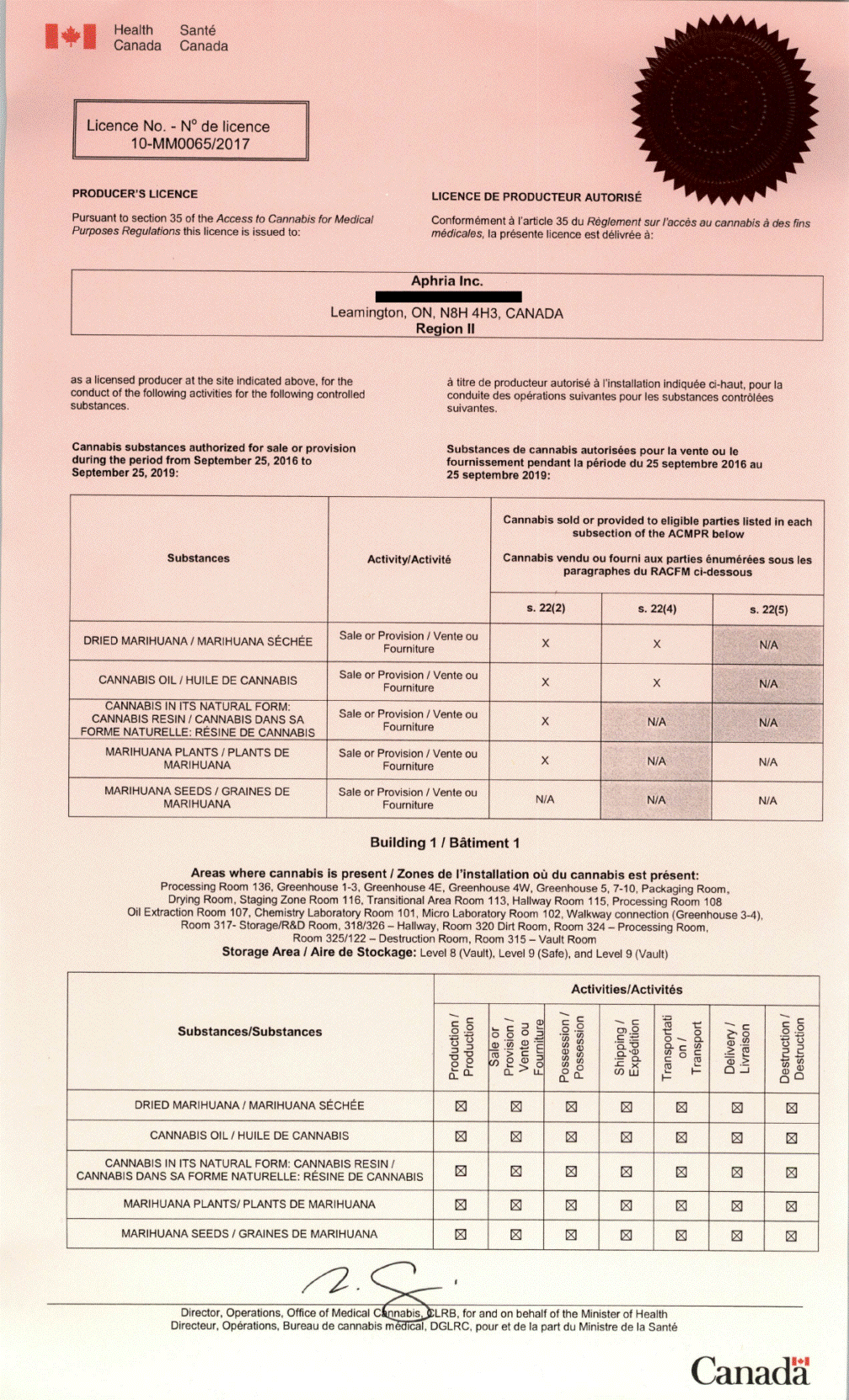
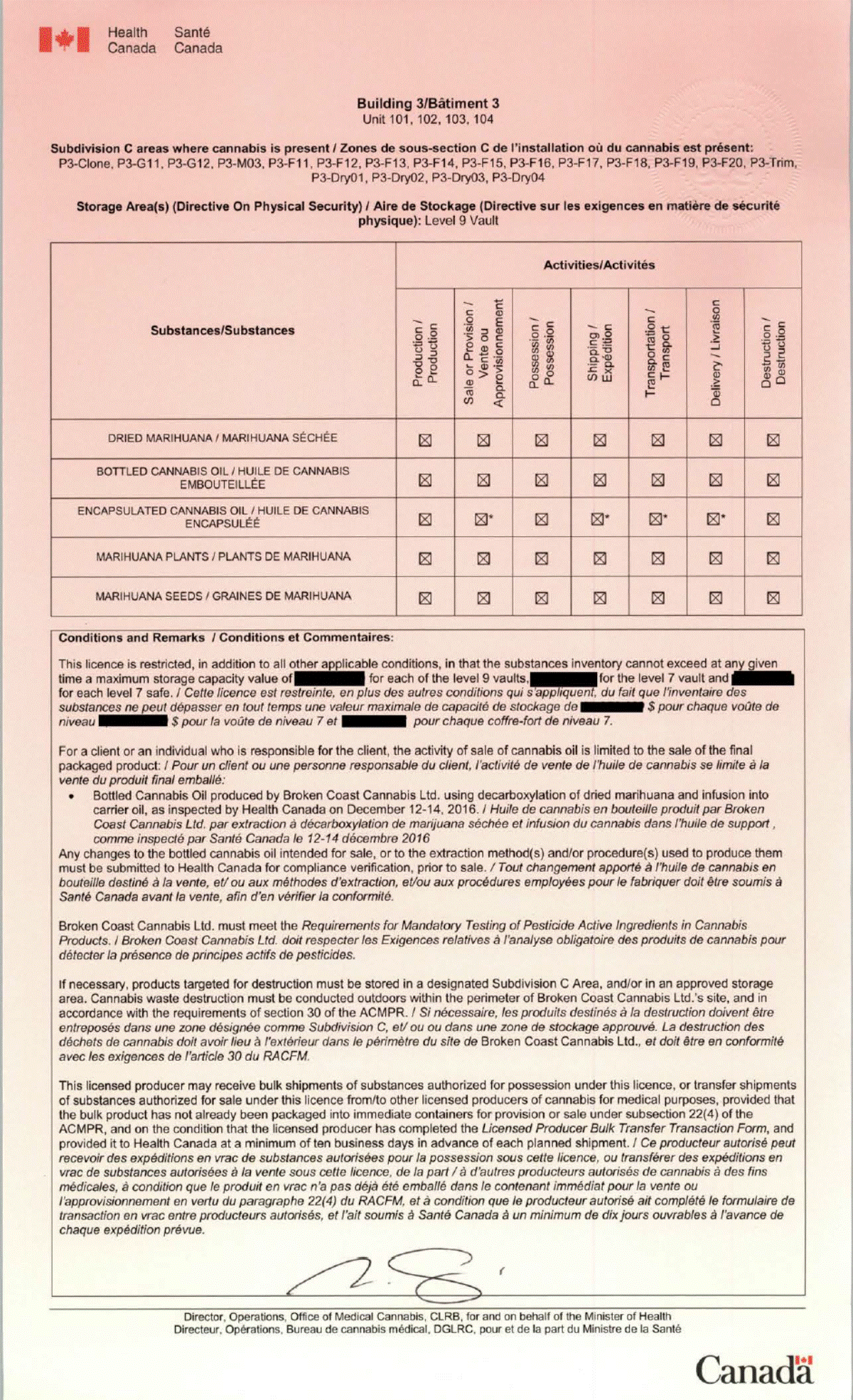
Aphria Inc.
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED MAY 31, 2018 AND MAY 31, 2017
(Expressed in Canadian Dollars, unless otherwise noted)

July 31, 2018
Independent Auditor’s Report
To the Shareholders of
Aphria Inc.
We have audited the accompanying consolidated financial statements of Aphria Inc. and its subsidiaries, which comprise the consolidated statements of financial position as at May 31, 2018 and May 31, 2017 and the consolidated statements of income and comprehensive income, changes in equity and cash flows for the years then ended, and the related notes, which comprise a summary of significant accounting policies and other explanatory information.
Management’s responsibility for the consolidated financial statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards. Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a basis for our audit opinion.
PricewaterhouseCoopers LLP
245 Ouellette Avenue, Suite 300, Windsor, Ontario, Canada N9A 7J4
T: +1 519 985 8900, F: +1 519 258 5457
“PwC” refers to PricewaterhouseCoopers LLP, an Ontario limited liability partnership.
Opinion
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Aphria Inc. and its subsidiaries as at May 31, 2018 and May 31, 2017 and their financial performance and their cash flows for the years then ended in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board.
(Signed) “PricewaterhouseCoopers LLP”
Chartered Professional Accountants, Licensed Public Accountants
Windsor, Ontario, Canada
Aphria Inc.
Consolidated Statements of Financial Position
(In thousands of Canadian dollars)
|
|
|
|
|
May 31, |
|
May 31, |
| ||
|
|
|
Note |
|
2018 |
|
2017 |
| ||
|
Assets |
|
|
|
|
|
|
| ||
|
Current assets |
|
|
|
|
|
|
| ||
|
Cash and cash equivalents |
|
|
|
$ |
59,737 |
|
$ |
79,910 |
|
|
Marketable securities |
|
4 |
|
45,062 |
|
87,347 |
| ||
|
Accounts receivable |
|
|
|
3,386 |
|
826 |
| ||
|
Other current assets |
|
5 |
|
14,384 |
|
5,571 |
| ||
|
Inventory |
|
6 |
|
22,150 |
|
3,887 |
| ||
|
Biological assets |
|
7 |
|
7,331 |
|
1,408 |
| ||
|
Due from related parties |
|
8 |
|
— |
|
464 |
| ||
|
Assets held for sale |
|
13 |
|
40,620 |
|
— |
| ||
|
Current portion of convertible notes receivable |
|
12 |
|
1,942 |
|
— |
| ||
|
|
|
|
|
194,612 |
|
179,413 |
| ||
|
Capital assets |
|
9 |
|
303,151 |
|
72,455 |
| ||
|
Intangible assets |
|
10 |
|
226,444 |
|
1,891 |
| ||
|
Convertible notes receivable |
|
12 |
|
16,129 |
|
1,534 |
| ||
|
Interest in equity investees |
|
13 |
|
4,966 |
|
28,376 |
| ||
|
Long-term investments |
|
14 |
|
46,028 |
|
27,788 |
| ||
|
Deferred tax asset |
|
15 |
|
— |
|
3,315 |
| ||
|
Goodwill |
|
11 |
|
522,762 |
|
1,200 |
| ||
|
|
|
|
|
$ |
1,314,092 |
|
$ |
315,972 |
|
|
Liabilities |
|
|
|
|
|
|
| ||
|
Current liabilities |
|
|
|
|
|
|
| ||
|
Accounts payable and accrued liabilities |
|
|
|
$ |
31,517 |
|
$ |
5,874 |
|
|
Income taxes payable |
|
15 |
|
3,584 |
|
— |
| ||
|
Deferred revenue |
|
|
|
2,607 |
|
2,800 |
| ||
|
Current portion of promissory note payable |
|
17 |
|
610 |
|
878 |
| ||
|
Current portion of long-term debt |
|
18 |
|
2,140 |
|
765 |
| ||
|
Current portion of derivative liability |
|
13 |
|
3,396 |
|
— |
| ||
|
|
|
|
|
43,854 |
|
10,317 |
| ||
|
Long-term liabilities |
|
|
|
|
|
|
| ||
|
Promissory note payable |
|
17 |
|
— |
|
366 |
| ||
|
Long-term debt |
|
18 |
|
28,337 |
|
31,420 |
| ||
|
Derivative liability |
|
13 |
|
9,055 |
|
— |
| ||
|
Deferred tax liability |
|
15 |
|
59,253 |
|
— |
| ||
|
|
|
|
|
140,499 |
|
42,103 |
| ||
|
Shareholders’ equity |
|
|
|
|
|
|
| ||
|
Share capital |
|
19 |
|
1,113,981 |
|
274,317 |
| ||
|
Warrants |
|
20 |
|
1,375 |
|
445 |
| ||
|
Share-based payment reserve |
|
|
|
22,006 |
|
3,230 |
| ||
|
Accumulated other comprehensive loss |
|
|
|
(801 |
) |
— |
| ||
|
Non-controlling interest |
|
22 |
|
9,580 |
|
— |
| ||
|
Retained earnings (deficit) |
|
|
|
27,452 |
|
(4,123 |
) | ||
|
|
|
|
|
1,173,593 |
|
273,869 |
| ||
|
|
|
|
|
$ |
1,314,092 |
|
$ |
315,972 |
|
Nature of operations (Note 1), Commitments (Note 30), Subsequent events (Note 31)
|
Approved on behalf of the Board: |
|
|
|
|
|
|
|
“John Cervini” |
|
“Cole Cacciavillani” |
|
Signed: Director |
|
Signed: Director |
The accompanying notes are an integral part of these consolidated financial statements
Aphria Inc.
Consolidated Statements of Income and Comprehensive Income
(In thousands of Canadian dollars, except share and per share amounts)
|
|
|
|
|
For the year ended |
| ||||
|
|
|
Note |
|
2018 |
|
2017 |
| ||
|
Revenue |
|
|
|
$ |
36,917 |
|
$ |
20,438 |
|
|
Production costs |
|
6 |
|
8,692 |
|
4,585 |
| ||
|
Other costs of sales |
|
|
|
313 |
|
— |
| ||
|
Gross profit before fair value adjustments |
|
|
|
27,912 |
|
15,853 |
| ||
|
Fair value adjustment on sale of inventory |
|
6 |
|
10,327 |
|
3,561 |
| ||
|
Fair value adjustment on growth of biological assets |
|
7 |
|
(23,302 |
) |
(5,005 |
) | ||
|
Gross profit |
|
|
|
40,887 |
|
17,297 |
| ||
|
Operating expenses: |
|
|
|
|
|
|
| ||
|
General and administrative |
|
23 |
|
13,901 |
|
4,678 |
| ||
|
Share-based compensation |
|
24 |
|
17,874 |
|
2,399 |
| ||
|
Selling, marketing and promotion |
|
|
|
11,873 |
|
6,664 |
| ||
|
Amortization |
|
|
|
3,985 |
|
956 |
| ||
|
Research and development |
|
|
|
490 |
|
492 |
| ||
|
Impairment of intangible asset |
|
|
|
— |
|
3,500 |
| ||
|
Transaction costs |
|
|
|
5,192 |
|
— |
| ||
|
|
|
|
|
53,315 |
|
18,689 |
| ||
|
|
|
|
|
(12,428 |
) |
(1,392 |
) | ||
|
Non-operating items: |
|
|
|
|
|
|
| ||
|
Consulting revenue |
|
17 |
|
1,244 |
|
512 |
| ||
|
Foreign exchange gain |
|
|
|
124 |
|
483 |
| ||
|
(Loss) gain on marketable securities |
|
4 |
|
(2,155 |
) |
209 |
| ||
|
(Loss) gain on sale of capital assets |
|
9 |
|
(191 |
) |
11 |
| ||
|
Gain on dilution of ownership in equity investee |
|
13 |
|
7,535 |
|
— |
| ||
|
(Loss) gain from equity investees |
|
13 |
|
(9,295 |
) |
210 |
| ||
|
Gain on sale of equity investee |
|
13 |
|
26,347 |
|
— |
| ||
|
Deferred gain recognized |
|
|
|
1,304 |
|
— |
| ||
|
Finance income, net |
|
25 |
|
5,012 |
|
728 |
| ||
|
Unrealized gain on embedded derivatives |
|
12 |
|
4,135 |
|
— |
| ||
|
Gain on long-term investments |
|
26 |
|
26,675 |
|
3,571 |
| ||
|
Unrealized loss on derivative liability |
|
13 |
|
(12,451 |
) |
— |
| ||
|
|
|
|
|
48,284 |
|
5,724 |
| ||
|
Income before income taxes |
|
|
|
35,856 |
|
4,332 |
| ||
|
Income taxes |
|
15 |
|
6,408 |
|
134 |
| ||
|
Net income |
|
|
|
29,448 |
|
4,198 |
| ||
|
Other comprehensive loss |
|
|
|
|
|
|
| ||
|
Other comprehensive loss from equity investee |
|
13 |
|
(801 |
) |
— |
| ||
|
Net comprehensive income |
|
|
|
$ |
28,647 |
|
$ |
4,198 |
|
|
Total comprehensive income is attributable to: |
|
|
|
|
|
|
| ||
|
Owners of Aphria Inc. |
|
|
|
28,867 |
|
4,198 |
| ||
|
Non-controlling interest |
|
22 |
|
(220 |
) |
— |
| ||
|
|
|
|
|
$ |
28,647 |
|
$ |
4,198 |
|
|
Weighted average number of common shares - basic |
|
|
|
161,026,463 |
|
104,341,319 |
| ||
|
Weighted average number of common shares - diluted |
|
|
|
165,914,000 |
|
111,427,893 |
| ||
|
Earnings per share - basic |
|
27 |
|
$ |
0.18 |
|
$ |
0.04 |
|
|
Earnings per share - diluted |
|
27 |
|
$ |
0.18 |
|
$ |
0.04 |
|
The accompanying notes are an integral part of these consolidated financial statements
Aphria Inc.
Consolidated Statements of Changes in Equity
(In thousands of Canadian dollars, except share amounts)
|
|
|
Number of |
|
Share capital |
|
Warrants |
|
Share-based |
|
Accumulated |
|
Non- |
|
Retained |
|
Total |
| |||||||
|
Balance at May 31, 2016 |
|
70,053,933 |
|
$ |
40,917 |
|
$ |
694 |
|
$ |
1,724 |
|
$ |
— |
|
$ |
— |
|
$ |
(8,321 |
) |
$ |
35,014 |
|
|
Share issuance - August 2016 bought deal |
|
17,250,000 |
|
31,959 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
31,959 |
| |||||||
|
Share issuance - November 2016 bought deal |
|
10,062,500 |
|
37,263 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
37,263 |
| |||||||
|
Share issuance - February 2017 bought deal |
|
11,500,000 |
|
53,869 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
53,869 |
| |||||||
|
Share issuance - May 2017 bought deal |
|
13,269,252 |
|
81,323 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
81,323 |
| |||||||
|
Income tax recovery on share issuance costs |
|
— |
|
3,448 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
3,448 |
| |||||||
|
Share issuance - warrants exercised |
|
15,251,165 |
|
23,647 |
|
(608 |
) |
— |
|
— |
|
— |
|
— |
|
23,039 |
| |||||||
|
Share issuance - options exercised |
|
1,053,095 |
|
1,534 |
|
— |
|
(558 |
) |
— |
|
— |
|
— |
|
976 |
| |||||||
|
Share issuance - intangible asset acquisition |
|
38,759 |
|
100 |
|
359 |
|
— |
|
— |
|
— |
|
— |
|
459 |
| |||||||
|
Share-based payments |
|
100,000 |
|
257 |
|
— |
|
2,064 |
|
— |
|
— |
|
— |
|
2,321 |
| |||||||
|
Shares held in escrow for services not yet earned |
|
50,000 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| |||||||
|
Net comprehensive income for the year |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
4,198 |
|
4,198 |
| |||||||
|
Balance at May 31, 2017 |
|
138,628,704 |
|
$ |
274,317 |
|
$ |
445 |
|
$ |
3,230 |
|
$ |
— |
|
$ |
— |
|
$ |
(4,123 |
) |
$ |
273,869 |
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
Non- |
|
|
|
|
| |||||||
|
|
|
|
|
|
|
|
|
Share based |
|
other |
|
controlling |
|
Retained |
|
|
| |||||||
|
|
|
Number of |
|
Share-based |
|
Warrants |
|
payment |
|
comprehensive |
|
interest |
|
earnings |
|
|
| |||||||
|
|
|
common shares |
|
(Note 19) |
|
(Note 20) |
|
reserve |
|
loss |
|
(Note 22) |
|
(deficit) |
|
Total |
| |||||||
|
Balance at May 31, 2017 |
|
138,628,704 |
|
$ |
274,317 |
|
$ |
445 |
|
$ |
3,230 |
|
$ |
— |
|
$ |
— |
|
$ |
(4,123 |
) |
$ |
273,869 |
|
|
Share issuance - November 2017 bought deal |
|
12,689,675 |
|
86,661 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
86,661 |
| |||||||
|
Share issuance - January 2018 bought deal |
|
8,363,651 |
|
109,000 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
109,000 |
| |||||||
|
Share issuance - Broken Coast acquisition |
|
14,373,675 |
|
214,168 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
214,168 |
| |||||||
|
Share issuance - Nuuvera acquisition |
|
31,226,910 |
|
411,258 |
|
1,015 |
|
12,133 |
|
— |
|
— |
|
— |
|
424,406 |
| |||||||
|
Share issuance - warrants exercised |
|
2,388,636 |
|
3,767 |
|
(85 |
) |
— |
|
— |
|
— |
|
— |
|
3,682 |
| |||||||
|
Share issuance - options exercised |
|
2,493,623 |
|
11,559 |
|
— |
|
(7,230 |
) |
— |
|
— |
|
— |
|
4,329 |
| |||||||
|
Share issuance - deferred share units |
|
5,050 |
|
62 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
62 |
| |||||||
|
Income tax recovery on share issuance costs |
|
— |
|
3,002 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
3,002 |
| |||||||
|
Share-based payments |
|
— |
|
— |
|
— |
|
15,780 |
|
— |
|
— |
|
— |
|
15,780 |
| |||||||
|
Shares held in escrow earned in exchange for services |
|
— |
|
187 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
187 |
| |||||||
|
Share-based payments rescinded |
|
— |
|
— |
|
— |
|
(1,907 |
) |
— |
|
— |
|
1,907 |
|
— |
| |||||||
|
Non-controlling interest |
|
— |
|
— |
|
— |
|
— |
|
— |
|
9,800 |
|
— |
|
9,800 |
| |||||||
|
Net comprehensive income for the year |
|
— |
|
— |
|
— |
|
— |
|
(801 |
) |
(220 |
) |
29,668 |
|
28,647 |
| |||||||
|
Balance at May 31, 2018 |
|
210,169,924 |
|
$ |
1,113,981 |
|
$ |
1,375 |
|
$ |
22,006 |
|
$ |
(801 |
) |
$ |
9,580 |
|
$ |
27,452 |
|
$ |
1,173,593 |
|
The accompanying notes are an integral part of these consolidated financial statements
Aphria Inc.
Consolidated Statements of Cash Flows
(In thousands of Canadian dollars)
|
|
|
|
|
For the year ended |
| ||||
|
|
|
Note |
|
2018 |
|
2017 |
| ||
|
Cash generated from (used in) operating activities: |
|
|
|
|
|
|
| ||
|
Net income for the year |
|
|
|
$ |
29,448 |
|
$ |
4,198 |
|
|
Adjustments for: |
|
|
|
|
|
|
| ||
|
Future income taxes |
|
15 |
|
3,658 |
|
134 |
| ||
|
Fair value adjustment on sale of inventory |
|
6 |
|
10,327 |
|
3,561 |
| ||
|
Fair value adjustment on growth of biological assets |
|
7 |
|
(23,302 |
) |
(5,005 |
) | ||
|
Loss (gain) on marketable securities |
|
4 |
|
2,155 |
|
(209 |
) | ||
|
Unrealized foreign exchange gain |
|
12 |
|
(94 |
) |
— |
| ||
|
Amortization |
|
9,10 |
|
6,678 |
|
1,942 |
| ||
|
Loss (gain) on sale of capital assets |
|
9 |
|
191 |
|
(11 |
) | ||
|
Impairment of intangible asset |
|
10 |
|
— |
|
3,500 |
| ||
|
Accretion interest on convertible note receivable |
|
12 |
|
(1,808 |
) |
(34 |
) | ||
|
Unrealized gain on embedded derivatives |
|
12 |
|
(4,135 |
) |
— |
| ||
|
Gain on dilution of ownership in equity investee |
|
13 |
|
(7,535 |
) |
— |
| ||
|
Loss (gain) from equity investees |
|
13 |
|
9,295 |
|
(210 |
) | ||
|
Gain on sale of equity investee |
|
13 |
|
(26,347 |
) |
— |
| ||
|
Deferred gain on recognized |
|
|
|
(1,304 |
) |
— |
| ||
|
Consulting revenue |
|
17 |
|
(1,244 |
) |
(512 |
) | ||
|
Other non-cash items |
|
|
|
(63 |
) |
71 |
| ||
|
Share-based compensation |
|
24 |
|
17,874 |
|
2,399 |
| ||
|
Gain on long-term investments |
|
26 |
|
(26,675 |
) |
(3,571 |
) | ||
|
Unrealized loss on derivative liability |
|
13 |
|
12,451 |
|
— |
| ||
|
Transaction costs |
|
|
|
5,192 |
|
— |
| ||
|
Change in non-cash working capital |
|
28 |
|
(10,411 |
) |
(928 |
) | ||
|
|
|
|
|
(5,649 |
) |
5,325 |
| ||
|
Cash provided by financing activities: |
|
|
|
|
|
|
| ||
|
Share capital issued, net of cash issuance costs |
|
|
|
195,661 |
|
204,408 |
| ||
|
Share capital issued on warrants and options exercised |
|
|
|
8,011 |
|
24,014 |
| ||
|
Proceeds from non-controlling interest |
|
|
|
9,800 |
|
— |
| ||
|
Advances from related parties |
|
8 |
|
11,386 |
|
388 |
| ||
|
Repayment of amounts due to related parties |
|
8 |
|
(10,890 |
) |
(852 |
) | ||
|
Proceeds from long-term debt |
|
18 |
|
— |
|
32,825 |
| ||
|
Repayment of long-term debt |
|
18 |
|
(7,622 |
) |
(644 |
) | ||
|
|
|
|
|
206,346 |
|
260,139 |
| ||
|
Cash used in investing activities: |
|
|
|
|
|
|
| ||
|
Investment in marketable securities |
|
4 |
|
(7,365 |
) |
(109,269 |
) | ||
|
Proceeds from disposal of marketable securities |
|
4 |
|
47,495 |
|
22,131 |
| ||
|
Investment in capital and intangible assets, net of shares issued |
|
9,10 |
|
(216,699 |
) |
(67,722 |
) | ||
|
Proceeds from disposal of capital assets |
|
9 |
|
431 |
|
33 |
| ||
|
Convertible notes advances |
|
12 |
|
(14,001 |
) |
(1,500 |
) | ||
|
Repayment of convertible notes receivable |
|
|
|
640 |
|
— |
| ||
|
Repayment of promissory notes receivable |
|
|
|
— |
|
568 |
| ||
|
Investment in long-term investments and equity investees |
|
|
|
(51,692 |
) |
(53,464 |
) | ||
|
Proceeds from disposal of long-term investments and equity investees |
|
|
|
43,077 |
|
7,196 |
| ||
|
Net cash paid on business acquisitions |
|
11 |
|
(22,756 |
) |
— |
| ||
|
|
|
|
|
(220,870 |
) |
(202,027 |
) | ||
|
Net (decrease) increase in cash and cash equivalents |
|
|
|
(20,173 |
) |
63,437 |
| ||
|
Cash and cash equivalents, beginning of year |
|
|
|
79,910 |
|
16,473 |
| ||
|
Cash and cash equivalents, end of year |
|
|
|
$ |
59,737 |
|
$ |
79,910 |
|
The accompanying notes are an integral part of these consolidated financial statements
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
1. Nature of operations
Aphria Inc. (the “Company” or “Aphria”) was continued in Ontario.
Pure Natures Wellness Inc. (o/a Aphria) (“PNW”), a wholly-owned subsidiary of the Company, is licensed to produce and sell medical cannabis under the provisions of the Access to Cannabis for Medical Purposes Regulations (“ACMPR”). In February 2018, the Company acquired Broken Coast Cannabis Ltd. (“Broken Coast”) (Note 11). Broken Coast is licensed to produce and sell medical cannabis under the provision of the Access to Cannabis for Medical Purposes Regulations (“ACMPR”). In March 2018, the Company acquired Nuuvera Inc. (“Nuuvera”) (Note 11), Nuuvera is an international organization with a focus on building a global cannabis brand, with operations in Germany, Italy, Spain, Malta, and Lesotho.
1974568 Ontario Ltd. (“Aphria Diamond”) is a 51% majority owned subsidiary of the Company, incorporated in November 2017. This entity is the Company’s venture with Double Diamond Farms. Aphria Diamond has applied for its cultivation licence under the provisions of the ACMPR.
The registered office of the Company is located at 5300 Commerce Court West, 199 Bay Street, Toronto, Ontario.
The Company’s common shares are listed under the symbol “APH” on the Toronto Stock Exchange (“TSX”) and under the symbol “APHQF” on the United States OTCQB Venture Market exchange.
These consolidated financial statements were approved by the Company’s Board of Directors on July 31, 2018.
2. Basis of preparation
(a) Statement of compliance
The policies applied in these consolidated financial statements are prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”) and Interpretations of the IFRS Interpretations Committee (“IFRIC”).
(b) Basis of measurement
These consolidated financial statements have been prepared on the going concern basis, under the historical cost convention except for certain financial instruments that are measured at fair value and biological assets that are measured at fair value less costs to sell, as detailed in the Company’s accounting policies.
(c) Functional currency
The Company and its subsidiaries’ functional currency, as determined by management is Canadian dollars. These consolidated financial statements are presented in Canadian dollars.
(d) Foreign currency translation
All figures presented in the consolidated financial statements are reflected in Canadian dollars, which is the functional currency of the Company and all of its subsidiaries.
Foreign currency transactions are translated into Canadian dollars at exchange rates in effect on the date of the transactions. Monetary assets and liabilities denominated in foreign currencies are translated to Canadian dollars at the foreign exchange rate applicable at the statement of financial position date. Realized and unrealized exchange gains and losses are recognized through profit and loss.
The assets and liabilities of foreign operations, including marketable securities, long-term investments and promissory notes payable, are translated in Canadian dollars at year-end exchange rates. Income and expenses, and cash flows of foreign operations are translated into Canadian dollars using average exchange rates. Exchange differences resulting from translating foreign operations are recognized in other comprehensive income and accumulated in equity.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
(e) Basis of consolidation
Subsidiaries are entities controlled by the Company. Control exists when the Company has the power, directly and indirectly, to govern the financial and operating policies of an entity and be exposed to the variable returns from its activities. The financial statements of subsidiaries are included in the consolidated financial statements from the date that control commences until the date that control ceases.
|
Subsidiaries |
|
Jurisdiction of incorporation |
|
Ownership interest |
|
|
Pure Natures Wellness Inc. (o/a Aphria) |
|
Ontario, Canada |
|
100 |
% |
|
Aphria (Arizona) Inc. |
|
Arizona, United States |
|
100 |
% |
|
Cannan Growers Inc. |
|
British Columbia, Canada |
|
100 |
% |
|
Nuuvera Inc. |
|
Ontario, Canada |
|
100 |
% |
|
Nuuvera Holdings Ltd. |
|
Ontario, Canada |
|
100 |
% |
|
ARA — Avanti Rx Analytics Inc. |
|
Ontario, Canada |
|
100 |
% |
|
Avalon Pharmaceuticals Inc. |
|
Ontario, Canada |
|
100 |
% |
|
2589671 Ontario Inc. |
|
Ontario, Canada |
|
100 |
% |
|
2589674 Ontario Inc. |
|
Ontario, Canada |
|
100 |
% |
|
Nuuvera Israel Ltd. |
|
Tel Aviv, Israel |
|
100 |
% |
|
Nuuvera Deutschland GmbH |
|
Hamburg, Germany |
|
100 |
% |
|
FL-Group |
|
Genoa, Italy |
|
100 |
% |
|
Broken Coast Cannabis Ltd. |
|
British Columbia, Canada |
|
99.86 |
% |
|
Nuuvera Malta Ltd. |
|
Valletta, Malta |
|
90 |
% |
|
ASG Pharma Ltd. |
|
Valletta, Malta |
|
90 |
% |
|
1974568 Ontario Ltd. |
|
Ontario, Canada |
|
51 |
% |
Intragroup balances, and any unrealized gains and losses or income and expenses arising from transactions with jointly controlled entities are eliminated to the extent of the Company’s interest in the entity.
The Company treats transactions with non-controlling interests that do not result in a loss of control as transactions with equity owners of the Company. A change in ownership interest results in an adjustment between the carrying amounts of the controlling and non-controlling interests to reflect their relative interests in the subsidiary. Any difference between the amount of the adjustment to non-controlling interests and any consideration paid or received is recognized in a separate reserve within equity attributable to the owners of the Company.
(e) Amalgamation
Effective June 1, 2017, CannWay Pharmaceuticals Ltd. (“CannWay”), a wholly-owned subsidiary of the Company, was amalgamated with Pure Natures Wellness Inc. (o/a Aphria). The Company has historically presented all balances and activities of CannWay as a fully consolidated entity for financial statement presentation purposes. As of the date of amalgamation, CannWay did not have any assets or outstanding liabilities. There are no material changes to be considered prospectively or to the comparative consolidated statements as a result of the amalgamation.
(f) Interest in equity investees
The Company’s interest in equity investees is comprised of its interest in Liberty Health Sciences Inc. (“Liberty”) and Althea Company Pty Ltd. (“Althea”). During the year, the Company entered into an agreement which has changed the classification of its investment in Liberty from equity investee to assets held for sale (Note 13).
In accordance with IFRS 10, associates are those in which the Company has significant influence, but not control or joint control over the financial and accounting policies.
Interests in associates are accounted for using the equity method in accordance with IAS 28. They are recognized initially at cost, which includes transaction costs. After initial recognition, the consolidated financial statements include the Company’s share of the profit or loss and other comprehensive income (“OCI”) of equity investees until the date on which significant influence ceases.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
If the Company’s share of losses in an equity investment equals or exceeds its interest in the entity, including any other unsecured long-term receivables, the group does not recognize further losses, unless it has incurred obligations or made payments on behalf of the other entity.
Unrealized gains on transactions between the Company and its associates are eliminated to the extent of the Company’s interest in these entities. Unrealized losses are also eliminated unless the transaction provides evidence of an impairment of the asset transferred.
The carrying amount of equity investments is tested for impairment in accordance with the policy described in Note 3(j).
3. Significant accounting policies
The significant accounting policies used by the Company are as follows:
a. Revenue
Revenue is recognized at the fair value of consideration received or receivable. Revenue from the sale of goods is recognized when all the following conditions have been satisfied, which are generally met once the products are shipped to customers.
· The Company has transferred the significant risks and rewards of ownership of the goods to the purchaser;
· The Company retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold;
· The amount of revenue can be measured reliably;
· It is probable that the economic benefits associated with the transaction will flow to the entity; and
· The costs incurred or to be incurred in respect of the transaction can be measured reliably.
The Company recognizes revenue from consulting services on a straight-line basis over the term of its consulting agreement with a third party as the services are provided.
Amounts disclosed as revenue are net of allowances, discounts and rebates.
b. Cash and cash equivalents
Cash and cash equivalents are comprised of cash and highly liquid investments that are readily convertible into known amounts of cash and are subject to insignificant risk of changes in value.
c. Marketable securities
Marketable securities are comprised of liquid investments in federal, provincial and/or corporate bonds with maturities less than 3.5 years. Marketable securities are recognized initially at fair value and subsequently adjusted to fair value through profit or loss (“FVTPL”).
d. Inventory
Inventory is valued at the lower of cost and net realizable value. Cost is determined using the weighted average method. Inventories of harvested cannabis are transferred from biological assets into inventory at their fair value at harvest less costs to sell, which is deemed to be their cost. Any subsequent post-harvest costs are capitalized to inventory to the extent that cost is less than net realizable value. Net realizable value is determined as the estimated selling price in the ordinary course of business less estimated costs to sell. Packaging and supplies are initially valued at cost.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
e. Biological assets
The Company’s biological assets consist of medical cannabis plants which are not yet harvested. These biological assets are measured at fair value less costs to sell. The Company capitalizes all related direct and indirect costs of production to the biological assets to fair value less costs to sell at each reporting date. At the point of harvest, the biological assets are transferred to inventory at their fair value less costs to sell.
Gains or losses arising from changes in fair value less cost to sell are included in the results of operations of the related period.
f. Assets held for sale
Assets and liabilities held for disposal are no longer depreciated and are presented separately in the statement of financial position at the lower of their carrying amount and fair value less costs to sell. An asset is regarded as held for sale if its carrying amount will be recovered principally through a sale transaction, rather than through continuing use. For this to be the case, the asset must be available for immediate sale and its sale must be highly probable.
g. Capital assets
Capital assets are stated at cost, net of accumulated amortization and accumulated impairment losses, if any.
Amortization is calculated using the following terms and methods:
|
Asset type |
|
Amortization method |
|
Amortization term |
|
Land |
|
Not amortized |
|
No term |
|
Production facility |
|
Straight-line |
|
20 years |
|
Equipment |
|
Straight-line |
|
3 – 10 years |
|
Leasehold improvements |
|
Straight-line |
|
Over lease term |
|
Construction in progress |
|
Not amortized |
|
No term |
An item of equipment is derecognized upon disposal or when no future economic benefits are expected from its use. Any gain or loss arising on derecognition of the asset (calculated as the difference between the net disposal proceeds and the carrying value of the asset) is included in the consolidated statements of income and comprehensive income in the year the asset is derecognized.
The assets’ residual values, useful lives and methods of amortization are reviewed at each financial year end, and adjusted prospectively if appropriate.
h. Intangible assets
Intangible assets are stated at cost, net of accumulated amortization and accumulated impairment losses, if any.
Amortization is calculated using the following terms and methods:
|
Asset type |
|
Amortization method |
|
Amortization term |
|
Customer relationships |
|
Straight-line |
|
3 years |
|
Corporate website |
|
Straight-line |
|
2 years |
|
Licences, permits & applications |
|
Straight-line |
|
90 months – indefinite |
|
Non-compete agreements |
|
Straight-line |
|
Over term of non-compete |
|
Tokyo Smoke licensing agreement |
|
Straight-line |
|
5 years |
|
Intellectual property, trademarks & brands |
|
Straight-line |
|
15 months – 20 years |
The estimated success of applications, useful life and amortization method are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Following initial recognition, intangible assets with indefinite useful lives are carried at cost less any accumulated impairment losses.
i. Goodwill
Goodwill represents the excess of the purchase price paid for the acquisition of subsidiaries over the fair value of the net tangible and intangible assets acquired. Following initial recognition, goodwill is measured at cost less any accumulated impairment losses.
j. Impairment of non-financial assets
Goodwill and intangible assets that have an indefinite useful life are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Other assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
For the purpose of testing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash-generating unit, or “CGU”). An impairment loss is recognized for the amount, if any, by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of the asset’s fair value less cost to sell and the value in use (being the present value of expected future cash flows of the asset or CGU). Where an impairment loss subsequently reverses, the carrying amount of the asset is increased to the lesser of the revised estimate of recoverable amount and the carrying amount that would have been recorded had no impairment loss been previously recognized.
k. Income taxes
Income tax expense consisting of current and deferred tax expense is recognized in the consolidated statements of income and comprehensive income. Current tax expense is the expected tax payable on the taxable income for the year, using tax rates enacted or substantively enacted at year end, adjusted for amendments to tax payable with regards to previous years.
Deferred tax assets and liabilities and the related deferred income tax expense or recovery are recognized for deferred tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using the enacted or substantively enacted tax rates expected to apply when the asset is realized or the liability settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that substantive enactment occurs.
A deferred tax asset is recognized to the extent that it is probable that future taxable income will be available against which the asset can be utilized.
Deferred tax assets and liabilities are offset when there is a legally enforceable right to set off current tax assets against current tax liabilities and when they relate to income taxes levied by the same taxation authority and the Company intends to settle its current tax assets and liabilities on a net basis.
l. Earnings per share
Basic earnings per share is calculated using the weighted average number of common shares outstanding during the year. The dilutive effect on earnings per share is calculated presuming the exercise of outstanding options, warrants and similar instruments. It assumes that the proceeds of such exercise would be used to repurchase common shares at the average market price during the year. However, the calculation of diluted loss per share excludes the effects of various conversions and exercise of options and warrants that would be anti-dilutive.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
m. Share-based compensation
The Company has a stock option plan in place. The Company measures equity settled share-based payments based on their fair value at the grant date and recognizes compensation expense over the vesting period based on the Company’s estimate of equity instruments that will eventually vest. Fair value is measured using the Black-Scholes option pricing model. Expected forfeitures are estimated at the date of grant and subsequently adjusted if further information indicates actual forfeitures may vary from the original estimate. Any revisions are recognized in the consolidated statements of income and comprehensive income such that the cumulative expense reflects the revised estimate.
n. Research and development
Research costs are expensed as incurred. Development expenditures are capitalized only if development costs can be measured reliably, the product or process is technically and commercially feasible, future economic benefits are probable, and the Company intends to and has sufficient resources to complete development to use or sell the asset. Other development expenditures that do not meet the above criteria are recognized in the consolidated statements of income and comprehensive income as incurred.
o. Financial instruments
Financial assets and other financial liabilities are classified into one of four categories:
· FVTPL;
· held-to-maturity (“HTM”);
· available for sale (“AFS”); and
· loans and receivables.
(i) FVTPL financial assets
Financial assets are classified as FVTPL when the financial asset is held for trading or it is designated as FVTPL. Financial assets classified as FVTPL are stated at fair value with any resulting gain or loss recognized in the consolidated statements of income and comprehensive income. Transaction costs are expensed as incurred.
(ii) HTM investments
HTM investments are recognized on a trade-date basis and are initially measured at fair value, including transaction costs and subsequently at amortized cost.
(iii) AFS financial assets
AFS financial assets are those non-derivative financial assets that are designated as available for sale or are not classified in any of the other categories. Gains and losses arising from changes in fair value are recognized in other comprehensive income.
(iv) Loans and receivables
Loans and receivables are financial assets having fixed or determinable payments that are not quoted in an active market. They are initially recognized at the transaction value and subsequently carried at amortized cost less, when material, a discount to reduce the loans and receivables to fair value.
(v) Impairment of financial assets
Financial assets, other than those at FVTPL, are assessed for indicators of impairment at the end of each reporting period. Financial assets are impaired when there is objective evidence that, as a result of one or more events that occurred after the initial recognition of the financial asset, the estimated future cash flows of the investment have been impacted.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
The carrying amount of all financial assets, excluding trade receivables, is directly reduced by the impairment loss. The carrying amount of trade receivables is reduced through the use of an allowance account. When a trade receivable is considered uncollectible, it is written off against the allowance account. Subsequent recoveries of amounts previously written off are credited against the allowance account. Changes in the carrying amount of the allowance account are recognized in the consolidated statements of income and comprehensive income. With the exception of AFS equity instruments, if, in a subsequent period, the amount of the impairment loss decreases and the decrease relates to an event occurring after the impairment was recognized; the previously recognized impairment loss is reversed through the consolidated statements of income and comprehensive income.
(vi) Financial liabilities and other financial liabilities
Financial liabilities are classified as either financial liabilities at FVTPL or other financial liabilities. Financial liabilities at FVTPL are stated at fair value, with changes being recognized through the consolidated statements of income and comprehensive income. Other financial liabilities are initially measured at fair value, net of transaction costs, and are subsequently measured at amortized cost using the effective interest method, with interest expense recognized on an effective yield basis.
(vii) Embedded derivatives
Embedded derivatives are separated from the host contract and accounted for separately if certain criteria are met. Derivatives are initially measured at fair value; any directly attributable transaction costs are recognised in profit or loss as incurred. Subsequent to initial recognition, derivatives are measured at fair value and changes therein are recognised in profit or loss. The Company has convertible loans receivables whereby balances can be converted into equity and a share purchase agreement resulting in an obligation to sell shares at an 18% discount on the market price, based on a 10 day volume weighted trading price (Note 13).
(viii) Classification of financial instruments
Cash and cash equivalents — FVTPL
Marketable securities — FVTPL
Accounts receivables — loans and receivables
Other receivables — loans and receivables
Convertible note receivable — AFS
Embedded derivative — derivative financial instruments
Long-term investments — FVTPL
Accounts payable and accrued liabilities — other financial liabilities
Promissory note payable — other financial liabilities
Long-term debt — other financial liabilities
Derivative liability — derivative financial instruments
(ix) Determination on fair value of long-term investments
All long-term investments (other than Level 3 warrants) are initially recorded at the transaction price, being the fair value at the time of acquisition. Thereafter, at each reporting period, the fair value of an investment is adjusted using one or more of the valuation indicators described below. Warrants of private companies are carried at their intrinsic value.
p. Critical accounting estimates and judgments
The preparation of financial statements requires management to make judgments, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, and revenue and expenses. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the review affects both current and future periods.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Long-term investments
The determination of fair value of the Company’s long-term investments at other than initial cost are subject to certain limitations. Financial information for private companies in which the Company has investments may not be available and, even if available, that information may be limited and/or unreliable.
Use of the valuation approach described below may involve uncertainties and determinations based on the Company’s judgment and any value estimated from these techniques may not be realized or realizable.
Company-specific information is considered when determining whether the fair value of a long-term investment should be adjusted upward or downward at the end of each reporting period. In addition to company-specific information, the Company will take into account trends in general market conditions and the share performance of comparable publicly-traded companies when valuing long-term investments.
The fair value of long-term investments may be adjusted if:
· There has been a significant subsequent equity financing provided by outside investors at a valuation different than the current value of the investee company, in which case the fair value of the investment is set to the value at which that financing took place;
· There have been significant corporate, political, or operating events affecting the investee company that, in management’s opinion, have a material impact on the investee company’s prospects and therefore its fair value. In these circumstances, the adjustment to the fair value of the investment will be based on management’s judgment and any value estimated may not be realized or realizable;
· The investee company is placed into receivership or bankruptcy;
· Based on financial information received from the investee company, it is apparent to the Company that the investee company is unlikely to be able to continue as a going concern;
· Important positive/negative management changes by the investee company that the Company’s management believes will have a positive/negative impact on the investee company’s ability to achieve its objectives and build value for shareholders.
Adjustment to the fair value of a long-term investment will be based upon management’s judgment and any value estimated may not be realized or realizable. The resulting values for non-publicly traded investments may differ from values that would be realized if a ready market existed.
Biological assets and inventory
Management is required to make a number of estimates in calculating the fair value less costs to sell of biological assets and harvested cannabis inventory. These estimates include a number of assumptions such as estimating the stage of growth of the cannabis, harvesting costs, sales price, and expected yields.
Estimated useful lives, impairment considerations and amortization of capital and intangible assets
Amortization of capital and intangible assets is dependent upon estimates of useful lives based on management’s judgment.
Goodwill and indefinite life intangible asset impairment testing requires management to make estimates in the impairment testing model. On an annual basis, the Company tests whether goodwill and indefinite life intangible assets are impaired.
Impairment of definite long-lived assets is influenced by judgment in defining a CGU and determining the indicators of impairment, and estimates used to measure impairment losses
The recoverable value of goodwill, indefinite and definite long-lived assets is determined using discounted future cash flow models, which incorporate assumptions regarding future events, specifically future cash flows, growth rates and discount rates.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Share-based compensation
The fair value of share-based compensation expenses are estimated using the Black-Scholes option pricing model and rely on a number of estimates, such as the expected life of the option, the volatility of the underlying share price, the risk free rate of return, and the estimated rate of forfeiture of options granted.
Business combinations
Judgement is used in determining whether an acquisition is a business combination or an asset acquisition. In determining the allocation of the purchase price in a business combination, including any acquisition-related contingent consideration, estimates including market based and appraisal values are used. The contingent consideration is measured at its acquisition-date fair value and included as part of the consideration transferred in a business combination. Contingent consideration that is classified as equity is not remeasured at subsequent reporting dates and its subsequent settlement is accounted for within equity. Contingent consideration that is classified as an asset or liability is remeasured at subsequent reporting dates in accordance with IAS 39, or IAS 37, as appropriate, with the corresponding gain or loss being recognized in profit or loss.
The Company measures all assets acquired and liabilities assumed at their acquisition-date fair values. Non-controlling interests in the acquiree are measured on the basis of the non-controlling interests’ proportionate share of this equity in the acquiree’s identifiable net assets. Acquisition-related costs are recognized as expenses in the periods in which the costs are incurred and the services are received (except for the costs to issue debt or equity securities which are recognized according to specific requirements). The excess of the aggregate of (a) the consideration transferred to obtain control, the amount of any non-controlling interest in the acquire over (b) the net of the acquisition-date amounts of the identifiable assets acquired and the liabilities assumed, is recognized as goodwill as of the acquisition date.
q. New standards and interpretations issued but not yet adopted
IFRS 9 - Financial Instruments; Classification and Measurement, effective for annual periods beginning on or after January 1, 2018, with early adoption permitted, introduces new requirements for the classification, measurement and derecognition of financial instruments and introduces a new impairment model for financial assets. The Company is assessing the impact of the standard on its convertible notes receivable and its investments where it holds less than significant influence. The Company is currently completing its assessment of the impact of this new standard.
The new standard also introduces expanded disclosure requirements and changes in presentation. These are expected to change the nature and extent of the Company’s disclosures about its financial instruments particularly in the period of the adoption of the new standard.
The Company will apply the new rules retrospectively from June 1, 2018 with the practical expedients permitted under the standards. Comparatives will not be restated.
IFRS 15 - Revenue from Contracts with Customers; effective for annual periods beginning on or after January 1, 2018, with early adoption permitted, specifies how and when to recognize revenue and enhances relevant disclosures to be applied to all contracts with customers. The Company continues to assess the impact of the standard, with a focus on consulting contracts and royalty fees.
The Company intends to adopt the standard using the modified retrospective approach which means that the cumulative impact of adoption will be recognized in retained earnings as of June 1, 2018 and that comparatives will not be restated.
IFRS 16 — Leases; in January 2016, the IASB issued IFRS 16, which specifies how an IFRS reporter will recognise, measure, present and disclose leases. The standard provides a single lessee accounting model, requiring lessees to recognise assets and liabilities for all leases unless the lease term is 12 months or less or the underlying asset has a low value. Lessors continue to classify leases as operating or finance, with IFRS 16’s approach to lessor accounting substantially unchanged from its predecessor, IAS 17. IFRS 16 is effective for annual reporting periods beginning on or after January 1, 2019, and a lessee shall either apply IFRS 16 with full retrospective effect or alternatively not restate comparative information but recognise the cumulative effect of initially applying IFRS 16 as an adjustment to opening equity at the date of initial application. Early adoption is permitted if IFRS 15 has also been adopted. Based on its current assets, interests and investments, no significant impact is anticipated from the new standard.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
There are no other standards that are not yet effective and that would be expected to have a material impact on the Company in the current or future reporting periods and on foreseeable future transactions.
The Company has reclassified certain immaterial items on the comparative consolidated statements of financial position, consolidated statements of income and comprehensive income, and consolidated statements of cash flows to improve clarity.
4. Marketable securities
Marketable securities are classified as fair value through profit or loss, and are comprised of:
|
|
|
S&P rating at |
|
Interest |
|
Maturity |
|
May 31, |
|
May 31, |
| ||
|
Fixed Income: |
|
|
|
|
|
|
|
|
|
|
| ||
|
Molson Coors Brewing Company |
|
BBB- |
|
3.950 |
% |
10/06/17 |
|
$ |
— |
|
$ |
1,116 |
|
|
Ford Motor Credit Co. LLC |
|
BBB |
|
3.320 |
% |
12/19/17 |
|
— |
|
1,988 |
| ||
|
Goldman Sachs & Co. LLC |
|
A+ |
|
3.375 |
% |
2/01/18 |
|
— |
|
5,078 |
| ||
|
The Manufacturer’s Life Insurance Company |
|
AA- |
|
2.819 |
% |
2/26/18 |
|
— |
|
1,472 |
| ||
|
Canadian Western Bank |
|
A- |
|
2.531 |
% |
3/22/18 |
|
— |
|
3,039 |
| ||
|
Ford Motor Credit Co. LLC |
|
BBB |
|
3.700 |
% |
8/02/18 |
|
1,015 |
|
1,037 |
| ||
|
Sobeys Inc. |
|
BB+ |
|
3.520 |
% |
8/08/18 |
|
3,040 |
|
3,078 |
| ||
|
Royal Bank of Canada |
|
AA- |
|
2.770 |
% |
12/11/18 |
|
— |
|
5,180 |
| ||
|
Canadian Western Bank |
|
A- |
|
3.077 |
% |
1/14/19 |
|
1,528 |
|
1,535 |
| ||
|
Sun Life Financial Inc. |
|
A |
|
2.770 |
% |
5/13/19 |
|
3,018 |
|
3,064 |
| ||
|
Ford Motor Credit Co. LLC |
|
BBB |
|
3.140 |
% |
6/14/19 |
|
5,101 |
|
5,207 |
| ||
|
Canadian Natural Resources Ltd. |
|
BBB+ |
|
3.050 |
% |
6/19/19 |
|
— |
|
2,054 |
| ||
|
Canadian Western Bank |
|
A- |
|
3.463 |
% |
12/17/19 |
|
1,025 |
|
1,028 |
| ||
|
Laurentian Bank of Canada |
|
BBB |
|
2.500 |
% |
1/23/20 |
|
3,003 |
|
6,099 |
| ||
|
Enercare Solutions Inc. |
|
BBB |
|
4.600 |
% |
2/03/20 |
|
3,974 |
|
4,008 |
| ||
|
Enbridge Inc. |
|
BBB+ |
|
4.530 |
% |
3/09/20 |
|
5,203 |
|
5,395 |
| ||
|
Central 1 Credit Union |
|
A |
|
1.870 |
% |
3/16/20 |
|
— |
|
5,020 |
| ||
|
Choice Properties REIT |
|
BBB |
|
3.600 |
% |
4/20/20 |
|
5,091 |
|
5,237 |
| ||
|
Penske Truck Leasing Co., L.P. |
|
BBB |
|
2.950 |
% |
6/12/20 |
|
— |
|
5,145 |
| ||
|
Westcoast Energy Inc. |
|
BBB+ |
|
4.570 |
% |
7/02/20 |
|
5,293 |
|
5,430 |
| ||
|
Bank of Montreal (USD) |
|
A+ |
|
1.400 |
% |
4/10/18 |
|
— |
|
4,052 |
| ||
|
Citigroup Inc. (USD) |
|
BBB+ |
|
2.050 |
% |
12/17/18 |
|
3,914 |
|
4,081 |
| ||
|
Royal Bank of Canada (USD) |
|
AA- |
|
1.625 |
% |
4/15/19 |
|
3,857 |
|
4,040 |
| ||
|
Wells Fargo & Company (USD) |
|
A |
|
2.150 |
% |
1/30/20 |
|
— |
|
3,964 |
| ||
|
|
|
|
|
|
|
|
|
$ |
45,062 |
|
$ |
87,347 |
|
The cost of marketable securities as at May 31, 2018 was $45,863 (May 31, 2017 — $87,138). During the year ended May 31, 2018, the company divested of certain marketable securities for proceeds of $47,495 (2017 - $22,131), resulting in a (loss) gain on disposal of $(608) (2017 - $35), and re-invested $7,365 (2017 - $109,269). During the year ended May 31, 2018, the Company recognized a (loss) gain of $(2,155) (2017 - $209) on its marketable securities portfolio, of which $(1,547) (2017 - $174) represented unrealized fair value adjustments.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
5. Other current assets
Other current assets are comprised of:
|
|
|
May 31, |
|
May 31, |
| ||
|
|
|
2018 |
|
2017 |
| ||
|
HST receivable |
|
$ |
10,840 |
|
$ |
3,675 |
|
|
Accrued interest |
|
831 |
|
701 |
| ||
|
Credit card receivable |
|
170 |
|
103 |
| ||
|
Prepaid assets |
|
1,720 |
|
1,060 |
| ||
|
Other |
|
823 |
|
32 |
| ||
|
|
|
$ |
14,384 |
|
$ |
5,571 |
|
6. Inventory
Inventory is comprised of:
|
|
|
Capitalized |
|
Fair value |
|
|
May 31, |
|
May 31, |
| ||||
|
Harvested cannabis |
|
$ |
4,111 |
|
$ |
8,220 |
|
|
$ |
12,331 |
|
$ |
2,507 |
|
|
Harvested cannabis trim |
|
810 |
|
1,467 |
|
|
2,277 |
|
421 |
| ||||
|
Cannabis oil |
|
2,660 |
|
3,918 |
|
|
6,578 |
|
682 |
| ||||
|
Packaging and supplies |
|
964 |
|
— |
|
|
964 |
|
277 |
| ||||
|
|
|
$ |
8,545 |
|
$ |
13,605 |
|
|
$ |
22,150 |
|
$ |
3,887 |
|
During the year ended May 31, 2018, the Company recorded $8,313 (2017 - $4,585) of production costs. Included in production costs for the year ended May 31, 2018 is $241 of cannabis oil conversion costs (2017 - $99), $236 related to the cost of accessories (2017 - $58), and amortization of $1,715 (2017 - $986). The Company also included $978 of amortization which remains in inventory for the year ended May 31, 2018 related to capital assets utilized in production. During the year ended May 31, 2018, the Company expensed $10,327 (2017 —$3,561) of fair value adjustments on the growth its biological assets included in inventory sold.
The Company holds 3,221.3 kilograms of harvested cannabis (May 31, 2017 — 668.5 kgs), 702.0 kilograms of harvested cannabis trim (May 31, 2017 — 140.1 kgs) and 7,724.7 litres of cannabis oils or 1,716.6 kilograms equivalent (May 31, 2017 — 1,091.3 litres or 181.9 kilograms equivalent) at May 31, 2018.
7. Biological assets
Biological assets are comprised of:
|
|
|
Amount |
| |
|
Balance as at May 31, 2016 |
|
$ |
698 |
|
|
Changes in fair value less costs to sell due to biological transformation |
|
5,005 |
| |
|
Production costs capitalized |
|
4,188 |
| |
|
Transferred to inventory upon harvest |
|
(8,483 |
) | |
|
Balance as at May 31, 2017 |
|
$ |
1,408 |
|
|
Changes in fair value less costs to sell due to biological transformation |
|
23,302 |
| |
|
Purchased as part of business acquisition |
|
826 |
| |
|
Production costs capitalized |
|
12,143 |
| |
|
Transferred to inventory upon harvest |
|
(30,348 |
) | |
|
Balance at May 31, 2018 |
|
$ |
7,331 |
|
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
The Company values medical cannabis plants at cost, which approximates fair value from the date of initial clipping from mother plants until half way through the flowering cycle of the plants. Measurement of the biological transformation of the plant at fair value less costs to sell begins in the fourth week prior to harvest and is recognized evenly until the point of harvest. The number of weeks in the growing cycle is between twelve and sixteen weeks from propagation to harvest. The Company has determined the fair value less costs to sell of harvested cannabis and harvested cannabis trim to be $3.75 and $3.00 per gram respectively, upon harvest for greenhouse produced cannabis and $4.25 and $3.50 per gram respectively, upon harvest for indoor produced cannabis.
The effect of the fair value less cost to sell over and above historical cost was an increase in non-cash value of biological assets and inventory of $23,302 during the year ended May 31, 2018 (2017 — $5,005).
The fair value of biological assets is determined using a valuation model to estimate expected harvest yield per plant applied to the estimated price per gram less processing and selling costs. Only when there is a material change from the expected fair value used for cannabis does the Company make any adjustments to the fair value used. During the year, there was no material change to these inputs and therefore there has been no change in the determined fair value per plant.
In determining the fair value of biological assets, management has made the following estimates in this valuation model:
· The harvest yield is between 40 grams and 80 grams per plant;
· The selling price is between $2.50 and $10.00 per gram;
· Processing costs include drying and curing, testing, post-harvest overhead allocation, packaging and labelling costs between $0.30 and $0.80 per gram;
· Selling costs include shipping, order fulfilment, patient acquisition and patient maintenance costs between $0.00 and $3.00 per gram;
Sales price used in the valuation of biological assets is based on the average selling price of all cannabis products and can vary based on different strains being grown as well as the proportion of sales derived from wholesale compared to retail. Selling costs vary depending on methods of selling and are considered based on the expected method of selling and the determined additional costs which would be incurred. Expected yields for the cannabis plant is also subject to a variety of factors, such as strains being grown, length of growing cycle, and space allocated for growing. Management reviews all significant inputs based on historical information obtained as well as based on planned production schedules.
Management has quantified the sensitivity of the inputs and determined the following:
· Selling price per gram — a decrease in the average selling price per gram by 5% would result in the biological asset value decreasing by $267 (2017 - $25) and inventory decreasing by $1,040 (2017 - $180)
· Harvest yield per plant — a decrease in the harvest yield per plant of 5% would result in the biological asset value decreasing by $179 (2017 - $15)
These inputs are level 3 on the fair value hierarchy, and are subject to volatility in market prices and several uncontrollable factors, which could significantly affect the fair value of biological assets in future periods.
8. Related party transactions
The Company funds a small portion of the Canadian operating costs of Liberty, for which Liberty reimburses the Company quarterly. Additionally, the Company purchases certain electrical generation equipment and pays rent to a company owned by a director. The balance owing from related parties as at May 31, 2018 was $nil (May 31, 2017 - $464). These parties are related as they are corporations that are controlled by certain officers and directors of the Company.
During the year ended May 31, 2018, related party corporations charged or incurred expenditures on behalf of the Company (including rent) totaling $276 (2017 - $388). Included in this amount was rent of $45 charged during the year ended May 31, 2018 (2017 - $49).
The Company funded the start-up costs and operations of Liberty Health Sciences Inc., a related party through an equity investment.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
|
|
|
Amount |
| |
|
Balance due to (from) related parties as at May 31, 2017 |
|
$ |
(464 |
) |
|
Related party charges in the year |
|
276 |
| |
|
Payments to related parties in the year |
|
(276 |
) | |
|
Non-cash payments made on behalf of related parties in the year |
|
(32 |
) | |
|
Payments made on behalf of related parties in the year |
|
(10,614 |
) | |
|
Repayments made by related parties in the year |
|
11,110 |
| |
|
Balance at May 31, 2018 |
|
$ |
— |
|
During the year ended May 31, 2018, the Company entered into a definitive agreement with respect to the sale of Aphria’s subsidiary Aphria (Arizona) Inc., its sole holdings being the minority interests in Copperstate and CSF, to Liberty for a purchase price of $20,000 (Note 14). Subsequent to entering into this definitive agreement, the existing investors in Copperstate and CSF exercised their right of first refusal to purchase the minority interests on the same terms. Subsequent to year-end, this transaction closed.
During the year ended May 31, 2018, the Company purchased capital assets for $995 from a company controlled by a director. During the prior year, the Company purchased 36 acres of farm land, with 9 acres of greenhouses located thereon, from F.M. and Cacciavillani Farms Ltd., a company controlled by a director, for $6,100. The purchase price was allocated as follows: (i) $1,300 to land; (ii) $3,550 to greenhouse infrastructure; and, (iii) $1,250 to licences, permits & applications — intangible assets.
Key management personnel compensation for the year ended May 31, 2018 and 2017 was comprised of:
|
|
|
For the year ended |
| ||||
|
|
|
2018 |
|
2017 |
| ||
|
Salaries |
|
$ |
1,699 |
|
$ |
829 |
|
|
Short-term employment benefits (included in office and general) |
|
70 |
|
84 |
| ||
|
Share-based compensation |
|
3,235 |
|
594 |
| ||
|
|
|
$ |
5,004 |
|
$ |
1,507 |
|
Directors and officers of the Company control 8.5% or 17,902,125 of the voting shares of the Company.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
9. Capital assets
|
|
|
|
|
Production |
|
|
|
Leasehold |
|
Construction |
|
Total capital |
| ||||||
|
|
|
Land |
|
Facility |
|
Equipment |
|
improvements |
|
in process |
|
assets |
| ||||||
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
At May 31, 2016 |
|
$ |
— |
|
$ |
— |
|
$ |
3,499 |
|
$ |
4,812 |
|
$ |
65 |
|
$ |
8,376 |
|
|
Additions |
|
10,725 |
|
4,018 |
|
1,700 |
|
16 |
|
49,958 |
|
66,417 |
| ||||||
|
Transfers |
|
104 |
|
12,152 |
|
174 |
|
(4,566 |
) |
(7,864 |
) |
— |
| ||||||
|
Disposals |
|
— |
|
— |
|
(33 |
) |
— |
|
— |
|
(33 |
) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
At May 31, 2017 |
|
10,829 |
|
16,170 |
|
5,340 |
|
262 |
|
42,159 |
|
74,760 |
| ||||||
|
Business acquisitions |
|
854 |
|
6,992 |
|
2,860 |
|
1,388 |
|
5,947 |
|
18,041 |
| ||||||
|
Additions |
|
12,716 |
|
47,149 |
|
4,759 |
|
15 |
|
151,899 |
|
216,538 |
| ||||||
|
Transfers |
|
105 |
|
29,338 |
|
2,990 |
|
— |
|
(32,433 |
) |
— |
| ||||||
|
Disposals |
|
— |
|
(207 |
) |
— |
|
— |
|
(415 |
) |
(622 |
) | ||||||
|
At May 31, 2018 |
|
$ |
24,504 |
|
$ |
99,442 |
|
$ |
15,949 |
|
$ |
1,665 |
|
$ |
167,157 |
|
$ |
308,717 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Accumulated depreciation |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
At May 31, 2016 |
|
$ |
— |
|
$ |
— |
|
$ |
554 |
|
$ |
513 |
|
$ |
— |
|
$ |
1,067 |
|
|
Amortization |
|
|
|
458 |
|
717 |
|
74 |
|
— |
|
1,249 |
| ||||||
|
Transfers |
|
— |
|
525 |
|
— |
|
(525 |
) |
— |
|
— |
| ||||||
|
Disposals |
|
— |
|
— |
|
(11 |
) |
— |
|
— |
|
(11 |
) | ||||||
|
At May 31, 2017 |
|
— |
|
983 |
|
1,260 |
|
62 |
|
— |
|
2,305 |
| ||||||
|
Amortization |
|
— |
|
1,517 |
|
1,697 |
|
47 |
|
— |
|
3,261 |
| ||||||
|
At May 31, 2018 |
|
$ |
— |
|
$ |
2,500 |
|
$ |
2,957 |
|
$ |
109 |
|
$ |
— |
|
$ |
5,566 |
|
|
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
At May 31, 2016 |
|
$ |
— |
|
$ |
— |
|
$ |
2,945 |
|
$ |
4,299 |
|
$ |
65 |
|
$ |
7,309 |
|
|
At May 31, 2017 |
|
$ |
10,829 |
|
$ |
15,187 |
|
$ |
4,080 |
|
$ |
200 |
|
$ |
42,159 |
|
$ |
72,455 |
|
|
At May 31, 2018 |
|
$ |
24,504 |
|
$ |
96,942 |
|
$ |
12,992 |
|
$ |
1,556 |
|
$ |
167,157 |
|
$ |
303,151 |
|
During the year ended May 31, 2018, the Company sold assets that were not yet in use prior to disposal with a cost of $622 (2017 - $33) and a net book value of $622 (2017 - $22), for proceeds of $431 (2017 - $33), resulting in a loss (gain) on sale of capital assets of $191 (2017 - $(11)).
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
10. Intangible assets
|
|
|
Customer |
|
Corporate |
|
Licences, |
|
Non-compete agreements |
|
Tokyo Smoke |
|
Intellectual |
|
Total |
| |||||||
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
At May 31, 2016 |
|
$ |
— |
|
$ |
162 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
4,428 |
|
$ |
4,590 |
|
|
Additions |
|
|
|
56 |
|
1,250 |
|
— |
|
459 |
|
— |
|
1,765 |
| |||||||
|
At May 31, 2017 |
|
— |
|
218 |
|
1,250 |
|
— |
|
459 |
|
4,428 |
|
6,355 |
| |||||||
|
Business acquisitions |
|
11,730 |
|
39 |
|
137,920 |
|
1,930 |
|
— |
|
76,190 |
|
227,809 |
| |||||||
|
Additions |
|
— |
|
152 |
|
— |
|
— |
|
— |
|
9 |
|
161 |
| |||||||
|
At May 31, 2018 |
|
$ |
11,730 |
|
$ |
409 |
|
$ |
139,170 |
|
$ |
1,930 |
|
$ |
459 |
|
$ |
80,627 |
|
$ |
234,325 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Accumulated depreciation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
At May 31, 2016 |
|
$ |
— |
|
$ |
88 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
184 |
|
$ |
272 |
|
|
Amortization |
|
|
|
68 |
|
153 |
|
— |
|
57 |
|
414 |
|
692 |
| |||||||
|
Impairment |
|
— |
|
— |
|
— |
|
— |
|
— |
|
3,500 |
|
3,500 |
| |||||||
|
At May 31, 2017 |
|
— |
|
156 |
|
153 |
|
— |
|
57 |
|
4,098 |
|
4,464 |
| |||||||
|
Amortization |
|
1,274 |
|
100 |
|
124 |
|
314 |
|
92 |
|
1,513 |
|
3,417 |
| |||||||
|
At May 31, 2018 |
|
$ |
1,274 |
|
$ |
256 |
|
$ |
277 |
|
$ |
314 |
|
$ |
149 |
|
$ |
5,611 |
|
$ |
7,881 |
|
|
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
At May 31, 2016 |
|
$ |
— |
|
$ |
74 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
4,244 |
|
$ |
4,318 |
|
|
At May 31, 2017 |
|
$ |
— |
|
$ |
62 |
|
$ |
1,097 |
|
$ |
— |
|
$ |
402 |
|
$ |
330 |
|
$ |
1,891 |
|
|
At May 31, 2018 |
|
$ |
10,456 |
|
$ |
153 |
|
$ |
138,893 |
|
$ |
1,616 |
|
$ |
310 |
|
$ |
75,016 |
|
$ |
226,444 |
|
11. Business Acquisitions
Acquisition of Broken Coast Cannabis Ltd.
On February 13, 2018, the Company entered into a share purchase agreement to purchase all of the shares of Cannan Growers Inc. (“Cannan”), a holding company owning shares of Broken Coast Cannabis Ltd. (“Broken Coast”), and to acquire the remaining shares, for a combined total of 99.86%, of the issued and outstanding shares of Broken Coast. The combined purchase price was $214,168 satisfied through the issuance of an aggregate 14,373,675 common shares. The share purchase agreement entitled the Company to control Broken Coast effective on February 1, 2018, which became the effective acquisition date.
The Company is in the process of determining the fair value of the net assets acquired and, as a result, the fair value of the net assets acquired may be subject to adjustments pending completion of final valuations and post closing adjustments. The table below summarizes the preliminary estimated fair value of the assets acquired and the liabilities assumed at the acquisition date:
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
|
|
|
Note |
|
Number of shares |
|
Share price |
|
Amount |
| ||
|
Consideration paid |
|
|
|
|
|
|
|
|
| ||
|
Shares issued |
|
(i) |
|
14,373,675 |
|
$ |
14.90 |
|
$ |
214,168 |
|
|
Total consideration paid |
|
|
|
|
|
|
|
$ |
214,168 |
| |
|
|
|
|
|
|
|
|
|
|
| ||
|
Net assets acquired |
|
|
|
|
|
|
|
|
| ||
|
Current assets |
|
|
|
|
|
|
|
|
| ||
|
Cash and cash equivalents |
|
|
|
|
|
|
|
2,007 |
| ||
|
Accounts receivable |
|
|
|
|
|
|
|
299 |
| ||
|
Other current assets |
|
|
|
|
|
|
|
43 |
| ||
|
Inventory |
|
|
|
|
|
|
|
2,572 |
| ||
|
Biological assets |
|
|
|
|
|
|
|
826 |
| ||
|
Long-term assets |
|
|
|
|
|
|
|
|
| ||
|
Capital assets |
|
|
|
|
|
|
|
13,298 |
| ||
|
Customer relationships |
|
|
|
|
|
|
|
11,730 |
| ||
|
Corporate website |
|
|
|
|
|
|
|
39 |
| ||
|
Licences, permits & applications |
|
|
|
|
|
|
|
6,320 |
| ||
|
Non-competition agreements |
|
|
|
|
|
|
|
1,930 |
| ||
|
Trademark & brands |
|
|
|
|
|
|
|
72,490 |
| ||
|
Goodwill |
|
|
|
|
|
|
|
145,794 |
| ||
|
Total assets |
|
|
|
|
|
|
|
257,348 |
| ||
|
Current liabilities |
|
|
|
|
|
|
|
|
| ||
|
Accounts payable and accrued liabilities |
|
|
|
|
|
|
|
10,455 |
| ||
|
Income taxes payable |
|
|
|
|
|
|
|
922 |
| ||
|
Long-term liabilities |
|
|
|
|
|
|
|
|
| ||
|
Deferred tax liability |
|
|
|
|
|
|
|
25,889 |
| ||
|
Long-term debt |
|
|
|
|
|
|
|
5,914 |
| ||
|
Total liabilities |
|
|
|
|
|
|
|
43,180 |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Total net assets acquired |
|
|
|
|
|
|
|
$ |
214,168 |
| |
(i) Share price based on the price of the shares on February 1, 2018.
The amount of net income and comprehensive income of Broken Coast since the acquisition date included in these consolidated financial statements was $1,837. Net income and comprehensive net income for the Company would have been higher by approximately $2,268 if the acquisition had taken place on June 1, 2017. In connection with this transaction, the Company expensed transaction costs to date of $1,643.
Acquisition of Nuuvera Corp.
On March 23, 2018, the Company completed the previously announced definitive arrangement agreement (the “Arrangement Agreement”) pursuant to which the Company acquired, by way of a court-approved plan of arrangement, under the Business Corporations Act (Ontario) (the “Transaction”), 100% of the issued and outstanding common shares (on a fully diluted basis) of Nuuvera for a total consideration of $0.62 in cash plus 0.3546 of an Aphria share for each Nuuvera share held. All of Nuuvera’s outstanding options were exchanged for an equivalent option granted pursuant to Aphria’s stock option plan (each, a “Replacement Option”) to purchase from Aphria the number of common shares (rounded to the nearest whole share) equal to: (i) the exchange ratio multiplied by (ii) the number of Nuuvera shares subject to such Nuuvera Option. Each such Replacement Option shall provide for an exercise price per common share (rounded to the nearest whole cent) equal to: (i) the exercise price per Nuuvera share purchasable pursuant to such Nuuvera Option; divided by (ii) the exchange ratio.
The Company is in the process of assessing the fair value of the net assets acquired and, as a result, the fair value of the net assets acquired may be subject to adjustments pending completion of final valuations and post closing adjustments. The table below summarizes the preliminary estimated fair value of the assets acquired and the liabilities assumed at the acquisition date:
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
|
|
|
Note |
|
Number of shares |
|
Share price |
|
Amount |
| ||
|
Consideration paid |
|
|
|
|
|
|
|
|
| ||
|
Cash |
|
|
|
|
|
|
|
$ |
54,604 |
| |
|
Shares issued |
|
(i) |
|
31,226,910 |
|
$ |
13.17 |
|
411,258 |
| |
|
Warrants outstanding |
|
(ii) |
|
1,345,866 |
|
|
|
1,015 |
| ||
|
Replacement options issued |
|
(Ii) |
|
1,280,330 |
|
|
|
12,133 |
| ||
|
|
|
|
|
|
|
|
|
479,010 |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Fair value of previously held investment |
|
|
|
|
|
|
|
|
| ||
|
Shares held by Aphria |
|
(i) |
|
1,878,738 |
|
$ |
14.92 |
|
28,028 |
| |
|
Warrants held by Aphria |
|
(ii) |
|
322,365 |
|
|
|
243 |
| ||
|
|
|
|
|
|
|
|
|
28,271 |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Total fair value of consideration |
|
|
|
|
|
|
|
$ |
507,281 |
| |
|
|
|
|
|
|
|
|
|
|
| ||
|
Net assets acquired |
|
|
|
|
|
|
|
|
| ||
|
Current assets |
|
|
|
|
|
|
|
|
| ||
|
Cash and cash equivalents |
|
|
|
|
|
|
|
35,033 |
| ||
|
Accounts receivable |
|
|
|
|
|
|
|
464 |
| ||
|
Other current assets |
|
|
|
|
|
|
|
1,142 |
| ||
|
Inventory |
|
|
|
|
|
|
|
401 |
| ||
|
Long-term assets |
|
|
|
|
|
|
|
|
| ||
|
Capital assets |
|
|
|
|
|
|
|
4,743 |
| ||
|
Intellectual property, trademarks & brands |
|
|
|
|
|
|
|
3,700 |
| ||
|
Licences, permits & applications |
|
|
|
|
|
|
|
131,600 |
| ||
|
Goodwilll |
|
|
|
|
|
|
|
375,768 |
| ||
|
Total assets |
|
|
|
|
|
|
|
552,851 |
| ||
|
Current liabilities |
|
|
|
|
|
|
|
|
| ||
|
Accounts payable and accrued liabilities |
|
|
|
|
|
|
|
9,547 |
| ||
|
Long-term liabilities |
|
|
|
|
|
|
|
|
| ||
|
Deferred tax liability |
|
|
|
|
|
|
|
36,023 |
| ||
|
Total liabilities |
|
|
|
|
|
|
|
45,570 |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Total net assets acquired |
|
|
|
|
|
|
|
$ |
507,281 |
| |
(i) Share price based on the price of the shares on March 23, 2018; shares held by Aphria include the cash consideration paid.
(ii) Options and warrants are valued using the Black-Scholes option pricing model using the following assumptions: the risk-free rate of 2.19%; expected life of 1- 10 years; volatility of 30% based on volatility used for similar instruments on the open market; forfeiture rate of nil; dividend yield of nil; and the exercise price of $2.52 - $20.30.
The amount of net loss and comprehensive loss of Nuuvera since the acquisition date included in these consolidated financial statements was $3,677. Net income and comprehensive net income for the Company would have been lower by approximately $19,611 if the acquisition had taken place on June 1, 2017. In connection with this transaction, the Company expensed transaction costs to date of $3,439.
Included in goodwill is $1,200 from the acquisition of CannWay, $145,794 from the acquisition of Broken Coast and $375,768 from the acquisition of Nuuvera.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
12. Convertible notes receivable
|
|
|
Notes receivable |
|
Embedded derivatives |
| ||||||||
|
|
|
May 31, |
|
May 31, |
|
May 31, |
|
May 31, |
| ||||
|
|
|
2018 |
|
2017 |
|
2018 |
|
2017 |
| ||||
|
CannaRoyalty Corp. |
|
$ |
— |
|
$ |
1,361 |
|
$ |
— |
|
$ |
173 |
|
|
Copperstate Farms Investors, LLC |
|
1,942 |
|
— |
|
— |
|
— |
| ||||
|
HydRx Farms Ltd. (d/b/a Scientus Pharma) |
|
8,719 |
|
— |
|
7,410 |
|
— |
| ||||
|
|
|
10,661 |
|
1,361 |
|
7,410 |
|
173 |
| ||||
|
Deduct - current portion |
|
(1,942 |
) |
— |
|
— |
|
— |
| ||||
|
|
|
$ |
8,719 |
|
$ |
1,361 |
|
$ |
7,410 |
|
$ |
173 |
|
CannaRoyalty Corp.
During the year, the Company’s note receivable from CannaRoyalty Corp. (“CR”) increased by $139 representing the recognition of accretion interest on the note and the embedded derivative increased by $1,175, representing the change in fair value of the conversion feature on the note. Prior to year-end, the Company converted the notes, and transferred $1,500 from notes receivable and $1,348 from embedded derivatives to long-term investments (Note 14).
Copperstate Farms Investors, LLC
On August 31, 2017, the Company lent Copperstate Farms Investors, LLC (“CSF”) $2,000 USD ($2,501 CAD) in exchange for a senior secured convertible loan. The convertible debenture bears interest at 9%, was originally due on May 15, 2018 (“Maturity Date”). The loan was pre-payable at any time by CSF, however no principal payments were due prior to the Maturity Date. If at least $500 USD of the outstanding loan balance was not repaid by February 28, 2018, then an automatic conversion would be triggered for $500 USD plus any accrued but unpaid interest, net of any repayments towards the principal, of the loan balance at $500 USD per unit of CSF. If the outstanding loan balance was not repaid before the Maturity Date, an automatic conversion would be triggered for the remaining loan balance at $500 USD per unit of CSF. The convertible loan was secured by a first charge on CSF’s greenhouse assets and real property located in Snowflake, Arizona. Since the option to settle payments in membership units was solely at the discretion of CSF, no embedded derivative was recognized. Prior to February 28, 2018, the Company received $500 USD as a partial repayment of the convertible note receivable.
On May 15, 2018, the Company entered into an amendment agreement with CSF which extended the maturity date and automatic conversion date to June 30, 2018, which was subsequently extended into July. As at May 31, 2018, the convertible note receivable totalled $1,500 USD ($1,942 CAD). Subsequent to year-end, this note was paid in full.
HydRx Farms Ltd. (d/b/a Scientus Pharma)
On August 14, 2017, Aphria lent $11,500 to Scientus Pharma (“SP”) as a convertible debenture. The convertible debenture bears interest at 8%, paid semi-annually, matures in two years and includes the right to convert the debenture into common shares of SP at $2.75 per common share at any time before maturity. SP maintains the option of forced conversion of the convertible debenture if the common shares of SP trade on a stock exchange at a value of $3.02 or more for 30 consecutive days.
The option to settle payments in common shares represents an embedded derivative in the form of a call option to the Company. The fair value of the derivative asset related to the convertible note is $7,410 at May 31, 2018.
During the year, the Company’s note receivable from SP increased by $1,669 representing the recognition of accretion interest on the note and the embedded derivative increased by $2,960, representing the change in fair value of the conversion feature on the note.
As at May 31, 2018, the convertible note receivable totalled $16,129.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
During the year, the Company lent a total of $14,001 in convertible notes, recognized total accretion interest revenue of $1,808, and recorded an unrealized gain on embedded derivatives of $4,135.
The fair value for the embedded derivatives was determined using the Black-Scholes option pricing model using the following assumptions: the risk-free rate of 0.85-1.15%; expected life of the convertible note; volatility of 70% based on comparable companies; forfeiture rate of nil; dividend yield of nil; and, the exercise price of the respective conversion feature.
13. Interest in equity investees
|
|
|
May 31, |
|
May 31, |
| ||
|
|
|
2018 |
|
2017 |
| ||
|
Associated company |
|
|
|
|
| ||
|
Liberty Health Sciences Inc. |
|
$ |
— |
|
$ |
28,376 |
|
|
Althea Company Pty Ltd. |
|
4,966 |
|
— |
| ||
|
|
|
$ |
4,966 |
|
$ |
28,376 |
|
Liberty Health Sciences Inc.
|
|
|
May 31, |
|
May 31, |
| ||
|
Reconciliation to carrying amount: |
|
|
|
|
| ||
|
Opening balance |
|
$ |
28,376 |
|
$ |
— |
|
|
Investment |
|
— |
|
28,166 |
| ||
|
Transfer of fair value of SecureCom shares on reverse takeover |
|
1,664 |
|
— |
| ||
|
Gain on account of dilution of ownership |
|
7,535 |
|
— |
| ||
|
Share of reported net (loss) income |
|
(9,281 |
) |
210 |
| ||
|
Share of reported comprehensive loss |
|
(801 |
) |
— |
| ||
|
Equity investee sold |
|
(6,873 |
) |
— |
| ||
|
Transfer to assets available for sale |
|
(20,620 |
) |
— |
| ||
|
Closing balance |
|
$ |
— |
|
$ |
28,376 |
|
During the year ended May 31, 2018, the Company entered into a share purchase agreement (the “Transaction”) to sell 26,716,025 common shares of Liberty in exchange for $33,395. The 26,716,025 common shares sold represented all the Company’s shares in Liberty that were not subject to Canadian Securities Exchange (“CSE”) escrow requirements at the time of the Transaction. The Transaction also included a call/put obligation (“Obligation Agreement”) for the 80,148,077 remaining shares in Liberty held by the Company, which are currently subject to the CSE mandatory escrow requirements. As each new tranche of shares becomes freely trading, the Obligation Agreement results in the buyers acquiring the newly freely trading shares at an 18% discount to the market price of Liberty, based on Liberty’s 10 day volume weighted trading price.
The Transaction includes an opt-out for Aphria’s benefit, in the event that the Toronto Stock Exchange amends their regulations such that it permits investments by Canadian companies in U.S. based cannabis businesses, and in such instance the Obligation Agreement would be automatically terminated. In exchange for the opt-out, the Company agrees to pay the buyers a $2,500 termination fee.
Based on the terms of the Obligation Agreement, the Company determined that the remaining shares held in Liberty meet the requirements under IFRS 5 and have been reclassified as held for sale. The Company has ceased accounting for the investment as an equity investment as of November 30, 2017 and transferred the carrying value $20,620 to assets held for sale. Also included in assets held for sale is $20,000 of long-term investments (Note 14). The Company recorded a derivative liability, and unrealized loss on derivative liability of $12,451 as a result of the 18% discount to the market price of Liberty, based on Liberty’s 10 day volume weighted trading price in the Obligation Agreement.
Based on its closing share price of $0.87 as at May 31, 2018, the Liberty shares held by Aphria have a fair value net of the 18% discount, of $57,178, which is $49,009 higher than the carrying value recorded in assets held for sale net of the derivative liability.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
The Company used a Monte-Carlo simulation to estimate the fair value of the derivative liability, using the following assumptions: risk-free rate of 1%; expected life of 0.4 — 2.4 years; volatility of 60% based on comparable companies; forfeiture rate of 0%; and, dividend yield of nil.
Prior to completion of the Transaction and reclassification of the investment to assets held for sale, Liberty reported a net loss $24,671 and a net comprehensive loss of $(26,798) for the period from May 1, 2017 to November 30, 2017. In accordance with the equity method, Aphria recorded a loss of $9,281 and an other comprehensive loss of $801 for the year ended May 31, 2018, from its investee relative to its ownership of the outstanding common shares at the time. The Company also recorded a gain on dilution of ownership in equity investee of $7,535 for the year ended May 31, 2018. No further loss from equity investee or gain on dilution of ownership in equity investee has been recorded in the year due to the reclassification of the investment from equity investment to asset held for sale.
Althea Company Pty Ltd. (“Althea”)
In February 2018, the Company entered into a subscription agreement with Althea for the purchase of 2,500 common shares for a total cost of $2,500 AUD ($2,483 CAD) (Note 14). On March 21, 2018 the Company acquired an additional 2,000 common shares for $2,500 AUD ($2,497 CAD). As a result of this transaction, the Company’s interest in Althea grew to 37.5%. Upon obtaining 37.5% interest in Althea, the Company determined they had significant influence, and transferred $5,000 AUD ($4,980 CAD) from long-term investments to equity accounted investee (Note 14).
The following table summarizes, in aggregate, the financial information of the Company’s associate as included in their own financial statements.
|
|
|
March 31, |
|
May 31, 2017 |
| ||
|
Current assets |
|
$ |
3,857 |
|
$ |
— |
|
|
Non-current assets |
|
3 |
|
— |
| ||
|
Current liabilities |
|
14 |
|
— |
| ||
|
Non-current liabilities |
|
— |
|
— |
| ||
|
Net assets |
|
$ |
3,874 |
|
$ |
— |
|
For the period from March 21, 2018 to March 31, 2018 the investee, Althea, reported a net loss of $41 AUD on its financial statements. In accordance with the equity method, the Company recorded a loss of $14, for the year ended May 31, 2018, from its investee relative to its ownership of the outstanding common shares at the time.
|
|
|
May 31, |
|
May 31, |
| ||
|
Reconciliation to carrying amount: |
|
|
|
|
| ||
|
Opening balance |
|
$ |
— |
|
$ |
— |
|
|
Transfer from long-term investments |
|
2,483 |
|
— |
| ||
|
Cash contributions, net of share issuance costs |
|
2,497 |
|
— |
| ||
|
Share of reported net (loss) income |
|
(14 |
) |
— |
| ||
|
Closing balance |
|
$ |
4,966 |
|
$ |
— |
|
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
14. Long-term investments
|
|
|
Cost |
|
|
Fair value |
|
Investment |
|
Divesture/ |
|
|
Subtotal |
|
Change in |
|
Fair value |
| |||||||
|
Level 1 on fair value hierarchy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
CannaRoyalty Corp. |
|
$ |
1,380 |
|
|
$ |
1,793 |
|
$ |
2,848 |
|
$ |
(1,793 |
) |
|
$ |
2,848 |
|
$ |
917 |
|
$ |
3,765 |
|
|
Kalytera Therapeutics, Inc. |
|
3,014 |
|
|
1,111 |
|
— |
|
(1,111 |
) |
|
— |
|
— |
|
— |
| |||||||
|
MassRoots, Inc. |
|
508 |
|
|
562 |
|
— |
|
(232 |
) |
|
330 |
|
(166 |
) |
164 |
| |||||||
|
SecureCom Mobile Inc. |
|
520 |
|
|
1,664 |
|
— |
|
(1,664 |
) |
|
— |
|
— |
|
— |
| |||||||
|
Tetra Bio-Pharma Inc. |
|
2,300 |
|
|
9,500 |
|
— |
|
— |
|
|
9,500 |
|
(2,700 |
) |
6,800 |
| |||||||
|
Canabo Medical Inc. |
|
1,160 |
|
|
316 |
|
— |
|
(316 |
) |
|
— |
|
— |
|
— |
| |||||||
|
Hiku Brands Company Ltd. |
|
— |
|
|
— |
|
8,775 |
|
1,000 |
|
|
9,775 |
|
3,783 |
|
13,558 |
| |||||||
|
Nuuvera Inc. |
|
— |
|
|
— |
|
8,423 |
|
(8,423 |
) |
|
— |
|
— |
|
— |
| |||||||
|
Scythian Biosciences Corp. |
|
— |
|
|
— |
|
9,349 |
|
— |
|
|
9,349 |
|
(746 |
) |
8,603 |
| |||||||
|
National Access Cannabis |
|
— |
|
|
— |
|
1,093 |
|
— |
|
|
1,093 |
|
(383 |
) |
710 |
| |||||||
|
|
|
8,882 |
|
|
14,946 |
|
30,488 |
|
(12,539 |
) |
|
32,895 |
|
705 |
|
33,600 |
| |||||||
|
Level 2 on fair value hierarchy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Hiku Brands Company Ltd. |
|
— |
|
|
— |
|
2,336 |
|
— |
|
|
2,336 |
|
(430 |
) |
1,906 |
| |||||||
|
Nuuvera Inc. |
|
— |
|
|
— |
|
1,627 |
|
(1,627 |
) |
|
— |
|
— |
|
— |
| |||||||
|
Scythian Biosciences Corp. |
|
— |
|
|
— |
|
3,153 |
|
— |
|
|
3,153 |
|
(2,492 |
) |
661 |
| |||||||
|
|
|
— |
|
|
— |
|
7,116 |
|
(1,627 |
) |
|
5,489 |
|
(2,922 |
) |
2,567 |
| |||||||
|
Level 3 on fair value hierarchy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Copperstate Farms, LLC |
|
1,755 |
|
|
1,755 |
|
— |
|
— |
|
|
1,755 |
|
3,545 |
|
5,300 |
| |||||||
|
Copperstate Farms Investors, LLC |
|
7,539 |
|
|
7,560 |
|
1,868 |
|
— |
|
|
9,428 |
|
5,272 |
|
14,700 |
| |||||||
|
Resolve Digital Health Inc. |
|
718 |
|
|
1,000 |
|
— |
|
— |
|
|
1,000 |
|
2,300 |
|
3,300 |
| |||||||
|
Resolve Digital Health Inc. |
|
282 |
|
|
242 |
|
— |
|
— |
|
|
242 |
|
1,674 |
|
1,916 |
| |||||||
|
Green Acre Capital Fund |
|
300 |
|
|
285 |
|
1,300 |
|
— |
|
|
1,585 |
|
457 |
|
2,042 |
| |||||||
|
Scythian Biosciences Inc. |
|
2,000 |
|
|
2,000 |
|
— |
|
(2,000 |
) |
|
— |
|
— |
|
— |
| |||||||
|
TS BrandCo Holdings Inc. |
|
— |
|
|
— |
|
1,000 |
|
(1,000 |
) |
|
— |
|
— |
|
— |
| |||||||
|
Nuuvera Inc. |
|
— |
|
|
— |
|
6,979 |
|
(6,979 |
) |
|
— |
|
— |
|
— |
| |||||||
|
Green Tank Holdings Corp. |
|
— |
|
|
— |
|
650 |
|
— |
|
|
650 |
|
(3 |
) |
647 |
| |||||||
|
Althea Company Pty Ltd. |
|
— |
|
|
— |
|
2,483 |
|
(2,483 |
) |
|
— |
|
— |
|
— |
| |||||||
|
IBBZ Krankenhaus GmbH |
|
— |
|
|
— |
|
1,956 |
|
— |
|
|
1,956 |
|
— |
|
1,956 |
| |||||||
|
|
|
12,594 |
|
|
12,842 |
|
16,236 |
|
(12,462 |
) |
|
16,616 |
|
13,245 |
|
29,861 |
| |||||||
|
|
|
21,476 |
|
|
27,788 |
|
53,840 |
|
(26,628 |
) |
|
55,000 |
|
11,028 |
|
66,028 |
| |||||||
|
Deduct - assets held for sale |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(20,000 |
) | |||||||
|
|
|
$ |
21,476 |
|
|
$ |
27,788 |
|
$ |
53,840 |
|
$ |
(26,628 |
) |
|
$ |
55,000 |
|
$ |
11,028 |
|
$ |
46,028 |
|
The fair value attached to warrants in both Level 2 and Level 3 were determined using the Black-Scholes option pricing model using the following assumptions: risk-free rate of 0.75-1.70% on the date of grant; expected life of 1 and 2 years; volatility of 70% based on comparable companies; forfeiture rate of 0%; dividend yield of nil; and, the exercise price of the respective warrant.
CannaRoyalty Corp. (“CR”)
During the year, the Company converted its convertible debt into 750,000 shares of CR, and transferred $2,850 from convertible notes receivable (Note 12). In addition, the Company sold 1,100,000 common shares in CR (Note 26). As at May 31, 2018, the Company holds 750,000 common shares at a cost of $1,500, with a fair value of $3,765.
Kalytera Therapeutics, Inc.
During the year ended May 31, 2018, the Company sold its 6,172,000 common shares in Kalytera Therapeutics, Inc. (Note 26).
MassRoots, Inc.
During the year ended May 31, 2018, the Company sold 350,000 common shares in MassRoots, Inc. (Note 26). The Company holds 500,000 common shares at a cost of $251 USD ($304 CAD), with a fair value of $127 USD ($164 CAD) as at May 31, 2018.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
SecureCom Mobile Inc. (“SecureCom”)
In July 2017, SecureCom amalgamated with DFMMJ and was re-named Liberty. As a result, the Company transferred the fair value of its investment in SecureCom into its investment in Liberty recognized as Interest in equity investees (Note 13).
Tetra Bio-Pharma Inc.
The Company owns 10,000,000 common shares at a cost of $2,300, with a fair value of $6,800 as at May 31, 2018.
Canabo Medical Inc.
During the year ended May 31, 2018, the Company sold its 800,000 common shares in Canabo Medical Inc. (Note 26).
Hiku Brands Company Ltd (formerly TS BrandCo Holdings Inc.)
In June 2017, the Company entered into a subscription agreement with TS BrandCo Holdings Inc. (“Tokyo Smoke”) for the purchase of 140,845 common shares, for a total cost of $1,000. In January 2018, TS BrandCo Holdings Inc. merged with DOJA Cannabis Company Ltd. and renamed the reporting issuer Hiku Brands Company Ltd. (“Hiku”). As part of the merger, each common share of Tokyo Smoke was exchanged for 13 common shares of Hiku. Subsequently, the Company contributed $10,000 as an equity investment in Hiku for 7,194,244 common shares and 7,194,244 common share purchase warrants, exercisable at $2.10 per warrant at any time for a period expiring two years from the date of issuance. The Company also entered into a supply agreement with Hiku. As part of the consideration for the supply agreement, the Company received 799,361 common shares and 799,361 common share purchase warrants, exercisable at $2.10 per warrant at any time for a period expiring two years from the date of issuance. As a result of these transactions, the Company holds 9,824,590 common shares and 7,993,605 common share purchase warrants at a cost of $12,111, with a fair value of $15,464 as at May 31, 2018.
Subsequent to year-end, all the issued and outstanding common shares of Hiku were acquired by a third party. The Company maintains the supply agreements identified previously.
Nuuvera Inc. (“Nuuvera”)
In August 2017, the Company entered into a subscription agreement with Nuuvera for the purchase of 2,000,000 common shares, for a total cost of $2,029. In November 2017, the Company purchased an additional 1,980,000 common shares for $4,950. In January 2018, the Company sold 500,000 common shares for gross proceeds of $2,945 (Note 26). In January 2018, Nuuvera began trading on the TSX-Venture Exchange.
In February 2018, the Company purchased an additional 1,818,190 units for $10,050. Each unit is comprised of one common share and one half of one common share purchase warrant. Each whole common share purchase warrant is exercisable to purchase one common share at a price of $7.20 per share for a period of 24 months.
In March 2018, the Company acquired 100% of the issued and outstanding common shares of Nuuvera (Note 11).
Scythian Biosciences Inc. (“Scythian”)
In August 2017, the Company’s subscription receipts converted to common shares. As part of the conversion, Scythian consolidated its shares on a 20:1 basis. On August 8, 2017, Scythian began trading on the TSX-Venture Exchange. In November, 2017, the Company sold its 250,000 common shares in Scythian (Note 26).
In February 2018, the Company purchased 672,125 units of Scythian for $12,502. Each unit is comprised of one common share and one common share purchase warrant. Each common share purchase warrant is exercisable to purchase one common share at a price of $22.00 per share for a period of 24 months. In April 2018, Scythian declared a 4:1 stock split. As a result, the Company received an additional 2,016,375 common shares and each common share purchase warrant is exercisable to purchase four common shares at a price of $22.00 per warrant for a period of 24 months. The Company holds 2,688,500 common shares and 672,125 common share purchase warrants at a cost of $12,502, with a fair value of $9,264 as at May 31, 2018.
National Access Cannabis
In March 2018, the Company acquired 1,000,000 common shares of National Access Cannabis for $1,093. The Company owns 1,000,000 common shares at a cost of $1,093, with a fair value of $710 as at May 31, 2018.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Copperstate Farms, LLC (“Copperstate”) and Copperstate Farms Investors, LLC (“CSF”)
In July 2017, the Company purchased an additional 2,668 membership units in CSF for $1,334 USD ($1,668 CAD). The Company owns 5,000 membership units in Copperstate for total cost of $1,300 USD ($1,755 CAD), with a fair value of $5,300 and owns 13,868 membership units in CSF for a total cost of $7,094 USD ($9,407 CAD) with a fair value of $14,700 as at May 31, 2018.
During the year ended May 31, 2018, the Company entered into a definitive agreement with respect to the sale of Aphria’s subsidiary Aphria (Arizona) Inc. and its sole holdings being the minority interests in Copperstate and CSF to Liberty for a purchase price of $20,000. Subsequent to entering into this definitive agreement, the existing investors in Copperstate and CSF exercised their right of first refusal to purchase the minority interests on the same terms. The fair value has been determined by the sale price from the definitive agreement with Liberty. As a result of the definitive agreement, the Company has recorded the total value of $20,000 as held for sale (Note 13). Subsequent to year-end, the Company received the $20,000 from the sale of the shares of Copperstate and CSF.
Resolve Digital Health Inc. (“Resolve”)
During the year, the Company received an additional 200,002 penalty units comprised of 200,002 common shares and 200,002 common share purchase warrants, exercisable at $0.65 per warrant at any time for a period expiring December 1, 2021. The warrants contain a forced conversion provision if Resolve trades on a public stock exchange at a price of more than $1.30 for a period of at least 30 days. The Company owns 2,200,026 common shares and 2,200,026 warrants at a total cost of $1,000, with a fair value of $5,216 as at May 31, 2018. The Company determined the fair value of its investment based on Resolve’s most recent financing.
Green Acre Capital Fund
The Company committed $2,000 to the expected $25,000 fund and as of the balance sheet date has funded $1,600. The Company determined that the fair value of its investment, based on its proportionate share of net assets, was $2,042 as at May 31, 2018.
Green Tank Holdings Corp. (“Green Tank”)
In November 2017, the Company entered into a subscription agreement with Green Tank Holdings Corp. for the purchase of 98,425 preferred shares, for a total cost of $500 USD ($650 CAD). The Company determined the fair value of its investment, based on Green Tank’s most recent financing at the same price, is equal to its carrying value. The Company recognized a loss from the change in fair value of $3 due to changes in the foreign exchange rate.
Althea Company Pty Ltd. (“Althea”)
In February 2018, the Company entered into a subscription agreement with Althea for the purchase of 2,500 common shares, for a total cost of $2,500 AUD ($2,483 CAD). Part of the consideration was satisfied through a promissory note (Note 17). On March 21, 2018, the Company acquired an additional 2,000 common shares for a total cost of $2,500 AUD ($2,497 CAD). As a result of the second investment, the Company has a 37.5% interest in Althea, and has determined that it exercises significant influence on Althea. Accordingly, the cost of this investment has been recorded as interest in equity investees (Note 13).
IBBZ Krankenhaus GmbH Klinik Hygiea (“Krankenhaus”)
In May 2018, the Company acquired a 25.1 % interest in Krankenhaus, which is the owner and operator of Berlin-based Schöneberg Hospital, for €1,294 ($1,956 CAD). Through this investment the Company is entitled to 5% of the net income (loss) for the years 2018 to 2021, and 10% of the net income (loss) for the period thereafter. The Company determined that the fair value of its investment, based on Krankenhaus’ most recent financing at the same price, is equal to its carrying value.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
15. Income taxes and deferred income taxes
A reconciliation of income taxes at the statutory rate with the reported taxes is as follows:
|
|
|
For the year ended |
| ||||
|
|
|
2018 |
|
2017 |
| ||
|
Income before income taxes |
|
$ |
35,856 |
|
$ |
4,332 |
|
|
Statutory rate |
|
26.5 |
% |
26.5 |
% | ||
|
Expected income tax expense at combined basic federal and provincial tax rate |
|
9,502 |
|
1,148 |
| ||
|
Effect on income taxes of: |
|
|
|
|
| ||
|
Permanent differences |
|
65 |
|
— |
| ||
|
Non-deductible share-based compensation and other expenses |
|
4,737 |
|
659 |
| ||
|
Non-taxable portion of losses (gains) |
|
(7,162 |
) |
(534 |
) | ||
|
Utilization of tax attributes not previously recognized |
|
— |
|
(876 |
) | ||
|
Deductible share issuance costs |
|
— |
|
(286 |
) | ||
|
Other |
|
(768 |
) |
23 |
| ||
|
Tax assets not recognized |
|
34 |
|
— |
| ||
|
|
|
$ |
6,408 |
|
$ |
134 |
|
|
Income tax expense is comprised of: |
|
|
|
|
| ||
|
Current |
|
$ |
2,750 |
|
$ |
— |
|
|
Future |
|
3,658 |
|
134 |
| ||
|
|
|
$ |
6,408 |
|
$ |
134 |
|
The following table summarizes the components of deferred tax:
|
|
|
May 31, |
|
May 31, |
| ||
|
Deferred tax assets |
|
|
|
|
| ||
|
Non-capital loss carry forward |
|
$ |
4,567 |
|
$ |
1,313 |
|
|
Capital loss carry forward |
|
405 |
|
381 |
| ||
|
Share issuance and financing fees |
|
5,443 |
|
3,448 |
| ||
|
Unrealized loss |
|
916 |
|
— |
| ||
|
Other |
|
27 |
|
34 |
| ||
|
Deferred tax liabilities |
|
|
|
|
| ||
|
Net book value in excess of undepreciated capital cost |
|
(1,017 |
) |
(164 |
) | ||
|
Intangible assets in excess of tax costs |
|
(64,120 |
) |
(194 |
) | ||
|
Unrealized gain |
|
(1,097 |
) |
(914 |
) | ||
|
Biological assets and inventory in excess of tax costs |
|
(4,377 |
) |
(589 |
) | ||
|
Net deferred tax (liabilities) assets |
|
$ |
(59,253 |
) |
$ |
3,315 |
|
16. Bank indebtedness
The Company secured an operating line of credit in the amount of $1,000 which bears interest at the lender’s prime rate plus 75 basis points. As of the May 31, 2018, the Company has not drawn on the line of credit. The operating line of credit is secured by a first charge on the property at 265 Talbot St. West, Leamington, Ontario and a first ranking position on a general security agreement.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
17. Promissory note payable
|
|
|
May 31, |
|
May 31, |
| ||
|
|
|
2018 |
|
2017 |
| ||
|
Note payable to Copperstate Farms, LLC - $1,300 USD ($1,755), opening balance, bearing nominal interest, two-year term, repayable in eight quarterly instalments of $162 USD |
|
$ |
1,244 |
|
$ |
1,539 |
|
|
Reduction of Promissory note payable balance with respect to consulting services provided |
|
(1,244 |
) |
(295 |
) | ||
|
Balance remaining |
|
— |
|
1,244 |
| ||
|
Deduct - principal portion included in current liabilities |
|
— |
|
(878 |
) | ||
|
|
|
$ |
— |
|
$ |
366 |
|
On May 15, 2018, the Company entered into an amendment agreement with CSF which resulted in the Company no longer expecting to provide any further consulting services. Accordingly, the Company has recorded the remaining balance of the loan as consulting revenue.
During the year ended May 31, 2018, the Company entered into a promissory note with Althea for $700 AUD ($686), as part of the purchase of Althea common shares (Note 14). The note is due and payable on December 31, 2020. The Company reached an agreement with Althea where the promissory note amount will be used by Althea to purchase products from the Company in connection with a supply agreement entered into in September 2017.
|
|
|
May 31, |
|
May 31, |
| ||
|
Note payable to Althea Company Pty Ltd - $700 AUD ($686), opening balance, non-interest bearing, due and payable on December 31, 2020 |
|
$ |
686 |
|
$ |
— |
|
|
Reduction of Promissory note payable balance with respect to products provided |
|
(63 |
) |
— |
| ||
|
Foreign exchange (gain) loss |
|
(13 |
) |
|
| ||
|
Balance remaining |
|
610 |
|
— |
| ||
|
Deduct - principal portion included in current liabilities |
|
(610 |
) |
— |
| ||
|
|
|
$ |
— |
|
$ |
— |
|
18. Long-term debt
|
|
|
May 31, |
|
May 31, |
| ||
|
|
|
2018 |
|
2017 |
| ||
|
Term loan - $25,000 - 3.95%, compounded monthly, 5 year term with a 15-year amortization, repayable in equal monthly installments of $188 including interest, due in April 2022 |
|
$ |
24,107 |
|
$ |
25,000 |
|
|
Term loan - $1,250 - 3.99%, 5-year term, with a 10-year amortization, repayable in equal monthly instalments of $13 including interest, due in July 2021 |
|
1,057 |
|
1,164 |
| ||
|
Mortgage payable - $3,750 - 3.95%, 5-year term, with a 20-year amortization, repayable in equal monthly instalments of $23 including interest, due in July 2021 |
|
3,515 |
|
3,645 |
| ||
|
Vendor take-back mortgage owed to related party - $2,850 - 6.75%, 5-year term, repayable in equal monthly instalments of $56 including interest, due in June 2021 |
|
1,869 |
|
2,396 |
| ||
|
|
|
30,548 |
|
32,205 |
| ||
|
Deduct - unamortized financing fees |
|
(71 |
) |
(20 |
) | ||
|
- principal portion included in current liabilities |
|
(2,140 |
) |
(765 |
) | ||
|
|
|
$ |
28,337 |
|
$ |
31,420 |
|
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Total long-term debt repayments are as follows:
|
Next 12 months |
|
$ |
2,140 |
|
|
2 years |
|
2,241 |
| |
|
3 years |
|
2,348 |
| |
|
4 years |
|
23,819 |
| |
|
Balance of obligation |
|
$ |
30,548 |
|
The term loan of $24,107 was entered into on May 9, 2017 and is secured by a first charge on the property at 265 Talbot Street West, Leamington Ontario, a first position on a general security agreement, and an assignment of fire insurance to the lender. Principal payments started on the term loan in March 2018.
The term loan of $1,057 and mortgage payable of $3,515 were entered into on July 22, 2016 and are secured by a first charge on the property at 265 Talbot St. West, Leamington, Ontario and a first position on a general security agreement.
The vendor take-back mortgage payable of $1,869, owed to a director of the Company, was entered into on June 30, 2016 in conjunction with the acquisition of the property at 265 Talbot St. West. The mortgage is secured by a second charge on the property at 265 Talbot St. West, Leamington, Ontario.
The Company acquired term loans of $3,000 and $1,201, and a mortgage payable of $1,713 as part of the acquisition of Broken Coast (Note 11). These loans and mortgages were paid in full during the year.
19. Share capital
The Company is authorized to issue an unlimited number of common shares. As at May 31, 2018, the Company has issued 210,169,924 shares, of which 1,777,971 shares were held and subject to various escrow agreements.
|
Common Shares |
|
Number of |
|
Amount |
| |
|
Balance at May 31, 2017 |
|
138,628,704 |
|
$ |
274,317 |
|
|
November 2017 bought deal, net of cash issuance costs |
|
12,689,675 |
|
86,661 |
| |
|
January 2018 bought deal, net of cash issuance costs |
|
8,363,651 |
|
109,000 |
| |
|
Broken Coast acquisition |
|
14,373,675 |
|
214,168 |
| |
|
Nuuvera acquisition |
|
31,226,910 |
|
411,258 |
| |
|
Warrants exercised |
|
2,388,636 |
|
3,767 |
| |
|
Options exercised |
|
2,493,623 |
|
11,559 |
| |
|
Deferred share units exercised |
|
5,050 |
|
62 |
| |
|
Income tax recovery on share issuance costs |
|
— |
|
3,002 |
| |
|
Shares held in escrow earned in exchange for services |
|
— |
|
187 |
| |
|
|
|
210,169,924 |
|
$ |
1,113,981 |
|
a) Throughout the year, 2,388,636 warrants with exercise prices ranging from $1.50 to $1.75 were exercised for a value of $3,767 including any cash consideration.
b) Throughout the year, 2,493,623 shares were issued from the exercise of stock options with exercise prices ranging from $0.60 to $9.05 for a value of $11,559, including any cash consideration.
c) Throughout the year, 5,050 shares were issued in accordance with the deferred share unit plan to former directors of the Company.
d) In January 2017, the Company issued 112,500 common shares in escrow pursuant to a third party consulting agreement for greenhouse related services, net of cash issuance costs. At May 31, 2018, all 112,500 common shares of the shares in escrow have been released.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
e) In November 2017, the Company closed a bought deal financing in which it issued 12,689,675 common shares at a purchase price of $7.25 per share for $86,661, net of cash issuance costs.
f) In January 2018, the Company closed a bought deal financing in which it issued 8,363,651 common shares at a purchase price of $13.75 per share for $109,000 net of cash issuance costs.
g) In February 2018, the Company completed the acquisition of Broken Coast (Note 11) in which it issued 14,373,675 common shares at a deemed price of $14.90 per share for $214,168.
h) In March 2018, the Company completed the acquisition of Nuuvera (Note 11) in which it issued 31,226,910 common shares at a deemed price of $13.17 per share for $411,258.
i) During the year, the Company recognized a $3,002 income tax recovery on share issuance costs.
20. Warrants
The warrant details of the Company are as follows:
|
Type of warrant |
|
Expiry date |
|
Number of |
|
Weighted |
|
Amount |
| ||
|
Warrant |
|
December 11, 2018 |
|
36,003 |
|
1.75 |
|
— |
| ||
|
Warrant |
|
December 2, 2019 |
|
1,261,269 |
|
1.50 |
|
— |
| ||
|
Warrant |
|
September 26, 2021 |
|
200,000 |
|
3.14 |
|
360 |
| ||
|
Nuuvera warrant |
|
February 14, 2020 |
|
1,345,866 |
|
20.30 |
|
1,015 |
| ||
|
|
|
|
|
2,843,138 |
|
$ |
10.52 |
|
$ |
1,375 |
|
|
|
|
May 31, 2018 |
|
May 31, 2017 |
| ||||||
|
|
|
Number of |
|
Weighted |
|
Number of |
|
Weighted |
| ||
|
Outstanding, beginning of the period |
|
3,885,908 |
|
$ |
1.61 |
|
18,721,987 |
|
$ |
1.51 |
|
|
Expired during the period |
|
— |
|
— |
|
(50,305 |
) |
1.20 |
| ||
|
Issued during the period |
|
1,345,866 |
|
20.30 |
|
465,391 |
|
2.35 |
| ||
|
Exercised during the period |
|
(2,388,636 |
) |
1.54 |
|
(15,251,165 |
) |
1.51 |
| ||
|
Outstanding, end of the period |
|
2,843,138 |
|
$ |
10.52 |
|
3,885,908 |
|
$ |
1.61 |
|
In March 2018, the Company completed the acquisition of Nuuvera (Note 11) in which it reserved 1,345,866 common shares for issuance to the holders of certain common share purchase warrants of Nuuvera (“Nuuvera Warrants”). There are 3,795,450 Nuuvera Warrants, exercisable for Nuuvera shares at an exercise price of $7.20 per share, the Nuuvera shares would convert to 0.3546 Aphria shares and $0.62 cash.
21. Stock options
The Company adopted a stock option plan under which it is authorized to grant options to officers, directors, employees and consultants enabling them to acquire common shares of the Company. The maximum number of common shares reserved for issuance of stock options that can be granted under the plan is 10% of the issued and outstanding common shares of the Company. The options granted can be exercised for up to a maximum of 10 years and vest as determined by the Board of Directors. The exercise price of each option can not be less than the market price of the common shares on the date of grant.
The Company recognized a share-based compensation expense of $15,780 during the year ended May 31, 2018 (2017 - $2,064), including $4,570 of options granted as part of the acquisition of Broken Coast. The total fair value of options granted during the year was $28,912 (2017 - $4,222), including $9,509 of options granted as part of the acquisition of Broken Coast.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
|
|
|
May 31, 2018 |
|
May 31, 2017 |
| ||||||
|
|
|
Number of |
|
Weighted |
|
Number of |
|
Weighted |
| ||
|
Outstanding, beginning of the period |
|
5,926,001 |
|
$ |
1.99 |
|
4,975,000 |
|
$ |
0.84 |
|
|
Exercised during the period |
|
(2,637,363 |
) |
2.30 |
|
(1,121,999 |
) |
1.05 |
| ||
|
Issued during the period |
|
6,703,330 |
|
11.12 |
|
2,253,000 |
|
3.99 |
| ||
|
Cancelled during the period |
|
(1,035,773 |
) |
11.77 |
|
(180,000 |
) |
1.09 |
| ||
|
Outstanding, end of the period |
|
8,956,195 |
|
$ |
7.60 |
|
5,926,001 |
|
$ |
1.99 |
|
|
Exercisable, end of the period |
|
4,507,696 |
|
$ |
4.04 |
|
3,919,542 |
|
$ |
1.36 |
|
In June 2017, the Company issued 250,000 stock options at an exercise price of $5.44 per share, exercisable for 5 years to officers of the company. 83,333 vested immediately and the remainder vest over 2 years.
In July 2017, the Company issued 1,015,000 stock options at an exercise price of $5.24 per share, exercisable for 3 years to employees, officers and consultants of the company. 688,333 vested immediately and the remainder vest over 2 years.
In October 2017, the Company issued 533,000 stock options at an exercise price of $6.90 per share, exercisable for 3 to 5 years to employees, officers and consultants of the company. 244,330 vested immediately and the remainder vest over 2 years.
In November 2017, the Company issued 330,000 stock options at an exercise price of $9.05 - $9.28 per share, exercisable for 3 years to employees and consultants of the company. 109,998 vested immediately and the remainder vest over 2 years.
In December 2017, the Company issued 100,000 stock options at an exercise price of $14.06 per share, exercisable for 3 years to employees of the company. 33,333 vested immediately and the remainder vest over 2 years.
In January 2018, the Company issued 1,000,000 stock options at an exercise price of $20.19 per share, exercisable for 3 years as part of the acquisition of Broken Coast. All of the options vest over 3 years.
In January 2018, the Company issued 725,000 stock options at an exercise price of $21.70 - $22.89 per share, exercisable for 3 — 5 years to employees, officers and consultants of the company. 171,662 vested immediately and the remainder vest over 2 — 3 years.
In March 2018, as part of the acquisition of Nuuvera, the Company issued 1,280,914 replacement options at an exercise price of $2.52 - $14.38, exercisable for 7 — 10 years to former option holders of Nuuvera. 1,211,197 vested immediately and the remainder vest over 8 months.
In March 2018, the Company issued 160,000 stock options at an exercise price of $12.39 - $14.39 per share, exercisable for 3 years to employees of the company. 23,332 vested immediately and the remainder vest over 1 — 3 years.
In April 2018, the Company issued 1,310,000 stock options at an exercise price of $9.98 - $11.40 per share, exercisable for 3 years to employees, officers and consultants of the company. 133,333 vested immediately and the remainder vest over 1 — 3 years.
In May 2018, directors and officers of the Company rescinded 541,000 stock options at an exercise price of $6.90 — $21.70 per share. As at that date, $1,907 was recorded as share based compensation. The Company has reclassified $1,907 from contributed surplus to retained earnings as part of this forfeiture.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
The outstanding option details of the Company are as follows:
|
Expiry date |
|
Weighted |
|
Number of |
|
Vested and |
| |
|
October 2018 |
|
$ |
1.17 |
|
20,000 |
|
20,000 |
|
|
November 2018 |
|
$ |
1.49 |
|
20,000 |
|
20,000 |
|
|
December 2018 |
|
$ |
1.30 |
|
20,000 |
|
20,000 |
|
|
April 2019 |
|
$ |
1.67 |
|
30,000 |
|
30,000 |
|
|
June 2019 |
|
$ |
0.60 |
|
1,480,000 |
|
1,480,000 |
|
|
September 2019 |
|
$ |
3.00 |
|
42,365 |
|
35,915 |
|
|
October 2019 |
|
$ |
3.47 |
|
13,400 |
|
6,733 |
|
|
November 2019 |
|
$ |
3.90 |
|
837,052 |
|
516,709 |
|
|
December 2019 |
|
$ |
5.25 |
|
500,000 |
|
133,332 |
|
|
January 2020 |
|
$ |
5.72 |
|
20,668 |
|
5,667 |
|
|
April 2020 |
|
$ |
7.92 |
|
133,334 |
|
88,333 |
|
|
June 2020 |
|
$ |
5.44 |
|
216,668 |
|
49,999 |
|
|
July 2020 |
|
$ |
5.24 |
|
761,658 |
|
456,967 |
|
|
September 2020 |
|
$ |
0.85 |
|
185,000 |
|
185,000 |
|
|
October 2020 |
|
$ |
6.90 |
|
381,000 |
|
94,330 |
|
|
November 2020 |
|
$ |
9.05 |
|
270,000 |
|
83,332 |
|
|
November 2020 |
|
$ |
9.28 |
|
50,000 |
|
16,666 |
|
|
December 2020 |
|
$ |
14.06 |
|
100,000 |
|
33,333 |
|
|
January 2021 |
|
$ |
21.70 |
|
10,000 |
|
3,333 |
|
|
January 2021 |
|
$ |
22.89 |
|
150,000 |
|
33,330 |
|
|
January 2021 |
|
$ |
22.08 |
|
50,000 |
|
16,666 |
|
|
March 2021 |
|
$ |
14.39 |
|
90,000 |
|
23,332 |
|
|
March 2021 |
|
$ |
11.40 |
|
300,000 |
|
— |
|
|
March 2021 |
|
$ |
9.98 |
|
200,000 |
|
— |
|
|
March 2021 |
|
$ |
12.39 |
|
50,000 |
|
— |
|
|
April 2021 |
|
$ |
11.40 |
|
710,000 |
|
100,000 |
|
|
April 2021 |
|
$ |
11.45 |
|
100,000 |
|
33,333 |
|
|
May 2021 |
|
$ |
20.19 |
|
1,000,000 |
|
— |
|
|
June 2021 |
|
$ |
1.40 |
|
193,336 |
|
100,000 |
|
|
August 2021 |
|
$ |
1.64 |
|
110,000 |
|
69,993 |
|
|
October 2022 |
|
$ |
6.90 |
|
74,000 |
|
74,000 |
|
|
July 2027 |
|
$ |
2.52 |
|
328,369 |
|
268,048 |
|
|
November 2027 |
|
$ |
6.29 |
|
250,693 |
|
250,693 |
|
|
December 2027 |
|
$ |
6.29 |
|
99,482 |
|
99,482 |
|
|
March 2028 |
|
$ |
12.29 |
|
119,378 |
|
119,378 |
|
|
March 2028 |
|
$ |
14.38 |
|
39,792 |
|
39,792 |
|
|
Outstanding, end of the period |
|
$ |
7.60 |
|
8,956,195 |
|
4,507,696 |
|
The Company used the Black-Scholes option pricing model to determine the fair value of options granted using the following assumptions: risk-free rate of 0.75-1.70% on the date of grant; expected life of 3 – 10 years; volatility of 70% based on comparable companies; forfeiture rate of 0%; dividend yield of nil; and, the exercise price of the respective option.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
22. Non-controlling interest
The following tables summarise the information relating to the Company’s subsidiary, Aphria Diamond, before intercompany eliminations.
|
|
|
May 31, |
|
May 31, |
| ||
|
Current assets |
|
$ |
7,313 |
|
$ |
— |
|
|
Non-current assets |
|
83,207 |
|
— |
| ||
|
Current liabilities |
|
(10,085 |
) |
— |
| ||
|
Non-current liabilities |
|
(60,884 |
) |
— |
| ||
|
Net assets |
|
19,551 |
|
— |
| ||
|
Non-controlling interest % |
|
49 |
% |
— |
| ||
|
Non-controlling interest |
|
$ |
9,580 |
|
$ |
— |
|
|
|
|
For the year ended |
| ||||
|
|
|
May 31, |
| ||||
|
|
|
2018 |
|
2017 |
| ||
|
Revenue |
|
$ |
— |
|
$ |
— |
|
|
Total expenses |
|
(449 |
) |
— |
| ||
|
Net loss and comprehensive loss |
|
(449 |
) |
— |
| ||
|
Non-controlling interest % |
|
49 |
% |
— |
| ||
|
|
|
$ |
(220 |
) |
$ |
— |
|
23. General and administrative expenses
|
|
|
For the year ended |
| ||||
|
|
|
2018 |
|
2017 |
| ||
|
Executive compensation |
|
$ |
1,794 |
|
$ |
829 |
|
|
Consulting fees |
|
1,154 |
|
220 |
| ||
|
Office and general |
|
3,562 |
|
1,336 |
| ||
|
Professional fees |
|
2,951 |
|
608 |
| ||
|
Salaries and wages |
|
3,295 |
|
1,142 |
| ||
|
Travel and accommodation |
|
889 |
|
464 |
| ||
|
Rent |
|
256 |
|
79 |
| ||
|
|
|
$ |
13,901 |
|
$ |
4,678 |
|
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
24. Share-based compensation
Share-based compensation is comprised of:
|
|
|
For the year ended |
| ||||
|
|
|
May 31, |
| ||||
|
|
|
2018 |
|
2017 |
| ||
|
Amounts charged to share-based payment reserve in respect of share-based compensation |
|
$ |
15,780 |
|
$ |
2,064 |
|
|
Share-based compensation accrued in the prior period |
|
(44 |
) |
44 |
| ||
|
Share-based compensation issued on behalf of a related party |
|
(32 |
) |
— |
| ||
|
Shares for services compensation |
|
187 |
|
263 |
| ||
|
Deferred share units expensed in the period |
|
1,983 |
|
28 |
| ||
|
|
|
$ |
17,874 |
|
$ |
2,399 |
|
During the year, the Company issued 480,090 deferred share units to certain directors and officers of the Company, under the terms of the Company’s Deferred Share Unit Plan. In May 2018, directors and officers of the Company forfeited 312,000 deferred share units which were granted during the year.
25. Finance income, net
Finance income, net, is comprised of:
|
|
|
For the year ended |
| ||||
|
|
|
May 31, |
| ||||
|
|
|
2018 |
|
2017 |
| ||
|
Interest income |
|
$ |
6,362 |
|
$ |
1,115 |
|
|
Interest expense |
|
(1,350 |
) |
(387 |
) | ||
|
|
|
$ |
5,012 |
|
$ |
728 |
|
26. Gain on long-term investments
Gain on long-term investments for the year ended May 31, 2018 is comprised of:
|
|
|
|
|
Opening fair |
|
|
Gain (loss) |
|
Change in |
|
|
|
| |||||
|
Investment |
|
Proceeds |
|
value / cost |
|
|
on disposal |
|
fair value |
|
|
Total |
| |||||
|
Level 1 on fair value hierarchy |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
CannaRoyalty Corp. - shares |
|
$ |
4,389 |
|
$ |
1,793 |
|
|
$ |
2,596 |
|
$ |
— |
|
|
$ |
2,596 |
|
|
Kalytera Therapeutics, Inc. - shares |
|
763 |
|
1,111 |
|
|
(348 |
) |
— |
|
|
(348 |
) | |||||
|
MassRoots, Inc. - shares |
|
102 |
|
232 |
|
|
(130 |
) |
— |
|
|
(130 |
) | |||||
|
Canabo Medical Inc. - shares |
|
433 |
|
316 |
|
|
117 |
|
— |
|
|
117 |
| |||||
|
Nuuvera Inc. - shares and warrants |
|
31,216 |
|
17,029 |
|
|
14,187 |
|
— |
|
|
14,187 |
| |||||
|
Scythian Biosciences Inc. - shares |
|
1,225 |
|
2,000 |
|
|
(775 |
) |
— |
|
|
(775 |
) | |||||
|
Long-term investments (Note 14) |
|
— |
|
— |
|
|
— |
|
11,028 |
|
|
11,028 |
| |||||
|
Year ended May 31, 2018 |
|
$ |
38,128 |
|
$ |
22,481 |
|
|
$ |
15,647 |
|
$ |
11,028 |
|
|
$ |
26,675 |
|
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
27. Earnings per share
The calculation of earnings per share for the year ended May 31, 2018 was based on the net income attributable to common shareholders of $29,448 (2017 — $4,198) and a weighted average number of common shares outstanding of 161,026,463 (2017 — 104,341,319) calculated as follows:
|
|
|
2018 |
|
2017 |
| ||
|
Basic earnings per share: |
|
|
|
|
| ||
|
Net income for the period |
|
$ |
29,448 |
|
$ |
4,198 |
|
|
Average number of common shares outstanding during the period |
|
161,026,463 |
|
104,341,319 |
| ||
|
Earnings per share - basic |
|
$ |
0.18 |
|
$ |
0.04 |
|
|
|
|
2018 |
|
2017 |
| ||
|
Diluted earnings per share: |
|
|
|
|
| ||
|
Net income for the period |
|
$ |
29,448 |
|
$ |
4,198 |
|
|
Average number of common shares outstanding during the period |
|
161,026,463 |
|
104,341,319 |
| ||
|
“In the money” warrants outstanding during the period |
|
1,293,890 |
|
2,697,681 |
| ||
|
“In the money” options outstanding during the period |
|
3,593,647 |
|
4,388,893 |
| ||
|
|
|
165,914,000 |
|
111,427,893 |
| ||
|
Earnings per share - diluted |
|
$ |
0.18 |
|
$ |
0.04 |
|
28. Change in non-cash working capital
Change in non-cash working capital is comprised of:
|
|
|
For the year ended |
| ||||
|
|
|
2018 |
|
2017 |
| ||
|
Decrease (increase) in accounts receivable |
|
$ |
(1,797 |
) |
$ |
953 |
|
|
Decrease (increase) in other current assets |
|
(7,628 |
) |
(5,284 |
) | ||
|
Decrease (increase) in inventory, net of fair value adjustment |
|
(7,045 |
) |
3,057 |
| ||
|
Decrease (increase) in biological assets, net of fair value adjustment |
|
(367 |
) |
(4,188 |
) | ||
|
Increase (decrease) in accounts payable and accrued liabilities |
|
3,764 |
|
4,534 |
| ||
|
Increase (decrease) in income taxes payable |
|
2,662 |
|
— |
| ||
|
|
|
$ |
(10,411 |
) |
$ |
(928 |
) |
29. Financial risk management and financial instruments
Financial instruments
The Company has classified its cash and cash equivalents, marketable securities, long-term investments, and embedded derivatives as fair value through profit or loss (“FVTPL”), accounts receivable and other current assets as loans and receivables, and accounts payable and accrued liabilities, promissory notes payable, and long-term debt as other financial liabilities. The convertible notes receivable are accounted for on an amortized cost basis.
The carrying values of accounts receivable and other current assets, accounts payable and accrued liabilities, and promissory notes payable approximate their fair values due to their short periods to maturity.
The Company’s long-term debt of $30,548 is subject to fixed interest rates. The Company’s long-term debt is valued based on discounting the future cash outflows associated with the long-term debt. The discount rate is based on the incremental premium above market rates for Government of Canada securities of similar duration. In each period thereafter, the incremental premium is held constant while the Government of Canada security is based on the then current market value to derive the discount rate. The fair value of the Company’s long-term debt in repayment as at May 31, 2018 was $29,725.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Fair value hierarchy
Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of inputs used in making the measurements. Cash and cash equivalents are Level 1. The hierarchy is summarized as follows:
Level 1 quoted prices (unadjusted) in active markets for identical assets and liabilities
Level 2 inputs that are observable for the asset or liability, either directly (prices) or indirectly (derived from prices) from observable market data
Level 3 inputs for assets and liabilities not based upon observable market data
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
May 31, |
| ||||
|
Financial assets at FVTPL |
|
|
|
|
|
|
|
|
| ||||
|
Cash and cash equivalents |
|
$ |
59,737 |
|
$ |
— |
|
$ |
— |
|
$ |
59,737 |
|
|
Marketable securities |
|
45,062 |
|
— |
|
— |
|
45,062 |
| ||||
|
Embedded derivatives (note 12) |
|
— |
|
— |
|
7,410 |
|
7,410 |
| ||||
|
Long-term investments |
|
33,600 |
|
2,567 |
|
29,861 |
|
66,028 |
| ||||
|
Outstanding, end of the period |
|
$ |
138,399 |
|
$ |
2,567 |
|
$ |
37,271 |
|
$ |
178,237 |
|
|
|
|
|
|
|
|
|
|
May 31, |
| ||||
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
2017 |
| ||||
|
Financial assets at FVTPL |
|
|
|
|
|
|
|
|
| ||||
|
Cash and cash equivalents |
|
$ |
79,910 |
|
$ |
— |
|
$ |
— |
|
$ |
79,910 |
|
|
Marketable securities |
|
87,347 |
|
— |
|
— |
|
87,347 |
| ||||
|
Embedded derivatives |
|
— |
|
— |
|
173 |
|
173 |
| ||||
|
Long-term investments |
|
14,946 |
|
— |
|
12,842 |
|
27,788 |
| ||||
|
Outstanding, end of the period |
|
$ |
182,203 |
|
$ |
— |
|
$ |
13,015 |
|
$ |
195,218 |
|
The following table presents the changes in level 3 items for the years ended May 31, 2018 and May 31, 2017:
|
|
|
Unlisted |
|
Trading |
|
Total |
| |||
|
Closing balance May 31, 2017 |
|
$ |
12,842 |
|
$ |
173 |
|
$ |
13,015 |
|
|
Acquisitions |
|
16,236 |
|
4,450 |
|
20,686 |
| |||
|
Reclassification to Level 1 |
|
(9,979 |
) |
(1,348 |
) |
(11,327 |
) | |||
|
Reclassification to equity Investee |
|
(2,483 |
) |
— |
|
(2,483 |
) | |||
|
Unrealized gain on fair value |
|
13,245 |
|
4,135 |
|
17,380 |
| |||
|
Closing balance May 31, 2018 |
|
$ |
29,861 |
|
$ |
7,410 |
|
$ |
37,271 |
|
Investments in Scythian Biosciences Corp., TS BrandCo Holdings Inc. and Nuuvera Inc., originally classified as a Level 3 investment, were reclassified subsequent to the investee going public. During the year ended May 31, 2018, the Company sold its shares in Scythian Biosciences Corp. The Company converted the CannaRoyalty Corp. notes, and transferred $1,348 from embedded derivatives to long-term investments.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Financial risk management
The Company has exposure to the following risks from its use of financial instruments: credit; liquidity; currency rate; and, interest rate price.
(a) Credit risk
The maximum credit exposure at May 31, 2018 is the carrying amount of cash and cash equivalents, marketable securities, accounts receivable and other current assets and promissory notes receivable. The Company does not have significant credit risk with respect to customers. All cash and cash equivalents are placed with major Canadian financial institutions. Marketable securities are placed with major Canadian investment banks and are represented by investment grade corporate bonds.
The Company mitigates its credit risk and volatility on its marketable securities through its investment policy, which permits investments in Federal or Provincial government securities, Provincial utilities or bank institutions and Investment grade corporate bonds.
|
|
|
Total |
|
0-30 days |
|
31-60 days |
|
61-90 days |
|
90+ days |
| |||||
|
Trade receivables |
|
$ |
3,386 |
|
$ |
1,622 |
|
$ |
1,005 |
|
$ |
227 |
|
$ |
532 |
|
|
|
|
|
|
48 |
% |
29 |
% |
7 |
% |
16 |
% | |||||
(b) Liquidity risk
As at May 31, 2018, the Company’s financial liabilities consist of accounts payable and accrued liabilities, which has contractual maturity dates within one year, promissory note payable, which has a contractual maturity within 15 months and long-term debt, which has contractual maturities over the next five years. The Company manages its liquidity risk by reviewing its capital requirements on an ongoing basis. Based on the Company’s working capital position at May 31, 2018, management regards liquidity risk to be low.
(c) Currency rate risk
As at May 31, 2018, a portion of the Company’s financial assets and liabilities held in USD consist of marketable securities, convertible notes receivable, long-term investments and a promissory note payable. The Company’s objective in managing its foreign currency risk is to minimize its net exposure to foreign currency cash flows by transacting, to the greatest extent possible, with third parties in Canadian dollars. The Company does not currently use foreign exchange contracts to hedge its exposure of its foreign currency cash flows as management has determined that this risk is not significant at this point in time.
The Company is exposed to unrealized foreign exchange risk through its convertible notes receivable and long-term investments. A 1% change in the foreign exchange rate would result in an unrealized gain or loss of approximately $28.
(d) Interest rate price risk
The Company manages interest rate risk by restricting the type of investments and varying the terms of maturity and issuers of marketable securities. Varying the terms to maturity reduces the sensitivity of the portfolio to the impact of interest rate fluctuations.
(e) Capital management
The Company’s objectives when managing its capital are to safeguard its ability to continue as a going concern, to meet its capital expenditures for its continued operations, and to maintain a flexible capital structure which optimizes the cost of capital within a framework of acceptable risk. The Company manages its capital structure and adjusts it in light of changes in economic conditions and the risk characteristics of the underlying assets. To maintain or adjust its capital structure, the Company may issue new shares, issue new debt, or acquire or dispose of assets. The Company is not subject to externally imposed capital requirements.
Aphria Inc.
Notes to the Consolidated Financial Statements
For the years ended May 31, 2018 and May 31, 2017
(In thousands of Canadian dollars, except share and per share amounts)
Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable. There have been no changes to the Company’s capital management approach in the year. The Company considers its cash and cash equivalents and marketable securities as capital.
30. Commitments
The Company has a lease commitment until December 31, 2018 for rental of office space from a related party. The Company has an option to extend this lease for two additional 5 year periods. Subsequent to year-end, the Company entered into a new lease for rental office space from December 2018 until November 30, 2028. The Company has lease commitments for the use of two motor vehicles expiring September 2019 and August 2020 in the amounts payable of $9 and $20, respectively. In April of 2017, the Company indemnified the landlord of the office space leased by Liberty with annual rent from $180 to $190 expiring June 2023. The Company has agreed to contribute an additional $400 to Green Acre Capital Fund. The Company has committed purchase orders outstanding at May 31, 2018 related to capital asset expansion of $30,360, all of which are expected to be paid within the next year. Minimum payments payable over the next five years are as follows:
|
|
|
Years ending May 31, |
| |
|
2019 |
|
$ |
30,914 |
|
|
2020 |
|
22 |
| |
|
2021 |
|
3 |
| |
|
|
|
$ |
30,939 |
|
31. Subsequent events
Subsequent to year-end, the Company completed the forming of CannInvest Africa Ltd. (“CannInvest”), with the South African Verve Group of Companies. Through the combination of a share-for-share swap and cash payment of $4.05 million, the Company obtained a 50% ownership in CannInvest which in turn acquired a 60% interest in Verve Dynamics Inc. (“Verve”), a licensed producer of medical cannabis extracts in Lesotho.
Subsequent to year-end, the Company closed a bought deal and issued 21,835,510 common shares for gross proceeds of $258,751.
Subsequent to year-end, the Company’s Malta based subsidiary, ASG Pharma Ltd. (“ASG”), received the first import license for medical cannabis issued by the Malta Medicines Authority. The license will allow ASG to import medical cannabis for analytical testing and research and is an important step that will enable ASG to become a cornerstone in testing, research and development of medical cannabis in Europe.
Subsequent to year-end, the Company announced the proposed acquisition of industry-leading companies in Colombia, Argentina, Jamaica and a right of first offer and refusal in respect of Brazil through a definitive share purchase agreement with Scythian. The Company expects to issue 15,678,310 shares, and assume $1,000 of existing debt in connection with the proposed acquisition.
Subsequent to year-end, Aphria Inc. amalgamated with its previously wholly-owned subsidiary, Pure Natures Wellness Inc., pursuant to a short form, vertical amalgamation. The resulting entity retained the name “Aphria Inc.”
Subsequent to year-end, the Company amended its Obligation Agreement, where the Company will accept a 30-day promissory note to settle the next tranche of Liberty shares owned by the Company that became freely trading on July 26, 2018. The Company also paid $480 to enter into a standstill agreement, whereby the purchaser of the Liberty shares will not sell the newly acquired shares for 18 months from the date of purchase. The purchaser also granted the Company an option to buy back the shares at $1.00 per share, subject to certain downside risk protection which results in the purchaser sharing a portion of the difference between the share price on the day the option is exercised and the exercise price, provided the share price exceeds $1.25.
Subsequent to year-end, the Company committed to a $15,000 investment in Green Acre Capital Fund II to be launched before December 2018.