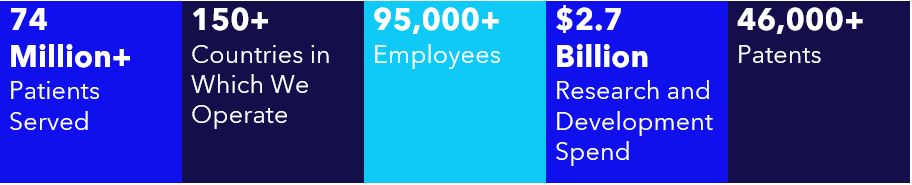
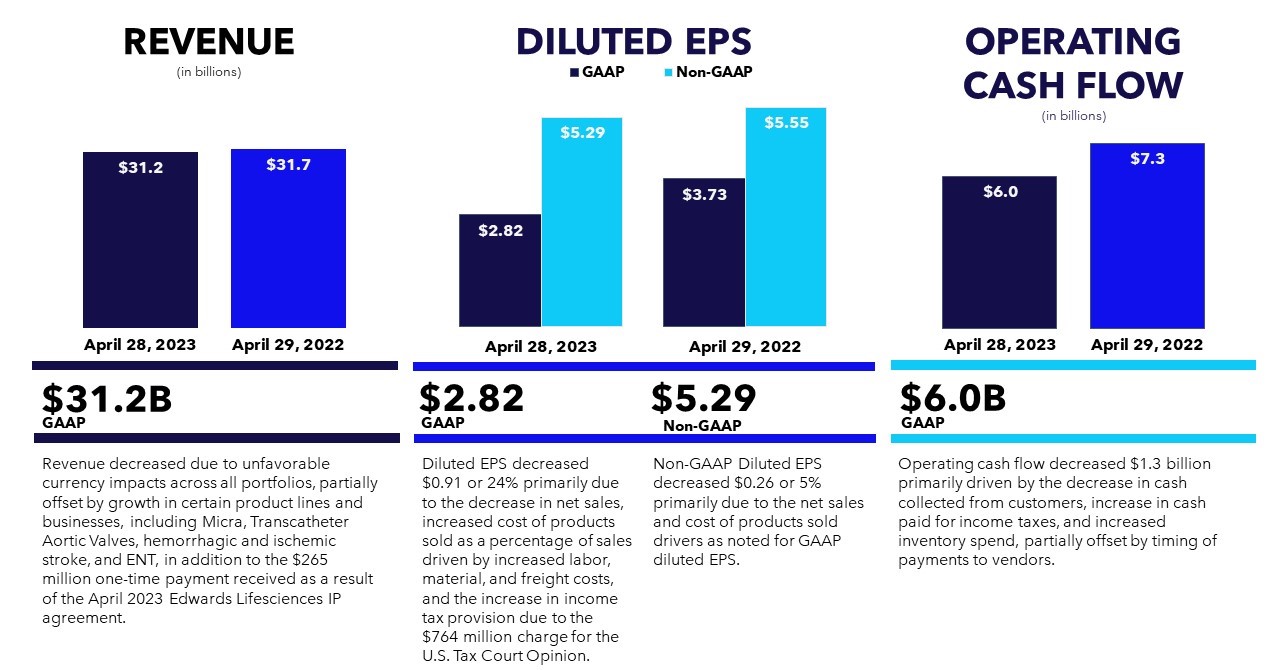
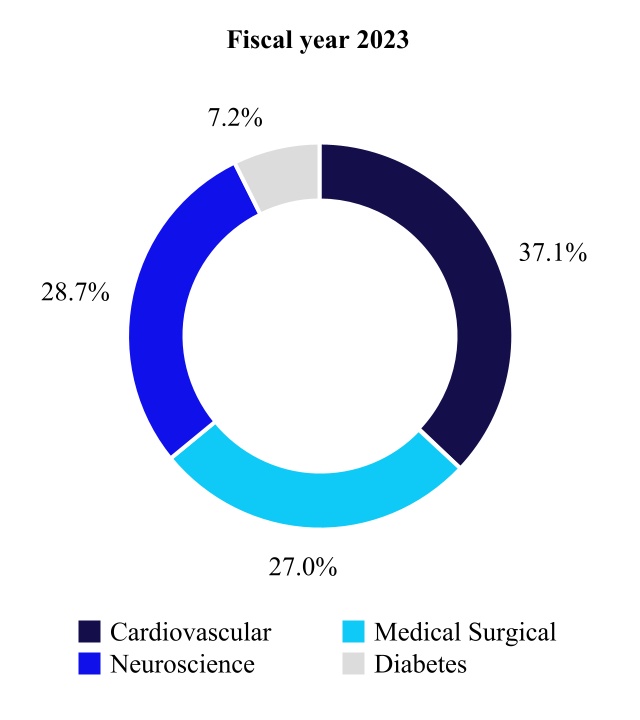
00016131034/28falseFY2023D02 XH02http://fasb.org/us-gaap/2022#AccountingStandardsUpdate201613MemberP3YP3YP4YP2Yhttp://fasb.org/us-gaap/2022#DebtCurrenthttp://fasb.org/us-gaap/2022#DebtCurrentP10YP6YP3YP5YP8YP10YP5YP6YP8YP12YP12YP9YP12YP10YP12YP20YP30YP20YP30YP20YP30YP20YP30YP30YP30YP30YP30YP30Yhttp://fasb.org/us-gaap/2022#LongTermDebtAndCapitalLeaseObligationshttp://fasb.org/us-gaap/2022#LongTermDebtAndCapitalLeaseObligationshttp://fasb.org/us-gaap/2022#InterestExpensehttp://fasb.org/us-gaap/2022#OtherAssetsCurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2022#OtherAssetsCurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2022#OtherOperatingIncomeExpenseNethttp://fasb.org/us-gaap/2022#OtherOperatingIncomeExpenseNetP10YP4YP1Yhttp://fasb.org/us-gaap/2022#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2022#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesNoncurrent00016131032022-04-302023-04-280001613103us-gaap:CommonStockMember2022-04-302023-04-280001613103mdt:SeniorNotes2019Due2025Member2022-04-302023-04-280001613103mdt:SeniorNotes2020Due2025Member2022-04-302023-04-280001613103mdt:SeniorNotes2022Due2025Member2022-04-302023-04-280001613103mdt:SeniorNotes2019Due2027Member2022-04-302023-04-280001613103mdt:SeniorNotes2020Due2028Member2022-04-302023-04-280001613103mdt:SeniorNotes2022Due2028Member2022-04-302023-04-280001613103mdt:SeniorNotes2019Due20311.625PercentMember2022-04-302023-04-280001613103mdt:SeniorNotes2019Due20311.00PercentMember2022-04-302023-04-280001613103mdt:SeniorNotes2022Due2031Member2022-04-302023-04-280001613103mdt:SeniorNotes2020Due2032Member2022-04-302023-04-280001613103mdt:SeniorNotes2022Due2034Member2022-04-302023-04-280001613103mdt:SeniorNotes2019Due20392.250PercentMember2022-04-302023-04-280001613103mdt:SeniorNotes2019Due20391.50PercentMember2022-04-302023-04-280001613103mdt:SeniorNotes2020Due2040Member2022-04-302023-04-280001613103mdt:SeniorNotes2019Due2049Member2022-04-302023-04-280001613103mdt:SeniorNotes2020Due2050Member2022-04-302023-04-2800016131032022-10-28iso4217:USD00016131032023-06-16xbrli:shares00016131032021-05-012022-04-2900016131032020-04-252021-04-30iso4217:USDxbrli:shares00016131032023-04-2800016131032022-04-290001613103us-gaap:CommonStockMember2020-04-240001613103us-gaap:AdditionalPaidInCapitalMember2020-04-240001613103us-gaap:RetainedEarningsMember2020-04-240001613103us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-04-240001613103us-gaap:ParentMember2020-04-240001613103us-gaap:NoncontrollingInterestMember2020-04-2400016131032020-04-240001613103us-gaap:RetainedEarningsMember2020-04-252021-04-300001613103us-gaap:ParentMember2020-04-252021-04-300001613103us-gaap:NoncontrollingInterestMember2020-04-252021-04-300001613103us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-04-252021-04-300001613103us-gaap:CommonStockMember2020-04-252021-04-300001613103us-gaap:AdditionalPaidInCapitalMember2020-04-252021-04-3000016131032019-04-272020-04-240001613103us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2020-04-240001613103srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2020-04-240001613103us-gaap:CommonStockMember2021-04-300001613103us-gaap:AdditionalPaidInCapitalMember2021-04-300001613103us-gaap:RetainedEarningsMember2021-04-300001613103us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-04-300001613103us-gaap:ParentMember2021-04-300001613103us-gaap:NoncontrollingInterestMember2021-04-3000016131032021-04-300001613103us-gaap:RetainedEarningsMember2021-05-012022-04-290001613103us-gaap:ParentMember2021-05-012022-04-290001613103us-gaap:NoncontrollingInterestMember2021-05-012022-04-290001613103us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-05-012022-04-290001613103us-gaap:CommonStockMember2021-05-012022-04-290001613103us-gaap:AdditionalPaidInCapitalMember2021-05-012022-04-290001613103us-gaap:CommonStockMember2022-04-290001613103us-gaap:AdditionalPaidInCapitalMember2022-04-290001613103us-gaap:RetainedEarningsMember2022-04-290001613103us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-290001613103us-gaap:ParentMember2022-04-290001613103us-gaap:NoncontrollingInterestMember2022-04-290001613103us-gaap:RetainedEarningsMember2022-04-302023-04-280001613103us-gaap:ParentMember2022-04-302023-04-280001613103us-gaap:NoncontrollingInterestMember2022-04-302023-04-280001613103us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-302023-04-280001613103us-gaap:CommonStockMember2022-04-302023-04-280001613103us-gaap:AdditionalPaidInCapitalMember2022-04-302023-04-280001613103us-gaap:CommonStockMember2023-04-280001613103us-gaap:AdditionalPaidInCapitalMember2023-04-280001613103us-gaap:RetainedEarningsMember2023-04-280001613103us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-280001613103us-gaap:ParentMember2023-04-280001613103us-gaap:NoncontrollingInterestMember2023-04-280001613103srt:MinimumMember2022-04-302023-04-280001613103srt:MaximumMember2022-04-302023-04-280001613103us-gaap:ShippingAndHandlingMember2022-04-302023-04-280001613103us-gaap:ShippingAndHandlingMember2021-05-012022-04-290001613103us-gaap:ShippingAndHandlingMember2020-04-252021-04-300001613103mdt:CardiacRhythmandHeartFailureDivisionMembermdt:CardiovascularMember2022-04-302023-04-280001613103mdt:CardiacRhythmandHeartFailureDivisionMembermdt:CardiovascularMember2021-05-012022-04-290001613103mdt:CardiacRhythmandHeartFailureDivisionMembermdt:CardiovascularMember2020-04-252021-04-300001613103mdt:StructuralHeartAndAorticDivisionMembermdt:CardiovascularMember2022-04-302023-04-280001613103mdt:StructuralHeartAndAorticDivisionMembermdt:CardiovascularMember2021-05-012022-04-290001613103mdt:StructuralHeartAndAorticDivisionMembermdt:CardiovascularMember2020-04-252021-04-300001613103mdt:CoronaryAndPeripheralVascularDivisionMembermdt:CardiovascularMember2022-04-302023-04-280001613103mdt:CoronaryAndPeripheralVascularDivisionMembermdt:CardiovascularMember2021-05-012022-04-290001613103mdt:CoronaryAndPeripheralVascularDivisionMembermdt:CardiovascularMember2020-04-252021-04-300001613103mdt:CardiovascularMember2022-04-302023-04-280001613103mdt:CardiovascularMember2021-05-012022-04-290001613103mdt:CardiovascularMember2020-04-252021-04-300001613103mdt:MedicalSurgicalPortfolioMembermdt:SurgicalInnovationsDivisionMember2022-04-302023-04-280001613103mdt:MedicalSurgicalPortfolioMembermdt:SurgicalInnovationsDivisionMember2021-05-012022-04-290001613103mdt:MedicalSurgicalPortfolioMembermdt:SurgicalInnovationsDivisionMember2020-04-252021-04-300001613103mdt:MedicalSurgicalPortfolioMembermdt:RespiratoryGastrointestinalAndRenalDivisionMember2022-04-302023-04-280001613103mdt:MedicalSurgicalPortfolioMembermdt:RespiratoryGastrointestinalAndRenalDivisionMember2021-05-012022-04-290001613103mdt:MedicalSurgicalPortfolioMembermdt:RespiratoryGastrointestinalAndRenalDivisionMember2020-04-252021-04-300001613103mdt:MedicalSurgicalPortfolioMember2022-04-302023-04-280001613103mdt:MedicalSurgicalPortfolioMember2021-05-012022-04-290001613103mdt:MedicalSurgicalPortfolioMember2020-04-252021-04-300001613103mdt:CranialAndSpinalTechnologiesDivisionMembermdt:NeurosciencePortfolioMember2022-04-302023-04-280001613103mdt:CranialAndSpinalTechnologiesDivisionMembermdt:NeurosciencePortfolioMember2021-05-012022-04-290001613103mdt:CranialAndSpinalTechnologiesDivisionMembermdt:NeurosciencePortfolioMember2020-04-252021-04-300001613103mdt:SpecialtyTherapiesDivisionMembermdt:NeurosciencePortfolioMember2022-04-302023-04-280001613103mdt:SpecialtyTherapiesDivisionMembermdt:NeurosciencePortfolioMember2021-05-012022-04-290001613103mdt:SpecialtyTherapiesDivisionMembermdt:NeurosciencePortfolioMember2020-04-252021-04-300001613103mdt:NeuromodulationDivisionMembermdt:NeurosciencePortfolioMember2022-04-302023-04-280001613103mdt:NeuromodulationDivisionMembermdt:NeurosciencePortfolioMember2021-05-012022-04-290001613103mdt:NeuromodulationDivisionMembermdt:NeurosciencePortfolioMember2020-04-252021-04-300001613103mdt:NeurosciencePortfolioMember2022-04-302023-04-280001613103mdt:NeurosciencePortfolioMember2021-05-012022-04-290001613103mdt:NeurosciencePortfolioMember2020-04-252021-04-300001613103mdt:DiabetesOperatingUnitMember2022-04-302023-04-280001613103mdt:DiabetesOperatingUnitMember2021-05-012022-04-290001613103mdt:DiabetesOperatingUnitMember2020-04-252021-04-300001613103country:USmdt:CardiovascularMember2022-04-302023-04-280001613103country:USmdt:CardiovascularMember2021-05-012022-04-290001613103country:USmdt:CardiovascularMember2020-04-252021-04-300001613103mdt:CardiovascularMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2022-04-302023-04-280001613103mdt:CardiovascularMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2021-05-012022-04-290001613103mdt:CardiovascularMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2020-04-252021-04-300001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:CardiovascularMember2022-04-302023-04-280001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:CardiovascularMember2021-05-012022-04-290001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:CardiovascularMember2020-04-252021-04-300001613103country:USmdt:MedicalSurgicalPortfolioMember2022-04-302023-04-280001613103country:USmdt:MedicalSurgicalPortfolioMember2021-05-012022-04-290001613103country:USmdt:MedicalSurgicalPortfolioMember2020-04-252021-04-300001613103mdt:MedicalSurgicalPortfolioMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2022-04-302023-04-280001613103mdt:MedicalSurgicalPortfolioMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2021-05-012022-04-290001613103mdt:MedicalSurgicalPortfolioMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2020-04-252021-04-300001613103mdt:MedicalSurgicalPortfolioMembermdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember2022-04-302023-04-280001613103mdt:MedicalSurgicalPortfolioMembermdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember2021-05-012022-04-290001613103mdt:MedicalSurgicalPortfolioMembermdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember2020-04-252021-04-300001613103country:USmdt:NeurosciencePortfolioMember2022-04-302023-04-280001613103country:USmdt:NeurosciencePortfolioMember2021-05-012022-04-290001613103country:USmdt:NeurosciencePortfolioMember2020-04-252021-04-300001613103mdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMembermdt:NeurosciencePortfolioMember2022-04-302023-04-280001613103mdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMembermdt:NeurosciencePortfolioMember2021-05-012022-04-290001613103mdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMembermdt:NeurosciencePortfolioMember2020-04-252021-04-300001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:NeurosciencePortfolioMember2022-04-302023-04-280001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:NeurosciencePortfolioMember2021-05-012022-04-290001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:NeurosciencePortfolioMember2020-04-252021-04-300001613103country:USmdt:DiabetesOperatingUnitMember2022-04-302023-04-280001613103country:USmdt:DiabetesOperatingUnitMember2021-05-012022-04-290001613103country:USmdt:DiabetesOperatingUnitMember2020-04-252021-04-300001613103mdt:DiabetesOperatingUnitMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2022-04-302023-04-280001613103mdt:DiabetesOperatingUnitMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2021-05-012022-04-290001613103mdt:DiabetesOperatingUnitMembermdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2020-04-252021-04-300001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:DiabetesOperatingUnitMember2022-04-302023-04-280001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:DiabetesOperatingUnitMember2021-05-012022-04-290001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMembermdt:DiabetesOperatingUnitMember2020-04-252021-04-300001613103country:US2022-04-302023-04-280001613103country:US2021-05-012022-04-290001613103country:US2020-04-252021-04-300001613103mdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2022-04-302023-04-280001613103mdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2021-05-012022-04-290001613103mdt:JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember2020-04-252021-04-300001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember2022-04-302023-04-280001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember2021-05-012022-04-290001613103mdt:MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember2020-04-252021-04-300001613103mdt:OtherAccruedExpensesMember2023-04-280001613103us-gaap:AccountsReceivableMember2023-04-280001613103mdt:OtherAccruedExpensesMember2022-04-290001613103us-gaap:AccountsReceivableMember2022-04-290001613103us-gaap:OtherLiabilitiesMember2023-04-280001613103us-gaap:OtherLiabilitiesMember2022-04-290001613103mdt:IntersectENTMember2022-05-130001613103mdt:IntersectENTMember2022-05-132022-05-130001613103us-gaap:TechnologyBasedIntangibleAssetsMembermdt:IntersectENTMember2022-05-130001613103mdt:IntersectENTMemberus-gaap:CustomerRelatedIntangibleAssetsMember2022-05-130001613103mdt:IntersectENTMemberus-gaap:TradeNamesMember2022-05-130001613103mdt:AfferaIncMember2022-08-302022-08-300001613103mdt:AfferaIncMember2022-08-3000016131032022-08-300001613103mdt:OtherAcquisitionsMember2023-04-280001613103us-gaap:TechnologyBasedIntangibleAssetsMembermdt:OtherAcquisitionsMember2023-04-280001613103us-gaap:TechnologyBasedIntangibleAssetsMembermdt:OtherAcquisitionsMember2022-04-302023-04-280001613103mdt:OtherAcquisitionsMember2022-04-302023-04-280001613103mdt:AllBusinessAcquisitionsMember2022-04-290001613103us-gaap:TechnologyBasedIntangibleAssetsMembermdt:AllBusinessAcquisitionsMember2022-04-290001613103us-gaap:TechnologyBasedIntangibleAssetsMembersrt:MinimumMembermdt:AllBusinessAcquisitionsMember2021-05-012022-04-290001613103us-gaap:TechnologyBasedIntangibleAssetsMembermdt:AllBusinessAcquisitionsMembersrt:MaximumMember2021-05-012022-04-290001613103mdt:AllBusinessAcquisitionsMember2022-04-290001613103mdt:InProcessResearchDevelopmentMember2021-05-012022-04-290001613103us-gaap:FairValueInputsLevel3Membermdt:RevenueAndOtherPerformanceBasedPaymentsMemberus-gaap:FairValueMeasurementsRecurringMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MeasurementInputDiscountRateMembersrt:MinimumMembermdt:RevenueAndOtherPerformanceBasedPaymentsMemberus-gaap:FairValueMeasurementsRecurringMember2023-04-28xbrli:pure0001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MeasurementInputDiscountRateMembermdt:RevenueAndOtherPerformanceBasedPaymentsMemberus-gaap:FairValueMeasurementsRecurringMembersrt:MaximumMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MeasurementInputDiscountRateMembermdt:RevenueAndOtherPerformanceBasedPaymentsMemberus-gaap:FairValueMeasurementsRecurringMembersrt:WeightedAverageMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMembermdt:ProductDevelopmentAndOtherMilestoneBasedPaymentsMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MeasurementInputDiscountRateMembersrt:MinimumMemberus-gaap:FairValueMeasurementsRecurringMembermdt:ProductDevelopmentAndOtherMilestoneBasedPaymentsMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MeasurementInputDiscountRateMemberus-gaap:FairValueMeasurementsRecurringMembermdt:ProductDevelopmentAndOtherMilestoneBasedPaymentsMembersrt:MaximumMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MeasurementInputDiscountRateMemberus-gaap:FairValueMeasurementsRecurringMembermdt:ProductDevelopmentAndOtherMilestoneBasedPaymentsMembersrt:WeightedAverageMember2023-04-280001613103mdt:RenalCareSolutionsMembermdt:MozarcMemberus-gaap:DisposalGroupNotDiscontinuedOperationsMember2022-05-252022-05-250001613103mdt:RenalCareSolutionsMembermdt:MozarcMemberus-gaap:DisposalGroupNotDiscontinuedOperationsMember2022-05-250001613103mdt:RenalCareSolutionsMembermdt:MozarcMembermdt:MozarcMember2022-05-250001613103mdt:RenalCareSolutionsMemberus-gaap:OtherOperatingIncomeExpenseMember2022-04-302023-04-280001613103mdt:RenalCareSolutionsMembermdt:MozarcMember2023-04-280001613103mdt:EnterpriseExcellenceMember2023-04-280001613103mdt:SimplificationRestructuringProgramMember2023-04-280001613103mdt:PreTaxChargesMembermdt:GlobalRestructuringProgramMember2023-04-280001613103us-gaap:CostOfSalesMember2022-04-302023-04-280001613103us-gaap:CostOfSalesMember2021-05-012022-04-290001613103us-gaap:CostOfSalesMember2020-04-252021-04-300001613103us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-04-302023-04-280001613103us-gaap:SellingGeneralAndAdministrativeExpensesMember2021-05-012022-04-290001613103us-gaap:SellingGeneralAndAdministrativeExpensesMember2020-04-252021-04-300001613103us-gaap:RestructuringChargesMember2022-04-302023-04-280001613103us-gaap:RestructuringChargesMember2021-05-012022-04-290001613103us-gaap:RestructuringChargesMember2020-04-252021-04-300001613103mdt:IncrementalDefinedBenefitDefinedContributionAndPostRetirementChargesMember2022-04-302023-04-280001613103mdt:IncrementalDefinedBenefitDefinedContributionAndPostRetirementChargesMember2020-04-252021-04-300001613103us-gaap:EmployeeSeveranceMember2021-04-300001613103mdt:AssociatedCostsMember2021-04-300001613103mdt:AssetWriteDownsMember2021-04-300001613103us-gaap:OtherRestructuringMember2021-04-300001613103us-gaap:EmployeeSeveranceMember2021-05-012022-04-290001613103mdt:AssociatedCostsMember2021-05-012022-04-290001613103mdt:AssetWriteDownsMember2021-05-012022-04-290001613103us-gaap:OtherRestructuringMember2021-05-012022-04-290001613103us-gaap:EmployeeSeveranceMember2022-04-290001613103mdt:AssociatedCostsMember2022-04-290001613103mdt:AssetWriteDownsMember2022-04-290001613103us-gaap:OtherRestructuringMember2022-04-290001613103us-gaap:EmployeeSeveranceMember2022-04-302023-04-280001613103mdt:AssociatedCostsMember2022-04-302023-04-280001613103mdt:AssetWriteDownsMember2022-04-302023-04-280001613103us-gaap:OtherRestructuringMember2022-04-302023-04-280001613103us-gaap:EmployeeSeveranceMember2023-04-280001613103mdt:AssociatedCostsMember2023-04-280001613103mdt:AssetWriteDownsMember2023-04-280001613103us-gaap:OtherRestructuringMember2023-04-280001613103mdt:CardiovascularMember2021-05-012021-07-300001613103mdt:CardiovascularMemberus-gaap:CostOfSalesMember2021-05-012021-07-300001613103us-gaap:OtherOperatingIncomeExpenseMembermdt:CardiovascularMember2021-05-012021-07-300001613103mdt:CardiovascularMember2022-01-292022-04-290001613103mdt:CardiovascularMember2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:InvestmentsMemberus-gaap:FairValueInputsLevel1Member2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:OtherAssetsMemberus-gaap:FairValueInputsLevel1Member2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:CorporateDebtSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:CorporateDebtSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:CorporateDebtSecuritiesMember2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMember2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMember2023-04-280001613103us-gaap:MortgageBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-04-280001613103us-gaap:MortgageBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMember2023-04-280001613103us-gaap:MortgageBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignGovernmentDebtSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:ForeignGovernmentDebtSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:ForeignGovernmentDebtSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:CertificatesOfDepositMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:CertificatesOfDepositMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:CertificatesOfDepositMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:AssetBackedSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:AssetBackedSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:AssetBackedSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel2Member2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:AuctionRateSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:InvestmentsMemberus-gaap:AuctionRateSecuritiesMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:OtherAssetsMemberus-gaap:AuctionRateSecuritiesMember2023-04-280001613103us-gaap:InvestmentsMember2023-04-280001613103us-gaap:OtherAssetsMember2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2022-04-290001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:InvestmentsMemberus-gaap:FairValueInputsLevel1Member2022-04-290001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:OtherAssetsMemberus-gaap:FairValueInputsLevel1Member2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:CorporateDebtSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:CorporateDebtSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:CorporateDebtSecuritiesMember2022-04-290001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-04-290001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMember2022-04-290001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMember2022-04-290001613103us-gaap:MortgageBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-04-290001613103us-gaap:MortgageBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMember2022-04-290001613103us-gaap:MortgageBackedSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignGovernmentDebtSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:ForeignGovernmentDebtSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:ForeignGovernmentDebtSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:CertificatesOfDepositMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:CertificatesOfDepositMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:CertificatesOfDepositMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:AssetBackedSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMemberus-gaap:AssetBackedSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMemberus-gaap:AssetBackedSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel2Member2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:InvestmentsMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:OtherAssetsMember2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:AuctionRateSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:InvestmentsMemberus-gaap:AuctionRateSecuritiesMember2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:OtherAssetsMemberus-gaap:AuctionRateSecuritiesMember2022-04-290001613103us-gaap:InvestmentsMember2022-04-290001613103us-gaap:OtherAssetsMember2022-04-290001613103us-gaap:CorporateDebtSecuritiesMember2023-04-280001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-04-280001613103us-gaap:MortgageBackedSecuritiesMember2023-04-280001613103us-gaap:AssetBackedSecuritiesMember2023-04-280001613103us-gaap:AuctionRateSecuritiesMember2023-04-280001613103us-gaap:CorporateDebtSecuritiesMember2022-04-290001613103us-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-04-290001613103us-gaap:MortgageBackedSecuritiesMember2022-04-290001613103us-gaap:AssetBackedSecuritiesMember2022-04-290001613103us-gaap:AuctionRateSecuritiesMember2022-04-290001613103mdt:EquityInvestmentsMember2022-04-302023-04-280001613103mdt:EquityInvestmentsMember2021-05-012022-04-290001613103mdt:MozarcMember2023-04-010001613103us-gaap:NotesPayableToBanksMember2023-04-280001613103us-gaap:NotesPayableToBanksMember2022-04-290001613103mdt:SeniorNotes2019Due2021Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2021Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2021Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2021Member2022-04-290001613103mdt:SeniorNotes2019Due2021FloatingMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2021FloatingMember2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2021FloatingMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2021FloatingMember2022-04-290001613103mdt:SeniorNotes2020Due20230000PercentMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due20230000PercentMember2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due20230000PercentMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due20230000PercentMember2022-04-290001613103mdt:A2015CommercialPaperProgramMemberus-gaap:CommercialPaperMember2015-01-260001613103us-gaap:CommercialPaperMember2022-04-290001613103us-gaap:CommercialPaperMember2023-04-280001613103us-gaap:CommercialPaperMember2022-04-302023-04-280001613103us-gaap:CommercialPaperMember2021-05-012022-04-290001613103srt:MinimumMemberus-gaap:CommercialPaperMember2022-04-290001613103mdt:AmendedandRestatedRevolvingCreditAgreementMemberus-gaap:LineOfCreditMember2020-12-120001613103mdt:AmendedandRestatedRevolvingCreditAgreementMemberus-gaap:LineOfCreditMember2020-12-122020-12-120001613103mdt:AmendedandRestatedRevolvingCreditAgreementMemberus-gaap:LineOfCreditMember2022-04-290001613103mdt:AmendedandRestatedRevolvingCreditAgreementMemberus-gaap:LineOfCreditMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2025Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2025Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2025Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2026Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2026Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2026Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:A2625PercentThreeYear2022SeniorNotesMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:A2625PercentThreeYear2022SeniorNotesMember2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:A2625PercentThreeYear2022SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2026Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2026Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2026Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2027Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2027Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2027Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2017Due2027Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2017Due2027Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2017Due2027Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:A4250PercentFiveYear2023SeniorNotesMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:A4250PercentFiveYear2023SeniorNotesMember2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:A4250PercentFiveYear2023SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:A3000PercentSixYear2022SeniorNotesMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:A3000PercentSixYear2022SeniorNotesMember2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:A3000PercentSixYear2022SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2029Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2029Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2029Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2031Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2031Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2031Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2032Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2032Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2032Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:A3125PercentNineYear2022SeniorNotesMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:A3125PercentNineYear2022SeniorNotesMember2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:A3125PercentNineYear2022SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2033Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2033Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2033Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:A4500PercentTenYear2023SeniorNotesMember2023-04-280001613103us-gaap:SeniorNotesMembermdt:A4500PercentTenYear2023SeniorNotesMember2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:A4500PercentTenYear2023SeniorNotesMember2022-04-290001613103mdt:A3375PercentTwelveYear2022SeniorNotesMemberus-gaap:SeniorNotesMember2023-04-280001613103mdt:A3375PercentTwelveYear2022SeniorNotesMemberus-gaap:SeniorNotesMember2022-04-302023-04-280001613103mdt:A3375PercentTwelveYear2022SeniorNotesMemberus-gaap:SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2035Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2035Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2035Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotesCIFSA2007Due2038Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotesCIFSA2007Due2038Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotesCIFSA2007Due2038Member2022-04-290001613103mdt:SeniorNotes2019Due2039Memberus-gaap:SeniorNotesMember2023-04-280001613103mdt:SeniorNotes2019Due2039Memberus-gaap:SeniorNotesMember2022-04-302023-04-280001613103mdt:SeniorNotes2019Due2039Memberus-gaap:SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2009Due2039Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2009Due2039Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2009Due2039Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2040Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2040Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2040Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2010Due2040Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2010Due2040Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2010Due2040Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2041Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2041Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2020Due2041Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2012Due2042Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2012Due2042Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2012Due2042Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2013Due2043Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2013Due2043Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2013Due2043Member2022-04-290001613103mdt:SeniorNotes2014Due2044Memberus-gaap:SeniorNotesMember2023-04-280001613103mdt:SeniorNotes2014Due2044Memberus-gaap:SeniorNotesMember2022-04-302023-04-280001613103mdt:SeniorNotes2014Due2044Memberus-gaap:SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2045Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2045Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2015Due2045Member2022-04-290001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2050Member2023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2050Member2022-04-302023-04-280001613103us-gaap:SeniorNotesMembermdt:SeniorNotes2019Due2050Member2022-04-290001613103mdt:SeniorNotes2020Due2051Memberus-gaap:SeniorNotesMember2023-04-280001613103mdt:SeniorNotes2020Due2051Memberus-gaap:SeniorNotesMember2022-04-302023-04-280001613103mdt:SeniorNotes2020Due2051Memberus-gaap:SeniorNotesMember2022-04-290001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoMembermdt:SeniorNotes2020Member2020-09-30mdt:trancheiso4217:EUR0001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoMembermdt:SeniorNotes2020Member2020-09-012020-09-300001613103us-gaap:SeniorNotesMembermdt:MedtronicIncAndCIFSASeniorNotesMember2020-10-012020-10-310001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2020-10-012020-10-310001613103us-gaap:SeniorNotesMembermdt:MedtronicIncCIFSAandMedtronicLuxcoSeniorNotesMember2020-10-012020-10-310001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2021-03-012021-03-310001613103us-gaap:SeniorNotesMembermdt:MedtronicIncCIFSAandMedtronicLuxcoSeniorNotesMember2020-04-252021-04-300001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoMembermdt:MedtronicLuxcoSeniorNotesMember2022-09-300001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2022-09-300001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2022-09-012022-09-300001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2022-12-012022-12-310001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2023-03-310001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2023-03-012023-03-310001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoMembermdt:MedtronicLuxcoSeniorNotesMember2023-03-310001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2023-03-312023-03-310001613103mdt:MedtronicLuxcoMemberus-gaap:MediumTermNotesMember2022-05-02iso4217:JPY0001613103mdt:MedtronicLuxcoMemberus-gaap:MediumTermNotesMember2022-05-022022-05-020001613103mdt:MedtronicLuxcoMembermdt:TokyoInterBankOfferedRateTIBORMemberus-gaap:MediumTermNotesMember2022-05-022022-05-020001613103mdt:MedtronicLuxcoMemberus-gaap:MediumTermNotesMember2022-05-310001613103mdt:MedtronicLuxcoMemberus-gaap:MediumTermNotesMember2022-06-300001613103us-gaap:SeniorNotesMembermdt:MedtronicIncSeniorNotesMember2022-05-022022-05-020001613103us-gaap:SeniorNotesMembermdt:MedtronicLuxcoSeniorNotesMember2022-05-022022-05-020001613103us-gaap:SeniorNotesMembermdt:MedtronicIncCIFSAandMedtronicLuxcoSeniorNotesMember2022-07-292022-07-290001613103us-gaap:ForeignExchangeContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-302023-04-280001613103us-gaap:ForeignExchangeContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:ForeignExchangeContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:NetInvestmentHedgingMemberus-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:NetInvestmentHedgingMemberus-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:NetInvestmentHedgingMembermdt:ForeignCurrencyDenominatedDebtMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:NetInvestmentHedgingMembermdt:ForeignCurrencyDenominatedDebtMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2023-04-280001613103us-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2022-04-290001613103srt:EuropeMemberus-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:NetInvestmentHedgingMemberus-gaap:ForeignExchangeContractMembercountry:JPus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:OtherOperatingIncomeExpenseMemberus-gaap:ForeignExchangeContractMember2022-04-302023-04-280001613103us-gaap:OtherOperatingIncomeExpenseMemberus-gaap:ForeignExchangeContractMember2021-05-012022-04-290001613103us-gaap:OtherOperatingIncomeExpenseMemberus-gaap:ForeignExchangeContractMember2020-04-252021-04-300001613103us-gaap:ForeignExchangeContractMemberus-gaap:CostOfSalesMember2022-04-302023-04-280001613103us-gaap:ForeignExchangeContractMemberus-gaap:CostOfSalesMember2021-05-012022-04-290001613103us-gaap:ForeignExchangeContractMemberus-gaap:CostOfSalesMember2020-04-252021-04-300001613103us-gaap:ForeignExchangeContractMembercurrency:EUR2022-04-302023-04-280001613103us-gaap:ForeignExchangeContractMembercurrency:EUR2021-05-012022-04-290001613103us-gaap:ForeignExchangeContractMembercurrency:EUR2020-04-252021-04-300001613103us-gaap:ForeignExchangeContractMember2022-04-302023-04-280001613103us-gaap:ForeignExchangeContractMember2021-05-012022-04-290001613103us-gaap:ForeignExchangeContractMember2020-04-252021-04-300001613103us-gaap:TotalReturnSwapMemberus-gaap:OtherOperatingIncomeExpenseMember2022-04-302023-04-280001613103us-gaap:TotalReturnSwapMemberus-gaap:OtherOperatingIncomeExpenseMember2021-05-012022-04-290001613103us-gaap:TotalReturnSwapMemberus-gaap:OtherOperatingIncomeExpenseMember2020-04-252021-04-300001613103us-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:ForeignExchangeContractMembermdt:OtherAccruedExpensesMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:ForeignExchangeContractMembermdt:OtherAccruedExpensesMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:OtherAssetsMemberus-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:OtherAssetsMemberus-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:ForeignExchangeContractMemberus-gaap:OtherLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:ForeignExchangeContractMemberus-gaap:OtherLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:DesignatedAsHedgingInstrumentMember2023-04-280001613103us-gaap:DesignatedAsHedgingInstrumentMember2022-04-290001613103us-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2023-04-280001613103us-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2022-04-290001613103us-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMembermdt:OtherAccruedExpensesMember2023-04-280001613103us-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMembermdt:OtherAccruedExpensesMember2022-04-290001613103us-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:TotalReturnSwapMemberus-gaap:NondesignatedMember2023-04-280001613103us-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:TotalReturnSwapMemberus-gaap:NondesignatedMember2022-04-290001613103us-gaap:TotalReturnSwapMemberus-gaap:NondesignatedMembermdt:OtherAccruedExpensesMember2023-04-280001613103us-gaap:TotalReturnSwapMemberus-gaap:NondesignatedMembermdt:OtherAccruedExpensesMember2022-04-290001613103us-gaap:NondesignatedMember2023-04-280001613103us-gaap:NondesignatedMember2022-04-290001613103us-gaap:FairValueInputsLevel1Member2023-04-280001613103us-gaap:FairValueInputsLevel1Member2022-04-290001613103us-gaap:ForeignExchangeContractMember2023-04-280001613103us-gaap:ForeignExchangeContractMember2022-04-290001613103us-gaap:TotalReturnSwapMember2022-04-290001613103mdt:CardiovascularMember2021-04-300001613103mdt:MedicalSurgicalPortfolioMember2021-04-300001613103mdt:NeurosciencePortfolioMember2021-04-300001613103mdt:DiabetesOperatingUnitMember2021-04-300001613103mdt:CardiovascularMember2022-04-290001613103mdt:MedicalSurgicalPortfolioMember2022-04-290001613103mdt:NeurosciencePortfolioMember2022-04-290001613103mdt:DiabetesOperatingUnitMember2022-04-290001613103mdt:MedicalSurgicalPortfolioMember2023-04-280001613103mdt:NeurosciencePortfolioMember2023-04-280001613103mdt:DiabetesOperatingUnitMember2023-04-280001613103mdt:RenalCareBusinessRCSMembermdt:MedicalSurgicalMember2022-07-290001613103mdt:RenalCareBusinessRCSMembermdt:MedicalSurgicalMember2022-04-302023-04-280001613103us-gaap:CustomerRelatedIntangibleAssetsMember2023-04-280001613103us-gaap:CustomerRelatedIntangibleAssetsMember2022-04-290001613103mdt:PurchasedTechnologyAndPatentsMember2023-04-280001613103mdt:PurchasedTechnologyAndPatentsMember2022-04-290001613103us-gaap:TrademarksAndTradeNamesMember2023-04-280001613103us-gaap:TrademarksAndTradeNamesMember2022-04-290001613103us-gaap:OtherIntangibleAssetsMember2023-04-280001613103us-gaap:OtherIntangibleAssetsMember2022-04-290001613103us-gaap:InProcessResearchAndDevelopmentMember2023-04-280001613103us-gaap:InProcessResearchAndDevelopmentMember2022-04-290001613103us-gaap:EquipmentMember2023-04-280001613103us-gaap:EquipmentMember2022-04-290001613103srt:MinimumMemberus-gaap:EquipmentMember2022-04-302023-04-280001613103us-gaap:EquipmentMember2022-04-302023-04-280001613103srt:MaximumMemberus-gaap:EquipmentMember2022-04-302023-04-280001613103us-gaap:ComputerSoftwareIntangibleAssetMember2023-04-280001613103us-gaap:ComputerSoftwareIntangibleAssetMember2022-04-290001613103us-gaap:ComputerSoftwareIntangibleAssetMembersrt:MaximumMember2022-04-302023-04-280001613103us-gaap:LandAndLandImprovementsMember2023-04-280001613103us-gaap:LandAndLandImprovementsMember2022-04-290001613103us-gaap:LandAndLandImprovementsMembersrt:MaximumMember2022-04-302023-04-280001613103mdt:BuildingsAndLeaseholdImprovementsMember2023-04-280001613103mdt:BuildingsAndLeaseholdImprovementsMember2022-04-290001613103srt:MaximumMembermdt:BuildingsAndLeaseholdImprovementsMember2022-04-302023-04-280001613103us-gaap:ConstructionInProgressMember2023-04-280001613103us-gaap:ConstructionInProgressMember2022-04-29iso4217:EURxbrli:shares0001613103us-gaap:SeriesAPreferredStockMember2023-04-2800016131032019-03-310001613103mdt:TwoThousandTwentyOneStockAwardAndIncentivePlanMember2023-04-280001613103us-gaap:EmployeeStockOptionMember2022-04-302023-04-280001613103us-gaap:EmployeeStockOptionMember2021-05-012022-04-290001613103us-gaap:EmployeeStockOptionMember2020-04-252021-04-300001613103us-gaap:RestrictedStockMember2022-04-302023-04-280001613103us-gaap:RestrictedStockMember2021-05-012022-04-290001613103us-gaap:RestrictedStockMember2020-04-252021-04-300001613103us-gaap:PerformanceSharesMember2022-04-302023-04-280001613103us-gaap:PerformanceSharesMember2021-05-012022-04-290001613103us-gaap:PerformanceSharesMember2020-04-252021-04-300001613103mdt:EmployeesStockPurchasePlanMember2022-04-302023-04-280001613103mdt:EmployeesStockPurchasePlanMember2021-05-012022-04-290001613103mdt:EmployeesStockPurchasePlanMember2020-04-252021-04-300001613103us-gaap:ResearchAndDevelopmentExpenseMember2022-04-302023-04-280001613103us-gaap:ResearchAndDevelopmentExpenseMember2021-05-012022-04-290001613103us-gaap:ResearchAndDevelopmentExpenseMember2020-04-252021-04-300001613103us-gaap:EmployeeStockOptionMember2022-04-290001613103us-gaap:EmployeeStockOptionMember2023-04-280001613103us-gaap:RestrictedStockUnitsRSUMembersrt:MaximumMember2022-04-302023-04-280001613103srt:MinimumMemberus-gaap:RestrictedStockUnitsRSUMember2022-04-302023-04-280001613103us-gaap:RestrictedStockMember2022-04-290001613103us-gaap:RestrictedStockMember2023-04-280001613103us-gaap:PerformanceSharesMembersrt:MinimumMember2022-04-302023-04-280001613103us-gaap:PerformanceSharesMembersrt:MaximumMember2022-04-302023-04-280001613103us-gaap:PerformanceSharesMember2022-04-290001613103us-gaap:PerformanceSharesMember2023-04-280001613103mdt:EmployeesStockPurchasePlanMember2023-04-280001613103us-gaap:ForeignCountryMember2023-04-280001613103us-gaap:ForeignCountryMembersrt:SubsidiariesMember2023-04-280001613103us-gaap:DomesticCountryMember2023-04-280001613103us-gaap:StateAndLocalJurisdictionMember2023-04-280001613103us-gaap:ForeignCountryMember2022-04-302023-04-280001613103us-gaap:ForeignCountryMember2021-05-012022-04-290001613103us-gaap:ForeignCountryMember2020-04-252021-04-300001613103us-gaap:RestrictedStockUnitsRSUMember2022-04-302023-04-280001613103us-gaap:RestrictedStockUnitsRSUMember2021-05-012022-04-290001613103us-gaap:RestrictedStockUnitsRSUMember2020-04-252021-04-300001613103us-gaap:EmployeeStockOptionMember2022-04-302023-04-280001613103us-gaap:EmployeeStockOptionMember2021-05-012022-04-290001613103us-gaap:EmployeeStockOptionMember2020-04-252021-04-300001613103us-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembercountry:US2021-04-300001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2021-04-300001613103us-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-302023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembercountry:US2021-05-012022-04-290001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-302023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2021-05-012022-04-290001613103us-gaap:PensionPlansDefinedBenefitMember2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMember2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembercountry:US2020-04-252021-04-300001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2020-04-252021-04-300001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMembercountry:US2023-04-280001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMembercountry:US2022-04-290001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2021-04-300001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMembercountry:US2021-04-300001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMemberus-gaap:ForeignPlanMember2023-04-280001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMemberus-gaap:ForeignPlanMember2022-04-290001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2021-04-300001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMemberus-gaap:ForeignPlanMember2021-04-300001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-302023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMembercountry:US2022-04-302023-04-280001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2021-05-012022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMembercountry:US2021-05-012022-04-290001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2020-04-252021-04-300001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMembercountry:US2020-04-252021-04-300001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-302023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMemberus-gaap:ForeignPlanMember2022-04-302023-04-280001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2021-05-012022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMemberus-gaap:ForeignPlanMember2021-05-012022-04-290001613103srt:MinimumMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2020-04-252021-04-300001613103us-gaap:PensionPlansDefinedBenefitMembersrt:MaximumMemberus-gaap:ForeignPlanMember2020-04-252021-04-300001613103us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:DefinedBenefitPlanDebtSecurityMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103mdt:OtherPlanAssetsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:DefinedBenefitPlanDebtSecurityMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:DefinedBenefitPlanDebtSecurityMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103mdt:OtherPlanAssetsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103mdt:OtherPlanAssetsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103mdt:PartnershipsMembersrt:MinimumMember2022-04-302023-04-280001613103mdt:PartnershipsMembersrt:MaximumMember2022-04-302023-04-28mdt:fund0001613103us-gaap:PrivateEquityFundsMember2023-04-280001613103us-gaap:PrivateEquityFundsMember2022-04-302023-04-280001613103srt:MinimumMembermdt:RealAssetInvestmentMember2022-04-302023-04-280001613103srt:MaximumMembermdt:RealAssetInvestmentMember2022-04-302023-04-280001613103us-gaap:DefinedBenefitPlanRealEstateMember2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ShortTermInvestmentsMembercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2023-04-280001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2023-04-280001613103us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:MutualFundMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:EquityFundsMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FixedIncomeFundsMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMemberus-gaap:FairValueInputsLevel1Membercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2023-04-280001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ShortTermInvestmentsMembercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2022-04-290001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ShortTermInvestmentsMembercountry:US2022-04-290001613103us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:MutualFundMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:EquityFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:EquityFundsMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FixedIncomeFundsMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMemberus-gaap:FairValueInputsLevel1Membercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2022-04-290001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembermdt:PartnershipUnitsMembercountry:US2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Membercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103mdt:PartnershipUnitsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2021-04-300001613103mdt:PartnershipUnitsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2021-05-012022-04-290001613103mdt:PartnershipUnitsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-290001613103mdt:PartnershipUnitsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-04-302023-04-280001613103mdt:PartnershipUnitsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2023-04-280001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2023-04-280001613103mdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103mdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:FairValueInputsLevel2Membermdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:FairValueInputsLevel3Membermdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMembermdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2023-04-280001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2022-04-290001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembermdt:RegisteredInvestmentCompanyMember2022-04-290001613103mdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103mdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:FairValueInputsLevel2Membermdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:FairValueInputsLevel3Membermdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMembermdt:InsuranceContractMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:FairValueInputsLevel3Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-04-290001613103us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-04-302023-04-280001613103us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-05-012022-04-290001613103us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2020-04-252021-04-300001613103us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-04-280001613103us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-04-290001613103country:US2005-05-01mdt:plan0001613103mdt:PersonalInvestmentAccountMember2022-04-302023-04-280001613103mdt:PersonalInvestmentAccountMember2021-05-012022-04-290001613103mdt:PersonalInvestmentAccountMember2020-04-252021-04-300001613103mdt:MedtronicCoreContributionMember2022-04-302023-04-280001613103mdt:MedtronicCoreContributionMember2021-05-012022-04-290001613103mdt:MedtronicCoreContributionMember2020-04-252021-04-300001613103us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-04-240001613103us-gaap:AccumulatedTranslationAdjustmentMember2020-04-240001613103us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2020-04-240001613103us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-04-240001613103us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2020-04-240001613103us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-04-252021-04-300001613103us-gaap:AccumulatedTranslationAdjustmentMember2020-04-252021-04-300001613103us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2020-04-252021-04-300001613103us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-04-252021-04-300001613103us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2020-04-252021-04-300001613103us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-04-300001613103us-gaap:AccumulatedTranslationAdjustmentMember2021-04-300001613103us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2021-04-300001613103us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-04-300001613103us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2021-04-300001613103us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-05-012022-04-290001613103us-gaap:AccumulatedTranslationAdjustmentMember2021-05-012022-04-290001613103us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2021-05-012022-04-290001613103us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-05-012022-04-290001613103us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2021-05-012022-04-290001613103us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-04-290001613103us-gaap:AccumulatedTranslationAdjustmentMember2022-04-290001613103us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2022-04-290001613103us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-04-290001613103us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2022-04-290001613103us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-04-302023-04-280001613103us-gaap:AccumulatedTranslationAdjustmentMember2022-04-302023-04-280001613103us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2022-04-302023-04-280001613103us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-04-302023-04-280001613103us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2022-04-302023-04-280001613103us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-04-280001613103us-gaap:AccumulatedTranslationAdjustmentMember2023-04-280001613103us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2023-04-280001613103us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-04-280001613103us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2023-04-280001613103mdt:ColibriMember2023-02-082023-02-080001613103mdt:PelvicMeshLitigationMemberus-gaap:DamagesFromProductDefectsMember2022-04-302023-04-28mdt:subsidiarymdt:manufacturer0001613103mdt:PelvicMeshLitigationMemberus-gaap:DamagesFromProductDefectsMember2015-04-252016-04-29mdt:claim0001613103mdt:PelvicMeshLitigationMemberus-gaap:DamagesFromProductDefectsMember2017-05-012017-05-310001613103mdt:PelvicMeshLitigationMemberus-gaap:DamagesFromProductDefectsMember2023-04-28mdt:claimant0001613103mdt:PelvicMeshLitigationMemberus-gaap:SubsequentEventMemberus-gaap:DamagesFromProductDefectsMember2023-06-072023-06-070001613103mdt:HerniaMeshLitigationMemberus-gaap:SubsequentEventMemberus-gaap:PendingLitigationMember2023-06-072023-06-07mdt:plantiff0001613103mdt:HerniaMeshLitigationMemberus-gaap:SubsequentEventMemberstpr:MAus-gaap:PendingLitigationMember2023-06-072023-06-070001613103stpr:MNmdt:HerniaMeshLitigationMemberus-gaap:SubsequentEventMemberus-gaap:PendingLitigationMember2023-06-072023-06-070001613103mdt:HerniaMeshLitigationMemberus-gaap:SubsequentEventMemberus-gaap:PendingLitigationMember2023-06-070001613103stpr:CAus-gaap:SubsequentEventMembermdt:DiabetesPumpRetainerRingLitigationMember2023-05-302023-05-30mdt:segment0001613103mdt:CardiovascularMemberus-gaap:OperatingSegmentsMember2022-04-302023-04-280001613103mdt:CardiovascularMemberus-gaap:OperatingSegmentsMember2021-05-012022-04-290001613103mdt:CardiovascularMemberus-gaap:OperatingSegmentsMember2020-04-252021-04-300001613103mdt:MedicalSurgicalPortfolioMemberus-gaap:OperatingSegmentsMember2022-04-302023-04-280001613103mdt:MedicalSurgicalPortfolioMemberus-gaap:OperatingSegmentsMember2021-05-012022-04-290001613103mdt:MedicalSurgicalPortfolioMemberus-gaap:OperatingSegmentsMember2020-04-252021-04-300001613103mdt:NeurosciencePortfolioMemberus-gaap:OperatingSegmentsMember2022-04-302023-04-280001613103mdt:NeurosciencePortfolioMemberus-gaap:OperatingSegmentsMember2021-05-012022-04-290001613103mdt:NeurosciencePortfolioMemberus-gaap:OperatingSegmentsMember2020-04-252021-04-300001613103mdt:DiabetesOperatingUnitMemberus-gaap:OperatingSegmentsMember2022-04-302023-04-280001613103mdt:DiabetesOperatingUnitMemberus-gaap:OperatingSegmentsMember2021-05-012022-04-290001613103mdt:DiabetesOperatingUnitMemberus-gaap:OperatingSegmentsMember2020-04-252021-04-300001613103us-gaap:OperatingSegmentsMember2022-04-302023-04-280001613103us-gaap:OperatingSegmentsMember2021-05-012022-04-290001613103us-gaap:OperatingSegmentsMember2020-04-252021-04-300001613103us-gaap:MaterialReconcilingItemsMember2022-04-302023-04-280001613103us-gaap:MaterialReconcilingItemsMember2021-05-012022-04-290001613103us-gaap:MaterialReconcilingItemsMember2020-04-252021-04-300001613103mdt:CardiovascularMemberus-gaap:OperatingSegmentsMember2023-04-280001613103mdt:CardiovascularMemberus-gaap:OperatingSegmentsMember2022-04-290001613103mdt:MedicalSurgicalPortfolioMemberus-gaap:OperatingSegmentsMember2023-04-280001613103mdt:MedicalSurgicalPortfolioMemberus-gaap:OperatingSegmentsMember2022-04-290001613103mdt:NeurosciencePortfolioMemberus-gaap:OperatingSegmentsMember2023-04-280001613103mdt:NeurosciencePortfolioMemberus-gaap:OperatingSegmentsMember2022-04-290001613103mdt:DiabetesOperatingUnitMemberus-gaap:OperatingSegmentsMember2023-04-280001613103mdt:DiabetesOperatingUnitMemberus-gaap:OperatingSegmentsMember2022-04-290001613103us-gaap:OperatingSegmentsMember2023-04-280001613103us-gaap:OperatingSegmentsMember2022-04-290001613103us-gaap:CorporateNonSegmentMember2023-04-280001613103us-gaap:CorporateNonSegmentMember2022-04-290001613103us-gaap:CorporateNonSegmentMember2022-04-302023-04-280001613103us-gaap:CorporateNonSegmentMember2021-05-012022-04-290001613103us-gaap:CorporateNonSegmentMember2020-04-252021-04-300001613103country:IE2022-04-302023-04-280001613103country:IE2021-05-012022-04-290001613103country:IE2020-04-252021-04-300001613103country:IE2023-04-280001613103country:IE2022-04-290001613103country:US2023-04-280001613103country:US2022-04-290001613103mdt:TotalOtherCountriesExcludingUnitedStatesandIrelandMember2022-04-302023-04-280001613103mdt:TotalOtherCountriesExcludingUnitedStatesandIrelandMember2021-05-012022-04-290001613103mdt:TotalOtherCountriesExcludingUnitedStatesandIrelandMember2020-04-252021-04-300001613103mdt:TotalOtherCountriesExcludingUnitedStatesandIrelandMember2023-04-280001613103mdt:TotalOtherCountriesExcludingUnitedStatesandIrelandMember2022-04-290001613103mdt:TotalOtherCountriesExcludingIrelandMember2022-04-302023-04-280001613103mdt:TotalOtherCountriesExcludingIrelandMember2021-05-012022-04-290001613103mdt:TotalOtherCountriesExcludingIrelandMember2020-04-252021-04-300001613103mdt:TotalOtherCountriesExcludingIrelandMember2023-04-280001613103mdt:TotalOtherCountriesExcludingIrelandMember2022-04-290001613103us-gaap:AllowanceForCreditLossMember2022-04-290001613103us-gaap:AllowanceForCreditLossMember2022-04-302023-04-280001613103us-gaap:AllowanceForCreditLossMember2023-04-280001613103us-gaap:AllowanceForCreditLossMember2021-04-300001613103us-gaap:AllowanceForCreditLossMember2021-05-012022-04-290001613103us-gaap:AllowanceForCreditLossMember2020-04-240001613103us-gaap:AllowanceForCreditLossMember2020-04-252021-04-300001613103us-gaap:InventoryValuationReserveMember2022-04-290001613103us-gaap:InventoryValuationReserveMember2022-04-302023-04-280001613103us-gaap:InventoryValuationReserveMember2023-04-280001613103us-gaap:InventoryValuationReserveMember2021-04-300001613103us-gaap:InventoryValuationReserveMember2021-05-012022-04-290001613103us-gaap:InventoryValuationReserveMember2020-04-240001613103us-gaap:InventoryValuationReserveMember2020-04-252021-04-300001613103us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2022-04-290001613103us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2022-04-302023-04-280001613103us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2023-04-280001613103us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2021-04-300001613103us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2021-05-012022-04-290001613103us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2020-04-240001613103us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2020-04-252021-04-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | | | | |
| ☒ | Annual report pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934. For the fiscal year ended April 28, 2023. |
| | |
| ☐ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
For the transition period from __________ to __________ |
Commission File No. 1-36820
 ®
®Medtronic plc
(Exact name of registrant as specified in its charter) | | | | | | | | |
| Ireland | | 98-1183488 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
20 On Hatch, Lower Hatch Street
Dublin 2, Ireland
(Address of principal executive offices)
+353 1 438-1700
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Ordinary shares, par value $0.0001 per share | MDT | New York Stock Exchange |
| 0.250% Senior Notes due 2025 | MDT/25 | New York Stock Exchange |
| 0.000% Senior Notes due 2025 | MDT/25A | New York Stock Exchange |
| 2.625% Senior Notes due 2025 | MDT/25B | New York Stock Exchange |
| 1.125% Senior Notes due 2027 | MDT/27 | New York Stock Exchange |
| 0.375% Senior Notes due 2028 | MDT/28 | New York Stock Exchange |
| 3.000% Senior Notes due 2028 | MDT/28A | New York Stock Exchange |
| 1.625% Senior Notes due 2031 | MDT/31 | New York Stock Exchange |
| 1.000% Senior Notes due 2031 | MDT/31A | New York Stock Exchange |
| 3.125% Senior Notes due 2031 | MDT/31B | New York Stock Exchange |
| 0.750% Senior Notes due 2032 | MDT/32 | New York Stock Exchange |
| 3.375% Senior Notes due 2034 | MDT/34 | New York Stock Exchange |
| 2.250% Senior Notes due 2039 | MDT/39A | New York Stock Exchange |
| 1.500% Senior Notes due 2039 | MDT/39B | New York Stock Exchange |
| 1.375% Senior Notes due 2040 | MDT/40A | New York Stock Exchange |
| 1.750% Senior Notes due 2049 | MDT/49 | New York Stock Exchange |
| 1.625% Senior Notes due 2050 | MDT/50 | New York Stock Exchange |
Securities registered pursuant to section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Smaller reporting company ☐ Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☒ No ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Aggregate market value of voting and non-voting common equity of Medtronic plc held by non-affiliates of the registrant as of October 28, 2022, based on the closing price of $86.82 as reported on the New York Stock Exchange: approximately $115.5 billion. Number of Ordinary Shares outstanding on June 16, 2023: 1,330,405,428
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2023 Annual General Meeting are incorporated by reference into Part III hereof.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, and other written reports of Medtronic plc, organized under the laws of Ireland (together with its consolidated subsidiaries, Medtronic, the Company, or we, us, or our), and oral statements made by or with the approval of one of the Company’s executive officers from time to time, may include “forward-looking” statements. All statements other than statements of historical fact contained in this Annual Report on Form 10-K, including statements regarding our future results of operations and financial position, business strategy and plans, objectives of management for future operations and current expectations or forecasts of future results, are forward-looking statements. These statements involve known and unknown risks, uncertainties, and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. Our forward-looking statements may include statements related to our growth and growth strategies, developments in the markets for our products, therapies and services, financial results, product development launches and effectiveness, research and development strategy, regulatory approvals, competitive strengths, the potential or anticipated direct or indirect impact of COVID-19 ("COVID-19" or the "pandemic") on our business, results of operations and/or financial condition, restructuring and cost-saving initiatives, intellectual property rights, litigation and tax matters, governmental proceedings and investigations, mergers and acquisitions, divestitures, market acceptance of our products, therapies and services, accounting estimates, financing activities, ongoing contractual obligations, working capital adequacy, value of our investments, our effective tax rate, our expected returns to shareholders, and sales efforts. In some cases, such statements may be identified by the use of terminology such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “looking ahead,” “may,” “plan,” “possible,” “potential,” “project,” “should,” “will,” and similar words or expressions. Forward-looking statements in this Annual Report include, but are not limited to, statements regarding: our ability to drive long-term shareholder value; development and future launches of products and continued or future acceptance of products, therapies and services in our segments; expected timing for completion of research studies relating to our products; market positioning and performance of our products, including stabilization of certain product markets; divestitures and the potential benefits thereof; the costs and benefits of integrating previous acquisitions; anticipated timing for United States (U.S.) Food and Drug Administration (U.S. FDA) and non-U.S. regulatory approval of new products; increased presence in new markets, including markets outside the U.S.; changes in the market and our market share; acquisitions and investment initiatives, including the timing of regulatory approvals as well as integration of acquired companies into our operations; the resolution of tax matters; the effectiveness of our development activities in reducing patient care costs and hospital stay lengths; our approach towards cost containment; our expectations regarding healthcare costs, including potential changes to reimbursement policies and pricing pressures; our expectations regarding changes to patient standards of care; our ability to identify and maintain successful business partnerships; the elimination of certain positions or costs related to restructuring initiatives; outcomes in our litigation matters and governmental proceedings and investigations; general economic conditions; the adequacy of available working capital and our working capital needs; our payment of dividends and redemption of shares; the continued strength of our balance sheet and liquidity; our accounts receivable exposure; and the potential impact of our compliance with governmental regulations and accounting guidance.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, results of operations, financial condition, and/or cash flows. These forward-looking statements speak only as of the date of this Annual Report on Form 10-K and are subject to a number of risks, uncertainties and assumptions described in the “Risk Factors” section and elsewhere in this Annual Report on Form 10-K. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on these forward-looking statements as predictions of future events. One must carefully consider forward-looking statements and understand that such forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, and involve a variety of risks and uncertainties, known and unknown, including, among others, those discussed in the sections entitled “Government Regulation” within “Item 1. Business” and “Item 1A. Risk Factors” in this Annual Report on Form 10-K, as well as those related to:
•competition in the medical device industry;
•delays in regulatory approvals;
•public health crises;
•reduction or interruption in our supply;
•failure to complete or achieve the intended benefits of acquisitions or divestitures;
•adverse regulatory action;
•laws and governmental regulations;
•litigation results;
•quality problems;
•healthcare policy changes;
•cybersecurity incidents;
•international operations, including the impact of armed conflicts;
•self-insurance;
•commercial insurance;
•changes in applicable tax rates;
•positions taken by taxing authorities;
•decreasing selling prices and pricing pressure;
•liquidity shortfalls;
•fluctuations in currency exchange rates;
•inflation; or
•disruption of our current plans and operations.
Consequently, no forward-looking statement may be guaranteed, and actual results may vary materially from those projected in the forward-looking statements. We intend to take advantage of the Safe Harbor provisions of the Private Securities Litigation Reform Act of 1995 regarding our forward-looking statements and are including this sentence for the express purpose of enabling us to use the protections of the safe harbor with respect to all forward-looking statements. While we may elect to update these forward-looking statements at some point in the future, whether as a result of any new information, future events, or otherwise, we have no current intention of doing so except to the extent required by applicable law.
PART I
Item 1. Business
Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company. Medtronic was founded in 1949 and today serves healthcare systems, physicians, clinicians, and patients in more than 150 countries worldwide. We remain committed to a mission written by our founder in 1960 that directs us “to contribute to human welfare by the application of biomedical engineering in the research, design, manufacture, and sale of products to alleviate pain, restore health, and extend life.”
Our Mission — to alleviate pain, restore health, and extend life — empowers insight-driven care and better outcomes for our world. We remain committed to being recognized as a company of dedication, honesty, integrity, and service. Building on this strong foundation, we are embracing our role as a healthcare technology leader and evolving our business strategy in four key areas:
•Leveraging our pipeline to accelerate revenue growth: The combination of our good end markets, recent product launches and robust pipeline is expected to continue accelerating our growth over both the near-and long-term. We aim to bring inventive and disruptive technology to large healthcare opportunities which enables us to better meet patient needs. Patients around the world deserve access to our life-saving products, and we are driven to use our local presence and scale to increase the adoption of our products and services in markets around the globe.
•Serving more patients by accelerating innovation driven growth and delivering shareholder value: We listen to our patients and customers to better understand the challenges they face. From the patient journey, to creating agile partnerships that produce novel solutions, to making it easier for our customers to deploy our therapies — everything we do is anchored in deep insight, and creates simpler, superior experiences.
•Creating and disrupting markets with our technology: We are confident in our ability to maximize new technology, artificial intelligence (AI), and data and analytics to tailor therapies in real-time, facilitating remote monitoring and care delivery that conveniently manages conditions, and creates new standards of care.
•Empowering our operating units to be more nimble and more competitive: Our operating model, which was effective February 2021, simplified our organization to accelerate decision making, improve commercial execution, and more effectively leverage the scale of our company.
We have four operating and reportable segments that primarily develop, manufacture, distribute, and sell device-based medical therapies and services: the Cardiovascular Portfolio, the Medical Surgical Portfolio, the Neuroscience Portfolio, and the Diabetes Operating Unit. For more information regarding our segments, please see Note 19 to the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K.
CARDIOVASCULAR PORTFOLIO
The Cardiovascular Portfolio is made up of the Cardiac Rhythm & Heart Failure, Structural Heart & Aortic, and Coronary & Peripheral Vascular divisions. The primary medical specialists who use our Cardiovascular products include electrophysiologists, implanting cardiologists, heart failure specialists, cardiovascular, cardiothoracic, and vascular surgeons, and interventional cardiologists and radiologists.
Cardiac Rhythm & Heart Failure
Our Cardiac Rhythm & Heart Failure division includes the following Operating Units: Cardiac Rhythm Management; Cardiac Ablation Solutions; and Cardiovascular Diagnostics and Services. The division develops, manufactures, and markets products for the diagnosis, treatment, and management of heart rhythm disorders and heart failure. Our products include implantable devices, leads and delivery systems, products for the treatment of atrial fibrillation (AF), products designed to reduce surgical site infections, and information systems for the management of patients with Cardiac Rhythm & Heart Failure devices. Principal products and services offered include:
•Implantable cardiac pacemakers including the Azure MRI SureScan, Adapta, Advisa MRI SureScan, and the Micra Transcatheter Pacing System. The Micra Transcatheter Pacing System, which is leadless and does not have a subcutaneous device pocket like a conventional pacemaker, includes the Micra VR and the Micra AV device families. Both of these pacemakers treat patients with atrioventricular block.
•Implantable cardioverter defibrillators (ICDs), including the Visia AF MRI SureScan, Evera MRI SureScan, Primo MRI, and the Cobalt and Crome portfolio of BlueSync-enabled ICDs, as well as defibrillator leads, including the Sprint Quattro Secure lead.
•Implantable cardiac resynchronization therapy devices (CRT-Ds and CRT-Ps) including the Claria/Amplia/Compia family of MRI Quad CRT-D SureScan systems and the Cobalt and Crome portfolio of BlueSync-enabled CRT-Ds, as well as the Percepta/Serena/Solara family of MRI Quad CRT-P SureScan systems.
•Cardiac ablation products include a full suite of electrophysiology solutions to treat patients with arrhythmias, including paroxysmal and persistent AF. The portfolio includes the Arctic Front Advanced Cardiac Cryoablation System, the DiamondTemp Ablation system, a temperature controlled, irrigated radiofrequency ablation system, Sphere 9 catheter, the first of its kind with high density mapping capabilities combined with radio frequency and pulsed field energies to deliver ablation lesions, and Affera Mapping and Navigation System with Prism-1 software aimed at integrating clinical information to improve patient outcomes.
•Insertable cardiac monitoring systems, including the Reveal LINQ and LINQ II. These devices are for patients who experience transient symptoms such as dizziness, palpitation, syncope (fainting) and chest pain, which may indicate a cardiac arrhythmia that requires long-term monitoring or ongoing management. The LINQ II device offers improved device longevity, remote programming, unmatched accuracy and a streamlined workflow with AccuRhythm AI algorithms to reduce clinic workload and data burden.
•TYRX products, including the Cardiac and Neuro Absorbable Antibacterial Envelopes, which are designed to stabilize electronic implantable devices and help prevent infection associated with implantable pacemakers and defibrillators.
•Remote monitoring services and patient-centered software to enable efficient care coordination as well as services related to hospital operational efficiency.
•Medtronic stopped the distribution and sale of the HVAD System in June 2021. We continue a support program for patients with HVAD devices, and for caregivers and healthcare professionals who participate in their care.
Structural Heart & Aortic
Our Structural Heart & Aortic division includes the following Operating Units: Structural Heart & Aortic and Cardiac Surgery. The division includes therapies to treat heart valve disorders and aortic disease. Our devices include products for the repair and replacement of heart valves, perfusion systems, positioning and stabilization systems for beating heart revascularization surgery, surgical ablation products, and comprehensive line of products and therapies to treat aortic disease, such as aneurysms, dissections, and transections. Principal products offered include:
•CoreValve family of aortic valves, including the Evolut PRO, Evolut PRO+, Evolut FX TAVR systems for transcatheter aortic valve replacement.
•Surgical valve replacement and repair products for damaged or diseased heart valves, including both tissue and mechanical valves; blood-handling products that form a circulatory support system to maintain and monitor blood circulation and coagulation status, oxygen supply, and body temperature during arrested heart surgery; and surgical ablation systems and positioning and stabilization technologies.
•Endovascular stent grafts and accessories, including the Endurant II Stent Graft System for the treatment of abdominal aortic aneurysms, the Valiant Captivia Thoracic Stent Graft System for thoracic endovascular aortic repair procedures, and the Heli-FX EndoAnchor System.
•Transcatheter Pulmonary Valves, including Harmony Transcatheter Pulmonary Valve (TPV) and Delivery Catheter System and Melody TPV/Ensemble II Delivery System.
Coronary & Peripheral Vascular
Our Coronary & Peripheral Vascular division includes the following Operating Units: Coronary & Renal Denervation and Peripheral Vascular Health. The division is comprised of a comprehensive line of products and therapies to treat coronary artery disease as well as peripheral vascular disease and venous disease. Our products include coronary stents and related delivery systems, including a broad line of balloon angioplasty catheters, guide catheters, guide wires, diagnostic catheters, and accessories, peripheral drug coated balloons, stent and angioplasty systems, carotid embolic protection systems for the treatment of vascular disease outside the heart, and products for superficial and deep venous disease. Principal products offered include:
•Percutaneous Coronary Intervention products including our Onyx Frontier and Resolute Onyx drug-eluting stents, Euphora balloons, and Launcher guide catheters.
•Percutaneous angioplasty balloons including the IN.PACT family of drug-coated balloons, vascular stents including the Abre venous stent, directional atherectomy products including the HawkOne directional atherectomy system, and other procedure support tools.
•Products to treat superficial venous diseases in the lower extremities including the ClosureFast radiofrequency ablation system and the VenaSeal Closure System.
MEDICAL SURGICAL PORTFOLIO
The Medical Surgical Portfolio includes the Surgical and Respiratory, Gastrointestinal, & Renal divisions. Products and therapies of this group are used primarily by healthcare systems, physicians' offices, ambulatory care centers, and other alternate site healthcare providers. While less frequent, some products and therapies are also used in home settings.
Surgical Innovations
Our Surgical Innovations division includes the following Operating Units: Surgical Innovations and Surgical Robotics. The division develops, manufactures, and markets advanced and general surgical products, including advanced stapling devices, vessel sealing instruments, wound closure products, electrosurgery products, AI-powered surgical video and analytics platform, and robotic-assisted surgery products, hernia mechanical devices, mesh implants, gynecology products, lung health and visualization, and therapies to treat diseases and conditions that are typically, but not exclusively, addressed by surgeons. Principal products and services offered include:
•Advanced stapling and energy products, including the Tri-Staple technology platform for endoscopic stapling, including the Endo GIA reloads and reinforced reloads with Tri-Staple Technology and the Endo GIA ultra universal stapler; the Signia Powered Stapling System; the LigaSure Exact Dissector and L-Hook Laparoscopic Sealer/Divider; and the Sonicision 7 curved jaw cordless ultrasonic dissection system.
•Electrosurgical hardware and instruments, including the Valleylab FT10 energy platform, the Valleylab LS10 generator, and the Force TriVerse electrosurgical pencils.
•Robotic and digital surgery technologies including, the Hugo robotic-assisted surgery (RAS) system designed for a broad range of soft-tissue procedures, and Touch Surgery Enterprise, the first-of-its-kind AI-powered surgical video management solution for the operating room.
•Products designed for the treatment of hernias, including the AbsorbaTack absorbable mesh fixation device for hernia repair, the Symbotex composite mesh for surgical laparoscopic and open ventral hernia repair, and ProGrip Laparoscopic Self-Fixating Mesh, a self-gripping, biocompatible solution for inguinal hernias.
•Suture and wound closure products, including the V-Loc barbed sutures, the Polysorb braided absorbable sutures, and the Monosof absorbable monofilament nylon sutures.
Respiratory, Gastrointestinal, & Renal
Our Respiratory, Gastrointestinal, & Renal division includes the following Operating Units: Respiratory Interventions; Patient Monitoring; and Gastrointestinal. The division develops, manufactures, and markets products in the emerging fields of minimally invasive gastrointestinal and hepatologic diagnostics and therapies, patient monitoring, and respiratory interventions including airway management and ventilation therapies. Effective April 1, 2023, we have contributed our Renal Care Solutions (RCS) business as part of an agreement with DaVita to form a new, independent kidney care-focused medical device company (“Mozarc Medical”). Principal products and services offered include:
•Gastrointestinal and endoscopy products, including the GI Genius intelligent endoscopy module, the PillCam capsule endoscopy systems, the Bravo calibration-free reflux testing systems, the Endoflip Impedance Planimetry System, the Emprint ablation system with Thermosphere Technology, the ManoScan Bravo system, the Barrx platform through ablation with the Barrx 360 Express catheter, the Cool-tip radiofrequency ablation system, the HET Bipolar System, the Beacon delivery system, and the Nexpowder endoscopic hemostasis system.
•Airway, ventilation, and inhalation therapies products, including the Puritan Bennett 980 and 840 ventilators, the Newport e360 and HT70 ventilators, the TaperGuard Evac tube, Shiley Endotracheal Tubes, Shiley Tracheostomy Tubes, McGRATH MAC video laryngoscopes, and DAR Filters.
•Products focused on patient monitoring, including Nellcor pulse oximetry monitors and sensors, Microstream capnography monitors, Bispectral Index (BIS) brain monitoring technology, INVOS cerebral/somatic oximetry systems, Vital Sync remote monitoring, WarmTouch convective warming, and the RespArray patient monitor.
NEUROSCIENCE PORTFOLIO
The Neuroscience Portfolio is made up of the Cranial & Spinal Technologies, Specialty Therapies, and Neuromodulation divisions. The primary medical specialists who use the products of this group include spinal surgeons, neurosurgeons, neurologists, pain management specialists, anesthesiologists, orthopedic surgeons, urologists, urogynecologists, interventional radiologists, and ear, nose, and throat specialists.
Cranial & Spinal Technologies
Our Cranial & Spinal Technologies division and Operating Unit develops, manufactures, and markets an integrated portfolio of devices and therapies for surgical technologies designed to improve the precision and workflow of neuro procedures, and a comprehensive line of medical devices and implants used in the treatment of the spine and musculoskeletal system. The division also provides biologic solutions for the orthopedic markets and offers unique and highly differentiated imaging, navigation, power instruments, and robotic guidance systems used in spine and cranial procedures. Principal products and services offered include:
•Neurosurgery products, including platform technologies, implant therapies, and advanced energy products through the Aible spine technology ecosystem. This includes our StealthStation S8 Navigation System, Stealth Autoguide cranial robotic guidance platform, O-arm Imaging System, Mazor X robotic guidance systems used in robot-assisted spine procedures, UNiD Adaptive Spine Intelligence AI-driven technology, and our Midas Rex surgical drills, including our MR8 high-speed drill system.
•Products to treat a variety of conditions affecting the spine, including degenerative disc disease, spinal deformity, spinal tumors, fractures of the spine, and stenosis. These products include our CATALYFT PL expandable interbody spacers, CD Horizon ModuLeX spinal system, and T2 STRATOSPHERE Expandable Corpectomy System. These products can also include titanium interbody implants and surface technologies, such as our Adaptix interbody system and incorporated Titan Interbody Fusion Device with nanoLOCK technology.
•Products that facilitate less invasive thoracolumbar surgeries, including the CD HORIZON SOLERA VOYAGER Percutaneous Fixation System and various retractor systems to access the spine through smaller incisions.
•Products to treat conditions in the cervical region of the spine, including the ZEVO Anterior Cervical Plate System, the INFINITY OCT System, and PRESTIGE LP Cervical Artificial Discs.
•Biologic solutions products, including our INFUSE Bone Graft (InductOs in the European Union (E.U.)), which contains a recombinant human bone morphogenetic protein-2, rhBMP-2, for certain spinal, trauma, and oral maxillofacial applications.
•Demineralized bone matrix products, including MAGNIFUSE, GRAFTON/GRAFTON PLUS, and the MASTERGRAFT family of synthetic bone graft products – Matrix, Putty, Strip, and Granules.
Specialty Therapies
Our Specialty Therapies division includes the following Operating Units: Neurovascular; Ear, Nose, and Throat (ENT); and Pelvic Health. The division develops, manufactures, and markets products and therapies to treat patients afflicted with acute ischemic and hemorrhagic stroke, diseases of ENT, and patients suffering from overactive bladder, (non-obstructive) urinary retention, and chronic fecal incontinence. Principal products and services offered include:
•Neurovascular products to treat diseases of the vasculature in and around the brain. This includes coils, neurovascular stent retrievers, and flow diversion products, as well as access and delivery products to support procedures. Products also include the Pipeline Flex Embolization Device with Shield Technology, endovascular treatments for large or giant wide-necked brain aneurysms, the portfolio of Solitaire revascularization devices for treatment of acute ischemic stroke, the Riptide Aspiration
System, the Onyx Liquid Embolic System, and a portfolio of associated access catheters including our React aspiration catheters also for the treatment of acute ischemic stroke.
•ENT products, including the Straightshot M5 Microdebrider Handpiece, the Integrated Power Console (IPC) system, NIM Vital Nerve Monitoring Systems, Propel and Sinuva Sinus Implants from the acquisition of Intersect ENT, StealthStation ENT and StealthStation FlexENT Navigation Systems, as well as products for hearing restoration.
•Pelvic health products, including our InterStim X and InterStim II recharge-free neurostimulators, InterStim Micro rechargeable neurostimulators, and SureScan MRI leads. Our NURO System delivers Percutaneous Tibial Neuromodulation therapy to treat overactive bladder and associated symptoms of urinary urgency, urinary frequency, and urge incontinence.
Neuromodulation
Our Neuromodulation division and Operating Unit develops, manufactures, and markets spinal cord stimulation and brain modulation systems, implantable drug infusion systems for chronic pain, as well as interventional products. Principal products and services offered include:
•Spinal cord stimulation products, including rechargeable and recharge-free devices and a large selection of leads used to treat chronic back and/or limb pain and chronic pain resulting from diabetic peripheral neuropathy. This includes the Intellis (rechargeable) and Vanta (recharge-free) Spinal Cord Stimulation Systems, with AdaptiveStim and SureScan MRI Technology, DTM (differential target multiplexed) proprietary waveform, the Evolve workflow algorithm, and Snapshot reporting.
•Brain modulation products, including those for the treatment of the disabling symptoms of Parkinson's disease, essential tremor, refractory epilepsy, severe, treatment-resistant obsessive-compulsive disorder (approved under a Humanitarian Device Exemption (HDE) in the U.S.), and chronic, intractable primary dystonia (approved under a HDE in the U.S.). Specifically, this includes our family of Activa neurostimulators, including Activa SC (single-channel primary cell battery), Activa PC (dual channel primary cell battery), and Activa RC (dual channel rechargeable battery), as well as Percept PC neurostimulator and SenSight directional lead system with the proprietary BrainSense technology.
•Implantable drug infusion systems, including our SynchroMed II Implantable Infusion System, that deliver small quantities of drug directly into the intrathecal space surrounding the spinal cord.
•Interventional products, including the Kyphon Balloon, the Kyphon V Premium, and Kyphon Assist systems and the OsteoCool RF Tumor ablation system.
•The Accurian nerve ablation system, which conducts radio frequency ablation of nerve tissues.
DIABETES OPERATING UNIT
The Diabetes Operating Unit develops, manufactures, and markets products and services for the management of Type 1 and Type 2 diabetes. The primary medical specialists who use and/or prescribe our Diabetes products are endocrinologists and primary care physicians.
Principal products and services offered include:
•Insulin pumps and consumables, including the MiniMed 770G system and MiniMed 780G system, which are all powered by SmartGuard technology. The MiniMed 770G and 780G system provides smartphone and Bluetooth connectivity, continuously delivers background insulin, monitors sugar levels, and an expanded age indication to ages two and up. The MiniMed 780G further reduces patient burden by including automatic correction boluses, meal-time detection system, and an adjustable glucose target down to 100 mg/dl.
•Continuous glucose monitoring (CGM) systems and sensors, including the Guardian Connect smart CGM system, the Guardian Sensor 3, and the Guardian Sensor 4, are products worn by patients capturing glucose data to reveal patterns and potential problems, such as hyperglycemic and hypoglycemic episodes.
•The InPen smart insulin pen system combines a reusable Bluetooth-enabled insulin pen with an intuitive mobile app that helps users administer the appropriate insulin dose. The InPen application integrates with our CGM data to provide real-time CGM readings alongside insulin dose information.
HUMAN CAPITAL
Medtronic Workforce Overview
Medtronic’s employees deliver on our Mission every day. We empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. We strive to be the employer of choice for the best and brightest global talent, where employees can grow and develop fulfilling careers. We aspire to create a truly inclusive, diverse, and equitable workplace that fosters innovation and creativity, and where every employee feels a sense of belonging and well-being. Medtronic has 95,000+ full-time employees, of which forty-three percent are based in the U.S. or Puerto Rico.
Inclusion, Diversity & Equity
We believe that improving health for people from all walks of life depends on our ability to unleash the creative power of our diverse global employees. By breaking down barriers to Inclusion, Diversity and Equity (ID&E), we open doors for everyone, driving progress and prosperity around the world. We integrate ID&E principles throughout our Company to ensure every operating unit, team, and leader recognizes and celebrates the value of diverse experiences and backgrounds. As of the end of fiscal year 2023, 40 percent of our U.S. workforce is ethnically diverse; women comprise 51 percent of our global workforce; 43 percent of our manager and above employees are women; and 28 percent of our U.S. managers are ethnically diverse. Additionally, Medtronic employee resource groups (ERGs) are employee-led affinity groups that provide career development and networking opportunities for members and strengthen ties between employees of many different backgrounds, cultures, and interests. In fiscal year 2023, there were 13 ERGs and Diversity Networks across 300+ Network and ERG chapters in 70 countries with more than 35,000 members.
Pay Equity
In our most recent reported period available, in the United States, we have achieved 100% pay equity for gender for the third consecutive year and 100% pay equity for ethnically diverse employees. Globally we have achieved 99% pay equity for gender. We are actively working to close any remaining pay gaps by continuing to expand the annual pay equity analyses for each country we operate in.
Workforce Compensation
Our compensation framework is designed to celebrate the value and contributions of our employees. We are committed to transparent communications on compensation. Our competitive approach to compensation reflects industry benchmarks and local market standards. Our programs include annual and long-term equity-based incentives that provide the means to share in the Company’s success, based on business and individual performance. To attract the best leaders, we offer competitive benefits and cash and equity incentives. We reward high-performing employees with an ownership stake in the Company through restricted stock, and all employees have the opportunity to purchase stock at a significant discount.
Learning & Development
The skills and dedication of our employees drive our business performance. Our comprehensive professional development programs empower our people to build rewarding careers and help us attract world-class talent from global and diverse populations. Our suite of professional development programs ensures that our employees, regardless of level, location, language or learning preferences, have access to opportunities to develop and grow. Our investment in employee development has contributed to more than 32 percent of our open roles being filled with internal employees.
We have shifted away from degree requirements to focus on skills-based certification for certain roles within Medtronic. Additionally, as members of the Multiple Pathways Initiative, we have used a skills-based approach to offering opportunities to expanded pools of external talent that have previously been held back due to lack of access to undergraduate education. Internally, employees can now participate through MAPS (Medtronic Advancement Pathways and Skill-building) in undergraduate courses from top-tier universities to enhance or obtain new skills, at no cost to the employee. Our change in approach has opened up opportunities for employees who have been otherwise restricted from career advancement due to degree requirements.
Employee Engagement and Culture
Through our Organizational Health Survey, we gain valuable insight into the Medtronic employee experience and identify where we can improve in key priority areas: 1) Employee Engagement, 2) Inclusion, 3) Innovation, 4) Ethics and 5) quality culture as part of our commitment to Put Patients First in our everyday decisions and actions. In our most recent survey ending in the fourth quarter of fiscal year 2023, more than 82 percent of our employees responded. Medtronic carefully reviews and implements actions based on employee feedback in order to partner and create an inclusive, innovative and supportive environment.
To enable our transformation to be the global healthcare technology leader, we introduced a reinvigorated and revived culture. The Medtronic Mindset builds on our core values of integrity, quality, inclusion, and collaboration. It urges us to act boldly, compete to win, move with speed and decisiveness, foster belonging, and deliver results… the right way. Our renewed culture helps us meet the needs of our patients and customers, and ensures our Mission endures for many years to come.
Health & Safety
As a large, global employer, it is our responsibility to maintain a safe workplace and support the well-being of our employees.
Medtronic has a comprehensive approach to providing robust support for our employees and their families in natural disasters, public health crises, civil unrest and war, bereavement, and other challenging events. Along with other programs, the Medtronic Employee Assistance Program and the Medtronic Employee Emergency Assistance Fund have historically supported employees and their families when faced with difficult times by providing a variety of services such as mental health, safety, and financial resources and support at no cost. These programs have proven invaluable in navigating our employees through unique challenges, including in fiscal year 2023. The Medtronic Employee Emergency Assistance Fund is supported by donations from employees and the Medtronic Foundation, and over the last five years has provided over $6 million in grants to employees experiencing unexpected events creating a financial hardship.
For more information on Human Capital Management at Medtronic, please refer to our 2022 Integrated Performance Report(1) as well as Medtronic’s 2022 Global Inclusion, Diversity and Equity Report(1) available on our company website.
CORPORATE SUSTAINABILITY GOALS
We see possibilities to further increase our positive impact in the world. We have identified three focus areas for our environmental, social, and governance (ESG) efforts to drive measurable impact on issues including: protecting our planet, accelerating access to healthcare technology, and advancing ID&E. In fiscal year 2022, we set new performance targets across the following areas: Patient Safety & Product Quality; Inclusion, Diversity & Equity; Climate Stewardship; Product Stewardship; and Access & Innovation. More information about our ESG focus areas, including progress we have made to date toward achieving them, is included in our Integrated Performance Report.(1)
(1)The contents of our Integrated Performance Report and our Global Inclusion, Diversity, and Equity Report are referenced for general information only and are not incorporated by reference in the Form 10-K.
OTHER FACTORS IMPACTING OUR OPERATIONS
Public Health Crises
The global COVID-19 pandemic, together with the preventative and precautionary measures taken by businesses, communities, and governments, have impacted, and may continue to impact significant aspects of our Company and business, including future procedural volumes, supply constraints, healthcare staffing, and resulting impacts on demand for our products and therapies. If there are significant outbreaks of other contagious diseases or other global public health crises, we may face similar impacts. See “Item 1A. Risk Factors” in this Annual Report on Form 10-K.
Research and Development
The markets in which we participate are subject to rapid technological advances and innovations. Constant improvement of existing products and introduction of new products is necessary to maintain market leadership. Our research and development (R&D) efforts are directed toward maintaining or achieving technological leadership in the markets we serve to help ensure that patients using our devices and therapies receive the most advanced and effective treatment possible. We remain committed to developing technological enhancements and new indications for existing products, and less invasive and new technologies for new and emerging markets to address unmet patient needs. That commitment leads to our initiation and participation in hundreds of clinical trials each fiscal year as the demand for clinical and economic evidence remains high. Furthermore, our development activities are intended to help reduce patient care costs and the length of hospital stays in the future. We have not engaged in significant customer or government-sponsored research.
Our R&D activities include improving existing products and therapies, expanding their indications and applications for use, developing new therapies and procedures, and entering into arrangements with third parties to fund the development of certain technologies. We continue to focus on optimizing innovation, improving our R&D productivity, driving growth in emerging markets, generating clinical evidence, and assessing our R&D programs based on their ability to address unmet clinical needs, produce better patient outcomes, and create new standards of care.
Intellectual Property and Litigation
We rely on a combination of patents, trademarks, tradenames, copyrights, trade secrets, and agreements (non-disclosure and non-competition agreements) to protect our business and proprietary technology. In addition, we have entered into exclusive and non-exclusive licenses relating to a wide array of third-party technologies. In the aggregate, these intellectual property assets and licenses are of material
importance to our business; however, we believe that no single intellectual property asset or license is material in relation to our business as a whole.
We operate in an industry characterized by extensive patent litigation. Patent litigation may result in significant damage awards and injunctions that could prevent the manufacture and sale of affected products or result in significant royalty payments in order to continue selling the products. At any given time, we are generally involved as both a plaintiff and a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time.
Sales and Distribution
We sell our medical devices and therapies through a combination of direct sales representatives and independent distributors globally. Additionally, a portion of the Company's revenue is generated from consignment inventory maintained at hospitals. Our medical supply products are used primarily in hospitals, surgical centers, and alternate care facilities, such as home care and long-term care facilities, and are marketed to materials managers, group purchasing organizations (GPOs) and integrated delivery networks (IDNs). We often negotiate with GPOs and IDNs, which enter into supply contracts for the benefit of their member facilities. Our four largest markets are the U.S., Western Europe, China, and Japan. Emerging markets are an area of increasing focus and opportunity, as we believe they remain under-penetrated.
Our marketing and sales strategy is focused on rapid, cost-effective delivery of high-quality products to a diverse group of customers worldwide. To achieve this objective, our marketing and sales teams are organized around physician specialties. This focus enables us to develop highly knowledgeable and dedicated sales representatives who are able to foster strong relationships with physicians and other customers and enhance our ability to cross-sell complementary products.
We are not dependent on any single customer for more than 10 percent of our total net sales.
Competition, Industry, and Cost Containment
We compete in both the therapeutic and diagnostic medical markets in more than 150 countries throughout the world. These markets are characterized by rapid change resulting from technological advances, innovations and scientific discoveries. Our product lines face a mix of competitors ranging from large manufacturers with multiple business lines to small manufacturers offering a limited selection of products. In addition, we face competition from providers of other medical therapies, such as pharmaceutical companies.
Major shifts in industry market share have occurred in connection with product corrective actions, physician advisories, safety alerts, results of clinical trials to support superiority claims, and publications about our products, reflecting the importance of product quality, product efficacy and quality systems in the medical device industry. In the current environment of managed care, economically motivated customers, consolidation among healthcare providers, increased competition, declining reimbursement rates, and national and provincial tender pricing, competitively priced product offerings are essential to our business. In order to continue to compete effectively, we must continue to create or acquire advanced technology, incorporate this technology into proprietary products, obtain regulatory approvals in a timely manner, maintain high-quality manufacturing processes, and successfully market these products.
Government and private sector initiatives to limit the growth of healthcare costs, including price regulation, competitive pricing, bidding and tender mechanics, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements, are continuing in many countries where we do business, including the U.S. These initiatives put increased emphasis on the delivery of more cost-effective medical devices and therapies. Government programs, including Medicare and Medicaid, private healthcare insurance and managed-care plans have attempted to control costs by limiting the amount of reimbursement they will pay for particular procedures or treatments, tying reimbursement to outcomes, shifting to population health management, and other mechanisms. Hospitals, which purchase our technology, are also seeking to reduce costs through a variety of mechanisms, including, for example, centralized purchasing, and in some cases, limiting the number of vendors that may participate in the purchasing program. Hospitals are also aligning interests with physicians through employment and other arrangements, such as gainsharing, where a hospital agrees with physicians to share any realized cost savings resulting from changes in practice patterns such as device standardization. This has created an increased level of price sensitivity among customers for our products.
Production and Availability of Raw Materials
We manufacture products at manufacturing facilities located in various countries throughout the world. We purchase many of the components and raw materials used in manufacturing our products from numerous suppliers in various countries. Certain components and raw materials are available only from a sole supplier. We work closely with our suppliers to help ensure continuity of supply while maintaining high quality and reliability. Generally, we have been able to obtain adequate supplies of such raw materials and components. However, due to the U.S. FDA’s manufacturing requirements, we may not be able to quickly establish additional or replacement sources for certain components or materials if we experience a sudden or unexpected reduction or interruption in supply and are unable to develop alternative sources.
For additional information related to our manufacturing facilities refer to “Item 2. Properties” in this Annual Report on Form 10-K.
Government Regulation
Our operations and products are subject to extensive regulation by numerous government agencies, including the U.S. FDA, European regulatory authorities such as the Medicines and Healthcare products Regulatory Agency in the United Kingdom, the Health Products Regulatory Authority in the Republic of Ireland and the Federal Institute for Drugs and Medical Devices in Germany, the China National Medical Product Administration (NMPA), and other government agencies inside and outside the U.S. To varying degrees, each of these agencies requires us to comply with laws and regulations governing the development, testing, manufacturing, labeling, marketing, distribution and post-marketing surveillance of our products. Our business is also affected by patient and data privacy laws and government payer cost containment initiatives, as well as environmental health and safety laws and regulations.
Product Approval and Monitoring
Many countries where we sell products are subjected to approval and other regulatory requirements regarding performance, safety, and quality of our products. Authorization to commercially distribute a new medical device in the U.S. is generally obtained in one of two primary ways. The first, known as pre-market notification or the 510(k) process, requires us to demonstrate that our medical device is substantially equivalent to a legally marketed medical device. The second, more rigorous process, known as pre-market approval, requires us to independently demonstrate that a medical device is safe and effective for its intended use. This process is generally much more time-consuming and expensive than the 510(k) process.
In the E.U., conformity with the marketing authorization requirements is represented by the CE Mark. To obtain a CE Mark, defined products must meet minimum standards of performance, safety, and quality (i.e., the essential requirements), and then, according to their classification, comply with one or more of a selection of conformity assessment routes. The competent authorities of the E.U. countries separately regulate the clinical research for medical devices and the market surveillance of products once they are placed on the market. The Medical Device Regulation was published by the E.U. in 2017, and it imposes significant additional pre-market and post-market requirements (EU MDR). The regulation provided an implementation period and became effective on May 26, 2021. The European Commission recently extended the implementation period to the end of 2027 for high-risk devices and to the end of 2028 for medium and low risk devices.
The global regulatory environment is increasingly stringent and unpredictable. While harmonization of global regulations has been pursued, requirements continue to differ among countries. We expect this global regulatory environment will continue to evolve, which could impact the cost, the time needed to approve, and ultimately, our ability to maintain existing approvals or obtain future approvals for our products. Regulations of the U.S. FDA and other regulatory agencies in and outside the U.S. impose extensive compliance and monitoring obligations on our business. These agencies review our design and manufacturing processes, labeling, record keeping, and manufacturers’ required reports of adverse experiences and other information to identify potential problems with marketed products. We are also subject to periodic inspections for compliance with applicable quality system regulations, which govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, and servicing of finished medical devices intended for human use. In addition, the U.S. FDA and other regulatory bodies, both in and outside the U.S. (including the Federal Trade Commission, the Office of the Inspector General of the Department of Health and Human Services, the U.S. Department of Justice, and various state Attorneys General), monitor the promotion and advertising of our products. Any adverse regulatory action, depending on its magnitude, may limit our ability to effectively market and sell our products, limit our ability to obtain future pre-market approvals or result in a substantial modification to our business practices and operations. For additional information, see "Item 1A. Risk Factors" We are subject to extensive and complex laws and governmental regulations and any adverse regulatory action may materially adversely affect our financial condition and business operations.
Trade Regulations
The movement of products, services, and investment across borders subjects us to extensive trade regulations. A variety of laws and regulations in the countries in which we transact business apply to the sale, shipment and provision of goods, services and technology across borders. These laws and regulations govern, among other things, our import, export and other business activities. We are also subject to the risk that these laws and regulations could change in a way that would expose us to additional costs, penalties or liabilities. Some governments also impose economic sanctions against certain countries, persons or entities. In addition to our need to comply with such regulations in connection with our direct activities, we also sell and provide goods, technology and services to agents, representatives and distributors who may export such items to customers and end-users. If we, or the third parties through which we do business, are not in compliance with applicable import, export control or economic sanctions laws and regulations, we may be subject to civil or criminal enforcement action, and varying degrees of liability. Such actions may disrupt or delay sales of our products or services or result in restrictions on our distribution and sales of products or services that may materially impact our business.
Anti-Boycott Laws
Under U.S. laws and regulations, U.S. companies and their subsidiaries and affiliates outside the U.S. are prohibited from participating or agreeing to participate in unsanctioned foreign boycotts in connection with certain business activities, including the sale, purchase, transfer, shipping or financing of goods or services within the U.S. or between the U.S. and countries outside of the U.S. If we, or certain third
parties through which we sell or provide goods or services, violate anti-boycott laws and regulations, we may be subject to civil or criminal enforcement action and varying degrees of liability.
Data Privacy and Security Laws and Regulations
As a business with a significant global footprint, compliance with evolving regulations and standards in data privacy and cybersecurity has resulted, and may continue to result, in increased costs, new compliance challenges, and the threat of increased regulatory enforcement activity. Our business relies on the secure electronic transmission, storage and hosting of sensitive information, including personal information, protected health information, financial information, intellectual property and other sensitive information related to our customers and workforce.
Our global operational footprint comes with the obligation for compliance and adherence to individual data security, confidentiality and breach notification laws at the State Level, Federal Level, and International Level. Examples of those laws include, in the U.S., the Health Insurance Portability and Accountability Act of 1996 (HIPAA), as amended, the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH), and various State privacy laws that have become effective recently. We are also subject to various other country-specific requirements around the world, such as the General Data Protection Regulation (GDPR) in the European Economic Area, the United Kingdom’s version of the same, and China's Personal information Protection Law (PIPL).
Because the laws and regulations continue to expand, differ from jurisdiction to jurisdiction, and are subject to evolving (and at times inconsistent) governmental interpretation, compliance with these laws and regulations may require significant additional cost expenditures or changes in products or business that increase competition or reduce revenue. Noncompliance could result in the imposition of fines, penalties, or orders to stop noncompliant activities, or withdrawal of noncompliant products from a market.
Regulations Governing Reimbursement
The delivery of our devices is subject to regulation by the U.S. Department of Health and Human Services (HHS) and comparable state and non-U.S. agencies responsible for reimbursement and regulation of healthcare items and services. U.S. laws and regulations are imposed primarily in connection with federally funded healthcare programs, such as the Medicare and Medicaid programs, as well as the government’s interest in regulating the quality and cost of healthcare. Other governments also impose regulations in connection with their healthcare reimbursement programs and the delivery of healthcare items and services.
U.S. federal healthcare laws apply when we or customers submit claims for items or services that are reimbursed under federally-funded healthcare programs, including laws related to kickbacks, false claims, self-referrals or other healthcare fraud. There are often similar state false claims, anti-kickback, and anti-self-referral and insurance laws that apply to state Medicaid and other healthcare programs and private third-party payers. In addition, as a manufacturer of U.S. FDA-approved devices reimbursable by federal healthcare programs, we are subject to the Physician Payments Sunshine Act, which requires us to annually report certain payments and other transfers of value we make to U.S.-licensed physicians or U.S. teaching hospitals. Any failure to comply with these laws and regulations could subject us or our officers and employees to criminal and civil financial penalties.
Implementation of legislative or regulatory reforms to reimbursement systems, or adverse decisions relating to our products by administrators of these systems in coverage or reimbursement, could significantly reduce reimbursement or result in the denial of coverage, which could have an impact on the acceptance of and demand for our products and the prices that our customers are willing to pay for them.
Environmental Health and Safety Laws
We are also subject to various environmental health and safety laws and regulations both within and outside the U.S. Like other companies in our industry, our manufacturing and other operations involve the use and transportation of substances regulated under environmental health and safety laws including those related to the transportation of hazardous materials.
Available Information
We maintain a website at www.medtronic.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (Exchange Act) are made available under the “Our Company – Investors” caption and “Financials – SEC Filings” subcaption of our website as soon as reasonably practicable after we electronically file them with, or furnish them to, the Securities and Exchange Commission (SEC).
Information relating to our corporate governance, including our Principles of Corporate Governance, Code of Conduct (including our Code of Ethics for Senior Financial Officers and any related amendments or waivers), Code of Business Conduct and Ethics for Members of the Board of Directors, and information concerning our executive officers, directors and Board committees (including committee charters) is available through our website at www.medtronic.com under the “Our Company – Governance” caption. Information relating to transactions in Medtronic securities by directors and officers is available through our website at www.medtronic.com under the “Our Company – Investors” caption and the “Financials – SEC Filings” subcaption.
Our website and the information contained on or connected to our website are not incorporated by reference into this Form 10-K.
The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers, including the Company, that file electronically with the SEC. The public may obtain any documents that we file with the SEC at http://www.sec.gov. We file annual reports, quarterly reports, proxy statements, and other documents with the SEC under the Exchange Act.
Item 1A. Risk Factors
Investing in our securities involves a variety of risks and uncertainties, known and unknown, including, among others, those discussed below. Each of the following risks should be carefully considered, together with all the other information included in this Annual Report on Form 10-K, including our consolidated financial statements and the related notes and in our other filings with the SEC. Furthermore, additional risks and uncertainty not presently known to us or that we currently believe to be immaterial may also adversely affect our business. Our business, results of operations, financial condition, and cash flow and prospects could be materially and adversely affected by any of these risks or uncertainties.
Business and Operational Risks
We operate in a highly competitive industry and we may be unable to compete effectively.
We compete in both the therapeutic and diagnostic medical markets in more than 150 countries throughout the world. These markets are characterized by rapid change resulting from technological advances, innovations and scientific discoveries. In the product lines in which we compete, we face a range of competitors from large companies with multiple business lines to small, specialized manufacturers that offer a limited selection of niche products. Development by other companies of new or improved products, processes, technologies, or the introduction of reprocessed products or generic versions when our proprietary products lose their patent protection may make our existing or planned products less competitive. In addition, we face competition from providers of alternative medical therapies, such as pharmaceutical companies.
We believe our ability to compete depends upon many factors both within and beyond our control, including:
•product performance and reliability,
•product technology and innovation,
•product quality and safety,
•breadth of product lines,
•product support services,
•customer support,
•cost-effectiveness and price,
•reimbursement approval from healthcare insurance providers, and
•changes to the regulatory environment.
Competition may increase as additional companies enter our markets or modify their existing products to compete directly with ours. In addition, academic institutions, governmental agencies and other public and private research organizations also may conduct research, seek patent protection and establish collaborative arrangements for discovery, research, clinical development and marketing of products similar to ours. These companies and institutions compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring necessary product technologies. From time to time we have lost, and may in the future lose, market share in connection with product problems, physician advisories, safety alerts and publications about our products, which highlights the importance of product quality, product efficacy and quality systems to our business. In the current environment of managed care, consolidation among healthcare providers, increased competition, declining reimbursement rates, and national and provincial tender pricing, as recently experienced in China, competitively priced product offerings are essential to our success. Further, our continued growth and success depend on our ability to develop, acquire and market new and differentiated products, technologies and intellectual property, and as a result we also face competition for marketing, distribution, and collaborative development agreements, establishing relationships with academic and research institutions and licenses to intellectual property. In order to continue to compete effectively, we must continue to create, invest in or acquire advanced technology, incorporate this technology into our proprietary products, obtain regulatory approvals in a timely manner, and manufacture and successfully market our products. Given these factors, we cannot guarantee that we will be able to compete effectively or continue our level of success.
Public health crises have had, and may continue to have, an adverse effect on certain aspects of our business, results of operations, financial condition, and cash flows. The nature and extent of future impacts are highly uncertain and unpredictable.
Our global operations and interactions with healthcare systems, providers and patients around the world expose us to risks associated with public health crises, including epidemics and pandemics such as COVID-19. In particular, the preventative and precautionary measures that we and other businesses, communities, and governments have taken to mitigate the spread of the disease has led to restrictions on, and disruptions in, business and personal activities in certain countries and regions, including China, which comprises approximately seven percent of our total revenues. These restrictions have reduced customer demand for certain of our products. We expect medical procedure rates to continue to vary by therapy and country, and could be impacted by regional COVID-19 case volumes, healthcare system staffing shortages and supply chain issues that affect their ability to provide care, patients’ ability or willingness to schedule deferrable procedures, travel restrictions, transportation limitations, quarantine restrictions, vaccine and booster immunization rates, and new COVID-19 variants.
Together with the preventative and precautionary measures being taken, as well as the corresponding need to adapt to new and improved methods of conducting business, such as increased remote monitoring, COVID-19 has had, and may continue to have, an adverse impact on certain aspects of our Company and business, including the demand for and supply of certain of our products, operations, supply chains and distribution systems, and our ability to generate cash flow. Some of our products are more sensitive to reductions in deferrable and emergent medical procedures, and certain medical procedures have been and may continue to be suspended or postponed. It is not possible to predict the timing of deferrable medical procedures and, to the extent individuals and hospital systems de-prioritize, delay or cancel these procedures, our business, results of operations, financial condition, and cash flows could continue to be negatively affected.
Reduction or interruption in supply or other manufacturing difficulties may adversely affect our manufacturing operations and related product sales.
The manufacture of our products requires the timely delivery of a sufficient amount of quality components and materials and is highly exacting and complex, due in part to strict regulatory requirements. We manufacture the majority of our products and procure important third-party services, such as sterilization services, at numerous facilities worldwide. We purchase many of the components, raw materials and services needed to manufacture these products from numerous suppliers in various countries. We seek to maintain continuity of supply by use of multiple options for sourcing where possible. We have generally been able to obtain adequate supplies of such raw materials, components and services, although global shortages of certain components such as semiconductors and resins have recently caused, and may in the future cause, disruptions to our product manufacturing supply chain. In addition, for reasons of quality assurance, cost effectiveness, or availability, certain components, raw materials and services needed to manufacture our products are obtained from a sole supplier. Although we work closely with our suppliers to try to ensure continuity of supply while maintaining high quality and reliability, the supply of these components, raw materials and services may be interrupted or insufficient. In addition, due to the stringent regulations and requirements of regulatory agencies, including the U.S. FDA, regarding the manufacture of our products, we may not be able to quickly establish additional or replacement sources. Additionally, many regulatory agencies are imposing regulatory requirements on safe use of chemicals and their potential impact on health and the environment which also may impact supply constraints. Furthermore, the prices of commodities and other materials used in our products, which are often volatile and outside of our control, could adversely impact our supply. We use resins, other petroleum-based materials and pulp as raw materials in some of our products, and the prices of oil and gas also significantly affect our costs for freight and utilities. A reduction or interruption in supply, and an inability to develop alternative sources for such supply, could adversely affect our ability to manufacture our products in a timely or cost-effective manner and could result in lost sales.
Other disruptions in the manufacturing process or product sales and fulfillment systems for any reason, including infrastructure, information and equipment malfunction, failure to follow specific protocols and procedures, supplier or Company facility shut-downs, defective raw materials, labor shortages, natural disasters such as hurricanes, tornadoes, earthquakes, or wildfires, property damage or facility closures from riots or public protests, and other environmental factors and the impact of epidemics, pandemics, or other public health crises, and actions by businesses, communities and governments in response, could lead to launch delays, product shortages, unanticipated costs, lost revenues and damage to our reputation. For example, in the past we have experienced a global information technology systems interruption that affected our customer ordering, distribution, and manufacturing processes, and we have been adversely impacted by, and may continue to be adversely impacted by, the global COVID-19 pandemic and the responses of governments and of our partners, including suppliers, manufacturers, distributors and other businesses. Furthermore, any failure to identify and address manufacturing problems prior to the release of products to our customers could result in quality or safety issues.
In addition, many of our products require sterilization before sale and several of our key products are manufactured or sterilized at a particular facility, with limited alternate facilities. If an event occurs that results in damage to or closure of one or more of such facilities, such as the Illinois Environmental Protection Agency's decision to close a supplier's sterilization facility in February 2019, we may be unable to manufacture or sterilize the relevant products to the required quality specifications or at all. Because of the time required to approve and license a manufacturing or sterilization facility, a third-party may not be available on a timely basis to replace production capacity in the event manufacturing or sterilization capacity is lost.
Our research and development efforts rely upon investments and investment collaborations, and we cannot guarantee that any previous or future investments or investment collaborations will be successful.
Our Mission is to provide a broad range of therapies to restore patients to fuller, healthier lives, which requires a wide variety of technologies, products and capabilities. The rapid pace of technological development in the medical industry and the specialized expertise required in different areas of medicine make it difficult for one company alone to develop a broad portfolio of technological solutions. In addition to internally generated growth through our research and development efforts, historically we have relied, and expect to continue to rely, upon investments and investment collaborations to provide us access to new technologies both in areas served by our existing businesses as well as in new areas.
We expect to make future investments where we believe that we can stimulate the development or acquisition of new technologies and products to further our strategic objectives and strengthen our existing businesses. Investments and investment collaborations in and with medical technology companies are inherently risky, and we cannot guarantee that any of our previous or future investments or investment collaborations will be successful or will not materially adversely affect our business, results of operations, financial condition and cash flows.
The continuing development of many of our products depends upon us maintaining strong relationships with healthcare professionals.
If we fail to maintain our working relationships with healthcare professionals, many of our products may not be developed and marketed in line with the needs and expectations of the professionals who use and support our products, which could cause a decline in our earnings and profitability. The research, development, marketing and sales of many of our new and improved products depends on our maintaining working relationships with healthcare professionals. We rely on these professionals to provide us with considerable knowledge and experience regarding the development, marketing and sale of our products. Physicians assist us as researchers, marketing and product consultants, inventors and public speakers. In addition, as a result of the COVID-19 pandemic, our access to these professionals has been limited at times, and travel restrictions, shutdowns and similar measures have impacted our ability to maintain these relationships, thereby affecting our ability to develop, market and sell new and improved products. If we are unable to maintain strong relationships with these professionals, the development and marketing of our products could suffer, which could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
We have debt obligations that create risk.
We are required to use a portion of our operating cash flow to pay interest or principal on our outstanding indebtedness instead of for other corporate purposes, including funding future expansion of our business. We may also incur additional indebtedness in the future to supplement our existing liquidity and cash generated from operations to satisfy our needs for working capital and capital expenditures, to pursue growth initiatives, and to make returns of capital to shareholders. Over the course of the past fiscal year, interest rate increases in the U.S. and Europe, and recent disruptions in the financial services industry, caused periods of tightened credit availability and volatility in borrowing terms. At the time we may incur such additional indebtedness, or refinance or restructure existing indebtedness, we may be unable to obtain capital market financing with similar terms and currency denomination to our existing indebtedness, or at all, which could have a material adverse effect on our business and results of operations. At any time, the value of our debt outstanding will fluctuate based on several factors including foreign currency exchange rate and interest rate movements.
Failure to integrate acquired businesses into our operations successfully, or challenges related to the Company's strategic initiatives, including divestitures, as well as liabilities or claims relating to such acquired businesses or divestitures, could adversely affect our business.
As part of our strategy to develop and identify new products and technologies and optimize our portfolio of products, we have made several significant acquisitions and divestitures in recent years, and may make additional acquisitions and divestitures in the future. Our integration of the operations of acquired businesses requires significant efforts, including the coordination of information technologies, research and development, sales and marketing, operations, manufacturing, and finance. These efforts result in additional expenses and involve significant amounts of management’s time that cannot then be dedicated to other projects. Our failure to manage and coordinate the growth of acquired companies successfully could also have an adverse impact on our business. Further, acquired businesses may have liabilities, or be subject to claims, litigation or investigations that we did not anticipate or which exceed our estimates at the time of the acquisition. In addition, we cannot be certain that the businesses we acquire will become profitable or remain so. Factors that will affect the success of our acquisitions include:
•the presence or absence of adequate internal controls and/or significant fraud in the financial systems of acquired companies,
•our ability or inability to integrate information technology systems of acquired companies in a secure and reliable manner,
•liabilities, claims, litigation, investigations, or other adverse developments relating to acquired businesses or the business practices of acquired companies, including investigations by governmental entities, potential FCPA or product liability claims or other unanticipated liabilities,
•any decrease in customer loyalty and product orders caused by dissatisfaction with the combined companies’ product lines and sales and marketing practices, including price increases,
•our ability to retain key employees, and
•the ability to achieve synergies among acquired companies, such as increasing sales of the integrated company’s products, achieving cost savings, and effectively combining technologies to develop new products.
We also could experience negative effects on our business, results of operations, financial condition, and cash flows from acquisition-related charges, amortization of intangible assets and asset impairment charges.
In addition, the potential exists that expected strategic benefits from any planned or completed divestiture by the Company may not be realized or may take longer to realize than expected, including but not limited to:
•The Company’s ability to consummate the planned separation of the combined Patient Monitoring and Respiratory Interventions businesses from the Medical Surgical Portfolio,
•The Company’s ability to realize the anticipated benefits from the recent contribution of half of the Company’s RCS business to Mozarc Medical,
•The Company’s performance under various transaction service agreements that have or may be executed as part of a divestiture.
Legal and Regulatory Risks
We are subject to extensive and complex laws and governmental regulations and any adverse regulatory action may materially adversely affect our financial condition and business operations.
Our medical devices and technologies, as well as our business activities, are subject to a complex set of regulations and rigorous enforcement, including by the U.S. FDA, U.S. Department of Justice, Health and Human Services Office of the Inspector General, and numerous other federal, state, and non-U.S. governmental authorities. To varying degrees, each of these agencies requires us to comply with laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of our products. As a part of the regulatory process of obtaining marketing clearance for new products and new indications for existing products, we conduct and participate in numerous clinical trials with a variety of study designs, patient populations, and trial endpoints. Unfavorable clinical data from existing or future clinical trials may adversely impact our ability to obtain product approvals, our position in, and share of, the markets in which we participate, and our business, results of operations, financial condition, and cash flows. We cannot guarantee that we will be able to obtain or maintain marketing clearance for our new products or enhancements or modifications to existing products, and the failure to maintain approvals or obtain approval or clearance could have a material adverse effect on our business, results of operations, financial condition and cash flows. Even if we are able to obtain approval or clearance, it may:
•take a significant amount of time,
•require the expenditure of substantial resources,
•involve stringent clinical and pre-clinical testing, as well as increased post-market surveillance,
•involve modifications, repairs or replacements of our products, and
•limit the proposed uses of our products.
Both before and after a product is commercially released, we have ongoing responsibilities under the U.S. FDA and other applicable non-U.S. government agency regulations. For instance, many of our facilities and procedures and those of our suppliers are also subject to periodic inspections by the U.S. FDA to assess compliance with applicable regulations. The results of these inspections can include inspectional observations on the U.S. FDA’s Form 483, warning letters, or other forms of enforcement. If the U.S. FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical products are ineffective or pose an unreasonable health risk, the U.S. FDA could detain or seize adulterated or misbranded medical products, order a recall, repair, replacement, or refund of such products, refuse to grant pending pre-market approval applications or require certificates of non-U.S. governments for exports, and/or require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health, and in certain rare circumstances, ban medical devices. The U.S. FDA and other non-U.S. government agencies may also assess civil or criminal penalties against us, our officers or employees and impose operating restrictions on a company-wide basis. The U.S. FDA may also recommend prosecution to the U.S. Department of Justice. Any adverse regulatory action, depending on its magnitude, may restrict us from effectively marketing and selling our products and limit our ability to obtain future pre-market clearances or approvals, and could result in a substantial modification to our business practices and operations. Furthermore, we occasionally receive subpoenas or other requests for information from various governmental agencies around the world, and while these investigations typically relate primarily to financial arrangements with healthcare providers, regulatory compliance and product promotional practices, we cannot predict the timing,
outcome or impact of any such investigations. Any adverse outcome in one or more of these investigations could include the commencement of civil and/or criminal proceedings, substantial fines, penalties, and/or administrative remedies, including exclusion from government reimbursement programs and/or entry into Corporate Integrity Agreements (CIAs) with governmental agencies. In addition, resolution of any of these matters could involve the imposition of additional, costly compliance obligations. These potential consequences, as well as any adverse outcome from government investigations, could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
In addition, the U.S. FDA has taken the position that device manufacturers are prohibited from promoting their products other than for the uses and indications set forth in the approved product labeling, and any failure to comply could subject us to significant civil or criminal exposure, administrative obligations and costs, and/or other potential penalties from, and/or agreements with, the federal government.
Governmental regulations in the U.S. and outside the U.S. are constantly changing and may become increasingly stringent. In the E.U, for example, the Medical Device Regulation which became effective in May 2021 includes significant additional pre-market and post-market requirements. Penalties for regulatory non-compliance could be severe, including fines and revocation or suspension of a company’s business license, mandatory price reductions and criminal sanctions. The development and implementation of future laws and regulations may have a material adverse effect on us.
Our failure to comply with laws and regulations relating to reimbursement of healthcare goods and services may subject us to penalties and adversely impact our reputation, business, results of operations, financial condition and cash flows.
Our devices, products and therapies are purchased principally by hospitals or physicians that typically bill various third-party payers, such as governmental healthcare programs (e.g., Medicare, Medicaid and comparable non-U.S. programs), private insurance plans and managed care plans, for the healthcare services provided to their patients. The ability of our customers to obtain appropriate reimbursement for products and services from third-party payers is critical because it affects which products customers purchase and the prices they are willing to pay. As a result, our devices, products and therapies are subject to regulation regarding quality and cost by HHS, including the Centers for Medicare & Medicaid Services (CMS), as well as comparable state and non-U.S. agencies responsible for reimbursement and regulation of health are goods and services, including laws and regulations related to fair competition, kickbacks, false claims, self-referrals and healthcare fraud. Many states have similar laws that apply to reimbursement by state Medicaid and other funded programs as well as in some cases to all payers. In certain circumstances, insurance companies attempt to bring a private cause of action against a manufacturer for causing false claims. In addition, as a manufacturer of U.S. FDA-approved devices reimbursable by federal healthcare programs, we are subject to the Physician Payments Sunshine Act, which requires us to annually report certain payments and other transfers of value we make to U.S.-licensed physicians or U.S. teaching hospitals. Any failure to comply with these laws and regulations could subject us or our officers and employees to criminal and civil financial penalties.
We are also subject to risks relating to changes in government and private medical reimbursement programs and policies, and changes in legal regulatory requirements in the U.S. and around the world. Implementation of further legislative or administrative reforms to these reimbursement systems, or adverse decisions relating to coverage of or reimbursement for our products by administrators of these systems, could have an impact on the acceptance of and demand for our products and the prices that our customers are willing to pay for them.
We are substantially dependent on patent and other proprietary rights and failing to protect such rights or to be successful in litigation related to our rights or the rights of others may result in our payment of significant monetary damages and/or royalty payments, negatively impacting our ability to sell current or future products.
We are substantially dependent on patent and other proprietary rights and rely on a combination of patents, trademarks, tradenames, copyrights, trade secrets, and agreements (such as employee, non-disclosure and non-competition agreements) to protect our business and proprietary intellectual property. We also operate in an industry characterized by extensive patent litigation. Patent litigation can result in significant damage awards and injunctions that could prevent our manufacture and sale of affected products or require us to pay significant royalties in order to continue to manufacture or sell affected products. At any given time, we are generally involved as both a plaintiff and a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time. While it is not possible to predict the outcome of patent litigation, it is possible that the results of such litigation could require us to pay significant monetary damages and/or royalty payments, negatively impact our ability to sell current or future products, or that enforcement actions to protect our patent and proprietary rights against others could be unsuccessful, any of which could have a material adverse impact on our business, results of operations, financial condition, and cash flows. In addition, any public announcements related to litigation or administrative proceedings initiated or threatened against us could cause our stock price to decline.
While we intend to defend against any threats to our intellectual property, our patents, trademarks, tradenames, copyrights, trade secrets or agreements (such as employee, non-disclosure and non-competition agreements) may not adequately protect our intellectual property. Further, pending patent applications may not result in patents being issued to us, patents issued to or licensed by us may be challenged or circumvented by competitors and such patents may be found invalid, unenforceable or too limited in scope to protect our technology or provide us with any competitive advantage. In addition, our patents will expire over time, our ability to protect novel business models is uncertain, and infringement may go undetected. Third parties could obtain patents that may require us to negotiate licenses to conduct our
business, and such licenses may not be available on reasonable terms or at all. In addition, license agreements could be terminated. We also rely on non-disclosure and non-competition agreements with certain employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. We cannot be certain that these agreements will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information, or that third parties will not otherwise gain access to our trade secrets or proprietary knowledge.
In addition, the laws of certain countries in which we market or manufacture some of our products do not protect our intellectual property rights to the same extent as the laws of the U.S., which could make it easier for competitors to capture market position. For example, business in China comprises approximately seven percent of our total revenues. This may increase our vulnerability to our technology being reverse engineered or our trade secrets being compromised. If we are unable to protect our intellectual property in China or other countries, it could have a material adverse effect on our business, results of operations, financial condition, and cash flows. Competitors also may harm our sales by designing products that substantially mirror the capabilities of our products or technology without infringing our intellectual property rights.
Quality problems could lead to recalls or safety alerts, product liability claims, reputational harm, adverse verdicts or costly settlements, and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Quality is extremely important to us and our customers due to the impact on patients, and the serious and potentially costly consequences of adverse product performance. Our business exposes us to potential product liability risks that are inherent in the design, manufacture, and marketing of medical devices. In addition, many of our products are often used in intensive care settings with seriously ill patients and some of the medical devices we manufacture and sell are designed to be implanted in the human body for long periods of time or indefinitely. Component failures, manufacturing nonconformances, design issues, off-label use, or inadequate disclosure of product-related risks or product-related information with respect to our products, if they were to occur, could result in an unsafe condition or injury to, or death of, a patient. These problems could lead to recall of, or issuance of a safety alert relating to, our products, and could result in product liability claims and lawsuits, including class actions, which could ultimately result, in certain cases, in the removal from the body of such products and claims regarding costs associated therewith. Due to the strong name recognition of the Medtronic brand, a material adverse event involving one of our products could result in diminished market acceptance and demand for all products within that brand, and could harm our reputation and ability to market products in the future. Further, we may be exposed to additional potential product liability risks related to products designed, manufactured and/or marketed in response to the COVID-19 pandemic, and unpredictable or accelerated changes in demand for certain of our products in connection with COVID-19 and its related impacts could increase the risk of regulatory enforcement actions, product defects or related claims, as well as adversely impact our customer relationships and reputation.
Strong product quality is critical to the success of our goods and services. If we fall short of these standards and our products are the subject of recalls or safety alerts, our reputation could be damaged, we could lose customers and our revenue and results of operations could decline. Our success also can depend on our ability to manufacture to exact specification precision-engineered components, subassemblies and finished devices from multiple materials. If our components fail to meet these standards or fail to adapt to evolving standards, our reputation, competitive advantage and market share could be harmed. In certain situations, we may undertake a voluntary recall of products or temporarily shut down production lines based on performance relative to our own internal safety and quality monitoring and testing data.
Any of the foregoing problems, including future product liability claims or recalls, regardless of their ultimate outcome, could harm our reputation and have a material adverse effect on our business, results of operations, financial condition and cash flows.
Healthcare policy changes may have a material adverse effect on us.
There have been and continue to be actions and proposals by several governments, regulators and third-party payers globally, including the U.S. federal and state governments, to control healthcare costs and, more generally, to reform healthcare systems. Certain of these actions and proposals, among other things, limit the prices we are able to charge for our products or the amounts of reimbursement available for our products, increase the importance of our ability to compete on cost, and could limit the acceptance and availability of our products. These actions and proposals could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We rely on the proper function, security and availability of our information technology systems and data, as well as those of third parties throughout our global supply chain, to operate our business, and a breach, cyber-attack or other disruption to these systems or data could materially and adversely affect our business, results of operations, financial condition, cash flows, reputation or competitive position.
We are increasingly dependent on sophisticated information technology systems to operate our business. That technology includes systems that could be used to process, transmit and store sensitive data. Additionally, many of our products and services include integrated software and information technology that collects data regarding patients or connects to other internal systems. One of the most prevalent attacks on large organizations has been ransomware which can have a devastating impact on an organization’s operations. Our ransomware readiness program has required and will continue to require investment and will not guarantee that we will be immune from an incident or be able to respond rapidly enough to prevent a negative impact on our business. Like all organizations, we routinely experience attempted interference with the integrity of, and interruptions in, our technology systems via events such as cyber-attacks, malicious intrusions, or other
breakdowns. The consequences could mean data breaches, interference with the integrity of our products and data, compromise of intellectual property or other proprietary information, or other significant disruptions. Furthermore, we rely on third-party vendors to supply and/or support certain aspects of our information technology systems and resulting products. These third-party systems could also become vulnerable to cyber-attack, malicious intrusions, breakdowns, interference, or other significant disruptions, and may contain defects in design or manufacture or other problems that could result in system disruption or compromise the information security of our own systems. Medtronic is constantly monitoring geopolitical events or issues (i.e., U.S.-China tensions) which may increase cybersecurity risks on a global basis, and we take appropriate measures to counter any threats. Lastly, we continue to grow in part through new business acquisitions and, as a result, may face risks associated with defects and vulnerabilities in acquired businesses’ systems, or difficulties or other breakdowns or disruptions in connection with the integration of the acquisitions into our information technology systems.
Our worldwide operations mean that we are subject to laws and regulations, including data protection and cybersecurity laws and regulations, in many jurisdictions. The variety of U.S. and international privacy and cybersecurity laws and regulations impacting our operations are described in “Item 1. Business" – Other Factors Impacting Our Operations – Data Privacy and Security Laws and Regulations. Any data security breaches, cyber-attacks, malicious intrusions or significant disruptions could result in actions by regulatory bodies and/or civil litigation, any of which could materially and adversely affect our business, results of operations, financial condition, cash flows, reputation, or competitive position.
In addition, our information technology systems require an ongoing commitment of significant resources to maintain, protect, and enhance existing systems and develop new systems. This enables us to keep pace with continuing changes in information processing technology, evolving legal and regulatory standards, the increasing need to protect patient and customer information, changes in the techniques used to obtain unauthorized access to data and information systems, and the information technology needs associated with our changing products and services. There can be no assurance that our extensive efforts (including, but not limited to, consolidating, protecting, upgrading, and expanding our systems and capabilities, continuing to build security into the design of our products, and developing new systems to keep pace with continuing changes in information processing technology, including, but not limited to, generative artificial intelligence platforms) will be successful or that additional systems issues will not arise in the future.
If our information technology systems, products or services or sensitive data are compromised, there are many consequences that could result. Consequences include, but are not limited, to patients or employees being exposed to financial or medical identity theft or suffering a loss of product functionality, losing existing customers or have difficulty attracting new customers, experiencing difficulty preventing, detecting, and controlling fraud, being exposed to the loss or misuse of confidential information, having disputes with customers, physicians, and other healthcare professionals, suffering regulatory sanctions or penalties under federal laws, state laws, or the laws of other jurisdictions, experiencing increases in operating expenses or an impairment in our ability to conduct our operations, incurring expenses or losing revenues as a result of a data privacy breach, product failure, information technology outages or disruptions, or suffering other adverse consequences including lawsuits or other legal action and damage to our reputation.
The failure to comply with anti-corruption laws could materially adversely affect our business and result in civil and/or criminal sanctions.
The U.S. Foreign Corrupt Practices Act (FCPA), the Irish Criminal Justice (Corruption Offences) Act 2018, and similar anti-corruption laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business and to ensure adequate internal controls, books, and records. Because of the predominance of government-administered healthcare systems in many jurisdictions around the world, many of our customer relationships outside of the U.S. are with governmental entities and are therefore potentially subject to such laws. We also participate in public-private partnerships and other commercial and policy arrangements with governments around the globe.
Global enforcement of anti-corruption laws has increased in recent years, including investigations and enforcement proceedings leading to assessment of significant fines and penalties against companies and individuals. Our international operations create a risk of unauthorized payments or offers of payments by one of our employees, consultants, sales agents, or distributors. We maintain various controls aligned with legal requirements to prevent and prohibit improper practices, including policies, programs, and training for our employees and third party intermediaries acting on our behalf. However, existing safeguards and any future improvements may not always be effective, and our employees, consultants, sales agents or distributors may engage in conduct for which we could be held responsible. In addition, regulators could seek to hold us liable for conduct committed by companies in which we invest or that we acquire. Any alleged or actual violations of these regulations may subject us to government scrutiny, criminal or civil sanctions and other liabilities, including exclusion from government contracting, and could disrupt our business, adversely affect our reputation and result in a material adverse effect on our business, results of operations, financial condition and cash flows.
Laws and regulations governing international business operations could adversely impact our business.
The U.S. Department of the Treasury’s Office of Foreign Assets Control (OFAC) and the U.S. Commerce Department’s Bureau of Industry and Security (BIS) administer certain laws and regulations that restrict U.S. persons and, in some instances, non-U.S. persons, in conducting activities, transacting business with, or making investments in, certain countries, governments, entities and individuals subject to U.S.
economic sanctions or export restrictions. Our international operations subject us to these laws and regulations, which are complex, restrict our business dealings with certain countries, governments, entities, and individuals, and are constantly changing. Further restrictions may be enacted, amended, enforced or interpreted in a manner that materially impacts our operations.
From time to time, certain of our subsidiaries have limited business dealings in countries subject to comprehensive sanctions, including Iran, Syria, Cuba, and the region of Crimea, as well as Russia and Belarus. Certain of our subsidiaries sell medical devices, and may provide related services, to distributors and other purchasing bodies in such countries/region. These business dealings represent an insignificant amount of our consolidated revenues and income, but expose us to a heightened risk of violating applicable sanctions regulations. Violations of these regulations are punishable by civil penalties, including fines, denial of export privileges, injunctions, asset seizures, debarment from government contracts and revocations or restrictions of licenses, as well as criminal fines and imprisonment. We have established policies and procedures designed to assist with our compliance with such laws and regulations. However, there can be no assurance that our policies and procedures will prevent us from violating these regulations in every transaction in which we may engage, and such a violation could adversely affect our reputation, business, results of operations, financial condition, and cash flows.
Climate change, or legal, regulatory or market measures to address climate change may materially adversely affect our financial condition and business operations.
Climate change resulting from increased concentrations of carbon dioxide and other greenhouse gases in the atmosphere presents risks to our current and future operations from natural disasters and extreme weather conditions, such as hurricanes, tornadoes, earthquakes, wildfires or flooding. Such extreme weather conditions and other conditions caused by or related to climate change could increase our operational costs, pose physical risks to our facilities and adversely impact our supply chain, including: manufacturing and distribution networks, the availability and cost of raw materials and components, energy supply, transportation, or other inputs necessary for the operation of our business. The impacts of climate change on global water resources may result in water scarcity, which could impact our ability to access sufficient quantities of water in certain locations and result in increased costs. Concerns over climate change could have an impact on customer demand for our products and result in new legal or regulatory requirements designed to mitigate the effects of climate change on the environment. Although it is difficult to predict and adequately prepare to meet the challenges to our business posed by climate change, if new laws or regulations are more stringent than current legal or regulatory requirements, we may experience increased compliance burdens and costs to meet the regulatory obligations as well as adverse impacts on raw material sourcing, manufacturing operations and the distribution of our products.
We are subject to environmental laws and regulations and the risk of environmental liabilities, violations and litigation.
We are subject to environmental, health, and safety laws, and regulations concerning, among other things, the generation, handling, transportation, and disposal of hazardous substances or wastes, the remediation of hazardous substances or materials at various sites, and emissions or discharges into the land, air or water. We are further subject to numerous laws and regulations concerning, among other things, chemical constituents in medical products and end-of-life disposal and take-back programs for medical devices. Our operations and those of certain third-party suppliers involve the use of substances subject to these laws and regulations, primarily those used in manufacturing and sterilization processes. If we or our suppliers violate these environmental laws and regulations, facilities could be shut down and violators could be fined, or otherwise sanctioned. New laws and regulations, violations of these laws or regulations, stricter enforcement of existing requirements, or the discovery of previously unknown contamination could require us to incur costs or could become the basis for new or increased liabilities that could be material.
We are subject to risks related to our environmental, social and governance (ESG) practices and initiatives.
There is an increased focus from our stakeholders, as well as regulatory authorities in the U.S., European Union (EU) and other global jurisdictions in which we operate, on ESG practices and disclosure. If we do not succeed, or are perceived to have fallen short, in any number of ESG matters, such as environmental stewardship, inclusion, diversity and equity (ID&E) initiatives, supply chain practices, good corporate governance, workplace conduct and support for local communities, or if we do not effectively respond to new or revised legal, regulatory or reporting requirements concerning climate change or other sustainability concerns, we may be subject to regulatory fines and penalties, our reputation or the reputation of our brands may suffer, we may be unable to attract and retain top talent, and our stock price may be negatively affected. In addition, enhanced ESG laws, regulations and expectations in the jurisdictions in which we do business may increase compliance burdens and costs for third parties throughout our global supply chain, which could cause disruption in the sourcing, manufacturing and distribution of our products and adversely affect our business, financial condition or results of operations.
Further, we have made several public disclosures of objectives and targets (targets) relating to product stewardship, ID&E, patient safety and product quality, access and innovation, and climate stewardship, including our ambition to be carbon neutral in our operations by 2030 and to achieve net zero emissions by 2045. Although we intend to achieve these targets, we may be required to expend significant resources to do so, which could increase our operational costs. In addition, there can be no assurance of the extent to which any of our targets will be achieved, or that any future investments we make to achieve such targets will meet investor, legal and/or any other regulatory expectations and requirements. If we are unable to meet our targets, we may face litigation and could incur regulatory fines and penalties or adverse
publicity and reaction from investors, advocacy groups or other stakeholders that may adversely impact our business, demand for our products and services, and/or our financial condition and results of operations.
Our insurance program may not be adequate to cover future losses.
We have elected to self-insure most of our insurable risks across the Company, and we made this decision based on cost and availability factors in the insurance marketplace. We manage and maintain a portion of our self-insured program through a wholly-owned captive insurance company. We continue to maintain a directors and officers liability insurance policy with third-party insurers that provides coverage for the directors and officers of the Company. We continue to monitor the insurance marketplace to evaluate the value of obtaining insurance coverage for other categories of losses in the future. Although we believe, based on historical loss trends, that our self-insurance program accruals and our existing insurance coverage will be adequate to cover future losses, historical trends may not be indicative of future losses. The absence of third-party insurance coverage for other categories of losses increases our exposure to unanticipated claims and these losses could have a material adverse impact on our business, results of operations, financial condition and cash flows.
Changes in tax laws or exposure to additional income tax liabilities could have a material impact on our business, results of operations, financial condition and cash flows.
We are subject to income taxes, as well as non-income based taxes, in the U.S., Ireland, and various other jurisdictions in which we operate. The tax laws in the U.S., Ireland and other countries in which we and our affiliates do business could change on a prospective or retroactive basis, and any such changes could have a material impact on our business, results of operations, financial condition, and cash flows.
The Organization for Economic Cooperation and Development (OECD) secured agreement from 142 countries to push forward with proposals to fundamentally rewrite International Tax rules which will likely impact the amount of tax multinationals such as Medtronic pay in the future. Certain countries have already enacted or are in the process of enacting legislation in line with guidance provided by the OECD. Ireland is subject to EU Directives and as a consequence has committed to enact legislation by December 31st 2023. As a result the first year Medtronic is expected to be impacted by these changes is fiscal year 2025.
The aggressive nature of the timeline set by the OECD may mean that all implications for business may not have been fully worked through or fully understood before rules are finalized. We continue to monitor the implications potentially resulting from this guidance. This action together with other legislative changes in many countries on the mandatory sharing of company information (financial and operational) with taxing authorities on a local and global basis under various information sharing initiatives, could lead to disagreements between jurisdictions associated with the proper allocation of profits between such jurisdictions.
We are subject to ongoing tax audits in the various jurisdictions in which we operate. Tax authorities may disagree with certain positions we have taken and assess additional taxes. We regularly assess the likely outcomes of these audits in order to determine the appropriateness of our tax provision. However, there can be no assurance that we will accurately predict the outcomes of these audits, and the actual outcomes of these audits could have a material impact on our business, results of operations, financial condition, and cash flows.
We have recorded reserves for potential payments of tax to various tax authorities related to uncertain tax positions. However, the calculation of such tax liabilities involves the application of complex tax laws, regulations and treaties (where applicable) in many jurisdictions. Therefore, any dispute with a tax authority may result in a payment that is significantly different from current estimates. If payment of these amounts ultimately proves to be less than the recorded amounts, the reversal of the liabilities generally would result in tax benefits being recognized in the period when we determine the liabilities are no longer necessary. If our estimate of tax liabilities proves to be less than the amount for which it is ultimately liable, we would incur additional charges, and such charges could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
The Medtronic, Inc. tax court proceeding outcome could have a material adverse impact on our financial condition.
In March 2009, the IRS issued its audit report for Medtronic Inc. for fiscal years 2005 and 2006. Medtronic, Inc. reached agreements with the IRS on some, but not all matters related to these fiscal years. The remaining unresolved issue for fiscal years 2005 and 2006 relates to the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico, which is one of our key manufacturing sites. The Tax Court issued its opinion on August 18, 2022, and it remains subject to appeal by either or both parties. At this time, the Company is evaluating whether to file an appeal. An adverse outcome in this matter could materially and adversely affect our business, results of operations, financial condition, and cash flows. See Note 18 to the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K.
Future potential changes to the U.S. tax laws could result in us being treated as a U.S. corporation for U.S. federal tax purposes, and the IRS may not agree with the conclusion that we should be treated as a foreign corporation for U.S. federal income tax purposes.
Because Medtronic plc is organized under the laws of Ireland, we would generally be classified as a foreign corporation under the general rule that a corporation is considered tax resident in the jurisdiction of its organization or incorporation for U.S. federal income tax purposes. Even so, the IRS may assert that we should be treated as a U.S. corporation (and, therefore, a U.S. tax resident) for U.S. federal income tax purposes pursuant to Section 7874 of the U.S. Internal Revenue Code of 1986, as amended (the Code). In addition, a retroactive change to
U.S. tax laws in this area could change this classification. If we were to be treated as a U.S. corporation for federal tax purposes, we could be subject to substantially greater U.S. tax liability than currently contemplated as a non-U.S. corporation.
Legislative or other governmental action relating to the denial of U.S. federal or state governmental contracts to U.S. companies that redomicile abroad could adversely affect our business.
Various U.S. federal and state legislative proposals that would deny governmental contracts to U.S. companies that move their corporate location abroad may affect us. We are unable to predict the likelihood that, or final form in which, any such proposed legislation might become law, the nature of the regulations that may be promulgated under any future legislative enactments, or the effect such enactments and increased regulatory scrutiny may have on our business.
Risks Relating to Our Jurisdiction of Incorporation
We are incorporated in Ireland, and Irish law differs from the laws in effect in the U.S. and may afford less protection to holders of our securities.
Our shareholders may have more difficulty protecting their interests than would shareholders of a corporation incorporated in a jurisdiction of the United States. It may not be possible to enforce court judgments obtained in the U.S. against us in Ireland based on the civil liability provisions of the U.S. federal or state securities laws. In addition, there is some uncertainty as to whether the courts of Ireland would recognize or enforce judgments of U.S. courts obtained against us or our directors or officers based on the civil liabilities provisions of the U.S. federal or state securities laws or hear actions against us or those persons based on those laws. We have been advised that the U.S. currently does not have a treaty with Ireland providing for the reciprocal recognition and enforcement of judgments in civil and commercial matters. Therefore, a final judgment for the payment of money rendered by any U.S. federal or state court based on civil liability, whether or not based solely on U.S. federal or state securities laws, would not automatically be enforceable in Ireland.
As an Irish company, we are governed by the Irish Companies Act 2014, which differs in some material respects from laws generally applicable to U.S. corporations and shareholders, including, among others, differences relating to interested director and officer transactions and shareholder lawsuits. Likewise, the duties of directors and officers of an Irish company generally are owed to the company only. Shareholders of Irish companies generally do not have a personal right of action against directors or officers of the company and may exercise such rights of action on behalf of the company only in limited circumstances. Accordingly, holders of our securities may have more difficulty protecting their interests than would holders of securities of a corporation incorporated in the U.S.
As an Irish public limited company, certain capital structure decisions require shareholder approval, which may limit Medtronic’s flexibility to manage its capital structure.
Under Irish law, our authorized share capital can be increased by an ordinary resolution of our shareholders and the directors may issue new ordinary or preferred shares, without shareholder approval, once authorized to do so by our articles of association or by an ordinary resolution of our shareholders. Additionally, subject to specified exceptions, Irish law grants statutory preemption rights to existing shareholders where shares are being issued for cash consideration but allows shareholders to disapply such statutory preemption rights either in our articles of association or by way of special resolution. Such disapplication can either be generally applicable or be in respect of a particular allotment of shares. Accordingly, at our 2022 Annual General Meeting, our Shareholders authorized our Board of Directors to issue up to 33% of our issued ordinary shares and further authorized our Board of Directors to issue up to 10% of such shares for cash without first offering them to our existing shareholders (provided that with respect to 5% of such shares, such allotment is to be used for the purposes of a specified capital investment). Both of these authorizations will expire on June 8, 2024, unless renewed by shareholders for a further period. We anticipate seeking new authorizations at our 2023 Annual General Meeting and in subsequent years. We cannot provide any assurance that these authorizations will always be approved, which could limit our ability to issue equity and thereby adversely affect the holders of our securities.
A transfer of our shares, other than ones effected by means of the transfer of book-entry interests in the Depository Trust Company, may be subject to Irish stamp duty.
Transfers of our shares effected by means of the transfer of book entry interests in the Depository Trust Company (DTC) will not be subject to Irish stamp duty. However, if a shareholder holds our shares directly rather than beneficially through DTC, any transfer of shares could be subject to Irish stamp duty (currently at the rate of 1% of the higher of the price paid or the market value of the shares acquired). Payment of Irish stamp duty is generally a legal obligation of the transferee. The potential for stamp duty could adversely affect the price of shares.
In certain limited circumstances, dividends we pay may be subject to Irish dividend withholding tax and dividends received by Irish residents and certain other shareholders may be subject to Irish income tax.
In certain limited circumstances, dividend withholding tax (currently at a rate of 25%) may arise in respect of dividends paid on our shares. A number of exemptions from dividend withholding tax exist such that shareholders resident in the U.S. and other specified countries that have a tax treaty with Ireland may be entitled to exemptions from dividend withholding tax.
Shareholders resident in the U.S. that hold their shares through DTC will not be subject to dividend withholding tax, provided the addresses of the beneficial owners of such shares in the records of the brokers holding such shares are recorded as being in the U.S. (and such brokers have further transmitted the relevant information to a qualifying intermediary appointed by us). However, other shareholders may be subject to dividend withholding tax, which could adversely affect the price of their shares.
Shareholders entitled to an exemption from Irish dividend withholding tax on dividends received from us will not be subject to Irish income tax in respect of those dividends unless they have some connection with Ireland other than their shareholding in our Company (for example, they are resident in Ireland). Shareholders who are not resident nor ordinarily resident in Ireland, but who receive dividends subject to Irish dividend withholding tax, will generally have no further liability to Irish income tax on those dividends.
Our shares received by means of a gift or inheritance could be subject to Irish capital acquisitions tax.
Irish capital acquisitions tax (CAT) could apply to a gift or inheritance of our shares irrespective of the place of residence, ordinary residence or domicile of the parties. This is because our shares will be regarded as property situated in Ireland. The person who receives the gift or inheritance has primary liability for CAT. Gifts and inheritances passing between spouses are exempt from CAT. Children currently have a tax-free threshold of €335,000 in respect of taxable gifts or inheritances received from their parents. Irish Revenue typically updates the amount of this tax-free threshold on an annual basis.
Economic and Industry Risks
Changes in the prices of our goods and services and/or inflationary costs may have a material adverse effect on our business, results of operations, financial condition and cash flows.
We have experienced, and may continue to experience, decreasing prices for certain of our goods and services due to pricing pressure from managed care organizations and other third-party payers on our customers, increased market power of our customers as the medical device industry consolidates and increased competition among medical engineering and manufacturing services providers. We have also recently experienced, and may continue to experience, rising costs due to inflation. If the prices for our goods and services change or inflation continues to rise, we may be unable to sufficiently reduce our expenses or offset rising costs through increased prices to customers. As a result, our business, results of operations, financial condition and cash flows may be adversely affected.
We are subject to a variety of risks associated with global operations that could adversely affect our profitability and operating results.
We develop, manufacture, distribute and sell our products globally. We intend to continue to expand our operations and to pursue growth opportunities outside the U.S., especially in emerging markets. Operations in different countries including emerging markets could expose us to additional and greater risks and potential costs, including:
•fluctuations in currency exchange rates,
•healthcare reform legislation,
•the need to comply with different regulatory regimes worldwide that are subject to change and that could restrict our ability to manufacture and sell our products,
•local product preferences and product requirements,
•longer-term receivables than are typical in the U.S.,
•economic sanctions, export controls, trade protection measures, tariffs and other border taxes, and import or export licensing requirements,
•less intellectual property protection in some countries outside the U.S. than exists in the U.S.,
•different labor regulations and workforce instability,
•political and economic instability, including as a result of wars and insurrections,
•the expiration and non-renewal of foreign tax rulings and/or grants,
•potentially negative consequences from changes in or interpretations of tax laws, and
•economic instability and inflation, recession or interest rate fluctuations.
The ongoing global economic competition and trade tensions between the U.S. and China present risk to Medtronic. Although we have been able to mitigate some of the impact on Medtronic from increased duties imposed by both sides (through petitioning both governments for tariff exclusions and other mitigations), the risk remains of additional tariffs and other kinds of restrictions. Tariff exclusions awarded to Medtronic by the U.S. Government require periodic renewal, and policies for granting exclusions could shift. The U.S. and China, which
comprises approximately seven percent of our total revenues, could impose other types of restrictions such as limitations on government procurement or technology export restrictions, which could affect Medtronic’s access to the markets.
The Russia-Ukraine conflict and resulting sanctions and export restrictions are creating barriers to doing business in Russia and Belarus and adversely impacting global supply chains. While we have no manufacturing, distribution or direct material suppliers in the region, we continue to closely monitor the potential raw material/sub-tier supplier impact in both Russia and Ukraine. Materials like palladium and neon, which are both dependent on Russia supply, are part of broader semiconductor shortages in industry. Additional sanctions, export restrictions, and potential countermeasures within Russia may lead to greater uncertainty and geopolitical shifts in Asia that could cause additional adverse impacts on global supply chains and our business, results of operations, financial condition, and cash flows.
More generally, several governments including the U.S. have raised the possibility of policies to induce “re-shoring” of supply chains, less reliance on imported supplies, and greater national production. Examples include potential “Buy America” requirements in the U.S. If such steps triggered retaliation in other markets restricting access to foreign products in purchases by their government-owned healthcare systems, the result could be a significant impact on Medtronic.
Other significant changes or disruptions to international trade arrangements, such as termination or modifications of other existing trade agreements, may adversely affect our business, results of operations, financial condition and cash flows. In addition, a significant amount of our trade receivables are with national healthcare systems in many countries. Repayment of these receivables is dependent upon the political and financial stability of those countries. In light of these global economic fluctuations, we continue to monitor the creditworthiness of customers. Failure to receive payment of all or a significant portion of these receivables could adversely affect our business, results of operations, financial condition and cash flows.
The COVID-19 pandemic, and the responses of business and governments to the pandemic, have at times resulted in reduced availability of air transport, port closures, increased border controls or closures, increased transportation costs and increased security threats to our supply chain, and countries may continue to close borders, impose prolonged quarantines, and further restrict travel and other activities. Our business could be adversely impacted if we are unable to successfully manage these and other risks of global operations.
Finally, changes in currency exchange rates may impact the reported value of our revenues, expenses, and cash flows. We cannot predict changes in currency exchange rates, the impact of exchange rate changes, nor the degree to which we will be able to manage the impact of currency exchange rate changes.
Instability in the financial sector could adversely affect our revenues, results of operation, or financial condition.
Recent disruptions in the financial services industry caused periods of tightened credit availability and volatility in borrowing terms. If these conditions were to recur or worsen, we may experience reduced demand for a number of our products. In addition, we could experience loss of sales and profits due to delayed payments or insolvency of healthcare professionals, hospitals and other customers, suppliers and vendors facing liquidity issues. As a result, our business and liquidity may be adversely impacted, and we may be compelled to take additional measures to preserve our cash flow.
Consolidation in the healthcare industry could have an adverse effect on our revenues and results of operations.
Many healthcare industry companies, including healthcare systems, distributors, manufacturers, providers, and insurers, are consolidating or have formed strategic alliances. As the healthcare industry consolidates, competition to provide goods and services to industry participants will become more intense. Further, this consolidation creates larger enterprises with greater negotiating power, which they can use to negotiate price concessions. If we must reduce our prices because of industry consolidation, or if we lose customers as a result of consolidation, our business, results of operations, financial condition, and cash flows could be adversely affected.
Healthcare industry cost-containment measures could result in reduced sales of our medical devices and medical device components.
Most of our customers, and the healthcare providers to whom our customers supply medical devices, rely on third-party payers, including government programs and private health insurance plans, to reimburse some or all of the cost of the procedures in which medical devices that incorporate components we manufacture or assemble are used. The continuing efforts of governmental authorities, insurance companies and other payers of healthcare costs to contain or reduce these costs could lead to patients being unable to obtain approval for payment from these third-party payers. If third-party payer payment approval cannot be obtained by patients, sales of finished medical devices that include our components may decline significantly and our customers may reduce or eliminate purchases of our components. The cost-containment measures that healthcare providers are instituting, both in the U.S. and outside of the U.S., could harm our ability to operate profitably. For example, managed care organizations have successfully negotiated volume discounts for pharmaceuticals, and GPOs and IDNs have also concentrated purchasing decisions for some customers, which has led to downward pricing pressure for medical device companies, including us.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Medtronic's principal executive office is located in Ireland and is leased by the Company, while its main operational offices are located in the Minneapolis, Minnesota metropolitan area and are owned by the Company.
The Company's total manufacturing and research space is approximately 9.8 million square feet. Approximately 34 percent of the manufacturing or research facilities are owned by Medtronic and the remaining balance is leased. The following is a summary of the Company's largest manufacturing facilities by location:
| | | | | | | | |
| Location Country or State | | Square Feet (in thousands) |
| Connecticut | | 1,138 | |
| Puerto Rico | | 811 | |
| Mexico | | 762 | |
| China | | 708 | |
| Minnesota | | 623 | |
| Ireland | | 446 | |
| Dominican Republic | | 395 | |
| Arizona | | 294 | |
| Switzerland | | 283 | |
| California | | 260 | |
| Colorado | | 259 | |
| Florida | | 255 | |
| France | | 249 | |
| Massachusetts | | 245 | |
| Italy | | 230 | |
Medtronic also maintains sales and administrative offices in the U.S. at five locations in five states and outside the U.S. at 119 locations in 62 countries. A majority of these locations are leased. The Company is using substantially all of its currently available productive space to develop, manufacture, and market its products. The Company's facilities are well-maintained, suitable for their respective uses, and adequate for current needs.
Item 3. Legal Proceedings
In accordance with Item 103 of Regulation S-K, we have adopted a $1 million disclosure threshold for proceedings under environmental laws to which a governmental authority is a party, as we believe matters under this threshold are not material to the Company. A discussion of the Company’s legal proceedings and other loss contingencies are described in Note 18 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Medtronic’s Common Equity, Related Shareholder Matters, and Issuer Purchases of Equity Securities
The Company’s ordinary shares are listed on the New York Stock Exchange under the symbol “MDT.”
The following table provides information about the shares repurchased by the Company during the fourth quarter of fiscal year 2023: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fiscal Period | | Total Number of
Shares Purchased | | Average Price
Paid per Share | | Total Number of Shares
Purchased as a Part of
Publicly Announced
Program | | Maximum Approximate Dollar Value of Shares that may yet be Purchased Under the Program |
| 1/28/2023-2/24/2023 | | 257,425 | | | $ | 84.48 | | | 257,425 | | | $ | 2,446,440,933 | |
| 2/25/2023-3/31/2023 | | 448,355 | | | 80.19 | | | 448,355 | | | 2,410,488,558 | |
| 4/1/2023-4/28/2023 | | 389,900 | | | 83.02 | | | 389,900 | | | 2,378,119,960 | |
| Total | | 1,095,680 | | | $ | 82.20 | | | 1,095,680 | | | $ | 2,378,119,960 | |
In March 2019, the Company's Board of Directors authorized the repurchase of $6.0 billion of the Company's ordinary shares. There is no specific time-period associated with these repurchase authorizations. For additional discussion, see Note 11 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
On June 16, 2023, there were approximately 21,589 shareholders of record of the Company’s ordinary shares. Ordinary cash dividends declared and paid totaled $0.68 per share for each quarter of fiscal year 2023 and $0.63 per share for each quarter of fiscal year 2022. On May 25, 2023, the Company announced an increase in Medtronic's cash dividends for the first quarter of fiscal year 2024, raising the amount to $0.69 per share.
Stock Performance Graph
The following graph compares the cumulative total shareholder return on Medtronic’s ordinary shares with the cumulative total shareholder return on the Standard & Poor’s (S&P) 500 Index and the S&P 500 Health Care Equipment Index for the last five fiscal years. The graph assumes that $100 was invested at market close on April 27, 2018 in Medtronic’s ordinary shares, the S&P 500 Index, and the S&P 500 Health Care Equipment Index and that all dividends were reinvested.
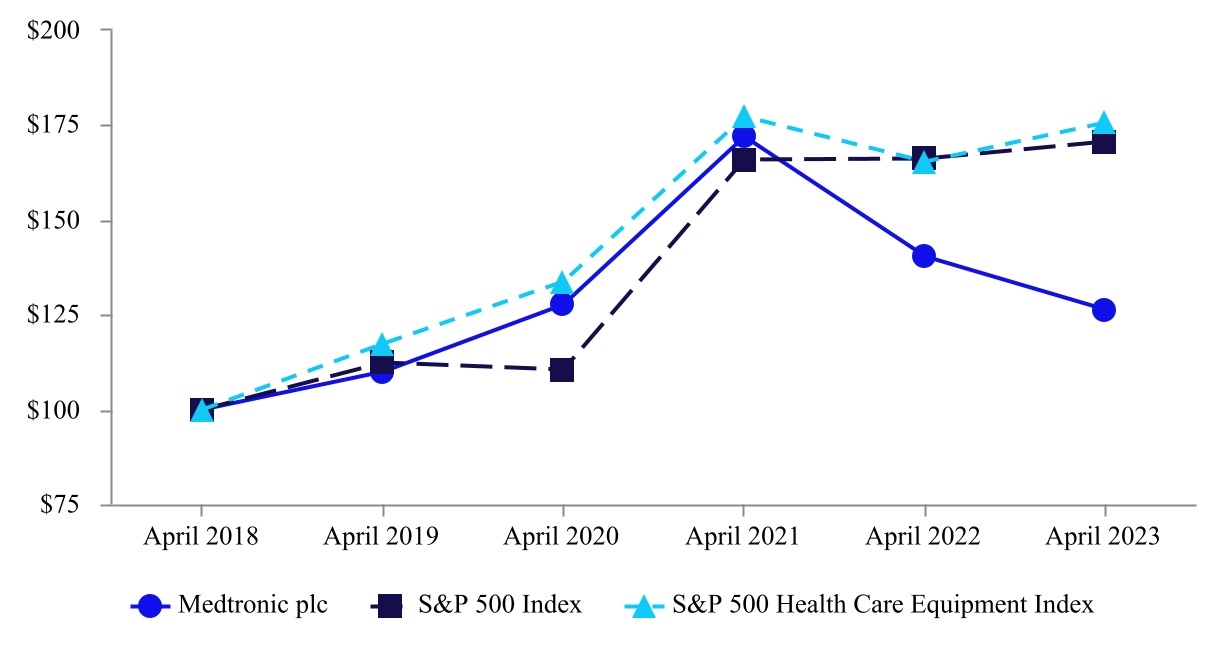
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Company/Index | | April 2018 | | April 2019 | | April 2020 | | April 2021 | | April 2022 | | April 2023 |
| Medtronic plc | | $ | 100.00 | | | $ | 109.85 | | | $ | 127.59 | | | $ | 171.99 | | | $ | 140.18 | | | $ | 126.27 | |
| S&P 500 Index | | 100.00 | | | 112.33 | | | 110.58 | | | 165.75 | | | 166.10 | | | 170.53 | |
| S&P 500 Health Care Equipment Index | | 100.00 | | | 117.36 | | | 133.57 | | | 177.12 | | | 165.24 | | | 175.54 | |
For information on the Company's equity compensation plans, see "Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters" in this Annual Report on Form 10-K.
Irish Restrictions on Import and Export of Capital
Except as indicated below, there are no restrictions on non-residents of Ireland dealing in Irish domestic securities, which includes ordinary shares of Irish companies. Except as indicated below, dividends and redemption proceeds also continue to be freely transferable to non-resident holders of such securities. The Financial Transfers Act, 1992 provides that the Irish Minister for Finance can make provision for the restriction of financial transfers between Ireland and other countries. For the purposes of this Act, “financial transfers” include all transfers which would be movements of capital or payments within the meaning of the treaties governing the E.U. if they had been made between Member States of the E.U. This Act and underlying E.U. regulations provide for the restriction of financial transfers to certain countries, organizations, and people including the Al-Qaeda network and the Taliban, Afghanistan, Belarus, Burma (Myanmar), Democratic People’s Republic of Korea, Democratic Republic of Congo, Iran, Iraq, Lebanon, Libya, Republic of Guinea, Republic of Guinea-Bissau, Russia, Somalia, Sudan, Syria, Tunisia, certain persons and groups in Ukraine and Zimbabwe.
Any transfer of, or payment in respect of, a share or interest in a share involving the government of any country that is currently the subject of United Nations or E.U. sanctions, any person or body controlled by any of the foregoing, or by any person acting on behalf of the foregoing, may be subject to restrictions pursuant to such sanctions as implemented into Irish law.
Irish Taxes Applicable to U.S. Holders
Dividends paid by Medtronic will generally be subject to Irish dividend withholding tax (currently at a rate of 25 percent) unless an exemption applies.
Dividends paid to U.S. residents will not be subject to Irish dividend withholding tax provided that:
•in the case of a beneficial owner of Medtronic shares held in the Depository Trust Company (DTC), the address of the beneficial owner in the records of his or her broker is in the United States and this information is provided by the broker to the Company’s qualifying intermediary; or
•in the case of a record owner, the record owner has provided to the Company’s transfer agent a valid U.S. Certification of Residence (Form 6166) or valid Irish Non-Resident Form V2.
Irish income tax may also arise with respect to dividends paid on Medtronic’s ordinary shares. A U.S. resident who meets one of the exemptions from dividend withholding tax described above and who does not hold Medtronic shares through a branch or agency in Ireland through which a trade is carried on generally will not have any Irish income tax liability on a dividend paid by Medtronic. In addition, if a U.S. shareholder is subject to the dividend withholding tax, the withholding payment discharges any Irish income tax liability, provided the shareholder furnishes to the Irish Revenue authorities a statement of the dividend withholding tax imposed.
While the U.S./Ireland Double Tax Treaty contains provisions regarding withholding, due to the wide scope of the exemptions from dividend withholding tax available under Irish domestic law, it would generally be unnecessary for a U.S. resident shareholder to rely on the treaty provisions.
Item 6. Reserved
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
UNDERSTANDING OUR FINANCIAL INFORMATION
The following discussion and analysis provides information management believes to be relevant to understanding the financial condition and results of operations of the Company. The discussion focuses on our financial results for the fiscal year ended April 28, 2023 (fiscal year 2023) and the fiscal year ended April 29, 2022 (fiscal year 2022). A discussion on our results of operations for fiscal year 2022 as compared to the year ended April 30, 2021 (fiscal year 2021) is included in Part II, Item 7. "Management's Discussion and Analysis of Financial Condition and Results of Operations" of our Annual Report on Form 10-K for the year ended April 29, 2022, filed with the SEC on June 23, 2022, and is incorporated by reference into this Form 10-K. You should read this discussion and analysis along with our consolidated financial statements and related notes thereto at April 28, 2023 and April 29, 2022 and for fiscal years 2023, 2022, and 2021, which are presented within "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K. Amounts reported in millions within this annual report are computed based on the amounts in thousands, and therefore, the sum of the components may not equal the total amount reported in millions due to rounding. Additionally, certain columns and rows within tables may not sum due to rounding.
Financial Trends
Throughout this Management’s Discussion and Analysis, we present certain financial measures that facilitate management's review of the operational performance of the Company and as a basis for strategic planning; however, such financial measures are not presented in our financial statements prepared in accordance with accounting principles generally accepted in the United States (U.S.) (U.S. GAAP). These financial measures are considered "non-GAAP financial measures" and are intended to supplement, and should not be considered as superior to, financial measures presented in accordance with U.S. GAAP. We believe that non-GAAP financial measures provide information useful to investors in understanding the Company's underlying operational performance and trends and may facilitate comparisons with the performance of other companies in the medical technologies industry.
As presented in the GAAP to Non-GAAP Reconciliations section below, our non-GAAP financial measures exclude the impact of amortization of intangible assets and certain charges or benefits that contribute to or reduce earnings and that may affect financial trends and include certain charges or benefits that result from transactions or events that we believe may or may not recur with similar materiality or impact to our operations in future periods (Non-GAAP Adjustments).
In the event there is a Non-GAAP Adjustment recognized in our operating results, the tax cost or benefit attributable to that item is separately calculated and reported. Because the effective rate can be significantly impacted by the Non-GAAP Adjustments that take place during the period, we often refer to our tax rate using both the effective rate and the non-GAAP nominal tax rate (Non-GAAP Nominal Tax Rate). The Non-GAAP Nominal Tax Rate is calculated as the income tax provision, adjusted for the impact of Non-GAAP Adjustments, as a percentage of income before income taxes, excluding Non-GAAP Adjustments.
Free cash flow is a non-GAAP financial measure calculated by subtracting property, plant, and equipment additions from operating cash flows.
Refer to the “GAAP to Non-GAAP Reconciliations," "Income Taxes," and "Free Cash Flow" sections for reconciliations of the non-GAAP financial measures to their most directly comparable financial measures prepared in accordance with U.S. GAAP.
EXECUTIVE LEVEL OVERVIEW
The following is a summary of revenue, diluted earnings per share, and cash flow for fiscal years 2023 and 2022:
GAAP to Non-GAAP Reconciliations
The tables below present reconciliations of our Non-GAAP financial measures to the most directly comparable financial measures prepared in accordance with U.S. GAAP for fiscal years 2023 and 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fiscal year ended April 28, 2023 |
| (in millions, except per share data) | Income Before Income Taxes | | Income Tax Provision (Benefit) | | Net Income Attributable to Medtronic | | Diluted EPS | | Effective Tax Rate |
| GAAP | $ | 5,364 | | | $ | 1,580 | | | $ | 3,758 | | | $ | 2.82 | | | 29.5 | % |
| Non-GAAP Adjustments: | | | | | | | | | |
| Amortization of intangible assets | 1,698 | | | 255 | | | 1,443 | | | 1.08 | | | 15.0 | |
Restructuring and associated costs (1) | 647 | | | 139 | | | 507 | | | 0.38 | | | 21.5 | |
Acquisition-related items (2) | 110 | | | 21 | | | 89 | | | 0.07 | | | 19.1 | |
Divestiture and separation-related items (3) | 235 | | | 8 | | | 227 | | | 0.17 | | | 3.4 | |
Certain litigation charges, net (4) | (30) | | | (8) | | | (23) | | | (0.02) | | | 26.7 | |
(Gain)/loss on minority investments (5) | (33) | | | 2 | | | (29) | | | (0.02) | | | (6.1) | |
Medical device regulations (6) | 150 | | | 30 | | | 120 | | | 0.09 | | | 20.0 | |
Debt redemption premium and other charges (7) | 53 | | | 11 | | | 42 | | | 0.03 | | | 20.8 | |
Certain tax adjustments, net (8) | — | | | (910) | | | 910 | | | 0.68 | | | — | |
| Non-GAAP | $ | 8,194 | | | $ | 1,128 | | | $ | 7,045 | | | $ | 5.29 | | | 13.8 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fiscal year ended April 29, 2022 |
| (in millions, except per share data) | Income Before Income Taxes | | Income Tax Provision (Benefit) | | Net Income Attributable to Medtronic | | Diluted EPS | | Effective Tax Rate |
| GAAP | $ | 5,517 | | | $ | 456 | | | $ | 5,039 | | | $ | 3.73 | | | 8.3 | % |
| Non-GAAP Adjustments: | | | | | | | | | |
| Amortization of intangible assets | 1,733 | | | 266 | | | 1,467 | | | 1.09 | | | 15.3 | |
Restructuring and associated costs (1) | 335 | | | 54 | | | 281 | | | 0.21 | | | 16.1 | |
Acquisition-related items (2) | (43) | | | 5 | | | (48) | | | (0.04) | | | (11.6) | |
| Certain litigation charges | 95 | | | 17 | | | 78 | | | 0.06 | | | 17.9 | |
(Gain)/loss on minority investments (5) | (12) | | | — | | | (9) | | | (0.01) | | | — | |
Medical device regulations (6) | 102 | | | 16 | | | 86 | | | 0.06 | | | 15.7 | |
MCS impairment / costs (9) | 881 | | | 220 | | | 661 | | | 0.49 | | | 25.0 | |
Certain tax adjustments, net (10) | — | | | 50 | | | (50) | | | (0.04) | | | — | |
| Non-GAAP | $ | 8,609 | | | $ | 1,084 | | | $ | 7,505 | | | $ | 5.55 | | | 12.6 | % |
(1)Associated costs include costs incurred as a direct result of the restructuring program, such as salaries for employees supporting the program and consulting expenses.
(2)The charges primarily include business combination costs and changes in fair value of contingent consideration.
(3)The charges predominantly include non-cash pre-tax impairments, primarily related to goodwill, changes in the carrying value of the disposal group, and other associated costs, as a result of the April 1, 2023 sale of half of the Company's Renal Care Solutions (RCS) business; charges related to the impending separation of the Patient Monitoring and Respiratory Interventions businesses within our Medical Surgical Portfolio in the fourth quarter of fiscal year 2023; and charges related to an exit of a business which are primarily comprised of inventory write-downs.
(4)Certain litigation includes $35 million income related to the one-time payment received as a result of the Intellectual Property Agreement entered into with Edwards Lifesciences on April 12, 2023.
(5)We exclude unrealized and realized gains and losses on our minority investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations.
(6)The charges represent estimated incremental costs of complying with the new European Union medical device regulations for previously registered products and primarily include charges for contractors supporting the project and other direct third-party expenses. We consider these costs to be duplicative of previously incurred costs and /or one-time costs, which are limited to a specific period.
(7)The charges relate to the early redemption of approximately $2.3 billion of debt and were recorded within interest expense, net within the consolidated statements of income.
(8)The charge primarily relates to a $764 million reserve adjustment that was a direct result of the U.S. Tax Court opinion, issued on August 18, 2022, on the previously disclosed litigation regarding the allocation of income between Medtronic, Inc. and its wholly owned subsidiary operating in Puerto Rico. Additional charges relate to the reduction of deferred tax assets due to the disallowance of certain interest deductions and the change in the reporting currency for certain carryover attributes, and the amortization on previously established deferred tax assets from intercompany intellectual property transactions.
(9)The charges relate to the Company’s June 2021 decision to stop the distribution and sale of the Medtronic HVAD System within the Mechanical Circulatory Support Operating Unit (MCS). The charges included $515 million of non-cash impairments, primarily related to $409 million of intangible asset impairments, as well as $366 million for commitments and obligations in connection with the decision, including patient support obligations, restructuring, and other associated costs. Medtronic is committed to serving the needs of patients currently implanted with the HVAD System.
(10)The net benefit primarily relates to the deferred tax impact associated with a step up in tax basis for Swiss Cantonal purposes and a change in tax rates on deferred taxes associated with intellectual property, which are partially offset by the amortization on previously established deferred tax assets from intercompany intellectual property transactions and a charge related to a change in the Company's permanent reinvestment assertion on certain historical earnings.
Free Cash Flow
Free cash flow, a non-GAAP financial measure, is calculated by subtracting additions to property, plant, and equipment from net cash provided by operating activities. Management uses this non-GAAP financial measure, in addition to U.S. GAAP financial measures, to evaluate our operating results. Free cash flow should be considered supplemental to, and not a substitute for, our reported financial results prepared in accordance with U.S. GAAP. Reconciliations between net cash provided by operating activities (the most comparable U.S. GAAP measure) and free cash flow are as follows:
| | | | | | | | | | | |
| Fiscal Year |
| (in millions) | 2023 | | 2022 |
| Net cash provided by operating activities | $ | 6,039 | | | $ | 7,346 | |
| Additions to property, plant, and equipment | (1,459) | | | (1,368) | |
| Free cash flow | $ | 4,580 | | | $ | 5,978 | |
Refer to the Summary of Cash Flows section for drivers of the change in cash provided by operating activities.
NET SALES
Segment and Division
The charts below illustrate the percent of net sales by segment for fiscal years 2023 and 2022:
The table below includes net sales by segment and division for fiscal years 2023 and 2022: | | | | | | | | | | | | | | | | | |
| | Net Sales by Fiscal Year | | Percent Change |
| (in millions) | 2023 | | 2022 | |
| Cardiac Rhythm & Heart Failure | $ | 5,835 | | | $ | 5,908 | | | (1) | % |
| Structural Heart & Aortic | 3,363 | | | 3,055 | | | 10 | |
| Coronary & Peripheral Vascular | 2,375 | | | 2,460 | | | (3) | |
| Cardiovascular | 11,573 | | | 11,423 | | | 1 | |
| Surgical Innovations | 5,663 | | | 6,060 | | | (7) | |
| Respiratory, Gastrointestinal, & Renal | 2,770 | | | 3,081 | | | (10) | |
| Medical Surgical | 8,433 | | | 9,141 | | | (8) | |
| Cranial & Spinal Technologies | 4,451 | | | 4,456 | | | — | |
| Specialty Therapies | 2,815 | | | 2,592 | | | 9 | |
| Neuromodulation | 1,693 | | | 1,735 | | | (2) | |
| Neuroscience | 8,959 | | | 8,784 | | | 2 | |
| Diabetes | 2,262 | | | 2,338 | | | (3) | |
| Total | $ | 31,227 | | | $ | 31,686 | | | (1) | % |
Segment and Market Geography
The charts below illustrate the percent of net sales by market geography for fiscal years 2023 and 2022:
The table below includes net sales by market geography for each of our segments for fiscal years 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S.(1) | | Non-U.S. Developed Markets(2) | | Emerging Markets(3) |
| (in millions) | Fiscal Year 2023 | | Fiscal Year 2022 | | % Change | | Fiscal Year 2023 | | Fiscal Year 2022 | | % Change | | Fiscal Year 2023 | | Fiscal Year 2022 | | % Change |
| Cardiovascular | $ | 5,848 | | | $ | 5,545 | | | 5 | % | | $ | 3,564 | | | $ | 3,866 | | | (8) | % | | $ | 2,161 | | | $ | 2,012 | | | 7 | % |
| Medical Surgical | 3,658 | | | 3,862 | | | (5) | | | 3,080 | | | 3,373 | | | (9) | | | 1,694 | | | 1,905 | | | (11) | |
| Neuroscience | 6,018 | | | 5,753 | | | 5 | | | 1,658 | | | 1,801 | | | (8) | | | 1,283 | | | 1,229 | | | 4 | |
| Diabetes | 849 | | | 974 | | | (13) | | | 1,106 | | | 1,085 | | | 2 | | | 307 | | | 279 | | | 10 | |
| Total | $ | 16,373 | | | $ | 16,135 | | | 1 | % | | $ | 9,408 | | | $ | 10,126 | | | (7) | % | | $ | 5,446 | | | $ | 5,426 | | | — | % |
(1)U.S. includes the United States and U.S. territories.
(2)Non-U.S. developed markets include Japan, Australia, New Zealand, Korea, Canada, and the countries within Western Europe.
(3)Emerging markets include the countries of the Middle East, Africa, Latin America, Eastern Europe, and the countries of Asia that are not included in the non-U.S. developed markets, as defined above.
The decline in net sales for fiscal year 2023 was primarily driven by unfavorable currency impacts, impact of volume-based procurement tenders and COVID-19 resurgence in China, as well as supply chain challenges in certain businesses, particularly in the first quarter of fiscal year 2023. Currency had an unfavorable impact of $1.2 billion on non-U.S. developed markets and $262 million on emerging markets. The decline in net sales was partially offset by growth in certain product lines and businesses, including Micra, Transcatheter Aortic Valve replacements (TAVR), hemorrhagic and ischemic stroke, and ENT, in addition to the $265 million one-time payment received as a result of the Intellectual Property Agreement entered into with Edwards Lifesciences, as further discussed in the Cardiovascular net sales section below.
Looking ahead, a number of macro-economic and geopolitical factors could negatively impact our business, including without limitation:
•Competitive product launches and pricing pressure, geographic macro-economic risks including fluctuations in currency exchange rates, general price inflation, rising interest rates, reimbursement challenges, impacts from changes in the mix of our product offerings, delays in product registration approvals, and replacement cycle challenges;
•National and provincial/state tender decisions, including around pricing, for certain products, particularly in China;
•The uncertain and uneven impact of COVID-19 on future procedural volumes, supply constraints including certain electronic components and semiconductors, healthcare staffing in certain regions, and resulting impacts on demand for our products and therapies; and
•The sanctions and other measures being imposed in response to the Russia-Ukraine conflict are having, and could continue to have impacts on revenue and supply chain. The financial impact of the conflict in fiscal year 2023, including on accounts receivable and inventory reserves, was not material, and for fiscal year 2023, the business of the Company in these countries represented less than 1% of the Company's consolidated revenues and assets. Although the implications of this conflict are difficult to predict at this time, the ongoing conflict may increase pressure on the global economy and supply chains, resulting in increased future volatility risk for our business operations and performance.
Cardiovascular
Cardiovascular products include pacemakers, insertable cardiac monitors, cardiac resynchronization therapy devices, implantable cardioverter defibrillators (ICD), leads and delivery systems, electrophysiology catheters, products for the treatment of atrial fibrillation, information systems for the management of patients with Cardiac Rhythm & Heart Failure devices, products designed to reduce surgical site infections, coronary and peripheral stents and related delivery systems, balloons and related delivery systems, endovascular stent graft systems, heart valve replacement technologies, cardiac tissue ablation systems, and open heart and coronary bypass grafting surgical products. Cardiovascular also includes Care Management Services and Cath Lab Managed Services (CLMS) within the Cardiac Rhythm & Heart Failure division. Cardiovascular net sales for fiscal year 2023 were $11.6 billion, an increase of 1 percent as compared to fiscal year 2022. The net sales increase was primarily due to the strong performance of Micra, TAVR, and Diagnostics, partially offset by unfavorable currency impact of $569 million and supply chain challenges in certain businesses.
The charts below illustrate the percent of Cardiovascular net sales by division for fiscal years 2023 and 2022:
Cardiac Rhythm & Heart Failure (CRHF) net sales decreased 1 percent in fiscal year 2023 as compared to fiscal year 2022. The decrease was driven by Cardiac Ablation Solutions experiencing competitive pressures in Western Europe, as well as the pending volume-based procurement (VBP) tenders in China, offset by continued adoption of Micra AV, TYRX antibacterial envelopes, LINQ II implants, and growth from Arctic Front cryoblation catheters in the U.S.
Structural Heart & Aortic (SHA) net sales increased 10 percent in fiscal year 2023 as compared to fiscal year 2022. The increase was led by growth in transcatheter aortic valve replacement (TAVR), including the U.S. and Japan. Results include $265 million of revenue from a one-time payment received as a result of the Intellectual Property Agreement (agreement) entered into with Edwards Lifesciences (Edwards) on April 12, 2023. As part of this agreement, Edwards will also pay the Company royalty payments tied to future net sales of certain Edwards products. Net sales growth was negatively impacted by a field corrective action with the Harmony Transcatheter Pulmonary Valve and Delivery Catheter System.
Coronary & Peripheral Vascular (CPV) net sales decreased 3 percent in fiscal year 2023 as compared to fiscal year 2022. The net sales declines were driven by market procedural volumes in Coronary remaining below pre-COVID levels in several major markets, headwinds related to U.S. hospital contrast shortages early in fiscal year 2023, and declines in Peripheral Vascular Health due to competitors re-entering the market and supply chain challenges. Net sales declines were partially offset by strong demand combined with improved product availability of the SpiderFX embolic protection device (EPD) and strong performance of our superficial venous product portfolio, including the VenaSeal system.
In addition to the macro-economic and geopolitical factors described in the Net Sales section, looking ahead, we expect Cardiovascular could be affected by the following:
•Continued growth of our Micra transcatheter pacing system. Our portfolio consists of Micra VR and Micra AV, which offer leadless pacing therapy to approximately 45 percent of pacemaker patients. We expect the launch of next generation Micra AV2/VR2 in the first quarter of fiscal year 2024 will continue to support adoption of leadless pacing, as it extends the capability of the Micra portfolio by adding significant battery longevity and programming simplicity.
•Continued acceptance and growth from the Azure XT and S SureScan pacing systems and the 3830 lead. Azure pacemakers feature Medtronic-exclusive BlueSync technology, which enables automatic, secure wireless remote monitoring with increased device longevity. The 3830 lead, previously labeled for His-bundle pacing, has now been expanded to include left bundle branch area pacing.
•Growth of the Cobalt and Crome portfolio of ICDs and CRT-Ds.
•Growth of the CRT-P quadripolar pacing system.
•Continued growth, adoption, and utilization of the TYRX Envelope for implantable devices.
•Continued acceptance and expansion of the LINQ II cardiac monitor. During the third quarter of fiscal year 2022, we launched two AccuRhythm AI algorithms on the LINQ II platform to significantly reduce false positive alerts for Atrial Fibrillation and pause episodes while retaining sensitivity for true positive detection and reduce clinic workload and burden. AccuRhythm AI launched in Europe during the first quarter of fiscal year 2023.
•Continued growth of Arctic Front cryoablation for treatment of atrial fibrillation.
•Acceptance and growth of the Affera Mapping/Navigation System and Sphere 9 mapping/ablation catheter. The system was launched under a limited market release in the fourth quarter of fiscal year 2023 in Western Europe.
•Continued acceptance and growth of the self-expanding CoreValve Evolut transcatheter aortic valve replacement platform. This includes Evolut PRO which provides enhanced hemodynamics, reliable delivery, enhanced durability, advanced sealing, and Evolut FX, a system designed to improve the overall procedural experience through enhancements in deliverability, implant visibility, and deployment stability.
•Continued expansion and training of field support to increase coverage in the U.S. centers performing TAVR procedures.
•Continued acceptance and growth of the Onyx Frontier DES platform. The platform launched in the U.S. in the first quarter of fiscal year 2023 and in select international countries in the second quarter of fiscal year 2023. Onyx Frontier is a drug-eluding stent (DES) that introduces an enhanced delivery system and is used for complex percutaneous coronary intervention (PCI).
•Continued acceptance of the VenaSeal Closure System in the U.S. The VenaSeal Closure System is a unique non-thermal solution to address superficial venous disease that provides improved patient comfort, reduces the recovery time, and eliminates the risk of thermal nerve injury.
•Acceptance and growth of IN.PACT 018 drug-coated balloon (DCB). The product was launched in the U.S. under limited market release in the first quarter of fiscal year 2023 with full market release in the third quarter of fiscal year 2023. IN.PACT 018 adds to the existing IN.PACT Admiral DCB portfolio and is used to treat femoropopliteal disease.
•Market and competitive pressure on sales of the Abre venous self-expanding stent system. Abre is designed for the unique challenges of venous disease. Now backed by 36 months of data, it offers easy deployment and delivers demonstrated endurance, to give patients freedom of movement.
•Our ability to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline, which include, but are not limited to, the Symplicity Spyral Multi-Electrode Renal Denervation Catheter, Pulse Field Ablation, a novel energy source that is non-thermal, and Aurora Extravascular ICD.
Medical Surgical
Medical Surgical’s products span the entire continuum of patient care from diagnosis to recovery, with a focus on diseases of the gastrointestinal tract, lungs, pelvic region, kidneys, obesity, and preventable complications. The products include those for advanced and general surgical products, surgical stapling devices, vessel sealing instruments, wound closure, electrosurgery products, hernia mechanical devices, mesh implants, advanced ablation, interventional lung, ventilators, airway products, renal care products, and sensors and monitors for pulse oximetry, capnography, level of consciousness and cerebral oximetry. Medical Surgical’s net sales for fiscal year 2023 were $8.4 billion, a decrease of 8 percent as compared to fiscal year 2022. The net sales decrease was primarily driven by unfavorable currency impact of $454 million, provincial volume-based procurement (VBP) stapling tenders in China, and a decline in ventilator sales due to the high COVID-19 demand in the corresponding period in the prior fiscal year. Supply chain disruptions, particularly in Surgical Innovations, also contributed to the net sales decrease for fiscal year 2023.
The charts below illustrate the percent of Medical Surgical net sales by division for fiscal years 2023 and 2022:
Surgical Innovations net sales for fiscal year 2023 decreased 7 percent as compared to fiscal year 2022. The net sales decline was led by Advanced Surgical instruments, driven by global supply chain challenges, including resins, semiconductors, and packaging trays, which impacted energy and stapling products, and provincial VBP stapling tenders and COVID-19 lockdowns in China. These declines were partially offset by growth in Advanced Energy in the fourth quarter of fiscal year 2023.
Respiratory, Gastrointestinal, & Renal (RGR) net sales for fiscal year 2023 decreased 10 percent as compared to fiscal year 2022. RGR net sales declines were largely due to declines in ventilator demand when compared to the corresponding period in the prior fiscal year as demand dropped below pre-pandemic levels, as well as declines in RCS driven by product availability challenges in the first three quarters of fiscal year 2023, and only two months of sales in the fourth quarter of fiscal year 2023 as a result of the April 1, 2023 contribution of half of the Company's RCS business to form Mozarc Medical. These declines were partially offset by growth in Gastrointestinal driven by strength in sales of GI Genius.
In addition to the macro-economic and geopolitical factors described in the Net Sales section, looking ahead we expect Medical Surgical could be affected by the following:
•The pending separation of the combined Patient Monitoring and Respiratory Interventions businesses from the Medical Surgical Portfolio. The Company announced its intention to pursue a separation in October 2022 and expects to complete the separation 18 to 24 months from the announcement date.
•Acceptance and continued growth of Open-to-MIS (minimally invasive surgery) techniques and tools through our efforts to transition open surgery to MIS. Open-to-MIS initiative focuses on capturing the market opportunity that exists in transitioning open procedures to MIS, whether through traditional MIS, advanced instrumentation, or robotics. Through our approach, in parallel, we also expand our presence and optimize open surgery in current open surgery markets.
•Continued global acceptance and future growth of powered stapling and energy platform.
•Our ability to execute ongoing strategies addressing the competitive pressure of reprocessing vessel sealing disposables and growth of our surgical soft tissue robotics procedures in the U.S.
•Our ability to create markets and drive products and procedures into emerging markets with our high quality and cost-effective surgical products designed for customers in emerging markets. An example is our ValleyLab LS10 single channel vessel sealing generator, which is compatible with our line of LigaSure instruments and designed for simplified use and affordability.
•Acceptance of less invasive standards of care in gastrointestinal and hepatology products, including products that span the care continuum from diagnostics to therapeutics. Recently launched products include GI Genius and PillCam capsule endoscopy.
•Expanding the use of less invasive treatments and furthering our commitment to improving options for women with abnormal uterine bleeding. Our expanded and strengthened surgical offerings complement our global gynecology business.
•Global adoption of robotic-assisted surgery and installations of Hugo robotic assisted surgery (RAS) system for urologic, bariatric, gynecologic, and general surgery procedures. This includes continued integration and adoption of Touch Surgery Enterprise with the first artificial intelligence powered surgical videos and analytics platform to make it easier to train and discover new techniques within the robotics platform. The Hugo RAS system, which received CE Mark in October 2021, as well as secured additional regulatory approvals outside the U.S., is designed to help reduce unwanted variability, improve patient outcomes, and, by extension, lower per procedure cost.
•Our ability to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline, which include, but are not limited to, our Hugo RAS system in the U.S., Signia power stapling devices, and our next-gen Ligasure and Sonicision vessel sealing devices.
Neuroscience
Neuroscience's products include various spinal implants, bone graft substitutes, biologic products, image-guided surgery and intra-operative imaging systems, robotic guidance systems used in the robot-assisted spine procedures, and systems that incorporate advanced energy surgical instruments. Neuroscience's products also focus on therapies to treat the diseases of the vasculature in and around the brain, including coils, neurovascular stents, and flow diversion products, as well as products to treat ear, nose, and throat (ENT), and the treatment of overactive bladder, urinary retention, fecal incontinence. Neuroscience also manufactures products related to implantable neurostimulation therapies and drug delivery systems for the treatment of chronic pain, movement disorders, and epilepsy. Neuroscience’s net sales for fiscal year 2023 were $9.0 billion, an increase of 2 percent as compared to fiscal year 2022. The net sales increase was primarily due to growth in U.S. Core Spine, Neurovascular, ENT, and continued supply risk mitigation, partially offset by unfavorable currency impact of $281 million and supply chain challenges in certain businesses.
The graphs below illustrate the percent of Neuroscience net sales by division for fiscal years 2023 and 2022:
Cranial & Spinal Technologies (CST) net sales for fiscal year 2023 were flat as compared to fiscal year 2022 as growth within U.S. Core Spine was offset by net sales declines in Biologics and unfavorable currency impact. The growth in U.S. Core Spine products was driven by the continued adoption of the Aible ecosystem of spine products. The net sales increase was also attributable to strong sales of StealthStation Navigation and Midas Rex powered surgical instruments. The decline in net sales in Biologics was due to supply chain challenges throughout the year.
Specialty Therapies (Specialty) net sales for fiscal year 2023 increased 9 percent as compared to fiscal year 2022. The increase was driven by growth in hemorrhagic and ischemic stroke, flow diversion and access delivery products. The net sales increase was also driven by benefits from the May 2022 acquisition of Intersect ENT.
Neuromodulation (NM) net sales for fiscal year 2023 decreased 2 percent as compared to fiscal year 2022. The decline in net sales was largely due to declines of Brain Modulation replacement devices and supply chain challenges in Interventional, which has recently seen improvements in product availability. The decline was partially offset by growth within Pain Stim and to a lesser extent, Targeted Drug Delivery.
In addition to the macro-economic and geopolitical factors described in the Net Sales section, looking ahead we expect Neuroscience could be affected by the following:
•Continued adoption and growth of our integrated solutions through the Aible offering, which integrates spinal implants with enabling technologies (StealthStation, O-arm Imaging Systems, and Midas), Mazor robotics, and UNiD Adaptive Spine Intelligence AI-driven technology for surgical planning and personalized spinal implants.
•Market acceptance and continued global adoption of innovative new spine products and procedural solutions within our CST operating unit, such as Catalyft PL, ModuLeX, CD Horizon Voyager System, and our Infinity OCT System, as well as continued growth from Titan spine titanium interbody implants with Nanolock technology.
•Continued growth of Pipeline Embolization Devices, endovascular treatments for large or giant wide-necked brain aneurysms.
•Continued acceptance and growth of the Solitaire X revascularization device for treatment of acute ischemic stroke and our React Catheter and Riptide aspiration system.
•Strengthening our position in the ENT market as a result of the May 2022 acquisition of Intersect ENT, a global ENT medical technology leader. The acquisition expands Neuroscience's portfolio of products used during ENT procedures and combined with the Company's navigation, powered instruments, and existing tissue health products, offers a broader suite of solutions to assist surgeons treating patients who suffer from chronic rhinosinusitis (CRS).
•Continued acceptance and growth of our Pelvic Health and ENT therapies, including our InterStim therapy with InterStim X and InterStim II recharge-free neurostimulators and InterStim Micro rechargeable neurostimulator for patients suffering from overactive bladder, (non-obtrusive) urinary retention, and chronic fecal incontinence, and capital equipment sales of the Stealth Station ENT surgical navigation system and intraoperative NIM nerve monitoring system.
•Market acceptance and growth from SCS therapy for treating chronic pain and Diabetic Peripheral Neuropathy (DPN) on the Intellis rechargeable neurostimulator and Vanta recharge-free neurostimulator.
•Continued acceptance and growth of our Percept family of DBS devices with proprietary BrainSense technology for objectifying and personalizing the treatment of Parkinson's Disease, epilepsy, and other movement disorders.
•Ongoing obligations under the U.S. FDA consent decree entered in April 2015 relating to the SynchroMed drug infusion system and the Neuromodulation quality system. The U.S. FDA lifted its distribution requirements on our implantable drug pump in October 2017 and its warning letter in November 2017.
•Our ability to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline, which include our closed-loop Percept devices with adaptive DBS (aDBS) and Inceptiv Neurostimulator, as well as our hemorrhagic stroke intravascular device, and our next-generation spine enabling technologies.
Diabetes
Diabetes' products include insulin pumps, continuous glucose monitoring (CGM) systems, consumables, and smart insulin pen systems. Diabetes' sales for fiscal year 2023 were $2.3 billion, a decrease of 3 percent as compared to fiscal year 2022. The decrease in net sales was primarily driven by unfavorable currency impact of $133 million and declines in the U.S. The net sales declines were partially offset by strong international growth primarily driven by the continued international expansion of the MiniMed 780G insulin pump system and integrated CGM. During April 2023, the U.S. FDA lifted the warning letter received in December 2021.
In addition to the macro-economic and geopolitical factors described in the Net Sales section, looking ahead we expect Diabetes could be affected by the following:
•Continued acceptance and growth for the MiniMed 780G insulin pump system, which is powered by SmartGuard technology and features the added benefits of meal detection technology that automatically adjusts and corrects sugar level levels every five minutes. The global adoption of sensor-augmented insulin pump systems has resulted in strong sensor attachment rates. The MiniMed 780G insulin pump system with the Guardian 4 Sensor was approved by the U.S. FDA in late April 2023.
•Continued acceptance and growth of the Guardian Connect CGM system, which displays glucose information directly to a smartphone to help ensure patients have access to their glucose levels seamlessly and discretely. The Guardian Connect CGM system is available on both Apple iOS and Android devices.
•Market acceptance and growth of our InPen smart pen system, which allows users to have their Medtronic CGM readings in real-time alongside insulin dose information, all in one view.
•Continued pump, CGM, and consumable competition in an expanding global market.
•Changes in medical reimbursement policies and programs, along with additional payor coverage on insulin pumps.
•Our ability to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline, including our next-generation sensor Simplera, which has been submitted to the U.S. FDA.
COSTS AND EXPENSES
The following is a summary of cost of products sold, research and development, and selling, general, and administrative expenses as a percent of net sales:
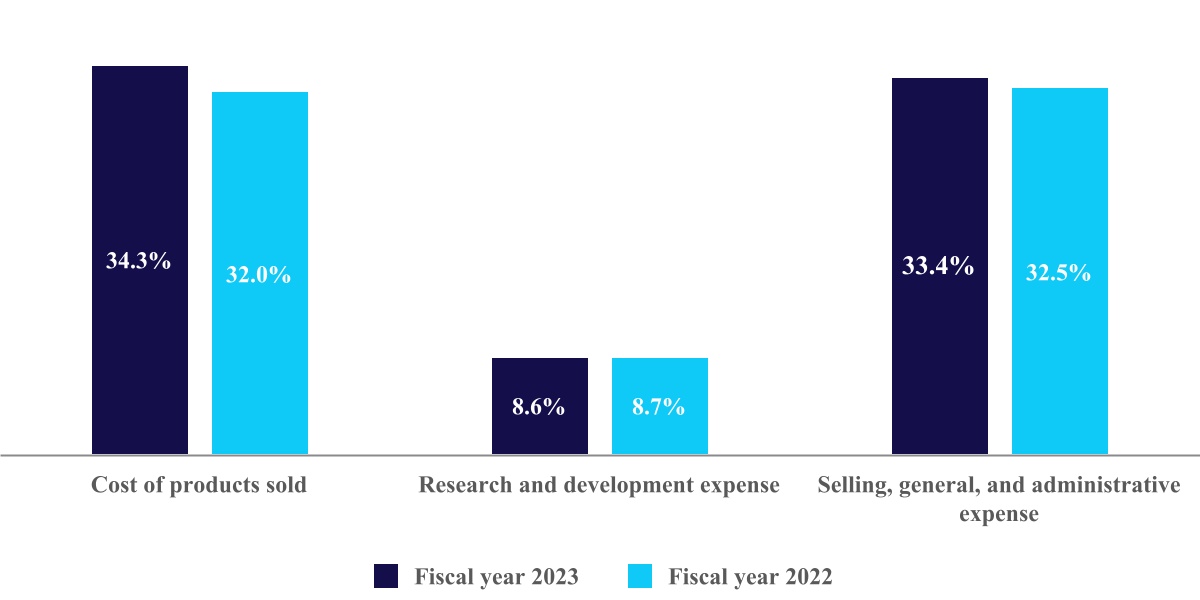
Cost of Products Sold Cost of products sold for fiscal year 2023 was $10.7 billion as compared to $10.1 billion for fiscal year 2022. The increase in cost of products sold as a percentage of net sales was primarily attributable to increased labor and direct material manufacturing costs, predominantly due to inflationary pressures and supply chain challenges, increased freight due to higher fuel costs and expedited shipments for backorders resulting from supply chain challenges, as well as increased inventory reserves. Fiscal year 2022 included $58 million of inventory write-downs associated with our June 2021 decision to stop the distribution and sale of Medtronic's HVAD System (MCS charges). Looking forward, our cost of products sold likely will be further negatively impacted by inflation and higher labor and direct material costs. We continue to focus on reducing our costs of production through supplier management, manufacturing improvements, and optimizing our manufacturing network.
Research and Development Expense We remain committed to deliver the best possible experiences for patients, physicians, and caregivers we serve; to create technologies that expand what’s possible across the human body to transform lives; to turn data and insights into real action to serve patient needs, improving care; and to expand healthcare access and deliver positive outcomes. Research and development expense for fiscal years 2023 and 2022 was $2.7 billion. Fiscal year 2022 included $101 million of expense related to acquisitions of, and license payments for, technology not approved by regulators, primarily in our Diabetes segment.
Selling, General, and Administrative Expense Our goal is to continue to leverage selling, general, and administrative expense initiatives. Selling, general, and administrative expense primarily consists of salaries and wages, other administrative costs, such as professional fees and marketing expenses, certain acquisition, divestiture and separation-related costs, and restructuring expenses. Selling, general, and administrative expense for fiscal year 2023 was $10.4 billion as compared to $10.3 billion for fiscal year 2022. The increase in selling, general, and administrative expense as a percentage of net sales was primarily driven by employee travel, and to a lesser extent by lower net sales, partially offset by a reduction in professional services.
The following is a summary of other costs and expenses (income):
| | | | | | | | | | | |
| Fiscal Year |
| (in millions) | 2023 | | 2022 |
| Amortization of intangible assets | $ | 1,698 | | | $ | 1,733 | |
| Restructuring charges, net | 375 | | | 60 | |
| Certain litigation charges, net | (30) | | | 95 | |
| Other operating (income) expense, net | (131) | | | 862 | |
| Other non-operating income, net | (515) | | | (318) | |
| Interest expense, net | 636 | | | 553 | |
Amortization of Intangible Assets Amortization of intangible assets includes the amortization expense of our definite-lived intangible assets, consisting of purchased patents, trademarks, tradenames, customer relationships, purchased technology, and other intangible assets.
Restructuring Charges, Net
In fiscal years 2023 and 2022, restructuring costs primarily related to Enterprise Excellence and Simplification restructuring programs, both of which were substantially completed as of the end of this fiscal year.
In the fourth quarter of fiscal year 2023, we incurred $0.3 billion of restructuring charges primarily related to employee termination benefits to support cost reduction initiatives. These charges were incremental to charges incurred under our Enterprise Excellence and Simplification programs noted above.
For all programs, employee-related costs primarily consist of termination benefits provided to employees who have been involuntarily terminated and voluntary early retirement benefits. Associated costs primarily include salaries and wages of employees that are fully-dedicated to restructuring programs and consulting fees.
For additional information about our restructuring programs, refer to Note 4 of the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K.
Certain Litigation Charges, Net We classify specified certain litigation charges and gains related to significant legal matters as certain litigation charges, net in the consolidated statements of income. For additional information, refer to Note 18 of the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Other Operating (Income) Expense, Net Other operating (income) expense, net primarily includes royalty expense, currency remeasurement and derivative gains and losses, Puerto Rico excise taxes, changes in the fair value of contingent consideration, MCS charges, RCS charges, impairment charges, income from funded research and development arrangements, and commitments to the Medtronic Foundation and Medtronic LABS.
The change in other operating (income) expense, net was primarily driven by MCS charges recorded in fiscal year 2022. The MCS charges of $823 million primarily included $409 million of intangible asset impairments and $366 million for commitments and obligations, including customer support obligations, restructuring, and other associated costs. Additionally, the change was driven by the net currency impact of remeasurement expense and our hedging programs, which resulted in a net gain of $466 million combined for fiscal year 2023 as compared to a net gain of $70 million for fiscal year 2022. During fiscal year 2023, the Company also recorded non-cash pre-tax charges of $136 million, primarily related to impairments of goodwill and changes in the carrying value of the disposal group, as a result of the April 1, 2023 sale of half of the Company's RCS business. Additional information regarding the MCS and RCS charges is described in Note 4 and Note 3, respectively, of the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K.
Other Non-Operating Income, Net Other non-operating income, net includes the non-service component of net periodic pension and postretirement benefit cost, investment gains and losses, and interest income.
The increase in other non-operating income, net for fiscal year 2023 is primarily attributable to an increase in interest income driven by higher returns on investments and an increase in rates on our global liquidity structures. Interest income was $386 million and $186 million for fiscal year 2023 and 2022, respectively.
Interest Expense, Net Interest expense, net includes interest incurred on our outstanding borrowings, amortization of debt issuance costs and debt premiums or discounts, amortization of amounts excluded from the effectiveness assessment of certain net investment hedges, and charges recognized in connection with the early redemption of senior notes.
The increase in interest expense, net for fiscal year 2023 was primarily due to an increase in rates on our global liquidity structures, the issuance of four tranches of Euro-denominated Senior Notes with an aggregate principal of €3.5 billion in September 2022, and the $53 million charge incurred as a result of the early redemption of approximately $2.3 billion of senior notes during the first quarter of fiscal year 2023. The Company's commercial paper activity and the issuance of two tranches of USD-denominated Senior Notes with an aggregate principal of $2.0 billion in March 2023 also increased interest expense, net. Partially offsetting the increase for fiscal year 2023 was $107 million in after-tax unrealized gains representing amounts excluded from the effectiveness assessment of certain net investment hedges, and benefits from foreign exchange rates.
INCOME TAXES | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | |
| Income tax provision | $ | 1,580 | | | $ | 456 | | | |
| Income before income taxes | 5,364 | | | 5,517 | | | |
| Effective tax rate | 29.5 | % | | 8.3 | % | | |
| | | | | |
| Non-GAAP income tax provision | $ | 1,128 | | | $ | 1,084 | | | |
| Non-GAAP income before income taxes | 8,194 | | | 8,609 | | | |
| Non-GAAP Nominal Tax Rate | 13.8 | % | | 12.6 | % | | |
| | | | | |
| Difference between the effective tax rate and Non-GAAP Nominal Tax Rate | (15.7) | % | | 4.3 | % | | |
On August 18, 2022, the U.S. Tax Court (Tax Court) issued its opinion on the previously disclosed litigation regarding the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico for fiscal years 2005 and 2006 (Opinion). While the Opinion rejected the IRS’s position and the Tax Court determined the methodology advanced by Medtronic was appropriate for purposes of determining the intercompany royalty rate between Puerto Rico and the U.S., it determined that the royalty rate should be higher, thereby increasing income allocated to the U.S. and consequently subject to U.S. tax. This case relates only to fiscal years 2005 and 2006. The Opinion remains subject to appeal by either or both parties. We have assumed the Tax Court findings will be applied for all years following fiscal year 2006. As a result, the Company recorded a $764 million net tax charge during the three months ended October 28, 2022 to recognize the estimated tax impact of the Tax Court Opinion.
Our effective tax rate for fiscal year 2023 was 29.5 percent, as compared to 8.3 percent in fiscal year 2022. The increase in our effective tax rate primarily relates to the certain tax adjustments discussed below and year-over-year changes in operational results by jurisdiction.
Our Non-GAAP Nominal Tax Rate for fiscal year 2023 was 13.8 percent, as compared to 12.6 percent in fiscal year 2022. The increase in our Non-GAAP Nominal Tax Rate primarily relates to year-over-year changes in operational results by jurisdiction.
During fiscal year 2023, we recognized $110 million of operational tax benefits. The operational tax benefits included an $11 million cost from the impact of stock-based compensation, and a $121 million benefit associated with the resolution of certain income tax audits, finalization of certain tax returns, changes to uncertain tax position reserves, and changes to certain deferred income tax balances.
During fiscal year 2022, we recognized $89 million of operational tax benefits. The operational tax benefits included a $46 million benefit from excess tax benefits associated with stock-based compensation, and a $43 million net benefit associated with the resolution of certain income tax audits, finalization of certain tax returns, changes to uncertain tax position reserves, and changes to certain deferred income tax balances.
An increase in our Non-GAAP Nominal Tax Rate of one percent would result in an additional income tax provision for fiscal years 2023 and 2022 of approximately $82 million and $86 million, respectively.
Certain Tax Adjustments
During fiscal year 2023, the net cost from certain tax adjustments of $910 million, recognized in income tax provision in the consolidated statement of income, included the following:
•A net cost of $764 million associated with a reserve adjustment that was a direct result of the U.S. Tax Court opinion, issued on August 18, 2022, on the previously disclosed litigation regarding the allocation of income between Medtronic, Inc. and its wholly owned subsidiary operating in Puerto Rico.
•A cost of $55 million related to the disallowance of certain interest deductions.
•A cost of $30 million related to the change in reporting currency for certain carryover attributes.
•A cost of $28 million associated with the amortization of the previously established deferred tax assets from intercompany intellectual property transactions.
•A net cost of $33 million primarily associated with the sale of half of the Company’s RCS business
During fiscal year 2022, the net benefit from certain tax adjustments of $50 million, recognized in income tax provision in the consolidated statement of income, included the following:
•A benefit of $82 million associated with a step up in tax basis for Swiss Cantonal purposes.
•A benefit of $82 million related to a change in tax rates on intangible assets.
•A cost of $47 million associated with the amortization of the previously established deferred tax assets from intercompany intellectual property transactions.
•A cost of $41 million associated with a change in the Company’s permanent reinvestment assertion on certain historical earnings.
•A net cost of $26 million primarily associated with an intercompany sale of assets.
Certain tax adjustments will affect the comparability of our operating results between periods. Therefore, we consider these Non-GAAP Adjustments. Refer to the "Executive Level Overview" section of this Management's Discussion and Analysis for further discussion of these adjustments.
Subsequent to year-end, on June 1, 2023 the Israeli Central-Lod District Court issued its decision in Medtronic Ventor Technologies Ltd v. Kfar Saba Assessing Office. The court determined that there was a deemed taxable transfer of intellectual property. At this time, the Company is evaluating the impact of the decision and whether or not it will appeal. The Company has currently estimated a potential income tax charge, including interest, of approximately $200 million.
LIQUIDITY AND CAPITAL RESOURCES
We are currently in a strong financial position, and we believe our balance sheet and liquidity as of April 28, 2023 provide us with flexibility, and our cash, cash equivalents, and current investments, along with our credit facility and related commercial paper programs will satisfy our foreseeable operating needs.
Our liquidity and capital structure are evaluated regularly within the context of our annual operating and strategic planning processes. We consider the liquidity necessary to fund our operations, which includes working capital needs, investments in research and development, property, plant, and equipment, and other operating costs. We also consider capital allocation alternatives that balance returning value to shareholders through dividends and share repurchases, satisfying maturing debt, and acquiring businesses and technology.
Summary of Cash Flows
The following is a summary of cash provided by (used in) operating, investing, and financing activities, the effect of exchange rate changes on cash and cash equivalents, and the net change in cash and cash equivalents: | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 |
| Cash provided by (used in): | | | |
| Operating activities | $ | 6,039 | | | $ | 7,346 | |
| Investing activities | (3,493) | | | (1,659) | |
| Financing activities | (4,960) | | | (5,336) | |
| Effect of exchange rate changes on cash and cash equivalents | 243 | | | (231) | |
| Net change in cash and cash equivalents | $ | (2,171) | | | $ | 121 | |
Operating Activities The $1.3 billion decrease in net cash provided was primarily driven by a decrease in cash collected from customers, an increase in cash paid for income taxes and an increase in spend on inventory. The decrease in net cash provided was partially offset by timing of payments to vendors. The decrease in cash collected from customers was primarily related to timing of sales, slower collections and supply chain challenges, as compared to the prior fiscal year. The increase in cash paid for income taxes was due to estimated income tax payments including a cash deposit associated with the U.S. Tax Court Opinion, and the increase in spend for inventory was due to inflationary impacts to direct labor and material costs. For more information about the tax cash deposit paid, refer to Note 13 of the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Investing Activities The $1.8 billion increase in net cash used was primarily attributable to an increase in cash paid for acquisitions of $1.8 billion as compared to fiscal year 2022. For more information on the acquisitions, refer to Note 3 of the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Financing Activities The $376 million decrease in net cash used as compared to fiscal year 2022 was primarily driven by a decrease of $1.9 billion in share repurchases, offset by debt transactions. In the fourth quarter of fiscal year 2023, the Company issued two tranches of USD-denominated Senior Notes resulting in cash proceeds of approximately $2.0 billion, net of discounts and issuance costs. The Company used the net proceeds to repay in full the ¥297 billion Fiscal 2023 Loan Agreement discussed below for $2.3 billion of total consideration. In the second quarter of fiscal year 2023, the Company issued four tranches of Euro-denominated Senior Notes for approximately
$3.4 billion. The Company used a portion of the net proceeds to repay at maturity €750 million of Medtronic Luxco Senior Notes for $772 million of total consideration in December 2022 and €2.8 billion of Medtronic Luxco Senior Notes for $2.9 billion of total consideration in March 2023. In the first quarter of fiscal year 2023, the Company issued short-term borrowings of approximately $2.3 billion under the Fiscal 2023 Loan Agreement and used the proceeds to fund the early redemption of senior notes for total consideration of $2.3 billion. For more information on the issuance and redemption of senior notes and the Term Loan, refer to the Debt and Capital section.
Debt and Capital
Our capital structure consists of equity and interest-bearing debt. We primarily utilize unsecured senior debt obligations to meet our financing needs and, to a lesser extent, bank borrowings. From time to time, we may repurchase our outstanding debt obligations in the open market or through privately negotiated transactions.
Total debt at April 28, 2023 was $24.4 billion, as compared to $24.1 billion at April 29, 2022. The increase in total debt was driven by issuance of Euro-denominated and USD-denominated Senior Notes, and fluctuations in exchange rates, offset by repayment of Euro-denominated and USD-denominated Senior Notes.
In May 2022, we entered into a term loan agreement (Fiscal 2023 Loan Agreement) with Mizuho Bank, Ltd. for an aggregate principal amount of up to ¥300 billion with a term of 364 days. In May and June 2022, Medtronic Luxco borrowed an aggregate of ¥297 billion, or approximately $2.3 billion, of the term loan, under the Fiscal 2023 Loan Agreement. The Company used the net proceeds of the borrowings to fund the early redemption of $1.9 billion of Medtronic Inc. Senior Notes for $1.9 billion of total consideration, and $368 million of Medtronic Luxco Senior Notes for $376 million of total consideration. The Company recognized a total loss on debt extinguishment of $53 million within interest expense, net in the consolidated statements of income during fiscal year 2023, which primarily included cash premiums and accelerated amortization of deferred financing costs and debt discounts and premiums. During the fourth quarter of fiscal year 2023, the Company repaid the term loan in full, including interest.
In September 2022, we issued four tranches of Euro-denominated Senior Notes with an aggregate principal of €3.5 billion, with maturities ranging from fiscal year 2026 to 2035, resulting in cash proceeds of approximately $3.4 billion, net of discounts and issuance costs. The Company used the net proceeds to repay at maturity €750 million of 0.000% Medtronic Luxco Senior Notes for $772 million of total consideration in December 2022 and €1.5 billion of 0.375% Medtronic Luxco Senior Notes and €1.25 billion of 0.000% Medtronic Luxco Senior Notes for $2.9 billion of total consideration in March 2023.
In March 2023, Medtronic Luxco issued two tranches of USD-denominated Senior Notes with an aggregate principal of $2.0 billion, with maturities ranging from 2028 to 2033, resulting in cash proceeds of approximately $2.0 billion, net of discounts and issuance costs. The Company used the net proceeds supplemented by additional cash to repay the ¥297 billion Fiscal 2023 Loan Agreement discussed above for $2.3 billion of total consideration.
We repurchase our ordinary shares on occasion as part of our focus on returning value to our shareholders. In March 2019, the Company's Board of Directors authorized the repurchase of $6.0 billion of the Company's ordinary shares. There is no specific time period associated with these repurchase authorizations. During fiscal years 2023 and 2022, we repurchased a total of 6 million and 22 million shares, respectively, under these programs at an average price of $91.31 and $113.11, respectively. At April 28, 2023, we had approximately $2.4 billion remaining under the share repurchase program authorized by our Board of Directors.
For more information on credit arrangements, see Note 6 of the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Liquidity
Our liquidity sources at April 28, 2023 included $1.5 billion of cash and cash equivalents and $6.4 billion of current investments. Additionally, we maintain commercial paper programs and a Credit Facility.
Our investments primarily include available-for-sale debt securities, including U.S. and non-U.S. government and agency securities, corporate debt securities, mortgage-backed securities, certificates of deposit, and other asset-backed securities. See Note 5 to the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K for additional information regarding fair value measurements.
We maintain multicurrency commercial paper programs for short-term financing, which allow us to issue unsecured commercial paper notes on a private placement basis up to a maximum aggregate amount outstanding at any time of $3.5 billion. At both April 28, 2023 and April 29, 2022, we had no commercial paper outstanding. The issuance of commercial paper reduces the amount of credit available under our existing line of credit, as explained below.
We also have a $3.5 billion five-year syndicated credit facility (Credit Facility), which expires in December 2027. At each anniversary date of the Credit Facility, we can request a one-year extension of the maturity date. The Credit Facility provides backup funding for the commercial paper programs and may also be used for general corporate purposes. The Credit Facility provides us with the ability to increase our borrowing capacity by an additional $1.0 billion at any time during the term of the agreement. At April 28, 2023 and April 29, 2022, no amounts were outstanding under the Credit Facility.
Interest rates on advances of our Credit Facility are determined by a pricing matrix based on our long-term debt ratings assigned by Standard & Poor's Ratings Services (S&P) and Moody's Investors Service (Moody’s). Facility fees are payable on the Credit Facility and are determined in the same manner as the interest rates. We are in compliance with all covenants related to the Credit Facility.
The following table is a summary of our S&P and Moody's long-term debt ratings and short-term debt ratings:
| | | | | | | | | | | | | | |
| | Agency Rating (1) |
| | April 28, 2023 | | April 29, 2022 |
| Standard & Poor's Ratings Services | | | | |
| Long-term debt | | A | | A |
| Short-term debt | | A-1 | | A-1 |
| Moody's Investors Service | | | | |
| Long-term debt | | A3 | | A3 |
| Short-term debt | | P-2 | | P-2 |
(1) Agency ratings are subject to change, and there may be no assurance that an agency will continue to provide ratings and/or maintain its current ratings. A security rating is not a recommendation to buy, sell or hold securities, and may be subject to revision or withdrawal at any time by the rating agency, and each rating should be evaluated independently of any other rating.
S&P and Moody's long-term debt ratings and short-term debt ratings at April 28, 2023 were unchanged as compared to the ratings at April 29, 2022. We do not expect the S&P and Moody's ratings to have a significant impact on our liquidity or future flexibility to access additional liquidity given our balance sheet, Credit Facility, and related commercial paper programs.
Contractual Obligations and Cash Requirements
We have future contractual obligations and other minimum commercial commitments that are entered into in the normal course of business, some of which are recorded in our consolidated balance sheet. We believe our off-balance sheet arrangements do not have a material current or anticipated future effect on our consolidated earnings, financial position, and/or cash flows.
Presented below is a summary of our off-balance sheet contractual obligations and other minimum commercial commitments at April 28, 2023, as well as long-term contractual obligations reflected in the balance sheet at April 28, 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Maturity by Fiscal Year |
| (in millions) | | Total | | 2024 | | 2025 | | 2026 | | 2027 | | 2028 | | Thereafter |
| Contractual obligations related to off-balance sheet arrangements: | | | | | | | | | | | | | | |
Commitments to fund minority investments, milestone payments, and royalty obligations(1) | | $ | 397 | | | $ | 155 | | | $ | 91 | | | $ | 93 | | | $ | 38 | | | $ | 18 | | | $ | 3 | |
Interest payments(2) | | 7,476 | | | 531 | | | 522 | | | 522 | | | 505 | | | 487 | | | 4,908 | |
Other(3) | | 2,022 | | | 688 | | | 396 | | | 300 | | | 278 | | | 239 | | | 122 | |
| | | | | | | | | | | | | | |
Contractual obligations reflected in the balance sheet(4): | | | | | | | | | | | | | | |
Debt obligations(5) | | $ | 24,553 | | | $ | 20 | | | $ | 7 | | | $ | 2,750 | | | $ | 1,652 | | | $ | 1,005 | | | $ | 19,119 | |
| Operating leases | | 1,160 | | | 204 | | | 171 | | | 144 | | | 121 | | | 94 | | | 426 | |
Contingent consideration(6) | | 206 | | | 28 | | | 49 | | | 73 | | | 56 | | | — | | | — | |
Tax obligations(7) | | 1,320 | | | 330 | | | 440 | | | 550 | | | — | | | — | | | — | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
(1)Includes commitments related to the funding of minority investments, estimated milestone payments, and royalty obligations. While it is not certain if and/or when payments will be made, the maturity dates included in the table reflect our best estimates.
(2)Includes the contractual interest payments on our outstanding debt and excludes the impacts of debt premium and discount amortization. See Note 6 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information on our debt agreements.
(3)Includes inventory purchase commitments, research and development, and other arrangements that are legally binding and specify minimum purchase quantities or spending amounts. These purchase commitments do not exceed our projected requirements and are in the normal course of business. Excludes open purchase orders with a remaining term of less than one year.
(4)Excludes defined benefit plan obligations, guarantee obligations, uncertain tax positions, non-current tax liabilities, and litigation settlements for which we cannot make a reliable estimate of the period of cash settlement. For further information, see Notes 13, 15, and 18 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
(5)Includes the current and non-current portion of our Senior Notes and bank borrowings. Excludes debt premium and discounts and commercial paper. See Note 6 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information on our debt agreements.
(6)Includes the fair value of our current and non-current portions of contingent consideration. While it is not certain if and/or when payments will be made, the maturity dates included in this table reflect our best estimates.
(7)Represents the tax obligations associated with the transition tax that resulted from U.S. Tax Reform. The transition tax will be paid over an eight-year period and will not accrue interest. See Note 13 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for further information.
In the normal course of business, we periodically enter into agreements that require us to indemnify customers or suppliers for specific risks, such as claims for injury or property damage arising as a result of our products or the negligence of our personnel or claims alleging that our products infringe third-party patents or other intellectual property. Our maximum exposure under these indemnification provisions is unable to be estimated, and we have not accrued any liabilities within our consolidated financial statements or included any indemnification provisions in the table above. Historically, we have not experienced significant losses on these types of indemnification agreements.
Note 18 to the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K provides information regarding amounts we have accrued related to legal matters. In accordance with U.S. GAAP, we record a liability in our consolidated financial statements for these matters when a loss is known or considered probable and the amount can be reasonably estimated. Actual settlements may be different than estimated and could have a material effect on our consolidated earnings, financial position, and/or cash flows.
We record tax liabilities in our consolidated financial statements for amounts that we expect to repatriate from subsidiaries (to the extent the repatriation would be subject to tax); however, no tax liabilities are recorded for amounts we consider to be permanently reinvested. We expect to have access to the majority of our cash flows in the future. In addition, we continue to evaluate our legal entity structure supporting our business operations, and to the extent such evaluation results in a change to our overall business structure, we may be required to accrue for additional tax obligations.
Beyond the contractual obligations and other minimum commercial commitments outlined above, we have recurring cash requirements arising from the normal operation of our business that include capital expenditures, research and developments costs, and other operational costs.
We believe our balance sheet and liquidity provide us with flexibility, and our cash, cash equivalents, current investments, Credit Facility and related commercial paper programs, as well as our ability to generate operating cash flows, will satisfy our current and future contractual obligations and cash requirements. We regularly review our capital needs and consider various investing and financing alternatives to support our requirements.
ACQUISITIONS
EOFlow Co. Ltd Acquisition
Subsequent to fiscal year 2023, on May 25, 2023, the Company entered into a set of definitive agreements to acquire EOFlow Co. Ltd. (EOFlow), manufacturer of the EOPatch device – a tubeless, wearable and fully disposable insulin delivery device. The acquisition expands the Diabetes segment portfolio of products. To the extent that all the public shares participate in the tender offer, the total consideration for the acquisition of the shares in EOFlow would be KRW 971 billion, or $738 million, at exchange rates on May 25, 2023. The acquisition is expected to close in the second half of calendar year 2023 subject to the satisfaction of the minimum tender condition and certain customary closing conditions, including receipt of required regulatory clearances.
Additional information regarding acquisitions is included in Note 3 of the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" within this Annual Report on Form 10-K.
CRITICAL ACCOUNTING ESTIMATES
We have used various accounting policies to prepare the consolidated financial statements in accordance with U.S. GAAP. Our significant accounting policies are disclosed in Note 1 to the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K.
The preparation of the consolidated financial statements, in conformity with U.S. GAAP, requires us to use judgment in making estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, and expenses. These estimates reflect our best judgment about economic and market conditions and the potential effects on the valuation and/or carrying value of assets and liabilities based upon relevant information available. We base our estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
Our critical accounting estimates include the following:
Revenue Recognition The Company sells its products through direct sales representatives and independent distributors. Additionally, a portion of the Company's revenue is generated from consignment inventory maintained at hospitals and royalty and intellectual property arrangements. The Company recognizes revenue when control is transferred to the customer. For products sold through direct sales representatives and independent distributors, control is transferred upon shipment or upon delivery, based on the contract terms and legal requirements. For consignment inventory, control is transferred when the product is used or implanted. Payment terms vary depending on the country of sale, type of customer, and type of product.
The amount of revenue recognized reflects sales rebates and returns, which are estimated based on sales terms, historical experience, and trend analysis. In estimating rebates, the Company considers the lag time between the point of sale and the payment of the rebate claim, the stated rebate rates, and other relevant information. The Company records adjustments to rebates and returns reserves as increases or decreases of revenue.
Litigation Contingencies We are involved in a number of legal actions involving product liability, intellectual property and commercial disputes, shareholder related matters, environmental proceedings, tax disputes, and governmental proceedings and investigations. The outcomes of these legal actions are not completely within our control and may not be known for prolonged periods of time. In some actions, the enforcement agencies or private claimants seek damages, as well as other civil or criminal remedies (including injunctions barring the sale of products that are the subject of the proceeding), that could require significant expenditures or result in lost revenues or limit our ability to conduct business in the applicable jurisdictions. Estimating probable losses from our litigation and governmental proceedings is inherently difficult, particularly when the matters are in early procedural stages, with incomplete scientific facts or legal discovery; involve unsubstantiated or indeterminate claims for damages; potentially involve penalties, fines, or punitive damages; or could result in a change in business practice. The Company records a liability in the consolidated financial statements for loss contingencies when a loss is known or considered probable, and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. Our significant legal proceedings
are discussed in Note 18 to the consolidated financial statements in "Item 8. Financial Statements and Supplementary Data" in this Annual Report on Form 10-K.
Income Tax Reserves We establish reserves when, despite our belief that our tax return positions are fully supportable, we believe that certain positions are likely to be challenged and that we may or may not prevail. Under U.S. GAAP, if we determine that a tax position is more likely than not of being sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that is greater than 50 percent likely of being realized upon settlement. We presume that all tax positions will be examined by a taxing authority with full knowledge of all relevant information. The calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax regulations in a multitude of jurisdictions across our global operations. We regularly monitor our tax positions and tax liabilities. We reevaluate the technical merits of our tax positions and recognize an uncertain tax benefit, or derecognize a previously recorded tax benefit, when there is (i) a completion of a tax audit, (ii) effective settlement of an issue, (iii) a change in applicable tax law including a tax case or legislative guidance, or (iv) the expiration of the applicable statute of limitations. These reserves are subject to a high degree of estimation and management judgment. Although we believe that we have adequately reserved for liabilities resulting from tax assessments by taxing authorities, positions taken by these tax authorities could have a material impact on our effective tax rate, consolidated earnings, financial position and/or cash flows.
Valuation of Intangible Assets and Goodwill When we acquire a business, the assets acquired and liabilities assumed are recorded at their respective fair values at the acquisition date. Goodwill is the excess of the purchase price over the estimated fair value of net assets of acquired businesses. Intangible assets primarily include patents, trademarks, tradenames, customer relationships, purchased technology, and in-process research and development. Determining the fair value of intangible assets acquired as part of a business combination requires us to make significant estimates. These estimates include the amount and timing of projected future cash flows of each project or technology, the discount rate used to discount those cash flows to present value, and the assessment of the asset’s life cycle. The estimates could be impacted by legal, technical, regulatory, economic, and competitive risks.
The test for impairment of goodwill requires us to make several estimates related to projected future cash flows to determine the fair value of the goodwill reporting units. Our estimates associated with the goodwill impairment test are considered critical due to the amount of goodwill recorded on our consolidated balance sheets and the judgment required in determining fair value. We assess the impairment of goodwill at the reporting unit level annually as of the first day of the third quarter and whenever an event occurs or circumstances change that would indicate that the carrying amount may be impaired.
We also test definite-lived intangible assets for impairment when an event occurs or circumstances change that would indicate the carrying amount of the assets or asset group may be impaired. We assess the impairment of indefinite-lived intangible assets annually in the third quarter and whenever an event occurs or circumstances change that would indicate that the carrying amount may be impaired.
Our tests for goodwill and intangible assets are based on future cash flows that require significant judgment with respect to future revenue and expense growth rates, appropriate discount rates, asset groupings, and other assumptions and estimates. We use estimates that are consistent with the highest and best use of the assets based on a market participant's view of the assets being evaluated. Actual results may differ from our estimates due to a number of factors including, among others, changes in competitive conditions, timing of regulatory approval, results of clinical trials, changes in worldwide economic conditions, and fluctuations in currency exchange rates.
NEW ACCOUNTING PRONOUNCEMENTS
Information regarding new accounting pronouncements is included in Note 1 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
SUPPLEMENTAL GUARANTOR FINANCIAL INFORMATION
Medtronic plc and Medtronic Global Holdings S.C.A. (Medtronic Luxco), a wholly-owned subsidiary guarantor, each have provided full and unconditional guarantees of the obligations of Medtronic, Inc., a wholly-owned subsidiary issuer, under the Senior Notes (Medtronic Senior Notes) and full and unconditional guarantees of the obligations of Covidien International Finance S.A. (CIFSA), a wholly-owned subsidiary issuer, under the Senior Notes (CIFSA Senior Notes). The guarantees of the CIFSA Senior Notes are in addition to the guarantees of the CIFSA Senior Notes by Covidien Ltd. and Covidien Group Holdings Ltd., both of which are wholly-owned subsidiary guarantors of the CIFSA Senior Notes. Medtronic plc and Medtronic, Inc. each have provided a full and unconditional guarantee of the obligations of Medtronic Luxco under the Senior Notes (Medtronic Luxco Senior Notes). The following is a summary of these guarantees:
Guarantees of Medtronic Senior Notes
•Parent Company Guarantor - Medtronic plc
•Subsidiary Issuer - Medtronic, Inc.
•Subsidiary Guarantor - Medtronic Luxco
Guarantees of Medtronic Luxco Senior Notes
•Parent Company Guarantor - Medtronic plc
•Subsidiary Issuer - Medtronic Luxco
•Subsidiary Guarantor - Medtronic, Inc.
Guarantees of CIFSA Senior Notes
•Parent Company Guarantor - Medtronic plc
•Subsidiary Issuer - CIFSA
•Subsidiary Guarantors - Medtronic Luxco, Covidien Ltd., and Covidien Group Holdings Ltd. (CIFSA Subsidiary Guarantors)
The following tables present summarized financial information for the fiscal year ended April 28, 2023 for the obligor groups of Medtronic and Medtronic Luxco Senior Notes, and CIFSA Senior Notes. The obligor group consists of the parent company guarantor, subsidiary issuer, and subsidiary guarantors for the applicable senior notes. The summarized financial information is presented after elimination of (i) intercompany transactions and balances among the guarantors and issuers and (ii) equity in earnings from and investments in any subsidiary that is a non-guarantor or issuer.
The summarized results of operations information for the fiscal year ended April 28, 2023 was as follows:
| | | | | | | | | | | |
| (in millions) | Medtronic & Medtronic Luxco Senior Notes (1) | | CIFSA Senior Notes (2) |
| Net sales | $ | 2,847 | | | $ | — | |
| Operating profit (loss) | 608 | | | (407) | |
| Loss before income taxes | (913) | | | (1,868) | |
| Net loss attributable to Medtronic | (1,705) | | | (1,861) | |
The summarized balance sheet information for the fiscal year ended April 28, 2023 was as follows:
| | | | | | | | | | | |
| (in millions) | Medtronic & Medtronic Luxco Senior Notes (1) | | CIFSA Senior Notes (2) |
Total current assets(3) | $ | 23,198 | | | $ | 8,344 | |
Total noncurrent assets(4) | 5,897 | | | 3 | |
Total current liabilities(5) | 33,854 | | | 25,184 | |
Total noncurrent liabilities(6) | 59,624 | | | 66,449 | |
| Noncontrolling interests | 182 | | | 182 | |
(1)The Medtronic Senior Notes and Medtronic Luxco Senior Notes obligor group consists of the following entities: Medtronic plc, Medtronic Luxco, and Medtronic, Inc. Refer to the guarantee summary above for further details.
(2)The CIFSA Senior Notes obligor group consists of the following entities: Medtronic plc, Medtronic Luxco, CIFSA, and CIFSA Subsidiary Guarantors. Please refer to the guarantee summary above for further details.
(3)Includes receivables due from non-guarantor subsidiaries of $22.5 billion and $8.3 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(4)Includes loans receivable due from non-guarantor subsidiaries of $20 million for Medtronic & Medtronic Luxco Senior Notes. No loans receivable due from non-guarantor subsidiaries for CIFSA Senior Notes.
(5)Includes payables due to non-guarantor subsidiaries of $31.8 billion and $25.0 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(6)Includes loans payable due to non-guarantor subsidiaries of $33.1 billion and $46.7 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
CURRENCY EXCHANGE RATE RISK
Due to the global nature of our operations, we are exposed to currency exchange rate changes, which may cause fluctuations in earnings and cash flows. Fluctuations in the currency exchange rates of currency exposures that are unhedged, such as in certain emerging markets, may result in future earnings and cash flow volatility. The gross notional amount of all currency exchange rate derivative instruments outstanding at April 28, 2023 and April 29, 2022 was $22.0 billion and $13.8 billion, respectively. At April 28, 2023, these contracts were in a net unrealized gain position of $132 million. Additional information regarding our currency exchange rate derivative instruments is included in Note 7 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
A sensitivity analysis of changes in the fair value of all currency exchange rate derivative contracts at April 28, 2023 and April 29, 2022 indicates that, if the U.S. dollar uniformly strengthened/weakened by 10 percent against all currencies, it would have the following impact on the fair value of these contracts:
| | | | | | | | | | | | | | |
| | Increase (decrease) |
| (in millions) | | April 28, 2023 | | April 29, 2022 |
| 10% appreciation in the U.S. dollar | | $ | 1,548 | | | $ | 903 | |
| 10% depreciation in the U.S. dollar | | (1,548) | | | (903) | |
Any gains and losses on the fair value of derivative contracts would generally be offset by gains and losses on the underlying transactions. These offsetting gains and losses are not reflected in the above analysis.
INTEREST RATE RISK
We are subject to interest rate risk on our short-term investments and our borrowings. We manage interest rate risk in the aggregate, while focusing on our immediate and intermediate liquidity needs. Our debt portfolio at April 28, 2023 was comprised of debt predominantly denominated in U.S. dollars and Euros, of which substantially all is fixed rate debt. We are also exposed to interest rate changes affecting our investments in interest rate sensitive instruments, which include our marketable debt securities.
A sensitivity analysis of the impact on our interest rate-sensitive financial instruments of a hypothetical 10 basis point change in interest rates, as compared to interest rates at April 28, 2023 and April 29, 2022, would have the following impact on the fair value of these instruments:
| | | | | | | | | | | | | | |
| | Increase (decrease) |
| (in millions) | | April 28, 2023 | | April 29, 2022 |
| 10 basis point increase in interest rates | | $ | 63 | | | $ | 53 | |
| 10 basis point decrease in interest rates | | (63) | | | (53) | |
For a discussion of current market conditions and the impact on our financial condition and results of operations, see the “Liquidity” section of the Management's Discussion and Analysis in "Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations" in this Annual Report on Form 10-K. For additional discussion of market risk, see Notes 5 and 7 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Item 8. Financial Statements and Supplementary Data
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Medtronic plc
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Medtronic plc and its subsidiaries (the “Company”) as of April 28, 2023 and April 29, 2022, and the related consolidated statements of income, of comprehensive income, of equity and of cash flows for each of the three years in the period ended April 28, 2023, including the related notes and schedule of valuation and qualifying accounts for each of the three years in the period ended April 28, 2023 appearing under Item 15 (a)(1) (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of April 28, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of April 28, 2023 and April 29, 2022, and the results of its operations and its cash flows for each of the three years in the period ended April 28, 2023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of April 28, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Income Tax Reserve for the Uncertain Tax Position Related to Puerto Rico Manufacturing
As described in Notes 13 and 18 to the consolidated financial statements, management records reserves for uncertain tax positions related to unresolved matters with the Internal Revenue Service (IRS) and other taxing authorities. A remaining unresolved issue with the IRS relates to the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico, which is one of the Company's manufacturing sites. These reserves are subject to a high degree of estimation and management judgment. During fiscal year 2023, management recognized an increase of $764 million associated with the August 18, 2022 U.S. Tax Court (Tax Court) Opinion on the previously disclosed litigation related to the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico for fiscal years 2005 and 2006 (Opinion). While the Opinion rejected the IRS’s position and the Tax Court determined the methodology advanced by Medtronic was appropriate for purposes of determining the intercompany royalty rate between Puerto Rico and the U.S., the Tax Court determined that the royalty rate should be higher, thereby increasing income allocated to the U.S. and consequently subject to U.S. tax. This case relates only to fiscal years 2005 and 2006. The Opinion remains subject to appeal by either or both parties. The Company has assumed the Tax Court findings will be applied for all years following fiscal year 2006. Total reserves relating to uncertain tax positions as of April 28, 2023 were $2.682 billion, of which the Puerto Rico manufacturing reserve makes up a significant portion.
The principal considerations for our determination that performing procedures relating to the income tax reserve for the uncertain tax position related to Puerto Rico manufacturing is a critical audit matter are (i) the significant judgment by management when determining the reserve, including a high degree of estimation uncertainty relative to the unresolved issue with the IRS involving one of the Company’s manufacturing sites; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating audit evidence related to management’s measurement of the income tax reserve for the uncertain tax position related to Puerto Rico manufacturing, as the nature of the evidence is often highly subjective; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the recognition of the income tax reserves for uncertain tax positions, as well as controls over measurement of the reserve for the uncertain tax position related to Puerto Rico manufacturing. These procedures also included, among others (i) testing management’s process for determining the reserve and (ii) evaluating the reasonableness of the measurement of the reserve, including underlying assumptions used in management’s calculations. Evaluating the reasonableness of the measurement of the reserve included evaluating whether the methodology and assumptions used by the Company were consistent with the Tax Court’s ruling. Professionals with specialized skill and knowledge were used to assist in evaluating the application of tax laws related to the ruling and the underlying assumptions used in management’s calculations.
| | |
/s/ PricewaterhouseCoopers LLP |
Minneapolis, Minnesota |
| June 22, 2023 |
We have served as the Company’s auditor since 1963.
Medtronic plc
Consolidated Statements of Income | | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions, except per share data) | 2023 | | 2022 | | 2021 |
| Net sales | $ | 31,227 | | | $ | 31,686 | | | $ | 30,117 | |
| Costs and expenses: | | | | | |
| Cost of products sold, excluding amortization of intangible assets | 10,719 | | | 10,145 | | | 10,483 | |
| Research and development expense | 2,696 | | | 2,746 | | | 2,493 | |
| Selling, general, and administrative expense | 10,415 | | | 10,292 | | | 10,148 | |
| Amortization of intangible assets | 1,698 | | | 1,733 | | | 1,783 | |
| Restructuring charges, net | 375 | | | 60 | | | 293 | |
| Certain litigation charges, net | (30) | | | 95 | | | 118 | |
| Other operating (income) expense, net | (131) | | | 862 | | | 315 | |
| Operating profit | 5,485 | | | 5,752 | | | 4,484 | |
| Other non-operating income, net | (515) | | | (318) | | | (336) | |
| Interest expense, net | 636 | | | 553 | | | 925 | |
| Income before income taxes | 5,364 | | | 5,517 | | | 3,895 | |
| Income tax provision | 1,580 | | | 456 | | | 265 | |
| Net income | 3,784 | | | 5,062 | | | 3,630 | |
| Net income attributable to noncontrolling interests | (26) | | | (22) | | | (24) | |
| Net income attributable to Medtronic | $ | 3,758 | | | $ | 5,039 | | | $ | 3,606 | |
| Basic earnings per share | $ | 2.83 | | | $ | 3.75 | | | $ | 2.68 | |
| Diluted earnings per share | $ | 2.82 | | | $ | 3.73 | | | $ | 2.66 | |
| Basic weighted average shares outstanding | 1,329.8 | | | 1,342.4 | | | 1,344.9 | |
| Diluted weighted average shares outstanding | 1,332.8 | | | 1,351.4 | | | 1,354.0 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Statements of Comprehensive Income
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Net income | $ | 3,784 | | | $ | 5,062 | | | $ | 3,630 | |
| | | | | |
| Other comprehensive (loss) income, net of tax: | | | | | |
| Unrealized (loss) gain on investment securities | (49) | | | (301) | | | 92 | |
| Translation adjustment | (240) | | | (2,086) | | | 1,699 | |
| Net investment hedge | (596) | | | 2,299 | | | (1,694) | |
| Net change in retirement obligations | 32 | | | 574 | | | 505 | |
| Unrealized (loss) gain on cash flow hedges | (381) | | | 727 | | | (519) | |
| Other comprehensive (loss) income | (1,234) | | | 1,213 | | | 83 | |
| Comprehensive income including noncontrolling interests | 2,549 | | | 6,274 | | | 3,713 | |
| Comprehensive income attributable to noncontrolling interests | (26) | | | (16) | | | (32) | |
| Comprehensive income attributable to Medtronic | $ | 2,524 | | | $ | 6,258 | | | $ | 3,681 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Balance Sheets | | | | | | | | | | | | | | |
| (in millions) | | April 28, 2023 | | April 29, 2022 |
| ASSETS | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 1,543 | | | $ | 3,714 | |
| Investments | | 6,416 | | | 6,859 | |
Accounts receivable, less allowances and credit losses of $176 and $230, respectively | | 5,998 | | | 5,551 | |
| Inventories, net | | 5,293 | | | 4,616 | |
| Other current assets | | 2,425 | | | 2,318 | |
| Total current assets | | 21,675 | | | 23,059 | |
| Property, plant, and equipment, net | | 5,569 | | | 5,413 | |
| Goodwill | | 41,425 | | | 40,502 | |
| Other intangible assets, net | | 14,844 | | | 15,595 | |
| Tax assets | | 3,477 | | | 3,403 | |
| Other assets | | 3,959 | | | 3,008 | |
| Total assets | | $ | 90,948 | | | $ | 90,981 | |
| LIABILITIES AND EQUITY | | | | |
| Current liabilities: | | | | |
| Current debt obligations | | $ | 20 | | | $ | 3,742 | |
| Accounts payable | | 2,662 | | | 2,276 | |
| Accrued compensation | | 1,949 | | | 2,121 | |
| Accrued income taxes | | 840 | | | 704 | |
| Other accrued expenses | | 3,581 | | | 3,551 | |
| Total current liabilities | | 9,051 | | | 12,394 | |
| Long-term debt | | 24,344 | | | 20,372 | |
| Accrued compensation and retirement benefits | | 1,093 | | | 1,113 | |
| Accrued income taxes | | 2,360 | | | 2,087 | |
| Deferred tax liabilities | | 708 | | | 884 | |
| Other liabilities | | 1,727 | | | 1,410 | |
| Total liabilities | | 39,283 | | | 38,260 | |
| Commitments and contingencies (Notes 3, 16, and 18) | | | | |
| Shareholders’ equity: | | | | |
Ordinary shares— par value $0.0001, 2.6 billion shares authorized, 1,330,809,036 and 1,330,743,395 shares issued and outstanding, respectively | | — | | | — | |
| Additional paid-in capital | | 24,590 | | | 24,566 | |
| Retained earnings | | 30,392 | | | 30,250 | |
| Accumulated other comprehensive loss | | (3,499) | | | (2,265) | |
| Total shareholders’ equity | | 51,483 | | | 52,551 | |
| Noncontrolling interests | | 182 | | | 171 | |
| Total equity | | 51,665 | | | 52,722 | |
| Total liabilities and equity | | $ | 90,948 | | | $ | 90,981 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Statements of Equity | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Ordinary Shares | | Additional Paid-in Capital | | Retained
Earnings | | Accumulated
Other
Comprehensive
Loss | | Total
Shareholders’
Equity | | Noncontrolling Interests | | Total Equity |
| (in millions, except per share data) | | Number | | Par Value | | | | | | |
| April 24, 2020 | | 1,341 | | | $ | — | | | $ | 26,165 | | | $ | 28,132 | | | $ | (3,560) | | | $ | 50,737 | | | $ | 135 | | | $ | 50,872 | |
| Net income | | — | | | — | | | — | | | 3,606 | | | — | | | 3,606 | | | 24 | | | 3,630 | |
| Other comprehensive income | | — | | | — | | | — | | | — | | | 75 | | | 75 | | | 8 | | | 83 | |
Dividends to shareholders ($2.32 per ordinary share) | | — | | | — | | | — | | | (3,120) | | | — | | | (3,120) | | | — | | | (3,120) | |
| Issuance of shares under stock purchase and award plans | | 8 | | | — | | | 382 | | | — | | | — | | | 382 | | | — | | | 382 | |
| Repurchase of ordinary shares | | (4) | | | — | | | (559) | | | — | | | — | | | (559) | | | — | | | (559) | |
| Stock-based compensation | | — | | | — | | | 344 | | | — | | | — | | | 344 | | | — | | | 344 | |
| Changes to noncontrolling ownership interests | | — | | | — | | | (13) | | | — | | | — | | | (13) | | | 7 | | | (6) | |
| Cumulative effect of change in accounting principle | | — | | | — | | | — | | | (24) | | | — | | | (24) | | | — | | | (24) | |
| April 30, 2021 | | 1,345 | | | $ | — | | | $ | 26,319 | | | $ | 28,594 | | | $ | (3,485) | | | $ | 51,428 | | | $ | 174 | | | $ | 51,602 | |
| Net income | | — | | | — | | | — | | | 5,039 | | | — | | | 5,039 | | | 22 | | | 5,062 | |
| Other comprehensive income (loss) | | — | | | — | | | — | | | — | | | 1,219 | | | 1,219 | | | (6) | | | 1,213 | |
Dividends to shareholders ($2.52 per ordinary share) | | — | | | — | | | — | | | (3,383) | | | — | | | (3,383) | | | — | | | (3,383) | |
| Issuance of shares under stock purchase and award plans | | 7 | | | — | | | 329 | | | — | | | — | | | 329 | | | — | | | 329 | |
| Repurchase of ordinary shares | | (21) | | | — | | | (2,442) | | | — | | | — | | | (2,442) | | | — | | | (2,442) | |
| Stock-based compensation | | — | | | — | | | 359 | | | — | | | — | | | 359 | | | — | | | 359 | |
| Changes to noncontrolling ownership interests | | — | | | — | | | 1 | | | — | | | — | | | 1 | | | (19) | | | (18) | |
| | | | | | | | | | | | | | | | |
| April 29, 2022 | | 1,331 | | | $ | — | | | $ | 24,566 | | | $ | 30,250 | | | $ | (2,265) | | | $ | 52,551 | | | $ | 171 | | | $ | 52,722 | |
| Net income | | — | | | — | | | — | | | 3,758 | | | — | | | 3,758 | | | 26 | | | 3,784 | |
| Other comprehensive (loss) income | | — | | | — | | | — | | | — | | | (1,234) | | | (1,234) | | | — | | | (1,234) | |
Dividends to shareholders ($2.72 per ordinary share) | | — | | | — | | | — | | | (3,616) | | | — | | | (3,616) | | | — | | | (3,616) | |
| Issuance of shares under stock purchase and award plans | | 6 | | | — | | | 236 | | | — | | | — | | | 236 | | | — | | | 236 | |
| Repurchase of ordinary shares | | (6) | | | — | | | (571) | | | — | | | — | | | (571) | | | — | | | (571) | |
| Stock-based compensation | | — | | | — | | | 355 | | | — | | | — | | | 355 | | | — | | | 355 | |
| Changes to noncontrolling ownership interests | | — | | | — | | | 5 | | | — | | | — | | | 5 | | | (15) | | | (10) | |
| April 28, 2023 | | 1,331 | | | $ | — | | | $ | 24,590 | | | $ | 30,392 | | | $ | (3,499) | | | $ | 51,483 | | | $ | 182 | | | $ | 51,665 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Statements of Cash Flows | | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Operating Activities: | | | | | |
| Net income | $ | 3,784 | | | $ | 5,062 | | | $ | 3,630 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | |
| Depreciation and amortization | 2,697 | | | 2,707 | | | 2,702 | |
| Provision for credit losses | 73 | | | 58 | | | 128 | |
| Deferred income taxes | (226) | | | (604) | | | (422) | |
| Stock-based compensation | 355 | | | 359 | | | 344 | |
| Loss on debt extinguishment | 53 | | | — | | | 308 | |
| | | | | |
| Asset impairment charges | — | | | 515 | | | — | |
| | | | | |
| Other, net | 270 | | | 138 | | | 251 | |
| Change in operating assets and liabilities, net of acquisitions and divestitures: | | | | | |
| Accounts receivable, net | (576) | | | (477) | | | (761) | |
| Inventories, net | (939) | | | (560) | | | 78 | |
| Accounts payable and accrued liabilities | 696 | | | 213 | | | 531 | |
| Other operating assets and liabilities | (148) | | | (65) | | | (549) | |
| Net cash provided by operating activities | 6,039 | | | 7,346 | | | 6,240 | |
| Investing Activities: | | | | | |
| Acquisitions, net of cash acquired | (1,867) | | | (91) | | | (994) | |
| | | | | |
| Additions to property, plant, and equipment | (1,459) | | | (1,368) | | | (1,355) | |
| Purchases of investments | (7,514) | | | (9,882) | | | (11,808) | |
| Sales and maturities of investments | 7,343 | | | 9,692 | | | 11,345 | |
| Other investing activities, net | 4 | | | (10) | | | (54) | |
| Net cash used in investing activities | (3,493) | | | (1,659) | | | (2,866) | |
| Financing Activities: | | | | | |
| Change in current debt obligations, net | — | | | — | | | (311) | |
| Proceeds from short-term borrowings (maturities greater than 90 days) | 2,284 | | | — | | | 2,789 | |
| Repayments from short-term borrowings (maturities greater than 90 days) | (2,279) | | | — | | | (2,853) | |
| Issuance of long-term debt | 5,409 | | | — | | | 7,172 | |
| Payments on long-term debt | (6,012) | | | (1) | | | (7,367) | |
| Dividends to shareholders | (3,616) | | | (3,383) | | | (3,120) | |
| Issuance of ordinary shares | 308 | | | 429 | | | 474 | |
| Repurchase of ordinary shares | (645) | | | (2,544) | | | (652) | |
| Other financing activities | (409) | | | 163 | | | (268) | |
| Net cash used in financing activities | (4,960) | | | (5,336) | | | (4,136) | |
| Effect of exchange rate changes on cash and cash equivalents | 243 | | | (231) | | | 215 | |
| Net change in cash and cash equivalents | (2,171) | | | 121 | | | (547) | |
| Cash and cash equivalents at beginning of period | 3,714 | | | 3,593 | | | 4,140 | |
| Cash and cash equivalents at end of period | $ | 1,543 | | | $ | 3,714 | | | $ | 3,593 | |
| Supplemental Cash Flow Information | | | | | |
| Cash paid for: | | | | | |
| Income taxes | $ | 1,548 | | | $ | 996 | | | $ | 1,250 | |
| Interest | 606 | | | 540 | | | 582 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Notes to Consolidated Financial Statements
1. Summary of Significant Accounting Policies
Nature of Operations Medtronic plc (Medtronic or the Company) is the leading global healthcare technology company– alleviating pain, restoring health, and extending life for millions of people around the world. The Company provides innovative products and therapies to serve healthcare systems, physicians, clinicians, and patients. Medtronic was founded in 1949 and is headquartered in Dublin, Ireland.
Principles of Consolidation The consolidated financial statements include the accounts of Medtronic plc, its wholly-owned subsidiaries, entities for which the Company has a controlling financial interest, and variable interest entities for which the Company is the primary beneficiary. Intercompany transactions and balances have been fully eliminated in consolidation. Amounts reported in millions within this annual report are computed based on the amounts in thousands, and therefore, the sum of the components may not equal the total amount reported in millions due to rounding. Additionally, certain columns and rows within tables may not sum due to rounding.
Use of Estimates The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States (U.S.) (U.S. GAAP) requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Estimates are used when accounting for items such as income taxes, contingencies, intangible asset, and liability valuations. Actual results may or may not differ from those estimates.
Fiscal Year-End The Company utilizes a 52/53-week fiscal year, ending the last Friday in April, for the presentation of its consolidated financial statements and related notes thereto at April 28, 2023 and April 29, 2022 and for each of the three fiscal years ended April 28, 2023 (fiscal year 2023), April 29, 2022 (fiscal year 2022), and April 30, 2021 (fiscal year 2021). Fiscal year 2021 was a 53-week year, with the extra week having occurred in the first fiscal month of the first quarter.
Cash Equivalents The Company considers highly liquid investments with maturities of three months or less from the date of purchase to be cash equivalents. These investments are carried at cost, which approximates fair value.
Investments The Company invests in marketable debt and equity securities, investments for which the Company has elected the fair value option, investments that do not have readily determinable fair values, and investments accounted for under the equity method.
Marketable debt securities are classified and accounted for as available-for-sale. These investments are recorded at fair value in the consolidated balance sheets. The change in fair value for available-for-sale securities is recorded, net of taxes, as a component of accumulated other comprehensive loss on the consolidated balance sheets. The Company determines the appropriate classification of its investments in marketable debt securities at the time of purchase and reevaluates such determinations at each balance sheet date. The classification of marketable debt securities as current or long-term is based on the nature of the securities and the availability for use in current operations consistent with the Company's management of its capital structure and liquidity.
Certain of the Company’s investments in marketable equity securities and other securities are long-term, strategic investments in companies that are in various stages of development and are included in other assets on the consolidated balance sheets. Marketable equity securities are recorded at fair value in the consolidated balance sheets. The change in fair value of marketable equity securities is recognized within other non-operating income, net in the consolidated statements of income. At each reporting period, the Company makes a qualitative assessment considering impairment indicators to evaluate whether the investment is impaired. Equity method investments for which the Company has elected the fair value option are valued using a discounted cash flow methodology, taking into consideration various assumptions including discount rate and all pertinent financial information available related to the investees, including historical financial statements and projected future cash flows. Equity investments that do not have readily determinable fair values are measured using the measurement alternative at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for an identical or similar investment of the same issuer. Equity securities accounted for under the equity method are initially recorded at the amount of the Company’s investment and are adjusted each period for the Company’s share of the investee’s income or loss and dividends paid. Securities accounted for under the equity method are reviewed quarterly for changes in circumstance or the occurrence of events that suggest other than temporary impairment has occurred.
Accounts Receivable and Allowance for Doubtful Accounts and Credit Losses The Company grants credit to customers in the normal course of business and maintains an allowance for doubtful accounts for potential credit losses. When evaluating allowances for doubtful accounts, the Company considers various factors, including historical experience and customer-specific information. Uncollectible accounts are written-off against the allowance when it is deemed that a customer account is uncollectible.
Inventories Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. The Company reduces the carrying value of inventories for items that are potentially excess, obsolete, or slow-moving based on changes in customer demand, technology developments, or other economic factors.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Property, Plant, and Equipment Property, plant, and equipment is stated at cost and depreciated over the useful lives of the assets using the straight-line method. Additions and improvements that extend the lives of the assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. The Company assesses property, plant, and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of property, plant, and equipment asset groupings may not be recoverable. The cost of interest that is incurred in connection with significant ongoing construction projects is capitalized using a weighted average interest rate. These costs are included in property, plant, and equipment and amortized over the useful life of the related asset. Upon retirement or disposal of property, plant, and equipment, the costs and related amounts of accumulated depreciation or amortization are eliminated from the asset and accumulated depreciation accounts. The difference, if any, between the net asset value and the proceeds, is recognized in earnings.
Goodwill and Intangible Assets Goodwill is the excess of the purchase price over the estimated fair value of net assets of acquired businesses. The Company assesses goodwill for impairment annually in the third quarter of the fiscal year and whenever an event occurs or circumstances change that would indicate the carrying amount may be impaired. Impairment testing for goodwill is performed at a reporting unit level. The test for impairment of goodwill requires the Company to make several estimates related to projected future cash flows to determine the fair value of the goodwill reporting units. The Company calculates the excess of each reporting unit's fair value over its carrying amount, including goodwill, utilizing a discounted cash flow analysis. Internal operational budgets and long-range strategic plans are used as a basis for the cash flow analysis. The Company also utilizes assumptions for working capital, capital expenditures, and terminal growth rates. The discount rate applied to the cash flow analysis is based on the weighted average cost of capital (“WACC”) for each reporting unit. An impairment loss is recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit.
Intangible assets include patents, trademarks, tradenames, customer relationships, purchased technology, and in-process research and development (IPR&D). Intangible assets with a definite life are amortized on a straight-line basis with estimated useful lives typically ranging from three to 20 years. Amortization is recognized within amortization of intangible assets in the consolidated statements of income. Intangible assets with a definite life are tested for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset group, which includes intangible assets, may not be recoverable. When events or changes in circumstances indicate that the carrying amount of an asset group, which includes intangible assets, may not be recoverable, the Company calculates the excess of an asset group's carrying value over its undiscounted future cash flows. If the carrying value is not recoverable, an impairment loss is recognized based on the amount by which the carrying value exceeds the fair value. The fair value of an asset group, which includes intangible assets, is estimated by utilizing a discounted cash flow analysis.
Acquired IPR&D represents the fair value assigned to those research and development projects that were acquired in a business combination for which the related products have not received regulatory approval and have no alternative future use. IPR&D is capitalized at its fair value as an indefinite-lived intangible asset, and any development costs incurred after the acquisition are expensed as incurred. The fair value of IPR&D is determined by estimating the future cash flows of each project and discounting the net cash flows back to their present values. Upon achieving regulatory approval or commercial viability for the related product, the indefinite-lived intangible asset is accounted for as a definite-lived asset and is amortized on a straight-line basis over the estimated useful life. If the project is not completed or is terminated or abandoned, the Company may have an impairment related to the IPR&D, which is charged to expense. Indefinite-lived intangible assets are tested for impairment annually in the third quarter of the fiscal year and whenever events or changes in circumstances indicate that the carrying amount may be impaired. Impairment is calculated as the excess of the asset’s carrying value over its fair value. Fair value is generally determined using a discounted future cash flow analysis. IPR&D with no alternative future use acquired outside of a business combination is expensed immediately.
Contingent Consideration Certain of the Company’s business combinations involve potential payment or receipt of future consideration that is contingent upon the achievement of certain product development milestones and/or contingent on the acquired business reaching certain performance milestones. The Company records contingent consideration at fair value at the date of acquisition or divestiture based on the consideration expected to be transferred, estimated as the probability-weighted future cash flows, discounted back to present value. The fair value of contingent consideration is measured using projected payment dates, discount rates, probabilities of payment, and projected revenues (for revenue-based considerations). Projected revenues are based on the Company’s most recent internal operational budgets and long-range strategic plans. The discount rate used is determined at the time of measurement in accordance with accepted valuation methodologies. Changes in projected revenues, probabilities of payment, discount rates, and projected payment dates may result in adjustments to the fair value measurements. Contingent consideration is remeasured each reporting period using Level 3 inputs, and the change in fair value, including accretion for the passage of time, is recognized as income or expense within other operating (income) expense, net in the consolidated statements of income. Contingent consideration payments made or received soon after the acquisition date are classified as investing activities in the consolidated statements of cash flows. Contingent consideration payments not made or received soon after the acquisition date that are related to the acquisition date fair value are reported as financing activities in the consolidated statements of cash flows, and amounts paid or received in excess of the original acquisition date fair value are reported as operating activities in the consolidated statements of cash flows.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Self-Insurance The Company self-insures the majority of its insurable risks, including medical and dental costs, disability coverage, physical loss to property, business interruptions, workers’ compensation, comprehensive general, and product liability. Insurance coverage is obtained for risks required to be insured by law or contract. The Company uses claims data and historical experience, as applicable, to estimate liabilities associated with the exposures that the Company has self-insured.
Retirement Benefit Plan Assumptions The Company sponsors various retirement benefit plans, including defined benefit pension plans, post-retirement medical plans, defined contribution savings plans, and termination indemnity plans, covering substantially all U.S. employees and many employees outside the U.S. See Note 15 for assumptions used in determining pension and post-retirement benefit costs and liabilities.
Derivatives The Company recognizes all derivative financial instruments in its consolidated financial statements at fair value in accordance with authoritative guidance on derivatives and hedging, and presents assets and liabilities associated with derivative financial instruments on a gross basis in the consolidated financial statements. For derivative instruments that are designated and qualify as hedging instruments, the hedging instrument must be designated as a cash flow hedge or hedges of net investments, based upon the exposure being hedged. See Note 7 for more information on the Company's derivative instruments and hedging programs.
Fair Value Measurements The Company follows the authoritative guidance on fair value measurements and disclosures with respect to assets and liabilities that are measured at fair value on both a recurring and nonrecurring basis. Fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The authoritative guidance also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability, based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The hierarchy is broken down into three levels defined as follows:
•Level 1 - Inputs are quoted prices in active markets for identical assets or liabilities.
•Level 2 - Inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly.
•Level 3 - Inputs are unobservable for the asset or liability.
Financial assets that are classified as Level 1 securities include highly liquid government bonds within U.S. government and agency securities and marketable equity securities for which quoted market prices are available. In addition, the Company classifies currency forward contracts as Level 1 since they are valued using quoted market prices in active markets which have identical assets or liabilities.
The valuation for most fixed maturity securities are classified as Level 2. Financial assets that are classified as Level 2 include corporate debt securities, government and agency securities, other asset-backed securities, certificate of deposits, and mortgage-backed securities whose value is determined using inputs that are observable in the market or may be derived principally from, or corroborated by, observable market data such as pricing for similar securities, recently executed transactions, cash flow models with yield curves, and benchmark securities. In addition, total return swaps are included in Level 2 as the Company uses inputs other than quoted prices that are observable for the asset. The Level 2 derivative instruments are primarily valued using standard calculations and models that use readily observable market data as their basis.
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies, or similar techniques, and at least one significant model assumption or input is unobservable. Financial assets that are classified as Level 3 include certain investment securities for which there is limited market activity such that the determination of fair value requires significant judgment or estimation, equity method investments for which the Company has elected the fair value option, and auction rate securities. The investment securities with limited market activity are valued using third-party pricing sources that incorporate transaction details such as contractual terms, maturity, timing, and amount of expected future cash flows, as well as assumptions about liquidity and credit valuation adjustments by market participants. The fair value of auction rate securities is estimated by the Company using a discounted cash flow model, which incorporates significant unobservable inputs. The significant unobservable inputs used in the fair value measurement of the Company’s auction rate securities are years to principal recovery and the illiquidity premium that is incorporated into the discount rate. Valuation techniques for investments valued using the fair value option are included in the "Investments" section above. For goodwill, other intangible assets, and IPR&D, inputs used in the fair value analysis fall within Level 3 of the fair value hierarchy due to the use of significant unobservable inputs to determine fair value.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Certain investments for which the fair value is measured using the net asset value per share (or its equivalent) practical expedient are excluded from the fair value hierarchy. Financial assets for which the fair value is measured using the net asset value per share practical expedient include certain debt funds, equity and fixed income commingled trusts, and registered investment companies.
Revenue Recognition The Company sells its products through direct sales representatives and independent distributors. Additionally, a portion of the Company's revenue is generated from consignment inventory maintained at hospitals and royalty and intellectual property arrangements. The Company recognizes revenue when control is transferred to the customer. For products sold through direct sales representatives and independent distributors, control is transferred upon shipment or upon delivery, based on the contract terms and legal requirements. For consignment inventory, control is transferred when the product is used or implanted. Payment terms vary depending on the country of sale, type of customer, and type of product.
If a contract contains more than one performance obligation, the transaction price is allocated to each performance obligation based on relative standalone selling price. Shipping and handling is treated as a fulfillment activity rather than a promised service, and therefore, is not considered a performance obligation. Taxes assessed by a governmental authority that are both imposed on, and concurrent with, a specific revenue producing transaction and collected by the Company from customers (for example, sales, use, value added, and some excise taxes) are not included in revenue. For contracts that have an original duration of one year or less, the Company uses the practical expedient applicable to such contracts and does not adjust the transaction price for the time value of money.
The amount of revenue recognized reflects sales rebates and returns, which are estimated based on sales terms, historical experience, and trend analysis. In estimating rebates, the Company considers the lag time between the point of sale and the payment of the rebate claim, the stated rebate rates, and other relevant information. The Company records adjustments to rebates and returns reserves as increases or decreases of revenue.
The Company records a deferred revenue liability if a customer pays consideration, or the Company has the right to invoice, before the Company transfers a good or service to the customer. Deferred revenue primarily represents remote monitoring services and equipment maintenance, for which consideration is received at the same time as consideration for the device or equipment. Revenue related to remote monitoring services and equipment maintenance is recognized over the service period as time elapses.
Shipping and Handling Shipping and handling costs incurred to physically move product from the Company's premises to the customer's premises are recognized in selling, general, and administrative expense in the consolidated statements of income and were $351 million, $354 million, and $308 million in fiscal years 2023, 2022, and 2021, respectively. Other shipping and handling costs incurred to store, move, and prepare products for shipment are recognized in cost of products sold in the consolidated statements of income.
Research and Development Research and development costs are expensed when incurred. Research and development costs include costs of research, engineering, and technical activities to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include pre-approval regulatory and clinical trial expenses and license payments for technology not yet approved by regulators.
Contingencies The Company records a liability in the consolidated financial statements for loss contingencies when a loss is known or considered probable, and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed.
Income Taxes The Company has deferred taxes that arise as a result of the different treatment of transactions for U.S. GAAP and income tax accounting, known as temporary differences. The Company records the tax effect of these temporary differences as deferred tax assets and deferred tax liabilities. Deferred tax assets generally represent items that may be used as a tax deduction or credit in a tax return in future years for which the Company has already recognized the tax benefit in the consolidated statements of income. The Company establishes valuation allowances for deferred tax assets when the amount of expected future taxable income is not likely to support the use of the deduction or credit. Deferred tax liabilities generally represent tax expense for which payment has been deferred or expense has already been taken as a deduction on the Company’s tax return but has not yet been recognized as an expense in the consolidated statements of income. See Footnote 13 for more information on the Company's uncertain tax positions and tax policies.
Other Operating (Income) Expense, Net Other operating (income) expense, net primarily includes royalty expense, currency remeasurement and derivative gains and losses, Puerto Rico excise taxes, changes in fair value of contingent consideration, changes in amounts accrued for certain contingent liabilities for a past acquisition, MCS charges, RCS charges, impairment charges, income from funded research and development arrangements, and commitments to the Medtronic Foundation and Medtronic LABS.
Other Non-Operating Income, Net Other non-operating income, net includes the non-service component of net periodic pension and post-retirement benefit cost, investment gains and losses, and interest income.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Currency Translation Assets and liabilities of non-U.S. dollar functional currency entities are translated to U.S. dollars at period-end exchange rates, and the currency impacts arising from the translation of the assets and liabilities are recorded as a cumulative translation adjustment, a component of accumulated other comprehensive loss, on the consolidated balance sheets. Elements of the consolidated statements of income are translated at the average monthly currency exchange rates in effect during the period. Currency transaction gains and losses are included in other operating (income) expense, net in the consolidated statements of income.
Stock-Based Compensation The Company measures stock-based compensation expense at the grant date based on the fair value of the award and recognizes the compensation expense over the requisite service period, which is generally the vesting period. The amount of stock-based compensation expense recognized during a period is based on the portion of the awards that are expected to vest. The Company estimates pre-vesting forfeitures at the time of grant and revises the estimates in subsequent periods.
Recently Adopted Accounting Standards
For fiscal year 2023, there were no newly adopted accounting standards that had a material impact to our consolidated financial statements.
2. Revenue
The Company's revenues are principally derived from device-based medical therapies and services related to cardiac rhythm disorders, cardiovascular disease, renal disease, neurological disorders and diseases, spinal conditions and musculoskeletal trauma, chronic pain, urological and digestive disorders, ear, nose, and throat conditions, and diabetes conditions as well as advanced and general surgical care products, respiratory and monitoring solutions, and neurological surgery technologies. The Company's primary customers include healthcare systems, clinics, third-party healthcare providers, distributors, and other institutions, including governmental healthcare programs and group purchasing organizations.
The table below illustrates net sales by segment and division for fiscal years 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| | Net Sales by Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Cardiac Rhythm & Heart Failure | $ | 5,835 | | | $ | 5,908 | | | $ | 5,584 | |
| Structural Heart & Aortic | 3,363 | | | 3,055 | | | 2,834 | |
| Coronary & Peripheral Vascular | 2,375 | | | 2,460 | | | 2,354 | |
| Cardiovascular | 11,573 | | | 11,423 | | | 10,772 | |
| Surgical Innovations | 5,663 | | | 6,060 | | | 5,438 | |
| Respiratory, Gastrointestinal, & Renal | 2,770 | | | 3,081 | | | 3,298 | |
| Medical Surgical | 8,433 | | | 9,141 | | | 8,737 | |
| Cranial & Spinal Technologies | 4,451 | | | 4,456 | | | 4,288 | |
| Specialty Therapies | 2,815 | | | 2,592 | | | 2,307 | |
| Neuromodulation | 1,693 | | | 1,735 | | | 1,601 | |
| Neuroscience | 8,959 | | | 8,784 | | | 8,195 | |
| Diabetes | 2,262 | | | 2,338 | | | 2,413 | |
| Total | $ | 31,227 | | | $ | 31,686 | | | $ | 30,117 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The table below illustrates net sales by market geography and segment for fiscal years 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S.(1) | | Non-U.S. Developed Markets(2) | | Emerging Markets(3) |
| (in millions) | Fiscal Year 2023 | | Fiscal Year 2022 | | Fiscal Year 2021 | | Fiscal Year 2023 | | Fiscal Year 2022 | | Fiscal Year 2021 | | Fiscal Year 2023 | | Fiscal Year 2022 | | Fiscal Year 2021 |
| Cardiovascular | $ | 5,848 | | | $ | 5,545 | | | $ | 5,248 | | | $ | 3,564 | | | $ | 3,866 | | | $ | 3,752 | | | $ | 2,161 | | | $ | 2,012 | | | $ | 1,773 | |
| Medical Surgical | 3,658 | | | 3,862 | | | 3,650 | | | 3,080 | | | 3,373 | | | 3,320 | | | 1,694 | | | 1,905 | | | 1,766 | |
| Neuroscience | 6,018 | | | 5,753 | | | 5,456 | | | 1,658 | | | 1,801 | | | 1,724 | | | 1,283 | | | 1,229 | | | 1,015 | |
| Diabetes | 849 | | | 974 | | | 1,171 | | | 1,106 | | | 1,085 | | | 1,019 | | | 307 | | | 279 | | | 222 | |
| Total | $ | 16,373 | | | $ | 16,135 | | | $ | 15,526 | | | $ | 9,408 | | | $ | 10,126 | | | $ | 9,815 | | | $ | 5,446 | | | $ | 5,426 | | | $ | 4,777 | |
(1)U.S. includes the United States and U.S. territories.
(2)Non-U.S. developed markets include Japan, Australia, New Zealand, Korea, Canada, and the countries within Western Europe.
(3)Emerging markets include the countries of the Middle East, Africa, Latin America, Eastern Europe, and the countries of Asia that are not included in the non-U.S. developed markets, as defined above.
At April 28, 2023, $1.1 billion of rebates were classified as other accrued expenses, and $555 million of rebates were classified as a reduction of accounts receivable in the consolidated balance sheet. At April 29, 2022, $981 million of rebates were classified as other accrued expenses, and $548 million of rebates were classified as a reduction of accounts receivable in the consolidated balance sheet. During fiscal year 2023, adjustments to rebate and return reserves recognized in revenue that were included in the rebate and return reserves at the beginning of the period were not material.
Deferred Revenue and Remaining Performance Obligations
Deferred revenue at April 28, 2023 and April 29, 2022 was $405 million and $399 million, respectively. At April 28, 2023 and April 29, 2022, $314 million and $305 million was included in other accrued expenses, respectively, and $91 million and $94 million was included in other liabilities, respectively. During the fiscal year ended April 28, 2023, the Company recognized $240 million of revenue that was included in deferred revenue as of April 29, 2022.
Remaining performance obligations include goods and services that have not yet been delivered or provided under existing, noncancellable contracts with minimum purchase commitments. At April 28, 2023, the estimated revenue expected to be recognized in future periods related to unsatisfied performance obligations for executed contracts with an original duration of one year or more was approximately $0.6 billion. The Company expects to recognize revenue on the majority of these remaining performance obligations over the next three years.
3. Acquisitions and Dispositions
The Company had acquisitions during fiscal years 2023 and 2022 that were accounted for as business combinations. The assets and liabilities of businesses acquired were recorded and consolidated on the acquisition date at their respective fair values. Goodwill resulting from business combinations is largely attributable to future, yet to be defined technologies, new customer relationships, existing workforce of the acquired businesses, and synergies expected to arise after the Company's acquisition of these businesses. The pro forma impact of acquisitions during fiscal years 2023 and 2022 was not significant, either individually or in the aggregate, to the consolidated results of the Company. The results of operations of acquired businesses have been included in the Company’s consolidated statements of income since the date each business was acquired. Purchase price allocation adjustments for fiscal years 2023 and 2022 business combinations were not significant.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Fiscal Year 2023
Intersect ENT
On May 13, 2022, the Company acquired Intersect ENT, a global ear, nose, and throat (ENT) medical technology leader. The acquisition expands the Neuroscience segment portfolio of products used during ENT procedures, and combined with the Company's navigation, powered instruments, and existing tissue health products, offers a broader suite of solutions to assist surgeons treating patients who suffer from chronic rhinosinusitis (CRS). Total consideration, net of cash acquired, for the transaction, in which the Company acquired all outstanding shares of Intersect ENT for $28.25 per share, was $1.2 billion consisting of $1.1 billion of cash and $98 million previously held investments in Intersect ENT. Based upon a preliminary acquisition valuation, the Company acquired $615 million of goodwill, $635 million of technology-based intangible assets, $35 million of customer-related intangible assets, and $13 million of tradenames with estimated useful lives of 20 years. The goodwill is not deductible for tax purposes.
Revenue and net loss attributable to Intersect ENT since the date of acquisition as well as costs incurred in connection with the acquisition included in the consolidated statements of income were not significant for fiscal year 2023.
Affera, Inc.
On August 30, 2022, the Company acquired Affera, Inc. (Affera) a privately-held company focused on the development of cardiac mapping and navigation systems and catheter-based cardiac ablation technologies. The acquisition expands the Cardiovascular segment suite of advanced cardiac ablation products and accessories, including its first cardiac mapping and navigation platform. Total consideration, net of cash acquired for the transaction, was $904 million. Based upon a preliminary acquisition valuation, the Company acquired $660 million of goodwill and $300 million of in-process research and development, which was capitalized into intangible assets during the fourth quarter of fiscal year 2023. The goodwill is not deductible for tax purposes. The Company recognized $201 million of non-cash contingent consideration liabilities in connection with the acquisition, which are comprised of product development milestone-based payments.
Revenue and net loss attributable to Affera since the date of acquisition as well as costs incurred in connection with the acquisition included in the consolidated statements of income were not significant for fiscal year 2023.
The acquisition date fair values of the assets acquired and liabilities assumed were as follows:
| | | | | | | | | | | |
| (in millions) | Intersect ENT | | Affera |
| Cash and cash equivalents | $ | 39 | | | $ | 66 | |
| Inventory | 32 | | | — | |
| Goodwill | 615 | | | 660 | |
| Other intangible assets | 683 | | | 300 | |
| Other assets | 40 | | | 1 | |
| Total assets acquired | 1,408 | | | 1,027 | |
| | | | |
| Current liabilities | 63 | | | 2 | |
| Deferred tax liabilities | 51 | | | 53 | |
| Other liabilities | 18 | | | 1 | |
| Total liabilities assumed | 131 | | | 56 | |
| Net assets acquired | $ | 1,277 | | | $ | 970 | |
Other acquisitions
For acquisitions other than Intersect ENT and Affera, the acquisition date fair value of net assets acquired during fiscal year 2023 was $123 million. Based upon preliminary valuations, assets acquired were primarily comprised of $66 million of goodwill and $57 million of technology-based intangible assets with estimated useful lives of 16 years. The goodwill is deductible for tax purposes. The Company recognized $73 million of non-cash contingent consideration liabilities in connection with these acquisitions during fiscal year 2023, which are comprised of revenue and product development milestone-based payments.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Fiscal Year 2022
The acquisition date fair value of net assets acquired during fiscal year 2022 was $125 million, consisting of $154 million of assets acquired and $29 million of liabilities assumed. Assets acquired were primarily comprised of $50 million of technology-based intangible assets with estimated useful lives ranging from 15 to 16 years, and $80 million of goodwill. The goodwill is not deductible for tax purposes. The Company recognized $31 million of non-cash contingent consideration liabilities in connection with business combinations during fiscal year 2022, which are comprised of revenue and product development milestone-based payments.
Acquired In-Process Research & Development (IPR&D)
IPR&D with no alternative future use acquired outside of a business combination is expensed immediately. During fiscal year 2023, IPR&D acquired in connection with asset acquisitions was not significant. During fiscal year 2022, the Company acquired $101 million of IPR&D in connection with asset acquisitions of technology not yet approved by regulators, which was recognized in research and development expense in the consolidated statements of income.
Contingent Consideration
Certain of the Company’s business combinations involve potential payment of future consideration that is contingent upon the achievement of certain product development milestones and/or contingent on the acquired business reaching certain performance milestones. A liability is recorded for the estimated fair value of the contingent consideration on the acquisition date. The fair value of the contingent consideration is remeasured at each reporting period, and the change in fair value is recognized within other operating (income) expense, net in the consolidated statements of income.
The fair value of contingent consideration at April 28, 2023 and April 29, 2022 was $206 million and $119 million, respectively. At April 28, 2023, $34 million was recorded in other accrued expenses, and $171 million was recorded in other liabilities on the consolidated balance sheets. At April 29, 2022, $35 million was reflected in other accrued expenses, and $84 million was reflected in other liabilities on the consolidated balance sheets.
The following table provides a reconciliation of the beginning and ending balances of contingent consideration:
| | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 |
| Beginning Balance | $ | 119 | | | $ | 270 | |
| Purchase price contingent consideration | 274 | | | 31 | |
| Purchase price allocation adjustments | — | | | 7 | |
| Payments | (154) | | | (86) | |
| Change in fair value | (24) | | | (103) | |
| Divestiture-related and other | (8) | | | — | |
| Ending Balance | $ | 206 | | | $ | 119 | |
The recurring Level 3 fair value measurements of contingent consideration for which a liability is recorded include the following significant unobservable inputs:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | Fair Value at April 28, 2023 | | | | Unobservable Input | | Range | | Weighted Average (1) |
| Revenue and other performance-based payments | | $ | 80 | | | | | Discount rate | | 11.2% - 27.2% | | 17.5% |
| | | | Projected fiscal year of payment | | 2024 - 2027 | | 2025 |
| Product development and other milestone-based payments | | $ | 126 | | | | | Discount rate | | 3.9% - 5.5% | | 4.1% |
| | | | Projected fiscal year of payment | | 2024 - 2027 | | 2026 |
(1) Unobservable inputs were weighted by the relative fair value of the contingent consideration liability. For projected fiscal year of payment, the amount represents the median of the inputs and is not a weighted average.
Renal Care Solutions disposition
On May 25, 2022, the Company and DaVita Inc. (“DaVita”) entered into a definitive agreement for the Company to sell half of its Renal Care Solutions (RCS) business, and on April 1, 2023, completed the transaction. This sale is part of an agreement between Medtronic and DaVita to form a new, independent kidney care-focused medical device company (“Mozarc Medical” or "Mozarc") with equal equity
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
ownership. RCS was part of the Company’s Medical Surgical portfolio. At closing, the Company received $45 million cash consideration, recorded non-cash contingent consideration receivables valued at $195 million due based on the achievement of certain revenue, regulatory, and profitability milestones, and retained a 50% non-controlling equity interest in Mozarc valued at $307 million. For the contingent consideration receivables, the maximum consideration the Company could receive in the future is $300 million based on the achievement of the aforementioned milestones, with potential payouts starting in fiscal year 2025 through 2029. The Company recorded total non-cash pre-tax charges of $136 million in fiscal year 2023, primarily related to impairment of goodwill and changes in the carrying amount of the disposal group, recognized in other operating (income) expense, net in the consolidated statements of income. Refer to Note 9 to the consolidated financial statements for additional information on the goodwill impairment. Refer to Note 5 to the consolidated financial statements for additional information on the Company’s retained 50% equity investment in Mozarc as a result of this transaction.
The Company determined that the sale of the RCS business did not meet the criteria to be classified as discontinued operations.
4. Restructuring Charges
In fiscal years 2023, 2022 and 2021, restructuring costs primarily related to Enterprise Excellence and Simplification restructuring programs, both of which were substantially completed as of the end of this fiscal year. Enterprise Excellence was designed to leverage the company’s global size and scale to focus on global operations, and functional and commercial optimization, and had total pre-tax charges of $1.8 billion. Simplification was designed to focus the organization on accelerating innovation, enhancing customer experience, driving revenue growth and winning market share, and had total pre-tax charges of $0.5 billion.
In addition, in the fourth quarter of fiscal year 2023, we incurred $0.3 billion of restructuring charges primarily related to employee termination benefits to support cost reduction initiatives. These charges were incremental to charges incurred under our Enterprise Excellence and Simplification programs noted above.
For all programs, employee-related costs primarily consist of termination benefits provided to employees who have been involuntarily terminated and voluntary early retirement benefits. Associated costs primarily include salaries and wages of employees that are fully-dedicated to restructuring programs and consulting fees.
The following table presents the classification of restructuring costs in the consolidated statements of income:
| | | | | | | | | | | | | | | | | |
| Fiscal year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Cost of products sold | $ | 97 | | | $ | 117 | | | $ | 128 | |
| Selling, general, and administrative expenses | 173 | | | 158 | | | 196 | |
| Restructuring charges, net | 375 | | | 60 | | | 293 | |
| Total restructuring and associated costs | $ | 647 | | | $ | 335 | | | $ | 617 | |
(1) In fiscal years 2023 and 2021, restructuring charges, net included $94 million and $97 million, respectively, of incremental defined benefit, defined contribution, and post-retirement related expenses for employees that accepted voluntary early retirement packages.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The following table summarizes the activity related to restructuring programs for fiscal years 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Employee Termination Benefits(1) | | Associated Costs(2) | | Asset
Write-downs | | Other
Costs | | Total |
| April 30, 2021 | $ | 123 | | | $ | 22 | | | $ | — | | | $ | 1 | | | $ | 146 | |
| Charges | 80 | | | 274 | | | — | | | — | | | 354 | |
| Cash payments | (109) | | | (269) | | | — | | | — | | | (378) | |
| | | | | | | | | |
Accrual adjustments(3) | (13) | | | — | | | — | | | — | | | (13) | |
| April 29, 2022 | 81 | | | 27 | | | — | | | 1 | | | 110 | |
| Charges | 285 | | | 271 | | | 1 | | | 7 | | | 564 | |
| Cash payments | (150) | | | (274) | | (1) | | | (6) | | | (433) | |
| | | | | | | | | |
Accrual adjustments(3) | (11) | | | — | | | — | | | (1) | | | (12) | |
| April 28, 2023 | $ | 204 | | | $ | 23 | | | $ | — | | | $ | 1 | | | $ | 230 | |
(1)In fiscal years 2023, restructuring charges, net included $94 million of incremental defined benefit, defined contribution, and post-retirement related expenses for employees that accepted voluntary early retirement packages. These costs are not included in the table summarizing restructuring charges above, as they are associated with costs that are accounted for under the pension and post-retirement rules.
(2)Associated costs include costs incurred as a direct result of the restructuring program, such as salaries for employees supporting the program and consulting expenses.
(3)Accrual adjustments relate to certain employees identified for termination finding other positions within the Company or contract terminations being settled for less than originally estimated.
Mechanical Circulatory Support (MCS)
In June 2021, the Company announced the decision to stop the distribution and sale of the Medtronic HVAD System in light of a growing body of observational clinical comparisons indicating a lower frequency of neurological adverse events and mortality with another circulatory support device available to patients compared to the HVAD system. In connection with this decision, the Company recorded charges of $726 million (MCS charges) within the Cardiovascular segment during the first quarter of fiscal year 2022, including $58 million recognized in costs of products sold and $668 million recognized within other operating (income) expense, net in the consolidated statement of income. The charges included $515 million of non-cash impairments and write-downs primarily related to $409 million of intangible asset impairments and $58 million of inventory write-downs. The Company also recorded charges of $211 million for commitments and obligations associated with the decision, which included charges for patient support obligations, restructuring, and other associated costs. During the fourth quarter of fiscal year 2022, the Company recorded additional charges of $155 million within other operating (income) expense, net primarily related to incremental commitments and obligations associated with the exit of the business. As of April 28, 2023, accruals were recorded in the consolidated balance sheet for these obligations, with $84 million reflected in other accrued expenses and $88 million recorded in other liabilities.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
5. Financial Instruments
Debt Securities
The Company holds investments in marketable debt securities that are classified and accounted for as available-for-sale and are remeasured on a recurring basis. The following tables summarize the Company's investments in available-for-sale debt securities by significant investment category and the related consolidated balance sheet classification at April 28, 2023 and April 29, 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| April 28, 2023 |
| Valuation | | Balance Sheet Classification |
| (in millions) | Cost | | Unrealized
Gains | | Unrealized
Losses | | Fair Value | | Investments | | Other Assets |
| Level 1: | | | | | | | | | | | |
| U.S. government and agency securities | $ | 527 | | | $ | — | | | $ | (22) | | | $ | 505 | | | $ | 505 | | | $ | — | |
| Level 2: | | | | | | | | | | | |
| Corporate debt securities | 4,140 | | | 6 | | | (162) | | | 3,984 | | | 3,984 | | | — | |
| U.S. government and agency securities | 879 | | | — | | | (45) | | | 834 | | | 834 | | | — | |
| Mortgage-backed securities | 560 | | | — | | | (54) | | | 506 | | | 506 | | | — | |
| Non-U.S. government and agency securities | 15 | | | — | | | — | | | 15 | | | 15 | | | — | |
| Certificates of deposit | 10 | | | — | | | — | | | 10 | | | 10 | | | |
| Other asset-backed securities | 580 | | | — | | | (19) | | | 561 | | | 561 | | | — | |
| Total Level 2 | 6,185 | | | 6 | | | (281) | | | 5,911 | | | 5,911 | | | — | |
| Level 3: | | | | | | | | | | | |
| Auction rate securities | 36 | | | — | | | (3) | | | 33 | | | — | | | 33 | |
| Total available-for-sale debt securities | $ | 6,748 | | | $ | 6 | | | $ | (305) | | | $ | 6,449 | | | $ | 6,416 | | | $ | 33 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| April 29, 2022 |
| Valuation | | Balance Sheet Classification |
| (in millions) | Cost | | Unrealized
Gains | | Unrealized
Losses | | Fair Value | | Investments | | Other Assets |
| Level 1: | | | | | | | | | | | |
| U.S. government and agency securities | $ | 533 | | | $ | 1 | | | $ | (15) | | | $ | 518 | | | $ | 518 | | | $ | — | |
| Level 2: | | | | | | | | | | | |
| Corporate debt securities | 4,457 | | | 4 | | | (140) | | | 4,321 | | | 4,321 | | | — | |
| U.S. government and agency securities | 910 | | | — | | | (41) | | | 869 | | | 869 | | | — | |
| Mortgage-backed securities | 592 | | | — | | | (35) | | | 558 | | | 558 | | | — | |
| Non-U.S. government and agency securities | 17 | | | — | | | — | | | 17 | | | 17 | | | — | |
| Certificates of deposit | 20 | | | — | | | — | | | 20 | | | 20 | | | |
| Other asset-backed securities | 567 | | | — | | | (11) | | | 556 | | | 556 | | | — | |
| | | | | | | | | | | |
| Total Level 2 | 6,563 | | | 4 | | | (227) | | | 6,341 | | | 6,341 | | | — | |
| Level 3: | | | | | | | | | | | |
| Auction rate securities | 36 | | | — | | | (3) | | | 33 | | | — | | | 33 | |
| Total available-for-sale debt securities | $ | 7,131 | | | $ | 5 | | | $ | (245) | | | $ | 6,893 | | | $ | 6,859 | | | $ | 33 | |
The amortized cost of debt securities excludes accrued interest, which is reported in other current assets in the consolidated balance sheets.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The following tables present the gross unrealized losses and fair values of the Company’s available-for-sale debt securities that have been in a continuous unrealized loss position deemed to be temporary, aggregated by investment category at April 28, 2023 and April 29, 2022:
| | | | | | | | | | | | | | | | | | | | | | | |
| | April 28, 2023 |
| | Less than 12 months | | More than 12 months |
| (in millions) | Fair Value | | Unrealized
Losses | | Fair Value | | Unrealized
Losses |
| Corporate debt securities | $ | 286 | | | $ | (4) | | | $ | 2,901 | | | $ | (158) | |
| U.S. government and agency securities | 89 | | | (3) | | | 821 | | | (64) | |
| Mortgage-backed securities | 26 | | | (1) | | | 460 | | | (53) | |
| Other asset-backed securities | — | | | — | | | 545 | | | (19) | |
| Auction rate securities | — | | | — | | | 33 | | | (3) | |
| Total | $ | 401 | | | $ | (8) | | | $ | 4,760 | | | $ | (297) | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | April 29, 2022 |
| | Less than 12 months | | More than 12 months |
| (in millions) | Fair Value | | Unrealized
Losses | | Fair Value | | Unrealized
Losses |
| Corporate debt securities | $ | 222 | | | $ | (1) | | | $ | 2,993 | | | $ | (139) | |
| U.S. government and agency securities | — | | | — | | | 945 | | | (56) | |
| Mortgage-backed securities | — | | | — | | | 507 | | | (35) | |
| Other asset-backed securities | — | | | — | | | 526 | | | (11) | |
| Auction rate securities | — | | | — | | | 33 | | | (3) | |
| Total | $ | 222 | | | $ | (1) | | | $ | 5,004 | | | $ | (244) | |
The Company reviews the fair value hierarchy classification on a quarterly basis. Changes in the ability to observe valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy. There were no transfers into or out of Level 3 during the fiscal years ended April 28, 2023 and April 29, 2022. When a determination is made to classify an asset or liability within Level 3, the determination is based upon the significance of the unobservable inputs to the overall fair value measurement.
Activity related to the Company’s available-for-sale debt securities portfolio is as follows:
| | | | | | | | | | | | | | | | | |
| (in millions) | April 28, 2023 | | April 29, 2022 | | April 30, 2021 |
| Proceeds from sales and maturities | $ | 7,321 | | | $ | 9,611 | | | $ | 10,420 | |
| Gross realized gains | 10 | | | 15 | | | 15 | |
| Gross realized losses | (43) | | | (18) | | | (14) | |
During the fiscal year ended April 30, 2021, the Company had proceeds from maturities of investments classified as held to maturity of $911 million.
The April 28, 2023 balance of available-for-sale debt securities by contractual maturity is shown in the following table. Within the table, maturities of mortgage-backed securities have been allocated based upon timing of estimated cash flows assuming no change in the current interest rate environment. Actual maturities may differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties.
| | | | | |
| (in millions) | April 28, 2023 |
| Due in one year or less | $ | 1,267 | |
| Due after one year through five years | 3,704 | |
| Due after five years through ten years | 803 | |
| Due after ten years | 676 | |
| Total debt securities | $ | 6,449 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Equity Securities, Equity Method Investments, and Other Investments
The Company holds investments in equity securities with readily determinable fair values, equity method investments for which the Company has elected the fair value option, equity investments without readily determinable fair values, investments accounted for under the equity method, and other investments. Equity securities with readily determinable fair values are included in Level 1 of the fair value hierarchy, as they are measured using quoted market prices. Equity method investments for which the Company has elected the fair value option are included within Level 3 of the fair value hierarchy due to the use of significant unobservable inputs to determine fair value. To determine the fair value of these investments, the Company uses a discounted cash flow methodology, taking into consideration various assumptions including discount rate, and all pertinent financial information available related to the investees, including historical financial statements and projected future cash flows. Equity investments that do not have readily determinable fair values, and that are not accounted for via the fair value option, are included within Level 3 of the fair value hierarchy, as they are measured using the measurement alternative at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for an identical or similar investment of the same issuer.
The following table summarizes the Company's equity and other investments at April 28, 2023 and April 29, 2022, which are classified as other assets in the consolidated balance sheets:
| | | | | | | | | | | | | | |
| (in millions) | | April 28, 2023 | | April 29, 2022 |
| Investments with readily determinable fair value (marketable equity securities) | | $ | 115 | | | $ | 64 | |
| Investments for which the fair value option has been elected | | 531 | | | — | |
| Investments without readily determinable fair values | | 872 | | | 732 | |
| Equity method and other investments | | 89 | | | 85 | |
| Total equity and other investments | | $ | 1,607 | | | $ | 881 | |
Gains and losses on the Company's portfolio of equity and other investment are recognized in other non-operating income, net in the consolidated statements of income. During the fiscal year ended April 28, 2023, there were $56 million of net unrealized gains on equity securities and other investments still held at April 28, 2023. During the fiscal year ended April 29, 2022, there were $8 million of net unrealized gains on equity securities and other investments still held at April 29, 2022.
Interest income is recognized in other non-operating income, net, in the consolidated statements of income. During the fiscal year ended April 28, 2023, there was $386 million of interest income. During the fiscal year ended April 29, 2022, there was $186 million of interest income.
Mozarc Medical Investment
On April 1, 2023 the Company sold half of its RCS business to Mozarc, and as a result of the transaction the Company retained a 50% equity interest in Mozarc. Please refer to Note 3 to the consolidated financial statements for additional information on this transaction. Although the equity investment provides the Company with the ability to exercise significant influence over Mozarc, the Company has elected the fair value option to account for this equity investment. The Company believes the fair value option best reflects the economics of the underlying transaction. Under the fair value option, changes in the fair value of the investment are recognized through earnings each reporting period in other non-operating income, net in the consolidated statements of income.
The following table provides a reconciliation of the beginning and ending balances of the Mozarc investment for which the Fair Value Option has been elected:
| | | | | |
| (in millions) | Fiscal Year 2023 |
| Beginning Balance | $ | — | |
| Initial valuation | 307 | |
| Additional cash investment | 224 | |
| |
| Ending Balance | $ | 531 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
6. Financing Arrangements
Current debt obligations consisted of the following: | | | | | | | | | | | |
| (in millions) | April 28, 2023 | | April 29, 2022 |
| Bank borrowings | $ | 13 | | | $ | 12 | |
0.000 percent three-year 2019 senior notes | — | | | 798 | |
0.375 percent four-year 2019 senior notes | — | | | 1,596 | |
0.000 percent two-year 2020 senior notes | — | | | 1,330 | |
| Finance lease obligations | 7 | | | 6 | |
| | | |
| Current debt obligations | $ | 20 | | | $ | 3,742 | |
Bank Borrowings Outstanding bank borrowings at April 28, 2023 and April 29, 2022 were not significant.
Commercial Paper On January 26, 2015, Medtronic Global Holdings S.C.A. (Medtronic Luxco), an entity organized under the laws of Luxembourg, entered into various agreements pursuant to which Medtronic Luxco may issue United States Dollar-denominated unsecured commercial paper notes (the 2015 CP Program) on a private placement basis, and on January 31, 2020 Medtronic Luxco entered into various agreements pursuant to which Medtronic Luxco may issue Euro-denominated unsecured commercial paper notes (the 2020 CP Program) on a private placement basis. The maximum aggregate amount outstanding at any time under the 2015 CP Program and the 2020 CP Program together may not exceed the equivalent of $3.5 billion. The Company and Medtronic, Inc. have guaranteed the obligations of Medtronic Luxco under the 2015 CP Program and the 2020 CP Program.
There was no commercial paper outstanding at April 28, 2023 and April 29, 2022. During fiscal year 2023, the weighted average original maturity of the commercial paper outstanding was approximately 22 days and the weighted average interest rate was 4.34 percent. During fiscal year 2022, the weighted average original maturity of the commercial paper outstanding was approximately 15 days and the weighted average interest rate was 0.70 percent. The issuance of commercial paper reduces the amount of credit available under the Company's existing credit facility, defined below.
Line of Credit On December 12, 2022, Medtronic Luxco, as borrower, entered into an amendment to its amended and restated credit agreement (Credit Facility), by and among Medtronic, Medtronic, Inc., Medtronic Luxco, the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent and issuing bank, extending the maturity date of the Credit Facility to December 2027.
The Credit Facility provides for a $3.5 billion five-year unsecured revolving credit facility (Credit Facility). At each anniversary date of the Credit Facility, we can request a one-year extension of the maturity date. The Credit Facility provides the Company with the ability to increase its borrowing capacity by an additional $1.0 billion at any time during the term of the agreement. The Company and Medtronic, Inc. have guaranteed the obligations of the borrowers under the Credit Facility, and Medtronic Luxco will also guarantee the obligations of any designated borrower. The Credit Facility includes a multi-currency borrowing feature for certain specified foreign currencies. At April 28, 2023 and April 29, 2022, no amounts were outstanding under the Credit Facility.
Interest rates on advances on the Credit Facility are determined by a pricing matrix based on the Company’s long-term debt ratings, assigned by Standard & Poor’s Ratings Services and Moody’s Investors Service. Facility fees are payable on the Credit Facility and are determined in the same manner as the interest rates. The Company is in compliance with all covenants related to the Credit Facility.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The Company's long-term debt obligations consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | April 28, 2023 | | April 29, 2022 |
| (in millions, except interest rates) | Maturity by Fiscal Year | | Amount | | Effective Interest Rate | | Amount | | Effective Interest Rate |
3.500 percent ten-year 2015 senior notes | 2025 | | $ | — | | | — | % | | $ | 1,890 | | | 3.74 | % |
0.250 percent six-year 2019 senior notes | 2026 | | 1,097 | | | 0.44 | | | 1,064 | | | 0.45 | |
2.625 percent three-year 2022 senior notes | 2026 | | 549 | | | 2.86 | | | — | | | — | |
0.000 percent five-year 2020 senior notes | 2026 | | 1,097 | | | 0.23 | | | 1,064 | | | 0.25 | |
1.125 percent eight-year 2019 senior notes | 2027 | | 1,646 | | | 1.25 | | | 1,596 | | | 1.26 | |
3.350 percent ten-year 2017 senior notes | 2027 | | — | | | — | | | 368 | | | 3.53 | |
4.250 percent five-year 2023 senior notes | 2028 | | 1,000 | | | 4.42 | | | — | | | — | |
3.000 percent six-year 2022 senior notes | 2029 | | 1,097 | | | 3.09 | | | — | | | — | |
0.375 percent eight-year 2020 senior notes | 2029 | | 1,097 | | | 0.51 | | | 1,064 | | | 0.52 | |
1.625 percent twelve-year 2019 senior notes | 2031 | | 1,097 | | | 1.75 | | | 1,064 | | | 1.75 | |
1.000 percent twelve-year 2019 senior notes | 2032 | | 1,097 | | | 1.06 | | | 1,064 | | | 1.06 | |
3.125 percent nine-year 2022 senior notes | 2032 | | 1,097 | | | 3.25 | | | — | | | — | |
0.750 percent twelve-year 2020 senior notes | 2033 | | 1,097 | | | 0.81 | | | 1,064 | | | 0.81 | |
4.500 percent ten-year 2023 senior notes | 2033 | | 1,000 | | | 4.62 | | | — | | | — | |
3.375 percent twelve-year 2022 senior notes | 2035 | | 1,097 | | | 3.44 | | | — | | | — | |
4.375 percent twenty-year 2015 senior notes | 2035 | | 1,932 | | | 4.47 | | | 1,932 | | | 4.47 | |
6.550 percent thirty-year 2007 CIFSA senior notes | 2038 | | 253 | | | 4.67 | | | 253 | | | 4.67 | |
2.250 percent twenty-year 2019 senior notes | 2039 | | 1,097 | | | 2.34 | | | 1,064 | | | 2.35 | |
6.500 percent thirty-year 2009 senior notes | 2039 | | 158 | | | 6.56 | | | 158 | | | 6.56 | |
1.500 percent twenty-year 2019 senior notes | 2040 | | 1,097 | | | 1.58 | | | 1,064 | | | 1.59 | |
5.550 percent thirty-year 2010 senior notes | 2040 | | 224 | | | 5.58 | | | 224 | | | 5.58 | |
1.375 percent twenty-year 2020 senior notes | 2041 | | 1,097 | | | 1.46 | | | 1,064 | | | 1.47 | |
4.500 percent thirty-year 2012 senior notes | 2042 | | 105 | | | 4.54 | | | 105 | | | 4.54 | |
4.000 percent thirty-year 2013 senior notes | 2043 | | 305 | | | 4.09 | | | 305 | | | 4.09 | |
4.625 percent thirty-year 2014 senior notes | 2044 | | 127 | | | 4.67 | | | 127 | | | 4.67 | |
4.625 percent thirty-year 2015 senior notes | 2045 | | 1,813 | | | 4.69 | | | 1,813 | | | 4.69 | |
1.750 percent thirty-year 2019 senior notes | 2050 | | 1,097 | | | 1.87 | | | 1,064 | | | 1.88 | |
1.625 percent thirty-year 2020 senior notes | 2051 | | 1,097 | | | 1.75 | | | 1,064 | | | 1.76 | |
| | | | | | | | | |
| Finance lease obligations | 2024-2036 | | 57 | | | 9.91 | | | 56 | | | 9.15 | |
| Debt discount, net | 2026-2051 | | (64) | | | — | | | (52) | | | — | |
| Deferred financing costs | 2026-2051 | | (124) | | | — | | | (109) | | | — | |
| Long-term debt | | | $ | 24,344 | | | | | $ | 20,372 | | | |
Senior Notes The Company has outstanding unsecured senior obligations, described as senior notes in the tables above (collectively, the Senior Notes). The Senior Notes rank equally with all other unsecured and unsubordinated indebtedness of the Company. The Company is in compliance with all covenants related to the Seniors Notes.
In September 2020, Medtronic Global Holdings S.C.A. (Medtronic Luxco) issued six tranches of Euro-denominated Senior Notes with an aggregate principal of €6.3 billion, with maturities ranging from fiscal year 2023 to fiscal year 2051, resulting in cash proceeds of approximately $7.2 billion, net of discounts and issuance costs. The Company used the net proceeds of the offering to fund the early redemption of $4.3 billion of Medtronic Inc. and CIFSA Senior Notes and €1.5 billion of Medtronic Luxco Senior Notes for $6.3 billion of total consideration in October 2020. Additionally, the Company used the proceeds to repay its €750 million floating rate senior notes at maturity in March 2021. The Company recognized a loss on debt extinguishment of $308 million in fiscal year 2021, which primarily
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
included cash premiums and accelerated amortization of deferred financing costs and debt discounts and premiums. The loss was recognized in interest expense, net in the consolidated statement of income.
In September 2022, Medtronic Luxco issued four tranches of Euro-denominated Senior Notes with an aggregate principal of €3.5 billion, with maturities ranging from fiscal year 2026 to 2035, resulting in cash proceeds of approximately $3.4 billion, net of discounts and issuance costs. The Company used the net proceeds to repay at maturity €750 million of Medtronic Luxco Senior Notes for $772 million of total consideration in December 2022 and €2.8 billion of Medtronic Luxco Senior Notes for $2.9 billion of total consideration in March 2023.
In March 2023, Medtronic Luxco issued two tranches of USD-denominated Senior Notes with an aggregate principal of $2.0 billion, with maturities ranging from fiscal year 2028 to 2033, resulting in cash proceeds of approximately $2.0 billion, net of discounts and issuance costs. The Company used the net proceeds supplemented by additional cash to repay the ¥297 billion Fiscal 2023 Loan Agreement discussed below for $2.3 billion of total consideration.
The Euro-denominated debt issued in September 2020 and September 2022 is designated as a net investment hedge of certain of the Company's European operations. Refer to Note 7 for additional information regarding the net investment hedge.
Term Loan Agreements In May 2022, Medtronic Luxco entered into a term loan agreement (Fiscal 2023 Loan Agreement) by and among Medtronic Luxco, Medtronic plc, Medtronic, Inc., and Mizuho Bank, Ltd. as administrative agent and as lender. The Fiscal 2023 Loan Agreement provides an unsecured term loan in an aggregate principal amount of up to ¥300 billion with a term of 364 days. Borrowings under the Fiscal 2023 Loan Agreement bear interest at the TIBOR Rate (as defined in the Fiscal 2023 Loan Agreement) plus a margin of 0.40% per annum. Medtronic plc and Medtronic, Inc. guaranteed the obligations of Medtronic Luxco under the Fiscal 2023 Loan Agreement. In May and June 2022, Medtronic Luxco borrowed an aggregate of ¥297 billion, or approximately $2.3 billion, of the term loan, under the Fiscal 2023 Loan Agreement. The Company used the net proceeds of the borrowings to fund the early redemption of $1.9 billion of Medtronic Inc.'s 3.500% Senior Notes due 2025 for $1.9 billion of total consideration, and $368 million of Medtronic Luxco's 3.350% Senior Notes due 2027 for $376 million of total consideration. The Company recognized a total loss on debt extinguishment of $53 million within interest expense, net in the consolidated statements of income during fiscal year 2023, which primarily includes cash premiums and accelerated amortization of deferred financing costs and debt discounts and premiums. During the fourth quarter of fiscal year 2023, the Company repaid the term loan in full, including interest.
Contractual maturities of debt for the next five fiscal years and thereafter, excluding deferred financing costs and debt discount, net, are as follows: | | | | | |
| (in millions) | |
| 2024 | $ | 20 | |
| 2025 | 7 | |
| 2026 | 2,750 | |
| 2027 | 1,652 | |
| 2028 | 1,005 | |
| Thereafter | 19,119 | |
| Total | $ | 24,553 | |
Financial Instruments Not Measured at Fair Value
At April 28, 2023, the estimated fair value of the Company’s Senior Notes was $21.7 billion compared to a principal value of $24.5 billion. At April 29, 2022, the estimated fair value was $22.9 billion compared to a principal value of $24.2 billion. The fair value was estimated using quoted market prices for the publicly registered Senior Notes, which are classified as Level 2 within the fair value hierarchy. The fair values and principal values consider the terms of the related debt and exclude the impacts of debt discounts and hedging activity.
7. Derivatives and Currency Exchange Risk Management
The Company uses derivative instruments and foreign currency denominated debt to manage the impact that currency exchange rate and interest rate changes have on reported financial statements. The Company does not enter into derivative contracts for speculative purposes.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Cash Flow Hedges
The Company uses foreign currency forward exchange contracts designated as cash flow hedges to manage its exposure to the variability of future cash flows that are denominated in a foreign currency.
At inception, foreign currency forward contracts are designated as a cash flow hedge. Changes in the fair value of these derivatives are reported as a component of accumulated other comprehensive loss until the hedged transaction affects earnings. When the hedged transaction affects earnings, the gain or loss on the derivative is reclassified to earnings. Amounts excluded from the measurement of hedge effectiveness are recognized in earnings on a straight-line basis over the term of the hedge. Cash flows are reported as operating activities in the consolidated statements of cash flows.
The Company's cash flow hedges will mature within the subsequent three-year period. At April 28, 2023 and April 29, 2022, the Company had $93 million and $474 million in after-tax unrealized gains, respectively, associated with cash flow hedging instruments recorded in accumulated other comprehensive loss. The Company expects that $140 million of after-tax net unrealized gains at April 28, 2023 will be recognized in the consolidated statements of income over the next 12 months.
Net Investment Hedges
The Company uses derivative instruments and foreign currency denominated debt to manage foreign currency risk associated with its net investment in foreign operations. The derivative instruments that the Company uses for this purpose may include foreign currency forward exchange contracts used on a standalone basis or in combination with option collars and standalone cross currency interest rate contracts.
For instruments that are designated as net investment hedges, the gains or losses are reported as a component of accumulated other comprehensive loss. The gains or losses are reclassified into earnings upon a liquidation event or deconsolidation of the foreign subsidiary. Amounts excluded from the assessment of effectiveness are recognized in interest expense, net on a straight-line basis over the term of the hedge. During the twelve months ended April 28, 2023, the Company recognized $107 million of after-tax unrealized gains related to excluded components in interest expense, net. The cash flows related to the Company’s derivative instruments designated as net investment hedges are reported as investing activities in the consolidated statements of cash flows. Cash flows attributable to amounts excluded from the assessment of effectiveness are reported as operating activities in the consolidated statements of cash flows.
Undesignated Derivatives
The Company uses foreign currency forward exchange contracts to offset the Company’s exposure to the change in the value of non-functional currency denominated assets, liabilities, and cash flows.
These foreign currency forward exchange rate contracts are not designated as hedges at inception, and therefore, changes in the fair value of these contracts are recognized in the consolidated statements of income. Cash flows related to the Company’s undesignated derivative contracts are reported in the consolidated statements of cash flows based on the nature of the derivative instrument.
Outstanding Instruments
The following table presents the contractual amounts of the Company's outstanding instruments:
| | | | | | | | | | | | | | |
| | As of |
| (in billions) | Designation | April 28, 2023 | | April 29, 2022 |
| Currency exchange rate contracts | Cash flow hedge | $ | 9.1 | | | $ | 8.8 | |
Currency exchange rate contracts(1) | Net investment hedge | 7.2 | | | — | |
Foreign currency-denominated debt(2) | Net investment hedge | 17.6 | | | 17.0 | |
| Currency exchange rate contracts | Undesignated | 5.8 | | | 4.9 | |
(1)At April 28, 2023, includes derivative contracts with a notional value of €4.5 billion, or $4.9 billion, designated as hedges of a portion of our net investment in certain European operations and derivative contracts with a notional value of ¥297 billion, or $2.2 billion, designated as hedges of a portion of our net investment in certain Japanese operations. These derivative contracts mature in fiscal years 2024 through 2033.
(2)At April 28, 2023, includes €16.0 billion, or $17.6 billion, of outstanding Euro-denominated debt as hedges of a portion our net investment in foreign operations. This debt matures in fiscal years 2026 through 2051.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Gains and Losses on Hedging Instruments and Derivatives not Designated as Hedging Instruments
The amount of the gains and losses on our hedging instruments and the classification of those gains and losses within our consolidated financial statements for fiscal years 2023, 2022, and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Gain) Loss Recognized in Accumulated Other Comprehensive Income | | (Gain) Loss Reclassified into Income | | |
| | | |
| Fiscal Year | | Fiscal Year | | Location of (Gain) Loss in Income Statement |
| (in millions) | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | |
| Cash flow hedges | | | | | | | | | | | | | |
| Currency exchange rate contracts | $ | (161) | | | $ | (953) | | | $ | 519 | | | $ | (703) | | | $ | (144) | | | $ | (17) | | | Other operating (income) expense, net |
| Currency exchange rate contracts | (79) | | | 18 | | | 108 | | | (3) | | | 61 | | | 15 | | | Cost of products sold |
| Net investment hedges | | | | | | | | | | | | | |
| Foreign currency-denominated debt | 524 | | | (2,299) | | | 1,694 | | | — | | | — | | | — | | | N/A |
| Currency exchange rate contracts | 73 | | | — | | | — | | | — | | | — | | | — | | | N/A |
| Total | $ | 356 | | | $ | (3,234) | | | $ | 2,321 | | | $ | (706) | | | $ | (83) | | | $ | (2) | | | |
The amount of the gains and losses on our derivative instruments not designated as hedging instruments and the classification of those gains and losses within our consolidated financial statements for fiscal years 2023, 2022, and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (Gain) Loss Recognized in Income | | |
| Fiscal Year | | Location of (Gain) Loss in Income Statement |
| (in millions) | 2023 | | 2022 | | 2021 | |
| Derivatives not designated as hedging instruments | | | | | | | |
| Currency exchange rate contracts | $ | 31 | | | $ | (54) | | | $ | 247 | | | Other operating (income) expense, net |
| Total return swaps | 1 | | | 1 | | | (81) | | | Other operating (income) expense, net |
| Total | $ | 32 | | | $ | (53) | | | $ | 166 | | | |
Balance Sheet Presentation
The following tables summarize the balance sheet classification and fair value of derivative instruments included in the consolidated balance sheets at April 28, 2023 and April 29, 2022. The fair value amounts are presented on a gross basis, and are segregated between derivatives that are designated and qualify as hedging instruments and those that are not designated and do not qualify as hedging instruments, and are further segregated by type of contract within those two categories.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value - Assets | | Fair Value - Liabilities |
| (in millions) | April 28, 2023 | | April 29, 2022 | | Balance Sheet Classification | | April 28, 2023 | | April 29, 2022 | | Balance Sheet Classification |
| Derivatives designated as hedging instruments | | | | | | | | | | | |
| Currency exchange rate contracts | $ | 318 | | | $ | 481 | | | Other current assets | | $ | 109 | | | $ | 43 | | | Other accrued expenses |
| | | | | | | | | | | |
| Currency exchange rate contracts | 33 | | | 168 | | | Other assets | | 117 | | | 16 | | | Other liabilities |
| Total derivatives designated as hedging instruments | 351 | | | 649 | | | | | 226 | | | 60 | | | |
| Derivatives not designated as hedging instruments | | | | | | | | | | | |
| Currency exchange rate contracts | 17 | | | 46 | | | Other current assets | | 10 | | | 49 | | | Other accrued expenses |
| Total return swaps | — | | | — | | | Other current assets | | — | | | 20 | | | Other accrued expenses |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Total derivatives not designated as hedging instruments | 17 | | | 46 | | | | | 10 | | | 69 | | | |
| Total derivatives | $ | 368 | | | $ | 695 | | | | | $ | 236 | | | $ | 129 | | | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The following table provides information by level for the derivative assets and liabilities that are measured at fair value on a recurring basis:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| April 28, 2023 | | April 29, 2022 | | | |
| (in millions) | Derivative Assets | | Derivative Liabilities | | Derivative Assets | | Derivative Liabilities | | | |
| Level 1 | $ | 368 | | | $ | 236 | | | $ | 695 | | | $ | 109 | | | | |
| Level 2 | — | | | — | | | — | | | 20 | | | | |
| Total | $ | 368 | | | $ | 236 | | | $ | 695 | | | $ | 129 | | | | |
The Company has elected to present the fair value of derivative assets and liabilities within the consolidated balance sheets on a gross basis, even when derivative transactions are subject to master netting arrangements and may otherwise qualify for net presentation. The cash flows related to collateral posted and received are reported gross as investing and financing activities, respectively, in the consolidated statements of cash flows.
The following tables provide information as if the Company had elected to offset the asset and liability balances of derivative instruments, netted in accordance with various criteria as stipulated by the terms of the master netting arrangements with each of the counterparties. Derivatives not subject to master netting arrangements are not eligible for net presentation.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | April 28, 2023 |
| | | | Gross Amount Not Offset on the Balance Sheet | | |
| (in millions) | | Gross Amount of Recognized Assets (Liabilities) | | Financial Instruments | | Cash Collateral (Received) Posted | | Net Amount |
| Derivative assets: | | | | | | | | |
| Currency exchange rate contracts | | $ | 368 | | | $ | (189) | | | $ | (11) | | | $ | 168 | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Derivative liabilities: | | | | | | | | |
| Currency exchange rate contracts | | (236) | | | 189 | | | — | | | (48) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Total | | $ | 132 | | | $ | — | | | $ | (11) | | | $ | 121 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | April 29, 2022 |
| | | | Gross Amount Not Offset on the Balance Sheet | | |
| (in millions) | | Gross Amount of Recognized Assets (Liabilities) | | Financial Instruments | | Cash Collateral (Received) Posted | | Net Amount |
| Derivative assets: | | | | | | | | |
| Currency exchange rate contracts | | $ | 695 | | | $ | (109) | | | $ | (254) | | | $ | 332 | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Derivative liabilities: | | | | | | | | |
| Currency exchange rate contracts | | (109) | | | 109 | | | — | | | — | |
| | | | | | | | |
| Total return swaps | | (20) | | | — | | | — | | | (20) | |
| | (129) | | | 109 | | | — | | | (20) | |
| Total | | $ | 566 | | | $ | — | | | $ | (254) | | | $ | 312 | |
Concentrations of Credit Risk
Financial instruments, which potentially subject the Company to significant concentrations of credit risk, consist principally of interest-bearing investments, derivative contracts, and trade accounts receivable. Global concentrations of credit risk with respect to trade accounts receivable are limited due to the large number of customers and their dispersion across many geographic areas. The Company monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The Company maintains cash and cash equivalents, investments, and certain other financial instruments (including currency exchange rate and interest rate derivative contracts) with various major financial institutions. The Company performs periodic evaluations of the relative credit standings of these financial institutions and limits the amount of credit exposure with any one institution. In addition, the Company has collateral credit agreements with its primary derivatives counterparties. Under these agreements, either party is required to post eligible collateral when the market value of transactions covered by the agreement exceeds specific thresholds, thus limiting credit exposure for both parties. As of April 28, 2023 and April 29, 2022, the Company received net cash collateral of $11 million and $254 million, respectively, from its counterparties. Cash collateral posted is recorded as a reduction in cash and cash equivalents, with the offset recorded as an increase in other current assets in the consolidated balance sheets. Cash collateral received is recorded as an increase in cash and cash equivalents with the offset recorded in other accrued expenses in the consolidated balance sheets.
8. Inventories
Inventory balances, net of reserves, were as follows:
| | | | | | | | | | | |
| (in millions) | April 28, 2023 | | April 29, 2022 |
| Finished goods | $ | 3,440 | | | $ | 3,070 | |
| Work-in-process | 789 | | | 682 | |
| Raw materials | 1,063 | | | 864 | |
| Total | $ | 5,293 | | | $ | 4,616 | |
9. Goodwill and Other Intangible Assets
Goodwill
The following table presents the changes in the carrying amount of goodwill by segment:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Cardiovascular | | Medical Surgical | | Neuroscience | | Diabetes | | Total |
| April 30, 2021 | $ | 7,209 | | | $ | 21,195 | | | $ | 11,300 | | | $ | 2,257 | | | $ | 41,961 | |
| Goodwill as a result of acquisitions | 55 | | | — | | | 26 | | | — | | | 80 | |
| Purchase accounting adjustments | 21 | | | 3 | | | 3 | | | (2) | | | 25 | |
| Currency translation and other | (125) | | | (1,241) | | | (196) | | | (1) | | | (1,563) | |
| April 29, 2022 | 7,160 | | | 19,957 | | | 11,132 | | | 2,254 | | | 40,502 | |
| Goodwill as a result of acquisitions | 726 | | | — | | | 615 | | | — | | | 1,340 | |
| Purchase accounting adjustments | (6) | | | — | | | 2 | | | — | | | (5) | |
| Sale of RCS business | — | | | (208) | | | — | | | — | | | (208) | |
| Currency translation and other | (6) | | | (170) | | | (30) | | | 1 | | | (204) | |
| April 28, 2023 | $ | 7,873 | | | $ | 19,579 | | | $ | 11,718 | | | $ | 2,255 | | | $ | 41,425 | |
As a result of the agreement with DaVita, as disclosed in Note 3, the Company allocated $208 million of goodwill to the RCS business that met the criteria to be classified as held for sale during the first quarter of fiscal year 2023 and was subsequently sold on April 1, 2023. Upon allocation, a goodwill impairment test was performed for the RCS business, and the Company recognized $61 million of goodwill impairment charges during fiscal year 2023. The goodwill impairment charges are recognized in other operating (income) expense, net in the consolidated statements of income. The Company did not recognize any goodwill impairment charges during fiscal years 2022 or 2021.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Intangible Assets
The following table presents the gross carrying amount and accumulated amortization of intangible assets:
| | | | | | | | | | | | | | | | | | | | | | | |
| April 28, 2023 | | April 29, 2022 |
| (in millions) | Gross Carrying Amount | | Accumulated Amortization | | Gross Carrying Amount | | Accumulated Amortization |
| Definite-lived: | | | | | | | |
| Customer-related | $ | 16,956 | | | $ | (7,979) | | | $ | 16,953 | | | $ | (7,005) | |
| Purchased technology and patents | 11,659 | | | (6,277) | | | 10,802 | | | (5,667) | |
| Trademarks and tradenames | 486 | | | (280) | | | 473 | | | (266) | |
| Other | 116 | | | (69) | | | 80 | | | (69) | |
| Total | $ | 29,217 | | | $ | (14,605) | | | $ | 28,308 | | | $ | (13,006) | |
| Indefinite-lived: | | | | | | | |
| IPR&D | $ | 232 | | | $ | — | | | $ | 293 | | | $ | — | |
The Company did not recognize any definite-lived intangible asset impairment charges during fiscal year 2023. During fiscal year 2022, the Company recognized $409 million of definite-lived intangible asset impairment charges in connection with MCS within the Cardiovascular Portfolio. The intangible asset impairment charge primarily related to purchased technology and patents. Refer to Note 4 Restructuring Charges for additional information on what led to the impairment. During fiscal year 2021, the Company recognized $30 million of definite-lived intangible asset impairment charges in connection with the abandonment of certain intangible assets within the Neuroscience segment. Definite-lived intangible asset impairment charges are recognized in other operating (income) expense, net in the consolidated statements of income.
Indefinite-lived intangible asset impairment charges were not significant for fiscal year 2023 or 2022. During fiscal year 2021, the Company recognized $45 million of indefinite-lived intangible asset impairment charges related to the abandonment of certain IPR&D projects in the Neuroscience segment. Indefinite-lived intangible asset impairment charges are recognized in other operating (income) expense, net in the consolidated statements of income. Due to the nature of IPR&D projects, the Company may experience future delays or failures to obtain regulatory approvals to conduct clinical trials, failures of such clinical trials, delays or failures to obtain required market clearances, other failures to achieve a commercially viable product, or the discontinuation of certain projects, and as a result, may recognize impairment losses in the future.
Amortization Expense
Intangible asset amortization expense was $1.7 billion for fiscal years 2023 and 2022 and $1.8 billion for fiscal year 2021. Estimated aggregate amortization expense by fiscal year based on the current carrying value and remaining estimated useful lives of definite-lived intangible assets at April 28, 2023, excluding any possible future amortization associated with acquired IPR&D which has not met technological feasibility, is as follows:
| | | | | |
| (in millions) | Amortization
Expense |
| 2024 | $ | 1,676 | |
| 2025 | 1,654 | |
| 2026 | 1,641 | |
| 2027 | 1,616 | |
| 2028 | 1,565 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
10. Property, Plant, and Equipment
Property, plant, and equipment balances and corresponding estimated useful lives were as follows:
| | | | | | | | | | | | | | | | | |
| (in millions) | April 28, 2023 | | April 29, 2022 | | Estimated Useful Lives
(in years) |
| Equipment | $ | 6,707 | | | $ | 6,489 | | | Generally 2-7, up to 15 |
| Computer software | 2,952 | | | 2,617 | | | Up to 5 |
| Land and land improvements | 162 | | | 170 | | | Up to 20 |
| Buildings and leasehold improvements | 2,487 | | | 2,351 | | | Up to 40 |
| Construction in progress | 1,754 | | | 1,737 | | | — | |
| Property, plant, and equipment | 14,062 | | | 13,365 | | | |
| Less: Accumulated depreciation | (8,493) | | | (7,952) | | | |
| Property, plant, and equipment, net | $ | 5,569 | | | $ | 5,413 | | | |
Depreciation expense of $999 million, $974 million, and $919 million was recognized in fiscal years 2023, 2022, and 2021, respectively.
11. Shareholders’ Equity
Share Capital Medtronic plc is authorized to issue 2.6 billion Ordinary Shares, $0.0001 par value; 40 thousand Euro Deferred Shares, €1.00 par value; 127.5 million Preferred Shares, $0.20 par value; and 500 thousand A Preferred Shares, $1.00 par value.
Euro Deferred Shares The authorized share capital of the Company includes 40 thousand Euro Deferred Shares, with a par value of €1.00 per share. At April 28, 2023, no Euro Deferred Shares were issued or outstanding.
Preferred Shares The authorized share capital of the Company includes 127.5 million of Preferred Shares, with a par value of $0.20 per share. At April 28, 2023, no Preferred Shares were issued or outstanding.
A Preferred Shares The authorized share capital of the Company includes 500 thousand A Preferred Shares, with a par value of $1.00 per share. At April 28, 2023, no A Preferred Shares were outstanding.
Dividends The timing, declaration, and payment of future dividends to holders of the Company's ordinary shares falls within the discretion of the Company's Board of Directors and depends upon many factors, including the statutory requirements of Irish law, the Company's earnings and financial condition, the capital requirements of the Company's businesses, industry practice and any other factors the Board of Directors deems relevant.
Ordinary Share Repurchase Program Shares are repurchased on occasion to support the Company’s stock-based compensation programs and to return capital to shareholders. During fiscal years 2023 and 2022, the Company repurchased approximately 6 million and 22 million shares, respectively, at an average price of $91.31 and $113.11, respectively.
In March 2019, the Company's Board of Directors authorized $6.0 billion for repurchase of the Company's ordinary shares. There is no specific time-period associated with these repurchase authorizations. At April 28, 2023, the Company had used $3.6 billion of the $6.0 billion authorized under the repurchase program, leaving approximately $2.4 billion available for future repurchases. The Company accounts for repurchases of ordinary shares using the par value method and shares repurchased are cancelled.
12. Stock Purchase and Award Plans
In fiscal year 2023, the Company granted stock awards under the 2021 Medtronic plc Long Term Incentive Plan (2021 Plan). The 2021 Plan provides for the grant of non-qualified and incentive stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, and other stock and cash-based awards. At April 28, 2023, there were approximately 108 million shares available for future grants under the 2021 Plan.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Stock-Based Compensation Expense The following table presents the components and classification of stock-based compensation expense recognized for stock options, restricted stock, performance share units, and employee stock purchase plan (ESPP) in fiscal years 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Stock options | $ | 77 | | | $ | 70 | | | $ | 72 | |
| Restricted stock | 166 | | | 184 | | | 185 | |
| Performance share units | 74 | | | 66 | | | 49 | |
| Employee stock purchase plan | 38 | | | 39 | | | 38 | |
| Total stock-based compensation expense | $ | 355 | | | $ | 359 | | | $ | 344 | |
| | | | | |
| Cost of products sold | $ | 36 | | | $ | 36 | | | $ | 35 | |
| Research and development expense | 39 | | | 40 | | | 38 | |
| Selling, general, and administrative expense | 280 | | | 283 | | | 272 | |
| Total stock-based compensation expense | 355 | | | 359 | | | 344 | |
| Income tax benefits | (60) | | | (62) | | | (59) | |
| Total stock-based compensation expense, net of tax | $ | 295 | | | $ | 297 | | | $ | 285 | |
Stock Options Options are granted at the exercise price, which is equal to the closing price of the Company’s ordinary shares on the grant date. The majority of the Company’s options are non-qualified options with a ten-year life and a four-year ratable vesting term. The Company uses the Black-Scholes option pricing model (Black-Scholes model) to determine the fair value of stock options at the grant date. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, risk-free interest rates, volatility of the Company’s stock price, and expected dividends.
The following table provides the weighted average fair value of options granted to employees and the related assumptions used in the Black-Scholes model:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| | 2023 | | 2022 | | 2021 |
| Weighted average fair value of options granted | $ | 17.76 | | | $ | 22.83 | | | $ | 16.15 | |
| Assumptions used: | | | | | |
| Expected life (years) | 6.0 | | 6.0 | | 6.0 |
| Risk-free interest rate | 2.70 | % | | 0.90 | % | | 0.33 | % |
| Volatility | 24.05 | % | | 23.04 | % | | 24.17 | % |
| Dividend yield | 2.92 | % | | 1.95 | % | | 2.36 | % |
The following table summarizes stock option activity during fiscal year 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Options
(in thousands) | | Wtd. Avg.
Exercise
Price | | Wtd. Avg. Remaining Contractual Term (in years) | | Aggregate Intrinsic Value (in millions) |
Outstanding at April 29, 2022 | 28,263 | | | $ | 92.00 | | | | | |
| Granted | 5,470 | | | 92.96 | | | | | |
| Exercised | (1,513) | | | 59.15 | | | | | |
| Expired/Forfeited/Cancelled | (1,354) | | | 102.93 | | | | | |
Outstanding at April 28, 2023 | 30,866 | | | 93.30 | | | 5.1 | | $ | 154 | |
Expected to vest at April 28, 2023 | 8,685 | | | 103.48 | | | 8.5 | | 1 | |
Exercisable at April 28, 2023 | 21,468 | | | 88.90 | | | 3.7 | | 153 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The following table summarizes the total cash received from the issuance of new shares upon stock option award exercises, the total intrinsic value of options exercised, and the related tax benefit during fiscal years 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Cash proceeds from options exercised | $ | 77 | | | $ | 209 | | | $ | 277 | |
| Intrinsic value of options exercised | 42 | | | 174 | | | 205 | |
| Tax benefit related to options exercised | 9 | | | 40 | | | 47 | |
Unrecognized compensation expense related to outstanding stock options at April 28, 2023 was $92 million and is expected to be recognized over a weighted average period of 2.4 years.
Restricted Stock Restricted stock units are expensed over the vesting period and are subject to forfeiture if employment terminates prior to the lapse of the restrictions. The expense recognized for restricted stock units is equal to the grant date fair value, which is equal to the closing stock price on the date of grant. Restricted stock units either have a four-year ratable vesting term or cliff vest after three years. Restricted stock units are not considered issued or outstanding ordinary shares of the Company. Dividend equivalent units are accumulated on restricted stock units during the vesting period.
The following table summarizes restricted stock activity during fiscal year 2023:
| | | | | | | | | | | |
| | Units
(in thousands) | | Wtd. Avg.
Grant
Price |
Nonvested at April 29, 2022 | 5,370 | | | $ | 108.92 | |
| Granted | 2,862 | | | 91.83 | |
| Vested | (2,471) | | | 103.75 | |
| Forfeited/Cancelled | (572) | | | 105.33 | |
Nonvested at April 28, 2023 | 5,189 | | | 102.34 | |
The following table summarizes the weighted-average grant date fair value of restricted stock granted, total fair value of restricted stock vested and related tax benefit during fiscal years 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| Fiscal Year |
| (in millions, except per share data) | 2023 | | 2022 | | 2021 |
| Weighted-average grant-date fair value per restricted stock | $ | 91.83 | | | $ | 127.47 | | | $ | 99.48 | |
| Fair value of restricted stock vested | 256 | | | 194 | | | 280 | |
| Tax benefit related to restricted stock vested | 45 | | | 52 | | | 65 | |
Unrecognized compensation expense related to restricted stock as of April 28, 2023 was $338 million and is expected to be recognized over a weighted average period of 2.6 years.
Performance Share Units Beginning in fiscal year 2021, the Company granted performance share units to officers and key employees. Performance share units typically cliff vest after three years. The awards include three metrics: relative total shareholder return (rTSR), revenue growth, and return on investor capital (ROIC). rTSR is considered a market condition metric, and the expense is determined at the grant date and will not be adjusted even if the market condition is not met. Revenue growth and ROIC are considered performance metrics, and the expense is recorded over the performance period, which will be reassessed each reporting period based on the probability of achieving the various performance conditions. The number of shares earned at the end of the three-year period will vary, based on only actual performance, from 0% to 200% of the target number of performance share units granted. Performance share units are subject to forfeiture if employment terminates prior to the lapse of the restrictions. Performance share units are not considered issued or outstanding ordinary shares of the Company. Dividend equivalent units are accumulated on performance share units for each component of the award during the vesting period.
The Company calculates the fair value of the performance share units for each component individually. The fair value of the rTSR metric will be determined using the Monte Carlo valuation model. The fair value of the revenue growth and ROIC metrics are equal to the closing stock price on the grant date.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The following table summarizes performance share unit activity during fiscal year 2023:
| | | | | | | | | | | |
| | Units
(in thousands) | | Wtd. Avg.
Grant
Price |
Nonvested at April 29, 2022 | 1,581 | | | $ | 138.95 | |
| Granted | 1,204 | | | 98.17 | |
| | | |
Performance adjustments (1) | (515) | | | 129.58 | |
| Forfeited/Cancelled | (227) | | | 124.53 | |
Nonvested at April 28, 2023 | 2,043 | | | 119.88 | |
(1)Performance adjustments are adjustments to grants where the performance period has ended and actual performance is known.
The following table summarizes the weighted-average grant date fair value of performance share units granted, total fair value of performance share units vested and related tax benefit during fiscal year 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| Fiscal Year |
| (in millions, except per share data) | 2023 | | 2022 | | 2021 |
| Weighted-average grant-date fair value per performance share units | $ | 98.17 | | | $ | 149.16 | | | $ | 129.04 | |
| Fair value of performance share units vested | — | | | — | | | — | |
| Tax benefit related to performance share units vested | — | | | — | | | — | |
Unrecognized compensation expense related to performance share units as of April 28, 2023 was $84 million and is expected to be recognized over a weighted average period of 1.8 years.
Employees Stock Purchase Plan The Medtronic plc Amended and Restated 2014 Employees Stock Purchase Plan allows participating employees to purchase the Company's ordinary shares at a discount through payroll deductions. The expense recognized for shares purchased under the Company’s ESPP is equal to the 15 percent discount the employee receives. Employees purchased 3 million shares at an average price of $69.92 per share in fiscal year 2023. At April 28, 2023, approximately 4 million ordinary shares were available for future purchase under the ESPP.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
13. Income Taxes
The income tax provision is based on income before income taxes reported for financial statement purposes. The components of income before income taxes, based on tax jurisdiction, are as follows:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| U.S. | $ | 1,295 | | | $ | 436 | | | $ | (358) | |
| International | 4,069 | | | 5,081 | | | 4,253 | |
| Income before income taxes | $ | 5,364 | | | $ | 5,517 | | | $ | 3,895 | |
The income tax provision consists of the following: | | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Current tax expense: | | | | | |
| U.S. | $ | 1,303 | | | $ | 467 | | | $ | 287 | |
| International | 530 | | | 599 | | | 439 | |
| Total current tax expense | 1,833 | | | 1,066 | | | 726 | |
| Deferred tax (benefit) expense: | | | | | |
| U.S. | (336) | | | (402) | | | (625) | |
| International | 83 | | | (209) | | | 165 | |
| Net deferred tax benefit | (253) | | | (611) | | | (461) | |
Income tax provision | $ | 1,580 | | | $ | 456 | | | $ | 265 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Tax assets (liabilities), shown before jurisdictional netting of deferred tax assets (liabilities), are comprised of the following:
| | | | | | | | | | | |
| (in millions) | April 28, 2023 | | April 29, 2022 |
| Deferred tax assets: | | | |
| Intangible assets | $ | 2,259 | | | $ | 2,334 | |
| Net operating loss, capital loss, and credit carryforwards | 10,803 | | | 5,982 | |
| Capitalization of research and development | 971 | | | 597 | |
| Other accrued liabilities | 458 | | | 483 | |
| Accrued compensation | 312 | | | 332 | |
| Pension and post-retirement benefits | 66 | | | 66 | |
| Stock-based compensation | 141 | | | 146 | |
| Inventory | 135 | | | 146 | |
| Lease obligations | 150 | | | 92 | |
| Federal and state benefit on uncertain tax positions | 79 | | | 60 | |
| Interest limitation | 377 | | | 386 | |
| Unrealized gain on available-for-sale securities and derivative financial instruments | 39 | | | — | |
| Other | 277 | | | 374 | |
| Gross deferred tax assets | 16,067 | | | 10,998 | |
| Valuation allowance | (11,311) | | | (6,583) | |
| Total deferred tax assets | 4,756 | | 4,415 |
| Deferred tax liabilities: | | | |
| Intangible assets | (1,551) | | | (1,488) | |
| Realized loss on derivative financial instruments | (70) | | | (66) | |
| Right of use leases | (147) | | | (89) | |
| | | |
| Accumulated depreciation | (109) | | | (121) | |
| Outside basis difference of subsidiaries | (119) | | | (129) | |
| Other | (80) | | | (70) | |
| Total deferred tax liabilities | (2,076) | | | (1,963) | |
| Prepaid income taxes | 480 | | | 474 | |
| Income tax receivables | 494 | | | 358 | |
| Tax assets, net | $ | 3,654 | | | $ | 3,284 | |
| Reported as (after valuation allowance and jurisdictional netting): | | | |
| Other current assets | $ | 885 | | | $ | 765 | |
| Tax assets | 3,477 | | | 3,403 | |
| Deferred tax liabilities | (708) | | | (884) | |
| Tax assets, net | $ | 3,654 | | | $ | 3,284 | |
No deferred taxes have been provided on the approximately $83.7 billion and $79.3 billion of undistributed earnings of the Company’s subsidiaries at April 28, 2023 and April 29, 2022, respectively, since these earnings have been, and under current plans will continue to be, permanently reinvested in these subsidiaries. Due to the number of legal entities and jurisdictions involved, the complexity of the legal entity structure of the Company, and the complexity of the tax laws in the relevant jurisdictions, the Company believes it is not practicable to estimate, within any reasonable range, the amount of additional taxes which may be payable upon distribution of these undistributed earnings.
At April 28, 2023, the Company had approximately $43.4 billion of net operating loss carryforwards in certain non-U.S. jurisdictions, of which $20.3 billion have no expiration, and the remaining $23.1 billion will expire during fiscal years 2024 through 2040. Included in these net operating loss carryforwards are $16.2 billion of net operating losses generated in fiscal year 2008 as a result of the receipt of a favorable tax ruling from certain non-U.S. taxing authorities; and $17 billion of net operating losses generated during fiscal year 2023 as a result of an intercompany reorganization. The Company has recorded a full valuation allowance against these net operating losses, as
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
management does not believe that it is more likely than not that these net operating losses will be utilized. Certain of the remaining non-U.S. net operating loss carryforwards of $10.2 billion have a valuation allowance recorded against the carryforwards, as management does not believe that it is more likely than not that these net operating losses will be utilized.
At April 28, 2023, the Company had $545 million of U.S. federal net operating loss carryforwards, of which $359 million have no expiration. The remaining loss carryforwards will expire during fiscal years 2024 through 2036. For U.S. state purposes, the Company had $1.8 billion of net operating loss carryforwards at April 28, 2023, $207 million of which have no expiration. The remaining U.S. state loss carryforwards will expire during fiscal years 2024 through 2043.
At April 28, 2023, the Company also had $347 million of tax credits available to reduce future income taxes payable, of which $146 million have no expiration. The remaining credits will expire during fiscal years 2024 through 2042.
The Company has established valuation allowances of $11.3 billion and $6.6 billion at April 28, 2023 and April 29, 2022, respectively, primarily related to the uncertainty of the utilization of certain deferred tax assets which are primarily comprised of tax loss and credit carryforwards in various jurisdictions. The increase in the valuation allowance during fiscal year 2023 is primarily related to the generation of certain net losses resulting from an intercompany reorganization. These valuation allowances would result in a reduction to the income tax provision in the consolidated statements of income if they are ultimately not required.
The Company’s effective income tax rate varied from the U.S. federal statutory tax rate as follows:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| | 2023 | | 2022 | | 2021 |
| U.S. federal statutory tax rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| Increase (decrease) in tax rate resulting from: | | | | | |
| U.S. state taxes, net of federal tax benefit | 0.1 | | | 0.2 | | | (1.1) | |
| Research and development credit | (1.9) | | | (1.3) | | | (2.3) | |
| Puerto Rico excise tax | (1.0) | | | (1.1) | | | (2.0) | |
| International | (8.2) | | | (11.2) | | | (12.6) | |
| Stock based compensation | 0.2 | | | (0.8) | | | (0.8) | |
| Interest on uncertain tax positions | 0.7 | | | 0.5 | | | 0.9 | |
| Base erosion anti-abuse tax | — | | | 0.9 | | | 0.5 | |
| Foreign derived intangible income benefit | (1.2) | | | (1.0) | | | (1.9) | |
| Certain tax adjustments | 17.0 | | | (0.9) | | | (1.0) | |
| Legal entity restructuring | — | | | — | | | 1.8 | |
| U.S. tax on foreign earnings | 2.5 | | | 2.2 | | | 3.4 | |
| Other, net | 0.3 | | | (0.2) | | | 0.9 | |
| Effective tax rate | 29.5 | % | | 8.3 | % | | 6.8 | % |
During fiscal year 2023, the net cost from certain tax adjustments of $910 million, recognized in income tax provision in the consolidated statement of income, included the following:
•A net cost of $764 million associated with the August 18, 2022 U.S. Tax Court (Tax Court) Opinion on the previously disclosed litigation regarding the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico for fiscal years 2005 and 2006 (Opinion). While the Opinion rejected the IRS’s position and the Tax Court determined the methodology advanced by Medtronic was appropriate for purposes of determining the intercompany royalty rate between Puerto Rico and the U.S., it determined that the royalty rate should be higher, thereby increasing income allocated to the U.S. and consequently subject to U.S. tax. This case relates only to fiscal years 2005 and 2006. The Opinion remains subject to appeal by either or both parties. The Company has assumed the Tax Court findings will be applied for all years following fiscal year 2006.
•A cost of $55 million related to the disallowance of certain interest deductions.
•A cost of $30 million related to the change in reporting currency for certain carryover attributes.
•A cost of $28 million associated with the amortization of the previously established deferred tax assets from intercompany intellectual property transactions.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
•A net cost of $33 million primarily associated with the sale of half of the Company’s RCS business.
During fiscal year 2022, the net benefit from certain tax adjustments of $50 million, recognized in income tax provision in the consolidated statement of income, included the following:
•A benefit of $82 million associated with a step up in tax basis for Swiss Cantonal purposes.
•A benefit of $82 million related to a change in tax rates on intangible assets.
•A cost of $47 million associated with the amortization of the previously established deferred tax assets from intercompany intellectual property transactions.
•A cost of $41 million associated with a change in the Company’s permanent reinvestment assertion on certain historical earnings.
•A net cost of $26 million primarily associated with an intercompany sale of assets.
During fiscal year 2021, the net benefit from certain tax adjustments of $41 million, recognized in income tax provision in the consolidated statement of income, included the following:
•A net benefit of $106 million associated with the resolution of an audit at the IRS Appellate level for fiscal years 2012, 2013, and 2014. The issues resolved relate to the utilization of certain net operating losses and the allocation of income between Medtronic, Inc. and its wholly owned subsidiary operating in Puerto Rico for businesses that are not the subject of the U.S. Tax Court Case for fiscal years 2005 and 2006.
•A net cost of $73 million related to a tax basis adjustment of previously established deferred tax assets from intercompany intellectual property transactions. The cumulative amount of deferred tax benefit previously recognized from intercompany intellectual property transactions and recorded as Certain Tax Adjustments is $1.5 billion. The corresponding deferred tax assets will be amortized over a period of approximately 20 years.
•A cost of $50 million associated with the amortization of the previously established deferred tax assets from intercompany intellectual property transactions.
•A net cost of $25 million associated with an internal restructuring and intercompany sale of assets.
•A benefit of $83 million related to the capitalization of certain research and development costs for U.S. income tax purposes and the establishment of a deferred tax asset at the U.S. federal statutory tax rate.
Subsequent to year-end, on June 1, 2023 the Israeli Central-Lod District Court issued its decision in Medtronic Ventor Technologies Ltd v. Kfar Saba Assessing Office. The court determined that there was a deemed taxable transfer of intellectual property. At this time, the Company is evaluating the impact of the decision and whether or not it will appeal. The Company has currently estimated a potential income tax charge, including interest, of approximately $200 million.
Currently, the Company’s operations in Puerto Rico, Singapore, Dominican Republic, Costa Rica, and China have various tax holidays and tax incentive grants. The tax reductions as compared to the local statutory rate favorably impacted earnings by $115 million, $248 million, and $301 million in fiscal years 2023, 2022, and 2021, respectively, and diluted earnings per share by $0.09, $0.18, and $0.22, in fiscal years 2023, 2022, and 2021, respectively. The tax holidays are conditional upon the Company meeting certain thresholds required under statutory law. The tax incentive grants, unless extended, will expire between fiscal years 2024 and 2035. The Company’s historical practice has been to renew, extend, or obtain new tax incentive grants upon expiration of existing tax incentive grants. If the Company is not able to renew, extend, or obtain new tax incentive grants, the expiration of existing tax incentive grants could have a material impact on the Company’s financial results in future periods. The tax incentive grants which expired during fiscal year 2023 did not have a material impact on the Company's consolidated financial statements.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The Company had $2.7 billion, $1.7 billion, and $1.7 billion of gross unrecognized tax benefits at April 28, 2023, April 29, 2022, and April 30, 2021, respectively. A reconciliation of the beginning and ending amount of unrecognized tax benefits for fiscal years 2023, 2022, and 2021 is as follows:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Gross unrecognized tax benefits at beginning of fiscal year | $ | 1,661 | | | $ | 1,668 | | | $ | 1,862 | |
| Gross increases: | | | | | |
| Prior year tax positions | 980 | | | 1 | | | 88 | |
| Current year tax positions | 89 | | | 40 | | | 62 | |
| Gross decreases: | | | | | |
| Prior year tax positions | (12) | | | (29) | | | (106) | |
| Settlements | (4) | | | (8) | | | (216) | |
| Statute of limitation lapses | (32) | | | (11) | | | (21) | |
| Gross unrecognized tax benefits at end of fiscal year | 2,682 | | | 1,661 | | | 1,668 | |
| Cash advance paid to taxing authorities | (918) | | | (859) | | | (859) | |
| Gross unrecognized tax benefits at end of fiscal year, net of cash advance | $ | 1,764 | | | $ | 802 | | | $ | 809 | |
If all of the Company’s unrecognized tax benefits at April 28, 2023, April 29, 2022, and April 30, 2021 were recognized, $2.5 billion, $1.6 billion, and $1.6 billion would impact the Company’s effective tax rate, respectively. Although the Company believes that it has adequately reserved for liabilities resulting from tax assessments by taxing authorities, positions taken by these tax authorities could have a material impact on the Company’s effective tax rate in future periods. The Company has recorded gross unrecognized tax benefits, net of cash advance, of $1.8 billion as a noncurrent liability. The Company estimates that within the next 12 months it is reasonably possible that its uncertain tax positions, excluding interest, could decrease by as much as $10 million, net as a result of statute of limitation lapses.
The Company recognizes interest and penalties related to income tax matters in income tax provision in the consolidated statements of income and records the liability in the current or noncurrent accrued income taxes in the consolidated balance sheets, as appropriate. The Company had $61 million, $117 million, and $99 million of accrued gross interest and penalties at April 28, 2023, April 29, 2022, and April 30, 2021, respectively. During fiscal years 2023, 2022, and 2021, the Company recognized gross interest income of $55 million, expense of $17 million, and income of $44 million, respectively, in income tax provision in the consolidated statements of income.
The Company reserves for uncertain tax positions related to unresolved matters with the IRS and other taxing authorities. These reserves are subject to a high degree of estimation and management judgment. Resolution of these significant unresolved matters, or positions taken by the IRS or other tax authorities during future tax audits, could have a material impact on the Company’s financial results in future periods. The Company continues to believe that its reserves for uncertain tax positions are appropriate and that it has meritorious defenses for its tax filings and will vigorously defend them during the audit process, appellate process, and through litigation in courts, as necessary.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The major tax jurisdictions where the Company conducts business which remain subject to examination are as follows:
| | | | | | | | |
| Jurisdiction | | Earliest Year Open |
| United States - federal and state | | 2005 |
| Australia | | 2018 |
| Brazil | | 2018 |
| Canada | | 2013 |
| China | | 2015 |
| Costa Rica | | 2019 |
| Dominican Republic | | 2019 |
| France | | 2020 |
| Germany | | 2014 |
| India | | 2002 |
| Ireland | | 2012 |
| Israel | | 2010 |
| Italy | | 2018 |
| Japan | | 2019 |
| Korea | | 2022 |
| Luxembourg | | 2018 |
| Mexico | | 2014 |
| Puerto Rico | | 2014 |
| Singapore | | 2018 |
| Switzerland | | 2010 |
| United Kingdom | | 2019 |
See Note 18 for additional information regarding the status of current tax audits and proceedings.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
14. Earnings Per Share
Basic earnings per share is computed based on the weighted average number of ordinary shares outstanding. Diluted earnings per share is computed based on the weighted number of ordinary shares outstanding, increased by the number of additional shares that would have been outstanding had the potentially dilutive ordinary shares been issued, and reduced by the number of shares the Company could have repurchased with the proceeds from issuance of the potentially dilutive shares. Potentially dilutive ordinary shares include stock-based awards granted under stock-based compensation plans and shares committed to be purchased under the employee stock purchase plan.
The table below sets forth the computation of basic and diluted earnings per share:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions, except per share data) | 2023 | | 2022 | | 2021 |
| Numerator: | | | | | |
| Net income attributable to ordinary shareholders | $ | 3,758 | | | $ | 5,039 | | | $ | 3,606 | |
| Denominator: | | | | | |
| Basic – weighted average shares outstanding | 1,329.8 | | | 1,342.4 | | | 1,344.9 | |
| Effect of dilutive securities: | | | | | |
| Employee stock options | 1.5 | | | 6.6 | | | 6.6 | |
| Employee restricted stock units | 1.0 | | | 1.6 | | | 2.1 | |
| Employee performance share units | 0.5 | | | 0.8 | | | 0.5 | |
| Diluted – weighted average shares outstanding | 1,332.8 | | | 1,351.4 | | | 1,354.0 | |
| | | | | |
| Basic earnings per share | $ | 2.83 | | | $ | 3.75 | | | $ | 2.68 | |
| Diluted earnings per share | $ | 2.82 | | | $ | 3.73 | | | $ | 2.66 | |
The calculation of weighted average diluted shares outstanding excludes options to purchase approximately 23 million, 5 million, and 4 million ordinary shares in fiscal year 2023, 2022, and 2021, respectively because their effect would have been anti-dilutive on the Company’s earnings per share.
15. Retirement Benefit Plans
The Company sponsors various retirement benefit plans, including defined benefit pension plans, post-retirement medical plans, defined contribution savings plans, and termination indemnity plans, covering substantially all U.S. employees and many employees outside the U.S. The net expense related to these plans was $494 million, $459 million, and $668 million in fiscal years 2023, 2022, and 2021, respectively.
In the U.S., the Company maintains qualified pension plans designed to provide guaranteed minimum retirement benefits to all eligible U.S. participants. Pension coverage for non-U.S. employees is provided, to the extent deemed appropriate, through separate plans. In addition to the benefits provided under the qualified pension plan, retirement benefits associated with wages in excess of the IRS allowable limits are provided to certain employees under a non-qualified plan. U.S. and Puerto Rico employees are also eligible to receive a medical benefit component, in addition to normal retirement benefits, through the Company’s post-retirement benefits.
At April 28, 2023 and April 29, 2022, the funded status of the Company’s benefit plans was $103 million overfunded and $74 million overfunded, respectively.
During fiscal years 2023 and 2021, the Company offered certain eligible U.S. employees voluntary early retirement packages, resulting in charges of $94 million and $97 million, respectively, primarily related to U.S. pension benefits. The charges were recognized in restructuring charges, net in the consolidated statements of income. See Note 4 for additional information on restructuring charges.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Defined Benefit Pension Plans The change in benefit obligation and funded status of the Company’s U.S. and Non-U.S. pension benefits are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Pension Benefits(2) | | Non-U.S. Pension Benefits |
| | Fiscal Year | | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2023 | | 2022 |
| Accumulated benefit obligation at end of year: | $ | 3,348 | | | $ | 3,396 | | | $ | 1,422 | | | $ | 1,638 | |
| Change in projected benefit obligation: | | | | | | | |
| Projected benefit obligation at beginning of year | $ | 3,526 | | | $ | 3,979 | | | $ | 1,740 | | | $ | 2,294 | |
| Service cost | 77 | | | 98 | | | 43 | | | 64 | |
| Interest cost | 142 | | | 102 | | | 38 | | | 26 | |
| Employee contributions | — | | | — | | | 9 | | | 12 | |
| Plan curtailments, settlements, and amendments | (19) | | | — | | | (8) | | | (11) | |
Actuarial (gain) loss(1) | (210) | | | (513) | | | (303) | | | (394) | |
| Benefits paid | (140) | | | (141) | | | (63) | | | (48) | |
Special termination benefits(3) | 74 | | | — | | | — | | | — | |
| Currency exchange rate changes and other | — | | | — | | | 43 | | | (203) | |
| Projected benefit obligation at end of year | $ | 3,451 | | | $ | 3,526 | | | $ | 1,499 | | | $ | 1,740 | |
| Change in plan assets: | | | | | | | |
| Fair value of plan assets at beginning of year | $ | 3,559 | | | $ | 3,660 | | | $ | 1,732 | | | $ | 1,900 | |
| Actual return on plan assets | (43) | | | 15 | | | (163) | | | (12) | |
| Employer contributions | 22 | | | 24 | | | 57 | | | 70 | |
| Employee contributions | — | | | — | | | 9 | | | 12 | |
| Plan settlements | — | | | — | | | (8) | | | (1) | |
| Benefits paid | (140) | | | (141) | | | (63) | | | (48) | |
| Currency exchange rate changes and other | — | | | — | | | 50 | | | (188) | |
| Fair value of plan assets at end of year | $ | 3,398 | | | $ | 3,559 | | | $ | 1,614 | | | $ | 1,732 | |
| Funded status at end of year: | | | | | | | |
| Fair value of plan assets | $ | 3,398 | | | $ | 3,559 | | | $ | 1,614 | | | $ | 1,732 | |
| Benefit obligations | 3,451 | | | 3,526 | | | 1,499 | | | 1,740 | |
| Over (under) funded status of the plans | (53) | | | 33 | | | 115 | | | (8) | |
| Recognized asset (liability) | $ | (53) | | | $ | 33 | | | $ | 115 | | | $ | (8) | |
Amounts recognized on the consolidated
balance sheets consist of: |
| Non-current assets | $ | 221 | | | $ | 313 | | | $ | 350 | | | $ | 240 | |
| Current liabilities | (24) | | | (21) | | | (6) | | | (6) | |
| Non-current liabilities | (250) | | | (259) | | | (228) | | | (242) | |
| Recognized asset (liability) | $ | (53) | | | $ | 33 | | | $ | 115 | | | $ | (8) | |
Amounts recognized in accumulated other
comprehensive loss: |
| Prior service cost (credit) | $ | (19) | | | $ | — | | | $ | (3) | | | $ | (4) | |
| Net actuarial loss | 891 | | | 854 | | | 76 | | | 161 | |
| Ending balance | $ | 873 | | | $ | 854 | | | $ | 73 | | | $ | 157 | |
(1)Actuarial gains and losses result from changes in actuarial assumptions (such as changes in the discount rate and revised mortality rates). The actuarial gain in fiscal year 2023 and 2022 was primarily related to increases in discount rates.
(2)As of April 24, 2020, the Company announced the freezing of the U.S. pension benefits beginning Plan year 2028. Employees will continue to earn benefits as required by the Medtronic Retirement Plan until April 30, 2027, after which date benefits will no longer be earned and employees will earn benefits through the Medtronic Savings and Investment Plan.
(3)This represents a portion of the total voluntary early retirement package charges for fiscal year 2023.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
In certain countries outside the U.S., fully funding pension plans is not a common practice, as funding provides no income tax benefit. Consequently, certain pension plans were partially funded at April 28, 2023 and April 29, 2022. U.S. and non-U.S. pension plans with accumulated benefit obligations in excess of plan assets consist of the following:
| | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 |
| Accumulated benefit obligation | $ | 731 | | | $ | 830 | |
| Projected benefit obligation | 772 | | | 880 | |
| Plan assets at fair value | 301 | | | 356 | |
U.S. and non-U.S. pension plans with projected benefit obligations in excess of plan assets consist of the following: | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 |
| Projected benefit obligation | $ | 1,285 | | | $ | 907 | |
| Plan assets at fair value | 776 | | | 379 | |
The net periodic benefit cost of the plans includes the following components: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Pension Benefits | | Non-U.S. Pension Benefits |
| | Fiscal Year | | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| Service cost | $ | 77 | | | $ | 98 | | | $ | 106 | | | $ | 43 | | | $ | 64 | | | $ | 70 | |
| Interest cost | 142 | | | 102 | | | 109 | | | 38 | | | 26 | | | 28 | |
| Expected return on plan assets | (224) | | | (226) | | | (242) | | | (58) | | | (64) | | | (59) | |
| Amortization of prior service cost | — | | | — | | | 1 | | | (1) | | | (1) | | | (1) | |
| Amortization of net actuarial loss | 20 | | | 64 | | | 69 | | | 2 | | | 22 | | | 25 | |
| Settlement and curtailment (gain) loss | — | | | — | | | — | | | 2 | | | (10) | | | 1 | |
| Special termination benefits | 74 | | | — | | | 73 | | | — | | | — | | | — | |
| Net periodic benefit cost | $ | 89 | | | $ | 39 | | | $ | 116 | | | $ | 26 | | | $ | 37 | | | $ | 64 | |
The other changes in plan assets and projected benefit obligations recognized in other comprehensive income for fiscal year 2023 are as follows:
| | | | | | | | | | | |
| (in millions) | U.S. Pension
Benefits | | Non-U.S.
Pension
Benefits |
| Net actuarial loss (gain) | $ | 58 | | | $ | (82) | |
| Prior service cost (credit) | (19) | | | — | |
| Amortization of prior service credit | — | | | 1 | |
| Amortization and settlement recognition of actuarial loss | (20) | | | (4) | |
| Effect of exchange rates | — | | | 3 | |
| Total recognized in other comprehensive income | 19 | | | (82) | |
| Total recognized in net periodic benefit cost and other comprehensive income | $ | 108 | | | $ | (57) | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The actuarial assumptions are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Pension Benefits | | Non-U.S. Pension Benefits |
| | Fiscal Year | | Fiscal Year |
| | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| Critical assumptions – projected benefit obligation: | | | | | | | | | | | |
| Discount rate | 4.73% - 4.99% | | 4.23% - 4.48% | | 2.80% - 3.50% | | 1.30% - 10.70% | | 0.60% - 25.40% | | 0.30% - 13.30% |
| Rate of compensation increase | 3.90 | % | | 4.83 | % | | 4.83 | % | | 2.75 | % | | 2.70 | % | | 2.90 | % |
| Critical assumptions – net periodic benefit cost: | | | | | | | | | | | |
Discount rate – benefit obligation | 4.23% - 4.48% | | 2.80% - 3.46% | | 3.10% - 3.70% | | 0.60% - 25.40% | | 0.25% - 12.80% | | 0.30% - 13.90% |
Discount rate – service cost | 4.12% - 4.51% | | 2.50% - 3.51% | | 2.60% - 3.90% | | 0.60% - 25.40% | | 0.24% - 12.80% | | 0.30% - 13.90% |
Discount rate – interest cost | 3.90% - 4.23% | | 2.08% - 2.87% | | 2.80% - 3.20% | | 0.60% - 25.40% | | 0.08% - 12.80% | | 0.30% - 13.90% |
| Expected return on plan assets | 5.30% - 7.20% | | 5.60% - 7.40% | | 7.50 | % | | 3.48 | % | | 3.67 | % | | 3.78 | % |
| Rate of compensation increase | 3.90 | % | | 3.90% - 4.83% | | 3.90 | % | | 2.70 | % | | 2.90 | % | | 2.91 | % |
The Company utilizes a full yield curve approach methodology to estimate the service and interest cost components of net periodic pension cost and net periodic post-retirement benefit cost for the Company’s pension and other post-retirement benefits. The full yield curve approach applies specific spot rates along the yield curve to their underlying projected cash flows in estimation of the cost components. The current yield curves represent high quality, long-term fixed income instruments.
The expected long-term rate of return on plan assets assumptions are determined using a building block approach, considering historical averages and real returns of each asset class. In certain countries, where historical returns are not meaningful, consideration is given to local market expectations of long-term returns.
Retirement Benefit Plan Investment Strategy The Company sponsors trusts that hold the assets for U.S. pension plans and other U.S. post-retirement benefit plans, primarily retiree medical benefits. For investment purposes, the Medtronic U.S. pension and other U.S. post-retirement benefit plans employ similar investment strategies with different asset allocation targets.
The Company has a Qualified Plan Committee (the Plan Committee) that sets investment guidelines for U.S. pension plans and other U.S. post-retirement benefit plans with the assistance of external consultants. These guidelines are established based on market conditions, risk tolerance, funding requirements, and expected benefit payments. The Plan Committee also oversees the investment allocation process, selects the investment managers, and monitors asset performance. As pension liabilities are long-term in nature, the Company employs a long-term total return approach to maximize the long-term rate of return on plan assets for a prudent level of risk. An annual analysis on the risk versus the return of the investment portfolio is conducted to justify the expected long-term rate of return assumption.
The investment portfolios contain a diversified allocation of investment categories, including equities, fixed income securities, hedge funds, and private equity. Securities are also diversified in terms of domestic and international, short- and long-term, growth and value styles, large cap and small cap stocks, and active and passive management.
Outside the U.S., pension plan assets are typically managed by decentralized fiduciary committees. There is significant variation in policy asset allocation from country to country. Local regulations, funding rules, and financial and tax considerations are part of the funding and investment allocation process in each country. The weighted average target asset allocations at April 28, 2023 for the plans are 42% equity securities, 34% debt securities, and 24% other.
The plans did not hold any investments in the Company’s ordinary shares at April 28, 2023 or April 29, 2022.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The Company’s U.S. plans target asset allocations at April 28, 2023, compared to the U.S. plans actual asset allocations at April 28, 2023 and April 29, 2022 by asset category, are as follows:
| | | | | | | | | | | | | | | | | |
| U.S. Plans | Target Allocation | | Actual Allocation |
| | April 28, 2023 | | April 28, 2023 | | April 29, 2022 |
| Asset Category: | | | | | |
| Equity securities | 34 | % | | 36 | % | | 36 | % |
| Debt securities | 51 | | | 46 | | | 45 | |
| Other | 15 | | | 19 | | | 19 | |
| Total | 100 | % | | 100 | % | | 100 | % |
Strong performance on equity securities during the fiscal year resulted in asset allocations different than targets. Management expects to move the allocations closer to target over the intermediate term.
Retirement Benefit Plan Asset Fair Values The following is a description of the valuation methodologies used for retirement benefit plan assets measured at fair value:
Short-term investments: Valued at the closing price reported in the active markets in which the individual security is traded.
Mutual funds: Comprised of investments in equity and fixed income securities held in pooled investment vehicles. The valuations of mutual funds are based on the respective net asset values which are determined by the fund daily at market close. The net asset values are calculated based on the valuation of the underlying assets which are determined using observable inputs. The net asset values are publicly reported.
Equity commingled trusts: Comprised of investments in equity securities held in pooled investment vehicles. The valuations of equity commingled trusts are based on the respective net asset values which are determined by the fund daily at market close. The net asset values are calculated based on the valuation of the underlying assets which are determined using observable inputs. The net asset values are not publicly reported, and funds are valued at the net asset value practical expedient.
Fixed income commingled trusts: Comprised of investments in fixed income securities held in pooled investment vehicles. The valuations of fixed income commingled trusts are based on the respective net asset values which are determined by the fund daily at market close. The net asset values are calculated based on the valuation of the underlying assets which are determined using observable inputs. The net asset values are not publicly reported, and funds are valued at the net asset value practical expedient.
Partnership units: Valued based on the year-end net asset values of the underlying partnerships. The net asset values of the partnerships are based on the fair values of the underlying investments of the partnerships. Quoted market prices are used to value the underlying investments of the partnerships, where the partnerships consist of the investment pools which invest primarily in common stocks. Partnership units include partnerships, private equity investments, and real estate investments. Partnerships primarily include long/short equity and absolute return strategies. These investments may be redeemed monthly with notice periods ranging from 45 to 95 days. At April 28, 2023, there are no funds in the process of liquidation. Private equity investments consist of common stock and debt instruments of private companies. For private equity funds, the sum of the unfunded commitments at April 28, 2023 is $233 million, and the estimated liquidation period of these funds is expected to be one to 15 years. Real estate investments consist of illiquid real estate holdings. These investments have investment and liquidation periods expected to total 3 years to 10 years in aggregate. At April 28, 2023, there are no real estate investments in the process of liquidation. Valuation procedures are utilized to arrive at fair value if a quoted market price is not available for a partnership investment.
Registered investment companies: Valued at net asset values which are not publicly reported. The net asset values are calculated based on the valuation of the underlying assets. The underlying assets are valued at the quoted market prices of shares held by the plan at year-end in the active market on which the individual securities are traded.
Insurance contracts: Comprised of investments in collective (group) insurance contracts, consisting of individual insurance policies. The policyholder is the employer, and each member is the owner/beneficiary of their individual insurance policy. These policies are a part of the insurance company’s general portfolio and participate in the insurer’s profit-sharing policy on an excess yield basis.
The methods described above may produce fair values that may not be indicative of net realizable value or reflective of future fair values. Furthermore, while the Company believes its valuation methodologies are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine fair value of certain financial instruments could result in a different fair value measurement at the reporting date.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The following tables provide information by level for the retirement benefit plan assets that are measured at fair value, as defined by U.S. GAAP. Certain investments for which the fair value is measured using the net asset value per share (or its equivalent) practical expedient are not presented within the fair value hierarchy. The fair value amounts presented for these investments are intended to permit reconciliation to the total fair value of plan assets at April 28, 2023 and April 29, 2022.
U.S. Pension Benefits
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value at | | | | |
| | Fair Value Measurements
Using Inputs Considered as | | Investments Measured at Net Asset Value |
| (in millions) | April 28, 2023 | Level 1 | | Level 2 | | Level 3 | |
| Short-term investments | $ | 114 | | | $ | 114 | | | $ | — | | | $ | — | | | $ | — | |
| Mutual funds | 114 | | | 114 | | | — | | | — | | | — | |
| Equity commingled trusts | 1,211 | | | — | | | — | | | — | | | 1,211 | |
| Fixed income commingled trusts | 968 | | | — | | | — | | | — | | | 968 | |
| Partnership units | 992 | | | — | | | — | | | 992 | | | — | |
| $ | 3,398 | | | $ | 227 | | | $ | — | | | $ | 992 | | | $ | 2,179 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value at | | Fair Value Measurements
Using Inputs Considered as | | Investments Measured at Net Asset Value |
| (in millions) | April 29, 2022 | Level 1 | | Level 2 | | Level 3 | |
| Short-term investments | $ | 73 | | | $ | 73 | | | $ | — | | | $ | — | | | $ | — | |
| Mutual funds | 125 | | | 125 | | | — | | | — | | | — | |
| Equity commingled trusts | 1,281 | | | — | | | — | | | — | | | 1,281 | |
| Fixed income commingled trusts | 1,069 | | | — | | | — | | | — | | | 1,069 | |
| Partnership units | 1,011 | | | — | | | — | | | 1,011 | | | — | |
| $ | 3,559 | | | $ | 197 | | | $ | — | | | $ | 1,011 | | | $ | 2,350 | |
The following tables provide a reconciliation of the beginning and ending balances of U.S. pension benefit assets measured at fair value that used significant unobservable inputs (Level 3):
| | | | | |
| (in millions) | Partnership Units |
April 30, 2021 | $ | 860 | |
| Total realized gains, net | 28 | |
| Total unrealized gains, net | 72 | |
| Purchases and sales, net | 51 | |
April 29, 2022 | 1,011 | |
| Total realized gains, net | 67 | |
| Total unrealized gains, net | 151 | |
| Purchases and sales, net | (238) | |
April 28, 2023 | $ | 992 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Non-U.S. Pension Benefits | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value at | | Fair Value Measurements
Using Inputs Considered as | | Investments Measured at Net Asset Value |
| (in millions) | April 28, 2023 | Level 1 | | Level 2 | | Level 3 | |
| Registered investment companies | $ | 1,571 | | | $ | — | | | $ | — | | | $ | — | | | $ | 1,571 | |
| Insurance contracts | 44 | | | — | | | — | | | 44 | | | — | |
| $ | 1,614 | | | $ | — | | | $ | — | | | $ | 44 | | | $ | 1,571 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value at | | Fair Value Measurements
Using Inputs Considered as | | Investments Measured at Net Asset Value |
| (in millions) | April 29, 2022 | Level 1 | | Level 2 | | Level 3 | |
| Registered investment companies | $ | 1,689 | | | $ | — | | | $ | — | | | $ | — | | | $ | 1,689 | |
| Insurance contracts | 43 | | | — | | | — | | | 43 | | | — | |
| $ | 1,732 | | | $ | — | | | $ | — | | | $ | 43 | | | $ | 1,689 | |
Non-U.S. pension benefit assets that are valued using significant unobservable inputs (Level 3) was $44 million and $43 million as of April 28, 2023 and April 29, 2022, respectively. The decrease in the fair value of the assets was due to insurance contracts being sold.
There were no transfers into or out of Level 3 for both the U.S. and non-U.S. pension plans during the fiscal years ended April 28, 2023 and April 29, 2022.
Retirement Benefit Plan Funding It is the Company’s policy to fund retirement costs within the limits of allowable tax deductions. During fiscal year 2023, the Company made discretionary contributions of approximately $22 million to the U.S. pension plan. Internationally, the Company contributed approximately $57 million for pension benefits during fiscal year 2023. The Company anticipates that it will make contributions of $24 million and $43 million to its U.S. pension benefit plans and non-U.S. pension benefit plans, respectively, in fiscal year 2024. Based on the guidelines under the U.S. Employee Retirement Income Security Act of 1974 and the various guidelines which govern the plans outside the U.S., the majority of anticipated fiscal year 2024 contributions will be discretionary. The Company believes that pension assets, returns on invested pension assets, and Company contributions will be able to meet its pension and other post-retirement obligations in the future.
Retiree benefit payments, which reflect expected future service, are anticipated to be paid as follows:
| | | | | | | | | | | |
| (in millions) | Gross Payments |
| Fiscal Year | U.S. Pension Benefits | | Non-U.S. Pension Benefits |
| 2024 | $ | 168 | | | $ | 64 | |
| 2025 | 178 | | | 62 | |
| 2026 | 188 | | | 62 | |
| 2027 | 200 | | | 68 | |
| 2028 | 213 | | | 69 | |
| 2029 – 2033 | 1,171 | | | 411 | |
| | | |
Post-retirement Benefit Plans The net periodic benefit cost associated with the Company’s post-retirement benefit plans was income of $11 million, $20 million, and $6 million in fiscal years 2023, 2022, and 2021, respectively. The Company’s projected benefit obligation for all post-retirement benefit plans was $261 million and $276 million at April 28, 2023 and April 29, 2022, respectively. The Company’s fair value of plan assets for all post-retirement benefit plans was $302 million and $325 million at April 28, 2023 and April 29, 2022, respectively. The post-retirement benefit plan assets at both April 28, 2023 and April 29, 2022 primarily comprised of equity and fixed commingled trusts, consistent with the U.S. retirement benefit plan assets outlined in the fair value leveling tables above.
Defined Contribution Savings Plans The Company has defined contribution savings plans that cover substantially all U.S. employees and certain non-U.S. employees. The general purpose of these plans is to provide additional financial security during retirement by providing employees with an incentive to make regular savings. Company contributions to the plans are based on employee contributions and Company performance. Expense recognized under these plans was $390 million, $403 million, and $495 million in fiscal years 2023, 2022, and 2021, respectively.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Effective May 1, 2005, the Company froze participation in the original defined benefit pension plan in the U.S. and implemented two new plans: an additional defined benefit pension plan, the Personal Pension Account (PPA), and a new defined contribution plan, the Personal Investment Account (PIA). Employees in the U.S. hired on or after May 1, 2005 but before January 1, 2016 had the option to participate in either the PPA or the PIA. Participants in the PPA receive an annual allocation of their salary and bonus on which they will receive an annual guaranteed rate of return, which is based on the ten-year Treasury bond rate. Participants in the PIA also receive an annual allocation of their salary and bonus; however, they are allowed to determine how to invest their funds among identified fund alternatives. The cost associated with the PPA is included in U.S. Pension Benefits in the tables presented earlier. The defined contribution cost associated with the PIA was approximately $43 million, $48 million, and $50 million in fiscal years 2023, 2022, and 2021, respectively.
Effective January 1, 2016, the Company froze participation in the existing defined benefit (PPA) and contribution (PIA) pension plans in the U.S. and implemented a new form of benefit under the existing defined contribution plan for legacy Covidien employees and employees in the U.S. hired on or after January 1, 2016 or rehired after July 1, 2020. Participants in the Medtronic Core Contribution (MCC) also receive an annual allocation of their salary and bonus and are allowed to determine how to invest their funds among identified fund alternatives. The defined contribution cost associated with the MCC was approximately $93 million, $83 million, and $73 million and in fiscal years 2023, 2022, and 2021, respectively.
16. Leases
The Company leases office, manufacturing, and research facilities and warehouses, as well as transportation, data processing, and other equipment. The Company determines whether a contract is a lease or contains a lease at inception date. Upon commencement, the Company recognizes a right-of-use asset and lease liability. Right-of-use assets represent the Company's right to use the underlying asset for the lease term. Lease liabilities are the Company's obligation to make the lease payments arising from a lease. As the Company’s leases typically do not provide an implicit rate, the Company’s lease liabilities are measured on a discounted basis using the Company's incremental borrowing rate. Lease terms used in the recognition of right-of-use assets and lease liabilities include only options to extend the lease that are reasonably certain to be exercised. Additionally, lease terms underlying the right-of-use assets and lease liabilities consider terminations that are reasonably certain to be executed.
The Company's lease agreements include leases that have both lease and associated nonlease components. The Company has elected to account for lease components and the associated nonlease components as a single lease component. The consolidated balance sheets do not include recognized assets or liabilities for leases that, at the commencement date, have a term of twelve months or less and do not include an option to purchase the underlying asset that is reasonably certain to be exercised. The Company recognizes such leases in the consolidated statements of income on a straight-line basis over the lease term. Additionally, the Company recognizes variable lease payments not included in its lease liabilities in the period in which the obligation for those payments is incurred. Variable lease payments for fiscal year 2023, 2022, and 2021 were not material.
The Company's lease agreements include leases accounted for as operating leases and those accounted for as finance leases. The right-of-use assets, lease liabilities, lease costs, cash flows, and lease maturities associated with the Company's finance leases were not material to the consolidated financial statements at April 28, 2023 or April 29, 2022 or for fiscal year 2023, 2022 and 2021. Finance lease right-of-use assets are included in property, plant, and equipment, net, and finance lease liabilities are included in current debt obligations and long-term debt on the consolidated balance sheets.
The following table summarizes the balance sheet classification of the Company's operating leases and amounts of the right-of-use assets and lease liabilities at April 28, 2023 and April 29, 2022:
| | | | | | | | | | | | | | | | | |
| (in millions) | Balance Sheet Classification | | April 28, 2023 | | April 29, 2022 |
| Right-of-use assets | Other assets | | $ | 1,041 | | | $ | 854 | |
| Current liability | Other accrued expenses | | 180 | | | 167 | |
| Non-current liability | Other liabilities | | 869 | | | 703 | |
The following table summarizes the weighted-average remaining lease term and weighted-average discount rate for the Company's operating leases at April 28, 2023, April 29, 2022, and April 30, 2021:
| | | | | | | | | | | | | | | | | | | | |
| | April 28, 2023 | | April 29, 2022 | | April 30, 2021 |
| Weighted-average remaining lease term | | 9.1 Years | | 7.3 Years | | 7.5 years |
| Weighted-average discount rate | | 2.4% | | 2.0% | | 2.3% |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The following table summarizes the components of total operating lease cost for fiscal year 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Operating lease cost | | $ | 211 | | | $ | 195 | | | $ | 216 | |
| Short-term lease cost | | 62 | | | 65 | | | 35 | |
| Total operating lease cost | | $ | 273 | | | $ | 260 | | | $ | 251 | |
The following table summarizes the cash paid for amounts included in the measurement of operating lease liabilities and right-of-use assets obtained in exchange for operating lease liabilities for fiscal year 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Cash paid for amounts included in the measurement of operating lease liabilities | | $ | 210 | | | $ | 174 | | | $ | 216 | |
| Right-of-use assets obtained in exchange for operating lease liabilities | | 417 | | | 78 | | | 230 | |
The following table summarizes the maturities of the Company's operating leases at April 28, 2023:
| | | | | | | | |
(in millions)
Fiscal Year | | Operating Leases |
| 2024 | | $ | 204 | |
| 2025 | | 171 | |
| 2026 | | 144 | |
| 2027 | | 121 | |
| 2028 | | 94 | |
| Thereafter | | 426 | |
| Total expected lease payments | | 1,160 | |
| Less: Imputed interest | | (111) | |
| Total lease liability | | $ | 1,049 | |
The Company makes certain products available to customers under lease arrangements, including arrangements whereby equipment is placed with customers who then purchase consumable products to accompany the use of the equipment. Income arising from arrangements where the Company is the lessor is recognized within net sales in the consolidated statements of income and the Company's net investments in sales-type leases are included in other current assets and other assets in the consolidated balance sheets. Lessor income and the related assets and lease maturities were not material to the consolidated financial statements at or for the fiscal year ended April 28, 2023 and April 29, 2022.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
17. Accumulated Other Comprehensive Loss
The following table provides changes in accumulated other comprehensive loss (AOCI), net of tax, and by component: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Unrealized (Loss) Gain on Investment Securities | | Cumulative Translation Adjustments | | Net Investment Hedges | | Net Change in Retirement Obligations | | Unrealized (Loss) Gain on Cash Flow Hedges | | Total Accumulated Other Comprehensive (Loss) Income |
| April 24, 2020 | $ | — | | | $ | (2,210) | | | $ | 236 | | | $ | (1,852) | | | $ | 266 | | | $ | (3,560) | |
| Other comprehensive income (loss) before reclassifications | 92 | | | 1,691 | | | (1,694) | | | 432 | | | (541) | | | (20) | |
| Reclassifications | — | | | — | | | — | | | 73 | | | 22 | | | 95 | |
| Other comprehensive income (loss) | 92 | | | 1,691 | | | (1,694) | | | 505 | | | (519) | | | 75 | |
| | | | | | | | | | | |
| April 30, 2021 | 92 | | | (519) | | | (1,458) | | | (1,347) | | | (253) | | | (3,485) | |
| Other comprehensive income (loss) before reclassifications | (304) | | | (2,080) | | | 2,299 | | | 514 | | | 781 | | | 1,210 | |
| Reclassifications | 3 | | | — | | | — | | | 60 | | | (54) | | | 9 | |
| Other comprehensive income (loss) | (301) | | | (2,080) | | | 2,299 | | | 574 | | | 727 | | | 1,219 | |
| | | | | | | | | | | |
| April 29, 2022 | (209) | | | (2,599) | | | 841 | | | (773) | | | 474 | | | (2,265) | |
| Other comprehensive income (loss) before reclassifications | (78) | | | (240) | | | (596) | | | 26 | | | 184 | | | (704) | |
| Reclassifications | 29 | | | — | | | — | | | 6 | | | (565) | | | (530) | |
| Other comprehensive income (loss) | (49) | | | (240) | | | (596) | | | 32 | | | (381) | | | (1,234) | |
| | | | | | | | | | | |
| April 28, 2023 | $ | (258) | | | $ | (2,839) | | | $ | 245 | | | $ | (741) | | | $ | 93 | | | $ | (3,499) | |
The income tax on gains and losses on investment securities in other comprehensive income before reclassifications during fiscal years 2023, 2022, and 2021 was a benefit of $21 million, a benefit of $51 million, and an expense of $31 million, respectively. During fiscal years 2023, 2022, and 2021, realized gains and losses on investment securities reclassified from AOCI were reduced by income taxes of $9 million, $1 million and $2 million, respectively. When realized, gains and losses on investment securities reclassified from AOCI are recognized within other non-operating income, net. Refer to Note 5 for additional information.
During fiscal years 2023, 2022, and 2021, the income tax on cumulative translation adjustment was a benefit of $5 million, a benefit of $8 million, and an expense of $7 million, respectively.
During fiscal years 2023, 2022, and 2021, there were no tax impacts on net investment hedges. Refer to Note 7 for additional information.
The net change in retirement obligations in other comprehensive income includes amortization of net actuarial losses included in net periodic benefit cost. The income tax on the net change in retirement obligations in other comprehensive income before reclassifications during fiscal years 2023, 2022, and 2021 resulted in an expense of $6 million, $134 million, and $115 million, respectively. During fiscal years 2023, 2022, and 2021, the gains and losses on defined benefit and pension items reclassified from AOCI were reduced by income taxes of $9 million, $20 million, and $16 million, respectively. When realized, net gains and losses on defined benefit and pension items reclassified from AOCI are recognized within other non-operating income, net. Refer to Note 15 for additional information.
The income tax on unrealized gains and losses on cash flow hedges in other comprehensive income before reclassifications during fiscal years 2023, 2022, and 2021 was an expense of $56 million, an expense of $152 million, and a benefit of $87 million, respectively. Amounts reclassified from AOCI related to cash flow hedges included income taxes of $133 million, $26 million, and $14 million for fiscal years 2023, 2022, and 2021, respectively. When realized, gains and losses on currency exchange rate contracts reclassified from AOCI are recognized within other operating (income) expense, net or cost of products sold. Refer to Note 7 for additional information.
18. Commitments and Contingencies
Legal Matters
The Company and its affiliates are involved in a number of legal actions from time to time involving product liability, employment, intellectual property and commercial disputes, shareholder related matters, environmental proceedings, tax disputes, and governmental proceedings and investigations, including those described below. With respect to governmental proceedings and investigations, like other companies in our industry, the Company is subject to extensive regulation by national, state, and local governmental agencies in the United States and in other jurisdictions in which the Company and its affiliates operate. As a result, interaction with governmental agencies is
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
ongoing. The Company’s standard practice is to cooperate with regulators and investigators in responding to inquiries. The outcomes of legal actions are not within the Company’s complete control and may not be known for prolonged periods of time. In some actions, the enforcement agencies or private claimants seek damages, as well as other civil or criminal remedies (including injunctions barring the sale of products that are the subject of the proceeding), that could require significant expenditures, result in lost revenues, or limit the Company's ability to conduct business in the applicable jurisdictions.
The Company records a liability in the consolidated financial statements on an undiscounted basis for loss contingencies related to legal actions when a loss is known or considered probable and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. When determining the estimated loss or range of loss, significant judgment is required. Estimates of probable losses resulting from litigation and governmental proceedings involving the Company are inherently difficult to predict, particularly when the matters are in early procedural stages with incomplete scientific facts or legal discovery, involve unsubstantiated or indeterminate claims for damages, potentially involve penalties, fines or punitive damages, or could result in a change in business practice. The Company classifies certain specified litigation charges and gains related to significant legal matters as certain litigation charges, net in the consolidated statements of income. During fiscal years 2023, 2022, and 2021, the Company recognized a net $30 million of certain litigation income and $95 million and $188 million of certain litigation charges, respectively. At April 28, 2023 and April 29, 2022, accrued litigation was approximately $0.3 billion. The ultimate cost to the Company with respect to accrued litigation could be materially different than the amount of the current estimates and accruals and could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows. The Company includes accrued litigation in other accrued expenses and other liabilities on the consolidated balance sheets. While it is not possible to predict the outcome for most of the legal matters discussed below, the Company believes it is possible that the costs associated with these matters could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows.
Intellectual Property Matters
At any given time, the Company is involved in litigation relating to patents, trademarks, copyrights, trade secrets, and other intellectual property (IP) rights, and licenses, acquisitions or other agreements relating to such rights. This litigation includes, but is not limited to, alleged infringement or misappropriation of IP rights, or breach of obligations related to IP rights, or other claims asserted by competitors, individuals, or, consistent with a growing trend across technology-intensive industries, other entities created specifically to fund IP litigation. While the outcome of these litigation matters is inherently uncertain, it is possible that the results of such litigation could require the Company to pay significant monetary damages and/or royalty payments, and negatively impact the Company's ability to sell current or future products, which could have a material adverse impact on the Company's business, results of operations, financial condition, and cash flows.
Colibri
The Company is a defendant in patent litigation brought by Colibri Heart Valve LLC (Colibri) in the U.S. District Court for the Central District of California. Colibri alleges infringement of one patent by the Company’s Evolut family of transcatheter aortic valve replacement devices. The patent asserted by Colibri has expired. On February 8, 2023, a jury returned a verdict against the Company for approximately $106 million. The Company has strong arguments to appeal the verdict and is pursuing its appeal. The Company has not recognized an expense in connection with this matter because it does not currently believe a loss is probable.
Product Liability Matters
Pelvic Mesh Litigation
The Company is currently involved in litigation in various state and federal courts against manufacturers of pelvic mesh products alleging personal injuries resulting from the implantation of those products. Two subsidiaries of Covidien supplied pelvic mesh products to one of the manufacturers, C.R. Bard (Bard), named in the litigation. The litigation includes a federal multi-district litigation in the U.S. District Court for the Northern District of West Virginia and cases in various state courts and jurisdictions outside the U.S. Generally, complaints allege design and manufacturing claims, failure to warn, breach of warranty, fraud, violations of state consumer protection laws and loss of consortium claims. In fiscal year 2016, Bard paid the Company $121 million towards the settlement of 11,000 of these claims. In May 2017, the agreement with Bard was amended to extend the terms to apply to up to an additional 5,000 claims. That agreement does not resolve the dispute between the Company and Bard with respect to claims that do not settle, if any. As part of the agreement, the Company and Bard agreed to dismiss without prejudice their pending litigation with respect to Bard’s obligation to defend and indemnify the Company. The Company estimates law firms representing approximately 16,200 claimants have asserted or may assert claims involving products
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
manufactured by Covidien’s subsidiaries. As of June 7, 2023, the Company had reached agreements to settle approximately 15,900 of these claims. The Company's accrued expenses for this matter are included within accrued litigation as discussed above.
Hernia Mesh Litigation
Starting in fiscal year 2020, plaintiffs began filing lawsuits against certain subsidiaries of the Company in U.S. state and federal courts that allege personal injury from hernia mesh products sold by those subsidiaries. As of June 7, 2023, the Company and certain of its subsidiaries have been named as defendants in lawsuits filed on behalf of approximately 7,240 individual plaintiffs, and certain plaintiffs’ law firms have advised the Company that they may file additional cases in the future. Approximately 6,284 plaintiffs have filed lawsuits in a coordinated proceeding in Massachusetts state court, where they have been consolidated before a single judge. Approximately 476 plaintiffs have filed lawsuits in a coordinated action in Minnesota state court, and there are approximately 445 actions coordinated in a federal Multidistrict Litigation in the U.S. District Court for the District of Massachusetts. The pending lawsuits relate almost entirely to hernia mesh products that have not been subject to recalls, withdrawals, or other adverse regulatory action. The Company has not recorded an expense related to damages in connection with these matters because any potential loss is not currently probable and reasonably estimable. Additionally, the Company is unable to reasonably estimate the range of loss, if any, that may result from these matters.
Diabetes Pump Retainer Ring Litigation
Starting in fiscal year 2021, plaintiffs began filing lawsuits against the Diabetes Operating Unit in U.S. state and federal courts alleging personal injury from Series 600 insulin pumps with allegedly defective clear retainer rings that were subject to field corrective actions in 2019 and 2021. As of May 30, 2023, 64 individual plaintiffs have filed lawsuits, and certain plaintiffs’ law firms have notified the Company that they may file additional lawsuits in the future on behalf of thousands of additional claimants. Most of the filed suits are coordinated in California state court. The Company has not recorded an expense related to damages in connection with these matters because any potential loss is not currently probable and reasonably estimable. Additionally, the Company is unable to reasonably estimate the range of loss, if any, that may result from these matters.
Environmental Proceedings
The Company is a successor to several investigation and cleanup actions at various stages related to environmental remediation matters at a number of sites, including in Orrington, Maine. These projects relate to a variety of activities, including removal of solvents, metals and other hazardous substances from soil and groundwater. The ultimate cost of site cleanup and timing of future cash flows is difficult to predict given uncertainties regarding the extent of the required cleanup, the interpretation of applicable laws and regulations, and alternative cleanup methods.
The Company is also a successor to a party named in a lawsuit filed in the U.S. District Court for the District of Maine in the early 2000's by the Natural Resources Defense Council and the Maine People's Alliance relating to mercury contamination of the Penobscot River and Bay and options for remediating such contamination. In March 2021, the parties notified the court that they had agreed on a settlement in principle of all issues in this matter, and in September 2022 the parties filed a joint motion for final approval by the court. In October 2022, the court issued a final order approving the settlement and the parties are working with consultants on implementation of remedial activities. The final court order did not result in a change to the Company's previous accrual for this matter.
The Company's accrued expenses for these various environmental proceedings are included within accrued litigation as discussed above.
Income Taxes
In March 2009, the IRS issued its audit report on Medtronic, Inc. for fiscal years 2005 and 2006. Medtronic, Inc. reached agreement with the IRS on some, but not all matters related to these fiscal years. The remaining unresolved issue for fiscal years 2005 and 2006 relates to the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico, which is one of the Company's key manufacturing sites. The U.S. Tax Court (Tax Court) reviewed this dispute, and in June 2016, issued an opinion with respect to the allocation of income between the parties for fiscal years 2005 and 2006 whereby it generally rejected the IRS’s position, but also made certain modifications to the Medtronic, Inc. tax returns as filed. In April 2017, the IRS filed a Notice of Appeal to the U.S. Court of Appeals for the Eighth Circuit regarding the Tax Court opinion. Oral argument for the Appeal occurred in March 2018. The U.S. Court of Appeals issued its opinion in August 2018 and remanded the case back to the Tax Court for additional factual findings, which it concluded in June 2021. The Tax Court issued its opinion on August 18, 2022, and it remains subject to appeal by either or both parties. At this time, the Company is evaluating whether to file an appeal.
The IRS has issued its audit reports on Medtronic, Inc. for fiscal years 2007 through 2016. Medtronic, Inc. and the IRS have reached agreement on all significant issues except for the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico for the businesses that are the subject of the U.S. Tax Court matter for fiscal years 2005 and 2006.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Medtronic, Inc.’s fiscal years 2017, 2018, and 2019 U.S. federal income tax returns are currently being audited by the IRS.
Covidien LP (a wholly owned subsidiary of Medtronic plc) has either reached agreement with the IRS or the statute of limitations has lapsed on its U.S. federal income tax returns through fiscal year 2019.
Although it is not possible to predict the outcome for most of the income tax matters discussed above, the Company believes it is possible that charges associated with these matters could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows.
Refer to Note 13 for additional discussion of income taxes.
Guarantees
In the normal course of business, the Company and/or its affiliates periodically enter into agreements that require one or more of the Company and/or its affiliates to indemnify customers or suppliers for specific risks, such as claims for injury or property damage arising as a result of the Company or its affiliates’ products, the negligence of the Company's personnel, or claims alleging that the Company's products infringe on third-party patents or other intellectual property. The Company also offers warranties on various products. The Company’s maximum exposure under these guarantees is unable to be estimated. Historically, the Company has not experienced significant losses on these types of guarantees.
The Company believes the ultimate resolution of the above guarantees is not expected to have a material effect on the Company’s consolidated earnings, financial position, and/or cash flows.
19. Segment and Geographic Information
There were no changes to the reportable segments during the fiscal year ended April 28, 2023. The Company's four principal operating and reportable segments are as follows: Cardiovascular Portfolio, Medical Surgical Portfolio, Neuroscience Portfolio, and Diabetes Operating Unit.
The Company's management has chosen to organize the entity based upon therapy solutions provided by each segment. The four principal segments are strategic businesses that are managed separately, as each one develops and manufactures products and provides services oriented toward targeted therapy solutions.
The primary products and services from which the Cardiovascular Portfolio segment derives its revenues include products for the diagnosis, treatment, and management of cardiac rhythm disorders and cardiovascular disease, as well as services to diagnose, treat, and manage heart and vascular-related disorders and diseases.
The primary products and services from which the Medical Surgical Portfolio segment derives its revenues include those focused on diseases of the respiratory system, gastrointestinal tract, lungs, pelvic region, kidneys, obesity, and other preventable complications.
The primary products and services from which the Neuroscience Portfolio segment derives its revenues include those focused on neurostimulation therapies and drug delivery systems for the treatment of chronic pain, as well as various areas of the spine and brain, along with pelvic health and conditions of the ear, nose, and throat.
The primary products from which the Diabetes Operating Unit segment derives its revenues include those focused on diabetes management, including insulin pumps, continuous glucose monitoring systems and sensors, and smart insulin pens.
As of the beginning of fiscal year 2024, the Company realigned the operating segment structure as a result of changes in segment leadership and how the Company makes operating decisions and assesses business performance. We continue to have four reportable segments: Cardiovascular Portfolio, Medical Surgical Portfolio, Neuroscience Portfolio, and Diabetes Operating Unit; however, the transition activities from the previously divested businesses in the Medical Surgical Portfolio segment are now in an all other reporting segment.
Segment disclosures are on a performance basis, consistent with internal management reporting. Net sales of the Company's segments include end-customer revenues from the sale of products the segment develops, manufactures, and distributes. Refer to Note 2 for discussion on net sales by segment. There are certain corporate and centralized expenses that are not allocated to the segments. The Company's management evaluates the performance of the segments and allocates resources based on net sales and segment operating profit. Segment operating profit represents income before income taxes, excluding interest income or expense, amortization of intangible assets, centralized distribution costs, non-operating income or expense items, certain corporate charges, and other items not allocated to the segments.
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
The accounting policies of the segments are the same as those described in Note 1. Certain depreciable assets may be recorded by one segment, while the depreciation expense is allocated to another segment. The allocation of depreciation expense is based on the proportion of the assets used by each segment.
Segment Operating Profit
| | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| (in millions) | 2023 | | 2022 | | 2021 |
| Cardiovascular | $ | 4,435 | | | $ | 4,512 | | | $ | 3,850 | |
| Medical Surgical | 2,856 | | | 3,572 | | | 3,021 | |
| Neuroscience | 3,617 | | | 3,765 | | | 3,162 | |
| Diabetes | 378 | | | 583 | | | 598 | |
| Segment operating profit | 11,286 | | | 12,432 | | | 10,632 | |
| Interest expense | (636) | | | (553) | | | (925) | |
| Other non-operating income, net | 515 | | | 318 | | | 336 | |
| Amortization of intangible assets | (1,698) | | | (1,733) | | | (1,783) | |
| Corporate | (1,763) | | | (1,724) | | | (1,577) | |
| Currency | 465 | | | 70 | | | (47) | |
| Centralized distribution costs | (1,624) | | | (1,822) | | | (1,830) | |
| Restructuring and associated costs | (647) | | | (335) | | | (617) | |
| Acquisition-related items | (110) | | | 43 | | | 15 | |
| Divestiture and separation-related items | (235) | | | — | | | — | |
| Certain litigation charges, net | 30 | | | (95) | | | (118) | |
| Impairment of abandoned intangible assets | — | | | — | | | (76) | |
| MCS impairment / costs | — | | | (881) | | | — | |
| IPR&D charges | — | | | (101) | | | (31) | |
| | | | | |
| | | | | |
| Medical device regulations | (150) | | | (102) | | | (83) | |
| Commitments to the Medtronic Foundation and Medtronic LABS | (70) | | | — | | | — | |
| Income before income taxes | $ | 5,364 | | | $ | 5,517 | | | $ | 3,895 | |
Total Assets and Depreciation Expense | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total Assets | | Depreciation Expense |
| (in millions) | April 28, 2023 | | April 29, 2022 | | 2023 | | 2022 | | 2021 |
| Cardiovascular | $ | 16,051 | | | $ | 14,490 | | | $ | 212 | | | $ | 214 | | | $ | 212 | |
| Medical Surgical | 36,248 | | | 36,940 | | | 202 | | | 200 | | | 195 | |
| Neuroscience | 18,346 | | | 16,917 | | | 267 | | | 265 | | | 236 | |
| Diabetes | 3,930 | | | 3,797 | | | 80 | | | 67 | | | 53 | |
| Segments | 74,575 | | | 72,144 | | | 761 | | | 746 | | | 696 | |
| Corporate | 16,373 | | | 18,837 | | | 238 | | | 228 | | | 223 | |
| Total | $ | 90,948 | | | $ | 90,981 | | | $ | 999 | | | $ | 974 | | | $ | 919 | |
Medtronic plc
Notes to Consolidated Financial Statements (Continued)
Geographic Information
Net sales are attributed to the country based on the location of the customer taking possession of the products or in which the services are rendered. Geographic property, plant, and equipment are attributed to the country based on the physical location of the assets.
The following table presents net sales for fiscal years 2023, 2022, and 2021, and property, plant, and equipment, net at April 28, 2023 and April 29, 2022 for the Company's country of domicile, countries with significant concentrations, and all other countries:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net sales | | Property, plant, and equipment, net |
| (in millions) | 2023 | | 2022 | | 2021 | | April 28, 2023 | | April 29, 2022 |
| Ireland | $ | 98 | | | $ | 101 | | | $ | 100 | | | $ | 184 | | | $ | 177 | |
| | | | | | | | | |
| United States | 16,373 | | | 16,135 | | | 15,526 | | | 4,083 | | | 3,821 | |
| Rest of world | 14,756 | | | 15,450 | | | 14,491 | | | 1,302 | | | 1,415 | |
| Total other countries, excluding Ireland | 31,129 | | | 31,585 | | | 30,017 | | | 5,385 | | | 5,236 | |
| Total | $ | 31,227 | | | $ | 31,686 | | | $ | 30,117 | | | $ | 5,569 | | | $ | 5,413 | |
No single customer represented over 10 percent of the Company’s consolidated net sales in fiscal years 2023, 2022, or 2021.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended (the Exchange Act)) and changes in the Company’s internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) as of the end of the period covered by this report. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of the period covered by this annual report, our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act) are effective.
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting for the Company (as defined in Exchange Act Rule 13a-15(f)). Management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation, management concluded that the Company’s internal control over financial reporting was effective as of April 28, 2023. The effectiveness of the Company's internal control over financial reporting as of April 28, 2023 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Changes in Internal Control Over Financial Reporting
During the quarter ended April 28, 2023, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) under the Exchange Act) that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
Item 9B. Other Information
As reported in our Quarterly Reports on Form 10-Q for the second and third quarters of fiscal year 2023, Medtronic has engaged in certain activities that it is required to disclose pursuant to Section 13(r)(1)(D)(ii) of the Securities Exchange Act of 1934, as amended. In particular, during the second and third quarters of fiscal year 2023, Medtronic engaged in certain regulatory activities involving Russia’s Federal Security Service (“FSB”) related to its medical devices that were expressly authorized by the U.S. Government under applicable economic sanctions regulations.
During the second and third quarters of fiscal year 2023, in the normal course of business and consistent with the OFAC authorizations as in effect at the time, Medtronic Russia filed a total of four notifications with the FSB, as required under local Russian law for the import of medical devices that make use of encryption functionality. These activities did not directly result in any revenues or profits for Medtronic. Medtronic did not engage in these activities during the first and fourth quarters of fiscal year 2023. To the extent that notifications with the FSB remain permissible under U.S. law, Medtronic may decide to continue engaging in such activities for the limited purposes of complying with local law requirements in Russia.
PART III
Part III of this Annual Report on Form 10-K incorporates information by reference from the Company's 2023 definitive proxy statement, which will be filed no later than 120 days after April 28, 2023.
Item 10. Directors, Executive Officers, and Corporate Governance
The sections entitled “Proposal 1 — Election of Directors — Directors and Nominees,” “Corporate Governance — Committees of the Board and Meetings,” and “Share Ownership Information — Delinquent Section 16(a) Report” in the Company's Proxy Statement for our 2023 Annual General Meeting of Shareholders, which will be filed no later than 120 days after April 28, 2023, are incorporated herein by reference.
Set forth below are the names and ages of our Executive Officers of Medtronic, as well as information regarding their positions with Medtronic, their periods of service in these capacities, and their business experiences. There are no family relationships among any of the officers named, nor is there any arrangement or understanding pursuant to which any person was selected as an officer.
The following table shows the name, age, and position as of April 28, 2023 of each of our Executive Officers:
| | | | | | | | | | | | | | |
| Name | | Age | | Position with the Company |
| Geoffrey S. Martha | | 53 | | Chairman and Chief Executive Officer |
| Ivan K. Fong | | 61 | | Executive Vice President, General Counsel and Corporate Secretary of the Company |
| Robert ten Hoedt | | 62 | | Executive Vice President and President, Global Regions |
| Karen L. Parkhill | | 57 | | Executive Vice President and Chief Financial Officer |
| Sean Salmon | | 58 | | Executive Vice President and President, Cardiovascular Portfolio |
| Gregory L. Smith | | 59 | | Executive Vice President, Global Operations and Supply Chain |
| Brett Wall | | 58 | | Executive Vice President and President, Neuroscience Portfolio |
| Robert J. White | | 60 | | Executive Vice President and President, Medical Surgical Portfolio |
Geoffrey S. Martha, age 53, is Chairman of the Board of Directors and Chief Executive Officer of Medtronic. Mr. Martha assumed the role of CEO on April 27, 2020 and became Chairman of the Board on December 11, 2020. Prior to his role as Chairman and CEO, he served as President of Medtronic from November 2019 through April 2020 and joined the Board of Directors in November 2019. Previously, Mr. Martha served as Executive Vice President and President, Restorative Therapies Group, a role he held since August 2015. Mr. Martha previously served as Senior Vice President of Strategy and Business Development of the Company beginning in January 2015 and of Medtronic, Inc. beginning in August 2011. Prior to that, he served as Managing Director of Business Development at GE Healthcare from April 2007 to July 2011; General Manager for GE Capital Technology Finance Services from November 2003 to March 2007; Senior Vice President, Business Development for GE Capital Vendor Financial Services from February 2002 to October 2003; General Manager for GE Capital Colonial Pacific Leasing from February 2001 to January 2002; and Vice President, Business Development for Potomac Federal, the GE Capital federal financing investment bank from May 1998 to January 2001.
Ivan K. Fong, age 61, has been Executive Vice President, General Counsel and Corporate Secretary of the Company since February 2022. Prior to that, he held several leadership positions at 3M Company from 2012 to 2022, including Executive Vice President, Chief Legal and Policy Officer and Secretary. Prior to joining 3M Company, Mr. Fong served as General Counsel of the U.S. Department of Homeland Security from 2009 to 2012. Prior to his role with the U.S. Government, he was Chief Legal Officer and Secretary for Cardinal Health, Inc from 2005 to 2009. Mr. Fong currently serves on the Board of Cboe Global Markets.
Robert ten Hoedt, age 62, is Executive Vice President and President of the Global Regions. He previously served as Executive Vice President and President, EMEA Region of the Company since January 2015 and of Medtronic, Inc. since May 2014, as well as President, APAC Region since March 2022. Prior to that, he was Senior Vice President and President, EMEA and Canada from 2009 to 2014; Vice President CardioVascular Europe and Central Asia from 2006 to 2009; Vice President and General Manager, Vitatron from 1999 to 2006; Gastro-Uro leader from 1994 to 1999; and Marketing Manager, Neurological from 1991 to 1994.
Karen L. Parkhill, age 57, joined the Company as Executive Vice President and Chief Financial Officer in June 2016. From 2011 to 2016, Ms. Parkhill served as Vice Chairman and Chief Financial Officer of Comerica Incorporated. Ms. Parkhill was a member of Comerica’s Management Executive Committee and the Comerica Bank Board of Directors. Prior to joining Comerica, Ms. Parkhill worked for J.P. Morgan Chase & Co. in various capacities from 1992 to 2011, including serving as Chief Financial Officer of the Commercial Banking business from 2007 to 2011. Ms. Parkhill is also a current member of the Board of Directors for American Express.
Sean Salmon, age 58, has been Executive Vice President and President of Medtronic's Cardiovascular Portfolio since January 2021. Mr. Salmon previously served as Executive Vice President and President of the Diabetes Operating Unit (previously known as Diabetes Group) from October 2019 to May 2022. Prior to that, he served as Senior Vice President and President of Coronary and Structural Heart Business
within the Cardiac and Vascular Group of the Company beginning in July 2014. Mr. Salmon is a seasoned leader who has been with Medtronic since 2004 and spent the past 16 years in increasingly senior levels of management. Prior to joining Medtronic, Mr. Salmon worked at CR Bard and Johnson & Johnson.
Gregory Smith, age 59, is Executive Vice President, Global Operations and Supply Chain, a position he has held since April 2021. Prior to joining Medtronic, he was Executive Vice President of U.S. Supply Chain at Walmart. In addition, Mr. Smith served as Senior Vice President, Global Operations at The Goodyear Tire & Rubber Company, and held leadership roles at ConAgra Foods, United Signature Foods, VDK Frozen Foods and Quaker Oats.
Brett Wall, age 58, is Executive Vice President and President of Medtronic’s Neuroscience Portfolio. Mr. Wall previously served as Senior Vice President and President of the Brain Therapies division of Medtronic within the Restorative Therapies Group from March 2016 to November 2019. Prior to that, Mr. Wall served as SVP and President of Medtronic’s Neurovascular business. Prior to joining Medtronic, he served as Covidien’s SVP and President of Neurovascular as well as Senior Vice President and President of the International Vascular Therapies business for Covidien. Mr. Wall also served as Senior Vice President and President, International at ev3, Inc. From 2000 to 2008, Brett held various marketing and sales positions with ev3, Inc. and Micro Therapeutics, Inc. Mr. Wall has also worked at Boston Scientific as Director of Marketing, Cardiovascular, Asia Pacifica and Marketing Manager, Japan, from September 1995 to September 2000.
Robert J. White, age 60, is Executive Vice President and President, Medical Surgical Portfolio. Since 2017, Mr. White has served as Executive Vice President and Group President of the Minimally Invasive Therapies Group of Medtronic. Prior to that, he was Senior Vice President and President, Asia Pacific from January 2015 to December 2017. He had served as President, Emerging Markets, President, Respiratory and Monitoring Solutions and Vice President and General Manager of Patient Monitoring at Covidien. He also held various leadership positions at GE Healthcare and IBM. Mr. White is also a current member of the Board of Directors of Smith & Nephew plc.
Item 11. Executive Compensation
The sections entitled “Corporate Governance — Director Compensation,” “Corporate Governance — Committees of the Board and Meetings,” “Compensation Discussion and Analysis,” and “Executive Compensation” in Medtronic's Proxy Statement for the Company's 2023 Annual General Meeting of Shareholders, which will be filed no later than 120 days after April 28, 2023, are incorporated herein by reference. The section entitled “Compensation Committee Report” in Medtronic's Proxy Statement for the Company's 2023 Annual General Meeting of Shareholders, which will be filed no later than 120 days after April 28, 2023, is furnished herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters
The sections entitled “Share Ownership Information – Significant Shareholders,” “Share Ownership Information – Beneficial Ownership of Management,” and “Executive Compensation — Equity Compensation Plan Information” in Medtronic's Proxy Statement for the Company's 2023 Annual General Meeting of Shareholders, which will be filed no later than 120 days after April 28, 2023, are incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The sections entitled “Corporate Governance — Director Independence” and “Corporate Governance — Related Party Transactions and Other Matters” in Medtronic's Proxy Statement for the Company's 2023 Annual General Meeting of Shareholders, which will be filed no later than 120 days after April 28, 2023, are incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
The sections entitled “Corporate Governance — Committees of the Board and Meetings” and “Audit and Non-Audit Fees” in Medtronic's Proxy Statement for the Company's 2023 Annual General Meeting of Shareholders, which will be filed no later than 120 days after April 28, 2023, are incorporated herein by reference.
PART IV
Item 15. Exhibits and Financial Statement Schedules | | | | | |
| (a) | 1. Financial Statement Schedule |
| |
| | Schedule II. Valuation and Qualifying Accounts — years ended April 28, 2023, April 29, 2022, and April 30, 2021. |
| |
MEDTRONIC PLC AND SUBSIDIARIES
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS
(in millions) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Additions | | Deductions | | |
| Balance at
Beginning of
Fiscal Year | | Charges to Income | | Charges to Other Accounts | | Other Changes (Debit) Credit | | Balance
at End of
Fiscal Year |
| Allowance for doubtful accounts: | | | | | | | | | |
| Fiscal year ended April 28, 2023 | $ | 230 | | | $ | 73 | | | $ | — | | | $ | (127) | | (a) | $ | 176 | |
| Fiscal year ended April 29, 2022 | 241 | | | 58 | | | — | | | (69) | | (a) | 230 | |
| Fiscal year ended April 30, 2021 | 208 | | | 128 | | | — | | | (95) | | (a) | 241 | |
| | | | | | | | | |
| Inventory reserve: | | | | | | | | | |
| Fiscal year ended April 28, 2023 | $ | 628 | | | $ | 271 | | | $ | — | | | $ | (231) | | (b) | $ | 669 | |
| Fiscal year ended April 29, 2022 | 629 | | | 156 | | | — | | | (157) | | (b) | 628 | |
| Fiscal year ended April 30, 2021 | 544 | | | 483 | | | — | | | (398) | | (b) | 629 | |
| | | | | | | | | |
| Deferred tax valuation allowance: | | | | | | | | | |
| Fiscal year ended April 28, 2023 | $ | 6,583 | | | $ | 4,779 | | | $ | 39 | | (c) | $ | (63) | | (d) | $ | 11,311 | |
| | | | | 1 | (e) | (27) | | (f) | |
| Fiscal year ended April 29, 2022 | 5,822 | | | 884 | | | (19) | | (e) | (103) | | (d) | 6,583 | |
| | | | | | | | | | |
| Fiscal year ended April 30, 2021 | 5,482 | | | 342 | | | 170 | | (e) | (172) | | (d) | 5,822 | |
| | | | | | | | | |
| | | | | |
| (a) Primarily consists of uncollectible accounts written off, less recoveries. |
| (b) Primarily reflects utilization of the inventory reserve. |
| (c) Reflects the impact from acquisitions. |
| (d) Primarily reflects carryover attribute utilization and expiration. |
| (e) Primarily reflects the effects of currency fluctuations. |
| (f) Primarily reflects the impacts from tax rate changes. |
| | | | | | | | | | | | | | | | | |
| All other schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto. |
| | | | | |
| | | | | |
| 2. Exhibits |
| | | | | | | | | | | | |
| Exhibit No. | | Description | |
| 3.1 | | | |
| | | | |
| 3.2 | | | |
| | | | |
| 4.1 | | | |
| | | | |
| | | | | | | | | | | | |
| 4.2 | | | |
| | | | |
| 4.3 | | | |
| | | | |
| 4.4 | | | |
| | | | |
| 4.5 | | | |
| | | | |
| 4.6 | | | |
| | | | |
| 4.7 | | | |
| | | | |
| 4.8 | | Seventh Supplemental Indenture, dated as of January 26, 2015, by and among Medtronic plc, Medtronic, Inc., Medtronic Global Holdings S.C.A. and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 4.2 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820). | |
| | | | |
| 4.9 | | | |
| | | | |
| 4.10 | | First Supplemental Indenture, dated December 10, 2014, between Medtronic, Inc. and Wells Fargo Bank, National Association (including Form of Floating Rate Senior Notes due 2020, Form of 1.500% Senior Notes due 2018, Form of 2.500% Senior Notes due 2020, Form of 3.150% Senior Notes due 2022, Form of 3.500% Senior Notes due 2025, Form of 4.375% Senior Notes due 2035 and Form of 4.625% Senior Notes due 2045) (incorporated by reference to Exhibit 4.2 of Medtronic, Inc.’s Current Report on Form 8-K filed with the Commission on December 10, 2014, File No. 001-07707). | |
| | | | |
| 4.11 | | | |
| | | | |
| 4.12 | | | |
| | | | |
| 4.13 | | | |
| | | | |
| 4.14 | | | |
| | | | |
| 4.15 | | | |
| | | | |
| | | | | | | | | | | | |
| 4.16 | | | |
| | | | |
| 4.17 | | | |
| | | | |
| 4.18 | | | |
| | | | |
| 4.19 | | Ninth Supplemental Indenture, dated as of January 26, 2015, by and among Medtronic plc, Medtronic Global Holdings S.C.A., Covidien public limited company, Covidien International Finance S.A., Covidien Ltd. and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.5 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820). | |
| | | | |
| 4.20 | | Senior Indenture, dated as of March 28, 2017, by and among Medtronic plc, Medtronic Global Holdings S.C.A., Medtronic, Inc., and Wells Fargo Bank, N.A. (incorporated by reference to Exhibit 4.1 to Medtronic plc’s Current Report on Form 8-K, filed on March 28, 2017, File No. 001-36820).
| |
| | | | |
| 4.21 | | First Supplemental Indenture, dated as of March 28, 2017, by and among Medtronic plc, Medtronic Global Holdings S.C.A., Medtronic, Inc., and Wells Fargo Bank, N.A. (incorporated by reference to Exhibit 4.2 to Medtronic plc’s Current Report on Form 8-K, filed on March 28, 2017, File No. 001-36820).
| |
| | | | |
| 4.22 | | Second Supplemental Indenture, dated as of March 7, 2019, by and among Medtronic plc, Medtronic Global Holdings S.C.A., Medtronic, Inc., Wells Fargo Bank, N.A., and Elavon Financial Services DAC, UK Branch (incorporated by reference to Exhibit 4.1 to Medtronic plc’s Current Report on Form 8-K, filed on March 7, 2019, File No. 001-36820). | |
| | | | |
| 4.23 | | Third Supplemental Indenture, dated as of July 2, 2019, among Medtronic Global Holdings S.C.A., Medtronic, Inc. and Medtronic plc, Wells Fargo Bank, N.A., as trustee, and Elavon Financial Services DAC (incorporated by reference to Exhibit 4.1 to Medtronic plc' Current Report on Form 8-K, filed July 2, 2019, File No. 001-36820). | |
| | | | |
| 4.24 | | Fourth Supplemental Indenture, dated as of September 29, 2020, among Medtronic Global Holdings S.C.A., Medtronic, Inc. and Medtronic plc, Wells Fargo Bank, N.A., as trustee, and Elavon Financial Services DAC, as paying agent (including the forms of the 2023 Notes, the 2025 Notes, the 2028 Notes, the 2032 Notes, the 2040 Notes and the 2050 Notes) (incorporated by reference to Exhibit 4.1 to Medtronic plc' Current Report on Form 8-K, filed September 29, 2020, File No. 001-36820). | |
| | | | |
| #4.25 | | | |
| | | | |
| 10.1 | | Amended and Restated Credit Agreement, dated as of December 12, 2018, by and among Medtronic Global Holdings, SCA, certain subsidiaries named therein, Medtronic, Inc., Medtronic PLC, the lenders from time to time party thereto, and Bank of America, N.A. as Administration Agent (incorporated by reference to Exhibit 10.1 to Medtronic plc’s Current Report on Form 8-K, filed on December 13, 2018, File No. 001-36820). | |
| | | | |
| 10.2 | | Amendment No. 1 and Extension Agreement to the Amended and Restated Credit Agreement, dated as of December 12, 2019, among Medtronic Global Holdings S.C.A., Medtronic, Inc., Medtronic PLC, the Lenders party thereto and Bank of America, N.A., as Administrative Agent (incorporated by reference to Exhibit 10.1 to Medtronic plc’s Current Report on Form 10-Q, filed on February 28, 2020, File No. 001-36820). | |
| | | | |
| 10.3 | | Term Loan Agreement, dated as of May 12, 2020, among Medtronic Global Holdings S.C.A., Medtronic, Inc., Medtronic PLC, the Lenders party thereto and Mizuho Bank, LTD., as Administrative Agent (incorporated by reference to Exhibit 10.1 to Medtronic plc’s Current Report on Form 8-K, filed on May 12, 2020, File No. 001-36820). | |
| | | | |
| | | | | | | | | | | | |
| 10.4 | | | |
| | | | |
| 10.5 | | | |
| | | | |
| *10.6 | | | |
| | | | |
| *10.7 | | | |
| | | | |
| *10.8 | | | |
| | | | |
| *10.9 | | | |
| | | | |
| *10.10 | | | |
| | | | |
| *10.11 | | | |
| | | | |
| *10.12 | | | |
| | | | |
| *10.13 | | | |
| | | | |
| *10.14 | | | |
| | | | |
| *10.15 | | | |
| | | | |
| *10.16 | | | |
| | | | |
| *10.17 | | | |
| | | | |
| *10.18 | | | |
| | | | |
| *10.19 | | | |
| | | | |
| *10.20 | | | |
| | | | |
| *10.21 | | | |
| | | | |
| | | | | | | | | | | | |
| *10.22 | | | |
| | | | |
| *10.23 | | | |
| | | | |
| *10.24 | | | |
| | | | |
| *10.25 | | | |
| | | | |
| *10.26 | | | |
| | | | |
| *10.27 | | | |
| | | | |
| *10.28 | | | |
| | | | |
| *10.29 | | | |
| | | | |
| *10.30 | | | |
| | | | |
| *10.31 | | | |
| | | | |
| *10.32 | | | |
| | | | |
| *10.33 | | | |
| | | | |
| *10.34 | | | |
| | | | |
| *10.35 | | | |
| | | | |
| *10.36 | | | |
| | | | |
| *10.37 | | | |
| | | | |
| *10.38 | | | |
| | | | |
| *10.39 | | | |
| | | | |
| | | | | | | | | | | | |
| *10.40 | | | |
| | | | |
| *10.41 | | | |
| | | | |
| *10.42 | | | |
| | | | |
| *10.43 | | | |
| | | | |
| *10.44 | | | |
| | | | |
| *10.45 | | | |
| | | | |
| *10.46 | | | |
| | | | |
| *10.47 | | | |
| | | | |
| *10.48 | | | |
| | | | |
| *10.49 | | | |
| | | | |
| *10.50 | | | |
| | | | |
| *10.51 | | | |
| | | | |
| *10.52 | | | |
| | | | |
| *10.53 | | | |
| | | | |
| *10.54 | | | |
| | | | |
| *10.55 | | | |
| | | | |
| *10.56 | | | |
| | | | |
| | | | | | | | | | | | |
| *10.57 | | | |
| | | | |
| *10.58 | | | |
| | | | |
| *10.59 | | | |
| | | | |
| *10.60 | | | |
| | | | |
| *10.61 | | | |
| | | | |
| *10.62 | | | |
| | | | |
| *10.63 | | | |
| | | | |
| *10.64 | | | |
| | | | |
| *10.65 | | | |
| | | | |
| *10.66 | | | |
| | | | |
| *10.67 | | | |
| | | | |
| *10.68 | | | |
| | | | |
| *10.69 | | | |
| | | | |
| *10.70 | | | |
| | | | |
| *10.71 | | | |
| | | | |
| *10.72 | | | |
| | | | |
| | | | | | | | | | | | |
| *10.73 | | | |
| | | | |
| *10.74 | | | |
| | | | |
| *10.75 | | | |
| | | | |
| 10.76 | | Amendment No. 3 and Extension Agreement to the Amended and Restated Credit Agreement, dated as of December 13, 2021, by and among Medtronic Global Holdings S.C.A., certain subsidiaries of Medtronic plc from time to time party thereto, Medtronic, Inc., Medtronic plc, the lenders from time to time party thereto and Bank of America N.A., as administrative agent. (incorporated by reference to Exhibit 10.01 to Medtronic plc’s Current Report on Form 8-K, filed on December 14, 2021, File No. 001-36820). | |
| | | | |
| *10.77 | | | |
| | | | |
| *10.78 | | | |
| | | | |
| *10.79 | | | |
| | | | |
| *10.80 | | | |
| | | | |
| *10.81 | | | |
| | | | |
| *10.82 | | | |
| | | | |
| 10.83 | | | |
| | | | |
| 10.84 | | | |
| | | | |
| #10.85 | | | |
| | | | |
| #10.86 | | | |
| | | | |
| #21 | | | |
| | | | |
| #22 | | | |
| | | | |
| #23 | | | |
| | | | |
| #24 | | | |
| | | | |
| #31.1 | | | |
| | | | |
| #31.2 | | | |
| | | | |
| #32.1 | | | |
| | | | |
| #32.2 | | | |
| | | | |
| #101.SCH | | XBRL Taxonomy Extension Schema Document | |
| | | | | | | | | | | | |
| | | | |
| #101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document | |
| | | | |
| #101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document | |
| | | | |
| #101.LAB | | XBRL Taxonomy Extension Label Linkbase Document | |
| | | | |
| #101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document | |
| | | | |
| #104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |
| | | | | | | | |
| *Exhibits that are management contracts or compensatory plans or arrangements. |
| #Filed herewith |
|
Item 16. Form 10-K Summary
Registrants may voluntarily include a summary of information required by Form 10-K under this Item 16. The Company has not elected to include such summary information.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| | Medtronic plc |
| | | |
| Dated: June 22, 2023 | By: | /s/ Geoffrey S. Martha |
| | | Geoffrey S. Martha |
| | | Chairman and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, the report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | |
| | Medtronic plc |
| | | |
| Dated: June 22, 2023 | By: | /s/ Geoffrey S. Martha |
| | | Geoffrey S. Martha |
| | | Chairman and Chief Executive Officer |
| | | (Principal Executive Officer) |
| | | |
| | | |
| Dated: June 22, 2023 | By: | /s/ Karen L. Parkhill |
| | | Karen L. Parkhill |
| | | Executive Vice President and Chief Financial Officer |
| | (Principal Financial Officer) |
| | |
| Dated: June 22, 2023 | By: | /s/ Jennifer M. Kirk |
| | Jennifer M. Kirk |
| | Senior Vice President, Global Controller and Chief Accounting Officer |
| | (Principal Accounting Officer) |
| | |
| | | |
| | Directors |
| | | |
| | | Richard H. Anderson* |
| | Craig Arnold* |
| | Scott C. Donnelly* |
| | Lidia Fonseca* |
| | Andrea J. Goldsmith, Ph.D.* |
| | | Randall J. Hogan* |
| | Kevin E. Lofton* |
| | Geoffrey S. Martha |
| | Elizabeth G. Nabel, M.D.* |
| | | Denise M. O’Leary* |
| | | Kendall J. Powell* |
*Ivan K. Fong, by signing his name hereto, does hereby sign this document on behalf of each of the above named directors of the registrant pursuant to powers of attorney duly executed by such persons.
| | | | | | | | |
| Dated: June 22, 2023 | By: | /s/ Ivan K. Fong |
| | Ivan K. Fong |
 ®
® ®
®