Trillium Therapeutics Inc.: Exhibit 99.2 - Filed by newsfilecorp.com

MANAGEMENT’S DISCUSSION AND ANALYSIS
FOR THE THREE AND NINE MONTHS ENDED
SEPTEMBER 30,
2018 AND 2017
Dated: November 13, 2018
2488 Dunwin Drive
Mississauga, Ontario, L5L 1J9
www.trilliumtherapeutics.com
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
ABOUT THIS MANAGEMENT’S DISCUSSION AND ANALYSIS
All references in this management’s discussion and analysis, or
MD&A to “the Company”, “Trillium”, “we”, “us”, or “our” refer to Trillium
Therapeutics Inc. and the subsidiaries through which it conducts its business,
unless otherwise indicated or the context requires otherwise.
The following MD&A is prepared as of November 13, 2018 for
Trillium Therapeutics Inc. for the three and nine months ended September 30,
2018 and 2017, and should be read in conjunction with the unaudited interim
condensed consolidated financial statements for the three and nine months ended
September 30, 2018 and 2017, and the annual audited consolidated financial
statements and accompanying notes for the years ended December 31, 2017 and
2016, which have been prepared by management in accordance with International
Financial Reporting Standards, or IFRS as issued by the International Accounting
Standards Board, or IASB. Our IFRS accounting policies are set out in note 3 of
the annual audited consolidated financial statements for the years ended
December 31, 2017 and 2016. All amounts are in thousands of Canadian dollars,
except per share amounts and unless otherwise indicated. References to “U.S. $”
are to United States dollars.
CAUTIONARY STATEMENT ABOUT FORWARD-LOOKING STATEMENTS
This MD&A contains forward-looking statements within the
meaning of applicable securities laws. All statements contained herein that are
not clearly historical in nature are forward-looking, and the words
“anticipate”, “believe”, “expect”, “estimate”, “may”, “will”, “could”,
“leading”, “intend”, “contemplate”, “shall” and similar expressions are
generally intended to identify forward-looking statements. Forward-looking
statements in this MD&A include, but are not limited to, statements with
respect to:
| |
• |
our expected future loss and accumulated
deficit levels; |
| |
• |
our projected financial position and estimated
cash burn rate; |
| |
• |
our requirements for, and the ability to
obtain, future funding on favorable terms or at all; |
|
• |
our projections for the SIRPαFc development
plans and progress of each of our products and technologies, particularly
with respect to the timely and successful completion of studies and trials
and availability of results from such studies and trials; |
|
• |
our plans to focus development of TTI-621 on
patients with cutaneous T cell lymphoma based on our early clinical
results; |
| |
• |
our expectations about our products’ safety and
efficacy; |
|
• |
our expectations regarding our ability to
arrange for and scale up the manufacturing of our products and
technologies; |
|
• |
our expectations regarding the progress, and
the successful and timely completion, of the various stages of the
regulatory approval process; |
| |
• |
our expectations about the timing of achieving
milestones and the cost of our development programs; |
|
• |
our observations and expectations regarding the relative
low binding of SIRPαFc to red blood cells, or RBCs, compared to anti-CD47
monoclonal antibodies and proprietary CD47-blocking agents and the
potential benefits to patients; |
| |
• |
our ability to intensify the dose of TTI-621
with the goal of achieving increased blockade of CD47; |
|
• |
our expectation that we will achieve levels of
TTI-622 in patients sufficient to obtain sustained CD47 blockade; |
|
• |
our expectation that TTI-622 is likely to be
more effective in combination with agents that provide additional “eat”
signals to macrophages or other forms of immune activation; |
| |
• |
our plans to market, sell and distribute our
products and technologies; |
| |
• |
our expectations regarding the acceptance of
our products and technologies by the market; |
| |
• |
our ability to retain and access appropriate
staff, management and expert advisers; |
|
• |
our expectations about the differentiated
nature and discovery research capabilities of Fluorinov Pharma Inc., or
Fluorinov; |
- 1 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
|
• |
our ability to generate future product
development programs with improved pharmacological properties and
acceptable safety profiles using Fluorinov technology; |
|
• |
our expectations about whether various clinical
and regulatory milestones with an existing Fluorinov compound will be
achieved; |
|
• |
our expectations of the final quantum and form
of any future contingent milestone payments related to the Fluorinov
acquisition; |
|
• |
our expectations of the ability to secure the requisite
approvals (including approvals from the Toronto Stock Exchange, or TSX,
and the NASDAQ Stock Market, or NASDAQ) with respect to the issuance of
any common shares in satisfaction of future milestone payments; |
| |
• |
our ability to secure strategic partnerships
with larger pharmaceutical and biotechnology companies; |
|
• |
our strategy to acquire and develop new
products and technologies and to enhance the safety and efficacy of
existing products and technologies; |
|
• |
our expectations with respect to existing and future
corporate alliances and licensing transactions with third parties, and the
receipt and timing of any payments to be made by us or to us in respect of
such arrangements; and |
| |
• |
our strategy with respect to the protection of
our intellectual property. |
All forward-looking statements reflect our beliefs and
assumptions based on information available at the time the assumption was made.
These forward-looking statements are not based on historical facts but rather on
management’s expectations regarding future activities, results of operations,
performance, future capital and other expenditures (including the amount, nature
and sources of funding thereof), competitive advantages, business prospects and
opportunities.
By its nature, forward-looking information involves numerous
assumptions, inherent risks and uncertainties, both general and specific, known
and unknown, that contribute to the possibility that the predictions, forecasts,
projections or other forward-looking statements will not occur. In evaluating
forward-looking statements, readers should specifically consider various
factors, including the risks outlined under the heading “Risk Factors” in this
MD&A. Some of these risks and assumptions include, among others:
|
• |
substantial fluctuation of losses from quarter
to quarter and year to year due to numerous external risk factors, and
anticipation that we will continue to incur significant losses in the
future; |
| |
• |
uncertainty as to our ability to raise
additional funding to support operations; |
| |
• |
our ability to generate product revenue to
maintain our operations without additional funding; |
|
• |
the risks associated with the development of
our product candidates which are at early stages of development; |
|
• |
positive results from preclinical and early
clinical research are not necessarily predictive of the results of
later-stage clinical trials; |
| |
• |
reliance on third parties to plan, conduct and
monitor our preclinical studies and clinical trials; |
|
• |
our product candidates may fail to demonstrate
safety and efficacy to the satisfaction of regulatory authorities or may
not otherwise produce positive results; |
|
• |
risks related to filing Investigational New
Drug applications, or INDs, to commence clinical trials and to continue
clinical trials if approved; |
| |
• |
the risks of delays and inability to complete
clinical trials due to difficulties enrolling patients; |
| |
• |
competition from other biotechnology and
pharmaceutical companies; |
|
• |
our reliance on the capabilities and experience
of our key executives and scientists and the resulting loss of any of
these individuals; |
| |
• |
our ability to fully realize the benefits of
acquisitions; |
| |
• |
our ability to adequately protect our
intellectual property and trade secrets; |
| |
• |
our ability to source and maintain licenses
from third-party owners; |
| |
• |
the risk of patent-related litigation; and
|
| |
• |
our expectations regarding our status as a
passive foreign investment company, or PFIC, |
all as further and more fully described under the heading “Risk
Factors” in this MD&A.
- 2 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Although the forward-looking statements contained in this
MD&A are based upon what our management believes to be reasonable
assumptions, we cannot assure readers that actual results will be consistent
with these forward-looking statements.
Any forward-looking statements represent our estimates only as
of the date of this MD&A and should not be relied upon as representing our
estimates as of any subsequent date. We undertake no obligation to update any
forward-looking statement or statements to reflect events or circumstances after
the date on which such statement is made or to reflect the occurrence of
unanticipated events, except as may be required by securities legislation.
BUSINESS
Overview
We are a clinical stage immuno-oncology company developing
innovative therapies for the treatment of cancer. Our lead program, TTI-621, is
a SIRPαFc fusion protein that consists of the extracellular CD47-binding domain
of human signal regulatory protein alpha, or SIRPα, linked to the Fc region of a
human immunoglobulin G1, or IgG1. It is designed to act as a soluble decoy
receptor, preventing CD47 from delivering its inhibitory (“do not eat”) signal.
Neutralization of the inhibitory CD47 signal enables the activation of
macrophage anti-tumor effects by pro-phagocytic (“eat”) signals. The IgG1 Fc
region of TTI-621 may also assist in the activation of macrophages by engaging
Fc receptors. Two phase 1 clinical trials evaluating TTI-621 are ongoing. In
these trials, TTI-621 has shown single agent activity by both local and/or
systemic delivery in multiple B- and T-cell lymphoma indications and has been
well tolerated in over 180 patients to date.
We are also developing a second SIRPαFc fusion protein,
TTI-622. TTI-622 consists of the extracellular CD47-binding domain of human
SIRPα linked to a human immunoglobulin G4, or IgG4 Fc region, which has a
decreased ability to engage Fc receptors than an IgG1 Fc. We initiated a phase 1
clinical trial for TTI-622 in June 2018. Both SIRPαFc fusion proteins enable
CD47 blockade with different levels of Fc receptor engagement on macrophages and
thus may find unique applications.
We also have a proprietary medicinal chemistry platform, using
unique fluorine chemistry, which permits the creation of new chemical entities
with improved pharmacological properties from validated drugs and drug
candidates. Our most advanced preclinical program stemming from this platform is
an epidermal growth factor receptor, or EGFR antagonist with increased uptake
and retention in the brain. In addition, a number of compounds directed at
undisclosed immuno-oncology targets are currently in the discovery phase.
Our Strategy
Our goal is to become a leading innovator in the field of
oncology by targeting immune-regulatory pathways that tumor cells exploit to
evade the host immune system. We believe we have the most differentiated and
comprehensive approach to targeting CD47, with the development of two SIRPαFc
fusion proteins, monotherapy and combination therapy approaches, and both
intravenous and intratumoral administration. We intend to:
|
• |
Rapidly advance the clinical development of
TTI-621. Because CD47 is highly expressed by multiple liquid and
solid tumors, and high expression is correlated with worse clinical
outcomes, we believe SIRPαFc has potential to be effective in a variety of
cancers. In our clinical trials to date, we have rapidly enrolled over 186
patients with multiple tumor types where TTI-621 may provide clinical
benefit to find specific malignancies of interest for further development.
|
| |
|
|
|
• |
Focus our TTI-621 clinical program on promising
cancer indications. From our broad clinical approach, we found a
number of cancers where we saw positive responses in patients. We are
particularly interested in our initial results treating mycosis fungoides,
a predominant form of cutaneous T cell lymphoma, or CTCL, and we are
focusing our near-term efforts on patients with T cell malignancies
broadly to include both CTCL and peripheral T cell lymphoma, or PTCL,
patients. |
- 3 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
|
• |
Expand our portfolio of
SIRPαFc constructs through advancement of
TTI-622. Our expertise in designing fusion proteins allows us to
explore alternative approaches to blocking CD47 that may be advantageous
for certain applications. We began testing TTI-622 in a phase 1 clinical
trial in June 2018 as a second and differentiated approach to block CD47.
We expect TTI-622 may be of particular interest when used in combination
with other anti-cancer drugs, including immunomodulatory agents.
|
| |
|
|
|
• |
Build a pipeline of novel oncology products using
our proprietary medicinal chemistry platform. We have several
preclinical and discovery stage assets developed using our proprietary
fluorine chemistry platform. We plan to advance these novel oncology
products for internal development or out-license. |
Our CD47 Clinical Pipeline

SIRPαFc
Blocking the CD47 “do not eat” signal using a
SIRPαFc decoy receptor
The immune system is the body’s mechanism to identify and
eliminate pathogens, and can be divided into the innate immune system and the
adaptive immune system. The innate immune system is the body’s first line of
defense to identify and eliminate pathogens and consists of proteins and cells,
such as macrophages, that identify and provide an immediate response to
pathogens. The adaptive immune system is activated by, and adapts to, pathogens,
creating a targeted and durable response. Cancer cells often have the ability to
reduce the immune system’s ability to recognize and destroy them.
Macrophages are a type of white blood cell that can ingest and
destroy (phagocytose) other cells. Macrophage activity is controlled by both
positive “eat” and negative “do not eat” signals. Recently, a role for
macrophages in the control of tumors has been described. Tumor cells may express
“eat” signals (e.g., calreticulin) that make themselves visible to macrophages.
To counterbalance this increased visibility the tumor cells often express high
levels of CD47, which transmits a “do not eat” signal by binding SIRPα on the
surface of macrophages. Elevated expression of CD47 has been observed across a
range of hematological and solid tumors. In many cases, high CD47 expression was
shown to have negative clinical consequences, correlating with more aggressive
disease and poor survival.
Our lead program, TTI-621, is a novel SIRPαFc fusion protein
that harnesses the innate immune system by blocking the activity of CD47.
TTI-621 is a protein that consists of the CD47-binding domain of human SIRPα
linked to the Fc region of IgG1. It is designed to act as a soluble decoy
receptor, preventing CD47 from delivering its inhibitory signal. Neutralization
of the inhibitory CD47 signal enables the activation of macrophage anti-tumor
effects by the pro-phagocytic “eat” signals. The IgG1 Fc region of TTI-621 may
also assist in the activation of macrophages by engaging Fc receptors. A second
SIRPαFc fusion protein, TTI-622, entered phase 1 testing in June 2018. TTI-622
consists of the same CD47-binding domain of human SIRPα and is
linked to the Fc region of IgG4. The IgG4 Fc region of TTI-622 is expected to
have a decreased ability to engage activating Fc receptors compared to an IgG1
Fc, and thus provide a more modest “eat” signal to macrophages, allowing for
greater tolerability and higher CD47 blockade but lower potency. TTI-622 will
allow us to assess how higher CD47 blockade with an IgG4-based agent in patients
compares to lower CD47 blockade with an IgG1-based drug (TTI-621).
- 4 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
In preclinical studies, TTI-621 and TTI-622 frequently
triggered significant macrophage-mediated tumor cell phagocytosis in vitro
compared to control treatment. In vivo, both fusion proteins exhibited
anti-tumor activity in human xenograft models.
In addition to their direct anti-tumor activity, macrophages
can also function as antigen-presenting cells and stimulate antigen-specific T
cells. Thus, it is possible that increasing tumor cell phagocytosis after
SIRPαFc exposure may result in enhanced adaptive immunity. In support of this,
CD47 antibody blockade has been recently shown to augment antigen presentation
and prime an anti-tumor cytotoxic T cell response in immune-competent mice. In
2016, we presented data demonstrating that TTI-621 can augment antigen-specific
T cell responses in vitro. CD47 blockade has also been reported to promote
tumor-specific T cell responses through a dendritic cell-based mechanism,
although the effect of SIRPαFc on dendritic cells is currently unknown.
The figure below illustrates how SIRPαFc blocks the CD47 “do
not eat” signal and engages activating Fc receptors on macrophages, leading to
tumor cell phagocytosis, increased antigen presentation and enhanced T cell
responses.
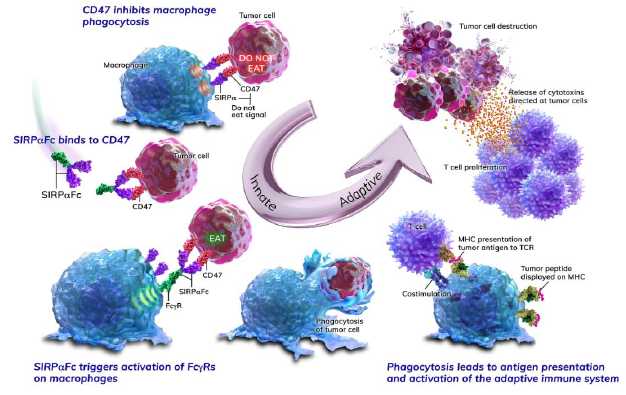
By inhibiting the CD47 “do not eat” signal, we believe SIRPαFc
has the ability to promote the macrophage-mediated killing of tumor cells in a
broad variety of cancers both as a monotherapy and in combination with other
immune therapies. Both SIRPαFc fusion proteins enable CD47 blockade with
different levels of Fc receptor engagement on macrophages and thus may find
unique applications.
- 5 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Combination Therapy
We believe that SIRPαFc enhancement of macrophage activity, and
possibly T cell responses, could be synergistic with other immune-mediated
therapies. Since many cancer antibodies work at least in part by activating
cells of the innate immune system, it may be possible to enhance the potency of
these agents by blocking the negative “do not eat” CD47 signal that tumor cells
deliver to macrophages. In fact, we have observed anti-tumor activity when
combining SIRPαFc with Rituxan® in both preclinical studies and in B lymphoma
patients. We hypothesize that SIRPαFc may act synergistically with other
immunological agents, including T cell checkpoint inhibitors (e.g. pembrolizumab
and nivolumab), cancer vaccines, oncolytic viruses or chimeric antigen receptor,
or CAR T cells. We are currently testing several of these combinations in
clinical trials.
SIRPαFc Clinical Development
– TTI-621
We are recruiting patients in two ongoing phase 1 clinical
trials; one with intratumoral injection and one with intravenous infusion. These
trials were designed to establish a safe dosing level, characterize safety,
pharmacokinetics, and pharmacodynamics and treat a broad range of malignancies
searching for evidence of antitumor activity.
Intratumoral administration – TTI-621
In a multi-center, open-label phase 1 trial, TTI-621 is being
delivered by intratumoral injection in patients with relapsed and refractory,
percutaneously-accessible cancers. In the escalation phase, patients were
enrolled in sequential dose cohorts to receive intratumoral injections of
TTI-621 that increased in dose and dosing frequency to characterize safety,
pharmacokinetics, pharmacodynamics and preliminary evidence of antitumor
activity. In addition, detailed evaluation of serial, on-treatment tumor
biopsies of both injected and non-injected cancer lesions is being performed to
help characterize the tumor microenvironment. Preliminary data from the
escalation phase were reported at the American Society of Hematology
59th Annual Meeting in December 2017. In September 2018, updated data
from 23 patients with relapsed/refractory mycosis fungoides/Sézary syndrome, 20
of whom only received induction therapy consisting of 1-6 injections over 2
weeks were presented at the European Organisation for Research and Treatment of
Cancer, Cutaneous Lymphoma Task Force, or EORTC CLTF, meeting. Local delivery of
TTI-621 was well tolerated, with no treatment-related > Grade 3 adverse
events or dose-limiting toxicity observed. Reductions in Composite Assessment of
Index Lesion Severity, or CAILS, scores, which measure local lesion responses,
were observed in 89% of patients, with 44% exhibiting reductions of 50% or
greater. These responses occurred rapidly within the 2-week induction period.
Similar CAILS scores changes were seen in adjacent non-injected lesions,
suggesting locoregional effects that were not confined to the site of injection.
Evidence of a systemic effect was observed in 1 of 2 patients receiving
continuation monotherapy beyond the 2-week induction therapy. In addition, data
suggest a combination effect with pegylated IFN-alpha-2a. Collectively, the data
demonstrate that CTCL appears biologically responsive to intratumoral injections
of TTI-621.
We have amended the protocol for this trial to focus on
recruiting additional patients with T cell malignancies, and specifically CTCL,
to determine if the preliminary results will be seen in a larger patient
population. Patients are currently being enrolled in the expansion phase of the
trial in which they receive 10 mg of TTI-621 three times per week for two weeks
followed by weekly dosing, to further characterize safety and efficacy. In
addition, patients may receive intratumoral TTI-621 in combination with other
anti-cancer therapies (anti-PD-1 or anti-PD-L1, pegylated interferon α2a,
talimogene laherparepvec or radiation). We have modified this trial to allow for
the increase in the size of each cohort from 12 to 40 patients based on early
signs of clinical benefit.
Intravenous administration – TTI-621
We are enrolling patients with advanced hematologic
malignancies in a phase 1b clinical trial with intravenous administration of
TTI-621. The most recent data from the ongoing expansion phase were reported at
the 16th Annual Discovery on Target conference in September 2018. Based on an
expanded data set of 163 patients, weekly infusions of TTI-621 were shown to be
well tolerated. Thrombocytopenia was the most frequent grade 3 or higher
treatment-emergent adverse event, occurring in 20% of patients. Platelet
reductions, however, were shown to be transient and pre-dose platelet levels
remained steady during the course of the study. Notably, the reversible thrombocytopenia did not lead to an increased risk of bleeding
and had no impact on drug delivery, nor was there a significant impact of
TTI-621 on hemoglobin levels. Monotherapy efficacy was observed in patients with
mycosis fungoides (19% overall response rate, or ORR, n=21), peripheral T-cell
lymphoma, or PTCL (25% ORR, n=12), and diffuse large B-cell lymphoma, or DLBCL
(25% ORR, n=8), and in DLBCL patients when combined with rituximab (25% ORR,
n=24). This clinical activity was observed in patients receiving relatively low
doses of drug (0.2 mg/kg for monotherapy or 0.1 mg/kg in combination with
rituximab). Dose intensification beyond 0.2 mg/kg is currently ongoing, and
doses of 0.5 mg/kg have been well tolerated for up to 27 weeks.
- 6 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
We are focusing our near-term efforts on patients with CTCL and
PTCL, following the early signals of efficacy observed in the intratumoral
trial. These patients are being enrolled in separate cohorts that will be
evaluated using a Simon 2-stage design with a maximum of 35 subjects in each
cohort. We also introduced a standardized intra-subject dose intensification
schedule for all newly enrolled subjects to increase drug exposure.
TTI-621 has recently been granted an Orphan Drug Designation by
the U.S. Food and Drug Administration, or FDA for the treatment of cutaneous T
cell lymphoma. Orphan Drug Designation qualifies the sponsor of the drug
candidate for various development incentives, which may include tax credits for
qualified clinical testing, an exemption from fees under the Prescription Drug
User Fee Act, and a seven-year marketing exclusivity period following approval.
SIRPαFc Clinical Development
– TTI-622
A second SIRPαFc fusion protein, TTI-622, is in clinical
development. A two-part, multicenter, open-label, phase 1a/1b study of TTI-622
in patients with advanced relapsed or refractory lymphoma or multiple myeloma
was initiated in June 2018. In the phase 1a dose-escalation part, patients will
be enrolled in sequential dose cohorts to receive TTI-622 once weekly to
characterize safety, tolerability, pharmacokinetics, and to determine the
maximum tolerated dose. In the phase 1b part, patients will be treated with
TTI-622 in combination with rituximab, a proteasome inhibitor-containing
regimen, or a PD-1 inhibitor. Rituximab and proteasome inhibitors may provide
additional “eat” signals that could enhance the efficacy of TTI-622. A PD-1
inhibitor may help amplify any anti-tumor T cell response generated by TTI-622.
TTI-622 consists of the same extracellular CD47-binding domain
of human SIRPα as TTI-621 but has a different Fc region (IgG4 Fc instead of IgG1
Fc), which provides a more modest “eat” signal than IgG1 due to more limited
interactions with activating Fc receptors. Preclinical studies suggest that
IgG4-based SIRPαFc fusion proteins have greater tolerability but lower potency
than IgG1-based fusion proteins. We therefore expect to achieve higher levels of
TTI-622 in patients compared to TTI-621, leading to greater and more sustained
CD47 blockade. Thus, TTI-622 will allow us to assess how higher CD47 blockade
with an IgG4-based agent in patients compares to lower CD47 blockade with the
IgG1-based TTI-621. Due to the lower potency of the IgG4 Fc, we expect that
TTI-622 is likely to be more effective in combination with agents that provide
additional “eat” signals to macrophages or other forms of immune activation.
Preclinical TTI-622 data were recently reported at the 2018
Annual Meeting of the American Association for Cancer Research. The data
demonstrate that TTI-622 induces the phagocytosis of a broad panel of tumor
cells derived from patients with both hematological and solid tumors. As a
monotherapy, TTI-622 treatment resulted in decreased tumor growth and improved
survival in a B cell lymphoma xenograft model, and enhanced the efficacy of
cetuximab (anti-EGFR) and daratumumab (anti-CD38) antibodies in solid and
hematological xenograft models, respectively. Unlike CD47-blocking antibodies,
TTI-622 bound minimally to RBCs and did not induce hemagglutination in vitro. We
believe that this property could give TTI-622 best-in-class status among
IgG4-based blocking agents currently in clinical development.
SIRPαFc Key Takeaways
|
• |
Multiple clinical approaches. We believe we
have the most systematic and comprehensive approach to CD47 with two decoy
receptors in development with different Fc functions, monotherapy and
combination therapy approaches, and intravenous and intratumoral delivery
modalities. |
- 7 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
|
• |
Demonstrated clear signals of activity.
TTI-621 monotherapy has produced positive signals of activity in CTCL,
PTCL and DLBCL patients. A signal of activity was also seen in DLBCL
patients when combined with rituximab. |
| |
|
|
| |
• |
Tolerability and safety. TTI-621
has been well tolerated in over 180 patients to date. |
| |
|
|
|
• |
Clear paths forward. We are focusing our
development on intratumoral monotherapy and combination therapy in CTCL;
intravenous monotherapy in both CTCL and PTCL; and intravenous combination
therapy in B-cell lymphoma. |
SIRPαFc
Competition
There are a number of companies developing blocking agents to
the CD47-SIRPα axis, which can be broadly classified into six groups which
include, but are not limited to:
|
• |
CD47-specific antibodies: Forty
Seven Inc (phase 2); Celgene Corporation, Surface Oncology, Innovent
Biologics (Suzhou) Co. (phase 1); Arch Oncology, I-Mab Biopharma, Phanes
Therapeutics (preclinical) |
| |
• |
CD47 bispecific antibodies:
Novimmune SA and Hummingbird BioSciences (preclinical) |
| |
• |
Mutated high affinity
SIRPαFc: ALX Oncology (phase 1) |
| |
• |
SIRPα-specific
antibody: OSE Immunotherapeutics (preclinical) |
| |
• |
SIRPαFc-agonist fusion
protein: Shattuck Labs (preclinical) |
| |
• |
Small molecule inhibitor:
Aurigene Discovery Technologies (preclinical) |
We believe that TTI-621 has advantages over other CD47 blocking
agents. TTI-621’s IgG1 Fc maximizes potency by delivering an activating signal
to macrophages through Fc receptors. With this higher potency, we believe that
TTI-621 has a higher likelihood of monotherapy activity and therefore is not
dependent upon a combination with another IgG1 antibody. TTI-621 could also have
the potential to be used to treat tumors where no anti-cancer antibody is
available.
We have also demonstrated that our SIRPαFc fusion proteins
exhibit minimal binding to RBCs in contrast to CD47-specific antibodies and a
mutated high affinity SIRPαFc. We believe that this property confers several
possible advantages including avoidance of drug-induced anemia, avoidance of the
“antigen sink effect” (i.e. removal of drug from circulation by RBCs) and
non-interference with laboratory blood typing tests. It should be noted that
TTI-622 shares the same CD47-binding domain as TTI-621 and preclinical studies
have shown that it also exhibits minimal binding to human RBCs. Thus, we
anticipate that TTI-622, like TTI-621, will not induce anemia in patients.
Fluorine Chemistry Platform
Our medicinal chemistry platform uses proprietary
fluorine-based chemistry yield new chemical entities. We believe the potency
and/or safety of both existing pharmacophores and historically inaccessible
chemical structures may be enhanced using our technology. This chemistry
platform has been utilized to establish several preclinical programs including
an EGFR inhibitor, and a number of compounds directed at undisclosed
immuno-oncology targets are currently in the discovery phase.
EGFR Inhibitor (TTI-2341)
A combination of molecular design, novel fluorine-based
chemical synthesis, and extensive biological testing led to the identification
of TTI-2341, a novel brain-penetrant, second generation, covalent EGFR
inhibitor. EGFR is a validated drug target in oncology but the use of EGFR
inhibitors has been limited by two factors. First, toxicities can arise from
indiscriminate reactivity with off-target proteins. Second, the low central
nervous system, or CNS penetration of existing EGFR inhibitors limits their use
for CNS indications such as glioblastoma multiforme and brain metastasis from
lung cancer. The incorporation of fluorine into small molecules is known to
minimize the formation of highly reactive metabolites and improve blood brain
barrier penetration and thus this strategy has the potential to overcome the
major limitations of existing EGFR inhibitors.
- 8 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
In preclinical studies we have benchmarked TTI-2341 against a
second- and third-generation EGFR inhibitor (both approved for the treatment of
non-small cell lung cancer). This comparison demonstrated that TTI-2341 achieves
superior free drug exposure levels in the brain. We are currently evaluating
different options for TTI-2341 development, including possible partnerships.
Plan of Operations
We are advancing our intratumoral and intravenous clinical
trials of TTI-621 with a focus on CTCL and PTCL. We are pursuing our combination
therapy strategy incorporating combination treatment cohorts in our TTI-621
clinical trials and our TTI-622 phase 1 trial has been initiated. We also
continue to advance our small molecule program in internal development and
pursue partnering activities.
Legal Proceedings
To our knowledge, there have not been any legal or arbitration
proceedings, including those relating to bankruptcy, receivership or similar
proceedings, those involving any third party, and governmental proceedings
pending or known to be contemplated, which may have, or have had in the recent
past, significant effect on our financial position or profitability.
Also, to our knowledge, there have been no material proceedings
in which any director, any member of senior management, or any of our affiliates
is either a party adverse to us or any of our subsidiaries or has a material
interest adverse to us or any of our subsidiaries.
RESULTS OF OPERATIONS
For the three and nine months ended September 30, 2018 and
2017
Overview
Since inception, we have incurred losses while advancing the
research and development of our products. Net loss for the nine months ended
September 30, 2018 of $33,933 was lower than the loss of $34,430 for the nine
months ended September 30, 2017. The net loss was lower due mainly to a net
foreign currency gain of $1,484 for the current period compared to a net foreign
currency loss of $4,909 in the prior year period, and lower manufacturing costs,
partially offset by higher clinical trial expenses and an amendment to the
SIRPαFc license agreement, where the sublicense revenue sharing provisions were
removed in return for a payment to the licensors of $3,000 in the form of
369,621 common shares.
Net loss for the three months ended September 30, 2018 of
$13,052 was higher than the loss of $11,337 for the three months ended September
30, 2017 mainly due to higher research and development expenses of $2,477 with
two active phase 1 trials for TTI-621, and the initiation of a phase 1 trial for
TTI-622. The increase in research and development expenses was partially offset
by a decrease in net foreign currency losses of $805.
Research and Development
Research and development expenses by program for the three and
nine months ended September 30, 2018 and 2017 were as follows:
- 9 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
| |
|
Three months |
|
|
Three months |
|
|
Nine months |
|
|
Nine months |
|
| |
|
ended |
|
|
ended |
|
|
ended |
|
|
ended |
|
| |
|
September 30, |
|
|
September 30, |
|
|
September 30, |
|
|
September 30, |
|
| |
|
2018 |
|
|
2017 |
|
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| SIRPαFc |
|
10,382
|
|
|
6,446 |
|
|
29,620
|
|
|
22,049 |
|
| Small molecule programs |
|
370 |
|
|
1,827 |
|
|
3,170 |
|
|
5,236 |
|
| Other |
|
- |
|
|
2 |
|
|
25 |
|
|
39 |
|
| Total(1) |
|
10,752 |
|
|
8,275 |
|
|
32,815 |
|
|
27,324 |
|
Note:
| (1) |
Research and development expenditures in the above table
include all direct and indirect costs for the programs, personnel costs,
intellectual property, amortization, share-based compensation and research
and development overhead, and is net of government assistance. Research
and development overhead costs have been allocated to the programs based
mainly on personnel time spent on the programs. |
During 2018 and 2017, most of our resources were focused on the
development of our SIRPαFc program. For the nine months ended September 30,
2018, SIRPαFc research and development costs were higher than the same period in
the prior year due to higher clinical trial expenses with three active phase 1
clinical trials and the amendment to the SIRPαFc license agreement, partially
offset by lower SIRPαFc manufacturing and reduced activity relating to academic
collaborations.
Small molecule program expenses were lower than the prior year
period as we completed most of our targeted preclinical development studies for
the bromodomain and EGFR inhibitors in 2017, while 2018 activities were focused
on our immuno-oncology discovery programs.
Components of research and development expenses for the three
months ended September 30, 2018 and 2017 were as follows:
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| Research and development
programs excluding the below items |
|
7,375 |
|
|
4,754 |
|
| Salaries, fees and short-term benefits |
|
1,988 |
|
|
1,829 |
|
| Share-based compensation |
|
636 |
|
|
574 |
|
| Amortization of intangible assets |
|
585 |
|
|
965 |
|
| Depreciation of property and
equipment |
|
202 |
|
|
202 |
|
| Tax
credits |
|
(34 |
) |
|
(49 |
) |
| |
|
10,752 |
|
|
8,275 |
|
Components of research and development expenses for the nine
months ended September 30, 2018 and 2017 were as follows:
- 10 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| Research and development
programs excluding the below items |
|
19,850
|
|
|
16,590 |
|
| Salaries, fees and short-term benefits |
|
6,396 |
|
|
5,294 |
|
| License agreement amendment
|
|
3,000 |
|
|
- |
|
| Share-based compensation |
|
1,393 |
|
|
2,198 |
|
| Amortization of intangible
assets |
|
1,754 |
|
|
2,895 |
|
| Change in fair value of contingent
consideration |
|
- |
|
|
(146 |
) |
| Depreciation of property and
equipment |
|
606 |
|
|
606 |
|
| Tax credits |
|
(184 |
) |
|
(113 |
) |
| |
|
32,815 |
|
|
27,324 |
|
The increase in research and development program expenses for
the three months ended September 30, 2018 compared to the same period last year
was due mainly to an increase in SIRPαFc clinical trial expenses of $2,162 and
an increase in manufacturing costs of $771. Salaries, fees and short-term
benefits increased in the three months ended September 30, 2018 due to higher
staffing and salaries compared to the same period in 2017. Share-based
compensation costs increased in the three months ended September 30, 2018
compared to the same period in the prior year due to a greater number of stock
options granted. Amortization of intangible assets decreased as we extended our
estimate of the life of our small molecule platform intangible asset to a
remaining useful life of approximately three years. Depreciation of property and
equipment and tax credits were comparable to the prior year period.
The increase in research and development program expenses for
the nine months ended September 30, 2018 over the same period last year was due
mainly to an increase in SIRPαFc clinical trial expenses of $5,689, partially
offset by a decrease in SIRPαFc manufacturing costs of $1,038 and lower activity
related to academic collaborations. Salaries, fees, and short-term benefits
increased in the nine months ended September 30, 2018 due to higher staffing and
salaries compared to the same period in 2017. For the nine months ended
September 30, 2018, we incurred an expense of $3,000 relating to the SIRPαFc
license agreement amendment. Share-based compensation costs decreased mainly due
to an increase in stock option forfeitures and an increase in the expected
forfeiture rate. Amortization of intangible assets decreased as we extended our
estimate of the life of our small molecule platform intangible asset to a
remaining useful life of approximately three years. The estimate of the fair
value of contingent consideration remained unchanged for the nine months ended
September 30, 2018. Depreciation of property and equipment was comparable to the
prior year period. Tax credits increased compared to the prior year period due
to an increase in eligible expenses.
General and Administrative
Components of general and administrative expenses for the three
months ended September 30, 2018 and 2017 were as follows:
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| General and administrative
expenses excluding the below items |
|
372 |
|
|
326 |
|
| Salaries, fees and short-term benefits |
|
640 |
|
|
514 |
|
| Change in fair value of
deferred share units |
|
6 |
|
|
53 |
|
| Share-based compensation |
|
82 |
|
|
76 |
|
| |
|
1,100 |
|
|
969 |
|
- 11 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Components of general and administrative expenses for the nine
months ended September 30, 2018 and 2017 were as follows:
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| General and administrative
expenses excluding the below items |
|
1,435 |
|
|
1,062 |
|
| Salaries, fees and short-term benefits |
|
1,887 |
|
|
1,435 |
|
| Change in fair value of
deferred share units |
|
(204 |
) |
|
(144 |
) |
| Share-based compensation |
|
258 |
|
|
254 |
|
| |
|
3,376 |
|
|
2,607 |
|
General and administrative expenses for the three months ended
September 30, 2018 of $372 were higher than the prior year comparable period
mainly due to higher professional fees incurred. Salaries, fees and short-term
benefits increased mainly due to higher staffing levels and higher
director-related fees. The change in the fair value of deferred share units was
a result of fluctuations in our share price during the respective periods.
Share-based compensation expense was comparable to the prior year period.
General and administrative expenses for the nine months ended
September 30, 2018 of $1,435 were higher than the prior year comparable period
mainly due to higher professional fees and listing fees incurred related to a
prospectus supplement filing and the 2018 Stock Option Plan. Salaries, fees and
short-term benefits increased mainly due to higher staffing levels and the
issuance of DSUs. The change in the fair value of deferred share units was a
result of fluctuations in the Company’s share price during the respective
periods. Share-based compensation expense was comparable to the prior year
period.
Finance income and costs, foreign exchange gains and
losses, and income taxes
Finance income for the three and nine months ended September
30, 2018 of $309 and $814, respectively, were higher than the prior year
comparable period amounts of $227 and $468 due mainly to higher average cash and
marketable securities balances and higher interest rates.
Finance costs for the three and nine months ended September 30,
2018 were comparable to the prior year periods.
During the three months ended September 30, 2018, we recorded a
net foreign currency loss of $1,498, compared to $2,303 for the comparative
period in 2017. The net foreign currency loss in the current period reflected a
weakening of the U.S. dollar versus the Canadian dollar while holding net U.S.
dollar denominated assets. During the nine months ended September 30, 2018, we
recorded a net foreign currency gain of $1,484, compared to a net foreign
currency loss of $4,909 for the nine months ended September 30, 2017.
Liquidity and Capital Resources
Cash, working capital, and debt
Since inception, we have financed our operations primarily from
sales of equity, proceeds from the exercise of warrants and stock options and
from interest income on funds available for investment. Our primary capital
needs are for funds to support our scientific research and development
activities including staffing, facilities, manufacturing, preclinical studies,
clinical trials, administrative costs and for working capital.
We have experienced operating losses and cash outflows from
operations since incorporation, will require ongoing financing in order to
continue our research and development activities and we have not earned
significant revenue or reached successful commercialization of our products. Our
future operations are dependent upon our ability to finance our cash
requirements which will allow us to continue our research and development
activities and the commercialization of our products. There can be no assurance
that we will be successful in continuing to finance our operations.
- 12 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
In June 2017, we completed an underwritten public offering of
common shares and non-voting convertible preferred shares in the United States.
In the offering, we sold 2,949,674 common shares and 3,250,000 Series II
Non-Voting Convertible First Preferred Shares at a price of U.S. $5.00 per
share. The gross proceeds from this offering were $41,847 (U.S. $30,998) before
deducting offering expenses of $2,856.
The Series II Non-Voting Convertible First Preferred Shares
sold in the offering are non-voting and are convertible into common shares, on a
one-for-one basis (subject to adjustment), at any time at the option of the
holder, subject to certain restrictions on conversion. Holders may not convert
Series II Non-Voting Convertible First Preferred Shares into common shares if,
after giving effect to the exercise of conversion, the holder and its joint
actors would have beneficial ownership or direction or control over common
shares in excess of 4.99% of the then outstanding common shares. This limit may
be raised at the option of the holder on 61 days’ prior written notice: (i) up
to 9.99%, (ii) up to 19.99%, subject to clearance of a personal information form
submitted by the holder to the TSX, and (iii) above 19.99%, subject to approval
by the TSX and shareholder approval.
In connection with the acquisition of Series II First Preferred
Shares in this offering at the public offering price by an existing
institutional shareholder, we entered into an investment agreement with such
shareholder. The investment agreement provides this shareholder the right, but
not the obligation, for so long as it beneficially owns at least 10% of the
adjusted share capital of the Company, calculated on a fully-diluted basis, to
nominate one person for election to our board of directors, subject to meeting
applicable legal and stock exchange requirements and we have the obligation to
appoint such director, whose term will run until the next annual meeting of
shareholders. Thereafter, we are required to nominate such director to be a
director at any meeting of shareholders called for the purposes of electing
directors and to use commercially reasonable efforts to ensure that such
director is elected to the board of directors, including soliciting proxies in
support of his or her election and taking the same actions taken by us to ensure
the election of the other nominees selected by the board of directors for
election to the board of directors. In addition, until such time as the existing
shareholder exercises its right to nominate a member of our board of directors,
and so long as the existing shareholder’s nominee is not an employee, officer,
director or limited partner of such shareholder, then such shareholder shall
have the right, but not the obligation, to appoint an observer to our board of
directors, who must be an employee, officer or director of such shareholder. The
observer will have the right to receive notice of and attend the meetings of the
board of directors, and will have the right to address the board of directors at
any of its meetings, but will not have any right to vote at any meeting of the
board of directors. In addition, we have agreed to provide this existing
shareholder with certain registration rights in the event that such shareholder
and its joint actors are deemed to be “affiliates” for purposes of applicable
U.S. securities laws.
In December 2017, we completed a non-brokered private placement
financing and sold 1,950,000 common shares and 400,000 Series II Non-Voting
Convertible First Preferred Shares at a price of U.S. $8.50 per share yielding
gross proceeds of $25,338 (U.S. $19,975) before deducting offering expenses of
$1,784.
On January 5, 2018, we filed a base shelf prospectus with the
British Columbia, Alberta, Manitoba, Ontario and Nova Scotia securities
commissions in Canada and a Form F-10 registration statement with the United
States Securities and Exchange Commission, or SEC, that provides that we may
sell under the prospectus from time to time over the following 25 months up to
U.S. $150 million, in one or more offerings, of common shares, First Preferred
shares, warrants to purchase common shares, subscription receipts, or units
comprising a combination of common shares, First Preferred shares and/or
warrants.
On June 19, 2018 we filed a prospectus supplement to the base
prospectus included in our U.S. registration statement on Form F-10 declared
effective on January 8, 2018. We also entered into a sales agreement with Cowen
and Company, LLC, or the Agent, pursuant to which we may, at our discretion and
from time to time during the term of the sales agreement, sell, through the
Agent, acting as agent and/or principal, such number of common shares of
Trillium as would result in aggregate gross proceeds to us of up to U.S. $25
million. Sales of common shares through the Agent, acting as agent, will be made
through “at the market” issuances on NASDAQ at the market price prevailing at
the time of each sale, and, as a result, sale prices may vary. No common shares
will be offered or sold on the TSX or any other trading markets in Canada.
- 13 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Our cash and cash equivalents and marketable securities, and
working capital at September 30, 2018 were $52,095 and $41,817, respectively
compared to $81,791 and $68,900, respectively at December 31, 2017. The decrease
in cash and cash equivalents and marketable securities was due mainly to cash
used in operations of $30,847, net of an unrealized foreign exchange gain of
$1,296. The decrease in working capital was due mainly to cash used in
operations, increases to amounts receivable and prepaid expenses, and a decrease
to accounts payable and accrued liabilities due to clinical trial payments.
We are indebted to the Federal Economic Development Agency for
Southern Ontario, or FedDev, under a non-interest-bearing contribution agreement
and are making monthly repayments of $10 through November 2019. As at September
30, 2018 and December 31, 2017, the balance repayable was $126 and $211
respectively. The loan payable was discounted using an estimated market interest
rate of 15%. Interest expense accretes on the discounted loan amount until it
reaches its face value at maturity.
As at September 30, 2018 and December 31, 2017, we had a
deferred lease inducement of $384 and $407, respectively, for our facility
lease. The inducement benefit is being recognized over the expected term of the
lease.
As at September 30, 2018 and December 31, 2017, we had a
long-term liability of $801 and $801, respectively, related to contingent
consideration on the acquisition of Fluorinov.
Cash flows from operating activities
Cash used in operating activities increased to $30,847 for the
nine months ended September 30, 2018, compared to $20,448 for the nine months
ended September 30, 2017, due mainly to higher research and development costs
offset by an unrealized foreign exchange gain of $1,296, and a decrease in
accounts payable and accrued liabilities of $1,979 compared to an increase of
$4,019 in the comparable period.
Cash flows from investing activities
Cash provided from investing activities totaled $14,422 for the
nine months ended September 30, 2018, compared to cash used of $39,165 for the
nine months ended September 30, 2017. The change was due to the maturities of
marketable securities in the nine months ended September 30, 2018.
Cash flows from financing activities
Cash used in financing activities totaled $86 for the nine
months ended September 30, 2018, compared to $38,894 provided by financing
activities in the prior year period. This change was due to an underwritten
public offering of common shares and non-voting convertible preferred shares in
June 2017.
- 14 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Contractual Obligations and Contingencies
We enter into research, development and license agreements in
the ordinary course of business where we receive research services and rights to
proprietary technologies. Milestone and royalty payments that may become due
under various agreements are dependent on, among other factors, clinical trials,
regulatory approvals and ultimately the successful development of a new drug,
the outcome and timing of which is uncertain.
Under the license agreement for SIRPαFc, we have future
contingent milestones payable of $25 related to successful patent grants, $200
and $300 on the first patient dosed in phase 2 and 3 clinical trials
respectively, regulatory milestones on their first achievement totaling $5,000,
and low single digit royalties payable on net sales. In a June 2018 amendment to
the license agreement, the sublicense revenue sharing provisions were removed in
return for a payment to the licensors of $3,000 in the form of 369,621 common
shares.
Under two agreements with Catalent pursuant to which we
acquired the right to use a proprietary expression system for the manufacture of
two SIRPαFc constructs, we have future contingent milestones on pre-marketing
approval of up to U.S. $875 and aggregate sales milestone payments of up to U.S.
$28,750 for each agreement.
In connection with our acquisition of all the outstanding
shares of Fluorinov, we are obligated to pay up to $35,000 of additional future
payments that are contingent on us achieving certain clinical and regulatory
milestones with an existing Fluorinov compound. We will also have an obligation
to pay royalty payments on future sales of such compounds.
We periodically enter into research and license agreements with
third parties that include indemnification provisions customary in the industry.
These guarantees generally require us to compensate the other party for certain
damages and costs incurred as a result of claims arising from research and
development activities undertaken by or on our behalf. In some cases, the
maximum potential amount of future payments that could be required under these
indemnification provisions could be unlimited. These indemnification provisions
generally survive termination of the underlying agreement. The nature of the
indemnification obligations prevents us from making a reasonable estimate of the
maximum potential amount we could be required to pay. Historically, we have not
made any indemnification payments under such agreements and no amount has been
accrued in our unaudited interim condensed consolidated financial statements
with respect to these indemnification obligations.
Other than as disclosed below, we did not have any contractual
obligations relating to long-term debt obligations, capital (finance) lease
obligations, operating lease obligations, purchase obligations or other
long-term liabilities reflected on our balance sheet as at September 30,
2018:
| |
|
Payment due by period |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Less than |
|
|
1 to 3 |
|
|
3 to 5 |
|
|
More than |
|
| Contractual Obligations(1)(2) |
|
Total |
|
|
1 year |
|
|
years |
|
|
years |
|
|
5 years |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Long-Term Debt
Obligations(3) |
$ |
134 |
|
$ |
115 |
|
$ |
19 |
|
$ |
- |
|
$ |
- |
|
| Operating Lease Obligations(4) |
|
1,893 |
|
|
286 |
|
|
508 |
|
|
527 |
|
|
572 |
|
| Purchase
Obligations(5) |
|
21,165 |
|
|
15,080 |
|
|
5,992 |
|
|
93 |
|
|
- |
|
| Other Long-Term Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Reflected on our Balance
Sheet(6) |
|
1,124 |
|
|
323 |
|
|
- |
|
|
608 |
|
|
193 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
$ |
24,316 |
|
$ |
15,804 |
|
$ |
6,519 |
|
$ |
1,228 |
|
$ |
765 |
|
Notes:
| |
(1) |
Contractual obligations in the above table do not include
amounts in accounts payable and accrued liabilities on our balance sheet
as at the current reporting date. |
- 15 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
| |
(2) |
Contingent milestones under the SIRPαFc license agreement
and the Catalent expression system agreements are not included in the
above table. |
| |
(3) |
Amounts due to FedDev repayable in equal monthly
installments of $10 through November 2019. |
| |
(4) |
Includes operating lease obligations for laboratory and
office facilities. |
| |
(5) |
Purchase obligations include all non-cancellable
contracts, and all cancellable contracts with $100 or greater remaining
committed at the period end including agreements related to the conduct of
our clinical trials, preclinical studies and manufacturing
activities. |
| |
(6) |
Includes $801 of contingent consideration related to
potential future payments of up to $35,000 based on the achievement of
clinical and regulatory milestones with an existing Fluorinov
compound. |
Description of Share Capital
The continuity of the number of our issued and outstanding
common and preferred shares from December 31, 2017 to the date of this MD&A
is presented below:
| |
|
Number of Series I |
|
|
Number of Series II |
|
|
Number of |
|
| |
|
Preferred Shares(1) |
|
|
Preferred Shares(2) |
|
|
Common Shares |
|
| |
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2017
|
|
52,325,827 |
|
|
4,368,403 |
|
|
13,147,404 |
|
| Issued to amend SIRPαFc license |
|
- |
|
|
- |
|
|
369,621 |
|
| Preferred share conversion |
|
(35,154,286 |
) |
|
- |
|
|
1,171,806 |
|
| Balance at September 30, 2018 and the date of this MD&A |
|
17,171,541 |
|
|
4,368,403 |
|
|
14,688,831 |
|
Notes:
| |
(1) |
Convertible at a ratio of 30 Series I Preferred Shares
for one common share. |
| |
(2) |
Convertible at a ratio of one Series II Preferred Share
for one common share. |
Share capital issued – for the nine months ended
September 30, 2018
During the nine months ended September 30, 2018, 369,621 common
shares were issued on the renegotiation of the SIRPαFc license agreement, and
1,171,806 common shares were issued on the conversion of preferred shares.
Warrants
The continuity of the number of issued and outstanding warrants
from December 31, 2017 to the date of this MD&A is presented below:
| |
|
Preferred |
|
|
Common Share |
|
| |
|
Warrants(1) |
|
|
Warrants(2) |
|
| |
|
|
|
|
|
|
| Balance at December 31, 2017
|
|
1,190,476 |
|
|
69,073,031 |
|
| Expired |
|
- |
|
|
(8,240,455 |
) |
| Balance at September 30, 2018 and the date of this
MD&A |
|
1,190,476 |
|
|
60,832,576 |
|
Notes:
| |
(1) |
Preferred Warrants are exercisable at $8.40 per warrant
for one common share or one Series II Preferred Share. |
| |
(2) |
These warrants are exercisable at a ratio of 30 warrants
for one common share. |
- 16 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
The following table shows the number of common share purchase
warrants outstanding, the exercise prices and the number of common shares
issuable on exercise of the warrants and the exercise price per common share for
30 warrants at September 30, 2018:
| |
|
|
|
|
|
|
|
Number of |
|
|
Exercise |
|
| |
|
|
|
|
|
|
|
Common Shares |
|
|
Price per |
|
| |
|
Number of |
|
|
Exercise |
|
|
Issuable |
|
|
Common Share |
|
| Expiry date |
|
Warrants |
|
|
Price |
|
|
on Exercise |
|
|
(30 Warrants |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| December 2018 |
|
60,832,576 |
|
$ |
0.28 |
|
|
2,027,753 |
|
$ |
8.40 |
|
The following table shows the number of Preferred Warrants
outstanding and their exercise price to acquire either one common share or one
Series II Preferred Share at the option of the holder at December 31, 2017:
| |
|
Number of |
|
|
|
|
| |
|
Preferred |
|
|
Exercise |
|
| Expiry date |
|
Warrants |
|
|
Price |
|
| |
|
|
|
|
|
|
| December 2018 |
|
1,190,476 |
|
$ |
8.40 |
|
Stock Options
The 2018 Stock Option Plan was approved by our shareholders at
the annual meeting held on June 1, 2018. Options granted are equity-settled,
have a vesting period of four years and have a maximum term of ten years. The
total number of common shares available for issuance under the 2018 Stock Option
Plan is 3,894,501. As at September 30, 2018, we were entitled to issue an
additional 2,064,271 stock options under the 2018 Stock Option Plan.
The continuity of the number of issued and outstanding stock
options from December 31, 2017 to the date of this MD&A is presented below:
| |
|
Number of |
|
|
Weighted Average |
|
| |
|
Options |
|
|
Exercise Price |
|
| |
|
|
|
|
|
|
| Balance at December 31, 2017
|
|
1,746,982 |
|
$ |
12.87 |
|
| Granted |
|
212,000 |
|
|
7.92 |
|
| Forfeited |
|
(128,356 |
)
|
|
12.98 |
|
| Expired |
|
(396 |
) |
|
13.98 |
|
| Balance at September 30, 2018
|
|
1,830,230 |
|
|
12.29 |
|
| Granted |
|
870,600 |
|
|
4.23 |
|
| Balance at the date of this MD&A |
|
2,700,830 |
|
|
9.69 |
|
Deferred Share Unit Plan
The board of directors approved a cash-settled DSU plan, or the
Cash-Settled DSU Plan, on November 9, 2016. There were 112,500 DSUs issued for
the nine months ended September 30, 2018. The fair values of DSUs under this
plan as at September 30, 2018 and December 31, 2017 were $1,257 and $1,349,
respectively. As at September 30, 2018, there were 159,898 DSUs outstanding
under this plan.
- 17 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Fully Diluted Share Capital
The number of issued and outstanding common shares, Series I
First Preferred Shares, Series II First Preferred Shares, common share purchase
warrants, Preferred Warrants, and stock options on a fully converted basis as at
September 30, 2018 was as follows:
| |
|
Number of Common |
|
| |
|
Share Equivalents |
|
| |
|
|
|
| Common shares |
|
14,688,831 |
|
| Series I First Preferred Shares |
|
572,385 |
|
| Series II First Preferred
Shares |
|
4,368,403 |
|
| Warrants (exercisable for common shares) |
|
2,027,753 |
|
| Preferred Warrants
(exercisable for common shares or Series II Preferred Shares) |
|
1,190,476 |
|
| Stock options |
|
1,830,230 |
|
| Total |
|
24,678,078 |
|
Trend Information
Historical patterns of expenditures cannot be taken as an
indication of future expenditures. The amount and timing of expenditures and
therefore liquidity and capital resources vary substantially from period to
period depending on the number of research and development programs being
undertaken at any one time, the stage of the development programs, the timing of
significant expenditures for manufacturing, toxicology and pharmacology studies
and clinical trials, and the availability of funding from investors and
prospective commercial partners.
Selected Quarterly Financial Information
|
Q3-2018
$ |
Q2-2018
$ |
Q1-2018
$ |
Q4-2017
$ |
| Revenue |
- |
- |
- |
- |
| Research and development expenses |
10,752 |
12,722 |
9,341 |
9,811 |
| General and administrative expenses |
1,100 |
1,247 |
1,029 |
1,254 |
| Net loss for the period |
13,052 |
12,316 |
8,565 |
10,658 |
| Basic and diluted net loss per share |
0.91 |
0.92 |
0.65 |
0.91 |
| Cash and cash equivalents and marketable securities |
52,095 |
64,698 |
73,920 |
81,791 |
|
Q3-2017
$ |
Q2-2017
$ |
Q1-2017
$ |
Q4-2016
$ |
| Revenue |
- |
- |
- |
- |
| Research and development expenses |
8,275 |
8,851 |
10,198 |
9,262 |
| General and administrative expenses |
969 |
684 |
954 |
917 |
| Net loss for the period |
11,337 |
11,642 |
11,451 |
9,023 |
| Basic and diluted net loss per share |
1.05 |
1.33 |
1.46 |
1.15 |
| Cash and cash equivalents and marketable securities |
64,297 |
72,618 |
41,347 |
50,473 |
- 18 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
The net losses for the first and second quarters of 2017 were
higher due to higher personnel costs, SIRPαFc clinical trial costs, and
preclinical work on the bromodomain inhibitor and EGFR inhibitor programs. The
net loss for the third and fourth quarters of 2017 reflected continued focus on
the SIRPαFc development program, and lower small molecule expenses relative to
the first and second quarters of 2017. The decrease in net loss in the first
quarter of 2018 reflected a higher net foreign currency gain. The increase in
net loss in the second quarter of 2018 reflected higher clinical development
expenses and the license agreement amendment payment, partially offset by a net
foreign currency gain. The increase in net loss in the third quarter of 2018
reflected higher clinical development costs.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have, or
are reasonably likely to have, a current or future effect on our financial
condition, revenues or expenses, results of operations, liquidity, capital
expenditures or capital resources that are material to investors.
Implications of Being an Emerging Growth Company
We are an “emerging growth company” under the U.S. Jumpstart
Our Business Startups Act of 2012, or the JOBS Act, and will continue to qualify
as an “emerging growth company” until the earliest to occur of: (a) the last day
of the fiscal year during which we have total annual gross revenues of $1.07
billion (as such amount is indexed for inflation every 5 years by the SEC) or
more; (b) the last day of our fiscal year following the fifth anniversary of the
date of the first sale of our common shares pursuant to an effective
registration statement under the U.S. Securities Act of 1933 which is December
31, 2019; (c) the date on which we have, during the previous 3-year period,
issued more than $1.0 billion in non-convertible debt; or (d) the date on which
we are deemed to be a “large accelerated filer”, as defined in Rule 12b–2 of the
U.S. Securities Exchange Act of 1934, or the Exchange Act.
Generally, a company that registers any class of its securities
under Section 12 of the Exchange Act is required to include in the second and
all subsequent annual reports filed by it under the Exchange Act, a management
report on internal control over financial reporting and, subject to an exemption
available to companies that meet the definition of a “smaller reporting company”
in Rule 12b-2 under the Exchange Act, an auditor attestation report on
management’s assessment of the company’s internal control over financial
reporting. However, for so long as we continue to qualify as an emerging growth
company, we will be exempt from the requirement to include an auditor
attestation report in our annual reports filed under the Exchange Act, even if
we do not qualify as a “smaller reporting company”. In addition, Section
103(a)(3) of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, has been
amended by the JOBS Act to provide that, among other things, auditors of an
emerging growth company are exempt from any rules of the Public Company
Accounting Oversight Board requiring mandatory audit firm rotation or a
supplement to the auditor’s report in which the auditor would be required to
provide additional information about the audit and the financial statements of
the company.
Any U.S. domestic issuer that is an emerging growth company is
able to avail itself of the reduced disclosure obligations regarding executive
compensation in periodic reports and proxy statements, and to not present to its
shareholders a non-binding advisory vote on executive compensation, obtain
approval of any golden parachute payments not previously approved, or present
the relationship between executive compensation actually paid and our financial
performance. So long as we are a foreign private issuer, we are not subject to
such requirements, and will not become subject to such requirements even if we
were to cease to be an emerging growth company.
As a reporting issuer under the securities legislation of the
Canadian provinces of Ontario, British Columbia, Manitoba, Nova Scotia and
Alberta, we are required to comply with all new or revised accounting standards
that apply to Canadian public companies. Pursuant to Section 107(b) of the JOBS
Act, an emerging growth company may elect to utilize an extended transition
period for complying with new or revised accounting standards for public
companies until such standards apply to private companies. We have elected not
to utilize this extended transition period.
- 19 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Critical Accounting Estimates
The preparation of financial statements in conformity with IFRS
requires management to make judgments, estimates and assumptions that affect the
application of accounting policies and the reported amounts of assets and
liabilities, revenue and expenses, related disclosures of contingent assets and
liabilities and the determination of our ability to continue as a going concern.
Actual results could differ materially from these estimates and assumptions. We
review our estimates and underlying assumptions on an ongoing basis. Revisions
are recognized in the period in which the estimates are revised and may impact
future periods.
The areas involving a higher degree of judgment or complexity,
or areas where assumptions and estimates are significant to the financial
statements have been set out in note 2 of our annual audited consolidated
financial statements for the year ended December 31, 2017.
Accounting Policies
Our significant accounting policies are outlined in our annual
audited consolidated financial statements for the year ended December 31, 2017.
This MD&A should be read in conjunction with the annual audited consolidated
financial statements for the year ended December 31, 2017.
New standards, amendments and interpretations adopted
during 2018
IFRS 9 Financial Instruments
As at January 1, 2018, we adopted IFRS 9 Financial
Instruments, or IFRS 9. We have elected to not restate comparative periods
in the year of initial application of IFRS 9 relating to the transition for
classification, measurement and impairment. As a result, the comparative
information provided continues to be accounted for on a basis consistent with
those followed in the most recent annual consolidated financial statements.
IFRS 9 replaces the provisions of IAS 39 Financial
Instruments: Recognition and Measurement that relate to the recognition,
classification and measurement of financial assets and financial liabilities,
derecognition of financial instruments, impairment of financial assets and hedge
accounting. IFRS 9 also significantly amends other standards dealing with
financial instruments such as IFRS 7 Financial Instruments: Disclosures.
We assessed the classification and measurement of financial
instruments we held at the date of initial application of IFRS 9 (January 1,
2018) and have classified our financial instruments into the appropriate IFRS 9
categories. There were no changes to the carrying value of our financial
instruments resulting from this reclassification and accordingly there was no
impact to our opening balance of deficit as at January 1, 2018 as a result of
the adoption of IFRS 9.
IFRS 15 Revenue from Contracts with Customers
As at January 1, 2018, we adopted IFRS 15 Revenue from
Contracts with Customers, or IFRS 15, which covers principles for reporting
about the nature, amount, timing and uncertainty of revenue and cash flows
arising from contracts with customers. The adoption of this standard did not
have an impact on the unaudited interim condensed consolidated financial
statements.
New standards and interpretations not yet
effective
IFRS 16 Leases
In January 2016, the IASB issued IFRS 16 Leases, or IFRS
16, its new leases standard that requires lessees to recognize assets and
liabilities for most leases on their balance sheets. Lessees applying IFRS 16
will have a single accounting model for all leases, with certain exemptions. The
new standard will be effective for annual periods beginning on or after January
1, 2019 with limited early application permitted. We plan to adopt IFRS 16 with
the cumulative effect of initial application recognized as an adjustment to
opening equity on January 1, 2019. We are continuing to assess the impact of this standard and will
disclose the estimated financial effects of the adoption of IFRS 16 in our 2018
annual audited consolidated financial statements.
- 20 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Other accounting standards or amendments to existing accounting
standards that have been issued, but have future effective dates, are either not
applicable or are not expected to have a significant impact on our unaudited
interim condensed consolidated financial statements.
RISK FACTORS
The following information sets forth material risks and
uncertainties that may affect our business, including our future financing and
operating results and could cause our actual results to differ materially from
those contained in forward-looking statements we have made in this MD&A. The
risks and uncertainties below are not the only ones we face. Additional risks
and uncertainties not presently known to us or that we believe to be immaterial
may also adversely affect our business. Further, if we fail to meet the
expectations of the public market in any given period, the market price of our
common shares could decline. We operate in a highly competitive environment that
involves significant risks and uncertainties, some of which are outside of our
control.
Risks Related to Our Financial Position and Need for
Additional Capital
We expect to incur future losses and we may never become
profitable.
We have incurred losses of $33,933, $45,088, and $31,733 for
the nine months ended September 30, 2018 and the years ended December 31, 2017,
and 2016, respectively, and expect to incur an operating loss for the year
ending December 31, 2018. We have an accumulated deficit since inception through
September 30, 2018 of $176,044. We believe that operating losses will continue
as we are planning to incur significant costs associated with the clinical
development of SIRPαFc. Our net losses have had and will continue to have an
adverse effect on, among other things, our shareholders’ equity, total assets
and working capital. We expect that losses will fluctuate from quarter to
quarter and year to year, and that such fluctuations may be substantial. We
cannot predict when we will become profitable, if at all.
We will require additional capital to finance our
operations, which may not be available to us on acceptable terms, or at all. As
a result, we may not complete the development and commercialization of our
product candidates or develop new product candidates.
As a research and development company, our operations have
consumed substantial amounts of cash since inception. We expect to spend
substantial funds to continue the research, development and testing of our
product candidates and to prepare to commercialize products subject to approval
of the FDA, in the U.S. and similar approvals in other jurisdictions. We will
also require significant additional funds if we expand the scope of our current
clinical plans or if we were to acquire any new assets and advance their
development. Therefore, for the foreseeable future, we will have to fund all of
our operations and development expenditures from cash on hand, equity or debt
financings, through collaborations with other biotechnology or pharmaceutical
companies or through financings from other sources. We expect that our existing
cash and cash equivalents and marketable securities as at September 30, 2018 of
$52,095 will enable us to fund our current operating plan requirements for at
least the next twelve months. Additional financing will be
required to meet our longer-term liquidity needs. If we do not succeed in raising additional
funds on acceptable terms, we might not be able to complete planned preclinical
studies and clinical trials or pursue and obtain approval of any product
candidates from the FDA and other regulatory authorities. It is possible that
future financing will not be available or, if available, may not be on favorable
terms. The availability of financing will be affected by the achievement of our
corporate goals, the results of scientific and clinical research, the ability to
obtain regulatory approvals, the state of the capital markets generally and with
particular reference to drug development companies, the status of strategic
alliance agreements and other relevant commercial considerations. If adequate
funding is not available, we may be required to delay, reduce or eliminate one
or more of our product development programs, or obtain funds through corporate
partners or others who may require us to relinquish significant rights to
product candidates or obtain funds on less favorable terms than we would
otherwise accept. To the extent that external sources of capital become limited
or unavailable or available on onerous terms, our intangible assets and our
ability to continue our clinical development plans may become impaired, and our assets, liabilities, business, financial
condition and results of operations may be materially or adversely affected.
- 21 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
We currently have no product revenue and will not be able
to maintain our operations and research and development without additional
funding.
To date, we have generated no product revenue and cannot
predict when and if we will generate product revenue. Our ability to generate
product revenue and ultimately become profitable depends upon our ability, alone
or with partners, to successfully develop our product candidates, obtain
regulatory approval, and commercialize products, including any of our current
product candidates, or other product candidates that we may develop, in-license
or acquire in the future. We do not anticipate generating revenue from the sale
of products for the foreseeable future. We expect our research and development
expenses to increase in connection with our ongoing activities, particularly as
we advance our product candidates through clinical trials.
We are exposed to the financial risk related to the
fluctuation of foreign exchange rates and the degrees of volatility of those
rates.
We may be adversely affected by foreign currency fluctuations.
To date, we have been primarily funded through issuances of equity, proceeds
from the exercise of warrants and stock options and from interest income on
funds available for investment, which are denominated both in Canadian and U.S.
dollars. Also, a significant portion of our expenditures are in U.S. dollars,
and we are therefore subject to foreign currency fluctuations which may, from
time to time, impact our financial position and results of operations.
Risks Related to Our Business and Our Industry
Our prospects depend on the success of our product
candidates which are at early stages of development, and we may not generate
revenue for several years, if at all, from these products.
Given the early stage of our product development, we can make
no assurance that our research and development programs will result in
regulatory approval or commercially viable products. To achieve profitable
operations, we, alone or with others, must successfully develop, gain regulatory
approval, and market our future products. We currently have no products that
have been approved by the FDA, Health Canada, or HC, or any similar regulatory
authority. To obtain regulatory approvals for our product candidates being
developed and to achieve commercial success, clinical trials must demonstrate
that the product candidates are safe for human use and that they demonstrate
efficacy. While we have commenced phase 1 trials for SIRPαFc, we have not yet
completed a phase 1 clinical trial or subsequent required clinical trials for
any of our product candidates.
Many product candidates never reach the stage of clinical
testing and even those that do have only a small chance of successfully
completing clinical development and gaining regulatory approval. Product
candidates may fail for a number of reasons, including, but not limited to,
being unsafe for human use or due to the failure to provide therapeutic benefits
equal to or better than the standard of treatment at the time of testing.
Unsatisfactory results obtained from a particular study relating to a research
and development program may cause us or our collaborators to abandon commitments
to that program.
We acquired several preclinical and discovery research programs
in our acquisition of Fluorinov, including certain assets relating to the
treatment of CNS disorders. While we conducted extensive due diligence before
making this acquisition, our assessment of the Fluorinov technologies may not be
accurate. Therefore, our expectations about whether various clinical and
regulatory milestones with an existing Fluorinov compound or development of a
future program on the Fluorinov development platform will be achieved may not be
borne out fully or at all. We have made a commitment to use commercially
reasonable efforts to monetize the Fluorinov CNS assets and, if successful, to
share the net proceeds with the Fluorinov vendors. As this is not our core
competency, our efforts to monetize these assets or any other Fluorinov assets
may not be successful. We can make no assurances that toxicology, or other
preclinical, studies will yield results that will allow us to proceed with
clinical trials in humans.
- 22 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
The early stage of our product development makes it
particularly uncertain whether any of our product development efforts will prove
to be successful and meet applicable regulatory requirements, and whether any of
our product candidates will receive the requisite regulatory approvals, be
capable of being manufactured at a reasonable cost or be successfully marketed.
If we are successful in developing our current and future product candidates
into approved products, we will still experience many potential obstacles such
as the need to develop or obtain manufacturing, marketing and distribution
capabilities. If we are unable to successfully commercialize any of our
products, our financial condition and results of operations may be materially
and adversely affected.
Positive results from preclinical and early clinical
research of TTI-621 and TTI-622 are not necessarily predictive of the results of
later clinical trials of TTI-621 or TTI-622. If we cannot replicate the positive
results from preclinical and early clinical research in our later clinical
trials, we may be unable to successfully develop, obtain regulatory approval for
and commercialize TTI-621 or TTI-622.
Positive results of preclinical and early clinical research of
TTI-621 and TTI-622 may not be indicative of the results that will be obtained
in later-stage clinical trials. For example, we have focused our near-term
clinical product development on T cell malignancies based on preliminary results
of our intratumoral trial which were presented at the American Society of
Hematology meeting in December 2017 and updated results presented at the EORTC
CLTF meeting in September 2018. There can be no assurance that the preliminary
results we have seen in a small number of mycosis fungoides patients will be
reproducible in a larger population of patients. We can make no assurance that
any future studies, if undertaken, will yield favorable results.
Many companies in the pharmaceutical and biotechnology
industries have suffered significant setbacks in later-stage clinical trials
after achieving positive results in early-stage development, and we cannot be
certain that we will not face similar setbacks. These setbacks have been caused
by, among other things, preclinical findings made while clinical trials were
underway or safety or efficacy observations made in clinical trials, including
previously unreported adverse events. Moreover, preclinical and clinical data
are often susceptible to varying interpretations and analyses, and many
companies that believed their product candidates performed satisfactorily in
preclinical studies and clinical trials nonetheless failed to obtain FDA
approval. If we fail to produce positive results in our future clinical trials
of TTI-621 or TTI-622, the development timeline and regulatory approval and
commercialization prospects for our leading product candidates, and,
correspondingly, our business and financial prospects, would be materially
adversely affected.
We rely and will continue to rely on third parties to
plan, conduct and monitor our preclinical studies and clinical trials, and their
failure to perform as required could cause substantial harm to our
business.
We rely and will continue to rely on third parties to conduct a
significant portion of our preclinical and clinical development activities.
Preclinical activities include in vivo studies providing access to specific
disease models, pharmacology and toxicology studies, and assay development.
Clinical development activities include trial design, regulatory submissions,
clinical patient recruitment, clinical trial monitoring, clinical data
management and analysis, safety monitoring and project management. If there is
any dispute or disruption in our relationship with third parties, or if they are
unable to provide quality services in a timely manner and at a feasible cost,
our active development programs will face delays. Further, if any of these third
parties fails to perform as we expect or if their work fails to meet regulatory
requirements, our testing could be delayed, cancelled or rendered
ineffective.
We rely on contract manufacturers over whom we have
limited control. If we are subject to quality, cost or delivery issues with the
preclinical and clinical grade materials supplied by contract manufacturers, our
business operations could suffer significant harm.
We have limited manufacturing experience and rely on contract
manufacturing organizations, or CMOs to manufacture our product candidates for
larger preclinical studies and clinical trials. We rely on CMOs for
manufacturing, filling, packaging, storing and shipping of drug product in
compliance with current Good Manufacturing Practice, or cGMP, regulations
applicable to our products. The FDA ensures the quality of drug products by
carefully monitoring drug manufacturers’ compliance with cGMP regulations. The
cGMP regulations for drugs contain minimum requirements for the methods,
facilities and controls used in manufacturing, processing and packing of a drug
product.
- 23 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
We contracted with Catalent for the manufacture of the SIRPαFc
protein to supply drug substance for our phase 1 clinical trials. The
manufacture of recombinant proteins uses well established processes including a
protein expression system. Catalent is producing SIRPαFc using their proprietary
GPEx® expression system. We believe that Catalent has the capacity, the systems,
and the experience to supply SIRPαFc for our phase 1 clinical trials and we may
consider using Catalent for manufacturing for later clinical trials. However,
since the Catalent manufacturing facility where SIRPαFc is being produced does
not support commercial manufacturing, it has not yet been inspected by the FDA.
Any manufacturing failures, delays or compliance issues could cause delays in
the conduct of SIRPαFc preclinical studies and clinical trials.
There can be no assurances that CMOs will be able to meet our
timetable and requirements. We have not contracted with alternate suppliers for
SIRPαFc drug substance production in the event Catalent is unable to scale up
production, or if Catalent otherwise experiences any other significant problems.
If we are unable to arrange for alternative third-party manufacturing sources on
commercially reasonable terms or in a timely manner, we may be delayed in the
development of our product candidates. Further, CMOs must operate in compliance
with cGMP and failure to do so could result in, among other things, the
disruption of product supplies. Our dependence upon third parties for the
manufacture of our products may adversely affect our profit margins and our
ability to develop and deliver products on a timely and competitive basis.
We require commercial scale and quality manufactured
product to be available for pivotal or registration clinical trials. If we do
not have commercial grade drug supply when needed, we may face delays in
initiating or completing pivotal trials and our business operations could suffer
significant harm.
To date, our product has been manufactured in small quantities
for pre-clinical studies and clinical trials by third-party manufacturers. In
order to commercialize our product, we need to manufacture commercial quality
drug supply for use in registration clinical trials. Most, if not all, of the
clinical material used in Phase III/pivotal/registration studies must be derived
from the defined commercial process including scale, manufacturing site, process
controls and batch size. If we have not scaled up and validated the commercial
production of our product prior to the commencement of pivotal clinical trials,
we may have to employ a bridging strategy during the trial to demonstrate
equivalency of early stage material to commercial drug product, or potentially
delay the initiation or completion of the trial until drug supply is available.
The manufacturing of commercial quality drug product requires significant
efforts including, but not limited to scale-up of production to anticipated
commercial scale, process characterization and validation, analytical method
validation, identification of critical process parameters and product quality
attributes, multiple process performance and validation runs, has long lead
times and is very expensive. If we do not have commercial drug supply available
when needed for pivotal clinical trials, our regulatory and commercial progress
may be delayed and we may incur increased product development cost. This may
have a material adverse effect on our business, financial condition and
prospects, and may delay marketing of the product.
If clinical trials of our product candidates fail to
demonstrate safety and efficacy to the satisfaction of regulatory authorities or
do not otherwise produce positive results, we would incur additional costs or
experience delays in completing, or ultimately be unable to complete, the
development and commercialization of our product candidates.
Before obtaining marketing approval from regulatory authorities
for the sale of our product candidates, we must conduct preclinical studies in
animals and extensive clinical trials in humans to demonstrate the safety and
efficacy of the product candidates. Clinical testing is expensive and difficult
to design and implement, can take many years to complete and has uncertain
outcomes. The outcome of preclinical studies and early clinical trials may not
predict the success of later clinical trials, and interim results of a clinical
trial do not necessarily predict final results. A number of companies in the
pharmaceutical and biotechnology industries have suffered significant setbacks
in advanced clinical trials due to lack of efficacy or unacceptable safety
profiles, notwithstanding promising results in earlier trials. We do not know
whether the clinical trials we may conduct will demonstrate adequate efficacy
and safety to result in regulatory approval to market any of our product
candidates in any jurisdiction. A product candidate may fail for safety or
efficacy reasons at any stage of the testing process. A major risk we face is
the possibility that none of our product candidates under development will
successfully gain market approval from the FDA or other regulatory authorities, resulting in us being unable to derive
any commercial revenue from them after investing significant amounts of capital
in their development.
- 24 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
If we experience delays in clinical testing, we will be
delayed in commercializing our product candidates, and our business may be
substantially harmed.
We cannot predict whether any clinical trials will begin as
planned, will need to be restructured, or will be completed on schedule, or at
all. Our product development costs will increase if we experience delays in
clinical testing. Significant clinical trial delays could shorten any periods
during which we may have the exclusive right to commercialize our product
candidates or allow our competitors to bring products to market before us, which
would impair our ability to successfully commercialize our product candidates
and may harm our financial condition, results of operations and prospects. The
commencement and completion of clinical trials for our products may be delayed
for a number of reasons, including delays related, but not limited, to:
| |
• |
failure by regulatory authorities to grant
permission to proceed or placing the clinical trial on hold; |
| |
• |
patients failing to enroll or remain in our
trials at the rate we expect; |
|
• |
suspension or termination of clinical trials by
regulators for many reasons, including concerns about patient safety or
failure of our CMOs to comply with cGMP requirements; |
| |
• |
any changes to our manufacturing process that
may be necessary or desired; |
| |
• |
delays or failure to obtain clinical supply
from CMOs of our products necessary to conduct clinical trials; |
| |
• |
product candidates demonstrating a lack of
safety or efficacy during clinical trials; |
|
• |
patients choosing an alternative treatment for
the indications for which we are developing any of our product candidates
or participating in competing clinical trials; |
|
• |
patients failing to complete clinical trials
due to dissatisfaction with the treatment, side effects or other reasons;
|
| |
• |
reports of clinical testing on similar
technologies and products raising safety and/or efficacy concerns; |
| |
• |
competing clinical trials and scheduling
conflicts with participating clinicians; |
|
• |
clinical investigators not performing our clinical trials
on their anticipated schedule, dropping out of a trial, or employing
methods not consistent with the clinical trial protocol, regulatory
requirements or other third parties not performing data collection and
analysis in a timely or accurate manner; |
|
• |
failure of our contract research organizations,
or CROs, to satisfy their contractual duties or meet expected deadlines;
|
|
• |
inspections of clinical trial sites by regulatory
authorities or Institutional Review Boards, or IRBs, or ethics committees
finding regulatory violations that require us to undertake corrective
action, resulting in suspension or termination of one or more sites or the
imposition of a clinical hold on the entire study; |
|
• |
one or more IRBs or ethics committees
rejecting, suspending or terminating the study at an investigational site,
precluding enrollment of additional subjects, or withdrawing its approval
of the trial; or |
| |
• |
failure to reach agreement on acceptable terms
with prospective clinical trial sites. |
Our product development costs will increase if we experience
delays in testing or approval or if we need to perform more or larger clinical
trials than planned. Additionally, changes in regulatory requirements and
policies may occur, and we may need to amend study protocols to reflect these
changes. Amendments may require us to resubmit our study protocols to regulatory
authorities or IRBs or ethics committees for re-examination, which may impact
the cost, timing or successful completion of that trial. Delays or increased
product development costs may have a material adverse effect on our business,
financial condition and prospects.
We may not be able to file INDs to commence additional
clinical trials on the timelines we expect, and even if we are able to, the FDA
may not permit us to proceed in a timely manner, or at all.
Prior to commencing clinical trials in the United States for
any of our product candidates, we may be required to have an allowed IND for
each product candidate and to file additional INDs prior to initiating any
additional clinical trials for SIRPαFc. We believe that the data from previous
studies will support the filing of additional INDs, to enable us to undertake
additional clinical studies as we have planned. However, submission of an IND
may not result in the FDA allowing further clinical trials to begin and, once
begun, issues may arise that will require us to suspend or terminate such clinical trials. Additionally, even
if relevant regulatory authorities agree with the design and implementation of
the clinical trials set forth in an IND, these regulatory authorities may change
their requirements in the future. Failure to submit or have effective INDs and
commence or continue clinical programs will significantly limit our opportunity
to generate revenue.
- 25 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
If we have difficulty enrolling patients in clinical
trials, the completion of the trials may be delayed or cancelled.
As our product candidates advance from preclinical testing to
clinical testing, and then through progressively larger and more complex
clinical trials, we will need to enroll an increasing number of patients that
meet our eligibility criteria. There is significant competition for recruiting
cancer patients in clinical trials, and we may be unable to enroll the patients
we need to complete clinical trials on a timely basis or at all. The factors
that affect our ability to enroll patients are largely uncontrollable and
include, but are not limited to, the following:
| |
• |
size and nature of the patient population;
|
| |
• |
eligibility and exclusion criteria for the
trial; |
| |
• |
design of the study protocol; |
| |
• |
competition with other companies for clinical
sites or patients; |
| |
• |
the perceived risks and benefits of the product
candidate under study; |
| |
• |
the patient referral practices of physicians;
and |
| |
• |
the number, availability, location and
accessibility of clinical trial sites. |
If we are unable to successfully develop companion
diagnostics for our therapeutic product candidates, or experience significant
delays in doing so, we may not achieve marketing approval or realize the full
commercial potential of our therapeutic product candidates.
We may develop companion diagnostics for our therapeutic
product candidates. We expect that, at least in some cases, regulatory
authorities may require the development and regulatory approval of a companion
diagnostic as a condition to approving our therapeutic product candidates. We
have limited experience and capabilities in developing or commercializing
diagnostics and plan to rely in large part on third parties to perform these
functions. We have not begun to develop companion diagnostics for any of our
therapeutic product candidates.
Companion diagnostics are subject to regulation by the FDA, HC,
and comparable foreign regulatory authorities as medical devices and may require
separate regulatory approval or clearance prior to commercialization. If we, or
any third parties that we engage to assist us, are unable to successfully
develop companion diagnostics for our therapeutic product candidates, or
experience delays in doing so, our business may be substantially harmed.
Regulatory approval processes are lengthy, expensive and
inherently unpredictable. Our inability to obtain regulatory approval for our
product candidates would substantially harm our business.
Our development and commercialization activities and product
candidates are significantly regulated by a number of governmental entities,
including the FDA, HC, and comparable authorities in other countries. Regulatory
approvals are required prior to each clinical trial and we may fail to obtain
the necessary approvals to commence or continue clinical testing. We must comply
with regulations concerning the manufacture, testing, safety, effectiveness,
labeling, documentation, advertising, and sale of products and product
candidates and ultimately must obtain regulatory approval before we can
commercialize a product candidate. The time required to obtain approval by such
regulatory authorities is unpredictable but typically takes many years following
the commencement of preclinical studies and clinical trials. Any analysis of
data from clinical activities we perform is subject to confirmation and
interpretation by regulatory authorities, which could delay, limit or prevent
regulatory approval. Even if we believe results from our clinical trials are
favorable to support the marketing of our product candidates, the FDA or other
regulatory authorities may disagree. In addition, approval policies,
regulations, or the type and amount of clinical data necessary to gain approval
may change during the course of a product candidate’s clinical development and
may vary among jurisdictions. We have not obtained regulatory approval for any
product candidate and it is possible that none of our existing product
candidates or any future product candidates will ever obtain regulatory
approval.
- 26 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
We could fail to receive regulatory approval for our product
candidates for many reasons, including, but not limited to:
| |
• |
disagreement with the design or implementation
of our clinical trials; |
| |
• |
failure to demonstrate that a product candidate
is safe and effective for its proposed indication; |
| |
• |
failure of clinical trials to meet the level of
statistical significance required for approval; |
| |
• |
failure to demonstrate that a product
candidate’s clinical and other benefits outweigh its safety risks; |
| |
• |
disagreement with our interpretation of data
from preclinical studies or clinical trials; |
|
• |
the insufficiency of data collected from clinical trials
of our product candidates to support the submission and filing of a
biologic license application, or BLA, or other submission to obtain
regulatory approval; |
|
• |
deficiencies in the manufacturing processes or
the failure of facilities of CMOs with whom we contract for clinical and
commercial supplies to pass a pre-approval inspection; or |
|
• |
changes in the approval policies or regulations
that render our preclinical and clinical data insufficient for approval.
|
A regulatory authority may require more information, including
additional preclinical or clinical data to support approval, which may delay or
prevent approval and our commercialization plans, or we may decide to abandon
the development program. If we were to obtain approval, regulatory authorities
may approve any of our product candidates for fewer or more limited indications
than we request, may grant approval contingent on the performance of costly
post-marketing clinical trials, or may approve a product candidate with a label
that does not include the labeling claims necessary or desirable for the
successful commercialization of that product candidate. Moreover, depending on
any safety issues associated with our product candidates that garner approval,
the FDA may impose a risk evaluation and mitigation strategy, thereby imposing
certain restrictions on the sale and marketability of such products.
We may not achieve our publicly announced milestones
according to schedule, or at all.
From time to time, we may announce the timing of certain events
we expect to occur, such as the anticipated timing of results from our clinical
trials. These statements are forward-looking and are based on the best estimates
of management at the time relating to the occurrence of such events. However,
the actual timing of such events may differ from what has been publicly
disclosed. The timing of events such as initiation or completion of a clinical
trial, filing of an application to obtain regulatory approval, or announcement
of additional clinical trials for a product candidate may ultimately vary from
what is publicly disclosed. These variations in timing may occur as a result of
different events, including the nature of the results obtained during a clinical
trial or during a research phase, timing of the completion of clinical trials,
problems with a CMO or a CRO or any other event having the effect of delaying
the publicly announced timeline. We undertake no obligation to update or revise
any forward-looking information, whether as a result of new information, future
events or otherwise, except as otherwise required by law. Any variation in the
timing of previously announced milestones could have a material adverse effect
on our business plan, financial condition or operating results and the trading
price of common shares.
We face competition from other biotechnology and
pharmaceutical companies and our financial condition and operations will suffer
if we fail to effectively compete.
The biotechnology and pharmaceutical industries are intensely
competitive and subject to rapid and significant technological change. Our
competitors include large, well-established pharmaceutical companies,
biotechnology companies, and academic and research institutions developing
cancer therapeutics for the same indications we are targeting and competitors
with existing marketed therapies. Many other companies are developing or
commercializing therapies to treat the same diseases or indications for which
our product candidates may be useful. Although there are no approved therapies
that specifically target the CD47 pathway, some competitors use therapeutic
approaches that may compete directly with our product candidates. For example,
SIRPαFc is in direct competition with CD47 blocking antibodies from Forty Seven
Inc., Celgene Corporation, Novimmune SA and others.
- 27 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Many of our competitors have substantially greater financial,
technical and human resources than we do and have significantly greater
experience than us in conducting preclinical testing and human clinical trials
of product candidates, scaling up manufacturing operations and obtaining
regulatory approvals of products. Accordingly, our competitors may succeed in
obtaining regulatory approval for products more rapidly than we do. Our ability
to compete successfully will largely depend on:
|
• |
the efficacy and safety profile of our product
candidates relative to marketed products and other product candidates in
development; |
|
• |
our ability to develop and maintain a
competitive position in the product categories and technologies on which
we focus; |
|
• |
the time it takes for our product candidates to
complete clinical development and receive marketing approval; |
| |
• |
our ability to obtain required regulatory
approvals; |
| |
• |
our ability to commercialize any of our product
candidates that receive regulatory approval; |
|
• |
our ability to establish, maintain and protect
intellectual property rights related to our product candidates; and |
|
• |
acceptance of any of our product candidates
that receive regulatory approval by physicians and other healthcare
providers and payers. |
Competitors have developed and may develop technologies that
could be the basis for products that challenge the discovery research
capabilities of Fluorinov. Some of those products may have an entirely different
approach or means of accomplishing the desired therapeutic effect than our
product candidates and may be more effective or less costly than our product
candidates. The success of our competitors and their products and technologies
relative to our technological capabilities and competitiveness could have a
material adverse effect on the future preclinical studies and clinical trials of
our product candidates, including our ability to obtain the necessary regulatory
approvals for the conduct of such clinical trials. This may further negatively
impact our ability to generate future product development programs using
Fluorinov technology.
If we are not able to compete effectively against our current
and future competitors, our business will not grow and our financial condition
and operations will substantially suffer.
We heavily rely on the capabilities and experience of our
key executives and scientists and the loss of any of them could affect our
ability to develop our products.
The loss of Dr. Niclas Stiernholm, our President and Chief
Executive Officer, or other key members of our staff, could harm us. We have
employment agreements with Dr. Stiernholm and other key members of our staff,
although such employment agreements do not guarantee their retention. We also
depend on our scientific and clinical collaborators and advisors, all of whom
have outside commitments that may limit their availability to us. In addition,
we believe that our future success will depend in large part upon our ability to
attract and retain highly skilled scientific, managerial, medical,
manufacturing, clinical and regulatory personnel, particularly as we expand our
activities and seek regulatory approvals for clinical trials. We enter into
agreements with our scientific and clinical collaborators and advisors, key
opinion leaders and academic partners in the ordinary course of our business. We
also enter into agreements with physicians and institutions who will recruit
patients into our clinical trials on our behalf in the ordinary course of our
business. Notwithstanding these arrangements, we face significant competition
for these types of personnel from other companies, research and academic
institutions, government entities and other organizations. We cannot predict our
success in hiring or retaining the personnel we require for continued growth.
The loss of the services of any of our executive officers or other key personnel
could potentially harm our business, operating results or financial condition.
Our employees may engage in misconduct or other improper
activities, including noncompliance with regulatory standards and requirements,
which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other
misconduct. Misconduct by employees could include failures to comply with FDA
regulations, provide accurate information to the FDA, comply with manufacturing
standards we have established, comply with federal and state healthcare
fraud and abuse laws and regulations, report financial information or data
accurately or disclose unauthorized activities to us. In particular, sales,
marketing and business arrangements in the healthcare industry are subject to
extensive laws and regulations intended to prevent fraud, kickbacks,
self-dealing, and other abusive practices. These laws and regulations may
restrict or prohibit a wide range of pricing, discounting, marketing and
promotion, sales commission, customer incentive programs and other business
arrangements. Employee misconduct could also involve the improper use of
information obtained in the course of clinical trials, which could result in
regulatory sanctions and serious harm to our reputation. If any such actions are
instituted against us, and we are not successful in defending ourselves or
asserting our rights, those actions could have a substantial impact on our
business and results of operations, including the imposition of substantial
fines or other sanctions.
- 28 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
The failure to fully realize the benefits of our
acquisition of Fluorinov may adversely affect our future results. In
January 2016, we acquired all of the outstanding capital stock of Fluorinov, a
small molecule medicinal chemistry company with preclinical oncology assets and
a potential discovery platform. The success of our acquisition of Fluorinov will
depend, in part, on our ability to fully realize the anticipated benefits from
combining our business with Fluorinov’s business. However, to realize these
anticipated benefits, we must continue the research and development activities
previously undertaken by Fluorinov as a stand-alone company. If we are unable to
achieve these objectives, the anticipated benefits of our acquisition of
Fluorinov may not be realized fully or at all or may take longer to realize than
expected.
We may expand our business through the acquisition of
companies or businesses or by entering into collaborations or by in-licensing
product candidates, each of which could disrupt our business and harm our
financial condition.
We have in the past and may in the future seek to expand our
pipeline and capabilities by acquiring one or more companies or businesses,
entering into collaborations, or in-licensing one or more product candidates.
Acquisitions, collaborations and in-licenses involve numerous risks, including,
but not limited to:
| |
• |
substantial cash expenditures; |
| |
• |
technology development risks; |
| |
• |
potentially dilutive issuances of equity
securities; |
|
• |
incurrence of debt and contingent liabilities,
some of which may be difficult or impossible to identify at the time of
acquisition; |
| |
• |
difficulties in assimilating the operations of
the acquired companies; |
| |
• |
potential disputes regarding contingent
consideration; |
| |
• |
diverting our management’s attention away from
other business concerns; |
| |
• |
entering markets in which we have limited or no
direct experience; and |
| |
• |
potential loss of our key employees or key
employees of the acquired companies or businesses. |
We have experience in making acquisitions, entering
collaborations, and in-licensing product candidates, however, we cannot provide
assurance that any acquisition, collaboration or in-license will result in
short-term or long-term benefits to us. We may incorrectly judge the value or
worth of an acquired company or business or in-licensed product candidate. In
addition, our future success would depend in part on our ability to manage the
rapid growth associated with some of these acquisitions, collaborations and
in-licenses. We cannot provide assurance that we would be able to successfully
combine our business with that of acquired businesses, manage a collaboration or
integrate in-licensed product candidates. Furthermore, the development or
expansion of our business may require a substantial capital investment by
us.
Negative results from clinical trials or studies of
others and adverse safety events involving the targets of our products may have
an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various
aspects of biopharmaceutical products are conducted by academic researchers,
competitors or others. The results of these studies or trials, when published,
may have a significant effect on the market for the biopharmaceutical
product that is the subject of the study. The publication of negative results of
studies or clinical trials or adverse safety events related to our product
candidates, or the therapeutic areas in which our product candidates compete,
could adversely affect our share price and our ability to finance future
development of our product candidates, and our business and financial results
could be materially and adversely affected.
- 29 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
We face the risk of product liability claims, which could
exceed our insurance coverage and produce recalls, each of which could deplete
our cash resources.
We are exposed to the risk of product liability claims alleging
that use of our product candidates caused an injury or harm. These claims can
arise at any point in the development, testing, manufacture, marketing or sale
of our product candidates and may be made directly by patients involved in
clinical trials of our product candidates, by consumers or healthcare providers
or by individuals, organizations or companies selling our products. Product
liability claims can be expensive to defend, even if the product or product
candidate did not actually cause the alleged injury or harm.
Insurance covering product liability claims becomes
increasingly expensive as a product candidate moves through the development
pipeline to commercialization. We currently maintain clinical trial liability
insurance coverage of $10,000. However, there can be no assurance that such
insurance coverage is or will continue to be adequate or available to us at a
cost acceptable to us or at all. We may choose or find it necessary under our
collaborative agreements to increase our insurance coverage in the future. We
may not be able to secure greater or broader product liability insurance
coverage on acceptable terms or at reasonable costs when needed. Any liability
for damages resulting from a product liability claim could exceed the amount of
our coverage, require us to pay a substantial monetary award from our own cash
resources and have a material adverse effect on our business, financial
condition and results of operations. Moreover, a product recall, if required,
could generate substantial negative publicity about our products and business,
inhibit or prevent commercialization of other products and product candidates or
negatively impact existing or future collaborations.
If we are unable to maintain product liability insurance
required by our third parties, the corresponding agreements would be subject to
termination, which could have a material adverse impact on our
operations.
Some of our licensing and other agreements with third parties
require or might require us to maintain product liability insurance. If we
cannot maintain acceptable amounts of coverage on commercially reasonable terms
in accordance with the terms set forth in these agreements, the corresponding
agreements would be subject to termination, which could have a material adverse
impact on our operations.
Risks Related to Intellectual Property
If we are unable to adequately protect and enforce our
intellectual property, our competitors may take advantage of our development
efforts or acquired technology and compromise our prospects of marketing and
selling our key products.
We control two patent families relating to SIRPα. One family
relates to the use of SIRPα to treat cancer. The other family relates to our
drug as a composition of matter, SIRPαFc. We have also recently filed for patent
protection covering additional inventions relating to SIRPα, including
anti-cancer drug combination therapies that utilize SIRPαFc.
Our small molecule portfolio embraces patent filings that cover
numerous different inventions. With the exception of one process scheme, these
patent filings each claim a family of small molecule drugs as compositions of
matter, together with claims for their production and their medical uses. These
drugs target cancer for the most part, and some related medical end-uses.
Our success will depend in part upon our ability to protect our
intellectual property and proprietary technologies and upon the nature and scope
of the intellectual property protection we receive. For example, some of our
patent portfolio covers primarily methods of medical use but not compositions of
matter. The ability to compete effectively and to achieve partnerships will depend on our ability to
develop and maintain proprietary aspects of our technology and to operate
without infringing on the proprietary rights of others. The presence of such
proprietary rights of others could severely limit our ability to develop and
commercialize our products, to conduct our existing research and could require
financial resources to defend litigation, which may be in excess of our ability
to raise such funds. There is no assurance that our pending patent applications
or those that we intend to acquire will be approved in a form that will be
sufficient to protect our proprietary technology and gain or keep any
competitive advantage that we may have or, once approved, will be upheld in any
post-grant proceedings brought by any third parties.
- 30 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
The patent positions of pharmaceutical companies can be highly
uncertain and involve complex legal, scientific and factual questions for which
important legal principles remain unresolved. Patents issued to us or our
respective licensors may be challenged, invalidated or circumvented. To the
extent our intellectual property, including licensed intellectual property,
offers inadequate protection, or is found to be invalid or unenforceable, we are
exposed to a greater risk of direct competition. If our intellectual property
does not provide adequate protection against our competitors’ products, our
competitive position could be adversely affected, as could our business,
financial condition and results of operations. Both the patent application
process and the process of managing patent disputes can be time consuming and
expensive, and the laws of some foreign countries may not protect our
intellectual property rights to the same extent as do the laws of Canada and the
United States.
We will be able to protect our intellectual property from
unauthorized use by third parties only to the extent that our proprietary
technologies, key products, and any future products are covered by valid and
enforceable intellectual property rights including patents or are effectively
maintained as trade secrets, and provided we have the funds to enforce our
rights, if necessary.
If we lose our licenses from third-party owners we may be
unable to continue a substantial part of our business.
We are party to licenses that give us rights to intellectual
property that is necessary or useful for a substantial part of our business.
Pursuant to our exclusive license agreement with the University Health Network
and the Hospital for Sick Children under which we license certain patent rights
for our key products and their uses, we are required to use commercially
reasonable efforts to commercialize products based on the licensed rights and
pay milestone payments, royalties on net sales, and an annual maintenance
fee.
We have also entered into agreements allowing us to manufacture
SIRPαFc using Catalent’s proprietary GPEx® expression system. The consideration
includes payments at the time we successfully reach a series of development and
sales milestones. We may also enter into licenses in the future to access
additional third-party intellectual property.
If we fail to pay annual maintenance fees, development and
sales milestones, or it is determined that we did not use commercially
reasonable efforts to commercialize licensed products, we could lose our
licenses which could have a material adverse effect on our business and
financial condition.
We may require additional third-party licenses to
effectively develop and manufacture our key products and are currently unable to
predict the availability or cost of such licenses.
A substantial number of patents have already been issued to
other biotechnology and pharmaceutical companies. To the extent that valid
third-party patent rights cover our products or services, we or our strategic
collaborators would be required to seek licenses from the holders of these
patents in order to manufacture, use or sell these products and services, and
payments under them would reduce our profits from these products and services.
We are currently unable to predict the extent to which we may wish or be
required to acquire rights under such patents, the availability and cost of
acquiring such rights, and whether a license to such patents will be available
on acceptable terms or at all. There may be patents in the U.S. or in foreign
countries or patents issued in the future that are unavailable to license on
acceptable terms. Our inability to obtain such licenses may hinder or eliminate
our ability to manufacture and market our products.
- 31 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Changes in patent law and its interpretation could
diminish the value of patents in general, thereby impairing our ability to
protect our product candidates.
As is the case with other biotechnology and pharmaceutical
companies, our success is heavily dependent on intellectual property rights,
particularly patents. Obtaining and enforcing patents in the biopharmaceutical
industry involves technological and legal complexity, and obtaining and
enforcing biopharmaceutical patents is costly, time-consuming and inherently
uncertain. The U.S. Supreme Court has ruled on several patent cases in recent
years, either narrowing the scope of patent protection available in certain
circumstances or weakening the rights of patent owners in certain situations. In
addition to increasing uncertainty with regard to our and our licensors’ or
collaborators’ ability to obtain patents in the future, this combination of
events has created uncertainty with respect to the value of patents, once
obtained. Depending on decisions by the U.S. Congress, the federal courts, and
the U.S. Patent and Trademark Office, or USPTO, the laws and regulations
governing patents could change in unpredictable ways that would weaken our and
our licensors’ or collaborators’ ability to obtain new patents or to enforce
existing patents and patents we and our licensors or collaborators may obtain in
the future.
Recent patent reform legislation could increase the
uncertainties and costs surrounding the prosecution of our and our licensors’ or
collaborators’ patent applications and the enforcement or defense of our or our
licensors’ or collaborators’ issued patents. On September 16, 2011, the
Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law.
The Leahy-Smith Act includes a number of significant changes to U.S. patent law.
These include provisions that affect the way patent applications are prosecuted
and may also affect patent litigation. The USPTO recently developed new
regulations and procedures to govern administration of the Leahy-Smith Act, and
many of the substantive changes to patent law associated with the Leahy-Smith
Act, and in particular, the first to file provisions, only became effective on
March 16, 2013. Accordingly, it is not clear what, if any, impact the
Leahy-Smith Act will have on the operation of our business. However, the
Leahy-Smith Act and its implementation could increase the uncertainties and
costs surrounding the prosecution of our or our licensors’ or collaborators’
patent applications and the enforcement or defense of our or our licensors’ or
collaborators’ issued patents, all of which could have a material adverse effect
on our business and financial condition.
Litigation regarding patents, patent applications, and
other proprietary rights may be expensive, time consuming and cause delays in
the development and manufacturing of our key products.
Our success will depend in part on our ability to operate
without infringing the proprietary rights of third parties. The pharmaceutical
industry is characterized by extensive patent litigation. Other parties may
have, or obtain in the future, patents and allege that the use of our
technologies infringes these patent claims or that we are employing their
proprietary technology without authorization.
In addition, third parties may challenge or infringe upon our
existing or future patents. Proceedings involving our patents or patent
applications or those of others could result in adverse decisions regarding:
| |
• |
the patentability of our inventions relating to
our key products; and/or |
| |
• |
the enforceability, validity, or scope of
protection offered by our patents relating to our key products.
|
If we are unable to avoid infringing the patent rights of
others, we may be required to seek a license, defend an infringement action, or
challenge the validity of the patents in court. Regardless of the outcome,
patent litigation is costly and time consuming. In some cases, we may not have
sufficient resources to bring these actions to a successful conclusion. In
addition, if we do not obtain a license, develop or obtain non-infringing
technology, fail to defend an infringement action successfully or have infringed
patents declared invalid, we may:
| |
• |
incur substantial monetary damages; |
| |
• |
encounter significant delays in bringing our
key products to market; and/or |
|
• |
be precluded from participating in the
manufacture, use or sale of our key products or methods of treatment
requiring licenses. |
Even if we are successful in these proceedings, we may incur
substantial costs and divert management time and attention in pursuing these
proceedings, which could have a material adverse effect on us.
- 32 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Our reliance on third parties requires us to share our
trade secrets, which increases the possibility that a competitor will discover
them.
Because we rely on third parties to develop our products, we
must share trade secrets with them. We seek to protect our proprietary
technology in part by entering into confidentiality agreements and, if
applicable, material transfer agreements, collaborative research agreements,
consulting agreements or other similar agreements with our collaborators,
advisors, employees and consultants prior to beginning research or disclosing
proprietary information. These agreements typically restrict the ability of our
collaborators, advisors, employees and consultants to publish data potentially
relating to our trade secrets. Our academic and clinical collaborators typically
have rights to publish data, provided that we are notified in advance and may
delay publication for a specified time in order to secure our intellectual
property rights arising from the collaboration. In other cases, publication
rights are controlled exclusively by us, although in some cases we may share
these rights with other parties. We may also conduct joint research and
development programs which may require us to share trade secrets under the terms
of research and development collaboration or similar agreements. Despite our
efforts to protect our trade secrets, our competitors may discover our trade
secrets, either through breach of these agreements, independent development or
publication of information including our trade secrets in cases where we do not
have proprietary or otherwise protected rights at the time of publication. A
competitor’s discovery of our trade secrets may impair our competitive position
and could have a material adverse effect on our business and financial
condition.
Risks Related to Our Common Shares
Our common share price has been volatile in recent years,
and may continue to be volatile.
The market prices for securities of biopharmaceutical
companies, including ours, have historically been volatile. In the nine months
ended September 30, 2018, our common shares traded on the TSX at a high of
$11.44 and a low of $6.34 per share and on the NASDAQ at a high of U.S. $9.16
and a low of U.S. $4.80 per share. In the year ended December 31, 2017, our
common shares traded on the TSX at a high of $15.68 and a low of $5.26 per share
and on the NASDAQ at a high of U.S. $13.30 and a low of U.S. $4.15 per share. A
number of factors could influence the volatility in the trading price of our
common shares, including changes in the economy or in the financial markets,
industry related developments, the results of product development and
commercialization, changes in government regulations, and developments
concerning proprietary rights, litigation and cash flow. Our quarterly losses
may vary because of the timing of costs for manufacturing, preclinical studies
and clinical trials. Also, the reporting of adverse safety events involving our
products and public rumors about such events could cause our share price to
decline or experience periods of volatility. Each of these factors could lead to
increased volatility in the market price of our common shares. In addition,
changes in the market prices of the securities of our competitors may also lead
to fluctuations in the trading price of our common shares.
We have never paid dividends and do not expect to do so
in the foreseeable future.
We have not declared or paid any cash dividends on our common
or preferred shares to date. The payment of dividends in the future will be
dependent on our earnings and financial condition in addition to such other
factors as our board of directors considers appropriate. Unless and until we pay
dividends, shareholders may not receive a return on their shares. There is no
present intention by our board of directors to pay dividends on our shares.
We may issue additional common shares to the former
shareholders of Fluorinov as a result of our satisfaction of certain milestones,
resulting in share ownership dilution.
Under the terms of our agreements with Fluorinov and its former
shareholders, at our discretion up to 50% of any future contingent payments can
be satisfied through the issuance of our common shares, provided that the
aggregate number of common shares issuable under such payments will not exceed
1,558,447 common shares, which amount represented 19.99% of the outstanding
common shares at the time of execution of the acquisition, unless shareholder
approval has first been obtained.
Issuing additional common shares to the former shareholders of
Fluorinov in satisfaction of contingent consideration dilutes the ownership
interests of holders of our common shares on the dates of such issuances. If we
are unable to realize the strategic, operational and financial benefits
anticipated from our acquisition of Fluorinov, our shareholders may experience
dilution of their ownership interests in our company upon any such future
issuances of our common shares without receiving any commensurate benefit.
- 33 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Future sales or issuances of equity securities and the
conversion of outstanding securities to common shares could decrease the value
of the common shares, dilute investors’ voting power, and reduce our earnings
per share.
We may sell additional equity securities in future offerings,
including through the sale of securities convertible into equity securities, to
finance operations, acquisitions or projects, and issue additional common shares
if outstanding warrants or stock options are exercised, or preferred shares are
converted to common shares, which may result in dilution. See the information in
the section of this MD&A entitled “Description of Share Capital” for details
of our outstanding securities convertible into common shares. Subject to receipt
of any required regulatory approvals, subscribers of the December 2013 private
placement who purchased a minimum of 10% of the securities sold under the
offering received rights to purchase our securities in future financings to
enable each such shareholder to maintain their percentage holding in our common
shares for so long as the subscriber holds at least 10% of the outstanding
common shares on a fully-diluted basis. Shareholders who do not have this future
financing participation right may be disadvantaged in participating in such
financings.
Our board of directors has the authority to authorize certain
offers and sales of additional securities without the vote of, or prior notice
to, shareholders. Based on the need for additional capital to fund expected
expenditures and growth, it is likely that we will issue additional securities
to provide such capital. Such additional issuances may involve the issuance of a
significant number of common shares at prices less than the current market price
for our common shares.
Sales of substantial amounts of our securities, or the
availability of such securities for sale, as well as the issuance of substantial
amounts of our common shares upon conversion of outstanding convertible equity
securities, could adversely affect the prevailing market prices for our
securities and dilute investors’ earnings per share. A decline in the market
prices of our securities could impair our ability to raise additional capital
through the sale of securities should we desire to do so.
U.S. holders of 10% or more of the voting power of our
common shares may be subject to U.S. federal income taxation at ordinary income
tax rates on undistributed earnings and profits.
There is a risk that we will be classified as a controlled
foreign corporation, or CFC, for U.S. federal income tax purposes. We will
generally be classified as a CFC if more than 50% of our outstanding shares,
measured by reference to voting power or value, are owned (directly, indirectly
or by attribution) by “U.S. Shareholders.” For this purpose, a “U.S.
Shareholder” is any U.S. person that owns directly, indirectly or by
attribution, 10% or more of the voting power of our outstanding shares. If we
are classified as a CFC, a U.S. Shareholder may be subject to U.S. income
taxation at ordinary income tax rates on all or a portion of our undistributed
earnings and profits attributable to “subpart F income” and may also be subject
to tax at ordinary income tax rates on any gain realized on a sale of common
shares, to the extent of our current and accumulated earnings and profits
attributable to such shares. The CFC rules are complex and U.S. Shareholders of
our common shares are urged to consult their own tax advisors regarding the
possible application of the CFC rules to them in their particular circumstances.
We are likely a “passive foreign investment company,”
which may have adverse U.S. federal income tax consequences for U.S.
shareholders.
U.S. investors should be aware that we believe we were
classified as a PFIC during the tax years ended December 31, 2017 and 2016, and
based on current business plans and financial expectations, we believe that we
will be a PFIC for the current tax year and may be a PFIC in future tax years.
If we are a PFIC for any year during a U.S. shareholder’s holding period of our
common shares, then such U.S. shareholder generally will be required to treat
any gain realized upon a disposition of our common shares, or any so-called
“excess distribution” received on our common shares, as ordinary income, and to
pay an interest charge on a portion of such gain or distributions, unless the
shareholder makes a timely and effective “qualified electing fund” election, or
QEF Election, or a “mark-to-market” election with respect to our shares. A U.S.
shareholder who makes a QEF Election generally must report on a current basis
its share of our net capital gain and ordinary earnings for any year in which we
are a PFIC, whether or not we distribute any amounts to our shareholders. A U.S.
shareholder who makes the mark-to-market election generally must include as
ordinary income each year the excess of the fair market value of the common
shares over the shareholder’s adjusted tax basis therein. Each U.S. shareholder
should consult its own tax advisors regarding the PFIC rules and the U.S.
federal income tax consequences of the acquisition, ownership and disposition of
our common shares.
- 34 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
The effect of comprehensive U.S. tax reform legislation
on the Company is uncertain.
On December 22, 2017, the U.S. government enacted H.R. 1, “An
Act to provide for reconciliation pursuant to titles II and V of the concurrent
resolution on the budget for fiscal year 2018” (informally titled the “Tax Cuts
and Jobs Act”). Among a number of significant changes to the U.S. federal income
tax rules, the Tax Cuts and Jobs Act reduces the marginal U.S. corporate income
tax rate from 35% to 21%, limits the deduction for net interest expense, shifts
the United States toward a more territorial tax system, and imposes new taxes to
combat erosion of the U.S. federal income tax base, such as a one-time tax on
earnings of certain foreign subsidiaries that were previously tax deferred and a
new minimum tax on foreign earnings. The effects of the Tax Cuts and Jobs Act on
our company, whether adverse or favorable, are uncertain, and may not become
evident for some period of time, but could have a material adverse effect on our
business, financial position or results from operations.
It may be difficult for non-Canadian investors to obtain
and enforce judgments against us because of our Canadian incorporation and
presence.
We are a corporation existing under the laws of the Province of
Ontario, Canada. Several of our directors and officers, and several of the
experts are residents of Canada, and all or a substantial portion of their
assets, and a substantial portion of our assets, are located outside the United
States. Consequently, although we have appointed an agent for service of process
in the United States, it may be difficult for holders of our securities who
reside in the United States to effect service within the United States upon
those directors and officers, and the experts who are not residents of the
United States. It may also be difficult for holders of our securities who reside
in the United States to realize in the United States upon judgments of courts of
the United States predicated upon our civil liability and the civil liability of
our directors, officers and experts under the United States federal securities
laws. Investors should not assume that Canadian courts (i) would enforce
judgments of United States courts obtained in actions against us or such
directors, officers or experts predicated upon the civil liability provisions of
the United States federal securities laws or the securities or “blue sky” laws
of any state or jurisdiction of the United States or (ii) would enforce, in
original actions, liabilities against us or such directors, officers or experts
predicated upon the United States federal securities laws or any securities or
“blue sky” laws of any state or jurisdiction of the United States. In addition,
the protections afforded by Canadian securities laws may not be available to
investors in the United States.
If there are substantial sales of our common shares, the
market price of our common shares could decline.
Sales of substantial numbers of our common shares could cause a
decline in the market price of our common shares. Any sales by existing
shareholders or holders who exercise their warrants or stock options may have an
adverse effect on our ability to raise capital and may adversely affect the
market price of our common shares.
We are an “emerging growth company,” and we cannot be
certain if the reduced reporting requirements applicable to emerging growth
companies will make our common shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS
Act. For as long as we continue to be an emerging growth company, we may take
advantage of exemptions from various reporting requirements that are applicable
to other public companies that are not emerging growth companies, including not
being required to comply with the auditor attestation requirements of Section
404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding
executive compensation in our periodic reports and exemptions from the
requirements of holding a nonbinding advisory vote on executive compensation and
shareholder approval of any golden parachute payments not previously approved.
We could be an emerging growth company until the last day of our fiscal year
following the fifth anniversary of the date of the first sale of our common
shares pursuant to an effective registration statement under the U.S. Securities Act of 1933, which is December 31,
2019, although circumstances could cause us to lose that status earlier. We
cannot predict if investors will find our common shares less attractive because
we may rely on these exemptions. If some investors find our common shares less
attractive as a result, there may be a less active trading market for our common
shares and our share price may be more volatile.
- 35 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
Any failure to maintain an effective system of internal
controls may result in material misstatements of our consolidated financial
statements or cause us to fail to meet our reporting obligations or fail to
prevent fraud; and in that case, our shareholders could lose confidence in our
financial reporting, which would harm our business and could negatively impact
the price of our common shares.
Effective internal controls are necessary for us to provide
reliable financial reports and prevent fraud. If we fail to maintain an
effective system of internal controls, we might not be able to report our
financial results accurately or prevent fraud; and in that case, our
shareholders could lose confidence in our financial reporting, which would harm
our business and could negatively impact the price of our common shares. While
we believe that we have sufficient personnel and review procedures to allow us
to maintain an effective system of internal controls, we cannot provide
assurance that we will not experience potential material weaknesses in our
internal control. Even if we conclude that our internal control over financial
reporting provides reasonable assurance regarding the reliability of financial
reporting and the preparation of consolidated financial statements for external
purposes in accordance with IFRS as issued by the IASB, because of its inherent
limitations, internal control over financial reporting may not prevent or detect
fraud or misstatements. Failure to implement required new or improved controls,
or difficulties encountered in their implementation, could harm our results of
operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our
internal control over financial reporting, we may not be able to produce
reliable financial reports or help prevent fraud. Our failure to achieve and
maintain effective internal control over financial reporting could prevent us
from complying with our reporting obligations on a timely basis, which could
result in the loss of investor confidence in the reliability of our consolidated
financial statements, harm our business and negatively impact the trading price
of our common shares.
As a foreign private issuer, we are not subject to
certain United States securities law disclosure requirements that apply to a
domestic United States issuer, which may limit the information which would be
publicly available to our shareholders.
As a foreign private issuer, we are not required to comply with
all the periodic disclosure requirements of the Exchange Act, and therefore,
there may be less publicly available information about us than if we were a
United States domestic issuer. For example, we are not subject to the proxy
rules in the United States and disclosure with respect to our annual meetings
will be governed by Canadian requirements.
Our charter documents and certain Canadian legislation
could delay or deter a change of control, limit attempts by our shareholders to
replace or remove our current management and limit the market price of our
common shares.
Our authorized preferred shares are available for issuance from
time to time at the discretion of our board of directors, without shareholder
approval. Our articles grant our board of directors the authority to determine
the special rights and restrictions granted to or imposed on any unissued series
of preferred shares, and those rights may be superior to those of our common
shares. Further, the Investment Canada Act subjects any acquisition of control
of a company by a non-Canadian to government review if the value of the assets
as calculated pursuant to the legislation exceeds a threshold amount or in other
circumstances determined at the discretion of the Canadian government. A
reviewable acquisition may not proceed unless the relevant minister is satisfied
that the investment is likely to be of net benefit to Canada and the Canadian
government is satisfied that no other important concerns arise from the
acquisition of control. Any of the foregoing could prevent or delay a change of
control and may deprive or limit strategic opportunities to our shareholders to
sell their shares.
- 36 -
| TRILLIUM THERAPEUTICS INC. |
| |
| Management’s
Discussion and Analysis |
DISCLOSURE CONTROLS AND INTERNAL CONTROLS OVER FINANCIAL
REPORTING
We have implemented a system of internal controls that we
believe adequately protects our assets and is appropriate for the nature of our
business and the size of our operations. Our internal control system was
designed to provide reasonable assurance that all transactions are accurately
recorded, that transactions are recorded as necessary to permit preparation of
financial statements in accordance with IFRS, and that our assets are
safeguarded.
These internal controls include disclosure controls and
procedures designed to ensure that information required to be disclosed by us is
accumulated and communicated as appropriate to allow timely decisions regarding
required disclosure.
Internal control over financial reporting means a process
designed by or under the supervision of the Chief Executive Officer and the
Chief Financial Officer to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of financial statements
for external purposes in accordance with IFRS as issued by the IASB. The
internal controls are not expected to prevent and detect all misstatements due
to error or fraud.
There were no changes in our internal control over financial
reporting that occurred during the three months ended September 30, 2018 that
have materially affected, or are reasonably likely to materially affect, our
internal control over financial reporting.
ADDITIONAL INFORMATION
Additional information regarding our company can be found on
SEDAR at www.sedar.com, and on EDGAR at www.sec.gov/edgar.shtml.
- 37 -



