Exhibit 99.1

Nasdaq: LGVN www.longeveron.com H.C. Wainwright BioConnect 2022 Virtual Conference Cell Therapy for Aging - Related Disease & Life - Threatening Conditions

Forward Looking Statements 2 Certain statements in this presentation that are not historical facts are forward - looking statements that reflect management's current expectations, assumptions, and estimates of future performance and economic conditions, and involve risks and uncertainties that could cause actual results to differ materially from those anticipated by the statements made herein . Forward - looking statements are generally identifiable by the use of forward - looking terminology such as "believe," "expects," "may," "looks to," "will," "should," "plan," "intend," "on condition," "target," "see," "potential," "estimates," "preliminary," or "anticipates" or the negative thereof or comparable terminology, or by discussion of strategy or goals or other future events, circumstances, or effects . Moreover, forward - looking statements in this release include, but are not limited to, statements about the ability of our clinical trials to demonstrate safety and efficacy of our product candidates, and other potentially positive or negative results ; the timing, focus and potential outcomes of our ongoing and future preclinical studies and clinical trials ; the size of the market opportunity for our product candidates, the beneficial characteristics, safety, efficacy and therapeutic effects of our product candidates ; our ability to obtain and maintain regulatory approval of our product candidates, our plans and ability to obtain or protect intellectual property rights, including extensions of existing patent terms where available and our ability to avoid infringing the intellectual property rights of others . Further information relating to factors that may impact the Company's results and forward - looking statements are disclosed in the Company's filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10 - K filed with the SEC on March 30 , 2021 . The forward - looking statements contained in this presentation are made as of the date of this presentation, and the Company disclaims any intention or obligation, other than imposed by law, to update or revise any forward - looking statements, whether as a result of new information, future events, or otherwise .

Longe v e r o n A t - a - G l an c e (N AS D A Q: L G V N) 3 Biotechnology Regenerative Medicine / Cell Therapy 2014 February 12, 2021 $19.0 million (September 30, 2021) $20.5 million private placement (gross proceeds; December 2021) Industry S ec tor Found e d IPO Last Reported Cash Recent Financing Location Miami, Florida (Corporate headquarters, manufacturing, research and development)

Cell Therapy for Aging - Related Disease & Life - Threatening Conditions 4 • Lomecel - B Œ : medicinal signaling cells (MSCs) from young, healthy donors (bone marrow) • Manufactured in Longeveron’s cGMP facility • Ongoing Phase 2 trials in Hypoplastic Left Heart Syndrome (HLHS) and Alzheimer’s disease • Clinical safety and effect data in three indications/conditions: • Hypoplastic Left Heart Syndrome (Ph. 1 data): W ell - tolerated; no Major Adverse Cardiac Events (MACE) • Orphan Drug & Rare Pediatric Disease designations from US FDA • Alzheimer’s disease (Ph. 1 data): Well - tolerated; no evidence of ARIA; exploratory cognitive function & biomarker data • Aging Frailty (Ph. 2b data): Well - tolerated; significant dose - dependent increase in walking distance (6 minute walk test) • Anticipated* near - term events: » Q1 2022 : » Q1 2022 : » Q1/Q2 2022 : Enroll first patient in Alzheimer’s Disease Phase 2a Trial Initiate Japan Phase 2 Aging Frailty Trial Top line data from Aging Frailty Influenza Vaccine Phase 1/2 “HERA” Trial *Anticipated event timeline, subject to adjustment and change. ARIA=amyloid related imaging abnormalities; NIA=National Institute on Aging; NHLBI=National Heart, Lung and Blood Institute; MSCRF - Maryland Stem Cell Research Fund

Experienced and Successful Leadership Geoff Green, MBA Chief Executive Officer 25+ years in life sciences, with leadership roles in public and private companies, and experience in corporate strategy, operations, business development, and clinical affairs. Previously President and Acting CEO of DOR BioPharma, (now Soligenix (NAS: SNGX)), VP of Business Dev. & Clinical Affairs at Accu - Break Pharmaceuticals, VP of Operations at Partikula. B.A. in biology from Kenyon College; M.B.A. from Barry University’s Andreas School of Business. Joshua M. Hare, MD Co - Founder & Chief Science Officer Co - founded Longeveron in 2014 from the University of Miami, where he is also the Founding Director of the University’s Interdisciplinary Stem Cell Institute (ISCI). Graduate of the University of Pennsylvania & Johns Hopkins University School of Medicine. Served as a research fellow at Harvard University and is Board Certified in cardiovascular medicine. Paul Lehr, JD General Counsel 25+ years with legal & executive positions in corporate and research settings. Former President of the Pritikin Longevity Institute and CEO of National YoungArts Foundation.. BA from Brown University, and JD from University of Florida. James Clavijo Chief Financial Officer 25+ years of experience as a CFO for pharmaceutical, healthcare and manufacturing companies. Served as Chief Financial Officer for Aeterna Zentaris (NASDAQ:AEZS) & Tri - source Pharma & as Chief Accounting Officer at Soligenix (NASDAQ: SNGX) Dan Gincel, PhD Senior VP, Strategic Collaborations & Scientific Affairs 20+ years leadership experience overseeing development and commercialization of regenerative medicines, including cell therapies, as well as establishing public - private collaborations and corporate partnerships. VP of University Partnerships and a member of the Executive Leadership team at the Maryland Technology Development Corporation (TEDCO). Lisa McClain - Moss Vice President, Manufacturing Leads Longeveron’s cell therapy manufacturing and product development activity. Previously held leadership roles at Cognate BioServices and St. Jude’s Children Research Hospital. Anthony Oliva, PhD Senior Scientist 20+ years experience in basic research, as well as regulatory and clinical affairs. Leads grant strategy, and other areas of scientific research. B.A. in Biological Sciences from the University of Chicago; Ph.D. in Neuroscience from Baylor College of Medicine; Post - doctoral research at Oregon Health & Science University.
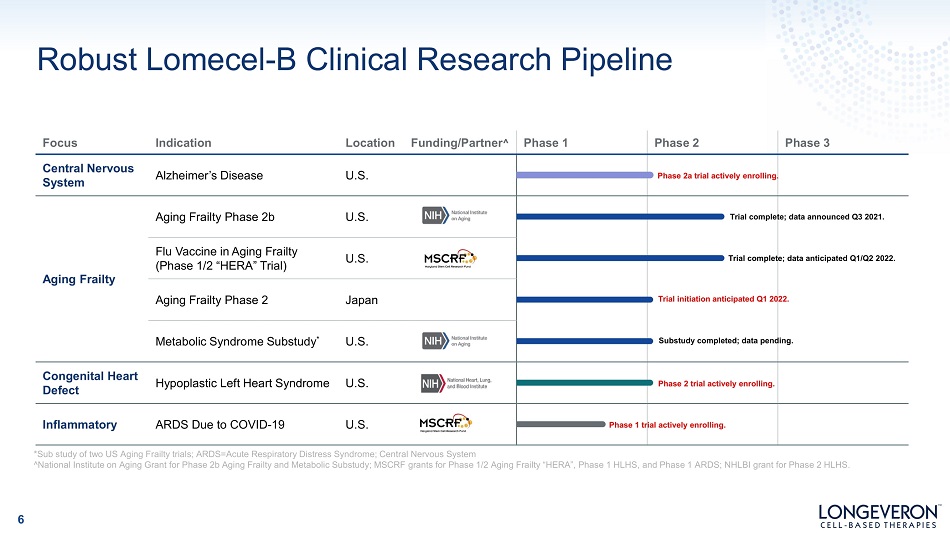
Robust Lomecel - B Clinical Research Pipeline *Sub study of two US Aging Frailty trials; ARDS=Acute Respiratory Distress Syndrome; Central Nervous System ^National Institute on Aging Grant for Phase 2b Aging Frailty and Metabolic Substudy; MSCRF grants for Phase 1/2 Aging Frailty “HERA”, Phase 1 HLHS, and Phase 1 ARDS; NHLBI grant for Phase 2 HLHS. Focus Indication Location Funding/Partner ^ Phase 1 Phase 2 Phase 3 C e nt ral N er v ou s System Alzheimer’s Disease U.S. Phase 2a trial actively enrolling. Aging Frailty Aging Frailty Phase 2b U.S. T r i a l c o m pl et Trial complet Trial initiation anticipated Q1 202 Substudy completed; data pendi e; data announced Q3 2021. e; data anticipated Q1/Q2 2022. 2. ng. Fl u V acc i ne i n A g i ng Fr a il ty (Phase 1/2 “HERA” Trial) U.S. Aging Frailty Phase 2 Japan Metabolic Syndrome Substudy * U.S. Congenital Heart Defect Hypoplastic Left Heart Syndrome U.S. Phase 2 trial actively enrolling. Inflammatory ARDS Due to COVID - 19 U.S. Phase 1 tr ial actively enrolling. 6
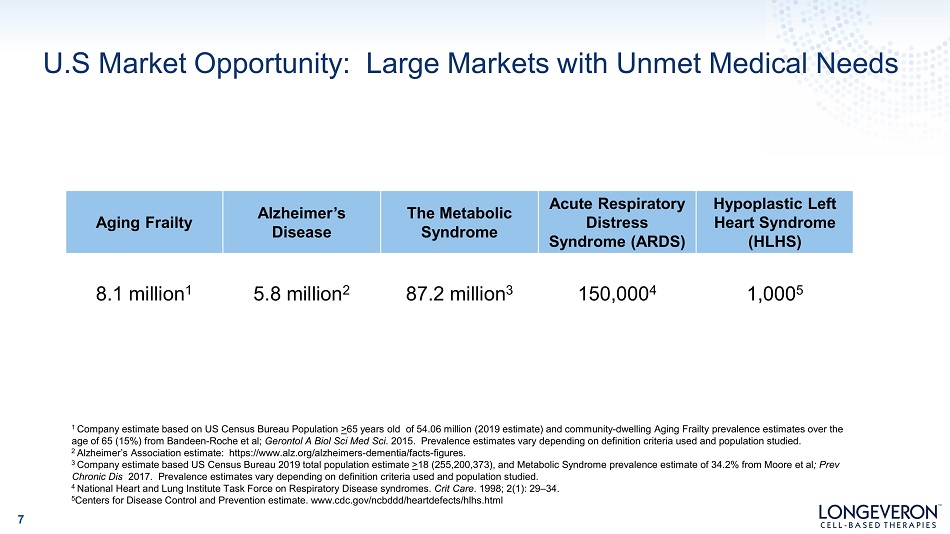
U.S Market Opportunity: Large Markets with Unmet Medical Needs 7 Aging Frailty A l z heime r ’ s Disease The Metabolic Syndrome Acute Respiratory Distress Syndrome (ARDS) Hypoplastic Left Heart Syndrome (HLHS) 8.1 million 1 5.8 million 2 87.2 million 3 150,000 4 1,000 5 1 Company estimate based on US Census Bureau Population > 65 years old of 54.06 million (2019 estimate) and community - dwelling Aging Frailty prevalence estimates over the age of 65 (15%) from Bandeen - Roche et al; Gerontol A Biol Sci Med Sci . 2015. Prevalence estimates vary depending on definition criteria used and population studied. 2 Alzheimer’s Association estimate: https:// www.alz.org/alzheimers - dementia/facts - figures. 3 Company estimate based US Census Bureau 2019 total population estimate > 18 (255,200,373), and Metabolic Syndrome prevalence estimate of 34.2% from Moore et al ; Prev Chronic Dis 2017. Prevalence estimates vary depending on definition criteria used and population studied. 4 National Heart and Lung Institute Task Force on Respiratory Disease syndromes. Crit Care . 1998; 2(1): 29 – 34. 5 Centers for Disease Control and Prevention estimate. www.cdc.gov/ncbddd/heartdefects/hlhs.html

Lomecel - B Medicinal Signaling Cells (MSC) Therapeutics 8
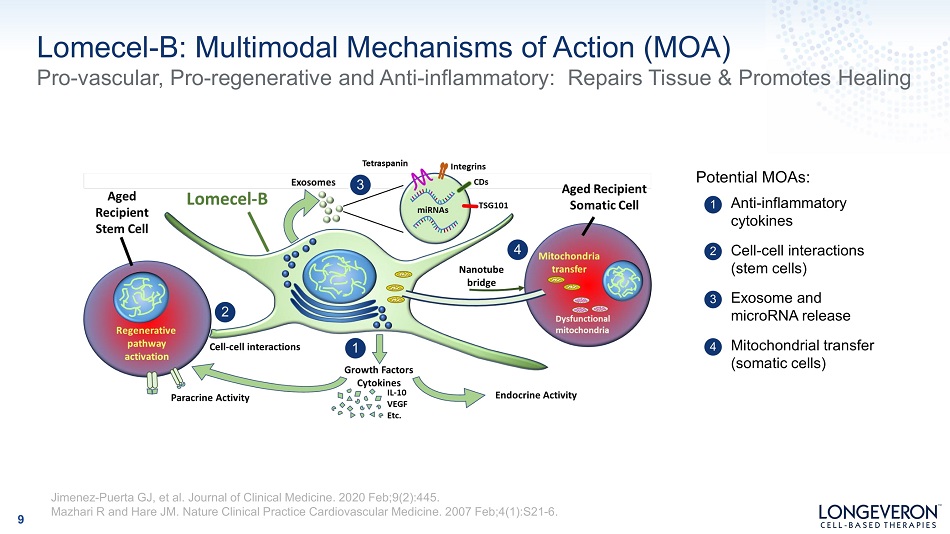
Lomecel - B: Multimodal Mechanisms of Action (MOA) Pro - vascular, Pro - regenerative and Anti - inflammatory: Repairs Tissue & Promotes Healing 9 1 A n ti - i n fl a mm a t o ry cytokines 2 3 Exosome and m i c r o RN A r e l ease 4 Mitochondrial transfer (somatic cells) Cell - cell interactions (stem cells) 1 2 3 4 Potential MOAs: Jimenez - Puerta GJ, et al. Journal of Clinical Medicine. 2020 Feb;9(2):445. Mazhari R and Hare JM. Nature Clinical Practice Cardiovascular Medicine. 2007 Feb;4(1):S21 - 6.
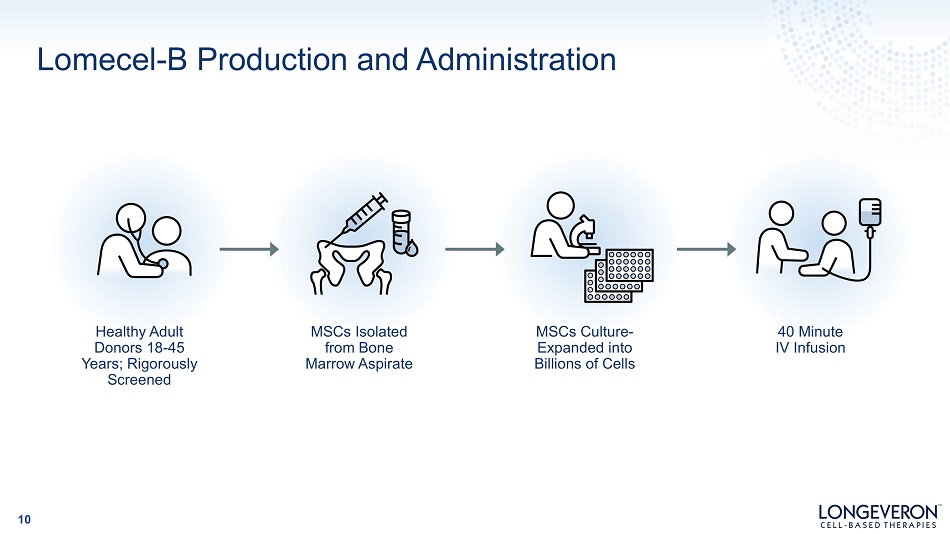
Lomecel - B Production and Administration 10 H ea lt h y A du lt Donors 18 - 45 Y ea r s ; R i go r ous ly Screened MSCs Isolated from Bone M a rr o w A sp ir a te MSCs Culture - Expanded into Billions of Cells 40 Minute IV Infusion
Lomecel - B: Proprietary, Scalable, “Off - the - Shelf” Cellular Therapy 11 • Robust quality assurance processes ensure batch - to - batch consistency • Cryogenically stored long - term, easy to prepare and administer • Delivered systemically or locally • In response to inflammatory mediators, MSCs home to sites of tissue damage 1 • Not perceived by the host as foreign (Immuno - evasive) 2 Ke y Advan t ages of Lomecel - B 1 Rustad KC, and Gurtner GC. Advances in wound care. 2012 Aug 1;1(4):147 - 52. 2 Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature biotechnology. 2014 Mar;32(3):252 - 60.

Lomecel - B Clinical Research Hypoplastic Left Heart Syndrome 12
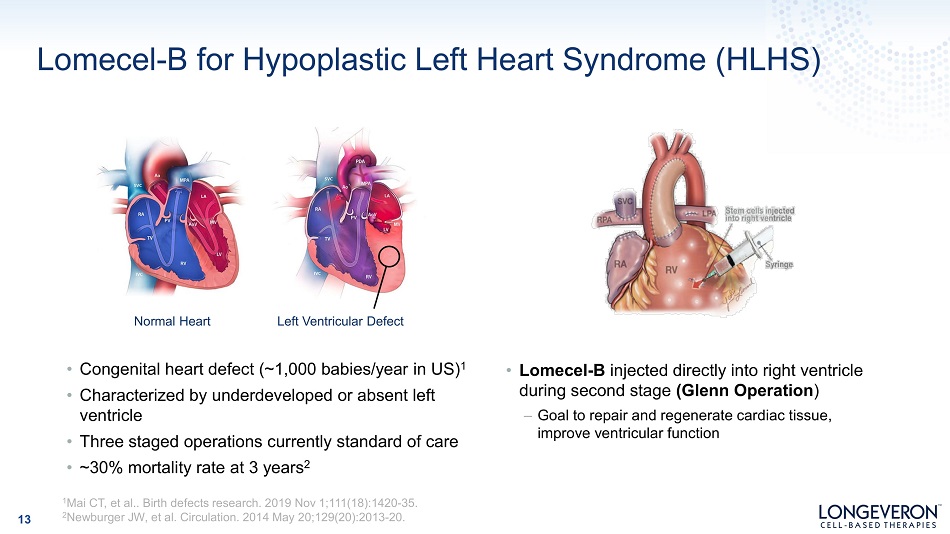
Lomecel - B for Hypoplastic Left Heart Syndrome (HLHS) Normal Heart Left Ventricular Defect • Congenital heart defect (~1,000 babies/year in US) 1 • Characterized by underdeveloped or absent left ventricle • Three staged operations currently standard of care • ~30% mortality rate at 3 years 2 • Lomecel - B injected directly into right ventricle during second stage (Glenn Operation ) – Goal to repair and regenerate cardiac tissue, improve ventricular function 13 1 Mai CT, et al.. Birth defects research. 2019 Nov 1;111(18):1420 - 35. 2 Newburger JW, et al. Circulation. 2014 May 20;129(20):2013 - 20.
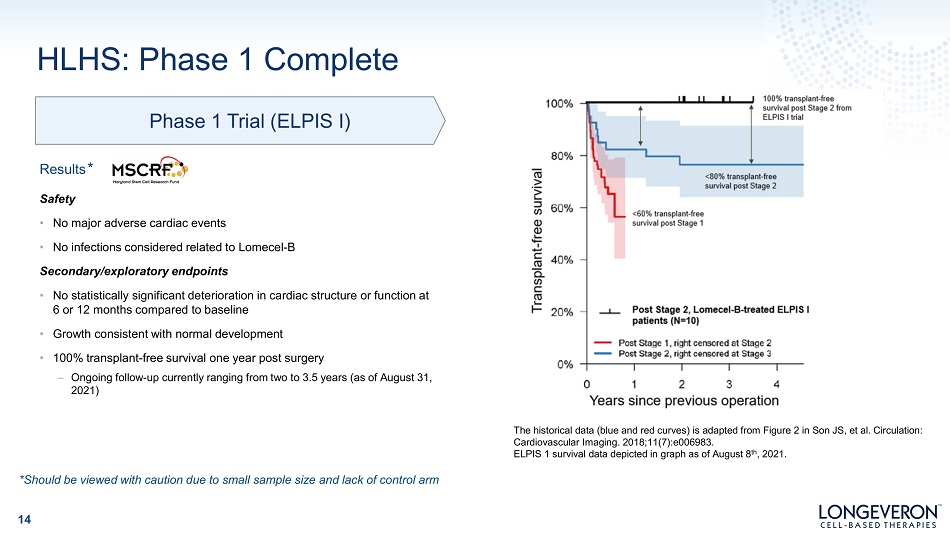
Phase 1 Trial (ELPIS I) HLHS: Phase 1 Complete Results * Safety • No major adverse cardiac events • No infections considered related to Lomecel - B Secondary/exploratory endpoints • No statistically significant deterioration in cardiac structure or function at 6 or 12 months compared to baseline • Growth consistent with normal development • 100% transplant - free survival one year post surgery – Ongoing follow - up currently ranging from two to 3.5 years (as of August 31, 2021) *Should be viewed with caution due to small sample size and lack of control arm The historical data (blue and red curves) is adapted from Figure 2 in Son JS, et al. Circulation: Cardiovascular Imaging. 2018;11(7):e006983. ELPIS 1 survival data depicted in graph as of August 8 th , 2021. 14
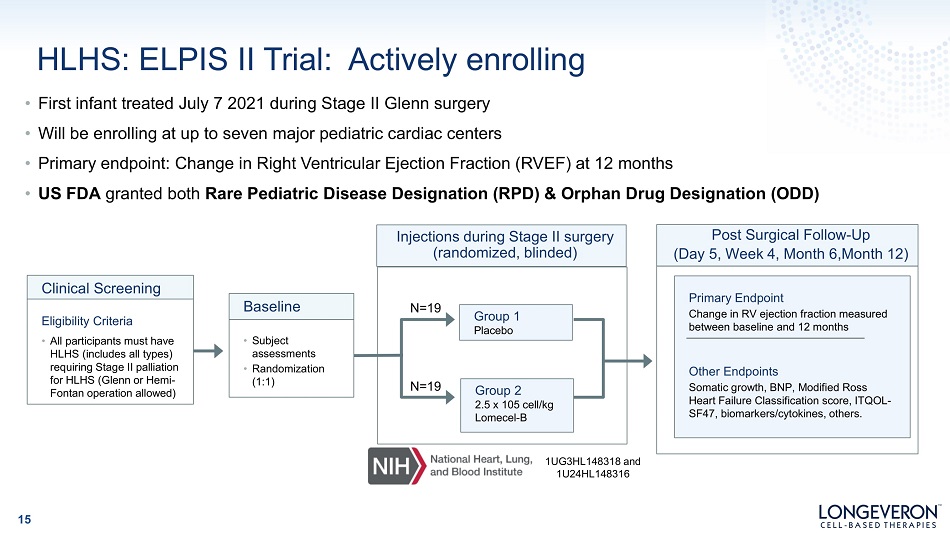
HLHS: ELPIS II Trial: Actively enrolling • First infant treated July 7 2021 during Stage II Glenn surgery • Will be enrolling at up to seven major pediatric cardiac centers • Primary endpoint: Change in Right Ventricular Ejection Fraction (RVEF) at 12 months • US FDA granted both Rare Pediatric Disease Designation (RPD) & Orphan Drug Designation (ODD) Group 1 Placebo Injections during Stage II surgery (randomized, blinded) Clinical Screening Eligibility Criteria • All participants must have HLHS (includes all types) requiring Stage II palliation for HLHS (Glenn or Hemi - Fontan operation allowed) • Subject as s es sm en t s • R ando m i z a t i on (1:1) Baseline Primary Endpoint Change in RV ejection fraction measured between baseline and 12 months Other Endpoints Somatic growth, BNP, Modified Ross Heart Failure Classification score, ITQOL - SF47, biomarkers/cytokines, others. Post Surgical Follow - Up (Day 5, Week 4, Month 6,Month 12) N =19 Group 2 2.5 x 105 cell/kg Lomecel - B N =19 1UG3HL148318 and 1U24HL148316 15

Lomecel - B Clinical Research Alzheimer’s Disease 16
Alzheimer’s Disease: Targeting Neuroinflammation 17 • Chronic neuroinflammation a hallmark of dysregulated microglia, cause of nerve cell death • Systemic inflammation likely contributes to disease progression • MSCs in animal models of Alzheimer’s: – Cross the blood brain barrier (BBB) – ↓ pro - inflammatory; ↑ anti - inflammatory biomarkers – Improve immune functioning – Promote neurogenesis – Improve endothelial function Pro - inflammatory state appears requisite for disease, neurodegeneration and neuronal death: Lue et al Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer's disease neurodegeneration. J Neuropathol Exp Neurol. 1996;55(10):1083 - 8. Epub 1996/10/01 Pimplikar. Neuroinflammation in Alzheimer's disease: from pathogenesis to a therapeutic target. J Clin Immunol. 2014;34 Suppl 1:S64 - 9. Epub 2014/04/09. doi: 10.1007/s10875 - 014 - 0032 - 5. Heneka et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388 - 405. Epub 2015/03/21. doi: 10.1016/S1474 - 4422(15)70016 - 5. Neves et al. Intravenous administration of mesenchymal stem cells reduces Tau phosphorylation and inflammation in the 3xTg - AD mouse model of Alzheimer's disease. Experimental neurology. Kim et al. The preventive and therapeutic effects of intravenous human adipose - derived stem cells in Alzheimer's disease mice. PloS one. 2012;7(9):e45757. Shin et al. Mesenchymal stem cells enhance autophagy and increase beta - amyloid clearance in Alzheimer disease models. Autophagy. 2014;10(1):32 - 44. Lee JK. Soluble CCL5 derived from bone marrow - derived mesenchymal stem cells and activated by amyloid beta ameliorates Alzheimer's disease in mice by recruiting bone marrow - induced microglia immune responses. Stem Cells. 2012;30(7):1544 - 55. Bae et al. Bone marrow - derived mesenchymal stem cells contribute to the reduction of amyloid - beta deposits and the improvement of synaptic transmission in a mouse model of pre - dementia Alzheimer's disease. Curr Alzheimer Res. 2013;10(5):524 - 31.
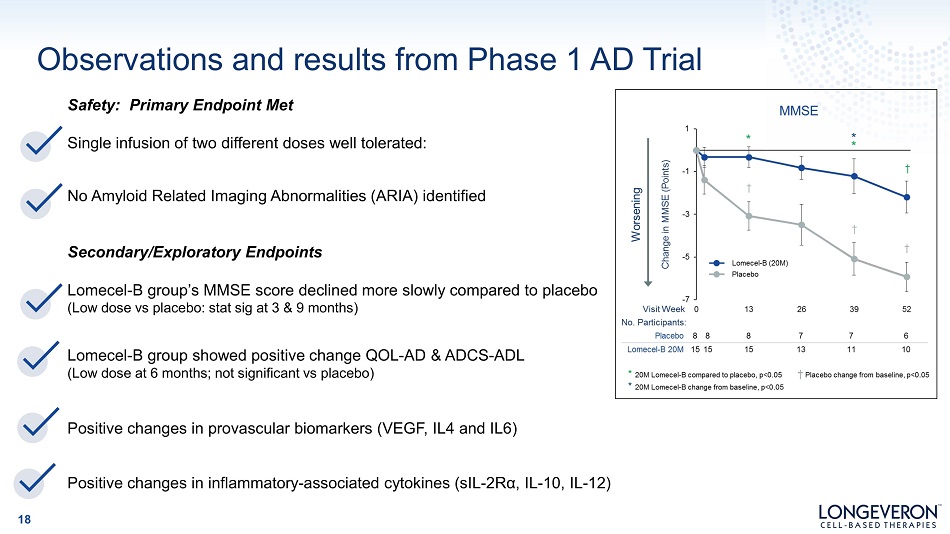
Safety: Primary Endpoint Met Single infusion of two different doses well tolerated: No Amyloid Related Imaging Abnormalities (ARIA) identified Secondary/Exploratory Endpoints Lomecel - B group’s MMSE score declined more slowly compared to placebo (Low dose vs placebo: stat sig at 3 & 9 months) Lomecel - B group showed positive change QOL - AD & ADCS - ADL (Low dose at 6 months; not significant vs placebo) Positive changes in provascular biomarkers (VEGF, IL4 and IL6) Positive changes in inflammatory - associated cytokines (sIL - 2Rα, IL - 10, IL - 12) Observations and results from Phase 1 AD Trial 18
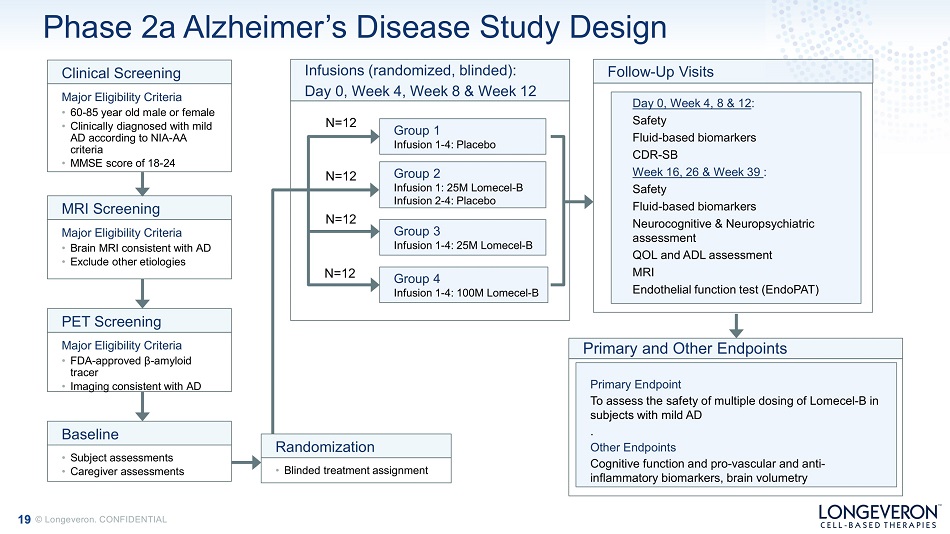
19 © Longeveron. CONFIDENTIAL Phase 2a Alzheimer’s Disease Study Design Group 1 Infusion 1 - 4: Placebo Group 2 Infusion 1: 25M Lomecel - B Infusion 2 - 4: Placebo Group 3 Infusion 1 - 4: 25M Lomecel - B Infusions (randomized, blinded): Day 0, Week 4, Week 8 & Week 12 Clinical Screening Major Eligibility Criteria • 60 - 85 year old male or female • Clinically diagnosed with mild AD according to NIA - AA criteria • MMSE score of 18 - 24 MRI Screening Major Eligibility Criteria • Brain MRI consistent with AD • Exclude other etiologies PET Screening Major Eligibility Criteria • FDA - approved β - amyloid tracer • Imaging consistent with AD • Subject assessments • Caregiver assessments Baseline Day 0, Week 4, 8 & 12 : Safety Fluid - based biomarkers CDR - SB Week 16, 26 & Week 39 : Safety Fluid - based biomarkers Neurocognitive & Neuropsychiatric assessment QOL and A D L a s se s s m e n t MRI Endothelial function test (EndoPAT) Follow - Up Visits N =12 N =12 N =12 Group 4 Infusion 1 - 4: 100M Lomecel - B N =12 • Blinded treatment assignment Randomization Primary Endpoint To assess the safety of multiple dosing of Lomecel - B in sub j e c ts w i th m il d AD . Other Endpoints Cognitive function and pro - vascular and anti - inflammatory biomarkers, brain volumetry Primary and Other Endpoints

Lomecel - B Robust Clinical Program Aging Frailty 20
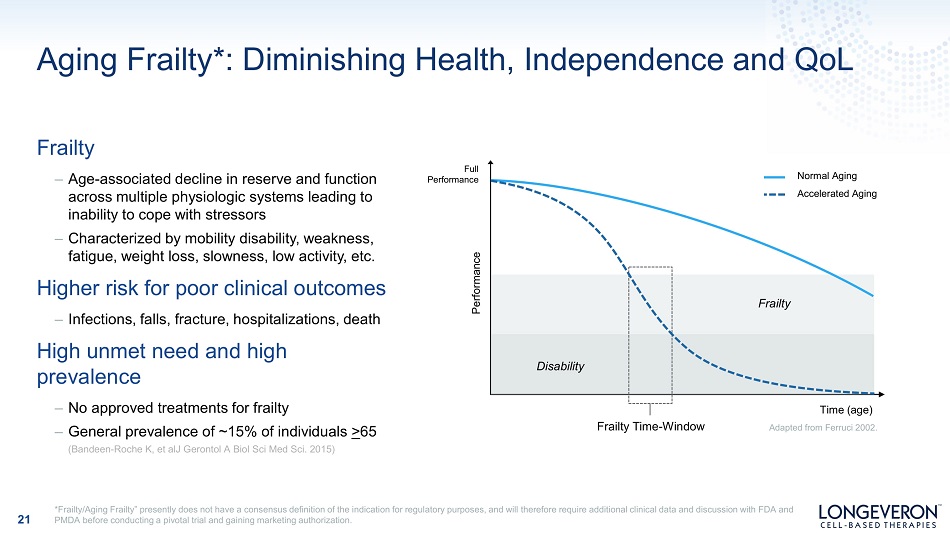
Aging Frailty*: Diminishing Health, Independence and QoL Frailty – Age - associated decline in reserve and function across multiple physiologic systems leading to inability to cope with stressors – Characterized by mobility disability, weakness, fatigue, weight loss, slowness, low activity, etc . Higher risk for poor clinical outcomes – Infections, falls, fracture, hospitalizations, death High unmet need and high prevalence – No approved treatments for frailty – General prevalence of ~15% of individuals > 65 (Bandeen - Roche K, et alJ Gerontol A Biol Sci Med Sci. 2015) 21 Performance Normal Aging A cc e l e r ate d Ag i ng Fr a il ty Disability Time (age) Adapted from Ferruci 2002. Frailty Time - Window Full P erfor m an c e *Frailty/Aging Frailty” presently does not have a consensus definition of the indication for regulatory purposes, and will therefore require additional clinical data and discussion with FDA and PMDA before conducting a pivotal trial and gaining marketing authorization.
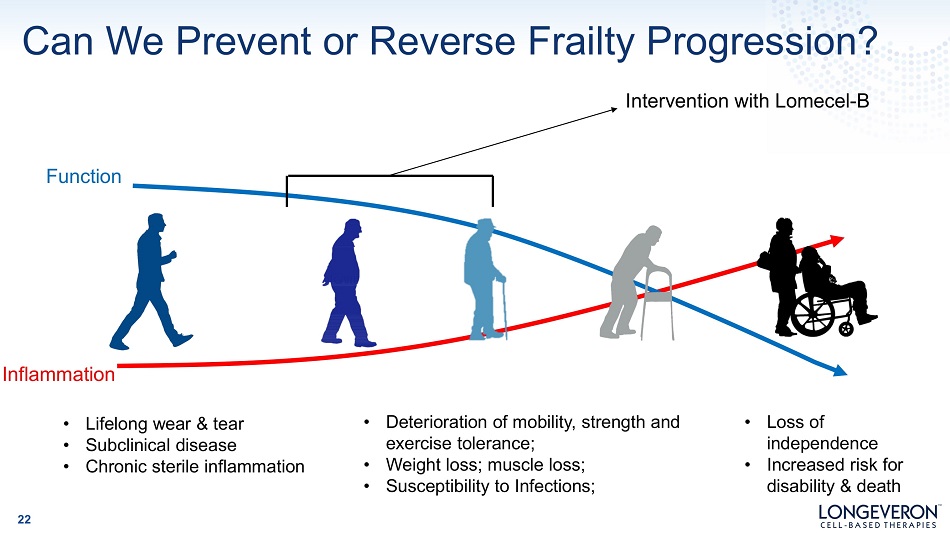
Can We Prevent or Reverse Frailty Progression? Inflammation • Lifelong wear & tear • Subclinical disease • Chronic sterile inflammation • Deterioration of mobility, strength and exercise tolerance; • Weight loss; muscle loss; • Susceptibility to Infections; • Loss of i ndependen c e • Increased risk for disability & death F un c ti on Intervention with Lomecel - B 22
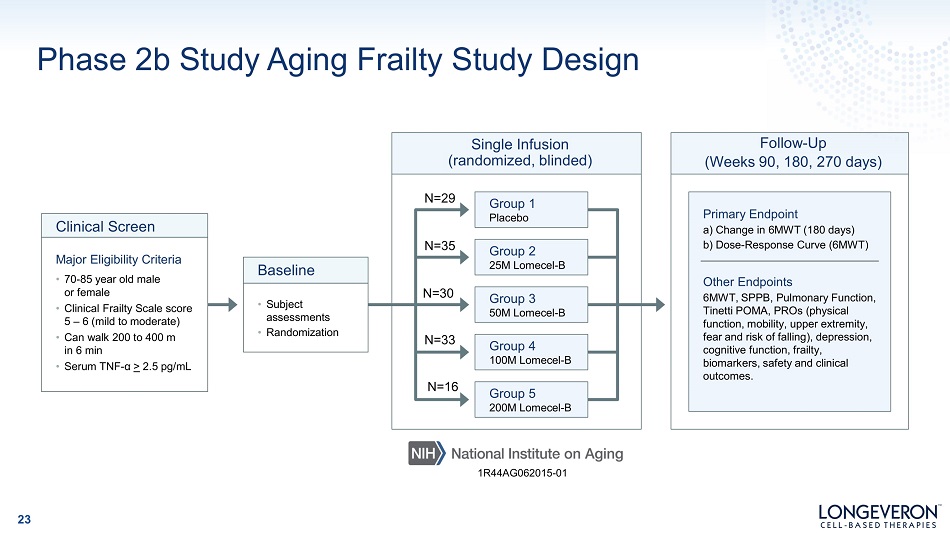
Phase 2b Study Aging Frailty Study Design Group 1 Placebo Group 2 25M Lomecel - B Group 3 50M Lomecel - B Single Infusion (randomized, blinded) Clinical Screen Major Eligibility Criteria • 70 - 85 year old male or female • Clinical Frailty Scale score 5 – 6 (mild to moderate) • Can walk 200 to 400 m in 6 min • Serum TNF - α > 2.5 pg/mL • Subject as s es sm en ts • Randomization Baseline Primary Endpoint a) Change in 6MWT (180 days) b) Dose - Response Curve (6MWT) Other Endpoints 6MWT, SPPB, Pulmonary Function, Tinetti POMA, PROs (physical function, mobility, upper extremity, fear and risk of falling), depression, cognitive function, frailty, biomarkers, safety and clinical outcomes. Follow - Up (Weeks 90, 180, 270 days) N =29 N =35 N =30 Group 4 100M Lomecel - B Group 5 200M Lomecel - B N =33 N =16 1R44AG062015 - 01 23

Lomecel - B Improved Walking Distance Over 9 Months 24 * Change from baseline; p<0.05 ** Change from baseline; p<0.01 # Versus placebo; p<0.05 ## Versus placebo; p<0.01 Statistically - significant dose response at 180 days.
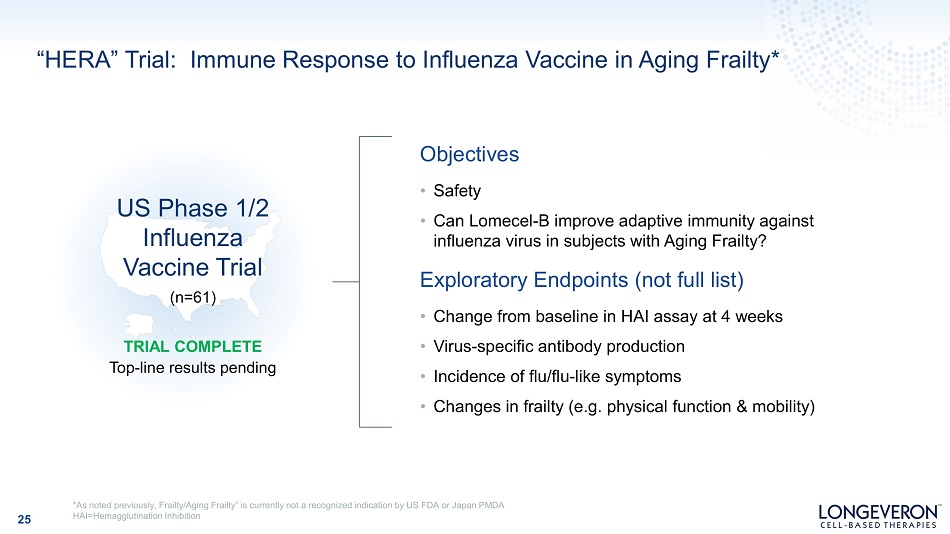
“HERA” Trial: Immune Response to Influenza Vaccine in Aging Frailty* 25 *As noted previously, Frailty/Aging Frailty” is currently not a recognized indication by US FDA or Japan PMDA HAI=Hemagglutination Inhibition O b j ec ti v es • Safety • Can Lomecel - B improve adaptive immunity against influenza virus in subjects with Aging Frailty? Exploratory Endpoints (not full list) • Change from baseline in HAI assay at 4 weeks • Virus - specific antibody production • Incidence of flu/flu - like symptoms • Changes in frailty (e.g. physical function & mobility) TRIAL COMPLETE Top - line results pending US Phase 1/2 Influenza Vaccine Trial (n=61)

Aging Frailty* Clinical Trial in Japan 26 Objectives • Safety • Can Lomecel - B improve exercise tolerance and mobility? Efficacy Endpoints • Change in 6MWT; grip strength, walking speed, patient - reported outcomes biomarkers, fear and risk of falling, depression, sexual function, clinical outcomes, others Japanese National Center for Geriatrics and Gerontology - sponsored trial • NCGG (Nagoya) and Juntendo University (Tokyo) selected as clinical sites Clinical Trial Notification (CTN) accepted by PMDA TRIAL PLANNING ONGOING Anticipated to start in 2022 Japan Phase 2 Trial (n=45) *Frailty/Aging Frailty” presently does not have a consensus definition of the indication for regulatory purposes, and will therefore require additional clinical data and discussion with FDA and PMDA before conducting a pivotal trial and gaining marketing authorization.
Intellectual Property & Barriers to Competition Proprietary Manufacturing Process • Exclusively licensed from University of Miami • Improved and optimized by Longeveron • In - house manufacturing to maintain proprietary trade secrets Company - Owned Patent Applications • Allogeneic MSCs for use as a vaccine adjuvant • Allogeneic MSCs for improving humoral immunity • Allogeneic MSCs to treat sexual dysfunction • MSC protein expression from stimuli (potency assay) • MSCs to treat Alzheimer’s disease • Other applications currently confidential Licensed Patents & Applications • Monitoring cardiomyopathy therapy • CD271+ MSCs for cardiac repair – License is for use of CD271+ cells for aging - related disorders 27 Trademarks and Trademark Applications • Applications pending in multiple jurisdictions for LONGEVERON and LOMECEL - B
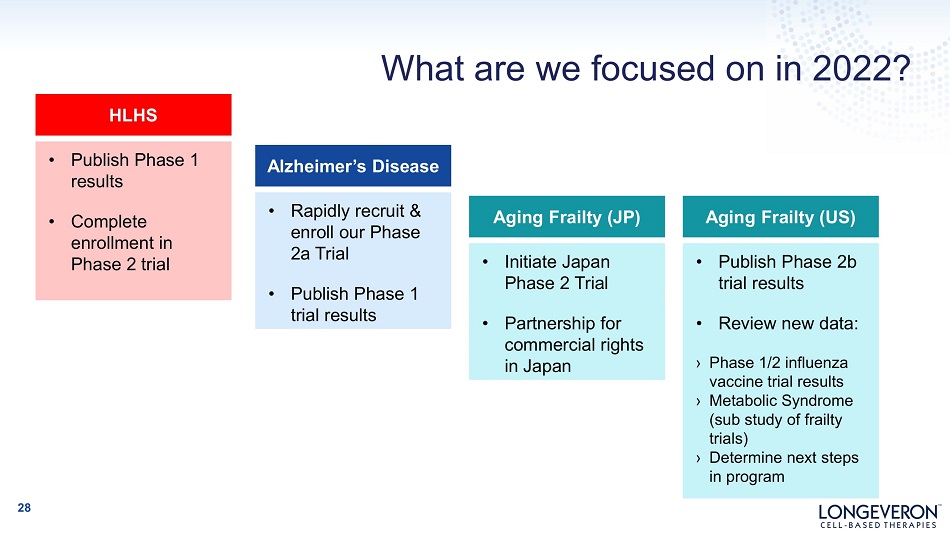
What are we focused on in 2022? 28 Alzheimer’s Disease • Rapidly recruit & enroll our Phase 2 a Trial • Publish Phase 1 trial results Aging Frailty (JP) • Initiate Japan Phase 2 Trial • Partnership for commercial rights in Japan Aging Frailty (US) • Publish Phase 2b trial results • Review new data: › Phase 1/2 influenza vaccine trial results › Metabolic Syndrome (sub study of frailty trials) › Determine next steps in program HLHS • Publish Phase 1 results • Complete enrollment in Phase 2 trial

S umma ry Leader in regenerative medicine for aging - related and life - threatening diseases and conditions Well - characterized allogeneic MSC product in development Robust clinical pipeline with advanced trials in Alzheimer’s disease, aging frailty, HLHS and other indications State - of - the - art GMP facility for stringent manufacturing Lomecel - B well - tolerated to date; early clinical data in 3 indications Proven management, scientific, and manufacturing teams Well - capitalized into 2024 29

Thank You 30 Website : www.longeveron.com Chief Financial Officer : James Clavijo jclavijo@Longeveron.com Investor Relations : Brendan Payne (212) 362 - 1200 Brendan.payne@sternir.com