UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One) | |
Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
For the fiscal year ended December 31, 2019
OR
Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
For the transition period from to
Commission file number: 001-36440

(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation) | (I.R.S. Employer Identification No.) | |||||
(Address of principal executive offices) | (Zip code) | |||||
Registrant’s telephone number, including area code: (844 ) 428-2667
Securities registered pursuant to Section 12(b) of the Act:
(Title of each class) | (Trading Symbol) | (Name of each exchange on which registered) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
☒ | Accelerated filer | ☐ | ||
Non-accelerated filer | ☐ | Emerging growth company | ||
Smaller reporting company | ||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of common stock held by non-affiliates or registrant on June 30, 2019 was $2,076,443,618 .
As of February 18, 2020, there were 47,755,212 shares of Avanos Medical, Inc. common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information contained in the definitive Proxy Statement for the Avanos Annual Meeting of Stockholders to be held on April 23, 2020 is incorporated by reference into Part III.
AVANOS MEDICAL, INC.
TABLE OF CONTENTS
Part I | Page | |
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Part II | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
Part III | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Part IV | ||
Item 15. | ||
PART I
ITEM 1. BUSINESS
Overview
Avanos Medical, Inc. is a medical technology company focused on delivering clinically superior breakthrough medical device solutions to improve patients’ quality of life. Headquartered in Alpharetta, Georgia, Avanos is committed to addressing some of today’s most important healthcare needs, such as reducing the use of opioids while helping patients move from surgery to recovery. We develop, manufacture and market clinically superior solutions in more than 90 countries. Unless the context indicates otherwise, the terms “Avanos,” “Company,” “we,” “our” and “us” refer to Avanos Medical, Inc. and its consolidated subsidiaries.
We conduct our business in one operating and reportable segment that provides our medical device products to healthcare providers and patients with manufacturing facilities in the United States, Mexico, France, Germany and Tunisia. We provide a portfolio of innovative product offerings focused on chronic care and pain management to improve patient outcomes and reduce the cost of care.
Chronic Care is a portfolio of products that includes (i) digestive health products and (ii) respiratory health products. The digestive health product category accounted for 38% of our consolidated net sales in the year ended December 31, 2019 and 37% in each of the years ended December 31, 2018 and 2017, respectively. Digestive health products include our MIC-KEY enteral feeding tubes, CORPAK patient feeding solutions and our recently acquired NEOMED neonatal and pediatric feeding solutions. The respiratory health product category accounted for 21% of our consolidated net sales in the year ended December 31, 2019 and 22% in each of the years ended December 31, 2018 and 2017, respectively. Respiratory health products include closed airway suction systems and other airway management devices under the BALLARD, MICROCUFF and recently-acquired ENDOCLEAR brands.
Pain Management is a portfolio of products that includes (i) acute pain products and (ii) interventional pain solutions. The acute pain product category accounted for 27%, 26% and 28% of our consolidated net sales in the years ended December 31, 2019, 2018 and 2017, respectively. Acute pain includes ON-Q and our recently acquired AMBIT surgical pain pumps and GAME READY cold and compression therapy systems. Our interventional pain product category accounted for 14%, 15% and 13% of our consolidated net sales in the years ended December 31, 2019, 2018 and 2017, respectively. Interventional pain provides minimally invasive pain relieving therapies, such as our COOLIEF pain relief therapy.
On April 30, 2018, we closed the sale of our Surgical and Infection Prevention (“S&IP”) business, which included the name “Halyard Health” (and all variations thereof and related intellectual property rights) and our information technology (“IT”) system (the “Divestiture”) pursuant to an Amended and Restated Purchase Agreement dated April 30, 2018 (“Purchase Agreement”) by and among us and certain of our affiliates and Owens & Minor, Inc. (“Buyer”). The purchase price paid for the Divestiture was $710.0 million plus certain adjustments as provided in the Purchase Agreement, and resulted in a gain of $89.9 million. A portion of the proceeds were used to retire our senior secured term loan (see Note 9, “Debt”). The remaining net proceeds will continue to be invested in the business through acquisitions (see Note 2, “Business Acquisitions”) and organic growth. See “Discontinued Operations” in Note 7 to the consolidated financial statements in Item 8 of this report.
As a focused Medical Devices business, we operate in attractive end-markets. We will deploy a dual-track growth strategy focused on product development and M&A while right-sizing the cost structure of our operations to create a scalable and cost efficient infrastructure. We are engaged in a multi-year restructuring plan to reduce dis-synergies and corporate costs. See “Restructuring” in Note 3 to the consolidated financial statements in Item 8 of this report.
As a result of the Divestiture, the results of operations from our S&IP business are reported as “Income from discontinued operations, net of tax” through April 30, 2018.
On September 19, 2019, we acquired substantially all the assets of Endoclear, LLC (“Endoclear”). The initial purchase price was $3.5 million plus future contingent payments with an estimated fair value of $5.5 million, subject to certain adjustments as defined in the purchase agreement. Endoclear develops and markets airway management devices that are complementary to our existing respiratory health portfolio.
On August 7, 2019, we acquired substantially all the assets of Summit Medical Products, Inc. (“Summit”) for $15.6 million plus future contingent payments with an estimated fair value of $1.7 million, subject to certain adjustments as defined in the purchase agreement. Summit develops and markets the ambIT® family of ambulatory electronic infusion pumps, with annual net sales of approximately $7.0 million in 2018. The accompanying consolidated income statement includes approximately $3.3 million of net sales of ambIT® products in the year ended December 31, 2019.
On April 16, 2019, we acquired a minority interest in NeoMed, Inc. (“NeoMed”) for $7.0 million. NeoMed is a market-leading medical device company that is focused on specialized feeding and medication dosing for low birth weight, neonatal and
1 | ||
pediatric patients. On July 8, 2019, we acquired all of the outstanding shares of NeoMed for a purchase price of $33.5 million, which includes the base purchase price of $28.0 million plus certain agreed-upon items at the closing date, net of cash acquired, and subsequently adjusted for certain items as defined in the purchase agreement. NeoMed’s net sales were $37.0 million in 2018. NeoMed’s net sales for the period from July 8, 2019 through December 31, 2019 were $19.7 million and are included in the accompanying consolidated income statement.
On July 1, 2018, we acquired Cool Systems, Inc. for $65.7 million, net of cash acquired, which was based on a purchase price of $65.0 million plus certain adjustments as provided in the purchase agreement. Cool Systems is marketed as Game Ready® and is hereinafter referred to as “Game Ready.” In the year ended December 31, 2019, the purchase price allocation for Game Ready was finalized, resulting in a $1.9 million reduction of goodwill. The goodwill arising from the Game Ready acquisition is now $18.7 million.
The address of our principal executive offices is 5405 Windward Parkway, Suite 100 South, Alpharetta, Georgia 30004, and our telephone number is (844) 428-2667.
Sales and Marketing
We direct our primary sales and marketing efforts toward hospitals and other healthcare providers to highlight the unique benefits and competitive differentiation of our branded products. We work directly with physicians, nurses, professional societies, hospital administrators and healthcare group purchasing organizations (“GPOs”) to collaborate and educate on emerging practices and clinical techniques. These marketing programs are delivered directly to healthcare providers. Additionally, we provide marketing programs to our strategic distribution partners throughout the world.
Distribution
While our products are generally marketed directly to hospitals and other healthcare providers, they are generally sold through third-party wholesale distributors, with some sales directly to healthcare facilities and other end-user customers. In 2019, approximately 53% of our net sales in North America were made through distributors. Globally, no single customer accounted for 10% or more of our consolidated net sales in the year ended December 31, 2019. Sales to Owens & Minor, Inc. accounted for approximately 10% of our net sales in the years ended December 31, 2018 and 2017, respectively, under standard terms and conditions of sale.
Outside North America, sales are made either directly to end-user customers or through distributors, depending on the market served. In 2019, approximately 85% of our net sales outside North America were made through wholesalers or distributors.
We utilize distribution centers in North America, Europe, Australia and Japan. No material portion of our business is subject to renegotiation of profits or termination of contracts at the election of the government.
Group Purchasing Organizations
We enter into agreements with GPOs which allows for the sale of our products to their members, whether sold directly by us or through independent wholesale distributors. GPOs negotiate pricing and volume purchasing discounts for hospitals, physician practices and other health care providers and institutions. Under our agreements with GPOs, we pay a fee based on sales of our products to GPO members, which is recorded as a reduction of net sales. Excluding our recent acquisitions previously described under “Overview,” approximately 32% of our 2019 global net sales, including sales to wholesale distributors, were contracted through four major national GPOs.
Competition
While no single company competes with us across the breadth of our offerings, we face significant competition in U.S. and international markets.
There are a variety of treatment means and alternative clinical practices to address the management of surgical and interventional pain management and respiratory and digestive health. We face competition from these alternative treatments, as well as improvements and innovations in products and technologies by our competitors.
Competitors for our products are fragmented by particular product category, and the individual markets for these products are highly competitive. Major competitors include, among others:
• | Digestive Health: Boston Scientific Corporation, Cook Medical, and Applied Medical Technology, Inc. |
• | Respiratory Health: Becton, Dickinson and Company, Stryker Corporation, Medline Industries, Inc. and Smiths Medical |
• | Acute Pain Management: B. Braun Medical Inc., Pacira Pharmaceuticals, Inc., Teleflex Incorporated, Medtronic plc, Ambu A/S, Baxter International, Inc., Pajunk Medical Systems and Leventon |
2 | ||
• | Interventional Pain Management: Boston Scientific Corporation, Abbott Laboratories, Medtronic plc and Stryker Corporation |
In developing and emerging markets, alternative clinical practices and different standards of care are our primary competition.
While we believe that the number of procedures using our products will grow due, in part, to increasing global access to healthcare, we expect that our ability to compete with other providers of similar products will be impacted by rapid technological advances, pricing pressures and third-party reimbursement practices. We continue to defend our market positions and have launched eleven new products in 2019. We believe that our key product characteristics, such as proven efficacy, reliability and safety, coupled with our core competencies such as our innovative ability to launch new products, efficient manufacturing processes, established distribution network, field sales organization and customer service, are important factors that distinguish us from our competitors.
Research and Development
We continuously engage in research and development to commercialize new products and enhance the effectiveness, reliability and safety of our existing products. We incurred $37.7 million in 2019, $41.8 million in 2018 and $38.2 million in 2017 on research and development for new products and processes, and to improve existing products and processes. These amounts consisted primarily of salaries and related expenses for personnel, product trial costs, outside laboratory and license fees, the costs of laboratory equipment and facilities, and asset write-offs for equipment associated with unsuccessful product launches. We intend to continue our research and development efforts as a key strategy for growth.
We collaborate with physicians to develop solutions that seek to accelerate the global adoption of our therapies and procedures. We are investing to expand the indications for use of our pain products with clinical research and studies and associated new product developments. We are expanding our portfolio with customer-preferred product enhancements, such as next generation cooled radiofrequency generators and a full line of needles, kits and accessories for continuous peripheral nerve block procedures.
Intellectual Property
Patents, trademarks and other proprietary rights are very important to our business. We also rely upon trade secrets, manufacturing know-how, continuing technological innovations and licensing opportunities to maintain and improve our competitive position. We review third-party proprietary rights, including patents and patent applications, as available, in an effort to develop an effective intellectual property strategy, avoid infringement of third-party proprietary rights, identify licensing opportunities and monitor the intellectual property owned by others.
We hold numerous patents and have numerous patent applications pending in the United States and other countries that relate to the technology used in many of our products. We utilize patents in our acute pain management, interventional pain management, respiratory health and digestive health products. These patents generally expire between 2020 and 2038. None of the patents we license from third parties are material to our business.
Under an agreement that we have with Buyer, we may continue to distribute products bearing the “Halyard Health” or “Halyard” brands through the end of 2020 as we continue rebranding efforts to ensure our customers’ transition from the Halyard brand.
We consider the patents and trademarks which we own and the trademarks under which we sell certain of our products, as a whole, to be material to our business. However, we do not consider our business to be materially dependent upon any individual patent or trademark.
Raw Materials
We use a wide variety of raw materials and other inputs in our production processes. We base our purchasing decisions on quality assurance, cost effectiveness and regulatory requirements, and we work closely with our suppliers to assure continuity of supply while maintaining high quality and reliability. We primarily purchase these materials from external suppliers, some of which are single-source suppliers.
Regulatory Matters
The development, manufacture, marketing, sale, promotion and distribution of our products are subject to comprehensive government regulation. Government regulation by various national, regional, federal, state and local agencies, both in the United States and other countries, addresses (among other matters) inspection of, and controls over, research and laboratory procedures, clinical investigations, product approvals and manufacturing, labeling, packaging, marketing and promotion, pricing and reimbursement, sampling, distribution, quality control, post-market surveillance, record keeping, storage and disposal practices. Our operations are also affected by trade regulations in many countries that limit the import of raw materials and finished products and by laws and regulations that seek to prevent corruption and bribery in the marketplace (including the
3 | ||
U.S. Foreign Corrupt Practices Act and the United Kingdom Bribery Act, which provide guidance on corporate interactions with government officials) and require safeguards for the protection of personal data. In addition, we are subject to laws and regulations pertaining to healthcare fraud and abuse, including state and federal anti-kickback and false claims laws in the United States.
Compliance with these laws and regulations is costly and materially affects our business. Among other effects, healthcare regulations substantially increase the time, difficulty and costs incurred in obtaining and maintaining approval to market newly developed and existing products. For example, in the United States, before we can market a new medical product, or market a new use for, claim for or significant modification to an existing product, we generally must first receive clearance under Section 510(k) of the Food, Drug and Cosmetic Act (“510(k) clearance”) from the United States Food and Drug Administration (“FDA”). In order for us to obtain 510(k) clearance, the FDA must determine that our proposed product is substantially equivalent to a device legally on the market, known as a predicate device, with respect to intended use, technology, safety and effectiveness. Similarly, most major markets for medical devices outside the United States also require clearance, approval or compliance with certain standards before a product can be commercially marketed. For instance, the European Commission, or EC, has harmonized national regulations for the control of medical devices through European Medical Device Directives with which manufacturers must comply. Under these regulations, manufacturing plants must have received certification of conformity from a notified body in order to be able to sell products within the member states of the European Union. Certification allows manufacturers to stamp the products of certified plants with a “CE” mark. Products covered by the EC regulations that do not bear the CE mark may not be sold or distributed in the European Union.
We expect compliance with these regulations to continue to require significant technical expertise and capital investment to ensure compliance. Failure to comply can delay the release of a new product or result in regulatory and enforcement actions, the seizure or recall of a product, the suspension or revocation of the authority necessary for a product’s production and sale, and other civil or criminal sanctions, including fines and penalties.
In addition to regulatory initiatives, our business can be affected by ongoing studies of the utilization, safety, efficacy, and outcomes of healthcare products and their components that are regularly conducted by industry participants, government agencies, and others. These studies can call into question the utilization, safety, and efficacy of previously marketed products. In some cases, these studies have resulted, and may in the future result, in the discontinuance of, or limitations on, marketing of such products domestically or worldwide, and may give rise to claims for damages from persons who believe they have been injured as a result of their use.
Access to healthcare products continues to be a subject of investigation and action by governmental agencies, legislative bodies, and private organizations in the United States and other countries. A major focus is cost containment. Efforts to reduce healthcare costs are also being made in the private sector, notably by healthcare payors and providers, which have instituted various cost reduction and containment measures. We expect insurers and providers to continue attempts to reduce the cost of healthcare products. Outside the United States, many countries control the price of healthcare products directly or indirectly, through reimbursement, payment, pricing, coverage limitations, or compulsory licensing. Budgetary pressures in the United States and in other countries may also heighten the scope and severity of pricing pressures on our products for the foreseeable future.
We expect debate to continue during the next several years at all government levels worldwide over the marketing, availability, method of delivery, and payment for healthcare products and services. We believe that future legislation and regulation in the markets we serve could affect access to healthcare products and services, increase rebates, reduce prices or the rate of price increases for healthcare products and services, change healthcare delivery systems, create new fees and obligations, or require additional reporting and disclosure. It is not possible to predict the extent to which we or the healthcare industry in general might be affected by the matters discussed above.
Since we market our products worldwide, certain products of a local nature and variations of product lines must also meet other local regulatory requirements. Certain additional risks are inherent in conducting business outside the United States, including price and currency exchange controls, changes in currency exchange rates, limitations on participation in local enterprises, expropriation, nationalization, and other governmental action.
Demand for many of our existing and new medical devices is, and will continue to be, affected by the extent to which government healthcare programs and private health insurers reimburse our customers for patients’ medical expenses in the countries where we do business. Statutory and regulatory requirements for Medicaid, Medicare, and other government healthcare programs govern provider reimbursement levels. From time to time, legislative changes are made to government healthcare programs that impact our business, and the federal and/or state governments may continue to enact measures in the future aimed at containing or reducing reimbursement levels for medical expenses paid for in whole or in part with government funds. We cannot predict the nature of such measures or their impact on our business, results of operations, financial condition and cash flows. Any reduction in the amount of reimbursements received by our customers could harm our business by reducing their selection of our products and the prices they are willing to pay.
4 | ||
Employee and Labor Relations
In our worldwide operations, we had approximately 4,700 employees as of December 31, 2019. We believe that we have good relations with our employees.
Environmental, Health and Safety Matters
Our operations are subject to federal, state, provincial and local laws, regulations and ordinances relating to various environmental, health and safety matters. Our operations are in compliance with, or we are taking actions designed to ensure compliance with, these laws, regulations and ordinances. However, the nature of our operations exposes us to the risk of claims concerning non-compliance with environmental, health and safety laws or standards, and there can be no assurance that material costs or liabilities will not be incurred in connection with those claims. We are not currently named as a party in any judicial or administrative proceeding relating to environmental, health and safety matters.
While we have incurred in the past several years, and will continue to incur, capital and operating expenditures in order to comply with environmental, health and safety laws, regulations and ordinances, we believe that our future cost of compliance with environmental, health and safety laws, regulations and ordinances, and our exposure to liability for environmental, health and safety claims will not have a material adverse effect on our business, results of operations, financial condition or cash flows. However, future events, such as changes in existing laws and regulations, or contamination of sites owned, operated or used for waste disposal by us (including currently unknown contamination and contamination caused by prior owners and operators of such sites or other waste generators) may give rise to additional costs which could have a material adverse effect on our financial condition, results of operations or liquidity.
Available Information
We make financial information, news releases and other information available on our corporate website at www.avanos.com. Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 are available free of charge on our corporate website as soon as reasonably practicable after we file these reports and amendments with, or furnish them to, the SEC. The information contained on or connected to our website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered part of this or any other report filed with the SEC. Stockholders may also contact Stockholder Services, 5405 Windward Parkway, Suite 100 South, Alpharetta, Georgia 30004 or call (844) 428-2667 to obtain a hard copy of these reports without charge.
ITEM 1A. RISK FACTORS
Our business faces many risks and uncertainties. Any of the risks discussed below, as well as factors described in other places in this Annual Report on Form 10-K, or in our other filings with the SEC, could adversely affect our business, consolidated financial position, results of operations or cash flows. In addition, these items could cause our future results to differ from those in any of our forward-looking statements. These risks are not the only ones we face. Other risks that we do not presently know about or that we presently believe are not material could also adversely affect us.
Risks Related to our Business and Industry
We face strong competition. Our failure to compete effectively could have a material adverse effect on our business.
Our industry is highly competitive. We compete with many domestic and foreign companies ranging from small start-up enterprises that might sell only a single or limited number of competitive products or compete only in a specific market segment, to companies that are larger and more established than us, have a broad range of competitive products, participate in numerous markets and have access to significantly greater financial and marketing resources than we do. We are also subject to potential competition from new technologies or new market entrants. Competitive factors include price, alternative clinical practices, innovation, quality and reputation. Our failure to compete effectively could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We may not be successful in developing, acquiring or marketing competitive products and technologies.
Our industry is characterized by extensive research and development and rapid technological advances. The future success of our business will depend, in part, on our ability to design, acquire and manufacture new competitive products and enhance existing products. Accordingly, we commit substantial time, funds and other resources to new product development, including research and development, acquisitions, licenses, clinical trials and physician education. We make these substantial expenditures without any assurance that our products will obtain regulatory clearance or reimbursement approval, acquire adequate intellectual property protection or receive market acceptance. Development by our competitors of improved products, technologies or enhancements may make our products, or those we develop, license or acquire in the future, obsolete or less competitive which could negatively impact our net sales. Our failure to successfully develop, acquire or market competitive
5 | ||
new products or enhance existing products could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We cannot guarantee that any of our strategic acquisitions, investments or alliances will be successful.
We intend to supplement our growth through strategic acquisitions of, investments in and alliances with new medical technologies. The success of any acquisition, investment or alliance may be affected by a number of factors, including our ability to identify and then properly assess and value the potential business opportunity or to successfully integrate any business we may acquire into our existing business. These types of transactions may require more resources and investments than originally anticipated, may divert management’s attention from our existing business, may result in exposure to unexpected liabilities of the acquired business, and may not result in the expected benefits, savings or synergies. There can be no assurance that we will be able to identify and successfully make strategic acquisitions of, investments in and alliances with new medical technologies or that any past or future acquisition, investment or alliance will be cost-effective, profitable or successful.
We may be unable to attract and retain key employees necessary to be competitive.
Our ability to compete effectively depends upon our ability to attract and retain executives and other key employees, including people in technical, marketing, sales, and research and development positions. Competition for experienced employees, particularly for persons with specialized skills, can be intense. Our ability to recruit such talent will depend on a number of factors, including compensation and benefits, work location and work environment. If we cannot effectively recruit and retain qualified executives and employees, our business could be materially adversely affected.
As we complete and stabilize our information technology infrastructure transition, we could incur substantial additional costs and experience temporary business interruption.
Pursuant to the sale of our Surgical & Infection Prevention business in 2018, we transferred ownership of our then existing information technology infrastructure to the Buyer and replaced such information technology infrastructure with new systems to support our critical business functions, including systems relating to accounting and reporting, accounts payable and receivable, manufacturing process control, customer service, inventory control and distribution. Although we have successfully completed the replacement process, we continue to work to stabilize the new system. We may incur temporary interruptions in business operations if we cannot effectively stabilize our existing transactional and operational systems and data centers. In addition, the stabilization process may result in (i) disruption to unrelated parts of our business, (ii) loss of employees or customers, (iii) exposure to unanticipated liabilities or (iv) assumption of ongoing obligations and liabilities following the completion of the process. Our failure to avoid operational interruptions, losses of employees or customers, or unanticipated liabilities as we seek to stabilize our information technology infrastructure could disrupt or have a material adverse effect on our business, financial condition, results of operations and cash flows.
Breaches of our information technology systems could have a material adverse effect on our business.
We rely on information technology systems to process, transmit and store electronic information in our day-to-day operations. Our information technology systems may be subjected to computer viruses or other malicious codes, unauthorized access attempts and cyber- or phishing-attacks. We also store certain information with third parties that could be subject to these types of attacks. These attacks could result in our intellectual property and other confidential information, including personal health information, being lost or stolen, disruption of our operations, loss of reputation and other negative consequences, such as increased costs for security measures or remediation costs and diversion of management attention. While we will continue to implement additional protective measures to reduce the risk of and detect future cyber incidents, cyber-attacks are becoming more sophisticated and frequent, and the techniques used in such attacks change rapidly. There can be no assurances that our protective measures will prevent future attacks that could have a material adverse effect on our business.
We may be unable to protect our intellectual property rights or may infringe the intellectual property rights of others.
We rely on patents, trademarks, trade secrets and other intellectual property assets in the operation of our business. Our efforts to protect our intellectual property and proprietary rights may not be sufficient. We cannot be sure that pending patent applications will result in the issuance of patents or that patents issued or licensed to us will remain valid or prevent competitors from introducing similar competing technologies. Our ability to enforce and protect our intellectual property rights may be limited in certain countries outside of the United States in which we operate, which could make it easier for our competitors to develop or distribute similar competing technologies in those jurisdictions. In addition, our competitive position may be adversely affected by expirations of our significant patents, which would allow competitors to freely use our technology to compete with us.
We operate in an industry characterized by extensive patent litigation and competitors may claim that our products infringe their intellectual property rights. Resolution of patent litigation or other intellectual property claims is inherently unpredictable, typically time consuming and costly and can result in significant damage awards and injunctions that could prevent the manufacture and sale of the affected products or require us to make significant royalty payments in order to continue selling the
6 | ||
affected products. Any one of these could have a material adverse effect on our business, results of operations, financial condition and cash flows. At any given time we are involved as either a plaintiff or a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time. We can expect to face additional claims of patent infringement in the future.
Our customers depend on third-party coverage and reimbursements. The failure of healthcare programs to provide coverage and reimbursement, or reductions in levels of reimbursement, could have a material adverse effect on our business.
The ability of our customers to obtain coverage and reimbursements for products they purchase from us is important to our business. Demand for many of our existing and new medical products is, and will continue to be, affected by the extent to which government healthcare programs and private health insurers reimburse our customers for patients’ medical expenses in the countries where we do business. Any reduction in the amount of reimbursements received by our customers could harm our business by reducing their selection of our products and the prices they are willing to pay.
In addition, as a result of their purchasing power, third-party payors are implementing cost-cutting measures such as seeking discounts, price reductions or other incentives from medical products suppliers and imposing limitations on coverage and reimbursements for medical technologies and procedures. These trends could compel us to reduce prices for our existing products and potential new products and could cause a decrease in the size of the market or a potential increase in competition that could have a material adverse effect on our business, results of operations, financial condition and cash flows.
An inability to obtain key components, raw materials or manufactured products from third parties may have a material adverse effect on our business.
We depend on the availability of various components, raw materials and manufactured products supplied by others for our operations, including suppliers who are based in or source their materials from China. If the capabilities of our suppliers and third-party manufacturers are limited or stopped, due to quality, regulatory or other reasons, including natural disasters, pandemics (or other health emergencies such as the recent “Coronavirus” outbreak), government actions, prolonged power or equipment failures or labor dispute, it could negatively impact our ability to manufacture or deliver our products and could expose us to regulatory actions. Further, for quality assurance or cost effectiveness, we purchase from sole suppliers certain components and raw materials. Although there are other sources in the market place for these items, we may not be able to quickly establish additional or replacement sources for certain components or materials due to regulations and requirements of the FDA and other regulatory authorities regarding the manufacture of our products. The loss of any sole supplier or any sustained supply interruption that affects our ability to manufacture or deliver our products in a timely or cost effective manner could have a material adverse effect on our business, results of operations, financial condition and cash flows.
An interruption in our ability to manufacture products may have a material adverse effect on our business.
Many of our key products are manufactured at single locations, with limited alternate facilities, including in certain cases by third-party manufacturers who are based in China. If one or more of these facilities experience damage, or if these manufacturing capabilities are otherwise limited or stopped due to quality, regulatory or other reasons, including natural disasters, pandemics (or other health emergencies such as the recent “Coronavirus” outbreak), government actions, prolonged power or equipment failures or labor dispute, it may not be possible to timely manufacture the relevant products at previous levels or at all. A reduction or interruption in any of these manufacturing processes could have a material adverse effect on our business, results of operations, financial condition and cash flows.
An interruption in distribution or transportation may have a material adverse effect on our business.
We rely on various transportation channels for global distribution of our products through shipping ports located throughout the world. Labor unrest, political instability, the outbreak of pandemics (or other health emergencies such as the recent “Coronavirus” outbreak), trade restrictions, transport capacity and costs, port security, weather conditions, natural disasters or other events could slow port activities and could adversely affect our business by interrupting product shipments and may increase our transportation costs if we are forced to use more expensive shipping alternatives.
We face significant uncertainty in the healthcare industry due to government healthcare reform in the United States and elsewhere.
The U.S. Congress, regulatory agencies and certain state legislatures, as well as international legislators and regulators, periodically review and assess alternative healthcare delivery systems and payment methods with an objective of ultimately reducing healthcare costs and expanding access. We cannot predict with certainty what healthcare initiatives, if any, will be implemented by states or foreign governments or what ultimate effect federal healthcare reform or any future legislation or regulation may have on our customers’ purchasing decisions regarding our products. However, the implementation of new legislation and regulation may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business, results of operations, financial condition and cash flows.
7 | ||
In addition, the United States Government periodically evaluates the potential repeal and potential replacement of all or parts of the 2010 Patient Protection and Affordable Care Act (sometimes colloquially known as “Obamacare”). Any such repeal or replacement may have a material adverse effect on our business, results of operations, financial condition and cash flows.
We are subject to extensive government regulation, which may require us to incur significant expenses to ensure compliance.
Many of our products are subject to extensive regulation in the United States by the FDA and other regulatory authorities and by comparable government agencies in other countries concerning the development, design, approval, manufacture, labeling, importing and exporting and sale and marketing of many of our products. Furthermore, our facilities are subject to periodic inspection by the FDA and other federal, state and foreign government authorities, which require manufacturers of medical devices to adhere to certain regulations, including the FDA’s Quality System Regulation, which requires periodic audits, design controls, quality control testing and documentation procedures, as well as complaint evaluations and investigation. Regulations regarding the development, manufacture and sale of medical products are evolving and subject to future change. We cannot predict what impact those regulatory changes may have on our business. Failure to comply with applicable regulations could lead to manufacturing shutdowns, product shortages, delays in product manufacturing, product seizures, recalls, operating restrictions, withdrawal or suspension of required licenses, and prohibitions against exporting of products to, or importing products from, countries outside the United States and may require significant resources to resolve. Any one or more of these events could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We are subject to healthcare fraud and abuse laws and regulations that could result in significant liability, require us to change our business practices or restrict our operations in the future.
We are subject to various U.S. federal, state and local laws targeting fraud and abuse in the healthcare industry, including the Food Drug and Cosmetic Act, anti-kickback and false claims laws. Violations of these laws are punishable by criminal or civil sanctions, including substantial fines, imprisonment and exclusion from participation in healthcare programs such as Medicare and Medicaid. These laws and regulations are wide ranging and subject to changing interpretation and application, which could restrict our sales or marketing practices. Furthermore, since many of our customers rely on reimbursement from Medicare, Medicaid and other governmental programs to cover a substantial portion of their expenditures, our exclusion from such programs as a result of a violation of these laws could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We must obtain clearance or approval from the appropriate regulatory authorities prior to introducing a new product or a modification to an existing product. The regulatory clearance process may result in substantial costs, delays and limitations on the types and uses of products we can bring to market, any of which could have a material adverse effect on our business.
In the United States, before we can market a new product, or a new use of, or claim for, or significant modification to, an existing product, we generally must first receive clearance or approval from the FDA and certain other regulatory authorities. Most major markets for medical devices outside the United States also require clearance, approval or compliance with certain standards before a product can be commercially marketed. The process of obtaining regulatory clearances and approvals to market a medical device can be costly and time consuming, involve rigorous pre-clinical and clinical testing, require changes in products or result in limitations on the indicated uses of products. There can be no assurance that these clearances and approvals will be granted on a timely basis, or at all. In addition, once a medical device has been cleared or approved, a new clearance or approval may be required before the medical device may be modified, its labeling changed or marketed for a different use. Medical devices are cleared or approved for one or more specific intended uses and promoting a device for an off-label use could result in government enforcement action. Furthermore, a product approval or clearance can be withdrawn or limited due to unforeseen problems with the medical device or issues relating to its application. The regulatory clearance and approval process may result in, among other things, delayed, if at all, realization of product net sales, substantial additional costs and limitations on the types of products we may bring to market or their indicated uses, any one of which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We may incur product liability losses, litigation liability, product recalls, safety alerts or regulatory action associated with our products which can be costly and disruptive to our business.
The risk of product liability claims is inherent in the design, manufacture and marketing of medical products of the type we produce and sell. A number of factors could result in an unsafe condition or injury to, or death of, a patient with respect to the products that we manufacture or sell, including physician technique and experience in performing the relevant surgical procedure, component failures, manufacturing flaws, design defects or inadequate disclosure of product-related risks or information.
In addition to product liability claims and litigation, an unsafe condition or injury to, or death of, a patient associated with our products could lead to a recall of, or issuance of a safety alert relating to, our products, or suspension or delay of regulatory product approvals or clearances, product seizures or detentions, governmental investigations, civil or criminal sanctions or
8 | ||
injunctions to halt manufacturing and distribution of our products. Any one of these could result in significant costs and negative publicity resulting in reduced market acceptance and demand for our products and harm our reputation. In addition, a recall or injunction affecting our products could temporarily shut down production lines or place products on a shipping hold.
All of the foregoing types of legal proceedings and regulatory actions are inherently unpredictable and, regardless of the outcome, could disrupt our business, result in substantial costs or the diversion of management attention and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Economic conditions have affected and may continue to adversely affect our business, results of operations, financial condition and cash flows.
Disruptions in the financial markets and other macro-economic challenges affecting the economy and the economic outlook of the United States, Europe, Japan, China and other parts of the world may have an adverse impact on our results of operations, financial condition and cash flows. Economic conditions and depressed levels of consumer and commercial spending have caused and may continue to cause our customers to reduce, modify, delay or cancel plans to purchase our products, and we have observed certain hospitals delaying and prioritizing purchasing decisions, which has had and may continue to have a material adverse effect on our business, results of operations, financial condition and cash flows.
In addition, as a result of economic conditions, our customers inside and outside the United States, including foreign governmental entities or other entities that rely on government healthcare systems or government funding, may be unable to pay their obligations on a timely basis or to make payment in full. If our customers’ cash flow or operating and financial performance deteriorate or fail to improve, or if our customers are unable to make scheduled payments or obtain credit, they may not be able to pay, or may delay payment of accounts receivable owed to us. These conditions also may have an adverse effect on certain of our suppliers who may reduce output or change terms of sales, which could cause a disruption in our ability to produce our products. Any inability of current and/or potential customers to pay us for our products or any demands by our suppliers for different payment terms may have a material adverse effect on our business, results of operations, financial condition and cash flows.
Currency exchange rate fluctuations could have a material adverse effect on our business and results of operations.
Due to our international operations, we transact business in many foreign currencies and are subject to the effects of changes in foreign currency exchange rates, including the Mexican peso, Japanese yen, Australian dollar and the Euro. Our financial statements are reported in U.S. dollars with international transactions being translated into U.S. dollars. If the U.S. dollar strengthens in relation to the currencies of other countries where we sell our products, our U.S. dollar reported net sales and income will decrease. Additionally, we incur significant costs in foreign currencies and a fluctuation in those currencies’ value can negatively impact manufacturing and selling costs. While we have in the past engaged, and may in the future engage, in various hedging transactions in attempts to minimize the effects of foreign currency exchange rate fluctuations, there can be no assurance that these hedging transactions will be effective. Changes in the relative values of currencies occur regularly and could have an adverse effect on our business, results of operations, financial condition and cash flows.
We are exposed to price fluctuations of key commodities, which may negatively impact our results of operations.
We rely on product inputs in the manufacture of our products. Prices of oil and gas affect our distribution and transportation costs. Prices of these commodities are volatile and have fluctuated significantly in recent years, which has contributed to, and in the future may continue to contribute to, fluctuations in our results of operations. Our ability to hedge commodity price volatility is limited. Furthermore, due to competitive dynamics, the cost containment efforts of our customers and third-party payors, and contractual limitations, particularly with respect to products we sell under group purchasing agreements, which generally set pricing for a three-year term, we may be unable to pass along commodity-driven cost increases through higher prices. If we cannot fully offset cost increases through other cost reductions, or recover these costs through price increases or surcharges, we could experience lower margins and profitability which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Cost-containment efforts of our customers, healthcare purchasing groups, third-party payors and governmental organizations could adversely affect our sales and profitability.
Many of our customers are members of GPOs, or integrated delivery networks (“IDNs”). GPOs and IDNs negotiate pricing arrangements with healthcare product manufacturers and distributors and offer the negotiated prices to affiliated hospitals and other members. Although we are the sole contracted supplier to certain GPOs for certain product categories, members of the GPO are generally free to purchase from other suppliers, and such contract positions can offer no assurance that sales volumes of those products will be maintained. In addition, initiatives sponsored by government agencies and other third-party payors to limit healthcare costs, including price regulation and competitive bidding for the sale of our products, are ongoing in markets where we sell our products. Pricing pressure has also increased in our markets due to consolidation among healthcare providers, trends toward managed care, governments becoming payors of healthcare expenses and regulation relating to reimbursements.
9 | ||
The increasing leverage of organized buying groups and consolidated customers and pricing pressure from third-party payors may reduce market prices for our products, thereby reducing our profitability and have a material adverse effect on our business, results of operations, financial condition and cash flows.
We are subject to political, economic and regulatory risks associated with doing business outside of the United States.
Most of our manufacturing facilities are outside the United States in Mexico, France, Germany and Tunisia. We also may use contract manufacturers outside the United States from time to time and may source many of our raw materials and components from foreign suppliers. We distribute and sell our products in over 90 countries. In 2019, approximately 23% of our net sales were generated outside of North America and we expect this percentage will grow over time. Our operations outside of the United States are subject to risks that are inherent in conducting business internationally, including compliance with both United States and foreign laws and regulations that apply to our international operations. These laws and regulations include robust data privacy requirements, labor relations laws that may impede employer flexibility, tax laws, anti-competition regulations, import, customs and trade restrictions, export requirements, economic sanction laws, environmental, health and safety laws, anti-bribery laws such as the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws in other jurisdictions. Given the high level of complexity of these laws, there is a risk that some provisions may be violated inadvertently or through fraudulent or negligent behavior of individual employees, our failure to comply with certain formal documentation requirements or otherwise. In addition, these laws are subject to changes, which may require additional resources or make it more difficult for us to comply with these laws. Violations of the laws and regulations governing our international operations could result in fines or criminal sanctions against us, our officers or our employees, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to manufacture or distribute our products in one or more countries and could have a material adverse effect on our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, our business, results of operations, financial condition and cash flows. Our success depends, in part, on our ability to anticipate and prevent or mitigate these risks and manage difficulties as they arise.
We may be subject to trade protection measures that are being contemplated by the United States Government and other governments around the world, as well as potential disruptions in trade agreements such as the exit of the United Kingdom from the European Union. These measures and disruptions may result in new or higher tariffs, import-export restrictions and taxes. Changes in, or revised interpretations of import-export laws or international trade agreements, along with new or increased tariffs, trade restrictions or taxation on income earned or goods manufactured outside the United States may have a material adverse effect on our business, financial condition, results of operations and cash flows.
In addition to the foregoing, engaging in international business inherently involves a number of other difficulties and risks, including:
• | different local medical practices, product preferences and product requirements, |
• | price and currency controls and exchange rate fluctuations, |
• | cost and availability of international shipping channels, |
• | longer payment cycles in certain countries other than the United States, |
• | minimal or diminished protection of intellectual property in certain countries, |
• | uncertainties regarding judicial systems, including difficulties in enforcing agreements through certain non-U.S. legal systems, |
• | political instability and actual or anticipated military or political conflicts, expropriation of assets, economic instability and the impact on interest rates, inflation and the credit worthiness of our customers, and |
• | difficulties and costs of staffing and managing non-U.S. operations. |
These risks and difficulties, individually or in the aggregate, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We have a significant amount of debt that could adversely affect our business.
Our $250 million of 6.25% senior unsecured notes, and any use of the borrowing capacity under our revolving line of credit, could have important consequences to us and our investors, including:
• | requiring a substantial portion of our cash flow from operations to make interest payments on this debt, |
• | reducing the cash flow available to fund capital expenditures and other corporate purposes and to grow our business, |
• | increasing our vulnerability to general adverse economic and industry conditions, |
10 | ||
• | increasing the risk of a future downgrade of our credit rating, which could increase future debt costs and limit the future availability of debt financing, |
• | limiting our ability to borrow additional funds as needed or take advantage of business opportunities as they arise, and |
• | limiting our flexibility in planning for, or reacting to, changes in our business and the industry and placing us at a competitive disadvantage to our competitors that may not be as highly leveraged. |
To the extent that we incur additional indebtedness, the risks described above could increase. In addition, our actual cash requirements in the future may be greater than expected. Our cash flow from operations may not be sufficient to repay all of the outstanding debt as it becomes due, and we may not be able to borrow money, sell assets or otherwise raise funds on acceptable terms, or at all, to refinance our debt.
We may need additional financing in the future to meet our capital needs or to make acquisitions and such financing may not be available on favorable terms, if at all.
We intend to continue our research and development activities and make acquisitions. Accordingly, we may need to seek additional debt or equity financing. We may be unable to obtain any desired additional financing on terms favorable to us, if at all. If we lose a previously assigned credit rating or adequate funds are not available on acceptable terms, we may be unable to fund our expansion, successfully develop or enhance products or respond to competitive pressures, any of which could negatively affect our business.
Risks Related to Ownership of Avanos Common Stock
We cannot guarantee that our stock price will not decline or fluctuate significantly.
The price at which Avanos common stock trades has and may continue to fluctuate significantly. The market price, or fluctuations in price, for Avanos common stock may be negatively influenced by many factors, including:
• | actual or unanticipated fluctuations in our quarterly and annual operating results, |
• | our failure to achieve the quarterly financial results forecast provided from time to time by the securities analysts who cover our stock, |
• | the outcome of litigation and enforcement actions, |
• | developments generally affecting the healthcare industry, |
• | changes in market valuations of comparable companies, |
• | the amount of our indebtedness, |
• | general economic, industry and market conditions, |
• | the depth and liquidity of the market for Avanos common stock, |
• | price fluctuations in key commodities, |
• | fluctuations in interest and currency exchange rates, |
• | our dividend policy, and |
• | perceptions of or speculations by the press or investment community. |
These and other factors may lower the market price of Avanos common stock, regardless of our actual financial condition or operating performance.
We have no present intention to pay dividends on Avanos common stock.
We have no present intention to pay dividends on Avanos common stock. Any determination to pay dividends to holders of Avanos common stock will be at the discretion of our Board of Directors and will depend on many factors, including our financial condition, results of operations, projections, liquidity, earnings, legal requirements, restrictions in our debt agreements and other factors that our Board of Directors deems relevant.
The percentage of ownership of existing stockholders in Avanos may be diluted in the future.
In the future, a stockholder’s percentage ownership in Avanos may be diluted because of equity issuances for acquisitions, capital market transactions or otherwise, including equity awards that we may grant to our directors, officers and employees. In addition, our compensation committee has, and we anticipate that they will continue in the future to, grant stock options or other equity based awards to our employees. These awards will have a dilutive effect on existing stockholders and on our earnings per share, which could adversely affect the market price of shares of Avanos common stock.
11 | ||
In addition, our certificate of incorporation authorizes us to issue, without the approval of Avanos stockholders, one or more classes or series of preferred stock having such designation, powers, preferences and relative, participating, optional and other special rights, including preferences over Avanos common stock with respect to dividends and distributions, as our Board of Directors generally may determine. If our Board of Directors were to approve the issuance of preferred stock in the future, the terms of one or more classes or series of such preferred stock could dilute the voting power or reduce the value of Avanos common stock. Similarly, the repurchase or redemption rights or liquidation preferences we could assign to Avanos preferred stock could affect the residual value of Avanos common stock.
Certain provisions of our certificate of incorporation and by-laws and of Delaware law may make it difficult for stockholders to change the composition of our Board of Directors and may discourage hostile takeover attempts which some of our stockholders may consider to be beneficial.
Certain provisions contained in our certificate of incorporation and by-laws and those contained in Delaware law may have the effect of delaying or preventing changes in control if our Board of Directors determines that such changes in control are not in the best interests of us and our stockholders. These provisions include, among other things, the following:
• | the division of our Board of Directors into three classes, each with three-year staggered terms, |
• | the ability of our Board of Directors to issue shares of preferred stock and to determine the price and other terms, including preferences and voting rights, of those shares without stockholder approval, |
• | the inability of our stockholders to call a special meeting of stockholders, |
• | stockholder action may be taken only at a special or regular meeting of stockholders, |
• | advance notice procedures for nominating candidates to our Board of Directors or presenting matters at stockholder meetings, |
• | stockholder removal of directors only for cause and only by a supermajority vote, |
• | the ability of our Board of Directors, and not our stockholders, to fill vacancies on our Board of Directors, and |
• | supermajority voting requirements to amend our by-laws and certain provisions of our certificate of incorporation and to engage in certain types of business combinations. |
While these provisions have the effect of encouraging persons seeking to acquire control of our company to negotiate with our Board of Directors, they could enable the Board of Directors to hinder or frustrate a transaction that some, or a majority, of the stockholders might believe to be in their best interests and, in that case, may prevent or discourage attempts to remove and replace incumbent directors. We are also subject to Delaware laws that could have similar effects. One of these laws prohibits us from engaging in a business combination with a significant stockholder unless specific conditions are met.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We own or lease operating facilities located throughout the world that handle manufacturing production, assembly, research, quality assurance testing, distribution and packaging of our products. We believe our facilities are suitable and adequate for our present operations. We lease our principal executive offices that are located in Alpharetta, Georgia. The locations of our principal medical device production facilities owned or leased by us around the world are as follows:
Location | Country | Owned/Leased | ||
Nogales | Mexico | Owned | ||
Nogales | Mexico | Leased | ||
Tucson, Arizona | USA | Leased | ||
Magdalena | Mexico | Leased | ||
Tijuana | Mexico | Leased | ||
Weinheim | Germany | Leased | ||
Marseille | France | Leased | ||
Sousse | Tunisia | Leased | ||
12 | ||
ITEM 3. LEGAL PROCEEDINGS
We are subject to various legal proceedings, claims and governmental inspections, audits or investigations pertaining to issues such as contract disputes, product liability, tax matters, patents and trademarks, advertising, governmental regulations, employment and other matters, including the matters described below. Under the terms of the distribution agreement we entered into with Kimberly-Clark Corporation (“Kimberly-Clark”) prior to the spin-off, legal proceedings, claims and other liabilities that are primarily related to our business are our responsibility and we are obligated to indemnify and hold Kimberly-Clark harmless for such matters (“Indemnification Obligation”). For the years ended December 31, 2019, 2018 and 2017, we have incurred $22.5 million, $15.6 million and $20.5 million, respectively, related to these matters.
Surgical Gown Litigation and Related Matters
Bahamas Surgery Center
We have an Indemnification Obligation for the matter styled Bahamas Surgery Center, LLC v. Kimberly-Clark Corporation and Halyard Health, Inc., No. 2:14-cv-08390-DMG-SH (C.D. Cal.) (“Bahamas”), filed on October 29, 2014. In that case, the plaintiff brought a putative class action asserting claims for common law fraud (affirmative misrepresentation and fraudulent concealment) and violation of California’s Unfair Competition Law (“UCL”) in connection with our marketing and sale of MicroCool surgical gowns.
On April 7, 2017, a jury returned a verdict for the plaintiff, finding that Kimberly-Clark was liable for $4 million in compensatory damages (not including prejudgment interest) and $350 million in punitive damages, and that Avanos was liable for $0.3 million in compensatory damages (not including prejudgment interest) and $100 million in punitive damages. Subsequently, the court also ruled on the plaintiff’s UCL claim and request for injunctive relief. The court found in favor of the plaintiff on the UCL claim but denied the plaintiff’s request for restitution. The court also denied the plaintiff’s request for injunctive relief.
On May 25, 2017, we filed post-trial motions seeking, among other things to have the award of punitive damages reduced. On April 11, 2018, the court issued an Amended Judgment in favor of the plaintiff and against us and Kimberly-Clark. The judgment against us is now $0.4 million in compensatory damages and pre-judgment interest and $1.3 million in punitive damages. The judgment against Kimberly-Clark is $3.9 million in compensatory damages, $2.3 million in pre-judgment interest and $19.4 million in punitive damages.
On April 12, 2018, we filed a notice of appeal to the Ninth Circuit Court of Appeals. We intend to continue our vigorous defense of the Bahamas matter.
Kimberly-Clark Corporation
We have notified Kimberly-Clark that we have reserved our rights to challenge any purported obligation to indemnify Kimberly-Clark for the punitive damages awarded against them. In connection with our reservation of rights, on May 1, 2017, we filed a complaint in the matter styled Halyard Health, Inc. v. Kimberly-Clark Corporation, Case No. BC659662 (County of Los Angeles, Superior Court of California). In that case, we seek a declaratory judgment that we have no obligation, under the Distribution Agreement or otherwise, to indemnify, pay, reimburse, assume, or otherwise cover punitive damages assessed against Kimberly-Clark in the Bahamas matter, or any Expenses or Losses (as defined in the distribution agreement) associated with an award of punitive damages. On May 2, 2017, Kimberly-Clark filed a complaint in the matter styled Kimberly-Clark Corporation v. Halyard Health, Inc., Case No. 2017-0332-AGB (Court of Chancery of the State of Delaware). In that case, Kimberly-Clark seeks a declaratory judgment that (1) we must indemnify them for all damages, including punitive damages, assessed against them in the Bahamas matter, (2) we have anticipatorily and materially breached the Distribution Agreement by our failure to indemnify them, and (3) we are estopped from asserting, or have otherwise waived, any claim that we are not required to indemnify them for all damages, including punitive damages, that may be awarded in the Bahamas matter.
On May 26, 2017, we moved to dismiss or stay Kimberly-Clark’s Delaware complaint, and on June 16, 2017, Kimberly-Clark moved for summary judgment. On September 12, 2017, the Delaware court granted our motion to stay Kimberly-Clark’s complaint and therefore did not take any action on Kimberly-Clark’s motion for summary judgment. On May 30, 2018, Kimberly-Clark moved to quash service of summons we served on Kimberly-Clark in California for lack of personal jurisdiction. On December 12, 2018, the court granted Kimberly-Clark’s motion. On December 18, 2018, we filed a notice of appeal to the California Court of Appeal. On December 6, 2019, the appellate court affirmed the lower court’s ruling, finding that it did not have personal jurisdiction over Kimberly-Clark. We intend to continue our vigorous defense of the matter.
Government Investigation
In June 2015, we were served with a subpoena from the Department of Veterans Affairs Office of the Inspector General (“VA OIG”) seeking information related to the design, manufacture, testing, sale and promotion of MicroCool and other Company surgical gowns, and, in July 2015, we also became aware that the subpoena and an earlier VA OIG subpoena served on
13 | ||
Kimberly-Clark requesting information about gown sales to the federal government are related to a United States Department of Justice (“DOJ”) investigation. In May 2016, April 2017 and September 2018, we received additional subpoenas from the DOJ seeking further information related to Company gowns. The Company is cooperating with the DOJ investigation.
Shahinian
On October 12, 2016, after the DOJ and various States declined to intervene, a qui tam matter was unsealed and a complaint was subsequently served on us in a matter styled U.S. ex rel. Shahinian, et al. v. Kimberly-Clark Corporation, No. 2:14-cv-08313-JAK-JPR (C. D. Cal.) (“Shahinian”), filed on October 27, 2014. The case alleges, among other things, violations of the federal and various state False Claims Acts in connection with the marketing and sale of certain surgical gowns. On March 8, 2017, Kimberly-Clark moved to dismiss the Shahinian complaint, and on July 14, 2017, the California court granted Kimberly-Clark’s motion. The plaintiff then filed a second amended complaint, and on August 11, 2017, Kimberly-Clark moved to dismiss that one as well. The plaintiff then filed a third amended complaint. On January 18, 2018, Kimberly-Clark moved to dismiss that one too. On September 30, 2018, the court granted Kimberly-Clark’s motion with prejudice. On November 13, 2018, Shahinian filed a notice of appeal to the Ninth Circuit Court of Appeals.
We may have an Indemnification Obligation for the Shahinian matter under the distribution agreement with Kimberly-Clark and have notified Kimberly-Clark that we reserve our rights to challenge the obligation to indemnify Kimberly-Clark for any damages or penalties which are not indemnifiable under applicable law or public policy. We intend to continue our vigorous defense of the matter.
Kromenaker
On March 17, 2017, the DOJ submitted a filing declining to intervene in another qui tam matter, and the complaint was unsealed and subsequently served on Kimberly-Clark and Avanos. That matter is styled U.S. ex rel. Kromenaker v. Kimberly-Clark Corporation and Halyard Health, Inc., No. 1:15-cv-04413-SCJ (N. D. Ga.) (“Kromenaker”), filed on December 21, 2015. In that case, the plaintiff alleges, among other things, violations of the federal False Claims Act in connection with the marketing and sale of certain products, including feminine hygiene products, surgical gowns and endotracheal tubes. On June 12, 2017 Kimberly-Clark and Avanos moved to dismiss the complaint. On August 21, 2017, Kromenaker filed an amended complaint, and Kimberly-Clark and Avanos filed motions to dismiss it. On March 27, 2019, the court granted Kimberly-Clark’s and our motion to dismiss. On April 24, 2019, Kromenaker filed a motion with the trial court seeking to have the court alter, amend, or vacate the dismissal. On January 14, 2020, the court denied Kromenaker’s motion, effectively dismissing the case.
We may have an Indemnification Obligation for certain parts of this matter under the distribution agreement with Kimberly-Clark and have notified Kimberly-Clark that we reserve our rights to challenge the obligation to indemnify Kimberly-Clark for any damages or penalties which are not indemnifiable under applicable law or public policy. We intend to continue our vigorous defense of this matter.
Jackson
We were served with a complaint in a matter styled Jackson v. Halyard Health, Inc., Robert E. Abernathy, Steven E. Voskuil, et al., No. 1:16-cv-05093-LTS (S.D.N.Y.), filed on June 28, 2016. In that case, the plaintiff brings a putative class action against the Company, our former Chief Executive Officer, our former Chief Financial Officer and other defendants, asserting claims for violations of the Securities Exchange Act, Sections 10(b) and 20(a). The plaintiff alleges that the defendants made misrepresentations and failed to disclose certain information about the safety and effectiveness of our MicroCool gowns and thereby artificially inflated the Company’s stock prices during the respective class periods. The alleged class period for purchasers of Kimberly-Clark securities who subsequently received Avanos securities is February 25, 2013 to October 21, 2014, and the alleged class period for purchasers of Avanos securities is October 21, 2014 to April 29, 2016. On February 16, 2017, we moved to dismiss the case. On March 30, 2018, the court granted our motion to dismiss and entered judgment in our favor. On April 27, 2018, the plaintiff filed a Motion for Relief from the Judgment and for Leave to Amend. On April 1, 2019, the court denied the plaintiff’s motion. On May 1, 2019, Jackson appealed the dismissal of the action to the 2nd Circuit Court of Appeals. We intend to continue our vigorous defense of this matter.
Richardson, Chiu and Pick
We were also served with a complaint in a matter styled Margaret C. Richardson Trustee of the Survivors Trust Dated 6/12/84 for the Benefit of the H&M Richardson Revocable Trust v. Robert E. Abernathy, Steven E. Voskuil, et al., No. 1:16-cv-06296 (S. D. N. Y.) (“Richardson”), filed on August 9, 2016. In that case, the plaintiff sues derivatively on behalf of Avanos Medical, Inc., and alleges that the defendants breached their fiduciary duty, were unjustly enriched, and violated Section 14(A) of the Securities and Exchange Act in connection with our marketing and sale of MicroCool gowns. We were also served with a complaint in a matter styled Kai Chiu v. Robert E. Abernathy, Steven E. Voskuil, et al, No. 2:16-cv-08768 (C.D. Cal.), filed on November 23, 2016. In that case, the plaintiff sues derivatively on behalf of Avanos Medical, Inc., and makes allegations and brings causes of action similar to those in Richardson, but the plaintiff also adds causes of action for abuse of control, gross
14 | ||
mismanagement, and waste of corporate assets. We were also served with a complaint in a matter styled Lukas Pick v. Robert E. Abernathy, Steven E. Voskuil, et al. No. e:18-cv-00295 (D. Del.), filed of February 21, 2018. In that case, the plaintiff sues derivatively on behalf of Avanos Medical, Inc., and makes allegations and brings causes of action similar to those in Richardson and Chiu. We intend to continue our vigorous defense of this matter.
Patent Litigation
We operate in an industry characterized by extensive patent litigation and competitors may claim that our products infringe upon their intellectual property. Resolution of patent litigation or other intellectual property claims is typically time consuming and costly and can result in significant damage awards and injunctions that could prevent the manufacture and sale of the affected products or require us to make significant royalty payments in order to continue selling the affected products. At any given time we may be involved as either a plaintiff or a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time.
General
While we maintain general and professional liability, product liability and other insurance, our insurance policies may not cover all of these matters and may not fully cover liabilities arising out of these matters. In addition, we may be obligated to indemnify our directors and officers against these matters.
We record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. For any matters that are reasonably possible to result in loss and for which no possible loss or range of loss is disclosed in this report, management has determined that it is unable to estimate the possible loss or range of loss because, in each case, at least the following facts applied: (a) early stage of the proceedings; (b) indeterminate (or unspecified) damages; and (c) significant factual issues yet to be resolved, or such amounts have been determined to be immaterial. At present, although the results of litigation and claims cannot be predicted with certainty, we believe that the ultimate resolution of these matters will not materially impact our liquidity, access to capital markets or ability to conduct our daily operations.
As of December 31, 2019, we have an accrued liability for the matters described herein, and reasonably possible losses have been disclosed. The accrued liability is included in “Accrued Expenses” in the accompanying consolidated balance sheet. Our estimate of these liabilities is based on facts and circumstances existing at this time, along with other variables. Factors that may affect our estimate include, but are not limited to: (i) changes in the number of lawsuits filed against us, including the potential for similar, duplicate or “copycat” lawsuits filed in multiple jurisdictions, including lawsuits that bring causes or action or allege violations of law with regard to additional products; (ii) changes in the legal costs of defending such claims; (iii) changes in the nature of the lawsuits filed against us, (iv) changes in the applicable law governing any legal claims against us; (v) a determination that our assumptions used in estimating the liability are no longer reasonable; and (vi) the uncertainties associated with the judicial process, including adverse judgments rendered by courts or juries. Thus, the actual amount of these liabilities for existing and future claims could be materially different than the accrued amount. Additionally, the above matters, regardless of the outcome, could disrupt our business and result in substantial costs and diversion of management attention.
Environmental Compliance
We are subject to federal, state and local environmental protection laws and regulations with respect to our business operations and are operating in compliance with, or taking action aimed at ensuring compliance with, these laws and regulations. None of our compliance obligations with environmental protection laws and regulations, individually or in the aggregate, is expected to have a material adverse effect on our business, financial condition, results of operations or liquidity.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
15 | ||
EXECUTIVE OFFICERS OF THE REGISTRANT
The names and ages of our executive officers as of February 25, 2020, together with certain biographical information, are as follows:
Name | Position | |
Joseph F. Woody | Chief Executive Officer | |
Michael C. Greiner | Senior Vice President and Chief Financial Officer | |
David E. Ball | Senior Vice President - Global Supply Chain & Procurement | |
Arjun R. Sarker | Senior Vice President - International | |
John W. Wesley | Senior Vice President and General Counsel | |
Joseph F. Woody, age 54, was appointed as Chief Executive Officer on June 26, 2017. Mr. Woody has more than 20 years of experience in the healthcare sector. Prior to joining the Company, Mr. Woody served as Director, President and Chief Executive Officer of Acelity Holdings, Inc. (“Acelity”), a global advanced wound care and regenerative medicine company, from August 2015 until April 2017. Prior to that, Mr. Woody served as President and Chief Executive Officer for the combined organization of Kinetic Concepts, Inc. (“KCI”), LifeCell Corporation (“LifeCell”), and Systagenix Wound Management B.V., which became Acelity, from September 2013 until August 2015. Prior to that, Mr. Woody served in leadership roles at KCI and LifeCell from November 2011 until September 2013, having been promoted to President and Chief Executive Officer of KCI in January 2012 and interim Chief Executive Officer of LifeCell in April 2013. Previously, Mr. Woody served as global president of Vascular Therapies for Covidien plc, and global president for Smith & Nephew Advanced Wound Management, and he held other leadership positions at Alliance Imaging, Inc., Acuson and GE Medical Systems.
Michael C. Greiner, age 47, was appointed as Senior Vice President and Chief Financial Officer on January 1, 2020. Mr. Greiner brings to Avanos more than 20 years of experience in corporate finance, accounting, treasury, and M&A strategy development and execution. He most recently served as Executive Vice President and CFO for AngioDynamics, Inc., a publicly listed medical device company (NASDAQ: ANGO), where he played an integral role in transforming and optimizing its product portfolio through both internal development and M&A. Prior to that, Mr. Greiner was the CFO at Extreme Reach, Inc., a cloud-based enterprise platform for brand advertising, responsible for all finance and human resource operations. Earlier in his career, Mr. Greiner held several senior executive roles, including Senior Vice President corporate finance and Chief Accounting Officer at Cimpress N.V. (formerly known as Vistaprint N.V.), global controller for GE’s Water and Processing Technologies division, as well as leadership roles at Bausch & Lomb and Wyeth.
David E. Ball, age 61, was appointed as Senior Vice President, Global Supply Chain & Procurement on December 17, 2018. His significant operations and R&D leadership experience includes more than 30 years in a variety of manufacturing, service, engineering, and quality positions within GE Healthcare, GE Transportation Systems, Hill-Rom, as well as Harris Corp.’s Communications and Aerospace Systems divisions. Prior to joining Avanos, Dave served as Senior Vice President of Operations for Acelity, where he led the company’s global manufacturing operations and oversaw its inventory, supply chain, procurement and facilities functions. In that role, he was instrumental in optimizing Acelity’s cost structure and sustaining the savings throughout the company’s expansion.
Arjun R. Sarker, age 54, was appointed as Senior Vice President - International as of April 2, 2018. Mr. Sarker joined the Company in January 2017 as Vice President and General Manager of the Company’s Asia-Pacific business. Prior to joining the Company, from 2007 to 2017, he held various leadership roles in general management and finance at Medtronic/Covidien. Prior to that, he worked at Honeywell in its specialty materials portfolio, at a British distribution group and at a public accounting firm. He is a former member of the advisory board of CFO Asia magazine, a regular panelist at Economist CFO roundtables and was co-chairman of the Medical Devices committee in AMCHAM India.
John W. Wesley, age 61, is our Senior Vice President of Legal and Government Relations. Prior to joining the Company in 2014, he had been serving as Kimberly-Clark’s Vice President, Deputy General Counsel and Corporate Secretary since 2009. He joined Kimberly-Clark in May 2000 as Senior Counsel, Corporate Affairs and has held a variety of positions, overseeing corporate transactions and corporate governance matters. Prior to joining Kimberly-Clark, he was a partner at the Dallas law firm of Carrington, Coleman, Sloman & Blumenthal, L.L.P., where he specialized in corporate, securities, corporate finance, mergers and acquisitions and general, commercial and business law.
16 | ||
PART II
ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Avanos common stock is listed on the New York Stock Exchange (“NYSE”) under the ticker symbol “AVNS”. We did not pay any dividends on our common stock in the years ended December 31, 2019 and 2018 and we do not expect to pay any cash dividends on our common stock in the foreseeable future.
As of February 18, 2020, we had 12,576 holders of record of our common stock.
For information relating to securities authorized for issuance under equity compensation plans, see Part III, Item 12 of this Form 10-K.
Performance
The following graph compares the cumulative total return of our common stock from December 31, 2014 through December 31, 2019 with the cumulative return of companies comprising the Standard and Poor’s S&P MidCap 400 Index and the S&P 500 Health Care Equipment and Services Index. The graph plots the change in value of an initial investment of $100 in each of our common stock, the S&P MidCap 400 Index and the S&P 500 Health Care Equipment and Services Index over the indicated time periods and assumes reinvestment of all dividends, if any, paid on the securities. We have not paid any cash dividends, and therefore, the cumulative total return calculation for us is based solely upon stock price appreciation and not upon reinvestment of cash dividends. The stock price performance shown on the graph is not necessarily indicative of future price performance.
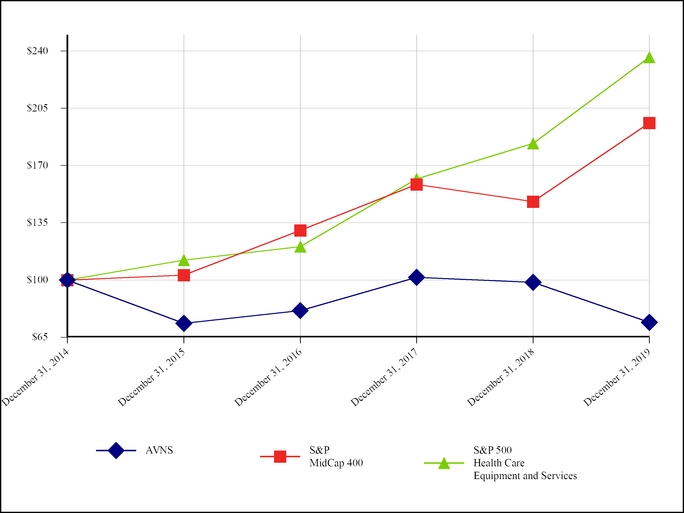
The preceding chart is based on the following data:
17 | ||
AVNS | S&P MidCap 400 | S&P 500 Health Care Equipment and Services | |||||||||
December 31, 2014 | $ | 100.00 | $ | 100.00 | $ | 100.00 | |||||
December 31, 2015 | 73.48 | 102.95 | 112.14 | ||||||||
December 31, 2016 | 81.33 | 130.27 | 120.43 | ||||||||
December 31, 2017 | 101.56 | 158.39 | 161.84 | ||||||||
December 31, 2018 | 98.50 | 147.89 | 183.43 | ||||||||
December 31, 2019 | 74.11 | 195.99 | 236.26 | ||||||||
ITEM 6. | SELECTED FINANCIAL DATA |
The following Selected Financial Data has been revised to reflect discontinued operations (see “Discontinued Operations” in Note 7 to the consolidated financial statements in Item 8 of this report). The Selected Financial Data as of December 31, 2019 and 2018 and for each of the years ended December 31, 2019, 2018 and 2017 have been derived from our audited consolidated financial statements which are included in Item 8 of this report. Selected Financial Data as of December 31, 2017, 2016 and 2015 and for each of the years ended December 31, 2016 and 2015 has been derived from our consolidated financial information but is not included in Item 8 of this report. The following Selected Financial Data is not necessarily indicative of future performance and should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Item 8, “Financial Statements and Supplementary Data” in this annual report on Form 10-K (in millions, except per-share amounts):
Year Ended December 31, | |||||||||||||||||||
2019 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||
Income Statement Data: | |||||||||||||||||||
Net Sales | $ | 697.6 | $ | 652.3 | $ | 611.6 | $ | 566.2 | $ | 509.0 | |||||||||
Operating Income (Loss) | (55.7 | ) | 0.5 | (43.1 | ) | (107.1 | ) | (135.7 | ) | ||||||||||
Loss from Continuing Operations | (45.9 | ) | (8.5 | ) | (32.1 | ) | (83.3 | ) | (101.2 | ) | |||||||||
Income (Loss) from Discontinued Operations, net of tax | — | 66.0 | 111.4 | 123.1 | (325.1 | ) | |||||||||||||
Net Income (Loss)(a)(b)(c)(d)(e) | (45.9 | ) | 57.5 | 79.3 | 39.8 | (426.3 | ) | ||||||||||||
Basic Earnings (Loss) Per Share: | |||||||||||||||||||
Continuing Operations | $ | (0.96 | ) | $ | (0.18 | ) | $ | (0.69 | ) | $ | (1.79 | ) | $ | (2.17 | ) | ||||
Discontinued Operations | — | 1.40 | 2.38 | 2.64 | (6.98 | ) | |||||||||||||
Basic Earnings (Loss) Per Share | (0.96 | ) | 1.22 | 1.69 | 0.85 | (9.15 | ) | ||||||||||||
Diluted Earnings (Loss) Per Share: | |||||||||||||||||||
Continuing Operations | $ | (0.96 | ) | $ | (0.18 | ) | $ | (0.69 | ) | $ | (1.79 | ) | $ | (2.17 | ) | ||||
Discontinued Operations | — | 1.40 | 2.38 | 2.64 | (6.98 | ) | |||||||||||||
Diluted Earnings (Loss) Per Share | (0.96 | ) | 1.22 | 1.69 | 0.85 | (9.15 | ) | ||||||||||||
______________________________
(a) | Net loss in 2019 includes $56.3 million of post divestiture transition charges, $20.2 million of post divestiture restructuring and IT charges (see “Restructuring” in Note 3 to the consolidated financial statements in Item 8 of this report), $13.1 million related to acquisition and integration activities (see “Business Acquisition” in Note 2 to the consolidated financial statements in Item 8 of this report), and $22.5 million of legal expenses for certain litigation matters (see “Commitments and Contingencies” in Note 14 to the consolidated financial statements in Item 8 of this report). |
(b) | Net income in 2018 includes $15.7 million of post divestiture restructuring and IT charges, $9.2 million of post divestiture transition expenses, $1.3 million of charges related to the acquisition and integration activities and $15.6 million of legal expenses for certain litigation matters. |
(c) | Net income in 2017 includes $12.8 million, net of tax, of costs related to legal expenses and litigation, $12.4 million, net of tax, of Divestiture-related charges, $4.7 million, net of tax, related to the integration of Corpak, $3.2 million, net of tax, of restructuring charges and a $10.1 million tax benefit as a result of recent passage of tax reform legislation. |
(d) | Net income in 2016 includes $14.1 million, net of tax, of spin-related transition expenses, $12.6 million, net of tax, of costs related to legal expenses and litigation and $10.9 million, net of tax, of costs related to our acquisition of Corpak. |
(e) | Net loss in 2015 includes a $474.0 million goodwill impairment charge, $32.8 million, net of tax, of spin-related transition expenses and $10.6 million, net of tax, of costs related to legal expenses and litigation partially offset by a $8.4 million net gain on the disposal of one of our exam glove manufacturing facilities in Thailand. |
18 | ||
As of December 31, | |||||||||||||||||||
2019 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||
Balance Sheet Data: | |||||||||||||||||||
Cash and cash equivalents | $ | 205.3 | $ | 384.5 | $ | 219.7 | $ | 113.7 | $ | 129.5 | |||||||||
Property, Plant and Equipment, net | 184.5 | 154.1 | 109.9 | 109.3 | 115.9 | ||||||||||||||
Total Assets | 1,799.6 | 1,833.4 | 2,195.9 | 2,071.8 | 2,000.2 | ||||||||||||||
Debt | 248.1 | 247.7 | 580.9 | 579.0 | 578.1 | ||||||||||||||
Stockholders’ Equity | $ | 1,265.2 | $ | 1,297.2 | $ | 1,215.4 | $ | 1,102.5 | $ | 1,055.3 | |||||||||
19 | ||
ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Introduction
Avanos is a medical technology company focused on delivering clinically superior breakthrough medical device solutions to improve patients’ quality of life. We are committed to addressing some of today’s most important healthcare needs, such as reducing the use of opioids while helping patients move from surgery to recovery. We develop, manufacture and market clinically superior solutions in more than 90 countries.
This Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is intended to provide investors with an understanding of our recent performance, financial condition and prospects and should be read in conjunction with the consolidated financial statements contained in Item 8, “Financial Statements and Supplementary Data” in this annual report on Form 10-K. The following will be discussed and analyzed:
• | Business Acquisitions |
• | Post-Divestiture Transition |
• | Restructuring Activities |
• | Discontinued Operations |
• | Results of Operations and Related Information |
• | Potential Impact of COVID-19 (Coronavirus) |
• | Unaudited Quarterly Data |
• | Liquidity and Capital Resources |
• | Critical Accounting Policies and Use of Estimates |
• | Legal Matters |
• | Information Concerning Forward-Looking Statements |
Business Acquisitions
On September 19, 2019, we acquired substantially all the assets of Endoclear, LLC (“Endoclear”). The initial purchase price was $3.5 million plus future contingent payments with an estimated fair value of $5.5 million, subject to certain adjustments as defined in the purchase agreement. Endoclear develops and markets airway management devices that are complementary to our existing respiratory health portfolio.
On August 7, 2019, we acquired substantially all the assets of Summit Medical Products, Inc. (“Summit”) for $15.6 million plus future contingent payments with an estimated fair value of $1.7 million, subject to certain adjustments as defined in the purchase agreement. Summit develops and markets the ambIT® family of ambulatory electronic infusion pumps, with annual net sales of approximately $7.0 million. Net sales of ambIT® products for the period from August 7, 2019 through December 31, 2019 were approximately $3.3 million and are included in the accompanying consolidated income statement.
On April 16, 2019, we acquired a minority interest in NeoMed, Inc. (“NeoMed”) for $7.0 million. NeoMed is a market-leading medical device company that is focused on specialized feeding and medication dosing for low birth weight, neonatal and pediatric patients. On July 8, 2019, we acquired all of the outstanding shares of NeoMed for a purchase price of $33.5 million, which includes the base purchase price of $28.0 million plus certain agreed-upon items at the closing date, net of cash acquired, and subsequently adjusted for certain items as defined in the purchase agreement. NeoMed’s net sales were $37.0 million in 2018. NeoMed’s net sales for the period from July 8, 2019 through December 31, 2019 were $19.7 million and are included in the accompanying consolidated income statement.
On July 1, 2018, we acquired Cool Systems, Inc. for $65.7 million, net of cash acquired, which was based on a purchase price of $65.0 million plus certain adjustments as provided in the purchase agreement. Cool Systems is marketed as Game Ready® and is hereinafter referred to as “Game Ready.” In the twelve months ended December 31, 2019, the purchase price allocation for Game Ready was finalized, resulting in a $1.9 million reduction of goodwill. The goodwill arising from the Game Ready acquisition is now $18.7 million.
Post Divestiture Transition
Following last year’s divestiture of the Surgical and Infection Prevention (“S&IP”) business (the “Divestiture”), we have been engaged in certain commercial agreements, including transition services agreements (“TSA”), the majority of which are complete. The remainder will terminate by the end of the third quarter of 2020.
20 | ||
For the year ended December 31, 2019, we have incurred additional $56.3 million of costs related to the Divestiture, which is included in “Cost of products sold,” “Selling and general expenses” and “Other expense, net” in the consolidated income statement in Item 8 of this report. For the year ended December 31, 2018, excluding a gain of $89.9 million we incurred $26.6 million of costs related to the Divestiture, consisting primarily of professional fees for legal, due diligence, consulting, tax and accounting services.
Restructuring Activities
Post-Divestiture Restructuring Plan
In conjunction with the Divestiture, we began a multi-phase restructuring plan (the “Plan”) intended to align our organizational structure, information technology platform, supply chain and distribution channels to be more appropriate for the size and scale of our remaining Medical Devices business. Each phase of the restructuring plan is described below:
Organizational Alignment: The first phase of the Plan aligned our organizational and management structure for our remaining Medical Devices business following the Divestiture. In the year ended December 31, 2019, we incurred $2.7 million of costs, primarily for employee retention, severance and benefits, that are included in “Cost of products sold” and “Selling and general expenses” in the accompanying consolidated income statement. In the year ended December 31, 2018, we incurred $9.3 million of costs.
As of December 31, 2019, this phase of the Plan was substantially complete. Plan-to-date expenses were $17.4 million, of which $10.5 million was for employee retention, severance and benefits and the remainder for third-party services and other costs.
Information Technology Systems: In the second phase of the Plan, we migrated to an IT platform that is more appropriate for our business and size (the “ITS Plan”). In the year ended December 31, 2019, we incurred $15.1 million of costs which are included in “Selling and general expenses” in the accompanying consolidated income statements compared to $6.4 million in the year ended December 31, 2018.
As of December 31, 2019, the ITS Plan was complete. Plan-to-date, we incurred $21.5 million of costs that were expensed as incurred and $54.1 million of costs that were capitalized, including $5.0 million of capitalized internal labor costs and $2.2 million of capitalized interest.
Cost Transformation: In June 2019, the third and final phase of the Plan was approved. This third phase relates to optimizing the Company’s procurement, manufacturing, and supply chain operations (the “Cost Transformation”). The Company expects to incur between $11.0 million and $13.0 million to execute the Cost Transformation, primarily consulting and other expenses that will be expensed as incurred. The Company also expects to spend between $8.0 million to $12.0 million of incremental capital through 2021 in support of the Cost Transformation. The Company expects to complete the Cost Transformation by the end of 2021. In the year ended December 31, 2019, we have incurred $2.3 million of costs related to Cost Transformation.
Integration of Business Acquisitions: During the third quarter of 2019, we initiated activities to integrate the asset and business acquisitions described in Note 2, “Business Acquisitions” into our operations, and where appropriate, re-align our organization accordingly. We expect to incur up to $17.0 million of costs, primarily for employee retention, severance and benefits and lease termination costs. In the year ended December 31, 2019, we incurred $9.1 million for employee retention, severance and benefits. We expect the integration of our acquisitions will be substantially complete by the end of 2020.
Discontinued Operations
As a result of the Divestiture, the results of operations from our S&IP business are reported as “Income from discontinued operations, net of tax” through April 30, 2018 and the related assets and liabilities are classified as “held for sale” as of December 31, 2018 in the consolidated financial statements in Item 8 of this report. See “Discontinued Operations” in Note 7 to the consolidated financial statements in Item 8 of this report.
Net sales from discontinued operations were $351.1 million for the period from January 1, 2018 to April 30, 2018 and $1,012.7 million for the year ended December 31, 2017.
Results of Operations and Related Information
Use of Non-GAAP Measures
In this section, we present “Adjusted Operating Profit (Loss)” which is a profitability measure that is not calculated in accordance with accounting principles generally accepted in the United States (“GAAP”) and is therefore referred to as a non-GAAP financial measure. We provide this non-GAAP measure because we use it to measure our operational performance and provide greater insight into our ongoing business operations. This measure is not intended to be, and should not be, considered separately from, or an alternative to, the most directly comparable GAAP financial measure. A reconciliation of “Adjusted
21 | ||
Operating Profit (Loss)” to the most directly comparable GAAP financial measure is provided in the “Operating Profit (Loss)” table below.
Net Sales
Our net sales are summarized in the following table for the years ended December 31, 2019, 2018 and 2017 (in millions):
Year Ended December 31, | |||||||||||||||||
2019 | 2018 | Change | 2017 | Change | |||||||||||||
Chronic care | $ | 413.7 | $ | 386.0 | 7.2 | % | $ | 360.8 | 7.0 | % | |||||||
Pain management | 283.9 | 266.3 | 6.6 | 250.8 | 6.2 | ||||||||||||
Total Net Sales | $ | 697.6 | $ | 652.3 | 6.9 | % | $ | 611.6 | 6.7 | % | |||||||
Total | Volume(a) | Pricing/Mix | Currency | Other(b) | |||||||||||||
Net Sales - percentage change 2019 vs. 2018 | 7 | % | 8 | % | (1 | )% | — | % | — | % | |||||||
Net Sales - percentage change 2018 vs. 2017 | 7 | % | 6 | % | — | % | — | % | 1 | % | |||||||
______________________________
(a) | Volume in 2019 includes incremental sales from Game Ready, NeoMed and Summit. Volume in 2018 includes incremental sales from Game Ready. |
(b) | Other includes rounding. |
Product Category Descriptions
Chronic care is a portfolio of products that include (i) digestive health products such as our Mic-Key enteral feeding tubes, Corpak patient feeding solutions and our recently acquired NeoMed neonatal and pediatric feeding solutions and (ii) respiratory health products such as closed airway suction systems and other airway management devices under the Ballard, Microcuff and recently acquired Endoclear brands.
Pain management is a portfolio of non-opioid pain solutions including (i) acute pain products such as On-Q and ambIT surgical pain pumps and Game Ready cold and compression therapy systems and (ii) interventional pain solutions, which provides minimally invasive pain relieving therapies, such as our Coolief pain relief therapy.
Net Sales - 2019 Compared to 2018
Net sales increased by 7% to $697.6 million for the year ended December 31, 2019 primarily due to volume. Incremental volume from the NeoMed, Summit and Game Ready acquisitions contributed 7% of the volume growth. Volume growth also came from organic growth in interventional pain products, digestive health and respiratory health, but was mostly offset by lower volume in acute pain which was affected this year by an industry-wide drug shortage, pre-fill disruption and consolidation of IV infusion customers.
Net Sales - 2018 Compared to 2017
Net sales increased by 7% to $652.3 million for the year ended December 31, 2018 as a result from a corresponding increase in sales volume. The acquisition of Game Ready, included in pain management, contributed 3% of the volume growth with the remainder from stronger demand for our interventional pain therapies and chronic care portfolios. Partially offsetting this growth was a decline in Acute Pain volume, which was affected by an industry-wide drug shortage, prefill disruption and consolidation of IV infusion customers.
Net Sales by Geographic Region
The factors causing organic volume growth were consistent throughout our geographic regions. Net sales by region is presented in the table below (in millions):
Year Ended December 31, | |||||||||||||||||
(in millions) | 2019 | 2018 | Change | 2017 | Change | ||||||||||||
North America | $ | 534.7 | $ | 505.3 | 5.8 | % | $ | 473.4 | 6.7 | % | |||||||
EMEA | 95.8 | 87.3 | 9.7 | 84.0 | 3.9 | ||||||||||||
Asia Pacific and Latin America | 67.1 | 59.7 | 12.4 | 54.2 | 10.1 | ||||||||||||
Total Net Sales | $ | 697.6 | $ | 652.3 | 6.9 | % | $ | 611.6 | 6.7 | % | |||||||
22 | ||
Gross Profit
Our gross profit and gross profit margin is summarized in the table below for the years ended December 31, 2019, 2018 and 2017 (in millions):
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Net Sales | $ | 697.6 | $ | 652.3 | $ | 611.6 | |||||
Gross Profit | 402.2 | 390.9 | 336.9 | ||||||||
Gross Profit Margin | 57.7 | % | 59.9 | % | 55.1 | % | |||||
Gross Profit - 2019 vs, 2018
Gross profit margin decreased to 58% for the year ended December 31, 2019 primarily due to higher post-divestiture and transition charges, higher distribution expenses due to the recent IT implementation as well as recent acquisitions with a lower margin.
Gross Profit - 2018 vs. 2017
Gross profit margin rose to 60% for the year ended December 31, 2018 driven primarily by costs that were historically presented as a component of the divested S&IP business. In the year ended December 31, 2018, there were $15.5 million of such costs, for the period from January 1, 2018 through the date of the Divestiture on April 30, 2018 compared to $45.0 million for the full year in 2017. These costs were not specifically identifiable and related to the S&IP business and accordingly, were not allocated to discontinued operations. Other factors that affect gross profit margin such as material costs, distribution, overhead and others impacted gross margin by less than 1% in the year ended December 31, 2018.
Adjusted Operating Profit (Loss)
The results of the S&IP business is reported in “Income from discontinued operations, net of tax” in the consolidated income statement for the periods from January 1, 2018 to April 30, 2018 and the year ended December 31, 2017, and are excluded from the operating (loss) profit presented in the table below. In accordance with GAAP, only costs specifically identifiable and attributable to a business to be disposed may be allocated to discontinued operations. Accordingly, for the periods from January 1, 2018 to April 30, 2018 and the year ended December 31, 2017, our operating losses were driven by certain costs that were historically presented as a component of the S&IP business but were included in continuing operations. These costs, on a pre-tax basis, were $37.0 million in the year ended December 31, 2018 and $115.8 million in 2017.
A reconciliation of adjusted operating profit (loss), a non-GAAP measure, to operating profit (loss) is provided in the table below (in millions):
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Operating profit (loss), as reported (GAAP) | $ | (55.7 | ) | $ | 0.5 | $ | (43.1 | ) | |||
Post divestiture restructuring and IT charges | 20.2 | 15.7 | 5.0 | ||||||||
Post divestiture transition charges | 56.3 | 9.2 | — | ||||||||
Acquisition and integration-related charges | 13.1 | 1.3 | 7.6 | ||||||||
Litigation and legal | 22.5 | 15.6 | 20.5 | ||||||||
Spin-related transition charges | — | — | 0.5 | ||||||||
Policy changes | — | — | (6.0 | ) | |||||||
Intangibles amortization | 20.0 | 20.0 | 20.7 | ||||||||
Adjusted Operating Profit (Loss) (non-GAAP) | $ | 76.4 | $ | 62.3 | $ | 5.2 | |||||
The items noted in the table above are described below:
Restructuring and IT charges: As previously described under “Restructuring Activities,” we have incurred $20.2 million and $15.7 million of costs, respectively, related to the Plan for the years ended December 31, 2019 and 2018. These costs are primarily for consulting and other services along with employee severance and benefits.
Post-divestiture transition costs: We incurred $56.3 million of costs related to transition activities following the Divestiture of the S&IP business. As previously noted under “Divestiture of the S&IP Business,” we incurred a total of $26.6 million related
23 | ||
to the Divestiture, excluding a gain of $89.9 million. Amounts incurred prior to the Divestiture and the gain are included in “Income from discontinued operations, net of tax” in the consolidated income statements in Item 8 of this report.
Acquisition and integration-related costs: We incurred $13.1 million of costs in connection with the acquisition and integration of EndoClear, Summit, NeoMed and Game Ready, as previously described under “Business Acquisitions.” Additional details are provided in “Business Acquisitions” in Note 2 to the consolidated financial statements in Item 8 of this report.
Spin-related costs: There were no spin-related activities or costs in 2019 or 2018, and were not material in 2017.
Litigation and legal: We incurred $22.5 million, $15.6 million and $20.5 million of expenses for certain litigation matters in the years ended December 31, 2019, 2018 and 2017, respectively. See “Commitments and Contingencies” in Note 14 to the consolidated financial statements in Item 8 of this report.
Intangibles Amortization: Intangibles amortization is related primarily to intangibles acquired in prior business acquisitions and was $20.0 million, $20.0 million and $20.7 million, respectively, in the years ended December 31, 2019, 2018 and 2017.
Adjusted Operating Profit excludes certain items, as applicable, for the relevant time periods as indicated in the “Operating Profit” table above. The excluded items include:
• | Expenses associated with post divestiture restructuring activities, including IT-related charges. |
• | Expenses associated with post-divestiture transition activities. |
• | Transaction and other expenses associated with the Divestiture. |
• | Acquisition and integration charges related to the acquisitions described earlier in “Business Acquisitions.” |
• | Prior year acquisition and integration charges related to the acquisition of CORPAK MedSystems, Inc. |
• | Prior year transition costs related to the separation from Kimberly-Clark Corporation, which included costs to establish our capabilities as a stand-alone entity, rebranding and other supply-chain transition costs. |
• | Expenses associated with certain litigation matters. |
• | Prior year charges associated with internal policy changes. |
• | Amortization of intangible assets associated with prior business acquisitions. |
Interest Expense
Interest expense was $15.0 million, $26.4 million and $31.6 million in the years ended December 31, 2019, 2018 and 2017, respectively. During 2018, we paid $339.0 million to retire our senior secured term loan, resulting in an early extinguishment loss of $4.8 million which was included in interest expense. Accordingly, interest expense was lower in 2019 compared to 2018 and 2017. In the years ended December 31, 2019, 2018 and 2017, $1.8 million, $1.5 million and $0.8 million, respectively, of interest was capitalized on long-term capital projects. Interest expense consists of interest accrued and amortization of debt discount and issuance costs on our long-term debt. See “Debt” in Note 9 to the consolidated financial statements in Item 8 of this report for further discussion of our indebtedness.
Provision for Income Taxes
Our overall effective tax rate was a 28% benefit for the year ended December 31, 2019 compared to a benefit of 53% in 2018 and 56% in 2017. The primary driver in the change in our effective tax rate are the effects of the Tax Cuts and Jobs Act of 2017. See “Income Taxes” in Note 10 to the consolidated financial statements in Item 8 of this report for further details regarding our income taxes.
Potential Impact of COVID-19 (Coronavirus)
The recent widespread outbreak of COVID-19 virus in central China could negatively impact our supply chain operations and our ability to meet demand for our products. We source both finished goods, such as our NeoMed line of enteral feeding devices for neonatal and pediatric patients, and components used in manufacturing other medical devices from various facilities in China. Government restrictions on travel and transportation and other efforts to curb the spread of the virus could lead to shortages and back orders if they persist into the second quarter 2020 and beyond. We are diligently working with our suppliers and transportation partners to minimize any potential supply disruptions. While recognizing that the situation is dynamic and changing, we currently believe that we will be able to successfully manage through the supply issues with limited disruptions to our business.
24 | ||
Unaudited Quarterly Data
2019 | 2018 | ||||||||||||||||||||||||||||||
(in millions, except per-share amounts) | Fourth | Third | Second | First | Fourth | Third | Second | First | |||||||||||||||||||||||
Net Sales | $ | 189.8 | $ | 171.4 | $ | 172.2 | $ | 164.2 | $ | 169.9 | $ | 165.1 | $ | 160.9 | $ | 156.4 | |||||||||||||||
Gross Profit | 109.7 | 95.0 | 98.7 | 98.8 | 100.4 | 104.7 | 94.7 | 91.1 | |||||||||||||||||||||||
Operating (Loss) Profit (a)(b) | (3.2 | ) | (18.1 | ) | (9.8 | ) | (24.6 | ) | (8.3 | ) | 7.0 | 8.8 | (7.0 | ) | |||||||||||||||||
Net (Loss) Income from Continuing Operations | (6.1 | ) | (11.5 | ) | (8.0 | ) | (20.3 | ) | (2.7 | ) | 4.2 | 1.3 | (11.3 | ) | |||||||||||||||||
Income from Discontinued Operations, net of tax | — | — | — | — | 0.5 | — | 34.0 | 31.5 | |||||||||||||||||||||||
Net (Loss) Income | $ | (6.1 | ) | $ | (11.5 | ) | $ | (8.0 | ) | $ | (20.3 | ) | $ | (2.2 | ) | $ | 4.2 | $ | 35.3 | $ | 20.2 | ||||||||||
Basic Earnings (Loss) Per Share: | |||||||||||||||||||||||||||||||
Continuing Operations | $ | (0.13 | ) | $ | (0.24 | ) | $ | (0.17 | ) | $ | (0.43 | ) | $ | (0.06 | ) | $ | 0.09 | $ | 0.03 | $ | (0.24 | ) | |||||||||
Discontinued Operations | — | — | — | — | 0.01 | — | 0.72 | 0.67 | |||||||||||||||||||||||
Net (loss) income | (0.13 | ) | (0.24 | ) | (0.17 | ) | (0.43 | ) | (0.05 | ) | 0.09 | 0.75 | 0.43 | ||||||||||||||||||
Diluted Earnings (Loss) Per Share: | |||||||||||||||||||||||||||||||
Continuing Operations | $ | (0.13 | ) | $ | (0.24 | ) | $ | (0.17 | ) | $ | (0.43 | ) | $ | (0.06 | ) | $ | 0.09 | $ | 0.03 | $ | (0.24 | ) | |||||||||
Discontinued Operations | — | — | — | — | 0.01 | — | 0.70 | 0.67 | |||||||||||||||||||||||
Net (loss) income | (0.13 | ) | (0.24 | ) | (0.17 | ) | (0.43 | ) | (0.05 | ) | 0.09 | 0.73 | 0.43 | ||||||||||||||||||
______________________________
(a) | Operating loss in 2019 includes $56.3 million of post-Divestiture transition charges, $22.5 million of expenses related to legal matters (see “Commitments and Contingencies” in Note 14 to the consolidated financial statements in Item 8 of this report), $20.2 million of restructuring charges (see “Restructuring” in Note 3 to the consolidated financial statements in Item 8 of this report), $13.1 million of acquisition-related expenses (see “Business Acquisition” in Note 2 to the consolidated financial statements in Item 8 of this report). |
(b) | Operating profit in 2018 includes $37.0 million of costs historically presented as a component of the S&IP business (see “Discontinued Operations” in Note 7 to the consolidated financial statements in Item 8 of this report), $15.7 million of restructuring charges, $15.6 million of expenses related to legal matters, $9.2 million of post-Divestiture transition charges and $1.3 million of acquisition-related expenses. |
25 | ||
Liquidity and Capital Resources
General
Our primary sources of liquidity are cash on hand provided by operating activities and amounts available under our revolving credit facility. Cash provided by operations has been and is expected to remain a primary source of funds. Cash provided by operations has historically generated sufficient cash to fund our investments in working capital and capital expenditures. As of December 31, 2019, $77.7 million of our $205.3 million of cash and cash equivalents was held by foreign subsidiaries. We consider the undistributed earnings of our foreign subsidiaries to be indefinitely reinvested and currently do not have plans to repatriate such earnings. See further discussion below in “Critical Accounting Policies and Use of Estimates” under “Income Taxes.” We do not expect restrictions on repatriation of cash held outside of the United States to have a material effect on our overall liquidity, financial condition or results of operations for the foreseeable future. We believe that our ability to generate cash from domestic and international operations and the borrowing capacity under our available credit facilities are adequate to fund our requirements for working capital, capital expenditures and other investments necessary to grow our business for the foreseeable future for both our domestic and international operations.
Cash and equivalents decreased by $179.2 million to $205.3 million as of December 31, 2019 compared to $384.5 million last year. The decrease was driven by $74.5 million used in operations, $57.5 million used to acquire assets and businesses and $50.6 million of capital expenditures.
Cash and equivalents increased by $164.8 million to $384.5 million as of December 31, 2018 compared to $219.7 million as of December 31, 2017. The increase was driven by $754.3 million received from the Divestiture partially offset $339.0 million used to retire our senior secured term loan, $145.6 million used in operations, including $98.0 million used to settle Divestiture-related net liabilities, $65.7 million used for the Game Ready acquisition and $49.1 million of capital expenditures.
Operating Activities
Operating activities used $74.5 million in the year ended December 31, 2019, primarily driven by higher inventory and lower accounts payable.
Operating activities used $145.6 million in the year ended December 31, 2018. Changes in operating assets and liabilities used $143.4 million, which includes $98.0 million used to settle Divestiture-related net liabilities and the remainder primarily related to higher inventories. Net income was driven lower by $26.6 million of Divestiture-related costs and $15.7 million of restructuring costs.
Investing Activities
Investing activities used $108.1 million in the year ended December 31, 2019, including $57.5 million used to acquire assets and businesses as described in “Business Acquisitions,” and capital expenditures of $50.6 million.
Investing activities provided $639.5 million in the year ended December 31, 2018, consisting primarily of $754.3 million received from the Divestiture partially offset by $65.7 million used in our acquisition of Game Ready and $49.1 million of capital expenditures.
Financing Activities
Financing activities provided $1.5 million in the year ended December 31, 2019, and was primarily proceeds from the exercise of stock options partially offset by purchases of treasury stock.
Financing activities used $324.4 million in the year ended December 31, 2018 and included $339.0 million used to retire our senior secured term loan partially offset by $17.1 million of net proceeds received from the exercise of stock options.
Long-Term Debt
As of December 31, 2019, total debt was $248.1 million, net of unamortized discount, on our Senior Unsecured Notes (“Notes”) that mature on October 15, 2022.
Following a divestiture of significant assets, such as the Divestiture, the credit agreement allows re-investment of the net proceeds into the business through acquisition of another business or through capital expenditures for a period of one year following the divestiture. We were required to offer to redeem a portion of the Notes at par value to the extent re-investments were not made by May 1, 2019. Accordingly, $130.5 million of the Notes were offered for redemption in the second quarter of 2019, of which $0.2 million were redeemed.
We retired our senior secured term loan following the Divestiture last year. Notwithstanding the retirement of our senior secured term loan, the senior secured revolving credit facility (“Revolving Credit Facility”) remains and is secured by substantially all of our assets located in the United States and a certain percentage of our foreign subsidiaries’ capital stock. The Revolving Credit Facility matures on October 30, 2023.
26 | ||
To the extent we remain in compliance with certain financial covenants in our credit agreement, funds under the Revolving Credit Facility are available for our working capital and other liquidity requirements. As of December 31, 2019, we had no borrowings outstanding and letters of credit of $0.7 million issued under the Revolving Credit Facility.
For further information regarding our debt arrangements, see “Debt” in Note 9 to the consolidated financial statements in Item 8 of this report.
Obligations
The following table presents our total contractual obligations for which cash flows are fixed or determinable as of December 31, 2019 (in millions):
Payments Due by Period | |||||||||||||||||||
Total | Less than 1 Year | 1-3 Years | 3-5 Years | More than 5 Years | |||||||||||||||
Debt | $ | 249.8 | $ | — | $ | 249.8 | $ | — | $ | — | |||||||||
Interest payments on long-term debt | 46.8 | 15.6 | 31.2 | — | — | ||||||||||||||
Operating lease obligation | 91.2 | 15.0 | 28.3 | 20.1 | 27.8 | ||||||||||||||
Open purchase orders(a) | 78.6 | 77.9 | 0.7 | — | — | ||||||||||||||
Pension obligations | 4.3 | 0.2 | 0.6 | 0.8 | 2.7 | ||||||||||||||
Other commitments(b) | 0.5 | — | — | — | 0.5 | ||||||||||||||
Total contractual obligations | $ | 471.2 | $ | 108.7 | $ | 310.6 | $ | 20.9 | $ | 31.0 | |||||||||
______________________________
(a) | The open purchase orders displayed in the table represent amounts that we anticipate will become payable within the next year for goods and services that we have negotiated for delivery. The table does not include payments that are discretionary or for which timing is uncertain. |
(b) | Other commitments is primarily uncertain tax positions. See “Income Taxes” in Note 10 to the consolidated financial statements in Item 8 of this report. |
Critical Accounting Policies and Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of net sales and expenses during the reporting period. The critical accounting policies we used in the preparation of the consolidated and financial statements are those that are important both to the presentation of our financial condition and results of operations and require significant judgments by management with regard to estimates used. The critical judgments by management relate to distributor rebate accruals, future cash flows associated with impairment testing for goodwill and long-lived assets, loss contingencies and deferred income taxes and potential tax assessments.
Use of Estimates
We prepare our consolidated financial statements in accordance with GAAP, which requires that we make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of net sales and expenses during the reporting periods. Estimates are used in accounting for, among other things, certain amounts included in discontinued operations, certain amounts included in assets and liabilities held for sale, distributor rebate accruals, future cash flows associated with impairment testing for goodwill and long-lived assets, loss contingencies, and deferred tax assets and potential income tax assessments. Actual results could differ from these estimates, and the effect of the change could be material to our financial statements. Changes in these estimates are recorded when known.
Revenue Recognition
Sales revenue is recognized at the time of product shipment or delivery of our products to unaffiliated customers, depending on shipping terms. Accordingly, control of the products transfers to the customer in accordance with the transaction’s shipping terms. Sales revenue is recognized for the amount of considerations that we expect to be entitled to receive in exchange for our products. Sales are reported net of returns, rebates, incentives, each as described below, and freight allowed. Taxes imposed by governmental authorities on our revenue-producing activities with customers, such as sales taxes and value-added taxes, are excluded from net sales.
Our contracts provide for forms of variable consideration including rebates. We provide for rebates to distributors for estimated historical differences between list prices and average end-user customer prices and the quantity of products expected to be sold to specific end-user customers. We maintain a liability for the estimated rebates.
Loss Contingencies
The outcome of loss contingencies, legal proceedings, indemnification matters and claims brought against us is subject to uncertainty. An estimated loss contingency is accrued by a charge to earnings if it is probable that an asset has been impaired or
27 | ||
a liability has been incurred and the amount can be reasonably estimated. Determination of whether to accrue a loss requires evaluation of the probability of an unfavorable outcome and the ability to make a reasonable estimate. Changes in these estimates could affect the timing and amount of accrual of loss contingencies.
Income Taxes
We recognize tax benefits in our financial statements when our uncertain tax positions are more likely than not to be sustained upon audit. The amount we recognize is measured as the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement.
We recognize deferred tax assets for deductible temporary differences, operating loss carry-forwards and tax credit carry-forwards. We record valuation allowances to reduce deferred tax assets to amounts that are more likely than not to be realized. In assessing the need for a valuation allowance, we consider both positive and negative evidence related to the likelihood of realization of the deferred tax assets. The weight given to the positive and negative evidence is commensurate with the extent to which the evidence may be objectively verified. As such, it is generally difficult for positive evidence regarding projected future taxable income exclusive of reversing taxable temporary differences to outweigh objective negative evidence of recent financial reporting losses. This assessment, which is completed on a taxing jurisdiction basis, takes into account a number of types of evidence, including the nature, frequency, and severity of current and cumulative financial reporting losses, sources of future taxable income, taxable income in prior carryback year(s) and tax planning strategies.
If it is determined that we would be able to realize deferred tax assets in the future in excess of our net recorded amount, an adjustment to the net deferred tax asset would increase income in the period that such determination was made. Likewise, should we determine that we would not be able to realize all or part of the net deferred tax assets in the future, an adjustment to the net deferred tax asset would decrease income in the period such determination was made. We regularly evaluate the need for valuation allowances against its deferred tax assets.
As of December 31, 2019, we have accumulated undistributed earnings generated by our foreign subsidiaries of approximately $41.0 million. Certain earnings were previously subject to tax due to the one-time transition tax of the Act. Any additional impacts due with respect to the previously-taxed earnings, if repatriated, would generally be limited to foreign withholding tax, U.S. state income tax and the tax effect of certain foreign exchange adjustments. We intend, however, to indefinitely reinvest these earnings and expect future U.S. cash generation to be sufficient to meet U.S. cash needs. At this time, the determination of deferred tax liabilities on the amount of financial reporting over tax basis is not practicable.
Legal Matters
A description of legal matters can be seen in Item 3. Legal Proceedings.
Information Concerning Forward-Looking Statements
This annual report on Form 10-K and other materials we have filed or furnished or will file or furnish with the SEC (as well as information included in our oral or other written statements) contain, or will contain, certain “forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, regarding business strategies, market potential, future financial performance and other matters. Forward-looking statements include all statements that do not relate solely to historical or current facts, and can generally be identified by the use of words such as “may,” “believe,” “will,” “expect,” “project,” “estimate,” “anticipate,” “plan” or “continue” and similar expressions, among others. The matters discussed in these forward-looking statements are based on the current plans and expectations of our management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those projected, anticipated or implied in the forward-looking statements. These factors include, but are not limited to:
• | general economic conditions particularly in the United States, |
• | fluctuations in global equity and fixed-income markets, |
• | the competitive environment, |
• | the loss of current customers or the inability to obtain new customers, |
• | litigation and enforcement actions, |
• | price fluctuations in key commodities, |
• | fluctuations in currency exchange rates, |
• | disruption in supply of raw materials or the distribution of finished goods, |
• | changes in governmental regulations that are applicable to our business, |
28 | ||
• | changes in asset valuations including write-downs of assets such as inventory, accounts receivable or other assets for impairment or other reasons, and |
• | the other matters described under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” |
Where, in any forward-looking statement, an expectation or belief as to future results or events is expressed, such expectation or belief is based on the current plans and expectations of our management and expressed in good faith and believed to have a reasonable basis, but there can be no assurance that the expectation or belief will result or be achieved or accomplished.
29 | ||
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to risks such as changes in foreign currency exchange rates and commodity prices. A variety of practices are employed to manage these risks, including derivative instruments where deemed appropriate. Derivative instruments are used only for risk management purposes and not for speculation. All foreign currency derivative instruments are entered into with major financial institutions. Our credit exposure under these arrangements is limited to agreements with a positive fair value at the reporting date. Credit risk with respect to the counterparties is actively monitored but is not considered significant.
Presented below is a description of our risk together with a sensitivity analysis, performed annually, based on selected changes in market rates and prices. These analyses reflect management’s view of changes which are reasonably possible to occur over a one-year period. Also included is a description of our commodity price risk.
Interest Rate Risk
Our Revolving Credit Facility that allows for borrowings up to $250.0 million is subject to a variable interest rate based on LIBOR. As of December 31, 2019, a one percentage point increase in LIBOR could result in $2.5 million of incremental interest expense if the Revolving Credit Facility was fully drawn for the entire year.
Foreign Currency Risk
Foreign currency risk is managed by the systematic use of foreign currency forward and swap contracts for a limited portion of our exposure. The use of these instruments allows the management of transactional exposures to exchange rate fluctuations because the gains or losses incurred on the derivative instruments will offset, in whole or in part, losses or gains on the underlying foreign currency exposure.
Foreign currency contracts and transactional exposures are sensitive to changes in foreign currency exchange rates. An annual test is performed to quantify the effects that possible changes in foreign currency exchange rates would have on annual operating profit based on our foreign currency contracts and transactional exposures at the current year-end. The balance sheet effect is calculated by multiplying each affiliate’s net monetary asset or liability position by a 10% change in the foreign currency exchange rate versus the U.S. dollar. The results of these sensitivity tests are presented in the following paragraph.
As of December 31, 2019, a 10% unfavorable change in the exchange rate of the U.S. dollar against the prevailing market rates of foreign currencies involving balance sheet transactional exposures would have an effect of $0.7 million to our consolidated financial position, results of operations and cash flows. These hypothetical losses on transactional exposures are based on the difference between the December 31, 2019 rates and the assumed rates.
The translation of the balance sheets of non-U.S. operations from local currencies into U.S. dollars is also sensitive to changes in foreign currency exchange rates. Consequently, an annual test is performed to determine if changes in currency exchange rates would have a significant effect on the translation of the balance sheets of non-U.S. operations into U.S. dollars. These translation gains or losses are recorded as unrealized translation adjustments (“UTA”) within stockholders’ equity. The hypothetical change in UTA is calculated by multiplying the net assets of these non-U.S. operations by a 10% change in the currency exchange rates.
As of December 31, 2019, a 10% unfavorable change in the exchange rate of the U.S. dollar against the prevailing market rates of our foreign currency translation exposures would have reduced stockholders’ equity by approximately $16.8 million. These hypothetical adjustments in UTA are based on the difference between the December 31, 2019 exchange rates and the assumed rates. In the view of management, the above UTA adjustments resulting from these assumed changes in foreign currency exchange rates are not material to our consolidated financial position because they would not affect our cash flow.
Commodity Price Risk
We are subject to commodity price risk for certain raw materials used in the manufacture of our products. As previously discussed under “Risk Factors,” increases in commodities prices could adversely affect our earnings if selling prices are not adjusted or if such adjustments significantly trail the increases in commodities prices.
Our energy, manufacturing and transportation costs are affected by various market factors including the availability of supplies of particular forms of energy, energy prices and local and national regulatory decisions. As previously discussed in “Risk Factors,” there can be no assurance we will be fully protected against substantial changes in the price or availability of energy sources. In addition, we are subject to price risk for utilities and manufacturing inputs, which are used in our manufacturing operations.
30 | ||
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED INCOME STATEMENTS
(in millions, except per share amounts)
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Net Sales | $ | $ | $ | ||||||||
Cost of products sold | |||||||||||
Gross Profit | |||||||||||
Research and development | |||||||||||
Selling and general expenses | |||||||||||
Other expense, net | |||||||||||
Operating (Loss) Income | ( | ) | ( | ) | |||||||
Interest income | |||||||||||
Interest expense | ( | ) | ( | ) | ( | ) | |||||
Loss Before Income Taxes | ( | ) | ( | ) | ( | ) | |||||
Income tax benefit | |||||||||||
Loss from Continuing Operations | ( | ) | ( | ) | ( | ) | |||||
Income from discontinued operations, net of tax | |||||||||||
Net (Loss) Income | $ | ( | ) | $ | $ | ||||||
(Loss) Earnings Per Share | |||||||||||
Basic: | |||||||||||
Continuing operations | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Discontinued operations | |||||||||||
Basic (Loss) Earnings Per Share | $ | ( | ) | $ | $ | ||||||
Diluted: | |||||||||||
Continuing operations | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Discontinued operations | |||||||||||
Diluted (Loss) Earnings Per Share | $ | ( | ) | $ | $ | ||||||
See Notes to the Consolidated Financial Statements.
31 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
(in millions)
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Net (Loss) Income | $ | ( | ) | $ | $ | ||||||
Other Comprehensive Income (Loss), Net of Tax | |||||||||||
Defined benefit plans | ( | ) | |||||||||
Unrealized currency translation adjustments | ( | ) | |||||||||
Cash flow hedges | ( | ) | |||||||||
Total Other Comprehensive Income (Loss), Net of Tax | ( | ) | |||||||||
Comprehensive (Loss) Income | $ | ( | ) | $ | $ | ||||||
See Notes to the Consolidated Financial Statements.
32 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in millions, except share data)
As of December 31, | |||||||
2019 | 2018 | ||||||
ASSETS | |||||||
Current Assets | |||||||
Cash and cash equivalents | $ | $ | |||||
Accounts receivable, net of allowances | |||||||
Inventories | |||||||
Prepaid and other current assets | |||||||
Total Current Assets | |||||||
Property, Plant and Equipment, net | |||||||
Operating Lease Right of Use Assets | — | ||||||
Goodwill | |||||||
Other Intangible Assets, net | |||||||
Deferred Tax Assets | |||||||
Other Assets | |||||||
TOTAL ASSETS | $ | $ | |||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current Liabilities | |||||||
Current portion of operating lease obligation | $ | $ | — | ||||
Trade accounts payable | |||||||
Accrued expenses | |||||||
Total Current Liabilities | |||||||
Long-Term Debt | |||||||
Operating Lease Obligation | — | ||||||
Deferred Tax Liabilities | |||||||
Other Long-Term Liabilities | |||||||
Total Liabilities | |||||||
Commitments and Contingencies | |||||||
Stockholders’ Equity | |||||||
Preferred stock - $0.01 par value - authorized 20,000,000 shares, none issued | |||||||
Common stock - $0.01 par value - authorized 300,000,000 shares, 47,734,206 outstanding at December 31, 2019 and 47,444,340 outstanding at December 31, 2018 | |||||||
Additional paid-in capital | |||||||
Accumulated deficit | ( | ) | ( | ) | |||
Treasury stock | ( | ) | ( | ) | |||
Accumulated other comprehensive loss | ( | ) | ( | ) | |||
Total Stockholders’ Equity | |||||||
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | $ | |||||
See Notes to the Consolidated Financial Statements.
33 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
(in millions, shares in thousands)
Common Stock Issued | Additional Paid-in Capital | Retained Earnings (Accumulated Deficit) | Treasury Stock | Accumulated Other Comprehensive Income (Loss) | Total Stockholders’ Equity | ||||||||||||||||||||||||
Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||
Balance at December 31, 2016 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||||
Net income | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock upon the exercise or redemption of share-based awards | — | — | — | — | — | ||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | |||||||||||||||||||||||
Purchases of treasury stock | — | — | — | — | ( | ) | — | ( | ) | ||||||||||||||||||||
Other comprehensive income, net of tax | — | — | — | — | — | — | |||||||||||||||||||||||
Balance at December 31, 2017 | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||
Net income | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock upon the exercise or redemption of share-based awards | — | — | — | — | — | ||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | |||||||||||||||||||||||
Purchases of treasury stock | — | — | — | — | ( | ) | — | ( | ) | ||||||||||||||||||||
Other comprehensive loss, net of tax | — | — | — | — | — | — | ( | ) | ( | ) | |||||||||||||||||||
Balance at December 31, 2018 | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||
Net loss | — | — | — | ( | ) | — | — | — | ( | ) | |||||||||||||||||||
Issuance of common stock upon the exercise or redemption of share-based awards | — | — | — | — | — | ||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | |||||||||||||||||||||||
Purchases of treasury stock | — | — | — | — | ( | ) | — | ( | ) | ||||||||||||||||||||
Other comprehensive income, net of tax | — | — | — | — | — | — | |||||||||||||||||||||||
Balance at December 31, 2019 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||||
See Notes to the Consolidated Financial Statements.
34 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED CASH FLOW STATEMENTS
(in millions)
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Operating Activities | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ||||||
Depreciation and amortization | |||||||||||
Stock-based compensation | |||||||||||
Net non-cash gain on Divestiture | ( | ) | |||||||||
Net losses on asset dispositions | |||||||||||
Changes in operating assets and liabilities, net of acquisition | |||||||||||
Accounts receivable | ( | ) | ( | ) | |||||||
Inventories, net of allowance | ( | ) | ( | ) | ( | ) | |||||
Prepaid expenses and other assets | ( | ) | ( | ) | |||||||
Accounts payable | ( | ) | ( | ) | |||||||
Accrued expenses | ( | ) | |||||||||
Deferred income taxes and other | ( | ) | ( | ) | ( | ) | |||||
Cash (Used in) Provided by Operating Activities | ( | ) | ( | ) | |||||||
Investing Activities | |||||||||||
Capital expenditures | ( | ) | ( | ) | ( | ) | |||||
Acquisition of assets and businesses, net of cash acquired | ( | ) | ( | ) | |||||||
Proceeds from the Divestiture | |||||||||||
Proceeds from dispositions of property | |||||||||||
Cash (Used in) Provided by Investing Activities | ( | ) | ( | ) | |||||||
Financing Activities | |||||||||||
Debt repayments | ( | ) | ( | ) | |||||||
Debt issuance costs | ( | ) | |||||||||
Purchase of treasury stock | ( | ) | ( | ) | ( | ) | |||||
Proceeds from the exercise of stock options | |||||||||||
Cash Provided by (Used in) Financing Activities | ( | ) | |||||||||
Effect of Exchange Rate Changes on Cash and Cash Equivalents | ( | ) | |||||||||
(Decrease) Increase in Cash and Cash Equivalents | ( | ) | |||||||||
Cash and Cash Equivalents - Beginning of Year | |||||||||||
Cash and Cash Equivalents - End of Year | $ | $ | $ | ||||||||
Supplemental Cash Flow Disclosure: | |||||||||||
Cash paid for income taxes | $ | $ | $ | ||||||||
Cash paid for interest | $ | $ | $ | ||||||||
Supplemental Noncash Disclosure | |||||||||||
Capital expenditures included in accounts payable or accrued expenses | $ | $ | $ | ||||||||
See Notes to the Consolidated Financial Statements.
35 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Accounting Policies
Avanos Medical, Inc. is a medical technology company focused on delivering clinically superior breakthrough medical device solutions to improve patients’ quality of life. Headquartered in Alpharetta, Georgia, Avanos is committed to addressing some of today’s most important healthcare needs, such as reducing the use of opioids while helping patients move from surgery to recovery. We develop, manufacture and market clinically superior solutions in more than 90 countries. References to “Avanos,” “Company,” “we,” “our” and “us” refer to Avanos Medical, Inc. and its consolidated subsidiaries.
The consolidated financial statements include our net assets, results of our operations and cash flows. All intercompany transactions and accounts within our consolidated businesses have been eliminated. The consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”).
Preparation of consolidated financial statements in accordance with GAAP requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of net sales and expenses during the reporting periods. Estimates are used in accounting for, among other things, distributor rebate accruals, future cash flows associated with impairment testing for goodwill and long-lived assets, loss contingencies, and deferred tax assets and potential income tax assessments. Actual results could differ from these estimates, and the effect of the change could be material to our financial statements. Changes in these estimates are recorded when known.
Cash Equivalents
Cash equivalents are short-term investments with an original maturity date of three months or less. We maintain cash balances and short-term investments in excess of insurable limits in a diversified group of major banks that are selected and monitored based on ratings by the major rating agencies in accordance with our treasury policy.
Inventories and Distribution Costs
Most U.S. inventories are valued at the lower of cost, using the Last-In, First-Out (“LIFO”) method, or market. The balance of the U.S. and non-U.S. inventories are valued at the lower of cost (determined on the First-In, First-Out (“FIFO”) or weighted-average cost methods) or market. Distribution costs are classified as cost of products sold.
Property, Plant and Equipment and Depreciation
Property, plant and equipment are stated at cost and depreciated on the straight-line method. Buildings are depreciated over their estimated useful lives, primarily 40 years. Machinery and equipment are depreciated over their estimated useful lives, primarily ranging from 16 to 20 years. Leasehold improvements are depreciated over the assets’ estimated useful lives, or the remaining lease term, whichever is shorter. Purchases of computer software, including external costs and certain internal costs (including payroll and payroll-related costs of employees) directly associated with developing significant computer software applications for internal use, are capitalized. Computer software costs are amortized on the straight-line method over the estimated useful life of the software, which is generally three to nine years . Depreciation expense is recorded in cost of products sold, research and development and selling and general expenses.
Estimated useful lives are periodically reviewed, and when warranted, changes are made to them. Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that their carrying amount may not be recoverable. An impairment loss would be indicated when estimated undiscounted future cash flows from the use and eventual disposition of an asset group, which are identifiable and largely independent of the cash flows of other asset groups, are less than the carrying amount of the asset group. Measurement of an impairment loss would be based on the excess of the carrying amount of the asset group over its fair value. Fair value is measured using discounted cash flows or independent appraisals, as appropriate. When property is sold or retired, the cost of the property and the related accumulated depreciation are removed from the consolidated balance sheet and any gain or loss on the transaction is included in income.
Goodwill and Other Intangible Assets
Goodwill is tested for impairment annually and whenever events and circumstances indicate that impairment may have occurred. The evaluation of goodwill involves comparing the current fair value of a reporting unit to its carrying value, including goodwill. We operate as a single operating segment with one reporting unit, and accordingly, our annual goodwill impairment test was based on an evaluation of the fair value of our Company as a whole, using a combination of income and market capitalization approaches. We completed the required annual goodwill impairment test as of July 1, 2019, and the fair value was substantially in excess of net asset carrying value.
36 | ||
Intangible assets with finite lives are amortized over their estimated useful lives and reviewed for impairment whenever events or changes in circumstances indicate that their carrying amount may not be recoverable. Estimated useful lives range from 7 to 30 years for trademarks, 7 to 17 years for patents and acquired technologies, and 2 to 16 years for other intangible assets. An impairment loss would be indicated when estimated undiscounted future cash flows from the use of the asset are less than its carrying amount. An impairment loss would be measured as the difference between the fair value (based on discounted future cash flows) and the carrying amount of the asset.
Sales revenue is recognized at the time of product shipment or delivery of our products to unaffiliated customers, depending on shipping terms. Accordingly, control of the products transfers to the customer in accordance with the transaction’s shipping terms. Sales revenue is recognized for the amount of consideration that we expect to be entitled to receive in exchange for our products. Sales are reported net of returns, rebates, incentives, each as described below, and freight allowed. Taxes imposed by governmental authorities on our revenue-producing activities with customers, such as sales taxes and value-added taxes, are excluded from net sales.
We provide medical products to distributors or end-user customers under supply agreements under which customers may place purchase orders for a variety of our products at specified pricing over a specified term, usually three years . While our sales and marketing efforts are directed to hospitals or other healthcare providers, our products are generally sold through third-party distribution channels.
Under our contracts with customers, our performance obligations are normally limited to shipment or delivery of products to a customer upon receipt of a purchase order. We bill our customers, depending on shipping terms, upon shipment or delivery of the products to the customer.
Amounts billed are typically due within 30 days, with a 1 % discount allowed for distributors if payments are made within 15 days. We estimate cash discounts based on historical experience and record the cash discounts as an allowance to trade receivables. The differences between estimated and actual cash discounts are normally not material.
We allow for returns with a specified period of time following customers’ receipt of the goods and estimate an allowance to trade receivables for returns based on historical experience. The differences between estimated and actual returns are normally not material.
Our contracts provide for forms of variable consideration including rebates, incentives and pricing tiers, each of which are described below:
Rebates - We provide for rebates on gross sales to distributors for estimated historical differences between list prices and average end-user customer prices. We maintain a liability for the estimated rebates.
Incentives - Incentives include fees paid to group purchasing organizations (“GPOs”) or distributors in conjunction with the sales of our products to end-user customers. We estimate our incentive liability based on historical experience. Differences between estimated and actual incentives are normally not material.
Pricing tiers - In certain of our contracts, pricing is dependent on volumes purchased. Pricing is lower for customers who purchase higher volumes. Customers are placed in a pricing tier based on expected purchase volume, which is developed primarily using the customer’s purchase history. Depending on the customer’s purchases, we may move the customer up or down a tier. Pricing in the new pricing tier is applied to purchase orders prospectively. There are no retrospective adjustments based on movements between pricing tiers.
See Note 5, “Supplemental Balance Sheet Information” for disclosure of our allowances for cash discounts, sales returns and doubtful accounts, and accrued rebates and incentives as of December 31, 2019 and 2018.
As of December 31, 2019, we had no single customer who individually accounted for more than 10% of our consolidated accounts receivable balance, and only one such customer as of December 31, 2018. The provision for doubtful accounts was not material in each of the years ended December 31, 2019, 2018 and 2017.
Foreign Currency Translation
The income statements of foreign operations are translated into U.S. dollars at rates of exchange in effect each month. The balance sheets of these operations are translated at period-end exchange rates, and the differences from historical exchange rates are reflected as unrealized translation adjustments in other comprehensive income.
Research and Development
Research and development expenses are expensed as incurred. Research and development expenses consist primarily of salaries and related expenses for personnel, product trial costs, outside laboratory and license fees, the costs of laboratory equipment and facilities and asset write-offs for equipment that does not reach success in product manufacturing certifications.
37 | ||
Stock-Based Compensation
Income Taxes
We account for income taxes under the asset and liability method of accounting, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. Under this method, changes in tax rates and laws are recognized in income in the period such changes are enacted. The provision for federal, state, and foreign income taxes is calculated on income before income taxes based on current tax law and includes the cumulative effect of any changes in tax rates from those used previously in determining deferred tax assets and liabilities. Such provision differs from the amounts currently payable because certain items of income and expense are recognized in different reporting periods for financial reporting purposes than for income tax purposes. Recording the provision for income taxes requires management to make significant judgments and estimates for matters whose ultimate resolution may not become known until the final resolution of an examination by the Internal Revenue Service (IRS) or state and foreign agencies. If it is more likely than not that some portion, or all, of a deferred tax asset will not be realized, a valuation allowance is recognized.
Recording liabilities for uncertain tax positions involves judgment in evaluating our tax positions and developing the best estimate of the taxes ultimately expected to be paid. We include any related tax penalties and interest in income tax expense.
As of December 31, 2019, we have accumulated undistributed earnings generated by our foreign subsidiaries of approximately $41.0 million. Certain earnings were previously subject to tax due to the one-time transition tax of the Act. Any additional impacts due with respect to the previously-taxed earnings, if repatriated, would generally be limited to foreign withholding tax, U.S. state income tax and the tax effect of certain foreign exchange adjustments. We intend, however, to indefinitely reinvest these earnings and expect future U.S. cash generation to be sufficient to meet U.S. cash needs. At this time, the determination of deferred tax liabilities on the amount of financial reporting over tax basis is not practicable.
We recognize the funded status of our defined benefit as an asset or a liability on our balance sheet. Actuarial gains or losses are a component of our other comprehensive income, which is then included in our accumulated other comprehensive income. Pension expenses are recognized over the period in which the employee renders service and becomes eligible to receive benefits. We make assumptions (including the discount rate and expected rate of return on plan assets) in computing the pension expense and obligations.
Recently Adopted Pronouncements
Effective January 1, 2019, we adopted Accounting Standards Update (“ASU”) No. 2016-02, Leases (“Topic 842”), using the transition method provided in ASU No. 2018-11, Leases (Topic 842) - Targeted Improvements, which allows for initial application on the date of adoption with recognition of a cumulative-effect adjustment, if applicable, to the opening balance of retained earnings. As of December 31, 2018, all our existing leases were operating leases, and accordingly, no adjustment to beginning retained earnings was required. In addition, we elected to use all available expedients allowed under ASU 2018-11. Other prior period amounts are not adjusted and continue to be reported under Topic 840, the previous lease guidance.
Topic 842 replaces the former guidance in Topic 840 and requires the recognition of right-of-use (“ROU”) assets and liabilities for leases with terms of more than twelve months. The recognition, measurement and presentation of expenses and cash flows arising from leases depend primarily on its classification as a finance or an operating lease, with the classification criteria for distinguishing between the two types being similar to the classification for distinguishing between capital and operating leases under Topic 840. In addition to recognition of ROU assets and liabilities, disclosures regarding lease obligations are required to help financial statement users better understand the amount, timing and uncertainty of cash flows arising from leases.
As a result of Topic 842 adoption, we have operating lease liabilities of $77.3 million and corresponding ROU assets of $64.0 million as of December 31, 2019. For other disclosures regarding our lease obligations, see “Leases” in Note 6 herein.
38 | ||
Effective January 1, 2019, we adopted ASU No. 2018-02, Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income. This ASU is intended to help companies reclassify certain stranded income tax effects in accumulated other comprehensive income (“AOCI”) resulting from the Tax Cuts and Jobs Act of 2017 (the “Act”), which was enacted in December 2017. ASU 2018-02 provides for the elimination of stranded tax effects of the Act by allowing reclassification of stranded tax effects from AOCI to retained earnings. We elected not to reclassify stranded tax effects from AOCI to retained earnings, and accordingly, adoption of this ASU did not have a material effect on our financial position, results of operations and cash flows.
Recently Issued Pronouncements
In December 2019, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. This ASU removes certain exceptions for recognizing deferred taxes for investments, performing intraperiod allocation and calculating income taxes in interim periods. The ASU also adds guidance to reduce complexity in certain areas, including recognizing deferred taxes for tax goodwill and allocating taxes to members of a consolidated group. This ASU is effective for annual periods and interim periods within those annual periods beginning after December 15, 2020, with early adoption permitted. We do not expect adoption of this ASU to have a material effect on our financial position, results of operations or cash flows.
In May 2019, the FASB issued ASU No. 2019-05, Financial Instruments – Credit Losses (Topic 326): Target Transition Relief. This ASU provides transition relief for entities adopting the new credit losses standard ASU No. 2016-13, Financial Instruments – Credit Losses (Topic 326). Upon adoption of ASU No. 2016-13, an entity is allowed to irrevocably elect the fair value option on certain financial assets that were previously measured at amortized cost basis. ASU No. 2019-05 is effective concurrent with the adoption of ASU No. 2016-13. For entities that have adopted ASU No. 2016-13, the amendment in ASU No. 2019-05 are effective for annual periods and interim periods within those annual periods beginning after December 15, 2019, with early adoption permitted. For entities that elect the fair value option, the difference between the carrying amount and the fair value of the financial asset would be recognized through a cumulative-effect adjustment to opening retained earnings as of the date an entity adopted ASU No. 2016-13. Changes in fair value of that financial asset would subsequently be reported in current earnings. Certain disclosures are required. We do not expect adoption of this ASU to have a material effect on our financial position, results of operations or cash flows.
In August 2018, the FASB issued ASU No. 2018-15, Intangibles – Goodwill and Other – Internal Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That is a Service Contract. This ASU is intended to reduce complexity by aligning the requirements for capitalizing implementation costs incurred in cloud-based arrangements with the requirements for capitalization of costs incurred to develop internal-use software. Any implementation costs in cloud-based arrangements would then be amortized over the term of the service contract. This ASU is effective for annual periods, and interim periods within those annual periods beginning after December 15, 2019, with early adoption permitted. We do not expect adoption of this ASU to have a material effect on our financial position, results of operations or cash flows.
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework – Changes to the Disclosure Requirements for Fair Value Measurement. This ASU removes certain disclosure requirements regarding the amounts and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy and the policy for timing of transfers between the levels. The ASU also adds disclosure requirements regarding unrealized gains and losses included in Other Comprehensive Income for recurring Level 3 fair value measurements and regarding the range and weighted average of unobservable inputs used in Level 3 fair value measurements. This ASU is effective for annual periods and interim periods within those annual periods beginning after December 15, 2019. The removal of certain disclosures is to be applied retrospectively for all periods presented, but the additional required disclosures are to be prospectively applied, and early application is permitted. We do not expect any transfers between Level 1 and Level 2 of the fair value hierarchy, and as of December 31, 2019, we have no assets or liabilities with fair value measurements in Level 3 of the fair value hierarchy. Accordingly, we do not expect adoption of this ASU to have a material effect on our financial position, results of operations or cash flows.
Note 2. Business Acquisitions
Endoclear LLC
On September 19, 2019, we acquired substantially all the assets of Endoclear, LLC (“Endoclear”). The initial purchase price was $3.5 million plus future contingent payments with an estimated fair value of $5.5 million, subject to certain adjustments as defined in the purchase agreement. Endoclear develops and markets airway management devices that are complementary to our existing respiratory health portfolio.
39 | ||
Summit Medical Products, Inc.
On August 7, 2019, we acquired substantially all the assets of Summit Medical Products, Inc. (“Summit”) for $15.6 million plus future contingent payments with an estimated fair value of $1.7 million, subject to certain adjustments as defined in the purchase agreement. Summit develops and markets the ambIT® family of ambulatory electronic infusion pumps, with annual net sales of approximately $7.0 million. Net sales of ambIT® products for the period from August 7, 2019 through December 31, 2019 were approximately $3.3 million and are included in the accompanying consolidated income statement.
NeoMed, Inc.
On April 16, 2019, we acquired a minority interest in NeoMed, Inc. (“NeoMed”) for $7.0 million. NeoMed is a market-leading medical device company that is focused on specialized feeding and medication dosing for low birth weight, neonatal and pediatric patients. On July 8, 2019, we acquired all of the outstanding shares of NeoMed for a purchase price of $33.5 million, which includes the base purchase price of $28.0 million plus certain agreed-upon items at the closing date, net of cash acquired, and subsequently adjusted for certain items as defined in the purchase agreement. NeoMed’s net sales were $37.0 million in 2018. NeoMed’s net sales for the period from July 8, 2019 through December 31, 2019 were $19.7 million and are included in the accompanying consolidated income statement.
Cool Systems, Inc.
On July 1, 2018, we acquired Cool Systems, Inc. for $65.7 million, net of cash acquired, which was based on a purchase price of $65.0 million plus certain adjustments as provided in the purchase agreement. Cool Systems is marketed as Game Ready® and is hereinafter referred to as “Game Ready.” In the year ended December 31, 2019, the purchase price allocation for Game Ready was finalized, resulting in a $1.9 million reduction of goodwill.
Purchase Price Allocation
We accounted for each of the acquisitions described above under the acquisition method of accounting for business combinations. Accordingly, the purchase price paid is allocated to the underlying net assets in proportion to their respective fair values. Any excess of the purchase price over the estimated fair values is recorded as goodwill. The allocation of the purchase price for each of the acquisitions described above is as follows (in millions):
EndoClear(a) | Summit(a) | NeoMed(a)(b) | Game Ready(c) | ||||||||||||
Current assets acquired net of liabilities assumed | $ | $ | $ | $ | |||||||||||
Property, plant and equipment | |||||||||||||||
Identifiable intangible assets | |||||||||||||||
Other noncurrent assets (liabilities), net | ( | ) | |||||||||||||
Deferred tax liabilities | ( | ) | ( | ) | |||||||||||
Goodwill | |||||||||||||||
Total | $ | $ | $ | $ | |||||||||||
_______________________________________________
(a) | The EndoClear, Summit and NeoMed transactions closed during the third quarter of 2019. Accordingly, the purchase price allocations in the table above are preliminary. |
(b) | The current assets acquired net of liabilities assumed in the NeoMed acquisition included $ |
(c) | Game Ready was acquired on July 1, 2018 and the purchase price allocation was finalized on June 30, 2019. |
The identifiable intangible assets include the following (in millions):
EndoClear | Summit | NeoMed | Game Ready | Weighted Average Useful Lives (Yrs) | |||||||||||||
Trademarks | $ | $ | $ | $ | |||||||||||||
Patents and acquired technologies | |||||||||||||||||
Other | |||||||||||||||||
Total | $ | $ | $ | $ | |||||||||||||
40 | ||
The following unaudited pro forma financial information is presented in the table below for the years ended December 31, 2019 and 2018 as if the acquisitions had occurred on January 1 in the year preceding the respective acquisitions (in millions, except per share amounts):
Year Ended December 31, | |||||||||||
2019 (Unaudited) | 2018 (Unaudited) | 2017 (Unaudited) | |||||||||
Net sales | $ | $ | $ | ||||||||
Net (loss) income | ( | ) | |||||||||
Earnings per share: | |||||||||||
Basic | $ | ( | ) | $ | $ | ||||||
Diluted | ( | ) | |||||||||
The pro forma financial information has been adjusted to include the effects of the acquisitions, including acquisition-related costs, amortization of acquired intangibles and related tax effects. The pro-forma financial information is not necessarily indicative of the results of operations that would have been achieved.
We have initiated activities to integrate the assets and businesses acquired into our operations, which are described in Note 3, “Restructuring Activities.” We expect the integration of our acquisitions will be substantially complete by the end of 2020.
Note 3. Restructuring
Post-Divestiture Restructuring Plan
In conjunction with the Divestiture, we began a multi-phase restructuring plan (the “Plan”) intended to align our organizational structure, information technology platform, supply chain and distribution channels to be more appropriate for the size and scale of our remaining Medical Devices business. Each phase of the restructuring plan is described below:
Organizational Alignment: The first phase of the Plan aligned our organizational and management structure for our remaining Medical Devices business following the Divestiture. In the year ended December 31, 2019, we incurred $2.7 million of costs, primarily for employee retention, severance and benefits, that are included in “Cost of products sold” and “Selling and general expenses” in the accompanying consolidated income statement. In the year ended December 31, 2018, we incurred $9.3 million of costs.
As of December 31, 2019, this phase of the Plan was substantially complete. Plan-to-date expenses were $17.4 million, of which $10.5 million was for employee retention, severance and benefits and the remainder for third-party services and other costs.
Information Technology Systems: In the second phase of the Plan, we migrated to an IT platform that is more appropriate for our business and size (the “ITS Plan”). In the year ended December 31, 2019, we incurred $15.1 million of costs which are included in “Selling and general expenses” in the accompanying consolidated income statements compared to $6.4 million in the year ended December 31, 2018.
As of December 31, 2019, the ITS Plan was complete. Plan-to-date, we incurred $21.5 million of costs that were expensed as incurred and $54.1 million of costs that were capitalized, including $5.0 million of capitalized internal labor costs and $2.2 million of capitalized interest.
Cost Transformation: In June 2019, the third and final phase of the Plan was approved. This third phase relates to optimizing the Company’s procurement, manufacturing, and supply chain operations (the “Cost Transformation”). The Company expects to incur between $11.0 million and $13.0 million to execute the Cost Transformation, primarily consulting and other expenses that will be expensed as incurred. The Company also expects to spend between $8.0 million to $12.0 million of incremental capital through 2021 in support of the Cost Transformation. The Company expects to complete the Cost Transformation by the end of 2021. In the year ended December 31, 2019, we have incurred $2.3 million of costs related to Cost Transformation.
Integration of Business Acquisitions: During the third quarter of 2019, we initiated activities to integrate the asset and business acquisitions described in Note 2, “Business Acquisitions” into our operations, and where appropriate, re-align our organization accordingly. We expect to incur up to $17.0 million of costs, primarily for employee retention, severance and benefits and lease termination costs. In the year ended December 31, 2019, we incurred $9.1 million for employee retention, severance and benefits. We expect the integration of our acquisitions will be substantially complete by the end of 2020.
41 | ||
Restructuring Liability
We have a liability for employee retention, severance and benefits associated with our restructuring activities, which is summarized below (in millions):
As of December 31, | |||||||
2019 | 2018 | ||||||
Balance, beginning of year | |||||||
Charges and adjustments, net | |||||||
Payments | ( | ) | ( | ) | |||
Balance, end of year | $ | $ | |||||
Note 4. Goodwill
We test goodwill for impairment annually (as of July 1) or more frequently whenever events or circumstances more likely than not indicate that the fair value of the reporting unit may be below its carrying amount. We operate as a single operating segment with one reporting unit, and accordingly, our annual goodwill impairment test was based on an evaluation of the fair value of our Company as a whole.
We completed our annual impairment test as of July 1, 2019, and based on a combination of income and market capitalization approaches, we determined that our fair value substantially exceeds the net carrying value of our reporting unit.
The changes in the carrying amount of goodwill are as follows (in millions):
Balance at December 31, 2017 | $ | ||
Goodwill acquired(a) | |||
Currency translation adjustment | ( | ) | |
Balance at December 31, 2018 | |||
Goodwill acquired(b) | |||
Purchase accounting adjustment(a) | ( | ) | |
Currency translation adjustment | |||
Balance at December 31, 2019 | $ | ||
_____________________________________________
(a) | We acquired $ |
(b) |
42 | ||
Note 5. Supplemental Balance Sheet Information
Accounts Receivable
Accounts receivable consist of the following (in millions):
As of December 31, | |||||||
2019 | 2018 | ||||||
Accounts Receivable | $ | $ | |||||
Allowances and doubtful accounts | |||||||
Doubtful accounts | ( | ) | ( | ) | |||
Sales discounts | ( | ) | ( | ) | |||
Sales returns | ( | ) | |||||
Accounts receivable, net | $ | $ | |||||
The provision for doubtful accounts and subsequent recoveries of bad debts were not material in each of the years ended December 31, 2019, 2018 and 2017.
Inventories
Inventories at the lower of cost (determined on the LIFO/FIFO or weighted-average cost methods) or market consists of the following (in millions):
As of December 31, | |||||||||||||||||||||||
2019 | 2018 | ||||||||||||||||||||||
LIFO | Non- LIFO | Total | LIFO | Non- LIFO | Total | ||||||||||||||||||
Raw Materials | $ | $ | $ | $ | $ | $ | |||||||||||||||||
Work in process | |||||||||||||||||||||||
Finished goods | |||||||||||||||||||||||
Supplies and other | |||||||||||||||||||||||
Excess of FIFO or weighted-average cost over LIFO cost | ( | ) | — | ( | ) | ( | ) | — | ( | ) | |||||||||||||
Total | $ | $ | $ | $ | $ | $ | |||||||||||||||||
The provision for obsolescence has not been material in each of the years ended December 31, 2019, 2018 and 2017.
Property, Plant and Equipment
Property, plant and equipment consists of the following (in millions):
As of December 31, | |||||||
2019 | 2018 | ||||||
Land | $ | $ | |||||
Buildings and leasehold improvements | |||||||
Machinery and equipment | |||||||
Construction in progress | |||||||
Less accumulated depreciation | ( | ) | ( | ) | |||
Total | $ | $ | |||||
Property, plant and equipment includes $1.8 million and $1.5 million of interest that was capitalized in the years ended December 31, 2019 and 2018, respectively. There were $11.2 million and $16.9 million of capital expenditures in accounts payable as of December 31, 2019 and 2018, respectively.
43 | ||
Depreciation expense was $16.9 million, $13.5 million and $18.8 million, respectively, in the years ended December 31, 2019, 2018 and 2017.
Intangible Assets
Intangible assets subject to amortization consist of the following (in millions):
As of December 31, | ||||||||||||||||||||||||
2019 | 2018 | |||||||||||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | |||||||||||||||||||
Trademarks | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||||||
Patents and acquired technologies | ( | ) | ( | ) | ||||||||||||||||||||
Other | ( | ) | ( | ) | ||||||||||||||||||||
Total | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||||||
Amortization expense for intangible assets was $20.0 million, $20.0 million and $20.7 million for the years ended December 31, 2019, 2018 and 2017, respectively.
We estimate amortization expense for the next five years and beyond will be as follows (in millions):
For the years ending December 31, | ||||
2020 | $ | |||
2021 | ||||
2022 | ||||
2023 | ||||
2024 | ||||
Thereafter | ||||
Total | $ | |||
Accrued Expenses
Accrued expenses consist of the following (in millions):
As of December 31, | |||||||
2019 | 2018 | ||||||
Accrued rebates | $ | $ | |||||
Accrued salaries and wages | |||||||
Accrued taxes and other | |||||||
Other | |||||||
Total | $ | $ | |||||
Other Long-Term Liabilities
Other long-term liabilities consist of the following (in millions):
As of December 31, | |||||||
2019 | 2018 | ||||||
Taxes payable | $ | $ | |||||
Accrued compensation benefits | |||||||
Other | |||||||
Total | $ | $ | |||||
44 | ||
Note 6. Leases
Our lease obligations relate primarily to our principal executive offices along with various manufacturing, warehouse and distribution facilities located throughout the world. For leases with terms greater than twelve months, we record an ROU asset and corresponding lease obligation. As of December 31, 2019, all our leasing arrangements were operating leases. Many of our leases include escalating rent payments, renewal options and termination options, which are considered in our determination of straight-line rent expense when appropriate. Many of our leases also include additional amounts for common area maintenance and taxes. We have elected not to separate lease and non-lease components in the determination of straight-line rent expense. For a majority of our leases, an implicit lease rate is not available. Accordingly, we use a rate that approximates our incremental secured borrowing rate.
The table below summarizes information related to ROU assets and lease liabilities that are included in the accompanying consolidated balance sheet (dollars in millions):
As of December 31, 2019 | |||
Assets | |||
Operating lease right-of-use assets | $ | ||
Liabilities | |||
Current portion of operating lease liabilities | |||
Operating lease liabilities | |||
Total Operating Lease Liabilities | $ | ||
Weighted average remaining lease term | |||
Weighted average discount rate | % | ||
The table below summarizes costs and cash flows arising from our lease arrangements for the year ended December 31, 2019 (in millions):
Year Ended December 31, 2019 | |||
Operating lease cost | $ | ||
Short-term lease cost | |||
Variable lease cost | |||
Total lease cost | $ | ||
Cash paid for amounts included in the measurement of lease liabilities | $ | ||
Right-of-use assets obtained in exchange for new operating lease liabilities | $ | ||
The future minimum obligations under operating leases having non-cancelable terms in excess of one year for the next five years and beyond will be (in millions):
For the years ending December 31, | Amount | |||
2020 | $ | |||
2021 | ||||
2022 | ||||
2023 | ||||
2024 | ||||
Thereafter | ||||
Future minimum obligations | $ | |||
45 | ||
Note 7. Discontinued Operations
The results of operations from our former S&IP business are reported in the accompanying consolidated income statements as “Income from Discontinued Operations, net of tax” in the years ended December 31, 2018 and 2017. The remaining business is managed with one operating segment, the Medical Devices business.
The following table summarizes the financial results of our discontinued operations for the years ended December 31, 2018 and 2017 (in millions):
Year Ended December 31, | |||||||
2018 | 2017 | ||||||
Net Sales | $ | $ | |||||
Cost of products sold | |||||||
Research and development | |||||||
Selling, general and other expenses | |||||||
Gain on Divestiture | ( | ) | |||||
Other expense (income), net | ( | ) | |||||
Income from discontinued operations before income taxes | |||||||
Tax provision from discontinued operations | ( | ) | ( | ) | |||
Income from discontinued operations, net of tax | $ | $ | |||||
In accordance with GAAP, only expenses specifically identifiable and related to a business to be disposed may be allocated to discontinued operations. Accordingly, certain expenses that were historically presented as a component of the S&IP were kept in continuing operations. These expenses, on a pre-tax basis, were $37.0 million in the year ended December 31, 2018 and $115.8 million in 2017.
The following table provides operating and investing cash flow information for our discontinued operations (in millions):
Year Ended December 31, | ||||||
2018 | 2017 | |||||
Operating Activities: | ||||||
Depreciation and amortization | $ | |||||
Stock-based compensation expense | ( | ) | ||||
Investing Activities: | ||||||
Capital expenditures | ||||||
Operating and investing cash flow information for the year ended December 31, 2018 represents activity from January 1, 2018 until the Divestiture closed on April 30, 2018.
Note 8. Fair Value Information
The following fair value information is based on a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The three levels in the hierarchy used to measure fair value are:
Level 1: Unadjusted quoted prices in active markets accessible at the reporting date for identical assets and liabilities.
Level 2: Quoted prices for similar assets or liabilities in active markets. Quoted prices for identical or similar assets and liabilities in markets that are not considered active or financial instruments for which all significant inputs are observable, either directly or indirectly.
Level 3: Prices or valuations that require inputs that are significant to the valuation and are unobservable.
A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
46 | ||
The following table includes the fair value of our financial instruments for which disclosure of fair value is required (in millions):
Fair Value Hierarchy Level | December 31, 2019 | December 31, 2018 | |||||||||||||||
Carrying Amount | Estimated Fair Value | Carrying Amount | Estimated Fair Value | ||||||||||||||
Assets | |||||||||||||||||
Cash and cash equivalents | 1 | $ | $ | $ | $ | ||||||||||||
Liabilities | |||||||||||||||||
Senior unsecured notes | 1 | ||||||||||||||||
Cash equivalents are recorded at cost, which approximates fair value due to their short-term nature.
The fair value of our senior unsecured notes is determined using observable market prices based on trading activity on a primary exchange. For the years ended December 31, 2019 and 2018, there were no transfers among Level 1, 2 or 3 fair value determinations. Transfers between levels occur when there are changes in the observability of inputs. Changes between levels are assumed to occur at the beginning of the year.
Note 9. Debt
As of December 31, 2019 and 2018, our debt balances were as follows (in millions):
Weighted- Average Interest Rate | Maturities | As of December 31, | |||||||||
2019 | 2018 | ||||||||||
Senior Unsecured Notes | 2022 | ||||||||||
Unamortized Debt Discounts and Issuance Costs | ( | ) | ( | ) | |||||||
Total Debt, net | $ | $ | |||||||||
Senior Unsecured Notes
The Senior Unsecured Notes (“Notes”) will mature on October 15, 2022 and interest accrues at a rate of 6.25 % per annum payable semi-annually in arrears on April 15 and October 15 of each year. The Notes are guaranteed, jointly and severally, by each of our domestic subsidiaries that guarantees the Senior Credit Facilities. Unamortized debt discount and issuance costs are being amortized to interest expense over the life of the credit agreement using the interest method, resulting in an effective interest rate of 6.51 % as of December 31, 2019.
Following a divestiture of significant assets, such as the Divestiture, the credit agreement allows re-investment of the net proceeds into the business through acquisition of another business or through capital expenditures for a period of one year following the divestiture. We were required to offer to redeem a portion of the Notes at par value to the extent re-investments were not made by May 1, 2019. Accordingly, $130.5 million of the Notes were offered for redemption in the second quarter of 2019, of which $0.2 million were redeemed.
Revolving Credit Facility
We have a senior secured revolving credit facility (“Revolving Credit Facility”) that matures on October 30, 2023 which allows for borrowings up to $250.0 million, with a letter of credit sub-facility in an amount of $75.0 million and a swingline sub-facility in an amount of $25.0 million.
Borrowings under the Revolving Credit Facility will bear interest, at our option, at either (i) a reserve-adjusted LIBOR rate, plus a margin ranging between 1.50 % to 2.25 % per annum, depending on our consolidated total leverage ratio, or (ii) the base rate plus a margin ranging between 0.50 % to 1.25 % per annum, depending on our consolidated total leverage ratio. The unused portion of our Revolving Credit Facility will be subject to a commitment fee equal to (i) 0.25 % per annum, when our consolidated total leverage ratio is less than 2.25 to 1.00 and (ii) 0.375 % per annum, otherwise.
To the extent we remain in compliance with certain financial covenants in our credit agreement, we have the ability to access our Revolving Credit Facility. As of December 31, 2019, we had no borrowings outstanding and letters of credit of $0.7 million issued under the Revolving Credit Facility.
Debt Covenants
The Revolving Credit Facility and the Notes are subject to similar covenants that, among other things, limit our ability and the ability of certain of our subsidiaries to:
47 | ||
• | incur additional indebtedness, guarantee indebtedness or issue disqualified stock or, in the case of our restricted subsidiaries, preferred stock; |
• | pay dividends on, repurchase or make distributions in respect of our capital stock; |
• | make certain investments or acquisitions; |
• | sell, transfer or otherwise convey certain assets; |
• | create liens; |
• | enter into agreements restricting certain subsidiaries’ ability to pay dividends or make other intercompany transfers; |
• | consolidate, merge, sell or otherwise dispose of all or substantially all of our and our subsidiaries’ assets; |
• | enter into transactions with affiliates; and |
• | prepay certain kinds of indebtedness. |
Pursuant to the restrictive covenants that limit our ability to pay dividends, we have the ability to pay dividends, repurchase stock and make investments up to an “Available Amount,” as defined in the credit agreement governing the Senior Credit Facilities, provided that we are in compliance with all required covenants, there are no events of default and upon meeting certain financial ratios.
As of December 31, 2019, we were in compliance with all of our debt covenants. As of December 31, 2019, our repayment requirements in the next five years includes the $249.8 million Notes due on October 15, 2022.
Note 10. Income Taxes
Our income taxes are calculated using the asset and liability method of accounting, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities.
The provision for income taxes includes federal, state and foreign taxes currently payable and those deferred because of net operating losses and temporary differences between the consolidated financial statements and tax bases of assets and liabilities.
The components of (loss) income before income taxes, and the provision (benefit) for income taxes are as follows (in millions):
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Loss before income taxes | |||||||||||
United States | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Foreign | ( | ) | |||||||||
Total | ( | ) | ( | ) | ( | ) | |||||
Income tax provision (benefit): | |||||||||||
Current: | |||||||||||
United States | ( | ) | ( | ) | ( | ) | |||||
State | ( | ) | ( | ) | ( | ) | |||||
Foreign | |||||||||||
Total | ( | ) | ( | ) | ( | ) | |||||
Deferred: | |||||||||||
United States | ( | ) | ( | ) | |||||||
State | ( | ) | ( | ) | |||||||
Foreign | ( | ) | ( | ) | ( | ) | |||||
Total | ( | ) | ( | ) | |||||||
Total income tax benefit | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
On December 22, 2017, new federal tax reform, the Tax Cuts and Jobs Act (the “Act”), was enacted in the United States, resulting in significant changes from previous tax law. The new legislation reduced the federal corporate income tax rate to 21% from 35% effective January 1, 2018. In the fourth quarter of 2017, we recorded a provisional estimate of a net $10.0 million benefit related to the Act. The provisional estimate included a $16.0 million benefit related to the re-measurement of certain
48 | ||
deferred tax assets and liabilities based on the rates at which they are expected to reverse offset by a $7.0 million one-time transition tax expense on the mandatory deemed repatriation of cumulative foreign earnings of $101 million. We also recorded a $1.0 million benefit related to the treatment of current year cash dividends in relation to the repatriation tax.
On December 22, 2017, Staff Accounting Bulletin No. 118 (“SAB 118”) was issued to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Act. In accordance with SAB 118, we determined the provisional estimates recorded in December 2017 were reasonable estimates through September 30, 2018.
Furthermore, during the fourth quarter of 2018 we recorded discrete tax benefits of $3.9 million related to new guidance issued during 2018 and certain tax planning actions taken in anticipation of the Act. As of December 31, 2018, our accounting for the Act was complete.
The Act subjects a U.S. shareholder to tax on Global Intangible Low Tax Income (“GILTI”) earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740, No. 5, Accounting for GILTI, states that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense only. The Company has elected to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense.
As of December 31, 2019, we have accumulated undistributed earnings generated by our foreign subsidiaries of approximately $41.0 million. Certain earnings were previously subject to tax due to the one-time transition tax of the Act. Any additional impacts due with respect to the previously-taxed earnings, if repatriated, would generally be limited to foreign withholding tax, U.S. state income tax and the tax effect of certain foreign exchange adjustments. We intend, however, to indefinitely reinvest these earnings and expect future U.S. cash generation to be sufficient to meet U.S. cash needs. At this time, the determination of deferred tax liabilities on the amount of financial reporting over tax basis is not practicable.
Major differences between the federal statutory rate and the effective tax rate are as follows:
Year Ended December 31, | ||||||||
2019 | 2018 | 2017 | ||||||
Federal statutory rate | % | % | % | |||||
Rate of state income taxes, net of federal tax benefit | ( | ) | ||||||
Statutory rate other than U.S. statutory rate | ( | ) | ( | ) | ||||
Foreign derived intangible income | ||||||||
Foreign tax credit carryback | ||||||||
Valuation allowance | ( | ) | ( | ) | ( | ) | ||
Uncertain tax positions | ||||||||
Transaction related expenses | ( | ) | ||||||
GILTI inclusion | ( | ) | ||||||
Nondeductible officer’s compensation | ( | ) | ( | ) | ||||
U.S. federal research and development credit | ||||||||
Share based compensation windfall tax deduction | ( | ) | ||||||
Impacts of U.S. federal tax reform | ||||||||
Other, net | ( | ) | ( | ) | ( | ) | ||
Effective tax rate | % | % | % | |||||
49 | ||
The following is a summary of the significant components of the Company’s deferred tax assets and liabilities (in millions):
As of December 31, | ||||||||
2019 | 2018 | |||||||
Deferred tax assets | ||||||||
Accrued liabilities | $ | $ | ||||||
Interest limitation | ||||||||
Stock-based compensation | ||||||||
Net Operating Losses | ||||||||
Operating Lease Right of Use Asset | — | |||||||
Other | ||||||||
Valuation allowance | ( | ) | ( | ) | ||||
Total deferred tax assets | ||||||||
Deferred tax liabilities | ||||||||
Intangibles, net | ||||||||
Operating Lease Obligations | — | |||||||
Inventories | ||||||||
Property, plant and equipment, net | ||||||||
Other | ||||||||
Total deferred tax liabilities | ||||||||
Net deferred tax assets (liabilities) | $ | $ | ||||||
Valuation allowances increased $0.1 million during the year ended December 31, 2019. Valuation allowances at the end of 2019 and 2018 primarily relate to tax credits and income tax loss carryforwards.
Realization of income tax loss carryforwards is dependent on generating sufficient taxable income prior to expiration of these carryforwards. Although realization is not assured, we believe it is more likely than not that all of the deferred tax assets, net of applicable valuation allowances, will be realized. The amount of the deferred tax assets considered realizable could be reduced or increased due to changes in the tax environment or if estimates of future taxable income change during the carryforward period.
At December 31, 2019, we have credit carryforwards for federal income tax purposes of $2.0 million, all of which will expire between 2023 and 2028. We also have net operating loss carryforwards for federal income tax purposes of $93.0 million, of which $28.5 million will expire between 2027 and 2037. The remaining net operating losses are available for carryforward indefinitely.
At December 31, 2019, we have credit carryforwards for state income tax purposes of $3.3 million, of which $2.0 million will expire in 2025. The rest will expire between 2026 and 2028. We also have net operating loss carryforwards for state income tax purposes of $78.0 million, some of which will expire between 2020 and 2034 and others that will remain available for carryforward indefinitely. We also have certain foreign subsidiaries with net operating loss carryforwards for income tax purposes of $19.7 million, of which $2.3 million will expire in 2020 and $3.4 million will expire in 2029. The remaining net operating losses are available for carryforward indefinitely.
50 | ||
A reconciliation of the beginning and ending amount of unrecognized tax benefit is as follows (in millions):
As of December 31, | |||||||
2019 | 2018 | ||||||
Beginning of year | $ | $ | |||||
Gross increases for tax positions of prior years | |||||||
Gross decreases for tax positions of prior years | |||||||
Decreases for settlements with taxing authorities | ( | ) | |||||
Decreases for lapse of the applicable statute of limitations | ( | ) | |||||
End of year | $ | $ | |||||
The amount, if recognized, that would affect our effective tax rate as of December 31, 2019 and 2018 is $0.4 million for both years.
We classify interest and penalties on uncertain tax benefits as income tax expense. As of December 31, 2019 and 2018, before any tax benefits, we had $0.3 million and $0.2 million, respectively of accrued interest and penalties on unrecognized tax benefits.
During the next twelve months, we do not expect the resolution of any tax audits which could potentially reduce unrecognized tax benefits by a material amount. In addition, an expiration of the statute of limitations for a tax year in which we have recorded uncertain tax benefits will occur in the next twelve months.
Note 11. Employee Benefit Plans
Defined Contribution Plans
Eligible employees participate in our defined contribution plans. Our 401(k) plan and supplemental plan provide for a matching contribution of a U.S. employee’s contributions and accruals, subject to predetermined limits. Avanos also has defined contribution pension plans for certain employees outside the U.S. in which eligible employees may participate. We recognized $8.4 million, $7.6 million and $7.4 million, respectively, of expense for our matching contributions to the 401(k) plan in the years ended December 31, 2019, 2018 and 2017, respectively. Our matching contributions to the 401(k) plan are recognized in cost of products sold, research and development and selling and general expenses in our consolidated income statements.
Defined Benefit Plans
Certain plans in our international operations are our direct obligation, and therefore, the related funded status has been recorded within our consolidated balance sheet. These plans are primarily unfunded and the aggregated projected benefit obligation was $4.3 million and $2.8 million as of December 31, 2019 and 2018, respectively. Net periodic pension cost for the years ended December 31, 2019, 2018 and 2017 was $0.5 million, $0.6 million and $0.6 million, respectively. Over the next ten years, we expect gross benefit payments to be $1.1 million in total for the years 2020 through 2024, and $1.7 million in total for the years 2025 through 2029.
51 | ||
Note 12. Accumulated Other Comprehensive Income
The changes in the components of Accumulated Other Comprehensive Income (“AOCI”), net of tax, are as follows (in millions):
Unrealized Translation | Cash Flow Hedges | Defined Benefit Pension Plans | Accumulated Other Comprehensive Income | ||||||||||||
Balance, December 31, 2016 | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
Other comprehensive income | |||||||||||||||
Balance, December 31, 2017 | ( | ) | ( | ) | ( | ) | |||||||||
Other comprehensive (loss) income | ( | ) | ( | ) | ( | ) | |||||||||
Balance, December 31, 2018 | ( | ) | ( | ) | |||||||||||
Other comprehensive income (loss) | ( | ) | |||||||||||||
Balance, December 31, 2019 | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | |||||
The net changes in the components of AOCI, including the tax effect, are as follows (in millions):
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Unrealized translation | $ | $ | ( | ) | $ | ||||||
Defined benefit pension plans | ( | ) | |||||||||
Tax effect | ( | ) | ( | ) | |||||||
Defined benefit pension plans, net of tax | ( | ) | |||||||||
Cash flow hedges | ( | ) | |||||||||
Tax effect | ( | ) | |||||||||
Cash flow hedges, net of tax | ( | ) | |||||||||
Change in AOCI | $ | $ | ( | ) | $ | ||||||
Note 13. Stock-Based Compensation
The Avanos Medical, Inc. Equity Participation Plan and the Avanos Medical, Inc. Outside Directors’ Compensation Plan (together, the “Equity Plans”) provide for awards of stock options, stock appreciation rights, restricted stock (and in certain limited cases, unrestricted stock), restricted stock units, performance units and cash awards to eligible employees (including officers who are employees), directors, advisors and consultants of Avanos or its subsidiaries. A maximum of 4.9 million shares of Avanos common stock may be issued under the Equity Plans, and there are 1.4 million shares remaining available for issuance as of December 31, 2019.
Aggregate stock-based compensation expense under the Equity Plans was $10.4 million, $10.5 million and $12.6 million for the years ended December 31, 2019, 2018 and 2017, respectively, which includes amounts allocated to discontinued operations in 2018 and 2017. See Note 7 for stock-based compensation included in discontinued operations in 2018 and 2017. Stock-based compensation expense described by award type below refers to expense in continuing operations only. Stock-based compensation expense is included in cost of sales, research and development expenses and selling and general expenses.
Stock Options
Stock options are granted at an exercise price equal to the fair market value of our common stock on the date of grant. Stock options are generally subject to graded vesting whereby options vest 30 % at the end of each of the first two 12-month periods following the grant and 40 % at the end of the third 12-month period and have a term of 10 years.
The fair value of stock option awards was determined using a Black-Scholes option-pricing model utilizing a range of assumptions related to volatility, risk-free interest rate, expected term and dividend yield. Expected volatility was based on historical weekly closing stock price volatility for a peer group of companies. The risk-free interest rate was based on the U.S.
52 | ||
Treasury yield curve in effect at the time of grant. The expected term was based on historical observed settlement behavior. The dividend yield was based on the expectation that no dividends are expected to be paid on our common stock.
The weighted-average fair value of options granted in the years ended December 31, 2019, 2018 and 2017 was $11.60 , $13.69 , and $9.07 , respectively, based on the following assumptions:
Year Ended December 31, | |||||
2019 | 2018 | 2017 | |||
Volatility | 24% to 25% | ||||
Risk-free rate | 1.7% to 1.8% | ||||
Expected term (Years) | |||||
Dividend Yield | |||||
Stock-based compensation expense related to stock options was $2.9 million, $2.8 million and $3.0 million for the years ended December 31, 2019, 2018 and 2017, respectively.
A summary of stock option activity is presented below:
Shares (in thousands) | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value (in millions) | |||||||||
Outstanding at December 31, 2018 | $ | |||||||||||
Granted | ||||||||||||
Exercises | ( | ) | ||||||||||
Forfeitures | ( | ) | ||||||||||
Outstanding at December 31, 2019 | $ | $ | ||||||||||
Vested and exercisable at December 31, 2019 | $ | $ | ||||||||||
The following table summarizes information about options outstanding as of December 31, 2019:
Options Outstanding | Options Exercisable | ||||||||||||
Range of Exercise Prices | Shares (in thousands) | Weighted-Average Remaining Contractual Term (Years) | Shares (in thousands) | Weighted-Average Exercise Price | |||||||||
$ | to | $ | $ | ||||||||||
$ | to | $ | |||||||||||
$ | |||||||||||||
$ | |||||||||||||
In the year ended December 31, 2019, options with an aggregate intrinsic value of $1.4 million were exercised. The tax effects for exercises in 2019 were not material. In the year ended December 31, 2018, options with an intrinsic value of $11.7 million were exercised resulting in an excess tax benefit of $1.8 million, and in the year ended December 31, 2017, options with an intrinsic value of $1.4 million were exercised resulting in an excess tax benefit of $0.4 million. For stock options outstanding at December 31, 2019, we expect to recognize an additional $3.5 million of expense over the remaining average service period of one year .
Restricted Share Units
Restricted shares, time-vested restricted share units and performance-based restricted share units granted to employees and directors are valued at the closing market price of our common stock on the grant date with vesting conditions determined upon approval of the award.
Stock-based compensation expense related to restricted stock units was $3.7 million, $4.8 million and $3.3 million for the years ended December 31, 2019, 2018 and 2017, respectively. A summary of restricted share unit activity is presented below:
53 | ||
Shares (in thousands) | Weighted Average Fair Value | |||||
Outstanding at December 31, 2018 | $ | |||||
Granted | ||||||
Vested | ( | ) | ||||
Forfeited | ( | ) | ||||
Outstanding at December 31, 2019 | $ | |||||
We also issue restricted share units for which vesting is conditioned on meeting a defined measure of total shareholder return (“TSR units”) over a restricted period of three years . Total shareholder return is measured as our stock price performance over the restricted period compared to defined group of peer companies. The expense recognition for TSR units differs from awards with service or performance conditions in that the expense is recognized over the restricted period regardless of whether the total shareholder return target is met or not, while expense for awards with service and performance conditions is recognized based on the number of awards expected to vest. The fair value of TSR units is determined using a Monte Carlo simulation with a volatility assumption based on the average stock-price volatility for a peer group of companies over the restricted period. The volatility assumption was 29 % for awards granted in 2019, and 27 % for awards granted in 2018 and 25 % for awards granted in 2017. The weighted average fair value per TSR unit was $52.36 , $69.41 and $42.24 for awards granted in 2019, 2018 and 2017, respectively. Stock-based compensation expense related to TSR units was $3.8 million, $4.4 million and $4.8 million for the years ended December 31, 2019, 2018 and 2017, respectively.
A summary of TSR unit activity is presented below.
Shares (in thousands) | Weighted Average Fair Value | |||||
Outstanding at December 31, 2018 | $ | |||||
Granted | ||||||
Vested | ( | ) | ||||
Forfeited | ( | ) | ||||
Outstanding at December 31, 2019 | $ | |||||
For TSR units outstanding at December 31, 2019, we expect to recognize an additional $6.8 million of expense over the weighted average remaining restricted period of one year .
Employee Stock Purchase Plan
Note 14. Commitments and Contingencies
Legal Matters
We are subject to various legal proceedings, claims and governmental inspections, audits or investigations pertaining to issues such as contract disputes, product liability, tax matters, patents and trademarks, advertising, governmental regulations, employment and other matters, including the matters described below. Under the terms of the distribution agreement we entered into with Kimberly-Clark Corporation (“Kimberly-Clark”) prior to the spin-off, legal proceedings, claims and other liabilities that are primarily related to our business are our responsibility and we are obligated to indemnify and hold Kimberly-Clark harmless for such matters (“Indemnification Obligation”). For the years ended December 31, 2019, 2018 and 2017, we have incurred $22.5 million, $15.6 million and $20.5 million, respectively, related to these matters.
Surgical Gown Litigation and Related Matters
54 | ||
Bahamas Surgery Center
We have an Indemnification Obligation for the matter styled Bahamas Surgery Center, LLC v. Kimberly-Clark Corporation and Halyard Health, Inc., No. 2:14-cv-08390-DMG-SH (C.D. Cal.) (“Bahamas”), filed on October 29, 2014. In that case, the plaintiff brought a putative class action asserting claims for common law fraud (affirmative misrepresentation and fraudulent concealment) and violation of California’s Unfair Competition Law (“UCL”) in connection with our marketing and sale of MicroCool surgical gowns.
On April 7, 2017, a jury returned a verdict for the plaintiff, finding that Kimberly-Clark was liable for $4 million in compensatory damages (not including prejudgment interest) and $350 million in punitive damages, and that Avanos was liable for $0.3 million in compensatory damages (not including prejudgment interest) and $100 million in punitive damages. Subsequently, the court also ruled on the plaintiff’s UCL claim and request for injunctive relief. The court found in favor of the plaintiff on the UCL claim but denied the plaintiff’s request for restitution. The court also denied the plaintiff’s request for injunctive relief.
On May 25, 2017, we filed post-trial motions seeking, among other things to have the award of punitive damages reduced. On April 11, 2018, the court issued an Amended Judgment in favor of the plaintiff and against us and Kimberly-Clark that substantially reduced the punitive damages awards. The judgment against us is now $0.4 million in compensatory damages and pre-judgment interest and $1.3 million in punitive damages. The judgment against Kimberly-Clark is $3.9 million in compensatory damages, $2.3 million in pre-judgment interest and $19.4 million in punitive damages.
On April 12, 2018, we filed a notice of appeal to the Ninth Circuit Court of Appeals. We intend to continue our vigorous defense of the Bahamas matter.
Kimberly-Clark Corporation
We have notified Kimberly-Clark that we have reserved our rights to challenge any purported obligation to indemnify Kimberly-Clark for the punitive damages awarded against them. In connection with our reservation of rights, on May 1, 2017, we filed a complaint in the matter styled Halyard Health, Inc. v. Kimberly-Clark Corporation, Case No. BC659662 (County of Los Angeles, Superior Court of California). In that case, we seek a declaratory judgment that we have no obligation, under the Distribution Agreement or otherwise, to indemnify, pay, reimburse, assume, or otherwise cover punitive damages assessed against Kimberly-Clark in the Bahamas matter, or any Expenses or Losses (as defined in the distribution agreement) associated with an award of punitive damages. On May 2, 2017, Kimberly-Clark filed a complaint in the matter styled Kimberly-Clark Corporation v. Halyard Health, Inc., Case No. 2017-0332-AGB (Court of Chancery of the State of Delaware). In that case, Kimberly-Clark seeks a declaratory judgment that (1) we must indemnify them for all damages, including punitive damages, assessed against them in the Bahamas matter, (2) we have anticipatorily and materially breached the Distribution Agreement by our failure to indemnify them, and (3) we are estopped from asserting, or have otherwise waived, any claim that we are not required to indemnify them for all damages, including punitive damages, that may be awarded in the Bahamas matter.
On May 26, 2017, we moved to dismiss or stay Kimberly-Clark’s Delaware complaint, and on June 16, 2017, Kimberly-Clark moved for summary judgment. On September 12, 2017, the Delaware court granted our motion to stay Kimberly-Clark’s complaint and therefore did not take any action on Kimberly-Clark’s motion for summary judgment. On May 30, 2018, Kimberly-Clark moved to quash service of summons we served on Kimberly-Clark in California for lack of personal jurisdiction. On December 12, 2018, the court granted Kimberly-Clark’s motion. On December 18, 2018, we filed a notice of appeal to the California Court of Appeal. On December 6, 2019, the appellate court affirmed the lower court’s ruling, finding that it did not have personal jurisdiction over Kimberly-Clark. We intend to continue our vigorous defense of the matter.
Government Investigation
In June 2015, we were served with a subpoena from the Department of Veterans Affairs Office of the Inspector General (“VA OIG”) seeking information related to the design, manufacture, testing, sale and promotion of MicroCool and other Company surgical gowns, and, in July 2015, we also became aware that the subpoena and an earlier VA OIG subpoena served on Kimberly-Clark requesting information about gown sales to the federal government are related to a United States Department of Justice (“DOJ”) investigation. In May 2016, April 2017 and September 2018, we received additional subpoenas from the DOJ seeking further information related to Company gowns. The Company is cooperating with the DOJ investigation.
Shahinian
On October 12, 2016, after the DOJ and various States declined to intervene, a qui tam matter was unsealed and a complaint was subsequently served on us in a matter styled U.S. ex rel. Shahinian, et al. v. Kimberly-Clark Corporation, No. 2:14-cv-08313-JAK-JPR (C. D. Cal.) (“Shahinian”), filed on October 27, 2014. The case alleges, among other things, violations of the federal and various state False Claims Acts in connection with the marketing and sale of certain surgical gowns. On March 8, 2017, Kimberly-Clark moved to dismiss the Shahinian complaint, and on July 14, 2017, the California court granted Kimberly-Clark’s motion. The plaintiff then filed a second amended complaint, and on August 11, 2017, Kimberly-Clark
55 | ||
moved to dismiss that one as well. The plaintiff then filed a third amended complaint. On January 18, 2018, Kimberly-Clark moved to dismiss that one too. On September 30, 2018, the court granted Kimberly-Clark’s motion with prejudice. On November 13, 2018, Shahinian filed a notice of appeal to the Ninth Circuit Court of Appeals.
We may have an Indemnification Obligation for the Shahinian matter under the distribution agreement with Kimberly-Clark and have notified Kimberly-Clark that we reserve our rights to challenge the obligation to indemnify Kimberly-Clark for any damages or penalties which are not indemnifiable under applicable law or public policy. We intend to continue our vigorous defense of the matter.
Kromenaker
On March 17, 2017, the DOJ submitted a filing declining to intervene in another qui tam matter, and the complaint was unsealed and subsequently served on Kimberly-Clark and Avanos. That matter is styled U.S. ex rel. Kromenaker v. Kimberly-Clark Corporation and Halyard Health, Inc., No. 1:15-cv-04413-SCJ (N. D. Ga.) (“Kromenaker”), filed on December 21, 2015. In that case, the plaintiff alleges, among other things, violations of the federal False Claims Act in connection with the marketing and sale of certain products, including feminine hygiene products, surgical gowns and endotracheal tubes. On June 12, 2017, Kimberly-Clark and Avanos moved to dismiss the complaint. On August 21, 2017, Kromenaker filed an amended complaint, and Kimberly-Clark and Avanos filed motions to dismiss it. On March 27, 2019, the court granted Kimberly-Clark’s and our motion to dismiss. On April 24, 2019, Kromenaker filed a motion with the trial court seeking to have the court alter, amend, or vacate the dismissal. On January 14, 2020, the court denied Kromenaker’s motion, effectively dismissing the case.
We may have an Indemnification Obligation for certain parts of this matter under the distribution agreement with Kimberly-Clark and have notified Kimberly-Clark that we reserve our rights to challenge the obligation to indemnify Kimberly-Clark for any damages or penalties which are not indemnifiable under applicable law or public policy. We intend to continue our vigorous defense of this matter.
Jackson
We were served with a complaint in a matter styled Jackson v. Halyard Health, Inc., Robert E. Abernathy, Steven E. Voskuil, et al., No. 1:16-cv-05093-LTS (S.D.N.Y.), filed on June 28, 2016. In that case, the plaintiff brings a putative class action against the Company, our former Chief Executive Officer, our former Chief Financial Officer and other defendants, asserting claims for violations of the Securities Exchange Act, Sections 10(b) and 20(a). The plaintiff alleges that the defendants made misrepresentations and failed to disclose certain information about the safety and effectiveness of our MicroCool gowns and thereby artificially inflated the Company’s stock prices during the respective class periods. The alleged class period for purchasers of Kimberly-Clark securities who subsequently received Avanos securities is February 25, 2013 to October 21, 2014, and the alleged class period for purchasers of Avanos securities is October 21, 2014 to April 29, 2016. On February 16, 2017, we moved to dismiss the case. On March 30, 2018, the court granted our motion to dismiss and entered judgment in our favor. On April 27, 2018, the plaintiff filed a Motion for Relief from the Judgment and for Leave to Amend. On April 1, 2019, the court denied the plaintiff’s motion. On May 1, 2019, Jackson appealed the dismissal of the action to the 2nd Circuit Court of Appeals. We intend to continue our vigorous defense of this matter.
Richardson, Chiu and Pick
We were also served with a complaint in a matter styled Margaret C. Richardson Trustee of the Survivors Trust Dated 6/12/84 for the Benefit of the H&M Richardson Revocable Trust v. Robert E. Abernathy, Steven E. Voskuil, et al., No. 1:16-cv-06296 (S. D. N. Y.) (“Richardson”), filed on August 9, 2016. In that case, the plaintiff sues derivatively on behalf of Avanos Medical, Inc., and alleges that the defendants breached their fiduciary duty, were unjustly enriched, and violated Section 14(A) of the Securities and Exchange Act in connection with our marketing and sale of MicroCool gowns. We were also served with a complaint in a matter styled Kai Chiu v. Robert E. Abernathy, Steven E. Voskuil, et al, No. 2:16-cv-08768 (C.D. Cal.), filed on November 23, 2016. In that case, the plaintiff sues derivatively on behalf of Avanos Medical, Inc., and makes allegations and brings causes of action similar to those in Richardson, but the plaintiff also adds causes of action for abuse of control, gross mismanagement, and waste of corporate assets. We were also served with a complaint in a matter styled Lukas Pick v. Robert E. Abernathy, Steven E. Voskuil, et al. No. e:18-cv-00295 (D. Del.), filed on February 21, 2018. In that case, the plaintiff sues derivatively on behalf of Avanos Medical, Inc., and makes allegations and brings causes of action similar to those in Richardson and Chiu. We intend to continue our vigorous defense of this matter.
Patent Litigation
We operate in an industry characterized by extensive patent litigation and competitors may claim that our products infringe upon their intellectual property. Resolution of patent litigation or other intellectual property claims is typically time consuming and costly and can result in significant damage awards and injunctions that could prevent the manufacture and sale of the affected products or require us to make significant royalty payments in order to continue selling the affected products. At any
56 | ||
given time we may be involved as either a plaintiff or a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time.
General
While we maintain general and professional liability, product liability and other insurance, our insurance policies may not cover all of these matters and may not fully cover liabilities arising out of these matters. In addition, we may be obligated to indemnify our directors and officers against these matters.
We record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. For any matters that are reasonably possible to result in loss and for which no possible loss or range of loss is disclosed in this report, management has determined that it is unable to estimate the possible loss or range of loss because, in each case, at least the following facts applied: (a) early stage of the proceedings; (b) indeterminate (or unspecified) damages; and (c) significant factual issues yet to be resolved, or such amounts have been determined to be immaterial. At present, although the results of litigation and claims cannot be predicted with certainty, we believe that the ultimate resolution of these matters will not materially impact our liquidity, access to capital markets or ability to conduct our daily operations.
As of December 31, 2019, we have an accrued liability for the matters described herein, and reasonably possible losses have been disclosed. The accrued liability is included in “Accrued Expenses” in the accompanying consolidated balance sheet. Our estimate of these liabilities is based on facts and circumstances existing at this time, along with other variables. Factors that may affect our estimate include, but are not limited to: (i) changes in the number of lawsuits filed against us, including the potential for similar, duplicate or “copycat” lawsuits filed in multiple jurisdictions, including lawsuits that bring causes or action or allege violations of law with regard to additional products; (ii) changes in the legal costs of defending such claims; (iii) changes in the nature of the lawsuits filed against us, (iv) changes in the applicable law governing any legal claims against us; (v) a determination that our assumptions used in estimating the liability are no longer reasonable; and (vi) the uncertainties associated with the judicial process, including adverse judgments rendered by courts or juries. Thus, the actual amount of these liabilities for existing and future claims could be materially different than the accrued amount. Additionally, the above matters, regardless of the outcome, could disrupt our business and result in substantial costs and diversion of management attention.
Environmental Compliance
We are subject to federal, state and local environmental protection laws and regulations with respect to our business operations and are operating in compliance with, or taking action aimed at ensuring compliance with, these laws and regulations. None of our compliance obligations with environmental protection laws and regulations, individually or in the aggregate, is expected to have a material adverse effect on our business, financial condition, results of operations or liquidity.
Note 15. Earnings Per Share (“EPS”)
Basic EPS is calculated by dividing net income by the weighted average number of common shares outstanding during each period. Diluted earnings per share is calculated by dividing net income by the number of common shares outstanding and the effect of all dilutive common stock equivalents outstanding during each period, as determined using the treasury stock method. The calculation of basic and diluted EPS for each of the three years ended December 31, 2019, 2018 and 2017 is set forth in the following table (in millions, except per share amounts):
57 | ||
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Loss from continuing operations | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Income from discontinued operations, net of tax | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ||||||
Weighted Average Shares Outstanding: | |||||||||||
Basic weighted average shares outstanding | |||||||||||
Dilutive effect of stock options and restricted share unit awards | |||||||||||
Diluted weighted average shares outstanding | |||||||||||
Earnings (Loss) Per Share: | |||||||||||
Basic: | |||||||||||
Continuing Operations | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Discontinued Operations | |||||||||||
Basic (Loss) Earnings Per Share | $ | ( | ) | $ | $ | ||||||
Diluted: | |||||||||||
Continuing operations | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Discontinued operations | |||||||||||
Diluted (Loss) Earnings Per Share | $ | ( | ) | $ | $ | ||||||
Restricted share units (“RSUs”) contain provisions allowing for the equivalent of any dividends paid on common stock during the restricted period to be reinvested into additional RSUs at the then fair market value of the common stock on the date dividends are paid. Such awards are to be included in the EPS calculation under the two-class method. Currently we do not anticipate any cash dividends for the foreseeable future and our outstanding RSU awards are not material in comparison to our weighted average shares outstanding. Accordingly, all EPS amounts reflect shares as if they were fully vested and the disclosures associated with the two-class method are not presented herein.
For the year ended December 31, 2019, 1.1 million of potentially dilutive stock options and restricted share unit awards were excluded from the computation of earnings per share as their effect would have been anti-dilutive.
Note 16. Business and Products Information
We conduct our business in one operating and reportable segment that provides our medical device products to healthcare providers and patients in more than 90 countries with manufacturing facilities in the United States, Mexico, France, Germany and Tunisia.
We provide a portfolio of innovative product offerings focused on pain management and respiratory and digestive health to improve patient outcomes and reduce the cost of care. Our management evaluates net sales by product category within our single reportable segment as follows (in millions):
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Chronic care | $ | $ | $ | ||||||||
Pain management | |||||||||||
Total Net Sales | $ | $ | $ | ||||||||
Chronic care is focused on (i) digestive health products such as our Mic-Key enteral feeding tubes and Corpak patient feeding solutions and (ii) respiratory health products such as our Ballard closed airway suction systems and oral care kits.
Pain management is focused on non-opioid solutions including (i) acute pain products such as On-Q and ambIT surgical pain pumps and Game Ready cold and compression therapy systems and (ii) interventional pain solutions, which provides minimally invasive pain relieving therapies, such as our Coolief pain therapy.
58 | ||
For the year ended December 31, 2019, 2018 and 2017, net sales to external customers in the United States were $481 million, $457 million and $467 million, respectively. Globally, no single customer accounted for 10% or more of our consolidated net sales in the year ended December 31, 2019. Net sales to one customer accounted for approximately 10 % of consolidated net sales in each of the years ended December 31, 2018 and 2017, respectively.
Due to the nature of our business, we receive purchase orders for products under supply agreements which are normally fulfilled within three to four weeks. Our performance obligations under purchase orders are satisfied and revenue is recognized at a point in time, which is upon shipment or upon delivery of our products, depending on shipping terms. Accordingly, we normally do not have transactions that give rise to material unfulfilled performance obligations.
Property, plant and equipment held domestically and in foreign countries is as follows (in millions):
As of December 31, | |||||||
2019 | 2018 | ||||||
Domestic | $ | $ | |||||
Foreign | |||||||
Total Property, Plant and Equipment | $ | $ | |||||
Note 17. Supplemental Guarantor Financial Information
In October 2014, Avanos Medical, Inc. (referred to below as “Parent”) issued the Notes (described in Note 9, “Debt”). The Notes are guaranteed, jointly and severally by each of our domestic subsidiaries that guarantees the Senior Credit Facilities (each, a “Guarantor Subsidiary” and collectively, the “Guarantor Subsidiaries”). The guarantees are full and unconditional, subject to certain customary release provisions as defined in the Indenture dated October 17, 2014. Each Guarantor Subsidiary is directly or indirectly 100 %-owned by Avanos Medical, Inc. Each of the guarantees of the Notes is a general unsecured obligation of each Guarantor and ranks equally in right of payment with all existing and future indebtedness and all other obligations (except subordinated indebtedness) of each Guarantor.
The following condensed consolidating balance sheets as of December 31, 2019 and 2018 and the condensed consolidating statements of income and cash flows for the years ended December 31, 2019, 2018 and 2017 provide condensed consolidating financial information for Avanos Medical, Inc. (“Parent”), the Guarantor Subsidiaries on a combined basis, the Non-Guarantor Subsidiaries on a combined basis and the Parent and its subsidiaries on a consolidating basis.
The Parent and the Guarantor Subsidiaries use the equity method of accounting to reflect ownership interests in subsidiaries which are eliminated upon consolidation. Eliminating entries in the following condensed consolidating financial information represent adjustments to (i) eliminate intercompany transactions between or among the Parent, the Guarantor Subsidiaries and the Non-Guarantor Subsidiaries and (ii) eliminate the investments in subsidiaries.
59 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING INCOME AND COMPREHENSIVE INCOME STATEMENTS
(in millions)
Year Ended December 31, 2019 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
Net Sales | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Cost of products sold | ( | ) | |||||||||||||||||
Gross Profit | |||||||||||||||||||
Research and development expenses | |||||||||||||||||||
Selling and general expenses | |||||||||||||||||||
Other expense (income), net | ( | ) | |||||||||||||||||
Operating (Loss) Profit | ( | ) | ( | ) | ( | ) | |||||||||||||
Interest income | ( | ) | |||||||||||||||||
Interest expense | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||
(Loss) Income Before Income Taxes | ( | ) | ( | ) | ( | ) | |||||||||||||
Income tax benefit (provision) | ( | ) | |||||||||||||||||
Equity in earnings of consolidated subsidiaries | ( | ) | |||||||||||||||||
Net (Loss) Income | ( | ) | ( | ) | ( | ) | |||||||||||||
Total other comprehensive income, net of tax | ( | ) | |||||||||||||||||
Comprehensive (Loss) Income | $ | ( | ) | $ | ( | ) | $ | $ | $ | ( | ) | ||||||||
60 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING INCOME AND COMPREHENSIVE INCOME STATEMENTS
(in millions)
Year Ended December 31, 2018 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
Net Sales | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Cost of products sold | ( | ) | |||||||||||||||||
Gross Profit | |||||||||||||||||||
Research and development expenses | |||||||||||||||||||
Selling and general expenses | |||||||||||||||||||
Other expense (income), net | ( | ) | ( | ) | |||||||||||||||
Operating (Loss) Profit | ( | ) | ( | ) | |||||||||||||||
Interest income | ( | ) | |||||||||||||||||
Interest expense | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||
(Loss) Income Before Income Taxes | ( | ) | ( | ) | ( | ) | |||||||||||||
Income tax benefit (provision) | ( | ) | |||||||||||||||||
Equity in earnings of consolidated subsidiaries | ( | ) | |||||||||||||||||
Income (Loss) from Continuing Operations | ( | ) | ( | ) | |||||||||||||||
(Loss) Income from discontinued operations, net of tax | ( | ) | ( | ) | |||||||||||||||
Net Income | ( | ) | |||||||||||||||||
Total other comprehensive (loss) income, net of tax | ( | ) | ( | ) | ( | ) | |||||||||||||
Comprehensive Income | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
61 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING INCOME AND COMPREHENSIVE INCOME STATEMENTS
(in millions)
Year Ended December 31, 2017 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
Net Sales | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Cost of products sold | ( | ) | |||||||||||||||||
Gross Profit | |||||||||||||||||||
Research and development expenses | |||||||||||||||||||
Selling and general expenses | |||||||||||||||||||
Other (income) expense, net | ( | ) | |||||||||||||||||
Operating (Loss) Income | ( | ) | ( | ) | ( | ) | |||||||||||||
Interest income | ( | ) | |||||||||||||||||
Interest expense | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||
(Loss) Income Before Income Taxes | ( | ) | ( | ) | ( | ) | |||||||||||||
Income tax benefit (provision) | ( | ) | |||||||||||||||||
Equity in earnings of consolidated subsidiaries | ( | ) | |||||||||||||||||
Income (Loss) from Continuing Operations | ( | ) | ( | ) | |||||||||||||||
(Loss) Income from discontinued operations, net of tax | ( | ) | |||||||||||||||||
Net Income | ( | ) | |||||||||||||||||
Total other comprehensive income, net of tax | ( | ) | |||||||||||||||||
Comprehensive Income | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
62 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING BALANCE SHEETS
(in millions)
As of December 31, 2019 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
ASSETS | |||||||||||||||||||
Current Assets | |||||||||||||||||||
Cash and cash equivalents | $ | $ | $ | $ | $ | ||||||||||||||
Accounts receivable, net | ( | ) | |||||||||||||||||
Inventories | |||||||||||||||||||
Prepaid and other current assets | |||||||||||||||||||
Total Current Assets | ( | ) | |||||||||||||||||
Property, Plant and Equipment, Net | |||||||||||||||||||
Operating Lease Right of Use Assets | |||||||||||||||||||
Investment in Consolidated Subsidiaries | ( | ) | |||||||||||||||||
Goodwill | |||||||||||||||||||
Other Intangible Assets, net | |||||||||||||||||||
Other Assets | |||||||||||||||||||
TOTAL ASSETS | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
LIABILITIES AND EQUITY | |||||||||||||||||||
Current Liabilities | |||||||||||||||||||
Current portion of operating lease liabilities | $ | $ | $ | $ | $ | ||||||||||||||
Trade accounts payable | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Accrued expenses | |||||||||||||||||||
Total Current Liabilities | ( | ) | |||||||||||||||||
Long-Term Debt | |||||||||||||||||||
Operating Lease Obligation | |||||||||||||||||||
Other Long-Term Liabilities | |||||||||||||||||||
Total Liabilities | ( | ) | |||||||||||||||||
Total Equity | ( | ) | |||||||||||||||||
TOTAL LIABILITIES AND EQUITY | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
63 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING BALANCE SHEETS
(in millions)
As of December 31, 2018 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
ASSETS | |||||||||||||||||||
Current Assets | |||||||||||||||||||
Cash and cash equivalents | $ | $ | $ | $ | $ | ||||||||||||||
Accounts receivable, net | ( | ) | |||||||||||||||||
Inventories | |||||||||||||||||||
Prepaid and other current assets | ( | ) | |||||||||||||||||
Total Current Assets | ( | ) | |||||||||||||||||
Property, Plant and Equipment, Net | |||||||||||||||||||
Investment in Consolidated Subsidiaries | ( | ) | |||||||||||||||||
Goodwill | |||||||||||||||||||
Other Intangible Assets, net | |||||||||||||||||||
Other Assets | |||||||||||||||||||
TOTAL ASSETS | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
LIABILITIES AND EQUITY | |||||||||||||||||||
Current Liabilities | |||||||||||||||||||
Trade accounts payable | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Accrued expenses | ( | ) | |||||||||||||||||
Total Current Liabilities | ( | ) | |||||||||||||||||
Long-Term Debt | |||||||||||||||||||
Other Long-Term Liabilities | |||||||||||||||||||
Total Liabilities | ( | ) | |||||||||||||||||
Total Equity | ( | ) | |||||||||||||||||
TOTAL LIABILITIES AND EQUITY | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
64 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS
(in millions)
Year Ended December 31, 2019 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
Operating Activities | |||||||||||||||||||
Cash Provided by (Used in) Operating Activities | $ | $ | ( | ) | $ | $ | $ | ( | ) | ||||||||||
Investing Activities | |||||||||||||||||||
Capital expenditures | ( | ) | ( | ) | ( | ) | |||||||||||||
Acquisition of business, net of cash acquired | ( | ) | ( | ) | |||||||||||||||
Intercompany contributions | ( | ) | |||||||||||||||||
Cash (Used in) Provided by Investing Activities | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||
Financing Activities | |||||||||||||||||||
Intercompany contributions | ( | ) | ( | ) | |||||||||||||||
Debt repayments | ( | ) | ( | ) | |||||||||||||||
Purchase of treasury stock | ( | ) | ( | ) | |||||||||||||||
Proceeds and excess tax benefits from the exercise of stock options | |||||||||||||||||||
Cash (Used in) Provided by Financing Activities | ( | ) | ( | ) | |||||||||||||||
Effect of Exchange Rate on Cash and Cash Equivalents | |||||||||||||||||||
(Decrease) Increase in Cash and Cash Equivalents | ( | ) | ( | ) | |||||||||||||||
Cash and Cash Equivalents, Beginning of Period | |||||||||||||||||||
Cash and Cash Equivalents, End of Period | $ | $ | $ | $ | $ | ||||||||||||||
65 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS
(in millions)
Year Ended December 31, 2018 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
Operating Activities | |||||||||||||||||||
Cash (Used in) Provided by Operating Activities | $ | ( | ) | $ | ( | ) | $ | $ | $ | ( | ) | ||||||||
Investing Activities | |||||||||||||||||||
Capital expenditures | ( | ) | ( | ) | ( | ) | |||||||||||||
Acquisition of business, net of cash acquired | ( | ) | ( | ) | |||||||||||||||
Proceeds from the Divestiture | |||||||||||||||||||
Dividend received from subsidiaries | ( | ) | |||||||||||||||||
Intercompany contributions | ( | ) | |||||||||||||||||
Cash Provided by Investing Activities | ( | ) | |||||||||||||||||
Financing Activities | |||||||||||||||||||
Intercompany contributions | ( | ) | |||||||||||||||||
Debt repayments | ( | ) | ( | ) | |||||||||||||||
Debt issuance costs | ( | ) | ( | ) | |||||||||||||||
Purchase of treasury stock | ( | ) | ( | ) | |||||||||||||||
Proceeds and excess tax benefits from the exercise of stock options | |||||||||||||||||||
Cash dividends paid to Guarantor | ( | ) | |||||||||||||||||
Cash Used in Financing Activities | ( | ) | ( | ) | ( | ) | |||||||||||||
Effect of Exchange Rate on Cash and Cash Equivalents | ( | ) | ( | ) | |||||||||||||||
Increase in Cash and Cash Equivalents | ( | ) | |||||||||||||||||
Cash and Cash Equivalents, Beginning of Period | |||||||||||||||||||
Cash and Cash Equivalents, End of Period | $ | $ | $ | $ | $ | ||||||||||||||
66 | ||
AVANOS MEDICAL, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS
(in millions)
Year Ended December 31, 2017 | |||||||||||||||||||
Parent | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Eliminations | Consolidated | |||||||||||||||
Operating Activities | |||||||||||||||||||
Cash (Used in) Provided by Operating Activities | $ | ( | ) | $ | $ | $ | $ | ||||||||||||
Investing Activities | |||||||||||||||||||
Capital expenditures | ( | ) | ( | ) | ( | ) | |||||||||||||
Proceeds from property dispositions | |||||||||||||||||||
Intercompany contributions | ( | ) | |||||||||||||||||
Cash Used in Investing Activities | ( | ) | ( | ) | ( | ) | |||||||||||||
Financing Activities | |||||||||||||||||||
Intercompany contributions | ( | ) | ( | ) | |||||||||||||||
Purchase of treasury stock | ( | ) | ( | ) | |||||||||||||||
Proceeds and excess tax benefits from the exercise of stock options | |||||||||||||||||||
Cash Provided by (Used in) Financing Activities | ( | ) | ( | ) | |||||||||||||||
Effect of Exchange Rate on Cash and Cash Equivalents | |||||||||||||||||||
Increase in Cash and Cash Equivalents | |||||||||||||||||||
Cash and Cash Equivalents, Beginning of Period | |||||||||||||||||||
Cash and Cash Equivalents, End of Period | $ | $ | $ | $ | $ | ||||||||||||||
67 | ||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Avanos Medical, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Avanos Medical, Inc. and subsidiaries (the “Company”) as of December 31, 2019 and 2018, the related consolidated statements of income, comprehensive income (loss), stockholders’ equity, and cash flows, for each of the three years in the period ended December 31, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 25, 2020, expressed an unqualified opinion on the Company’s internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the US federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Net Sales/Accrued Expenses-Refer to Notes 1 and 5 to the financial statements
Critical Audit Matter Description
The Company generally distributes its products through wholesale distributors, and in many cases, discounts to the net selling prices are determined based on the contractual arrangements that the Company has with its end-user groups’ purchasing organizations. The Company’s contracts provide for variable consideration, including rebates. Sales are reported net of distributor rebates, which are estimated based on the historical difference between list prices and average end-user contract prices and the quantity of products expected to be sold to end users. Total rebates due to customers that were not settled as of December 31, 2019 was $51.1 million and is included in accrued expenses.
The Company must make certain judgments to estimate the liability for rebates as of the fiscal year end. The judgment of determining the liability includes estimating the quantity of products to be sold to end-user customers and determining the difference in the product’s list price and the average end-user customers’ prices. Due to the extent of subjectivity in management’s estimation, our audit in this area involves especially subjective judgment and an increased extent of effort.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to management’s estimates of rebates included the following, among others:
• | We tested effectiveness of controls related to the accounting for rebates, including those over the estimates of quantity of products to be sold to end-user customers and the difference in the product’s list price and the average end-user prices. |
• | We tested the accuracy and evaluated the relevance of the historical rebate data as an input to the estimated rebates by agreeing rebate rates to contractual arrangements. |
• | We performed a historical trend analysis of rebates paid as a percentage of gross sales. |
68 | ||
• | We evaluated management’s ability to accurately estimate rebates by comparing management’s historical estimates to rebates paid. |
• | We evaluated whether the estimated rebates were consistent with evidence obtained in other areas of the audit. |
Accrued Expenses/Loss Contingencies-Refer to Note 13 to the financial statements
Critical Audit Matter Description
The medical device industry is subject to comprehensive government regulation, and in the normal course of business, liabilities may arise from product-specific and general legal proceedings, and other government investigations. Given the highly complex nature of certain legal proceedings and claims, including the surgical gown litigation and related indemnification obligations under the terms of the distribution agreement with Kimberly-Clark Corporation (“Kimberly-Clark”), management applies significant judgment when considering the likelihood and estimate of loss to record or disclose for each of these legal matters. The Company has disclosed a potential range of loss of related to the surgical gown litigation which includes the judgment against the Company and the judgment against Kimberly-Clark. The judgment against the Company is $0.4 million in compensatory damages and pre-judgment interest and $1.3 million in punitive damages. The judgment against Kimberly-Clark is $3.9 million in compensatory damages, $2.3 million in pre-judgment interest and $19.4 million in punitive damages. The Company has accrued its best estimate of probable loss related to these matters within accrued expenses.
The most significant judgment related to accounting for loss contingencies associated with the surgical gown litigation is the estimate of the Company’s obligation to indemnify Kimberly-Clark, for which the Company has notified Kimberly-Clark that it reserved the rights to challenge any purported obligation to indemnify Kimberly-Clark for the punitive damages awarded against them. Given the complexity of this area, the degree of management’s judgments and estimates, and the nature of the evidence required to address these matters, we consider loss contingencies to be a critical audit matter.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to management’s estimates of loss contingencies, specifically management’s estimate related to its obligation to indemnify Kimberly-Clark related to the surgical gown litigation, and the reserved rights to challenge any purported obligation related to indemnification of punitive damages awarded against Kimberly-Clark, included the following, among others:
• | We tested the effectiveness of controls related to loss contingencies, including those over the estimate of indemnification obligations. |
• | We evaluated management’s judgments in connection with recording and disclosing loss contingencies through inquiries of management and inspection of minutes of the Board of Directors and management meetings. |
• | We obtained and evaluated the Company’s distribution agreement with Kimberly-Clark, which governs the Company’s indemnification obligations. |
• | We obtained letters from internal and external counsel regarding potential outcomes of outstanding legal proceedings and claims, including the proceedings related to the surgical gown litigation and related indemnification obligations. |
• | We evaluated the Company’s disclosures related to loss contingencies. |
/s/ DELOITTE & TOUCHE LLP |
Deloitte & Touche LLP |
Atlanta, Georgia |
February 25, 2020 |
We have served as the Company’s auditor since 2013.
69 | ||
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2019. The term "disclosure controls and procedures," as defined in Rule 13a-15 under the Securities Exchange Act of 1934, as amended (or the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
Based on our evaluation, our chief executive officer and chief financial officer believe that, as of December 31, 2019, our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2019. The scope of management’s evaluation included all of our businesses except for the businesses acquired with NeoMed, Inc., which was acquired in July 2019 and whose financial statements constitute 2% of our consolidated net assets, 3% of our consolidated net sales and 0% of our consolidated net income as of and for the year ended December 31, 2019. For further information, see “Business Acquisition” in Note 2 to the consolidated financial statements in Item 8 of this report. Management’s evaluation was based on the criteria related to internal control over financial reporting described in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2019.
Deloitte & Touche LLP, the independent registered public accounting firm that audited the consolidated financial statements included in this Form 10-K, has issued a report, included herein, on the effectiveness of the Company's internal control over financial reporting as of December 31, 2019.
Changes in Internal Control Over Financial Reporting
IT System Implementation and Stabilization
As of December 31, 2019, the Company was stabilizing its recently-implemented new global IT platform. The new IT platform and related applications were designed to support business activities following the divestiture of the S&IP business and post-divestiture network separation. The new IT platform affects many of the processes that constitute the Company’s internal control over financial reporting. The Company expects improvement in internal control over financial reporting overall, but the implementation was not in response to any identified deficiency.
70 | ||
Except as noted above, there have been no changes in our internal control over financial reporting that occurred during our fourth fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
71 | ||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
Avanos Medical, Inc.
Atlanta, GA
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of Avanos Medical, Inc. and subsidiaries (the “Company”) as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2019, of the Company and our report dated February 25, 2020, expressed an unqualified opinion on those consolidated financial statements.
As described in Management’s Annual Report on Internal Control over Financial Reporting, management excluded from its assessment the internal control over financial reporting at NeoMed, Inc., which was acquired in July 2019 and whose financial statements constitute 2% of consolidated net assets, 3% of consolidated net sales and 0% of consolidated net income of the consolidated financial statement amounts as of and for the year ended December 31, 2019. Accordingly, our audit did not include the internal control over financial reporting at NeoMed, Inc.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ DELOITTE & TOUCHE LLP |
Deloitte & Touche LLP |
Atlanta, Georgia |
February 25, 2020 |
72 | ||
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following sections of our 2020 Proxy Statement for the Annual Meeting of Stockholders (the “2020 Proxy Statement”) are incorporated in this Item 10 by reference:
• | “The Nominees” and “Directors Continuing in Office” under “Proposal 1. Election of Directors,” which identifies our directors and nominees for our Board of Directors. |
• | “Other Information—Section 16(a) Beneficial Ownership Reporting Compliance.” |
• | “Corporate Governance—Other Corporate Governance Policies and Practices–Code of Conduct,” which describes our Code of Conduct. |
• | “Other Information—Stockholder Nominations for Board of Directors,” which describes the procedures by which stockholders may nominate candidates for election to our Board of Directors. |
• | “Corporate Governance—Board Committees–Audit Committee,” which identifies members of the Audit Committee of our Board of Directors and an audit committee financial expert. |
Information regarding our executive officers is reported under the caption “Executive Officers of the Registrant” in Part I of this Report.
We believe we are in compliance with all applicable corporate governance requirements of the New York Stock Exchange, the Securities and Exchange Commission, the Sarbanes-Oxley Act of 2002 and the provisions of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 that have become effective as of the date of this Annual Report on Form 10-K.
ITEM 11. EXECUTIVE COMPENSATION
The information in the sections of the 2020 Proxy Statement captioned “Compensation Discussion and Analysis,” “Compensation Tables,” “Director Compensation” and “Corporate Governance—Compensation Committee Interlocks and Insider Participation” is incorporated in this Item 11 by reference.
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information in the section of the 2020 Proxy Statement captioned “Other Information—Security Ownership Information” is incorporated in this Item 12 by reference.
Equity Compensation Plan Information
The following table gives information about our common stock that may be issued upon the exercise of options, warrants and rights under all of our equity compensation plans as of December 31, 2019.
Number of securities to be issued upon exercise of outstanding options, warrants, and rights (in thousands) (a) | Weighted average exercise price of outstanding options, warrants, and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (in thousands) (c) | |||
Equity compensation plans approved by stockholders(1) | 1,999(2) | $41.70 | 1,406 | ||
(1) | Includes (a) the Avanos Medical, Inc. Equity Participation Plan (the “Employee Plan”), effective November 1, 2014 and (b) the Avanos Medical, Inc. Outside Directors’ Compensation Plan, effective November 1, 2014 (the “Director Plan”). |
(2) | Includes 538 restricted share units granted under the Employee Plan (including shares that may be issued pursuant to outstanding performance-based restricted share units, assuming the target award is met; actual shares issued may vary, depending on actual performance). Upon vesting, a share of Avanos common stock is issued for each restricted share unit. Column (a) also includes 168 restricted share units granted under the Director Plan. Under the Director Plan, upon retirement from, or any other termination of service from the Board, a share of Avanos common stock is issued for each restricted share unit. Column (b) does not take these awards into account because they do not have an exercise price. |
73 | ||
Avanos Medical, Inc. Outside Directors’ Compensation Plan
In 2014, our Board of Directors and our stockholders approved the Director Plan. A maximum of 400,000 shares of our common stock is available for grant under this plan. The Board may grant awards in the form of stock options, stock appreciation rights, restricted stock, restricted share units or any combination of cash, stock options, stock appreciation rights, restricted stock or restricted share units under this plan.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information in the sections of the 2020 Proxy Statement captioned “Other Information—Transactions with Related Persons” and “Corporate Governance—Director Independence” is incorporated in this Item 13 by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information in the sections of the 2020 Proxy Statement captioned “Principal Accounting Firm Fees” and “Audit Committee Approval of Audit and Non-Audit Services” under “Proposal 2. Ratification of Auditors” is incorporated in this Item 14 by reference.
74 | ||
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) | Documents filed as part of this report. |
1. | Financial statements. |
The financial statements are set forth under Item 8 of this report on Form 10-K.
2. | Financial statement schedules. |
The following information is filed as part of this Form 10-K and should be read in conjunction with the financial statements contained in Item 8:
•Report of Independent Registered Public Accounting Firm
All other schedules have been omitted because they were not applicable or because the required information has been included in the financial statements or notes thereto.
3. | Exhibits |
Exhibit Number | Description | |
101.INS | XBRL Instance Document | |
101.SCH | XBRL Taxonomy Extension Schema Document | |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |
75 | ||
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
AVANOS MEDICAL, INC. | ||
February 25, 2020 | By: | /s/ Michael C. Greiner |
Michael C. Greiner | ||
Senior Vice President and | ||
Chief Financial Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
/s/ Joseph F. Woody | Chief Executive Officer and Director (principal executive officer) | February 25, 2020 | |
Joseph F. Woody | |||
/s/ Michael C. Greiner | Senior Vice President and Chief Financial Officer (principal financial officer) | February 25, 2020 | |
Michael C. Greiner | |||
/s/ Renato Negro | Vice President and Controller (principal accounting officer) | February 25, 2020 | |
Renato Negro | |||
Directors
Gary D. Blackford |
John P. Byrnes |
Ronald W. Dollens |
Heidi Kunz |
William A. Hawkins III |
Patrick J. O’Leary |
Maria Sainz |
Dr. Julie Shimer |
By: | /s/ S. Ross Mansbach | February 25, 2020 | |
S. Ross Mansbach Attorney-in-Fact | |||
76 | ||