinical trials and related preparatory work and the expected timing of the availability of results of the clinical trials;
•our ability to recruit and enroll suitable patients in our clinical trials;
•the potential attributes and benefits of our product candidates;
•our ability to obtain and maintain regulatory approval for our product candidates, and any related restrictions, limitations or warnings in the label of an approved product candidate;
•our ability to obtain funding for our operations, including funding necessary to complete further development, approval and, if approved, commercialization of our product candidates;
•the period over which we anticipate our existing cash and cash equivalents will be sufficient to fund our operating expenses and capital expenditure requirements;
•the potential for our business development efforts to maximize the potential value of our portfolio;
•our ability to identify, in-license or acquire additional product candidates;
•our ability to maintain the license agreements underlying our product candidates;
•our ability to compete with other companies currently marketing or engaged in the development of treatments for the indications that we are pursuing for our product candidates;
•our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates and the duration of such protection;
•our ability to contract with and rely on third parties to assist in conducting our clinical trials and manufacture our product candidates;
•the development and expansion of our own manufacturing facility in Indianapolis, Indiana and the ability of this facility to provide adequate production capacity to meet future clinical and commercial demands for our product candidates;
•the size and growth potential of the markets for our product candidates, and our ability to serve those markets, either alone or in partnership with others;
•the rate and degree of market acceptance of our product candidates, if approved;
•the pricing and reimbursement of our product candidates, if approved;
•regulatory developments in the United States ("U.S.") and foreign countries;
•the impact of laws and regulations;
•our ability to attract and retain key scientific, medical, commercial or management personnel;
•our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
•our financial performance;
•the level of activity in the trading market for our common stock, par value $0.0001 per share ("Common Stock") and the volatility of the market price of our Common Stock;
•the effect of the COVID-19 coronavirus ("COVID-19") pandemic and Russo-Ukrainian conflict on the foregoing; and
• other factors detailed under the section entitled “Risk Factors.”
Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. Some of these risks and uncertainties may in the future be amplified by the COVID-19 outbreak and/or the Russo-Ukrainian conflict and there may be additional risks that we consider immaterial or which are unknown. It is not possible to predict or identify all such risks. Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and
4
uncertainties related to them and to the risk factors. We do not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Summary of Risk Factors
Our business is subject to numerous material and other risks that you should be aware of before making an investment decision. These risks are described more fully under the heading "Risk Factors" in Part I, Item 1A of this Form 10-K. These risks include, among others the following:
•Risks related to our financial condition and capital requirements, including, among others, that:
◦We have incurred significant losses for most of our operating history and may not be able to achieve or sustain revenues or profitability in the future.
◦We will require substantial additional financing, which may not be available on acceptable terms, or at all.
◦We may be exposed to risks related to the global economic slowdown, inflation, rising interest rates and the prospects for recession, as well as recent and potential future disruptions in access to bank deposits or lending commitments due to bank failures.
◦We may be exposed to financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates.
◦We have a limited operating history.
•Risks related to the development of our product candidates, including, among others, that:
◦Our approach to the discovery and development of product candidates based on our proprietary radioligand targeted therapies represents a novel approach to radiation therapy.
◦We are very early in our development efforts and we may not achieve research, development and commercialization goals in the time frames that we publicly estimate.
◦We may be unable to obtain regulatory approval for our product candidates under applicable regulatory requirements.
◦Clinical development involves a lengthy and expensive process with uncertain outcomes. We may encounter difficulties enrolling patients in our clinical trials.
◦General political, economic and business conditions, including the effects to mitigate pandemic diseases such as COVID-19 and the ongoing Russo-Ukrainian conflict may continue to adversely affect our business and financial results.
◦We currently have a minimal marketing and sales organization and have no experience in marketing products.
•Risks related to our manufacturing operations, including, among others, that:
◦Our product candidates are radioligands and the manufacture of our product candidates is complex.
◦Our ability to maintain regulatory approvals for our manufacturing facilities, could delay our development plans or commercialization efforts.
◦Any damage, destruction or interruption of production at our manufacturing facility could negatively affect us.
◦Any failure to perform proper quality control and quality assurance would have a material adverse effect on our business and financial results.
•Risks related to our reliance on third parties, including, among others, that:
◦While we operate our own manufacturing facility, we currently rely, and will likely continue to rely, on third parties to manufacture additional supply of our lead product candidates for our ongoing clinical trial and our preclinical studies, as well as any preclinical studies or clinical trials of our future product candidates that we may conduct.
◦We may be unable to obtain a sufficient supply of radioisotopes to support clinical development or at a commercial scale.
◦We rely on third parties to conduct our clinical trials of 177Lu-PNT2002, 177Lu-PNT2003, and PNT2004 radioligands and plan to rely on third parties to conduct future clinical trials.
◦We may not be successful in our efforts to carry out our obligations under our collaborations for our manufacturing scale up; for instance, without limitation, failure to complete in a timely manner or at all our contractual obligations to Lantheus.
◦The collaboration and license agreements (the "Lantheus License Agreements") with Lantheus Holdings Inc. ("Lantheus") are important to our business. If Lantheus is unable to develop and commercialize 177Lu-PNT2002 or 177Lu-PNT2003, if we or Lantheus fail to adequately perform under the Lantheus License Agreements, or if we or Lantheus terminate the Lantheus License Agreements, our business would be adversely affected.
5
◦We may form or seek collaborations or strategic alliances in the future.
•Risks related to government regulation, including, among others, that:
◦The Food and Drug Administration ("FDA") regulatory approval process is lengthy and time-consuming, and we may experience significant delays in the clinical development and regulatory approval of our product candidates.
◦Even if we receive regulatory approval of our product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review.
◦Failure to obtain or maintain adequate coverage and reimbursement for newly-approved products could limit our ability to market those product candidates.
◦Our relationships with healthcare providers and physicians and third-party payors are subject to complex and extensive healthcare laws and regulations.
◦Healthcare legislative reform measures, policy changes and constraints on national budget social security systems may have a material adverse effect on our business and results of operations.
◦Our ability to comply with data privacy laws and regulations, and uncertainties regarding potential significant breaches of data privacy.
•Risks related to our intellectual property, including, among others, that:
◦If we are unable to obtain and maintain patent protection for any product candidates we develop and for our technology, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize products and technology similar or identical to ours.
◦If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively in its market.
◦If we fail to comply with our obligations under our patent licenses with third parties, we could lose license rights that are important to our business.
◦We may not be able to protect our intellectual property and proprietary rights throughout the world.
◦Issued patents covering our product candidates or technologies could be found invalid or unenforceable if challenged in court.
◦If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
◦Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
◦Intellectual property rights and regulatory exclusivity rights do not necessarily address all potential threats.
•Risks related to employee matters and managing growth, including, among others, that:
◦Our management’s focus and resources may be diverted from operational matters and other strategic opportunities as a result of the strategic collaboration and exclusive license agreements with Lantheus.
◦We are highly dependent on our key personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
◦We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
◦Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
•Risks relating to ownership of our Common Stock, including, among others, that:
◦We do not know whether an active, liquid and orderly trading market will develop for our Common Stock or what the market price of our Common Stock will be and, as a result, it may be difficult for you to sell your Common Stock.
◦The price of our Common Stock may be volatile, and you could lose all or part of your investment.
◦Future sales and issuances of our Common Stock or rights to purchase our Common Stock, including pursuant to the 2021 Equity Incentive Plan ("Equity Incentive Plan"), could result in additional dilution of the percentage ownership of our stockholders and could cause the price of our Common Stock to fall.
◦We do not intend to pay dividends on our Common Stock, so any returns will be limited to the value of our Common Stock.
•Risks related to our organizational structure, including, among others, that:
◦Delaware law and our governing documents contain certain provisions, including anti-takeover provisions, which limit the ability of stockholders to take certain actions and could delay or discourage takeover attempts that stockholders may consider favorable.
◦Our bylaws designate a state or federal court located within the State of Delaware as the sole and exclusive forum for substantially all disputes between us and our stockholders.
6
PART I
Item 1. Business
Overview
POINT Biopharma Global Inc. is a next-generation radioligand company. Our mission is to accelerate the discovery, development and global access to targeted radioligand therapies. In pursuit of this mission, we have built a platform for the clinical development and commercialization of next-generation radioligands. Our platform consists of a strong pipeline of potential first-in-class and best-in-class assets, internal research and development expertise, in-house manufacturing capabilities, and a secured supply for rare medical isotopes like actinium-225 ("225Ac") and lutetium-177 ("177Lu").
Our product pipeline consists of late-stage programs in prostate cancer (PNT2002) and neuroendocrine tumors (PNT2003), as well as an early-stage portfolio of next-generation programs (for example, PNT2001, PNT2004). Our late-stage programs are 177Lu-based radioligands. For our early-stage development programs, we plan to evaluate the utility of both 177Lu and 225Ac, as well as other isotopes that may be considered for use in radioligands.
Our management team brings decades of combined experience in targeted radioligand therapy clinical development and manufacturing. Our 100% company-owned radioligand manufacturing facility, located in Indianapolis, Indiana, is currently operational and supplying 177Lu-PNT2002 drug product for our phase 3 SPLASH trial. We have also assembled one of the radiopharmaceutical industry’s most redundant isotope supply chains, with in-house manufacturing of isotopes such as no-carrier-added ("n.c.a.") 177Lu expected to commence by the end of 2023.
With recent innovations in the production and purification of medical isotopes, radioligand development is progressing faster than ever before; we believe POINT is well-positioned to be a leader in this rapidly advancing field.
Strategic Collaboration with Lantheus Holdings Inc. (NASDAQ: LNTH)
In November 2022, POINT announced strategic collaboration and exclusive license agreements (the "Lantheus License Agreements") with Lantheus Holdings Inc. ("Lantheus") for exclusive worldwide rights for 177Lu-PNT2002 and 177Lu-PNT2003, excluding certain territories (Japan, South Korea, Singapore, Indonesia, and China including Hong Kong, Macau and Taiwan). The collaboration pairs POINT's expertise in next-generation radioligand therapy development and manufacturing with Lantheus’ commercial leadership in prostate-specific membrane antigen - positron emission tomography ("PSMA-PET") and radiopharmaceuticals.
In December 2022, closing conditions for the transaction, including Hart-Scott-Rodino antitrust clearance, were met. For 177Lu-PNT2002, POINT has received a $250 million upfront payment. POINT will also receive an additional payment of up to $250 million upon U.S. regulatory approval, and, once certain return on investment financial thresholds have been achieved and other conditions met, royalties of 20% on all net sales (prior to which there is a period of sales in which the royalty may be based on only a portion of the gross profit), as well as the potential for up to an additional $1.3 billion in various net sales milestone payments. For 177Lu-PNT2003, POINT has received a $10 million upfront payment, and will receive up to an additional $30 million upon U.S. regulatory approval, royalties of 15% on net sales, as well as the potential for up to an additional $275 million in various net sales milestone payments.
Joint steering committees have been formed to oversee the clinical studies, regulatory filings, manufacturing, and commercial readiness for both 177Lu-PNT2002 and 177Lu-PNT2003. As per the strategic collaboration and exclusive license agreements, Lantheus will be responsible for all subsequent development of the assets post initial regulatory filings within the U.S., and for all development efforts in their territories.
Product Pipeline Summary:
PNT2002 Program
177Lu-PNT2002 is a late-stage prostate-specific membrane antigen ("PSMA") targeted radioligand therapy currently in a phase 3 trial sponsored by POINT. 177Lu-PNT2002 combines a PSMA-specific ligand, PSMA-I&T, with the beta-emitting radioisotope 177Lu ("177Lu-PSMA-I&T").
The multi-center, randomized, open label phase 3 Study evaluating metastatic castration-resistant Prostate cancer ("mCRPC") using 177Lu-PNT2002 PSMA therapy After Second-line Hormonal treatment ("SPLASH") trial assesses 177Lu-
7
PNT2002 in participants with PSMA-expressing mCRPC who have progressed on androgen receptor pathway inhibitor, ("ARPI"), therapy and are ineligible or averse to chemotherapy. The study has two phases: 1) a 25-participant safety and dosimetry lead-in study (with follow-up ongoing) and 2) an approximately 400-participant randomized study (which is still ongoing). The randomization phase of the study started enrolling in September 2021. In December 2022, the target enrollment for randomization was achieved on schedule with more than 390 participants randomized across 55 SPLASH trial sites in North America, the European Union (the "E.U."), and the United Kingdom ("U.K."). We expect to report top line data from SPLASH in the second half of 2023.
In November 2022, POINT announced a strategic collaboration and exclusive license agreements for the commercialization of 177Lu-PNT2002 with Lantheus. Under the Lantheus License Agreements, Lantheus will be responsible for all subsequent development of the asset post initial regulatory filings within the U.S., and for all development efforts in their territories.
PNT2003 Program
177Lu-PNT2003 is a late-stage somatostatin-targeted radioligand in development for the treatment of neuroendocrine tumors. 177Lu-PNT2003 combines a somatostatin-specific radioligand called DOTATATE with n.c.a. 177Lu. 177Lu-PNT2003’s use of n.c.a. 177Lu could avoid the need for clinics to maintain costly dedicated waste streams, providing a unique advantage over the currently approved radioligand product for the gastroenteropancreatic neuroendocrine tumors ("GEP-NETs") indication. The clinical trial involving 177Lu-PNT2003 is being sponsored by the University Health Network ("UHN") with collaboration from Canadian Molecular Probe Consortium ("CanProbe"), Ozmosis Research Inc., and Cancer Care Ontario (OZM-067; NCT02743741). The last patient last dose for OZM-067 occurred in July 2021, all participants completed the primary follow-up period in the second quarter of 2022; long-term follow-up is ongoing.
In November 2022, POINT announced strategic collaboration and exclusive license agreements for the commercialization of 177Lu-PNT2003 with Lantheus. Following the strategic collaboration announcement, POINT received the OZM-067 clinical trial data sets from the trial sponsor, UHN. POINT will facilitate the analysis of the data sets. Under the Lantheus License Agreements, Lantheus will be responsible for all subsequent development of the asset post initial regulatory filings within the U.S., and for all development efforts in their territories.
PNT2004 Program
PNT2004 is a fibroblast activation protein-α ("FAP") targeting program being developed for use in multiple tumor types. FAP is a compelling pan cancer target for imaging and therapy that is expressed in >90% of epithelial tumors. In cancer, FAP is over-expressed on cancer associated fibroblasts ("CAF"s), as well as on certain cancer cells of mesenchymal origin, and its expression is understood to be involved in mechanisms driving tumor progression and resistance to chemo and immunotherapy. FAP is a 170 kDa membrane bound prolyl endopeptidase that is expressed almost exclusively to pathological conditions with known effects on cell proliferation, migration, and invasion. POINT believes the D-ala-boroPro warhead, which is the core of the PNT2004 program, has the potential to generate best-in-class FAP-targeting ligands. 177Lu-PNT6555, the clinical lead, has demonstrated compelling tumor retention and normal tissue clearance in preclinical models, enabling delivery of large doses of tumor killing radiation.
POINT filed a clinical trial application (CTA) with Health Canada at the end of the first quarter of 2022 for 177Lu-PNT6555. A No Objection Letter, or authorization to proceed, was received from Health Canada in May 2022. The phase 1 clinical trial commenced in July 2022 in Canada and uses a gallium-68 (68Ga)-based PNT6555 molecular imaging agent to select patients to receive a n.c.a. 177Lu-based PNT6555 therapeutic agent. The FAPi Radioligand OpeN-Label, phase 1 study to evaluate safety, Tolerability and dosImetry of 177Lu-PNT6555; a dose Escalation study for tReatment of patients with select solid tumors, ("FRONTIER"), protocol evaluates PNT6555 with dosing starting at 4 gigabecquerel ("GBq") and each subsequent dose level increasing by 4 GBq, also allowing for 2 GBq dose de-escalations. Estimated enrollment across the multiple dosing levels is up to 30 participants in five FAP-avid cancer indications: colorectal, pancreatic, esophageal, melanoma, and soft tissue sarcoma.
Enrollment in cohort 2 began in December 2022 and cohort 3 enrollment is expected to begin in the second quarter 2023. A total of six participants have been dosed with 177Lu PNT6555 to date. We anticipate data from the full FRONTIER trial to be available in the first half of 2024.
In November, 2022, preclinical data supporting the combination of 177Lu-PNT6555 with immunotherapy was released via a press release. The preclinical study focused on assessing the potential of the lead candidate in the PNT2004 clinical program, 177Lu-PNT6555, in combination with anti-PD-1 immunotherapy. The CT26-mFAP tumor model used expresses low levels of FAP, grows aggressively, and is insensitive to anti-PD-1 immunotherapy. The study found that combination
8
treatment with 177Lu-PNT6555 and anti-PD-1 resulted in a significant survival benefit, as compared to either treatment independently.
PNT2001 Program
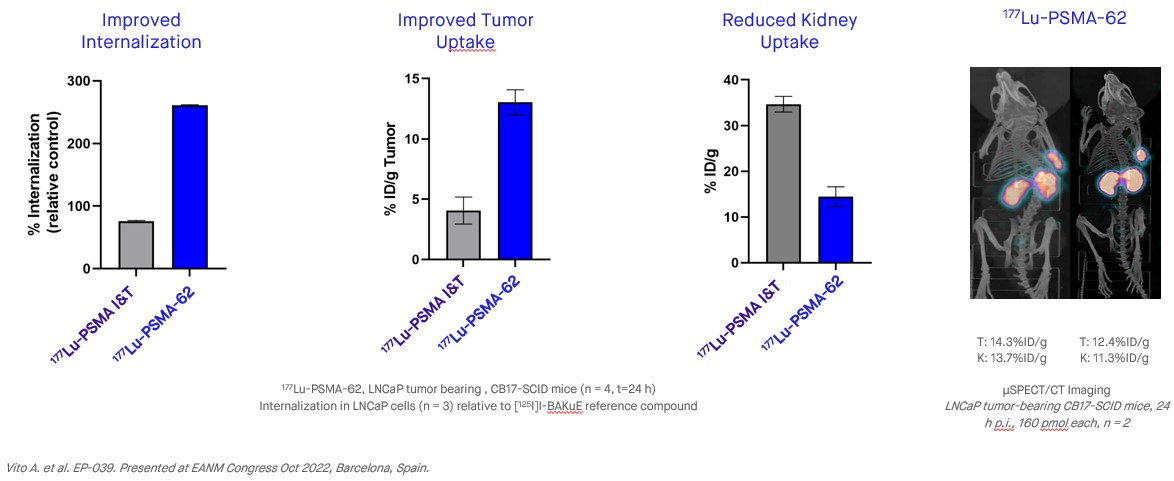
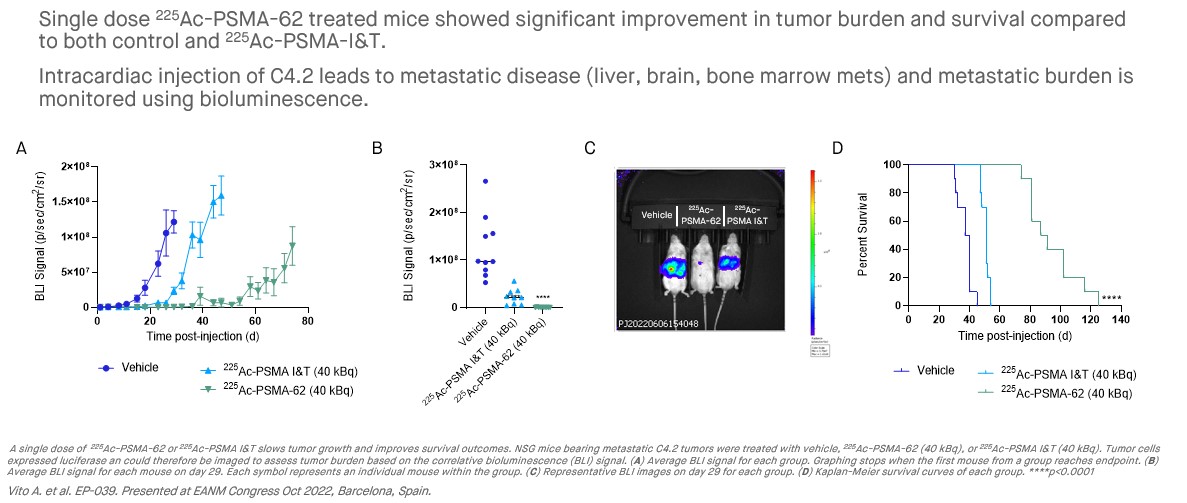
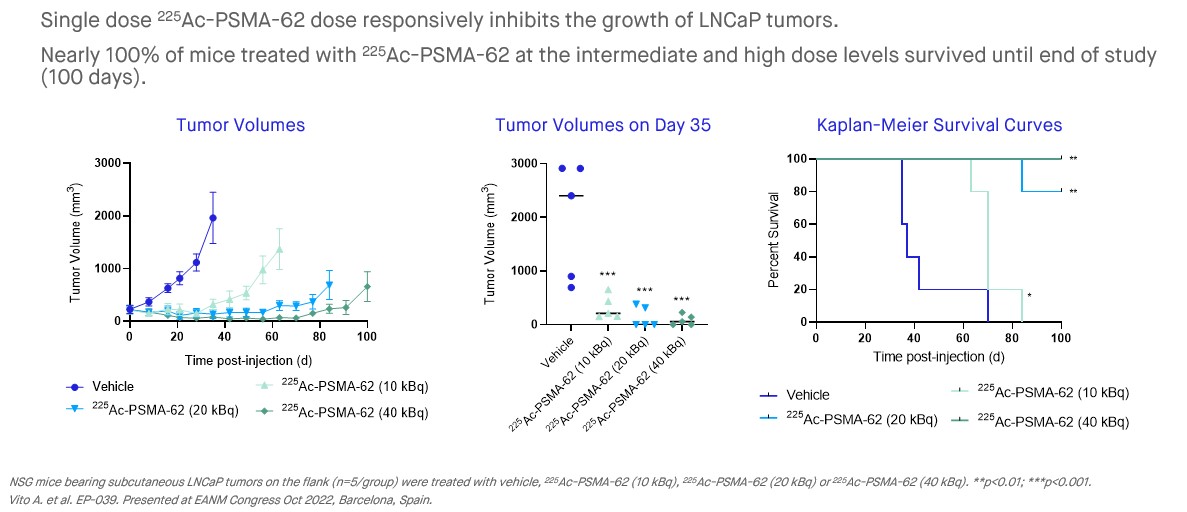
The PNT2001 program encompasses a next-generation PSMA-targeting ligand family which leverages linker technology that promotes increased internalization of the ligand which has translated into increased tumor accumulation in preclinical models versus PSMA-I&T. The lead candidate in the program, 225Ac-PSMA-62, shows compelling efficacy as a single dose in preclinical tumor models, with potent anti-tumor activity using 225Ac, while also having an improved biodistribution profile versus the PSMA ligands that are currently approved, or in later-stage development. This profile could result in increased efficacy at a lower dose, potentially allowing for a reduction in the radioligand dose needed and improved patient outcomes.
In October 2022, POINT published new preclinical data from the PNT2001 program. The new PNT2001 data were shared in E-poster #039, “Development and characterization of a next-generation 225Ac-PMSA radioligand,” at the 35th Annual Congress of the European Association of Nuclear Medicine (EANM) in Barcelona, Spain.
POINT is planning to advance the candidate into investigational new drug ("IND")-enabling studies with the aim of pursuing clinical development of 225Ac-PSMA-62 in the biochemically recurrent, oligometastatic, and post-177Lu-PSMA mCRPC populations. POINT is targeting an IND/CTA submission to support initiation of the first-in-human, phase 1 study in the first half 2023.
CanSEEK
CanSEEK™ is a prodrug technology that has the potential to prevent target engagement outside of the tumor microenvironment ("TME"), which could significantly improve the therapeutic index of targeted radioligand therapy. CanSEEK™ is based on the (d)-Ala-Pro FAP substrate technology, which works by preventing a radioligand from binding to the target receptor until it has been activated by FAP in the TME. Multiple (d)-Ala-Pro substrate enabled ligands are being studied preclinically against different targets. If successful, the platform has the potential to significantly improve the precision and safety of radioligands. POINT’s CanSEEKTM technology is the subject of licenses from both Bach Biosciences, LLC ("Bach Biosciences") and Avacta Life Sciences Limited ("Avacta").
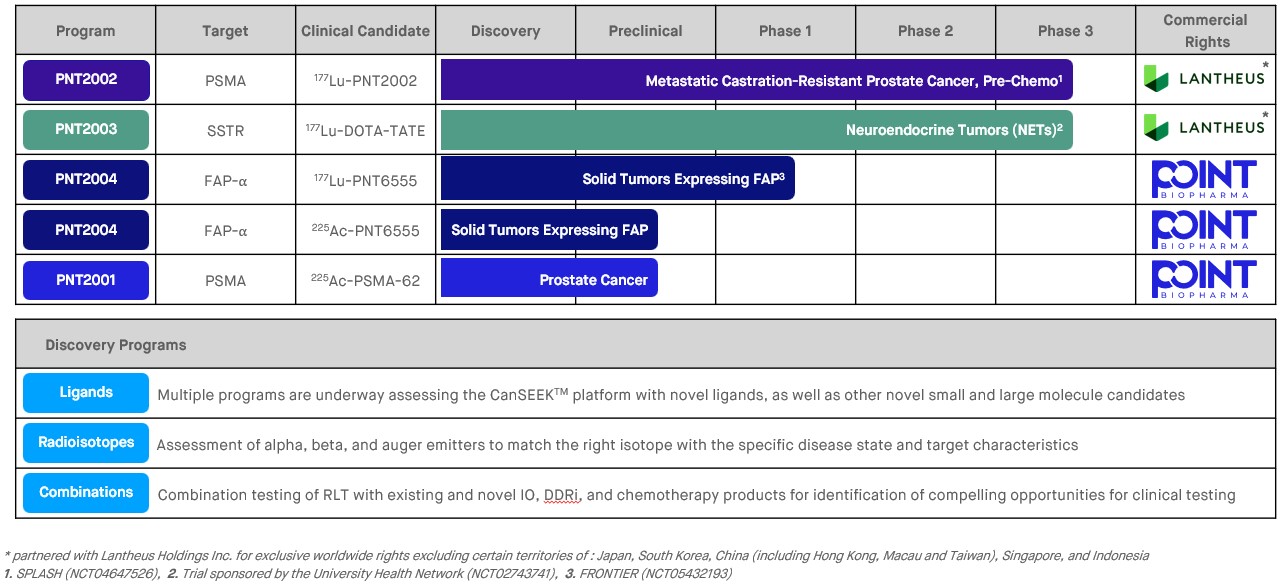
Our Product Candidates
POINT is advancing multiple radioligands based on peptides and small molecules combined with beta- and alpha-emitting radioisotopes for the treatment of various cancers. Our current pipeline is summarized in the diagram below:
9
*mCRPC = metastatic castration resistant prostate cancer, PSMA = prostate specific membrane antigen, SSTR = somatostatin receptors, FAP = fibroblast activation protein, RLT = radioligand therapy, IO = immuno-oncology, DDRi = DNA damage response inhibitor
PNT2002 Program
Background on Prostate Cancer and PSMA targeting
The incidence of prostate cancer is increasing as the U.S. population ages into older at-risk groups. The American Cancer Society estimates that there will be approximately-288,300 new prostate cancer cases and over 34,700 deaths due to prostate cancer in the U.S. in 2023. Prostate cancer is the most common cancer diagnosed in men, and with a significant mortality rate, is the second leading cause of cancer death in males in the U.S. In Europe, across the E.U.-27 countries, the European Cancer Information System estimated that there were approximately 335,000 new prostate cancer cases and over 69,000 deaths as a result of prostate cancer in 2020. In these countries, prostate cancer is the most common cancer in males and is the third leading cause of cancer death in men.
PSMA is a unique membrane-bound glycoprotein that is over-expressed in prostate cancer but has low expression in normal healthy tissue. Furthermore, PSMA is highly expressed in all states of prostate cancer, including primary and poorly differentiated, metastatic and castrate-resistant disease. This unique expression of PSMA provides the opportunity to design treatments that can be precisely targeted to safely enable the delivery of highly potent drug payloads, while sparing normal healthy tissue. In addition, companion imaging agents are approved and can be used to identify individuals whose disease over-expresses PSMA. In December 2020, the FDA approved the first drug for positron emission tomography ("PET") imaging of PSMA-positive lesions in individuals with prostate cancer, 68Ga-PSMA-11. In May 2021, the FDA approved the second PET imaging agent for PSMA imaging, 18F-DCFPyL, commercialized by Lantheus Medical Imaging. Subsequently, in December 2021, the FDA approved Telix Pharmaceuticals’ kit for the preparation of 68Ga-PSMA-11. POINT believes the approval of PSMA-PET agents will facilitate broad access and permit the identification of individuals who are likely to benefit from PSMA-targeted treatments, including 177Lu-PNT2002.
Although the five-year survival rate of local and regional prostate cancer is nearly 100%, more aggressive forms of the disease, representing 22% of individuals at the time of initial diagnosis, have a substantially poorer prognosis, with a five-year survival rate of only 30%. While these more aggressive forms of prostate cancer can initially be treated, nearly all of these individuals experience a recurrence in tumor growth that results in the subsequent development of mCRPC. mCRPC is the most advanced form of the disease and recent estimates suggest a potential incidence of approximately 62,000 new cases of first line mCRPC in the U.S. per year. Individuals with mCRPC have a poor prognosis, from the time of diagnosis of mCRPC, the median overall survival is only 21.2 months (George et al., 2020). mCRPC represents nearly all prostate cancer-specific deaths.
ARPI therapies such as Zytiga® (abiraterone), Xtandi® (enzalutamide), Nubeqa® (darolutamide), or Erleada® (apalutamide) are now standard of care for both non-mCRPC and mCRPC following progression and failure on initial hormone therapy. Peak global annual sales of Johnson & Johnson’s Erleada® were $968 million in the U.S. plus an additional $913 million in international markets for a total of $1.88 billion in 2022. Astellas reported global annual sales of Xtandi® for year ended March 2022 exceeding ¥534.3 billion (approximately $3.9 billion). Finally, Bayer reported that Nubeqa® reached sales of €219.0 million (approximately $250 million) in 2021, driven by U.S. market over-performance, signaling a change in clinical preferences.
In mCRPC, median radiographic progression-free survival ("rPFS") for first-line use of abiraterone was 16.5 months, with ~20% of participants having progressed by the sixth month. Median time that particpants received enzalutamide was 16.6 months with 16% having progressed by the sixth month. For most individuals, progression is inevitable and as a result there is a large unmet need upon failure of ARPI therapy. In spite of progression while on ARPI, a majority of individuals are offered an ARPI switch to avoid the increasingly toxic profile of chemotherapy. Two recent studies have shown that, when patients are switched to a second-line ARPI therapy, rPFS is only approximately four months (de Bono J, et al. N Engl J Med 2020; 382:2091-102, and Powles T, et al. Nature 2022;28:144–53). As a result, there continues to be a significant need for novel treatment options with the potential to modify or ultimately cure the disease.
POINT's Solution: 177Lu-PNT2002
177Lu-PNT2002 combines a PSMA-specific ligand with the beta-emitting radioisotope 177Lu. 177Lu-PNT2002’s ligand is referred to as “PSMA-I&T” in academic literature and has been used for many years in research and compassionate use
10
settings. PSMA-I&T was developed by Dr. Hans-Jürgen Wester at the Technische Universität München. We created the PNT2002 program by leveraging the data collected in an academic study on PSMA-I&T (Baum RP, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy), in combination with issued patents and provisional patents pertaining to the formation of the radioligand active pharmaceutical ingredient, the manufacturing process and the formulation of the finished product.
Current standard of care for individuals with castration-resistant prostate cancer who have failed androgen deprivation therapy ("ADT") and ARPI is chemotherapy. The side effects of chemotherapy are significant including neuropathies, nausea, diarrhea, decreased mental capacity and increased risk of infections. 177Lu-PNT2002 has the potential to offer an alternative with significantly fewer side effects compared to chemotherapy, leading to a higher quality of life.
A prospective clinical study by Baum, et al. focused on the safety and efficacy of 177Lu-PSMA-I&T in individuals with progressive mCRPC and increasing levels of prostate-specific antigen ("PSA"). Fifty-six participants were administered a dose of 3.6 to 8.7 GBq, per cycle for one to six cycles, and monitored for morphologic response, response in PSA levels, and overall change in clinical symptoms. Analysis with contrast-enhanced computed tomography ("CT") determined that 20%, 52%, and 28% of participants had partial remission, stable disease, and progressive disease, respectively. The PSA levels decreased in 80.4% of participants, with the median PSA level reduced from 43.2 ng/mL to 23.8 ng/mL pre- and post-therapy. Progression-free survival ("PFS") was a median of 13.7 months and the median overall survival was not reached after a 28-month follow-up period.
Participants were monitored for side effects for two to four days after each treatment, and vital signs were recorded during administration of the therapy. No clinically significant adverse events were reported during the early monitoring period (i.e., during therapy and for up to four days thereafter) or at the 28-month follow-up time point. Leukocytopenia (grades 1 or 2) occurred only in participants who had received prior long-term chemotherapy. Events of mild, reversible dry mouth, which resolved within three months, occurred in two participants after the third and fourth cycles. No evidence of nephrotoxicity was observed and no clinically significant changes in pre- to post-dosing lab parameters were identified.
POINT held regulatory discussions with the FDA in 2020, leveraging data in the literature. The FDA provided feedback on protocol design, dosing strategy, dosimetry, control arm, imaging agents and statistical approach regarding the clinical development plans for 177Lu-PNT2002. Leveraging feedback from the FDA, POINT filed an IND in December 2020 to evaluate the clinical efficacy and safety of 177Lu-PNT2002. The IND was accepted in December 2020. Regulatory discussions were also held with Health Canada in December 2020. Following these discussions, POINT submitted a clinical trial application ("CTA") for the study and received a No Objection Letter from Health Canada in January 2021. CTAs were also filed and are active in four other countries.
Clinical Development and Next Steps
POINT initiated participant recruitment for our phase 3 SPLASH trial in February 2021, and we expect to report top line data from SPLASH in the second half of 2023. The randomization phase of the study started enrolling in September 2021, and in December 2022 the target enrollment for randomization was completed on schedule with more than 390 participants randomized across 55 SPLASH trial sites in North America, Europe, and the U.K. All participants undergo PSMA imaging as part of screening to confirm PSMA expression eligibility, as evaluated by central review. The study commenced with dosing 27 participants in the safety and dosimetry lead-in and proceeded to a randomization treatment phase in approximately 400 participants in September 2021 who were randomized in a 2:1 ratio to: 177Lu-PNT2002 (Arm A) versus enzalutamide (160 mg orally qd) or abiraterone (1000 mg orally qd with: 5 mg bid prednisone or 0.5 mg qd dexamethasone) (Arm B). POINT anticipates that Arm B will result in a median PFS of approximately 5 months based on results in previous clinical studies (3.7 months, De Bono et al. N Engl J Med 2020; 382:2091-2102; 4.2 months: Powles T, et al. Nature 2022;28:144–53.).
Participants in Arm A will receive a 6.8 GBq dose of 177Lu-PNT2002 for 4 cycles, 8 weeks apart, for a maximum cumulative dose of 27.2 GBq. Participants in Arm B may be eligible to crossover to 177Lu-PNT2002 every 8 weeks for 4 cycles after radiographic progression per central review. All participants will be followed in long-term follow-up for at least 5 years from the first therapeutic dose, or until death or loss to follow-up. The primary endpoint of the trial is rPFS. Key secondary endpoints include objective response rate ("ORR") and overall survival.
11
In February 2022, POINT published dosimetry data from the 27 participants dosed in the safety and dosimetry lead-in cohort for the Company’s phase 3 SPLASH trial evaluating 177Lu-PNT2002 for the treatment of mCRPC at the 2022 SNMMI Mid-Winter and ACNM Annual Meeting on February 25-27, 2022. The abstract was made public on the first day of the conference. Radiation dosimetry of 177Lu-PNT2002 was calculated in 27 participants with mCRPC based on biodistribution data from planar whole-body conjugate imaging at 1, 24, 48, 72, and 168 hours and, for 7 of them, SPECT/CT imaging at 48-72 hours post injection of their first cycle of 177Lu-PNT2002 (6.8±10% GBq). Data from the abstract titled “Dosimetry Results from the SPLASH Trial” (Abstract #: MWMA2244) demonstrated the following:
•Organs receiving the largest absorbed doses were the lacrimal glands at 1.2 Gy/GBq, followed by the kidneys at 0.73 Gy/GBq.
•The average dose to the salivary glands and red marrow was 0.34 Gy/GBq and 0.034 Gy/GBq, respectively.
•For a cumulative administered activity of 27.2 GBq, (four cycles of 6.8 GBq) the kidneys would receive a cumulative absorbed dose of 19.9 Gy, and the red marrow, 0.91 Gy.
•SPECT/CT vs planar-based kidney dosimetry was consistent across most participants (±20%) where SPECT/CT images were available with a mean kidney absorbed dose difference of 1%.
In April 2022, POINT dosed the first E.U. participant in the SPLASH trial, and in September 2022, the Company published a poster at ESMO Congress 2022 containing updated efficacy and safety data from the 27-participant safety and dosimetry lead-in cohort for the phase 3 SPLASH trial (NCT04647526) evaluating 177Lu-PNT2002 for the treatment of metastatic castration-resistant prostate cancer (mCRPC). The poster is titled “Efficacy and Safety of 177Lu-PNT2002 Prostate-specific Membrane Antigen (PSMA) Therapy in Metastatic Castration Resistant Prostate Cancer (mCRPC): Initial Results from SPLASH” (e-Poster #1400P). Key findings highlighted in the poster include:
•Median rPFS was 11.5 months, as compared to the control arm benchmarks of 3.5–4.2 months for individuals with progressive mCRPC post-ARPI failure receiving similar treatment (de Bono J, et al. N Engl J Med 2020; 382:2091-102.; Powles T, et al. Nature 2022;28:144–53.)
•Median overall survival had not been reached with an 11.7-month median duration of follow-up from time of enrollment.
•A radiographic objective response was achieved in 60% of the 10 participants with evaluable disease at baseline
•84.8% of individuals imaged met PSMA eligibility criteria.
•From a median baseline PSA (ng/mL) of 22 (range 0.3–701.0), 11 (42%) participants achieved a PSA50 response.
•177Lu-PNT2002 was well tolerated with no treatment-related deaths and few treatment-related adverse events of grade 3 or higher.
•Treatment-related adverse events occurring in >10% of participants included dry mouth (25.9% of participants; all grade 1), fatigue (22.2%; grades 1-2), nausea (18.5%; grades 1-2), and anaemia (14.8%; grades 1-3).
In November 2022, POINT announced strategic collaboration and exclusive license agreements with Lantheus for exclusive worldwide rights for 177Lu-PNT2002, excluding certain territories (Japan, South Korea, Singapore, Indonesia, and China including Hong Kong, Macau and Taiwan).
In December 2022, closing conditions for the transaction, including Hart-Scott-Rodino antitrust clearance, were met. For 177Lu-PNT2002, POINT has received a $250 million upfront payment. POINT will receive an additional payment up to $250 million upon U.S. regulatory approval, and, once certain return on investment financial thresholds have been achieved and other conditions met, royalties of 20% on net sales, as well as the potential for up to an additional $1.3 billion in various net sales milestone payments.
Joint steering committees have been formed to oversee the clinical studies, regulatory filings, manufacturing, and commercial readiness for 177Lu-PNT2002. As per the strategic collaboration and exclusive license agreement, Lantheus will be responsible for all subsequent development of the asset post initial regulatory filings within the U.S., and for all development efforts in their territories.
The randomization phase of the study started enrolling in September 2021 and in December 2022 the target enrollment for randomization was completed on schedule with more than 390 participants randomized across 55 SPLASH trial sites in North America, Europe, and the United Kingdom ("U.K."). We expect to report top line data from SPLASH in the second half of 2023.
PNT2003 Program
12
Background on Neuroendocrine Tumors and Somastatin Targeting
Neuroendocrine tumors ("NETs") are rare, heterogenous tumors that originate in neuroendocrine cells, and frequently arise in the gastroenteropancreatic and respiratory systems. NETs are the second most common neoplasm of the diffuse endocrine system. Because of their low incidence and prevalence among the population, NETs are considered an orphan disease. However, there has been a trend towards rising incidence in recent years, especially for NETs located in the lungs, small intestine and rectum.
According to an analysis performed using Surveillance, Epidemiology, and End Results Program research data, there was a prevalence of 187,000 people with NETs in the U.S. in 2016 and an estimate of 33,000 newly diagnosed cases. The American Society of Clinical Oncology estimates that 50% of the NETs originate in the gastrointestinal tract and pancreas, 30% in the lung and the remainder in various sites including the adrenal glands, thyroid and pituitary, among others.
GEP-NETs are a rare type of tumor that can form in the pancreas or in other parts of the gastrointestinal tract, including the stomach, small intestine, colon, rectum and appendix. GEP-NETs, sometimes called carcinoid tumors or islet cell tumors, usually form in cells that secrete hormones and can either be benign or malignant. Non-GEP-NETs, includes neuroendocrine tumors of the lung, thyroid, adrenal, ovary, kidney, pituitary, and unknown origin. The five- and ten-year overall survival for GEP-NETs is ~22 – ~56% and ~7 – ~28%, respectively, while individuals with metastatic disease have significantly worse ten-year overall survival across all NET subtypes than those with local disease. The five- and ten-year overall survival rates for lung NETs, which make up the majority of non-GEP-NETs, are 33.7% and 17.3%, respectively, while for NETs of unknown origin (second-largest non-GEP NET grouping), five- and ten-year overall survival rates are 38% and 22%, respectively.
NETs are characterized by over-expression of somatostatin receptors, which are protein receptors that sense molecules outside of the cell and activate cellular responses to somatostatin. Somatostatin is an important regulator of the endocrine system, and the over-expression of these receptors in NETs, particularly the SSTR2 receptor, creates a target for radioligand therapy to bind to and provide treatment.
NETs are difficult to diagnose because individuals often remain asymptomatic in the early stages of the cancer, and symptoms that are exhibited are not unique to NETs. According to some reports, over 50% of people with NETs have metastases at diagnosis, upon which NETs cannot be effectively treated with surgery alone and there is a need for alternative therapeutics to provide additional treatment.
NETs are characteristically insensitive to chemotherapeutic agents due to their slow proliferation. In generally small studies (n = 20-85 GEP-NET participants), combination chemotherapy using various anti-proliferative agents (e.g., fluorouracil, streptozotocin, doxorubicin, dacarbazine, interferon-alpha) has yielded typical response rates of less than 40%, duration of response as low as 3 – 8 months and median overall survival of 10 – 40 months.
There are a limited number of therapeutic approaches targeting somatostatin in NETs. Novartis’ Sandostatin®, which generated $1.2 billion in 2022 sales, is a somatostatin analogue that controls clinical syndromes associated with NETs but does not treat the metastatic NETs themselves. Ipsen S.A.’s Somatuline®, which generated €1.2 billion in 2022 sales, was approved in December 2014 for the treatment of adult with differentiated, locally advanced or metastatic GEP-NETs. Novartis’ Lutathera® is another radioligand therapy using 177Lu-DOTATATE that was approved in January 2018 for the treatment of somatostatin receptor positive gastreoenteropancreatic neuroendocrine tumors in adults. Lutathera® received an orphan drug designation from the FDA and is the first available FDA-approved peptide receptor radionuclide therapy. Novartis reported sales of $471 million for Lutathera® in 2022. While these therapies have brought treatment options to people with GEP-NETs, the scope of the approved indications creates a significant need for a novel approach to treatments for people with non-GEP-NETs.
The options for people with non-GEP-NETs are more limited. Afinitor® (everolimus) is approved for lung NETs. If a lung NET is detected early enough it will be treated first with a somatostatin analogue ("SSA") and then a combination of SSA and Afinitor® upon progression. However, Afinitor® has a challenging side effect profile with 42% of participants with NETs in the pivotal trial experiencing serious adverse events, including death. Following treatment with SSA and Afinitor®, there is no clear treatment paradigm for lung NETs, leaving those individuals and their physicians choosing between clinical trials or chemotherapy. With lung NET cancers accounting for nearly 30% of all NETs, this represents a large unmet need.
POINT's Solution: 177Lu-PNT2003
177Lu-PNT2003, also known as 177Lu-octreotate ("177Lu-DOTATATE"), is a somatostatin-targeted radioligand therapy in development for the treatment of individuals with somatostatin receptor-positive neuroendocrine tumors, targeting both
13
GEP-NETs and non-GEP-NETs. 177Lu-PNT2003 is the subject of a completed multi-center trial in Canada (OZM-067; NCT02743741) sponsored by UHN and designed to evaluate 177Lu-PNT2003's safety and efficacy across individuals with NETs who have positive somatostatin receptor expression identified by 68Ga-DOTATATE PET.
POINT licensed worldwide rights to the clinical data and the intellectual property for the radioligand compound from CanProbe in December 2020. CanProbe’s intellectual property for 177Lu-PNT2003 covers methods of preparing, pharmaceutical compositions and methods of treatment of various indications including neuroendocrine tumors.
Although 177Lu-PNT2003 uses the same molecular entity as Novartis’ Lutathera®, 177Lu-PNT2003 uses a different type of 177Lu, called n.c.a. 177Lu. By using n.c.a. 177Lu, 177Lu-PNT2003 will not contain any metastable lutetium-177m ("177mLu"), an impurity introduced into Lutathera® via the production method of its carrier-added 177Lu. The introduction of the 177mLu impurity in Lutathera® is problematic for hospitals and clinics as 177mLu is a radionuclide with a half-life of 5+ months, which necessitates dedicated waste streams and other burdensome overhead for physicians and clinics. POINT believes 177Lu-PNT2003’s use of n.c.a. 177Lu will therefore provide a competitive advantage. 177Lu-PNT2003 could be further differentiated from Lutathera® in the indication it is targeting (non-GEP-NETs), which is not included in Lutathera’s current label and for which there are limited treatment options.
Clinical Development and Next Steps
177Lu-PNT2003 is currently the subject of a completed open-label, single-arm study designed to evaluate the safety and efficacy of 177Lu-PNT2003 across individuals with NETs who have positive somatostatin receptor expression identified by 68Ga-DOTATATE PET. The interim data received from UHN in December 2020 included 167 participants across GEP-NETs as well as neuroendocrine tumors of other tissue origins including lung, thyroid, adrenal, ovary, kidney, pituitary, and unknown origin, collectively referred to as non-GEP-NETs. The breakdown of participants was 33 non-GEP-NET participants and 134 GEP-NET participants. The last patient last dose for OZM-067 occurred in July 2021, all participants completed the primary follow-up in the second quarter of 2022, and long term follow-up is still ongoing.
In November 2022, POINT announced strategic collaboration and exclusive license agreements with Lantheus for exclusive worldwide rights for 177Lu-PNT2003, excluding certain territories (Japan, South Korea, Singapore, Indonesia, and China including Hong Kong, Macau and Taiwan). For 177Lu-PNT2003, POINT has received a $10 million upfront payment, and will receive up to an additional $30 million upon U.S. regulatory approval, royalties of 15% on net sales, as well as the potential for up to an additional $275 million in various net sales milestone payments. Joint steering committees have been formed to oversee the clinical studies, regulatory filings, manufacturing, and commercial readiness for 177Lu-PNT2003.
In late 2022, POINT received the OZM-067 clinical trial data sets from the trial sponsor. POINT will facilitate the analysis of the data sets. As per the strategic collaboration and exclusive license agreement, Lantheus will be responsible for all subsequent development of the asset post initial regulatory filings within the U.S., and for all development efforts in their territories.
PNT2004 Program
Background on Fibroblast Activation Protein-α and Fibroblast Activation Protein-α Inhibitors
An exciting development in cancer research has been the identification of fibroblast activation protein (FAP) as a potential target for imaging and treatment of various pathological conditions including fibrosis, arthritis and cancer. Several tumors, including breast, colon, prostate and pancreatic, develop a dense fibrous tissue comprised of CAFs around and within the tumor. CAFs can contribute up to 90% of the tumor mass. The CAFs of malignant tumors express FAP, a type II membrane-bound glycoprotein with peptidase activity. FAP is unique in that it is expressed in over 90% of epithelial cancers, but generally is not present on healthy cells. Given that FAP is expressed in the majority of cancers and that FAP-positive CAFs are involved in tumor progression and supporting an immunosuppressive tumor microenvironment, it is believed that targeting this protein is a potential strategy to effectively treat malignant tumors.
Initial imaging studies of 68Ga-FAP inhibitors (“FAPI”) PET/CT suggested that these tracers are suitable for diagnostic cancer solutions and radioligand therapies. It was determined that even lesions that were already unequivocally identified radiologically could be characterized in additional detail through FAPIs. A retrospective analysis reviewing the development of quinoline-based FAPIs, conducted by Kratochwil and published in the Journal of Nuclear Medicine in 2019, illustrated that the technology has promising clinical applications. The study concluded that several cancers, including lung, breast, esophagus, pancreatic, head-neck, and colorectal cancer, presented high uptake on FAPI. The over-expression of FAP in cancers which have limited treatment options and low five-year survival rates present a significant market opportunity in an area of unmet need.
14
POINT's Solution: PNT2004 Program
Most targeted radioligand therapies are designed to target a limited number of cancers by targeting a receptor that is present on tumor cells of a specific type of tissue, such as prostate, but absent in all other healthy tissues. As FAP is highly expressed on a wide range of solid tumors, a FAP-targeted radioligand offers the potential for a tissue agnostic opportunity that could enable the precise treatment of a variety of solid tumors independent of tissue origin. The PNT2004 program consists of a family of FAP-targeted radioligands invented by Dr. William Bachovchin at Tufts University. POINT licensed worldwide rights to this family of FAP-targeted radioligands from Bach Biosciences in April 2020.
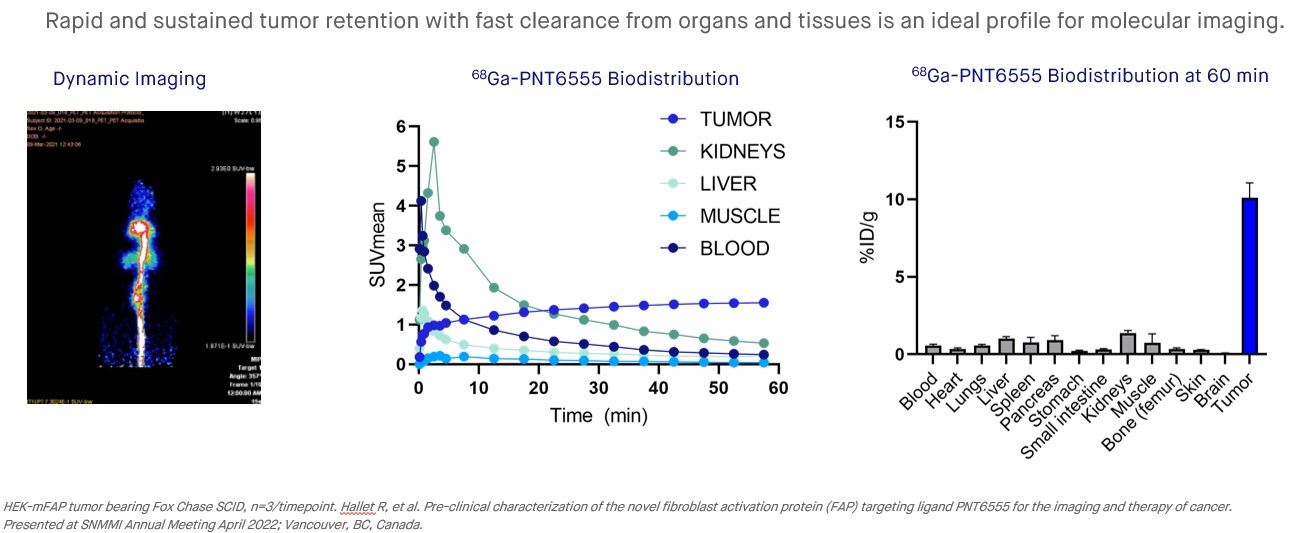
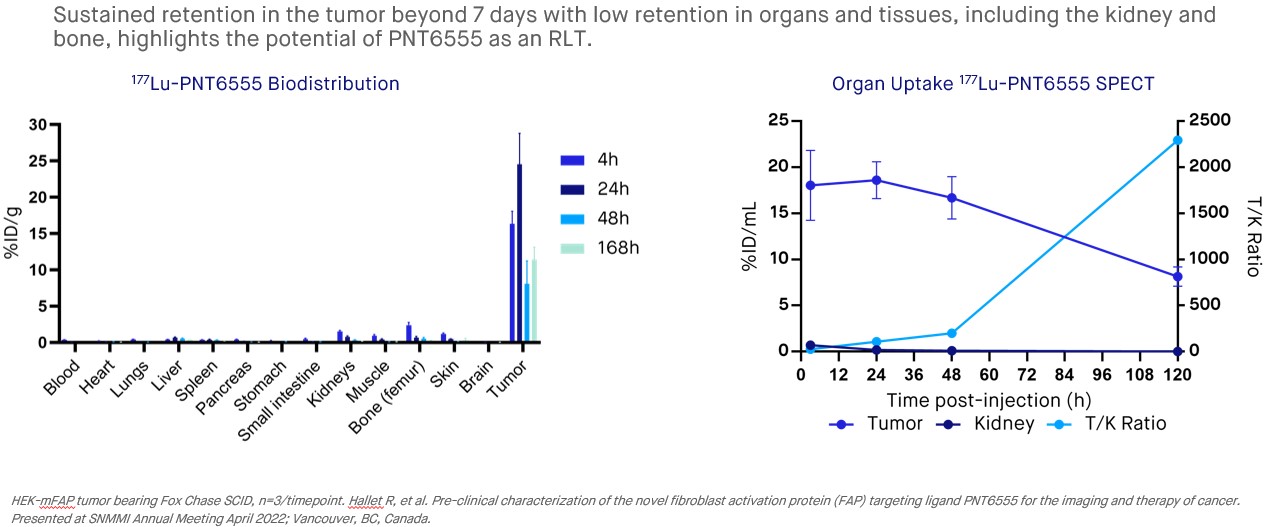
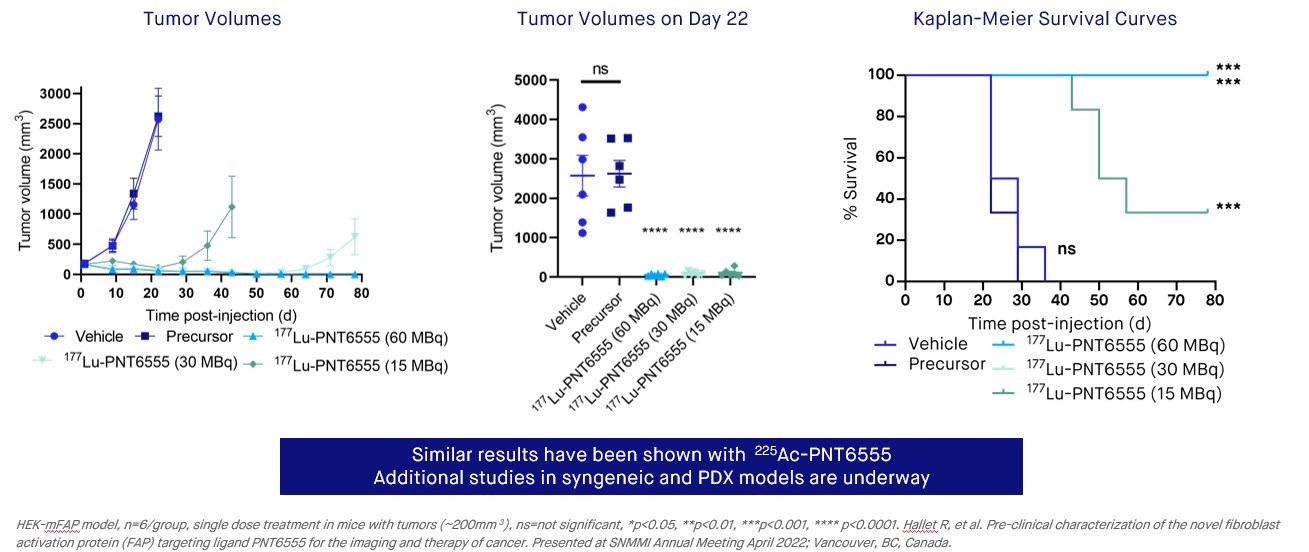
A clinical candidate, 177Lu-PNT6555, was identified from a screen of FAP inhibitors for our PNT2004 program. The clinical candidate was characterized in preclinical studies using Fox Chase SCID mice bearing HEK-mFAP tumor xenografts. In studies with 177Lu, the compound was found to have a significant amount of the injected dose accumulate in xenograft tissue (~16% of the injected dose/gram at 4 hours, %ID/g), with normal tissues showing little accumulation. After 24 hours the amounts of 177Lu-labeled compound in healthy tissue were less than 2% ID/g, with ~24% ID/g accumulating in xenograft tissue, and after 7 days ~11% ID/g remained in xenograft tissue. In survival studies, mice bearing tumors of approximately 200 mm3 were dosed with the 177Lu-labeled compound (15 MBq, 30 MBq and 60 MBq) or control. Anti-tumor activity was found at all tested dose levels and the response was dose-dependent. Complete regressions occurred in 6 of 6 mice in the 60 MBq cohort and 3 of 6 in the 30 MBq cohort. Prolonged survival was found in all dose cohorts versus the controls, with 100% survival in the 30 MBq and 60 MBq dose cohorts surviving until day 80.
15
Clinical Development and Next Steps
The Company completed a pre-CTA meeting with Health Canada in December 2021 regarding the development pathway and clinical study design for the phase 1 trial. A CTA with Health Canada was filed at the end of the first quarter of 2022 for PNT6555, and a No Objection Letter was received from Health Canada in May 2022. The phase 1 clinical trial is called FRONTIER: “FAPi Radioligand OpeN-Label, phase 1 Study to Evaluate Safety, Tolerability and DosImetry of [Lu-177]-PNT6555; A Dose Escalation Study for Treatment of Patients with Select Solid Tumors”.
In April 2022, preclinical data for the program was presented in a poster at the American Association for Cancer Research (AACR) 2022 Annual Meeting. The poster was titled “Preclinical Characterization of the Novel Fibroblast Activation Protein (FAP)-Targeting Ligand PNT6555 for the Imaging and Therapy of Cancer” (Abstract ID: 3554, Session: Preclinical Radiotherapeutics). The presented data concluded:
•In preclinical xenograft models: 68Ga-PNT6555 is an effective imaging agent, with strong tumor targeting, low background in normal tissues and rapid clearance via urinary excretion, and 177Lu-PNT6555 shows prolonged tumor retention out to 168 hours post-injection.
•Efficacy studies with 177Lu-PNT6555 or 225Ac-PNT6555 demonstrate compelling and dose-responsive inhibition of HEK-mFAP tumor growth.
FRONTIER commenced in July 2022 in Canada using a 68Ga-based PNT6555 molecular imaging agent to select participants to receive a n.c.a. 177Lu-based PNT6555 therapeutic agent. FRONTIER's clinical protocol evaluates PNT6555 in ~30 participants in five FAP-avid cancer indications: colorectal, pancreatic, esophageal, melanoma, and soft tissue sarcoma.
Dosing in the FRONTIER phase 1 trial started at 4 GBq, with each subsequent dose level increasing by 4 GBq, also allowing 2 GBq dose de-escalations. Each 177Lu PNT6555 dose is followed by a 6-week interval. 68Ga-PNT6555 PET/CT imaging is conducted approximately 90 minutes post injection, and dosimetry is assessed at the time of first 177Lu-PNT6555 dose and at each subsequent cycle. The primary objective of the study is to determine the maximum tolerated dose, and the recommended phase 2 dose.
In November, 2022, preclinical data supporting the combination of 177Lu-PNT6555 with immunotherapy was released via a press release. The preclinical study focused on assessing the potential of the lead candidate in the PNT2004 clinical program, 177Lu-PNT6555, in combination with anti-PD-1 immunotherapy. The CT26-mFAP tumor model used expresses low levels of FAP, grows aggressively, and is insensitive to anti-PD-1 immunotherapy. The study found that combination treatment with 177Lu-PNT6555 and anti-PD-1 resulted in a significant survival benefit, as compared to either treatment independently.
Enrollment in cohort 2 began in December 2022 and cohort 3 enrollment is expected to begin in the second quarter 2023. A total of six participants have been dosed with 177Lu-PNT6555 to date. We anticipate data from the full FRONTIER trial to be available in the first half of 2024.
16
PNT2001 — Next-generation PSMA-Targeted Radioligand Therapy
Background
POINT estimates that there were 269,000 new cases of prostate cancer in 2022. Of these, approximately 4% will be newly diagnosed with metastatic hormone-sensitive prostate cancer ("mHSPC"), another 34% will progress with biochemically recurrent disease and finally another 26% will have their case misdiagnosed from traditional imaging methods. Therefore, POINT estimates that nearly 40,000 individuals diagnosed in 2022 will require lifelong ADT. These individuals often live 15 years or more on androgen deprivation therapy and suffer with erectile dysfunction, loss of bone density and bone fractures, loss of muscle mass and physical strength, weight gain, mood swings and fatigue. There is therefore an unmet need in this population to delay the need for lifelong androgen deprivation therapy in prostate cancer.
PSMA is expressed in approximately 80% of prostate cancer tumors and is present at all stages of the disease. PSMA expression significantly increases in locally advanced cases, lymph node metastases, and distant metastases as compared to primary tumors. Given the expression of PSMA throughout all stages of the disease, there is potential for PSMA-targeting radioligands to help delay the need for ADT. Radioligand development programs that are in late-stage development, including 177Lu-PNT2002, may not be suitable for the treatment of mHSPC due to off-target accumulation in kidney which creates the potential for delayed renal toxicity which is more likely to manifest in this population given these individuals live for 15 years or more; therefore, there is an opportunity for next-generation PSMA-targeted radioligands with less off-target accumulation to fill this gap.
POINT's Solution: PNT2001 Program
PNT2001 is a next-generation PSMA radioligand family of drug candidates licensed from Scintomics GmbH which have an improved biodistribution profile compared to current-generation PSMA-targeted radioligands, such as 177Lu-PNT2002, and therefore has the potential to deliver increased efficacy and be used more broadly. As demonstrated in preclinical animal model studies, PNT2001 ligands have unique linker technology that enables increased tumor accumulation, potentially enabling lower doses of radioisotope to reduce off-target toxicity while allowing for the same level of tumor kill.
Preclinical studies have resulted in the identification of a lead candidate, 225Ac-PSMA-62, which, as compared to other late-stage PSMA-targeted radioligands, demonstrates potent anti-tumor activity using 225Ac, while also having an improved biodistribution profile. POINT has advanced 225Ac-PSMA-62 into IND-enabling studies with the aim of pursuing clinical development with 225Ac-PSMA-62 in the biochemically recurrent, oligometastatic, and post-177Lu-PSMA mCRPC settings.
Clinical Development and Next Steps
In October 2022, POINT published additional preclinical data for the PNT2001 program. The new data were shared at the 35th Annual Congress of the European Association of Nuclear Medicine (EANM) in Barcelona, Spain in E-poster #039, titled “Development and characterization of a next-generation 225Ac-PMSA radioligand”. An IND/CTA submission for 225Ac-PSMA-62 is projected for the second quarter of 2023, with the first participant expected for 225Ac-PSMA-62's phase 1 clinical trial by the end of 2023.
17
CanSEEKTM
18
Unlike POINT's other product candidates, CanSEEKTM is not a radioligand. It is a prodrug technology platform which can be applied to a wide variety of radioligands to refine their targeting precision. The goal of CanSEEKTM is to enable the creation of tumor-activated radioligand therapies, which have higher therapeutic indexes then their non-tumor-activated counterparts.
Background
One of the most commonly raised concerns about radiation therapy is that off-tissue delivery of radiation can damage otherwise healthy tissue. For example, with PSMA-targeted therapies, kidney and salivary gland uptake are of concern. These concerns are amplified when patient life expectancy is long, as the effects of radiation toxicity can take long periods of time to manifest. The risk of damaging healthy tissue may also increase as new higher energy radioisotopes like 225Ac are used, increasing the importance of off-target damage mitigation strategies.
POINT's solution: CanSEEKTM
Due to POINT's interest in introducing radioligand therapies earlier in the treatment setting with a wider variety of radioisotopes, the Company began investigating technologies that could be used to increase the therapeutic index of radioligands. These investigations lead to FAP-activated radioligands invented by Dr. William Bachovchin of Tufts University / Bach Biosciences, who had also previously invented a general FAP-activated prodrug technology to increase the therapeutic index of chemotherapy drugs like doxorubicin. Dr. Bachovchin’s technology had been licensed to Avacta, a U.K.-based life sciences firm, who subsequently branded the technology as pre|CISIONTM. POINT’s CanSEEKTM technology is the subject of licenses from both Bach Biosciences and Avacta.
CanSEEKTM could decrease the risks associated with off-target delivery of radioligands by limiting their ability to bind to receptors on healthy cells. The way CanSEEKTM achieves this is by using the presence of fibroblast-activation proteins, which are expressed in over 90% of epithelial cancers but not expressed in healthy cells, as the switch to activate the radioligands. The following image visually explains how CanSEEKTM works:
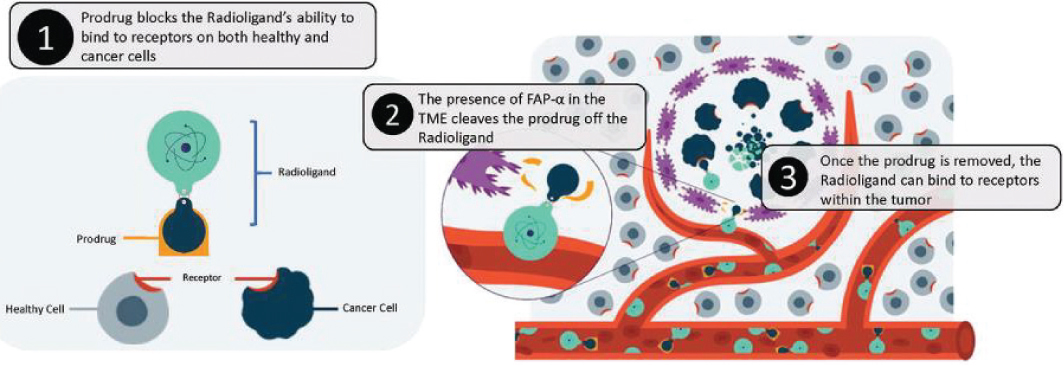
CanSEEKTM is first conjugated with the radioligand, which effectively blocks the radioligand from binding to receptors. The presence of FAP in the tumor microenvironment enables tumor-specific activation of the radioligand. Once activated, the radioligand can once again bind to cancer cell receptors. If the radioligand does not enter the tumor microenvironment, the radioligand is eliminated from the body without accumulating in off-target tissues expressing the receptor.
Preclinical Data and Next Steps
POINT has not published substantial preclinical data from CanSEEKTM. Multiple (d)-Ala-Pro substrate enabled-ligands are currently being studied preclinically against different targets.
POINT's Radioligand Platform: Manufacturing, Supply Chain, and Pipeline Expansion
Although radioligand therapies have existed for over 50 years, very few radioligand therapies are available today. POINT's founders believe the primary reason for this is that radioligands have very little in common with other drug classes, making it more difficult for traditional pharmaceutical companies to develop them.
19
POINT was founded to capitalize on this opportunity, by building a new pharmaceutical company focused on the unique requirements of radioligand development and commercialization.
Isotope Supply
The first of these unique requirements is access to rare medical isotopes. POINT has built one of the industry’s most resilient medical isotope supply chains, which includes both external supply partners as well as internal isotope manufacturing capabilities expected to commence by the end of 2023.
Manufacturing
The second requirement is the ability and licensing to handle and manufacture radioactive drugs. POINT owns and operates an 80,000 square foot radioligand manufacturing facility in Indianapolis, Indiana. In April 2021, the Nuclear Regulatory Commission issued a materials license for POINT's facility, and POINT began supplying doses to the SPLASH trial in January 2022. The location of the facility, near several high-volume transport hubs, such as FedEx’s hub for radioactive shipments, will enable our facility to service North America and the E.U., as well as many international destinations. POINT expects this facility will provide adequate production capacity to meet future commercial demands for their product candidates, if approved. POINT also relies, and expects to continue to relying, on third parties to package, label, store and distribute their investigational product candidates.
The specifications of the Indianapolis facility were designed to meet the future needs of the Company, and give POINT a unique competitive position in the industry. These design decisions include:
•Scale: The facility is one of the largest radioligand production facilities in the world. The facility has been designed in a modular fashion which enables additional production lines to be enabled only when needed. The large scale of POINT's facility also is expected to enable the implementation of a level of automation not commonly found in the radioligand industry, with the intention overtime to lower costs of production while simultaneously increasing the throughput of production.
•Isotope agnostic: Although POINT's pipeline of product candidates primarily uses the 177Lu radioisotope, their facility is designed and licensed to support a wide variety of radioisotopes, include betas, gammas and alphas such as 225Ac. External supply of 225Ac has been secured through the U.S. Department of Energy Isotope Program (managed by the Office of Isotope R&D and Production), TerraPower, Ionetix, and NorthStar Medical Radioisotopes.
•Internal isotope production: In addition to manufacturing the finished radioligand product, POINT's facility is designed to support manufacturing of radioisotopes in-house, for example purifying n.c.a. 177Lu from irradiated 176Yb. For 177Lu production specifically, POINT has already executed a supply agreement for 176Yb and licensed technology for the purification of n.c.a. 177Lu, as well as secured access to neutrons from nuclear reactors. The ability to produce Their own n.c.a. 177Lu is expected to both lower the cost of goods sold of POINT drugs, and further increase the resilience of the Company's supply chain. With internal isotope manufacturing capabilities, POINT intends to use in-house manufactured 177Lu isotopes, as well as externally sourced 177Lu, to ensure supply redundancy and uninterrupted supply for the manufacture of their radioligand therapies. External supply of 177Lu has been secured through ITM Medical Isotopes and Isotopia.
Pipeline Expansion
The final requirement is the technical expertise to develop new radioligands. Developing new radioligands is technically difficult - for example, the radiation emitted by the radioisotope can destroy the ligand. POINT has assembled a team of radioligand experts with experience in the discovery and development of new radioligands to further expand POINT's pipeline of product candidates. POINT is currently focused on expanding its pipeline by identifying and assessing novel ligands and working with new radioisotopes, as well as investigating combinations with other drug classes such as immune-oncology, DNA damage response inhibitors and chemotherapies.
Commercialization
None of POINT’s product candidates have received the regulatory approvals required to begin commercialization. In November 2022, POINT announced strategic collaboration and exclusive license agreements for the commercialization of 177Lu-PNT2002 and 177Lu-PNT2003 with Lantheus. If marketing approvals are obtained, Lantheus will commercialize both 177Lu-PNT2002 and 177Lu-PNT2003. In preparation, POINT and Lantheus have formed a joint steering committee to oversee the commercial readiness for both 177Lu-PNT2002 and 177Lu-PNT2003.
20
POINT intends to retain significant development and commercial rights to their other product candidates and, if marketing approval is obtained, to either commercialize their investigational products on their own or with a partner. The decision as to whether to commercialize a product candidate independently or with a partner will depend on several factors, such as the clinical data, the competitive landscape and the size of the addressable patient population, as well as the size of the commercial infrastructure and manufacturing requirements.
Competition
The biotechnology and pharmaceutical industries are characterized by the rapid advancement of novel technologies, intense competition, and a strong emphasis on intellectual property. While POINT believes that their technology and scientific expertise and manufacturing capabilities provide them with competitive advantages, their face potential competition from multiple sources, including large pharmaceutical companies, specialty pharmaceutical and biotechnology companies, academic institutions, government agencies and public and private research organizations.
Radioligands are being explored in several different settings, including both commercial and academic clinical trials. Results from these trials, combined with recent product approvals, have garnered continued interest in the space by both large pharmaceutical companies and specialized biotechnology companies, which are developing both early-stage and later-stage candidates.
Given the nature of POINT's product candidates, POINT considers our most direct competitors to be other companies focusing on beta-based radiopharmaceuticals, both in development and already approved. There are multiple companies, including Lantheus, Novartis AG, and Q BioMed Inc., with approved beta-based radiopharmaceutical products using isotopes such as 131I, 177Lu, 89Sr and 90Y. Novartis’ Lutathera® and Pluvicto® are prominent beta-based radioligands, and other beta-based radiopharmaceuticals are in various stages of clinical development by companies including Novartis AG, Curium SAS, Nordic Nanovector, Cellectar Biosciences, ITM Isotope Technologies Munich SE, Clovis Oncology and Y-mAbs Therapeutics, Inc., Actinium Pharmaceuticals, Inc., Lantheus, Blue Earth Therapeutics, and Clarity Pharmaceuticals.
There are also several companies developing alpha-based radiopharmaceuticals for the treatment of cancer, including Bayer AG, or Bayer, Novartis AG, or Novartis, Telix Pharmaceuticals Limited, Actinium Pharmaceuticals, Inc., RadioMedix, Inc., Orano Med, Viewpoint Molecular Targeting, Aktis Oncology, Fusion Pharmaceuticals, Inc., RayzeBio, Inc., Aktis Oncology, Inc., Convergent Therapeutics, Janssen, ARTBIO, and Curie Therapeutics, Inc. These companies use various alpha-emitting isotopes such as 223Ra, 225Ac, 212Pb and 227Th. Most alpha-based radiopharmaceuticals are in clinical development, with Bayer’s Xofigo® being the only approved alpha particle-based therapy. Xofigo® was approved in 2013 for the treatment of symptomatic bone metastases in people with castration-resistant prostate cancer.
For POINT's product candidates 177Lu-PNT2002 and 225Ac-PSMA-62 of the PNT2001 program, the Company is aware of several competing therapies targeting metastatic prostate cancer. POINT's closest approved competitor is Novartis’ Pluvicto®, which uses 177Lu for the treatment of late-stage metastatic castration-resistant prostate cancer and is currently approved for use in the U.S. and Europe. POINT is aware of the following companies with prostate cancer preclinical and clinical development programs: Janssen, CTT, Novartis, BlueEarth, Curium, Telix, and Lantheus. In addition, POINT expects to face indirect competition from established treatments of prostate cancer, including Jevtana® (Sanofi), Zytiga® & Erleada® (Johnson & Johnson), Xtandi® (Astellas Pharma and Pfizer), Lynparza® (AstraZeneca), docetaxel, and Xofigo® & Nubeqa® (Bayer). While POINT believes 177Lu-PNT2002 and 225Ac-PSMA-62 could have significant competitive advantages compared to established treatments for prostate cancer (such as being used at different stages of disease), the Company may still face competition from these more established treatments.
For POINT's product candidate 177Lu-PNT2003, the company is aware of several competing therapies targeting neuroendocrine tumors. Novartis’ Lutathera®, which was approved in 2018, uses 177Lu for the treatment of individuals with somatostatin receptor-positive gastroenteropancreatic neuroendocrine cancers. The Company is aware of the following companies with neuroendocrine tumor, radioligand preclinical and clinical development programs: ITM, RayzeBio, Inc and Radiomedix. POINT also faces potential competition from other treatments targeting neuroendocrine tumors such as Sandostatin® and Afinitor® (Novartis), Somatuline® (Ipsen) and Sutent® (Pfizer). While POINT believes 177Lu-PNT2003 has significant advantages (such as its usage of n.c.a. 177Lu) compared to conventional approaches to neuroendocrine tumors, the Company may still face competition from these more established treatments.
For POINT's product candidates 68Ga-PNT6555 and 177Lu-PNT6555, of the PNT2004 program competitive threats include those candidates that are in development or currently approved for whatever indications that POINT's product candidates may be developed. FRONTIER's clinical protocol evaluates 177Lu-PNT6555 in ~30 participants in five FAP-avid cancer indications: colorectal, pancreatic, esophageal, melanoma, and soft tissue sarcoma. There are other early-stage radioligand programs targeting FAP, including Clovis Oncology, Novartis, Philogen Spa and SOFIE, which may directly
21
compete with POINT's candidate as either an imaging agent and/or therapeutic agent. However, given the early development stage of these product candidates, it is unclear whether they will be in direct competition with the product candidates from the PNT2004 program, 68Ga-PNT6555 and 177Lu-PNT6555.
CanSEEKTM, POINT's tumor microenvironment targeting prodrug platform, is too early-stage to identify direct competitors. While there are some companies such as Fusion Pharmaceuticals with early-stage technologies that hope to enhance the safety of actinium-based radioligands, the goals of our tumor microenvironment targeting prodrug platform extend beyond 225Ac-focused programs to increase the therapeutic index of radioligands regardless of the isotope used.
Many of the companies that POINT is competing against have significantly greater financial resources and expertise in research and development, manufacturing, and marketing approved products than POINT does. Furthermore, the merger and acquisition or other strategic transactions involving these companies may result in resources being concentrated among a small number of POINT's competitors. If POINT's competition develops a product that is safer or more effective than theirs or brings a product to market faster than they can, POINT could see a negative impact on their commercial success. The key competitive factors affecting the success of the Company's product candidates, if approved, are their efficacy, safety, convenience for doctors and patients, price, competitive branded and generic products, and reimbursement from government and other third-party payors.
Intellectual Property
POINT's intellectual property is critical to our business and our success depends, in large part, on our ability to maintain and develop a robust intellectual property portfolio for our technology and product candidates, as well as on our ability to defend and enforce our intellectual property rights. We generally seek to protect our product candidates and technology by seeking patent protection that would enhance clinical development and commercial success. We intend to continue relying upon patent protection, exclusivity pursuant to the Hatch-Waxman Act, trade secrets, know-how, continuing technological innovations and licensing opportunities.
As of December 31, 2022, our patent portfolio of owned and exclusively licensed patent filings included ten different patent families with pending and issued patents in the U.S. and other major markets, with several of the existing patent families still affording additional opportunities for worldwide filings. Our portfolio includes both in-licensed and internally developed intellectual property to protect our programs. Estimated expiry dates for our patent portfolio range from 2038 to 2043, with additional potential patent term adjustment and patent term extensions available in major markets and various other jurisdictions to extend the duration of patent protection.
PNT2002 Program
POINT has a patent portfolio of (currently) 26 pending and issued patents covering 177Lu-PNT2002, which includes two issued U.S. Patents and filings in another 21 countries including in the major market countries of North and South America, Asia and Europe (in the form of an European Patent Office ("EPO") application which if granted can later be activated across Europe and the U.K.). Our issued U.S. Patents cover our proprietary method for manufacturing 177Lu-PNT2002 providing high purity and extended shelf life, as well as our proprietary formulations of 177Lu-PNT2002. We intend to pursue the same or similar claims in the other countries in which the patent application has been filed. This family of patent applications also disclose additional patentable subject matter including to pharmaceutical compositions, methods of treatment and the process for production and formulation of 177Lu-PNT2002 to achieve such high purities and stability for up to five days. The patents from this family are expected to expire in July 2041, with any available patent term extensions (if applicable) added to that date.
PNT2004 Program
POINT obtained an exclusive sublicense from Bach Biosciences, to the rights in a patent portfolio currently including 29 pending patent applications covering the PNT2004 family of FAPi-targeted radioligands. This portfolio includes several pending U.S. filings and filings in another 27 countries including in the major market countries of North and South America, Asia and Europe (in the form of an EPO application which if granted can later be activated across Europe and the U.K.). The licensed patent portfolio discloses and claims (as new chemical entities) small-molecule radioligand and imaging agents based on a FAP-specific inhibitor. The pending patent applications cover, in addition to the new compositions-of-matter, pharmaceutical compositions, and methods for diagnosing, imaging or treating/reducing tissue over-expressing FAP in an animals (including humans). The patents from this family are expected to expire in March 2041, with any available patent term extensions (if applicable) added to that date.
PNT2003 Program
22
POINT obtained an exclusive license from CanProbe, Centre for Probe Development and Commercialization ("CPDC") and The University Health Network ("UHN") to the rights in a patent portfolio currently including 24 pending and issued patents covering 177Lu-PNT2003, which includes an issued U.S. Patent and filings in another 21 countries including in the major market countries of North and South America, Asia, Middle East and the E.U. (in the form of an EPO application which, if granted, can later be activated across Europe and the U.K.). Our issued U.S. Patent covers our proprietary formulation of 177Lu-PNT2003, which provides for a high purity and extended shelf life of the product. We intend to pursue the same or similar claims in the other countries in which the patent application has been filed. This family of applications includes additional support for patentable subject matter relating to the radioligand 177Lu-PNT2003 compound, including relating to methods of preparing the compound, pharmaceutical compositions and methods of treatment of various indications including neuroendocrine tumors using 177Lu-PNT2003. The patents from this family are expected to expire in August 2041, with any available patent term extensions (if applicable) added to that date.
PNT2001 Program
POINT obtained an exclusive license from Scintomics GmbH to a patent portfolio covering the new chemical entity underlying the PNT2001 program. This family of patents has nationalized and the license to POINT is for major markets including the U.S., Canada, the E.U. and Australia, as well as other countries. The patents cover, as new chemical entities, new amide-based radioligand compounds which bind PSMA and target PSMA-expressing tumor cells. In addition to the composition-of-matter claims, the compounds are disclosed and claimed for use in pharmaceutical or diagnostic compositions for diagnosing and/or treating cancer. The compounds may also be used for imaging and performing endoradiotherapy in diseases involving PSMA. The patents in this family will expire in December 2038, with any available patent term extensions (if applicable) added to that date.
CanSEEKTM Tumor Microenvironment-Targeting Technology Platform
POINT obtained an exclusive sublicense from Bach Biosciences to a patent portfolio of 11 (currently) pending applications. The portfolio has been filed in major market jurisdictions including the U.S., Canada, Japan, China, South Korea, Israel and Europe (in the form of an EPO application which, if granted, can later be activated across Europe and the U.K.). The pending applications cover FAP-activated radiotheranostics that enable the delivery of radiodiagnostics and radiotherapeutics selectively to the tumor microenvironment for tumors expressing the FAP enzyme. This includes radiotherapeutics designed to target other molecules or receptors in the tumor microenvironment, such as PSMA. The pending patent application also discloses FAP-activated theranostic prodrugs, compositions comprising them, and a method of treating a disorder characterized by FAP up-regulation. The patents from this family are expected to expire in December 2041, with any available patent term extensions (if applicable) added to that date.
POINT also has a non-exclusive sublicense from Avacta to a patent portfolio directed to FAP-prodrugs generally (originally licensed from Bach Biosciences to Avacta) which includes pending patent applications in major markets, and issued patents in the U.S. That license, while a freedom-to-operate license for FAP-activated radioligands generally, does include a grant of an exclusive license to POINT for FAP-activated PSMA radioligands.
License Agreements
Strategic Collaboration and License Agreements for the Commercialization of 177Lu-PNT2002 and 177Lu-PNT2003
In November 2022, POINT announced strategic collaboration and exclusive license agreements for the commercialization of 177Lu-PNT2002 and 177Lu-PNT2003 with Lantheus. In exchange for Lantheus receiving exclusive worldwide rights, excluding certain territories (Japan, South Korea, Singapore, Indonesia, and China including Hong Kong, Macau and Taiwan), for 177Lu-PNT2002, POINT received a $250 million upfront payment. POINT will receive an additional payment up to $250 million upon U.S. regulatory approval, and, once certain return on investment financial thresholds have been achieved and other conditions met, royalties of 20% on net sales, as well as the potential for up to an additional $1.3 billion in various net sales milestone payments. For 177Lu-PNT2003, POINT received a $10 million upfront payment and will receive up to an additional $30 million upon U.S. regulatory approval and royalties of 15% on net sales, as well as the potential for up to an additional $275 million in various net sales milestone payments.
Lantheus and POINT will work together to prepare and Lantheus will submit the regulatory filings in the U.S. Under the Lantheus License Agreements, Lantheus will be responsible for all subsequent development of the assets post initial regulatory filings within the U.S., and for all development efforts in their territories.
In December 2022, closing conditions for the transaction, including Hart-Scott-Rodino antitrust clearance, were met and POINT received a total of $260 million in upfront payments prior to the end of 2022.
23
License Agreement with Bach Biosciences for PNT2004
In April 2020, we entered into a sublicense and collaboration agreement with Bach Biosciences to develop and commercialize radioligands, as amended April 14, 2020, January 5, 2021, September 24, 2021 and May 6, 2022 (collectively the “BACH Agreement”). The BACH Agreement grants to POINT Biopharma Inc. an exclusive, sublicensable, worldwide license under Bach Biosciences patent rights to use, develop, manufacture and commercialize any products arising from the licensed technology. Pursuant to the amendment dated September 24, 2021 (the "Third Amendment"), POINT Biopharma Inc. exercised its option (the “Commercialization Option”) under the BACH Agreement to acquire a worldwide exclusive, royalty-bearing license to commercialize any products and processes from uses of patent rights for FAP-targeted radioligands. The Third Amendment also amended the Sublicense Agreement to provide the Company with the first option (the “Invention Option”) to acquire a worldwide exclusive royalty-bearing license to Bach Biosciences’s patent rights, materials and know-how with respect to new inventions directed to FAP-targeted radioligands. As partial consideration for the exercise of the Commercialization Option and the grant of the Invention Option under the Third Amendment, POINT Biopharma Inc. paid, upon execution of the Sublicense Agreement, an option exercise fee of $3.3 million. Pursuant to the amendment dated May 6, 2022, (the “Fourth Amendment”), the Company and Bach Biosciences agreed to remove all regulatory and sales milestones which would have been payable from the Company to Bach Biosciences, as well as to reduce the royalty rate payable by Company to Bach Biosciences by fifty percent (one-half). In signing the Fourth Amendment, the Company agreed to pay a one-time amendment fee to Bach Biosciences of $2.0 million, and to extend the duration of the Company’s sponsored research relationship relating to the generation of new FAPi-targeting drugs with Bach Biosciences and Tufts Medical School by an additional two (2) years at the current sponsorship rate. We are also obligated to pay a low-teens percentage royalty related to the annual net sales of each licensed products or licensed process covered by a valid claim, but reduced to a single digit percentage royalty related to net sales in the absence of a valid claim by us and any of its affiliates and sublicensees based on its global sales. Royalties will be paid by POINT on a country-by-country basis beginning upon the first commercial sale in such country. There is also an additional low- teens to mid-twenties percentage sublicense fee payable to Bach Biosciences for monetary payments arising from a grant of a sub-license to a sub-licensee or in the form of other benefits, depending on the specified development stage of the product.
We have the right to terminate the BACH Agreement, upon 90 days' prior written notice to Bach Biosciences. If POINT or Bach Biosciences fails to comply with any of its respective obligations or otherwise breaches the agreement, the other party may terminate the agreement. The BACH Agreement expires upon the cessation of commercialization of the last licensed product by us. During the year ended December 31, 2022, we have made a payment to Bach Biosciences of $2.0 million related to the Fourth Amendment fee discussed above.
Concurrently in April 2020, we entered into a research agreement with Bach Biosciences for a period of five years where Bach Biosciences is contracted to perform research on our behalf, with respect to the BACH Agreement. Under the research agreement we will make payments to Bach Biosciences in accordance with an agreed upon payment schedule, upon which any sums payable will be credited against the option exercise fee under the sublicense agreement. During the year ended December 31, 2022, we made payments in connection with the sponsored research agreements in the amount of $1.8 million.
License Agreement with CanProbe for PNT2003
In December 2020 we entered into a license agreement with CanProbe (“CanProbe Agreement”). Under the CanProbe Agreement, we were granted an exclusive, sublicensable, worldwide license under CanProbe’s patent rights to use, develop, manufacture and commercialize any products arising from the patent. We are obligated to make aggregate milestone payments to CanProbe of up to $2.4 million ($3.3 million CAD) upon the achievement of receiving marketing authorization milestones for specified territories. On November 8, 2022, POINT entered into a first amendment to the CanProbe Agreement, between the POINT; CanProbe; CPDC; and UHN, collectively the (“Parties”) pursuant to which certain amendments have been made to our royalty obligations. We are obligated to pay a single digit royalty related to the annual net sales by us and any of our affiliates and sublicenses. We will pay royalties on a country-by country basis beginning upon the first commercial sale in such country. There is also an additional low-teens percentage fee payable to CanProbe for monetary payments arising from a grant of a sublicense to a sublicensee or in the form of other benefits. In the event it is necessary for us or our sublicensees to sell the product in a sub-territory or to obtain a license and to pay royalties to one or more third parties on net-sales, and if the aggregate royalty burden payable is greater than a high single digit percent of net-sales, then we may reduce the royalty fees or sub-licensing fees for sales of such product by 50% of royalties actually paid to the third party on net sales of the product in the territory in the same royalty period.
We have the right to terminate the CanProbe Agreement, upon 90 days' prior written notice. CanProbe may also terminate the agreement for specified territories under certain conditions. If POINT or CanProbe fails to comply with any of its obligations or otherwise breaches the agreement, the other party may terminate the agreement. During the year ended December 31, 2022, we paid $1.0 million in royalty payments in connection with this agreement
24
License Agreement with Scintomics GmbH for PNT2001
In November 2019, we entered into a sublicense agreement with Scintomics GmbH (“SCI Agreement”). Under the SCI Agreement, we were granted an exclusive, sublicensable, worldwide (other than the Middle East and Asia) license under SCI's patent rights to use, develop, manufacture and commercialize any products arising from SCI's patent rights related to PSMA ligands for imaging and endoradiotherapy. Under the SCI Agreement, we are obligated to make aggregate milestone payments to SCI of up to $25.4 million (€23.5 million) upon the achievement of specified development and regulatory milestones. We are also obligated to pay a low-teens percentage royalty related to the annual net sales by us and any of our affiliates and sublicensees. We will pay royalties on a country-by-country basis beginning upon the first commercial sale in such country. There is also an additional low thirties percentage fee payable to SCI for monetary payments arising from the grant of a sublicense to a sublicensee or in the form of other benefits
We have the right to terminate the SCI Agreement, subject to five months’ prior notice. If POINT or SCI fails to comply with any of its respective obligations or otherwise breaches the agreement, the other party may terminate the agreement upon written notification. During the year ended December 31, 2022, we made a payment to SCI of approximately $1.1 million upon the achievement of a specified development milestone.
Second License Agreement with Bach Biosciences for Tumor Microenvironment Targeting Technology
In December 2020, we entered into a sublicense and collaboration agreement with Bach Biosciences to develop and commercialize synthetic compounds that leverage a proprietary technology platform (“Second BACH Agreement”). Under the Second BACH Agreement, we were granted an exclusive, sublicensable and worldwide license under Bach Biosciences’s patent rights to use, develop, manufacture and commercialize any products arising from the patent related to the synthetic compounds. We are obligated to make aggregate milestone payments to Bach Biosciences of up to $3.0 million for the first product developed, upon the achievement of specified development and regulatory milestones and of up to $45.0 million upon the achievement of specified sales milestones. For subsequent products, we are obligated to make a milestone payment to Bach Biosciences of up to $1.0 million for major market regulatory approval and of up to $45.0 million upon the achievement of specified sales milestones. We are also obligated to pay a low-teens percentage royalty related to net sales of each licensed product or licensed process covered by a valid claim, but reduced to a single digit percentage royalty related to net sales in the absence of a valid claim. Royalty payments will be reduced in an amount equal to 100% of royalty fees paid to Avacta for the same licensed product. We will pay royalties on a country-by-country basis beginning upon the first commercial sale in such country. There is also an additional low- teens to mid-twenties percentage sublicense fee payable to Bach Biosciences for monetary payments arising from a grant of a sub-license to a sub-licensee or in the form of other benefits, depending on the specified development stage of the product.
We have the right to terminate the Second BACH Agreement upon 90 days' prior written notice to Bach Biosciences. If we fail or Bach Biosciences fails to comply with any of its obligations or otherwise breaches the agreement, the other party may terminate the agreement. The Second BACH Agreement expires upon the cessation of commercialization of the last licensed product by us. The royalty term will expire on a licensed product-by-licensed product or licensed process-by-licensed process and country-by-country basis, the time period commencing on the first commercial sale of such licensed product or licensed process in such country and continuing until the later of (i) the expiration of the last to expire valid claim covering the licensed product or licensed process in such country, or (ii) 10 years after the first commercial sale of the licensed product or licensed process in such country.
On January 22, 2021, we entered into a research agreement with Bach Biosciences for a period of three years under which Bach Biosciences is contracted to perform research on our behalf, with respect to the Second BACH Agreement. During the year ended December 31, 2022, we made payments in connection with the sponsored research agreement in the amount of $1.0 million.
License agreement with Avacta for Tumor Microenvironment Targeting Technology
In December 2020, we entered into an agreement with Avacta (the "Avacta Agreement") which is directly related to the Second BACH Agreement above. Under the Avacta Agreement, we became a sublicensee of Avacta’s license for using the intellectual property of developing and marketing FAP-activated radioligand agents. Under this agreement, we obtained an exclusive license of Avacta’s patent rights to use, develop, manufacture and commercialize any products arising from the patent.
We are further obligated to make aggregate milestone payments to Avacta of up to $4.5 million, upon the achievement of specified development milestones for our first product and up to $3.0 million each for any additional products developed with the technology upon reaching the specified development milestones. In addition, we are obligated to pay a milestone payment of $5.0 million for each product for the regulatory milestone being approved in specified territories. We are also
25
obligated to pay a single digit percentage royalty (subject to a reduction on certain conditions) related to the annual net sales by us, our affiliates or our sublicensees for each licensed product or license process and a single digit percentage royalty on a specified product arising out of the patents. The royalty rate will be reduced by 50% for net sales occurring in the U.S. if there is no valid claim at the time of sale. There is also an additional single digit percentage fee payable to Avacta for monetary payments arising from a grant of a sublicense to a sublicensee or in the form of other benefits. We are responsible for all costs and expenses incurred related to the development, manufacture, regulatory approval and commercialization of all licensed products.
We have the right to terminate the Avacta Agreement, subject to 90 days' prior notice to Avacta. If POINT or Avacta fails to comply with any of its respective obligations or otherwise breaches the agreement, the other party may terminate the agreement. The Avacta Agreement will expire on a licensed product-by-licensed product or licensed process-by-licensed process and country-by-country basis, upon the expiration of the royalty term in such country. The royalty term will expire on a licensed product-by-licensed product or licensed process-by-licensed process and country-by-country basis, with the royalty term commencing on the first commercial sale of such licensed product or licensed process in such country and continuing until the later of (i) the expiration of the last to expire valid claim covering the licensed product or licensed process in such country, or (ii) 10 years after the first commercial sale of the licensed product or licensed process in such country. During the year ended December 31, 2022, we did not make any payments to Avacta in connection to this license agreement.
License agreement with Belgian Nuclear Research Centre
On June 30, 2021, we entered into a license agreement (the “SCK-CEN Agreement”) with the Belgian Nuclear Research Centre (“SCK-CEN”). Under the SCK-CEN Agreement, we were granted a worldwide, royalty-bearing, non-exclusive, sublicensable license under SCK-CEN’s patent rights to develop, make, have made, use and import n.c.a. 177Lu using SCK-CEN Technology. We are obligated to make aggregate milestone payments to SCK-CEN of up to $0.1 million (€0.1 million) upon the achievement of certain technology implementation milestones. We are also obligated to make aggregate minimum royalty payments of $7.6 million (€7.1 million) over the course of eight years commencing in 2023 with an annual cap of €6.3 million over the same term. During the year ended December 31, 2022, we paid $34.0 thousand upon the achievement of specified development milestones in connection to this license agreement.
Business Combination
On June 30, 2021 (the “Closing Date”), POINT consummated a business combination transaction (the “Business Combination”) with Therapeutics Acquisition Corp., d/b/a Research Alliance Corp. I, a Delaware corporation (“RACA”), pursuant to the terms of the Business Combination Agreement, dated as of March 15, 2021 (the “Business Combination Agreement”), by and among RACA, Bodhi Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of RACA (“Merger Sub”), and POINT Biopharma Inc. Pursuant to the Business Combination Agreement, on the Closing Date, (i) Merger Sub merged with and into POINT Biopharma Inc. (the “Merger”), with POINT Biopharma Inc. as the surviving company in the Merger as a wholly-owned subsidiary of RACA and (ii) RACA changed its name to “POINT Biopharma Global Inc.”
In accordance with the terms and subject to the conditions of the Business Combination Agreement, at the effective time of the Merger (the “Effective Time”), (i) each share and vested equity award of POINT Biopharma Inc. outstanding as of immediately prior to the Effective Time was exchanged for shares of the Common Stock or comparable vested equity awards that are exercisable for shares of Common Stock, as applicable, based on an implied POINT Biopharma Inc. vested equity value of $585 million (which results in a conversion ratio of approximately 3.59:1); (ii) all unvested equity awards of POINT Biopharma Inc. were exchanged for comparable unvested equity awards that are exercisable for shares of Common Stock, determined based on the same exchange ratio at which the vested equity awards were exchanged for shares of Common Stock; and (iii) each share of Class A common stock, par value $0.0001 per share, of RACA (“Class A Common Stock”) and each share of Class B common stock, par value $0.0001 per share, of RACA (“Class B Common Stock”) that was issued and outstanding immediately prior to the Effective Time became one share of Common Stock following the consummation of the Business Combination.
In addition, concurrently with the execution of the Business Combination Agreement, on March 15, 2021, RACA entered into subscription agreements (the “Subscription Agreements”) with certain investors (the “PIPE Investors”), pursuant to which the PIPE Investors agreed to subscribe for and purchase, and RACA agreed to issue and sell to the PIPE Investors, an aggregate of 16,500,000 shares of Class A Common Stock at a price of $10.00 per share, for aggregate gross proceeds of $165 million (the “PIPE Financing”). The PIPE Financing was consummated concurrently with the closing of the Business Combination. We received net proceeds of approximately $260.0 million consisting of proceeds of the PIPE
26
Financing and the proceeds remaining in RACA’s trust account. Transaction costs of approximately $27.0 million consisted of investment banker, legal, audit, tax, accounting, consulting, insurance, board retainer fees and listing fees.
Government Regulation
Government authorities in the U.S. at the federal, state and local level extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug products, such as those we are developing and any other product candidates we may develop. These laws and regulations include, but are not limited to the Comprehensive Environmental Response, Compensation, and Liability Act (“CERCLA”), which imposes strict, joint and several liability on current and former owners and operators of sites and on persons who disposed of or arranged for the disposal of hazardous substances found at such sites, including releases of radioactive materials, regardless of the lawfulness of the original activities that led to the contamination, the Low-level Radioactive Waste Policy Act ("LLRW Policy Act"), which requires the safe disposal of mildly radioactive materials that cannot be decayed in storage, U.S. Nuclear Regulatory Commission ("NRC") regulations concerning various irradiated and radioactive, materials, and health regulations from the U.S. Occupational Safety and Health Administration, which limit exposures to hazardous substances, including radioactive materials, in the workplace and impose various worker safety requirements.
We, along with third-party contractors, are also required to comply with the various preclinical, clinical, and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of the PNT2001, PNT2002, PNT2003, and PNT2004 programs, or CanSEEKTM or any future drug candidate. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and effectiveness must be obtained, organized into a format specific for each regulatory authority, submitted for review and approved by the regulatory authority.
FDA Drug Development and Approval Process
In the U.S., the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations. Drugs also are subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state and local statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with applicable U.S. requirements at any time during the product development process, approval process or post-market may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, product recalls or market withdrawals, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
Our product candidates and any future drug product candidates we may develop must be approved by the FDA through a new drug application (“NDA”), the vehicle through which drug sponsors formally propose that the FDA approve a new drug for sale and marketing in the U.S. NDAs can be divided into the following four categories:
1.A standalone NDA is an application submitted under section 505(b)(1) and approved under section 505(c) of the FDCA that contains full reports of investigations of safety and effectiveness that were conducted by or for the applicant or for which the applicant has a right of reference or use.
2.A 505(b)(2) application is an NDA submitted under section 505(b)(1) and approved under section 505(c) of the FDCA that contains full reports of investigations of safety and effectiveness, where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use.
3.An abbreviated new drug application ("ANDA") is an application submitted and approved under section 505(j) of the FDCA for a drug product that is a duplicate of a previously approved drug product. An ANDA relies on FDA’s finding that the previously approved drug product, i.e., the reference listed drug (RLD) is safe and effective. An ANDA generally must contain information to show that the proposed generic product (1) is the same as the RLD with respect to the active ingredient(s), conditions of use, route of administration, dosage form, strength, and labeling (with certain permissible differences) and (2) is bioequivalent to the RLD. An ANDA may
27
not be submitted if clinical investigations are necessary to establish the safety and effectiveness of the proposed drug product.
4.A petitioned ANDA is a type of ANDA for a drug product that differs from the RLD in its dosage form, route of administration, strength, or active ingredient (in a product with more than one active ingredient) and for which FDA has determined, in response to a petition submitted under section 505(j)(2)(C) of the FDCA (suitability petition), that studies are not necessary to establish the safety and effectiveness of the proposed drug product. A petitioned ANDA is generally expected to provide the same therapeutic effect as the listed drug that was relied on as the basis of the suitability petition.
There are other preliminary steps that must be conducted prior to the submission of a NDA. The FDA review and approval process for drugs generally involves the following:
•completion of extensive preclinical studies in accordance with applicable regulations, including studies conducted in accordance with good laboratory practices (“GLP”) requirements;
•submission to the FDA of an IND application, which must become effective before human clinical trials may begin and must be updated annually or when significant changes are made;
•approval by an Institutional Review Board (“IRB”) or independent ethics committee at each clinical trial site before each trial may be initiated;
•performance of adequate and well-controlled human clinical trials in accordance with applicable IND regulations, good clinical practice (“GCP”) requirements and other clinical trial-related regulations to establish the safety and effectiveness of the investigational drug for each proposed indication;
•preparation and submission of the NDA to the FDA after completion of all pivotal clinical trials that includes substantial evidence of safety and effectiveness from results of nonclinical and clinical trials, and satisfactory completion of an FDA Advisory Committee review, if applicable;
•a determination by the FDA within 60 days of its receipt of the NDA to accept the filing for review;
•satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities where the drug will be produced to assess compliance with current good manufacturing practices (“cGMP”) requirements;
•potential FDA audit of the clinical trial sites that generated the data in support of the NDA; and
•FDA review and approval of the NDA, including (if deemed necessary by FDA) consideration of the views of any FDA Advisory Committee, prior to any commercial marketing or sale of the drug for the intended indications in the U.S.
The preclinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, or at all.
Preclinical Studies, Development and IND Submission
Preclinical studies include laboratory evaluation of product chemistry and formulation, as well as in vitro and animal studies to assess the potential for adverse events and in some cases to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations for safety/toxicology studies.
An IND sponsor must submit the results of its preclinical studies, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical trials, among other things, to the FDA as part of the IND. An IND is a request for authorization from the FDA to administer an investigational product to humans, and must become effective before human clinical trials may begin. Some long-term preclinical testing may continue after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless, before that time, the FDA raises concerns or questions related to one or more proposed clinical trials and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
28
Clinical Trials
Clinical trials involve the administration of the investigational drug to humans under the supervision of qualified investigators, who are generally physicians not employed by or under the trial sponsor’s control, in accordance with GCP and regulations governing the protection of human research participants. All research participants must provide voluntary informed consent for their participation in any clinical trial.
Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, participant selection and exclusion criteria and the parameters to be used to monitor participant safety and assess efficacy. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed and approved by an IRB for each institution at which the clinical trial will be conducted to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial participant or his or her legal representative, and must monitor the clinical trial until completed. The IRB is responsible for ensuring that human participants' rights and privacy are maintained. Regulatory authorities, the IRB, or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board (“DSMB”), which provides authorization for whether or not a study may move forward at designated check-points based on access to certain data from the study. The DSMB may halt the clinical trial if it determines that there is an unacceptable safety risk for participants or other grounds, such as no demonstration of efficacy. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries.
A sponsor who wishes to conduct a clinical trial outside of the U.S. may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of an NDA. The FDA will accept a well-designed and well-conducted foreign clinical trial not conducted under an IND if the study was conducted in accordance with GCP requirements, and the FDA is able to validate the data through an onsite inspection (if deemed necessary).
Clinical trials generally are conducted in three sequential phases, known as phase 1, phase 2 and phase 3. Trials and phases may overlap or be combined.
•Phase 1 clinical trials generally involve a small number of healthy volunteers or disease-affected individuals who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, side effect tolerability and safety of the product candidate.
•Phase 2 clinical trials involve studies in disease-affected individuals to determine the dose required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, possible adverse effects and safety risks are identified and a preliminary evaluation of efficacy is conducted. For oncologic diseases with high unmet medical need phase 2 trials with primary endpoints considered a validated surrogate for clinical effectiveness may support accelerated approval. Multiple phase 2 clinical trials may be conducted to obtain information prior to beginning larger, confirmatory phase 3 clinical trials.
•Phase 3 clinical trials generally involve a large number of disease-affected individuals at multiple sites and are designed to provide the statistically significant data necessary to demonstrate or confirm the effectiveness of the product candidate for its intended use, to demonstrate or confirm its safety in use, to establish the overall benefit/risk relationship of the product candidate and to provide an adequate basis for product labeling.
Post-approval trials, sometimes referred to as phase 4 clinical trials, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of people in the intended therapeutic indication. In some cases, the FDA may require phase 4 clinical trials as a condition for approval of the NDA.
Progress reports detailing the results of the clinical trials, among other information, must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected suspected adverse events, findings from other studies or animal or in vitro testing that suggest a significant risk for human participants and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure.
29
Phase 1, phase 2 and phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research participants are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to humans.
Concurrent with clinical trials, companies usually complete additional animal studies and must develop additional information about the chemistry and physical characteristics of the drug as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product and, among other things, companies must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidates do not undergo unacceptable deterioration over their shelf life.
NDA Submission, Review and Approval Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of nonclinical studies and clinical trials are submitted to the FDA as part of the NDA requesting approval to market the product for one or more indications. The NDA must include all relevant data available from pertinent preclinical and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing and controls, and proposed labeling, among other things. The submission of a NDA requires payment of a substantial application user fee to the FDA (unless a waiver or exemption applies).
Once a NDA has been submitted, the FDA’s goal is to review standard applications within ten months after it accepts the application for filing (a 60-day process) or, if the application qualifies for priority review, six months after the FDA accepts the application for filing. In both standard and priority reviews, the review process can be significantly extended by FDA requests for additional information or clarification.
The FDA reviews a NDA to determine, among other things, whether a drug is safe and effective and that any facility in which it is manufactured, processed, packed, or held meets standards designed to assure the product’s continued safety and effectiveness. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs. If the FDA determines that the application, manufacturing processes, or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the NDA does not satisfy the regulatory criteria for approval.
After the FDA evaluates the NDA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be produced, the FDA may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A complete response letter will describe all of the deficiencies that the FDA has identified in the NDA, except that, where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the complete response letter without first conducting required inspections, testing submitted product lots, and/or reviewing proposed labeling. In issuing the complete response letter, the FDA may recommend actions that the applicant might undertake to resolve any findings and place the NDA in condition for approval, including requests for additional information or clarification. The FDA may delay or refuse approval of a NDA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or effectiveness of a product.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may include limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy (“REMS”), to ensure the benefits of the product outweigh its risks. REMS is a safety strategy to manage a known or potential serious risk associated with a product and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Even if approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more phase 4 post-market studies and surveillance to further assess and monitor
30
the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post-marketing studies.
Expedited Development and Review Programs
The FDA offers a number of expedited development and review programs for qualifying product candidates. The fast track program is intended to expedite or facilitate the process for reviewing new drugs that meet certain criteria. Specifically, new drugs are eligible for fast track designation if they are intended to treat a serious or life-threatening condition and preclinical or clinical data demonstrate the potential to address unmet medical needs for the condition. Fast track designation applies to both the product and the specific indication for which it is being studied. For products containing new molecular entities, priority review designation means the FDA’s goal is to take action on the marketing application within six months of the 60-day filing date (compared with ten months under standard review). The FDA may also review sections of the NDA for a fast track product on a rolling basis before the complete application is submitted, provided that the sponsor and FDA agree on a schedule for the submission of the application sections, and the sponsor pays any required user fees upon submission of the first section of the NDA. The review clock does not begin until the final section of the NDA is submitted.
Additionally, a drug may be eligible for designation as a “breakthrough therapy” if the product is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over currently approved therapies on one or more clinically significant endpoints. The benefits of breakthrough therapy designation include the same benefits as fast track designation, as well as more intensive FDA interaction and guidance beginning as early as phase 1 and an organizational commitment to expedite the development and review of the product, including involvement of senior managers.
Additionally, products studied for their safety and effectiveness in treating serious or life-threatening diseases or conditions may receive accelerated approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of accelerated approval, the FDA generally requires the sponsor to perform adequate and well-controlled post-marketing clinical studies to verify and describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. Post-marketing adequate and well-controlled studies would usually be already underway at the time of NDA approval. Among other reforms, the Food and Drug Omnibus Reform Act of 2022 (“FDORA”), part of the Consolidated Appropriations Act, 2023, which was signed into law on December 29, 2022, expanded the FDA’s authority with regard the accelerated approval pathway for drugs and biologics. The statute requires the FDA to specify the conditions for any post-approval studies by the date of the accelerated approval (e.g., enrollment targets), and permits the FDA to require, as appropriate, that post-marketing confirmatory studies be underway prior to approval or within a specific time period following approval. Accelerated approval sponsors must submit periodic progress reports (every 180 days) on required post-approval trials and related conditions. The FDORA also outlines FDA procedures for withdrawing accelerated approval of a drug or indication approved under accelerated approval on an expedited basis. As an additional condition of accelerated approval, the FDA generally requires the pre-approval of promotional materials, which could adversely affect the timing of the commercial launch of a product. The FDA is expected to issue several guidance documents regarding accelerated approval and post-approval studies by mid-2024.
Fast track designation, priority review, and breakthrough therapy designation do not change the standards for approval, but may expedite the development or approval process. Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S., or more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making the product available in the U.S. for this type of disease or condition will be recovered from sales of the product.
Orphan designation must be requested before submitting a NDA. After the FDA grants orphan designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not convey any advantage in, or automatically shorten the duration of, the regulatory review or approval process. However, the orphan designation allows the sponsor to receive tax credits and a user fee waiver.
31
If a product that has orphan designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. A designated orphan product may not receive orphan exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the U.S. may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of people with the rare disease or condition.
Pediatric Information
Section 504 of the FDA Reauthorization Act of 2017 ("FDARA") amended section 505B of the FDCA to require—for original applications submitted on or after August 18, 2020—pediatric investigations of certain targeted cancer drugs with new active ingredients, based on molecular mechanism of action rather than clinical indication. Specifically, if an original NDA is for a new active ingredient, and the drug that is the subject of the application is intended for treatment of an adult cancer and directed at a molecular target that the FDA determines to be substantially relevant to the growth or progression of a pediatric cancer, reports on the molecularly targeted pediatric cancer investigation must be submitted with the marketing application, unless FDA formally waives or defers the requirement after submission of an initial Pediatric Study Plan.
Post-Approval Requirements
Following approval of a new product, the manufacturer and the approved product are subject to continuing regulation by the FDA, addressing, among other things, monitoring and record-keeping activities, reporting of adverse experiences, product sampling and distribution, complying with promotion and advertising requirements, which include restrictions on promoting products for unapproved uses or patient populations (known as “off-label use”) and limitations on industry-sponsored scientific and educational activities. Although physicians may prescribe legally available products for off-label uses, manufacturers may not market or promote such uses. Prescription drug promotional materials must be submitted to the FDA in conjunction with their first use. Further, most modifications to the drug, including changes in indications, labeling or manufacturing processes or facilities, are subject to FDA review and approval. There also are continuing user fee requirements, under which the FDA assesses an annual program fee for each product identified in an approved NDA.
The FDA may also place other conditions on approvals including the requirement for REMS to assure the safe use of the product. If the FDA concludes REMS is needed, the sponsor of the NDA must submit a proposed REMS. The FDA will not approve the NDA without an approved REMS, if required. REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following initial marketing. Product approvals may also require post-marketing requirements ("PMRs"), which include studies and clinical trials that sponsors are required to conduct under one or more statutes or regulations, and/or post-marketing commitments ("PMCs"), which are studies or clinical trials that a sponsor has agreed to conduct but that are not required by a statute or regulation.
FDA regulations require that products be manufactured in specific facilities and in accordance with cGMP regulations. Even with our facility in Indianapolis, Indiana, being operational, we may also rely on third parties for the production of clinical supply of our product candidates in accordance with cGMP regulations. These manufacturers must comply with cGMP regulations that require, among other things, quality control and quality assurance, the maintenance of records and documentation and the investigation and correction of any deviations from cGMP. Manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. The discovery of violations, including failure to conform to cGMP regulations, could result in enforcement actions, and the discovery of post-approval problems with a product may result in restrictions on a product, manufacturer or holder of an approved NDA, including recall.
32
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, but are not limited to:
•restrictions on the marketing or manufacturing of a product, mandated modification of promotional materials or the issuance of corrective information, issuance by the FDA or other regulatory authorities of safety alerts, Dear Healthcare Provider letters, press releases or other communications containing warnings or other safety information about the product, or complete withdrawal of the product from the market or product recalls;
•fines, warning or untitled letters or holds on post-approval clinical studies;
•refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of existing product approvals;
•product seizure or detention, or refusal by the FDA to permit the import or export of products; and
•injunctions or the imposition of civil or criminal penalties.
U.S. Healthcare Laws and Regulation
The pharmaceutical industry is subject to extensive and complex federal and state laws and regulations, including those related to healthcare fraud and abuse, privacy and security of health information and other personal data, transparency of financial relationships, registration of manufacturers and distributors, and marketing. These laws and regulations are broadly applicable, may vary across jurisdictions, and are administered by several different government agencies, including the FDA, the U.S. Department of Health and Human Services (“HHS”), the Centers for Medicare and Medicaid Services (“CMS”), and the U.S. Department of Justice (“DOJ”). Further, these laws and regulations are subject to change, enforcement practices may evolve, and it is difficult to predict the impact of new laws and regulations. Noncompliance with applicable laws and regulations may result in the imposition of civil and criminal penalties that could adversely affect our operations and financial condition.
Fraud and Abuse
The federal Anti-Kickback Statute prohibits providers and others from directly or indirectly soliciting, receiving, offering or paying any remuneration with the intent of generating referrals or orders for items or services covered by a federal healthcare program. Courts have interpreted this statute broadly and have held that there is a violation of the Anti-Kickback Statute if just one purpose of the remuneration is to generate referrals, even if there are other lawful purposes. Furthermore, knowledge of the law or the intent to violate the law is not required. Violations of the Anti-Kickback Statute may be punished by criminal fines, imprisonment, substantial civil monetary penalties, damages of up to three times the total amount of the remuneration and/or exclusion from participation in federal health care programs, including Medicare and Medicaid. In addition, submission of a claim for services or items generated in violation of the Anti-Kickback Statute may be subject to additional penalties under the federal False Claims Act (“FCA”) as a false or fraudulent claim.
Federal civil and criminal false claims laws, including the civil FCA, govern the submission of claims for reimbursement and prohibit individuals and entities from making false claims or statements. The FCA may be enforced by the federal government directly or by a private individual, acting as a “whistleblower,” on behalf of the federal government. There are many potential bases for liability under the FCA, including knowingly, submitting a false claim for reimbursement to the federal government or submitting claims for services or items generated in violation of the Anti-Kickback Statute. Parties can be held liable under the FCA even when they do not directly submit claims to government payors if they are deemed to cause the submission of false or fraudulent claims. If a defendant is determined to have violated the FCA, they may be required to pay three times the actual damages sustained by the government, plus mandatory civil penalties for each separate false claim, and may be excluded from participation in federal healthcare programs. Additionally, individuals or entities may be subject to criminal penalties under the criminal FCA.
Criminal and civil penalties may be imposed under other statutes that prohibit various forms of fraud and abuse. For example, the federal Civil Monetary Penalties Law (“CMP Law”), which requires a lower burden of proof than some other fraud and abuse laws, provides for penalties for a variety of healthcare fraud violations. These include, but are not limited
33
to, the offering or transfer of remuneration to a federal healthcare program beneficiary knowing that such action is likely to influence the beneficiary’s selection of a particular provider of items or services payable by federal healthcare programs, unless an exception applies. The CMP Law authorizes the HHS Office of Inspector General (“OIG”) to seek civil monetary penalties and exclusion from federal healthcare programs.
Many states have similar fraud and abuse laws, such as state anti-kickback and false claims laws, which may impose additional liability for the types of acts prohibited by federal law. Some state laws apply to healthcare items or services that are reimbursed by non-governmental third-party payors, including private insurers.
Privacy and Security
Privacy and security regulations issued pursuant to the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”), restrict the use and disclosure of individually identifiable health information (“protected health information”), provide for individual privacy-related rights, require safeguards for protected health information and require notification of breaches of unsecure protected health information. Entities subject to HIPAA include health plans, healthcare clearinghouses, and most healthcare providers. Entities that handle protected health information on behalf of covered entities (known as business associates) are required to comply with certain provisions of the security and privacy regulations. Although we are not a covered entity or business associate, we are impacted by HIPAA indirectly. For example, HIPAA restricts the use and disclosure of protected health information for research, which may limit our ability to receive protected health information that is not redacted from covered entities and business associates. We may be required to agree to privacy and security terms as a condition of receiving data, and failure to comply with those terms could result in civil and criminal penalties.
There are several other laws and legislative and regulatory initiatives at the federal and state levels addressing privacy and security of personal information. These laws vary, and noncompliance may result in penalties or other disincentives. For example, the California Consumer Privacy Act of 2018 (the “CCPA”), which was significantly modified by the California Privacy Rights Act, gives California residents expanded rights to access and require deletion of their personal information, opt out of certain personal information sharing and receive detailed information about how their personal information is used. In addition to civil penalties for violations, the laws provide for a private right of action for data breaches. Although information collected, used or disclosed in research, including clinical trials, and information governed by HIPAA are currently exempt from the CCPA, our other personal information collection practices may be subject to the CCPA and similar state laws. Some other states also afford consumers expanded privacy protections, and several privacy bills have been proposed at both the federal and state level that may result in additional legal requirements that impact our business. Foreign laws, including for example the E.U.’s General Data Protection Regulation (“GDPR”), also govern the privacy and security of health information in some circumstances, and may differ significantly from HIPAA.
The costs associated with developing and maintaining systems to comply with evolving data privacy and security laws, defending against privacy and security related claims or enforcement actions and paying any assessed fines can be substantial.
Sunshine Laws
The federal government and certain state governments have enacted laws, sometimes referred to as “sunshine laws” that aim to increase transparency in financial relationships between manufacturers of drugs, devices, biologicals, or medical supplies and healthcare professionals and teaching hospitals. These laws and implementing regulations generally require manufacturers to disclose payments and other transfers of value provided to healthcare physicians (defined to include doctors, dentists, optometrists, podiatrists, and chiropractors) and teaching hospitals and to disclose ownership and investment interests held by healthcare professionals and their immediate family members. These reporting obligations extend to include transfers of value made to certain non-physician providers such as physician assistants and nurse practitioners. Under the federal Physician Payments Sunshine Act, CMS collects data submitted by manufacturers of drugs, devices, biologics and medical supplies covered by federal healthcare programs and publishes the data on its public Open Payments website. In addition, some states have sunshine laws that are broader than the federal law by requiring the reporting of a larger set of payments or activities and including additional types of healthcare professionals or entities. Failure to comply with sunshine laws may result in sanctions, including civil monetary penalties.
Pharmaceutical Industry Compliance
Manufacturers and distributors of drug products are subject to state laws requiring registration with the state, which may extend to manufacturers and distributors that ship products into a state even if they do not have a place of business within the state. The federal government and some state governments also require manufacturers and distributors to
34
maintain records regarding the history of products in the chain of distribution. Federal law requires manufacturers to provide product tracing information to subsequent supply chain partners. The federal Drug Supply Chain Security Act (“DSCSA”) governs the system of tracing certain prescription drugs as they are distributed in the U.S., with the goal of protecting consumers from drugs that may be counterfeit, contaminated, stolen, or adulterated. This law requires manufacturers to, prior to or at the time of each transfer of ownership of a drug, provide the next owner with transaction history and other information and to only engage in transactions with authorized trading partners. In the event of a recall or an inquiry regarding a potentially illegitimate product, manufacturers must be able to provide information regarding the transaction history and other information about the products. Violations of the DSCSA may result in fines or imprisonment. Many states have similar laws and enforce recordkeeping and licensure requirements.
Some state laws require pharmaceutical companies to comply with the HHS OIG Compliance Program Guidance for Pharmaceutical Manufacturers, which is guidance designed to help companies prevent fraud and abuse within federal healthcare programs, and other relevant government-issued compliance guidance. Several states require pharmaceutical and biotechnology companies to publicly disclose sales information, marketing expenditures, drug pricing, clinical trials and other activities. Several states require pharmaceutical and biotechnology companies to establish marketing compliance programs, require registration of sales representatives, prohibit manufacturers from acquiring certain physician prescribing data for use in sales and marketing activities and prohibit certain other sales and marketing practices. In addition, various federal and state consumer protection and unfair competition laws may also apply. Violation of laws governing the pharmaceutical industry may result in penalties such as criminal fines, imprisonment, civil fines, exclusion from participation in federal healthcare programs and additional reporting obligations and oversight.
Federal price reporting laws require many pharmaceutical manufacturers to calculate and report certain pricing metrics, such as average sales price (“ASP”) and best price, which may be used in the calculation of reimbursement and discounts on approved products. Penalties may apply if these metrics are not submitted timely and accurately.
Reimbursement and Pricing
Payment for healthcare items and services in the U.S. comes primarily from third-party payors, including government healthcare programs, such as Medicare and Medicaid, and commercial payors. Coverage and reimbursement for pharmaceutical products can differ significantly between payors.
Sales of pharmaceutical products are materially affected by the extent to which third-party payors provide coverage and establish adequate reimbursement levels for the product. A decision by a third-party payor to not cover a product may impact utilization. At the same time, one payor’s determination to provide coverage for a product does not mean that other payors will also provide coverage for the product. Securing coverage and reimbursement for a product (often two separate processes) may involve conducting expensive pharmacoeconomic studies to demonstrate medical necessity and cost-effectiveness of a product, in addition to the studies required to obtain FDA or comparable regulatory approvals. Drugs administered under the supervision of a physician often have high price points, which may make obtaining adequate reimbursement from payors particularly difficult.
The containment of healthcare costs has become a priority of both commercial payors and government healthcare programs. Third-party payors are increasingly challenging prices, examining medical necessity and reviewing cost-effectiveness of medical products and services as they seek to control costs. For example, payors may limit coverage to specific products on an approved list, known as a formulary, which might not include all of the approved products for a particular indication, or they may require substitution of generic products. Adoption of, or enhancement of existing, price controls and other cost-containment measures may limit revenue. Drug prices may also be reduced by discounts or rebates required by payors and future relaxation of laws that presently restrict imports of drugs from other countries.
The marketability of any drug candidate for which we receive regulatory approval for commercial sale may be negatively impacted if commercial payors and government healthcare programs do not provide adequate coverage and reimbursement. There is no guarantee that coverage or adequate reimbursement will be available for any of our products or product candidates, even if the product candidates are FDA-approved. If adequate coverage and reimbursement is not available, we may not be able to realize an appropriate return on our investment in product development.
Government Reimbursement
We expect that reimbursement from Medicare and Medicaid will eventually be a significant part of our revenues. The Medicare and Medicaid programs are highly regulated and subject to frequent and substantial changes resulting from legislation, regulations and administrative and judicial interpretations of existing law. Drug manufacturers must offer
35
discounted pricing or rebates on purchases of pharmaceutical products under various government healthcare programs and may also be required to report specific prices to government agencies under these programs.
Medicare is a federal health insurance program for persons age 65 and over, some disabled persons, and persons with end-stage renal disease. Medicare Part B generally covers medically necessary outpatient care, including physician services and a limited number of outpatient prescription drugs under limited conditions. Medicare Part B typically covers drugs that are not self-administered by a patient, including injectable and infused drugs and oral cancer drugs. Drugs that are not covered under Medicare Part B may be covered under Part D, an optional prescription drug benefit for Medicare beneficiaries that is administered by private companies.
Medicaid is a medical assistance program for eligible needy persons that is funded jointly by federal and state governments. Medicaid programs are operated by state agencies under plans approved by the federal government. Reimbursement methodologies, eligibility requirements and covered services vary from state to state. Although pharmacy coverage is an optional benefit under federal law, all states currently provide coverage for outpatient prescription drugs. Pharmaceutical manufacturers participating in Medicaid are required to participate in the 340B Drug Pricing Program, which requires them to sell outpatient drugs at discounted prices to certain safety net providers that serve vulnerable or underserved populations. Manufacturers participating in Medicaid are also required to participate in the Medicaid Drug Rebate Program (“MDRP”), a program intended to help offset the federal and state costs of outpatient prescription drugs. Under this program, manufacturers agree to rebate a specified portion of the Medicaid payment for the drug to the states, and states share the rebates with the federal government based on their federal medical assistance percentage, which is the share of Medicaid spending in each state paid for by the federal government. When a manufacturer enters into an MDRP rebate agreement with HHS, Medicaid agrees to cover nearly all FDA-approved drugs from that manufacturer, effectively creating an open formulary. An MDRP rebate agreement is also required in order for payment to be made available under Medicare Part B for a manufacturer’s covered outpatient drugs.
Drug Pricing Policies and Related Reforms
In recent years, the U.S. Congress and certain state legislatures have considered and passed a large number of laws intended to result in significant changes to the healthcare industry, including proposals targeted at reducing the price of pharmaceutical products and limiting coverage and reimbursement for drugs and other medical products. For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the "Affordable Care Act"), affects how health care services are covered, delivered and reimbursed through expanded health insurance coverage, reduced growth in Medicare program spending, and the establishment and expansion of value-based purchasing programs. The law also imposes price transparency requirements and established the Patient-Centered Outcomes Research Institute, which focuses on comparative clinical effectiveness research. In addition, the Affordable Care Act contains several provisions relevant to pharmaceutical manufacturers and that may impact our potential product candidates, including expansion of the 340B program, expansion of manufacturers’ rebate liability under the MDRP, and measures intended to reduce Medicare Part D enrollees’ out-of-pocket liability.
There is uncertainty regarding the ultimate effect of the Affordable Care Act, particularly as it has been, and continues to be, subject to legislative and regulatory changes and court challenges. However, President Biden has indicated that his administration intends to protect and strengthen the Affordable Care Act and Medicaid programs. There is also uncertainty regarding the potential impact of other reform efforts at the federal and state levels. For example, some members of Congress have proposed measures that would expand government-sponsored coverage, such as single-payor proposals (commonly referred to as “Medicare for All”) and changes to Medicare age requirements. These proposals could lead to increased coverage levels and utilization of services. However, the impact and timing of additional reform initiatives is unclear.
In recent years, there has been heightened governmental and public scrutiny over the manner in which pharmaceutical manufacturers set prices for their products. This has resulted in proposed and enacted federal and state legislation and regulations, executive orders and other initiatives and proposals designed to increase transparency in product pricing, review the relationship between pricing and manufacturer patient programs and reform government reimbursement methodologies for pharmaceutical products. For example, the Inflation Reduction Act, passed in August 2022, requires drug manufacturers to pay rebates if prices for certain drugs increase faster than the rate of inflation (beginning in 2023), caps annual out-of-pocket drug costs for Medicare Part D beneficiaries (effective in 2024), and allows the federal government to negotiate drug prices with pharmaceutical manufacturers for a select number of high-cost drugs (prices to take effect beginning in 2026), among other drug pricing provisions. In February 2023, CMS announced that its Innovation Center will test new payment models intended to lower drug prices, including the Accelerating Clinical Evidence Model, which will focus on drugs that receive accelerated approval from the FDA, and the Cell & Gene Therapy Access Model, which will test a partnership approach between CMS, drug manufacturers, and state Medicaid agencies for administering outcomes-based agreements to help Medicaid beneficiaries access high-cost specialty drugs. In addition, HHS, the
36
Department of Labor and the Department of the Treasury issued a Transparency in Coverage final rule in 2020 that, for plan years beginning on or after January 1, 2022, requires health plans to disclose on a public website the negotiated rates and historical net pricing for prescription drugs and to provide consumers with personalized cost-sharing information. The agencies also issued an interim final rule in November 2021, implementing certain reporting requirements imposed by the No Surprises Act, requiring health plans to disclose, among other things, information on the most frequently dispensed and costliest drugs, as well as prescription drug rebates paid by drug manufacturers to plans, issuers, third-party administrators and pharmacy benefit managers. CMS announced its intention to start enforcement of the transparency requirements beginning July 1, 2022. Beginning in 2023, the agencies will issue biennial public reports on prescription drug pricing trends and the impact of prescription drug costs on premiums and out-of-pocket costs.
Some other efforts related to drug pricing reform involve importing drugs from other countries or using international pricing benchmarks. For example, in 2020, HHS and the FDA issued a final rule to allow FDA-authorized programs to import certain prescription drugs from Canada, although the rule excludes several types of prescription drugs such as radioactive drugs and biologics and imaging drugs. Some states, such as Florida, are pursuing their own initiative to import drugs for state health care programs.
In addition, HHS OIG issued a final rule in 2020 that amends the discount safe harbor of the federal Anti-Kickback Statute to exclude rebates from drug manufacturers to Medicare Part D plan sponsors, adds a safe harbor to protect point-of-sale reductions from a drug manufacturer to a Part D or Medicaid managed care organization, and adds a safe harbor to protect certain drug manufacturer payments to pharmacy benefit managers. However, this rule was subject to legal challenges, and the Inflation Reduction Act prohibits implementation of the rule prior to January 1, 2032.
The Biden administration and certain members of Congress have indicated their intent to continue to pursue drug pricing reforms. Proposals include allowing additional importation of prescription drugs from other countries and modifying the design of the Medicare Part D program. Some states also have passed legislation and issued regulations designed to lower prescription drug costs. These and other initiatives at the federal and state levels, if enacted and implemented, may directly or indirectly affect pricing of our product candidates.
Additionally, the Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017, (the “Right to Try Act”) provides a federal framework for eligible patients to access certain investigational drugs that are the subject of an active IND application submitted to the FDA, but that have not been approved or licensed by the FDA for any use. The Right to Try Act allows patients to seek treatment without participating in a clinical trial and without obtaining FDA permission under the FDA Expanded Access Program (i.e., compassionate use). Manufacturers are not obligated under the Right to Try Act to make products available to eligible patients Manufacturers and sponsors that provide an eligible investigational drug under the Right to Try Act must submit an annual summary to the FDA of the use of any drugs supplied under the statute. Most states have enacted similar laws.
The FDORA, which was enacted in December 2022, provides for several changes to the FDA’s regulatory framework, including changes to the accelerated approvals pathway and requirements intended to modernize clinical trials, including through diversity action plans. The legislation also created new programs, including an emerging technology program that is intended to support the adoption of innovative approaches to drug design and manufacturing.
Canadian Drug Development and Approval Process
In accordance with the Food and Drugs Act and associated Regulations, manufacturers of prescription drug products must receive authorization from Health Canada before prescription drug products may be marketed and sold. There is no assurance that Health Canada will issue an authorization for a product.
In order to obtain authorization, a manufacturer must file a regulatory submission with evidence of safety, efficacy and quality of the proposed drug product. While Health Canada’s review of the evidence and a manufacturer’s response to Health Canada’s inquiries can take as long as several years from the date that the manufacturer files its regulatory submission, it can often be completed within 1 year.
The typical regulatory process for prescription drug approval from pre-market to post-market in Canada, involves:
•preclinical studies, using for example, laboratory studies involving cell or tissue samples, or tests conducted on animals, to collect preliminary safety and efficacy data;
•clinical trials on humans, which require authorization by Health Canada to collect further safety and efficacy data;
37
•a drug submission with Health Canada, including review by Health Canada that can take between one and two years;
•a market authorization decision by Health Canada and issuance of a notice of compliance (NOC) and drug identification number (DIN); and
•public access to the drug product through submission to provincial and territorial public drug benefit programs, subject to post-marketing surveillance, inspection and investigation by Health Canada.
Employees
As of December 31, 2022, we had 129 employees. We also utilize the services of several consultants. We believe our relationship with our employees and consultants is good.
Item 1A. Risk Factors
Investing in our securities involves a high degree of risk. The following risk factors will apply to our business and operations. These risk factors are not exhaustive and investors are encouraged to perform their own investigation with respect to the business, prospects, financial condition and operating results of POINT. You should carefully consider the following risk factors in addition to the other information included elsewhere in this Form 10-K, including our consolidated financial statements and the related notes and "Management’s Discussion and Analysis of Financial Condition and Results of Operations" before deciding whether to invest in our securities. We may face additional risks and uncertainties that are not presently known to us, or that we currently deem immaterial, which may also impair our business, prospects, financial condition or operating results. The occurrence of one or more of the events or circumstances described in these risk factors, alone or in combination with other events or circumstances, may have a material adverse effect on the our business, reputation, revenue, financial condition, results of operations and future prospects, in which event the market price of our Common Stock could decline, and you could lose part or all of your investment. Unless otherwise indicated, reference in this section and elsewhere in this Form 10-K to our business being adversely affected, negatively impacted or harmed will include an adverse effect on, or a negative impact or harm to, our business, reputation, financial condition, results of operations, revenue and our future prospects. This Form 10-K also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of a number of factors, including the risks described below. See the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Risks Related to POINT’s Financial Condition and Capital Requirements
POINT has incurred significant losses for most of its operating history and may not be able to achieve or sustain revenues or profitability in the future.
Investment in drug product development is a highly speculative undertaking and entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval and become commercially viable. POINT is still in the early stages of development of its product candidates and its lead product candidates are still in clinical trials. POINT has no products licensed for commercial sale and has not generated any revenue to date, and POINT continues to incur significant research and development and other expenses related to its ongoing operations.
For the years ended December 31, 2021 and 2022, POINT reported net loss of $45.9 million and net income $98.3 million, respectively. Following the year end December 31, 2022, POINT expects to incur significant losses for the foreseeable future, and POINT could experience substantial additional losses to the extent that POINT:
•continues its research and development efforts and submits NDAs, for POINT’s lead product candidates and submits INDs, for its other product candidates;
•conducts preclinical studies and clinical trials for POINT’s current and future product candidates;
•seeks to identify additional product candidates;
•acquires or in-licenses other product candidates and technologies;
•continues to expand POINT’s manufacturing facility and obtain additional regulatory approvals of the facility;
38
•adds operational, financial and management information systems and personnel, including personnel to support the development of POINT’s product candidates and help it comply with its obligations as a public company;
•hires and retains additional personnel, such as clinical, regulatory, quality control, scientific, commercial and administrative personnel;
•seeks marketing approvals for any product candidates that successfully complete clinical trials;
•establishes a sales, marketing and distribution infrastructure and scale-up manufacturing capabilities, whether alone or with third parties, to commercialize product candidates for which POINT may obtain regulatory approval, if any;
•expands, maintains and protects POINT’s intellectual property portfolio;
•continues to experience delays or interruptions from the impact of COVID-19; and
•competes with technological and market developments;
Because of the numerous risks and uncertainties associated with drug product development, POINT is unable to accurately predict the timing or amount of increased expenses it will incur or when, if ever, it will be able to sustain profitability. Even if POINT succeeds in commercializing one or more of its product candidates, POINT will continue to incur substantial research and development, manufacturing and other expenditures to develop, seek regulatory approval for, and market additional product candidates. POINT may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect POINT’s business. The size of POINT’s future net losses will depend, in part, on the rate of future growth of its expenses and its ability to generate revenue. POINT’s prior losses and expected future losses have had and will continue to have an adverse effect on POINT’s stockholders’ equity and working capital.
POINT will still require substantial additional financing, which may not be available on acceptable terms, or at all. A failure to obtain this necessary capital when needed could force POINT to delay, limit, reduce or terminate its product development or commercialization efforts.
As of December 31, 2022, POINT had cash, cash equivalents and investments totaling $541.3 million and accumulated earnings of $39.0 million. POINT’s operations have consumed substantial amounts of cash since inception. POINT expects to continue to spend substantial amounts to continue the clinical development of its remaining PSMA-targeted radioligand program (PNT2001), fibroblast activation protein-targeted radioligand program (PNT2004), and future clinical trials for its other product candidates and to continue to identify new product candidates.
POINT will require significant additional amounts of cash in order to launch and commercialize its current and future product candidates to the extent that such launch and commercialization are not the responsibility of Lantheus or a collaborator that POINT may contract with in the future. In addition, other unanticipated costs may arise in the course of POINT’s development efforts. Because the design and outcome of POINT’s planned and anticipated clinical trials is highly uncertain, POINT cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of any product candidate POINT develops.
POINT’s future capital requirements depend on many factors, including:
•the scope, progress, results and costs of researching, developing and if marketing approval is received commercializing product candidates from the PNT2001 and PNT2004 programs, and its other product candidates;
•the timing of, and the costs involved in, obtaining marketing approvals for its current and future product candidates;
•the number of future product candidates and potential additional indications that it may pursue and their development requirements;
•the timing of and costs involved in, completing the construction of POINT’s manufacturing facility and obtaining all the regulatory approvals of such facility;
•the cost of manufacturing its product candidates for clinical trials in preparation for regulatory approval and in preparation for commercialization;
39
•the cost and availability of 177Lu and 225Ac or any other medical isotope it may incorporate into its product candidates;
•the cost and availability of ytterbium-176 (“176Yb”) or any other raw material necessary to manufacture medical isotopes internally;
•if approved, the costs of commercialization activities for any approved product candidate to the extent such costs are not the responsibility of any future collaborators, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities;
•receipt of regulatory approval and revenue, if any, received from commercial sales for any approved indications for any of its product candidates;
•the extent to which it in-licenses or acquires rights to other products, product candidates or technologies;
•its headcount growth and associated costs as it expands its research and development capabilities and establishes a commercial infrastructure;
•the continued impact of delays or interruptions from COVID-19;
•the costs of preparing, filing and prosecuting patent applications and maintaining and protecting its intellectual property rights, including enforcing and defending intellectual property related claims; and
•the costs of operating as a public company.
POINT cannot be certain that additional funding will be available on acceptable terms, or at all. If POINT is unable to raise additional capital in sufficient amounts or on terms acceptable to it, POINT may have to significantly delay, scale back or discontinue the development or commercialization of its product candidates or other research and development initiatives. Any of its current or future license agreements may also be terminated if it is unable to meet the payment or other obligations under the agreements.
POINT currently anticipates that, based on its existing research and development programs and expectations related to the build out of its manufacturing facility, POINT’s existing cash, cash equivalents and investments will enable POINT to fund its operating expenses and capital expenditure requirements into 2026. POINT’s estimate may prove to be wrong, and POINT could use its available capital resources sooner than currently expected. Further, changing circumstances, some of which may be beyond POINT’s control, could cause POINT to consume capital significantly faster than it currently anticipates, and POINT may need to seek additional funds sooner than planned.
Unstable market and economic conditions and adverse developments with respect to financial institutions and associated liquidity risk may have serious adverse consequences on our business, financial condition and stock price.
The global economic slowdown, inflation, rising interest rates and the prospects for recession, as well as recent and potential future disruptions in access to bank deposits or lending commitments due to bank failure, could materially and adversely affect our liquidity, our business and financial condition. The recent closures of Silicon Valley Bank and Signature Bank and their placement into receivership with the Federal Deposit Insurance Corporation (“FDIC") created bank-specific and broader financial institution liquidity risk and concerns. Although the Department of the Treasury, the Federal Reserve, and the FDIC jointly released a statement that depositors at Silicon Valley Band and Signature Bank would have access to their funds, even those in excess of the standard FDIC insurance limits, future adverse developments with respect to specific financial institutions or the broader financial services industry may lead to market-wide liquidity shortages. The failure of any bank in which we deposit our funds could reduce the amount of cash we have available for our operations or delay our ability to access such funds. Any such failure may increase the possibility of a sustained deterioration of financial market liquidity, or illiquidity at clearing, cash management and/or custodial financial institutions. In the event we have a commercial relationship with a bank that has failed or is otherwise distressed, we may experience delays or other issues in meeting our financial obligations. If other banks and financial institutions enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our cash and cash equivalents and investments may be threatened and could have a material adverse effect on our business and financial condition.
POINT’s limited operating history may make it difficult for you to evaluate the success of its business to date and to assess its future viability.
40
POINT is a clinical-stage precision oncology company with a limited operating history. POINT was founded to advance the development and commercialization of radioligand therapies for the treatment of cancer in September 2019. POINT may have access to additional experience through its collaboration with Lantheus, but this collaboration is limited in its scope. Consequently, any predictions you make about POINT’s future success or viability may not be as accurate as they could be if POINT had a longer operating history.
POINT may be exposed to financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates.
POINT may be adversely affected by foreign currency fluctuations. POINT’s reporting currency is the U.S. dollar. The functional currency of POINT’s subsidiary in Canada and its two subsidiaries in the U.S. are also the U.S. dollar. To date, POINT has been primarily funded through issuances of equity that have been denominated in U.S. dollars. However, certain expenditures in connection with the production of POINT’s clinical trial products and other expenditures are paid in Canadian dollars and therefore, POINT is subject to foreign currency fluctuations that may, from time to time, impact its financial positions and results of operations.
Risks Related to the Development of POINT’s Product Candidates
POINT’s approach to the discovery and development of product candidates based on its proprietary radioligand targeted therapies represents a novel approach to radiation therapy, which creates significant and potentially unpredictable challenges for it.
POINT’s future success depends on the successful development of its product candidates, which are designed to treat cancers using targeted radioligand therapies, representing a novel approach to radioligand therapy. 177Lu oncology therapy is relatively new, and only two 177Lu therapies have been approved in the U.S. or the E.U. and only a limited number of clinical trials of products based on 177Lu therapies have commenced. There are currently no approved therapies which use 225Ac. Global supply of 225Ac is also currently limited and may not be capable of expanding sufficiently to enable commercial volume manufacturing of 225Ac therapies. As such, it is difficult to accurately predict the developmental challenges POINT may incur for its product candidates as they proceed through product discovery or identification, preclinical studies and clinical trials. In addition, there may be long-term effects from treatment, including late radiation toxicity, with any of POINT’s current or future product candidates that it cannot predict at this time. It is difficult for POINT to predict the time and cost of the development of its product candidates, and it cannot predict whether the application of its technology, or any similar or competitive technologies, will result in the identification, development, and regulatory approval of any product candidates. There can be no assurance that any development problems POINT experiences in the future related to its technology or any of its research programs will not cause significant delays or unanticipated costs, or that such development problems can be solved at all. Any of these factors may prevent POINT from completing its preclinical studies and clinical trials that it may initiate or commercializing any product candidates it may develop on a timely or profitable basis, if at all. In addition, the success of POINT’s targeted radioligand therapies, including its lead product candidates, will depend on several factors, including the following:
•sourcing clinical and, if successfully approved for commercial sale, commercial supplies for the materials used to manufacture its product candidates;
•establishing manufacturing capabilities to produce adequate amounts of its product candidates;
•utilizing imaging analogues or other companion diagnostics to visualize tumor uptake in advance of administering its product candidates, which may increase the risk of adverse side effects;
•educating medical personnel regarding the potential side effect profile of its product candidates;
•facilitating patient access to the limited number of facilities able to administer its product candidates, if licensed;
•using medicines to manage adverse side effects of its product candidates that may not adequately control the side effects or that may have detrimental impacts on the efficacy of the treatment; and
•establishing sales and marketing capabilities upon obtaining any regulatory approval to gain market acceptance of POINT’s novel therapies.
41
POINT is very early in its development efforts. If POINT is unable to advance its product candidates through clinical development, obtain regulatory approval and ultimately commercialize its product candidates, or if it experiences significant delays in doing so, POINT’s business will be materially harmed.
POINT is very early in its development efforts. In November 2022, POINT entered into an agreement with Lantheus pursuant to which Lantheus was granted exclusive world-wide licenses (excluding certain countries in Asia) to commercialize 177Lu-PNT2002 and 177Lu-PNT2003. The success of POINT’s product candidates will depend on several factors, including the following:
•Lantheus' ability to successfully commercialize 177Lu-PNT2002 and 177Lu-PNT2003:
•successful initiation and completion of preclinical studies;
•successful initiation of clinical trials;
•successful participant enrollment in, and completion, of clinical trials;
•receipt and related terms of marketing approvals from applicable regulatory authorities;
•obtaining and maintaining patent and trade secret protection and regulatory exclusivity for its product candidates, and its methods of manufacturing or using those product candidates
•making and maintaining arrangements with third-party manufacturers, or establishing manufacturing capabilities, for both clinical and commercial supplies of its product candidates;
•establishing sales, marketing and distribution capabilities and successfully launching commercial sales of its product candidates, if and when approved, whether alone or in collaboration with others;
•acceptance of its product candidates, if and when approved, by patients, the medical community and third-party payors;
•effectively competing with other cancer therapies;
•avoidance of any delays or interruptions in its preclinical studies, clinical trials and supply chain due to the COVID-19 pandemic or other supply chain disruptions;
•obtaining and maintaining third-party coverage and adequate reimbursement; and
•maintaining a continued acceptable safety profile of its products following regulatory approval.
If POINT does not achieve one or more of these factors in a timely manner or at all, it could experience significant delays or be unable to successfully commercialize its product candidates, which would materially harm its business.
Clinical development involves a lengthy and expensive process with uncertain outcomes, and results of earlier studies and trials may not be predictive of future clinical trial results. If POINT’s preclinical studies and clinical trials are not sufficient to support regulatory approval of any of its product candidates, it may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development of such product candidate.
POINT cannot be certain that its preclinical study and clinical trial results will be sufficient to support regulatory approval of its product candidates. Clinical testing is expensive and can take many years to complete, and its outcomes are inherently uncertain. Human clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. POINT’s clinical trials may not be conducted as planned or completed on schedule, if at all, and failure can occur at any time during the preclinical study or clinical trial process. Despite promising preclinical or clinical results, any product candidate can unexpectedly fail at any stage of preclinical or clinical development. The historical failure rate for product candidates in POINT’s industry is high.
POINT may experience delays in obtaining the FDA’s authorization to initiate clinical trials. Additionally, POINT cannot be certain that preclinical studies or clinical trials for its product candidates will begin on time, not require redesign, enroll an adequate number of participants on time, or be completed on schedule, if at all. Clinical trials can be delayed or terminated for a variety of reasons, including delays or failures related to:
•the availability of financial resources to commence and complete the planned trials;
42
•limited number of, and competition for, suitable sites to conduct POINT’s clinical trials;
•the FDA or similar foreign regulatory authorities disagreeing as to the design or implementation of POINT’s clinical trials or imposing a clinical hold on a clinical trial;
•delays in obtaining regulatory approval or authorization to commence a clinical trial, including delays or issues relating to POINT’s use of imaging analogues or any future companion diagnostics it may develop;
•reaching agreement on acceptable terms with prospective contract research organizations ("CROs") and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites;
•obtaining and maintaining IRB or ethics committee approval at each clinical trial site;
•termination of POINT’s clinical trials by an IRB at one or more clinical trial sites;
•recruiting an adequate number of suitable patients to participate in a clinical trial;
•having participants complete a clinical trial or return for post-treatment follow-up;
•clinical trial sites deviating from clinical trial protocol or dropping out of a clinical trial;
•having third-party contractors fail to complete their obligations in a timely manner or failing to comply with applicable regulatory requirements;
•addressing participants safety concerns that arise during the course of a clinical trial;
•access or travel to clinical sites as a result of COVID-19;
•adding a sufficient number of clinical trial sites;
•access to raw materials, such as radioisotopes;
•obtaining sufficient product supply of POINT’s product candidates for use in preclinical studies or clinical trials from third-party suppliers; or
•lack of efficacy evidenced during clinical trials, which risk may be heightened given the advanced state of disease and lack of response to prior therapies of participants in certain clinical trials.
If POINT is required to conduct additional clinical trials or other testing of its product candidates beyond those that it currently contemplates, for example, should FDA require additional clinical data to evaluate the potential for late radiation toxicity, if it is unable to successfully complete clinical trials of its product candidates or other testing, if the results of these trials or tests are not positive or are not as positive as POINT expects or if there are safety concerns, POINT’s business and results of operations may be adversely affected and it may incur significant additional costs.
If POINT experiences delays in the completion, or termination, of any preclinical study or clinical trial of its product candidates, the commercial prospects of its product candidates may be harmed, and its ability to generate revenues from any of these product candidates will be delayed or not realized at all. In addition, any delays in completing POINT’s preclinical studies or clinical trials may increase its costs, slow down the development of its product candidates and approval process and jeopardize its ability to commence product sales and generate revenues. Any of these occurrences may significantly harm POINT’s business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of POINT’s product candidates. If one or more of POINT’s product candidates generally prove to be ineffective, unsafe or commercially unviable, POINT’s entire pipeline and targeted radioligand therapies would have little, if any, value, which would have a material and adverse effect on POINT’s business, financial condition, results of operations and prospects.
POINT may not achieve research, development and commercialization goals in the time frames that it publicly estimates, which could have an adverse impact on POINT’s business and could cause its stock price to decline.
43
POINT sets goals, and make public statements regarding its expectations, regarding the timing of certain accomplishments, developments and milestones under its research and development programs. The actual timing of these events can vary significantly due to a number of factors, including, without limitation, the amount of time, effort and resources committed to POINT’s programs by it and any collaborators, COVID-19 related delays and the uncertainties inherent in the regulatory approval process. As a result, there can be no assurance that POINT or any collaborators will make regulatory submissions or receive regulatory approvals as planned or that POINT or any collaborators will be able to adhere to POINT’s current schedule for the achievement of key milestones under any of its programs. If POINT or any collaborators fail to achieve one or more of the milestones described above as planned, POINT’s business could be materially adversely affected and the price of its Common Stock could decline.
The commercial success of POINT’s product candidates will depend upon competitive products, public perception of radioligands and the degree of their market acceptance by physicians, patients, healthcare payors and others in the medical community.
Adverse events in clinical trials of POINT’s product candidates or in clinical trials of others developing similar products and the resulting negative publicity, as well as any other adverse events in the field of radioligands that may occur in the future, could result in a decrease in demand for POINT’s current product candidates or any product candidates that it may develop. If public perception is influenced by claims that radioligands or specific therapies within radioligands are unsafe or less safe than available alternatives, POINT’s product candidates may not be accepted by the general public or the medical community.
In particular, the future commercial success of POINT’s product candidates, as applicable, depends and will depend upon, among other things, these product candidates gaining and maintaining acceptance by physicians, patients, third-party payors and other members of the medical community as efficacious and cost-effective alternatives to competing products and treatments. If any of POINT’s product candidates do not achieve and maintain an adequate level of acceptance, POINT may not generate material sales of that product candidate or be able to successfully commercialize it. The degree of market acceptance of POINT’s product candidates will depend on a number of factors, including:
•POINT’s ability to provide acceptable evidence of safety and efficacy;
•the prevalence and severity of any side effects and any contraindications, drug interactions or other limitations included in the product labeling of any product candidates that may receive regulatory approval;
•publicity concerning its product candidates or competing products and treatments;
•availability, relative cost and relative efficacy of alternative and competing treatments;
•the ability to offer its product candidates, if approved, for sale at competitive prices;
•the relative convenience and ease of administration of its product candidates;
•the willingness of the target patient population to try new product candidates and of physicians to prescribe these product candidates;
•the strength of marketing and distribution support; and
•the sufficiency of coverage or reimbursement by third parties.
If POINT’s product candidates, if approved, do not become widely accepted by potential customers, physicians, patients, third-party payors and other members of the medical community, such a lack of acceptance could have a material adverse effect on POINT’s business, financial condition and results of operations.
POINT may be unable to obtain regulatory approval for its product candidates under applicable regulatory requirements. The denial or delay of any such approval would delay commercialization of POINT’s product candidates and adversely impact its potential to generate revenue, its business and its results of operations.
The research, testing, manufacturing, labeling, licensure, sale, marketing and distribution of drug products are subject to extensive regulation by the FDA and similar regulatory authorities in the U.S. and other countries, and such regulations differ from country to country. POINT is not permitted to market its product candidates in the U.S. or in any foreign countries until they receive the requisite marketing approval from the applicable regulatory authorities of such jurisdictions.
44
The FDA and similar foreign regulatory authorities can delay, limit or deny marketing authorization of POINT’s product candidates for many reasons, including:
•its inability to demonstrate to the satisfaction of the FDA or similar foreign regulatory authority that any of its product candidates are safe and effective;
•the FDA’s or the applicable foreign regulatory agency’s disagreement with its trial protocols, trial designs or the interpretation of data from preclinical studies or clinical trial;
•its inability to demonstrate that the clinical and other benefits of any of its product candidates outweigh any safety or other perceived risks;
•the FDA’s or the applicable foreign regulatory agency’s requirement for additional preclinical studies or clinical trial;
•the results of clinical trials may not meet the level of statistical significance required by the FDA or similar foreign regulatory authorities for marketing approval, or that regulatory agencies may require it to include a larger number of participants than POINT anticipated;
•the FDA’s or the applicable foreign regulatory agency’s failure to approve the manufacturing processes or facilities of third-party manufacturers upon which it relies;
•the quality of its product candidates or other materials necessary to conduct preclinical studies or clinical trials of its product candidates, including any potential companion diagnostics, (imaging radioligand for participant selection) may be insufficient or inadequate;
•the potential for approval policies or regulations of the FDA or similar foreign regulatory authorities to significantly change in a manner rendering its clinical data insufficient for marketing approval; or
•the data collected from clinical trials of its product candidates may not be sufficient to the satisfaction of the FDA or comparable foreign regulatory authorities to support the submission of a NDA or other comparable submission in foreign jurisdictions or to obtain approval of its product candidates in the U.S. or elsewhere.
Any of these factors, many of which are beyond POINT’s control, may result in POINT failing to obtain regulatory approval to market any of its product candidates, which would significantly harm its business, results of operations and prospects. Of the large number of drug products in development, only a small percentage successfully complete the FDA or similar regulatory approval processes and are commercialized. Even if POINT eventually completes clinical testing and receives marketing authorization from the FDA or similar foreign regulatory authorities for any of its product candidates, the FDA or similar foreign regulatory agency may grant approval contingent on the performance of costly additional clinical trials which may be required after approval. The FDA or similar foreign regulatory agency also may approve POINT’s product candidates for a more limited indication or a narrower patient population than POINT originally requested, and the FDA or similar other foreign regulatory agency, may not approve its product candidates with the labeling that it believes is necessary or desirable for the successful commercialization of such product candidates.
In addition, even if the trials are successfully completed, preclinical and clinical data are often susceptible to varying interpretations and analyses, and POINT cannot guarantee that the FDA or similar foreign regulatory authorities will interpret the results as POINT does, and more preclinical studies and clinical trials could be required before POINT submits its product candidates for approval. To the extent that the results of the clinical trials are not satisfactory to the FDA or similar foreign regulatory authorities for support of a marketing application, approval of POINT’s product candidates may be significantly delayed, or it may be required to expend significant additional resources, which may not be available to it, to conduct additional clinical trials in support of potential approval of its product candidates.
Any delay in obtaining, or inability to obtain, applicable regulatory approval would delay or prevent commercialization of POINT’s product candidates and would materially adversely impact its business and prospects.
POINT’s preclinical studies and clinical trial may fail to adequately demonstrate the safety or effectiveness of any of POINT’s product candidates, which would prevent or delay development, regulatory approval and commercialization.
Before obtaining regulatory approvals for the commercial sale of POINT’s product candidates, including its lead product candidates, POINT must demonstrate through lengthy, complex and expensive preclinical studies and clinical trials that its product candidates are both safe and effective for use in each target indication. Preclinical studies and clinical trials
45
are expensive and can take many years to complete, and their outcomes are inherently uncertain. Failure can occur at any time during the preclinical study and clinical trial processes, and, because POINT’s product candidates are in an early stage of development, there is a high risk of failure and POINT may never succeed in developing marketable products.
Any preclinical studies or clinical trials that POINT may conduct may not demonstrate the safety or effectiveness necessary to obtain regulatory approval to market its product candidates. If the results of POINT’s ongoing or future preclinical studies and clinical trials are inconclusive, if POINT does not meet the clinical endpoints with statistical and clinically meaningful significance, or if there are safety concerns associated with POINT’s product candidates, POINT may be prevented or delayed in obtaining marketing approval for such product candidates. In some instances, there can be significant variability in results between different preclinical studies and clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the clinical trial protocols and the rate of dropout among clinical trial participants.
In addition, for POINT’s phase 3 clinical trial of 177Lu-PNT2002 (SPLASH; NCT04647526), UHN's clinical trial of 177Lu-PNT2003 (OZM-067; NCT02743741) and any future clinical trials that may be completed for other product candidates, POINT cannot guarantee that the FDA will interpret the results as POINT does, and more trials could be required before it submits its product candidates for approval. To the extent that the results of the trials are not satisfactory to the FDA to support a marketing application, approval of POINT’s product candidates may be significantly delayed or prevented entirely, or it may be required to expend significant additional resources, which may not be available to it, to conduct additional trials in support of potential approval of its product candidates.
The results of preclinical studies and early-stage clinical trials may not be predictive of future results. Initial success in POINT’s ongoing clinical trials may not be indicative of results obtained when these trials are completed or in later-stage trials.
The results of preclinical studies may not be predictive of the results of clinical trials, and the results of any early-stage clinical trials POINT commences may not be predictive of the results of the later-stage clinical trials. In addition, initial success in clinical trials may not be indicative of results obtained when such trials are completed. There can be no assurance that any of POINT’s current or future clinical trials will ultimately be successful or support further clinical development of any of its product candidates. There is a high failure rate for drugs proceeding through clinical trials.
A number of companies in the pharmaceutical industry have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in POINT’s clinical development could have a material adverse effect on its business and operating results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses and many companies that believed their product candidates performed satisfactorily in preclinical studies or clinical trials nonetheless failed to obtain FDA approval or approval from foreign regulatory authorities.
Interim, “top line” and preliminary data from POINT’s clinical trials that it announces or publishes from time to time may change as more participant data becomes available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, POINT may publish interim, “top line” or preliminary data from its clinical trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a full analysis of all data related to the particular trial. POINT has included certain interim data obtained from UHN in this Form 10-K, which is also subject to change following a full analysis of all data related to the particular trial. POINT also makes assumptions, estimations, calculations and conclusions as part of its analyses of data, and it may not have received or had the opportunity to fully and carefully evaluate all data. For example, POINT’s ongoing trials of 177Lu-PNT2002 and 177Lu-PNT2003 are open-label trials and it may decide to disclose interim, “top line,” or preliminary safety data at certain points in their development. Such data from clinical trials that POINT may complete are subject to the risk that one or more of the clinical outcomes may materially change as participant enrollment continues and more participant data become available. Interim, “top line” or preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data POINT previously published. As a result, interim, “top line,” and preliminary data should be viewed with caution until the final data are available. Adverse differences between interim, “top line” or preliminary data and final data could significantly harm POINT’s reputation and business prospects.
In addition, the information POINT chooses to publicly disclose regarding a particular study or clinical trial is distilled from a large body of raw data and you or others may not agree with what POINT determines is the material or otherwise appropriate information to include in its disclosures, and any information POINT determines not to disclose may ultimately
46
be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular drug, product candidate or its business. If the interim, “top line,” or preliminary data that POINT reports differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, POINT’s ability to obtain approval for and commercialize its product candidates, its business, prospects, financial condition and results of operations may be harmed.
POINT has never commercialized a product candidate and may experience delays or unexpected difficulties in obtaining regulatory approval for its current and future product candidates.
POINT has never obtained regulatory approval for, or commercialized, a drug. It is possible that the FDA may refuse to accept any or all of POINT’s planned NDAs for substantive review or may conclude after review of POINT’s data that its application is insufficient to obtain regulatory approval for any product candidates. If the FDA does not approve any of POINT’s planned NDAs, it may require that POINT conduct additional costly clinical trials, preclinical studies or manufacturing validation studies before it will reconsider POINT’s applications. Depending on the extent of these or any other FDA- required studies, approval of any NDA or other application that POINT submits may be significantly delayed, possibly for several years, or may require POINT to expend more resources than it has available. Any failure or delay in obtaining regulatory approvals would prevent POINT from commercializing its product candidates, generating revenues and achieving and sustaining profitability. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA to approve any NDA or other application that POINT submits. If any of these outcomes occur, POINT may be forced to abandon the development of its product candidates, which would materially adversely affect its business and could potentially cause it to cease operations. POINT faces similar risks for its applications in foreign jurisdictions.
POINT’s product candidates may cause adverse events, undesirable side effects or have other properties that could halt their preclinical or clinical development, prevent, delay, or cause the withdrawal of their regulatory approval, limit their commercial potential, or result in significant negative consequences, including death of patients. If any of POINT’s product candidates receive marketing approval and POINT, or others, later discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, POINT’s ability, or that of any collaborators, to market the drug could be compromised.
As with most drug products, use of POINT’s product candidates could be associated with undesirable side effects or adverse events which can vary in severity from minor reactions to death and in frequency from infrequent to prevalent. Undesirable side effects or unacceptable toxicities caused by POINT’s product candidates could cause it or regulatory authorities to interrupt, delay, or halt clinical trials.
Treatment-related undesirable side effects or adverse events could also affect participant recruitment or the ability of enrolled participants to complete the trial, or could result in potential product liability claims. In addition, these side effects may not be appropriately or timely recognized or managed by the treating medical staff, particularly outside of the research institutions that collaborate with POINT. POINT expects to have to educate and train medical personnel using its product candidates to understand their side effect profiles, both for its clinical trial for 177Lu-PNT2002 (SPLASH; NCT04647526), UHN's clinical trial of 177Lu-PNT2003 (OZM-067; NCT02743741), and for any future clinical trials and upon any commercialization of any product candidates. Inadequate training in recognizing or managing the potential side effects of POINT’s product candidates could result in adverse events to patients, including death. Additionally, there could be later discovery of longer-term undesirable side effects associated with POINT’s product candidates, including the potential discovery of late radiation toxicity. Any of these occurrences may materially and adversely harm POINT’s business, financial condition, results of operations and prospects.
Clinical trials of POINT’s product candidates must be conducted in carefully defined subsets of patients who have agreed to enter into clinical trials. Consequently, it is possible that POINT’s clinical trials, or those of any collaborator, may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify undesirable side effects. If one or more of POINT’s product candidates receives marketing approval and POINT, or others, discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, including during any long-term follow-up observation period recommended or required for patients who receive treatment using POINT’s products, a number of potentially significant negative consequences could result, including:
•regulatory authorities may withdraw approvals of such product, seize the product, or seek an injunction against its manufacture or distribution;
•POINT, or any collaborators, may be required to recall the product, change the way such product is administered to patients or conduct additional clinical trials;
47
•additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular product;
•regulatory authorities may require additional warnings on the label, such as a “black box” warning or a contraindication, or impose distribution or use restrictions;
•POINT, or any collaborators, may be required to create a Risk Evaluation and Mitigation Strategy, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers, and/or other elements to assure safe use;
•POINT, or any collaborators, may be subject to fines, injunctions or the imposition of civil or criminal penalties;
•POINT, or any collaborators, could be sued and held liable for harm caused to patients;
•the drug may become less competitive; and
•POINT’s reputation may suffer.
Any of the foregoing could prevent POINT from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm POINT’s business, results of operations, and prospects, and could adversely impact its financial condition, results of operations or the market price of its common shares.
COVID-19, escalating military fighting between Russia and Ukraine, terrorism or other geopolitical events may continue to adversely affect POINT’s business and financial results.
POINT’s business has been and may continue to be adversely affected by health epidemics such as the COVID-19 pandemic in regions where it has clinical trial sites or other business operations, and COVID-19 may continue to cause significant disruption in the operations of third-party manufacturers and CROs upon whom POINT relies.
In an attempt to contain the spread and impact of the COVID-19 pandemic, travel bans and restrictions, quarantines, shelter-in-place orders and other limitations on business activity have been implemented globally. Specifically, the U.S. government-imposed travel restrictions on travel between the U.S., Europe and certain other countries, and other countries have imposed travel restrictions against those traveling to or from the U.S. and other countries. Although some restrictions aimed at minimizing the spread of COVID-19 have been eased or lifted in the U.S. and other countries, in response to local surges and new waves of infection, some countries, states, and local governments may reinstitute these restrictions from time to time. POINT has a principal executive office in Toronto, Ontario and a manufacturing facility in Indianapolis, Indiana.
In response to public health directives and orders and to help minimize the risk of the virus to POINT’s employees, POINT has taken precautionary measures, including implementing work-from-home policies for certain employees. The effects of the public health directives and orders and POINT’s work-from-home policies may negatively impact productivity, disrupt its business and delay its clinical programs and timelines and any future clinical trials, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on POINT’s ability to conduct its business in the ordinary course. These and similar, and perhaps more severe, disruptions in POINT’s operations could negatively impact its business, financial condition and results of operations, including its ability to obtain financing.
Quarantines, shelter-in-place and similar government orders, or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could occur, related to COVID-19, the identification of emerging variants or other infectious diseases could impact personnel at third-party manufacturing facilities in the U.S. and other countries, or the availability or cost of materials, which would disrupt POINT’s supply chain.
In addition, POINT’s clinical trials of 177Lu-PNT2002, and any future clinical trials, have been and may be further affected by the COVID-19 pandemic, including:
•delays or difficulties in enrolling participants in the clinical trial, including participant inability to comply with clinical trial protocols if quarantines impede participant movement or interrupt healthcare services;
•delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff;
48
•diversion or prioritization of healthcare resources away from the conduct of clinical trials and towards the COVID-19 pandemic, including the diversion of hospitals serving as its clinical trial sites and hospital staff supporting the conduct of its clinical trials, who, as healthcare providers, may have heightened exposure to COVID-19 and adversely impact its clinical trial operations;
•interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal, state or provincial governments, employers and others; and
•limitations in employee resources that would otherwise be focused on the conduct of its clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people.
For POINT’s clinical trials that it may conduct at sites outside the U.S., in addition to the risks listed above, it has also experienced, and may also in the future experience, the following adverse impacts:
•delays in receiving approval from local regulatory authorities to initiate its planned clinical trials;
•delays in clinical sites receiving the supplies and materials needed to conduct its clinical trials;
•interruption in global shipping that may affect the transport of clinical trial materials, such as investigational drug products and comparator drugs used in its clinical trials;
•changes in local regulations as part of a response to the COVID-19 outbreak, which may require it to change the ways in which its clinical trials are conducted, which may result in unexpected costs, or to discontinue the clinical trials altogether;
•delays in necessary interactions with local regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; and
•the refusal of the FDA to accept data from clinical trials in these affected geographies.
Additionally, our clinical trials may be adversely affected by the current or anticipated impact of military conflict, including escalating military fighting between Russia and Ukraine, terrorism or other geopolitical events. The U.S. and other nations in response to the Russo-Ukrainian conflict have announced economic sanctions which may have an adverse effect on our business, financial condition and results of operations. Our SPLASH trial has vendor staff in Ukraine, and any political instability in the region may disrupt resourcing assigned to our trial and negatively impact or business. We are monitoring the potential impact of the COVID-19 pandemic and the Russo-Ukrainian conflict on our business.
The global COVID-19 pandemic and emergence of variants continues to evolve. The extent to which the COVID-19 pandemic may impact POINT’s business and clinical trials will depend on future developments, which are uncertain and cannot be predicted with confidence, such as the duration of any outbreak, travel restrictions and social distancing in the U.S. and other countries, business closures or business disruptions, the effectiveness of actions taken in the U.S. and other countries to contain and treat the disease, and the efficacy and the ability to widely distribute vaccines.
The market opportunities for POINT’s product candidates may be smaller than POINT anticipated or may be limited to those patients who are ineligible for or have failed prior treatments. If POINT encounters difficulties enrolling participants in its clinical trials, its clinical development activities could be delayed or otherwise adversely affected.
POINT’s current and future target patient populations are based on its beliefs and estimates regarding the competitive environment and incidence or prevalence of certain types of cancers that may be addressable by its product candidates, which is derived from a variety of sources, including scientific literature and surveys of clinics. POINT’s estimates may prove to be incorrect and the number of potential patients may turn out to be lower than expected. Even if POINT obtains significant market share for its product candidates, because the potential target populations could be small, POINT may never achieve profitability without obtaining regulatory approval for additional indications, including use of its product candidates for front-line and second-line therapy.
POINT may initially seek approval of some of its product candidates as second- or third-line therapies for patients who have failed other approved treatments. Subsequently, for those product candidates that prove to be sufficiently beneficial, if any, POINT would expect to seek approval as a second-line therapy and potentially as a front-line therapy, but there is no guarantee that its product candidates, even if approved for third-line therapy, would be approved for second-line or front-
49
line therapy. In addition, POINT may have to conduct additional clinical trials prior to gaining approval for second-line or front-line therapy.
POINT may encounter difficulties enrolling participants in its clinical trials, and its clinical development activities could thereby be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on POINT’s ability to enroll a sufficient number of participants who remain in the trial until its conclusion. POINT may experience difficulties in participant enrollment in its clinical trials for a variety of reasons, including:
•the size and nature of the patient population;
•the participant eligibility criteria defined in the protocol;
•the size of the trial population required for analysis of the trial’s primary endpoints;
•the proximity of participants to trial sites;
•the design of the trial;
•its ability to recruit clinical trial investigators with the appropriate competencies and experience;
•competing clinical trials for similar therapies or other new therapeutics not involving its product candidates and or related technologies;
•clinicians’ and patients’ perceptions as to the potential advantages and side effects of the type of targeted radioligand therapy of the product candidate being studied in relation to other available therapies, including any new drugs or treatments that may be approved for the indications it is investigating;
•its ability to obtain and maintain patient consents; and
•the risk that participants enrolled in clinical trials will not complete a clinical trial.
In addition, POINT’s clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as POINT’s product candidates, and this competition will reduce the number and types of patients available to it, because some patients who might have opted to enroll in POINT’s trials may instead opt to enroll in a trial being conducted by one of its competitors. POINT may conduct some of its clinical trials at the same clinical trial sites that some of its competitors use, which will reduce the number of patients who are available for its clinical trials at such clinical trial sites. Moreover, because POINT’s product candidates represent a departure from more commonly used methods for cancer treatment, potential participants and their doctors may be inclined to only use conventional therapies, such as chemotherapy and external beam radiation, rather than enroll in any future clinical trial.
Even if POINT is able to enroll a sufficient number of participants in its clinical trials, delays in participant enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect POINT’s ability to advance the development of its product candidates.
POINT currently has a minimal marketing and sales organization and has no experience in marketing products. If POINT is unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell its product candidates, if approved for commercial sale, it may not be able to generate product revenue.
POINT currently has a very minimal in-house sales, marketing or distribution capabilities and has no experience in marketing products. POINT intends to further develop its in-house marketing organization and sales force, which will require significant capital expenditures, management resources and time. POINT will have to compete with other pharmaceutical companies to recruit, hire, train and retain marketing and sales personnel.
If POINT is unable or decides not to establish internal sales, marketing and distribution capabilities, it will pursue collaborative arrangements regarding the sales and marketing of its product candidates, if approved and licensed. However, there can be no assurance that POINT will be able to establish or maintain such collaborative arrangements, or if it is able to do so, that they will have effective sales forces. In connection with 177Lu-PNT2002 and 177Lu-PNT2003, POINT has elected to enter into the License agreements with Lantheus, pursuant to which Lantheus will market and sell 177Lu-
50
PNT2002 and 177Lu-PNT2003, if they are approved. Any revenue POINT receives will depend upon the efforts of Lantheus and any other third parties, which may not be successful. POINT may have little or no control over the marketing and sales efforts of such third parties and its revenue from product sales may be lower than if it had commercialized its product candidates itself. POINT also faces competition in its search for third parties to assist it with the sales and marketing efforts of its product candidates.
There can be no assurance that POINT will be able to develop in-house sales and distribution capabilities or establish or maintain relationships with third-party collaborators to commercialize any product in the U.S. or overseas for which it is able to obtain regulatory approval.
If POINT evolves from a company primarily involved in clinical development to a company also involved in commercialization, it may encounter difficulties in managing its growth and expanding its operations successfully.
If POINT is able to advance its product candidates through clinical trials, it will need to expand its development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for it. If POINT’s operations expand, it expects that it may need to manage additional relationships with such third parties, as well as additional collaborators and suppliers.
Maintaining these relationships and managing POINT’s future growth will impose significant added responsibilities on members of its management and other personnel. POINT must be able to: manage its development efforts effectively; manage its clinical trials effectively; hire, train and integrate additional management, development, administrative and sales and marketing personnel; improve its managerial, development, operational and finance systems; and expand its facilities, all of which may impose a strain on its administrative and operational infrastructure. POINT may also begin to expand its capabilities or enter into contractual relationships during the later stage clinical trial or regulatory approval process, and then have to reduce its capabilities or terminate those relationships if the trials or approval processes are terminated.
Due to POINT's limited access to capital and the significant resources required for the development of its product candidates, POINT must prioritize development of certain of its product candidates over others. In doing so, POINT may expend its limited resources to pursue a particular product candidate and forgo the opportunity ti capitalize on product candidates or indications that may ultimately be more profitable or for which there is a greater likelihood of success.
POINT has limited resources and access to capital and, as a result, must decide which product candidates to pursue and the amount of resources to allocate to each. POINT’s decisions regarding the allocation of research, collaboration, management, financial and other resources toward the development of its lead product candidates may not result in the development of viable commercial products, and may divert resources away from opportunities with a greater likelihood of commercial success. Such decisions may cause POINT to fail to capitalize on profitable market opportunities. Further, POINT’s spending on current and future research and development programs and other future product candidates for specific indications may not yield any commercially viable future product candidates. If POINT does not accurately evaluate the commercial potential or target market for a particular future product candidate or misreads certain trends in the biopharmaceutical industry, it may relinquish valuable rights to those future product candidates through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for POINT to retain sole development and commercialization rights to such future product candidates.
POINT currently conducts and may in the future conduct clinical trials for its product candidates outside the U.S., and the FDA and similar foreign regulatory authorities may not accept data from such trials.
POINT is currently conducting clinical trials in Canada, Europe, and the U.K. and may in the future choose to conduct additional clinical trials outside the U.S., including in Australia and other foreign jurisdictions. The acceptance of trial data from clinical trials conducted outside the U.S. by the FDA may be subject to certain conditions. In cases where data from clinical trials conducted outside the U.S. are intended to serve as the sole basis for marketing approval in the U.S., the FDA will generally not approve the application on the basis of foreign data alone unless (i) the data are applicable to the U.S. population and U.S. medical practice; (ii) the trials were performed by clinical investigators of recognized competence and (iii) the data may be considered valid without the need for an on-site inspection by the FDA or, if the FDA considers such an inspection to be necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means. Additionally, the FDA’s clinical trial requirements, including sufficient size of patient populations and statistical powering, must be met. Many foreign regulatory bodies have similar approval requirements. In addition, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. There can be no assurance that the FDA or any similar foreign regulatory authority will accept data from trials conducted outside of the U.S. or the applicable jurisdiction. If the FDA or any similar foreign regulatory authority does not accept such data, it would result in the need for additional trials, which would be costly and time-consuming and delay aspects of POINT’s business plan, and
51
which may result in its product candidates not receiving approval or clearance for commercialization in the applicable jurisdiction.
If POINT’s competitors develop and market products that are more effective, safer or less expensive than its product candidates, its commercial opportunities will be negatively impacted.
The pharmaceutical industry is highly competitive, and POINT faces significant competition from many companies that are researching and marketing products designed to address various types of cancer and other indications it treats or may treat in the future. POINT is currently developing cancer therapeutics that will compete with other drugs and therapies that currently exist or are being developed. Also, certain of POINT’s product candidates may be clinically developed not as an initial first line therapy but as a therapy for patients whose tumors have developed resistance to first line chemotherapy, which limits its potential addressable market. Products POINT may develop in the future are also likely to face competition from other drugs and therapies.
Many of POINT competitors have significantly greater financial, manufacturing, marketing and drug development resources than POINT does. Large pharmaceutical companies, in particular, have extensive experience in clinical testing and in obtaining regulatory approvals for drugs. Additional mergers and acquisitions in the pharmaceutical industry may result in even more resources being concentrated by POINT’s competition. Competition may increase further as a result of advances in the commercial applicability of technologies currently being developed and a greater availability of capital investment in those fields. These companies may also have significantly greater research and marketing capabilities than POINT does. In addition, many universities and private and public research institutes are active in cancer research, the results of which may result in direct competition with POINT’s product candidates.
In certain instances, the drugs which will compete with POINT’s product candidates are widely available or established, with existing standards of care. To compete effectively with these drugs, POINT’s product candidates will need to demonstrate advantages that lead to improved clinical safety or efficacy compared to these competitive products. POINT cannot assure you that it will be able to achieve competitive advantages versus alternative drugs or therapies. If POINT’s competitors’ market products are more effective, safer or less expensive than POINT’s product candidates or reach the market sooner than POINT’s product candidates, POINT may not achieve commercial success.
POINT believes that its ability to successfully compete will depend on, among other things:
•its ability to design and successfully execute appropriate clinical trials;
•its ability to recruit and enroll participants for its clinical trials;
•the results of its clinical trials and the efficacy and safety of its product candidates;
•the speed at which it develops its product candidates;
•achieving and maintaining compliance with regulatory requirements applicable to its business;
•the timing and scope of regulatory approvals, including labeling;
•adequate levels of reimbursement under private and governmental health insurance plans, including Medicare;
•its ability to protect intellectual property rights related to its product candidates;
•its ability to commercialize and market any of its product candidates that may receive regulatory approval;
•its ability to have any partners manufacture and sell commercial quantities of any approved product candidates to the market;
•acceptance of its product candidates by physicians, other healthcare providers and patients; and
•the cost of treatment in relation to alternative therapies.
In addition, the pharmaceutical industry is characterized by rapid technological change. POINT’s future success will depend in large part on its ability to maintain a competitive position with respect to these technologies. POINT’s competitors may render POINT’s technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in POINT’s drug discovery process
52
that it believes it derives from its research approach and proprietary technologies. Also, because POINT’s research approach integrates many technologies, it may be difficult for POINT to stay abreast of the rapid changes in each technology. If POINT fails to stay at the forefront of technological change, it may be unable to compete effectively.
Risks Related to POINT’s Manufacturing Operations
POINT’s product candidates are radioligands and the manufacture of its product candidates is complex. POINT has constructed a manufacturing facility with the intent to manufacture most, if not all, of any approved drugs itself.
POINT’s product candidates are radioligands and the process of manufacturing them is complex, highly regulated and subject to multiple risks. The facility constructed by POINT to manufacture its product candidates and future approved drugs is subject to applicable laws, regulations, and GMP. These regulations govern manufacturing processes and procedures, including record keeping and the implementation and operation of quality management systems to control and assure the quality of investigational products and products approved for sale.
POINT has built a manufacturing facility in Indianapolis, Indiana and may build additional manufacturing facilities in other markets to expand its manufacturing capacity. These facilities may encounter unanticipated delays and expenses due to a number of factors, including regulatory requirements. If construction, regulatory evaluation, and/or approval of POINT’s current or future facility(s) is delayed, POINT may not be able to manufacture sufficient quantities of its drug candidates, if approved, which could limit POINT’s development and commercialization activities and its opportunities for growth. Cost overruns associated with constructing or maintaining POINT’s current or future facility(s) could require POINT to raise additional funds from other sources.
To produce POINT’s drug candidates in the quantities that it believes will be required to meet anticipated market demand, if approved, POINT will need to increase or “scale up” the production process by a significant factor over expected initial levels of production. A significant part of the scaling up process will include seeking ways to increase the automation and semi-automation of POINT’s production process, which will require additional research and development, investment, potential new regulatory approvals, and cooperation with third-parties, some of which may not be successful. If POINT is unable or delayed in scaling up, or if the cost of doing so is not economically feasible for POINT, POINT may not be able to produce its drug candidates, if approved, in a sufficient quantity to meet future demand.
Any problems with receiving and maintaining regulatory approvals for POINT’s manufacturing facilities, could delay its development plans or commercialization efforts.
POINT’s manufacturing facilities are subject to ongoing, periodic inspection by various regulatory authorities, including the NRC, as well as the FDA, Health Canada and other comparable regulatory agencies to ensure compliance with cGMP. POINT’s failure to follow and document its adherence to such cGMP or other regulatory requirements may lead to significant delays in the availability of product candidates for clinical or, in the future, commercial use, and may result in the termination of or a hold on a clinical trial, or may delay or prevent filing or approval of marketing applications for POINT’s drug candidates or the commercialization of its drugs, if approved. POINT also may encounter manufacturing problems with the following:
•achieving adequate or clinical-grade materials that meet FDA, European Medicines Agency ("EMA), National Medical Products Administration (China) ("NMPA"), Health Canada, Therapeutic Goods Administration (Australia) ("TGA") or other comparable regulatory agency standards or specifications with consistent and acceptable production yield and costs;
•shortages of qualified personnel, raw materials or key contractors; and
•ongoing compliance with GMP and other requirements of the FDA, EMA, NMPA, TGA or other comparable regulatory agencies.
Failure to comply with applicable regulations could also result in sanctions being imposed on POINT, including fines, injunctions, civil penalties, a requirement to suspend or put on hold one or more of POINT’s clinical trials, failure of regulatory authorities to grant marketing approval of POINT’s drug candidates, delays, suspension or withdrawal of approvals, supply disruptions, license revocation, seizures, or recalls of POINT’s drug candidates, operating restrictions and civil or criminal prosecutions, any of which could harm POINT’s business.
Damage to, destruction of or interruption of production at POINT’s manufacturing facilities would negatively affect its business and prospects.
53
If POINT’s manufacturing facilities or the equipment in them is damaged or destroyed, POINT may not be able to quickly or inexpensively replace its manufacturing capacity or replace it at all. In the event of a temporary or protracted loss of the facilities or equipment, POINT might not be able to transfer manufacturing to a third party. Even if POINT could transfer manufacturing to a third party, the shift would likely be expensive and time-consuming, particularly since the new facility would need to comply with the necessary regulatory requirements and POINT would need regulatory agency approval before selling any of POINT’s drug candidates, if approved, manufactured at that new facility. Such an event could delay POINT’s clinical trials or reduce POINT’s product sales if and when POINT is able to commercialize one or more of its drug candidates. Any interruption in manufacturing operations at POINT’s manufacturing facilities could result in its inability to satisfy the demands of its clinical trials or commercialization. Any disruption that impedes POINT’s ability to manufacture its drugs in a timely manner could materially harm its business, financial condition and operating results.
Any failure to perform proper quality control and quality assurance would have a material adverse effect on POINT’s business and financial results.
The manufacturing of POINT’s drug candidates, if approved, and any future approved drugs is subject to applicable laws, regulations, and cGMP. These regulations govern manufacturing processes and procedures, including record keeping and the implementation and operation of quality management systems to control and assure the quality of investigational products and products approved for sale. POINT and/or its third party manufacturers apply stringent quality controls at each stage of its production process to comply with these requirements. POINT and/or its third party manufacturers perform extensive tests throughout the manufacturing processes to ensure the safety and effectiveness of its drug candidates. POINT and/or its third party manufacturers may, however, detect instances in which an unreleased product was produced without adherence to its manufacturing procedures or the raw material used in the production process was not collected to store in accordance with the cGMP or other regulations, resulting in a determination that the implicated products should be destroyed. In addition, if POINT and/or its third party manufacturers fail to comply with relevant quality control requirements under laws and cGMP, POINT could experience a disruption in the supply of POINT’s product candidates, which could delay or prevent further sales of such product candidates, which could have a material adverse effect on POINT’s business and financial results. In addition, quality issues may arise during scale-up activities. If POINT and/or its third party manufacturers are unable to successfully ensure consistent and high quality of its product candidates during large-volume production, the sales of its product candidates may not be able to be promoted, which could have a material adverse effect on its business and financial results. In addition, quality issues may arise during scale-up activities. If POINT is unable to successfully ensure consistent and high quality of its product candidates during large-volume production, the sales of its products may not be able to be promoted, which could have a material adverse effect on its business and financial results.
POINT has limited experience as a company managing a manufacturing facility.
POINT’s operation of its manufacturing facility requires significant resources, and POINT, as a company, has limited experience managing a manufacturing facility. In part because of its inexperience, POINT may have unacceptable or inconsistent product quality success rates and yields, and may be unable to maintain adequate quality control, quality assurance, and qualified personnel. In addition, if POINT switches from its current contract manufacturers to its own manufacturing facility for one or more of its product candidates in the future, it may need to conduct additional preclinical studies to bridge its modified product candidates to earlier versions. Failure to successfully operate its manufacturing facility could adversely affect the commercial viability of POINT’s product candidates.
Risks Related to POINT’s Reliance on Third Parties
Although POINT operates its own manufacturing facility, it currently relies, and will likely continue to rely, on third parties to manufacture additional supply of its lead product candidates for its ongoing clinical trial and its preclinical studies, as well as any preclinical studies or clinical trials of its future product candidates that it may conduct.
Although POINT operates its own manufacturing facility, it still relies, and is likely to continue to rely on third parties to manufacture additional supply of its product candidates. If POINT’s third-party suppliers fail to comply with relevant quality control requirements under laws and cGMP or contaminations are discovered in POINT’s product candidates or in the manufacturing facilities in which its product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Any such event could cause a disruption in the supply of POINT’s product candidates, which could delay or prevent further sales of such product candidates, which could have a material adverse effect on POINT’s business and financial results.
POINT’s third-party suppliers’ failure to achieve and maintain high manufacturing standards, in accordance with applicable regulatory requirements, or the incidence of manufacturing errors, could result in patient injury or death, product shortages, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems
54
that could seriously harm POINT’s business. Contract manufacturers often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel.
If any contract manufacturer with whom POINT contracts fails to perform its obligations, POINT may be forced to manufacture the materials itself sooner than expected, but POINT may not have the capabilities or resources to do so and may need to enter into an agreement with a different contract manufacturer. However, POINT may not be able to enter into such an agreement on terms as favorable to it. In either scenario, POINT’s clinical trials supply could be delayed as it establishes alternative supply sources. In some cases, such as under the CPDC Clinical Supply Agreement, the technical skills required to manufacture POINT’s product candidates may be unique or proprietary to the original contract manufacturer and POINT may have difficulty, or there may be contractual restrictions prohibiting POINT from, transferring such skills to a back-up or alternate supplier, or POINT may be unable to transfer such skills at all. In addition, if POINT is required to change contract manufacturers for any reason, it will be required to verify that the new contract manufacturers maintains facilities and procedures that comply with quality standards and with all applicable regulations. POINT will also need to verify, such as through a manufacturing comparability study, that any new manufacturing process will produce POINT’s product candidate according to the specifications previously submitted to the FDA or another regulatory authority. The delays associated with the verification of a new contract manufacturer or transition to POINT’s own manufacturing earlier than expected could negatively affect POINT’s ability to develop product candidates or commercialize POINT’s product candidates in a timely manner or within budget. Furthermore, a contract manufacturer, such as the CPDC, may possess technology related to the manufacture of POINT’s product candidate that such contract manufacturer owns independently. This would increase POINT’s reliance on such contract manufacturer or require POINT to obtain a license from such contract manufacturer in order to have another contractor manufacture its product candidates. In addition, changes in manufacturers often involve changes in manufacturing procedures and processes, which could require that POINT conducts bridging studies between its prior clinical supply used in its clinical trials and that of any new manufacturer. POINT may be unsuccessful in demonstrating the comparability of clinical supplies which could require the conduct of additional clinical trials.
POINT may be unable to obtain a sufficient supply of radioisotopes to support clinical development or at commercial scale.
POINT currently relies on third-party entities for the supply of its raw materials and for manufacturing. N.c.a. 177Lu is a key component of some of POINT’s product candidates. POINT’s current suppliers of n.c.a. 177Lu are located in Germany and Israel and POINT may encounter issues with importing n.c.a. 177Lu into the U.S., including on account of any shipping interruptions or delays due to the COVID-19 pandemic. To date, POINT has obtained n.c.a. 177Lu for the clinical trials of 177Lu-PNT2002 and 177Lu-PNT2003 from Isotopia Molecular Imaging LTD and ITG Isotope Technologies Garching GmbH. The isotopes for these targeted radioligand therapies are shipped to either POINT’s CMO, the Centre for Probe Development and Commercialization located in southern Ontario, Canada, or POINT’s Indianapolis, IN facility.
Currently, POINT believes there is sufficient supply of the needed radioisotopes to advance the ongoing 177Lu-PNT2002 and 177Lu-PNT2004 clinical trials, support additional trials it may undertake and for commercialization of its product candidates. POINT continually evaluates manufacturers and suppliers of its radioisotopes and intends to have redundant suppliers prior to the commercial launch of 177Lu-PNT2002 and 177Lu-PNT2003, if approved.
POINT’s third-party suppliers may not perform their contracted services or may breach or terminate their agreements with POINT. POINT’s suppliers are subject to regulations and standards that are overseen by regulatory and government agencies and POINT has no control over its suppliers’ compliance to these standards. Failure to comply with regulations and standards may result in their inability to supply isotopes could result in delays in POINT’s clinical trials, which could have a negative impact on its business.
POINT also intends to produce n.c.a. 177Lu in-house. For the in-house production of n.c.a. 177Lu, POINT has secured access to a sufficient North American supply of 176Yb, obtained a license to n.c.a. 177Lu purification technology and has contracted with multiple research reactors to irradiate ytterbium-176. However, if POINT is not able to establish the n.c.a. 177Lu purification technology or expand its reactor network, or if the supplier of 176Yb is unable to produce sufficient product, POINT would be required to continue to rely on third-party suppliers as it currently does.
POINT relies on third parties to conduct the clinical trials of 177Lu-PNT2002 and 177Lu-PNT2003 and plans to rely on third parties to conduct future clinical trials. If these third parties do not properly and successfully carry out their contractual duties or meet expected deadlines, POINT may not be able to obtain regulatory approval of or commercialize its product candidates.
55
POINT depends and will continue to depend on independent investigators and collaborators, such as medical institutions, CROs, contract manufacturing organizations ("CMOs") and strategic partners to conduct and supply product candidates for its preclinical studies and clinical trials, including the clinical trials of 177Lu-PNT2003 and 177Lu-PNT2002. POINT expects to negotiate budgets and contracts with CROs, trial sites and CMOs, which may result in delays to its development timelines and increased costs. POINT will rely heavily on these third parties over the course of its clinical trials, and POINT controls only certain aspects of their activities. As a result, POINT will have less direct control over the conduct, timing and completion of these clinical trials and the management of data developed through clinical trials than would be the case if POINT were relying entirely upon its own staff. Nevertheless, POINT is responsible for ensuring that each of its studies is conducted in accordance with applicable protocol, legal and regulatory requirements and scientific standards, and its reliance on third parties does not relieve it of its regulatory responsibilities. POINT and these third parties are required to comply with GCPs, which are regulations and guidelines enforced by the FDA and similar foreign regulatory authorities for product candidates in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If POINT or any of these third parties fail to comply with applicable GCP regulations, the clinical data generated in POINT’s clinical trials may be deemed unreliable and the FDA or similar foreign regulatory authorities may require POINT to perform additional clinical trials before approving its marketing applications. POINT cannot assure you that, upon inspection, such regulatory authorities will determine that any of its clinical trials comply with the GCP regulations. In addition, POINT’s clinical trials must be conducted with drug products produced under cGMP regulations, and will require a large number of test participants. POINT’s failure or any failure by these third parties to comply with these regulations or to recruit a sufficient number of participants may require POINT to repeat clinical trials, which would delay the regulatory approval process. Moreover, POINT’s business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
Any third parties conducting POINT’s clinical trials are not and will not be POINT’s employees and, except for remedies available to POINT under its agreements with such third parties, POINT cannot control whether or not they devote sufficient time and resources to POINT’s ongoing, clinical and preclinical product candidates. These third parties may also have relationships with other commercial entities, including POINT’s competitors, for whom they may also be conducting clinical trials or other drug development activities, which could affect their performance on POINT’s behalf. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to POINT’s clinical protocols or regulatory requirements or for other reasons, POINT’s clinical trials may be extended, delayed or terminated and POINT may not be able to complete development of, obtain regulatory approval of or successfully commercialize its product candidates. As a result, POINT’s financial results and the commercial prospects for its product candidates would be harmed, its costs could increase and its ability to generate revenue could be delayed.
Switching or adding third parties to conduct POINT’s clinical trials involves substantial cost and requires extensive management time and focus and may ultimately be unsuccessful. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact POINT’s ability to meet its desired clinical development timelines.
POINT depends in part on UHN, CanProbe, Ozmosis Research Inc. and Cancer Care Ontario and other third-party collaborators to advance clinical development of 177Lu-PNT2003.
UHN is currently sponsoring the clinical trials involving 177Lu-PNT2003 (OZM-067; NCT02743741). The advancement of 177Lu-PNT2003 depends in part on the continued sponsorship by UHN, as POINT’s resources and capital would not be sufficient to conduct these trials on its own. UHN is not obligated to continue sponsorship of any clinical trials involving POINT’s product candidates and could stop their support at any time. If this or other third-party sponsors ceased their support for POINT’s product candidates, its ability to advance clinical development of product candidates could be limited and POINT may not be able to pursue the number of different indications for its product candidates that are currently being pursued.
Even if UHN continues to sponsor clinical trials of POINT’s product candidates, POINT’s reliance on their support subjects it to numerous risks. For example, POINT has limited control over the design, execution or timing of the clinical trials and limited visibility into the day-to-day activities, including with respect to how the sponsor is providing and administering POINT product candidates. If a clinical trial sponsored by a third party has a failure due to poor design of the trial, errors in the way the clinical trial is executed or for any other reason, or if the sponsor fails to comply with applicable regulatory requirements or if there are errors in the reported data, it could represent a major set-back for the development and approval of POINT’s product candidates, even if POINT was not directly involved in the trial and even if the clinical trial failure was not related to the underlying safety or efficacy of the product candidate. In addition, these third-party sponsors could decide to de-prioritize clinical development of POINT’s product candidates in relation to other projects,
56
which could adversely affect the timing of further clinical development. POINT is also subject to various confidentiality obligations with respect to the clinical trials sponsored by third party sponsors, which could prevent POINT from disclosing current information about the progress or results from these trials until the applicable sponsor publicly discloses such information or permits POINT to do so. This may make it more difficult to evaluate POINT’s business and prospects at any given POINT in time and could also impair its ability to raise capital on its desired timelines.
POINT intends to rely on its collaboration arrangement with Lantheus for the development and commercialization of its 177Lu-PNT2002 and 177Lu-PNT2003, and may form or seek collaborations or strategic alliances or enter into additional licensing arrangements in the future. Such arrangements, if unsuccessful, could result in delays and other obstacles in the development, manufacture or commercialization of POINT's product candidates and could materially harm its results of operations.
POINT expects to depend on Lantheus for the commercialization of 177Lu-PNT2002 and 177Lu-PNT2003. If the collaboration is not successful, we may not be able to realize the market potential of those product candidates. Lantheus has certain commercialization rights for 177Lu-PNT2002 and 177Lu-PNT2003 under the Lantheus License Agreements. Such arrangements with any third parties, generally provide us with shared or limited control over the amount and timing of resources that our collaborators dedicate to the development or potential commercialization of any product candidates we may seek to develop with them.
Our ability to generate revenue from these arrangements with commercial entities will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. We cannot predict the success of our current collaborations or any collaboration that we may enter into.
In addition, POINT may form or seek other strategic alliances, create joint ventures or collaborations, or enter into additional licensing arrangements with third parties that it believes will complement or augment its development and commercialization efforts with respect to its product candidates and any future product candidates that it may develop. Any of these relationships may require POINT to incur non-recurring and other charges, increase its near and long-term expenditures, issue securities that dilute its existing stockholders or disrupt its management and business.
Further, POINT faces significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. POINT may not be successful in its efforts to establish a strategic partnership or other alternative arrangements for its product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view POINT’s product candidates as having the requisite potential to demonstrate safety and effectiveness and obtain marketing approval.
Further, POINT's collaboration with Lantheus, and any similar future collaboration involving POINT’s product candidates are subject to numerous risks, which may include the following:
•collaborators have significant discretion in determining the efforts and resources that they will apply to a collaboration;
•collaborators may not pursue development and commercialization of POINT’s product candidates or may elect not to continue or renew development or commercialization of POINT’s product candidates based on clinical trial results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities;
•collaborators may cease development in therapeutic areas which are the subject of its collaboration with POINT;
•collaborators may change the success criteria for a particular product candidate, thereby delaying or ceasing development of such product candidate;
•collaborators may significantly delay the initiation or conduct of certain activities which could delay POINT’s receipt of milestone payments tied to such activities, thereby impacting POINT’s ability to fund its own activities;
•collaborators may fail to successfully commercialize a product candidate;
•collaborators may fail to obtain the requisite regulatory approval of a product candidate;
•collaborators may encounter regulatory, resource or quality issues and be unable to meet demand requirements;
•collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
57
•collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with POINT’s product candidates;
•a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to their marketing and distribution;
•collaborators may not properly maintain or defend POINT’s intellectual property rights or may use POINT’s intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate POINT’s intellectual property or proprietary information or expose POINT to potential liability;
•disputes may arise between POINT and a collaborator that cause the delay or termination of the research, development or commercialization of POINT’s product candidates, or that result in costly litigation or arbitration that diverts management attention and resources;
•collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates; and
•collaborators may own or co-own intellectual property covering POINT’s product candidates that results from POINT collaborating with them, and in such cases, POINT would not have the exclusive right to commercialize such intellectual property.
As a result, if POINT enters into additional collaboration agreements and strategic partnerships or license its product candidates, it may not be able to realize the benefit of such transactions if POINT is unable to successfully integrate them with its existing operations and company culture, which could delay its timelines or otherwise adversely affect its business. POINT also cannot be certain that, following a strategic transaction or license, it will achieve the revenue or specific net income that justifies such transaction. Any delays in entering into new collaborations or strategic partnership agreements related to its product candidates could delay the development and commercialization of its product candidates in certain geographies for certain indications, which would harm its business, prospects, financial condition and results of operations.
If POINT or the third parties with which it contracts fail to comply with environmental, health and safety laws and regulations, POINT may be subject to fines or penalties or incur costs that could have a material effect on the success of its business.
POINT’s manufacturing, research and development and other activities may involve the controlled use of potentially hazardous substances, including chemical materials, by POINT or the third parties with which it contracts. The use of radioligand therapies involves the inherent risk of exposure from radiation, which can alter or harm healthy cells in the body. POINT and such third parties are subject to federal, state, provincial and local laws and regulations in the U.S., Canada and other foreign jurisdictions governing the generation, use, manufacture, storage, handling, treatment, and disposal of hazardous and regulated materials, and the emission and discharge of such materials into the ground, air and water. These laws and regulations include, but are not limited to the CERCLA, which imposes strict, joint and several liability on current and former owners and operators of sites and on persons who disposed of or arranged for the disposal of hazardous substances found at such sites, including releases of radioactive materials, regardless of the lawfulness of the original activities that led to the contamination, the LLRW Policy Act, which requires the safe disposal of mildly radioactive materials that cannot be decayed in storage, NRC regulations concerning various irradiated and radioactive, materials, and health regulations from the U.S. Occupational Safety and Health Administration, which limit exposures to hazardous substances, including radioactive materials, in the workplace and impose various worker safety requirements. Although POINT believes that its and such third-parties’ procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, POINT cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. As a result of any such contamination or injury, POINT may incur liability or local, city, state, provincial or federal authorities may curtail the use of these materials and interrupt POINT’s business operations. In the event of an accident, POINT could be held liable for damages or penalized with fines, and the liability could exceed POINT’s resources. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair POINT’s research, development and production efforts, which could harm its business, prospects, financial condition, or results of operations. POINT will maintain insurance coverage for injuries resulting from the hazardous materials it uses; however, future claims may exceed the amount of its coverage. Also, POINT does not currently have insurance coverage for pollution cleanup and removal. Currently the costs of complying with such federal, state, provincial, local and foreign environmental regulations are not significant, and consist
58
primarily of waste disposal expenses. However, they could become expensive, and current or future environmental laws or regulations may impair POINT’s research, development, production and commercialization efforts.
POINT currently relies and is likely to rely in the future on outside scientists and their third-party research institutions for research and development and early clinical testing of our product candidates. These scientists and institutions may have other commitments or conflicts of interest, which could limit our access to their expertise and adversely affect the timing of the IND filings and our ability to conduct future planned clinical trials.
We currently have limited internal research and development capabilities and we have not and are not currently conducting any independent clinical trials. Therefore, we currently rely on third-party research institutions for both capabilities.
Some of the outside scientists who conduct the clinical testing of our current product candidates, and who conduct the research and development upon which our product candidate pipeline depends, may not be our employees; rather they serve as either independent contractors or the primary investigators under research and other agreements that we have entered into with their sponsoring academic or research institutions. Such scientists and collaborators may have other commitments that would limit their availability to us, and other research being conducted by their institutions may at times receive higher priority than research on the programs we are funding. We typically have less control of the research, clinical trial protocols and participant enrollment than we might with activity led by our employees. Further, though our scientific advisors generally agree not to do competing work, if an actual or potential conflict of interest between their work for us and their work for another entity arises, we may lose their services. These factors could adversely affect the timing of our IND filings and our ability to conduct future planned clinical trials. It is also possible that some of our valuable proprietary knowledge may become publicly known through these scientific advisors if they breach their confidentiality agreements with us, which would cause competitive harm to, and have a material adverse effect on, our business.
Risks Related to Government Regulation
The FDA regulatory approval process is lengthy and time-consuming, and POINT may experience significant delays in the clinical development and regulatory approval of its product candidates.
POINT has not previously submitted a NDA to the FDA or similar marketing applications to similar foreign regulatory authorities. A NDA must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety and effectiveness for each desired indication. The NDA must also include significant information regarding the manufacturing controls for the product.
Securing regulatory approval also requires the submission of information about the radioligand manufacturing process and inspection of manufacturing facilities by the relevant regulatory authority. The FDA or similar foreign regulatory authorities may fail to approve POINT’s manufacturing processes or facilities, whether run by POINT or its CMOs. In addition, if POINT makes manufacturing changes to its product candidates in the future, POINT may need to conduct additional preclinical studies and/or clinical trials to bridge its modified product candidates to earlier versions.
Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may ultimately lead to the denial of regulatory approval of POINT’s product candidates.
POINT may seek orphan drug designation for product candidates it develops, and POINT may be unsuccessful or may be unable to maintain the benefits associated with orphan drug designation, including the potential for market exclusivity.
As part of POINT’s business strategy, it has sought orphan drug designation for 177Lu-PNT2003 and may seek orphan drug designation for other product candidates it develops, and it may be unsuccessful. Regulatory authorities in some jurisdictions, including the U.S. and Europe, may designate drugs for relatively small patient populations as orphan drugs.
Generally, if a drug with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the drug is entitled to a period of marketing exclusivity, which precludes the EMA or the FDA from approving another marketing application for the same drug and for the same indication during the period of exclusivity, except in limited circumstances.
Even if POINT obtains orphan drug exclusivity for a product candidate, such exclusivity may not effectively protect the product candidate from competition because different therapies can be approved for the same condition and the same therapies can be approved for different conditions but used off-label. Even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In addition, a designated orphan
59
drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. Moreover, orphan drug exclusive marketing rights in the U.S. may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. Orphan drug designation neither shortens the development time or regulatory review time of a drug nor gives the drug any advantage in the regulatory review or approval process. While POINT has sought orphan drug designation for 177Lu-PNT2003 and may seek orphan drug designation for other applicable indications for its current and any future product candidates, POINT may never receive such designations. Even if POINT does receive such designation, there is no guarantee that it will enjoy the benefits of that designation.
A breakthrough therapy designation by the FDA, even if granted for any of POINT’s product candidates, may not lead to a faster development or regulatory review or approval process and it does not increase the likelihood that POINT’s product candidates will receive marketing approval.
POINT may seek breakthrough therapy designation for some or all of its future product candidates. A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For product candidates that have been designated as breakthrough therapies, sponsors may obtain more frequent interaction with and communication with the FDA to help to identify the most efficient path for clinical development. Therapies designated as breakthrough therapies by the FDA may also be eligible for other expedited approval programs, including accelerated approval.
Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if POINT believes one of its product candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a breakthrough therapy designation for a product candidate may not result in a faster development process, review or approval and does not assure ultimate approval by the FDA. In addition, even if one or more of POINT’s product candidates qualify as a breakthrough therapy, the FDA may later decide that the product candidate no longer meets the conditions for qualification. As such, even though POINT could seek breakthrough therapy designation for 177Lu-PNT2002 and/or 177Lu-PNT2003 and some or all of its future product candidates for the treatment of certain cancers, there can be no assurance that POINT will receive breakthrough therapy designation or that even if POINT does receive it, that such designation will have a material impact on POINT’s development program.
A fast-track designation by the FDA, even if granted for 177Lu-PNT2002, 177Lu-PNT2003 or any other future product candidates, may not lead to a faster development or regulatory review or approval process and does not increase the likelihood that POINT’s product candidates will receive marketing approval.
If a drug is intended for the treatment of a serious or life-threatening condition and the product demonstrates the potential to address unmet medical needs for this condition, the sponsor may apply for FDA fast track designation for a particular indication. POINT may seek fast track designation for certain of its current or future product candidates, but there is no assurance that the FDA will grant this status to any of POINT’s proposed product candidates. If granted, fast track designation makes a product eligible for more frequent interactions with FDA to discuss the development plan and clinical trial design, as well as rolling review of the application, which means that the company can submit completed sections of its marketing application for review prior to completion of the entire submission. Marketing applications of product candidates with fast-track designation may qualify for priority review under the policies and procedures offered by the FDA, but the fast-track designation does not assure any such qualification or ultimate marketing approval by the FDA. The FDA has broad discretion whether or not to grant fast track designation, so even if POINT believes a particular product candidate is eligible for this designation, there can be no assurance that the FDA would decide to grant it. Even if POINT does receive fast track designation, POINT may not experience a faster development process, review or approval compared to conventional FDA procedures, and receiving a fast-track designation does not provide any assurance of ultimate FDA approval. In addition, the FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from POINT’s clinical development program. In addition, the FDA may withdraw any fast-track designation at any time.
Accelerated approval by the FDA, even if granted for 177Lu-PNT2002, 177Lu-PNT2003 or any other future product candidates, may not lead to a faster development process.
POINT may seek accelerated approval for product candidates. A product may be eligible for accelerated approval if it treats a serious or life-threatening condition and generally provides a meaningful advantage over available therapies. In
60
addition, it must demonstrate an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality ("IMM") that is reasonably likely to predict an effect on IMM or other clinical benefit. As a condition of accelerated approval, the FDA generally requires that the sponsor of the drug perform adequate and well-controlled post-marketing clinical trials. The FDORA expands the FDA’s authority with regard to the accelerated approval pathway for drugs and biologics, including by permitting the FDA to require that post-marketing confirmatory studies be underway prior to approval or within a specific time period following approval. The FDORA also mandates periodic progress reports from sponsors on post-approval trials and related conditions. As an additional condition of accelerated approval, the FDA generally requires pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product.
If POINT is unable to successfully develop, validate and obtain regulatory approval for companion diagnostic tests for its product candidates that require or would commercially benefit from such tests, or experiences significant delays in doing so, it may not realize the full commercial potential of these product candidates.
In connection with the clinical development of POINT’s product candidates for certain indications, POINT may work with collaborators to develop or obtain access to in vitro or in vivo companion diagnostic tests to identify patient subsets within a disease category who may derive selective and meaningful benefit from POINT’s product candidates. Such companion diagnostics would be used during POINT’s clinical trials as well as in connection with the commercialization of its product candidates. To be successful, POINT or its collaborators will need to address a number of scientific, technical, regulatory and logistical challenges. The FDA and similar foreign regulatory authorities regulate in vitro companion diagnostics as medical devices and, under that regulatory framework, will likely require the conduct of clinical trials to demonstrate the safety and effectiveness of any diagnostics POINT may develop, which POINT expects will require separate regulatory clearance or approval prior to commercialization.
POINT may rely on third parties for the design, development and manufacture of companion diagnostic tests for its therapeutic product candidates that may require such tests. If POINT enters into such collaborative agreements, it will be dependent on the sustained cooperation and effort of its future collaborators in developing and obtaining approval for these companion diagnostics. It may be necessary to resolve issues, such as selectivity/specificity, analytical validation, reproducibility or clinical validation of companion diagnostics, during the development and regulatory approval processes. Moreover, even if data from preclinical studies and early clinical trials appear to support development of a companion diagnostic for a product candidate, data generated in later clinical trials may fail to support the analytical and clinical validation of the companion diagnostic. POINT and its future collaborators may encounter difficulties in developing, obtaining regulatory approval for, manufacturing and commercializing companion diagnostics similar to those POINT faces with respect to its therapeutic candidates themselves, including issues with achieving regulatory clearance or approval, production of sufficient quantities at commercial scale and with appropriate quality standards, and in gaining market acceptance. If POINT is unable to successfully develop companion diagnostics for these therapeutic product candidates, or experiences delays in doing so, the development of these therapeutic product candidates may be adversely affected, these therapeutic product candidates may not obtain marketing approval, and POINT may not realize the full commercial potential of any of these therapeutics that obtain marketing approval. As a result, POINT’s business, results of operations and financial condition could be materially harmed. In addition, a diagnostic company with whom POINT contracts may decide to discontinue selling or manufacturing the companion diagnostic test that POINT anticipates using in connection with development and commercialization of its product candidates or POINT’s relationship with such diagnostic company may otherwise terminate. POINT may not be able to enter into arrangements with another diagnostic company to obtain supplies of an alternative diagnostic test for use in connection with the development and commercialization of POINT’s product candidates or do so on commercially reasonable terms, which could adversely affect and/or delay the development or commercialization of its therapeutic candidates.
Obtaining and maintaining regulatory approval of POINT’s product candidates in one jurisdiction does not mean that it will be successful in obtaining regulatory approval of its product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of POINT’s product candidates in one jurisdiction does not guarantee that it will be able to obtain or maintain regulatory approval in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, similar foreign regulatory authorities must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval and licensure procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the U.S., including additional preclinical studies or clinical trials, as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the U.S., a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that POINT intends to charge for its product candidates is also subject to approval.
61
POINT may also submit marketing applications in other countries. Regulatory authorities in jurisdictions outside of the U.S. have requirements for approval of product candidates with which POINT must comply prior to marketing in those jurisdictions. Obtaining similar foreign regulatory approvals and compliance with similar foreign regulatory requirements could result in significant delays, difficulties and costs for POINT and could delay or prevent the introduction of its product candidates in certain countries. If POINT fails to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, POINT’s target market will be reduced and its ability to realize the full market potential of its product candidates will be harmed.
Even if POINT receives regulatory approval of its product candidates, POINT will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and POINT may be subject to penalties if it fails to comply with regulatory requirements or experiences unanticipated problems with its product candidates.
Following potential approval of any of POINT’s current or future product candidates, the FDA or similar foreign regulatory authorities may impose significant restrictions on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly and time-consuming post-approval studies, post-market surveillance or clinical trials to monitor the safety and efficacy of the product. The FDA may also require a REMS in order to license POINT’s product candidates, which could entail requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. In addition, if the FDA or a similar foreign regulatory authority approves POINT’s product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping for POINT’s product candidates will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and GCPs, for any clinical trials that POINT conducts post-approval. Later discovery of previously unknown problems with POINT’s product candidates, including adverse events of unanticipated severity or frequency, or with POINT’s third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
•restrictions on the marketing or manufacturing of POINT’s product candidates, withdrawal of the product candidates from the market or voluntary or mandatory product recalls;
•revisions to the labeling, including limitation on approved uses or the addition of additional warnings, contraindications or other safety information, including boxed warnings;
•imposition of a REMS which may include distribution or use restrictions;
•requirements to conduct additional post-market clinical trials to assess the safety of the product candidates;
•fines, warning or untitled letters or holds on clinical trials;
•refusal by the FDA to approve pending applications or supplements to approved applications filed by POINT or suspension or revocation of license approvals;
•product seizure or detention, or refusal to permit the import or export of POINT’s product candidates; and
•injunctions or the imposition of civil or criminal penalties.
The FDA’s and similar regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of POINT’s product candidates. POINT cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. For example, certain policies of the Biden administration may impact POINT’s business and industry. Namely, the Biden administration is expected to take several executive actions that could increase FDA enforcement actions. It is difficult to predict how these executive actions will be implemented, and the extent to which they will impact the FDA’s exercise of its regulatory authority. If POINT is slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if POINT is not able to maintain regulatory compliance, POINT may lose any marketing approval that it may have obtained and it may not achieve or sustain profitability.
Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products and services from being developed, approved or commercialized in a timely manner, which could negatively impact POINT’s business.
62
The ability of the FDA to review and approve new products can be affected by a variety of factors, including, but not limited to, government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, statutory, regulatory, and policy changes and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the agency have fluctuated in recent years as a result of the factors identified. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved or cleared by necessary government agencies, which would adversely affect POINT’s business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities.
Separately, in response to the global pandemic of COVID-19, on March 10, 2020, the FDA announced its intention to postpone inspections of manufacturing facilities and products, and regulatory authorities outside the U.S. may have adopted similar restrictions or other policy measures in response to the COVID-19 pandemic. The FDA has since resumed some prioritized domestic inspections based on a rating system. Additionally, as of June 23, 2020, the FDA noted it is continuing to ensure timely reviews of applications for medical products during the COVID-19 pandemic in line with its user fee performance goals. On July 16, 2020, FDA noted that it is continuing to expedite oncology product development with its staff teleworking full-time. In addition, on April 15, 2021, the FDA issued a guidance document in which the FDA outlined plans to conduct voluntary remote interactive evaluations of certain drug manufacturing facilities and clinical research sites. According to the guidance, the FDA intends to request such remote interactive evaluations in situations where an in-person inspection would not be prioritized, deemed mission-critical or is otherwise limited by travel restrictions, but where the FDA determines that a remote evaluation would still be appropriate. However, FDA may not be able to continue its current pace and approval timelines could be extended, including where a pre-approval inspection or an inspection of clinical sites is required and due to the COVID-19 pandemic and travel restrictions FDA is unable to complete such required inspections during the review period. In 2020, several companies announced receipt of complete response letters due to the FDA’s inability to complete required inspections for their applications. Regulatory authorities outside the U.S. may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic.
If a prolonged government shutdown occurs, or if global health concerns prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process regulatory submissions, which could have a material adverse effect on POINT’s business. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process POINT’s regulatory submissions, which could have a material adverse effect on POINT’s business.
Failure to obtain or maintain adequate coverage and reimbursement for any of POINT’s product candidates, if approved, could limit POINT’s ability to market those product candidates and decrease its ability to generate revenue.
In the U.S. and markets in other countries, patients generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. Adequate coverage and reimbursement for products and related treatments from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors are critical to new product acceptance, will affect POINT’s ability to successfully commercialize its product candidates, and also will impact utilization patterns. Sales of current or future product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of its product candidates will be paid by various third-party payors. If adequate coverage and reimbursement are not available, POINT may not be able to successfully commercialize its product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow POINT to establish or maintain pricing sufficient to realize a sufficient return on its investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products and coverage may be more limited than the purposes for which the medicine is approved by the FDA or comparable foreign regulatory authorities. Securing coverage and reimbursement for a product (often two separate processes) can be costly and time-consuming, and we may encounter significant delays. Factors payors consider in determining reimbursement are based on whether the product is: (i) a covered benefit under its health plan; (ii) safe, effective and medically necessary; (iii) appropriate for the specific patient; (iv) cost-effective; and (v) neither experimental nor investigational. In the U.S., the principal decisions about coverage and reimbursement for new medicines are typically made by CMS. CMS decides whether, and to what extent, a new medicine will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. However, one payor’s determination to provide coverage for a product does not mean that other payors will also provide coverage for the product.
63
The containment of healthcare costs has become a priority of both government healthcare programs and commercial payors. Third-party payors are increasingly challenging prices, examining medical necessity and reviewing cost-effectiveness of medical products and services as they seek to control costs. Many pharmaceutical manufacturers must calculate and report certain price reporting metrics to the government, such as average sales price, or ASP, and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely. List prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future laws or regulations limiting or changing pharmaceutical practices, such as relaxation of laws that presently restrict imports of drugs from other countries. POINT cannot be sure that reimbursement will be available for any product candidate that it commercializes. Further, eligibility for coverage and reimbursement does not necessarily mean that a payor will cover a drug in all appropriate cases or at a rate that covers POINT’s costs, including research and development, intellectual property, manufacturing and sale and distribution expenses, and POINT may not be able to realize an appropriate rate of return on investment.
In addition, in certain foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the E.U. provides options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. To obtain reimbursement or pricing approval, certain countries with the E.U. may require the completion of clinical trials that compare the cost effectiveness of a particular product candidate to currently available therapies. A Member State may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of POINT’s product candidates. Historically, products launched in the E.U. do not follow the same price structures as in the U.S. and, generally, prices in the E.U. tend to be significantly lower.
POINT’s relationships with healthcare providers and third-party payors are subject to complex and extensive healthcare laws and regulations, including fraud and abuse laws, which could expose POINT to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
POINT is subject to complex and extensive laws and regulations, including those related to healthcare fraud and abuse, privacy and security of health information and other personal data, transparency of financial relationships, registration of manufacturers and distributors, and marketing. These laws and regulations may constrain our business and the financial relationships through which we sell, market and distribute our products. They are broadly applicable, may vary across jurisdictions, and are administered by several different government agencies, such as the FDA, HHS, CMS, and the DOJ. The potentially applicable federal, state and foreign healthcare laws and regulations laws that may impact our operations, and which are described in more detail in the section titled “Business — Government Regulation — U.S. Healthcare Laws and Regulation,” include, but are not limited to:
•the federal Anti-Kickback Statute, which prohibits providers and others from directly or indirectly soliciting, receiving, offering or paying any remuneration with the intent of generating referrals or orders for items or services covered by a federal healthcare program;
•various laws specific to the pharmaceutical industry, including the federal Drug Supply Chain Security Act, laws requiring registration of drug manufacturers and distributors, those requiring disclosure of drug pricing, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs, and those restricting certain sales and marketing practices;
•federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
•federal civil and criminal false claims laws, including the civil FCA, which govern the submission of claims for reimbursement and prohibit individuals and entities from making false claims or statements
•the federal CMP Law and other statutes that prohibit various forms of fraud and abuse, including improper patient inducements;
•HIPAA, which restricts the use and disclosure of protected health information and may limit our ability to obtain and further use and disclose patient identifiable information for such activities as research;
64
•the federal Physician Payment Sunshine Act, which aims to increase transparency in financial relationships between manufacturers of drugs, devices, biologicals, or medical supplies and healthcare professional and teaching hospitals; and
•various state fraud and abuse laws, including state anti-kickback and false claims laws, which are often broad in scope and may apply regardless of payor.
Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies, healthcare providers and other third parties, including charitable foundations, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. State and federal authorities have aggressively targeted pharmaceutical companies for alleged violations of anti-fraud statutes, based on improper research or consulting contracts with doctors, certain marketing arrangements with pharmacies and other healthcare providers that rely on volume-based pricing, off-label marketing schemes, and other improper promotional practices. Companies targeted in such prosecutions have paid substantial fines, have been ordered to implement extensive corrective action plans, and have in many cases become subject to consent decrees severely restricting the manner in which they conduct their business, among other consequences. Additionally, federal and state regulators have brought criminal actions against individual employees responsible for alleged violations. If POINT becomes the target of such an investigation or prosecution based on its contractual relationships with providers or institutions, or its marketing and promotional practices, POINT could face similar or other sanctions, which would materially harm its business.
Responding to investigations can be time-and resource-consuming and can divert management’s attention from the business. Any such investigation or settlement could increase POINT’s costs or otherwise have an adverse effect on its business. While we endeavor to comply with applicable laws and regulations, we cannot guarantee that our practices are fully compliant or that courts or regulatory agencies will not interpret laws and regulations in ways that could adversely affect our practices. Further, the laws and regulations governing our business are subject to change, enforcement practices may evolve, and it is difficult to predict the impact of new laws and regulations. These changes could subject us to allegations of impropriety or illegality, require restructuring of relationships or otherwise require changes to our operations and materially affect business in an adverse way. Violation of applicable healthcare laws and regulations, including fraud and abuse laws, could result in penalties, including administrative, civil and criminal penalties, damages, fines, disgorgement, the exclusion from participation in federal and state healthcare programs, individual imprisonment, reputational harm, and the curtailment or restructuring of its operations, as well as additional reporting obligations and oversight pursuant to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws. An investigation or an action for violation of these laws, even if successfully defended, could cause POINT to incur significant legal expenses and divert management’s attention from the operation of the business. Efforts to ensure that POINT’s business arrangements will comply with applicable healthcare laws may involve substantial costs.
Also, the Foreign Corrupt Practices Act and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. POINT’s internal control policies and procedures may not protect POINT from reckless or negligent acts committed by its employees, future distributors, partners, collaborators or agents. Violations of these laws, or allegations of such violations, could result in fines, penalties or prosecution and have a negative impact on POINT’s business, results of operations and reputation.
Healthcare policy changes, including those resulting from healthcare reform initiatives and government budgetary constraints, may have a material adverse effect on POINT’s business and results of operations.
In recent years, the U.S. Congress and certain state legislatures have considered and passed a large number of laws intended to result in significant changes to the healthcare industry, including proposals targeted at reducing the price of pharmaceutical products and limiting coverage and reimbursement for drugs and other medical products. For example, the Affordable Care Act, affects how health care services are covered, delivered and reimbursed through expanded health insurance coverage, reduced growth in Medicare program spending, and the establishment and expansion of value-based purchasing programs. The law also imposes price transparency requirements and establishes the Patient-Centered Outcomes Research Institute, which focuses on comparative clinical effectiveness research. In addition, the Affordable Care Act contains several provisions relevant to pharmaceutical manufacturers and that may impact to POINT’s potential product candidates, including expansion of the 340B program, expansion of manufacturers’ rebate liability under the MDRP, and measures intended to reduce Medicare Part D enrollees’ out-of-pocket liability.
There is uncertainty regarding the ultimate effect of the Affordable Care Act, particularly as it has been, and continues to be, subject to legislative and regulatory changes and court challenges. However, President Biden has indicated that his administration intends to protect and strengthen the Affordable Care Act and Medicaid programs. There is also uncertainty regarding the potential impact of other reform efforts at the federal and state levels. For example, some members of
65
Congress have proposed measures that would expand government-sponsored coverage, such as single-payor proposals (commonly referred to as “Medicare for All”) and changes to Medicare age requirements. These proposals could lead to increased coverage levels and utilization of services. The impact and timing of additional reform initiatives is unclear, but these efforts could have an adverse effect on our business or require us to modify certain aspects of our operations.
In recent years, there has been heightened governmental and public scrutiny over the manner in which pharmaceutical manufacturers set prices for their products. This has resulted in proposed and enacted federal and state legislation and regulations, executive orders, and other initiatives and proposals designed to increase transparency in product pricing, review the relationship between pricing and manufacturer patient programs and reform government reimbursement methodologies for pharmaceutical products. For example, the Inflation Reduction Act requires drug manufacturers to pay rebates if prices for certain drugs increase faster than the rate of inflation (beginning in 2023), caps annual out-of-pocket drug costs for Medicare Part D beneficiaries (effective in 2024), and allows the federal government to negotiate drug prices with pharmaceutical manufacturers for a select number of high-cost drugs (prices to take effect beginning in 2026), among other drug pricing provisions. Some other efforts related to drug pricing reform involve importing drugs from other countries or using international pricing benchmarks. For example, in 2020, HHS and the FDA issued a final rule to allow FDA-authorized programs to import certain prescription drugs from Canada, although the rule excludes several types of prescription drugs such as radioactive drugs and biologics and imaging drugs. Some states, such as Florida, are pursuing their own initiative to import drugs for state health care programs.
The Biden administration and certain members of Congress have indicated their intent to continue to pursue drug pricing reforms. Proposals include allowing additional importation of prescription drugs from other countries and modifying the design of the Medicare Part D program. Some states also have passed legislation and issued regulations designed to lower prescription drug costs. These and other initiatives at the federal and state levels, if enacted and implemented, may directly or indirectly affect pricing of POINT’s product candidates, our revenues, our competitive position, and our relationships with patients, payors, and ancillary providers.
POINT’s employees, independent contractors, consultants, commercial collaborators, principal investigators, vendors and other agents may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
POINT is exposed to the risk that its employees, independent contractors, consultants, commercial collaborators, principal investigators, vendors and other agents may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless or negligent conduct or disclosure of unauthorized activities to POINT that violates applicable regulations, including those laws requiring the reporting of true, complete and accurate information to regulatory agencies, manufacturing standards and U.S. federal and state healthcare laws and regulations. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. POINT could face liability under the Anti-Kickback Statute and similar U.S. state laws. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, referrals, customer incentive programs and other business arrangements. Misconduct by these parties could also involve the improper use of individually identifiable information, including, without limitation, information obtained in the course of clinical trials, which could result in significant regulatory sanctions and serious harm to POINT’s reputation. Further, should violations include promotion of unapproved (off-label) uses of one or more of POINT’s product candidates, POINT could face significant regulatory sanctions for unlawful promotion, as well as substantial penalties under the FCA and similar state laws. Similar concerns could exist in jurisdictions outside of the U.S. as well.
POINT has adopted a code of conduct applicable to all of its employees, but it is not always possible to identify and deter misconduct by employees and other third parties. The precautions POINT takes to detect and prevent misconduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting POINT from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against POINT, and POINT is not successful in defending itself or asserting its rights, those actions could have a significant impact on its business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, additional reporting requirements and oversight if POINT becomes subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of POINT’s operations, any of which could adversely affect POINT’s ability to operate its business, financial condition and results of operations.
If POINT’s security measures are breached or unauthorized access to protected health information or other personal information is otherwise obtained or if POINT fails to comply with applicable privacy and security laws and regulations, its reputation may be harmed, and it may incur significant expenses and liabilities.
66
Unauthorized access to, or security breaches of, POINT’s systems and databases could result in unauthorized access to data and information and loss, compromise or corruption of such data and information. Present and future CROs, contractors and consultants also could experience breaches of security leading to the exposure of confidential and personal information. Cyber incidents have been increasing in sophistication and frequency and can include third parties gaining access to employee or customer data using stolen or inferred credentials, computer malware, viruses, spamming, phishing attacks, ransomware, card skimming code, and other deliberate attacks and attempts to gain unauthorized access. Because the techniques used by computer programmers who may attempt to penetrate and sabotage POINT’s network security or its website change frequently and may not be recognized until launched against a target, POINT may be unable to anticipate these techniques or there may be a delay in detection of a breach. Breaches may also be caused by user error and failure to follow security policies and procedures. It is possible that unauthorized access to customer data may be obtained through inadequate use of security controls by customers, suppliers or other vendors. While POINT is not currently aware of any impact that the SolarWinds supply chain attack had on its business, this is a recent event, and the scope of the attack is yet unknown. Therefore, there is residual risk that POINT may experience a security breach arising from the SolarWinds supply chain attack.
Health plans, healthcare clearinghouses and most healthcare providers, including research institutions from which POINT obtains patient health information, are subject to privacy and security regulations promulgated under HIPAA. Entities that handle protected health information on behalf of covered entities, known as business associates, are required to comply with certain provisions of the security and privacy regulations. POINT is not currently classified as a covered entity or business associate under HIPAA and thus is not directly subject to its requirements or penalties. However, even if there is no direct liability under HIPAA, any person may be prosecuted under HIPAA’s criminal provisions. Consequently, depending on the facts and circumstances, POINT could face substantial criminal penalties if it knowingly receives protected health information from a HIPAA-covered healthcare provider, research institution or other party that has not satisfied HIPAA’s requirements for disclosure of individually identifiable health information.
POINT may receive and maintain personal information, including health information, throughout the clinical trial process, in the course of its research collaborations, and directly from individuals (or their healthcare providers) who enroll in POINT’s patient assistance programs. As a result, health privacy laws, data breach notification laws, consumer protection laws and genetic testing laws may apply directly to POINT’s operations and/or those of POINT’s collaborators and may impose restrictions on POINT’s collection, use and dissemination of individuals’ health and other personal information. These laws may apply to a broader class of information than the health information protected by HIPAA. Further, individuals about whom POINT or its collaborators obtain health information, as well as the providers who share this information with POINT, may have statutory or contractual rights that limit POINT’s ability to use and disclose the information.
In the event of a security breach, POINT could suffer loss of business, severe reputational damage adversely affecting investor or patient confidence, regulatory investigations and orders, litigation, indemnity obligations, damages for contract breach, penalties for violation of applicable laws or regulations, significant costs for remediation and other liabilities. For example, the loss of preclinical study or clinical trial data from completed or future preclinical studies or clinical trials could result in delays in POINT’s regulatory approval efforts and significantly increase POINT’s costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, POINT’s data or applications, or inappropriate disclosure of confidential or proprietary information, POINT could incur liability and the further development and commercialization of POINT’s product candidates could be delayed.
POINT has incurred and expects to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws and to prevent security breaches, including costs related to deploying additional personnel and protection technologies, training employees, and engaging third-party solution providers and consultants. Claims that POINT has violated individuals’ privacy rights or breached its contractual obligations, even if POINT is not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm POINT’s business. Although POINT expends significant resources to create security protections that shield personal information against potential theft and security breaches, such measures cannot provide absolute security. Moreover, as POINT outsources more of its information systems to vendors and relies more on cloud-based information systems, the related security risks will increase, and POINT will need to expend additional resources to protect its technology and information systems and may be adversely affected by security breaches experienced by POINT’s vendors. POINT maintains a limited amount of cyber liability insurance, POINT cannot be certain that its coverage will be adequate for liabilities actually incurred or that insurance will continue to be available to POINT on economically reasonable terms, or at all.
POINT is subject to certain U.S. and foreign anti-corruption, anti-money laundering, export control, sanctions and other trade laws and regulations. POINT can face serious consequences for violations.
67
Among other matters, U.S. and foreign anti-corruption, anti-money laundering, export control, sanctions and other trade laws and regulations, which are collectively referred to as Trade Laws, prohibit companies and their employees, agents, CROs, legal counsel, accountants, consultants, contractors and other partners from authorizing, promising, offering, providing, soliciting or receiving, directly or indirectly, corrupt or improper payments or anything else of value to or from recipients in the public or private sector. Violations of Trade Laws can result in substantial criminal fines and civil penalties, imprisonment, the loss of trade privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences. POINT has direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. POINT plans to engage third parties for clinical trials and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals and POINT can be held liable for the corrupt or other illegal activities of our personnel, agents or partners, even if POINT does not explicitly authorize or have prior knowledge of such activities.
Changes in tax law could adversely affect POINT's business and financial condition
The rules dealing with U.S. federal, state, and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service ("IRS") and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application), including with respect to net operating losses and research and development tax credits, could adversely affect POINT or holders of POINT Common Stock. In recent years, many such changes have been made and changes are likely to continue to occur in the future. Future changes in tax laws could have a material adverse effect on POINT’s business, cash flow, financial condition or results of operations. POINT urges investors to consult with their legal and tax advisers regarding the implications of potential changes in tax laws on an investment in POINT Common Stock.
Risks Related to POINT’s Intellectual Property
If POINT is unable to obtain and maintain patent protection for any product candidates it develops and for its technology, or if the scope of the patent protection obtained is not sufficiently broad, its competitors could develop and commercialize products and technology similar or identical to POINT’s, and POINT’s ability to commercialize any product candidates it may develop, and its technology may be adversely affected.
POINT’s success depends, in large part, on its ability to seek, obtain, maintain, enforce and defend patent rights in the U.S. and other countries with respect to its product candidates. POINT and its licensors have sought and intend to continue to seek to protect POINT’s proprietary position by filing patent applications in the U.S. and one or more countries outside the U.S. related to POINT’s product candidates and technologies that are important to POINT’s business. However, the risks associated with patent rights generally apply to patent rights that POINT owns, has licensed now or licenses in the future.
The patent prosecution process is expensive, time-consuming and complex, and POINT and its licensors may not be able to file, prosecute, maintain, enforce, defend or license all necessary or desirable patents and patent applications at a reasonable cost or in a timely manner.
Changes in either the patent laws or their interpretation in the U.S. and other countries may diminish POINT’s ability to protect its inventions, obtain, maintain and enforce its intellectual property rights, and more generally, could affect the value of POINT’s intellectual property rights or narrow the scope of POINT’s owned or licensed patents. POINT cannot predict with certainty whether patent applications POINT and its licensors are currently pursuing will issue as patents or whether the claims of any issued patents will provide sufficient competitive advantage.
It is also possible that POINT will fail to identify patentable aspects of its research and development output before it is too late to obtain patent protection. Although POINT enters into non-disclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of POINT’s research and development output, such as POINT’s employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing POINT’s ability to seek patent protection.
POINT is a party to a number of intellectual property license agreements which are important to its business, and POINT may enter into one or more additional license agreements and other intellectual property agreements in the future. POINT’s existing license agreements impose, and POINT expects that future license agreements will impose, various diligence, development and commercialization timelines, milestone payments, royalties and other obligations. If POINT fails to comply with obligations under these agreements, the licensor may have the right to terminate the license.
The patent position of pharmaceutical and biotechnology companies generally is highly uncertain, involves complex legal and factual questions and has, in recent years, been the subject of much litigation. As a result, the issuance, scope,
68
validity, enforceability and commercial value of POINT’s patent rights are highly uncertain. POINT’s current and future owned and licensed patent rights may not result in patents being issued which protect POINT’s technology or product candidates, effectively prevent others from commercializing competitive technologies and products or otherwise provide any competitive advantage. In fact, patent applications may not issue as patents at all. Even assuming patents issue from patent applications in which POINT has rights, changes in either the patent laws or interpretation of the patent laws in the U.S. and other jurisdictions may diminish the value of POINT’s patents or narrow the scope of patent protection.
Other parties have developed products and technologies that may be related or competitive to those of POINT and such parties may have filed or may file patent applications, or may have received or may receive patents, claiming inventions that may overlap or conflict with those claimed in POINT’s patent applications or issued patents. POINT may not be aware of all third-party intellectual property rights potentially relating to POINT’s current or future product candidates or technologies. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and in other jurisdictions are typically not published until 18 months after filing, or, in some cases, not at all. Therefore, POINT cannot know with certainty whether certain of POINT’s owned or licensed patent applications are the first filed for patent protection of the disclosed inventions. As a result, the issuance, scope, validity, enforceability and commercial value of POINT’s patent rights cannot be predicted with any certainty.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if patent applications POINT owns or licenses do issue as patents, they may not issue in a form that will provide meaningful protection, prevent competitors or other third parties from competing with POINT or otherwise provide competitive advantage. Any patents that POINT owns or licenses now or in the futures may be challenged, narrowed, circumvented, or invalidated by third parties. Consequently, POINT does not know whether any of its product candidates and technology will be protectable or remain protected by valid and enforceable patents. Competitors or other third parties may be able to circumvent POINT’s patents by developing similar or alternative technologies or products in a non-infringing manner. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, POINT’s intellectual property may not provide sufficient rights to exclude others from commercializing products similar or identical to POINT’s.
The degree of patent protection required to successfully compete in the marketplace may be unavailable or severely limited in some cases and may not adequately protect POINT’s rights or permit POINT to gain or keep any competitive advantage. POINT cannot provide any assurances that any of the patents or patent applications included in POINT’s patent rights include or will include, claims with a scope sufficient to protect POINT product candidates and technologies or otherwise provide any competitive advantage. In addition, the laws of foreign countries may not protect POINT’s proprietary rights to the same extent as the laws of the U.S.. Furthermore, patents have a limited lifespan. In the U.S., the natural expiration of a patent is generally twenty years after it is filed. Certain extensions may be available; however, the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, POINT’s patent rights may not provide adequate and continuing patent protection sufficient to exclude others from commercializing products similar or identical to a POINT product candidate.
Even if POINT has patent protection that is expected to maintain some competitive advantage, third parties, including competitors, may challenge the validity, enforceability or scope thereof, which may result in POINT owned or licensed patents being narrowed, invalidated or held unenforceable. In litigation, a competitor could claim that one or more POINT patents are not valid or enforceable for a number of reasons. If a court agrees, POINT would lose its rights to those challenged patents.
Even if issued, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and POINT’s licensed patents may be challenged in the courts or patent offices in the U.S. and abroad. For example, POINT may be subject to a third party submission of prior art to the USPTO challenging the validity of one or more claims of POINT’s licensed patents. Such submissions may also be made prior to a patent’s issuance, precluding the granting of a patent based on one of the pending patent applications included in POINT patent rights. POINT or a POINT licensor may become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review, or interference proceedings challenging one or more patents included in the POINT patent rights. For example, competitors may claim that they have filed one or more patent applications before the filing date of the patents or patent applications included in POINT’s patent rights. A competitor may also assert that POINT is infringing their patents and that POINT therefore cannot practice its technology. Competitors may also contest patents or patent applications included in POINT’s patent rights by showing that the claimed subject matter was not patent-eligible, was not novel, was obvious or that the patent claims failed any other requirement for patentability or enforceability. In addition, POINT may in the future be subject to claims by its or its licensors’ current or former employees or consultants asserting an ownership right in the patents or patent applications included in the POINT patent rights as an inventor or co-inventor, as a result of the work they performed.
69
An adverse determination in any such submission or proceeding may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit POINT’s ability to stop others from using or commercializing similar technology and therapeutics, without payment to POINT, or could limit the duration of the patent protection covering POINT’s technology and product candidates. Such challenges may also result in POINT’s inability to manufacture or commercialize its product candidates without infringing third party patent rights, and POINT may be required to obtain a license from third parties, which may not be available on commercially reasonable terms or at all, or POINT may need to cease the development, manufacture and commercialization of one or more of its product candidates. In addition, if the breadth or strength of protection provided by the patents and patent applications included in POINT’s patent rights is threatened, it could dissuade companies from collaborating with POINT to license, develop or commercialize current or future product candidates. Any of the foregoing would result in a material adverse effect on POINT’s business, financial condition, results of operations or prospects. Such proceedings also may result in substantial cost and require significant time from POINT’s scientists and management, even if the eventual outcome is favorable to POINT.
Even if they are unchallenged, the patents and pending patent applications included in POINT’s patent rights may not provide POINT with any meaningful protection or prevent competitors from designing around POINT’s patent claims to circumvent POINT’s patent rights by developing similar or alternative technologies or therapeutics in a non-infringing manner. If the patent protection provided by the patents and patent applications POINT owns or licenses is not sufficiently broad to impede such competition, POINT’s ability to successfully commercialize its product candidates and technologies could be negatively affected, which would have a material adverse effect on POINT’s business, financial conditions, results of operations and prospects.
If POINT fails to comply with its obligations under its patent licenses with third parties, POINT could lose license rights that are important to its business.
POINT is a party to various license agreements, pursuant to which POINT in-licenses patent and patent applications for use in one or more of its product candidates. These existing licenses impose various diligence, milestone payment, royalty, insurance and other obligations on POINT. If POINT fails to comply with these obligations, the licensors may have the right to terminate the licenses, in which event POINT would not be able to develop or market the product candidates covered by such licensed intellectual property.
POINT relies on certain of its licensors to file and prosecute patent applications and maintain patents and otherwise protect the intellectual property POINT licenses from them and may continue to do so in the future. POINT has limited control over these activities or any other intellectual property that may be related to its in-licensed intellectual property.
For example, POINT cannot be certain that such activities by these licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. POINT has limited control over the manner in which its licensors initiate an infringement proceeding against a third-party infringer of the intellectual property rights, or defend certain of the intellectual property that is licensed to POINT.
It is possible that any licensors’ infringement proceeding or defense activities may be less vigorous than had POINT conducted them itself.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies, and POINT’s patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on patents and/or applications will be due to be paid to the USPTO and various government patent agencies outside of the U.S. over the lifetime of POINT’s owned and licensed patents and/or applications. POINT relies on its outside counsel or its licensing partners to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. government patent agencies require compliance with several procedural, documentary, fee payment and other similar provisions during the patent application process. POINT employs reputable law firms and other professionals to help comply and POINT is also dependent on its licensors to take the necessary action to comply with these requirements with respect to POINT’s licensed intellectual property. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market and this circumstance could have a material adverse effect on POINT’s business.
POINT may not be able to protect its intellectual property and proprietary rights throughout the world.
70
Filing, prosecuting, maintaining, enforcing and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and POINT’s intellectual property rights in some countries outside the U.S. could be less extensive than those in the U.S. The laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S. even in jurisdictions where POINT does pursue patent protection. Consequently, POINT and its licensors may not be able to prevent third parties from practicing POINT inventions in all countries outside the U.S., even in jurisdictions where POINT pursues patent protection, or from selling or importing products made using POINT inventions in and into the U.S. or other jurisdictions. Competitors may use POINT technologies in jurisdictions where POINT has not pursued and obtained patent protection to develop their own products and may export otherwise infringing products to territories where POINT has patent protection, but where enforcement is not as strong as it is in the U.S. These products may compete with POINT product candidates and its patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property rights, particularly those relating to biotechnology products, which could make it difficult to stop the infringement of POINT patents, if pursued and obtained, or marketing of competing products in violation of POINT intellectual property and proprietary rights generally. Proceedings to enforce POINT’s intellectual property and proprietary rights in foreign jurisdictions could (i) result in substantial costs and divert efforts and attention from other aspects of POINT’s business, (ii) put POINT patents at risk of being invalidated or interpreted narrowly and its patent applications at risk of not issuing and (iii) provoke third parties to assert claims against POINT. POINT may not prevail in any lawsuits that POINT initiates and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, POINT’s efforts to enforce intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that POINT develops or licenses.
Issued patents covering POINT product candidates or technologies could be found invalid or unenforceable if challenged in court.
If POINT or one of its licensing partners initiates legal proceedings against a third party to enforce a patent covering a product candidate, assuming such patents have or do issue, the defendant could counterclaim that the patent covering POINT’s product candidate is invalid or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, written description, non-enablement or failure to claim patent eligible subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld information material to patentability from the USPTO, or made a misleading statement, during prosecution. Third parties also may raise similar claims before administrative bodies in the U.S. or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, inter partes review, interference proceedings, derivation proceedings and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation or cancellation of or amendment to its patents in such a way that they no longer cover a POINT product candidate. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, POINT cannot be certain that there is no invalidating prior art, of which the patent examiner, POINT or a licensing partner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, POINT could lose at least part, and perhaps all, of the patent protection on a product candidate. Such a loss of patent protection could have a material adverse impact on POINT’s business, financial condition, results of operations and prospects.
If POINT is unable to protect the confidentiality of its trade secrets, its business and competitive position would be harmed.
In addition to the protection afforded by patents, POINT relies on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that POINT elects not to patent, processes for which patents are difficult to enforce and any other elements of the discovery and development processes of product candidates and technologies that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect and some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. POINT seeks to protect its proprietary technology and processes, in part, by entering into confidentiality agreements with its employees, consultants, scientific advisors and contractors. However, POINT may not be able to prevent the unauthorized disclosure or use of its technical know-how or other trade secrets by the parties to these agreements, despite the existence generally of confidentiality agreements and other contractual restrictions. Monitoring unauthorized uses and disclosures is difficult and POINT cannot not know whether steps taken to protect its proprietary technologies will be effective. If any POINT employees, collaborators, CROs, contract manufacturers, consultants, advisors and other third parties who are parties to these agreements breaches or violates the terms of any of these agreements, POINT may not have adequate remedies for any such breach or violation. Enforcing a claim that a party
71
illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. As a result, POINT could lose its trade secrets. POINT cannot guarantee that it has entered into such agreements with each party that may have or have had access to its trade secrets or proprietary technology and processes. POINT also seeks to preserve the integrity and confidentiality of its data and trade secrets by maintaining physical security of its premises and physical and electronic security of its information technology systems. While POINT has confidence in these individuals, organizations and systems, agreements and security measures, they may still be breached, and POINT may not have adequate remedies for any breach.
In addition, POINT trade secrets may otherwise become known or be independently discovered by competitors. If any of POINT trade secrets were to be lawfully obtained or independently developed by a competitor, POINT would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with POINT. If POINT trade secrets are not adequately protected so as to protect against competitors’ products and technologies, POINT’s competitive position could be adversely affected.
Third parties may initiate legal proceedings alleging that POINT is infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of POINT’s business.
POINT’s commercial success depends upon its ability to develop, manufacture, market and sell current and future product candidates without infringing, misappropriating or otherwise violating the proprietary rights and intellectual property of third parties. The pharmaceutical industry is characterized by extensive and complex litigation regarding patents and other intellectual property rights. POINT or POINT’s licensors may in the future become party to, or be threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to a POINT product candidate, including interference proceedings, post grant review and inter partes review before the USPTO. Competitors or other third parties may assert infringement claims against POINT, alleging that POINT therapeutics, manufacturing methods, formulations or administration methods are covered by their patents.
POINT cannot be certain or guarantee that a court would hold that a POINT product candidate does not infringe an existing patent or a patent that may be granted in the future. Furthermore, because patent applications can take many years to issue, may be confidential for 18 months or more after filing and can be revised before issuance, there may be applications now pending which may later result in issued patents that may be infringed by the manufacture, use, sale or importation of a POINT product candidate and POINT may or may not be aware of such patents.
It is also possible that POINT has failed to identify relevant third-party patents or applications. It can be difficult for industry participants, including POINT, to identify all third-party patent rights that may be relevant to POINT product candidates and technologies because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. In addition, POINT may be unaware of one or more issued patents that would be infringed by the manufacture, sale or use of a current or future product candidate, or POINT may incorrectly conclude that a third-party patent is invalid, unenforceable or not infringed by POINT’s activities. Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover POINT’s technologies.
Third parties may assert infringement claims against POINT based on existing patents or patents that may be granted in the future, regardless of their merit. Even if it is believed such claims are without merit, there is no assurance that POINT would be successful in defending such claims. A court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, which could materially and adversely affect POINT’s ability to commercialize a product candidate covered by the asserted third-party patents. In order to successfully challenge the validity of any such U.S. patent in federal court, POINT would need to overcome a presumption of validity. As this burden is a high one requiring presentation of clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction would invalidate the claims of any such U.S. patent. Similarly, there is no assurance that a court of competent jurisdiction would find that a POINT product candidate did not infringe a third party patent.
Patent and other types of intellectual property litigation can involve complex factual and legal questions, and their outcome is uncertain. If POINT is found, or POINT believes there is a risk to be found, to infringe, misappropriate or otherwise violate a third party’s intellectual property rights, and POINT is unsuccessful in demonstrating that such intellectual property rights are invalid or unenforceable, POINT could be required or may choose to obtain a license from such third party to continue developing, manufacturing and marketing a product candidate. However, POINT may not be able to obtain any required license on commercially reasonable terms or at all. Even if POINT were able to obtain a license, it could be non-exclusive, thereby giving competitors and other third parties access to the same technologies licensed to POINT, and it could require POINT to make substantial licensing and royalty payments. POINT could be forced, including by court order, to cease developing, manufacturing and commercializing a product candidate. In addition, POINT could be found liable for monetary damages, including treble damages and attorneys’ fees, if POINT is found to have willfully
72
infringed a patent or other intellectual property right. A finding of infringement, misappropriation or other violation of intellectual property rights could prevent POINT from manufacturing and commercializing one or more product candidate or force POINT to cease some or all of its business operations, which could materially harm POINT’s business, financial condition, results of operations and prospects. Claims that POINT has misappropriated the confidential information or trade secrets of third parties could have a similar material adverse impact on POINT’s business, financial condition, results of operations and prospects.
Intellectual property litigation could cause POINT to spend substantial resources and distract POINT’s personnel from their normal responsibilities.
Litigation or other legal proceedings relating to intellectual property claims, with or without merit, is unpredictable and generally expensive and time-consuming. Competitors may infringe POINT patents or the patents of POINT’s licensing partners, or POINT may be required to defend against claims of infringement. To counter infringement or unauthorized use claims or to defend against claims of infringement can be expensive and time consuming. Even if resolved in POINT’s favor, litigation or other legal proceedings relating to intellectual property claims may cause POINT to incur significant expenses and could distract technical and management personnel from their normal responsibilities. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of POINT’s confidential information could be compromised by disclosure during this type of litigation.
POINT may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some competitors may be able to sustain the costs of such litigation or proceedings more effectively than POINT can because of their greater financial resources and more mature and developed intellectual property portfolios. Accordingly, POINT may not be able to prevent third parties from infringing or misappropriating or successfully challenging POINT intellectual property rights.
POINT may be subject to claims asserting that its employees, consultants or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what POINT regards as its own intellectual property.
Certain of POINT’s employees, consultants or advisors are currently, or were previously, employed at universities or other biotechnology or pharmaceutical companies, including POINT’s competitors or potential competitors, as well as POINT’s academic partners. Although POINT tries to ensure that its employees, consultants and advisors do not use the proprietary information or know-how of others in their work for POINT, POINT may be subject to claims that these individuals or POINT have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If POINT fails in defending any such claims, in addition to paying monetary damages, POINT may lose valuable intellectual property rights or personnel. An inability to incorporate such technologies or features would have a material adverse effect on POINT’s business and may prevent POINT from successfully commercializing its product candidates. Moreover, any such litigation or the threat thereof may adversely affect POINT’s ability to hire employees or contract with independent contractors. A loss of key personnel or their work product could hamper or prevent POINT’s ability to commercialize a product candidate, which could have an adverse effect on POINT’s business, results of operations and financial condition. Even if POINT is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while it is POINT’s policy to require employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to POINT, POINT may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that POINT regards as its own. Moreover, even when POINT does obtain agreements assigning intellectual property to POINT, the assignment agreements may be breached, and POINT may be forced to bring claims against third parties, or defend claims that they may bring against POINT, to determine the ownership of what POINT regards as its intellectual property. Moreover, individuals executing agreements with POINT may have preexisting or competing obligations to a third party, such as an academic institution, and thus an agreement with POINT may be ineffective in perfecting ownership of inventions developed by that individual. Disputes about the ownership of intellectual property that POINT may own may have a material adverse effect on POINT’s business, financial condition, results of operations and prospects.
If patent term extension is not obtained for POINT product candidates, POINT’s business may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of POINT product candidates, one or more U.S. patents that POINT owns or licenses may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments. The Hatch-Waxman
73
Amendments permit a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process based on the first regulatory approval for a particular drug or biologic. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended. However, POINT may not be granted an extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than requested. In addition, to the extent POINT wishes to pursue patent term extension based on a patent in-licensed from a third party, POINT would need the cooperation of that third party. If POINT is unable to obtain patent term extension or the term of any such extension is less than requested, competitors may be able to enter the market sooner, and POINT’s business, financial condition, results of operations and prospects could be materially harmed.
Intellectual property rights and regulatory exclusivity rights do not necessarily address all potential threats.
The degree of future protection afforded by POINT intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect POINT’s business or permit POINT to maintain its competitive advantage. For example:
•POINT, or its current or future license partners or collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent applications that POINT owns or licenses;
•POINT, or its current and future license partners or collaborators, might not have been the first to file patent applications covering certain inventions;
•others may independently develop similar or alternative technologies or duplicate any of POINT technologies without infringing POINT’s owned or licensed intellectual property rights;
•others may circumvent POINT regulatory exclusivities, such as by pursuing approval of a competitive product candidate via the traditional approval pathway based on their own clinical data, rather than relying on the abbreviated pathway provided for generic applicants;
•it is possible that POINT’s pending patent applications will not lead to issued patents;
•issued patents that POINT holds rights to now or in the future may be held invalid or unenforceable, including as a result of legal challenges by competitors;
•POINT competitors might conduct research and development activities in countries where POINT does not have patent rights and then use the information learned from such activities to develop competitive products for sale in major commercial markets;
•the patents or other intellectual property rights of others may have an adverse effect on POINT’s business; and
•POINT may choose not to file a patent for certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
Should any of these events occur, they could have a material adverse effect on POINT’s business, financial condition, results of operations and prospects.
Changes in patent law in the U.S. and in non-U.S. jurisdictions could diminish the value of patents in general, thereby impairing POINT’s ability to protect its products.
As is the case with other pharmaceutical companies, POINT’s success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. In recent years, U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to POINT’s ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents, particularly those directed to pharmaceutical products and uses, could change in unpredictable ways that would weaken POINT’s ability to obtain new patents or to enforce its existing patents and patents that it might obtain in the future. POINT cannot predict
74
how these decisions or any future decisions by the U.S. Congress, the federal courts or the USPTO may impact the value of its patents. Similarly, any adverse changes in the patent laws of other jurisdictions could have a material adverse effect on POINT’s business and financial condition.
Risks Related to Employee Matters and Managing Growth
POINT is highly dependent on its key personnel, and if it is not successful in attracting and retaining highly qualified personnel, it may not be able to successfully implement its business strategy.
POINT’s ability to compete in the highly competitive pharmaceutical industry depends upon its ability to attract and retain highly qualified managerial, scientific and medical personnel. POINT is highly dependent on its management, scientific and medical personnel, including Dr. Joe McCann, PhD, POINT’s Chief Executive Officer. The loss of the services of any of POINT’s executive officers, other key employees and other scientific and medical advisors, and an inability to find suitable replacements could result in delays in product development and harm POINT’s business.
POINT has its corporate headquarters and manufacturing facility in Indianapolis, Indiana and a regional office in Toronto, Ontario. These regions are headquarters to many other pharmaceutical companies and many academic and research institutions. Competition for skilled personnel in POINT’s market is intense and may limit its ability to hire and retain highly qualified personnel on acceptable terms or at all. Changes to U.S, Canadian, or similar foreign immigration and work authorization laws and regulations, including those that restrain the flow of scientific and professional talent, can be significantly affected by political forces and levels of economic activity. POINT’s business may be materially adversely affected if legislative or administrative changes to U.S., Canadian, or similar foreign immigration or visa laws and regulations impair POINT’s hiring processes and goals or projects involving personnel who are not U.S. or Canadian citizens.
To encourage valuable employees to remain at POINT, in addition to salary and cash incentives, POINT has provided stock options that vest over time and performance stock units that vest upon completion of certain performance conditions. The value to employees of stock options that vest over time and of performance share units may be significantly affected by movements in POINT’s share price that are beyond its control, and may at any time be insufficient to counteract more lucrative offers from other companies. Despite POINT’s efforts to retain valuable employees, members of POINT’s management, scientific and development teams may terminate their employment with POINT on short notice. Although POINT has employment agreements with its key employees, some of these employment agreements provide for at-will employment, which means that some of POINT’s employees could leave its employment at any time, with or without notice. POINT’s success also depends on its ability to continue to attract, retain and motivate highly skilled junior, mid-level and senior managers as well as junior, mid-level and senior scientific and medical personnel.
POINT will need to grow the size of its organization, and it may experience difficulties in managing this growth.
As of December 31, 2022, POINT had 129 full-time employees. As POINT’s strategy develops, POINT expects to need additional managerial, operational, sales, marketing, financial and other personnel, as well as additional facilities to expand its operations. Future growth would impose significant added responsibilities on members of management, including:
•identifying, recruiting, integrating, maintaining and motivating additional employees;
•managing POINT’s internal development efforts effectively, including the clinical and FDA review process for its product candidates, while complying with POINT’s contractual obligations to contractors and other third parties; and
•improving POINT’s operational, financial and management controls, reporting systems and procedures.
POINT’s future financial performance and its ability to commercialize its product candidates will depend, in part, on its ability to effectively manage any future growth, and its management may also have to divert a disproportionate amount of its attention away from day-to-day activities in order to devote a substantial amount of time to managing these growth activities.
POINT currently relies, and for the foreseeable future will continue to rely, in substantial part on certain independent organizations, advisors and consultants to provide certain services. There can be no assurance that the services of independent organizations, advisors and consultants will continue to be available to POINT on a timely basis when needed, or that it can find qualified replacements. In addition, if POINT is unable to effectively manage its outsourced activities or if the quality or accuracy of the services provided by consultants is compromised for any reason, POINT’s clinical trials may be extended, delayed or terminated, and it may not be able to obtain regulatory approval of its product candidates or
75
otherwise advance its business. There can be no assurance that POINT will be able to manage its existing consultants or find other competent outside contractors and consultants on economically reasonable terms, or at all. If POINT is not able to effectively expand its organization by hiring new employees and expanding its groups of consultants and contractors, or POINT is not able to effectively build out new facilities to accommodate this expansion, POINT may not be able to successfully implement the tasks necessary to further develop and commercialize its product candidates and, accordingly, may not achieve its research, development and commercialization goals.
If product liability lawsuits are brought against POINT, it may incur substantial liabilities and may be required to limit commercialization of its product candidates.
POINT faces an inherent risk of product liability as a result of the planned clinical testing of its product candidates and will face an even greater risk if POINT commercializes any product candidates. For example, POINT may be sued if its product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If POINT cannot successfully defend itself against product liability claims, POINT may incur substantial liabilities or be required to limit commercialization of its product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
•decreased demand for POINT’s current product candidates or future product candidates that it may develop;
•injury to POINT’s reputation;
•withdrawal of clinical trial participants;
•initiation of investigations by regulators;
•costs to defend the related litigation;
•a diversion of management’s time and POINT’s resources;
•substantial monetary awards to trial participants or patients;
•product recalls, withdrawals or labeling, marketing or promotional restrictions;
•loss of revenue;
•exhaustion of any available insurance and POINT’s capital resources; and
•the inability to commercialize any product candidate.
Failure to obtain or retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of product candidates POINT develops, alone or with corporate collaborators. Although POINT has clinical trial insurance, its insurance policies also have various exclusions, and it may be subject to a product liability claim for which it has no coverage. POINT may have to pay any amounts awarded by a court or negotiated in a settlement that exceed its coverage limitations or that are not covered by its insurance, and it may not have, or be able to obtain, sufficient capital to pay such amounts. Even if POINT’s agreements with any future corporate collaborators entitle POINT to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Unstable market and economic conditions may have serious adverse consequences on POINT’s business, financial condition and share price.
As widely reported, global credit and financial markets have experienced extreme volatility and disruptions in the past several years, especially due to the impacts of the COVID-19 pandemic, escalating military fighting between Russia and Ukraine, terrorism and other geopolitical events, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. The U.S. and other nations, in response to the Russo-Ukranian conflict have announced economic sanctions which may have an adverse effect on the global financial markets. Adverse developments that affect financial institutions, such as events involving liquidity that are rumored or actual, have in the past and may in the future lead to market-wide liquidity problems. Recent and potential future disruptions in access to bank deposits or lending commitments due to bank
76
failure may reduce our ability to access our cash and cash equivalents as well as debt capital or financing on preferable terms, which may adversely affect our future capital requirements. There can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. POINT’s general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on POINT’s growth strategy, financial performance and share price and could require POINT to delay or abandon clinical development plans.
Risks Related to Our Organizational Structure
Delaware law and POINT’s governing documents contain certain provisions, including anti-takeover provisions, that limit the ability of stockholders to take certain actions and could delay or discourage takeover attempts that stockholders may consider favorable.
Our governing documents and the Delaware General Corporation Law (“DGCL”), contain provisions that could have the effect of rendering more difficult, delaying, or preventing an acquisition deemed undesirable by our board of directors ("Board"), and, therefore, depress the trading price of the Common Stock. These provisions could also make it difficult for stockholders to take certain actions, including electing directors who are not nominated by the current members of the Board or taking other corporate actions, including effecting changes in POINT’s management. Among other things, our governing documents include provisions regarding:
•the ability of the Board to issue shares of preferred stock, including “blank check” preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquirer;
•the limitation of the liability of, and the indemnification of, POINT’s directors and officers;
•a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of stockholders after such date and could delay the ability of stockholders to force consideration of a stockholder proposal or to take action, including the removal of directors;
•the classification of the board into three classes, each class which is elected for a three year term and only one class is up for re-election each year
•the requirement that a special meeting of stockholders may be called only by a majority of the entire Board, which could delay the ability of stockholders to force consideration of a proposal or to take action, including the removal of directors;
•controlling the procedures for the conduct and scheduling of Board and stockholder meetings;
•the ability of the Board to amend the bylaws, which may allow the Board to take additional actions to prevent an unsolicited takeover and inhibit the ability of an acquirer to amend the bylaws to facilitate an unsolicited takeover attempt; and
•advance notice procedures with which stockholders must comply to nominate candidates to the Board or to propose matters to be acted upon at a stockholders’ meeting, which could preclude stockholders from bringing matters before annual or special meetings of stockholders and delay changes in the Board, and also may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of POINT.
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in the Board or management.
In addition, our Certificate of Incorporation includes a provision substantially similar to Section 203 of the DGCL, which may prohibit certain stockholders holding 15% or more of POINT’s outstanding capital stock from engaging in certain business combinations with POINT for a specified period of time.
POINT’s bylaws designate a state or federal court located within the State of Delaware as the sole and exclusive forum for substantially all disputes between POINT and its stockholders, which could limit POINT’s stockholders’ ability to obtain a favorable judicial forum for disputes with POINT or its directors, officers, stockholders, employees or agents.
77
POINT’s bylaws provide that, unless POINT consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for state law claims for (i) any derivative action or proceeding brought on behalf of POINT, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of POINT to POINT or POINT’s stockholders, (iii) any action asserting a claim arising pursuant to any provision of the DGCL or its Certificate of Incorporation or bylaws, (iv) any action to interpret, apply, enforce or determine the validity of its Certificate of Incorporation or bylaws, or (v) any action asserting a claim against POINT governed by the internal affairs doctrine. The forgoing provisions will not apply to any claims arising under the Exchange Act or the Securities Act and, unless POINT consents in writing to the selection of an alternative forum, the U.S. District Court for the District of Delaware will be the sole and exclusive forum for resolving any action asserting a claim arising under the Securities Act of 1933, as amended (the "Securities Act").
This choice of forum provision in POINT’s bylaws may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with POINT or any of POINT’s directors, officers, or other employees, which may discourage lawsuits with respect to such claims. There is uncertainty as to whether a court would enforce such provisions, and the enforceability of similar choice of forum provisions in other companies’ charter documents has been challenged in legal proceedings. It is possible that a court could find these types of provisions to be inapplicable or unenforceable, and if a court were to find the choice of forum provision contained in its Certificate of Incorporation to be inapplicable or unenforceable in an action, POINT may incur additional costs associated with resolving such action in other jurisdictions, which could harm POINT’s business, results of operations and financial condition.
Risks Relating to Ownership of POINT’s Common Stock
POINT does not know whether an active, liquid and orderly trading market will develop for its common shares or what the market price of its common shares will be and, as a result, it may be difficult for you to sell your common shares.
Although, POINT’s Common Stock is listed on Nasdaq, an active trading market for its shares may never develop or be sustained. You may not be able to sell your shares quickly or at the market price if trading in POINT’s Common Stock is not active. Further, an inactive market may also impair POINT’s ability to raise capital by selling POINT’s Common Stock and may impair POINT’s ability to enter into strategic partnerships or acquire companies or products by using POINT’s Common Stock as consideration.
The price of POINT Common Stock may be volatile, and you could lose all or part of your investment.
The trading price of POINT Common Stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond POINT’s control, including limited trading volume. These factors include:
•the results of POINT’s ongoing, planned or any future preclinical studies, clinical trials or clinical development programs;
•the commencement, enrollment or results of clinical trials of POINT’s product candidates or any future clinical trials POINT may conduct, or changes in the development status of POINT’s product candidates;
•adverse results or delays in preclinical studies and clinical trials;
•POINT’s decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial;
•any delay in POINT’s regulatory filings or any adverse regulatory decisions, including failure to receive regulatory approval of POINT’s product candidates;
•changes in laws or regulations applicable to POINT’s product candidates, including but not limited to clinical trial requirements for approvals;
•adverse developments concerning POINT’s manufacturers or its manufacturing plans;
•POINT’s inability to obtain adequate product supply for any licensed product or inability to do so at acceptable prices;
•POINT’s inability to establish collaborations, if needed;
•POINT’s failure to commercialize its product candidates;
78
•departures of key scientific or management personnel;
•unanticipated serious safety concerns related to the use of POINT’s product candidates;
•introduction of new products or services offered by POINT or its competitors;
•announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by POINT or its competitors;
•POINT’s ability to effectively manage its growth;
•the size and growth of POINT’s initial cancer target markets;
•POINT’s ability to successfully treat additional types of cancers or at different stages;
•actual or anticipated variations in quarterly operating results;
•POINT’s cash position;
•POINT’s failure to meet the estimates and projections of the investment community or that POINT may otherwise provide to the public;
•publication of research reports about POINT or its industry, or positive or negative recommendations or withdrawal of research coverage by securities analysts;
•changes in the market valuations of similar companies;
•activities of similar companies;
•overall performance of the equity markets;
•sales of POINT Common Stock by POINT or its stockholders in the future;
•trading volume of POINT Common Stock;
•changes in accounting practices;
•ineffectiveness of POINT’s internal controls;
•disputes or other developments relating to proprietary rights, including patents, litigation matters and POINT’s ability to obtain patent protection for its technologies;
•significant lawsuits, including patent or stockholder litigation;
•general political and economic conditions; and
•other events or factors, many of which are beyond POINT’s control.
In addition, the stock market in general, and The Nasdaq Stock Market and pharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of POINT Common Stock, regardless of POINT’s actual operating performance. In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management’s attention and resources, which would harm POINT’s business, financial condition and results of operations.
Future sales and issuances of Common Stock or rights to purchase Common Stock, including pursuant to the Equity Incentive Plan and future exercise of registration rights, could result in additional dilution of the percentage ownership of POINT’s stockholders and could cause POINT’s share price to fall.
79
POINT expects that significant additional capital will be needed in the future to continue its planned operations, including conducting clinical trials, expanded research and development activities, and costs associated with operating as a public company. To raise capital, POINT may sell common shares, convertible securities or other equity securities in one or more transactions at prices and in a manner POINT determines from time to time. If POINT sells common shares, convertible securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to POINT’s existing stockholders, and new investors could gain rights, preferences, and privileges senior to the holders of POINT’s common shares.
Pursuant to the Equity Incentive Plan, POINT is authorized to grant equity awards to its employees, directors and consultants. Initially, the aggregate number of shares of POINT Common Stock that may be issued pursuant to share awards under the Equity Incentive Plan is equal to ten percent (10%) of the issued and outstanding shares of POINT Common Stock, inclusive of rollover options and exercised shares, as of immediately following the Effective Time. This shall be cumulatively increased annually on the first day of each fiscal year beginning with the 2022 fiscal year in an amount equal to four percent (4%) of shares of POINT Common Stock outstanding on the last day of the immediately preceding fiscal year or a lesser number of shares determined by the Board. As a result, on January 1, 2023, an additional 4,225,990 shares were added to the Equity Incentive Plan. Unless the Board elects not to increase the number of shares available for future grants each year, POINT’s stockholders may experience additional dilution, which could cause POINT’s share price to fall. In 2023, the Board allowed the number of shares to increase per the terms of the Equity Incentive Plan.
Pursuant to our Amended and Restated Registration and Stockholder Rights Agreement, certain stockholders of POINT can demand that POINT register their registrable securities under certain circumstances and will each also have piggyback registration rights for these securities. In addition, following the closing of the Business Combination, POINT was required to file and maintain an effective registration statement under the Securities Act covering such securities and certain other securities of POINT. The registration of these securities permit the public sale of such securities, subject to certain contractual restrictions imposed by the Amended and Restated Registration and Stockholder Rights Agreement and the Business Combination Agreement. The presence of these additional shares of Common Stock trading in the public market may have an adverse effect on the market price of POINT’s securities.
POINT does not intend to pay dividends on its Common Stock, so any returns will be limited to the value of POINT Common Stock.
POINT currently anticipates that it will retain future earnings for the development, operation and expansion of its business and does not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, POINT may enter into agreements that prohibit it from paying cash dividends without prior written consent from POINT’s contracting parties, or which other terms prohibiting or limiting the amount of dividends that may be declared or paid on Common Stock. Any return to stockholders will therefore be limited to the appreciation of their POINT Common Stock, which may never occur.
POINT is an emerging growth company, and it cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make its Common Stock less attractive to investors.
POINT is an emerging growth company, as defined in the Jumpstart Our Business Startups Act ("JOBS Act"). For as long as POINT continues to be an emerging growth company, POINT may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 ("Sarbanes-Oxley Act"), reduced disclosure obligations regarding executive compensation in POINT’s periodic reports and proxy statements, exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved, and an exemption from compliance with the requirement of the Public Accounting Oversight Board regarding the communication of critical audit matters in the auditor’s report on the financial statements. POINT could be an emerging growth company for up to five years following the year in which RACA completed its initial public offering, although circumstances could cause POINT to lose that status earlier. POINT will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the date of the completion of RACA’s initial public offering, (b) in which POINT has total annual gross revenue of at least $1.07 billion or (c) in which POINT is deemed to be a large accelerated filer, which requires the market value of POINT Common Stock that are held by non-affiliates to exceed $700 million as of the prior June 30th, and (2) the date on which POINT has issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. POINT has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies
80
until the earlier of the date POINT (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, POINT will not be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies and POINT’s financial statements may not be comparable to other public companies that comply with new or revised accounting pronouncements as of public company effective dates. POINT may choose to early adopt any new or revised accounting standards whenever such early adoption is permitted for private companies.
Further, even after POINT no longer qualifies as an emerging growth company, it may still qualify as a “smaller reporting company,” which would allow POINT to take advantage of many of the same exemptions from disclosure requirements, including reduced disclosure obligations regarding executive compensation in POINT’s periodic reports and proxy statements.
POINT cannot predict if investors will find its Common Stock less attractive because POINT may rely on these exemptions. If some investors find POINT Common Stock less attractive as a result, there may be a less active trading market for POINT Common Stock and POINT’s share price may be more volatile.
POINT is incurring and will continue to incur significant increased costs as a public company, and POINT’s management is required to devote substantial time to compliance initiatives.
As a public company, POINT faces increased legal, accounting, administrative and other costs and expenses as a public company that it did not incur as a private company. The Sarbanes-Oxley Act, including the requirements of Section 404, as well as rules and regulations subsequently implemented by the Securities and Exchange Commission (“SEC”), the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and the rules and regulations promulgated and to be promulgated thereunder, the PCAOB and the securities exchanges, impose additional reporting and other obligations on public companies. Compliance with public company requirements have increased costs and made certain activities more time-consuming. A number of those requirements require POINT to carry out activities it had not done previously. In addition, additional expenses associated with SEC reporting requirements have been and will continue to be incurred. Furthermore, if any issues in complying with those requirements are identified (for example, if the auditors identify a material weakness or significant deficiency in the internal control over financial reporting), POINT could incur additional costs rectifying those issues, and the existence of those issues could adversely affect POINT’s reputation or investor perceptions of it. It may also be more expensive to obtain director and officer liability insurance. Risks associated with POINT’s status as a public company may make it more difficult to attract and retain qualified persons to serve on the board of directors or as executive officers. The additional reporting and other obligations imposed by these rules and regulations will increase legal and financial compliance costs and the costs of related legal, accounting and administrative activities. These increased costs will require POINT to divert a significant amount of money that could otherwise be used to expand the business and achieve strategic objectives. Advocacy efforts by stockholders and third parties may also prompt additional changes in governance and reporting requirements, which could further increase costs.
Because POINT has significant operations in Canada, it may be difficult to serve legal process or enforce judgments against POINT.
While POINT is incorporated in Delaware and has operations in Indianapolis, Indiana, it also maintains significant operations in Canada. In addition, while many of POINT’s directors and officers reside in the U.S., several of them reside outside of the U.S. Accordingly, service of process upon POINT may be difficult to obtain within the U.S.. Furthermore, because certain of POINT’s assets are located outside the U.S., any judgment obtained in the U.S. against POINT, including one predicated on the civil liability provisions of the U.S. federal securities laws, may not be collectible within the U.S.. Therefore, it may not be possible to enforce those actions against POINT.
Furthermore, it may not be possible to subject foreign persons or entities to the jurisdiction of the courts in the U.S. Similarly, to the extent that POINT’s assets are located in Canada, investors may have difficulty collecting from it any judgments obtained in the U.S. courts and predicated on the civil liability provisions of U.S. securities provisions.
If POINT fails to establish and maintain an effective system of internal control over financial reporting, POINT may not be able to accurately report its financial results or prevent fraud, which may cause investors to lose confidence in POINT’s financial and other public reporting and may lead to a decline in the price of POINT Common Stock.
Effective internal control over financial reporting is necessary for POINT to provide reliable financial reports and, together with adequate disclosure controls and procedures, is designed to prevent fraud. Ensuring that POINT has adequate internal financial and accounting controls and procedures in place so that it can produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could cause POINT to fail to meet its reporting obligations. In addition, any testing by POINT conducted in connection with Section 404 of the Sarbanes-
81
Oxley Act or any subsequent testing by its independent registered public accounting firm, may reveal deficiencies in POINT’s internal control over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to its financial statements or identify other areas for further attention or improvement. Inferior internal controls or any failure to remediate any significant deficiencies or material weaknesses could lead POINT to fail to meet its reporting obligations, result in material misstatements in POINT’s financial statements or cause investors to lose confidence in POINT’s reported financial information, each of which could have a negative effect on the trading price of POINT Common Stock.
POINT has established its internal controls and procedures for compliance with Section 404 and applicable U.S. laws. POINT will be required to disclose changes made in its internal controls and procedures on a quarterly basis and its management will be required to assess the effectiveness of these controls annually. However, for as long as POINT is an emerging growth company, or EGC, its independent registered public accounting firm will not be required to attest to the effectiveness of its internal controls over financial reporting pursuant to Section 404. An independent assessment of the effectiveness of POINT’s internal control over financial reporting could detect problems that its management’s assessment might not. Undetected material weaknesses in POINT’s internal control over financial reporting could lead to restatements of its financial statements and require POINT to incur the expense of remediation.
There can be no assurance that POINT will be able to comply with the continued listing standards of Nasdaq.
If Nasdaq delists shares of POINT Common Stock from trading on its exchange for failure to meet Nasdaq’s listing standards, POINT and its stockholders could face significant material adverse consequences including:
•a limited availability of market quotations for POINT’s securities;
•reduced liquidity for POINT’s securities;
•a determination that POINT Common Stock is a “penny stock” which will require brokers trading in POINT Common Stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for POINT’s securities;
•a limited amount of news and analyst coverage; and
•a decreased ability to issue additional securities or obtain additional financing in the future.
Reports published by analysts, including projections in those reports that differ from POINT’s actual results, could adversely affect the price and trading volume of POINT Common Stock.
Securities research analysts may establish and publish their own periodic projections for POINT. These projections may vary widely and may not accurately predict the results POINT actually achieves. POINT’s share price may decline if POINT’s actual results do not match the projections of these securities research analysts. Similarly, if one or more of the analysts who write reports on POINT downgrades POINT Common Stock or publishes inaccurate or unfavorable research about POINT’s business, POINT’s share price could decline. If one or more of these analysts ceases coverage of POINT or fails to publish reports on POINT regularly, POINT’s share price or trading volume could decline.
We may be adversely affected by the effects of uncertainty in macroeconomic conditions such as rising inflation, supply chain disruptions and concerns regarding a potential global recession.
Our operations and performance depend on worldwide economic conditions. These conditions have been adversely impacted by continued global economic concerns over rising inflation rates, supply chain disruptions, a potential recession, disruptions in access to bank deposits or lending commitments due to bank failures and other monetary and financial uncertainties. Rising inflation may adversely affect our liquidity, business, financial condition and results of operations by increasing our overall cost structure. The existence of inflation in the economy has resulted in, and may continue to result in, higher interest rates and capital costs, supply shortages, increased costs of labor, components, manufacturing and shipping, as well as weakening exchange rates and other similar effects. In response to recent inflationary pressure, the U.S. Federal Reserve and other global central banks have raised interest rates in 2022, and further interest rate increases are anticipated. These increases have led to concerns of a potential global recession. In addition, there have been recent bank failures such as Silicon Valley Bank and Signature Bank. Additional such failures, fears of such failures, or other bank distresses could cause disruptions in access to bank deposits or lending commitments. Any such events are likely to result in significant disruption of global financial markets, which may reduce our ability to access capital on favorable terms or at all. In addition, a recession, depression or other sustained adverse market event could materially and adversely affect our business and the value of our Common Stock. Although we may take measures to mitigate these events, if these measures
82
are not effective, our business, financial condition, results of operations and liquidity could be materially adversely affected. Even if such measures are effective, there could be a difference between the timing of when these beneficial actions impact our results of operations and when the costs are incurred.
Actions of activist stockholders against us could be disruptive and potentially costly and the possibility that activist stockholders may seek changes that contest, or conflict with, our strategic direction could cause uncertainty about the strategic direction of our business.
Activist stockholders may from time to time attempt to effect changes in our strategic direction and, in furtherance thereof, may seek changes in how we are governed. While our board of directors and management strive to maintain constructive, ongoing communications with all of our stockholders, including activist stockholders, and welcomes their views and opinions with the goal of working together constructively to enhance value for all stockholders, activist campaigns that contest, or conflict with, our strategic direction could have an adverse effect on us because:
•responding to proxy contests or other actions by activist stockholders, or adverse proxy advisory firm recommendations, can be costly and time-consuming, disrupting operations and diverting the attention of management and employees;
•perceived uncertainties as to future direction may result in the loss of potential acquisitions, collaborations or licensing opportunities, and may make it more difficult to attract and retain qualified personnel and business partners; and
•if individuals are elected to our board of directors with a specific agenda, it may adversely affect our ability to effectively and timely implement our strategic plan and create additional value for our stockholders.
These actions could cause our stock price to decrease and experience periods of increased volatility.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our principal executive offices are located in the U.S. in Indianapolis, Indiana, including an office space occupying 10,500 square feet and a manufacturing facility occupying 70,200 square feet. We also lease an additional location in Toronto, Canada. See Note 11 to our consolidated financial statements included elsewhere in this Form 10-K, which information is incorporated herein by reference for further discussion surrounding mortgages on our owned properties. We believe our existing facilities are sufficient for our ongoing needs, and that, if we require additional space, we will be able to obtain suitable additional or alternative facilities on commercially reasonable terms.
Item 3. Legal Proceedings
We are not currently a party to any material legal proceedings. From time to time, we may become involved in litigation or legal proceedings relating to claims arising from the ordinary course of business.
Item 4. Mine Safety Disclosures
Not applicable.
83
PART II
Item 5, Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our Common Stock is listed on the Nasdaq Capital Market under the symbol “PNT”. On March 22, 2023, the closing price of our Common Stock was $6.85 per share.
Holders of Our Common Stock
As of March 22, 2023, we had 43 holders of record of our Common Stock. Certain shares are held in “street” name and accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We currently intend to retain all available funds and any future earnings to fund the growth and development of our business. We have never declared or paid any cash dividends on our capital stock. We do not intend to pay cash dividends to our stockholders in the foreseeable future. Investors should not purchase our Common Stock with the expectation of receiving cash dividends.
Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions, and other factors that our board of directors may deem relevant.
Recent Sales of Unregistered Securities
None.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Item 6. Reserved
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our audited consolidated financial statements and notes thereto for the periods ended December 31, 2022 and 2021 (the “2022 Financial Statements”) included elsewhere in this Form 10-K. Certain of the information contained in this discussion and analysis or set forth elsewhere in this Form 10-K, including information with respect to plans and strategy for our business, constitutes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the section entitled “Risk Factors” in Part I, Item 1A of this Form 10-K, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. You should carefully read the section entitled “Risk Factors” to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section titled “Cautionary Note Regarding Forward-Looking Statements”. We do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Unless otherwise indicated or the context otherwise requires, references in this Management’s Discussion and Analysis of Financial Condition and Results of Operations section to "POINT," the "Company," “we,” “us,” “our” and other similar terms refer to POINT Biopharma Global Inc. and its consolidated subsidiaries.
Overview
Introduction
84
We are a globally focused radioligand company building a platform for the clinical development and commercialization of radioligands that fight cancer. We have a pipeline of product candidates and early-stage development programs, in-house manufacturing capabilities, and a secured supply for rare medical isotopes like 225Ac and 177Lu.
Our team brings decades of combined experience in radioligand clinical development and manufacturing. In a space where supply chain is often overlooked, the Company has carved out a unique advantage for itself: a 100% Company-owned facility, located in Indianapolis, Indiana, which includes an office space occupying 10,500 square feet and a manufacturing facility occupying 70,200 square feet, and which we believe has the capacity for expansion to commercially supply both North America and Europe with large volumes. Furthermore, management has leveraged their prior relationships to assemble resilient radioisotope supply chains for the Company, which even includes manufacturing the Company's own n.c.a. 177Lu isotope in-house.
Our predecessor was incorporated on September 18, 2019 as POINT Theranostics Inc. under the DGCL and subsequently amended its name to “POINT Biopharma Inc.” on November 22, 2019. Subsequent to the Business Combination, POINT Biopharma Inc. became a wholly-owned subsidiary of POINT Biopharma Global Inc. on June 30, 2021.
Business Combination
On June 30, 2021, POINT Biopharma Inc. consummated the Business Combination with RACA, pursuant to the terms of the Business Combination Agreement, dated as of March 15, 2021, by and among RACA, Bodhi Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of RACA, and POINT Biopharma Inc. Pursuant to the Business Combination Agreement, on the Closing Date, (i) Merger Sub merged with and into POINT Biopharma Inc., with POINT Biopharma Inc. as the surviving company in the Merger as a wholly-owned subsidiary of RACA and (ii) RACA changed its name to “POINT Biopharma Global Inc.”
In accordance with the terms and subject to the conditions of the Business Combination Agreement, at the Effective Time of the Merger, (i) each share and vested equity award of POINT Biopharma Inc. outstanding as of immediately prior to the Effective Time was exchanged for shares of the Common Stock of POINT or comparable vested equity awards that are exercisable for shares of Common Stock, as applicable, based on an implied POINT Biopharma Inc. vested equity value of $585 million (which results in a conversion ratio of approximately 3.59:1); (ii) all unvested equity awards of POINT Biopharma Inc. were exchanged for comparable unvested equity awards that are exercisable for shares of Common Stock, determined based on the same exchange ratio at which the vested equity awards were exchanged for shares of Common Stock; and (iii) each share of Class A Common Stock of RACA and each share of Class B Common Stock, of RACA that was issued and outstanding immediately prior to the Effective Time became one share of Common Stock following the consummation of the Business Combination.
In addition, concurrently with the execution of the Business Combination Agreement, on March 15, 2021, RACA entered into Subscription Agreements with certain investors, pursuant to which the PIPE Investors agreed to subscribe for and purchase, and RACA agreed to issue and sell to the PIPE Investors, an aggregate of 16,500,000 shares of Class A Common Stock at a price of $10.00 per share, for aggregate gross proceeds of $165 million. The PIPE Financing was consummated concurrently with the closing of the Business Combination. We received net proceeds of approximately $260.0 million consisting of proceeds of the PIPE Financing and the proceeds remaining in RACA’s trust account. Transaction costs of approximately $27.0 million consisted of investment banker, legal, audit, tax, accounting, consulting, insurance, board retainer fees and listing fees.
In September 2022, POINT closed a previously announced underwritten public offering of approximately 15,500,000 shares of Common Stock at a public offering price of $9.00 per share (the "2022 Public Offering"). The gross proceeds to the Company from the 2022 Public Offering, before deducting underwriting discounts and commissions and other estimated offering expenses, were approximately $140.0 million.
Recent Developments
PNT2002: 177Lu-based PSMA-targeted radioligand therapy
In February 2022 POINT published dosimetry data at the 2022 SNMMI Mid-Winter & ACNM Annual Meeting on February 25-27, 2022, from the 27 participants dosed in the safety and dosimetry lead-in cohort for the Company’s phase 3 SPLASH trial (NCT04647526) evaluating 177Lu-PNT2002 for the treatment of metastatic castration-resistant prostate cancer (mCRPC). The abstract was made public on the first day of the conference. Radiation dosimetry of 177Lu-PNT2002 was calculated for the 27 participants based on biodistribution data from planar whole-body conjugate imaging at 1, 24, 48, 72, and 168 hours and, for 7 of them, SPECT/CT imaging at 48-72 hours post injection of their first cycle of 177Lu-PNT2002 (6.8±10% GBq). Data from the abstract titled “Dosimetry Results from the SPLASH Trial” (Abstract #: MWMA2244) demonstrated the following:
85
•Organs receiving the largest absorbed doses were the lacrimal glands at 1.2 Gy/GBq, followed by the kidneys at 0.73 Gy/GBq.
•The average dose to the salivary glands and red marrow was 0.34 Gy/GBq and 0.034 Gy/GBq, respectively.
•For a cumulative administered activity of 27.2 GBq (four cycles of 6.8 GBq) the kidneys would receive a cumulative absorbed dose of 19.9 Gy, and the red marrow, 0.91 Gy.
•SPECT/CT vs planar-based kidney dosimetry was consistent across most participants (±20%) where SPECT/CT images were available with a mean kidney absorbed dose difference of 1%.
In April 2022, POINT dosed the first E.U. participant in the SPLASH trial, and in September 2022, the Company published a poster at ESMO Congress 2022 containing updated efficacy and safety data from the 27-participant safety and dosimetry lead-in cohort for the phase 3 SPLASH trial. The poster, titled “Efficacy and Safety of 177Lu-PNT2002 Prostate-specific Membrane Antigen (PSMA) Therapy in Metastatic Castration Resistant Prostate Cancer (mCRPC): Initial Results from SPLASH” (e-Poster #1400P). Key findings highlighted in the poster include:
•Median radiographic progression-free survival (rPFS) was 11.5 months, as compared to the control arm benchmarks of 3.5–4.2 months for individuals with progressive mCRPC post-ARPI failure receiving similar treatment (de Bono J, et al. N Engl J Med 2020; 382:2091-102.; Powles T, et al. Nature 2022;28:144–53).
•Median overall survival had not been reached with an 11.7-month median duration of follow-up from time of enrollment
•A radiographic objective response was achieved in 60% of the 10 participants with evaluable disease at baseline
•84.8% of individuals imaged met PSMA eligibility criteria
•From a median baseline PSA (ng/mL) of 22 (range 0.3–701.0), 11 (42%) participants achieved a PSA50 response
•177Lu-PNT2002 was well tolerated with no treatment-related deaths and few treatment-related adverse events of grade 3 or higher
•Treatment-related adverse events occurring in >10% of participants included dry mouth (25.9% of participants; all grade 1), fatigue (22.2%; grades 1-2), nausea (18.5%; grades 1-2), and anaemia (14.8%; grades 1-3)
In November 2022, POINT announced strategic collaboration and exclusive license agreements with Lantheus for exclusive worldwide rights for 177Lu-PNT2002, excluding certain territories (Japan, South Korea, Singapore, Indonesia, and China including Hong Kong, Macau and Taiwan).
In December 2022, closing conditions for the transaction, including Hart-Scott-Rodino antitrust clearance, were met. For 177Lu-PNT2002, POINT has received a $250 million upfront payment. POINT will receive an additional payment of up to $250 million upon U.S. regulatory approval, and, once certain return on investment financial thresholds have been achieved and other conditions met, royalties of 20% on net sales, as well as the potential for up to an additional $1.3 billion in various net sales milestone payments.
Joint steering committees have been formed to oversee the clinical studies, regulatory filings, manufacturing, and commercial readiness for 177Lu-PNT2002. As per the strategic collaboration and exclusive license agreement, Lantheus will be responsible for all subsequent development of the asset post initial regulatory filings within the U.S., and for all development efforts in their territories.
The randomization phase of the study started enrolling in September 2021, and in December 2022 the target enrollment for randomization was completed on schedule with more than 390 participants randomized across 55 SPLASH trial sites in North America, Europe, and the U.K. We expect to report top line data from SPLASH in the second half of 2023.
PNT2003: n.c.a. 177Lu-labelled somatostatin-targeted radioligand
PNT2003’s use of n.c.a. 177Lu enables administration in outpatient clinics without requiring the clinic to maintain costly dedicated waste streams, providing a unique advantage over the currently approved radioligand product for the GEP-NETs indication.
The last patient last dose for OZM-067 occurred in July 2021, all participants completed the primary follow-up in the second quarter of 2022, and long term follow-up is still ongoing.
In November 2022, POINT announced strategic collaboration and exclusive license agreements with Lantheus for exclusive worldwide rights for 177Lu-PNT2003, excluding certain territories (Japan, South Korea, Singapore, Indonesia, and China including Hong Kong, Macau and Taiwan). For 177Lu-PNT2003, POINT has received a $10 million upfront payment, and will receive up to an additional $30 million upon U.S. regulatory approval, royalties of 15% on net sales, as
86
well as the potential for up to an additional $275 million in various net sales milestone payments. Joint steering committees have been formed to oversee the clinical studies, regulatory filings, manufacturing, and commercial readiness for 177Lu-PNT2003.
In late 2022, POINT received the OZM-067 clinical trial data sets from the trial sponsor. POINT will facilitate the analysis of the data sets. As per the strategic collaboration and exclusive license agreement, Lantheus will be responsible for all subsequent development of the asset post initial regulatory filings within the U.S., and for all development efforts in their territories.
PNT2004: fibroblast activation protein-alpha (FAP-alpha) inhibitor
The Company completed a pre-CTA meeting with Health Canada in December 2021 regarding the development pathway and clinical study design for a phase 1 trial. A CTA with Health Canada was filed at the end of the first quarter of 2022 for PNT6555, and a No Objection Letter was received from Health Canada in May 2022. The phase 1 clinical trial is called FRONTIER: “FAPi Radioligand OpeN-Label, Phase 1 Study to Evaluate Safety, Tolerability and DosImetry of [Lu-177]-PNT6555; A Dose Escalation Study for TReatment of Patients with Select Solid Tumors”.
In April 2022, preclinical data for the program was presented in a poster at the American Association for Cancer Research (AACR) 2022 Annual Meeting. The poster was titled “Preclinical Characterization of the Novel Fibroblast Activation Protein (FAP)-Targeting Ligand PNT6555 for the Imaging and Therapy of Cancer” (Abstract ID: 3554, Session: Preclinical Radiotherapeutics). The presented data concluded:
•In preclinical xenograft models: 68Ga-PNT6555 is an effective imaging agent, with strong tumor targeting, low background in normal tissues and rapid clearance via urinary excretion, and 177Lu-PNT6555 shows prolonged tumor retention out to 168 hours post-injection.
•Efficacy studies with 177Lu-PNT6555 or 225Ac-PNT6555 demonstrate compelling and dose-responsive inhibition of HEK-mFAP tumor growth.
FRONTIER commenced in July 2022 in Canada using a 68Ga-based PNT6555 molecular imaging agent to select participants to receive a n.c.a. 177Lu-based PNT6555 therapeutic agent. FRONTIER's clinical protocol evaluates PNT6555 in approximately 30 participants in five FAP-avid cancer indications: colorectal, pancreatic, esophageal, melanoma, and soft tissue sarcoma.
Dosing in the FRONTIER phase 1 trial started at 4 GBq, with each subsequent dose level increasing by 4 GBq, also allowing 2 GBq dose de-escalations. Each 177Lu-PNT6555 dose is followed by a 6-week interval. 68Ga-PNT6555 PET/CT imaging is conducted approximately 90 minutes post injection, and dosimetry is assessed at the time of first 177Lu-PNT6555 dose and at each subsequent cycle. The primary objective of the study is to determine the maximum tolerated dose, and the recommended phase 2 dose.
In November, 2022, preclinical data supporting the combination of 177Lu-PNT6555 with immunotherapy was released via a press release. The preclinical study focused on assessing the potential of the lead candidate in the PNT2004 clinical program, 177Lu-PNT6555, in combination with anti-PD-1 immunotherapy. The CT26-mFAP tumor model used expresses low levels of FAP, grows aggressively, and is insensitive to anti-PD-1 immunotherapy. The study found that combination treatment with 177Lu-PNT6555 and anti-PD-1 resulted in a significant survival benefit, as compared to either treatment independently.
Enrollment in cohort 2 of FRONTIER began in December 2022 and the Company expects enrollment in cohort 3 enrollment to begin in second quarter of 2023. A total of six participants have been dosed with 177Lu-PNT6555 to date. We anticipate data from the full FRONTIER trial to be available in the first half of 2024.
PNT2001: 225Ac-labelled next-generation PSMA-targeted radioligand
The PNT2001 program leverages linker technology that promotes increased tumor accumulation. Preclinical studies have resulted in the identification of a lead candidate, 225Ac-PSMA-62, which, as compared to other late-stage PSMA-targeted radioligands, demonstrates potent anti-tumor activity using 225Ac, while also having an improved biodistribution profile. POINT has advanced 225Ac-PSMA-62 into IND-enabling studies with the aim of pursuing clinical development with 225Ac-PSMA-62 in the biochemically recurrent, oligometastatic, and post-177Lu-PSMA mCRPC settings.
In October 2022, POINT published additional preclinical data for the PNT2001 program, and shared the data at the 35th Annual Congress of the European Association of Nuclear Medicine (EANM) in Barcelona, Spain in E-poster #039, titled “Development and Characterization of a Next-generation 225Ac-PMSA Radioligand”. The Company projects an IND/
87
CTA submission for 225Ac-PSMA-62 for the second quarter of 2023, with the first participant expected for 225Ac-PSMA-62's phase 1 clinical trial by the end of 2023.
CanSEEKTM: Tumor Microenvironment (TME) Targeting Technology
The goal of the CanSEEKTM program is to significantly improve the precision and safety of radioligands. Based on the (d)-Ala-Pro FAP-α substrate technology, CanSEEKTM prevents a radioligand from binding to receptors until it has been activated by FAP-α in the tumor microenvironment. If successful, CanSEEKTM could significantly improve the therapeutic index of targeted radioligands. Multiple (d)-Ala-Pro substrate-enabled ligands are being studied preclinically against different targets.
POINT’s CanSEEKTM technology is the subject of licenses from both Bach Biosciences and Avacta.
Risks and Liquidity
Drug research and development is very expensive and involves a high degree of risk. Only a small number of research and development programs result in the commercialization of a product. We will not generate revenue from product sales unless and until we successfully complete clinical development, are able to obtain regulatory approval for and successfully commercialize the product candidates we are currently developing or may develop. We currently do not have any product candidates approved for commercial sale.
Our product candidates, currently under development or that we may develop, will require significant additional research and development efforts, including extensive clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel infrastructure and extensive compliance and reporting capabilities. There can be no assurance that our research and development activities will be successfully completed, that adequate protection for our licensed or developed technology will be obtained and maintained, that products developed will obtain necessary regulatory approval or that any approved products will be commercially viable.
If we obtain regulatory approval for one or more of our product candidates, we expect to incur significant expenses related to developing our commercialization capabilities to support product sales, marketing, and distribution activities, either alone or in collaboration with others. Prior to the Lantheus License Agreements, the Company had incurred significant net losses and had funded operations through the Business Combination and equity financings. Operating losses and negative cash flows are expected to be incurred again in the future.
We believe that the net proceeds from the Business Combination and PIPE Financing, the 2022 Public Offering, the Lantheus License Agreements, together with our available resources and existing cash, cash equivalents and investments, are sufficient to fund our operating expenses and capital expenditure requirements into 2026. However, this does not reflect the possibility that we may not be able to access a portion of our existing cash, cash equivalents and investments due to market conditions. If additional banks and financial institutions enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our cash and cash equivalents and investments may be threatened and could have a material adverse effect on our business and financial condition.
We are subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, successful discovery and development of our product candidates, development by competitors of new technological innovations, dependence on key personnel, the ability to attract and retain qualified employees, protection of proprietary technology, compliance with governmental regulations, the impact of COVID-19, the ability to secure additional capital to fund operations and commercial success of our product candidates. Product candidates currently under development will require extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel, and infrastructure and extensive compliance-reporting capabilities. Even if our drug development efforts are successful, it is uncertain when, if ever, we will realize significant revenue from product sales.
We anticipate that our expenses will increase significantly in connection with our ongoing activities, as we:
•advance our clinical-stage product candidates 177Lu-PNT2002 and 177Lu-PNT6555 through clinical development;
•advance our preclinical-stage product candidate 225Ac-PSMA-62, along with candidates developed with our CanSEEKTM Prodrug Platform, into clinical development;
•seek to identify, acquire, and develop additional product candidates, including through business development efforts to invest in or in-license other technologies or product candidates;
88
•hire additional clinical, quality control, medical, scientific, and other technical personnel to support our clinical operations;
•expand our operational, financial and management systems, and increase personnel to support our operations;
•meet the requirements and demands of being a public company;
•maintain, expand, and protect our intellectual property portfolio;
•make milestone, royalty or other payments due under various in-license or collaboration agreements;
•seek regulatory approvals for any product candidates that successfully complete clinical trials; and
•undertake any pre-commercialization activities to establish sales, marketing, and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own or jointly with third parties.
COVID-19 Pandemic and other geopolitical events
The COVID-19 pandemic has caused many governments to implement measures to slow the spread of the outbreak through quarantines, travel restrictions, heightened border security and other measures. The U.S. administration has announced that it intends to end the public health emergency declaration as a result of COVID-19 on May 11, 2023. Although the impact has decreased and many of these measures have been terminated, COVID-19 may continue to have an impact on the global economy as well as businesses and capital markets around the world.
Further, general macroeconomic trends, including rising inflation rates, sustained supply chain disruptions, and any resulting recession, depression or other sustained adverse market event could materially and adversely affect our business, financial condition, results of operations and the value of our Common Stock.
Additionally, financial markets may be adversely affected by the current or anticipated impact of military conflict, including escalating military fighting between Russia and Ukraine, terrorism or other geopolitical events. The U.S. and other nations in response to the Russo-Ukrainian conflict have announced economic sanctions which may have an adverse effect on the global financial markets, which, in turn, could have an adverse effect on our business, financial condition and results of operations. The Company's SPLASH trial has vendor staff in Ukraine, and any political instability in the region may disrupt resourcing assigned to the trial and negatively impact our business.
We are monitoring the potential impact of the COVID-19 pandemic and the impact of the Russo-Ukrainian conflict on our business and financial results. To date, we have not experienced any material business disruptions or incurred any impairment losses in the carrying values of our assets as a result of these events and we are not aware of any specific related event or circumstance that would require us to revise our estimates reflected in the 2022 Financial Statements.
Components of Operating Results
Revenue from Product Sales
To date, we do not have any approved product candidates and such, have not generated any revenue from product sales, and we do not expect to generate any revenue from the sale of products for the foreseeable future. If our development efforts for our current or future product candidates are successful and result in regulatory approval, we may generate revenue in the future from product sales. We cannot predict if, when or to what extent we will generate revenue from the commercialization and sale of our product candidates. We may never succeed in obtaining regulatory approval for any of our product candidates.
Revenue from Lantheus License Agreements
On November 11, 2022, POINT entered into the Lantheus License Agreements with affiliates of Lantheus pursuant to which Lantheus was granted exclusive world-wide licenses (excluding certain countries in Asia) to commercialize the Company's PNT2002 and PNT2003 late-stage product radioligand therapies. POINT will be responsible for completing the SPLASH trial and the parties will work together to file the NDA with the costs incurred in connection with the FDA submission being borne by Lantheus. Thereafter, Lantheus will be responsible for all additional clinical and regulatory costs in the U.S., as well as all costs for development, clinical trials and regulatory approval in the rest of its territories outside the U.S., except Asia. On December 15, 2022, we announced the consummation of the agreements.
89
In connection with the PNT2002 Agreement, we received an up-front payment of $250.0 million on December 22, 2022. We will also receive a regulatory milestone payment upon FDA approval of up to $250.0 million, $530.0 million on various Lantheus annual net sales milestones up to $600.0 million, plus up to an additional $750.0 million in payments on various Lantheus annual net sales in excess of $600.0 million. Once certain financial thresholds have been achieved, we will receive a royalty rate of 20.0 percent on all net sales. In the event Lantheus sub-licenses the product outside the U.S., we will be entitled to 40.0 percent of all sublicense income (i.e., up-front, milestone and royalty payments) received by Lantheus. In addition, we will also be entitled to milestone payments upon E.U., Middle East and Africa approval of $25.0 million for the E.U., $4.0 million for the Middle East and $2.0 million for any African country.
In connection with the PNT2003 Agreement, we received an up-front payment of $10.0 million on December 22, 2022. We will also receive a regulatory milestone payment upon FDA approval of up to $30.0 million, net sales milestone payments of up to $275.0 million on various Lantheus annual net sales milestones, and a royalty rate of 15.0 percent of net sales. In the event Lantheus sub-licenses the product outside the U.S., we will be entitled to 40.0 percent of all sublicense income (i.e., up-front, milestone and royalty payments) received by Lantheus. Any costs incurred in connection with the FDA submission and development costs will be the responsibility of Lantheus. For any sublicensing of PNT2003, we are obligated to pay the original sublicensor of PNT2003 a low double digit percent royalty fee on any upfront and milestone payments and a low-single digit percent royalty fee based on such sub-licensing royalties. In addition, we will also be entitled to milestone payments upon E.U., Middle East and Africa approval of $2.5 million for the E.U., $1 million for the Middle East and $1 million for any African country.
During the year ended December 31, 2022, we recognized $226.6 million in revenue in connection with the Lantheus License Agreements in our consolidated statement of operations and comprehensive income (loss).
Research and Development
We support our drug discovery and development efforts through the commitment of significant resources to our preclinical and clinical development activities. Research and development expenses consist of costs incurred in performing research and development activities, including costs for salaries and bonuses, employee benefits, subcontractors, facility-related expenses, share-based compensation, third-party license fees, laboratory supplies, and external costs of outside vendors engaged to conduct discovery, preclinical and clinical development activities and clinical trials as well as to manufacture clinical trial materials, and other costs. We recognize external research and development costs based on an evaluation of the services performed to date of specific tasks using information provided to us by our service providers.
Non-refundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. Such prepaid expenses are recognized as an expense when the goods have been delivered or the related services have been performed, or when it is no longer expected that the goods will be delivered, or the services rendered.
Upfront payments under our license agreements are expensed as research and development expense upon receipt of the license. Milestone payments under these license agreements are accrued, with a corresponding expense being recognized, in the period in which the milestone is determined to be probable of achievement and the related amount is reasonably estimable.
We may be entitled to investment tax credits in connection with our research and development costs. These investment tax credits are non-refundable tax credits and are accounted for in accordance with our accounting policies.
We expect that our research and development expenses will substantially increase in connection with our planned preclinical and clinical development activities, both in the near-term and beyond as we continue to invest in activities to develop our product candidates and preclinical programs and as certain product candidates advance into later stages of development. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size, scope, and duration of later-stage clinical trials. Furthermore, the process of conducting the necessary clinical trials to obtain regulatory approval is costly and time-consuming, and the successful development of our product candidates is highly uncertain. As a result, we cannot accurately estimate or know the nature, timing and costs that will be necessary to complete the preclinical and clinical development for any of our product candidates or when and to what extent we may generate revenue from the commercialization and sale of any of our product candidates or achieve profitability.
The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors that include, but are not limited to:
•per patient trial costs;
90
•the number of patients that participate in the trials;
•the number of sites included in the trials;
•the countries in which the trials are conducted;
•the length of time required to enroll eligible patients;
•the number of doses that patients receive;
•the drop-out or discontinuation rates of patients;
•potential additional safety monitoring or other studies requested by regulatory agencies;
•the duration of patient follow-up; and
•the efficacy and safety profile of our product candidates.
Changes in any of these assumptions could significantly impact the cost and timing associated with the development of our product candidates. Additionally, future competition and commercial and regulatory factors beyond our control may also impact our clinical development programs and plans.
General and Administrative
We expense general and administrative costs as incurred. General and administrative expenses consist primarily of salaries, benefits, and share-based compensation. General and administrative expenses also include legal fees incurred relating to corporate and patent matters, professional fees incurred for accounting, auditing, tax and administrative consulting services, insurance costs, and facilities expenses.
We estimate and accrue for services provided by third parties related to the above expenses by monitoring the status of services provided and receiving estimates from our service providers. We reassess and adjust our accruals as actual costs become known or as additional information becomes available.
We expect our general and administrative expenses will increase over the next several years as we increase our headcount to support the continued development of our product candidates. We also anticipate that we will incur increased accounting, audit, legal, regulatory, compliance and director and officer insurance costs as well as investor, public relations and other expenses associated with being a public company.
Income Taxes
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the 2022 Financial Statements or our tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. We assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent we believe, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
We account for uncertainty in income taxes recognized in the 2022 Financial Statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more likely than not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the 2022 Financial Statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
91
Results of Operations
Comparison of the Year Ended December 31, 2022 and the Year Ended December 31, 2021
The following table summarizes our results of operations for the year ended December 31, 2022, and for the year ended December 31, 2021:
| For the year ended December 31, 2022 | For the year ended December 31, 2021 | Change | ||||||||||||||||||||||||
| (In U.S. dollars) | $ | % | ||||||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||||||
| License revenue | 225,165,000 | — | 225,165,000 | 100.0 | % | |||||||||||||||||||||
| Other revenue | 1,414,563 | — | 1,414,563 | 100.0 | % | |||||||||||||||||||||
| Total revenue | 226,579,563 | — | 226,579,563 | 100.0 | % | |||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||
| Research and development | 82,050,392 | 33,505,392 | 48,545,000 | 144.9 | % | |||||||||||||||||||||
| General and administrative | 19,006,876 | 12,006,438 | 7,000,438 | 58.3 | % | |||||||||||||||||||||
| Total operating expenses | 101,057,268 | 45,511,830 | 55,545,438 | 122.0 | % | |||||||||||||||||||||
| Income (loss) from operations | 125,522,295 | (45,511,830) | 171,034,125 | (375.8 | %) | |||||||||||||||||||||
| Other income (expenses): | ||||||||||||||||||||||||||
| Investment income (finance costs) | 4,469,320 | (11,840) | 4,481,160 | (37,847.6 | %) | |||||||||||||||||||||
| Foreign currency loss | (405,527) | (73,153) | (332,374) | 454.4 | % | |||||||||||||||||||||
| Total other income (expenses) | 4,063,793 | (84,993) | 4,148,786 | (4,881.3) | % | |||||||||||||||||||||
| Income (loss) before provision for income taxes | 129,586,088 | (45,596,823) | 175,182,911 | (384.2 | %) | |||||||||||||||||||||
| Provision for income taxes | (31,292,875) | (305,658) | (30,987,217) | 10,137.9 | % | |||||||||||||||||||||
| Net income (loss) | 98,293,213 | (45,902,481) | 144,195,694 | (314.1 | %) | |||||||||||||||||||||
| Net income (loss) per common share: | ||||||||||||||||||||||||||
| Basic | $ | 1.04 | $ | (0.62) | ||||||||||||||||||||||
| Diluted | $ | 1.02 | $ | (0.62) | ||||||||||||||||||||||
| Weighted average common shares outstanding: | ||||||||||||||||||||||||||
| Basic | 94,601,214 | 73,850,822 | ||||||||||||||||||||||||
| Diluted | 96,034,506 | 73,850,822 | ||||||||||||||||||||||||
Revenue
Revenue was $226.6 million for the year ended December 31, 2022 generated in connection with the license and services provided under the Lantheus License Agreements. We did not recognize any commercial revenue for product sales for the year ended December 31, 2022. No revenue was recognized for the year ended December 31, 2021.
Research and Development
The following table summarizes the components of research and development expense for the year ended December 31, 2022, and for the year ended December 31, 2021:
92
| For the year ended December 31, 2022 | For the year ended December 31, 2021 | Change | ||||||||||||||||||||||||
| (In U.S. dollars) | $ | % | ||||||||||||||||||||||||
| Research and development expenses: | ||||||||||||||||||||||||||
| Clinical trial | 35,185,416 | 11,042,144 | 24,143,272 | 218.6 | % | |||||||||||||||||||||
| Salaries and benefits | 20,204,287 | 7,688,852 | 12,515,435 | 162.8 | % | |||||||||||||||||||||
| Contract manufacturing | 14,498,475 | 6,442,844 | 8,055,631 | 125.0 | % | |||||||||||||||||||||
| Sponsored research & product licenses | 6,839,462 | 7,544,596 | (705,134) | (9.3 | %) | |||||||||||||||||||||
| Depreciation and overhead | 4,956,128 | — | 4,956,128 | 100.0 | % | |||||||||||||||||||||
| Regulatory consulting | 366,624 | 786,956 | (420,332) | (53.4 | %) | |||||||||||||||||||||
| Total | 82,050,392 | 33,505,392 | 48,545,000 | 144.9 | % | |||||||||||||||||||||
For the year ended December 31, 2022 as compared to the year ended December 31, 2021, the increase in research and development expense was primarily due to increases in (a) costs incurred in clinical trials and contract manufacturing as we continue to increase the scale of our trials and operations, (b) personnel costs as the Company continues to expand its research and development headcount, most notably in our Indianapolis manufacturing facility and (c) depreciation and overhead related to our manufacturing facility. This was partially offset by decreased costs associated with our licensing agreements and related sponsored research in connection with our product candidates both preclinical and clinical, which required up-front payments in the prior year period. Although the Company does not currently track its research and development expenditures by product, it intends to begin tracking such expenditures by product.
General and administrative
For the year ended December 31, 2022, as compared to the year ended December 31, 2021, the increase in general and administrative expenses was primarily due to increased (a) personnel costs as the Company continues to expand its finance, information technology, human resources and other administrative headcount, and (b) insurance, legal, professional and consulting services, each in support of the growth in scale of the operations of the Company.
Other Income (Expenses)
For the year ended December 31, 2022, other expenses consist primarily of (a) interest income earned on the Company's cash, cash equivalents and investments, partially offset by (b) foreign exchange losses primarily associated with foreign currency transactions occurring within the Company’s Canadian subsidiary. For the year ended December 31, 2021, other expenses consisted of a foreign exchange loss associated with foreign currency transactions primarily occurring within the Company’s Canadian subsidiary.
Provision for Income Taxes
For the year ended December 31, 2022, income tax expense consisted of tax related to the out-licensed income received from the Lantheus License Agreements as well as taxes owing in Canada in relation to taxable income generated through management and research and development services performed by the Canadian subsidiary of the Company. For the year ended December 31, 2021, income tax expense consisted of taxes owing in Canada in relation to taxable income generated through management and research and development services performed by the Canadian subsidiary of the Company.
Liquidity and Capital Resources
Sources of Liquidity and Capital
Prior to the Lantheus License Agreements, the Company had incurred significant net losses and had funded operations through the Business Combination and equity financings. Operating losses and negative cash flows are expected to be incurred again in the future. We are subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, successful discovery and development of our product candidates, development by competitors of new technological innovations, dependence on key personnel, the ability to attract and retain qualified employees, protection of proprietary technology, compliance with governmental regulations, the impact of COVID-19, the ability to secure additional capital to fund operations and commercial success of our product candidates. Product candidates currently under development will require extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel,
93
and infrastructure and extensive compliance-reporting capabilities. Even if our drug development efforts are successful, it is uncertain when, if ever, we will realize significant revenue from product sales.
Net income totaled $98.3 million for the year ended December 31, 2022 compared to a net loss of $45.9 million for the year ended December 31, 2021. The net income generated for the year ended December 31, 2022 is attributed to $226.6 million of revenue recognized in connection with the Lantheus License Agreements which was partially offset by operating costs incurred.
As of December 31, 2022, we did not have any lease or other contractual obligations. We currently have a facility lease that is a month-to-month arrangement.
On June 30, 2021, we received net proceeds of approximately $260.0 million in connection with the Business Combination consisting of proceeds of the PIPE Financing and the proceeds remaining in RACA’s trust account. On September 16, 2022, we issued 13,900,000 shares of Common Stock in connection with the 2022 Public Offering, resulting in net proceeds of approximately $117.3 million, excluding certain costs incurred in connection with the S-3 and on October 3, 2022, we issued an additional 1,589,779 shares of Common Stock for net proceeds to the Company of approximately $13.4 million as a result of an underwriters exercising their option to purchase additional shares pursuant to the underwriting agreement. On December 22, 2023, we received $260.0 million in connection with the Lantheus License Agreements. We intend to use the net proceeds from these transactions to fulfill our obligations under the Lantheus License Agreements, for general corporate purposes, funding of development programs, payment of milestones pursuant to our license agreements, general and administrative expenses, licensing of additional product candidates and to support our working capital needs.
Future Funding Requirements
Our primary use of cash is to fund operating expenses, primarily related to our research and development activities. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our outstanding accounts payable, accrued expenses and prepaid expenses.
Operating losses and negative cash flows are expected to be incurred again in the future. We may require additional capital to meet operational needs and capital requirements for clinical trials, other research and development expenditures, and business development activities. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials and preclinical studies.
Our future funding requirements will depend on many factors, including, but not limited to:
•the scope, progress, results and costs of researching and developing our current product candidates, as well as other additional product candidates we may develop and pursue in the future;
•the timing of, and the costs involved in, obtaining marketing approvals for our product candidates and any other additional product candidates we may develop and pursue in the future;
•the number of future product candidates that we may pursue and their development requirements;
•subject to receipt of regulatory approval, the costs of commercialization activities for our product candidates, to the extent such costs are not the responsibility of any future collaborators, including the costs and timing of establishing product sales, marketing, distribution, and manufacturing capabilities;
•subject to receipt of regulatory approval, revenue, if any, received from commercial sales of our product candidates or any other additional product candidates we may develop and pursue in the future;
•the achievement of milestones that trigger payments under our various license agreements;
•the extent to which we in-license or acquire rights to other products, product candidates or technologies;
•our ability to establish collaboration arrangements for the development of our product candidates on favorable terms, if at all;
•our headcount growth and associated costs as we expand our research and development and establish a commercial infrastructure;
94
•the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights, including enforcing and defending intellectual property related claims; and
•the costs of operating as a public company.
Cash, cash equivalents and investments totaled $541.3 million as of December 31, 2022, which is anticipated to fund operations into 2026. We have based this estimate on current assumptions that may change or prove to be wrong, and we could utilize our available capital resources sooner than we expect. In addition, our cash, cash equivalents and investments are subject to the economic pressures or uncertainties associated with local or global economic recessions, ongoing geopolitical events. The failure of one or more of the financial institutions in which our cash, cash equivalents or investments are held, any resulting inability for us to obtain the return of our funds from any of those financial institutions, or any other adverse condition suffered by those financial institutions, could impact access to our cash, cash equivalents or investments and could adversely impact our operating liquidity and financial performance.
On July 11, 2022 the SEC declared effective our shelf registration statement on Form S-3 (the “S-3”) that allows the Company to offer and sell to the public up to $400.0 million of our Common Stock, preferred stock, debt securities, warrants to purchase our Common Stock, preferred stock or debt securities, subscription rights to purchase our Common Stock, preferred stock or debt securities and/or units consisting of some or all of these securities, from time to time in one or more offerings. The details of any future offerings, along with the use of proceeds from any securities offered, will be described in a prospectus supplement or other offering materials, at the time of offering. Also covered under the S-3 as part of the $400.0 million total amount is an at-the-market offering (“ATM”) of up to $150.0 million of our Common Stock pursuant to a distribution agreement with Piper Sandler & Co. No shares of Common Stock were issued in connection with the ATM as of December 31, 2022. Pursuant to the registration statement on Form S-3, on September 16, 2022 we issued 13,900,000 shares of Common Stock in connection with a public offering at the price of $9.00 per share, or an aggregate of $125.1 million, resulting in net proceeds of approximately $117.3 million, excluding certain transaction costs incurred in connection with filing the S-3 and on October 3, 2022, we issued an additional 1,589,779 shares of Common Stock at a public offering price of $9.00 per share for net proceeds to the Company of approximately $13.4 million as a result of an underwriters exercising their option to purchase additional shares pursuant to the underwriting agreement.
Until such time as we can generate substantial product revenue, if ever, we expect to finance our operations through a combination of equity offerings, debt financings, collaborations, strategic alliances and marketing, distribution or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, our stockholders' ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect rights of our stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or drug candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce or terminate our research, product development or future commercialization efforts, or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Going Concern
We assess and determine our ability to continue as a going concern in accordance with the provisions of ASC Topic 205-40, Presentation of Financial Statements — Going Concern. We have determined that the Company will continue as a going concern, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business.
For additional information on risks associated with our substantial capital requirements, please read the section entitled “Risk Factors” in Part I, Item 1A of this Form 10-K.
Working Capital
Working capital is defined as current assets less current liabilities.
The following table summarizes our total working capital and current assets and liabilities as of December 31, 2022 and December 31, 2021:
95
| As at December 31, 2022 | As at December 31, 2021 | Change | ||||||||||||||||||||||||
| (In U.S. dollars) | $ | % | ||||||||||||||||||||||||
| Current assets | 530,822,730 | 243,846,556 | 286,976,174 | 117.7 | % | |||||||||||||||||||||
| Current liabilities | 79,738,440 | 7,979,964 | 71,758,476 | 899.2 | % | |||||||||||||||||||||
| Total working capital | 451,084,290 | 235,866,592 | 215,217,698 | 91.2 | % | |||||||||||||||||||||
The increase in working capital as of December 31, 2022, primarily reflects (a) an increase in cash and investments following the receipt of $260.0 million in connection with the Lantheus License Agreements, (b) proceeds of approximately $116.9 million from the issuance of Common Stock net of approximately $8.2 million of transaction costs, which also includes certain costs incurred in connection with filing the S-3, and (c) proceeds of approximately $13.4 million from the issuance of Common Stock net of approximately $0.9 million of transaction costs as a result of an underwriters exercising their option to purchase additional shares pursuant to the underwriting agreement in the underwritten public share offering in September 2022. The increase in working capital as of December 31, 2022 was partially offset by cash used for (a) operating expenses, including, personnel costs and research and development costs as we advance our clinical trials and continue to expand our pipeline, and (b) capital expenditures for equipment and machinery used in our manufacturing facility in Indiana.
Cash Flows: Comparison of the Year Ended December 31, 2022 and Year Ended December 31, 2021
The following table summarizes our sources and uses of cash for the years ended December 31, 2022 and December 31, 2021:
| For the Year Ended December 31, 2022 | For the Year Ended December 31, 2021 | Change | ||||||||||||||||||||||||
| (In U.S. dollars) | $ | % | ||||||||||||||||||||||||
| Net cash flows provided by (used in) operating activities | 183,518,622 | (44,698,758) | 228,217,380 | (510.6 | %) | |||||||||||||||||||||
| Net cash flows used in investing activities | (266,264,986) | (8,802,182) | (257,462,804) | 2,925.0 | % | |||||||||||||||||||||
| Net cash flows provided by financing activities | 130,358,744 | 281,770,182 | (151,411,438) | (53.7 | %) | |||||||||||||||||||||
| Net increase in cash and cash equivalents | 47,612,380 | 228,269,242 | (180,656,862) | (79.1 | %) | |||||||||||||||||||||
Cash flows provided by (used in) operating activities
Net cash flows provided by (used in) operating activities represent the cash receipts and disbursements related to all of our activities other than investing and financing activities. We believe that the net proceeds from the Lantheus License Agreements, the 2022 Public Offering and the Business Combination and PIPE Financing, together with our available resources and existing cash and cash equivalents, are sufficient to fund our operating expenses and capital expenditure requirements into 2026.
The significant increase in cash provided by operating activities for the year ended December 31, 2022 compared to the year ended December 31, 2021 was primarily due to $226.6 million of revenue recognized in connection with the Lantheus License Agreements, which was partially offset by (a) increased operating expenses as we grow our operations and further the development of our pipeline, as described above, (b) continued costs and pre-payments made in connection with our clinical trials.
Cash flows used in Investing Activities
For the years ended December 31, 2022 and December 31, 2021, cash used in investing activities reflected $266.3 million and $8.8 million, respectively. The increase in cash used in investing activities relates to (a) the Company's investment of its available cash resources into fixed income investments and (b) increased capital expenditures for purchases in connection with our Indianapolis manufacturing facility.
Cash flows provided by Financing Activities
For the year ended December 31, 2022, net cash provided by financing activities totaled $130.4 million, which consisted primarily of the net proceeds received from the issuance of Common Stock in the 2022 Public Offering as discussed above.
96
For the year ended December 31, 2021, net cash provided by financing activities totaled $281.8 million, which consisted primarily of the net proceeds received (a) in connection with the Business Combination and related PIPE Financing and (b) from the exercise of warrants and stock options, each as discussed above.
Contractual Obligations and Other Commitments
The Company in the normal course of business enters into various services and supply agreements in connection with its clinical trials to ensure the supply of certain product and product lines during the Company’s clinical phase. These agreements often have minimum purchase commitments and generally terminate upon the termination of the clinical trial. For additional information, see Note 14 to the 2022 Financial Statements included elsewhere in this Form 10-K. For additional information related to our license agreements, please also see Note 15 to the 2022 Financial Statements included elsewhere in this Form 10-K.
Off-balance sheet arrangements
We do not have any off-balance sheet arrangements or holdings in any variable interest entities.
Critical Accounting Policies and Estimates
This management’s discussion and analysis of our financial condition and results of operations is based on our 2022 Financial Statements, which have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”).
The preparation of the 2022 Financial Statements in conformity with U.S. GAAP requires us to make estimates, judgments and assumptions that may affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the 2022 Financial Statements and the reported amounts of expenses during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. Our significant accounting policies are outlined in Note 2 to the 2022 Financial Statements included elsewhere in this Form 10-K.
Revenue Recognition
The Company recognizes revenue in accordance with ASC Topic 606, Revenue From Contracts With Customers (“ASC 606”). Under ASC 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements within the scope of ASC 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price, including variable consideration, if any; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue as the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that the entity will collect the consideration to which it is entitled in exchange for the goods or services it transfers to the customer.
At contract inception, once the contract is determined to be within the scope of ASC 606, the Company assesses whether the promised goods or services within each contract are distinct and, therefore, represent a separate performance obligation. Goods and services that are determined not to be distinct are combined with other promised goods and services until a distinct bundle is identified. In determining whether goods or services are distinct, the Company evaluates certain criteria, including whether (i) the customer can benefit from the good or service either on its own or together with other resources that are readily available to the customer (capable of being distinct) and (ii) the good or service is separately identifiable from other goods or services in the contract (distinct in the context of the contract).
The Company then determines the transaction price, which is the amount of consideration it expects to be entitled from a customer in exchange for the promised goods or services for each performance obligation and recognizes the associated revenue as each performance obligation is satisfied. The Company’s estimate of the transaction price for each contract includes all variable consideration to which it expects to be entitled. If it is probable that a significant revenue reversal would not occur, the associated variable consideration is included in the transaction price.
ASC 606 requires the Company to allocate the arrangement consideration on a relative standalone selling price basis for each performance obligation after determining the transaction price of the contract and identifying the performance obligations to which that amount should be allocated. The relative standalone selling price is defined in the revenue standard as the price at which an entity would sell a promised good or service separately to a customer. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation as each
97
performance obligation is satisfied, either at a point in time or over time, and if over time, recognition is based on the use of an output or input method.
Fair Value Measurements
Certain of our assets and liabilities are carried at fair value under U.S. GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies, and similar techniques.
Share-Based Compensation
We determine the fair value of each award issued under our equity-based compensation plan on the date of grant. Compensation expense for service-based stock option awards is recognized on a straight-line basis for the entire award over the requisite service period, with the amount of compensation expense recognized at any date at least equaling the portion of the grant-date fair value of the award that is vested at that date.
We elected to account prospectively for forfeitures as they occur rather than apply an estimated forfeiture rate to share-based compensation expense. We classify share-based compensation expense in our consolidated financial statements in the same manner in which the award recipient’s salary and related costs are classified or in which the award recipient’s service payments are classified, as applicable.
We estimate the fair value of the stock option awards on the date of grant using the Black-Scholes-Merton option pricing model which includes certain judgments and estimates including the expected life of the stock options as well the risk-free rate, dividend yield, and volatility, each estimated over the expected life of the stock options. Because we lack sufficient company-specific historical and implied volatility, we determined the volatility for stock options granted based on an analysis of reported data for a peer group of companies. We will continue to apply this method until a sufficient amount of historical information regarding the volatility of our own share price becomes available. As we do not have a history of stock option exercises, the expected life of the stock options has been determined as the period to expiry of the stock option. The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected life of the stock options.
The expected dividend yield is assumed to be zero as we have never paid dividends and do not have current plans to pay any dividends on our common shares.
Recently adopted accounting standards and recent accounting pronouncements
For a discussion of new accounting standard updates adopted by us as well as recent accounting pronouncements for accounting standard updates not yet effective and their respective impact and expected impact on our consolidated financial statements or disclosures, please see Note 2 to the 2022 Financial Statements included elsewhere in this Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Concentration risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash, cash equivalents, and investments. The Company’s cash equivalents and investments as of December 31, 2022 consisted of money market funds, U.S. and Canadian government agency securities, corporate bonds and commercial paper. The
98
Company does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships. The Company currently holds all its cash, cash equivalents and investments with two financial institutions. These financial institutions are large and high-quality investment grade institutions. The Company will continue to monitor for changes in credit quality in relation to the counterparties to which it has relationships.
Foreign currency risk
The Company does not have significant operating subsidiaries or significant investments in foreign countries as of December 31, 2022 and 2021. The Company is subject to foreign currency exposure on its cash balances, accounts payable and accrued liabilities denominated in currencies other than the U.S. dollar, expenses incurred from certain supplier and service agreements denominated in Canadian dollars and Euro, as well as salaries and wages in respect of the Company’s Canadian employees. The Company does not currently manage its foreign currency exposure through hedging programs and will continue to monitor its foreign currency assets and liabilities and evaluate the needs for these programs in the future. During the years ended December 31, 2022 and 2021, the Company recorded $0.4 million and $0.2 million, respectively, of foreign currency losses in the consolidated statements of operations. A 10% increase or decrease in current exchange rates would not have a material effect on our financial results.
Interest rate risk
As of December 31, 2022, we had an aggregate cash, cash equivalents, restricted cash and investments balance of $541.3 million which consisted of cash, money market funds, U.S. and Canadian government agency debt securities, corporate bonds, municipal bonds and commercial paper. The Company has a policy of investing in highly-rated securities, whose maturities are, at December 31, 2022, primarily less than one year. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, a 10% increase or decrease in interest rates would not have a material effect on the fair market value of our investment portfolio. We have the ability to hold our investments until maturity, and therefore, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investment portfolio. The Company does not enter into investments for trading or speculative purposes.
The Company does not currently manage interest rate exposure through hedging programs. The Company will continue to monitor and evaluate the need for interest rate hedging programs in the future.
99
Item 8. Financial Statements and Supplementary Data
Our 2022 Financial Statements, together with the report of our independent registered public accounting firm, appear in this Form 10-K beginning on page F-1 and are incorporated by reference into this Item 8.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosures
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The Company’s management, with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act, as of December 31, 2022. Based on that evaluation, the Company’s Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures were effective in recording, processing, summarizing and reporting, on a timely basis, information required to be disclosed by the Company in the reports that we file or submit under the Exchange Act as of December 31, 2022.
Management’s Report on Internal Control over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. Our systems of internal controls are designed under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed our internal control over financial reporting as of December 31, 2022. This assessment was based on criteria established in the 2013 Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on this assessment, we have concluded that, as of December 31, 2022, our internal control over financial reporting was effective.
Our internal controls over financial reporting continues to be updated as necessary to accommodate modifications to our business processes and accounting procedures. There has been no change in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the last fiscal quarter of the fiscal year for which this Form 10-K is filed that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
Not applicable.
Item 9C. Disclosure Regarding Foreign Jurisdiction that Prevent Inspections.
Not applicable.
100
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Incorporated herein by reference to our definitive proxy statement for our 2023 annual meeting of stockholders, which we will file within 120 days of the end of the fiscal year ended December 31, 2022.
Item 11. Executive Compensation
Incorporated herein by reference to our definitive proxy statement for our 2023 annual meeting of stockholders, which we will file within 120 days of the end of the fiscal year ended December 31, 2022.
Item 12. Security Ownership of Certain Beneficial Owners and Management
Incorporated herein by reference to our definitive proxy statement for our 2023 annual meeting of stockholders, which we will file within 120 days of the end of the fiscal year ended December 31, 2022.
Item 13. Certain Relationships and Related Person Transactions
Incorporated herein by reference to our definitive proxy statement for our 2023 annual meeting of stockholders, which we will file within 120 days of the end of the fiscal year ended December 31, 2022.
Item 14. Principal Accounting Fees and Services
Incorporated herein by reference to our definitive proxy statement for our 2023 annual meeting of stockholders, which we will file within 120 days of the end of the fiscal year ended December 31, 2022.
101
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)Financial Statements
For a list of the financial statements included herein, see Index to the 2022 Financial Statements on page F-1 of this Annual Report, incorporated into this Item by reference.
(b)Financial Statement Schedule
Financial statement schedules have been omitted because they are either not required or not applicable or the information is included in the 2022 Financial Statements.
(c)Exhibits
The exhibits required by Item 601 of Regulation S-K and Item 15(b) of this Annual Report are listed in the Exhibit Index below. Certain of the exhibits listed in the Exhibit Index are incorporated by reference herein.
102
Exhibit Index
| Exhibit Number | Description | |||||||
| 2.1† | ||||||||
| 3.1 | ||||||||
| 3.2 | ||||||||
| 4.1 | ||||||||
| 10.1 | ||||||||
| 10.2† | ||||||||
| 10.3# | ||||||||
| 10.4# | ||||||||
| 10.5# | ||||||||
| 10.6# | ||||||||
| 10.7# | ||||||||
| 10.8# | ||||||||
| 10.9#* | ||||||||
| 10.10# | ||||||||
| 10.11# | ||||||||
| 10.12# | ||||||||
| 10.13# | ||||||||
| 10.14# | ||||||||
| 10.15# | ||||||||
| 10.16†† | ||||||||
103
| Exhibit Number | Description | |||||||
| 10.17††* | ||||||||
| 10.18†† | ||||||||
| 10.19 | ||||||||
| 10.20# | ||||||||
| 10.21†† | ||||||||
| 10.22†† | ||||||||
| 10.23†† | ||||||||
| 10.24†† | ||||||||
| 10.25†† | ||||||||
| 10.26# | ||||||||
| 10.27†† | ||||||||
| 10.28†† | ||||||||
| 10.29†† | ||||||||
| 21.1 | ||||||||
| 23.1* | ||||||||
| 31.1* | ||||||||
| 31.2* | ||||||||
| 32* | ||||||||
| 101.INS* | XBRL Instance Document | |||||||
| 101.SCH* | XBRL Taxonomy Extension Schema Document | |||||||
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document | |||||||
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document | |||||||
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document | |||||||
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document | |||||||
* Filed herewith.
104
# Indicates a management contract or any compensatory plan, contract or arrangement.
† Schedules and exhibits to this Exhibit omitted pursuant to Regulation S-K Item 601(b)(2). The Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request.
†† Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit.
(d)Financial Statement Schedules
All schedules have been omitted as not applicable or not required under the rules of Regulation S-X.
Item 16. Form 10-K Summary
None.
105
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
POINT BIOPHARMA GLOBAL INC.
| Date: March 27, 2023 | By: | /s/ Bill Demers | ||||||
| Name: | Bill Demers | |||||||
| Title: | Chief Financial Officer | |||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||||||
| /s/ Joe McCann | Chief Executive Officer (Principal Executive Officer) and Director | March 27, 2023 | ||||||
| Dr. Joe McCann, Ph.D. | ||||||||
| /s/ Allan C. Silber | Executive Chair and Director | March 27, 2023 | ||||||
| Allan C. Silber | ||||||||
| /s/ Bill Demers | Chief Financial Officer (Principal Financial and Accounting Officer) | March 27, 2023 | ||||||
| Bill Demers | ||||||||
| /s/ Neil Fleshner | Chief Medical Officer and Director | March 27, 2023 | ||||||
| Dr. Neil Fleshner | ||||||||
| /s/ Rajesh K. Malik | Director | March 27, 2023 | ||||||
| Dr. Rajesh K. Malik, M.D. | ||||||||
| /s/ Jonathan Ross Goodman | Director | March 27, 2023 | ||||||
| Jonathan Ross Goodman | ||||||||
| /s/ Gerald Hogue | Director | March 27, 2023 | ||||||
| Gerald Hogue | ||||||||
| /s/ David C. Lubner | Director | March 27, 2023 | ||||||
| David C. Lubner | ||||||||
| /s/ Yael Margolin | Director | March 27, 2023 | ||||||
| Dr. Yael Margolin, Ph.D. | ||||||||
106
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Audited Annual Consolidated Financial Statements | Page | ||||
| F-2 | |||||
| F-3 | |||||
| F-4 | |||||
| F-5 | |||||
| F-6 | |||||
| F-7 | |||||
| F-8 | |||||
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of POINT Biopharma Global Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of POINT Biopharma Global Inc. and Subsidiaries (collectively the "Company") as of December 31, 2022 and 2021 and the related consolidated statements of operations, comprehensive income (loss), stockholders' equity (deficit), and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the consolidated financial statements).
In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2022 and 2021, and the related consolidated results of its operations and cash flows for each of the two years in the period ended December 31, 2022, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
The Company's management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on the Company's consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Armanino LLP
We have served as the Company’s auditor since 2021.
March 27, 2023
F-2
POINT BIOPHARMA GLOBAL INC.
Consolidated Balance Sheets
(In U.S. dollars)
| December 31, 2022 | December 31, 2021 | ||||||||||
| $ | $ | ||||||||||
| ASSETS | |||||||||||
| Current assets | |||||||||||
| Cash and cash equivalents | |||||||||||
| Short-term investments | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Non-current assets | |||||||||||
| Long-term investments | |||||||||||
| Property, plant and equipment, net | |||||||||||
| Total non-current assets | |||||||||||
| Total assets | |||||||||||
| LIABILITIES & STOCKHOLDERS’ EQUITY | |||||||||||
| Current liabilities | |||||||||||
| Accounts payable | |||||||||||
| Accrued liabilities | |||||||||||
| Deferred revenue | |||||||||||
| Income taxes payable | |||||||||||
| Total current liabilities | |||||||||||
| Deferred tax liability | |||||||||||
| Long-term income taxes payable | |||||||||||
| Deferred revenue, net of current portion | |||||||||||
| Total liabilities | |||||||||||
| Commitments and contingencies (Notes 14 and 15) | |||||||||||
| Stockholders' equity | |||||||||||
Common Stock, par value $ | |||||||||||
| Additional paid-in capital | |||||||||||
| Retained earnings (accumulated deficit) | ( | ||||||||||
| Accumulated other comprehensive loss | ( | ||||||||||
| Total stockholders' equity | |||||||||||
| Total liabilities and stockholders' equity | |||||||||||
See accompanying Notes to the Consolidated Financial Statements
F-3
POINT BIOPHARMA GLOBAL INC.
Consolidated Statements of Operations
For the years ended December 31, 2022 and December 31, 2021
(In U.S. dollars)
| For The Year Ended December 31, 2022 | For The Year Ended December 31, 2021 | ||||||||||
| $ | $ | ||||||||||
| Revenue | |||||||||||
| License revenue | |||||||||||
| Other revenue | |||||||||||
| Total revenue | |||||||||||
| Operating expenses | |||||||||||
| Research and development | |||||||||||
| General and administrative | |||||||||||
| Total operating expenses | |||||||||||
| Income (loss) from operations | ( | ||||||||||
| Other income (expenses) | |||||||||||
| Investment income (finance costs) | ( | ||||||||||
| Foreign currency loss | ( | ( | |||||||||
| Total other income (expenses) | ( | ||||||||||
| Income (loss) before provision for income taxes | ( | ||||||||||
| Provision for income taxes | ( | ( | |||||||||
| Net income (loss) | ( | ||||||||||
| Net income (loss) per common share: | |||||||||||
| Basic | $ | $ | ( | ||||||||
| Diluted | $ | $ | ( | ||||||||
| Weighted average common shares outstanding: | |||||||||||
| Basic | |||||||||||
| Diluted | |||||||||||
See accompanying Notes to the Consolidated Financial Statements
F-4
POINT BIOPHARMA GLOBAL INC.
Consolidated Statements of Comprehensive Income (Loss)
For the years ended December 31, 2022 and December 31, 2021
(In U.S. dollars)
| For The Year Ended December 31, 2022 | For The Year Ended December 31, 2021 | ||||||||||
| $ | $ | ||||||||||
| Net income (loss) | $ | $ | ( | ||||||||
| Other comprehensive loss, net of tax | |||||||||||
| Net unrealized loss on available-for-sale debt securities | ( | ||||||||||
Total comprehensive income (loss) | $ | $ | ( | ||||||||
See accompanying Notes to the Consolidated Financial Statements
F-5
POINT BIOPHARMA GLOBAL INC.
Consolidated Statements of Stockholders’ Equity (Deficit)
For the years ended December 31, 2022 and December 31, 2021
(In U.S. dollars, except share amounts)
| Common Stock | Additional Paid-in Capital | Retained Earnings (Accumulated Deficit) | Accumulated Other Comprehensive Loss | Total Equity | ||||||||||||||||||||||||||||||||||
| # | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||||||
| Balance at December 31, 2020, effect of the Business Combination (refer to Note 4) | ( | |||||||||||||||||||||||||||||||||||||
| Issuance of shares of Common Stock in connection with exercise of warrants | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of shares of Common Stock in connection with stock option exercises | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of shares of Common Stock, net of direct and incremental costs in connection with the Business Combination (refer to Note 4) | — | — | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||
| Balance at December 31, 2021 | ( | |||||||||||||||||||||||||||||||||||||
| Issuance of shares of Common Stock, net of direct and incremental costs | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of shares of Common Stock in connection with stock option exercises | — | — | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of taxes | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||
| Balance at December 31, 2022 | ( | |||||||||||||||||||||||||||||||||||||
See accompanying Notes to the Consolidated Financial Statements
F-6
POINT BIOPHARMA GLOBAL INC.
Consolidated Statements of Cash Flows
For the years ended December 31, 2022 and December 31, 2021
(In U.S. dollars)
| For the Year Ended December 31, 2022 | For the Year Ended December 31, 2021 | ||||||||||
| $ | $ | ||||||||||
| Cash flows from operating activities | |||||||||||
| Net income (loss): | ( | ||||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
| Depreciation on property, plant and equipment | |||||||||||
| Deferred income taxes | |||||||||||
| Share-based compensation expense | |||||||||||
| Amortization of debt issuance costs | |||||||||||
| Accretion of discounts net of amortization of premiums on investments | ( | ||||||||||
| Changes in operating assets and liabilities | |||||||||||
| Prepaid expenses and other current assets | ( | ( | |||||||||
| Accounts payable | ( | ||||||||||
| Accrued liabilities | |||||||||||
| Deferred revenue | |||||||||||
| Income taxes payable | |||||||||||
| Amount due to related party within accrued liabilities | ( | ||||||||||
| Change in accrued interest and dividends within investments | |||||||||||
| Net cash provided by (used in) operating activities | ( | ||||||||||
| Cash flows from investing activities | |||||||||||
| Purchase of investments, net of sales and maturities | ( | ||||||||||
| Purchase of property, plant and equipment | ( | ( | |||||||||
| Net cash used in investing activities | ( | ( | |||||||||
| Cash flows from financing activities | |||||||||||
| Issuance of shares of Common Stock, net of direct and incremental costs paid | — | ||||||||||
| Issuance of shares of Common Stock in connection with stock option exercises | |||||||||||
| Repayment of mortgage payable | ( | ||||||||||
| Issuance of shares of Common Stock in connection with exercise of warrants | |||||||||||
| Issuance of shares of Common Stock in connection with the Business Combination (see Note 4), net of costs incurred by RACA and direct and incremental costs paid | — | ||||||||||
| Net cash provided by financing activities | |||||||||||
| Net increase in cash and cash equivalents | |||||||||||
| Cash and cash equivalents, beginning of period | |||||||||||
Cash and cash equivalents, end of period | |||||||||||
| Supplemental disclosure of cash flow information: | |||||||||||
| Cash paid for income taxes | ( | ( | |||||||||
| Cash paid for interest on mortgage payable | ( | ||||||||||
| Non-cash investment activities: | |||||||||||
| Purchase of property, plant and equipment recorded in accounts payable and accrued liabilities | |||||||||||
See accompanying Notes to the Consolidated Financial Statements
F-7
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
1. Nature of business
Formation and organization
POINT Biopharma Global Inc., together with its consolidated subsidiaries ("POINT" or the “Company”), is a globally focused radioligand company building a platform for the clinical development and commercialization of radioligands that fight cancer. On September 18, 2019, POINT Theranostics Inc. was incorporated under the General Corporation Law of the State of Delaware (the "DGCL") and amended its name to “POINT Biopharma Inc.” on November 22, 2019. On June 30, 2021, following the Business Combination (as defined below), POINT Biopharma Inc. became a wholly-owned subsidiary of POINT Biopharma Global Inc. Under the terms of the Business Combination Agreement (as defined below), stockholders of POINT Biopharma Inc. received approximately 3.59 shares of common stock, par value $0.0001 per share, of the Company (“Common Stock”) in exchange for each common share of Point Biopharma Inc. Also in connection with the closing of the Business Combination, RACA (as defined below) consummated the sale of an aggregate of 16,500,000 shares of Class A common stock, par value $0.0001 per share, of RACA (“Class A Common Stock”) in a private placement at a price of $10.00 per share, for aggregate gross proceeds of $165.0 million (“PIPE Financing”). In accordance with the terms of the Business Combination Agreement, upon the closing of the Business Combination (as defined below), each share of Class A Common Stock and each share of Class B common stock, par value $0.0001 per share, of RACA (“Class B Common Stock”) was converted into one share of Common Stock of the Company. For additional information on the Business Combination, please see Note 4.
The Company was founded on a mission to make radioligand therapy applicable to more cancers and available to more people, thereby improving the lives of cancer patients and their families everywhere.
The Company has four wholly-owned subsidiaries, POINT Biopharma Inc., POINT Biopharma USA Inc. and West 78th Street, LLC, each located in the U.S., and POINT Biopharma Corp., located in Canada. The Company’s headquarters is located at 4850 West 78th Street, Indianapolis, Indiana, 46268.
2. Summary of significant accounting policies
Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and include the accounts of the Company and its wholly-owned subsidiaries, POINT Biopharma Inc., POINT Biopharma Corp., POINT Biopharma USA, Inc. and West 78th Street, LLC, for financial information and pursuant to the rules and regulations of the SEC. All intercompany accounts and transactions have been eliminated in consolidation.
Impact of Covid-19 and other geopolitical events
The COVID-19 pandemic has caused many governments to implement measures to slow the spread of the outbreak through quarantines, travel restrictions, heightened border security and other measures. The U.S. administration has announced that it intends to end the public health emergency declaration as a result of COVID-19 on May 11, 2023. Although the impact has decreased and many of these measures have been terminated, COVID-19 may continue to have an impact on the global economy as well as businesses and capital markets around the world.
Further, general macroeconomic trends, including rising inflation rates, sustained supply chain disruptions, and any resulting recession, depression or other sustained adverse market event could materially and adversely affect our business, financial condition, results of operations and the value of our Common Stock.
Additionally, financial markets may be adversely affected by the current or anticipated impact of military conflict, including escalating military fighting between Russia and Ukraine, terrorism or other or macroeconomic geopolitical events. The U.S. and other nations in response to the Russo-Ukrainian conflict have announced economic sanctions which
F-8
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
may have an adverse effect on the global financial markets. The Company's SPLASH trial has vendor staff in Ukraine, and any political instability in the region may disrupt resourcing assigned to the trial and negatively impact our business.
The Company is monitoring the potential impact of the COVID-19 pandemic and the potential impact of the Russo-Ukrainian conflict and other macroeconomic events on its business and consolidated financial statements. To date, the Company has not experienced any material business disruptions or incurred any impairment losses in the carrying values of its assets as a result of these events and it is not aware of any specific related event or circumstance that would require it to revise its estimates reflected in these consolidated financial statements.
Risks and uncertainties
Prior to the Lantheus License Agreements (as defined in Note 3 below), the Company had incurred significant net losses and had funded operations through the Business Combination and equity financings. Operating losses and negative cash flows are expected to be incurred again in the future. The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, successful discovery and development of its product candidates, regulatory approval of its product candidates, development by competitors of new technological innovations, dependence on key personnel, the ability to attract and retain qualified employees, protection of proprietary technology, compliance with governmental regulations, the impact of macroeconomic disruptions, such as those arising from the COVID-19 coronavirus and the Russo-Ukrainian conflict, the ability to secure additional capital to fund operations and commercial success of its product candidates. Product candidates currently under development will require extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of capital, adequate personnel, and infrastructure and extensive compliance-reporting capabilities. Even if the Company’s drug development efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, related disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenue and expenses for the periods presented. Significant estimates and assumptions reflected in these consolidated financial statements include, but are not limited to, the allocation of consideration to the performance obligations as well as the recognition of revenue with respect to performance obligations recognized over time each in connection with the Lantheus License Agreements, the accrual of research and development expenses and the valuations of stock options and warrants. The Company bases its estimates on historical experience, known trends and other market-specific or other relevant factors that it believes to be reasonable under the circumstances. On an ongoing basis, management evaluates its estimates when there are changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known. Actual results may differ from those estimates or assumptions.
Foreign currency and currency translation
The reporting currency of the Company is the U.S. dollar. The functional currency of the Company’s legal entities is also the U.S. dollar. As a result, the Company records no cumulative translation adjustments related to translation of unrealized foreign exchange gains or losses.
Realized foreign exchange gains and losses that arise from exchange rate changes on transactions denominated in a currency other than the local currency are included in other income (expense), net in the consolidated statements of operations, as incurred.
Account balances denominated in a currency other than the local currency are translated at the year-end spot rate with the unrealized exchanged gains and losses included in other income (expense) net in the consolidated statements of operations.
Fair value of financial instruments
Cash and cash equivalents are carried at fair value. Other financial instruments, including accounts payable and mortgage payable, are carried at amortized cost, which approximates fair value given their short-term nature.
F-9
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
Fair value measurements
Certain assets and liabilities of the Company are carried at fair value under U.S. GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
•Level 1 — Quoted prices in active markets for identical assets or liabilities. Cash and cash equivalents fall within level 1 of the fair value hierarchy.
•Level 2 — Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data.
•Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques.
Cash and cash equivalents
The Company considers all highly liquid instruments with an original maturity of three months or less as cash equivalents.
Investments
The Company determines the appropriate classification of its investments in debt securities at the time of purchase and re-evaluates such determination at each balance sheet date. The Company classifies its investments as current or non-current based on each instrument’s underlying maturity date. Investments with original maturities of greater than three months and less than one year are classified as current and are included in short-term investments in the consolidated balance sheets. Investments with remaining maturities greater than one year from the balance sheet date are classified as non-current and are included in long-term investments in the consolidated balance sheets. The Company’s investments are classified as available-for-sale and reported at fair value. Unrealized gains and losses are included in other comprehensive income (loss) as a component of stockholders’ equity until realized. Amortization and accretion of premiums and discounts are recorded in finance income (expense). Realized gains and losses on debt securities are included in other income (expense), net.
The Company evaluates its investments with unrealized losses for other-than-temporary impairment. If any adjustment to fair value reflects a decline in the value of the investment, the Company considers all available evidence to evaluate the extent to which the decline is other-than-temporary in nature. For any adjustment the Company considers to be other-than-temporary, the Company reduces the investment to fair value through a charge to the statement of operations. No such adjustments were necessary during the periods presented. Recent and potential future disruptions in access to bank deposits or lending commitments due to bank failure could have a negative impact on the valuation of our investments.
Leases
Currently, the Company only holds short term leases and has elected to apply the short-term lease exemption. For all future lease arrangements, at the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present in the arrangement and in accordance with the guidance of ASC 842. Operating lease liabilities and their corresponding right-of-use assets are initially recorded based on the present value of lease payments over the expected remaining lease term. Certain adjustments to the right-of-use asset may be required for items such as incentives received. The interest rate implicit in lease contracts is typically not readily determinable. As a result, the Company utilizes its incremental borrowing rate to discount lease payments, which reflects the fixed rate at which the Company could borrow, on a collateralized basis, the amount of the lease payments in the same
F-10
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
currency, for a similar term, and in a similar economic environment. The Company currently does not have financing leases.
The Company has elected not to recognize leases with an original term of one year or less on the consolidated balance sheets. Options to renew or early terminate a lease are included in the initial lease term of a lease when there is reasonable certainty that the option will be applied.
The Company’s lease expense is recognized in the consolidated statements of operations according to its use in either research and development expenses or general administrative expenses. Currently, all lease expense is recorded in general and administrative expense.
Warrants
Common share purchase warrants entitle the holder to acquire common shares of the Company at a specified price for a specified period of time, which are classified as equity. Warrants are measured at the date of issuance using the Black-Scholes-Merton option pricing model. On January 28, 2021, all outstanding warrants to purchase common shares of the Company were exercised resulting in cash proceeds of $20.0 million.
Revenue Recognition
The Company recognizes revenue in accordance with ASC Topic 606, Revenue From Contracts With Customers (“ASC 606”). Under ASC 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements within the scope of ASC 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price, including variable consideration, if any; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue as the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that the entity will collect the consideration to which it is entitled in exchange for the goods or services it transfers to the customer.
At contract inception, once the contract is determined to be within the scope of ASC 606, the Company assesses whether the promised goods or services within each contract are distinct and, therefore, represent a separate performance obligation. Goods and services that are determined not to be distinct are combined with other promised goods and services until a distinct bundle is identified. In determining whether goods or services are distinct, the Company evaluates certain criteria, including whether (i) the customer can benefit from the good or service either on its own or together with other resources that are readily available to the customer (capable of being distinct) and (ii) the good or service is separately identifiable from other goods or services in the contract (distinct in the context of the contract).
The Company then determines the transaction price, which is the amount of consideration it expects to be entitled from a customer in exchange for the promised goods or services for each performance obligation and recognizes the associated revenue as each performance obligation is satisfied. The Company’s estimate of the transaction price for each contract includes all variable consideration to which it expects to be entitled. If it is probable that a significant revenue reversal would not occur, the associated variable consideration is included in the transaction price.
ASC 606 requires the Company to allocate the arrangement consideration on a relative standalone selling price basis for each performance obligation after determining the transaction price of the contract and identifying the performance obligations to which that amount should be allocated. The relative standalone selling price is defined in the revenue standard as the price at which an entity would sell a promised good or service separately to a customer. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation as each performance obligation is satisfied, either at a point in time or over time, and if over time, recognition is based on the use of an output or input method.
Research and development costs
Research and development costs are expensed as incurred. Research and development expenses consist of costs incurred in performing research and development activities, including costs for salaries and bonuses, employee benefits, subcontractors, facility-related expenses, depreciation and amortization, share-based compensation, third-party license fees, laboratory supplies, and external costs of outside vendors engaged to conduct discovery, preclinical and clinical
F-11
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
development activities and clinical trials as well as to manufacture clinical trial materials, and other costs. The Company recognizes external research and development costs based on an evaluation of the progress to completion of specific tasks using information provided to the Company by its service providers.
Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. Such prepaid expenses are recognized as an expense when the goods have been delivered or the related services have been performed, or when it is no longer expected that the goods will be delivered, or the services rendered.
Upfront payments under license agreements are expensed as research and development expense upon receipt of the license, and annual maintenance fees under license agreements are expensed in the period in which they are incurred. Milestone payments under license agreements are accrued, with a corresponding expense being recognized, in the period in which the milestone is determined to be probable of achievement and the related amount is reasonably estimable.
Acquired in-process research and development costs
The Company has entered into various research, development and manufacturing contracts with research institutions and other companies. These agreements are generally cancelable, and related costs are recorded as research and development expenses as incurred. The Company records accruals for estimated ongoing research, development and manufacturing costs.
The upfront payments to acquire a new drug compound, as well as subsequent milestone payments, are immediately expensed as acquired in-process research and development, provided that the drug has not achieved regulatory approval for marketing and, absent obtaining such approval, has no alternative future use. Once regulatory approval is received, payments to acquire rights, and the related milestone payments, are capitalized and the amortization of such assets recorded to product cost of sales.
Share-based compensation expense
The Company recognizes share-based compensation expense for all share-based awards made to employees, directors and consultants based on estimated fair values. The Company determines share-based compensation on the grant date using the Black-Scholes-Merton option pricing model. The value of the award is recognized as expense on a straight-line basis over the requisite service period. ASC 718, Stock Compensation ("ASC 718") allows for forfeitures to be recognized in the period in which they occur. ASC 718 aligns the accounting for share-based payments to non-employees with that of employees, with certain exceptions.
Income taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
The Company accounts for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more likely than not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
F-12
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
Net income (loss) per share
Basic net income (loss) per share attributable to common stockholders is computed by dividing the net income (loss) attributable to common stockholders by the weighted-average number of common shares outstanding for the period.
Diluted net income (loss) attributable to common stockholders is computed by adjusting net income (loss) attributable to common stockholders to reallocate undistributed earnings based on the potential impact of dilutive securities. Diluted net income (loss) per share attributable to common stockholders is computed by dividing the diluted net income (loss) attributable to common stockholders by the weighted-average number of common shares outstanding for the period, including potential dilutive common shares. For the purpose of this calculation, outstanding warrants, stock options and performance share units are considered potential dilutive common shares.
Segment information
The Company manages its operations as a single operating segment for the purposes of assessing performance and making operating decisions. The Company’s focus is on the development of radioligand therapy for the treatment of cancer.
Recent accounting pronouncements
Debt with Conversion and Other Options
The FASB has issued ASU 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”). ASU 2020-06 simplifies the accounting for convertible instruments, such as convertible debt or convertible preferred stock, by eliminating two potential methods in accounting for the embedded conversion feature. The standard also removes certain conditions previously used to evaluate whether a freestanding financial instrument, or certain types of embedded features, are considered to be settled in the issuer’s own equity. Finally, ASU 2020-06 requires that an entity use the if-converted method in calculating the effects of convertible instruments on diluted earnings per share, with one limited exception. The amendments in this ASU are effective for the Company for fiscal years beginning after December 15, 2023. The Company early adopted the provisions of ASU 2020-06 on January 1, 2022 and there was no material impact to its consolidated financial statements.
Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options
The FASB has issued ASU 2021-04, Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40). ASU 2021-04 provides guidance that an entity should treat a modification of the terms or conditions or an exchange of a freestanding equity-classified written call option that remains equity classified after modification or exchange as an exchange of the original instrument for a new instrument. The standard also provides guidance on how an entity should measure and recognize the effect of a modification or an exchange of a freestanding equity-classified written call option that remains equity classified. The amendments in this ASU are effective for the Company for fiscal years beginning after December 15, 2021. The Company adopted the provisions of ASU 2021-04 on January 1, 2022 and there was no material impact to its consolidated financial statements.
3. Revenue
F-13
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
In December 2022, closing conditions for the transaction, including Hart-Scott-Rodino antitrust clearance, were met. For PNT2002, POINT has received a $250 million upfront payment. POINT will receive an additional payment of up to $250 million upon U.S. regulatory approval, and, once certain return on investment financial thresholds have been achieved and other conditions met, royalties of 20 % on all net sales (prior to which there is a period of sales in which the royalty may be based on only a portion of the gross profit), as well as the potential for up to an additional $1.3 billion in various net sales milestone payments. For PNT2003, POINT has received a $10 million upfront payment, and will receive up to an additional $30 million upon U.S. regulatory approval, royalties of 15 % on net sales, as well as the potential for up to an additional $275 million in various net sales milestone payments.
The Company is responsible for completing the SPLASH trial and the parties will work together to file the New Drug Application (“NDA”) with the costs incurred in connection with the U.S. Food and Drug Administration ("FDA") submission being borne by Lantheus. Thereafter, Lantheus will be responsible for all additional clinical and regulatory costs in the U.S., as well as all costs for development, clinical trials and regulatory approval in the rest of its territories outside the U.S., except Asia.
To determine the appropriate amount of revenue to be recognized under ASC 606, the Company performed the following steps: (i) identify the promised goods or services in the contract, (ii) determine whether the promised goods or services are performance obligations, including whether they are distinct in the context of the contract, (iii) measure the transaction price, including the constraint on variable consideration, (iv) allocate the transaction price to the performance obligations and (v) recognize revenue when (or as) the Company satisfies each performance obligation.
In connection with the PNT2002 Agreement the Company identified the following performance conditions: (i) the license it conveyed to Lantheus with respect to certain intellectual property, (ii) service provided to complete the SPLASH trial, support the NDA submission and participate in joint steering activities and (iii) manufacturing activities. The Company determined the transaction price under ASC 606 at the inception of the PNT2002 Agreement to be the $250 million upfront payment and has allocated this to the first two performance obligations based on a relative standalone selling price basis. The standalone selling prices for the first two performance obligations were determined using adjusted market and expected cost plus a margin assessments, respectively. The Company concluded that variable consideration associated with the product manufacturing relates solely to the manufacturing activities performance obligation on the basis that it believes that the expected margin associated with this consideration is in line with market standards and specifically relate to the Company's efforts to satisfy its manufacturing obligations.
In connection with the PNT2003 Agreement the Company identified the following performance conditions: (i) the license it conveyed to Lantheus with respect to certain intellectual property, (ii) service provided to complete the necessary submissions for regulatory approval and participate in joint steering activities and (iii) manufacturing activities. The Company determined the transaction price under ASC 606 at the inception of the PNT2003 Agreement to be the $10 million upfront payment and has allocated this to the first two performance obligations based on a relative standalone selling price basis. The standalone selling prices for the first two performance obligations were determined using adjusted market and expected cost plus a margin assessments, respectively. The Company concluded that variable consideration associated with the product manufacturing relates solely to the manufacturing activities performance obligation on the basis that it believes that the expected margin associated with this consideration is in line with market standards and specifically relate to the Company's efforts to satisfy its manufacturing obligations.
The Company does not include variable consideration to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will occur. Variable consideration in the PNT2002 Agreement and PNT2003 Agreement consists of:
•Potential future regulatory milestone payments. The Company concluded that this variable consideration is constrained considering that achievement of the milestones are outside its control and contingent upon the future success of clinical trials and regulatory approval by the FDA and in respect of other territories outside the U.S..
•Potential future milestone payments in connection with certain sales targets as well as any future royalties. The Company concluded that these payments qualify for the royalty exception. Under the royalty exception, sales-based royalties are recognized at the later of when (1) the subsequent sale or usage occurs or (2) the performance obligation to which some or all of the sales- or usage-based royalty has been allocated is satisfied (in whole or in part). That is, an entity does not estimate the amount of a sales-based royalty at contract inception; rather, revenue would be recognized when the subsequent sales occur (under the assumption that the associated performance obligation has been satisfied or partially satisfied).
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
•Potential payments for the manufacturing and supply of commercial product. The Company concluded that this variable consideration is constrained as it is contingent upon future regulatory approvals and the execution of a manufacturing and supply agreement.
The estimate of the Company’s variable consideration to be included in the transaction price will be updated at each reporting date as a change in estimate. For the potential future regulatory milestone payments, the Company utilizes the most likely amount approach to determine the amounts recognized and timing of recognition. For the potential payments for manufacturing and supply of commercial product, the Company utilizes the expected value approach to determine the amounts recognized and timing of recognition. Once the constraint is removed, the milestone payments will be accounted for and allocated to the performance obligations.
For the licenses conveyed to Lantheus, the Company recognized revenue at a point in time upon execution and regulatory approval of the Lantheus License Agreements. The Company concluded that the licenses represent that of functional intellectual property as each has significant standalone functionality and derives a substantial portion of their utility from that standalone functionality.
For the obligations to complete the SPLASH trial, support the NDA submission and participate in joint steering activities in connection with the PNT2002 Agreement as well as the obligations to complete the necessary submissions for regulatory approval and participate in joint steering activities for the PNT2003 Agreement, the Company recognizes revenue using the cost-to-cost method, which it concluded best depicts the transfer of control to the customer. Under the cost-to-cost method, the extent of progress towards completion is measured based on the ratio of actual costs incurred to the total estimated costs expected upon satisfying the identified performance obligation. Under this method, revenue is recorded as a percentage of the estimated transaction price based on the extent of progress towards completion.
The following table presents the Company’s contract liabilities as of December 31, 2022 and December 31, 2021:
| December 31, 2022 | December 31, 2021 | ||||||||||
| Deferred revenue | |||||||||||
| Deferred revenue, current | $ | $ | |||||||||
| Deferred revenue, net of current portion | |||||||||||
| Total | $ | $ | |||||||||
At inception of the Lantheus License Agreements, deferred revenue $34.8 million was recognized in connection with future performance. During the year ended December 31, 2022, the Company recognized $1.4 million in revenue for services performed. The current portion of deferred revenue reflects the Company’s estimate of the revenue it expects to recognize within the next 12 months. The Company expects to recognize the remainder of the deferred revenue in subsequent periods through the year ending December 31, 2028. No contract assets, were recognized in connection with the Lantheus License Agreements, including costs incurred in obtaining the agreements.
4. Business Combination
On March 15, 2021, POINT Biopharma Inc. entered into a definitive business combination agreement (the “Business Combination Agreement”) with Therapeutics Acquisition Corp. (NASDAQ:RACA), d/b/a Research Alliance Corp. I (“RACA”), a special purpose acquisition company sponsored by RA Capital Management L.P., that was created for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization, or similar business combination with one or more businesses. On June 30, 2021 (the “Closing Date”), Bodhi Merger Sub, Inc., a wholly-owned subsidiary of RACA, merged with and into POINT Biopharma Inc. (the “Business Combination”), with POINT Biopharma Inc. as the surviving company in the Business Combination and, after giving effect to such Business Combination, POINT Biopharma Inc. became a wholly-owned subsidiary of RACA. RACA was then renamed “POINT Biopharma Global Inc.”
In accordance with the terms of the Business Combination Agreement, upon the closing of the Business Combination:
(i)each share and vested equity award of POINT Biopharma Inc. outstanding as of immediately prior to the Closing Date was converted into shares of Common Stock of the Company or comparable vested equity
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
awards that are exercisable for shares of Common Stock of the Company, based on an implied vested equity value of $585.0 million (which is equal to a conversion ratio of approximately 3.59 -for-1);
(ii)all unvested equity awards of POINT Biopharma Inc. were converted into comparable equity awards that are exercisable for shares of Common Stock of the Company, determined based on the same conversion ratio at which the vested equity awards are converted into shares of Common Stock of the Company; and
(iii)each share of RACA Class A Common Stock and each share of RACA Class B Common Stock that was issued and outstanding immediately prior to the Closing Date became one share of Common Stock of the Company.
In connection with the Business Combination, the Company consummated the PIPE Financing, pursuant to which it received $165.0 million in exchange for 16,500,000 shares of Common Stock of the Company.
After giving effect to the Business Combination, there were 90,121,794
We accounted for the Business Combination as a reverse recapitalization, in accordance with U.S. GAAP. POINT Biopharma Inc. is treated as the accounting acquirer (legal acquiree), while RACA is the accounting acquiree (legal acquirer) for financial reporting purposes. This determination is primarily based on the fact that the former POINT Biopharma Inc. stockholders retained a majority of the voting power of the Company and comprise a majority of the governing body of the Company, and the former POINT Biopharma Inc. senior management comprise substantially all of the senior management of the Company. Accordingly, for accounting purposes, the Business Combination is treated as the equivalent of POINT Biopharma Inc. issuing shares for the net assets of RACA, accompanied by a recapitalization. The net assets of RACA are stated at historical costs. No
In connection with the Business Combination, the Company incurred underwriting fees and other costs considered to be direct or incremental to the proceeds raised in connection with the Business Combination and PIPE Financing totaling approximately $21.9 million, consisting of costs incurred by RACA prior to the completion of the Business Combination as well as investment banker, legal, audit, tax, accounting and listing fees. These amounts are reflected within additional paid-in capital in the consolidated balance sheet as of December 31, 2022.
Summary of net proceeds
The following table summarizes the elements of the net proceeds from the Business Combination:
| Recapitalization | |||||
| Cash - RACA Trust and cash (net of redemptions) | $ | ||||
| Cash - PIPE Financing | |||||
| Less: Underwriting fees, costs incurred by RACA and other direct and incremental costs, each paid prior to December 31, 2021 | ( | ||||
| Net proceeds from the Business Combination, net of costs incurred by RACA and direct and incremental costs paid per the statement of cash flows | $ | ||||
The net proceeds noted above exclude approximately $4.7 million in transaction costs that were not considered direct and incremental to the raising of capital. These costs consist of corporate expenses in the normal course of business comprised of accounting, consulting, insurance and board retainer fees. These costs were recorded as incurred in accordance with the nature of the services received.
Summary of shares of Common Stock issued
F-16
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
| Number of Shares | |||||
| RACA Class A and Class B shares outstanding prior to the Business Combination | |||||
| Class A shares issued pursuant to the PIPE Financing | |||||
| Common Stock issued upon conversion of RACA Class A Common Stock and Class B Common Stock and PIPE Financing shares | |||||
| Common Stock issued upon conversion of POINT Biopharma Inc. common shares | |||||
| Total shares of Common Stock outstanding immediately following the Business Combination | |||||
5. Cash, cash equivalents and investments
Cash, cash equivalents and investments consisted of the following:
| As of December 31, 2022 | As of December 31, 2021 | ||||||||||
| Cash | $ | $ | |||||||||
| Cash equivalents: | |||||||||||
| Money market funds | |||||||||||
| Commercial paper | |||||||||||
| Total cash and cash equivalents | |||||||||||
| Short-term investments | |||||||||||
| Commercial paper | |||||||||||
| Corporate bonds | |||||||||||
| U.S. Government agency debt securities | |||||||||||
| Asset backed securities | |||||||||||
| Total short-term investments | |||||||||||
| Long-term investments | |||||||||||
| Asset backed securities | |||||||||||
| Total long-term investments | |||||||||||
| Total cash, cash equivalents and investments | $ | $ | |||||||||
Available-for-sale investments
The amortized cost, gross unrealized gains, gross unrealized losses and fair value of available-for-sale investments by type of security as at December 31, 2022, were as follows:
| Amortized Cost | Unrealized Gains | Unrealized Losses | Fair Value | Current | Non-current | ||||||||||||||||||||||||||||||
| Commercial paper | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||
| Asset backed securities | ( | ||||||||||||||||||||||||||||||||||
| Corporate bonds | ( | ||||||||||||||||||||||||||||||||||
| U.S. Government agency debt securities | ( | ||||||||||||||||||||||||||||||||||
| Total available-for-sale securities | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||
The following table summarizes the fair value of available-for-sale investments based on maturities as of December 31, 2022:
F-17
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
| Amortized Cost | Fair Value | ||||||||||
| Maturing within one year | $ | $ | |||||||||
| Maturing between one and five years | |||||||||||
| Total | $ | $ | |||||||||
6. Fair value measurements
We measure fair value based on the prices that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Fair value measurements are based on a three-tier hierarchy that prioritizes the inputs used to measure fair value. These tiers include the following:
Level 1: Quoted prices (unadjusted) in active markets for identical assets or liabilities that are accessible at the measurement date. The fair value hierarchy gives the highest priority to Level 1 inputs.
Level 2: Observable prices that are based on inputs not quoted on active markets, but corroborated by market data. These inputs include quoted prices for similar assets or liabilities; quoted market prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3: Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs.
The following tables present information about the Company’s financial assets and liabilities as of December 31, 2022 that are measured at fair value on a recurring basis and indicates the level of the fair value hierarchy used to determine such fair values (in thousands):
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||
| Cash equivalents: | |||||||||||||||||||||||
| Money market mutual fund | $ | $ | $ | $ | |||||||||||||||||||
| Commercial paper | |||||||||||||||||||||||
| Available-for-sale debt securities: | |||||||||||||||||||||||
| Commercial paper | |||||||||||||||||||||||
| Asset backed securities | |||||||||||||||||||||||
| Corporate bonds | |||||||||||||||||||||||
| U.S. Government agency debt securities | |||||||||||||||||||||||
| Total | $ | $ | $ | $ | |||||||||||||||||||
Certain of our available-for-sale debt securities, including U.S. Government agency debt securities, are valued using inputs observable in active markets for identical securities and are therefore classified as Level 1 within the fair value hierarchy.
We did not have any financial liabilities measured at fair value on a recurring basis as of December 31, 2022.
There have been no transfers of assets or liabilities between the fair value measurement levels.
7. Prepaid expenses and other current assets
F-18
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
| As at December 31, 2022 | As at December 31, 2021 | ||||||||||
| Prepaid clinical trial expenses | $ | $ | |||||||||
| Insurance | |||||||||||
| Canadian harmonized sales tax receivable | |||||||||||
| Deposit on production equipment | |||||||||||
| Other | |||||||||||
Total | $ | $ | |||||||||
8. Property, plant and equipment, net
Property, plant and equipment consisted of the following:
| As at December 31, 2022 | As at December 31, 2021 | ||||||||||
| Land and building | $ | $ | |||||||||
| Property in development | |||||||||||
| Machinery and equipment | |||||||||||
| Furniture and fixtures | |||||||||||
| Computer equipment | |||||||||||
| Less: Accumulated depreciation | ( | ||||||||||
| Property, plant and equipment, net | $ | $ | |||||||||
On July 2020, the Company purchased land and a building in Indianapolis, Indiana (which has been expanded to approximately 81,000 square feet) for the purpose of retrofitting the existing building into a state-of-the-art, Good Manufacturing Practices ("GMP") compliant facility that will support the Company’s drug manufacturing operations. The purchase of the property was financed by a mortgage that was repaid on July 29, 2021 (see Note 11).
The Company commenced the manufacture of clinical supply in the Indianapolis manufacturing facility in January 2022; however, construction continues on the facility to expand capacity. The Company has determined this to be the date upon which its property, plant and equipment was available for its intended use. Property, plant and equipment that have finite lives are recorded at cost less accumulated depreciation and impairment losses. Depreciation is expensed from the month the particular asset is available for its intended use, using the straight-line method over the estimated useful life of such asset at the following rates, which in each case are intended to reduce the carrying value of the asset to the estimated residual value:
| Asset Category | Estimated Useful Life | ||||
| Computer equipment | |||||
| Machinery and equipment | |||||
| Furniture and fixtures | |||||
| Building | |||||
9. Accounts payable
F-19
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
| As at December 31, 2022 | As at December 31, 2021 | ||||||||||
| Accounts payable | $ | $ | |||||||||
| Other payables | |||||||||||
Total | $ | $ | |||||||||
10. Accrued expenses
Accrued liabilities consisted of the following:
| As at December 31, 2022 | As at December 31, 2021 | ||||||||||
| Accrued research and development costs | $ | $ | |||||||||
| Accrued personnel costs | |||||||||||
| Accrued corporate legal fees and other professional services | |||||||||||
| Accrued costs for purchase of property, plant and equipment | |||||||||||
| Other accrued costs | |||||||||||
| Total | $ | $ | |||||||||
11. Mortgage payable
On July 10, 2020, the Company obtained a mortgage loan in the amount of $3,562,500 (the “Mortgage”) for the purpose of purchasing its manufacturing facility and related land located in Indianapolis, Indiana (the “Property”). The Mortgage was collateralized by a first charge over the Property. As part of the financing, the Company incurred $17,194 of costs and fees from the lender that were capitalized and recorded as finance costs over the life of the Mortgage. On July 29, 2021, the loan on the manufacturing facility in Indianapolis, Indiana was repaid and the related mortgage on the Company's facility in Indianapolis, Indiana was released.
Prior to its repayment, the Mortgage bore interest at 2.85 % plus a minimum rate of 1-month LIBOR, subject to a LIBOR floor of 0.25 %. The Mortgage required quarterly interest payments, which commenced on October 1, 2020, with the principal amount due at maturity on January 10, 2022.
For the year ended December 31, 2021, the Company recorded $63,195 in interest costs which have been capitalized within property, in development, and recorded amortization of debt issuance costs of $11,840 through finance costs.
12. Stockholders’ equity
Common and Preferred Stock
The Company is authorized to issue 430,000,000 shares of Common Stock, with a par value of $0.0001 per share, as well as 20,000,000 of shares of preferred stock, with a par value of $0.0001 per share (“Preferred Stock”). The figures below are presented giving effect to a retroactive application of the Business Combination which resulted in a conversion of the previous POINT Biopharma Inc. common shares to shares of Common Stock of the Company at a conversion ratio of approximately 3.59 :1. The par value of previous POINT Biopharma Inc. common shares was $0.001 . See Note 4 for additional details.
During the year ended December 31, 2022, the Company issued 15,527,947 shares of Common Stock, including (a) 15,489,779 shares of Common Stock in connection with an underwritten public offering at the price of $9.00 per share pursuant to a registration statement on Form S-3 previously filed and declared effective by the Securities and Exchange Commission, resulting in total gross proceeds of $139.4 million (excluding approximately $9.1 million of issuance costs) and (b) 38,168 shares of Common Stock in connection with the exercise of stock options issued to non-employee consultants, resulting in total cash proceeds of $0.1 million.
F-20
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
During the year ended December 31, 2021, the Company issued 35,474,138 shares of Common Stock, including (a) 32,539,769 of shares of Common Stock in connection with the Business Combination and PIPE Financing (see Note 4), (b) 800,000 shares of common stock of POINT Biopharma Inc. (exchanged for 2,869,799 shares of Common Stock) in connection with the exercise of warrants, resulting in cash proceeds of $20.0 million and (c) 18,000 shares of common stock of POINT Biopharma Inc. (exchanged for 64,570 shares of Common Stock) in connection with the exercise of stock options issued to a non-employee consultant, resulting in cash proceeds of $0.5 million.
As of December 31, 2022, the number of total issued and outstanding shares of Common Stock was 105,649,741 90,121,794
Each share of Common Stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are entitled to receive dividends, if any, as may be declared by the Company’s board of directors. During the year ended December 31, 2022, no nil
The Company’s board of directors has the authority to issue shares of Preferred Stock from time to time on terms it may determine, to divide shares of Preferred Stock into one or more series and to fix the designations, preferences, privileges, and restrictions of Preferred Stock, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preference, sinking fund terms, and the number of shares constituting any series or the designation of any series to the fullest extent permitted by the DGCL. During the year ended December 31, 2022, no shares of Preferred Stock have been issued by the Company (December 31, 2021 — nil ).
13. Share-based compensation
Equity Incentive Plans
In March 2020, the board of directors of POINT Biopharma Inc. approved the 2020 Equity Incentive Plan (the “2020 EIP”). The 2020 EIP provided for the granting of incentive and non-qualified stock options, stock appreciation rights, restricted stock units, performance awards and other stock-based awards to employees, directors, and consultants of POINT Biopharma Inc. Effective as of June 30, 2021, in connection with the Business Combination, the Company’s board of directors adopted the POINT Biopharma Global Inc. 2021 Equity Incentive Plan (the “2021 EIP”) to replace the 2020 EIP and allow the Company to grant equity and equity-based incentive awards to officers, employees, non-employee directors and consultants of the Company. Upon the closing of the Business Combination, the Company assumed the outstanding equity awards under the 2020 EIP and each outstanding option to acquire common shares of POINT Biopharma Inc. (whether vested or unvested) under the 2020 EIP was substituted with a substantially equivalent option to acquire shares of Common Stock of the Company based on the conversion ratio for the POINT Biopharma Inc. common shares in the Business Combination and remained outstanding under the 2020 EIP. No further grants may be made under the 2020 EIP. The 2021 EIP provides that the number of shares reserved and available for issuance under the 2021 EIP will automatically increase each January 1, beginning on January 1, 2022, by 4 % of the number of outstanding shares of Common Stock on the immediately preceding December 31, or such lesser amount as determined by the Company's board of directors. As of December 31, 2022, the number of shares of Common Stock authorized for issuance under the 2021 EIP was 7,925,052 . On January 1, 2023, the number of shares of Common Stock available under the 2021 EIP increased by 4,225,990 .
Stock options
The Company concluded that the replacement stock options issued in connection with the Business Combination did not require accounting for effects of the modification under the ASC 718 as it was concluded that (a) the fair value of the modified award is the same as the fair value of the original award immediately before the original award was modified, (b) there are no changes to the vesting conditions of the award, and (c) there is no change to the classification of the award.
Stock options generally vest over a four-year period, with 25 % vesting after the 1st year anniversary and the remaining options vesting ratably over the remaining three years . All employee stock options generally expire 6 years from the date of the grant.
F-21
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
Share-based compensation expense for the years ended December 31, 2022 and 2021 was recognized in the Consolidated Statements of Operations as follows:
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
| Research and development | $ | $ | |||||||||
| General and administrative | |||||||||||
| Total share-based compensation expense | $ | $ | |||||||||
The Company did not
Stock option valuation and activity
The fair value of stock option grants is estimated using the Black-Scholes-Merton option-pricing model. The Company lacks sufficient company-specific historical and implied volatility information. Therefore, it estimates its expected share volatility based on the historical volatility of a publicly traded set of peer companies and expects to continue to do so until such time as it has adequate historical data regarding the volatility of its own traded share price. For options with service-based vesting conditions, the expected term of the Company’s stock options has been determined utilizing the “simplified” method for awards that qualify as “plain-vanilla” options. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future.
The following table presents the assumptions used in the Black-Scholes-Merton option-pricing model to determine the grant date fair value of stock options granted:
| Year ended December 31, 2022 | Year ended December 31, 2021 | |||||||
| Risk-free interest rate | ||||||||
| Expected term (in years) | ||||||||
| Expected volatility | ||||||||
| Expected dividend yield | ||||||||
F-22
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
| Number of Shares (#) | Weighted Average Exercise Price ($) | Weighted- Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value ($) | ||||||||||||||||||||
| Outstanding as of December 31, 2021 | |||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( | ||||||||||||||||||||||
| Forfeited | ( | ||||||||||||||||||||||
| Expired | ( | ||||||||||||||||||||||
| Outstanding as of December 31, 2022 | |||||||||||||||||||||||
| Vested and expected to vest as of December 31, 2022 | |||||||||||||||||||||||
| Options exercisable as of December 31, 2022 | |||||||||||||||||||||||
The aggregate intrinsic value in the table above represents the pretax intrinsic value, which is calculated as the difference between the exercise price of the stock options and the fair value of the Common Stock.
During the year ended December 31, 2022, 1,948,614 stock options were granted to employees and directors of the Company, with a weighted average grant date fair value of $4.590 . The vesting terms of these stock options are such that 25 % of the options vest on the one-year anniversary of the date of grant and the remaining 75 % of such stock options vest ratably over the remaining three years . On June 6, 2022, the terms of 128,070 stock options previously granted to directors of the Company were amended to reduce the vesting period to one year . This amendment was accounted for as a modification and there was no material impact to stock-based compensation recorded.
During the year ended December 31, 2021, 1,614,683 stock options were granted, including: (a) 1,255,959 stock options granted to employees and directors of the Company, with a weighted average grant date fair value of $4.466 and (b) 358,724 stock options to a non-employee consultant of the Company, with a weighted average grant date fair value of $3.885 . The vesting terms of the stock options granted to employees and directors are such that 25 % vest on the one-year anniversary of the date of grant and the remaining 75 % vest ratably over the remaining three years . The vesting terms of the grant to the non-employee consultant were such that 25 % of the options vested immediately upon grant, 10 % vesting on the first anniversary of the date of the grant and the remaining 65 % vesting based on certain performance milestones. Upon completion of the Business Combination, the remaining 269,043 unvested stock options immediately vested and all remaining unrecognized stock-based compensation expense associated with these stock options was recorded.
During the year ended December 31, 2022, non-employee consultants of the Company exercised 38,168 stock options with an intrinsic value of $0.3 million, resulting in the issuance of 38,168 shares of Common Stock for cash proceeds of $0.1 million.
During the year ended December 31, 2021, a non-employee consultant of the Company exercised 64,570 stock options with an intrinsic value of $nil , resulting in the issuance of 64,570 shares of Common Stock for cash proceeds of $0.5 million.
As of December 31, 2022, the unrecognized share-based compensation expense related to unvested options, was $10.5 million and the estimated weighted average remaining vesting period was 2.0 years. As of December 31, 2021, the unrecognized share-based compensation expense related to unvested options, was $5.8 million and the estimated weighted average remaining vesting period was 2.4 years.
Performance Share Units
During the year ended December 31, 2022, 146,044 (December 31, 2021 - nil ) performance share units ("PSUs") were granted to employees of the Company, with a grant date fair value of $6.61 per unit based on the closing share price of the Company's Common Stock on the date of grant. The vesting terms of these PSUs are such that 100 % vest upon the regulatory approval of PNT2002 by the FDA. The Company did not record any stock based compensation expense related to these PSUs on the basis that they cannot be considered probable to vest as the regulatory approval requirement is outside the control of the Company and the clinical trial remains ongoing.
F-23
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
14. Commitments and contingencies
Indianapolis facility commitments
The Company is party to certain agreements for the continuing expansion of the manufacturing capabilities of the Indianapolis facility. Effective in the second quarter of 2022, the Company entered into an agreement for the design and build of a commercial manufacturing line. As of December 31, 2022, the Company is committed to aggregate future payments of approximately $18.3 million in connection with these agreements. During the years ended December 31, 2022 and December 31, 2021 approximately $8.7 million and $6.1 million, respectively, has been recorded within property, plant and equipment in connection with these agreements.
Clinical trial and commercial commitments
The Company, in the normal course of business, enters into various services and supply agreements in connection with its clinical trials to ensure the supply of certain product and product lines during the Company’s clinical phase. These agreements often have minimum purchase commitments and generally terminate upon the termination of the clinical trial. Minimum purchase commitments under these agreements include individual commitments up to $1.4 million. Aggregate remaining minimum commitments amount to approximately $3.1 million with payments ranging from to five years or upon completion of the clinical trial, if earlier. The Company recorded research and development expenses in connection with its supply agreements of approximately $12.1 million during the year ended December 31, 2022 (year ended December 31, 2021 – $3.6 million).
The Company also has supply agreements with third parties to purchase certain products for use in the Company’s full scale production process. The Company is committed to purchase a minimum quantity of product in the amount of approximately $109.3 million ($148.3 million CAD) over the remaining contract term. The purchase commitments are contingent upon the completion of certain milestones by the third-party suppliers. The Company recorded $0.1 million in connection with this agreement during the year ended December 31, 2022 (year ended December 31, 2021 – $nil ).
The Company also has an agreement with a third party to provide certain services in connection with the Company’s SPLASH clinical phase study. The agreement expires on the date of the completion or termination of the clinical trial. The remaining minimum purchase commitment under this agreement is approximately $23.6 million with payments that range from to five years . The Company recorded research and development expenses in connection with this agreement of approximately $23.5 million during the year ended December 31, 2022 (year ended December 31, 2021 – $8.9 million).
15. License agreements
License agreement with Scintomics GMBH (“SCI”)
In November 2019, the Company entered into a sublicense agreement with SCI (“SCI Agreement). Under the SCI Agreement, the Company was granted an exclusive, sublicensable, worldwide (other than the Middle East and Asia) license under SCI’s patent rights to use, develop, manufacture and commercialize any products arising from SCI’s patent rights related to PSMA ligands for imaging and endoradiotherapy. Under the SCI Agreement, the Company is obligated to make aggregate milestone payments to SCI of up to $25.4 million (€23.5 million), upon the achievement of specified development and regulatory milestones. The Company is also obligated to pay a low-teens percentage royalty related to the annual net sales by the Company and any of its affiliates and sublicensees. Royalties will be paid by the Company on a country-by-country basis beginning upon the first commercial sale in such country. There is also an additional low thirties percentage fee payable to SCI for monetary payments arising from the grant of a sublicense to a sublicensee or in the form of other benefits. The Company has the right to terminate the agreement, subject to a prior notice of five months . If the Company or SCI fails to comply with any of its obligations or otherwise breaches the agreement, the other party may terminate the agreement upon written notification.
During the year ended December 31, 2022, the Company made a payment to SCI of approximately $1.1 million upon the achievement of a specified development milestone and recognized this amount as a research and development expense. During the year ended December 31, 2021 the Company did not make any payments to SCI or recognize any research and development expenses under the SCI Agreement.
Research and license agreements with Bach Sciences LLC (“Bach Biosciences”)
F-24
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
First BACH Agreement
In April 2020, the Company entered into a sublicense and collaboration agreement with Bach Biosciences to develop and commercialize a radioligand agent as amended on April 14, 2020, January 5, 2021, September 24, 2021, and May 6, 2022 (collectively, the “BACH Agreement"). The BACH Agreement grants to POINT Biopharma Inc. an exclusive, sublicensable, worldwide license under Bach Biosciences' patent rights to use, develop, manufacture and commercialize any products arising from the licensed technology. Pursuant to the September 24, 2021 amendment (the "Third Amendment"), POINT Biopharma Inc. exercised its option (the “Commercialization Option”) under the BACH Agreement to acquire a worldwide exclusive, royalty-bearing license to commercialize any products and processes from uses of patent rights for FAP-targeted radioligands. The Third Amendment also amended the Sublicense Agreement to provide the Company with the first option (the “Invention Option”) to acquire a worldwide exclusive royalty-bearing license to Bach Biosciences' patent rights, materials and know-how with respect to new inventions directed to FAP-targeted radioligands. As partial consideration for the exercise of the Commercialization Option and the grant of the Invention Option under the Third Amendment, POINT Biopharma Inc. paid, upon execution of the BACH Agreement, an option exercise fee of $3.3 million. Pursuant to the May 6, 2022 amendment (the “Fourth Amendment”), the Company and Bach Biosciences agreed to remove all regulatory and sales milestones which would have been payable from the Company to Bach Biosciences, as well as to reduce the royalty rate payable by Company to Bach Biosciences by fifty percent (one-half). In signing the Fourth Amendment, the Company agreed to pay a one-time amendment fee to Bach Biosciences of $2.0 million, and to extend the duration of the Company’s sponsored research relationship relating to the generation of new FAPi-targeting drugs with Bach Biosciences and Tufts Medical School by an additional two years at the current sponsorship rate. The Company is also obligated to pay a low-teens percentage royalty related to the annual net sales of each licensed products or licensed process covered by a valid claim, but reduced to a single digit percentage royalty related to net sales in the absence of a valid claim by the Company and any of its affiliates and sublicensees based on its global sales. Royalties will be paid by the Company on a country-by-country basis beginning upon the first commercial sale in such country. There is also an additional low- teens to mid-twenties percentage sublicense fee payable to Bach Biosciences for monetary payments arising from a grant of a sub-license to a sub-licensee or in the form of other benefits, depending on the specified development stage of the product.
In April 2020, we entered into a research agreement with Bach Biosciences for a period of 5 years where Bach Biosciences is contracted to perform research on our behalf, with respect to the BACH Agreement. During the year ended December 31, 2022, the Company made a payment to Bach Biosciences of $2.0 million related to the Fourth Amendment fee discussed above and recognized this amount as research and development expenses in its consolidated statements of operations. During the year ended December 31, 2021, a payment of $3.3 million was recorded as research and development expenses. During the year ended December 31, 2022, the Company also made payments for the sponsored research agreements in the amount of $1.8 million (December 31, 2021 – $1.3 million), which are recognized as research and development expenses in the Company’s consolidated statements of operations.
Second BACH Agreement
In December 2020, the Company entered into a sublicense and collaboration agreement with Bach Biosciences to develop and commercialize compounds that leverage a proprietary technology platform (“Second BACH Agreement’”). Under the Second BACH Agreement, the Company was granted an exclusive, sublicensable, worldwide license under Bach Bioscience’s patent rights to use, develop, manufacture and commercialize any products arising from the patent related to the synthetic compound. The Company is obligated to make aggregate milestone payments to Bach Biosciences of up to $3.0 million for the first product developed, upon the achievement of specified development and regulatory milestones and of up to $45.0 million upon the achievement of specified sales milestones. For subsequent products, the Company is obligated to make a milestone payment to Bach Biosciences of up to $1.0 million for major market regulatory approval and of up to $45.0 million upon the achievement of specified sales milestones. The Company is also obligated to pay a low-teens percentage royalty related to net sales of each licensed product or licensed process covered by a valid claim, which reduces to a single digit percentage royalty related to net sales in the absence of a valid claim. Royalty payments will be reduced in an amount equal to 100 % of royalty fees paid to Avacta (defined below) for the same licensed product. Royalties will be paid by the Company on a country-by-country basis beginning upon the first commercial sale in such country. There is also an additional low-teens to mid-twenties percentage sublicense fee payable to Bach Biosciences for monetary payments arising from a grant of a sub-license to a sub-licensee or in the form of other benefits, depending on the specified development stage of the product.
In January 2021, we entered into a research agreement with Bach Biosciences for a period of three years where Bach Biosciences is contracted to perform research on our behalf, with respect to the Second BACH Agreement. During the year
F-25
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
ended December 31, 2022, the Company made payments in connection with the sponsored research agreement in the amount of $1.0 million as a research and development expense. During the year ended December 31, 2021, the Company made payments in connection with the sponsored research agreement in the amount of $0.8 million and $0.2 million for the exclusive commercialization option upfront fee, both of which were recorded within research and development expense.
License agreement with Avacta Life Sciences Limited (“Avacta”)
In December 2020, the Company entered into an agreement with Avacta (“Avacta Agreement”), which is directly related to the Second BACH Agreement described above. Under the Avacta Agreement, the Company became a sublicensee of Avacta’s license for using intellectual property related to developing and marketing FAP-activated radioligand agents. Under this agreement, the Company obtained an exclusive license of Avacta’s patent rights to use, develop, manufacture and commercialize any products arising from the patent. The Company has the right to grant sublicenses of its rights. The Company is obligated to make aggregate milestone payments to Avacta of up to $4.5 million, upon the achievement of specified development milestones for its first product and up to $3.0 million each for any additional products developed with the technology upon reaching the specified development milestones. In addition, the Company is obligated to pay a milestone payment of $5.0 million for each product for the regulatory milestone being approved in specified territories. There is also an additional single digit percentage fee payable to Avacta for monetary payments arising from a grant of a sublicense to a sublicensee or in the form of other benefits. The Company is also obligated to pay a single digit percentage royalty (subject to a reduction on certain conditions) related to the annual net sales by the Company, its affiliates or its sublicensees for each licensed product or license process and a single digit percentage royalty on a specified product arising out of the patents. The royalty rate will be reduced by 50 % for net sales occurring in the U.S. if there is no valid claim at the time of sale. There is also an additional single digit percentage fee payable to Avacta for monetary payments arising from a grant of a sublicense to a sublicensee or in the form of other benefits.
During the year ended December 31, 2022, the Company did not make any payments to Avacta or recognize any research and development expenses under the Avacta Agreement (December 31, 2021 – the last three installments of the initial license fee of $0.8 million as a research and development expense).
License agreement with Canadian Molecular Probe Consortium (“CanProbe”)
In December 2020, the Company entered into a license agreement with CanProbe (“CanProbe Agreement”). Under the CanProbe Agreement, the Company was granted an exclusive, sublicensable and worldwide license under CanProbe’s patent rights to use, develop, manufacture and commercialize any products arising from a patent associated with the process for the production of 177Lu. The Company is obligated to make aggregate milestone payments to CanProbe of up to $2.4 million ($3.3 million CAD) upon the achievement of receiving marketing authorization milestones for specified territories. On November 8, 2022, POINT entered into a first amendment to the CanProbe Agreement, between the Company; the Canadian Molecular Probe Consortium; the Centre for Probe Development and Commercialization; and The University Health Network, collectively the (“Parties”) pursuant to which certain amendments have been made to the Company's royalty obligations. The Company is obligated to pay a single digit royalty related to the annual net sales by the Company and any of its affiliates and sublicensees. Royalties will be paid by the Company on a country-by-country basis beginning upon the first commercial sale in such country. There is also an additional low-teens percentage fee payable to CanProbe for monetary payments arising from a grant of a sublicense to a sublicensee or in the form of other benefits. In the event it is necessary for the Company or its sublicensees to sell the product in a sub-territory or to obtain a license and to pay royalties to one or more third parties on net sales, and if the aggregate royalty burden payable is greater than a high single digit percent of net sales, then the Company may reduce the royalty fees or sub-licensing fees for sales of such product by 50 % of royalties actually paid to the third party on net sales of the product in the territory in the same royalty period.
During the year ended December 31, 2022, the Company paid $1.0 million in royalty payments in connection with this agreement (December 31, 2021 – nil ).
License agreement with Belgian Nuclear Research Centre (“SCK-CEN”)
On June 30, 2021, the Company entered into a license agreement with the Belgian Nuclear Research Centre (“SCK-CEN”). Under the SCK-CEN Agreement, the Company was granted a worldwide, royalty-bearing, non-exclusive,
F-26
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
16. Income taxes
Domestic and international pre-tax income (loss) consists of the following:
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
| U.S. | $ | $ | ( | ||||||||
| Canada | |||||||||||
| Income (loss) before provision for income taxes | $ | $ | ( | ||||||||
Income tax expense attributable to operations is comprised of the following:
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
| Current income tax: | |||||||||||
| Federal | $ | $ | |||||||||
| State | ( | ||||||||||
| Foreign | |||||||||||
| Total current expense | |||||||||||
| Deferred income tax: | |||||||||||
| Federal | |||||||||||
| State | |||||||||||
| Foreign | ( | ||||||||||
| Total deferred expense | ( | ||||||||||
| Total provision for income tax | $ | $ | |||||||||
A reconciliation of the U.S. federal statutory income tax rate to the Company’s effective income tax rate is as follows:
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
Provision for income taxes at the Company’s statutory tax rate of | $ | $ | ( | ||||||||
| State income taxes, net of federal tax benefit | ( | ||||||||||
| Change in valuation allowance | |||||||||||
| Research and development credits | ( | ||||||||||
| Permanent items and other | |||||||||||
Provision for income taxes at the Company’s effective income tax rate | $ | $ | |||||||||
F-27
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
| Net operating loss carryforwards | $ | $ | |||||||||
| License agreements | |||||||||||
| Capitalized research expenses | |||||||||||
| Start-up costs | |||||||||||
| Share based compensation | |||||||||||
| Reserves and accruals | |||||||||||
| Tax credits | |||||||||||
| Other | |||||||||||
| Total deferred tax assets | |||||||||||
| Valuation allowance | ( | ( | |||||||||
| Net deferred tax assets | |||||||||||
| Deferred tax liabilities | |||||||||||
| Depreciation | ( | ||||||||||
| Unrealized exchange gain | ( | ||||||||||
| Total deferred tax liabilities | ( | ( | |||||||||
| Net deferred tax liabilities | $ | $ | ( | ||||||||
Deferred income tax assets and liabilities are recorded for differences between the financial statement and tax basis of the assets and liabilities that will result in taxable or deductible amounts in the future based on enacted laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Currently, management expects to generate losses for the foreseeable future as a result of the Company's continued focus on the development of its existing research programs. In addition, the federal jurisdiction and state jurisdictions in which the Company operates do not allow for carryback of operating losses. As a result of the expectation of future losses and the lack of carryback potential, the Company has concluded that its deferred tax assets are not realizable. As a result, the Company has recognized a valuation allowance against its deferred tax assets.
As of December 31, 2022, the Company has fully utilized its federal net operating loss carryforwards and has state net operating loss carryforwards of approximately $8.3 million. The state net operating losses will begin to expire in 2030. As of December 31, 2022, the Company has $0.4 million of state research and development credits that begin to expire in 2029. As of December 31, 2022, the Company has $0.4 million of Indiana Hoosier Business tax credits that begin to expire in 2029.
Pursuant to the Internal Revenue Code (IRC) Sections 382 and 383, annual use of the Company's net operating losses and research and development credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The Company completed an ownership change analysis pursuant to IRS Section 382 through 2022 and ownership changes were identified, however, the changes did not result in a reduction in net operating loss carryforwards or research and development credit carryforwards. If ownership changes occur in the future, the amount of remaining tax attribute carryforwards available to offset taxable income and income tax expense in future years may be restricted or eliminated. If eliminated, the related asset would be removed from deferred tax assets with corresponding reduction in the valuation allowance.
Uncertain tax positions are evaluated based upon the facts and circumstances that exist at each reporting period. Subsequent changes in judgement based upon new information may lead to changes in recognition, derecognition, and
F-28
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
measurement. Adjustment may result, for example, upon resolution of an issue with the taxing authorities or expiration of a statute of limitations barring an assessment for an issue. The Company recognizes a tax benefit from an uncertain tax position when it is more likely than not that the position will be sustained upon examination by tax authorities.
The following table summarizes the changes to the Company’s gross unrecognized tax benefits for the years ended December 31, 2022 and 2021, respectively:
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
| Unrecognized tax benefits, beginning of year and period | $ | $ | |||||||||
| Additions for tax positions of current year positions | |||||||||||
| Additions for tax positions of prior year positions | |||||||||||
| Unrecognized tax benefits, end of year and period | $ | $ | |||||||||
As of December 31, 2022, the Company has unrecognized tax benefits of $1.5 million of which $1.5 million will affect the effective tax rate if recognized when the Company no longer has a valuation allowance offsetting its deferred tax assets. As of December 31, 2021, the Company had no unrecognized tax benefits. The Company is subject to taxation in the United States, Canada and various state jurisdictions. All of the Company’s tax years from inception are subject to examination by federal, and state tax authorities. The Company’s practice is to recognize interest and penalties related to income tax matters in income tax expense.
The Company had no accrued interest or penalties related to income tax matters in the Company’s consolidated balance sheet at December 31, 2022, and has not recognized interest or penalties in the Company’s consolidated statement of operations and consolidated statement of comprehensive income (loss) for the year ended December 31, 2022. Further, the Company is not currently under examination by any federal, state or local tax authority.
The Company does not record U.S. income taxes on the undistributed earnings of its foreign subsidiaries based upon the Company's intention to permanently reinvest undistributed earnings to ensure sufficient working capital and further expansion of existing operations outside the U.S. In the event the Company is required to repatriate funds from outside of the U.S., such repatriation would be subject to local laws, customs, and tax consequences.
The Tax Cut and Jobs Act subjects a U.S. stockholder to tax on global intangible low-taxed income ("GILTI") earned by certain foreign subsidiaries. The Company has elected to account for GILTI in the year the tax is incurred in accordance with the FASB Staff Q&A, Topic 740, No. 5, Accounting for Global Intangible Low-Taxed Income, which states that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) was passed into law. The CARES Act includes several significant business tax provisions including modification to the taxable income limitation for utilization of net operating losses incurred in 2019 to 2021, an increase to the limitation on deductibility of certain business interest expense, bonus depreciation for purchases of qualified improvement property and special deductions on certain corporate charitable contributions. The Company analyzed the provisions of the CARES Act and determined there was no impact to its income tax provision for the years ended December 31, 2022 and 2021.
The Inflation Reduction Act (IRA) was signed into law on August 16, 2022. The IRA introduces a 15% corporate alternative minimum tax (CAMT) for corporations whose average annual adjusted financial statement income (AFSI) for any consecutive three-tax-year period ending after December 31, 2021 and preceding the tax year exceeds $1.0 billion and a 1% excise tax on stock repurchases made by publicly traded U.S. corporations. Since the Company does not meet the book income threshold to be subject to CAMT, the excise tax is not an ASC 740 tax, they are not expected to have any impact. The other tax law updates are not expected to have any material impact to the Company's consolidated financial statements and related disclosures. The Company will continue to evaluate the impact and will monitor in future quarters.
The CHIPS and Science Act was signed into law on August 9, 2022. The Act introduces the advanced manufacturing investment tax credit, a 25% tax credit for investments in semiconductor manufacturing. It also includes incentives for
F-29
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
manufacturing semiconductors, as well as specialized tooling equipment required in the semiconductor manufacturing process. The Company is not currently claiming any such tax credits, as such the tax law updates are not expected to have any material impact to the Company's consolidated financial statements and related disclosures.
Enacted in 2017, the Tax Cuts and Jobs Act (“TCJA”) included significant changes in tax law including a change to Internal Revenue Code section 174 regarding the deductibility of research and experimentation expenses (“R&E expenses”). The section 174 tax law change had a delayed effective date and became effective for the Company in fiscal year 2022. New section 174 requires that companies capitalize and amortize R&E expenses performed in the U.S. over five years and further provides for a fifteen-year amortization period for R&E expenses incurred outside the U.S. This has impacted our effective tax rate and our cash tax payable in 2022, and it may also impact our effective tax rate and our cash tax liability in future years.
17. Net earnings (loss) per share
Basic earnings (loss) per share is computed by dividing the earnings (loss) available to common stockholders by the weighted-average number of shares of Common Stock outstanding during the period. Diluted earnings (loss) per share is computed by dividing earnings (loss) available to common stockholders by the weighted-average number of shares of Common Stock outstanding during the period increased to include the number of additional shares of Common Stock that would have been outstanding if the potentially dilutive securities had been issued, using the treasury stock method.
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
| Numerator: | |||||||||||
| Net income (loss) attributable to common stockholders | $ | $ | ( | ||||||||
| Denominator: | |||||||||||
| Weighted-average basic common shares outstanding | |||||||||||
| Effect of dilutive stock options | |||||||||||
| Weighted-average diluted shares | |||||||||||
| Basic income (loss) per share | $ | $ | ( | ||||||||
| Diluted income (loss) per share | $ | $ | ( | ||||||||
For the year ended December 31, 2022, the Company’s stock options have been included in the computation of diluted net income per share where those stocks options are in-the-money. The Company's performance share units are considered contingently issuable shares for the purpose of the computation of earnings (loss) per share and have been excluded on the basis that as at December 31, 2022, the performance condition for vesting had not been achieved.
For the year ended December 31, 2021, the Company’s potentially dilutive securities, which include stock options and warrants, have been excluded from the computation of diluted net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted-average number of shares of Common Stock outstanding used to calculate both basic and diluted net loss per share attributable to common stockholders is the same.
The Company excluded the 3,411,611 and 3,825,751 stock options to purchase Common Stock for the years ended December 31, 2022 and December 31, 2021, respectively, from the computation of diluted net loss per share attributable to common stockholders for the periods indicated because including them would have had an anti-dilutive effect.
18. Financial instruments risk management
Concentration risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash, cash equivalents, and investments. The Company’s cash equivalents and investments as of December 31, 2022 consisted of money market funds, U.S. and Canadian government agency securities, corporate bonds and commercial paper. The Company does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships. The Company currently holds all its cash, cash equivalents and investments with two financial
F-30
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
institutions. These financial institutions are large and high-quality investment grade institutions. The Company will continue to monitor for changes in credit quality in relation to the counterparties to which it has relationships.
Foreign currency risk
The Company does not have significant operating subsidiaries or significant investments in foreign countries as of December 31, 2022 and 2021. The Company is subject to foreign currency exposure on its cash balances, accounts payable and accrued liabilities denominated in currencies other than the U.S. dollar, expenses incurred from certain supplier and service agreements denominated in Canadian dollars and Euro, as well as salaries and wages in respect of the Company’s Canadian employees. The Company does not currently manage its foreign currency exposure through hedging programs and will continue to monitor its foreign currency assets and liabilities and evaluate the needs for these programs in the future. During the years ended December 31, 2022 and 2021, the Company recorded $0.4 million and $0.1 million, respectively, of foreign currency losses in the consolidated statements of operations. A 10 % increase or decrease in current exchange rates would not have a material effect on our financial results.
Interest rate risk
As of December 31, 2022, we had an aggregate cash, cash equivalents, restricted cash and investments balance of $541.3 million which consisted of cash, money market funds, U.S. and Canadian government agency debt securities, corporate bonds, municipal bonds and commercial paper. The Company has a policy of investing in highly-rated securities, whose maturities are, at December 31, 2022, primarily less than one year. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, a 10 % increase or decrease in interest rates would not have a material effect on the fair market value of our investment portfolio. We have the ability to hold our investments until maturity, and therefore, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investment portfolio. The Company does not enter into investments for trading or speculative purposes.
The Company was previously subject to interest rate risk under its Mortgage loan. The Mortgage bore interest at 2.85 % plus a minimum rate of 1-month LIBOR, subject to a LIBOR floor of 0.25 %. The Mortgage required quarterly interest payments, which commenced on October 1, 2020, with the principal amount originally due at maturity on January 10, 2022. On July 29, 2021, the mortgage loan was repaid in full and the related mortgage on our facility in Indianapolis, Indiana was released. For more details around the Mortgage see Note 11.
The Company does not currently manage interest rate exposure through hedging programs. The Company will continue to monitor and evaluate the need for interest rate hedging programs in the future.
19. Related party transactions
The Company recognized expenses in connection with related party transactions in the consolidated statements of operations as follows:
| Year ended December 31, 2022 | Year ended December 31, 2021 | ||||||||||
| Consulting fees on business activities to Board member | $ | $ | |||||||||
| Reimbursement to Board member for occupancy costs | |||||||||||
| $ | $ | ||||||||||
Transactions with related parties are in the normal course of operations and have been measured at their agreed upon exchange amount.
During the year ended December 31, 2022, the Company received consulting services for research and development from a Board member. As of December 31, 2022, $0.1 million is recorded within accrued liabilities in relation to this consulting arrangement (December 31, 2021 - $0.1 million).
F-31
POINT BIOPHARMA GLOBAL INC.
December 31, 2022 and December 31, 2021
Notes to the consolidated financial statements (in U.S. dollars)
20. Employee benefit plans
The Company maintains a retirement plan, which is qualified under section 401(k) of the Internal Revenue Code for its U.S. employees. The plan allows eligible employees to defer, at the employee’s discretion, pretax compensation up to the IRS annual limits. The Company matched contributions up to 50 % of the first 4 % of the eligible employee’s compensation or the maximum amount permitted by law. Total expense for contributions made to U.S. employees was $0.2 million for the year ended December 31, 2022 (December 31, 2021 - $0.05 million).
The Company also sponsors a registered retirement savings plan, which is registered with the Canada Revenue Agency ("CRA") for its Canadian employees. The plan allows eligible employees to defer, at the employee’s discretion, pretax compensation up to the individuals cumulative limits as determined by the CRA. The Company matched contributions up to 50 % of the first 4 % of the eligible employee’s compensation. Total expense for contributions made to Canadian employees was $0.2 million CAD for the year ended December 31, 2022 (December 31, 2021 - $nil ).
21. Subsequent events
University Health Network (“UHN”) Agreement
On February 2, 2023, Point Biopharma Corp., a wholly owned subsidiary of POINT, entered into a Facility Agreement with UHN (the “UHN Agreement”), a not-for-profit corporation incorporated under the laws of Canada. Pursuant to the UHN Agreement, which will become effective on April 1, 2023, POINT is authorized to access and utilize a 7,700 square foot, licensed research and development space with cGMP manufacturing suites (the “Facility”), which the Company will use to develop and expand its pipeline of next-generation radioligands.
The initial term of the UHN Agreement will run for five years from the effective date, with an option to renew for additional two year terms thereafter, subject to certain conditions described in the UHN Agreement. The agreement does not transfer any title in the Facility to POINT. The access fee for the Facility is expected to be approximately $4.0 million for the first two years of the term, after which the access fee is subject to a Consumer Price Index adjustment. POINT will also bear variable costs for services such as radiation safety, equipment service, IT, engineering and other services.
During the term, POINT shall be responsible for day-to-day management, activities and decision-making regarding the Facility. General governance of the Facility will be exercised by POINT and UHN through a joint committee. The joint committee, which will meet quarterly, will have a minimum of six (6) people and will be comprised of an equal number of members from each of POINT and UHN.
Lease Agreement
On March 10, 2023, West 78th Street, LLC, a wholly owned subsidiary of POINT, entered into a Lease Agreement (the "Lease") for the lease of approximately 103,000 square feet of building space which the Company will utilize as additional office space and will develop the property as needed. The initial term of the lease is from July 1, 2023 through July 31, 2033 with an option for two additional ten year renewal periods at the Company's discretion and subject to certain conditions as described in the Lease. The agreement does not transfer any title in to the Company but provides for the right of first offer to purchase in the event of a proposed sale, subject to certain conditions as described in the Lease.
Over the initial term of the Lease, the Company will be required to make annual payments increasing from approximately $0.7 million to approximately $1.0 million in annual payments including certain estimated variable costs as described in the Lease. Annual payments in connection with the renewal options will be at the then market rate for a similar lease.
F-32