
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
For
the quarterly period ended
For the transition period from ____________ to ____________
Commission
File Number:

| (Exact name of registrant as specified in its charter) |
| (State or other jurisdiction of incorporation) | (IRS Employer Identification Number) |
(Address of principal executive offices)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| N/A | N/A | N/A |
Securities registered under Section 12(g) of the Act:
Common Stock, $0.001 par value
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate
by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant
was required to submit and post such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| ☐ | Large accelerated filer | ☐ | Accelerated filer |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
On March 31, 2023, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant had an undetermined value as the registrant’s common stock was not trading on any exchange, nor was it quoted for trading on the OTC Link ATS or any other over-the-counter market or alternative trading system.
The number of the registrant’s shares of common stock issued, issuable and outstanding was as of May 12, 2024.
GENVOR INCORPORATED
INDEX
| Page | ||
| PART I. FINANCIAL INFORMATION | ||
| Item 1. | Financial Statements | 3 |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 4 |
| Item 3. | Quantitative and Qualitative Disclosures about Market Risks | 11 |
| Item 4. | Controls and Procedures | 11 |
| PART II. OTHER INFORMATION | ||
| Item 1. | Legal Proceedings | 12 |
| Item 1A. | Risk Factors | 12 |
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 12 |
| Item 3. | Defaults Upon Senior Securities | 13 |
| Item 4. | Mine Safety Disclosures | 13 |
| Item 5. | Other Information | 13 |
| Item 6. | Exhibits | 14 |
| SIGNATURES | 15 | |
| 2 |
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements
Genvor Incorporated
Index to Financial Statements
| 3 |
Genvor Incorporated
Condensed Consolidated Balance Sheets
| March 31, | September 30, | |||||||
| 2024 | 2023 | |||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses | ||||||||
| Total current assets | ||||||||
| Fixed assets, net | ||||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Convertible notes payable | $ | $ | ||||||
| Accounts payable and accrued expenses | ||||||||
| Due to related party | ||||||||
| SBA loan | ||||||||
| Total current liabilities | ||||||||
| Total liabilities | ||||||||
| Commitments and contingencies (Note 6) | ||||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $ par value, shares authorized | ||||||||
| Preferred stock - series A, shares authorized, and shares issued as of March 31, 2024 and September 30, 2023, respectively, and and shares outstanding as of March 31, 2024 and September 30, 2023, respectively | ||||||||
| Preferred stock – series B, shares authorized, and shares issued as of March 31, 2024 and September 30, 2023, respectively, and outstanding as of March 31, 2024 and September 30, 2023, respectively | ||||||||
| Common stock, $ par value, shares authorized, | ||||||||
| Treasury stock, and shares of series B preferred stock at March 31, 2024 and September 30, 2023, respectively | ( | ) | ( | ) | ||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ deficit | ( | ) | ( | ) | ||||
| Total liabilities and stockholders’ deficit | $ | $ | ||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-1 |
Genvor Incorporated
Condensed Consolidated Statements of Operations
(unaudited)
| For
the Three Months Ended March 31, | For
the Six Months Ended March 31, | |||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| Revenue | $ | $ | $ | $ | ||||||||||||
| Operating expenses | ||||||||||||||||
| Professional fees | ||||||||||||||||
| Payroll related expenses | ||||||||||||||||
| Research and development | ||||||||||||||||
| Stock-based compensation | ||||||||||||||||
| Marketing expenses | ||||||||||||||||
| Investor and public relations | ||||||||||||||||
| Other general and administrative expenses | ||||||||||||||||
| Total operating expenses | ||||||||||||||||
| Operating loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Other income (expense) | ||||||||||||||||
| Interest expense | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Penalties | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Amortization of debt discount | ( | ) | ( | ) | ||||||||||||
| Gain on settlement of liabilities, net | ||||||||||||||||
| Total other income (expense) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Basic and diluted net loss per common share | $ | ) | $ | ) | $ | ) | $ | ) | ||||||||
| Basic and diluted weighted average common shares outstanding | ||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-2 |
Genvor Incorporated
Condensed Consolidated Statements of Changes in Stockholders’ Deficit
For the Six Months Ended March 31, 2024
(unaudited)
| Series A | Series B | Additional | Accumu- | |||||||||||||||||||||||||||||||||||||
| Preferred Stock | Preferred Stock | Common Stock | Treasury | Paid-in | lated | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Stock | Capital | Deficit | Total | |||||||||||||||||||||||||||||||
| Balance, September 30, 2022 | $ | $ | $ | $ | $ | $ | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Conversion of common stock into series B preferred stock | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||
| Sale of common stock | - | - | ||||||||||||||||||||||||||||||||||||||
| Net loss for the period ended December 31, 2022 | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||
| Balance, December 31, 2022 | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||
| Sale of common stock | - | - | ||||||||||||||||||||||||||||||||||||||
| Net loss for the period ended March 31, 2023 | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||
| Balance, March 31, 2023 | $ | $ | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||||||||||||
| Balance, September 30, 2023 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||||||||||
| Sale of common stock | - | - | ||||||||||||||||||||||||||||||||||||||
| Issuance of common stock erroneously omitted from prior year | - | - | ( | ) | ||||||||||||||||||||||||||||||||||||
| Double issuance of common stock |
- |
|
- |
|
| ( | ) |
|
| |||||||||||||||||||||||||||||||
| Issuance of warrants for services | - | - | - | |||||||||||||||||||||||||||||||||||||
| Issuance of warrants for conversion of note payable | - | - | - | |||||||||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of note payable | - |
- |
|
| ||||||||||||||||||||||||||||||||||||
| Net loss for the period ended December 31, 2023 | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||
| Issuance of common stock for services | - | - | ||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of note payable | - | - | ||||||||||||||||||||||||||||||||||||||
| Sale of common stock | - | - | ||||||||||||||||||||||||||||||||||||||
| Issuance of warrants for services | - | - | - | |||||||||||||||||||||||||||||||||||||
| Cancellation of common stock | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||
| Net loss for the period ended March 31, 2024 | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||
| Balance, March 31, 2024 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-3 |
Genvor Incorporated
Condensed Consolidated Statements of Cash Flow
For the Six Months Ended March 31,
(unaudited)
| 2024 | 2023 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation expense | ||||||||
| Stock-based compensation | ||||||||
| Late fee capitalized into notes payable | ||||||||
| Gain on settlement of liabilities, net | ( | ) | ||||||
| Amortization of debt discount | ||||||||
| Changes in assets and liabilities: | ||||||||
| Prepaid expenses | ||||||||
| Other current assets | ( | ) | ||||||
| Accounts payable and accrued expenses | ( | ) | ||||||
| Due to related party | ||||||||
| USDA CRADA liability | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from notes payable | ||||||||
| Proceeds from sale of common stock | ||||||||
| Net cash provided by financing activities | ||||||||
| Net decrease in cash | ( | ) | ( | ) | ||||
| Cash at beginning of period | ||||||||
| Cash at end of period | $ | $ | ||||||
| Cash paid for interest | $ | $ | ||||||
| Cash paid for taxes | $ | $ | ||||||
| Non-cash investing and financing activities: | ||||||||
| Conversion of note payable into common stock | $ | $ | ||||||
| Conversion of notes payable into warrants | $ | $ | ||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-4 |
GENVOR INCORPORATED
Notes to Condensed Consolidated Financial Statements
March 31, 2024
(unaudited)
NOTE 1 – ORGANIZATION AND BASIS OF PRESENTATION
Company Background
On May 27, 2022, Genvor Incorporated, formerly known as Allure Worldwide, Inc. (the “Company” or “Genvor” or “we”), a Nevada corporation, Genvor Acquisition, Corp., a Delaware corporation and a wholly owned subsidiary of the Company (“Merger Sub”), and Genvor Inc., a Delaware corporation (“Old Genvor”), completed their previously announced merger transaction pursuant to which the Company acquired Old Genvor (the “Acquisition”), and Old Genvor became a wholly-owned subsidiary of the Company. The Acquisition was completed pursuant to an Exchange Agreement, dated as of January 11, 2021 (the “Acquisition Agreement”), pursuant to which Old Genvor was to be acquired by the Company as its wholly owned subsidiary and each share of Old Genvor common stock would be exchanged for a share of the Company’s common stock, and a merger agreement, dated March 2, 2022 (the “Merger Agreement”), pursuant to which Merger Sub merged with and into Old Genvor, with Old Genvor continuing as a wholly owned subsidiary of the Company and the surviving corporation of the merger, and each share of Old Genvor being converted into the right to receive a share of the Company (the “Merger”). After closing of the Merger, the Company was renamed “Genvor Incorporated.” Genvor develops plant-based defense technology designed to help farmers achieve global food security.
During May 2019, Old Genvor acquired Nexion Biosciences LLC (“NBLLC”) from a founder for nominal consideration as a wholly owned subsidiary. NBLLC was formed in the state of Delaware on December 28, 2018. The condensed consolidated financial statements of the Company include the accounts of Genvor Incorporated, Old Genvor, and its wholly owned subsidiary NBLLC. Intercompany accounts and transactions have been eliminated upon consolidation.
Nature of Operations
The Company’s business plan is that Genvor will be continuing its research and development addressing plant-based defense technology which then can be commercialized to help farmers and growers globally to overcome potentially catastrophic losses resulting from plant disease, toxins, bacteria, and fungi that destroy their crops. These solutions can result in greater crop yields and economic savings, which can assist in overcoming world-wide food scarcity.
Basis of Presentation
The accompanying unaudited condensed consolidated financial information as of and for the six months ended March 31, 2024, and 2023 has been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) for interim financial information and with the instructions to Quarterly Report on Form 10-Q and Article 10 of Regulation S-X. In the opinion of management, such financial information includes all adjustments (consisting only of normal recurring adjustments) considered necessary for a fair presentation of our financial position at such date and the operating results and cash flows for such periods. Operating results for the six months ended March 31, 2024, are not necessarily indicative of the results that may be expected for the entire year or for any other subsequent interim period.
Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been omitted pursuant to the rules of the U.S. Securities and Exchange Commission, or the SEC. These unaudited financial statements and related notes should be read in conjunction with the audited financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K for the year ended September 30, 2023, as filed with the SEC.
Principles of Consolidation
The condensed consolidated financial statements include the accounts of the Company and its wholly owned subsidiary. All significant intercompany balances and transactions have been eliminated in the consolidation. The condensed consolidated financial statements included herein, presented in accordance with U.S. GAAP and stated in United States dollars, have been prepared by the Company, pursuant to the rules and regulations of the Securities and Exchange Commission.
Liquidity and Going Concern
The
accompanying condensed consolidated financial statements have been prepared assuming the Company will continue as a going concern, which
contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. At March
31, 2024, the Company had an accumulated deficit of $
While the Company is currently developing its products and technologies, the Company’s cash position may not be significant enough to support the Company’s daily operations. Management intends to raise additional funds by way of additional public and/or private offerings of its stock. Management believes that the actions presently being taken to further implement its business plan, develop its products and technologies, and generate revenues should provide the opportunity for the Company to continue as a going concern. While the Company believes in the viability of its strategy to generate revenues and in its ability to raise additional funds in the future, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon the Company’s ability to further implement its business plan and generate cash flows from financing activities or operating activities. The financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
| F-5 |
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash Flow Reporting
The Company follows Accounting Standards Codification (“ASC 230”), Statement of Cash Flows, for cash flow reporting, classifies cash receipts and payments according to whether they stem from operating, investing, or financing activities and provides definitions of each category, and uses the indirect or reconciliation method (“indirect method”) as defined by ASC 230, Statement of Cash Flows, to report net cash flow from operating activities by adjusting net income to reconcile it to net cash flow from operating activities by removing the effects of (a) all deferrals of past operating cash receipts and payments and all accruals of expected future operating cash receipts and payments and (b) all items that are included in net income that do not affect operating cash receipts and payments.
Cash
Cash
is comprised of cash balances. Cash is held at major financial institutions and is subject to credit risk to the extent that those balances
exceed applicable Federal Deposit Insurance Corporation (“FDIC”) insurance amounts of $
The Company maintains its cash balances at one financial institution that is insured by the Federal Deposit Insurance Corporation. At March 31, 2024, the Company’s cash balances were not in excess of federally insured limits.
Fixed Assets
Furniture and equipment are stated at cost. Depreciation is provided by the straight-line method over the useful lives of the related assets, approximately seven years. Expenditures for minor enhancements and maintenance are expensed as incurred.
Fair Value of Financial Instruments
The book values of cash and accounts payable approximate their respective fair values due to the short-term nature of these instruments. The fair value hierarchy under U.S. GAAP distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs).
The hierarchy consists of three levels
| ● | Level one — Quoted market prices in active markets for identical assets or liabilities; | |
| ● | Level two — Inputs other than level one inputs that are either directly or indirectly observable; and | |
| ● | Level three — Unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
Determining which category an asset or liability falls within the hierarchy requires significant judgment. We evaluate our hierarchy disclosures each quarter.
Financial Instruments
The Company’s financial instruments include cash and cash equivalents, payables, and accrued interest and short-term and long-term notes payable and are accounted for under the provisions of ASC 825, Financial Instruments. The carrying amount of these financial instruments, as reflected in the accompanying condensed consolidated balance sheets approximates fair value.
Long-lived Assets
The Company’s long-lived assets and other assets (consisting of furniture, equipment, and a patent) are reviewed for impairment in accordance with the guidance of the ASC 360, Property, Plant, and Equipment, and ASC 205, Presentation of Financial Statements. The Company tests for impairment losses on long-lived assets used in operations whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. The recoverability of an asset to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. Impairment evaluations involve management’s estimates on asset useful lives and future cash flows. Actual useful lives and cash flows could be different from those estimated by management, which could have a material effect on our reporting results and financial positions. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary. During the six months ended March 31, 2024, and 2023, the Company had not experienced impairment losses on its long-lived assets.
Research and Development
The
Company expenses the cost of research and development as incurred. Research and development expenses consist primarily of professional
service costs associated with the development of plant-based defense technology products. For the six months ended March 31, 2024, and
2023, the Company had $
Patents
Any patent costs for internally developed patents will be expensed as incurred. Costs to maintain and defend patents are recorded as administrative expenses in the statement of operations.
Purchased patents are recorded at cost and reviewed for impairment in accordance with the guidance of the ASC 360,
| F-6 |
Income Taxes
The Company accounts for income taxes in accordance with FASB ASC 740, Income Taxes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and loss carryforwards and their respective tax bases.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income (loss) in the years in which those temporary differences are expected to be recovered or settled.
The effect of a change in tax rules on deferred tax assets and liabilities is recognized in operations in the year of change. A valuation allowance is recorded when it is “more likely-than-not” that a deferred tax asset will not be realized.
Tax benefits of uncertain tax positions are recognized only if it is more likely than not that the Company will be able to sustain a position taken on an income tax return. The Company has no liability for uncertain tax positions as of March 31, 2024. Interest and penalties, if any, related to unrecognized tax benefits would be recognized as interest expense. The Company does not have any accrued interest or penalties associated with unrecognized tax benefits, nor was any significant interest expense recognized during the six months ended March 31, 2024.
Stock-Based Compensation
The Company accounts for stock-based instruments issued to employees in accordance with ASC Topic 718, Compensation – Stock Compensation, and Certain Redeemable Financial Instruments. ASC Topic 718 requires companies to recognize in the statement of operations the grant-date fair value of stock options and other equity-based compensation issued to employees. The value of the portion of an award that is ultimately expected to vest is recognized as an expense over the requisite service periods using the straight-line attribution method.
The Company measures compensation cost for all employee stock-based awards at their fair values on the date of grant. Stock-based awards issued to non-employees are measured at their fair values on the date of grant and are re-measured at each reporting period through their vesting dates, as applicable. The fair value of stock-based awards is recognized as expense over the service period, net of estimated forfeitures, using the straight-line method.
Basic
net loss per common share is computed using the weighted average number of common shares outstanding. Diluted earnings per share (“EPS”)
include additional dilution from common stock equivalents, such as stock issuable pursuant to the exercise of stock options, warrants
and convertible notes. Common stock equivalents are not included in the computation of diluted earnings per share when the Company reports
a loss because to do so would be anti-dilutive for the periods presented. The Company had total potential additional dilutive securities
outstanding at March 31, 2024 and 2023 of $
Recent Accounting Pronouncements
Recently Issued Accounting Standards: Management does not believe that any recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on the accompanying financial statements.
NOTE 3 – BORROWINGS
Commercial Loan
On
April 9, 2020, the Company received a loan from the Small Business Administration pursuant to the Paycheck Protection Program (“PPP”)
in the principal amount of $
Payable for Patent
Notes Payable
From time to time, the Company’s subsidiary, Old Genvor, enters into unsecured notes payable with individual investors. Only Noteholder E (below) has security in the form of a personal guarantee by the CEO and prior consultant (Note 6). The terms of these notes are listed below. Several of the notes are convertible into shares of the Company’s common stock as detailed in the following schedule.
| Interest | Loan | |||||||||||||||
| Noteholder | Origination | Maturity | Rate | Balance | ||||||||||||
| Brent Lilienthal (a) (b) | % | $ | ||||||||||||||
| Mel Wentz (a) (b) | % | |||||||||||||||
| $ | ||||||||||||||||
| (a) |
| (b) |
The
notes do not have default provisions except for Mel Wentz receives a default penalty of $
| F-7 |
The Company is currently disputing amounts claimed to be owed to two noteholders, Brent Lilienthal, and Mel Wentz, under state usury laws (See Note 6).
On
September 13, 2023, the Company entered into a convertible promissory note with Barkley Capital LLC for $
On
November 11, 2023, John Hare converted the $
On
December 15, 2023, R. Kirk Huntsman converted the $
On
March 9, 2024, Barkley converted the $
During
the year ended September 30, 2023, $
Interest
expense totaled $
NOTE 4 – STOCKHOLDERS’ DEFICIT
Preferred Stock
The authorized preferred stock of the Company consists of shares with a $ par value.
Series A Preferred Stock
On
August 10, 2022, the Company designated shares of its preferred stock as Series A Preferred Stock (“Series A”).
The preferred stock was issued on August 16, 2022, as follows: Bradley White (former Chief Executive Officer), shares; Dr. Clayton Yates (Chief Scientific Officer and Chairman), shares; and Dr. Jesse Jaynes (Chief Research Officer and Director), shares. See Note 7.
On September 28, 2023, as part of the Settlement Agreement with Bradley White (see Notes 6 and 7), Mr. White returned to the Company for cancellation of shares of Series A preferred stock.
As of March 31, 2024, and September 30, 2023, there were and shares of Series A preferred stock issued and outstanding, respectively.
Series B Preferred Stock
On
October 19, 2022, the Company filed a Certificate of Designation with the State of Nevada to designate its Series B Preferred Stock (“Series
B”). The designation authorized shares of Series B.
On October 19, 2022, the following shareholders converted shares of common stock of the Company into shares of Series B to modify the common shares outstanding to reduce the outstanding common stock issued by the Company, as follows:
| Name | Common Exchanged | Series B Issued | ||||||
| Jaynes Investment LLC (a) | ||||||||
| ACT Holdings LLC (a) | ||||||||
| LASB Family Trust (a) | ||||||||
| Jesse Michael Jaynes (a) | ||||||||
| Bradley White (a) | ||||||||
| PJ Advisory Group | ||||||||
| Total | ||||||||
| (a) |
The conversion of the common stock into Series B was valued at par, respectively, offset to additional paid-in capital. Series B is convertible into common stock into the original amount of common stock converted therefore there is no change in the amount of common stock outstanding on a fully diluted basis.
On September 28, 2023, as part of the Settlement Agreement with Bradley White (see Notes 6 and 7), Mr. White returned to the Company for cancellation of shares of Series B preferred stock.
As of March 31, 2024, and September 30, 2023, there were and shares of Series B preferred stock issued and outstanding, respectively.
| F-8 |
Common Stock
The authorized common stock of the Company consists of shares with a $ par value. All common stock shares are non-assessable and have one vote per share.
On
April 21, 2022, the Company issued shares of common stock to an individual under a transfer and exchange agreement for a note receivable
held in NBFL (see Note 3). At the transfer date, the latest sale of common stock was at $, accordingly the shares were valued at
$
In connection with the Merger (see Notes 1 and 8), the founding shareholders of the Company cancelled shares of common stock, retaining 5%, or shares of common stock, as of June 30, 2022. The cancellation is presented in the accompanying statements of changes in stockholders’ deficit within the line item “Retroactive application of recapitalization.”
During
July 2022, the Company entered into a transfer and exchange agreement with an individual to issue shares of common stock for the
note receivable held in NBFL. Since NBFL had minimal assets and was dissolved during the year ended December 31, 2019, the note receivable
was immediately written-off. Based on the latest SPA price per share, the stock was valued at $ per share, or $
On September 8, 2022, the Company issued shares of common stock to a prior Nexion contractor. This was regarding a claim against the predecessor management and the Company opted as a settlement to issue the common stock.
Shares Issued for Services
On
January 1, 2024, the Company issued shares of common stock for services valued at $
On
January 16, 2024, the Company issued shares of common stock valued at $
On
January 17, 2024, the Company issued shares of common stock valued at $
On
January 17, 2024, the Company issued shares of common stock for services valued at $
On
February 2, 2024, the Company issued shares of common stock for services valued at $
On
February 5, 2024, the Company issued shares of common stock for services valued at $
On
February 16, 2024, the Company issued shares of common stock valued at $
On
February 17, 2024, the Company issued shares of common stock valued at $
On
March 2, 2024, the Company issued shares of common stock for services valued at $
On
March 5, 2024, the Company issued shares of common stock for services valued at $
On
March 11, 2024, the Company issued shares of common stock for services valued at $
On
March 16, 2024, the Company issued shares of common stock valued at $
On
March 17, 2024, the Company issued shares of common stock valued at $2
Stock Issued for Cash
On
November 17, 2022, the Company issued shares of common stock to an investor for $
On
May 3, 2023, the Company issued shares of common stock to an investor for $
On
May 12, 2023, the Company issued shares of common stock to an investor for $
| F-9 |
On
May 29, 2023, the Company issued shares of common stock to an investor for $
On
July 12, 2023, the Company issued shares of common stock to an investor for $
On
July 13, 2023, the Company issued shares of common stock to an investor for $
On
July 14, 2023, the Company issued shares of common stock to an investor for $
On
July 17, 2023, the Company issued shares of common stock to an investor for $
On
August 25, 2023, the Company issued shares of common stock to an investor for $
On
September 16, 2023, the Company issued shares of common stock for the settlement of a debt and accrued interest for $
On
September 19, 2023, the Company issued shares of common stock to an investor for $
On
November 1, 2023, the Company issued shares of common stock to an investor for $
On
November 1, 2023, the Company issued shares of common stock to an investor for $
On
November 1, 2023, the Company issued shares of common stock to an investor for $
On
November 6, 2023, the Company issued shares of common stock to an investor for $
On
November 8, 2023, the Company issued shares of common stock to an investor for $
On
November 8, 2023, the Company issued shares of common stock to an investor for $
On
November 8, 2023, the Company issued shares of common stock to an investor for $
On
November 8, 2023, the Company issued shares of common stock to an investor for $
On
November 10, 2023, the Company issued shares of common stock to an investor for $
On
November 13, 2023, the Company issued shares of common stock to an investor for $
On
November 14, 2023, the Company issued shares of common stock to an investor for $
On
December 8, 2023, the Company issued shares of common stock to an investor for $
On
December 11, 2023, the Company issued shares of common stock to an investor for $
On
December 13, 2023, the Company issued shares of common stock to an investor for $
On
December 14, 2023, the Company issued shares of common stock to an investor for $
On
December 20, 2023, the Company issued shares of common stock to an investor for $
On
December 26, 2023, the Company issued shares of common stock to an investor for $
On
January 8, 2024, the Company issued shares of common stock to an investor for $
On
January 16, 2024, the Company issued shares of common stock to an investor for $
On
February 29, 2024, the Company issued shares of common stock to an investor for $
On
March 14, 2024, the Company issued shares of common stock to an investor for $
On
March 26, 2024, the Company issued shares of common stock to an investor for $
Other Stock Issuances
On
June 14, 2023, the Company issued shares of common stock related to the conversion of a note payable for $
On
July 1, 2023, the Company issued shares of common stock related to the conversion of a note payable and accrued interest for $
On
October 16, 2023, the Company issued shares of common stock related to a sale of common stock in the prior year for $
On October 19, 2023, the Company issued shares of common stock, which were a double issuance.
On
December 15, 2023, the Company issued shares of common stock related to the conversion of a note payable for $
On
March 9, 2024, the Company issued shares of common stock related to the conversion of a note payable for $
| F-10 |
Stock Cancellation
On January 16, 2024, a shareholder agreed to return shares of common stock that they received incorrectly in a prior year.
Stock Options and Warrants
During the year ended September 30, 2023, the Company issued warrants for common stock of the Company. The issuance was for the following:
| ● | Services
- warrants for common stock with an exercise price of $ | |
| ● | Services
by related party – warrants for common stock with an exercise price of $ | |
| ● | Settlement
of debt – warrants for common stock with an exercise price of $ | |
| ● | Conversion
of notes payable and accrued interest – warrants for common stock with an exercise price of $ |
During the six months ended March 31, 2024, the Company issued warrants for common stock of the Company. The issuance was for the following:
| ● | Services
– warrants for common stock with an exercise price of $ | |
| ● | Services
by a related party – warrants for common stock with an exercise price of $ | |
| ● | Conversion
of notes payable – warrants for common stock with an exercise price of $ |
NOTE 5 – FEDERAL INCOME TAX
No provision for federal, state or foreign income taxes has been recorded for the six months ended March 31, 2024, and 2023. The Company has incurred net operating losses for all of the periods presented and has not reflected any benefit of such net operating loss carryforwards in the accompanying condensed financial statements due to uncertainty around utilizing these tax attributes within their respective carryforward periods. The Company has recorded a full valuation allowance against all of its deferred tax assets as it is not more likely than not that such assets will be realized in the near future. The Company’s policy is to recognize interest expense and penalties related to income tax matters as income tax expense. For the six months ended March 31, 2024, and 2023, the Company has not recognized any interest or penalties related to income taxes.
NOTE 6 – COMMITMENTS AND CONTINGENCIES
From time to time, the Company may be involved in litigation in the ordinary course of business. The Company is not currently involved in any litigation that we believe could have a material adverse effect on its financial condition or results of operations except as noted.
The Company is currently disputing amounts claimed to be owed to two noteholders, Brent Lilienthal, and Mel Wentz, under state usury laws (see Note 3).
On February 7, 2024, the Company filed suit against Justin Kimbrough and Prosperity Consultants, LLC, in the 14th Judicial District Court for Dallas County, Texas (case no. DC-24-02022), alleging fraud, conversion, unjust enrichment and other causes of action arising from the defendants’ improper receipt of shares of Company common stock under agreements which required the defendants to provide services to the Company and which services the defendants ultimately never provided. The Company is seeking monetary damages and for a constructive trust to be imposed on defendants’ shares of Company common stock and for them to be returned to the Company.
On April 12, 2024, the Company filed suit against Richard Saied, in the 192nd Judicial District Court for Dallas County, Texas (case no. DC-24-05442), alleging fraud, conversion, unjust enrichment and other causes of action arising from the defendant’s improper receipt of shares of Company common stock under an agreement which required the defendant to provide services to the Company and which services the defendant ultimately never provided. The Company is seeking monetary damages and for a constructive trust to be imposed on defendant’s shares of Company common stock and for them to be returned to the Company.
Subscription Agreement and Cash Held in Escrow
On
February 20, 2019, the Company entered into a subscription escrow agreement (the “Trust Agreement”) with Branch Banking
and Trust Company (“BB&T”). This Trust Agreement was established for the subscription agreement proceeds raised and
escrowed pursuant to the Company’s prior Rule 419 S-1 offering. The balance held in trust at March 31, 2024 and September 30,
2023 totaled $
Consulting Agreements
On
October 5, 2023, the Company entered into an Interim CEO & Executive Consultant Agreement (the “Executive Consulting Agreement”)
with Judith S. Miller, pursuant to which Judith S. Miller would serve as the Company’s Interim CEO, and with the Executive Consulting
Agreement intended to be considered effective as of June 20, 2023, the date of Ms. Miller’s original appointment as Interim CEO
of the Company. Under the Executive Consulting Agreement, which can be terminated at any time with or without cause by the Company and
upon 30 days’ advance written notice by Ms. Miller, Ms. Miller will act as the Interim CEO of the Company and, among other management
duties, assist the Company in recruiting a full-time CEO and/or agricultural biotechnology management professional. Following the appointment
of a full-time CEO, Ms. Miller will be retained as an executive consultant for a period of 6 months thereafter. For the six months ended
March 31, 2024, Ms. Miller earned $
| F-11 |
Research and Development Agreement
During September 2020, the Company assumed a Cooperative Research and Development Agreement (“CRADA”) with the United States Department of Agriculture (“USDA”), Agricultural Research Service (“ARS”). Under this agreement, the Company committed to funding the remaining amount due. As of March 31, 2024, there are no balances due.
Settlement Agreement
On
September 28, 2023, the Company entered into a Settlement Agreement with Bradley White, former CEO and director of the Company, who
was terminated on June 20, 2023. As part of the Settlement Agreement, Mr. White was to receive a total settlement of $
NOTE 7 – RELATED PARTY TRANSACTIONS
Consulting Agreement
On
October 5, 2023, the Company entered into an Interim CEO & Executive Consultant Agreement (the “Executive Consulting Agreement”)
with Judith S. Miller, pursuant to which Judith S. Miller would serve as the Company’s Interim CEO, and with the Executive Consulting
Agreement intended to be considered effective as of June 20, 2023, the date of Ms. Miller’s original appointment as Interim CEO
of the Company. Under the Executive Consulting Agreement, which can be terminated at any time with or without cause by the Company and
upon 30 days’ advance written notice by Ms. Miller, Ms. Miller will act as the Interim CEO of the Company and, among other management
duties, assist the Company in recruiting a full-time CEO and/or agricultural biotechnology management professional. Following the appointment
of a full-time CEO, Ms. Miller will be retained as an executive consultant for a period of 6 months thereafter. For the six months ended
March 31, 2024, Ms. Miller earned $
As
of March 31, 2024, Ms. Miller was owed $
Share Issuances to the Board of Directors
The Company issued Series A preferred stock on August 16, 2022, as follows: Bradley White (former Chief Executive Officer), shares; Dr. Clayton Yates (Chief Scientific Officer and Chairman), shares; and Dr. Jesse Jaynes (Chief Research Officer and Director), shares. See Note 4.
On October 19, 2022, the following shareholders converted shares of common stock of the Company into shares of Series B to modify the common shares outstanding to reduce the outstanding common stock issued by the Company, as follows:
| Name | Common Exchanged | Series B Issued | ||||||
| Jaynes Investment LLC (a) | ||||||||
| ACT Holdings LLC (a) | ||||||||
| LASB Family Trust (a) | ||||||||
| Jesse Michael Jaynes (a) | ||||||||
| Bradley White (a) | ||||||||
| PJ Advisory Group | ||||||||
| Total | ||||||||
| (a) |
On September 28, 2023, as part of the Settlement Agreement, Bradley White returned for cancellation shares of Series A preferred stock and shares of Series B preferred stock.
On
January 17, 2024, the Company issued warrants for common stock to Ms. Miller for a contractual milestone. The warrants were valued
at $
On February 16, 2024, the Company issued shares
of common stock valued at $
On March 16, 2024, the Company issued shares
of common stock valued at $
Payables to Related Parties and Share Issuances to Related Parties
Chad Pawlak
As
of March 31, 2024, Mr. Pawlak, the Company’s CEO, is due $
On
January 17, 2024, the Company issued shares of common stock valued at $
| F-12 |
On
February 17, 2024, the Company issued shares of common stock valued at $
On
March 17, 2024, the Company issued shares of common stock valued at $
Judith Miller
As
of March 31, 2024, Ms. Miller was owed $
On
January 16, 2024, the Company issued shares of common stock valued at $
On
February 16, 2024, the Company issued shares of common stock valued at $
On
March 16, 2024, the Company issued shares of common stock valued at $
For
the six months ended March 31, 2024, Ms. Miller has been issued warrants for common stock with an exercise price of $
Robert Bubeck
During
2018, Robert Bubeck, former CEO, paid $
On December 30, 2023, the Company issued Robert Bubeck warrants for common stock.
Settlement Agreement
On
September 28, 2023, the Company entered into a Settlement Agreement with Bradley White, former CEO and director of the Company, who
was terminated on June 20, 2023. As part of the Settlement Agreement, Mr. White was to receive a total settlement of $
Related Party Agreements
On
January 17, 2024, Ms. Miller resigned as the Company’s Interim Chief Executive Officer and was appointed as a member of the Company’s
Board of Directors, as the Chief Business Officer of the Company, and as the Interim Chief Financial Officer of the Company. Pursuant
to the Miller Employment Agreement, which supersedes Ms. Miller’s prior Executive Consulting Agreement with the Company dated June
20, 2023, Ms. Miller will act as Chief Business Officer and Interim Chief Financial Officer of the Company until the agreement is terminated
in accordance with its terms, and Ms. Miller will be compensated as follows:
On
January 17, 2024, the Company executed an advisor agreement with Dr. Jesse Jaynes, a director of the Company (the “Jaynes Advisor
Agreement”). Dr. Jaynes will be compensated as follows:
On
January 17, 2024, the Company executed an advisor agreement with Dr. Clayton Yates, a director of the Company (the “Yates Advisor
Agreement”). Dr. Yates will be compensated as follows:
| F-13 |
On
January 17, 2024, the Company appointed Chad Pawlak as Chief Executive Officer of the Company. Pursuant to the Pawlak Employment
Agreement, Mr. Pawlak will act as Chief Executive Officer of the Company until the agreement is terminated in accordance with its
terms, and Mr. Pawlak will be compensated as follows: (i) Mr. Pawlak will receive a base salary of $
Effective as of January 17, 2024, the Company entered into (i) indemnification agreements with each of its officers and directors, Mr. Pawlak, Ms. Miller, Dr. Jaynes and Dr. Yates (the “Indemnification Agreements”), (ii) an employment agreement with Mr. Pawlak (the “Pawlak Employment Agreement”), (iii) an employment agreement with Ms. Miller (the “Miller Employment Agreement”), (iv) a science advisor agreement with Dr. Jaynes (the “Jaynes Advisor Agreement”), and (v) a science advisor agreement with Dr. Yates (the “Yates Advisor Agreement”).
NOTE 8 – INTELLECTUAL PROPERTIES
The Company was granted a patent (#11083775) on August 10, 2021, by the United States Patent and Trademark Office. The patent was assigned by the inventors to the Company and The United States of America, as represented by the Secretary of Agriculture.
NOTE 9 – SUBSEQUENT EVENTS
The Company has evaluated subsequent events from the condensed consolidated balance sheet through the date of this filing and determined there were no events to disclose except the following.
On April 5, 2024, the Company issued shares
of common stock to an investor for $
On
April 16, 2024, the Company issued shares of common stock to an investor for $
On April 16, 2024, the Company issued shares
of common stock valued at $
On April 17, 2024, the Company issued shares
of common stock valued at $
| F-14 |
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of Rule 175 of the Securities Act of 1933, as amended, and Rule 3b-6 of the Securities Act of 1934, as amended, that involve substantial risks and uncertainties. These forward-looking statements are not historical facts, but rather are based on current expectations, estimates and projections about our industry, our beliefs and our assumptions. Words such as “anticipate,” “expects,” “intends,” “plans,” “believes,” “seeks” and “estimates” and variations of these words and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to risks, uncertainties, and other factors, some of which are beyond our control and difficult to predict and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this Form 10-Q. Investors should carefully consider all of such risks before making an investment decision with respect to the Company’s stock. The following discussion and analysis should be read in conjunction with our financial statements and summary of selected financial data for Genvor Incorporated. Such discussion represents only the best present assessment from our Management.
Company Overview
Genvor Incorporated (the “Company”) was incorporated in Florida on September 26, 2018, as Allure Worldwide, Inc., and as of November 18, 2019, redomiciled to Nevada. On June 24, 2022, the Company changed its name to from Allure Worldwide, Inc. to Genvor Incorporated.
The Company’s subsidiary, Genvor Inc. was incorporated under the laws of the State of Delaware on April 4, 2019, as “Nexion Biosciences Inc.,” and on January 22, 2020, its name was changed to “Genvor Inc.” Genvor Inc. develops plant-based defense technology designed to help farmers achieve global food security.
During May 2019, Genvor Inc. acquired Nexion Biosciences LLC (“NBLLC”) from a founder for nominal consideration. NBLLC was formed in the State of Delaware on December 28, 2018.
The Company was originally formed with the intention of seeking to acquire the assets or shares of an entity actively engaged in business which generates revenues, in exchange for its securities. On January 11, 2021, the Company entered into an Exchange Agreement (the “Purchase Agreement”) with Genvor Inc., a Delaware corporation (“Genvor”) to acquire (the “Acquisition”) Genvor. On March 2, 2022, the Company and Genvor entered into a merger agreement (the “Merger Agreement”) to consummate the Acquisition, and pursuant to which a wholly-owned subsidiary of the Company, Genvor Acquisition Corp., a Delaware corporation, would merge (the “Merger”) with and into Genvor, with each share of Genvor common stock issued immediately prior to the time of the merger automatically converted into the right to receive one share of common stock of the Company.
On May 27, 2022, the Acquisition closed, Merger Subsidiary merged with and into Genvor, each share of Genvor was exchanged for the right to receive one share of Company common stock, 35,261,871 shares of Company common stock were issued to Genvor’s pre-merger shareholders (the “Merger Shares”), constituting a change of control of the Company, and Genvor became a wholly owned subsidiary of the Company. As a result of these transactions, the Company had 55,261,871 issued and outstanding common shares upon the closing of the share exchange with Genvor, and subsequently the Company’s original founding shareholders cancelled 18,144,112 shares of Company common stock in connection with the Acquisition.
As a result of the Acquisition, the Company’s business plan is that Genvor will be continuing its research and development addressing plant-based defense technology ich then can be commercialized to help farmers and growers globally to overcome potentially catastrophic losses resulting from plant disease, toxins, bacteria, and fungi that destroy their crops. These solutions can result in greater crop yields and economic savings, which can assist in overcoming world-wide food scarcity.
The Company’s technology was developed by two university scientists, Dr. Clayton Yates, and Dr. Jesse Jaynes, who shared a mission to develop crop protection technology designed to defend against crop diseases affecting both animals and humans alike. The Company’s technology is currently being advanced by the USDA in corn seed varieties and with U.S. Sugar in citrus trees.
The Company’s headquarters is located at 201 S. Elliott Road, Suite 538, Chapel Hill, NC. 27514.
The following Management Discussion and Analysis should be read in conjunction with the financial statements and accompanying notes included in this Form 10-Q.
| 4 |
Genvor’s Operations
Overview
Genvor’s technology was developed by two university scientists, Dr. Clayton Yates, and Dr. Jesse Jaynes, who share a common mission to develop crop protection technology to defend against deadly crop diseases, which ultimately impact both animals and humans alike. Genvor’s technology is currently being advanced by the USDA in corn seed varieties.
Dr. Jaynes has, over decades, perfected techniques for synthesizing and modifying anti-microbial peptides (“AMPs”).1
Genvor is a research and development (“R&D”) company, advancing the next generation of sustainable plant health solutions, which are designed to fight debilitating and deadly plant diseases, such as bacteria and fungi, in a broad spectrum of crop types through its proprietary library of patented anti-microbial peptides, or AMPs. These AMPs can be applied to plants in the form of non-chemical seed traits (“Transgenic” seed traits) – which imbue seeds with the desired traits, allowing plants to naturally produce Genvor’s AMPs – as well as a topical spray (“Bio-pesticide”) application. The AMPs are intended to help farmers worldwide achieve healthier and more productive crops, boosting yields and overcoming potentially catastrophic losses.
With critical global crop losses directly caused by plant disease exceeding $220 billion annually,2 solutions utilizing peptide technology are actively being evaluated worldwide. Genvor’s proprietary library of patented peptides, utilizing only naturally occurring substances, has been proven to enhance a plant’s ability to defend itself. In addition to crop disease defense, Genvor has created nutritional enhancement peptides (“NEPs”) which increase protein output by 5-10x in poultry and swine feed, such as corn or sweet potato, intended to deliver higher economic yields for farmers. Genvor’s approach does not use any harsh chemicals, which upon adoption should lead to reduced greenhouse gas emissions while conserving topsoil as compared to traditional petrochemical-based solutions. These technologies should translate into substantial savings for potential customers, while concurrently providing a more sustainable solution for organic or environmentally conscious growers.
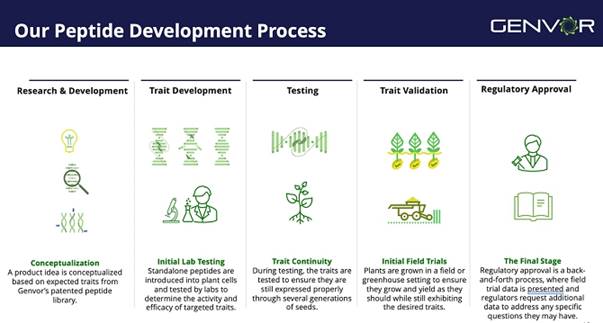
Below is a summary of Genvor’s peptide development process.
1 See, e.g., Rajasekaran, K. Jaynes, J.M. and Cary, J.W. (2009) Transgenic Expression of Lytic Peptides in Food and Feed Crops to Control Phytopathogens and pre-harvest Mycotoxin Contamination. In: Mycotoxin Prevention and Control in Agriculture, Chapter 9, pp 119-142. American Chemical Society Symposium Vol. 1031.
2 https://www.fao.org/news/story/en/item/1402920/icode/.
| 5 |
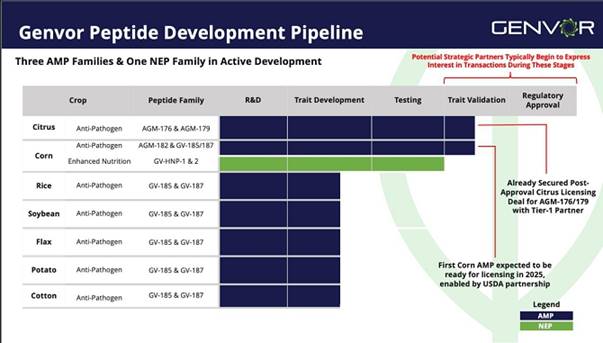
Commercialization of seed traits for corn, soy and cotton is among the Company’s immediate goals, while simultaneously developing an effective topical spray, and continued research into the nutritional enhancement of feed for swine and poultry. Genvor protects its comprehensive library of 24 proprietary peptides with two issued United States patents, and a third patent pending covering an additional 16 peptides. The following chart describes Genvor’s peptide development pipeline, showing the relative stages of different peptides and crop applications:
Addressing a $220 Billion Global Economic and Health Risk with Proven Solutions
Food production must double by 2050 to meet the food demand of the global population’s expected growth to 9.6 billion people. Annual crop losses due to plant pathogens and viruses are now estimated to exceed $220 billion globally.3 Alarming to the United States Food and Drug Administration (“FDA”) is the fungi “Aspergillus Flavus,” which produces Aflatoxins, a toxic carcinogenic compound known to cause liver cancer in humans and animals. Humans infected with Aspergillus Flavus often have reduced or compromised immune systems. Contamination of corn with acutely toxic and carcinogenic aflatoxin is a major human and livestock health risk, estimated to cost the U.S. corn industry between $52 million and $1.7 billion annually.4
The below image shows an infected crop leaf, a common sight for farmers facing these types of crop contamination issues:

Aflatoxin contamination causes market rejection of infected crops, as well as animal and human health impacts. The USDA has imposed strict guidelines for crop inspection and discovery of diseased crops caused by Aflatoxins. Both planted fields and harvested crops found to be contaminated exceeding permitted testing levels must be destroyed, at a loss to the farmer, who is often exposed to catastrophic economic losses as a result. The guidelines in the European Union are stricter than in the United States, creating a critical market need for sustainable and effective plant health solutions to combat such plant disease.5
Industry observers have noted that seed trait expression of AMPs is a promising approach to providing resistance to aflatoxin infection in corn.6 Testing of Genvor’s AMPs by the US Department of Agriculture over a period of six years, the results of which were published in May 2018 and March 2023, showed promising results in defense against aflatoxins, as seeds infused with Genvor AMPs showed a 70% reduction in aflatoxin contamination, making them promising candidates for genetic engineering the next-generation of disease-resistant crops.7
Application of Peptides in Plant Protection
Plant pathogens attack crops and lead to serious adverse impacts on their growth. Traditional chemical fungicides are effective in preventing diseases caused by plant pathogens; however, their long-term continuous use has led to plant pathogens developing resistance to those fungicides, and their residues present a risk of harm to humans and the environment. Industry observers believe that more sustainable methods to control plant diseases are urgently needed.8 Naturally occurring AMPs mediate the innate host defense and can be used as immune inducers. Given their high specificity, rapid degradation, and efficacy, AMPs are expected to be a promising first line of defense against fungi, viruses, and bacteria. Some industry observers believe that peptides will likely become mainstream tools for plant protection in the future.9
3 Id.
4 Broad-Spectrum Antimicrobial Activity of Synthetic Peptides GV185 and GV187, Rebecca R. Sweany, Jeffrey W. Cary, Jesse M. Jaynes and Kanniah Rajasekaran, March 20, 2023.
5 Id.
6 Science Direct – Advanced Agrochem: Volume 2, Issue 1, March 2023, Pages 58-78, Peptides, new tools for plant protection in eco-agriculture. Yi-Meng Zhang, De-Xing Ye, Yan Liu, Xin-Yuan Zhang, Yuan-Lin Zhou, Li Zhang, Xin-Ling Yang.
7 Broad-Spectrum Antimicrobial Activity of Synthetic Peptides GV185 and GV187, Rebecca R. Sweany, Jeffrey W. Cary, Jesse M. Jaynes and Kanniah Rajasekaran, March 20, 2023.
8 Donley N. The USA lags behind other agricultural nations in banning harmful pesticides. Environ Health Glob Access Sci Source. 2019;18(1):44.
9 Science Direct – Advanced Agrochem: Volume 2, Issue 1, March 2023, Pages 58-78, Peptides, new tools for plant protection in eco-agriculture. Yi-Meng Zhang, De-Xing Ye, Yan Liu, Xin-Yuan Zhang, Yuan-Lin Zhou, Li Zhang, Xin-Ling Yang.
| 6 |
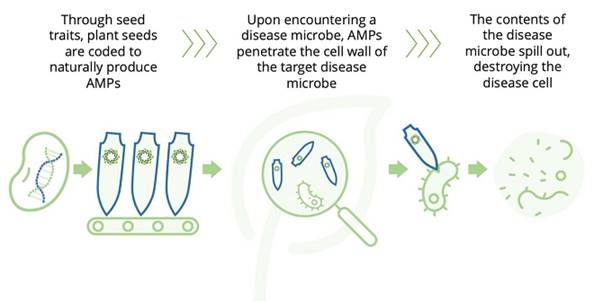
The following is an illustration that shows the mode of action of Genvor’s peptides against a disease cell:
Genvor’s AMP Technology has Created Significant Economic Opportunities in a Broad Spectrum of Plant Types
The Aflatoxin problem has created significant opportunities for companies developing the technology needed to defend against Aflatoxins. AMPs, such as those developed by Genvor, are a safer alternative to fungicides. Pesticides or fungicides with a chemical composition are known to degrade the environment, are inefficient to apply, persist in crops and livestock, and are less effective as plant pathogen resistance increases. AMPs kill microorganisms directly, resistance to them is rare and should remain so given their mode of action, and AMPs can be manufactured via a non-GMO process.
Genvor’s peptides have proven effective on corn and show broad spectrum effectiveness for other crop types10 for most known bacteria and fungi within a single product solution. This broad-spectrum efficacy should be more efficient for farmers compared to the toxic fungicide alternatives, which are currently used to combat a single fungi or bacteria type. Genvor’s technology can be delivered into plants by both bioengineered seed traits, as well as through bio-fungicide (topical spray) application.11 There is no evidence of pathogens developing resistance to a Genvor’s designed AMPs. At this point in time, AMPs show a likelihood of killing pathogens in berries, corn, cannabis, wheat, cotton, citrus, and peanuts.
What are Peptides Exactly?
Peptides are broadly utilized in medicine, cosmetics, healthcare products, animal nutrition and health, and plant nutrition and protection. In recent years, they’ve become active research subjects to protect plants from bacteria, viruses, pests, and weeds, as antimicrobial and immune inducers, plant growth regulators, insecticides, and herbicides. This is due to their extensive raw material sources, excellent activity, and ideal environmental compatibility.
Peptides are short-chain biomolecules of between 2 and 50 amino acids, linked by peptide bonds. Based on their sources, peptides can be categorized as natural or artificially synthesized. Most natural peptides are from animals, plants, and microorganisms. Both natural and synthetic peptides can be produced through chemical synthesis, biological fermentation, gene recombination and other methods. Peptides are ubiquitous in living organisms and modulate many physiological processes, making them a common research subject in medicine, cosmetics, and agriculture.12
How do Peptides Work Against Diseases?
Through seed traits, plant seeds are genetically coded to produce AMPs. Upon encountering a disease microbe, AMPs penetrate the cell wall of the target disease microbe, causing the contents of the disease microbe to spill out, destroying the disease cell. The same effect is foreseen in the utilization of the topical spray.
Delivery by Transgenic Seed Traits
In North America, the seed trait market is currently estimated to exceed $10 billion. The adoption of crop seeds with enhanced traits has been staggering with over 75% of U.S. corn, cotton and soybeans being produced using seeds with enhanced traits.13
10 Broad-Spectrum Antimicrobial Activity of Synthetic Peptides GV185 and GV187, Rebecca R. Sweany, Jeffrey W. Cary, Jesse M. Jaynes and Kanniah Rajasekaran, March 20, 2023.
11 Id.
12 Science Direct – Advanced Agrochem: Volume 2, Issue 1, March 2023, Pages 58-78, Peptides, new tools for plant protection in eco-agriculture. Yi-Meng Zhang, De-Xing Ye, Yan Liu, Xin-Yuan Zhang, Yuan-Lin Zhou, Li Zhang, Xin-Ling Yang.
13 https://www.fortunebusinessinsights.com/industry-reports/genetically-modified-seeds-market-100389.
| 7 |
The following image shows corn with Genvor’s seed traits, which is currently growing in a USDA facility for study:

Corn with Genvor Seed Traits Growing in USDA Facility for Study
Delivery by Topical Spray (Bio-fungicide)
The rising global demand for organic foods, the trend in the reduction of chemical residues, stricter import and supermarket standards, shorter pre-harvest intervals, a push for sustainability, growing food scarcity, the phase out of synthetic agricultural chemicals, along with the market’s demand for additional modes of action to manage resistance, has resulted in biologicals being one of the fastest-growing sectors in the crop protection market, increasing at twice the compound annual growth rate of the crop protection market as a whole.14 The global agricultural biologicals market was approximately $9.5 billion in 2019 and is expected to grow to approximately $19.7 billion by 2026.15 Unlike synthetic chemicals, bio-fungicides are derived from configurations of amino acids and proteins occurring in nature; therefore they do not contaminate soil, water, turf, beneficial insects, birds, fish, and non-targeted plants. 16
USDA Partnership – Benefits of a CRADA
Genvor’s AMPs gained the attention of the USDA in its pursuit of solutions for plant disease in corn, the U.S.’s largest crop. Based on over 30 years of research by Genvor founder Dr. Jesse Jaynes, Genvor was awarded a Cooperative Research and Development Agreement (“CRADA”) in 2018 to develop and commercialize disease resistance and nutritional enhancement in corn seed based upon Genvor’s proven technology. A CRADA expands expertise and speeds development of many technologies that are now used by farmers or found in the grocery store through access to USDA resources such as advanced laboratories, increasing the chances that research outcomes are adopted commercially to maximize impact, driving a significantly lower overhead rate than a university’s research program, and creating a multi-disciplinary research team to increase technical breadth and depth of the lab. In addition, a CRADA provides access to the USDA regulatory team for registrations and processes.
Genvor’s Peptides are proven effective in corn seed traits.
As a result of the USDA and Genvor partnership through the CRADA, the effectiveness of Genvor’s solutions in protecting against Aflatoxins in corn seed has been established, with the probability that this technology can effectively be applied to other crop types.17 Genvor is expecting an extension of the CRADA to continue the studies of Genvor’s 4th generation of its peptides (GNV-185 and GNV-187) towards commercialization. The Company now has the technology to move its superior AMPs from the research lab and greenhouse to the field, with the goal of commercially producing Genvor’s AMPs at a desirable price point for cost-effective and broad-based agricultural use.
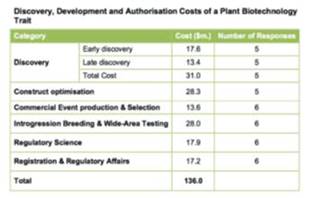
What is a USDA CRADA Worth to Genvor?
In the United States, it often takes eight years and $136 million to develop and bring a new seed trait through regulatory to the marketplace.18 The value of a CRADA, utilizing the USDA’s existing labs, know-how, research, fields, greenhouses, and oversight to develop a Seed Trait for corn, is significant as it allows Genvor to bring seed traits to market with substantially less capital investment. For its CRADA in corn, Genvor’s contribution was under $700,000, primarily for a dedicated scientist to work onsite at the USDA facility over the years. Genvor is expecting to continue R&D of its anti-microbial and nutritional enhancement peptides (“NEPs”) for poultry and swine feed through additional CRADAs with the USDA. In addition, Genvor is planning to apply for a CRADA for Aflatoxins in peanuts. The USDA partnership is based upon the confidence and proof of concept found in Genvor’s solutions.
14 https://www.researchandmarkets.com/reports/5317983/agricultural-biologicals-market-forecasts-from.
15 Id.
16 Id
17 Broad-Spectrum Antimicrobial Activity of Synthetic Peptides GV185 and GV187, Rebecca R. Sweany, Jeffrey W. Cary, Jesse M. Jaynes and Kanniah Rajasekaran, March 20, 2023.
18 https://geneticliteracyproject.org/gmo-faq/what-does-it-take-to-bring-a-new-gm-product-to-market/.
| 8 |
Below is a chart showing the average surveyed development costs of bringing a new plant trait to market in the United States:
Business Strategy: Licensing First and Leveraging R&D with Third Parties
Genvor’s business model is to remain capital-light, focused on leveraging its third-party research and development through its initial CRADA with the USDA, and any subsequent CRADAs that may be granted, while also adopting a licensing approach to reduce “cash burn” or the need for significant manufacturing and marketing overhead. Genvor is highly scalable and capital efficient with minimal overhead relative to peers, allowing the Company to focus on core research and development competency. Genvor does not expect revenue until the end of 2025, through the licensing and distribution of either or both the topical spray and seed trait, utilizing licensing and royalty opportunities.
Citrus Licensing with Royalty
Genvor has established an initial licensing and royalty agreement with Southern Gardens Citrus, a division of U.S. Sugar, operating 6,600 acres of orange groves in Florida, utilizing Genvor’s AGM-176 and AGM-179 peptides, proving the effectiveness of these peptides on the crippling problems of canker on citrus crops. While 5+ years of studies showed the effectiveness of Genvor’s AGM-176 and AGM-179, Southern Gardens has not yet taken the usage of these solutions through regulatory approval for commercialization, which should generate royalty revenue to Genvor following receipt of such approval.
Discussions with Additional Third-Party Crop Growers
Genvor is in discussions with other major growers of other crop types regarding the potential funding of Genvor’s costs of research and development through to seed traits, topical spray, or both – all as a solution to fight toxins, bacteria or fungi that damage various spectrums of crop types. A licensing and royalty agreement with those growers are likely outcomes of any R&D funding agreement.
Financing Needs
Genvor is currently exploring financing options to generate the cash needed to pay for overhead, marketing, and production of peptides to be used in R&D, contributions to the CRADA and regulatory costs to take existing technology through the process. Additional financing may be required in the event self-funded R&D is needed. There are no plans currently to create Genvor’s own labs, greenhouses or fields or its research with the availability of utilizing existing labs, scientific and regulatory professionals – chiefly through its CRADA with the USDA.
Competition
Below are some of Genvor’s agriscience competitors.
Invaio Sciences - Peptide maSAMP (https://www.invaio.com/) is used to control citrus Huanglongbing, a destructive disease.
Plant Health Care - PREtec technology (https://www.planthealthcare.com/), from the American Plant Health Care (PHC) company, was patented in the United States in 2019. Its unique immune-inducing peptides and its mixtures with other products have been recognized for strengthening plant resistance to disease and stress, as well as promoting plant growth. All PREtec peptides are variants of natural proteins and break down rapidly in the environment, leaving no harmful residues on the crop or in the environment. In 2021, PHC launched PHC279 with PREtec technology in Brazil and sold it under the name of Saori™. This product is used as a seed treatment to prevent Asian soybean rust.
PHYTOTECH LABS FLG22 (https://phytotechlab.com/), from Phytotech, induces the natural immune response. Its sequence was derived from the highly conserved N-terminal region of Pseudomonas aeruginosa flagellin. FLG22 and its derivatives induce defense responses in Lycopersicon esculentum and Arabidopsis thaliana and have elicitor activity. Many immune induction peptides are in development.
CEV, SA (CEV) (https://cev.com.pt/ established in 2006 with the objective of developing a new patented protein-based bio-fungicide. The active ingredient is a polypeptide, named BLAD, which is extracted from the germinated seeds of lupines. BLAD’s multi-site targets cell walls, cell membrane and cell metabolism.
Syngenta Global https://www.syngenta.com/en Syngenta is a world market leader in crop protection products, developing and producing herbicides, insecticides, fungicides and seed treatment products for farmers and growers.
Hello Nature - https://www.hello-nature.com/us/ offers a range of biotechnological and certified solutions for modern and sustainable agriculture including natural bio-stimulants of vegetal origin, beneficial microbials and organic fertilizers.
| 9 |
Sym Agro - https://sym-agro.com/ serves horticultural and agricultural specialty markets with an assortment of fertilizers, fungicides, biologics, and pesticides. ProBlad® Verde fungicide is available in the US for use on a variety of crops, including stone fruit, cane berries, strawberries, pome fruits, grapes, almonds, leafy greens, herbs, and tomatoes.
Innatrix – Products under development in their pipeline: InnaLB™ (Potato Late Blight) - Peptide product to stop late blight infection, by interfering with critical late blight effectors, InnaNema™ (Soybean Cyst Nematode) Seed treatment product to stop nematode infection and reproduction process on soybean roots, by RNAi technology, and nnaHLB™ (Citrus Greening) Peptide product to stop citrus greening infection by interfering with critical citrus greening effector.
Recent Activity
The Company has actively participated in two prominent biotechnology conferences and one significant industry event this quarter. Moreover, productive engagements with USDA partners have enhanced our ongoing multi-year studies on seed traits in corn, focusing on combatting a wide array of pathogens, including aflatoxin. The company has reached an agreement to broaden its research collaboration with the USDA, encompassing barley studies at its Midwest research facility.
Our outreach efforts extended to major agricultural enterprises, facilitating discussions on potential partnership opportunities to bring Genvor’s peptide portfolio to market. Multiple product formats are currently under evaluation and development, with our steadfast commitment to the license-first business model. The company is collaborating with several contract manufacturing firms to develop efficient and cost-effective fermentation systems. This initiative aims to meet the manufacturing requirements of commercial partnerships and ensure the ability to offer economically viable pricing that aligns with market expectations in the global agricultural sector.
Exploration of international animal health research collaborations is underway, including discussions with a leading animal health research company. Concurrently, we are intensifying efforts in non-GMO product development and expediting the innovation of novel peptides.
Reports to Security Holders
The Company is required to file reports pursuant to Section 15(d) of the Securities Exchange Act of 1934 and is required to furnish its stockholders with annual reports containing consolidated financial statements audited by its independent registered public accounting firm and to make available quarterly reports containing unaudited condensed consolidated financial statements for each of the first three quarters of each fiscal year ending September 30th. The Company files Quarterly Reports on Form 10-Q, Annual Reports on Form 10-K and Current Reports on Form 8-K with the Securities and Exchange Commission. The Company may also file additional documents with the Commission if those documents become necessary in the course of its operations.
Available Information
All reports of the Company filed with the SEC are available free of charge through the SEC’s website at www.sec.gov. In addition, the public may read and copy materials filed by the Company at the SEC’s Public Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. The public may also obtain additional information on the operation of the Public Reference Room by calling the Commission at 1-800-SEC-0330.
Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the condensed consolidated financial statements and notes thereto for the three and six months ended March 31, 2024, and 2023, and related management discussion herein.
Our financial statements are stated in U.S. Dollars and are prepared in accordance with generally accepted accounting principles of the United States (“U.S. GAAP”).
Going Concern Qualification
Several conditions and events cast substantial doubt about the Company’s ability to continue as a going concern. The Company has incurred cumulative net losses of $19,842,789 from its inception to March 31, 2024, and requires capital for its contemplated operational and marketing activities to take place. The Company’s ability to raise additional capital through debt or future issuances of capital stock is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. The ability to successfully resolve these factors raises substantial doubt about the Company’s ability to continue as a going concern.
For the Three Months Ended March 31, 2024, and 2023
Revenues
We did not earn any revenues during the three months ended March 31, 2024, and 2023.
Operating Loss
During the three months ended March 31, 2024, and 2023, the Company had an operating loss of $747,400 and $155,282, respectively. For the three months March 31, 2024, the primary expenses were stock-based compensation of $406,250 and professional fees of $177,453.
Net Loss
The Company incurred a net loss of $772,055 and $221,212, respectively, during the three months ended March 31, 2024, and 2023, primarily as a result of the increasing operating loss described previously.
| 10 |
For the Six Months Ended March 31, 2024, and 2023
Revenues
We did not earn any revenues during the six months ended March 31, 2024, and 2023.
Operating Loss
During the six months ended March 31, 2024, and 2023, the Company had an operating loss of $2,052,477 and $268,944, respectively. For the six months March 31, 2024, the primary expenses were stock-based compensation of $1,313,350 and professional fees of $445,039.
Net Loss
The Company incurred a net loss of $2,123,482 and $400,831, respectively, during the six months ended March 31, 2024, and 2023, primarily as a result of the increasing operating loss described previously.
Liquidity and Capital Resources
Based upon our current financial condition, we do not have sufficient cash to operate our business at the current level for the next twelve months. We intend to fund operations through debt and/or equity financing arrangements, which may be insufficient to fund expenditures or other cash requirements. We plan to seek additional financing in a private equity offering to secure funding for operations. There can be no assurance that we will be successful in raising additional funding. If we are not able to secure additional funding, the implementation of our business plan will be impaired. There can be no assurance that such additional financing will be available to us on acceptable terms or at all.
Working Capital
Cash Flow
We fund our operations with cash received from advances from officers and related parties and issuances of equity or notes payable.
Cash Flows from Operating Activities
For the three months ended March 31, 2024, as compared to the three months ended March 31, 2023, cash used in operating expenses increased due to payments of accounts payable, as well as due to an increase in professional fees.
Cash Flows from Investing Activities
For the three months ended March 31, 2024, no cashflows were provided by or used in investing activities.
Cash Flows from Financing Activities
For the three months ended March 31, 2024, cash was raised through the sale of common stock.
Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on the Company’s financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not Applicable.
ITEM 4. CONTROLS AND PROCEDURES.
Disclosure Controls and Procedures
The Securities and Exchange Commission defines the term “disclosure controls and procedures” to mean the company’s controls and other procedures of an issuer that are designed to ensure that information required to be disclosed in the reports that it files or submits under the Securities Exchange Act of 1934 (the “Exchange Act”) is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Securities Exchange Act of 1934 is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. The Company maintains such a simple system of controls and procedures in an effort to ensure that all information which it is required to disclose in the reports it files under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified under the SEC’s rules and forms and that information required to be disclosed is accumulated and communicated to principal executive and principal financial officers to allow timely decisions regarding disclosure.
As of the end of the period covered by this report, we carried out an evaluation, under the supervision and with the participation of our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures. Based on this evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were not effective to provide reasonable assurance of achieving the objectives of timely alerting them to material information required to be included in our periodic SEC reports and of ensuring that such information is recorded, processed, summarized, and reported with the time periods specified. Our chief executive officer and chief financial officer also concluded that our disclosure controls and procedures were not effective as of the end of the period covered by this report to provide reasonable assurance of the achievement of these objectives.
Changes in Internal Control over Financial Reporting
There were no changes in the Company’s internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Rule 13a-15 or 15d-15 of the Exchange Act that occurred during the quarter ended March 31, 2024, that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
| 11 |
PART II - OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS.
The Company is not a party to any significant pending legal proceedings other than as disclosed below, and no other such proceedings are known to be contemplated. No director, officer or affiliate of the Company, and no owner of record or beneficial owner of more than 5.0% of the securities of the Company, or any associate of any such director, officer or security holder is a party adverse to the Company or has a material interest adverse to the Company in reference to pending litigation.
On February 7, 2024, the Company filed suit against Justin Kimbrough and Prosperity Consultants, LLC, in the 14th Judicial District Court for Dallas County, Texas (case no. DC-24-02022), alleging fraud, conversion, unjust enrichment and other causes of action arising from the defendants’ improper receipt of shares of Company common stock under agreements which required the defendants to provide services to the Company and which services the defendants ultimately never provided. The Company is seeking monetary damages and for a constructive trust to be imposed on defendants’ shares of Company common stock and for them to be returned to the Company.
On April 12, 2024, the Company filed suit against Richard Saied, in the 192nd Judicial District Court for Dallas County, Texas (case no. DC-24-05442), alleging fraud, conversion, unjust enrichment and other causes of action arising from the defendant’s improper receipt of shares of Company common stock under an agreement which required the defendant to provide services to the Company and which services the defendant ultimately never provided. The Company is seeking monetary damages and for a constructive trust to be imposed on defendant’s shares of Company common stock and for them to be returned to the Company.
ITEM 1A. RISK FACTORS.
Not applicable.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
On January 1, 2024, the Company issued 3,750 shares of common stock for services valued at $15,000.
On January 8, 2024, the Company issued 8,000 shares of common stock to an investor for $8,000.
On January 16, 2024, the Company issued 115,000 shares of common stock to an investor for $115,000.
On January 16, 2024, the Company issued 25,000 shares of common stock valued at $25,000 to Good Works Funding, LLC, an entity controlled by Judith Miller, the CBO, and a director of the Company, for services as defined in her employment agreement.
On January 17, 2024, the Company issued 50,000 shares of common stock valued at $50,000 to Chad Pawlak, the CEO of the Company, for services as defined in his employment agreement.
On January 17, 2024, the Company issued 25,000 shares of common stock for services valued at $25,000.
On February 2, 2024, the Company issued 1,250 shares of common stock for services valued at $1,250.
On February 5, 2024, the Company issued 6,250 shares of common stock for services valued at $6,250.
On February 16, 2024, the Company issued 25,000 shares of common stock valued at $25,000 to Good Works Funding, LLC, an entity controlled by Judith Miller, the CBO, and a director of the Company, for services as defined in her employment agreement.
On February 17, 2024, the Company issued 25,000 shares of common stock valued at $25,000 to Chad Pawlak, the CEO of the Company, for services as defined in his employment agreement.
On February 29, 2024, the Company issued 50,000 shares of common stock to an investor for $50,000.
On March 2, 2024, the Company issued 1,429 shares of common stock for services valued at $1,429.
On March 5, 2024, the Company issued 13,393 shares of common stock for services valued at $13,393.
On March 11, 2024, the Company issued 25,000 shares of common stock for services valued at $25,000.
On March 14, 2024, the Company issued 50,000 shares of common stock to an investor for $50,000,
On March 16, 2024, the Company issued 25,000 shares of common stock valued at $25,000 to Good Works Funding, LLC, an entity controlled by Judith Miller, the CBO, and a director of the Company, for services as defined in her employment agreement.
On March 17, 2024, the Company issued 25,000 shares of common stock valued at $25,000 to Chad Pawlak, the CEO of the Company, for services as defined in his employment agreement.
On April 5, 2024, the Company issued 10,000 shares of common stock to an investor for $10,000.
On April 16, 2024, the Company issued 50,000 shares of common stock to an investor for $50,000.
On April 16, 2024, the Company issued 25,000 shares of common stock valued at $25,000 to Good Works Funding, LLC, an entity controlled by Judith Miller, the CBO and a director of the Company, for services as defined in her employment agreement.
On April 17, 2024, the Company issued 25,000 shares of common stock valued at $25,000 to Chad Pawlak, the CEO of the Company, for services as defined in his employment agreement.
| 12 |
On March 26, 2024, the Company issued 25,000 shares of common stock to an investor for $25,000.
During the six months ended March 31, 2024, the Company issued 1,192,800 warrants to purchase common stock of the Company. The issuances were for the following:
| ● | Services – 392,800 warrants for common stock with an exercise price of $0.001, valued at $392,800. | |
| ● | Services by a related party – 600,000 warrants for common stock with an exercise price of $0.001, valued at $600,000. | |
| ● | Conversion of notes payable – 300,000 warrants for common stock with an exercise price of $0.001, valued at $300,000. |
On April 15, 2024, the Company issued 50,000 shares of common stock to an investor for $50,000,
The forgoing securities were issued in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities Act of 1933, as amended, and Rule 506(b) promulgated thereunder, as there was no general solicitation, and the transactions did not involve a public offering.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES.
None.
ITEM 4. MINE SAFETY DISCLOSURES.
None.
ITEM 5. OTHER INFORMATION.
None.
| 13 |
ITEM 6. EXHIBITS.
* Certain schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule or exhibit will be furnished supplementally to the Securities and Exchange Commission upon request; provided, however that the Company may request confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, for any schedule or Exhibit so furnished.
** Filed herewith.
*** XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections.
| 14 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| GENVOR INCORPORATED | ||
| Date: May 13, 2024 | By: | /s/ Chad Pawlak |
| Chad Pawlak | ||
| Chief Executive Officer, | ||
| Date: May 13, 2024 | By: | /s/ Judith S. Miller |
| Judith S. Miller | ||
| Interim Chief Financial Officer |
| 15 |