| Maximum Severity of Treatment-Related AEs (TRAEs) |
Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade | ||||||||||||||||||||
| AST elevation |
8(7) | — | — | — | 8(7) | ||||||||||||||||||||
| Electrocardiogram QT prolonged |
1(1) | — | — | — | 1(1) | ||||||||||||||||||||
| TRAEs leading to dose reduction*, n (%) |
— | 10(9) | 5(5) | † | — | 15(14) | |||||||||||||||||||
| TRAEs leading to treatment discontinuation, n (%) |
— | — | — | 1 | (1)^ | 1(1) | |||||||||||||||||||
AE, adverse event; ALT, alanine transaminase; AST, aspartate transferase; TRAEs, treatment-related adverse events.
‡ Includes preferred terms of dermatitis acneiform, rash maculopapular, rash, rash pustular, erythema, rash erythematous; multiple types of rash may have occurred in the same patient.
* The most common reason for dose reduction was rash.
† Grade 3 TRAEs leading to reduction were rash (n=4), including one patient with a dose reduction due to rash and decreased appetite, and stomatitis (n=1).
^ One Grade 4 TRAE occurred in a patient with PDAC at the 80 mg dose level who had a large intestine perforation at the site of an invasive tumor that reduced in size while on treatment.
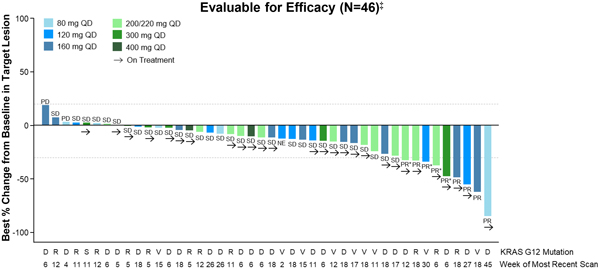
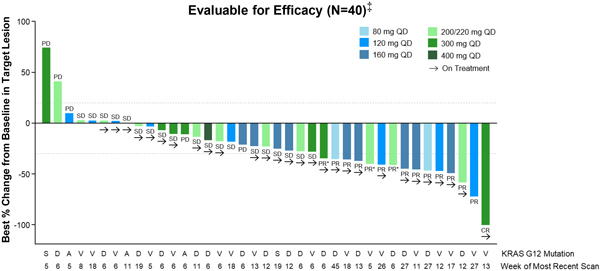
Clinical activity was evaluated as of the Data Cut-off Date in patients with NSCLC (n=40) and PDAC (n=46) who had received the first dose of RMC-6236 at least eight weeks prior to the Data Cut-off Date (n=86). Confirmed objective responses included tumors harboring KRAS mutations G12D, G12V or G12R, and disease control was observed across all KRAS mutations, including G12A and G12S.
As of the Data Cut-off Date, RMC-6236 demonstrated preliminary evidence of clinical activity in efficacy-evaluable NSCLC patients (Figure 1).
Figure 1. RMC-6236-001: Change in tumor burden from efficacy-evaluable KRASG12X NSCLC patients
Data extracted October 12, 2023.
QD, daily dosing; PD, progressive disease; SD, stable disease; PR, partial response; PR*, unconfirmed PR per Response Evaluation Criteria in Solid Tumors (“RECIST”) 1.1; CR, complete response.
‡ Patients who received first dose of RMC-6236 at least eight weeks prior to data extract date.
Among the efficacy evaluable NSCLC patients, the objective response rate was 38 percent, with one patient achieving a complete response (“CR”) as a best response and 14 patients achieving a partial response (“PR”) (including three unconfirmed PRs) (Table 2). The disease control rate (“DCR”) in this NSCLC population was 85 percent.