false--12-31FY201810-K000161385965531659YesfalseLarge Accelerated FilerPRA Health Sciences, Inc.falsefalseNoYesP6MP1DP12MP0Y00.0110000000001000000000636239506539452663623950653945260.0125P5YP1Y1000001000000000046700002303000269900016490006220009600010070000.011000000001000000000000P7YP7YP3YP5YP1YP3YP1YP2Y83.252.9475.8116.42116.1111.7382.9975.37
0001613859
2018-01-01
2018-12-31
0001613859
2018-06-30
0001613859
2019-02-22
0001613859
2018-12-31
0001613859
2017-12-31
0001613859
2017-01-01
2017-12-31
0001613859
2016-01-01
2016-12-31
0001613859
prah:DirectCostsMember
2017-01-01
2017-12-31
0001613859
prah:ReimbursableInvestigatorFeesMember
2018-01-01
2018-12-31
0001613859
prah:DirectCostsMember
2018-01-01
2018-12-31
0001613859
prah:ReimbursableOutOfPocketCostsMember
2017-01-01
2017-12-31
0001613859
prah:ReimbursableOutOfPocketCostsMember
2018-01-01
2018-12-31
0001613859
prah:DirectCostsMember
2016-01-01
2016-12-31
0001613859
prah:ReimbursableInvestigatorFeesMember
2016-01-01
2016-12-31
0001613859
prah:ReimbursableOutOfPocketCostsMember
2016-01-01
2016-12-31
0001613859
prah:ReimbursableInvestigatorFeesMember
2017-01-01
2017-12-31
0001613859
2015-12-31
0001613859
us-gaap:CommonStockMember
2017-01-01
2017-12-31
0001613859
us-gaap:CommonStockMember
2015-12-31
0001613859
us-gaap:RetainedEarningsMember
2016-12-31
0001613859
us-gaap:CommonStockMember
2018-01-01
2018-12-31
0001613859
us-gaap:CommonStockMember
2018-12-31
0001613859
us-gaap:NoncontrollingInterestMember
2018-01-01
2018-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2016-01-01
2016-12-31
0001613859
us-gaap:CommonStockMember
2016-01-01
2016-12-31
0001613859
us-gaap:RetainedEarningsMember
2018-12-31
0001613859
us-gaap:RetainedEarningsMember
2018-01-01
2018-12-31
0001613859
2016-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2017-01-01
2017-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2018-01-01
2018-12-31
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-12-31
0001613859
us-gaap:RetainedEarningsMember
2017-01-01
2017-12-31
0001613859
us-gaap:RetainedEarningsMember
2015-12-31
0001613859
us-gaap:RetainedEarningsMember
2017-12-31
0001613859
us-gaap:NoncontrollingInterestMember
2017-01-01
2017-12-31
0001613859
us-gaap:CommonStockMember
2017-12-31
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-01-01
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2016-01-01
2016-12-31
0001613859
us-gaap:CommonStockMember
2018-01-01
0001613859
us-gaap:RetainedEarningsMember
2018-01-01
0001613859
us-gaap:NoncontrollingInterestMember
2018-01-01
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2017-01-01
2017-12-31
0001613859
2018-01-01
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2017-12-31
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2015-12-31
0001613859
us-gaap:NoncontrollingInterestMember
2016-12-31
0001613859
us-gaap:CommonStockMember
2016-12-31
0001613859
us-gaap:NoncontrollingInterestMember
2015-12-31
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-01-01
2018-12-31
0001613859
us-gaap:RetainedEarningsMember
2016-01-01
2016-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2015-12-31
0001613859
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2016-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2016-12-31
0001613859
us-gaap:NoncontrollingInterestMember
2018-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2018-01-01
0001613859
us-gaap:NoncontrollingInterestMember
2017-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2018-12-31
0001613859
us-gaap:AdditionalPaidInCapitalMember
2017-12-31
0001613859
prah:SecondaryPublicOfferingMember
2017-01-01
2017-12-31
0001613859
prah:KohlbergKravisRobertsCoLpAndCertainExecutiveOfficersMember
prah:SecondaryPublicOfferingMember
2017-01-01
2017-12-31
0001613859
prah:KohlbergKravisRobertsCoLpAndCertainExecutiveOfficersMember
prah:SecondaryPublicOfferingMember
2018-01-01
2018-12-31
0001613859
prah:KohlbergKravisRobertsCoLPMember
2018-01-01
2018-12-31
0001613859
prah:SecondaryPublicOfferingMember
2018-01-01
2018-12-31
0001613859
prah:SecondaryPublicOfferingMember
2016-01-01
2016-12-31
0001613859
prah:KohlbergKravisRobertsCoLpAndCertainExecutiveOfficersMember
prah:SecondaryPublicOfferingMember
2016-01-01
2016-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
us-gaap:MeasurementInputPriceVolatilityMember
2018-12-31
0001613859
us-gaap:AccountingStandardsUpdate201409Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
0001613859
us-gaap:AccountingStandardsUpdate201609Member
us-gaap:RetainedEarningsMember
2017-12-31
0001613859
2019-01-01
2018-12-31
0001613859
srt:MinimumMember
2018-01-01
2018-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsNonrecurringMember
2018-12-31
0001613859
prah:EDSPRandSSMember
prah:ClinicalResearchMember
2018-12-31
0001613859
prah:AccountingStandardsUpdate201609ExcessTaxBenefitsComponentMember
2017-12-31
0001613859
us-gaap:ServiceMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2016-01-01
2016-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2018-01-01
2018-12-31
0001613859
us-gaap:AccountingStandardsUpdate201609Member
2017-12-31
0001613859
us-gaap:ServiceMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2017-01-01
2017-12-31
0001613859
prah:ReimbursementMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2017-01-01
2017-12-31
0001613859
srt:MaximumMember
2018-01-01
2018-12-31
0001613859
prah:ServiceInKindMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
prah:DataSolutionsMember
2018-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2018-01-01
2018-12-31
0001613859
srt:MaximumMember
2019-01-01
2018-12-31
0001613859
prah:ReimbursableInvestigatorFeesMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2017-01-01
2017-12-31
0001613859
prah:ServiceInKindMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:ReimbursementMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2016-01-01
2016-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
us-gaap:MeasurementInputDiscountRateMember
2018-12-31
0001613859
us-gaap:SecuredDebtMember
2018-05-31
0001613859
prah:ReimbursableInvestigatorFeesMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2016-01-01
2016-12-31
0001613859
prah:DirectCostsMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2018-01-01
2018-12-31
0001613859
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2018-01-01
2018-12-31
0001613859
prah:ReimbursableOutOfPocketCostsMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2018-01-01
2018-12-31
0001613859
prah:AccountingStandardsUpdate201409ImpactFromAdoptionOfASC606Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
2018-12-31
0001613859
prah:ReimbursableInvestigatorFeesMember
prah:AccountingStandardsUpdate201409ImpactFromAdoptionOfASC606Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
2018-12-31
0001613859
prah:DirectCostsMember
prah:AccountingStandardsUpdate201409ImpactFromAdoptionOfASC606Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
2018-12-31
0001613859
prah:ReimbursementMember
prah:AccountingStandardsUpdate201409ReclassificationFromAdoptionOfASC606Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
2018-12-31
0001613859
prah:ReimbursableOutOfPocketCostsMember
prah:AccountingStandardsUpdate201409ImpactFromAdoptionOfASC606Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2018-01-01
2018-12-31
0001613859
prah:AccountingStandardsUpdate201409ReclassificationFromAdoptionOfASC606Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
2018-12-31
0001613859
prah:ReimbursableInvestigatorFeesMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2018-01-01
2018-12-31
0001613859
prah:ReimbursementMember
us-gaap:CalculatedUnderRevenueGuidanceInEffectBeforeTopic606Member
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
prah:AccountingStandardsUpdate201409ReclassificationFromAdoptionOfASC606Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
2017-01-01
2017-12-31
0001613859
us-gaap:EmployeeStockOptionMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
2016-01-01
2016-12-31
0001613859
prah:NextrialsIncMember
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
prah:ParallelSixMember
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
prah:ParallelSixMember
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2016-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2016-12-31
0001613859
prah:NextrialsIncMember
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-01-01
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
prah:AccruedExpensesAndOtherCurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:OtherNoncurrentLiabilitiesMember
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueInputsLevel2Member
prah:ContingentConsiderationMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:MarketableSecuritiesMember
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:MarketableSecuritiesMember
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:MarketableSecuritiesMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueInputsLevel1Member
prah:ContingentConsiderationMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:ContingentConsiderationMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
prah:ContingentConsiderationMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:MarketableSecuritiesMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:CustomerAMember
us-gaap:SalesRevenueServicesNetMember
us-gaap:CustomerConcentrationRiskMember
2017-01-01
2017-12-31
0001613859
prah:CustomerAMember
us-gaap:SalesRevenueServicesNetMember
us-gaap:CustomerConcentrationRiskMember
2016-01-01
2016-12-31
0001613859
prah:CustomerBMember
us-gaap:SalesRevenueServicesNetMember
us-gaap:CustomerConcentrationRiskMember
2016-01-01
2016-12-31
0001613859
prah:CustomerAMember
us-gaap:AccountsReceivableMember
us-gaap:CustomerConcentrationRiskMember
2017-01-01
2017-12-31
0001613859
prah:CustomerAMember
us-gaap:AccountsReceivableMember
us-gaap:CustomerConcentrationRiskMember
2018-01-01
2018-12-31
0001613859
prah:CustomerBMember
us-gaap:AccountsReceivableMember
us-gaap:CustomerConcentrationRiskMember
2018-01-01
2018-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001613859
srt:MinimumMember
2019-01-01
2018-12-31
0001613859
srt:MaximumMember
prah:FurnitureAndEquipmentMember
2018-01-01
2018-12-31
0001613859
srt:MinimumMember
prah:FurnitureAndEquipmentMember
2018-01-01
2018-12-31
0001613859
srt:MaximumMember
prah:ComputerEquipmentAndSoftwareMember
2018-01-01
2018-12-31
0001613859
srt:MinimumMember
prah:ComputerEquipmentAndSoftwareMember
2018-01-01
2018-12-31
0001613859
prah:A2praCorporationJointVentureMember
2018-12-31
0001613859
prah:A2praCorporationJointVentureMember
us-gaap:ScenarioForecastMember
2019-01-01
2019-03-31
0001613859
prah:PRAWuXiJointVentureMember
2016-05-06
2016-05-06
0001613859
prah:WuxipraJointVentureHongKongMember
2016-05-06
2016-05-06
0001613859
prah:A2praCorporationJointVentureMember
2017-12-31
0001613859
prah:PRAWuXiJointVentureMember
2016-05-06
0001613859
prah:A2HealthcareCorporationMember
prah:A2praCorporationJointVentureMember
2018-12-31
0001613859
prah:A2praCorporationJointVentureMember
2018-01-01
2018-12-31
0001613859
prah:A2praCorporationJointVentureMember
2016-01-01
2016-12-31
0001613859
prah:A2praCorporationJointVentureMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2018-01-01
2018-12-31
0001613859
prah:ParallelSixMember
2017-05-10
0001613859
prah:ParallelSixMember
us-gaap:ComputerSoftwareIntangibleAssetMember
2017-05-10
2017-05-10
0001613859
prah:ParallelSixMember
us-gaap:OtherIntangibleAssetsMember
2017-05-10
2017-05-10
0001613859
prah:ParallelSixMember
us-gaap:ComputerSoftwareIntangibleAssetMember
2017-05-10
0001613859
prah:ParallelSixMember
us-gaap:OtherIntangibleAssetsMember
2017-05-10
0001613859
prah:TakedaPraDevelopmentCenterKkMember
2017-06-01
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:AcquisitionRelatedCostsMember
2017-12-31
0001613859
prah:TakedaPraDevelopmentCenterKkMember
2017-06-01
2017-06-01
0001613859
prah:NextrialsIncMember
prah:ContingentEarnOutPaymentsMilestoneOneMember
2016-03-18
0001613859
prah:TakedaPraDevelopmentCenterKkMember
2017-01-01
2017-12-31
0001613859
prah:NextrialsIncMember
prah:ContingentEarnOutPaymentsMember
2016-03-18
2016-03-18
0001613859
prah:ParallelSixMember
prah:ContingentEarnOutPaymentsSalesTargetsMember
2017-05-10
2017-05-10
0001613859
prah:NextrialsIncMember
prah:ContingentEarnOutPaymentsMember
2017-10-01
2017-12-31
0001613859
prah:ParallelSixMember
2018-01-01
2018-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2017-09-06
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:AcquisitionRelatedCostsMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:ContingentEarnOutPaymentsMember
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2017-09-06
2018-12-31
0001613859
prah:ParallelSixMember
us-gaap:FairValueInputsLevel3Member
2017-05-10
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2017-09-06
2017-09-06
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:ContingentEarnOutPaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2017-09-06
0001613859
prah:NextrialsIncMember
prah:ContingentEarnOutPaymentsMember
2017-01-01
2017-12-31
0001613859
prah:TakedaPharmaceuticalDataServicesIncMember
2017-06-01
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:ContingentEarnOutPaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001613859
prah:TakedaPharmaceuticalCompanyLtdMember
prah:TakedaPraDevelopmentCenterKkMember
2017-06-01
2017-06-01
0001613859
prah:NextrialsIncMember
prah:ContingentEarnOutPaymentsMember
us-gaap:FairValueInputsLevel3Member
2016-03-18
0001613859
prah:NextrialsIncMember
2016-03-18
0001613859
prah:ParallelSixMember
2017-05-10
2017-05-10
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:AcquisitionrelatedCostsModificationorExtinguishmentofDebtMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:FairValueInputsLevel3Member
2018-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:AcquisitionrelatedCostsTransactionMember
2017-01-01
2017-12-31
0001613859
prah:WuxipraJointVentureHongKongMember
2016-05-06
0001613859
prah:NextrialsIncMember
2016-03-18
2016-03-18
0001613859
prah:ParallelSixMember
prah:ContingentEarnOutPaymentsSalesTargetsMember
2017-05-10
0001613859
prah:ParallelSixMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2018-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:FairValueInputsLevel3Member
2018-02-28
0001613859
prah:TakedaPharmaceuticalCompanyLtdMember
prah:TDCJointVentureMember
2017-06-01
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2017-12-31
0001613859
prah:NextrialsIncMember
prah:ContingentEarnOutPaymentsMember
2016-03-18
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2017-10-01
2017-12-31
0001613859
prah:TakedaPharmaceuticalDataServicesIncMember
2017-06-01
2017-06-01
0001613859
prah:NextrialsIncMember
prah:ContingentEarnOutPaymentsMilestoneTwoMember
2016-03-18
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:CustomerRelationshipsMember
2017-09-06
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:DatabasesMember
2017-09-06
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:CustomerRelationshipsMember
2017-09-06
2017-09-06
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:DatabasesMember
2017-09-06
2017-09-06
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:TradeNamesMember
2017-09-06
2017-09-06
0001613859
prah:SymphonyHealthSolutionsCorporationMember
us-gaap:TradeNamesMember
2017-09-06
0001613859
prah:NextrialsIncMember
us-gaap:ComputerSoftwareIntangibleAssetMember
2016-03-18
0001613859
prah:NextrialsIncMember
us-gaap:ComputerSoftwareIntangibleAssetMember
2016-03-18
2016-03-18
0001613859
us-gaap:AccountingStandardsUpdate201409Member
us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Member
2018-12-31
0001613859
prah:ComputerEquipmentAndSoftwareMember
2018-12-31
0001613859
prah:FurnitureAndEquipmentMember
2018-12-31
0001613859
prah:FurnitureAndEquipmentMember
2017-12-31
0001613859
prah:ComputerEquipmentAndSoftwareMember
2017-12-31
0001613859
us-gaap:LeaseholdImprovementsMember
2018-12-31
0001613859
us-gaap:LeaseholdImprovementsMember
2017-12-31
0001613859
prah:TakedaPharmaceuticalDataServicesIncMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
prah:ParallelSixMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
prah:ClinicalResearchMember
2018-12-31
0001613859
prah:DataSolutionsMember
2017-12-31
0001613859
prah:TakedaPharmaceuticalDataServicesIncMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
prah:ParallelSixMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
prah:ClinicalResearchMember
2017-12-31
0001613859
prah:TakedaPharmaceuticalDataServicesIncMember
2017-01-01
2017-12-31
0001613859
prah:ClinicalResearchMember
2016-12-31
0001613859
prah:TDCJointVentureMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
prah:DataSolutionsMember
2016-12-31
0001613859
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
prah:TDCJointVentureMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
prah:ParallelSixMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
prah:SymphonyHealthSolutionsCorporationMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
prah:TDCJointVentureMember
2017-01-01
2017-12-31
0001613859
prah:ParallelSixMember
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
prah:DataSolutionsMember
2018-12-31
0001613859
us-gaap:DatabasesMember
2018-12-31
0001613859
prah:CustomerBacklogMember
2017-12-31
0001613859
us-gaap:CustomerRelationshipsMember
2017-12-31
0001613859
us-gaap:NoncompeteAgreementsMember
2018-12-31
0001613859
us-gaap:DatabasesMember
2017-12-31
0001613859
us-gaap:CustomerRelationshipsMember
2018-12-31
0001613859
us-gaap:TradeNamesMember
2017-12-31
0001613859
us-gaap:TradeNamesMember
2018-12-31
0001613859
us-gaap:NoncompeteAgreementsMember
2017-12-31
0001613859
prah:CustomerBacklogMember
2018-12-31
0001613859
prah:PatientListAndOtherIntangiblesMember
2017-12-31
0001613859
prah:PatientListAndOtherIntangiblesMember
2018-12-31
0001613859
prah:FirstLienTermLoanMember
2017-12-31
0001613859
prah:FirstLienTermLoanMember
2018-12-31
0001613859
us-gaap:SecuredDebtMember
2017-12-31
0001613859
us-gaap:SecuredDebtMember
2018-12-31
0001613859
srt:MaximumMember
prah:CreditFacilities2016Member
us-gaap:LondonInterbankOfferedRateLIBORMember
2018-01-01
2018-12-31
0001613859
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2018-01-01
2018-12-31
0001613859
prah:CreditFacilities2016Member
prah:FirstLienTermLoanMember
2018-01-01
2018-12-31
0001613859
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2016-12-06
0001613859
prah:CreditFacilities2016Member
2017-09-06
2017-09-06
0001613859
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2018-12-31
0001613859
prah:CreditFacilities2016Member
2017-01-01
2017-12-31
0001613859
prah:TermLoansCreditFacilityBorrowingsAndAccountsReceivableFinancingAgreementMember
2018-12-31
0001613859
us-gaap:SecuredDebtMember
2018-05-30
2018-05-30
0001613859
prah:CreditFacilities2013Member
prah:FirstLienTermLoanMember
2016-01-01
2016-12-31
0001613859
prah:CreditFacilities2016Member
prah:FirstLienTermLoanMember
2016-12-06
0001613859
prah:CreditFacilities2016Member
prah:FirstLienTermLoanMember
2017-01-01
2017-12-31
0001613859
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2017-12-28
0001613859
srt:MinimumMember
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2018-01-01
2018-12-31
0001613859
srt:MinimumMember
prah:CreditFacilities2016Member
us-gaap:BaseRateMember
2018-01-01
2018-12-31
0001613859
us-gaap:SecuredDebtMember
2018-01-01
2018-12-31
0001613859
prah:TermLoansCreditFacilityBorrowingsAndAccountsReceivableFinancingAgreementMember
2017-12-31
0001613859
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2017-12-31
0001613859
srt:MaximumMember
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2018-01-01
2018-12-31
0001613859
us-gaap:SecuredDebtMember
us-gaap:LondonInterbankOfferedRateLIBORMember
2018-01-01
2018-12-31
0001613859
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2016-12-31
0001613859
us-gaap:SecuredDebtMember
2017-01-01
2017-12-31
0001613859
us-gaap:SecuredDebtMember
2018-05-31
2018-05-31
0001613859
srt:MinimumMember
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
us-gaap:LondonInterbankOfferedRateLIBORMember
2018-01-01
2018-12-31
0001613859
srt:MaximumMember
prah:CreditFacilities2016Member
us-gaap:BaseRateMember
2018-01-01
2018-12-31
0001613859
prah:CreditFacilities2016Member
2016-12-06
0001613859
srt:MinimumMember
prah:CreditFacilities2016Member
us-gaap:LondonInterbankOfferedRateLIBORMember
2018-01-01
2018-12-31
0001613859
us-gaap:SecuredDebtMember
2016-03-31
0001613859
us-gaap:SecuredDebtMember
us-gaap:BaseRateMember
2018-01-01
2018-12-31
0001613859
prah:CreditFacilities2016Member
us-gaap:RevolvingCreditFacilityMember
2016-12-06
2016-12-06
0001613859
us-gaap:EmployeeStockOptionMember
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
2017-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeOneMember
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeThreeMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeOneMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeTwoMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeTwoMember
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeFourMember
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeFourMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:ExercisePriceRangeThreeMember
2018-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
us-gaap:SellingGeneralAndAdministrativeExpensesMember
2018-01-01
2018-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
prah:TransactionRelatedCostsMember
2018-01-01
2018-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
prah:DirectCostsMember
2018-01-01
2018-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
us-gaap:SellingGeneralAndAdministrativeExpensesMember
2016-01-01
2016-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
prah:TransactionRelatedCostsMember
2017-01-01
2017-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
prah:DirectCostsMember
2017-01-01
2017-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
prah:TransactionRelatedCostsMember
2016-01-01
2016-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
2016-01-01
2016-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
2018-01-01
2018-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
2017-01-01
2017-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
prah:DirectCostsMember
2016-01-01
2016-12-31
0001613859
prah:StockOptionsRestrictedStockAwardsAndRestrictedStockUnitsMember
us-gaap:SellingGeneralAndAdministrativeExpensesMember
2017-01-01
2017-12-31
0001613859
prah:VestingBasedOnMarketConditionsRateOneMember
us-gaap:EmployeeStockOptionMember
2013-12-01
2013-12-31
0001613859
prah:VestingBasedOnServiceMember
us-gaap:EmployeeStockOptionMember
prah:TransactionRelatedCostsMember
2018-01-01
2018-12-31
0001613859
prah:VestingBasedOnMarketConditionsRateOneMember
us-gaap:EmployeeStockOptionMember
2017-01-01
2017-12-31
0001613859
prah:VestingBasedOnMarketConditionsRateTwoMember
us-gaap:EmployeeStockOptionMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockMember
2018-12-31
0001613859
prah:VestingBasedOnServiceMember
us-gaap:EmployeeStockOptionMember
prah:TransactionRelatedCostsMember
2016-01-01
2016-12-31
0001613859
prah:VestingBasedOnMarketConditionsRateOneMember
us-gaap:EmployeeStockOptionMember
2018-01-01
2018-12-31
0001613859
prah:VestingBasedOnMarketConditionsRateOneMember
us-gaap:EmployeeStockOptionMember
2016-03-02
2016-03-02
0001613859
prah:VestingBasedOnMarketConditionsRateOneMember
us-gaap:EmployeeStockOptionMember
2016-01-01
2016-12-31
0001613859
prah:VestingBasedOnServiceMember
us-gaap:EmployeeStockOptionMember
prah:TransactionRelatedCostsMember
2017-01-01
2017-12-31
0001613859
us-gaap:EmployeeStockMember
2018-01-01
2018-12-31
0001613859
prah:VestingBasedOnMarketConditionsRateTwoMember
us-gaap:EmployeeStockOptionMember
2016-01-01
2016-12-31
0001613859
prah:RestrictedStockAwardsRsasAndRestrictedStockUnitsRsusMember
prah:EmployeeMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:OmnibusPlanForKeyEmployees2014Member
2018-01-01
2018-12-31
0001613859
prah:RestrictedStockAwardsRsasAndRestrictedStockUnitsRsusMember
us-gaap:DirectorMember
us-gaap:ShareBasedCompensationAwardTrancheOneMember
2018-01-01
2018-12-31
0001613859
prah:VestingBasedOnServiceMember
us-gaap:EmployeeStockOptionMember
2018-01-01
2018-12-31
0001613859
2013-09-23
2013-09-23
0001613859
prah:RestrictedStockAwardsRsasAndRestrictedStockUnitsRsusMember
us-gaap:DirectorMember
us-gaap:ShareBasedCompensationAwardTrancheTwoMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
prah:StockIncentivePlan2018Member
2018-01-01
2018-12-31
0001613859
us-gaap:EmployeeStockOptionMember
2013-09-23
2013-09-23
0001613859
prah:VestingBasedOnMarketConditionsRateTwoMember
us-gaap:EmployeeStockOptionMember
2016-11-16
2016-11-16
0001613859
2018-05-31
0001613859
prah:VestingBasedOnMarketConditionsRateTwoMember
us-gaap:EmployeeStockOptionMember
2017-01-01
2017-12-31
0001613859
prah:RestrictedStockAwardsRsasAndRestrictedStockUnitsRsusMember
2018-01-01
2018-12-31
0001613859
prah:RestrictedStockAwardsRsasAndRestrictedStockUnitsRsusMember
2017-12-31
0001613859
prah:RestrictedStockAwardsRsasAndRestrictedStockUnitsRsusMember
2018-12-31
0001613859
prah:VestingBasedOnMarketConditionsRateTwoMember
us-gaap:EmployeeStockOptionMember
2013-12-01
2013-12-31
0001613859
2017-10-01
2017-12-31
0001613859
2018-10-01
2018-12-31
0001613859
us-gaap:StateAndLocalJurisdictionMember
2018-12-31
0001613859
prah:EmployeeHealthInsuranceMember
2018-01-01
2018-12-31
0001613859
prah:EmployeeHealthInsuranceMember
2018-12-31
0001613859
us-gaap:EmploymentContractsMember
prah:SeniorVicePresidentMember
2018-01-01
2018-12-31
0001613859
prah:LitigationWithCityOfSaoPauloBrazilMember
2018-12-31
0001613859
prah:GeneralBusinessInsuranceMember
2018-01-01
2018-12-31
0001613859
prah:EmployeeHealthInsuranceMember
2017-12-31
0001613859
us-gaap:EmploymentContractsMember
us-gaap:VicePresidentMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmploymentContractsMember
us-gaap:ExecutiveVicePresidentMember
2018-01-01
2018-12-31
0001613859
prah:LitigationWithCityOfSaoPauloBrazilMember
2018-01-01
2018-12-31
0001613859
us-gaap:EmploymentContractsMember
us-gaap:ChiefExecutiveOfficerMember
2018-01-01
2018-12-31
0001613859
us-gaap:InterestRateContractMember
us-gaap:CashFlowHedgingMember
2017-01-01
2017-12-31
0001613859
us-gaap:InterestRateContractMember
us-gaap:CashFlowHedgingMember
2016-01-01
2016-12-31
0001613859
us-gaap:InterestRateContractMember
us-gaap:CashFlowHedgingMember
2018-01-01
2018-12-31
0001613859
us-gaap:OtherNoncurrentAssetsMember
us-gaap:FairValueInputsLevel2Member
us-gaap:DesignatedAsHedgingInstrumentMember
2017-12-31
0001613859
us-gaap:OtherAssetsMember
us-gaap:FairValueInputsLevel2Member
us-gaap:DesignatedAsHedgingInstrumentMember
2018-12-31
0001613859
us-gaap:InterestRateSwapMember
2013-10-02
0001613859
us-gaap:InterestRateSwapMember
2018-01-05
0001613859
prah:InterestRateSwapTwoMember
2015-06-30
0001613859
prah:InterestRateSwapTwoMember
2018-01-01
2018-12-31
0001613859
prah:InterestRateSwapTwoMember
2016-12-01
2016-12-31
0001613859
us-gaap:InterestRateSwapMember
us-gaap:DesignatedAsHedgingInstrumentMember
2018-01-05
0001613859
us-gaap:InterestRateSwapMember
2018-01-01
2018-12-31
0001613859
srt:MaximumMember
us-gaap:InterestRateSwapMember
2013-10-02
2013-10-02
0001613859
us-gaap:InterestRateSwapMember
2015-07-01
2015-09-30
0001613859
prah:InterestRateSwapTwoMember
us-gaap:DesignatedAsHedgingInstrumentMember
2018-01-05
0001613859
us-gaap:InterestRateSwapMember
2017-01-01
2017-12-31
0001613859
prah:InterestRateSwapTwoMember
2018-01-05
0001613859
us-gaap:InterestRateSwapMember
2016-01-01
2016-12-31
0001613859
srt:MinimumMember
us-gaap:InterestRateSwapMember
2013-10-02
2013-10-02
0001613859
us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember
2018-12-31
0001613859
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2015-12-31
0001613859
us-gaap:AccumulatedNetGainLossFromCashFlowHedgesAttributableToNoncontrollingInterestMember
2015-12-31
0001613859
us-gaap:AccumulatedNetGainLossFromCashFlowHedgesAttributableToNoncontrollingInterestMember
2017-01-01
2017-12-31
0001613859
us-gaap:AccumulatedNetGainLossFromCashFlowHedgesAttributableToNoncontrollingInterestMember
2018-01-01
2018-12-31
0001613859
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2017-12-31
0001613859
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2018-12-31
0001613859
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2017-01-01
2017-12-31
0001613859
us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember
2016-12-31
0001613859
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2016-12-31
0001613859
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2016-01-01
2016-12-31
0001613859
us-gaap:AccumulatedNetGainLossFromCashFlowHedgesAttributableToNoncontrollingInterestMember
2016-12-31
0001613859
us-gaap:AccumulatedNetGainLossFromCashFlowHedgesAttributableToNoncontrollingInterestMember
2016-01-01
2016-12-31
0001613859
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2018-01-01
2018-12-31
0001613859
us-gaap:AccumulatedNetGainLossFromCashFlowHedgesAttributableToNoncontrollingInterestMember
2018-12-31
0001613859
us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember
2015-12-31
0001613859
us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember
2017-12-31
0001613859
us-gaap:AccumulatedNetGainLossFromCashFlowHedgesAttributableToNoncontrollingInterestMember
2017-12-31
0001613859
currency:CAD
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2018-01-01
2018-12-31
0001613859
currency:GBP
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2018-01-01
2018-12-31
0001613859
currency:GBP
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2016-01-01
2016-12-31
0001613859
currency:CAD
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2016-01-01
2016-12-31
0001613859
currency:RUB
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2017-01-01
2017-12-31
0001613859
currency:EUR
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2017-01-01
2017-12-31
0001613859
currency:EUR
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2016-01-01
2016-12-31
0001613859
currency:RUB
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2018-01-01
2018-12-31
0001613859
currency:CAD
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2017-01-01
2017-12-31
0001613859
currency:EUR
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2018-01-01
2018-12-31
0001613859
currency:GBP
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2017-01-01
2017-12-31
0001613859
currency:RUB
us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember
2016-01-01
2016-12-31
0001613859
country:US
2018-12-31
0001613859
country:GB
2018-12-31
0001613859
country:NL
2017-12-31
0001613859
country:NL
2018-12-31
0001613859
prah:OtherAmericasMember
2017-12-31
0001613859
prah:OtherEuropeAfricaAsiaPacificMember
2017-12-31
0001613859
srt:AmericasMember
2017-12-31
0001613859
srt:AmericasMember
2018-12-31
0001613859
prah:OtherAmericasMember
2018-12-31
0001613859
country:GB
2017-12-31
0001613859
country:US
2017-12-31
0001613859
prah:EuropeAfricaAsiaPacificMember
2017-12-31
0001613859
prah:OtherEuropeAfricaAsiaPacificMember
2018-12-31
0001613859
prah:EuropeAfricaAsiaPacificMember
2018-12-31
0001613859
us-gaap:ServiceMember
prah:OtherEuropeAfricaAsiaPacificMember
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
country:GB
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
prah:EuropeAfricaAsiaPacificMember
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
srt:AmericasMember
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
country:US
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
prah:OtherAmericasMember
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
country:NL
2018-01-01
2018-12-31
0001613859
us-gaap:ServiceMember
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
country:NL
2016-01-01
2016-12-31
0001613859
us-gaap:ServiceMember
srt:AmericasMember
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
prah:OtherEuropeAfricaAsiaPacificMember
2016-01-01
2016-12-31
0001613859
us-gaap:ServiceMember
country:US
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
country:US
2016-01-01
2016-12-31
0001613859
prah:ReimbursementMember
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
srt:AmericasMember
2016-01-01
2016-12-31
0001613859
us-gaap:ServiceMember
2016-01-01
2016-12-31
0001613859
us-gaap:ServiceMember
prah:OtherEuropeAfricaAsiaPacificMember
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
country:GB
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
prah:EuropeAfricaAsiaPacificMember
2016-01-01
2016-12-31
0001613859
us-gaap:ServiceMember
prah:OtherAmericasMember
2016-01-01
2016-12-31
0001613859
us-gaap:ServiceMember
prah:EuropeAfricaAsiaPacificMember
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
country:GB
2016-01-01
2016-12-31
0001613859
prah:ReimbursementMember
2016-01-01
2016-12-31
0001613859
us-gaap:ServiceMember
country:NL
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
prah:OtherAmericasMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
us-gaap:MaterialReconcilingItemsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
us-gaap:MaterialReconcilingItemsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
prah:DataSolutionsMember
2016-01-01
2016-12-31
0001613859
us-gaap:MaterialReconcilingItemsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
prah:ClinicalResearchMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ClinicalResearchMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
prah:DataSolutionsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
prah:ClinicalResearchMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
prah:DataSolutionsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
prah:ClinicalResearchMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
prah:DataSolutionsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DataSolutionsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableOutOfPocketCostsMember
prah:ClinicalResearchMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
prah:DataSolutionsMember
2017-01-01
2017-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
prah:ClinicalResearchMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
prah:DataSolutionsMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:DirectCostsMember
2016-01-01
2016-12-31
0001613859
us-gaap:OperatingSegmentsMember
prah:ReimbursableInvestigatorFeesMember
2018-01-01
2018-12-31
0001613859
us-gaap:OperatingSegmentsMember
us-gaap:ServiceMember
prah:ClinicalResearchMember
2017-01-01
2017-12-31
0001613859
us-gaap:ServiceMember
2017-04-01
2017-06-30
0001613859
2017-01-01
2017-03-31
0001613859
2017-07-01
2017-09-30
0001613859
prah:ReimbursementMember
2017-04-01
2017-06-30
0001613859
us-gaap:ServiceMember
2017-07-01
2017-09-30
0001613859
us-gaap:ServiceMember
2017-10-01
2017-12-31
0001613859
2017-04-01
2017-06-30
0001613859
prah:ReimbursementMember
2017-01-01
2017-03-31
0001613859
us-gaap:ServiceMember
2017-01-01
2017-03-31
0001613859
prah:ReimbursementMember
2017-10-01
2017-12-31
0001613859
prah:ReimbursementMember
2017-07-01
2017-09-30
0001613859
2018-01-01
2018-03-31
0001613859
2018-04-01
2018-06-30
0001613859
2018-07-01
2018-09-30
0001613859
prah:SymphonyHealthSolutionsCorporationMember
2017-07-01
2017-09-30
0001613859
prah:VestingBasedOnServiceMember
us-gaap:EmployeeStockOptionMember
prah:TransactionRelatedCostsMember
2017-07-01
2017-09-30
0001613859
prah:TransactionRelatedCostsMember
prah:SecondaryPublicOfferingMember
2017-07-01
2017-09-30
iso4217:USD
xbrli:shares
xbrli:pure
prah:segment
xbrli:shares
iso4217:USD
prah:derivative
prah:employee
prah:reportable_segments
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10‑K
|
| |
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2018
or
|
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission file number: 001‑36732
PRA Health Sciences, Inc.
(Exact name of registrant as specified in its charter)
|
| |
Delaware | 46‑3640387 |
(State or other jurisdiction of | (I.R.S. Employer |
incorporation or organization) | Identification No.) |
4130 ParkLake Avenue, Suite 400, Raleigh, NC 27612
(Address of principal executive offices) (Zip Code)
(919) 786‑8200
Registrant’s telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act:
|
| | |
Title of each class | | Name of each exchange on which registered |
Common Stock, par value $0.01 per share | | Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well‑known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S‑T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S‑K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10‑K or any amendment to this Form 10‑K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b‑2 of the Exchange Act.
|
| | | | |
Large accelerated filer ☒ | Accelerated filer ☐ | Non-accelerated filer ☐ | Smaller reporting company ☐ | Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Act). Yes ☐ No ☒
The aggregate market value of the voting and non‑voting common equity held by non‑affiliates of the registrant, based upon the closing sale price as reported on the Nasdaq Global Select Market on June 30, 2018, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $4.7 billion. For purposes of this computation, shares of the registrant’s common stock held by affiliates, including executive officers, directors and certain holders known to the registrant, have been excluded.
Indicate the number of shares outstanding of each of the issuer’s classes of Common Stock, as of the latest practicable date.
|
| | |
Class | | Number of Shares Outstanding |
Common Stock $0.01 par value | | 65,531,659 shares outstanding as of February 22, 2019 |
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement to be filed with the Securities and Exchange Commission relating to the 2019 Annual Meeting of Stockholders are incorporated herein by reference into Part III of this Annual Report on Form 10‑K to the extent stated herein. Such Proxy Statement will be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
PRA HEALTH SCIENCES, INC.
ANNUAL REPORT ON FORM 10‑K
FOR FISCAL YEAR ENDED DECEMBER 31, 2018
TABLE OF CONTENTS
FORWARD‑LOOKING STATEMENTS
This Annual Report on Form 10‑K, or this report, contains forward‑looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Such forward‑looking statements reflect, among other things, our current expectations and anticipated results of operations, all of which are subject to known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements, market trends, or industry results to differ materially from those expressed or implied by such forward‑looking statements. Therefore, any statements contained herein that are not statements of historical fact may be forward‑looking statements and should be evaluated as such. Without limiting the foregoing, the words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “should,” “targets,” “will” and the negative thereof and similar words and expressions are intended to identify forward‑looking statements. These forward‑looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors” in Part I, Item 1A of this report, and speak only as of the date hereof. Unless legally required, we assume no obligation to update any such forward‑looking information to reflect actual results or changes in the factors affecting such forward‑looking information.
Market and Industry Data and Forecasts
This report includes data, forecasts and information obtained from industry publications and surveys and other information available to us. Forecasts and other metrics included in this report to describe our industry are inherently uncertain and speculative in nature, and actual results for any period may materially differ. Estimates and forecasts involve uncertainties and risks and are subject to change based on various factors, including those discussed above under “Forward-Looking Statements.” While we are not aware of any misstatements regarding the third-party industry data presented in this report, we have not independently verified any of the data from third-party sources, nor have we ascertained the underlying assumptions relied upon therein.
The ISR 2018 Market Report, as defined below, represents research opinion or viewpoints published by a market research firm Industry Standard Research. Such opinions or viewpoints should not be construed as statements of fact. The ISR 2018 Market Report speaks as of its original publication date (and not as of the date of this report) and the opinions expressed in the ISR 2018 Market Report are subject to change without notice. ISR does not endorse any vendor, product or service depicted in its research publications.
Website and Social Media Disclosure
We use our website (www.prahs.com) as a channel of distribution of company information. The information we post through this channel may be deemed material. Accordingly, investors should monitor this channel, in addition to following our press releases, Securities and Exchange Commission, or SEC, filings and public conference calls and webcasts. The contents of our website are not, however, a part of this report.
Part I
Item 1. Business
Overview
We are one of the world’s leading global contract research organizations, or CROs, by revenue, providing outsourced clinical development and data solution services to the biotechnology and pharmaceutical industries. We believe we are one of a select group of CROs with the expertise and capability to conduct clinical trials across major therapeutic areas on a global basis. Our therapeutic expertise includes areas that are among the largest in pharmaceutical development, and we focus in particular on oncology, immunology, central nervous system, inflammation, respiratory, cardiometabolic and infectious diseases. We believe that we further differentiate ourselves from our competitors through our investments in medical informatics and clinical technologies designed to enhance efficiencies, improve study predictability and provide better transparency for our clients throughout their clinical development processes. Our Data Solutions segment allows us to better serve our clients across their entire product lifecycle by (i) improving clinical trial design, recruitment, and execution; (ii) creating real-world data solutions based on the use of medicines by actual patients in normal situations; and (iii) increasing the efficiency of biotechnology and pharmaceutical companies' commercial organizations through enhanced analytics and outsourcing services.
Our global clinical development platform includes more than 70 offices across North America, Europe, Asia, Latin America, South Africa, Australia and the Middle East and more than 16,400 employees worldwide. Since 2000, we have participated in more than 3,800 clinical trials worldwide, worked on marketed drugs across several therapeutic areas and conducted the pivotal or supportive trials that led to U.S. Food and Drug Administration, or FDA, or international regulatory approval of more than 85 drugs.
We offer flexible clinical development service offerings, which include embedded and functional outsourcing services in addition to traditional, project‑based clinical trial services. Our Strategic Solutions offerings provide Embedded Solutions™ and functional outsourcing services in which our teams are fully integrated within the client’s internal clinical development operations and are responsible for managing functions across the entire breadth of the client’s drug development pipeline. We believe that our Strategic Solutions offerings represent an innovative alternative to the traditional, project‑based approach and allow our clients to maintain greater control over their clinical development processes. Our flexible clinical development service offerings expand our addressable market beyond the traditional outsourced clinical development market to include the clinical development spending that biopharmaceutical companies historically have retained in‑house.
Over the past 30 years, we have developed strong client relationships and have performed services for more than 300 biotechnology and pharmaceutical clients. Our Strategic Solutions offerings have significantly expanded our relationships with large pharmaceutical companies in recent years, which has allowed us to pursue strategic alliances with these companies due to our global presence, broad therapeutic expertise and flexible clinical development service offerings. Additionally, we believe that we have built a reputation as a strategic partner of choice for biotechnology and small‑ to mid‑sized pharmaceutical companies as a result of our competitively-differentiated platform and our long‑term track record of serving these companies.
CRO Industry
CROs provide drug development services, regulatory and scientific support, and infrastructure and staffing support to provide their clients with the flexibility to supplement their in‑house capabilities or to provide a fully outsourced solution. The CRO industry has grown from providing limited clinical trial services in the 1970s to a full-service industry characterized by broad relationships with clients and by service offerings that encompass the entire drug development process. Today, CROs provide a comprehensive range of clinical services, including protocol design and management and monitoring of Phase I through Phase IV clinical trials, data management, laboratory testing, medical and safety reviews and statistical analysis. In addition, CROs provide services that generate high quality and timely data in support of applications for regulatory approval of new drugs or reformulations of existing drugs as well as new and existing marketing claims. CROs leverage selected information technologies and procedures to efficiently capture, manage and analyze the large streams of data generated during a clinical trial.
Drug development processes
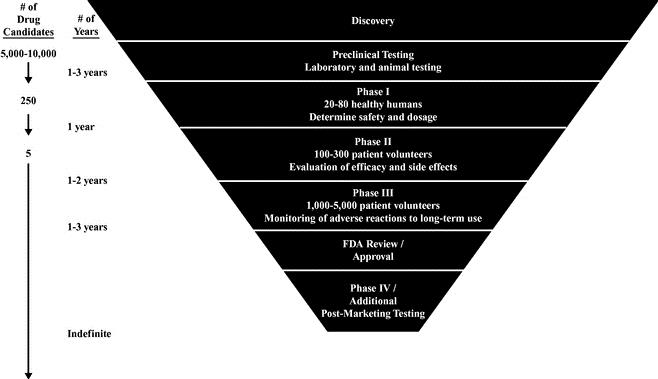
Discovering and developing new drugs is an expensive and time‑consuming process and is highly regulated and monitored through approval processes that vary by region. Before a new prescription drug reaches commercialization, it must undergo extensive pre‑clinical and clinical testing and regulatory review, to verify that the drug is safe and effective.
A drug is first tested in pre‑clinical studies, which can take several years to complete. When a new molecule is synthesized or discovered, it is tested for therapeutic value using various animal and tissue models. If the drug warrants further development, additional studies are completed and an investigational new drug application, or IND, is submitted to the FDA. Once the IND becomes effective, the drug may proceed to the human clinical trial phase which generally consists of the following interrelated phases, which may overlap:
Stages of Clinical Development
Market trends
Industry Standard Research, or ISR, a market research firm, estimated in its “2018 CRO Market Size Projections 2017-2022” report, or ISR 2018 Market Report, that the size of the worldwide CRO market was approximately $34 billion in 2017 and will grow at a 7.5% CAGR to $49 billion in 2022. This growth will be driven by an increase in the amount of research and development expenditure and levels of clinical development outsourcing by biopharmaceutical companies.
Increased R&D spending
ISR estimates in its 2018 Market Report that research and development, or R&D, expenditures by biopharmaceutical companies were approximately $293 billion in 2017 and will grow approximately 3% per year through 2022. Of this amount, approximately $121 billion was spent on development, including $86 billion on Phase I through IV clinical development. Growth drivers of R&D spending among biopharmaceutical companies include the need to replenish lost revenues resulting from the patent expirations of a large number of high‑profile drugs in recent years which has resulted in the need for biopharmaceutical companies to increase their R&D expenditures to eventually fill this revenue void with new drug approvals, and a healthy capital-raising environment among biotechnology companies in recent years. We believe biotechnology companies primarily use the capital to fund clinical trials, and due to the general lack of existing infrastructure, these trials are often contracted to CROs.
Higher outsourcing penetration
ISR estimates in its 2018 Market Report that approximately 40% of Phase I through IV of clinical development spend is outsourced to CROs, and the levels of penetration are expected to increase to approximately 47% by 2022. We believe this increase in outsourcing is due to several factors, including the need to maximize R&D productively, the increasing burden of clinical trial complexity, and the desire to pursue simultaneous registration in multiple countries.
| |
• | Maximizing Productivity and Reducing Cost—Productivity within the biopharmaceutical industry has declined over the past several years and the cost of developing a new drug has significantly increased. The combined impact of declining R&D productivity and increased development costs has translated into significant pressure on margins and short‑term earnings for biopharmaceutical companies. We believe that the need for these companies to maximize |
productivity and lower costs will lead them to partner increasingly with CROs that can improve efficiency, and increase flexibility and speed across their clinical operations.
| |
• | Increasing Clinical Trial Complexity—Over the last decade, the burden of clinical trial complexity has been increasingly difficult to manage due to requirements from regulatory authorities worldwide for greater amounts of clinical trial and safety data to support the approval of new drugs, and requirements for adherence to increasingly complex and diverse regulations and guidelines. In an effort to minimize potential risks, these regulatory agencies also typically require a greater amount of post‑approval information and monitoring of drugs on the market. To balance the conflicting demands of a growing market with the need to control R&D expenses, biopharmaceutical companies partner with CROs that can provide services designed to generate high-quality and timely data in support of regulatory approvals of new drugs or the reformulations of existing drugs, as well as support of post‑approval regulatory requirements. |
| |
• | Simultaneous Multi‑Country Registration—Given their desire to maximize efficiency and global market penetration to achieve higher potential returns on their R&D expenditures, biopharmaceutical companies are increasingly pursuing simultaneous, rather than sequential, regulatory new drug submissions and approvals in multiple countries. However, most biotechnology and small‑ to mid‑sized pharmaceutical companies do not possess the capability or capacity to simultaneously conduct large‑scale clinical trials in more than one country. In addition, establishing and maintaining internal global infrastructure to pursue multiple regulatory approvals in different therapeutic categories and jurisdictions can be costly. |
Our History and Corporate Information
PRA Health Sciences, Inc. was incorporated in Delaware in June 2013. Our wholly‑owned subsidiary, PRA Holdings, Inc., or PRA Holdings, was incorporated in Delaware in July 2007 and its predecessors date back to 1982. Our qualified and experienced clinical and scientific staff has been delivering clinical drug development services to our clients for more than 30 years and our service offerings now encompass the spectrum of the clinical drug development process. See Note 4 to our audited consolidated financial statements found elsewhere in this Annual Report on Form 10-K for additional information with respect to our recent acquisitions.
Our Competitive Strengths
Global CRO platform
We are one of the largest CROs in the world by revenue focused on executing clinical trials on a global basis. Our global clinical development platform includes more than 70 offices across North America, Europe, Asia, Latin America, South Africa, Australia and the Middle East and over 16,400 employees worldwide. We are dedicated to the seamless execution of integrated clinical trials on multiple continents concurrently. We believe our global presence and scale are important differentiators as biopharmaceutical companies are increasingly focused on greater patient access for increasingly complex clinical trials and gaining regulatory approval for new products in multiple jurisdictions simultaneously.
Broad and flexible service offering
We believe that we are one of a select group of CROs capable of providing both traditional, project‑based CRO services as well as embedded and functional outsourcing services. Our broad and flexible service offering allows us to meet the clinical research needs of a wide range of clients, from small biotechnology companies to large pharmaceutical companies. Through more than 30 years of experience, we have developed significant expertise executing complex drug development projects that span Phase I through Phase IV clinical trials. Our Product Registration offerings consist primarily of traditional, project‑based CRO services, where we have gained the reputation as a strategic partner of choice to biotechnology and pharmaceutical companies. Our Strategic Solutions offerings primarily cater to the needs of large pharmaceutical companies that seek to maintain greater control over their clinical trial processes.
Therapeutic expertise in large segments of drug development
Our therapeutic expertise encompasses areas that are among the largest in pharmaceutical development, including oncology, immunology, central nervous system, inflammation and infectious diseases. We have participated in more than 2,300 clinical trials in these key areas since 2005, accounting for a substantial majority of our total clinical trials during this period. We employ drug development experts with extensive experience across numerous therapeutic areas in preparing development plans, establishing study and protocol designs, identifying investigative sites and patients and submitting regulatory filings. Our
staff is highly experienced and includes approximately 750 Ph.Ds, 600 medical doctors and 275 doctors of pharmacy worldwide.
Innovative approach to clinical trials using medical informatics
We are committed to being an industry leader in developing global, scalable and sustainable solutions for our clients. We aim to continuously improve our systems and processes by investing in medical informatics, technology, analytics and IT infrastructure. Our information delivery system enables rapid, web‑based delivery of clinical trial data to clients and project teams. We believe our proprietary analysis and application of this data are key differentiators and allow us to identify more productive investigative sites and speed up overall patient enrollment, thereby decreasing drug development timelines. We have invested in and acquired large databases of aggregated patient medical data, which we refer to as medical informatics, to better understand patient distribution and location. Specifically, we have acquired data sources that give us significant amounts of information about patient populations within the United States to enhance enrollment, including medical claims data, hospital master charge data, pharmacy data, laboratory data and payor data. Capitalizing on our investments in medical informatics, we have the capability to identify potential patient populations by location, diagnostic code, treating physician, medications, date diagnosed, last treatment and other relevant metrics. Our medical informatics suite includes physician, hospital and pharmacy databases that cover more than 280 million patient lives and approximately 10 billion patient and pharmacy claims in the United States.
Leading enabler of integrated health data and analytics
The acquisition of Symphony Health supports our commitment to enhancing the future of clinical development with best-in-class technology solutions which enable deep, data-driven insights to optimize global clinical studies and drug commercialization.
Diversified and attractive client base
Over the past 30 years, we have developed strong client relationships and have performed services for more than 300 biotechnology and pharmaceutical clients. We have significantly expanded our relationships with large pharmaceutical companies in recent years, which has allowed us to pursue strategic alliances with these companies due to our global presence, broad therapeutic expertise and flexible clinical development service offerings. Additionally, we believe that we have built a reputation as a strategic partner of choice for biotechnology and small‑ to mid‑sized pharmaceutical companies as a result of our competitively differentiated platform and our long‑term track record of serving these companies. Our client relationships are also broad and diversified, and in the year ended December 31, 2018 our top 10 clients represented 56% of revenue, with our largest client representing approximately 9% of revenue and our largest single study accounting for approximately 3% of our revenue.
Innovative management team
We are led by a dedicated and experienced executive management team with an average of more than 20 years of experience across the global clinical research, pharmaceutical and life sciences industries. This team has been responsible for building our global platform, successfully integrating our acquisitions, developing our advanced IT‑enabled infrastructure and realizing our significant growth in revenue and earnings over the past five years.
Our Growth Strategy
Leverage our strong market position within the biotechnology and small‑ to mid‑sized pharmaceutical market
We believe our long‑term track record serving biotechnology and small‑ to mid‑sized pharmaceutical companies has resulted in our earning a reputation as a strategic partner of choice for these companies. We believe that biotechnology and small‑ to mid‑sized pharmaceutical companies rely on full service CROs to deliver fast, effective and thorough support throughout the clinical development and regulatory processes, as these companies generally lack a global clinical development infrastructure. We intend to leverage our strong relationships with biotechnology and small‑ to mid‑sized pharmaceutical companies to capture additional business from these companies. In particular, we believe the CRO strategic alliances that have become prevalent with large pharmaceutical companies over the past several years will increasingly be utilized by biotechnology and small‑ to mid‑sized pharmaceutical companies. We believe we are well-positioned to take advantage of these opportunities given the depth of our relationships and our proven track record serving these customers.
Build deeper and broader relationships with large pharmaceutical companies
Large pharmaceutical companies have increasingly focused on partnering with multi‑national CROs that offer a wide array of global therapeutic and service capabilities. We have invested significantly in our global scale and infrastructure over the past several years to enhance our status as a service provider for these companies. Our acquisition of RPS significantly increased the depth of our relationships with large pharmaceutical companies. We intend to continue to expand our relationships beyond the Embedded Solutions provided through our Strategic Solutions offering to include traditional, project‑based clinical trial services.
Expand our leading therapeutic expertise in existing and new areas
We believe that our therapeutic expertise in all clinical phases of drug development is critical to the proper design and management of clinical trials and we intend to continue to capitalize on our strong market positions in several large therapeutic categories. We have established, and will continue to refine, our scientific and therapeutic business development initiatives, which link our organization to key clinical opinion leaders and medical informatics data to more effectively leverage therapeutic expertise throughout our client engagement. Specifically, we believe that oncology, central nervous system, inflammation and infectious diseases, which together represent the majority of all drug candidates currently in clinical development by biotechnology and pharmaceutical companies, will be significant drivers of our growth. In the area of oncology, we believe that the growth of targeted therapies, companion diagnostics and personalized medicine will continue to drive drug development. With the aging demographics, we believe we will see significant growth in the area of dementia and Alzheimer’s research and drug development, which is complemented by our specialty and focus in neurology. Additionally, we believe that development of niche therapeutic drugs (orphan drugs) will continue to see considerable growth moving forward and we have a dedicated staff focused on the design and conduct of trials for these drugs.
Continue to enhance our tech-enabled CRO engagement model
The acquisition of Symphony Health has provided us with rich data insights that will allow us to customize our clinical studies to be as unique as the patients who they are designed around. By creatively harnessing the power of our technology and data assets, we are redefining the clinical development process for a more patient-centric future.
Continue to realize strategic benefits from recent acquisitions
We believe we will continue to realize strategic benefits from the acquisitions we have completed over the past five years, resulting in additional revenue growth and margin improvements. We believe that our strategic acquisitions are complementary to our customer base and expect to generate incremental revenue growth by cross‑selling our full set of services to our existing and new customers, thereby expanding the scope of our customer relationships and generating additional revenue.
Pursue selective and complementary acquisition strategy
We are a selectively acquisitive company focused on growing our core service offerings, therapeutic capabilities and geographic reach into areas of high market growth. We have acquired 21 companies since 1997 and have established programs to help us identify acquisition targets and integrate them successfully. Our acquisition strategy is driven by our comprehensive commitment to serve client needs and we are continuously assessing the market for potential opportunities.
Service Offerings
We have two reportable segments: Clinical Research and Data Solutions. Our Clinical Research segment encompasses a broad array of services across the spectrum of clinical development programs. Our Data Solutions segment provides data, analytics, technology, and consulting solutions to the life sciences market. The offerings of our two segments complement each other and can provide enhanced value to our clients when delivered together, with each driving demand for the other.
For financial information regarding our segments, see Part II, Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Segment Results of Operations" and Note 20 to our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Clinical Research
We perform a broad array of services across the spectrum of clinical development programs, from the filing of INDs and similar regulatory applications to conducting all phases of clinical trials. Our core service offerings include:
| |
• | Product Registration, which includes Phase IIb through III product registration trials and Phase IV trials, inclusive of post‑marketing commitments and registries; |
| |
• | Strategic Solutions, which provides Embedded Solutions and functional outsourcing services, in which our teams are fully integrated within the client’s internal clinical development operations and responsible for managing functions across the entire breadth of the client’s drug development pipeline; and |
| |
• | Early Development Services, which includes Phase I through Phase IIa clinical trials and bioanalytical laboratory services. |
We provide many back office services to clients as well, including processing the payments to investigators and volunteers. We also collaborate with third‑party vendors for services such as imaging, central lab and patient recruitment services.
Product Registration
Our Product Registration offerings encompass the design, management and implementation of study protocols for Phase II through Phase III clinical trials, which are the critical building blocks of product development programs, as well as Phase IV, or post‑approval, clinical trials. We have extensive resources and expertise to design and conduct studies on a global basis, develop integrated global product databases, collect and analyze trial data and prepare and submit regulatory submissions in the United States, Europe and other jurisdictions.
A typical full‑scale program or project may involve the following components:
| |
• | clinical program development, review and consultation and lifecycle management planning; |
| |
• | design of the clinical protocol and electronic case report forms, or CRFs; |
| |
• | feasibility studies for investigator interest and patient access and availability; |
| |
• | patient recruitment and retention services; |
| |
• | investigator and site analysis for selection and qualification; |
| |
• | investigator handbook and meetings; |
| |
• | investigational site support and clinical monitoring; |
| |
• | patient medical and safety management; |
| |
• | medical and scientific publications; and |
| |
• | preparation of regulatory filings. |
As described below, we offer a suite of product registration service offerings to our clients to address the several components involved in conducting a full‑scale program or project.
Clinical Trial Management—Our clinical trial management services, used by biotechnology and pharmaceutical clients, may be performed exclusively by us or in collaboration with the client’s internal staff or other CROs. With our broad clinical trial management capabilities, we conduct single site studies, multi‑site U.S. and international studies and global studies
on multiple continents. Through our electronic trial master file, we can create, collect, store, edit and retrieve any electronic document in any of our office locations worldwide, enabling our global project teams to work together efficiently regardless of where they are physically located and allowing seamless transfer of work to a more efficient locale.
Project Management—Our project management group manages the development process, setting specific targets and utilizing various metrics to ensure that a project moves forward in the right trajectory, resources are used optimally and client satisfaction is met. This group also oversees the implementation of a work breakdown structure, communication plan, and a risk and contingency program for each study. We believe that the management structure of our service delivery model sets us apart in the industry. Each individual project is assigned a director of project delivery and key strategic accounts are also assigned a general partner. As a member of the senior management team, the general partner works with the director of project delivery, the project management group and client representatives to ensure the highest level of client satisfaction. With approximately 370 project directors and project managers, we match our project management personnel to projects based on experience and study specific parameters.
Regulatory Affairs—Our team of global regulatory professionals has extensive experience working with biotechnology and pharmaceutical companies and regulatory authorities worldwide. Our regulatory affairs group is comprised of an internal network of local regulatory experts who are native speakers in countries across North America, Latin America, Western and Eastern Europe, Africa and Asia Pacific. Regulatory team members and local regulatory experts act as clients’ representatives for submissions and direct communications with regulatory authorities in all regions. The group’s regulatory expertise enables rapid study start‑up and facilitates competitive product development plans and effective submission strategies.
Therapeutic Expertise—Our therapeutic expertise group provides scientific and medical expertise and patient access and retention services worldwide across a broad range of therapeutic areas. Our broad experience throughout various therapeutic areas allows us to offer a more complete global service offering to our clients. Our diverse therapeutic expertise group leverages best‑in‑class data assets to assist our clients with the design and implementation of entire clinical development programs and our current and potential clients increasingly seek partners who can provide these capabilities. We provide clients with therapeutic expertise in the design and implementation of high‑quality product development programs and help them achieve key development milestones in a cost and time effective manner. Our therapeutic expertise is used by both emerging biotechnology companies that lack clinical development infrastructure and pharmaceutical companies that have limited internal medical resources or are exploring new therapeutic areas.
Clinical Operations—Our clinical operations group provides clients with a full set of study site management and monitoring services in approximately 90 countries worldwide, through our highly experienced team of clinical research associates and specialists. This experience includes knowledge of local regulations, medical practices, safety and individual therapeutic areas. We provide our clients with fully trained and locally based clinical teams led by experienced clinical team managers that initiate site start‑up, monitor activities and review data. Based in the Americas, Europe, Asia Pacific and Africa, these teams work from a strategic foundation that combines reliance on proven, consistent processes with the flexibility to adapt innovative ideas and technologies. Given our expertise executing clinical trials around the world, we are positioned to meet our clients’ diverse needs and expectations. Our study start‑up services group, a unit within clinical operations, manages the key components of rapid site activation and investigational site set‑up for clinical trials by utilizing our global and region specific expertise.
Data and Programming Services—Our global data and programming services group offers an innovative suite of technologies that gather and organize clinical trial data. We employ industry leading electronic data capture technologies and innovative delivery systems to produce high quality and standardized data and reports. We focus on evaluating a client’s needs, presenting optimal solutions for each trial and implementing the chosen solution effectively during project execution. To support these goals, we have built a group of technological experts in drug research that has a strong foundation in data management fundamentals and core programming abilities.
Safety and Risk Management—Our dedicated safety and risk management group helps clients design, implement and operationalize the proper safety procedures from development through to post‑marketing, allowing for clear assessment and the communication of patient safety profiles. We have centralized drug safety centers in Mannheim, Germany; Swansea, United Kingdom; Charlottesville, Virginia, United States (with a satellite center in Lenexa, Kansas); Sao Paulo, Brazil; and Singapore. Centers are staffed with experienced drug safety associates. These associates are responsible for integrating an effective risk minimization strategy for a drug product and generating useable information through ongoing risk evaluation. Our safety and risk management team provides risk mitigation strategies for our clients at all stages of the drug development cycle along with core signal detection capabilities.
Biostatistics and Medical Writing—Our global biostatistics and medical writing operations integrate our biostatistics, medical writing, pharmacokinetics and regulatory publishing groups. With a staff of industry experienced and therapeutically trained biostatisticians and medical writers, we offer clients expertise in statistical analysis, data pooling and regulatory reporting. This global team provides specialist consulting expertise and support to clients from the first stage of protocol design through post‑marketing surveillance and Phase IV studies. For publishing, we use a specialized electronic system that enables us to seamlessly assemble, manage and publish complex documents in compliance with applicable regulatory guidelines.
Quality Assurance Services—Our global quality assurance group is staffed by a team of experienced professionals in the Americas, Europe and Asia Pacific. Our quality assurance department is entirely separate from and independent of the personnel engaged in the direction and conduct of clinical trials. The objective of the quality assurance group is the global promotion of ongoing quality awareness and continuous improvement of our processes. This group serves these efforts by performing audits on the processes and systems used in the management of clinical trials to ensure compliance with study protocol and applicable regulatory requirements. This group has performed audits for a wide range of medical indications and in all phases of clinical trials across the globe.
Late-Phase Services—Our global late-phase services group supports global and regional post‑approval trials with management locations centralized in Pennsylvania, Germany and Singapore. Our experienced late‑phase services team assists clients with the post‑marketing process by helping identify trends and signals in large populations as well as planning and conducting safety surveillance studies, large‑sample trials, registries, restricted access programs, risk management programs, diagnostic trials and biomarker research. The team consists of industry leading strategic experts, operational specialists and epidemiologists who work with clients to identify post‑marketing research objectives and goals and translate them into comprehensive study designs.
Strategic Solutions
Our Strategic Solutions offerings allow biotechnology and pharmaceutical companies to execute their internally‑managed development portfolio with greater flexibility and to leverage their existing infrastructure to minimize redundancy. These offerings provide a broad spectrum of solutions that allow for the efficient management and execution of critical clinical development functions for pharmaceutical clients. These services are embedded or integrated within the client’s internal clinical development operations to support the entire breadth of the client’s drug development pipeline. By embedding our employees within our clients’ infrastructure, we create a strategic and interdependent relationship that allows us to anticipate our clients’ clinical trial demands and efficiently deploy our skilled clinical professionals to meet our clients’ needs. Clinical functions supported by this service offering include study start‑up activities, site monitoring, study management, data management, biostatistics, regulatory and product safety. We focus our solutions primarily on our clients’ Phase II through Phase IV development programs. While traditional, project‑based CRO offerings target the outsourced component of biopharmaceutical industry spending, our Strategic Solutions offerings address the total Phase II through IV development market. We pioneered the embedded services model described below, and we have extensive experience helping customers re‑align their operating model to more efficiently manage their development portfolio with greater flexibility and control.
Our Strategic Solutions offerings include:
Embedded Solutions—We believe we are the only company in the industry to offer a strategically scalable, fully‑embedded clinical development solution. Our Embedded Solutions model is designed to merge clinical operations expertise, management, infrastructure and support to create a flexible and integrated operating model. The goal of our Embedded Solutions model is to enable our clients’ internally‑managed development processes to be executed with greater flexibility. These solutions can be further enhanced by leveraging our systems and technology as required. In our Embedded Solutions model, we typically work with our partners to assist in redesigning existing systems and processes to drive greater efficiency, speed and quality and to implement innovative approaches and enhanced technology. We employ a strong joint governance structure and robust metrics to measure and ensure strong quality, cycle time, productivity and service‑level performance.
Functional Services Provider Solutions—Our functional services provider offering provides dedicated capacity management within a single operating platform and within one function or across multiple functions and geographies. While the customer provides direction and functional management, we provide resources and line management, training and support. We also utilize business level metrics to help ensure that staff are deployed with the relevant experience and are producing consistent, repeatable results.
Staff Augmentation Solutions—Our staff augmentation solutions offering provides clients with the ability to address their dynamic staffing needs by supplying access to resources qualified to meet their clinical development needs. This allows
clients to maintain flexibility while also reducing fixed costs. In order to rapidly attract and recruit qualified employees for these situations, we have assembled what we believe is the largest team in the industry focused on personnel recruitment. These individual professionals are hired as our employees and are managed by our teams, minimizing co‑employment related issues. The customer has the ability to define the resources required according to the therapeutic‑ and disease‑specific experience required. These resources can be on site at the customer’s facility, at our offices, or regionally based.
Custom‑Built Development Solutions—Our custom‑built development solutions are designed to offer people, process, systems and development expertise that enable the efficient internal development of a company’s product portfolio with greater control and flexibility, accelerated development timelines and substantially reduced costs. With the client’s core leadership in control, we help to build the development team our clients need, while enabling them to maintain the flexibility to be nimble during the development lifecycle.
Commercialization Services—Through our commercialization services offering, we assist our clients in addressing the challenge of commercializing products. We do this by deploying professionals who are knowledgeable in launch preparation and product lifecycle management. We assist customers in managing the product lifecycle by working with them to create concise messaging, engage thought leadership and health care providers, generate consumer enthusiasm for the product, and prepare for post‑marketing commitments. Our commercialization services offering utilizes our flexible service model and, as such, can be delivered as an Embedded Solution, through our functional service provider model, or through staff augmentation.
Early Development Services
Our Early Development Services, or EDS, offerings include a full range of services for Phase I and Phase IIa studies as well as bioanalytical analysis. We have conducted studies for major pharmaceutical companies in Europe, the United States and Japan, as well as for many smaller and emerging biotechnology companies. We have also built direct relationships with a large base of available subjects, including healthy volunteers and patient populations with specific medical conditions.
Acquisitions in recent years have allowed us to significantly expand our Phase I to Phase II services. This includes offerings focused on the conduct and design of early stage patient population studies, and therapeutically focused in human abuse liability, or HAL, addiction, pain, psychiatric, neurological, pediatric and infectious disease services. We are one of the largest providers of patient population for Phase I and confined Phase II to Phase III services in the United States, and are one of only a few CROs in the world that has the ability to design and conduct HAL studies, a regulatory‑required study for central nervous system compounds. We believe this enables us to provide our clients with a full range of Phase I to Phase II clinical research services in specialized patient populations for both inpatient and outpatient settings.
EDS also supports a variety of additional services, ranging from protocol development to data management and pharmacy services, including manufacturing of investigational medicinal products. Our state‑of‑the‑art laboratories provide pharmacokinetics, the branch of pharmacology concerned with the movement of drugs within the body, and pharmacodynamics, the branch of pharmacology concerned with the effects of drugs and the mechanism of their action analyses, including biomarkers, as needed. Our safety laboratory supports our own clinics and also acts as a central lab for medium sized Phase II trials. We also provide clinical study reports, statistical analysis, medical writing and regulatory support.
We focus on high‑end Phase I studies and specialize in more complex types of studies in which safety, intelligent design, and a wide range of pharmacodynamics assessments are critical factors. We believe our Phase I team is a leader in new developments, such as microdosing studies, pain models, HAL studies and multi‑purpose protocols with adaptive designs. We have developed extensive methodologies enabling us to conduct studies with pharmacokinetics and/or pharmacodynamics objectives.
We have more than 1,200 early development specialists working in five clinical pharmacology units located across four different countries, including the United States, the Netherlands and countries in Central and Eastern Europe. We are equipped with the technologies and infrastructure for high‑quality, efficient studies on a wide range of drugs and indications. Over the past five years, we have conducted more than 700 high‑level, complex early development clinical trials and more than 250 bioanalytical studies per year over the previous five years.
Phase I through IIa Studies—For in‑house Phase I studies, we offer more than 420 beds worldwide and accommodate volunteers in our state‑of‑the‑art clinical pharmacology units, some of which are hospital-based. At these centers, volunteers are under constant medical supervision by a team of highly experienced medical professionals. We have a pool of more than 100,000 study participants (both healthy volunteers and various specific patient populations).
In addition to in‑house studies, we use an innovative “unit‑on‑demand” business model that brings a Phase I center to patients. This model establishes a Phase I study environment in central medical facilities that specialize in the treatment of the target patient population. Physicians can recruit high volumes of patients using extensive networks of referring specialists and general practitioners. The studies occur in single center and multi‑national settings. We have also built an extensive patient network and database in areas including depression, schizophrenia, diabetes and hepatitis C. In addition to conducting Phase I and IIa studies in subjects, these sites act as investigative sites in Phase IIb and III trials.
We also offer full pharmacy capabilities and we operate a manufacturing site that complies with applicable current Good Manufacturing Practice regulations and is designed for fast and flexible manufacturing of small batches of investigational medicinal product for studies. In addition, dedicated data management professionals who can process clinical data into specific deliverables are integrated in each clinical pharmacology unit.
Since a large proportion of drug compounds do not succeed in Phase I, we utilize IND trials that include “microdose” or “low‑dose” studies to screen multiple candidates at an early stage and minimize the number of failing clinical product candidates. We have been closely involved in the field of microdose studies over the past ten years and have conducted more than 30 microdose studies.
Bioanalytical Laboratory—We offer clients two state‑of‑the‑art bioanalytical laboratories located in Assen, the Netherlands, and Lenexa, Kansas, United States. These bioanalytical laboratories have been harmonized with respect to standard operating procedures, work instructions and equipment. This provides a high level of consistency, continuity and efficiency. It also provides our clients with the ability to run studies in either laboratory, depending on the requirements of the study, and ensures that they will receive the same high level of service. Both bioanalytical laboratories are located within close proximity to their respective Phase I clinical pharmacology unit, ensuring rapid sample processing for critical dose escalation decision making involving pharmacokinetic assays. Both facilities include laboratories for mass spectrometry and ultra‑ performance liquid chromatography, typically applied to small molecule analysis. For large molecules, such as biologicals and biomarkers, our laboratories operate a wide variety of specialized assays, including ligand binding assays with a variety of detection methodologies and immunogenicity. In our fully licensed isotope laboratory, bioanalytical support is provided for mass balance and microdosing studies. The laboratories, combined with expert and highly educated staff, provide a full range of analytical services throughout the development process.
Data Solutions
Our Data Solutions segment provides data, analytics, technology, and consulting solutions to the life sciences market. We have proprietary sources of data about pharmaceutical transactions that we purchase from pharmaceutical retailers, prescribers, payers and institutional users. The data is anonymized and includes details on the patient, the location where they purchased the drug or therapy, and the payer. The details on the patient, although anonymous, are tracked in such a way as to allow analysis of therapies and purchasing over a long term. They also include demographic data such as age, gender, race and diagnoses. The data is refreshed monthly.
The core service offerings of our Data Solutions segment include:
Market Intelligence Services
Targeting and Compensation - Prescription and drug sales data services used primarily to compensate sales representatives. This data includes dispensed prescription data, non-retail pharmacy drug purchasing data and healthcare demographic and affiliations data.
Pharmaceutical Audit Suite - National-level prescription and sales data services used primarily for market research. Data subscriptions include all products and therapeutic areas and are primarily accessed on-line through our business intelligence tool.
Consulting & Services
Brand Analytics - Anonymized patient-level data sets and services that enable a variety of commercial analytics, including patient compliance, persistency, product switching, share and counts, and diagnosis. The most significant offering is PatientSource, a comprehensive patient-level data set, providing a detailed view of patient treatment activity in a client-defined disease category. PatientSource includes data regarding prescribers, patients and payer dynamics.
Managed Markets - A suite of prescription claims-based data products and analytic tools that leverage our exclusive claims lifecycle data to understand managed markets' influence on product demand.
Commercial Effectiveness - A professional services unit providing offerings that enable clients to optimize promotion spend and activities. Offerings include digital promotional measurement, advanced targeting, patient journey, and market landscape.
Scientific Studies/Clinical Hubs - A unit providing services that include clinically-oriented data hubs and health economics studies to pharmaceutical companies' medical affairs or health economics divisions. Our team provides real world evidence data to support the assessment of the clinical effectiveness of drugs.
Apps & Technology
Health Data Services - Technology-enabled products and services that allow clients to access and analyze effectively Symphony Health and integrated third-party data.
Clients and Suppliers
We serve a wide range of client types, including biotechnology and pharmaceutical companies. We have developed numerous strategic relationships in the last five years. In the year ended December 31, 2018, we derived 48% of our revenue from large pharmaceutical companies, 16% of our revenue from small‑ to mid‑sized pharmaceutical companies, 18% of our revenue from large biotechnology companies, 17% of our revenue from all other biotechnology companies and 1% of our revenue from non-pharmaceutical companies. In 2018 our top five clients represented approximately 36% of revenue; this revenue was derived from a combination of fixed‑fee contracts, fee‑for‑service contracts and time and materials contracts. No individual client or project accounted for 10% or more of revenue for the year ended December 31, 2018.
We utilize a number of suppliers in our business, including data suppliers, central laboratory services, drug storage and shipping, foreign language translation services and information technology. In 2018, our largest individual supplier was paid $23.3 million. In addition, our top 10 suppliers together received payments during 2018 of approximately $137.1 million. We believe that we will continue to be able to meet our current and future supply needs.
Sales and Marketing
We have a proven sales team with the ability to build relationships with new clients and to grow within existing clients. Critical to our sales process is the involvement of our operations and global scientific and medical affairs teams who contribute their knowledge to project implementation strategies presented in client proposals. These teams also work closely with the sales team to build long‑term relationships with biotechnology and pharmaceutical companies. Our therapeutic expertise team supports the sales effort by developing robust service offerings in its core therapeutic areas, which link our organization to key clinical opinion leaders, global investigator networks and best‑in‑class vendors. We rely heavily on our past project performance, qualified teams, medical informatics data and therapeutic expertise in winning new business.
Our approach to proposal development, led by seasoned proposal developers in conjunction with insight from our drug development experts, allows us to submit proposals that address client requirements in a creative and tailored manner. Proposal teams conduct research on competing drugs and conduct feasibility studies among potential investigators to assess their interest and patient availability for proposals and presentations. Our proprietary, automated estimation system allows for rapid and accurate creation of project budgets, which forms the initial basis for business management of budgets subsequent to award of the study.
Competition
Our Clinical Research business competes primarily with other full‑service CROs and in‑house research and development departments of pharmaceutical and established biotech companies. Our principal traditional CRO competitors are ICON plc, IQVIA Holdings Inc., Laboratory Corporation of America Holdings, PAREXEL International Corporation, Pharmaceutical Product Development LLC, and Syneos Health, Inc.
CROs compete on the basis of a number of factors, including reliability, past performance, expertise and experience in specific therapeutic areas, scope of service offerings, strengths in various geographic markets, technological capabilities, ability to manage large scale global clinical trials, and price.
The CRO industry remains highly fragmented, with several hundred smaller, limited service providers and a small number of full‑service companies with global capabilities. We believe there are significant barriers to becoming a global provider offering a broad range of services and products. These barriers include:
| |
• | the cost and experience necessary to develop broad therapeutic expertise; |
| |
• | the ability to manage large, complex international clinical programs; |
| |
• | the ability to deliver high‑quality services consistently for large drug development projects; |
| |
• | the experience to prepare regulatory submissions on a global basis; and |
| |
• | the infrastructure and knowledge to respond to the global needs of clients. |
Our Data Solutions business competes with a diverse set of businesses. We generally compete with other information, analytics, technology, services and consulting companies, as well as with government agencies, private payers and other healthcare companies that provide their data directly to others. Our offerings compete with a number of firms, including IQVIA Inc., OptumHealth, Cognizant Technology Solutions, and ZS Associates.
Backlog
Our studies and projects are performed over varying durations, ranging from several months to several years. Backlog represents anticipated service revenue from contracted new business awards that either have not started or are in process but have not been completed for our Clinical Research segment. Canceled contracts and scope reductions are removed from backlog as they occur. Our backlog at December 31, 2018, 2017 and 2016 was approximately $4.2 billion, $3.5 billion and $2.9 billion, respectively. Cancellations totaled $378.8 million, $366.0 million and $290.6 million for the years ended December 31, 2018, 2017 and 2016, respectively.
We believe our backlog as of any date is not necessarily a meaningful indicator of our future results for a variety of reasons. First, studies vary in duration. For instance, some studies that are included in our backlog may be completed in 2019, while others may be completed in later years. Second, the scope of studies may change, which may either increase or decrease the amount of backlog. Third, studies may be terminated or delayed at any time by the client or regulatory authorities. Delayed contracts remain in our backlog until a determination of whether to continue, modify or cancel the study is made.
We had $2,644.8 million, $2,413.7 million and $2,076.5 million in net new business awards for our Clinical Research segment in the years ended December 31, 2018, 2017, and 2016, respectively. Net new business represents gross new business awards less cancellations for the period.
We exclude our Data Solutions segment from backlog and new business awards due to the short term nature of its contracts.
For more details regarding risks related to our backlog, see “Risk Factors—Our backlog may not convert to service revenue at the historical conversion rate.”
Intellectual Property
We develop and use proprietary methodologies, analytics, systems, technologies and other intellectual property throughout our business, including a number of patents as well as other proprietary information regarding our methodologies, technologies, systems and analytics. We rely upon a combination of legal, technical, and administrative safeguards to protect our proprietary and confidential information and trade secrets, and patent, copyright and trademark laws to protect other intellectual property rights. We also hold various federal trademark registrations and pending applications in the United States and other jurisdictions, including PRA Health Sciences, Nextrials, Parallel 6, and Symphony Health. Trademarks and service marks generally may be renewed indefinitely so long as they are in use and/or their registrations are properly maintained, and so long as they have not been found to have become generic. The technology and other intellectual property rights owned and licensed by us are important to our business, although our management believes that our business, as a whole, is not dependent upon any one intellectual property or group of such properties.
Government Regulation
In the United States, the FDA governs the conduct of clinical trials of drug products in human subjects, the form and content of regulatory applications, including, but not limited to, IND applications for human clinical testing, and the development, approval, manufacture, safety, labeling, storage, record keeping, and marketing of drug products. The FDA has similar authority and similar requirements with respect to the clinical testing of biological products and medical devices. In the European Union, or EU, similar laws and regulations apply which may vary slightly from one member state to another and are enforced by the European Medicines Agency or respective national member states’ authorities, depending on the case.
Governmental regulation directly affects our business. Increased regulation leads to more complex clinical trials and an increase in potential business for us. Conversely, a relaxation in the scope of regulatory requirements, such as the introduction of simplified marketing applications for pharmaceutical and biological products, could decrease the business opportunities available to us.
We must perform our clinical drug and biologic services in compliance with applicable laws, rules and regulations, including “Good Clinical Practices,” or GCP, which govern, among other things, the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials. Before a human clinical trial may begin, the manufacturer or sponsor of the clinical product candidate must file an IND with the FDA, which contains, among other things, the results of preclinical tests, manufacturer information, and other analytical data. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Each clinical trial must be conducted in accordance with an effective IND. In addition, under GCP, each human clinical trial we conduct is subject to the oversight of an independent institutional review board, or IRB, which is an independent committee that has the regulatory authority to review, approve and monitor a clinical trial. The FDA, the IRB, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the study subjects are being exposed to an unacceptable health risk. In the EU, we must perform our clinical drug services in compliance with similar laws and regulations.
In order to comply with GCP and other regulations, we must, among other things:
| |
• | comply with specific requirements governing the selection of qualified investigators; |
| |
• | obtain specific written commitments from the investigators; |
| |
• | obtain IRB review and approval of the clinical trial; |
| |
• | verify that appropriate patient informed consent is obtained before the patient participates in a clinical trial; |
| |
• | ensure adverse drug reactions resulting from the administration of a drug or biologic during a clinical trial are medically evaluated and reported in a timely manner; |
| |
• | monitor the validity and accuracy of data; |
| |
• | verify drug or biologic accountability; |
| |
• | instruct investigators and study staff to maintain records and reports; and |
| |
• | permit appropriate governmental authorities access to data for review. |
We must also maintain reports in compliance with applicable regulatory requirements for each study for auditing by the client and regulatory authorities.
A failure to comply with applicable regulations relating to the conduct of clinical trials or the preparation of marketing applications could lead to a variety of sanctions. For example, violations of GCP could result, depending on the nature of the violation and the type of product involved, in the issuance of a warning letter, suspension or termination of a clinical study, refusal of the FDA to approve clinical trial or marketing applications or withdrawal of such applications, injunction, seizure of investigational products, civil penalties, criminal prosecutions, or debarment from assisting in the submission of new drug applications.
We monitor our clinical trials to test for compliance with applicable laws and regulations in the United States and the non‑U.S. jurisdictions in which we operate. We have adopted standard operating procedures that are designed to satisfy regulatory requirements and serve as a mechanism for controlling and enhancing the quality of our clinical trials. In the United States, our procedures were developed to ensure compliance with GCP and associated guidelines. Within Europe, all work is carried out in accordance with the Guideline for Good Clinical Practice ICH E6 (R2) adopted by the European Medicines Agency as EMA/CHMP/ICH/135/95. In order to facilitate global clinical trials, we have implemented common standard operating procedures across our regions to assure consistency whenever feasible.
The Standards for Privacy of Individually Identifiable Health Information, or the Privacy Rule, and the Security Rule, issued under the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health, or HITECH, Act of 2009, collectively HIPAA, as well as applicable state privacy and security laws and regulations, restrict the use and disclosure of certain protected health information, or PHI, and establish standards to protect individuals’ electronic PHI that is created, received, used or maintained by certain entities. Under the Privacy Rule, “covered entities” may not use or disclose PHI without the authorization of the individual who is the subject of the PHI, unless such use or disclosure is specifically permitted by the Privacy Rule or required by law.
We are not a covered entity under HIPAA. However, in connection with our clinical development activities, we do receive PHI from covered entities subject to HIPAA. In order for those covered entities to disclose PHI to us, the covered entity must obtain an authorization from the research subject that meets the Privacy Rule requirements, or make such disclosure pursuant to an exception to the Privacy Rule’s authorization requirement. We are both directly and indirectly affected by the privacy provisions surrounding individual authorizations because many investigators with whom we are involved in clinical trials are directly subject to them as a HIPAA “covered entity” and because we obtain identifiable health information from third parties that are subject to such regulations. Because of amendments to the HIPAA data security and privacy rules, there are some instances where we may be a HIPAA “business associate” of a “covered entity,” meaning that we may be directly liable for any breaches in PHI and other HIPAA violations. As part of our research activities, we require covered entities that perform research activities on our behalf to comply with HIPAA, including the Privacy Rule’s authorization requirement, and applicable state privacy and security laws and regulations.
In Europe, the European Union General Data Protection Regulation, or the EU GDPR, requires organizations working with the personal data of EU citizens to have established processes related to its collection and use. Organizations must have objective evidence of compliance (Principle of Accountability) with the EU GDPR. The penalties for non-compliance are significant, including up to four percent of an organization's global annual revenue. There are also administrative penalties where transfers of personal data may be stopped. As PRA is a global organization, such a disruption in data transfers could pose significant operational challenges.
We maintain applicable registrations with the Drug Enforcement Administration, or DEA, that enable us to use controlled substances in connection with our research services. Controlled substances are those drugs and drug products that appear on one of five schedules promulgated and administered by DEA under the Controlled Substances Act. This act governs, among other things, the distribution, recordkeeping, handling, security, and disposal of controlled substances. Our DEA registrations authorize us to receive, conduct testing on, and distribute controlled substances in Schedules II through V. A failure to comply with the DEA’s regulations governing these activities could lead to a variety of sanctions, including the revocation or the denial of a renewal of our DEA registration, injunctions, or civil or criminal penalties.
Environmental Regulation and Liability
We are subject to various laws and regulations relating to the protection of the environment and human health and safety in the countries in which we do business, including laws and regulations governing the management and disposal of hazardous substances and wastes, the cleanup of contaminated sites and the maintenance of a safe workplace. Our operations include the use, generation, and disposal of hazardous materials and medical wastes. We may, in the future, incur liability under environmental statutes and regulations for contamination of sites we own or operate (including contamination caused by prior owners or operators of such sites), the off‑site disposal of hazardous substances and for personal injuries or property damage arising from exposure to hazardous materials from our operations. We believe that we have been and are in substantial compliance with all applicable environmental laws and regulations and that we currently have no liabilities under such environmental requirements that could reasonably be expected to materially harm our business, results of operations or financial condition.
Liability and Insurance
We may be liable to our clients for any failure to conduct their studies properly according to the agreed‑upon protocol and contract. If we fail to conduct a study properly in accordance with the agreed‑upon procedures, we may have to repeat a study or a particular portion of the services at our expense, reimburse the client for the cost of the services and/or pay additional damages.
At our clinical pharmacology units, we study the effects of drugs on healthy volunteers. In addition, in our clinical business we, on behalf of our clients, contract with physicians who render professional services, including the administration of the substance being tested, to participants in clinical trials, many of whom are seriously ill and are at great risk of further illness or death as a result of factors other than their participation in a trial. As a result, we could be held liable for bodily injury, death, pain and suffering, loss of consortium, or other personal injury claims and medical expenses arising from a clinical trial. In addition, we sometimes engage the services of vendors necessary for the conduct of a clinical trial, such as laboratories or medical diagnostic specialists. Because these vendors are engaged as subcontractors, we are responsible for their performance and may be held liable for damages if the subcontractors fail to perform in the manner specified in their contract.
To reduce our potential liability, and as a requirement of the GCP regulations, informed consent is required from each volunteer and patient. In addition, our clients provide us with contractual indemnification for all of our service related contracts. These indemnities generally do not, however, protect us against certain of our own actions such as those involving negligence or misconduct. Our business, financial condition and operating results could be harmed if we were required to pay damages or incur defense costs in connection with a claim that is not indemnified, that is outside the scope of an indemnity or where the indemnity, although applicable, is not honored in accordance with its terms.
We maintain errors, omissions, and professional liability insurance in amounts we believe to be appropriate. This insurance provides coverage for vicarious liability due to negligence of the investigators who contract with us, as well as claims by our clients that a clinical trial was compromised due to an error or omission by us. If our insurance coverage is not adequate, or if insurance coverage does not continue to be available on terms acceptable to us, our business, financial condition, and operating results could be materially harmed.
Seasonality
Although our business is not generally seasonal, our Clinical Research segment typically experiences a slight decrease in its revenue growth rate during the fourth quarter due to holiday vacations and a similar decrease in new business awards in the first quarter due to our clients’ budgetary cycles and vacations during the year‑end holiday period. Our Data Solutions segment usually experiences an increase in revenue during the fourth quarter as many pharmaceutical companies use a portion of funds remaining in their annual budgets to purchase its data offerings.
Employees
As of December 31, 2018, we had over 16,400 employees, of which approximately 43% were in the United States, approximately 34% were in Europe, approximately 3% were in Canada, and approximately 20% were in Africa, Latin America, and Asia Pacific. Some of our employees located outside of the United States are represented by workers council or labor unions. We believe that our employee relations are satisfactory. Approximately 40% of employees hold a Master’s level degree or higher. We have approximately 1,800 employees that hold a Ph.D, M.D. or other doctorate level degrees.
Available Information
We are subject to the informational requirements of the Exchange Act and, in accordance therewith, file reports, including annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission, or the SEC. Copies of our annual reports on Form 10‑K, quarterly reports on Form 10‑Q, current reports on Form 8‑K and our proxy statements for our annual meetings of stockholders, and any amendments or supplements to those reports, as well as Section 16 reports filed by our insiders, are available free of charge on our website as soon as reasonably practicable after we file the reports with, or furnish the reports to the SEC. Our website address is http://www.prahs.com, and our investor relations website is located at investor.prahs.com. Information on our website is not incorporated by reference herein. In addition, the SEC maintains an Internet site (http://www.sec.gov) containing reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
Item 1A. Risk Factors
You should consider carefully the risks and uncertainties described below together with the other information included in this Annual Report on Form 10‑K, including our consolidated financial statements and related notes thereto. The occurrence of any of the following risks may materially and adversely affect our business, financial condition, results of operations and future prospects, which could in turn materially affect the price of our common stock.
The potential loss, delay or non‑renewal of our contracts, or the non‑payment by our clients for services that we have performed, could adversely affect our results.
We routinely experience termination, cancellation and non‑renewals of contracts by our clients in the ordinary course of business, and the number of cancellations can vary significantly from year to year.
Most of our clients for traditional, project‑based clinical trial services can terminate our contracts without cause upon 30 to 60 days’ notice. For example, our cancellation percentage for traditional, project‑based Phase I through IV trials was 17% and 18% for the years ended December 31, 2018 and 2017, respectively. Our traditional, project‑based clients may delay, terminate or reduce the scope of our contracts for a variety of reasons beyond our control, including but not limited to:
| |
• | decisions to forgo or terminate a particular clinical trial; |
| |
• | lack of available financing, budgetary limits or changing priorities; |
| |
• | actions by regulatory authorities; |
| |
• | production problems resulting in shortages of the drug being tested; |
| |
• | failure of the drug being tested to satisfy safety requirements or efficacy criteria; |
| |
• | unexpected or undesired clinical results; |
| |
• | insufficient patient enrollment in a trial; |
| |
• | insufficient investigator recruitment; |
| |
• | decisions to downsize product development portfolios; |
| |
• | dissatisfaction with our performance, including the quality of data provided and our ability to meet agreed upon schedules; |
| |
• | shift of business to another CRO or internal resources; |
| |
• | product withdrawal following market launch; or |
| |
• | shut down of our clients’ manufacturing facilities. |
In addition, our clients for our Strategic Solutions offerings may elect not to renew our contracts for a variety of reasons beyond our control, including in the event that we are unable to provide staff sufficient in number or experience as required for a project.
In the event of termination, our contracts often provide for fees for winding down the study, but these fees may not be sufficient for us to maintain our profit margins, and termination or non‑renewal may result in lower resource utilization rates, including with respect to personnel who we are not able to place on another client engagement.
Clinical trials can be costly and a material portion of our revenue is derived from emerging biotechnology and small to mid‑sized pharmaceutical companies, which may have limited access to capital. In addition, we provide services to such companies before they pay us for some of our services. There is a risk that we may initiate a clinical trial for a client, and the client subsequently becomes unwilling or unable to fund the completion of the trial. In such a situation, notwithstanding the client’s ability or willingness to pay for or otherwise facilitate the completion of the trial, we may be legally or ethically bound to complete or wind down the trial at our own expense.
Because the contracts included in our backlog can generally be terminated without cause, we do not believe that our backlog as of any date is necessarily a meaningful predictor of future results. In addition, we may not realize the full benefits of our backlog of contractually committed services if our clients cancel, delay or reduce their commitments under our contracts with them. For the reasons described above, the loss or delay of a large contract or the loss or delay of multiple contracts or a
clients' non-payment for services could adversely affect our service revenue and profitability. In addition, the terminability of our contracts puts increased pressure on our quality control efforts, since not only can our contracts be terminated by clients as a result of poor performance, but any such termination may also affect our ability to obtain future contracts from the client involved and others. We believe the risk of loss or delay of multiple contracts is even greater in those cases where we are party to broader partnering arrangements with global biopharmaceutical companies.
We bear financial risk if we underprice our fixed‑fee contracts or overrun cost estimates, and our financial results can also be adversely affected by failure to receive approval for change orders or delays in documenting change orders.
Most of our traditional, project‑based Phase I through IV contracts are fixed‑fee contracts. We bear the financial risk if we initially underprice our contracts or otherwise overrun our cost estimates. In addition, contracts with our clients are subject to change orders, which we commonly experience and which occur when the scope of work we perform needs to be modified from that originally contemplated by our contract with the client. Modifications can occur, for example, when there is a change in a key trial assumption or parameter, a significant change in timing or a change in staffing needs. Furthermore, if we are not successful in converting out‑of‑scope work into change orders under our current contracts, we bear the cost of the additional work. Such underpricing, significant cost overruns or delay in documentation of change orders could have a material adverse effect on our business, results of operations, financial condition or cash flows.
Our backlog may not convert to revenue at the historical conversion rate.
Backlog represents anticipated revenue from contracted new business awards, excluding reimbursable out-of-pocket costs or reimbursable investigator fees, that either have not started or are in process but have not been completed. Our backlog was $4.2 billion, $3.5 billion, and $2.9 billion at December 31, 2018, 2017, and 2016, respectively. Our revenue conversion rate is based on a financial and operational analysis performed by our project management teams and represents the level of effort expected to be expended at a specific point in time. Once work begins on a project, revenue is recognized over the duration of the project. Projects may be terminated or delayed by the client or delayed by regulatory authorities for reasons beyond our control. To the extent projects are delayed, the timing of our revenue could be affected. In the event that a client cancels a contract, we generally would be entitled to receive payment for all services performed up to the cancellation date and subsequent client authorized services related to terminating the canceled project. Generally, however, we have no contractual right to the full amount of the revenue reflected in our backlog in the event of a contract cancellation. The duration of the projects included in our backlog, and the related revenue recognition, range from a few months to many years. Our backlog may not be indicative of our future results, and we may not realize all the anticipated future revenue reflected in our backlog. A number of factors may affect the realization of our revenue from backlog, including:
| |
• | the size, complexity and duration of the projects; |
| |
• | the cancellation or delay of projects; and |
| |
• | change in the scope of work during the course of a project. |
Fluctuations in our reported backlog levels also result from the fact that we may receive a small number of relatively large orders in any given reporting period that may be included in our backlog. Revenue recognition on larger, more global projects could be slower than on smaller, less global projects for a variety of reasons, including but not limited to, an extended period of negotiation between the time the project is awarded to us and the actual execution of the contract, as well as an increased time frame for obtaining the necessary regulatory approvals.
The relationship of backlog to realized revenues is indirect and may vary over time. As we increasingly compete for and enter into large contracts that are more global in nature, there can be no assurance about the rate at which our backlog will convert into revenue. A decrease in this conversion rate would mean that the rate of revenue recognized on contracts may be slower than what we have experienced in the past, which could materially and adversely impact our revenue and results of operations on a quarterly and annual basis. Additionally, delayed projects will remain in backlog and will not generate revenue at the rate originally expected, which could impair our cash flows and results of operations in the short term. Because of these large orders, our backlog in that reporting period may reach levels that may not be sustained in subsequent reporting periods.
Our operating margins and profitability will be adversely affected if we are unable to either achieve efficiencies in our operating expenses or grow revenues at a rate faster than expenses.
We operate in a highly competitive environment and experience competitive pricing pressure. To achieve our operating margins over the last three years, we have implemented initiatives to control the rate of growth of our operating expenses. We will continue to utilize these initiatives in the future with a view to offsetting these pricing pressures; however, we cannot be certain that we will be able to achieve the efficiency gains necessary to maintain or grow our operating margins or
that the magnitude of our growth in service revenue will be faster than the growth in our operating costs. If we are unable to grow our service revenue at a faster rate than our operating costs, our operating margins will be adversely affected. Our initiatives and any future cost initiatives may also adversely affect us, as they may decrease employee morale or make it more difficult for us to meet operational requirements.
If we are unable to attract suitable investigators and patients for our clinical trials, our clinical development business may suffer.
The recruitment of investigators and patients for clinical trials is essential to our business. Patients typically include people from the communities in which the clinical trials are conducted. Our clinical development business could be adversely affected if we are unable to attract suitable and willing investigators or patients for clinical trials on a consistent basis. For example, if we are unable to engage investigators to conduct clinical trials as planned or enroll sufficient patients in clinical trials, we may need to expend additional funds to obtain access to resources or else be compelled to delay or modify the clinical trial plans, which may result in additional costs to us. These considerations might result in our being unable to successfully achieve our projected development timelines, or potentially even lead us to consider the termination of ongoing clinical trials or development of a product.
Our embedded and functional outsourcing solutions could subject us to significant employment liability.
With our embedded and functional outsourcing services, we place employees at the physical workplaces of our clients. The risks of this activity include claims of errors and omissions, misuse or misappropriation of client proprietary information, theft of client property and torts or other claims under employment liability, co‑employment liability or joint employment liability. We have policies and guidelines in place to reduce our exposure to such risks, but if we fail to follow these policies and guidelines we may suffer reputational damage, loss of client relationships and business, and monetary damages.
If we lose the services of key personnel or are unable to recruit experienced personnel, our business could be adversely affected.
Our success substantially depends on the collective performance, contributions and expertise of our senior management team and other key personnel, including qualified management, professional, scientific and technical operating staff and qualified sales representatives for our contract sales services. There is significant competition for qualified personnel in the biopharmaceutical services industry, particularly those with higher educational degrees, such as a medical degree, a Ph.D or an equivalent degree. The departure of any key executive, the payment of increased compensation to attract and retain qualified personnel, or our inability to continue to identify, attract and retain qualified personnel or replace any departed personnel in a timely fashion, may impact our ability to grow our business and compete effectively in our industry and may negatively affect our ability to meet financial and operational goals. Furthermore, clients or other companies seeking to develop in‑house capabilities may hire some of our senior management or key employees. We cannot assure you that a court would enforce the non‑competition provisions in our employment agreements.
Changes in accounting standards may adversely affect our financial statements.
From time to time the Financial Accounting Standards Board, or FASB, and SEC issue new or revised guidance that we are required to adopt. It is possible that future accounting standards may require changes to our current accounting treatment and may require us to make changes to our accounting systems and processes. These changes could have a material impact on our business, results of operations and financial condition. See Note 2 to our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K for details regarding recently implemented accounting standards and recently issued accounting pronouncements and the potential impact they may have on the Company.
Our effective income tax rate may fluctuate for different reasons, including the U.S. Tax Cuts and Jobs Act enacted in 2017, which may adversely affect our operations, earnings and earnings per share.
Our effective income tax rate is influenced by our projected profitability in the various taxing jurisdictions in which we operate. The global nature of our business increases our tax risks. In addition, as a result of increased funding needs by governments resulting from fiscal stimulus measures, revenue authorities in many of the jurisdictions in which we operate are known to have become more active in their tax collection activities. Changes in the distribution of profits and losses among taxing jurisdictions may have a significant impact on our effective income tax rate, which in turn could have an adverse effect on our net income and earnings per share. The application of tax laws in various taxing jurisdictions, including the United States, is subject to interpretation, and tax authorities in various jurisdictions may have diverging and sometimes conflicting
interpretations of the application of tax laws. Changes in tax laws or tax rulings in the United States or other tax jurisdictions in which we operate, could materially impact our effective tax rate.
Factors that may affect our effective income tax rate include, but are not limited to:
| |
• | the requirement to exclude from our quarterly worldwide effective income tax calculations losses in jurisdictions where no income tax benefit can be recognized; |
| |
• | actual and projected full year pre‑tax income, including differences between actual and anticipated income before taxes in various jurisdictions; |
| |
• | changes in tax laws, or in the interpretation or application of tax laws, in various taxing jurisdictions, including the U.S. Tax Cuts and Jobs Act; |
| |
• | audits or other challenges by taxing authorities; and |
| |
• | the establishment of valuation allowances against a portion or all of certain deferred income tax assets if we determined that it is more likely than not that future income tax benefits will not be realized. |
In addition, our effective income tax rate is influenced by U.S. tax law which has been substantially modified by the U.S. Tax Cuts and Jobs Act. The following provisions of the U.S. Tax Cuts and Jobs Act could have an adverse effect on our tax rate if it is determined that the provisions are applicable to the Company:
| |
• | global intangible low-taxed income; |
| |
• | limitations on the U.S. deductions for net business interest; |
| |
• | base erosion anti-abuse provisions; and |
| |
• | performance-based compensation subject to $1 million limit. |
These changes may cause fluctuations in our effective income tax rate that could adversely affect our results of operations and cause fluctuations in our earnings and earnings per share.
Our business depends on the continued effectiveness and availability of our information systems, including the information systems we use to provide our services to our clients, and failures of these systems may materially limit our operations.
Due to the global nature of our business and our reliance on information systems to provide our services, we have increased, and intend to continue to increase, our use of web‑enabled and other integrated information systems in delivering our services. We also provide access to similar information systems to certain of our clients in connection with the services we provide them. As the breadth and complexity of our information systems continue to grow, we will increasingly be exposed to the risks inherent in the development, integration and ongoing operation of evolving information systems, including:
| |
• | disruption, impairment or failure of data centers, telecommunications facilities or other key infrastructure platforms; |
| |
• | security breaches of, cyberattacks on and other failures or malfunctions in our critical application systems or their associated hardware; and |
| |
• | excessive costs, excessive delays or other deficiencies in systems development and deployment. |
The materialization of any of these risks may impede the processing of data, the delivery of databases and services, and the day‑to‑day management of our business and could result in the corruption, loss or unauthorized disclosure of proprietary, confidential or other data. While we have disaster recovery plans in place, they might not adequately protect us in the event of a system failure. Despite any precautions we take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, information system security breaches and similar events at our various computer facilities could result in interruptions in the flow of data to our servers and from our servers to our clients. Corruption or loss of data may result in the need to repeat a trial at no cost to the client, but at significant cost to us, or result in the termination of a contract or damage to our reputation. Additionally, significant delays in system enhancements or inadequate performance of new or upgraded systems once completed could damage our reputation and harm our business. Finally, long‑term disruptions in the infrastructure caused by events such as natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism, particularly involving cities in which we have offices, could adversely affect our business.
Although we carry property and business interruption insurance, our coverage might not be adequate to compensate us for all losses that may occur.
A failure or breach of our IT systems or technology could result in sensitive customer information being compromised or otherwise significantly disrupt our business operations, which would negatively materially affect our reputation and/or results of operations.
In the current environment, there are numerous and evolving risks to cybersecurity and privacy, including criminal hackers, hacktivists, state-sponsored intrusions, industrial espionage, employee malfeasance and human or technological error. High-profile security breaches at other companies and in government agencies have increased in recent years, and security industry experts and government officials have warned about the risks of hackers and cyberattacks targeting businesses such as ours. Computer hackers and others routinely attempt to breach the security of technology products, services and systems, and to fraudulently induce employees, customers, or others to disclosure information or unwittingly provide access to systems or data. Unauthorized disclosure of sensitive or confidential data, whether through system failure or employee negligence, fraud or misappropriation, could damage our reputation and cause us to lose clients. Similarly, unauthorized access to or through our information systems or those we develop for our clients, whether by our employees or third parties, including a cyberattack by computer programmers and hackers who may develop and deploy viruses, worms or other malicious software programs, could result in negative publicity, loss of client confidence, significant remediation costs, time-consuming and costly regulatory investigations, legal liability and damage to our reputation, and could have a material adverse effect on our results of operations. In addition, our liability insurance might not be sufficient in type or amount to adequately cover us against claims related to security breaches, cyberattacks and other related breaches. To date, cybersecurity attacks directed at us have not had a material impact on our financial results. Our clients are also increasingly requiring cybersecurity protections and mandating cybersecurity standards in our products, and we may incur additional costs to comply with such demands. While we have certain cybersecurity safeguards in place designed to protect and preserve the integrity of our information technology systems, due to the evolving nature of security threats, however, the impact of any future incidents cannot be predicted.
Upgrading the information systems that support our operating processes and evolving the technology platform for our services pose risks to our business.
Continued efficient operation of our business requires that we implement standardized global business processes and evolve our information systems to enable this implementation. We have continued to undertake significant programs to optimize business processes with respect to our services. Our inability to effectively manage the implementation and adapt to new processes designed into these new or upgraded systems in a timely and cost‑effective manner may result in disruption to our business and negatively affect our operations.
We have entered into agreements with certain vendors to provide systems development and integration services that develop or license to us the IT platform for programs to optimize our business processes. If such vendors fail to perform as required or if there are substantial delays in developing, implementing and updating the IT platform, our client delivery may be impaired, and we may have to make substantial further investments, internally or with third parties, to achieve our objectives. Additionally, our progress may be limited by parties with existing or claimed patents who seek to enjoin us from using preferred technology or seek license payments from us.
Meeting our objectives is dependent on a number of factors which may not take place as we anticipate, including obtaining adequate technology-enabled services, creating IT‑enabled services that our clients will find desirable and implementing our business model with respect to these services. Also, increased IT‑related expenditures may negatively impact our profitability.
Our operations might be affected by the occurrence of a natural disaster or other catastrophic event.
We depend on our clients, investigators, laboratories and other facilities for the continued operation of our business. Although we have contingency plans in place for natural disasters or other catastrophic events, these events, including terrorist attacks, pandemic flu, hurricanes and ice storms, could nevertheless disrupt our operations or those of our clients, investigators and collaboration partners, which could also affect us. In particular, our headquarters are in Raleigh, North Carolina where hurricanes might occur. Even though we carry business interruption insurance policies and typically have provisions in our contracts that protect us in certain events, we might suffer losses as a result of business interruptions that exceed the coverage available under our insurance policies or for which we do not have coverage. Any natural disaster or catastrophic event affecting us or our clients, investigators or collaboration partners could have a significant negative impact on our operations and financial performance.
We may be adversely affected by client concentration or concentration in therapeutic classes in which we conduct clinical trials.
We derive a substantial portion of our revenues from a limited number of large clients. In 2018, we derived 36% of our revenue from our top five clients. In addition, almost 43% of our backlog, as of December 31, 2018, is concentrated among five clients. If any large client decreases or terminates its relationship with us, our business, results of operations or financial condition could be materially adversely affected.
Additionally, we conduct multiple clinical trials for different clients in single therapeutic classes, particularly in the areas of oncology and immunology. Conducting multiple clinical trials for different clients in a single therapeutic class involving drugs with the same or similar chemical action has in the past, and may in the future, adversely affect our business if some or all of the trials are canceled because of new scientific information or regulatory judgments that affect the drugs as a class or if industry consolidation results in the rationalization of drug development pipelines.
Our business is subject to international economic, political and other risks that could negatively affect our results of operations and financial condition.
We have significant operations in non‑U.S. countries that may require complex arrangements to deliver services on global contracts for our clients. Additionally, we have established operations in locations remote from our most developed business centers. As a result, we are subject to heightened risks inherent in conducting business internationally, including the following:
| |
• | conducting a single trial across multiple countries is complex, and issues in one country, such as a failure to comply with local regulations or restrictions, may affect the progress of the trial in the other countries, for example, by limiting the amount of data necessary for a trial to proceed, resulting in delays or potential cancellation of contracts, which in turn may result in loss of revenue; |
| |
• | non‑U.S. countries could enact legislation or impose regulations or other restrictions, including unfavorable labor regulations or tax policies, which could have an adverse effect on our ability to conduct business in or expatriate profits from those countries; |
| |
• | tax rates in certain non‑U.S. countries may exceed those in the United States and non‑U.S. earnings may be subject to withholding requirements or the imposition of tariffs, exchange controls or other restrictions, including restrictions on repatriation; |
| |
• | certain non‑U.S. countries are expanding or may expand their regulatory framework with respect to patient informed consent, protection and compensation in clinical trials, which could delay or inhibit our ability to conduct trials in such jurisdictions or which could materially increase the risks associated with performing trials in such jurisdictions; |
| |
• | the regulatory or judicial authorities of non‑U.S. countries may not enforce legal rights and recognize business procedures in a manner to which we are accustomed or would reasonably expect; |
| |
• | we may have difficulty complying with a variety of laws and regulations in non‑U.S. countries, some of which may conflict with laws in the United States; |
| |
• | changes in political and economic conditions may lead to changes in the business environment in which we operate, as well as changes in non‑U.S. currency exchange rates; |
| |
• | a prolonged shutdown of the U.S. federal government may hinder the growth of the U.S. economy, thus negatively affecting our business; |
| |
• | the adoption and expansion of trade restrictions, the occurrence or escalation of a “trade war,” or other governmental action related to tariffs or trade agreements or policies among the governments of the United States and other countries, such as Mexico or China, could adversely impact demand for our services, our costs, our clients, and the U.S. economy; |
| |
• | regulatory changes and economic conditions leading up to and following “Brexit” (the United Kingdom’s exit from the European Union), including uncertainties as to its timing and its effect on trade laws, tariffs and taxes, could create instability and volatility in the global financial and currency markets, conflicting or redundant regulatory regimes in Europe (such as the European Medicines Agency ("EMA") relocation from the United Kingdom to the Netherlands) and political instability; |
| |
• | clients in non‑U.S. jurisdictions may have longer payment cycles, and it may be more difficult to collect receivables in non‑U.S. jurisdictions; and |
| |
• | natural disasters, pandemics or international conflict, including terrorist acts, could interrupt our services, endanger our personnel or cause project delays or loss of trial materials or results. |
These risks and uncertainties could negatively impact our ability to, among other things, perform large, global projects for our clients. Furthermore, our ability to deal with these issues could be affected by applicable U.S. laws and the need to protect our assets. In addition, we may be more susceptible to these risks as we enter and continue to target growth in emerging countries and regions, including India, China, Eastern Europe and Latin America, which may be subject to a relatively higher risk of political instability, economic volatility, crime, corruption and social and ethnic unrest, all of which are exacerbated in many cases by a lack of an independent and experienced judiciary and uncertainties in how local law is applied and enforced. The materialization of any such risks could have an adverse impact on our financial condition and results of operations.
Due to the global nature of our business, we may be exposed to liabilities under the Foreign Corrupt Practices Act and various non‑U.S. anti‑corruption laws, and any allegation or determination that we violated these laws could have a material adverse effect on our business.
We are required to comply with the U.S. Foreign Corrupt Practices Act, or FCPA, and other U.S. and non‑U.S. anti‑corruption laws, which prohibit companies from engaging in bribery, including corruptly or improperly offering, promising, or providing money or anything else of value to non‑U.S. officials and certain other recipients. In addition, the FCPA imposes certain books, records, and accounting control obligations on public companies and other issuers. We operate in parts of the world in which corruption can be common and compliance with anti‑bribery laws may conflict with local customs and practices. Our global operations face the risk of unauthorized payments or offers being made by employees, consultants, sales agents, and other business partners outside of our control or without our authorization. It is our policy to implement safeguards to prohibit these practices by our employees and business partners with respect to our operations. However, irrespective of these safeguards, or as a result of monitoring compliance with such safeguards, it is possible that we or certain other parties may discover or receive information at some point that certain employees, consultants, sales agents, or other business partners may have engaged in corrupt conduct for which we might be held responsible. Violations of the FCPA or other non‑U.S. anti‑corruption laws may result in restatements of, or irregularities in, our financial statements as well as severe criminal or civil sanctions, and we may be subject to other liabilities, which could negatively affect our business, operating results and financial condition. In some cases, companies that violate the FCPA may be debarred by the U.S. government and/or lose their U.S. export privileges. Changes in anti‑corruption laws or enforcement priorities could also result in increased compliance requirements and related costs which could adversely affect our business, financial condition and results of operations. In addition, the U.S. or other governments may seek to hold us liable for successor liability FCPA violations or violations of other anti‑corruption laws committed by companies in which we invest or that we acquired or will acquire.
If we are unable to successfully develop and market new services or enter new markets, our growth, results of operations and financial condition could be adversely affected.
A key element of our growth strategy is the successful development and marketing of new services and entering new markets that complement or expand our existing business. As we develop new services or enter new markets, including services targeted at participants in the broader healthcare industry, we may not have or adequately build the competencies necessary to perform such services satisfactorily, may not receive market acceptance for such services or may face increased competition. If we are unable to succeed in developing new services, entering new markets or attracting a client base for our new services or in new markets, we will be unable to implement this element of our growth strategy, and our future business, reputation, results of operations and financial condition could be adversely affected.
If we fail to perform our services in accordance with contractual requirements, government regulations and ethical considerations, we could be subject to significant costs or liability and our reputation could be adversely affected.
We contract with biotechnology and pharmaceutical companies to perform a wide range of services to assist them in bringing new drugs to market. Our services include monitoring clinical trials, laboratory analysis, electronic data capture, patient recruitment, data analytics, technology solutions and other related services. Such services are complex and subject to contractual requirements, government regulations, and ethical considerations. For example, we are subject to regulation by the FDA and comparable non‑U.S. regulatory authorities relating to our activities in conducting pre‑clinical and clinical trials. The clinical trial process must be conducted in accordance with regulations promulgated by the FDA under the Federal Food, Drug and Cosmetic Act, which requires the drug to be tested and studied in certain ways. In the United States, before human clinical testing may begin, a manufacturer must file an IND with the FDA. Further, an IRB for each medical center proposing to participate in the clinical trial must review and approve the protocol for the clinical trial before the medical center’s investigators participate. Once initiated, clinical trials must be conducted pursuant to and in accordance with the applicable IND, the requirements of the relevant IRBs, and GCP regulations. Similarly, before clinical trials begin, a drug is tested in pre‑clinical studies that are expected to comply with Good Laboratory Practice requirements. We are also subject to regulation by the DEA which regulates the distribution, recordkeeping, handling, security, and disposal of controlled substances. If we fail to perform our services in accordance with these requirements, regulatory authorities may take action against us. Such actions may include injunctions or failure to grant marketing approval of products, imposition of clinical holds or delays, suspension or withdrawal of approvals, rejection of data collected in our studies, license revocation, product seizures or recalls, operational restrictions, civil or criminal penalties or prosecutions, damages or fines. Clients may also bring claims against us for breach of our contractual obligations and patients in the clinical trials and patients taking drugs approved on the basis of those trials may bring personal injury claims against us. Any such action could have a material adverse effect on our results of operations, financial condition and reputation.
Such consequences could arise if, among other things, the following occur:
Improper performance of our services. The performance of clinical development services is complex and time‑consuming. For example, we may make mistakes in conducting a clinical trial that could negatively impact or obviate the usefulness of the trial or cause the results of the trial to be reported improperly. If the trial results are compromised, we could be subject to significant costs or liability, which could have an adverse impact on our ability to perform our services and our reputation would be harmed. As examples:
| |
• | non‑compliance generally could result in the termination of ongoing clinical trials or the disqualification of data for submission to regulatory authorities; |
| |
• | compromise of data from a particular trial, such as failure to verify that adequate informed consent was obtained from patients, could require us to repeat the trial under the terms of our contract at no further cost to our client, but at a potentially substantial cost to us; and |
| |
• | breach of a contractual term could result in liability for damages or termination of the contract. |
Large clinical trials can cost tens of millions of dollars, and while we endeavor to contractually limit our exposure to such risks, improper performance of our services could have a material adverse effect on our financial condition, damage our reputation and result in the cancellation of current contracts by the affected client or other current clients or failure to obtain future contracts from the affected client or other current or potential clients.
Investigation of clients. From time to time, one or more of our clients are investigated by regulatory authorities or enforcement agencies with respect to regulatory compliance of their clinical trials, programs or the marketing and sale of their drugs. In these situations, we have often provided services to our clients with respect to the clinical trials, programs or activities being investigated, and we are called upon to respond to requests for information by the authorities and agencies. There is a risk that either our clients or regulatory authorities could claim that we performed our services improperly or that we are responsible for clinical trial or program compliance. If our clients or regulatory authorities make such claims against us and prove them, we could be subject to damages, fines or penalties. In addition, negative publicity regarding regulatory compliance of our clients’ clinical trials, programs or drugs could have an adverse effect on our business and reputation.
If we fail to comply with certain healthcare laws, including fraud and abuse laws, we could face substantial penalties and our business, results of operations, financial condition and prospects could be adversely affected.
Even though we do not order healthcare services or bill directly to Medicare, Medicaid or other third‑party payors, certain federal and state healthcare laws and regulations pertaining to fraud and abuse are and will be applicable to our business. We could be subject to healthcare fraud and abuse laws of both the federal government and the states in which we conduct our business. Because of the breadth of these laws and the narrowness of available statutory and regulatory exceptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If we or our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, imprisonment and the curtailment or restructuring of our operations, any of which could materially adversely affect our ability to operate our business and our financial results.
Our services could subject us to potential liability that may adversely affect our results of operations and financial condition.
Our business involves the testing of new drugs on patients in clinical trials. Our involvement in the clinical trial and development process creates a risk of liability for personal injury to or death of patients, particularly those with life‑threatening illnesses, resulting from adverse reactions to the drugs administered during testing or after regulatory approval. For example, we may be sued in the future by individuals alleging personal injury due to their participation in clinical trials and seeking damages from us under a variety of legal theories. If we are required to pay damages or incur defense costs in connection with any personal injury claim that is outside the scope of indemnification agreements we have with our clients, if any indemnification agreement is not performed in accordance with its terms or if our liability exceeds the amount of any applicable indemnification limits or available insurance coverage, our financial condition, results of operations and reputation could be materially and adversely affected. We might also not be able to obtain adequate insurance or indemnification for these types of risks at reasonable rates in the future.
We also contract with physicians to serve as investigators in conducting clinical trials. Investigators are typically located at hospitals, clinics or other sites and supervise the administration of the investigational drug to patients during the course of a clinical trial. If the investigators commit errors or make omissions during a clinical trial that result in harm to trial patients or if the investigators commit errors or make omissions in the administration of a drug to a patient, claims for personal injury or products liability damages may result. Additionally, if the investigators engage in fraudulent or negligent behavior, trial data may be compromised, which may require us to repeat the clinical trial or subject us to liability or regulatory action. We do not believe we are legally responsible for the medical care rendered by such third‑party investigators, and we would vigorously defend any claims brought against us. However, it is possible we could be found liable for claims with respect to the actions of third‑party investigators.
Some of our services involve direct interaction with clinical trial patients and operation of Phase I and IIa clinical facilities, which could create potential liability that may adversely affect our results of operations and financial condition.
We operate facilities where Phase I to IIa clinical trials are conducted, which ordinarily involve testing an investigational drug on a limited number of individuals to evaluate its safety, determine a safe dosage range and identify side effects. Failure to operate such a facility in accordance with applicable regulations could result in disruptions to our operations. Additionally, we face risks associated with adverse events resulting from the administration of such drugs and the professional malpractice of medical care providers. We also directly employ nurses and other trained employees who assist in implementing the testing involved in our clinical trials, such as drawing blood from subjects. Any professional malpractice or negligence by such investigators, nurses or other employees could potentially result in liability to us in the event of personal injury to or death of a subject in clinical trials. This liability, particularly if it were to exceed the limits of any indemnification agreements and insurance coverage we may have, may adversely affect our financial condition, results of operations and reputation.
Our insurance may not cover all of our indemnification obligations and other liabilities associated with our operations.
We maintain insurance designed to provide coverage for ordinary risks associated with our operations and our ordinary indemnification obligations. The coverage provided by such insurance may not be adequate for all claims we may make or may be contested by our insurance carriers. If our insurance is not adequate or available to pay liabilities associated with our operations, or if we are unable to purchase adequate insurance at reasonable rates in the future, our profitability may be adversely impacted.
Exchange rate fluctuations may affect our results of operations and financial condition.
During 2018, approximately 16% of our revenue and 35% of our operating expenses were denominated in currencies other than the U.S. dollar, particularly the Euro and the British pound. Because a portion of our revenue and expenses are denominated in currencies other than the U.S. dollar and our financial statements are reported in U.S. dollars, changes in non‑U.S. currency exchange rates could significantly affect our results of operations and financial condition.
The revenue and expenses of our non‑U.S. operations are generally denominated in local currencies and translated into U.S. dollars for financial reporting purposes. Accordingly, exchange rate fluctuations will affect the translation of non‑U.S. results into U.S. dollars for purposes of reporting our consolidated results.
We are subject to non‑U.S. currency transaction risk for fluctuations in exchange rates during the period of time between the consummation and cash settlement of a transaction. We earn revenue from our service contracts over a period of several months and, in some cases, over several years. Accordingly, exchange rate fluctuations during this period may affect our profitability with respect to such contracts.
We may limit these risks through exchange rate fluctuation provisions stated in our service contracts, or we may hedge our transaction risk with non‑U.S. currency exchange contracts or options. We have not, however, hedged any of our non‑U.S. currency transaction risk, and we may experience fluctuations in financial results from our operations outside the United States and non‑U.S. currency transaction risk associated with our service contracts.
If we do not keep pace with rapid technological changes, our services may become less competitive or obsolete.
The biopharmaceutical industry generally, and drug development and clinical research more specifically, are subject to rapid technological changes. Our current competitors or other businesses might develop technologies or services that are more effective or commercially attractive than, or render obsolete, our current or future technologies and services. If our competitors introduce superior technologies or services and if we cannot make enhancements to remain competitive, our competitive position would be harmed. If we are unable to compete successfully, we may lose clients or be unable to attract new clients, which could lead to a decrease in our revenue and financial condition.
Our relationships with existing or potential clients who are in competition with each other may adversely impact the degree to which other clients or potential clients use our services, which may adversely affect our results of operations.
The biopharmaceutical industry is highly competitive, with companies each seeking to persuade payors, providers and patients that their drug therapies are more cost‑effective than competing therapies marketed or developed by competing firms. In addition to the adverse competitive interests that biopharmaceutical companies have with each other, these companies also have adverse interests with respect to drug selection and reimbursement with other participants in the healthcare industry, including payors and providers. Biopharmaceutical companies also compete to be first to the market with new drug therapies. We regularly provide services to biopharmaceutical companies that compete with each other, and we sometimes provide services to such clients regarding competing drugs in development. Our existing or future relationships with our biopharmaceutical clients have in the past deterred, and may continue to deter, other biopharmaceutical clients from using our services or, in certain instances, have resulted in our clients seeking to place limits on our ability to serve their competitors and other industry participants. In addition, our further expansion into the broader healthcare market may adversely impact our relationships with biopharmaceutical clients, and such clients may elect not to use our services, reduce the scope of services that we provide to them or seek to place restrictions on our ability to serve clients in the broader healthcare market with interests that are adverse to theirs. Any loss of clients or reductions in the level of revenues from a client could have a material adverse effect on our results of operations, business and prospects.
The biopharmaceutical services industry is fragmented and highly competitive.
The biopharmaceutical services industry is fragmented and highly competitive and if we do not compete successfully, our business will suffer. We often compete for business with other biopharmaceutical services companies, universities, niche providers and discovery and development departments within our clients, some of which are large biopharmaceutical services companies in their own right with greater resources than ours. As part of our business model, we have formed preferred vendor relationships. These relationships generally are not contractual and are subject to change at any time. As a result of these relationships, we may find reduced access to certain potential clients due to preferred vendor arrangements with other competitors. There are few barriers to entry for smaller specialized companies. Because of their size and focus, these companies might compete effectively against larger companies like us, which could have a material adverse impact on our business. Additionally, the industry is highly fragmented, with numerous smaller specialized companies and a handful of
full‑service companies with global capabilities similar to ours. Increased competition has led to price and other forms of competition, which may result in acceptance of less favorable contract terms that could adversely affect our operating results. As a result of competitive pressures, in recent years our industry has experienced consolidation. This trend is likely to produce more competition from the resulting larger companies for both clients and acquisition candidates.
If we are unable to manage our joint ventures and identify, acquire and integrate future acquisitions and joint ventures with our existing business, services and technologies, our business, results of operations and financial condition could be adversely impacted.
We have historically grown our business both organically and through acquisitions, and we anticipate that a portion of our future growth may come from acquiring existing businesses, services or technologies and entering into strategic alliances and joint ventures. The success of any acquisition will depend upon, among other things, our ability to effectively integrate acquired personnel, operations, products and technologies into our business, to obtain regulatory approvals, and to retain the key personnel and clients of our acquired businesses. Failure to successfully integrate any acquired business may result in reduced levels of revenue, earnings or operating efficiency than might have been achieved if we had not acquired such businesses. In addition, any future acquisitions could result in the incurrence of additional debt and related interest expense, contingent liabilities and amortization expenses related to intangible assets, which could have a material adverse effect on our business, financial condition, operating results and cash flow.
The success of any joint venture will involve, among other things, learning about new markets and regulations, ensuring quality controls are adequate and not inadvertently creating competitors. We may be unable to identify suitable acquisition opportunities, properly evaluate the price of such acquisitions or obtain any necessary financing on commercially acceptable terms. We may also spend time and money investigating and negotiating with potential acquisition targets and strategic alliance partners but not complete the transaction. Acquisitions involve other risks, including, among others, the assumption of additional liabilities and expenses, difficulties and expenses in connection with integrating the acquired companies and achieving the expected benefits, issuances of potentially dilutive securities or debt, loss of key employees of the acquired companies, transaction costs, diversion of management’s attention from other business concerns and, with respect to the acquisition of non‑U.S. companies, the inability to overcome differences in non‑U.S. business practices, language and customs. Our failure to identify potential acquisitions, complete targeted acquisitions and integrate completed acquisitions or identify and manage strategic alliances or joint ventures could have a material adverse effect on our business, financial condition and results of operations.
We have a significant amount of goodwill and intangible assets on our balance sheet, and our results of operations may be adversely affected if we fail to realize the full value of our goodwill and intangible assets.
Our balance sheet reflects goodwill and intangibles assets of $1,494.8 million and $704.4 million, respectively, as of December 31, 2018. Collectively, goodwill and intangibles assets represented 69% of our total assets as of December 31, 2018. In accordance with generally accepted accounting principles, or GAAP, goodwill and indefinite-lived intangible assets are not amortized, but are subject to a periodic impairment evaluation. We assess the realizability of our indefinite-lived intangible assets and goodwill annually and conduct an interim evaluation whenever events or changes in circumstances, such as operating losses or a significant decline in earnings associated with the acquired business or asset, indicate that these assets may be impaired. In addition, we review long‑lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets might not be recoverable. If indicators of impairment are present, we evaluate the carrying value in relation to estimates of future undiscounted cash flows. Our ability to realize the value of the goodwill and intangible assets will depend on the future cash flows of the businesses we have acquired, which in turn depend in part on how well we have integrated these businesses into our own business. The carrying amount of the goodwill could be impaired if there is a downturn in our business or our industry or other factors that affect the fair value of our business, in which case a charge to earnings would become necessary. If we are not able to realize the value of the goodwill and intangible assets, we may be required to incur material charges relating to the impairment of those assets. Such impairment charges could materially and adversely affect our operating results and financial condition. See Note 2 to our consolidated financial statements included elsewhere in this Annual Report on Form 10‑K for a further discussion of our goodwill and intangible asset impairment testing.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
Under Sections 382 and 383 of the U.S. Internal Revenue Code, if a corporation undergoes an “ownership change” (generally defined as a greater than 50 percentage point change, by value, in the aggregate stock ownership of certain stockholders over a three‑year period), the corporation’s ability to use its pre‑change net operating loss carryforwards to offset its future taxable income and other pre‑change tax attributes may be limited. We have experienced at least one ownership change in the past. We may experience additional ownership changes in the future. In addition, future changes in our stock ownership (including future sales by KKR) could result in additional ownership changes. Any such ownership changes could limit our ability to use our net operating loss carryforwards to offset any future taxable income and other tax attributes. State and non‑U.S. tax laws may also impose limitations on our ability to utilize net operating loss carryforwards and other tax attributes.
Our business could be harmed if we are unable to manage our growth effectively.
We believe that sustained growth places a strain on operational, human and financial resources. To manage our growth, we must continue to improve our operating and administrative systems and to attract and retain qualified management, professional, scientific and technical operating personnel. We believe that maintaining and enhancing both our systems and personnel at reasonable cost are instrumental to our success. We cannot assure you that we will be able to enhance our current technology or obtain new technology that will enable our systems to keep pace with industry developments and the sophisticated needs of our clients. The nature and pace of our growth introduces risks associated with quality control and client dissatisfaction due to delays in performance or other problems. In addition, non‑U.S. operations involve the additional risks of assimilating differences in non‑U.S. business practices, hiring and retaining qualified personnel and overcoming language barriers. Failure to manage growth effectively could have an adverse effect on our business.
Our operations involve the use and disposal of hazardous substances and waste which can give rise to liability that could adversely impact our financial condition.
We conduct activities that have involved, and may continue to involve, the controlled use of hazardous materials and the creation of hazardous substances, including medical waste and other highly regulated substances. Although we believe that our safety procedures for handling the disposal of such materials generally comply with the standards prescribed by non‑U.S., state and federal laws and regulations, our operations nevertheless pose the risk of accidental contamination or injury caused by the release of these materials and/or the creation of hazardous substances, including medical waste and other highly regulated substances. In the event of such an accident, we could be held liable for damages and cleanup costs which, to the extent not covered by existing insurance or indemnification, could harm our business. In addition, other adverse effects could result from such liability, including reputational damage resulting in the loss of additional business from certain clients.
We rely on third parties to provide certain data and other information to us. Our suppliers or providers might increase our cost to obtain, restrict our use of or refuse to license data, which could lead to our inability to access certain data or provide certain services and, as a result, materially and adversely affect our operating results and financial condition.
Our services are derived from, or include, the use of data we collect from third parties. We have several data suppliers that provide us a broad and diverse scope of information that we collect, use in our business and sell.
We generally enter into long-term contractual arrangements with many of our data suppliers. At the time we enter into a new data supply contract or renew an existing contract, suppliers may increase our cost to obtain and use the data provided by such supplier, increase restrictions on our ability to use or sell such data, or altogether refuse to license the data to us. Also, our data suppliers may fail to meet or adhere to our quality control standards or fail to deliver the data to us. Although no single supplier is material to our business, if suppliers that collectively provide a significant amount of the data we receive or use were to increase our costs to obtain or use such data, further restrict our access to or use of such data, fail to meet or adhere to our quality control standards, refuse to provide data or fail to deliver data to us, our ability to provide data-dependent services to our clients may be adversely impacted, which could have a material adverse effect on our business, results of operations, financial condition or cash flow.
We rely on third parties for important products and services, services and licenses to certain technology and intellectual property rights and we might not be able to continue to obtain such products, services and licenses.
We depend on certain third parties to provide us with products and services critical to our business. Such services include, among others, suppliers of drugs for patients participating in trials, suppliers of kits for use in our laboratories, suppliers of reagents for use in our testing equipment and providers of maintenance services for our equipment. The failure of
any of these third parties to adequately provide the required products or services, or to do so in compliance with applicable regulatory requirements, could have a material adverse effect on our business.
Some of our services rely on intellectual property, technology and other similar property owned and/or controlled by third parties. Our licenses to this property and technology could terminate or expire and we might not be able to replace these licenses in a timely manner. Also, we might not be able to renew these licenses on similar terms and conditions. Failure to renew these licenses, or renewals of these licenses on less advantageous terms, could have a material adverse effect on our business, results of operations, financial condition or cash flow.
We have only a limited ability to protect our intellectual property rights, and these rights are important to our success.
Our success depends, in part, upon our ability to develop, use and protect our proprietary methodologies, analytics, systems, technologies and other intellectual property. We rely upon a combination of trade secrets, confidentiality policies, nondisclosure, invention assignment and other contractual arrangements, and copyright, trademark and trade secret laws, to protect our intellectual property rights. Existing laws of the various countries in which we provide services or solutions offer only limited protection of our intellectual property rights, and the protection in some countries may be very limited. The laws of some foreign countries, especially developing countries, do not protect intellectual property rights to the same extent as federal and state laws in the United States. Additionally, both in developed and developing countries, these laws are subject to change at any time and certain agreements may not be fully enforceable, which could further restrict our ability to protect our innovations.
Our intellectual property rights may not prevent competitors from independently developing services similar to, or duplicative of, ours. For instance, unauthorized parties may attempt to copy or reverse engineer certain aspects of our products that we consider proprietary or our proprietary information may otherwise become known or may be independently developed by our competitors or other third parties. Further, the steps we take in this regard might not be adequate to prevent or deter infringement or other misappropriation of our intellectual property by competitors, former employees or other third parties, and we might not be able to detect unauthorized use of, or take appropriate and timely steps to enforce, our intellectual property rights. Enforcing our rights might also require considerable time, money and oversight, and we may not be successful in enforcing our rights. It may not be possible to enforce intellectual property rights effectively in some countries at all or to the same extent as in the United States and other countries, and many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions.
Depending on the circumstances, we might need to grant a specific client greater rights in intellectual property developed in connection with a contract than we otherwise generally do. In certain situations, we might forgo all rights to the use of intellectual property we create, which would limit our ability to reuse that intellectual property for other clients. Any limitation on our ability to provide a service or solution could cause us to lose revenue generating opportunities and require us to incur additional expenses to develop or license new or modified solutions for future projects.
Our business has experienced substantial expansion and contraction in the past and we might not properly manage any expansion or contraction in the future.
Rapid expansion or contraction, both of which we have experienced, could strain our operational, human and financial resources and facilities. If we fail to properly manage any changes, our expenses might grow more than revenue and our results of operations and financial condition might be negatively affected. In order to manage expansion or contraction, we must, among other things, do the following:
| |
• | continue to improve our operating, administrative and information systems; |
| |
• | accurately predict our future personnel, resource and facility needs to meet our commitments; |
| |
• | track the progress of ongoing projects; and |
| |
• | attract and retain qualified management, sales, professional, scientific and technical operating personnel. |
In addition, we have numerous business groups, subsidiaries and divisions. If we cannot properly manage these groups, subsidiaries or divisions, it will disrupt our operations. We also face additional risks in expanding our non‑U.S. operations. Specifically, we might find it difficult to:
| |
• | assimilate differences in non‑U.S. business practices and regulations; |
| |
• | properly integrate systems and operating procedures; |
| |
• | hire and retain qualified personnel; and |
| |
• | overcome language and cultural barriers. |
Outsourcing trends in the biopharmaceutical industry and changes in aggregate spending and R&D budgets could adversely affect our operating results and growth rate.
We provide services to the biopharmaceutical industry and our revenues depend on the outsourcing trends and R&D expenditures in the industry. Economic factors and industry trends that affect biopharmaceutical companies affect our business. Biopharmaceutical companies continue to seek long‑term strategic collaborations with global CROs with favorable pricing terms. Competition for these collaborations is intense and we may decide to forego an opportunity or we may not be selected, in which case a competitor may enter into the collaboration and our business with the client, if any, may be limited. In addition, if the biopharmaceutical industry reduces its outsourcing of clinical trials or such outsourcing fails to grow at projected rates, our operations and financial condition could be materially and adversely affected. All of these events could adversely affect our business, results of operations or financial condition.
Consolidation in the biopharmaceutical industry could lead to a reduction in our revenues.
Several large biopharmaceutical companies have completed mergers and acquisitions that will consolidate the outsourcing trends and R&D expenditures into fewer companies. Large pharmaceutical companies represent a significant portion of our customer base. The pharmaceutical industry is currently undergoing a period of increased merger activity. As a result of this and future consolidations, our client diversity may decrease and our business may be adversely affected. In addition, consolidation and other factors in the biopharmaceutical industry, may slow decision making by our clients or result in the delay or cancellation of clinical trials.
We may be affected by healthcare reform and potential additional reforms.
Numerous government bodies are considering or have adopted various healthcare reforms and may undertake, or are in the process of undertaking, efforts to control growing healthcare costs through legislation, regulation and voluntary agreements with medical care providers and biopharmaceutical companies. We are uncertain as to the effects of these reforms on our business and are unable to predict what legislative proposals, if any, will be adopted in the future. If regulatory cost containment efforts limit the profitability of new drugs, our clients may reduce their R&D spending, which could reduce the business they outsource to us. Similarly, if regulatory requirements are relaxed or simplified drug approval procedures are adopted, the demand for our services could decrease.
Government bodies may also adopt healthcare legislation or regulations that are more burdensome than existing regulations. For example, product safety concerns and recommendations by the Drug Safety Oversight Board could change the regulatory environment for drug products, and new or heightened regulatory requirements may increase our expenses or limit our ability to offer some of our services. Additionally, new or heightened regulatory requirements may have a negative impact on the ability of our clients to conduct industry-sponsored clinical trials, which could reduce the need for our services. Furthermore, a relaxation of the scope of regulatory requirements, such as the introduction of simplified marketing applications for pharmaceuticals and biologics, could decrease the business opportunities available to us.
Actions by regulatory authorities or clients to limit the scope of or withdraw an approved drug from the market could result in a loss of revenue.
Government regulators have the authority, after approving a drug, to limit its indication for use by requiring additional labeled warnings or to withdraw the drug’s approval for its approved indication based on safety concerns. Similarly, clients may act to voluntarily limit the availability of approved drugs or withdraw them from the market after we begin our work. If we are providing services to clients for drugs that are limited or withdrawn, we may be required to narrow the scope of or terminate our services with respect to such drugs, which would prevent us from earning the full amount of service revenue anticipated under the related service contracts.
Current and proposed laws and regulations regarding the protection of personal data could result in increased risks of liability or increased cost to us or could limit our service offerings.
The confidentiality, collection, use and disclosure of personal data, including clinical trial patient‑specific information, are subject to governmental regulation generally in the country in which the personal data was collected or used. For example, U.S. federal regulations under HIPAA generally require individuals’ written authorization, in addition to any required informed consent, before PHI may be used for research and such regulations specify standards for de‑identifications and for limited data
sets. We may also be subject to applicable state privacy and security laws and regulations in states in which we operate. We are both directly and indirectly affected by the privacy provisions surrounding individual authorizations because many investigators with whom we are involved in clinical trials are directly subject to them as a HIPAA “covered entity” and because we obtain PHI from third parties that are subject to such regulations. Because of amendments to the HIPAA data security and privacy rules, there are some instances where we may be a HIPAA “business associate” of a “covered entity,” meaning that we may be directly liable for any breaches in PHI and other HIPAA violations. These amendments may subject us to HIPAA’s enforcement scheme, which, as amended, can result in up to $1.5 million in annual civil penalties for each HIPAA violation.
In the EU and other jurisdictions, personal data includes any information that relates to an identified or identifiable natural person with health information carrying additional obligations, including obtaining the explicit consent from the individual for collection, use or disclosure of the information. In addition, we are subject to laws and regulations with respect to cross‑border transfers of such data out of certain jurisdictions in which we operate, including the EU. If we are unable to transfer data between and among countries and regions in which we operate, it could affect the manner in which we provide our services or adversely affect our financial results. The United States, the EU and its member states, and other countries where we have operations continue to issue new privacy and data protection rules and regulations that relate to personal data and health information. For instance, the EU GDPR, which took effect in 2018, imposes more stringent data protection requirements and will provide for greater penalties for noncompliance. With respect to our operations in Europe, the EU GDPR may increase our responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with the GDPR. Federal, state and non‑U.S. governments may propose or have adopted additional legislation governing the collection, possession, use or dissemination of personal data, such as personal health information, and personal financial data as well as security breach notification rules for loss, theft or unauthorized use of such data that results in significant harm to individuals.
Failure to comply with these data protection and privacy regulations and rules in various jurisdictions, or to resolve any serious privacy or security complaints, could subject us to regulatory sanctions, criminal prosecution or civil liability. Additional legislation or regulation of this type might, among other things, require us to implement new security measures and processes or bring within the legislation or regulation de‑identified health or other personal data, each of which may require substantial expenditures or limit our ability to offer some of our services. Additionally, if we violate applicable laws, regulations or duties relating to the use, privacy or security of personal data, we could be subject to civil liability or criminal prosecution, be forced to alter our business practices and suffer reputational harm.
The biopharmaceutical industry has a history of patent and other intellectual property litigation, and we might be involved in costly intellectual property lawsuits.
The biopharmaceutical industry has a history of intellectual property litigation, and these lawsuits will likely continue in the future. Accordingly, we may face patent infringement suits by companies that have patents for similar business processes or other suits alleging infringement of their intellectual property rights. Legal proceedings relating to intellectual property could be expensive, take significant time and divert management’s attention from other business concerns, regardless of the outcome of the litigation. If we do not prevail in an infringement lawsuit brought against us, we might have to pay substantial damages, and we could be required to stop the infringing activity or obtain a license to use technology on unfavorable terms.
Circumstances beyond our control could cause the CRO industry to suffer reputational or other harm that could result in an industry‑wide reduction in demand for CRO services, which could harm our business.
Demand for our services may be affected by perceptions of our clients regarding the CRO industry as a whole. For example, other CROs could engage in conduct that could render our clients less willing to do business with us or any CRO. Although to date no event has occurred causing material industry‑wide reputational harm, one or more CROs could engage in or fail to detect malfeasance, such as inadequately monitoring sites, producing inaccurate databases or analysis, falsifying patient records, and performing incomplete lab work, or take other actions that would reduce the confidence of our clients in the CRO industry. As a result, the willingness of biopharmaceutical companies to outsource R&D services to CROs could diminish and our business could thus be harmed materially by events outside our control.
Our substantial indebtedness could adversely affect our financial condition and prevent us from fulfilling our debt obligations and may otherwise restrict our activities.
As of December 31, 2018, we had total indebtedness of $1,086.5 million, which consisted of: $170.0 million principal amount of variable rate accounts receivable financing due in 2021, $916.5 million principal amount of variable rate first lien term loans due in 2021, or the 2016 First Lien Term Loan, and no borrowings under our revolving line of credit, or the 2016
Revolver. The 2016 First Lien Term Loan and 2016 Revolver, which were amended in September 2017 and December 2017, are collectively known as the 2016 Credit Facilities.
Specifically, our high level of debt could have important consequences to our business and financial condition, including:
| |
• | making it more difficult for us to satisfy our obligations with respect to our debt; |
| |
• | limiting our ability to obtain additional financing to fund future working capital, capital expenditures, investments or acquisitions or other general corporate requirements; |
| |
• | requiring a substantial portion of our cash flows to be dedicated to debt service payments instead of other purposes, thereby reducing the amount of cash flow available for working capital, capital expenditures, investments or acquisitions and other general corporate purposes; |
| |
• | increasing our vulnerability to adverse changes in general economic, industry and competitive conditions; |
| |
• | exposing us to the risk of increased interest rates as certain of our borrowings, including borrowings under the 2016 Credit Facilities and accounts receivable financing agreement, are at variable rates of interest; |
| |
• | limiting our flexibility in planning for and reacting to changes in the industry in which we compete; |
| |
• | placing us at a disadvantage compared to other, less leveraged competitors; and |
| |
• | increasing our cost of borrowing. |
Despite our level of indebtedness, we may incur more debt and undertake additional obligations. Incurring such debt or undertaking such additional obligations could further exacerbate the risks to our financial condition.
Although the credit agreement governing the 2016 Credit Facilities, as amended, contains restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of qualifications and exceptions, and the indebtedness incurred in compliance with these restrictions could increase. To the extent new debt is added to our current debt levels, the risks to our financial condition would increase.
If we do not comply with the covenants in our financing agreements, we may not have the funds necessary to pay all of our indebtedness that could become due.
The credit agreement governing the 2016 Credit Facilities and the accounts receivable financing agreement, as amended, require us to comply with certain covenants. In particular, our credit agreement prohibits us from incurring any additional indebtedness, except in specified circumstances, or amending the terms of agreements relating to certain existing junior indebtedness, if any, in a manner materially adverse to the lenders under our credit agreement without their respective approval. Further, our credit agreement and the accounts receivable financing agreement contain customary covenants, including covenants that restrict our ability to acquire and dispose of assets, engage in mergers or reorganizations, pay dividends or make investments. A violation of any of these covenants could cause an event of default under our financing agreements.
If we default on our financing agreements, all outstanding amounts could become immediately due and payable. If any of the holders of our indebtedness accelerate the repayment of such indebtedness, there can be no assurance that we will have sufficient assets to repay our indebtedness. If we were unable to repay those amounts, the holders of our secured indebtedness could proceed against the collateral granted to them to secure that indebtedness. Any acceleration of amounts due or the substantial exercise by the lenders of their rights under applicable security documents would likely have a material adverse effect on us.
We may not be able to generate sufficient cash to service all of our indebtedness, and may be forced to take other actions to satisfy our obligations under our indebtedness that may not be successful.
Our ability to satisfy our debt obligations will depend upon, among other things:
| |
• | our future financial and operating performance, which will be affected by prevailing economic conditions and financial, business, regulatory and other factors, many of which are beyond our control; and |
| |
• | the future availability of borrowings under our 2016 Credit Facilities, which depends on, among other things, our compliance with the covenants in those facilities. |
It cannot be assured that our business will generate sufficient cash flow from operations, or that future borrowings will be available under our 2016 Credit Facilities or otherwise, in an amount sufficient to fund our liquidity needs.
If our cash flows and capital resources are insufficient to service our indebtedness, we may be forced to reduce or delay capital expenditures, sell assets, seek additional capital or restructure or refinance our indebtedness. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. In addition, the terms of existing or future debt agreements, may restrict us from adopting some of these alternatives. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. We may not be able to consummate those dispositions for fair market value or at all and any proceeds that we could realize from any such dispositions may not be adequate to meet our debt service obligations then due.
Interest rate fluctuations may affect our results of operations and financial condition.
Because all of our debt is variable‑rate debt, fluctuations in interest rates could have a material effect on our business. We currently utilize derivative financial instruments such as interest rate swaps to hedge our exposure to interest rate fluctuations, but such instruments may not be effective in reducing our exposure to interest fluctuations, and we may discontinue utilizing them at any time. As a result, we may incur higher interest costs if and when interest rates increase. These higher interest costs could have a material adverse impact on our financial condition and the levels of cash we maintain for working capital.
The interest rates of our term loans are priced using a spread over LIBOR.
LIBOR, the London Interbank Offered Rate, is the basic rate of interest used in lending between banks on the London interbank market and is widely used as a reference for setting the interest rate on loans globally. We typically use LIBOR as a reference rate in our term loans such that the interest due to our creditors pursuant to a term loan extended to us is calculated using LIBOR. Most of our term loan agreements contain a stated minimum value for LIBOR.
On July 27, 2017, the United Kingdom’s Financial Conduct Authority, which regulates LIBOR, announced that it intends to phase out LIBOR by the end of 2021. It is unclear if at that time whether or not LIBOR will cease to exist or if new methods of calculating LIBOR will be established such that it continues to exist after 2021. The U.S. Federal Reserve, in conjunction with the Alternative Reference Rates Committee, a steering committee comprised of large U.S. financial institutions, is considering replacing U.S. dollar LIBOR with a new index calculated by short-term repurchase agreements, backed by Treasury securities (“SOFR”). SOFR is observed and backward-looking, which stands in contrast with LIBOR under the current methodology, which is an estimated forward-looking rate and relies, to some degree, on the expert judgment of submitting panel members. Given that SOFR is a secured rate backed by government securities, it will be a rate that does not take into account bank credit risk (as is the case with LIBOR). Whether or not SOFR attains market traction as a LIBOR replacement tool remains in question. As such, the future of LIBOR at this time is uncertain. At this time, due to a lack of consensus existing as to what rate or rates may become accepted alternatives to LIBOR, it is impossible to predict the effect of any such alternatives on our liquidity. However, if LIBOR ceases to exist, we may need to renegotiate our credit agreements that utilize LIBOR as a factor in determining the interest rate to replace LIBOR with the new standard that is established. Additionally, these changes may have an adverse impact on the value of any LIBOR-based marketable securities, loans and derivatives that are included in our financial assets and liabilities.
KKR continues to have influence over us, including some control over decisions that require the approval of stockholders, which could limit your ability to influence the outcome of matters submitted to stockholders for a vote.
KKR beneficially owned approximately 10.2% of our common stock as of December 31, 2018. Even though KKR does not control a majority of our outstanding voting power, two directors previously nominated by KKR remain on our board of directors and KKR may nominate up to 10% of our board provided it maintains ownership over 5% of our outstanding voting power. As such, KKR has the ability to exercise some control over certain corporate actions that require or may be taken by board approval, including major corporate transactions, changes in the size of our board, the amendment of our bylaws and other changes to corporate governance. Additionally, as a stockholder, KKR may be able to exercise some control over matters requiring stockholder approval, including :
| |
• | the election and removal of directors and the size of our board of directors; |
| |
• | any amendment of our certificate of incorporation or bylaws; or |
| |
• | the approval of mergers and other significant corporate transactions, including a sale of substantially all of our assets. |
Provisions of our corporate governance documents and Delaware law could make any change in our board of directors or in control of our company more difficult.
Our amended and restated certificate of incorporation, our amended and restated bylaws and Delaware law contain provisions, such as provisions authorizing, without a vote of stockholders, the issuance of one or more series of preferred stock, that could make it difficult or expensive for a third party to pursue a tender offer, change in control or takeover attempt that is opposed by our management and board of directors even if such a transaction would be beneficial to our stockholders. We also have a staggered board of directors that could make it more difficult for stockholders to change the composition of our board of directors in any one year. These anti‑takeover provisions could substantially impede the ability of public stockholders to change our management or board of directors.
Our operating results and share price may be volatile, which could cause the fair value of our stockholders’ investments to decline.
Securities markets worldwide have experienced, and are likely to continue to experience, significant price and volume fluctuations. This market volatility, as well as general economic, market or political conditions, could subject the market price of our shares to wide price fluctuations regardless of our operating performance. Our operating results and the trading price of our shares may fluctuate in response to various factors, including:
| |
• | market conditions in the broader stock market; |
| |
• | actual or anticipated fluctuations in our quarterly financial and operating results; |
| |
• | introduction of new products or services by us or our competitors; |
| |
• | the public’s reaction to our press releases, our other public announcements and our filings with the SEC; |
| |
• | changes in, or failure to meet, earnings estimates or recommendations by research analysts who track our common stock or the stock of other companies in our industries; |
| |
• | strategic actions by us, our customers or our competitors, such as acquisitions or restructurings; |
| |
• | changes in accounting standards, policies, guidance, interpretations or principles; |
| |
• | issuance of new or changed securities analysts’ reports or recommendations or termination of coverage of our common stock by securities analysts; |
| |
• | sales, or anticipated sales, of large blocks of our stock; |
| |
• | the granting or exercise of employee stock options; |
| |
• | volume of trading in our common stock; |
| |
• | additions or departures of key personnel; |
| |
• | regulatory or political developments; |
| |
• | litigation and governmental investigations; |
| |
• | changing economic conditions; |
| |
• | defaults on our indebtedness; and |
| |
• | exchange rate fluctuations. |
These and other factors, many of which are beyond our control, may cause our operating results and the market price and demand for our shares to fluctuate substantially. While we believe that operating results for any particular quarter are not necessarily a meaningful indication of future results, fluctuations in our quarterly operating results could limit or prevent investors from readily selling their shares and may otherwise negatively affect the market price and liquidity of our shares. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management from our business, which could significantly harm our profitability and reputation.
A significant portion of our total outstanding shares may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of our common stock into the public market could occur at any time. We have 65,394,526 outstanding shares of common stock as of December 31, 2018. Certain of our stockholders have demand registration rights and “piggyback” registration rights with respect to future registered offerings of our common stock. KKR and other stockholders, who collectively owned 10.2% of our common stock as of December 31, 2018, may sell shares of our common stock. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. We also registered all shares of common stock that we may issue under our equity compensation plans and they can be freely sold in the public market upon issuance, subject to restrictions on transfer contained in management stockholders agreements entered into between certain recipients of equity compensation and KKR and restrictions on sales by our affiliates under Rule 144 under the Securities Act. As restrictions on transfer end, the market price of our stock could decline if the holders of currently restricted shares sell them or are perceived by the market as intending to sell them.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We lease a facility for our corporate headquarters in Raleigh, North Carolina. We also lease other offices in North America, Europe, Africa, Latin America, Australia and Asia. In 2018, our total rental expense for our facilities and offices was approximately $39.6 million. We do not own any real estate. We believe that our properties, taken as a whole, are in good operating condition and are suitable for our business operations.
Item 3. Legal Proceedings
We are currently involved, as we are from time to time, in legal proceedings that arise in the ordinary course of our business. We believe that we have adequately accrued for these liabilities and that there is no other litigation pending that could materially harm our results of operations and financial condition. See "Contingent Liabilities" under Note 2 and Note 13 to our consolidated financial statements included elsewhere in this Annual Report on Form 10‑K for a further discussion of our current legal proceedings.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information for Common Stock
Our common stock trades on the NASDAQ under the symbol “PRAH.”
Holders of Record
On February 22, 2019, we had approximately 123 common stockholders of record. This number does not include beneficial owners for whom shares are held by nominees in street name.
Recent Sales of Unregistered Securities
There were no unregistered sales of equity securities in 2018 that have not been previously reported.
Purchases of Equity Securities by the Issuer
None.
Stock Performance Graph
This performance graph shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or incorporated by reference into any filing of PRA Health Sciences, Inc.
The following graph shows a comparison from November 13, 2014 (the date our common stock commenced trading on the NASDAQ) through December 31, 2018 of the cumulative total return for our common stock, the Nasdaq Composite Index and the Nasdaq Health Care Index.
The graph assumes that $100 was invested at the market close on November 13, 2014 in the common stock of PRA Health Sciences, Inc., the Nasdaq Composite Index and the Nasdaq Health Care Index, and assumes reinvestments of dividends, if any. The stock price performance of the following graph is not necessarily indicative of future stock price performance.
Item 6. Selected Financial Data
The following tables set forth, for the periods and at the dates indicated, our selected historical consolidated financial data. We have derived the selected consolidated financial data for the years ended December 31, 2016, 2017 and 2018, and as of December 31, 2017 and 2018, from our audited consolidated financial statements appearing elsewhere in this Annual Report on Form 10‑K. We have derived the selected consolidated financial data for the years ended December 31, 2014 and 2015, and as of December 31, 2014, 2015 and 2016 from our consolidated financial statements not appearing elsewhere in this Annual Report on Form 10‑K. Our historical results are not necessarily indicative of the results we may achieve in any future period.
Historical results are not necessarily indicative of the results to be expected in the future.
You should read the following information together with the more detailed information contained in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the accompanying notes appearing elsewhere in this Annual Report on Form 10‑K.
|
| | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
(in thousands, except per share data) | 2014 | | 2015 | | 2016 | | 2017 | | 2018 |
Consolidated statement of operations data: | | | | | | | | | |
Revenue: | | | | | | | | | |
Service revenue (1) | $ | 1,266,596 |
| | $ | 1,375,847 |
| | | | | | |
Reimbursement revenue (1) | 192,990 |
| | 238,036 |
| | | | | | |
Total revenue (1) | 1,459,586 |
| | 1,613,883 |
| | $ | 1,811,711 |
| | $ | 2,259,389 |
| | $ | 2,871,922 |
|
Operating expenses: | | | | | | | | | |
Direct costs (exclusive of depreciation and amortization) | 859,218 |
| | 886,528 |
| | 1,032,688 |
| | 1,283,868 |
| | 1,500,226 |
|
Reimbursable out-of-pocket costs | 192,990 |
| | 238,036 |
| | 231,688 |
| | 311,015 |
| | 308,291 |
|
Reimbursable investigator fees (1) | — |
| | — |
| | — |
| | — |
| | 262,114 |
|
Selling, general and administrative | 253,970 |
| | 246,417 |
| | 269,893 |
| | 321,987 |
| | 371,795 |
|
Transaction-related costs | — |
| | — |
| | 44,834 |
| | 87,709 |
| | 35,817 |
|
Depreciation and amortization | 96,564 |
| | 77,952 |
| | 69,506 |
| | 78,227 |
| | 112,247 |
|
Loss on disposal of fixed assets | 5 |
| | 652 |
| | 753 |
| | 358 |
| | 120 |
|
Income from operations | 56,839 |
| | 164,298 |
| | 162,349 |
| | 176,225 |
| | 281,312 |
|
Interest expense, net | (81,939 | ) | | (61,747 | ) | | (54,913 | ) | | (46,729 | ) | | (57,399 | ) |
Loss on modification or extinguishment of debt | (25,036 | ) | | — |
| | (38,178 | ) | | (15,023 | ) | | (952 | ) |
Foreign currency gains (losses), net | 10,538 |
| | 14,048 |
| | 24,029 |
| | (39,622 | ) | | (1,043 | ) |
Other (expense) income, net | (2,254 | ) | | (1,434 | ) | | 607 |
| | (304 | ) | | (371 | ) |
(Loss) income before income taxes and equity in (losses) income of unconsolidated joint ventures | (41,852 | ) | | 115,165 |
| | 93,894 |
| | 74,547 |
| | 221,547 |
|
(Benefit from) provision for income taxes | (8,154 | ) | | 30,004 |
| | 28,494 |
| | (12,623 | ) | | 67,232 |
|
(Loss) income before equity in (losses) income of unconsolidated joint ventures | (33,698 | ) | | 85,161 |
| | 65,400 |
| | 87,170 |
| | 154,315 |
|
Equity in (losses) income of unconsolidated joint ventures, net of tax | (2,044 | ) | | (3,396 | ) | | 2,775 |
| | 123 |
| | 143 |
|
Net (loss) income | (35,742 | ) | | 81,765 |
| | 68,175 |
| | 87,293 |
| | 154,458 |
|
Net income attributable to noncontrolling interest | — |
| | — |
| | — |
| | (366 | ) | | (553 | ) |
Net (loss) income attributable to PRA Health Sciences, Inc. | $ | (35,742 | ) | | $ | 81,765 |
| | $ | 68,175 |
| | $ | 86,927 |
| | $ | 153,905 |
|
Net (loss) income per share: | | | | | | | | | |
Basic | $ | (0.83 | ) | | $ | 1.36 |
| | $ | 1.12 |
| | $ | 1.39 |
| | $ | 2.40 |
|
Diluted | $ | (0.83 | ) | | $ | 1.29 |
| | $ | 1.06 |
| | $ | 1.32 |
| | $ | 2.32 |
|
Cash dividends declared per common stockholders | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
|
Weighted average common shares outstanding: | | | | | | | | | |
Basic | 42,897 |
| | 59,965 |
| | 60,759 |
| | 62,437 |
| | 64,123 |
|
Diluted | 42,897 |
| | 63,207 |
| | 64,452 |
| | 65,773 |
| | 66,341 |
|
Cash flow data: | | | | | | | | | |
Net cash provided by operating activities | $ | 34,034 |
| | $ | 152,428 |
| | $ | 160,047 |
| | $ | 220,408 |
| | $ | 329,792 |
|
Net cash used in investing activities | (11,472 | ) | | (71,686 | ) | | (34,614 | ) | | (687,420 | ) | | (55,473 | ) |
Net cash (used in) provided by financing activities | (5,956 | ) | | (42,444 | ) | | (101,595 | ) | | 507,009 |
| | (319,512 | ) |
Other financial data: | | | | | | | | | |
Backlog (at period end) (2) | $ | 2,141,112 |
| | $ | 2,440,123 |
| | $ | 2,934,823 |
| | $ | 3,535,611 |
| | $ | 4,224,225 |
|
Net new business (3) | 1,493,652 |
| | 1,696,635 |
| | 2,076,484 |
| | 2,413,730 |
| | 2,644,791 |
|
| | | | | | | | | |
| | | | | | | | | |
| As of December 31, |
| 2014 | | 2015 | | 2016 | | 2017 | | 2018 |
| | | | | | | | | |
Consolidated balance sheet data | | | | | | | | | |
Cash and cash equivalents | $ | 85,192 |
| | $ | 121,065 |
| | $ | 144,623 |
| | $ | 192,229 |
| | $ | 144,221 |
|
Accounts receivable and unbilled services, net | 338,781 |
| | 415,077 |
| | 439,053 |
| | 627,003 |
| | 568,099 |
|
Working capital | 22,367 |
| | 43,796 |
| | 60,538 |
| | (94,592 | ) | | (116,519 | ) |
Total assets | 2,214,484 |
| | 2,228,743 |
| | 2,190,391 |
| | 3,358,046 |
| | 3,186,467 |
|
Total long-term debt, net | 924,444 |
| | 889,514 |
| | 797,052 |
| | 1,225,397 |
| | 1,082,384 |
|
Total liabilities | 1,537,669 |
| | 1,526,021 |
| | 1,461,139 |
| | 2,421,565 |
| | 2,135,047 |
|
Total stockholders' equity | 676,815 |
| | 702,722 |
| | 729,252 |
| | 936,481 |
| | 1,051,420 |
|
Total liabilities and stockholders' equity | 2,214,484 |
| | 2,228,743 |
| | 2,190,391 |
| | 3,358,046 |
| | 3,186,467 |
|
| |
(1) | On January 1, 2018, we adopted Accounting Standards Codification, or ASC, Topic 606, "Revenue from Contracts with Customers," or ASC 606, using the modified retrospective method for all contracts that were not completed as of January 1, 2018. Comparative prior period information continues to be accounted for under the accounting standards in effect for the period presented. The revenue captions for the years ended December 31, 2017 and 2016 have been recast to conform with the presentation of a single revenue total in the consolidated statement of operations as opposed to separate line items. Previously, the year ended December 31, 2017 included service revenue of $1,948.4 million and reimbursement revenue of $311.0 million. The year ended December 31, 2016 included service revenue of $1,580.0 million and reimbursement revenue of $231.7 million. |
| |
(2) | Our backlog consists of anticipated service revenue for our Clinical Research segment from new business awards that either have not started or are but have not been completed. Backlog varies from period to period depending upon new business awards and contract increases, cancellations and the amount of service revenue recognized under existing contracts. |
| |
(3) | For our Strategic Solutions offering, the value of new business awards is the anticipated service revenue to be recognized in the corresponding quarter of the next fiscal year. For the remainder of our business, net new business is the value of services awarded during the period from projects under signed contracts, letters of intent and, in some cases, pre‑contract commitments that are supported by written communications, adjusted for contracts that were modified or canceled during the period. New business awards are for our Clinical Research segment. |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our “Selected Financial Data” and the consolidated financial statements and the related notes included elsewhere in “Financial Statements and Supplementary Data.” Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business, includes forward‑looking statements that involve risks and uncertainties. You should read the “Risk Factors” section of this Annual Report on Form 10‑K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward‑looking statements contained in the following discussion and analysis.
Overview
We are one of the world’s leading global CROs, by revenue, providing outsourced clinical development services to the biotechnology and pharmaceutical industries. We believe we are one of a select group of CROs with the expertise and capability to conduct clinical trials across major therapeutic areas on a global basis. Our therapeutic expertise includes areas that are among the largest in pharmaceutical development, and we focus in particular on oncology, immunology, central nervous system inflammation, respiratory, cardiometabolic and infectious diseases. We believe that we further differentiate ourselves from our competitors through our investments in medical informatics and clinical technologies designed to enhance efficiencies, improve study predictability and provide better transparency for our clients throughout their clinical development processes. Our Data Solutions segment allows us to better serve our clients across their entire product lifecycle by (i) improving clinical trial design, recruitment, and execution; (ii) creating real-world data solutions based on the use of medicines by actual patients in normal situations; and (iii) increasing the efficiency of healthcare companies' commercial organizations through enhanced analytics and outsourcing services.
How We Assess the Performance of Our Business
We are managed through two reportable segments, (i) Clinical Research and (ii) Data Solutions. Our chief operating decision maker uses segment profit as the primary measure of each segment's operating results in order to allocate resources and in assessing the Company's performance. In addition to our GAAP financial measures, we review various financial and operational metrics. For our Clinical Research segment we review new business awards, cancellations, and backlog.
Our gross new business awards for the years ended December 31, 2018, 2017 and 2016 were $3,023.6 million, $2,779.8 million and $2,367.1 million, respectively. New business awards arise when a client selects us to execute its trial and is documented by written or electronic correspondence or for our Strategic Solutions offering when the amount of revenue expected to be recognized is measurable. The number of new business awards can vary significantly from year to year, and awards can have terms ranging from several months to several years. For our Strategic Solutions offering, the value of a new business award is the anticipated service revenue to be recognized in the corresponding quarter of the next fiscal year. For the remainder of our business, the value of a new award is the anticipated service revenue over the life of the contract, which does not include reimbursement activity or investigator fees.
In the normal course of business, we experience contract cancellations, which are reflected as cancellations when the client provides us with written or electronic correspondence that the work should cease. During the years ended December 31, 2018, 2017 and 2016 we had $378.8 million, $366.0 million, and $290.6 million, respectively, of cancellations for which we received correspondence from the client. The number of cancellations can vary significantly from year to year. The value of the cancellation is the remaining amount of unrecognized service revenue, less the estimated effort to transition the work back to the client.
Our backlog consists of anticipated service revenue from new business awards that either have not started or are in process but have not been completed. Backlog varies from period to period depending upon new business awards and contract modifications, cancellations, and the amount of service revenue recognized under existing contracts. Our backlog at December 31, 2018, 2017 and 2016 was $4.2 billion, $3.5 billion, and $2.9 billion, respectively.
Industry Trends
ISR estimated in its 2018 Market Report that the size of the worldwide CRO market was approximately $34 billion in 2017 and will grow at a 7.5% CAGR to $49 billion in 2022. This growth will be driven by an increase in the amount of research and development expenditures and higher levels of clinical development outsourcing by biopharmaceutical companies.
Sources of Revenue
Total revenue is comprised of revenue from the provision of our services and revenue from reimbursed expenses, and effective January 1, 2018, also includes reimbursable investigator grants, that are incurred while providing our services. We do not have any material product revenue.
On January 1, 2018, we adopted ASC 606 “Revenue from Contracts with Customers,” using the modified retrospective method for all contracts that were not completed as of January 1, 2018. Thus, in this Item 7 and elsewhere in this report, we report 2018 fiscal year revenue under the modified retrospective method of ASC 606, and continue to report comparative prior period information for the 2017 and 2016 fiscal years under the accounting standards in effect for those periods. See Note 2 to our audited consolidated financial statements found elsewhere in this Annual Report on Form 10-K for additional details regarding our sources of revenue and changes associated with the adoption of ASC 606.
Costs and Expenses
Our costs and expenses are comprised primarily of our direct costs, selling, general and administrative costs, depreciation and amortization and income taxes. In addition, we monitor and measure costs as a percentage of revenue, excluding reimbursement revenue from out-of-pocket costs and investigator fees, rather than total revenue, as we believe this is a more meaningful comparison and better reflects the operations of our business.
Direct Costs (Exclusive of Depreciation and Amortization)
For our Clinical Research segment, direct costs consist primarily of labor‑related charges. They include elements such as salaries, benefits and incentive compensation for our employees. In addition, we utilize staffing agencies to procure primarily part time individuals to perform work on our contracts. Labor-related charges as a percentage of the Clinical Research segment's total direct costs were 96.0%, 96.4%, and 96.6% for the years ended December 31, 2018, 2017 and 2016, respectively. The cost of labor procured through staffing agencies is included in these percentages and represents 4.5%, 5.6%, and 5.1% of the Clinical Research segment's total direct costs for the years ended December 31, 2018, 2017 and 2016, respectively. Our remaining direct costs are items such as travel, meals, postage and freight, patient costs, medical waste and supplies. The total of all these items as a percentage of the Clinical Research segment's total direct costs were 4.0%, 3.6%, and 3.4% for the year ended December 31, 2018, 2017 and 2016, respectively.
For our Data Solutions segment, direct costs consist primarily of data costs. Data costs as a percentage of the Data Solutions segment's total direct costs were 73.0% and 71.0% for the years ended December 31, 2018 and 2017, respectively. Labor-related charges, such as salaries, benefits and incentive compensation for our employees, were 20.3% and 23.0% of the Data Solutions segment's total direct costs for the years ended December 31, 2018 and 2017, respectively. Our remaining direct costs are items such as travel, meals, and supplies and were 6.7% and 6.0% of the Data Solutions segment's total direct costs for the years ended December 31, 2018 and 2017, respectively.
Historically, direct costs have increased with an increase in revenues. The future relationship between direct costs and revenue may vary from historical relationships. Excluding the adoption of ASC 606, direct costs as a percentage of revenue were 65.3%, 65.9%, and 65.4% during the years ended December 31, 2018, 2017, and 2016, respectively. Several factors will cause direct costs to decrease as a percentage of revenue. Deployment of our billable staff in an optimally efficient manner has the greatest impact on our ratio of direct cost to revenue. The most effective deployment of our staff is when they are fully engaged in billable work and are accomplishing contract related activities at a rate that meets or exceeds budgeted targets. We also seek to optimize our efficiency by performing work using the employee with the lowest cost. Generally, the following factors may cause direct costs to increase as a percentage of revenue: our staff are not fully deployed, as is the case when there are unforeseen cancellations or delays, or when our staff are accomplishing tasks at levels of effort that exceed budget, such as rework; as well as pricing pressure from increased competition.
Reimbursable Out-of-Pocket Costs and Reimbursable Investigator Fees
We incur out-of-pocket costs that are reimbursable by our customers. As is customary in our industry, we also routinely enter into separate agreements on behalf of our clients with independent physician investigators in connection with clinical trials. We do not pay independent physician investigators until funds are received from the applicable clients. We include these out-of-pocket costs as reimbursable out-of-pocket expenses and these investigator fees as reimbursable investigator fees in our consolidated condensed statements of operations. Reimbursable costs and investigator fees are not included in our backlog because they are pass-through costs to our clients.
We believe that the fluctuations in reimbursement costs and the associated revenue are not meaningful to our economic performance given that such costs are passed through to the client. The reimbursable costs are included in our measure of progress for our long-term contracts.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist of administration payroll and benefits, marketing expenditures, and overhead costs such as information technology and facilities costs. These expenses also include central overhead costs that are not directly attributable to our operating business and include certain costs related to insurance, professional fees and property.
Transaction-Related Costs
Transaction-related costs consist of expenses incurred with our secondary offerings, transaction-related stock-based compensation awards, revaluations of contingent consideration related to business combinations and other transaction costs, the closing of our accounts receivable financing agreement and the subsequent amendment to the agreement, fees associated with the Incremental Borrowing (defined below), and our refinancing of the 2013 Credit Facilities (defined below).
Loss on Modification or Extinguishment of Debt
The loss on modification or extinguishment of debt during the year ended December 31, 2018 is related to previously capitalized unamortized debt financing costs that were expensed as a result of voluntary debt repayments made during the year. Loss on modification or extinguishment of debt for the year ended December 31, 2017 was associated with the September 2017 incremental borrowing under the 2016 Credit Facilities, or the Incremental Borrowing, redemption of our 9.5% senior notes due 2023, or Senior Notes, and the December 2017 amendment to the 2016 Credit Facilities, or the 2017 Refinancing. Loss on extinguishment of debt for the year ended December 31, 2016 was associated with our cash tender offer on Senior Notes and the refinancing of our variable rate first lien term loan due 2020, or 2013 First Lien Term Loan, and revolving line of credit, or 2013 Revolver, collectively known as the 2013 Credit Facilities.
Depreciation and Amortization
Depreciation represents the depreciation charged on our fixed assets. The charge is recorded on a straight‑line method, based on estimated useful lives of three to seven years for computer hardware and software and five to seven years for furniture and equipment. Leasehold improvements are depreciated over the lesser of the life of the lease term or the useful life of the improvements. Amortization expense consists of amortization recorded on acquisition‑related intangible assets. Customer relationships, backlog, databases and finite‑lived trade names are amortized on an accelerated basis, which coincides with the period of economic benefit we expect to receive. All other finite‑lived intangibles are amortized on a straight‑line basis. In accordance with GAAP, we do not amortize goodwill and indefinite‑lived intangible assets.
Income Taxes
Because we conduct operations on a global basis, our effective tax rate has and will continue to depend upon the geographic distribution of our pre‑tax earnings among several different taxing jurisdictions. Our effective tax rate can also vary based on changes in the tax rates of the different jurisdictions. Our effective tax rate is also impacted by tax credits and the establishment or release of deferred tax asset valuation allowances and tax reserves, as well as significant non‑deductible items such as portions of transaction‑related costs.
Foreign subsidiaries are taxed separately in their respective jurisdictions. We have foreign net operating loss carryforwards in some jurisdictions. The carryforward periods for these losses vary from four years to an indefinite carryforward period depending on the jurisdiction. Our ability to offset future taxable income with the net operating loss carryforwards may be limited in certain instances, including changes in ownership.
Business Combinations
We have completed and will continue to consider strategic business combinations to enhance our capabilities and offerings in certain areas. In September 2017, we acquired Symphony Health, which has enhanced our ability to serve customers throughout the clinical research process with technologies that provide data and analytics. Additionally, in May 2017, we acquired Parallel 6, Inc., or Parallel 6, which has allowed us to offer our customers technologies that provide improved efficiencies by reducing study durations and costs through integrated operational management.
These transactions were accounted for as business combinations and the acquired results of operations are included in our consolidated financial information since the acquisition date.
See Note 4 to our audited consolidated financial statements found elsewhere in this Annual Report on Form 10-K for additional information with respect to these and other smaller acquisitions.
Joint Ventures
In June 2017, we closed on a joint venture transaction with Takeda Pharmaceutical Company Ltd., or Takeda, that enables us to provide clinical trial delivery and pharmacovigilance services as a strategic partner of Takeda Japan. The joint venture was effectuated through the creation of a new legal entity, Takeda PRA Development Center KK, or TDC joint venture. The TDC joint venture is based in Japan and is owned by us (50%) and Takeda (50%).
See Note 3 and Note 4 to our audited consolidated financial statements found elsewhere in this Annual Report on Form 10-K for additional information with respect to the joint ventures.
Exchange Rate Fluctuations
The majority of our foreign operations transact in the Euro, or EUR, or British pound, or GBP. As a result, our revenue and expenses are subject to exchange rate fluctuations with respect to these currencies. We have translated these currencies into U.S. dollars using the following average exchange rates:
|
| | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
U.S. dollars per: | | | | | |
Euro | 1.18 |
| | 1.13 |
| | 1.11 |
|
British pound | 1.33 |
| | 1.29 |
| | 1.35 |
|
Results of Operations
Consolidated Results of Operations for the Year Ended December 31, 2018 Compared to the Year Ended December 31, 2017
|
| | | | | | | | | | | | | | | | |
| | | | Change | | |
| | Year Ended December 31, 2017 | | $ Change | | Adoption of ASC 606 (1) | | Year Ended December 31, 2018 |
(in thousands) | | | | | | | | |
Revenue | | | | | | | | |
Service revenue | | $ | 1,948,374 |
| | $ | 348,475 |
| | $ | — |
| | |
Reimbursement revenue - out-of-pocket costs | | 311,015 |
| | (2,724 | ) | | — |
| | |
Total revenue | | 2,259,389 |
| | 345,751 |
| | 266,782 |
| | $ | 2,871,922 |
|
Operating expenses | | | | | | | | |
Direct costs (exclusive of depreciation and amortization) | | 1,283,868 |
| | 216,358 |
| | — |
| | 1,500,226 |
|
Reimbursable out-of-pocket costs | | 311,015 |
| | (2,724 | ) | | — |
| | 308,291 |
|
Reimbursable investigator fees | | — |
| | — |
| | 262,114 |
| | 262,114 |
|
Selling, general and administrative | | 321,987 |
| | 49,808 |
| | — |
| | 371,795 |
|
Transaction-related costs | | 87,709 |
| | (51,892 | ) | | — |
| | 35,817 |
|
Depreciation and amortization | | 78,227 |
| | 34,020 |
| | — |
| | 112,247 |
|
Loss on disposal of fixed assets | | 358 |
| | (238 | ) | | — |
| | 120 |
|
Income from operations | | 176,225 |
| | 100,419 |
| | 4,668 |
| | 281,312 |
|
Interest expense, net | | (46,729 | ) | | (10,670 | ) | | — |
| | (57,399 | ) |
Loss on modification or extinguishment of debt | | (15,023 | ) | | 14,071 |
| | — |
| | (952 | ) |
Foreign currency losses, net | | (39,622 | ) | | 38,579 |
| | — |
| | (1,043 | ) |
Other expense, net | | (304 | ) | | (67 | ) | | — |
| | (371 | ) |
Income before income taxes and equity in income of unconsolidated joint ventures | | 74,547 |
| | 142,332 |
| | 4,668 |
| | 221,547 |
|
(Benefit from) provision for income taxes | | (12,623 | ) | | 78,438 |
| | 1,417 |
| | 67,232 |
|
Income before equity in income of unconsolidated joint ventures | | 87,170 |
| | 63,894 |
| | 3,251 |
| | 154,315 |
|
Equity in income of unconsolidated joint ventures, net of tax | | 123 |
| | 20 |
| | — |
| | 143 |
|
Net income | | 87,293 |
| | 63,914 |
| | 3,251 |
| | 154,458 |
|
Net income attributable to noncontrolling interest | | (366 | ) | | (187 | ) | | — |
| | (553 | ) |
Net income attributable to PRA Health Sciences, Inc. | | $ | 86,927 |
| | $ | 63,727 |
| | $ | 3,251 |
| | $ | 153,905 |
|
(1) See Note 2, Significant Accounting Policies, to our consolidated financial statements for information about the adoption of ASC 606. |
Revenue increased by $612.5 million, or 27.1%, from $2,259.4 million during the year ended December 31, 2017 to $2,871.9 million during the year ended December 31, 2018. Revenue for the year ended December 31, 2018 includes $266.8 million in reimbursable investigator fees and adjustments to revenue as a result of the adoption of ASC 606. Excluding the impact of the adoption of ASC 606 and reimbursement revenue, revenue increased $348.5 million. Revenue for the year ended December 31, 2018, excluding the impact of the adoption of ASC 606 and reimbursement revenue, benefited from an increase in billable hours, an increase in the effective rate of hours billed on our studies, a favorable impact of $10.1 million from foreign currency exchange rate fluctuations, and an increase of $159.0 million due to the acquisition of Symphony Health, which was completed on September 6, 2017. The growth in revenue and the increase in billable hours were due largely to the increase in our backlog as we entered the year, the type of services we are providing on our active studies, which was driven by the life cycles of projects that were active during the period, the growth in new business awards as a result of higher demand for our services across the industries we serve, more effective sales efforts and the growth in the overall CRO market. The increase in our effective rate of hours billed on our studies is attributable to the contract pricing terms on our current mix of active studies and the mix of clients and services that we provide to those clients.
Direct costs increased by $216.4 million, or 16.9%, from $1,283.9 million during the year ended December 31, 2017 to $1,500.2 million during the year ended December 31, 2018. Salaries and related benefits in our Clinical Research segment
increased $89.3 million as we continue to hire billable staff to ensure appropriate staffing levels for our current studies and future growth and an unfavorable impact of $4.6 million from foreign currency exchange rate fluctuations. The addition of our Data Solutions segment resulted in $113.2 million of incremental direct costs during 2018. Excluding the impact of the adoption of ASC 606 and reimbursement revenue, direct costs as a percentage of revenue decreased from 65.9% during the year ended December 31, 2017 to 65.3% during the year ended December 31, 2018. The decrease in direct costs as a percentage of revenue was primarily due to the increased utilization of our staff.
Reimbursable out-of-pocket costs decreased by $2.7 million from $311.0 million during the year ended December 31, 2017 to $308.3 million during the year ended December 31, 2018. Reimbursable investigator fees were $262.1 million during the year ended December 31, 2018. Reimbursable investigator fees were recorded on a net basis prior to our adoption of ASC 606, and therefore we did not record any reimbursable investigator fees during the year ended December 31, 2017. We believe that the fluctuations in reimbursable costs from period to period are not meaningful to our underlying performance over the full terms of the relevant contracts.
Selling, general and administrative expenses increased by $49.8 million, or 15.5%, from $322.0 million during the year ended December 31, 2017 to $371.8 million during the year ended December 31, 2018. Excluding the impact of the adoption of ASC 606 and reimbursement revenue, selling, general and administrative expenses as a percentage of revenue decreased from 16.5% during the year ended December 31, 2017 to 16.2% during the year ended December 31, 2018. The decrease in selling, general and administrative expenses as a percentage of revenue is primarily related to our continued efforts to effectively leverage our selling and administrative functions.
During the year ended December 31, 2018, we incurred transaction-related expenses of $35.8 million. These costs consist of $32.6 million for changes in the estimated fair value of contingent consideration related to our recent acquisitions, $1.4 million related to Symphony retention bonuses that will be reimbursed by the seller, $0.5 million of of third-party costs incurred in connection with our August 2018 secondary offering, $0.8 million of stock-based compensation expense related to the release of a portion of the transfer restrictions on vested options, and $0.5 million of third-party fees associated with the amendment to our accounts receivable financing agreement. During the year ended December 31, 2017, we incurred transaction-related expenses of $87.7 million. These costs consisted of $75.0 million of contingent consideration related to our recent acquisitions, $6.4 million of fees in connection with the acquisition of Symphony Health, $5.3 million of stock-based compensation expense related to the release of a portion of the transfer restrictions on vested options, and $1.0 million of third-party costs incurred in connection with our August 2017 secondary offering of common stock.
Depreciation and amortization expense increased by $34.0 million, or 43.5%, from $78.2 million during the year ended December 31, 2017 to $112.2 million during the year ended December 31, 2018. The increase in depreciation and amortization expense is primarily due to the continued amortization of our acquired intangibles, which increased as a result of the Symphony Health acquisition in September 2017.
Interest expense, net increased by $10.7 million from $46.7 million during the year ended December 31, 2017 to $57.4 million during the year ended December 31, 2018. Interest expense on borrowings under our 2016 Credit Facilities increased by $17.5 million, primarily due to the Incremental Borrowing to fund the Symphony Health acquisition and interest expense on borrowings under our Accounts Receivable Financing Agreement increased by $1.9 million. This was offset by an $8.9 million decrease associated with repayment of our Senior Notes in 2017.
Loss on modification or extinguishment of debt was $1.0 million during the year ended December 31, 2018 compared to $15.0 million during the year ended December 31, 2017. The loss on modification or extinguishment of debt during the year ended December 31, 2018 is related to previously capitalized unamortized debt financing costs that were expensed as a result of voluntary debt repayments made during the year. The loss on modification or extinguishment of debt during the year ended December 31, 2017 is related to the Incremental Borrowing, the 2017 Refinancing, and the extinguishment of the Senior Notes.
Foreign currency losses, net changed by $38.6 million from $39.6 million during the year ended December 31, 2017 to $1.0 million during the year ended December 31, 2018. Foreign exchange gains and losses are due to fluctuations in the U.S. dollar, gains or losses that arise in connection with the revaluation of short-term inter-company balances between our domestic and international subsidiaries, and gains or losses from foreign currency transactions, such as those resulting from the settlement of third-party accounts receivables and payables denominated in a currency other than the local currency of the entity making the payment. The decrease in foreign currency losses, net during the year ended December 31, 2018 is primarily due to the reclassification of certain intercompany balances that were deemed to be of a long-term investment nature.
Provision for income taxes increased by $79.9 million from a benefit of $12.6 million during the year ended December 31, 2017 to a provision of $67.2 million during the year ended December 31, 2018. Our effective tax rate was 30.3% during the year ended December 31, 2018, and an effective tax benefit rate of 16.9% during the year ended December 31, 2017. The effective tax rate for the year ended December 31, 2018 was primarily attributable to (i) the U.S. tax related to amounts included for Global Intangible Low-taxed Income ("GILTI"), (ii) the U.S. tax related to inclusions of the amounts related to Base Erosion and Anti-abuse Tax (“BEAT”), and (iii) the increase in fair value of the earn-out liability related to the stock acquisition of Symphony Health, which is not deductible for tax but instead increases stock basis for tax purposes. The effective tax rate for the year ended December 31, 2017 was primarily attributable to (i) the benefit realized from the tax deduction of stock awards in excess of the amount recognized in the financial statements per guidance under ASU No. 2016-09, "Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting," (ii) the release of the valuation allowance against the federal net deferred tax assets, and (iii) the decrease in the net U.S. deferred tax liabilities due to the Tax Cuts and Jobs Act enacted December 22, 2017.
Consolidated Results of Operations for the Year Ended December 31, 2017 Compared to the Year Ended December 31, 2016
|
| | | | | | | |
| Year Ended December 31, |
(in thousands) | 2017 | | 2016 |
Revenue | | | |
Service revenue | $ | 1,948,374 |
| | $ | 1,580,023 |
|
Reimbursement revenue | 311,015 |
| | 231,688 |
|
Total revenue | 2,259,389 |
| | 1,811,711 |
|
Operating expenses | | | |
Direct costs (exclusive of depreciation and amortization) | 1,283,868 |
| | 1,032,688 |
|
Reimbursable out-of-pocket costs | 311,015 |
| | 231,688 |
|
Selling, general and administrative | 321,987 |
| | 269,893 |
|
Transaction-related costs | 87,709 |
| | 44,834 |
|
Depreciation and amortization | 78,227 |
| | 69,506 |
|
Loss on disposal of fixed assets | 358 |
| | 753 |
|
Income from operations | 176,225 |
| | 162,349 |
|
Interest expense, net | (46,729 | ) | | (54,913 | ) |
Loss on modification or extinguishment of debt | (15,023 | ) | | (38,178 | ) |
Foreign currency gains, net | (39,622 | ) | | 24,029 |
|
Other income (expense), net | (304 | ) | | 607 |
|
Income before income taxes and equity in income (losses) of unconsolidated joint ventures | 74,547 |
| | 93,894 |
|
Provision for income taxes | (12,623 | ) | | 28,494 |
|
Income before equity in income (losses) of unconsolidated joint ventures | 87,170 |
| | 65,400 |
|
Equity in income (losses) of unconsolidated joint ventures, net of tax | 123 |
| | 2,775 |
|
Net income | 87,293 |
| | 68,175 |
|
Net income attributable to noncontrolling interest | (366 | ) | | — |
|
Net income attributable to PRA Health Sciences, Inc. | $ | 86,927 |
| | $ | 68,175 |
|
Service revenue increased by $368.4 million, or 23.3%, from $1,580.0 million during the year ended December 31, 2016 to $1,948.4 million during the year ended December 31, 2017. Service revenue for the year ended December 31, 2017 benefited from an increase in billable hours and an increase in the effective rate of the hours billed on our studies, an increase of $90.5 million due to the acquisition of Symphony Health, which was completed on September 6, 2017, and by a favorable impact of $7.1 million from foreign currency exchange rate fluctuations. The growth in service revenue and the increase in billable hours were due largely to the increase in our backlog as we entered the year, the type of services we are providing on our active studies, which was driven by the life cycles of projects that were active during the period, the growth in new business awards as a result of higher demand for our services across the industries we serve, and more effective sales efforts and the growth in the overall CRO market. New business awards arise when a client selects us to execute its trial. The number of awards can vary significantly from period to period and our studies have terms ranging from several months to several years. The increase in our effective rate of the hours billed on our studies is attributable to the contract pricing terms on our current mix of active studies and the mix of clients and the services that we provide to those clients.
Direct costs increased by $251.2 million, or 24.3%, from $1,032.7 million during the year ended December 31, 2016 to $1,283.9 million during the year ended December 31, 2017. Salaries and related benefits in our Clinical Research segment increased $184.3 million as we continue to hire billable staff to ensure appropriate staffing levels for our current studies and future growth and an unfavorable impact of $6.4 million from foreign currency exchange rate fluctuations. The addition of our Data Solutions segment resulted in $52.2 million of incremental direct costs during 2017. Direct costs as a percentage of service revenue increased from 65.4% during the year ended December 31, 2016 to 65.9% during the year ended December 31, 2017. The increase in direct costs as a percentage of revenue was primarily due to the aforementioned increase in salaries and related benefits.
Selling, general and administrative expenses increased by $52.1 million, or 19.3%, from $269.9 million during the year ended December 31, 2016 to $322.0 million during the year ended December 31, 2017. Selling, general and administrative expenses as a percentage of service revenue decreased from 17.1% during the year ended December 31, 2016 to 16.5% during the year ended December 31, 2017. The decrease in selling, general and administrative expenses as a percentage of service revenue is primarily related to our continued efforts to effectively leverage our selling and administrative functions.
During the year ended December 31, 2017, we incurred transaction-related expenses of $87.7 million. These costs consist of $6.4 million of fees incurred in connection with the acquisition of Symphony Health, $5.3 million of stock-based compensation expense related to the release of a portion of the transfer restrictions on vested options and $1.0 million of third-party costs incurred in connection with our August 2017 secondary offering. The Company also recognized changes in the fair value of contingent consideration of $75.0 million related to our recent acquisitions. During the year ended December 31, 2016, we incurred transaction-related expenses of $44.8 million. These costs consist of $10.1 million of stock-based compensation expense associated with the release of the transfer restrictions on a portion of shares issuable upon exercise of vested service-based options in connection with the announcement of our March, May, and November 2016 secondary offerings. These costs also include $32.0 million of stock-based compensation expense related to the vesting and release of the transfer restrictions of certain performance-based stock options. In addition, we incurred $2.7 million of third-party fees associated with the secondary offerings and the closing of our accounts receivable financing agreement.
Depreciation and amortization expense increased by $8.7 million, or 12.5%, from $69.5 million during the year ended December 31, 2016 to $78.2 million during the year ended December 31, 2017. Depreciation and amortization expense as a percentage of service revenue was 4.4% during the year ended December 31, 2016 and 4.0% during the year ended December 31, 2017. The decrease in depreciation and amortization expense as a percentage of service revenue is primarily due the continued decline in amortization of our acquired intangibles, which are amortized on an accelerated basis.
Interest expense, net decreased by $8.2 million from $54.9 million during the year ended December 31, 2016 to $46.7 million during the year ended December 31, 2017. The cash tender of our Senior Notes during March 2016 and refinancing of our variable first lien term loans in December 2016 contributed to a 1.2% decrease in the weighted average interest rate and resulted in a $12.5 million reduction in interest expense. The $550.0 million Incremental Borrowing during the year ended December 31, 2017 to fund the Symphony Health acquisition contributed to a $5.9 million increase in interest expense. Additionally, interest expense decreased by $2.3 million due to lower amortization of debt issuance costs, which was partially offset by an increase of $0.9 million related to the amortization of our terminated interest rate swaps and interest expense on our current interest rate swap.
Loss on modification or extinguishment of debt was $15.0 million during the year ended December 31, 2017 compared to $38.2 million during the year ended December 31, 2016. The loss on modification or extinguishment of debt during the year ended December 31, 2017 is related to the Incremental Borrowing, the 2017 Refinancing, and the extinguishment of the Senior Notes. The Incremental Borrowing was to fund the acquisition of Symphony Health and we recognized $3.1 million in fees in loss on modification of debt. The 2017 Refinancing was to reduce the interest rate margin and amend the payment schedule on the 2016 First Lien Term Loan as well as increase the 2016 Revolver's borrowing capacity, which resulted in a $0.6 million loss on modification of debt. The voluntary redemption of the remaining Senior Notes resulted in a $11.3 million loss on extinguishment of debt, which consists of $9.2 million early payment premium and $2.1 million write-off of unamortized debt issuance cost. The $38.2 million loss on extinguishment of debt incurred during the year ended December 31, 2016 was associated with our cash tender offer on our Senior Notes and our refinancing of the 2013 Credit Facilities. The loss of $21.5 million due to our cash tender offer consisted of a $17.4 million early tender premium, a $3.7 million write-off of unamortized debt issuance cost and $0.4 million of fees associated with the transaction. The refinancing of our 2013 Credit Facilities resulted in a $16.7 million loss on extinguishment of debt, which consisted of the write-off of $15.8 million write-off of unamortized debt issuance costs and $0.9 million of fees associated with the transaction.
Foreign currency (losses) gains, net changed by $63.7 million from foreign currency gains of $24.0 million during the year ended December 31, 2016 to foreign currency losses of $39.6 million during the year ended December 31, 2017. The foreign currency gains and losses are due to fluctuations in the U.S. dollar, gains or losses that arise in connection with the revaluation of short-term inter-company balances between our domestic and international subsidiaries, and gains or losses from foreign currency transactions, such as those resulting from the settlement of third-party accounts receivables and payables denominated in a currency other than the local currency of the entity making the payment. During the year ended December 31, 2017, foreign currency losses were primarily due to the U.S. dollar weakening against the EUR, GBP, Canadian dollar, or CAD, and Russian ruble, or RUB by 13.7%, 9.3%, 7.1% and 6.2%, respectively. During the year ended December 31, 2016, the foreign currency gains were primarily a result of the weakening of the GBP against the U.S. dollar by 16.7% following the decision by voters in the United Kingdom, to approve a referendum to exit the European Union, commonly referred to as Brexit, in June 2016.
(Benefit from) provision for income taxes decreased by $41.1 million from a provision of $28.5 million during the year ended December 31, 2016 to a benefit of $12.6 million during the year ended December 31, 2017. Our effective tax benefit rate was 16.9% during the year ended December 31, 2017, and an effective tax rate of 30.3% during the year ended December 31, 2016. The change in the effective tax rate was primarily attributable to (i) the benefit realized from the tax deduction of stock awards in excess of the amount recognized in the financial statements per guidance under ASU No. 2016-09, "Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting", (ii) the release of the valuation allowance against the federal net deferred tax assets, and (iii) the decrease in the net U.S. deferred tax liabilities due to the Tax Cuts and Jobs Act enacted December 22, 2017.
Segment Results of Operations for the Year Ended December 31, 2018 Compared to the Year Ended December 31, 2017
Clinical Research
|
| | | | | | | | | | | | | | | | |
| | | | Change | | |
| | Year Ended December 31, 2017 | | $ Change | | Adoption of ASC 606 (See Note 2) | | Year Ended December 31, 2018 |
(in thousands) | | | | | | | | |
Service revenue | | $ | 1,857,876 |
| | $ | 189,460 |
| | $ | — |
| |
|
Reimbursement revenue - out-of-pocket costs | | 311,015 |
| | (2,724 | ) | | — |
| |
|
Total revenue | | 2,168,891 |
| | 186,736 |
| | 266,782 |
| | 2,622,409 |
|
Segment profit | | $ | 626,186 |
| | $ | 86,347 |
| | $ | 4,668 |
| | $ | 717,201 |
|
Revenue increased by $453.5 million, or 20.9%, from $2,168.9 million during the year ended December 31, 2017 to $2,622.4 million during the year ended December 31, 2018. Revenue for the year ended December 31, 2018 includes $266.8 million in revenue as a result of the adoption of ASC 606. Excluding the impact of the adoption of ASC 606 and reimbursement revenue, revenue increased by $189.5 million the year ended December 31, 2018, benefiting from an increase in billable hours and an increase in the effective rate of hours billed on our studies. The growth in revenue and the increase in billable hours were due largely to the increase in our backlog as we entered the year, the type of services we are providing on our active studies, which was driven by the life cycles of projects that were active during the period, the growth in new business awards as a result of higher demand for our services across the industries we serve, and more effective sales efforts and the growth in the overall CRO market. The increase in our effective rate of the hours billed on our studies is attributable to the contract pricing terms on our current mix of active studies and the mix of clients and services that we provide to those clients.
Segment profit increased by $91.0 million, or 14.5%, from $626.2 million during the year ended December 31, 2017 to $717.2 million during the year ended December 31, 2018 primarily due to an increase in revenue. Excluding the impact of the adoption of ASC 606 and reimbursement revenue, segment profit as a percentage of revenue increased from 33.7% during the year ended December 31, 2017 to 35.0% for the same period in 2018. The increase in segment profit is primarily due to the increased utilization of our staff.
Data Solutions
|
| | | | | | | | | | | | | | | | |
| | | | Change | | |
| | Year Ended December 31, 2017 | | $ Change | | Adoption of ASC 606 (See Note 2) | | Year Ended December 31, 2018 |
(in thousands) | | | | | | | | |
Revenue | | $ | 90,498 |
| | $ | 159,015 |
| | $ | — |
| | $ | 249,513 |
|
Segment profit | | 38,320 |
| | 45,770 |
| | — |
| | 84,090 |
|
The Company acquired Symphony Health on September 6, 2017. The Company recognized $249.5 million of revenue and $165.4 million in direct costs during the year ended December 31, 2018. See Note 4 to our consolidated financial statements found elsewhere in this Annual Report on Form 10-K for additional information about the acquisition.
Segment Results of Operations for the Year Ended December 31, 2017 Compared to the Year Ended December 31, 2016
Clinical Research
|
| | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| 2017 | | 2016 | | 2017 vs. 2016 |
(in thousands) |
Service revenue | $ | 1,857,876 |
| | $ | 1,580,023 |
| | $ | 277,853 |
| | 17.6 | % |
Segment profit | 626,186 |
| | 547,335 |
| | 78,851 |
| | 14.4 | % |
Segment profit % | 33.7 | % | | 34.6 | % | | (0.9 | )% | | |
Service revenue increased by $277.9 million, or 17.6%, from $1,580.0 million during the year ended December 31, 2016 to $1,857.9 million during the year ended December 31, 2017. Service revenue for the year ended December 31, 2017 benefited from an increase in billable hours and an increase in the effective rate of hours billed on our studies. The growth in service revenue and the increase in billable hours were due largely to the increase in our backlog as we entered the year, the type of services we were providing on our active studies, which was driven by the life cycles of projects that were active during the period, the growth in new business awards as a result of higher demand for our services across the industries we serve, and more effective sales efforts and the growth in the overall CRO market. New business awards arise when a client selects us to execute its trial. The number of awards can vary significantly from period to period and our studies have terms ranging from several months to several years. The increase in our effective rate of the hours billed on our studies is attributable to the contract pricing terms on our current mix of active studies and the mix of clients and the services that we provide to those clients.
Segment profit increased by $78.9 million, or 14.4%, from $547.3 million during the year ended December 31, 2016 to $626.2 million during the year ended December 31, 2017 primarily due to an increase in revenue. Segment profit as a percentage of revenue decreased from 34.6% during the year ended December 31, 2016 to 33.7% for the same period in 2017. Segment profit as a percentage of revenue decreased primarily due to an increase in labor-related costs of $184.3 million, as we continued to hire billable staff to ensure appropriate staffing levels for our current studies and our future growth.
Data Solutions
|
| | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| 2017 | | 2016 | | 2017 vs. 2016 |
(in thousands) |
Service revenue | $ | 90,498 |
| | $ | — |
| | $ | 90,498 |
| | n/a |
Segment profit | 38,320 |
| | — |
| | 38,320 |
| | n/a |
Segment profit % | 42.3 | % | | — |
| | n/a |
| | |
The Company acquired Symphony Health on September 6, 2017, in conjunction with the acquisition the Company expanded its reporting segments. The Company recognized $90.5 million of revenue and $52.2 million in direct costs during the period between September 6, 2017 and December 31, 2017 for our Data Solutions segment. See Note 4 to our consolidated financial statements found elsewhere in this Annual Report on Form 10-K for additional information about the acquisition.
Liquidity and Capital Resources
We assess our liquidity in terms of our ability to generate cash to fund our operating, investing and financing activities. Our principal source of liquidity is operating cash flows. As of December 31, 2018, we had approximately $144.2 million of cash and cash equivalents of which $63.2 million was held by our foreign subsidiaries. Our expected primary cash needs on both a short and long‑term basis are for capital expenditures, expansion of services, geographic expansion, debt repayments, and other general corporate purposes. We have historically funded our operations and growth, including acquisitions, with cash flow from operations, borrowings, and issuances of equity securities. We expect to continue expanding our operations through internal growth and strategic acquisitions and investments. We expect these activities will be funded from existing cash, cash flow from operations and, if necessary or appropriate, borrowings under our existing or future credit facilities. Our sources of liquidity could be affected by our dependence on a small number of industries and clients, compliance with regulations, international risks, and personal injury, environmental or other material litigation claims.
Cash Collections
Cash collections from accounts receivable were $2,894.4 million during the year ended December 31, 2018, including $293.6 million of funds received from customers to pay independent physician investigators, or investigators, as compared to $2,495.5 million during the year ended December 31, 2017, including $257.1 million of funds received from customers to pay investigators, $2,074.1 million during the year ended December 31, 2016, including $248.2 million of funds received from customers to pay investigators. The increase in cash collections is related to our increase in revenue, driven by an increase in new business awards and backlog.
Discussion of Cash Flows
Cash Flow from Operating Activities
During the year ended December 31, 2018, net cash provided by operations was $329.8 million, compared to $220.4 million in 2017. Cash provided by operating activities increased over the prior year primarily due to increased cash flows from our operating performance, as well as a decrease in cash outflows from working capital, offset by the portion of acquisition related earn-out payments being classified as an outflow from operating activities. The changes in working capital were driven by changes in our accounts receivable, unbilled services and advanced billings accounts, as a result of an improvement in our days sales outstanding as compared to the prior year.
Cash provided by operating activities increased by $60.4 million during the year ended December 31, 2017 as compared to 2016. The increase in operating cash flow reflects increased cash flows from our operating performance and a slight decrease in cash outflows primarily from working capital. Net income after non-cash adjustments increased $51.5 million as compared to the prior year and outflows from working capital decreased by $8.9 million. The decrease in working capital outflows was primarily driven by positive changes in operating assets and liabilities attributable to the timing and payment of invoices offset by an increase in our days sales outstanding during the year ended December 31, 2017.
Cash Flow from Investing Activities
Net cash used in investing activities was $55.5 million during the year ended December 31, 2018, compared to $687.4 million in 2017. There were no cash outflows from acquisitions during the year ended December 31, 2018 compared to $625.3 million of net cash outflows during the year ended December 31, 2017. Additionally, cash outflows from capital expenditures decreased from $61.3 million during the year ended December 31, 2017 to $55.9 million during the same period in 2018.
Net cash used in investing activities was $687.4 million during the year ended December 31, 2017, compared to $34.6 million for the same period of 2016. The net cash outflows from acquisitions increased from $4.3 million during the year ended December 31, 2016 to $625.3 million during the same period in 2017. Additionally, capital expenditures increased by $28.2 million compared to the prior year, which is partially offset by $3.7 million received from the sale of our ownership stake in the WuXiPRA joint venture during the year ended December 31, 2016.
Cash Flow from Financing Activities
Net cash used in financing activities was $319.5 million during the year ended December 31, 2018 compared to net cash provided by financing activities of $507.0 million for the same period of 2017. During the year ended December 31, 2018, our long-term debt balance, including borrowings under our revolving line of credit, decreased by $265.9 million due to payments on our debt compared to a $516.0 million increase in our debt during the year ended December 31, 2017. Cash outflows from financing activities during the year ended December 31, 2018 also increased due to the portion of acquisition related earn-out payments classified as a financing activity.
Net cash provided by financing activities during the year ended December 31, 2017 was $507.0 million compared to $101.6 million of net cash used in financing activities for the same period of 2016. During the year ended December 31, 2017 our long-term debt balance, including borrowing under the revolving line of credit, increased by $516.0 million; these borrowings were used to fund the acquisition of Symphony Health. During the year ended December 31, 2016, our total debt decreased by $77.6 million due to voluntary principal payments on our long-term debt.
Inflation
Our long‑term contracts, those in excess of one year, generally include an inflation or cost of living adjustment for the portion of the services to be performed beyond one year from the contract date. As a result, we expect that inflation generally will not have a material adverse effect on our operations or financial condition. Historically our projection of inflation contained within our contracts has not significantly impacted our operating income. Should inflation be in excess of the estimates within our contracts our operating margins would be negatively impacted if we were unable to negotiate contract modifications with our clients.
Indebtedness
2016 Credit Facilities
The 2016 Credit Facilities provide senior secured financing of up to $1,400.0 million, consisting of:
| |
• | the 2016 First Lien Term Loan in an aggregate principal amount of up to $1,175.0 million; and |
| |
• | the 2016 Revolver in an aggregate principal amount of up to $225.0 million. |
The above amounts reflect the $550.0 million Incremental Borrowing associated with the acquisition of Symphony Health and the $100.0 million new revolving credit commitment received as part of the 2017 Refinancing. Refer to Note 9 - Revolving credit facilities and long‑term debt for further information on those transactions.
The borrower of the 2016 First Lien Term Loan and the 2016 Revolver is Pharmaceutical Research Associates, Inc., a wholly-owned subsidiary of PRA Health Sciences, Inc. The 2016 Revolver includes borrowing capacity available for letters of credit up to $25.0 million and for up to $20.0 million of borrowings on same‑day notice, referred to as swingline loans.
The 2016 Credit Facilities provide that we have the right at any time to request incremental term loans and/or revolving commitments in an aggregate principal amount of up to (a) $275.0 million, plus (b) all voluntary prepayments and corresponding voluntary commitment reductions of the Senior Secured Credit Facilities, other than from proceeds of long-term indebtedness, prior to the date of any such incurrence, plus (c) an additional amount which, after giving effect to the incurrence of such amount, we would not exceed a consolidated net first lien secured leverage to consolidated EBITDA ratio of 3.0 to 1.0 pro forma for such incremental facilities, minus (d) the sum of (i) the aggregate principal amount of new term loan commitments and new revolving credit commitments incurred and (ii) the aggregate principal amount of certain other indebtedness incurred. The lenders under these facilities are not under any obligation to provide any such incremental commitments or loans, and any such addition of or increase in commitments or loans is subject to certain customary conditions precedent.
Interest Rate and Fees
Borrowings under the 2016 First Lien Term Loan and the 2016 Revolver bear interest at a rate equal to, at our option, either (a) LIBOR for the relevant interest period, plus an applicable margin; provided that, solely with respect to the 2016 First Lien Term Loan, LIBOR shall be deemed to be no less than 0.00% per annum or (b) an adjusted base rate, or the ABR, plus an applicable margin.
The applicable margin on our 2016 First Lien Term Loan is based on our ratio of total debt to EBITDA per the table below:
|
| | | | | | | | | | |
Pricing Level | | Total indebtedness to EBITDA Ratio | | Letter of Credit Fees | | ABR Margin Rate | | Adjusted LIBOR Margin Rate | | Commitment Fees |
I | | > 3.75x | | 2.00% | | 1.00% | | 2.00% | | 0.40% |
II | | < 3.75x but > 3.00x | | 1.75% | | 0.75% | | 1.75% | | 0.35% |
III | | < 3.00x but > 2.25x | | 1.50% | | 0.50% | | 1.50% | | 0.30% |
IV | | < 2.25x but > 1.50x | | 1.25% | | 0.25% | | 1.25% | | 0.25% |
V | | < 1.50x | | 1.00% | | —% | | 1.00% | | 0.20% |
In addition to paying interest on outstanding principal under the 2016 Revolver, we are required to pay a commitment fee to the lenders under the 2016 Revolver in respect of the unutilized commitments thereunder. The commitment fee rate will be based on the ratio of total indebtedness to EBITDA on a given date. We are also required to pay customary letter of credit fees.
As of December 31, 2018, 2017, and 2016, the weighted average interest rate on the 2016 First Lien Term Loan was 3.88%, 3.45% and 2.70%, respectively.
Prepayments
The 2016 Credit Facilities require us to prepay outstanding term loans, subject to certain exceptions, with:
| |
• | 100% of the net cash proceeds of the incurrence or issuance of certain debt; and |
| |
• | 100% of the net cash proceeds of $5.0 million of certain non-ordinary course asset sales and casualty and condemnation events, subject to reinvestment rights and certain other exceptions. |
The foregoing mandatory prepayments will be applied first to accrued interest and fees and second, to the scheduled installments of principal of the 2016 Credit Facilities in direct order of maturity.
We may voluntarily repay outstanding loans under the 2016 Credit Facilities at any time without premium or penalty, subject to reimbursements of the lenders’ redeployment costs actually incurred in the case of a prepayment of LIBOR borrowings other than on the last day of the relevant interest period.
Amortization and Final Maturity
The 2016 First Lien Term Loan, including the Incremental Borrowing and the 2017 Refinancing, is a floating rate term loan with scheduled, fixed quarterly principal payments of $7.2 million to be made quarterly until September 30, 2021. Our voluntary prepayments of $195.6 million during 2018 fully satisfied all required quarterly principal payments through maturity, with the remaining $916.5 million principal payment due at December 6, 2021.
We have the option of 1, 2, 3 or 6-month borrowing terms under the 2016 Revolver. Principal amounts outstanding under the 2016 Revolver are due and payable in full at maturity, on or about December 6, 2021.
Guarantee and Security
All obligations of the borrower under the 2016 Credit Facilities are unconditionally guaranteed by us and all our material, wholly‑owned U.S. restricted subsidiaries with customary exceptions, including where providing such guarantees is not permitted by law, regulation or contract or would result in material adverse tax consequences.
All obligations of the borrower under the 2016 Credit Facilities, and the guarantees of such obligations, are secured, subject to permitted liens and other exceptions, by substantially all of the assets of the borrower and each guarantor, including but not limited to: (i) a perfected pledge of all of the capital stock issued by the borrower and each guarantor and (ii) perfected security interests in substantially all other tangible and intangible assets of the borrower and the guarantors (subject to certain exceptions and exclusions).
Certain Covenants and Events of Default
The 2016 Credit Facilities contain a number of covenants that, among other things, restrict, subject to certain exceptions, our ability to:
| |
• | make investments and acquisitions; |
| |
• | incur or guarantee additional indebtedness; |
| |
• | enter into mergers or consolidations and other fundamental changes; |
| |
• | conduct sales and other dispositions of property or assets; |
| |
• | enter into sale-leaseback transactions or hedge agreements; |
| |
• | prepay subordinated debt; |
| |
• | pay dividends or make other payments in respect of capital stock; |
| |
• | change the line of business; |
| |
• | enter into transactions with affiliates; |
| |
• | enter into burdensome agreements with negative pledge clauses and clauses restriction; and |
| |
• | subsidiary distributions. |
Our 2016 Credit Facilities contain customary events of default (subject to exceptions, thresholds and grace periods), including, without limitation: (i) nonpayment of principal or interest; (ii) failure to perform or observe covenants; (iii) inaccuracy or breaches of representations and warranties; (iv) cross‑defaults with certain other indebtedness; (v) certain bankruptcy related events; (vi) impairment of certain security interests in collateral, guarantees or invalidity or unenforceability of certain 2016 Credit Facilities documents; (vii) monetary judgment defaults; (viii) certain ERISA matters; and (ix) certain change of control events.
The 2016 Credit Facilities require us to maintain a consolidated total debt to consolidated EBITDA ratio of 4.25 to 1.0 and consolidated EBITDA to fixed charges no less than 3.0 to 1.0 for any four consecutive fiscal quarters for which financial statements have been provided to the administrative agent as required by the Senior Secured Credit Agreement. Following a qualified material acquisition, the 2016 Credit Facilities allows us to increase its Consolidated Total Debt to Consolidated EBITDA Ratio to 5.25 to 1.00; provided that (i) such ratio in respect of each quarter shall be reduced by 0.25 to 1.00, (ii) in no event shall such ratio be lower than 4.25 to 1.00 and (iii) such an increase pursuant to this shall be permitted no more than once during any period of 24 consecutive months.
The 2016 Credit Facilities also contain certain customary affirmative covenants and events of default, including a change of control.
Accounts Receivable Financing Agreement
We entered into an accounts receivable financing agreement with PNC Bank, National Association, as administrative agent and lender on March 22, 2016. On May 31, 2018, we amended our accounts receivable financing agreement. The amendment increased the agreement's borrowing capacity, decreased the applicable margin, and extended the termination date to May 31, 2021, unless terminated earlier pursuant to its terms.
We may borrow up to $200.0 million under the accounts receivable financing agreement, secured by liens on our accounts receivables and other assets. We are liable for customary representations, warranties, covenants and indemnities. In addition, we have guaranteed the performance of the obligations and will guarantee the obligations of any additional servicer that may become party to the accounts receivable financing agreement. As of December 31, 2018, the outstanding balance was $170.0 million.
The accounts receivable financing agreement matures on May 31, 2021, unless terminated earlier pursuant to its term.
Interest Rate and Fees
Loans under the accounts receivable financing agreement will accrue interest at either a reserve-adjusted LIBOR or a base rate, plus 1.25%. As of December 31, 2018, 2017 and 2016, the weighted average interest rate on the accounts receivable financing agreement was 3.72%, 2.96% and 2.31%, respectively. We may prepay loans upon one business day prior notice and may terminate the accounts receivable financing agreement with 15 days’ prior notice.
Covenants and Events of Default
The accounts receivable financing agreement contains various customary representations and warranties and covenants, and default provisions that provide for the termination and acceleration of the commitments and loans under the accounts receivable financing agreement in circumstances including, but not limited to, failure to make payments when due, breach of representations, warranties or covenants, certain insolvency events or failure to maintain the security interest in the trade receivables, and defaults under other material indebtedness.
Contractual Obligations and Commercial Commitments
The following table summarizes our future minimum payments for all contractual obligations and commercial commitments for years subsequent to the year ended December 31, 2018:
|
| | | | | | | | | | | | | | | | | | | |
| Payments Due by Period |
| Less than 1 year | | 1 - 3 years | | 3 - 5 years | | More than 5 years | | Total |
| (in thousands) |
Principal payments on long-term debt (1) | $ | — |
| | $ | 1,086,533 |
| | $ | — |
| | $ | — |
| | $ | 1,086,533 |
|
Interest payments on long-term debt (1) | 42,461 |
| | 78,694 |
| | — |
| | — |
| | 121,155 |
|
Service purchase commitments (2) | 89,455 |
| | 123,872 |
| | 14,086 |
| | 68 |
| | 227,481 |
|
Operating leases | 43,675 |
| | 78,417 |
| | 54,473 |
| | 90,978 |
| | 267,543 |
|
Less: sublease income | (157 | ) | | (314 | ) | | (119 | ) | | — |
| | (590 | ) |
Uncertain income tax positions (3) | — |
| | — |
| | — |
| | — |
| | — |
|
Contingent consideration on acquisition (4) | 83,249 |
| | — |
| | — |
| | — |
| | 83,249 |
|
Total | $ | 258,683 |
| | $ | 1,367,202 |
| | $ | 68,440 |
| | $ | 91,046 |
| | $ | 1,785,371 |
|
| |
(1) | Principal payments are based on the terms contained in our credit agreements. Principal payments include payments on the first lien term debt and the accounts receivable financing agreement. Interest payments are based on the interest rate in effect on December 31, 2018. |
| |
(2) | Service purchase commitments are defined as agreements to purchase goods or services that are enforceable and legally binding and that specify all significant terms, including fixed or minimum quantities to be purchased. |
| |
(3) | As of December 31, 2018, our liability related to uncertain income tax positions was approximately $12.9 million; the entire amount has been excluded from the table as we are unable to predict when these liabilities will be paid due to the uncertainties in timing of the settlement of the income tax positions. |
| |
(4) | Represents contingent payments associated with our acquisitions. |
Off‑Balance Sheet Arrangements
We have no off‑balance sheet arrangements. The term “off‑balance sheet arrangement” generally means any transaction, agreement or other contractual arrangement to which an entity unconsolidated with us is a party, under which we have any obligation arising under a guarantee contract, derivative instrument or variable interest or a retained or contingent interest in assets transferred to such entity or similar arrangement that serves as credit, liquidity or market risk support for such assets.
Recent Accounting Pronouncements
For information on new accounting pronouncements and the impact, if any, on our financial position or results of operations, see Note 2 to our audited consolidated financial statements found elsewhere in this Annual Report on Form 10-K.
Critical Accounting Policies and Estimates
In preparing our financial statements in conformity with GAAP, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Our actual results could differ from those estimates. We believe that the following are some of the more critical judgment areas in the application of our accounting policies that affect our financial condition and results of operations. We have discussed the application of these critical accounting policies with our board of directors.
Revenue Recognition
Revenue is generated from contracts with customers. Revenue is recognized when control of the performance obligation is transferred to the customer, in an amount that reflects the consideration we expect to be entitled to receive in exchange for those services. Our long term fixed-fee arrangements for clinical research services are considered a single performance obligation because we provide a highly-integrated service. Revenue is recognized for the single performance obligation over time due to our right to payment for work performed to date. We generally use the cost-to-cost measure of progress for our contracts because it best depicts the transfer of control to the customer as the performance obligation is fulfilled. For this method, we compare the contract costs incurred to date to the estimated total contract costs through completion. Contract costs consist primarily of direct labor and other reimbursable project-related costs such as travel, third-party vendor costs and investigator fees. We review the total current estimated costs on each project to determine if these estimates are still accurate and, if necessary, we adjust the total estimated costs for each project. During our contract review process, we review each contract’s performance to date, current cost trends, and circumstances specific to each study. The original or current cost estimates are reviewed and if necessary the estimates are adjusted and refined to reflect any changes in the anticipated performance under the study. As the work progresses, original estimates might be deemed incorrect due to, among other things, revisions in the scope of work or patient enrollment rate, and a contract modification might be negotiated with the customer to cover additional costs. If not, we bear the risk of costs exceeding our original estimates. We assume that actual costs incurred to date under the contract are a valid basis for estimating future costs. Should our assumption of future cost trends fluctuate significantly, future margins could be reduced. In the past, we have had to commit unanticipated resources to complete projects, resulting in lower margins on those projects. Should our actual costs exceed our estimates on fixed price contracts, future margins could be reduced, absent our ability to negotiate a contract modification. We accumulate information on each project to refine our bidding process. Historically, the majority of our estimates and assumptions have been materially correct, but these estimates might not continue to be accurate in the future. Clinical research services delivered under fee-for-service arrangements are recognized over time. Revenue from time and materials contracts is recognized as hours are incurred.
Our Data Solutions segment provides data reports and analytics to customers based on agreed-upon specifications. If a customer requests more than one type of data report or series of data reports within a contract, each distinct type of data report is a separate performance obligation. When multiple performance obligations exist, the transaction price is allocated to performance obligations on a relative standalone selling price basis. In cases where we contract to provide a series of data reports, or in some cases data, we recognize revenue over time using the ‘units delivered’ output method as the data or reports are delivered. Certain Data Solutions arrangements include upfront customization or consultative services for customers. Under these arrangements, we contract with a customer to carry out a specific study, ultimately resulting in delivery of a custom report or data product. These arrangements are a single performance obligation given the integrated nature of the service being provided. We typically recognize revenue under these contracts over time, using an output-based measure, generally time elapsed, to measure progress and transfer of control of the performance obligation to the customer.
Allowance for Doubtful Accounts
Included in “Accounts receivable and unbilled services, net” on our consolidated balance sheets is an allowance for doubtful accounts. Generally, before we do business with a new client, we perform a credit check, as our allowance for doubtful accounts requires that we make an accurate assessment of our customers’ creditworthiness. Approximately 17% of our client base is small- to mid-sized biotech companies, creating a heightened risk related to the creditworthiness for a portion of our client base. We manage and assess our exposure to bad debt on each of our contracts. We age our billed accounts receivable and assess exposure by client type, by aged category, and by specific identification. After all attempts to collect a receivable have failed, the receivable is written off against the allowance. Historically, we have not had any write‑offs in excess of our allowance. If, at December 31, 2018, our aged accounts receivable balance greater than 90 days were to increase by 10% (for the U.S. operations), no material adjustments to bad debt expense would be required.
Income Taxes
Changes in judgment as to recognition or measurement of tax positions can materially affect the estimate of our effective tax rate and, consequently, our operating results. We consider many factors when evaluating and estimating our tax positions and tax benefits, which may require periodic adjustments and may not accurately anticipate actual outcomes.
We have to use estimates and judgments in calculating certain tax liabilities and determining the recoverability of certain deferred tax assets, which arise from net operating losses, tax credit carry forwards and temporary differences between the tax and financial statement recognition of revenue and expense. We are also required to reduce our deferred tax assets by a valuation allowance if, based on the weight of available evidence, it is more likely than not that some portion or all of the recorded deferred tax assets will not be realized in future periods.
On December 22, 2017, the Tax Cuts and Jobs Act of 2017 (the “Act”) was signed into U.S. law making significant changes to the Internal Revenue Code, including but not limited to, a corporate tax rate decrease from 35% to 21% effective for tax years beginning after December 31, 2017, the transition of U.S international taxation of worldwide income to a territorial system, and a one-time transition tax on the mandatory deemed repatriation of cumulative foreign earnings as of December 31, 2017. Additionally, Staff Accounting Bulletin No. 118 (“SAB 118”) was issued to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Act. We calculated our best estimate of the impact of the Act and recorded a provisional amount per the guidance under SAB 118.
We calculated our best estimate of the impact of the Act in our year-end income tax provision and recorded $0.2 million as additional income tax expense in the fourth quarter of 2017, the period in which the legislation was enacted. This provisional amount related to the remeasurement of certain deferred tax assets, deferred tax liabilities, and U.S. uncertain tax positions, based on the rates at which they are expected to reverse in the future, was a benefit of $41.7 million. The provisional amount related to the one-time transition tax on the mandatory deemed repatriation of foreign earnings was $77.6 million based on cumulative foreign earnings of $392.5 million. We also recorded a provisional tax benefit of $35.7 million related to the utilization of foreign tax credits against the one-time transition tax. In addition, we recorded a valuation allowance against an estimated $12.8 million of excess foreign tax credits related to the transition tax inclusion.
We completed our analysis of the impact of the Act which resulted in an additional tax benefit of $0.6 million in the fourth quarter of 2018 and a total tax provision of $3.0 million related to the impact of the Act for the year ended December 31, 2018. The total tax provision included a $14.5 million provision related to adjustments to the transition tax and a $11.5 million benefit related to the remeasurement of certain deferred tax assets and liabilities. Additionally, we have elected to treat any potential GILTI inclusions as a period cost.
In evaluating our ability to recover our deferred tax assets, in full or in part, we consider all available positive and negative evidence, including our past operating results, the existence of cumulative losses in the most recent fiscal years and our forecast of future taxable income on a jurisdiction‑by‑jurisdiction basis. In determining future taxable income, assumptions include the amount of state, federal and international pretax operating income, international transfer pricing policies, the reversal of temporary differences and the implementation of feasible and prudent tax planning strategies. These assumptions require significant judgment about the forecasts of future taxable income and are consistent with the plans and estimates we use to manage the underlying businesses. Based on our analysis of the above factors, we determined that a valuation allowance of $9.8 million was required as of December 31, 2018 relating to state net operating loss carryforwards, foreign net operating loss carryforwards, certain foreign deferred tax assets and state tax credit carryforwards. Changes in our assumptions could result in an adjustment to the valuation allowance, up or down, in the future.
In addition, the calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax regulations in a multitude of jurisdictions. We determine our liability for uncertain tax positions globally under the provisions in the FASB's Accounting Standards Codification, or ASC, 740, Income Taxes. As of December 31, 2018, we had recorded a liability for uncertain tax positions of $12.9 million. If events occur such that payment of these amounts ultimately proves to be unnecessary, the reversal of liabilities would result in tax benefits being recognized in the period when we determine the liabilities are no longer necessary. If our calculation of liability related to uncertain tax positions proves to be more or less than the ultimate assessment, a tax expense or benefit to expense, respectively, would result. The total liability reversal that could affect the tax rate is $12.9 million.
Stock‑Based Compensation
In accordance with the ASC 718, Stock Compensation, as modified and supplemented, we estimate the value of employee stock options on the date of grant using either the Black‑Scholes model for all options with a service condition or a lattice model for options with market and performance conditions. The determination of fair value of stock‑based payment
awards on the date of grant using an option‑pricing model is affected by the stock price of similar entities as well as assumptions regarding a number of highly complex and subjective variables. These variables include the expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. The Black‑Scholes and lattice models require extensive actual employee exercise behavior data and the use of a number of complex assumptions including expected volatility, risk‑free interest rate, expected dividends, and expected life. In developing our assumption, we take into account the following:
| |
• | We use the historical volatilities of a selected peer group as we do not have sufficient history to estimate the volatility of our common share price. We calculate expected volatility based on reported data for selected reasonably similar publicly traded companies for which the historical information is available. For the purpose of identifying peer companies, we consider characteristics such as industry, length of trading history, similar vesting terms and in‑the‑money option status. We plan to continue to use the guideline peer group volatility information until the historical volatility of our common shares is relevant to measure expected volatility for future award grants. |
| |
• | The risk‑free interest rate assumption is based upon observed interest rates appropriate for the term of our employee stock options. |
| |
• | The dividend yield assumption is based on the history and expectation of dividend payouts. |
| |
• | For those options valued using the Black-Scholes model, the expected life is based upon the guidance provided by the FASB. For those options with a market condition valued under the lattice model, the expected life varies depending on the target stock price that triggers vesting. |
| |
• | We account for forfeitures as they occur. |
Due to the absence of an active market for our common stock prior to our initial public offering, or IPO, in 2014, the fair value of our common stock on the date of the grant was determined in good faith by our Board of Directors with the assistance of management, based on a number of factors consistent with the methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation. Subsequent to the IPO, the fair value of our common stock is based upon the market price of our common stock on the date of the grant as listed on the NASDAQ.
Long‑Lived Assets, Goodwill and Indefinite‑Lived Intangible Assets
As a result of our acquisitions we have recorded goodwill and other identifiable finite and indefinite‑lived acquired intangibles. The identification and valuation of these intangible assets at the time of acquisition require significant management judgment and estimates.
We review long‑lived asset groups for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset group might not be recoverable. If indicators of impairment are present, we evaluate the carrying value of property and equipment in relation to estimates of future undiscounted cash flows. As a result of our acquisitions we have recorded goodwill and other identifiable finite and indefinite‑lived acquired intangibles. The identification and valuation of these intangible assets at the time of acquisition require significant management judgment and estimates. In connection with acquisitions, valuations were completed and value was assigned to identifiable finite‑lived and indefinite‑lived intangible assets and goodwill, based on the purchase price of the transactions.
We test goodwill for impairment on at least an annual basis by comparing the carrying value to the estimated fair value of our reporting units. On October 1, 2018, we reviewed goodwill for impairment and our analysis indicated that the fair value of goodwill exceeded the carrying value and, therefore, no impairment exists. When evaluating for impairment, we may first perform a qualitative assessment to determine whether it is more likely than not that a reporting unit or indefinite-lived intangible asset is impaired. If we do not perform a qualitative assessment, or if it determines that it is not more likely than not that the fair value of the reporting unit or indefinite-lived intangible asset exceeds its carrying amount, we will calculate the estimated fair value of the reporting unit’s or indefinite-lived intangible asset. Our decision to perform a qualitative impairment assessment for an individual reporting unit in a given year is influenced by a number of factors, inclusive of the size of the reporting unit's goodwill, the significance of the excess of the reporting unit's estimated fair value over carrying value at the last quantitative assessment date, the amount of time in between quantitative fair value assessments and the date of acquisition. During 2018, as part of our annual impairment analysis, we performed the qualitative assessment for approximately $1.0 billion, or 68.1% of our goodwill balance of $1.5 billion, which relates to our EDS, PR, and SS business units, and for our indefinite-lived trade name intangible asset balances.
If we do not perform a qualitative assessment, goodwill impairment is determined by comparing the fair value of each reporting unit, determined using various valuation techniques, with the primary technique being a discounted cash flow analysis, to its carrying value. This process is inherently subjective and dependent upon the estimates and assumptions we make. In determining the expected future cash flows of our company, we assume that we will continue to enter into new contracts, execute the work on these contracts profitably, collect receivables from customers, and thus generate positive cash flows. In addition, our analysis could be impacted by future adverse change such as future declines in our operating results, a further significant slowdown in the worldwide economy or pharmaceutical and biotechnology industry or failure to meet the performance projections included in our forecast. We performed our impairment test for the Data Solutions operating segment during the fourth quarter of 2018. It was concluded that the estimated fair value of the Data Solutions operating segment exceeded its carrying value by approximately $200.0 million, or 34.6%.
Fair Value Measurements
We record certain assets and liabilities at fair value. Fair value is defined as a price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. A three‑level hierarchy that prioritizes the inputs used to measure fair value is further described in Note 2 to our audited consolidated financial statements included elsewhere in this Annual Report on Form 10‑K.
Fair Value Measurements on a Recurring Basis
At December 31, 2017, we used Level 3 inputs to measure liabilities totaling $50.6 million. The liabilities relate to contingent consideration issued in connection with our acquisition of Symphony Health.
All derivatives are measured at fair value and recognized as either assets or liabilities on the consolidated balance sheets. The fair value of our interest rate swaps, measured using Level 2 inputs, were assets of $3.3 million and $0.4 million at December 31, 2018 and 2017, respectively.
No other liabilities or assets are remeasured at fair value.
Dividend History
We have not declared or paid dividends during 2018, 2017 and 2016.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk is the potential loss arising from adverse changes in market rates and prices, such as foreign currency exchange rates, interest rates and other relevant market rate or price changes. In the ordinary course of business, we are exposed to various market risks, including changes in foreign currency exchange rates and interest rates, and we regularly evaluate our exposure to such changes. Our overall risk management strategy seeks to balance the magnitude of the exposure and the cost and availability of appropriate financial instruments.
Interest Rate Risk
We are subject to market risk associated with changes in interest rates. In September 2015, we entered into an interest rate swap with a notional value of $250.0 million to hedge our variable rate term debt. This swap matured in September 2018.
On January 5, 2018, we entered into two new interest rate swaps in order to manage our cash flow exposure to variable rate debt and also to replace the interest rate swap that matured in September 2018. The first interest rate swap has an aggregate notional amount of $375.0 million and a fixed payment rate of 2.2% offsetting a one-month LIBOR variable rate with an effective date of January 8, 2018, and a maturity date of December 6, 2020. The second interest rate swap has an aggregate notional amount of $250.0 million and a fixed payment rate of 2.3% offsetting a one-month LIBOR variable rate with an effective date of September 6, 2018, and a maturity date of September 6, 2020.
At December 31, 2018, we had $461.5 million outstanding under our 2016 First Lien Term Loans and accounts receivable financing agreement that were not covered by an interest rate swap and therefore subject to variable interest rates.
Each quarter percentage point increase or decrease in the variable rate would result in our interest expense changing by approximately $1.2 million per year under our unhedged variable rate debt.
Foreign Exchange Risk
Since we operate on a global basis, we are exposed to various foreign currency risks. First, our consolidated financial statements are denominated in U.S. dollars, but a significant portion of our revenue is generated in the local currency of our foreign subsidiaries. Accordingly, changes in exchange rates between the applicable foreign currency and the U.S. dollar will affect the translation of each foreign subsidiary’s financial results into U.S. dollars for purposes of reporting consolidated financial results. A hypothetical change of 10% in average exchange rates used to translate all foreign currencies to U.S. dollars would have impacted income (loss) before income taxes and equity in income (losses) of unconsolidated joint ventures by approximately $34.4 million for the year ended December 31, 2018. The process by which each foreign subsidiary’s financial results are translated into U.S. dollars is as follows: income statement accounts are translated at average exchange rates for the period; balance sheet asset and liability accounts are translated at end of period exchange rates; and equity accounts are translated at historical exchange rates. Translation of the balance sheet in this manner affects the stockholders’ equity account, referred to as the cumulative translation adjustment account. This account exists only in the foreign subsidiary’s U.S. dollar balance sheet and is necessary to keep the foreign balance sheet stated in U.S. dollars in balance. Accumulated currency translation adjustments recorded as a separate component of stockholders’ equity were $(158.3) million and $(117.2) million at December 31, 2018 and 2017, respectively. We do not have significant operations in countries in which the economy is considered to be highly‑inflationary.
In addition, two specific risks arise from the nature of the contracts we enter into with our clients, which from time to time are denominated in currencies different than the particular subsidiary’s local currency. These risks are generally applicable only to a portion of the contracts executed by our foreign subsidiaries providing clinical services. The first risk occurs as revenue recognized for services rendered is denominated in a currency different from the currency in which the subsidiary’s expenses are incurred. As a result, the subsidiary’s earnings can be affected by fluctuations in exchange rates.
The second risk results from the passage of time between the invoicing of clients under these contracts and the ultimate collection of client payments against such invoices. Because the contract is denominated in a currency other than the subsidiary’s local currency, we recognize a receivable at the time of invoicing for the local currency equivalent of the foreign currency invoice amount. Changes in exchange rates from the time the invoice is prepared until payment from the client is received will result in our receiving either more or less in local currency than the local currency equivalent of the invoice amount at the time the invoice was prepared and the receivable established. This difference is recognized by us as a foreign currency transaction gain or loss, as applicable, and is reported in foreign currency gains (losses), net in our consolidated statements of operations. Historically, fluctuations in exchange rates from those in effect at the time contracts were executed have not had a material effect on our consolidated financial results.
Item 8. Financial Statements and Supplementary Data
Management’s Report on Internal Control Over Financial Reporting
Management of PRA Health Sciences, Inc. (the “Company”) is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external reporting purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company, (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America, and that receipts and expenditures are being made only in accordance with authorizations of management and directors of the Company, and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements in the consolidated financial statements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2018. In making these assessments, management used the framework established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control — Integrated Framework (2013). Based on management’s assessment and the criteria in the COSO framework, management has concluded that the Company’s internal control over financial reporting as of December 31, 2018 was effective.
The Company’s independent registered public accounting firm has issued a report on the Company’s internal control over financial reporting. This report appears in this Annual Report on Form 10-K.
|
| | |
/s/ Colin Shannon | | /s/ Michael J. Bonello |
| | |
Colin Shannon | | Michael J. Bonello |
President, Chief Executive Officer and Chairman of the Board of Directors | | Executive Vice President and Chief Financial Officer |
(Principal Executive Officer) | | (Principal Financial Officer) |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of PRA Health Sciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of PRA Health Sciences, Inc. and subsidiaries (the "Company") as of December 31, 2018 and 2017, and the related consolidated statements of operations, comprehensive income (loss), changes in stockholders’ equity, and cash flows, for each of the three years in the period ended December 31, 2018, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018, in conformity with the accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 28, 2019, expressed an unqualified opinion on the Company's internal control over financial reporting.
Adoption of new Accounting Standards
As discussed in Note 2 to the financial statements, the Company adopted Accounting Standards Codification (ASC) Topic 606, “Revenue from Contracts with Customers” on January 1, 2018 using the modified retrospective method and Accounting Standards Update No. 2016-09, “Compensation-Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting” on January 1, 2017.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Deloitte & Touche LLP
Raleigh, North Carolina
February 28, 2019
We have served as the Company's auditor since 2013.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of PRA Health Sciences, Inc.
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of PRA Health Sciences, Inc. and subsidiaries (the “Company”) as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2018, of the Company and our report dated February 28, 2019 expressed an unqualified opinion on those financial statements and included an explanatory paragraph regarding the Company’s adoption of Accounting Standards Codification (ASC) Topic 606, “Revenue from Contracts with Customers.”
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management’s Report on Internal Control Over Financial Reporting.” Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Deloitte & Touche LLP
Raleigh, North Carolina
February 28, 2019
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except share amounts)
|
| | | | | | | |
| December 31, |
| 2018 | | 2017 |
ASSETS | | | |
Current assets: | | | |
Cash and cash equivalents | $ | 144,221 |
| | $ | 192,229 |
|
Restricted cash | 488 |
| | 661 |
|
Accounts receivable and unbilled services, net | 568,099 |
| | 627,003 |
|
Prepaid expenses and other current assets | 66,605 |
| | 55,580 |
|
Income taxes receivable | 2,942 |
| | 1,551 |
|
Total current assets | 782,355 |
| | 877,024 |
|
Fixed assets, net | 154,764 |
| | 143,070 |
|
Goodwill | 1,494,762 |
| | 1,512,424 |
|
Intangible assets, net | 704,446 |
| | 783,836 |
|
Deferred tax assets | 8,954 |
| | 8,939 |
|
Investment in unconsolidated joint ventures | — |
| | 407 |
|
Deferred financing fees | 1,373 |
| | 1,844 |
|
Other assets | 39,813 |
| | 30,502 |
|
Total assets | $ | 3,186,467 |
| | $ | 3,358,046 |
|
LIABILITIES AND STOCKHOLDERS' EQUITY | | | |
Current liabilities: | | | |
Current portion of borrowings under credit facilities | $ | — |
| | $ | 91,500 |
|
Current portion of long-term debt | — |
| | 28,789 |
|
Accounts payable | 43,734 |
| | 64,635 |
|
Accrued expenses and other current liabilities | 369,477 |
| | 303,875 |
|
Income taxes payable | 44,306 |
| | 13,606 |
|
Advanced billings | 441,357 |
| | 469,211 |
|
Total current liabilities | 898,874 |
| | 971,616 |
|
Deferred tax liabilities | 100,712 |
| | 112,181 |
|
Long-term debt, net | 1,082,384 |
| | 1,225,397 |
|
Other long-term liabilities | 53,077 |
| | 112,371 |
|
Total liabilities | 2,135,047 |
| | 2,421,565 |
|
Commitments and contingencies (Note 13) |
|
| |
|
|
Stockholders' equity: | | | |
Preferred stock (100,000,000 authorized shares; $0.01 par value) | | | |
Issued and outstanding -- none | — |
| | — |
|
Common stock (1,000,000,000 authorized shares; $0.01 par value) | | | |
Issued and outstanding -- 65,394,526 and 63,623,950 at December 31, 2018 and 2017, respectively | 654 |
| | 636 |
|
Additional paid-in capital | 960,535 |
| | 905,423 |
|
Accumulated other comprehensive loss | (170,659 | ) | | (136,470 | ) |
Retained earnings | 254,500 |
| | 161,182 |
|
Equity attributable to PRA Health Sciences, Inc. stockholders | 1,045,030 |
| | 930,771 |
|
Noncontrolling interest | 6,390 |
| | 5,710 |
|
Total stockholders' equity | 1,051,420 |
| | 936,481 |
|
Total liabilities and stockholders' equity | $ | 3,186,467 |
| | $ | 3,358,046 |
|
The accompanying notes are an integral part of the consolidated financial statements.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Revenue: | $ | 2,871,922 |
| | $ | 2,259,389 |
| | $ | 1,811,711 |
|
Operating expenses: | | | | | |
Direct costs (exclusive of depreciation and amortization) | 1,500,226 |
| | 1,283,868 |
| | 1,032,688 |
|
Reimbursable out-of-pocket costs | 308,291 |
| | 311,015 |
| | 231,688 |
|
Reimbursable investigator fees | 262,114 |
| | — |
| | — |
|
Selling, general and administrative | 371,795 |
| | 321,987 |
| | 269,893 |
|
Transaction-related costs | 35,817 |
| | 87,709 |
| | 44,834 |
|
Depreciation and amortization | 112,247 |
| | 78,227 |
| | 69,506 |
|
Loss on disposal of fixed assets | 120 |
| | 358 |
| | 753 |
|
Income from operations | 281,312 |
| | 176,225 |
| | 162,349 |
|
Interest expense, net | (57,399 | ) | | (46,729 | ) | | (54,913 | ) |
Loss on modification or extinguishment of debt | (952 | ) | | (15,023 | ) | | (38,178 | ) |
Foreign currency (losses) gains, net | (1,043 | ) | | (39,622 | ) | | 24,029 |
|
Other (expense) income, net | (371 | ) | | (304 | ) | | 607 |
|
Income before income taxes and equity in income of unconsolidated joint ventures | 221,547 |
| | 74,547 |
| | 93,894 |
|
Provision for (benefit from) income taxes | 67,232 |
| | (12,623 | ) | | 28,494 |
|
Income before equity in income of unconsolidated joint ventures | 154,315 |
| | 87,170 |
| | 65,400 |
|
Equity in income of unconsolidated joint ventures, net of tax | 143 |
| | 123 |
| | 2,775 |
|
Net income | 154,458 |
| | 87,293 |
| | 68,175 |
|
Net income attributable to noncontrolling interest | (553 | ) | | (366 | ) | | — |
|
Net income attributable to PRA Health Sciences, Inc. | $ | 153,905 |
| | $ | 86,927 |
| | $ | 68,175 |
|
Net income per share attributable to common stockholders: | | | | | |
Basic | $ | 2.40 |
| | $ | 1.39 |
| | $ | 1.12 |
|
Diluted | $ | 2.32 |
| | $ | 1.32 |
| | $ | 1.06 |
|
Weighted average common shares outstanding: | | | | | |
Basic | 64,123 |
| | 62,437 |
| | 60,759 |
|
Diluted | 66,341 |
| | 65,773 |
| | 64,452 |
|
The accompanying notes are an integral part of the consolidated financial statements.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(in thousands)
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Net income | $ | 154,458 |
| | $ | 87,293 |
| | $ | 68,175 |
|
Other comprehensive income (loss): | | | | | |
Foreign currency translation adjustments net of tax $4,670, $0, and $0 | (41,042 | ) | | 83,814 |
| | (95,019 | ) |
Unrealized gains (losses) on derivative instruments, net of income taxes of $1,007, $96, and $(622) | 2,152 |
| | 149 |
| | (978 | ) |
Reclassification adjustments: | | | | | |
Losses on derivatives included in net income, net of income taxes, $1,649, $2,699, and $2,303 | 4,828 |
| | 4,156 |
| | 3,618 |
|
Comprehensive income (loss) | 120,396 |
| | 175,412 |
| | (24,204 | ) |
Comprehensive income attributable to noncontrolling interest | (680 | ) | | (269 | ) | | — |
|
Comprehensive income (loss) attributable to PRA Health Sciences, Inc. | $ | 119,716 |
| | $ | 175,143 |
| | $ | (24,204 | ) |
The accompanying notes are an integral part of the consolidated financial statements.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(in thousands)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Accumulated Other Comprehensive Loss (Note 16) | | Retained Earnings | | Non-controlling Interest | | |
| Common Stock | | Additional Paid-in Capital | | | | | |
| Shares | | Amount | | | | | | Total |
Balance at December 31, 2015 | 60,245 |
| | $ | 602 |
| | $ | 828,347 |
| | $ | (132,307 | ) | | $ | 6,080 |
| | $ | — |
| | $ | 702,722 |
|
Exercise of common stock options | 1,303 |
| | 13 |
| | 642 |
| | — |
| | — |
| | — |
| | 655 |
|
Stock-based compensation | 50 |
| | 1 |
| | 49,232 |
| | — |
| | — |
| | — |
| | 49,233 |
|
Income tax benefit from stock-based award activities | — |
| | — |
| | 846 |
| | — |
| | — |
| | — |
| | 846 |
|
Net income | — |
| | — |
| | — |
| | — |
| | 68,175 |
| | — |
| | 68,175 |
|
Other comprehensive loss, net of tax | — |
| | — |
| | — |
| | (92,379 | ) | | — |
| | — |
| | (92,379 | ) |
Balance at December 31, 2016 | 61,598 |
| | 616 |
| | 879,067 |
| | (224,686 | ) | | 74,255 |
| | — |
| | 729,252 |
|
Exercise of common stock options | 1,904 |
| | 19 |
| | 8,072 |
| | — |
| | — |
| | — |
| | 8,091 |
|
Issuance of common stock | 5 |
| | — |
| | 375 |
| | — |
| | — |
| | — |
| | 375 |
|
Stock-based compensation | 117 |
| | 1 |
| | 17,909 |
| | — |
| | — |
| | — |
| | 17,910 |
|
Non-controlling interest related to Takeda joint venture | — |
| | — |
| | — |
| | — |
| | — |
| | 5,441 |
| | 5,441 |
|
Net income | — |
| | — |
| | — |
| | — |
| | 86,927 |
| | 366 |
| | 87,293 |
|
Other comprehensive income, net of tax | — |
| | — |
| | — |
| | 88,216 |
| | — |
| | (97 | ) | | 88,119 |
|
Balance at December 31, 2017 | 63,624 |
| | 636 |
| | 905,423 |
| | (136,470 | ) | | 161,182 |
| | 5,710 |
| | 936,481 |
|
Impact to retained earnings from adoption of ASC 606 | — |
| | — |
| | — |
| | — |
| | (60,587 | ) | | — |
| | (60,587 | ) |
Balance at January 1, 2018 | 63,624 |
| | 636 |
| | 905,423 |
| | (136,470 | ) | | 100,595 |
| | 5,710 |
| | 875,894 |
|
Exercise of common stock options and employee stock purchase plan purchases | 1,626 |
| | 16 |
| | 30,535 |
| | — |
| | — |
| | — |
| | 30,551 |
|
Stock award distributions net of shares for tax withholding | 145 |
| | 2 |
| | (5,339 | ) | | — |
| | — |
| | — |
| | (5,337 | ) |
Stock-based compensation | — |
| | — |
| | 29,916 |
| | — |
| | — |
| | — |
| | 29,916 |
|
Net income | — |
| | — |
| | — |
| | — |
| | 153,905 |
| | 553 |
| | 154,458 |
|
Other comprehensive loss, net of tax | — |
| | — |
| | — |
| | (34,189 | ) | | — |
| | 127 |
| | (34,062 | ) |
Balance at December 31, 2018 | 65,395 |
| | $ | 654 |
| | $ | 960,535 |
| | $ | (170,659 | ) | | $ | 254,500 |
| | $ | 6,390 |
| | $ | 1,051,420 |
|
The accompanying notes are an integral part of the consolidated financial statements.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands) |
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Cash flows from operating activities: | | | | | |
Net income | $ | 154,458 |
| | $ | 87,293 |
| | $ | 68,175 |
|
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | |
Depreciation and amortization | 112,247 |
| | 78,227 |
| | 69,506 |
|
Amortization of debt issuance costs and discount | 2,111 |
| | 2,108 |
| | 4,433 |
|
Amortization of terminated interest rate swaps | 7,146 |
| | 6,684 |
| | 4,961 |
|
Stock-based compensation expense | 29,143 |
| | 12,616 |
| | 7,067 |
|
Non-cash transaction related stock-based compensation expense | 773 |
| | 5,294 |
| | 42,166 |
|
Unrealized foreign currency (gains) losses | (3,307 | ) | | 39,700 |
| | (24,499 | ) |
Loss on modification or extinguishment of debt | 952 |
| | 15,023 |
| | 38,178 |
|
Loss on disposal of fixed assets | 120 |
| | 358 |
| | 753 |
|
Change in acquisition-related contingent consideration | 34,538 |
| | 74,969 |
| | (527 | ) |
Equity in income of unconsolidated joint ventures | (143 | ) | | (123 | ) | | (2,775 | ) |
Excess tax benefit from stock-based compensation | — |
| | — |
| | (846 | ) |
Deferred income taxes | 11,665 |
| | (75,915 | ) | | (10,469 | ) |
Other reconciling items | 30 |
| | 763 |
| | (605 | ) |
Changes in operating assets and liabilities, net of acquired assets and assumed liabilities: | | | | | |
Accounts receivable and unbilled services | (17,017 | ) | | (136,330 | ) | | (31,313 | ) |
Prepaid expenses and other assets | (18,931 | ) | | 1,762 |
| | (10,071 | ) |
Accounts payable and other liabilities | 31,579 |
| | 35,792 |
| | (1,474 | ) |
Income taxes | 5,241 |
| | 10,640 |
| | 7,308 |
|
Advanced billings | 14,216 |
| | 61,547 |
| | 79 |
|
Payment of acquisition-related contingent consideration | (35,029 | ) | | — |
| | — |
|
Net cash provided by operating activities | 329,792 |
| | 220,408 |
| | 160,047 |
|
Cash flows from investing activities: | | | | | |
Purchase of fixed assets | (55,880 | ) | | (61,318 | ) | | (33,143 | ) |
Proceeds from the sale of fixed assets | 43 |
| | 56 |
| | 10 |
|
Cash received (paid) for interest on interest rate swap | 181 |
| | (874 | ) | | (913 | ) |
Cash received from the sale of marketable securities | 183 |
| | — |
| | — |
|
Acquisition of Symphony Health Solutions Corporation, net of cash acquired | — |
| | (521,182 | ) | | — |
|
Payment of Symphony Health Solutions Corporation contingent consideration | — |
| | (67,781 | ) | | — |
|
Acquisition of Parallel 6, Inc., net of cash acquired | — |
| | (38,859 | ) | | — |
|
Acquisition of Takeda PRA Development Center KK, net of cash acquired | — |
| | 2,680 |
| | — |
|
Acquisition of Takeda Pharmaceutical Data Services, Ltd., net of cash acquired | — |
| | (142 | ) | | — |
|
Acquisition of Nextrials, Inc., net of cash acquired | — |
| | — |
| | (4,268 | ) |
Distributions from unconsolidated joint ventures | — |
| | — |
| | 3,700 |
|
Net cash used in investing activities | (55,473 | ) | | (687,420 | ) | | (34,614 | ) |
Cash flows from financing activities: | | | | | |
Proceeds from issuance of long-term debt | — |
| | 550,000 |
| | 625,000 |
|
Repayment of long-term debt | (224,394 | ) | | (125,513 | ) | | (822,559 | ) |
Proceeds from accounts receivable financing agreement | 60,000 |
| | 20,000 |
| | 120,000 |
|
Repayment on accounts receivable financing agreement | (10,000 | ) | | (20,000 | ) | | — |
|
Borrowings on line of credit | — |
| | 121,500 |
| | 110,000 |
|
Repayments of line of credit | (91,500 | ) | | (30,000 | ) | | (110,000 | ) |
Payment of debt prepayment and debt extinguishment costs | — |
| | (9,226 | ) | | (17,824 | ) |
Payment for debt issuance costs | — |
| | (6,588 | ) | | (7,713 | ) |
Excess tax benefit from stock-based compensation | — |
| | — |
| | 846 |
|
Proceeds from stock issued under employee stock purchase plan and stock option exercises | 31,382 |
| | 7,236 |
| | 655 |
|
Taxes paid related to net shares settlement of equity awards | (5,337 | ) | | — |
| | — |
|
Payment of acquisition-related contingent consideration | (79,663 | ) | | (400 | ) | | — |
|
Net cash (used in) provided by financing activities | (319,512 | ) | | 507,009 |
| | (101,595 | ) |
Effects of foreign exchange changes on cash, cash equivalents, and restricted cash | (2,988 | ) | | 3,555 |
| | (625 | ) |
Change in cash, cash equivalents, and restricted cash | (48,181 | ) | | 43,552 |
| | 23,213 |
|
Cash, cash equivalents, and restricted cash, beginning of year | 192,890 |
| | 149,338 |
| | 126,125 |
|
Cash, cash equivalents, and restricted cash, end of year | $ | 144,709 |
| | $ | 192,890 |
| | $ | 149,338 |
|
The accompanying notes are an integral part of the consolidated financial statements.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(1) Basis of Presentation
Description of Business
PRA Health Sciences, Inc. and its subsidiaries, or the Company, is a full-service global contract research organization providing a broad range of product development and data solution services to pharmaceutical and biotechnology companies around the world. The Company’s integrated services include data management, statistical analysis, clinical trial management, and regulatory and drug development consulting.
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States, or GAAP.
Secondary Offerings
During 2018, 2017 and 2016, Kohlberg Kravis Roberts & Co. L.P., or KKR, and certain executive officers of the Company sold a total of 6,500,000, 10,000,000 and 17,500,000 shares, respectively, of the Company’s common stock as part of secondary offerings. The Company incurred professional fees in connection with the secondary offerings of $0.5 million, $1.0 million and $1.3 million during years ended December 31, 2018, 2017 and 2016, respectively. The fees are included in transaction-related costs in the accompanying consolidated statement of operations. As of December 31, 2018, KKR owned 10.2% of the Company’s outstanding common stock.
(2) Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts and operations of the Company, its subsidiaries and investments in which the Company has control. Amounts pertaining to the non-controlling ownership interests held by third parties in the operating results and financial position of the Company’s majority-owned subsidiaries are reported as non-controlling interests. Intercompany accounts and transactions have been eliminated in consolidation.
Variable Interest Entities
Financial Accounting Standards Board’s, or FASB, accounting guidance concerning variable interest entities, or VIE, addresses the consolidation of business enterprise to which the usual condition of consolidation (ownership of a majority voting interest) does not apply. This guidance focuses on controlling financial interests that may be achieved through arrangements that do not involve voting interests. The guidance requires an assessment of who the primary beneficiary is and whether the primary beneficiary should consolidate the VIE. The primary beneficiary is identified as the variable interest holder that has both the power to direct the activities of the variable interest entity that most significantly impacts the entity’s economic performance and the obligation to absorb losses or the right to receive benefits from the entity that could potentially be significant to the variable interest entity. Application of the VIE consolidation requirements may require the exercise of significant judgment by management.
Takeda PRA Development Center KK
The Company entered into a joint venture with Takeda Pharmaceutical Company Ltd. during 2017. For further discussion on the joint venture, refer to Note 4, Business Combinations.
Accounts Receivable Financing Agreement
On March 22, 2016, the Company entered into a receivable financing agreement, which the Company refers to as the "accounts receivable financing agreement," to securitize certain of its accounts receivable. This agreement was subsequently amended on May 31, 2018. Under the accounts receivable financing agreement, certain of the Company’s U.S. accounts receivable and unbilled services balances are sold by certain of its consolidated subsidiaries to another of its consolidated
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
subsidiaries, a wholly-owned bankruptcy-remote special purpose entity, or SPE. The SPE in turn may borrow up to $200.0 million from a third-party lender, secured by liens on the receivables and other assets of the SPE.
The Company retains the servicing of the securitized accounts receivable portfolio and has a variable interest in the SPE by holding the residual equity. The Company determined that the SPE is a VIE and it is the primary beneficiary because (i) the Company’s servicing responsibilities for the securitized portfolio gives it the power to direct the activities that most significantly impact the performance of the VIE and (ii) its variable interest in the VIE gives it the obligation to absorb losses and the right to receive residual returns that could potentially be significant. As a result, the Company has consolidated the VIE within its financial statements.
Refer to Note 9, Revolving Credit Facilities and Long-Term Debt, for additional information regarding the accounts receivable financing agreement.
Risks and Other Factors
The Company’s revenues are dependent on research and development expenditures of the pharmaceutical and biotechnology industries. Any significant reduction in research and development expenditures by the pharmaceutical and biotechnology industries could have a material adverse effect on the Company and its results of operations.
Clients of the Company generally may terminate contracts without cause upon 30 to 60 days’ notice. While the Company generally negotiates deposit payments and early termination fees up front, such terminations could significantly impact the future level of staff utilization and have a material adverse effect on the Company and the results of future operations.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. In particular, the Company’s primary method of revenue recognition requires estimates of costs to be incurred to fulfill existing long-term contract obligations. Actual results could differ from those estimates. Estimates are also used when accounting for certain items such as allowance for doubtful accounts, depreciation and amortization, asset impairment, certain acquisition-related assets and liabilities including contingent consideration, income taxes, fair value determinations, and contingencies.
Reportable Segments
The Company is managed through two reportable segments, Clinical Research and Data Solutions. Clinical Research, which primarily serves biopharmaceutical clients, provides outsourced clinical research and clinical trial related services. Data Solutions provides data and analytics, technology solutions and real-world insights and services to companies in the pharmaceutical industry.
The Clinical Research segment is solely focused on the execution of clinical trials on a global basis. The Company has considered whether the delivery of the different types of capabilities in various stages of clinical development constitute separate products or lines of service in accordance with Accounting Standards Codification, or ASC, Topic 280, “Segment Reporting,” or ASC 280, and has concluded that there are substantial similarities and overlaps in the capabilities delivered at each stage of clinical development, with the primary differences between the Early Development Services, or EDS, compared to the Product Registration, or PR, and Strategic Solutions, or SS, relating to the points during the life cycle of a clinical trial at which such capabilities are delivered. After review and analysis of the operating characteristics of each service offering and using the aggregation characteristics under ASC 280, the Company has concluded that the services provided are similar across most characteristics.
The Company's operations consist of two reportable segments, which represents management's view of the Company's operations based on its management and internal reporting structure. The Company considered the guidance in ASC 350, “Intangibles—Goodwill and Other,” which notes that a reporting unit is an operating segment or one level below an operating segment. PR, EDS, and SS are the business units that are one level below the Company’s Clinical Research operating segment and the Company determined that they meet the definition of “components,” as discrete financial information exists and this
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
information is regularly reviewed by management. The Data Solutions operating segment does not have any material components.
Business Combinations
Business combinations are accounted for using the acquisition method and, accordingly, the identifiable assets acquired, the liabilities assumed, and any non-controlling interest in the acquiree are recorded at their estimated fair values on the date of the acquisition. Goodwill represents the excess of the purchase price over the estimated fair value of the net assets acquired, including the amount assigned to identifiable intangible assets.
Contingent Liabilities
The Company provides for contingent liabilities when (1) it is probable that an asset has been impaired or a liability has been incurred at the date of the consolidated financial statements and (2) the amount of the loss can be reasonably estimated. Disclosure in the notes to the consolidated financial statements is required for loss contingencies that do not meet both these conditions if there is a reasonable possibility that a loss may have been incurred. The Company expenses, as incurred, the costs of defending legal claims against the Company.
Cash Equivalents
The Company considers all highly-liquid investments purchased with an original maturity of three months or less to be cash equivalents. As of December 31, 2018 and 2017, substantially all of the Company’s cash and cash equivalents were held in or invested with large financial institutions. Certain bank deposits may at times be in excess of the Federal Deposit Insurance Corporation insurance limits.
Restricted cash
The Company receives cash advances from its customers to be used for the payment of investigator costs and other pass-through expenses. The terms of certain customer contracts require that such advances be maintained in separate escrow accounts; these accounts are not commingled with the Company’s cash and cash equivalents and are presented separately in the consolidated balance sheets as restricted cash.
The following table provides a reconciliation of cash, cash equivalents, and restricted cash reported within the consolidated balance sheets that sum to the total of the same amounts shown in the consolidated statements of cash flows (in thousands):
|
| | | | | | | | | | | |
| December 31, |
| 2018 | | 2017 | | 2016 |
Cash and cash equivalents | $ | 144,221 |
| | $ | 192,229 |
| | $ | 144,623 |
|
Restricted cash | 488 |
| | 661 |
| | 4,715 |
|
Total cash, cash equivalents, and restricted cash | $ | 144,709 |
| | $ | 192,890 |
| | $ | 149,338 |
|
Accounts Receivable and Unbilled Services
Accounts receivable represent amounts for which invoices have been sent to clients based upon contract terms. Unbilled services represent amounts earned for services that have been rendered but for which customers have not been billed. Unbilled services where the Company’s right to bill is conditioned on something other than the passage of time are contract assets and are separately disclosed in Note 5, Accounts Receivable, Unbilled Services, and Advanced Billings.
Allowances for Doubtful Accounts
The Company maintains an allowance for estimated losses resulting from the inability of its customers to make required payments. The Company performs credit reviews of each customer, monitors collections and payments from customers, and determines the allowance based upon historical experience and specific customer collection issues. The
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Company ages billed accounts receivable and assesses exposure by customer type, by aged category, and by specific identification. After all attempts to collect a receivable have failed, the receivable is written off against the allowance or, to the extent unreserved, to bad debt expense.
Advanced Billings
Advanced billings, also referred to as contract liabilities, consist of advanced payments and billings on a contract in excess of revenue recognized. These amounts represent consideration received or unconditionally due from a customer prior to transferring services to the customer under the terms of the service contract. These balances are reported net of contract assets on a contract-by-contract basis at the end of each reporting period.
In order to determine revenue recognized in the period from advanced billings liabilities, the Company first allocates revenue from the customer contract to the individual advanced billings liability balance outstanding at the beginning of the period until the revenue exceeds that balance.
Fixed Assets
Fixed assets and software purchased or developed for internal use are recorded at cost and are depreciated on a straight-line basis over the following estimated useful lives:
|
| |
Furniture, fixtures and equipment | 5-7 years |
Computer hardware and software | 3-7 years |
Leasehold improvements | Lesser of the life of the lease or useful life of the improvements |
Internal Use Software
The Company accounts for internal use software in accordance with the guidance in ASC 350‑40, “Internal-Use Software," which requires certain direct costs and interest costs incurred during the application stage of development to be capitalized and amortized over the useful life of the software.
Derivative Financial Instruments
The Company utilizes interest rate swaps to manage changes in market conditions related to debt obligations. All derivatives are measured at fair value and recognized as either assets or liabilities on the consolidated balance sheets. Derivatives that are not determined to be effective hedges are adjusted to fair value with a corresponding effect on earnings. Changes in the fair value of derivatives that are designated and determined to be effective as part of a hedge transaction have no immediate effect on earnings and depending on the type of hedge, are recorded either as part of other comprehensive loss and will be included in earnings in the period in which earnings are affected by the hedged item, or are included in earnings as an offset to the earnings impact of the hedged item. Any ineffective portion of hedges is reported in earnings as it occurs. Amounts previously recorded in accumulated other comprehensive loss related to these interest rate swaps will be reclassified into earnings over the term of the previously hedged borrowing using the swaplet method. The Company has elected the accounting policy that cash flows associated with interest rate derivative contracts are classified as cash flows from investing activities.
Contingent Consideration
The consideration for the Company’s acquisitions may include potential future earn-out payments that are contingent upon the occurrence of particular events. These payments might be based on the achievement of future revenue or earnings milestones. The Company records a contingent consideration obligation for such contingent payments at fair value on the acquisition date. The Company estimates the fair value of contingent consideration obligations through valuation models designed to estimate the probability of such contingent payments based on various assumptions and incorporating estimated success rates. Estimated payments are discounted using present value techniques to arrive at an estimated fair value at the balance sheet date. Changes in the fair value of the contingent consideration obligations, excluding adjustments that qualify as measurement period adjustments, are recognized within the Company’s consolidated statements of operations. Changes in the fair value of the contingent consideration obligations can result from changes to one or multiple inputs, including adjustments to the discount rates, changes in the amount or probability of achieving certain revenue or earnings targets. These fair value
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
measurements are based on significant inputs not observable in the market. Substantial judgment is employed in determining the appropriateness of these assumptions as of the acquisition date and for each subsequent period. Accordingly, changes in assumptions or actual results could have a material impact on the amount of contingent consideration expense the Company records in any given period.
Fair Value Measurements
The Company records certain assets and liabilities at fair value. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy that prioritizes the inputs used to measure fair value is described below. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| |
• | Level 1—Quoted prices in active markets for identical assets or liabilities. |
| |
• | Level 2—Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data. |
| |
• | Level 3—Unobservable inputs that are supported by little or no market activity. This includes certain pricing models, discounted cash flow methodologies and similar techniques that use significant unobservable inputs. |
The carrying amount of financial instruments, including cash and cash equivalents, accounts receivable, unbilled services, accounts payable and advanced billings, approximate fair value due to the short maturities of these instruments.
Recurring Fair Value Measurements
The following table summarizes the fair value of the Company’s financial assets that are measured on a recurring basis as of December 31, 2018 (in thousands):
|
| | | | | | | | | | | | | | | |
| Level 1 | | Level 2 | | Level 3 | | Total |
Assets: | | | | | | | |
Interest rate swaps | $ | — |
| | $ | 3,318 |
| | $ | — |
| | $ | 3,318 |
|
Total | $ | — |
| | $ | 3,318 |
| | $ | — |
| | $ | 3,318 |
|
The following table summarizes the fair value of the Company’s financial assets and liabilities that are measured on a recurring basis as of December 31, 2017 (in thousands):
|
| | | | | | | | | | | | | | | |
| Level 1 | | Level 2 | | Level 3 | | Total |
Assets: | | | | | | | |
Interest rate swap | $ | — |
| | $ | 428 |
| | $ | — |
| | $ | 428 |
|
Marketable securities | 393 |
| | — |
| | — |
| | 393 |
|
Total | $ | 393 |
| | $ | 428 |
| | $ | — |
| | $ | 821 |
|
Liabilities: | | | | | | | |
Contingent consideration | $ | — |
| | $ | — |
| | $ | 50,644 |
| | $ | 50,644 |
|
Total | $ | — |
| | $ | — |
| | $ | 50,644 |
| | $ | 50,644 |
|
Until the finalization of the earn-out period, the Company values contingent consideration using models that include significant unobservable Level 3 inputs, such as projected financial performance over the earn-out period along with estimates for market volatility and the discount rate applicable to potential cash payments. Interest rate swaps are measured at fair value using a market approach valuation technique. The valuation is based on an estimate of net present value of the expected cash flows using relevant mid-market observable data inputs and based on the assumption of no unusual market conditions or forced liquidation.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The following table summarizes the changes in Level 3 financial liabilities measured on a recurring basis (in thousands):
|
| | | | | | | | |
| | Contingent consideration - Accrued expenses and other current liabilities | | Contingent consideration - Other long-term liabilities |
Balance at December 31, 2016 | | $ | 1,735 |
| | $ | 1,019 |
|
Initial estimate of Symphony Health contingent consideration | | 90,394 |
| | 18,390 |
|
Initial estimate of Parallel 6, Inc. contingent consideration | | — |
| | 8,350 |
|
Payments on Nextrials contingent consideration | | (400 | ) | | — |
|
Payments on Symphony Health contingent consideration | | (67,788 | ) | | — |
|
Measurement period adjustments | | 24,388 |
| | 14,279 |
|
Changes in fair value included in earnings | | 66,363 |
| | 8,606 |
|
Transfer out | | (114,692 | ) | | — |
|
Balance at December 31, 2017 | | — |
| | 50,644 |
|
Reclassification adjustment | | 50,644 |
| | (50,644 | ) |
Change in fair value recognized in transaction-related costs | | 34,538 |
| | — |
|
Transfer out | | (85,182 | ) | | — |
|
Balance at December 31, 2018 | | $ | — |
| | $ | — |
|
The $85.2 million transfer out during the year ended December 31, 2018 represents the year-end 2018 earn-out payment to the sellers of Symphony Health Solutions Corporation, or Symphony Health, as the earn-out period concluded on December 31, 2018.
The $114.7 million transfer out during the year ended December 31, 2017 represents the year-end 2017 earn-out payment to the sellers of Symphony Health that was calculated using the settlement formula at December 31, 2017. The remaining $50.6 million balance at December 31, 2017, which was valued using a Monte Carlo simulation, relates to the 2018 earn-out payment to Symphony Health and was based on its future adjusted earnings before interest, taxes, depreciation and amortization, or Adjusted EBITDA. Key assumptions include (1) a discount rate of 8%, (2) a volatility rate of 32%, and (3) probability adjusted level of Adjusted EBITDA of $56.5 million for the year ended December 31, 2018. Refer to Note 4, Business Combinations, for additional discussion of the Symphony Health acquisition.
Non-recurring Fair Value Measurements
Certain assets and liabilities are carried on the accompanying consolidated balance sheets at cost and are not remeasured to fair value on a recurring basis. These assets include finite-lived intangible assets, which are tested when a triggering event occurs, and goodwill and identifiable indefinite-lived intangible assets, which are tested for impairment annually on October 1 or when a triggering event occurs.
As of December 31, 2018, assets carried on the balance sheet and not remeasured to fair value on a recurring basis totaling approximately $2,199.2 million were identified as Level 3. These assets are comprised of goodwill of $1,494.8 million and identifiable intangible assets, net of $704.4 million.
Refer to Note 9, Revolving Credit Facilities and Long-Term Debt, for additional information regarding the fair value of long-term debt balances.
Impairment of Long-Lived Assets
The Company reviews the recoverability of its long-lived asset groups, including furniture and equipment, computer hardware and software, leasehold improvements, and other finite-lived intangibles, when events or changes in circumstances occur that indicate the carrying value of the asset group may not be recoverable. The assessment of possible impairment is based on the Company’s ability to recover the carrying value of the asset group from the expected future pre-tax cash flows (undiscounted and without interest charges) of the related operations. If these cash flows are less than the carrying value of such
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
asset, an impairment loss is recognized for the difference between estimated fair value and carrying value. The Company’s primary measure of fair value is based on discounted cash flows. The measurement of impairment requires the Company to make estimates of these cash flows related to long-lived assets, as well as other fair value determinations.
Goodwill and Other Intangibles
Goodwill and indefinite-lived intangible assets are tested for impairment annually or more frequently if an event or circumstance indicates that an impairment loss may have been incurred. Separate intangible assets that have finite useful lives are amortized over their estimated useful lives or over the period in which economic benefit is received. The Company’s primary finite-lived intangibles are customer relationships, customer backlog, and acquired databases, which are amortized on an accelerated basis, which coincides with the period of economic benefit received by the Company.
The Company reviews the carrying value of goodwill to determine whether impairment may exist on an annual basis or whenever it has reason to believe goodwill may not be recoverable. The annual impairment test of goodwill is performed during the fourth quarter of each fiscal year. The Company did not have an impairment for any of the years presented.
When evaluating for impairment, the Company may first perform a qualitative assessment to determine whether it is more likely than not that a reporting unit or indefinite-lived intangible asset is impaired. If the Company does not perform a qualitative assessment, or if it determines that it is not more likely than not that the fair value of the reporting unit or indefinite-lived intangible asset exceeds its carrying amount, the Company will calculate the estimated fair value of the reporting unit or indefinite-lived intangible asset. The Company’s decision to perform a qualitative impairment assessment for an individual reporting unit in a given year is influenced by a number of factors, inclusive of the size of the reporting unit's goodwill, the significance of the excess of the reporting unit's estimated fair value over carrying value at the last quantitative assessment date, the amount of time in between quantitative fair value assessments and the date of acquisition. During 2018, as part of the Company’s annual impairment analysis, the Company performed the qualitative assessment for approximately $1.0 billion, or 68.1% of its goodwill balance of $1.5 billion, which relates to its EDS, PR, and SS business units, and for its indefinite-lived trade name intangible asset balances.
If the Company does not perform a qualitative assessment, goodwill impairment is determined by the Company using a two-step process. The first step of the goodwill impairment test is used to identify potential impairment by comparing the fair value of each reporting unit, determined using various valuation techniques, with the primary technique being a discounted cash flow analysis, to its carrying value. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test is performed to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of the reporting unit’s goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit’s goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized. The Company performed its impairment test for the Data Solutions operating segment during the fourth quarter of 2018. It was concluded that the estimated fair value of the Data Solutions operating segment exceeded its carrying value by approximately $200.0 million, or 34.6%.
Revenue Recognition
As discussed further below under Recently Implemented Accounting Standards, on January 1, 2018, the Company adopted Accounting Standards Codification, or ASC, Topic 606, "Revenue from Contracts with Customers," or ASC 606, using the modified retrospective method for all contracts that were not completed as of January 1, 2018. Comparative prior period information continues to be accounted for under the accounting standards in effect for the period presented. Accordingly, the Company has included our revenue recognition policies and disclosures for the year ended December 31, 2018 and the policies applicable for the years ended December 31, 2017 and 2016 are described below.
Revenue Recognition Policies for the year ended December 31, 2018
All revenue is generated from contracts with customers. Revenue is recognized when control of the performance obligation is transferred to the customer, in an amount that reflects the consideration the Company expects to be entitled to receive in exchange for those services. Revenue recognition is determined through the application of the following steps:
| |
• | identification of the contract, or contracts, with a customer; |
| |
• | identification of the performance obligations in the contract; |
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
| |
• | determination of the transaction price; |
| |
• | allocation of the transaction price to the performance obligations in the contract; and |
| |
• | recognition of revenue when, or as, the Company satisfies a performance obligation. |
Clinical Research
The Company generally enters into contracts with customers to provide clinical research services with payments based on either fixed‑fee, time and materials, or fee‑for‑service arrangements. The Company is also entitled to reimbursement for investigator fees and out-of-pocket costs associated with these services. At contract inception, the Company assesses the services promised in the contracts with customers to identify the performance obligations in the arrangement. The Company’s long term fixed-fee arrangements for clinical research services are considered a single performance obligation because the Company provides a highly-integrated service. A single performance obligation requires the inclusion of investigator fees and out-of-pocket costs in both the contract revenue value and in the cost used to measure progress in transferring control to the customer.
The inclusion of investigator fees and out-of-pocket costs in the measurement of progress under these long-term fixed-fee contracts as part of a single performance obligation can create a timing difference between amounts the Company is entitled to receive in reimbursement for costs incurred and the amount of revenue recognized related to such costs on individual projects, which is recognized as unbilled services. The magnitude of this timing difference compared to historical accounting is dependent on the relative size and progress of the direct service portion of the arrangement compared to the progress of the reimbursable investigator fees and reimbursable out-of-pocket costs relative to their respective forecasted costs over the life of the project.
Revenue is recognized for the single performance obligation over time due to the Company’s right to payment for work performed to date. The contracts generally provide for the right to invoice the customer as work progresses, either based on units performed or the achievement of billing milestones. The Company generally uses the cost-to-cost measure of progress for the Company's contracts because it best depicts the transfer of control to the customer as the performance obligation is fulfilled. For this method, the Company compares the contract costs incurred to date to the estimated total contract costs through completion. As part of the client proposal and contract negotiation process, the Company develops a detailed project budget for the direct costs and reimbursable costs based on the scope of the work, the complexity of the study, the geographical location involved and the Company’s historical experience.
The estimated total contract costs at the project level are reviewed and revised periodically throughout the life of the contract, with adjustments to revenue resulting from such revisions being recorded on a cumulative basis in the period in which the revisions are identified. Contract costs consist primarily of direct labor and other reimbursable project-related costs such as travel, third-party vendor costs and investigator fees.
The Company establishes pricing based on the Company’s internal pricing guidelines, discount agreements, if any, and negotiations with the client. The transaction price is the contractually defined amount that includes adjustment for variable consideration such as reimbursable costs, discounts, and bonus or penalties, which are estimable.
A majority of the Company’s contracts undergo modifications over the contract period and the Company’s contracts provide for these modifications. During the modification process, the Company recognizes revenue to the extent it incurs costs, provided that a contractual understanding has been reached.
Fixed-fee arrangements for Phase I and Phase II(a) clinical services and bio-analytical services are short-term contracts for accounting purposes as these contracts are cancelable and the termination penalties for exiting these contracts are not substantive. The Company generally bills for services on a milestone basis. The transaction price, representing the value of the services to be provided over the entire contract inclusive of all costs for which the Company is a principal, is the contractually defined amount that includes adjustment for variable consideration, such as reimbursable expenses and discounts, which are estimable. When multiple performance obligations exist, the transaction price is allocated to the performance obligations on a relative standalone selling price basis. Given the highly integrated nature of the services provided, most contracts represent a single performance obligation. Due to the Company's right to payment for work performed, revenue is recognized over time as services are delivered.
Clinical research services delivered under fee-for-service arrangements are recognized over time. The services are accounted for as a single performance obligation that is a series of distinct services with substantially the same pattern of
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
transfer to the customer. Clinical research services provided in these types of arrangements are typically linked to the delivery of resources billed at contractual rates, such rates being dependent on the role and the tenure of the resource provided. The fee-for-service is typically billed one month in arrears, which generally results in an unbilled services asset at period-end. In addition, out-of-pocket costs are reimbursed by the customer. Fees are allocated to each distinct month of service using time elapsed as a measure of progress toward the satisfaction of the performance obligation and variable consideration is allocated to the period in which it is incurred.
Revenue from time and materials contracts is recognized as hours are incurred.
The Company often offers volume discounts to certain of its large customers based on annual volume, which is variable consideration that is considered in the contract value. The Company records an estimate of the volume rebate as a reduction of the transaction price based on the estimated total rebates to be earned by the customers for the period.
Data Solutions
The Company provides data reports and analytics to customers based on agreed-upon specifications, including the timing of delivery, which is typically either weekly, monthly, or quarterly. If a customer requests more than one type of data report or series of data reports within a contract, each distinct type of data report is a separate performance obligation. The contracts provide for the Company to be compensated for the value of each deliverable. The transaction price is determined using list prices, discount agreements, if any, and negotiations with the customers, and generally includes any out-of-pocket expenses. Typically, the Company bills in advance of services being provided with the amount being recorded as advanced billings.
When multiple performance obligations exist, the transaction price is allocated to performance obligations on a relative standalone selling price basis. In cases where the Company contracts to provide a series of data reports, or in some cases data, the Company recognizes revenue over time using the “units delivered” output method as the data or reports are delivered. Expense reimbursements are recorded to revenue as the expenses are incurred as they relate directly to the services performed.
Certain Data Solutions arrangements include upfront customization or consultative services for customers. These arrangements often include payments based on the achievement of certain contractual milestones. Under these arrangements, the Company contracts with a customer to carry out a specific study, ultimately resulting in delivery of a custom report or data product. These arrangements are a single performance obligation given the integrated nature of the service being provided. The Company typically recognizes revenue under these contracts over time, using an output-based measure, generally time elapsed, to measure progress and transfer of control of the performance obligation to the customer. Expense reimbursements are recorded to revenue as the expenses are incurred as they relate directly to the service performed.
The Company's Data Solutions segment enters into contracts with some of its larger data suppliers that involve non-monetary terms. The Company will issue purchase credits to be used toward the data supplier's purchase of the Company's services. In exchange, the Company receives monetary discounts on the data received from the data suppliers. The fair value of the revenue earned from the customer purchases is determined based on similar product offerings to other customers. At the end of the contract year, any unused customer purchase credits may be forfeited or carried over to the next contract year based on the terms of the data supplier contract. For the years ended December 31, 2018 and 2017, the Company recognized service in kind revenue of $21.8 million and $5.8 million, respectively, from these transactions, which is included in revenue in the accompanying consolidated condensed statements of operations. The cost of data acquired under these arrangements is included in direct costs.
Significant Judgments and Estimates
Accounting for the Company’s long term contracts requires estimates of future costs to be incurred to fulfill the contract obligations.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Due to the nature of the work required to be performed by the Company to fulfill performance obligations, the estimation of total revenue and cost at completion is complex, subject to many variables and requires significant judgment. The Company's long-term contracts may contain incentive fees, penalties, or other provisions that can either increase or decrease the transaction price. The Company estimates variable consideration at the most likely amount to which the Company expects to be entitled. The Company includes estimated amounts in the transaction price to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is resolved. The Company's estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on an assessment of the anticipated performance and information that is available to the Company. Judgment is also required to identify performance obligations and in determining the relative standalone selling price of those obligations, specifically for the Data Solutions segment. The estimates and assumptions are evaluated on an ongoing basis and adjusted, as needed, using historical experience and contract specific factors. Actual results could differ significantly from these estimates.
Performance Obligations
Revenue recognized for the year ended December 31, 2018 from services completed in prior periods was $79.1 million. This primarily relates to adjustments attributable to changes in estimates such as estimated total contract costs, and from contract modifications on long-term fixed price contracts executed in the current period, which result in changes to the transaction price.
The Company does not disclose the value of the transaction price allocated to unsatisfied performance obligations on contracts that have an original contract term of less than one year. These contracts are short in duration and revenue recognition generally follows the delivery of the promised services. The total transaction price for the undelivered performance obligation on contracts with an original initial contract term greater than one year is $4.8 billion as of December 31, 2018. This amount includes reimbursement revenue and investigator fees. The Company expects to recognize revenue over the remaining contract term of the individual projects, with contract terms generally ranging from one to five years.
Revenue Recognition Policies for the year ended December 31, 2017 and 2016
Revenue for services is recognized only after persuasive evidence of an arrangement exists, the sales price is determinable, services have been rendered, and collectability is reasonably assured.
Once these criteria have been met, the Company recognizes revenue for the services provided on fixed-fee contracts in the Clinical Research segment based on the proportional performance methodology, which determines the proportion of outputs or performance obligations that have been completed or delivered compared to the total contractual outputs or performance obligations. To measure performance, the Company compares the contract costs incurred to estimated total contract costs through completion. The estimated total contract costs are reviewed and revised periodically throughout the life of the contract, with adjustments to revenue resulting from such revisions being recorded on a cumulative basis in the period in which the revisions are first identified. Contract costs consist primarily of direct labor and other project-related costs. The Company recognizes revenue for services provided on fixed-fee contracts in the Data Solutions segment either ratably as earned over the contract period, for subscription-based services, or upon delivery, for one-time delivery of data solutions or reports. Revenue from time and materials contracts is recognized as hours are incurred. Revenues and the related costs of fee-for-service contracts are recognized in the period in which services are performed.
In the Clinical Research segment, a majority of contracts undergo modifications over the contract period and the Company’s contracts provide for these modifications. During the modification process, the Company recognizes revenue to the extent it incurs costs, provided client acceptance and payment is deemed reasonably assured.
The Company records an estimate of the annual volume rebate as a reduction of revenue throughout the period based on the estimated total rebate to be earned for the period.
The Company incurs out-of-pocket costs that are reimbursable by its customers. The Company includes out-of-pocket costs both as reimbursement revenue and as reimbursable out-of-pocket costs in the consolidated statements of operations.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
As is customary in the industry, the Company routinely enters into separate agreements on behalf of its clients with independent physician investigators in connection with clinical trials. The funds received for investigator fees are netted against the related cost because such fees are the obligation of the Company’s clients, without risk or reward to the Company. The Company is not obligated either to perform the service or to pay the investigator in the event of default by the client. In addition, the Company does not pay the independent physician investigator until funds are received from the client. Total payments to investigators were $262.1 million, $250.9 million, and $249.6 million and for the years ended December 31, 2018, 2017, and 2016, respectively. Prior to the year ended December 31, 2018, the Company did not recognize revenue for investigator fees and recognized revenue and the related expense for reimbursable out-of-pocket costs. Upon adoption of ASC 606, all investigator and other reimbursable expenses are included within revenue as part of one performance obligation, as described above.
The revenue captions for the years ended December 31, 2017 and 2016 have been recast to conform with the presentation of a single revenue total in the consolidated statement of operations as opposed to separate line items. Previously, the year ended December 31, 2017 included service revenue of $1,948.4 million, reimbursement revenue of $311.0 million, and excluded $250.9 million in investigator fees that were reported net of costs incurred. The year ended December 31, 2016 included service revenue of $1,580.0 million and reimbursement revenue of $231.7 million, and excluded $249.6 million in investigator fees that were reported net of costs incurred.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist of cash and cash equivalents, accounts receivable, unbilled services, and derivatives. As of December 31, 2018, substantially all of the Company’s cash and cash equivalents were held in or invested with large financial institutions. Accounts receivable include amounts due from pharmaceutical and biotechnology companies. The Company establishes an allowance for potentially uncollectible receivables. In management’s opinion, there is no additional material credit risk beyond amounts provided for such losses.
Revenue from individual customers greater than 10% of consolidated revenue in the respective periods was as follows:
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 (1) | | 2016 (1) |
Customer A | * | | 10.3 | % | | 11.0 | % |
Customer B | * | | * |
| | 10.4 | % |
(1) As noted in the Revenue Recognition section of this Note, the Company adopted ASC 606 on January 1, 2018 using the modified retrospective method. Comparative prior period amounts were calculated using service revenue only.
* Less than 10%
Accounts receivable and unbilled receivables from individual customers that were equal to or greater than 10% of consolidated accounts receivable and unbilled receivables at the respective dates were as follows:
|
| | | | | |
| December 31, |
| 2018 | | 2017 |
Customer A | 12.2 | % | | 11.5 | % |
Customer B | 11.4 | % | | * |
|
* Less than 10%
Foreign Currency
The assets and liabilities of the Company’s foreign subsidiaries are translated into U.S. dollars at exchange rates in effect as of the end of the period. Equity activities are translated at the spot rate effective at the date of the transaction. Revenue and expense accounts and cash flows of these operations are translated at average exchange rates prevailing during the period the transactions occurred. Translation gains and losses are included as an adjustment to the accumulated other comprehensive loss account in stockholders’ equity. In addition, gains or losses related to the Company’s intercompany loans payable and
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
receivable denominated in a foreign currency other than the subsidiary’s functional currency that are deemed to be of a long-term investment nature are remeasured to cumulative translation and recorded in accumulated other comprehensive loss in the consolidated balance sheets.
Translation gains and losses from foreign currency transactions, such as those resulting from the settlement and revaluation of foreign receivables and payables, are included in the determination of net income. These amounts are included in foreign currency (losses) gains, net in the consolidated statements of operations.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets are recognized for future deductible temporary differences, along with net operating loss carryforwards and credit carryforwards, if it is more likely than not that the tax benefits will be realized. To the extent a deferred tax asset cannot be recognized under the preceding criteria, a valuation allowance is established to reduce the deferred tax asset to the amount that is more likely than not to be realized. Deferred tax liabilities are recognized for future taxable temporary differences. Deferred tax assets and liabilities are measured using enacted tax rates in effect for the year in which those temporary differences are expected to be recovered or settled.
There are inherent uncertainties related to the interpretation of tax regulations in the jurisdictions in which the Company transacts business. The judgments and estimates made at a point in time may change based on the outcome of tax audits, as well as changes to, or further interpretations of, regulations. Income tax expense is adjusted in the period in which these events occur, and these adjustments are included in the Company’s consolidated statement of operations. If such changes take place, there is a risk that the Company’s effective tax rate may increase or decrease in any period. A company must recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution.
Stock-Based Compensation
The primary type of stock-based compensation utilized by the Company is stock options. Stock options are awards which allow the employee to purchase shares of the Company’s stock at a fixed price. The Company measures compensation cost at the grant date, based on fair value of the award, and recognizes it as expense over the employees’ requisite service period.
The fair value of each option issued during these periods was estimated on the date of grant using the Black-Scholes option pricing model for service condition awards with the following weighted average assumptions:
|
| | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Risk-free interest rate | 2.8 | % | | 1.9 | % | | 1.5 | % |
Expected life, in years | 6.3 |
| | 6.3 |
| | 6.3 |
|
Dividend yield | N/A |
| | N/A |
| | N/A |
|
Volatility | 28.9 | % | | 29.7 | % | | 31.2 | % |
The risk-free interest rate is based on the United States Treasury yield curve in effect at the time of the grant. The expected life represents the period of time the grants are expected to be outstanding. The Company uses the historical volatilities of a selected peer group as it does not have sufficient history to estimate the volatility of its common share price. The Company calculates expected volatility based on reported data for selected reasonably similar publicly traded companies for which the historical information is available. For the purpose of identifying peer companies, the Company considers characteristics such as industry, length of trading history, similar vesting terms and in-the-money option status.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Due to the absence of an active market for the Company’s common shares prior to the Company’s initial public offering, or IPO, the fair value of the Company's common shares for purposes of determining the exercise price for award grants was determined in good faith by the Company’s Board of Directors, or Board, with the assistance and upon the recommendation of management based on a number of market factors, including: the common shares underlying the award involved illiquid securities in a private company; results of operations and financial position; and the market performance of publicly traded companies compared to the Company.
The Company accounts for its stock-based compensation for restricted share awards and restricted share units, or collectively RSAs/RSUs, based on the closing market price of the Company’s common stock on the trading day immediately prior to the grant date, and recognizes it as expense over the employees’ requisite service period.
Net Income Per Share
The calculation of net income per share, or EPS, is based on the weighted average number of common shares or common stock equivalents outstanding during the applicable period. The dilutive effect of common stock equivalents is excluded from basic earnings per share and is included in the calculation of diluted earnings per share, unless the effect of inclusion would be anti-dilutive.
Debt Issuance Costs
Debt issuance costs relating to the Company’s long-term debt are recorded as a direct reduction of long-term debt; these costs are deferred and amortized to interest expense using the effective interest method, over the respective terms of the related debt. Debt issuance costs relating to the Company’s revolving credit facilities are recorded as an asset; these costs are deferred and amortized to interest expense using the straight-line method.
Compensated Absences
The Company accrues for the costs of compensated absences to the extent that the employee’s right to receive payment relates to service already rendered, the obligation vests or accumulates, payment is probable and the amount can be reasonably estimated. The Company’s policies related to compensated absences vary by jurisdiction and obligations are recorded net of estimated forfeiture due to turnover when reasonably predictable.
Operating Leases
The Company records rent expense for operating leases, some of which have escalating rent over the term of the lease, on a straight-line basis over the initial effective lease term. The Company begins recognition of rent expense on the date of initial possession, which is generally when the Company enters the space and begins to make improvements in preparation for its intended use. Some of the Company’s facility leases provide for concessions by the landlords, including payments for leasehold improvements considered tenant assets, free rent periods, and other lease inducements. The Company reflects these concessions as deferred rent in the accompanying consolidated financial statements. The Company accounts for the difference between rent expense and rent paid as deferred rent. For tenant allowances for improvements considered to be tenant assets, rent holidays and other lease incentives, the Company records a deferred rent liability at the inception of the lease term and amortizes the deferred rent over the term of the lease as a reduction to rent expense. For tenant allowances considered to be property owner assets, the payment is treated as a reimbursement for the cost of the lessor asset.
Transaction-related Cost
Transaction-related costs consist primarily of: (1) the change in the fair value of acquisition-related contingent consideration; (2) costs incurred in connection with due diligence performed in connection with acquisitions; (3) costs associated with the accounts receivable financing agreement; and (4) secondary offering costs, which consist of stock-based compensation expense related to the release of the transfer restrictions on vested options and third-party fees incurred in connection with the offerings.
Recently Implemented Accounting Standards
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Upon the adoption of ASC 606 on January 1, 2018, the Company recorded a net decrease in opening retained earnings of $60.6 million as of January 1, 2018 due to the cumulative impact of adopting ASC 606. The Company decreased unbilled services by $67.4 million, increased deferred tax assets by $18.3 million, increased accrued expenses by $50.8 million, and decreased advanced billings by $39.3 million as of January 1, 2018. The adoption of ASC 606 had no net impact on the Company’s consolidated statement of cash flows.
The impact of adoption of ASC 606 on the Company's consolidated condensed statements of operations for the year ended December 31, 2018 is as follows (in thousands):
|
| | | | | | | | | | | | | | | | |
| | As Reported | | Reclassification from adoption of ASC 606 | | Impact from adoption of ASC 606 | | Balances without adoption of ASC 606 |
Revenue: | | | | | | | | |
Revenue | | $ | 2,871,922 |
| | $ | (2,605,140 | ) | | $ | (266,782 | ) | | $ | — |
|
| | | | | | | | |
Service revenue | | — |
| | 2,296,849 |
| | — |
| | 2,296,849 |
|
Reimbursement revenue | | — |
| | 308,291 |
| | — |
| | 308,291 |
|
Total revenue | | 2,871,922 |
| | — |
| | (266,782 | ) | | 2,605,140 |
|
Operating expenses: | | | | | | | | |
Direct costs (exclusive of depreciation and amortization) | | 1,500,226 |
| | — |
| | — |
| | 1,500,226 |
|
Reimbursable out-of-pocket costs | | 308,291 |
| | — |
| | — |
| | 308,291 |
|
Reimbursable investigator fees | | 262,114 |
| | — |
| | (262,114 | ) | | — |
|
Selling, general and administrative expenses | | 371,795 |
| | — |
| | — |
| | 371,795 |
|
Transaction-related costs | | 35,817 |
| | — |
| | — |
| | 35,817 |
|
Depreciation and amortization | | 112,247 |
| | — |
| | — |
| | 112,247 |
|
Loss on disposal of fixed assets, net | | 120 |
| | — |
| | — |
| | 120 |
|
Income from operations | | $ | 281,312 |
| | $ | — |
| | $ | (4,668 | ) | | $ | 276,644 |
|
In January 2018, the FASB released guidance on the accounting for tax on the global intangible low-taxes income (“GILTI”) provisions of the Tax Cuts and Jobs Act (the “Act”). The GILTI provisions impose a minimum tax on certain foreign earnings. In the first quarter of 2018, the Company elected to treat the related GILTI inclusions as a period cost.
In March 2016, the FASB issued Accounting Standards Update, or ASU, No. 2016-09, “Compensation-Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting.” This update includes provisions intended to simplify various aspects of accounting for share-based compensation. In addition, ASU No. 2016-09 went into effect for public companies for annual periods beginning after December 15, 2016. The Company adopted this ASU beginning with the first quarter of 2017. The adoption of this ASU had the following effects on the consolidated financial statements:
Income taxes - The standard requires excess tax benefits and tax deficiencies to be recorded as income tax benefit or expense in the statement of operations. The Company applied the modified retrospective adoption approach beginning in 2017 and recorded a cumulative-effect adjustment to retained earnings and reduced its deferred tax liabilities by $12.6 million with an offsetting increase to the valuation allowance of $12.6 million. As such, the net impact to retained earnings was zero. This adjustment relates to tax assets that had previously arisen from tax deductions for equity compensation expenses that were greater than the compensation recognized for financial reporting. During the year ended December 31, 2017, the Company recorded $33.7 million in 2017 excess tax benefits associated with equity awards within (benefit from) provision for income taxes on the consolidated statement of operations.
Forfeitures – The standard provides an accounting policy election to account for forfeitures as they occur. The Company made this accounting policy election and the modified retrospective adoption for this component of the standard did not have a material impact on its financial statements.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Statements of Cash Flows - Cash flows related to excess tax benefits are no longer separately classified as a financing activity apart from other income tax cash flows. The Company adopted this component of the standard on a prospective basis.
Earnings Per Share - The Company uses the treasury stock method to compute diluted earnings per share, unless the effect would be anti-dilutive. Under this method, the Company is no longer required to estimate the tax rate and apply it to the dilutive share calculation for determining the dilutive earnings per share. The Company adopted this component of the standard on a prospective basis.
In January 2017, the FASB issued ASU No. 2017-01, “Business Combinations: Clarifying the Definition of a business,” which clarifies that when substantially all of the fair value of the gross assets acquired (or disposed of) is concentrated in a single identifiable asset or group of similar identifiable assets, it should be treated as an acquisition or disposal of an asset. The amendments to ASU No. 2017-01 were effective for fiscal years beginning after December 15, 2017. The adoption of ASU No. 2017-01 did not have a material impact on the Company's consolidated financial statements.
In May 2017, the FASB issued ASU No. 2017-09, “Compensation-Stock Compensation: Scope of Modification Accounting,” which provides guidance about what changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in ASC Topic 718, “Stock Compensation.” The amendments to ASU No. 2017-09 were effective on January 1, 2018. The adoption of ASU No. 2017-09 did not have a material impact on the Company's consolidated financial statements.
Recently Issued Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, “Leases,” which revises the accounting related to lessee accounting. Under the new guidance, lessees will be required to recognize a lease liability and a right-of-use asset for all leases with terms greater than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the statement of operations. In July 2018, the FASB issued ASU 2018-11, “Leases (Topic 842): Targeted Improvements,” which approved a transition method that provides the option to use the effective date as the date of initial application on transition. The Company will adopt ASU 2016-02 in the first quarter of 2019 and plans to elect this transition method, and as a result, the Company will not adjust comparative information for prior periods. The Company is progressing with its preparation for the adoption and implementation of this new accounting standard and related changes in internal controls. The Company anticipates that the adoption of this standard will have a material impact on its consolidated balance sheet as a lease liability associated with the remaining lease payments will be recorded on the consolidated balance sheet for all long-term leases, with recognition of a right-of-use asset. The adoption is not expected to have a material impact on the consolidated statements of operations or cash flows. The majority of the Company’s lease portfolio represents leases of real estate for its facilities. The Company’s future noncancelable lease obligations as of December 31, 2018 are disclosed in Note 13: Commitments and Contingencies, on an undiscounted basis.
In January 2017, the FASB issued ASU No. 2017-04, “Intangibles-Goodwill and Other: Simplifying the Test for Goodwill Impairment,” in order to simplify the subsequent measurement of goodwill by eliminating the Step 2 goodwill impairment test. Under the amendments in this ASU, an entity should perform its annual or interim goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity will then recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. The amendments to ASU No. 2017-04 are effective for fiscal years beginning after December 15, 2019, with early adoption permitted. The adoption of ASU No. 2017-04 is not expected to have a material impact on the Company's consolidated financial statements.
In August 2017, the FASB issued ASU No. 2017-12, "Derivatives and Hedging (Topic 815): Targeted Improvements to Accounting for Hedging Activities," in order to simplify certain aspects of hedge accounting and improve disclosures of hedging arrangements. ASU No. 2017-12 eliminates the need to separately measure and report hedge ineffectiveness and generally requires the entire change in fair value of a hedging instrument to be presented in the same income statement line as the hedged item. Entities must apply the amendments to cash flow and net investment hedge relationships that exist on the date of adoption using a modified retrospective approach. The presentation and disclosure requirements must be applied prospectively. The amendments to ASU No. 2017-12 are effective for fiscal years beginning after December 15, 2018. The adoption of ASU No. 2017-12 will not have a material impact on the Company's consolidated financial statements.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In February 2018, the FASB issued ASU No. 2018-02, "Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income." The amendments in this update allow a reclassification from accumulated other comprehensive income to retained earnings for stranded tax effects resulting from the Tax Cuts and Jobs Act. The amendments in this update also require entities to disclose their accounting policy for releasing income tax effects from accumulated other comprehensive income. The amendments to ASU No. 2018-02 are effective for the reporting period beginning after December 15, 2018, and interim periods therein. Early adoption is permitted. The Company is currently assessing the potential impact of ASU No. 2018-02 on the Company's consolidated financial statements.
In August 2018, the FASB issued ASU No. 2018-15, "Customer's Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract," in order to expand on the FASB's guidance of capitalized costs incurred in a cloud computing arrangement. The amendments in this update require an entity (customer) in a hosting arrangement that is a service contract to follow the guidance in Subtopic 350-40 to determine which implementation costs to capitalize as an asset related to the service contract and which costs to expense. The amendments to ASU No. 2018-15 are effective for the reporting period beginning after December 15, 2019, and interim periods therein, with early adoption permitted. The adoption of ASU No. 2018-15 is not expected to have a material impact on the Company's consolidated financial statements.
In October 2018, the FASB issued ASU No. 2018-16, "Inclusion of the Secured Overnight Financing Rate (SOFR) Overnight Index Swap (OIS) Rate as a Benchmark Interest Rate for Hedge Accounting Purposes", which amends ASC 815, Derivatives and Hedging. This ASU adds the OIS rate based on SOFR to the list of permissible benchmark rates for hedge accounting purposes. The Company will adopt ASU No. 2018-16 concurrent with adoption of ASU No. 2017-12 in the first quarter of 2019. The adoption will not have a material effect on the Company’s consolidated financial statements.
(3) Joint Ventures
The Company entered into a joint venture with Takeda Pharmaceutical Company Ltd. during 2017. For further discussion on the joint venture, refer to Note 4, Business Combinations.
On May 6, 2016, the Company and WuXi AppTec (Shanghai) Co., Ltd., or WuXi, finalized an agreement to dissolve the WuXiPRA joint venture. Under the agreement, the Company sold its 49% portion of the joint venture located in mainland China for $4.0 million, which subsequently became a wholly owned subsidiary of WuXi. The portion of the joint venture located in Hong Kong became a wholly owned subsidiary of the Company and was acquired for $0.3 million. As a result of the transaction, the Company recognized a $3.3 million gain on the sale, which is recorded in the equity in income of unconsolidated joint ventures in the accompanying consolidated statement of operations.
The Company entered into a joint venture agreement with A2 Healthcare Corporation (formerly part of Asklep, Inc.) in 2013. The joint venture was dissolved in October 2018. The Company expects to receive approximately $0.5 million in proceeds from the dissolution in the first quarter of 2019. This balance is recorded in prepaid expenses and other current assets on the consolidated balance sheet at December 31, 2018. The joint venture provided research and development outsourcing solutions in Japan to the biopharmaceutical and medical device industries. This joint venture was based in Tokyo, Japan and was owned by the Company (49%) and Asklep (51%). On October 17, 2014, the joint venture changed its name from RPS Asklep, Inc. to A2PRA Corporation, or A2PRA. The Company recorded increases to the investment balance totaling $0.1 million during each of the years ended December 31, 2018, 2017, and 2016, for the Company’s equity in the venture’s net income for the period, which is recorded in the equity in income of unconsolidated joint venture, net of tax in the Company's consolidated statement of operations. The investment was adjusted for the Company’s equity in the venture’s net income (loss), cash contributions, distributions, and other adjustments required by the equity method of accounting. The investment in A2PRA totaled $0.4 million at December 31, 2017.
(4) Business Combinations
Symphony Health Solutions, Inc.
On September 6, 2017, the Company acquired all of the outstanding equity interest of Symphony Health, a provider of data and analytics to help professionals understand the full market lifecycle of products offered for sale by companies in the
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
pharmaceutical industry, for $539.4 million in cash and contingent consideration, which was not capped, in the form of potential earn-out payments based on a multiple of future earnings for the years ended December 31, 2017 and 2018. With this acquisition, the Company expects to enhance its ability to serve customers throughout the clinical research process with technologies that provide data and analytics.
The liability associated with the expected payment of the earn-out was preliminarily valued at $108.8 million at the acquisition date. During the fourth quarter of 2017, the Company recorded a measurement period adjustment to increase the fair value of the contingent consideration at the acquisition date to $147.5 million based on the Company's finalized assessment of earnings forecasts as of the acquisition date. This measurement period adjustment was reflected as a corresponding increase to goodwill as of the acquisition date. The fair value of the contingent consideration was determined by using a Monte Carlo simulation that includes significant unobservable inputs such as a risk-adjusted discount rate and projected future earnings over the earn-out periods. As the fair value was based on significant inputs not observed in the market and thus represented a Level 3 measurement.
The Company reassesses the fair value of expected contingent consideration and the corresponding liability each reporting period until the end of the earn-out period. Changes in the fair value of the contingent consideration subsequent to the acquisition date, excluding adjustments that qualify as measurement period adjustments, are recognized in earnings in the period of such change. The Company recorded $34.5 million and $85.7 million to transaction-related costs in the consolidated statements of operations during the years ended December 31, 2018 and 2017, respectively, associated with changes in the fair value of the earn-out liability. The Company made a preliminary 2017 earn-out payment, totaling $67.8 million, to the former owners of Symphony Health during the fourth quarter of 2017. During February 2018, the Company made the year-end 2017 earn-out payment, which totaled $114.7 million. During April 2018, the Company and the sellers of Symphony Health finalized the 2017 earn-out calculation and the actual 2017 earn-out payment was adjusted to $112.8 million. As a result, the Company recorded a $1.9 million reduction to transaction-related costs during the year ended December 31, 2018, and a $1.9 million reduction to accrued expenses and other current liabilities in the consolidated balance sheet as of December 31, 2018. As of December 31, 2018, the earn-out liability totaled $83.2 million, which is included in accrued expenses and other current liabilities in the consolidated balance sheet.
The acquisition of Symphony Health was accounted for as a business combination and, accordingly, the assets acquired and the liabilities assumed have been recorded at their respective fair values as of the acquisition date. In connection with the acquisition, the Company recorded approximately $476.9 million of goodwill, which was assigned to the Data Solutions segment and is not deductible for income tax purposes. The goodwill is attributable to the workforce of the acquired business and expected synergies with the Company’s existing operations. The Company incurred $6.4 million in acquisition related costs that are included in transaction-related costs in the consolidated statements of operations for the year ended December 31, 2017. During the year ended December 31, 2018, the Company incurred $1.4 million of expenses associated with transaction-related retention incentives that are included in transaction-related costs in the consolidated statement of operations.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The Company’s purchase price allocation is as follows (in thousands):
|
| | | | | | |
| | Purchase Price Allocation | | Weighted Amortization Period |
Cash and cash equivalents | | $ | 26,297 |
| | |
Accounts receivable and unbilled services | | 39,132 |
| | |
Other current assets | | 23,726 |
| | |
Fixed assets | | 12,340 |
| | |
Customer relationships | | 190,100 |
| | 10 years |
Database | | 137,100 |
| | 3 years |
Tradename | | 2,000 |
| | 2 years |
Accounts payable and accrued expenses | | (42,222 | ) | | |
Advanced billings | | (66,846 | ) | | |
Deferred tax liabilities | | (104,869 | ) | | |
Other long-term liabilities | | (6,740 | ) | | |
Estimated fair value of net assets acquired | | 210,018 |
| | |
Purchase price, including contingent consideration and working capital adjustment | | 686,877 |
| | |
Total goodwill | | $ | 476,859 |
| | |
The results of operations for Symphony Health are included in the consolidated financial statements of the Company from the date of acquisition. During year ended December 31, 2017, Symphony Health's revenue and net income totaled $90.5 million and $6.3 million, respectively.
Since December 31, 2017, goodwill increased by $0.9 million, as a result of adjustments made to the acquired advanced billings balance.
The following unaudited pro-forma information assumes the acquisition of Symphony Health occurred as of the beginning of 2016. This pro-forma financial information is not necessarily indicative of operating results if the acquisition had been completed at the date indicated, nor is it necessarily an indication of future operating results.
|
| | | | | | | |
| December 31, |
(in thousands, except per share amounts) | 2017 | | 2016 |
Total revenue | $ | 2,408,770 |
| | $ | 2,011,544 |
|
Net income attributable to PRA Health Sciences, Inc. | 104,700 |
| | 45,836 |
|
Net income per share: | | | |
Basic | $ | 1.68 |
| | $ | 0.75 |
|
Diluted | $ | 1.59 |
| | $ | 0.71 |
|
The unaudited pro-forma financial information for the year ended December 31, 2017 includes the following non-recurring adjustments:
| |
• | a $6.4 million increase to transaction-related costs incurred by the Company during the year ended December 31, 2017 attributable to the transaction, with a corresponding $2.5 million increase to the benefit from income taxes. |
| |
• | a $3.1 million increase to loss on the modification or extinguishment of long-term debt incurred by the Company during the year ended December 31, 2017 attributable to the above transaction, with a corresponding $1.2 million increase to the benefit from income taxes. |
Takeda Transactions
On June 1, 2017, the Company acquired all of the outstanding shares of Takeda Pharmaceutical Data Services, Ltd., or TDS, from Takeda Pharmaceutical Company Ltd., or Takeda, for $0.7 million in cash. The Company recorded approximately
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
$1.0 million of goodwill, which is assigned to the Clinical Research segment and is not deductible for income tax purposes. Pro-forma results of operations and a complete purchase price allocation have not been presented because the results of this acquisition did not have a material effect on the Company's consolidated financial statements.
On June 1, 2017, the Company and Takeda also closed on a joint venture transaction that enables the Company to provide clinical trial delivery and pharmacovigilance services as a strategic partner of Takeda in Japan. The joint venture transaction was effectuated through the creation of a new legal entity, Takeda PRA Development Center KK, or the TDC joint venture. The Company paid $5.4 million for a 50% equity interest in the TDC joint venture, which represents 50% of the fair value of the net assets and workforce that Takeda contributed to the joint venture. The joint venture provides services including clinical trial monitoring, project management, regulatory strategy and submissions, data management, biostatistics, drug safety reporting, and medical monitoring. The Company is required to buy-out Takeda’s 50% interest in the TDC joint venture in May 2019.
The Company determined that the TDC joint venture is a VIE in which the Company is the primary beneficiary. Accordingly, the Company accounted for the $5.4 million contribution to the TDC joint venture as a business combination and consolidated the VIE in its financial statements with a noncontrolling interest for the 50% portion owned by Takeda. The assets acquired and the liabilities assumed have been recorded at their respective estimated fair values as of June 1, 2017. The Company recorded approximately $2.7 million of goodwill, which is assigned to the Clinical Research segment and is not deductible for income tax purposes. The goodwill is primarily attributable to the assembled workforce. The Company incurred $0.6 million in acquisition related costs that are included in selling, general and administrative expenses in the consolidated statements of operations for the year ended December 31, 2017.
The Company’s fair value of the net assets acquired as part of the TDC joint venture transaction at the closing date of the business combination is as follows (in thousands):
|
| | | | |
| | Purchase Price Allocation |
Cash and cash equivalents | | $ | 8,120 |
|
Other current assets | | 1,671 |
|
Other non-current assets | | 799 |
|
Accounts payable and accrued expenses | | (2,380 | ) |
Estimated fair value of net assets acquired | | 8,210 |
|
PRA purchase price | | 5,440 |
|
Fair value of Takeda's noncontrolling interest | | 5,440 |
|
Total goodwill | | $ | 2,670 |
|
The Company has not disclosed post-acquisition or pro-forma revenue and earnings attributable to the TDC joint venture as they did not have a material effect on the Company’s consolidated financial statements.
Parallel 6, Inc.
On May 10, 2017, the Company acquired all of the outstanding equity interest of Parallel 6, Inc., or Parallel 6, a developer of technologies for improving patient enrollment, engagement, and management of clinical trials, for $39.0 million in cash and contingent consideration in the form of a potential earn-out payment of up to $10.0 million. The earn-out payment is contingent upon the achievement of certain external software sales targets during the 18-month period following closing. With this acquisition, the Company expects to enhance its ability to serve customers throughout the clinical research process with technologies that provide improved efficiencies by reducing study durations and costs through integrated operational management.
The fair value of the earn-out as of the acquisition date was $8.4 million, which was determined by using a Monte Carlo simulation that includes significant unobservable inputs such as a risk-adjusted discount rate and projected external software sales of Parallel 6 over the earn-out period. As the fair value was based on significant inputs not observed in the market and thus represented a Level 3 measurement. During the fourth quarter of 2017, the Company determined that the
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
external software sales targets likely would not be met. Therefore the Company released the $8.4 million contingent consideration liability, which is recorded within transaction-related costs in the consolidation statements of operations.
The acquisition of Parallel 6 was accounted for as a business combination and, accordingly, the assets acquired and the liabilities assumed have been recorded at their respective fair values as of the acquisition date. In connection with the acquisition, the Company recorded approximately $31.3 million of goodwill, which was assigned to the Clinical Research segment and is not deductible for income tax purposes. The goodwill is attributable to the workforce of the acquired business and expected synergies with the Company’s existing information technology operations. The Company incurred $1.3 million in acquisition related costs that are included in selling, general and administrative expenses in the consolidated statements of operations for the year ended December 31, 2017.
The Company’s purchase price allocation is as follows (in thousands):
|
| | | | | | |
| | Purchase Price Allocation | | Weighted Amortization Period |
Cash and cash equivalents | | $ | 132 |
| | |
Accounts receivable and unbilled services | | 929 |
| | |
Other current assets | | 26 |
| | |
Software intangible | | 15,500 |
| | 5 years |
Other intangibles | | 920 |
| | 5 years |
Accounts payable and accrued expenses | | (780 | ) | | |
Advanced billings | | (692 | ) | | |
Other long-term liabilities | | (31 | ) | | |
Estimated fair value of net assets acquired | | 16,004 |
| | |
Purchase price, including contingent consideration | | 47,339 |
| | |
Total goodwill | | $ | 31,335 |
| | |
Since December 31, 2017, goodwill decreased by $1.1 million, primarily as a result of adjustments to acquired income tax balances. The Company has not disclosed post-acquisition or pro-forma revenue and earnings attributable to Parallel 6 as they did not have a material effect on the Company’s consolidated financial statements.
Acquisition of Nextrials
On March 18, 2016, the Company acquired all of the outstanding shares of Nextrials, Inc., or Nextrials, a developer of web-based software which integrates electronic health records with clinical trials, for $4.8 million in cash and contingent consideration in the form of potential earn-out payments of up to $3.0 million. Earn-out payments of $2.0 million and $1.0 million were contingent upon the achievement of project milestones and certain external software sales targets, respectively, during the 30-month period following closing. The Company recognized a liability of approximately $2.3 million as the estimated acquisition date fair value of the earn-out. The fair value was based on significant inputs not observed in the market and thus represented a Level 3 measurement. Changes in the fair value of the earn-out subsequent to the acquisition date were recognized in earnings in the period of the change. The Company made a payment of $0.4 million during the year ended December 31, 2017 as a result of the achievements of certain project milestones. During the fourth quarter of 2017, the Company determined that the remaining project milestone and external software sales targets likely would not be met. Therefore, the Company released the remaining $1.4 million contingent consideration liability, which is recorded within transaction-related costs in the consolidated statements of operations. With this acquisition, the Company expects to enhance its ability to serve customers throughout the clinical research process with technologies that include improved efficiencies by reducing study durations and costs through integrated operational management.
The acquisition of Nextrials was accounted for as a business combination and, accordingly, the assets acquired and the liabilities assumed have been recorded at their respective fair values as of the acquisition date. In connection with the acquisition, the Company recorded approximately $4.3 million of goodwill, which is not deductible for income tax purposes. The goodwill is attributable to the workforce of the acquired business and expected synergies with the Company’s existing information technology operations.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The Company’s purchase price allocation is as follows (in thousands):
|
| | | | | |
| Purchase
Price
Allocation | | Weighted
Amortization
Period |
Cash and cash equivalents | $ | 94 |
| | |
Accounts receivable and unbilled services | 211 |
| | |
Other current assets | 96 |
| | |
Property, plant and equipment | 111 |
| | |
Software intangible | 5,574 |
| | 5 years |
Accounts payable and accrued expenses | (1,585 | ) | | |
Other long-term liabilities | (1,663 | ) | | |
Estimated fair value of net assets acquired | 2,838 |
| | |
Purchase price, including contingent consideration | 7,145 |
| | |
Total goodwill | $ | 4,307 |
| | |
Pro forma information is not provided as the acquisition did not have a material effect on the Company’s consolidated results.
Acquisition of WuXiPRA’s Hong Kong Operations
As noted in Note 3, the Company acquired WuXiPRA’s Hong Kong operations for $0.3 million when the joint venture was dissolved on May 6, 2016. The acquisition was accounted for as a business combination and, accordingly, the assets acquired and the liabilities assumed were recorded at their respective fair values as of the acquisition date. In connection with the acquisition, the Company recorded approximately $0.6 million of goodwill, which is attributable to the workforce of the acquired business. Pro forma information is not provided as the acquisition did not have a material effect on the Company’s consolidated results.
(5) Accounts Receivable, Unbilled Services, and Advanced Billings
Accounts receivable and unbilled services include service revenue, reimbursement revenue, and amounts associated with work performed by investigators. Accounts receivable and unbilled services were (in thousands):
|
| | | | | | | |
| December 31, |
| 2018 | | 2017 |
Accounts receivable | $ | 437,001 |
| | $ | 457,676 |
|
Unbilled services | 133,147 |
| | 170,760 |
|
Total accounts receivable and unbilled services | 570,148 |
| | 628,436 |
|
Less allowance for doubtful accounts | (2,049 | ) | | (1,433 | ) |
Total accounts receivable and unbilled services, net | $ | 568,099 |
| | $ | 627,003 |
|
Unbilled services as of December 31, 2018 includes $66.6 million of contract assets where the Company’s right to bill is conditioned on criteria other than the passage of time. There were no impairment losses on contract assets during the year ended December 31, 2018.
A rollforward of the allowance for doubtful accounts is as follows (in thousands):
|
| | | | | | | | | | | |
| December 31, |
| 2018 | | 2017 | | 2016 |
Beginning balance | $ | 1,433 |
| | $ | 1,203 |
| | $ | 2,641 |
|
Charged (credited) to income from operations | 605 |
| | 255 |
| | (652 | ) |
Write-offs, recoveries and the effects of foreign currency exchange | 11 |
| | (25 | ) | | (786 | ) |
Ending balance | $ | 2,049 |
| | $ | 1,433 |
| | $ | 1,203 |
|
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Advanced billings were as follows (in thousands):
|
| | | | | | | | |
| | December 31, |
| | 2018 | | 2017 |
Advanced billings | | $ | 441,357 |
| | $ | 469,211 |
|
The $27.9 million decrease in advanced billings from December 31, 2017 to December 31, 2018 was primarily due to the adoption of ASC 606. During the year ended December 31, 2018, the Company recognized revenue of $393.2 million related to advanced billings recorded as of January 1, 2018.
(6) Fixed Assets
The carrying amount of fixed assets is as follows (in thousands):
|
| | | | | | | |
| December 31, |
| 2018 | | 2017 |
Leasehold improvements | $ | 58,300 |
| | $ | 49,548 |
|
Computer hardware and software | 172,346 |
| | 139,861 |
|
Furniture and equipment | 45,962 |
| | 44,325 |
|
Total fixed assets | 276,608 |
| | 233,734 |
|
Accumulated depreciation | (121,844 | ) | | (90,664 | ) |
Total fixed assets, net | $ | 154,764 |
| | $ | 143,070 |
|
All U.S. fixed assets are included as collateral for the payment and performance in full of the term loans pledged by the Company and its subsidiaries.
Depreciation expense was $40.6 million, $29.0 million, and $24.1 million for the years ended December 31, 2018, 2017 and 2016, respectively.
(7) Goodwill and Intangible Assets
Goodwill
The changes in the carrying amount of goodwill are as follows (in thousands):
|
| | | | | | | | | | | |
| Clinical Research | | Data Solutions | | Consolidated |
Balance at December 31, 2016 | $ | 971,980 |
| | $ | — |
| | $ | 971,980 |
|
Acquisition of Symphony Health | — |
| | 475,981 |
| | 475,981 |
|
Acquisition of Parallel 6 | 32,452 |
| | — |
| | 32,452 |
|
Acquisition of TDC joint venture | 2,670 |
| | — |
| | 2,670 |
|
Acquisition of TDS | 966 |
| | — |
| | 966 |
|
Currency translation | 28,375 |
| | — |
| | 28,375 |
|
Balance at December 31, 2017 | 1,036,443 |
| | 475,981 |
| | 1,512,424 |
|
Adjustments to Symphony Health purchase price allocation | — |
| | 878 |
| | 878 |
|
Adjustments to Parallel 6 purchase price allocation | (1,117 | ) | | — |
| | (1,117 | ) |
Currency translation | (17,423 | ) | | — |
| | (17,423 | ) |
Balance at December 31, 2018 | $ | 1,017,903 |
| | $ | 476,859 |
| | $ | 1,494,762 |
|
There are no accumulated impairment charges as of December 31, 2018 and 2017.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Intangible Assets
Intangible assets consist of the following (in thousands):
|
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2018 | | December 31, 2017 |
| Gross Amount | | Accumulated Amortization | | Net Amount | | Gross Amount | | Accumulated Amortization | | Net Amount |
Customer relationships | $ | 555,915 |
| | $ | (103,248 | ) | | $ | 452,667 |
| | $ | 565,638 |
| | $ | (72,133 | ) | | $ | 493,505 |
|
Customer backlog | 120,934 |
| | (120,934 | ) | | — |
| | 123,746 |
| | (120,583 | ) | | 3,163 |
|
Trade names (finite-lived) | 28,505 |
| | (12,810 | ) | | 15,695 |
| | 28,558 |
| | (9,265 | ) | | 19,293 |
|
Patient list and other intangibles | 44,474 |
| | (30,939 | ) | | 13,535 |
| | 44,474 |
| | (24,226 | ) | | 20,248 |
|
Database | 137,100 |
| | (32,561 | ) | | 104,539 |
| | 137,100 |
| | (7,544 | ) | | 129,556 |
|
Non-competition agreements | 2,679 |
| | (2,679 | ) | | — |
| | 2,767 |
| | (2,706 | ) | | 61 |
|
Total finite-lived intangible assets | 889,607 |
| | (303,171 | ) | | 586,436 |
| | 902,283 |
| | (236,457 | ) | | 665,826 |
|
Trade names (indefinite-lived) | 118,010 |
| | — |
| | 118,010 |
| | 118,010 |
| | — |
| | 118,010 |
|
Total intangible assets | $ | 1,007,617 |
| | $ | (303,171 | ) | | $ | 704,446 |
| | $ | 1,020,293 |
| | $ | (236,457 | ) | | $ | 783,836 |
|
The Company conducts its annual impairment test of indefinite‑lived intangibles during the fourth quarter of the fiscal year. For the periods ended December 31, 2018, 2017 and 2016, the Company concluded that the fair value of indefinite‑lived intangibles exceeded the carrying value and, therefore, no impairment exists. Amortization expense was $71.6 million, $49.2 million and $45.4 million for the years ended December 31, 2018, 2017 and 2016, respectively.
Estimated amortization expense related to finite‑lived intangible assets for the next five years and thereafter is as follows (in thousands):
|
| | | |
2019 | $ | 68,583 |
|
2020 | 68,971 |
|
2021 | 63,868 |
|
2022 | 49,485 |
|
2023 | 37,748 |
|
2024 and thereafter | 297,781 |
|
Total | $ | 586,436 |
|
(8) Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following (in thousands):
|
| | | | | | | |
| December 31, |
| 2018 | | 2017 |
Compensation, including bonuses, fringe benefits and payroll taxes | $ | 133,758 |
| | $ | 125,658 |
|
Acquisition-related contingent consideration | 83,249 |
| | 114,692 |
|
Accrued data costs | 17,422 |
| | 15,669 |
|
Accrued reimbursable out-of-pocket and investigator costs | 89,317 |
| | — |
|
Other | 42,751 |
| | 44,591 |
|
Interest | 2,980 |
| | 3,265 |
|
Total accrued expenses and other current liabilities | $ | 369,477 |
| | $ | 303,875 |
|
Prior to the adoption of ASC 606, accrued reimbursable out-of-pocket and investigator costs were included as a component of advanced billings.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) Revolving Credit Facilities and Long‑Term Debt
Long‑term debt consists of the following (in thousands):
|
| | | | | | | |
| December 31, |
| 2018 | | 2017 |
Term loans, first lien | $ | 916,533 |
| | $ | 1,140,927 |
|
Accounts receivable financing agreement | 170,000 |
| | 120,000 |
|
Total debt | 1,086,533 |
| | 1,260,927 |
|
Less current portion of long-term debt | — |
| | (28,789 | ) |
Total long-term debt | 1,086,533 |
| | 1,232,138 |
|
Less debt issuance costs | (4,149 | ) | | (6,741 | ) |
Total long-term debt, net | $ | 1,082,384 |
| | $ | 1,225,397 |
|
Principal payments on long‑term debt are due as follows (in thousands):
|
| | | |
2019 | $ | — |
|
2020 | — |
|
2021 | 1,086,533 |
|
Total | $ | 1,086,533 |
|
2016 Credit Facilities
On December 6, 2016, the Company through its wholly-owned subsidiary, Pharmaceutical Research Associates, Inc., entered into a credit agreement providing for senior secured credit facilities, or the 2016 Credit Facilities, totaling $750.0 million. The 2016 Credit Facilities were comprised of a $625.0 million first lien term loan due 2021, or the 2016 First Lien Term Loan, and a five-year $125.0 million revolving line of credit, or the 2016 Revolver.
The proceeds from the 2016 Credit Facilities were used to repay the then outstanding 2013 First Lien Term Loan (defined below). In accordance with the guidance in ASC 470‑50, “Debt—Modifications and Extinguishments,” or ASC 470-50, the debt repayment was accounted for as a partial debt extinguishment. The repayment resulted in a $16.7 million loss on extinguishment of debt, consisting of $15.8 million write-off of unamortized debt issuance costs and $0.9 million of fees associated with the transaction, which is included in loss on modification or extinguishment of debt in the consolidated statements of operations for the year ended December 31, 2016.
On September 6, 2017, the Company borrowed $550.0 million, or the Incremental Borrowing, pursuant to an incremental amendment to the credit agreement governing the 2016 Credit Facilities. The incremental borrowing provisions of the credit agreement allow for, among other things, additional borrowings in the event of a material acquisition by the Company. The proceeds of the Incremental Borrowing were primarily used to fund the acquisition of Symphony Health. In accordance with the guidance in ASC 470-50, the Incremental Borrowing was accounted for as a debt modification. The Incremental Borrowing resulted in a $3.1 million loss on modification of debt, which consists of fees associated with the transaction for the year ended December 31, 2017.
On December 28, 2017, the Company amended the credit agreement governing the 2016 Credit Facilities, or the 2017 Refinancing, to refinance the 2016 First Lien Term Loan which reduced the interest rate margin and amended the payment schedule applicable to the 2016 First Lien Term Loan. The 2017 Refinancing also increased the Company's borrowing capacity under the 2016 Revolver from $125.0 million to $225.0 million and modified the definition of permitted investments and refreshed the capacity for incremental credit facilities under the Credit Agreement. In accordance with the guidance in ASC 470-50, the 2017 Refinancing was accounted for as a debt modification. As a result of the debt modification, the Company recognized a loss of modification of debt totaling $0.6 million, which was recorded during the year ended December 31, 2017.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
As collateral for borrowings under the 2016 Credit Facilities, the Company granted a pledge on primarily all of its assets, and the stock of wholly‑owned U.S. restricted subsidiaries. The Company is also subject to certain financial covenants, which require the Company to maintain certain debt‑to‑EBITDA and interest expense-to-EBITDA ratios. The 2016 Credit Facilities also contain covenants that, among other things, restrict the Company’s ability to create liens, make investments and acquisitions, incur or guarantee additional indebtedness, enter into mergers or consolidations and other fundamental changes, conduct sales and other dispositions of property or assets, enter into sale-leaseback transactions or hedge agreements, prepay subordinated debt, pay dividends or make other payments in respect of capital stock, change the line of business, enter into transactions with affiliates, enter into burdensome agreements with negative pledge clauses, and make subsidiary distributions. After giving effect to the applicable restrictions on the payment of dividends under the 2016 Credit Facilities, subject to compliance with applicable law, as of December 31, 2018 and 2017, all amounts in retained earnings were free of restriction and were available for the payment of dividends. The Company does not expect to pay dividends in the foreseeable future. The Company does not expect these covenants to restrict its liquidity, financial condition or access to capital resources in the foreseeable future. The 2016 Credit Facilities also contain customary representations, warranties, affirmative covenants, and events of default.
2016 First Lien Term Loan
The 2016 First Lien Term Loan, including the Incremental Borrowing and as modified by the 2017 Refinancing, is a floating rate term loan. During 2018, the Company made an additional $195.6 million in voluntary principal payments. In accordance with the guidance in ASC 470-50, the debt repayments were accounted for as a partial debt extinguishment. The repayments resulted in the write-off of $1.0 million in unamortized debt issuance costs, which is included in loss on modification or extinguishment of debt in the consolidated statements of operations for the year ended December 31, 2018. The Company’s voluntary prepayments made during 2018 fully satisfied all required quarterly principal payments through maturity.
The variable interest rate is a rate equal to the London Interbank Offered Rate, or LIBOR, or the adjusted base rate, or ABR, at the election of the Company, plus a margin based on the ratio of total indebtedness to EBITDA. The margin ranges from 1.00% to 2.00%, in the case of LIBOR loans, and 0.00% to 1.00%, in the case of ABR loans. The Company has the option of 1, 2, 3 or 6 month base interest rates. As of December 31, 2018 and 2017, the weighted average interest rate on the first lien term loan was 3.88% and 3.45%, respectively. There are no prepayment penalties.
2016 Revolver
The Company’s 2016 Revolver, as modified by the 2017 Refinancing, provides for $225.0 million of potential borrowings and expires on December 6, 2021. The interest rate on the 2016 Revolver is based on the LIBOR with a 0% LIBOR floor or ABR, at the election of the Company, plus an applicable margin based on the leverage ratio of the Company. The Company, at its discretion, may elect interest periods of 1, 2, 3 or 6 months. The Company is required to pay to the lenders a commitment fee for unused commitments of 0.2% to 0.4% based on the Company’s debt-to-EBITDA ratio. At December 31, 2018, there were no outstanding borrowings under the 2016 Revolver; the Company had $91.5 million in outstanding borrowings under the 2016 Revolver at December 31, 2017. In addition, at December 31, 2018 and 2017, the Company had $5.4 million and $4.9 million, respectively, in letters of credit outstanding, which are secured by the 2016 Revolver.
Accounts Receivable Financing Agreement
In March 2016, the Company entered into the $140.0 million accounts receivable financing agreement. The initial borrowings in 2016 were used to repay amounts outstanding on the Company’s previous revolving credit facility that were used to fund the cash tender offer for the Senior Notes.
On May 31, 2018, the Company amended its accounts receivable financing agreement. The amendment increased the agreement's borrowing capacity to $200.0 million, decreased the applicable margin from 1.60% to 1.25%, and extended the maturity date to May 31, 2021, unless terminated earlier pursuant to its terms. The Company had $170.0 million and $120.0 million outstanding on the accounts receivable financing agreement as of December 31, 2018 and 2017, respectively. The additional borrowings during 2018 were used to repay amounts outstanding on the Company's 2016 Revolver. At December 31, 2018, there was $30.0 million of remaining capacity available under the accounts receivable financing agreement.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Loans under the accounts receivable financing agreement accrue interest at either a reserve-adjusted LIBOR or a base rate, plus 1.25%. The Company may prepay loans upon one business day prior notice and may terminate the accounts receivable financing agreement with 15 days’ prior notice. As of December 31, 2018 and 2017, the weighted average interest rate on the accounts receivable financing agreement was 3.72% and 2.96%, respectively.
The accounts receivable financing agreement contains various customary representations and warranties and covenants, and default provisions which provide for the termination and acceleration of the commitments and loans under the agreement in circumstances including, but not limited to, failure to make payments when due, breach of representations, warranties or covenants, certain insolvency events or failure to maintain the security interest in the trade receivables, and defaults under other material indebtedness.
Fair Value of Debt
The estimated fair value of the Company’s debt and outstanding borrowings under its revolving credit facilities was $1,084.2 million and $1,352.4 million at December 31, 2018 and 2017, respectively, and was determined based on Level 2 inputs, which are primarily based on rates at which the debt is traded among financial institutions adjusted for the Company’s credit standing.
(10) Stockholders’ Equity
Authorized Shares
The Company is authorized to issue up to one billion shares of common stock, with a par value of $0.01. The Company is authorized to issue up to one hundred million shares of preferred stock, with a par value of $0.01.
(11) Stock-Based Compensation
Stock Option and RSA/RSU Activity
On September 23, 2013 and in connection with the acquisition of the Company by KKR, the Board of Directors approved the formation of the 2013 Stock Incentive Plan for Key Employees of Pinnacle Holdco Parent, Inc. and its subsidiaries, or the 2013 Plan. The 2013 Plan allowed for the issuance of stock options and other stock-based awards as permitted by applicable laws. The number of shares available for grant under the 2013 Plan was 12.5% of the outstanding shares at closing on a fully diluted basis. The Company rolled over 2,052,909 stock options under the 2013 Plan. The fair value of the options that were rolled over equaled the fair value of the options in the predecessor company and, therefore, there was no additional stock-based compensation expense recorded.
All stock options granted under the 2013 Plan are subject to transfer restrictions of the stock option’s underlying shares once vested and exercised. This lack of marketability was included as a discount, calculated using the Finnerty Model, when determining the grant date value of these options. In conjunction with the secondary offerings during 2018, 2017, and 2016, the transfer restrictions on such shares issuable upon exercise of vested options granted under the 2013 Plan were released. The release of the transfer restrictions is considered a modification under ASC 718, “Stock Compensation.” As a result of these modifications, the Company incurred approximately $0.7 million, $3.7 million, and $10.1 million of incremental compensation expense associated with service-based options during the years ended December 31, 2018, 2017, 2016, respectively, which is included in transaction-related costs in the accompanying consolidated statements of operations.
On November 23, 2014 and in connection with the IPO, the Board of Directors approved the formation of the 2014 Omnibus Plan for Key Employees, or the 2014 Plan. The 2014 Plan allowed for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws.
The 2018 Stock Incentive Plan, or the 2018 Plan, was approved by stockholders at the annual meeting on May 31, 2018. The 2018 Plan allows for the issuance of stock options, stock appreciation rights, restricted shares and restricted stock units, other stock-based awards, and performance compensation awards as permitted by applicable laws. The 2018 Plan authorized the issuance of 2,000,000 shares of common stock plus all shares that remained available under the 2014 Plan on May 31, 2018 (which included shares carried over from the 2013 Plan).
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Generally, the Company grants stock options with exercise prices greater than or equal to the fair market value of the Company’s common stock on the date of grant. The stock option compensation cost calculated under the fair value approach is recognized on a pro-rata basis over the vesting period of the stock options (usually five years under the 2013 Plan and four years under the 2014 Omnibus Plan and 2018 Plan). Most stock option grants are subject to graded vesting as services are rendered and have a contractual life of ten years. The Board and the Compensation Committee have the discretion to determine different vesting schedules.
In December 2013, the Company granted certain employees market-based options under the Plan that vest only upon the achievement of a specified internal rate of return from a liquidity event (“2.0x Options” and “2.5x Options”). At the time of grant, no compensation expense was recorded as the 2.0x Options and 2.5x Options vest upon a liquidity event, which is not considered probable until the date it occurs. On January 20, 2016, the Compensation Committee of the Board of Directors adopted a resolution to adjust the vesting criteria for all 2.0x Options granted and still outstanding on such date. Under the revised vesting criteria, the 2.0x Options vest upon the announcement of a secondary offering. The Company did not record compensation expense on the January 20, 2016 modification date as the Company determined the modification resulted in Type IV Improbable-to-Improbable modification as the secondary offering was deemed improbable since the event was outside of the Company’s control and could not be considered probable until the date it occurred. On March 2, 2016, the Company announced a secondary offering of shares by KKR and certain management stockholders, and it became probable that the 2.0x Options would vest. Due to the modification of the terms of the 2.0x Options, the Company calculated the fair value of these options using the Black-Scholes option pricing model with the following assumptions: expected life of 2.92 years; risk-free rate of 1.04%; volatility of 45%; dividend yield of 0%; and a Finnerty discount of approximately 16%. In total, 835,551 2.0x Options held by current employees were modified. As a result of this modification, and the modifications associated with the transfer restrictions releases noted above, the Company incurred approximately $0.1 million, $0.8 million and $25.7 million of incremental compensation expense associated with the 2.0x Options during the years ended December 31, 2018, 2017 and 2016, respectively, which is included in transaction-related costs in the accompanying consolidated statements of operations.
On November 16, 2016, the 2.5x Options vested upon the achievement of a specified internal rate of return and multiple on invested capital in connection with the closing of a secondary offering of shares by KKR. In total, 809,755 2.5x Options held by current employees vested. The Company incurred approximately $0.1 million, $0.8 million and $6.4 million of incremental compensation expense associated with the vesting and transfer restriction release of the 2.5x Options during the years ended December 31, 2018, 2017 and 2016, respectively, which is included in transaction-related costs in the accompanying consolidated statements of operations.
As of December 31, 2018, there was $95.2 million of unrecognized compensation cost related to unvested stock-based compensation, which is expected to be recognized over a weighted average period of two years. The total fair value of options vested during the years ended December 31, 2018, 2017 and 2016 was $14.6 million, $5.2 million and $27.3 million, respectively.
Aggregated information regarding the Company’s option plans is summarized below:
|
| | | | | | | | | | | | |
| Options | | Wtd. Average Exercise Price | | Wtd. Average Remaining Contractual Life | | Intrinsic Value (in millions) |
Outstanding at December 31, 2017 | 5,245,625 |
| | $ | 39.14 |
| | 7.6 | | $ | 272.4 |
|
Granted | 1,569,000 |
| | 98.22 |
| | | | |
Exercised (1) | (1,690,088 | ) | | 21.93 |
| | | | |
Expired/forfeited | (482,937 | ) | | 68.79 |
| | | | |
Outstanding at December 31, 2018 | 4,641,600 |
| | $ | 62.29 |
| | 7.8 | | $ | 149.7 |
|
Exercisable at December 31, 2018 | 1,612,983 |
| | $ | 23.61 |
| | 5.6 | | $ | 110.2 |
|
(1) During the year ended December 31, 2018, of the 1,690,088 shares exercised, 140,428 were withheld from the option holders to cover the exercise price of the options being exercised.
The weighted average fair value of service-based options granted was $34.08, $25.24 and $15.57 during the years ended December 31, 2018, 2017 and 2016, respectively.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Selected information regarding the Company’s stock options as of December 31, 2018 is as follows:
|
| | | | | | | | | | | | | | | | | | | |
Options Outstanding | | Options Exercisable |
Exercise Price | | Number of Options | | Wtd Average Remaining Life (in Years) | | Wtd. Average Exercise Price | | Number of Options | | Wtd. Average Remaining Life (in Years) | | Wtd. Average Exercise Price |
$ | 2.94 - 11.73 | | 1,142,281 |
| | 5.0 | | $ | 11.55 |
| | 1,142,281 |
| | 5.0 | | $ | 11.55 |
|
$ | 16.42 - 75.37 | | 794,119 |
| | 7.2 | | $ | 47.34 |
| | 280,252 |
| | 6.4 | | $ | 37.17 |
|
$ | 75.81 - 82.99 | | 1,415,950 |
| | 8.7 | | $ | 76.82 |
| | 190,450 |
| | 8.6 | | $ | 75.99 |
|
$ | 83.25 - 116.11 | | 1,289,250 |
| | 9.6 | | $ | 100.50 |
| | — |
| | — | | $ | — |
|
The Company’s RSAs/RSUs will settle in shares of the Company’s common stock on the applicable vesting date. Most RSAs/RSUs granted to employees vest 100% on the third anniversary of the date of grant. RSAs/RSUs granted to the Company's non-employee directors vest 50% on the first anniversary of the date of grant and 50% on the second anniversary of the date of grant.
Activity related to the Company’s RSAs/RSUs in 2018 is as follows:
|
| | | | | | | | | | |
| Awards | | Wtd. Average Grant-Date Fair Value | | Intrinsic Value (millions) |
Unvested at December 31, 2017 | 309,538 |
| | $ | 46.76 |
| | $ | 28.2 |
|
Granted | 240,228 |
| | 94.51 |
| | |
Forfeited | (49,000 | ) | | 85.92 |
| | |
Vested | (156,516 | ) | | 31.62 |
| | |
Unvested at December 31, 2018 | 344,250 |
| | $ | 81.39 |
| | $ | 31.7 |
|
Employee Stock Purchase Plan
In April 2017, the Board of Directors approved the PRA Health Sciences, Inc. 2017 Employee Stock Purchase Plan, or ESPP, which was approved by the Company’s shareholders on June 1, 2017. The ESPP allows eligible employees to authorize payroll deductions of up to 15% of their base salary or wages to be applied toward the purchase of shares of the Company’s common stock on the last trading day of the offering period. Participating employees will purchase shares of the Company's common stock at a discount of up to 15% on the lesser of the closing price of the Company's common stock on the NASDAQ Global Select Market (i) on the first trading day of the offering period or (ii) the last day of any offering period. The aggregate number of shares of the Company’s common stock that may be issued under the ESPP may not exceed 3,000,000 shares and no one employee may purchase any shares under the ESPP having a collective fair market value greater than $25,000 in any one calendar year. Offering periods under the ESPP will generally be in six month increments, commencing on January 1 and July 1 of each calendar year with the compensation committee having the right to establish different offering periods. The Company's first offering period commenced on January 1, 2018 and the Company recognized stock-based compensation expense of $3.3 million associated with the ESPP during the year ended December 31, 2018. As of December 31, 2018, there have been 76,116 shares issued and 2,923,884 shares reserved for future issuance under the ESPP.
Stock-based Compensation Expense
Stock-based compensation expense related to employee stock plans is summarized below (in thousands):
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Direct costs | $ | 9,508 |
| | $ | 3,552 |
| | $ | 1,813 |
|
Selling, general and administrative | 19,635 |
| | 9,064 |
| | 5,254 |
|
Transaction-related costs | 773 |
| | 5,294 |
| | 42,166 |
|
Total stock-based compensation expense | $ | 29,916 |
| | $ | 17,910 |
| | $ | 49,233 |
|
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(12) Income Taxes
The components of income before income taxes and equity in income (losses) of unconsolidated joint ventures are as follows (in thousands):
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Domestic | $ | 45,672 |
| | $ | (52,083 | ) | | $ | (61,226 | ) |
Foreign | 175,875 |
| | 126,630 |
| | 155,120 |
|
| $ | 221,547 |
| | $ | 74,547 |
| | $ | 93,894 |
|
The components of the provision for (benefit from) income taxes were as follows (in thousands):
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Current: | |
| | |
| | |
|
Federal | $ | 14,793 |
| | $ | 30,084 |
| | $ | 151 |
|
State | 776 |
| | 2,607 |
| | 1,842 |
|
Foreign | 39,998 |
| | 30,601 |
| | 36,970 |
|
Total current income tax expense | 55,567 |
| | 63,292 |
| | 38,963 |
|
Deferred: | | | | | |
Federal | 14,224 |
| | (70,041 | ) | | (2,230 | ) |
State | 1,403 |
| | (1,203 | ) | | (451 | ) |
Foreign | (3,962 | ) | | (4,671 | ) | | (7,788 | ) |
Total deferred income tax expense (benefit) | 11,665 |
| | (75,915 | ) | | (10,469 | ) |
Total income tax expense (benefit) | $ | 67,232 |
| | $ | (12,623 | ) | | $ | 28,494 |
|
Income taxes computed at the statutory U.S. federal income tax rate are reconciled to the provision for (benefit from) income taxes as follows:
|
| | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Statutory federal income tax rate | 21.0 | % | | 35.0 | % | | 35.0 | % |
State income taxes, net of federal benefit | 0.8 | % | | (5.5 | )% | | 0.3 | % |
Impact of the U.S. Tax Cuts and Jobs Act of 2017: | | | | | |
Rate change | (5.2 | )% | | (56.0 | )% | | — | % |
U.S. minimum tax on foreign entities | 3.3 | % | | — | % | | — | % |
Base erosion anti-abuse tax | 8.4 | % | | — | % | | — | % |
Tax on foreign earnings: | | | | | |
Foreign rate differential | 0.8 | % | | (20.3 | )% | | (17.7 | )% |
Foreign earnings taxed in the U.S. | 7.9 | % | | 60.7 | % | | 17.5 | % |
Foreign dividends | — | % | | 5.2 | % | | — | % |
Research and development credits | (2.6 | )% | | (3.3 | )% | | (3.9 | )% |
Stock-based compensation | (9.6 | )% | | (39.9 | )% | | 1.9 | % |
Nondeductible contingent consideration | 3.1 | % | | 35.4 | % | | — | % |
Valuation allowance | 0.4 | % | | (28.0 | )% | | — | % |
Change in liability for uncertain tax positions | 0.4 | % | | (3.2 | )% | | — | % |
Nondeductible expenses | 1.0 | % | | 2.2 | % | | 0.1 | % |
Other | 0.6 | % | | 0.8 | % | | (2.9 | )% |
Effective income tax rate | 30.3 | % | | (16.9 | )% | | 30.3 | % |
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Components of the deferred tax assets and liabilities were as follows (in thousands):
|
| | | | | | | |
| December 31, |
| 2018 | | 2017 |
Net operating loss carryforwards | $ | 15,741 |
| | $ | 48,603 |
|
Accruals and reserves | 13,496 |
| | 15,943 |
|
Equity based compensation | 8,821 |
| | 7,447 |
|
Prepaid expenses and other | 15,809 |
| | 13,492 |
|
Deferred and unbilled revenue | 55,771 |
| | 24,937 |
|
Tax credits | 2,645 |
| | 15,111 |
|
| 112,283 |
| | 125,533 |
|
Valuation allowance | (9,824 | ) | | (25,226 | ) |
Total deferred tax assets (net of valuation allowance) | 102,459 |
| | 100,307 |
|
Identified intangibles | (177,845 | ) | | (190,115 | ) |
Depreciable, amortizable and other property | (16,372 | ) | | (13,434 | ) |
Deferred tax liabilities | (194,217 | ) | | (203,549 | ) |
Net deferred tax liability | $ | (91,758 | ) | | $ | (103,242 | ) |
Long-term deferred tax asset | $ | 8,954 |
| | $ | 8,939 |
|
Long-term deferred tax liability | $ | (100,712 | ) | | $ | (112,181 | ) |
The Company’s foreign subsidiaries are taxed separately in their respective jurisdictions. As of December 31, 2018, the Company has cumulative foreign net operating loss carryforwards of approximately $9.4 million. In addition, the Company has federal net operating loss carryforwards of approximately $9.4 million and state net operating loss carryforwards of approximately $316.4 million.
The carryforward periods for the Company’s net operating losses vary from four years to an indefinite number of years depending on the jurisdiction. The Company’s ability to offset future taxable income with net operating loss carryforwards may be limited in certain instances, including changes in ownership.
On December 22, 2017, the Act was signed into U.S. law making significant changes to the Internal Revenue Code. Changes include, but are not limited to, a corporate tax rate decrease from 35% to 21% effective for tax years beginning after December 31, 2017, the transition of U.S international taxation of worldwide income to a territorial system, and a one-time transition tax on the mandatory deemed repatriation of cumulative foreign earnings as of December 31, 2017. Additionally, Staff Accounting Bulletin No. 118 (“SAB 118”) was issued to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Act.
The Company calculated its best estimate of the impact of the Act in its year-end income tax provision and recorded $0.2 million as additional income tax expense in the fourth quarter of 2017, the period in which the legislation was enacted. This provisional amount related to the remeasurement of certain deferred tax assets, deferred tax liabilities, and U.S. uncertain tax positions, based on the rates at which they are expected to reverse in the future, was a benefit of $41.7 million. The provisional amount related to the one-time transition tax on the mandatory deemed repatriation of foreign earnings was $77.6 million based on cumulative foreign earnings of $392.5 million. The Company also recorded a provisional tax benefit of $35.7 million related to the utilization of foreign tax credits against the one-time transition tax. In addition, the Company has recorded a valuation allowance against an estimated $12.8 million of excess foreign tax credits related to the transition tax inclusion.
During the year ended December 31, 2018, the Company completed its analysis of the impact of the Act which resulted in an additional tax benefit of $0.6 million in the fourth quarter of 2018 and a total tax provision of $3.0 million related to the impact of the Act for the year ended December 31, 2018. The total tax provision included a $14.5 million provision related to adjustments to the transition tax and a $11.5 million benefit related to the remeasurement of certain deferred tax assets and liabilities. Additionally, the Company has elected to treat any potential GILTI inclusions as a period cost.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The application of the Act and the related regulations is complex and requires significant judgment, particularly with respect to the GILTI and the base erosion and anti-abuse tax (“BEAT”) provisions. If the structure of the Company’s arrangements changes or new regulations are issued that clarify or change the application of these or other provisions of the Act, these changes could have a material effect on the Company’s tax provision.
The Company also has state income tax credit carryforwards available to potentially offset future state income tax of $2.4 million. The state credits begin expiring in 2022. The Company has a $1.9 million valuation allowance against the benefits of these credits.
In determining the extent to which a valuation allowance for deferred tax assets is required, the Company evaluates all available evidence including projections of future taxable income, carry-back opportunities, reversal of certain deferred tax liabilities, and other tax‑planning strategies. The valuation allowance at December 31, 2018 relates to certain foreign net operating losses, certain foreign deferred tax assets, certain state net operating losses and state tax credit carryforwards.
A reconciliation of the beginning and ending amount of gross unrecognized income tax benefits is presented below (in thousands):
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Beginning balance | $ | 7,911 |
| | $ | 12,432 |
| | $ | 11,729 |
|
Additions based on tax positions related to current year | 764 |
| | 1,641 |
| | 1,196 |
|
Additions for income tax positions of prior years | 1,065 |
| | 400 |
| | 542 |
|
Impact of changes in exchange rates | (58 | ) | | 427 |
| | (127 | ) |
Impact of change in federal tax rate | 4,236 |
| | (3,536 | ) | | — |
|
Settlements with tax authorities | (180 | ) | | (108 | ) | | (559 | ) |
Reductions for income tax positions for prior years | (456 | ) | | (3,174 | ) | | (349 | ) |
Reductions due to lapse of applicable statute of limitations | (391 | ) | | (171 | ) | | — |
|
Ending balance | $ | 12,891 |
| | $ | 7,911 |
| | $ | 12,432 |
|
As of December 31, 2018, 2017, and 2016, the total gross unrecognized tax benefits were $12.9 million, $7.9 million, and $12.4 million, respectively. As of December 31, 2018, the total amount of gross unrecognized tax benefits which, if recognized, would impact the Company’s effective tax rate is $12.9 million. The Company anticipates changes in total unrecognized tax benefits due to the expiration of statute of limitations within the next 12 months. Specifically, adjustments related to certain foreign tax exposures are expected to be resolved in various jurisdictions. A reasonable estimate of the change in the total gross unrecognized tax benefit expected to be recognized as a result is $1.0 million as of the balance sheet date.
The Company’s policy for recording interest and penalties associated with uncertain tax positions is to record such items as a component of income tax expense. The Company recorded an increase of $0.6 million, a decrease of $0.8 million, and an increase of $0.1 million during the years ended December 31, 2018, 2017 and 2016, respectively. As of December 31, 2018, the Company has a total of $2.2 million recognized on uncertain tax positions. To the extent interest and penalties are not incurred with respect to uncertain tax positions, amounts accrued will be reduced and reflected as a reduction in income tax expense.
The Company has analyzed filing positions in all of the significant federal, state and foreign jurisdictions where the Company is required to file income tax returns. The only periods subject to examination by the major tax jurisdictions where the Company does business are the 2010 through 2017 tax years.
As of December 31, 2018, the Company has accumulated undistributed earnings generated by our foreign subsidiaries of approximately $528.5 million. Because $363.4 million of such earnings have previously been subject to the one-time transition tax on foreign earnings required by the 2017 Tax Act, and 2018 earnings were subject to GILTI inclusion, any additional taxes due with respect to such earnings or the excess of the amount for financial reporting over the tax basis of the Company's foreign investments would generally be limited to foreign withholding taxes and state taxes and it is not practicable to calculate the deferred tax liability. The Company intends to indefinitely reinvest these earnings.
A rollforward of the deferred tax asset valuation allowance accounts is as follows (in thousands):
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Beginning balance | $ | 25,226 |
| | $ | 21,689 |
| | $ | 23,205 |
|
Additions - excess benefit offset to NOL change | — |
| | 12,623 |
| | — |
|
Additions - purchase accounting | — |
| | 219 |
| | — |
|
Additions - other comprehensive income | — |
| | — |
| | — |
|
Additions - charged to expense | 1,428 |
| | 12,863 |
| | 3,421 |
|
Additions - U.S. federal tax rate change | — |
| | 1,330 |
| | — |
|
Deductions - charged to expense (including translation adjustments) | (16,830 | ) | | (23,498 | ) | | (4,937 | ) |
Ending balance | $ | 9,824 |
| | $ | 25,226 |
| | $ | 21,689 |
|
The valuation allowance at December 31, 2018 is primarily related to state loss carryforwards, state credit carryforwards, certain foreign deferred tax assets, and loss carryforwards in various foreign jurisdictions.
(13) Commitments and Contingencies
Operating Leases
The Company leases office space under operating lease agreements expiring at various times through 2036. The Company has sublease agreements for certain facilities to reduce rent expense and accommodate expansion needs. The subleases expire at various times through 2023. The Company also leases certain office equipment under the terms of operating leases expiring at various times through 2023.
Rent expense under operating leases, net of sublease rental income, was $39.6 million, $37.0 million and $31.9 million for the years ended December 31, 2018, 2017 and 2016, respectively.
Future minimum lease commitments on non‑cancelable operating leases are as follows (in thousands):
|
| | | | | | | | | | | |
Years Ended December 31, | Leases | | Sublease Rental Income | | Net Total |
2019 | $ | 43,675 |
| | $ | (157 | ) | | $ | 43,518 |
|
2020 | 40,948 |
| | (157 | ) | | 40,791 |
|
2021 | 37,469 |
| | (157 | ) | | 37,312 |
|
2022 | 30,238 |
| | (80 | ) | | 30,158 |
|
2023 | 24,235 |
| | (39 | ) | | 24,196 |
|
2024 and thereafter | 90,978 |
| | — |
| | 90,978 |
|
Total | $ | 267,543 |
| | $ | (590 | ) | | $ | 266,953 |
|
Employment Agreements
The Company has entered into employment and non‑compete agreements with certain management employees. In the event of termination of employment for certain instances, employees will receive severance payments for base salary and benefits for a specified period (six months for vice presidents, nine months for senior vice presidents and 12 months for executive vice presidents, the president and chief executive officer). Each employment agreement also contains provisions that restrict the employee’s ability to compete directly with the Company for a comparable period after employment terminates. In addition, stock option grant agreements for these employees provide the Company with the right to repurchase from the employee, or the employee with the right to sell to the Company, stock owned by the employee in certain limited instances of termination.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Legal Proceedings
The Company is involved in legal proceedings from time to time in the ordinary course of its business, including employment claims and claims related to other business transactions. Although the outcome of such claims is uncertain, management believes that these legal proceedings will not have a material adverse effect on the financial condition or results of future operations of the Company.
The Company is currently a party to litigation with the City of Sao Paulo, Brazil. The dispute relates to whether the export of services provided by the Company is subject to a local tax on services. The Company has not recorded a liability associated with the claim, which totaled $4.9 million at December 31, 2018, given that it is not deemed probable the Company will incur a loss related to this case. However, a deposit totaling $4.9 million has been made to the Brazilian court in order to annul the potential tax obligation and to avoid the accrual of additional interest and penalties. This balance is recorded in other assets on the consolidated balance sheets. In June 2015, the Judiciary Court of Justice of the State of Sao Paulo ruled in the favor of the Company; however, the judgment was appealed by the City of Sao Paulo. The Company expects to recover the full amount of the deposit when the case is settled. In September 2017, a judge from the Superior Court of Justice of Brazil denied relief to the City of Sao Paulo's appeal and upheld the lower court's ruling in the favor of the Company for the years 2005 to 2012, and in the period from January to October 2013. The judge from the Superior Court of Justice of Brazil also ruled that the Company must appeal the lower court's verdict for October 2013 and the subsequent periods as the Judiciary Court of Justice of the State of Sao Paulo only reviewed the facts that pertained to the period before October 2013.
Insurance
The Company currently maintains insurance for risks associated with the operation of its business, provision of professional services, and ownership of property. These policies provide coverage for a variety of potential losses, including, without limitation, loss or damage to property, bodily injury, general commercial liability, professional errors and omissions, and medical malpractice.
The Company’s retentions and deductibles associated with these insurance policies range up to a maximum of $0.5 million.
Employee Health Insurance
The Company is self‑insured for health insurance for employees within the United States. The Company maintains stop‑loss insurance on a “claims made” basis for expenses in excess of $0.3 million per member per year. As of December 31, 2018 and 2017, the Company maintained a reserve of approximately $5.1 million and $5.0 million, respectively, included in accrued expense and other current liabilities on the consolidated balance sheets, to cover open claims and estimated claims incurred but not reported.
(14) Employee Benefit Plans
Defined contribution or profit sharing style plans are offered in Australia, Belgium, Germany, Hong Kong, India, Israel, Japan, the Netherlands, New Zealand, the Philippines, South Africa, Spain, Sweden, Thailand, and the United Kingdom. In some cases, these plans are required by local laws or regulations.
The Company maintains 401(k) plans in the United States, which cover substantially all employees of its U.S. subsidiaries. The Company matches participant's contributions at varying amounts, subject to a maximum contribution of 6% of the participant's compensation. The employer contributions to the 401(k) plans were approximately $13.6 million, $11.9 million and $9.9 million for the years ended December 31, 2018, 2017 and 2016, respectively.
As a result of the Takeda transactions during 2017, the Company maintains defined benefit pension plans sponsored by certain TDC and TDS subsidiaries in Japan and Germany. The funded status of the defined benefit pension plans, which represents the difference between the projected benefit obligation and the fair value of plan assets, is calculated on a plan-by-plan basis. The funded status of the plan in Japan, which covers approximately 106 employees, totaled $0.8 million and $0.8 million at December 31, 2018 and 2017, respectively, and was recorded in other assets on the consolidated balance sheets. The unfunded status of the plan in Germany, which covers eight employees, totaled $0.9 million and $0.7 million at December 31, 2018 and 2017, respectively, and was recorded in other long-term liabilities on the consolidated balance sheets. Additional
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
disclosures regarding these defined benefit pension plans have been excluded due to their immateriality. The Company did not have defined benefit pension plans prior to 2017.
(15) Derivatives
The Company is exposed to certain risks relating to its ongoing business operations. The primary risk that the Company seeks to manage by using derivative instruments is interest rate risk. Accordingly, the Company has instituted interest rate hedging programs that are accounted for in accordance with ASC 815, “Derivatives and Hedging.” The interest rate hedging program is a cash flow hedge program designed to minimize interest rate volatility. The Company swaps the difference between fixed and variable interest amounts calculated by reference to an agreed-upon notional principal amount, at specified intervals. The Company also employed an interest rate cap that would have compensated the Company if variable interest rates had risen above a pre-determined rate. The Company’s interest rate contracts are designated as hedging instruments.
On October 2, 2013, the Company entered into interest rate swap agreements with an aggregate notional principal amount of $620.0 million, or the 2013 Swaps. The interest rate swaps were set to begin on September 23, 2015. The interest rate swaps were to be used to hedge the Company’s variable rate debt. The interest rate swaps had maturity dates ranging from one to five years. During the third quarter of 2015, the Company paid $32.9 million to terminate the 2013 Swaps. Amounts previously recorded in accumulated other comprehensive loss related to these interest rate swaps, totaling $29.6 million, are being reclassified into earnings over the term of the previously hedged borrowing using the swaplet method. For the terminated swaps, the Company reclassified $6.8 million, $6.3 million and $4.7 previously recorded in accumulated other comprehensive loss into interest expense during the years ended December 31, 2018, 2017 and 2016, respectively.
Subsequent to the termination of all existing interest rate swaps, the Company entered into a new interest rate swap agreement with a notional principal amount of $250.0 million and a fixed three-month LIBOR of 1.48%, or the 2015 Swap. The interest rate swap began on September 23, 2015 and matured on September 23, 2018. The interest rate swap was being used to hedge the Company’s variable rate debt.
In conjunction with the closing of the 2016 Credit Facilities in December 2016, the 2015 Swap was amended to modify the fixed rate, repricing dates and embedded floor, or the Modified 2015 Swap. The Company re-designated the Modified 2015 Swap against the refinanced debt under the 2016 Credit Facilities. As a result of the re-designation, all amounts previously recorded in accumulated other comprehensive loss related to the 2015 Swap, totaling $0.8 million, were frozen and were amortized into earnings over the term of the previously hedged borrowing using the swaplet method. The Company reclassified $0.4 million previously recorded in accumulated other comprehensive loss into interest expense associated with the 2015 Swap during the years ended December 31, 2018 and 2017. The closing of the 2016 Credit Facilities did not impact the amortization of the losses frozen in accumulated other comprehensive loss associated with the 2013 Swaps.
On January 5, 2018, the Company entered into two interest rate swaps in order to manage its cash flow exposure to variable rate debt and also to replace the Modified 2015 Swap, which was scheduled to mature in September 2018. The first interest rate swap has an aggregate notional amount of $375.0 million and a fixed payment rate of 2.2% offsetting a one-month LIBOR variable rate with an effective date of January 8, 2018, and a maturity date of December 6, 2020. The second interest rate swap has an aggregate notional amount of $250.0 million and a fixed payment rate of 2.3% offsetting a one-month LIBOR variable rate with an effective date of September 6, 2018, and a maturity date of September 6, 2020.
The following table presents the notional amounts and fair values (determined using level 2 inputs) of the Company’s derivatives as of December 31, 2018 and 2017 (in thousands):
|
| | | | | | | | | | | | | | | | | |
| Balance Sheet Classification | | December 31, 2018 | | December 31, 2017 |
| | Notional amount | | Asset | | Notional amount | | Asset |
Derivatives in an asset position: | Other current assets | |
|
| |
|
| | $ | 250,000 |
| | $ | 428 |
|
| Other assets | | $ | 625,000 |
| | $ | 3,318 |
| | | | |
The Company records the effective portion of any change in the fair value of derivatives designated as hedging instruments under ASC 815 to other accumulated other comprehensive loss in the consolidated balance sheets, net of deferred taxes, and will later reclassify into earnings when the hedged item affects earnings or is no longer expected to occur. Gains and losses from the ineffective portion of any hedge are recognized in earnings immediately. For other derivative contracts that do
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
not qualify or no longer qualify for hedge accounting, changes in the fair value of the derivatives are recognized in earnings each period.
The table below presents the effect of the Company's derivatives on the consolidated statements of operations and comprehensive (loss) income (in thousands):
|
| | | | | | | | | | | |
| Years Ended December 31, |
Derivatives in Cash Flow Hedging Relationships (Interest Rate Contracts) | 2018 | | 2017 | | 2016 |
Amount of pre-tax gain (loss) recognized in other comprehensive income (loss) on derivatives | $ | 3,159 |
| | $ | 245 |
| | $ | (1,600 | ) |
Amount of loss recognized in other (expense) income, net on derivatives (ineffective portion) | — |
| | — |
| | 1 |
|
Amount of loss reclassified from accumulated other comprehensive loss into interest expense, net on derivatives | (6,477 | ) | | (6,855 | ) | | (5,921 | ) |
The Company expects that $4.4 million of unrealized losses will be reclassified out of accumulated other comprehensive loss and into interest expense, net over the next 12 months.
(16) Accumulated Other Comprehensive Loss
Below is a summary of the components of accumulated other comprehensive loss (in thousands):
|
| | | | | | | | | | | |
| Foreign Currency Translation | | Derivative Instruments | | Total |
Balance at December 31, 2015 | $ | (106,072 | ) | | $ | (26,235 | ) | | $ | (132,307 | ) |
Other comprehensive loss before reclassifications, net of tax | (95,019 | ) | | (978 | ) | | (95,997 | ) |
Reclassification adjustments, net of tax | — |
| | 3,618 |
| | 3,618 |
|
Balance at December 31, 2016 | (201,091 | ) | | (23,595 | ) | | (224,686 | ) |
Other comprehensive income before reclassifications, net of tax | 83,911 |
| | 149 |
| | 84,060 |
|
Reclassification adjustments, net of tax | — |
| | 4,156 |
| | 4,156 |
|
Balance at December 31, 2017 | (117,180 | ) | | (19,290 | ) | | (136,470 | ) |
Other comprehensive (loss) income before reclassifications, net of tax | (41,169 | ) | | 2,152 |
| | (39,017 | ) |
Reclassification adjustments, net of tax | — |
| | 4,828 |
| | 4,828 |
|
Balance at December 31, 2018 | $ | (158,349 | ) | | $ | (12,310 | ) | | $ | (170,659 | ) |
Foreign Currency Translation
The change in the foreign currency translation adjustment during the year ended December 31, 2018 was primarily due to the movements in the British pound, or GBP, Euro, or EUR, Canadian dollar, or CAD, and Russian ruble, or RUB, exchange rates against the U.S. dollar, or USD. The USD strengthened by 5.6%, 4.5%, 7.9% and 17.1% versus the GBP, EUR, CAD and RUB, respectively, during the year ended December 31, 2018. The movement in the GBP, EUR, CAD and RUB represented $12.4 million, $15.2 million, $3.2 million and $4.6 million, respectively, of the $41.2 million loss recorded to accumulated other comprehensive loss during the year ended December 31, 2018.
The change in the foreign currency translation adjustment during the year ended December 31, 2017 was primarily due to the movements in the GBP, EUR, CAD, and RUB exchange rates against the USD. The USD depreciated by 9.3%, 13.7%, 7.1% and 6.2% versus the GBP, EUR, CAD and RUB respectively, during the year ended December 31, 2017. The movement in the GBP, EUR, CAD and RUB represented $46.0 million, $31.0 million, $3.5 million and $1.9 million, respectively, of the $83.9 million income recorded to accumulated other comprehensive loss during the year ended December 31, 2017.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The change in the foreign currency translation adjustment during the year ended December 31, 2016 was primarily due to the movements in the GBP, EUR, CAD, and RUB exchange rates against the USD. The USD strengthened by 16.7% and 3.6% versus the GBP and EUR, respectively, during the year ended December 31, 2016, and the USD depreciated by 3.1% and 19.5% against the CAD and RUB, respectively, during the same period. The movement in the GBP and EUR represented $90.2 million and $8.4 million, respectively, of the $95.0 million loss recorded to accumulated other comprehensive loss during the year ended December 31, 2016. The overall change was partially offset by gains in the CAD and RUB, representing $1.1 million and $4.0 million of the adjustment, respectively.
Accumulated earnings of the Company’s U.K. subsidiary totaling $375.4 million have been previously taxed in the U.S. or were deemed to have been repatriated as part of the one-time transition tax under the Act enacted December 22, 2017. The Company has deemed a corresponding amount of intercompany accounts between its U.S. and U.K. subsidiaries to be of a long-term investment nature; these balances have been remeasured to foreign currency translation adjustment during the year ended December 31, 2018.
Derivative Instruments
See Note 15 for further information on changes to accumulated other comprehensive loss related to the derivative instruments.
(17) Net Income Per Share
Basic net income per share is calculated by dividing net income by the weighted average number of common shares outstanding for the applicable period. Diluted net income per share is calculated after adjusting the denominator of the basic net income per share calculation for the effect of all potentially dilutive common shares, which in the Company’s case, includes shares issuable under the stock option and incentive award plan.
The following table reconciles the basic to diluted weighted average shares outstanding (in thousands):
|
| | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Basic weighted average common shares outstanding | 64,123 |
| | 62,437 |
| | 60,759 |
|
Effect of dilutive stock options and RSAs/RSUs | 2,218 |
| | 3,336 |
| | 3,693 |
|
Diluted weighted average common shares outstanding | 66,341 |
| | 65,773 |
| | 64,452 |
|
Anti-dilutive shares | 1,620 |
| | 741 |
| | 305 |
|
The anti-dilutive shares disclosed above were calculated using the treasury stock method. The treasury stock method calculates dilution assuming the exercise of all in-the-money options and vesting of RSAs/RSUs, reduced by the repurchase of shares with the proceeds from the assumed exercises, and unrecognized compensation expense for outstanding awards.
(18) Supplemental Cash Flow Information
The following table presents the Company’s supplemental cash flow information (in thousands):
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Cash paid during the period for: | | | | | |
Income taxes, net of refunds | $ | 43,127 |
| | $ | 47,829 |
| | $ | 27,644 |
|
Interest | 48,911 |
| | 48,330 |
| | 48,156 |
|
Non-cash investing and financing activities: | | | | | |
Issuance of common stock for the acquisition of Value Health Solutions, Inc. | — |
| | 369 |
| | — |
|
Accrued fixed assets purchases | 10,312 |
| | 3,962 |
| | 2,644 |
|
Cashless exercises of stock options | 12,390 |
| | 13,252 |
| | 9,456 |
|
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Additionally, the acquisition date fair value of contingent consideration liabilities recorded during the year ended December 31, 2017 totaled $155.8 million. Refer to Note 2 - Significant Accounting Polices and Note 4 - Business Combinations for further information.
(19) Operations by Geographic Area
The table below presents certain enterprise‑wide information about the Company’s operations by geographic area for the years ended December 31, 2018, 2017 and 2016. The Company attributes revenues to geographical locations based upon where the services are performed.
The Company’s operations within each geographical region are further broken down to show each country which accounts for 10% or more of the totals (in thousands):
|
| | | |
| Year Ended |
| December 31, 2018 |
Revenue: | |
Americas: | |
|
United States | $ | 1,962,509 |
|
Other | 47,116 |
|
Americas | 2,009,625 |
|
Europe, Africa, and Asia-Pacific | |
United Kingdom | 689,345 |
|
Netherlands | 115,778 |
|
Other | 57,174 |
|
Europe, Africa, and Asia-Pacific | 862,297 |
|
Total revenue | $ | 2,871,922 |
|
|
| | | | | | | |
| Years Ended December 31, |
| 2017 | | 2016 |
Service Revenue (1): | | | |
Americas: | | | |
United States | $ | 1,310,772 |
| | $ | 1,063,625 |
|
Other | 42,227 |
| | 33,320 |
|
Americas | 1,352,999 |
| | 1,096,945 |
|
Europe, Africa, and Asia-Pacific | | | |
United Kingdom | 479,623 |
| | 394,363 |
|
Netherlands | 79,555 |
| | 68,118 |
|
Other | 36,197 |
| | 20,597 |
|
Europe, Africa, and Asia-Pacific | 595,375 |
| | 483,078 |
|
Total service revenue | 1,948,374 |
| | 1,580,023 |
|
Reimbursement revenues | 311,015 |
| | 231,688 |
|
Total revenue | $ | 2,259,389 |
| | $ | 1,811,711 |
|
(1) As noted in Note 2, the Company adopted ASC 606 on January 1, 2018 using the modified retrospective method. Comparative prior period amounts continue to be reported under the accounting standards in effect for the period presented.
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
|
| | | | | | | |
| December 31, |
| 2018 | | 2017 |
Long-lived assets: | | | |
Americas: | | | |
United States | $ | 118,860 |
| | $ | 107,952 |
|
Other | 950 |
| | 1,714 |
|
Americas | 119,810 |
| | 109,666 |
|
Europe, Africa, and Asia-Pacific | | | |
United Kingdom | 4,153 |
| | 4,182 |
|
Netherlands | 18,321 |
| | 15,876 |
|
Other | 12,480 |
| | 13,346 |
|
Europe, Africa, and Asia-Pacific | 34,954 |
| | 33,404 |
|
Total long-lived assets | $ | 154,764 |
| | $ | 143,070 |
|
(20) Segments
The Company is managed through two reportable segments, (i) Clinical Research and (ii) Data Solutions. In accordance with the provisions of ASC 280, "Segment Reporting", the Company's chief operating decision-maker has been identified as the Chief Executive Officer, who reviews operating results to make decisions about allocating resources and assessing performance for the entire company.
| |
• | Clinical Research Segment: The Clinical Research segment, which primarily serves biopharmaceutical clients, provides outsourced clinical research and clinical trial related services. |
| |
• | Data Solutions Segment: The Data Solutions segment provides data and analytics, technology solutions and real-world insights and services primarily to the Company’s life science clients. |
The Company's chief operating decision maker uses segment profit as the primary measure of each segment's operating results in order to allocate resources and in assessing the Company's performance. Asset information by segment is not presented, as this measure is not used by the chief operating decision maker to assess the Company's performance. The Company’s reportable segment information is presented below (in thousands):
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
|
| | | | | | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 | | 2016 |
Revenue: | | | | | |
Clinical Research | $ | 2,622,409 |
| | $ | 2,168,891 |
| | $ | 1,811,711 |
|
Data Solutions | 249,513 |
| | 90,498 |
| | — |
|
Total revenue | 2,871,922 |
| | 2,259,389 |
| | 1,811,711 |
|
Direct costs (exclusive of depreciation and amortization): | | | | | |
Clinical Research | 1,334,803 |
| | 1,231,690 |
| | 1,032,688 |
|
Data Solutions | 165,423 |
| | 52,178 |
| | — |
|
Total direct costs (exclusive of depreciation and amortization) | 1,500,226 |
| | 1,283,868 |
| | 1,032,688 |
|
Reimbursable out-of-pocket costs: | | | | | |
Clinical Research | 308,291 |
| | 311,015 |
| | 231,688 |
|
Data Solutions | — |
| | — |
| | — |
|
Total reimbursable out-of-pocket costs | 308,291 |
| | 311,015 |
| | 231,688 |
|
Reimbursable investigator fees: | | | | | |
Clinical Research | 262,114 |
| | — |
| | — |
|
Data Solutions | — |
| | — |
| | — |
|
Total reimbursable investigator fees | 262,114 |
| | — |
| | — |
|
Segment profit: | | | | | |
Clinical Research | 717,201 |
| | 626,186 |
| | 547,335 |
|
Data Solutions | 84,090 |
| | 38,320 |
| | — |
|
Total segment profit | $ | 801,291 |
| | $ | 664,506 |
| | $ | 547,335 |
|
Less expenses not allocated to segments: | | | | | |
Selling, general and administrative | 371,795 |
| | 321,987 |
| | 269,893 |
|
Transaction-related costs | 35,817 |
| | 87,709 |
| | 44,834 |
|
Depreciation and amortization | 112,247 |
| | 78,227 |
| | 69,506 |
|
Loss on disposal of fixed assets, net | 120 |
| | 358 |
| | 753 |
|
Consolidated income from operations | 281,312 |
| | 176,225 |
| | 162,349 |
|
Interest expense, net | (57,399 | ) | | (46,729 | ) | | (54,913 | ) |
Loss on modification or extinguishment of debt | (952 | ) | | (15,023 | ) | | (38,178 | ) |
Foreign currency (losses) gains, net | (1,043 | ) | | (39,622 | ) | | 24,029 |
|
Other (expense) income, net | (371 | ) | | (304 | ) | | 607 |
|
Consolidated income before income taxes and equity in income of unconsolidated joint ventures | $ | 221,547 |
| | $ | 74,547 |
| | $ | 93,894 |
|
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(21) Quarterly Financial Data (unaudited)
The following table summarizes the Company’s unaudited quarterly results of operations (in thousands, except per share data:
|
| | | | | | | | | | | | | | | |
| 2018 |
| First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter |
Revenue | $ | 701,837 |
| | $ | 722,841 |
| | $ | 717,596 |
| | $ | 729,648 |
|
Income from operations (1) | 71,948 |
| | 73,796 |
| | 38,812 |
| | 96,756 |
|
Provision for income taxes | 17,654 |
| | 17,490 |
| | 20,248 |
| | 11,840 |
|
Income before equity in gains of unconsolidated joint ventures | 39,187 |
| | 42,236 |
| | 1,810 |
| | 71,082 |
|
Equity in income of unconsolidated joint ventures | 28 |
| | 46 |
| | 44 |
| | 25 |
|
Net income | 39,215 |
| | 42,282 |
| | 1,854 |
| | 71,107 |
|
Net (income) loss attributable to non-controlling interests | (234 | ) | | (305 | ) | | (359 | ) | | 345 |
|
Net income attributable to PRA Health Sciences, Inc. | 38,981 |
| | 41,977 |
| | 1,495 |
| | 71,452 |
|
Comprehensive income (loss) | 61,294 |
| | 8,185 |
| | (31 | ) | | 50,948 |
|
Comprehensive (income) loss attributable to noncontrolling interest | (582 | ) | | (48 | ) | | (193 | ) | | 143 |
|
Comprehensive income (loss) attributable to PRA Health Sciences, Inc. | $ | 60,712 |
| | $ | 8,137 |
| | $ | (224 | ) | | $ | 51,091 |
|
Basic earnings per share (2) | $ | 0.61 |
| | $ | 0.66 |
| | $ | 0.02 |
| | $ | 1.10 |
|
Diluted earnings per share (2) | $ | 0.59 |
| | $ | 0.64 |
| | $ | 0.02 |
| | $ | 1.07 |
|
|
| | | | | | | | | | | | | | | |
| 2017 |
| First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter |
Service revenue | $ | 427,080 |
| | $ | 457,942 |
| | $ | 494,550 |
| | $ | 568,802 |
|
Reimbursement revenue | 60,680 |
| | 75,782 |
| | 87,459 |
| | 87,094 |
|
Total revenue | 487,760 |
| | 533,724 |
| | 582,009 |
| | 655,896 |
|
Income from operations (3) | 49,986 |
| | 64,850 |
| | 57,776 |
| | 3,613 |
|
Provision for (benefit from) income taxes (4) | 7,883 |
| | 10,193 |
| | (18,241 | ) | | (12,458 | ) |
Income (loss) before equity in gains of unconsolidated joint ventures (5) | 25,182 |
| | 29,632 |
| | 48,582 |
| | (16,226 | ) |
Equity in gains of unconsolidated joint ventures | 42 |
| | 26 |
| | 24 |
| | 31 |
|
Net income (loss) | 25,224 |
| | 29,658 |
| | 48,606 |
| | (16,195 | ) |
Net (income) loss attributable to non-controlling interests | — |
| | (112 | ) | | (401 | ) | | 147 |
|
Net income (loss) attributable to PRA Health Sciences, Inc. | 25,224 |
| | 29,546 |
| | 48,205 |
| | (16,048 | ) |
Comprehensive income (loss) | 42,552 |
| | 63,892 |
| | 75,348 |
| | (6,380 | ) |
Comprehensive (income) loss attributable to noncontrolling interest | — |
| | (50 | ) | | (373 | ) | | 154 |
|
Comprehensive income (loss) attributable to PRA Health Sciences, Inc. | $ | 42,552 |
| | $ | 63,842 |
| | $ | 74,975 |
| | $ | (6,226 | ) |
Basic earnings (loss) per share (2) | $ | 0.41 |
| | $ | 0.47 |
| | $ | 0.77 |
| | $ | (0.25 | ) |
Diluted earnings (loss) per share (2) | $ | 0.39 |
| | $ | 0.45 |
| | $ | 0.73 |
| | $ | (0.25 | ) |
| |
(1) | During the three months ended September 30, 2018, the Company recorded $42.6 million of transaction-related costs associated with the change in fair value of contingent consideration. During the three months ended March 31, 2018, the Company recorded an $11.6 million reduction to transaction-related costs associated with the change in fair value of contingent consideration. |
| |
(2) | The sum of the quarterly per share amounts may not equal per share amounts reported for year‑to‑date periods. This is due to changes in the number of weighted average shares outstanding and the effects of rounding for each period. |
PRA HEALTH SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
| |
(3) | During the three months ended December 31, 2017, the Company recorded $75.0 million of transaction-related costs associated with the change in fair value of contingent consideration. During the three months ended September 30, 2017, transaction-related costs consisted of $6.4 million of fees incurred in connection with the acquisition of Symphony Health, $5.3 million of stock-based compensation expense related to the release of the transfer restrictions on vested options, and $1.0 million of third-party fees incurred in connection with the August 2017 secondary offering. These amounts were offset by a $1.0 million adjustment to the change in fair value of contingent consideration. |
| |
(4) | During the three months ended September 30, 2017 and December 31, 2017, the Company recorded a benefit from income taxes of $18.2 million and $12.5 million, respectively. The benefit was due primarily to (i) the benefit realized from the tax deduction of stock awards in excess of the amount recognized in the financial statements per guidance under ASU No. 2016-09, “Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting”, (ii) the release of the valuation allowance against the federal net deferred tax assets, and additionally during the three months ended December 31, 2017 (iii) the U.S. federal rate decrease effect on an overall net deferred tax liability due to the recent law changes in the Tax Cuts and Jobs Act. |
| |
(5) | During the three months ended September 30, 2017 and December 31, 2017, the Company recorded a loss on extinguishment of debt of $3.1 million and $11.9 million, respectively. The loss on extinguishment of debt recorded during the three months ended September 30, 2017 related to the Incremental Borrowings on the Company’s term debt. The loss on extinguishment of debt recorded during the three months ended December 31, 2017 related to the refinancing of the Company’s 2016 Credit Facilities and the redemption of the Company's Senior Notes. Refer to Note 9, Current Borrowings and Long-Term Debt, for additional information regarding the items noted above. |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
As of December 31, 2018, we carried out an evaluation under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures. Regulations under the Exchange Act require public companies, including us, to maintain “disclosure controls and procedures,” which are defined in Rule 13a‑15(e) and Rule 15d‑15(e) of the Exchange Act to mean a company’s controls and other procedures that provide reasonable assurance that information required to be disclosed in reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to management, including our principal executive officer and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosures. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Based upon our evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report to accomplish their objectives at a reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting
Our management’s report on internal control over financial reporting is set forth in Part II, Item 8 of this Annual Report on Form 10-K and is incorporated herein by reference.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting during the quarter ended December 31, 2018 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item will be included in our definitive proxy statement (or the “2019 Proxy Statement”) to be filed with the SEC within 120 days of the end of our fiscal year covered by this Annual Report and is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this item will be included in our 2019 Proxy Statement to be filed with the SEC within 120 days of the end of our fiscal year covered by this Annual Report and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item will be included in our 2019 Proxy Statement to be filed with the SEC within 120 days of the end of our fiscal year covered by this Annual Report and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item will be included in our 2019 Proxy Statement to be filed with the SEC within 120 days of the end of our fiscal year covered by this Annual Report and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services
The information required by this item will be included in our 2019 Proxy Statement to be filed with the SEC within 120 days of the end of our fiscal year covered by this Annual Report and is incorporated herein by reference.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(1) Financial Statements
The following financial statements and supplementary data are included in Item 8 of this annual report:
(2) Financial Statement Schedules
The information required to be submitted in the Financial Statement Schedules for PRA Health Sciences, Inc. and subsidiaries has either been shown in the financial statements or notes, or is not applicable or required under Regulation S‑X; therefore, those schedules have been omitted.
(3) Exhibits
The exhibits listed in the accompanying Exhibit Index following the signature page are filed or furnished as a part of this report and are incorporated herein by reference.
Item 16. Form 10-K Summary
None.
EXHIBIT INDEX
|
| | | |
Exhibit Number | | Description of Exhibit |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
|
| | | |
| | |
| | Agreement and Plan of Merger, dated as of August 3, 2017, by and among Pharmaceutical Research Associates, Inc., Symphony Health Solutions Corporation, Skyhook Merger Sub, Inc., and STG III, L.P. (incorporated by reference to Exhibit 2.1 to the Registrant's Current Report on Form 8-K filed on August 7, 2017). |
| | Joinder Agreement, dated as September 6, 2017, by and among of Pharmaceutical Research Associates, Inc., PRA Health Sciences, Inc., each of the subsidiaries from time to time party thereto, Wells Fargo Bank, National Association, as administrative agent and collateral agent and other agents and lenders party thereto. (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on September 11, 2017). |
| | |
| |
|
| | First Amendment and Second Joinder Agreement, dated as of December 28, 2017, by and among Pharmaceutical Research Associates, Inc. (the “Borrower”), PRA Health Sciences, Inc., each of the subsidiaries of the Borrower from time to time party thereto, Wells Fargo Bank, National Association, as administrative agent and collateral agent and other agents and lenders party thereto (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on December 29, 2017). |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
101* | | The following financial information from PRA Health Sciences, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2018 formatted in XBRL: (i) Consolidated Balance Sheets as of December 31, 2018 and December 31, 2017, (ii) Consolidated Statements of Operations for the years ended December 31, 2018, 2017 and 2016, (iii) Consolidated Statements of Comprehensive Income (Loss) for the years ended December 31, 2018, 2017 and 2016, (iv) Consolidated Statements of Cash Flows for the years ended December 31, 2018, 2017 and 2016, and (v) Notes to Consolidated Financial Statements
|
* Filed herewith.
** This document has been identified as a management contract or compensatory plan or arrangement.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf on February 28, 2019 by the undersigned, thereunto duly authorized.
|
| | | |
| PRA Health Sciences, Inc. |
| By: | /s/ Michael J. Bonello |
| | Name: | Michael J. Bonello |
| | Title: | Executive Vice President and Chief Financial Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENT that the undersigned officers and directors of PRA Health Sciences, Inc. do hereby constitute and appoint Colin Shannon and Michael J. Bonello, and each of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities indicated on February 28, 2019.
|
| | |
Signature | | Capacity |
| | |
/s/ Colin Shannon | | |
Colin Shannon | | President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) |
| | |
/s/ Michael J. Bonello | | |
Michael J. Bonello
| | Executive Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) |
| | |
/s/ Jeffrey T. Barber | | |
Jeffrey T. Barber | | Director |
| | |
/s/ Max C. Lin | | |
Max C. Lin | | Director |
| | |
/s/ James C. Momtazee | | |
James C. Momtazee | | Director |
| | |
/s/ Matthew P. Young | | |
Matthew P. Young | | Director |
| | |
/s/ Linda S. Grais | | |
Linda S. Grais | | Director |
| | |
/s/ Alexander G. Dickinson | | Director |
Alexander G. Dickinson | | |