UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
(Mark One)
|
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE QUARTERLY PERIOD ENDED
or
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
Commission file number:
(Exact name of registrant as specified in its charter)
|
|
|
|
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
|
|
|
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
(
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
|
|
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
Accelerated filer |
|
☒ |
|
|
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
☐ |
Smaller reporting company |
|
|
|
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
As of August 3, 2020, the registrant had
CUE BIOPHARMA, INC.
TABLE OF CONTENTS
2
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
Cue Biopharma, Inc.
Consolidated Balance Sheets
(Unaudited in thousands, except share amounts)
|
|
|
June 30, 2020 |
|
|
December 31, 2019 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
|
|
|
$ |
|
|
|
Marketable securities |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets |
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
|
|
|
|
|
|
|
Operating lease right-of-use |
|
|
|
|
|
|
|
|
|
Deposits |
|
|
|
|
|
|
|
|
|
Restricted cash, long term |
|
|
|
|
|
|
|
|
|
Other long term assets |
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
|
|
|
$ |
|
|
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
|
|
|
$ |
|
|
|
Accrued expenses |
|
|
|
|
|
|
|
|
|
Research and development contract liability, current portion |
|
|
|
|
|
|
|
|
|
Operating lease liability, current portion |
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
|
|
|
|
|
|
|
Research and development contract liability, net of current portion |
|
|
|
|
|
|
|
|
|
Operating lease liability, net of current portion |
|
|
|
|
|
|
|
|
|
Total liabilities |
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
Preferred Stock, $ outstanding at June 30, 2020 and December 31, 2019, respectively |
|
|
|
|
|
|
|
|
|
Common stock, $ and December 31, 2019, respectively |
|
|
|
|
|
|
|
|
|
Additional paid in capital |
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income/ (loss) |
|
|
|
|
|
|
( |
) |
|
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
|
Total stockholders’ equity |
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity |
|
$ |
|
|
|
$ |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
3
Cue Biopharma, Inc.
Consolidated Statements of Operations and Other Comprehensive Loss
(Unaudited in thousands, except share and per share amounts)
|
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
Collaboration revenue |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Other income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (expense) income |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Total other income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss Before Income Taxes |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Income Tax Expense |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
Unrealized gains (losses) from available-for-sale securities |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
Net loss per common share – basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
Weighted average common shares outstanding – basic and diluted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
4
Cue Biopharma, Inc.
Consolidated Statements of Stockholders’ Equity
(Unaudited in thousands, except share and per share amounts
|
For the three months ended June 30, 2020 and 2019: |
|
|||||||||||||||||||||||
|
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated Other |
|
|
|
|
|
|
Total |
|
||||||||
|
|
|
Shares |
|
|
Par Value |
|
|
Paid-in Capital |
|
|
Comprehensive Income |
|
|
Accumulated Deficit |
|
|
Stockholders’ Equity |
|
||||||
|
Balance, April 1, 2019 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
|
Issuance of common stock from public offerings, net of underwriter discounts |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Exercise of stock options |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Unrealized gains from available-for-sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Balance at June 30, 2019 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, April 1, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
Issuance of common stock from public offerings, net of underwriter discounts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Exercise of stock options |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Issuance of common stock upon exercise of warrants, net |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Restricted stock awards released |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Restricted stock withheld to cover taxes |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Unrealized losses from available-for-sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Balance at June 30, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the six months ended June 30, 2020 and 2019: |
|
|||||||||||||||||||||||
|
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated Other |
|
|
|
|
|
|
Total |
|
||||||||
|
|
|
Shares |
|
|
Par Value |
|
|
Paid-in Capital |
|
|
Comprehensive Income |
|
|
Accumulated Deficit |
|
|
Stockholders’ Equity |
|
||||||
|
Balance, December 31, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
Common stock issued in initial public offering |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Costs incurred in connection with initial public offering |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Common stock to be issued pursuant to license agreements |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Proceeds from sale of placement agent warrants |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Fair value of warrants issued in connection with initial public offering of common stock |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Exercise of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Balance, December 31, 2018 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
|
Issuance of common stock from public offerings, net of underwriter discounts |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Exercise of stock options |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Unrealized gains from available-for-sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Balance at June 30, 2019 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
Issuance of common stock from public offerings, net of underwriter discounts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Exercise of stock options |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Issuance of common stock upon exercise of warrants, net |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Restricted stock awards released |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Restricted stock withheld to cover taxes |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Unrealized gains from available-for-sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Balance at June 30, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
See accompanying notes to consolidated financial statements.
5
Cue Biopharma, Inc.
Consolidated Statements of Cash Flows
(Unaudited in thousands)
|
|
|
Six Months Ended June 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
|
|
|
|
|
|
|
Change in operating lease right-of-use asset |
|
|
( |
) |
|
|
|
|
|
Other non cash income |
|
|
— |
|
|
|
( |
) |
|
Amortization of premium/discount on purchased securities |
|
|
|
|
|
|
( |
) |
|
Loss on disposal of fixed asset |
|
|
— |
|
|
|
|
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Account receivable |
|
|
( |
) |
|
|
( |
) |
|
Prepaid expenses and other current assets |
|
|
( |
) |
|
|
( |
) |
|
Operating lease liability |
|
|
|
|
|
|
( |
) |
|
Accounts payable |
|
|
|
|
|
|
|
|
|
Accrued expenses |
|
|
|
|
|
|
|
|
|
Research and development contract liability |
|
|
( |
) |
|
|
|
|
|
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
( |
) |
|
|
( |
) |
|
Cash received from sale of fixed asset |
|
|
— |
|
|
|
|
|
|
Redemption of short term investments |
|
|
— |
|
|
|
|
|
|
Purchases of marketable securities |
|
|
( |
) |
|
|
— |
|
|
Net cash (used in) provided by investing activities |
|
|
( |
) |
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from the public offering of common stock, net of underwriter's commission and fees |
|
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
|
|
|
|
|
|
|
|
Restricted stock buy-back at vesting to cover taxes |
|
|
( |
) |
|
|
— |
|
|
Net cash provided by financing activities |
|
|
|
|
|
|
|
|
|
Net increase in cash, cash equivalents, and restricted cash |
|
|
|
|
|
|
|
|
|
Cash, cash equivalents, and restricted cash at beginning of period |
|
|
|
|
|
|
|
|
|
Cash, cash equivalents, and restricted cash at end of period |
|
$ |
|
|
|
$ |
|
|
|
Supplemental disclosures of non-cash investing activities: |
|
|
|
|
|
|
|
|
|
Income Taxes |
|
$ |
— |
|
|
$ |
( |
) |
The accompanying notes are an integral part of these consolidated financial statements.
6
Cue Biopharma, Inc.
Notes to Consolidated Financial Statements (Unaudited)
For the three and six months ended June 30, 2020 and 2019
|
1. |
Organization and Basis of Presentation |
Cue Biopharma, Inc. (the “Company”) was incorporated in the State of Delaware on
The Company is a clinical-stage biopharmaceutical company engineering a novel class of injectable biologics designed to selectively engage and modulate targeted T cells within the body to treat a broad range of cancers, chronic infectious diseases, and autoimmune disorders.
The Company is in the development stage and has incurred recurring losses and negative cash flows from operations. As of June 30, 2020, the Company had unrestricted cash, cash equivalents, and marketable securities of approximately $
|
2. |
Summary of Significant Accounting Policies |
Basis of Presentation
The accompanying unaudited consolidated financial statements as of June 30, 2020, and for the three and six months ended June 30, 2020, and 2019, have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission (the “SEC”) and Generally Accepted Accounting Principles in the United States (“U.S. GAAP”) for financial information, which prescribes elimination of all significant intercompany accounts and transactions in the accounts of the Company and its wholly owned subsidiary, Cue Biopharma Securities Corporation, Inc., which was formed in December 2018 and incorporated in the Commonwealth of Massachusetts. In the opinion of management, these financial statements reflect all adjustments which are necessary for a fair statement of the Company’s financial position and results of its operations, as of and for the periods presented. These financial statements should be read in conjunction with the financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K filed with the SEC on March 12, 2020, as amended on Form 10-K/A filed with the SEC on March 12, 2020 and April 29, 2020.
Interim results for the three and six months ended June 30, 2020 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2020, or any future periods.
7
Public Offerings
In March 2020, the Company entered into an “at-the-market” (ATM) equity offering sales agreement (the “March 2020 ATM Agreement”) with Stifel Nicolaus & Company, Inc. (“Stifel”) to sell shares of the Company’s common stock for aggregate gross proceeds of up to $
.
In June 2020, the Company entered into another ATM equity offering sales agreement with Stifel (the “June 2020 ATM Agreement”) to sell shares of the Company’s common stock for aggregate gross proceeds of up to $
Consolidation
The accompanying consolidated financial statements include the Company and its wholly owned subsidiary, Cue Biopharma Securities Corporation, Inc. The Company has eliminated all intercompany transactions.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Significant estimates include estimates related to collaboration revenue, the accounting for potential liabilities and accrued expenses, the assumptions utilized in valuing stock-based compensation issued for services, the realization of deferred tax assets, and the useful life with respect to long-lived assets and intangibles. Actual results could differ from those estimates.
The COVID-19 outbreak, which the World Health Organization has classified as a pandemic, has prompted governments and regulatory bodies throughout the world to issue “stay-at-home” or similar orders, and enact restrictions on the performance of “non-essential” services, public gatherings and travel.
The extent to which COVID-19 impacts the Company’s business and financial results will depend on numerous evolving factors including, but not limited to: the magnitude and duration of COVID-19, the extent to which it will impact worldwide macroeconomic conditions, the speed of the anticipated recovery, access to capital markets, and governmental and business reactions to the pandemic. The Company assessed certain accounting matters that generally require consideration of forecasted financial information in context with the information reasonably available to the Company and the unknown future impacts of COVID-19 as of June 30, 2020 and through the date of the filing of this Quarterly Report on Form 10-Q. The accounting matters assessed included, but were not limited to estimates related to collaboration revenue, the accounting for potential liabilities and accrued expenses, the assumptions utilized in valuing stock-based compensation issued for services, the realization of deferred tax assets, and assessments of impairment related to long-lived assets and intangibles. The Company’s future assessment of the magnitude and duration of COVID-19, as well as other factors, could result in additional material impacts to the Company’s consolidated financial statements in future reporting periods.
Despite the Company’s efforts, the ultimate impact of COVID-19 depends on factors beyond the Company’s knowledge or control, including the duration and severity of the outbreak, as well as third-party actions taken to contain its spread and mitigate its public health effects. As a result, the Company is unable to estimate the extent to which COVID-19 will negatively impact its financial results or liquidity.
8
Cash Concentrations
The Company maintains its cash balances with a financial institution in federally insured accounts and may periodically have cash balances in excess of insurance limits. The Company maintains its accounts with a financial institution with a high credit rating. The Company has not experienced any losses to date and believes that it is not exposed to any significant credit risk on cash.
Cash and Cash Equivalents
The Company considers all highly liquid investments with a maturity of three months or less at the date of purchase to be cash equivalents. The Company currently invests available cash in money market funds.
Marketable Securities
Marketable securities consist of investments with original maturities greater than ninety days and less than one year from the balance sheet date. The Company classifies all of its investments as available-for-sale securities. Accordingly, these investments are recorded at fair value, which is based on quoted market prices. Unrealized gains and losses are recognized and determined on a specific identification basis and are included in other comprehensive loss. Realized gains and losses are determined on a specific identification basis and are included in other income (loss) on the income statement. Amortization and accretion of discounts and premiums is recorded in interest income. The Company has invested available cash in United States Treasury obligations.
Restricted Cash
The Company had $
Property and Equipment
Property and equipment is recorded at cost. Major improvements are capitalized, while maintenance and repairs are charged to expense as incurred. Gains and losses from disposition of property and equipment are included in income and expense when realized. Amortization of leasehold improvements is provided using the straight-line method over the shorter of the lease term or the useful life of the underlying assets.
|
Laboratory equipment |
|
|
|
Computer and office equipment |
|
|
|
Furniture and fixtures |
|
|
The Company recognizes depreciation and amortization expense in general and administrative expenses and in research and development expenses in the Company’s statements of operations, depending on how each category of property and equipment is utilized in the Company’s business activities.
Trademark
Trademark consists of the Company’s right, title and interest to the CUE BIOLOGICS Mark, and any derivative mark incorporating CUE, throughout the world, together with all associated goodwill and common law rights appurtenant thereto, including, but not limited to, any right, title and interest in any corporate name, company name, business, name, trade name, dba, domain name, or other source identifier incorporating CUE.
The Company has classified the trademark as a component of other long-term assets, having a useful life of
9
Revenue Recognition
The Company recognizes collaboration revenue under certain of the Company’s license or collaboration agreements that are within the scope of ASC 606. The Company’s contracts with customers typically include promises related to licenses to intellectual property and research and development services. If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenue from non-refundable, up-front fees allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgement to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. Accordingly, the transaction price is generally comprised of a fixed fee due at contract inception and variable consideration in the form of milestone payments due upon the achievement of specified events and tiered royalties earned when customers recognize net sales of licensed products. The Company measures the transaction price based on the amount of consideration to which it expects to be entitled in exchange for transferring the promised goods and/or services to the customer. The Company utilizes the “most likely amount” method to estimate the amount of variable consideration, to predict the amount of consideration to which it will be entitled for its one open contract. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. At the inception of each arrangement that includes development and regulatory milestone payments, the Company evaluates whether the associated event is considered probable of achievement and estimates the amount to be included in the transaction price using the most likely amount method. Currently, the Company has one contract with an option to acquire additional goods and/or services in the form of additional research and development services for additional product candidates which it evaluated and determined was not a material right related to such agreement and so was not included in the transaction price.
Research and Development Expenses
Research and development expenses consist primarily of compensation costs, fees paid to consultants, outside service providers and organizations (including research institutes at universities), facility costs, and development and clinical trial costs with respect to the Company’s product candidates.
Research and development expenses incurred under contracts are expensed ratably over the life of the underlying contracts, unless the achievement of milestones, the completion of contracted work, or other information indicates that a different pattern of performance is more appropriate. Other research and development expenses are charged to operations as incurred.
Nonrefundable advance payments are recognized as an expense as the related services are performed. The Company evaluates whether it expects the services to be rendered at each quarter end and year end reporting date. If the Company does not expect the services to be rendered, the advance payment is charged to expense. Nonrefundable advance payments for research and development services are included in prepaid and other current assets on the balance sheet. To the extent that a nonrefundable advance payment is for contracted services to be performed within 12 months from the reporting date, such advance is included in current assets; otherwise, such advance is included in non-current assets.
The Company evaluates the status of its research and development agreements and contracts, and the carrying amount of the related assets and liabilities, at each quarter end and year end reporting date, and adjusts the carrying amounts and their classification on the balance sheet as appropriate.
Patent Expenses
The Company is the exclusive worldwide licensee of, and has patent applications pending for, numerous domestic and foreign patents. Due to the significant uncertainty associated with the successful development of one or more commercially viable product candidates based on the Company’s research efforts and any related patent applications, all patent costs, including patent-related legal fees, filing fees and other costs are charged to expense as incurred. For the three and six months ended June 30, 2020, patent expenses were $
Licensing Fees and Costs
Licensing fees and costs consist primarily of costs relating to the acquisition of the Company’s license agreement with the Albert Einstein College of Medicine (“Einstein”), including related royalties, maintenance fees, milestone payments and product development costs. Licensing fees and costs are charged to expense as incurred.
10
Long-Lived Assets
The Company reviews long-lived assets, consisting of property and equipment, for impairment at each fiscal year end or when events or changes in circumstances indicate the carrying value of these assets may exceed their current fair values. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized for the amount by which the carrying amount of the asset exceeds the fair value of the assets. Assets to be disposed of are separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell and are no longer depreciated. The Company has not historically recorded any impairment to its long-lived assets. In the future, if events or market conditions affect the estimated fair value to the extent that a long-lived asset is impaired, the Company will adjust the carrying value of these long-lived assets in the period in which the impairment occurs. During the three and six months ended June 30, 2020, there were
Leases
In February 2016, the FASB issued ASU 2016-02, Leases (ASC 842), which supersedes the existing guidance for lease accounting, Leases (Topic 840). ASU 2016-02 requires a lessee to record a right-of-use asset and a corresponding lease liability, for most lease arrangements on the balance sheet. Under the standard, disclosure of key information about leasing arrangements to assist users of the financial statements with assessing the amount, timing and uncertainty of cash flows arising from leases are required. The new standard is effective for fiscal years beginning after December 15, 2018.
The standard permits two transition methods, (1) to apply the new lease requirements at the beginning of the earliest period presented, or (2) to apply the new lease requirements at the effective date. The Company adopted ASC 842 as of January 1, 2019 using the effective date method, in which we did not restate prior periods. Upon adoption, the Company elected the package of practical expedients permitted under the transition guidance within ASC 842, which among other things, allowed it to carry forward the historical lease classification.
The adoption of ASC 842 on January 1, 2019 resulted in the recognition of approximately $
Stock-Based Compensation
The Company periodically issues stock based awards to officers, directors, employees, Scientific and Clinical Advisory Board members, non-employees and consultants for services rendered. Such issuances vest and expire according to terms established at the issuance date.
Stock-based payments to officers, directors, members of the Company’s Scientific and Clinical Advisory Board, non-employees and outside consultants and employees, including grants of employee stock options, are recognized in the financial statements based on their grant date fair values. Stock option grants, which are generally time-vested, are measured at the grant date fair value and charged to operations on a straight-line basis over the service period, which generally approximates the vesting term. The Company also grants performance-based awards periodically to officers of the Company. The Company recognizes compensation costs related to performance awards over the requisite service period if and when the Company concludes that it is probable that the performance condition will be achieved.
The fair value of stock options and restricted stock units is determined utilizing the Black-Scholes option-pricing model, which is affected by several variables, including the risk-free interest rate, the expected dividend yield, the life of the equity award, the exercise price of the stock option as compared to the fair value of the common stock on the grant date, and the estimated volatility of the common stock over the term of the equity award.
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. Until the Company has established a trading market for its common stock, estimated volatility is based on the average historical volatilities of comparable public companies in a similar industry. The expected dividend yield is based on the current yield at the grant date; the Company has never declared or paid dividends and has no plans to do so for the foreseeable future. As permitted by Staff Accounting Bulletin No. 107, due to the Company’s lack of trading history and option activity, management utilizes the simplified method to estimate the expected term of options at the date of grant. The exercise price is determined based on the fair value of the Company’s common stock at the date of grant. The Company accounts for forfeitures as they occur.
11
The Company recognizes the fair value of stock-based compensation in general and administrative expenses and in research and development expenses in the Company’s statements of operations, depending on the type of services provided by the recipient of the equity award.
Comprehensive Income (Loss)
Components of comprehensive income or loss, including net income or loss, are reported in the financial statements in the period in which they are recognized. Other comprehensive income or loss is defined as the change in equity during a period from transactions and other and other events and circumstances from non-owner sources. Net income (loss) and other comprehensive income (loss) are reported net of any related tax effect to arrive at comprehensive income (loss). Comprehensive income (loss) includes net income (loss) as well as changes in stockholders’ equity that result from transactions and economic events other than those with stockholders. The Company’s only element of other comprehensive income (loss) in all periods presented was unrealized gain or loss on available-for-sale securities.
Earnings (Loss) Per Share
The Company’s computation of earnings (loss) per share (“EPS”) for the respective periods includes basic and diluted EPS. Basic EPS is measured as the income (loss) attributable to common stockholders divided by the weighted average number of common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares that would result from the exercise of outstanding stock options and warrants as if they had been exercised at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. Basic and diluted loss per common share is the same for all periods presented because all outstanding stock options and warrants are anti-dilutive.
At June 30, 2020, the Company excluded the outstanding securities summarized below, which entitle the holders thereof to acquire shares of common stock, from its calculation of earnings per share, as their effect would have been anti-dilutive.
|
|
|
June 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Common stock warrants |
|
|
|
|
|
|
|
|
|
Common stock options |
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
|
|
|
Fair Value of Financial Instruments
The authoritative guidance with respect to fair value established a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three levels and requires that assets and liabilities carried at fair value be classified and disclosed in one of three categories, as presented below.
Level 1. Observable inputs such as quoted prices in active markets for an identical asset or liability that the Company has the ability to access as of the measurement date. Financial assets and liabilities utilizing Level 1 inputs include active-exchange traded securities and exchange-based derivatives.
Level 2. Inputs, other than quoted prices included within Level 1, which are directly observable for the asset or liability or indirectly observable through corroboration with observable market data. Financial assets and liabilities utilizing Level 2 inputs include fixed income securities, non-exchange-based derivatives, mutual funds, and fair-value hedges.
Level 3. Unobservable inputs in which there is little or no market data for the asset or liability which requires the reporting entity to develop its own assumptions. Financial assets and liabilities utilizing Level 3 inputs include infrequently traded non-exchange-based derivatives and commingled investment funds and are measured using present value pricing models.
The Company determines the level in the fair value hierarchy within which each fair value measurement falls in its entirety, based on the lowest level input that is significant to the fair value measurement in its entirety. In determining the appropriate levels, the Company performs an analysis of the assets and liabilities at each reporting period end.
The Company had approximately $
12
The carrying value of financial instruments (consisting of cash, a certificate of deposit, accounts payable, accrued compensation and accrued expenses) is considered to be representative of their respective fair values due to the short-term nature of those instruments.
Recent Accounting Pronouncements
In June 2016, the FASB issued Accounting Standard Update (ASU) No. 2016-13, Financial Instruments- Credit Losses: Measurement of Credit Losses on Financial Instruments (Topic 326) (CECL). The new standard requires entities to measure all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions and reasonable and supportable forecasts. The new standard is effective for annual reporting periods beginning after December 15, 2022, including interim reporting periods within each annual reporting period for smaller reporting companies. The Company is still evaluating the impact of ASU 2016-13 on the Company’s consolidated financial statements; however, it does not expect the impact to be material.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. The pronouncement is effective for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. ASU No. 2019-12 is effective for us beginning in fiscal 2021. We are currently in the process of evaluating the effects of this pronouncement on our financial statements.
Management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
|
3. |
Fair Value |
The Company accounts for its financial assets and liabilities using fair value measurements. The authoritative accounting guidance defines fair value, establishes a framework for measuring fair value under generally accepted accounting principles and enhances disclosures about fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
The following table presents information about the Company’s assets that are measured at fair value on a recurring basis as of June 30, 2020 and December 31, 2019, and indicate the level of the fair value hierarchy utilized to determine such fair value:
|
|
|
Fair Value Measurements as of June 30, 2020 |
|
|||||||||||||
|
|
|
(in thousands) |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Fair Value |
|
||||
|
Cash equivalents |
|
$ |
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
Marketable securities |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Total |
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements as of December 31, 2019 |
|
|||||||||||||
|
|
|
(in thousands) |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Fair Value |
|
||||
|
Cash equivalents |
|
$ |
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
Marketable securities |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Total |
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
As of June 30, 2020, the Company reported approximately $
The carrying values of accounts receivable, prepaid expenses, other current assets, accounts payable and accrued expenses approximate their fair value due to the short-term nature of these balances.
13
|
4. |
Marketable Securities |
As of June 30, 2020 and December 31, 2019, the fair value of available-for-sale marketable securities by type of security was as follows:
|
|
|
June 30, 2020 |
|
|||||||||||||
|
(In thousands) |
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Fair Value |
|
||||
|
U.S. Treasury Securities |
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2019 |
|
|||||||||||||
|
(In thousands) |
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Fair Value |
|
||||
|
U.S. Treasury Securities |
|
$ |
|
|
|
$ |
— |
|
|
$ |
( |
) |
|
$ |
|
|
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
( |
) |
|
$ |
|
|
At June 30, 2020, there were $
|
5. |
Property and Equipment |
Property and equipment as of June 30, 2020 and December 31, 2019 consisted of the following:
|
|
|
June 30, 2020 |
|
|
December 31, 2019 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Computer equipment |
|
$ |
|
|
|
$ |
|
|
|
Laboratory equipment |
|
|
|
|
|
|
|
|
|
Furniture and fixtures |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less: Accumulated depreciation |
|
|
( |
) |
|
|
( |
) |
|
Total property and equipment, net |
|
$ |
|
|
|
$ |
|
|
Depreciation expense for the six months ended June 30, 2020 and 2019 was approximately $
14
|
6. |
Stock-Based Compensation |
Stock Option Valuation
For stock options requiring an assessment of value during the six months ended June 30, 2020, the fair value of each stock option award was estimated using the Black-Scholes option-pricing model utilizing the following assumptions:
|
|
|
June 30, 2020 |
|
Risk-free interest rate |
|
|
|
Expected dividend yield |
|
|
|
Expected volatility |
|
|
|
Expected life |
|
|
|
|
|
|
|
|
|
June 30, 2019 |
|
Risk-free interest rate |
|
|
|
Expected dividend yield |
|
|
|
Expected volatility |
|
|
|
Expected life |
|
|
A summary of stock option activity for the six months ended June 30, 2020 is as follows:
|
|
|
Number of Shares |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Contractual Life (in Years) |
|
|||
|
Stock options outstanding at December 31, 2019 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Stock options outstanding at June 30, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock options exercisable at June 30, 2020 |
|
|
|
|
|
$ |
|
|
|
|
|
|
The Company recognized approximately $
The intrinsic value of exercisable but unexercised in-the-money stock options at June 30, 2020, was approximately $
Option Amendments- Modification of Incentive Stock Options
During the six months ended June 30, 2020, the following event resulted in the amendment to terms of outstanding stock option awards:
On January 22, 2020, an employee who had an employment agreement in place under which the employee was employed in a particular role and that prescribed certain separation benefits to the employee was separated from that role. Per the employment agreement, upon termination (i) all unvested stock options would accelerate and become vested as of the termination date, and (ii) the options would remain exercisable, to the extent applicable, following the date of termination for the period prescribed in the equity award plan. As of January 21, 2020, the terminated employee held outstanding options to purchase an aggregate of
15
award plan, to 12 months from the date of the termination. In May 2020, the final separation agreement was amended to extend the post-employment option exercise period from
The Company calculated the change in stock-based compensation cost associated with the previously described stock option modifications pursuant to the applicable guidance in FASB ASC 718, Compensation—Stock Compensation. The change in compensation cost was determined by calculating the difference between (a) the estimated fair value of each option award immediately prior to the modifications and (b) the estimated fair value of each option award immediately after the modifications. The fair value of each option award immediately prior to and immediately after modification was estimated using the Black-Scholes option-pricing model to determine an incremental fair value, consistent with and in accordance with the Company’s existing accounting policy for stock compensation (see Note 2). The total additional compensation cost associated with the previously described modifications was determined to be approximately $
Restricted Stock Units
On October 3, 2019, the Company granted
On February 5, 2020, the Company granted
The following table summarizes the RSU activity under the 2016 Omnibus Incentive Plan for the six months ended June 30, 2020:
|
Restricted Securities |
|
Number of Shares |
|
|
Weighted Average Grant Date Fair Value Per Share |
|
||
|
Nonvested balance as of December 31, 2019 |
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Vested/Released |
|
|
( |
) |
|
|
|
|
|
Forfeited |
|
|
|
|
|
|
|
|
|
Nonvested balance at June 30, 2020 |
|
|
|
|
|
$ |
|
|
The Company recognized approximately $
Stock-based Compensation
Stock-based compensation for the three and six months ended June 30, 2020 and 2019 was included in the consolidated statement of operations and other comprehensive loss as follows:
|
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
(in thousands) |
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
General and administrative |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Research and development |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
16
|
7. |
Warrants |
The Company had
Each tranche of warrants was evaluated under ASC 480, Distinguishing Liabilities from Equity, and ASC 815, Derivatives and Hedging, and the Company determined that equity classification was appropriate.
The following table summarizes common stock warrant activity for the six months ended June 30, 2020:
|
|
|
Warrant Issued June 15, 2015- Tranche 1 |
|
|
Warrant Issued December 27, 2017- Tranche 2 |
|
|
Total |
|
|||
|
Shares remaining to be issued as of December 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued via cashless exercises |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Withheld as payment to cover issued shares |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Balance at June 30, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
8. |
Revenue Recognition |
The Company recognizes collaboration revenue under certain of the Company’s license or collaboration agreements that are within the scope of ASC 606. The Company’s contracts with customers typically include promises related to licenses to intellectual property and research and development services. If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenue from non-refundable, up-front fees allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgement to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. The Company’s contracts may include options to acquire additional goods and/or services.
The terms of the Company’s arrangements with customers typically include the payment of one or more of the following: (i) Non-refundable, up-front payment, (ii) Development, regulatory and commercial milestone payments, (iii) Future options and (iv) Royalties on net sales of licensed products. Accordingly, the transaction price is generally comprised of a fixed fee due at contract inception and variable consideration in the form of milestone payments due upon the achievement of specified events and tiered royalties earned when customers recognize net sales of licensed products. The Company measures the transaction price based on the amount of consideration to which it expects to be entitled in exchange for transferring the promised goods and/or services to the customer. The Company utilizes the “most likely amount” method to estimate the amount of variable consideration, to predict the amount of consideration to which it will be entitled for its one open contract. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Milestone payments that are not within the control of the Company or the licensee, such as those dependent upon receipt of regulatory approval, are not considered to be probable of achievement until the triggering event occurs. At the end of each reporting period, the Company reevaluates the probability of achievement of each milestone and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net loss in the period of adjustment.
For arrangements that include sales-based royalties, including milestone payments based upon the achievement of a certain level of product sales, the Company recognizes revenue upon the later of: (i) When the related sales occur or (ii) When the performance obligation to which some or all of the payment has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized any development, regulatory or commercial milestones or royalty revenue resulting from any of its collaboration arrangements. Consideration that would be received for optional goods and/or services is excluded from the transaction price at contract inception.
17
The Company allocates the transaction price to each performance obligation identified in the contract on a relative standalone selling price basis, when applicable. However, certain components of variable consideration are allocated specifically to one or more particular performance obligations in a contact to the extent both of the following criteria are met: (i) The terms of the payment relate specifically to the efforts to satisfy the performance obligation or transfer the distinct good or service and (ii) Allocating the variable amount of consideration entirely to the performance obligation or the distinct good or service is consistent with the allocation objective of the standard whereby the amount allocated depicts the amount of consideration to which the entity expects to be entitled in exchange for transferring the promised goods or services. The Company develops assumptions that require judgement to determine the standalone selling price for each performance obligation identified in each contract. The key assumptions utilized in determining the standalone selling price for each performance obligation may include forecasted revenues, development timelines, estimated research and development costs, discount rates, likelihood of exercise and probabilities of technical and regulatory success.
Revenue is recognized based on the amount of the transaction price that is allocated to each respective performance obligation when or as the performance obligation is satisfied by transferring a promised good and/or service to the customer. For performance obligations that are satisfied over time, the Company recognizes revenue by measuring the progress toward complete satisfaction of the performance obligation using a single method of measuring progress which depicts the performance in transferring control of the associated goods and/or services to the customer. The Company uses input methods to measure the progress toward the complete satisfaction of performance obligations satisfied over time. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net loss in the period of adjustment. The Company measures progress toward satisfaction of the performance obligation overtime as effort is expended.
As it relates to the Collaboration Agreement with Merck Sharp & Dohme Corp. (“Merck”) the Company recognized the upfront payment associated with its one open contract as a contract liability upon receipt of payment as it requires deferral of revenue recognition to a future period until the Company performs its obligations under the arrangement. Amounts expected to be recognized as revenue within the
The Company does not believe that any variable consideration should be included in the transaction price at June 30, 2020. Such assessment considered the application of the constraint to ensure that estimates of variable consideration would be included in the transaction price only to the extent the Company had a high degree of confidence that revenue would not be reversed in a subsequent reporting period. The Company will re-evaluate the transaction price, including the estimated variable consideration included in the transaction price and all constrained amounts, in each reporting period and as other changes in circumstances occur. For the three months ended June 30, 2020 and 2019, the Company recorded approximately $
On November 6, 2018, the Company entered into a Collaboration Agreement with LG Chem Life Sciences (“LG Chem”) related to the development of the Company’s Immuno-STATs focused in the field of oncology. Pursuant to the Collaboration Agreement the Company granted LG Chem an exclusive license to develop, manufacture and commercialize the Company’s lead product, CUE-101, as well as Immuno-STATs that target T-cells against two additional cancer antigens, in certain Asian countries (collectively, the “LG Chem Territory”). LG Chem has the option to elect one additional Immuno-STAT for an oncology target within two years of the agreement for a worldwide development and commercialization license, and Cue Biopharma will retain an option to co-develop and co-commercialize the additional program worldwide. Cue Biopharma retains rights to develop and commercialize all assets included in the agreement in the United States and in global markets outside of Asia. In exchange for the licenses and other rights granted to LG Chem under the Collaboration Agreement, LG Chem made a $
18
On May 16, 2019, LG Chem paid the Company a $
Aside from the $2.5 million milestone payment, the Company does not believe that any variable consideration should be included in the transaction price as of June 30, 2020. Such assessment considered the application of the constraint to ensure that estimates of variable consideration would be included in the transaction price only to the extent the Company had a high degree of confidence that revenue would not be reversed in a subsequent reporting period. The Company will re-evaluate the transaction price, including the estimated variable consideration included in the transaction price and all constrained amounts, in each reporting period and as other changes in circumstances occur. For the three and six months ended June 30, 2020, the Company recognized revenue of approximately $
The Company considered the capitalization of contract costs under the guidance in ASC 340-40, Other Assets and Deferred Costs: Contracts with Customers. There were no contract costs identified in the Collaboration Agreement with Merck. As it related to the LG Chem agreement, the Company capitalized license expenses of approximately $
|
9. |
Commitments and Contingencies |
Einstein License Agreement
In 2015, the Company entered into the Einstein License with Einstein for certain patent rights relating to the Company’s core technology platform for the engineering of biologics to control T-cell activity, precision, immune-modulatory drug candidates, and
The Company’s remaining commitments with respect to the Einstein License are based on the attainment of future milestones. The aggregate amount of milestone payments made under the Einstein License may equal up to $
Collaboration Agreement with Merck
On November 14, 2017, the Company entered into the Collaboration Agreement with Merck for a partnership to research and develop certain of the Company’s proprietary biologics that target certain autoimmune disease indications (the “Initial Indications”). We view this Collaboration Agreement as a component of our development strategy since it will allow us to advance our autoimmune programs in partnership with a world class pharmaceutical company, while also continuing our focus on our more advanced cancer programs. The research program outlined in the Collaboration Agreement entails (1) our research, discovery and development of certain Immuno-STAT™ drug candidates up to the point of demonstration of certain biologically relevant effects (“Proof of Mechanism”) and (2) the further development by Merck of the Immuno-STAT™ drug candidates that have demonstrated Proof of Mechanism (the “Proposed Product Candidates”) up to the point of demonstration of all or substantially all of the properties outlined in such Proposed Product Candidates’ profiles as described in the Collaboration Agreement.
In exchange for the licenses and other rights granted to Merck under the Collaboration Agreement, Merck paid to the Company a $
19
milestone payments, as well as tiered royalties, if all research, development, regulatory and commercial milestones agreed upon by both parties are successfully achieved. Excluding the up-front payment described above, the Company is eligible to earn up to $
Collaboration Agreement with LG Chem Life Sciences
On November 6, 2018, the Company entered into a Collaboration Agreement with LG Chem related to the development of the Company’s Immuno-STATs focused in the field of oncology. Pursuant to the Collaboration Agreement the Company granted LG Chem an exclusive license to develop, manufacture and commercialize the Company’s lead product, CUE-101, as well as Immuno-STATs that target T-cells against two additional cancer antigens, in certain Asian countries (collectively, the “LG Chem Territory”). LG Chem has the option to elect one additional Immuno-STAT for an oncology target within two years of the agreement for a worldwide development and commercialization license, and Cue Biopharma will retain an option to co-develop and co-commercialize the additional program worldwide. Cue Biopharma retains rights to develop and commercialize all assets included in the agreement in the United States and in global markets outside of Asia. In exchange for the licenses and other rights granted to LG Chem under the Collaboration Agreement, LG Chem made a $
On May 16, 2019, LG Chem paid the Company a $
Contingencies
The Company accrues for contingent liabilities to the extent that the liability is probable and estimable. There are no accruals for contingent liabilities in these consolidated financial statements.
The Company may be subject to various legal proceedings from time to time as part of its business. As of June 30, 2020, the Company was not a party to any legal proceedings or threatened legal proceedings, the adverse outcome of which, individually or in the aggregate, would have a material adverse effect on its business, financial condition or results of operations.
|
10. |
Leases |
The Company leases approximately
The Company adopted ASC 842 as of January 1, 2019 using the effective date method, in which we did not restate prior periods. Upon adoption, the Company elected the package of practical expedients permitted under the transition guidance within ASC 842, which among other things, allowed it to carry forward the historical lease classification. The Company does not allocate consideration in its leases to lease and non-lease components and does not record leases on its balance sheets with terms of 12 months or less.
The Company uses its estimated incremental borrowing rate, which is derived from information available at the lease commencement date, in determining the present value of lease payments. The Company’s incremental borrowing rate represents the rate of interest that it would have to pay to borrow over a similar term an amount equal to the lease payments in a similar economic environment.
The adoption of ASC 842 on January 1, 2019 resulted in the recognition of approximately $
20
either an operating or a finance lease and recognize a related right-of-use asset and lease liability on its balance sheet upon commencement.
On January 18, 2018, the Company entered into an operating lease agreement for its laboratory and office space in Cambridge, Massachusetts for the period from
On June 18, 2018, the Company entered into an amended lease agreement that provided the Company with a reduction in rental fees for its office and laboratory space in exchange for prepayment of a portion of the fees. This amendment was effective beginning on
On September 20, 2018, the Company entered into an operating lease for additional laboratory space in Cambridge, Massachusetts for the period from
On September 16, 2019, the Company entered into an amendment to the Additional Laboratory Lease that removed one holding room from the additional laboratory space. The amendment was effective beginning on
On June 24, 2020, the “Company entered into a second amendment to the Laboratory and Office Lease.
At June 30, 2020, the Company recorded approximately $
Future minimum lease payments under these leases at June 30, 2020 are as follows:
|
Year |
|
(in thousands) |
|
|
|
2020 |
|
|
|
|
|
2021 |
|
|
|
|
|
2022 |
|
|
|
|
|
Total lease payment |
|
$ |
|
|
|
Less: present value discount |
|
|
( |
) |
|
Total |
|
$ |
|
|
21
Total rent expense of approximately $
|
Other information (in thousands) |
|
Three Months Ended June 30, 2020 |
|
Six Months Ended June 30, 2020 |
|
||
|
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
|
|
|
Operating cash flows from operating leases |
|
$ |
|
|
$ |
|
|
|
Operating lease cost |
|
$ |
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average discount rate |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
Weighted average remaining lease term |
|
|
|
|
|
||
The Company recorded a right of use asset of approximately $
|
11. |
Subsequent Events |
In June 2020, the Company entered into the June 2020 ATM Agreement with Stifel to sell shares of the Company’s common stock for aggregate gross proceeds of up to $
On July 15, 2020, the Company filed a Certificate of Amendment (the “Certificate of Amendment”) to its Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware effecting an amendment to increase the number of authorized shares of the Company’s common stock from
22
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations of Cue Biopharma, Inc. (“Cue,” “we”, “us” “our” or the “Company”) should be read in conjunction with our financial statements and accompanying notes included in this Quarterly Report on Form 10-Q and the financial statements and accompanying notes thereto for the fiscal year ended December 31, 2019 and the related Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our Annual Report on Form 10-K filed by us with the Securities and Exchange Commission, or SEC, on March 12, 2020, as amended on Form 10-K/A filed by us with the SEC on March 12, 2020 and on Form 10-K/A filed by us with the SEC on April 29, 2020.
Forward-Looking Statements
This Quarterly Report on Form 10-Q contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “would,” “could,” “seek,” “intend,” “plan,” “goal,” “project,” “estimate,” “anticipate,” “strategy,” “future,” “likely” or other comparable terms. All statements other than statements of historical facts included in this Quarterly Report on Form 10-Q regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding anticipated results of our drug development efforts, including study results, our expectations regarding regulatory developments and expected future operating results. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, our limited operating history, limited cash and a history of losses; our ability to achieve profitability; potential setbacks in our research and development efforts including negative or inconclusive results from our preclinical studies; our ability to secure required Food and Drug Administration (“FDA”) or other governmental approvals for our product candidates and the breadth of any approved indication; adverse effects on our business caused by public health pandemics, including COVID-19; negative or inconclusive results from our clinical studies or serious and unexpected drug-related side effects or other safety issues experienced by participants in our clinical trials; delays and changes in regulatory requirements, policy and guidelines including potential delays in submitting required regulatory applications to the FDA; our reliance on licensors, collaborators, contract research organizations, suppliers and other business partners; our ability to obtain adequate financing to fund our business operations in the future; and the other risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our most recently filed Annual Report on Form 10-K and any subsequently filed Quarterly Report(s) on Form 10-Q. Any forward-looking statement made by us in this report is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Overview
Cue Biopharma is a clinical-stage biopharmaceutical company engineering a novel class of injectable biologics to selectively engage and modulate targeted T cells within the body. Our proprietary Immuno-STAT™ (Selective Targeting and Alteration of T Cells) platform is engineered to selectively engage and modulate disease relevant T cells directly within the body which we believe will allow us to harness the fullest potential of an individual’s intrinsic immune repertoire for restoring health while avoiding the deleterious side-effects of broad immune activation (for immuno-oncology or infectious disease indications) or broad immune suppression (for autoimmunity and inflammation). In addition to the selective control of T cell activity, we believe Immuno-STATs offer several key points of potential differentiation over competing approaches, including modularity and versatility providing broad disease coverage, manufacturability, and convenient administration.
Through protein engineering, we leverage the modular and versatile nature of the Immuno-STAT platform to design therapeutics for selective immune modulation in cancer, chronic infectious disease, and autoimmune disease. To address the needs of these clinical indications, we have developed three biologic series, CUE-100, CUE-200, and CUE-300, each possessing distinct signaling modules that underscore unique biological mechanisms that may be applied across many diseases. The CUE-100 series exploits engineered IL-2 in context of the core Immuno-STAT framework for activation of tumor-specific T cells, while the CUE-200 series is focused on co-stimulatory T cell signaling pathways including activation signals CD80 and/or 4-1BBL. The CUE-300 series is being developed for autoimmune diseases for selective modulation of the autoreactive T cell repertoire.
23
The Company’s product candidates are in various stages of clinical and preclinical development, and while we believe that these candidates hold tremendous potential value, the Company’s activities are subject to significant risks and uncertainties. The Company has not yet commenced any commercial revenue-generating operations, has limited cash flows from operations, and will need to raise additional capital to fund its growth and ongoing business operations.
Our Immuno-STAT Pipeline
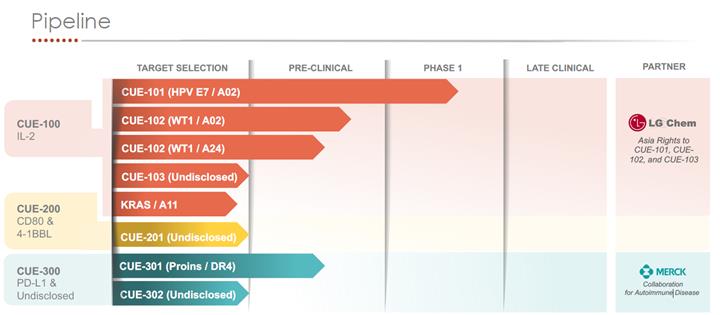
The pipeline snapshot above details our current portfolio assets and their stages of development. CUE-101 is our most advanced clinical stage asset, currently being dosed in a Ph 1 monotherapy trial for human papilloma virus (HPV)-driven head and neck cancer. CUE-102 focuses on Wilm’s tumor-1 (WT1) as the tumor antigen.
We have made significant progress furthering the advancement of the CUE-100 series. We dosed the first patient in September 2019 in a Phase 1 clinical trial of CUE-101 for the treatment of HPV16-driven recurrent/metastatic (“R/M”) head and neck squamous cell carcinoma (“HNSCC”). This monotherapy Phase 1 clinical trial focuses on patients with R/M HNSCC who have likely received several lines of systemic therapy including checkpoint inhibitors. During the second half of 2020 we plan to expand this Phase 1 clinical trial to evaluate the combination of CUE-101 with Merck’s anti-PD-1 therapy KEYTRUDA® (pembrolizumab) as a first-line therapy in R/M HNSCC as well as to initiate a submission to the FDA for a neoadjuvant study. Enabled by the company’s proprietary Immuno-STAT platform, CUE-101 is the company’s lead biologic drug candidate, designed to directly engage and activate T cells in the body to target HPV-driven cancers. The CUE-101 program is representative of the IL2-based CUE-100 series for which we have generated a robust preclinical data package, including activation of HPV specific T cells from human blood. These data were recently published in a peer-reviewed journal (Quayle et al., Clinical Cancer Research 2020, https://clincancerres.aacrjournals.org/content/early/2020/01/15/1078-0432.CCR-19-3354).
We are also currently advancing a pipeline of additional promising preclinical candidates that we believe hold the potential to treat multiple cancers. Emerging data from our CUE-102 (WT1) program recently presented at NYAS Frontiers in Cancer Immunotherapy demonstrates early evidence of selective T cell expansion and continues developing towards IND enabling studies. In addition to oncology, we have made recent advances with the CUE-300 series for autoimmune disorders, and expect to further develop a growing pipeline of promising autoimmune candidates during the second half of 2020 and throughout 2021. Furthermore, we also have the potential of developing a pipeline of Immuno-STATs for treating chronic infectious diseases with the CUE-200 series. In addition to the Immuno-STATs described above, we are developing a next generation platform, termed Neo-STAT, that we believe has the potential to significantly enhance our productivity and platform flexibility by generating a stable “peptide-less” or “empty” Immuno-STAT scaffold of the CUE-100 series, to which peptides of interest may be covalently attached.
Coronavirus (“COVID-19”) Pandemic
The COVID-19 outbreak, which the World Health Organization has classified as a pandemic, has prompted governments and regulatory bodies throughout the world to issue “stay-at-home” or similar orders, and enact restrictions on the performance of “non-essential” services, public gatherings and travel.
24
Beginning in March 2020, we undertook precautionary measures intended to help minimize the risk of virus transmission to our employees, including the establishment of remote working standards, pausing all non-essential travel worldwide for our employees, and limiting employee attendance at industry events and in-person work-related meetings, to the extent those events and meetings are continuing. To date, we do not believe these actions have had a significant negative impact on our productivity or our operations. However, these actions or additional measures we may undertake may ultimately delay progress our developmental goals or otherwise negatively affect our business. In addition, third-party actions taken to contain its spread and mitigate its public health effects of COVID-19 may negatively affect our business.
Plan of Operation
Our technology is in the development phase. We believe that our licensed platforms have the potential for creating a diverse pipeline of strong product candidates addressing multiple medical indications. The Company intends to maximize the value and probability of commercialization of its Immuno-STAT™ product candidates by focusing on research, testing, optimizing, conducting pilot studies, performing early stage clinical development and partnering for more extensive, later stages of clinical development, as well as seeking extensive patent protection and intellectual property development.
Since we are a development stage company, the majority of our business activities to date and our planned future activities will be devoted to further research and development.
A fundamental part of our corporate development strategy is to establish one or more strategic partnerships with leading pharmaceutical or biotechnology organizations that will allow the Company to more fully exploit the potential of its technology platform, such as those described below under the headings “Collaboration Agreement with Merck” and “Collaboration with LG Chem”.
Critical Accounting Policies and Significant Judgements and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of our financial statements, and the reported revenue and expenses during the reported periods. We evaluate these estimates and judgments, including those described below, on an ongoing basis. We base our estimates on historical experience, known trends and events, contractual milestones and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe that the estimates, assumptions and judgments involved in the accounting policies described in Management’s Discussion and Analysis of Financial Condition and Results of Operations in Item 7 of our Annual Report on Form 10-K for the year ended December 31, 2019, have the greatest potential impact on our financial statements, so we consider them to be our critical accounting policies and estimates. There were no material changes to our critical accounting policies and estimates during the six months ended June 30, 2020.
Revenue Recognition
The Company adopted Accounting Standards Codification, Topic 606, Revenue from Contracts with Customers (“ASC 606”), during 2018. The Company generates revenue solely through collaboration arrangements with strategic partners for the development and commercialization of product candidates. The core principle of ASC 606 is that an entity should recognize revenue to depict the transfer of promised goods and/or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods and/or services. To determine the appropriate amount of revenue to be recognized for arrangements that the Company determines are within the scope of ASC 606, the Company performs the following steps: (i) Identify the contract(s) with the customer, (ii) Identify the performance obligations in the contract, (iii) Determine the transaction price, (iv) Allocate the transaction price to the performance obligations in the contract and (v) Recognize revenue when (or as) each performance obligation is satisfied.
The Company recognizes collaboration revenue under certain of the Company’s license or collaboration agreements that are within the scope of ASC 606. The Company’s contracts with customers typically include promises related to licenses to intellectual property and research and development services. If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenue from non-refundable, up-front fees allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgement to assess the nature of the combined
25
performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. Accordingly, the transaction price is generally comprised of a fixed fee due at contract inception and variable consideration in the form of milestone payments due upon the achievement of specified events and tiered royalties earned when customers recognize net sales of licensed products. The Company measures the transaction price based on the amount of consideration to which it expects to be entitled in exchange for transferring the promised goods and/or services to the customer. The Company utilizes the “most likely amount” method to estimate the amount of variable consideration, to predict the amount of consideration to which it will be entitled for its one open contract. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. At the inception of each arrangement that includes development and regulatory milestone payments, the Company evaluates whether the associated event is considered probable of achievement and estimates the amount to be included in the transaction price using the most likely amount method. Currently, the Company has one contract with an option to acquire additional goods and/or services in the form of additional research and development services for additional product candidates.
Research and Development Costs
Research and development expenses consist primarily of compensation costs, fees paid to consultants, outside service providers and organizations (including research institutes at universities), facility costs, and development and clinical trial costs with respect to the Company’s product candidates.
Research and development expenses incurred under contracts are expensed ratably over the life of the underlying contracts, unless the achievement of milestones, the completion of contracted work, or other information indicates that a different pattern of performance is more appropriate. Other research and development expenses are charged to operations as incurred. Payments made pursuant to research and development contracts are initially recorded as research and development contract advances in the Company’s balance sheet and then charged to research and development expenses in the Company’s statement of operations as those contract services are performed. Expenses incurred under research and development contracts in excess of amounts advanced are recorded as research and development contract liabilities in the Company’s balance sheet, with a corresponding charge to research and development expenses in the Company’s statement of operations.
Nonrefundable advance payments for future research and development activities pursuant to an executory contractual arrangement are recorded as advances as described above. Nonrefundable advance payments are recognized as an expense as the related services are performed. The Company evaluates whether it expects the services to be rendered at each quarter end and year end reporting date. If the Company does not expect the services to be rendered, the advance payment is charged to expense. To the extent that a nonrefundable advance payment is for contracted services to be performed within 12 months from the reporting date, such advance is included in current assets; otherwise, such advance is included in non-current assets.
The Company evaluates the status of its research and development agreements and contracts, and the carrying amount of the related assets and liabilities, at each quarter end and year end reporting date, and adjusts the carrying amounts and their classification on the balance sheet as appropriate.
Stock-Based Compensation
The Company periodically issues stock based-awards to officers, directors, employees, Scientific and Clinical Advisory Board members, non-employees and consultants for services rendered. Such issuances vest and expire according to terms established at the issuance date.
Stock-based payments to officers, directors and employees, including grants of employee stock options, are recognized in the financial statements based on their grant date fair values. Stock option grants, which are generally time-vested, are measured at the grant date fair value and charged to operations on a straight-line basis over the service period, which generally approximates the vesting term. The Company also grants performance-based awards periodically to officers of the Company. The Company recognizes compensation costs related to performance awards over the requisite service period if and when the Company concludes that it is probable that the performance condition will be achieved. Stock options granted to members of the Company’s Scientific and Clinical Advisory Board, non-employees and outside consultants are valued at the grant date fair value and charged to operations on a straight-line basis over the service period, which generally approximates the vesting term.
The fair value of stock options and restricted stock units is determined utilizing the Black-Scholes option-pricing model, which is affected by several variables, including the risk-free interest rate, the expected dividend yield, the life of the equity award, the exercise price of the stock option as compared to the fair value of the common stock on the grant date, and the estimated volatility of the common stock over the term of the equity award.
26
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. Until the Company has established a trading market for its common stock that approximates the expected term of the granted options, estimated volatility is based on the average historical volatilities of comparable public companies in a similar industry. The expected dividend yield is based on the current yield at the grant date; the Company has never declared or paid dividends and has no plans to do so for the foreseeable future. As permitted by Staff Accounting Bulletin No. 107, due to the Company’s lack of trading history and option activity, management utilizes the simplified method to estimate the expected term of options at the date of grant. The exercise price is determined based on the fair value of the Company’s common stock at the date of grant. The Company accounts for forfeitures as they occur.
The Company recognizes the fair value of stock-based compensation in general and administrative expenses and in research and development expenses in the Company’s statements of operations, depending on the type of services provided by the recipient of the equity award.
Recent Accounting Pronouncements and Adopted Standards
A discussion of recent accounting pronouncements is included in Note 2 to the consolidated financial statements in this Quarterly Report on Form 10-Q.
Significant Contracts and Agreements Related to Research and Development Activities
Einstein License Agreement
On January 14, 2015, the Company entered into a license agreement, as amended and restated on July 31, 2017 (the “Einstein License”), with Einstein for certain patent rights relating to the Company’s core technology platform for the engineering of biologics to control T cell activity, precision, immune-modulatory drug candidates, and two supporting technologies that enable the discovery of costimulatory signaling molecules (ligands) and T cell targeting peptides.
The Company holds an exclusive worldwide license, with the right to sublicense, import, make, have made, use, provide, offer to sell, and sell all products, processes and services that use the patents covered by the Einstein License, including certain technology received from Einstein related thereto (the “Licensed Products”). Under the Einstein License, the Company is required to:
|
|
• |
Pay royalties and amounts based on certain percentage of proceeds, as defined in the Einstein License, from sales of Licensed Products and sublicense agreements. |
|
|
• |
Pay escalating annual maintenance fees, which are non-refundable, but are creditable against the amount due to Einstein for royalties. |
|
|
• |
Make significant payments based upon the achievement of certain milestones, as defined in the Einstein License. For the three and six months ended June 30, 2020, none of these milestones had been achieved by the Company. |
|
|
• |
Incur minimum product development costs per year until the first commercial sale of the first Licensed Product. |
The Company was in compliance with its obligations under the Einstein License at June 30, 2020.
The Einstein License expires upon the expiration of the last obligation to make royalty payments to Einstein which may be due with respect to certain Licensed Products, unless terminated earlier under the provisions thereof. The Einstein License includes certain termination provisions if the Company fails to meet its obligations thereunder.
The Company accounts for the costs incurred in connection with the Einstein License in accordance with ASC 730, Research and Development. For the three and six months ended June 30, 2020 and 2019, costs incurred with respect to the Einstein License aggregate $18,750 and $37,500, respectively, for such period. Such costs are included in research and development costs in the statement of operations.
Pursuant to the Einstein License, the Company issued to Einstein 671,572 shares of common stock of the Company in connection with the consummation of the initial public offering of its common stock on December 27, 2017.
Collaboration Agreement with Merck
On November 14, 2017, the Company entered into an Exclusive Patent License and Research Collaboration Agreement (the “Merck Agreement”) with Merck Sharp & Dohme Corp. (“Merck”) for a partnership to research and develop certain of the Company’s proprietary biologics that target certain autoimmune disease indications (the “Initial Indications”). We view the Merck Agreement as a component of our development strategy since it will allow us to advance our autoimmune programs in partnership
27
with a world class pharmaceutical company, while also continuing our focus on our more advanced cancer programs. The research program outlined in the Merck Agreement entails (1) our research, discovery and development of certain Immuno-STAT™ drug candidates up to the point of demonstration of certain biologically relevant effects (“Proof of Mechanism”) and (2) the further development by Merck of the Immuno-STAT™ drug candidates that have demonstrated Proof of Mechanism (the “Proposed Product Candidates”) up to the point of demonstration of all or substantially all of the properties outlined in such Proposed Product Candidates’ profiles as described in the Merck Agreement.
For the purposes of this collaboration, the Company granted to Merck under the Merck Agreement an exclusive license under certain of its patent rights, including a sublicense of patent rights licensed from Einstein, to the extent applicable to the specific Immuno-STAT™ that are elected to be developed by Merck. So long as Merck continues product development on a Proposed Product Candidate, the Company is restricted from conducting any development activities within the Initial Indication covered by such Proposed Product Candidate other than pursuant to the Merck Agreement.
In exchange for the licenses and other rights granted to Merck under the Merck Agreement, Merck paid to the Company a $2.5 million nonrefundable up-front payment. Additionally, the Company may be eligible to receive funding in developmental milestone payments, as well as tiered royalties, if all research, development, regulatory and commercial milestones agreed upon by both parties are successfully achieved. Excluding the up-front payment described above, the Company is eligible to earn up to $101 million for the achievement of certain research and development milestones, $120 million for the achievement of certain regulatory milestones and $150 million for the achievement of certain commercial milestones, in addition to tiered royalties on sales, if all pre-specified milestones associated with multiple products across the primary disease indication areas are achieved. The Merck Agreement requires the Company to use the first $2.5 million of milestone payments we receive under the agreement to fund contract research. The amount of the royalty payments is a percentage of product sales ranging in the single digits based on the amount of such sales. For the three and six months ended June 30, 2020, the Company recorded approximately $118,000 and $173,000, respectively, in collaboration revenue related to this agreement.
The term of the Merck Agreement extends until the expiration of all royalty obligations following a product candidate’s receipt of marketing authorization, at which point Merck’s licenses and sublicenses granted under the agreement shall become fully paid-up, perpetual licenses and sublicenses, as applicable. Royalties on each product subject to the Merck Agreement shall continue on a country-by-country basis until the expiration of the later of: (1) the last-to-expire patent claiming the compound on which such product is based and (2) a period of ten years after the first commercial sale of such product in such country.
Notwithstanding the foregoing, Merck may terminate the Merck Agreement at any time upon 30 days’ notice to the Company. The Merck Agreement may also be terminated by either party if the other party is in breach of its obligations thereunder and fails to cure such breach within 90 days after notice or by either party if the other party files for bankruptcy or other similar insolvency proceedings.
Collaboration Agreement with LG Chem
Effective November 6, 2018, we entered into the LG Chem Agreement with LG Chem, related to the development of Immuno-STATs focused in the field of oncology.
Pursuant to the LG Chem Agreement, we granted LG Chem an exclusive license to develop, manufacture and commercialize our lead product, CUE-101, as well as Immuno-STATs that target T-cells against two additional cancer antigens (“Product Candidates”), in Australia, Japan, Republic of Korea, Singapore, Malaysia, Vietnam, Thailand, Philippines, Indonesia, China (including Macau and Hong Kong) and Taiwan (collectively, the “LG Chem Territory”). On December 20, 2018, the Company reported the selection of Wilms Tumor 1 (“WT1”) as the first target antigen for a Product Candidate under the LG Chem Agreement. We retain rights to develop and commercialize all assets included in the LG Chem Agreement in the United States and in global markets outside of the LG Chem Territory. Under the LG Chem Agreement, we will engineer the selected Immuno-STATs for up to three alleles, which are expected to include the predominant alleles in the LG Chem Territory, thereby enhancing Cue’s market reach by providing for greater patient coverage of populations in global markets, while LG Chem will establish a chemistry, manufacturing and controls (“CMC”) process for the development and commercialization of selected Product Candidates. In addition, LG Chem has the option to select one additional Immuno-STAT for an oncology target (an “Additional Immuno-STAT”) within two years of the effective date of the LG Chem Agreement for an exclusive worldwide development and commercialization license. If LG Chem exercises this option, then the parties will execute a license and collaboration agreement (“Global License and Collaboration Agreement”) setting forth the terms and conditions relating to such arrangement. We will retain an option to co-develop and co-commercialize the additional program worldwide.
Under the terms of the LG Chem Agreement, LG Chem paid us a $5.0 million non-refundable, non-creditable upfront payment and purchased approximately $5.0 million of shares of our common stock at a price per share equal to a twenty percent (20%) premium to the volume weighted-average closing price per share over the thirty (30) trading day period immediately prior to the effective date of the LG Chem Agreement. We are also eligible to receive additional aggregate payments of approximately $400
28
million if certain research, development, regulatory and commercial milestones are successfully achieved. On May 16, 2019, the Company earned a $2.5 million milestone payment for the FDA acceptance of the IND for the Company’s lead drug candidate, CUE-101, pursuant to the LG Chem Agreement. In addition, the LG Chem Agreement also provides that LG Chem will pay us tiered single-digit royalties on net sales of commercialized Product Candidates (“Collaboration Products”) in the LG Chem Territory on a product-by-product and country-by-country basis, until the later of expiration of patent rights in a country, the expiration of regulatory exclusivity in such country, or ten years after the first commercial sale of a Collaboration Product in such country, subject to certain royalty step-down provisions set forth in the LG Chem Agreement.
Pursuant to the LG Chem Agreement, the parties will share research costs related to Collaboration Products, and LG Chem will provide CMC process development for selected Product Candidates and potentially additional downstream manufacturing capabilities, including clinical and commercial supply for Collaboration Products. In return for performing CMC process development, LG Chem is eligible to receive low-single digit royalty payments on the sales of Collaboration Products sold in all countries outside the LG Chem Territory. Furthermore, should the parties enter into a Global License and Collaboration Agreement for an Additional Immuno-STAT, LG Chem will pay us a one-time, non-refundable, non-creditable upfront payment and we will be eligible to receive up to approximately $470 to $675 million in fees and milestone payments as well as tiered royalty payments on future global sales that range from high-single digits to mid-double digit teens in the United States and mid-single to low-double digits outside of the United States. The amount of fees and milestone payments, as well as whether we receive royalty payments, will depend on when LG Chem nominates the Additional Immuno-STAT Biologic, the number of alleles selected by LG Chem and whether we exercise our option to co-develop and co-commercialize the additional program worldwide, in which case we would share costs and profits instead of receiving royalties and post-option-exercise milestones. For the three and six months ended June 30, 2020, the Company recognized approximately $957,000 and $1,802,000, respectively, in collaboration revenue related to this agreement.
The LG Chem Agreement includes various representations, warranties, covenants, indemnities and other customary provisions. LG Chem may terminate the LG Chem Agreement for convenience or change of control of the Company on a program-by-program, product-by-product or country-by-country basis, or in its entirety, at any time following the notice period set forth in the LG Chem Agreement. Either party may terminate the LG Chem Agreement, in its entirety or on a program-by-program, product-by-product or country-by-country basis, in the event of an uncured material breach. The LG Chem Agreement is also terminable by either party (i) upon the bankruptcy, insolvency or liquidation of the other party or (ii) for certain activities involving the challenge of certain patents controlled by the other party. Unless earlier terminated, the LG Chem Agreement will expire on a product-by-product and country-by-country basis upon the expiration of the applicable royalty term.
Results of Operations
Collaboration Revenue
The Company has not generated commercial revenue from product sales. To date, the Company has generated collaboration revenue from the Merck Agreement and the LG Chem Agreement.
Operating Expenses
The Company generally recognizes operating expenses as they are incurred in two general categories, general and administrative expenses and research and development expenses. The Company’s operating expenses also include non-cash components related to depreciation and amortization of property and equipment and stock-based compensation, which are allocated, as appropriate, to general and administrative expenses and research and development expenses.
General and administrative expenses consist of salaries and related expenses for executive, legal, finance, human resources, information technology and administrative personnel, as well as professional fees, insurance costs, and other general corporate expenses. Management expects general and administrative expenses to increase in future periods as the Company adds personnel and incurs additional expenses related to an expansion of its research and development activities and its operation as a public company, including higher legal, accounting, insurance, compliance, compensation and other expenses.
Research and development expenses consist primarily of compensation expenses, fees paid to consultants, outside service providers and organizations (including research institutes at universities), facility expenses, and development and clinical trial expenses with respect to the Company’s product candidates. The Company charges research and development expenses to operations as they are incurred. Management expects research and development expenses to increase in the future as the Company increases its efforts to develop technology for potential future products based on its technology and research.
29
Three and Six Months Ended June 30, 2020 and 2019
The Company’s statements of operations for the three and six months ended June 30, 2020 and 2019, as discussed herein are presented below.
|
|
|
Three Months Ended |
|
|
Six Months Ended |
|
||||||||||
|
|
|
June 30, |
|
|
June 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
|
|
(in thousands) |
|
|
(in thousands) |
|
||||||||||
|
Collaboration revenue |
|
$ |
1,075 |
|
|
$ |
1,055 |
|
|
$ |
1,975 |
|
|
$ |
1,425 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
3,898 |
|
|
|
3,419 |
|
|
|
7,887 |
|
|
|
6,864 |
|
|
Research and development |
|
|
8,119 |
|
|
|
6,867 |
|
|
|
18,025 |
|
|
|
15,220 |
|
|
Total operating expenses |
|
|
12,017 |
|
|
|
10,286 |
|
|
|
25,912 |
|
|
|
22,084 |
|
|
Loss from operations |
|
|
(10,942 |
) |
|
|
(9,231 |
) |
|
|
(23,937 |
) |
|
|
(20,659 |
) |
|
Other income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
134 |
|
|
|
96 |
|
|
|
337 |
|
|
|
210 |
|
|
Other (expense) income |
|
|
(25 |
) |
|
|
26 |
|
|
|
(51 |
) |
|
|
71 |
|
|
Total other income |
|
|
109 |
|
|
|
122 |
|
|
|
286 |
|
|
|
281 |
|
|
Loss Before Income Taxes |
|
$ |
(10,833 |
) |
|
$ |
(9,109 |
) |
|
$ |
(23,651 |
) |
|
$ |
(20,378 |
) |
|
Income tax expense |
|
|
— |
|
|
|
(413 |
) |
|
|
— |
|
|
|
(413 |
) |
|
Net Loss |
|
$ |
(10,833 |
) |
|
$ |
(9,522 |
) |
|
$ |
(23,651 |
) |
|
$ |
(20,791 |
) |
|
Unrealized gains (losses) from available-for-sale securities |
|
|
(94 |
) |
|
|
3 |
|
|
|
165 |
|
|
|
11 |
|
|
Comprehensive loss |
|
$ |
(10,927 |
) |
|
$ |
(9,519 |
) |
|
$ |
(23,486 |
) |
|
$ |
(20,780 |
) |
|
Net loss per common share – basic and diluted |
|
$ |
(0.38 |
) |
|
$ |
(0.46 |
) |
|
$ |
(0.86 |
) |
|
$ |
(1.00 |
) |
|
Weighted average common shares outstanding – basic and diluted |
|
|
28,221,537 |
|
|
|
20,821,390 |
|
|
|
27,391,081 |
|
|
|
20,770,173 |
|
Collaboration Revenue
Collaboration revenue was $1,075,000 and $1,055,000 for the three months ended June 30, 2020 and 2019, respectively. The Company recognized collaboration revenue of approximately $1,975,000 and $1,425,000 for the six months ended June 30, 2020 and 2019, respectively. All collaboration revenue recognized was related to the performance of services under our Collaboration Agreements with Merck and LG Chem.
General and Administrative
General and administrative expenses totaled approximately $3,898,000 and $3,419,000 for the three months ended June 30, 2020 and 2019, respectively. This increase of approximately $479,000 was due primarily to stock-based compensation related to executive management and legal fees offset by a decrease in travel expenses as the COVID-19 pandemic hampered business travel throughout the second quarter. We expect our general and administrative expenses to increase as we continue to expand our operations.
General and administrative expenses for the three months ended June 30, 2020 consisted of expenses related to employee and board compensation of approximately $891,000, stock based compensation of $995,000, professional and consulting fees of $1,375,000, rent of $259,000, insurance expense of $64,000, depreciation and amortization of $26,000, investor relations of $173,000, and other expenses of $115,000. General and administrative expenses for the three months ended June 30, 2019 included expenses related to employee and board compensation of approximately $798,000, professional and consulting fees of $1,412,000, rent of $270,000, depreciation and amortization of $26,000, insurance expense of $208,000, stock-based compensation of $402,000, travel of $115,000, and other expenses of $282,000.
General and administrative expenses totaled approximately $7,887,000 and $6,864,000 for the six months ended June 30, 2020 and 2019, respectively. This increase of approximately $1,023,000 was due primarily to the growth of the Company and its activities. We expect our general and administrative expenses to continue to increase as we expand our operations.
General and administrative expenses for the six months ended June 30, 2020 consisted of expenses related to employee and board compensation of approximately $1,937,000, stock based compensation of $2,011,000, professional and consulting fees of $2,754,000, rent of $516,000, insurance expense of $127,000, depreciation and amortization of $50,000, travel of $25,000, other
30
expenses of $249,000, and investor relations of $218,000. General and administrative expenses for the six months ended June 30, 2019 consisted of expenses related to employee and board compensation of approximately $1,875,000, stock based compensation of $962,000, professional and consulting fees of $2,524,000, rent of $536,000, insurance expense of $363,000, depreciation and amortization of $37,000, travel of $106,000, and other expenses of $461,000.
Research and Development
Research and development expenses totaled approximately $8,119,000 and $6,867,000 for the three months ended June 30, 2020 and 2019, respectively. This increase of approximately $1,252,000 was due primarily to the increase in laboratory and drug substance manufacturing costs, stock based compensation and clinical expenses, offset by a decrease in travel expenses as the COVID-19 pandemic hampered business travel throughout the second quarter. We expect our research and development expenses to increase as we expand our clinical development activities.
Research and development expenses for the three months ended June 30, 2020 included expenses related to employee and Scientific and Clinical Advisory Board compensation of approximately $2,131,000, stock-based compensation of $1,530,000, depreciation and amortization of $246,000, research and laboratory expenses of $1,860,000, clinical expenses of $1,038,000, rent of $820,000, other professional fees of $147,000, licensing fees of $24,000, insurance expense of $161,000, and other expenses of $162,000. Research and development expenses for the three months ended June 30, 2019 included expenses related to employee and Scientific and Clinical Advisory Board compensation of $1,777,000, depreciation and amortization of $239,000, stock-based compensation of $915,000, research and laboratory expenses of $1,491,000, rent of $895,000, other professional fees of $245,000, licensing fees of $294,000, travel expenses of $78,000 and other expenses of $220,000.
Research and development expenses totaled approximately $18,025,000 and $15,220,000 for the six months ended June 30, 2020 and 2019, respectively. This increase of approximately $2,805,000 was due primarily to the growth of the Company and its activities. We expect our research and development expenses to continue to increase as we expand our development activities.
Research and development expenses for the six months ended June 30, 2020 included expenses related to employee and Scientific and Clinical Advisory Board compensation of approximately $4,117,000, stock-based compensation of $3,688,000 depreciation and amortization of $485,000, research and laboratory expenses of $4,892,000, clinical expenses of $2,032,000, rent of $1,644,000, other professional fees of $267,000, licensing fees of $50,000, travel expenses of $24,000, insurance expense of 321,000, and other expenses of $445,000. Research and development expenses for the six months ended June 30, 2019 included expenses related to employee and Scientific and Clinical Advisory Board compensation of approximately $3,585,000, stock-based compensation of $2,123,000 depreciation and amortization of $435,000, research and laboratory expenses of $4,274,000, clinical expenses of $713,000, rent of $1,796,000, other professional fees of $1,133,000, licensing fees of $614,000, travel expenses of $100,000 and other expenses of $447,000.
Interest Income
Interest income was approximately $134,000 for the three months ended June 30, 2020, as compared to $96,000 for the three months ended June 30, 2019. Interest income was approximately $337,000 for the six months ended June 30, 2020, as compared to $210,000 for the six months ended June 30, 2019. The increase in interest income was due to the investment of an increased portion of the Company’s cash in cash equivalents and marketable securities during 2020.
Other Income and Expense
Other income and expense for the three months ended June 30, 2020 was comprised of approximately $25,000 in expense resulting from amortization of discounts received on certain of the Company’s marketable securities, compared to $26,000 in amortization of discounts received for the three months ended June 30, 2019. Other income and expense was approximately $51,000 for the six months ended June 30, 2020 in expense, as compared to $71,000 for the six months ended June 30, 2019 in income, comprised of expense resulting from the amortization of discounts received on certain of the Company’s marketable securities.
Liquidity and Capital Resources
The Company has financed its working capital requirements primarily through private and public offerings of equity securities and cash received from Merck and LG Chem under the respective collaboration agreements. At June 30, 2020, the Company had unrestricted cash, cash equivalents, and marketable securities totaling approximately $84,928,000 available to fund the Company’s ongoing business activities. Additional information concerning the Company’s financial condition and results of operations is provided in the financial statements included in this report.
31
The amounts that the Company actually spends for any specific purpose may vary significantly and will depend on a number of factors, including, but not limited to, the Company’s research and development activities and programs, clinical testing, regulatory approval, market conditions, and changes in or revisions to the Company’s business strategy and technology development plans. Investors will be relying on the judgment of the Company’s management regarding the application of the proceeds from the sale of the Company’s common stock.
The Company believes that its existing cash resources will be sufficient to fund the Company’s projected operating requirements for at least the next 12 months from the issuance of this report based on current operating plans. Until the Company is able to generate sustainable revenues that generate operating profitability and positive operating cash flows, the Company expects to finance its future cash needs through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. However, there can be no assurances that the Company will be able to obtain additional financing on acceptable terms and in the amounts necessary to fully fund its future operating requirements, if at all. If the Company is unable to obtain sufficient cash resources to fund its operations, the Company may be forced to reduce or discontinue its operations entirely.
In March 2020, the Company entered into an at-the-market equity offering sales agreement with Stifel Nicolaus & Company, Inc. (“Stifel”) to sell shares of the Company’s common stock for aggregate gross proceeds of up to $35 million, from time to time, through an “at-the-market” equity offering program under which Stifel acts as sales agent. As of June 30, 2020, the Company had sold a total of 1,824,901 common shares under the sales agreement for proceeds of approximately $34.3 million, net of commissions paid, but excluding estimated transaction expenses. Due to the issuance and sale of all the shares of common stock subject thereto, the March 2020 sales agreement terminated in accordance with its terms.
In June 2020, the Company entered into an at-the-market equity offering sales agreement with Stifel to sell shares of the Company’s common stock for aggregate gross proceeds of up to $40 million, from time to time, through an “at-the-market” equity offering program under which Stifel acts as sales agent. The sales agreement will terminate upon the earliest of (a) the sale of $40 million of shares of the Company’s common stock or (b) the termination of the sales agreement by the Company or Stifel. As of June 30, 2020, the Company had sold 350,000 shares of common stock under the sales agreement for proceeds of approximately $8.1 million, net of commissions paid, but excluding estimated transaction expenses.
If the Company issues additional equity securities to raise funds, the ownership percentage of the Company’s existing stockholders would be reduced. New investors may demand rights, preferences or privileges senior to those of existing holders of the Company’s common stock. If the Company issues debt securities, the Company may be required to grant security interests in its assets, could have substantial debt service obligations, and lenders may have a senior position (compared to stockholders) in any potential future bankruptcy or liquidation of the Company. Additionally, corporate collaboration and licensing arrangements may require us to incur non-recurring and other charges, give up certain rights relating to our intellectual property and research and development activities, increase our near and long-term expenditures, issue securities that dilute our existing stockholders, issue debt which may require liens on our assets and which will increase our monthly expense obligations, or disrupt our management and business.
The following table summarizes our changes in cash, cash equivalents, and restricted cash for the six months ended June 30, 2020 and 2019:
|
|
Six Months Ended |
|
|||||
|
|
June 30, |
|
|||||
|
|
2020 |
|
|
2019 |
|
||
|
(in thousands) |
|
|
|
|
|
|
|
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
Operating activities |
$ |
(17,808 |
) |
|
$ |
(15,001 |
) |
|
Investing activities |
|
(10,090 |
) |
|
|
14,608 |
|
|
Financing activities |
|
43,357 |
|
|
|
4,004 |
|
|
Net increase in cash, cash equivalents, and restricted cash |
$ |
15,459 |
|
|
$ |
3,611 |
|
32
Operating Activities
During the six months ended June 30, 2020, the Company used cash of approximately $17,808,000 in operating activities, as compared to approximately $15,001,000, in operating activities during the six months ended June 30, 2019, an increase of approximately $2,807,000. Cash used in operating activities during the six months ended June 30, 2020 consisted primarily of our net loss of approximately $23,651,000, and reflected the following changes in account balances: a decrease of approximately $1,337,000 in prepaid expenses, $473,000 in accounts receivable, $698,000 in research and development contract liabilities, and $2,813,000 in change in operating lease right-of-use asset, offset by $535,000 in depreciation and amortization, and $5,699,000 in stock based compensation, premium/discount on purchased securities of $55,000, operating lease liability of $2,636,000, accounts payable of $406,000, and accrued expenses of approximately $1,833,000. Cash used in operating activities during the six months ended June 30, 2019, consisted primarily of our net loss of approximately $20,650,000, and increases of approximately $522,000 in accounts receivable, $438,000 in prepaid expenses and other current assets, and $1,524,000 in operating lease liability, offset by decreases of approximately $254,000 in accounts payable, $792,000 in accrued expenses, and $1,597,000 in research and development contract liabilities, and non-cash charges of operating lease right of use amortization of approximately $2,013,000, depreciation of $410,000, and stock based compensation of $3,085,000.
Investing Activities
During the six months ended June 30, 2020, the Company’s investing activities used $10,090,000 in cash, compared to cash provided by investing activities of approximately $14,608,000 during the six months ended June 30, 2019, a decrease of $24,698,000. Cash used in investing activities during the six months ended June 30, 2020 consisted primarily of approximately $10,000,000 for the purchase of short-term investments, offset by a discount on securities purchased of approximately $51,000, and the purchase of property and equipment of approximately $141,000. Cash provided by investing activities during the six months ended June 30, 2019 consisted primarily of approximately $127,500 cash received for the sale of lab equipment and $14,500,000 for the redemption of short-term investments, offset by the purchase of property and equipment of approximately $20,000.
Financing Activities
During the six months ended June 30, 2020, the Company generated cash from financing activities of approximately $43,357,000, compared to approximately $4,004,000 in financing activities during the six months ended June 30, 2019, an increase of approximately $39,353,000. Cash from financing activities during the six months ended June 30, 2020, consisted of cash proceeds from the common stock sold through our at-the-market sales agreement with Stifel of approximately $42,353,000, net of underwriting commissions and fees, the exercise of common stock options of approximately $1,080,000, and cash used for restricted stock buy-back at vesting of approximately $76,000. Cash from financing activities during the six months ended June 30, 2019, consisted of cash proceeds from the common stock sold through our at-the-market sales agreement with Stifel of approximately $3,715,000, net of underwriting commissions and fees, and the exercise of common stock options of approximately $288,000.
Principal Commitments
Einstein License Agreement
The Company’s commitments with respect to the Einstein License are summarized above at “Significant Contracts and Agreements Related to Research and Development Activities”.
Off-balance sheet arrangements
At June 30, 2020, the Company did not have any transactions, obligations or relationships that could be considered off-balance sheet arrangements.
33
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company, we are not required to provide the information required by this Item 3.
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We are responsible for maintaining disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Disclosure controls and procedures are controls and other procedures designed to ensure that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and our principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Based on our management’s evaluation (with the participation of our principal executive officer and our principal financial officer) of our disclosure controls and procedures as required by Rule 13a-15 under the Exchange Act, our principal executive officer and our principal financial officer have concluded that our disclosure controls and procedures were effective as of June 30, 2020, the end of the period covered by this report.
Inherent Limitations on Effectiveness of Controls
Our management, including our principal executive officer and our principal financial officer, do not expect that our disclosure controls or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of a simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of control effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the three months ended June 30, 2020 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
34
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
We are not currently a party to any material legal proceedings.
ITEM 1A. RISK FACTORS
We operate in a rapidly changing environment that involves a number of risks that could materially affect our business, financial condition or future results, some of which are beyond our control. The occurrence of any of these risks could harm our business, financial condition, results of operations and/or growth prospects or cause our actual results to differ materially from those contained in forward-looking statements we have made in this report and those we may make from time to time. In evaluating the Company and its business, you should carefully consider the information included in this Quarterly Report on Form 10-Q and in other documents we file with the SEC and the risk factors previously disclosed in “Part I, Item 1A. Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2019. Except as described below, there have been no material changes to such risk factors as of June 30, 2020.
The outbreak of the novel strain of coronavirus, SARS-CoV-2, which causes COVID-19, could adversely impact our business, including our preclinical studies and clinical trials.
Public health crises such as pandemics or similar outbreaks could adversely impact our business. In December 2019, a novel strain of coronavirus, SARS-CoV-2, which causes coronavirus disease 2019 (“COVID-19”), surfaced in Wuhan, China. Since then, COVID-19 has spread to countries around the world and has been declared a pandemic by the World Health Organization. The full impact of the outbreak is uncertain at this time and continues to evolve globally. Beginning in March 2020, we undertook precautionary measures to help minimize risk of virus transmission to our employees, including the establishment of remote working standards, pausing all non-essential travel worldwide for our employees, and limiting employee attendance at industry events and in-person work-related meetings, to the extent those events and meetings are continuing. We may take additional measures, any of which could negatively affect our business.
As a result of the COVID-19 outbreak, or similar pandemics, we have and may in the future experience disruptions that could severely impact our business, preclinical studies and clinical trials, including:
|
|
• |
supply chain disruptions making it difficult to order and receive materials needed for development of our product candidates; |
|
|
• |
government responses including orders that make it difficult to remain open for business, and other seen and unforeseen actions taken by government agencies; |
|
|
• |
absenteeism or loss of employees at our Company, or at our collaborator companies, due to health reasons or government restrictions, that are needed to develop, validate, manufacture and perform other necessary functions for our operations; |
|
|
• |
delays or difficulties in enrolling patients in our clinical trials; |
|
|
• |
delays or disruptions in non-clinical experiments due to unforeseen circumstances at contract research organizations and vendors along their supply chain; |
|
|
• |
increased rates of patients withdrawing from our clinical trials following enrollment as a result of contracting COVID-19, being forced to quarantine, or otherwise; |
|
|
• |
interruption of key clinical trial activities due to limitations on travel imposed or recommended by federal or state governments, employers and others or interruption of clinical trial subject visits and study procedures; |
|
|
• |
interruption or delays in the operations of the FDA and comparable foreign regulatory agencies, which may impact approval timelines; |
|
|
• |
equipment failures, loss of utilities and other disruptions that could impact our operations or render them inoperable; and |
|
|
• |
effects of a local or global recession or depression that could harm the international banking system, and limit access to capital by the Company. |
Despite our efforts to manage and remedy these impacts to the Company, their ultimate impact depends on factors beyond our knowledge or control, including the duration and severity of the outbreak, as well as third-party actions taken to contain its spread and mitigate its public health effects. Additionally, the anticipated economic consequences of the COVID-19 pandemic have adversely impacted financial markets, resulting in high share price volatility, reduced market liquidity, and substantial declines in the market prices of the securities of many publicly traded companies. Volatile or declining markets for equities could adversely affect our ability to raise capital when needed through the sale of shares of common stock or other equity or equity-linked securities. If these market
35
conditions persist when we need to raise capital, and if we are able to sell shares of our common stock under then prevailing market conditions, we might have to accept lower prices for our shares and issue a larger number of shares than might have been the case under better market conditions, resulting in significant dilution of the interests of our stockholders.
These and other factors arising from the COVID-19 pandemic could worsen in the United States or locally at the location of our offices or clinical trials, each of which could further adversely impact our business generally, and could have a material adverse impact on our operations and financial condition and results.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
During the three months ended June 30, 2020, the Company issued an aggregate of 227,184 shares of its common stock upon the cashless exercise of outstanding warrants with an exercise price of $2.70 per share and an aggregate of 50,995 shares of its common stock upon the cashless exercise of outstanding warrants with an exercise price of $9.38 per share. The Company relied upon the exemption from the registration requirements of the Securities Act of 1933, as amended, by virtue of Section 3(a)(9) thereof for securities issued in an exchange by the issuer with its existing security holder exclusively where no commission or other remuneration is paid or given directly or indirectly for soliciting such exchange.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
None.
36
ITEM 6. EXHIBITS
|
|
|
Incorporated by Reference |
||||
|
Exhibit Number |
Exhibit Description |
Filed Herewith |
Form |
Exhibit |
Filing Date |
Registration/File No. |
|
Amended and Restated Certificate of Incorporation of the Registrant |
|
8-K |
3.1 |
12/27/17 |
001-38327 |
|
|
|
S-1 |
3.5 |
12/05/17 |
333-220550 |
||
|
Certificate of Amendment to the Company’s Amended and Restated Certificate of Incorporation |
|
8-K |
3.1 |
07/15/20 |
001-38327 |
|
|
|
|
|
|
|
|
|
|
Second Amendment to License Agreement between the Registrant and MIL 21E, LLC. |
|
8-K |
10.1 |
06/26/20 |
001-38327 |
|
|
Certification Pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 |
X |
|
|
|
|
|
|
Certification Pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 |
X |
|
|
|
|
|
|
X |
|
|
|
|
||
|
101.INS |
Inline eXtensible Business Reporting Language (XBRL) Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
X |
|
|
|
|
|
101.SCH |
Inline XBRL Taxonomy Extension Schema Document |
X |
|
|
|
|
|
101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
X |
|
|
|
|
|
101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document |
X |
|
|
|
|
|
101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document |
X |
|
|
|
|
|
101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
X |
|
|
|
|
|
104 |
The cover page from the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2020, has been formatted in Inline XBRL. |
X |
|
|
|
|
37
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
Cue Biopharma, Inc. |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Dated: August 7, 2020 |
|
By: |
|
/s/ Daniel R. Passeri |
|
|
|
|
|
|
|
|
|
|
|
Daniel R. Passeri Chief Executive Officer and Director (Principal Executive Officer) |
|
|
|
|
|
|
|
Dated: August 7, 2020 |
|
By: |
|
/s/ Kerri-Ann Millar |
|
|
|
|
|
|
|
|
|
|
|
Kerri-Ann Millar Vice President, Finance (Principal Financial and Accounting Officer) |
38