Table of Contents
As filed with the Securities and Exchange Commission on July 6, 2020.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Vir Biotechnology, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2836 | 81-2730369 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) 499 Illinois Street, Suite 500 San Francisco, California 94158 (415) 906-4324 |
(I.R.S. Employer Identification Number) |
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
George Scangos, Ph.D.
President and Chief Executive Officer
Vir Biotechnology, Inc.
499 Illinois Street, Suite 500
San Francisco, California 94158
(415) 906-4324
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Laura A. Berezin Charles S. Kim Kristin VanderPas Cooley LLP 3175 Hanover Street Palo Alto, California 94304 (650) 843-5000 |
Brian J. Cuneo B. Shayne Kennedy Drew Capurro Latham & Watkins LLP 140 Scott Drive Menlo Park, California 94025 (650) 328-4600 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. ☐
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of securities being registered |
Proposed maximum aggregate offering price(1)(2) |
Amount of registration fee (2) | ||
| Common Stock, $0.0001 par value per share |
$287,500,000 | $37,317.50 | ||
|
| ||||
|
| ||||
| (1) | Includes additional shares that the underwriters have the option to purchase. |
| (2) | Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JULY 6, 2020
PRELIMINARY PROSPECTUS
6,200,000 Shares

Common Stock
We are offering 6,200,000 shares of our common stock.
Our common stock is listed on The Nasdaq Global Select Market under the trading symbol “VIR.” The last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020 was $40.32 per share.
We are an “emerging growth company” as defined under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements.
Investing in our common stock involves a high degree of risk. See the section titled “Risk Factors” beginning on page 17.
| Per Share |
Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds to us before expenses |
$ | $ | ||||||
| (1) | See the section titled “Underwriting” for additional information regarding compensation payable to the underwriters. |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
We have granted the underwriters an option for a period of 30 days to purchase up to 930,000 additional shares of common stock at the public offering price, less the underwriting discounts and commissions.
The underwriters expect to deliver the shares against payment in New York, New York on , 2020.
| Goldman Sachs & Co. LLC | BofA Securities | Cowen | Barclays | |||
| Needham & Company | ||||||
Prospectus dated , 2020
Table of Contents
| Page | ||||
| 1 | ||||
| 17 | ||||
| 20 | ||||
| 21 | ||||
| 22 | ||||
| 24 | ||||
| 25 | ||||
| 27 | ||||
| 29 | ||||
| 33 | ||||
| Material U.S. Federal Income Tax Consequences to Non-U.S. Holders |
38 | |||
| 42 | ||||
| 48 | ||||
| 48 | ||||
| 48 | ||||
| 49 | ||||
Neither we nor the underwriters have authorized anyone to provide any information or to make any representations other than those contained or incorporated by reference in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained or incorporated by reference in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus and any such free writing prospectus outside the United States.
Unless the context otherwise requires, the terms “Vir,” “the company,” “we,” “us,” “our” and similar references in this prospectus refer to Vir Biotechnology, Inc. and its consolidated subsidiaries.
i
Table of Contents
This summary highlights selected information contained elsewhere in this prospectus or incorporated by reference herein. Before investing in our common stock, you should carefully read this entire prospectus, including the information incorporated by reference herein, especially the matters discussed in the information set forth under the sections titled “Risk Factors” in this prospectus and in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, and the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and notes thereto included in our Annual Report on Form 10-K for the year ended December 31, 2019 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, each of which are incorporated by reference herein.
Business Summary
Our mission is to create a world without infectious disease
We are a clinical-stage immunology company focused on combining immunologic insights with cutting-edge technologies to treat and prevent serious infectious diseases. Infectious diseases are one of the leading causes of death worldwide and can cause trillions of dollars of direct and indirect economic burden each year – as evidenced by the current coronavirus disease 2019, or COVID-19, pandemic. We believe that now is the time to apply the recent and remarkable advances in immunology to combat infectious diseases. Our approach begins with identifying the limitations of the immune system in combating a particular pathogen, the vulnerabilities of that pathogen and the reasons why previous approaches have failed. We then bring to bear powerful technologies that we believe, individually or in combination, will lead to effective therapies.
We have assembled four technology platforms, focused on antibodies, T cells, innate immunity and small interfering ribonucleic acid, or siRNA, through internal development, collaborations and acquisitions. Our current development pipeline consists of product candidates targeting severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2, the virus that causes COVID-19, hepatitis B virus, or HBV, influenza A, human immunodeficiency virus, or HIV, and tuberculosis, or TB. For SARS-CoV-2, VIR-7831, a SARS-CoV-2- neutralizing monoclonal antibody, or mAb, is planned to start a Phase 2/3 clinical trial program in August 2020 and we anticipate initial clinical data to be available before the end of the year. VIR-7832, a vaccinal SARS-CoV-2-neutralizing mAb, is planned to initiate a Phase 2 clinical trial later this year. VIR-2703, a SARS-CoV-2-targeting siRNA, is in preclinical studies. For HBV, VIR-2218, an HBV-targeting siRNA, is currently in an ongoing Phase 2 clinical trial. Initial Phase 2 data have demonstrated substantial, durable, and dose dependent reduction of hepatitis B virus surface antigen, or HBsAg, and VIR-2218 has been generally well-tolerated. We recently initiated a Phase 2 clinical trial to combine VIR-2218 with pegylated interferon-alpha, or PEG-IFN-α, an approved immune modulatory agent. In addition, we recently initiated a Phase 1 clinical trial for VIR-3434, an HBV-neutralizing mAb. For influenza A, VIR-2482, a mAb designed for the prevention of influenza A, is currently in a Phase 1/2 clinical trial and has been generally well-tolerated. For HIV, VIR-1111, an HIV T cell vaccine based on HCMV, is planned to initiate a Phase 1 trial in the second half of this year. We have built an industry-leading team that has deep experience in immunology, infectious diseases and product development. Given the global impact of infectious diseases, we are committed to developing cost-effective treatments that can be delivered at scale.
Our Technology Platforms
Our four current technology platforms are designed to stimulate and enhance the immune system by exploiting critical observations of natural immune processes. We are using our platforms to advance our current product candidates and generate additional product candidates for multiple indications.
1
Table of Contents
Antibody Platform: We have established a robust method for capitalizing on unusually successful immune responses naturally occurring in people who are protected from, or have recovered from, infectious diseases. We identify rare antibodies from survivors that have the potential to treat and prevent rapidly evolving and/or previously untreatable pathogens via direct pathogen neutralization and immune system stimulation. The fully-human antibodies that we discover may also be modified to enhance their therapeutic potential. We have applied these methods to identify mAbs for a range of pathogens including Ebola, HBV, influenza A and influenza B virus, SARS-CoV-2, RSV and malaria, and bacterial pathogens, including clostridium difficile, Staphylococcus aureus, Klebsiella pneumoniae, and Acinetobacter spp.
T Cell Platform: We are exploiting the unique immunology of human cytomegalovirus, or HCMV, a commonly occurring virus in humans, as a vaccine vector to potentially treat and prevent infection by pathogens refractory to current vaccine technologies. This approach is based on fundamental observations made in non-human primates, or NHPs, with rhesus cytomegalovirus, or RhCMV. We believe that this platform may also have applicability beyond infectious diseases, to areas such as cancer.
Innate Immunity Platform: Moving beyond more traditional approaches that are used to evoke adaptive immunity or that directly target pathogens, where the development of resistance can occur, we plan to target host proteins as a means of creating host-directed therapies with high barriers to resistance. We believe that by leveraging the power of innate immunity, we can create medicines that break the “one-drug-for-one-bug” paradigm to produce “one-drug-for-multiple-bugs.”
siRNA Platform: We are harnessing the power of siRNA to inhibit pathogen replication, eliminate key host factors necessary for pathogen survival and remove microbial immune countermeasures. Our collaboration with Alnylam Pharmaceuticals, Inc., or Alnylam, includes VIR-2218 for HBV, VIR-2703 for SARS-CoV-2 and up to seven additional programs for infectious diseases.
Our Development Pipeline
Our current product candidates are summarized in the chart below:
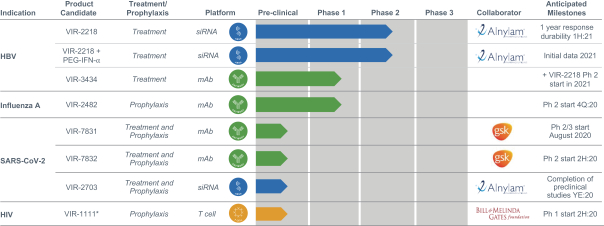
| * | VIR-1111 is a vaccine designed to establish proof of concept in Phase (Ph) 1 clinical trial to determine whether unique immune response observed in NHPs can be replicated in humans; ultimately, any candidates we advance as a potential HIV vaccine will require modifications to VIR-1111 before further clinical development. |
2
Table of Contents
SARS-CoV-2: The substantial impact of viral outbreaks and the need for global preparedness have been highlighted by the current COVID-19 pandemic. As of June 19, 2020, the virus had spread to 188 countries, there were over 8.5 million recorded infections and over 450,000 recorded deaths. We have moved rapidly to address this global health challenge. Our focus is on treating and preventing SARS-CoV-2, as well as potential future coronavirus outbreaks. To do so, we are taking multiple approaches: antibodies (VIR-7831 and VIR-7832), siRNA (VIR-2703), applying our innate immunity platform to identify cellular host genes necessary for virus replication, and vaccines. We anticipate that the initial registration populations for our product candidates will include those at high risk of contracting COVID-19 and those in need of treatment for COVID-19.
VIR-7831 and VIR-7832 are SARS-CoV-2-neutralizing mAbs. For VIR-7831, we plan to submit an Investigational New Drug Application, or IND, and thereafter commence a Phase 2/3 clinical trial program in August 2020. VIR-7832 is planned to initiate a Phase 2 clinical trial later this year. Both VIR-7831 and VIR-7832 are based on a parent antibody, S309, which was derived from samples previously gathered for research on pan-coronavirus-neutralizing mAbs. S309 has demonstrated high affinity and avidity for the SARS-CoV-2 spike protein and the ability to potently neutralize SARS-CoV-2 in multiple live-virus cellular assays. S309 binds to an epitope on SARS-CoV-2 that is shared with SARS-CoV-1 (also commonly known as ‘SARS’), indicating that the epitope is highly conserved. We believe the conservation of this epitope will make it more difficult for escape mutants to develop and result in a high barrier to resistance. S309 also exhibits potent effector function in vitro, potentially allowing the engagement and recruitment of immune cells to kill off already infected cells. VIR-7831 and VIR-7832 have both been engineered with “LS” mutations within the Fc region of the mAbs, for the purpose of increasing lung tissue bioavailability and extending their half-life. VIR-7832 has been further engineered with “XX2” mutations in the Fc region of the mAb to potentially allow it to function as a T cell vaccine. We anticipate initial clinical data from our Phase 2/3 trial of VIR-7831 to be available before the end of the year.
Our Phase 2/3 trial program for VIR-7831 will be comprised of the following:
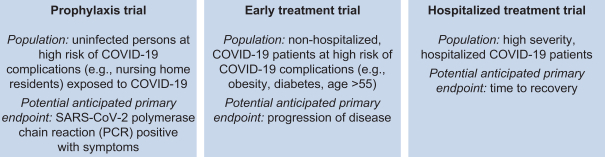
To accelerate the progress of VIR-7831 and VIR-7832, we have signed a number of collaboration agreements to aid in their manufacture and potential commercialization. Specifically, we are collaborating on clinical manufacturing with WuXi Biologics (Hong Kong) Limited, or WuXi, and Biogen Inc., or Biogen. We are collaborating on commercial manufacturing with WuXi and Samsung Biologics Co., Ltd., or Samsung, and we anticipate commercial supply of approximately 10-15 million doses in 2021, depending on titer, yield and dose amount. And we are collaborating on potential commercialization with WuXi for greater China and GlaxoSmithKline plc, or GSK, for all other countries. See the section titled “Recent SARS-CoV-2 Activities” for a description of these and other collaborations.
VIR-2703 is an inhaled SARS-CoV-2-targeting siRNA for which we are conducting preclinical studies that are expected to be completed by the end of 2020. In vitro, VIR-2703 has demonstrated the ability to significantly reduce SARS-CoV-2 live virus replication. It is designed to degrade the viral genome, leading to inhibition of viral protein synthesis and blocking the production of infectious virus. It targets a nucleic acid sequence in the SARS-CoV-2 genome that is highly conserved amongst currently available viral sequences and is also conserved in SARS-CoV-1. VIR-2703 leverages Alnylam’s latest advances in lung delivery of siRNAs and is the first development candidate selected in our expanded collaboration with Alnylam for SARS-CoV-2 and other coronaviruses.
3
Table of Contents
HBV: Approximately 290 million people globally are chronically infected with HBV and approximately 900,000 of them die from HBV-associated complications each year. There is a significant unmet medical need for more effective therapies that lead to life-long control of the virus after a finite duration of therapy, which is the definition of a functional cure. For a registrational trial to demonstrate a functional cure, the formal endpoint accepted by the U.S. Food and Drug Administration, or the FDA, is undetectable HBsAg, defined as less than 0.05 international units per milliliter, or IU/ml, as well as HBV DNA less than the lower limit of quantification, in the blood six months after the end of therapy.
We are developing VIR-2218 and VIR-3434 for the functional cure of HBV. Each of these product candidates has the potential to stimulate an effective immune response and also has direct antiviral activity against HBV. We believe that a functional cure for HBV will require an effective immune response, in addition to antiviral activity, based on the observation that severe immunosuppression can reactivate HBV disease. While monotherapy with VIR-2218 and VIR-3434 may provide a functional cure in some patients, we believe combination therapy will be necessary for a functional cure in many patients. We are planning trials that combine VIR-2218 and VIR-3434, which we believe have the potential to act in concert by removing potentially tolerogenic HBV proteins and stimulating new HBV specific T cells. We also recently initiated a trial that combines VIR-2218 with PEG-IFN-α and are evaluating additional combinations with other immunotherapy agents and direct acting antiviral agents. We anticipate that the initial registration population for these product candidates will be patients chronically infected with HBV.
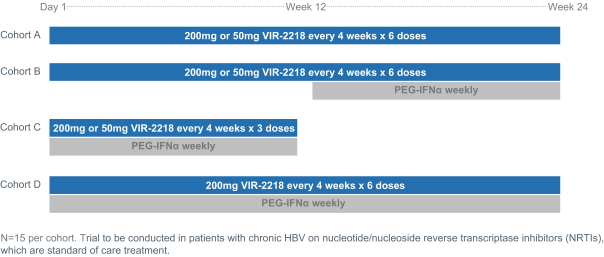
VIR-2218 is a subcutaneously administered HBV-targeting siRNA that is currently in a Phase 2 clinical trial. By targeting a conserved region of the HBV genome, it is designed to inhibit the production of all HBV proteins: X, polymerase, S, and core. Suppression of HBV proteins, particularly HBsAg, is hypothesized to remove the inhibition of T cell and B cell activity directed against HBV, allowing VIR-2218 to potentially result in a functional cure. VIR-2218 was the first siRNA in the clinic to include Alnylam’s ESC+ technology, which has the potential to enhance the therapeutic index. In total, 37 healthy volunteers have received VIR-2218 and 12 healthy volunteers have received placebo. In addition, 24 patients with chronic HBV on nucleotide/nucleoside reverse transcriptase inhibitors, or NRTIs, have received VIR-2218, and eight patients with chronic HBV on NRTIs have received placebo. The data suggest that VIR-2218 is generally well-tolerated in healthy volunteers given as a single dose up to 900 mg and in patients given as two doses of 20 mg, 50 mg, 100 mg or 200 mg each dose. The data also demonstrate substantial, dose dependent reductions in HBsAg in patients at doses ranging from 20 mg to 200 mg, which are durable at the higher doses for at least six months. We recently initiated a Phase 2 combination trial of VIR-2218 and PEG-IFN-α, and anticipate initial clinical data to be available in 2021.
The trial design for the Phase 2 combination trial of VIR-2218 and PEG-IFN-α is shown below:
4
Table of Contents
VIR-3434 is a subcutaneously administered HBV-neutralizing mAb currently in a Phase 1 clinical trial. By targeting a conserved region of HBsAg, it is designed to block entry of all 10 genotypes of HBV into liver cells called hepatocytes and reduce the level of virions and subviral particles in the blood. We have also engineered VIR-3434 to have an extended half-life and to potentially function as a therapeutic T cell vaccine for chronic HBV infection. These modifications are intended to enhance its potential to result in an HBV functional cure. We anticipate clinical data from our Phase 1 trial will enable us to initiate a Phase 2 clinical trial of VIR-3434 in combination with VIR-2218 in 2021.
Influenza: On average, each year the influenza virus infects 5% to 10% of the world’s population and results in an estimated 500,000 deaths. In the 2017-2018 flu season, it is now estimated that 61,000 people died from influenza in the United States alone. Influenza vaccines have historically had limited success, with an average efficacy of 40%. This limited efficacy results from incomplete coverage against seasonal strains and the lack of an effective immune response in many individuals after receiving the vaccine. We are developing VIR-2482 as a universal prophylactic for influenza A and have designed it to overcome both limitations of flu vaccines, which we believe will lead to meaningfully higher levels of protection. We anticipate that the initial registration population for VIR-2482 will be individuals at high risk of influenza A complications, such as the elderly with chronic lung disease or congestive heart failure.
VIR-2482 is an intramuscularly administered influenza A-neutralizing mAb currently in a Phase 1/2 clinical trial. In vitro, VIR-2482 has been shown to cover all major strains of influenza A that have arisen since the 1918 Spanish flu pandemic. We believe that VIR-2482 has the potential to provide superior protection to flu vaccines and be able to be used year after year because it has broad strain coverage as opposed to the limited strain coverage generated by vaccines. We also believe that it provides passive immunity rather than relying on a person to generate active immunity via a functional immune response, an ability that is known to decline with age. VIR-2482 has been engineered to increase lung tissue bioavailability and to extend its half-life so that a single intramuscular dose has the potential to last the entire flu season, which is typically five to six months long. VIR-2482 is estimated to have a half-life of 58 days based on preliminary data. VIR-2482 has been generally well-tolerated in the approximately 100 healthy volunteers dosed in the Phase 1 portion of the clinical trial. We anticipate initiating the Phase 2 portion of the clinical trial in the northern hemisphere in the fourth quarter of 2020, followed by a second northern hemisphere season if necessary. Data from an interim analysis of the first flu season of the Phase 2 clinical trial are anticipated to be available in the first half of 2021.
HIV: Each year there are approximately 1.8 million new cases of HIV and approximately 1.0 million HIV-related deaths globally. Current prevention approaches such as behavioral modification and pharmacological intervention have had only a modest effect on HIV transmission globally, leaving a high unmet medical need for a safe and effective vaccine for the billions of individuals who are or may become sexually active. VIR-1111 is a proof of concept HIV vaccine designed to elicit a type of immune response that is different from other vaccines. We anticipate the initial registration population for our eventual HIV vaccine will be individuals at high risk of contracting HIV.
VIR-1111 is a subcutaneously administered HIV T cell vaccine based on HCMV for which we plan to submit an IND in the second half of 2020 and thereafter commence a Phase 1 clinical trial. VIR-1111 has been designed to elicit T cells that recognize HIV epitopes that are different from those recognized by prior HIV vaccines and to stimulate a different and specific type of T cell immune response to HIV, known as an HLA-E restricted immune response. An HLA-E restricted immune response has been shown to be associated with protection of NHPs from simian immunodeficiency virus, or SIV, the NHP equivalent of HIV. VIR-1111 is a vaccine designed solely to establish proof of concept in a Phase 1 clinical trial to determine whether the unique immune response observed in NHPs can be replicated in humans.
5
Table of Contents
TB: Globally, nearly two billion people are latently infected with TB, and each year there are approximately 10 million new active cases of TB and approximately 1.6 million TB-related deaths. There is a high unmet medical need for a safe and effective vaccine that prevents active pulmonary TB in adolescents and adults, as they represent the key sources of TB transmission and are the primary contributors to overall disease burden. VIR-2020 is a vaccine designed to provide a type of immune response that is different from other vaccines and lead to meaningful levels of protection from active TB. We anticipate that the initial registration population for VIR-2020 will be people at high risk of developing active TB, such as those who have latent TB infection.
VIR-2020 is a subcutaneously administered TB T cell vaccine based on HCMV for which we plan to submit an IND in 2023 and thereafter commence a Phase 1 clinical trial. VIR-2020 is designed to stimulate T cells that reside in the lung and to recognize TB epitopes that are different from those recognized by prior TB vaccines. In preclinical studies, a T cell vaccine based on RhCMV has been shown to provide protection of NHPs from TB.
Recent SARS-CoV-2 Activities
Since February 2020, we have entered into a number of collaboration agreements to accelerate the development, manufacture, and potential commercialization of therapies to treat and prevent SARS-CoV-2 and other coronaviruses. We have also made substantial efforts to protect our intellectual property in this area, as evidenced by our recent expansion of our patent portfolio.
Development and Commercialization
GSK Collaboration Agreement
In June 2020, we entered into a definitive collaboration agreement with Glaxo Wellcome UK Limited and Beecham S.A. of GSK (and collectively referred to as GSK), pursuant to which we agreed to collaborate to research, develop and commercialize products for the prevention, treatment and prophylaxis of diseases caused by SARS-CoV-2 and potentially other coronaviruses. The collaboration is focused on the development and commercialization of three types of collaboration products under three programs: (1) antibodies targeting SARS-CoV-2, and potentially other coronaviruses, or the Antibody Program; (2) vaccines targeting SARS-CoV-2, and potentially other coronaviruses, or the Vaccine Program, and (3) products based on genome-wide CRISPR screening of host targets expressed in connection with exposure to SARS-CoV-2, or the Functional Genomics Program. The initial antibodies under the Antibody Program will be VIR-7831 and VIR-7832, which have demonstrated high affinity for the SARS-CoV-2 spike protein and are highly potent in neutralizing SARS-CoV-2 in live-virus cellular assays.
We are primarily responsible for the development and clinical manufacturing activities for the Antibody Program, and for conducting the initial development activities directed to a vaccine in the Vaccine Program. GSK will be primarily responsible for the commercialization activities for the Antibody Program (except in connection with sales of antibody products licensed to WuXi in greater China), the later-stage development, manufacturing and commercialization activities for the Vaccine Program and the development, manufacturing and commercialization activities for the Functional Genomics Program. We and GSK are required to use commercially reasonable efforts to conduct the activities assigned to each party under each development plan and to seek and obtain regulatory approval for collaboration products that arise from such activities in the United States and specified major markets. Subject to an opt-out mechanism, we and GSK will share all development costs, manufacturing costs and costs and expenses for the commercialization of the collaboration products, with us bearing 72.5% of such costs for the antibody products, 27.5% of such costs for the vaccine products, and we and GSK sharing equally all such costs for the functional genomics products, and all profits will be shared in the same ratios. If we and GSK elect to conduct a technology transfer of manufacturing technology under our agreements with WuXi (as further described below) and Biogen, we will bear 72.5% of the costs related to such
6
Table of Contents
manufacturing technology transfer and for commercial manufacturing of the antibody products under such agreements with WuXi and Biogen, and GSK will bear 27.5% of such costs. The parties will also share the committed costs for the reservation of manufacturing capacity for the drug substance for antibody products in the foregoing ratio under our agreement with Samsung as well as such costs relating to committed manufacturing capacity for antibody products as are approved by the joint steering committee from time to time.
On an antibody product-by-antibody product basis, we have a co-promotion right with respect to such antibody product in the United States, pursuant to which we will have the right to perform up to 20% of details in connection with such antibody product. GSK will lead commercialization and book all sales and is required to use commercially reasonable efforts to commercialize each collaboration product following regulatory approval in the United States and specified major markets. This definitive agreement superseded and replaced the April 2020 preliminary agreement with GSK. In connection with the GSK collaboration, we also entered into a stock purchase agreement in April 2020, pursuant to which we issued 6,626,027 shares of our common stock to an affiliate of GSK at a price per share of $37.73, for an aggregate purchase price of approximately $250.0 million.
Expansion of Alnylam Collaboration and License Agreement
In March and April 2020, we entered into two further amendments to our collaboration and license agreement with Alnylam, dated October 16, 2017, to expand our existing collaboration of five infectious disease targets to nine, including one targeting SARS-CoV-2 and potentially other coronaviruses, and up to three targeting human host factors for SARS-CoV-2.
Pursuant to both recent amendments, we and Alnylam will each be responsible for pre-clinical development costs incurred by such party in performing its allocated responsibilities under an agreed-upon initial pre-clinical development plan for each of the four new targets. We and Alnylam will equally share costs incurred in connection with the manufacture of non-GMP drug product required for pre-clinical development prior to filing of an IND. Following the completion of initial pre-clinical development activities, if we exercise our option to progress one or more candidates arising from the coronavirus program into further development, we will be responsible for conducting all development, manufacturing and commercialization activities at our sole expense, subject to Alnylam’s right to opt-in, during a specified period, to share equally with us the profits and losses in connection with development and commercialization of a coronavirus product.
Manufacturing
Consistent with our corporate manufacturing strategy of building internal capabilities in chemistry, manufacturing and control, or CMC, and working with contract development and manufacturing organizations, or CDMOs, to supply clinical and commercial batches of our product candidates, we have entered into the following agreements to date in support of our SARS-CoV-2 program:
WuXi Manufacturing Agreements
In February 2020, we entered into a development and manufacturing collaboration agreement with WuXi, for the clinical development, manufacturing, and commercialization of our proprietary antibodies developed for SARS-CoV-2. Under the agreement, WuXi will conduct cell-line development, process and formulation development, and initial manufacturing for clinical development. WuXi will have the right to commercialize products incorporating such antibodies in greater China pursuant to an exclusive license granted for the selected antibodies that have been developed. We will have the right to commercialize such products in all other markets worldwide.
7
Table of Contents
WuXi will perform mutually agreed development and manufacturing activities, under individual statements of work. In addition, the parties agreed that WuXi will pay us tiered royalties at percentages ranging from the high single-digits to mid-teens on annual net sales of all products sold by WuXi in greater China.
On June 15, 2020, we entered into a binding letter of intent with WuXi, pursuant to which WuXi will perform certain development and manufacturing services for our SARS-CoV-2 antibody program. Under the terms of the letter of intent, we have committed to purchase a firm and binding capacity reservation for the manufacture of a specified number of batches of drug substance of our SARS-CoV-2 antibody in 2020 and 2021. In addition, we have the right to order an additional specified number of batches of drug substance, provided we make such election by a specified date in the fourth calendar quarter in 2020. WuXi is obligated to reserve such manufacturing slots on a non-cancellable basis, and will manufacture the agreed number of batches of drug substance in accordance with an agreed manufacturing schedule. We are obligated to pay a total of approximately $130.0 million for such capacity reservation, if all batches are manufactured, inclusive of estimated raw material costs, with between 70% and 80% of the batch production fees owed to WuXi on a take-or-pay basis regardless of whether we utilize such manufacturing slots. The amounts will be payable during 2020 and 2021 and invoiced on a per-batch basis. The SARS-CoV-2 antibody drug substance contemplated to be manufactured in accordance with the terms of the letter of intent will be utilized in connection with progressing the development and commercialization of the SARS-CoV-2 antibody product under our collaboration with GSK.
We and WuXi will continue to negotiate additional terms in a definitive commercial manufacturing and supply agreement and will use commercially reasonable efforts to execute such definitive agreement before July 30, 2020.
We will bear 72.5% of the costs under the development and manufacturing collaboration agreement and letter of intent with WuXi and GSK will bear 27.5% of such costs pursuant to our collaboration agreement with GSK, subject to certain conditions and exceptions.
Biogen Clinical Development and Manufacturing Agreement
In May 2020, we entered into a clinical development and manufacturing agreement with Biogen pursuant to which Biogen will perform process development activities and specified manufacturing services under agreed statements of work for certain pre-commercial and clinical supply of our SARS-CoV-2 mAbs. We also agreed to collaborate with Biogen to develop highly productive clonal cell lines and clinical and commercial manufacturing processes for our SARS-CoV-2 mAbs. These processes are designed to be transferrable to global biomanufacturing facilities designed for advanced biologics production. Under the agreement, Biogen will conduct cGMP clinical manufacturing in the United States and provide technical support to facilitate process transfer to Samsung, and potentially other large-scale biomanufacturing facilities in the United States and other regions of the world to enable us to obtain reliable supply of a potential commercial product.
Under the terms of the Biogen agreement, we have agreed to pay fees for Biogen’s performance of services as provided in each applicable statement of work, including costs to third parties on a pass-through basis. We entered into three statements of work with Biogen for the process development and certain clinical manufacturing services simultaneously with the execution of the agreement, with the cost of activities under such agreed statements of work totaling approximately $13.8 million.
The Biogen agreement provides us the right to request a technology transfer of all manufacturing technology and processes developed under the agreement to us or any third party designated by us to conduct manufacturing of a SARS-CoV-2 antibody using such technology, including applicable licenses to us under Biogen’s relevant intellectual property rights. In connection with any such technology transfer, we have also agreed to pay an “access fee” to Biogen for each successful batch of SARS-CoV-2 antibody drug substance manufactured using
8
Table of Contents
certain improvements relating to increases in batch yield developed under the agreement, whether such manufacturing is performed by us, our affiliates, or third parties. If we successfully manufacture all batches of SARS-CoV-2 antibody drug substance for which we are currently committed under the Samsung letter agreement, based on our current working assumptions of manufacturing yield per batch, the access fee payable to Biogen in connection with the Samsung manufacturing will total approximately $100.0 million.
We will bear 72.5% of the costs under the Biogen agreement and GSK will bear 27.5% of such costs pursuant to our collaboration agreement with GSK, subject to certain conditions and exceptions.
Samsung Manufacturing Agreement
In April 2020, we entered into a binding letter agreement with Samsung pursuant to which Samsung will perform development and manufacturing services for our SARS-CoV-2 mAbs. Under the terms of the letter agreement, we have committed to purchase a firm and binding capacity reservation for a specified number of drug substance manufacturing slots in 2021 and 2022. Samsung will reserve such manufacturing slots on a non-cancellable, non-adjustable basis and will not offer such manufacturing slots under our capacity reservation to third parties. We are obligated to pay a total of approximately $362.0 million for such capacity reservation on a take-or-pay basis regardless of whether such manufacturing slots are utilized by us. The amounts will be payable during 2021 and 2022 and invoiced on a per-batch basis, with shortfalls invoiced at the end of the year in which such shortfall occurs. Samsung began performing services for us upon execution of the letter agreement, and we agreed to pay fees and out-of-pocket costs for the services performed thereunder. Samsung is expected to commence manufacturing on our behalf as early as October 2020 with the first engineering run.
We will bear 72.5% of the costs under the Samsung letter agreement and GSK will bear 27.5% of such costs pursuant to our collaboration agreement with GSK, subject to certain conditions and exceptions.
We continue to negotiate a definitive agreement with Samsung, to expand upon the letter agreement, and agreed to use best efforts to execute such definitive agreement before July 31, 2020.
Intellectual Property
VIR-7831
Our VIR-7831 intellectual property portfolio includes multiple United States provisional patent applications. These applications include composition of matter claims, pharmaceutical composition claims, and method of treatment claims. The 20-year term of any patents issuing from these provisional patent applications is presently estimated to expire in 2041, absent any available patent term adjustments or extensions.
Licensed Patents
Our VIR-7831 intellectual property portfolio also includes patents and patent applications that we have non- exclusively licensed from Xencor, Inc., or Xencor. As of February 15, 2020, these patents and applications include seven issued patents in the United States directed to composition of matter claims, methods of extending antibody serum half-life claims, pharmaceutical composition claims and process (methods of producing) claims. The 20-year term of these patents is presently estimated to expire between 2021 and 2025, absent any available patent term adjustments or extensions. Additionally, as of February 15, 2020, these patents and applications include 70 issued patents in Australia, Austria, Belgium, Canada, China, Croatia, Czech Republic, Estonia, Finland, France, Germany, Hungary, Iceland, India, Ireland, Israel, Italy, Japan, South Korea, Lithuania, Luxembourg, Malta, Monaco, Netherlands, Poland, Russia, Slovenia, Spain, Sweden, Switzerland, Turkey and the United Kingdom directed to composition of matter claims, pharmaceutical composition claims, method of
9
Table of Contents
treatment claims, composition for use in treatment claims and process (methods of producing) claims. The 20-year term of these patents is presently estimated to expire between 2021 and 2028, absent any available patent term adjustments or extensions.
The patents and applications we have non-exclusively licensed from Xencor also include, as of February 15, 2020, a pending patent application in the United States and five patent applications pending in Brazil, Canada, China, Europe and Russia directed to composition of matter claims, pharmaceutical composition claims, composition for use in treatment claims, and process (methods of producing) claims. The 20-year term of any patents issuing from these patent applications is presently estimated to expire between 2021 and 2028, absent any available patent term adjustments or extensions.
VIR-7832
Our VIR-7832 intellectual property portfolio includes multiple United States provisional patent applications. These applications include composition of matter claims, pharmaceutical composition claims, and method of treatment claims. The 20-year term of any patents issuing from these provisional patent applications is presently estimated to expire in 2041, absent any available patent term adjustments or extensions.
Licensed Patents
Our VIR-7832 intellectual property portfolio includes a patent family that we have exclusively licensed from Rockefeller, which includes, as of February 15, 2020, one pending patent application in the United States, one pending PCT patent application and one pending patent application in Europe. The applications in this family include composition of matter claims, pharmaceutical composition claims, method of treatment claims, composition for use in treatment claims and process (methods of producing) claims. The 20-year term of any patents issuing from the application in this family is presently estimated to expire in 2038, absent any available patent term adjustments or extensions.
Our VIR-7832 intellectual property portfolio also includes patents and patent applications that we have non- exclusively licensed from Xencor. As of February 15, 2020, these patents and applications include seven issued patents in the United States directed to composition of matter claims, methods of extending antibody serum half-life claims, pharmaceutical composition claims and process (methods of producing) claims. The 20-year term of these patents is presently estimated to expire between 2021 and 2025, absent any available patent term adjustments or extensions. Additionally, as of February 15, 2020, these patents and applications include 70 issued patents in Australia, Austria, Belgium, Canada, China, Croatia, Czech Republic, Estonia, Finland, France, Germany, Hungary, Iceland, India, Ireland, Israel, Italy, Japan, South Korea, Lithuania, Luxembourg, Malta, Monaco, Netherlands, Poland, Russia, Slovenia, Spain, Sweden, Switzerland, Turkey and the United Kingdom directed to composition of matter claims, pharmaceutical composition claims, method of treatment claims, composition for use in treatment claims and process (methods of producing) claims. The 20-year term of these patents is presently estimated to expire between 2021 and 2028, absent any available patent term adjustments or extensions.
The patents and applications we have non-exclusively licensed from Xencor also include, as of February 15, 2020, a pending patent application in the United States and five patent applications pending in Brazil, Canada, China, Europe and Russia directed to composition of matter claims, pharmaceutical composition claims, composition for use in treatment claims, and process (methods of producing) claims. The 20-year term of any patents issuing from these patent applications is presently estimated to expire between 2021 and 2028, absent any available patent term adjustments or extensions.
10
Table of Contents
VIR-2703
Licensed Patents
Our VIR-2703 intellectual property portfolio includes multiple United States provisional patent applications that we have exclusively licensed from Alnylam. These applications include composition of matter claims, pharmaceutical composition claims, and method of treatment claims. The 20-year term of any patents issuing from these provisional patent applications is presently estimated to expire in 2041, absent any available patent term adjustments or extensions.
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making a decision to invest in our common stock. These risks are more fully described in the section titled “Risk Factors” immediately following this prospectus summary. These risks include, among others, the following:
| • | We have incurred significant net losses since inception and anticipate that we will continue to incur substantial net losses for the foreseeable future and may never achieve or maintain profitability. |
| • | Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability. |
| • | Even after this offering, we will require substantial additional funding to finance our operations. If we are unable to raise capital when needed, we could be forced to delay, reduce or terminate certain of our development programs or other operations. |
| • | Our pursuit of a potential therapy for COVID-19, the disease caused by the virus SARS-CoV-2, is at an early stage, and we are committing substantial financial resources and personnel and making substantial capital commitments with third parties in furtherance thereof. |
| • | Our future success is substantially dependent on the successful clinical development, regulatory approval and commercialization of our product candidates in a timely manner. If we are not able to obtain required regulatory approvals, we will not be able to commercialize our product candidates and our ability to generate product revenue will be adversely affected. |
| • | We are a party to strategic collaboration and license agreements pursuant to which we are obligated to make substantial payments upon achievement of milestone events and, in certain cases, have relinquished important rights over the development and commercialization of certain current and future product candidates. We also intend to explore additional strategic collaborations, which may never materialize or may require that we relinquish rights to and control over the development and commercialization of our product candidates. |
| • | Success in preclinical studies or earlier clinical trials may not be indicative of results in future clinical trials and we cannot assure you that any ongoing, planned or future clinical trials will lead to results sufficient for the necessary regulatory approvals. |
| • | Clinical product development involves a lengthy and expensive process. We may incur additional costs and encounter substantial delays or difficulties in our clinical trials. |
| • | Our business could be materially adversely affected by the effects of health pandemics or epidemics, including the current COVID-19 pandemic and future outbreaks of the disease. |
| • | We intend to rely on third parties to produce clinical and commercial supplies of our product candidates. |
11
Table of Contents
| • | If we are unable to obtain and maintain patent protection for our product candidates and technology, or if the scope of the patent protection obtained is not sufficiently broad or robust, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our product candidates and technology may be adversely affected. |
| • | We are highly dependent on our key personnel, and if we are not able to retain these members of our management team or recruit and retain additional management, clinical and scientific personnel, our business will be harmed. |
Our Corporate Information
We were incorporated under the laws of the State of Delaware on April 7, 2016. Our principal executive offices are located at 499 Illinois Street, Suite 500, San Francisco, California 94158, and our telephone number is (415) 906-4324. Our corporate website address is www.vir.bio. Information contained on or accessible through our website is not a part of this prospectus or the registration statement of which it forms a part, and the inclusion of our website address in this prospectus is an inactive textual reference only.
“Vir Biotechnology,” “Vir Bio,” “Vir.Bio,” the Vir logo and other trademarks, trade names or service marks of Vir Biotechnology, Inc. appearing in this prospectus or the documents incorporated by reference herein are the property of Vir Biotechnology, Inc. All other trademarks, trade names and service marks appearing in this prospectus or incorporated by reference herein are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus or the documents incorporated by reference herein may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert their rights thereto.
Implications of Being an Emerging Growth Company
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, enacted in April 2012. For so long as we remain an emerging growth company, we are permitted and intend to rely on certain exemptions from various public company reporting requirements, including not being required to have our internal control over financial reporting audited by our independent registered public accounting firm pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and any golden parachute payments not previously approved. In particular, in this prospectus, we have provided only two years of audited consolidated financial statements and have not included all of the executive compensation-related information that would be required if we were not an emerging growth company. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
We would cease to be an “emerging growth company” upon the earliest to occur of: (i) December 31, 2024; (ii) the last day of the fiscal year in which we have $1.07 billion or more in annual revenue; (ii) the date on which we first qualify as a large accelerated filer under the rules of the U.S. Securities and Exchange Commission, or the SEC; and (iii) the date on which we have, in any three-year period, issued more than $1.0 billion in non-convertible debt securities. We may choose to take advantage of some but not all of these reduced reporting burdens.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of some accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting standards, and therefore we will not be subject to the same requirements to adopt new or revised accounting standards as other public companies that are not emerging growth companies.
12
Table of Contents
The Offering
| Common stock to be offered by us |
6,200,000 shares |
| Underwriters’ option to purchase additional shares |
930,000 shares |
| Common stock to be outstanding immediately after this offering |
122,499,280 shares (or approximately 123,429,280 shares if the underwriters exercise in full their option to purchase additional shares of common stock) |
| Use of proceeds |
We estimate that the net proceeds from this offering will be approximately $234.0 million (or approximately $269.2 million if the underwriters exercise in full their option to purchase up to 930,000 additional shares of common stock), based on the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| We currently intend to use the net proceeds from this offering, together with our existing cash, cash equivalents and short-term investments, to fund the research and development of our product candidates and development programs, including VIR-7831, VIR-7832, VIR-2703, VIR-2218, VIR-2218 with PEG-IFN-α, VIR-3434 and VIR-2482, and the remainder for commercial manufacturing and launch preparation for our SARS-CoV-2 antibodies, our other clinical trials and preclinical programs, as well as for working capital and other general corporate purposes. |
| The intended uses set forth above include any related milestone payments that may be due from us under the applicable license and collaboration agreements. In addition, we expect that the current grants from the Bill & Melinda Gates Foundation will fund the manufacture and early clinical development of VIR-1111 and VIR-2020. |
See the section titled “Use of Proceeds” for additional information.
| Risk factors |
You should read the section titled “Risk Factors” for a discussion of factors to consider carefully, together with all the other information included in this prospectus and incorporated by reference herein, before deciding to invest in our common stock. |
| Nasdaq Global Select Market symbol |
“VIR” |
13
Table of Contents
The number of shares of our common stock to be outstanding after this offering is based on 116,299,280 shares of common stock (excluding 1,457,432 shares of unvested restricted common stock) outstanding as of March 31, 2020 on a pro forma basis, and after giving effect to the subsequent issuances after March 31, 2020 of an aggregate of 7,948,912 shares of our common stock as described below, and excludes:
| • | 7,959,416 shares of our common stock issuable upon the exercise of outstanding stock options as of March 31, 2020, with a weighted-average exercise price of $7.30 per share; |
| • | 1,884,693 shares of our common stock issuable upon the exercise of outstanding stock options granted subsequent to March 31, 2020, with a weighted-average exercise price of $30.36 per share; |
| • | 7,611,513 shares of our common stock reserved for future issuance under our 2019 Equity Incentive Plan, or the 2019 Plan, as of March 31, 2020; and |
| • | 2,377,244 shares of our common stock reserved for future issuance under our 2019 Employee Stock Purchase Plan, or ESPP, as of March 31, 2020. |
Unless otherwise indicated, all information contained in this prospectus, including the number of shares of common stock that will be outstanding after this offering, assumes or gives effect to:
| • | the issuance of 6,626,027 shares of our common stock to Glaxo Group Limited, or GGL, on April 29, 2020 at a purchase price per share of $37.73, or approximately $250.0 million; |
| • | the issuance of 1,111,111 shares of our common stock to Alnylam on May 6, 2020 upon the achievement of a development milestone pursuant to a collaboration and license agreement; |
| • | the issuance of 211,774 shares of common stock to Takeda Ventures, Inc., or Takeda, on May 26, 2020 upon the cashless exercise of a warrant to purchase 244,444 shares; |
| • | no exercise of the outstanding options after March 31, 2020; and |
| • | no exercise by the underwriters of their option to purchase up to 930,000 additional shares of our common stock. |
14
Table of Contents
Summary Consolidated Financial Data
The following tables summarize our consolidated financial data for the periods and as of the dates set forth below. You should read the following summary consolidated financial data together with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes thereto included in our Annual Report on Form 10-K for the year ended December 31, 2019 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, each of which are incorporated by reference herein. We have derived the summary consolidated statements of operations data for the years ended December 31, 2018 and 2019 from our audited consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2019, which are incorporated by reference herein. We derived the summary consolidated statements of operations data for the three months ended March 31, 2019 and 2020, and the summary consolidated balance sheet data as of March 31, 2020, from our unaudited interim condensed consolidated financial statements included in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, which are incorporated by reference herein. Our unaudited consolidated interim financial statements were prepared on a basis consistent with our audited consolidated financial statements and include, in management’s opinion, all adjustments, consisting of normal recurring adjustments, that we consider necessary for a fair presentation of the financial information set forth in those financial statements. Our historical results are not necessarily indicative of the results that may be expected in the future and our results for the three months ended March 31, 2020 are not necessarily indicative of results to be expected for the full fiscal year or any other period.
| Year Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| 2018 | 2019 | 2019 | 2020 | |||||||||||||
| (in thousands, except share and per share data) |
||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||
| Revenue: |
||||||||||||||||
| Grant revenue |
$ | 9,800 | $ | 7,380 | $ | 3,644 | $ | 5,231 | ||||||||
| Contract revenue |
868 | 711 | 17 | 487 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
10,668 | 8,091 | 3,661 | 5,718 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
100,229 | 148,472 | 25,872 | 64,979 | ||||||||||||
| General and administrative |
29,131 | 37,598 | 8,559 | 12,649 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
129,360 | 186,070 | 34,431 | 77,628 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(118,692 | ) | (177,979 | ) | (30,770 | ) | (71,910 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
2,540 | 8,511 | 2,245 | 1,755 | ||||||||||||
| Other income (expense), net |
(212 | ) | (5,061 | ) | (145 | ) | (7,069 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense) |
2,328 | 3,450 | 2,100 | (5,314 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before benefit from (provision for) income taxes |
(116,364 | ) | (174,529 | ) | (28,670 | ) | (77,224 | ) | ||||||||
| Benefit from (provision for) income taxes |
480 | (154 | ) | — | (16 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (115,884 | ) | $ | (174,683 | ) | $ | (28,670 | ) | $ | (77,240) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share, basic and diluted(1) |
$ | (15.12) | $ | (5.76) | $ | (3.19) | $ | (0.71) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares outstanding, basic and diluted(1) |
7,666,463 | 30,349,920 | 9,001,158 | 108,387,913 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | See Notes 2 and 13 to our audited consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2019 and Note 12 to our unaudited condensed consolidated financial statements included in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, each of which are incorporated by reference herein, for explanations of the |
15
Table of Contents
| calculations of our basic and diluted net loss per share and the weighted-average number of shares outstanding used in the computation of the per share amounts. |
| As of March 31, 2020 | ||||||||||||
| Actual | Pro Forma(1) | Pro Forma As Adjusted(2)(3) |
||||||||||
| (in thousands) | ||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||
| Cash, cash equivalents and short-term investments |
$ | 355,611 | $ | 605,611 | $839,596 | |||||||
| Working capital(4) |
321,145 | 571,145 | 805,130 | |||||||||
| Total assets |
477,114 | 727,114 | 961,099 | |||||||||
| Accumulated deficit |
(445,759 | ) | (445,759 | ) | (445,759) | |||||||
| Total stockholders’ equity |
380,333 | 630,333 | 864,318 | |||||||||
| (1) | The pro forma column reflects: (i) the issuance of 6,626,027 shares of our common stock to GGL on April 29, 2020 at a purchase price per share of $37.73, or approximately $250.0 million; (ii) the issuance of 1,111,111 shares of our common stock to Alnylam on May 6, 2020 upon the achievement of a development milestone pursuant to a collaboration and license agreement; and (iii) the issuance of 211,774 shares of common stock to Takeda on May 26, 2020 upon the cashless exercise of a warrant to purchase 244,444 shares. |
| (2) | The pro forma as adjusted column reflects (i) the pro forma adjustments set forth in footnote (1) above and (ii) the sale of 6,200,000 shares of our common stock in this offering at the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| (3) | The pro forma as adjusted information discussed above is illustrative only and will depend on the actual public offering price and other terms of this offering determined at pricing. Each $1.00 increase or decrease in the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, would increase or decrease, as applicable, each of our pro forma as adjusted cash, cash equivalents and short-term investments, working capital, total assets and total stockholders’ equity by approximately $5.8 million, assuming the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase or decrease of 1.0 million shares of common stock offered by us would increase or decrease, as applicable, each of our pro forma as adjusted cash, cash equivalents and short-term investments, working capital, total assets and total stockholders’ equity by approximately $37.9 million, assuming the assumed public offering price of $40.32 per share remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| (4) | We define working capital as current assets less current liabilities. See our unaudited condensed consolidated financial statements and the related notes included in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, which is incorporated by reference herein, for further details regarding our current assets and current liabilities. |
16
Table of Contents
Investing in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below, as well as the risks and uncertainties set forth under the heading “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, which is incorporated by reference herein, and all of the other information in this prospectus and the documents incorporated by reference herein before deciding whether to purchase shares of our common stock. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that event, the price of our common stock could decline, and you could lose part or all of your investment.
Risks Related to This Offering
We have broad discretion in the use of our cash, cash equivalents and short-term investments, including the net proceeds from this offering, and may use them ineffectively, in ways with which you do not agree or in ways that do not increase the value of your investment.
Our management will have broad discretion in the application of our cash, cash equivalents and short-term investments, including the net proceeds from this offering, and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in additional operating losses that could have a negative impact on our business, cause the price of our common stock to decline and delay the development of our product candidates. Pending their use, we may invest our cash, cash equivalents and short-term investments, including the net proceeds from this offering, in a manner that does not produce income or that loses value. See the section titled “Use of Proceeds” for additional information.
We will require substantial additional funding to finance our operations. If we are unable to raise additional capital when needed, we could be forced to delay, reduce or terminate certain of our development programs or other operations.
Based on our current operating plan, we expect our operating expenses to increase in future periods relative to our historical spend, and we believe that the net proceeds from this offering, together with our existing cash, cash equivalents and short-term investments, will fund our operations through at least the next 12 months. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned. Moreover, it is particularly difficult to estimate with certainty our future expenses given the dynamic and rapidly evolving nature of our business and the COVID-19 pandemic environment generally. The expected net proceeds from this offering, together with our cash, cash equivalents and short-term investments, will not be sufficient for us to fund any of our product candidates through regulatory approval, and we will need to raise additional capital to complete the development and commercialization of our product candidates and fund certain of our existing manufacturing and other commitments. We expect to finance our cash needs through public or private equity or debt financings, third-party (including government) funding and marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements, or any combination of these approaches. Our future capital requirements will depend on many factors, including:
| • | the timing, progress and results of our ongoing preclinical studies and clinical trials of our product candidates; |
| • | the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials of other product candidates that we may pursue; |
17
Table of Contents
| • | our ability to establish and maintain collaboration, license, grant and other similar arrangements, and the financial terms of any such arrangements, including timing and amount of any future milestones, royalty or other payments due thereunder; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs and timing of future commercialization activities, including product manufacturing, marketing, sales and distribution, for any of our product candidates for which we receive marketing approval; |
| • | the revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval; |
| • | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; |
| • | any expenses needed to attract, hire and retain skilled personnel; |
| • | the costs of operating as a public company; and |
| • | the extent to which we acquire or in-license other companies’ product candidates and technologies. |
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenue, if any, will be derived from sales of products that we do not expect to be commercially available for several years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives.
The COVID-19 pandemic continues to rapidly evolve and has already resulted in a significant disruption of global financial markets. If the disruption persists and deepens, we could experience an inability to access additional capital, which could in the future negatively affect our capacity for certain corporate development transactions or our ability to make other important, opportunistic investments. Adequate additional financing may not be available to us on acceptable terms, or at all. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or altogether terminate our research and development programs or future commercialization efforts, which may adversely affect our business, financial condition, results of operations and prospects. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
If you purchase shares of common stock in this offering, you will suffer immediate dilution of your investment.
The public offering price of our common stock will be substantially higher than the pro forma, as adjusted net tangible book value per share of our common stock as of March 31, 2020. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our pro forma, as adjusted net tangible book value per share immediately after this offering. Based on the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, you will experience immediate dilution of $33.69 per share, representing the difference between our pro forma, as adjusted net tangible book value per share after this offering and the public offering price per share. After this offering, we will also have outstanding options to purchase common stock. To the extent these outstanding options are exercised, there will be further dilution to investors in this offering. See the section titled “Dilution” for additional information.
18
Table of Contents
Future sales and issuances of our capital stock or rights to purchase capital stock could result in additional dilution of the percentage ownership of our stockholders and could cause the price of our common stock to decline.
We may issue additional securities following the closing of this offering. Future sales and issuances of our capital stock or rights to purchase our capital stock could result in substantial dilution to our existing stockholders. We may sell common stock, convertible securities, and other equity securities in one or more transactions at prices and in a manner as we may determine from time to time. If we sell any such securities in subsequent transactions, investors may be materially diluted. New investors in such subsequent transactions could gain rights, preferences, and privileges senior to those of holders of our common stock.
Future sales of our common stock in the public market could cause the market price of our common stock to decline.
Sales of a substantial number of shares of our common stock in the public market, or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that such sales may have on the prevailing market price of our common stock.
As of March 31, 2020, we had outstanding a total of 108,350,368 shares of common stock (without giving effect to any of the share issuances that occurred after March 31, 2020). All of our outstanding shares as of such date were eligible for sale in the public market, other than shares and options held by directors, executive officers, and other affiliates that are subject to volume limitations under Rule 144 of the Securities Act of 1933, as amended, or the Securities Act, and various vesting agreements.
Future sales also could cause the trading price of our common stock to decline and make it more difficult for investors to sell shares of our common stock.
19
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents we have filed with the SEC that are incorporated by reference herein contain “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These statements relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. All statements other than statements of historical facts contained in this prospectus or in the documents incorporated by reference herein, including statements regarding our strategy, future financial condition, future operations, research and development, planned clinical trials and preclinical studies, technology platforms, the timing and likelihood of regulatory filings and approvals for our product candidates, our ability to commercialize our product candidates, the potential benefits of collaborations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “positioned,” “potential,” “predict,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of known and unknown risks, uncertainties and assumptions described in the section titled “Risk Factors” and in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section in our Annual Report on Form 10-K for the year ended December 31, 2019 and in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, which are incorporated by reference herein, and elsewhere in this prospectus and the documents incorporated by reference herein. Other sections of this prospectus and the documents incorporated by reference herein may include additional factors that could harm our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements.
In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus or the documents incorporated by reference herein, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur at all. You should refer to the section titled “Risk Factors” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act do not protect any forward-looking statements that we make in connection with this offering.
You should read this prospectus and the documents that we incorporate by reference in this prospectus and the documents that we have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus and in the documents incorporated by reference herein by these cautionary statements.
20
Table of Contents
Certain market, industry and competitive data contained or incorporated by reference in this prospectus were obtained from our own internal estimates and research, as well as from publicly available information, reports of governmental agencies and industry publications and surveys. In some cases, we do not expressly refer to the sources from which this data is derived. All of the market and industry data used in this prospectus is inherently subject to uncertainties and involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such information. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section titled “Risk Factors” in this prospectus. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
21
Table of Contents
We estimate that the net proceeds to us from this offering will be approximately $234.0 million (or approximately $269.2 million if the underwriters exercise in full their option to purchase up to 930,000 additional shares of common stock), based on the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase or decrease in the assumed public offering price of $40.32 per share would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $5.8 million, assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase or decrease of 1.0 million shares of common stock offered by us, would increase or decrease, as applicable, the net proceeds to us by approximately $37.9 million, assuming the assumed public offering price of $40.32 per share remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds from this offering, together with our existing cash, cash equivalents and short-term investments, as follows:
| • | to fund the following clinical trials through completion: |
| • | VIR-7831 – our planned IND for SARS-CoV-2 and assuming regulatory clearance, subsequent Phase 2/3 clinical trial, |
| • | VIR-7832 – our planned IND or equivalent submission for SARS-CoV-2 and assuming regulatory clearance, subsequent Phase 2 |
| • | VIR-2703 – our ongoing preclinical studies for SARS-CoV-2, |
| • | VIR-2218 – our ongoing Phase 2 clinical trial of VIR-2218 and Phase 2 combination trial of VIR-2218 and PEG-IFN-α for HBV, |
| • | VIR-3434 – our ongoing Phase 1 clinical trial for HBV, and |
| • | VIR-2482 – our ongoing Phase 1/2 clinical trial for influenza A, including the Phase 2 portion of the trial in the northern hemisphere that we anticipate initiating in the fourth quarter of 2020; and |
| • | any remainder for commercial manufacturing and launch preparation for our SARS-CoV-2 antibodies, our other clinical trials and preclinical programs, as well as for working capital and other general corporate purposes. |
The intended uses set forth above include any related milestone payments that may be due from us under the applicable license and collaboration agreements. In addition, we expect that the current grants from the Bill & Melinda Gates Foundation will fund the manufacture and early clinical development of VIR-1111 and VIR-2020.
Based on our current operating plan, we expect our operating expenses to increase in future periods relative to our historical spend, and we believe that the net proceeds from this offering, together with our existing cash, cash equivalents and short-term investments, will fund our operations through at least the next 12 months.
This expected use of the net proceeds from this offering represents our intentions based on our current plans and business conditions, which could change in the future as our plans and business conditions evolve. Further, due to the uncertainties inherent in the drug development process, it is difficult to estimate with certainty the amounts of the net proceeds from this offering that may be used for the above purposes. Moreover, it is particularly difficult to estimate with certainty our future expenses given the dynamic and rapidly evolving nature of our business and the COVID-19 pandemic environment generally.
22
Table of Contents
Our management will have broad discretion over the use of the net proceeds from this offering, and our investors will be relying on the judgment of our management regarding the application of the net proceeds of this offering. The amounts and timing of our expenditures will depend upon numerous factors including the results of our research and development efforts, the timing and success of preclinical studies and any ongoing clinical trials or clinical trials we may commence in the future, the timing of regulatory submissions and the amount of cash obtained through current and any future collaborations.
The expected net proceeds from this offering, together with our cash, cash equivalents and short-term investments, will not be sufficient for us to fund any of our product candidates through regulatory approval and commercialization, and we will need to raise additional capital to complete the development and commercialization of our product candidates and fund certain of our existing manufacturing and other commitments. We expect to finance our cash needs through a combination of equity offerings, debt financings and potential collaborations, and license and development agreements. We have based these estimates on assumptions that may prove to be incorrect, and we could expend our available capital resources at a rate greater than we currently expect.
Pending the use of the net proceeds from this offering as described above, we intend to invest the net proceeds in a variety of capital preservation instruments, including short-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
23
Table of Contents
We have never declared or paid cash dividends on our capital stock, and we do not currently intend to pay any cash dividends on our capital stock in the foreseeable future. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business. Any future determination related to dividend policy will be made at the discretion of our board of directors, subject to applicable laws, and will depend upon, among other factors, our results of operations, financial condition, contractual restrictions and capital requirements. In addition, our ability to pay cash dividends on our capital stock in the future may be limited by the terms of any future debt or preferred securities we issue or any credit facilities we enter into.
24
Table of Contents
The following table sets forth our cash, cash equivalents and short-term investments, and our capitalization as of March 31, 2020 on:
| • | an actual basis; |
| • | a pro forma basis to reflect: (i) the issuance of 6,626,027 shares of our common stock to GGL on April 29, 2020 at a purchase price per share of $37.73, or approximately $250.0 million, (ii) the issuance of 1,111,111 shares of our common stock to Alnylam on May 6, 2020 upon the achievement of a development milestone pursuant to a collaboration and license agreement, and (iii) the issuance of 211,774 shares of common stock to Takeda on May 26, 2020 upon the cashless exercise of a warrant to purchase 244,444 shares; and |
| • | a pro forma as adjusted basis giving effect to the pro forma adjustments discussed above, and giving further effect to the sale of 6,200,000 shares of our common stock in this offering at the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read this table together with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our consolidated financial statements and the related notes included in our condensed consolidated financial statements in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, which are incorporated by reference herein.
| As of March 31, 2020 | ||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted(1) |
||||||||||
| (in thousands, except share and per share amounts) |
||||||||||||
| Cash, cash equivalents and short-term investments |
$ | 355,611 | $ | 605,611 | $ | 839,596 | ||||||
|
|
|
|
|
|
|
|||||||
| Stockholders’ equity: |
||||||||||||
| Preferred stock, $0.0001 par value per share; 10,000,000 shares authorized as of March 31, 2020; no shares issued or outstanding, actual, pro forma, or pro forma as adjusted |
— | — | — | |||||||||
| Common stock, $0.0001 par value per shares; 300,000,000 shares authorized as of March 31, 2020; 108,350,368 shares issued and outstanding as of March 31, 2020, actual; 116,299,280 shares issued and outstanding, pro forma, and 122,499,280 shares issued and outstanding, pro forma as adjusted |
11 | 12 | 12 | |||||||||
| Additional paid-in capital |
825,833 | 1,075,832 | 1,309,817 | |||||||||
| Accumulated other comprehensive income |
248 | 248 | 248 | |||||||||
| Accumulated deficit |
(445,759 | ) | (445,759 | ) | (445,759 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity |
380,333 | 630,333 | 864,318 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 380,333 | $ | 630,333 | $ | 864,318 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | The pro forma as adjusted information discussed above is illustrative only and will depend on the actual public offering price and other terms of this offering determined at pricing. Each $1.00 increase or decrease in the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, would increase or decrease, as applicable, each of our pro forma as adjusted cash, cash equivalents and short-term investments, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $5.8 million, assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase or decrease of 1.0 million shares of common stock offered by us would increase or decrease, as applicable, each of our pro forma as adjusted cash, cash equivalents and |
25
Table of Contents
| short-term investments, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $37.9 million, assuming that the assumed public offering price of $40.32 per share remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The number of shares of our common stock to be outstanding after this offering is based on 116,299,280 shares of common stock (excluding 1,457,432 shares of unvested restricted common stock) outstanding as of March 31, 2020 on a pro forma basis, and after giving effect to the subsequent issuances after March 31, 2020 of an aggregate of 7,948,912 shares of our common stock as described above, and excludes:
| • | 7,959,416 shares of our common stock issuable upon the exercise of outstanding stock options as of March 31, 2020, with a weighted-average exercise price of $7.30 per share; |
| • | 1,884,693 shares of our common stock issuable upon the exercise of outstanding stock options granted subsequent to March 31, 2020, with a weighted-average exercise price of $30.36 per share; |
| • | 7,611,513 shares of our common stock reserved for future issuance under the 2019 Plan as of March 31, 2020; and |
| • | 2,377,244 shares of our common stock reserved for future issuance under the ESPP as of March 31, 2020. |
26
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our historical net tangible book value as of March 31, 2020 was $328.0 million, or $3.03 per share of our common stock. Our historical net tangible book value represents our total tangible assets less total liabilities. Historical net tangible book value per share is our historical net tangible book value divided by the number of shares of our common stock (excluding 1,457,432 shares of unvested restricted common stock) outstanding as of March 31, 2020.
Our pro forma net tangible book value as of March 31, 2020 was $578.0 million, or $4.97 per share of our common stock, based on the total number of shares of our common stock outstanding as of March 31, 2020 on a pro forma basis to reflect issuances of our common stock after March 31, 2020 as described below. Pro forma net tangible book value per share represents our total tangible assets less our total liabilities, divided by the number of shares of our common stock (excluding 1,457,432 shares of unvested restricted common stock) outstanding as of March 31, 2020 after giving effect to:
| • | the issuance of 6,626,027 shares of our common stock to GGL on April 29, 2020 at a purchase price per share of $37.73, or approximately $250.0 million; |
| • | the issuance of 1,111,111 shares of our common stock to Alnylam on May 6, 2020 upon the achievement of a development milestone pursuant to a collaboration and license agreement; and |
| • | the issuance of 211,774 shares of common stock to Takeda on May 26, 2020 upon the cashless exercise of a warrant to purchase 244,444 shares. |
After giving effect to the sale of 6,200,000 shares of common stock in this offering at the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of March 31, 2020 would have been $812.0 million, or $6.63 per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $1.66 per share to our existing stockholders and an immediate dilution of $33.69 per share to new investors participating in this offering.
The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share |
$ | 40.32 | ||||||
| Historical net tangible book value per share as of March 31, 2020 |
$ | 3.03 | ||||||
| Pro forma increase in net tangible book value per share as of March 31, 2020 attributable to the pro forma transactions described above |
1.94 | |||||||
|
|
|
|||||||
| Pro forma net tangible book value per share as of March 31, 2020 |
4.97 | |||||||
| Increase in pro forma net tangible book value per share attributable to new investors participating in this offering |
1.66 | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after this offering |
6.63 | |||||||
|
|
|
|||||||
| Dilution per share to new investors participating in this offering |
$ | 33.69 | ||||||
|
|
|
The pro forma as adjusted information discussed above is illustrative only and will depend on the actual public offering price and other terms of this offering determined at pricing. Each $1.00 increase or decrease in the assumed public offering price of $40.32 per share, which was the last reported sale price of our common stock on The Nasdaq Global Select Market on July 2, 2020, would increase or decrease, as applicable, our pro
27
Table of Contents
forma as adjusted net tangible book value per share after this offering by $0.05 per share and the dilution per share to new investors participating in this offering by $0.95 per share, assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, an increase of 1.0 million in the number of shares of common stock offered by us would increase the pro forma as adjusted net tangible book value after this offering by $0.25 per share and decrease the dilution per share to new investors participating in this offering by $0.25 per share, and a decrease of 1.0 million shares of common stock offered by us would decrease the pro forma as adjusted net tangible book value by $0.26 per share, and increase the dilution per share to new investors in this offering by $0.26 per share, assuming that the assumed public offering price of $40.32 per share remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise in full their option to purchase up to 930,000 additional shares of common stock from us, the pro forma as adjusted net tangible book value per share after giving effect to this offering would be $6.86 per share, representing an immediate increase to existing stockholders of $1.89 per share, and dilution to new investors participating in this offering of $33.46 per share.
The foregoing discussion and tables above are based on 116,299,280 shares of common stock (excluding 1,457,432 shares of unvested restricted common stock) outstanding as of March 31, 2020 on a pro forma basis, and after giving effect to the subsequent issuances after March 31, 2020 of an aggregate of 7,948,912 shares of our common stock as described above, and excludes:
| • | 7,959,416 shares of our common stock issuable upon the exercise of outstanding stock options as of March 31, 2020, with a weighted-average exercise price of $7.30 per share; |
| • | 1,884,693 shares of our common stock issuable upon the exercise of outstanding stock options granted subsequent to March 31, 2020, with a weighted-average exercise price of $30.36 per share; |
| • | 7,611,513 shares of our common stock reserved for future issuance under the 2019 Plan as of March 31, 2020; and |
| • | 2,377,244 shares of our common stock reserved for future issuance under the ESPP as of March 31, 2020. |
To the extent that any outstanding options are exercised, new options or other equity awards are issued under our equity incentive plans, or we issue additional equity or convertible debt securities in the future, there will be further dilution to new investors participating in this offering.
28
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions since January 1, 2017 and any currently proposed transactions, to which we were or are to be a participant, in which (i) the amount involved exceeded or will exceed $120,000 and (ii) any of our directors, executive officers or holders of more than 5% of our capital stock, or any affiliate or member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest, other than compensation and other arrangements that are described under the sections titled “Executive Compensation” and “Director Compensation” included in our Definitive Proxy Statement on Schedule 14A for our 2020 Annual Meeting of Stockholders, which is incorporated by reference herein.
We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that we would pay or receive, as applicable, in arm’s-length transactions.
Convertible Preferred Stock Financings
Series A-1 Convertible Preferred Stock Financing
In September 2016, we entered into a Series A-1 preferred stock purchase agreement with various investors, pursuant to which we issued shares of our Series A-1 convertible preferred stock at a price per share of $4.50. We held a second closing in March 2017, at which time we issued an additional 3,333,333 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $15.0 million. Two additional closings occurred in June 2017, at which time we issued an aggregate of 24,571,107 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $110.6 million. The fifth closing occurred in July 2017, at which time we issued an additional 6,641,111 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $29.9 million.
In August 2017, the Series A-1/B Purchase Agreement was amended and restated, or the A&R Purchase Agreement, pursuant to which we issued an aggregate of 25,843,330 shares of Series A-1 convertible preferred stock at $4.50 per share for gross proceeds of $116.3 million in five closings. The first two closings occurred in August 2017, at which time we issued an aggregate of 21,111,110 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $95.0 million. Two additional closings occurred in September 2017, at which time we issued an aggregate of 3,968,270 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $17.9 million. The fifth closing occurred in October 2017, at which time we issued an aggregate of 763,950 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $3.4 million. In June 2018, the A&R Purchase Agreement was amended, or the Amended A&R Purchase Agreement, pursuant to which we issued an aggregate of 3,222,220 shares of Series A-1 convertible preferred stock at $4.50 per share for gross proceeds of $14.5 million in two closings. The first closing occurred in June 2018, at which time we issued an additional 2,777,776 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $12.5 million. The second closing occurred in July 2018, at which time we issued an additional 444,444 shares of our Series A-1 convertible preferred stock for gross cash proceeds of $2.0 million.
The table below sets forth the number of shares of our Series A-1 convertible preferred stock purchased by our executive officers, directors, holders of more than 5% of our capital stock and their affiliated entities or immediate family members. Each share of Series A-1 convertible preferred stock in the table below converted into one share of our common stock in October 2019 upon the closing of our initial public offering.
29
Table of Contents
| Name | Series A-1 Convertible Preferred Stock (#) |
Aggregate Cash Purchase Price ($) |
||||||
| Entities affiliated with ARCH Venture Partners(1) |
24,444,442 | 110,000,000 | ||||||
| SVF Endurance (Cayman) Limited(2) |
15,555,555 | 70,000,000 | ||||||
| Alta Partners NextGen Fund I, L.P.(3) |
1,666,666 | 7,500,000 | ||||||
| (1) | Kristina Burow and Robert Nelsen, members of our Board of Directors, were designated to our Board in 2016 by ARCH Venture Fund IX, L.P., or ARCH IX, which is an affiliate of ARCH Venture Fund IX Overage, L.P., or ARCH Overage, and their affiliated funds. ARCH Venture Partners IX, L.P., or ARCH IX LP, is the sole general partner of ARCH IX, and ARCH IX Overage LP is the sole general partner of ARCH Overage. Mr. Nelsen is a managing director of ARCH Venture Partners IX, LLC, or ARCH IX LLC, the sole general partner of ARCH IX LP and ARCH IX Overage LP. Ms. Burow holds an interest in each of ARCH IX LP and ARCH IX Overage LP. Dr. Daniel, a former member of our Board of Directors, is a venture partner of ARCH, which is an affiliate of ARCH IX and ARCH Overage, and their affiliated funds. Dr. Parrish, our Chief Business Officer, is a venture partner of ARCH, which is an affiliate of ARCH IX and ARCH Overage, and their affiliated funds. |
| (2) | SVF Endurance (Cayman) Limited is a wholly owned subsidiary of SoftBank Vision Fund (AIV M1) L.P., SVF. Dipchand Nishar, a member of our Board of Directors, was designated to our Board in 2017 by SVF. Mr. Nishar is Senior Managing Partner at SoftBank Investment Advisers, an affiliate of SVF. |
| (3) | Robert More, a member of our board of directors, is a managing director of Alta Partners NextGen Fund I Management, LLC, or APNG I Management. APNG I Management is the general partner of Alta Partners NextGen Fund I, L.P., or APNG I. |
Series B Convertible Preferred Stock Financing
In January 2019, we issued an aggregate of 18,202,213 shares of Series B convertible preferred stock at $18.00 per share for gross proceeds of $327.6 million in two closings pursuant to an Amended and Restated Series A-1 and Series B Preferred Stock Purchase Agreement. Both closings occurred in January 2019.
The table below sets forth the number of shares of our Series B convertible preferred stock purchased by our executive officers, directors, holders of more than 5% of our capital stock and their affiliated entities or immediate family members. Each share of Series B convertible preferred stock in the table below converted into one share of our common stock upon the completion of our initial public offering.
| Name |
Series B Convertible Preferred Stock (#) |
Aggregate Cash Purchase Price ($) |
||||||
| Entities affiliated with ARCH Venture Partners(1) |
2,777,777 | 50,000,000 | ||||||
| SVF Endurance (Cayman) Limited(2) |
6,111,111 | 110,000,000 | ||||||
| Alta Partners NextGen Fund I, L.P.(3) |
277,777 | 5,000,000 | ||||||
| (1) | Ms. Burow and Mr. Nelsen, members of our Board of Directors, were designated to our Board in 2016 by ARCH IX, which is an affiliate of ARCH Overage, and their affiliated funds. ARCH IX LP, is the sole general partner of ARCH IX, and ARCH IX Overage LP is the sole general partner of ARCH Overage. Mr. Nelsen is a managing director of ARCH IX LLC, the sole general partner of ARCH IX LP and ARCH IX Overage LP. Ms. Burow holds an interest in each of ARCH IX LP and ARCH IX Overage LP. Dr. Daniel, a former member of our Board of Directors, is a venture partner of ARCH, which is an affiliate of ARCH IX and ARCH Overage, and their affiliated funds. Dr. Parrish, our Chief Business Officer, is a venture partner of ARCH, which is an affiliate of ARCH IX and ARCH Overage, and their affiliated funds. |
| (2) | SVF Endurance (Cayman) Limited is a wholly owned subsidiary of SVF. Mr. Nishar, a member of our Board of Directors, was designated to our Board in 2017 by SVF. Mr. Nishar is Senior Managing Partner at SoftBank Investment Advisers, an affiliate of SVF. |
| (3) | Mr. More, a member of our Board of Directors, is a managing director of APNG I Management. APNG I Management is the general partner of APNG I. |
30
Table of Contents
Relationships with Klaus Frueh
In June 2016, we entered into a consulting agreement with Klaus Frueh, Ph.D., a former member of our Board of Directors and a stockholder, pursuant to which Dr. Frueh agreed to provide certain consulting, advisory and related services within the field of immune programming on an exclusive basis, in exchange for a consulting fee of $150,000 per year. Unless we terminate the agreement earlier, the consulting agreement will terminate in September 2021. We paid Dr. Frueh an aggregate of $150,000 pursuant to the consulting agreement during 2019.
Investors’ Rights, Management Rights, Voting and Co-Sale Agreements
In connection with our convertible preferred stock financings, we entered into investors’ rights, management rights, voting and right of first refusal and co-sale agreements containing registration rights, information rights, rights of first offer, voting rights and rights of first refusal, among other things, with certain holders of our capital stock. The holders of more than 5% of our capital stock that are party to these agreements are entities affiliated with ARCH Venture Partners and SVF. In connection with our acquisition of TomegaVax, former stockholders of TomegaVax became parties to the investors’ rights, voting and right of first refusal and co-sale agreements. Our directors who are parties to these agreements are Dr. Frueh, who resigned from our Board in October 2019, and Mr. More.
These stockholder agreements terminated upon the closing of our initial public offering in October 2019, except for the registration rights granted under our investors’ rights agreement, which will terminate upon the earliest of (i) the closing of a deemed liquidation event, as defined in our amended and restated certificate of incorporation as currently in effect; (ii) with respect to each stockholder, the date when such stockholder can sell all of its registrable shares without limitation during a three-month period without registration pursuant to Rule 144 of the Securities Act, or Rule 144, or another similar exemption under the Securities Act; and (iii) five years after the completion of our initial public offering.
Certain Loan Transactions
In January 2017, we issued two promissory notes to Dr. Scangos, our President, Chief Executive Officer and a member of our Board of Directors, and Vicki Sato, Ph.D., Chairman of our Board of Directors, for principal amounts of $2.9 million and $0.2 million, respectively, with an interest rate of 1.97% per annum, to allow Dr. Scangos and Dr. Sato to purchase 3,338,222 shares and 286,133 shares of our restricted stock, respectively, pursuant to their respective restricted stock purchase agreements. The principal and accrued interest outstanding on each of these promissory notes was approximately $3.0 million and $0.3 million for Dr. Scangos and Dr. Sato, respectively, as of July 31, 2019. These loans were repaid in full in August 2019.
Employment of an Immediate Family Member
Jennifer Scangos, the daughter of Dr. Scangos, our President, Chief Executive Officer and a member of our board of directors, is employed by us as a legal counsel. For the years ended December 31, 2017 and 2018, Ms. Scangos earned $9,394 and $153,282, respectively, in base salary and bonus which was in line with similar roles at the Company. For the year ended December 31, 2019, Ms. Scangos earned $139,000 and $24,325, respectively, in base salary and bonus, which was in line with compensation we pay to employees in similar roles. Ms. Scangos has received and continues to be eligible to receive equity awards and benefits on the same general terms and conditions as applicable to unrelated employees in similar positions.
Collaboration with Brii Biosciences
In May 2018, we entered into an option and license agreement with Brii Bio Parent and Brii Bio, pursuant to which we granted, and were granted, an exclusive option with respect to up to four collaboration programs for the development and commercialization of therapeutic products for infectious diseases. Dr. Scangos, our
31
Table of Contents
President, Chief Executive Officer and a member of our Board of Directors, and Mr. Nelsen, a member of our Board of Directors, served at the time and currently serve as directors of Brii Bio Parent and Brii Bio. We agreed to pay Brii Bio an option exercise fee for each licensed Brii Bio program up to $50.0 million, and milestone payments and royalties for net sales of licensed products in the United States arising from the selected collaboration programs. Brii Bio agreed to pay us an option exercise fee for each licensed Vir program up to $20.0 million, and milestone payments and royalties for net sales of licensed products in greater China arising from the selected collaboration programs. On June 12, 2020, following our achievement of proof of concept for VIR-2218, Brii Bio notified us of the exercise of its option to obtain exclusive rights to develop and commercialize compounds and products arising from VIR-2218 in greater China. Brii Bio paid us a $20.0 million option exercise fee in connection with the option exercise, half of which we will pay to Alnylam in connection with our collaboration and license agreement with Alnylam.
Other Transactions
We have entered into offer letter agreements with our executive officers that, among other things, provide for certain compensatory and change in control benefits, as well as severance benefits. For a description of these agreements with our named executive officers, see the sections titled “Executive Compensation” and “Director Compensation” included in our Definitive Proxy Statement on Schedule 14A for our 2020 Annual Meeting of Stockholders, which is incorporated by reference herein.
We have also granted stock options and restricted stock to our executive officers and certain of our directors. For a description of these equity awards, see the sections titled “Executive Compensation” and “Director Compensation” included in our Definitive Proxy Statement on Schedule 14A for our 2020 Annual Meeting of Stockholders, which is incorporated by reference herein.
Indemnification Agreements
We have entered into indemnification agreements with each of our current directors and executive officers. Our amended and restated certificate of incorporation and our amended and restated bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by applicable law.
Other than as described above under this section “Certain Relationships and Related Party Transactions” and in the documents incorporated by reference herein, since January 1, 2017, we have not entered into any transactions, nor are there any currently proposed transactions, between us and a related person where the amount involved exceeds, or would exceed, $120,000, and in which any related person had or will have a direct or indirect material interest. We believe the terms of the transactions described above were comparable to terms we could have obtained in arm’s length dealings with unrelated third parties.
32
Table of Contents
The following description of our capital stock is based upon our amended and restated certificate of incorporation and our amended and restated bylaws. This summary does not purport to be complete and is subject to, and is qualified in its entirety by express reference to, the applicable provisions of our amended and restated certificate of incorporation and our amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus is part. We encourage you to read our amended and restated certificate of incorporation, our amended and restated bylaws and the applicable provisions of the Delaware General Corporation Law, or the DGCL for more information.
General
Our amended and restated certificate of incorporation states that our authorized capital stock consists of 300,000,000 shares of common stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share.
Common Stock
Voting Rights
Each holder of common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders. The affirmative vote of holders of at least 66 2/3% of the voting power of all of the then-outstanding shares of capital stock, voting as a single class, is required to amend certain provisions of our amended and restated certificate of incorporation, including provisions relating to amending our amended and restated bylaws, the classified structure of our board of directors, the size of our board of directors, removal of directors, director liability, vacancies on our board of directors, special meetings, stockholder notices, actions by written consent and exclusive jurisdiction.
Dividends
The holders of our common stock are entitled to receive ratably any dividends that our board of directors may declare out of funds legally available for that purpose on a non-cumulative basis.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities.
Rights and Preferences
Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock.
Preferred Stock
Under our amended and restated certificate of incorporation, our board of directors has the authority, without further action by the stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding. Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible
33
Table of Contents
acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control that may otherwise benefit holders of our common stock and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock.
Registration Rights Under Amended and Restated Investors’ Rights Agreement
Certain holders of shares of our common stock issued upon conversion of previously outstanding convertible preferred stock are entitled to certain rights with respect to registration of such shares under the Securities Act. These shares are referred to as registrable securities. The holders of these registrable securities possess registration rights pursuant to the terms of our amended and restated investors’ rights agreement and are described in additional detail below. The registration of shares of our common stock pursuant to the exercise of the registration rights described below would enable the holders to trade these shares without restriction under the Securities Act when the applicable registration statement is declared effective. We will pay the registration expenses, other than underwriting discounts, selling commissions and stock transfer taxes, of the shares registered pursuant to the piggyback and Form S-3 registrations described below.
Generally, in an underwritten offering, the managing underwriter, if any, has the right, subject to specified conditions and limitations, to limit the number of shares the holders may include. The demand, piggyback and Form S-3 registration rights described below will expire no later than five years after the completion of our initial public offering, or with respect to any particular holder, at such time that such holder can sell its shares under Rule 144 of the Securities Act during any three-month period.
Demand Registration Rights
Certain holders of our common stock issued upon conversion of previously outstanding convertible preferred stock are entitled to certain demand registration rights. Certain major investors holding, collectively, a majority of registrable securities may, on not more than two occasions, request that we register all or a portion of their shares, subject to certain specified exceptions.
Piggyback Registration Rights
Certain holders of our common stock issued upon conversion of previously outstanding convertible preferred stock are entitled to their rights to notice of this offering and to include their shares of registrable securities in this offering. The requisite percentage of these stockholders have waived all such stockholders’ rights to notice of this offering and to include their shares of registrable securities in this offering. In the event that we propose to register any of our securities under the Securities Act in another offering, either for our own account or for the account of other security holders, the holders of registrable securities will be entitled to certain “piggyback” registration rights allowing them to include their shares in such registration, subject to specified conditions and limitations.
S-3 Registration Rights
Certain holders of our common stock issued upon conversion of previously outstanding convertible preferred stock will be entitled to certain Form S-3 registration rights. Certain major investors holding at least 10% of registrable securities may, on not more than two registrations on Form S-3 within any 12-month period, request that we register all or a portion of their shares on Form S-3 if we are qualified to file a registration statement on Form S-3, subject to specified exceptions. Such request for registration on Form S-3 must cover securities with an aggregate offering price which equals or exceeds $5.0 million, net of selling expenses. The right to have such shares registered on Form S-3 is further subject to other specified conditions and limitations.
Stock Purchase Agreement with Glaxo Group Limited
On April 5, 2020, concurrently with the execution of the Preliminary Collaboration Agreement, we entered into a stock purchase agreement, or the Stock Purchase Agreement, with GGL, an affiliate of GSK, pursuant to
34
Table of Contents
which GGL purchased 6,626,027 shares of our common stock on April 29, 2020, or the GGL Shares, at a purchase price of $37.73 per share, or approximately $250.0 million.
Pursuant to the terms of the Stock Purchase Agreement, GGL has agreed not to, without our prior written consent and subject to certain conditions and exceptions, among other things, directly or indirectly acquire additional shares of our common stock, seek or propose a tender or exchange offer, merger or other business combination involving us, solicit proxies or consents with respect to any matter, or undertake other specified actions related to the potential acquisition of additional equity interests in us. Such restrictions will expire on the one-year anniversary of the effective date of the Stock Purchase Agreement.
The Stock Purchase Agreement also provides that until the first anniversary of the effective date of such agreement, GGL will hold and not sell any of the GGL Shares, subject to certain exceptions. We agreed to register the GGL Shares for resale following expiration of the one-year lock-up period if Rule 144 under the Securities Act is not available for such resale without any volume or manner of sale restrictions.
Anti-Takeover Provisions of Delaware Law and Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| • | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| • | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
Section 203 defines a “business combination” to include the following:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| • | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; and |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
35
Table of Contents
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Among other things, our amended and restated certificate of incorporation and amended and restated bylaws will:
| • | permit our board of directors to issue up to 10,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in control; |
| • | provide that the authorized number of directors may be changed only by resolution of our board of directors; |
| • | provide that our board of directors will be classified into three classes of directors; |
| • | provide that, subject to the rights of any series of preferred stock to elect directors, directors may only be removed for cause, which removal may be effected, subject to any limitation imposed by law, by the holders of at least 66 2/3% of the voting power of all of our then-outstanding shares of the capital stock entitled to vote generally at an election of directors; |
| • | provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| • | require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent or electronic transmission; |
| • | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder’s notice; |
| • | provide that special meetings of our stockholders may be called only by the chairman of our board of directors, our chief executive officer or president or by our board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors, and not by our stockholders; and |
| • | not provide for cumulative voting rights, therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose. |
The amendment of any of these provisions would require approval by the holders of at least 66 2/3% of the voting power of all of our then-outstanding common stock entitled to vote generally in the election of directors, voting together as a single class.
The combination of these provisions will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Because our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
36
Table of Contents
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to reduce our vulnerability to hostile takeovers and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts. We believe that the benefits of these provisions, including increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company, outweigh the disadvantages of discouraging takeover proposals, because negotiation of takeover proposals could result in an improvement of their terms.
Choice of Forum
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware (or, if and only if the Court of Chancery of the State of Delaware lacks subject matter jurisdiction, any state court located within the State of Delaware or, if and only if all such state courts lack subject matter jurisdiction, the federal district court for the District of Delaware) is the sole and exclusive forum for the following types of actions or proceedings under Delaware statutory or common law: (i) any derivative action or proceeding brought on our behalf; (ii) any action or proceeding asserting a claim of breach of a fiduciary duty owed by any of our current or former directors, officers or other employees to us or our stockholders; (iii) any action or proceeding asserting a claim against us or any of our current or former directors, officers or other employees, arising out of or pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws; (iv) any action or proceeding to interpret, apply, enforce or determine the validity of our certificate of incorporation or our bylaws; and (v) any action asserting a claim against us or any of our directors, officers or other employees governed by the internal affairs doctrine. This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the U.S. federal courts have exclusive jurisdiction. Nothing in our amended and restated certificate of incorporation precludes stockholders that assert claims under the Securities Act from bringing such claims in state or federal court, subject to applicable law. Our amended and restated certificate of incorporation further provides that the federal district courts of the United States is the exclusive forum for resolving any complaint asserting a cause of action under the Securities Act, unless we consent in writing to the selection of an alternative forum.
Listing
Our common stock is listed on The Nasdaq Global Select Market under the trading symbol “VIR.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent’s address is 250 Royall Street, Canton, Massachusetts 02021.
37
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following is a general discussion of the material U.S. federal income tax consequences applicable to non-U.S. holders (as defined herein) with respect to their purchase, ownership and disposition of shares of our common stock issued pursuant to this offering. All prospective non-U.S. holders of our common stock should consult their own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the purchase, ownership and disposition of our common stock. In general, a non-U.S. holder means a beneficial owner of our common stock (other than a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) that is not, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized in the United States or under the laws of the United States or of any state thereof or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust if (i) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons have the authority to control all of the trust’s substantial decisions or (ii) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person. |
We assume in this discussion that a non-U.S. holder holds shares of our common stock as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder’s individual circumstances, nor does it address any estate or gift tax consequences, or any aspects of U.S. state, local or non-U.S. taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as holders that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below), corporations that accumulate earnings to avoid U.S. federal income tax, tax-exempt or governmental organizations, banks, financial institutions, insurance companies, brokers, dealers or traders in securities, commodities or currencies, tax-qualified retirement plans, holders subject to the alternative minimum tax or the Medicare contribution tax on net investment income, holders holding our common stock as part of a hedge, straddle or other risk reduction strategy, conversion transaction, synthetic security or other integrated investment, holders deemed to sell our common stock under the constructive sale provisions of the Code, controlled foreign corporations, passive foreign investment companies, accrual method taxpayers subject to special tax accounting rules under Section 451(b) of the Code, and U.S. expatriates and certain former U.S. citizens or long-term residents.
In addition, this discussion does not address the tax treatment of partnerships (or entities or arrangements that are treated as partnerships for U.S. federal income tax purposes) or persons that hold their common stock through such partnerships. If a partnership, including any entity or arrangement treated as a partnership for U.S. federal income tax purposes, holds shares of our common stock, the U.S. federal income tax treatment of a partner in such partnership will generally depend upon the status of the partner, the activities of the partnership and certain determinations made at the partner level. Such partners and partnerships should consult their own tax advisors regarding the tax consequences of the purchase, ownership and disposition of our common stock.
There can be no assurance that a court or the Internal Revenue Service, or the IRS, will not challenge one or more of the tax consequences described herein, and we have not obtained, nor do we intend to obtain, a ruling with respect to the U.S. federal income tax consequences to a non-U.S. holder of the purchase, ownership or disposition of our common stock.
Distributions on Our Common Stock
As described in the section titled “Dividend Policy,” we do not currently intend to pay any cash dividends on our capital stock in the foreseeable future. If we do make distributions of cash or property on our common
38
Table of Contents
stock, such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder’s investment, up to such holder’s adjusted tax basis in the common stock. Any remaining excess will be treated as capital gain from the sale or exchange of such common stock subject to the tax treatment described below in “Gain on Sale, Exchange or Other Disposition of Our Common Stock.” Any distributions will also be subject to the discussion below under the heading “Foreign Accounts.”
Dividends paid to a non-U.S. holder will generally be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the U.S. federal income tax rates applicable to “United States persons” (as defined in the Code). Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence.
To claim a reduction or exemption from withholding, a non-U.S. holder of our common stock generally will be required to provide (i) a properly executed IRS Form W-8BEN (in the case of individuals) or IRS Form W-8BEN-E (in the case of entities), or successor form, and satisfy applicable certification and other requirements to claim the benefit of an applicable income tax treaty between the United States and such holder’s country of residence, or (ii) a properly executed IRS Form W-8ECI stating that dividends are not subject to withholding because they are effectively connected with such non-U.S. holder’s conduct of a trade or business within the United States. The tax forms referred to above must be provided to us or our paying agent prior to the payment of dividends and must be updated periodically. In the case of a non-U.S. holder that is an entity, Treasury Regulations and any relevant tax treaty provide rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. Non-U.S. holders are urged to consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
Gain on Sale, Exchange or Other Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding, in general, a non-U.S. holder will not be subject to any U.S. federal income or withholding tax on any gain realized upon such holder’s sale, exchange or other disposition of shares of our common stock unless:
| • | the gain is effectively connected with a U.S. trade or business of the non-U.S. holder and, if an applicable income tax treaty so provides, is attributable to a permanent establishment or a fixed base maintained in the United States by such non-U.S. holder, in which case the non-U.S. holder generally will be taxed at the U.S. federal income tax rates applicable to “United States persons” (as defined in the Code) and, if the non-U.S. holder is a foreign corporation, the branch profits tax described above in “Distributions on Our Common Stock” may also apply; |
39
Table of Contents
| • | the non-U.S. holder is a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the net gain derived from the disposition, which may be offset by U.S. source capital losses of the non-U.S. holder, if any (even though the individual is not considered a resident of the United States), provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses; or |
| • | our common stock constitutes a U.S. real property interest because we are, or have been, at any time during the five-year period preceding such disposition (or the non-U.S. holder’s holding period, if shorter) a U.S. real property holding corporation. Generally, a corporation is a U.S. real property holding corporation only if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus the fair market value of its other assets used or held for use in a trade or business. We do not believe that we are, or have been, a U.S. real property holding corporation, or that we are likely to become one in the future. However, because the determination of whether we are a U.S. real property holding corporation depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a U.S. real property holding corporation in the future. Even if we are or become a U.S. real property holding corporation, provided that our common stock is “regularly traded” (as defined by applicable Treasury Regulations), on an established securities market, our common stock will be treated as a U.S. real property interest only with respect to a non-U.S. holder that holds more than 5% of our outstanding common stock, actually or constructively, during the shorter of the five-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock. In such case, such non-U.S. holder generally will be taxed on its net gain derived from the disposition at the U.S. federal income tax rates applicable to “United States persons” (as defined in the Code). No assurance can be provided that our common stock will continue to be regularly traded on an established securities market for purposes of the rules described above. |
Backup Withholding and Information Reporting
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on our common stock paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. holders will have to comply with specific certification procedures to establish that the holder is not a “United States person” (as defined in the Code) in order to avoid backup withholding at the applicable rate with respect to dividends on our common stock. A non-U.S. holder generally will not be subject to U.S. backup withholding with respect to payments of dividends on our common stock if it certifies its non-U.S. status by providing a valid IRS Form W-8BEN (in the case of individuals), IRS Form W-8BEN-E (in the case of entities) or IRS Form W-8ECI, or successor form, or otherwise establishes an exemption; provided the applicable withholding agent does not have actual knowledge or reason to know such non-U.S. holder is a “United States person,” as defined in the Code.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in fact, a U.S. person. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
40
Table of Contents
Copies of information returns that are filed with the IRS may be made available under the provisions of a specific treaty or agreement to the tax authorities of the country in which the non-U.S. holder resides or is incorporated.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder may be allowed as a credit against the non-U.S. holder’s U.S. federal income tax liability, if any, and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Accounts
Withholding taxes may be imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the Foreign Account Tax Compliance Act, or FATCA) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends on our common stock paid to a “foreign financial institution” (as specifically defined for this purpose), unless such institution enters into an agreement with the U.S. government to, among other things, withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise qualifies for an exemption from these rules. A U.S. federal withholding tax of 30% also applies to dividends on our common stock paid to a “non-financial foreign entity” (as defined in the Code), unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or provides information regarding substantial direct and indirect U.S. owners of the entity, or otherwise qualifies for an exemption from these rules. The withholding provisions described above currently apply to dividends paid on our common stock. Under the applicable Treasury Regulations and administrative guidance, withholding under FATCA would have applied to payments of gross proceeds from the sale or other disposition of stock on or after January 1, 2019, although under proposed Treasury Regulations (the preamble to which specifies that taxpayers are permitted to rely on such proposed Treasury Regulations pending finalization), no withholding applies with respect to payments of gross proceeds. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS TAX ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY RECENT AND PROPOSED CHANGE IN APPLICABLE LAWS.
41
Table of Contents
We and the underwriters named below have entered into an underwriting agreement with respect to the shares being offered. Subject to certain conditions, each underwriter has severally agreed to purchase the number of shares indicated in the following table. Goldman Sachs & Co. LLC is the sole representative of the underwriters.
| Underwriters | Number of Shares |
|||
| Goldman Sachs & Co. LLC |
||||
| BofA Securities, Inc. |
||||
| Cowen and Company, LLC |
||||
| Barclays Capital Inc. |
||||
| Needham & Company, LLC |
||||
|
|
|
|||
| Total |
6,200,000 | |||
|
|
|
|||
The underwriters are committed to take and pay for all of the shares being offered, if any are taken, other than the shares covered by the option described below unless and until this option is exercised.
The underwriters have an option to buy up to an additional 930,000 shares from us to cover sales by the underwriters of a greater number of shares than the total number set forth in the table above. They may exercise that option for 30 days. If any shares are purchased pursuant to this option, the underwriters will severally purchase shares in approximately the same proportion as set forth in the table above.
The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters by us. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Paid by Us | No Exercise | Full Exercise | ||||||
| Per Share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. After the initial offering of the shares, the representative may change the offering price and the other selling terms. The offering of the shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
We and our directors, our executive officers and certain affiliated stockholders have agreed with the underwriters, subject to certain limited exceptions, not to dispose of or hedge any of their common stock or securities convertible into or exchangeable for shares of common stock during the period from the date of this prospectus continuing through the date 90 days after the date of this prospectus, except with the prior written consent of Goldman Sachs & Co. LLC.
Our common stock is listed on The Nasdaq Global Select Market under the trading symbol “VIR.”
In connection with the offering, the underwriters may purchase and sell shares of common stock in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering, and a short position represents the amount of such sales that have not been covered by subsequent purchases. A “covered short position” is a short position that is not greater than the amount of additional shares for which the underwriters’ option described above may be exercised. The underwriters may cover any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to cover the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase additional shares pursuant to the option described above.
42
Table of Contents
“Naked” short sales are any short sales that create a short position greater than the amount of additional shares for which the option described above may be exercised. The underwriters must cover any such naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representative has repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of our common stock, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of the common stock. As a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. The underwriters are not required to engage in these activities and may end any of these activities at any time. These transactions may be effected on the New York Stock Exchange, The Nasdaq Global Select Market or relevant exchange, in the over-the-counter market or otherwise. In connection with the offering, underwriters and selling group members may engage in passive market making transactions in the common stock on The Nasdaq Global Select Market in accordance with Rule 103 of Regulation M under the Exchange Act during the period before the commencement of offers or sales of common stock and extending through the completion of distribution. A passive market maker must display its bids at a price not in excess of the highest independent bid of the security. However, if all independent bids are lowered below the passive market maker’s bid that bid must be lowered when specified purchase limits are exceeded.
We estimate that our share of the total expenses of the offering, excluding underwriting discounts and commissions, will be approximately $1,000,000. We will reimburse the underwriters for certain of their expenses incurred in connection with this offering in an amount up to $25,000.
We have agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. Certain of the underwriters and their respective affiliates have provided, and may in the future provide, a variety of these services to the issuer and to persons and entities with relationships with the issuer, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the issuer (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with the issuer. The underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
43
Table of Contents
Selling Restrictions
European Economic Area and United Kingdom
In relation to each Member State of the European Economic Area and the United Kingdom (each a “Relevant State”), no shares have been offered or will be offered pursuant to this offering to the public in that Relevant State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that offers of shares may be made to the public in that Relevant State at any time under the following exemptions under the Prospectus Regulation:
| • | to any legal entity which is a qualified investor as defined in the Prospectus Regulation; |
| • | to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of the representative for any such offer; or |
| • | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, provided that no such offer of shares shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation and each person who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and the company that it is a “qualified investor” within the meaning of Article 2(e) of the Prospectus Regulation. In the case of any shares being offered to a financial intermediary as that term is used in the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant State to qualified investors as so defined or in circumstances in which the prior consent of the underwriters have been obtained to each such proposed offer or resale. |
For the purposes of this provision, the expression an “offer to the public” in relation to shares in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
This European Economic Area selling restriction is in addition to any other selling restrictions set out below.
United Kingdom
In the United Kingdom, this prospectus is only addressed to and directed as qualified investors who are (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the Order); or (ii) high net worth entities and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). Any investment or investment activity to which this prospectus relates is available only to relevant persons and will only be engaged with relevant persons. Any person who is not a relevant person should not act or relay on this prospectus or any of its contents.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument
44
Table of Contents
31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption form, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Hong Kong
The shares may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), or (ii) to “professional investors” as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for six months after that corporation has acquired the shares under Section 275 of the SFA except: (i) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (ii) where such transfer arises from an offer in that corporation’s securities pursuant
45
Table of Contents
to Section 275(1A) of the SFA, (iii) where no consideration is or will be given for the transfer, (iv) where the transfer is by operation of law, (v) as specified in Section 276(7) of the SFA, or (vi) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”).
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for six months after that trust has acquired the shares under Section 275 of the SFA except: (i) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (ii) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (iii) where no consideration is or will be given for the transfer, (iv) where the transfer is by operation of law, (v) as specified in Section 276(7) of the SFA, or (vi) as specified in Regulation 32.
Singapore Securities and Futures Act Product Classification—Solely for the purposes of its obligations pursuant to Sections 309B(1)(a) and 309B(1)(c) of the SFA, we have determined, and hereby notify all relevant persons (as defined in Section 309A of the SFA) that the common shares are “prescribed capital markets products” (as defined in the Securities and Futures (Capital Markets Products) Regulations 2018) and Excluded Investment Products (as defined in MAS Notice SFA 04-N12: Notice on the Sale of Investment Products and MAS Notice FAA-N16: Notice on Recommendations on Investment Products).
Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended), or the FIEA. The securities may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
Israel
In the State of Israel this prospectus shall not be regarded as an offer to the public to purchase shares of common stock under the Israeli Securities Law, 5728 – 1968, which requires a prospectus to be published and authorized by the Israel Securities Authority, if it complies with certain provisions of Section 15 of the Israeli Securities Law, 5728–1968, including, inter alia, if: (i) the offer is made, distributed or directed to not more than 35 investors, subject to certain conditions (“Addressed Investors”); or (ii) the offer is made, distributed or directed to certain qualified investors defined in the First Addendum of the Israeli Securities Law, 5728 – 1968, subject to certain conditions (“Qualified Investors”). The Qualified Investors shall not be taken into account in the count of the Addressed Investors and may be offered to purchase securities in addition to the 35 Addressed Investors. The company has not and will not take any action that would require it to publish a prospectus in accordance with and subject to the Israeli Securities Law, 5728 – 1968. We have not and will not distribute this prospectus or make, distribute or direct an offer to subscribe for our common stock to any person within the State of Israel, other than to Qualified Investors and up to 35 Addressed Investors.
Qualified Investors may have to submit written evidence that they meet the definitions set out in of the First Addendum to the Israeli Securities Law, 5728 – 1968. In particular, we may request, as a condition to be offered common stock, that Qualified Investors will each represent, warrant and certify to us and/or to anyone acting on our behalf: (i) that it is an investor falling within one of the categories listed in the First Addendum to the Israeli Securities Law, 5728 – 1968; (ii) which of the categories listed in the First Addendum to the Israeli Securities
46
Table of Contents
Law, 5728 – 1968 regarding Qualified Investors is applicable to it; (iii) that it will abide by all provisions set forth in the Israeli Securities Law, 5728 – 1968 and the regulations promulgated thereunder in connection with the offer to be issued common stock; (iv) that the shares of common stock that it will be issued are, subject to exemptions available under the Israeli Securities Law, 5728 – 1968: (1) for its own account; (2) for investment purposes only; and (3) not issued with a view to resale within the State of Israel, other than in accordance with the provisions of the Israeli Securities Law, 5728 – 1968; and (v) that it is willing to provide further evidence of its Qualified Investor status. Addressed Investors may have to submit written evidence in respect of their identity and may have to sign and submit a declaration containing, inter alia, the Addressed Investor’s name, address and passport number or Israeli identification number.
Switzerland
The securities will not be offered, directly or indirectly, to the public in Switzerland and this prospectus does not constitute a public offering prospectus as that term is understood pursuant to article 652a or 1156 of the Swiss Federal Code of Obligations.
Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission, or ASIC, in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001, or the Corporations Act, and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons, or the Exempt Investors, who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority, or DFSA. This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The shares to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the shares offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
47
Table of Contents
The validity of the issuance of our common stock offered in this prospectus will be passed upon for us by Cooley LLP, Palo Alto, California. Certain legal matters in connection with this offering will be passed upon for the underwriters by Latham & Watkins LLP, Menlo Park, California.
Ernst & Young LLP, independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2019, as set forth in their report, which is incorporated by reference in this prospectus and elsewhere in the registration statement. Our financial statements are incorporated by reference in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1, including exhibits and schedules, under the Securities Act, with respect to the shares of common stock being offered by this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You may read our SEC filings, including this registration statement, over the Internet at the SEC’s website at www.sec.gov. We are subject to the information reporting requirements of the Exchange Act and have filed reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for review on the web site of the SEC referred to above. We also maintain a website at www.vir.bio, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on or accessible through our website is not a part of this prospectus or the registration statement of which it forms a part, and the inclusion of our website address in this prospectus is an inactive textual reference only.
48
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (File No. 001-39083):
| • | our Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on March 26, 2020; |
| • | the portions of our Definitive Proxy Statement on Schedule 14A for our 2020 Annual Meeting of Stockholders specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on April 6, 2020 and as supplemented on May 8, 2020; |
| • | our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, filed with the SEC on May 12, 2020; |
| • | our Current Reports on Form 8-K (File No. 001-39083) filed with the SEC on January 23, 2020, January 30, 2020, February 12, 2020, March 9, 2020, April 6, 2020, April 7, 2020, April 10, 2020, April 30, 2020, May 22, 2020, May 29, 2020, June 15, 2020, June 19, 2020 and July 6, 2020; and |
| • | the description of the Common Stock contained in our Registration Statement on Form 8-A filed with the SEC on October 9, 2019, including any amendments or reports filed for the purpose of updating such description. |
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the SEC pursuant to the Exchange Act shall be incorporated by reference into this prospectus.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Vir Biotechnology, Inc., Attn: Investor Relations, 499 Illinois Street, Suite 500, San Francisco, California 94158.
You also may access these filings on our website at www.vir.bio. Information contained on or accessible through our website is not a part of this prospectus or the registration statement of which it forms a part, and the inclusion of our website address in this prospectus is an inactive textual reference only.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
49
Table of Contents
6,200,000 Shares

Common Stock
PRELIMINARY PROSPECTUS
| Goldman Sachs & Co. LLC | BofA Securities | Cowen | Barclays | |||
| Needham & Company | ||||||
, 2020
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by Vir Biotechnology, Inc., or the Registrant, in connection with the sale of our common stock being registered. All amounts are estimates except for the Securities and Exchange Commission, or SEC, registration fee, and the Financial Industry Regulatory Authority, or FINRA, filing fee.
| Item | Amount Paid or to Be Paid |
|||
| SEC registration fee |
$ | 37,318 | ||
| FINRA filing fee |
43,625 | |||
| Printing expenses |
75,000 | |||
| Legal fees and expenses |
600,000 | |||
| Accounting fees and expenses |
175,000 | |||
| Miscellaneous expenses |
69,057 | |||
|
|
|
|||
| Total |
$ | 1,000,000 | ||
|
|
|
|||
Item 14. Indemnification of Directors and Officers.
As permitted by Section 102 of the Delaware General Corporation Law, we have adopted provisions in our amended and restated certificate of incorporation and amended and restated bylaws that limit or eliminate the personal liability of our directors for a breach of their fiduciary duty of care as a director. The duty of care generally requires that, when acting on behalf of the corporation, directors exercise an informed business judgment based on all material information reasonably available to them. Consequently, a director will not be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for:
| • | any breach of the director’s duty of loyalty to us or our stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | any act related to unlawful stock repurchases, redemptions or other distributions or payment of dividends; or |
| • | any transaction from which the director derived an improper personal benefit. |
These limitations of liability do not affect the availability of equitable remedies such as injunctive relief or rescission. Our amended and restated certificate of incorporation also authorizes us to indemnify our officers, directors and other agents to the fullest extent permitted under Delaware law.
As permitted by Section 145 of the Delaware General Corporation Law, our amended and restated bylaws provide that:
| • | we may indemnify our directors, officers and employees to the fullest extent permitted by the Delaware General Corporation Law, subject to limited exceptions; |
| • | we may advance expenses to our directors, officers and employees in connection with a legal proceeding to the fullest extent permitted by the Delaware General Corporation Law, subject to limited exceptions; and |
| • | the rights provided in our bylaws are not exclusive. |
II-1
Table of Contents
Our amended and restated certificate of incorporation and our amended and restated bylaws provide for the indemnification provisions described above and elsewhere herein. We have entered or will enter into, and intend to continue to enter into, separate indemnification agreements with our directors and officers that may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. These indemnification agreements generally require us, among other things, to indemnify our officers and directors against certain liabilities that may arise by reason of their status or service as directors or officers, other than liabilities arising from willful misconduct. These indemnification agreements also generally require us to advance any expenses incurred by the directors or officers as a result of any proceeding against them as to which they could be indemnified. These indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of our officers and directors for liabilities, including reimbursement of expenses incurred, arising under the Securities Act of 1933, as amended, or the Securities Act.
The Registrant has purchased and currently intends to maintain insurance on behalf of each and every person who is or was a director or officer of the Registrant against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
The form of underwriting agreement for this public offering provides for indemnification by the underwriters of us and our officers and directors who sign this registration statement for specified liabilities, including matters arising under the Securities Act.
Item 15. Recent Sales of Unregistered Securities.
Since May 31, 2017, we have made the following sales of unregistered securities:
Equity Plan-Related Issuances
| 1. | Since May 31, 2017, we have granted to certain of our directors, employees and consultants options to purchase 7,714,906 shares of our common stock with per share exercise prices ranging from $1.49 to $10.40 under our 2016 Equity Incentive Plan, as amended or 2016 Plan. |
| 2. | Since May 31, 2017, we have issued to certain of our directors, employees and consultants an aggregate of 1,902,016 shares of our common stock at per share purchase prices ranging from $0.86 to $10.40 pursuant to exercises of options under the 2016 Plan for an aggregate purchase price of $3.7 million. |
| 3. | In October 2019, we granted to certain of our directors and employees options to purchase 306,441 shares of our common stock with a per share exercise price of $20.00 under our 2019 Equity Incentive Plan. |
Sale of Common Stock
| 4. | In October 2017, we issued 1,111,111 shares of our common stock to one accredited investor as partial consideration for entry into a collaboration and license agreement and the investor granting us certain license rights. |
| 5. | In August 2019, we issued 38,888 shares of our common stock to one accredited investor as partial consideration for entry into a license agreement and the investor granting us certain license rights. |
| 6. | In April 2020, we issued and sold 6,626,027 shares of our common stock to one accredited investor pursuant to a stock purchase agreement at a cash purchase price per share of $37.73, or approximately $250.0 million. |
| 7. | In May 2020, we issued 1,111,111 shares of our common stock to one accredited investor as consideration for achieving a specified development milestone under a collaboration and license agreement. |
| 8. | In May 2020, we issued 211,774 shares of our common stock to one accredited investor upon the exercise of an outstanding warrant to acquire 244,444 shares pursuant to the cashless exercise provisions contained therein. |
II-2
Table of Contents
Sale of Preferred Stock
| 9. | Between May 2017 and July 2018, we issued and sold an aggregate of 60,277,749 shares of Series A-1 convertible preferred stock to 13 accredited investors at a purchase price per share of $4.50 for an aggregate purchase price of $271.3 million. |
| 10. | In January 2019, we issued and sold an aggregate of 18,202,213 shares of Series B convertible preferred stock to 20 accredited investors at a purchase price per share of $18.00 for an aggregate purchase price of $327.6 million. |
Acquisitions
| 11. | In August 2017, we issued to 23 accredited investors, including certain of our employees and consultants, 1,666,656 shares of our common stock in connection with our acquisition of a company. |
| 12. | In January 2018, we issued to 35 accredited investors, including certain of our consultants, an aggregate of 555,537 shares of Series A-2 convertible preferred stock in connection with our acquisition of a company. |
| 13. | In February 2018, we issued to two accredited investors an aggregate of 188,333 shares of Series A-2 convertible preferred stock in connection with our acquisition of a company. |
The offers, sales and issuances of the securities described in paragraphs (1) through (3) were deemed to be exempt from registration under Rule 701 promulgated under the Securities Act as transactions under compensatory benefit plans and contracts relating to compensation, or under Section 4(a)(2) of the Securities Act as a transaction by an issuer not involving a public offering. The recipients of such securities were our directors, employees or bona fide consultants and received the securities under our equity incentive plans. Appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions had adequate access, through employment, business or other relationships, to information about us.
The offers, sales and issuances of the securities described in paragraphs (4) through (13) were deemed to be exempt under Section 4(a)(2) of the Securities Act or Rule 506 of Regulation D under the Securities Act as a transaction by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor within the meaning of Rule 501 of Regulation D under the Securities Act and had adequate access, through employment, business or other relationships, to information about us. No underwriters were involved in these transactions.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
The exhibits listed below are filed as part of this registration statement.
II-3
Table of Contents
II-4
Table of Contents
II-5
Table of Contents
II-6
Table of Contents
II-7
Table of Contents
| + | Indicates a management contract or compensatory plan. |
| † | Certain portions of this exhibit (indicated by “[***]”) have been omitted pursuant to confidential treatment. |
II-8
Table of Contents
(b) Financial Statement Schedules.
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the consolidated financial statements or notes thereto, which are incorporated herein by reference.
Item 17. Undertakings.
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
1. For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
2. For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-9
Table of Contents
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Francisco, State of California on July 6, 2020.
| VIR BIOTECHNOLOGY, INC. | ||
| By: | /s/ George Scangos | |
| George Scangos, Ph.D. | ||
| President and Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints George Scangos, Ph.D., and Howard Horn, and each of them, as his or her true and lawful attorneys-in-fact and agents, each with the full power of substitution, for him or her and in his or her name, place or stead, in any and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments), and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act, and all post-effective amendments thereto, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their, his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement on Form S-1 has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ George Scangos George Scangos, Ph.D. |
President, Chief Executive Officer and Director (Principal Executive Officer) | July 6, 2020 | ||
| /s/ Howard Horn Howard Horn |
Chief Financial Officer and Secretary (Principal Financial and Accounting Officer) |
July 6, 2020 | ||
| /s/ Vicki Sato, Ph.D. Vicki Sato, Ph.D. |
Chairman of the Board of Directors | July 6, 2020 | ||
| /s/ Kristina Burow Kristina Burow |
Director | July 6, 2020 | ||
| /s/ Robert More Robert More |
Director | July 6, 2020 | ||
| /s/ Robert Nelsen Robert Nelsen |
Director | July 6, 2020 | ||
| /s/ Dipchand Nishar Dipchand Nishar |
Director | July 6, 2020 | ||
II-10
Table of Contents
| Signature |
Title |
Date | ||
| /s/ Robert Perez Robert Perez |
Director | July 6, 2020 | ||
| /s/ Saira Ramasastry Saira Ramasastry |
Director | July 6, 2020 | ||
| /s/ Phillip Sharp Phillip Sharp, Ph.D. |
Director | July 6, 2020 | ||
II-11


