As filed with the U.S. Securities and Exchange Commission on May 3, 2021
Registration No. 333—
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
VIVOS THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 8011 | 81-3224056 | ||
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
9137 Ridgeline Boulevard, Suite 135,
Highlands Ranch, Colorado 80129
(866) 908-4867
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
R. Kirk Huntsman
Chief Executive Officer
Vivos Therapeutics, Inc.
9137 Ridgeline Boulevard, Suite 135,
Highlands Ranch, Colorado 80129
(866) 908-4867
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
Barry I. Grossman, Esq. Lawrence A. Rosenbloom, Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas, 11th Floor New York, NY 10105 Phone: (212) 370-1300 Fax: (212) 370-1300 |
Christopher J. Barry, Esq. David F. Marx, Esq. Dorsey & Whitney LLP 701 Fifth Avenue, Suite 6100 Seattle, WA 98104-7043 Phone: (206) 903-8815 Fax: (206) 903-8820 |
Approximate date of proposed sale to public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on the Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: [X]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering: [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering: [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering: [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an “emerging growth company”. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | [ ] | Accelerated filer | [ ] |
| Non-accelerated filer | [X] | Smaller reporting company | [X] |
| Emerging growth company | [X] | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. [ ]
CALCULATION OF REGISTRATION FEE
| Title of Class of Securities to be Registered | Amount Being Registered(2) | Amount of Registration Fee | ||||||
| Shares of common stock, par value $0.0001 per share (1) | $ | 23,000,000 | $ | 2,509.30 | ||||
| Representative’s warrant to purchase common stock (3) | — | — | ||||||
| Shares of common stock underlying representative’s warrant (4) | $ | 2,012,500 | $ | 219.56 | ||||
| Total | $ | 25,012,500 | $ | 2,728.86 |
| (1) | Estimated solely for the purpose of calculating the registration fee under Rule 457(o) of the Securities Act of 1933, as amended (the “Securities Act”). Includes shares of common stock that are issuable upon the exercise of the underwriters’ over-allotment option. | |
| (2) | Pursuant to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminate number of shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. | |
| (3) | In accordance with Rule 457(g) under the Securities Act, because the shares of the registrant’s common stock underlying the representative’s warrant are registered hereby, no separate registration fee is required with respect to the warrant registered hereby. | |
| (4) | The warrant issued to the representative of the underwriters is exercisable at a per share exercise price equal to 125% of the offering price. As estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the representative’s warrant is $2,012,500 (which is equal to 125% of 7% of $23,000,000). |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission acting pursuant to Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not the solicitation of an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | Subject to Completion, dated May 3, 2021 |

2,962,963 shares
Vivos Therapeutics, Inc.
Common Stock
We are offering 2,962,963 shares of our common stock on an underwritten, firm commitment basis based on an assumed offering price of $6.75 per share (which was the last reported sale price of our common stock on the Nasdaq Capital Market (or Nasdaq) on April 29, 2021). Our common stock is listed on Nasdaq under the symbol “VVOS”. On April 30, 2021, the last reported sales price of a share of our common stock on Nasdaq was $6.95.
The assumed offering price used throughout this prospectus has been included for illustration purposes only. The actual offering price may differ materially from the assumed price used in the prospectus and will be determined by negotiations between us and the representatives of the underwriters and may not be indicative of prices that will prevail in the trading market.
We have granted the underwriters an option to buy up to an additional 15% or (444,444 shares based on the assumed offering price) of common stock from us to cover over-allotments. The underwriters may exercise this option at any time and from time to time during the 45-day period from the date of the closing of the offering.
We are an “emerging growth company,” as that term is used in the Jumpstart Our Business Startups Act of 2012, and will be subject to reduced public company reporting requirements.
| Per share | Total | |||||||
| Offering price | $ | $ | ||||||
| Underwriting discounts and commissions (1) | $ | $ | ||||||
| Offering proceeds to us, before expenses | $ | $ | | |||||
(1) Does not include additional items of compensation payable to Roth Capital Partners, LLC, the representative of the underwriters, which includes a warrant to purchase five (5%) of the aggregate number of shares issued in this offering (which amount may be increased to up to 7% at our discretion), with an exercise price equal to 125% of the price per share sold in this offering. We have also agreed to reimburse the underwriters for certain accountable expenses incurred by them. See “Underwriting.”
Investing in our common stock is highly speculative and involves a significant degree of risk. See “Risk Factors” beginning on page 12 of this prospectus for a discussion of information that should be considered before making a decision to purchase our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver our shares of common stock to purchasers on or about May 3, 2021.
Sole Book Running Manager
Roth Capital Partners
Co-Managers
National Securities Corporation |
Craig-Hallum |
The date of this prospectus is May 3, 2021
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or in any related free-writing prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus or any free-writing prospectus. We are offering to sell, and seeking offers to buy, our shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is current only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the common stock.
We have not taken any action to permit a public offering of the common stock outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of the common stock and the distribution of the prospectus outside the United States.
We are responsible for the disclosure in this prospectus. However, this prospectus includes industry data that we obtained from internal surveys, market research, publicly available information and industry publications. The market research, publicly available information and industry publications that we use generally state that the information contained therein has been obtained from sources believed to be reliable. The information contained herein represents the most recently available data from the relevant sources and publications and we believe remains reliable. We did not fund and are not otherwise affiliated with any of the sources cited in this prospectus. Forward-looking information obtained from these sources is subject to the same qualifications and additional uncertainties regarding the other forward-looking statements in this prospectus.
We own or have rights to trademarks or trade names that we use in connection with the operation of our business, including our corporate names, logos and website names. In addition, we own or have the rights to copyrights, trade secrets and other proprietary rights that protect the content of our products. This prospectus may also contain trademarks, service marks and trade names of other companies, which are the property of their respective owners. Our use or display of third parties’ trademarks, service marks, trade names or products in this prospectus is not intended to, and should not be read to, imply a relationship with or endorsement or sponsorship of us. Solely for convenience, some of the copyrights, trade names and trademarks referred to in this prospectus are listed without their ©, ® and ™ symbols, but we will assert, to the fullest extent under applicable law, our rights to our copyrights, trade names and trademarks. All other trademarks are the property of their respective owners.
This summary of the prospectus highlights material information concerning our business and this offering. This summary does not contain all of the information that you should consider before making your investment decision. You should carefully read the entire prospectus, including the information presented under the section entitled “Risk Factors” and the financial data and related notes, before making an investment decision. This summary contains forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from future results contemplated in the forward-looking statements as a result of factors such as those set forth in “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements.”
In this prospectus, unless the context indicates otherwise, the terms “we,” “our,” “ours” “us” or similar terminology refer to Vivos Therapeutics, Inc. and its consolidated subsidiaries.
Overview
We are a revenue stage medical technology company focused on the development and commercialization of innovative treatment alternatives for patients with sleep disordered breathing (SDB), including mild-to-moderate obstructive sleep apnea (OSA). We believe our products and technology represent a significant improvement in the treatment of mild-to-moderate OSA versus other treatments such as continuous positive airway pressure (or CPAP) or palliative oral appliance therapies. We call our alternative and advanced treatment the Vivos System.
The Vivos System is an advanced therapeutic protocol, which combines the use of customized oral appliance specifications developed by Vivos and prescribed by specially trained dentists in cooperation with their medical colleagues. The Vivos System features our Mandibular Repositioning Nighttime Appliance (or mRNA appliance®), which incorporates the same patented technology built into our Daytime Nighttime Appliance (or DNA appliance®). We also separately market our own pre-formed guide and rescue appliances which are not a part of the Vivos System (which we refer to collectively as Vivos Guides or Guides).
We believe the Vivos System technology represents the first non-surgical, non-invasive and cost-effective treatment for people with mild-to-moderate OSA. Combining technologies and protocols that alter the size, shape and position of the tissues of a patient’s upper airway, the Vivos System opens airway space and can significantly reduce symptoms and conditions associated with mild-to-moderate OSA. Published studies have shown that using our customized appliances and clinical protocols led to significantly lower Apnea Hypopnea Index scores and improve other conditions associated with OSA. Our patented oral appliances have proven effective (within the scope of the U.S. Food and Drug Administration (or FDA) cleared uses) in over 15,000 patients treated worldwide by more than 1,200 trained dentists.
The House of Delegates of the American Dental Association in 2017 adopted a policy statement describing the important role dentists can play in helping identify patients at greater risk of sleep related breathing disorders. By focusing our business model around dentists, we fulfill this role by training dentists and providing the support to use the Vivos System with their patients that suffer from mild-to-moderate OSA. Our program to train dentists and offer them other value-added services is called the Vivos Integrated Practice (VIP) program. The VIP program provides dentists with a strong economic incentive to provide this treatment and prescribe the Vivos System, together with practice support services.
Sleep apnea is a serious and chronic disease that negatively impacts a patient’s sleep, health and quality of life. According to a 2019 article published in Chest Physician, it is estimated that OSA afflicts 54 million adults in the U.S. alone, and according to a 2016 report by Frost & Sullivan, OSA has an annual societal cost of over $149.6 billion. According to the study “Global Prevalence of Obstructive Sleep Apnea (OSA)” conducted by an international panel of leading researchers, nearly 1 billion people worldwide have sleep apnea.
The Vivos System is estimated to be effective in approximately 80% of cases of obstructive sleep apnea. Approximately 1 billion people globally suffer from OSA, and as many as 80% remain undiagnosed. Research has shown that when left untreated, OSA increases the risk of comorbidities, such as high blood pressure, heart failure, stroke, diabetes, dementia and other debilitating, life-threatening diseases.
| 1 |
In February 2021, we launched VivoScoreTM Powered by SleepImage®, a 510(k) cleared diagnostic technology for home sleep apnea testing featuring what we believe to be significant commercial advantages over existing HSAT products and technologies in the market. We believe VivoScore may enable healthcare providers to more efficiently screen, diagnose and initiate treatment for OSA in their patients which could result in more patients being treated with our Vivos System. While we anticipate increased revenue from VivoScore due to an expected increase in total patients tested and a corresponding increase in patient enrollment in Vivos System treatment, in arriving at this conclusion, we are relying on the results of a pilot test we conducted and other feedback from VIPs, which may or may not prove reliable on a broader scale.
Our Mission
Our mission is to rid the world of OSA. We believe we are well-positioned with what we consider to be a disruptive technology in our Vivos System aimed at treating mild-to-moderate OSA, with a clear first-mover strategy in penetrating the dental market as a means of treating OSA, compelling economics at each level of the delivery chain, and a talented team of experienced professionals who are passionate about what we do and driven to deliver results.
Our Market Opportunity
Estimates from publicly available information vary as to the extent of obstructive sleep apnea in the United States, but we believe the market is significant. According to a 2010 publicly available analysis from researchers at the Harvard Medical School Division of Sleep Medicine, mild obstructive sleep apnea is defined by an apnea-hypopnea index (or AHI) of between 5 and 15 and has a prevalence of 8-11% of the adult population in the United States. A 2004 study published in the Journal of the American Medical Association stated the prevalence of mild obstructive sleep apnea is one in five adults. Based on our analysis of the available public information, we estimate that approximately 15% of the adult population in the United States and Canada suffers from mild-to-moderate OSA. Based on the estimated total adult population of 284 million in the United States and Canada, we believe the total addressable United States and Canadian market is approximately 43 million adults. Our estimates set forth below relating to the intended uses of the Vivos System are also based in part upon data found in the study Oral Appliance Treatment for Obstructive Sleep Apnea: An Update, published publicly by the National Institutes of Health in 2014. Targeted treatment projections identified by this method of sleep titration were found to result in effective treatment in 87% of patients predicted to be successfully treated of OSA in an initial study. To be conservative and based on available data and our internal market analysis, we estimate that over 80% of individuals diagnosed with OSA in the North American addressable market may be candidates for the Vivos System, leaving us with a total addressable consumer market of over 43.2 million adults.
We currently charge clinicians an average sales price of approximately $1,600 per adult case for the Vivos System. There are approximately 160,000 qualified general dentists in the United States and Canada who could potentially offer the Vivos System to their patients. Based on the addressable US and Canadian consumer market described above and average sales price, we believe the addressable consumer market for adults in the United States and Canada is approximately $69 billion.
Our Treatment Alternative for OSA – the Vivos System
The Vivos System is a non-invasive, non-surgical, non-pharmaceutical, multi-disciplinary treatment modality for the treatment of mild to moderate OSA. The proprietary and virtually painless Vivos System enhances and increases the upper airway and offers patients what we believe to be an effective treatment alternative based on clinical retrospective data showing that some patients diagnosed with mild-to-moderate OSA, snoring and SDB symptoms are improving. Based on VIP and patient feedback we have received, we believe initial therapeutic benefits from using the device are often achieved relatively quickly (in days or weeks) and final clinical results are typically achieved in 12 to 24 months), all at a relatively low cost to consumers ranging between $7,000 and $10,000 for adults and $3,500 to $6,000 for children (costs vary by provider) when compared to other options such as surgery.
| 2 |
We believe that the Vivos System alters the size, shape and position of the tissues that surround and comprise the functional space known as the upper airway. This belief is based on retrospective raw data with validated before and after sleep studies and Cone Beam Computerized Tomography (CBCT) scans from treating clinicians and patient testimony. As the Vivos System treatment process progresses, the airway expands, with many patients reporting a significant reduction of their mild-to-moderate OSA symptoms. Our primary product used in the Vivos System is the mRNA appliance®, a specifically designed, custom oral appliance that is worn primarily in the evening hours and overnight and is available for adults. The total treatment time can range from 12 to 24 months with 18 months being the approximate mean treatment time. Our appliances require periodic adjustments some of which can be performed by the patient and others that are typically rendered at the dental office where treatment was initiated. Through the course of treatment with the Vivos System, patients have reported a variety of outcomes, including:
| ● | Reduction of snoring, | |
| ● | Reduction in AHI level and/or other indicators of mild-to-moderate OSA, | |
| ● | Relief of mild-to-moderate OSA symptoms, | |
| ● | Restoration and improvement of normal (nasal) breathing, | |
| ● | Improvement in overall sleep quality, | |
| ● | Reduction in the need for other lifetime treatment options such as CPAP, | |
| ● | Restoration and maintenance of proper facial symmetry and alignment, | |
| ● | Craniofacial and orthodontic correction, | |
| ● | Resolution of TMJ pain, clicking, and locking, and | |
| ● | Facial aesthetic improvement, including a broader smile and reduced ‘gummy smile’ |
Our Growth Strategy
Our goal is to be the global leader in providing a clinically effective non-surgical, non-invasive, non-pharmaceutical, and low-cost alternative for patients with sleep disordered breathing, including mild-to-moderate OSA. We believe the following strategies will play a critical role in achieve this goal and our future growth:
| ● | Expand our North American (U.S. and Canada) sales and marketing organization to drive adoption of our Vivos System. We intend to rapidly and efficiently grow our sales and marketing organization in order to target and expand our network of Vivos Integrated Practices. | |
| ● | Drive medical and dental community awareness of Vivos System. We intend to continue to promote awareness of the value proposition of the Vivos System through training and educating dentists, physicians, and other healthcare providers. |
| ● | Continue to establish indirect marketing channels. We have entered and plan to expand strategic alliances within the medical and dental communities to increase awareness of our products. | |
| ● | Build patient awareness of the Vivos System. We also plan to continue building patient awareness through our direct-to-patient marketing initiatives which we anticipate will include celebrity endorsements, paid search, radio, television, social media, company sponsored events, corporate wellness programs, and online video. | |
| ● | Invest in research and development to drive innovation and expand indications. We are committed to ongoing research and development and we intend to invest in our business to further improve our products and validate our value proposition. | |
| ● | Pursue strategically adjacent markets and international opportunities. We believe there is a significant opportunity for our products outside the United States. We have begun an initial assessment of the development and commercialization of the Vivos System for markets outside of North America, and we plan to conduct further strategic evaluation of such markets as we expand our market penetration throughout the United States and Canada. |
| 3 |
Our Revenue Model
Our revenue is derived from three primary sources, namely (1) VIP enrollment and training fees (comprised of one-time, up-front fees, as well as optional renewal fees after 12 months); (2) recurring Vivos System and Vivos Guides sales; and (3) recurring fees from practice management programs and strategic initiatives, each as described below:
| ● | VIP office training and enrollment fees. | |
| ● | Recurring Vivos System and Guide sales. | |
| ● | Recurring VIP Subscription Fees. | |
| ● | The Institute for Craniofacial Sleep Medicine. Our Institute for Craniofacial Sleep Medicine (ICSM) provides advanced post-graduate education and certification in the emerging science of Pneumopedics® and product-specific training for the use of Vivos products and services. Revenue from such courses is not material at the present time. | |
| ● | The Airway Intelligence Service (AIS.) This service provides a complete resource for VIPs to help simplify the diagnostic and appliance design matrix and expedite the treatment planning process. AIS is provided as part of the price of each appliance and is not a separate revenue stream. | |
| ● | Billing Intelligence Services (BIS). This complete billing solution includes a comprehensive integrated revenue cycle management software system that allows dentists to focus on running their practice and delivering the best care for their patients. Our medical billing service generates recurring subscription fees from participating VIPs. | |
| ● | AireO2 Patient Management Software. This management software enables healthcare professionals to diagnose, treat and monitor patients with OSA and its related conditions more effectively. Developed in collaboration with Lyon Dental, AireO2 contains features that enhance a VIP’s billing services and practice management systems. AireO2 is a complement to our BIS software system. On April 14, 2021, we entered into an asset purchase agreement with Lyon Management and Consulting, LLC and its affiliates to acquire certain medical billing and practice management software, licenses and contracts, including the software underlying AireO2. The asset acquisition allows us to expand and enhance our current medical billing practice through our BIS division. The terms of the purchase include $225,000 of cash and the issuance of a warrant to purchase 25,000 shares of our common stock at a price of $8.90 per share for three years. The vesting of the warrant is as follows: 5,000 shares vested immediately upon issuance of the warrant, 10,000 shares vest and become exercisable on April 14, 2022 and 10,000 shares vest and become exercisable on April 14, 2023. | |
| ● | Medical Integration Division (MID). In 2020, we launched our MID to assist VIP practices to establish clinical collaboration ties to local primary care physicians, sleep specialists, ENTs, pediatricians, pulmonologists and other healthcare professionals who routinely see or treat patients with sleep and breathing disorders. The primary objective of our MID is to promote the Vivos System to the medical profession and thus facilitate the potential for more SDB and OSA patients gaining access to the Vivos System. The MID seeks to fulfill that objective by meeting with VIP dentists and physicians in their local areas to establish physician practices using the trademarked name “Pneusomnia Craniofacial Sleep Medicine Center” (Pneusomnia Center). These independent medical practices will be managed by our company under a management and development agreement which pays us six (6%) percent of all net revenue from sleep-related services. We have built into our core MID business model a great degree of flexibility, such that elements of each Pneusomnia Center as described above may change and be adapted to local state laws and regulations, and entity formation laws as any such alterations do not violate any state or federal statutes or regulations. We believe our early market response from MID activities has been promising, and in March 2021 we announced the opening of the first Pneusomnia Center in Del Mar, California as well as plans to open additional Pneusomnia Centers in several other cities in the U.S. However, it remains too early to predict the eventual impact on our overall revenue. If successful, the MID is expected to enhance the overall practice level economics for independent VIP offices and generate additional lines of recurring revenue for us. | |
| ● | MyoCorrect (Orofacial Myofunctional Therapy) Program. In March 2021, we introduced orofacial myofunctional therapy (or OMT) as a service under the name MyoCorrect. Through MyoCorrect, dentists enrolled in the VIP program will have access to trained therapists who provide OMT via telemedicine technology. This OMT therapy will be a component of obstructive sleep apnea treatment in conjunction with our Vivos System oral appliances and protocols. OMT, which is given by a certified OMT therapist, involves exercises and other techniques aimed at strengthening the tongue and orofacial muscles by teaching individuals how to engage the muscles to the appropriate position. |
| 4 |
Our Competitive Strengths
We believe that the Vivos System has numerous advantages that, taken together, set us apart from the competition and position us for success in the marketplace:
| ● | Significant barriers to entry: We believe that third parties seeking to compete directly with us have significant barriers to entry for the following reasons: competitors must offer a treatment modality with similar features, capabilities, research support, FDA regulatory clearances, and successful clinical outcomes in the market; then establish a comprehensive educational training program featuring other clinical professionals with actual experience and success using that particular treatment modality to properly educate dentists on all clinical aspects of use with patients; then develop and promulgate the systems and best practices required to successfully integrate the treatment of mild-to-moderate OSA using this novel treatment modality in a dental practice; then establish and provide, by recruitment and otherwise, ongoing clinical mentoring and support to dentists engaged in treating their patients for mild-to-moderate OSA and related conditions (clinical mentors are limited and may be hard to find); and finally, assisting the dentists with case selection, case acceptance, patient financing, and medical insurance reimbursement. We believe we have strategically and effectively addressed each and every one of the aforementioned barriers to entry, and thus have created a novel and compelling single-source value proposition for dentists seeking to deliver OSA treatment to their patients. | |
| ● | Vivos System insurance reimbursement: Most major commercial insurance payers reimburse for our adult treatment in the United States. The average level of reimbursement is approximately 50% (with coverage ranging from 5% to 70%), although medical insurance is never a guarantee of payment, and patient deductibles and policy restrictions will vary. | |
| ● | Body of published research and strong patient outcomes: Together with our network of trained dentists, we have developed a body of clinical and patient data over approximately ten years and an estimated delivery of approximately 15,000 appliances that demonstrates the safety, effectiveness, therapy adherence (patient compliance), and benefits of the Vivos System for its 510(k) cleared and registered uses. The documented and reported benefits of treatment with the Vivos System have been consistent across reports from dentists, and have been highlighted in approximately 55 published studies, case reports, and articles, most of which have been peer reviewed. We believe this favorable data provides us with a significant competitive advantage and will continue to support increased adoption. |
| ● | First mover advantage: Our business model is the first to focus on dentists screening patients for mild-to-moderate OSA and SDB, referring patients to physicians for diagnosis, with the dentists then serving as the primary source of treatment using the Vivos System for such patients. | |
| ● | Differentiated products: To our knowledge, only the Vivos System offers a truly differentiated, non-invasive treatment option that actually works on a common root cause of OSA. Older oral appliances are typically less expensive, but do not reshape the upper airway like the Vivos System, and therefore require nightly use over a lifetime, and have a number of other disadvantages. | |
| ● | Intellectual property portfolio and research and development capabilities: We have a comprehensive patent portfolio to protect our intellectual property and technology, with five design patents that expire between 2023 through 2029 and two utility patents expiring in 2029 and 2030. We also own two Canadian patents and one European patent that has been validated in Belgium, Switzerland, Germany, Denmark, Spain, France, United Kingdom, Hungary, Italy and the Netherlands, all of which expire in 2029. Our U.S. trademark portfolio consists of ten registered marks and three pending trademark applications. Extensive online and in-person training, multiple touch point support systems, specific fabrication materials, customized appliance designs, and multi-disciplinary treatment protocols are all considered proprietary trade secrets and competitive advantages with no known counterparts. |
| ● | Extensive Training and Support Systems: We believe our extensive online and in-person clinical and business systems training program offered through our ICSM is unmatched anywhere in dentistry and is a clear competitive strength that would be difficult to replicate |
| ● | Targeted approach to market development: We have established a systematic and scalable approach to actively and consistently engage with our primary target audience of U.S. and Canadian dentists. In addition, our recently launched MID is actively targeting physicians and other relevant healthcare providers in order to build awareness and collaborative patient options at our VIP practices. | |
| ● | Marketplace acceptance: Patient access to the Vivos System at a VIP practice is rapidly becoming readily available, and active VIP providers can now be found in almost all major US cities and in many cities in Canada. |
| 5 |
Our Financial Condition
Since our inception, we have not been profitable and have incurred significant losses and cash flow deficits. For the fiscal years ended December 31, 2020 and 2019, we reported net losses of $12,056,877 and $10,754,319 respectively, and negative cash flow from operating activities of $5,680,294 and $5,340,480, respectively. As of December 31, 2020, we had an aggregate accumulated deficit of $35,334,728. We anticipate that we will continue to report losses and negative cash flow.
Recent Developments
Estimated preliminary unaudited financial results as of and for the three months ended March 31, 2021. Our financial results as of and for the three months ended March 31, 2021 are not yet complete and will not be available until after the completion of this offering. Accordingly, we are presenting ranges, rather than specific amounts, for certain estimated preliminary unaudited financial results set forth below as of and for the three months ended March 31, 2021. The unaudited estimated financial results set forth below are preliminary and subject to revision based upon the completion of our quarter-end financial closing processes. Our estimated preliminary unaudited financial results set forth below are forward-looking statements based solely on information available to us as of the date of this prospectus. As a result, our actual results as of and for the three months ended March 31, 2021 may differ materially from the estimated preliminary unaudited financial results set forth below upon the completion of our financial closing procedures, or upon occurrence of other developments that may arise prior to the time our financial results are finalized. You should not place undue reliance on these preliminary estimates. For additional information, see “Forward-looking statements” and “Risk factors.” Our estimated preliminary unaudited financial results contained in this prospectus have been prepared in good faith by, and are the responsibility of, our management based upon our internal reporting as of and for the three months ended March 31, 2021. Plante & Moran, PLLC has not audited, reviewed, compiled or performed any procedures with respect to the preliminary financial results. Accordingly, Plante & Moran, PLLC does not express an opinion or any other form of assurance with respect thereto.
Based on information currently available to us as of the date of this prospectus, we currently expect that our total revenue for the three months ended March 31, 2021 will range between $3.2 million to $3.5 million and net loss will range between $3.2 million to $3.7 million.
Summary of Risks Affecting Our Business
Investing in our common stock is highly speculative and involves significant risks and uncertainties. You should carefully consider the risks and uncertainties discussed under the section titled “Risk Factors” elsewhere in this prospectus before making a decision to invest in our common stock. Certain of the key risks we face include, without limitation:
| ● | Our business has a limited operating history on which you can evaluate our past performance and future prospects. | |
| ● | We have a history of operating losses and may never achieve cash flow positive or profitable results of operations. | |
| ● | We will need to raise additional capital to fund and grow our business even following this offering. Such funding, even if obtained, could result in substantial dilution or significant debt service obligations. We may not be able to obtain additional capital on commercially reasonable terms in a timely manner, which could adversely affect our liquidity, financial position, and ability to continue operations. | |
| ● | We have identified a material weakness in our internal control over financial reporting. | |
| ● | Substantial portion of our future revenue is from sales of a single product (the Vivos System), which leaves us reliant on the commercial viability of the Vivos System. | |
| ● | Our future operating results may vary significantly from quarter to quarter, which may adversely affect the price of our common stock. | |
| ● | We may not be able to successfully implement our growth strategies for our VIPs, which could harm our business, financial condition and results of operations. | |
| ● | Further clinical studies of our Vivos System may adversely impact our ability to generate revenue if they do not demonstrate that the Vivos System is effective for new indications. | |
| ● | Our business and results of operations may be impacted by the extent to which patients using the Vivos System achieve adequate levels of third-party insurance reimbursement. | |
| ● | Our products and third-party contract manufacturing activities are subject to governmental regulation that could prevent us from selling our Vivos System or introducing new and/or improved products in the United States or internationally. | |
| ● | We face significant competition in the market for treating sleep breathing disorders, and we may be unable to manage competitive pressures. | |
| ● | We may not be able to protect our patents and proprietary technology and may become subject to intellectual property claims or litigation. |
| 6 |
| ● | We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to maintain adequate product liability insurance. | |
| ● | If we are unable to comply, or have not fully complied, with federal and state healthcare fraud and abuse laws, false claims laws, health information privacy and security laws, and other healthcare laws and regulations, we could face substantial penalties. | |
| ● | The misuse or off-label use of the Vivos System could result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business. | |
| ● | We may pursue acquisitions of complementary businesses or technologies, which could divert the attention of management and which may not be integrated successfully into our existing business. |
| ● | The loss of access to our Vivos System technology would terminate or delay the further development of our products, injure our reputation or force us to pay higher fees. | |
| ● | Our failure to obtain government approvals, or to comply with ongoing governmental regulations relating to our technologies and products, could delay or limit introduction of our products and result in failure to achieve revenue or maintain our ongoing business. | |
| ● | We cannot assure that we will be able to complete any required clinical trial programs successfully within any specific time period, and if such clinical trials take longer to complete than we project, our ability to execute our current business strategy will be adversely affected. | |
| ● | Modifications to the Vivos System may require additional FDA approvals which, if not obtained, could force us to cease marketing and/or recall the modified device until we obtain new approvals. | |
| ● | We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, the agency can institute a wide variety of enforcement actions which may materially affect our business operations. | |
| ● | Treatment with the Vivos System has only been available for a relatively limited time, and we do not know whether there will be significant post-treatment regression or relapse. | |
| ● | Our new Medical Integration Division business line may implicate federal and state laws involving the practice of medicine and related anti-kickback and similar laws. | |
| ● | The market for our common stock is new and may not develop to provide you with adequate liquidity. | |
| ● | The market price of our common stock may be highly volatile resulting in substantial losses for investors. | |
| ● | There is a risk of significant future sales by our stockholders that are currently subject to lock-up agreements which expire in June 2021. Such sales could cause the price of our stock price to fall considerably and may adversely impact our ability to raise funds in new stock offerings. Other future sales of other shares of our common stock could have a similar adverse effect on us. | |
| ● | Our failure to meet the continuing listing requirements of The Nasdaq Capital Market could result in a de-listing of our securities. | |
| ● | Our officers and directors may have the ability to exert significant influence over our affairs, including the outcome of matters requiring stockholder approval. |
| 7 |
| ● | We will have considerable discretion over the allocation of the use of proceeds from this offering, and we may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield a return. | |
| ● | You will experience immediate and substantial dilution as a result of this offering, and will likely experience additional dilution in the future. |
Emerging Growth Company under the JOBS Act
As a company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we have elected to take advantage of reduced reporting requirements and are relieved of certain other significant requirements that are otherwise generally applicable to public companies. As an emerging growth company:
| ● | we may present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations; | |
| ● | we are exempt from the requirement to obtain an attestation and report from our auditors on whether we maintained effective internal control over financial reporting under the Sarbanes-Oxley Act; | |
| ● | we are permitted to provide less extensive disclosure about our executive compensation arrangements; and | |
| ● | we are not required to give our stockholders non-binding advisory votes on executive compensation or golden parachute arrangements. |
We may take advantage of these provisions until December 31, 2025 (the last day of the fiscal year following the fifth anniversary of our initial public offering) if we continue to be an emerging growth company. We would cease to be an emerging growth company if we have more than $1.07 billion in annual revenue, have more than $700 million in market value of our shares held by non-affiliates or issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced burdens. We have elected to provide two years of audited financial statements. Additionally, we have elected to take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act.
Corporate Information
Our principal offices are located at 9137 Ridgeline Boulevard, Suite 135, Highlands Ranch, Colorado 80129, and our telephone number is (866) 908-4867. Our website is www.vivoslife.com. Our website and the information on or that can be accessed through such website are not part of this prospectus.
| 8 |
THE OFFERING
| Shares of common stock offered by us: | 2,962,963 shares of common stock based on the assumed offering price | |
| Number of shares of common stock outstanding after this offering: (1) | 21,175,082 shares of common stock (or 21,619,526 shares of common stock if the underwriters exercise their option to purchase additional shares in full based on the assumed offering price) | |
| Over-allotment option: | We have granted the underwriters the right to purchase up to 15% or (444,444 additional shares based on the assumed offering price) of common stock from us at the offering price less the underwriting discount within 45 days from the date of the closing of the offering to cover over-allotments. | |
| Representative’s warrant: | We will issue to Roth Capital Partners, LLC, the representative of the underwriters, upon closing of this offering, a compensation warrant, or the Representative’s Warrant, entitling Roth Capital Partners, LLC to purchase 5% of the aggregate number of shares of common stock issued in this offering, with an exercise price equal to 125% of the price per share sold in this offering ; provided, however, that this amount may be increased from 5% to up to 7% at our discretion. The Representative’s Warrant has a term of five years commencing on the effective date of registration and will be exercisable 180 days after the effective date of the registration statement relating to this offering. | |
| Use of proceeds: | While we will have broad discretion on the allocation of the use of net proceeds of this offering, we currently expect to utilize such proceeds for (i) promotion, distribution and related expenses associated with our VivoScore home sleep apnea test; (ii) sales and marketing expenses generally; (ii), sales and support staff; (iv) research and development expenses; (v) software development and enterprise resource planning implementation; and (vi) working capital and general corporate purposes. We may also use proceeds from this offering to acquire complimentary technologies, products or businesses, although we are not a party to any letters of intent or definitive agreement for any such acquisition. See “Use of Proceeds”. | |
| Nasdaq Capital Market symbol: | Our common stock is listed on the Nasdaq Capital Market under the symbol “VVOS”. | |
| Risk factors: | Investing in our common stock is highly speculative and involves a significant degree of risk. As an investor you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section beginning on page 12. |
(1)The number of shares of our common stock outstanding before and after this offering, as set forth in the table above, is based on 18,212,119 shares outstanding as of the date of this prospectus and excludes as of that date:
| ● | 2,302,345 shares of common stock underlying options to purchase shares of our common stock issued and outstanding as of December 31, 2020 with a weighted average exercise price of $4.84 per share; | |
| ● | an additional 145,000 shares of common stock underlying options to purchase shares of our common stock issued subsequent to December 31, 2020 with an exercise price of $7.50 per share; |
| 9 |
| ● | 1,199,195 shares of common stock issuable upon the exercise of 1,199,195 common stock warrants associated with our previously outstanding Series B Preferred Stock issued in 2020 at an exercise price of $7.50 per share; | |
| ● | 402,500 shares of our common stock underlying a warrant issued to the representative of the underwriters in connection with our December 2020 initial public offering with an exercise price of $7.50 per share; | |
| ● | up to 148,149 shares of our common stock underlying the Representative’s Warrant to be issued to the representative of the underwriters in connection with this offering based on the assumed offering price (or up to 170,371 shares of our common stock to the underwriters if the over-allotment option to purchase shares of common stock is exercised in full based on the assumed offering price), which amounts may be increased, at our discretion, to up to 207,408 and 238,519 respectively (based on the assumed offering price); | |
| ● | 325,000 warrants which were issued to certain shareholders in November 2020 (see “Management—October 2020 Derivative Demand and Settlement”) at an exercise price of $7.50 per share; |
| ● | 295,000 warrants to purchase common stock issued to contractors and consultants subsequent to December 31, 2020 with an exercise price of $7.50 per share, which includes 200,000 warrants issued in connection with the establishment of our MyoCorrect Program (see “Business—Our Revenue Model— MyoCorrect (Orofacial Myofunctional Therapy) Program”), for further information; | |
| ● | 25,000 warrants to purchase common stock issued in connection with the acquisition of Lyon Management and Consulting, LLC subsequent to December 31, 2020 with an exercise price of $8.90 per share, for the purpose to acquire certain medical billing and practice management software, licenses and contracts, including the software underlying AireO2 (see “Business—Our Revenue Model—AireO2 Patient Management Software”), for further information; and | |
| ● | 33,334 warrants to purchase common stock issued to an investor of convertible notes. The warrants are exercisable on a cash basis at an exercise price of $1.50 per share. |
Unless otherwise indicated, all information in this prospectus:
| ● | assumes no exercise of the Representative’s Warrant; | |
| ● | assumes no exercise of the underwriters’ over-allotment option to purchase an additional 444,444 shares of common stock from us. |
| 10 |
SUMMARY HISTORICAL CONSOLIDATED FINANCIAL DATA
The following table presents our summary historical consolidated financial data as of and for the years ended December 31, 2020 and 2019.
Historical results are included for illustrative and informational purposes only and are not necessarily indicative of results we expect in future periods. You should read the following summary financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes appearing elsewhere in this prospectus.
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Statement of Operations Data | ||||||||
| Total revenue | $ | 13,066,237 | $ | 11,393,277 | ||||
| Cost of sales | (2,653,429 | ) | (2,736,034 | ) | ||||
| Gross profit | 10,412,808 | 8,657,243 | ||||||
| Total operating expenses | (22,452,616 | ) | (19,234,476 | ) | ||||
| Loss from operations | (12,039,808 | ) | (10,577,233 | ) | ||||
| Interest expense | (96,681 | ) | (137,876 | ) | ||||
| Interest income | 79,612 | 21,133 | ||||||
| Loss on sale of business | — | (60,343 | ) | |||||
| Loss before income taxes | (12,056,877 | ) | (10,754,319 | ) | ||||
| Income tax expense | — | — | ||||||
| Net loss | $ | (12,056,877 | ) | $ | (10,754,319 | ) | ||
| Warrant beneficial conversion feature expense | (3,597,585 | ) | — | |||||
| Preferred stock accretion | (2,333,333 | ) | (1,000,000 | ) | ||||
| Net loss attributable to common stockholders | (17,987,795 | ) | (11,754,319 | ) | ||||
| Net loss per common share, basic and diluted | $ | (1.40 | ) | $ | (0.95 | ) | ||
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Balance Sheet Data | ||||||||
| Cash and cash equivalents | $ | 18,205,668 | $ | 469,353 | ||||
| Working capital (1) | 12,571,266 | (7,109,528 | ) | |||||
| Total assets | 25,327,469 | 7,551,537 | ||||||
| Total liabilities | 8,410,110 | 9,177,929 | ||||||
| Total stockholders’ equity | $ | 16,917,359 | $ | (2,943,059 | ) | |||
| (1) | Working capital represents total current assets less total current liabilities. |
| 11 |
Investing in our common stock is highly speculative and involves a significant degree of risk. Before you invest in our securities, you should give careful consideration to the following risk factors, in addition to the other information included in this this prospectus, including our financial statements and related notes, before deciding whether to invest in our securities. The occurrence of any of the adverse developments described in the following risk factors could materially and adversely harm our business, financial condition, results of operations or prospects. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Business and Industry
Our business has a limited operating history on which you can evaluate our past performance and future prospects.
Our business was formed only in 2016, and therefore you have limited historical data on which to evaluate our company. This is particularly true because our current VIP-focused business model was only commenced in mid-2018. Therefore, you have even more limited historical operating data on which to evaluate the results of and prospects for our current business model.
We have a history of operating losses and may never achieve cash flow positive or profitable results of operations.
Since our inception, we have not been profitable and have incurred significant losses and cash flow deficits. For the fiscal years ended December 31, 2020 and 2019, we reported net losses of $12,056,877 and $10,754,319 respectively, and negative cash flow from operating activities of $5,680,294 and $5,340,480, respectively. As of December 31, 2020, we had an aggregate accumulated deficit of $35,334,728. We anticipate that we will continue to report losses and negative cash flow. There is therefore a risk that we will be unable to operate our business in a manner that generate positive cash flow or profit, and our failure to operate our business profitably would damage our reputation and stock price. Our independent auditors issued an audit opinion with respect to our consolidated financial statements for the year ended December 31, 2019 that indicated that there was a substantial doubt about our ability to continue as a going concern, and this may occur again if we do not achieve positive results of operations in the future.
We will need to raise additional capital to fund and grow our business even following this offering. Such funding, even if obtained, could result in substantial dilution or significant debt service obligations. We may not be able to obtain additional capital on commercially reasonable terms in a timely manner, which could adversely affect our liquidity, financial position, and ability to continue operations.
In order to fund and grow our business, we will need to obtain additional financing even following this offering, either through borrowings, private offerings, public offerings, or some type of business combination, such as a merger, or buyout, and there can be no assurance that we will be successful in such pursuits. We may be unable to acquire the additional funding necessary to fund our growth or to continue operating. Accordingly, if we are unable to generate adequate cash from operations, and if we are unable to find sources of funding, it may be necessary for us to sell one or more lines of business or all or a portion of our assets, enter into a business combination, or reduce or eliminate operations. These possibilities, to the extent available, may be on terms that result in significant dilution to our shareholders or that result in our investors losing all of their investment in our company.
If we are able to raise additional capital, we do not know what the terms of any such capital raising would be. In addition, any future sale of our equity securities would dilute the ownership and control of your shares and could be at prices substantially below prices at which our shares currently trade. Our inability to raise capital, coupled with our inability to generate adequate cash from operations, could require us to significantly curtail or terminate our operations. We may seek to increase our cash reserves through the sale of additional equity or debt securities. The sale of convertible debt securities or additional equity securities could result in additional and potentially substantial dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations and liquidity and ability to pay dividends. In addition, our ability to obtain additional capital on acceptable terms is subject to a variety of uncertainties. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all. Any failure to raise additional funds on favorable terms could have a material adverse effect on our liquidity and financial condition.
| 12 |
We have identified a material weakness in our internal control over financial reporting.
Prior to our initial public offering in December 2020, we were a private company and had limited accounting and financial reporting personnel and other resources with which to address our internal controls and related procedures. In connection with the audit of our consolidated financial statements for the years ended December 31, 2020 and 2019, we and our independent registered public accounting firm identified a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness in our case arose from an accumulation of significant deficiencies which amounted to a material weakness in internal controls. Such significant deficiencies identified included insufficient supporting documentation and inadequate review of certain journal entries, segregation of duties, and inadequate application of accounting guidance. If we are unable to remedy our material weakness, or if we generally fail to establish and maintain effective internal controls appropriate for a public company, we may be unable to produce timely and accurate financial statements, and we may conclude that our internal control over financial reporting is not effective, which could adversely impact our investors’ confidence and our stock price.
We will not be successful if our Vivos System is not sufficiently adopted by the medical and dental communities, including independent practitioners and dental service organizations (DSOs) for the treatment of craniofacial deficiencies that are often associated with SDB and mild-to-moderate OSA.
We believe that the Vivos System is the first commercially available product based on our proprietary technology for the treatment of craniofacial deficiencies that are often associated with SDB and mild-to-moderate OSA. Our success depends both on the sufficient acceptance and adoption by the medical/dental community of our Vivos System as a non-invasive treatment for the treatment of craniofacial deficiencies that are often associated with SDB and mild-to-moderate OSA, and heightening public awareness of the prevalence of mild-to-moderate OSA to increase the number of undiagnosed patients with SDB and mild-to-moderate OSA who seek treatment. Currently, a relatively limited number of dentists and other medical clinicians provide treatment with the Vivos System. We cannot predict how quickly, if at all, the medical/dental community will accept our Vivos System, or, if accepted, the extent of its use. For us to be successful:
| ● | our dentist customers and referring physicians must believe that the Vivos System offers meaningful clinical and economic benefits for the treating provider and for the patient as compared to the other surgical and non-surgical procedures or devices currently being used to treat individuals with SDB or mild-to-moderate OSA and referring physicians must write a prescription for the use of the Vivos System; | |
| ● | our dentist customers must use our Vivos System to treat craniofacial deficiencies that are often associated with SDB and mild-to-moderate OSA either as a stand-alone treatment or in combination with procedures to treat other areas of upper airway obstruction, and achieve acceptable clinical outcomes in the patients they treat; | |
| ● | our dentist customers must believe patients will pay for the Vivos System out-of-pocket, and patients must believe that paying out-of-pocket for treatment in the Vivos System is the best alternative to either doing nothing or entering into another treatment option; and | |
| ● | our dentist customers must be willing to pay us for the right to become VIPs and to commit the time and resources required to learn the new clinical and technical skills and invest in the technology required to treat patients with SDB or mild-to-moderate OSA using the Vivos System. |
Studies have shown that a significant percentage of people who have SDB or OSA remain undiagnosed and therefore do not seek treatment, or those who are diagnosed with SDB or OSA may be reluctant to seek treatment or incur significant costs of treatment given the less severe nature of their condition, the potentially negative lifestyle effects of traditional treatments, and the lack of awareness of new treatment options. If we are unable to increase public awareness of the prevalence of SDB or OSA due to untreated craniofacial deficiencies or if the medical/dental community is slow to adopt, or fails to adopt, the Vivos System as a treatment for individuals with SDB or mild-to-moderate OSA, we would suffer a material adverse effect on our business, financial condition and results of operations.
| 13 |
Our VIP program is a relatively new business model for us, and management has limited experience operating this model.
Our VIP program is a relatively new business model for us, and members of our management team have limited experience operating our company through this model. As a result, our historical financial results may not be comparable to future results. Also, we are subject to many risks associated with this new business model that we are unable to presently identify, such as pricing, competition, marketing and regulatory risks. Moreover, our ability to onboard new VIPs may be impeded by the investments VIPs must make in adapting their practices to the use of the Vivos System. We cannot assure you that management will be able to recruit and adopt new VIPs. Any such failure may have an adverse impact on our business, financial condition and results of operations.
We expect to derive a substantial portion of our future revenue from sales of a single product (the Vivos System) through our VIPs and the offering of related services, which leaves us reliant on the commercial viability of the Vivos System.
Currently, our primary product is our Vivos System. Our secondary source of revenue is our clinical training and practice support programs, including Billing Intelligence Services, Airway Intelligence System, AireO2, VivoScore and MyoCorrect. We expect that sales of our Vivos System and our services to our VIPs related to the use of such product will account for a significant majority of our revenue for the foreseeable future. We currently market and sell our Vivos System primarily in the United States and Canada, with a very limited presence a in very few select countries such as South Korea, Australia, Japan and India. Because the Vivos System is different from current surgical and non-surgical treatments for SDB or OSA, we cannot assure you that dentists in corroboration with physicians will use the Vivos System or become VIPs, and demand for our Vivos System may decline or may not increase as quickly as we expect. Also, we cannot assure you that the Vivos System will compete effectively as a treatment alternative to other more well-known and well-established therapies, such as CPAP, mandibular advancement, or palatal surgical procedures. Since our Vivos System and other oral appliances currently represent our only products, and since our VIP program is our primary means of commercialization, we are significantly reliant on the level of recurring sales of the Vivos System and other oral appliances, and decreased or lower than expected sales or recruitment and maintenance of new VIPs would cause us to lose all or substantially all of our revenue.
We face risks relating to public health conditions such as the COVID-19 pandemic, which could adversely affect our dentist customers, our business and our results of operations.
Our business and prospects has been and could be materially adversely affected by the COVID-19 pandemic or recurrences of COVID-19 (such as has occurred in the fall of 2020) or any other similar diseases in the future. Material adverse effects from COVID-19 and similar diseases could result in numerous known and currently unknown ways including from quarantines and lockdowns which impair our marketing and sales efforts to dentists or other medical professionals. During the COVID-19 pandemic, dental offices throughout the U.S. and Canada shut down for extended periods of time (and may be shut down again due to recurrences of COVID-19), thus negatively impacting our product revenues. The pandemic and reactions to the pandemic or future outbreaks of COVID-19 could also impair the timing of obtaining necessary consents and approvals from the FDA, as its employees could also be under such quarantines and lockdowns and their time could be mandatorily required to be allocated to more immediate global and domestic concerns relating to COVID-19. In addition, we purchase materials for our products from suppliers located in affected areas, and we may not be able to procure required components or secure manufacturing capability. The effects of the COVID-19 pandemic have also placed travel restrictions on us and our VIPs, as well as temporary closures of the facilities of our suppliers and our VIPs as non-essential medical and dental procedures have been limited, which could also adversely impact our business. In addition, a significant outbreak of contagious diseases in the human population could result in a widespread health crisis that could adversely affect the economies and financial markets of many countries, resulting in an economic downturn that could reduce the demand for our products and impair our business prospects including as a result of being unable to raise additional capital on acceptable terms to us, if at all.
We may not be able to successfully implement our growth strategy for our VIPs on a timely basis or at all, which could harm our business, financial condition and results of operations.
The growth of our VIP base depends on our ability to execute our plan to recruit and enroll new VIPs. Our ability to recruit and enroll VIPs depends on many factors, including our ability to:
| ● | achieve brand awareness in new and existing markets; |
| 14 |
| ● | convince potential VIPs of the value of our products and services and to make the required investments in becoming a VIP and using the Vivos System; | |
| ● | manage costs, which could give rise to delays or cost overruns; | |
| ● | recruit, train, and retain qualified dentists, dental hygienists, physicians, physician assistants, medical technologists and other staff in our local markets; | |
| ● | obtain favorable reimbursement rates for services rendered at VIP offices; | |
| ● | outperform competitors; and | |
| ● | maintain adequate information systems and other operational system capabilities. |
Further, applicable laws, rules and regulations (including licensure requirements) could negatively impact our ability to recruit and enroll VIPs.
Accordingly, we may not be able to achieve our planned growth or, even if we are able to grow our VIP base as planned, any new VIPs may not be profitable or otherwise perform as planned. Failure to successfully implement our growth strategy would likely have an adverse impact on our business, financial condition and results of operations.
The long-term success of our VIP program is highly dependent on our ability to successfully identify, recruit and enroll target dental practices.
To achieve our growth strategy, we will need to identify, recruit and enroll new VIPs and have them operate on a profitable basis. We take into account numerous factors in identifying target markets where we can enter or expand.
The number and timing of new VIPs enrolled during any given period may be negatively impacted by a number of factors including, without limitation:
| ● | the identification and availability of attractive practices to be VIPs; | |
| ● | our ability to successfully identify and address pertinent risks and benefits during the onboarding process; | |
| ● | the proximity of VIPs to one of our or our competitors’ existing centers; | |
| ● | our VIP’s ability to obtain required governmental licenses, permits and authorizations on a timely basis; and | |
| ● | our VIP’s ability to recruit qualified dentists, dental hygienists, physicians, physician assistants, medical technologists and other personnel to staff their practices using the Vivos System. |
If we are unable to find and onboard attractive VIPs in existing markets or new markets, our revenue and profitability may be harmed, we may not be able to implement our growth strategy and our financial results may be negatively affected.
Our future operating results are difficult to predict and may vary significantly from quarter to quarter, which may adversely affect the price of our common stock.
Our limited history of sales of our Vivos System, together with our history of losses, make prediction of future operating results difficult. You should not rely on our past revenue growth as any indication of future growth rates or operating results. Our valuation and the price of our securities likely will fall in the event our operating results do not meet the expectations of analysts and investors. Comparisons of our quarterly operating results are an unreliable indication of our future performance because they are likely to vary significantly based on many factors, including:
| ● | our inability to attract demand for and obtain acceptance of our Vivos System for the treatment of craniofacial deficiencies that are often associated with SDB and mild-to-moderate OSA by both physicians/dentists and patients; |
| 15 |
| ● | the success of alternative therapies and surgical procedures to treat individuals with SDB, and the possible future introduction of new products and treatments for SDB; | |
| ● | our ability to maintain current pricing for our Vivos System; | |
| ● | our ability to expand by adding additional VIPs in leading major metro areas; | |
| ● | the expansion and rate of success of our marketing and advertising efforts to both consumers and dentists, and the rate of success of our direct sales force in the United States and internationally; | |
| ● | failure of third-party contract manufacturers to deliver products or provide services in a cost effective and timely manner; | |
| ● | our failure to develop, find or market new products; | |
| ● | the successful completion of current and future clinical studies, and the possibility that the results of any future study may be adverse to our product and services, or reveal some heretofore unknown risk to patients from treatment in the Vivos System; the failure by us to make professional presentation and publication of positive outcomes data from these clinical studies, and the increased adoption of the Vivos System by dentists as a result of the data from these clinical studies; | |
| ● | actions relating to ongoing FDA compliance; | |
| ● | the size and timing of orders from dentists and independent distributors; | |
| ● | our ability to obtain reimbursement for the Vivos System for the treatment of craniofacial conditions that are often associated with SDB and OSA in the future from third-party healthcare insurers; |
| ● | the willingness of patients to pay out-of-pocket for treatment in the Vivos System or other Vivos oral appliances, in the absence of reimbursement from third-party healthcare insurers, for the treatment of craniofacial conditions that are often associated with SDB and OSA; decisions by one or more commercial health insurance companies to preclude, deny, limit, reduce, eliminate, or curtain reimbursement for treatment in whole or part by the Vivos System; | |
| ● | unanticipated delays in the development and introduction of our future products and/or our inability to control costs; | |
| ● | the effects of global or local pandemics or epidemics and governmental responses, such as COVID-19; | |
| ● | seasonal fluctuations in revenue due to the elective nature of sleep-disordered breathing treatments, including the Vivos System, as well as seasonal fluctuations resulting from adverse weather conditions, earthquakes, floods or other acts of nature in certain areas or regions that result in power outages, transportation interruptions, damages to one or more of our facilities, food shortages, or other events which may cause a temporary or long-term disruption in patient priorities, finances, or other matters; and | |
| ● | general economic conditions as well as those specific to our customers and markets. |
Therefore, you should expect that our results of operations will be difficult to predict, which will make an investment in our company uncertain.
| 16 |
Our MID program is a new business offering for us, and it may not perform as anticipated or may take longer than expected to gain acceptance.
Begun only in 2020, our MID is a new business offering for us, and the model is yet unproven. As a result, actual results may be lower than expected due to lower than expected referrals or other factors. Also, we are subject to many risks associated with this new business model that we are unable to presently identify, such as pricing, competition, marketing and regulatory risks. If we fail to adequately identify and respond to such risks in a timely manner, on our business, financial condition and results of operations could be adversely affected.
VivoScore is a new technology which may not be utilized by VIPs to the degree anticipated.
VivoScore is a relatively new technology. New technologies often take longer to gain acceptance within the medical and dental communities. If medical and dental care providers do not utilize this new technology, or if VivoScore is not as effective as anticipated, the financial results of VivoScore may be lower than currently expected. Also, we are subject to many risks associated with this new technology that we are unable to presently identify, such as pricing, competition, marketing and regulatory risks. If we fail to adequately identify and respond to such risks in a timely manner, on our business, financial condition and results of operations could be adversely affected.
We may not be able to respond in a timely and cost-effective manner to changes in consumer preferences.
The Vivos System is subject to changing consumer preferences. A shift in consumer preferences away from the product we offer would result in significantly reduced revenue. Our future success depends in part on our ability to anticipate and respond to changes in consumer preferences. Failure to anticipate and respond to changing consumer preferences in the products we market could lead to, among other things, lower sales of products, significant markdowns or write-offs of inventory, increased product returns and lower margins. If we are not successful in anticipating and responding to changes in consumer preferences, our results of operations in future periods will be materially adversely impacted.
Further clinical studies of our Vivos System may adversely impact our ability to generate revenue if they do not demonstrate that our Vivos System is clinically effective for currently specified or expanded indications or if they are not completed in a timely manner.
We have conducted, and continue to conduct, a number of clinical studies of the use of our Vivos System and other Vivos oral appliances to treat patients with SDB or mild-to-moderate OSA due to craniofacial deficiencies in the United States and Canada. We are involved in a number of ongoing clinical studies evaluating clinical outcomes from the use of the Vivos System and other Vivos oral appliances, including prospective, randomized, placebo-controlled studies, as well as clinical studies that are structured to obtain additional clearances from the FDA for expanded clinical indications for use of our Vivos System.
We cannot assure you that these clinical studies will continue to demonstrate that our Vivos System provides clinical effectiveness for individuals diagnosed with SDB or mild-to-moderate OSA, nor can we assure you that the use of our Vivos System will prove to be safe and effective in clinical studies under United States or international regulatory guidelines for any expanded indications. Additional clinical studies of our Vivos System may identify significant clinical, technical or other obstacles that will have to be overcome prior to obtaining clearance from the applicable regulatory bodies to market our Vivos System for such expanded indications. If further studies of our Vivos System indicate that the Vivos System is not a safe and effective treatment of SDB or mild-to-moderate OSA, our ability to market our Vivos System, and generate substantial revenue from additional sales of our Vivos Systems, may be materially limited.
Individuals selected to participate in these further clinical studies must meet certain anatomical and other criteria to participate. We cannot assure you that an adequate number of individuals can be enrolled in clinical studies on a timely basis. Further, we cannot assure you that the clinical studies will be completed as planned. A delay in the analysis and publication of the positive outcomes data from these clinical studies, or the presentation or publication of negative outcomes data from these clinical studies, including data related to approval of our Vivos System for expanded indications, may materially impact our ability to increase revenue through sales and negatively impact our stock price.
| 17 |
Our business and results of operations may be impacted by the extent to which patients using the Vivos System achieve adequate levels of third-party insurance reimbursement.
Whenever practical, the Vivos System is paid for primarily out-of-pocket by patients, with any available health insurance coverage being reimbursed if and as paid at a later date, where the patient is being treated for SDB or mild-to-moderate OSA.
The cost of treatments for SDB or OSA, such as CPAP, and most surgical procedures generally are covered and reimbursed in whole or part by third-party healthcare insurers. The Vivos System is a customized and highly specialized combination of oral appliances and clinical protocols, some of which currently qualify for reimbursement for the treatment of mild-to-moderate OSA and SDB. Our ability to generate revenue from additional sales of our Vivos System for the treatment of SDB or OSA may be materially limited by the extent to which reimbursement of the Vivos System for the treatment of mild-to-moderate OSA and SDB is available in the future. In addition, third-party healthcare insurers are increasingly challenging the prices charged for medical products and procedures. In the event that we are successful in our efforts to obtain reimbursement for the Vivos System, any changes in this reimbursement system could materially affect our ability to continue to grow our business.
Reimbursement and healthcare payment systems in international markets vary significantly by country and reimbursement for the Vivos System may not be available at all under either government or private reimbursement systems. If we are unable to achieve reimbursement approvals in international markets, it could have a negative impact on market acceptance of our Vivos System and potential revenue growth in the markets in which these approvals are sought.
Our products and third-party contract manufacturing activities are subject to extensive governmental regulation that could prevent us from selling our Vivos System or introducing new and/or improved products in the United States or internationally.
Our products and third-party contract manufacturing activities are subject to extensive regulation by a number of governmental agencies, including the FDA and comparable international regulatory bodies. We are required to:
| ● | obtain clearance from the FDA and certain international regulatory bodies before we can market and sell our products; | |
| ● | satisfy all content requirements for the sales and promotional materials associated with the Vivos System; and | |
| ● | undergo rigorous inspections of our facilities, manufacturing and quality control processes, records and documentation. |
Compliance with the rules and regulations of these various regulatory bodies may delay or prevent us from introducing any new models of our Vivos System or other new products. In addition, government regulations may be adopted that could prevent, delay, modify or rescind regulatory clearance or approval of our products.
Our manufacturing partners are further required to demonstrate compliance with the FDA’s quality system regulations. The FDA enforce their quality system regulations through pre-approval and periodic post-approval inspections by representatives from the FDA. These regulations relate to product testing, vendor qualification, design control and quality assurance, as well as the maintenance of records and documentation. If we fail to conform to these regulations, the FDA may take actions that could seriously harm our business. These actions include sanctions, including temporary or permanent suspension of our operations, product recalls and marketing restrictions. A recall or other regulatory action could substantially increase our costs, damage our reputation and materially affect our operating results.
Our products are currently not recommended by most pulmonologists, who are integral to the diagnosis and treatment of sleep breathing disorders.
The majority of patients being treated today for SDB or OSA, domestically and internationally, are initially referred to pulmonologists by their primary care physicians. Pulmonologists typically administer a polysomnogram, or overnight sleep study, to diagnose the presence and severity of SDB or OSA. If an individual is diagnosed with SDB or OSA by a pulmonologist, the pulmonologist typically prescribes CPAP as the therapy of choice. Although we offer the Vivos System through our VIPs, our domestic sales organization does not generally call on pulmonologists or third-party sleep centers to sell our Vivos System, and we do not believe that most pulmonologists today would recommend the Vivos System to their patients with SDB or mild-to-moderate OSA. We cannot predict the extent to which pulmonologists will, in the future, endorse or recommend the Vivos System to their SDB or mild-to-moderate OSA patients, even for those patients who are unwilling or unable to comply with CPAP therapy.
| 18 |
We face significant competition in the rapidly changing market for treating sleep breathing disorders, and we may be unable to manage competitive pressures.
The market for treating sleep disordered breathing, including sleep apnea in people of all ages, is highly competitive and evolving rapidly. We compete as a second-line therapy in the OSA treatment market for patients with mild to moderate OSA. According to the American Sleep Apnea Association, over 100 different oral appliances are FDA cleared for the treatment of snoring and obstructive sleep apnea. The Vivos System must compete with more established products, treatments and surgical procedures, which may limit our growth and negatively affect our business. Many of our competitors have an established presence in the field of treating SDB and have established relationships with pulmonologists, sleep clinics and ear, nose and throat specialists, which play a significant role in determining which product, treatment or procedure is recommended to the patient. We believe certain of our competitors are attempting to develop innovative approaches and new products for diagnosing and treating SDB or OSA and other sleep disordered breathing conditions. We cannot predict the extent to which ENTs, oral maxillofacial surgeons, primary care physicians or pulmonologists would or will recommend our Vivos System over new or other established devices, treatments or procedures.
Moreover, we are in the early stages of implementing our business plan and have limited resources with which to market, develop and sell our Vivos System. Many of our competitors have substantially greater financial and other resources than we do, including larger research and development staffs who have more experience and capability in conducting research and development activities, testing products in clinical trials, obtaining regulatory approvals and manufacturing, marketing, selling and distributing products. Some of our competitors may achieve patent protection, regulatory approval or product commercialization more quickly than we do, which may decrease our ability to compete. If we are unable to be competitive in the market for OSA and SDB, our revenue will decline, which would negatively affect our results of operations.
Our Vivos System may become obsolete if we are unable to anticipate and adapt to rapidly changing technology.
The medical device industry is subject to rapid technological innovation and, consequently, the life cycle of any particular product can be short. Alternative products, procedures or other discoveries and developments to treat SDB and OSA may render our Vivos System obsolete. Furthermore, the greater financial and other resources of many of our competitors may permit them to respond more rapidly than we can to technological advances. If we fail to develop new technologies, products or procedures to upgrade or improve our existing Vivos System to respond to a changing market before our competitors are able to do so, our ability to market our products and generate substantial revenue may be limited.
Our international sales are subject to a number of risks that could seriously harm our ability to successfully commercialize our Vivos System in international markets.
We do not have significant international sales outside of Canada, although we hope to more broadly introduce our Vivos Systems into international markets. Our ability to generate international sales is subject to several risks, including:
| ● | our ability to obtain appropriate regulatory approvals to market the Vivos System in certain countries; | |
| ● | our ability to identify new independent third-party distributors in international markets where we do not currently have distributors; | |
| ● | the impact of recessions in economies outside the United States; | |
| ● | greater difficulty in negotiating with socialized medical systems, maintaining profit margins comparable to those achieved in the United States, collecting accounts receivable, and longer collection periods; |
| 19 |
| ● | unexpected changes in regulatory requirements, tariffs or other trade barriers; | |
| ● | weaker intellectual property rights protection in some countries; | |
| ● | potentially adverse tax consequences; and | |
| ● | political and economic instability. |
The occurrence of any of these events could seriously harm our future international sales and our ability to successfully commercialize our products in international markets, thereby limiting our growth and revenue.
There are risks associated with outsourced production that may result in a decrease in profit to us.
We outsource the manufacture of substantially all of our products to third-party manufacturers on a case-by-case basis. By law, the selection of the manufacturer is at the sole discretion of the treating dentist. However, we select our approved and certified manufacturers by training and screening them in advance based on their capabilities, supply capacity, reputation, regulatory registration and compliance, and other relevant traits. Most of these manufacturers are located in the U.S., but at least one important manufacturer is located in South Korea, and other smaller manufacturers are located in Canada. Nonetheless, the possibility of delivery delays, product defects, import or customs blockages, and other production-side risks stemming from outsourcers cannot be eliminated. In particular, inadequate production capacity among outsourced manufacturers could result in our being unable to supply enough product amid periods of high product demand, the opportunity costs of which could be substantial.
We do not have any long-term contracts with manufacturers, suppliers or other service providers for our products. Our business would be harmed if manufacturers and service providers are unable to deliver products or provide services in a timely and cost-effective manner, or if we are unable to timely fulfill orders.
We do not have any long-term contracts with manufacturers, suppliers or other service providers for our products. We do not anticipate that this will change. As a result, if any manufacturer or supplier is unable, either temporarily or permanently, to manufacture or deliver products or provide services to us in a timely and cost-effective manner, it could have an adverse effect on our financial condition and results of operations. Our ability to provide effective customer service and efficiently fulfill orders for merchandise depends, to a large degree, on the efficient and uninterrupted operation of the manufacturing and related call centers, distribution centers, and management information systems, some of which are run by third parties. Any material disruption or slowdown in manufacturing, order processing or fulfillment systems resulting from strikes or labor disputes, telephone down times, electrical outages, mechanical problems, human error or accidents, fire, natural disasters, adverse weather conditions or comparable events could cause delays in our ability to receive and fulfill orders and may cause orders to be lost or to be shipped or delivered late. As a result, these disruptions could adversely affect our financial condition or results of operations in future periods.
The failure of large U.S. customers or Dental Service Organizations (DSO) to pay for their purchases of Vivos System products and services on a timely basis could reduce our future sales revenue and negatively impact our liquidity.
The timing and extent of our future growth in sales revenue depends, in part, on our ability to continue to increase the number of U.S. dentists using the Vivos System, as well as expanding the number of Vivos Systems used by these physicians/dentists. To the extent one or more of our large U.S. dentist customers or DSO groups fails to pay us for Vivos Systems on a timely basis, we may be required to discontinue selling to these organizations and find new customers, which could reduce our future sales revenue and negatively impact our liquidity.
We depend on our patents and proprietary technology, which we may not be able to protect.
Our success depends, in part, on our ability to obtain and maintain patent protection for our Vivos System components and the confidentiality of proprietary clinical protocols. Our success further depends on our ability to obtain and maintain trademark protection for our name and mark; to preserve our trade secrets and know-how; and to operate without infringing the intellectual property rights of others.
| 20 |
We cannot assure investors that we will continue to innovate and file new patent applications, or that if filed any future patent applications will result in granted patents We cannot assure you that any of our patents pending will result in issued patents, that any current or future patents will not be challenged, invalidated or circumvented, that the scope of any of our patents will exclude competitors or that the patent rights granted to us will provide us any competitive advantage or protect our products. The patent position of device companies, including ours, is generally uncertain and involves complex legal and factual considerations and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies, protocols and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
Any patents we have obtained or do obtain may be challenged by re-examination or otherwise invalidated or eventually found unenforceable. Both the patent application process and the process of managing patent disputes can be time consuming and expensive. If we were to initiate legal proceedings against a third party to enforce a patent related to one of our products, the defendant in such litigation could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity and/or unenforceability are commonplace, as are validity challenges by the defendant against the subject patent or other patents before the United States Patent and Trademark Office (or USPTO). Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement, failure to meet the written description requirement, indefiniteness, and/or failure to claim patent eligible subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent intentionally withheld material information from the USPTO, or made a misleading statement, during prosecution. Additional grounds for an unenforceability assertion include an allegation of misuse or anticompetitive use of patent rights, and an allegation of incorrect inventorship with deceptive intent. Third parties may also raise similar claims before the USPTO even outside the context of litigation. The outcome is unpredictable following legal assertions of invalidity and unenforceability. With respect to the validity question, for example, we cannot be certain that no invalidating prior art existed of which we and the patent examiner were unaware during prosecution. These assertions may also be based on information known to us or the USPTO. If a defendant or third party were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the claims of the challenged patent. Such a loss of patent protection would or could have a material adverse impact on our business.
The standards that the USPTO (and foreign equivalents) use to grant patents are not always applied predictably or uniformly and can change. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in device patents. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others.
However, there can be no assurance that our technology will not be found in the future to infringe upon the rights of others or be infringed upon by others. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products or product candidates infringe. For example, pending applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our product infringes. In such a case, others may assert infringement claims against us, and should we be found to infringe upon their patents, or otherwise impermissibly utilize their intellectual property, we might be forced to pay damages, potentially including treble damages, if we are found to have willfully infringed on such parties’ patent rights. In addition to any damages we might have to pay, we may be required to obtain licenses from the holders of this intellectual property. We may fail to obtain any of these licenses or intellectual property rights on commercially reasonable terms. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products, which could materially harm our business and the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation. Conversely, we may not always be able to successfully pursue our claims against others that infringe upon our technology. Thus, the proprietary nature of our technology or technology licensed by us may not provide adequate protection against competitors.
In addition to patents, we rely on trademarks to protect the recognition of our company and product in the marketplace. We also rely on trade secrets, know-how, and proprietary knowledge that we seek to protect, in part, through confidentiality agreements with employees, consultants and others. We cannot assure you that our proprietary information will not be shared, our confidentiality agreements will not be breached, that we will have adequate remedies for any breach, or that our trade secrets will not otherwise become known to or independently developed by competitors.
| 21 |
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information and disclosure of our trade secrets or proprietary information could compromise any competitive advantage that we have, which could have a materially adverse effect on our business.
Our success depends, in part, on our ability to protect our proprietary rights to the technologies used in our products and our proprietary clinical protocols. We depend heavily upon confidentiality agreements with our officers, employees, consultants and subcontractors to maintain the proprietary nature of our technology and our proprietary clinical protocols. These measures may not afford us complete or even sufficient protection, and may not afford an adequate remedy in the event of an unauthorized disclosure of confidential information. If we fail to protect and/or maintain our intellectual property, third parties may be able to compete more effectively against us, we may lose our technological or competitive advantage, and/or we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property. In addition, others may independently develop technology similar to ours, otherwise avoiding the confidentiality agreements, or produce patents that would materially and adversely affect our business, prospects, financial condition and results of operations in which event and you could lose all of your investment.
We may face intellectual property infringement claims that would be costly to resolve.
There has been substantial litigation regarding patent and other intellectual property rights in the medical device industry, and our competitors and others may initiate intellectual property litigation, including as a means of competition. Intellectual property litigation is complex and expensive, and outcomes are difficult to predict. We cannot assure you that we will not become subject to patent infringement claims or litigation, or interference proceedings, to determine the priority of inventions. Litigation or regulatory proceedings also may be necessary to enforce our patent or other intellectual property rights. We may not always have the financial resources to assert patent infringement suits or to defend ourselves from claims. An adverse result in any litigation could subject us to liabilities, or require us to seek licenses from or pay royalties to others that may be substantial. Furthermore, we cannot predict the extent to which the necessary licenses would be available to us on satisfactory terms, if at all.
Our failure to secure trademark registrations could adversely affect our ability to market our products and operate our business.
Our trademark applications in the United States and any other jurisdictions where we may file may not be allowed registration, and we may not be able to maintain or enforce our registered trademarks. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in corresponding foreign agencies, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our applications and/or registrations, and our applications and/or registrations may not survive such proceedings. Failure to secure such trademark registrations in the United States and in foreign jurisdictions could adversely affect our ability to market our products and our business.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the medical device industry, we may employ individuals who were previously employed at other companies similar to ours, including our competitors or potential competitors. We may become subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
| 22 |
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing and marketing of medical devices. This risk exists even if a device is cleared or approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA or an applicable foreign regulatory authority. Our Vivos System is designed to affect, and any future products will be designed to affect, important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our Vivos System could result in patient injury or death. The medical device industry has historically been subject to extensive litigation over product liability claims, and we cannot offer any assurance that we will not face product liability suits. We may be subject to product liability claims if our Vivos System causes, or merely appears to have caused, patient injury or death. In addition, an injury that is caused by the activities of our suppliers, such as those who provide us with components and raw materials, may be the basis for a claim against us. Product liability claims may be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with our Vivos System, among others. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | costs of litigation; | |
| ● | distraction of management’s attention from our primary business; | |
| ● | the inability to commercialize our Vivos System or new products; | |
| ● | decreased demand and brand reputation for our Vivos System; | |
| ● | product recalls or withdrawals from the market; | |
| ● | withdrawal of clinical trial participants; | |
| ● | substantial monetary awards to patients or other claimants; or | |
| ● | loss of sales. |
Any recall or market withdrawal of our products may delay the supply of those products to our customers and may impact our reputation. We can provide no assurance that we will be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future or that these efforts will have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Such recalls and withdrawals may also be used by our competitors to harm our reputation for safety or be perceived by patients as a safety risk when considering the use of our products, either of which could have a material adverse effect on our business, financial condition and results of operations.
We may not be able to maintain adequate product liability insurance.
Our product liability and clinical study liability insurance is subject to deductibles and coverage limitations. Our product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, coverage may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at an acceptable cost or on acceptable terms or otherwise protect against potential product liability claims, we could be exposed to significant liabilities. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations.
We bear the risk of warranty claims on the Vivos System.
We bear the risk of warranty claims on our Vivos System. We may not be successful in claiming recovery under any warranty or indemnity provided to us by our suppliers or vendors in the event of a successful warranty claim against us by a customer or that any recovery from such vendor or supplier would be adequate. In addition, warranty claims brought by our customers related to third-party components may arise after our ability to bring corresponding warranty claims against such suppliers expires, which could result in costs to us.
| 23 |
We depend on a few suppliers for key components, making us vulnerable to supply shortages and price fluctuation.
We purchase components for our Vivos System from a variety of vendors on a purchase order basis; we have no long-term supply contracts with any of our vendors. While it is our goal to have multiple sources to procure certain key components, in some cases it is not economically practical or feasible to do so. To mitigate this risk, we maintain an awareness of alternate supply sources that could provide our currently single-sourced components with minimal or no modification to the current version of our Vivos System, practice supply chain management, maintain safety stocks of critical components and have arrangements with our key vendors to manage the availability of critical components. Despite these efforts, if our vendors are unable to provide us with an adequate supply of components in a timely manner, or if we are unable to locate qualified alternate vendors for components at a reasonable cost, the cost of our products would increase, the availability of our products to our customers would decrease and our ability to generate revenue could be materially limited.
Our sales and marketing efforts may not be successful.
We currently market and sell our Vivos System to a limited number of licensed professionals, primarily general dentists. Less than 1% of the general dentists in the U.S. have been trained and certified in the Vivos System. The commercial success of our Vivos System ultimately depends upon a number of factors, including the number of dentists who use the Vivos System, the number of Vivos Systems used by these dentists, the number of patients who become aware of the Vivos System by self-referral or referrals by their primary care physicians, the number of patients who elect to use the Vivos System, and the number of patients who, having successfully used the Vivos System, endorse and refer the Vivos System to other potential patients. The Vivos System may not gain significant increased market acceptance among physicians/dentists who use it or who refer their patients, other patients, third-party healthcare insurers and managed care providers. We believe that primary care physicians typically elect to refer individuals with SDB to pulmonologists or other physicians who treat sleep disordered breathing, and these physicians may not recommend the Vivos System to patients for any number of reasons, including safety and clinical efficacy, the availability of alternative procedures and treatment options, or inadequate levels of reimbursement. In addition, while positive patient experiences can be a significant driver of future sales, it is impossible to influence the manner in which this information is transmitted and received, the choices potential patients may make and the recommendations that treating physicians make to their patients.
Although we sell our product directly to our corporate-owned and partner clinics, our experience in marketing and selling our Vivos System or VIP program through a direct sales organization in the United States is limited. We may not be able to maintain a suitable sales force in the United States or train up a suitable number of VIPs, or enter into or maintain satisfactory marketing and distribution arrangements with others. Our marketing and sales efforts may not be successful in increasing awareness and sales of our Vivos System. In addition, other marketing efforts like MID and VivoScore may not increase revenue to the extent we currently anticipate.
The failure to educate or train a sufficient number of physicians and dentists in the use of our Vivos System could reduce the market acceptance of our Vivos System and reduce our revenue.
It is critical to the success of our sales efforts that there is an increasing number of dentists familiar with, trained in, and proficient in the use of our Vivos System. Currently, dentists learn to use the Vivos System through hands-on, on-site training or virtual training by our representatives. However, to receive this training, dentists must be aware of the Vivos System as a treatment option for SDB or mild-to-moderate OSA and be interested in using the Vivos System in their practice. We cannot predict the extent to which dentists will dedicate the time and energy necessary for adequate training in the use of our Vivos System, have the knowledge of or experience in the clinical outcomes of the Vivos System or feel comfortable enough using the Vivos System to recommend it to their patients. Even if a dentist is well versed in the Vivos System, he or she may be unwilling to require patients to pay for the Vivos System out-of-pocket. If dentists do not continue to accept and recommend the Vivos System, our revenue could be materially and adversely affected.
| 24 |
We rely on third-party suppliers and contract manufacturers for the manufacture and assembly of our products, and a loss or degradation in performance of these suppliers and contract manufacturers could have a material adverse effect on our business, financial condition and results of operations.
We rely on third-party suppliers and contract manufacturers for the raw materials and components used in our Vivos System and to manufacture and assemble our products. Any of our other suppliers or our third-party contract manufacturers may be unwilling or unable to supply the necessary materials and components or manufacture and assemble our products reliably and at the levels we anticipate or that are required by the market. Our ability to supply our products commercially and to develop any future products depends, in part, on our ability to obtain these materials, components and products in accordance with regulatory requirements and in sufficient quantities for commercialization and clinical testing. While our suppliers and contract manufacturers have generally met our demand for their products and services on a timely basis in the past, we cannot guarantee that they will in the future be able to meet our demand for their products, either because of acts of nature, the nature of our agreements with those manufacturers or our relative importance to them as a customer, and our manufacturers may decide in the future to discontinue or reduce the level of business they conduct with us. If we are required to change contract manufacturers due to any change in or termination of our relationships with these third parties, or if our manufacturers are unable to obtain the materials they need to produce our products at consistent prices or at all, we may lose sales, experience manufacturing or other delays, incur increased costs or otherwise experience impairment to our customer relationships. We cannot guarantee that we will be able to establish alternative relationships on similar terms, without delay or at all.
Establishing additional or replacement suppliers for any of these materials, components or services, if required, could be time-consuming and expensive, may result in interruptions in our operations and product delivery, may affect the performance specifications of our Vivos System or could require that we modify its design. Even if we are able to find replacement suppliers or third-party contract manufacturers, we will be required to verify that the new supplier or third-party manufacturer maintains facilities, procedures and operations that comply with our quality expectations and applicable regulatory requirements.
If our third-party suppliers fail to deliver the required commercial quantities of materials on a timely basis and at commercially reasonable prices, and we are unable to find one or more replacement suppliers capable of production at a substantially equivalent cost in substantially equivalent volumes and quality on a timely basis, the continued commercialization of our Vivos System, the supply of our products to customers and the development of any future products will be delayed, limited or prevented, which could have material adverse effect on our business, financial condition and results of operations.
Damage to our reputation or our brand could negatively impact our business, financial condition and results of operations.
We must grow the value of our brand to be successful. We intend to develop a reputation based on the high quality of our products and services, trained clinic personnel, as well as on our particular culture and the experience of our patients with our VIPs. If we do not make investments in areas such as marketing and advertising, as well as personnel training, the value of our brand may not increase or may be diminished. Any incident, real or perceived, regardless of merit or outcome, that adversely affects our brand, such as, but not limited to, patient disability or death due to malpractice or allegations of malpractice, failure to comply with federal, state, or local regulations, including allegations or perceptions of non-compliance or failure to comply with ethical and operational standards, could significantly reduce the value of our brand, expose us to negative publicity and damage our overall business and reputation.
Our marketing activities may not be successful.
We incur costs and expend other resources in our marketing efforts to attract and retain VIPs. Our marketing activities are principally focused on increasing brand awareness in the communities in which we provide services. As we onboard VIP providers, we expect to undertake aggressive marketing campaigns to increase community awareness about our presence and our service capabilities. We conduct our targeted marketing efforts in neighborhoods through channels such as direct mail, billboards, radio advertisements, physician open houses, community sponsorships and various social media. If we are not successful in these efforts, we will have incurred expenses without materially increasing revenue.
The SDB and OSA market is highly competitive, including competition for patients, strategic relationships, and commercial payor contracts.
The market for providing treatment for SDB and OSA is highly competitive. Our VIP offices and our VIPs face competition from existing facilities providing treatment for SDB and OSA, depending on the type of patient and geographic market. Our VIPs compete on the basis of our product (the Vivos System), quality, price, accessibility, and overall experience. We compete with national, regional, and local enterprises, many of which have greater financial and other resources available to them, greater access to dentists and physicians or greater access to potential patients. We also compete on the basis of our multistate, regional footprint, which we believe will be of value to both employers and third-party payors. As a result of the differing competitive factors within the markets in which we operate and will operate, the individual results of our VIP offices may be volatile. If we are unable to compete effectively with any of these entities or groups, or we are unable to implement our business strategies, there could be a material adverse effect on our business, prospects, results of operations and financial condition.
| 25 |
We have limited clinical evidence to support patient compliance with the use our products is superior to competitive products.
We believe based on our experiences to date that our non-surgical treatment of limited duration is preferable relative to CPAP or other oral appliance or surgical therapies, resulting in improved patient compliance. However, we have limited clinical evidence to support our beliefs that patient compliance in the use of our products is superior to competitive products. If actual patient compliance as studied in a clinical trial (should we conduct one) proves less than what we had anticipated, the acceptance of the Vivos System in the marketplace, and our revenues and overall results of operations, may be adversely impacted.
Government healthcare programs may reduce reimbursement rates, which could adversely affect sales of the Vivos System and demand for dental practitioners from becoming or remaining VIPs.
In recent years, new legislation has been proposed and adopted at both the federal and state level that is effecting major changes in the healthcare system. Any change in the laws, regulations, or policies governing the healthcare system could adversely affect reimbursement rates, which could adversely affect sales of the Vivos System and thus adversely affect our operations and financial condition. Enacted in 2010, the Affordable Care Act (or ACA) seeks to expand healthcare coverage, while increasing quality and limiting costs. The ACA substantially changes the way healthcare is financed by both governmental and commercial payors. As a result of the ACA or the adoption of additional federal and state healthcare reforms measures there could be limits to the amounts that federal and state governments will pay for healthcare services, which could result in reduced demand for, or profitability of, the Vivos System and for dental practitioners from becoming or remaining VIPs.
Significant uncertainty exists as to the reimbursement status of healthcare products. The regulations that govern marketing approvals, pricing and reimbursement for medical devices vary widely from country to country. In the United States, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010, is significantly changing the way healthcare is financed by both governmental and private insurers. While we cannot predict what impact on federal reimbursement policies this law or any amendment to it will continue to have in general or specifically on the Vivos System or any product that we commercialize, the ACA or any such amendment may result in downward pressure on reimbursements, which could negatively affect market acceptance of the Vivos System. In addition, although the United States Supreme Court has upheld the constitutionality of most of the ACA, several states have not implemented certain sections of the ACA, including 19 that have rejected the expansion of Medicaid eligibility for low income citizens, and some members of the U.S. Congress are still working to repeal the ACA. In addition, the United States Supreme Court has recently determined to hear another case challenging the constitutionality of the ACA. President Trump and the Republican majority in the U.S. Senate have also been seeking to repeal or replace all or portions of the ACA but to date they have been unable to agree on any such legislation.
The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain fees mandated by the ACA, including the so-called “Cadillac” tax on certain high cost employer- sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. The Cadillac tax was repealed in 2019 and is no longer simply delayed. Congress may still consider other legislation to repeal and replace elements of the ACA. We expect that the ACA, as currently enacted or as it may be amended or repealed in the future, and other healthcare reform measures that may be adopted in the future, could have a material adverse effect on our industry generally and on our ability to successfully commercialize our products. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or our collaborators are not able to maintain regulatory compliance, our products may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
| 26 |
If payments from commercial or governmental payors are significantly delayed, reduced or eliminated, our business, prospects, results of operations and financial condition could be adversely affected.
We will depend upon revenue from sales of the Vivos System, and in turn on reimbursement from third-party payors for the Vivos System. The amount that our VIPs receive in payment for the Vivos System may be adversely affected by factors we do not control, including federal or state regulatory or legislative changes, cost-containment decisions and changes in reimbursement schedules of third-party payors. Any reduction or elimination of these payments could have a material adverse effect on our business, prospects, results of operations and financial condition.
Additionally, the reimbursement process is complex and can involve lengthy delays. Also, third-party payors may reject, in whole or in part, requests for reimbursement based on determinations that certain amounts are not reimbursable under plan coverage, that services provided were not medically necessary, that additional supporting documentation is necessary, or for other reasons. Retroactive adjustments by third-party payors may be difficult or cost prohibitive to appeal, and such changes could materially reduce the actual amount we receive from our VIPs. Delays and uncertainties in the reimbursement process may be out of our control and may adversely affect our business, prospects, results of operations and financial condition.
Significant changes in our payor mix resulting from fluctuations in the types of patients seen by our VIPs could have a material adverse effect on our business, prospects, results of operations and financial condition.
Our results may change from period to period due to fluctuations in our VIPs’ payor mix. Payor mix refers to the relative amounts we receive from the mix of persons or entities that pay or reimburse our VIPs for healthcare services. Because we believe that our VIPs will receive a higher payment rate from commercial payors than from governmental payors or self-pay patients, a significant shift in our payor mix toward a higher percentage of self-pay or patients whose treatment is paid in whole or part by a governmental payor, could occur for reasons beyond our control and could lessen demand for the Vivos System, which in turn could have a material adverse effect on our business, prospects, results of operations and financial condition.
Failure by our Billing Intelligence Service to bill timely or accurately for billable services rendered by participating VIP providers could have a negative impact on our revenue and cash flow.
Billing for medical services rendered in connection with the Vivos System treatment is often complex and time consuming. The practice of providing dental or medical services in advance of payment or prior to assessing a patient’s ability to pay for such services may have a significant negative impact on a VIP provider’s patient service revenue, bad debt expense and cash flow. Not all of our VIPs subscribe to our Billing Intelligence Service program. For VIPs who do subscribe, we bill numerous payors, including various forms of commercial health insurance providers on their behalf. Billing requirements that must be met prior to receiving payment for services rendered often vary by payor. Self-pay patients and third-party payors may fail to pay for services even if they have been properly billed. Reimbursement is typically dependent on providing the proper procedure and diagnosis codes, supportive documentation to show medical necessity. Medical insurance is never a guarantee of payment.
Additional factors that could affect our ability to collect from insurers for the services rendered by our participating VIP providers include:
| ● | disputes among payors as to which party is responsible for payment; | |
| ● | variations in coverage among various payors for similar services; | |
| ● | the difficulty of adherence to specific compliance requirements, coding and various other procedures mandated by responsible parties; | |
| ● | the institution of new coding standards; and | |
| ● | failure to properly credential our dentists to enable them to bill various payors. |
The complexity associated with billing for our services may lead to delays in cash collections by our VIPs, resulting in increased carrying costs associated with the aging of our accounts receivable as well as the increased potential for bad debt expense.
| 27 |
We may incur costs resulting from security risks in connection with the electronic data processing by our partner banks.
Because we accept electronic payment cards for payments at our facilities and the facilities of our VIPs, we may incur costs resulting from related security risks in connection with the electronic processing of confidential information by our partner banks. Recently, several large national banks have experienced potential or actual breaches in which similar data has been or may have been stolen. Such occurrences could cause patient dissatisfaction resulting in decreased visits or could also distract our management team from the management of the day-to-day operations.
Our relationships with VIPs, other healthcare providers, and third-party payors will be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, health information privacy and security laws, and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Healthcare providers (including our VIPs), physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation of the Vivos System. Our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors may subject us to various federal and state fraud and abuse laws and other health care laws, including, without limitation, the federal Anti-Kickback Statute, the federal civil and criminal false claims laws and the law commonly referred to as the Physician Payments Sunshine Act and regulations. These laws will impact, among other things, our clinical research, sales, marketing and educational programs. In addition, we may be subject to patient privacy laws by both the federal government and the states in which we conduct or may conduct our business. The laws that will affect our operations include, but are not limited to:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, in return for the purchase, recommendation, leasing or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. This statute has been interpreted to apply to arrangements between medical device manufacturers on the one hand, and physicians and patients on the other. The Patient Protection and Affordable Care Act, as amended (or the PPACA), amended the intent requirement of the federal Anti-Kickback Statute and, as a result, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it; |
| ● | federal civil and criminal false claims laws, including, without limitation, the False Claims Act, and civil monetary penalty laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other government payors that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. The PPACA provides, and recent government cases against medical device manufacturers support, the view that federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, may implicate the False Claims Act; | |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 (or HIPAA), which created new federal criminal statutes that prohibit a person from knowingly and willfully executing a scheme or making false or fraudulent statements to defraud any healthcare benefit program, regardless of the payor (e.g., public or private); | |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (or HITECH), and its implementing regulations, and as amended again by the final HIPAA omnibus rule, Modifications to the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules Under HITECH and the Genetic Information Nondiscrimination Act; Other Modifications to HIPAA, published in January 2013, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization by entities subject to the rule, such as health plans, health care clearinghouses and health care providers, and their respective business associates; |
| 28 |
| ● | federal transparency laws, including the federal Physician Payments Sunshine Act, which is part of the PPACA, that require certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services (or CMS), information related to: (i) payments or other “transfers of value’’ made to physicians and teaching hospitals; and (ii) ownership and investment interests held by physicians and their immediate family members; | |
| ● | state and foreign law equivalents of each of the above federal laws, state laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and state laws that require medical device companies to comply with the specific industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or to adopt compliance programs as prescribed by state laws and regulations, or that otherwise restrict payments that may be made to healthcare providers; and | |
| ● | state and foreign laws that govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws.
It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion of our products from government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations.
The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. The shifting compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance and/or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
The misuse or off-label use of the Vivos System may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
We train our marketing personnel and direct sales force to not promote the Vivos System for uses outside of the FDA-cleared indications for use, known as off-label uses. We cannot, however, prevent a medical professional from using the Vivos System off label when, in their independent professional medical judgment, he or she deems it appropriate. There may be increased risk of injury or other side effects to patients if physicians attempt to use the Vivos System off-label. Furthermore, the use of the Vivos System for indications other than those cleared by the FDA or cleared by any foreign regulatory body may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
| 29 |
Given that we are aware that, notwithstanding our training guidelines, our VIPs may use our DNA device off-label, there is a risk that we could face regulatory scrutiny as a result of such use. If the FDA or any foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance or imposition of an untitled letter, which is used for violations that do not necessitate a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action under other regulatory authority, such as false claims laws, if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment of our operations.
In addition, dentists may misuse our Vivos System or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If our Vivos System is misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Similarly, in an effort to decrease costs, physicians may also reuse our Vivos System despite it being intended for a single use or may purchase reprocessed Vivos Systems from third-party processors in lieu of purchasing a new Vivos System from us, which could result in product failure and liability. Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizeable damage awards against us that may not be covered by insurance.
We may pursue acquisitions of complementary businesses or technologies, which could divert the attention of management and which may not be integrated successfully into our existing business.
We may pursue acquisitions or licenses of technology to, among other things, expand the scope of products services we provide. We cannot guarantee that we will identify suitable acquisition candidates, that acquisitions will be completed on acceptable terms or that we will be able to integrate successfully the operations of any acquired business into our existing business. The acquisitions could be of significant size and involve operations in multiple jurisdictions. The acquisition and integration of another business or technology would divert management attention from other business activities, including our core business. This diversion, together with other difficulties we may incur in integrating an acquired business or technology, could have a material adverse effect on our business, financial condition and results of operations. In addition, we may borrow money or issue capital stock to finance acquisitions. Such borrowings might not be available on terms as favorable to us as our current borrowing terms and may increase our leverage, and the issuance of capital stock could dilute the interests of our stockholders.
Our business is seasonal, which impacts our results of operations.
We believe that the patient volumes of our VIPs will be sensitive to seasonal fluctuations in urgent care and primary care activity. Typically, winter months see a higher occurrence of influenza, bronchitis, pneumonia and similar illnesses; however, the timing and severity of these outbreaks vary dramatically. Additionally, as consumers shift toward high deductible insurance plans, they are responsible for a greater percentage of their bill, particularly in the early months of the year before other healthcare spending has occurred, which may lead to lower than expected patient volume or an increase in bad debt expense during that period. Our quarterly operating results may fluctuate significantly in the future depending on these and other factors.
We could be subject to lawsuits for which we are not fully insured.
Healthcare providers have become subject to an increasing number of lawsuits alleging malpractice and related legal theories such as negligent hiring, supervision and credentialing. Some of these lawsuits involve large claim amounts and substantial defense costs. We generally procure professional liability insurance coverage for our affiliated medical professionals and professional and corporate entities. We are currently insured under policies in amounts management deems appropriate, based upon the nature and risk of our business. Our medical professionals are also required to provide their own medical malpractice insurance coverages. Nevertheless, there are exclusions and exceptions to coverage under each insurance policy that may make coverage for any claim unavailable, future claims could exceed the limits of available insurance coverage, existing insurers could become insolvent and fail to meet their obligations to provide coverage for such claims, and such coverage may not always be available with sufficient limits and at reasonable cost to insure us adequately and economically in the future. One or more successful claims against us not covered by, or exceeding the coverage of, our insurance could have a material adverse effect on our business, prospects, results of operations and financial condition. Moreover, in the normal course of our business, we may be involved in other types of lawsuits, claims, audits and investigations, including those arising out of our billing and marketing practices, employment disputes, contractual claims and other business disputes for which we may have no insurance coverage. Furthermore, for our losses that are insured or reinsured through commercial insurance providers, we are subject to the financial viability of those insurance companies. Although we believe our commercial insurance providers are currently creditworthy, they may not remain so in the future. The outcome of these matters could have a material adverse effect on our financial position, results of operations, and cash flows.
| 30 |
We depend on certain key personnel.
We substantially rely on the efforts of our current senior management, including our founder and Chief Medical Officer, Dr. G. Dave Singh, our co-founder, Chairman of the Board and Chief Executive Officer, R. Kirk Huntsman and our Chief Financial Officer, Brad Amman. Our business would be impeded or harmed if we were to lose their services. In addition, if we are unable to attract, train and retain highly skilled technical, managerial, product development, sales and marketing personnel, we may be at a competitive disadvantage and unable to develop new products or increase revenue. The failure to attract, train, retain and effectively manage employees could negatively impact our research and development, sales and marketing and reimbursement efforts. In particular, the loss of sales personnel could lead to lost sales opportunities as it can take several months to hire and train replacement sales personnel. Uncertainty created by turnover of key employees could adversely affect our business.
Members of our board of directors and our executive officers will have other business interests and obligations to other entities.
Neither our directors nor our executive officers will be required to manage our business as their sole and exclusive function and they may have other business interests and may engage in other activities in addition to those relating to us, provided that such activities do not compete with the business of our company or otherwise breach their agreements with us. We are dependent on our directors and executive officers to successfully operate our company. Their other business interests and activities could divert time and attention from operating our business.
We will need to carefully manage our expanding operations to achieve sustainable growth.
To achieve increased revenue levels, complete clinical studies and develop future products, we believe that we will be required to periodically expand our operations, particularly in the areas of sales and marketing, clinical research, reimbursement, research and development, manufacturing and quality assurance. As we expand our operations in these areas, management will face new and increased responsibilities. To accommodate any growth and compete effectively, we must continue to upgrade and improve our information systems, as well as our procedures and controls across our business, and expand, train, motivate and manage our work force. Our future success will depend significantly on the ability of our current and future management to operate effectively. Our personnel, systems, procedures and controls may not be adequate to support our future operations. If we are unable to effectively manage our expected growth, this could have a material adverse effect on our business, financial condition and results of operations.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery and anti-kickback laws with respect to our activities outside the United States.
We distribute our products to locations within and outside the United States in Canada. Our business plan also anticipates VIP offices outside the United States and Canada. The U.S. Foreign Corrupt Practices Act, and other similar anti-bribery and anti-kickback laws and regulations, generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. As we expect to expand our international operations in the future, we will become increasingly subjected to these laws and regulations. We cannot assure you that we will be successful in preventing our agents from taking actions in violation of these laws or regulations. Such violations, or allegations of such violations, could disrupt our business and result in a material adverse effect on our financial condition, results of operations and cash flows.
| 31 |
There is no guarantee that our PPP loan will be forgiven in whole or in part.
In May 2020, we received loan proceeds in the amount of approximately $1,265,000 under the Paycheck Protection Program (or PPP), established as part of the Coronavirus Aid, Relief and Economic Security (CARES) Act, which provides economic relief to businesses in response to the COVID-19 pandemic. The loan and accrued interest are forgivable after 24 weeks as long as we use the loan proceeds for eligible purposes, including payroll, benefits, rent and utilities, and our employee head count remains consistent with our baseline period over the 24-week period after the loan was received. The amount of loan forgiveness will be reduced if we terminate employees or reduce salaries during the 24-week period. The unforgiven portion of the PPP loan is payable over two years at an interest rate of 1%, with a deferral of payments for the first six months. While we believe that our use of the loan proceeds will meet the conditions for forgiveness of the loan, there is a risk that the loan will not be forgiven or that we will take actions that could cause us to be ineligible for forgiveness of the loan, there is a risk that (i) the loan will not be forgiven, in whole or in part, (ii) we will take actions that could cause us to be ineligible for forgiveness of the loan, in whole or in part or (iii) we may be required to repay the loan, in whole or in part, upon event of default under the loan or upon a breach of applicable PPP regulations (including upon a change of ownership in our company that may have occurred as a result of our initial public offering).
Risks Related to Our Products and Regulation
We depend in large part on our Vivos System technology, and the loss of access to this technology would terminate or delay the further development of our products, injure our reputation or force us to pay higher fees.
We depend, in large part, on our Vivos System technology. The loss of this key technology would seriously impair our business and future viability, and could result in delays in developing, introducing or maintaining our products until equivalent technology, if available, is identified, licensed and integrated. In addition, any defects in the Vivos System technology or other technologies we gain access to in the future could prevent the implementation or impair the functionality of our products, delay new product introductions or injure our reputation. If we are required to acquire or enter into license agreements with third parties for replacement technologies, we could be subject to higher fees, milestone or royalty payments, assuming we could access such technologies at all.
Our failure to obtain government approvals, including required FDA approvals, or to comply with ongoing governmental regulations relating to our technologies and products could delay or limit introduction of our products and result in failure to achieve revenue or maintain our ongoing business.
Our development activities and the manufacture and marketing of the Vivos System are subject to extensive regulation for safety, efficacy and quality by numerous government authorities in the United States and abroad. Before receiving FDA or foreign regulatory clearance to market our products which are not presently approved, we will have to demonstrate that these products are safe and effective in the patient population and for the diseases that are to be treated. Clinical trials, manufacturing and marketing of medical devices are subject to the rigorous testing and approval process of the FDA and equivalent foreign regulatory authorities. The Federal Food, Drug and Cosmetic Act and other federal, state and foreign statutes and regulations govern and influence the testing, manufacture, labeling, advertising, distribution and promotion of medical devices. As a result, regulatory approvals for our products not yet approved or that we may develop in the future can take a number of years or longer to accomplish and require the expenditure of substantial financial, managerial and other resources.
Clinical trials that may be required to support regulatory submissions in the United States are expensive. We cannot assure that we will be able to complete any required clinical trial programs successfully within any specific time period, and if such clinical trials take longer to complete than we project, our ability to execute our current business strategy will be adversely affected.
Conducting clinical trials is a lengthy, time-consuming and expensive process. Before obtaining regulatory approvals for the commercial sale of any products, we must demonstrate through clinical trials the safety and effectiveness of our products. We have incurred, and we will continue to incur, substantial expense for, and devote a significant amount of time to, product development, pilot trial testing, clinical trials and regulated, compliant manufacturing processes.
Even if completed, we do not know if these trials will produce statistically significant or clinically meaningful results sufficient to support an application for marketing approval. If and how quickly we complete clinical trials is dependent in part upon the rate at which we are able to advance the rate of patient enrollment, and the rate to collect, clean, lock and analyze the clinical trial database.
| 32 |
Patient enrollment in trials is a function of many factors. These include the design of the protocol; the size of the patient population; the proximity of patients to and availability of clinical sites; the eligibility criteria for the study; the perceived risks and benefits of the product candidate under study; the medical investigators’ efforts to facilitate timely enrollment in clinical trials; the patient referral practices of local physicians; the existence of competitive clinical trials; and whether other investigational, existing or new products are available or cleared for the indication. If we experience delays in patient enrollment and/or completion of our clinical trial programs, we may incur additional costs and delays in our development programs and may not be able to complete our clinical trials on a cost-effective or timely basis. Accordingly, we may not be able to complete the clinical trials within an acceptable time frame, if at all. If we fail to enroll and maintain the number of patients for which the clinical trial was designed, the statistical power of that clinical trial may be reduced, which would make it harder to demonstrate that the product candidate being tested in such clinical trial is safe and effective. Further, if we or any third party have difficulty enrolling a sufficient number of patients in a timely or cost-effective manner to conduct clinical trials as planned, or if enrolled patients do not complete the trial as planned, we or a third party may need to delay or terminate ongoing clinical trials, which could negatively affect our business.
The results of our clinical trials may not support either further clinical development or the commercialization of any new product candidates or modifications to existing products.
Even if our ongoing or contemplated clinical trials are completed as planned, their results may not support either the further clinical development or the commercialization of any new product candidates or modifications of existing products. The FDA or government authorities may not agree with our conclusions regarding the results of our clinical trials. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and the results from any later clinical trials may not replicate the results of prior clinical trials and pre-clinical testing. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for indicated uses. This failure would cause us to abandon a product candidate or a modification to any existing product and may delay development of other product candidates. Any delay in, or termination of, our clinical trials will delay the filing of our 510(k)’s and, ultimately, our ability to commercialize our product candidates and generate product revenue. Each Class I and Class II medical device marketed in the U.S. must receive a 510(k) clearance from the FDA. A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent (or SE), to a legally marketed device. Companies must compare their device to one or more similar legally marketed devices, commonly known as “predicates”, and make and support their substantial equivalency claims. The submitting company may not proceed with product marketing until it receives an order from the FDA declaring a device substantially equivalent. The substantially equivalent determination is usually made within 90 days, based on the information submitted by the applicant.
In addition, we or the FDA may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the FDA finds deficiencies in the conduct of these trials. A number of companies in the medical technology industry have suffered significant setbacks in advanced clinical trials despite promising results in earlier trials. In the end, we may be unable to develop marketable products.
Modifications to the Vivos System may require additional FDA approvals which, if not obtained, could force us to cease marketing and/or recall the modified device until we obtain new approvals.
After a device receives a 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a Premarket approval (or PMA). PMA is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Class III devices are those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury. Currently we do not market devices within this Class III category nor do we intend to in the foreseeable future. However, the FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified devices until 510(k) clearance or PMA approval is obtained. We cannot assure you that the FDA would agree with any of our decisions not to seek 510(k) clearance or PMA approval. If the FDA requires us to seek 510(k) clearance or PMA approval for any modification, we also may be required to cease marketing and/or recall the modified device until we obtain a new 510(k) clearance or PMA approval.
Our DNA appliance® currently has a pending 510(k) application to include additional indications of use for the treatment of mild-to-moderate OSA, snoring, and SDB in adults. This use would require the DNA appliance® to be registered as a Class II device. We have validated this 510(k) request with retrospective clinical data. This DNA appliance® 510(k) review and approval process is expected to take another three to six months, meaning we would expect to hear from the FDA in 2021. However, it is possible that we may not receive this FDA additional clearance.
| 33 |
Also, in March 2021, we submitted a 510(k) for Class II clearance to the FDA for our mmRNA device with indications to treat mild-to-moderate OSA, SDB and Snoring in adults. We cannot assure you that the FDA will approve our 510(k) Class II approval or we will receive PMA approval. Further, we cannot assure you that our mmRNA appliance® will be added to the CMS Medicare list of approved sleep appliances , both in general and in the event that Class II approval is not obtained for the mmRNA device (which is a prerequisite for inclusion in the CMS Medicare list of approved sleep appliances).
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, the agency can institute a wide variety of enforcement actions which may materially affect our business operations.
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, the agency can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
| ● | fines, injunctions and civil penalties; | |
| ● | recall, detention or seizure of our products; |
| ● | the issuance of public notices or warnings; | |
| ● | operating restrictions, partial suspension or total shutdown of production; | |
| ● | refusing our requests for a 510(k) clearance of new products; | |
| ● | withdrawing a 510(k) clearance already granted; and | |
| ● | criminal prosecution. |
We have received an FDA warning letter in the past when such a letter was received by our subsidiary BioModeling Solutions, Inc. (“BioModeling” or “BMS”) in January 2018 following a routine FDA audit. In its letter, the FDA noted matters such as inadequate documentation of certain FDA-required procedures, not keeping certain records and materials in paper format and in triplicate, and using certain descriptive words and phrases on its website and in marketing materials that were unapproved in advance by FDA. While we believe these issues have been resolved, to date the FDA has made no definitive statement that the matters raised by such letter have been satisfactorily resolved.
The FDA also has the authority to request repair, replacement or refund of the cost of any medical device manufactured or distributed by us. Our failure to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our financial condition and results of operations.
Treatment with the Vivos System has only been available for a relatively limited time, and we do not know whether there will be significant post-treatment regression or relapse.
Patient treatment using the FDA registered DNA appliance began in 2009, while treatment for mild-to-moderate OSA using the FDA cleared mRNA appliance began in 2014. Both began under the prior business model of our predecessor (and now subsidiary) BMS, and well before our formation. Under the BMS model, the independent treating dentists generated and maintained all records of treatment and ordered their appliances directly from one of the BMS designated labs. Thus, with the exception of specific patients who participated in studies, clinical trials or case reports, we have had limited visibility into patient records which might contain data on this subject. Therefore, we have limited empirical data to support our view that the risk of post treatment regression or relapse is not significant. To the extent a material number of patients who were treated with the Vivos System were to be found to experience post-treatment relapse or regression, it could pose a significant risk to our brand, the willingness or ability of physicians to prescribe and dentists to use our products and the willingness of patients to engage in treatment with our products and could thus have a material adverse effect on our results of operations.
| 34 |
We are subject to potential risks associated with the need to comply with state or other dental support organization laws.
Our core VIP business model does not involve any form of joint ownership, operational control, or employment of licensed professionals by our company. Thus, we are not typically regarded as a “dental support organization” (or DSO) under the laws of the various states within the United States or in Canada, in which we conduct most of our business. However, we do operate two retail treatment clinics in Colorado wherein we do employ dentists under a provider network model consistent with Colorado law. In that respect, for Colorado only, we may be regarded as a DSO. Nevertheless, if we were deemed to be a DSO in any jurisdiction, it could make it difficult or impossible for us to recruit and retain qualified dentists as VIPs, as some state dental boards are sometimes adverse to corporate DSOs operating in their states. Moreover, where such DSO-provider relationships are permitted, such regulations may impose significant constraints on the structure and financial arrangements that are permissible between us and our affiliated dentists in a particular state.
In jurisdictions where laws allow DSOs to operate (which includes almost all U.S. states and Canada), a growing number of dentists are affiliating with corporate DSOs. In those cases, the DSO may not allow their affiliated dentists to offer our products and services or to become VIPs. Thus, the overall number of dentists who are prospects to become VIPs and utilize our products and services may be reduced, which would impair our ability to generate revenue from our core VIP business model.
Our new Medical Integration Division business line may implicate federal and state laws involving the practice of medicine and related anti-kickback and similar laws.
Our MID was launched in 2020 to assist VIP practices in establishing clinical collaboration ties to local primary care physicians, sleep specialists, ENTs, pediatricians and other healthcare professionals who routinely see or treat patients with sleep and breathing disorders. The primary objective of our MID is to promote the Vivos System to the medical profession and thus facilitate more patients being able to receive a treatment with the Vivos System. There is a risk, however, that our MID may implicate legal or regulatory compliance issues that may arise in the course of our activities, including various Federal healthcare statutes such as the Stark and anti-kickback laws as well as state-by-state regulations pertaining to inter-disciplinary ownership of professional corporations or other legal entities. We have conducted research, including obtaining advice from outside legal counsel, regarding the implications of these laws and regulations to MID and believe the MID’s operations will be in compliance with or will not implicate these laws and regulations. However, there is a risk that such laws and regulations (or similar laws and regulations adopted in the future) might be interpreted, reinterpreted, or modified in the future in such a way so as to impede or prevent us from continuing to develop or manage our MID, which could lead to our having to discontinue the MID and could leave us subject to regulatory scrutiny and sanction. No advice of counsel has been obtained with respect any potential operations of the MID in Canada.
We may not be able to prohibit or limit our dentists, physicians and other healthcare professionals from competing with us in our local markets.
In certain states in which we operate or intend to operate, non-compete, non-solicitation, and other negative covenants applicable to employment or ownership are judicially or statutorily limited in their effectiveness or are entirely unenforceable against dentists, physicians and other healthcare professionals. As a result, we may not be able to retain our provider relationships or protect our market share, operational processes or procedures, or limit insiders or VIPs from using competitive information against us or competing with us, which could have a material adverse effect on our business, financial condition and ability to remain competitive as our arrangements with our VIPs do not contain competitive restrictions.
Risks Related to this Offering and Our Securities Generally
The market for our common stock is new and may not develop to provide investors with adequate liquidity.
We only recently conducted our initial public offering in December 2020. Therefore, the market for our common stock is new, and we cannot assure you that an active trading market for our common stock will develop, or if it does develop, it may not be maintained. You may not be able to sell your common stock quickly or at the market price if trading in our securities is not active.
| 35 |
The market price of our common stock may be highly volatile, and you could lose all or part of your investment.
The market price of our common stock is likely to be volatile. This volatility may prevent you from being able to sell your securities at or above the price you paid for your securities. Our stock price could be subject to wide fluctuations in response to a variety of factors, which include:
| ● | whether we achieve our anticipated corporate objectives; | |
| ● | actual or anticipated fluctuations in our quarterly or annual operating results; | |
| ● | changes in our financial or operational estimates or projections; | |
| ● | our ability to implement our operational plans; | |
| ● | termination of lock-up agreements or other restrictions on the ability of our stockholders to sell shares in the future; | |
| ● | changes in the economic performance or market valuations of companies similar to ours; and | |
| ● | general economic or political conditions in the United States or elsewhere. |
In addition, the stock market in general, and the stock of publicly-traded medical technology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
There is a risk of significant future sales by our stockholders that are currently subject to lock-up agreements which expire in June 2021. Such sales could cause the price of our stock price to fall considerably and may adversely impact our ability to raise funds in new stock offerings. Other future sales of other shares of our common stock could have a similar adverse effect on us
Approximately 8,185,815 shares of common stock (representing approximately 45% of our currently outstanding shares) held by pre-initial public offering stockholders of our company (including 1,199,195 shares of common stock that were issued upon the conversion of shares of Series B Preferred Stock in connection with our initial public offering) were registered with the SEC pursuant to a resale prospectus included as part of our initial public offering registration statement. The holders of such shares have entered into “lock-up” agreements in favor of the representative of the underwriters of our initial public offering, and such lock-ups will expire on June 13, 2021. As such, following the expiration of such lock-ups, such holders will be free to sell their shares in the market. Such sales, should they occur in large volume and over a short period of time, could cause the price of our public stock to fall considerably, leading to losses by our investors and a potential inability of to raise funds in new stock offering.
Furthermore, options to purchase up to 2,447,345 shares of our common stock with a weighted average exercise price of $5.00 are outstanding, and we also have outstanding (i) a warrant issued to the representative of the underwriters of our initial public offering (exercisable for 402,500 shares of common stock at an exercise price of $7.50 per share), (ii) warrants associated with our previously outstanding Series B Preferred Stock (exercisable for 1,199,195 shares of common stock at an exercise price of $7.50 per share); (iii) 325,000 warrants issued to certain shareholders in November 2020 (see “Management—2020 Derivative Demand and Settlement”) at an exercise price of $7.50 per share; (iv) a warrant issued to a 2017 noteholder (exercisable for 33,334 shares of common stock) at an exercise price of $1.50 per share; and (v) 295,000 warrants issued to contractors and consultants subsequent to December 31, 2020 with an exercise price of $7.50 per share; (vi) 25,000 warrants to purchase common stock issued in connection with the acquisition of Lyon Management and Consulting, LLC subsequent to December 31, 2020 with an exercise price of $8.90 per share.
| 36 |
The exercise or conversion of any of these securities would result in additional dilution, and the sale of the shares issuable upon exercise or conversion of these securities could also lower the market price of our common stock.
We may also acquire or license other technologies or finance strategic alliances by issuing equity, which may result in additional dilution to our stockholders, and the sale of such securities could adversely affect the market price for our common stock.
You will experience immediate and substantial dilution as a result of this offering, and will likely experience additional dilution in the future.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of 2,962,963 shares of common stock offered in this offering based on an assumed public offering price of $6.75 per share, and after deducting underwriter discounts and commissions and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $5.23 per share, or 78% at the assumed public offering price, assuming no exercise of the underwriters’ over-allotment option. In addition, we have also issued and may in the future issue common stock or securities that will convert to common stock that could be dilutive.
We will have considerable discretion over the allocation of the use of proceeds from this offering, and we may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield a return.
We currently intend to use the net proceeds we receive from this offering primarily as described in “Use of Proceeds.” Our management will have considerable discretion in the application of the net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. Investors in this offering will need to rely upon the judgment of our management with respect to the use of proceeds. If we do not use the net proceeds that we receive in this offering effectively, our business, financial condition, results of operations and prospects could be harmed.
Our failure to meet the continuing listing requirements of The Nasdaq Capital Market could result in a de-listing of our securities.
If we fail to satisfy the continuing listing requirements of Nasdaq, such as the corporate governance, stockholders equity or minimum closing bid price requirements, Nasdaq may take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we would likely take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our securities, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements.
If our shares of common stock become subject to the penny stock rules, it would become more difficult to trade our shares.
The Securities and Exchange Commission (or SEC) has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not obtain or retain a listing on Nasdaq and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares. See “Certain Relationships and Related Party Transactions” for further information on the foregoing transactions with Dr. Singh.
| 37 |
There can be no assurance that we will ever provide liquidity to our investors through a sale of our company.
While acquisitions of medical technology companies like ours are not uncommon, potential investors are cautioned that no assurances can be given that any form of merger, combination, or sale of our company will take place relating to our company, or that any merger, combination, or sale, even if consummated, would provide liquidity or a profit for our investors. You should not invest in our company with the expectation that we will be able to sell the business in order to provide liquidity or a profit for our investors.
Our officers and directors may have the ability to exert significant influence over our affairs, including the outcome of matters requiring stockholder approval.
Our officers and directors and their affiliates (primarily Kirk Huntsman and Dr. G. Dave Singh) currently own shares, in the aggregate, representing approximately 29% of our outstanding voting capital stock. As a result, if these stockholders were to choose to act together, they have and will continue to be able to exert significant control over certain matters submitted to our stockholders for approval by having the ability to block certain proposals. For example, these persons, if they choose to act collectively, would have the ability to vote against and block a proposed merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
In addition, this concentration of voting power was evidenced in April 2020, when Mr. Huntsman, Dr. Singh and a small group of additional shareholders acted to remove three independent members of our board of directors and appoint new members of our board of directors. These shareholders could continue to exert this voting power.
Actions of activist shareholders could be disruptive and potentially costly and the possibility that activist shareholders may seek changes that conflict with our strategic direction could cause uncertainty about the strategic direction of our business.
Activist investors or other stockholders who disagree with our management may attempt to effect changes in our strategic direction and how our company is governed or may seek to acquire control over our company. Some investors (commonly known as “activist investors”) seek to increase short-term stockholder value by advocating corporate actions such as financial restructuring, increased borrowing, special dividends, stock repurchases, or even sales of assets or the entire company. Activist campaigns can also seek to change the composition of our board of directors, and campaigns that contest or conflict with our strategic direction could have an adverse effect on our results of operations and financial condition as responding to proxy contests and other actions by activist shareholders can disrupt our operations, be costly and time-consuming, and divert the attention of our board of directors and senior management from the pursuit of our business strategies. In addition, perceived uncertainties as to our future direction that can arise from potential changes to the composition of our board of directors sought by activists may lead to the perception of a change in the direction of the business, instability or lack of continuity which may be exploited by our competitors, may cause concern to our current or potential customers or other partners, may result in the loss of potential business opportunities and may make it more difficult to attract and retain qualified personnel and business partners. These types of actions could divert our management’s attention from our business or cause significant fluctuations in our stock price based on temporary or speculative market perceptions or other factors that do not necessarily reflect the underlying fundamentals and prospects of our business, all of which could have a material adverse effect on our company.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” or EGC, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. We will remain an EGC until the earlier of: (i) the last day of the fiscal year in which we have total annual gross revenue of $1.07 billion or more; (ii) the last day of the fiscal year following the fifth anniversary of the date of the completion of our initial public offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC. For so long as we remain an EGC, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, or Section 404; |
| 38 |
| ● | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; | |
| ● | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; | |
| ● | reduced disclosure obligations regarding executive compensation; and | |
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of reduced reporting burdens in this prospectus. In particular, we have not included all of the executive compensation information that would be required if we were not an EGC. We cannot predict whether investors will find our common stock less attractive if we rely on certain or all of these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a newly public company, and particularly after we are no longer an EGC, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act and rules subsequently implemented by the SEC and Nasdaq have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance.
Pursuant to Section 404, we will be required to furnish a report by our management on our internal control over financial reporting, including an attestation report on internal control over financial reporting issued by our independent registered public accounting firm, only if the criteria are met. However, while we remain an EGC, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Certain provisions of our Certificate of Incorporation may make it more difficult for a third party to effect a change-of-control.
Our certificate of incorporation authorizes the Board of Directors to issue up to 50,000,000 shares of preferred stock. The preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by the Board of Directors without further action by the stockholders. These terms may include preferences as to dividends and liquidation, conversion rights, redemption rights and sinking fund provisions. The issuance of any preferred stock could diminish the rights of holders of our common stock, and therefore could reduce the value of such common stock. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell assets to, a third party. The ability of the Board of Directors to issue preferred stock could make it more difficult, delay, discourage, prevent or make it more costly to acquire or effect a change-in-control, which in turn could prevent our stockholders from recognizing a gain in the event that a favorable offer is extended and could materially and negatively affect the market price of our common stock.
| 39 |
Our bylaws designate certain courts as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our bylaws provide that, unless we consent in writing to an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware) will be the exclusive forum for: (i) any derivative action or proceeding brought on behalf of the Company; (ii) any action asserting a claim for breach of a fiduciary duty owed by any director, officer, employee, or agent of ours to us or our stockholders; (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, the Certificate of Incorporation, or the bylaws; and (iv) any action asserting a claim governed by the internal affairs doctrine (the “Delaware Forum Provision”). Our bylaws further provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the sole and exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act (the “Federal Forum Provision”). In addition, our bylaws provide that any person or entity purchasing or otherwise acquiring any interest in shares of our common stock is deemed to have notice of and consented to the Delaware Forum Provision and the Federal Forum Provision.
Section 27 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the Delaware Forum Provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. We note, however, that there is uncertainty as to whether a court would enforce this provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
We recognize that the Delaware Forum Provision and the Federal Forum Provision in our bylaws may impose additional litigation costs on stockholders in pursuing any such claims, particularly if the stockholders do not reside in or near the State of Delaware. Additionally, the Delaware Forum Provision and the Federal Forum Provision may limit our stockholders’ ability to bring a claim in a forum that they find favorable for disputes with us or our directors, officers or employees, which may discourage such lawsuits against us and our directors, officers and employees even though an action, if successful, might benefit our stockholders. In addition, while the Delaware Supreme Court ruled in March 2020 that federal forum selection provisions purporting to require claims under the Securities Act be brought in federal court were “facially valid” under Delaware law, there is uncertainty as to whether other courts will enforce the Federal Forum Provision. If the Federal Forum Provision is found to be unenforceable, we may incur additional costs associated with resolving such matters. The Federal Forum Provision may also impose additional litigation costs on stockholders who assert that the provision is not enforceable or invalid. The Court of Chancery of the State of Delaware and the United States District Court may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may be located or would otherwise choose to bring the action, and such judgments may be more or less favorable to us than our stockholders.
Limitations on director and officer liability and indemnification of our officers and directors by us may discourage stockholders from bringing suit against an officer or director.
Our certificate of incorporation and bylaws provide that, to the fullest extent permitted by Delaware law, as it presently exists or may be amended from time to time, a director shall not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director. Under Delaware law, this limitation of liability does not extend to, among other things, acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or unlawful payments of dividends. These provisions may discourage stockholders from bringing suit against a director or officer for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director or officer.
| 40 |
We are responsible for the indemnification of our officers and directors.
Should our officers and/or directors require us to contribute to their defense, we may be required to spend significant amounts of our capital. Our certificate of incorporation and bylaws also provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney’s fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on behalf of our company. This indemnification policy could result in substantial expenditures, which we may be unable to recoup. If these expenditures are significant or involve issues which result in significant liability for our key personnel, we may be unable to continue operating as a going concern.
Our ability to use our net operating losses and research and development credit carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (or the Code), a corporation that undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership over a three-year period, is subject to limitations on its ability to utilize its pre-change net operating losses, or NOLs, and its research and development credit carryforwards to offset future taxable income. Our existing NOLs and research and development credit carryforwards may be subject to limitations arising from previous ownership changes, and if we undergo an ownership change, our ability to utilize NOLs and research and development credit carryforwards could be further limited by Sections 382 and 383 of the Code. In addition, our ability to deduct net interest expense may be limited if we have insufficient taxable income for the year during which the interest is incurred, and any carryovers of such disallowed interest would be subject to the limitation rules similar to those applicable to NOLs and other attributes. Future changes in our stock ownership, some of which might be beyond our control, could result in an ownership change under Section 382 of the Code. For these reasons, in the event we experience a change of control, we may not be able to utilize a material portion of the NOLs, research and development credit carryforwards or disallowed interest expense carryovers, even if we attain profitability.
The financial and operational projections that we may make from time to time are subject to inherent risks.
The projections that our management may provide from time to time (including, but not limited to, those relating to market sizes and other financial or operational matters) reflect numerous assumptions made by management, including assumptions with respect to our specific as well as general business, economic, market and financial conditions and other matters, all of which are difficult to predict and many of which are beyond our control. Accordingly, there is a risk that the assumptions made in preparing the projections, or the projections themselves, will prove inaccurate. There will be differences between actual and projected results, and actual results may be materially different from those contained in the projections. The inclusion of the projections in this prospectus should not be regarded as an indication that we or our management or representatives considered or consider the projections to be a reliable prediction of future events, and the projections should not be relied upon as such.
If we were to dissolve, the holders of our securities may lose all or substantial amounts of their investments.
If we were to dissolve as a corporation, as part of ceasing to do business or otherwise, we may be required to pay all amounts owed to any creditors before distributing any assets to the investors. There is a risk that in the event of such a dissolution, there will be insufficient funds to repay amounts owed to holders of any of our indebtedness and insufficient assets to distribute to our other investors, in which case investors could lose their entire investment.
An investment in our company may involve tax implications, and you are encouraged to consult your own advisors as neither we nor any related party is offering any tax assurances or guidance regarding our company or your investment.
The formation of our company and our financings, as well as an investment in our company generally, involves complex federal, state and local income tax considerations. Neither the Internal Revenue Service nor any state or local taxing authority has reviewed the transactions described herein, and may take different positions than the ones contemplated by management. You are strongly urged to consult your own tax and other advisors prior to investing, as neither we nor any of our officers, directors or related parties is offering you tax or similar advice, nor are any such persons making any representations and warranties regarding such matters.
| 41 |
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. This means that it is very unlikely that we will pay dividends on our shares of common stock. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our common stock adversely, the price of our common stock and trading volume could decline.
The trading market for our common stock may be influenced by the research and reports that securities or industry analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us change their recommendation regarding our common stock adversely, or provide more favorable relative recommendations about our competitors, the price of our common stock would likely decline. If any analyst who may cover us was to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our common stock or trading volume to decline.
In making your investment decision, you should understand that we and the underwriters have not authorized any other party to provide you with information concerning us or this offering.
You should carefully evaluate all of the information in this prospectus before investing in our common stock. We may receive media coverage regarding our company, including coverage that is not directly attributable to statements made by our officers, that incorrectly reports on statements made by our officers or employees, or that is misleading as a result of omitting information provided by us, our officers or employees. We and the underwriters have not authorized any other party to provide you with information concerning us or this offering, and you should not rely on this information in making an investment decision.
| 42 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that reflect our current expectations and views of future events. The forward-looking statements are contained principally in the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Readers are cautioned that known and unknown risks, uncertainties and other factors, including those over which we may have no control and others listed in the “Risk Factors” section of this prospectus, may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,” “potential,” “continue” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include statements relating to:
| ● | our ability to formulate and implement our business plan, including the recruitment of dentists to enroll in our Vivos Integrated Practice (VIP) program and utilize the Vivos System; | |
| ● | the understanding and adoption by dentists and other healthcare professionals of the Vivos System as a treatment for mild-to-moderate OSA; | |
| ● | our expectations concerning the effectiveness of treatment using the Vivos System and patient relapse after completion of treatment; | |
| ● | the potential financial benefits to VIP dentists from treating patients with the Vivos System; | |
| ● | our potential profit margin from enrollment of VIPs and sales of the Vivos System appliances; | |
| ● | our ability to property train VIPs in the use of the Vivos System and other services we offer in their dental practices; | |
| ● | our ability to implement effective sales, marketing and strategic initiatives to drive revenue growth (including, for example, our Medical Integration Division and VivoScore home sleep apnea test); | |
| ● | the viability of our current intellectual property; | |
| ● | acceptance by the marketplace of the products and services that we market; | |
| ● | government regulations and our ability to comply with government regulations; | |
| ● | our ability to retain key employees; | |
| ● | adverse changes in general market conditions for medical devices such as the Vivos System; | |
| ● | our ability to generate cash flow and profitability and continue as a going concern; | |
| ● | our future financing plans; and | |
| ● | our ability to adapt to changes in market conditions (including as a result of the COVID-19 pandemic) which could impair our operations and financial performance. |
These forward-looking statements involve numerous risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our actual results of operations or the results of other matters that we anticipate herein could be materially different from our expectations. Important risks and factors that could cause our actual results to be materially different from our expectations are generally set forth in “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business,” “Regulation” and other sections in this prospectus. You should thoroughly read this prospectus and the documents that we refer to with the understanding that our actual future results may be materially different from and worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements.
The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this prospectus and the documents that we refer to in this prospectus and have filed as exhibits to the registration statement, of which this prospectus forms a part, completely and with the understanding that our actual future results may be materially different from what we expect.
| 43 |
We estimate that the net proceeds from our issuance and sale of our common stock in this offering will be approximately $18,113,500, based upon an assumed public offering price of $6.75 (which was the closing price of our common stock on Nasdaq on April 29, 2021) and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their over-allotment option in full, we estimate that the net proceeds from this offering will be approximately $20,903,500.
We expect the net proceeds from this offering will allow us to fund our operations for at least 12 months following the closing of the offering. We intend to use the net proceeds from this offering as follows:
| ● | approximately $4,000,000 for promotion, distribution and related expenses associated with our VivoScore home sleep apnea test; | |
| ● | approximately $4,000,000 for sales and marketing expenses generally; | |
| ● | approximately $3,000,000 for sales and support staff; | |
| ● | approximately $2,000,000 for research and development expenses; | |
| ● | approximately $2,000,000 for software development including enterprise resource planning implementation; and | |
| ● | approximately $3,113,500 working capital and general corporate purposes. |
The foregoing expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions. However, the nature, amounts and timing of our actual expenditures may vary significantly depending on numerous factors. For example, we may also elect to use proceeds from this offering to acquire complimentary technologies, products or businesses, although we are not a party to any letters of intent or definitive agreements for any such acquisition. As a result, our management has and will retain broad discretion over the allocation of the net proceeds from this offering. We may find it necessary or advisable to use the net proceeds from this offering for other purposes, and we will have broad discretion in the application of net proceeds from this offering. Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant. Our future ability to pay cash dividends on our stock may also be limited by the terms of any future debt or preferred securities or future credit facility.
| 44 |
The following table shows our capitalization at December 31, 2020:
| ● | on an actual basis; and | |
| ● | on an as adjusted basis to reflect the sale of 2,962,963 shares of common stock by us in this offering at an assumed offering price of $6.75 per share (which was the closing price of our common stock on Nasdaq on April 29, 2021), after deducting the underwriters’ discounts and commissions and estimated offering expenses payable by us, assuming the underwriters do not exercise the over-allotment option. |
We derived this table from, and it should be read in conjunction with and is qualified in its entirety by reference to, our historical consolidated financial statements and the accompanying notes included elsewhere in this prospectus. You should also read this table in conjunction with “Selected Historical Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| As of December 31, 2020 | ||||||||
| Actual | As adjusted | |||||||
| Cash and cash equivalents | $ | 18,205,668 | $ | 36,319,168 | ||||
| Preferred stock, $0.0001 par value; 50,000,000 shares authorized | ||||||||
| Series A Convertible Redeemable Preferred Stock, none issued and outstanding, actual and as adjusted | - | - | ||||||
| Stockholders’ equity: | ||||||||
| Series B Convertible Preferred Stock, $.0001 par value, 1,200,000 authorized, none issued and outstanding, actual; no shares authorized, issued or outstanding, as adjusted | - | - | ||||||
| Common stock, $0.0001 par value; 200,000,000 shares authorized, 18,209,452 shares issued and outstanding, actual; 200,000,000 shares authorized, 21,172,415 shares issued and outstanding, as adjusted (based on the assumed offering price) | 1,821 | 2,117 | ||||||
| Additional paid-in capital | 52,250,266 | 70,363,470 | ||||||
| Accumulated deficit | (35,334,728 | ) | (35,334,728 | ) | ||||
| Total stockholders’ equity | $ | 16,917,359 | $ | 35,028,742 | ||||
The number of shares of common stock to be outstanding after the offering is based on 18,209,452 shares, which is the number of shares outstanding on December 31, 2020, and excludes the following:
| ● | 2,302,345 shares of common stock underlying options to purchase shares of our common stock with a weighted average exercise price of $4.84 per share; | |
| ● | an additional 145,000 shares of common stock underlying options to purchase shares of our common stock subsequent to December 31, 2020 with an exercise price of 7.50 per share; | |
| ● | an additional 2,667 shares of common stock which were issued to a consultant subsequent to December 31, 2020; | |
| ● | 402,500 shares of our common stock underlying a warrant issued to the representative of the underwriters in connection with our December 2020 initial public offering with an exercise price of $7.50 per share; | |
| ● | 1,199,195 shares of common stock issuable upon the exercise of 1,199,195 common stock warrants associated with our previously outstanding Series B Preferred Stock issued in 2020 at an exercise price of $7.50 per share; | |
| ● | up to 148,149 shares of our common stock underlying the Representative’s Warrant to be issued to the representative of the underwriters in connection with this offering based on the assumed offering price (or up to 170,371 shares of our common stock to the underwriters if the over-allotment option to purchase shares of common stock is exercised in full based on the assumed offering price), which amounts may be increased, at our discretion, to up to 207,408 and 238,519, respectively (based on the assumed offering price); | |
| ● | 325,000 warrants which were issued to certain shareholders in November 2020 (see “Management—October 2020 Derivative Demand and Settlement”) at an exercise price of $7.50 per share; | |
| ● | 295,000 warrants to purchase common stock issued to contractors and consultants subsequent to December 31, 2020 with an exercise price of $7.50 per share, which includes 200,000 warrants issued in connection with the establishment of our MyoCorrect Program (see “Business—Our Revenue Model— MyoCorrect (Orofacial Myofunctional Therapy) Program”), for further information; | |
| ● | 25,000 warrants to purchase common stock issued in connection with the acquisition of Lyon Management and Consulting, LLC subsequent to December 31, 2020 with an exercise price of $8.90 per share, for the purpose to acquire certain medical billing and practice management software, licenses and contracts, including the software underlying AireO2 (see “Business—Our Revenue Model—AireO2 Patient Management Software”), for further information; and | |
| ● | 33,334 warrants to purchase common stock issued to an investor of convertible notes. The warrants are exercisable on a cash basis at an exercise price of $1.50 per share. |
| 45 |
If you invest in our common stock, you will incur immediate dilution since the offering price per share you will pay in this offering is more than the net tangible book value per common share immediately after this offering.
The net tangible book value of our stockholders’ equity as of December 31, 2020 was $13,975,804 or $0.77 per share based upon 18,209,452 shares of common stock outstanding. Net tangible book value per share represents the amount of our total tangible assets reduced by the amount of our total liabilities, divided by the total number of shares of common stock outstanding. Tangible assets equal our total assets less goodwill and intangible assets.
The as adjusted net tangible book value of our stockholders’ equity as of December 31, 2020, based on the assumed offering price of $6.75 (which was the closing price of our common stock on Nasdaq on April 29, 2021), was $32,089,304 or $1.52 per share. The as adjusted net tangible book value gives effect to the sale of 2,962,963 shares of common stock in this offering at an assumed offering price of $6.75 per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
The following table illustrates this dilution on a per share basis to new investors:
| Assumed offering price per share | $ | 6.75 | ||
| Net tangible book value per share before this offering, as of December 31, 2020 | $ | 0.77 | ||
| Increase in net tangible book value per share attributable to new investors in this offering based on the assumed offering price | 0.75 | |||
| As adjusted net tangible book value per share after offering | 1.52 | |||
| Dilution in tangible book value per share to new investors | $ | 5.23 |
If the underwriters’ over-allotment option to purchase additional shares from us is exercised in full, and based on an assumed offering price of $6.75 per share, the as adjusted net tangible book value (deficit) per share after this offering would be approximately $1.61 per share.
The following table sets forth, on an as adjusted basis as of December 31, 2020, the difference between the number of shares of common stock purchased from us, the total cash consideration paid, and the average price per share paid by our existing shareholders and by new public investors before deducting estimated underwriters’ discounts and commissions and estimated offering expenses payable by us, using an assumed offering price of $6.75 per share:
| Shares Purchased | Total Cash Consideration | Average Price | ||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | ||||||||||||||||
| Existing shareholders | 18,209,452 | 69 | % | $ | 43,529,115 | 100 | % | $ | 2.39 | |||||||||||
| New investors from offering | 2,962,963 | 31 | % | $ | 20,000,000 | - | % | $ | 6.75 | |||||||||||
| Total | 21,172,415 | 100 | % | $ | 63,529,115 | 100 | % | $ | 3.00 | |||||||||||
The as adjusted information discussed above is illustrative only. Our net tangible book value following the completion of this offering is subject to adjustment based on the actual offering price of our common stock and other terms of this offering determined at pricing.
The foregoing discussion and tables excludes the following:
| ● | 2,302,345 shares of common stock underlying options to purchase shares of our common stock issued and outstanding as of December 31, 2020 with a weighted average exercise price of $4.84 per share; | |
| ● | an additional 145,000 shares of common stock underlying options to purchase shares of our common stock issued subsequent to December 31, 2020 with an exercise price of $7.50 per share; | |
| ● | an additional 2,667 shares of common stock which were issued to a consultant subsequent to December 31, 2020; | |
| ● | 402,500 shares of our common stock underlying a warrant issued to the representative of the underwriters in connection with our December 2020 initial public offering at an exercise price of $7.50 per share; | |
| ● | 1,199,195 shares of common stock issuable upon the exercise of 1,199,195 common stock warrants associated with our previously outstanding Series B Preferred Stock issued in 2020 at an exercise price of $7.50 per share; | |
| ● | up to 148,149 shares of our common stock underlying the Representative’s Warrant to be issued to the representative of the underwriters in connection with this offering based on the assumed offering price (or up to 170,371 shares of our common stock to the underwriters if the over-allotment option to purchase shares of common stock is exercised in full based on the assumed offering price), which amounts may be increased, at our discretion, to up to 207,408 and 238,519, respectively (based on the assumed offering price); | |
| ● | 325,000 warrants were issued to certain shareholders (see “Management—October 2020 Derivative Demand and Settlement”) in November 2020 at an exercise price of $7.50 per share; | |
●
|
295,000 warrants to purchase common stock issued to contractors and consultants subsequent to December 31, 2020 with an exercise price of $7.50 per share, which includes 200,000 warrants issued in connection with the establishment of our MyoCorrect Program (see “Business—Our Revenue Model— MyoCorrect (Orofacial Myofunctional Therapy) Program”), for further information; | |
| ● | 25,000 warrants to purchase common stock issued in connection with the acquisition of Lyon Management and Consulting, LLC subsequent to December 31, 2020 with an exercise price of $8.90 per share, for the purpose to acquire certain medical billing and practice management software, licenses and contracts, including the software underlying AireO2 (see “Business—Our Revenue Model—AireO2 Patient Management Software”), for further information; and | |
●
|
33,334 warrants to purchase common stock issued to an investor of convertible notes. The warrants are exercisable on a cash basis at an exercise price of $1.50 per share. |
| 46 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the accompanying notes thereto included elsewhere in this prospectus. The below discussion may contain forward-looking statements, as that term is defined in the federal securities laws, which are based upon current expectations but which involve significant risks and uncertainties. We caution you that these statements are not guarantees of future performance or events and are subject to a number of uncertainties, risks and other influences, many of which are beyond our control, which may influence the accuracy of the statements and the projections upon which the statements are based.
Please refer to the sections of this prospectus captioned “Risk Factors” and “Cautionary Note Regarding Forward Looking Statements” for important information to be read in conjunction with the below discussion.
Overview
We are a revenue stage medical technology company focused on the development and commercialization of a highly differentiated technology offering a clinically effective non-surgical, non-invasive, non-pharmaceutical, and low-cost solution for patients with SDB, including mild-to-moderate OSA. We offer novel and proprietary alternatives for treating mild-to-moderate OSA as well as certain craniofacial and anatomical anomalies known to be associated with OSA. We believe our products and technology represent a significant improvement in the treatment of mild-to-moderate OSA versus other treatments such as CPAP.
Our treatment for mild-to-moderate OSA involves specially designed and customized oral appliances and treatment protocols that we call the Vivos System. We believe the Vivos System technology represents the first non-surgical, non-invasive and cost-effective solution that normally does not require lifetime use of intervention for the hundreds of millions of people globally who suffer from mild-to-moderate OSA. We intend to more rapidly expand the use of the Vivos System by actively recruiting dentists and training them about OSA and the use and application of our products and technology to treat mild-to-moderate OSA. Our oral appliances have proven effective (within the scope of the U.S. Food and Drug Administration (or FDA) cleared uses as described below) in over 15,000 patients treated worldwide by more than 1,200 trained dentists.
On December 11, 2020, we completed our initial public offering by issuing 4,025,000 shares of common stock, at a public offering price of $6.00 per share, for net proceeds of approximately $21.6 million after deducting underwriting discounts and commissions and offering expenses payable by us.
Impact of COVID-19
The early 2020 outbreak of COVID-19 and its development into a pandemic in March 2020 has resulted in significant economic disruption globally. Actions taken by various governmental authorities, individuals and companies around the world to prevent the spread of COVID-19 through social distancing have restricted travel, many business operations, public gatherings and the overall level of individual movement and in-person interaction across the globe. This has significantly reduced global economic activity and resulted in a decline in demand across many industries.
Many of our VIPs and potential VIPs closed their offices for periods of time during 2020 as a result of COVID-19, although some remained open to specifically provide patients with our appliances and VIPs were deemed an essential business for health considerations in many jurisdictions. In the face of the pandemic and the potential for revenue reduction, we worked diligently to reduce expenses and maintain revenues during 2020. While revenue growth flattened in March and April 2020, expenses were reduced, and we aggressively expanded our network of healthcare providers familiar with our products by offering online continuing education courses which introduced many in the medical and dental communities to our product line. As a result, we determined no triggering events had occurred indicating no impairment needed as of December 31, 2020. However, even as we take action to face the challenges of the pandemic, since the situation with COVID-19 remains uncertain, we cannot predict with certainty the impact of the pandemic or local outbreaks thereof will have on our near- and longer-term results of operations.
| 47 |
Results of Operations
Year Ended December 31, 2020 Compared to Year Ended December 31, 2019
| Year ended | ||||||||||||
| December
31, 2020 | December
31, 2019 | Increase (Decrease) | ||||||||||
| Revenue | ||||||||||||
| Product revenue | $ | 4,889,840 | $ | 4,349,623 | $ | 540,217 | ||||||
| Service revenue | 8,176,397 | 7,043,654 | 1,132,743 | |||||||||
| Total revenue | 13,066,237 | 11,393,277 | 1,672,960 | |||||||||
| Cost of sales | (2,653,429 | ) | (2,736,034 | ) | (82,605 | ) | ||||||
| Gross profit | 10,412,808 | 8,657,243 | 1,755,565 | |||||||||
| Gross profit % | 80 | % | 76 | % | 4 | pp | ||||||
| Operating expenses | ||||||||||||
| General and administrative | (16,090,049 | ) | (16,172,505 | ) | (82,456 | ) | ||||||
| Sales and marketing | (2,314,023 | ) | (2,310,743 | ) | 3,280 | |||||||
| Settlement expense | (3,330,679 | ) | - | 3,330,679 | ||||||||
| Depreciation and amortization | (717,865 | ) | (751,228 | ) | (33,363 | ) | ||||||
| Operating loss | (12,039,808 | ) | (10,577,233 | ) | (1,462,575 | ) | ||||||
| Interest expense | (96,681 | ) | (137,876 | ) | 41,195 | |||||||
| Interest income | 79,612 | 21,133 | 58,479 | |||||||||
| Loss on sale of business | - | (60,343 | ) | 60,343 | ||||||||
| Net loss | $ | (12,056,877 | ) | $ | (10,754,319 | ) | $ | (1,302,558 | ) | |||
Revenue
Revenue increased $1.7 million, or 15%, to $13.1 million for the year ended December 31, 2020 compared to the year ended December 31, 2019. This increase was related to revenue from our VIP program along with the increase in the number of oral appliances sold. During the year ended December 31, 2020, we enrolled 248 VIPs for a total of $7,540,718. During the year ended December 31, 2019, we enrolled 204 VIPs for a total of $6,742,283. Additionally, BIS service revenues increased from $256,415 for the year ended December 31, 2019 to $620,094 for the year ended December 31, 2020. During the year ended December 31, 2020 we sold 8,135 total oral appliance arches for a total of $4,547,883 and for the year ended December 31, 2019 we sold 4,696 total oral appliance arches for a total of $2,917,095. The increase in appliance revenue is due to both volume and price increases.
Cost of Goods Sold and Gross Margin
Cost of goods sold decreased $0.1 million, on increased sales of $1.7 million. COVID-19 impacted our sales mix as many dental offices were closed for a good portion of April and May, resulting in having higher margin service revenues represent a larger portion of our overall revenues than our product revenues for the year ended December 31, 2020 as compared to the year ended December 31, 2019.
General and Administrative Expenses
General and administrative expenses decreased $0.1 million, for the year ended December 31, 2020 as compared to the year ended December 31, 2019. As a percentage of revenues, general and administrative expenses decreased to 123% of revenues for the year ended December 31, 2020 from 143% of revenues for the year ended December 31, 2019. This decrease as a percent of revenues was achieved as a result of scaling operations as our revenues grew and reducing payroll and travel expenses during the COVID-19 outbreak.
| 48 |
Sales and Marketing
Sales and marketing expense was flat for the year ended December 31, 2020 as compared to the year ended December 31, 2019. The primary reason for this decrease was the postponement until 2021 of our annual conference for VIPs due to the COVID-19 outbreak combined with the increase in revenues that drives sales and marketing expenses.
Settlement Expense
Settlement expense in 2020 resulted from the settlement of a shareholder demand in the fourth quarter of 2020. We issued 300,000 shares of common stock and 325,000 warrants to purchase common shares as a result of this settlement.
Depreciation and Amortization
Depreciation and amortization expense decreased approximately $33,000 for the year ended December 31, 2020 as compared to the year ended December 31, 2019, due primarily to the sale of one of our Vivos Centers in the fourth quarter of 2019.
Interest Expense
Interest expense decreased by approximately $41,000, for the year ended December 31, 2020 as compared to the year ended December 31, 2019 as a result of the convertible notes being exchanged into Series B Preferred Stock throughout 2020.
Interest Income
Interest income increased by approximately $58,000 for the year ended December 31, 2020 as compared to the year ended December 31, 2019 primarily due to interest on our note receivable related to the sale of one of our Vivos Centers in the fourth quarter of 2019.
Net Loss
We incurred a net loss of $12.1 million during the year ended December 31, 2020 as compared to a net loss of $10.8 million for the year ended December 31, 2019. The $1.3 million additional loss was primarily due to the settlement expense of $3.3 million offset by $1.8 million higher gross margin in 2020.
Year Ended December 31, 2019 Compared to the Year Ended December 31, 2018
| Year ended | ||||||||||||
| December
31, 2019 | December
31, 2018 | Increase (Decrease) | ||||||||||
| Revenue | ||||||||||||
| Product revenue | $ | 4,349,623 | $ | 1,848,375 | $ | 2,501,248 | ||||||
| Service revenue | 7,043,654 | 1,943,886 | 5,099,768 | |||||||||
| Total revenue | 11,393,277 | 3,792,261 | 7,601,016 | |||||||||
| Cost of sales | (2,736,034 | ) | (1,081,641 | ) | (1,654,393 | ) | ||||||
| Gross profit | 8,657,243 | 2,710,620 | 5,946,623 | |||||||||
| Gross profit % | 76 | % | 71 | % | 5 | pp | ||||||
| Operating expenses | ||||||||||||
| General and administrative | (16,172,505 | ) | (9,272,890 | ) | (6,899,615 | ) | ||||||
| Sales and marketing | (2,310,743 | ) | (1,163,239 | ) | (1,147,504 | ) | ||||||
| Depreciation and amortization | (751,228 | ) | (610,673 | ) | (140,555 | ) | ||||||
| Operating loss | (10,577,233 | ) | (8,336,182 | ) | (2,241,051 | ) | ||||||
| Interest expense | (137,876 | ) | (102,974 | ) | (34,902 | ) | ||||||
| Interest income | 21,133 | - | 21,133 | |||||||||
| Loss on sale of business | (60,343 | ) | - | (60,343 | ) | |||||||
| Net loss | $ | (10,754,319 | ) | $ | (8,439,156 | ) | $ | (2,315,163 | ) | |||
| 49 |
Revenue
Our revenue for the year ended December 31, 2019 increased $7,601,016, or 200%, to $11,393,277 from $3,792,261 for the year ended December 31, 2018. This increase was related to revenue from our VIP program that began during 2019 along with the increase in the number of oral appliances sold. During the year ended December 31, 2019, we enrolled 204 VIPs for a total of $6,742,283. During the year ended December 31, 2018, we enrolled 67 VIPs for a total of $1,251,679. During the year ended December 31, 2019 we sold 4,696 total oral appliance arches for a total of $2,917,095 and for the year ended December 31, 2018 we sold 2,201 total oral appliance arches for a total of $695,250. The increase in appliance revenue is due to both volume and price increases.
Cost of Sales
Cost of sales for the year ended December 31, 2019 increased $1,654,393, or 153%, to $2,736,034 from $1,081,641 for the year ended December 31, 2018 due to the relative increase in revenue. As a percentage of revenue, cost of sales was 24% for the year ended December 31, 2019 and 29% for the year ended December 31, 2018. The decrease in cost as percentage of revenue was due to a greater mix of higher margin VIP program revenue during the year ended December 31, 2019 over year ended December 31, 2018.
General and Administrative
General and administrative expenses increased $6,899,615, or 74%, for the year ended December 31, 2019 as compared to the year ended December 31, 2018. This increase relates primarily to payroll and benefits, consultants, travel and other costs associated with the growth of our business.
Sales and Marketing
Sales and marketing increased $1,147,504, or 99%, for the year ended December 31, 2019 as compared to the year ended December 31, 2018. The primary reason for this increase were additional commissions related to the increased service and product revenues, which increased 200%.
Depreciation and Amortization
Depreciation and amortization expense increased $140,555 for the year ended December 31, 2019 as compared to the year ended December 31, 2018, due almost entirely to the full year’s depreciation on furniture and equipment and leasehold improvements at the Vivos Centers in 2019 versus a partial year in 2018.
Interest Expense
Interest expense increased $34,902 for the year ended December 31, 2019 compared to the year ended December 31, 2018, primarily as a result of a convertible note offering that commenced in April 2019.
Net Loss
We incurred a net loss of $10,754,319 during the year ended December 31, 2019 as compared to $8,439,156 of net loss for the year ended December 31, 2018. A higher gross margin of $5,946,623 was offset by higher sales and marketing expenses and general and administrative expenses.
Liquidity and Capital Resources
As of December 31, 2020, we had cash and cash equivalents of $18,205,860 compared to cash and cash equivalents of $469,353 at December 31, 2019. In January 2020, we commenced a private placement offering that authorized the issuance of up to $15,000,000 of newly designated Series B Preferred Stock to accredited investors. As of October 1, 2020, we closed our Series B Preferred Stock offering after having received approximately $2,450,000 from the issuance of Series B Preferred Stock and exchanging approximately $2,944,000 in accrued principal and interest from our 2019 convertible notes into Series B Preferred Stock, whereas other 2019 convertible note holders elected to convert their notes into common stock. All Series B Preferred Stock converted into common stock in December 2020 in connection with our initial public offering.
| 50 |
In May 2020, we secured funding of $1,265,067 under the Paycheck Protection Program that was signed into law as part of the Coronavirus Aid, Relief and Economic Security (CARES) Act as a result of the COVID-19 pandemic. The promissory note contains an interest rate of 1.0% per year. Payments will be deferred for the first six months of the loan, then we must pay principal and interest monthly based on the unforgiven portion of the loan balance plus all accrued interest, beginning seven months from the month the note is dated. We anticipate seeking forgiveness of a significant portion of the loan amount under the provisions of the program as the amount borrowed has been used to pay compensation, rent and utilities. While we believe that our use of the loan proceeds will meet the conditions for forgiveness of the loan, there is a risk that the loan will not be forgiven or that we will take actions that could cause us to be ineligible for forgiveness of the loan, in whole or in part.
On December 11, 2020, we completed our initial public offering by issuing 4,025,000 common shares at a price of $6.00 per share, for net proceeds of approximately $21.6 million, after deducting underwriter discounts and commissions and offering expenses payable by us. Following our initial public offering, we made payments of $2.0 million to our founder and chief medical officer to redeem a portion of the outstanding Series A Preferred Stock. The remaining Series A Preferred Stock were redeemed in full as of December 31, 2020, and in early January, we made the final payment of $1.5 million to our founder and chief medical officer related to the December 2020 redemption of all outstanding remaining Series A Preferred Stock.
We believe that our existing cash resources will be sufficient to meet our capital requirements and fund our operations for at least the next 12 months. We may also seek liquidity through additional securities offerings or through borrowings under a new credit facility.
Cash Flows
The following table presents a summary of our cash flow for the periods indicated:
| 2020 | 2019 | |||||||
| Net cash provided by (used in): | ||||||||
| Operating activities | $ | (5,680,294 | ) | $ | (5,340,480 | ) | ||
| Investing activities | (120,252 | ) | 86,223 | |||||
| Financing activities | 23,536,861 | 4,468,887 | ||||||
| Increase (Decrease) in cash and cash equivalents | $ | 17,736,315 | $ | (785,370 | ) | |||
Net cash used in operations was $5,680,294 for the year ended December 31, 2020 compared to net cash used of $5,340,480 for the year ended December 31, 2019. The increase in cash used from operating activities was primarily driven by the increase in our net loss of $1.2 million.
Net cash used in investing activities consists of capital expenditures for property, plant and equipment and increased by approximately $206,000 from the year ended December 31, 2020 compared to cash provided by investing activities for the year ended December 31, 2019. For the year ended December 31, 2019, $250,000 in proceeds from the sale of a business were included in investing activities.
Net cash provided by financing activities for the year ended December 31, 2020 consisted of the more than $22.3 million in net proceeds from our initial public offering plus $2.5 million in proceeds from the sale of Series B Preferred Stock and $1.3 million in proceeds from the PPP loan offset by $2.2 million in redemptions on the Series A Preferred Stock. For the year ended December 31, 2019, $1.2 million was received from the issuance of common stock and $3.8 million was received from the proceeds of our convertible debt offering that was offset by $0.4 million in redemptions on Series A Preferred Stock.
| 51 |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as defined by applicable regulations of the SEC, that are reasonably likely to have a current or future material effect on our financial condition, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies Involving Management Estimates and Assumptions
Basis of Presentation and Consolidation
Our consolidated financial statements included as part of this prospectus, which include the accounts of our company and our wholly owned subsidiaries (BMS and First Vivos), are prepared in conformity with U.S. GAAP and the rules and regulations of the SEC related to annual and quarterly reports. All significant intercompany balances and transactions have been eliminated in consolidation. Certain information and note disclosures normally included in annual financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to those rules and regulations. The consolidated balance sheet as of December 31, 2020 included in this prospectus has been derived from our audited consolidated financial statements.
Use of Estimates
To prepare financial statements in conformity with U.S. GAAP, management must make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Concentration of Credit Risk and Significant Customers
Financial instruments, which potentially subject us to concentrations of credit risk, consist primarily of cash and cash equivalents and accounts receivable. We limit our exposure to credit loss by placing our cash with high credit quality financial institutions. Additionally, we have a diverse customer base and no single customer represented greater than ten percent of sales or accounts receivable for the years ended December 31, 2020 and December 31, 2019.
Accounts Receivable, Net
The accounts receivable in the accompanying consolidated financial statements are stated at the amounts management expects to collect. We perform credit evaluations of our customers’ financial condition and may require a prepayment for a portion of the services to be performed. We reduce accounts receivable by estimating an allowance that may become uncollectible in the future. Management determines the estimated allowance for uncollectible amounts based on its judgements in evaluating the aging of the receivables and the financial condition of our clients. Allowance for uncollectible receivables was $507,347 and $180,852 as of December 31, 2020 and 2019, respectively.
Intangible Assets, Net
Intangible assets consist of assets acquired from First Vivos and costs paid to third parties for work related to our patents. The identified intangible assets acquired from First Vivos are amortized using the straight-line method over the estimated life of the assets, which approximates 5 years. The costs paid to third parties for our assets are amortized using the straight-line method over the life of the underlying patents, which approximates 15 years commencing at which time the patent has been granted. We determined the fair value of the intangible assets using a discounted cash flow approach.
Goodwill
Goodwill is the excess of acquisition cost of an acquired entity over the fair value of the identifiable net assets acquired. Goodwill is not amortized, but tested for impairment annually or whenever indicators of impairment exist. These indicators may include a significant change in the business climate, legal factors, operating performance indicators, competition, sale or disposition of a significant portion of the business or other factors. We test for impairment annually after the close of the year. There was no impairment of goodwill recognized at December 31, 2020 or 2019.
| 52 |
Long-lived Asset Policy
We review and evaluate the recoverability of long-lived assets whenever events or changes in circumstances indicate that an asset’s carrying amount may not be recoverable. Such circumstances could include, but are not limited to (1) a significant decrease in the market value of an asset, (2) a significant adverse change in the extent or manner in which an asset is used, or (3) an adverse action or assessment by a regulator. We measure the carrying amount of the asset against the estimated undiscounted future cash flows associated with it. Should the sum of the expected future net cash flows be less than the carrying value of the asset being evaluated, an impairment loss would be recognized. The impairment loss would be calculated as the amount by which the carrying value of the asset exceeds its fair value. The fair value is measured based on quoted market prices, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including the discounted value of estimated future cash flows. The evaluation of asset impairment requires us to make assumptions about future cash flows over the life of the asset being evaluated. These assumptions require significant judgment and actual results may differ from assumed and estimated amounts. Our evaluation of long-lived assets completed for the years ended December 31, 2020 and 2019 resulted in no impairment loss.
Notes Receivable, Net
The notes receivable in the accompanying financial statements are stated at the amount management expects to collect. The current portion is what the Company expects to collect in the next twelve months and the long-term portion consists of the portion the Company expects to collect beyond twelve months. Periodically throughout the year, management evaluates the collectability of the note receivable based on its judgements of the operations and financial strength of underlying practice. The Company reduced notes receivable by estimating a discount based on market rates. The discount on notes receivable was $68,101 and $93,421 as of December 31, 2020 and 2019, respectively. Accretion on the discount and interest on the note is recorded in interest income.
Revenue Recognition
We adopted Accounting Standards Update No. 2014-09 (Topic 606) titled, “Revenue from Contracts with Customers” as of January 1, 2019 and relied upon transitional guidance provided for in 606-10- 65-1(f)(3) and do not disclose the transaction price allocated to the remaining performance obligations or an explanation of when we expect to recognize that amount as revenue.
We generate revenue from the sale of products and services. Revenue is recognized when control of the products or services is transferred to our customers in a way that reflects the consideration we expect to be entitled to in exchange for those products and services.
We determine revenue recognition through the following five-step model, which entails:
| 1) | identification of the promised goods or services in the contract; | |
| 2) | determination of whether the promised goods or services are performance obligations, including whether they are distinct in the context of the contract; | |
| 3) | measurement of the transaction price, including the constraint on variable consideration; | |
| 4) | allocation of the transaction price to the performance obligations; and | |
| 5) | recognition of revenue when, or as the Company satisfies each performance obligation. |
Service revenue
Service revenue is recognized when the underlying training or other services are performed. Unearned revenue reported on the balance sheet as contract liability represents the portion of fees paid by customers for services that have not yet been performed as of the reporting date and are recorded as the service is rendered. We recognize this revenue over the twelve-month life of the contract. Provisions for discounts are provided in the same period that the related revenue from the products and/or services is recorded.
| 53 |
We enter into programs that may provide for multiple element deliverables. Commencing in 2018, we began enrolling medical and dental professionals in a one-year program which includes training in a highly personalized, deep immersion workshop format which provides the dentist access to an onboarding team who is dedicated to creating a successful integrated practice. The key topics covered in training include case selection, clinical diagnosis, appliance design, adjunctive therapies, instructions on ordering our products, guidance on pricing, instruction on insurance reimbursement protocols and interacting with our proprietary software system and the many features on our website. The initial training and educational workshop is typically provided in the first month that a VIP enrolls. Since VIPs are able to begin generating revenue after the first training workshop, we recognize 50% of the service revenue in the second month of enrollment and the remaining 50% pro-rata throughout the following eleven months of the service contract. Ongoing support and additional training are provided throughout the year and include access to our proprietary Airway Intelligence Service (or AIS) which provides VIPs with resources to help simplify the diagnostic and treatment planning process. AIS is provided as part of the price of each appliance and is not a separate revenue stream. Following the year of training and support, a VIP may pay for seminars and training courses that meet the VIP’s needs on a subscription or a course by course basis. In addition to enrollment service revenue, we have more recently launched an additional service on a monthly subscription basis: Billing Intelligence Service (or BIS). Revenue for this service is recognized monthly during the month the service is rendered.
We identify all goods and services that are delivered separately under a sales arrangement and allocates revenue to each deliverable based on relative fair values. Fair values are generally established based on the relevant service period which approximates the prices for relevant training that would be charged if those services were sold separately. In general, revenues are separated between durable medical equipment (product revenue) and education and training services (service revenue). The allocated revenue for each deliverable is then recognized ratably based on relative fair values of the components of the sale. Revenue from training is recognized over the relevant service period (i.e., as we satisfy our performance obligations and creates value for the VIP). We also evaluate the impact of undelivered items on the functionality of delivered items for each sales transaction and, where appropriate, defer revenue on delivered items when that functionality has been affected. Functionality is determined to be met if the delivered products or services represent a separate earnings process.
From time to time we offer various discounts to our customers. These include the following:
| 1) | Discount for cash pay in full | |
| 2) | Conference or trade show incentives | |
| 3) | Negotiated concessions on annual enrollment fee |
The amount of the discount is determined up front prior to the sale. Accordingly, measurement is determined before the sale occurs and revenue is recognized based on the terms agreed upon between us and the VIP over the performance period. In rare circumstances, a discount has been given after the sale during a conference which is offering a discount to full price. In this situation revenue is measured and the change in transaction price is allocated over the remaining performance obligation.
The amount of consideration can vary by customer due to promotions and discounts authorized to incentivize a sale. Prior to the sale, the customer and us agree upon the amount of consideration that the customer will pay in exchange for the services we provide. The net consideration that the customer has agreed to pay is the expected value that is recognized as revenue over the service period. Any overpayments are refunded during the reporting period so that no refund liability is recognized. At the end of each reporting period, we update the transaction price to represent the circumstances present at the end of the reporting period and any changes in circumstances during the reporting period.
Product revenue
In addition to revenue from services, we also generate revenue from the sale of our patented oral devices and preformed guides, known as appliances or systems to our customer, the VIP. Revenue from the appliance sale is recognized when control of product is transferred to the VIP in an amount that reflects the consideration we expect to be entitled to in exchange for those products. The VIP in turn charges the VIP’s patient and/or patient’s insurance a fee for the appliance and for his or her professional services in measuring, fitting, installing the appliance and educating the patient as to its use. We are contracted with the VIP for the sale of the appliance and are not involved in the sale of the products and services from the VIP to the VIP’s patient.
| 54 |
Our appliances are visually similar to a retainer that is worn after braces are removed. Each appliance is specifically fitted to each patient. We utilize our network of certified VIPs throughout the country to sell the appliances to their customers as well as in two centers that we operate. We utilize third party contract manufacturers or labs to produce each appliance and preformed Guide. The manufacturer designated by us (of which there are several) produces the appliance in strict adherence to our patents, design files, protocols, processes and procedures and under the direction and specific instruction of us. The manufacturer then ships the appliance to the VIP who ordered the appliance from us. All of our contract manufacturers are required to follow our master design files in production of appliances or the lab will be in violation of the FDA’s rules and regulations. We performed an analysis under ASC Topic 606-10-55-36 through 55-40 and concluded it is the principal in the transaction and is reporting revenue gross. We bill the VIP provider the contracted price for the appliance which is recorded as product revenue. Product revenue is recognized once the appliance ships to the VIP provider under our direction.
Beginning in 2018, we operated three centers in Colorado and Utah. Effective October 1, 2019, we sold our center in Utah (see Note 4 to the financial statements included as part of this prospectus). Within each center, we utilize a team of medical professionals to measure, order and fit each appliance. Upon scheduling the patient (which is our customer in this case), the center takes a deposit and reviews the patient’s insurance coverage. Revenue is recognized differently for our owned centers than for our VIPs. We recognize revenue in the centers after the appliance is received from the manufacturer and once the appliance is fitted and provided to the patient.
We offer our clinical advisors (who help our VIPs with technical aspects of our products) discounts from our standard VIP pricing. This is done to help encourage our clinical advisors to purchase our products for their own practices. In addition, from time to time, we offer buy one, get one offers and other credits to incentivize our VIPs to embrace our products and increase volume within their practices.
Stock-Based Compensation
Our board of directors (or the compensation committee thereof) grants share-based payments to employees under our equity incentive plans described below. Historically, this is has come in the form of options to purchase shares of our common stock. Since November 2018, all stock options have been granted with an exercise price of $7.50 per share on post-reverse split basis. Exercise price of such stock options has been consistent with the price offered to private investors in the Company’s private placements during this period, which our board of directors or its compensation committee deemed to be the fair value of the underlying common stock.
From an accounting perspective, we account for share-based payments to employees by recognizing compensation expense based upon the estimated fair value of the awards on the date of grant. Absent a publicly traded market for our stock, we use the price paid for our stock in the most recent sales to third parties as the stock price input into our valuation model as of the date of grant. We determine the estimated grant fair value using the Black-Scholes option pricing model and recognize compensation costs ratably over the requisite service period which approximates the vesting period using the straight-line method. For options issued to consultants, we recognize the estimated fair value of options issued using the Black-Scholes option pricing model at the time the services are rendered.
The Black-Scholes model requires the input of certain subjective assumptions and the application of judgment in determining the fair value of the awards. The most significant assumptions and judgments include the expected volatility, risk-free interest rate, the expected dividend yield, and the expected term of the awards. The Company accounts for forfeitures as they occur.
The assumptions used in our option pricing model represent management’s best estimates. If factors change and different assumptions are used, our equity-based compensation expense could be materially different in the future. The key assumptions included in the model are as follows:
| ● | Share Price – We use the price of our stock sold to third parties in our offerings as the most available representation of fair value per share of common stock on date of grant. | |
| ● | Expected volatility — We determine the expected price volatility based on the historical volatilities of our peer group as we do not have a sufficient trading history for our common stock. Industry peers consist of several public companies in the bio-tech industry similar to us in size, stage of life cycle and financial leverage. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own stock price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be utilized in the calculation. |
| 55 |
| ● | Risk-free interest rate — The risk free rate was determined based on yields of U.S. Treasury Bonds of comparable terms. The volatility is based on analyzing the stock price and implied volatility of guideline companies. | |
| ● | Expected dividend yield — We have not previously issued dividends and do not anticipate paying dividends in the foreseeable future. Therefore, we used a dividend rate of zero based on our expectation of additional dividends. | |
| ● | Expected term — We estimate the expected term using the simplified method which is the average of the vesting term and the contractual term of the options. |
In 2017, our board of directors and shareholders approved the adoption of a stock and option award plan (the “2017 Plan”), under which shares were reserved for future issuance for options, restricted stock awards and other equity awards. The 2017 Plan permits grants of equity awards to employees, directors, consultants and other independent contractors. Our board of directors and shareholders have approved a total reserve of 1,333,333 shares for issuance under the 2017 Plan.
In 2019, our board of directors and shareholders approved the adoption of a stock and option award plan (the “2019 Plan”), under which shares were reserved for future issuance for options, restricted stock awards and other equity awards. The 2019 Plan permits grants of equity awards to employees, directors, consultants and other independent contractors. Our board of directors and shareholders have approved a total reserve of 333,334 shares for issuance under the 2019 Plan. On June 18, 2020, our shareholders approved an amendment and restatement of the 2019 Plan to increase the number shares or our common stock available for issuance thereunder by 833,333 share of common stock such that, after amendment and restatement of the 2019 Plan, and prior to any grants, 1,166,667 shares of common stock were available under the 2019 Plan.
Basic and Diluted Net Loss Per Share
Basic net loss per share is computed using the weighted average number of common shares outstanding during the period. Diluted net loss per common share is computed using the weighted average number of common shares outstanding and the weighted average dilutive potential common shares outstanding using the treasury stock method. However, for the years ended December 31, 2020 and 2019, diluted net loss per share is the same as basic net loss per share as the inclusion of weighted average shares of common stock issuable upon the exercise of outstanding warrants and stock options would be anti-dilutive. The numerator in the basic and diluted net loss per share calculation is the net loss attributable to common stockholders, which is the net loss for the year increased by the current year preferred stock dividends accrued.
The holder of our outstanding Series A Preferred Stock (Dr. G. Dave Singh, our founder and Chief Medical Officer) was entitled to participate in common stock dividends, if and when declared, on a one-to-one per-share basis. Accordingly, in periods in which we have net income, earnings per share will be computed using the two-class method whereby the pro rata dividends distributable to the holder of our Series A Preferred Stock will be deducted from earnings applicable to common stockholders, regardless of whether a dividend is declared for such undistributed earnings. For the years ended December 31, 2020 and 2019, we incurred a net loss and, accordingly, there were no undistributed earnings to allocate under the two-class method.
The following table summarizes outstanding common stock securities not included in the computation of diluted net loss per common share as their inclusion would be anti-dilutive:
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Common stock warrants | 1,960,029 | 83,334 | ||||||
| Common stock options | 2,302,345 | 1,900,000 | ||||||
| 56 |
Recent Accounting Pronouncements
We are an emerging growth company (“EGC”) as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), whereby we are not required to comply with new or revised financial accounting standards until the dates when private companies are required to comply with such standards. The JOBS Act provides that a company can elect to opt out of the extended transition periods and comply with the requirements that apply to non-EGC public companies but any such election to opt out is irrevocable. Presented below is a discussion of new accounting standards including deadlines for adoption assuming that the Company retains its designation as an EGC.
Standards Required to be Adopted in Future Years. The following accounting standards are not yet effective, and a decision has not been reached about whether we may elect to early adopt any of the standards:
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). This ASU requires us to recognize lease assets and lease liabilities on the balance sheet and also disclose key information about leasing arrangements. In July 2018, the FASB issued ASU No. 2018-11 Targeted Improvements, which provides lessees the option to adopt either (i) retrospectively to each prior reporting period presented upon initial adoption, or (ii) apply the new leasing standard to all open leases as of the adoption date by recognizing a cumulative-effect adjustment to accumulated deficit in the period of adoption without restating prior periods. The Company is still evaluating which transition approach will be implemented upon adoption of ASU No. 2016-02. ASU 2016-02 is effective for the Company beginning in the first quarter of 2022 and early adoption is permitted.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. ASU 2016-13 amends the guidance on the impairment of financial instruments. This guidance requires use of an impairment model (known as the “current expected credit losses”, or CECL model) that is based on expected losses rather than incurred losses. Under the new guidance, an entity recognizes, as an allowance, its estimate of expected credit losses. ASU 2016-13 is effective for the Company beginning in the first quarter of 2023. The Company is still evaluating the impact the adoption of ASU 2016-13 will have on its results of operations or financial position.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740), Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principles in Topic 740 and clarifies and amends existing guidance to improve consistent application. ASU 2019-12 is effective for the Company beginning in the first quarter of 2022. Early adoption is permitted, including adoption in an interim period. The adoption of this standard is not expected to have a material impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not currently expected to have a material impact on the Company’s financial statements upon adoption.
Recently Adopted Standards. The following recently issued accounting standards were adopted by the Company during the year ended December 31, 2020:
In June 2018, the FASB issued ASU 2018-07, Compensation — Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which expands the scope of Accounting Standards Codification (“ASC”) 718, Compensation—Stock Compensation to include share-based payment transactions for acquiring goods and services from non-employees. An entity should apply the requirements of ASC 718 to non-employee awards except for specific guidance on inputs to an option pricing model and the attribution of cost. The Company adopted this new guidance using the modified retrospective method effective on January 1, 2020. On the date of adoption, there were no outstanding awards granted to non-employees in transactions to acquire goods and services for which the measurement date had not yet occurred. Therefore, the adoption of this standard did not have any impact on the Company’s financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. ASU 2017-04 simplifies the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test and eliminating the requirement for a reporting unit with a zero or negative carrying amount to perform a qualitative assessment. Under ASU 2017-04, goodwill impairment testing is performed by comparing the fair value of a reporting unit with its carrying amount whereby an impairment charge is recognized for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized is not to exceed the total amount of goodwill allocated to that reporting unit. In addition, income tax effects are considered, if applicable. ASU 2017-04 is effective for annual and any interim impairment tests performed after December 15, 2022. Effective October 1, 2020, the Company early adopted this new guidance for its annual goodwill impairment testing whereby the adoption of this standard did not have any impact on the Company’s financial statements.
| 57 |
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurements (Topic 820): Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement. ASU 2018-13 modifies the disclosure requirements on fair value measurements. ASU 2018-13 was adopted effective for the Company beginning in the first quarter of 2020. The Company adopted ASU 2018-13 effective January 1, 2020. The adoption of this standard did have a material impact on the Company’s financial statements.
Internal Control Over Financial Reporting
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. GAAP. Under standards established by the Public Company Accounting Oversight Board, or PCAOB, a deficiency in internal control over financial reporting exists when the design or operation of a control does not allow management or personnel, in the normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis. The PCAOB defines a material weakness as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented, or detected and corrected, on a timely basis.
During the preparation of our consolidated financial statements for the fiscal years ended December 31, 2020 and 2019, we and our independent registered public accounting firm identified a material weakness in our internal control over financial reporting arising from an accumulation of significant deficiencies which amounted to a material weakness in internal controls. Such significant deficiencies identified included insufficient supporting documentation and inadequate review of certain journal entries, segregation of duties, and inadequate application of accounting guidance. We continue to implement processes and procedures to mitigate significant deficiencies and material weaknesses in the future, including the hiring of an Assistant Controller in first quarter 2021 to build our accounting team and help remediate our significant deficiencies which amounted to a material weakness in our internal control procedures over financial reporting.
See “Risk Factors—Risks Related to our Business and Industry —We have identified a material weakness in our internal control over financial reporting.”
Quantitative and Qualitative Disclosures About Market Risk
Trade Policy Risk. Certain of our products or components are manufactured outside the United States. Most products imported into the United States is subject to duty and restrictive quotas on the amount of products that can be imported from certain countries into the United States each year. Because of the duty rates and quotas, changes in U.S. trade policy as reflected in various legislation, trade preference programs and trade agreements have the potential to materially impact our sourcing strategy and the competitiveness of its contract manufacturers. We manage this risk by continually monitoring U.S. trade policy, analyzing the impact of changes in such policy and adjusting its manufacturing and sourcing strategy accordingly.
Foreign Currency Risk. We receive United States dollars for all of our product sales. Currently, all inventory purchases from our non-US contract manufacturers are also denominated in United States dollars; however, should we make purchases in foreign currencies in the future, purchase prices for our products may be impacted by fluctuations in the exchange rate between the United States dollar, which may have the effect of increasing our cost of goods in the future.
Commodity Price Risk. We are subject to commodity price risk arising from price fluctuations in the market prices of sourced titanium and steel products or the various raw materials components of its manufactured products. We are subject to commodity price risk to the extent that any fluctuations in the market prices of its purchased titanium and steel products and raw materials are not reflected by adjustments in selling prices of its products or if such adjustments significantly trail changes in these costs. We neither enter into significant long-term sales contracts nor enter into significant long-term purchase contracts. We do not engage in hedging activities with respect to such risk.
Credit Risk. Credit risk relates to the risk of loss resulting from non-performance or non-payment by counterparties pursuant to the terms of their contractual obligations. Risks surrounding counterparty performance and credit could ultimately impact the amount and timing of expected cash flows. Certain financial instruments potentially subject our company to a concentration of credit risk. These financial instruments consist primarily of cash and cash equivalents and accounts and vendor receivables. We place our cash and cash equivalents with high-credit, quality financial institutions. The balances in these accounts exceed the amounts insured by the Federal Deposit Insurance Corporation.
| 58 |
Overview
We are a revenue stage medical technology company focused on the development and commercialization of innovative treatment alternatives for patients with sleep disordered breathing (SDB), including mild-to-moderate obstructive sleep apnea (OSA). We believe our products and technology represent a significant improvement in the treatment of mild-to-moderate OSA versus other treatments such as continuous positive airway pressure (or CPAP) or palliative oral appliance therapies. We call our alternative and advanced treatment the Vivos System.
The Vivos System
The Vivos System is an advanced therapeutic protocol, which combines the use of customized oral appliance specifications developed by Vivos and prescribed by specially trained dentists in cooperation with their medical colleagues. We believe the Vivos System technology represents the first non-surgical, non-invasive and cost-effective treatment for people with mild-to-moderate OSA. Combining technologies and protocols that alter the size, shape and position of the tissues of a patient’s upper airway, the Vivos System opens airway space and can significantly reduce symptoms and conditions associated with mild-to-moderate OSA. Published studies have shown that using our customized appliances and clinical protocols led to significantly lower Apnea Hypopnea Index scores and improve other conditions associated with OSA. Our patented oral appliances have proven effective (within the scope of the U.S. Food and Drug Administration (or FDA) cleared uses) in over 15,000 patients treated worldwide by more than 1,200 trained dentists.
The Vivos System consists of combination of our patented oral appliance (the mRNA appliance®) with multi-disciplinary and proprietary clinical treatment protocols that has 510(k) clearance from the FDA as a Class II medical device for the treatment of snoring, mild-to-moderate OSA and SDB. We also market a specially designed and patented FDA Class I customized oral appliance (DNA appliance®) and a number of preformed pediatric oral appliances, which we call the Vivos Guides. For the treatment of mild-to-moderate OSA, the Vivos System and other Vivos products are typically delivered to patients by dentists specially trained to use the Vivos System to address certain craniofacial and morphological conditions commonly associated with SDB and mild-to-moderate OSA.
 |
 | |
| Vivos DNA appliance | Vivos mRNA appliance |
Sleep Apnea and the Role of Dentists in Treatment
The House of Delegates of the American Dental Association in 2017 adopted a policy statement describing the important role dentists can play in helping identify patients at greater risk of sleep related breathing disorders. By focusing our business model around dentists, we fulfill this role by training dentists and providing the support to use the Vivos System with their patients that suffer from mild-to-moderate OSA. Our program to train dentists and offer them other value-added services as described below is called the Vivos Integrated Practice (VIP) program. The VIP program provides dentists with a strong economic incentive to provide this treatment and prescribe the Vivos System, together with practice support services.
| 59 |
Sleep apnea is a serious and chronic disease that negatively impacts a patient’s sleep, health and quality of life. According to a 2019 article published in Chest Physician, it is estimated that OSA afflicts 54 million adults in the U.S. alone, and according to a 2016 report by Frost & Sullivan, OSA has an annual societal cost of over $149.6 billion. According to the study “Global Prevalence of Obstructive Sleep Apnea (OSA)” conducted by an international panel of leading researchers, nearly 1 billion people worldwide have sleep apnea.
The Vivos System is estimated to be effective in approximately 80% of cases of obstructive sleep apnea. Approximately 1 billion people globally suffer from OSA, and as many as 80% remain undiagnosed. Research has shown that when left untreated, OSA increases the risk of comorbidities, such as high blood pressure, heart failure, stroke, diabetes, dementia and other debilitating, life-threatening diseases.
Obstructive sleep apnea can range from mild to severe, based on a measurement system called the apnea-hypopnea index (AHI). The AHI is an index of the number of partial or complete airway blockages lasting 10 seconds or longer that a patient experiences in an hour. Studies have shown that the patented and proprietary technologies and protocols incorporated into the Vivos System technology alter the size, shape and position of the tissues that comprise the human airway. In 17 published, peer-reviewed studies (on which our founder and Chief Medical Officer was an author) that examined the impact of our technologies and protocols on the AHI scores of patients with varying degrees of OSA, patient AHI scores were reduced from a low of 38% to a high of 98.6%, with the mean AHI reduction shown in such studies being 67.4%. The results from published case reports and articles, together with patient-reported outcomes, have shown that our Vivos System therapy provides a significant reduction in the severity of patients’ mild-to-moderate OSA (as measured by industry standard indices such as the AHI among others), improvement in sleep-related quality of life, reduction in snoring, as well as a high patient compliance rates and a strong safety profile.
The treatment by a dentist of SBD and mild-to-moderate OSA with the Vivos System follows a required diagnosis of these conditions (typically through the use of either a polysomnogram (or PSG) or home sleep apnea test (or HSAT) by a medical doctor which is often provided by the sleep test provider.
VivoScoreTM, Powered by SleepImage®
In February 2021, we launched VivoScoreTM Powered by SleepImage®, a 510(k) cleared diagnostic technology for home sleep apnea testing featuring what we believe to be significant commercial advantages over existing HSAT products and technologies in the market. We believe VivoScore may enable healthcare providers to more efficiently screen, diagnose and initiate treatment for OSA in their patients which could result in more patients being treated with our Vivos System. While we anticipate increased revenue from VivoScore due to an expected increase in total patients tested and a corresponding increase in patient enrollment in Vivos System treatment, in arriving at this conclusion, we are relying on the results of a pilot test we conducted and other feedback from VIPs, which may or may not prove reliable on a broader scale.
VivoScore is a comprehensive home sleep apnea test that utilizes proprietary cardiopulmonary coupling technology developed by MyCardio LLC d/b/a SleepImage (“SleepImage”). VivoScore consists of a single-sensor ring recorder worn on the finger that works with a mobile phone application which facilitates a seamless data capture and upload and proprietary cloud-based algorithms to evaluate sleep quality and clinically diagnose sleep apnea. VivoScore test results have been shown to be comparable with overnight in-lab PSG tests. VivoScore creates comprehensive proprietary sleep quality measures, such as the Sleep Quality Index (or SQI), that we believe go beyond a mere clinical diagnosis for sleep apnea to more effectively manage treatment benefit and improve patient outcomes. With no consumables required, per test costs are significantly reduced, which is expected to allow for broad distribution and multi-night sleep evaluations using VivoScore that are often required by insurance carriers.
The SleepImage System, which is the underlining technology for VivoScore, has received 510(k) clearance for the purpose of evaluating sleep quality and to diagnose and manage sleep disordered breathing in both children and adults and may eliminate access and cost hurdles that may exist with other competing HSAT technology. Current estimates show that 80% of sleep apnea sufferers remain undiagnosed and untreated, creating a pressing need for an easy-to-use, clinical grade, low-cost HSAT for patients of all ages.
We are bringing VivoScore to market under a Licensing, Distribution, and Marketing agreement with SleepImage. This agreement is exclusive to our company with respect to white labeling of the VivoScore brand to the sleep dentistry market in the United States and Canada. Our agreement with SleepImage has an initial term of two (2) years and is subject to automatic one (1) year extensions, subject to the right of the parties to terminate the agreement prior to an extension. Either party also has the right to terminate the agreement (subject to applicable notice and cure periods) for customary matters such as breach of the Agreement or bankruptcy of a party. SleepImage also has the right to terminate the agreement under certain other circumstances, including a change of control of our company.
| 60 |
 |
 | |
| VivoScore home sleep apnea test | ||
Our Mission
Our mission is to rid the world of OSA. We believe we are well-positioned with what we consider to be a disruptive technology in our Vivos System aimed at treating mild-to-moderate OSA, with a clear first-mover strategy in penetrating the dental market as a means of treating OSA, compelling economics at each level of the delivery chain, and a talented team of experienced professionals who are passionate about what we do and driven to deliver results.
Our Market Opportunity
Estimates from publicly available information vary as to the extent of obstructive sleep apnea in the United States, but we believe the market is significant. According to a 2010 publicly available analysis from researchers at the Harvard Medical School Division of Sleep Medicine, mild obstructive sleep apnea is defined by an AHI between 5 and 15 and has a prevalence of 8-11% of the adult population in the United States. A 2004 study published in the Journal of the American Medical Association stated the prevalence of mild obstructive sleep apnea is one in five adults. Based on our analysis of the available public information, we estimate that approximately 15% of the adult population in the United States and Canada suffers from mild-to-moderate OSA. Based on the estimated total adult population of 284 million in the United States and Canada, we believe the total addressable United States and Canadian market is approximately 43 million adults. Our estimates set forth below relating to the intended uses of the Vivos System are also based in part upon data found in the study Oral Appliance Treatment for Obstructive Sleep Apnea: An Update, published publicly by the National Institutes of Health in 2014. Targeted treatment projections identified by this method of sleep titration were found to result in effective treatment in 87% of patients predicted to be successfully treated of OSA in an initial study. To be conservative and based on available data and our internal market analysis, we estimate that over 80% of individuals diagnosed with OSA in the North American addressable market may be candidates for the Vivos System, leaving us with a total addressable consumer market of over 43.2 million adults.
We currently charge clinicians an average sales price of approximately $1,600 per adult case for the Vivos System. There are approximately 160,000 qualified general dentists in the United States and Canada who could potentially offer the Vivos System to their patients. Based on the addressable US and Canadian consumer market described above and average sales price, we believe the addressable consumer market for adults in the United States and Canada is approximately $69 billion.
In addition, another published study, titled “Global Prevalence of Obstructive Sleep Apnea (OSA),” conducted by an international panel of leading researchers in 2007, reported that nearly 1 billion people worldwide have sleep apnea. Accordingly, we believe there is a substantial market opportunity for us outside the United States and Canada.
Our Treatment Alternative for OSA – the Vivos System
The Vivos System is a non-invasive, non-surgical, non-pharmaceutical, multi-disciplinary treatment modality for the treatment of mild to moderate OSA. The proprietary and virtually painless Vivos System enhances and increases the upper airway and offers patients what we believe to be an effective treatment alternative based on clinical retrospective data showing that some patients diagnosed with mild-to-moderate OSA, snoring and SDB symptoms are improving. Based on VIP and patient feedback we have received, we believe initial therapeutic benefits from using the device are often achieved relatively quickly (in days or weeks) and final clinical results are typically achieved in 12 to 24 months), all at a relatively low cost to consumers ranging between $7,000 and $10,000 for adults and $3,500 to $6,000 for children (costs vary by provider) when compared to other options such as surgery.
| 61 |
We believe that the Vivos System alters the size, shape and position of the tissues that surround and comprise the functional space known as the upper airway. This belief is based on retrospective raw data with validated before and after sleep studies and Cone Beam Computerized Tomography (CBCT) scans from treating clinicians and patient testimony. As the Vivos System treatment process progresses, the airway expands, with many patients reporting a significant reduction of their mild-to-moderate OSA symptoms. Our primary product used in the Vivos System is the mRNA appliance®, a specifically designed, custom oral appliance that is worn primarily in the evening hours and overnight and is available for adults. The total treatment time can range from 12 to 24 months with 18 months being the approximate mean treatment time. Our appliances require periodic adjustments some of which can be performed by the patient and others that are typically rendered at the dental office where treatment was initiated.
Patients who undergo treatment in the Vivos System will typically receive a customized mRNA appliance fitted to both the upper and lower arches. Alternatively, the VIP may prescribe an upper arch DNA appliance with the possibility of adding a lower arch DNA appliance later-on in treatment. Each case is priced accordingly, and patient fees are set by the treating dentist. It is not common for a given patient to be prescribed both an mRNA appliance and a DNA appliance. Irrespective of the Vivos device prescribed, each patient is given specific protocols and instructions for wear and maintenance, including the expected duration of daily wear (typically 14-16 hours per day including overnight). In addition to the oral appliance treatment, the patient may be referred for treatment by an orofacial myofunctional therapist, a chiropractor, an ear, nose and throat physician (“ENT”), and/or other healthcare providers for adjunctive therapy, as necessary. Each of these providers contributes to the overall treatment outcomes within the scope of their individual licensures. The Vivos System is a multi-specialty system that is collaborative with several related healthcare specialties such as those just listed.
Through the course of treatment with the Vivos System, patients have reported a variety of outcomes, including:
| ● | Reduction of snoring, | |
| ● | Reduction in AHI level and/or other indicators of mild-to-moderate OSA, | |
| ● | Relief of mild-to-moderate OSA symptoms, | |
| ● | Restoration and improvement of normal (nasal) breathing, | |
| ● | Improvement in overall sleep quality, | |
| ● | Reduction in the need for other lifetime treatment options such as CPAP, | |
| ● | Restoration and maintenance of proper facial symmetry and alignment, | |
| ● | Craniofacial and orthodontic correction, | |
| ● | Resolution of TMJ pain, clicking, and locking, and | |
| ● | Facial aesthetic improvement, including a broader smile and reduced ‘gummy smile’ |
The Vivos System has been specifically designed to promote the proper growth and development of the hard and soft tissues surrounding and comprising the oral cavity, nasal cavity, upper and lower jaws, and other tissues which together form and shape the airway. As these areas develop more fully using the Vivos System, a patient’s airway typically widens and expands (a process we call Pneumopedics®), enabling them to breathe properly through their nose. With a more open and less-obstructed airway and easier nocturnal breathing, the symptoms of SDB often diminish over time and patients often report no longer suffering from the adverse impacts of SDB or mild-to-moderate OSA. Use of the Vivos System is variable and case dependent but is typically recommended to be worn daily for 12 to 16 hours starting in the early evening and continuing overnight. During use, patients can typically talk (with minor difficulty), drink and swallow, but the device must be removed to eat. An example of the impact of Vivos System treatment on an upper airway is shown in the figures below depicting scans of the airway before and after treatment.
| 62 |
30-Year-Old Male | 14 Months
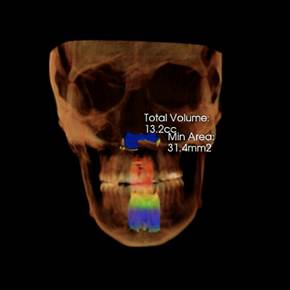 |
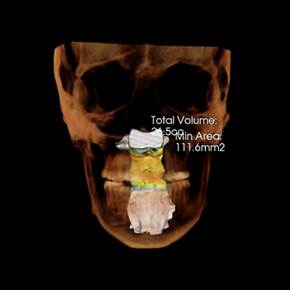 | |
| Before (March 2017) | After (May 2018) |
30-Year-Old Male – 14 Months Treatment. Before minimum airway area: 31.4mm2 – After minimum airway area: 111.6mm2. Before total airway volume: 13.22c – After total airway volume 26.5cc. (Imaging performed with no oral appliance in the mouth)
Often the cause of OSA is abnormal anatomical features of soft tissues and/or structures of the maxillomandibular skeleton that cause a disproportionate anatomy of the airway. Correcting the maxillo-mandibular skeletal and oral soft tissue structures can reduce obstruction of the upper airway, as shown above.
The Vivos System works to treat OSA as follows:
| ● | Published studies (including in the Austin Journal of Sleep Disorders by our Founder and Chief Medical Officer, Dr. Dave Singh, published October 16, 2014) have shown that the patented and proprietary technologies and protocols incorporated into the Vivos System alter the size, shape and position of the tissues that comprise the human airway, leading to lower AHI scores in patients with mild-to-moderate OSA. |
| ● | Our multi-disciplinary clinical approach often involves sleep specialist physicians, dentists, myofunctional therapists, chiropractors, and other healthcare providers. Each of these providers contributes to the overall treatment outcomes within the scope of their individual licensures. Our recently launched Medical Integration Division (MID) assists VIP practices establish clinical collaboration ties to local primary care physicians, sleep specialists, ENTs, pediatricians, pulmonologists and other healthcare professionals who routinely see or treat patients with sleep and breathing disorders. |
| ● | Retrospective evaluations of patients post treatment, as reported observationally by Vivos-trained clinicians, have not shown (where patient compliance with prescribed protocols has occurred) significant amounts of regression, resorption (a common type of dental injury or irritation that causes a loss of a part or parts of a tooth) or relapse in the majority of cases (although we have only very limited case report data to support this view). |
| 63 |
Our Growth Strategy
Our goal is to be the global leader in providing a clinically effective non-surgical, non-invasive, non-pharmaceutical, and low-cost alternative for patients with sleep disordered breathing, including mild-to-moderate OSA. We believe the following strategies will play a critical role in achieve this goal and our future growth:
| ● | Expand our North American (U.S. and Canada) sales and marketing organization to drive adoption of our Vivos System. We intend to rapidly and efficiently grow our sales and marketing organization in order to target and expand our network of Vivos Integrated Practices. | |
| ● | Drive medical and dental community awareness of Vivos System. We intend to continue to promote awareness of the value proposition of the Vivos System through training and educating dentists, physicians, and other healthcare providers. To accomplish this, we conduct regular online, national, regional and local training and educational programs for both the dental and medical communities. We intend to continue to publish additional clinical data in various industry and scientific journals and online and to present at various industry conferences. |
| ● | Continue to establish indirect marketing channels. We have entered and plan to expand strategic alliances within the medical and dental communities to increase awareness of our products. | |
| ● | Build patient awareness of the Vivos System. We also plan to continue building patient awareness through our direct-to-patient marketing initiatives which we anticipate will include celebrity endorsements, paid search, radio, television, social media, company sponsored events, corporate wellness programs, and online video. | |
| ● | Invest in research and development to drive innovation and expand indications. We are committed to ongoing research and development and we intend to invest in our business to further improve our products and validate our value proposition. We intend to invest in existing and next generation technologies to further improve our products and clinical outcomes, optimize patient acceptance and broaden the patient population that benefits from the Vivos therapy. We are in the early stage of initiating a prospective randomized clinical trial evaluating our mRNA appliance® evaluating the efficacy of the mRNA appliance® to treat mild-to-moderate OSA, SDB and snoring. The proposed study is described further below: | |
Proposed Study: Stanford University, Department of Sleep Medicine Purpose: To evaluate efficacy of the mRNA appliance® to treat mild-to-moderate OSA, SDB and snoring Design: Prospective randomized clinical trial. Trial duration: Approximately 30 months Randomization process: Case-control sample of 140 subjects, randomized on a 1:1 basis for continuous positive airway pressure (CPAP) or biomimetic device Inclusion criteria: Age over 21 years old to age 63; good compliance; good oral hygiene/dental health; sufficiently dentate in both arches Endpoints: AHI: RDI: ODI: SaPO2: %N3: %REM: Upper airway volume, Minimum cross-sectional area Lead investigator: Dr. Clete Kushida MD PhD Expected enrollment time: 6 months; proposed commencement of May 1, 2021 Expected date of completion: Summer 2023. Note: this study has received IRB approval from the Stanford University. | ||
| ● | Pursue strategically adjacent markets and international opportunities. We have trained dentists from many different countries all over the world. Obstructive sleep apnea is a disease that is prevalent worldwide, and we believe there is a significant opportunity for our products outside the United States. We have begun an initial assessment of the development and commercialization of the Vivos System for markets outside of North America, and we plan to conduct further strategic evaluation of such markets as we expand our market penetration throughout the United States and Canada. We also intend to explore strategic collaboration opportunities in Europe and the rest of the world in order to maximize the commercial potential and the availability of the Vivos System to patients. |
| 64 |
Our Revenue Model
Our revenue is derived from three primary sources, namely (1) VIP enrollment and training fees (comprised of one-time, up-front fees, as well as optional renewal fees after 12 months); (2) recurring Vivos System and Vivos Guides sales; and (3) recurring fees from practice management programs and strategic initiatives, each as described below:
VIP office training and enrollment fees. We derive revenue from one-time enrollment and training fees charged to new VIPs, which are dental practices specially trained by us in the use of the Vivos System. We have three VIP program pricing options which we refer to as Tier 1, Tier 2 and Tier 3. Our Tier 1 fees are currently set at $50,000 for the main practice provider plus $10,000 for each associate doctor (although such fees for the main practice provider can be discounted to $40,000, while the associate fees are not typically discounted and are the same across all tiers). Tier 2 pricing reflects a one-time enrollment fee of $25,000 coupled with a 30% price premium on appliances, and Tier 3 pricing reflects a $12,500 one-time enrollment fee coupled with a 50% price premium on appliances. The one-time enrollment fee provides VIPs with extensive clinical and business integration training, including training on matters such as billing and marketing, through our Institute for Craniofacial Sleep Medicine. For additional subscription fees described further below, VIPs can sign up for our Billing Intelligence Services (BIS) under which the VIPs outsource their medical credentialing, pre-authorizations, billing, and payer collections functions to us. On average, our revenue from VIP enrollment fees currently is approximately $28,000 per VIP.
A new VIP dentist typically achieves 2 to 4 new cases per month within 12 months after receiving training, with a mid-term target of 4 to 6 cases per month and a long-term target of 10 cases per month. At this average level of production and profit margin, VIP providers can expect to see a full payback of their investment well within 18 months after they complete their training. In addition to the Vivos training enrollment fees, all VIP practices are strongly advised to have Cone Beam Computerized Tomography (CBCT) equipment that meets certain criteria available at their practices. These machines have many uses in dentistry such as with implants, orthodontics, and routine diagnostics, and are critical in the diagnosis and treatment planning with the Vivos System. The return on such an investment is seen by the relatively high gross margins available to VIP providers. See “Recurring Vivos System and Guide Sales” below. According to the largest dental industry supplier, Henry Schein, within the typical general dental practice, there are well over 400 patients with OSA. After the first year, dentists may renew their access to the Institute for a reasonable monthly subscription fee.
Recurring Vivos System and Guide sales. Trained VIPs pay us an average adult case fee of approximately $1,600 per case, and $400 for a pediatric Guide case. We maintain average gross margins in excess of 70% on both adult and pediatric cases. In turn, VIP offices typically charge adult patients fees ranging from $7,000 to $10,000, and $3,500 to $6,000 for pediatric cases. We estimate that fully burdened costs to the VIP practice range from between $1,500 (pediatric Guides) and $3,000 (adult mRNA appliance®) per case. Thus, VIP providers also have compelling unit case economics with relatively high gross margins. In addition, VIP providers also have the option of incorporating MyoCorrect therapy into their treatment protocols. We expect this will add approximately $1,000 - $1,200 per OMT case to revenue with gross margins comparable to our other appliance product offerings.
Recurring VIP Subscription Fees. Ongoing renewal access to our Institute for Craniofacial Sleep Medicine (as described further below) and online training courses after first 12 months as a VIP are estimated at $595 per month and started in the first quarter of 2021. Due to our extensive use of online broadcasting and training delivery, we believe incremental training costs to scale and accommodate additional VIP providers will not be significant. Nevertheless, we do have costs associated with paying professional lecturers, acquiring and recording fresh new content, and constant upgrades to our curricula and course offerings. In addition, we do have an in person ICSM training facility currently under lease near Denver, Colorado with certain fixed and variable costs.
The Institute for Craniofacial Sleep Medicine. Our Institute for Craniofacial Sleep Medicine (ICSM) provides advanced post-graduate education and certification in the emerging science of Pneumopedics® and product-specific training for the use of Vivos products and services. Certain adjunctive courses, such as orofacial myofunctional training and certification are offered through the ICSM at an additional cost. Revenue from such courses is not material at the present time.
| 65 |
The Airway Intelligence Service (AIS.) This service provides a complete resource for VIPs to help simplify the diagnostic and appliance design matrix and expedite the treatment planning process. AIS is provided as part of the price of each appliance and is not a separate revenue stream. We believe that this value-added service included with every new case start is a major differentiator between our higher cost products and other lower cost oral appliances on the market.
Billing Intelligence Services (BIS). This complete billing solution includes a comprehensive integrated revenue cycle management software system that allows dentists to focus on running their practice and delivering the best care for their patients. Our medical billing service generates recurring subscription fees from participating VIPs (Silver Package: $795 setup fee and $795 per month, Gold Package: $1,800 setup fee and $895 per month, or Platinum Package: $2,800 set up fee and $995 per month) This important adjunctive service is priced competitively and allows VIP offices to outsource a key back-office function without adding one or more full time employees.
AireO2 Patient Management Software. This management software enables healthcare professional to diagnose, treat and monitor patients with OSA and its related conditions more effectively. Developed in collaboration with Lyon Dental, AireO2 contains features that enhance a VIP’s billing services and practice management systems. AireO2 is a complement to our BIS software system. On April 14, 2021, we entered into an asset purchase agreement with Lyon Management and Consulting, LLC and its affiliates to acquire certain medical billing and practice management software, licenses and contracts, including the software underlying AireO2. The asset acquisition allows us to expand and enhance our current medical billing practice through our BIS division. The terms of the purchase include $225,000 of cash and the issuance of a warrant to purchase 25,000 shares of our common stock at a price of $8.90 per share for three years. The vesting of the warrant is as follows: 5,000 shares vested immediately upon issuance of the warrant, 10,000 shares vest and become exercisable on April 14, 2022 and 10,000 shares vest and become exercisable on April 14, 2023.
Medical Integration Division (MID). In 2020, we launched our MID to assist VIP practices to establish clinical collaboration ties to local primary care physicians, sleep specialists, ENTs, pediatricians, pulmonologists and other healthcare professionals who routinely see or treat patients with sleep and breathing disorders. The primary objective of our MID is to promote the Vivos System to the medical profession and thus facilitate the potential for more SDB and OSA patients gaining access to the Vivos System, which we believe can improve quality of life and can reduce overall health risks experienced these patients. The MID seeks to fulfill that objective by meeting with VIP dentists and physicians in their local areas to establish physician practices using the trademarked name “Pneusomnia Craniofacial Sleep Medicine Center” (Pneusomnia Center). These independent medical practices will be set up as LLCs or subchapter S corporations owned by a small group of independent physicians, co-located in the dental practice of the VIP dentist, and managed by our company under a management and development agreement. The physicians will capitalize the physician owned medical practice through an initial investment (which totals approximately $100,000) and appoint a wholly-owned subsidiary of our company as Manager under a long-term Management Services Agreement which pays us six (6%) percent of all net revenue from sleep-related services. The treating dentist will sub-lease a portion of the space in his or her dental practice to the physician practice. He or she will also contract through a professional services agreement with the physician practice as a contract provider to treat patients at a fair market value rate to provide professional services. The difference between the fees paid by patients and the contract rate paid by the physician practice to the treating dentist is designed to give the physician practice a margin of profit that will allow the physician practice to pay expenses and potentially generate a cash flow for the physician owners. Owner doctors will receive profit distributions from their limited liability companies or subchapter S corporations based solely on their ownership percentage and will not be compensated for patient referrals in any way. We have built into our core MID business model a great degree of flexibility, such that elements of each Pneusomnia Center as described above may change and be adapted to local state laws and regulations, and entity formation laws as any such alterations do not violate any state or federal statutes or regulations.
We believe our early market response from MID activities has been promising, and in March 2021 we announced the opening of the first Pneusomnia Center in Del Mar, California as well as plans to open additional Pneusomnia Centers in several other cities in the U.S. However, it remains too early to predict the eventual impact on our overall revenue. If successful, the MID is expected to enhance the overall practice level economics for independent VIP offices and generate additional lines of recurring revenue for us.
MyoCorrect (Orofacial Myofunctional Therapy) Program. In March 2021, we introduced orofacial myofunctional therapy (or OMT) as a service under the name MyoCorrect. Through MyoCorrect, dentists enrolled in the VIP program will have access to trained therapists who provide OMT via telemedicine technology. This OMT therapy will be a component of obstructive sleep apnea treatment in conjunction with our Vivos System oral appliances and protocols. OMT, which is given by a certified OMT therapist, involves exercises and other techniques aimed at strengthening the tongue and orofacial muscles by teaching individuals how to engage the muscles to the appropriate position. Some VIPs have reported improvements in case acceptance, patient compliance, treatment times, and clinical outcomes when active OMT is incorporated into the Vivos System treatment protocols. Typically, VIPs who prescribe OMT for their patients have had to search out and contract with an independent certified OMT provider at costs between $1,600 and $2,500 depending on the region. MyoCorrect will be a part of the ecosystem of services provided by us to VIPs and leverages a telemedicine model that allows for the treatment to be delivered at a cost-effective initial price point of $1,000 - $1,200.
| 66 |
The launch of MyoCorrect follows our March 29, 2021 acquisition of certain assets from, and the entry into related agreements with, MyoCorrect, LLC and its affiliates, which affiliates include an existing VIP dentist who will administer the MyoCorrect program and a certified OMT therapist who has been hired by us to recruit and train OMT therapists and promote the MyoCorrect program throughout the VIP network. The VIP dentist and OMT therapist are also entitled to a fee for each MyoCorrect case (capped at an aggregate amount). We have also issued to the OMT therapist warrants to purchase 200,000 shares of our common stock with an exercise price of $7.50 per share. 25,000 of these warrants vested initially upon issuance, but the remainder only vest and become exercisable upon the achievement of pre-determined performance metrics related to the utilization of MyoCorrect.
Vivos Centers. Finally, we derive a relatively small amount of revenue from the management of two (2) clinics in Colorado (which we call the Vivos Centers) where dentists and other healthcare professionals treat patients using the Vivos System. As a company, we are not in the business of treating patients per se, as this occurs only through dentists and other professionals, operating within the scope of their respective licenses, who, among other services, prescribe and treat patients using the Vivos System and/or Vivos Guides. We thus have no direct control over patient intake or clinical care at our Vivos Centers. Our role is limited to training and educating dentists and their staff, and to fulfilling orders placed for the Vivos System and/or Vivos Guides.
While operating Vivos Centers through licensed dentists and other healthcare professionals was the main aspect of our business model prior to July 2018, the Vivos Centers are not currently our core business, but rather a means by which we derive hands-on assessments and field intelligence from the use and practice of the Vivos System in actual clinical settings. As such, we may dispose of one or more of the Vivos Centers in the future, as was the case in October 2019 when we sold one Vivos Center located in Orem, Utah. In our current business model, our core revenue drivers are enrollment and renewal fees from VIP clinical education and office training, sales of the Vivos System and other appliances, and subscription fees from BIS services as described above.
Patient Advantages
We believe the Vivos System offers the following patient advantages:
| ● | Reduce or possibly eliminate the need for surgery or lifetime CPAP or mandibular advancement therapy |
| ● | Non-invasive, non-surgical and non-pharmaceutical treatment of OSA |
| ● | Comfortable and easy to wear and to comply with treatment protocols |
| ● | No known material side effects (minor spacing between teeth, bite changes, etc. are all minor and easily addressed) |
| ● | Average treatment is 12 to 24 months for most cases |
| ● | Affordable (typically $7,000-$10,000 for an adult case and $3,500 to $6,000 for a child case) |
| ● | Adults covered by most major medical insurance plans up to 70% (average is about 50%) |
| ● | Treatment effective (for its FDA cleared uses) |
| ● | Restoration and maintenance of craniofacial symmetry |
| ● | Improved facial aesthetics (stronger jawline, reduce or eliminate “gummy” smiles) |
| ● | Near term benefits (no waiting for months to see improvements) |
| ● | U.S. patented 3D axial springs™ and screw mechanism for patient adjustment |
During the course of treatment with our Vivos System, patients have reported the following adverse effects that include, but are not necessarily limited to:
| ● | Excessive salivation or drooling (especially during the first few days or weeks of use) |
| ● | Changes in dental occlusion (typically corrected at the end of treatment with clear aligners) |
| ● | Increases in interproximal spacing between teeth (typically corrected with clear aligners or veneers) |
| ● | Minor mouth or tooth soreness or pain that often results from the use of any intraoral device |
| ● | TMJ or bite changes |
| 67 |
Patient Treatment Process
Most potential patients learn they may be a possible candidate for OSA therapy through physician referral, education and advertising campaigns, and/or dentist examinations. Some useful predictive information can be obtained from self-reported questionnaires given to the patient in advance of a formal evaluation, and this procedure may simplify the clinical assessment of patients. The most widely used of such questionnaires are the Berlin Questionnaire and the Multivariable Apnea Prediction Index.
If a VIP dentist determines that a patient may have OSA, they will refer the patient to complete either a VivoScore or other home sleep apnea test (which could be our VivoScore test) or a full polysomnography, which provides detailed information on sleep state, respiratory behavior and gas exchange abnormalities, in addition to a range of other variables including body position, heart rate and rhythm, and muscle tone and activity. The sleep test will be reviewed, and a diagnosis of the test will be given by a medical doctor; usually by a doctor that specializes in sleep, a pulmonologist or a cardiologist.
If a patient is diagnosed with sleep apnea from the reading of the home sleep apnea test or polysomnography test and is a candidate for oral appliance therapy, additional data will be recorded including a CBCT imaging scan. After obtaining a prescription from a physician, the VIP dentist will design a treatment plan and present the case to the patient. Upon treatment acceptance, the financial arrangements will be organized including insurance pre-authorization and/or any deposits and payment plan agreements. The VIP dentist will design the appliance(s) based upon treatment protocol and order the appliance through our cloud-based portal that we call Vivos Aire.
Fabrication of the Vivos System appliances usually takes between two to four weeks for delivery. Upon receipt of appliance(s) by the VIP dentist, the patient will visit the dentist for an appliance seating and delivery appointment. Routine follow-up lasts for the 12 to 24 months of treatment.
Upon determination of treatment completion, the patient will take a post-treatment home sleep apnea test, such as VivoScore, or a polysomnography test. Post treatment CBCT imaging will be taken to compete the patient’s treatment and records profile.
Competition
Our industry is subject to significant competition and rapid change from the introduction of new products and technologies and other activities of industry participants. We compete as a first-line therapy in the OSA treatment market for patients with mild to moderate OSA. There are several treatment options for patients with OSA depending on the level of severity of the disease, ranging from lifestyle changes to surgery. The goals of therapy are to resolve signs and symptoms of OSA, improve sleep quality, normalize and reduce the AHI, and generally increase SpO2 (blood oxygen saturation) levels. CPAP therapy is typically considered the first-line standard of care of therapy for adults with OSA; however, decreased patient adherence lessens the benefits of CPAP therapy. Common reasons cited for lack of adherence is trouble getting used to wearing the CPAP device, difficulty tolerating forced air, dry and stuffy nose, feeling claustrophobic, skin irritation, pressure sores, leaky mask, dry mouth, bothersome noise, chronic bacterial and respiratory infections, and lack of intimacy. According to published research, many patients with mild-to-moderate OSA, who prefer not to use CPAP, use mandibular advancement devices (or MAD) oral appliances as an alternative therapy; however, treatment with MADs comes with its own set of adverse side effects, including dry mouth, dental caries, TMJ pain and sounds, soft tissue and tongue irritation, excessive salivating, occlusal changes, damage to teeth or restorations, and tooth mobility, among other effects.
CPAP is a therapy often prescribed by medical doctors for patients with OSA. CPAP is delivered through a face or nasal mask that connects through a hose to a bedside air compressor. The CPAP machine forces air into the nasal passages at pressures high enough to overcome obstructions in the airway and facilitate normal breathing. The effectiveness of CPAP has been limited by low patient compliance due to claustrophobic sensations, discomfort with the constant air pressure, irritation from an ill-fitting mask, embarrassment in front of a bed partner, machine noise, skin irritation, dry mouth, sinus infections, nausea, acid reflux, and depression about having a sleep disorder. CPAP therapy is a palliative solution to OSA. It can relieve symptoms but does not address the underlying cause. When CPAP therapy is discontinued, patients typically revert back to having OSA.
Another palliative solution to OSA is a mandibular advance device. MADs are oral appliances used to treat mild-to-moderate OSA. MADs are used with the intent of moving the lower jaw and tongue base forward and/or preventing the tongue from moving back into the throat or the oropharynx. This specific action has the effect of opening the airway, thereby minimizing or preventing snoring and/or airflow compromise leading to OSA. Forward jaw posturing, maintained over several hours repeated daily, however, is not normal, and can lead to a number of adverse side effects, including but not limited to, dental caries, dry mouth, tooth discomfort, temporomandibular joint dysfunction (TMD or TMJD), craniofacial pain, muscular discomfort, malocclusion (bite changes), tooth movement, and more.
| 68 |
CPAP, MADs and other products on the market that non-surgically address SDB and OSA are palliative therapies that temporarily treat the symptoms only, which may worsen over time. We believe these therapies are not designed or intended to address or resolve the tissue obstruction(s) which, in the opinion of some researchers, constitutes the potential root cause(s) of the disorder in up to 98% of patients with OSA. CPAP and MADs require lifetime nightly use to be effective. Conversely, a number of published studies show that by addressing the potential root cause of OSA in many patients, we believe the Vivos System may offer patients the very real hope of a more effective solution to their OSA that can be accomplished in about 12 to 24 months.
The follow graphic depicts what we believe to be the competitive landscape for the Vivos System:
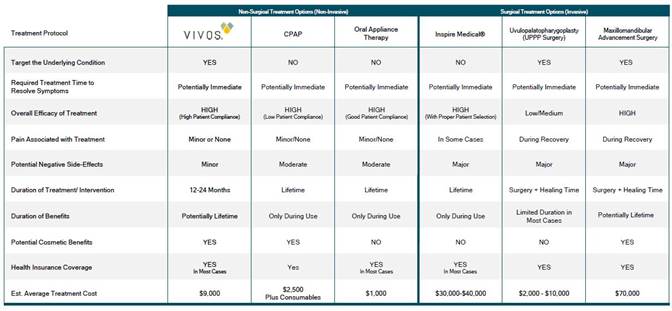
We believe that the leading SDB and OSA treatment modalities fall into the following categories:
| ● | Continuous Positive Airway Pressure (CPAP): This device is generally regarded as the first-line standard of care treatment of OSA by the medical community. However, according to published research, an estimated 29 to 83 percent of patients are nonadherent to CPAP therapy, with non-adherence defined as a mean of less than 4 hours of use per night. CPAP devices reportedly have 85% of the market share of those who are diagnosed with OSA according to Frost & Sullivan. |
| ● | Mandibular Advancement Devices (MADs): These oral appliances open the airway by moving the mandible (the lower jaw) forward and holding it there. This jaw position tends to open the airway and allows patients to breathe more freely during sleep. According to a published presentation, “Oral Appliances in Today’s Treatment of OSA and Snoring”, there are over 100 different brands and several configurations of MADs available through dentists, and an unknown number of over-the-counter devices (which purportedly treat snoring only). |
| ● | Other: Weight loss, position therapy, myofunctional therapy, certain orthodontic treatments, surgical implants such as Inspire, and maxillofacial surgery are other options to address OSA. |
We consider our primary competition, both within and outside of the United States, to be both CPAP and other oral appliance products (all of which represent variations on the same mandibular advancement device platform) typically delivered by licensed dentists, such as SomnoMed, DynaFlex, TAP, EMA, and Herbst (which are FDA cleared) as well as ALF, Homeoblock and FAGGA (which are not FDA cleared). According to the American Sleep Apnea Association, over 100 different oral appliances are FDA cleared for the treatment of snoring and OSA. We believe other emerging businesses are in the early stages of developing mandibular advancement or other oral appliance devices which incorporate novel technologies.
| 69 |
To a lesser extent, we also compete with surgical therapies such as Uvulopalatopharyngoplasty (UPPP), maxillomandibular advancement (MMA), robotic tongue reduction surgery, and Inspire Medical implants. While we compete with CPAP in general as an alternative treatment for mild-to-moderate OSA, we believe the Vivos System is a superior alternative given its relative safety, comfort, ease of use and the potential to resolve underlying conditions. In addition, the Vivos System is suitable for patients who cannot tolerate CPAP or for whom CPAP has not been effective. In certain cases, clinicians may temporarily treat patients using a combination of the Vivos System and CPAP.
As highlighted in the chart above, a patient who is diagnosed with OSA faces two primary treatment pathways—non-surgical and surgical. The Vivos System, CPAP, and mandibular advancement oral appliances are examples of non-surgical treatment options. Inspire Medical Systems implants, UPPP surgery, and Maxillomandibular Advancement surgery are examples of surgical treatment options. Each treatment option offers patients potential benefits and risks at a different price point.
We believe the Vivos System offers patients several important advantages. Treatment in the Vivos System is typically limited to a defined period of time (12-24 months), whereas both CPAP and oral appliance therapy require lifetime nightly use to be effective. Treatment in the Vivos System also addresses the underlying anatomical cause of the OSA, whereas both CPAP and oral appliances are palliative and effective only for temporary relief of symptoms while the devices are being used. Neither treatment purports to correct the underlying tissue and structural anomalies that give rise to the OSA condition in the first place. Long-term compliance in both alternative non-surgical protocols can be challenging. Yet once treatment in the Vivos System is complete, no further intervention is necessary, in most cases.
Inspire Medical Systems’ primary treatment for OSA involves surgical implant devices that seek to temporarily remove airway obstruction by moving the tongue forward via an electrical stimulation. These devices relieve OSA symptoms and lower AHI scores, but pose the added cost and risks of surgery, and must be used nightly over the patient’s lifetime in order to be effective. The Vivos System avoids the cost and risk of surgery, and is less costly for both patients and insurance carriers than surgical options. The Vivos System is thus far less dependent on insurance reimbursement for patients to be able to afford treatment.
We believe that the primary competitive factors in the OSA treatment market are:
| ● | company, product and brand recognition; |
| ● | product efficacy, safety, reliability and differentiation; |
| ● | third party medical / dental insurance reimbursement availability; |
| ● | dedicated practice development and clinical training teams; |
| ● | treatment time duration, product ease of use, patient compliance, and patient comfort; |
| ● | procedure costs to patients; |
| ● | quality and volume of clinical data; |
| ● | education of patients, dentists, physicians and sleep centers; |
| ● | sales force experience and access; |
| ● | technological innovation, product enhancements and speed of innovation; and |
| ● | pricing and revenue strategies. |
Most of the other OSA treatments against which we compete have a greater penetration into the OSA treatment market. Mandibular advancement oral appliances and a variety of surgical treatments are better known to ENT physicians, sleep centers, dentists, and the other physicians on whom we may rely for referrals, but we believe dentist and physician awareness of our Vivos System therapy is increasing.
| 70 |
Our Competitive Strengths
We believe that the Vivos System has numerous advantages that, taken together, set us apart from the competition and position us for success in the marketplace:
| ● | Significant barriers to entry: We believe that third parties seeking to compete directly with us have significant barriers to entry for the following reasons: competitors must offer a treatment modality with similar features, capabilities, research support, FDA regulatory clearances, and successful clinical outcomes in the market; then establish a comprehensive educational training program featuring other clinical professionals with actual experience and success using that particular treatment modality to properly educate dentists on all clinical aspects of use with patients; then develop and promulgate the systems and best practices required to successfully integrate the treatment of mild-to-moderate OSA using this novel treatment modality in a dental practice; then establish and provide, by recruitment and otherwise, ongoing clinical mentoring and support to dentists engaged in treating their patients for mild-to-moderate OSA and related conditions (clinical mentors are limited and may be hard to find); and finally, assisting the dentists with case selection, case acceptance, patient financing, and medical insurance reimbursement.
We believe we have strategically and effectively addressed each and every one of the aforementioned barriers to entry, and thus have created a novel and compelling single-source value proposition for dentists seeking to deliver OSA treatment to their patients. | |
| ● | Vivos System insurance reimbursement: Most major commercial insurance payers reimburse for our adult treatment in the United States. The average level of reimbursement is approximately 50% (with coverage ranging from 5% to 70%), although medical insurance is never a guarantee of payment, and patient deductibles and policy restrictions will vary. | |
| ● | Body of published research and strong patient outcomes: Together with our network of trained dentists, we have developed a body of clinical and patient data over approximately ten years and an estimated delivery of approximately 15,000 appliances that demonstrates the safety, effectiveness, therapy adherence (patient compliance), and benefits of the Vivos System for its FDA cleared and registered uses. The documented and reported benefits of treatment with the Vivos System have been consistent across reports from dentists, and have been highlighted in approximately 55 published studies, case reports, and articles, most of which have been peer reviewed. We believe this favorable data provides us with a significant competitive advantage and will continue to support increased adoption. |
| ● | First mover advantage: Our business model is the first to focus on dentists screening patients for mild-to-moderate OSA and SDB, referring patients to physicians for diagnosis, with the dentists then serving as the primary source of treatment using the Vivos System for such patients. In addition, we provide VIPs not only with our novel treatment technology and protocols, but also programs to support and incentivize broad case acceptance. We are the first company to offer individuals diagnosed with mild-to-moderate OSA access to the Vivos System via our VIP dentists across the United States and Canada, whereby patients can receive much-needed treatment that offers many of them a potentially better option than CPAP and/or MADs. We believe our focus provides us with a significant first mover advantage and momentum over future competitors, as we have an estimated 1,200 dentists trained in the proper use of the Vivos System. | |
| ● | Differentiated products: The dental profession’s historical and current contribution to the treatment of OSA has almost exclusively been via the fitting of MADs. To our knowledge, only the Vivos System offers a truly differentiated, non-invasive treatment option that actually works on a common root cause of the condition. MAD-type oral appliances are typically less expensive, but do not reshape the upper airway like the Vivos System, and therefore require nightly use over a lifetime, and have a number of other disadvantages. | |
| ● | Intellectual property portfolio and research and development capabilities: We have a comprehensive patent portfolio to protect our intellectual property and technology, with five design patents that expire between 2023 through 2029 and two utility patents expiring in 2029 and 2030. We also own two Canadian patents and one European patent that has been validated in Belgium, Switzerland, Germany, Denmark, Spain, France, United Kingdom, Hungary, Italy and the Netherlands, all of which expire in 2029. Our U.S. trademark portfolio consists of ten registered marks and three pending trademark applications. Extensive online and in-person training, multiple touch point support systems, specific fabrication materials, customized appliance designs, and multi-disciplinary treatment protocols are all considered proprietary trade secrets and competitive advantages with no known counterparts. |
| 71 |
| ● | Extensive Training and Support Systems: We believe our extensive online and in-person clinical and business systems training program offered through our Institute for Craniofacial Sleep Medicine (ICSM) is unmatched anywhere in dentistry and is a clear competitive strength that would be difficult to replicate. Our integrated network of clinical advisors, market advisors, and practice advisors is comprised of experienced and dedicated individuals with proven abilities to mentor, consult, and drive new case starts within the specific environment of a dental practice. The collective experience, training, and performance of such a broad network of individuals would be difficult to replicate and represents a core competitive strength. |
| ● | Targeted approach to market development: We have established a systematic and scalable approach to actively and consistently engage with our primary target audience of U.S. and Canadian dentists. In addition, our recently launched MID is actively targeting physicians and other relevant healthcare providers in order to build awareness and collaborative patient options at our VIP practices. Since the end of January 2020 our Continuing Education Department has offered over 200 education courses through continuing education Zoom seminars, with total registration of more than 44,000 medical and dental professionals with over 32,000 continuing education certificates distributed. Our sales force is focused on building long-lasting relationships with dentists as we support their practices through all aspects of the Vivos System treatment protocol. We highlight our compelling clinical data and value proposition to increase awareness and adoption by the medical community. We are confident that our approach to engagement across multiple channels will continue to drive increased awareness of and demand for our Vivos System. | |
| ● | Marketplace acceptance: Patient access to the Vivos System at a VIP practice is rapidly becoming readily available, and active VIP providers can now be found in almost all major US cities and in many cities in Canada. The Vivos System and other company products are in the marketplace, with growing acceptance among dentists and other healthcare providers. |
Sales and Marketing
We have established a methodical approach to market development which centers on active engagement directly with members of the medical community, including general dentists and medical doctors who treat SDB and OSA, to educate them on the Vivos System and its benefits. The goals of our sales and marketing efforts are (i) secure new VIP dentists provide them with the tools to treat patients with our products and (ii) more broadly educate the medical community regarding our products with a view towards expanding our number of VIPs as well as medical professionals who could refer SBD and OSA patients to our VIPs for treatment.
We sell the VIP Program to dentists through a direct sales force that primarily targets general dentists in the United States and Canada. Our VIP program was developed to train dentists to identify and treat conditions associated with SDB and mild-to-moderate sleep apnea. Our sales program to target medical doctors is our recently launched MID program, which was developed to assist VIP practices to establish clinical collaboration ties to local primary care physicians, sleep specialists, ENTs, pediatricians, pulmonologists and other healthcare professionals who routinely see or treat patients with sleep and breathing disorders.
We sell our VIP program to dentists in the United States and Canada. In countries outside of North America we typically offer a modified training and support program at a lower cost. We currently have approximately 10 direct sales representatives in the United States and Canada. Our direct sales force engages in sales efforts and promotional activities focused on referring physicians, as well as directly to the over 147,000 professionally active general dentists in the United States and the 13,000 general dentists in Canada.
Our current VIP sales organization is comprised of:
| ● | one Enrollment Specialist, who is the primary salesperson responsible for enrolling new VIPs; |
| ● | two Enrollment Support Staff members, who are responsible for organizing potential VIP appointments for Enrollment Specialist; |
| ● | three Business Development Associates, who are responsible for cultivating new business leads which are referred to the Enrollment Support Staff); |
| 72 |
| ● | one Outreach and Engagement Associate, who is responsible for engaging with potential VIPs in our sales process with surveys and offers of online courses with the purpose of leads to be referred to the Enrollment Support Staff members; and |
| ● | one Practice Advisory Onboarding Specialist, who is responsible for onboarding new VIPs to our training programs. |
Our MID sales organization is comprised of a Senior Vice President that leads the MID sales efforts and one Senior Director of Business Development. We plan on growing our MID sales organization by recruiting candidates that have extensive healthcare backgrounds, strong business development experience setting up physician owned medical facilities/practices and significant healthcare regulatory knowledge.
Our VivoScore sales organization is comprised of a Senior Vice President that leads the VivoScore sales efforts, one Director and two support employees. VivoScore is a strategic initiative that we anticipate will increase revenue due to an expected increase in total patients tested and a corresponding increase in patient enrollment in Vivos System treatment. We plan on growing our VivoScore sales organization by recruiting candidates that have extensive healthcare backgrounds, strong business development experience setting up physician owned medical facilities/practices and significant healthcare regulatory knowledge.
We utilize indirect and direct marketing channels to inform and educate dentists, medical doctors and healthcare professionals about the Vivos System. Our indirect marketing channels include strategic partners, industry key opinion leaders, trade shows and our own clinical advisor network. Our direct marketing channel includes outreach to prospective VIPs using digital advertising platforms including Facebook and Google ad placements. The objective of our indirect and direct marketing efforts are to bring dentists, medical doctors and healthcare professionals to our educational and training websites to learn about SDB, OSA and treatment alternatives.
We believe our dentist and medical doctor marketing efforts have been effective in facilitating contact via our Vivos introduction and online training webinars, particularly during the COVID-19 epidemic.
Potential Economics for Trained VIP Clinicians
Dentists that enroll in our VIP program have favorable economics. The actual incidence of dental patients with OSA will vary, but our conservative estimate would suggest the average dental practice sees 400-500 adult patients with a high risk of suffering from obstructive sleep apnea. Using these demographic figures, the economic potential per dentist may be calculated, based on a retail adult case fee of approximately $9,000, fully burdened VIP provider costs of approximately $3,000, and net profit of approximately $6,000, to be over $3,600,000 in annual gross revenue potential annually with over $2,400,000 in potential net profit. We believe based on our experience that dentists have seen accretive economic additions to their practices with the Vivos System, and thus the VIP program can likely add to the doctor’s take-home income. Our sales and clinical advisory dentists conduct training primarily in a highly personalized, deep immersion workshop format at our Institute for Craniofacial Sleep Medicine. The key topics covered in training include case selection, clinical diagnosis, treatment planning, appliance design, adjunctive therapies, instructions on ordering Vivos products, guidance on pricing, case acceptance, instruction on insurance reimbursement protocols and interacting with our proprietary software system and the many other features of our website. We present our training material in a manner we believe to be superior to most other dental training and experience. As a result, we are able to complete the initial training workshops, both online and in person, typically within just 15 days spread out over several weeks. Our success in training approximately 1,200 dentists confirms our belief that training represents a minimal barrier to adoption for most dentists.
Below is an illustrative model depicting the total additional revenue a dentist might receive by treating patients with the Vivos System. The potential patients with OSA is determined by using a calculation that results in a conservative estimate that 30% of patients of a dental practice patient may suffer from OSA (according to a 2019 article published in Chest Physician). The revenue treatment fee is estimated at $9,000 per patient. This illustration helps to explain why a dentist might want to become a trained VIP and use the Vivos System.
Number of Active Patients in Typical Dental Practice | Potential Patients with OSA | Potential Additional Revenue for Dentist | |||||||
| 1,250 | 375 | $ | 3,375,000 | ||||||
| 1,500 | 450 | $ | 4,050,000 | ||||||
| 1,750 | 525 | $ | 4,725,000 | ||||||
| 2,000 | 600 | $ | 5,400,000 | ||||||
| 2,250 | 675 | $ | 6,075,000 | ||||||
| 73 |
To facilitate the adoption of the Vivos System, we market the VIP Program, and as part of that offering, we often partner with equipment manufacturers to bundle training and equipment into a turn-key program financed by third party lenders for those dental practices who need to purchase additional equipment. The VIP Program fees are also often financed by third party lenders separate from any equipment purchases. Loan terms and payments will vary depending on the doctor’s credit, the interest rate, the amount financed, and the term of the loan. Generally, payments on such financing range from about $600 to $2,500 per month.
Insurance Reimbursement
Our mRNA appliance® is a custom fabricated appliance to treat mild-to-moderate OSA, SDB and snoring in adults. The mRNA can be billed in and out of network to most commercial payers under the E0486 CPT code. The E0486 is reimbursable by many major commercial medical payers following a medical diagnosis of OSA. Level of reimbursement is approximately 50% (ranging from 5% to 70%), although medical insurance is never a guarantee of payment, and patient deductibles and policy limitations may vary. A verification of benefits is required for all medical policies to check for validity of CPT code E0486 and oral appliance therapy (OAT). Pre-authorization may be required for reimbursement. Pre-Authorization requirements may vary based on the payer policies and patient’s insurance coverage. Although many patients pay for treatment out of pocket on a fee for service basis, the availability of health insurance coverage is an important consideration for many patients who desire treatment in the Vivos System. All medical policies have different reimbursement policies which may affect availability of reimbursement.
VIPs typically remain out of network with commercial health insurance payers, but this depends on the individual practice and the commercial payer guidelines in each state. As out of network providers, dentists can set their own fees and balance bill the patient for the cost of care not covered by the patient’s health insurance. The American Medical Association will provide fee ranges for all billable CPT codes. A dentist must set their own fees for the CPT codes billed in their office that are within their scope of practice. The Vivos System of appliances are reimbursable by Medicare or Medicaid.
The mRNA appliance® is not covered by Medicare or Medicaid due to not meeting approved design criteria by CMS. We have made modifications to the mRNA appliance® in order to meet CMS criteria for the billing code E0486 to Medicare. These slight modifications of the mRNA appliance® have provided the opportunity to create a new device called the mmRNA appliance® (Modified Mandibular Repositioning Nighttime Appliance). We have completed mechanical testing on the mmRNA appliance® and in March 2021 we announced that we submitted a 510(k) for Class II clearance to the FDA for the mmRNA with indications to treat mild-to-moderate OSA, SDB and Snoring in adults. Upon 510(k) Class II approval, we plan to submit an application to PDAC (Pricing, Coding, Analysis and Coding) for the mmRNA appliance® to be added to the CMS Medicare list of approved sleep appliances. We expect this process to take 3 to 6 months. We have not found the lack of inclusion on the current CMS Medicare list of approved sleep appliances to hinder market distribution or acceptance due to the fact that most dentists who work with the Vivos System are out of network with commercial payers and do not typically file for reimbursement under Medicare.
We have seen an increase in the ability for reimbursement for our other FDA registered oral appliances such as DNA appliances and Guides. These oral appliances are being pre-authorized and billed under an undefined CPT code only when medical necessity is present and documented properly. Pre-authorization with medical director review is required with a “letter of medical necessity” (LMN) to gain possible medical reimbursement. A dentist billing an undefined CPT code for a Class I or Class II oral appliance must proceed with caution. Billing an undefined CPT code for OAT must be supported with documented medical necessity and is reviewed by the medical director at the payor before being submitted for possible reimbursement. Typically, the dentist writes an LMN to explain the medical necessity, the subscriber’s request for oral appliance therapy and submit these for review to the medical directors at the payor. The plan medical directors will then review any craniofacial abnormalities, CT images, comorbidities, and any medical conditions the patient has be diagnosed with by a medical doctor. This documentation is how the dentist establishes medical necessity. Once pre-authorization is gained, then oral appliance therapy can be billed for a possible reimbursement from the medical payor. A dentist typically can gain reimbursement for OAT by the medical insurance as long as there is medical necessity present and documented.
| 74 |
Published Research
There are several studies in the medical literature on upper airway remodeling in pathologic conditions such as asthma, chronic obstructive pulmonary disease and similar conditions. In contrast, there is a dearth of studies that have documented pneumatization and physiologic upper airway remodeling. Advances in 3D digital technology, as well as an increased understanding of the human genome and epigenetics, has allowed us to make further advances in understanding of craniofacial phenomena. For example, while it was believed that sutures undergo closure in early adulthood, according to published research, it is now thought that populations of stem cells may persist to permit continued growth and development. Using this premise, the midfacial bone volume can be increased surgically or non-surgically. Since the roof of the mouth is the floor of the nose, the volume of the nasal airway can also be increased surgically or non-surgically. Therefore, using our patented, non-surgical protocols we targeted upper airways to address sleep disordered breathing. Using various assessment techniques, we found surface area, volumetric and functional changes of the upper airway. These treatment-induced changes might be described as physiologic remodeling of the upper airway (a process we have labeled and trademarked as Pneumopedics®) achieved through craniofacial epigenetics.
Since 2009, our technology has been the subject of approximately 55 peer-reviewed articles in the medical, dental and orthodontic literature. Of the 55, 27 of these articles are journal papers, with Dr. G. Dave Singh, our Chief Medical Officer, as first author on 22 of these papers. Of the 27, 17 of these articles describe the studies that examine the impact of our technology and protocols on the AHI scores of patients with varying degrees of OSA as described in “Overview” above. In addition, over 25 conference papers have been published as abstracts, with Dr. Singh as first author on 20 of these conference papers, and 19 independent dentists and 5 different sleep physicians are co-authors on these publications as well. The results published in these case reports and articles, together with patient-reported outcomes, have shown that our Vivos System therapy provides a significant reduction in the severity of patients’ mild-to-moderate OSA (as measured by industry standard indices such as the AHI, among others), improvement in sleep-related quality of life, reduction in snoring, as well as a high patient compliance rates and a strong safety profile.
Intellectual Property
To establish and protect our proprietary rights, we rely on a combination of patents, trademarks, copyrights, trade secrets, including know-how, license agreements, confidentiality procedures, non-disclosure agreements with third parties, employee disclosure and invention assignment agreements, and other contractual rights. Our intellectual property is important to achieving and maintaining our position in the market. We currently own five design patents that expire between 2023 through 2029 and two utility patents expiring in 2029 and 2030. We also own two Canadian patents and one European patent that has been validated in Belgium, Switzerland, Germany, Denmark, Spain, France, United Kingdom, Hungary, Italy and the Netherlands, all of which expire in 2029. Our U.S. trademark portfolio consists of ten registered marks and three pending trademark applications.
FDA Regulatory Status
The Vivos System features our Mandibular Repositioning Nighttime Appliance (or mRNA appliance®), which incorporates the same patented technology built into our Daytime Nighttime Appliance (DNA appliance®). We also separately market our own pre-formed guide and rescue appliances which are not a part of the Vivos System (which we refer to collectively as Vivos Guides or Guides). The regulatory status of our products is as follows:
| ● | Our mRNA appliance® has 510(k) clearance from the FDA as a Class II medical device for the treatment of snoring, mild-to-moderate OSA and SDB. | |
| ● | The DNA appliance® is an FDA-registered product, and is currently used by Vivos-trained clinicians accordingly. The DNA appliance® also currently has a pending 510(k) application to include additional indications of use for the treatment of mild-to-moderate OSA, snoring, and SDB in adults. We have validated this 510(k) request with retrospective clinical data. This DNA appliance® 510(k) review and approval process is expected to take another three to six months, meaning we would expect to hear from the FDA in 2021. However, it is possible that we may not receive this FDA additional clearance. Nevertheless, the DNA appliance® is exempt from 510(k) clearance as a Class I device. |
| 75 |
| We instruct all dentists prescribing the DNA appliance about the device’s approved indications of use and of the fact that the DNA appliance is a Class I FDA registered oral appliance. Dentists, as licensed clinicians within the scope of their practice, are free to diagnose, treat and prescribe the appropriate oral appliance therapy as they see fit, including uses which might be “off label”, based on their professional judgement. Given the fact that our dentists regularly prescribe the DNA appliance to treat conditions closely associated with OSA, we do not believe a failure to receive FDA Class II clearance would materially impact our results or financial condition. Any potential consequences of off-label use of the DNA appliance are the responsibility of the treating dentist; however, we may face consequences related to such off-label use. See “Risk Factors— The misuse or off-label use of the Vivos System may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.” | ||
| ● | The Guides are an FDA-registered product for orthodontic tooth positioning. |
We are conducting two separate Western Institutional Review Board (WIRB) approved pediatric clinical trials with seven private dental sites around the country. The purpose of the first study is to evaluate the safety and efficacy of the DNA appliance® to reduce SDB, including snoring, mild to moderate OSA, and Upper Airway Resistance Syndrome (or UARS), and to establish nasal breathing in children. The purpose of the second study is to evaluate the safety and efficacy of the Guides (which we call the Vivos Grow and Vivos Way appliances) to reduce SDB, including snoring, mild to moderate OSA, and Upper Airway Resistance Syndrome (or UARS). Upon completion of these WIRB pediatric clinical trials (expected to be completed in the next 12 to 18 months), we plan to submit two separate 510(k) applications to the FDA requesting pediatric clearances and indications of use for the DNA appliance® as well as the Guides.
The mRNA appliance® is cleared by the FDA as Class II sleep appliance to treat mild-to-moderate OSA, sleep disordered breathing and snoring in adults. Patients undergoing treatment are seeing improvement in the said cleared indications of use, but clinicians have also reported that they are seeing other comorbidities and medical conditions improve due to treatment. The mRNA appliance® (central to the Vivos System) and other Vivos appliances are made available to trained clinicians who exercise their independent clinical judgment with respect to their use and suitability as a part of an overall treatment protocol created for each individual patient.
We submitted a 510(k) Class II application to the U.S. Food and Drug Administration in March 2021 for our mmRNA oral appliance with indications to treat mild-to-moderate OSA, sleep-disordered breathing and snoring in adults. The mmRNA oral appliance (modified mandibular Repositioning Nighttime Appliance) is a new version of our existing mRNA appliance®, which is an FDA-cleared Class II oral appliance. Assuming the mmRNA’s 510(k) Class II approval, we expect to submit an application to a PDAC (Pricing, Data Analysis and Coding) contractor for the mmRNA to be added to the Centers for Medicare and Medicaid Services’ list of approved sleep apnea appliances.
In September of 2017 our subsidiary, BMS, was the subject of a routine FDA audit. It was the very first time the FDA had ever audited BMS. That audit resulted in certain findings that BMS was required to remediate, such as the inadequate documentation of certain FDA-required procedures, not keeping certain records and materials in paper format and in triplicate and using certain descriptive words and phrases on its website and in marketing materials that were unapproved in advance by FDA. We immediately hired a highly qualified FDA consultant and legal counsel with FDA expertise to assist BMS in preparing both a written response and a plan for maintaining compliance with FDA regulations and guidelines. In good faith, and based on documents provided by BMS, we believed BMS had filed its response to the original audit in a timely manner with FDA. However, in January 2018 BMS received a request for a response to an FDA Warning Letter that had been posted online at the FDA website for its alleged failure to reply in a timely manner to FDA and address the findings of the September audit. Prior to that request, BMS had never before seen or received any further notice of deficiency and no such Warning Letter. We discovered that this Warning Letter was the direct result of FDA never having received the BMS initial response, which we believed we had filed on September 27, 2017. Due to the local BMS office in Portland, Oregon being closed down on September 30, 2017 pursuant to a share exchange pursuant to which BMS became a subsidiary of our company (which transaction was accounted for as a merger as disclosed in the consolidated financial statements), all of which was fully disclosed to FDA, neither we nor BMS ever received any further notices from FDA as to them not having received the initial BMS response.
| 76 |
Immediately upon becoming aware of the miscommunication and deficiency, we and BMS notified the FDA of the error and provided the FDA with full documentation of our substantial efforts to fully comply with FDA rules and regulations. The FDA completed a second audit in April 2018, which examined the responses to the BMS findings and Warning Letter. We believe that this matter has been satisfactorily resolved, although no definitive statement to that effect has been made by FDA, nor has the Warning Letter been taken down. The FDA also audited our company (then known as Vivos BioTechnologies, Inc.) and issued one minor observation, to which we have responded and addressed.
In addition to the proactive steps previously mentioned, we engaged a consultant in October of 2017 who we hired as our Senior Vice President of Compliance in January 2018, revamped 100% of all website and marketing materials and literature, accelerated our efforts to address all of the findings of deficiencies from the September 2017 audit, and began filing additional documentation and requests to expand the current labeling restrictions and allow us to have greater latitude in using certain descriptive phrases such as Sleep Disordered Breathing in public communications.
We have validated a 510(k) request for the DNA appliance® with retrospective clinical data. This DNA appliance® 510(k) is under review and the approval process is expected to take three to six months, meaning we would expect to hear from the FDA in 2021. However, it is possible that we may not receive this FDA additional clearance. Nevertheless, the DNA appliance® is exempt from 510(k) clearance, as a Class I device.
Also see “Corporate History – Rescission Offering in 2018” below for more information relating to such FDA matter.
Manufacturing and Supply
We rely on third-party suppliers and manufacturers on a per order, or per item basis. Outsourcing manufacturing reduces our need for capital investment and reduces operational expenses. Additionally, outsourcing provides expertise and capacity necessary to scale up or down based on demand for our Vivos System. We select our manufacturing labs to ensure that our Vivos System appliances are safe and effective, adhere to all applicable regulations, are of the highest quality, and meet our supply needs. We also rely on third-party carriers and freight forwarders for product shipments, including shipments to and from our manufactures’ distribution facilities and customer distribution facilities.
Our Ongoing Clinical Research
We are committed to ongoing research and development and we intend to invest in our business to further improve our products and clinical outcomes, increase patient acceptance and comfort and broaden the patient population that can benefit from the Vivos System.
| ● | Commencing Q3 2021 – Biomimetic oral appliance therapy (BOAT) for the treatment of mild-to-moderate OSA in adults. The aim of this study is to investigate structural and functional effects of the novel BOAT protocol using the mRNA appliance® in the treatment of mild-to-moderate adult OSA. This study will test the hypothesis that treatment of the upper airway in the Vivos System is associated with functional improvements of sleep parameters in adults with mild-to-moderate OSA. | |
| ● | Commenced January 2019 – Treatment of SDB with an intraoral device in a pediatric population. Approved by WIRB as non-significant controlled clinical trials, we are conducting 2 separate clinical trials to evaluate the safety and efficacy of the DNA appliance® and the Vivos Guides (which we call the Vivos Grow and Vivos Way appliances) to reduce SDB in children, including snoring, mild-to-moderate OSA, and UARS. The WIRB is an independent Institutional Review Board located in Olympia, Washington that provides services for academic and non-academic institutions. WIRB is accredited by the Association for the Accreditation of Human Research Protection Programs. (AAHRPP) Clinical outcomes: Pediatric Sleep Questionnaire, reduction in sleep apnea and UARS using the AHI, Epworth Sleepiness Scale for Children and Adolescents, and changes in upper airway volume. |
Government Regulation
Our products and our operations are subject to extensive regulation by the FDA and other federal and state authorities in the United States, as well as comparable authorities in the EEA. Our products are subject to regulation as medical devices under the Federal Food, Drug, and Cosmetic Act, or FDCA, as implemented and enforced by the FDA. The FDA regulates the development, design, non-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, import, export, adverse event reporting, advertising, promotion, marketing and distribution, and import and export of medical devices to ensure that medical devices distributed domestically are safe and effective for their intended uses and otherwise meet the requirements of the FDCA.
| 77 |
In addition to U.S. regulations, we are subject to a variety of regulations in the EEA governing clinical trials and the commercial sales and distribution of our products. Whether or not we have or are required to obtain FDA clearance or approval for a product, we will be required to obtain authorization before commencing clinical trials and to obtain marketing authorization or approval of our products under the comparable regulatory authorities of countries outside of the United States before we can commence clinical trials or commercialize our products in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA clearance or approval.
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) premarket notification or pre-market approval (PMA). Under the FDCA, medical devices are classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to ensure its safety and effectiveness. Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be assured by adherence to the FDA’s General Controls for medical devices, which include compliance with the applicable portions of the QSR, facility registration and product listing, reporting of adverse medical events, and truthful and non-misleading labeling, advertising, and promotional materials. Class II devices are subject to the FDA’s General Controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents. While most Class I devices are exempt from the 510(k) premarket notification requirement, manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to a 510(k) premarket notification is generally known as 510(k) clearance. Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification demonstrating that the device is “substantially equivalent” to either a device that was legally marketed (for which the FDA has not required a PMA submission) prior to May 28, 1976, the date upon which the Medical Device Amendments of 1976 were enacted, or another commercially available device that was cleared to through the 510(k) process. The FDA has 90 days from the date of the pre-market equivalence acceptance to authorize or decline commercial distribution of the device. However, similar to the PMA process, clearance may take longer than this three-month window, as the FDA can request additional data. If the FDA resolves that the product is not substantially equivalent to a predicate device, then the device acquires a Class III designation, and a PMA must be approved before the device can be commercialized.
The Guides are registered with the FDA as Class I devices for orthodontic tooth positioning. The DNA appliance® is registered with the FDA as a Class I device for palatal expansion and is currently used by Vivos-trained clinicians accordingly. The DNA appliance® also currently has a pending 510(k) application to include additional indications of use for the treatment of mild-to-moderate OSA, snoring, and SDB in adults. This use would require the DNA appliance® to be registered as a Class II device. We have validated this 510(k) request with retrospective clinical data. This DNA appliance® 510(k) review and approval process is expected to take another three to six months, meaning we would expect to hear from the FDA in 2021. However, it is possible that we may not receive this FDA additional clearance. Nevertheless, the DNA appliance® is exempt from 510(k) clearance as a Class I device. Given the fact that our dentists regularly prescribe the DNA appliance to treat conditions closely associated with OSA, we do not believe a failure to receive FDA Class II clearance would materially impact our results or financial condition. The mRNA appliance® has 510(k) clearance from the FDA as a Class II medical device for the treatment of snoring, mild-to-moderate OSA and SDB.
Devices deemed by the FDA to pose the greatest risks, such as life-sustaining, life-supporting or some implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in Class III, requiring approval of a PMA. Some pre-amendment devices are unclassified, but are subject to the FDA’s premarket notification and clearance process in order to be commercially distributed. We do not have any Class III devices.
| 78 |
PMA Pathway
Class III devices require PMA approval before they can be marketed although some pre-amendment Class III devices for which the FDA has not yet required a PMA are cleared through the 510(k) process. The PMA process is more demanding than the 510(k) premarket notification process. In a PMA application, the manufacturer must demonstrate that the device is safe and effective, and the PMA application must be supported by extensive data, including data from preclinical studies and human clinical trials. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA application, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review of a PMA application, although in practice, the FDA’s review often takes significantly longer, and can take up to several years. An advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a preapproval inspection of the applicant or its third-party manufacturers.
The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA application constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). The FDA may approve a PMA application with post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution, and collection of long-term follow-up data from patients in the clinical study that supported a PMA approval or requirements to conduct additional clinical studies post-approval. The FDA may condition a PMA approval on some form of post-market surveillance when deemed necessary to protect the public health or to provide additional safety and efficacy data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Failure to comply with the conditions of approval can result in material adverse enforcement action, including withdrawal of the approval.
Certain changes to an approved device, such as changes in manufacturing facilities, methods, or quality control procedures, or changes in the design performance specifications, which affect the safety or effectiveness of the device, require submission of a new PMA application or a PMA supplement. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application and may not require as extensive clinical data or the convening of an advisory panel. Certain other changes to an approved device require the submission of a new PMA application, such as when the design change causes a different intended use, mode of operation, and technical basis of operation, or when the design change is so significant that a new generation of the device will be developed, and the data that were submitted with the original PMA application are not applicable for the change in demonstrating a reasonable assurance of safety and effectiveness.
Clinical Trials
Clinical trials are almost always required to support a PMA application and are sometimes required to support a 510(k) submission. All clinical investigations of investigational devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption, or IDE, regulations which govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies us that the investigation may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE for which it requires modification, the FDA may require a response on such deficiencies or permit a clinical trial to proceed under a conditional approval.
| 79 |
In addition, the study must be approved by, and conducted under the oversight of, an Institutional Review Board, or IRB, for each clinical site. The IRB is responsible for the initial and continuing review of the IDE, and may pose additional requirements for the conduct of the study. If an IDE application is approved by the FDA and one or more IRBs, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements. Acceptance of an IDE application for review does not guarantee that the FDA will allow the IDE to become effective and, if it does become effective, the FDA may or may not determine that the data derived from the trials support the safety and effectiveness of the device or warrant the continuation of clinical trials. An IDE supplement must be submitted to, and approved by, the FDA before a sponsor or investigator may make a change to the investigational plan that may affect its scientific soundness, study plan or the rights, safety or welfare of human subjects.
During a study, the sponsor is required to comply with the applicable FDA requirements, including, for example, trial monitoring, selecting clinical investigators and providing them with the investigational plan, ensuring IRB review, adverse event reporting, record keeping and prohibitions on the promotion of investigational devices or on making safety or effectiveness claims for them. The clinical investigators in the clinical study are also subject to FDA regulations and must obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of the investigational device, and comply with all reporting and recordkeeping requirements. Additionally, after a trial begins, we, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.
Post-market Regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
| ● | establishment registration and device listing with the FDA; | |
| ● | QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; | |
| ● | labeling and marketing regulations, which require that promotion is truthful, not misleading, fairly balanced and provide adequate directions for use and that all claims are substantiated, and also prohibit the promotion of products for unapproved or off-label uses and impose other restrictions on labeling; FDA guidance on off-label dissemination of information and responding to unsolicited requests for information; | |
| ● | the federal Physician Sunshine Act and various state and foreign laws on reporting remunerative relationships with health care customers; | |
| ● | the federal Anti-Kickback Statute (and similar state laws) prohibiting, among other things, soliciting, receiving, offering or providing remuneration intended to induce the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as Medicare or Medicaid. A person or entity does not have to have actual knowledge of this statute or specific intent to violate it to have committed a violation; | |
| ● | the federal False Claims Act (and similar state laws) prohibiting, among other things, knowingly presenting, or causing to be presented, claims for payment or approval to the federal government that are false or fraudulent, knowingly making a false statement material to an obligation to pay or transmit money or property to the federal government or knowingly concealing, or knowingly and improperly avoiding or decreasing, an obligation to pay or transmit money to the federal government. The government may assert that claim includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statute; | |
| ● | clearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices, or approval of a supplement for certain modifications to PMA devices; |
| 80 |
| ● | medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur; | |
| ● | correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; | |
| ● | complying with the new federal law and regulations requiring Unique Device Identifiers (UDI) on devices and also requiring the submission of certain information about each device to the FDA’s Global Unique Device Identification Database (GUDID); | |
| ● | the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and |
| ● | post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
We may be subject to similar foreign laws that may include applicable post-marketing requirements such as safety surveillance. Our manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. As a manufacturer, our facilities, records and manufacturing processes are subject to periodic scheduled or unscheduled inspections by the FDA. Our failure to maintain compliance with the QSR or other applicable regulatory requirements could result in the shut-down of, or restrictions on, our manufacturing operations and the recall or seizure of our products. The discovery of previously unknown problems with any of our products, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls or a public warning letter that could harm both our reputation and sales. Any potential consequences of off-label use of the DNA appliance are the responsibility of the treating dentist; however, we may face consequences related to such off-label use. See “Risk Factors— The misuse or off-label use of the Vivos System may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.”
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that we failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, which may result in any of the following sanctions:
| ● | warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties; | |
| ● | recalls, withdrawals, or administrative detention or seizure of our products; | |
| ● | operating restrictions or partial suspension or total shutdown of production; | |
| ● | refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products; | |
| ● | withdrawing 510(k) clearances or PMAs that have already been granted; | |
| ● | refusal to grant export or import approvals for our products; or | |
| ● | criminal prosecution. |
| 81 |
Regulation of Medical Devices in the EEA
There is currently no premarket government review of medical devices in the EEA (which is comprised of the 28 Member States of the EU plus Norway, Liechtenstein and Iceland). However, all medical devices placed on the market in the EEA must meet the relevant essential requirements laid down in Annex I of Directive 93/42/EEC concerning medical devices, or the Medical Devices Directive. There is also a directive specifically addressing Active Implantable Medical Devices (Directive 90/385/EEC). The most fundamental essential requirement is that a medical device must be designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users and others. In addition, the device must achieve the performances intended by the manufacturer and be designed, manufactured and packaged in a suitable manner. The European Commission has adopted various standards applicable to medical devices. These include standards governing common requirements, such as sterilization and safety of medical electrical equipment, and product standards for certain types of medical devices. There are also harmonized standards relating to design and manufacture. While not mandatory, compliance with these standards is viewed as the easiest way to satisfy the essential requirements as a practical matter. Compliance with a standard developed to implement an essential requirement also creates a rebuttable presumption that the device satisfies that essential requirement.
To demonstrate compliance with the essential requirements laid down in Annex I to the Medical Devices Directive, medical device manufacturers must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Conformity assessment procedures require an assessment of available clinical evidence, literature data for the product and post-market experience in respect of similar products already marketed. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can self-declare the conformity of its products with the essential requirements (except for any parts which relate to sterility or metrology), a conformity assessment procedure requires the intervention of a Notified Body. Notified bodies are often separate entities and are authorized or licensed to perform such assessments by government authorities. The notified body would typically audit and examine a product’s technical dossiers and the manufacturers’ quality system. If satisfied that the relevant product conforms to the relevant essential requirements, the notified body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE Mark to the device, which allows the device to be placed on the market throughout the EEA. Once the product has been placed on the market in the EEA, the manufacturer must comply with requirements for reporting incidents and field safety corrective actions associated with the medical device.
In order to demonstrate safety and efficacy for their medical devices, manufacturers must conduct clinical investigations in accordance with the requirements of Annex X to the Medical Devices Directive, Annex 7 of the Active Implantable Medical Devices Directive, and applicable European and International Organization for Standardization standards, as implemented or adopted in the EEA member states. Clinical trials for medical devices usually require the approval of an ethics review board and approval by or notification to the national regulatory authorities. Both regulators and ethics committees also require the submission of serious adverse event reports during a study and may request a copy of the final study report.
On April 5, 2017, the European Parliament passed the Medical Devices Regulation (Regulation 2017/745), which repeals and replaces the EU Medical Devices Directive and the Active Implantable Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member States, the regulations would be directly applicable, i.e., without the need for adoption of EEA member State laws implementing them, in all EEA member States and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation. The Medical Devices Regulation will however only become applicable three years after publication (in 2020). Once applicable, the new regulations will among other things:
| ● | strengthen the rules on placing devices on the market and reinforce surveillance once they are available; | |
| ● | establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| 82 |
| ● | improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; | |
| ● | set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; | |
| ● | strengthened rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
We are subject to regulations and product registration requirements in many foreign countries in which we may sell our products, including in the areas of:
| ● | design, development, manufacturing and testing; | |
| ● | product standards; | |
| ● | product safety; |
| ● | product safety reporting; | |
| ● | marketing, sales and distribution; | |
| ● | packaging and storage requirements; | |
| ● | labeling requirements; | |
| ● | content and language of instructions for use; | |
| ● | clinical trials; | |
| ● | record keeping procedures; | |
| ● | advertising and promotion; | |
| ● | recalls and field corrective actions; | |
| ● | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
| ● | import and export restrictions; | |
| ● | tariff regulations, duties and tax requirements; | |
| ● | registration for reimbursement; and | |
| ● | necessity of testing performed in country by distributors for licensees.
| |
| ● | The time required to obtain clearance required by foreign countries may be longer or shorter than that required for FDA clearance, and requirements for licensing a product in a foreign country may differ significantly from FDA requirements. |
The EU Medical Devices Regulation became effective in May 2020. The revised regulation includes further controls and requirements on the following activities:
| 83 |
| ● | high level of request for premarket clinical evidence for high risk devices; | |
| ● | increased scrutiny of technical files for implantable devices; | |
| ● | monitoring of notified bodies, by independent auditors; | |
| ● | increased requirements regarding vigilance and product traceability (specifically related to labeling requirements); and | |
| ● | increased regulation for non-traditional roles such as importer and distributor. |
Federal, State and Foreign Fraud and Abuse and Physician Payment Transparency Laws
In addition to FDA restrictions on marketing and promotion of drugs and devices, other federal and state laws restrict our business practices. These laws include, without limitation, foreign, federal, and state anti-kickback and false claims laws, as well as transparency laws regarding payments or other items of value provided to healthcare providers.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any good, facility, item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value, including stock, stock options, and the compensation derived through ownership interests.
Recognizing that the federal Anti-Kickback Statute is broad and may prohibit many innocuous or beneficial arrangements within the healthcare industry, the United State Department of Health and Human Services (“DHHS”) issued regulations in July 1991, which DHHS has referred to as “safe harbors.” These safe harbor regulations set forth certain provisions which, if met in form and substance, will assure medical device manufacturers, healthcare providers and other parties that they will not be prosecuted under the federal Anti-Kickback Statute. Additional safe harbor provisions providing similar protections have been published intermittently since 1991. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Our arrangements with physicians, hospitals and other persons or entities who are in a position to refer may not fully meet the stringent criteria specified in the various safe harbors. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not fall within an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the federal Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the federal Anti-Kickback Statute has been violated. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Moreover, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act (described below).
Violations of the federal Anti-Kickback Statute may result in civil monetary penalties up to $100,000 for each violation, plus up to three times the remuneration involved. Civil penalties for such conduct can further be assessed under the federal False Claims Act. Violations can also result in criminal penalties, including criminal fines of up to $100,000 and imprisonment of up to 10 years. Similarly, violations can result in exclusion from participation in government healthcare programs, including Medicare and Medicaid. Liability under the federal Anti-Kickback Statute may also arise because of the intentions or actions of the parties with whom we do business. While we are not aware of any such intentions or actions, we have only limited knowledge regarding the intentions or actions underlying those arrangements. Conduct and business arrangements that do not fully satisfy one of these safe harbor provisions may result in increased scrutiny by government enforcement authorities. The majority of states also have anti-kickback laws which establish similar prohibitions and, in some cases, may apply more broadly to items or services covered by any third-party payor, including commercial insurers and self-pay patients.
| 84 |
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. The federal civil False Claims Act also applies to false submissions that cause the government to be paid less than the amount to which it is entitled, such as a rebate. Intent to deceive is not required to establish liability under the civil federal civil False Claims Act.
In addition, private parties may initiate “qui tam” whistleblower lawsuits against any person or entity under the federal civil False Claims Act in the name of the government and share in the proceeds of the lawsuit. Penalties for federal civil False Claim Act violations include fines for each false claim, plus up to three times the amount of damages sustained by the federal government and, most critically, may provide the basis for exclusion from government healthcare programs, including Medicare and Medicaid. On May 20, 2009, the Fraud Enforcement Recovery Act of 2009, or FERA, was enacted, which modifies and clarifies certain provisions of the federal civil False Claims Act. In part, the FERA amends the federal civil False Claims Act such that penalties may now apply to any person, including an organization that does not contract directly with the government, who knowingly makes, uses or causes to be made or used, a false record or statement material to a false or fraudulent claim paid in part by the federal government. The government may further prosecute conduct constituting a false claim under the federal criminal False Claims Act. The criminal False Claims Act prohibits the making or presenting of a claim to the government knowing such claim to be false, fictitious or fraudulent and, unlike the federal civil False Claims Act, requires proof of intent to submit a false claim. When an entity is determined to have violated the federal civil False Claims Act, the government may impose civil fines and penalties ranging from $11,181 to $22,363 for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs.
The Civil Monetary Penalty Act of 1981 imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent, or offering or transferring remuneration to a federal healthcare beneficiary that a person knows or should know is likely to influence the beneficiary’s decision to order or receive items or services reimbursable by the government from a particular provider or supplier.
HIPAA also created additional federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Many foreign countries have similar laws relating to healthcare fraud and abuse. Foreign laws and regulations may vary greatly from country to country. For example, the advertising and promotion of our products is subject to EU Directives concerning misleading and comparative advertising and unfair commercial practices, as well as other EEA Member State legislation governing the advertising and promotion of medical devices. These laws may limit or restrict the advertising and promotion of our products to the general public and may impose limitations on our promotional activities with healthcare professionals. Also, many U.S. states have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs.
Additionally, there has been a recent trend of increased foreign, federal, and state regulation of payments and transfers of value provided to healthcare professionals or entities. The federal Physician Payments Sunshine Act imposes annual reporting requirements on certain drug, biologics, medical supplies and device manufacturers for which payment is available under Medicare, Medicaid or Children’s Health Insurance Program (“CHIP”), for payments and other transfers of value provided by them, directly or indirectly, to physicians (including physician family members), certain other healthcare providers, and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. A manufacturer’s failure to submit timely, accurately and completely the required information for all payments, transfers of value or ownership or investment interests may result in civil monetary penalties ranging from $1,000 to $10,000 for each payment or other transfer of value that Is not reported (up to a maximum per annual report of $150,000) and from $10,000 to $100,000 for each knowing failure to report (up to a maximum per annual report of $1,150,000). Manufacturers must submit reports by the 90th day of each calendar year. Certain foreign countries and U.S. states also mandate implementation of commercial compliance programs, impose restrictions on device manufacturer marketing practices and require tracking and reporting of gifts, compensation and other remuneration to healthcare professionals and entities. Additionally, there are criminal penalties if an entity intentionally makes false statement in such reports. With some exceptions, the information that manufacturers report is made publicly available.
| 85 |
Data Privacy and Security Laws
We are also subject to various federal, state and foreign laws that protect the confidentiality of certain patient health information, including patient medical records, and restrict the use and disclosure of patient health information by healthcare providers, such as HIPAA, as amended by HITECH, in the United States.
HIPAA established uniform standards governing the conduct of certain electronic healthcare transactions and requires certain entities, called covered entities, to comply with standards that include the privacy and security of protected health information, or PHI. HIPAA also requires business associates, such as independent contractors or agents of covered entities that have access to PHI in connection with providing a service to or on behalf of a covered entity, of covered entities to enter into business associate agreements with the covered entity and to safeguard the covered entity’s PHI against improper use and disclosure.
The HIPAA privacy regulations cover the use and disclosure of protected health information by covered entities as well as business associates, which are defined to include subcontractors that create, receive, maintain, or transmit protected health information on behalf of a business associate. They also set forth certain rights that an individual has with respect to his or her protected health information maintained by a covered entity, including the right to access or amend certain records containing protected health information, or to request restrictions on the use or disclosure of protected health information. The security regulations establish requirements for safeguarding the confidentiality, integrity, and availability of protected health information that is electronically transmitted or electronically stored. HITECH, among other things, established certain health information security breach notification requirements. A covered entity must notify any individual whose protected health information is breached according to the specifications set forth in the breach notification rule. The HIPAA privacy and security regulations establish a uniform federal “floor” and do not supersede state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing protected health information or insofar as such state laws apply to personal information that is broader in scope than protected health information as defined under HIPAA.
HIPAA requires the notification of patients, and other compliance actions, in the event of a breach of unsecured protected health information, or PHI. If notification to patients of a breach is required, such notification must be provided without unreasonable delay and in no event later than 60 calendar days after discovery of the breach. In addition, if the PHI of 500 or more individuals is improperly used or disclosed, we would be required to report the improper use or disclosure to DHHS, Office of Civil Rights, which would post the violation on its website, and to the media. Failure to comply with the HIPAA privacy and security standards can result in civil monetary penalties up to $59,522 per violation, not to exceed $1,785,651 per calendar year for non-compliance of an identical provision, and, in certain circumstances, criminal penalties with fines up to $250,000 per violation and/or imprisonment.
HIPAA authorizes state attorneys general to file suit on behalf of their residents for violations. Courts are able to award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to file suit against us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care cases in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI. In addition, HIPAA mandates that the Secretary of DHHS conduct periodic compliance audits of HIPAA covered entities, such as us, and their business associates for compliance with the HIPAA privacy and security standards. It also tasks DHHS with establishing a methodology whereby harmed individuals who were the victims of breaches of unsecured PHI may receive a percentage of the civil monetary penalty paid by the violator.
In the European Union, we may be subject to laws relating to our collection, control, processing and other use of personal data (i.e. data relating to an identifiable living individual). We process personal data in relation to our operations. We process data of both our employees and our customers, including health and medical information. The data privacy regime in the EU includes the EU Data Protection Directive (95/46/EC) regarding the processing of personal data and the free movement of such data, the E-Privacy Directive 2002/58/EC and national laws implementing each of them. Each EU Member State has transposed the requirements laid down by the Data Protection Directive and E-Privacy Directive into its own national data privacy regime and therefore the laws may differ by jurisdiction, sometimes significantly. We need to ensure compliance with the rules in each jurisdiction where we are established or are otherwise subject to local privacy laws.
| 86 |
The requirements include that personal data may only be collected for specified, explicit and legitimate purposes based on legal grounds set out in the local laws and may only be processed in a manner consistent with those purposes. Personal data must also be adequate, relevant, not excessive in relation to the purposes for which it is collected, be secure, not be transferred outside of the EEA unless certain steps are taken to ensure an adequate level of protection and must not be kept for longer than necessary for the purposes of collection. To the extent that we process, control or otherwise use sensitive data relating to living individuals (for example, patients’ health or medical information), more stringent rules apply, limiting the circumstances and the manner in which we are legally permitted to process that data and transfer that data outside of the EEA. In particular, in order to process such data, explicit consent to the processing (including any transfer) is usually required from the data subject (being the person to whom the personal data relates).
The new EU-wide General Data Protection Regulation, or GDPR, became applicable on May 25, 2018, replacing the current data protection laws issued by each EU member state based on the Directive 95/46/EC. Unlike the Directive (which needed to be transposed at national level), the GDPR text is directly applicable in each EU member state, resulting in a more uniform application of data privacy laws across the EU. The GDPR imposes onerous accountability obligations requiring data controllers and processors to maintain a record of their data processing and policies. It requires data controllers to be transparent and disclose to data subjects (in a concise, intelligible and easily accessible form) how their personal information is to be used, imposes limitations on retention of information, increases requirements pertaining to pseudonymized (i.e., key-coded) data, introduces mandatory data breach notification requirements and sets higher standards for data controllers to demonstrate that they have obtained valid consent for certain data processing activities. Fines for non-compliance with the GDPR are significant—the greater of EUR 20 million or 4% of global turnover. The GDPR provides that EU member states may introduce further conditions, including limitations, to the processing of genetic, biometric or health data, which could limit our ability to collect, use and share personal data, or could cause our compliance costs to increase, ultimately having an adverse impact on our business.
We are subject to the supervision of local data protection authorities in those jurisdictions where we are established or otherwise subject to applicable law.
We depend on a number of third parties in relation to our provision of our services, a number of which process personal data on our behalf. With each such provider we enter into contractual arrangements to ensure that they only process personal data according to our instructions, and that they have sufficient technical and organizational security measures in place. Where we transfer personal data outside the EEA, we do so in compliance with the relevant data export requirements. We take our data protection obligations seriously, as any improper disclosure, particularly with regard to our customers’ sensitive personal data, could negatively impact our business and/or our reputation.
Healthcare Reform
The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality or expanding access. Current and future legislative proposals to further reform healthcare or reduce healthcare costs may limit coverage of or lower reimbursement for the procedures associated with the use of our products. The cost containment measures that payors and providers are instituting and the effect of any healthcare reform initiative implemented in the future could impact our revenue from the sale of our products.
The implementation of the Affordable Care Act in the United States, for example, has changed healthcare financing and delivery by both governmental and private insurers substantially, and affected medical device manufacturers significantly. The Affordable Care Act imposed, among other things, a 2.3% federal excise tax, with limited exceptions, on any entity that manufactures or imports Class I, II and III medical devices offered for sale in the United States that began on January 1, 2013. Through a series of legislative amendments, the tax was suspended for 2016 through 2019. Absent further legislative action, the device excise tax will be reinstated on medical device sales starting January 1, 2020. The Affordable Care Act also provided incentives to programs that increase the federal government’s comparative effectiveness research and implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models. Additionally, the Affordable Care Act has expanded eligibility criteria for Medicaid programs and created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. We do not yet know the full impact that the Affordable Care Act will have on our business. There have been judicial and Congressional challenges to certain aspects of the Affordable Care Act, and we expect additional challenges and amendments in the future. Moreover, the Trump Administration and the U.S. Congress may take further action regarding the Affordable Care Act, including, but not limited to, repeal or replacement. Most recently, the Tax Cuts and Jobs Acts was enacted, which, among other things, removes penalties for not complying with the individual mandate to carry health insurance, beginning in 2019.
| 87 |
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. For example, the Budget Control Act of 2011, among other things, included reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2027 unless additional Congressional action is taken. Additionally, the American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
We expect additional state and federal healthcare reform measures to be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure.
Anti-Bribery and Corruption Laws
Our U.S. operations are subject to the FCPA. We are required to comply with the FCPA, which generally prohibits covered entities and their intermediaries from engaging in bribery or making other prohibited payments to foreign officials for the purpose of obtaining or retaining business or other benefits. In addition, the FCPA imposes accounting standards and requirements on publicly traded U.S. corporations and their foreign affiliates, which are intended to prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made. We also are subject to similar anticorruption legislation implemented in Europe under the Organization for Economic Co-operation and Development’s Convention on Combating Bribery of Foreign Public Officials in International Business Transactions.
Human Capital Resources
As of the date of this prospectus, we had 112 full-time employees and 6 part-time employees. None of our employees are represented by a union. We consider our relations with our employees to be good but we do have a Whistleblower Hotline setup for employees to confidentially report concerns. Of our current employees, approximately 9 are involved in senior management, 25 in sales and marketing, 2 in research, development and regulatory and 82 in finance and operations.
We value the importance of retention, growth and development of our employees and we believe we offer competitive compensation (including salary, incentive bonus, and equity) and benefits packages. We traditionally will benchmark compensation with external sources to verify positions are paid in-line with the market. Our corporate culture is built on passion – we believe in the company’s vision of ridding the world of sleep apnea and hire employees who want to share that same passion. We hold annual company-wide trainings and host regularly scheduled management meetings where management communicates notable corporate developments to be disseminated to employees, as well as a periodic corporate all hands meetings. We are always looking for additional ways to diversify our workforce. We will continue to promote a work environment that is based on the fundamental principles of human dignity, equality and mutual respect. In addition, we are committed to providing a safe and healthy work environment for all of our employees. In response to the COVID-19 pandemic, we have required personal protective equipment for patient-facing employees in addition to requiring daily health questionnaires and temperature checks. Many employees work remotely and we have limited travel as a result of the pandemic. We will continue to support our workforce during these unprecedented circumstances to ensure their safety and well-being.
| 88 |
Corporate History
Formation
We were originally organized on July 7, 2016 in Wyoming as Corrective BioTechnologies, Inc. On September 6, 2016, we changed our name from Corrective BioTechnologies, Inc. to Vivos BioTechnologies, Inc. On March 2, 2018, we changed our name from Vivos BioTechnologies, Inc. to Vivos Therapeutics, Inc. During our formation in 2016, we issued an aggregate of 933,334 shares of common stock to a group of our founders, including Summit Capital USA (now Upeva, Inc., 666,667 shares), Regal Capital Venture Partners LLC (166,667 shares) and Thomas P. Madden (100,000 shares) at a purchase price of $0.0003 per share (for an aggregate of $280 of proceeds).
Acquisition of BioModeling Solutions, Inc. and First Vivos, Inc.
In August and September 2016, we completed, by way of share exchange, an agreement to acquire the business and operations of (1) BMS (now a wholly-owned subsidiary), which was engaged in the manufacture and sale of our patented DNA appliance® and FDA cleared mRNA appliance® (collectively with special proprietary treatment protocols comprises the Vivos System), and (2) First Vivos, Inc., a Texas corporation (or First Vivos), which proposed to develop and operate a retail chain of Vivos Centers with specially trained dentists that offer the Vivos System and corroborating physicians. In connection with the share exchange with BMS, we issued 3,333,334 shares of common stock to the shareholders of BMS (including, but not limited to, Dr. G. Dave Singh, our founder and Chief Medical Officer, who received 3,219,705 shares) in exchange for 12,423,500 shares of BMS, which constitutes 100% ownership interest in BMS. In connection with the share exchange with First Vivos, we issued 3,333,334 shares of common stock to the shareholders of First Vivos (including, but not limited to, R. Kirk Huntsman, our co-founder, Chairman of the Board and Chief Executive Officer, who received 1,833,334 shares) in exchange for 5,000 shares of First Vivos, which constitutes 100% ownership interest in First Vivos.
The transaction was accounted for as a reverse acquisition and recapitalization, with BMS as the acquirer for financial reporting and accounting purposes. Upon the consummation of the acquisition, the historical financial statements of BMS became our historical financial statements and continued to be recorded at their historical carrying amounts.
Rescission Offering in 2018
On January 26, 2018, we offered fifteen (15) investors who invested from January 4, 2018 to February 9, 2018 a right to rescind their purchase of shares of common stock during such period and to receive a refund of the full purchase price paid for such shares due to inadvertent non-disclosure of our receipt of a Warning Letter from the FDA on January 12, 2018 requesting that we take prompt action to correct the violations discussed in the Warning Letter, and noting that our failure to do so may result in regulatory action being initiated by the FDA. See “FDA Regulatory Status” above for further information on FDA matter. None of such investors elected to rescind their purchase of such shares.
Issuance of Common Stock and Convertible Promissory Note in Connection with Acquisition of Orem Vivos Center and Empowered Dental Lab
On July 1, 2018, we issued 93,334 shares of common stock with a value of $7.50 per share (an aggregate value of $700,000) and a 6% convertible promissory note in the principal amount of $525,000 to a third party to acquire his dentistry clinic in Orem, Utah (total consideration of $1,225,000). On November 6, 2018, we entered into an asset purchase agreement with Empowered Dental Lab, LLC, a Utah limited liability company, under which we agreed to purchase certain inventory and assets from Empowered Dental Lab in exchange for consideration of 6,667 shares of common stock and a 6% convertible promissory note for $25,000, for total consideration of $75,000.
Adoption of Stock and Option Award Plan
On April 18, 2019, our stockholders approved the adoption of a stock and option award plan (the “2019 Plan”), under which 333,334 shares were reserved for future issuance for options, restricted stock awards and other equity awards. On June 18, 2020, our stockholders approved an amendment and restatement of the 2019 Plan to increase the number shares or our common stock available for issuance thereunder by 833,333 share of common stock such that, after amendment and restatement of the 2019 Plan, 1,166,667 shares of common stock will be available for issuance under the 2019 Plan. The 2019 Plan permits grants of equity awards to employees, directors, consultants and other independent contractors.
| 89 |
Approval of Transfer of Corporate Domicile and Reverse Stock Split
On April 18, 2019, our stockholders voted to authorize our board of directors to recapitalize our common stock by way of reverse stock split at a ratio of up to one for three. In addition, on such date, our shareholders also authorized our board of directors to transfer our corporate domicile from Wyoming to another U.S. state. Our board of directors elected not to implement the reverse stock split transfer of corporate domicile at that time.
Effective August 12, 2020, we transferred our corporate domicile and became a Delaware corporation pursuant to Section 17-16-1720 of the Wyoming Business Corporation Act and Section 265 of the Delaware General Corporation Law. As a result of the transfer of corporate domicile, each share of capital stock of Vivos Therapeutics, Inc., the Wyoming corporation (or Vivos Wyoming) became a share of capital stock of Therapeutics, Inc., the Delaware corporation (or Vivos Delaware) on a one-to-one basis, and such shares shall carry the same terms in all material respects as the shares of Vivos Wyoming. The transfer of corporate domicile has heretofore been approved by the board of directors and majority shareholders of Vivos Wyoming.
On July 30, 2020, prior to the transfer of our corporate domicile from Wyoming to Delaware, Vivos Wyoming implemented a one-for-three reverse stock split of our outstanding common stock pursuant to which holders of Vivos Wyoming’s outstanding common stock received one share of common stock for every three shares of common stock held. Unless the context expressly dictates otherwise, all references to share and per share amounts referred to in this prospectus reflect the reverse stock split.
Segment Information
We manage our business within one reportable segment. Segment information is consistent with how management reviews our business, makes investing and resource allocation decisions, and assesses our operating performance.
Seasonality
We believe that the patient volumes of our VIPs will be sensitive to seasonal fluctuations in urgent care and primary care activity. Typically, winter months see a higher occurrence of influenza, bronchitis, pneumonia and similar illnesses; however, the timing and severity of these outbreaks vary dramatically. Additionally, as consumers shift toward high deductible insurance plans, they are responsible for a greater percentage of their bill, particularly in the early months of the year before other healthcare spending has occurred, which may lead to lower than expected patient volume or an increase in bad debt expense during that period. Our quarterly operating results may fluctuate significantly in the future depending on these and other factors.
Corporate Information
Our principal offices are located at 9137 Ridgeline Boulevard, Suite 135, Highlands Ranch, Colorado 80129, and our telephone number is (866) 908-4867. Our website is www.vivoslife.com. Our website and the information on or that can be accessed through such website are not part of this prospectus. We were originally organized on July 7, 2016 as a Wyoming corporation under the name as Corrective BioTechnologies, Inc. On September 6, 2016, we changed our name from Corrective BioTechnologies, Inc. to Vivos BioTechnologies, Inc., and on March 2, 2018, we changed our name from Vivos BioTechnologies, Inc. to Vivos Therapeutics, Inc. Effective August 12, 2020, we transferred our corporate domicile from Wyoming to Delaware.
Available Information
We maintain a website at www.vivoslife.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The reference to our website address does not constitute incorporation by reference of the information contained on our website, and you should not consider the contents of our website in making an investment decision with respect to our common stock.
| 90 |
Legal Proceedings
From time to time, we are involved in various claims and legal actions arising in the ordinary course of business.
On June 5, 2020, we filed suit against Ortho-Tain, Inc. in the United States District Court for the District of Colorado seeking relief from certain false, threatening, and defamatory statements to our business affiliate, Benco Dental. We believe such statements have interfered with our business relationship and contract, causing us harm to our reputation, loss of goodwill, and unspecified monetary damages. On February 12, 2021, we amended our complaint to add claims for false advertising and unfair business practices, as well as additional variants of the original claims to address Ortho-Tain’s false advertising campaign against us in the fall of 2020. We are in the process of pursuing discovery for the expanded claims. Our complaint seeks permanent injunctive relief to prevent the defendant’s continued illegal defamatory statements and interference with our business relationships. We further seek declaratory relief to refute the defendant’s false allegations, as well as monetary damages to compensate us for harm caused by the defendant. Prior to filing suit, we worked collaboratively with legal counsel at Benco Dental to address and reasonably resolve this matter. Such efforts were unsuccessful. On February 26, 2021, Ortho-Tain, Inc. filed a Motion to Dismiss the amended complaint we filed against it in the United States District Court for the District of Colorado. While we are still evaluating the Motion to Dismiss, we believe such arguments made by Ortho-Tain, Inc. in the Motion to Dismiss lack merit.
On July 22, 2020 Ortho-Tain, Inc. filed a Complaint in the United States District Court for the Northern District of Illinois naming Vivos, along with our Chief Executive Officer, R. Kirk Huntsman, Benco Dental Supply Co., Dr. Brian Kraft, Dr. Ben Miraglia, and Dr. Mark Musso. The Ortho-Tain complaint addresses the same events as the suit we filed against Ortho-Tain, Inc. in June 2020 as described above. The Ortho-Tain complaint alleges violation of the Lanham Act and an alleged civil conspiracy among the defendants to violate the Lanham Act by an alleged false designation of origin related to a presentation given by Dr. Brian Kraft at an event sponsored by us and Benco Dental. Ortho-Tain also alleges that the actions of the defendants, including our company, diverted sales from Ortho-Tain, deprived Ortho-Tain of advertising value and resulted in a loss of goodwill to Ortho-Tain. However, Ortho-Tain does not attempt to measure any such damages or clearly articulate its losses, short of the broad allegations contained in its complaint. Ortho-Tain also alleges two separate breach of contract actions against Dr. Brian Kraft and our Chief Executive Officer, R. Kirk Huntsman. Ortho-Tain’s allegation of breach of contract against Mr. Huntsman, relates to a Non-Disclosure Agreement entered into in October 2013 with Mr. Huntsman’s prior entity, Xenith Practices, LLC, which Non-Disclosure Agreement expired pursuant to its terms in October 2016. We continue to evaluate the allegations, although we believe they lack merit and Ortho-Tain will be unable to establish actionable damages. On September 9, 2020, we moved to dismiss the claims against us. On October 23, 2020, we filed a motion requesting, in the alternative, that if the case is not dismissed, it be transferred to the Colorado action described above or stayed. Both motions are pending before the Court. If either motion is unsuccessful, we will defend the claims alleged by Ortho-Tain vigorously, and we do not believe that Ortho-Tain’s claims would materially impact our operations, nor would they amount to any material damages should Otho-Tain prevail.
There are no other legal proceedings currently pending against us, or known to be contemplated by any governmental agency, which we believe would have a material effect on our business, financial position or results of operations.
Properties
We lease approximately 3,231 rentable square feet of office space from an unaffiliated third party for our corporate office located at 9137 Ridgeline Boulevard, Suite 135, Highlands Ranch, Colorado. This lease expires on in May 2022. Terms of the office lease currently provide for a base rent payment of $4,712 per month. We also lease approximately 2,220 rentable square feet of space from an unaffiliated third party for one of our Vivos Centers located at 4795 Larimer Parkway, Johnstown, Colorado. This lease expires in February 2025. Terms of the office lease provide for a base rent payment of $3,608 per month and a share of the buildings operating expenses such as taxes and maintenance of $2,035 per month. We also lease 3,643 rentable square feet of space from an unaffiliated third party for our Vivos Center located at 9135 Ridgeline Boulevard, Highlands Ranch, Colorado. This lease expires in January 2029. Terms of the office provide for a base rent payment of $5,465 per month and a share of the building’s operating expenses such as taxes and maintenance of $3,273 per month. Effective May 20, 2019, we entered into a lease at 7001 Tower Road, Denver, Colorado for 14,732 rentable square feet for the Institute for Craniofacial Sleep Medicine. This leased facility is being built out as a training facility where our VIPs will be trained. We believe that these facilities are adequate for our current and near-term future needs.
| 91 |
The following table sets forth the names, positions and ages of our directors and executive officers as of the date of this prospectus. Our directors are elected by our stockholders at the annual meeting of the stockholders, and have been elected via written consent of a majority of stockholders, and serve until the next annual meeting of the stockholders or, in absence of such annual meeting, until their successors are elected and qualified. Officers are elected by our board of directors and their terms of office are at the discretion of our board, subject to applicable employment agreements.
| Name | Age | Positions Held | Initial Term of Office | |||
| R. Kirk Huntsman | 63 | Co-founder, Chairman of the Board and Chief Executive Officer | September 2016 | |||
| G. Dave Singh | 63 | Founder, Chief Medical Officer and Director | September 2016 | |||
| Bradford Amman | 59 | Chief Financial Officer, Secretary | October 2018 | |||
| Ralph E. Green | 81 | Director | June 2020 | |||
| Anja Krammer | 53 | Director | June 2020 | |||
| Mark F. Lindsay | 57 | Director | June 2020 | |||
| Leonard J. Sokolow | 64 | Director | June 2020 | |||
| Matthew Thompson | 59 | Director | June 2020 |
The biographical information concerning the directors and executive officers listed above is set forth below.
Executive Officers
R. Kirk Huntsman is a co-founder of our company and has served as our Chief Executive Officer and a director since September 2016. In June 2020, he was elected Chairman of the Board by our board of directors. In 1995, he founded Dental One (now Dental One Partners), which, as President and Chief Executive Officer he grew to become one of the leading DSOs (dental service organizations) in the country, with over 165 practices in 15 states. After a successful sale of Dental One to MSD Capital in 2008 and subsequent merger in 2009 with Dental Care Partners, Mr. Huntsman was appointed in 2010 as Chief Executive Officer of ReachOut Healthcare America, a Morgan Stanley Private Equity portfolio company. In 2012, he founded Xenith Practices, LLC, a DSO focused on rolling up larger independent general dental offices, which were sold in 2015. From January 2014 to September 2015, Mr. Huntsman founded and served as the Chief Executive Officer of Ortho Ventures, LLC, a US distributor of certain pediatric oral appliances with applications for pediatric sleep disordered breathing. Since November 2015, he has served as the Chief Executive Officer of First Vivos, Inc., which is now our wholly owned subsidiary. He was also a founding member of the Dental Group Practice Association (DGPA), now known as the Association of Dental Support Organizations (ADSO). He is the father of Todd Huntsman, Sr. Vice President, Product and Technology. He holds a BS degree in finance from Brigham Young University.
G. Dave Singh, DMD, Ph.D., DDSc. is the founder of our company and has served as our Chief Medical Officer and as a director since September 2016. Until June 2019, he also served as our President. Since January 2008, Dr. Singh served as the Chief Executive Officer of BioModeling Solutions, Inc., which became our wholly owned subsidiary. Dr. Singh is regarded as a leading professor in the field of SDB in all its many forms. He was awarded a grant by the British Society for Developmental Biology (University of Oxford, UK), and later was appointed to the Board of Examiners, Royal College of Surgeons of England. As an “outstanding professor” supported by Harvard University, University of Michigan, and University of Hawaii, he was invited to relocate to the US where he led a NIH-funded program of clinical craniofacial research. Currently, he is a Board Member of the American Sleep and Breathing Association and Member of the World Sleep Society. He has published over 200 articles in the peer-reviewed medical, dental and orthodontic literature, and 7 books/chapters. His pioneering research into epigenetic influencers on craniofacial growth and development led to the development of the patented DNA appliance® and mRNA appliance® technology. He holds a DDSc in orthodontics from University of Dundee, UK, a Ph.D. in Craniofacial Development from University of Bristol, UK, and a BDS/DMD in dentistry from University of Newcastle, UK. In 2020, Dr. Singh was given a lifetime achievement award as one of the world’s top 100 doctors in dentistry for his work on sleep apnea.
Bradford Amman has served as our Chief Financial Officer since October 2018. From January 2017 to October 2018, Mr. Amman served as the Chief Financial Officer and Chief Operations Officer of InLight Medical, a manufacturer and distributor of medical devices cleared by the FDA for increased circulation and reduced pain. Prior to InLight, from 2010 to 2017, he served as CereScan Corp.’s Chief Financial Officer. CereScan specializes in state-of-the-art functional brain imaging, utilizing a patented process, the latest generation functional imaging SPECT and PET cameras and the industry’s leading brain imaging software to assist in the diagnosis of a magnitude of brain-related conditions and disorders. Mr. Amman served as Chief Financial Officer of LifeVantage Corporation from 2006 to 2010, including during its initial public offering. Mr. Amman holds a Master of Business Administration from the University of Notre Dame and a BS in Accounting from the University of Denver.
| 92 |
Directors
Ralph E. Green, DDS, MBA joined our board in June 2020. He has devoted more than 35 years to senior level executive positions. Since 2003, Dr. Green has served as President and CEO of his proprietary dental practice. From 2003 to 2017 he served as Vice President of Clinical Affairs for ReachOut Healthcare America, a Morgan Stanley Private Equity company focused on Arizona’s underserved children’s population. From1997 through 2002, Dr. Green was President of Zila Pharmaceuticals Inc. where he was engaged in clinical trials, patent development and regulatory approval submissions. Dr. Green has done extensive research on bone growth and oral cancer. In the mid-1980’s, Bofors Nobel-Pharma selected Dr. Green to establish the Swedish Branemark Dental Implant in America, now known as Nobel Biocare, the global leader in dental implants with several billions in sales. In 1987, Dr. Green discovered and patented a method of activating the titanium implant surface to enhance its success rate. He started his own titanium implant company, OTC America, which was acquired after 18 months by Collagen Corporation, where he served as Senior Vice President. Following his tenure at Collagen, he started his own consulting firm, Biofusion Technology. He also served as Assistant Professor in the Tufts University School of Medicine and School of Dental Medicine in the 1970’s and 1980’s. Dr. Green has served as President-elect and director of the Dental Manufacturers of America. He was honored as a fellow in the Academy of International Dentistry in Nice, France. Dr. Green holds a DDS from the University of Iowa, an MBA from Boston University and a BA in Biology from Graceland University.
Anja Krammer joined our board in June 2020. In early 2020, Ms. Krammer was appointed as the Chief Executive Officer of Turn Biotechnologies, a development stage company focused on reversing aging and age-related diseases. From 2013 through 2018, she co-founded, served as President, Secretary and a director of BioPharmX, a specialty pharmaceutical company where she led the initial public offering onto the New York Stock Exchange in 2015. Ms. Krammer served as Principal/Founder of MBI, Inc., a management consulting firm beginning in January 1998. While at MBI, Inc., Ms. Krammer also served as Vice President Global Marketing from April 2006 to August 2008 for Reliant Technologies, a venture-backed startup in aesthetic medicine. From April 2004 to April 2006, Ms. Krammer served as Sr. Director of Strategic Marketing for Medtronic Corporation. From December 2000 to September 2001, Ms. Krammer was Vice President, Solutions Marketing for Getronics Corporation, a global IT services company. From April 1999 to December 2000, Ms. Krammer served as Vice President, Indirect Channel Sales and Worldwide Industry Partnership Marketing in the Itronix Division of Acterna Corporation, an optical communications company. Ms. Krammer’s other prior roles include serving as Director of Worldwide Marketing and Communications for Tektronix Corporation in its Color Printing and Imaging Division from October 1997 to April 1999. From October 1995 to October 1997, Ms. Krammer was Director of Worldwide Sales and Marketing with KeyTronic Corporation, a computer equipment manufacturer. Ms. Krammer holds a BAIS degree with a focus on Marketing/Management from the University of South Carolina and an International Trade Certificate from the University of Paris—Sorbonne.
Mark F. Lindsay joined our board in June 2020. Since 2008, he has served as a consultant and the director of the healthcare and pharmaceuticals practices group with the Livingston Group. From February 2001 through September 2008, Mr. Lindsay was with UnitedHealth Group, one of the world’s largest healthcare companies, where he held a number of senior positions including President of the AARP Pharmacy Services Division and Vice President of Public Communications and Strategy. In 2008, he served on President Obama’s transition team. From May 1996 through January 2001, Mr. Lindsay served in President Clinton’s White House as Assistant to the President for the Office of Management and Administration. His areas of responsibility included the White House Military Office, which managed Air Force One; The White House Communications Agency; the Medical Unit and Camp David; running the White House Operations; and the Executive Office of the President’s Office of Administration, which was responsible for finance, information systems, human resources, legal/appropriations and security. Mr. Lindsay’s office was responsible for the logistics of all domestic and international Presidential travel and special air missions. President Clinton selected Mr. Lindsay to be the operational lead for the White House’s 2001 transition preparation and execution. From 1994 through 1997, Mr. Lindsay served as senior legislative aid and counsel to Congressman Louis Stokes (D-OH). He worked closely with Democrats and the Congressional Black Caucus on a number of business and economic issues. He was also a member of Senator Hillary Clinton’s Minnesota Finance Committee for her 2008 Presidential campaign. Mr. Lindsay holds a graduate degree from Macalester College in St. Paul, Minnesota; a Juris Doctorate from Case Western Reserve University School of Law; a master’s degree in international Affairs from Georgetown University; and a graduate degree from the Advanced Management program at the University of Pennsylvania’s Wharton Business School. He is a member of the District of Columbia Bar.
| 93 |
Leonard J. Sokolow, joined our board in June 2020. Since 2015, Mr. Sokolow has been Chief Executive Officer and President of Newbridge Financial, Inc., a financial services holding company and Chairman of Newbridge Securities Corporation, its full service broker-dealer. From 2008 through 2012, he served as President and Vice Chairman of National Holdings Corporation, a publicly traded financial services company. From November 1999 until January 2008, Mr. Sokolow was Chief Executive Officer and President, and a member of the Board of Directors, of vFinance Inc., a publicly traded financial services company, which he cofounded. Mr. Sokolow was the Chairman of the Board of Directors and Chief Executive Officer of vFinance Inc. from January 2007 until July 2008, when it merged into National Holdings Corporation, a publicly traded financial services company. Mr. Sokolow was founder, chairman and chief executive officer of the Americas Growth Fund Inc., a closed-end 1940 Act management investment company, from 1994 to 1998. From 1988 until 1993, Mr. Sokolow was an Executive Vice President and the General Counsel of Applica Inc., a publicly traded appliance marketing and distribution company. From 1982 until 1988, Mr. Sokolow practiced corporate, securities and tax law and was one of the founding attorneys and a partner of an international boutique law firm. From 1980 until 1982, he worked as a Certified Public Accountant for Ernst & Young and KPMG Peat Marwick. Since June 2006, Mr. Sokolow has served on the Board of Directors of Consolidated Water Company Ltd. (NASDAQ: CWCO) and as Chairman of its Audit Committee; as well as a member of its Nominations and Corporate Governance Committee since 2011. Since January 2016 Mr. Sokolow has served as a member of the Board of Directors of SQL Technologies Corp., d/b/a Sky Technologies and Chairman of its Audit Committee and, since September 2016, Chairman of its Corporate Development Committee. The Audit Committee of Vivos has determined that Mr. Sokolow meets the statutory requirements to be identified as the audit committee financial expert.
Matthew Thompson, M.D. joined our board in June 2020. Since December 2016, Dr. Thompson has served as Chief Medical Officer of Endologix LLC. Dr. Thompson is an Adjunctive Professor at Stanford School of Medicine (since 2017) and contract surgeon and Visiting Professor at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University (since 2020). Prior to joining Endologix, Dr. Thompson served as Professor of Vascular Surgery at St. George’s University of London and St George’s Vascular Institute (2002-2016). Dr. Thompson’s awards include a Hunterian Professorship, the Moynihan traveling fellowship and the gold medal for the intercollegiate examination. Dr. Thompson is also the editor of the Oxford Textbook of Vascular Surgery and the Oxford Handbook of Vascular Surgery. Dr. Thompson was Chair of the National Specialized Commissioning Clinical Reference Group (2013-2016) for Vascular Services and is a founder of the British Society for Endovascular Therapy (2004). Dr. Thompson was a Council Member of the Vascular Society (2014-2017), and Chairman of the Vascular Society Annual Scientific Meeting (2014-2017). Dr Thompson was the clinical director for three London-wide service reconfigurations (cardiovascular disease, major trauma and emergency services) (2010-2013). Dr. Thompson trained at Cambridge University (1981-1984), St. Bartholomew’s Hospital (1984-1987), the University of Leicester (1994) and Adelaide (1998).
Directors and Executive Officers Qualifications
Although we have not formally established any specific minimum qualifications that must be met by each of our officers, we generally evaluate the following qualities: educational background, diversity of professional experience, including whether the person is a current or was a former chief executive officer or chief financial officer of a public company or the head of a division of a prominent international organization, knowledge of our business, integrity, professional reputation, independence, wisdom, and ability to represent the best interests of our shareholders.
The nominating and corporate governance committee of the board of directors prepare policies regarding director qualification requirements and the process for identifying and evaluating director candidates for adoption by the board of directors. The above-mentioned attributes, along with the leadership skills and other experiences of our officers and board of directors members described above, provide us with a diverse range of perspectives and judgment necessary to facilitate our goals of shareholder value appreciation through organic and acquisition growth.
Director Qualifications
R. Kirk Huntsman – Our board believes that Mr. Huntsman’s qualifications to serve on our board include his extensive experience in the dental industry, focusing on dental support organizations by integrating cutting-edge technology and better management practices.
| 94 |
G. Dave Singh, DMD, Ph.D., DDSc – Our board believes that Dr. Singh’s qualifications to serve on our board include his extensive experience in the treatment of craniofacial conditions that are often associated with SDB and OSA and experience in developing the patented Vivos System.
Ralph E. Green, DDS, MBA – Our board believes that Dr. Green’s qualifications to serve on our board include his extensive experience and relationships in the dental industry, his expertise with clinical trials and executive-level experience with pharmaceutical and dental implant firms.
Anja Krammer – Our board believes that Ms. Krammer’s qualifications to serve on our board include her experience as a director and chief executive officer, experience with startup enterprises, her successful leadership roles in securing capital markets funding, and her experience in the pharmaceutical industry.
Mark F. Lindsay – Our board believes that Mr. Lindsay’s qualifications to serve on our board include his director experience and his experience in legal, governmental, regulatory and business development within the healthcare industry.
Leonard J. Sokolow – Our board believes Mr. Sokolow’s qualifications include his experience as a director and principal executive officer, his legal, accounting, auditing and consulting background, and that he meets the statutory requirements to be identified as an “audit committee financial expert.”
Matthew Thompson, M.D. – Our board believes that Dr. Thompson’s qualifications to serve on our board include his executive-level experience with a publicly-traded medical technology firm and his extensive medical background.
Director Independence
Our Board of Directors has affirmatively determined that Ms. Krammer, Mr. Lindsay, Dr. Thompson, Dr. Green and Mr. Sokolow are “independent directors,” and Mr. Huntsman and Dr. Singh are “non-independent directors,” as defined by the applicable rules and regulations of the Nasdaq.
Board Leadership Structure and Board’s Role in Risk Oversight
R. Kirk Huntsman is our Chairman of the Board as well as our Chief Executive Officer. The Chairman has authority, among other things, to preside over board meetings and set the agenda for board meetings. Accordingly, the Chairman has substantial ability to shape the work of our board. We believe that the presence of five independent members of our board ensures appropriate oversight by the board of our business and affairs. However, no single leadership model is right for all companies and at all times. The board recognizes that depending on the circumstances, other leadership models, such as the appointment of a lead independent director, might be appropriate. Accordingly, the board may periodically review its leadership structure. In addition, the board holds executive sessions in which only independent directors are present.
Our board is generally responsible for the oversight of corporate risk in its review and deliberations relating to our activities. Our principal source of risk falls into two categories, financial and product commercialization. Our Audit Committee oversees management of financial risks; our board regularly reviews information regarding our cash position, liquidity and operations, as well as the risks associated with each. The board regularly reviews plans, results and potential risks related to our product offerings, growth, and strategies. Our Compensation Committee oversees risk management as it relates to our compensation plans, policies and practices for all employees including executives and directors, particularly whether our compensation programs may create incentives for our employees to take excessive or inappropriate risks which could have a material adverse effect on our company.
Committees of the Board of Directors
Our Board of Directors established an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. The composition and function of each committee are described below.
| 95 |
Audit Committee
The Audit Committee has three members that are independent directors, including Mr. Sokolow, Ms. Krammer and Dr. Green. Mr. Sokolow serves as the chair of the Audit Committee and satisfies the definition of “audit committee financial expert”. Our Audit Committee has adopted a written charter, a copy of this charter is posted on the Corporate Governance section of our website, at www.vivoslife.com (click “Investor Relations” and “Governance”). Our Audit Committee is authorized to:
| ● | approve and retain the independent auditors to conduct the annual audit of our financial statements; | |
| ● | review the proposed scope and results of the audit; | |
| ● | review and pre-approve audit and non-audit fees and services; | |
| ● | review accounting and financial controls with the independent auditors and our financial and accounting staff; | |
| ● | review and approve transactions between us and our directors, officers and affiliates; | |
| ● | recognize and prevent prohibited non-audit services; and | |
| ● | establish procedures for complaints received by us regarding accounting matters; oversee internal audit functions, if any. |
Compensation Committee
The Compensation Committee has three members that are independent directors, including Mr. Lindsay, Dr. Thompson and Dr. Green. Mr. Lindsay serves as the chair of the Compensation Committee. Our Compensation Committee has adopted a written charter, a copy of this charter is posted on the Corporate Governance section of our website, at www.vivoslife.com (click “Investor Relations” and “Governance”). Our Compensation Committee is authorized to:
| ● | review and determine the compensation arrangements for management; | |
| ● | establish and review general compensation policies with the objective to attract and retain superior talent, to reward individual performance and to achieve our financial goals; | |
| ● | review and determine our stock incentive and purchase plans; | |
| ● | oversee the evaluation of the Board of Directors and management; and | |
| ● | review the independence of any compensation advisers. |
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee has three members that are independent directors, including Dr. Thompson, Ms. Krammer and Mr. Sokolow. Dr. Thompson serves as the chair of the Nominating and Corporate Governance Committee. Our Nominating and Corporate Governance Committee has adopted a written charter, a copy of this charter is posted on the Corporate Governance section of our website, at www.vivoslife.com (click “Investor Relations” and “Governance”). The functions of our Governance Committee, among other things, include:
| ● | identifying individuals qualified to become board members and recommending directors; | |
| ● | nominating board members for committee membership; | |
| ● | developing and recommending to our board corporate governance guidelines; | |
| ● | reviewing and determining the compensation arrangements for directors; and | |
| ● | overseeing the evaluation of our board of directors and its committees and management. |
| 96 |
Compensation Committee Interlocks and Insider Participation
None of the members of our Compensation Committee, at any time, has been one of our officers or employees, or, during the last fiscal year, was a participant in a related-party transaction that is required to be disclosed. None of our executive officers currently serves, or in the past year has served, as a member of the Board of Directors or Compensation Committee of any entity that has one or more executive officers on our Board of Directors or Compensation Committee.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics is available at our website at www.vivoslife.com (click “Investor Relations” and “Governance”). We expect that any amendments to the code, or any waivers of its requirement, will be disclosed on our website.
July 2019 Director Resignation Agreements
On July 18, 2019, three directors of our company, Kelly J. McCrann, Paul Lajoie and Dan McKeon, each voluntarily resigned as members of the board of directors. The directors resigned after discussions with the board regarding the optimal size and composition of the board for purposes of our initial public offering and for thereafter operating as a public company. In addition, one director resigned due to the requirements of other professional commitments. In connection with such resignations, we entered into separate Resignation Agreements with each of the resigning directors. Pursuant to such Resignation Agreements, Paul Lajoie, Kelly J. McCrann and Dan McKeon each received options to purchase 8,334 shares of our common stock, which options have an exercise price of $7.50 per share and which expire on July 18, 2024. The Resignation Agreements contain customary confidentiality, non-disparagement and mutual release provisions. We do not believe that the Resignation Agreements are material to our company on an ongoing basis.
2020 Investigation and Recommendations of Joint Board Committee
In February 2020, an issue regarding stock sales by members of our senior management, was brought to the attention of the Audit Committee, and a recommendation was made by our then General Counsel that our company adopt a new formal written policy pertaining to such matters, which had not existed prior to this. Further, and in order to ascertain that no violations of securities law or ethics had occurred, an internal investigation was undertaken by a joint committee of our board consisting of the members of our board’s Audit Committee and Nominating and Corporate Governance Committee in accordance with authority delegated to such committees under their respective charters. With the input of internal and external counsel, the investigation concluded that no securities laws had been violated in connection with such sales, and further concluded that enhanced corporate governance (in the form of a formal written policy on private stock sales requiring prior approval of our internal or external legal counsel) should be implemented. Pursuant to the findings and recommendations of the joint committee, an insider stock resale policy and other organizational matters, including changing of duties of certain other employees, were formally adopted by the board on April 27, 2020 and these policies and organizational changes remain in place in all material respects. Notwithstanding the board’s approval of these changes, certain organizational matters that were adopted by the board, including relating to the Board’s oversight over employees, were deemed by Mr. Huntsman and, in certain instances, other members of the board to be inappropriate, impractical, and excessively intrusive in day-to-day management issues, and were opposed. Our board of directors adopted an Insider Trading policy appropriate for a publicly-traded company which is available at our website at www.vivoslife.com (click “Investor Relations” and “Governance”).
2020 Removal of Independent Directors and Reconstitution of the Board
On April 30, 2020, a group of our shareholders, representing a majority interest (including R. Kirk Huntsman and G. Dave Singh, who serve as our Chairman of the Board/Chief Executive Officer and Chief Medical Officer, respectively), acted by written consent to action under Wyoming law to remove all three independent directors then serving on our board of directors: Cody Teets, Carol Coughlin and Robert Mitchell. This action was taken because of disagreements on organizational matters as described above and further because such shareholders believed it to be in the best interest of our company to have a group of independent directors with different experiences, perspectives and skill sets as we transitioned from a private to a public company.
| 97 |
Following the removal of these three directors, the remaining directors appointed Gregg C.E. Johnson, a co-founder of our company who also served as our corporate secretary from 2016 to April 2020, to our board on an interim basis until our next Annual Meeting of Shareholders. Subsequent to their removal, two of the directors, Carol Coughlin and Robert Mitchell, voluntarily entered into Separation Agreements with our company in July 2020. Such Separation Agreements contained customary releases, confidentiality and non-disparagement provisions. As consideration for the entering the Separation Agreements, Ms. Coughlin and Mr. Mitchell each received an equity grant in the amount 16,667 shares and the ability to retain and exercise their previously granted and vested options, and we also committed to providing continued indemnification obligations consistent with our organizational documents and to retain director’s and officer’s insurance for a period of twenty-four months in connection with Ms. Coughlin’s and Mr. Mitchell’s prior service on the board. In August 2020, we also entered into a Separation Agreement with Cody Teets pursuant to which we are required to purchase from Ms. Teets and her affiliated entities 13,575 shares of Series B Preferred Stock and warrants to purchase common stock and 16,667 shares of common stock held for an aggregate purchase price of $325,000. In addition, pursuant to the Separation Agreement with Ms. Teets, since we did not close a qualified financing, as defined in the agreement of at least $3,000,000 of equity or equity-linked securities by October 28, 2020, Ms. Teets had the option of receiving a modified consideration package consisting of 16,667 shares of unrestricted, fully vested common stock, a grant of stock options to purchase 33,334 shares of common stock at a price of $7.50 that would be fully vested and exercisable and $22,000 in cash. In November 2020, Ms. Teets elected the modified consideration on her Separation Agreement. We do not believe that the Separation Agreements are material to our company on an ongoing basis.
As a result of the removal of these directors, our remaining board members assembled the slate of director nominees for election at our next annual meeting. Mr. Johnson did not stand for re-election. Our entire slate of directors was elected at our annual general meeting on June 18, 2020 and the current membership includes five independent directors from diverse backgrounds that will assist our business going forward.
October 2020 Derivative Demand and Settlement
On October 22, 2020, two minority stockholders of our company, Lazarus Asset Management, LLC and Paul Lajoie, a former director of our company (who we refer to as the Demanding Stockholders), sent a derivative demand to us through counsel asking our board of directors to review and investigate certain recent actions taken by our board of directors, or members thereof, and our senior management including (i) our pursuit of the initial public offering described in this prospectus, (ii) our board of directors’ previous rejection (on two occasions) of a “reverse merger” transaction proposal made by Lazarus Asset Management, LLC, (iii) purported mismanagement of our corporate assets, and (iv) various matters related to stock sales described above under the caption “2020 Investigation and Recommendations of Joint Board Committee” above and other matters, with the Demanding Stockholders asserting that these actions may have constituted breaches of fiduciary duties, gross corporate mismanagement, waste of corporate assets, material misrepresentations and/or insider self-dealing. After discussions with the Demanding Stockholders and their counsel, we ascertained that the Demanding Stockholders were acting for themselves and on behalf of an additional group of minority shareholders, (we refer to the Demanding Stockholders and all such other minority shareholders they acted on behalf of collectively as the Stockholder Group). In addition to Mr. Lajoie, the Stockholder Group included another former director of our company, Joe Womack.
While we believe that the assertions of the Demanding Stockholders lacked any merit in fact and in law, rather than expending resources investigating or litigating the claims of the Demanding Stockholders, and in order to proceed with our initial public offering, on November 6, 2020, without admitting or denying any claims asserted by the Demanding Stockholders, we entered into a Settlement and Release Agreement with each member of the Stockholder Group (which we refer to as the Settlement and Release Agreement). Pursuant to the Settlement and Release Agreement, all claims of the Demanding Stockholders were withdrawn with prejudice, and we and the Stockholder Group provided each other with full releases of any claims. In consideration of such withdrawal and releases, the members of the Stockholder Group have received: (i) an aggregate of 300,000 shares of our common stock, which shares are subject to a lock-up agreement on terms identical to those executed by other investors in connection with our initial public offering and further may not be sold by the members of the Stockholder Group until June 13, 2021, and thereafter the members of the Stockholder Group may only sell such shares at the rate of 20% of each Stockholder Group members’ respective pro rata portion of such shares per month and (ii) warrants to purchase an aggregate of 325,000 shares of our common stock. Such warrants (x) will be exercisable on a cash only basis at a strike price of $7.50, (y) will be exercisable for a period of 36 months, beginning June 13, 2021 and ending on June 13, 2024. In addition, each member of the Stockholder Group has executed a lock-up agreement in connection with our initial public offering with respect to any other securities of our company they may hold on terms identical to those executed by other investors in connection with our initial public offering. Finally, the Settlement and Release Agreement contains customary representations, warranties and covenants, including relating to confidentiality and non-disparagement, and we reimbursed the Demanding Stockholders for $50,000 of their legal fees associated with the demand letter we received on October 22, 2020 from them.
2021 Washington State Investigation
On April 13, 2021 the Washington State Department of Financial Institutions (or WSDFI) sent a letter and subpoena requesting that we produce certain documents and records. WSDFI is investigating certain sales of our common stock by a previous employee and independent contractor in Washington prior to our initial public offering. This subject matter in general (including activities of such previous employee and independent contractor) had been among the issues previously investigated by a joint committee of our Board of Directors and internal and external legal counsel as part of the process described above under “2020 Investigation and Recommendations of Joint Board Committee”. We are cooperating with the WSDFI investigation but it has not yet concluded.
Ortho Ventures Bankruptcy
Ortho Ventures, LLC was a Texas limited liability company controlled and operated by its managing member, R. Kirk Huntsman (our Chairman and Chief Executive Officer). Ortho Ventures was established as a single-product national distributor in the pediatric orthodontic appliance space. In August 2015, Ortho Ventures’ negotiations with its sole supplier (Ortho-Tain, Inc.) came to an impasse, and Ortho Ventures’ distribution rights were terminated. Ortho Ventures thus subsequently wound down and ceased operations. In September 2017, Ortho Ventures filed for Chapter 7 bankruptcy protection. The bankruptcy case was closed on October 30, 2018.
| 98 |
Summary Compensation Table
The following summary compensation table provides information regarding the compensation paid during our fiscal years ended December 31, 2020 and 2019 to our Chief Executive Officer (principal executive officer), our Chief Medical Officer, and our Chief Financial Officer (principal financial officer). We refer to these individuals as our “named executive officers”, or “NEOs”.
| Name and Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non- Equity Incentive Plan Compensation ($) | Non- qualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||||||||
| R. Kirk Huntsman,(1) | 2020 | $ | 251,784 | — | — | — | $ | 177,847 | (5) | — | $ | 25,705 | (6)(7) | $ | 455,336 | ||||||||||||||||||||
| Chief Executive Officer (principal executive officer) | 2019 | $ | 249,231 | — | — | — | $ | 56,982 | (5) | — | $ | 21,872 | (6) | $ | 328,085 | ||||||||||||||||||||
| G. Dave Singh (2) | 2020 | $ | 250,492 | — | — | — | $ | 32,987 | (5) | — | 15,028 | (6) | $ | 298,507 | |||||||||||||||||||||
| Chief Medical Officer | 2019 | $ | 249,231 | — | — | — | $ | 28,941 | (5) | — | $ | 16,235 | (6) | $ | 294,407 | ||||||||||||||||||||
| Bradford Amman (3) | 2020 | $ | 181,167 | — | — | — | $ | 65,348 | (5) | — | $ | 22,423 | (6)(7) | $ | 268,938 | ||||||||||||||||||||
| Chief Financial Officer (principal accounting officer) | 2019 | $ | 180,000 | — | — | $ | 98,727 | (4) | — | — | 18,493 | (6)(7) | $ | 297,220 | |||||||||||||||||||||
| (1) | Mr. Huntsman has served as Chief Executive Officer of our company since September 2016. Since November 2015, Mr. Kirk Huntsman served as Chief Executive Officer of First Vivos, Inc., a wholly owned subsidiary of our company, which we acquired in August 2016. |
| (2) | Dr. Singh has served as Chief Medical Officer of our company since September 2016 and served as our President from September 2016 to June 2019. Since July 2008, Dr. Singh served as Chief Executive Officer of BioModeling Solutions, Inc., a wholly owned subsidiary of our company, which we acquired in August 2016. |
| (3) | Mr. Amman joined our company as Chief Financial Officer in October 2018. In November 2019, Mr. Amman was granted stock options to purchase up to 16,667 shares of the common stock of Vivos Therapeutics, Inc. at an exercise price of $7.50 per share. |
| (4) | Stock option award value was based upon a Black-Scholes valuation calculation at the date of the stock option grant. We provide information regarding the assumptions used to calculate the value of all stock option awards made to named executive officers in Note 9 to our audited financial statements for the fiscal year ended December 31, 2020 and 2019. |
| (5) | Represents annual incentive compensation in accordance with terms of individual employment agreement, including estimated future compensation earned but not paid as of December 31, 2020 ($65,973 for Mr. Huntsman and $32,987 for Dr. Singh). |
| (6) | Includes company contributions towards health insurance premiums in 2020 and 2019 ($16,705 and $18,122 for Mr. Huntsman and $18,163 and $16,718 for Mr. Amman respectively). |
| (7) | Includes 2020 and 2019 company paid automobile expense reimbursement of $9,000 and $3,750 for Mr. Huntsman and $4,260 and $1,775 for Mr. Amman respectively. |
| 99 |
Employment Agreements
R. Kirk Huntsman
We entered into an amended employment agreement on October 8, 2020 (the Huntsman Effective Date) with R. Kirk Huntsman. The term of the employment agreement commenced on the Huntsman Effective Date and is subject to termination:
(i) for cause (as defined therein) by us or without cause by Mr. Huntsman, whereby Mr. Huntsman would be entitled to earned but unpaid compensation, bonuses and benefits through the date of termination and his option shares through the date of termination for cause will be deemed vested;
(ii) upon the death or disability of Mr. Huntsman, whereby Mr. Huntsman, upon disability, or Mr. Huntsman’s estate, upon death of Mr. Huntsman, will be entitled to receive all compensation and benefits through the date of death or disability as well as continue to receive incentive compensation (as set forth in the agreement) through the end of our fiscal year, as well as salary payable in periodic installments on regular paydays, at the rate then in effect for a period of six months (in addition to the incapacity period, as defined therein, if terminated upon disability) following termination (the “Extended Period”) and his option shares through the Extended Period will be deemed vested; or
(iii) without cause by us or for “Good Reason” (as defined therein) by Mr. Huntsman, whereby Mr. Huntsman would be entitled to receive all earned but unpaid compensation, bonuses and benefits through the date of termination as well as continue to receive incentive compensation (as set forth in the agreement) as well as salary payable in periodic installments on regular paydays, at the rate then in effect for a period of one year (if terminated without cause by us) or two years (if terminated upon Good Reason by Mr. Huntsman) following termination and all of his option shares will be deemed vested.
Pursuant to the terms of the employment agreement, in exchange for Mr. Huntsman’s services as Chief Executive Officer, we agreed to:
(i) pay Mr. Huntsman an annual base salary of $344,229 during the term of the employment agreement less taxes payable in accordance with employer’s normal policies, subject to adjustment by the Board at its sole discretion;
(ii) make Mr. Huntsman eligible for incentive cash compensation under a management by objectives incentive plan at 65% of base salary that shall be paid not less than frequently than annually when certain targets are met;
(iii) make available to Mr. Huntsman employee benefits available to regular full-time executive management employees of our company, including medical and dental insurance, pension and profit-sharing plans, 401(k) plans, incentive savings plans, group life insurance, salary continuation plans, disability coverage and other fringe benefits.;
(iv) make available to Mr. Huntsman other equity-based compensation awards under our equity incentive plans and otherwise, which equity awards may be granted pursuant to the authority and sole discretion of the Board, together with the Compensation Committee;
(v) make available to Mr. Huntsman paid cellular and high-speed internet access, at our expense, including monthly service charges and maintenance, for use on company business.
G. Dave Singh
We entered into an amended employment agreement on October 9, 2020 (the Singh Effective Date) with G. Dave Singh. The term of the employment agreement commenced on the Singh Effective Date and is subject to termination:
(i) for cause (as defined therein) by us or without cause by Dr. Singh, whereby Dr. Singh would be entitled to earned but unpaid compensation, bonuses and benefits through the date of termination and his option shares through the date of termination for cause will be deemed vested;
| 100 |
(ii) upon the death or disability of Dr. Singh, whereby Dr. Singh, upon disability, or Dr. Singh’s estate, upon death of Dr. Singh, will be entitled to receive all compensation and benefits through the date of death or disability as well as continue to receive incentive compensation (as set forth in the agreement) through the end of our fiscal year, as well as salary payable in periodic installments on regular paydays, at the rate then in effect for a period of six months (in addition to the incapacity period, as defined therein, if terminated upon disability) following termination (the “Extended Period”) and his option shares through the Extended Period will be deemed vested; or
(iii) without cause by us or for “Good Reason” (as defined therein) by Dr. Singh, whereby Dr. Singh would be entitled to receive all earned but unpaid compensation, bonuses and benefits through the date of termination as well as continue to receive incentive compensation (as set forth in the agreement) as well as salary payable in periodic installments on regular paydays, at the rate then in effect for a period of one year (if terminated without cause by us) or two years (if terminated upon Good Reason by Dr. Singh) following termination and all of his option shares will be deemed vested.
Pursuant to the terms of the employment agreement, in exchange for Dr. Singh’s services as Chief Medical Officer, we agreed to:
(i) pay Dr. Singh an annual base salary of $288,269 during the term of the employment agreement less taxes payable in accordance with employer’s normal policies, subject to adjustment by the board at its sole discretion;
(ii) make Dr. Singh eligible for incentive cash compensation under a management by objectives incentive plan at 35% of base salary that shall be paid not less than frequently than annually when certain targets are met;
(iii) make available to Dr. Singh employee benefits available to regular full-time executive management employees of our company including medical and dental insurance, pension and profit-sharing plans, 401(k) plans, incentive savings plans, group life insurance, salary continuation plans, disability coverage and other fringe benefits.; and
(iv) make available to Dr. Singh other equity-based compensation awards under our equity incentive plans and otherwise, which equity awards may be granted pursuant to the authority and sole discretion of the board, together with the Compensation Committee.
Bradford Amman
We entered into an amended employment agreement on October 8, 2020 (the Amman Effective Date) with Bradford Amman. The term of the employment agreement commenced on the Amman Effective Date and is subject to termination:
(i) for cause (as defined therein) by us or without cause by Mr. Amman, whereby Mr. Amman would be entitled to earned but unpaid compensation, bonuses and benefits through the date of termination and his option shares through the date of termination for cause will be deemed vested;
(ii) upon the death or disability of Mr. Amman, whereby Mr. Amman, upon disability, or Mr. Amman’s estate, upon death of Mr. Amman, will be entitled to receive all compensation and benefits through the date of death or disability as well as continue to receive incentive compensation (as set forth in the agreement) through the end of our fiscal year, as well as salary payable in periodic installments on regular paydays, at the rate then in effect for a period of six months (in addition to the incapacity period, as defined therein, if terminated upon disability) following termination (the “Extended Period”) and his option shares through the Extended Period will be deemed vested; or
(iii) without cause by us or for “Good Reason” (as defined therein) by Mr. Amman, whereby Mr. Amman would be entitled to receive all earned but unpaid compensation, bonuses and benefits through the date of termination as well as continue to receive incentive compensation (as set forth in the agreement) as well as salary payable in periodic installments on regular paydays, at the rate then in effect for a period of one year (if terminated without cause by us) or two years (if terminated upon Good Reason by Mr. Amman) following termination and all of his option shares will be deemed vested.
| 101 |
Pursuant to the terms of the employment agreement, in exchange for Mr. Amman’s services as Chief Financial Officer, we agreed to:
(i) pay Mr. Amman an annual base salary of $230,558 during the term of the employment agreement less taxes payable in accordance with employer’s normal policies, subject to adjustment by the board at its sole discretion;
(ii) make Mr. Amman eligible for incentive cash compensation under a management by objectives incentive plan at 35% of base salary that shall be paid not less than frequently than annually when certain targets are met;
(iii) make available to Mr. Amman employee benefits available to regular full-time executive management employees of our company including medical and dental insurance, pension and profit-sharing plans, 401(k) plans, incentive savings plans, group life insurance, salary continuation plans, disability coverage and other fringe benefits.;
(iv) make available to Mr. Amman other equity-based compensation awards under our equity incentive plans and otherwise, which equity awards may be granted pursuant to the authority and sole discretion of the board, together with the Compensation Committee; and
(v) make available to Mr. Amman paid cellular telephone and high-speed internet access, at our expense, including monthly service charges and maintenance, for use on company business.
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes the number of shares of common stock underlying outstanding equity incentive plan awards for each named executive officer as of December 31, 2020.
| Name | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | |||||||||||||
| R. Kirk Huntsman | 9/30/2017 | (1) | 333,334 | — | $ | 1.65 | 8/31/2021 | |||||||||||
| G. Dave Singh | — | — | — | — | — | |||||||||||||
| Bradford Amman | 11/8/2018 | (2) | 50,000 | 33,334 | 7.50 | 11/8/2023 | ||||||||||||
| 11/18/2019 | (2) | 6,667 | 10,000 | 7.50 | 11/18/2024 | |||||||||||||
| (1) | Stock option grants vests equally over 12 quarters with the first vesting tranche on the grant date and on the last day of each successive calendar quarter through June 30, 2020. |
| (2) | Stock option grant vests 20% on the grant date and 20% on each successive anniversary through the following four years. |
Director Compensation
Historically, our directors have not received compensation for their service except for option grants. We adopted a new director compensation program recommended by our corporate governance committee pursuant to which we would make equity-plan based awards to the directors (i) each of our non-employee directors will receive $48,000 cash compensation annually; (ii) chairs of our committees will receive $10,000 cash compensation annually; and (iii) members of our committees will receive $5,000 cash compensation annually. No additional compensation will be provided for attending committee meetings. Our corporate governance committee will continue to review and make recommendations to the board regarding compensation of directors, including equity-based plans. We will reimburse our non-employee directors for reasonable travel expenses incurred in attending board and committee meetings. We also intend to allow our non-employee directors to participate in our equity compensation plans.
| 102 |
Director Compensation Table
The following table sets forth information concerning the compensation of our directors for the fiscal year ended December 31, 2020:
Fees Earned or Paid In Cash | Stock Awards (9) | Option Awards (10) | Total | |||||||||||||
| Name | ($) | ($) | ($) | ($) | ||||||||||||
| Cody Teets (1) | — | 125,000 | 181,149 | 306,149 | ||||||||||||
| Carol Coughlin (2) | — | 125,000 | — | 125,000 | ||||||||||||
| Robert Mitchell (3) | — | 125,000 | — | 125,000 | ||||||||||||
| Leonard J. Sokolow (4) | 36,750 | — | 90,928 | 127,678 | ||||||||||||
| Matthew Thompson, M.D. (5) | 36,750 | — | 90,928 | 127,678 | ||||||||||||
| Mark F. Lindsay (6) | 33,833 | — | 90,928 | 124,761 | ||||||||||||
| Anja Krammer (7) | 33,833 | — | 90,928 | 124,761 | ||||||||||||
| Ralph E. Green, DDS, MBA (8) | 33,833 | — | 90,928 | 124,761 | ||||||||||||
| (1) | Ms. Teets commenced service as a member of the board on April 18, 2019 and was removed from our board of directors on April 30, 2020. As of December 31, 2020 Ms. Teets has 50,000 shares of common stock underlying vested and unvested options. |
| (2) | Ms. Coughlin commenced service as a member of the board on July 29, 2019 and was removed from our board of directors on April 30, 2020. As of December 31, 2020 Ms. Coughlin has 16,666 shares of common stock underlying vested and unvested options |
| (3) | Mr. Mitchell commenced service as a member of the board on July 29, 2019 and was removed from our board of directors on April 30, 2020. As of December 31, 2020 Mr. Mitchell has 16,666 shares of common stock underlying vested and unvested options. |
| (4) | Mr. Sokolow commenced service as a member of the board on June 19, 2020. As of December 31, 2020 Mr. Sokolow has 16,666 shares of common stock underlying vested and unvested options. |
| (5) | Mr. Thompson commenced service as a member of the board on June 19, 2020. As of December 31, 2020 Mr. Thompson has 16,666 shares of common stock underlying vested and unvested options. |
| (6) | Mr. Lindsay commenced service as a member of the board on June 19, 2020. As of December 31, 2020 Mr. Lindsay has 16,666 shares of common stock underlying vested and unvested options. |
| (7) | Ms. Krammer commenced service as a member of the board on June 19, 2020. As of December 31, 2020 Mr. Krammer has 16,666 shares of common stock underlying vested and unvested options. |
| (8) | Mr. Green commenced service as a member of the board on June 19, 2020. As of December 31, 2020 Mr. Green has 16,666 shares of common stock underlying vested and unvested options. |
| (9) | As consideration for the entering the Separation Agreements, Ms. Teets, Ms. Coughlin, and Mr. Mitchell each received an equity grant in the amount 16,667 shares of common stock at a price of $7.50 that are fully vested and exercisable. |
| (10) | Stock option award value was based upon a Black-Scholes valuation calculation at the date of the stock option grant. We provide information regarding the assumptions used to calculate the value of all stock option awards made to named executive officers in Note 9 to our audited financial statements for the fiscal year ended December 31, 2020. |
2017 Stock Option Plan
The 2017 Stock Option and Stock Issuance Plan (or the 2017 Plan) is intended to promote the interests of our company by providing eligible persons in our employ or service with the opportunity to acquire a proprietary interest, or otherwise increase their proprietary interest, in our company as an incentive for them to continue in such employ or service.
Individuals eligible to participate in the Plan are as follows:
1. employees,
2. non-employee members of the board of directors or the non-employee members of the board of directors of any parent or subsidiary, and
3. consultants and other independent contractors who provide services to us (or any parent or subsidiary)
| 103 |
The common stock issuable under the 2017 Plan shall be shares of authorized but unissued or reacquired common stock. The maximum number of shares of common stock which may be issued over the term of the 2017 Plan shall not exceed 1,333,333 shares.
The exercise price per share shall be fixed by the board of directors or its designated committee, as plan administrator, in accordance with the following provisions: the exercise price per share shall not be less than 100% of the Fair Market Value (as defined in the 2017 Plan) per share of common stock on the option grant date. If the person to whom the option is granted is a 10% stockholder, then the exercise price per share shall not be less than 110% of the Fair Market Value per share of common stock on the option grant date. The exercise price shall become immediately due and payable upon exercise of the option.
2019 Stock Option and Stock Issuance Plan
The 2019 Stock Option and Stock Issuance Plan (or the 2019 Plan) is intended to promote the interests of our company by providing eligible persons in our employ or service with the opportunity to acquire a proprietary interest, or otherwise increase their proprietary interest, in our company as an incentive for them to continue in such employ or service.
Individuals eligible to participate in the 2019 Plan are as follows:
1. employees,
2. non-employee members of the board of directors or the non-employee members of the board of directors of any parent or subsidiary, and
3. consultants and other independent contractors who provide services to us (or any parent or subsidiary)
The common stock issuable under the 2019 Plan shall be shares of authorized but unissued or reacquired common stock. The maximum number of shares of common stock which may be issued over the term of the 2019 Plan shall not exceed 1,166,667 shares.
The exercise price per share shall be fixed by the board of directors or its designated committee, as plan administrator, in accordance with the following provisions: the exercise price per share shall not be less than 100% of the Fair Market Value (as defined in the 2019 Plan) per share of common stock on the option grant date. If the person to whom the option is granted is a 10% stockholder, then the exercise price per share shall not be less than 110% of the Fair Market Value per share of common stock on the option grant date. The exercise price shall become immediately due and payable upon exercise of the option.
| 104 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than the executive and director compensation and other arrangements, which are described in the “Management” section of this prospectus, and the transactions described below, we are not a party to any related party transactions.
On May 4, 2017, we issued 1,000,000 shares of our Series A Preferred Stock to Dr. G. Dave Singh with a value of $5.00 per share in exchange for intellectual property of Dr. Singh with a value of $5,000,000. In 2018, we redeemed 200,000 shares of the 1,000,000 shares of Series A Preferred Stock held by Dr. G. Dave Singh for $5.00 per share (for an aggregate of $1,000,000). During 2019, Dr. Singh exercised his right to redeem 70,000 shares of the Series A Preferred Stock for $5.00 per share for a total of $350,000. During the first six months of 2020, Dr. Singh exercised his right to redeem 30,000 shares of the Series A Preferred Stock for $5.00 per share for a total of $150,000. On February 20, 2020, Dr. Singh requested the redemption of an additional 100,000 shares at $5.00 per share. On December 15, 2020, we redeemed all remaining outstanding shares of Series A Preferred Stock from Dr. Singh for $3,500,000. Our obligation to redeem Dr. Singh’s shares of Series A Preferred Stock was secured by a lien on certain intellectual property assets previously assigned by him to our company. The security agreement terminated upon our redemption of Dr. Singh’s Series A Preferred Stock.
We were a party to a management agreement with Upeva, Inc., a company for which our prior Secretary and a former member of the board of directors, Gregg C.E. Johnson serves as chief executive officer. In return for various legal and other consulting services, we paid Upeva a monthly fee of $10,000 until that arrangement terminated on May 1, 2020. As of December 31, 2020, we owed Upeva, Inc. approximately $10,000. This contract expired April 30, 2020 and was not renewed. Additionally, Mr. Johnson is the beneficial owner of 254,902 common shares of our company through Spire Family Holdings, L.P.
In 2018, the then Chair of our board of directors, Joseph Womack, agreed to guarantee the facility leases for our first two Vivos Centers. In return for providing these lease guarantees, we paid Mr. Womack $100,000. On July 1, 2018, Mr. Womack entered into a consulting agreement with us whereby he was paid $15,000 per month in return for certain executive work prescribed by R. Kirk Huntsman. This contract was terminated December 31, 2018.
On July 1, 2018, we entered into a merger agreement with TMJ & Sleep Therapy Centre of Utah, LLC (“TMJ”) operating as a center in Orem, Utah. TMJ is owned by an employee of ours. Effective October 1, 2019, we sold TMJ to an entity controlled by the spouse of an employee of ours for a total consideration of $1,225,000.
During the year ended December 31, 2020, Cody Teets, one of our former directors who held $200,000 in our convertible notes issued in 2019, exchanged her outstanding notes for 45,252 shares of our common stock.
For the year ended December 31, 2020 and 2019, options for the purchase of 429,012 and 503,333 shares, respectively, of our common stock were granted to our directors, officers, employees and consultants.
In late 2019, a voucher program was offered whereby any employee could pre-purchase a $30,000 VIP deposit with us that could be redeemed in full after February 15, 2020, subject to certain limitations, toward a VIP enrollment the employee brought forth in the future. The purpose of this program was to assist with cash flow constraints at the time. Thirteen vouchers totaling $390,000 were sold. For the year ended December 31, 2020, we redeemed each of the thirteen vouchers totaling $390,000. We include the balance in contract liabilities.
In July 2020, we entered into Separation Agreements with Robert Mitchell and Carol Coughlin. In August 2020, we entered into a Separation Agreement with Cody Teets. For a description of these agreements, see “Management————2020 Removal of Independent Directors and Reconstitution of the Board”.
On November 6, 2020, we entered into the Settlement and Release Agreement with the Stockholder Group, which included to former directors of our company, Paul Lajoie and Joe Womack. For a description of this agreement, see “Management—October 2020 Derivative Demand and Settlement.”
We have entered into indemnification agreements with each of our directors and entered into such agreements with certain of our executive officers. These agreements require us, among other things, to indemnify these individuals for certain expenses (including attorneys’ fees), judgments, fines and settlement amounts reasonably incurred by such person in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on behalf of us or that person’s status as a member of the board of directors to the maximum extent allowed under Wyoming law.
| 105 |
Policies and Procedures for Related Party Transactions
Pursuant to the written charter of our Audit Committee, the Audit Committee will be responsible for reviewing and approving, prior to our entry into any such transaction, all related party transactions and potential conflict of interest situations involving:
| ● | any of our directors, director nominees or executive officers; | |
| ● | any beneficial owner of more than 5% of our outstanding stock; and | |
| ● | any immediate family member of any of the foregoing. |
Our Audit Committee will review any financial transaction, arrangement or relationship that:
| ● | involves or will involve, directly or indirectly, any related party identified above; | |
| ● | would cast doubt on the independence of a director; | |
| ● | would present the appearance of a conflict of interest between us and the related party; or | |
| ● | is otherwise prohibited by law, rule or regulation. |
The Audit Committee will review each such transaction, arrangement or relationship to determine whether a related party has, has had or expects to have a direct or indirect material interest. Following its review, the Audit Committee will take such action as it deems necessary and appropriate under the circumstances, including approving, disapproving, ratifying, canceling or recommending to management how to proceed if it determines a related party has a direct or indirect material interest in a transaction, arrangement or relationship with us. Any member of the Audit Committee who is a related party with respect to a transaction under review will not be permitted to participate in the discussions or evaluations of the transaction; however, the Audit Committee member will provide all material information concerning the transaction to the Audit Committee. The Audit Committee will report its action with respect to any related party transaction to the board of directors.
| 106 |
The following table sets forth information about the beneficial ownership of our common stock as of the date of this prospectus, as adjusted to reflect the sale of 2,962,963 shares of our common stock in this offering based on the assumed offering price, which does not assume the underwriters exercise their option to purchase additional shares of our common stock, for:
| ● | each person known to us to be the beneficial owner of more than 5% of our common stock; | |
| ● | each named executive officer; | |
| ● | each of our directors; and | |
| ● | all of our named executive officers and directors as a group. |
Unless otherwise noted below, the address for each beneficial owner listed on the table is in care of Vivos Therapeutics, Inc., 9137 Ridgeline Blvd., Suite 135, Highlands Ranch, Colorado 80129. We have determined beneficial ownership in accordance with the rules of the SEC. We believe, based on the information furnished to us, that the persons and entities named in the tables below have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws. We have based our calculation of the percentage of beneficial ownership on 18,212,119 shares of our common stock outstanding as of the date of this prospectus.
In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed outstanding shares of common stock underlying convertible securities of our company held by that person that are currently exercisable or convertible or exercisable or convertible within 60 days as of the date of this prospectus. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
| Shares | Percentage of Shares | |||||||||||
| Beneficially Owned Prior to | Beneficially Owned | |||||||||||
| Name of Beneficial Owner | the Offering | Before Offering | After Offering | |||||||||
| G. Dave Singh (1) | 3,219,705 | 18 | % | 15 | % | |||||||
| R. Kirk Huntsman (2) | 2,083,834 | 11 | % | 10 | % | |||||||
| Bradford Amman (3) | 77,667 | * | % | * | % | |||||||
| Mark F. Lindsay (4) | 14,584 | * | % | * | % | |||||||
| Anja Krammer (5) | 14,584 | * | % | * | % | |||||||
| Ralph E. Green, DDS, MBA (6) | 14,584 | * | % | * | % | |||||||
| Leonard J. Sokolow (7) | 14,584 | * | % | * | % | |||||||
| Matthew Thompson, M.D. (8) | 14,584 | * | % | * | % | |||||||
| All executive officers and directors as a group (8 persons) (9) | 5,454,126 | 30 | % | 26 | % | |||||||
* Less than 1%.
| (1) | G. Dave Singh beneficially owns directly 3,219,705 shares of common stock through Himmat LP. Dr Singh and his wife are the members and managers of Himmat LP and may be deemed to have shared voting and dispositive power of all securities beneficially owned by Himmat LP. |
| (2) | R. Kirk Huntsman beneficially owns (i) indirectly 1,749,000 shares of common stock through Coronado V Partners, LLC and (ii) directly 333,334 shares of common stock issuable upon exercise of options held by him, of which all 333,334 are exercisable and, 1,500 shares of common stock purchased in December 2020 in the open market. R. Kirk Huntsman and his wife are the members and managers of Coronado V Partners, LLC. As such, Mr. Huntsman may be deemed to have shared voting and dispositive power of all securities beneficially owned by Coronado V Partners, LLC reported herein. |
| (3) | Includes 76,667 shares of common stock issuable upon exercise of options held by Bradford Amman, all of which are exercisable within 60 days and, 1,000 shares of common stock purchased in December 2020 in the open market. Excludes 123,333 shares of common stock underlying unvested options. |
| (4) | Includes 14,584 shares of common stock issuable upon exercise of options held by Mark F. Lindsay, all of which are exercisable within 60 days. Excludes 2,083 shares of common stock underlying unvested options. |
| (5) | Includes 14,584 shares of common stock issuable upon exercise of options held by Anja Krammer, all of which are exercisable within 60 days. Excludes 2,083 shares of common stock underlying unvested options. |
| (6) | Includes 14,584 shares of common stock issuable upon exercise of options held by Ralph E. Green, DDS, MBA, all of which are exercisable within 60 days. Excludes 2,083 shares of common stock underlying unvested options. |
| (7) | Includes 14,584 shares of common stock issuable upon exercise of options held by Leonard J. Sokolow, all of which are exercisable within 60 days. Excludes 2,083 shares of common stock underlying unvested options. |
| (8) | Includes 14,584 shares of common stock issuable upon exercise of options held by Matthew Thompson M.D., all of which are exercisable within 60 days. Excludes 2,083 shares of common stock underlying unvested options. |
| (9) | Includes: (i) 516,668 shares of common stock issuable upon exercise of options held by this group, of which 462,920 are exercisable within 60 days. Excludes 53,748 shares of common stock underlying unvested options. |
| 107 |
The following description of our capital stock is based upon our certificate of incorporation, our bylaws and applicable provisions of law, in each case as currently in effect. This discussion does not purport to be complete and is qualified in its entirety by reference to our certificate of incorporation, as amended, and our bylaws, copies of which have been filed with the SEC. We encourage you to read the Certificate of Incorporation, the Bylaws and the applicable provisions of the Delaware General Corporation Law for additional information.
Authorized Capital Stock
As of the date of this prospectus, pursuant to our certificate of incorporation, our authorized capital is 250,000,000 shares, of which (1) 200,000,000 shares are common stock, par value $0.0001 per share (or common stock) and (2) 50,000,000 shares are preferred stock, par value $0.0001 per share (or preferred stock), which may, at the sole discretion of our board of directors be issued in one or more series.
As of the date of this prospectus, 18,212,119 shares of common stock have been issued and are outstanding, No shares of preferred stock are current outstanding.
Our board may from time to time authorize by resolution the issuance of any or all shares of the common stock and the preferred stock authorized in accordance with the terms and conditions set forth in the certificate of incorporation for such purposes, in such amounts, to such persons, corporations, or entities, for such consideration and in the case of the preferred stock, in one or more series, all as the Board in its discretion may determine and without any vote or other action by the stockholders, except as otherwise required by law.
Common Stock
As of the date of this prospectus, there were approximately 525 holders of record of our common stock. This number does not include stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities. Each holder of common stock shall be entitled to one vote for each share of common stock held of record by such holder. The holders of shares of Common Stock shall not have cumulative voting rights. The common stock does not have cumulative voting rights. Therefore, holders of a majority of the shares of common stock voting for the election of directors can elect all of the directors. Holders of our common stock representing a majority of the voting power of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of stockholders. Subject to the rights of holders of any class of stock having preference over our common stock, holders of our common stock are entitled to share in all dividends that our board of directors, in its discretion, declares from legally available funds. In the event of a liquidation, dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over the common stock. Our common stock has no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to the common stock.
Our common stock is traded on The Nasdaq Capital Market under the trading symbol “VVOS.”
Warrants Associated with MyoCorrect
In connection with our March 29, 2021 acquisition of certain assets from, and the entry into related agreements with, MyoCorrect, LLC and its affiliates, we issued three year warrants to purchase 200,000 shares of our common stock with an exercise price of $7.50 per share. 25,000 of these warrants vested initially upon issuance, but the remainder only vest and become exercisable upon the achievement of pre-determined performance metrics related to the utilization of MyoCorrect. These warrants may be exercised only for cash, and the exercise price is subject to customary, stock-based anti-dilution protection.
| 108 |
Warrants Associated with Lyon Management & Consulting
In connection with our April 14, 2021 acquisition of certain assets from, and the entry into related agreements with, Lyon Management & Consulting, LLC and its affiliates, we issued three year warrants to purchase 25,000 shares of our common stock with an exercise price of $8.90 per share. 5,000 of these warrants vested initially upon issuance, but the remainder only vest and become exercisable at the end of each anniversary year following the issuance date. These warrants may be exercised only for cash, and the exercise price is subject to customary, stock-based anti-dilution protection.
Warrants Associated with Series B Preferred
There are presently outstanding warrants to purchase an aggregate of 1,199,195 shares of our common stock which were used to the holders of our previously outstanding Series B Preferred Stock (which converted to common stock in connection with our initial public offering). These warrants have an exercise price of $7.50 per share and have a term of five years ending on December 15, 2025. These warrants may be exercised only for cash, and the exercise price is subject to customary, stock-based anti-dilution protection.
Representative’s Warrant Issued in Connection with our Initial Public Offering
In connection with our Initial Public Offering, we issued warrants to the underwriter and its designees that provide for the purchase of 402,500 shares of common stock at an exercise price of $7.50 per share. The warrants are exercisable beginning on June 8, 2021, and expire on December 10, 2025.
November 2020 Warrants
In November 2020, we issued warrants to certain shareholders to purchase an aggregate of 325,000 shares of common stock. Such warrants are substantially similar to the Series B Warrants except such warrants will be exercisable for a period of 36 months, beginning six months after the consummation of our initial public offering and ending on the forty-second month anniversary of the consummation of our initial public offering. See “Management—October 2020 Derivative Demand and Settlement” for further information on the issuance of these warrants.
Warrants Associated with Convertible Notes
On June 13, 2017, we issued warrants to purchase an aggregate of 33,334 shares of common stock to an investor of convertible notes. The warrants are exercisable on a cash basis at an exercise price of $1.50 per share, are exercisable beginning on June 30, 2017, and expire on June 30, 2022.
Warrants Associated with Contractors and Consultants
There are presently outstanding warrants to purchase an aggregate of 95,000 shares of our common stock which are being held by contractors and consultants. These warrants have an exercise price of $7.50 per share. 45,000 of these warrants vested initially upon issuance, but the remainder only vest and become exercisable at the end of each anniversary following the issuance date. These warrants may be exercised only for cash, and the exercise price is subject to customary, stock-based anti-dilution protection.
Issuance of Stock Options
On July 1, 2017, we granted options to purchase 166,667 shares of common stock at an exercise price of $1.50 per share to Upeva, Inc. (which is owned and operated by Gregg Johnson, our former director and Secretary) pursuant to the terms of a consulting agreement. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 9,260 shares as of the date of grant and (ii) options to purchase 4,630 shares at the end of each calendar month following July 1, 2017.
On September 30, 2017, we granted options to purchase 333,334 shares of common stock at an exercise price of $1.65 per share to R. Kirk Huntsman in recognition of his service to our company. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 27,778 shares as of the date of grant and (ii) options to purchase 27,778 shares at the end of each calendar quarter following September 30, 2017.
| 109 |
Adoption of Vivos Therapeutics, Inc. 2017 Stock Option and Stock Issuance Plan
Our board of directors and shareholders adopted and approved on September 22, 2017 and February 9, 2018, respectively, the Vivos Therapeutics, Inc. 2017 Stock Option and Stock Issuance Plan, effective September 22, 2017, under which stock options and restricted stock may be granted to officers, directors, employees and consultants. Under the Plan, a total of 1,333,333 of common stock are reserved for issuance.
Issuance of Stock Options under 2017 Stock Option and Stock Issuance Plan
On September 30, 2017, we granted options to purchase 100,000 shares of common stock at an exercise price of $1.65 per share to each of Joe Womack, Kelly McCrann, Dr. Willis Pumphrey, and Dr. C. Michael Bennett (for an aggregate of 400,000 shares) in recognition of their service as members of our board of directors. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 8,334 shares as of the date of grant and (ii) options to purchase 8,334 shares at the end of each calendar quarter following September 30, 2017 that they serve as directors.
On September 30, 2017, we granted options to purchase 100,000 shares of common stock at an exercise price of $1.65 per share to Susan McCullough in recognition of her service to our company. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 8,334 shares as of the date of grant and (ii) options to purchase 8,334 shares at the end of each calendar quarter following September 30, 2017.
On January 1, 2018, we granted options to purchase 6,667 shares of common stock at an exercise price of $1.50 per share to Amanda Cruess pursuant to the terms of her employment agreement. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 417 shares as of the date of grant and (ii) options to purchase 417 shares at the end of each calendar quarter following January 1, 2018.
On February 9, 2018, we granted options to purchase 83,334 shares of common stock at an exercise price of $4.50 per share to Bryan Ferre in recognition of his service to our company. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 16,667 shares as of the date of grant and (ii) options to purchase 16,667 shares at the end of each year following February 9, 2018.
On February 9, 2018, we granted options to purchase 33,334 shares of common stock at an exercise price of $4.50 per share to Edward Loew. Such granted options to purchase 33,334 shares vested upon the closing of our initial public offering.
On February 9, 2018, we granted options to purchase up to 16,667 shares of common stock at an exercise price of $4.50 per share to each of four of the six advisors on our Board of Advisors (for an aggregate of 66,668 shares). Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 3,334 shares as of the date of grant and (ii) options to purchase 3,334 shares at the end of each year following the date of grant that they serve as advisors.
On April 30, 2018, we granted options to purchase up to 16,667 shares of common stock at an exercise price of $7.50 per share to each of two then incoming members of our board of directors, De Lyle Bloomquist and Chris Strong, and 16,667 to an incoming member of the Board of Advisors, Dr. Bhaskar Savani, (for an aggregate of 50,001 shares). Such granted options are subject to quarterly vesting of 4,167 shares through March 31, 2019.
On April 30, 2018, we granted options to purchase up to 3,334 shares of common stock at an exercise price of $7.50 per share to each of two employees, Dr. C. Michael Bennett and Lori Jones, (for an aggregate of 6,668 shares). Such granted options are subject to quarterly vesting of 667 shares through June 30, 2019.
On April 30, 2018, we granted options to purchase up to 8,334 shares of common stock at an exercise price of $7.50 per share to Cathryn H. Bonar, our compliance officer. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 1,667 shares as of the date of grant and (ii) options to purchase 1,667 shares at the end of each year following the date of grant.
On August 16, 2018, we granted options to purchase up to 66,667 shares of common stock at an exercise price of $7.50 per share to Edward Loew. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 13,334 shares as of the date of grant and (ii) options to purchase 13,334 shares at the end of each year following the date of grant.
On August 16, 2018, we granted options to purchase up to 166,667 shares of common stock at an exercise price of $7.50 per share to Joe Womack, then a member of our board of directors. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 33,334 shares as of the date of grant and (ii) options to purchase 33,334 shares at the end of each year following the date of grant. On March 20, 2019, 100,000 of the 166,667 options expired.
| 110 |
Effective October 22, 2018, we granted options to purchase 83,334 shares of common stock at an exercise price of $7.50 per share to Bradford Amman pursuant to the terms of his employment agreement. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 16,667 shares as of the date of grant and (ii) options to purchase 16,667 shares at the end of each calendar year beginning December 31, 2018. Effective November 18, 2020, we granted an option to purchase 16,667 shares of common stock at an exercise price of $7.50 per share. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 3,334 shares as of the date of grant and (ii) options to purchase 3,334 shares at the anniversary date of grant beginning November 18, 2020.
On November 9, 2018, we granted options to purchase up to 248,336 shares of common stock at an exercise price of $7.50 per share in the following amounts to each of the following officers and employees, 116,667 to Bryan Ferre, Chief Marketing Officer, 83,334 to Bradford Amman, Chief Financial Officer, 16,667 to Edward Loew, Chief Strategy Officer, 25,000 to Cathryn H. Bonar, Chief Compliance Officer, 3,334 to Michele Grasmick and 3,334 to Teri McKenna. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 49,667 shares as of the date of grant and (ii) options to purchase 49,667 shares at the end of each year following the date of grant.
On February 14, 2019, we granted options to purchase up to 50,000 shares of common stock at an exercise price of $7.50 per share to an employee, Corbin Cowan. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 10,000 shares as of the date of grant and (ii) options to purchase 10,000 shares at the end of each year following the date of grant.
On February 14, 2019, we granted options to purchase up to 16,667 shares of common stock at an exercise price of $7.50 per share to Jon Caufield, as a member of our Clinical Advisory Board. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 3,334 shares as of the date of grant and (ii) options to purchase 3,334 shares at the end of each year following the date of grant.
On May 7, 2019, we granted the options to purchase up to an aggregate of 188,334 shares of common stock at an exercise price of $7.50 per share to employees. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant.
On July 18, 2019, we granted the options to purchase up to an aggregate of 25,000 shares of common stock at an exercise price of $7.50 per share to outgoing directors. Such granted options are fully vested upon the date of grant.
On July 22, 2019, we granted the options to purchase up to an aggregate of 33,334 shares of common stock at an exercise price of $7.50 per share to two directors. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) 25% as of the date of grant and (ii) 25% at the end of each calendar quarter following the date of grant.
On August 15, 2019, we granted options to purchase up to an aggregate of 33,334 shares of common stock at an exercise price of $7.50 per share to an external contractor. Such granted options vest according to the following installments, 25% vesting immediately and 75% upon completion of performance obligations, completing endorsement videos, making qualified introductions and other duties.
On November 18, 2019, we granted options to purchase up to 16,667 shares of common stock at an exercise price of $7.50 per share to an officer of the company with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant.
On January 8, 2020, we granted options to purchase up to 23,334 shares of common stock at an exercise price of $7.50 per share in the following amounts to employees and consultants, 16,667 to an employee with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, and 6,667 to a contractor with immediate vesting on January 8, 2020 for services rendered.
| 111 |
On June 19, 2020, we granted options to five independent board members elected by stockholders to serve on the board for a one-year term. The options vest 50% on the date of grant and 12.5% quarterly on September 30, 2020, December 31, 2020, March 31, 2021 and June 19, 2021.
On July 9, 2020, we granted options to purchase up to 8,334 shares of common stock at an exercise price of $7.50 per share in the following amounts to employees and consultants, 1,667 to employees with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, and 6,667 to an advisor with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant.
Adoption of Vivos Therapeutics, Inc. 2019 Stock Option and Stock Issuance Plan
Our board of directors and shareholders adopted and approved on April 18, 2019, the Vivos Therapeutics, Inc. 2019 Stock Option and Stock Issuance Plan, effective April 18, 2019, under which stock options and restricted stock may be granted to officers, directors, employees and consultants. Under the Plan, a total of 333,334 of common stock are reserved for issuance. On June 18, 2020, our stockholders approved an amendment and restatement of the 2019 Plan to increase the number shares or our common stock available for issuance thereunder by 833,333 share of common stock such that, after amendment and restatement of the 2019 Plan, 1,166,667 shares of common stock will be available for issuance under the 2019 Plan.
On July 18, 2019, we granted options to purchase up to an aggregate of 25,000 shares of common stock at an exercise price of $7.50 per share to outgoing directors. Such granted options are fully vested upon the date of grant.
On July 22, 2019, we granted options to purchase up to an aggregate of 33,334 shares of common stock at an exercise price of $7.50 per share to two directors. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) 25% as of the date of grant and (ii) 25% at the end of each calendar quarter following the date of grant.
On October 22, 2019, we granted options to purchase up to an aggregate of 1,667 shares of common stock at an exercise price of $7.50 per share to an outside consultant. Such granted options are fully vested upon the date of grant.
On November 18, 2019, we granted options to purchase up to 121,667 shares of common stock at an exercise price of $7.50 per share in the following amounts to employees and consultants, 60,000 to employees with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, 51,667 to contractors with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, and 10,000 to a contractors over the service period with vesting of 50% upon grant and 50% six months following the grant.
On July 9, 2020, we granted options to purchase up to 217,334 shares of common stock at an exercise price of $7.50 per share in the following amounts to employees and consultants, 85,000 to employees with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, 54,000 to contractors with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant; 75,000 to advisors over the service period with vesting of 50% at the end of each year following the date of grant; and 3,334 to a former employee that vested on the date of grant.
On October 5, 2020, we granted options to purchase 96,668 shares of common stock at an exercise price of $7.50 per share in the following amounts to employees, a former board member and consultants, 50,000 to an employee with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, and 33,334 shares of common stock at an exercise price of $7.50 per share to a former director with immediate 100% vesting; and 12,334 shares of common stock at an exercise price of $7.50 per share to consultants with immediate 100% vesting.
On March 12, 2021, we granted options to purchase 145,000 shares of common stock at an exercise price of $7.50 per share in the following amounts to employees and consultants, 120,000 to two employees (100,000 and 20,000 respectively) with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, and 25,000 to a consultant with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant.
| 112 |
Piggyback Registration Rights
As of the date of this prospectus, the holders of 9,710,010 shares of our common stock, including shares which were issued upon the conversion of our previously outstanding Series B Preferred Stock and common stock warrants associated with the Series B Preferred Stock, are entitled to (or we have otherwise granted to certain parties, subject to such parties signing a lock-up agreement in connection with our initial public offering) piggyback registration rights from prior offerings. Such shares were registered for resale as part of the registration statement for our initial public offering.
Cash Dividends
As of the date of this prospectus, we have not paid any cash dividends to stockholders. The declaration of any future cash dividend will be at the discretion of our board of directors and will depend upon our earnings, if any, our capital requirements and financial position, the general economic conditions, and other pertinent conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
Anti-Takeover Effects of Certain Provisions of Our Bylaws
Provisions of our bylaws could make it more difficult to acquire us by means of a merger, tender offer, proxy contest, open market purchases, removal of incumbent directors and otherwise. These provisions, which are summarized below, are expected to discourage types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us to first negotiate with us. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging takeover or acquisition proposals because negotiation of these proposals could result in an improvement of their terms.
Vacancies. Newly created directorships resulting from any increase in the number of directors and any vacancies on the board of directors resulting from death, resignation, disqualification, removal or other cause shall be filled by a majority of the remaining directors on the board.
Bylaws. Our certificate of incorporation and bylaws authorizes the board of directors to adopt, repeal, rescind, alter or amend our bylaws without shareholder approval.
Removal. Except as otherwise provided, a director may be removed from office only by the affirmative vote of the holders of not less than a majority of the voting power of the issued and outstanding stock entitled to vote.
Calling of Special Meetings of Stockholders. Our bylaws provide that special meetings of stockholders for any purpose or purposes may be called at any time only by the board of directors or by our Secretary following receipt of one or more written demands from stockholders of record who own, in the aggregate, at least 15% the voting power of our outstanding stock then entitled to vote on the matter or matters to be brought before the proposed special meeting.
Effects of authorized but unissued common stock and blank check preferred stock. One of the effects of the existence of authorized but unissued common stock and undesignated preferred stock may be to enable our board of directors to make more difficult or to discourage an attempt to obtain control of our company by means of a merger, tender offer, proxy contest or otherwise, and thereby to protect the continuity of management. If, in the due exercise of its fiduciary obligations, the board of directors were to determine that a takeover proposal was not in our best interest, such shares could be issued by the board of directors without stockholder approval in one or more transactions that might prevent or render more difficult or costly the completion of the takeover transaction by diluting the voting or other rights of the proposed acquirer or insurgent stockholder group, by putting a substantial voting block in institutional or other hands that might undertake to support the position of the incumbent board of directors, by effecting an acquisition that might complicate or preclude the takeover, or otherwise.
| 113 |
In addition, our certificate of incorporation grants our board of directors broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common stock. The issuance also may adversely affect the rights and powers, including voting rights, of those holders and may have the effect of delaying, deterring or preventing a change in control of our company.
Cumulative Voting. Our certificate of incorporation does not provide for cumulative voting in the election of directors, which would allow holders of less than a majority of the stock to elect some directors.
Choice of Forum
Our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware) will be the exclusive forum for: (i) any derivative action or proceeding brought on behalf of us; (ii) any action asserting a claim for breach of a fiduciary duty owed by any director, officer, employee, or agent of ours or our stockholders; (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, the Certificate of Incorporation, or the bylaws; and (iv) any action asserting a claim governed by the internal affairs doctrine. In addition, our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Our bylaws further provide that any person or entity purchasing or otherwise acquiring any interest in our shares of capital stock shall be deemed to have notice of and consented to these forum selection clauses.
Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, our bylaws provide that the exclusive forum provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction.
We note, however, that there is uncertainty as to whether a court would enforce this provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
Indemnification of Directors and Officers
Our Certificate of Incorporation and bylaws provide that, to the fullest extent permitted by the laws of the State of Delaware, any officer or director of our company, who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he/she is or was or has agreed to serve at our request as a director, officer, employee or agent of our company, or while serving as a director or officer of our company, is or was serving or has agreed to serve at the request of our company as a director, officer, employee or agent (which includes service as a trustee, partner or manager or similar capacity) of another corporation, partnership, joint venture, trust, employee benefit plan or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity. For the avoidance of doubt, the foregoing indemnification obligation includes, without limitation, claims for monetary damages against Indemnitee to the fullest extent permitted under Section 145 of the Delaware General Corporation Law as in existence on the date hereof.
The indemnification provided shall be from and against expenses (including attorneys’ fees) actually and reasonably incurred by a director or officer in defending such action, suit or proceeding in advance of its final disposition, upon receipt of an undertaking by or on behalf of such person to repay all amounts advanced if it shall ultimately be determined by final judicial decision from which there is no further right to appeal that such person is not entitled to be indemnified for such expenses under our certificate of incorporation and bylaws or otherwise.
To the extent that indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling our company pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. If a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person of our company in the successful defense of any action, suit or proceeding) is asserted by any of our directors, officers or controlling persons in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of that issue.
Transfer Agent
The transfer agent and registrar, for our common stock is VStock Transfer, LLC. The transfer agent and registrar’s address is 18 Lafayette Place, Woodmere, New York 11598. The transfer agent’s telephone (212) 828-8436.
| 114 |
SHARES ELIGIBLE FOR FUTURE SALE
Shares Eligible for Future Sale
We cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock prevailing from time to time. Nevertheless, sales of substantial amounts of our common stock, including shares issued upon exercise of outstanding options or warrants, or the perception that these sales could occur in the public market after this offering could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through the sale of our equity securities.
Based on the number of shares of common stock outstanding as of the date of this prospectus, upon the closing of this offering at the assumed offering price, 21,175,082 shares of common stock will be outstanding.
| ● | all 2,962,963 shares sold in this offering (based on the assumed offering price) will be freely tradable, unless purchased by our officers, directors or other affiliates; | |
| ● | 67,858 shares are held by non-affiliates of ours that are not included in the resale prospectus contained in our initial public offering registration statement, and, with respect to such shares that are held for at least six months, may be sold under Rule 144 immediately; and | |
| ● | 5,913,002 shares are held by affiliates and non-affiliates of our company and are subject to 180 day lock-up agreements that were entered into in connection with our initial public offering which expire on June 13, 2021. | |
| ● | 300,000 shares are held by certain shareholders participating in the November 6, 2020 settlement and release agreement (see “Management—October 2020 Derivative Demand and Settlement”); are subject to 180 day lock-up agreements that were entered into in connection with our initial public offering which expire on June 13, 2021. |
Following the expiration of the applicable lock-up period referred to above, all shares will be eligible for resale pursuant to such registration statement or in compliance with Rule 144 or Rule 701 to the extent these shares have been released from any repurchase option that we may hold.
Rule 144
In general, under Rule 144 as currently in effect, any person who is or has been an affiliate of ours during the 90 days immediately preceding the sale and who has beneficially owned shares for at least six months is entitled to sell, within any three-month period commencing 90 days after the date of this prospectus, a number of shares that does not exceed the greater of:
| ● | 1% of the then-outstanding shares of common stock, which will equal approximately 21,175 shares immediately after this offering based on the assumed offering price; and | |
| ● | the average weekly trading volume during the four calendar weeks preceding the sale, subject to the filing of a Form 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us. A person who is not deemed to have been an affiliate of ours at any time during the 90 days immediately preceding the sale and who has beneficially owned his or her shares for at least six months is entitled to sell his or her shares under Rule 144 without regard to the limitations described above, subject only to the availability of current public information about us during the six months after the initial six-month holding period is met. After a non-affiliate has beneficially owned his or her shares for one year or more, he or she may freely sell his or her shares under Rule 144 without complying with any Rule 144 requirements.
| 115 |
We are unable to estimate the number of shares that will be sold under Rule 144, since this will depend on the market price for our common stock, the personal circumstances of the sellers and other factors. Prior to the offering, there has been no public market for the common stock, and there can be no assurance that a significant public market for the common stock will develop or be sustained after the offering. Any future sale of substantial amounts of the common stock in the open market may adversely affect the market price of the common stock offered by this prospectus.
Rule 701
In general, under Rule 701 under the Securities Act, any of our employees, directors, consultants or advisors who purchased shares from us in connection with a qualified compensatory stock or option plan or other written agreement and in compliance with Rule 701, is eligible to resell those shares 90 days after the effective date of this offering in reliance on Rule 144, but without compliance with the various restrictions, including the holding period, contained in Rule 144.
Lock-up Agreements
Our executive officers and directors have agreed that, subject to certain exceptions, for a period of 90 days after the date of this prospectus, they will not, without the prior written consent of Roth Capital Partners, LLC dispose of or hedge any shares or any securities convertible into or exchangeable for shares of our capital stock. There are no existing agreements between the underwriters and any person who will execute a lock-up agreement in connection with this offering providing consent to the sale of shares prior to the expiration of the lock-up period.
We previously agreed that, subject to certain exceptions, we will not, without the prior written consent of Roth Capital Partners, LLC, (i) offer, pledge, issue, sell, contract to sell, purchase, contract to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock; (ii) enter into any swap or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of shares of common stock; or (iii) file any registration statement with the SEC relating to the offering of any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock, for a period ending on September 10, 2021. There are no existing agreements between the underwriters and any person who will execute a lock-up agreement in connection with this offering providing consent to the sale of shares prior to the expiration of the lock-up period, other than with respect to the offering described in this prospectus.
Equity Incentive Plans
We intend to file registration statements on Form S-8 under the Securities Act after the closing of this offering to register the shares of our common stock that are issuable pursuant to our equity incentive plans. The registration statements are expected to be filed and become effective as soon as practicable after the completion of this offering. Accordingly, shares registered under the registration statements will be available for sale in the open market following their effective dates, subject to Rule 144 volume limitations and the lock-up arrangement described above, if applicable.
| 116 |
We have entered into an underwriting agreement with the several underwriters listed in the table below. Roth Capital Partners, LLC is the representative of the underwriters. We refer to the several underwriters listed in the table below as the ‘‘underwriters.’’ Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and the underwriters have agreed to purchase from us, shares of our common stock.
Pursuant to the terms and subject to the conditions contained in the underwriting agreement, we have agreed to sell to the underwriters named below, and each underwriter severally has agreed to purchase from us, the respective number of shares of common stock set forth opposite its name below:
| Underwriters | Number of Shares | |||
| Roth Capital Partners, LLC | ||||
National Securities Corporation | ||||
Craig-Hallum Capital Group LLC | ||||
| Total | ||||
All of the shares to be purchased by the underwriters will be purchased from us.
The underwriting agreement provides that the obligation of the underwriters to purchase the shares of common stock offered by this prospectus is subject to certain conditions. The underwriters are obligated to purchase all of the shares of common stock offered hereby if any of the shares are purchased.
We have granted the underwriters an option to buy up to an additional 15% (or 444,444 shares of common stock based on the assumed offering price) from us at the offering price, less the underwriting discounts and commissions, to cover over-allotments, if any. The underwriters may exercise this option at any time, in whole or in part, during the 45-day period after the date of this prospectus.
Discounts, Commissions and Expenses
The underwriters propose to offer the shares of common stock purchased pursuant to the underwriting agreement to investors at the offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $[●] per share. After this offering, the offering price and concession may be changed by the underwriters. No such change shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
In connection with the sale of the common stock to be purchased by the underwriters, the underwriters will be deemed to have received compensation in the form of underwriting commissions and discounts. The underwriters’ commissions and discounts will be 7% of the gross proceeds of this offering, or $0.47 per share of common stock, based on the assumed offering price per share set forth on the cover page of this prospectus.
We have also agreed to reimburse Roth Capital Partners, LLC at closing for expenses incurred by it in connection with the offering. We estimate that the total expenses of the offering payable by us, not including the underwriting discount and commissions or the expense reimbursement, will be approximately $486,500, which includes fees and expenses for which we have agreed to reimburse the underwriters up to an aggregate of $100,000. As of the date of this prospectus, no fees have been advanced.
| 117 |
The following table shows the underwriting discounts and commissions payable to the underwriters by us in connection with this offering (assuming both the exercise and non-exercise of the over-allotment option to purchase additional shares of common stock we have granted to the underwriters):
| Per Share | Total | |||||||||||||||
| Without Over- allotment | With Over- allotment | Without Over- allotment | With Over- allotment | |||||||||||||
| Offering price | $ | $ | $ | $ | ||||||||||||
| Underwriting discounts and commissions paid by us | $ | $ | $ | $ | ||||||||||||
| Proceeds to us, before expenses | $ | $ | $ | $ | ||||||||||||
(1) The underwriting discounts and commissions do not include the Representative Warrant described below or expense reimbursement as described above.
Indemnification
Pursuant to the underwriting agreement, we have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments that the underwriters or such other indemnified parties may be required to make in respect of those liabilities.
Representative’s Warrant
We have agreed to issue to Roth Capital Partners, LLC a warrant to purchase 148,149 shares of common stock (based on the assumed offering price) equal to five percent (5%) of the shares sold in the offering (or 170,371 shares of common stock if the underwriter exercises its option to purchase additional shares in full based on the assumed offering price); provided, however, that these amounts may be increased to up to 207,408 and 238,519, respectively (based on the assumed offering price), at our discretion; provided that the aggregate compensation will not exceed the maximum amount permitted by FINRA. Such Representative’s Warrant will be exercisable 180 days after the effective date of the registration statement of which this prospectus forms a part, shall have an exercise price equal to 125% of the price per share sold in this offering, will provide for cashless exercise and shall expire five (5) years after the effective date of the registration statement of which this prospectus forms a part. The Representative’s Warrant will be subject to FINRA Rule 5110(e)(1)(A) in that, except as otherwise permitted by FINRA rules, for a period of 180 days following the effective date of the registration statement of which this prospectus forms a part, the Representative’s Warrant shall not be (A) sold, transferred, assigned, pledged, or hypothecated, or (B) the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person except as permitted by FINRA Rule 5110(e)(2). In addition, holders of the Representative’s Warrant shall not (i) have more than one demand registration right at the Company’s expense, (ii) have the right to demand registration of the Representative’s Warrant or underlying shares more than five years from the earlier of (a) the effective date of the registration statement of which this prospectus forms a part or (b) the commencement of sales of the public offering contemplated by this prospectus, and (iii) have the right to piggyback registration with respect to the Representative’s Warrant or underlying shares more than seven years from the earlier of (y) the effective date of the registration statement of which this prospectus forms a part or (z) the commencement of sales of the public offering contemplated by this prospectus.
Lock-Up Agreements
We previously agreed not to (i) offer, pledge, issue, sell, contract to sell, purchase, contract to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock; (ii) enter into any swap or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of shares of common stock; or (iii) file any registration statement with the SEC relating to the offering of any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock, without the prior written consent of Roth Capital Partners, LLC for a period ending on September 10, 2021 (the “Lock-up Period”). This consent may be given at any time without public notice. These restrictions on future issuances are subject to exceptions for (i) the issuance of shares of our common stock sold in this offering, (ii) the issuance of shares of our common stock upon the exercise of outstanding options or warrants or certain conversions or redemptions of preferred stock and other outstanding convertible securities, and the vesting of restricted stock awards or units, (iii) the issuance of employee stock options not exercisable during the Lock-up Period and the grant of restricted stock awards or restricted stock units or shares of common stock pursuant to our equity incentive plans, (iv) the filing of a registration statement on Form S-8, and (v) the issuance of securities in one or more unregistered offerings pursuant to acquisitions or strategic transactions approved by a majority of our disinterested directors, provided that any such issuance shall only be to a person or entity (or to the equity-holders of an entity) which is, itself or through its subsidiaries, an operating company or an owner of an asset in a business synergistic with our business.
| 118 |
In addition, each of our directors and executive officers have entered into a lock-up agreement with the underwriters. Under the lock-up agreements, the directors, executive officers and these stockholders may not, directly or indirectly, sell, offer to sell, contract to sell, or grant any option for the sale (including any short sale), grant any security interest in, pledge, hypothecate, hedge, establish an open “put equivalent position” (within the meaning of Rule 16a-1(h) under the Securities Exchange Act of 1934, as amended, or the Exchange Act), or otherwise dispose of, or enter into any transaction which is designed to or could be expected to result in the disposition of, any shares of our common stock or securities convertible into or exchangeable for shares of our common stock, or publicly announce any intention to do any of the foregoing, without the prior written consent of Roth Capital Partners, LLC, for a period of 90 days from the closing date of this offering. Any consent by Roth Capital Partners, LLC to waive a lock-up restriction may be given at any time without public notice. These restrictions on future dispositions by our directors, executive officers and these stockholders are subject to exceptions for (i) one or more bona fide gift transfers of securities to immediate family members, (ii) transfers of securities to one or more immediate family members or trusts for bona fide estate planning purposes, (iii) transfers of securities by operation of law, including by will, intestacy or pursuant to a valid decree of divorce, or (iv) if such director, executive officer or stockholder controls a corporation, partnership, limited liability company or other business entity, any transfer of securities to any shareholder, partner or member of, or owner of similar equity interests in, such entity; provided that in each case the transferee agrees to be bound by these restrictions.
Electronic Distribution
This prospectus may be made available in electronic format on websites or through other online services maintained by the underwriters or by their affiliates. In those cases, prospective investors may view offering terms online and prospective investors may be allowed to place orders online. Other than this prospectus in electronic format, the information on the underwriters’ websites or our website and any information contained in any other websites maintained by the underwriters or by us is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon by investors.
Price Stabilization, Short Positions and Penalty Bids
In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act:
| ● | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. | |
| ● | Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment option and/or purchasing shares in the open market. | |
| ● | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. A naked short position occurs if the underwriters sell more shares than could be covered by the over-allotment option. This position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. | |
| ● | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be discontinued at any time.
| 119 |
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our shares of common stock. In addition, neither we nor the underwriters make any representation that the underwriter will engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Determination of Offering Price
The offering price will be determined by negotiation among us and the underwriters. The principal factors to be considered in determining the offering price include:
| ● | the information set forth in this prospectus and otherwise available to the representatives; | |
| ● | our history and prospects and the history and prospects for the industry in which we compete; | |
| ● | our past and present financial performance; | |
| ● | our prospects for future earnings and the present state of our development; | |
| ● | the general condition of the securities market at the time of this offering; | |
| ● | the recent market prices of, and demand for, publicly traded shares of our company and generally comparable companies; and | |
| ● | other factors deemed relevant by the underwriters and us. |
The assumed offering price set forth on the cover page of this prospectus is subject to change as a result of market conditions and other factors. Neither we nor the underwriters can assure investors that an active trading market will develop for our common stock or that the common stock will trade in the public market at or above the offering price.
Affiliations
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and their affiliates may from time to time in the future engage with us and perform services for us or in the ordinary course of their business for which they will receive customary fees and expenses. In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of us. The underwriters and their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect of these securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in these securities and instruments.
Nasdaq Capital Market Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “VVOS”.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
| 120 |
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI33-105 regarding underwriter conflicts of interest in connection with this offering.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area — Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| ● | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; | |
| ● | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); | |
| ● | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining our prior consent or any underwriter for any such offer; or | |
| ● | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall require us to publish a prospectus pursuant to Article 3 of the Prospectus Directive. |
| 121 |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2 and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), or ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with this offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| ● | to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| 122 |
| ● | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| ● | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| ● | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
New Zealand
The shares of common stock offered hereby have not been offered or sold, and will not be offered or sold, directly or indirectly in New Zealand and no offering materials or advertisements have been or will be distributed in relation to any offer of shares in New Zealand, in each case other than:
| ● | to persons whose principal business is the investment of money or who, in the course of and for the purposes of their business, habitually invest money; | |
| ● | to persons who in all the circumstances can properly be regarded as having been selected otherwise than as members of the public; | |
| ● | to persons who are each required to pay a minimum subscription price of at least NZ$500,000 for the shares before the allotment of those shares (disregarding any amounts payable, or paid, out of money lent by the issuer or any associated person of the issuer); or | |
| ● | in other circumstances where there is no contravention of the Securities Act 1978 of New Zealand (or any statutory modification or reenactment of, or statutory substitution for, the Securities Act 1978 of New Zealand). |
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissao do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
| 123 |
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. We may not render services relating to the securities within the United Arab Emirates, including the receipt of applications and/or the allotment or redemption of such shares.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
| 124 |
The validity of the securities offered by this prospectus will be passed upon for us by Ellenoff Grossman & Schole LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriters by Dorsey & Whitney LLP, Seattle, Washington.
Our balance sheets as of December 31, 2020 and 2019 and the related statement of operations, changes in statement of stockholders’ equity and statement of cash flows for the years ended December 31, 2020 and 2019, included in this prospectus have been audited by Plante & Moran, PLLC, independent registered public accounting firm, with respect thereto, and has been so included in reliance upon the report of such firm given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer you to the registration statement and the exhibits and schedules filed thereto. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. The SEC maintains an Internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the SEC. The address of that site is www.sec.gov.
Upon the completion of our initial public offering in December 2020, we became subject to the information and periodic reporting requirements of the Exchange Act and, in accordance therewith, we file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information is available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at www.vivoslife.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The reference to our website address does not constitute incorporation by reference of the information contained on our website, and you should not consider the contents of our website in making an investment decision with respect to our common stock.
| 125 |
VIVOS THERAPEUTICS, INC. AND SUBSIDIARIES
| 126 |
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors of
Vivos Therapeutics, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Vivos Therapeutics, Inc. and Subsidiaries (the “Company”), as of December 31, 2020 and 2019 and the related statements of operations, stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the years in the two-year period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
The Company’s management is responsible for these financial statements. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Plante & Moran, PLLC
We have served as the Company’s auditor since 2018.
Denver, Colorado
March 25, 2021
| F-1 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
Consolidated Balance Sheets
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 18,205,668 | $ | 469,353 | ||||
| Accounts receivable, net | 1,430,890 | 871,290 | ||||||
| Current portion of note receivable | 84,696 | 84,696 | ||||||
| Deferred offering costs | - | 263,814 | ||||||
| Prepaid expenses and other current assets | 673,061 | 295,002 | ||||||
| Total current assets | 20,394,315 | 1,984,155 | ||||||
| Property and equipment, net | 871,597 | 1,139,501 | ||||||
| Intangible assets, net | 270,121 | 689,151 | ||||||
| Note receivable, net - related party | 810,635 | 785,061 | ||||||
| Goodwill | 2,671,434 | 2,671,434 | ||||||
| Deposits | 309,367 | 282,235 | ||||||
| Total assets | $ | 25,327,469 | $ | 7,551,537 | ||||
| LIABILITIES AND STOCKHOLDER’S EQUITY | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 781,364 | $ | 1,083,422 | ||||
| Accounts payable – related party | 1,500,000 | - | ||||||
| Accrued expenses | 1,736,721 | 1,353,161 | ||||||
| Contract liability | 2,937,992 | 2,947,565 | ||||||
| Current portion of long-term debt | 866,972 | 3,709,535 | ||||||
| Total current liabilities | 7,823,049 | 9,093,683 | ||||||
| Long-term debt | 423,095 | - | ||||||
| Deferred rent | 163,966 | 84,246 | ||||||
| Total liabilities | 8,410,110 | 9,177,929 | ||||||
| Commitments and contingencies | ||||||||
| Convertible Redeemable Series A Preferred Stock - $0.0001 par value. 50,000,000 shares authorized, none and 730,000 shares issued and outstanding at December 31, 2020 and 2019, respectively | - | 1,316,667 | ||||||
| Stockholders’ equity | ||||||||
| Preferred Stock | ||||||||
| Series B, nonvoting - $0.0001 par value, 1,200,000 authorized, none issued and outstanding at December 31, 2020 and 2019, respectively | - | - | ||||||
| Common Stock | ||||||||
| Class A, voting - $0.0001 par value, 200,000,000 shares authorized, 18,209,452 and 12,444,165 issued and outstanding at December 31, 2020 and 2019, respectively | 1,821 | 1,244 | ||||||
| Additional paid-in capital | 52,250,266 | 20,333,548 | ||||||
| Accumulated deficit | (35,334,728 | ) | (23,277,851 | ) | ||||
| Total stockholders’ equity | 16,917,359 | (2,943,059 | ) | |||||
| Total liabilities and stockholders’ equity | $ | 25,327,469 | $ | 7,551,537 | ||||
See notes to consolidated financial statements.
| F-2 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
Consolidated Statements of Operations
| Year Ended | ||||||||
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Revenue | ||||||||
| Product revenue | $ | 4,889,840 | $ | 4,349,623 | ||||
| Service revenue | 8,176,397 | 7,043,654 | ||||||
| Total revenue | 13,066,237 | 11,393,277 | ||||||
| Cost of sales (exclusive of depreciation and amortization shown separately below) | 2,653,429 | 2,736,034 | ||||||
| Gross profit | 10,412,808 | 8,657,243 | ||||||
| Operating expenses | ||||||||
| General and administrative | 16,090,049 | 16,172,505 | ||||||
| Sales and marketing | 2,314,023 | 2,310,743 | ||||||
| Settlement | 3,330,679 | - | ||||||
| Depreciation and amortization | 717,865 | 751,228 | ||||||
| Total operating expenses | 22,452,616 | 19,234,476 | ||||||
| Operating loss before interest expense and income taxes | (12,039,808 | ) | (10,577,233 | ) | ||||
| Interest expense | (96,681 | ) | (137,876 | ) | ||||
| Loss on sale of business | - | (60,343 | ) | |||||
| Interest income | 79,612 | 21,133 | ||||||
| Loss before income taxes | (12,056,877 | ) | (10,754,319 | ) | ||||
| Income tax expense | - | - | ||||||
| Net loss | (12,056,877 | ) | (10,754,319 | ) | ||||
| Warrant beneficial conversion feature expense | (3,597,585 | ) | - | |||||
| Preferred stock accretion | (2,333,333 | ) | (1,000,000 | ) | ||||
| Net loss attributable to common stockholders | $ | (17,987,795 | ) | $ | (11,754,319 | ) | ||
| Net loss per share attributable to common stockholders (basic and diluted) | $ | (1.40 | ) | $ | (0.95 | ) | ||
| Weighted average number of shares of Common Stock outstanding (basic and diluted) | 12,869,266 | 12,331,280 | ||||||
See notes to consolidated financial statements.
| F-3 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders’ Equity
| Year Ended December 31, 2020 and 2019 | ||||||||||||||||||||||||||||
| Series B | Series B | Additional | Total | |||||||||||||||||||||||||
| Common Stock | Preferred | Preferred | Paid-in | Accumulated | Stockholders’ | |||||||||||||||||||||||
| Shares | Amount | Units | Amount | Capital | Deficit | Equity | ||||||||||||||||||||||
| Balance December 31, 2018 | 12,067,592 | $ | 1,207 | - | - | $ | 17,349,118 | $ | (12,523,532 | ) | $ | 4,826,793 | ||||||||||||||||
| Stock-based compensation expense | - | - | - | - | 1,987,275 | - | 1,987,275 | |||||||||||||||||||||
| Preferred stock accretion | - | - | - | - | (1,000,000 | ) | - | (1,000,000 | ) | |||||||||||||||||||
| Common stock sold for cash, net | 155,769 | 15 | - | - | 1,165,984 | - | 1,165,999 | |||||||||||||||||||||
| Common stock issued from exercise of stock options | 50,000 | 5 | - | - | 82,495 | - | 82,500 | |||||||||||||||||||||
| Common stock issued for convertible debt | 170,804 | 17 | - | - | 748,676 | - | 748,693 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (10,754,319 | ) | (10,754,319 | ) | |||||||||||||||||||
| Balance December 31, 2019 | 12,444,165 | $ | 1,244 | - | - | $ | 20,333,548 | $ | (23,277,851 | ) | $ | (2,943,059 | ) | |||||||||||||||
| Stock-based compensation expense | - | - | - | - | 2,172,197 | - | 2,172,197 | |||||||||||||||||||||
| Series A preferred stock accretion | - | - | - | - | (2,333,333 | ) | - | (2,333,333 | ) | |||||||||||||||||||
| Series B preferred stock issued for cash, net of issuance costs | - | - | 163,500 | 2,402,668 | - | - | 2,402,668 | |||||||||||||||||||||
| Series B preferred stock issued in exchange for convertible debt | - | - | 196,258 | 2,943,870 | - | - | 2,943,870 | |||||||||||||||||||||
| Exchange of Series B preferred stock into common shares, net of issuance costs | 1,199,195 | 120 | (359,758 | ) | (5,346,538 | ) | 5,346,418 | - | - | |||||||||||||||||||
| Issuance of common stock in initial public offering, net of issuance costs | 4,025,000 | 402 | - | - | 21,577,241 | - | 21,577,643 | |||||||||||||||||||||
| Common stock issued in settlement | 300,000 | 30 | - | - | 1,799,970 | - | 1,800,000 | |||||||||||||||||||||
| Common stock warrants issued in settlement | - | - | - | - | 1,530,679 | - | 1,530,679 | |||||||||||||||||||||
| Common stock issued to consultants for services | 88,111 | 9 | - | - | 677,494 | - | 677,503 | |||||||||||||||||||||
| Common stock issued for settlement of liability | 46,667 | 5 | - | - | 349,995 | - | 350,000 | |||||||||||||||||||||
| Conversion of convertible debt to common stock | 106,314 | 11 | - | - | 796,057 | - | 796,068 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (12,056,877 | ) | (12,056,877 | ) | |||||||||||||||||||
| Balance December 31, 2020 | 18,209,452 | $ | 1,821 | - | $ | - | $ | 52,250,266 | $ | (35,334,728 | ) | $ | 16,917,359 | |||||||||||||||
See notes to consolidated financial statements.
| F-4 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
| Year Ended | ||||||||
| Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (12,056,877 | ) | $ | (10,754,319 | ) | ||
| Adjustments to reconcile net loss to net cash: used in operating activities: | ||||||||
| Depreciation and amortization expense | 717,865 | 751,228 | ||||||
| Stock-based compensation expense | 2,172,197 | 1,987,275 | ||||||
| Common stock for settlements | 1,925,003 | 76,200 | ||||||
| Warrants issued for settlements | 1,530,679 | - | ||||||
| Common stock issued for services | 487,488 | - | ||||||
| Accretion of discount on convertible debt | - | 13,455 | ||||||
| Accretion of discount on note receivable | (25,574 | ) | (6,587 | ) | ||||
| Loss on sale of business | - | 60,343 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (559,600 | ) | (276,103 | ) | ||||
| Prepaid expenses and other current assets | (114,244 | ) | (271,877 | ) | ||||
| Deposits | (27,132 | ) | (258,331 | ) | ||||
| Accounts payable | (274,212 | ) | 547,620 | |||||
| Accrued expenses | 473,967 | 672,892 | ||||||
| Contract liability | (9,573 | ) | 2,058,057 | |||||
| Deferred rent | 79,719 | 59,667 | ||||||
| Net Cash Used In Operating Activities | (5,680,294 | ) | (5,340,480 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Acquisitions of property and equipment | (120,252 | ) | (175,599 | ) | ||||
| Proceeds from sale of business | - | 250,000 | ||||||
| Principal collections under note receivable | - | 11,822 | ||||||
| Net Cash Used In Investing Activities | (120,252 | ) | 86,223 | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance of common stock | 22,289,500 | 1,248,499 | ||||||
| Proceeds from issuance of debt | 1,265,067 | 3,759,535 | ||||||
| Redemption of preferred stock | (2,150,000 | ) | (350,000 | ) | ||||
| Proceeds from issuance of preferred stock | 2,452,500 | - | ||||||
| Payment for issuance costs | (245,206 | ) | (159,887 | ) | ||||
| Principal payments on debt | (75,000 | ) | (29,260 | ) | ||||
| Net Cash Provided by Financing Activities | 23,536,861 | 4,468,887 | ||||||
| Net increase (decrease) in cash and cash equivalents | 17,736,315 | (785,370 | ) | |||||
| Cash and cash equivalents, at beginning of period | 469,353 | 1,254,723 | ||||||
| Cash and cash equivalents, at end of period | $ | 18,205,668 | $ | 469,353 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION | ||||||||
| Cash paid for interest | $ | 33,169 | $ | 20,674 | ||||
| Cash paid for income taxes | ||||||||
| Accretion of redeemable preferred stock | - | 1,000,000 | ||||||
| Conversion of debt to common stock | 770,000 | 720,740 | ||||||
| Exchange of debt to Series B preferred stock | 2,943,870 | - | ||||||
| Exchange of Series B preferred stock into common shares | 5,346,538 | - | ||||||
| Common stock issued for payment of interest | 26,068 | 27,952 | ||||||
| Series B Preferred Stock issued for payment of interest | 102,422 | - | ||||||
| Series A Preferred Stock redemption included in accounts payable | 1,500,000 | - | ||||||
| Capital expenditures included in accounts payable | 2,400 | 91,719 | ||||||
See notes to consolidated financial statements.
| F-5 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 - ORGANIZATION, DESCRIPTION AND SIGNIFICANT ACCOUNTING POLICIES
Organization
BioModeling Solutions, Inc. (“BioModeling”) was organized on March 20, 2007 as an Oregon limited liability company, and subsequently incorporated in 2013. On August 16, 2016, BioModeling entered into a share exchange agreement (the “SEA”) with First Vivos, Inc. (“First Vivos”), and Vivos Therapeutics, Inc. (“Vivos”), a Wyoming corporation established on July 7, 2016 to facilitate this merger. Vivos was formerly named Corrective BioTechnologies, Inc. until its name changed on September 6, 2016 to Vivos Biotechnologies and on March 2, 2018 to Vivos Therapeutics, Inc. and had no substantial pre-combination business activities. First Vivos was incorporated in Texas on November 10, 2015. Pursuant to the SEA, all of the outstanding shares of common stock and warrants of BioModeling and all of the shares of commons stock of First Vivos were exchanged for newly issued shares of Class A common stock and warrants of Vivos, the legal acquirer, collectively the “Company”.
The transaction was accounted for as a reverse acquisition and recapitalization, with BioModeling as the acquirer for financial reporting and accounting purposes. Upon the consummation of the merger, the historical financial statements of BioModeling became the Company’s historical financial statements and continued to be recorded at their historical carrying amounts.
COVID-19
The early 2020 outbreak of COVID-19 and its development into a pandemic in March 2020 has resulted in significant economic disruption globally. Actions taken by various governmental authorities, individuals and companies around the world to prevent the spread of COVID-19 through social distancing have restricted travel, many business operations, public gatherings and the overall level of individual movement and in-person interaction across the globe. This has significantly reduced global economic activity and resulted in a decline in demand across many industries.
Many of the Company’s VIPs and potential VIPs closed their offices as a result of COVID-19, although some remained open to specifically provide patients with Company products as Company appliances and VIPs were deemed an essential business for health considerations in many jurisdictions. In the face of the pandemic and the results potential for revenue reduction, Company management worked diligently to reduce expenses and maintain revenues during 2020. While revenue growth flattened in March and April 2020, expenses were reduced and the Company aggressively expanded its network of healthcare providers familiar with its products by offering online continuing education courses which introduced many in the medical and dental communities to the Company’s product line. As a result of improving operating cash flows, the Company determined no triggering events had occurred indicating no impairment needed as of December 31, 2020.
Description of Business
The Company is engaged in the designing and selling of oral devices that assist with sleep and breathing disorders and hosting training seminars for medical and dental professionals on sleep and breathing disorders. The Company owns and operates three locations where Vivos systems are measured and fitted. The Company licenses its intellectual property to third-party manufacturers which fabricate appliance devices for orders requested by healthcare professionals, at a specified price per appliance.
Basis of Presentation and Consolidation
The accompanying consolidated financial statements, which include the accounts of the Company and its wholly owned subsidiaries (BioModeling and First Vivos), are prepared in conformity with generally accepted accounting principles in the United States of America (“U.S. GAAP”). All significant intercompany balances and transactions have been eliminated in consolidation.
On July 30, 2020, the Company effected a reverse stock split in which each common shareholder received one share of common stock for every three shares outstanding. On August 12, 2020, the Company reincorporated as a domestic Delaware corporation under Delaware General Corporate Law from Wyoming. All share and per share amounts have been adjusted to reflect the effect of these Reverse Stock Split.
Use of Estimates
To prepare financial statements in conformity with U.S. GAAP, management must make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
| F-6 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 - ORGANIZATION, DESCRIPTION AND SIGNIFICANT ACCOUNTING POLICIES (Continued)
Initial Public Offering
On December 11, 2020, the Company completed its initial public offering (“IPO”) by offering 4,025,000 common shares at a price of $6.00 per share, for net proceeds of approximately $21.6 million after deducting underwriting discounts and commissions and offering expenses payable by the Company. In connection with the IPO, our outstanding units of Series B preferred stock were automatically converted into an aggregate of 1,199,195 shares of common stock and 1,199,195 warrants to purchase an aggregate of 1,199,195 shares of common stock (see Note 9).
Payroll Protection Program Loan
On May 8, 2020, the Company received approximately $1,265,000 in funding through the U.S. Small Business Administration’s Payroll Protection Program (PPP) that was part of the Coronavirus Aid, Relief, and Economic Security (CARES) Act signed into law in March 2020. The interest rate on the loan is 1.00% per year and matures on May 5, 2022 and may be forgiven to the extent proceeds of the loan are used for eligible expenditures such as payroll and other expenses described in the CARES Act. The note is payable in monthly installments of principal and interest over 12 months, beginning 12 months from the date of the note (deferral period). The note might be repaid at any time with no payment penalty.
The Company used these funds to assist with payroll, rent and utilities. The Company has spent the funding in a manner in which it believes the entire balance of the outstanding promissory note will be eligible for forgiveness through the terms of the PPP. An application to forgive the entire amount was submitted with the lender in January 2021, however, there can be no assurance given that any portion of the PPP loan will be forgiven. Any request for forgiveness is subject to review and approval by the lender and the SBA, including review of qualifying expenditures, staffing and salary levels.
Currently, there is no guidance in U.S. GAAP that specifically addresses the accounting by an entity that obtains a forgivable loan from a government entity. In the absence of specific guidance, the Company believes that is acceptable to account for the PPP loan as a debt instrument under ASC 470, Debt and apply the interest method in ASC 835-30, Imputation of Interest, which considers the interest accrued during the payment deferral period allowed for the loan. The Company recognized the entire loan amount as a financial liability (current and noncurrent per ASC 470-10-45, Other Presentation), with interest accrued and expensed over the term of the loan (see Note 7). Additionally, any amount forgiven when the Company is legally released as the primary obligor under the loan, will be recognized in the income statement as a gain from extinguishment of the loan.
Cash and Cash Equivalents
We consider currency on hand, demand deposits and all highly liquid investments with an original or remaining maturity of three months or less to be cash and cash equivalents. As of December 31, 2020 and 2019, the Company had no cash equivalents and all cash amounts consisted of cash on deposit. As of December 31, 2020 and 2019, and from time to time during each year, the Company maintained balances in excess of federally insured limits.
| F-7 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 - ORGANIZATION, DESCRIPTION AND SIGNIFICANT ACCOUNTING POLICIES (Continued)
Concentration of Credit Risk and Significant Customers
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash and cash equivalents and accounts receivable. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions. Additionally, the Company has a diverse customer base and no single customer represented greater than ten percent of sales or accounts receivable for the years ended December 31, 2020 and 2019.
Accounts Receivable, Net
The accounts receivable in the accompanying financial statements are stated at the amounts management expects to collect. The Company performs credit evaluations of its customers’ financial condition and may require a prepayment for a portion of the services to be performed. The Company reduces accounts receivable by estimating an allowance that may become uncollectible in the future. Management determines the estimated allowance for uncollectible amounts based on its judgements in evaluating the aging of the receivables and the financial condition of our clients. Allowance for uncollectible receivables was $507,347 and $180,852 as of December 31, 2020 and 2019, respectively.
Property and Equipment, Net
Property and equipment are stated at historical cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, which ranges from 4 to 5 years. Amortization of leasehold improvements is recognized using the straight-line method over the shorter of the life of the improvement or the term of the respective leases which range between 5 and 7 years. The Company does not begin depreciating assets until they are placed in service.
Intangible Assets, Net
Intangible assets consist of assets acquired from First Vivos and costs paid to third parties for work related to the Company’s patents. The identified intangible assets acquired from First Vivos are amortized using the straight-line method over the estimated life of the assets, which approximates 5 years (See Note 5). The costs paid to third parties for the Companies’ assets are amortized using the straight-line method over the life of the underlying patents, which approximates 15 years. The Company initially determined the fair value of the intangible assets using a discounted cash flow approach.
Goodwill
Goodwill is the excess of acquisition cost of an acquired entity over the fair value of the identifiable net assets acquired (See Note 5). Goodwill is not amortized, but tested for impairment annually or whenever indicators of impairment exist. These indicators may include a significant change in the business climate, legal factors, operating performance indicators, competition, sale or disposition of a significant portion of the business or other factors. The Company tests for impairment annually. There was no impairment of goodwill recognized at December 31, 2020 or 2019.
| F-8 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 - ORGANIZATION, DESCRIPTION AND SIGNIFICANT ACCOUNTING POLICIES (Continued)
Long-lived Assets
The Company reviews and evaluates the recoverability of long-lived assets whenever events or changes in circumstances indicate that an asset’s carrying amount may not be recoverable. Such circumstances could include, but are not limited to, 1) a significant decrease in the market value of an asset, 2) a significant adverse change in the extent or manner in which an asset is used, or 3) an adverse action or assessment by a regulator. The Company measures the carrying amount of the asset against the estimated undiscounted future cash flows associated with it. Should the sum of the expected future net cash flows be less than the carrying value of the asset being evaluated, an impairment loss would be recognized. The impairment loss would be calculated as the amount by which the carrying value of the asset exceeds its fair value. The fair value is measured based on quoted market prices, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including the discounted value of estimated future cash flows. The evaluation of asset impairment requires the Company to make assumptions about future cash flows over the life of the asset being evaluated. These assumptions require significant judgment and actual results may differ from assumed and estimated amounts. The Company’s evaluation of long-lived assets completed for the years ended December 31, 2020 and 2019 resulted in no impairment loss.
Notes Receivable, net
The notes receivable in the accompanying financial statements are stated at the amount management expects to collect. The current portion is what the Company expects to collect in the next twelve months and the long-term portion consists of the portion the Company expects to collect beyond twelve months. The Company reduced notes receivable by estimating a discount based on market rates. The discount on notes receivable was $68,101 and $93,421 as of December 31, 2020 and 2019, respectively. Accretion on the discount and interest on the note is recorded in interest income.
Fair Value Measurements
Fair value is the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Fair value is estimated by applying the following hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement:
Level 1 - Quoted prices in active markets for identical assets or liabilities.
Level 2 - Observable inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 - Inputs that are generally unobservable and typically reflect management’s estimate of assumptions that market participants would use in pricing the asset or liability.
The Company believes that the fair value of cash, accounts receivable, accounts payable and accrued liabilities approximates their carrying values at December 31, 2020 and 2019 due to their short maturities. The Company also believes that the current and long-term portion of notes receivable and debt approximates their carrying value at December 31, 2020 and 2019 as its terms are commensurate with terms the Company can obtain from third parties.
| F-9 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 - ORGANIZATION, DESCRIPTION AND SIGNIFICANT ACCOUNTING POLICIES (Continued)
Share-Based Compensation
The Company accounts for share-based payments to employees by recognizing compensation expense based upon the estimated fair value of the awards on the date of grant. Absent a publicly traded market for our stock, the Company uses the price paid for our stock in the most recent sales to third parties as the stock price input into our valuation model as of the date of grant. The Company determines the estimated grant fair value using the Black-Scholes option pricing model and recognizes compensation costs ratably over the requisite service period which approximates the vesting period using the straight-line method. For options issued to consultants, the Company recognizes the estimated fair value of options issued using the Black-Scholes option pricing model at the time the services are rendered.
The Black-Scholes model requires the input of certain subjective assumptions and the application of judgment in determining the fair value of the awards. The most significant assumptions and judgments include the expected volatility, risk-free interest rate, the expected dividend yield, and the expected term of the awards. The Company accounts for forfeitures as they occur.
The assumptions used in our option pricing model represent management’s best estimates. If factors change and different assumptions are used, our equity-based compensation expense could be materially different in the future. The key assumptions included in the model are as follows:
| ● | Share price – Historically, we used the price of our stock sold to third parties in our offerings as the most available representation of fair value per share of common stock on date of grant. Beginning in 2021, we will use our publicly quoted market price on Nasdaq. | |
| ● | Expected volatility — We determine the expected price volatility based on the historical volatilities of our peer group as we do not have a sufficient trading history for our common stock. Industry peers consist of several public companies in the bio-tech industry similar to us in size, stage of life cycle and financial leverage. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own stock price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be utilized in the calculation. | |
| ● | Risk-free interest rate — The risk-free rate was determined based on yields of U.S. Treasury Bonds of comparable terms. The volatility is based on analyzing the stock price and implied volatility of guideline companies. | |
| ● | Expected dividend yield — We have not previously issued dividends and do not anticipate paying dividends in the foreseeable future. Therefore, we used a dividend rate of zero based on our expectation of not paying additional dividends. | |
| ● | Expected term — We estimate the expected term using the simplified method which is the average of the vesting term and the contractual term of the options. |
Research and Development
Costs related to research and development are expensed as incurred and include costs associated with research and development of new products and enhancements to existing products. There were no significant research and development costs incurred during the years ended December 31, 2020 or 2019.
| F-10 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 - ORGANIZATION, DESCRIPTION AND SIGNIFICANT ACCOUNTING POLICIES (Continued)
Income Taxes
The Company uses the asset and liability method to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax basis of assets and liabilities.
Deferred tax assets and liabilities are determined using the effective tax rates for the years in which the tax assets and liabilities are expected to be realized. A valuation allowance is established when it is more likely than not that the future realization of all or some of the deferred tax assets will not be achieved.
Basic and Diluted Net Loss Per Share
Basic net loss per share is computed using the weighted average number of common shares outstanding during the period. Diluted net loss per common share is computed using the weighted average number of common shares outstanding and the weighted average dilutive potential common shares outstanding using the treasury stock method. However, for the years ended December 31, 2019 and 2018, diluted net loss per share is the same as basic net loss per share as the inclusion of weighted average shares of common stock issuable upon the exercise of outstanding warrants and stock options would be anti-dilutive. The numerator in the basic and diluted net loss per share calculation is the net loss attributable to common stockholders, which is the net loss for the year increased by the current year preferred stock dividends accrued.
The holder of the Company’s outstanding Series A Preferred Stock (see Note 8) was entitled to participate in Common Stock dividends, if and when declared, on a one-to-one per-share basis. Accordingly, in periods in which the Company has net income, earnings per share will be computed using the two-class method whereby the pro rata dividends distributable to the holder of Series A Preferred Stock will be deducted from earnings applicable to common stockholders, regardless of whether a dividend is declared for such undistributed earnings. For the years ended December 31, 2020 and 2019, the Company incurred a net loss and, accordingly, there were no undistributed earnings to allocate under the two-class method.
The following table summarizes outstanding common stock securities not included in the computation of diluted net loss per common share as their inclusion would be anti-dilutive:
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Common Stock Warrants | 1,960,029 | 83,334 | ||||||
| Common Stock Options | 2,302,345 | 1,900,000 | ||||||
| F-11 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 - ORGANIZATION, DESCRIPTION AND SIGNIFICANT ACCOUNTING POLICIES (Continued)
Recent Accounting Pronouncements
The Company is an emerging growth company (“EGC”) as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), whereby the Company is not required to comply with new or revised financial accounting standards until the dates when private companies are required to comply with such standards. The JOBS Act provides that a company can elect to opt out of the extended transition periods and comply with the requirements that apply to non-EGC public companies but any such election to opt out is irrevocable. Presented below is a discussion of new accounting standards including deadlines for adoption assuming that the Company retains its designation as an EGC.
Standards Required to be Adopted in Future Years. The following accounting standards are not yet effective, and a decision has not been reached about whether the Company may elect to early adopt any of the standards:
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). This ASU requires the Company to recognize lease assets and lease liabilities on the balance sheet and also disclose key information about leasing arrangements. In July 2018, the FASB issued ASU No. 2018-11 Targeted Improvements, which provides lessees the option to adopt either (i) retrospectively to each prior reporting period presented upon initial adoption, or (ii) apply the new leasing standard to all open leases as of the adoption date by recognizing a cumulative-effect adjustment to accumulated deficit in the period of adoption without restating prior periods. The Company is still evaluating which transition approach will be implemented upon adoption of ASU No. 2016-02. ASU 2016-02 is effective for the Company beginning in the first quarter of 2022 and early adoption is permitted.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. ASU 2016-13 amends the guidance on the impairment of financial instruments. This guidance requires use of an impairment model (known as the “current expected credit losses”, or CECL model) that is based on expected losses rather than incurred losses. Under the new guidance, an entity recognizes, as an allowance, its estimate of expected credit losses. ASU 2016-13 is effective for the Company beginning in the first quarter of 2023. The Company is still evaluating the impact the adoption of ASU 2016-13 will have on its results of operations or financial position.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740), Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principles in Topic 740 and clarifies and amends existing guidance to improve consistent application. ASU 2019-12 is effective for the Company beginning in the first quarter of 2022. Early adoption is permitted, including adoption in an interim period. The adoption of this standard is not expected to have a material impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not currently expected to have a material impact on the Company’s financial statements upon adoption.
Recently Adopted Standards. The following recently issued accounting standards were adopted by the Company during the year ended December 31, 2020:
In June 2018, the FASB issued ASU 2018-07, Compensation — Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which expands the scope of Accounting Standards Codification (“ASC”) 718, Compensation—Stock Compensation to include share-based payment transactions for acquiring goods and services from non-employees. An entity should apply the requirements of ASC 718 to non-employee awards except for specific guidance on inputs to an option pricing model and the attribution of cost. The Company adopted this new guidance using the modified retrospective method effective on January 1, 2020. On the date of adoption, there were no outstanding awards granted to non-employees in transactions to acquire goods and services for which the measurement date had not yet occurred. Therefore, the adoption of this standard did not have any impact on the Company’s financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. ASU 2017-04 simplifies the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test and eliminating the requirement for a reporting unit with a zero or negative carrying amount to perform a qualitative assessment. Under ASU 2017-04, goodwill impairment testing is performed by comparing the fair value of a reporting unit with its carrying amount whereby an impairment charge is recognized for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized is not to exceed the total amount of goodwill allocated to that reporting unit. In addition, income tax effects are considered, if applicable. ASU 2017-04 is effective for annual and any interim impairment tests performed after December 15, 2022. Effective October 1, 2020, the Company early adopted this new guidance for its annual goodwill impairment testing whereby the adoption of this standard did not have any impact on the Company’s financial statements.
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurements (Topic 820): Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement. ASU 2018-13 modifies the disclosure requirements on fair value measurements. ASU 2018-13 was adopted effective for the Company beginning in the first quarter of 2020. The Company adopted ASU 2018-13 effective January 1, 2020. The adoption of this standard did have a material impact on the Company’s financial statements.
| F-12 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2 – REVENUE RECOGNITION
In May 2014, the FASB issued Accounting Standards Update No. 2014-09 (Topic 606) titled, “Revenue from Contracts with Customers.” Topic 606 supersedes the revenue recognition requirements in Topic 605 “Revenue Recognition” (Topic 605), and requires entities to recognize revenues when control of the promised goods or services is transferred to customers at an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services.
Revenue Recognition
The Company generates revenue from the sale of products and services. Revenue is recognized when control of the products or services is transferred to our customers in a way that reflects the consideration we expect to be entitled to in exchange for those products and services.
The Company determines revenue recognition through the following five-step model, which entails:
| 1) | identification of the promised goods or services in the contract; | |
| 2) | determination of whether the promised goods or services are performance obligations, including whether they are distinct in the context of the contract; | |
| 3) | measurement of the transaction price, including the constraint on variable consideration; | |
| 4) | allocation of the transaction price to the performance obligations; and | |
| 5) | recognition of revenue when, or as the Company satisfies each performance obligation. |
Service revenue
Service revenue is recognized when the underlying training or other services are performed. Unearned revenue reported on the balance sheet as contract liability represents the portion of fees paid by customers for services that have not yet been performed as of the reporting date and are recorded as the service is rendered. The Company recognizes this revenue over the twelve-month life of the contract. Provisions for discounts are provided in the same period that the related revenue from the products and/or services is recorded.
The Company enters into programs that may provide for multiple element deliverables. Commencing in 2018, the Company began enrolling medical and dental professionals in a one-year program which includes training in a highly personalized, deep immersion workshop format which provides the dentist access to a global team who is dedicated to creating a successful integrated practice. The key topics covered in training include case selection, clinical diagnosis, appliance design, adjunctive therapies, instructions on ordering Vivos products, guidance on pricing, instruction on insurance reimbursement protocols and interacting with our proprietary software system and the many features on the Company’s website. The initial training and educational workshop is typically provided in the first month that a Vivos Integrated Provider (“VIP” or “Provider”) enrolls. Since Providers are able to begin generating revenue after the first training workshop, we recognize 50% of the service revenue in the second month of enrollment and the remaining 50% prorata throughout the following eleven months of the service contract. Ongoing support and additional training is provided throughout the year and includes access to the Company’s proprietary Airway Intelligence Service (“AIS”) which provides the Provider with resources to help simplify the diagnostic and treatment planning process. AIS is provided as part of the price of each appliance and is not a separate revenue stream. Following the year of training and support, the Provider may pay for seminars and training courses that meet the Provider’s needs on a subscription or a course-by-course basis. In addition to enrollment service revenue, the Company has launched an additional service on a monthly subscription basis, its Billing Intelligence Service (“BIS”). Revenue for these services is recognized monthly during the month the services are rendered.
The Company identifies all goods and services that are delivered separately under a sales arrangement and allocates revenue to each deliverable based on relative fair values. Fair values are generally established based on the relevant service period which approximates the prices for relevant training that would be charged if those services were sold separately. In general, revenues are separated between durable medical equipment (product revenue) and education and training services (service revenue). The allocated revenue for each deliverable is then recognized ratably based on relative fair values of the components of the sale. Revenue from training is recognized over the relevant service period, i.e. as the Company satisfies its performance obligations and creates value for the Provider. The Company also evaluates the impact of undelivered items on the functionality of delivered items for each sales transaction and, where appropriate, defers revenue on delivered items when that functionality has been affected. Functionality is determined to be met if the delivered products or services represent a separate earnings process.
| F-13 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2 – REVENUE RECOGNITION (Continued)
From time to time we offer various discounts to our customers. These include the following:
1) Discount for cash pay in full
2) Conference or trade show incentives
3) Negotiated concessions on annual enrollment fee
The amount of the discount is determined up front prior to the sale. Accordingly, measurement is determined before the sale occurs and revenue is recognized based on the terms agreed upon between the Company and the customer over the performance period. In rare circumstances, a discount has been given after the sale during a conference which is offering a discount to full price. In this situation revenue is measured and the change in transaction price is allocated over the remaining performance obligation.
The amount of consideration can vary by customer due to promotions and discounts authorized to incentivize a sale. Prior to the sale, the customer and the Company agree upon the amount of consideration that the customer will pay in exchange for the services the Company provides. The net consideration that the customer has agreed to pay is the expected value that is recognized as revenue over the service period. Any overpayments are refunded during the reporting period so that no refund liability is recognized. At the end of each reporting period, the Company updates the transaction price to represent the circumstances present at the end of the reporting period and any changes in circumstances during the reporting period.
Product revenue
In addition to revenue from services, the Company also generates revenue from the sale of its patented oral devices and preformed guides, known as appliances or systems to its customer, the Provider. Revenue from the appliance sale is recognized when control of product is transferred to the Provider in an amount that reflects the consideration it expects to be entitled to in exchange for those products. The Provider in turn charges the Provider’s patient and or patient’s insurance a fee for the appliance and for his or her professional services in measuring, fitting, installing the appliance and educating the patient as to its use. The Company is contracted with the Provider for the sale of the appliance and is not involved in the sale of the products and services from the Provider to the Provider’s patient.
The appliance is similar to a retainer that is worn after braces are removed. Each appliance is unique and is fitted to the patient. The Company utilizes its network of certified dental Providers throughout the country to sell the appliances to their customers as well as in two centers that the Company operates. The Company utilizes third party contract manufacturers or labs to produce its unique, patented appliances and preformed guides. The manufacturer designated by the Company produces the appliance in strict adherence to the Company’s patents, design files, protocols, processes and procedures and under the direction and specific instruction of the Company, ships the appliance to the Provider who ordered the appliance from the Company. All of the Company’s contract manufacturers are required to follow the Company’s master design files in production of appliances or the lab will be in violation of the FDA’s rules and regulations. The Company performed an analysis under ASC Topic 606-10-55-36 through 55-40 and concluded it is the principal in the transaction and is reporting revenue gross. The Company bills the Provider the contracted price for the appliance which is recorded as product revenue. Product revenue is recognized once the appliance ships to the Provider under the direction of the Company.
Beginning in 2018, the Company operated three centers in Colorado and Utah. Effective October 1, 2019, the Company sold its center in Utah (see Note 3). Within each center, the Company utilizes a team of medical professionals to measure, order and fit each appliance. Upon scheduling the patient (which is the Company’s customer in this case), the center takes a deposit and reviews the patient’s insurance coverage. Revenue is recognized differently for our Company owned centers than for its Providers. The Company recognizes revenue in the centers after the appliance is received from the manufacturer and once the appliance is fitted and provided to the patient.
The Company offers its Clinical Advisors discounts from our standard Provider pricing. This is done to help encourage our Clinical Advisors, who help the Provider with technical aspects of our products, to purchase our products for their own practices. In addition, from time to time, we offer buy one get one offers and other credits to incentivize our Providers to embrace our products and increase volume within their practices.
| F-14 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2 – REVENUE RECOGNITION (Continued)
The Company’s revenue from contracts with customers is shown in the table below:
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Revenue | ||||||||
| Product revenue: | ||||||||
| Appliance sales to integrated providers | $ | 4,547,883 | $ | 2,917,095 | ||||
| Center revenue | 341,957 | 1,432,528 | ||||||
| Total product revenue | 4,889,840 | 4,349,623 | ||||||
| Service revenue | ||||||||
| VIP | 7,540,718 | 6,742,283 | ||||||
| Billing intelligence services | 620,094 | 256,415 | ||||||
| Sponsorship/seminar/other | 15,585 | 44,956 | ||||||
| Total service revenue | 8,176,397 | 7,043,654 | ||||||
| Total revenue | $ | 13,066,237 | $ | 11,393,277 | ||||
Costs of obtaining the contract
The Company does pay commissions to certain employees and others to incentivize sales growth. The Company recognizes these incremental costs of obtaining a contract as an expense when incurred since the amortization period of the asset that we would have otherwise recognized would be amortized over a period of less than one year.
Contract Balances
When timing of the Company’s delivery of product is different from the timing of the payments made by customers, the Company recognizes either a contract asset (performance precedes customer payment) or a contract liability (customer payment precedes performance). Contracts are often paid in arrears and are recognized as receivables after the Company considers whether a significant financing component exists.
Payment on product revenues is typically paid by credit card upfront. Payment on service revenues in 2020 and 2019 was sought up front and for training to be received, a minimum deposit is required. In some cases, the Company allowed installment plans to entice additional providers.
The opening and closing balances of the Company’s contract liability are as follows:
| 2020 | 2019 | |||||||
| Beginning balance, January 1 | $ | 2,947,565 | $ | 889,508 | ||||
| New contracts | 7,531,145 | 8,800,340 | ||||||
| Revenue recognized | (7,540,718 | ) | (6,742,283 | ) | ||||
| Ending balance, December 31 | $ | 2,937,992 | $ | 2,947,565 | ||||
| F-15 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
3 - BUSINESS DIVESTITURES
Divestitures
Effective October 1, 2019, the Company sold its center in Utah to an entity controlled by the spouse of an employee for total consideration of $1,225,000. Consideration included cash of $250,000 and a note receivable of $975,000. The note receivable has a stated interest rate of 6%. Based on market rates, the Company recorded a discount on the note receivable of approximately $100,000 that is being amortized monthly over a five-year period. Assets disposed of included goodwill of approximately $1,072,000, other intangible assets of $27,000 and tangible assets of approximately $86,000. The sale of the center resulted in recognizing a loss of approximately $60,000. The results of operations from this center were immaterial to the Company as a whole.
4 - PROPERTY AND EQUIPMENT, NET
Property and equipment consist of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Furniture and equipment | $ | 935,697 | $ | 908,957 | ||||
| Leasehold improvements | 519,378 | 519,378 | ||||||
| Construction in progress | 143,037 | 138,845 | ||||||
| Molds | 74,822 | 74,822 | ||||||
| Gross property and equipment | 1,672,934 | 1,642,002 | ||||||
| Less - Accumulated depreciation and amortization | (801,337 | ) | (502,501 | ) | ||||
| Net property and equipment | $ | 871,597 | $ | 1,139,501 | ||||
Leasehold improvements relate to the centers in Colorado. Total depreciation and amortization expense was $298,836 and $326,849 for the years ended December 31, 2020 and 2019, respectively.
| F-16 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
5 - INTANGIBLE ASSETS, NET AND GOODWILL
We amortize identifiable intangible assets on a straight-line basis over their estimated lives, which range from 5-15 years. As of December 31, 2020 and 2019, identifiable intangibles were as follows:
| December 31, 2020 | December 31, 2019 | |||||||
| Patents and developed technology | $ | 1,775,438 | $ | 1,775,438 | ||||
| Trade name | 330,000 | 330,000 | ||||||
| Other | 26,500 | 26,500 | ||||||
| 2,131,938 | 2,131,938 | |||||||
| Less - Accumulated amortization | (1,861,817 | ) | (1,442,787 | ) | ||||
| $ | 270,121 | $ | 689,151 | |||||
Amortization expense of identifiable intangible assets was $419,029 and $424,379 for the years ended December 31, 2020 and 2019, respectively. The estimated future amortization of identifiable intangible assets is as follows:
| 2021 | $ | 262,279 | ||
| 2022 | 1,029 | |||
| 2023 | 1,029 | |||
| 2024 | 1,029 | |||
| 2025 | 1,029 | |||
| Thereafter | 3,727 | |||
| Total | $ | 270,122 |
Goodwill of $2,671,434 at December 31, 2020 and 2019 was tested for impairment on December 31, 2020 and 2019, respectively and impairment was not required.
6 – ACCRUED EXPENSES
Accrued expenses consist of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Accrued payroll | $ | 1,024,931 | $ | 771,583 | ||||
| Accrued interest and other | 411,723 | 156,578 | ||||||
| Lab rebate liabilities | 300,067 | - | ||||||
| Accrued common stock subscriptions | - | 350,000 | ||||||
| Accrued consulting | - | 75,000 | ||||||
| Total accrued expenses | $ | 1,736,721 | $ | 1,353,161 | ||||
| F-17 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
7 - DEBT
The Company issued debt on April 19, 2017 and May 22, 2017 included stock warrants that allow the holders to purchase 33,334 and 16,667 shares of the Company’s common stock, respectively, at a price equal to the higher of a) $1.50/share or b) a 50% discount to the Company’s ten-day average stock price as quoted or listed on a national exchange. The warrants expire on the third anniversary from the date of the debt issuance. The debt issued on April 19, 2017 was converted into shares of the Company’s common stock at a conversion price of $1.50 per share on April 19, 2019. The debt issued on May 22, 2017 was converted into shares of the Company’s common stock at a conversion price of $1.50 per share on May 22, 2019.
On July 1, 2018, the Company issued convertible debt of $525,000 as part of the Merger Agreement with TMJ. The debt is convertible into shares of the Company’s common stock at a conversion rate of $7.50 per share. The interest rate on the debt is 6% and the maturity date is July 1, 2023. The debt was paid in full in 2019.
On November 6, 2018, the Company issued convertible debt of $25,000 as part of the asset purchase agreement with Empowered Dental Lab, LLC. The debt is convertible into shares of the Company’s common stock at a conversion rate of $7.50 per share. The interest rate on the debt is 10% per annum beginning July 1, 2020, and the maturity date was extended to December 31, 2020. The Company repaid this convertible debt plus interest in January 2021.
On April 18, 2019, the Company began offering 6% convertible notes (the “2019 Notes”) to accredited investors pursuant to SEC Rule 506(c). Upon the closing of an aggregate gross cash consideration to the Company of at least $10,000,000 (a “Qualified Financing”), the outstanding loan balance of the 2019 Notes (the “Loan Balance”) shall be automatically converted into that number or principal amount of the securities of the Company issued in the Qualified Financing (the “New Securities”) at a conversion price equal to (a) seventy-five percent (75%) of the price per share (or conversion price per share as the case may be) of New Securities paid by the investors in such Qualified Financing if the Qualified Financing occurs on or prior to December 31, 2019 and (b) fifty percent (50%) of the price per share (or conversion price per share as the case may be) of New Securities paid by the investors in such Qualified Financing if the Qualified Financing occurs after December 31, 2019; provided, however, that in no event for purposes of any mandatory conversion shall the Loan Balance be convertible at a price lower than $7.50 per share, which shall serve as a floor price. In any such conversion, the holders of the 2019 Notes shall be provided with all of the same rights, privileges and preferences (including contractual rights and protections such as pre-emptive rights, rights of first refusal, co-sale rights, information and registration rights) as are provided to the holders of the New Securities issued in such Qualified Financing. The Company incurred approximately $31,000 in issuance costs associated with the 2019 Notes. The maturity date of the 2019 Notes was March 31, 2020. One holder of a $75,000 note elected to be paid out the principal and interest which was repaid in December 2020. During the year ended December 31, 2020, holders of $2,943,870 exchanged outstanding principal and interest on the notes into Series B preferred units (see Note 9). Holders of $770,000 principal (plus $26,068 in accrued interest) exchanged their 2019 Notes into the Company Class A common stock.
On May 8, 2020, the Company received approximately $1,265,000 in funding through the U.S. Small Business Administration’s Payroll Protection Program (PPP) that was part of the Coronavirus Aid, Relief, and Economic Security Act signed into law in March 2020. The interest rate on the loan is 1.00% per year and matures on May 5, 2022. The Company used these funds to assist with payroll, rent and utilities. The Company has spent the funding in a manner in which it believes the entire balance of the outstanding promissory note will be eligible for forgiveness through the terms of the PPP. An application to forgive the entire amount was submitted with the lender in January 2021, however, there can be no assurance given that any portion of the PPP loan will be forgiven. Any request for forgiveness is subject to review and approval by the lender and the SBA, including review of qualifying expenditures, staffing and salary levels.
Included in interest expense for the year ended December 31, 2020 was $47,001 of interest on the 2019 Notes and $2,799 of interest on the Empowered Dental Lab convertible note. Included in interest expense for the year ended December 31, 2019 was $88,045 of accrued interest on 2019 convertible notes.
| F-18 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
7 –DEBT (Continued)
Outstanding debt was as follows:
| December 31, 2020 | December 31, 2019 | |||||||
| Principal balance of debt due December 31, 2020 | $ | 25,000 | $ | 25,000 | ||||
| 2019 Convertible Notes due March 31, 2020 | - | 3,684,535 | ||||||
| PPP loan maturing May 5, 2022 | 1,265,067 | - | ||||||
| Total debt | 1,290,067 | 3,709,535 | ||||||
| Less - Current portion of debt | (866,972 | ) | (3,709,535 | ) | ||||
| Long-term portion of debt | $ | 423,095 | $ | - | ||||
Expected future principal payments for outstanding debt are as follows:
| Year ending December 31: | ||||
| 2021 | $ | 866,972 | ||
| 2022 | 423,095 | |||
| Total expected future principal payments | $ | 1,290,067 | ||
8 – CONVERTIBLE REDEEMABLE PREFERRED STOCK
The Company’s Board of Directors may, from time to time, authorize the issuance of preferred stock from the 50,000,000 shares approved for issuance. Each issuance of preferred stock may have different voting, dividend, conversion, redemption, and liquidation preferences. In May 2017, the Company entered into a Definitive Purchase Agreement (the “DPA”) to acquire all of the licensed intellectual property, consisting primarily of patents, from its largest shareholder, current Chief Medical Officer and former majority shareholder of BioModeling. The Company’s Board of Directors previously authorized the issuance of 1 million shares of Series A convertible preferred stock (“Series A Preferred Stock”) with a stated value of $5 per share. Each share is convertible at any time into one share of Class A common stock and each share of Series A Preferred Stock is also entitled to one vote. The Series A Preferred Stock was redeemable at the Company’s option at any time for the stated value and at the option of the holder at 20% each year, commencing twelve months from the closing date with a limitation of $1 million in any twelve-month period unless authorized by the Board of Directors to be more in any twelve-month period.
In accordance with ASC 480, the Company has accounted for the Series A Preferred Stock as temporary equity. As such, the carrying value of the shares was accreted over time such that the carrying value of the shares was at least equal to the redemption value of the shares. The accretion was recorded as a debit to Additional Paid-In Capital and a credit to Preferred Stock. As a result of the IPO, the Company redeemed all remaining Series A Preferred Stock in December 2020 representing 700,000 shares and $3,500,000. During the years ended December 31, 2020 and 2019, the Company recognized $2,333,333 and $1,000,000 of accretion, respectively. During the years ended December 31, 2020 and 2019, the Company redeemed 730,000 and 70,000 shares, respectively, of the Series A Preferred Stock for $3,650,000 and $350,000, respectively.
| F-19 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
9 - STOCKHOLDERS’ EQUITY
Common Stock
The Company is authorized to issue 200,000,000 shares of common stock, par value of $0.0001 per share and 50,000,000 of preferred stock, par value of $0.0001 per share. Holders of the common stock are entitled to one vote for each share held. The Company’s Board of Directors may grant dividends to holders of the preferred stock and the common stock.
For the year ended December 31, 2020, the Company issued 4,025,000 shares of common stock for net proceeds of approximately $21.6 million. Offering costs associated with this stock issuance were approximately $700,000. The Company also issued 1,199,195 shares issued through the conversion of Series B preferred stock (the “Series B Preferred”). The Company issued 300,000 shares to settle a shareholder demand (see Note 10). The Company issued 106,314 shares for the conversion of convertible debt (see Note 7). Finally, the Company also issued 134,778 shares to consultants for services.
For the year ended December 31, 2019, the Company issued 376,574 shares of common stock for net proceeds of $1,997,192. Offering costs associated with this stock issuance were immaterial. Included in these amounts were 50,000 shares of common stock issued through option exercises for net proceeds of $82,500. The Company also issued 126,518 shares issued through the conversion of convertible debt for net proceeds of $250,475, and 44,286 shares through the conversion of a shareholder note for net proceeds of $498,218.
Preferred Stock – Series B
On January 9, 2020, the Company’s Board of Directors designated 1,200,000 shares of Series B Preferred. The terms of the Series B Preferred have a par value of $0.0001 per share and provide for an issuance price of $15.00 per share. The shares of Series B Preferred do not provide the holders with rights to demand redemption, dividends, or to vote as a class with the Company’s holders of common stock. Upon liquidation, the shares of Series B Preferred have priority over the holders of shares of common stock. The terms of the Series B Preferred provide for mandatory conversion to shares of common stock upon a sale of the Company or upon completion of a qualified financing for aggregate gross cash proceeds of at least $15.0 million. Upon a mandatory conversion event, the shares of Series B Preferred will convert to shares of common stock based on a conversion price equal to 75% of the price paid by investors in a sale of the Company or a qualified financing.
The Company commenced a private placement of units (the “Series B Units”) consisting of (i) one share of Series B Preferred, and (ii) one warrant to be issued for the number of shares of common stock into which to Series B Preferred stock is convertible upon a mandatory conversion event (the “Contingent Warrants”). The Contingent Warrants will provide for an exercise price equal to 125% of the price of the Company’s shares of common stock on the date of a mandatory conversion event. The Company reported no beneficial conversion on the Contingent Warrant as the warrant has a contingent beneficial conversion feature that is not calculated as a separate derivative until the contingent event has occurred. The private placement provides for the sale of units at an issuance price of $15.00 per unit for gross proceeds up to $15,000,000. The private placement also provides for an over-allotment option for the issuance of up to an additional $3,000,000 or 200,000 units. Based on the terms of the Series B Preferred, the Company has classified it within permanent equity in the accompanying consolidated balance sheet during 2020.
For the year ended December 31, 2020, the Company received gross proceeds of approximately $2,450,000 from the issuance of Series B Units resulting in the issuance of 163,500 shares of Series B Preferred stock. Additionally, holders of the 2019 Notes agreed to exchange an aggregate principal balance of $2,839,535 plus accrued interest of $104,335 into 196,258 shares of Series B Preferred. Offering costs associated with this issuance were approximately $50,000. As of December 31, 2020, all of the Series B stock was converted into 1,199,195 shares of common stock as the IPO triggered the mandatory conversion.
Stock Options
In 2017, the Company’s shareholders approved the adoption of a stock and option award plan (the “2017 Plan”), under which shares were reserved for future issuance for options, restricted stock awards and other equity awards. The 2017 Plan permits grants of equity awards to employees, directors, consultants and other independent contractors. The Company’s shareholders have approved a total reserve of 1,333,333 million shares for issuance under the 2017 Plan. In April 2019, the Company’s shareholders approved the adoption of a stock and option award plan (the “2019 Plan”), under which shares were reserved for future issuance for options, restricted stock awards and other equity awards. The 2019 Plan permits grants of equity awards to employees, directors, consultants and other independent contractors. The Company’s shareholders have approved a total reserve of 333,334 shares for issuance under the 2019 Plan. On June 18, 2020, the Company’s shareholders approved an amendment and restatement of the 2019 Plan to increase the number of shares of common stock available for issuance thereunder by 833,333 share of common stock such that, after amendment and restatement of the 2019 Plan, and prior to any grants, 1,166,667 shares of common stock were available under the 2019 Plan.
During the years ended December 31, 2020 and 2019, the Company issued stock options to purchase 429,012 and 503,333 shares at a weighted average exercise price of $7.50 per share of the Company’s common stock to certain members of the Board of Directors and certain employees. The stock options allow the holders to purchase shares of the Company’s common stock at prices between $1.50 and $7.50 per share. Options for the purchase of 26,667 shares of common stock expired as of December 31, 2020. The following table summarizes all stock options as of December 31, 2020 and 2019:
| F-20 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
9 - STOCKHOLDERS’ EQUITY (Continued)
Number of Stock Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life | Aggregate Intrinsic Value | |||||||||||||
| Options outstanding at December 31, 2018 | 1,803,334 | $ | 3.69 | 3.34 | $ | 4,551,196 | ||||||||||
| Granted | 503,333 | $ | 7.50 | 4.46 | - | |||||||||||
| Exercised | (50,000 | ) | $ | 1.65 | ||||||||||||
| Expired/terminated | (356,667 | ) | $ | 3.93 | ||||||||||||
| Options outstanding at December 31, 2019 | 1,900,000 | $ | 4.29 | 3.08 | $ | 6,695,876 | ||||||||||
| Granted | 429,012 | 7.50 | 4.55 | - | ||||||||||||
| Exercised | - | - | ||||||||||||||
| Expired/terminated | (26,667 | ) | $ | 7.50 | ||||||||||||
| Options outstanding at December 31, 2020 | 2,302,345 | $ | 4.84 | 1.33 | $ | 2,463,498 | ||||||||||
| Options exercisable at December 31, 2019 | 1,228,176 | $ | 3.99 | 1.65 | ||||||||||||
| Options exercisable at December 31, 2020 | 1,672,991 | $ | 4.10 | 2.46 | ||||||||||||
The Company accounts for share based payments by recognizing compensation expense based upon the estimated fair value of the awards on the date of grant. The Company determines the estimated grant fair value using the Black-Scholes option pricing model and recognizes compensation expense ratably over the requisite service period which approximates the vesting period using the straight-line method.
The weighted average assumptions used in the fair value calculations are as follows:
| 2020 | 2019 | |||||||
| Expected term (years) | 3.15 | 3.20 | ||||||
| Risk-free interest rate | 0.38 | % | 2.00 | % | ||||
| Expected volatility | 134 | % | 122 | % | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
During the years ended December 31, 2020 and 2019, the Company recognized approximately $2,172,000 and $1,987,000, respectively, of share-based compensation expense relating to the vesting of stock options. The options were valued using the Black-Scholes valuation method at the date of the grant and compensation expense is recognized over the vesting period. Unrecognized expense relating to these awards as of December 31, 2020 was approximately $3,441,030, which will be recognized over the weighted average remaining term of 2.38 years at December 31, 2020.
Warrants
During 2020 and in connection with the IPO, the Company issued warrants to the underwriter that provide for the purchase of 402,500 shares of common stock at an exercise price of $7.50 per share, are exercisable beginning on June 8, 2021, and expire on December 10, 2025.
Pursuant to the terms of the Series B Units and in connection with the IPO which qualified as a mandatory conversion event, 1,199,195 Contingent Warrants were provided for an exercise price equal to 125% of the price of the Company’s shares of common stock on the date of an MC event, or $7.50 per share based on the IPO price of $6.00.
On October 22, 2020, two minority stockholders initiated a derivative demand which resulted in a settlement and release agreement that was entered into on November 6, 2020 (See Note 10). Pursuant to the settlement, the Company issued warrants to purchase an aggregate of 325,000 shares of common stock (the “Settlement Warrants”). The Settlement Warrants are exercisable on a cash only basis at an exercise price of $7.50 per share, are exercisable beginning on June 13, 2021, and expire on May 6, 2024.
On June 13, 2017, the Company issued warrants to purchase an aggregate of 33,334 shares of common stock to an investor of convertible notes. The warrants are exercisable on a cash basis at an exercise price of $1.50 per share, are exercisable beginning on June 30, 2017, and expire on June 30, 2022.
| F-21 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
10 - RELATED PARTY TRANSACTIONS
The Company was a party to a management agreement with Upeva, Inc., a company for which the Company’s prior Secretary and one of the Company’s former board members serves as chief executive officer. In return for various legal and other consulting services, the Company paid Upeva a monthly fee of $10,000. This agreement terminated on April 30, 2020. As of December 31, 2020, the Company owed Upeva, Inc. approximately $10,000. Additionally, the former Secretary and director is the beneficial owner of 254,902 common shares of the Company through Spire Family Holdings, L.P.
During the year ended December 31, 2020, one of the Company’s former directors who held $200,000 in 2019 Notes exchanged her outstanding notes for Series B preferred units, which converted into 45,252 common shares.
During 2019, one of the Company’s directors and holder of the Company’s Series A preferred stock, exercised his right to redeem 70,000 shares of the Series A preferred stock for $5.00 per share for a total of $350,000. During 2020, one of the Company’s Directors and holder of the Company’s Series A preferred stock, exercised his right to redeem 730,000 shares of the Series A preferred stock for $5.00 per share for a total of $3,650,000.
In July 2020, two of the directors voluntarily entered into separation agreements with our company. Such agreements contained customary releases, confidentiality and non-disparagement provisions. As consideration for the entering the separation agreements, each director received an equity grant in the amount 16,667 shares and the ability to retain and exercise their previously granted and vested options, and the Company also committed to providing continued indemnification obligations consistent with organizational documents and to retain director’s and officer’s insurance for a period of twenty-four months in connection with two of the directors’ prior service on the board.
In August 2020, the Company also entered into a Separation Agreement with another director pursuant to which the Company is required to purchase from the director and her affiliated entities 13,575 shares of Series B Preferred Stock and warrants to purchase common stock and 16,667 shares of common stock held for an aggregate purchase price of $325,000. If the Company was unable to close a qualified financing, as defined in the agreement of at least $3,000,000 of equity or equity-linked securities by September 15, 2020 (as was extended up to October 28, 2020), a modified consideration would include 16,667 shares of unrestricted, fully vested common stock, a grant of stock options to purchase 33,334 shares of common stock at a price of $7.50 that will be fully vested and exercisable and $22,000 in cash. The Company recorded general and administrative expense and accrued expenses of approximately $286,000 for cash and equity issuances with this settlement. In November 2020, the Company granted this former director 16,667 shares of unrestricted, fully vested common stock, a grant of stock options to purchase 33,334 shares of common stock at a price of $7.50 that will be fully vested and exercisable and paid $47,000 in cash (including $25,000 for legal fees) to settle terms outlined in her separation agreement.
On October 22, 2020, two minority stockholders of the Company, Lazarus Asset Management, LLC and a former director of the Company (who we refer to as the Demanding Stockholders), sent a derivative demand to the Company through counsel asking the board of directors to review and investigate certain recent actions taken by the board of directors, or members thereof, and senior management including (i) pursuit of the initial public offering described in the Company’s filing on Form S-1, (ii) the board of directors’ previous rejection (on two occasions) of a “reverse merger” transaction proposal made by Lazarus Asset Management, LLC, (iii) purported mismanagement of corporate assets, and (iv) various matters related to stock sales and other matters. After discussions with the Demanding Stockholders and their counsel, the Company ascertained that the Demanding Stockholders were acting for themselves and on behalf of an additional group of minority shareholders, (we refer to the Demanding Stockholders and all such other minority shareholders they acted on behalf of collectively as the Stockholder Group).
| F-22 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
10 - RELATED PARTY TRANSACTIONS (Continued)
While the Company believes that the assertions of the Demanding Stockholders lacked any merit in fact and in law, rather than expending resources investigating or litigating the claims of the Demanding Stockholders, and in order to proceed with the Company’s initial public offering, on November 6, 2020, without admitting or denying any claims asserted by the Demanding Stockholders, the Company entered into a Settlement and Release Agreement with each member of the Stockholder Group (which the Company refers to as the Settlement and Release Agreement). Pursuant to the Settlement and Release Agreement, all claims of the Demanding Stockholders were withdrawn with prejudice, and the Company and the Stockholder Group provided each other with full releases of any claims. In consideration of such withdrawal and releases, the members of the Stockholder Group have received: (i) an aggregate of 300,000 shares of Company common stock and (ii) warrants to purchase an aggregate of 325,000 shares of common stock (see Note 9). Such warrants (x) will be exercisable on a cash only basis at a strike price of 125% of the public offering price per share in a Company qualified public offering of more than $10 million, (y) will be exercisable for a period of 36 months, beginning six months after the consummation of a qualified public offering and ending on the forty-second month anniversary of a Company qualified public offering. Finally, the Settlement and Release Agreement contains customary representations, warranties and covenants, including relating to confidentiality and non-disparagement, and the Company agreed to reimburse the Demanding Stockholders for up to $50,000 of their legal fees associated with the demand letter the Company received on October 22, 2020 from them.
In late 2019, a voucher program was offered whereby any employee could pre-purchase a $30,000 VIP deposit with the Company that could be redeemed in full after February 15, 2020, subject to certain limitations, toward a VIP enrollment the employee brought forth in the future. The purpose of this program was to assist with cash flow constraints at the time. Thirteen vouchers totaling $390,000 were sold. For the year ended December 31, 2020, the Company redeemed each of the thirteen vouchers totaling $390,000. The Company included the balance in contract liabilities.
11 - INCOME TAXES
Domestic and foreign components of loss before income tax are as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Domestic | $ | (12,071,603 | ) | $ | (10,768,069 | ) | ||
| Foreign | 14,726 | 13,750 | ||||||
| Total | (12,056,877 | ) | (10,754,319 | ) | ||||
Income tax expense (benefit) consists of the following:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Current income taxes | ||||||||
| Federal | $ | - | $ | - | ||||
| States | - | - | ||||||
| Total current income taxes | - | - | ||||||
| Deferred income taxes | ||||||||
| Federal | - | - | ||||||
| States | - | - | ||||||
| Total deferred income taxes | - | - | ||||||
| Total income tax expense (benefit) | $ | - | $ | - | ||||
Income tax expense (benefit) differed from amounts that would result from applying the US statutory income tax rates (21% for the year ended December 31, 2020 and 2019) to loss before income taxes as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| U.S. statutory income tax expense (benefit) | $ | (2,507,484 | ) | $ | (2,258,407 | ) | ||
| Permanent differences | 1,622,396 | 509,514 | ||||||
| State tax expenses | (180,724 | ) | (575,086 | ) | ||||
| Change in valuation allowance | 1,065,812 | 2,323,979 | ||||||
| Income tax expense | $ | - | $ | - | ||||
| F-23 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
11 - INCOME TAXES (Continued)
The principal components of deferred tax assets and liabilities at December 31, 2020 and 2019 were as follows:
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carry forwards | $ | 5,105,063 | $ | 4,372,081 | ||||
| Stock based compensation | 609,587 | 323,572 | ||||||
| Others | 335,882 | 181,700 | ||||||
| Total deferred tax assets before valuation allowance | 6,050,532 | 4,877,353 | ||||||
| Valuation allowance | (5,837,312 | ) | (4,771,500 | ) | ||||
| Total deferred tax assets after valuation allowance | 213,220 | 105,853 | ||||||
| Deferred tax liabilities: | ||||||||
| Property, equipment and intangibles | (213,220 | ) | (105,853 | ) | ||||
| Total deferred tax liabilities | (213,220 | ) | (105,853 | ) | ||||
| Net deferred tax assets and liabilities | $ | - | $ | - | ||||
Management assesses the available positive and negative evidence to estimate if sufficient future taxable income will be generated to use the existing deferred tax assets. A significant piece of objective negative evidence evaluated was the cumulative loss incurred since inception. Such objective evidence limits the ability to consider other subjective evidence such as our projections for future growth. On the basis of this evaluation, as of December 31, 2020, a valuation allowance of $5,837,312 has been recorded to record the deferred tax asset that is more likely than not to be realized. The net change during the year in the total valuation allowance is an increase of $1,065,812.
The Company has federal net operating loss carry forwards of $22,380,564. The Company has various state net operating loss carry forwards. The determination of the state net operating loss carry forwards is dependent upon the apportionment percentages and state laws that can change from year to year and impact the amount of such carry forwards. If federal net operating loss carry forwards are not utilized, $3,332,471 will begin to expire in 2036. The remaining federal net operating losses of $19,048,093 have no expiration.
Management does not believe that there are significant uncertain tax positions in 2020 or 2019. There are no interest and penalties related to uncertain tax positions in 2020 or 2019.
The Company files income tax returns in the United States federal and various state jurisdictions. The Company is no longer subject to income tax examinations for federal income taxes before 2016 or for states before 2015. Net operating loss carryforwards are subject to examination in the year they are utilized regardless of whether the tax year in which they are generated has been closed by statute. The amount subject to disallowance is limited to the NOL utilized. Accordingly, the Company may be subject to examination for prior NOL’s generated as such NOL’s are utilized. As of December 31, 2020, the Company had not filed its 2018 and 2019 foreign operation tax returns.
| F-24 |
VIVOS THERAPEUTICS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
12 – COMMITMENTS AND CONTINGENCIES
Leases
The Company leases office properties under various lease terms. Rent expense, including real estate taxes and related costs, for the years ended December 31, 2020 and 2019 aggregated approximately $458,497 and $309,086, respectively. In connection with some of the Company’s leases, lease incentives were granted. Deferred lease incentives are being amortized on a straight-line basis over the term of the lease.
Future rental payments over the term of the Company’s leases are as follows:
| Year Ending December 31, | ||||
| 2021 | 337,000 | |||
| 2022 | 417,415 | |||
| 2023 | 390,500 | |||
| 2024 | 403,542 | |||
| 2025 | 537,511 | |||
| Thereafter | 1,109,257 | |||
| Total | 3,195,225 | |||
Employment Agreements
During 2020, the Company entered into new employment agreements with its chief executive officer, chief medical officer and chief financial officer. The agreements include incentive compensation in the form of cash bonuses and stock options. The employment agreements require the continuation of salary and benefits for up to two years in the event the employee is terminated without cause.
Consulting Agreement
In August 2018, the Company entered into a consulting agreement with Pro Player Health Alliance, LLC. In accordance with the agreement, the consultant will provide business advisory and consulting services in exchange for cash and shares of the Company’s common stock. These shares will be held in escrow and distributed upon board approval as these services are performed and certain milestones are met. Total expense recognized for this agreement was approximately $0 and $151,000 for the years ended December 31, 2020 and 2019, respectively. Following the IPO, the Company issued 40,000 shares of common stock to settle a liability that had been established and recorded in accrued expenses.
Regulatory status
In September 2017, BioModeling was the subject of a routine FDA audit. The audit resulted in certain findings that BioModeling was required to remediate. On September 27, 2017, BioModeling believed that it had filed its response letter to the audit findings with the FDA. In January 2018, BioModeling received notice that the FDA had posted a Warning Letter on its website alleging failure by BioModeling to reply in a timely manner to the September 2017 audit findings. The Company and BioModeling immediately contacted the FDA in January 2018 and resubmitted the September 27, 2017 audit response letter. In April 2018, the FDA completed a second audit of BioModeling which focused on the September 2017 response letter and the Warning Letter. The Company believes that this issue has been satisfactorily resolved although no definitive statement to that effect has been made by the FDA.
13 - SUBSEQUENT EVENTS
In January 2021, the Company paid off the outstanding balance of a convertible note payable (see Note 7) issued in connection with an acquisition in 2018. $25,000 in principal amount on the convertible note plus interest of $4,741 was paid.
In January 2021, $1,500,000 in cash was paid to our founder and chief medical officer to fully redeem the remaining Series A preferred stock he held and had redeemed in December 2020. This amount was recorded in accounts payable at December 31, 2020.
In March 2021, the Company issued 145,000 stock options to certain employees and an officer.
| F-25 |
2,962,963 Shares
common stock

VIVOS THERAPEUTICS, INC.
PROSPECTUS
| Roth Capital Partners | ||
| National Securities Corporation | Craig-Hallum | |
May 3, 2021
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The table below lists various expenses payable in connection with the sale and distribution of the securities being registered hereby. All the expenses are estimates, except the Securities and Exchange Commission (“SEC”) registration fee. All such expenses will be borne by the Company.
| Type | Amount | |||
| SEC Registration Fee | $ | 3,000 | ||
| FINRA filing fee | 3,500 | |||
| The Nasdaq Capital Market initial listing Fee | 75,000 | |||
| Transfer agent registrar fees | 15.000 | |||
| Accounting fees and expenses | 125,000 | |||
| Legal Fees and expenses | 225,000 | |||
| Printing and engraving expenses | 20,000 | |||
| Miscellaneous expenses | 20,000 | |||
| Total expenses | $ | 486,500 |
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of such person being or having been a director, officer, employee or agent of the corporation. Section 145 of the Delaware General Corporation Law also provides that expenses (including attorneys’ fees) incurred by a director or officer in defending an action may be paid by a corporation in advance of the final disposition of an action if the director or officer undertakes to repay the advanced amounts if it is determined such person is not entitled to be indemnified by the corporation. The Delaware General Corporation Law provides that Section 145 is not exclusive of other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise. Our bylaws provide that, to the fullest extent permitted by law, we shall indemnify and hold harmless any person who was or is made or is threatened to be made a party or is otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative by reason of the fact that such person, or the person for whom he is the legally representative, is or was a director or officer of ours, against all liabilities, losses, expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such proceeding.
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its Certificate of Incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions, or (iv) for any transaction from which the director derived an improper personal benefit.
Our Certificate of Incorporation provides that we shall, to the maximum extent permitted from time to time under the law of the State of Delaware, indemnify and upon request shall advance expenses to any person who is or was a party or is threatened to be made a party to any threatened, pending or completed action, suit, proceeding or claim, whether civil, criminal, administrative or investigative, by reason of the fact that such person is or was or has agreed to be a director or officer of ours or while a director or officer is or was serving at our request as a director, officer, partner, trustee, employee or agent of any corporation, partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans, against expenses (including attorneys’ fees and expenses), judgments, fines, penalties and amounts paid in settlement incurred in connection with the investigation, preparation to defend or defense of such action, suit, proceeding or claim; provided, however, that the foregoing shall not require us to indemnify or advance expenses to any person in connection with any action, suit, proceeding or claim initiated by or on behalf of such person or any counterclaim against us initiated by or on behalf of such person. Such indemnification shall not be exclusive of other indemnification rights arising under any by-law, agreement, vote of directors or stockholders or otherwise and shall inure to the benefit of the heirs and legal representatives of such person. Any person seeking indemnification shall be deemed to have met the standard of conduct required for such indemnification unless the contrary shall be established. Any repeal or modification of our Certificate of Incorporation shall not adversely affect any right or protection of a director or officer of ours with respect to any acts or omissions of such director or officer occurring prior to such repeal or modification.
| II-1 |
Our bylaws provide we shall, to the fullest extent permitted under the laws of the State of Delaware, as amended and supplemented from time to time, indemnify each person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such party is or was, or has agreed to become, a director or officer of ours, or is or was serving, or has agreed to serve, at our request, as a director, officer or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, including any employee benefit plan, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such party or on such party’s behalf in connection with such action, suit or proceeding and any appeal therefrom.
Expenses incurred by such a person in defending a civil or criminal action, suit or proceeding by reason of the fact that such person is or was, or has agreed to become, a director or officer of ours, or is or was serving, or has agreed to serve, at our request, as a director, officer or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, including any employee benefit plan, or by reason of any action alleged to have been taken or omitted in such capacity shall be paid by us in advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of such person to repay such amount if it shall ultimately be determined that he is not entitled to be indemnified by us as authorized by relevant sections of the Delaware General Corporation Law. Notwithstanding the foregoing, we shall not be required to advance such expenses to a person who is a party to an action, suit or proceeding brought by us and approved by a majority of our Board of Directors that alleges willful misappropriation of corporate assets by such person, disclosure of confidential information in violation of such person’s fiduciary or contractual obligations to us or any other willful and deliberate breach in bad faith of such person’s duty to us or our stockholders.
We shall not indemnify any such person seeking indemnification in connection with a proceeding (or part thereof) initiated by such person unless the initiation thereof was approved by our Board of Directors.
The indemnification rights provided in our bylaws shall not be deemed exclusive of any other rights to which those indemnified may be entitled under any by-law, agreement or vote of stockholders or disinterested directors or otherwise, both as to action in their official capacities and as to action in another capacity while holding such office, continue as to such person who has ceased to be a director or officer, and inure to the benefit of the heirs, executors and administrators of such a person.
If the Delaware General Corporation Law is amended to expand further the indemnification permitted to indemnitees, then we shall indemnify such persons to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
We may, to the extent authorized from time to time by our Board of Directors, grant indemnification rights to other employees or agents of ours or other persons serving us and such rights may be equivalent to, or greater or less than, those set forth in our bylaws.
Our obligation to provide indemnification under our bylaws shall be offset to the extent of any other source of indemnification or any otherwise applicable insurance coverage under a policy maintained by us or any other person.
To assure indemnification under our bylaws of all directors, officers, employees or agents who are determined by us or otherwise to be or to have been “fiduciaries” of any employee benefit plan of ours that may exist from time to time, Section 145 of the Delaware General Corporation Law shall, for the purposes of our bylaws, be interpreted as follows: an “other enterprise” shall be deemed to include such an employee benefit plan, including without limitation, any plan of ours that is governed by the Act of Congress entitled “Employee Retirement Income Security Act of 1974,” as amended from time to time; we shall be deemed to have requested a person to serve an employee benefit plan where the performance by such person of his duties to us also imposes duties on, or otherwise involves services by, such person to the plan or participants or beneficiaries of the plan; and excise taxes assessed on a person with respect to an employee benefit plan pursuant to such Act of Congress shall be deemed “fines.”
| II-2 |
Our bylaws shall be deemed to be a contract between us and each person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that person is or was, or has agreed to become, a director or officer of ours, or is or was serving, or has agreed to serve, at our request, as a director, officer or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, including any employee benefit plan, or by reason of any action alleged to have been taken or omitted in such capacity, at any time while this bylaw is in effect, and any repeal or modification thereof shall not affect any rights or obligations then existing with respect to any state of facts then or theretofore existing or any action, suit or proceeding theretofore or thereafter brought based in whole or in part upon any such state of facts.
The indemnification provision of our bylaws does not affect directors’ responsibilities under any other laws, such as the federal securities laws or state or federal environmental laws.
We may purchase and maintain insurance on behalf of any person who is or was a director, officer or employee of ours, or is or was serving at our request as a director, officer, employee or agent of another company, partnership, joint venture, trust or other enterprise against liability asserted against him and incurred by him in any such capacity, or arising out of his status as such, whether or not we would have the power to indemnify him against liability under the provisions of this section. We currently maintain such insurance.
The right of any person to be indemnified is subject to our right, in lieu of such indemnity, to settle any such claim, action, suit or proceeding at our expense of by the payment of the amount of such settlement and the costs and expenses incurred in connection therewith.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling our company pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment of expenses incurred or paid by a director, officer or controlling person in a successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered herewith, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to the court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Item 15. Recent Sales of Unregistered Securities
The following is a summary of transactions by us within the past three years involving sales or our securities that were not registered under the Securities Act. All of the sales listed below were made pursuant to an exemption from registration afforded by Section 4(a)(2) of the Securities Act and/or Regulation D thereunder in that (i) none of the offers and sales constituted a public offering of securities and/or (ii) the securities were only offered and sold to accredited investors.
On January 1, 2018, Vivos Therapeutics granted options to purchase 6,667 shares of common stock at an exercise price of $1.50 per share to Amanda Cruess pursuant to the terms of her employment agreement with Vivos Therapeutics. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 417 shares as of the date of grant and (ii) options to purchase 417 shares at the end of each calendar quarter following January 1, 2018.
On February 9, 2018, Vivos Therapeutics granted options to purchase 83,334 shares of common stock at an exercise price of $4.50 per share to Bryan Ferre in recognition of his service. Such granted options are subject to graduated vesting in the following installments on each of the following dates: (i) options to purchase 16,667 shares as of the date of grant and (ii) options to purchase 16,667 shares at the end of each year following February 9, 2018.
| II-3 |
On February 9, 2018, Vivos Therapeutics granted options to purchase 83,334 shares of common stock at an exercise price of $4.50 per share to Edward Loew. Such granted options were subject to the following vesting: (i) options to purchase 33,334 shares shall vest upon the closing of an SEC Regulation A or S-1 initial public offering and (ii) the remaining options to purchase up to 50,000 shares shall vest at the rate of 1,667 options (to purchase 1,667 shares) per $1,000,000 raised in our initial public offering (up to a maximum offering amount of $50,000,000) over and above $20,000,000 upon the closing of an SEC Regulation A initial public offering. Options for the purchase of 50,000 were cancelled as Vivos Therapeutics did not pursue a Regulation A offering.
On February 9, 2018, Vivos Therapeutics granted options to purchase up to 16,667 shares of common stock at an exercise price of $4.50 per share to each of four of the six advisors on its Board of Advisors (for an aggregate of 66,667 shares). Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 3,334 shares as of the date of grant and (ii) options to purchase 3,334 shares at the end of each year following the date of grant that they serve as advisors.
On April 30, 2018, Vivos Therapeutics granted options to purchase up to 16,667 shares of common stock at an exercise price of $7.50 per share to each of two incoming members of the Board of Directors, De Lyle Bloomquist and Chris Strong, and 16,667 to an incoming member of the Board of Advisors, Dr. Bhaskar Savani, (for an aggregate of 50,000 shares). Such granted options were subject to quarterly vesting of 4,167 shares through March 31, 2019.
On April 30, 2018, Vivos Therapeutics granted options to purchase up to 3,334 shares of common stock at an exercise price of $7.50 per share to each of two employees, Dr. C. Michael Bennett and Lori Jones, (for an aggregate of 6,667 shares). Such granted options were subject to quarterly vesting of 667 shares through June 30, 2019.
On April 30, 2018, Vivos Therapeutics granted options to purchase up to 8,333 shares of common stock at an exercise price of $7.50 per share to Cathryn Bonar, Vivos Compliance Officer. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 1,667 shares as of the date of grant and (ii) options to purchase 1,667 shares at the end of each year following the date of grant.
On August 16, 2018, Vivos Therapeutics granted options to purchase up to 66,667 shares of common stock at an exercise price of $7.50 per share to Edward Loew. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 13,334 shares as of the date of grant and (ii) options to purchase 13,334 shares at the end of each year following the date of grant.
On August 16, 2018, Vivos Therapeutics granted options to purchase up to 166,667 shares of common stock at an exercise price of $7.50 per share to Joe Womack. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 33,334 shares as of the date of grant and (ii) options to purchase 33,334 shares at the end of each year following the date of grant. On March 20, 2019, 100,000 of the 166,667 options expired.
On November 9, 2018, Vivos Therapeutics granted options to purchase up to 248,334 shares of common stock at an exercise price of $7.50 per share in the following amounts to each of the following officers and employees, 116,667 to Bryan Ferre, Chief Marketing Officer, 83,334 to Brad Amman, Chief Financial Officer, 16,667 to Edward Loew, Chief Strategy Officer, 25,000 to Cathryn Bonar, Chief Compliance Officer, 3,334 to Michele Grasmick and 3,334 to Teri McKenna. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 49,667 shares as of the date of grant and (ii) options to purchase 49,667 shares at the end of each year following the date of grant.
On February 14, 2019, Vivos Therapeutics granted options to purchase up to 50,000 shares of common stock at an exercise price of $7.50 per share to an employee, Corbin Cowan. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 10,000 shares as of the date of grant and (ii) options to purchase 10,000 shares at the end of each year following the date of grant.
On February 14, 2019, Vivos Therapeutics granted options to purchase up to 16,667 shares of common stock at an exercise price of $7.50 per share to Jon Caufield, as a member of the Clinical Advisory Board. Such granted options are subject to the following vesting in the following installments on each of the following dates: (i) options to purchase 3,334 shares as of the date of grant and (ii) options to purchase 3,334 shares at the end of each year following the date of grant.
During the year ended December 31, 2018, Vivos Therapeutics issued 965,636 shares of common stock at an average purchase price of $6.66 per share (for aggregate proceeds of $6,445,195) to accredited investors in a private placement under Rule 506(c) of Regulation D of the Securities Act.
| II-4 |
On January 26, 2018, we offered thirteen (13) investors who invested from January 4, 2018 to February 9, 2018 a right to rescind their purchase of shares of common stock during such period and to receive a refund of the full purchase price paid for such shares due to inadvertent non-disclosure of our receipt of a Warning Letter from the FDA on January 12, 2018 requesting that we take prompt action to correct the violations discussed in the Warning Letter, and noting that our failure to do so may result in regulatory action being initiated by the FDA. See “Business– Regulatory Status” for further information on FDA matter. None of such investors elected to rescind their purchase of such shares.
During 2018, Vivos Therapeutics issued 50,000 shares of common stock to consultants in exchange for consulting services rendered by the consultants in 2018 with a value of $4.50 per share (for an aggregate value of $225,000).
In 2018, Vivos Therapeutics redeemed 200,000 shares of the 1,000,000 shares of Series A Preferred Stock held by Dr. G. Dave Singh for $5.00 per share (for an aggregate of $1,000,000).
On July 1, 2018, Vivos Therapeutics issued 93,334 shares of common stock with a value of $7.50 per share (an aggregate value of $700,000) and a 6% convertible promissory note in the principal amount of $525,000 to Dr. Michael Bennett to acquire TMJ & Sleep Therapy Centre of Utah, LLC (“TMJ”) operating as a clinic in Orem, Utah from Dr. Bennett (total consideration of $1,225,000)
On November 6, 2018, Vivos Therapeutics entered into an asset purchase agreement with Empowered Dental Lab, LLC, a Utah limited liability company. The Company agreed to purchase certain inventory and assets from Empowered Dental Lab in exchange for consideration of 6,667 shares of the Company’s common stock and a 6% convertible promissory note for $25,000, for total consideration of $75,000.
On April 18, 2019, we began offering 6% convertible notes to accredited investors pursuant to SEC Rule 506(c) (we refer to these notes as the 2019 Notes). Upon the closing of an offering by our company generating aggregate gross cash consideration to us of at least $10,000,000 (which we refer to as Qualified Financing), the outstanding loan balance of the 2019 Notes shall be automatically converted into that number or principal amount of our securities issued in the Qualified Financing at a conversion price equal to (a) seventy-five percent (75%) of the price per share (or conversion price per share as the case may be) of securities paid for by the investors in such Qualified Financing if the Qualified Financing occurs on or prior to the December 31, 2019 and (b) fifty percent (50%) of the price per share (or conversion price per share as the case may be) of securities paid for by the investors in such Qualified Financing if the Qualified Financing occurs after December 31, 2019; provided, however, that in no event for purposes of any mandatory conversion shall the loan balance be convertible at a price lower than $7.50 per share, which shall serve as a floor price. In any such conversion, the note holders shall be provided with all of the same rights, privileges and preferences (including contractual rights and protections such as pre-emptive rights, rights of first refusal, co-sale rights, information and registration rights) as are provided to the holders of the securities issued in the Qualified Financing. The outside maturity date of the 2019 Notes was March 31, 2020. As of the date of this registration statement, none of our 2019 convertible notes remain outstanding.
| II-5 |
In November 2020, Vivos Therapeutics issued warrants to certain shareholders to purchase an aggregate of 325,000 shares of common stock. Such warrants are substantially similar to the Series B Warrants except such warrants will be exercisable for a period of 36 months, beginning six months after the consummation of the initial public offering and ending on the forty-second month anniversary of the consummation of our initial public offering. See “Management—October 2020 Derivative Demand and Settlement” for further information on the issuance of these warrants.
On March 12, 2021, Vivos Therapeutics granted options to purchase up to 145,000 shares of common stock at an exercise price of $7.50 share in the following amounts to employees and consultants, 120,000 to two employees (100,000 and 20,000 respectively) with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant, and 25,000 to a consultant with standard vesting on each of the following dates: (i) 20% as of the date of grant and (ii) 20% at the end of each year following the date of grant.
On March 29, 2021 and as part of the acquisition of certain assets from, and the entry into related agreements with, MyoCorrect, LLC and its affiliates, Vivos Therapeutics issued three-year warrants to purchase 200,000 shares of our common stock with an exercise price of $7.50 per share. 25,000 of these warrants vested initially upon issuance, but the remainder only vest and become exercisable upon the achievement of pre-determined performance metrics related to the utilization of MyoCorrect. These warrants may be exercised only for cash, and the exercise price is subject to customary, stock-based anti-dilution protection.
On April 14, 2021 and as part of the acquisition of certain assets from, and the entry into related agreements with, Lyon Management & Consulting, LLC and its affiliates, we issued three year warrants to purchase 25,000 shares of our common stock with an exercise price of $8.90 per share. 5,000 of these warrants vested initially upon issuance, but the remainder only vest and become exercisable at the end of each anniversary year following the issuance date. These warrants may be exercised only for cash, and the exercise price is subject to customary, stock-based anti-dilution protection.
During the period from March 12, 2021 through March 30, 2021, Vivos Therapeutics issued warrants to purchase an aggregate of 95,000 shares of common stock to contractors and consultants in exchange for services. These warrants have an exercise price of $7.50 per share. 45,000 of these warrants vested initially upon issuance, but the remainder only vest and become exercisable at the end of each anniversary year following the issuance date. These warrants may be exercised only for cash, and the exercise price is subject to customary, stock-based anti-dilution protection.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits. See the Exhibit Index on the page immediately preceding the exhibits for a list of exhibits filed as part of this registration statement on Form S-1, which Exhibit Index is incorporated herein by reference.
EXHIBIT INDEX
| II-6 |
| * | Filed herewith |
| (1) | Incorporated by reference to the Company’s Registration Statement on Form S-1, filed with the SEC on October 9, 2020. |
| (2) | Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed with the SEC on November 19, 2020. |
| (3) | Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed with the SEC on October 26, 2020. |
| (4) | Incorporated by reference to the Company’s Annual Report on Form 10-K, filed with the SEC on March 25, 2021. |
| + | Certain portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K. The Company will furnish supplementally an unredacted copy of such exhibit to the U.S. Securities and Exchange Commission or its staff upon request. |
| † | Includes management contracts and compensation plans and arrangements |
(b) Financial Statement Schedules. None.
Item 17. Undertakings
| 1. | The undersigned registrant hereby undertakes: |
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
| II-7 |
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
3. Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
4. The undersigned hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. | |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| II-8 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the County of Douglas, State of Colorado, on May 3, 2021.
| VIVOS THERAPEUTICS, INC. | ||
| By: | /s/ R. Kirk Huntsman | |
| R. Kirk Huntsman | ||
| Chairman of the Board and Chief Executive Officer | ||
KNOW ALL PERSONS BY THESE PRESENTS that each individual whose signature appears below hereby constitutes and appoints R. Kirk Huntsman and Bradford Amman and each of them, as his or her true and lawful attorney-in-fact and agent with full power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments, including post-effective amendments, to this registration statement, and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act of 1933 increasing the number of shares for which registration is sought, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and all documents in connection therewith, making such changes in this registration statement as such attorney-in-fact and agent so acting deem appropriate, with the SEC, granting unto said attorney-in-fact and agent, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done with respect to the offering of securities contemplated by this registration statement, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agent or any of them, or his, her or their substitute or substitutes, may lawfully do or cause to be done or by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities indicated on May 3, 2021.
| Name | Title | |
| /s/ R. Kirk Huntsman | Chairman of the Board & Chief Executive Officer | |
| R. Kirk Huntsman | (Principal Executive Officer) | |
| /s/ Bradford Amman | Chief Financial Officer | |
| Bradford Amman | (Principal Financial Officer and Principal Accounting Officer) | |
| /s/ G. Dave Singh, DMD, Ph.D, DDSc | Chief Medical Officer and Director | |
| G. Dave Singh, DMD, Ph.D, DDSc | ||
| /s/ Ralph E. Green, DDS, MBA | Director | |
| Ralph E. Green, DDS, MBA | ||
| /s/ Anja Krammer | Director | |
| Anja Krammer | ||
| /s/ Mark F. Lindsay | Director | |
| Mark F. Lindsay | ||
| /s/ Leonard J. Sokolow | Director | |
| Leonard J. Sokolow | ||
| /s/ Matthew Thompson, MD | Director | |
| Matthew Thompson, MD |