UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
For
the fiscal year ended
For the transition period from ______ to ______
Commission
file number
(Exact name of registrant as specified in charter)
| (State or other jurisdiction of incorporation or organization) | I.R.S. Employer Identification No. |
New York, | ||
| (Address of principal executive offices) | (Zip code) |
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered | ||
| The |
Securities registered pursuant to Section 12(g) of the Act: None.
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate
by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Exchange Act) Yes ☐ No
The
aggregate market value of the voting stock and non-voting common equity held by non-affiliates of the registrant as of the last business
day of the registrant’s most recently completed second fiscal quarter ended June 30, 2024 was approximately $
As
of March 28, 2025, there were
Documents
Incorporated by Reference:
Table of Contents
i
Explanatory Note
Restatement of Previously Issued Annual and Quarterly Consolidated Financial Statements
As previously disclosed in a Current Report on Form 8-K filed by Hoth Therapeutics, Inc. (the “Company”) with the Securities and Exchange Commission (“SEC”) on March 25, 2025, certain of the Company’s previously filed interim unaudited and annual audited consolidated financial statements should no longer be relied upon and a restatement is required for these previously issued consolidated financial statements. During the preparation of our 2024 audited consolidated financial statements and notes thereto, we concluded that there were material errors related to recording of prepaid research and development and the timing of the related research and development expense in our previously issued audited consolidated financial statements as of and for the year ended December 31, 2023, and in our previously issued unaudited condensed consolidated financial statements as of and for each of the quarterly and year to date periods ended March 31, 2024 and 2023, June 30, 2024 and 2023, and September 30, 2024 and 2023. As a result of such errors, we concluded that the issued audited consolidated financial statements and unaudited condensed consolidated financial statements discussed above were materially misstated, and we have restated, herein, our previously issued audited consolidated financial statement and unaudited condensed consolidated financial statements for each of the quarterly and year to date periods ended March 31, 2024 and 2023, June 30, 2024 and 2023, and September 30, 2024 and 2023. The December 31, 2022 understatement of prepaid expenses and other current assets of $983,497 was corrected by increasing prepaid expenses and other current assets and decreasing accumulated deficit as of December 31, 2022 by $983,497, as reflected of the consolidated statements of changes in stockholders’ equity as of December 31, 2022.
The restatement corrections impact certain components within operating cash flows of our audited consolidated financial statements and unaudited condensed consolidated statements of cash flows. Total operating cash flows, investing activities, financing activities, and cash and cash equivalents are unchanged as a result of the restatements and as a result are not restated herein.
In this Annual Report on Form 10-K for the year ended December 31, 2024, we are restating (the “Restatement”) our previously issued (A) (i) audited consolidated balance sheet as of December 31, 2023, (ii) consolidated statement of operations and comprehensive loss for the year ended December 31, 2023 and (iii) consolidated statement of cash flows for the years ended December 2023, which are included in the Annual Report on Form 10-K for the year ended December 31, 2023 (collectively, the “Prior Annual Financial Statements”), and (B) (i) unaudited condensed consolidated balance sheets as of March 31, 2024 and 2023, June 30, 2024 and 2023, and September 30, 2024 and 2023, (ii) unaudited condensed consolidated statements of operations and comprehensive loss for the three months ended March 31, 2024 and 2023, three and six months ended June 30, 2024 and 2023, and three and nine months ended September 30, 2024 and 2023, and (iii) unaudited condensed consolidated statements of cash flows for the three months ended March 31, 2024 and 2023, six months ended June 30, 2024 and 2023, and nine months ended September 30, 2024 and 2023, which are included in each of the Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2024 and 2023, June 30, 2024 and 2023, and September 30, 2024 and 2023 (collectively, the “Prior Quarterly Financial Statements”).
The financial information that was previously filed or otherwise reported for the Prior Quarterly Financial Statements is superseded by the information in this Annual Report on Form 10-K, Note 8 - Restatement of Previously Issued Audited Consolidated Financial Statements and Unaudited Condensed Financial Statements. We have not filed and do not intend to file amendments to our Annual Report of Form 10-K and Quarterly Reports on Form 10-Q for any of the annual and quarterly periods in fiscal years 2024 and 2023. Accordingly, investors should rely only on the restated consolidated financial statements and related disclosures included in this Annual Report on Form 10-K for the year ended December 31, 2024 for the applicable periods or in future filings with the SEC (as applicable), and not on any previously issued or filed reports, earnings releases or similar communications including the consolidated financial statements.
Internal Control Considerations
In connection with the Restatement described above, management has determined that there was a material weakness in the Company’s operation of effective internal control over financial reporting related to the recording of prepaid research and development and the related timing of research and development expenses as of and for the periods indicated above. For a discussion of management’s considerations of the Company’s disclosures controls and procedures, internal controls over financial reporting, and the material weakness identified, see “Controls and Procedures” in Part II, Item 9A in this Annual Report on Form 10-K for the year ended December 31, 2024.
ii
CAUTIONARY NOTE ON FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Any statements in this Annual Report on Form 10-K about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and are forward-looking statements. These statements are often, but not always, made through the use of words or phrases such as “believe,” “will,” “expect,” “anticipate,” “estimate,” “intend,” “plan” and “would.” For example, statements concerning financial condition, possible or assumed future results of operations, growth opportunities, industry ranking, plans and objectives of management, markets for our common stock and future management and organizational structure are all forward-looking statements. Forward-looking statements are not guarantees of performance. They involve known and unknown risks, uncertainties and assumptions that may cause actual results, levels of activity, performance or achievements to differ materially from any results, levels of activity, performance or achievements expressed or implied by any forward-looking statement.
Any forward-looking statements are qualified in their entirety by reference to the risk factors discussed throughout this Annual Report on Form 10-K. Some of the risks, uncertainties and assumptions that could cause actual results to differ materially from estimates or projections contained in the forward-looking statements include, but are not limited to:
| ● | our business strategies; |
| ● | the timing of regulatory submissions; |
| ● | our ability to obtain and maintain regulatory approval of our existing product candidates and any other product candidates we may develop, and the labeling under any approval we may obtain; |
| ● | risks relating to the timing and costs of clinical trials and the timing and costs of other expenses; |
| ● | risks related to market acceptance of products; |
| ● | the ultimate impact of any public health crisis on our business, our clinical trials, our research programs, healthcare systems or the global economy as a whole; |
| ● | intellectual property risks; |
| ● | risks associated with our reliance on third-party organizations; |
| ● | our competitive position; |
| ● | our industry environment; |
| ● | our anticipated financial and operating results, including anticipated sources of revenues; |
| ● | risks related to the Restatement of our financial statements including risks of increased costs and the increased possibility of legal proceedings and regulatory inquiries, sanctions, or investigation; |
| ● | assumptions regarding the size of the available market, benefits of our products, product pricing and timing of product launches; |
| ● | management’s expectation with respect to future acquisitions; |
| ● | statements regarding our goals, intentions, plans and expectations, including the introduction of new products and markets; | |
| ● | general business and economic conditions, such as inflationary pressures and geopolitical conditions; and |
| ● | our cash needs and financing plans. |
The foregoing list sets forth some, but not all, of the factors that could affect our ability to achieve results described in any forward-looking statements. You should read this Annual Report on Form 10-K and the documents that we reference herein and have filed as exhibits to the Annual Report on Form 10-K, completely and with the understanding that our actual future results may be materially different from what we expect. You should assume that the information appearing in this Annual Report on Form 10-K is accurate as of the date hereof. Because the risk factors referred to on page 10 of the Annual Report on Form 10-K could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made, and except as required by law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of the information presented in this Annual Report on Form 10-K, and particularly our forward-looking statements, by these cautionary statements.
iii
RISK FACTOR SUMMARY
Our business is subject to significant risks and uncertainties that make an investment in us speculative and risky. Below we summarize what we believe are the principal risk factors, but these risks are not the only ones we face, and you should carefully review and consider the full discussion of our risk factors in the section titled “Risk Factors,” together with the other information in this Annual Report on Form 10-K. If any of the following risks actually occurs (or if any of those listed elsewhere in this Annual Report on Form 10-K occur), our business, reputation, financial condition, results of operations, revenue, and future prospects could be seriously harmed. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that adversely affect our business.
Risks Related to our Financial Position, Financial Reporting Matters and Need for Capital
| ● | We have generated no revenue from commercial sales and our future profitability is uncertain. If we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development. |
| ● | The Restatement of our financial statements may affect shareholder and investor confidence in us or harm our reputation, and may subject us to additional risks and uncertainties, including increased costs and the increased possibility of legal proceedings and regulatory inquiries, sanctions or investigations. |
Risks Related to Product Development, Regulatory Approval, Manufacturing and Commercialization
| ● | The marketing approval process is lengthy, time-consuming and inherently unpredictable, and if we are ultimately unable to obtain marketing approval for the product candidates we intend to develop, our business may be substantially harmed. |
| ● | We may encounter substantial delays in completing our clinical studies which in turn will require additional costs, or we may fail to demonstrate adequate safety and efficacy to the satisfaction of applicable regulatory authorities. If we are not able to obtain any required regulatory approvals for our product candidates, we will not be able to commercialize our product candidates and our ability to generate revenue will be limited. |
| ● | Conducting successful clinical studies may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. |
| ● | We rely on and intend to rely on third parties to conduct our clinical trials, to assist us with pre-clinical development and for manufacturing and marketing of our proposed product candidates. If we are not able to secure favorable arrangements with such third parties, or such third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our products and our business and financial condition could be harmed. |
| ● | We rely on and intend to rely on third parties to manufacture our clinical product supplies, and to produce and process our product candidates, if approved. Our commercialization of any of our product candidates could be stopped, delayed, or made less profitable if those third parties fail to obtain approval of government regulators, fail to provide us with sufficient quantities of drug products, devices, or device components, or fail to do so at acceptable quality levels or prices. |
| ● | Even if our product candidates are approved by regulatory authorities, if we or our suppliers fail to comply with ongoing U.S. Food and Drug Administration regulations or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market. |
| ● | Our revenue stream will depend upon third-party reimbursement. |
| ● | Our products will face significant competition, and if they are unable to compete successfully, our business will suffer. |
| ● | If we fail to comply with healthcare regulations, we could face substantial enforcement actions, including civil and criminal penalties and our business, operations and financial condition could be adversely affected. |
iv
Risks Related to our Intellectual Property Rights
| ● | Our business depends upon us securing and protecting critical intellectual property. Patent positions in our industry are highly uncertain and involve complex legal and factual questions. |
| ● | We rely upon licenses granted to us by various licensors, and if such licensors do not adequately defend such licenses, our business may be harmed. |
Risks Related to our Company
| ● | We have expanded and may continue to expand our business through the acquisition of rights to new drug candidates that could disrupt our business, harm our financial condition and may also dilute current shareholders’ ownership interests in our Company. |
| ● | If a product liability claim is successfully brought against us for uninsured liabilities, or such claim exceeds our insurance coverage, we could be forced to pay substantial damage awards that could materially harm our business. |
| ● | Significant disruptions of information technology systems or breaches of data security could adversely affect our business |
| ● | Any international operations we undertake may subject us to risks inherent with operations outside of the United States. |
Risks Related to our Common Stock
| ● | Unstable market and economic conditions and adverse developments with respect to financial institutions and associated liquidity risk may have serious adverse consequences on our business, financial condition and stock price. |
| ● | Future sales and issuances of our securities could result in additional dilution of the percentage ownership of our shareholders and could cause our share price to fall. |
| ● | We do not intend to pay cash dividends on our shares of common stock so any returns will be limited to the value of our shares. |
| ● | If we are unable to maintain listing of our securities on The Nasdaq Capital Market (“Nasdaq”) or any stock exchange, our stock price could be adversely affected and the liquidity of our stock and our ability to obtain financing could be impaired. |
| ● | Our Amended and Restated Bylaws provide that the Eighth Judicial District Court of Clark County, Nevada will be the sole and exclusive forum for certain disputes which could limit shareholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, employees or agents. |
| ● | We identified a material weakness in our internal control over financial reporting, which resulted in the restatement of certain or our financial statements. If we are unable to remediate this weakness, or if we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately or timely report our financial condition or operating results, which may adversely affect investor confidence in our Company and, as a result, the value of our common stock. |
v
PART I
Throughout this Annual Report on Form 10-K, the “Company,” “Hoth,” “we,” “us,” and “our” refers to Hoth Therapeutics, Inc., individually, or as the context requires, collectively with its subsidiaries, merveille.ai and Hoth Therapeutics Australia Pty Ltd.
ITEM 1. BUSINESS
Overview
We are a clinical-stage biopharmaceutical company focused on developing new generation therapies for unmet medical needs. We are focused on developing (i) a topical formulation for treating side effects from drugs used for the treatment of cancer (HT-001); (ii) a treatment for mast-cell derived cancers and anaphylaxis (HT-KIT); and (iii) a treatment and/or prevention for Alzheimer’s or other neuroinflammatory diseases (HT-ALZ). We also have assets being developed for (i) atopic dermatitis (also known as eczema) (BioLexa); (ii) a treatment for asthma and allergies using inhalational administration (HT-004); and (iii) a treatment for obesity, and obesity-related diseases and conditions (HT-VA).
Primary Development:
HT-001
On February 1, 2020, we entered into a patent license agreement with The George Washington University (“GW”) pursuant to which GW granted us a license to certain patent rights to, among other things, make, use, offer and sell certain licensed products throughout the world with respect to HT-001 which we intend to seek approval for use for treating dermatological side effects from epidermal growth factor receptor (“EGFR”) inhibitors, and potentially other drugs used for the treatment of cancer. HT-001 is a topical formulation under development for the treatment of patients with rash and skin disorders associated with initial and repeat courses of tyrosine kinase EGFR inhibitor therapy. EGFR inhibitors are used for the treatment of cancers with EGFR up-regulation (such as non-small cell lung cancer, pancreatic cancer, breast cancer and colon cancer); however, EGFR inhibitors are often associated with dose-limiting skin toxicities that can result in the interruption or reduction of treatment. HT-001 is targeted to treat these EGFR-induced skin disorders to allow patients to achieve the best potential outcomes of EGFR therapy. In November 2022, we submitted an Investigational New Drug (“IND”) application to the FDA with respect to HT-001 as a concomitant therapy with EGFR inhibitors, for a Phase 2a clinical trial in humans. We have engaged Worldwide Clinical Trials (“Worldwide”) as our clinical research organization to provide clinical management, data management, biostatistical, medical monitoring, pharmacovigilance, and other related services to support the CLEER-001 Phase 2a clinical trial in the United States. We received FDA approval to proceed with our clinical study on December 28, 2022. HT-001 has achieved positive interim preliminary clinical results from its open label cohort, and we are actively enrolling in both the open label and double-blind randomized cohorts.
We believe that the key elements for our market success with respect to HT-001 include:
| ● | To our knowledge, there are currently no drugs approved for the treatment of skin toxicities associated with EFGR inhibitor therapy and 49-100% of patients develop skin toxicities during EGFR inhibitory therapy; |
| ● | The main active ingredient of HT-001 is already approved in oral and IV dosage forms which supports pursuit of the 505(b)(2) regulatory pathway to reduce development time and cost; |
| ● | To our knowledge, there are no current topical formulations available using HT-001’s active ingredient so we believe that there is no direct market competition; and |
| ● | We have the potential to pursue other indications such as chronic pruritus, atopic dermatitis and other skin toxicities that develop from anti-cancer therapies using the HT-001 formulation. In January 2025, we acquired two provisional patent applications for additional indications that could be treated using the HT-001 formulation. |
1
HT-KIT
We have obtained from North Carolina State University (“NC State”) an exclusive, worldwide, royalty bearing license to certain intellectual property to, among other things, discover, develop, make, have made, use and sell certain licensed products and sell, use and practice certain licensed services with respect to cancer and anaphylaxis; this is being developed as HT-KIT. The HT-KIT drug is designed to more specifically target the receptor tyrosine kinase KIT in mast cells, which is required for the proliferation, survival and differentiation of bone marrow-derived hematopoietic stem cells. Mutations in the KIT pathway have been associated with several human cancers, such as gastrointestinal stromal tumors and mast cell-derived cancers (mast cell leukemia and mast cell sarcoma). Based on the initial proof-of-concept success, we intend to initially target mast cell neoplasms for development of HT-KIT, which is a rare, aggressive cancer with poor prognosis.
The same target, KIT, also plays a key role in mast cell-mediated anaphylaxis, a serious allergic reaction that is rapid in onset and may cause death. Anaphylaxis typically occurs after exposure to an external allergen that results in an immediate and severe immune response. We also intend to pursue the anaphylaxis indication for HT-KIT in parallel to cancer treatment.
On November 15, 2021, we entered into a sponsored research agreement with NC State to focus on characterizing the HT-KIT dose and dosing frequency for treatment of aggressive mastocytosis and mast cell neoplasms using humanized tumor mouse models. These preclinical studies are still ongoing, and the results inform the IND enabling studies also currently underway.
In December 2021, we submitted an Orphan Drug Designation (“ODD”) request to the U.S. Food and Drug Administration (“FDA”) for HT-KIT for the treatment of mastocytosis, and on March 10, 2022, we received such ODD. Drugs intended to treat orphan diseases (rare diseases that affect less than 200,000 people in the U.S.) are eligible to apply for ODD, which provides benefits such as 7-year marketing exclusivity and tax incentives to the sponsor during development and after approval. In September 2023, we submitted a pre-IND meeting request to the FDA with respect to HT-KIT as for the treatment of adult patients with advanced systemic mastocytosis (“AdvSM”), systemic mastocytosis with an associated hematological neoplasm (“SM-AHN”) and mast cell leukemia (“MCL”). In preparation for such pre-IND meeting, we prepared and submitted to the FDA our IND-opening clinical trial plan which includes two phase 1 trials conducted in patients. Based on the FDA’s feedback, we intend to advance our IND-enabling activities for HT-KIT as planned. We are currently conducting the analytical and animal toxicology studies required for our IND submission.
HT-ALZ
In November 2024, we were granted a patent by the United States Patent and Trademark Office for the use of the active ingredient of HT-001 to treat and prevent Alzheimer’s disease and other neuroinflammatory diseases.
We intend to develop HT-ALZ for use in patients following the Section 505(b)(2) regulatory pathway of the FDA rules. Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act (“FDCA”) was enacted to enable sponsors to seek New Drug Application (“NDA”) approval for novel repurposed drugs without the need for such sponsors to undertake time consuming and expensive pre-clinical safety studies and Phase 1 safety studies. Proceeding under this regulatory pathway, we will be able to rely upon publicly available data with respect to our active ingredient in our NDA submission to the FDA for marketing approval.
On June 7, 2021, we entered into a sponsored research agreement with Washington University in St. Louis to investigate the effects of HT-ALZ on behavioral and pathological markers of Alzheimer’s disease and to determine if HT-ALZ can improve learning and memory in an animal model of Alzheimer’s disease. Our study will also determine if behavior is improved utilizing HT-ALZ in blocking NK-1Rs. The study commenced in August 2021 and after positive initial preclinical results, a chronic dosing study in mice was initiated. We received further preclinical results from the chronic dosing study in 2023 and amended the SRA to conduct additional studies which concluded in 2024. In 2024, we initiated formulation development to develop prototypes of HT-ALZ. We plan to continue our work on optimizing our formulation as well as conduct additional animal studies to confirm the mechanism of action prior to submitting a pre-IND submission to FDA.
2
The BioLexa Platform
We have obtained an exclusive license from the University of Cincinnati to make, use, have made, import, offer for sale, and sell products based upon or involving the use of (i) topical compositions comprising a zinc chelator and gentamicin and (ii) zinc chelators to inhibit biofilm formation (the “BioLexa Platform” or “BioLexa”). The license enables us to develop the platform for any indications in humans. The BioLexa Platform is a proprietary, patented, drug compound platform for the treatment of eczema. It combines an FDA approved zinc chelator with one or more approved antibiotics in a topical dosage form to address unchecked eczema flare-ups by preventing the formation of infectious biofilms and the resulting clogging of sweat ducts. We intend to develop the BioLexa Platform for use in patients following the Section 505(b)(2) regulatory pathway of the FDA rules. Proceeding under this regulatory pathway, we will be able to rely upon publicly available data with respect to gentamicin and the zinc chelator in our NDA submission to the FDA for marketing approval.
In December 2020, we received approval from the Belberry Human Research Ethics Committee in Australia to conduct our Phase 1b clinical trial of BioLexa, and we have engaged Novotech (Australia) Pty Limited as our local clinical research organization in Australia to provide clinical management, data management, biostatistical, medical monitoring, pharmacovigilance, and other related services to support the first in human clinical trial of BioLexa. Phase 1b of the trial was initiated in 2021 and final dosing of patients concluded in September 2022. At this time, we do not anticipate conducting any further trials in Australia.
We believe that the key elements for our market success with respect to BioLexa include:
| ● | the proprietary formulation of two FDA-approved drugs to treat bacterial proliferation which may reduce development time and costs by giving us the ability to rely on safety and efficacy data from the two approved drugs; |
| ● | our proprietary formulation is not a topical corticosteroid, and provides a novel mechanism of action and potentially a preferred safety profile as a market differentiator; and |
| ● | the literature set forth below reaffirms the critical role that S. aureus plays in the development of atopic dermatitis flare-ups within the international medical community, supporting the targeted mechanism of action of BioLexa. |
Shi et al, “MRSA Colonization is Associated with Decreased Skin Commensal Bacteria in Atopic Dermatitis,” Invest Dermatol. 2018.
Blicharz, et al, “Staphylococcus aureus: an underestimated factor in the pathogenesis of atopic dermatitis?,” Adv Dermatol Allergol 2019.
Preclinical Development
HT-004
On November 20, 2019, we entered into a license agreement with NC State pursuant to which NC State granted us an exclusive license to, among other things, develop, make, use, offer and sell certain licensed products throughout the world with respect to HT-004 for treating allergic diseases. HT-004 is a potential disease-modifying agent that uses exon-skipping oligonucleotide-targeted methods to reduce mast cell responses to immunoglobulin E (IgE)-directed antigens, which is one of the key mechanisms in the pathophysiology of asthma, atopic dermatitis and other allergic diseases. HT-004 is currently under investigation for the treatment of asthma and allergies using inhalational administration.
In December 2019, we entered a sponsored research agreement with NC State for proof of principle in targeting allergic inflammation in the airways. Preclinical proof-of-concept data was generated in October 2020 supporting the efficacy of HT-004 after inhalational delivery in a mouse model. Critical proof-of-concept studies in a humanized mouse model were completed in 2023. Further preclinical studies are underway at NC State to study HT-004 in different animal models.
3
We believe that the key elements for our market success with respect to HT-004 include:
| ● | To our knowledge, there are currently no disease-modifying agents for asthma or allergy diseases; |
| ● | The active pharmaceutical ingredient in HT-004 is a novel molecular class that we believe would prevent generic competition after commercialization; |
| ● | HT-004 is being developed for inhalational administration by either inhaler or nebulizer for easy access at home by patients; and |
| ● | HT-004 is applicable for both adult and pediatric patient populations with asthma and/or allergies. |
HT-VA
On December 9, 2024, we entered into a license agreement with the Department of Veterans Affairs (the “VA”) pursuant to which the VA granted us an exclusive license to, among other things, develop, make, use, offer and sell certain licensed products throughout the world with respect to HT-VA for treating obesity and obesity conditions. HT-VA is a glial cell line derived neurotrophic factor (“GDNF”) being developed for the treatment of obesity and obesity-related diseases and conditions.
A GDNF family receptor alpha-1 (GFRα-1) agonist, important for the differentiation and survival of neurons, GDNF signals through its receptors, including Ret and GFR-1α, by activation of the PI3K and MAPK pathways. HT-VA is being formulated to treat or prevent obesity, metabolic syndrome, or insulin resistance by administering an effective amount of a pharmaceutical composition comprising of one or more GDNF receptor agonists.
Product Development Pipeline
The following table summarizes our product development pipeline.
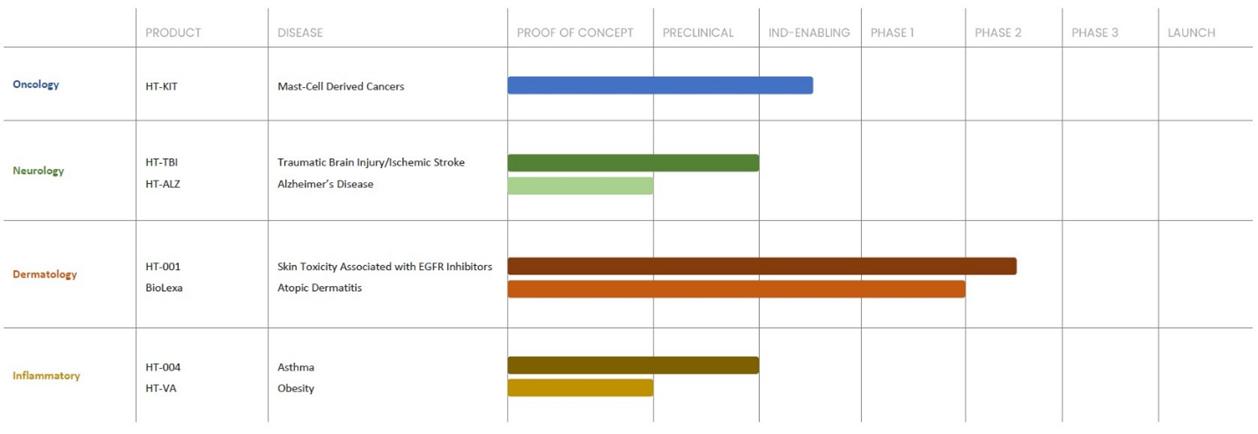
Competition
The biopharmaceutical industry utilizes rapidly advancing technologies and is characterized by intense competition. There is also a strong emphasis on intellectual property and proprietary products. In our segment of the biopharmaceutical industry, competition from different sources including major biopharmaceutical companies, academic institutions, government agencies, and public and private research institutions will continue. Many of our competitors have significantly greater financial resources and expertise in product candidate development and may have progressed further toward approval and marketing. In addition, smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
Manufacturing and Supply
We do not have any manufacturing capability and therefore we currently rely on and intend to continue to rely on contract manufacturing organizations to produce our product candidates in accordance with regulatory requirements.
4
Commercialization
Our success depends not only on the successful development and approval of our products candidates but also on the commercialization of our potential products. If and when our product candidates receive regulatory approval, we intend to engage third parties such as pharmaceutical and biotechnology companies for the commercialization of our products.
Intellectual Property Portfolio
Our goal is to obtain, maintain and enforce patent protection for our products, formulations, processes, methods and other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the U.S. and in other countries. Our policy is to actively seek the broadest intellectual property protection possible for our products, proprietary information and proprietary technology through a combination of contractual arrangements and patents, both in the U.S. and elsewhere in the world. In addition, we intend to actively pursue product life-cycle management initiatives to extend our market exclusivity.
We intend to cement our market exclusivity in conjunction with our formulation-development partners through additional patents based on the pharmaceutical and clinical characteristics of our product candidates in the proprietary formulation and through the introduction of line extensions such as combination drugs and new formulations.
In addition to any granted patents, our products may be eligible for market exclusivity to run concurrently with the term of the patent for three and a half years in the U.S. pursuant to the Hatch-Waxman Act and pediatric exclusivity guideline and up to ten years of market exclusivity in the E.U. which includes eight years of data exclusivity and two years of market exclusivity from the date we file an NDA or the European equivalent referred to as Marketing Authorization Application.
We currently have licenses to seven U.S. patents and one pending U.S. patent application, and we have licenses to three patents issued in Europe and Australia and five pending patent applications in foreign jurisdictions including Europe, Brazil, Canada and Hong Kong. We also hold one U.S. patent, eight pending U.S. patent applications (including six U.S. provisional patent applications), one European application and one pending PCT patent application.
In addition to patents, we rely on trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. However, trade secrets and know-how can be difficult to protect. We take measures to protect and maintain the confidentiality of proprietary information in order to protect aspects of the business that are not amenable to, or that we do not consider appropriate for, patent protection. We require employees, consultants, outside scientific partners, sponsored researchers and other advisors to execute confidentiality agreements with us on or prior to the commencement of employment or consulting relationships with us.
Government Regulations
Governmental authorities in the U.S. and other countries extensively regulate the research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing of pharmaceutical products, including biological products, and medical devices, such as those being developed by us. In the U.S., the FDA regulates such products under the FDCA and the Public Health Services Act and implements related regulations. Failure to comply with applicable FDA requirements, both before and after approval, may subject us to administrative and judicial sanctions, such as a delay in approving or refusal by the FDA to approve pending applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution.
U.S. Food and Drug Administration Regulations
United States Drug Development
In the United States, the FDA regulates drugs (including biological products, such as vaccines), medical devices and combinations of drugs and devices, or combination products, under the FDCA and its implementing regulations. These products are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, requests for voluntary product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement, or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
5
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| ● | completion of extensive pre-clinical laboratory tests, animal studies and formulation studies in accordance with applicable regulations, including the FDA’s Good Laboratory Practice regulations; |
| ● | submission to the FDA of an IND, which must become effective before human clinical trials may begin; |
| ● | performance of adequate and well-controlled human clinical trials in accordance with an applicable IND and other clinical study related regulations, referred to as good clinical practice (“GCP”), to establish the safety and efficacy of the proposed drug for its proposed indication; |
| ● | submission to the FDA of an NDA or biologics license application (“BLA”); |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with the FDA’s current good manufacturing practice (“cGMP”) requirements; |
| ● | potential FDA audit of the clinical trial sites that generated the data in support of the NDA or BLA; and |
| ● | FDA review and approval of the NDA or BLA prior to any commercial marketing or sale. |
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| ● | Phase 1. The product is initially introduced into a small number of healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, especially when the product is suspected or known to be unavoidably toxic, the initial human testing may be conducted in patients. |
| ● | Phase 2. Involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule. |
| ● | Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit relationship of the product and provide an adequate basis for product labeling. |
Post-approval trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 trials. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the clinical trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an Institutional Review Board (“IRB”), which oversees the conduct of clinical trials, can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product has been associated with unexpected serious harm to patients. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether a trial may move forward at designated check points based on access to certain data from the study. The clinical trial sponsor may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
FDA Review Process
The results of product development, pre-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the drug, proposed labeling and other relevant information, are submitted to the FDA as part of an NDA for a new drug, or BLA for a biological product, requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of a substantial user fee, and the sponsor of an approved NDA or BLA is also subject to an annual program user fee; although a waiver of such fee may be obtained under certain limited circumstances.
6
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information rather than accepting an NDA for filing. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act (“PDUFA”), the FDA’s goal to complete its substantive review of a standard NDA and respond to the applicant is ten months from the receipt of the NDA. The FDA does not always meet its PDUFA goal dates, and the review process is often significantly extended by FDA requests for additional information or clarification and may go through multiple review cycles.
The review and evaluation of an NDA or BLA by the FDA is extensive and time consuming and may take longer than originally planned to complete, and we may not receive a timely approval, if at all.
Before approving an NDA, the FDA will conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether they comply with cGMPs. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to ensure consistent production of the product within required specifications. In addition, before approving an NDA, the FDA may also audit data from clinical trials to ensure compliance with GCP requirements.
There is no assurance that the FDA will ultimately approve a product for marketing in the United States, and we may encounter significant difficulties or costs during the review process. If a product receives marketing approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling or may condition the approval of the NDA or BLA on other changes to the proposed labeling, development of adequate controls and specifications, or a commitment to conduct post-market testing or clinical trials and surveillance to monitor the effects of approved products. For example, the FDA may require Phase 4 clinical trials to further assess drug safety and effectiveness and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. The FDA may also place other conditions on approvals, including the requirement for a risk evaluation and mitigation strategy (“REMS”), to assure the safe use of the drug.
Section 505(b)(2) Regulatory Approval Pathway
Section 505(b)(2) of the FDCA provides an alternate regulatory pathway for approval of a new drug by allowing the FDA to rely on data not developed by the applicant. Specifically, Section 505(b)(2) permits the submission of an NDA where one or more of the investigations relied upon by the applicant for approval were not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon published literature and/or the FDA’s findings of safety and effectiveness for an approved drug already on the market. Approval or submission of a 505(b)(2) application, like those for abbreviated new drugs (“ANDAs”), may be delayed because of patent and/or exclusivity rights that apply to the previously approved drug.
A 505(b)(2) application may be submitted for a new chemical entity (“NCE”) when some part of the data necessary for approval is derived from studies not conducted by or for the applicant and when the applicant has not obtained a right of reference.
Section 505(b)(2) applications also may be entitled to marketing exclusivity if supported by appropriate data and information. Three-year new data exclusivity may be granted to the 505(b)(2) application if one or more clinical investigations conducted in support of the application, other than bioavailability/bioequivalence studies, were essential to the approval and conducted or sponsored by the applicant. Five years of marketing exclusivity may be granted if the application is for an NCE, and pediatric exclusivity is likewise available.
Orange Book Listing and Paragraph IV Certification
For NDA submissions, including those under Section 505(b)(2), applicants are required to list with the FDA certain patents with claims that cover the applicant’s product. Upon approval, each of the patents listed in the application is published in Approved Drug Products with Therapeutic Equivalence Evaluations, commonly referred to as the Orange Book. Any applicant who subsequently files an ANDA or 505(b)(2) NDA that references a drug listed in the Orange Book must certify to the FDA that (1) no patent information on the drug product that is the subject of the application has been submitted to the FDA; (2) such patent has expired; (3) the date on which such patent expires; or (4) such patent is invalid or will not be infringed upon by the manufacture, use or sale of the drug product for which the application is submitted. This last certification is known as a Paragraph IV Certification.
7
If an applicant has provided a Paragraph IV Certification to the FDA, the applicant must also send notice of the Paragraph IV Certification to the holder of the NDA for the approved drug and the patent owner once the application has been accepted for filing by the FDA. The NDA holder or patent owner may then initiate a patent infringement lawsuit in response to notice of the Paragraph IV Certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV Certification prevents the FDA from approving the ANDA or 505(b)(2) application until the earlier of 30 months from the date of the lawsuit, the applicant’s successful defense of the suit, or expiration of the patent.
Reimbursement
Potential sales of any of our product candidates, if approved, will depend, at least in part, on the extent to which such products will be covered by third-party payors, such as government health care programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly limiting coverage and/or reducing reimbursements for medical products and services. A third-party payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a drug product does not ensure that other payors will also provide coverage for the drug product. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our future revenues and results of operations. Decreases in third-party reimbursement or a decision by a third-party payor to not cover a product candidate, if approved, or any future approved products could reduce physician usage of our products, and have a material adverse effect on our sales, results of operations and financial condition.
In the United States, the Medicare Part D program provides a voluntary outpatient drug benefit to Medicare beneficiaries for certain products. We do not know whether our product candidates, if approved, will be eligible for coverage under Medicare Part D, but individual Medicare Part D plans offer coverage subject to various factors such as those described above. Furthermore, private payors often follow Medicare coverage policies and payment limitations in setting their own coverage policies. These policies, including government-funded health care as a whole, face uncertainty under the new Trump Administration.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States for which there is no reasonable expectation that the cost of developing and making available in the United States a drug or biologic for this type of disease or condition will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review or approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusive approval (or exclusivity), which means that the FDA may not approve any other applications, including a full NDA or BLA, to market the same drug for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the application user fee.
8
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Orphan drugs face additional risk due to the Drug Price Negotiation Program as part of the Inflation Reduction Act (“IRA”). The IRA currently excludes from the Drug Price Negotiation Program orphan drugs that treat one rare disease, which disincentivizes companies to test whether existing treatments for rare diseases also benefit other rare diseases. To expand and clarify the exclusion for orphan drugs under the Drug Price Negotiation Program, the ORPHAN Cures Act was introduced in 2023, which, if passed, would amend the IRA to exclude orphan drugs treating one or more rare diseases from pricing negotiations. This would incentivize critical follow-on investment into rare disease drug development. The ORPHAN Cures Act was recently re-introduced, has bi-partisan support, and could be passed in the near future.
Healthcare Laws and Regulations
Sales of our product candidates, if approved, or any other future product candidate will be subject to healthcare regulation and enforcement by the federal government and the states and foreign governments in which we might conduct our business. The healthcare laws and regulations that may affect our ability to operate include the following:
| ● | The federal Anti-Kickback Statute makes it illegal for any person or entity to knowingly and willfully, directly or indirectly, solicit, receive, offer, or pay any remuneration that is in exchange for or to induce the referral of business, including the purchase, order, lease of any good, facility, item or service for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value. |
| ● | Federal false claims and false statement laws, including the federal civil False Claims Act, prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, for payment to, or approval by, federal programs, including Medicare and Medicaid, claims for items or services, including drugs, that are false or fraudulent. | |
| ● | Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) created additional federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors or making any false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 and their implementing regulations, impose obligations on certain types of individuals and entities regarding the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information. | |
| ● | The federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services information related to payments or other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. |
Also, many states have similar laws and regulations, such as anti-kickback and false claims laws that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. Additionally, we may be subject to state laws that require pharmaceutical companies to comply with the federal government’s and/or pharmaceutical industry’s voluntary compliance guidelines, state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, as well as state and foreign laws governing the privacy and security of health information, many of which differ from each other in significant ways and often are not preempted by HIPAA.
Additionally, to the extent that our product is sold in a foreign country, we may be subject to similar foreign laws.
Employees
As of March 28, 2025, we employed a total of 2 full-time employees, 3 employee consultants, and 1 part-time employee. We are not a party to any collective bargaining agreements. We believe that we maintain good relations with our employees.
9
Our Corporate Information and History
We were incorporated as a Nevada corporation on May 16, 2017. On June 5, 2019, we formed our wholly owned subsidiary, Hoth Therapeutics Australia Pty Ltd, under the laws of the State of Victoria in Australia and, on October 4, 2023, we formed our wholly owned subsidiary, merveille.ai, under the laws of the State of Nevada. Our principal executive offices are located at 1177 Avenue of the Americas, 5th Floor, Suite 5066, New York, New York 10036 and our telephone number is (646) 756-2997.
Available Information
Our website address is www.hoththerapeutics.com. The contents of, or information accessible through, our website are not part of this Annual Report, and our website address is included in this document as an inactive textual reference only. We make our filings with the U.S. Securities and Exchange Commission (“SEC”), including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports, available free of charge on our website as soon as reasonably practicable after we file such reports with, or furnish such reports to, the SEC. The public may read and copy the materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Additionally, the SEC maintains an internet site that contains reports, proxy and information statements and other information. The address of the SEC’s website is www.sec.gov. The information contained in the SEC’s website is not intended to be a part of this Annual Report.
ITEM 1A. RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the following risk factors and the other information in this Annual Report before investing in our common stock. Our business and results of operations could be seriously harmed by any of the following risks. The risks set out below are not the only risks we face. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition and/or operating results. If any of the following events occur, our business, financial condition and results of operations could be materially adversely affected. In such case, the value and trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Financial Position, Financial Reporting Matters and Need for Capital
We have generated no revenue from commercial sales to date and our future profitability is uncertain.
We were incorporated in May 2017 and have a limited operating history and our business is subject to all of the risks inherent in the establishment of a new business enterprise. Our likelihood of success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with the development and expansion of a new business enterprise. Since inception, we have incurred losses and expect to continue to operate at a net loss for at least the next several years as we continue our research and development efforts, conduct clinical trials and develop manufacturing, sales, marketing and distribution capabilities. Our net losses for the years ended December 31, 2024 and 2023 were $8.2 million and $8.1 million, respectively, and our accumulated deficit as of December 31, 2024 and 2023 was $60.4 million and $52.2 million, respectively. There can be no assurance that the products under development by us will be approved for sale in the U.S. or elsewhere. Furthermore, there can be no assurance that if such products are approved they will be successfully commercialized, and the extent of our future losses and the timing of our profitability are highly uncertain. If we are unable to achieve profitability, we may be unable to continue our operations.
If we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development and you will likely lose your entire investment.
We will need to continue to seek capital from time to time to continue development of our product candidates. We cannot provide any assurances that any revenues that we may generate in the future will be sufficient to fund our ongoing operations. We believe that we will need to raise substantial additional capital to fund our operations and the development and commercialization of our product candidates.
10
Our business or operations may change in a manner that may consume available funds more rapidly than anticipated and substantial additional funding may be required to maintain operations, fund expansion, commercialize our product candidates, develop new or enhanced products, acquire complementary products, business or technologies or otherwise respond to competitive pressures and opportunities, such as a change in the regulatory environment or a change in preferred treatment modalities. In addition, we may need to accelerate the growth of our sales capabilities and distribution beyond what is currently envisioned, and this would require additional capital. However, we may not be able to secure funding on favorable terms, if at all.
If we cannot raise adequate funds to satisfy our capital requirements, we may have to delay, scale back or eliminate our research and development activities, clinical studies or operations. We may also be required to obtain funds through arrangements with collaborators, which arrangements may require us to relinquish rights to certain intellectual property, technologies or products that we otherwise would not consider relinquishing, including rights to future product candidates or certain major geographic markets. This could result in sharing revenues which we might otherwise retain for ourselves. Any of these actions may harm our business, financial condition and results of operations.
The amount of capital we may need depends on many factors, including the progress, timing and scope of our product development programs; the progress, timing and scope of our pre-clinical studies and clinical trials; the time and cost necessary to obtain regulatory approvals; the time and cost necessary to further develop manufacturing processes and arrange for contract manufacturing; our ability to enter into and maintain collaborative, licensing and other commercial relationships; and our partners’ commitment of time and resources to the development and commercialization of our products.
Even if we can raise additional funding, we may be required to do so on terms that are dilutive to you.
The capital markets have been unpredictable in the recent past for unprofitable companies such as ours. The amount of capital that a company such as ours is able to raise often depends on variables that are beyond our control. As a result, we may not be able to secure financing on terms attractive to us, or at all. If we are able to consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition and our continued viability will be materially adversely affected.
The Restatement of our financial statements may affect shareholder and investor confidence in us or harm our reputation, and may subject us to additional risks and uncertainties, including increased costs and the increased possibility of legal proceedings and regulatory inquiries, sanctions or investigations.
We have incurred, and may continue to incur, substantial unanticipated costs for accounting and legal fees in connection with, or related to, the Restatement. The Restatement could also subject us to other risks and uncertainties, including the increased possibility of legal proceedings and inquiries, sanctions, or investigations by the SEC or other regulatory authorities relating to the Restatement. Any of the foregoing may adversely affect our reputation, the accuracy and timing of our financial reporting, or our business, results of operations, liquidity, and financial condition, or cause shareholders and investors to lose confidence in the accuracy and completeness of our financial reports or cause the market price of our common stock to decline. Any such legal proceedings or regulatory inquiries, sanctions, or investigation, whether successful or not, could adversely affect our business, financial condition, and results of operations.
Risks Related to Product Development, Regulatory Approval, Manufacturing and Commercialization
We are dependent upon the clinical success of our licensed products and technologies. If we are unable to generate revenues from our licensed products and technologies, our ability to create shareholder value may be limited.
We do not currently generate revenues from any of our product candidates, and we may not be successful in obtaining regulatory approvals to commence our clinical trials. If we do not obtain such approvals, the time in which we expect to commence clinical programs for our product candidates will be extended and such extension may increase our expenses and our need for additional capital. Moreover, there is no guarantee that our clinical trials will be successful or that we will continue clinical development in support of an approval from the regulatory agencies for any indication. We note that most drug candidates never reach the clinical stage and even those that do commence clinical development have only a small chance of successfully completing clinical development and gaining regulatory approval. Therefore, our business currently depends entirely on the successful development, regulatory approval and commercialization of our product candidates, which may never occur.
11
The marketing approval process of the FDA is lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain marketing approval for the product candidates we intend to develop, our business may be substantially harmed.
None of the product candidates we intend to develop have gained marketing authorization, approval or clearance in the U.S. or elsewhere, and we cannot guarantee that we will ever have marketable products. Our business is substantially dependent on our ability to complete the development of, obtain marketing approval for, and successfully commercialize our product candidates in a timely manner. We cannot commercialize our product candidates in the United States or elsewhere without first obtaining approval from regulatory agencies such as the FDA to market each product candidate. Our product candidates could fail to receive marketing approval for many reasons, including among others:
| ● | the FDA or other regulatory agencies may disagree with the design or implementation of our clinical trials; |
| ● | the FDA could determine that we cannot rely on Section 505(b)(2) for any of our product candidates; and |
| ● | the FDA may determine that we have identified the wrong reference listed drug or drugs or that approval of our Section 505(b)(2) application for any of our product candidates is blocked by patent or non-patent exclusivity of the reference listed drug or drugs. |
In addition, the process of seeking regulatory clearance or approval to market the product candidates we intend to develop is expensive and time consuming and, notwithstanding the effort and expense incurred, clearance or approval is never guaranteed. If we are not successful in obtaining timely clearance or approval of our product candidates from the FDA or other foreign regulatory agencies, we may never be able to generate significant revenue and may be forced to cease operations. The NDA process is costly, lengthy and uncertain. Any NDA application filed by us will have to be supported by extensive data, including, but not limited to, technical, pre-clinical, clinical, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the product for its intended use.
Obtaining clearances or approvals from the FDA and from regulatory agencies in other countries is an expensive and time-consuming process and is uncertain as to outcome. The FDA and other agencies could ask us to supplement our submissions, collect non-clinical data, conduct additional clinical trials or engage in other time-consuming actions, or it could simply deny our applications. In addition, even if we obtain an NDA approval or pre-market approvals in other countries, the approval could be revoked or other restrictions imposed if post-market data demonstrates safety issues or lack of effectiveness. We cannot predict with certainty how, or when, the FDA or other regulatory agencies will act. If we are unable to obtain the necessary regulatory approvals, our financial condition and cash flow may be adversely affected, and our ability to grow domestically and internationally may be limited. Additionally, even if cleared or approved, our products may not be approved for the specific indications that are most necessary or desirable for successful commercialization or profitability.
We may encounter substantial delays in completing our clinical studies which in turn will require additional costs, or we may fail to demonstrate adequate safety and efficacy to the satisfaction of applicable regulatory authorities.
It is impossible to predict if or when any of our product candidates will prove safe or effective in humans or will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical studies to demonstrate the safety and efficacy of the product candidates in humans. Clinical testing is expensive, time-consuming and uncertain as to the outcome. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
| ● | delays in reaching, or failing to reach, a consensus with regulatory agencies on study design; |
| ● | delays in reaching, or failing to reach, agreement on acceptable terms with a sufficient number of prospective contract research organizations (“CROs”) and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | delays in obtaining required IRB or Ethics Committee (“EC”) approval at each clinical study site; | |
| ● | delays in recruiting a sufficient number of suitable patients to participate in our clinical studies; | |
| ● | imposition of a clinical hold by regulatory agencies, after an inspection of our clinical study operations or study sites; |
12
| ● | failure by our CROs, other third parties or us to adhere to clinical study, regulatory or legal requirements; |
| ● | failure to perform in accordance with the FDA’s GCP or applicable regulatory guidelines in other countries; |
| ● | delays in the testing, validation, manufacturing and delivery of sufficient quantities of our product candidates to the clinical sites; | |
| ● | delays in having patients complete participation in a study or return for post-treatment follow-up; | |
| ● | clinical study sites or patients dropping out of a study; | |
| ● | delay or failure to address any patient safety concerns that arise during the course of a trial; | |
| ● | unanticipated costs or increases in costs of clinical trials of our product candidates; | |
| ● | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; or | |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
We could also encounter delays if a clinical trial is suspended or terminated by us, by the IRBs or ECs of the institutions in which such trials are being conducted, by an independent Safety Review Board for such trial or by the FDA, Therapeutics Goods Administration (“TGA”), European Medicines Agency (“EMA”), or other regulatory authorities. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA, TGA, or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Any inability to successfully complete pre-clinical and clinical development could result in additional costs to us or impair our ability to generate revenues from product sales, regulatory and commercialization milestones and royalties. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates to earlier versions.
Clinical study delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
The outcome of pre-clinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Further, pre-clinical and clinical data are often susceptible to various interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in pre-clinical studies and clinical trials have nonetheless failed to obtain marketing approval. If the results of our clinical studies are inconclusive or if there are safety concerns or adverse events associated with our other product candidates, we may:
| ● | be delayed in obtaining marketing approval for our product candidates, if approved at all; |
| ● | obtain approval for indications or patient populations that are not as broad as intended or desired; | |
| ● | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
13
| ● | be required to change the way the product is administered; |
| ● | be required to perform additional clinical studies to support approval or be subject to additional post-marketing testing requirements; | |
| ● | have regulatory authorities withdraw their approval of a product or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy; | |
| ● | be sued; or | |
| ● | experience damage to our reputation. |
Additionally, our product candidates could potentially cause other adverse events that have not yet been predicted. The inclusion of ill patients in our clinical studies may result in deaths or other adverse medical events due to other therapies or medications that such patients may be using. As described above, any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our products.
If we are not able to obtain any required regulatory approvals for our product candidates, we will not be able to commercialize our product candidates and our ability to generate revenue will be limited.
We must successfully complete clinical trials for our product candidates before we can apply for marketing approval. Even if we complete our clinical trials, it does not assure marketing approval. Our pre-clinical trials may be unsuccessful, which would materially harm our business. Even if our initial pre-clinical trials are successful, we are required to conduct clinical trials to establish our product candidates’ safety and efficacy, before a marketing application (NDA or BLA or their foreign equivalents) can be filed with the FDA, the EMA, or comparable foreign regulatory authorities for marketing approval of our product candidates.
Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. Success in early phases of pre-clinical and clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. The research, testing, manufacturing, labeling, packaging, storage, approval, sale, marketing, advertising and promotion, pricing, export, import and distribution of drug products are subject to extensive regulation by the FDA, EMA, and other regulatory authorities in the United States, European Union, and other countries, where regulations differ from country to country. We are not permitted to market our product candidates as prescription pharmaceutical products in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. In the United States, the FDA generally requires the completion of clinical trials of each drug to establish its safety and efficacy and extensive pharmaceutical development to ensure its quality before an NDA is approved. Regulatory authorities in other jurisdictions impose similar requirements. Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA or other regulatory authorities and even fewer are eventually approved for commercialization. We have not submitted an NDA to the FDA or comparable applications to other regulatory authorities. If our development efforts for our product candidates, including regulatory approval, are not successful for their planned indications, or if adequate demand for our product candidates is not generated, our business will be materially adversely affected.
Our success depends on the receipt of regulatory approval and the issuance of such regulatory approvals is uncertain and subject to a number of risks, including the following:
| ● | the results of nonclinical or toxicology studies may not support the filing of an IND or foreign equivalent for our product candidates; | |
| ● | the FDA, EMA, or comparable foreign regulatory authorities or IRBs or ECs may disagree with the design or implementation of our clinical trials; |
14
| ● | we may not be able to provide acceptable evidence of our product candidates’ safety and efficacy; |
| ● | the results of our clinical trials may not be satisfactory or may not meet the level of statistical or clinical significance required by the FDA, EMA, or other regulatory agencies for marketing approval; | |
| ● | the dosing of our product candidates in a particular clinical trial may not be at an optimal level; | |
| ● | patients in our clinical trials may suffer adverse effects for reasons that may or may not be related to our product candidates; | |
| ● | the data collected from clinical trials may not be sufficient to support the submission of an NDA, BLA or other marketing application or to obtain regulatory approval in the United States or elsewhere; | |
| ● | the requirement for additional studies; | |
| ● | the FDA, EMA, or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; | |
| ● | the approval policies or regulations of the FDA, EMA, or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval; | |
| ● | the FDA, EMA, or comparable foreign regulatory authorities may disagree on the design or implementation of our clinical trials, including the methodology used in our studies, our chosen endpoints, our statistical analysis, or our proposed product indication; | |
| ● | our failure to demonstrate to the satisfaction of the FDA, EMA, or comparable regulatory authorities that a product candidate is safe and effective for its proposed indication; | |
| ● | we may fail to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| ● | immunogenicity might affect a product candidate’s efficacy and/or safety; | |
| ● | the FDA, EMA, or comparable foreign regulatory authorities may disagree with our interpretation of data from nonclinical studies or clinical trials; |
| ● | data collected from clinical trials of our product candidates may be insufficient to support the submission and filing of a marketing application or to obtain marketing approval. For example, the FDA may require additional studies to show that our product candidates are safe or effective; | |
| ● | we may fail to obtain approval of the manufacturing processes or facilities of third-party manufacturers with whom we contract for clinical and commercial supplies; | |
| ● | there may be changes in the approval policies or regulations that render our nonclinical and clinical data insufficient for approval; or | |
| ● | the FDA, EMA or comparable foreign regulatory authority may require more information, including additional nonclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program. |
Failure to obtain regulatory approval for our product candidates for the foregoing, or any other reasons, will prevent us from commercializing our product candidates, and our ability to generate revenue will be materially impaired. We cannot guarantee that regulators will agree with our assessment of the results of the clinical trials we intend to conduct in the future or that such trials will be successful. The FDA, EMA and other regulators have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional clinical trials, or pre-clinical or other studies. In addition, varying interpretations of the data obtained from pre-clinical and clinical testing could delay, limit or prevent regulatory approval of our product candidates.
15
We have only limited experience in filing the applications necessary to gain regulatory approvals and expect to rely on consultants and third-party CROs with expertise in this area to assist us in this process. Securing regulatory approvals to market a product requires the submission of pre-clinical, clinical, and/or pharmacokinetic data, information about product manufacturing processes and inspection of facilities, proposed product labeling and supporting information to the appropriate regulatory authorities for each therapeutic indication to establish a product candidate’s safety and efficacy for each indication. Our product candidates may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining regulatory approval or prevent or limit commercial use with respect to one or all intended indications.
The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon, among other things, the type, complexity and novelty of the product candidates involved, the jurisdiction in which regulatory approval is sought and the substantial discretion of the regulatory authorities. Regulatory approval through the FDA specifically may be further impacted or delayed by the ongoing cuts to the federal budget under the Trump Administration. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for a submitted product application may cause delays in the approval or rejection of an application. Regulatory approval obtained in one jurisdiction does not necessarily mean that a product candidate will receive regulatory approval in all jurisdictions in which we may seek approval, but the failure to obtain approval in one jurisdiction may negatively impact our ability to seek approval in a different jurisdiction. Failure to obtain regulatory marketing approval for our product candidates in any indication will prevent us from commercializing our product candidates, and our ability to generate revenue will be materially impaired.
If we are unable to submit an application for product candidate approval under Section 505(b)(2) of the FDCA or if we are required to generate additional data related to the safety and efficacy of a product candidate in order to obtain approval under Section 505(b)(2), we may be unable to meet our anticipated development and commercialization timelines.
We may seek marketing authorization in the United States under Section 505(b)(2) of the FDCA which permits use of a marketing application, referred to as a 505(b)(2) application, where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use. The FDA interprets this to mean that an applicant may rely for approval on such data as that found in published literature or the FDA’s finding of safety or effectiveness, or both, of a previously approved drug product owned by a third-party. There is no assurance that the FDA would find third-party data relied upon by us in a 505(b)(2) application sufficient or adequate to support approval and may require us to generate additional data to support the safety and efficacy of a product candidate. Consequently, we may need to conduct substantial new research and development activities beyond those we currently plan to conduct. Such additional new research and development activities would be costly and time-consuming and there is no assurance that such data generated from such additional activities would be sufficient to obtain approval.
If the data to be relied upon in a 505(b)(2) application is related to drug products previously approved by the FDA and covered by patents that are listed in the FDA’s Orange Book, we would be required to submit with our 505(b)(2) application a Paragraph IV Certification in which we must certify that we do not infringe the listed patents or that such patents are invalid or unenforceable, and provide notice to the patent owner or the holder of the approved NDA. The patent owner or NDA holder would have 45 days from receipt of the notification of our Paragraph IV Certification to initiate a patent infringement action against us. If an infringement action is initiated, the approval of our NDA would be subject to a stay of up to 30 months or more while we defend against such a suit. Approval of our product candidates under Section 505(b)(2) may therefore be delayed until patent exclusivity expires or until we successfully challenge the applicability of those patents to our product candidates. Alternatively, we may elect to generate sufficient clinical data so that we would no longer need to rely on third-party data, which would be costly and time consuming and there would be no assurance that such data generated from such additional activities would be sufficient to obtain approval.
We may not be able to obtain shortened review of our applications, and the FDA may not agree that a product candidate qualifies for marketing approval. If we are required to generate additional data to support approval, we may be unable to meet anticipated or reasonable development and commercialization timelines, may be unable to generate the additional data at a reasonable cost, or at all, and may be unable to obtain marketing approval. If the FDA changes its interpretation of Section 505(b)(2) allowing reliance on data in a previously approved drug application owned by a third-party, or there is a change in the law affecting Section 505(b)(2), this could delay or even prevent the FDA from approving any Section 505(b)(2) application that we submit.
16
We may not be able to obtain or maintain ODD or exclusivity for our product candidates.
Regulatory authorities in some jurisdictions, including the United States, may designate drugs for relatively small patient populations as “orphan drugs.” Under the Orphan Drug Act, the FDA may designate a drug candidate as an orphan drug if it is intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States, or if the disease or condition affects more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making a drug product available in the United States for the type of disease or condition will be recovered from sales of the product.
ODD entitles a party to financial incentives, such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. Additionally, if a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity. This means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in certain circumstances, including proving clinical superiority (i.e., another product is safer, more effective or makes a major contribution to patient care) to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity, or obtain approval for the same product but for a different indication than that for which the orphan product has exclusivity. In addition, exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan-designated indication or may be lost if the FDA later determines that the request for designation was materially defective.
Modifications to our products may require new drug approvals.
Once a particular product receives FDA approval or clearance, expanded uses or uses in new indications of our products may require additional human clinical trials and new regulatory approvals or clearances, including additional IND and NDA/BLA submissions or premarket approvals before we can begin clinical development, and/or prior to marketing and sales. If the FDA requires new clearances or approvals for a particular use or indication, we may be required to conduct additional clinical studies, which would require additional expenditures and harm our operating results. If the products are already being promoted for these new indications, we may also be subject to significant enforcement actions. Conducting clinical trials and obtaining clearances and approvals can be a time-consuming process, and delays in obtaining required future clearances or approvals could adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Conducting successful clinical studies may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit.
Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population; the nature of the trial protocol; the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects; the availability of appropriate clinical trial investigators; support staff; proximity of patients to clinical sites; ability to comply with the eligibility and exclusion criteria for participation in the clinical trial; and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of our product candidates or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competitive products.
17
Additional delays to the completion of clinical studies may result from modifications being made to the protocol during the clinical trial, if such modifications are warranted and/or required by the occurrences in the given trial.
Each modification to the protocol during a clinical trial has to be submitted to the FDA. This could result in the delay or halt of a clinical trial while the modification is evaluated. In addition, depending on the quantity and nature of the changes made, the FDA could take the position that the data generated by the clinical trial is not poolable because the same protocol was not used throughout the trial. This might require the enrollment of additional subjects, which could result in the extension of the clinical trial and the FDA delaying clearance or approval of a product. Any such delay could have a material adverse effect on our business and results of operations.
There can be no assurance that the data generated from our clinical trials using modified protocols will be acceptable to FDA.
There can be no assurance that the data generated using modified protocols will be acceptable to the FDA or that if future modifications during the trial are necessary, that any such modifications will be acceptable to the FDA. If the FDA believes that its prior approval is required for a particular modification, it can delay or halt a clinical trial while it evaluates additional information regarding the change.
Serious injury or death resulting from a failure of one of our drug candidates during clinical trials could also result in the FDA delaying our clinical trials or denying or delaying clearance or approval of a product candidate. Even though an adverse event may not be the result of the failure of our drug candidate, the FDA or an IRB could delay or halt a clinical trial for an indefinite period of time while an adverse event is reviewed, and likely would do so in the event of multiple such events.
Any delay or termination of our current or future clinical trials as a result of the risks summarized above, including delays in obtaining or maintaining required approvals from IRBs, delays in patient enrollment, the failure of patients to continue to participate in a clinical trial, and delays or termination of clinical trials as a result of protocol modifications or adverse events during the trials, may cause an increase in costs and delays in the filing of any product submissions with the FDA, delay the approval and commercialization of our products or result in the failure of the clinical trial, which could adversely affect our business, operating results and prospects.
We rely on and intend to rely on third-parties to conduct our clinical trials and to assist us with pre-clinical development. If these third-parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our products.
We do not have the ability to independently conduct our pre-clinical and clinical trials for our product candidates, and we must rely on third-parties, such as CROs, medical institutions, clinical investigators and contract laboratories to conduct such trials. If these third-parties do not successfully carry out their contractual duties or regulatory obligations, meet expected deadlines or need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize, our products on a timely basis, if at all. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control. The occurrence of any of the foregoing may adversely affect our business, operating results and prospects.
We rely on and intend to rely on third parties to manufacture our clinical product supplies, and to produce and process our product candidates, if approved. Our commercialization of any of our product candidates could be stopped, delayed, or made less profitable if those third parties fail to obtain approval of government regulators, fail to provide us with sufficient quantities of drug product, devices, or device components, or fail to do so at acceptable quality levels or prices.
We do not currently have, nor do we currently plan to develop, the infrastructure or capability internally to manufacture our clinical supplies for use in the conduct of our clinical trials, and we lack the resources and the capability to manufacture any of our product candidates, devices, or device components on a clinical or commercial scale. We currently rely on outside vendors to manufacture our clinical supplies of our product candidates and plan to continue relying on third parties to manufacture our product candidates, devices, or device components on a commercial scale, if approved. The prominent regulatory standard used by the FDA to ensure pharmaceutical quality is the Current Good Manufacturing Practice (“cGMP”). The FDA can and will take regulatory action against drug manufacturers based on lack of CGMP, which can cause production delays and incur additional costs. In particular, we rely upon single-sourced manufacturing with one third-party contract development and manufacturing organization (a “CDMO”), WuXi AppTec (“WuXi”), for HT-KIT.
In January 2024, the BIOSECURE Act (H.R. 7085) was introduced in the House of Representatives and a substantially similar bill (S.3558) was introduced in the Senate. Although the House of Representatives of the prior Congress (the 118th Congress) passed the BIOSECURE Act on September 9, 2024, the legislation ultimately did not become law in the 118th Congress. It is unclear whether the current Congress (the 119th Congress) will introduce the BIOSECURE Act or similar legislation in this congressional session. If these bills became law, or similar laws are passed, they would have the potential to severely restrict the ability of U.S. biopharmaceutical companies to contract with certain Chinese biotechnology companies “of concern” without losing the ability to contract with, or otherwise receive funding from, the U.S. government. We do business with companies in China and it is possible some of our contractual counterparties could be impacted by this legislation.
18
Our reliance on third-party manufacturers exposes us to the following additional risks:
| ● | We may be unable to identify manufacturers of our product candidates on acceptable terms or at all. |
| ● | Our third-party manufacturers might be unable to timely formulate and manufacture our product or produce the quantity and quality required to meet our clinical and commercial needs, if any. |
| ● | Contract manufacturers may not be able to execute our manufacturing procedures appropriately. |
| ● | Our future third-party manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store, and distribute our commercial products, if approved. |
| ● | Our reliance on single-sourced manufacturing with WuXi increases the risk that any problems or delays with WuXi could materially, negatively affect the development of HT-KIT. |
| ● | Manufacturers are subject to ongoing periodic unannounced inspection by the FDA and some state agencies to ensure strict compliance with cGMPs and other government regulations and corresponding foreign standards. We do not have control over third-party manufacturers’ compliance with these regulations and standards. |
| ● | We may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our product candidates. |
| ● | Our third-party manufacturers could breach or terminate their agreement with us. |
| ● | Our third-party manufacturers’ performance, available capacity and ability to manufacture clinical or commercial products may be impacted by mergers and or acquisitions. |
| ● | We and our third-party manufacturers may be impacted by global conflicts, including any potential conflict involving China and Taiwan, and any resulting trade sanctions. |
| ● | Foreign third-party manufacturers may be subject to U.S. legislation or investigations, trade restrictions and other foreign regulatory requirements, which could increase the cost or reduce the supply of HT-KIT, delay the procurement or supply of HT-KIT or delay clinical trials. |
Each of these risks could delay our clinical trials, as well as the approval, if any, of our product candidates by the FDA, or the commercialization of our product candidates, or could result in higher costs, or could deprive us of potential product revenue.
We currently rely on foreign CROs and CDMOs, including WuXi to manufacture HT-KIT, and will likely continue to rely on foreign CROs and CDMOs in the future. Foreign CDMOs may be subject to U.S. legislation or investigations, sanctions, trade restrictions and other foreign regulatory requirements, which could increase the cost or reduce the supply of HT-KIT, delay the procurement or supply of HT-KIT, delay or impact clinical trials and could adversely affect our financial condition and business prospects. While we believe we may be able to replace WuXi, this could be time-consuming and expensive, which may adversely affect our financial condition and business prospects.
19
The future results of our current or future clinical trials may not support our product candidate claims or may result in the discovery of unexpected adverse side effects.
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our drug candidate claims or that the FDA or foreign regulatory agencies will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our drug candidates are safe and effective for the proposed indicated uses. If the FDA or other regulatory agencies conclude that the clinical trials for any of our product candidates has failed to demonstrate safety and effectiveness, we would not receive clearance from the FDA or other regulatory agencies to market that product in the United States or internationally for the indications sought.
In addition, such an outcome could cause us to abandon the product candidate and might delay development of other product candidates. Any delay or termination of our clinical trials will delay the filing of any product submissions with the FDA and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile. In addition, our clinical trials may involve a relatively small patient population. Because of the small sample size, our results may not be indicative of future results.
Even if our product candidates are approved by regulatory authorities, if we or our suppliers fail to comply with ongoing FDA regulations or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
The manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for any product candidate for which we obtain regulatory approval will be subject to continued regulatory review, oversight and periodic inspections by the FDA. In particular, we and our suppliers are required to comply with FDA’s Quality System Regulations and International Standards Organization (“ISO”) regulations for the manufacture of our products and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval. Regulatory bodies, such as the FDA, enforce these regulations through periodic inspections. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, enforcement actions by the FDA.
If any of these actions were to occur it would harm our reputation and cause our product sales and profitability to suffer and may prevent us from generating revenue. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Even if regulatory clearance or approval of a product is granted, such clearance or approval may be subject to limitations on the intended uses for which the product may be marketed and reduce the potential to successfully commercialize the product and generate revenue from the product. If the FDA determines that the product promotional materials, labeling, training or other marketing or educational activities constitute promotion of an unapproved use, it could request that we or our commercialization partners cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider such training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with adverse event and pharmacovigilance reporting requirements, including the reporting of adverse events which occur in connection with, and whether or not directly related to, our products. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to recall, replace or refund the cost of any product we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
20
Our revenue stream will depend upon third-party reimbursement.
The commercial success of our products in both domestic and international markets will be substantially dependent on whether third-party coverage and reimbursement is available for patients that use our products. However, the availability of insurance coverage and reimbursement for newly approved therapies is uncertain, and therefore, third-party coverage may be particularly difficult to obtain even if our products are approved by the FDA as safe and efficacious. Patients using existing approved therapies are generally reimbursed all or part of the product cost by Medicare or other third-party payors. Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new drugs, and, as a result, they may not cover or provide adequate payment for these products. Submission of applications for reimbursement approval generally does not occur prior to the filing of an NDA for that product and may not be granted for as long as many months after NDA approval. In order to obtain reimbursement arrangements for these products, we or our commercialization partners may have to agree to a net sales price lower than the net sales price we might charge in other sales channels. The continuing efforts of government and third-party payors to contain or reduce the costs of healthcare may limit our revenue. Initial dependence on the commercial success of our products may make our revenues particularly susceptible to any cost containment or reduction efforts.
Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain for such product candidates.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell our product candidates. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
In the United States, the Medicare Modernization Act (“MMA”) changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, this legislation authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for our product candidates and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the “Health Care Reform Law”) is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The Health Care Reform Law revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the law imposed a significant annual fee on companies that manufacture or import branded prescription drug products.
21
The Health Care Reform Law remains subject to legislative efforts to repeal, modify or delay the implementation of the law. However, if the Health Care Reform Law is repealed or modified, or if implementation of certain aspects of the Health Care Reform Law are delayed, such repeal, modification or delay may materially adversely impact our business, strategies, prospects, operating results or financial condition. We are unable to predict the full impact of any repeal, modification or delay in the implementation of the Health Care Reform Law on us at this time. Due to the substantial regulatory changes that will need to be implemented by the Centers for Medicare & Medicaid Services and others, and the numerous processes required to implement these reforms, we cannot predict which healthcare initiatives will be implemented at the federal or state level, the timing of any such reforms, or the effect such reforms or any other future legislation or regulation will have on our business.
In addition, other legislative changes have been proposed and adopted in the United States since the Health Care Reform Law was enacted. We expect that additional federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, and in turn could significantly reduce the projected value of certain development projects and reduce or eliminate our profitability.
We are dependent on third parties for manufacturing and marketing of our proposed product candidates. If we are not able to secure favorable arrangements with such third parties, our business and financial condition could be harmed.
We will not manufacture any of our proposed product candidates for commercial sale nor do we have the resources necessary to do so. In addition, we currently do not have the capability to market our drug products ourselves. In addition to our internal sales force efforts, we have contracted with and intend to continue to contract with specialized manufacturing companies to manufacture our proposed product candidates and partner with larger pharmaceutical companies for commercialization of our products. In connection with our efforts to commercialize our proposed product candidates, we will seek to secure favorable arrangements with third parties to distribute, promote, market and sell our proposed product candidates. If our internal sales force is unable to successfully distribute, market and promote our product candidates and we are not able to secure favorable commercial terms or arrangements with third parties for the distribution, marketing, promotion and sales of our proposed product candidates, we may have to retain promotional and marketing rights and seek to develop the commercial resources necessary to promote or co-promote or co-market certain or all of our proposed drug candidates to the appropriate channels of distribution in order to reach the specific medical market that we are targeting. We may not be able to enter into any partnering arrangements on this or any other basis. If we are not able to secure favorable partnering arrangements or are unable to develop the appropriate resources necessary for the commercialization of our proposed product candidates, our business and financial condition could be harmed. In addition, we will have to hire additional employees or consultants, since our current employees have limited experience in these areas. Sufficient employees with relevant skills may not be available to us. Any increase in the number of our employees would increase our expense level and could have an adverse effect on our financial position.
In addition, we, or our potential commercial partners, may not successfully introduce our proposed product candidates or such candidates may not achieve acceptance by patients, health care providers and insurance companies. Further, it is possible that we may not be able to secure arrangements to manufacture, market, distribute, promote and sell our proposed product candidates at favorable commercial terms that would permit us to make a profit. To the extent that corporate partners conduct clinical trials, we may not be able to control the design and conduct of these clinical trials.
We may have conflicts with our partners that could delay or prevent the development or commercialization of our product candidates.
We may have conflicts with our partners, such as conflicts concerning the interpretation of pre-clinical or clinical data, the achievement of milestones, the interpretation of contractual obligations, payments for services, development obligations or the ownership of intellectual property developed during our collaboration. If any conflicts arise with any of our partners, such partner may act in a manner that is averse to our best interests. Any such disagreement could result in one or more of the following, each of which could delay or prevent the development or commercialization of our product candidates, and in turn prevent us from generating revenues: unwillingness on the part of a partner to pay us milestone payments or royalties we believe are due to us under a collaboration; uncertainty regarding ownership of intellectual property rights arising from our collaborative activities, which could prevent us from entering into additional collaborations; unwillingness by the partner to cooperate in the development or manufacture of the product, including providing us with product data or materials; unwillingness on the part of a partner to keep us informed regarding the progress of its development and commercialization activities or to permit public disclosure of the results of those activities; initiating of litigation or alternative dispute resolution options by either party to resolve the dispute; or attempts by either party to terminate the agreement.
22
Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and the revenue that we generate from its sales, if any, may be limited.
If approved for marketing, the commercial success of our product candidates will depend upon each product’s acceptance by the medical community, including physicians, patients and health care payors. The degree of market acceptance for any of our product candidates will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; | |
| ● | relative convenience, dosing burden and ease of administration; |
| ● | the prevalence and severity of any adverse effects; | |
| ● | the willingness of physicians to prescribe our product candidates, and the target patient population to try new therapies; | |
| ● | efficacy of our product candidates compared to competing products; | |
| ● | the introduction of any new products that may in the future become available targeting indications for which our product candidates may be approved; | |
| ● | new procedures or therapies that may reduce the incidences of any of the indications in which our product candidates may show utility; | |
| ● | pricing and cost-effectiveness; | |
| ● | the inclusion or omission of our product candidates in applicable therapeutic and vaccine guidelines; | |
| ● | the effectiveness of our own or any future collaborators’ sales and marketing strategies; | |
| ● | limitations or warnings contained in approved labeling from regulatory authorities; | |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government bodies regulating the pricing and usage of therapeutics; and | |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors, and patients, we may not generate sufficient revenue and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
23
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our product candidates successfully. For example, if the approval process takes too long, we may miss market opportunities thereby giving other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our product candidates not commercially viable. For example, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve any of our product candidates with a label that does not include the labeling claims necessary or desirable for the successful commercialization for that indication. Further, the FDA or comparable foreign regulatory authorities may place conditions on approvals or require risk management plans or a REMS to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS. The FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA may also require a REMS for an approved product when new safety information emerges. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of our product candidates. Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of our product candidates.
Our products will face significant competition, and if they are unable to compete successfully, our business will suffer.
Our product candidates face, and will continue to face, intense competition from large pharmaceutical companies, as well as academic and research institutions. We compete in an industry that is characterized by: (i) rapid technological change, (ii) evolving industry standards, (iii) emerging competition and (iv) new product introductions. Our competitors have and may develop products and technologies that will compete with our products and technologies. Because several competing companies and institutions have greater financial resources than us, they may be able to: (i) provide broader services and product lines, (ii) make greater investments in research and development and (iii) carry on larger research and development initiatives. Our competitors also have greater development capabilities than we do and have substantially greater experience in undertaking pre-clinical and clinical testing of products, obtaining regulatory approvals, and manufacturing and marketing pharmaceutical products. They also have greater name recognition and better access to customers than us.
Adverse events involving our products may lead the FDA or other regulatory agencies to delay or deny clearance for our products or result in product recalls that could harm our reputation, business and financial results.
Once a product receives clearance or approval, the agency has the authority to require the recall of commercialized products in the event of adverse side effects, material deficiencies or defects in design or manufacture. With respect to the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the product would cause serious injury or death. Manufacturers may, under their own initiative, recall a product if any material deficiency in a product is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of adverse side effects, impurities or other product contamination, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. In addition, the FDA requires that certain classifications of recalls be reported to FDA within ten working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA. We may initiate voluntary recalls or market withdrawal involving our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted.
If we fail to comply with healthcare regulations, we could face substantial enforcement actions, including civil and criminal penalties and our business, operations and financial condition could be adversely affected.
Sales of our product candidates, if approved, or any other future product candidate will be subject to healthcare regulation and enforcement by the federal government and the states and foreign governments in which we might conduct our business. The healthcare laws and regulations that may affect our ability to operate include the following:
| ● | the federal Anti-Kickback Statute makes it illegal for any person or entity to knowingly and willfully, directly or indirectly, solicit, receive, offer, or pay any remuneration that is in exchange for or to induce the referral of business, including the purchase, order, lease of any good, facility, item or service for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value, including gifts, discounts, credit arrangements, payments of cash, ownership interests and providing anything at less than its fair market value. Recognizing that the federal Anti- Kickback Statute is broad and may prohibit certain common activities within the healthcare industry, the Office of Inspector General for HHS has issued a series of statutory exceptions and regulatory “safe harbors.” However, these exceptions and safe harbors are drawn narrowly and require strict compliance in order to offer protection from prosecution under the federal Anti-Kickback Statute; |
24
| ● | the Omnibus Budget Reconciliation Act of 1993 (42 U.S.C. § 1395nn) (the “Stark Law”) prohibit referrals by a physician of “designated health services” which are payable, in whole or in part, by Medicare or Medicaid, to an entity in which the physician or the physician’s immediate family member has an investment interest or other financial relationship, subject to several exceptions. The Stark Law also prohibits billing for services rendered pursuant to a prohibited referral. Several states have enacted laws similar to the Stark Law. These state laws may cover all (not just Medicare and Medicaid) patients. Many federal healthcare reform proposals in the past few years have attempted to expand the Stark Law to cover all patients as well. We consider the Stark Law in planning our products, marketing and other activities, and believe that our operations are in compliance with the Stark Law. If we violate the Stark Law, our financial results and operations could be adversely affected. Penalties for violations include denial of payment for the services, significant civil monetary penalties, and exclusion from the Medicare and Medicaid programs; |
| ● | federal false claims and false statement laws, including the federal civil False Claims Act and the Civil Monetary Penalties Law (“CMPL”), prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, for payment to, or approval by, federal programs, including Medicare and Medicaid, claims for items or services, including drugs, that are false or fraudulent; | |
| ● | HIPAA, created additional federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors or making any false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services; | |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 and their implementing regulations, impose obligations on certain types of individuals and entities regarding the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information; | |
| ● | the FDCA which among other things, strictly regulates drug and biologics manufacturing, sales, distribution, prohibits the adulteration or misbranding of drugs and biologics prohibits manufacturers from marketing drug products for off-label use and regulates the distribution of drug samples; | |
| ● | the federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services information related to payments or other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and | |
| ● | the U.S. Foreign Corrupt Practices Act (“FCPA”) prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations. The future of FCPA enforcement remains uncertain, as there has already been a temporary enforcement suspension under the Trump Administration, and future changes are possible. |
Also, many states have similar laws and regulations, such as Stark Law, anti-kickback and false claims laws that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. Additionally, we may be subject to state laws that require pharmaceutical companies to comply with the federal government’s and/or pharmaceutical industry’s voluntary compliance guidelines, state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, as well as state and foreign laws governing the privacy and security of health information, many of which differ from each other in significant ways and often are not preempted by HIPAA.
The laws and regulations applicable to our business are complex, changing and often subject to varying interpretations. As a result, we may not be able to adhere to all applicable laws and regulations. Any violation or alleged violation of any of these laws or regulations by us could have a material adverse effect on our business, financial condition, cash flows and results of operations. We may be a party to various lawsuits, demands, claims, qui tam suits, government investigations and audits, of which any could result in, among other things, substantial financial penalties or awards against us, reputational harm, termination of relationships or contracts related to our business, mandated refunds, substantial payments made by us, required changes to our business practices, exclusion from future participation in Medicare and other healthcare programs, seizure of product and possible criminal penalties.
25
If we are found in violation of applicable laws or regulations, we could suffer severe consequences that would have a material adverse effect on our business, results of operations, financial condition, cash flows, reputation and stock price, including:
| ● | suspension or termination of our participation in federal healthcare programs; | |
| ● | criminal or civil liability, fines, damages or monetary penalties for violations of healthcare fraud and abuse laws, including the federal False Claims Act, CMPL, and Anti-Kickback Statute; | |
| ● | enforcement actions by governmental agencies or claims for monetary damages by patients under federal or state patient privacy laws, including HIPAA; |
| ● | repayment of amounts received in violation of law or applicable payment program requirements, and related monetary penalties; | |
| ● | mandated changes to our practices or procedures that materially increase operating expenses; | |
| ● | imposition of corporate integrity agreements that could subject us to ongoing audits and reporting requirements as well as increased scrutiny of our business practices; | |
| ● | termination of various relationships or contracts related to our business; and | |
| ● | harm to our reputation which could negatively affect our business relationships, decrease our ability to attract or retain patients and physicians, decrease access to new business opportunities and impact our ability to obtain financing, among other things. |
Responding to lawsuits and other proceedings as well as defending ourselves in such matters will continue to require management’s attention and cause us to incur significant legal expense. It is also possible that criminal proceedings may be initiated against us or individuals in our business in connection with investigations by the federal government.
Furthermore, to the extent that our product is sold in a foreign country, we may be subject to similar foreign laws.
If a third-party contract manufacturing organization (“CMO”) upon whom we rely to formulate and manufacture our product candidates does not perform, fails to manufacture according to our specifications or fails to comply with strict regulations, our pre-clinical studies or clinical trials could be adversely affected, and the development of our product candidates could be delayed or terminated, or we could incur significant additional expenses.
We do not own or operate any manufacturing facilities. We rely on and intend to continue to rely on CMOs to formulate and manufacture our pre-clinical and clinical materials. Our reliance on a CMO exposes us to a number of risks, any of which could delay or prevent the completion of our pre-clinical studies or clinical trials, or the regulatory approval or commercialization of our product candidates, result in higher costs, or deprive us of potential product revenues. Some of these risks include:
| ● | our CMO failing to develop an acceptable formulation to support later-stage clinical trials for, or the commercialization of, our product candidates; | |
| ● | our CMO failing to manufacture our product candidate according to our specifications, the FDA’s cGMP requirements, or otherwise manufacturing material that we or the FDA may deem to be unsuitable in our clinical trials; |
| ● | our CMO being unable to increase the scale of, increase the capacity for, or reformulate the form of our product candidates. We may experience a shortage in supply, or the cost to manufacture our products may increase to the point where it may adversely affect the cost of our product candidates. We cannot assure you that our CMO will be able to manufacture our product candidates at a suitable scale, or we will be able to find alternative manufacturers acceptable to us that can do so; |
26
| ● | our CMO placing a priority on the manufacture of their own products, or other customers’ products; | |
| ● | our CMO failing to perform as agreed upon or not remain in business; and | |
| ● | our CMOs’ plants being closed as a result of regulatory sanctions, natural disasters, health epidemics or otherwise. |
Manufacturers of pharmaceutical products are subject to ongoing periodic inspections by the FDA, the U.S. Drug Enforcement Administration and corresponding state and foreign agencies to ensure strict compliance with FDA mandated cGMPs, other government regulations and corresponding foreign standards. While we are obligated to audit their performance, we do not have control over our CMO’s compliance with these regulations and standards. Failure by any of our CMOs, or us, to comply with applicable regulations could result in sanctions being imposed on us or the CMOs. These sanctions may include fines, injunctions, civil penalties, failure of the government to grant pre-market approval of drugs, delays, suspension or withdrawal of approvals, seizures or recalls of product, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business.
In the event that we need to change our CMOs, our pre-clinical studies, clinical trials or the commercialization of our product candidates could be delayed, adversely affected or terminated, or such a change may result in significantly higher costs.
Various steps in the manufacture of our product candidates may need to be sole-sourced. In accordance with cGMP, changing manufacturers may require the re-validation of manufacturing processes and procedures, and may require further pre-clinical studies or clinical trials to show comparability between the materials produced by different manufacturers. Changing our current or future CMOs may be difficult for us and could be costly, which could result in our inability to manufacture our product candidates for an extended period of time and therefore a delay in the development of our product candidates. Further, in order to maintain our development timelines in the event of a change in our CMOs, we may incur significantly higher costs to manufacture our product candidates.
Healthcare Reform in the United States.
In the United States, there have been, and continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect the future results of pharmaceutical manufactures’ operations. In particular, there have been and continue to be a number of initiatives at the federal and state levels that seek to reduce healthcare costs. On the federal level, the Affordable Care Act (“ACA”) was enacted in March 2010, and included measures to significantly change the way healthcare is financed by both governmental and private insurers. Among the provisions of the ACA that have been of greatest importance to the pharmaceutical and biotechnology industry are the following:
| ● | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; | |
| ● | implementation of the federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act”; | |
| ● | a licensure framework for follow-on biologic products; | |
| ● | creation of Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; | |
| ● | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending; |
| ● | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, to 23.1% and 13% of the average manufacturer price for most branded and generic drugs, respectively and capped the total rebate amount for innovator drugs at 100% of the Average Manufacturer Price; | |
| ● | adoption of methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for certain drugs and biologics, including our product candidates, that are inhaled, infused, instilled, implanted or injected; |
27
| ● | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; | |
| ● | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for individuals with income at or below 133% of the federal poverty level, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| ● | creation of a Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and | |
| ● | expansion of the entities eligible for discounts under the Public Health program. |
Although there have been legal and political challenges to certain aspects of the ACA, the Biden Administration affirmed support for the law and, entered its own executive orders to enforce and strengthen it. Because of the volatility surrounding the implementation and enforcement of the ACA since its passage, and at this time, the full effect that the ACA would have on a pharmaceutical manufacturer remains unclear. This uncertainty is heightened by actions taken under the Trump Administration. On January 20, 2025, President Trump issued Executive Order 14148, which revoked Executive Order 14009 issued by President Biden on January 28, 2021, that had initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear what healthcare reform measures will be implemented by the Trump Administration, but significant changes are anticipated. The potential changes in patient coverage by government funded insurance may impact our pricing.
The first Trump Administration, on July 24, 2020 and September 13, 2020, announced several executive orders related to prescription drug pricing. As a result, the FDA concurrently released a final rule and guidance in September 2020 providing pathways for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, the HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Medicare Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a new safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. The implementation of the rule was delayed until 2032 by the Inflation Reduction Act of 2022. On November 20, 2020, CMS issued an interim final rule implementing President Trump’s Most Favored Nation executive order, which would tie Medicare Part B payments for certain physician-administered drugs to the lowest price paid in other economically advanced countries. The Most Favored Nation regulations mandate participation by identified Medicare Part B providers and will apply in all U.S. states and territories for a seven-year period beginning January 1, 2021, and ending December 31, 2027. As a result of litigation challenging the Most Favored Nation model, on December 27, 2021 CMS published a final rule that rescinds the Most Favored Nation model interim final rule. Further, in July 2021, the Biden administration released an executive order that included multiple provisions aimed at prescription drugs. In response to President Biden’s executive order, on September 9, 2021, the HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform. The plan sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. No legislation or administrative actions have been finalized to implement these principles. It is unclear how the current Trump Administration will further address drug pricing.
In August 2022, the Inflation Reduction Act of 2022 was signed into law by President Biden. The new legislation has implications for Medicare Part D, which is a program available to individuals who are entitled to Medicare Part A or enrolled in Medicare Part B to give them the option of paying a monthly premium for outpatient prescription drug coverage. Among other things, the Inflation Reduction Act of 2022 requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The Inflation Reduction Act of 2022 permits the Secretary of the HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. There is uncertainty surrounding this program with the new administration, especially in light of the administration’s budget cuts which impact an agency’s ability to regulate through guidance. Further, it is unclear how the new leadership of HHS, CMS, etc. will approach the issue of drug pricing.
In addition, we cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad, but the Trump administration has shown a tendency to govern through executive action. We expect that additional state and federal health care reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for health care products and services.
Further, there is uncertainty surrounding the applicability of the biosimilars provisions under the ACA. The FDA has issued several guidance documents, but no implementing regulations, on biosimilars. A number of biosimilar applications have been approved over the past few years. The regulations that are ultimately promulgated and their implementation are likely to have considerable impact on the way pharmaceutical manufacturers conduct their business and may require changes to current strategies. A biosimilar is a biological product that is highly similar to an approved drug notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biological product and the approved drug in terms of the safety, purity, and potency of the product.
28
Individual states have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm a pharmaceutical manufacturer’s business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce ultimate demand for certain products or put pressure on product pricing, which could negatively affect a pharmaceutical manufacturer’s business, results of operations, financial condition and prospects.
In addition, given past federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and biologics and the reform of the Medicare and Medicaid programs. While no one cannot predict the full outcome of any such legislation, it may result in decreased reimbursement for drugs and biologics, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm a pharmaceutical manufacturer’s ability to generate revenue. Increases in importation or re-importation of pharmaceutical products from foreign countries into the United States could put competitive pressure on a pharmaceutical manufacturer’s ability to profitably price products, which, in turn, could adversely affect business, results of operations, financial condition and prospects. The new administration’s recent introduction of tariffs on foreign nations may also have an impact on business operations. A pharmaceutical manufacturer might elect not to seek approval for or market products in foreign jurisdictions in order to minimize the risk of re-importation, which could also reduce the revenue generated from product sales. It is also possible that other legislative proposals having similar effects will be adopted.
Furthermore, regulatory authorities’ assessment of the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, changing policies and agency funding, staffing and leadership. We cannot be sure whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects. For example, average review times at the FDA for marketing approval applications can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes.
Our business may be adversely affected by cybersecurity threats, information systems interruptions and/or threats to our physical buildings.
It is essential to our business strategy that our technology and network infrastructure and our physical buildings remain secure and are perceived by our customers and corporate partners to be secure. Despite security measures, however, any network infrastructure may be vulnerable to cyber-attacks by hackers and other security threats. We may face cybersecurity threats that attempt to penetrate our network security, sabotage or otherwise disable our research, products and services, misappropriate our or our customers’ and partners’ proprietary information, which may include personally identifiable information, or cause interruptions or failures of our internal systems and services. Despite security measures, we also cannot guarantee security of our physical buildings. Physical building penetration or any cybersecurity threats could negatively affect our reputation, damage our network infrastructure and our ability to deploy our products and services, harm our relationship with customers and partners that are affected, and expose us to financial liability.
Although we continue to review and enhance our systems and cybersecurity controls, we may experience cybersecurity threats, including threats to our information technology infrastructure and attempts to gain access to our sensitive information, as do our customers and suppliers. Although we maintain information security policies and procedures to prevent, detect, and mitigate these threats, information system disruptions, equipment failures or cybersecurity attacks, such as unauthorized access, malicious software and other intrusions, could still occur and may lead to potential data corruption, exposure of proprietary and confidential information. Further, while we work cooperatively with our customers and suppliers to seek to minimize the impacts of cybersecurity threats, other security threats or business disruptions, in addition to our internal processes, procedures and systems, we must also rely on the safeguards put in place by those entities.
Any intrusion, disruption, breach or similar event may cause operational stoppages, fines, penalties, diminished competitive advantages through reputational damages and increased operational costs. The costs related to cybersecurity or other security threats or disruptions may not be fully mitigated by insurance or other means. In addition to existing risks, any adoption or deployment of new technologies may increase our exposure to risks, breaches, or failures, which could materially adversely affect our results of operations or financial condition.
29
Additionally, there are a number of state, federal and international laws protecting the privacy and security of health information and personal data. For example, HIPAA imposes limitations on the use and disclosure of an individual’s healthcare information by healthcare providers, healthcare clearinghouses, and health insurance plans, or, collectively, covered entities, and also grants individuals rights with respect to their health information. HIPAA also imposes compliance obligations and corresponding penalties for non-compliance on individuals and entities that provide services to healthcare providers and other covered entities. As part of the American Recovery and Reinvestment Act of 2009 (“ARRA”) the privacy and security provisions of HIPAA were amended. ARRA also made significant increases in the penalties for improper use or disclosure of an individual’s health information under HIPAA and extended enforcement authority to state attorneys general. As amended by ARRA and subsequently by the final omnibus rule adopted in 2013, HIPAA also imposes notification requirements on covered entities in the event that certain health information has been inappropriately accessed or disclosed, notification requirements to individuals, federal regulators, and in some cases, notification to local and national media. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with encryption or other standards developed by the U.S. Department of Health and Human Services. Most states have laws requiring notification of affected individuals and/or state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms, to ensure ongoing protection of personal information. Activities outside of the U.S. implicate local and national data protection standards, impose additional compliance requirements and generate additional risks of enforcement for non-compliance. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws, to protect against security breaches and hackers or to alleviate problems caused by such breaches.
Risks Related to Our Intellectual Property Rights
We rely upon licenses granted to us by various licensors, and if such licensors do not adequately defend such licenses, our business may be harmed.
We have entered into and may, in the future, enter into license and sublicense agreements with respect to our product candidates. We have limited control over the activities of our licensors, and we rely upon our licensors to protect their intellectual property, including the patents covered by our licenses. We cannot be certain that activities conducted by our licensors have been or will be conducted in compliance with applicable laws and regulations. Furthermore, we have no or limited control or input over whether, and in what manner, our licensors may enforce or defend the patents that we license against a third-party. Our licensors may defend the patents we license less vigorously than if we had enforced or defended the patents ourselves. Furthermore, our licensors may not necessarily seek enforcement in scenarios in which we would feel that enforcement was in our best interests. For example, our licensors may not enforce the patents against a competitor of ours who is not a direct competitor of such licensor. If our in-licensed intellectual property is found to be invalid or unenforceable, then our licensors may not be able to enforce the patents against a competitor of ours. Moreover, if we fail to meet our obligations under our license agreements, the licensor may terminate the license agreement. Furthermore, if we fail to meet our obligations under our sublicense agreements or our sublicensor fails to meet its obligations to the licensor, such licensor may terminate the license agreement thereby terminating our sublicense agreement.
Our business depends upon us securing and protecting critical intellectual property.
To the extent we develop intellectual property, our commercial success will depend in part on obtaining and maintaining patent, trade secret, copyright and trademark protection of our technologies in the United States and other jurisdictions as well as successfully enforcing and defending such intellectual property rights against third-party challenges. We will only be able to protect our intellectual property from unauthorized use by third parties to the extent that valid and enforceable intellectual property protection, such as patents or trade secrets, cover them. In particular, we place considerable emphasis on obtaining patent and trade secret protection for significant new technologies, products and processes. Furthermore, the degree of future protection of our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. Moreover, the degree of future protection of our proprietary rights is uncertain for products that are currently in the early stages of development because we cannot predict which of these products will ultimately reach the commercial market or whether the commercial versions of these products will incorporate proprietary technologies.
30
Patent positions in our industry are highly uncertain and involve complex legal and factual questions.
Patent positions in our industry are highly uncertain and involve complex legal and factual questions. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example, we or our licensors might not have been the first to make the inventions covered by our pending patent applications and issued patents, as applicable; we or our licensors might not have been the first to file patent applications for these inventions; others may independently develop similar or alternative technologies or duplicate any of our technologies; it is possible that none of our pending patent applications or the pending patent applications of our licensors will result in issued patents; our issued patents and issued patents of our licensors may not provide a basis for commercially viable technologies, or may not provide us with any competitive advantages, or may be challenged and invalidated by third parties; and, we may not develop additional proprietary technologies that are patentable. As a result, our owned and licensed patents may not be valid, and we may not be able to obtain and enforce patents and to maintain trade secret protection for the full commercial extent of our technology. The extent to which we are unable to do so could materially harm our business.
We and/or our licensors have applied for and will continue to apply for patents for certain products. Such applications may not result in the issuance of any patents, and any patents now held or that may be issued may not provide us with adequate protection from competition. Furthermore, it is possible that patents issued or licensed to us may be challenged successfully. In that event, if we have a preferred competitive position because of such patents, any preferred position held by us would be lost. If we are unable to secure or to continue to maintain a preferred position, we could become subject to competition from the sale of generic products. Failure to receive, inability to protect, or expiration of our patents for medical use, manufacture, conjugation and labeling of any of our product candidates may adversely affect our business and operations.
Patents issued or licensed to us may be infringed by the products or processes of others. The cost of enforcing our patent rights against infringers, if such enforcement is required, could be significant, and we may not have the financial resources to fund such litigation. Further, such litigation can go on for years and the time demands could interfere with our normal operations. There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical industry. We may become a party to patent litigation and other proceedings. The cost to us of any patent litigation, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation more effectively than we can because of their substantially greater financial resources. Litigation may also absorb significant management time.
Unpatented trade secrets, improvements, confidential know-how and continuing technological innovation are important to our scientific and commercial success. Although we attempt to and will continue to attempt to protect our proprietary information through reliance on trade secret laws and the use of confidentiality agreements with our corporate partners, collaborators, employees and consultants and other appropriate means, these measures may not effectively prevent disclosure of our proprietary information, and, in any event, others may develop independently, or obtain access to the same or similar information.
If we are found to be infringing on patents or trade secrets owned by others, we may be forced to cease or alter our product development efforts, obtain a license to continue the development or sale of our products, and/or pay damages.
Our manufacturing processes and potential products may violate proprietary rights of patents that have been or may be granted to competitors, universities or others, or the trade secrets of those persons and entities. As the pharmaceutical industry expands and more patents are issued, the risk increases that our processes and potential products may give rise to claims that they infringe the patents or trade secrets of others. These other persons could bring legal actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the affected product or process. If any of these actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to conduct clinical tests, manufacture or market the affected product or use the affected process. Required licenses may not be available on acceptable terms, if at all, and the results of litigation are uncertain. If we become involved in litigation or other proceedings, it could consume a substantial portion of our financial resources and the efforts of our personnel.
31
Our ability to protect and enforce any patents we may obtain does not guarantee that we will secure the right to commercialize such patents.
A patent is a limited monopoly right conferred upon an inventor, and his successors in title, in return for the making and disclosing of a new and non-obvious invention. This monopoly is of limited duration but, while in force, allows the patent holder to prevent others from making and/or using his invention. While a patent gives the holder this right to exclude others, it is not a license to commercialize the invention, where other permissions may be required for permissible commercialization to occur. For example, a drug cannot be marketed without the appropriate authorization from the FDA, regardless of the existence of a patent covering the product. Further, the invention, even if patented itself, cannot be commercialized if it infringes the valid patent rights of another party.
We rely on confidentiality agreements to protect our trade secrets. If these agreements are breached by our employees or other parties, our trade secrets may become known to our competitors.
We rely on trade secrets which we seek to protect through confidentiality agreements with our employees and other parties. If these agreements are breached, our competitors may obtain and use our trade secrets to gain a competitive advantage over us. We may not have any remedies against our competitors and any remedies that may be available to us may not be adequate to protect our business or compensate us for the damaging disclosure. In addition, we may have to expend resources to protect our interests from possible infringement by others.
Risks Related to the Company
We have expanded and may continue to expand our business through the acquisition of rights to new drug candidates that could disrupt our business, harm our financial condition and may also dilute current shareholders’ ownership interests in our Company.
Our business strategy includes expanding our products and capabilities, and we may seek acquisitions of additional drug candidates or technologies to do so. Acquisitions involve numerous risks, including substantial cash expenditures; potentially dilutive issuance of equity securities; incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; difficulties in assimilating the acquired technologies or the operations of the acquired companies; diverting our management’s attention away from other business concerns; risks of entering markets in which we have limited or no direct experience; and the potential loss of our key employees or key employees of the acquired companies.
We cannot assure you that any acquisition will result in short-term or long-term benefits to us. We may misjudge the value or worth of an acquired product, company or business. In addition, our future success would depend in part on our ability to manage the rapid growth associated with acquisitions. We cannot assure you that we will be able to make the combination of our business with that of acquired products, businesses or companies work or be successful. Furthermore, the development or expansion of our business or any acquired products, business or companies may require a substantial capital investment by us. We may not have these necessary funds, or they might not be available to us on acceptable terms, or at all. We may also seek to raise funds by selling shares of our preferred or common stock, which could dilute each current shareholder’s ownership interest in the Company.
Any international operations we undertake may subject us to risks inherent with operations outside of the United States.
We may seek to obtain market clearance in foreign markets that we deem to generate significant opportunities. However, even with the cooperation of a commercialization partner, conducting drug development in foreign countries involves inherent risks, including, but not limited to: difficulties in staffing, funding and managing foreign operations; unexpected changes in regulatory requirements; export restrictions; tariffs and other trade barriers; difficulties in protecting, acquiring, enforcing and litigating intellectual property rights; fluctuations in currency exchange rates; and potentially adverse tax consequences. If we were to experience any of the difficulties listed above, or any other difficulties, our international development activities and our overall financial condition may suffer and cause us to reduce or discontinue our international development and registration efforts.
32
We may not be successful in hiring and retaining key employees, including executive officers.
Our future operations and successes depend in large part upon the strength of our management team. We rely heavily on the continued service of each member of our management team. Accordingly, if any member of our management team were to terminate their employment with us, such departure may have a material adverse effect on our business. In addition, our future success depends on our ability to identify, attract, hire or engage, retain and motivate other well-qualified financial, managerial, technical, clinical and regulatory personnel. There can be no assurance that these professionals will be available in the market, or that we will be able to retain existing professionals or to meet or to continue to meet their compensation requirements. Furthermore, the cost base in relation to such compensation, which may include equity compensation, may increase significantly, which could have a material adverse effect on us. Failure to establish and maintain an effective management team and workforce could adversely affect our ability to operate, grow and manage our business.
Managing our growth as we expand operations may strain our resources.
We expect to grow rapidly in order to support additional, larger, and potentially international, pivotal clinical trials of our drug candidates, which will place a significant strain on our financial, managerial and operational resources. In order to achieve and manage growth effectively, we must continue to improve and expand our operational and financial management capabilities. Moreover, we will need to increase staffing and to train, motivate and manage our employees. All of these activities will increase our expenses and may require us to raise additional capital sooner than expected. Failure to manage growth effectively could harm our business, financial condition or results of operations.
If a product liability claim is successfully brought against us for uninsured liabilities, or such claim exceeds our insurance coverage, we could be forced to pay substantial damage awards that could materially harm our business.
The use of any of our existing or future product candidates in clinical trials and the sale of any approved pharmaceutical products may expose us to significant product liability claims. Any product liability insurance coverage we obtain may not protect us against any or all of the product liability claims that may be brought against us in the future. We may not be able to acquire or maintain adequate product liability insurance coverage at a commercially reasonable cost or in sufficient amounts or scope to protect us against potential losses. In the event a product liability claim is brought against us, we may be required to pay legal and other expenses to defend the claim, as well as uncovered damage awards resulting from a claim brought successfully against us. In the event our product candidate is approved for sale by the FDA or other regulatory agency and commercialized, we may need to substantially increase the amount of our product liability coverage. Defending any product liability claim, or claims, could require us to expend significant financial and managerial resources, which could have an adverse effect on our business.
Our business may be adversely affected by public health crises, such as pandemics and epidemics, which may have a material adverse effect on our business.
We are subject to the risks associated with public health crises, such as pandemics and epidemics. Any governmental lockdowns, quarantine requirements or other restrictions as a result of a pandemic or epidemic may cause shutdowns or other significant business disruptions, thereby effecting our ability to conduct our business in the manner presently planned which could have a material adverse effect on us. In addition, any pandemic or epidemic may impact the global economy which may have a material adverse effect on our business. For example, staffing issues related to a public health crises may disrupt our business operations, including our clinical trials. Site initiation, participant recruitment and enrollment, participant dosing, distribution of clinical trial materials, study monitoring and data analysis may be paused or delayed due to changes in hospital or university policies, federal, state or local regulations, prioritization of hospital resources toward other efforts, or other staffing issues related to any such health epidemic. Also, some participants and clinical investigators may not be able to comply with clinical trial protocols. For example, quarantines or other travel limitations (whether voluntary or required) stemming from a health epidemic may impede participant movement, affect sponsor access to study sites, or interrupt healthcare services, and we may be unable to conduct our clinical trials. In addition, if any third parties in the supply chain for materials used in the production of our product candidates are adversely impacted by a public health crises, our supply chain may be disrupted, limiting our ability to manufacture our product candidates for our clinical trials and research and development operations. Furthermore, we may be at risk of delaying, defaulting and/or not performing under existing agreements, which may increase our costs. These cost increases may not be fully recoverable or adequately covered by insurance. Infections and deaths related to a health epidemic may also disrupt the United States’ healthcare and healthcare regulatory systems which could divert healthcare resources away from or materially delay FDA review and/or approval of our product candidates.
33
The scope and duration of any future public health crisis, the pace at which government restrictions are imposed and lifted, global vaccination and booster rates, the speed and extent to which global markets fully recover from the disruptions caused by such public health crisis, and the impact of these factors on our business, financial condition and results of operations, will depend on future developments that are highly uncertain and cannot be predicted with confidence.
Significant disruptions of information technology systems or breaches of data security could adversely affect our business.
Our business is increasingly dependent on critical, complex, and interdependent information technology systems, including Internet-based systems, to support business processes as well as internal and external communications. These systems are also critical to enable remote working arrangements, which have been growing in importance. The size and complexity of our computer systems make us potentially vulnerable to IT system breakdowns, internal and external malicious intrusion, and computer viruses and ransomware, which may impact product production and key business processes. We also have outsourced significant elements of our information technology infrastructure and operations to third parties, which may allow them to access our confidential information and may also make our systems vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by such third parties or others.
In addition, our systems are potentially vulnerable to data security breaches - whether by employees or others - which may expose sensitive data to unauthorized persons. Data security breaches could lead to the loss of trade secrets or other intellectual property, result in demands for ransom or other forms of blackmail, or lead to the public exposure of personal information (including sensitive personal information) of our employees, clinical trial patients, customers, and others. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including industrial espionage or extortion) and expertise, including by organized criminal groups, “hacktivists,” nation states, and others. As a company with an increasingly global presence, our systems are subject to frequent attacks. There is the potential that our systems may be directly or indirectly affected as nation-states conduct global cyberwarfare.
Due to the nature of some of these attacks, there is a risk that an attack may remain undetected for a period of time. While we continue to make investments to improve the protection of data and information technology, and to oversee and monitor the security measures of our suppliers and/or service providers, there can be no assurance that our efforts will prevent service interruptions or security breaches. In addition, we depend in part on third-party security measures over which we do not have full control to protect against data security breaches.
If we or our suppliers and/or service providers fail to maintain or protect our information technology systems and data security effectively and in compliance with U.S. and foreign laws, or fail to anticipate, plan for, or manage significant disruptions to these systems, we or our suppliers and/or service providers could have difficulty preventing, detecting, or controlling such disruptions or security breaches, which could result in legal proceedings, liability under U.S. and foreign laws that protect the privacy of personal information, disruptions to our operations, government investigations, breach of contract claims, and damage to our reputation (in each case in the U.S. or globally), which could have a material adverse effect on our business, prospects, operating results, and financial condition.
34
Risks Related to Our Common Stock
The price of our common stock may fluctuate substantially.
You should consider an investment in our common stock to be risky, and you should invest in our common stock only if you can withstand a significant loss and wide fluctuations in the market value of your investment. Some factors that may cause the market price of our common stock to fluctuate, in addition to the other risks mentioned in this “Risk Factors” section and elsewhere in this Annual Report on Form 10-K, are:
| ● | sale of our common stock by our shareholders, executives, and directors; | |
| ● | volatility and limitations in trading volumes of our shares of common stock; |
| ● | our ability to obtain financings to conduct and complete research and development activities including, but not limited to, our clinical trials, and other business activities; | |
| ● | the timing and success of introductions of new products by us or our competitors or any other change in the competitive dynamics of our industry, including consolidation among competitors; | |
| ● | our ability to attract new customers; | |
| ● | our ability to secure resources and the necessary personnel to conduct clinical trials on our desired schedule; | |
| ● | commencement, enrollment or results of our clinical trials for our product candidates; | |
| ● | changes in the development status of our product candidates; | |
| ● | any delays or adverse developments or perceived adverse developments with respect to a regulatory agency’s review of our planned pre-clinical and clinical trials; |
| ● | any delay in our submission for studies or product approvals or adverse regulatory decisions, including failure to receive regulatory approval for our product candidates; | |
| ● | unanticipated safety concerns related to the use of our product candidates; | |
| ● | changes in our capital structure or dividend policy, future issuances of securities and sales of large blocks of common stock by our shareholders; |
| ● | our cash position; | |
| ● | announcements and events surrounding financing efforts, including debt and equity securities; | |
| ● | our inability to enter new markets or develop new products; | |
| ● | reputational issues; | |
| ● | announcements of acquisitions, partnerships, collaborations, joint ventures, new products, capital commitments, or other events by us or our competitors; | |
| ● | changes in general economic, political and market conditions in or any of the regions in which we conduct our business; | |
| ● | changes in industry conditions or perceptions; | |
| ● | analyst research reports, recommendations and changes in recommendations, price targets, and withdrawals of coverage; | |
| ● | departures and additions of key personnel; |
| ● | disputes and litigations related to intellectual properties, proprietary rights, and contractual obligations; | |
| ● | changes in applicable laws, rules, regulations, or accounting practices and other dynamics; |
35
| ● | actual or anticipated fluctuations in our operating results; | |
| ● | changes in market valuations of other similar companies; and | |
| ● | other events or factors, many of which may be out of our control, including, but not limited to, pandemics, war, or other acts of God. |
In addition, if the market for stocks in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
We may acquire other companies or technologies which could divert our management’s attention, result in dilution to our shareholders and otherwise disrupt our operations and adversely affect our operating results.
We may in the future seek to acquire or invest in businesses, applications and services or technologies that we believe could complement or expand our services, enhance our technical capabilities or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated.
In addition, we do not have any experience in acquiring other businesses. If we acquire additional businesses, we may not be able to integrate the acquired personnel, operations and technologies successfully, or effectively manage the combined business following the acquisition. We also may not achieve the anticipated benefits from the acquired business due to a number of factors, including:
| ● | inability to integrate or benefit from acquired technologies or services in a profitable manner; | |
| ● | unanticipated costs or liabilities associated with the acquisition; |
| ● | difficulty integrating the accounting systems, operations and personnel of the acquired business; | |
| ● | difficulties and additional expenses associated with supporting legacy products and hosting infrastructure of the acquired business; | |
| ● | difficulty converting the customers of the acquired business onto our platform and contract terms, including disparities in the revenue, licensing, support or professional services model of the acquired company; | |
| ● | diversion of management’s attention from other business concerns; | |
| ● | adverse effects to our existing business relationships with business partners and customers as a result of the acquisition; | |
| ● | the potential loss of key employees; | |
| ● | use of resources that are needed in other parts of our business; and | |
| ● | use of substantial portions of our available cash to consummate the acquisition. |
In addition, a significant portion of the purchase price of companies we acquire may be allocated to acquired goodwill and other intangible assets, which must be assessed for impairment at least annually. In the future, if our acquisitions do not yield expected returns, we may be required to take charges to our operating results based on this impairment assessment process, which could adversely affect our results of operations. Acquisitions could also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our operating results. In addition, if an acquired business fails to meet our expectations, our operating results, business and financial position may suffer.
36
Unstable market and economic conditions and adverse developments with respect to financial institutions and associated liquidity risk may have serious adverse consequences on our business, financial condition and stock price.
The global credit and financial markets have recently experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, inflationary pressure and interest rate changes, increases in unemployment rates and uncertainty about economic stability. The financial markets and the global economy may also be adversely affected by the current or anticipated impact of military conflict, terrorism or other geopolitical events. Sanctions imposed by the United States and other countries in response to such conflicts, may also adversely impact the financial markets and the global economy, and any economic countermeasures by the affected countries or others could exacerbate market and economic instability. Moreover, the 2023 closures of Silicon Valley Bank and Signature Bank and their placement into receivership with the Federal Deposit Insurance Corporation (“FDIC”) created bank-specific and broader financial institution liquidity risk and concerns. Although the Department of the Treasury, the Federal Reserve, and the FDIC jointly released a statement that depositors at SVB and Signature Bank would have access to their funds, even those in excess of the standard FDIC insurance limits, under a systemic risk exception, future adverse developments with respect to specific financial institutions or the broader financial services industry may lead to market-wide liquidity shortages, impair the ability of companies to access near-term working capital needs, and create additional market and economic uncertainty. We have significant cash balances at financial institutions which, throughout the year, regularly exceed the federally insured limit of $250,000. Any loss incurred or a lack of access to such funds could have a significant adverse impact on our financial condition, results of operations, and cash flow.
There can be no assurance that future credit and financial market instability and a deterioration in confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, liquidity shortages, volatile business environment or continued unpredictable and unstable market conditions. If the equity and credit markets deteriorate, or if adverse developments are experienced by financial institutions, it may cause short-term liquidity risk and make any necessary debt or equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our financial institutions, manufacturers and other third parties with whom we engage may be adversely affected by the foregoing risks, which may have a material adverse effect on our business.
Future sales and issuances of our securities could result in additional dilution of the percentage ownership of our shareholders and could cause our share price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations, including research and development, increased marketing, hiring new personnel, commercializing our products, and continuing activities as an operating public company. To the extent we raise additional capital by issuing equity securities, our shareholders may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing shareholders, and new investors could gain rights superior to our existing shareholders.
We do not intend to pay cash dividends on our shares of common stock so any returns will be limited to the value of our shares.
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors that our board of directors deems relevant. Therefore, any return to shareholders will be limited to the increase, if any, of our share price.
We are a “smaller reporting company”, and the reduced disclosure requirements applicable to smaller reporting companies may make our common stock less attractive to investors.
We are a “smaller reporting company” as defined in Rule 12b-2 under the Exchange Act. We would cease to be a smaller reporting company if (i) we have a public float of $250 million or more and have annual revenues in excess of $100 million or (ii) if we have a public float of $700 million or more, determined on an annual basis.
37
As a smaller reporting company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not smaller reporting companies. These exemptions include:
| ● | not being required to furnish a stock performance graph in our annual report; |
| ● | reduced disclosure obligations regarding executive compensation; |
| ● | being permitted to provide only two years of audited financial statements in our Annual Report on Form 10-K, with corresponding reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; and |
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. |
We cannot predict whether investors will find our common stock less attractive as a result of any reliance by us on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We may be at risk of securities class action litigation.
We may be at risk of securities class action litigation. In the past, biotechnology and pharmaceutical companies have experienced significant stock price volatility, particularly when associated with binary events such as clinical trials and product approvals. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business and result in a decline in the market price of our common stock.
We are currently listed on The Nasdaq Capital Market (“Nasdaq”). If we are unable to maintain listing of our securities on Nasdaq or any stock exchange, our stock price could be adversely affected and the liquidity of our stock and our ability to obtain financing could be impaired and it may be more difficult for our shareholders to sell their securities.
Although our common stock is currently listed on Nasdaq and we are in compliance with the exchange’s minimum listing requirement, we may not be able to continue to meet Nasdaq’s minimum listing requirements or those of any other national exchange. The Listing Rules of Nasdaq require listing issuers to comply with certain standards in order to remain listed on its exchange. If, for any reason, we should fail to maintain compliance with these listing standards and Nasdaq should delist our securities from trading on its exchange and we are unable to obtain listing on another national securities exchange, a reduction in some or all of the following may occur, each of which could have a material adverse effect on our shareholders:
| ● | the liquidity of our common stock; | |
| ● | the market price of our common stock; | |
| ● | our ability to obtain financing for the continuation of our operations; | |
| ● | the number of investors that will consider investing in our common stock; | |
| ● | the number of market makers in our common stock; | |
| ● | the availability of information concerning the trading prices and volume of our common stock; and | |
| ● | the number of broker-dealers willing to execute trades in shares of our common stock. |
Our Articles of Incorporation, as amended (“Articles of Incorporation”), our Amended and Restated Bylaws, and Nevada law may have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price to decline.
Our Articles of Incorporation, Amended and Restated Bylaws, and Nevada law could make it more difficult for a third-party to acquire us, even if closing such a transaction would be beneficial to our shareholders. We are authorized to issue up to 10,000,000 shares of preferred stock, none of which are outstanding as of March 28, 2025. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by shareholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. As of March 28, 2025, 5,000,000 shares of our preferred stock have been designated as Series A Preferred Stock of which 3,102,480 shares of Series A Preferred Stock were previously issued and converted into common stock at the time of our initial public offering and 1,897,520 shares of Series A Preferred Stock remain authorized. As of March 28, 2025, 2,000,000 shares of our preferred stock have been designated as Series B Preferred Stock of which 2,000,000 shares of Series B Preferred Stock were previously issued and redeemed. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third-party and thereby preserve control by the present management.
38
Provisions of our Articles of Incorporation, our Amended and Restated Bylaws and Nevada law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a shareholder might consider favorable. Such provisions may also prevent or frustrate attempts by our shareholders to replace or remove our management. In particular, the Articles of Incorporation, our Amended and Restated Bylaws and Nevada law, as applicable, among other things:
| ● | provide the board of directors with the ability to alter the Amended and Restated Bylaws without shareholder approval; | |
| ● | place limitations on the removal of directors; |
| ● | establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at shareholder meetings; and | |
| ● | provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum. |
Our Amended and Restated Bylaws provide that the Eighth Judicial District Court of Clark County, Nevada will be the sole and exclusive forum for certain disputes which could limit shareholders’ ability to obtain a favorable judicial forum for disputes with us or its directors, officers, employees or agents.
Our Amended and Restated Bylaws provide that unless we consent in writing to the selection of an alternative forum, the Eighth Judicial District Court of Clark County, Nevada shall be the sole and exclusive forum for state law claims with respect to: (i) any derivative action or proceeding brought in the name or right of us or on our behalf, (ii) any action asserting a claim for breach of any fiduciary duty owed by any director, officer, employee or agent to us or our shareholders, (iii) any action arising or asserting a claim arising pursuant to any provision of Nevada Revised Statutes Chapters 78 or 92A or any provision of our Articles of Incorporation or Amended and Restated Bylaws or (iv) any action asserting a claim governed by the internal affairs doctrine, including, without limitation, any action to interpret, apply, enforce or determine the validity of our Articles of Incorporation or Amended and Restated Bylaws. This exclusive forum provision would not apply to suits brought to enforce any liability or duty created by the Securities Act or the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. To the extent that any such claims may be based upon federal law claims, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
This choice of forum provision may limit a shareholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, other employees or agents and may result in increased costs to our shareholders, which may discourage such lawsuits against us and our directors, officers, other employees and agents. Alternatively, if a court were to find the choice of forum provision contained in our Amended and Restated Bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could have a material adverse effect on our business, results of operations, and financial condition.
General Risk Factors
If securities or industry analysts do not publish research or reports, or publish unfavorable research or reports about our business, our stock price and trading volume may decline.
The trading market for our common stock will rely in part on the research and reports that industry or financial analysts publish about us, our business, our markets and our competitors. We do not control these analysts. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of our common stock. Furthermore, if one or more of the analysts who do cover us downgrade our stock or if those analysts issue other unfavorable commentary about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our stock could decrease, which in turn could cause our stock price or trading volume to decline and may also impair our ability to expand our business with existing customers and attract new customers.
Financial reporting obligations of being a public company in the United States are expensive and time-consuming, and our management will be required to devote substantial time to compliance matters.
As a publicly traded company we incur significant legal, accounting and other expenses. The obligations of being a public company in the United States require significant expenditures and places significant demands on our management and other personnel, including costs resulting from public company reporting obligations under the Exchange Act and the rules and regulations regarding corporate governance practices, including those under Sarbanes-Oxley, the Dodd-Frank Wall Street Reform and Consumer Protection Act, and the listing requirements of Nasdaq. These rules require the establishment and maintenance of effective disclosure and financial controls and procedures, internal control over financial reporting and changes in corporate governance practices, among many other complex rules that are often difficult to implement, monitor and maintain compliance with. Moreover, despite reforms made possible by the JOBS Act, the reporting requirements, rules, and regulations will make some activities more time-consuming and costly, since we are no longer an “emerging growth company.” Our management and other personnel will need to devote a substantial amount of time to ensure that we comply with all of these requirements and to keep pace with new regulations, otherwise we may fall out of compliance and risk becoming subject to litigation or being delisted, among other potential problems.
39
We identified a material weakness in our internal control over financial reporting, which resulted in the restatement of our consolidated financial statements for several prior annual and quarterly and year-to-date periods. If remediation of this material weakness is not effective, or if we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately or timely report our financial condition or operating results, which may adversely affect investor confidence in our company and, as a result, the value of our common stock.
We identified a material weakness in our internal control over financial reporting as of March 21, 2025. As defined in the standards established by the U.S. Public Company Accounting Oversight Board, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness identified related to the proper classification of research and development expenses, which impacted our previously issued consolidated financial statements and condensed consolidated financial statements as of and for the years ended December 31, 2023, 2022 and 2021, and for each of the quarterly and year to date periods ended March 31, 2024 and 2023, June 30, 2024 and 2023, and September 30, 2024 and 2023. As further described in Note 8 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K, there were material amounts inappropriately classified as research and development expense which should have been classified as prepaid assets and other assets. We are taking steps to remediate the material weakness and are in the process of supplementing our existing internal controls related to the proper classification of research and development expenses. In response to the material weakness, we are enhancing our review procedures over significant contracts with contract manufacturing organizations and contract research organizations, augmenting existing staff and strengthening our review process. The incremental internal controls created to respond to this material weakness are being integrated into our internal controls testing plan and they will be tested during 2025 and beyond.
Although we plan to complete the above remediation process and associated evaluation and testing as quickly as possible, we may not be able to do so and our initiatives may prove not to be successful. If our remedial measures are insufficient to address the material weakness, or if additional material weaknesses or significant deficiencies in our internal control over financial reporting are discovered during the evaluation and testing process, we will be unable to assert that our internal control over financial reporting is effective and our independent registered public accounting firm will be unable to express an opinion on the effectiveness of our internal control. If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
The restatement of our prior quarterly financial statements may affect investor confidence and raise reputational issues and may subject us to additional risks and uncertainties, including increased professional costs and the increased possibility of legal proceedings and regulatory inquiries.
As discussed in Note 8 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K, we determined to restate our previously issued audited consolidated financial statements as of and for the years ended December 31, 2023, 2022 and 2021, and our unaudited condensed consolidated financial statements as of and for the years ended December 31, 2023, 2022 and 2021, and for each of the quarterly and year to date periods ended March 31, 2024 and 2023, June 30, 2024 and 2023, and September 30, 2024 and 2023, after we identified material amounts inappropriately classified as research and development expense which should have been classified as prepaid assets and other assets. As a result of this error and the resulting restatement of our consolidated financial statements and condensed consolidated financial statements for the impacted periods, we have incurred, and may continue to incur, unanticipated costs for accounting and legal fees in connection with or related to the restatement and have become subject to a number of additional risks and uncertainties, including the increased possibility of litigation and regulatory inquiries. Any of the foregoing may affect investor confidence in the accuracy of our financial disclosures and may raise reputational risks for our business, both of which could harm our business and financial results.
Failure to maintain effective internal controls could cause our investors to lose confidence in us and adversely affect the market price of our common stock. If our internal controls are not effective, we may not be able to accurately report our financial results or prevent fraud.
Section 404 of Sarbanes-Oxley requires annual management assessments of the effectiveness of our internal controls over financial reporting. If we fail to comply with the rules under Sarbanes-Oxley related to disclosure controls and procedures in the future, or, if we discover material weaknesses and other deficiencies in our internal controls over financial reporting, our stock price could decline significantly and raising capital could be more difficult. If material weaknesses or significant deficiencies are discovered or if we otherwise fail to achieve and maintain the adequacy of our internal controls, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of Sarbanes-Oxley. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important to prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could drop significantly.
40
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 1C. CYBERSECURITY
We operate in the biotechnology sector, which is subject to various cybersecurity risks that could adversely affect our business, financial condition, and results of operations, including intellectual property theft; fraud; extortion; harm to customers and/or corporate partners or customers; violation of privacy laws and other litigation and legal risk; and reputational risk.
Risk management is further managed through the use of an expert third party company to assist in managing relevant risks. We outsource our monitoring to a third-party provider whereby we benefit from a professionally managed network monitoring, management, maintenance, detection and response system and a 24/7 security operations center with both onsite and remote support services. Any cybersecurity incident would be reported to us promptly by our third-party provider and material and potentially material incidents would be assessed by management for remediation and future prevention and detection.
Our business depends on the availability, reliability, and security of our information systems, networks, data, and intellectual property. Any disruption, compromise, or breach of our network systems or infrastructure due to a cybersecurity threat or incident could adversely affect our operations, services, product development, and competitive position. They may also result in a breach of our contractual obligations or legal duties to protect the privacy and confidentiality of our customers’ and partners’ proprietary information, which may include personally identifiable information. Such a breach could expose us to business interruption, lost revenue, ransom payments, remediation costs, liabilities to affected parties, cybersecurity protection costs, lost assets, litigation, regulatory scrutiny and actions, reputational harm, customer dissatisfaction and harm to our relationships with corporate partners.
ITEM 2. PROPERTIES
Our executive office is located at 1177 Avenue of the Americas, 5th Floor, Suite 5066, New York, New York 10036. We currently lease such office for approximately $2,700 per month pursuant to a lease which terminates on February 28, 2026. We lease an additional office located at 720 Monroe Street, #E514, Hoboken, NJ 07030 for approximately $1,850 per month pursuant to a lease which expires on December 31, 2025. We believe that our existing facilities are suitable and adequate to meet our current needs. We intend to add new facilities or expand existing facilities as we add employees, and we believe that suitable additional or substitute space will be available as needed to accommodate any such expansion of our operations.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we may become involved in various lawsuits and legal proceedings, which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
41
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
On February 15, 2019, our common stock began trading on The Nasdaq Capital Market under the symbol “HOTH.” Prior to that time, there was no public market for our common stock.
Shareholders
As of March 28, 2025, there were 101 shareholders of record of our common stock. The actual number of holders of our common stock is greater than this number of record holders, and includes shareholders who are beneficial owners, but whose shares are held in street name by brokers or held by other nominees. This number of holders of record also does not include shareholders whose shares may be held in trust by other entities.
Dividend Policy
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development, operation and expansion of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors that our board of directors deems relevant.
Recent Sales of Unregistered Securities
None.
Issuers Purchases of Equity Securities
None.
ITEM 6. [RESERVED]
42
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing elsewhere in this Annual Report on Form 10-K. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this Annual Report on Form 10-K. All amounts in this report are in U.S. dollars, unless otherwise noted.
Overview
We are a clinical-stage biopharmaceutical company focused on developing new generation therapies for unmet medical needs. We are focused on developing (i) a topical formulation for treating side effects from drugs used for the treatment of cancer (HT-001); (ii) a treatment for mast-cell derived cancers and anaphylaxis (HT-KIT); and (iii) a treatment and/or prevention for Alzheimer’s or other neuroinflammatory diseases (HT-ALZ). We also have assets being developed for (i) atopic dermatitis (also known as eczema) (BioLexa); (ii) a treatment for asthma and allergies using inhalational administration (HT-004); and (iii) a treatment for obesity, and obesity-related diseases and conditions (HT-VA).
Results of Operations
Comparison of Our Results of Operations for the Years Ended December 31, 2024 and 2023
Operating Costs and Expenses
Research and Development Expenses
For the year ended December 31, 2024, research and development expenses were approximately $3.2 million. Specifically, during the year ended December 31, 2024, our research and development costs consisted primarily of the following costs for each of our key research and development projects: (i) HT-001, approximately $2.1 million related to manufacturing and clinical activities; (ii) HT-KIT, approximately $0.6 million related to manufacturing and preclinical activities; (iii) HT-ALZ, approximately $0.2 million related to preclinical studies; and (iv) HT-004, approximately $0.1 million related to sponsored research. In addition to the foregoing, we also incurred fees of approximately $0.2 million payable to members of our scientific advisory board for services.
For the year ended December 31, 2023, research and development expenses were approximately $3.9 million. Specifically, during the year ended December 31, 2023, our research and development costs consisted primarily of the following costs for each of our key research and development projects: (i) HT-001, approximately $2.0 million related to manufacturing and clinical activities; (ii) HT-KIT, approximately $1.6 million related to manufacturing and preclinical activities; (iii) HT-ALZ, approximately $65,000 related to preclinical studies; (iv) BioLexa, approximately $56,000 related to manufacturing; and (v) HT-004, approximately $59,000 related to sponsored research. In addition to the foregoing, we also incurred fees of approximately $0.2 million payable to members of our scientific advisory board for services.
We expect our research and development activities to increase as we develop our existing product candidates and potentially acquire new product candidates, reflecting increasing costs associated with the following:
| ● | employee-related expenses, which include salaries and benefits, and rent expenses; |
| ● | fees related to in-licensed products and technology; |
| ● | expenses incurred under agreements with CROs, investigative sites and consultants that conduct our clinical trials and a substantial portion of our pre-clinical activities; |
| ● | the cost of acquiring and manufacturing clinical trial materials; and |
| ● | costs associated with non-clinical activities and regulatory approvals. |
43
General and Administrative Expenses
For the year ended December 31, 2024, general and administrative expenses amounted to approximately $5.0 million as compared to $4.2 million for the year ended December 31, 2023, an increase of $0.8 million, or 17.9%. For the years ended December 31, 2024 and 2023, general and administrative expenses consisted of the following (rounded to the nearest $1,000):
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Compensation and related expenses | $ | 2,251,000 | $ | 1,570,000 | ||||
| Professional and consulting expenses | 1,902,000 | 2,089,000 | ||||||
| Rent expense | 53,000 | 39,000 | ||||||
| Other general and administrative expenses | 760,000 | 514,000 | ||||||
| Total | $ | 4,966,000 | $ | 4,212,000 | ||||
During the year ended December 31, 2024, the increase in general and administrative expenses of approximately $754,000 was primarily attributed to an increase other general and administrative expenses of approximately $246,000, which primarily consisted of an increase in conference fees of approximately $154,000, and an increase in travel expenses of approximately $32,000, an increase in compensation and related expenses of approximately $681,000, comprising of an increase in stock-based compensation of approximately $612,000 related to the issuance of stock options to executives and board of director members and an increase in health insurance, and an increase in rent of approximately $14,000, offset by a decrease in professional and consulting expenses of approximately $187,000.
We anticipate that our general and administrative expenses will increase in future periods, reflecting continued and increasing costs associated with:
| ● | support of our research and development activities; |
| ● | stock compensation granted to key employees and non-employees; |
| ● | support of business development activities; and |
| ● | increased professional fees and other costs associated with regulatory requirements that we are subject to. |
Other Income (Expenses), net
For the year ended December 31, 2024, net other income was approximately $27,000, which primarily resulted from $27,000 of dividend and interest income.
For the year ended December 31, 2023, net other expenses were approximately $0.1 million, which primarily resulted from $0.2 million of unrealized losses on marketable securities, partially offset by approximately $0.1 million of dividend income.
Net Loss
For the year ended December 31, 2024 and 2023, we incurred a net loss of approximately $8.2 million, or $1.28 per common share (basic and diluted), and $8.1 million, or $2.38 per common share (basic and diluted), respectively.
Liquidity and Capital Resources
To date we have funded our operations primarily through the sale of equity and debt securities. As of December 31, 2024, we had approximately $7.0 million in cash and cash equivalents, working capital of approximately $6.8 million and an accumulated deficit of approximately $60.4 million. Net cash used in operating activities was $7.0 million and $8.4 million for the years ended December 31, 2024 and 2023, respectively. We incurred net losses of approximately $8.2 million and $8.1 million for the years ended December 31, 2024 and 2023, respectively. We have incurred substantial operating losses since inception and expect to continue to incur significant operating losses for the foreseeable future as we continue our pre-clinical and clinical development of our product candidates. We have not yet commercialized any products and have never generated any revenue from product sales. We believe that our existing cash as of December 31, 2024 plus cash proceeds we received of $5,625,000 from exercise of warrants in January 2025 and cash proceeds we received of $1,470,435 from the sale of our common shares under the ATM Agreement during the period from January 7, 2025 to March 28, 2025 will enable us to fund our operating expenses and capital expenditure requirements for at least 12 months from the date that our audited financial statements are available to be issued.
44
During the year ended December 31, 2024, we issued 2,500,000 shares (the “Warrant Shares”) of our common stock upon the exercise of the 2,500,000 January 2023 Existing Warrants (as defined herein) for net proceeds of approximately $3.7 million, after deducting placement agent fees and other offering expenses of approximately $0.4 million. The Warrant Shares were issued as a result of a March 27, 2024 inducement offer agreement, which closed on April 1, 2024, with a holder (the “Holder”) of certain of our existing warrants (“January 2023 Existing Warrants”) to immediately exercise, for cash, an aggregate of 2,500,000 January 2023 Existing Warrants to purchase shares of our common stock at a reduced exercise price of $1.6775 per share.
On November 8, 2024, we entered into an At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”) under which we may offer and sell shares of our common stock having an aggregate sales price of up to $2,700,000 through Wainwright as the sales manager pursuant to our effective shelf registration statement on Form S-3, including an accompanying prospectus (File No. 333-272620), and a prospectus supplement dated November 8, 2024. Sales of shares of the Company’s common stock through Wainwright, if any, will be made by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415(a)(4) under the Securities Act of 1933, as amended. Wainwright will use commercially reasonable efforts to sell shares of the Company’s common stock from time to time, based on instructions from us (including any price, time or size limits or other parameters or conditions we may impose). We will pay Wainwright a commission equal to 3.0% of the aggregate gross proceeds from the sales of shares of the Company’s common stock sold through Wainwright under the ATM Agreement and will also reimburse Wainwright for certain specified expenses in connection with the ATM Agreement. The offering of shares pursuant to the ATM Agreement will terminate on the earlier of (1) the sale, pursuant to the ATM Agreement, of shares having an aggregate offering price of $2,700,000 and (2) the termination of the ATM Agreement by either us or Wainwright, as set forth therein. From November 8, 2024 to December 31, 2024 we issued 1,137,250 shares of our common stock for net proceeds of approximately $1.0 million pursuant to the ATM Agreement.
We have entered into certain license, sublicense, sponsored research and option agreements with third parties. Pursuant to such agreements, we may be required to make certain: (i) license maintenance fee payments; (ii) out-of-pocket expense payments, including, but not limited to, payments related to intellectual property and research related expenses; (iii) development and commercialization expense payments; (iv) annual and quarterly minimum payments; (v) diligence expense payments; and (vi) revenue interest payments. In addition, subject to the achievement of certain development and/or commercialization events, we may also be required to make certain: (i) minimum royalty payments, ranging from middle to high five figures, (ii) sales-based royalties and running royalties, ranging from low single digits to low double digits; and (iii) milestone payments, of up to approximately $30 million (if all milestones in all of our current agreements are achieved).
Additional funding will be necessary to fund our future clinical and pre-clinical activities. We may obtain additional financing through sales of our equity and debt securities or entering into strategic partnership arrangements, or a combination of the foregoing. There are no assurances that we will be successful in obtaining an adequate level of financing as and when needed to finance our operations on terms acceptable to us or at all, particularly in light of the economic downturn. If we are unable to secure adequate additional funding as and when needed, we may have to significantly delay, scale back or discontinue the development and commercialization of one or more of our product candidates.
Cash Flows from Operating Activities
For the year ended December 31, 2024, net cash used in operating activities was approximately $7.0 million, which primarily resulted from a net loss of approximately $8.2 million, offset by $0.8 million in stock-based compensation, a decrease in prepaid expense of $0.2 million and an increase in accounts payable and accrued expenses of $0.2 million.
For the year ended December 31, 2023, net cash used in operating activities was approximately $8.4 million, which primarily resulted from a net loss of approximately $8.1 million, a $0.3 million gain on termination of license agreement, offset by $0.2 million unrealized loss on marketable securities, $0.2 million stock-based compensation and changes in operating assets and liabilities of approximately $0.5 million.
Cash Flows from Investing Activities
The Company did not have any cash flows from investing activities for the years ended December 31, 2024 or December 31, 2023.
Cash Flows from Financing Activities
For the year ended December 31, 2024, net cash provided by financing activities was approximately $4.7 million, which primarily resulted from net proceeds from the issuance of common stock, common stock warrants, and prefunded warrants of $1.0 million and proceeds from the exercise of warrants of approximately $3.7 million.
For the year ended December 31, 2023, net cash provided by financing activities was approximately $11.3 million, which primarily resulted from net proceeds from the issuance of common stock, common stock warrants, and prefunded warrants.
45
Our ultimate success is dependent on our ability to obtain additional financing and generate sufficient cash flow to meet our obligations on a timely basis. We will require significant amounts of capital to sustain operations, and we will need to make the investments we need to execute our longer-term business plan to support new technologies and help advance innovation. Absent generation of sufficient revenue from the execution of our long-term business plan, we will need to obtain debt or equity financing, especially if we experience downturns in our business that are more severe or longer than anticipated, or if we experience significant increases in expense levels resulting from being a publicly traded company or from operations. Such additional debt or equity financing may not be available to us on favorable terms, if at all.
We plan to pursue our plans with respect to the research and development of our pre-clinical products which will require resources beyond those that we currently have, ultimately requiring additional capital from third-party sources. We currently do not expect to generate any revenue.
Critical Accounting Estimates
The preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts and related disclosures in the financial statements. Management considers an accounting estimate to be critical if:
| ● | it requires assumptions to be made that were uncertain at the time the estimate was made; and |
| ● | changes in the estimate or different estimates that could have been selected could have a material impact in our results of operations or financial condition. |
While we base our estimates and judgments on our experience and on various other factors that we believe to be reasonable under the circumstances, actual results could differ from those estimates and the differences could be material.
See Note 2 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K for an additional discussion of our significant accounting policies.
Stock-based compensation
The Company accounts for stock-based payment awards exchanged for services at the estimated grant date fair value of the award. Stock options issued under the Company’s long-term incentive plans are granted with an exercise price equal to no less than the market price of the Company’s stock at the date of grant and expire up to ten years from the date of grant. Options are generally issued fully vested. The Company accounts for forfeited awards as they occur.
The Company estimates the fair value of stock option grants using the Black-Scholes option pricing model and the assumptions used in calculating the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment.
Expected Term - The expected term of options represents the period that the Company’s stock-based awards are expected to be outstanding based on the simplified method, which is the half-life from vesting to the end of its contractual term.
Expected Volatility - The Company computes stock price volatility over expected terms based on its historical common stock trading prices.
Risk-Free Interest Rate - The Company bases the risk-free interest rate on the implied yield available on U.S. Treasury zero-coupon issues with an equivalent remaining term.
Expected Dividend - The Company has never declared or paid any cash dividends on its common shares and does not plan to pay cash dividends in the foreseeable future, and, therefore, uses an expected dividend yield of zero in its valuation models.
46
The Company grants restricted stock awards under its equity incentive plan. Restricted stock awards are granted to employees and non-employees. The restricted stock awards are measured based on the grant-date fair value. In general, the restricted stock awards vest over a service period of zero to three years. Stock-based compensation expense is generally recognized based on the straight-line basis over the requisite service period and forfeitures are accounted for as they occur.
The Company has issued warrants to non-employees. The warrants are measured based on the grant-date fair value. In general, the warrants vest over a term of zero to ten years. Stock-based compensation expense is generally recognized based on the straight-line basis over the vesting term.
Income taxes
Income taxes are recorded in accordance with Accounting Standards Codification (“ASC”) 740, Income Taxes (“ASC 740”) which provides for deferred taxes using an asset and liability approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of events that have been included in our consolidated financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between our financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Valuation allowances are provided, if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
We account for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, we recognize the tax benefit of tax positions to the extent that the benefit would more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances.
Recently Issued Accounting Standards Not Yet Effective or Adopted
Income Taxes (Topic 740)
In December 2023, the Financial Accounting Standards Board (“FASB”) issued guidance within Accounting Standards Update (“ASU”) 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The amendments in the ASU are intended to provide more transparency about income tax information through improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. The ASU requires disclosure in the rate reconciliation of specific categories as well as additional information for reconciling items that meet a quantitative threshold.
The ASU requires disclosure of the following information about income taxes paid on an annual basis:
| ● | Income taxes paid (net of refunds received), disaggregated by federal and state taxes and by individual jurisdictions in which income taxes paid (net of refunds received) is equal to or greater than five percent of total income taxes paid (net of refunds received). |
| ● | Income tax expense (or benefit) from continuing operations disaggregated by federal and state jurisdictions. |
The ASU is effective for annual periods beginning after December 15, 2024. The amendments should be applied on a prospective basis. The Company is evaluating the impact that the adoption of this ASU will have on the Company’s consolidated financial statements, as it may require additional disclosures in the notes to our condensed consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
The Company is not required to provide the information required by this Item as it is a “smaller reporting company,” as defined in Rule 12b-2 of the Exchange Act.
47
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Hoth
Therapeutics, Inc.
Consolidated Financial Statements
TABLE OF CONTENTS
F-1
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of
Hoth Therapeutics, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Hoth Therapeutics, Inc. (the “Company”) as of December 31, 2024 and 2023, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Restatement of Prior Period Consolidated Financial Statements and the Unaudited Interim Condensed Consolidated Financial Statements
As discussed in Note 8 to the consolidated financial statements, the accompanying 2023 consolidated financial statements have been restated to correct certain misstatements. Additionally, the Company has restated its unaudited interim condensed consolidated financial statements previously reported in the Forms 10-Q for the quarters ended March 31, 2023 and 2024, June 30, 2023 and 2024, and September 30, 2023 and 2024, as these interim periods were also affected by the errors. Our opinion is not modified with respect to the restatements.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Research and Development Expenses, Accrued Clinical Trial Liabilities, and Prepaid Research and Development Costs
F-2
Description of the Matter
The Company recognizes research and development expenses as incurred. Advance payments for future research and development activities are deferred and expensed as the related services are performed. The Company recognizes its clinical trial expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations (collectively, "CROs") that conduct and manage clinical trials on the Company's behalf.
The Company works closely with its CROs to reconcile prepaid research and development costs and accrued clinical trial prepaid expenses and liabilities by obtaining reporting from the CROs, discussing progress or stage of completion of services with internal personnel and external service providers, and comparing this information to payments made, invoices received, and the agreed-upon fees to be paid for such services in the applicable contract, statements of work, or purchase orders. The reconciliation of the amount of work completed is primarily based on the status and timing of services performed and the completion of project milestones.
We identified research and development expenses, accrued clinical trial liabilities, and prepaid research and development costs as a critical audit matter given the estimation involved in accounting for research and development expenses, accrued clinical trial liabilities, and prepaid research and development costs. In addition, as described in Note 8 to the consolidated financial statements, the Company identified errors in the accounting for advance payments to a clinical research organization (CRO) during the 2024 audit which arose from expensing advance payments in full upon payment rather than recording them as prepaid expenses and recognizing the expense as services were performed. The errors led to a material overstatement of research and development (R&D) expenses and an understatement of prepaid expenses in the affected periods. This required extensive audit effort related to the estimation of research and development expenses, accrued clinical trial liabilities and prepaid clinical expenses and the complexity involved in determining the completeness and accuracy of the restated annual and interim financial data.
How We Addressed the Matter in Our Audit
Our audit procedures related to research and development expenses, accrued clinical trial liabilities, and prepaid clinical expenses included selecting a sample of amounts recognized as research and development expense, accrued clinical trial liabilities and prepaid research and development expenses and performing the following procedures for each item selected:
| ● | We obtained and read related master service agreements, statements of work, purchase orders and/or other supporting agreements with the CROs. |
| ● | We performed corroborating inquiries with the Company's operations personnel responsible for the oversight of activities regarding the nature and status of work performed under the various CRO agreements. |
| ● | We inspected evidence from the third-party vendors regarding the payments made and the status and timing of services performed. In addition, we obtained confirmations from selected CROs related to billings incurred, balances due, work performed, and remaining advance balances. |
| ● | We compared the data and evidence obtained from internal and external sources to the inputs used in the Company's analysis and recalculated the related research and development expense, prepaid research and development expense, and the accrued clinical liabilities balance. |
| ● | We evaluated the Company’s process for identifying and correcting the prior period errors by testing the restated annual and quarterly amounts, including agreeing the corrected balances to underlying CRO contracts, payment records, and service performance timelines. |
/S/
We have served as the Company’s auditor since 2018.
March 28, 2025
PCAOB
ID No.
F-3
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2024 | 2023 | |||||||
| (As Restated) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Prepaid expenses and other current assets | ||||||||
| Total Current Assets | ||||||||
| NON-CURRENT ASSETS: | ||||||||
| Operating lease right-of-use asset, net | ||||||||
| Investment in joint ventures at fair value | ||||||||
| Total Non-Current Assets | ||||||||
| Total Assets | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Operating lease liability, current portion | ||||||||
| Total Current Liabilities | ||||||||
| LONG-TERM LIABILITIES: | ||||||||
| Operating lease liability, less current portion | ||||||||
| Total Long-Term Liabilities | ||||||||
| Total Liabilities | ||||||||
| Commitments and Contingencies (Note 6) | ||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||
| Preferred stock, $ | ||||||||
| Series A Convertible Preferred Stock, $ | ||||||||
| Series B Preferred Stock, $ | ||||||||
| Common stock, $ | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive income | ||||||||
| Total Stockholders’ Equity | ||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| For the Year Ended | ||||||||
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| (As Restated) | ||||||||
| NET REVENUES | $ | $ | ||||||
| OPERATING COSTS AND EXPENSES: | ||||||||
| Research and development expense | ||||||||
| General and administrative expenses | ||||||||
| Total operating expenses | ||||||||
| LOSS FROM OPERATIONS | ( |
) | ( |
) | ||||
| OTHER INCOME (EXPENSES), NET: | ||||||||
| Unrealized loss on marketable securities | ( |
) | ||||||
| Change in fair value of investment in joint venture | ( |
) | ||||||
| Dividend and interest income | ||||||||
| Total other income (expenses), net | ( |
) | ||||||
| NET LOSS | $ | ( |
) | $ | ( |
) | ||
| NET LOSS PER COMMON SHARE: | ||||||||
| Basic and diluted | $ | ( |
) | $ | ( |
) | ||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | ||||||||
| Basic and diluted | ||||||||
| COMPREHENSIVE LOSS: | ||||||||
| Net loss | $ | ( |
) | $ | ( |
) | ||
| Other comprehensive (loss) income: | ||||||||
| Foreign currency translation adjustment | ( |
) | ||||||
| Total comprehensive loss | $ | ( |
) | $ | ( |
) | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2024 AND 2023
| Accumulated | ||||||||||||||||||||||||
| Common Stock | Additional Paid-in | Accumulated Deficit | other Comprehensive | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | (As Restated) | Income (Loss) | Equity | |||||||||||||||||||
| Balance, December 31, 2022 (As Restated) | $ | $ | $ | ( | ) | $ | $ | 6,105,147 | ||||||||||||||||
| Exercise of warrants | 2,355 | |||||||||||||||||||||||
| Stock-based compensation | - | 216,428 | ||||||||||||||||||||||
| Common stock and warrants issued in private placement, net of offering costs | 11,314,998 | |||||||||||||||||||||||
| Vesting of restricted shares | - | |||||||||||||||||||||||
| Cumulative translation adjustment | - | 5,254 | ||||||||||||||||||||||
| Net loss | - | ( | ) | (8,106,122 | ) | |||||||||||||||||||
| Balance, December 31, 2023 (As Restated) | ( | ) | 9,538,060 | |||||||||||||||||||||
| Exercise of pre-funded warrants | ( | ) | - | |||||||||||||||||||||
| Stock-based compensation | - | 804,277 | ||||||||||||||||||||||
| Common shares issued for exercise of warrants, net of issuance costs | 3,682,300 | |||||||||||||||||||||||
| Common stock issued for cash, net | 1,060,719 | |||||||||||||||||||||||
| Vesting of restricted shares | - | |||||||||||||||||||||||
| Cumulative translation adjustment | - | ( | ) | (18,197 | ) | |||||||||||||||||||
| Net loss | - | ( | ) | (8,188,300 | ) | |||||||||||||||||||
| Balance, December 31, 2024 | $ | $ | $ | ( | ) | $ | $ | 6,878,859 | ||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Year Ended | ||||||||
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| (As Restated) | ||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Gain on termination of license agreement | ( | ) | ||||||
| Stock-based compensation | ||||||||
| Unrealized loss on marketable securities | ||||||||
| Change in fair value of investment in joint ventures | ( | ) | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses | ||||||||
| Accounts payable and accrued expenses | ( | ) | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance of common stock, common stock warrants and prefunded warrants, net of offering costs | ||||||||
| Proceeds from exercise of warrants, net of issuance costs | ||||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | ||||||||
| NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | ( | ) | ||||||
| Effect of exchange rate changes on cash and cash equivalents | ( | ) | ( | ) | ||||
| CASH AND CASH EQUIVALENTS - beginning of year | ||||||||
| CASH AND CASH EQUIVALENTS - end of year | $ | $ | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| ROU assets obtained in exchange for lease liability | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
NOTE 1 – Organization and Description of Business Operations
Hoth
Therapeutics, Inc. (together with its wholly-owned subsidiaries, merveille.ai and Hoth Therapeutics Australia Pty Ltd, the “Company”)
was incorporated under the laws of the State of Nevada on
Liquidity and Capital Resources
Accounting Standards Update (“ASU”) No. 2014-15, Presentation of Financial Statements - Going Concern, requires management to evaluate the Company’s ability to continue as a going concern one year beyond the filing date of the given financial statements. This evaluation requires management to perform two steps. First, management must evaluate whether there are conditions and events that raise substantial doubt about the entity’s ability to continue as a going concern. Second, if management concludes that substantial doubt is raised, management is required to consider whether it has plans in place to alleviate that doubt. Disclosures in the notes to the consolidated financial statements are required if management concludes that substantial doubt exists or that its plans alleviate the substantial doubt that was raised.
The Company has funded its operations from proceeds from the sale of equity and debt securities. The Company will require significant additional capital to make the investments it needs to execute its longer-term business plan. The Company’s ability to successfully raise sufficient funds through the sale of debt or equity securities when needed is subject to many risks and uncertainties and, even if it were successful, future equity issuances may result in dilution to its existing shareholders and future debt securities may contain covenants that limit the Company’s operations or ability to enter into certain transactions.
The Company believes its current cash is sufficient to fund operations for at least the next 12 months from the issuance date of these financial statements. However, the Company will need to raise additional funding, through strategic relationships, public or private equity or debt financings, grants or other arrangements, to develop and seek regulatory approvals for the Company’s current and future product candidates. If such funding is not available, or not available on terms acceptable to the Company, the Company’s current development plan and plans for expansion of its general and administrative infrastructure may be curtailed.
On November 8, 2024, the Company entered into
an At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”) under
which the Company may offer and sell shares of its common stock having an aggregate sales price of up to $
F-8
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
NOTE 2 – Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The Company’s consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”).
The accompanying consolidated financial statements include the accounts of the Company’s wholly-owned subsidiaries, merveille.ai which was incorporated under the laws of Nevada on October 4, 2023 and Hoth Therapeutics Australia Pty Ltd, which was incorporated under the laws of the State of Victoria in Australia on June 5, 2019. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of expenses during the reporting periods. The most significant estimates in the Company’s consolidated financial statements relate to stock-based compensation, the valuation of modified warrants, and the valuation allowance of deferred tax assets resulting from net operating losses. These estimates and assumptions are based on current facts, historical experience and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially and adversely from these estimates. To the extent there are material differences between the estimates and actual results, the Company’s future results of operations may be affected.
Cash and Cash Equivalents
The
Company considers all highly liquid investments purchased with original maturities of 90 days or less at acquisition to be cash equivalents.
Cash and cash equivalents consist of bank accounts and highly liquid money funds and totaled $
Concentrations of Credit Risk and Off-Balance Sheet Risk
The
Company has significant cash balances at financial institutions which, throughout the year, regularly exceed the federally insured limit
of $
Fair Value of Financial Instruments
Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurements, (“ASC-820”), provides guidance on the development and disclosure of fair value measurements. Under this accounting guidance, fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability.
The fair value of the Company’s assets and liabilities, which would qualify as financial instruments under ASC-Topic 820, approximates the carrying amounts represented in the Company’s consolidated balance sheets, primarily due to their short-term nature.
F-9
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
The accounting guidance classifies fair value measurements in one of the following three categories for disclosure purposes:
| Level 1: | Quoted prices in active markets for identical assets or liabilities. |
| Level 2: | Inputs other than Level 1 prices for similar assets or liabilities that are directly or indirectly observable in the marketplace. |
| Level 3: | Unobservable inputs which are supported by little or no market activity and values determined using pricing models, discounted cash flow methodologies, or similar techniques, as well as instruments for which the determination of fair value requires significant judgment or estimation. |
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement. During the years ended December 31, 2024 and 2023, there were no changes in valuation techniques or transfers between Level 1, Level 2, and Level 3.
Leases
The Company determines if an arrangement is a lease at inception and classifies its leases at commencement. Operating leases are presented as right-of-use (“ROU”) assets and the corresponding lease liabilities are included in operating lease liability, current and lease liability, on the Company’s consolidated balance sheets. ROU assets represent the Company’s right to use an underlying asset, and lease liabilities represent the Company’s obligation to make lease payments in exchange for the ability to use the asset for the duration of the lease term.
The Company has lease agreements which contain both lease and non-lease components, which it has elected to account for as a single lease component. As such, minimum lease payments include fixed payments for non-lease components within a lease agreement but exclude variable lease payments not dependent on an index or rate, such as common area maintenance, operating expenses, utilities, or other costs that are subject to fluctuation from period to period. Certain of the leases contain an option to extend the term of the lease. The option to extend a lease is included in the lease term only when it is reasonably certain that the Company will elect that option. Additionally, the Company does not record ROU assets or lease liabilities for short-term leases that have a term of twelve months or less at lease commencement.
ROU assets and lease liabilities are recognized at the commencement date and determined using the present value of the future minimum lease payments over the lease term. The Company uses an incremental borrowing rate based on an estimated rate of interest for collateralized borrowing since the Company’s leases do not include an implicit interest rate. The estimated incremental borrowing rate considers market data, actual lease economic environment, and the lease term at commencement date.
Investment in Joint Ventures
Ownership
interests in entities for which the Company has significant influence that are not consolidated are accounted for as equity method investments.
SEC Staff Announcement: Accounting for Limited Partnership Investments (codified in ASC 323-30-S99-1) guidance requires the use of the
equity method unless the investor’s interest “is so minor that the limited partner may have virtually no influence over partnership
operating and financial policies.” The SEC staff’s position is that investments in limited partnerships of greater than
F-10
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Prepaid Expenses
As of December 31, 2024 and 2023, prepaid expenses and other current assets consisted of the following:
| As of December 31, | ||||||||
| 2024 | 2023 | |||||||
| Prepaid clinical trial expenses | $ | $ | ||||||
| Prepaid insurance | ||||||||
| R&D credit receivable | ||||||||
| Other prepaid expenses | ||||||||
| $ | $ | |||||||
Accounts Payable
For the year ended December 31, 2023, the Company’s
subsidiary Hoth Therapeutics Australia Pty Ltd, recorded approximately a $
Research and Development Costs
Research and development costs, including acquired in-process research and development expenses for which there is no alternative future use, are expensed as incurred. Advance payments for goods and services that will be used in future research and development activities are accrued and then expensed when the activity has been performed or when the goods have been received rather than when the payment is made.
Stock-Based Compensation
The Company accounts for share-based payment awards exchanged for services at the estimated grant date fair value of the award. Stock options issued under the Company’s long-term incentive plans are granted with an exercise price equal to no less than the market price of the Company’s stock at the date of grant and expire up to ten years from the date of grant. Options are generally issued fully vested. The Company accounts for forfeited awards as they occur.
The Company estimates the fair value of stock option grants using the Black-Scholes option pricing model and the assumptions used in calculating the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment.
Expected Term - The expected term of options represents the period that the Company’s stock-based awards are expected to be outstanding based on the simplified method, which is the half-life from vesting to the end of its contractual term.
Expected Volatility - The Company computes stock price volatility over expected terms based on its historical common stock trading prices.
Risk-Free Interest Rate - The Company bases the risk-free interest rate on the implied yield available on U.S. Treasury zero-coupon issues with an equivalent remaining term.
Expected Dividend - The Company has never declared or paid any cash dividends on its common shares and does not plan to pay cash dividends in the foreseeable future, and, therefore, uses an expected dividend yield of zero in its valuation models.
The Company grants restricted stock awards under its equity incentive plan. Restricted stock awards are granted to employees and non-employees. The restricted stock awards are measured based on the grant-date fair value. In general, the restricted stock awards vest over a service period of zero to three years. Stock-based compensation expense is generally recognized based on the straight-line basis over the requisite service period and forfeitures are accounted for as they occur.
The Company has issued warrants to non-employees. The warrants are measured based on the grant-date fair value. In general, the warrants vest over a term of zero to ten years. Stock-based compensation expense is generally recognized based on the straight-line basis over the vesting term.
Income Taxes
Income taxes are recorded in accordance with ASC 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Valuation allowances are provided, if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit would more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances.
F-11
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Net Loss per Share
Net
loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the period.
Since the Company had a net loss in the periods presented, basic and diluted net loss per common share are the same.
| Year Ended December 31, | ||||||||
| Potentially dilutive securities | 2024 | 2023 | ||||||
| Warrants | ||||||||
| Options | ||||||||
| Non-vested restricted stock awards | ||||||||
| Total | ||||||||
Warrants
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and in accordance with ASC 480, “Distinguishing Liabilities from Equity” (“ASC 480”), and ASC 815, “Derivatives and Hedging” (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued warrants that meet all of the criteria for equity classification, the warrants are recorded as a component of additional paid-in capital at the time of issuance. For issued warrants that do not meet all the criteria for equity classification, the warrants are classified as liability and are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter.
Comprehensive Income (Loss)
Comprehensive income (loss) is composed of net loss and other comprehensive income (loss). During the years ended December 31, 2024 and 2023, other comprehensive (loss) income was attributable to foreign currency translation adjustments.
Foreign Currency
The reporting currency of the Company is the U.S. dollar. For the Company’s subsidiary with non-U.S. dollar functional currencies, assets and liabilities are translated into U.S. dollars at period-end exchange rates. Revenue and expenses are translated at the average exchange rates during the period. Equity transactions are translated using historical exchange rates. The resulting translation adjustments are recorded in accumulated other comprehensive income (loss) as a component of stockholders’ equity. Foreign currency translation adjustments arising from differences in exchange rates from period to period are recorded within "Accumulated other comprehensive income (loss)" in the consolidated balance sheets.
Segment Reporting
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (ASC 280): Improvements to Reportable Segment Disclosures (ASU 2023-07), which improves reportable segment disclosure requirements, primarily through enhanced disclosures about significant segment expenses among other disclosure requirements. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. The Company adopted ASU 2023-07 on January 1, 2024. The Company operates as a single operating segment as a clinical-stage biopharmaceutical company focused on developing new generation therapies for unmet medical needs. In accordance with ASC 280, the Company’s chief operating decision maker has been identified as the Chief Executive Officer, who reviews operating results to make decisions about allocating resources and assessing performance for the entire Company and decides how to allocate resources based on loss from operations, managing cash flows and evaluating research and development and general and administrative expenses. Existing guidance, which is based on a management approach to segment reporting, establishes requirements to report selected segment information quarterly and to report annually entity-wide disclosures about products and services, major customers, and the countries in which the entity holds material assets and reports revenue. All material operating units qualify for aggregation under “Segment Reporting” due to their similarities in economic characteristics such as nature of services and procurement processes. Since the Company operates in one segment, all financial information required by “Segment Reporting” can be found in the accompanying notes to consolidated financial statements.
Recent Accounting Pronouncements
Income Taxes (Topic 740)
In December 2023, the FASB issued guidance within ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The amendments in the ASU are intended to provide more transparency about income tax information through improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. The ASU requires disclosure in the rate reconciliation of specific categories as well as additional information for reconciling items that meet a quantitative threshold.
F-12
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
The ASU requires disclosure of the following information about income taxes paid on an annual basis:
| ● | Income taxes paid (net of refunds received), disaggregated by federal and state taxes and by individual jurisdictions in which income taxes paid (net of refunds received) is equal to or greater than five percent of total income taxes paid (net of refunds received). |
| ● | Income tax expense (or benefit) from continuing operations disaggregated by federal and state jurisdictions. |
The ASU is effective for annual periods beginning after December 15, 2024. The amendments should be applied on a prospective basis. The Company believes the adoption of this ASU will not have any impact on the Company’s consolidated financial statements.
Currently, management does not believe that any other recently issued, but not yet effective accounting pronouncements, if currently adopted, would have a material impact on the Company’s consolidated financial statements.
NOTE 3 – License Agreements
The following summarizes the Company’s research and development expenses for licenses acquired (including stock-based compensation) during the years ended December 31, 2024 and 2023:
| For the Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| The George Washington University | $ | $ | ||||||
| North Carolina State University | ||||||||
| Virginia Commonwealth University | ( | ) | ||||||
| U.S. Department of Veteran Affairs | ||||||||
| University of Cincinnati | ||||||||
| $ | $ | ( | ) | |||||
The George Washington University
During
the year ended December 31, 2024, the Company recorded expenses of $
During the year ended December 31, 2023, the Company
recorded expenses of $
North Carolina State University
During
the year ended December 31, 2024, the Company recorded expenses of $
During the year ended December 31, 2023, the Company did recognize any expenses for license fees associated with such license agreement.
Virginia Commonwealth University
During the year ended December 31, 2024, the Company did recognize any expenses for license fees associated with the exclusive license agreement (the “VCU License Agreement”) by and between the Company and Virginia Commonwealth University (“VCU”) dated May 18, 2020 that was terminated August 16, 2023.
During
the year ended December 31, 2023, the Company recognized a gain of $
F-13
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
U.S. Department of Veteran Affairs
During the year ended December 31, 2024, the
Company recognized expenses of $
During the year ended December 31, 2023, the Company did not recognize any expenses for license fees associated with such license agreement.
Chelexa Biosciences, Inc. and the University of Cincinnati
During
the years ended December 31, 2024 and 2023, the Company recognized expenses of $
NOTE 4 – Fair Value of Financial Assets and Liabilities
The following table presents the Company’s assets and liabilities that are measured at fair value on December 31, 2024 and 2023:
| Fair value measured on December 31, 2024 | ||||||||||||||||
| Total at December 31, 2024 | Quoted prices in active markets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Investment in joint ventures | $ | $ | $ | $ | ||||||||||||
| Fair value measured on December 31, 2023 | ||||||||||||||||
| Total at December 31, 2023 | Quoted prices in active markets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Investment in joint ventures | $ | $ | $ | $ | ||||||||||||
Level 3 Measurement
The following table sets forth a summary of the changes in the fair value of the Company’s Level 3 financial assets that are measured at fair value on a recurring basis for the years ended December 31, 2024 and 2023:
| Investment in joint venture for the year ended December 31, 2024 and 2023 | ||||||||
| For the Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Investment in joint ventures at fair value – beginning of year | $ | $ | ||||||
| Change in fair value of investment in joint ventures | ( | ) | ||||||
| Investment in joint ventures at fair value – end of year | $ | $ | ||||||
F-14
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Investment in Joint Ventures
The Company has elected to measure the investment in joint ventures using the fair value option at each reporting date. Under the fair value option, bifurcation of an embedded derivative is not necessary, and all related gains and losses on the host contract and derivative due to change in the fair value will be reflected in other income (expenses), net in the consolidated statements of operations and comprehensive loss.
The value at which the Company’s investment in joint ventures is carried on its books is adjusted to estimated fair value at the end of each quarter, taking into account general economic and stock market conditions and those characteristics specific to the underlying investments.
Investment in Zylö Therapeutics
In connection with the Company’s March 2020 underwritten public
offering of shares of its common stock, on May 4, 2020, the Company purchased
On
February 23, 2024, the Company acquired
The valuations reflect a probability-weighted present value of expected future investment returns considering certain possible outcomes and the rights of each class of Zylö’s and Atticus Pharma’s equity. The future values of the common stock under the various outcomes are discounted back to the valuation date at a risk-adjusted discount rate and probability weighted to determine the value for the Class B common stock. Significant unobservable inputs in the valuation include: (i) probabilities of each scenario, (ii) timing of occurrence, (iii) future valuation; (iv) and the risk-adjusted discount rate.
The
consolidated investment in Zylö was valued at $
F-15
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
NOTE 5 – Stockholders’ Equity
Preferred Stock
The
Company is authorized to issue up to
Series A Convertible Preferred Stock
The
shares of Series A Convertible Preferred Stock, par value $
Series B Preferred Stock
On November 2, 2022, the Company filed a Certificate
of Designation of the Series B Preferred Stock (the “Certificate of Designation”) with the Secretary of State of the State
of Nevada to create a new class of Series B Preferred Stock, par value $
F-16
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Warrants
2023
On
December 29, 2022, the Company entered into a securities purchase agreement with an accredited investor pursuant to which it sold (i)
The
measurement of fair value of the December Pre-Funded Warrants was determined utilizing a Black-Scholes model considering all relevant
assumptions current at January 3, 2023, the date of issuance (i.e., share price of $
The
measurement of fair value of the December Common Stock Warrants was determined utilizing a Black-Scholes model considering all relevant
assumptions current at January 3, 2023, the date of issuance (i.e., share price of $
As
a result of exercising the December Pre-Funded Warrants on various dates in February 2023, the investor exercised all the December Pre-Funded
Warrants for an aggregate of
In
addition, pursuant to the terms of the offering, the Company issued the designees of the placement agent, Wainwright, warrants to purchase
up to
On
September 13, 2023, the Company entered into a securities purchase agreement with certain institutional investors (the “September
Investors”) pursuant to which it sold (i)
F-17
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
The
measurement of fair value of the September Pre-Funded Warrants was determined utilizing a Black-Scholes model considering all relevant
assumptions current at September 15, 2023, the date of issuance (i.e., share price of $
The
measurement of fair value of the September Common Stock Warrants was determined utilizing a Black-Scholes model considering all relevant
assumptions current at September 15, 2023, the date of issuance (i.e., share price of $
On
various dates in September 2023, the September Investors exercised
In
addition, pursuant to the terms of the September offering, the Company issued designees of the placement agent, Wainwright warrants (the
“September Wainwright Warrants”) to purchase up to
2024
On
January 8, 2024, the Company issued
On
March 27, 2024, the Company entered into an inducement offer agreement with a holder (the “Holder”) of certain of the Company’s
existing warrants (the “January 2023 Existing Warrants”) to immediately exercise for cash an aggregate
As
an inducement to such exercise, the Company agreed to issue new unregistered warrants to purchase up to
F-18
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
The amendment to the January 2023 Existing Warrants on March 27, 2024 to lower the exercise price thereof was considered a modification of the January 2023 Existing Warrants under the guidance of ASU 2021-04. The modification is consistent with the “Equity Issuance” classification under that guidance as the reason for the modification was to induce the holders to cash exercise their warrants, resulting in the exercise of the January 2023 Existing Warrants on April 1, 2024.
On
March 27, 2024, the Company calculated the total fair value of the consideration for the modification of the January 2023 Existing Warrants,
which includes the incremental fair value of the January 2023 Existing Warrants (determined by comparing the fair values immediately
prior to and immediately after the modification). The fair values were calculated using the Black-Scholes option-pricing model, and the
Company determined that the total fair value of the consideration related to the modification of the January 2023 Existing Warrants amounted
to $
On
April 1, 2024, in connection with the March 27, 2024 inducement offer agreement with the Holder of the January 2023 Existing Warrants,
the Holder exercised the January 2023 Existing Warrants for cash at a reduced exercise price of $
On
April 1, 2024, in connection with the issuance of the April 2024 Inducement Warrants and the placement agent warrants, the Company calculated
the fair value of such warrants using the Black-Scholes option-pricing model, and the Company determined that the aggregate total fair
value of the April 2024 Inducement Warrants and placement agent warrants amounted to $
The fair value of the January 2023 Existing Warrants on the modification date and the fair value of the April 2024 Inducement Warrants were estimated using the Black-Scholes option-pricing model with the following assumptions:
| March
27, 2024 to April 1, 2024 | ||||
| Exercise price | $ | |||
| Term (years) | ||||
| Expected stock price volatility | ||||
| Risk-free rate of interest | ||||
F-19
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
A summary of warrant activity for the years ended December 31, 2024 and 2023 is as follows:
| Number of Warrants | Weighted Average Exercise Price | Total Intrinsic Value | Weighted Average Remaining Contractual Life (in years) | |||||||||||||
| Outstanding as of December 31, 2022 | $ | |||||||||||||||
| Issued | — | |||||||||||||||
| Exercised | ( | ) | — | — | ||||||||||||
| Outstanding as of December 31, 2023 | ||||||||||||||||
| Issued | — | — | ||||||||||||||
| Expired | ( | ) | — | — | ||||||||||||
| Exercised | ( | ) | — | — | ||||||||||||
| Outstanding as of December 31, 2024 | ||||||||||||||||
| Warrants exercisable as of December 31, 2024 | $ | $ | ||||||||||||||
The Company has determined that the warrants should be accounted for as a component of stockholders’ equity.
Common Shares
As
a result of exercising the December Pre-Funded Warrants on various dates in February 2023, the investor exercised all the December Pre-Funded
Warrants for an aggregate of
On
September 13, 2023, the Company entered into a securities purchase agreement with certain institutional investors pursuant to which it
sold (i)
On
January 8, 2024, the Company issued
During
the year ended December 31, 2024, the Company issued
On
November 8, 2024, the Company entered into the ATM Agreement with Wainwright under which the Company may offer and sell shares of
its common stock having an aggregate sales price of up to $
F-20
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
2018 Equity Incentive Plan
On
May 4, 2018, the Company’s board of directors adopted the Hoth Therapeutics, Inc. 2018 Equity Incentive Plan (the “2018 Plan”)
initially reserving
The
compensation committee of the board of directors increased the number of shares reserved pursuant to the Company’s 2018 Equity
Incentive Plan (“2018 Plan”) by
2022 Equity Incentive Plan
On
March 24, 2022, the Company’s board of directors adopted the Hoth Therapeutics, Inc. 2022 Omnibus Equity Incentive Plan (the “2022
Plan”) initially reserving
On
June 2, 2023, the Company’s board of directors approved the Hoth Therapeutics, Inc. Amended and Restated 2022 Omnibus Equity Incentive
Plan (the “Amended and Restated 2022 Plan”) which, among other things, increased the number of shares reserved under the
plan by
On
May 15, 2024, the Company’s compensation committee recommended, and the board of directors approved an increase to the number of
shares of common stock reserved for issuance under the Amended and Restated 2022 Plan by
Restricted Stock Awards
A summary of the Company’s restricted stock awards granted under the equity incentive plans during the years ended December 31, 2024 and 2023 is as follows:
| Number of Restricted Stock Awards | Weighted Average Grant Day Fair Value | |||||||
| Nonvested on December 31, 2022 | ||||||||
| Vested | ( | ) | ||||||
| Nonvested on December 31, 2023 | ||||||||
| Vested | ( | ) | ||||||
| Nonvested on December 31, 2024 | ||||||||
F-21
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
During
the years ended December 31, 2024 and 2023, the Company recognized stock-based compensation of $
Stock Options
On July 17, 2023, pursuant to and subject to the available number of
shares reserved under the 2022 Plan, the Company issued an aggregate of
On
January 5, 2024, pursuant to and subject to the available number of shares reserved under the Amended and Restated 2022 Plan, the Company
issued options to the Company’s employees and directors to purchase up to
On
August 19, 2024, pursuant to and subject to the available number of shares reserved under the Amended and Restated 2022 Plan, the Company
issued options to the Company’s employees and directors to purchase up to
The fair value of option grants was estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions:
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Exercise price | $ | $ | ||||||
| Term (years) | ||||||||
| Expected stock price volatility | % | % | ||||||
| Risk-free rate of interest | % | % | ||||||
A summary of option activity under the Company’s equity incentive plans for the years ended December 31, 2024 and 2023 is presented below:
| Number of Shares | Weighted Average Exercise Price | Total Intrinsic Value | Weighted Average Remaining Contractual Life (in years) | |||||||||||||
| Outstanding as of December 31, 2022 | $ | $ | ||||||||||||||
| Employee options issued | ||||||||||||||||
| Expired | ( | ) | — | |||||||||||||
| Outstanding as of December 31, 2023 | ||||||||||||||||
| Employee options issued | — | |||||||||||||||
| Expired | ( | ) | — | |||||||||||||
| Outstanding as of December 31, 2024 | $ | $ | ||||||||||||||
| Options vested and exercisable as of December 31, 2024 | $ | $ | ||||||||||||||
A summary of stock options outstanding at December 31, 2024 by price range is as follows:
| Options outstanding and exercisable | ||||||||||||
| Range of Exercise Prices | Number of Shares | Weighted Average Remaining Contractual Life (in years) | Weighted Average Exercise Price | |||||||||
| $ | | |||||||||||
| $ | ||||||||||||
| $ | ||||||||||||
| $ | ||||||||||||
F-22
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
All stock compensation associated with the amortization of employee stock option expense was recorded as a component of general and administrative expenses in the consolidated statements of operations and comprehensive loss.
Estimated
future stock-based compensation expense relating to unvested stock options is $
Stock Based Compensation
Stock based compensation expense for the years ended December 31, 2024 and 2023 was as follows:
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Employee stock option awards | $ | $ | ||||||
| Non-employee restricted stock awards | ||||||||
| Non-employee stock warrant awards (a) | ||||||||
| $ | $ | |||||||
| (a) |
For the years ended December 31, 2024 and 2023, the amount of stock-based compensation expense included within research and development and general and administrative expenses was as follows:
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| $ | $ | |||||||
NOTE 6 – Commitments and Contingencies
Office Lease
Effective
November 2023, the Company leased office space for a two-year term. The Company’s office lease contained a renewal option. The
Company evaluated several factors in assessing whether there is reasonable certainty that the Company will exercise its contractual renewal
option concluding that it is not reasonably certain to exercise such option. As it is not reasonably certain to be exercised, the Company
excluded the renewal term in determining the lease term used in calculating the right-of-use asset and lease liability. In December 2024,
the landlord notified the Company that it will be closing its operations at the Company’s location and offering to relocate the
Company to a new location. The Company agreed to relocate and accordingly, on December 9, 2024, the Company and the landlord entered
into a new lease agreement (the “December 2024 Lease”). Pursuant to the December 2024 Lease, effective December 20, 2024,
the Company leased office space for a term of 14 months, expiring on February 28, 2026. Pursuant to such lease agreement, the Company
is required to pay a monthly base rent of $
The table below presents certain information related to the Company’s lease costs, which are included in general and administrative expenses in the accompanying consolidated statements of operation and comprehensive loss:
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Operating lease expense | $ | $ | ||||||
| Short term lease expense | ||||||||
| Total lease cost | $ | $ | ||||||
F-23
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Right-of-use asset for operating leases were recorded in the consolidated balance sheets as follows:
| December 31, 2024 | December 31, 2023 | |||||||
| Office lease right-of-use asset | $ | $ | ||||||
| Less accumulated amortization | ( | ) | ||||||
| Total right-of-use asset, net | $ | $ | ||||||
Operating lease liability for operating leases were recorded in the consolidated balance sheets as follows:
| December 31, 2024 | December 31, 2023 | |||||||
| Current portion of operating lease liability | $ | $ | ||||||
| Long-term portion of operating lease liability | ||||||||
| Total operating lease liability | $ | $ | ||||||
Supplemental cash flow information related to the Company’s leases for the year ended December 31, 2024 were as follows:
| Cash paid for amounts included in the measurement of lease liabilities: | ||||
| Operating cash flows for operating leases | $ | |||
The
weighted-average remaining lease term for the operating lease is
As of December 31, 2024, future annual minimum lease payments required under operating leases are as follows:
| 2025 | $ | |||
| 2026 | ||||
| Total minimum lease payments | $ | |||
| Less: effects of discounting | ( | ) | ||
| Present value of future minimum lease payments | $ |
NOTE 7 – Income Taxes
The table below presents the components of the provision for taxes:
The Company’s provision is primarily driven by the full valuation allowance in 2024 and 2023.
| As of December 31, | ||||||||
| 2024 | 2023 | |||||||
| Current | (As Restated) | |||||||
| U.S. Federal | $ | $ | ||||||
| U.S. State | ||||||||
| U.S. Foreign | ||||||||
| Total current provision | ||||||||
| Deferred | - | - | ||||||
| U.S. Federal | ||||||||
| U.S. State | ||||||||
| U.S. Foreign | ||||||||
| Total deferred benefit | ||||||||
| Change in valuation allowance | ||||||||
| Total provision for income taxes | $ | $ | ||||||
F-24
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
At December 31, 2024 and 2023, the tax effects of the temporary differences and carryforwards that give rise to deferred tax assets consist of the following:
| As of December 31, | ||||||||
| 2024 | 2023 | |||||||
| Deferred tax assets | (As Restated) | |||||||
| Net operating loss carryforwards | $ | $ | ||||||
| Capitalized research costs | ||||||||
| Equity based compensation | ||||||||
| Licenses acquired | ||||||||
| Accruals and other temporary differences | ||||||||
| Total deferred tax assets | ||||||||
| Less valuation allowance | ( | ) | ( | ) | ||||
| Deferred tax assets, net of allowance | $ | $ | ||||||
A reconciliation of the statutory income tax rates and the Company’s effective tax rate for the years ended December 31, 2024 and 2023 is as follows:
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Statutory federal income tax rate | % | % | ||||||
| State taxes, net of federal benefit | % | % | ||||||
| Impact of non-U.S. earnings | % | % | ||||||
| Permanent items | % | % | ||||||
| Credits | % | % | ||||||
| Equity compensation | % | % | ||||||
| Foreign rate differential | % | % | ||||||
| Previous tax year adjustment | ( | )% | ( | )% | ||||
| Other | % | % | ||||||
| Change in valuation allowance | ( | )% | ( | )% | ||||
| Total | % | % | ||||||
The Company has determined, based upon available evidence, that it is more likely than not that the net deferred tax assets will not be realized and, accordingly, has provided a full valuation allowance against its net deferred tax assets.
F-25
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
As of December 31, 2024 and 2023, the Company
has Federal net operating loss carryforwards of approximately $
As
required by the 2017 Tax Cuts and Jobs Act and effective in 2022, the deferred tax asset as of December 31, 2024 and 2023, included $
On
August 16, 2022, the Inflation Reduction Act of 2022 (“IRA”) was signed into law. The IRA increased and modified the qualified
small business (“QSB”) payroll tax credit for increasing research activities. Provision 13902 of the IRA of 2022 increased
the maximum amount of payroll tax research credit that a QSB can elect to apply against payroll tax liability from $
The utilization of the Company’s net operating loss carryforwards and research tax credit carryovers could be subject to annual limitations under Section 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Code”), and similar state tax provisions, due to ownership change limitations that may have occurred previously or that could occur in the future. These ownership changes limit the amount of net operating loss carryforwards and other deferred tax assets that can be utilized to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382 and 383 of the Code, results from transactions increasing ownership of certain stockholders or public groups in the stock of the corporation by more than 50 percent points over a three-year period. The Company has not conducted an analysis of an ownership change under Section 382 of the Code. To the extent that a study is completed and an ownership change is deemed to occur, the Company’s net operating losses and tax credits could be limited.
At December 31, 2024 and 2023, the Company did not have any significant uncertain tax positions. The Company will recognize interest and penalties related to uncertain tax positions, as applicable, in income tax expense. As of December 31, 2024 and 2023, the Company had no accrued interest or penalties related to uncertain tax positions and no amounts have been recognized in the Company’s statements of operations. The Company does not anticipate a material change to unrecognized tax benefits in the next twelve months.
All of the Company’s tax years will remain open for examination by the Federal and state tax authorities from the date of utilization of the net operating loss.
Management asserts that its foreign earnings are permanently reinvested, and therefore, have not provided deferred taxes on foreign cash. Additionally, no additional income taxes have been provided for any remaining undistributed foreign earnings not subject to the transition tax, or any additional outside basis differences inherent in our foreign subsidiaries, as these amounts continue to be indefinitely reinvested in foreign operations. The Company will continue to monitor the foreign cash position as they maintain the assertion that foreign earnings are permanently reinvested.
F-26
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
NOTE 8 – Restatement of Previously Issued Audited and Unaudited Financial Statements
During the preparation of the Company’s 2024 audited consolidated financial statements and notes thereto, the Company concluded that there were material research and development expenses and related balance sheet errors in its previously issued audited consolidated financial statements as of and for the year ended December 31, 2023, 2022 and 2021, and there were material research and development expenses and related balance sheet errors in its previously issued unaudited condensed consolidated financial statements as of and for each of the quarterly and year to date periods ended March 31, 2024 and 2023, June 30, 2024 and 2023, and September 30, 2024 and 2023, relating to the recording of prepaid expenses, and the timing of recognition of research and development expenses.
| 1) | The Company noted the following items were improperly recorded as of December 31, 2023, 2022 and 2021, and for the year ended December 31, 2023 |
As of December 31, 2023, 2022 and
2021, the Company’s consolidated balance sheets did not reflect prepaid
expenses and other current assets related to advance payments made in 2022 and 2021 for clinical studies. These errors in the accounting
for prepaid expenses and other current assets and research and development expenses resulted in an understatement of prepaid assets and
other current assets of $
The December 31, 2022 understatement of
prepaid expenses and other current assets of $
| 2) | The Company noted the following items were improperly recorded as of March 31, 2024 and 2023, and during the three months ended March 31, 2024 and 2023: |
| ● | As
of March 31, 2024, prepaid expenses and other current assets were understated by $ |
| ● | As
of March 31, 2023, prepaid expenses and other current assets were understated by $ |
| 3) | The Company noted the following items were improperly recorded as of June 30, 2024 and 2023, and during the three and six months ended June 30, 2024 and 2023: |
| ● | As
of June 30, 2024, prepaid expenses and other current assets were understated by $ |
| ● | As
of June 30, 2023, prepaid expenses and other current assets were understated by $ |
| 4) | The Company noted the following items were improperly recorded as of September 30, 2024 and 2023, and during the three and nine months ended September 30, 2024 and 2023: |
| ● | As
of September 30, 2024, prepaid expenses and other current assets were understated by $ |
| ● | As
of September 30, 2023, prepaid expenses and other current assets were understated by $ |
F-27
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
For all periods presented, the errors in the accounting for research and development expenses resulted in an understatement of prepaid assets and other current assets and an understatement of research and development expenses, operating expenses and net losses for the periods presented, respectively.
As a result of such errors, the Company concluded that the previously issued 2023 consolidated financial statements and the previously issued interim periods during 2024 and 2023 were materially misstated and has restated herein its previously issued audited consolidated financial statements for the year ended December 31, 2023, and its unaudited condensed consolidated financial statements for each interim period within the fiscal years ended December 31, 2024 and 2023. The restatement corrections impact certain components within operating cash flows of the respective consolidated statements of cash flows. Total operating cash flows, investing activities, financing activities, and cash and cash equivalents are unchanged as a result of the restatements.
The following tables present the amounts previously reported, the restatement impact and the amount as restated. The 2024 and 2023 quarterly restatements will be effective with the filing of our future 2025 unaudited interim condensed financial statement filings in Quarterly Reports on Form 10-Q.
The values “as reported” on the following respective consolidated financial statements were derived from:
| 1) | Our Annual Report on Form 10-K for the year ended December 31, 2023 filed on March 28, 2024; |
| 2) | Our Quarterly Report on Form 10-Q for the period ended March 31, 2024 filed on May 14, 2024; |
| 3) | Our Quarterly Report on Form 10-Q for the period ended June 30, 2024 filed on August 9, 2024; |
| 4) | Our Quarterly Report on Form 10-Q for the period ended September 30, 2024 filed on November 12, 2024; |
| 5) | Our Quarterly Report on Form 10-Q for the period ended March 31, 2023 filed on May 15, 2023; |
| 6) | Our Quarterly Report on Form 10-Q for the period ended June 30, 2023 filed on August 11, 2023; and |
| 7) | Our Quarterly Report on Form 10-Q for the period ended September 30, 2023 filed on November 13, 2023. |
F-28
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Balance Sheet
| As of December 31, 2022 | ||||||||||||
| As Previously Reported | Restatement Impacts | As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | $ | $ | $ | |||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| CURRENT LIABILITIES: | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | $ | ( | ) | $ | $ | ( | ) | |||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
As of December 31, 2023 | ||||||||||||
| As Previously Reported | Restatement Impacts | As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | $ | $ | $ | |||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| CURRENT LIABILITIES: | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | $ | ( | ) | $ | $ | ( | ) | |||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
Consolidated Statement of Operations and Comprehensive Loss
| For the Year Ended December 31, 2023 | ||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| Research and development expense | $ | $ | $ | |||||||||
| Total operating expenses | ||||||||||||
| LOSS FROM OPERATIONS | ( |
) | ( |
) | ( |
) | ||||||
| NET LOSS | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| NET LOSS PER COMMON SHARE: | ||||||||||||
| Basic and diluted | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| COMPREHENSIVE LOSS: | ||||||||||||
| Total comprehensive loss | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
F-29
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Statements of Stockholders’ Equity
| For the Year Ended December 31, 2023 | ||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| Accunulated Deficit ACTIVITIES: | ||||||||||||
| Net loss | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses | ( |
) | ||||||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( |
) | ( |
) | ||||||||
Reconciliation of the Original and Restated Consolidated Statements of Cash Flows
| For the Year Ended December 31, 2023 | ||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses | ( |
) | ||||||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( |
) | ( |
) | ||||||||
Reconciliation of the Original and Restated Consolidated Balance Sheet
| As of March 31, 2024 | ||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | $ | $ | $ | |||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | $ | ( |
) | $ | $ | ( |
) | |||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
F-30
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Statement of Operations and Comprehensive Loss
| For the Three Months Ended March 31, 2024 |
||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| OPERATING COSTS AND EXPENSES: | ||||||||||||
| Research and development expense | $ | $ | $ | |||||||||
| General and administrative expenses | ||||||||||||
| Total operating expenses | ||||||||||||
| LOSS FROM OPERATIONS | ( |
) | ( |
) | ( |
) | ||||||
| NET LOSS | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| NET LOSS PER COMMON SHARE: | ||||||||||||
| Basic and diluted | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| COMPREHENSIVE LOSS: | ||||||||||||
| Total comprehensive loss | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Reconciliation of the Original and Restated Consolidated Statements of Cash Flows
| For the Three Months Ended March 31, 2024 |
||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| Prepaid expenses | ( |
) | ( |
) | ||||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( |
) | ( |
) | ||||||||
F-31
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Balance Sheet
| As of June 30, 2024 | ||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | $ | $ | $ | |||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | $ | ( |
) | $ | $ | ( |
) | |||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
Reconciliation of the Original and Restated Consolidated Statement of Operations and Comprehensive Loss
| For the Three Months Ended June 30, 2024 |
For the Six Months Ended June 30, 2024 |
|||||||||||||||||||||||
| As
Previously Reported |
Restatement Impacts |
As Restated | As
Previously Reported |
Restatement Impacts |
As Restated | |||||||||||||||||||
| OPERATING COSTS AND EXPENSES: | ||||||||||||||||||||||||
| Research and development expense | ||||||||||||||||||||||||
| Total operating expenses | ||||||||||||||||||||||||
| LOSS FROM OPERATIONS | ( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||
| NET LOSS | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
| NET LOSS PER COMMON SHARE: | ||||||||||||||||||||||||
| Basic and diluted | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
| COMPREHENSIVE LOSS: | ||||||||||||||||||||||||
| Total comprehensive loss | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
F-32
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Statements of Cash Flows
| For the Six Months Ended June 30, 2024 |
||||||||||||
| As Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses | ( |
) | ||||||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( |
) | ( |
) | ||||||||
Reconciliation of the Original and Restated Consolidated Balance Sheet
| As of September 30, 2024 | ||||||||||||
| As Previously Reported |
Restatement Impacts |
As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | $ | $ | $ | |||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | ( |
) | ( |
) | ||||||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
F-33
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Statement of Operations and Comprehensive Los
| For
the Three Months Ended September 30, 2024 | For
the Nine Months Ended September 30, 2024 | |||||||||||||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | As
Previously Reported | Restatement Impacts | As Restated | |||||||||||||||||||
| OPERATING COSTS AND EXPENSES: | ||||||||||||||||||||||||
| Research and development expense | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Total operating expenses | ||||||||||||||||||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| NET LOSS | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| NET LOSS PER COMMON SHARE: | ||||||||||||||||||||||||
| Basic and diluted | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| COMPREHENSIVE LOSS: | ||||||||||||||||||||||||
| Total comprehensive loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
Reconciliation of the Original and Restated Consolidated Statements of Cash Flows
| For
the Nine Months Ended September 30, 2024 | ||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Changes in operating assets and liabilities: | - | |||||||||||
| Prepaid expenses | ( | ) | ||||||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( | ) | ( | ) | ||||||||
F-34
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Balance Sheet
| As of March 31, 2023 | ||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | $ | $ | $ | |||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | $ | ( | ) | $ | $ | ( | ) | |||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
Reconciliation of the Original and Restated Consolidated Statement of Operations and Comprehensive Loss
| For
the Three Months Ended March 31, 2023 | ||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | ||||||||||
| OPERATING COSTS AND EXPENSES: | ||||||||||||
| Research and development expense | $ | $ | $ | |||||||||
| Total operating expenses | ||||||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ( | ) | ||||||
| NET LOSS | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| NET LOSS PER COMMON SHARE: | ||||||||||||
| Basic and diluted | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| COMPREHENSIVE LOSS: | ||||||||||||
| Total comprehensive loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
F-35
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Statements of Cash Flows
| For
the Three Months Ended March 31, 2023 | ||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses | ( | ) | ( | ) | ||||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( | ) | - | ( | ) | |||||||
Reconciliation of the Original and Restated Consolidated Balance Sheet
| As of June 30, 2023 | ||||||||||||
| As Previously Reported | Restatement Impacts | As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | ||||||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | $ | ( | ) | $ | $ | ( | ) | |||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
F-36
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Statement of Operations and Comprehensive Loss
| For
the Three Months Ended June 30, 2023 | For
the Six Months Ended June 30, 2023 | |||||||||||||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | As
Previously Reported | Restatement Impacts | As Restated | |||||||||||||||||||
| OPERATING COSTS AND EXPENSES: | ||||||||||||||||||||||||
| Research and development expense | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Total operating expenses | ||||||||||||||||||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| NET LOSS | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| NET LOSS PER COMMON SHARE: | ||||||||||||||||||||||||
| Basic and diluted | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| COMPREHENSIVE LOSS: | ||||||||||||||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| Total comprehensive loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
Reconciliation of the Original and Restated Consolidated Statements of Cash Flows
| For
the Six Months Ended June 30, 2023 | ||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Prepaid expenses | ( | ) | ( | ) | ||||||||
| NET CASH USED IN OPERATING ACTIVITIES | $ | ( | ) | $ | - | $ | ( | ) | ||||
F-37
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
Reconciliation of the Original and Restated Consolidated Balance Sheet
| As of September 30, 2023 | ||||||||||||
| As Previously Reported | Restatement Impacts | As Restated | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Prepaid expenses and other current assets | $ | $ | $ | |||||||||
| Total Current Assets | ||||||||||||
| Total Assets | $ | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||
| Accumulated deficit | $ | ( | ) | $ | $ | ( | ) | |||||
| Total Stockholders’ Equity | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | $ | $ | |||||||||
Reconciliation of the Original and Restated Consolidated Statement of Operations and Comprehensive Loss
| For
the Three Months Ended September 30, 2023 | For
the Nine Months Ended September 30, 2023 | |||||||||||||||||||||||
| As
Previously Reported | Restatement Impacts | As Restated | As
Previously Reported | Restatement Impacts | As Restated | |||||||||||||||||||
| OPERATING COSTS AND EXPENSES: | ||||||||||||||||||||||||
| Research and development expense | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Total operating expenses | ||||||||||||||||||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| NET LOSS | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| NET LOSS PER COMMON SHARE: | ||||||||||||||||||||||||
| Basic and diluted | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| COMPREHENSIVE LOSS: | ||||||||||||||||||||||||
| Total comprehensive loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
Reconciliation of the Original and Restated Consolidated Statements of Cash Flows
| For the Nine Months Ended September 30, 2023 | ||||||||||||
| As Previously Reported | Restatement Impacts | As Restated | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Prepaid expenses | ( | ) | ||||||||||
| NET CASH USED IN OPERATING ACTIVITIES | ( | ) | ( | ) | ||||||||
F-38
HOTH THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
NOTE 9 – Subsequent Events
The Company has evaluated subsequent events and transactions that occurred up to the date the consolidated financial statements were issued. Based upon this review, except for as noted below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the consolidated financial statements.
On January 6, 2025, the compensation committee
of the board of directors further increased the number of shares reserved for issuance under the 2018 Plan from
On January 7, 2025, the Company issued
On January 7, 2025 through March 5, 2025, pursuant
to the ATM Agreement (See Note 5), the Company issued an aggregate of
On January 13, 2025, the Company entered into
a Patent Application Acquisition Agreement with Med30, LLC (the “Seller”), whereby the Seller sold, conveyed, assigned and
transferred to the Company all of Seller’s right, title, and interest in and to certain patent applications and associated rights,
subject to the terms and conditions set forth in such agreement for a cash payment of $
On January 14, 2025, pursuant to and subject to
the available number of shares reserved under the 2018 Plan, the Company issued options to the Company’s Chief Executive Officer
to purchase up to
On December 23, 2024, the Company provided notice to Isoprene Pharmaceutical, Inc. (“Isoprene”) of its intent to terminate the exclusive license agreement (the “Isoprene Agreement”) by and between the Company and Isoprene dated July 2, 2021. The Isoprene Agreement terminated on March 23, 2025.
F-39
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls
Our management, with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2024, the end of the period covered by this Annual Report on Form 10-K. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost benefit relationship of possible controls and procedures. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of the period covered by this report, as a result of the material weaknesses in our internal control identified below, our disclosure controls and procedures were not effective to ensure that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in SEC’s rules and forms and (ii) accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosures.
Identified Material Weakness
In connection with the audit of our financial statements as of December 31, 2024 for the years ended December 31, 2024 and 2023, we identified a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness that we have identified relates to the proper classification of prepaid expenses and other current assets and research and development expenses, which impacted our previously issued consolidated financial statements as of and for the year ended December 31, 2023, and our previously issued unaudited condensed consolidated financial statements as of March 31, 2024 and 2023, June 30, 2024 and 2023 and September 30, 2024 and 2023, and for the three months ended March 31, 2024 and 2023, three and six months ended June 30, 2024 and 2023, and three and nine months ended September 30, 2024 and 2023.
48
Remediation Plan
Our management, with the oversight of the Audit Committee of the board of directors, has updated our internal processes and controls to strengthen their effectiveness and developed a remediation plan which includes the following actions:
| ● | Enhance our review procedures over significant contracts with contract research and clinical studies organizations; and | |
| ● | Strengthen our review process. |
We will not be able to conclude whether the actions we are taking will fully remediate the material weakness in our internal control over financial reporting until the updated controls have operated for a sufficient period of time and management has concluded, through testing, that such controls are operating effectively. We may also conclude that additional measures may be required to remediate the material weakness in our internal control over financial reporting, which may necessitate further action.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with GAAP. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
As of December 31, 2024, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control Integrated Framework 2013. Based on this assessment, our management concluded that, as of December 31, 2024, our internal control over financial reporting was not effective based on such criteria, due to the material weakness in our internal control over financial reporting described above.
Changes in Internal Control Over Financial Reporting
Other than as described above, there have been no changes in our internal control over financial reporting that occurred during our last fiscal quarter ended December 31, 2024 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. We are taking actions to remediate the material weakness described above, which may result in changes in our internal control over financial reporting in periods subsequent to December 31, 2024.
ITEM 9B. OTHER INFORMATION
During
our last fiscal quarter ended December 31, 2024,
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
49
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth the name, age and positions of our executive officers and directors as of March 28, 2025.
| NAME | AGE | POSITION | ||
| Robb Knie | 56 | President, Chief Executive Officer and Director | ||
| David Briones | 48 | Chief Financial Officer | ||
| Wayne Linsley | 68 | Director | ||
| David B. Sarnoff | 57 | Director | ||
| Graig Springer | 45 | Director | ||
| Jeff Pavell | 58 | Director |
The business background and certain other information about our directors and executive officers are set forth below.
Robb Knie
Robb Knie has served as President and Chief Executive Officer and as a director of the Company since May 2017 and served as our principal financial and accounting officer from June 2018 until March 2019. From October 2020 to January 2023, Mr. Knie served as the Chief Executive Officer, Chief Financial Officer and chairman of the board of directors of FoxWayne Enterprises Acquisition Corp. (“FoxWayne”), a special purpose acquisition corporation. Mr. Knie served as the President of Lifeline Industries Inc. since its inception in 1995. From 2002 to 2010 he was a Semiconductor Analyst for PAW Partners. From 1993 until 1995, Mr. Knie served as Northeast Regional Manager of American Express Financial Advisors. Mr. Knie has served as a board member for Nasdaq-listed companies. He has been featured on Bloomberg, The Wall Street Journal and Forbes Magazine as an Independent Equity Analyst. Mr. Knie has over 20 years of equity markets experience. Mr. Knie has been a member of the American Chemical Society, Institute of Electrical and Electronics Engineers, as well as The National Alliance for Youth Sports. We believe that Mr. Knie is qualified to serve as a director because of his business and leadership experience and experience as a board member of public companies in the healthcare industry.
David Briones
David Briones has served as Chief Financial Officer of the Company since March 2019 and has over 25 years of public accounting and executive level experience. He consults with various public companies in financial reporting, internal control development and evaluation, budgeting and forecasting. Since October 2010, he has served as the managing member and founder of Brio Financial Group, LLC, a full-service financial consulting firm that brings experienced finance and accounting expertise to both public and private companies. Since 2010, Mr. Briones has served over 75 companies as well as numerous banks, hedge funds, venture capital funds and private equity firms. In addition, from May 2018 until its dissolution in April 2021, Mr. Briones served as Executive Chair of Zovis Pharmaceuticals, and from September 2021 to December 2022, Mr. Briones served as Chief Financial Officer, Treasurer and Secretary and a member of the board of directors of Larkspur Healthcare Acquisition Corp. (Nasdaq: LSPR), a special purpose acquisition corporation that merged with ZyVersa Therapeutics Inc. From August 2013 to January 2020, Mr. Briones served as Chief Financial Officer of Petro River Oil Corp., an independent energy company focused on the exploration and development of conventional oil and gas assets, and from January 2018 to July 2020 (until the company’s initial public offering), Mr. Briones served as interim Chief Financial Officer of AdiTx Therapeutics, Inc. (Nasdaq: ADTX), a pre-clinical stage, life sciences company with a mission to prolong life and enhance life quality of transplanted patients. Prior to founding Brio Financial Group, LLC, Mr. Briones was an auditor with Bartolomei Pucciarelli, LLC in Lawrenceville, New Jersey and PricewaterhouseCoopers LLP in New York, New York. Since May 2020, Mr. Briones has served as a member of the board of directors of Unique Logistics International Inc (OTC Pink: UNQL). Mr. Briones received a Bachelor of Science degree in accounting from Fairfield University.
50
Wayne Linsley
Wayne D. Linsley has served as a director of the Company since April 2020. Mr. Linsley has been in business management for over 40 years. He possesses a wide and varied skillset including sales and sales management, finance (for both public and private companies), accounting, audit support and financial reporting. He has a bachelor’s in business administration from Siena College in Loudonville, New York. From 2009 to September 2021, he worked for a financial reporting firm that works with publicly traded companies. He has extensive knowledge of financial statements, MD&A, SEC filings (10-K, 10-Q, 8-K, etc.), Edgar, etc. He often negotiated on behalf of clients in such areas as audit fees, transfer agents, Edgar companies, etc. He currently serves as an independent director for DatChat Inc. (Nasdaq: DATS), serving as the chair of its audit committee, compensation committee and nominating and corporate governance committee, and Silo Pharma, Inc. (Nasdaq: SILO) serving as the chair of its audit committee and compensation committee. We believe Mr. Linsley is qualified to serve as a member of the board because of his business management experience.
David B. Sarnoff
David Sarnoff has served as a director of the Company since August 2018. Since May 2015, Mr. Sarnoff has served as the founder and Principal of Sarnoff Group, LLC, and since January 2019, he has served as the Director of Strategic Partnerships and Executive Leadership Coach at Loeb Leadership. In addition, since December 2021, Mr. Sarnoff has served as Adjunct Faculty at iCoach Global (formally known as iCoach New York) with respect to a professional coaching program affiliated with the Zicklin School of Business at Baruch College. From October 2003 until May 2015, Mr. Sarnoff served as the co-founder and Principal of Morandi, Taub & Sarnoff LLC, an executive search firm, and from July 1998 until October 2003 he served as a Legal Recruiter for Schneider Legal Search, Inc. From August 1994 until July 1998, Mr. Sarnoff served as a litigation associate attorney at Wachtel Missry LLP (formerly known as Gold & Wachtel LLP). Since July 2018, Mr. Sarnoff has served as a member of the advisory committee of the New Jersey Association of School Resource Officers. From January 2015 until January 2018, Mr. Sarnoff served as board President of Fort Lee Board of Education and served as a board member from January 2013 through January 2019. In September of 2020, Mr. Sarnoff was appointed to a three-year term on the Diversity, Equity & Inclusion Committee of the New York City Bar Association, and in September 2022, he was appointed as Co-Chair of that committee. Mr. Sarnoff received his Juris Doctor from Rutgers University School of Law and his Bachelor of Arts from Hofstra University. Mr. Sarnoff is admitted to the New York and New Jersey (retired status) state bars. We believe that Mr. Sarnoff is qualified to serve as a director because of his legal experience as well as his extensive experience in executive leadership and business development.
Graig Springer
Graig Springer has served as a director of the Company since February 2020. Since April 2021, Mr. Springer has served as Vice President for Brookfield Oaktree Wealth Solutions LLC (“Brookfield”) in their Legal and Regulatory Department, and from August 2020 to April 2021, he served as a consultant to Brookfield Public Securities Group LLC. From May 2019 to August 2019, Mr. Springer assisted with product development and governance at Invesco U.S., an investment management company, and from December 2013 to May 2019, he served in various capacities at OppenheimerFunds, Inc., an investment management company acquired by Invesco U.S., including distribution compliance and product development. In addition, Mr. Springer served on the Sub-Adviser Oversight Committee at OppenheimerFunds, Inc. Mr. Springer received his Bachelor of Arts from Columbia University and his Juris Doctor from Fordham University School of Law. Mr. Springer also holds a Series 7 and a Series 24 license. We believe that Mr. Springer is qualified to serve as a director because of his fifteen years of experience within the financial services industry overseeing and advising firms’ compliance with federal rules and regulations.
Jeff Pavell
Jeff Pavell has served as a director of the Company since December 2022. Since January 2017, Dr. Pavell has served as Chief of Rehabilitation Medicine at Englewood Health, and since November 2021, he has been on the teaching staff at New York-Presbyterian. In addition, since December 2020 he has been on the teaching staff at Hackensack Meridian School of Medicine at Seton Hall. Furthermore, since 2010, Dr. Pavell has served as a partner at Patient Care Associates, an outpatient surgical center, and since 2002, he has served as a Partner at the Physical Medicine and Rehabilitation Center, a private medical practice serving patients with spine, sports and occupational injuries. Dr. Pavell is a Board-Certified physician specializing in the field of physical medicine and rehabilitation. Dr. Pavell is also certified in pain medicine and specializes in the most advanced non-operative treatments for spine, sports and interventional pain medicines. Dr. Pavell received his Bachelor of Arts from Johns Hopkins University and his D.O. degree with honors from the New York College of Osteopathic Medicine. From January 2021 to January 2023, Dr. Pavell served as a member of the board of directors as well as chairman of the audit committee and a member of the compensation committee of FoxWayne, a special purpose acquisition corporation. Furthermore, since September 2022, Dr. Pavell has served as a director of Silo Pharma, Inc. (Nasdaq: SILO) as well as a member of the audit committee, compensation committee and chair of the nominating and corporate governance committee. We believe that Dr. Pavell is qualified to serve as a director due to his extensive experience practicing in the healthcare industry as well as his prior experience serving as a director for other public companies.
51
Family Relationships
There are no family relationships among any of our executive officers or directors.
Arrangements Between Officers and Directors
Except as set forth herein, to our knowledge, there is no arrangement or understanding between any of our officers or directors and any other person pursuant to which the officer or director was selected to serve as an officer or director.
Involvement in Certain Legal Proceedings
We are not aware of any of our directors or officers being involved in any legal proceedings in the past ten years relating to any matters in bankruptcy, insolvency, criminal proceedings (other than traffic and other minor offenses), or being subject to any of the items set forth under Item 401(f) of Regulation S-K.
Committees of Our Board of Directors
Our board of directors directs the management of our business and affairs, as provided by Nevada law, and conducts its business through meetings of the board of directors and its standing committees. We have a standing audit committee, compensation committee and nominating and corporate governance committee. In addition, from time to time, special committees may be established under the direction of the board of directors when necessary to address specific issues.
Our board of directors has determined that all of the members of the audit committee, the compensation committee and the nominating and corporate governance committee are independent as defined under the applicable rules of Nasdaq, including, in the case of all of the members of our audit committee, the independence requirements contemplated by Rule 10A-3 under the Exchange Act. In making such determination, the board of directors considered the relationships that each director has with our Company and all other facts and circumstances that the board of directors deemed relevant in determining director independence, including the beneficial ownership of our capital stock by each director.
Audit Committee
Our audit committee is responsible for, among other things:
| ● | approving and retaining the independent registered public accounting firm to conduct the annual audit of our consolidated financial statements; | |
| ● | reviewing the proposed scope and results of the audit; | |
| ● | reviewing and pre-approval of audit and non-audit fees and services; |
| ● | reviewing accounting and financial controls with the independent registered public accounting firm and our financial and accounting staff; | |
| ● | reviewing and approving transactions between us and our directors, officers and affiliates; |
52
| ● | establishing procedures for complaints received by us regarding accounting matters; | |
| ● | overseeing internal audit functions, if any; and | |
| ● | preparing the report of the audit committee that the rules of the Securities and Exchange Commission require to be included in our annual meeting proxy statement. |
Our audit committee consists of Wayne Linsley, David Sarnoff and Graig Springer, with Wayne Linsley serving as chair. Each member of our audit committee meets the financial literacy requirements of the Nasdaq rules. In addition, our board of directors has determined that Wayne Linsley qualifies as an “audit committee financial expert,” as such term is defined in Item 407(d)(5) of Regulation S-K.
Our board of directors adopted a written charter for the audit committee which is available on our website at www.hoththerapeutics.com.
Compensation Committee
Our compensation committee is responsible for, among other things:
| ● | reviewing and recommending the compensation arrangements for management, including the compensation for our president and chief executive officer; | |
| ● | establishing and reviewing general compensation policies with the objective to attract and retain superior talent, to reward individual performance and to achieve our financial goals; | |
| ● | administering our stock incentive plans; and | |
| ● | preparing the report of the compensation committee that the rules of the Securities and Exchange Commission require to be included in our annual meeting proxy statement. |
Our compensation committee currently consists of Wayne Linsley, Graig Springer and Jeff Pavell, with Wayne Linsley serving as chair.
Our board of directors adopted a written charter for the compensation committee which is available on our website at www.hoththerapeutics.com.
Nominating and Governance Committee
Our nominating and governance committee is responsible for, among other things:
| ● | identifying and nominating members of the board of directors; | |
| ● | developing and recommending to the board of directors a set of corporate governance principles applicable to our Company; and | |
| ● | overseeing the evaluation of our board of directors. |
Our nominating and corporate governance committee consists of Wayne Linsley, Graig Springer and David Sarnoff, with Graig Springer serving as chair.
Our board of directors adopted a written charter for the nominating and corporate governance committee which is available on our website at www.hoththerapeutics.com.
53
Scientific Advisory Board
In July 2017, the board of directors formed a Scientific Advisory Board (formerly known as the Technology Advisory Board). As of March 28, 2025, the members of such board are as follows: (i) Dr. Mario Lacouture, Dr. William Weglicki, and Dr. Adam Friedman as Medical Doctor members and (ii) Dr. Glenn Cruse, Dr. Carla Yuede, Dr. John Cirrito, and Sergio Traversa as Non-Medical Doctor members.
Code of Business Code and Ethics Conduct
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of the code is posted on our website at www.hoththerapeutics.com. Disclosure regarding any amendments to, or waivers from, provisions of the code of conduct and ethics that apply to our directors, principal executive and financial officers will be posted on the “Investors-Corporate Governance” section of our website at www.hoththerapeutics.com or will be included in a Current Report on Form 8-K, which we will file within four business days following the date of the amendment or waiver.
Insider Trading Policy
We
have
Changes in Nominating Procedures
None.
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth the compensation paid or accrued during the fiscal year ended December 31, 2024 and 2023 to our principal executive officer (the “named executive officer”):
| ● | Robb Knie, Chief Executive Officer and President |
| Name and Principal Position | Year | Salary ($) | Bonus ($)(1) | Stock Awards ($) | Option Awards ($)(2) | Non-Equity Incentive Plan Compensation ($) | Nonqualified deferred compensation earnings ($) | All Other Compensation ($)(3) | Total ($) | |||||||||||||||||||||||||||
| Robb Knie | 2024 | 450,000 | 200,000 | - | 449,685 | - | - | 127,107 | 1,226,792 | |||||||||||||||||||||||||||
| Chief Executive Officer and President | 2023 | 450,000 | 200,000 | - | 81,120 | - | - | 115,222 | 846,342 | |||||||||||||||||||||||||||
| (1) | Represents payments of discretionary bonuses for performance during the applicable years as determined by the board, and as further described below Bonus Arrangements. |
| (2) | Represents the aggregate grant date fair value of options granted for the fiscal year ended December 31, 2024 and December 31, 2023 as determined in accordance with FASB ASC Topic 718, rather than the amount paid to or realized by Robb Knie. See Note 6, “Stockholders’ Equity” in the notes to the Company’s consolidated financial statements for the fiscal year ended December 31, 2024 and December 31, 2023 included elsewhere in this Annual Report on Form 10-K for more information regarding the Company’s accounting for share-based compensation plans. |
| (3) | All other compensation represents the employer matching contributions to Robb Knie’s 401(k) account and the amounts received for his executive health or supplemental health insurance premiums. Mr. Knie received (i) an employer 401(k) contribution in the amount of $20,475 and $19,800 for fiscal years 2024 and 2023, respectively, and (ii) payments for executive health or supplemental medical insurance premiums in the amount of $106,632 and $95,422 for fiscal years 2024 and 2023, respectively. |
54
Employment Agreements
Robb Knie Employment Agreement
On March 28, 2023, we entered into an employment agreement (the “2023 Knie Employment Agreement”) with Robb Knie, pursuant to which Mr. Knie continues to serve as our Chief Executive Officer. The term of the 2023 Knie Employment Agreement will continue for a period of three years from the date of execution and automatically renews for successive one-year periods at the end of each term until either party delivers written notice of their intent not to review at least six months prior to the expiration of the then effective term. Mr. Knie’s base salary is $450,000 per year. Mr. Knie is eligible to receive an annual bonus of up to $350,000 per year at the discretion of the compensation committee of the Company, based upon the achievement of Company and individual performance targets established by the compensation committee. Under the 2023 Knie Employment Agreement, Mr. Knie is also entitled to receive equity-based compensation awards. In addition, the 2023 Knie Employment Agreement contains standard non-competition and non-solicitation provisions. Mr. Knie is also eligible to receive additional equity-based compensation awards as the Company may grant from time to time. The 2023 Knie Employment Agreement further provides for standard expense reimbursement, vacation time and other standard executive benefits.
Pursuant to the 2023 Knie Employment Agreement, in the event Mr. Knie’s employment is terminated without Cause (as defined in the 2023 Knie Employment Agreement), due to a non-renewal by the Company, he voluntarily resigns, or if he resigns for Good Reason (as defined in the 2023 Knie Employment Agreement), Mr. Knie is entitled to (i) a cash payment equal to the sum of (x) 24 months of his base salary at the then current rate (or 36 months if such termination occurs within 12 months of a Change in Control (as defined in the 2023 Knie Employment Agreement)) and (y) annual bonus in effect on his last day of employment; (ii) continuation of health benefits for a period of 24 months (or 36 months if such termination occurs within 12 months of a Change in Control); (iii) a lump sum payment equal to the amount of any annual bonus earned with respect to a prior fiscal year, but unpaid as of the date of termination; (iv) a lump sum payment equal to the amount of annual bonus that was accrued through the date of termination for the year in which employment ends; and (v) subject to Mr. Knie’s compliance with his restrictive covenants, the outstanding and unvested portion of any equity award will accelerate and immediately vest on the date of Mr. Knie’s termination.
In the event that Mr. Knie’s employment is terminated due to his death or disability, he will be entitled to receive (i) a lump sum payment equal to the amount of any annual bonus earned with respect to a prior fiscal year, but unpaid as of the date of termination; (ii) a lump sum payment equal to the amount of annual bonus that was accrued for the year in which employment ends; and (iii) the treatment of any equity awards in accordance with their respective equity award agreements.
In the event that Mr. Knie’s employment is terminated due to his non-renewal or resignation without Good Reason, he will be entitled to receive a lump sum payment equal to the amount of any annual bonus earned with respect to a prior fiscal year, but unpaid as of the date of termination.
Equity Grant Practices
2018 Equity Incentive Plan
On May 4, 2018, the Company’s board of directors adopted the Hoth Therapeutics, Inc. 2018 Omnibus Equity Incentive Plan (the “2018 Plan”). The 2018 Plan became effective on May 4, 2018 upon approval of the 2018 Plan by the Company’s shareholders at the Company’s annual meeting of shareholders. Pursuant to the 2018 Plan, the Company can grant stock options, stock appreciation rights, restricted stock, restricted stock units, deferred stock units, annual or long-term performance awards or other stock-based awards. As of December 31, 2024, the outstanding option awards under the 2018 Plan total 77,362 as described in the table under “Outstanding Equity Awards at December 31, 2024” below.
55
2022 Equity Incentive Plan
On March 24, 2022, the Company’s board of directors adopted the Hoth Therapeutics, Inc. 2022 Omnibus Equity Incentive Plan (the “2022 Plan”) initially reserving 96,000 shares of the Company’s common stock for issuance thereunder. The 2022 Plan became effective on June 23, 2022 upon approval of the 2022 Plan by the Company’s shareholders at the Company’s annual meeting of shareholders. On June 2, 2023, the Company’s board of directors approved the Hoth Therapeutics, Inc. Amended and Restated 2022 Omnibus Equity Incentive Plan (the “Amended and Restated 2022 Plan”) which was approved by stockholders on August 18, 2023. Pursuant to the Amended and Restated 2022 Plan, the Company can grant stock options, stock appreciation rights, restricted stock, restricted stock units, deferred stock units, annual or long-term performance awards or other stock-based awards. As of December 31, 2024, the outstanding option awards under the Amended and Restated 2022 Plan total 1,013,000 as described in the table under “Outstanding Equity Awards at December 31, 2024” below.
Bonus Arrangements
Pursuant to the terms of the executive employment agreements described above, the Company, through the board, has the discretion to determine the amounts of the annual incentive bonus payments which executives may receive Based on the review of the Company’s performance for calendar year 2024, the board, in its sole discretion, determined to pay the bonus to the named executive officer listed in the summary compensation table above.
401(k) Plan
The Company maintains a defined contribution employee retirement plan, or 401(k) plan, for its employees. The 401(k) plan is intended to qualify as a tax-qualified plan under Section 401(k) of the Code so that contributions to the 401(k) plan, and income earned on such contributions, are not taxable to participants until withdrawn or distributed from the 401(k) plan. The Company will match a participant’s contribution 100% up to 6% of their compensation, subject to statutory limits.
Perquisites
Perquisites are not a material component of compensation. In general, named executive officers do not receive reimbursements for meals, airlines, and travel costs, other than those costs allowed for all employees. During 2024, our named executive officer did not receive an allowance from the Company or any of the above or a reimbursement for any expense incurred for non-business purposes.
Outstanding Equity Awards at December 31, 2024
The following table provides information regarding option awards held by our named executive officer that were outstanding as of December 31, 2024. There were no stock awards or other equity awards outstanding as of December 31, 2024.
| Option Awards | ||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | ||||||||||
| Robb Knie | 10,000 | (1) | - | $ | 131.50 | 12/24/2029 | ||||||||
| 3,201 | (1) | - | $ | 76.25 | 7/21/2030 | |||||||||
| 9,000 | (1) | - | $ | 52.75 | 1/29/2031 | |||||||||
| 20,000 | (1) | - | $ | 14.75 | 3/16/2032 | |||||||||
| 40,000 | (1) | - | $ | 2.59 | 7/17/2033 | |||||||||
| 225,000 | (1) | - | $ | 1.36 | 1/5/2034 | |||||||||
| 325,000 | (1) | - | $ | 0.7548 | 8/19/2034 | |||||||||
| (1) | Stock options granted to Robb Knie vested in full immediately upon grant. |
56
Pay Versus Performance Disclosure
In accordance with the SEC’s disclosure requirements regarding pay versus performance (“PVP”), this section presents the SEC-defined “Compensation Actually Paid,” (“CAP”) of our NEO for each of the fiscal years ended December 31, 2024 and 2023, and our financial performance. Also required by the SEC, this section compares CAP to various measures used to gauge performance at HOTH for each such fiscal year.
Pay versus Performance Table — Compensation Definitions
Salary, Bonus, Stock Awards, and All Other Compensation are each calculated in the same manner for purposes of both CAP and Summary Compensation Table (“SCT”) values. The primary difference between the calculation of CAP and SCT total compensation is the calculation of the value of “Stock Awards,” with the table below describing the differences in how these awards are valued for purposes of SCT total and CAP:
| SCT Total | CAP | |||
| Stock Awards | Grant date fair value of stock and option awards granted during the year | Year over year change in the fair value of stock and option awards that are unvested as of the end of the year, or vested or were forfeited during the year |
Pay Versus Performance Table
| Year(1) | Summary Compensation Table Total for PEO | Compensation Actually Paid to PEO(2) | Average Summary Compensation Table Total for Non-PEO NEOs | Average Compensation Actually Paid to Non-PEO NEOs(2) | Value of Initial Fixed $100 Investment Based On Total Shareholder Return | Net Loss | ||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (h) | ||||||||||||||||||||
| 2024 | $ | 1,226,792 | $ | 1,226,792 | $ | - | $ | - | $ | 1.26 | $ | (7,786,842 | ) | |||||||||||||
| 2023 | $ | 846,342 | $ | 846,342 | $ | - | $ | - | $ | 2.43 | $ | (7,845,390 | ) | |||||||||||||
| 2022 | $ | 1,060,370 | $ | 1,060,370 | $ | - | $ | - | $ | 13.16 | $ | (11,371,953 | ) | |||||||||||||
| (1) | The PEO (CEO) in the 2024 and 2023 reporting year is Robb Knie. |
| (2) | The CAP was calculated beginning with the PEO’s SCT total. No amounts were deducted from or added to the applicable SCT total compensation. Since all equity awards were fully vested prior to 2022, no reconciliation with respect to equity awards for summary compensation numbers was required. |
57
Non-Employee Director Compensation
The following table presents the total compensation for each person who served as a non-employee member of our board of directors and received compensation for such service during the fiscal year ended December 31, 2024. Other than as set forth in the table and described more fully below, we did not pay any compensation, make any equity awards or non-equity awards to, or pay any other compensation to any of the non-employee members of our board of directors in 2024.
| Name | Fees earned or paid in cash ($) | Stock Awards ($) | Option Awards ($)(1)(2) | Non-Equity Incentive Plan Compensation ($) | Nonqualified deferred compensation earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
| Jeff Pavell | 50,000 | - | 43,355 | - | - | - | 93,355 | |||||||||||||||||||||
| David Sarnoff | 50,000 | - | 43,355 | - | - | - | 93,355 | |||||||||||||||||||||
| Graig Springer | 50,000 | - | 43,355 | - | - | - | 93,355 | |||||||||||||||||||||
| Wayne Linsley | 50,000 | - | 43,355 | - | - | - | 93,355 | |||||||||||||||||||||
| (1) | Amounts reported represent the aggregate grant date fair value for option awards granted in each respective year in accordance with FASB ASC Topic 718, excluding the effect of forfeitures. See Note 6, “Stockholders’ Equity” in the notes to the Company’s consolidated financial statements for the fiscal year ended 2024 included elsewhere in this Annual Report on Form 10-K for the year ended 2024 for more information regarding the Company’s accounting for share-based compensation plans. |
| (2) | On January 5, 2024, Jeff Pavell was granted ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $1.36, which options vested in full upon grant, and on August 19, 2024, he was granted additional ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $0.7548, which options vested in full upon grant. |
| On January 5, 2024, David Sarnoff was granted ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $1.36, which options vested in full upon grant, and on August 19, 2024, he was granted additional ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $0.7548, which options vested in full upon grant. |
| On January 5, 2024, Graig Springer was granted ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $1.36, which options vested in full upon grant, and on August 19, 2024, he was granted additional ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $0.7548, which options vested in full upon grant. | |
| On January 5, 2024, Wayne Linsley was granted ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $1.36, which options vested in full upon grant, and on August 19, 2024, he was granted additional ten-year options to purchase up to 25,000 shares of the Company’s common stock at an exercise price of $0.7548, which options vested in full upon grant. |
Non-Employee Director Compensation Policy
Our directors receive $50,000 cash compensation per year for their service on the board of directors, as well as reimbursement for out-of-pocket expenses with respect to such directors’ attendance at meetings of the board of directors of the Company.
Committee chairs receive an additional one-time $6,000 cash compensation upon appointment for their added services in such roles.
Company Policies and Practices Related to the Grant of Certain Equity Awards Close in Time to the Release of Material Nonpublic Information
The Compensation Committee last granted a stock option in January 2025. The Company does not grant stock options or similar awards to Section 16 Insiders, most SVPs, and other Vice Presidents and above who directly report to the CEO in anticipation of the release of material nonpublic information that is likely to result in changes to the price of the Company’s stock, such as a significant positive or negative earnings announcement, or time the public release of such information based on stock option grant dates. In addition, the Company does not grant stock options or similar awards during the four business days prior to or the one business day following the filing of our periodic reports or the filing or furnishing of a Current Report on Form 8-K that discloses material nonpublic information. These restrictions do not apply to RSUs or other types of equity awards that do not include an exercise price related to the market price of the Company’s stock on the date of grant.
The Company’s executive officers would not be permitted to choose the grant date for any stock option grants.
During fiscal 2024, the Company’s named executive officer was awarded stock options. The Company did not time the disclosure of material nonpublic information for the purpose of affecting the value of executive compensation.
58
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding beneficial ownership of shares of our common stock as of March 28, 2025 by (i) each person known to beneficially own more than 5% of our outstanding common stock, (ii) each of our directors, (iii) each of our named executive officers and (iv) all of our directors and named executive officers as a group. Except as otherwise indicated, the persons named in the table below have sole voting and investment power with respect to all shares beneficially owned, subject to community property laws, where applicable.
| Beneficial Owner(1) | Shares of Common Stock Beneficially Owned | Percentage(2) | ||||||
| Directors and Named Executive Officers: | ||||||||
| Robb Knie | 790,331 | (3) | 5.68 | % | ||||
| Wayne Linsley | 61,154 | (4) | * | |||||
| David Sarnoff | 63,420 | (5) | * | |||||
| Graig Springer | 320,067 | (6) | 2.37 | % | ||||
| Jeff Pavell | 62,575 | (7) | * | |||||
| All Named Executive Officers and Directors as a Group (5 persons) | 1,297,547 | 9.01 | % | |||||
| * | Represents beneficial ownership of less than 1%. |
| (1) | The address of each person is c/o Hoth Therapeutics, Inc., 1177 Avenue of the Americas, 5th Floor, Suite 5066, New York, New York 10036 unless otherwise indicated herein. |
| (2) | The calculation in this column is based upon 13,170,715 shares of common stock outstanding on March 28, 2025. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to the subject securities. Shares of common stock that are currently exercisable or convertible within 60 days of March 28, 2025 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage beneficial ownership of such person, but are not treated as outstanding for the purpose of computing the percentage beneficial ownership of any other person. |
| (3) | Includes options to purchase up to 732,200 shares of the Company’s common stock. |
| (4) | Includes options to purchase up to 61,020 shares of the Company’s common stock. |
| (5) | Includes options to purchase up to 62,420 shares of the Company’s common stock. |
| (6) | Includes (i) 134 shares of the Company’s common stock held by Graig Springer, (ii) options to purchase up to 61,020 shares of the Company’s common stock held by Graig Springer, (iii) 1,113 shares of the Company’s common stock held by Mr. Springer’s spouse and (iv) options to purchase up to 257,800 shares of the Company’s common stock held by Mr. Springer’s spouse. Mr. Springer’s spouse is an employee of the Company. |
| (7) | Includes options to purchase up to 57,500 shares of the Company’s common stock. |
59
Securities Authorized for Issuance Under Equity Compensation Plans
The following table summarizes information about our equity compensation plans as of December 31, 2024.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| Equity compensation plans approved by security holders | 1,090,362 | $ | 4.78 | 165,989 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 1,090,362 | 165,989 | ||||||||||
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The following includes a summary of transactions during our fiscal years ended December 31, 2024 and December 31, 2023 to which we have been a party, including transactions in which the amount involved in the transaction exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described elsewhere in this Annual Report on Form 10-K. We are not otherwise a party to a current related party transaction, and no transaction is currently proposed, in which the amount of the transaction exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years and in which a related person had or will have a direct or indirect material interest.
On September 13, 2023, we entered into a securities purchase agreement with certain investors, including Armistice Capital Master Fund Ltd. (“Armistice”)y pursuant to which Armistice (i) acquired (A) 384,500 shares of our common stock and (B) pre-funded warrants to purchase up to 165,500 shares of our common stock in a public offering and (ii) warrants to purchase up to 550,000 shares of our common stock in a concurrent private placement for aggregate gross proceeds to us from Armistice of $1,446,500, exclusive of placement agent commission and fees and other offering expenses. The closing of the offering occurred on September 15, 2023 pursuant to which we received gross proceeds of $2.89 million in the aggregate, prior to deducting placement agent’s fees and other offering expenses payable by us. Each pre-funded warrant is exercisable until exercised in full at an exercise price of $0.001 per share and may be exercised on a cashless basis. Each warrant is exercisable for a period of five years from the issuance date at an exercise price of $2.505 per share, subject to adjustment, and can, under certain circumstances, be exercised on a cashless basis.
60
On December 29, 2022, we entered into a securities purchase agreement with Armistice pursuant to which we agreed to sell an aggregate of (i) 140,000 shares (the “Shares”) of common stock, (ii) pre-funded warrants to purchase up to 1,860,000 shares (the “Pre-Funded Warrant Shares”) of common stock and (iii) warrants (the “January 2023 Warrants”) to purchase up to 2,500,000 shares (the “Warrant Shares” and together with the Shares and the Pre-Funded Warrant Shares, the “Registrable Securities”) of common stock at a purchase price of $5.00 per share and accompanying warrant (less $0.001 for each pre-funded warrant and accompanying warrant) in a private placement for aggregate gross proceeds of approximately $10 million, exclusive of placement agent commission and fees and other offering expenses. The closing of the offering occurred on January 3, 2023. Each common stock warrant was exercisable for a period of five and one-half years from the issuance date at an exercise price of $5.00 per share, subject to adjustment, and could, under certain circumstances, be exercised on a cashless basis. Each pre-funded warrant is exercisable until exercised in full at an exercise price of $0.001 per share and may be exercised on a cashless basis. In connection with the offering, we also entered into a registration rights agreement (the “Registration Rights Agreement”) with Armistice pursuant to which we filed a Registration Statement on Form S-3 covering the Registrable Securities on January 13, 2023, which registration statement was declared effective by the SEC on January 25, 2023. On March 27, 2024, we entered into an inducement offer agreement with Armistice to immediately exercise, for cash, all of the January 2023 Warrants at a reduced exercise price of $1.6775 per share for gross proceeds to us of approximately $4.2 million before deducting placement agent fees and other offering expenses payable by us. In accordance with the terms of an inducement offer agreement dated as of March 27, 2024 between us and Armistice, we shall only issue such number of shares of common stock issuable upon exercise of the January 2023 Warrants to Armistice that would not cause Armistice to exceed the maximum number of shares of common stock permitted thereunder, as directed by Armistice, with the balance of the shares of common stock issuable upon exercise of such warrants to be held in abeyance until notice from Armistice that the balance (or portion thereof) may be issued in compliance the limitations set forth in the inducement offer agreement, which abeyance was evidenced through the January 2023 Warrants which shall be deemed prepaid thereafter (including the cash payment in full of the exercise price), and exercised pursuant to a Notice of Exercise in the January 2023 Warrants (provided no additional exercise price shall be due and payable). As such, on April 1, 2024, we issued 485,000 shares of common stock to Armistice upon exercise of the January 2023 Warrants and 2,015,000 shares of common stock are held in abeyance for future issuance. As an inducement to exercise the January 2023 Warrants, we agreed to issue new unregistered warrants (the “New Warrants”) to purchase up to 3,750,000 shares of our common stock at an exercise price of $1.50 per share to Armistice. As of January 7, 2025, all of the New Warrants have been exercised.
Related Person Transaction Policy
We have adopted a formal policy regarding approval of transactions with related parties. For purposes of our policy only, a related person transaction is a transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we and any related person are, were or will be participants in which the amount involved exceeds the lesser of $120,000 or 1% of our total assets at the end of our last completed fiscal year. Transactions involving compensation for services provided to us as an employee or director are not covered by this policy. A related person is any executive officer, director or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, our management must present information regarding the related person transaction to our audit committee, or, if audit committee approval would be inappropriate, to another independent body of our board of directors, for review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related persons, the benefits to us of the transaction and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third-party or to or from employees generally. Under the policy, we will collect information that we deem reasonably necessary from each director, executive officer and, to the extent feasible, significant shareholder to enable us to identify any existing or potential related-person transactions and to effectuate the terms of the policy. In addition, under our code of business conduct and ethics, our employees and directors will have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest. In considering related person transactions, our audit committee, or other independent body of our board of directors, will take into account the relevant available facts and circumstances including, but not limited to:
| ● | the risks, costs and benefits to us; | |
| ● | the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated; | |
| ● | the availability of other sources for comparable services or products; and | |
| ● | the terms available to or from, as the case may be, unrelated third parties or to or from employees generally. |
61
The policy requires that, in determining whether to approve, ratify or reject a related person transaction, our audit committee, or other independent body of our board of directors, must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, our best interests and those of our shareholders, as our audit committee, or other independent body of our board of directors, determines in the good faith exercise of its discretion.
Director Independence
Our board of directors determined that a majority of the board during the year ended December 31, 2024 consisted of members who were “independent” as that term is defined under Nasdaq Listing Rule 5605(a)(2). The Board considered Wayne Linsley, David Sarnoff, Graig Springer and Jeff Pavell to be “independent.”
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees billed by WithumSmith+Brown, PC as described below:
| 2024 | 2023 | |||||||
| Audit Fees | $ | 209,029 | $ | 193,758 | ||||
| Audit Related Fees | - | - | ||||||
| Tax Fees | - | 9,800 | ||||||
| All Other Fees | - | - | ||||||
| Total | $ | 209,029 | $ | 203,558 | ||||
Audit Fees: Audit fees consist of fees billed for professional services performed by WithumSmith+Brown, PC for the audit of our annual consolidated financial statements, the review of interim consolidated financial statements, and related services that are normally provided in connection with registration statements. There were $209,029 and $193,758 of such fees incurred by the Company during the fiscal years ended December 31, 2024 and 2023, respectively.
Audit-Related Fees: Audit related fees consist of fees billed by an independent registered public accounting firm for assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements. There were no such fees incurred by the Company during the fiscal years ended December 31, 2024 and 2023.
Tax Fees: Tax fees consist of fees for professional services, including tax compliance, performed by WithumSmith+Brown, PC. There were $0 and $9,800 of such fees incurred by the Company during the fiscal years ended December 31, 2024 and 2023, respectively.
All Other Fees: There were no such fees incurred by the Company during the fiscal years ended December 31, 2024 and 2023.
Pre-Approval Policies and Procedures
In accordance with Sarbanes-Oxley, our audit committee charter requires the audit committee to pre-approve all audit and permitted non-audit services provided by our independent registered public accounting firm, including the review and approval in advance of our independent registered public accounting firm’s annual engagement letter and the proposed fees contained therein. The audit committee has the ability to delegate the authority to pre-approve non-audit services to one or more designated members of the audit committee. If such authority is delegated, such delegated members of the audit committee must report to the full audit committee at the next audit committee meeting all items pre-approved by such delegated members. In the fiscal years ended December 31, 2024 and 2023, all of the services performed by our independent registered public accounting firm were pre-approved by the audit committee.
62
PART IV
ITEM 15. EXHIBIT AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this report:
(1) Financial Statements:
The consolidated financial statements required by this Item are included beginning at page F-1.
(1) Financial Statement Schedules:
All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the consolidated financial statements or the notes thereto.
63
(b) Exhibits
EXHIBIT INDEX
64
65
66
| * | Filed herewith. |
| ** | Furnished herewith. |
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
ITEM 16. FORM 10-K SUMMARY
67
SIGNATURES
Pursuant to the requirements of Section 13 and 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on this 28th day of March 2025.
| HOTH THERAPEUTICS, INC. | |
| /s/ Robb Knie | |
| Robb Knie | |
| Chief Executive Officer, President and Director | |
| (Principal Executive Officer) | |
| /s/ David Briones | |
| David Briones | |
| Chief Financial Officer | |
| (Principal Financial and Accounting Officer) |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Robb Knie as his attorney-in-fact, with full power of substitution and resubstitution, for him in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Robb Knie | Chief Executive Officer, President and Director | March 28, 2025 | ||
| Robb Knie | (Principal Executive Officer) | |||
| /s/ David Briones | Chief Financial Officer | March 28, 2025 | ||
| David Briones | (Principal Financial and Accounting Officer) | |||
| /s/ Wayne Linsley | Director | March 28, 2025 | ||
| Wayne Linsley | ||||
| /s/ David B. Sarnoff | Director | March 28, 2025 | ||
| David B. Sarnoff | ||||
| /s/ Graig Springer | Director | March 28, 2025 | ||
| Graig Springer | ||||
| /s/ Jeff Pavell | Director | March 28, 2025 | ||
| Jeff Pavell |
68
