UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM
(Mark One)
|
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
OR
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _________ to _________
Commission File Number:
(Exact Name of Registrant as Specified in its Charter)
|
|
Not Applicable |
|
( State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer |
|
|
|
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|
|||
|
|
|
☒ |
|
Smaller reporting company |
|
|
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes
As of May 10, 2021, the registrant had
Table of Contents
|
|
|
Page |
|
PART I. |
|
|
|
Item 1. |
1 |
|
|
|
1 |
|
|
|
Condensed Consolidated Statements of Income (Loss) and Comprehensive Income (Loss) |
2 |
|
|
3 |
|
|
|
4 |
|
|
|
Notes to Unaudited Condensed Consolidated Financial Statements |
5 |
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
12 |
|
Item 3. |
23 |
|
|
Item 4. |
23 |
|
|
PART II. |
|
|
|
Item 1. |
25 |
|
|
Item 1A. |
25 |
|
|
Item 2. |
26 |
|
|
Item 3. |
26 |
|
|
Item 4. |
26 |
|
|
Item 5. |
26 |
|
|
Item 6. |
27 |
|
|
|
28 |
i
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements.
AbCellera Biologics Inc.
Condensed Consolidated Balance Sheets
(Expressed in thousands of U.S. dollars except share data)
(Unaudited)
|
|
|
December 31, 2020 |
|
|
March 31, 2021 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
|
|
|
$ |
|
|
|
Accounts receivable |
|
|
|
|
|
|
|
|
|
Accrued accounts receivable |
|
|
|
|
|
|
|
|
|
Other current assets |
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
|
|
|
|
|
|
|
Long term assets: |
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
|
|
|
|
|
|
|
Intangible assets |
|
|
|
|
|
|
|
|
|
Goodwill |
|
|
|
|
|
|
|
|
|
Investments in and loans to equity accounted investees |
|
|
|
|
|
|
|
|
|
Other long-term assets |
|
|
|
|
|
|
|
|
|
Total long-term assets |
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
|
|
|
$ |
|
|
|
Liabilities and shareholders' equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable and other liabilities |
|
$ |
|
|
|
$ |
|
|
|
Current portion of contingent consideration payable |
|
|
|
|
|
|
|
|
|
Income taxes payable |
|
|
|
|
|
|
|
|
|
Accrued royalties payable |
|
|
|
|
|
|
|
|
|
Deferred revenue |
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
|
|
|
|
|
|
|
Long-term liabilities: |
|
|
|
|
|
|
|
|
|
Operating lease liability |
|
|
|
|
|
|
|
|
|
Deferred revenue and grant funding |
|
|
|
|
|
|
|
|
|
Contingent consideration payable |
|
|
|
|
|
|
|
|
|
Deferred tax liability |
|
|
|
|
|
|
|
|
|
Other long-term liabilities |
|
|
|
|
|
|
|
|
|
Total long-term liabilities |
|
|
|
|
|
|
|
|
|
Total liabilities |
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
Shareholders' equity: |
|
|
|
|
|
|
|
|
|
Common shares: par value, unlimited authorized shares at December 31, 2020 and March 31, 2021: |
|
|
|
|
|
|
|
|
|
Additional paid-in capital |
|
|
|
|
|
|
|
|
|
Accumulated earnings |
|
|
|
|
|
|
|
|
|
Total shareholders' equity |
|
|
|
|
|
|
|
|
|
Total liabilities and shareholders' equity |
|
$ |
|
|
|
$ |
|
|
|
Subsequent events |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
1
AbCellera Biologics Inc.
Condensed Consolidated Statements of Income (Loss) and Comprehensive Income (Loss)
(Expressed in thousands of U.S. dollars except share and per share data)
(Unaudited)
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
Revenue: |
|
|
|
|
|
|
|
|
|
Research fees |
|
$ |
|
|
|
$ |
|
|
|
Licensing revenue |
|
|
- |
|
|
|
|
|
|
Milestone payments |
|
|
- |
|
|
|
|
|
|
Royalty revenue |
|
|
- |
|
|
|
|
|
|
Total revenue |
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Royalty fees |
|
|
- |
|
|
|
|
|
|
Research and development(1) |
|
|
|
|
|
|
|
|
|
Sales and marketing(1) |
|
|
|
|
|
|
|
|
|
General and administrative(1) |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
|
|
|
|
|
|
|
Income (loss) from operations |
|
|
( |
) |
|
|
|
|
|
Other (income) expense |
|
|
|
|
|
|
|
|
|
Other (income) expense |
|
|
|
|
|
|
( |
) |
|
Grants and incentives |
|
|
( |
) |
|
|
( |
) |
|
Total other income |
|
|
( |
) |
|
|
( |
) |
|
Net earnings (loss) before income tax |
|
|
( |
) |
|
|
|
|
|
Provision for income tax |
|
|
- |
|
|
|
|
|
|
Net earnings (loss) and comprehensive income (loss) for the period |
|
$ |
( |
) |
|
$ |
|
|
|
Net earnings (loss) per share attributable to common shareholders |
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
( |
) |
|
$ |
|
|
|
Diluted |
|
$ |
( |
) |
|
$ |
|
|
|
Weighted-average common shares outstanding |
|
|
|
|
|
|
|
|
|
Basic |
|
|
|
|
|
|
|
|
|
Diluted |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
1
2
AbCellera Biologics Inc.
Condensed Consolidated Statements of Stockholders’ Equity
(Expressed in thousands of U.S. dollars except share data)
(Unaudited)
|
|
|
Series A1 Preferred Shares |
|
|
Series A2 Preferred Shares |
|
|
Common Shares |
|
|
Additional Paid-in |
|
|
Accumulated Earnings |
|
|
Total Shareholders' |
|
||||||||||||||||||
|
(in thousands, except share data) |
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
(Deficit) |
|
|
Equity |
|
|||||||||
|
Balances as of December 31, 2020 |
|
|
- |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Shares issued under stock option plan |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
- |
|
|
|
|
|
|
Stock-based compensation expense |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
Reclassification of liability classified options |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
Net earnings |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
Balances as of March 31, 2021 |
|
|
- |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
Series A1 Preferred Shares |
|
|
Series A2 Preferred Shares |
|
|
Common Shares |
|
|
Additional Paid-in |
|
|
Accumulated Earnings |
|
|
Total Shareholders' |
|
||||||||||||||||||
|
(in thousands, except share data) |
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
(Deficit) |
|
|
Equity |
|
|||||||||
|
Balances as of December 31, 2019 |
|
|
|
|
|
$ |
|
|
|
|
- |
|
|
$ |
- |
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
Issuance of Series A2 preferred shares |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
Shares issued under stock option plan |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
- |
|
|
|
|
|
|
Share-based compensation expense |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
( |
) |
|
|
( |
) |
|
Balances as of March 31, 2020 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
3
AbCellera Biologics Inc.
Condensed Consolidated Statements of Cash Flows
(Expressed in thousands of U.S. dollars)
(Unaudited)
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
( |
) |
|
$ |
|
|
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation of property and equipment |
|
|
|
|
|
|
|
|
|
Amortization of intangible assets |
|
|
|
|
|
|
|
|
|
Amortization of operating lease right-of-use-assets |
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
|
|
|
|
|
|
|
Deferred tax expense |
|
|
- |
|
|
|
|
|
|
Other |
|
|
( |
) |
|
|
( |
) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts and accrued research fees receivable |
|
|
( |
) |
|
|
( |
) |
|
Accrued royalties receivable |
|
|
- |
|
|
|
|
|
|
Income taxes payable |
|
|
- |
|
|
|
( |
) |
|
Accounts payable and accrued liabilities |
|
|
( |
) |
|
|
( |
) |
|
Deferred revenue |
|
|
|
|
|
|
|
|
|
Accrued royalties payable |
|
|
- |
|
|
|
( |
) |
|
Other assets and liabilities |
|
|
( |
) |
|
|
( |
) |
|
Net cash (used in) provided by operating activities |
|
|
( |
) |
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
( |
) |
|
|
( |
) |
|
Purchase of intangible assets |
|
|
( |
) |
|
|
- |
|
|
Investment in equity investees |
|
|
- |
|
|
|
( |
) |
|
Net cash used in investing activities |
|
|
( |
) |
|
|
( |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
Repayment of long-term debt |
|
|
( |
) |
|
|
( |
) |
|
Proceeds from long-term debt |
|
|
|
|
|
|
- |
|
|
Payment of deferred financing fees |
|
|
( |
) |
|
|
- |
|
|
Short-term borrowings |
|
|
( |
) |
|
|
- |
|
|
Issuance of common shares pursuant to exercise of stock options |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of preferred shares - series A2 financing |
|
|
|
|
|
|
- |
|
|
Net cash provided by (used in) financing activities |
|
|
|
|
|
|
( |
) |
|
Effect of exchange rate changes on cash and cash equivalents |
|
|
|
|
|
|
( |
) |
|
Increase in cash and cash equivalents |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period |
|
$ |
|
|
|
$ |
|
|
|
Supplemental disclosure of non-cash investing and financing activities |
|
|
|
|
|
|
|
|
|
Property plant and equipment in accounts payable |
|
|
|
|
|
|
|
|
|
Right-of-use assets obtained in exchange for operating lease obligation |
|
|
|
|
|
|
|
|
|
Purchase of intangible assets in exchange for in-licensing agreement payable |
|
|
|
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
4
AbCellera Biologics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(Unaudited)
1. Nature of operations
AbCellera Biologics Inc.’s (the “Company”) mission is to improve health with technologies that transform the way that antibody-based therapies are discovered. The Company aims to become the centralized operating system for next generation antibody discovery. The Company’s full-stack, AI-powered drug discovery platform searches and analyzes the database of natural immune systems to find antibodies that can be developed as drugs. The Company believes its technology increases the speed and the probability of success of therapeutic antibody discovery, including enabling discovery against targets that may otherwise be intractable. Rather than advancing its own clinical pipeline of drug candidates, the Company forges partnerships with drug developers of all sizes, from large cap pharmaceutical to small biotechnology companies.
2.
The accompanying interim condensed consolidated financial statements of the Company have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the SEC for interim financial information. Accordingly, these financial statements do not include all the information and footnotes required for complete financial statements and should be read in conjunction with the audited consolidated financial statements of the Company and the accompanying notes thereto for the year ended December 31, 2020.
These unaudited interim condensed consolidated financial statements reflect all adjustments, consisting solely of normal recurring adjustments, which, in the opinion of management, are necessary for a fair presentation of results for the interim periods presented. The results of operations for the three months ended March 31, 2021 and 2020 are not necessarily indicative of results that can be expected for a full year. These unaudited interim condensed consolidated financial statements follow the same significant accounting policies as those described in the notes to the audited consolidated financial statements of the Company for the year ended December 31, 2020, except for the new accounting guidance adopted during the period (Note 3).
All amounts expressed in the consolidated financial statements of the Company and the accompanying notes thereto are expressed in thousands of U.S. dollars, except for share and per share data and where otherwise indicated. References to “$” are to U.S. dollars and references to “C$” and “CAD” are to Canadian dollars.
3. Significant accounting policies
Use of estimates
The preparation of the consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Areas of significant estimates include, but are not limited to, revenue recognition including estimated timing of completion of performance obligations and determining whether an option for additional goods or services represents a material right, recoverability of investment tax credits receivable, value of contingent consideration payable and the fair value of stock-based compensation awards. The Company bases its estimates on historical experience, known trends and other market-specific or other relevant factors that it believes to be reasonable under the circumstances. On an ongoing basis, management evaluates its estimates when there are changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known. Actual results could significantly differ from those estimates.
COVID-19 Pandemic
With the global spread of the ongoing COVID-19 pandemic, the Company has implemented business continuity plans designed to address and mitigate the impact of the COVID-19 pandemic on its employees and its business. The Company has taken measures to secure its research and development activities, while work in its laboratories and facilities has been re-organized to reduce risk of COVID-19 transmission. Given the global economic impact, the overall disruption of global healthcare systems and the other risks and uncertainties associated with the pandemic, the Company’s business, financial condition, and results of operations could be materially adversely affected. The Company continues to closely monitor the COVID-19 pandemic as it evolves its business continuity plans and response strategy. As of the date of these financial statements, the Company is not aware of any specific event or circumstance that would require the Company to update its estimates, assumptions and judgments or revise the carrying value of its assets or liabilities. Actual results could differ from these estimates, and any such differences may be material to the Company’s financial statements.
5
Recent accounting pronouncements not yet adopted
The Company has reviewed recent accounting pronouncements and concluded that they are either not applicable to the Company or that there was no material impact or no material impact is expected in the consolidated financial statements as a result of future adoption.
4. Net Earnings (Loss) per share
Basic and diluted net earnings (loss) per share attributable to common shareholders was calculated as follows:
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
|
|
(in thousands, except share and per share data) |
|
|||||
|
Basic earnings (loss) per share |
|
|
|
|
|
|
|
|
|
Net earnings (loss) |
|
$ |
( |
) |
|
$ |
|
|
|
Net earnings (loss) attributable to common shareholders - basic |
|
$ |
( |
) |
|
$ |
|
|
|
Weighted-average common shares outstanding - basic |
|
|
|
|
|
|
|
|
|
Net earnings (loss) per share attributable to common shareholders - basic |
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted earnings (loss) per share |
|
|
|
|
|
|
|
|
|
Net earnings (loss) attributable to common shareholders - diluted |
|
$ |
( |
) |
|
$ |
|
|
|
Weighted-average common shares outstanding - basic |
|
|
|
|
|
|
|
|
|
Stock options and RSUs |
|
|
- |
|
|
|
|
|
|
Weighted-average common shares outstanding - diluted |
|
|
|
|
|
|
|
|
|
Net earnings (loss) per share attributable to common shareholders - diluted |
|
$ |
( |
) |
|
$ |
|
|
The Company’s potentially dilutive securities, which include convertible preferred shares and stock options have been excluded from the computation of diluted net loss per share for the three months ended March 31, 2020 as the effect would be to reduce the net loss per share. Therefore, the weighted-average number of common shares outstanding for the three months ended March 31, 2020 used to calculate both basic and diluted net loss per share attributable to common shareholders is the same.
The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net earnings (loss) per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
Options to purchase common shares |
|
|
|
|
|
|
- |
|
|
Convertible preferred shares |
|
|
|
|
|
|
- |
|
|
Total potential common shares excluded |
|
|
|
|
|
|
- |
|
5. Other current assets
Other current assets consisted of the following:
|
|
|
|
|
December 31, 2020 |
|
|
March 31, 2021 |
|
||
|
|
|
|
|
(in thousands) |
|
|||||
|
Tax and investment tax credit receivable |
|
|
|
$ |
|
|
|
$ |
|
|
|
Prepaid expenses |
|
|
|
|
|
|
|
|
|
|
|
Materials and supplies |
|
|
|
|
|
|
|
|
|
|
|
Total other current assets |
|
|
|
$ |
|
|
|
$ |
|
|
6
6. Property and equipment, net
Property and equipment, net consisted of the following:
|
|
|
|
|
December 31, 2020 |
|
|
March 31, 2021 |
|
||
|
|
|
|
|
(in thousands) |
|
|||||
|
Computers |
|
|
|
$ |
|
|
|
$ |
|
|
|
Laboratory equipment |
|
|
|
|
|
|
|
|
|
|
|
Furniture and fixtures |
|
|
|
|
|
|
|
|
|
|
|
Leasehold improvements |
|
|
|
|
|
|
|
|
|
|
|
Operating lease right-of-use assets |
|
|
|
|
|
|
|
|
|
|
|
Property and equipment |
|
|
|
|
|
|
|
|
|
|
|
Less accumulated depreciation |
|
|
|
|
( |
) |
|
|
( |
) |
|
Property and equipment, net |
|
|
|
$ |
|
|
|
$ |
|
|
Depreciation expense on property and equipment for the three months ended March 31, 2020 and 2021 was $
7. Intangible assets:
Intangible assets consisted of the following:
|
|
|
March 31, 2021 |
|
|||||||||
|
|
|
Gross carrying amount |
|
|
Accumulated amortization |
|
|
Net book value |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
License |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Technology |
|
|
|
|
|
|
|
|
|
|
|
|
|
IPR&D |
|
|
|
|
|
|
- |
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
At March 31, 2021, amortization expense on intangible assets is estimated to be as follows for each of the next five years:
|
|
|
Amortization Expense |
|
|
|
|
|
(in thousands) |
|
|
|
2021 |
|
$ |
|
|
|
2022 |
|
|
|
|
|
2023 |
|
|
|
|
|
2024 |
|
|
|
|
|
2025 |
|
|
|
|
|
|
|
$ |
|
|
8. Investments in and loans to equity accounted investees
The Company has entered into
In the three months ended March 31, 2021, the Company made a commitment up to CAD$
7
9. Accounts payable and other liabilities
Accounts payable and other liabilities consisted of the following:
|
|
|
|
|
December 31, 2020 |
|
|
March 31, 2021 |
|
||
|
|
|
|
|
(in thousands) |
|
|||||
|
Accounts payable and accrued liabilities |
|
|
|
$ |
|
|
|
$ |
|
|
|
Liability for in-licensing agreement |
|
|
|
|
|
|
|
|
|
|
|
Operating lease liability |
|
|
|
|
|
|
|
|
|
|
|
Liability classified options |
|
|
|
|
|
|
|
|
|
|
|
Government remittances payable |
|
|
|
|
|
|
|
|
|
|
|
Current portion of deferred grant funding |
|
|
|
|
|
|
|
|
|
|
|
Current portion of long-term debt |
|
|
|
|
|
|
|
|
- |
|
|
Total accounts payable and other liabilities |
|
|
|
$ |
|
|
|
$ |
|
|
10. Shareholders’ equity
Sixth Amended and Restated Stock Option Plan:
The Company maintains the AbCellera Biologics Inc. Sixth Amended and Restated Stock Option Plan and our Pre-IPO Plan. Any awards granted under the Pre-IPO Plan will remain subject to the terms of our Pre-IPO Plan and applicable award agreements.
In March 2021, substantially all employee option holders whose awards were liability-classified elected to convert the currency of their option exercise price from Canadian dollars to U.S. dollars for administrative convenience. As a result of the modification, $
2020 Share Option and Incentive Plan:
Our 2020 Share Option and Incentive Plan, or 2020 Plan, was approved by our board of directors on November 18, 2020 and approved by our shareholders on December 1, 2020.
As of March 31, 2021, the number of shares available for issuance under the 2020 Plan was
2020 Employee Share Purchase Plan:
In December 2020, the Company’s Board of Directors approved the 2020 Employee Share Purchase Plan, or the 2020 ESPP. A total of
The following table summarizes the Company’s stock options granted in Canadian dollars under the Pre-IPO Plan since December 31, 2020:
|
|
|
Number of Shares |
|
|
Weighted- Average Exercise Price |
|
||
|
Outstanding as of December 31, 2020 |
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
- |
|
|
|
- |
|
|
Exercised |
|
|
( |
) |
|
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
Outstanding as of March 31, 2021 |
|
|
|
|
|
|
|
|
|
Options exercisable as of March 31, 2021 |
|
|
|
|
|
$ |
|
|
8
The following table summarizes the Company’s stock options granted under the 2020 Plan since December 31, 2020:
|
|
|
Number of Shares |
|
|
Weighted- Average Exercise Price |
|
||
|
Outstanding as of December 31, 2020 |
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
Outstanding as of March 31, 2021 |
|
|
|
|
|
$ |
|
|
|
Options exercisable as of March 31, 2021 |
|
|
- |
|
|
|
- |
|
As part of the 2020 Plan, restricted share units (RSUs) were available to be granted and offer holders are subject to a one year cliff vesting followed by monthly vesting thereafter. The following table summarizes the Company’s restricted share units granted under the 2020 Plan since December 31, 2020:
|
|
|
Number of Shares |
|
|
Weighted- Average Grant Date Fair Value |
|
||
|
Outstanding as of December 31, 2020 |
|
|
- |
|
|
$ |
- |
|
|
Granted |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
Outstanding as of March 31, 2021 |
|
|
|
|
|
$ |
|
|
Stock-based compensation:
Stock-based compensation expense was classified in the consolidated statements of income (loss) and comprehensive income (loss) as follows:
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Research and development |
|
$ |
|
|
|
$ |
|
|
|
General and administrative |
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
At December 31, 2020, there were
11. Revenue
The disaggregated revenue categories are presented on the face of the statement of income (loss) and comprehensive income (loss).
Deferred revenue
Deferred revenue represents payments received for performance obligations not yet satisfied and are presented as current or long-term in the accompanying balance sheets based on the expected timing of satisfaction of the underlying goods and/or services.
Deferred revenue outstanding in each respective period is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
December 31, 2019 |
|
|
March 31, 2020 |
|
|
December 31, 2020 |
|
March 31, 2021 |
|
||||
|
Deferred revenue |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
$ |
( |
) |
9
During the three months ended March 31, 2020 and 2021, the Company recognized $
In March of 2020, the Company entered into a research collaboration and license agreement with Eli Lilly pursuant to which the Company will perform discovery research for several targets for Eli Lilly to develop and commercialize. Under the agreement, the Company is entitled to receive an aggregate of up to $
The agreement resulted in initial upfront of $
Of the remaining deferred revenue balance of $
License revenue
For the licenses to our intellectual property the Company recognizes revenue from non-refundable, up-front fees when the license is transferred to the customer and the customer is able to use and benefit from the license. For the three months ended March 31, 2021, the Company recognized $
12. Financial instruments
The Company categorizes its financial assets and liabilities measured at fair value into a three-level hierarchy established by U.S. GAAP that prioritizes those inputs to valuation techniques used to measure fair value based on the degree to which they are observable. The three levels of the fair value hierarchy are as follows: Level 1 inputs are quoted prices in active markets for identical assets and liabilities; Level 2 inputs, other than quoted prices included within Level 1, are observable for the asset or liability either directly or indirectly; and Level 3 inputs are not observable in the market.
The Company’s financial instruments consist of cash and cash equivalents, accounts receivable, loans to related parties, accounts payable and accrued liabilities and royalties payable, operating lease obligations, long-term debt, and contingent consideration payable. The carrying values of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, and bank indebtedness approximate their fair values due to the immediate and short-term maturity of these financial instruments. The fair value of loans to related party approximate the carrying value as the interest rates approximate the rates applicable for non-related party loans.
At December 31, 2020 and March 31, 2021, the carrying value of long-term debt was $
Contingent consideration related to business acquisitions are recorded at fair value on the acquisition date and adjusted on a recurring basis for changes in its fair value. Changes in the fair value of contingent consideration liabilities can result from changes in anticipated payments and changes in assumed discount periods and rates. These inputs are unobservable in the market and therefore categorized as Level 3 inputs.
The following table presents the changes in fair value of the liability for contingent consideration:
|
(in thousands) |
|
Liability at beginning of the period |
|
|
Increase (decrease) in fair value of liability for contingent consideration |
|
Liability at end of the period |
|
||
|
Three months ended March 31, 2021 |
|
$ |
|
|
|
|
|
$ |
|
|
10
13. Commitments and contingencies
From time to time, the Company may become involved in routine litigation arising in the ordinary course of business. At each reporting date, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under the provisions of the authoritative guidance that addresses accounting for contingencies. The Company does not have contingency reserves established for any litigation liabilities and any of the costs related to such legal proceedings are expensed as incurred.
The Company may enter into certain agreements with strategic partners in the ordinary course of operations that may include contractual milestone payments related to the achievement of pre-specified research, development, regulatory and commercialization events and indemnification provisions, which are common in such agreements.
Pursuant to the agreements, the Company may be obligated to make research and development and regulatory milestone payments upon the occurrence of certain events and upon receipt of royalty payments in the low single-digits to mid-twenties based on certain net sales targets. During the three months ended March 31, 2021, the Company has expensed approximately $
During the quarter ended March 31, 2021 the Company entered into a lease for office and laboratory space in Sydney, Australia representing future lease payments of $
The Company entered into a lease for office and laboratory space in Vancouver, Canada, in conjunction with our
11
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q includes “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 and “forward-looking information” within the meaning of Canadian securities laws, or collectively, forward-looking statements. Forward-looking statements include statements that may relate to our plans, objectives, goals, strategies, future events, future revenue or performance, capital expenditures, financing needs and other information that is not historical information. Many of these statements appear, in particular, under the headings “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. Forward-looking statements can often be identified by the use of terminology such as “subject to”, “believe,” “anticipate,” “plan,” “expect,” “intend,” “estimate,” “project,” “may,” “will,” “should,” “would,” “could,” “can,” the negatives thereof, variations thereon and similar expressions, or by discussions of strategy. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. In particular, these forward-looking statements include, but are not limited to:
|
|
• |
our expectations regarding the rate and degree of market acceptance of our drug-discovery platform; |
|
|
• |
companies and technologies in our industry that we compete with; |
|
|
• |
our ability to manage and grow our business by expanding our sales to existing partners or introducing our drug-discovery platform to new partners; |
|
|
• |
our ability to provide our partners with a full solution from target to IND submission; |
|
|
• |
our expectations regarding the completion of our GMP facility and our manufacturing capabilities; |
|
|
• |
our ability to establish and maintain intellectual property protection for our technologies and workflows, including with respect to our intellectual property litigation with Berkeley Lights, or avoid or defend against claims of infringement; |
|
|
• |
our ability to attract, hire and retain key personnel and to manage our future growth effectively; |
|
|
• |
our ability to obtain additional financing in future offerings; |
|
|
• |
the volatility of the trading price of our common shares; |
|
|
• |
our ability to attract and retain key scientific and engineering personnel; |
|
|
• |
our expectations regarding the period during which we qualify as an emerging growth company under the JOBS Act; |
|
|
• |
business disruptions affecting our operations and the development of our platform due to the global COVID-19 pandemic; |
|
|
• |
our ability to remediate our material weaknesses; |
|
|
• |
our expectations regarding our PFIC status for our taxable year ended December 31, 2020 or any future taxable year; |
|
|
• |
our expectations regarding the Trianni acquisition and our ability to realize the intended benefits of such transaction; |
|
|
• |
our expectations regarding the use of proceeds from our initial public offering; |
|
|
• |
our expectations about market trends; and |
|
|
• |
our ability to predict and manage government regulation. |
We may not actually achieve the plans, intentions, or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions, and expectations disclosed in the forward-looking statements we make. Moreover, we operate in a competitive and rapidly changing environment. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this Quarterly Report. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, collaborations, joint ventures, or investments we may make or enter into.
12
You should read this Quarterly Report and the documents that we file with the Securities and Exchange Commission, or the SEC, with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this Quarterly Report are made as of the date of this Quarterly Report, and we do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Quarterly Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete. Our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
This Quarterly Report includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties as well as our own estimates of potential market opportunities. All of the market data used in this Quarterly Report involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research, and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions.
We express all amounts in this Quarterly Report on Form 10-Q in U.S. dollars, except where otherwise indicated. References to “$” and “US$” are to U.S. dollars and references to “C$” and “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this Quarterly Report on Form 10-Q to “AbCellera,” the “Company,” “we,” “us” and “our” refer to AbCellera Biologics Inc. and its consolidated subsidiaries.
Impact of COVID-19
At the onset of the pandemic in March 2020, the Company took proactive measures to protect the health and safety of our employees, business partners, vendors, and contractors. Some of the actions taken include the following:
|
|
• |
We implemented a comprehensive COVID-19 policy and communication platform and provided real-time updates company-wide relying on directives from local health authorities. As the situation progressed, we adapted accordingly, including adjusting all administrative staff to work from home. |
|
|
• |
We implemented protocols for employees necessary to carryout Company functions in the office and laboratory facilities including physical distancing, personal and protective equipment, signage, erecting barriers between desks and lab benches, and implementing space restrictions for different areas of the facilities. |
|
|
• |
Consistent with national and local health authorities, we restricted business travel and implemented procedures to control and monitor all office and facility access. |
|
|
• |
We have not been required to stop laboratory and research activities due to the COVID-19 pandemic. We will continue to adapt and apply new measures as required and as directed by local health authorities. |
Overview
We believe that the surest path to a better future is through technological advancement and that the new frontier of technology lies at the interface of computation, engineering and biology. Our mission is to improve health with technologies that transform the way that antibody-based therapies are discovered. We aim to become the centralized operating system for next generation antibody discovery.
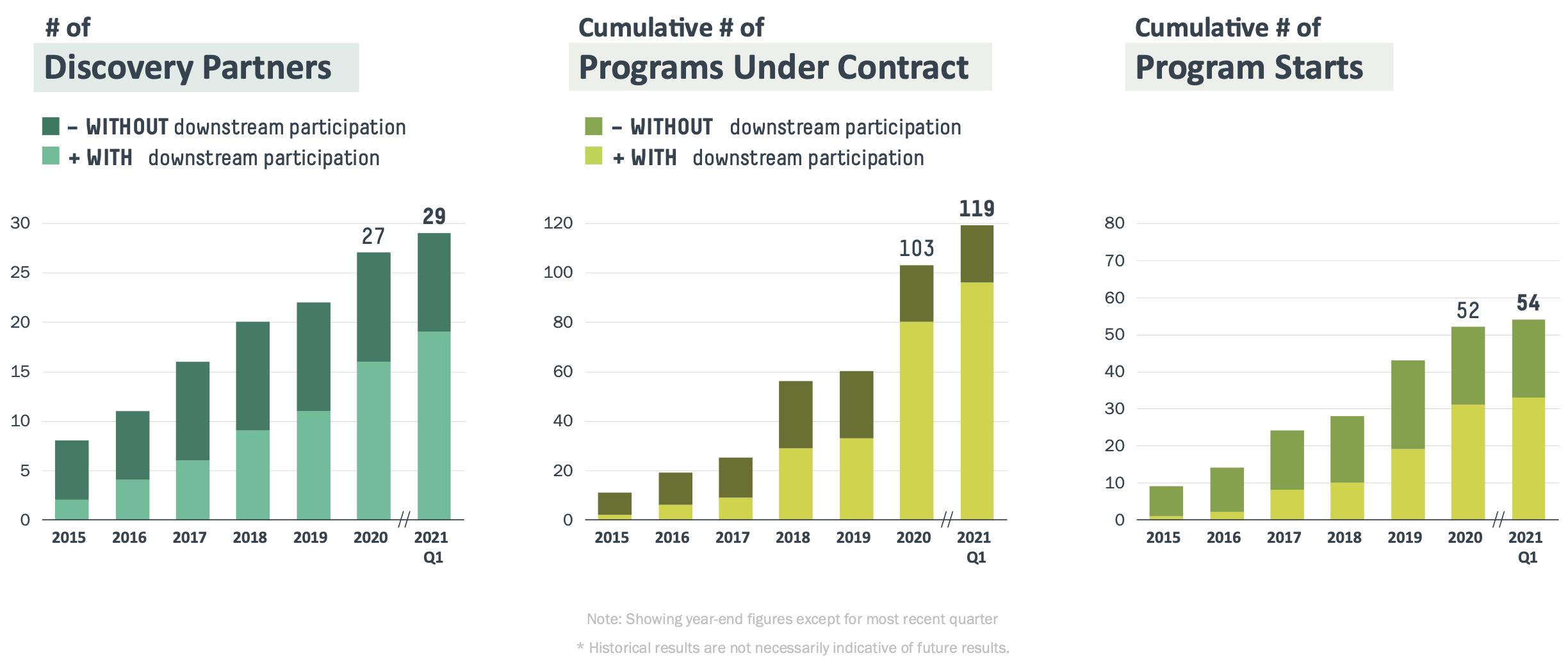
Our full-stack, artificial intelligence-, or AI, powered drug discovery platform searches and analyzes the database of natural immune systems to find antibodies that can be developed as drugs. We believe our technology increases the speed and the probability of success of therapeutic antibody discovery, including enabling discovery against targets that may otherwise be intractable. Rather than advancing our own clinical pipeline of drug candidates, we forge partnerships with drug developers of all sizes, from large cap pharmaceutical to small biotechnology companies. We empower them to move quickly, reduce cost and tackle the toughest problems in drug development As of March 31, 2021, we had 119 discovery programs that are either completed, in progress or under contract
13
with 29 partners. As a recent example, in a collaboration with Eli Lilly and Company, or Lilly, we applied our technology stack to co-develop bamlanivimab, a potential antibody therapy to treat and prevent COVID-19. Starting from a single blood sample obtained from a convalescent patient, we and our partners identified a viable antibody drug candidate within three weeks that advanced into clinical testing 90 days after initiation of the program. Lilly progressed into these clinical trials at a greatly accelerated pace as a result of the Coronavirus Treatment Acceleration Program, which is a special emergency program for possible coronavirus therapies created by the FDA in 2020 to expedite the development of potentially safe and effective life-saving treatments to combat the COVID-19 pandemic. With respect to other or future product candidates, there is no assurance that any of our partners or collaborators will be able to advance a product candidate into clinical development on this timeframe again in the future, or at all. We initiated our partnering program in 2015 and have only had this one program result in milestone and royalty payments to us to date and we have not yet had a program receive marketing approval.
We structure our agreements in a way that is designed to align our partners’ economic interests with our own. We forge partnerships with large cap pharmaceutical companies, biotechnology companies of all sizes and non-profit and government organizations. Our partners select a target and define the antibody properties needed for therapeutic development. We provide discovery solutions to partners that have a range of discovery capabilities, from the highly enabled to the less enabled. We enable discovery against targets that have traditionally been intractable, and we accelerate programs against less difficult targets.
Our deals emphasize participation in the success and upside of future antibody therapeutics. Our partnership agreements include near-term payments for technology access, research and intellectual property rights, and downstream payments in the form of clinical and commercial milestones, and royalties on net sales. Longer-term we are eligible to receive additional payments upon satisfaction of clinical and commercial milestones, which we refer to as milestone payments, as well as royalties on sales of products derived from antibodies that we discover for our partners. Our discovery partnerships generally include royalty payments on net sales in the single digit to low-double digit range.
We generated revenue of $4.7 million and $202.7 million for the three months ended March 31, 2020 and 2021, respectively. As of March 31, 2021, we had a total of 29 partners for whom we were conducting drug discovery activities. For the three months ended March 31, 2020, three of our partners accounted for 51%, 12%, and 11% of research fees revenue and five partners accounted for the remaining 26% of research fees revenue. For the three months ended March 31, 2021, three of our partners accounted for 36%, 24% and 20% of research fees revenue, and seven partners accounted for the remaining 20% of research fees revenue. For the three months ended March 31, 2021 we recognized a milestone payment and royalty revenue streams, totaling $178.5 million, exclusively from our partnership with Lilly. Our partnership with Lilly constituted one of the partnerships that generated 10% or more of our consolidated revenues during the one or more periods described above. With respect to the other partners, we do not believe the loss of any one or more of such partners would have a material adverse effect on us and our subsidiaries taken as a whole. We have also grown the number of programs that we have under contract with our partners, as illustrated by the following charts.
We incurred sales and marketing expenses of $0.5 million and $2.6 million for the three months ended March 31, 2020 and 2021, respectively. We are significantly increasing investment into our business development team and into marketing our solutions to new and existing partners.
14
We focus a substantial portion of our resources on research and development efforts towards deepening our technology and expertise along our technology stack, and we expect to continue to make significant investments in this area for the foreseeable future. We incurred research and development expenses of $4.1 million and $12.4 million for the three months ended March 31, 2020 and 2021, respectively. We incurred general and administrative expenses of $1.7 million and $6.4 million for the three months ended March 31, 2020 and 2021, respectively. We expect to continue to incur significant expenses, and we expect such expenses to increase substantially in connection with our ongoing activities, including as we:
|
|
• |
Invest in research and development activities to improve our technology stack and platform; |
|
|
• |
Market and sell our solutions to existing and new partners; |
|
|
• |
Expand and enhance operations to deliver programs, including investments in manufacturing; |
|
|
• |
Acquire businesses or technologies to support the growth of our business; |
|
|
• |
Attract, hire and retain qualified personnel; |
|
|
• |
Continue to establish, protect and defend our intellectual property and patent portfolio, including our ongoing litigation; and |
|
|
• |
Operate as a public company. |
To date, we have financed our operations primarily from revenue from our drug discovery partnerships in the form of research fees, government funding from grants, external borrowings, and from the issuance and sale of convertible preferred shares and notes, and common shares.
Our net loss for the three months ended March 31, 2020 was $2.1 million and our net earnings for the three months ended March 31, 2021 were $117.2 million. As of March 31, 2021, we had accumulated earnings of $231.4 million and we had cash and cash equivalents totaling $685.8 million.
Recent Developments
In March 2020, we entered into a discovery partnership agreement with Eli Lilly and Company, or Lilly, pursuant to which we will perform discovery research for a number of targets for Lilly that will result in antibodies for Lilly to develop and potentially commercialize. This partnership includes the licensing of bamlanivimab, a monoclonal antibody designed to block viral attachment of the COVID-19 virus and its entry into human cells as well as other candidate antibodies against COVID-19 discovered by AbCellera. On June 1, 2020, 90 days after program initiation, bamlanivimab moved to first-in-human testing and progressed to Phase 3 clinical trials by July 2020.
In December 2020, we completed AbCellera’s IPO on the Nasdaq. The Company completed the sale of 27,772,500 shares of its common shares in the IPO at a price to the public of $20.00 per share. The Company raised gross proceeds of $555.5 million, or aggregate net proceeds of $522.8 million, after deducting underwriting discounts and commissions and estimated offering expenses payable by the Company. Immediately prior to the completion of our IPO, our convertible preferred shares and notes were converted to common shares.
In February 2021, it was announced that bamlanivimab (LY-CoV555) 700 mg, a human antibody discovered by AbCellera and developed with Eli Lilly and Company (Lilly), administered with a second Lilly antibody, etesevimab (LY-CoV016) 1400 mg, has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the treatment of mild to moderate COVID-19 in patients aged 12 and older who are at high risk for progressing to severe COVID-19 and/or hospitalization. New protocols enable front-line clinicians to administer bamlanivimab alone, and bamlanivimab and etesevimab together, in as few as 16 minutes and 21 minutes, respectively.
In February 2021, we announced the appointment of Ester Falconer, Ph.D. as our Chief Technology Officer. As CTO, Dr. Falconer will lead our long-term strategy in the development, aggregation, and integration of technologies that improve the speed and success of therapeutic antibody discovery from target to investigational new drug application submission.
15
In March 2021, we entered agreements to expand our collaboration with Gilead Sciences, Inc. including a multi-year, multi-target antibody discovery collaboration and access to our humanized mouse technology, the Trianni Mouse®. Under the financial terms of the agreements, we will receive an upfront payment and we are eligible for milestone payments and royalties based on the development and commercialization of antibodies generated by the Company under this collaboration.
On March 5, 2021 the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive scientific opinion for bamlanivimab alone and bamlanivimab administered together with etesevimab. The opinion advises bamlanivimab alone and bamlanivimab administered together with etesevimab can be used for the treatment of confirmed COVID-19 in patients aged 12 years and older that do not require supplemental oxygen for COVID-19 and who are at high risk of progressing.
On March 10, 2021 Lilly reported Phase 3 clinical trials results that showed that bamlanivimab 700 mg and etesevimab 1400 mg together reduced COVID-19-related hospitalizations and deaths by 87% in high-risk patients recently diagnosed with COVID-19.
In April 2021, the Company announced it entered into a joint venture (Beedie JV) whereby we will invest in equal shares of a Vancouver building development to be leased exclusively by AbCellera for additional office and lab facilities for our future office headquarters.
In April 2021, Eli Lilly and Company (Lilly) requested the U.S. FDA revoke the Emergency Use Authorization (EUA) for bamlanivimab (LY-CoV555) 700 mg alone. Lilly made this request due to the evolving variant landscape in the U.S. and the full availability of bamlanivimab and etesevimab together. The request was not due to any new safety concern. This final step in Lilly's transition to only supply bamlanivimab and etesevimab for administration together in the U.S. for the treatment of COVID-19 – as planned with the FDA – followed the modification of contracts with the U.S. government to ensure adequate supply of etesevimab to be used together with bamlanivimab. The FDA announced that it had revoked the EUA for bamlanivimab 700 mg alone on April 16, 2020.
In April 2021, we entered multi-target collaboration agreement with Empirico Inc. The agreement will leverage the Company's hyper-scale datasets, machine learning, and advanced computation to both identify high-value, genetically-validated drug targets and discover novel therapeutic antibodies while Empirico will use its Precision Insights Platform, a human genetics-focused discovery platform, to select up to five therapeutic targets. Under the terms of the agreement, Empirico will have the rights to develop and commercialize novel antibodies resulting from the collaboration. AbCellera will receive research payments and is eligible to receive downstream clinical and commercial milestone payments and royalties on net sales of products from Empirico.
In May 2021, the Company announced that a second antibody from its collaboration with Lilly, LY-CoV1404, entered clinical trials in patients with mild-to-moderate COVID-19. Lilly expanded its ongoing BLAZE-4 trials to evaluate LY-CoV1404 alone and together with other monoclonal antibodies.
Key Factors Affecting Our Results of Operations and Future Performance
We believe that our financial performance has been, and in the foreseeable future will continue to be, primarily driven by multiple factors as described below, each of which presents growth opportunities for our business. These factors also pose important challenges that we must successfully address in order to sustain our growth and improve our results of operations. Our ability to successfully address these challenges is subject to various risks and uncertainties, including those described in Part II, Item 1A of this report, captioned “Risk Factors”.
|
|
• |
Securing additional programs under contract. Our potential to grow revenue, in both the near and long term, is dependent on our ability to secure additional programs under contract from new and existing partners. For existing partners, we seek to expand our relationships with them to cover multi-year, multi-target programs. Since our first commercial partnership in 2015, as of March 31, 2021, we had 119 discovery programs that are either completed, in progress or under contract with 29 partners. We are building our business development team across the major biotechnology geographic hubs in order to bring in new partners and new programs under contract, and we believe that we have a significant opportunity to continue to increase the number of partners who have programs based on our platform. Our ability to continue to grow our number of programs under contract is dependent upon our ability to educate the market and support the business through investment in our sales and marketing efforts and through further research and development to enhance our technological differentiation. |
|
|
• |
Our partners successfully developing and commercializing the antibodies that we discover. Until recently, we had generated nearly all of our revenue from research fees. We estimate that, based on the terms of our existing contracts and estimates of historical rates of success of antibody drug development, the vast majority of the potential value for each |
16
|
|
program under contract is represented by potential future milestone payments and royalties rather than research fees. As a result, we believe our business and our future results of operations will be highly reliant on the degree to which our partners successfully develop and commercialize the antibodies that we discover based on contracts with our partners. As our partners continue to advance development of the antibodies that we have discovered, we expect to start receiving additional milestone payments and royalties if any partners commence commercial sales of such antibodies. |
|
|
• |
Rate and timing of selecting and initiating discovery projects by our partners. Once programs are secured under contract, partners must select targets and agree on a detailed statement of work before we commence discovery research on any antibodies. The rate and timing of such selection and initiation differs from partner to partner. Because the vast majority of research fees that we are entitled to recognize under our partnerships depend on our delivery of antibodies for development by our partners, any delays by our partners in selecting targets and agreeing on statements of work will impact revenue recognition. |
|
|
• |
Investing in enhancements to our technology stack. Our ability to maintain and expand our partnerships is dependent on the advantages our technology stack delivers to our partners. We intend to maintain our leading position through research and development investments to refine and add capabilities in areas such as computation, protein engineering, immunization technologies, genetically engineered rodents and cell line selection. We have successfully closed and will continue to look for strategic technology acquisitions to improve, broaden and deepen our capabilities and expertise in antibody drug discovery and development, or those that offer opportunities to expand our partnership business into adjacent therapeutic modalities. We intend to devote substantial resources to continue to improve our technological differentiation which will impact our financial performance. |
|
|
• |
Scaling our operations to execute on discovery programs. As we secure additional programs under contract and as our partners initiate discovery programs, our operational capacity to execute such research activities may become strained. We are making significant investments in capital and time to increase our ability to address future growth, including building new headquarters, building a new small-scale manufacturing plant, investing in research and development and hiring more talented personnel across functions. We have new facilities under development scheduled to take occupancy in late 2021 and 2023 that are intended to materially expand capacity. As we expand our workforce, we expect a significant increase in our operating expenses, including stock-based compensation. |
Key Business Metrics
We regularly review the following key business metrics to evaluate our business, measure our performance, identify trends affecting our business, formulate financial projections and make strategic decisions. We believe that the following metrics are important to understand our current business. These metrics may change or may be substituted for additional or different metrics as our business develops. For example, as our business matures and to the extent programs are discontinued, we anticipate updating these metrics to reflect such changes.
|
Metric |
|
March 31, 2020 |
|
|
March 31, 2021 |
|
|
Change % |
|
|||
|
Number of discovery partners |
|
|
24 |
|
|
|
29 |
|
|
|
21 |
% |
|
Programs under contract, cumulative |
|
|
73 |
|
|
|
119 |
|
|
|
63 |
% |
|
Program starts, cumulative |
|
|
47 |
|
|
|
54 |
|
|
|
15 |
% |
|
Programs in the clinic |
|
|
- |
|
|
|
1 |
|
|
N/M |
|
|
Number of discovery partners represents the unique number of partners with whom we have executed partnership contracts. We view this metric as an indication of the competitiveness of our technology stack and our current level of market penetration. The metric also relates to our opportunities to secure programs under contract from existing customers through repeat business opportunities.
Programs under contract represent the number of antibody development programs that are under contract for delivery of discovery research activities. A program under contract is counted when a contract is executed with a partner under which we commit to discover antibodies against one selected target. A target is any relevant antigen for which a partner seeks our support in developing binding antibodies. We view this metric as an indication of commercial success and technological competitiveness. It further relates to revenue from technology access fees. The cumulative number of programs under contract with downstream participation is related to our ability to generate future revenue from milestone payments and royalties.
Program starts represent the number of unique programs under contract for which we have commenced the discovery effort. The discovery effort commences on the later of (i) the day on which we receive sufficient reagents to start discovery of antibodies against a target and (ii) the day on which the kick-off meeting for the program is held. We view this metric as an indication of our
17
operational capacity to execute on programs under contract. It is also an indication of the selection and initiation of discovery projects by our partners and the resulting near-term potential to earn research fees. Cumulatively, program starts with downstream participation indicate our total opportunities to earn downstream revenue from milestone fees and royalties in the mid- to long-term.
Programs in the clinic represent the count of unique molecules for which an Investigational New Drug, or IND, New Animal Drug or Pre-Market Approval, or PMA, application, or equivalents under other regulatory regimes, has been filed based on an antibody that was discovered by us. Where the date of such application is not known to us, the date of the first public announcement of clinical trials will be used instead for the purpose of this metric. We view this metric as an indication of our near- and mid-term potential revenue from milestone fees and potential royalty payments in the long term.
Components of Results of Operations
Revenue
Our revenue is comprised of partnership research fees, licensing revenue, development milestones, and royalty payments from commercial products. Research fees consist primarily of technology access fees, which are generally generated upon execution of our partnership agreements, and discovery research fees, which are generated through our performance of antibody discovery research for our partners. Licensing revenue is primarily from our licensing of our humanized rodent platform, Trianni™. Our partnership agreements also entitle us to receive payments upon the satisfaction of clinical, approval, and commercial milestones as well as royalties on our partners’ commercial sales of the molecules that we discover.
We expect revenue to increase over time as we secure additional programs under contract and conduct discovery efforts for our partners, and as our partners continue the development of the antibodies that we deliver. We expect that our revenue will fluctuate from period to period due to the timing of securing additional programs under contract, the inherently uncertain nature of the timing of milestone achievement and our dependence on the program decisions of our partners.
Operating Expenses
Royalty Fees. Royalty fees consist of certain contractual royalty payments to our strategic partners upon receipt of royalty revenue based on our customers third-party net sales. Royalty fees are not included in every program. For royalties received from Lilly for commercial sales of bamlanivimab, royalty fees are due to collaboration partners in AbCellera’s DARPA P3 (Pandemic Preparedness Program) project focused on rapid pandemic response. Royalty fees are recorded when the third-party sale occurs.
Research and Development Expenses. Research and development expenses primarily consist of salaries, benefits, incentive compensation, stock-based compensation, laboratory supplies, materials expenses for employees and contractors engaged in research and product development, and facilities expenses related to direct research and development activities. These expenses are exclusive of depreciation and amortization. Research and development activities consist of discovery research for partners as well as our internal platform development. We derive improvements to our technology stack from both types of activities.
We expect to continue to incur substantial research and development expenses as we conduct discovery research for our partners. In addition, we plan to continue to invest in research and development to enhance our solutions and offerings to our partners, including hiring additional employees and continuing research and development projects obtained through strategic technology acquisitions. As a result, we expect that our research and development expenses will continue to increase in absolute dollars in future periods and vary from period to period as a percentage of revenue.
Sales and Marketing Expenses. Our sales and marketing expenses consist primarily of salaries, benefits, and stock-based compensation costs for employees within our commercial sales functions, as well as marketing, travel expenses and information technology costs that are directly associated with sales and marketing efforts, such as client relationship management tools and other information technology data tools to provide insight into market segments and trends. This activity has been complemented with research and development staff attending a variety of scientific conferences, which has helped increase the business development pipeline. The associated expenses are included in research and development expenses as scientific conference attendance is primarily related to our research and development efforts. We expect our sales and marketing expenses to increase in absolute dollars as we expand our commercial sales, marketing and business development teams; increase our presence globally; and increase marketing activities to drive awareness and adoption of our platform.
General and Administrative Expenses. General and administrative expenses primarily consist of salaries, benefits and stock-based compensation costs for employees in our executive, accounting and finance, project management, corporate development, office administration, legal and human resources functions as well as professional services fees, such as consulting, audit, tax and legal fees, general corporate costs and allocated overhead expenses. We expect that our general and administrative expenses will continue to
18
increase in absolute dollars in future periods, primarily due to increased headcount to support anticipated growth in the business and due to incremental costs associated with operating as a public company, including costs to comply with the rules and regulations applicable to companies listed on a securities exchange and costs related to compliance and reporting obligations pursuant to the rules and regulations of the SEC and stock exchange listing standards, public relations, insurance and professional services. We expect these expenses to vary from period to period as a percentage of revenue.
Depreciation and Amortization. Depreciation expense consists of the depreciation of property and equipment used actively in the business, primarily by research and development activities. Amortization expense includes the amortization of intangible assets over their respective useful lives.
Other (Income) Expense. Other (Income) Expense consists of interest income earned on our cash balances, interest expense related to borrowings under any credit agreements, and foreign exchange (gain) loss due to fluctuation in exchange rates between the Canadian dollar and the U.S. dollar.
Grants and Incentives. Grants and incentives include cost recovery on activities that qualified for approved projects supported by grant funding or tax credits. Grants primarily include the benefit from programs administered by the Canadian government’s Ministry of Innovation, Science and Economic Development, such as their Industrial Research Assistance Program, and the Strategic Innovation Fund. To the extent that grant funding covers capital expenditures, a deferred credit is recorded on the balance sheet and recognized rateably over the benefit period of the related expenditure for which the grant was intended to compensate.
Results of operations
Comparison of the three months ended March 31, 2020 and 2021
The following table summarizes our unaudited results of operations data for the three months ended March 31, 2020 and 2021:
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
Revenue: |
|
|
|
|
|
|
|
|
|
Research fees |
|
$ |
4,657 |
|
|
$ |
3,986 |
|
|
Licensing revenue |
|
|
- |
|
|
|
20,259 |
|
|
Milestone payments |
|
|
- |
|
|
|
7,000 |
|
|
Royalty revenue |
|
|
- |
|
|
|
171,496 |
|
|
Total revenue |
|
|
4,657 |
|
|
|
202,741 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Royalty fees |
|
|
- |
|
|
|
20,010 |
|
|
Research and development(1) |
|
|
4,118 |
|
|
|
12,352 |
|
|
Sales and marketing(1) |
|
|
437 |
|
|
|
2,578 |
|
|
General and administrative(1) |
|
|
1,650 |
|
|
|
6,422 |
|
|
Depreciation and amortization |
|
|
574 |
|
|
|
3,305 |
|
|
Total operating expenses |
|
|
6,779 |
|
|
|
44,667 |
|
|
Income (loss) from operations |
|
|
(2,122 |
) |
|
|
158,074 |
|
|
Other (income) expense: |
|
|
|
|
|
|
|
|
|
Other (income) expense |
|
|
1,001 |
|
|
|
(265 |
) |
|
Grants and incentives |
|
|
(1,030 |
) |
|
|
(3,148 |
) |
|
Total other income |
|
|
(29 |
) |
|
|
(3,413 |
) |
|
Net earnings (loss) before income tax |
|
|
(2,093 |
) |
|
|
161,487 |
|
|
Provision for income tax |
|
|
- |
|
|
|
44,266 |
|
|
Net earnings (loss) for the year |
|
$ |
(2,093 |
) |
|
$ |
117,221 |
|
|
Net earnings (loss) per share attributable to common shareholders |
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.01 |
) |
|
$ |
0.43 |
|
|
Diluted |
|
$ |
(0.01 |
) |
|
$ |
0.37 |
|
|
Weighted-average common shares outstanding |
|
|
|
|
|
|
|
|
|
Basic |
|
|
151,859,924 |
|
|
|
269,697,212 |
|
|
Diluted |
|
|
151,859,924 |
|
|
|
320,282,747 |
|
|
(1) |
Amounts are exclusive of depreciation and amortization. Amounts include stock-based compensation as follows: |
19
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Research and development |
|
$ |
616 |
|
|
$ |
3,158 |
|
|
General and administrative |
|
|
608 |
|
|
|
1,316 |
|
|
Sales and marketing |
|
|
13 |
|
|
|
953 |
|
|
|
|
$ |
1,237 |
|
|
$ |
5,427 |
|
Revenue
|
|
|
Three months ended March 31, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|
||||
|
|
|
(in thousands, except percentages) |
|
|||||||||||||
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research fees |
|
$ |
4,657 |
|
|
$ |
3,986 |
|
|
$ |
(671 |
) |
|
|
-14 |
% |
|
Licensing revenue |
|
|
- |
|
|
|
20,259 |
|
|
|
20,259 |
|
|
N/A |
|
|
|
Milestone payments |
|
|
- |
|
|
|
7,000 |
|
|
|
7,000 |
|
|
N/A |
|
|
|
Royalty revenue |
|
|
- |
|
|
|
171,496 |
|
|
|
171,496 |
|
|
N/A |
|
|
|
Total revenue |
|
$ |
4,657 |
|
|
$ |
202,741 |
|
|
$ |
198,084 |
|
|
|
4253 |
% |
Revenue increased by $198.1 million from the three months ended March 31, 2020 to March 31, 2021. We received a milestone payment upon the first commercial sale in Europe by Lilly relating to molecule bamlanivimab for treatment of COVID-19 in the amount of $7.0 million. Royalty payments of $171.5 million are directly associated with the specified percentage of proceeds that Lilly received from the sales of bamlanivimab. We earned $20.3 million in licensing revenue related to the recently acquired Trianni humanized rodent platform business. Despite a significant increase in cumulative Programs Under Contract compared to the same period in the previous year, revenues associated to research fees decreased by $0.7 million for the three months ended March 31, 2021. The decrease was driven by a reduction in revenue from our DARPA COVID-19 antibody discovery program.
Operating Expenses
Royalty Fees
|
|
|
Three months ended March 31, |
|
|
Change |
|||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|||
|
|
|
(in thousands, except percentages) |
||||||||||||
|
Royalty fees |
|
$ |
— |
|
|
$ |
20,010 |
|
|
$ |
20,010 |
|
|
N/A |
Royalty fees for the three months ended March 31, 2021 were $20.0 million. These were directly attributable to the royalty revenues received by the Company from sales of bamlanivimab by Lilly due to AbCellera’s collaborators in pandemic response.
Research and Development
|
|
|
Three months ended March 31, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|
||||
|
|
|
(in thousands, except percentages) |
|
|||||||||||||
|
Research and development |
|
$ |
4,118 |
|
|
$ |
12,352 |
|
|
$ |
8,234 |
|
|
|
200 |
% |
Research and development expenses increased by $8.2 million, or 200%, from the three months ended March 31, 2020 to March 31, 2021. $5.9 million of the increase is due to the increase in compensation expense consistent with the increase in headcount. $2.3 million of the increase is attributed to an increase in research materials, facilities, supplies and services consistent with the overall increase in research and development activities.
20
Sales and Marketing
|
|
|
Three months ended March 31, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|
||||
|
|
|
(in thousands, except percentages) |
|
|||||||||||||
|
Sales and marketing |
|
$ |
437 |
|
|
$ |
2,578 |
|
|
$ |
2,141 |
|
|
|
490 |
% |
Sales and marketing expenses increased by $2.1 million, or 490%, from the three months ended March 31, 2020 to March 31, 2021. $1.1 million of the increase is due to the increase in compensation expense consistent with increased headcount. $0.8 million of the increase is attributable to a donation made to Surrey Hospital to fund a study related to bamlanivimab in Canada. Sales and marketing expenses related to travel were significantly lower for the three months ended March 31, 2021 due to continued COVID-19 related travel restrictions.
General and Administrative
|
|
|
Three months ended March 31, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|
||||
|
|
|
(in thousands, except percentages) |
|
|||||||||||||
|
General and administrative |
|
$ |
1,650 |
|
|
$ |
6,422 |
|
|
$ |
4,772 |
|
|
|
289 |
% |
General and administrative expenses increased by $4.8 million, or 289%, from the three months ended March 31, 2020 to March 31, 2021. $1.4 million of the increase was driven by increased headcount within the general and administrative function and the associated compensation expenses. $2.2 million is attributable to legal and corporate matters as a public company. $1.0 million of the increase in general and administrative expense is due to increased expenditures related to director and office insurance and increased general office expense.
Depreciation and Amortization
|
|
|
Three months ended March 31, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|
||||
|
|
|
(in thousands, except percentages) |
|
|||||||||||||
|
Depreciation and amortization |
|
$ |
574 |
|
|
$ |
3,305 |
|
|
$ |
2,731 |
|
|
|
476 |
% |
Depreciation and amortization expenses increased by $2.7 million, or 476%, from the three months ended March 31, 2020 to March 31, 2021. Amortization expense increased by $2.5 million due to the amortization of acquired intangible assets over their respective useful lives. Depreciation expense increased by $0.3 million due to the depreciation of equipment and facilities related to capital equipment purchases.
Other (Income) Expense
|
|
|
Three months ended March 31, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|
||||
|
|
|
(in thousands, except percentages) |
|
|||||||||||||
|
Other (income) expense |
|
$ |
1,001 |
|
|
$ |
(265 |
) |
|
$ |
(1,266 |
) |
|
|
-126 |
% |
Other (income) expense decreased by $1.3 million, or 126%, from the three months ended March 31, 2020 to March 31, 2021. Other (income) expense for 2020 included a foreign exchange loss of $0.9 million along with offsetting interest income and expense amounts. Other (income) expense for the three months ended March 31, 2021 included a foreign exchange loss of $0.5 million and a $1.1 million gain on fair value adjustments.
21
Grants and Incentives
|
|
|
Three months ended March 31, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2021 |
|
|
Amount |
|
|
% |
|
||||
|
|
|
(in thousands, except percentages) |
|
|||||||||||||
|
Grants and incentives |
|
$ |
(1,030 |
) |
|
$ |
(3,148 |
) |
|
$ |
(2,118 |
) |
|
|
206 |
% |
Grants and incentives increased by $2.1 million, or 206%, from the three months ended March 31, 2020 to March 31, 2021. This increase is attributable to increased research and development expenditures incurred during the quarter that are eligible for the SIF project.
Liquidity and Capital Resources
As of March 31, 2021, we had $685.8 million of cash and cash equivalents. The increase of $91.7 million since December 31, 2020 was driven primarily from cash flow from operations in the first quarter of 2021.
We have generated positive operating cash flow cumulatively since our inception in 2012 and in every year since 2018. We intend to significantly invest in our business, and as a result may incur operating losses in future periods. We will continue to invest in research and development efforts towards expanding our capabilities and expertise along our technology stack, the building of our business development team and marketing our solutions to new and existing partners, and the expansion of our future office headquarters, and related infrastructure, including execution of long-term office-lease arrangements. Based on our current business plan, we believe that our existing cash and cash equivalents and anticipated cash flows from operations, will be sufficient to meet our working capital and capital expenditure needs over at least the next 24 months following the date of this report.
Cash Flows
The following table summarizes our cash flows for the periods presented:
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2021 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(764 |
) |
|
$ |
109,545 |
|
|
Investing activities |
|
|
(5,583 |
) |
|
|
(15,839 |
) |
|
Financing activities |
|
|
87,738 |
|
|
|
(1,543 |
) |
|
Net increase in cash and cash equivalents |
|
$ |
81,391 |
|
|
$ |
92,163 |
|
Operating activities
Net cash (used in) provided by operating activities increased from cash used of $0.8 million in the three months ended March 31, 2020 to cash provided by operating activities of $109.5 million in the three months ended March 31, 2021. The increase resulted primarily from increased revenue from royalty and licensing streams, satisfaction of clinical milestones under our partnership with Lilly, and continued discovery research activities, as well as securing new multi-year, multi-target contracts with partners.
Investing activities
Net cash used in by investing activities increased from $5.6 million in the three months ended March 31, 2020 to $15.8 million in the three months ended March 31, 2021. Investing activities during the three months ended March 31, 2020 were directly attributed to the purchase of intangible assets. Investing activities during the three months ended March 31, 2021 are attributable to our investment in real estate, facilities and equipment in our Vancouver offices.
Financing activities
Net cash provide by financing activities was $87.7 million for the three months ended March 31, 2020. This was due to proceeds from our Series A2 financing. Net cash used by financing activities was $1.5 million for the three months ended March 31, 2021 due to repayment of long-term debt.
22
Critical Accounting Policies and Significant Judgements and Estimates
Detailed information about our critical accounting policies and estimates is set forth in Part II, Item 7 of our Annual Report on Form 10-K for the year ended December 31, 2020. There have been no significant changes to these policies during the three months ended March 31, 2021 other than for the inclusion of license revenue as disclosed in the condensed consolidated financial statements included elsewhere in this report.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Concentration of Credit Risk
For the three months ended March 31, 2020, three of our partners accounted for 51%, 12%, and 11% of research fees revenue and five partners accounted for the remaining 26% of research fees revenue. For the three months ended March 31, 2021, three of our partners accounted for 36%, 24% and 20% of our research fees revenue and seven partners accounted for the remaining 20% of research fees revenue. For the three months ended March 31, 2021 we recognized a clinical milestone payment and royalty revenues, totaling $173.8 million, exclusively from our partnership with Lilly. Our partnership with Lilly constituted one of the partnerships that generated 10% or more of our consolidated revenues during the one or more periods described above. With respect to the other partners, we do not believe the loss of any one or more of such partners would have a material adverse effect on us and our subsidiaries taken as a whole.
Interest Rate Risk
As of March 31, 2021, we had a cash and cash equivalents balance of $685.8 million, a majority of which was maintained in bank accounts and term deposits. Our primary exposure to market risk is to interest income volatility, which is affected by changes in the general level of interest rates. As such rates are at a near record low, a 10% change in the market interest rates would not have a material effect on our business, financial condition or results of operations.
Foreign Currency Risk
We are exposed to financial risks as a result of exchange rate fluctuations between the U.S. dollar and the Canadian dollar and the volatility of these rates. In the normal course of business, we earn revenue denominated in U.S. dollars and we incur expenses in Canadian denominated, U.S. denominated and Australian denominated dollars. Our reporting currency is the U.S. dollar. We hold a majority of our cash in U.S. dollars. To date, we have not entered into any hedging arrangements with respect to foreign currency risk. As our international operations grow, we will continue to reassess our approach to manage our risk relating to fluctuations in currency exchange rates.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, are designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures are designed to ensure that information required to be disclosed is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, to allow timely decisions regarding required disclosure.
Based upon our evaluation of the Company’s disclosure controls and procedures, as of March 31, 2021, the CEO and the CFO concluded that the disclosure controls were not effective, due to the material weakness in internal control over financial reporting disclosed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020. In light of this fact, our management has performed additional analyses, reconciliations, and other post-closing procedures and has concluded that, notwithstanding the material weakness in our internal control over financial reporting, the condensed consolidated financial statements included in this Quarterly Report on Form 10-Q fairly present, in all material respects, our financial position, results of operations and cash flows for the periods presented in conformity with GAAP.
Changes in Internal Control over Financial Reporting
We are taking actions to remediate the material weakness relating to our internal control over financial reporting, as described below. Except as otherwise described herein, there was no change in our internal control over financial reporting that occurred during the period covered by this Quarterly Report on Form 10-Q that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
23
Ongoing Remediation of Material Weakness in Internal Control over Financial Reporting
While management believes that progress has been made in enhancing internal controls as of March 31, 2021, and in the period since, the material weakness described in Part II, Item 9A, “Controls and Procedures” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, has not been fully remediated due to insufficient time to assess the design, fully implement remediation and assess operating effectiveness of the related controls. Management will continue to evaluate and work to improve our disclosure controls and procedures and internal control over financial reporting throughout 2021, and will make any further changes management deems appropriate. We expect that the remediation of the identified material weakness will be completed by the end of the fiscal year.
24
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
There have been no material changes to legal proceedings as set forth in the Form 10-K filed with the SEC on March 30, 2021.
Item 1A. Risk Factors.
There have been no material changes to the risk factors from the information provided in Item 1A. Risk Factors of our 2020 Annual Report on Form 10-K, with the exception of the below.
Our quarterly and annual operating results have fluctuated significantly in the past and may fluctuate significantly in the future, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations.
Our quarterly and annual operating results have fluctuated in the past and may fluctuate in the future, which makes it difficult for us to predict our future operating results. These fluctuations may occur due to a variety of factors, many of which are outside of our control, including, but not limited to:
|
|
• |
the level of demand for our antibody discovery platform and solutions, which may vary significantly; |
|
|
• |
the timing and cost of, and level of investment in, research, development and commercialization activities relating to our platform and technology, which may change from time to time; |
|
|
• |
the start and completion of programs in which our platform is utilized; |
|
|
• |
the relative reliability and robustness of our platform, including the data generation and computational tools within our technology stack; |
|
|
• |
the introduction of new technologies, platform features or software, by us or others in our industry; |
|
|
• |
expenditures that we may incur to acquire, develop or commercialize additional technologies; |
|
|
• |
expenditures involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including costs related to our intellectual property litigation with Berkeley Lights, and the outcome of this and any other future patent litigation we may be involved in; |
|
|
• |
the degree of competition in our industry and any change in the competitive landscape of our industry, including consolidation among our competitors or future partners; |
|
|
• |
natural disasters, outbreaks of disease or public health crises, such as the COVID-19 pandemic; |
|
|
• |
the timing and nature of any future acquisitions or strategic partnerships; |
|
|
• |
future accounting pronouncements or changes in our accounting policies; and |
|
|
• |
general social, political and economic conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors. |
For example, 2020 was the first year in which we received payments from a partner beyond upfront fees. The antibody, bamlanivimab developed by Eli Lilly and Company, or Lilly, has undergone clinical testing and has received Emergency Use Authorization from the FDA, and we have received associated milestone payments and royalties on net sales in 2020 and during the three months ended March 31, 2021. Lilly progressed into these clinical trials at a greatly accelerated pace as a result of the Coronavirus Treatment Acceleration Program, which is a special emergency program for possible coronavirus therapies created by the FDA in 2020 to expedite the development of potentially safe and effective life-saving treatments to combat the COVID-19 pandemic. With respect to other or future product candidates, there is no assurance that any of our partners or collaborators will be able to advance a product candidate through clinical development on this timeframe again in the future, or at all. We initiated our partnering program in 2015 and have only had this one program result in milestone and royalty payments to us to date and we have not yet had a program receive marketing approval. There is no guarantee that we will continue to generate the levels of revenue, particularly milestone and royalty revenues, from our partnerships as we have experienced in recent periods. For example, in April 2021, the FDA revoked the EUA for bamlanivimab as monotherapy, but maintained the EUA for bamlanivimab and etesevimab when administered together. Such decisions or similar decisions by regulatory agencies may adversely impact the payments that we receive from bamlanivimab in future periods. In addition, we have only recently begun to generate licensing revenue from our Trianni humanized
25
rodent platform. There can be no assurance that we will continue to generate or expand our licensing revenue from this product offering in future periods.
The effect of one of the factors discussed above, or the cumulative effects of a combination of factors discussed above, could result in large fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparing our operating results on a period-to-period basis may not be meaningful. Investors should not rely on our past results as an indication of our future performance.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
Sale of Unregistered Securities
There were no unregistered sales of the Company’s equity securities during the three months ended March 31, 2021.
Use of Proceeds from our Initial Public Offering
On December 15, 2020, we completed the initial public offering, or IPO, of our common shares pursuant to which we issued and sold 27,772,500 common shares at a price to the public of $20.00 per share, which included the exercise in full of the underwriters’ option to purchase additional common shares. The offer and sale of all of the common shares in our IPO was registered under the Securities Act pursuant to a registration statement on Form S-1, as amended (File No. 333-250838), which was declared effective by the SEC on December 10, 2020.
We received aggregate gross proceeds from our IPO of $555.5 million, or aggregate net proceeds of $522.8 million after deducting underwriting discounts and commissions and other offering costs. None of the underwriting discounts and commissions or offering expenses were incurred or paid, directly or indirectly, to any of our directors or officers or their associates or to persons owning 10% or more of our common shares or to any of our affiliates.
Cash used since the IPO is described elsewhere in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of our periodic reports filed with the SEC. There has been no material change in our planned use of the net proceeds from the IPO as described in the final prospectus for our IPO.
Item 3. Defaults Upon Senior Securities.
Not applicable.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
None.
26
Item 6. Exhibits.
The following exhibits are filed with this Quarterly Report on Form 10-Q:
|
Exhibit Number |
|
Description |
|
|
|
|
|
3.1 |
|
|
|
4.1 |
|
|
|
4.2 |
|
|
|
4.3 |
|
|
|
31.1* |
|
|
|
31.2* |
|
|
|
32.1** |
|
|
|
32.2** |
|
|
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
* |
Filed herewith. |
|
** |
The certifications furnished in Exhibit 32.1 and 32.2 hereto are deemed to be furnished with this Quarterly Report on Form 10-Q and will not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, except to the extent that the Registrant specifically incorporates them by reference |
27
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
AbCellera Biologics Inc. |
|
|
|
|
|
|
|
Date: May 14, 2021 |
|
By: |
/s/ Carl L.G. Hansen |
|
|
|
|
Carl L.G. Hansen, Ph.D. Chief Executive Officer (Principal Executive Officer)
|
|
|
|
|
|
|
Date: May 14, 2021 |
|
By: |
/s/ Andrew Booth |
|
|
|
|
Andrew Booth Chief Financial Officer |
|
|
|
|
(Principal Financial Officer) |
28