false2021Q20001682852December 31http://fasb.org/us-gaap/2021-01-31#PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortizationhttp://fasb.org/us-gaap/2021-01-31#PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortizationhttp://fasb.org/us-gaap/2021-01-31#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2021-01-31#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2021-01-31#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2021-01-31#OtherLiabilitiesCurrent00016828522021-01-012021-06-30xbrli:shares00016828522021-07-30iso4217:USD00016828522021-06-3000016828522020-12-31iso4217:USDxbrli:shares0001682852mrna:ProductSalesMember2021-04-012021-06-300001682852mrna:ProductSalesMember2020-04-012020-06-300001682852mrna:ProductSalesMember2021-01-012021-06-300001682852mrna:ProductSalesMember2020-01-012020-06-300001682852us-gaap:GrantMember2021-04-012021-06-300001682852us-gaap:GrantMember2020-04-012020-06-300001682852us-gaap:GrantMember2021-01-012021-06-300001682852us-gaap:GrantMember2020-01-012020-06-300001682852mrna:CollaborationArrangementMember2021-04-012021-06-300001682852mrna:CollaborationArrangementMember2020-04-012020-06-300001682852mrna:CollaborationArrangementMember2021-01-012021-06-300001682852mrna:CollaborationArrangementMember2020-01-012020-06-3000016828522021-04-012021-06-3000016828522020-04-012020-06-3000016828522020-01-012020-06-300001682852us-gaap:CommonStockMember2021-03-310001682852us-gaap:AdditionalPaidInCapitalMember2021-03-310001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-03-310001682852us-gaap:RetainedEarningsMember2021-03-3100016828522021-03-310001682852us-gaap:CommonStockMember2021-04-012021-06-300001682852us-gaap:AdditionalPaidInCapitalMember2021-04-012021-06-300001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-04-012021-06-300001682852us-gaap:RetainedEarningsMember2021-04-012021-06-300001682852us-gaap:CommonStockMember2021-06-300001682852us-gaap:AdditionalPaidInCapitalMember2021-06-300001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-06-300001682852us-gaap:RetainedEarningsMember2021-06-300001682852us-gaap:CommonStockMember2020-03-310001682852us-gaap:AdditionalPaidInCapitalMember2020-03-310001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-03-310001682852us-gaap:RetainedEarningsMember2020-03-3100016828522020-03-310001682852us-gaap:CommonStockMember2020-04-012020-06-300001682852us-gaap:AdditionalPaidInCapitalMember2020-04-012020-06-300001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-04-012020-06-300001682852us-gaap:RetainedEarningsMember2020-04-012020-06-300001682852us-gaap:CommonStockMember2020-06-300001682852us-gaap:AdditionalPaidInCapitalMember2020-06-300001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-06-300001682852us-gaap:RetainedEarningsMember2020-06-3000016828522020-06-300001682852us-gaap:CommonStockMember2020-12-310001682852us-gaap:AdditionalPaidInCapitalMember2020-12-310001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310001682852us-gaap:RetainedEarningsMember2020-12-310001682852us-gaap:CommonStockMember2021-01-012021-06-300001682852us-gaap:AdditionalPaidInCapitalMember2021-01-012021-06-300001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-06-300001682852us-gaap:RetainedEarningsMember2021-01-012021-06-300001682852us-gaap:CommonStockMember2019-12-310001682852us-gaap:AdditionalPaidInCapitalMember2019-12-310001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-310001682852us-gaap:RetainedEarningsMember2019-12-3100016828522019-12-310001682852us-gaap:CommonStockMember2020-01-012020-06-300001682852us-gaap:AdditionalPaidInCapitalMember2020-01-012020-06-300001682852us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-01-012020-06-300001682852us-gaap:RetainedEarningsMember2020-01-012020-06-30mrna:programmrna:clinic0001682852us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-12-310001682852us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2020-12-310001682852us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-01-012021-03-310001682852us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2021-01-012021-03-3100016828522021-01-012021-03-310001682852us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-03-310001682852us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2021-03-310001682852us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-04-012021-06-300001682852us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2021-04-012021-06-300001682852us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-06-300001682852us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2021-06-300001682852country:USmrna:ProductSalesMember2021-04-012021-06-300001682852country:USmrna:ProductSalesMember2021-01-012021-06-300001682852us-gaap:NonUsMembermrna:ProductSalesMember2021-04-012021-06-300001682852us-gaap:NonUsMembermrna:ProductSalesMember2021-01-012021-06-300001682852mrna:ProductSalesMember2021-06-300001682852mrna:ProductSalesMember2020-12-310001682852mrna:DefenseAdvancedResearchProjectsAgencyMember2020-09-012020-09-300001682852mrna:DefenseAdvancedResearchProjectsAgencyMembermrna:ContractOptionsMember2020-06-300001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2020-04-012020-04-300001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2020-07-31mrna:option0001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-03-310001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-04-012021-04-300001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-01-012021-03-310001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-06-300001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2016-09-012016-09-300001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-01-012021-06-300001682852mrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2016-09-300001682852mrna:ContractOptionsMembermrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-06-300001682852mrna:InitialProjectMembermrna:TheBillAndMelindaGatesFoundationMember2021-06-300001682852us-gaap:GrantMembermrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-04-012021-06-300001682852us-gaap:GrantMembermrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2020-04-012020-06-300001682852us-gaap:GrantMembermrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2021-01-012021-06-300001682852us-gaap:GrantMembermrna:BiomedicalAdvancedResearchAndDevelopmentAuthorityMember2020-01-012020-06-300001682852mrna:OtherGrantRevenueMemberus-gaap:GrantMember2021-04-012021-06-300001682852mrna:OtherGrantRevenueMemberus-gaap:GrantMember2020-04-012020-06-300001682852mrna:OtherGrantRevenueMemberus-gaap:GrantMember2021-01-012021-06-300001682852mrna:OtherGrantRevenueMemberus-gaap:GrantMember2020-01-012020-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMembermrna:AstraZenecaMember2021-04-012021-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMembermrna:AstraZenecaMember2020-04-012020-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMembermrna:AstraZenecaMember2021-01-012021-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMembermrna:AstraZenecaMember2020-01-012020-06-300001682852mrna:MerckMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-04-012021-06-300001682852mrna:MerckMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-04-012020-06-300001682852mrna:MerckMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-01-012021-06-300001682852mrna:MerckMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-01-012020-06-300001682852mrna:VertexMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-04-012021-06-300001682852mrna:VertexMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-04-012020-06-300001682852mrna:VertexMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-01-012021-06-300001682852mrna:VertexMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-01-012020-06-300001682852mrna:OtherCollaborativePartiesMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-04-012021-06-300001682852mrna:OtherCollaborativePartiesMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-04-012020-06-300001682852mrna:OtherCollaborativePartiesMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-01-012021-06-300001682852mrna:OtherCollaborativePartiesMembermrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-01-012020-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-04-012021-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-04-012020-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-01-012021-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-01-012020-06-300001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2020-12-310001682852mrna:CollaborationArrangementIncludingArrangementsWithAffiliateMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMemberus-gaap:CashAndCashEquivalentsMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMembermrna:CurrentMarketableSecuritiesMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMembermrna:NoncurrentMarketableSecuritiesMember2021-06-300001682852us-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CertificatesOfDepositMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CertificatesOfDepositMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USTreasurySecuritiesMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USTreasurySecuritiesMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CorporateDebtSecuritiesMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CorporateDebtSecuritiesMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852mrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852mrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMemberus-gaap:CashAndCashEquivalentsMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMembermrna:CurrentMarketableSecuritiesMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CashAndCashEquivalentsMembermrna:NoncurrentMarketableSecuritiesMember2020-12-310001682852us-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CertificatesOfDepositMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CertificatesOfDepositMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USTreasurySecuritiesMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USTreasurySecuritiesMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CorporateDebtSecuritiesMembermrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CorporateDebtSecuritiesMembermrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852mrna:CurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852mrna:NoncurrentMarketableSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2021-06-300001682852us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CertificatesOfDepositMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:CertificatesOfDepositMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001682852us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-06-300001682852us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2020-12-310001682852us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CertificatesOfDepositMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:CertificatesOfDepositMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001682852us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310001682852us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeContractMember2021-06-300001682852us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2021-06-300001682852us-gaap:NondesignatedMember2021-06-300001682852us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2020-12-310001682852us-gaap:NondesignatedMember2020-12-310001682852us-gaap:ForeignExchangeContractMemberus-gaap:OtherNonoperatingIncomeExpenseMember2021-04-012021-06-300001682852us-gaap:ForeignExchangeContractMemberus-gaap:OtherNonoperatingIncomeExpenseMember2021-01-012021-06-300001682852us-gaap:EquipmentMember2021-06-300001682852us-gaap:EquipmentMember2020-12-310001682852us-gaap:LeaseholdImprovementsMember2021-06-300001682852us-gaap:LeaseholdImprovementsMember2020-12-310001682852us-gaap:PropertyPlantAndEquipmentOtherTypesMember2021-06-300001682852us-gaap:PropertyPlantAndEquipmentOtherTypesMember2020-12-310001682852us-gaap:ComputerEquipmentMember2021-06-300001682852us-gaap:ComputerEquipmentMember2020-12-310001682852us-gaap:SoftwareDevelopmentMember2021-06-300001682852us-gaap:SoftwareDevelopmentMember2020-12-310001682852mrna:FinancingRightOfUseAssetMember2021-06-300001682852mrna:FinancingRightOfUseAssetMember2020-12-310001682852us-gaap:ConstructionInProgressMember2021-06-300001682852us-gaap:ConstructionInProgressMember2020-12-310001682852us-gaap:GrantMember2020-12-310001682852us-gaap:GrantMember2021-06-300001682852mrna:CollaborationArrangementMember2020-12-310001682852mrna:CollaborationArrangementMember2021-06-30mrna:campusutr:sqft0001682852mrna:CambridgeMassachusettsMember2021-06-300001682852mrna:NorwoodMassachusettsMembermrna:MTCSouthMember2016-08-31mrna:extension_period0001682852mrna:NorwoodMassachusettsMembermrna:MTCNorthMember2019-02-280001682852mrna:NorwoodMassachusettsMembermrna:MTCNorthMember2021-06-300001682852mrna:NorwoodMassachusettsMembermrna:MTCNorthMember2020-05-310001682852mrna:MTCEastMember2021-04-300001682852mrna:EmbeddedLeasesMember2021-06-300001682852mrna:EmbeddedLeasesMember2020-12-310001682852mrna:NorwoodMassachusettsMembermrna:MTCSouthMTCNorthAndMTCEastMember2021-06-30xbrli:pure0001682852mrna:NorwoodMassachusettsMembermrna:MTCSouthMember2021-01-012021-06-300001682852mrna:NorwoodMassachusettsMembermrna:MTCNorthMember2021-01-012021-06-300001682852mrna:NorwoodMassachusettsMembermrna:MTCEastMember2020-01-012020-06-300001682852mrna:EmbeddedLeasesMember2021-01-012021-06-300001682852mrna:PersonalizedMRNACancerVaccinesProductsMembermrna:PersonalizedMRNACancerVaccinesPCVCollaborationAndLicenseAgreementWithMerckSharpAndDohmeCorpMember2020-12-310001682852mrna:PersonalizedMRNACancerVaccinesProductsMembermrna:PersonalizedMRNACancerVaccinesPCVCollaborationAndLicenseAgreementWithMerckSharpAndDohmeCorpMember2021-06-300001682852mrna:SupplyAndManufacturingAgreementsMember2021-06-300001682852mrna:ClinicalServicesMember2021-06-300001682852mrna:ClinicalOperationsAndSupportCommitmentMember2021-06-300001682852mrna:ClinicalOperationsAndSupportCommitmentMember2020-12-3100016828522017-06-260001682852us-gaap:EmployeeStockOptionMember2021-06-300001682852mrna:StockOptionAndIncentivePlan2018Member2021-06-3000016828522020-01-012020-12-310001682852us-gaap:RestrictedStockUnitsRSUMember2020-12-310001682852us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-06-300001682852us-gaap:RestrictedStockUnitsRSUMember2021-06-300001682852us-gaap:PerformanceSharesMember2021-01-012021-03-310001682852srt:MinimumMemberus-gaap:PerformanceSharesMember2021-03-310001682852srt:MaximumMemberus-gaap:PerformanceSharesMember2021-03-310001682852us-gaap:EmployeeStockMember2021-06-300001682852us-gaap:EmployeeStockOptionMember2021-04-012021-06-300001682852us-gaap:EmployeeStockOptionMember2020-04-012020-06-300001682852us-gaap:EmployeeStockOptionMember2021-01-012021-06-300001682852us-gaap:EmployeeStockOptionMember2020-01-012020-06-300001682852mrna:RestrictedStockAndRestrictedStockUnitsRSUMember2021-04-012021-06-300001682852mrna:RestrictedStockAndRestrictedStockUnitsRSUMember2020-04-012020-06-300001682852mrna:RestrictedStockAndRestrictedStockUnitsRSUMember2021-01-012021-06-300001682852mrna:RestrictedStockAndRestrictedStockUnitsRSUMember2020-01-012020-06-300001682852us-gaap:EmployeeStockMember2021-04-012021-06-300001682852us-gaap:EmployeeStockMember2020-04-012020-06-300001682852us-gaap:EmployeeStockMember2021-01-012021-06-300001682852us-gaap:EmployeeStockMember2020-01-012020-06-300001682852us-gaap:CostOfSalesMember2021-04-012021-06-300001682852us-gaap:CostOfSalesMember2020-04-012020-06-300001682852us-gaap:CostOfSalesMember2021-01-012021-06-300001682852us-gaap:CostOfSalesMember2020-01-012020-06-300001682852us-gaap:ResearchAndDevelopmentExpenseMember2021-04-012021-06-300001682852us-gaap:ResearchAndDevelopmentExpenseMember2020-04-012020-06-300001682852us-gaap:ResearchAndDevelopmentExpenseMember2021-01-012021-06-300001682852us-gaap:ResearchAndDevelopmentExpenseMember2020-01-012020-06-300001682852us-gaap:GeneralAndAdministrativeExpenseMember2021-04-012021-06-300001682852us-gaap:GeneralAndAdministrativeExpenseMember2020-04-012020-06-300001682852us-gaap:GeneralAndAdministrativeExpenseMember2021-01-012021-06-300001682852us-gaap:GeneralAndAdministrativeExpenseMember2020-01-012020-06-300001682852us-gaap:EmployeeStockOptionMember2020-01-012020-06-300001682852us-gaap:RestrictedStockUnitsRSUMember2020-01-012020-06-30mrna:dose0001682852us-gaap:SubsequentEventMember2021-07-012021-08-050001682852us-gaap:SubsequentEventMember2021-08-02
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2021
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _ to _
Commission File Number: 001-38753
Moderna, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| | | | | | | | | | | |
| Delaware | | 81-3467528 |
| (State or Other Jurisdiction of Incorporation or Organization) | | (IRS Employer Identification No.) |
| | | |
| 200 Technology Square | | |
| Cambridge, | Massachusetts | | 02139 |
| (Address of Principal Executive Offices) | | (Zip Code) |
(617) 714-6500
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading symbol(s) | Name of each exchange on which registered |
| Common stock, par value $0.0001 per share | MRNA | The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | ☒ | | Accelerated filer o | | Non-accelerated filer o | | Smaller reporting company | ☐ |
| | | | | | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No x
As of July 30, 2021, there were 403,646,312 shares of the registrant’s common stock, par value $0.0001 per share, outstanding.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q (“Form 10-Q”), including the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains express or implied forward-looking statements that are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future operational or financial performance, and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by these forward-looking statements. Forward-looking statements in this Form 10-Q include, but are not limited to, statements about:
•our activities with respect to our COVID-19 vaccine, and our plans and expectations regarding future generations of our COVID-19 vaccine, including boosters, that we may develop in response to variants of the SARS-CoV-2 virus, ongoing clinical development, manufacturing and supply, pricing, commercialization, if approved, regulatory matters (including dosage for vaccines and authorization or approval for boosters) and third-party and governmental arrangements and potential arrangements;
•our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately, particularly with respect to the timely production and delivery of our COVID-19 vaccine, including any variant booster vaccine candidate, if authorized;
•our ability and the ability of third parties with whom we contract to successfully manufacture our commercial products at scale, as well as drug substances, delivery vehicles, development candidates, and investigational medicines for preclinical and clinical use;
•the scope of protection we are able to establish and maintain for intellectual property rights covering our commercial products, investigational medicines and technology;
•the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs;
•the ultimate impact of the current coronavirus pandemic, or the COVID-19 pandemic, or any other health epidemic, on our business, manufacturing, clinical trials, research programs, supply chain, regulatory review, healthcare systems or the global economy as a whole;
•risks related to the direct or indirect impact of the COVID-19 pandemic or any future large-scale adverse health event, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays in diagnoses, initiation or continuation of treatment for diseases that may be addressed by our development candidates and investigational medicines, or in patient enrollment in clinical trials, potential clinical trials, regulatory review or supply chain disruptions, and other potential impacts to our business, the effectiveness or timeliness of steps taken by us to mitigate the impact of the pandemic, and our ability to execute business continuity plans to address disruptions caused by the COVID-19 pandemic or future large-scale adverse health event;
•our anticipated next steps for our development candidates and investigational medicines that may be slowed down due to the impact of the COVID-19 pandemic, including our resources being significantly diverted towards our COVID-19 vaccine efforts, particularly if the federal government seeks to require us to divert such resources;
•our ability to identify research priorities and apply a risk-mitigated strategy to efficiently discover and develop development candidates and investigational medicines, including by applying learnings from one program to our other programs and from one modality to our other modalities;
•the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and investigational medicines;
•our ability to obtain and maintain regulatory approval of our investigational medicines;
•our ability to successfully commercialize any future products, if approved;
•the pricing and reimbursement of our investigational medicines, if approved;
•the implementation of our business model, and strategic plans for our business, investigational medicines, and technology;
•estimates of our future expenses, revenues, capital requirements, and our needs for additional financing;
•the potential benefits of strategic collaboration agreements, our ability to enter into strategic collaborations or arrangements, and our ability to attract collaborators with development, regulatory, and commercialization expertise;
•future agreements with third parties in connection with the commercialization of our investigational medicines, if approved;
•the size and growth potential of the markets for our investigational medicines, and our ability to serve those markets;
•our financial performance;
•the rate and degree of market acceptance of our investigational medicines;
•regulatory developments in the United States and foreign countries;
•our ability to produce our products or investigational medicines with advantages in turnaround times or manufacturing cost;
•the success of competing therapies that are or may become available;
•our ability to attract and retain key scientific or management personnel;
•the impact of laws and regulations;
•developments relating to our competitors and our industry; and
•other risks and uncertainties discussed in this Form 10-Q.
In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “could,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the section entitled “Risk Factors” and elsewhere in this Form 10-Q. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those expressed or implied by the forward-looking statements. No forward-looking statement is a promise or a guarantee of future performance.
The forward-looking statements in this Form 10-Q represent our views as of the date of this Form 10-Q. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Form 10-Q.
This Form 10-Q includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We have not independently verified the information contained in such sources.
NOTE REGARDING COMPANY REFERENCES
Unless the context otherwise requires, the terms “Moderna,” “the Company,” “we,” “us,” and “our” in this Form 10-Q refer to Moderna, Inc. and its consolidated subsidiaries.
ADDITIONAL INFORMATION
Our website, www.modernatx.com including the Investor Relations section, www.investors.modernatx.com; and corporate blog www.modernatx.com/moderna-blog; as well as our social media channels: Facebook, www.facebook.com/modernatx; Twitter, www.twitter.com/modernatx; and LinkedIn, www.linkedin.com/company/modernatx; contain a significant amount of information about us, including financial and other information for investors. We encourage investors to visit these websites and social media channels as information is frequently updated and new information is shared.
Table of Contents
| | | | | | | | |
PART I. | | Page |
| Item 1. | | |
| | |
| | |
| | |
| | |
| | |
| | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
PART II. | | |
Item 1. | | |
Item 1A. | | |
| Item 6. | | |
| | |
Item 1. Financial Statements
MODERNA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited, in millions, except per share data)
| | | | | | | | | | | |
| June 30, | | December 31, |
| 2021 | | 2020 |
Assets | | | |
Current assets: | | | |
Cash and cash equivalents | $ | 5,603 | | | $ | 2,624 | |
Investments | 2,387 | | | 1,984 | |
Accounts receivable | 2,020 | | | 1,391 | |
| Inventory | 643 | | | 47 | |
Prepaid expenses and other current assets | 316 | | | 252 | |
Total current assets | 10,969 | | | 6,298 | |
Investments, non-current | 4,207 | | | 639 | |
Property and equipment, net | 794 | | | 297 | |
| Right-of-use assets, operating leases | 104 | | | 90 | |
Restricted cash, non-current | 11 | | | 11 | |
| Deferred tax assets | 66 | | | — | |
Other non-current assets | 2 | | | 2 | |
Total assets | $ | 16,153 | | | $ | 7,337 | |
Liabilities and Stockholders’ Equity | | | |
Current liabilities: | | | |
Accounts payable | $ | 77 | | | $ | 18 | |
Accrued liabilities | 848 | | | 470 | |
Deferred revenue | 7,302 | | | 3,867 | |
Other current liabilities | 613 | | | 34 | |
Total current liabilities | 8,840 | | | 4,389 | |
Deferred revenue, non-current | 175 | | | 177 | |
| Operating lease liabilities, non-current | 105 | | | 97 | |
| Financing lease liabilities, non-current | 328 | | | 110 | |
Other non-current liabilities | 1 | | | 3 | |
| Total liabilities | 9,449 | | | 4,776 | |
| Commitments and contingencies (Note 12) | | | |
| Stockholders’ equity: | | | |
Preferred stock, par value $0.0001; 162 shares authorized as of June 30, 2021 and December 31, 2020; no shares issued or outstanding at June 30, 2021 and December 31, 2020 | — | | | — | |
Common stock, par value $0.0001; 1,600 shares authorized as of June 30, 2021 and December 31, 2020; 403 and 399 shares issued and outstanding as of June 30, 2021 and December 31, 2020, respectively | — | | | — | |
Additional paid-in capital | 4,931 | | | 4,802 | |
Accumulated other comprehensive income | 16 | | | 3 | |
| Retained earnings (accumulated deficit) | 1,757 | | | (2,244) | |
Total stockholders’ equity | 6,704 | | | 2,561 | |
Total liabilities and stockholders’ equity | $ | 16,153 | | | $ | 7,337 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited, in millions, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Revenue: | | | | | | | | |
| Product sales | | $ | 4,197 | | | $ | — | | | $ | 5,930 | | | $ | — | |
| Grant revenue | | 139 | | | 38 | | | 333 | | | 42 | |
| Collaboration revenue | | 18 | | | 29 | | | 28 | | | 33 | |
| Total revenue | | 4,354 | | | 67 | | | 6,291 | | | 75 | |
| Operating expenses: | | | | | | | | |
| Cost of sales | | 750 | | | — | | | 943 | | | — | |
| Research and development | | 421 | | | 152 | | | 822 | | | 267 | |
| Selling, general and administrative | | 121 | | | 37 | | | 198 | | | 61 | |
| Total operating expenses | | 1,292 | | | 189 | | | 1,963 | | | 328 | |
| Income (loss) from operations | | 3,062 | | | (122) | | | 4,328 | | | (253) | |
| Interest income | | 3 | | | 7 | | | 7 | | | 15 | |
| Other expense, net | | (2) | | | (2) | | | (12) | | | (3) | |
| Income (loss) before income taxes | | 3,063 | | | (117) | | | 4,323 | | | (241) | |
| Provision for income taxes | | 283 | | | — | | | 322 | | | — | |
| Net income (loss) | | $ | 2,780 | | | $ | (117) | | | $ | 4,001 | | | $ | (241) | |
| | | | | | | | |
| Earnings (loss) per share: | | | | | | | | |
| Basic | | $ | 6.93 | | | $ | (0.31) | | | $ | 9.98 | | | $ | (0.66) | |
| Diluted | | $ | 6.46 | | | $ | (0.31) | | | $ | 9.30 | | | $ | (0.66) | |
| | | | | | | | |
| Weighted average common shares used in calculation of earnings (loss) per share: | | | | | | | | |
| Basic | | 402 | | | 381 | | | 401 | | | 367 | |
| Diluted | | 431 | | | 381 | | | 430 | | | 367 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(Unaudited, in millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, | |
| | 2021 | | 2020 | | 2021 | | 2020 | |
| Net income (loss) | | $ | 2,780 | | | $ | (117) | | | $ | 4,001 | | | $ | (241) | | |
| Other comprehensive income (loss), net of taxes: | | | | | | | | | |
| Available-for-sales securities: | | | | | | | | | |
| Unrealized (losses) gains on available-for-sale debt securities | | (5) | | | 13 | | | (7) | | | 5 | | |
| Less: net realized (gains) losses on available-for-sale securities reclassified in net income (loss) | | (1) | | | 1 | | | (1) | | | 1 | | |
| Net (decrease) increase from available-for-sale debt securities | | (6) | | | 14 | | | (8) | | | 6 | | |
| Cash flow hedges: | | | | | | | | | |
| Unrealized gains on derivative instruments | | 21 | | | — | | | 21 | | | — | | |
| | | | | | | | | |
| | | | | | | | | |
Total other comprehensive income | | 15 | | | 14 | | | 13 | | | 6 | | |
| Comprehensive income (loss) | | $ | 2,795 | | | $ | (103) | | | $ | 4,014 | | | $ | (235) | | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
FOR THE THREE MONTHS AND SIX MONTHS ENDED JUNE 30, 2021 AND 2020
(Unaudited, in millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Retained Earnings (Accumulated Deficit) | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at March 31, 2021 | 401 | | | $ | — | | | $ | 4,860 | | | $ | 1 | | | $ | (1,023) | | | $ | 3,838 | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 2 | | | — | | | 31 | | | — | | | — | | | 31 | |
| Purchase of common stock under employee stock purchase plan | — | | | — | | | 5 | | | — | | | — | | | 5 | |
| Stock-based compensation | — | | | — | | | 35 | | | — | | | — | | | 35 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 15 | | | — | | | 15 | |
| Net income | — | | | — | | | — | | | — | | | 2,780 | | | 2,780 | |
| Balance at June 30, 2021 | 403 | | | $ | — | | | $ | 4,931 | | | $ | 16 | | | $ | 1,757 | | | $ | 6,704 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income (Loss) | | Accumulated Deficit | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at March 31, 2020 | 370 | | | $ | — | | | $ | 3,268 | | | $ | (6) | | | $ | (1,621) | | | $ | 1,641 | |
Proceeds from public offering of common stock, net of issuance costs of $1 | 18 | | | — | | | 1,303 | | | — | | | — | | | 1,303 | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 5 | | | — | | | 78 | | | — | | | — | | | 78 | |
| Purchase of common stock under employee stock purchase plan | — | | | — | | | 3 | | | — | | | — | | | 3 | |
| Stock-based compensation | — | | | — | | | 25 | | | — | | | — | | | 25 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 14 | | | — | | | 14 | |
| Net loss | — | | | — | | | — | | | — | | | (117) | | | (117) | |
| Balance at June 30, 2020 | 393 | | | $ | — | | | $ | 4,677 | | | $ | 8 | | | $ | (1,738) | | | $ | 2,947 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Retained Earnings (Accumulated Deficit) | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at December 31, 2020 | 399 | | | $ | — | | | $ | 4,802 | | | $ | 3 | | | $ | (2,244) | | | $ | 2,561 | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 4 | | | — | | | 59 | | | — | | | — | | | 59 | |
| Purchase of common stock under employee stock purchase plan | — | | | — | | | 5 | | | — | | | — | | | 5 | |
| Stock-based compensation | — | | | — | | | 65 | | | — | | | — | | | 65 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 13 | | | — | | | 13 | |
| Net income | — | | | — | | | — | | | — | | | 4,001 | | | 4,001 | |
| Balance at June 30, 2021 | 403 | | | $ | — | | | $ | 4,931 | | | $ | 16 | | | $ | 1,757 | | | $ | 6,704 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Accumulated Deficit | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at December 31, 2019 | 337 | | | $ | — | | | $ | 2,670 | | | $ | 2 | | | $ | (1,497) | | | $ | 1,175 | |
Proceeds from public offering of common stock, net of issuance costs of $2 | 48 | | | — | | | 1,853 | | | — | | | — | | | 1,853 | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 8 | | | — | | | 106 | | | — | | | — | | | 106 | |
| Purchase of common stock under employee stock purchase plan | — | | | — | | | 3 | | | — | | | — | | | 3 | |
| Stock-based compensation | — | | | — | | | 45 | | | — | | | — | | | 45 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 6 | | | — | | | 6 | |
| Net loss | — | | | — | | | — | | | — | | | (241) | | | (241) | |
| Balance at June 30, 2020 | 393 | | | $ | — | | | $ | 4,677 | | | $ | 8 | | | $ | (1,738) | | | $ | 2,947 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited, in millions)
| | | | | | | | | | | |
| Six Months Ended June 30, |
| 2021 | | 2020 |
Operating activities | | | |
| Net income (loss) | $ | 4,001 | | | $ | (241) | |
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | | |
Stock-based compensation | 65 | | | 45 | |
Depreciation and amortization | 84 | | | 15 | |
Amortization/accretion of investments | 13 | | | 2 | |
| | | |
| Deferred income taxes | (72) | | | — | |
Changes in assets and liabilities: | | | |
Accounts receivable | (629) | | | (28) | |
Prepaid expenses and other assets | (110) | | | (12) | |
| Inventory | (596) | | | — | |
Right-of-use assets, operating leases | (14) | | | (12) | |
Accounts payable | 44 | | | 12 | |
Accrued liabilities | 367 | | | 20 | |
Deferred revenue | 3,433 | | | 51 | |
Operating lease liabilities | 8 | | | 14 | |
Other liabilities | 440 | | | 4 | |
| Net cash provided by (used in) operating activities | 7,034 | | | (130) | |
Investing activities | | | |
Purchases of marketable securities | (6,559) | | | (903) | |
Proceeds from maturities of marketable securities | 860 | | | 517 | |
Proceeds from sales of marketable securities | 1,706 | | | 108 | |
Purchases of property and equipment | (65) | | | (25) | |
Net cash used in investing activities | (4,058) | | | (303) | |
Financing activities | | | |
| Proceeds from public offerings of common stock, net of issuance costs | — | | | 1,853 | |
Proceeds from issuance of common stock through equity plans, net | 64 | | | 106 | |
| | | |
| Changes in financing lease liabilities | (62) | | | — | |
Net cash provided by financing activities | 2 | | | 1,959 | |
| Net increase in cash, cash equivalents and restricted cash | 2,978 | | | 1,526 | |
Cash, cash equivalents and restricted cash, beginning of year | 2,636 | | | 248 | |
Cash, cash equivalents and restricted cash, end of period | $ | 5,614 | | | $ | 1,774 | |
Non-cash investing and financing activities | | | |
Purchases of property and equipment included in accounts payable and accrued liabilities | $ | 45 | | | $ | 9 | |
| Right-of-use assets obtained through finance lease modifications and reassessments | $ | 363 | | | $ | 14 | |
| Right-of-use assets obtained in exchange for financing lease liabilities | $ | 126 | | | $ | — | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Description of the Business
Moderna, Inc. (collectively, with its consolidated subsidiaries, any of Moderna, we, us, our, or the Company) was incorporated in Delaware on July 22, 2016. We are the successor in interest to Moderna LLC, a limited liability company formed under the laws of the State of Delaware in 2013. Our principal executive office is located at 200 Technology Square, Cambridge, MA.
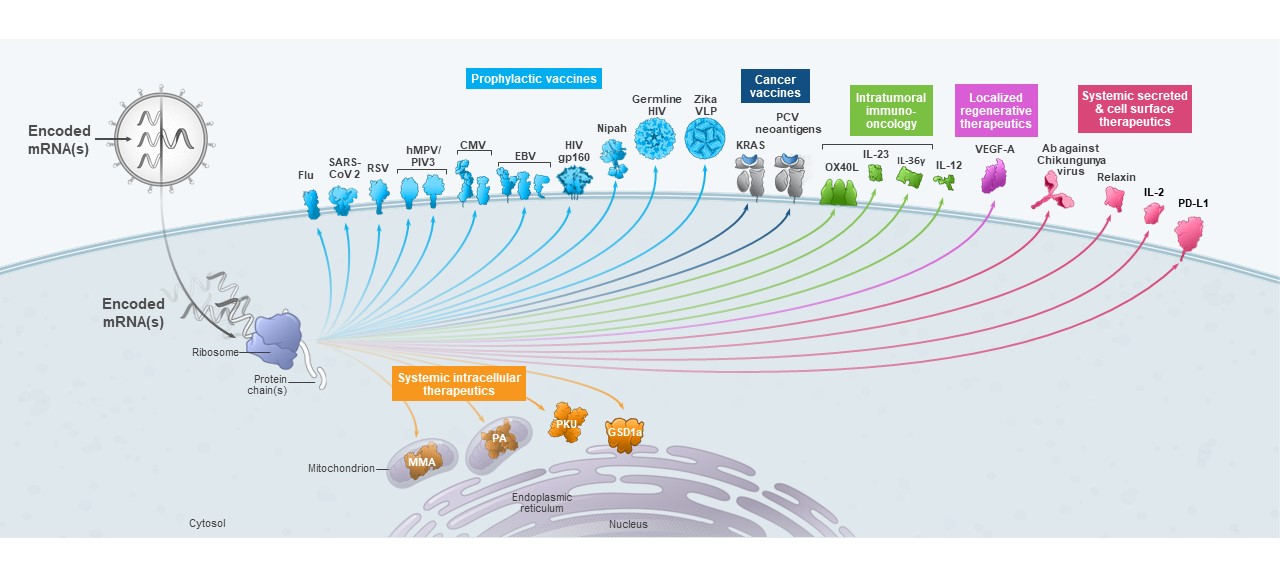
We are a biotechnology company creating a new generation of transformative medicines based on messenger RNA (mRNA), to improve the lives of patients. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane, or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases. Our platform builds on continuous advances in basic and applied mRNA science, delivery technology, and manufacturing, providing us the capability to pursue in parallel a robust pipeline of new development candidates. We are developing vaccines and therapeutics for infectious diseases, immuno-oncology, rare diseases, autoimmune and cardiovascular diseases, independently and with our strategic collaborators.
On December 18, 2020, we received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the emergency use of the Moderna COVID-19 Vaccine (also referred to as mRNA-1273) in individuals 18 years of age or older. We have also received authorization for our COVID-19 vaccine from health agencies in more than 50 countries and from the World Health Organization. Additional authorizations are currently under review in other countries. In addition, we have received authorization for our COVID-19 vaccine for use in adolescents in the European Union and Japan and have pending applications for authorization to administer the vaccine to adolescents with regulatory agencies in the United States and other countries. On June 1, 2021, we initiated the rolling submission process with the U.S. FDA for a Biologics License Application for our COVID-19 vaccine, and we currently anticipate completing our submission in August 2021.
As of June 30, 2021, we had 24 mRNA development programs in our portfolio with 14 having entered the clinic. We have incurred significant expenses in connection with the discovery, development and commercialization of our products, and we expect to continue to incur significant expenses for the foreseeable future. We anticipate that our expenses will increase significantly in connection with the ongoing development and commercialization of our COVID-19 vaccine and ongoing activities to support our platform research, drug discovery and clinical development, including development of any new generations of boosters and vaccines against variants of SARS-CoV-2, infrastructure and Research Engine and Early Development Engine (which includes our Moderna Technology Center), digital infrastructure, creation of a portfolio of intellectual property, and administrative support. We may finance our future cash needs that exceed our operating costs through a combination of public or private equity offerings, structured financings and debt financings, government funding arrangements, strategic alliances and marketing, manufacturing, distribution and licensing arrangements. We may be unable to raise additional funds or enter into such other agreements on favorable terms, or at all.
We believe that our cash, cash equivalents, and investments as of June 30, 2021 will be sufficient to enable us to fund our projected operations through at least the next 12 months from the issuance of these financial statements. We are subject to numerous risks and uncertainties associated with pharmaceutical development and commercialization, and we are unable to predict the timing or amount of expenses or if we will be able to maintain profitability. If we are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
2. Summary of Basis of Presentation and Recent Accounting Standards
Basis of Presentation and Principles of Consolidation
The accompanying unaudited condensed consolidated financial statements that accompany these notes have been prepared in accordance with U.S. generally accepted accounting principles (GAAP) and applicable rules and regulations of the Securities and Exchange Commission (SEC) for interim financial reporting, consistent in all material respects with those applied in our Annual Report on Form 10-K for the year ended December 31, 2020 (2020 Form 10-K). Any reference in these notes to applicable guidance is meant to refer to the authoritative accounting principles generally accepted in the United States as found in the Accounting Standard Codification (ASC) and Accounting Standards Update (ASU) of the Financial Accounting Standards Board (FASB). This report should be read in conjunction with the consolidated financial statements in our 2020 Form 10-K.
The condensed consolidated financial statements include the Company and its subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
The significant accounting policies used in preparation of these condensed consolidated financial statements for the three and six months ended June 30, 2021 are consistent with those described in our 2020 Form 10-K.
Use of Estimates
We have made estimates and judgments affecting the amounts reported in our condensed consolidated financial statements and the accompanying notes. We base our estimates on historical experience and various relevant assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods that are not readily apparent from other sources. Significant estimates relied upon in preparing these financial statements include, but are not limited to, critical accounting policies or estimates related to revenue recognition, research and development expenses, stock-based compensation, leases, fair value of financial instruments, derivative financial instruments, inventory, useful lives of property and equipment, income taxes and our valuation allowance on our deferred tax assets. The actual results that we experience may differ materially from our estimates.
Comprehensive Income (Loss)
Comprehensive income (loss) includes net income (loss) and other comprehensive income (loss) for the period. Other comprehensive income (loss) consists of unrealized gains/losses and gains/losses on our investments and derivatives designated as hedging instruments. Total comprehensive income (loss) for all periods presented has been disclosed in the condensed consolidated statements of comprehensive income (loss).
The components of accumulated other comprehensive income for the three and six months ended June 30, 2021 were as follows (in millions):
| | | | | | | | | | | | | | | | | |
| Unrealized Loss on Available-for-Sale Debt Securities | | Net Unrealized Gains on Derivatives Designated As Hedging Instruments | | Total |
| Accumulated other comprehensive income, balance at December 31, 2020 | $ | 3 | | | $ | — | | | $ | 3 | |
| Other comprehensive loss | (2) | | | — | | | (2) | |
| Accumulated other comprehensive income, balance at March 31, 2021 | 1 | | | — | | | 1 | |
| Other comprehensive (loss) income | (6) | | | 21 | | | 15 | |
| Accumulated other comprehensive income, balance at June 30, 2021 | $ | (5) | | | $ | 21 | | | $ | 16 | |
| | | | | |
| | | | | |
| | | | | |
Restricted Cash
We include our restricted cash balance in the cash, cash equivalents and restricted cash reconciliation of operating, investing and financing activities in the condensed consolidated statements of cash flows.
The following table provides a reconciliation of cash, cash equivalents and restricted cash in the condensed consolidated balance sheets that sum to the total of the same such amounts shown in the condensed consolidated statements of cash flows (in millions):
| | | | | | | | | | | | | | |
| | June 30, |
| | 2021 | | 2020 |
| Cash and cash equivalents | | $ | 5,603 | | | $ | 1,762 | |
| Restricted cash | | — | | | 1 | |
| Restricted cash, non-current | | 11 | | | 11 | |
Total cash, cash equivalents and restricted cash shown in the condensed consolidated
statements of cash flows | | $ | 5,614 | | | $ | 1,774 | |
Recently Issued Accounting Standards Not Yet Adopted
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies and adopted by us as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our condensed consolidated financial statements and disclosures.
3. Product Sales
In December 2020, we began selling our COVID-19 vaccine to the U.S. Government and international governments. Under the supply agreements with these governments, we received or billed for upfront deposits for our future vaccine supply, which are initially recorded as deferred revenue. We recognize revenue based on the fixed price per dose when control of the product has transferred and customer acceptance has occurred as applicable, unless such acceptance provisions are deemed perfunctory.
Product sales by customer geographic location was as follows (in millions):
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended
June 30, 2021 | | | | Six Months Ended
June 30, 2021 | | |
| United States | | $ | 2,093 | | | | | $ | 3,451 | | | |
| Rest of world | | 2,104 | | | | | 2,479 | | | |
| Total | | $ | 4,197 | | | | | $ | 5,930 | | | |
There were no product sales for the three and six months ended June 30, 2020. As of June 30, 2021, we had one commercial product authorized for use, our COVID-19 vaccine.
As of June 30, 2021 and December 31, 2020, we had deferred revenue of $7.2 billion and $3.8 billion, respectively, related to customer deposits, classified as current deferred revenue in our condensed consolidated balance sheets. Timing of product manufacturing, delivery, and receipt of marketing approval will determine the period in which revenue is recognized.
4. Grant Revenue
In September 2020, we entered into an agreement with the Defense Advanced Research Projects Agency (DARPA) for an award of up to $56 million to fund development of a mobile manufacturing prototype leveraging our existing manufacturing technology that is capable of rapidly producing vaccines and therapeutics. As of June 30, 2021, we have earned the committed funding of $5 million. An additional $51 million funding would be available if DARPA exercises additional contract options.
In April 2020, we entered into an agreement with the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), for an award of up to $483 million to accelerate development of mRNA-1273, our vaccine candidate against the novel coronavirus. In July 2020, we amended our agreement with BARDA to provide for an additional commitment of up to $472 million to support late-stage clinical development of mRNA-1273, including the execution of a 30,000 participant Phase 3 study in the U.S. We further amended the agreement in March 2021 to provide for an additional commitment of $63 million to further support late-stage clinical development, including Phase 2/3 mRNA-1273 pediatric studies. In April 2021, we entered into a further amendment to the BARDA agreement, increasing the amount of potential reimbursements by $236 million in connection with costs associated with the Phase 3 clinical trials for mRNA-1273 and pharmacovigilance efforts. The maximum award from BARDA, inclusive of the March 2021 and April 2021 amendments, was approximately $1.3 billion. Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure. All contract options have been exercised. As of June 30, 2021, the remaining available funding, net of revenue earned, was $421 million.
In September 2016, we received from BARDA an award of up to $126 million, subsequently adjusted to $117 million in 2021, to help fund our Zika vaccine program. Three of the four contract options have been exercised. As of June 30, 2021, the remaining available funding, net of revenue earned, was $59 million, with an additional $8 million available if the final contract option is exercised.
In January 2016, we entered a global health project framework agreement with the Bill and Melinda Gates Foundation (Gates Foundation) to advance mRNA-based development projects for various infectious diseases, including human immunodeficiency virus (HIV). As of June 30, 2021, the available funding, net of revenue earned, was $10 million, with up to an additional $80 million available, if additional follow-on projects are approved.
The following table summarizes grant revenue as of and for the periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| BARDA | $ | 134 | | | $ | 37 | | | $ | 326 | | | $ | 40 | |
| Other grant revenue | 5 | | | 1 | | | 7 | | | 2 | |
| Total grant revenue | $ | 139 | | | $ | 38 | | | $ | 333 | | | $ | 42 | |
5. Collaboration Agreements
We have entered into collaboration agreements with strategic collaborators to accelerate the discovery and advancement of potential mRNA medicines across therapeutic areas. As of June 30, 2021 and December 31, 2020, we had collaboration agreements with AstraZeneca plc (AstraZeneca), Merck & Co., Inc (Merck), Vertex Pharmaceuticals Incorporated and Vertex Pharmaceuticals (Europe) Limited (together, Vertex), and Chiesi Farmaceutici S.P.A. (Chiesi). Please refer to our 2020 Form 10-K under the heading “Third-Party Strategic Alliances” and Note 5 to our consolidated financial statements for further description of each of the collaboration agreements.
The following table summarizes our total consolidated revenue from our strategic collaborators for the periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| Collaboration Revenue by Strategic Collaborator: | 2021 | | 2020 | | 2021 | | 2020 |
| AstraZeneca | $ | 4 | | | $ | 16 | | | $ | 4 | | | $ | 17 | |
| Merck | 4 | | | 11 | | | 4 | | | 12 | |
| Vertex | 9 | | | 2 | | | 18 | | | 4 | |
| Other | 1 | | | — | | | 2 | | | — | |
| Total collaboration revenue | $ | 18 | | | $ | 29 | | | $ | 28 | | | $ | 33 | |
The following table presents changes in the balances of our receivables and contract liabilities related to our strategic collaboration agreements during the six months ended June 30, 2021 (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | Additions | | Deductions | | June 30, 2021 |
| Contract Assets: | | | | | | | | |
| Accounts receivable | | $ | 6 | | | $ | 17 | | | $ | (16) | | | $ | 7 | |
| Contract Liabilities: | | | | | | | | |
| Deferred revenue | | $ | 240 | | | $ | 18 | | | $ | (28) | | | $ | 230 | |
As of June 30, 2021, the aggregated amount of the transaction price allocated to performance obligations under our collaboration agreements that are unsatisfied or partially unsatisfied was $321 million.
6. Financial Instruments
Cash and Cash Equivalents and Investments
The following tables summarize our cash and available-for-sale securities by significant investment category at June 30, 2021 and December 31, 2020 (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | June 30, 2021 |
| | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses | | Estimated Fair Value | | Cash and
Cash
Equivalents | | Current
Marketable
Securities | | Non-
Current
Marketable
Securities |
| Cash and cash equivalents | | $ | 5,603 | | | $ | — | | | $ | — | | | $ | 5,603 | | | $ | 5,603 | | | $ | — | | | $ | — | |
| Available-for-sale: | | | | | | | | | | | | | | |
| Certificates of deposit | | 50 | | | — | | | — | | | 50 | | | — | | | 50 | | | — | |
| U.S. treasury bills | | 87 | | | — | | | — | | | 87 | | | — | | | 87 | | | — | |
| U.S. treasury notes | | 4,349 | | | — | | | (3) | | | 4,346 | | | — | | | 1,411 | | | 2,935 | |
| Corporate debt securities | | 2,113 | | | 1 | | | (3) | | | 2,111 | | | — | | | 839 | | | 1,272 | |
| | $ | 12,202 | | | $ | 1 | | | $ | (6) | | | $ | 12,197 | | | $ | 5,603 | | | $ | 2,387 | | | $ | 4,207 | |
| | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses | | Estimated Fair Value | | Cash and
Cash
Equivalents | | Current
Marketable
Securities | | Non-
Current
Marketable
Securities |
| Cash and cash equivalents | | $ | 2,624 | | | $ | — | | | $ | — | | | $ | 2,624 | | | $ | 2,624 | | | $ | — | | | $ | — | |
| Available-for-sale: | | | | | | | | | | | | | | |
| Certificates of deposit | | 239 | | | — | | | — | | | 239 | | | — | | | 215 | | | 24 | |
| U.S. treasury bills | | 492 | | | — | | | — | | | 492 | | | — | | | 492 | | | — | |
| U.S. treasury notes | | 87 | | | — | | | — | | | 87 | | | — | | | 38 | | | 49 | |
| Corporate debt securities | | 1,801 | | | 4 | | | — | | | 1,805 | | | — | | | 1,239 | | | 566 | |
| | $ | 5,243 | | | $ | 4 | | | $ | — | | | $ | 5,247 | | | $ | 2,624 | | | $ | 1,984 | | | $ | 639 | |
The amortized cost and estimated fair value of marketable securities by contractual maturity at June 30, 2021 and December 31, 2020 were as follows (in millions):
| | | | | | | | | | | | | | |
| | June 30, 2021 |
| | Amortized
Cost | | Estimated
Fair Value |
| Due in one year or less | | $ | 2,386 | | | $ | 2,387 | |
| Due after one year through five years | | 4,213 | | | 4,207 | |
| Total | | $ | 6,599 | | | $ | 6,594 | |
| | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Amortized
Cost | | Estimated
Fair Value |
| Due in one year or less | | $ | 1,981 | | | $ | 1,984 | |
| Due after one year through five years | | 638 | | | 639 | |
| Total | | $ | 2,619 | | | $ | 2,623 | |
In accordance with our investment policy, we place investments in investment grade securities with high credit quality issuers, and generally limit the amount of credit exposure to any one issuer. We evaluate securities for impairment at the end of each reporting period. Impairment is evaluated considering numerous factors, and their relative significance varies depending on the situation. Factors considered include whether a decline in fair value below the amortized cost basis is due to credit-related factors or noncredit-related factors, the financial condition and near-term prospects of the issuer, and our intent and ability to hold the investment to allow for an anticipated recovery in fair value. Any impairment that is not credit related is recognized in other comprehensive loss, net of
applicable taxes. A credit-related impairment is recognized as an allowance on the balance sheet with a corresponding adjustment to earnings. We did not recognize any impairment charges related to available-for-sale securities for the three and six months ended June 30, 2021 and 2020. We did not recognize any credit-related allowance to available-for-sale securities as of June 30, 2021 and December 31, 2020.
As of June 30, 2021 and December 31, 2020, we did not have material gross unrealized losses. We neither intend to sell these investments, nor do we believe that we are more-likely-than-not to conclude we will have to sell them before recovery of their carrying values. We also believe that we will be able to collect both principal and interest amounts due to us at maturity.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used to value the assets and liabilities:
•Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
•Level 2: Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability; or
•Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
The following tables summarize our financial assets and liabilities measured at fair value on a recurring basis as of June 30, 2021 and December 31, 2020 (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | Fair value at June 30, 2021 | | Fair Value Measurement Using |
| | | Level 1 | | Level 2 |
| Assets: | | | | | | |
| Money market funds | | $ | 3,838 | | | $ | 3,838 | | | $ | — | |
| Certificates of deposit | | 50 | | | — | | | 50 | |
| U.S. treasury bills | | 87 | | | — | | | 87 | |
| U.S. treasury notes | | 4,346 | | | — | | | 4,346 | |
| Corporate debt securities | | 2,111 | | | — | | | 2,111 | |
| Derivative instruments (Note 7) | | 30 | | | — | | | 30 | |
| Total | | $ | 10,462 | | | $ | 3,838 | | | $ | 6,624 | |
| Liabilities: | | | | | | |
| Derivative instruments (Note 7) | | $ | 2 | | | $ | — | | | $ | 2 | |
| | | | | | | | | | | | | | | | | | | | |
| | Fair value at December 31, 2020 | | Fair Value Measurement Using |
| | | Level 1 | | Level 2 |
| Assets: | | | | | | |
| Money market funds | | $ | 660 | | | $ | 660 | | | $ | — | |
| Certificates of deposit | | 239 | | | — | | | 239 | |
| U.S. treasury bills | | 492 | | | — | | | 492 | |
| U.S. treasury notes | | 87 | | | — | | | 87 | |
| Corporate debt securities | | 1,805 | | | — | | | 1,805 | |
| Total | | $ | 3,283 | | | $ | 660 | | | $ | 2,623 | |
As of June 30, 2021 and December 31, 2020, we did not have non-financial assets or liabilities measured at fair value on a recurring basis and did not have any Level 3 financial assets or financial liabilities.
7. Derivative Financial Instruments
We transact business in various foreign currencies and have international sales and expenses denominated in foreign currencies. Therefore, we are exposed to certain risks arising from both our business operations and economic conditions. Our risk management strategy includes the use of derivative financial instruments to hedge: (1) forecasted product sales that are denominated in foreign currencies and (2) foreign currency exchange rate fluctuations on monetary assets or liabilities denominated in foreign currencies. We do not enter into derivative financial contracts for speculative or trading purposes. We do not believe that we are exposed to more than a nominal amount of credit risk in our foreign currency hedges, as counterparties are large, global and well-capitalized financial institutions. We classify cash flows from our derivative transactions as cash flows from operating activities in our condensed consolidated statements of cash flows.
Cash Flow Hedges
We mitigate the foreign exchange risk arising from the fluctuations in foreign currency denominated product sales in Euro through a foreign currency cash flow hedging program, using forward contracts and foreign currency options that do not exceed 15 months in duration. We hedge these cash flow exposures to reduce the risk that our earnings and cash flows will be adversely affected by changes in exchange rates. To receive hedge accounting treatment, all hedging relationships are formally documented at the inception of the hedge, and the hedges must be highly effective in offsetting changes to future cash flows on hedged transactions. The derivative assets or liabilities associated with our hedging activities are recorded at fair value in other current assets or other current liabilities, respectively, in our condensed consolidated balance sheets. The gains or losses resulting from changes in the fair value of these hedges are initially recorded as a component of accumulated other comprehensive income (AOCI) in stockholders’ equity and subsequently reclassified to product sales in the period during which the hedged transaction affects earnings. In the event the underlying forecasted transaction does not occur, or it becomes probable that it will not occur, within the defined hedge period, we reclassify the gains or losses on the related cash flow hedge from AOCI to product sales. We evaluate hedge effectiveness at the inception of the hedge prospectively, and on an on-going basis both retrospectively and prospectively. If we do not elect hedge accounting, or the contract does not qualify for hedge accounting treatment, the changes in fair value from period to period are recorded in the same income statement line item as the hedged item. As of June 30, 2021, we had net deferred gains of $27 million on our foreign currency forward contracts included in AOCI that are expected to be recognized into product sales within the next 12 months.
Balance Sheet Hedges
We enter into foreign currency forward contracts to hedge fluctuations associated with foreign currency denominated monetary assets and liabilities, primarily accounts receivable in Euro and lease liabilities in Swiss Franc, that are not designated for hedge accounting treatment. Therefore, these forward contracts are accounted for as derivatives whereby the fair value of the contracts are reported as other current assets or other current liabilities in our condensed consolidated balance sheets, and gains and losses resulting from changes in the fair value are recorded as a component of other expense, net, in our condensed consolidated statements of operations. The gains and losses on these foreign currency forward contracts generally offset the gains and losses in the underlying foreign currency denominated assets and liabilities, which are also recorded to other expense, net, in our condensed consolidated statements of operations.
Total gross notional amount and fair value of our foreign currency derivatives were as follows (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | June 30, 2021 |
| | Notional Amount | | Fair Value |
| | | Asset (1) | | Liability (2) |
| Derivatives designated as cash flow hedging instruments: | | | | | | |
| Foreign currency forward contracts | | $ | 981 | | | $ | 27 | | | $ | — | |
| | | | | | |
| Derivatives not designated as hedging instruments: | | | | | | |
| Foreign currency forward contracts | | 308 | | | 3 | | | 2 | |
| Total derivatives | | $ | 1,289 | | | $ | 30 | | | $ | 2 | |
| | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Notional Amount | | Fair Value |
| | | Asset (1) | | Liability (2) |
| Derivatives not designated as hedging instruments: | | | | | | |
| Foreign currency forward contracts | | $ | 368 | | | $ | — | | | $ | — | |
| Total derivatives | | $ | 368 | | | $ | — | | | $ | — | |
_________
(1) As presented in the condensed consolidated balance sheets within other current assets.
(2) As presented in the condensed consolidated balance sheets within other current liabilities.
Gains (losses) on our foreign currency derivatives, net of tax, recognized in our condensed consolidated statements of comprehensive income (loss) for the three and six months ended June 30, 2021 were as follows (in millions):
| | | | | | | | | | | | | | |
| | Three Months Ended
June 30, 2021 | | Six Months Ended
June 30, 2021 |
| Derivatives in cash flow hedging relationships: | | | | |
| Foreign currency forward contracts | | $ | 21 | | | $ | 21 | |
The effect of our foreign currency derivatives in our condensed consolidated statements of operations for the three and six months ended June 30, 2021 was as follows (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | Statement of Operations Classification | | Three Months Ended
June 30, 2021 | | Six Months Ended
June 30, 2021 |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Derivatives not designated as hedging instruments: | | | | | | |
| Foreign currency forward contracts | | | | | | |
| Net realized and unrealized loss | | Other expense, net | | $ | (54) | | | $ | (19) | |
We had immaterial gains reclassified from AOCI to product sales during the three and six months ended June 30, 2021. There were no hedging activities for the three and six months ended June 30, 2020.
8. Inventory
Inventory as of June 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | | | |
| | June 30, | | December 31, | | |
| | 2021 | | 2020 | | |
| Raw materials | | $ | 378 | | | $ | 37 | | | |
| Work in progress | | 224 | | | 9 | | | |
| Finished goods | | 41 | | | 1 | | | |
| Total inventory | | $ | 643 | | | $ | 47 | | | |
9. Property and Equipment, Net
Property and equipment, net, as of June 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | |
| | June 30, | | December 31, |
| | 2021 | | 2020 |
Laboratory equipment | | $ | 142 | | | $ | 121 | |
Leasehold improvements | | 199 | | | 180 | |
| Furniture, fixtures and other | | 6 | | | 5 | |
Computer equipment and software | | 14 | | | 13 | |
Internally developed software | | 9 | | | 7 | |
| Right-of-use asset, financing (Note 11) | | 545 | | | 56 | |
Construction in progress | | 83 | | | 35 | |
| | 998 | | | 417 | |
Less: Accumulated depreciation | | (204) | | | (120) | |
Property and equipment, net | | $ | 794 | | | $ | 297 | |
Depreciation and amortization expense for the three months ended June 30, 2021 and 2020 was $69 million and $8 million, respectively. Depreciation and amortization expense for the six months ended June 30, 2021 and 2020 was $84 million and $15 million, respectively.
10. Other Balance Sheet Components
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets, as of June 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | |
| | June 30, | | December 31, |
| | 2021 | | 2020 |
| Down payments to manufacturing vendors | | $ | 138 | | | $ | 217 | |
| Other prepaid expenses | | 59 | | | 7 | |
| Value added tax receivable | | 51 | | | 7 | |
| Derivative assets | | 30 | | | — | |
| Other current assets | | 38 | | | 21 | |
Prepaid expenses and other current assets | | $ | 316 | | | $ | 252 | |
Accrued Liabilities
Accrued liabilities, as of June 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | |
| | June 30, | | December 31, |
| | 2021 | | 2020 |
| Clinical trials | | $ | 108 | | | $ | 98 | |
| Raw materials | | 184 | | | 78 | |
| Royalties | | 146 | | | — | |
| Development operations | | 102 | | | 29 | |
| Manufacturing | | 158 | | | 53 | |
| Other external goods and services | | 49 | | | 92 | |
| Compensation-related | | 62 | | | 95 | |
| Other | | 39 | | | 25 | |
| | | | |
| | | | |
Accrued liabilities | | $ | 848 | | | $ | 470 | |
Other Current Liabilities
Other current liabilities, as of June 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | |
| | June 30, | | December 31, |
| | 2021 | | 2020 |
| Lease liabilities - financing (Note 11) | | $ | 159 | | | $ | 24 | |
| Lease liabilities - operating (Note 11) | | 11 | | | 6 | |
| Income taxes payable | | 377 | | | — | |
| Other | | 66 | | | 4 | |
| Other current liabilities | | $ | 613 | | | $ | 34 | |
Deferred Revenue
The following table summarizes the activities in deferred revenue for the six months ended June 30, 2021 (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | Additions | | Deductions | | June 30, 2021 |
| Product sales | | $ | 3,799 | | | $ | 6,145 | | | $ | (2,705) | | | $ | 7,239 | |
| Grant revenue | | 5 | | | 10 | | | (7) | | | 8 | |
| Collaboration revenue | | 240 | | | 18 | | | (28) | | | 230 | |
| Total deferred revenue | | $ | 4,044 | | | $ | 6,173 | | | $ | (2,740) | | | $ | 7,477 | |
11. Leases
We have entered into various long-term non-cancelable lease arrangements for our facilities and equipment expiring at various times through 2035. Certain of these arrangements have free rent periods or escalating rent payment provisions. We recognize lease cost under such arrangements on a straight-line basis over the life of the leases. We have two campuses in Massachusetts, our Cambridge facility and our Moderna Technology Center, located in Norwood. We also leased other office spaces globally for our business operations.
Operating Leases
Cambridge facility
We occupy a multi-building campus in Technology Square in Cambridge, Massachusetts with a mix of offices and research laboratory space totaling approximately 261,000 square feet. Our Cambridge facility leases have expiry ranges from 2022 to 2029.
Finance Leases
Moderna Technology Center
We have an industrial technology center in Norwood, Massachusetts, Moderna Technology Center (MTC), which comprises three buildings, MTC South, MTC North, and MTC East.
In August 2016, we entered into a lease agreement for approximately 200,000 square feet of office, laboratory, and light manufacturing space (MTC South). The lease will expire in September 2032. We have the option to extend the term for two extension periods of ten years each at market-based rents.
In February 2019, we entered into a lease agreement for office and laboratory space of approximately 200,000 square feet (MTC North). The lease commenced in the second quarter of 2019 and had an initial expiration date of 2031. We have the option to extend the lease for up to four additional five-year terms. In May 2020, we entered into an amendment to the lease whereby we exercised an option available in the original lease to receive a tenant improvement allowance in the amount of $22 million to be paid back over the term of the lease with interest and extend the term of the lease to 2035.
In April 2021, we entered into a lease agreement for a 240,000 square foot building located on the same campus for expansion of our commercial and clinical activities (MTC East). The lease will expire in February 2034. We have the option to extend the term for two extension periods of five years each at market-based rents.
Embedded Leases
We have entered into multiple contract manufacturing service agreements with third parties which contain embedded leases within the scope of ASC 842. As of June 30, 2021 and December 31, 2020, we had lease liabilities of $251 million and $24 million, respectively, related to the embedded leases. As of June 30, 2021 and December 31, 2020, we had right-of-use assets of $296 million and zero, as certain embedded leases dedicated to our COVID-19 vaccine program were deemed to have no alternative use prior to the EUA from the FDA in December 2020.
Operating and financing lease right-of-use assets and lease liabilities as of June 30, 2021 and December 31, 2020 were as follows (in millions):
| | | | | | | | | | | | | | |
| | June 30, | | December 31, |
| | 2021 | | 2020 |
| Assets: | | | | |
Right-of-use assets, operating, net (1) (2) | | $ | 104 | | | $ | 90 | |
Right-of-use assets, financing, net (3) (4) | | 475 | | | 55 | |
| Total | | $ | 579 | | | $ | 145 | |
| | | | |
| Liabilities: | | | | |
| Current: | | | | |
Operating lease liabilities (5) | | $ | 11 | | | $ | 6 | |
Financing lease liabilities (5) | | 159 | | | 24 | |
| Total current lease liabilities | | 170 | | | 30 | |
| Non-current: | | | | |
| Operating lease liabilities, non-current | | 105 | | | 97 | |
| Financing lease liabilities, non-current | | 328 | | | 110 | |
| Total non-current lease liabilities | | $ | 433 | | | $ | 207 | |
| Total | | $ | 603 | | | $ | 237 | |
_______
(1) These assets are real estate related assets, which include land, office, and laboratory spaces.
(2) Net of accumulated depreciation.
(3) These assets are real estate assets related to the MTC South, MTC North, and MTC East leases as well as assets related to contract manufacturing service agreements.
(4) Included in property and equipment in the condensed consolidated balance sheets, net of accumulated depreciation.
(5) Included in other current liabilities in the condensed consolidated balance sheets.
Future minimum lease payments under our non-cancelable lease agreements at June 30, 2021, are as follows (in millions):
| | | | | | | | | | | | | | | | | |
Fiscal Year | Operating Leases (1) | | Financing Leases (1) |
| 2021 | (remainder of the year) | $ | 9 | | | $ | 100 | | |
| 2022 | | 23 | | | 174 | | |
| 2023 | | 23 | | | 19 | | |
| 2024 | | 16 | | | 19 | | |
| 2025 | | 17 | | | 19 | | |
| Thereafter | 94 | | | 600 | | |
Total minimum lease payments | 182 | | | 931 | | |
| Less amounts representing interest or imputed interest | (66) | | | (444) | | (2) |
Present value of lease liabilities | $ | 116 | | | $ | 487 | | |
______
(1) Includes optional extensions in the MTC South, MTC North, and MTC East lease terms which represent a total of $445 million undiscounted future lease payments.
(2) MTC South interest is based on an imputed interest rate of 17.2%. MTC North, MTC East, and the embedded lease interest is based upon incremental borrowing rates of 8.2%, 3.7%, and 0.6%, respectively.
12. Commitments and Contingencies
Strategic Collaborations
Under our strategic collaboration agreements, we are committed to perform certain research, development, and manufacturing activities. As part of our Personalized Cancer Vaccine (PCV) Agreement and PCV/SAV Agreement (which also relates to shared neoantigen mRNA cancer vaccine) with Merck, we are committed to perform certain research, development and manufacturing activities related to PCV products through an initial Phase 2 clinical trial up to a budgeted amount of $243 million for both periods as of June 30, 2021 and December 31, 2020. Please refer to our 2020 Form 10-K Note 5 to our consolidated financial statements.
Legal Proceedings
We are not currently a party to any material legal proceedings.
Indemnification Obligations
As permitted under Delaware law, we indemnify our officers, directors, and employees for certain events, occurrences while the officer, or director is, or was, serving at our request in such capacity. The term of the indemnification is for the officer’s or director’s lifetime.
We have standard indemnification arrangements in our leases for laboratory and office space that require us to indemnify the landlord against any liability for injury, loss, accident, or damage from any claims, actions, proceedings, or costs resulting from certain acts, breaches, violations, or non-performance under our leases.
We enter into indemnification provisions under our agreements with counterparties in the ordinary course of business, typically with business partners, contractors, clinical sites and customers. Under these provisions, we generally indemnify and hold harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of our activities. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited.
Through the three and six months ended June 30, 2021 and the year ended December 31, 2020, we had not experienced any losses related to these indemnification obligations, and no material claims were outstanding. We do not expect significant claims related to these indemnification obligations and, consequently, concluded that the fair value of these obligations is negligible, and no related reserves were established.
Purchase Commitments and Purchase Orders
We enter into agreements in the normal course of business with vendors and contract manufacturing organizations (CMOs) for raw materials and manufacturing services and with vendors for preclinical research studies, clinical trials and other goods or services. As of June 30, 2021, we had $1.5 billion of non-cancelable purchase commitments related to raw materials and manufacturing agreements, which are expected to be paid through 2022. As of June 30, 2021, we had $49 million of non-cancelable purchase commitments related to clinical services and other goods and services which are expected to be paid through 2024. These amounts represent our minimum contractual obligations, including termination fees.
In addition to purchase commitments, we have agreements with third parties for various services, including services related to clinical operations and support and contract manufacturing, for which we are not contractually able to terminate for convenience and avoid any and all future obligations to the vendors. Certain agreements provide for termination rights subject to termination fees or wind down costs. Under such agreements, we are contractually obligated to make certain payments to vendors, mainly, to reimburse them for their unrecoverable outlays incurred prior to cancellation. At June 30, 2021 and December 31, 2020, we had cancelable open purchase orders of $1.3 billion and $897 million, respectively, in total under such agreements for our significant clinical operations and support and contract manufacturing. These amounts represent only our estimate of those items for which we had a contractual commitment to pay at June 30, 2021 and December 31, 2020, assuming we would not cancel these agreements. The actual amounts we pay in the future to the vendors under such agreements may differ from the purchase order amounts.
Licenses to Patented Technology
On June 26, 2017, we entered into sublicense agreements with Cellscript, LLC and its affiliate, mRNA RiboTherapeutics, Inc. to sublicense certain patent rights. Pursuant to each agreement, we are required to pay certain license fees, annual maintenance fees, minimum royalties on future net sales and milestone payments contingent on achievement of certain development, regulatory and commercial milestones for specified products, on a product-by-product basis. Commercial milestone payments, up to $24 million, and royalties based on annual net sales of licensed products for therapeutic and prophylactic products are accounted for as additional expense of the related product sales in the period in which the corresponding sales occur. For the three and six months ended June 30, 2021, we recognized $148 million and $232 million, respectively, of royalty expenses associated with our product sales, which was recorded to cost of sales in our condensed consolidated statements of operations. We did not recognize any such royalties in the first half of 2020 as we did not have product sales during the period.
Additionally, we have other in-license agreements with third parties which require us to make future development, regulatory and commercial milestone payments for specified products associated with the agreements. The achievement of these milestones was not deemed probable as of June 30, 2021.
13. Stock-Based Compensation
As of June 30, 2021, we had a total of 60 million shares reserved for future issuance under our Equity Plans, of which 33 million shares were reserved for equity awards previously granted, and 27 million shares were available for future grants under the 2018 Equity Plan.
Options
The following table summarizes our option activity during the six months ended June 30, 2021:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Number of Options (in millions) | | Weighted-
Average
Exercise
Price per
Share | | Weighted-
Average
Grant Date Fair
Value per
Share | | Weighted-
Average
Remaining
Contractual
Term | | Aggregate Intrinsic Value (1) (in millions) |
| Outstanding at December 31, 2020 | | 34.06 | | | $ | 17.14 | | | $ | 9.12 | | | 6.7 years | | $ | 2,976 | |
Granted | | 1.13 | | | 173.82 | | | 76.63 | | | | | |
Exercised | | (3.86) | | | 15.31 | | | 8.60 | | | | | |
Canceled/forfeited | | (0.45) | | | 27.47 | | | 14.33 | | | | | |
| Outstanding at June 30, 2021 | | 30.88 | | | 22.98 | | | 11.59 | | | 6.3 years | | 6,547 | |
| Exercisable at June 30, 2021 | | 17.22 | | | 12.16 | | | 6.30 | | | 5.1 years | | 3,837 | |
| Expected to vest at June 30, 2021 | | 13.66 | | | 36.62 | | | 18.26 | | | 7.8 years | | 2,710 | |
_______
(1)Aggregate intrinsic value is calculated as the difference between the exercise price of the underlying options and the fair value of common stock for those options in the money as of June 30, 2021.
The total intrinsic value of options exercised was $575 million for the six months ended June 30, 2021. The aggregate intrinsic value represents the difference between the exercise price and the selling price received by option holders upon the exercise of stock options during the period. The total consideration recorded as a result of stock option exercises was approximately $59 million for the six months ended June 30, 2021.
Restricted Common Stock Units (RSUs) and Performance Stock Units (PSUs)
The following table summarizes our RSU and PSU activity during the six months ended June 30, 2021:
| | | | | | | | | | | |
| Units
(in millions) | | Weighted-Average Fair Value per Unit |
| Outstanding, non-vested at December 31, 2020 | 2.19 | | | $ | 30.85 | |
Issued | 0.55 | | | 170.03 | |
Vested | (0.35) | | | 25.63 | |
Canceled/forfeited | (0.07) | | | 37.99 | |
| Outstanding, non-vested at June 30, 2021 | 2.32 | | | 64.40 | |
The total fair value of restricted stock units vested during the six months ended June 30, 2021 was $9 million. The total intrinsic value of restricted stock units vested during the six months ended June 30, 2021 was $54 million.
During the first quarter of 2021, we granted PSUs to certain senior executives with vesting that is contingent upon the achievement of specified preestablished goals over the performance period, generally three years. The actual number of common shares ultimately issued is calculated by multiplying the number of PSUs by a payout percentage ranging from 0% to 200%. The estimated fair value of PSUs is based on the grant date fair value.
2018 Employee Stock Purchase Plan (ESPP)
We sold an immaterial number of shares under the ESPP during the six months ended June 30, 2021. As of June 30, 2021, 4 million shares were available for future issuance under the ESPP.
The following table presents the components and classification of stock-based compensation expense for the three and six months ended June 30, 2021 and 2020 as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
Options | | $ | 24 | | | $ | 21 | | | $ | 46 | | | $ | 39 | |
| RSUs and PSUs | | 10 | | | 3 | | | 17 | | | 5 | |
ESPP | | 1 | | | 1 | | | 2 | | | 1 | |
Total | | $ | 35 | | | $ | 25 | | | $ | 65 | | | $ | 45 | |
| | | | | | | | |
| Cost of sales | | $ | 8 | | | $ | — | | | $ | 12 | | | $ | — | |
| Research and development | | 15 | | | 15 | | | 29 | | | 27 | |
| Selling, general and administrative | | 12 | | | 10 | | | 24 | | | 18 | |
Total | | $ | 35 | | | $ | 25 | | | $ | 65 | | | $ | 45 | |
As of June 30, 2021, there was $336 million of total unrecognized compensation cost related to unvested stock-based compensation with respect to options, RSUs and PSUs granted. That cost is expected to be recognized over a weighted-average period of 3.0 years at June 30, 2021.
14. Income Taxes
We are subject to U.S. federal, state, and foreign income taxes. For the three and six months ended June 30, 2021, we recorded provisions for income taxes of $283 million and $322 million, respectively, compared to an insignificant amount for the same periods in 2020. Our effective tax rate for the three and six months ended June 30, 2021 was 9% and 7%, respectively, and was lower than the U.S. statutory rate primarily due to the benefit related to the release of the valuation allowance on the majority of our tax attributes, the benefit of the foreign derived intangible income deduction and other deferred tax assets, as well as a discrete item for excess tax benefits related to stock-based compensation. Our effective tax rate for the three and six months ended June 30, 2020 was lower than the U.S. statutory rate primarily due to the valuation allowance.
We recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities. These differences are measured using the enacted statutory tax rates that are expected to be in effect for the years in which differences are expected to reverse. On a periodic basis, we reassess any valuation allowances that we maintain on our deferred tax assets, weighing positive and negative evidence to assess the recoverability of the deferred tax assets. In the first quarter of 2021, we reassessed the valuation allowance noting the increase in positive evidence, including significant revenue growth, expectations regarding future profitability, and successful supply chain and manufacturing capabilities to meet global product demand. After assessing both the positive evidence and negative evidence, we determined it was more likely than not that we will realize the majority of our deferred tax assets. Therefore, we determined we should reverse the majority of our valuation allowance through the annual effective tax rate (AETR) with respect to amounts we expect to realize through current year income. In addition, for the three and six months ended June 30, 2021, we have recorded a discrete benefit of $49 million related to the release of the valuation allowance on deferred tax assets that we expect to utilize in future years. We maintain a valuation allowance on certain state tax attributes that we expect will expire prior to the utilization.
In March 2020, the Coronavirus Aid, Relief and Economic Security Act (CARES Act) was signed into law. The CARES Act includes provisions relating to several aspects of corporate income taxes. We do not currently expect the CARES Act to have a significant impact on our provision for income taxes.
We have reviewed the tax positions taken, or to be taken, in our tax returns for all tax years currently open to examination by a taxing authority. Unrecognized tax benefits represent the aggregate tax effect of differences between tax return positions and the benefits recognized in the financial statements. As of June 30, 2021 and December 31, 2020, we had an immaterial amount of gross unrecognized tax benefits, which would affect our tax rate if recognized. We do not expect that our unrecognized tax benefits will materially increase within the next twelve months. We accrue interest and penalties related to unrecognized tax benefits as a component of our provision for income taxes. We did not recognize any material interest or penalties related to uncertain tax positions during the three and six months ended June 30, 2021 and 2020.
We file U.S. federal income tax returns and income tax returns in various state, local and foreign jurisdictions. We are not currently subject to any tax assessment from an income tax examination in the United States or any other major taxing jurisdiction since inception.
15. Earnings (Loss) per Share
The computation of basic earnings (loss) per share (EPS) is based on the weighted-average number of our common shares outstanding. The computation of diluted EPS is based on the weighted-average number of our common shares outstanding and potential dilutive common shares outstanding during the period as determined by using the treasury stock method.
Basic and diluted EPS for the three and six months ended June 30, 2021 and 2020 were calculated as follows (in millions, except per share data):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Numerator: | | | | | | | | |
| Net income (loss) | | $ | 2,780 | | | $ | (117) | | | $ | 4,001 | | | $ | (241) | |
| Denominator: | | | | | | | | |
| Basic weighted-average common shares outstanding | | 402 | | | 381 | | | 401 | | | 367 | |
| Effect of dilutive securities | | 29 | | | — | | | 29 | | | — | |
| Diluted weighted-average common shares outstanding | | 431 | | | 381 | | | 430 | | | 367 | |
| | | | | | | | |
| Basic EPS | | $ | 6.93 | | | $ | (0.31) | | | $ | 9.98 | | | $ | (0.66) | |
| Diluted EPS | | $ | 6.46 | | | $ | (0.31) | | | $ | 9.30 | | | $ | (0.66) | |
The following common stock equivalents, presented based on amounts outstanding as of June 30, 2020 were excluded from the calculation of diluted net loss per share attributable to common stockholders for the periods indicated because their inclusion would have been anti-dilutive (in millions):
| | | | | | | | | |
| | | June 30, |
| | | 2020 |
Stock options | | | 40 | |
| | | |
Restricted common stock units | | | 2 | |
| | | 42 | |
16. Subsequent Events
Subsequent to June 30, 2021, we have entered into additional supply agreements with customers to provide up to 262 million doses of our COVID-19 vaccine and our updated variant booster vaccine candidate based on the initial confirmed volume, subject to modifications.
On August 2, 2021, our Board of Directors authorized a Share Repurchase Program of our common stock, which expires on August 2, 2023. Pursuant to the Share Repurchase Program, we may repurchase up to $1.0 billion of our outstanding common stock. The timing and actual number of shares repurchased depend on a variety of factors, including price, general business and market conditions, and other investment opportunities, and shares may be repurchased through open market purchases through the use of trading plans intended to qualify under Rule 10b5-1 under the Exchange Act.
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our unaudited financial information and related notes included in this Form 10-Q and our consolidated financial statements and related notes and other financial information in our Annual Report on Form 10-K for the year ended December 31, 2020, which was filed with the SEC on February 26, 2021 (the “2020 Form 10-K”). Some of the information contained in this discussion and analysis or set forth elsewhere in this Form 10-Q, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in Part II, Item 1A - Risk Factors in this Form 10-Q, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines to improve the lives of patients. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane, or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases. Our platform builds on continuous advances in basic and applied mRNA science, delivery technology, and manufacturing, providing us the capability to pursue in parallel a robust pipeline of new development candidates. We are developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, autoimmune diseases and cardiovascular diseases, independently and with our strategic collaborators.
Within our platform, we develop technologies that enable the development of mRNA medicines for diverse applications. When we identify technologies that we believe could enable a new group of potential mRNA medicines with shared product features, we call that group a “modality.” While the programs within a modality may target diverse diseases, they share similar mRNA technologies, delivery technologies, and manufacturing processes to achieve shared product features. The programs within a modality will also generally share similar pharmacology profiles, including the desired dose response, the expected dosing regimen, the target tissue for protein expression, safety and tolerability goals, and pharmaceutical properties. Programs within a modality often have correlated technology risk, but because they pursue diverse diseases they often have uncorrelated biology risk. We have created six modalities to date:
•prophylactic vaccines;
•systemic secreted and cell surface therapeutics;
•cancer vaccines;
•intratumoral immuno-oncology;
•localized regenerative therapeutics; and
•systemic intracellular therapeutics.
We have designated our prophylactic vaccines and systemic secreted and cell surface therapeutics modalities as our “core modalities”. In these core modalities, our strategy is to invest in additional development candidates using our accumulated innovations in technology, our process insights and our preclinical and clinical experience. Our exploratory modalities continue to be a critical part of advancing our strategy to maximize the application of our potential mRNA medicines.
Business Highlights
Moderna COVID-19 Vaccine
On December 18, 2020, the U.S. Food and Drug Administration (FDA) authorized the emergency use of the Moderna COVID-19 Vaccine in individuals 18 years of age or older. We have also received authorization for our COVID-19 vaccine from health agencies in more than 50 countries and from the World Health Organization. Additional authorizations are currently under review in other countries. In addition, we have received authorization for our COVID-19 vaccine for use in adolescents in the European Union and Japan and have pending applications for authorization to administer the vaccine to adolescents with regulatory agencies in the United States and other countries. On June 1, 2021, we initiated the rolling submission process with the U.S. FDA for a Biologics License Application for our COVID-19 vaccine and we currently anticipate completing our submission in August 2021.
We have entered into supply agreements with the U.S. Government, several other governments outside the United States and with UNICEF (on behalf of the COVAX Facility) for the supply of our COVID-19 vaccine. The agreements are generally subject to receipt of authorization or approval for the use and distribution of the vaccine from the relevant regulatory authority in each jurisdiction. Under these agreements, we are entitled to upfront deposits for our COVID-19 vaccine supply, initially recorded as deferred revenue. As of June 30, 2021, we had approximately $7.2 billion in deferred revenue in connection with the supply agreements with the U.S. Government and other customers, which will be recognized as revenue when revenue recognition criteria have been met.
For the second quarter of 2021, we delivered approximately 126 million doses of our COVID-19 vaccine to the U.S. government and approximately 73 million doses to other governments, and recognized $4.2 billion in product sales. For the first quarter of 2021, we delivered approximately 88 million doses of our COVID-19 vaccine to the U.S. government and approximately 14 million doses to other governments, and recognized $1.7 billion in product sales.
In the second quarter of 2021, we announced additional investments to facilitate the increased supply of our COVID-19 vaccine from our own and partnered manufacturing facilities, and an expansion of the Moderna Technology Center (MTC) in Norwood, Massachusetts, to more than double our facility space to help transform it from a production and lab space to an industrial technology center. As a result of these investments, we expect that we will supply between 800 million and 1 billion doses of our COVID-19 vaccine in 2021, at the 100 µg dose. We anticipate these investments will also increase our global 2022 capacity for the vaccine to up to 3 billion doses, in the event that our 2022 production is dedicated to 50 μg booster doses. If our 2022 production is dedicated to 100 μg doses, we expect our total potential capacity to be approximately 2 billion doses. The ultimate dosage for our 2022 supply is subject to ongoing internal review, and is also subject to further regulatory approval and authorization, among other factors. These investments are expected to facilitate a doubling of drug substance manufacturing from Lonza’s Switzerland-based facility, a more than doubling of formulation, fill and finish and drug substance manufacturing at Rovi’s Spain-based facility, as well as a 50% increase of drug substance at Moderna’s facilities in the U.S. When completed, the investments are expected to also result in an increase in safety stock of raw materials and finished product used to deliver committed volumes. These forecasted increases to our supply are subject in part to performance by our manufacturing partners, which will require ramping-up capabilities at their own facilities and the hiring of qualified manufacturing personnel.
Moderna COVID-19 Vaccine Clinical Studies
The final analysis of adjudicated cases from the Phase 3 clinical trial for mRNA-1273, which we refer to as the COVE Study, demonstrated efficacy of 93% through six months after the second dose of the vaccine. The final analysis also demonstrated greater than 98% efficacy against severe cases of COVID-19 and 100% efficacy against death caused by COVID-19 in the per protocol cohort. The final analysis also demonstrated consistency in our subgroup analysis, including analyses by gender, by race and by preexisting medical conditions. The safety profile for mRNA-1273 continues to be consistent with the Phase 3 data over the longer period of safety follow up and across population subgroups.
The Phase 2/3 TeenCOVE study of mRNA-1273 in adolescents ages 12-17 years has completed enrollment in the U.S. An initial analysis of 3,732 participants randomized 2:1 in the TeenCOVE study showed a vaccine efficacy rate of 93% in seronegative participants who received at least one injection (modified intent-to-treat cohort) in a secondary analysis. The median duration for follow-up in this analysis was 53 days following the second dose. mRNA-1273 was generally well tolerated. The majority of adverse events were mild or moderate in severity. No serious safety concerns have been identified to date. The most common solicited local adverse event was injection site pain. The most common solicited systemic adverse events after the second dose of mRNA-1273 were headache, fatigue, myalgia and chills. The Conditional Marketing Authorization (CMA) for our COVID-19 vaccine in the European Union has been expanded to include adolescents 12 years of age and older. In addition, the Japanese Ministry of Health, Labor and Welfare also approved our COVID-19 vaccine for ages 12 to 17. We have filed for an EUA for adolescents with the U.S. FDA as well as with additional regulatory agencies around the world.
The Phase 2/3 KidCOVE study of mRNA-1273 in the pediatric population ages 6 months-11 years is currently enrolling. We expect to enroll 12,000 healthy pediatric participants in the U.S. and Canada into this two-part, dose escalation study. In Part 1, each participant ages 2 years to less than 12 years may receive one of two dose levels (50 µg or 100 µg). Also in Part 1, each participant ages six months to less than 2 years may receive one of three dose levels (25 µg, 50 µg and 100 µg). An interim analysis will be conducted to determine which dose will be used in Part 2, the placebo-controlled expansion portion of the study.
Variant-Specific Booster Candidates
On February 24, we announced that we had completed manufacturing of clinical trial material for our variant-specific vaccine candidate, mRNA-1273.351, against the SARS-CoV-2 variant known as the Beta variant (or B.1.351, first identified in the Republic of South Africa) and that this vaccine had been shipped to the National Institutes of Health (NIH) for a Phase 1 clinical trial to be led and funded by the NIH’s National Institute of Allergy and Infectious Diseases. We are also developing a multivalent booster candidate, mRNA-1273.211, which combines mRNA-1273 (Moderna’s authorized vaccine against ancestral strains) and the Beta variant in a single vaccine.
Data from our Phase 2 study showed that a single 50 µg dose of mRNA-1273, mRNA-1273.351 or mRNA-1273.211 given as a booster to previously vaccinated individuals (n=20 per group) increased neutralizing antibody titer responses against SARS-CoV-2 and important variants of concern, including the Gamma variant (or P.1, first identified in Brazil), the Beta variant, and the Delta variant (B.1.617.2). Neutralizing antibody levels following the boost approached those observed after primary vaccination with two doses of 100 µg of mRNA-1273. These data have been submitted to a peer-reviewed journal for publication. Safety and tolerability profiles following third dose booster injections of 50 µg of mRNA-1273, mRNA-1273.351 or mRNA-1273.211 were generally comparable to those observed after the second dose of mRNA-1273 in the previously reported Phase 2 and Phase 3 studies. Our Phase 2 study to evaluate three approaches to boosting is ongoing. We are also in the process of developing a booster tailored to the Delta variant (mRNA-1273.617), and anticipate developing a multivalent booster—referred to as mRNA-1273.213—that combines mRNA-1273.617 with another COVID-19 candidate.
Our strategy on the dosing for boosters will be informed by ongoing clinical trials that assess mRNA-1273 at the 100 µg dose against the results seen at the 50 µg dose, before pursuing an approach with regulatory authorities.
Key Updates for our Other Development Candidates
•CMV vaccine (mRNA-1647): Our vaccine against cytomegalovirus, or CMV (mRNA-1647) is a vaccine combining six mRNAs in a single vial, which encode for two antigens located on the surface of CMV: five mRNAs encoding the subunits that form the membrane-bound pentamer complex and one mRNA encoding the full-length membrane-bound glycoprotein B (gB). Both the pentamer and gB are essential for CMV to infect barrier epithelial surfaces and gain access to the body. mRNA-1647 is designed to produce an immune response against both the pentamer and gB for the prevention of CMV infection.
Based on the interim analysis of the Phase 2 study, which we announced in April 2021, the 100 μg dose has been chosen for the Phase 3 pivotal study for mRNA-1647, which will evaluate the prevention of primary CMV infection in seronegative women ages 16-40 years. We plan to enroll approximately 8,000 participants from approximately 150 sites across the U.S., Europe and Asia-Pacific into the Phase 3 study, which has begun site initiation and is expected to start in the near-term. Moderna owns worldwide commercial rights for mRNA-1647.
•Respiratory syncytial virus (RSV) vaccine (mRNA-1345): mRNA-1345 is a vaccine against RSV encoding for a prefusion F glycoprotein, which elicits a superior neutralizing antibody response compared to the postfusion state. The Phase 1 study of mRNA-1345 to evaluate the tolerability, reactogenicity and immunogenicity of mRNA-1345 in younger adults, older adults, women of child bearing age and children is ongoing. All younger adult (ages 18-49 years) and older adult (ages 65-79 years) cohorts are fully enrolled. Dosing in the older adult cohort (ages 65-79 years) is ongoing. The age range of toddlers in this de-escalation Phase 1 study is 12-59 months and the pediatric cohort is ongoing, as are the cohorts of women of child-bearing potential. The U.S. FDA has granted Fast Track designation for mRNA-1345 in adults older than 60 years of age.
We also intend to evaluate the potential of combinations of mRNA-1345 with our vaccines against other respiratory pathogens in children and separately in older adults. There is no approved vaccine for RSV. Moderna owns worldwide commercial rights to mRNA-1345.
•Seasonal influenza (flu) (mRNA-1010, mRNA-10202 and mRNA-1030): In January 2021, we announced three development candidates for a seasonal influenza vaccine (mRNA-1010, mRNA-1020 and mRNA-1030). In July 2021, the first participants were dosed in the Phase 1/2 study of mRNA-1010, our first generation flu program. mRNA-1010 is a
quadrivalent seasonal influenza vaccine candidate targeting WHO recommendations including A H1N1, H3N2 and influenza B Yamagata and Victoria lineages. Our vision is to develop a respiratory vaccine for the adult and elderly populations combining seasonal flu, COVID-19 variant booster and RSV. Moderna owns worldwide commercial rights to mRNA-1010.
•Zika vaccine (mRNA-1893): mRNA-1893 is a vaccine against ZIKV. After administration of the vaccine, the mRNA is translated as a polyprotein and processed inside the cell to make a virus-like particle, or VLP. This process mimics the response of the cell after natural infection. This program is funded by BARDA. In 2020, we announced positive Phase 1 data showing that mRNA-1893 was well-tolerated at all dose levels, and safety and tolerability did not appear to be influenced by the serostatus of the participants at baseline. All dose levels of mRNA-1893 induced a strong neutralizing ZIKV-specific antibody response in baseline flavivirus seronegative participants. GMTs post-dose two were comparable to those in a small panel of Zika convalescent sera collected during the epidemic. In participants with pre-existing flavivirus antibodies, neutralizing antibody titers were boosted with a single dose of the vaccine as shown by the GMTs and the seroconversion rates. We have started a Phase 2 study that is expected to enroll approximately 800 participants in the United States and Puerto Rico. There is no approved vaccine for Zika.
•Human immunodeficiency virus (HIV) (mRNA-1644 and mRNA-1574): In January 2021, we announced two vaccine programs against HIV. mRNA-1644 is a novel approach to HIV vaccine strategy in humans designed to elicit broadly neutralizing HIV-1 antibodies (bNAbs) and is being developed in collaboration with the International AIDS Vaccine Initiative (IAVI) and the Gates Foundation. A Phase 1 study for mRNA-1644 will use iterative human testing to validate the approach and antigens and multiple novel antigens will be used for germline-targeting and immuno-focusing. A second approach, mRNA-1574, is being evaluated in collaboration with the National Institutes of Health (NIH) and includes multiple native-like trimer antigens.
•Propionic acidemia (PA) (mRNA-3927): Enrollment for the Phase 1/2 clinical trial for mRNA-3927, our therapy for the treatment of propionic acidemia, or PA, is ongoing. The Phase 1/2 study is designed to evaluate the safety and tolerability of mRNA-3927 in patients with PA. PA, is a rare, life-threatening, inherited metabolic disorder due to a defect in the mitochondrial enzyme propionyl-CoA carboxylase, or PCC. It primarily affects the pediatric population. There is no approved therapy for PA, including no approved enzyme replacement therapy. We have received Rare Pediatric Disease Designation and Orphan Drug Designation from the FDA and Orphan Drug Designation from the European Commission for the PA program. The FDA has also granted Fast Track designation to mRNA-3927. This is the first development candidate to enter the clinic in our intracellular therapeutics modality.
•Methylmalonic acidemia (MMA) (mRNA-3705): Site start-up activities ongoing for the Phase 1/2 study. The Phase 1/2 study is designed to evaluate the safety and tolerability of mRNA-3705 in patients with MMA. MMA is a rare, life-threatening, inherited metabolic disorder that is primarily caused by a defect in the mitochondrial enzyme methylmalonyl-coenzyme A mutase, or MUT. It primarily affects the pediatric population. There is no approved therapy that addresses the underlying disorder, including no approved enzyme replacement therapy, due to the complexity of the protein and its mitochondrial localization.
•IL-2 Mutein (mRNA-6231): mRNA-6231 is an mRNA-encoded IL-2 modified for the expansion of regulatory T cells. A Phase 1 study in healthy volunteers has dosed its first participants. It is also our first subcutaneously administered therapeutic program.
•Intratumoral Immuno-Oncology: We have decided to discontinue the development of our OX40L program (mRNA-2416), which was in a Phase 2 study in ovarian patients. We are continuing development of Triplet (mRNA-2752) which includes OX40L and two proinflammatory cytokines, IL-23, and IL-36γ, encapsulated in our proprietary LNP. The Phase 1 is ongoing and new expansion cohorts are currently enrolling and dosing patients.
Our Pipeline
The following chart shows our current pipeline of 23 development programs, grouped into modalities—first our two core modalities where we believe we have reduced the technology risk, followed by our four exploratory modalities in which we are continuing to investigate the clinical use of mRNA medicines.
Abbreviations: EBV, Epstein-Barr virus; PD-L1, programmed death-ligand-1; PKU, phenylketonuria; GSD1a, Glycogen storage disease type 1a; H7N9, H7N9 influenza vaccine; hMPV/PIV3, human metapneumovirus (hMPV)/parainfluenza virus type 3 (PIV3) vaccine; IL-2, Interleukin 2: IL-12, interleukin 12; IL-23, interleukin 23; IL-36γ, interleu; OX40L, wildtype OX40 ligand; VEGF-A, vascular endothelial growth factor A.
The breadth of biology addressable using mRNA technology is reflected in our current development pipeline of 23 programs. The diversity of proteins made from mRNA within our development pipeline is shown in the figure below.
We have developed six modalities, which are summarized as follows:
•Prophylactic vaccines: Our prophylactic vaccines modality currently includes ten programs, six of which have entered into clinical trials and demonstrated desired pharmacology, in the form of immunogenicity, in positive Phase 1 clinical trials: H7N9 vaccine (mRNA-1851), RSV vaccine (mRNA-1777), human metapneumovirus (hMPV)/parainfluenza virus type 3 (PIV3) vaccine (mRNA-1653), Zika vaccine (mRNA-1893), CMV vaccine (mRNA-1647), COVID-19 vaccine (mRNA-1273). We have ongoing Phase 1 trials for our RSV vaccine (mRNA-1345), flu vaccine (mRNA-1010) and hMPV/PIV3 vaccine (mRNA-1653). We have an ongoing Phase 2 study for our Zika vaccine (mRNA-1893) and CMV vaccine
(mRNA-1647). Our CMV vaccine is also expected to start a Phase 3 trial in the near-term. Our COVID-19 vaccine (mRNA-1273) is described in detail above. Our three preclinical programs within our prophylactic vaccines modality are for Epstein-Barr virus (mRNA-1189), Nipah virus (mRNA-1215) and HIV (mRNA-1644 and mRNA-1574). Two other vaccines being developed as part of public health programs―H10N8 vaccine (mRNA-1440) and Chikungunya vaccine (mRNA-1388)―also produced positive Phase 1 clinical trial results, but are not being further developed without government or other funding.
•Systemic secreted and cell surface therapeutics: We have four systemic secreted and cell surface therapeutics development candidates in our pipeline. Our secreted programs include our antibody against Chikungunya virus (mRNA-1944), Relaxin (mRNA-0184) for cardiac disorders, PD-L1 (mRNA-6981) for autoimmune hepatitis and IL-2 (mRNA-6231) for autoimmune disorders. Our antibody against Chikungunya virus (mRNA-1944) has had positive Phase 1 readouts to date. Our IL-2 program (mRNA-6231) is currently in a Phase 1 study, and is our first first autoimmune therapeutic candidate to enter the clinic. The remaining programs for Relaxin (mRNA-0184) and PD-L1 (mRNA-6981) are currently in preclinical development.
•Cancer vaccines: We are currently developing two programs within our cancer vaccines modality. Our personalized cancer vaccine program mRNA-4157 is being developed in collaboration with Merck and is in a multiple-arm Phase 1 trial and a randomized Phase 2 trial. Our second program within this modality, mRNA-5671, is a KRAS vaccine. Our strategic collaborator Merck has a Phase 1 clinical trial ongoing for mRNA-5671.
•Intratumoral immuno-oncology: We have two programs in this modality. Our first program, OX40L/IL-23/IL-36γ (Triplet) (mRNA-2752), is currently in a Phase 1 study that is designed as an open-label, multicenter study of intratumoral injections of Triplet (mRNA-2752) alone or in combination with durvalumab (anti-PD-L1) with sites in the United States and Israel. Our second program, IL-12 (MEDI1191), is being developed in collaboration with AstraZeneca. AstraZeneca is currently enrolling an open-label multicenter Phase 1 clinical trial of intratumoral injections of MEDI1191 alone and in combination with the checkpoint inhibitor, durvalumab.
•Localized regenerative therapeutics: Our localized VEGF-A program, AZD8601, which is being developed by AstraZeneca, has completed a Phase 1a/b trial to describe its safety, tolerability, protein production, and activity in diabetic patients. The study has met its primary objectives of describing safety and tolerability and secondary objectives of demonstrating protein production and changes in blood flow post AZD8601 administration. We believe these data provide clinical proof of mechanism for our mRNA technology outside of the vaccine setting. AstraZeneca has initiated a Phase 2a study of AZD8601 for VEGF-A for ischemic heart disease in patients undergoing coronary artery bypass grafting (CABG) surgery with moderately impaired systolic function, and the trial is ongoing.
•Systemic intracellular therapeutics: We have four systemic intracellular therapeutics development candidates in our pipeline. Our intracellular programs address propionic acidemia, or PA (mRNA-3927), methylmalonic acidemia, or MMA (mRNA-3705), phenylketonuria, or PKU (mRNA-3283), and glycogen storage disorder type 1a, or GSD1a (mRNA-3745). We have an ongoing Phase 1 clinical trial for PA (mRNA-3927). MMA (mRNA-3705), PKU (mRNA-3283) and GSD1a (mRNA-3745) are currently in preclinical development.
Financial Operations Overview
Revenue
The following table summarizes revenue for each period presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
Revenue: | | | | | | | | |
| Product sales | | $ | 4,197 | | | $ | — | | | $ | 5,930 | | | $ | — | |
| Grant revenue | | 139 | | | 38 | | | 333 | | | 42 | |
| Collaboration revenue | | 18 | | | 29 | | | 28 | | | 33 | |
Total revenue | | $ | 4,354 | | | $ | 67 | | | $ | 6,291 | | | $ | 75 | |
We began to record product sales for our COVID-19 vaccine subsequent to its authorization for emergency use by the FDA and Health Canada in December 2020. For the three months ended June 30, 2021, we recognized $4.2 billion of product sales from our COVID-19 vaccine, of which $2.1 billion was generated in the United States and $2.1 billion was generated from the rest of the world. For the six months ended June 30, 2021, we recognized $5.9 billion of product sales from our COVID-19 vaccine, of which $3.4 billion was generated in the United States and $2.5 billion was generated from the rest of the world.
Other than product sales, our revenue has been primarily derived from government-sponsored and private organizations including BARDA, DARPA and the Gates Foundation and from strategic alliances with AstraZeneca, Merck and Vertex to discover, develop, and commercialize potential mRNA medicines.
Grant revenue was comprised as follows for the periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| Grant revenue: | | | | | | | |
BARDA (1) | $ | 134 | | | $ | 37 | | | $ | 326 | | | $ | 40 | |
| Other | 5 | | | 1 | | | 7 | | | 2 | |
| Total grant revenue | $ | 139 | | | $ | 38 | | | $ | 333 | | | $ | 42 | |
_______
(1) For the three months ended June 30, 2021, $132 million of BARDA grant revenue was related to our mRNA-1273 program and $2 million was related to our Zika vaccine program. For the six months ended June 30, 2021, $322 million of BARDA grant revenue was related to our mRNA-1273 program and $4 million was related to our Zika vaccine program.
Collaborative revenue from our strategic alliances was comprised as follows for the periods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
Collaboration revenue: | | | | | | | |
AstraZeneca | $ | 4 | | | $ | 16 | | | $ | 4 | | | $ | 17 | |
Merck | 4 | | | 11 | | | 4 | | | 12 | |
Vertex | 9 | | | 2 | | | 18 | | | 4 | |
| Other | 1 | | | — | | | 2 | | | — | |
Total collaboration revenue | $ | 18 | | | $ | 29 | | | $ | 28 | | | $ | 33 | |
We expect our product sales to significantly increase in 2021 compared to 2020. As of June 30, 2021, we had signed supply agreements of approximately $19.2 billion for the future supply of our COVID-19 vaccine through 2022 and had deferred revenue of $7.2 billion associated with customer deposits received or billable under these agreements. Additional supply agreements have been agreed upon since June 30, 2021, and others are under discussion for 2021 and 2022 deliveries. In addition, we expect to continue to receive funding from our contract with BARDA. As of June 30, 2021, the remaining available funding, net of revenue, earned under our agreement with BARDA for the development of our mRNA-1273 vaccine was $421 million. To the extent that existing or potential future products generate revenue, our revenue may vary due to many uncertainties in the independent development of our mRNA medicines and pursuant to our strategic alliances and other factors.
Cost of sales
Cost of sales includes raw materials, personnel and facility and other costs associated with manufacturing our commercial product. These costs include production materials, production costs at our manufacturing facilities, third-party manufacturing costs, and final formulation and packaging costs. Cost of sales also includes shipping costs and royalties payable to third parties based on sales of our products.
Research and development expenses
The nature of our business and primary focus of our activities generate a significant amount of research and development costs.
Research and development expenses represent costs incurred by us for the following:
•cost to develop our platform;
•discovery efforts leading to development candidates;
•preclinical, nonclinical, and clinical development costs for our programs;
•cost to develop our manufacturing technology and infrastructure; and
•digital infrastructure costs.
The costs above comprise the following categories:
•personnel-related expenses, including salaries, benefits, and stock-based compensation expense;
•expenses incurred under agreements with third parties, such as consultants, investigative sites, contract research organizations (CROs) that conduct our preclinical studies and clinical trials, and in-licensing arrangements;
•expenses associated with developing manufacturing capabilities and acquiring materials for preclinical studies and clinical trials, including both internal manufacturing and third-party contract manufacturing organizations (CMOs);
•expenses incurred for the procurement of materials, laboratory supplies, and non-capital equipment used in the research and development process; and
•facilities, depreciation, and amortization, and other direct and allocated expenses incurred as a result of research and development activities.
We use our employee and infrastructure resources for the advancement of our platform, and for discovering and developing programs. Due to the number of ongoing programs and our ability to use resources across several projects, indirect or shared operating costs incurred for our research and development programs are generally not recorded or maintained on a program- or modality-specific basis. The following table reflects our research and development expenses, including direct program-specific expenses summarized by modality and indirect or shared operating costs summarized under other research and development expenses during the three and six months ended June 30, 2021 and 2020 (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Program expenses by modality: | | | | | | | | |
| Prophylactic vaccines | | $ | 241 | | | $ | 36 | | | $ | 489 | | | $ | 45 | |
| Cancer vaccines | | 7 | | | 12 | | | 24 | | | 16 | |
| Intratumoral immuno-oncology | | 7 | | | 1 | | | 13 | | | 3 | |
| Systemic secreted and cell surface therapeutics | | 1 | | | — | | | 2 | | | 1 | |
| Systemic intracellular therapeutics | | 6 | | | 6 | | | 11 | | | 13 | |
Total program-specific expenses by modality (1) | | 262 | | | 55 | | | 539 | | | 78 | |
| Other research and development expenses: | | | | | | | | |
| Discovery programs | | 15 | | | 11 | | | 28 | | | 21 | |
| Platform research | | 27 | | | 18 | | | 52 | | | 40 | |
| Technical development and unallocated manufacturing expenses | | 53 | | | 29 | | | 86 | | | 58 | |
| Shared discovery and development expenses | | 49 | | | 24 | | | 88 | | | 43 | |
| Stock-based compensation | | 15 | | | 15 | | | 29 | | | 27 | |
| Total research and development expenses | | $ | 421 | | | $ | 152 | | | $ | 822 | | | $ | 267 | |
__________
(1)Includes a total of 30 and 23 development candidates at June 30, 2021 and 2020, respectively. Program-specific expenses include external costs and allocated manufacturing costs of pre-launch inventory, mRNA supply and consumables, and are reflected as of the beginning of the period in which the program was internally advanced to development or removed if development was ceased.
A “modality” refers to a group of programs with common product features and the associated combination of enabling mRNA technologies, delivery technologies, and manufacturing processes. The program-specific expenses by modality summarized in the table above include expenses we directly attribute to our programs, which consist primarily of external costs, such as fees paid to outside consultants, central laboratories, investigative sites, and CROs in connection with our preclinical studies and clinical trials, CMOs, and allocated manufacturing costs of inventory, mRNA supply and consumables. Costs to acquire and manufacture inventory, mRNA supply for preclinical studies and clinical trials are recognized and included in unallocated manufacturing expenses when incurred, and subsequently allocated to program-specific manufacturing costs after completion of the program-specific production. The timing of allocating manufacturing costs to the specific program varies depending on the program development and production schedule. We generally do not allocate personnel-related costs, including stock-based compensation, costs associated with our general platform research, technical development, and other shared costs on a program-specific basis. These costs were therefore excluded from the summary of program-specific expenses by modality.
Discovery program expenses are costs associated with research activities for our programs in the preclinical discovery stage, and primarily consist of external costs for CROs and lab services, and allocated manufacturing cost of preclinical mRNA supply and consumables.
Platform research expenses are mainly costs to develop technical advances in mRNA science, delivery science, and manufacturing process design. These costs include personnel-related costs, computer equipment, facilities, preclinical mRNA supply and consumables, and other administrative costs to support our platform research. Technology development and unallocated manufacturing expenses are primarily related to non-program-specific manufacturing process development and manufacturing costs.
Shared discovery and development expenses are research and development costs such as personnel-related costs and other costs, which are not otherwise included in development programs, discovery programs, platform research, technical development and unallocated manufacturing expenses, stock-based compensation, and other expenses.
The largest component of our total operating expenses has historically been our investment in research and development activities, including preclinical and clinical development of our product candidates, development of our platform, mRNA technologies, and manufacturing technologies. We expense research and development costs as incurred and cannot reasonably estimate the nature, timing, and estimated costs required to complete the development of the development candidates and investigational medicines we are currently developing or may develop in the future. There are numerous risks and uncertainties associated with the research and development of such development candidates and investigational medicines, including, but not limited to:
•scope, progress, and expense of developing ongoing and future development candidates and investigational medicines;
•entry in and completion of related preclinical studies;
•enrollment in and completion of subsequent clinical trials;
•safety and efficacy of investigational medicines resulting from these clinical trials;
•changes in laws or regulations relevant to the investigational medicines in development;
•receipt of the required regulatory approvals; and
•commercialization, including establishing manufacturing and marketing capabilities.
As we continue to progress mRNA-1273 through the development process toward a Biologics License Application approval, indication expansion of mRNA-1273 and variant-specific vaccine candidates during the current pandemic, we expect to continue to incur significant additional expenses. At this time, the magnitude of these potential expenditures is not known. In connection with the BARDA agreement to accelerate development of mRNA-1273, significant grant revenue and expenses are expected in 2021. BARDA's funding is expected to offset those expenses that are covered under the BARDA agreement, subject to our obtaining reimbursement from BARDA. As of June 30, 2021, the remaining available funding, net of revenue, earned was $421 million.
Changes in expectations or outcomes of any of the known or unknown risks and uncertainties may materially impact our expected research and development expenditures. Continued research and development is central to the ongoing activities of our business. Investigational medicines in later stages of clinical development, such as our CMV vaccine (mRNA-1647) and our COVID-19 vaccine, generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect our research and development costs to continue to increase in the foreseeable future as our investigational medicines progress through the development phases and identify and develop additional programs. There are numerous factors associated with the successful commercialization of any of our investigational medicines, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time due to the early stage of development of our investigational medicines. Moreover, future commercial and regulatory factors beyond our control will impact our clinical development programs and plans.
Selling, general and administrative expenses
We started to incur sales and marketing expenses in the fourth quarter of 2020 to prepare for commercial operations in connection with the sale of our COVID-19 vaccine. Selling, general and administrative expenses consist primarily of personnel-related costs, including stock-based compensation, for executives, finance, legal, human resources, business development and other administrative and operational functions, professional fees, accounting and legal services, sales and marketing, information technology and facility-related costs, and expenses associated with obtaining and maintaining intellectual property, or IP. These costs relate to the operation of the business, unrelated to the research and development function, or any individual program.
We anticipate selling, general and administrative expenses will increase as we continue to expand the number of programs in development and to establish our commercial activities both within and outside the United States. We have already incurred additional expenses related to building out a regulatory, sales and marketing team to support the sale, marketing and distribution of our COVID-19 vaccine. If we obtain regulatory approval for any of our other investigational medicines, and do not enter into one or more third-party commercialization collaboration and manufacturing arrangements, we will incur significant additional expenses related to building out these functions.
We have a broad IP portfolio covering our development and commercialization of mRNA vaccine and therapeutic programs, including those related to mRNA design, formulation, and manufacturing platform technologies. We regularly file patent applications to protect
innovations arising from our research and development. We also hold trademarks and trademark applications in the United States and foreign jurisdictions. Costs to secure and defend our IP are expensed as incurred and are classified as selling, general and administrative expenses.
Interest income
Interest income consists of interest generated from our investments in cash and cash equivalents, money market funds, and high-quality fixed income securities.
Other expense, net
Other expense, net consists of interest expense, gains (losses) from the sale of investments in marketable securities, foreign currency transaction and remeasurement gains (losses), gains (losses) on foreign currency balance sheet hedges, and other income and expense unrelated to our core operations. Interest expense is primarily derived from our finance leases related to our Moderna Technology Center, and certain contract manufacturing service agreements.
We expect to continue to incur significant expenses as we continue our research and development and commercialization efforts. We expect our programs to mature and advance to later stage clinical development, and we expect expenses to increase as we seek regulatory approvals for our investigational medicines and commercialize any approved mRNA medicines. If we fail to sustain profitability on a continuing basis, we may incur losses in the future.
Critical accounting policies and significant judgments and estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our condensed consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these condensed consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets, liabilities, revenues, and expenses and the disclosure of contingent assets and liabilities in our condensed consolidated financial statements. We base our estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts, and experience. The effects of material revisions in estimates, if any, are reflected in the condensed consolidated financial statements prospectively from the date of change in estimates.
There have been no material changes in our critical accounting policies and estimates in the preparation of our condensed consolidated financial statements during the three months ended June 30, 2021 compared to those disclosed in our 2020 Form 10-K.
Recently issued accounting pronouncements
We have reviewed all recently issued standards and have determined that such standards will not have a material impact on our financial statements or do not otherwise apply to our operations.
Results of operations
The following table summarizes our condensed consolidated statements of operations for each period presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Change 2021 vs. 2020 |
| 2021 | | 2020 | | $ | | % |
Revenue: | | | | | | | |
| Product revenue | $ | 4,197 | | | $ | — | | | $ | 4,197 | | | 100% |
| Grant revenue | 139 | | | 38 | | | 101 | | | 266% |
| Collaboration revenue | 18 | | | 29 | | | (11) | | | (38)% |
Total revenue | 4,354 | | | 67 | | | 4,287 | | | 6,399% |
Operating Expenses: | | | | | | | |
| Cost of sales | 750 | | | — | | | 750 | | | 100% |
| Research and development | 421 | | | 152 | | | 269 | | | 177% |
| Selling, general and administrative | 121 | | | 37 | | | 84 | | | 227% |
Total operating expenses | 1,292 | | | 189 | | | 1,103 | | | 584% |
| Income (loss) from operations | 3,062 | | | (122) | | | 3,184 | | | 2,610% |
Interest income | 3 | | | 7 | | | (4) | | | (57)% |
| Other expense, net | (2) | | | (2) | | | — | | | —% |
| Income (loss) before income taxes | 3,063 | | | (117) | | | 3,180 | | | 2,718% |
| Provision for income taxes | 283 | | | — | | | 283 | | | 100% |
| Net income (loss) | $ | 2,780 | | | $ | (117) | | | $ | 2,897 | | | 2,476% |
| | | | | | | | | | | | | | | | | | | | | | | |
| Six Months Ended June 30, | | Change 2021 vs. 2020 |
| 2021 | | 2020 | | $ | | % |
Revenue: | | | | | | | |
| Product revenue | $ | 5,930 | | | $ | — | | | $ | 5,930 | | | 100% |
| Grant revenue | 333 | | | 42 | | | 291 | | | 693% |
| Collaboration revenue | 28 | | | 33 | | | (5) | | | (15)% |
Total revenue | 6,291 | | | 75 | | | 6,216 | | | 8,288% |
Operating Expenses: | | | | | | | |
| Cost of sales | 943 | | | — | | | 943 | | | 100% |
| Research and development | 822 | | | 267 | | | 555 | | | 208% |
| Selling, general and administrative | 198 | | | 61 | | | 137 | | | 225% |
Total operating expenses | 1,963 | | | 328 | | | 1,635 | | | 498% |
| Income (loss) from operations | 4,328 | | | (253) | | | 4,581 | | | 1,811% |
Interest income | 7 | | | 15 | | | (8) | | | (53)% |
| Other expense, net | (12) | | | (3) | | | (9) | | | 300% |
| Income (loss) before income taxes | 4,323 | | | (241) | | | 4,564 | | | 1,894% |
| Provision for income taxes | 322 | | | — | | | 322 | | | 100% |
| Net income (loss) | $ | 4,001 | | | $ | (241) | | | $ | 4,242 | | | 1,760% |
Revenue
Total revenue increased by $4.3 billion for the three months ended June 30, 2021, compared to the same period in 2020, due to increases in product sales and grant revenue. Product revenue was $4.2 billion for the three months ended June 30, 2021 from sales of our COVID-19 vaccine. For the three months ended June 30, 2021, we delivered approximately 126 million doses to the U.S. government and approximately 73 million doses to other governments of our COVID-19 vaccine. We did not have product sales until December 2020. Grant revenue increased by $101 million for the three months ended June 30, 2021, compared to the same period in 2020, mainly driven by an increase in revenue from BARDA related to our mRNA-1273 vaccine development.
Total revenue increased by $6.2 billion, for the six months ended June 30, 2021, compared to the same period in 2020, due to increases in product sales and grant revenue. Product revenue was $5.9 billion for the six months ended June 30, 2021 from sales of
our COVID-19 vaccine. For the six months ended June 30, 2021, we delivered approximately 215 million doses to the U.S. government and approximately 87 million doses to other governments. We did not have product sales until December 2020. Grant revenue increased by $291 million for the six months ended June 30, 2021, compared to the same period in 2020, primarily driven by an increase in revenue from BARDA related to our mRNA-1273 vaccine development.
Operating expenses
Cost of sales
We began capitalizing our COVID-19 vaccine inventory costs in December 2020, in connection with an EUA from the FDA, and based upon our expectation that these costs would be recoverable through commercialization of our COVID-19 vaccine. Prior to the capitalization of our COVID-19 vaccine inventory costs, such costs were recorded as research and development expenses in the period incurred. We expensed $242 million of pre-launch inventory costs in 2020. Our cost of sales was $750 million, or 18%, of our product sales, for the three months ended June 30, 2021, including third-party royalties of $148 million. Our cost of sales was $943 million, or 16%, of our product sales, for the six months ended June 30, 2021, including third-party royalties of $232 million. A portion of the inventory costs associated with our product sales for the six months ended June 30, 2021 was expensed previously. At the end of the first quarter of 2021, we had substantially utilized our zero-cost COVID-19 vaccine inventory. If inventory sold for the six months ended June 30, 2021 was valued at cost, our cost of sales for the period would have been $1.1 billion, or 19%, of our product sales. We expect that our cost of sales as a percentage of product sales will remain at a similar level for the second half of 2021.
Research and development expenses
Research and development expenses increased by $269 million, or 177%, for the three months ended June 30, 2021, compared to the same period in 2020. The increase was primarily attributable to an increase in clinical trial expenses of $217 million, and an increase in personnel, technology and facility-related costs of $24 million.
Research and development expenses increased by $555 million, or 208%, for the six months ended June 30, 2021, compared to the same period in 2020. The increase was primarily attributable to an increase in clinical trial expenses of $436 million, an increase in personnel-related costs of $37 million, an increase in manufacturing expenses related to our clinical trials of $30 million, and an increase in consulting and outside services of $29 million.
These increases for both the three and six month periods in 2021 were largely driven by increased mRNA-1273 clinical development and headcount.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by $84 million, or 227%, for the three months ended June 30, 2021, compared to the same period in 2020. The increase was mainly due to an increase in consulting and outside services of $33 million, an increase in personnel-related costs of $20 million, and an increase in marketing expenses of $15 million.
Selling, general and administrative expenses increased by $137 million, or 225%, for the six months ended June 30, 2021, compared to the same period in 2020. The increase was mainly due to an increase in consulting and outside services of $50 million, an increase in marketing, legal and licensing expenses of $32 million, and an increase in personnel-related costs of $31 million.
These increases for both the three and six month periods in 2021 were primarily driven by our COVID-19 vaccine commercialization-related activities and increased headcount.
Interest income
Interest income decreased by $4 million, or 57%, for the three months ended June 30, 2021, compared to the same period in 2020. Interest income decreased by $8 million, or 53%, for the six months ended June 30, 2021, compared to the same period in 2020. The decreases in interest income from our investments in marketable securities for both the three and six month periods in 2021 were mainly driven by an overall lower interest rate environment, partially offset by increased investment balances.
Other expense, net
The following table summarizes other expense, net for each period presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Change 2021 vs. 2020 |
| 2021 | | 2020 | | $ | | % |
| Gain on investments | $ | 2 | | | $ | 1 | | | $ | 1 | | | 100% |
| Interest expense | (5) | | | $ | (3) | | | (2) | | | 67% |
| Other income, net | 1 | | | — | | | 1 | | | 100% |
| Total other expense, net | $ | (2) | | | $ | (2) | | | $ | — | | | —% |
| | | | | | | | | | | | | | | | | | | | | | | |
| Six Months Ended June 30, | | Change 2021 vs. 2020 |
| 2021 | | 2020 | | $ | | % |
| Gain on investments | $ | 2 | | | $ | 1 | | | $ | 1 | | | 100% |
| Interest expense | (8) | | | $ | (4) | | | (4) | | | 100% |
| Other expense, net | (6) | | | — | | | (6) | | | 100% |
| Total other expense, net | $ | (12) | | | $ | (3) | | | $ | (9) | | | 300% |
Total other expense, net remained flat for the three months ended June 30, 2021, compared to the same period in 2020. Total other expense, net increased by $9 million, or 300%, for the six months ended June 30, 2021, compared to the same period in 2020. The increase in other expense, net for the six-month period in 2021 was primarily due to losses on foreign currency transactions and remeasurements, partially offset by our balance sheet hedge activities. Our interest expense is primarily related to our finance leases.
Income taxes
Our provision for income taxes for the three and six months ended June 30, 2021 was $283 million and $322 million, respectively, as compared to an insignificant amount for the same periods in 2020. Our effective tax rate for the three and six months ended June 30, 2021 was lower than the U.S. statutory rate primarily due to the benefit related to the release of the valuation allowance on the majority of our tax attributes, the benefit of the foreign derived intangible income deduction and other deferred tax assets, as well as a discrete item for excess tax benefits related to stock-based compensation. Our effective tax rate for the three and six months ended months ended June 30, 2020 was lower than the U.S. statutory rate primarily due to the valuation allowance.
On a periodic basis, we reassess any valuation allowances that we maintain on our deferred tax assets, weighing positive and negative evidence to assess the recoverability of the deferred tax assets. In the first quarter of 2021, we reassessed the valuation allowance noting the increase in positive evidence, including significant revenue growth, expectations regarding future profitability, and successful supply chain and manufacturing capabilities to meet global product demand. After assessing both the positive evidence and negative evidence, we determined it was more likely than not that we will realize the majority of our deferred tax assets. Therefore, in the first quarter of 2021, we released our valuation allowance on the majority of our federal and state net operating losses and other deferred tax assets through the annual effective tax rate (AETR) as income is earned, resulting in a reduction in the AETR. In addition, we have recorded a discrete benefit of $49 million related to the deferred tax assets that we expect to utilize in future years. As of June 30, 2021, we continue to maintain a valuation allowance on certain state tax attributes.
Liquidity and capital resources
As of June 30, 2021, we had cash, cash equivalents and investments of $12.2 billion. Cash, cash equivalents and investments are invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Investments, consisting primarily of government and corporate debt securities, are stated at fair value. As of June 30, 2021, we had current and non-current investments of approximately $2.4 billion and $4.2 billion, respectively.
We historically funded our operations primarily from the sale of equity instruments and from proceeds from certain strategic alliance arrangements and grant agreements. Starting in August 2020, we have entered into supply agreements with the U.S. Government and several governments outside the United States for the supply of our COVID-19 vaccine and receive upfront deposits. As of June 30, 2021, we had $7.2 billion in deferred revenue related to customer deposits received or billable. In addition, as of June 30, 2021, BARDA has committed to fund up to $1.3 billion for the clinical development and advancement of mRNA-1273 to FDA licensure and the scale-up of manufacturing processes of our COVID-19 vaccine. As of June 30, 2021, the remaining available funding from BARDA, net of revenue earned was $421 million.
We continue to work toward the large-scale technical development, manufacturing scale-up in several countries and larger scale deployment of our COVID-19 vaccine. To support the scale-up, we have expended and will need to continue to expend significant resources and capital.
Cash flow
The following table summarizes the primary sources and uses of cash for each period presented (in millions):
| | | | | | | | | | | |
| Six Months Ended June 30, |
| 2021 | | 2020 |
Net cash provided by (used in): | | | |
Operating activities | $ | 7,034 | | | $ | (130) | |
Investing activities | (4,058) | | | (303) | |
Financing activities | 2 | | | 1,959 | |
| Net increase in cash, cash equivalents and restricted cash | $ | 2,978 | | | $ | 1,526 | |
Operating activities
We derive cash flows from operations primarily from cash collected from customer deposits and accounts receivable related to our COVID-19 vaccine supply agreements as well as certain government-sponsored and private organizations and strategic alliances. Our cash flows from operating activities are significantly affected by our use of cash for operating expenses and working capital to support the business. Prior to 2020, we historically experienced negative cash flows from operating activities as we have invested in mRNA technologies, development pipeline, digital infrastructure, manufacturing technology and infrastructure.
Net cash provided by operating activities for the six months ended June 30, 2021 was $7.0 billion and consisted of net income of $4.0 billion and non-cash adjustments of $90 million, plus a net change in assets and liabilities of $2.9 billion. Non-cash items included depreciation and amortization of $84 million, deferred income taxes of $72 million, stock-based compensation of $65 million, and amortization of investment premium and discount of $13 million. The net change in assets and liabilities was mainly due to an increase in deferred revenue of $3.4 billion, an increase in other liabilities of $440 million, an increase in accrued liabilities of $367 million and an increase in accounts payable of $44 million, partially offset by an increase in accounts receivable of $629 million, an increase in inventory of $596 million, and an increase in prepaid expenses and other assets of $110 million.
Net cash used in operating activities for the six months ended June 30, 2020 was $130 million and consisted of net loss of $241 million and non-cash adjustments of $62 million, plus a net change in assets and liabilities of $49 million. Non-cash items primarily included stock-based compensation of $45 million and depreciation and amortization of $15 million. The net change in assets and liabilities was due to an increase in deferred revenue of $51 million, an increase in accrued liabilities of $20 million, an increase in operating lease liabilities of $14 million, an increase in accounts payable of $12 million and an increase in other liabilities of $4 million, partially offset by an increase in accounts receivable of $28 million, an increase in right-of-use assets related to operating leases of $12 million, and an increase in prepaid expenses and other assets of $12 million.
Investing activities
Our primary investing activities consist of purchases, sales, and maturities of our investments and capital expenditures for manufacturing, laboratory, computer equipment and software.
Net cash used in investing activities for the six months ended June 30, 2021 was $4.1 billion, which included purchases of marketable securities of $6.6 billion and purchases of property and equipment of $65 million, partially offset by proceeds from sales of marketable securities of $1.7 billion and proceeds from maturities of marketable securities of $860 million.
Net cash used in investing activities for the six months ended June 30, 2020 was $303 million, which included purchases of marketable securities of $903 million and purchases of property and equipment of $25 million, partially offset by proceeds from maturities of marketable securities of $517 million and proceeds from sales of marketable securities of $108 million.
Financing activities
We generated cash from financing activities of $2 million for the six months ended June 30, 2021, primarily from net proceeds from the issuance of common stock in connection with the exercise of stock options and employee stock purchases under our equity plans of $64 million, partially offset by changes in financing lease liabilities of $62 million.
We generated cash from financing activities of $2.0 billion for the six months ended June 30, 2020, primarily from net proceeds from equity offerings of $1.9 billion and net proceeds from the issuance of common stock in connection with the exercise of stock options under our equity plans of $106 million.
Operation and funding requirements
From our inception to the end of 2020, we incurred significant losses and negative cash flows from operations due to our significant research and development expenses. We generated net income in the first half of 2021 in connection with our product sales. We have retained earnings of $1.8 billion as of June 30, 2021. We expect our expenses to increase in connection with our ongoing activities, particularly as we continue research and development of our development candidates and clinical activities for our investigational medicines. We also expect our expenses to increase associated with manufacturing costs, including our arrangements with our international supply and manufacturing partners. Our ongoing work on mRNA-1273, including development of any new generations of boosters and vaccines against variants of SARS-CoV-2, will require significant cash outflows during 2021, most of which may not be reimbursed or otherwise paid for by our partners or collaborators.
We believe that our cash, cash equivalents, and investments as of June 30, 2021, together with cash expected to be generated from operations, will be sufficient to enable us to fund our projected operations, capital expenditures and stock repurchases through at least the next 12 months from the issuance of these financial statements. We are subject to all the risks related to the development and commercialization of novel medicines, and we may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors including expenses related to the ongoing coronavirus pandemic, which may adversely affect our business. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
If we are unable to sustain profitability on a continuing basis, we may be required to finance future cash needs through a combination of public or private equity offerings, structured financings and debt financings, government funding arrangements, potential future strategic alliances from which we receive upfront fees, milestone payments, and other forms of consideration, and marketing, manufacturing, distribution and licensing arrangements. If we are required to finance future cash needs, additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back, or discontinue the development or commercialization of one or more of our investigational medicines, or slow down or cease work on one or more of our programs. If we raise additional funds through the issuance of additional equity or debt securities, it could result in dilution to our existing stockholders or increased fixed payment obligations, and any such securities may have rights senior to those of our common stock. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise funds through strategic alliances or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs, or investigational medicines or grant licenses on terms that may not be favorable to us. Any of these events could significantly harm our business, financial condition, and prospects.
Contractual Obligations
As of June 30, 2021, other than disclosed at Note 11 and Note 12 to our condensed consolidated financial statements, there have been no material changes to our contractual obligations and commitments from those described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in our 2020 Form 10-K.
Off balance sheet arrangements
As of June 30, 2021, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
As of June 30, 2021 and December 31, 2020, we had cash, cash equivalents, and investments in marketable securities of $12.2 billion and $5.2 billion, respectively. Our investment portfolio is comprised of money market funds and marketable debt securities (including U.S. Treasury securities, debt securities of U.S. government agencies and corporate entities, and commercial paper), which are classified as available-for-sale securities. Our primary investment objectives are the preservation of capital and the maintenance of liquidity and our investment policy defines allowable investments based on quality of the institutions and financial instruments designed to minimize risk exposure. Our exposure to interest rate sensitivity is affected by changes in the general level of U.S. interest rates. Our available-for-sale securities are subject to interest rate risk and will fall in value if market interest rates increase. We generally hold investments in marketable debt securities to maturity to limit our exposure to interest rate risk. Due to the short-term maturities and low risk profiles of our investments, we do not anticipate a significant exposure to interest rate risk. If market interest rates were to increase immediately and uniformly by one percentage point from levels at June 30, 2021, the net fair value of our marketable securities would decrease by approximately $98 million.
Foreign Currency Risk
Our revenue generating activities and operations have been primarily denominated in U.S. dollars. As we expand internationally, our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. To help manage the exposure to foreign currency exchange rate fluctuations, we have implemented cash flow hedging and balance sheet hedging programs.
Cash Flow Hedging Activities
We hedge foreign currency product sales denominated in Euros, including the use of foreign exchange forward contracts or purchased options. We hedge our cash flow exposures to reduce the risk that our earnings and cash flows will be adversely affected by changes in exchange rates. These transactions are designated and qualify as cash flow hedges. Our foreign exchange contracts at June 30, 2021, carried at fair value, had maturities of up to six months.
Balance Sheet Hedging Activities
We use foreign currency forward contracts to mitigate foreign currency exchange risk associated with foreign currency-denominated monetary assets and liabilities. These contracts reduce the impact of currency exchange rate movements on our assets and liabilities. As of June 30, 2021, our outstanding balance sheet hedging derivatives, carried at fair value, had maturities of less than three months.
We enter into these foreign exchange contracts to hedge forecasted revenue in the normal course of business and accordingly, they are not speculative in nature. We believe the counterparties to our foreign currency forward contracts are creditworthy multinational commercial banks. While we believe the risk of counterparty nonperformance is not material, a sustained decline in the financial stability of financial institutions as a result of disruption in the financial markets could affect our ability to secure creditworthy counterparties for our foreign currency hedging programs.
Notwithstanding our efforts to mitigate some foreign currency exchange risks, there can be no assurance that our hedging activities will adequately protect us against the risks associated with foreign currency fluctuations. As of June 30, 2021, a hypothetical adverse movement of 10 percent in foreign currency exchange rates compared to the U.S. dollars across all maturities would have resulted in potential declines in the fair value on our foreign currency forward contracts used in cash flow hedging of approximately $95 million. As of June 30, 2021, a hypothetical adverse movement of 10 percent in foreign currency exchange rates compared to the U.S. dollars across all maturities would have resulted in potential declines in the fair value on our foreign currency forward contracts used in balance sheet hedging of approximately $6 million. We expect that any increase or decrease in the fair value of the balance sheet hedging portfolio would be substantially offset by increases or decreases in the underlying exposures being hedged.
Item 4. Controls and Procedures
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of June 30, 2021. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, or the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of June 30, 2021, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended June 30, 2021, which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, believes that our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving their objectives and are effective at the reasonable assurance level. However, our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by the collusion of two or more people or by a management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
PART II
Item 1. Legal Proceedings
From time to time, we may be subject to legal proceedings and claims in the ordinary course of business. We are not currently a party to any material legal proceedings.
Item 1A. Risk Factors
Investing in our common stock involves a high degree of risk. Information regarding risk and uncertainties related to our business appears in Part I, Item 1A. “Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2020, which was filed with the Securities and Exchange Commission, or the SEC, on February 26, 2021. There have been no material changes from the risk factors previously disclosed in the Annual Report on Form 10-K. You should carefully consider the risks and uncertainties described below, together with all of the other information contained in this Quarterly Report on Form 10-Q, including “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the condensed consolidated financial statements and the related notes. If any of the risks actually occur, it could harm our business, prospects, operating results and financial condition and future prospects. In such event, the market price of our common stock could decline and you could lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business
operations. This Quarterly Report on Form 10-Q also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this Quarterly Report.
Item 6. Exhibits
The Exhibits listed below are filed or incorporated by reference as part of this Form 10-Q.
| | | | | | | | |
| Exhibit No. | | Exhibit Index |
| | |
| 10.1*† | | Amendment Nos. P00004, P00005, P00006, P00007, P00008, P00009, P00010, P00011 and P00012 to Award Contract No. W911QY20C0100, by and between Moderna US Inc. and the Army Contracting Command of the U.S. Department of Defense, dated June 15, 2021 |
| 10.2*† | | |
| | |
| | |
| | |
| | |
| 31.1* | | |
| 31.2* | | |
| 32.1+ | | |
| 101.INS* | | XBRL Instance Document - The instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
| 101.SCH* | | XBRL Taxonomy Extension Schema Document |
| 101.CAL* | | XBRL Taxonomy Extension Calculation Document |
| 101.DEF* | | XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB* | | XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE* | | XBRL Taxonomy Extension Presentation Link Document |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101.*) |
| | | | | |
| † | Portions of this exhibit (indicated by asterisks) have been omitted in accordance with the rules of the Securities and Exchange Commission. |
+
| The certification furnished in Exhibit 32.1 hereto is deemed to accompany this Form 10-Q and will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. Such certification will not be deemed to be incorporated by reference into any filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Registrant specifically incorporates it by reference. |
| |
SIGNATURES
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| | | MODERNA, INC. |
| | | |
| Date: | | By: | /s/ Stéphane Bancel |
| August 5, 2021 | | | |
| | | Stéphane Bancel |
| | | Chief Executive Officer and Director |
| | | (Principal Executive Officer) |
| | | |
| Date: | | By: | /s/ David W. Meline |
| August 5, 2021 | | | |
| | | David W. Meline |
| | | Chief Financial Officer |
| | | (Principal Financial Officer) |
| | | |