Safe Harbor and Disclaimer This presentation contains forward-looking statements about future expectations, plans and prospects for Nabriva Therapeutics, including but not limited to statements about launch and commercialization of XENLETA for the treatment of CABP, the development of CONTEPO for cUTI, the clinical utility of XENLETA for CABP and of CONTEPO for cUTI, plans for and timing of the review of regulatory filings for CONTEPO, efforts to bring XENLETA and CONTEPO to market, the market opportunity for and the potential market acceptance of XENLETA for CABP and CONTEPO for cUTI, plans for distributing and timing of the availability of XENLETA, the development of XENLETA and CONTEPO for additional indications, the development of additional formulations of XENLETA and CONTEPO, plans to pursue research and development of other product candidates, the sufficiency of Nabriva Therapeutics’ existing cash resources and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “likely,” “will,” “would,” “could,” “should,” “continue,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: Nabriva Therapeutics’ ability to successfully implement its commercialization plans for XENLETA and whether market demand for XENLETA is consistent with its expectations, Nabriva Therapeutics’ ability to build and maintain a sales force and prepare for commercial launch of XENLETA on the timeline expected, or at all, the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory authorities, Nabriva Therapeutics’ reliance on third-party manufacturers for the commercial supply of XENLETA and third-party distributors to make XENLETA available to hospitals and the medical community and the ability of such third parties to comply with regulatory requirements, the uncertainties inherent in the initiation and conduct of clinical trials, availability and timing of data from clinical trials, whether results of early clinical trials or studies in different disease indications will be indicative of the results of ongoing or future trials, uncertainties associated with regulatory review of clinical trials and applications for marketing approvals, the availability or commercial potential of CONTEPO for the treatment of cUTI or of XENLETA for the treatment of CABP, the ability to retain and hire key personnel, the sufficiency of cash resources and need for additional financing and such other important factors as are set forth in Nabriva Therapeutics’ annual and quarterly reports and other filings on file with the U.S. Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent Nabriva Therapeutics’ views as of the date of this presentation. Nabriva Therapeutics anticipates that subsequent events and developments will cause its views to change. However, while Nabriva Therapeutics may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Nabriva Therapeutics’ views as of any date subsequent to the date of this presentation. 2

XENLETA Approval Introductory Comments Ted Schroeder Chief Executive Officer

XENLETA Receives U.S. FDA Approval XENLETA (lefamulin) injection and XENLETA (lefamulin) tablets received U.S. FDA approval for the treatment of adult patients with community-acquired bacterial pneumonia (CABP) caused by susceptible microorganisms XENLETA is the first IV and oral pleuromutilin antibiotic and the first novel antibacterial class to be approved for the treatment of CABP in nearly two decades Provides medical community with an important empiric monotherapy treatment option to address increasing resistance and safety concerns with currently approved agents Expected to Be Available for Patients and Physicians in Mid-September 2019 Key Highlights 4

XENLETA Prescribing Information Highlights Jennifer Schranz, MD Chief Medical Officer

Pneumonia A Leading Cause of Morbidity, Mortality and Healthcare Cost 6 #3 Cause of hospital readmission2 ~5MM Cases Annually8 1: el Bcheraoui C, Mokdad AH, Dwyer-Lindgren L, et al. Trends and Patterns of Differences in Infectious Disease Mortality Among US Counties, 1980-2014. JAMA. 2018;319(12):1248–1260. doi:10.1001/jama.2018.2089 2: Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009-2013: Statistical Brief #196. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; November 2015. http//www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.pdf. Accessed February 23, 2016. 3: HCUP Fast Stats - Most Common Diagnoses for Inpatient Stays – 2015 https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet 4: 2017 CHARTBOOK STATIC ANALYSES - Trends in mortality rates following admission for acute myocardial infarction, chronic obstructive pulmonary disease, heart failure, pneumonia, and acute ischemic stroke. Prepared for CMS by Yale New Haven Health Services Corporation - Center for Outcomes Research and Evaluation (YNHHSC/CORE) September 2017 5: Joya-Montosa Critical Care 2015 19(Suppl 1):P19. 6: AlOtair Journal of Taibah University Medical Sciences Volume 10, Issue 3, Sept. 2015, Pages 293-299. 7: File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–41. 8: National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS) 2009 - 2010. https://www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf (Last Accessed June 21, 2019). #5 Cause of total hospitalizations3 #1 Cause of infectious death1 Mortality rate ~$17B ~15% in hospital4 ~25 – 30% in ICU5,6 Direct costs of pneumonia7

New Treatment Options For CABP Are Needed Resistance, Safety & Convenience Concerns Related to First-Line Treatment Options1 7 Mid Atlantic 38.5% East North Central 44.3% West North Central 51.7% East South Central 50.5% West South Central 57.2% Mountain 33.2% Pacific 29.1% New England 38.6% Mid Atlantic 38.5% South Atlantic 43.7% CONCERNS WITH FIRST-LINE AGENTS THREAT OF ANTIMICROBIAL RESISTANCE Distribution of Azithromycin Resistance for S. pneumoniae Across the U.S. (2015-2018)2 FDA and EMA have issued class safety alerts for fluoroquinolone, including boxed warnings β-lactams limited by hypersensitivity and lack of IV-to-oral conversion (ceftriaxone) 1First-line treatment options per consensus guidelines are macrolides, fluoroquinolones, and/or β-lactams 2:Criteria as published by CLSI (2019) Dataset provided by JMI Laboratories and the SENTRY Antimicrobial Surveillance Program; available at sentry-mvp.jmilabs.com 7

XENLETA, USPI, August 2019 Paukner S; Riedl R. Cold Spring Harbor perspectives in medicine;2017:2157-1422 Waites KB, et al. Antimicrob Agents Chemother. 2017;61:pii: e02008-16. 8 P site A site PTC of the 23S rRNA of the large 50s ribosomal subunit XENLETA inhibits bacterial protein synthesis through multiple interactions with peptidyl transferase center (PTC) of the 50S ribosomal subunit No cross-resistance with β-lactams, fluoroquinolones, macrolides, and tetracyclines Low risk of resistance development due to multiple binding sites on the bacterial ribosome XENLETA Novel Mechanism of Action

CLINICAL TRIAL EXPERIENCE INDICATION 9 XENLETA is indicated for the treatment of adult patients with community-acquired bacterial pneumonia (CABP) caused by susceptible microorganisms XENLETA was approved based on two global, Phase 3 clinical trials (LEAP 1 & 2) that enrolled 1289 adult patients with PORT scores II to V CABP 1: Adjunctive linezolid in patients with known or suspected MRSA. XENLETA First Approved IV and Oral Pleuromutilin DOSAGE AND ADMINISTRATION Dosage Treatment Duration 150 mg every 12 hours by intravenous infusion over 60 minutes* 5 to 7 days 600 mg orally every 12 hours 5 days *With the option to switch to XENLETA Tablets 600 mg every 12 hours to complete the treatment course

XENLETA is contraindicated in patients with known hypersensitivity to XENLETA or pleuromutilins XENLETA tablets are contraindicated for use with CYP3A4 substrates that prolong the QT interval 10 CONTRAINDICATIONS WARNINGS AND PRECAUTIONS QT Prolongation: Avoid use in patients with known QT prolongation, ventricular arrhythmias including torsades de pointes, and patients receiving drugs that prolong the QT interval such as antiarrhythmic agents Embryo-Fetal Toxicity: Based on findings from animal studies, XENLETA may cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception Clostridium difficile-associated Diarrhea (CDAD): Class labeling XENLETA Favorable Safety Profile in Adult Patients with CABP ADVERSE REACTIONS OCCURRING IN >2% OF PATIENTS RECEIVING XENLETA XENLETA injection: administration site reactions, hepatic enzyme elevation, nausea, hypokalemia, insomnia, headache XENLETA tablets: diarrhea, nausea, vomiting, hepatic enzyme elevation

1806 KOLs engaged and profiled; scientific exchange with 2815 HCPs Over 1,300 KOL clinical insights captured to better understand the unmet need in cUTI and CABP 13 Advisory Boards to gain insights from HCPs (ID, ED, PharmD, Chest) in 2018/19 Health Economics and Outcomes Research (HEOR) Value determination, demonstration & communication Scientific Publications Lefamulin PK/PD Supplement LEAP 1 & LEAP 2 Scientific reach from 2010 116 abstracts/posters 25 manuscripts Medical Education Live symposia Web based and enduring materials Medical Information Call Center/Pharmacovigilance Standard response letters AMCP dossier Experienced Medical Science Liaisons: Profiling and Engaging Key Option Leaders (KOLs) 11 Nabriva’s Comprehensive Approach to Medical Affairs Scientific Leader Engagement, Data Dissemination and Medical Education HCPs: Healthcare Physicians Customer Insights What do HCPs value? What drives decision making? Scientific Communication Publication Plan Clinical Evidence Value of Antimicrobial Treatment Evidence Generation Registrational Trials Life Cycle Global Value Team

XENLETA In Vitro Antimicrobial Susceptibility Testing Will Be Available at Launch Research Use Only (RUO) materials for non-diagnostic, in vitro purposes available for up to 700 hospitals Disks (Mast) MIC test strips (Liofilchem) Automated device testing (Vitek-2, Sensititre); disks (BD, Thermofisher, Liofilchem) Nabriva Observational Bacterial EvaLuation program (NOBEL) 1-800-738-3344 or email: nobel-ruo@ihma.com August 2019 1Q20 4Q19 1H20 12

XENLETA Commercial Opportunity Francesco Maria Lavino Chief Commercial Officer

Pneumonia A Leading Cause of Morbidity, Mortality and Healthcare Cost 14 #3 Cause of hospital readmission2 ~5MM Cases Annually8 1: el Bcheraoui C, Mokdad AH, Dwyer-Lindgren L, et al. Trends and Patterns of Differences in Infectious Disease Mortality Among US Counties, 1980-2014. JAMA. 2018;319(12):1248–1260. doi:10.1001/jama.2018.2089 2: Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009-2013: Statistical Brief #196. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; November 2015. http//www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.pdf. Accessed February 23, 2016. 3: HCUP Fast Stats - Most Common Diagnoses for Inpatient Stays – 2015 https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet 4: 2017 CHARTBOOK STATIC ANALYSES - Trends in mortality rates following admission for acute myocardial infarction, chronic obstructive pulmonary disease, heart failure, pneumonia, and acute ischemic stroke. Prepared for CMS by Yale New Haven Health Services Corporation - Center for Outcomes Research and Evaluation (YNHHSC/CORE) September 2017 5: Joya-Montosa Critical Care 2015 19(Suppl 1):P19. 6: AlOtair Journal of Taibah University Medical Sciences Volume 10, Issue 3, Sept. 2015, Pages 293-299. 7: File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–41. 8: National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS) 2009 - 2010. https://www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf (Last Accessed June 21, 2019). #5 Cause of total hospitalizations3 #1 Cause of infectious death1 Mortality rate ~$17B ~15% in hospital4 ~25 – 30% in ICU5,6 Direct costs of pneumonia7

Outpatients Transition of Care In-Patients Prioritized Accounts Community Hospital Point of Care: Initial Nabriva Focus 1 2 3 A: Healthcare Cost and Utilization Project (HCUP) 2013, Age 18+ (projected to 2029) B: Community Data (CDC 2009-2010), Age 18+ (projected to 2029) C: Company Sponsored Market Research, Medical Marketing Economics, July 2016, (N=122) ED Treatments & Discharge ~ 0.9MM PatientsA,C Hospital Discharge ~ 2.4MM PatientsA,C Hospital In Patient ~ 3.8MM PatientsA,C Office Based ~ 2.3MM PatientsB Patients numbers approximately six years after launch & continue to grow until LOE, following the CABP epidemiology growth**~3.0MM Hospital Initiated Outpatients and ~0.9MM of ED Outpatients. 15 3 Distinct and Significant Opportunities in the Treatment of CABP Targeted Commercial Strategy to Accelerate Adoption and Market Uptake

IPSOS Research, March 2018 (funded by Nabriva) First Line Empiric Therapy Of CABP Ceftriaxone, Macrolides And Fluoroquinolones Are The Main Choices For All Adult Patients with CABP 16 Macrolides Fluoroquinolones Cephalosporins PCN/BLI Tetracyclines Vancomycin Oxazolidinones TMP/SMZ 0% 10% 20% 30% 40% EMERGENCY DEPARTMENT 0% 20% 40% 60% FLOOR 0% 20% 40% 60% ICU 0% 10% 20% 30% 40% COMMUNITY

Safety Issues: Box Warnings, FDA Ad Com Recommendations High Risk for C. difficile Infections Off target activity: Collateral Damage Pressure to reduce use Ceftriaxone IV only Admission required Need to change antibiotic for discharge (no Oral available) Macrolide resistance to S. pneumoniae and M. pneumoniae High Risk for C. difficile Infections with β-Lactams No MRSA coverage Ceftriaxone plus Macrolide Fluoroquinolones Current First-Line Treatment Options for CABP Have Limitations 17 30% of patients treated for CABP are receiving 2nd/3rd line Therapy A Hospitals on average lose money when using generics due to extended length of stay and readmission B A Company sponsored market research, Medical Marketing Economics, July 2016, (N=122) B Company sponsored market research, Avalere 2016 Analysis of CABP 2015 MedPAR data

Emerging Fluoroquinolone Safety Concerns FDA web site: Fluoroquinolone antimicrobial drugs information. EMA website: https://www.ema.europa.eu/en/news/fluoroquinolone-quinolone-antibiotics-prac-recommends-new-restrictions-use-following-review Communications Issued by FDA and EMA 18 2008 2011 2013 2015 2016 2018 July 2008 FDA Tendinitis and tendon rupture; “consider risk/benefit in individuals” Feb 2011 FDA Worsening symptoms in patients with myasthenia gravis Aug 2013 FDA Risk of peripheral neuropathy Nov 2015 FDA Advisory Committee meeting July 2018 FDA: Distinction between warning for mental health and CNS effects; include risk of coma with hypoglycemia July 2016 FDA “Serious side effects associated with FQs generally outweighs the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated UTI” Reserved for those without alternative treatment options. Adverse effects include tendons, muscles, joints, nerves, CNS May 2018 EMA: Recommendation to restrict the use of fluoroquinolone following a review of disabling and potentially long-lasting side effects Dec 2018 FDA: “fluoroquinolone antibiotics can increase the occurrence of rare but serious events of ruptures or tears in the main artery of the body, called the aorta” Oct 2018 EMA: New warning regarding risk of aortic aneurysm and dissection associated with fluoroquinolones for systemic and inhalation use

Fluoroquinolones Use Clear Trend of Reduced Use in the Hospital and Community 19 Source: IQVIA, NSP Audit Extended Units, All-Outlet (Hospital & Retail) Extended units are the number of tablets, capsules, milliliters, ounces, etc. of a product shipped in each unit. This number is calculated by multiplying the number of units by the product size. Increased fluoroquinolone restrictions, including specific limitations for CABP at some hospitals, are leading to a reduced use Fluoroquinolones remain the most commonly prescribed antibiotics in the hospital FQ Usage in the Hospital & Retail Combined has Declined in Recent Years 0 500,000,000 1,000,000,000 1,500,000,000 2,000,000,000 2,500,000,000 3,000,000,000 3,500,000,000 4,000,000,000 4,500,000,000 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 Extended Units Fluoroquinolones Volume Trends (Units)

The most important unmet needs in CABP New Drugs with Base: All Respondents: Total (n=291) across ID/ED/PCP/ICU/Pharm D/Hospitalists Responses on a 1-7 Scale: 1-2 is Lowest Need and 6-7 is Highest Need IPSOS Research, March 2018 (funded by Nabriva) The majority of respondents believe there is a high need for new CABP treatments Activity against multi-resistant pathogens Low propensity to develop resistance Easy IV to Oral conversion Good safety & tolerability profile 20 HCPs Believe There is An Extremely High Need For New CABP Treatment Options Differentiated Treatments Needed Both in the Hospital and Community Setting

Addressing Antimicrobial Resistance: MSDRG: certain diagnosis related group codes (ICD-10) that include an antimicrobial resistance (MDRO) will be designated as a complication or comorbidity, which generally results in assignment to a higher payment for inpatient stays. Increased DRG payments will begin on October 1st 2019 New NTAP Policy CMS has finalized an alternative and simplified New Technology Add-on Payment (NTAP) pathway for Antimicrobial Products designated by FDA as Qualified Infectious Disease Products (QIDPs) CMS is increasing the payment for QIDP antimicrobials granted NTAP from 50% to 75% The final rule will update Medicare Payment Policies for hospitals for fiscal year (FY) 2020 which begins on October 1st 2019 New NTAP applications will be filed by early October 2019 and, if granted, will be effective in October 2020 CMS Inpatient Prospective Payment System (IPPS), August 2, 2019 21 Healthcare Policy Reform Pathways for Increased Reimbursement for New Innovative Antibiotics Are Becoming a Reality

Complete Spectrum of Coverage of Main CABP Pathogens Monotherapy with appropriate spectrum for CABP aligned with principles of antimicrobial stewardship; Low risk of C. difficile infection Generally Well Tolerated Profile Low rates of discontinuations due to adverse events in Phase 3 clinical trials New Class for IV and Oral Use New Mechanism of Action Overcomes existing mechanism of resistance in vitro; Low propensity for development of bacterial resistance Convenient for Patients in Hospital, Transition of Care and the Community IV and bioequivalent oral formulations approved; Short course of monotherapy Excellent PK-PD Profile No loading dose; no dose adjustment for renal insufficiency; mild food interaction potential 22 XENLETA A Promising Treatment Option for CABP

The Right Spectrum, Right from the Start 23 1st Line Empiric Therapy for CABP Patients in the Emergency Department that can Avoid Hospitalization 1st Line Empiric Therapy for CABP Patients admitted to the Hospital with Co-Morbidities and limited options 1st Line Empiric Therapy for CABP Patients admitted to the Hospital then Transitioned to Oral and Discharged XENLETA Target Patient Population

Critical Success Factors for an Antibiotic Launch Preparation, Preparation, Preparation! 24

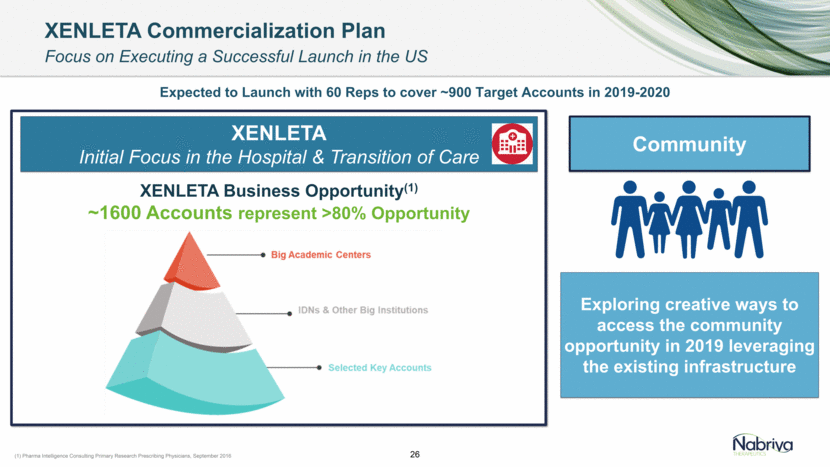
Focus on Transition of Care (Outpatients) Market Development identified accounts with: High macrolide or β-Lactams resistance Higher than average: C. difficile rate CABP mortality rate CABP readmission rate Reimbursement penalties for CABP Fluoroquinolone restrictions for CABP Higher incentive ($) to deflect admissions or to accelerate patients discharge on oral therapy XENLETA Hospital Initiated Business Opportunity(1) ~1,600 Accounts Represents >80% CABP Patients Symphony Data: Antibiotic Spend in US Hospitals, 2016 As of August 10, 2019 Priority Accounts >600 Accounts Profiled to Date(2) Nabriva’s Comprehensive Approach to Pre-Commercial Activities Focus on Identification of Priority Accounts and Maximization of Patients Access 25 Engagement with Key Payers (2) Meetings with Key Payer Accounts ~95% of the Targeted Lives Corporate Overview and Disease State Overview Clinical Review of LEAP 1 & 2 Topline Data (by Medical Affairs) Contracting Process initiated with the majority of Key Payers and contracts finalized with large, integrated health systems

XENLETA Initial Focus in the Hospital & Transition of Care XENLETA Commercialization Plan Focus on Executing a Successful Launch in the US Exploring creative ways to access the community opportunity in 2019 leveraging the existing infrastructure XENLETA Business Opportunity(1) ~1600 Accounts represent >80% Opportunity (1) Pharma Intelligence Consulting Primary Research Prescribing Physicians, September 2016 26 Expected to Launch with 60 Reps to cover ~900 Target Accounts in 2019-2020 Community
XENLETA Distribution Plan Specialty Distribution Model and Specialty Pharmacies Network to Support Transition of Care 27 Specialty Distributors McKesson Plasma and Biologics ASD Healthcare Cardinal SD 3PL Hospital Specialty Pharmacies (Supporting Transition of Care) Hospital Hospital Our Goal: XENLETA is available to clinicians in mid-September 2019

Nabriva Specialty Pharmacy Network Partners Removing Barriers to Ensure Patient Access to Oral XENLETA Focus on IV to Oral Transition and Aligned to Key Hospital Targets Direct-to-Patient Services, Meds to Beds and Home Fulfilment Consistent with XENLETA Dosing Schedule Expected to be available at: ~300 Specialty Retail IDN, Community and Medical Office Building Sites Bedside delivery at discharge, same day pick up and mail to home Facilities in 46 States, serving patients across 50 States 96% of US population covered 76 nationwide care management centers & 90 ambulatory Infusion Suites (IV) Home care infusion services (IV) and Mail to home service (Oral) Outpatient Hospital Pharmacies and Specialty Pharmacy Network will ensure continuity of care Cleveland Clinic* UPMC* Highmark* Kaiser* Geisinger* Local Outpatient Hospital Pharmacies * Listed Institutions are just examples of Hospitals that do have their own Outpatients Pharmacies to support Transition of Care 28

Nabriva Hub Services Removing Barriers to Ensure Patient Access to Oral XENLETA In Partnership with RX Cross-Road Multiple services available for providers and patients to ensure a smooth transition from Hospital to Home Available for eligible patients with commercial insurance Limited out-of-pocket to secure adherence No eligible patient will pay more than $50 / prescription 29

Closing Remarks Ted Schroeder Chief Executive Officer

XENLETA Receives U.S. FDA Approval Well-Positioned for a Successful Launch Key Drivers of Success 31 Product XENLETA has a novel mechanism of action with a complete spectrum of activity for the main CABP pathogens with a low risk of resistance Demonstrated efficacy as an empiric monotherapy with activity against multi-resistant pathogens Generally well-tolerated safety profile XENLETA offers short course IV or oral dosing flexibility People Highly experienced and talented HQ and field-based team Preparation Diverse and thorough preparation over the past two years focusing on: Medical education Profiling the right hospitals for launch Creating an innovative distribution network Optimizing payor coverage to help with patient access

Questions?



