UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
—————
FORM
——————
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _________ to _________
Commission File Number
| (Exact name of registrant as specified in its charter) |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
(Address
of principal executive offices) (Zip Code)
Registrant's telephone number, including
area code: + 1
|
Securities registered pursuant to Section 12(b) of the Act:
| ||||
| Title of Each Class | Name of each exchange on which registered | Trading Symbol | ||
| The |
||||
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is
a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is
not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing
requirements for the past 90 days.
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§
232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer", "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Smaller reporting company | |
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐
The aggregate market value of the registrant’s
common stock held by non-affiliates computed by reference to the closing price as of the last business day of the registrant’s most
recently completed second fiscal quarter (September 30, 2021) was approximately $
The number of shares outstanding of the registrant's common stock as of June 29, 2022, was .
Documents Incorporated by Reference:
None
NEMAURA MEDICAL INC.
INDEX TO ANNUAL REPORT ON FORM 10-K
| 2 |
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements contained in this Annual Report that are not historical facts constitute forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, and are intended to be covered by the safe harbors created by that Act. Reliance should not be placed on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which may cause actual results, performance, or achievements to differ materially from those expressed or implied. Any forward-looking statement speaks only as of the date made. We undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date on which they are made.
The words "believe," "anticipate," "design," "estimate," "plan," "predict," "seek," "expect," "intend," "may," "could," "should," "potential," "likely," "projects," "continue," "will," and "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are not guarantees of the future as there are a number of meaningful factors that could cause Nemaura Medical Inc.’s (“Nemaura Medical”) actual results to vary materially from those indicated by such forward-looking statements. These statements are based on certain assumptions made based on experience, expected future developments and other factors Nemaura Medical believes are appropriate in the circumstances. Factors which could cause actual results to differ from expectations, many of which are beyond Nemaura Medical’s control, include, but are not limited to, obtaining regulatory approval for our sugarBEAT® device, conducting successful clinical trials, executing agreements required to successfully advance the Company's objectives; retaining the management and scientific team to advance the product; overcoming adverse changes in market conditions and the regulatory environment; obtaining and enforcing intellectual property rights; obtaining adequate financing in the future through product licensing, public or private equity or debt financing or otherwise; dealing with general business conditions and competition; and other factors referenced herein in “Risk Factors.” Except as required by law, we do not assume any obligation to update any forward-looking statement. We disclaim any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
| 3 |
PART I
ITEM 1. BUSINESS.
Business Overview
We are a medical technology company that has developed sugarBEAT®, a non-invasive, flexible, continuous glucose monitoring system, for adjunctive use by persons with diabetes, and any person wishing to determine factors influencing their blood glucose profiles. SugarBEAT® consists of a disposable adhesive skin-patch containing a sensor, which is connected to a rechargeable wireless transmitter. The sensor takes a measurement of the glucose reading every 5 minutes and sends the data by low energy blue tooth to a smart device such as mobile phone (both android and iOS). An app on the smart device uses a proprietary algorithm to display true glucose values, after the data is calibrated using a minimum of one finger stick calibration. SugarBEAT® works by extracting glucose from the skin into a chamber in the patch that is in direct contact with an electrode-based sensor. The data is recorded on the application and can be viewed in real time as well as storing all historic data for later evaluation as desired. We believe sugarBEAT® may be utilized by any person with diabetes, whether Type 1 or Type 2 and also by any persons wishing to determine factors affecting their blood glucose profiles, and therefore their state of metabolic health in terms of insulin resistance.
On May 29, 2019, we announced that we had been awarded CE approval to allow sugarBEAT® to be legally sold in the European Union. CE approval is disclosed by the use of the CE mark, a manufacturers' declaration that the product meets the requirements of the applicable European laws. The European clinical trial program for sugarBEAT® evaluated 525 patient days across 75 Type 1 and Type 2 diabetic patients and was completed in December 2017. CE approval is the process to achieve a mandatory conformity marking for the sugarBEAT® device to allow it to be legally sold in the European Union. It is a manufacturers' declaration that the product meets the requirements of the applicable European laws. This approval is subject to an annual review of the underlying ISO 13485 accredited Quality Management System. The accreditation was successfully renewed in November 2021.
We also submitted a PMA (Premarket Approval) application to the U.S. Food and Drug Administration (the “FDA”) with the same label claim as achieved for CE approval, an adjunct device for glucose trending for persons with diabetes. The PMA is currently under review.
In July 2020, we filed a PMA application with the FDA to use sugarBEAT® as an adjunct to finger prick testing for blood glucose trending. We, along with other applicants, were then informed by the FDA that the approval process was subject to delays as a result of the FDA’s Center for Devices and Radiological Health (“CDRH”) being actively engaged in responding to the COVID-19 pandemic, which resulted in staff being reallocated to other approval requests associated with COVID-19. In April 2021 the FDA confirmed that it was recommencing its review of the PMA application, and in December 2021, the FDA’s Bio-monitoring research division conducted an audit of the clinical program submitted in support of the PMA application. A single 483 observation was raised, and the Company submitted a full response in January 2022. The FDA subsequently scheduled a pre-market inspection for the second calendar quarter of 2022, intended to cover the FDA’s Quality System/Current Good Manufacturing Practice regulations for Medical Devices (21 CFR Part 820).
In addition to this, Nemaura established that proBEATTM, which is based on the sugarBEAT® platform, can be classified under the Wellness guidance when it is used according to the FDA Wellness guidance notes, to provide prompts and educate users on factors affecting their blood sugar profiles. Nemaura launched proBEAT™ in the U.S. in December 2020, as part of a diabetes prevention and reversal program branded BEATdiabetes.life, having licensed a clinically validated weight loss program for the management of diabetes from Healthimation, LLC, which was originally developed at the Joslin Diabetes Center, an affiliate of Harvard Medical School. This program, together with proBEATTM, forms the BEATdiabetes.life program that is currently being developed for commercialization in the U.S. Key opinion leader (“KOL”) studies are ongoing and are intended to provide additional validation of and marketing support for the program in preparation for a broader U.S.-wide roll-out. We continue to monitor and respond to feedback received from these user-groups.
We believe there are additional applications for sugarBEAT® and the underlying BEAT technology platform, which may include:
| · | a web-server accessible by physicians and diabetes professionals to track the condition remotely, thereby reducing healthcare costs and managing the condition more effectively; |
| · | a complete virtual doctor that monitors a person's vital signs and transmits results via the web; |
| · | other patches using the BEAT technology platform to measure alternative analytes, including lactate, uric acid, lithium and drugs. This would be a step-change in the monitoring of conditions, particularly in the hospital setting. Lactate monitoring is currently used to determine the relative fitness of professional athletes and we completed preliminary studies demonstrating the application of the BEAT technology for continuous lactate monitoring; |
| · | a continuous temperature monitoring system which could have various applications, including use for individuals to monitor their temperature in connection with diagnosis and monitoring of symptoms of novel coronavirus (COVID-19); |
| · | monitoring disease progression in COVID-19 patients using continuous lactate monitoring (CLM). |
| 4 |
Our Business Strategy
We intend to lead in the discovery, development and commercialization of innovative and targeted diagnostic medical devices, and data-driven digital platforms, that improve disease monitoring, management and overall patient care. Specifically, we intend to focus on the monitoring of molecules that can be drawn out through the skin non-invasively using our technology platform. In addition to glucose, such molecules may include lactic acid monitoring and the monitoring of prescription drugs and blood biomarkers that may help in the diagnosis, prevention, or management of diseases, such as diabetes. We plan to take the following steps to implement our broad business strategy. Our key commercial strategies post-approval will first be implemented in Europe and then in parts of the Middle East and Asia, and then the U.S., as follows:
| - | Commercialize sugarBEAT® in the United Kingdom and Republic of Ireland. We intend to commercialize sugarBEAT® in the United Kingdom, and Republic of Ireland with MySugarWatch Limited (previously known as Dallas Burston Ethitronix Limited) (“MSW”), with whom we have an exclusive marketing rights agreement for these two countries. We have also signed a full commercial agreement with MySugarWatch (Europe) Limited (previously known as Dallas Burston Ethitronix (Europe) Limited) in May 2018 for all other European territories as part of an equal joint venture agreement. The joint venture intends to seek sub-license rights opportunities to one or more leading companies in the diabetes monitoring space, to leverage their network, infrastructure and resources. |
| - | Establish licensing or joint venture agreements with other parties to market sugarBEAT® in other geographies. We are in detailed discussions and negotiations with several other parties worldwide for licensing or joint venture agreements for the sale of the sugarBEAT® device and have signed commercial agreements with TP MENA for the Gulf Cooperation Council, and Al-Danah Medical for Qatar. |
| - | Seek FDA PMA approval of sugarBEAT®. The PMA application is currently in review by the FDA. |
| - | Expand the indications for which the sugarBEAT® device may be used. We believe that the sugarBEAT® device may offer significant benefits as compared to those found in the non-acute setting for the monitoring of other diseases. This includes monitoring of lactic acid for performance athletics, and the monitoring of drugs. We have completed initial proof of concept for lactate monitoring and now plan to explore the route to commercialization for well-being applications in athletic performance training, and plan to undertake further clinical programs to support clinical use of the device for lactate monitoring. |
| - | Expand our product pipeline through our proprietary platform technologies, acquisitions and strategic licensing arrangements. We intend to leverage our proprietary platform technologies to grow our portfolio of product candidates for the diagnosis of diabetes and other diseases. This includes digital platforms driven by data gathered by our sensors within the medical and wellbeing markets, such as for metabolic health monitoring. In addition, we intend to license our product and acquire products and technologies that are consistent with our research and development and business focus and strategies. This may include drug delivery products for the improved management of diabetes, for example improved insulin injector systems, and/or combination drug products for diabetes related drugs. |
Product Development
Management has extensive experience in regulatory and clinical development of diagnostic medical devices. We intend to take advantage of this experience in the field of diagnostic medical devices in an attempt to increase the probability of product approval. The overall regulatory process for diagnostic medical devices for diabetes is currently similar to those governing other diagnostic devices. The timelines are shorter than, for example, when new drugs or completely invasive diagnostic devices are trialed in clinics. We have successfully tested and evaluated the device for its clinical output, in this case the accuracy and safety with which it can trend blood glucose levels, based on which CE approval was granted by the Notified Body BSI. A PMA (pre-market approval) application was also submitted to the FDA and is currently under review. We continue to seek collaborations with future licensees and marketing partners to achieve our commercial growth milestones.
Market Opportunity for the Company's Products
According to the International Diabetes Federation Atlas 10th Edition 2021 (the "IDF"), there are approximately 537 million adults living with diabetes, representing 10.5% of the world’s population in this age group. This number is predicted to rise to 643 million (11.3%) by 2030 and to 783 million (12.2%) by 2045. Additionally an estimated 240 million people are living with undiagnosed diabetes worldwide, meaning almost one-in-two adults with diabetes are unaware they have the condition. The IDF identifies that almost 90% of people with undiagnosed diabetes live in low-and-middle income countries.
Statistics published by the IDF evidence the fact that diabetes is a huge and growing problem, and that whilst the costs to society are already high, they continue to escalate. In addition, the IDF also notes that Europe has the highest prevalence of children and adolescents with Type 1 diabetes, as well as the highest incidence annually. Europe is also reported as having the second highest average cost per person with diabetes ($3,086), with only North America and the Caribbean being higher ($8,208).
| 5 |
Statistical Data for Diabetes Globally
| 2021 | 2030 | 2045 | |
| Total world population | 7.9 billion | 8.6 billion | 9.5 billion |
| Adult population (20-79 years) | 5.1 billion | 5.7 billion | 6.4 billion |
| Diabetes (20 – 79 years) | |||
| Prevalence (%) | 10.5% | 11.3% | 12.2% |
| Number of people with diabetes | 536.6 million | 642.7 million | 783.2 million |
| Total health expenditure due to diabetes (2021 $) | $966 billion | $1,028 billion | $1,054 billion |
| Impaired Glucose Tolerance “IGT” (20 – 79 years) | |||
| Prevalence (%) | 10.6% | 11.0% | 11.4% |
| Number of people with IGT | 541.0 million | 622.7 million | 730.3 million |
| Type 1 diabetes (0 – 19 years) | |||
| Number of children / adolescents with Type 1 diabetes | 1.2 million | - | - |
| Number of newly diagnosed cases per year | 184,100 | - | - |
Type 1 diabetes, once known as juvenile diabetes or insulin-dependent diabetes, is a chronic condition in which the pancreas produces little or no insulin, a hormone needed to allow sugar (glucose) to enter cells to produce energy. The far more common Type 2 diabetes occurs when the body becomes resistant to the effects of insulin or doesn't make enough insulin.
Various factors may contribute to Type 1 diabetes including genetics and exposure to certain viruses. Although Type 1 diabetes typically appears during childhood or adolescence, it also can develop in adults.
Despite active research, Type 1 diabetes has no cure, although it can be managed. With proper treatment, people who have Type 1 diabetes can expect to live longer, healthier lives than they did in the past. Type 1 diabetes includes autoimmune Type 1 diabetes (Type 1a) which is characterized by having positive autoantibodies, as well as idiopathic Type 1 diabetes (Type 1b) where autoantibodies are negative, and c-peptide is low. Patients with Type 1 diabetes (insulin dependent) require long term treatment with exogenous insulin and these patients perform self-monitoring of blood glucose (SMBG) to calculate the appropriate dose of insulin. SMBG is done by using blood samples obtained by finger sticks but frequent SMBG does not detect all the significant deviations in blood glucose, specifically in patients who have rapidly fluctuating glucose levels.
Type 2 diabetes, once known as adult-onset or non-insulin-dependent diabetes, is a chronic condition that affects the way your body metabolizes sugar (glucose), your body's main source of fuel. With Type 2 diabetes, your body either resists the effects of insulin, a hormone that regulates the movement of sugar into your cells or doesn't produce enough insulin to maintain a normal glucose level. Untreated, Type 2 diabetes can be life-threatening.
More common in adults, Type 2 diabetes increasingly affects children as childhood obesity increases. Whilst there is currently no acknowledged cure for Type 2 diabetes, there is increasing evidence to suggest that it can be effectively managed by eating well, exercising and maintaining a healthy weight. If diet and exercise don't control the blood sugar, diabetes medications or insulin therapy may be required.
Each year, millions of patients undergo diabetes testing in the European Union and in the U.S. The main reason for this testing is to detect and evaluate diabetes in patients with symptoms of diabetes. These studies provide clinical benefit in the initial evaluation of patients with suspected but unproven diabetes, and in those patients in whom a diagnosis of diabetes has been established and information on prognosis or risk is required.
We believe that our market opportunity is a direct function of the number of persons tested, diagnosed and treated for Type 2 diabetes. The IDF indicates that the total world market opportunity for a continuous glucose monitoring device is in the billions of dollars and is projected to grow annually as incidences of diabetes continue to grow.
We do not believe it is possible to estimate the number of diabetes patients that undergo finger pricks or other types of invasive glucose monitoring. However, we are unaware of any product currently on the market that may allow for non-invasive continuous glucose monitoring. We believe the sugarBEAT® device may be readily adopted by the medical community for the assessment of a patient continuously.
We believe our non-invasive sugarBEAT® device possesses many significant advantages and may represent an ideal device for the detection of discordances in an individual's blood sugar levels. We believe the CE approved sugarBEAT® device may represent a best-in-class non-invasive continuous glucose monitoring device to reach those afflicted with diabetes. While we cannot estimate the market share that our sugarBEAT® device may capture, we believe that the sugarBEAT® device will capture a significant share of the non-invasive continuous glucose monitoring market, in-particular the market that has been established by the Abbott Freestyle Libre device for glucose trending, as well as be adopted by non-insulin dependent diabetics who have not historically used continuous glucose monitoring devices due to their invasiveness.
| 6 |
Commercialization Plan
Throughout the fiscal year ended March 31, 2022, we have continued to work with our UK Licensee, MSW, to provide support in the development of their go-to-market strategy which incorporates the utilization of our sugarBEAT® device into their own branded product offering. While COVID-19 did result in some short delays to MSW’s user assessment program, the overall feedback was positive, albeit the anticipated timetable for purchase orders to be placed by MSW was extended out, with the first order for 5,000 sugarBEAT® transmitters and 200,000 sugarBEAT® sensors not being placed until April 2021. Our focus continues to be to support and optimize MSW’s launch program, in line with which, we took the following actions during the fiscal year ended March 31, 2022:
| · | Entered into a new leased facility to provide additional capacity for commercial assembly to commence. |
| · | Increased headcount of production operatives to facilitate product manufacture. |
| · | Placed forward orders for raw materials to support scale-up and secure inventory of those items that are currently in short supply globally i.e. semi-conductors etc. |
| · | Appointed Benchmark Electronics Inc.as our CMO partner to facilitate future volume scale up of transmitter production via its FDA approved facility in Thailand. |
| · | Signed a new global agreement for the provision of our sugarBEAT® device with MySugarWatch DuoPack Limited (“MSW-DP”). Under the terms of the agreement, our CGM and sensors will be provided as Duo-Packs with prescription only medicines that are widely prescribed for people with Type 2 diabetes. The initial Duo-Pack presentation will be launched as the first of these medicines loses its patent protection in the fourth calendar quarter of 2022. |
| · | Commenced phased delivery of transmitters against the purchase order received from MSW in December 2021. |
We also advanced our plans to develop our go-to-market capabilities in the U.S., which included:
| · | Development of a US based standalone team is ongoing and this activity will continue for the foreseeable future. |
| · | In July 2020, we submitted a PMA application to the FDA for the sugarBEAT® device for glucose profiling as an adjunct to a finger-stick measurement. We, along with other applicants, were then informed by the FDA that the approval process was subject to delays as a result of the CDRH being actively engaged in responding to the pandemic caused by COVID-19 which resulted in staff being reallocated to other approval requests associated with COVID-19. In April 2021, the FDA confirmed that it would recommence its review of the PMA application and this is now ongoing and in-progress. |
| · | In December 2021, the FDA’s Bio-monitoring research division conducted an audit of the clinical program submitted in support of the PMA application. A single 483 observation was raised, and the Company submitted a full response in January 2022. |
| · | The FDA subsequently conducted a pre-market inspection for during the second calendar quarter of 2022, covering the FDA’s Quality System / Current Good Manufacturing Practice regulations for Medical Devices (21 CFR Part 820). Once again a single 483 observation was made, and this was responded to within the mandated time frame. The company continues its dialogue with the FDA with respect to the PMA submission and plans to provide further material updates as they arise in due course. |
In addition to this, we continue to explore commercialization opportunities in other key geographic markets, which includes engaging with the German regulatory authority (GBA) to establish how best to proceed with achieving reimbursement for sugarBEAT® in Germany, as well as continuing to engage in dialogue with additional potential licensees / distributors in other geographical territories.
Competitive Landscape
To the best of our knowledge, there are currently no other competing devices on the market that offer continuous glucose monitoring and profiling, non-invasively, with a single day sensor wear. We believe this positions us uniquely in a market where we can target persons with diabetes as well as those that are pre-diabetic. Additionally, we believe that this can also be used to improve outcomes in weight management and wellbeing markets. There are companies, such as Dexcom and Abbott, that currently offer Continuous Glucose Monitoring (CGM) sensors with 10 and 14 continuous day wear, respectively. These companies could be deemed future competitors were they to:
| – | develop and market products that are less expensive or more effective than our current and/or future products; |
| – | operate larger research and development programs or have substantially greater financial resources than we do; |
| – | initiate or withstand substantial price competition more successfully than we can; |
| – | have greater success in recruiting skilled technical and scientific workers from the limited pool of available talent; |
| – | more effectively negotiate third-party licenses and strategic relationships; and |
| – | take advantage of acquisition or other opportunities more readily than we can. |
| 7 |
We may compete for market share against these companies and potential newcomers in this general field. These potential competitors, either alone or together with their partners, may develop new products that will compete with ours, and these competitors may, and in certain cases do, operate larger research and development programs, or have substantially greater financial resources than we do.
As noted, while it is difficult to analyze our major competitors since currently there are no non-invasive diagnostic medical devices to continuously monitor blood glucose levels, we anticipate that specific companies may compete with us in the future.
Regulatory Requirements
Our device has undergone the applicable electrical safety testing and biocompatibility has been demonstrated against the relevant European Directives, Regulations and Standards. If and when new materials are introduced, they will undergo a biocompatibility risk assessment, and further testing where necessary. Batches of the device and patches were manufactured for human clinical studies that took place between November 2014 and December 2015. This was a functional watch device with a wire connection to a skin adhered sensor and electrode. Subsequent to studies conducted in India the device received a CE mark approval in February 2016. The device has since been upgraded to reduce it in size, include an enhanced sensor system and allow wireless communication from a body worn transmitter. This miniaturized wireless device achieved CE approval in May 2019, and a PMA was submitted to the U.S. FDA in July 2020 and is currently in review. An application for CE mark approval requires the Company to have an ISO13485 Quality Management System, covering the design, development and manufacture of a medical device. Nemaura Medical does not have this accreditation, and instead under the terms of a service contract dated April 4, 2018, with Nemaura Pharma Limited (“Pharma”), Nemaura Medical has outsourced the CE approval registration process to Pharma. Pharma, a related company, is controlled by our Chief Executive Officer, President, Chairman of the Board and majority shareholder, Dr D.F.H. Chowdhury. Under the terms of the service contract Pharma has undertaken all required activities to register the product for CE approval under a fee for service arrangement, while Nemaura Medical will retain full title and beneficial ownership of the CE mark, and all related intellectual property without any further payments or royalties becoming due other than the fee for service.
Intellectual Property
We believe that clear and extensive intellectual property relating to our technologies is central to long-term success and we intend to invest accordingly. This applies to both domestic and international patent coverage, and trade secrets, and trademarks.
The sugarBEAT® technology is protected by our portfolio of intellectual property comprised of issued and pending patents and trade secrets covering a range of claims, including the methods and apparatus for measuring glucose extracted from human skin in a non-invasive manner, devices for extracting glucose from the skin is a stable manner, devices for reducing background noise signals, algorithm for converting raw data in to glucose values to calibrate the device, and the formulation and process for preparation of the enzyme solution used in the sensor.
On May 8, 2014, NDM Technologies Limited, a related company, assigned the UK patent application 1208950.4 and International (PCT) patent application PCT/GB2013/051322 entitled "Cumulative Measurement of an Analyte" to Dermal Diagnostics Limited (“DDL”) for a nominal consideration.
Two additional patents were filed in 2018 relating to the sensor and device application, which are expected to provide further strength to the intellectual property position. Additional patents are intended to be filed in the future relating to the device and sensor, providing new intellectual property protection. Some of the recently filed patents and future patents may supersede previous intellectual property.
Additionally, we retain substantial trade secrets relating to aspects of the sensor manufacture process and the sensor formulation, which have taken over five years to develop, and will prove challenging to reverse engineer as it consists of formulation components in addition to processing methods in complex combinations that are unique to the final functional sensor. Patents will not be filed on this aspect of the technology to avoid any public dissemination of the know-how.
These patents and know-how cover aspects of the technology platform. Furthermore, the trademarks BEAT® and sugarBEAT® have been registered in multiple key global territories. Accordingly, all intellectual property essential to the sugarBEAT® product is owned by us, and not subject to royalty payments. We intend to take the lead in the preservation and/or prosecution of these patents and patent applications going forward as required. We intend to file additional patents as the development progresses, where deemed to be of value to protecting the technology platform and future modifications and improvements. Where patents cannot be secured, the intellectual property will be limited to know-how and trade secrets, and these will be diligently guarded.
| 8 |
Trade Secrets, Trademarks, and Patents Filed, Granted and Pending
| IP: Patent (Core Claim), Know-how, Trademark | Expiration Date | Jurisdictions in which Granted / Issued | Jurisdictions in which Pending | Ongoing Royalty or Milestone Payments | ||||
| Patent: Cumulative Measurement of an Analyte (1) | May 20, 2033 | Australia, France, Germany, Italy, Poland, Spain, Netherlands, UK, China, Japan, USA, Canada, UAE | Brazil, Qatar | None. Internal development | ||||
| Skin Prep Patch (2) | December 2, 2039 | N/A | UK, Europe, USA | None. Internal development | ||||
| Know-how: Sensor Formulation and manufacture processes | N/A | Trade Secret | N/A | None. Internal development | ||||
|
Trademark: BEAT |
Renewal due in 2026 | UK, Canada, China, EU, India, Japan, Norway, Russia, Singapore | Malaysia, Brazil, Mexico, Switzerland, Turkey | None. Internal development | ||||
| Trademark: sugarBEAT | Renewal due in 2025 | UK, Canada, Australia, Switzerland, China, Egypt, EU, Israel, India, Iran, Japan, North Korea, Morocco, Mexico, Norway, New Zealand, Russia, Singapore, Tunisia, Turkey, USA | N/A | None. Internal development | ||||
|
Sensors for metabolic health (3)
|
December 7, 2041 | N/A | UK | None. Internal development |
(1) This patent provides a formula for calculating the amount of glucose extracted over a defined period of time by deducting the difference between two readings to allow rapid sensing without needing to deplete the analyte being measured.
(2) This patent describes a device and method for preparing the skin for extraction of glucose.
(3) This patent has two sets of claims relating to the sensor.
Clinical Trials
Our clinical testing is conducted by contract clinical research organizations in various centers around the world to cover a wide demographic – including Asia and Europe – and is managed by our in-house management team.
We had 2 pre-submission meetings with the FDA in June 2016, to define the clinical roadmap. As a result, a detailed clinical plan was developed and approved internally and a clinical site in Europe was selected and audited and approved for commencement of clinical studies using the body worn transmitter device version of the sugarBEAT®. The study was completed, and a PMA application submitted to the FDA in July 2020.
The data from these studies was also submitted as part of the CE approval in Europe was received in May 2019.
Research and development
We spent $1,556,988 and $1,554,603 during the fiscal years ended March 31, 2022 and 2021, respectively, on research and development; management currently anticipated that spend in this area will remain reasonably consistent in the coming fiscal year.
Manufacturing
The manufacture and sale of CE certified medical devices are controlled and governed by guidelines stipulated in the International Organization for Standardization (ISO), more specifically ISO13485; sugarBEAT® will be manufactured and marketed according to ISO13485 quality standards.
In support of commercial sales of sugarBEAT® in the UK and EU we have worked with our manufacturing partner Nemaura Pharma, to scale-up manufacturing of the various sugarBEAT® components alongside facilities for final assembly and packaging. As part of this process, we have expanded our manufacturing and assembly capabilities by occupying additional space within our existing headquarters site at Loughborough University Science and Enterprise Park (LUSEP) in the UK.
We have entered into the following types of agreements with various manufacturing partners:
| – | Manufacturing agreements for the sensor manufacture |
| – | Manufacturing agreements for the patch manufacture |
| – | Manufacturing agreements for the CGM transmitter device and re-charging station manufacture |
| 9 |
Sales and Marketing
An Exclusive Marketing Rights agreement for the UK and Republic of Ireland was signed on March 31, 2014 with Dallas Burston Pharma, a Jersey (Channel Island) based company (“DB Pharma”) (subsequently updated in 2018 and again in 2021 to include a change in the company name to MySugarWatch Limited “MSW”), who has pharmaceutical product marketing operations in the UK and has demonstrated a very successful model for the marketing of prescription medical products directly to general practitioners. We received a non-refundable upfront payment of £1 million ($1.67 million at the then exchange rate) in return for providing MSW with the exclusive right to sell the sugarBEAT® device in the UK and Republic of Ireland, both direct to consumer and through prescriptions by general practitioners. The key terms of the Exclusive Marketing Rights Agreement were concluded in a Commercial Agreement signed in August 2015. This agreement was updated and re-issued in October 2019 to cover new IP / improvements to the technology.
In addition, a joint venture agreement was entered into with MySugar Watch (Europe) Limited (previously known as Dallas Burston Ethitronix (Europe) Limited) in May 2018, whereby we will share equally the costs and net profits of the sales of our sugarBEAT® system in all territories in Europe, with the exception of the United Kingdom, which is the subject of a separate agreement with MSW. This agreement was updated and re-issued in October 2019 to cover new IP/ improvements to the technology. Commercial agreements were signed in 2018 with TPMENA and Al-Danah Medical, for the Gulf Region (GCC) and Qatar respectively.
Regulatory matters
Government Regulation
Our business is subject to extensive federal, state, local and foreign laws and regulations, including those relating to the protection of the environment, and health and safety. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In addition, these laws and their interpretations are subject to change, or new laws may be enacted.
Both federal and state governmental agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. We believe that we have structured our business operations to comply with all applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise. We discuss below the statutes and regulations that are most relevant to our business.
United Kingdom and Wales and the European Union regulations
Government authorities in the United Kingdom and Wales and the European Union as well as other foreign countries extensively regulate, among other things, the research, development, testing, manufacture, labelling, promotion, advertising, distribution, sampling, marketing and import and export of medical devices, including patches and other pharmaceutical products. Our body worn transmitter devices in the United Kingdom and Wales will be subject to strict regulation and require regulatory approval prior to commercial distribution. The process of obtaining governmental approvals and complying with ongoing regulatory requirements requires the expenditure of substantial time and financial resources. In addition, statutes, rules, regulations and policies may change and new legislation or regulations may be issued that could delay such approvals. If we fail to comply with applicable regulatory requirements at any time during the product development process, approval process, or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include the authority's refusal to approve pending applications, withdrawals of approvals, clinical holds, warning letters, product recalls, product seizures, total or partial suspension of our operations, injunctions, fines, civil penalties or criminal prosecution. Any agency enforcement action could have a material adverse effect on us.
The European Commission on Public Health (the "ECPH") provides the regulation for the development and commercialization of new medical diagnostic devices. Any medical device placed on the European market must comply with the relevant legislation, notably with Directive 93/42/EEC for medical devices, with the active implantable devices Directive (90/385/EEC) or with the in vitro devices Directive (98/79/EC). From 26th May 2021, all newly approved medical devices must comply with the Medical Device Regulation (2017/745). Before manufacture / import, it must be determined whether the device in question falls under any of these Directives. All medical devices must fulfil the essential requirements set out in the above-mentioned directives. Where available, relevant standards may be used to demonstrate compliance with the essential requirements defined in the devices Directives.
Manufacturers also need to determine the appropriate conformity assessment route. For devices falling under Directive 93/42/EEC / Regulation 2017/745, other than custom-made devices and devices intended for clinical investigation, the conformity assessment route depends on the class of the device, to be determined in accordance with certain rules set forth in the directives / regulations. Once the applicable class or list has been determined, manufacturers need to follow the appropriate conformity assessment procedure. Subject to the type of the device, this may require manufacturers to have their quality systems and technical documentation reviewed by a Notified Body before they can place their products on the market. A Notified Body is a third-party body that can carry out a conformity assessment recognized by the European Union. The Notified Body will need to assure itself that relevant requirements have been met before issuing relevant certification. Manufacturers can then place the CE marking on their products to demonstrate compliance with the requirements.
| 10 |
The CE approval is the process of achieving a mandatory conformity marking for the sugarBEAT® device to allow it to be legally sold in the European Union. It is a manufacturers' declaration that the product meets the requirements of the applicable European laws. The process for the sugarBEAT® device CE submission and approval involved the following:
1. The device is classified depending on certain categories described by the European Directive with Class I products being low risk (e.g., band aid plasters), with Class III devices being the highest risk. The classes are Class I, IIa, IIb and III. Risk is based upon the potential harm to the patient should a problem arise with a product or its use. The sugarBEAT® device is classified as a IIb device.
2. A 'technical file' containing all of the information required to demonstrate that the product meets the essential requirements of the European directive will be prepared. This includes information relating to performance and safety of the device such as product specifications, labelling, instructions for use, risk analysis and specific test information/clinical evidence relating to the product that support the claims being made for the product.
3. Clinical evidence included in the technical file is expected to demonstrate that the device is safe and meets defined performance requirements. This clinical evidence can be in the form of literature data where substantial published data exists that utilizes the same technique for glucose extraction and measurement (albeit in a different device format), or data from actual clinical studies performed using the sugarBEAT® device. The first CE mark submission was based on literature evaluation of 3rd party published clinical data available in the public domain. The final CE mark submission has claims based on the clinical performance of the device, based on clinical studies described earlier herein. The clinical data showed that the sugarBEAT® device can trend blood glucose levels in a human subject by taking measurements every 5 minutes. The clinical trial data demonstrates the sugarBEAT® device blood glucose trend can be used to supplement normal finger prick measurements.
4. The technical file has been assessed by an independent inspector (the Notified Body), regulated by the competent authority, (Medicines and Healthcare products Regulatory Agency, MHRA in the United Kingdom). The Notified Body (an organization in the European Union that has been accredited by a member state to determine whether a medical device complies with the European medical device directives), will then notify The European Commission on Public Health (the "ECPH") of the approval and a certificate will be issued to the Company by the notified body and we will then be able to apply the CE mark to the device, and legally offer the product for sale in the European Economic Area (EEA). The CE mark has been issued as of May 2019 and the company is now able to offer the device for commercial sale in the EU.
5. The review of the technical file commenced in August 2018, and the final review and sign off was received in May 2019. Since the CE mark was approved, we have undergone routine inspections of our ISO 13485 Quality Management System in order to maintain our CE mark accreditation. An addendum was also submitted to the notified body and approval obtained, to include within the approved CE marked device, the iOS version of the smart device app that the transmitter connects to.
U.S. Food and Drug Administration regulation of medical devices
The US Food, Drug, and Cosmetic Act (the “FDCA”) and FDA regulations establish a comprehensive system for the regulation of medical devices intended for human use. sugarBEAT® is a medical device that is subject to these, as well as other federal, state, local and foreign, laws and regulations. The FDA is responsible for enforcing the laws and regulations governing medical devices in the United States.
The FDA classifies medical devices into one of three classes (Class I, Class II, or Class III) depending on their level of risk and the types of controls that are necessary to ensure device safety and effectiveness. The class assignment is a factor in determining the type of premarketing submission or application, if any, that will be required before marketing in the United States. SugarBEAT® falls under Class III.
| – | Class I devices present a low risk and are not life-sustaining or life-supporting. The majority of Class I devices are subject only to "general controls" (e.g., prohibition against adulteration and misbranding, registration and listing, good manufacturing practices, labelling, and adverse event reporting. General controls are baseline requirements that apply to all classes of medical devices.) |
| – | Class II devices present a moderate risk and are devices for which general controls alone are not sufficient to provide a reasonable assurance of safety and effectiveness. Devices in Class II are subject to both general controls and "special controls" (e.g., special labelling, compliance with performance standards, and post market surveillance. Unless exempted, Class II devices typically require FDA clearance before marketing, through the premarket notification (510(k)) process.) |
| – | Class III devices present the highest risk. These devices generally are life-sustaining, life-supporting, or for a use that is of substantial importance in preventing impairment of human health or present a potential unreasonable risk of illness or injury. Class III devices are devices for which general controls, by themselves, are insufficient and for which there is insufficient information to determine that application of special controls would provide a reasonable assurance of safety and effectiveness. Class III devices are subject to general controls and typically require FDA approval of a PMA application before marketing. |
Unless it is exempt from premarket review requirements, a medical device must receive marketing authorization from the FDA prior to being commercially marketed, distributed or sold in the United States. The most common pathways for obtaining marketing authorization are 510(k) clearance and PMA. After preliminary discussions with the FDA in June 2016 as part of a pre-submission meeting it was determined that the pathway for sugarBEAT® would be a PMA approval.
| 11 |
Premarket approval pathway
The PMA approval process requires an independent demonstration of the safety and effectiveness of a device. PMA is the most stringent type of device marketing application required by the FDA. PMA approval is based on a determination by the FDA that the PMA contains sufficient valid scientific evidence to ensure that the device is safe and effective for its intended use(s). A PMA application generally includes extensive information about the device including the results of clinical testing conducted on the device and a detailed description of the manufacturing process.
After a PMA application is accepted for review, the FDA begins an in-depth review of the submitted information. FDA regulations provide 180 days to review the PMA and make a determination; however, in reality, the review time is normally longer (e.g., 1-3 years). During this review period, the FDA may request additional information or clarification of information already provided. Also, during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the data supporting the application and provide recommendations to the FDA as to whether the data provides a reasonable assurance that the device is safe and effective for its intended use. In addition, the FDA generally will conduct a preapproval inspection of the manufacturing facility to ensure compliance with Quality System Regulation, which imposes comprehensive development, testing, control, documentation and other quality assurance requirements for the design and manufacturing of a medical device.
Based on its review, the FDA may (i) issue an order approving the PMA, (ii) issue a letter stating the PMA is "approvable" (e.g., minor additional information is needed), (iii) issue a letter stating the PMA is "not approvable," or (iv) issue an order denying PMA. A company may not market a device subject to PMA review until the FDA issues an order approving the PMA. As part of a PMA approval, the FDA may impose post-approval conditions intended to ensure the continued safety and effectiveness of the device including, among other things, restrictions on labelling, promotion, sale and distribution, and requiring the collection of additional clinical data. Failure to comply with the conditions of approval can result in materially adverse enforcement action, including withdrawal of the approval.
Most modifications to a PMA approved device, including changes to the design, labelling, or manufacturing process, require prior approval before being implemented. Prior approval is obtained through submission of a PMA supplement. The type of information required to support a PMA supplement and the FDA's time for review of a PMA supplement vary depending on the nature of the modification.
In February 2020 Nemaura announced that following discussions with the FDA, it was established that Nemaura may sell its CGM product with a digital service offering in the U.S. without FDA approval as a non-medical wellbeing application. Nemaura further announced that it intended to launch this product under the brand proBEATÔ in the U.S. in October to December 2020. The product enables users to wear the CGM device from which data will be sent to Nemaura’s servers in the cloud, from where data will be processed to provide users with educational material and insights into factors that can affect their sugar levels and tips for healthy lifestyle and diet, with a view to helping pre-diabetics and diabetics alike live healthier lives. A limited product launch commenced in the U.S. in December 2020 to enabled potential customers to register their interest utilizing proBEATÔ in conjunction with a digital program for weight loss targeted at persons with diabetes, under the brand BEATdiabetes.life.
Clinical trials
Clinical trials of medical devices in the U.S. are governed by the FDA's Investigational Device Exemption ("IDE") regulation. This regulation places significant responsibility on the sponsor of the clinical study including, but not limited to, choosing qualified investigators, monitoring the trial, submitting required reports, maintaining required records, and assuring investigators obtain informed consent, comply with the study protocol, control the disposition of the investigational device, submit required reports, etc.
Clinical trials of significant risk devices (e.g., implants, devices used in supporting or sustaining human life, devices of substantial importance in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health) require FDA and Institutional Review Board ("IRB") approval prior to starting the trial. FDA approval is obtained through submission of an IDE application. Clinical trials of non-significant risk ("NSR") devices (i.e., devices that do not meet the regulatory definition of a significant risk device) only require IRB approval before starting. The clinical trial sponsor is responsible for making the initial determination of whether a clinical study is significant risk or NSR; however, a reviewing IRB and/or FDA may review this decision and disagree with the determination.
An IDE application must be supported by appropriate data, such as performance data, animal and laboratory testing results, showing that it is safe to evaluate the device in humans and that the clinical study protocol is scientifically sound. There is no assurance that submission of an IDE will result in the ability to commence clinical trials. Additionally, after a trial begins, the FDA may place it on hold or terminate it if, among other reasons, it concludes that the clinical subjects are exposed to an unacceptable health risk.
As noted above, the FDA may require a company to collect clinical data on a device in the post-market setting.
The collection of such data may be required as a condition of PMA approval. The FDA also has the authority to order, via a letter, a post-market surveillance study for certain devices at any time after they have been cleared or approved.
| 12 |
Pervasive and continuing FDA regulation
After a device is placed on the market, regardless of its classification or premarket pathway, numerous additional FDA requirements generally apply. These include, but are not limited to:
| – | Establishment registration and device listing requirements; |
| – | Quality System Regulation ("QSR"), which governs the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labelling, storage, installation, and servicing of finished devices; |
| – | Labelling requirements, which mandate the inclusion of certain content in device labels and labelling, and generally require the label and package of medical devices to include a unique device identifier ("UDI"), and which also prohibit the promotion of products for uncleared or unapproved, i.e., "off-label," uses; |
| – | Medical Device Reporting ("MDR") regulation, which requires that manufacturers and importers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and |
| – | Reports of Corrections and Removals regulation, which requires that manufacturers and importers report to the FDA recalls (i.e., corrections or removals) if undertaken to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug and Cosmetic Act that may present a risk to health; manufacturers and importers must keep records of recalls that they determine to be non-reportable. |
The FDA enforces these requirements by inspection and market surveillance. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include, but is not limited to, the following sanctions:
| – | Untitled letters or warning letters; |
| – | Fines, injunctions and civil penalties; |
| – | Recall or seizure of our products; |
| – | Operating restrictions, partial suspension or total shutdown of production; |
| – | Refusing a request for 510(k) clearance or premarket approval of new products; |
| – | Withdrawing 510(k) clearance or premarket approvals that are already granted; and |
| – | Criminal prosecution. |
We would be subject to unannounced device inspections by the FDA, as well as other regulatory agencies overseeing the implementation of and compliance with applicable state public health regulations. These inspections may include our suppliers' facilities.
Other Regulation in the United Kingdom and Wales and the EU
Healthcare Reimbursement
Government and private sector initiatives to limit the growth of healthcare costs, including price regulation, competitive pricing, coverage and payment policies, and managed-care arrangements, are continuing in many countries where we do business, including the United Kingdom and Wales. These changes are causing the marketplace to put increased emphasis on the delivery of more cost-effective medical products. Government programs, private healthcare insurance and managed-care plans have attempted to control costs by limiting the amount of reimbursement they will pay for particular procedures or treatments. This has created an increasing level of price sensitivity among customers for products. Some third-party payers must also approve coverage for new or innovative devices or therapies before they will reimburse healthcare providers who use the medical devices or therapies. Even though a new medical product may have been cleared for commercial distribution, we may find limited demand for the product until reimbursement approval has been obtained from governmental and private third-party payers.
Environmental Regulation
We are also subject to various environmental laws and regulations both within and outside the United Kingdom and Wales. Like many other medical device companies, our operations involve the use of substances, including hazardous wastes, which are regulated under environmental laws, primarily manufacturing and sterilization processes. We do not expect that compliance with environmental protection laws will have a material impact on our consolidated results of operations, financial position or cash flow. These laws and regulations are all subject to change, however, and we cannot predict what impact, if any, such changes might have on our business, financial condition or results of operations.
| 13 |
Foreign Regulation
Whether or not we obtain regulatory approval for a product, we must obtain approval from the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for EC approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement also vary greatly from country to country.
In addition, regulatory approval of prices is required in most countries other than the United States. We face the risk that the prices which result from the regulatory approval process would be insufficient to generate an acceptable return to us or our collaborators.
EU General Data Protection Regulation
The EU General Data Protection Regulation (the “GDPR”) came into force in all EU Member States from May 25, 2018 and replaced previous EU data privacy laws. Although a number of basic existing principles will remain the same, the GDPR introduces new obligations on data controllers and rights for data subjects, including, among others:
| – | accountability and transparency requirements, which will require data controllers to demonstrate and record compliance with the GDPR and to provide more detailed information to data subjects regarding processing; |
| – | enhanced data consent requirements, which includes “explicit” consent in relation to the processing of sensitive data; |
| – | obligations to consider data privacy as any new products or services are developed and limit the amount of information collected, processed, stored and its accessibility; |
| – | constraints on using data to profile data subjects; |
| – | providing data subjects with personal data in a useable format on request and erasing personal data in certain circumstances; and |
| – | reporting of breaches without undue delay (72 hours where feasible). |
The GDPR also introduced new fines and penalties for a breach of requirements, including fines for serious breaches of up to the higher of 4% of annual worldwide revenue or €20m and fines of up to the higher of 2% of annual worldwide revenue or €10m (whichever is highest) for other specified infringements. The GDPR identifies a list of points to consider when imposing fines (including the nature, gravity and duration of the infringement).
The Company has assessed the implications of the GDPR on all personal data it holds and has implemented measures to ensure that personal data shall be:
| - | Processed lawfully, fairly and in a transparent manner in relation to the data subject. |
| - | Collected for a specified, explicit and legitimate purpose and not further processed in a manner that is incompatible with those purposes. |
| - | Adequate, relevant and limited to what is necessary in relation to the purposes for which they are processed. |
| - | Kept in a form which permits identification of data subjects for no longer than is necessary for the purposes for which the personal data is processed. |
| - | Processed in a manner that ensures appropriate security of the personal data, including protection against unauthorised or unlawful processing and against accidental loss, destruction or damage, using appropriate technical or organisational measures. |
| - | Maintained accurately and up to date and that every reasonable step is taken to ensure that personal data that is inaccurate, having regard to the purposes for which they are processed, are erased or rectified without delay. |
At the current stage of the Company’s development and, with being pre-revenue at this stage, the scope of data held, and consequently the impact of GDPR, is limited. Increased application of GDPR will be assessed and implemented prior to further Company developments that warrant additional GDPR measures. As the Company progresses with product commercialization, the extent to which GDPR will affect the Company will increase, which will require additional changes to the Company’s procedures and policies which could adversely impact operational and compliance costs. Further, there is a risk that the measures will not be implemented correctly or that individuals within the business will not be fully compliant with the new procedures. If there are breaches of these measures, the Company could face significant administrative and monetary sanctions as well as reputational damage which may have a material adverse effect on its operations, financial condition, and prospects.
| 14 |
Corporate Information
Our principal executive offices are located at 57 West 57th Street New York, NY 10019. Our website is located at www.nemauramedical.com and our telephone number is + 1 646-416-7912. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this Annual Report, and you should not consider it part of the Annual Report.
Human Capital Management
We believe that a diverse workforce is important to our success. We will continue to focus on the hiring, retention and advancement of women and underrepresented populations, and to cultivate an inclusive and diverse corporate culture. In the future, we intend to continue to evaluate our use of human capital measures or objectives in managing our business such as the factors we employ or seek to employ in the development, attraction and retention of personnel and maintenance of diversity in our workforce.
The success of our business is fundamentally connected to the well-being of our people. Accordingly, we are committed to the health, safety, and wellness of our employees. We provide our employees with access to a variety of flexible and convenient health and wellness programs, including benefits that provide protection and security so they can have peace of mind concerning events that may require time away from work or that impact their financial well-being; that support their physical and mental health by providing tools and resources to help them improve or maintain their health status and encourage engagement in healthy behaviors; and that offer choice where possible so they can customize their benefits to meet their needs and the needs of their families.
We also provide robust compensation and benefits programs to help meet the needs of our employees. We believe that we maintain a satisfactory working relationship with our employees and have not experienced any labor disputes. As of March 31, 2022, we had 37 personnel employed on our payroll, which equates to approximately 32 full-time equivalents.
Corporate History and Restructuring
We are a holding corporation that owns 100% of a diagnostic medical device company specializing in discovering, developing, and commercializing specialty medical devices. We were organized on December 24, 2013, under the laws of the State of Nevada. We own 100% of Dermal Diagnostic (Holdings) Limited, an England and Wales corporation formed on December 11, 2013. Dermal Diagnostics (Holdings) Limited owns 100% of the stock in Dermal Diagnostics Limited (“DDL”), an England and Wales corporation formed on January 20, 2009, and 100% of the stock in Trial Clinic Limited (“TCL”), an England and Wales corporation formed on January 12, 2011.
The following diagram illustrates Nemaura’s corporate structure as of March 31, 2022:
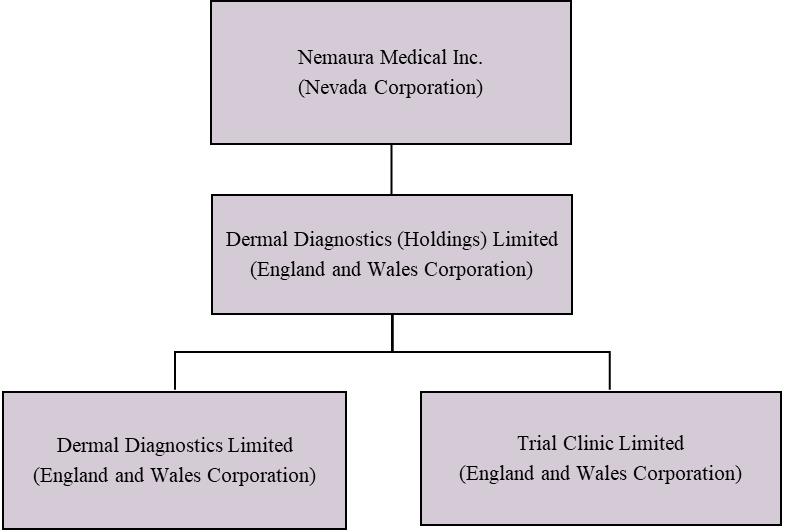
| 15 |
During the fiscal year ended March 31, 2021, the Board of Directors assessed the adequacy of the group’s organizational structure and concluded that an intermediary holding company, Region Green Limited, was no longer required as the entity had been effectively dormant since inception and no longer represented a requirement to be maintained. It was therefore determined that Region Green Limited should be unwound, with the assets held by Region Green Limited being transferred up to Nemaura Medical Inc. following which Region Green Limited would be dissolved.
The transfer of assets took place on March 5, 2021 and Region Green Limited was formally dissolved as of April 23, 2021.
In December 2013, we restructured the Company and re-domiciled as a domestic corporation in the United States. The corporate re-organization was accomplished to preserve the tax advantages under the laws of the England and Wales tax laws for the benefit of the shareholders of both Dermal Diagnostics Limited and Trial Clinic Limited.
DDL is a diagnostic medical device company headquartered in Loughborough, Leicestershire, England. DDL was founded on January 20, 2009, to engage in the discovery, development and commercialization of diagnostic medical devices. The Company’s initial focus has been on the development of a novel CGM device.
ATM Offering
On July 23, 2021, Company entered into an At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC (the “Agent”) pursuant to which the Company may offer and sell from time to time to or through the Agent shares of the Company’s common stock.
The offer and sale of shares of common stock through the Agent will be made pursuant to the Registration Statement on Form S-3 (File No. 333-230535), which was declared effective by the Securities and Exchange Commission (the “SEC”) on April 8, 2019, and a related prospectus supplement pursuant to which the Company offered shares of its common stock having an aggregate offering price of up to $100,000,000.
Under the ATM Agreement, the Company may offer and sell shares of common stock through the Agent by any method deemed to be an “at the market offering” as defined in Rule 415 of the Securities Act of 1933, as amended, including sales made directly on or through The Nasdaq Capital Market, sales made to or through a market maker other than on an exchange or otherwise, directly to the Agent as principal, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, and/or in any other method permitted by law. If the Company elects to utilize the ATM Agreement, the Agent would be obligated to use commercially reasonable efforts consistent with its normal trading and sales practices and applicable law and regulations to sell such shares in accordance with the Company’s instructions (including as to price, time or size limit or other parameters or conditions the Company may impose). The Company will pay the Agent a commission of 3.0% of the gross sales price of any shares of common stock sold under the ATM Agreement. The Company has also provided the Agent with customary indemnification rights and has agreed to reimburse the Agent for certain specified expenses up to $20,000 plus up to $2,500 per quarter while the ATM Agreement remains in effect.
The Company is not obligated to sell, and the Agent is not obligated to buy or sell, any shares of common stock under the ATM Agreement. The Company or the Agent may terminate the ATM Agreement by providing notice to the other party. The Company intends to use the net proceeds from any ATM offering for general corporate purposes, which include, but are not limited to, the targeted launch of sugarBEAT® into other European markets outside of the UK; the development of the subscription-based service for the U.S. under the Wellness category that was launched in December 2020; establishing a business-to-consumer offering for a metabolic health program; research and development of our BEAT platform for other, non, CGM purposes, such as Lactate monitoring, as well as potential acquisition of other companies, products or technologies that are complementary to the delivery of our mission. Accordingly, our management will have broad discretion as to the use of the net proceeds from any ATM offering under this agreement.
| 16 |
ITEM 1A. — RISK FACTORS
Investing in our securities is highly speculative and involves a high degree of risk. You should carefully consider the following risks and other information in this Annual Report on Form 10-K and our other SEC filings before deciding to invest. Additional risks and uncertainties that we are unaware of may become relevant to us. Any of the following risks could materially and adversely affect our business, results of operations or financial condition. In that event, the trading price of our common stock and warrants may decline, and you could lose all or part of your investment.
We will need to raise additional funds in order to finance the anticipated commercialization of our product by incurring indebtedness, through collaboration and licensing arrangements, or by issuing securities which may cause dilution to existing stockholders, or require us to relinquish rights to our technologies and our product.
Developing our product, conducting clinical trials, establishing manufacturing facilities and developing marketing and distribution capabilities is expensive. We will need to finance future cash needs through additional public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. We cannot be certain that additional funding will be available to us on acceptable terms, or at all. If adequate funds are not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or our commercialization efforts. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or our product or grant licenses on terms that are not favorable to us.
We have a limited operating history, and you should not rely on our historical financial data as an indicator of our future financial performance.
We have a limited operating history in the medical device industry. You should consider our business and prospects in light of the risks and difficulties we face with our limited operating history and should not rely on our past results as an indication of our future performance. In particular, we may face challenges in planning our growth strategy and forecasting market demand accurately as a result of our limited historical data and limited experience in implementing and evaluating our business strategies. If we are unable to successfully address these risks, difficulties and challenges as a result of our limited operating history, our ability to implement our strategic initiatives could be adversely affected, which may in turn have a material adverse effect on our business, financial condition, results of operations and prospects.
We have a history of losses and may not achieve or maintain profitability.
We have incurred net losses every year since our inception in 2009 and have not generated revenue from the period of our inception from product sales or licenses to date. As of March 31, 2022, we had an accumulated deficit of approximately $37.7 million. We expect to incur losses until our product is successfully launched and cannot be certain that we will ever achieve profitability. As a result, our business is subject to all of the risks inherent in the development of a new business enterprise, such as the risk that we may not obtain substantial additional capital needed to support the expenses of developing our technology and commercializing our potential products; develop a market for our potential products; successfully transition from a company with a research focus to a company capable of either manufacturing and selling potential products or profitably licensing our potential products to others; and/or attract and retain qualified management, technical and scientific staff.
Revenue generation from product sales has only commenced in the current fiscal year and may never become profitable.
To date, we have generated revenue for the first time in the current fiscal period for product sales. Our ability to generate and grow revenue depends on several factors, including our ability to support the market launch of our UK Licensee, successfully obtain regulatory approval in all key markets identified to commercialize our product pipeline. Even then, we will need to establish and maintain sales, marketing, distribution and to the extent we do not outsource manufacturing, manufacturing capabilities. We plan to rely on one or more strategic collaborators to help generate revenues in markets outside of Great Britain however, we cannot be sure that our collaborators, if any, will be successful. Our ability to generate revenue will also be impacted by certain challenges, risks and uncertainties frequently encountered in the establishment of new technologies and products in emerging markets and evolving industries. These challenges include our ability to:
| – | execute our business model; |
| – | create brand recognition; |
| – | manage growth in our operations; |
| – | create a customer base cost-effectively; |
| – | retain customers; |
| – | access additional capital when required; and |
| – | attract and retain key personnel. |
We cannot be certain that our business model will be successful or that it will successfully address these and other challenges, risks, and uncertainties. If we are unable to generate significant revenue, we may not become profitable, and we may be unable to continue our operations. Even if we are able to commercialize the sugarBEAT® device, we may not achieve profitability for at least several years, if at all, after generating material revenue.
| 17 |
Our substantial amount of indebtedness may adversely affect our cash flow and our ability to operate our business, remain in compliance with debt covenants and make payments on our indebtedness.
Our substantial level of indebtedness increases the possibility that we may be unable to generate cash sufficient to pay, when due, the principal of, interest on or other amounts due with respect to our indebtedness. Our indebtedness could have other important consequences to you as a stockholder. For example, it could:
| – | make it more difficult for us to satisfy our obligations with respect to our indebtedness and any failure to comply with the obligations of any of our debt instruments, including financial and other restrictive covenants, could result in an event of default under the senior secured credit facility and the senior subordinated note; |
| – | make is more vulnerable to adverse changes in general economic, industry and competitive conditions and adverse change in government regulation; |
| – | require us to dedicate a substantial portion of our cashflow from operations to payments on our indebtedness, thereby reducing the availability of our cashflows to fund working capital, capital expenditures, acquisitions and other general corporate purposes; |
| – | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| – | place us at a competitive disadvantage compared to our competitors that have less debt; and |
| – | limit our ability to borrow amounts for working capital, capital expenditures, acquisitions, debt service requirements, execution of our business strategy or other purposes. |
Risks Related to Our Product Candidate and Operations
We are largely dependent on the success of our sole product candidate, the sugarBEAT® device, and we may not be able to successfully commercialize this potential product.
We have incurred and will continue to incur significant costs relating to the development and marketing of our sole product candidate, the sugarBEAT® device. We have obtained approval to market this product in the EU, but it is not guaranteed that we will achieve this in any jurisdiction and we may never be able to obtain approval or, if approvals are obtained, to commercialize this product successfully in other territories.
If we fail to successfully commercialize our product(s) in multiple territories, we may be unable to generate sufficient revenue to sustain and grow our business, and our business, financial condition and results of operations will be adversely affected.
If we fail to obtain regulatory approval of the sugarBEAT® device or any of our other future products, we will be unable to commercialize these potential products.
The development, testing, manufacturing and marketing of our product is subject to extensive regulation by governmental authorities in Great Britain and the European Union. In particular, the process of obtaining CE approval by a Notified Body, a third party that can carry out a conformity assessment recognized by the European Union, is costly and time consuming, and the time required for such approval is uncertain. Our product must undergo rigorous preclinical and clinical testing and an extensive regulatory approval process mandated for the CE. Such regulatory review includes the determination of manufacturing capability and product performance. CE approval was granted by the European Notified Body BSI in May 2019, allowing the product to be made available for commercial sale. This approval is subject to an annual review of the underlying ISO 13485 accredited Quality Management System. The accreditation was successfully renewed in November 2021.
There can be no assurance that all necessary approvals will be granted for future products or that CE review or actions will not involve delays caused by requests for additional information or testing that could adversely affect the time to market for and sale of our product. Further failure to comply with applicable regulatory requirements can, among other things, result in the suspension of regulatory approval as well as possible civil and criminal sanctions.
Failure to enroll patients in our clinical trials may cause delays in developing the sugarBEAT® device or any of our future products.
We may encounter delays in the development and commercialization, or fail to obtain marketing approval, of the sugarBEAT® device or any other future products if we are unable to enroll enough patients to complete clinical trials. Our ability to enroll sufficient numbers of patients in our clinical trials depends on many factors, including the severity of illness of the population, the size of the patient population, the nature of the clinical protocol, the proximity of patients to clinical sites, and the eligibility criteria for the trial and competing clinical trials. Delays in any possible future patient enrollment, based on request by local regulatory agencies to conduct studies in their territory, may result in increased costs and harm our ability to complete our clinical trials and obtain regulatory approval.
| 18 |
Delays in clinical testing could result in increased costs to us and delay our ability to generate revenue.
Significant delays in clinical testing could materially, adversely impact our product development costs. We do not know whether planned clinical trials will begin on time, will need to be restructured or will be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence and continue a study, delays in reaching agreement on acceptable clinical study terms with prospective sites, delays in obtaining institutional review board approval to conduct a study at a prospective site and delays in recruiting patients to participate in a study.
Significant delays in testing or regulatory approvals for any of our current or future products, including the sugarBEAT® device, could prevent or cause delays in the commercialization of such product candidates, reduce potential revenues from the sale of such product candidates and cause our costs to increase.
Our clinical trials for any of our current or future products may produce negative or inconclusive results and we may decide, or regulators may require us, to conduct additional clinical and/or preclinical testing for these products or cease our trials.
We will only receive regulatory approval to commercialize a product candidate if we can demonstrate to the satisfaction of the applicable regulatory agency that the product is safe and effective. We do not know whether our future clinical trials will demonstrate safety and efficacy sufficiently to result in marketable products. Because our clinical trials for the sugarBEAT® device may produce negative or inconclusive results, we may decide, or regulators may require us, to conduct additional clinical and/or preclinical testing for this product or cease our clinical trials. If this occurs, we may not be able to obtain approval for this product or our anticipated time to market for this product may be substantially delayed and we may also experience significant additional development costs. We may also be required to undertake additional clinical testing if we change or expand the indications for our product.
If approved, the commercialization of our product, the sugarBEAT® device, may not be profitable due to the need to develop sales, marketing and distribution capabilities, or make arrangements with a third party to perform these functions.
In order for the commercialization of our potential product to be profitable, our product must be cost-effective and economical to manufacture on a commercial scale. Subject to regulatory approval, we expect to incur significant sales, marketing, distribution, and to the extent we do not outsource manufacturing, manufacturing expenses in connection with the commercialization of the sugarBEAT® device and our other potential products. We do not currently have a dedicated sales force and our current manufacturing capability has limited capacity, we also have limited experience in the sales, marketing and distribution of medical diagnostic device products. In order to commercialize the sugarBEAT® device or any of our other potential products that we may develop, we must develop sales, marketing and distribution capabilities or make arrangements with a third party to perform these functions. Developing a sales force is expensive and time-consuming, and we may not be able to develop this capacity. If we are unable to establish adequate sales, marketing and distribution capabilities, independently or with others, we may not be able to generate significant revenue and may not become profitable. Our future profitability will depend on many factors, including, but not limited to:
| – | the costs and timing of developing a commercial scale manufacturing facility or the costs of outsourcing the manufacturing of the sugarBEAT® device; |
| – | receipt of regulatory approval of the sugarBEAT® device; |
| – | the terms of any marketing restrictions or post-marketing commitments imposed as a condition of approval by regulatory authorities; |
| – | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| – | costs of establishing sales, marketing and distribution capabilities; |
| – | the effect of competing technological and market developments; and |
| – | the terms and timing of any collaborative, licensing and other arrangements that we may establish. |
Even if we receive regulatory approval for the sugarBEAT® device or any other product candidates, we may never receive significant revenues from any of them. To the extent that we are not successful in commercializing our potential products, we will incur significant additional losses.
Our proprietary rights may not adequately protect our intellectual property and product and if we cannot obtain adequate protection of our intellectual property and product, we may not be able to successfully market our product.
Our commercial success will depend in part on obtaining and maintaining intellectual property protection for our technologies and product. We will only be able to protect our technologies and product from unauthorized use by third parties to the extent that valid and enforceable patents cover them, or that other market exclusionary rights apply. While we have issued enforceable patents covering the sugarBEAT® device, the patent positions of companies like ours can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in Great Britain and the European Union. The general patent environment outside the United States involves significant uncertainty. Accordingly, we cannot predict the breadth of claims that may be allowed or that the scope of these patent rights would provide a sufficient degree of future protection that would permit us to gain or keep our competitive advantage with respect to this product and technology. Additionally, companies like ours are dependent on creating a pipeline of products. We may not be able to develop additional proprietary technologies or products that produce commercially viable products or that are themselves patentable.
| 19 |
Our issued patents may be subject to challenge and possibly invalidated by third parties. Changes in either the patent laws or in the interpretations of patent laws in Great Britain or the European Union or other countries may diminish the market exclusionary ability of our intellectual property.
In addition, others may independently develop similar or alternative technologies that may be outside the scope of our intellectual property. Should third parties obtain patent rights to similar technology, this may have an adverse effect on our business.
To the extent that consultants or key employees apply technological information independently developed by them or by others to our product, disputes may arise as to the proprietary rights of the information, which may not be resolved in our favor. Consultants and key employees that work with our confidential and proprietary technologies are required to assign all intellectual property rights in their discoveries to us. However, these consultants or key employees may terminate their relationship with us, and we cannot preclude them indefinitely from dealing with our competitors. If our trade secrets become known to competitors with greater experience and financial resources, the competitors may copy or use our trade secrets and other proprietary information in the advancement of their products, methods or technologies. If we were to prosecute a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming and the outcome would be unpredictable. In addition, courts in Great Britain and the European Union are sometimes less willing to protect trade secrets than courts in the United States. Moreover, if our competitors independently develop equivalent knowledge, we would lack any contractual claim to this information, and our business could be harmed.
Our ability to commercialize our product will depend on our ability to sell such products without infringing the patent or proprietary rights of third parties. If we are sued for infringing intellectual property rights of third parties, such litigation will be costly and time consuming and an unfavorable outcome would have a significant adverse effect on our business.
Our ability to commercialize our product will depend on our ability to sell such products without infringing the patents or other proprietary rights of third parties. Third-party intellectual property in the field of diagnostic medical devices is complicated, and third-party intellectual property rights in this field are continuously evolving. We have not performed searches for third-party intellectual property rights that may raise freedom-to-operate issues, and we have not obtained legal opinions regarding commercialization of our product other than patent research prior to the filing of our patent applications, and search and examination reports from the respective patent examination offices.
In addition, because patent applications are published months after their filing, and because applications can take several years to issue, there may be currently pending third-party patent applications that are unknown to us, which may later result in issued patents. If a third-party claim that we infringe on its patents or other proprietary rights, we could face a number of issues that could seriously harm our competitive position, including:
| – | infringement claims that, with or without merit, can be costly and time consuming to litigate, can delay the regulatory approval process and can divert management’s attention from our core business strategy; |
| – | substantial damages for past infringement which we may have to pay if a court determines that our products or technologies infringe upon a competitor’s patent or other proprietary rights; |
| – | if a license is available from a holder, we may have to pay substantial royalties or grant cross licenses to our patents or other proprietary rights; and |
| – | Re-designing our process so that it does not infringe the third-party intellectual property, which may not be possible, or which may require substantial time and expense including delays in bringing our own products to market. |
Such actions could harm our competitive position and our ability to generate revenue and could result in increased costs.
If our product, the sugarBEAT® device, does not gain market acceptance among physicians, patients and the medical community, we will be unable to generate significant revenue, if any.
The sugarBEAT® device that we developed may not achieve market acceptance among physicians, patients, third-party payers and others in the medical community. If we receive the regulatory approvals necessary for commercialization, the degree of market acceptance will depend upon a number of factors, including:
| – | limited indications of regulatory approvals; |
| – | the establishment and demonstration in the medical community of the clinical efficacy and safety of our product and its potential advantages over existing diagnostic medical devices; |
| – | the prevalence and severity of any side effects; |
| – | our ability to offer our product at an acceptable price; |
| – | the relative convenience and ease of use of our product; |
| – | the strength of marketing and distribution support; and |
| – | sufficient third-party coverage or reimbursement. |
| 20 |
The market may not accept the sugarBEAT® device based on any number of the above factors. If the sugarBEAT® device is approved, there may be other therapies available which directly compete for the same target market. The market may choose to continue utilizing the existing products for any number of reasons, including familiarity with or pricing of these existing products. The failure of any of our products to gain market acceptance could impair our ability to generate revenue, which could have a material adverse effect on our future business.
We have outsourced the bulk of the commercial manufacturing operations for the various components of the sugarBEAT®, with the exception of the Sensor chemistry which is being conducted in-house. The failure to find manufacturing partners or expand our internal manufacturing facility could have an adverse impact on our ability to grow our business.
We are largely dependent on third parties to supply our product according to our specifications, in sufficient quantities, on time, in compliance with appropriate regulatory standards and at competitive prices. We cannot be sure that we will be able to obtain an adequate supply of our product candidates on acceptable terms, or at all.
Manufacturers supplying diagnostic medical devices must comply with regulations which require, among other things, compliance with evolving regulations under Medical Device Directives stipulated under ISO13485. The manufacturing of products at any facility will be subject to strict quality control, testing and record keeping requirements, and continuing obligations regarding the submission of safety reports and other post-market information. Both the sensor and patch manufacturing facilities for the sugarBEAT® device are currently ISO13485 certified. We cannot guarantee that the facilities will continue to pass regulatory inspection, or that future changes to ISO13485 standards will not also affect the manufacture of the sensors and patches.
If we fail to attract and retain senior management, consultants, advisors and scientific and technical personnel, our product development and commercialization efforts could be impaired.
Our performance is substantially dependent on the performance of our senior management and key scientific and technical personnel, particularly Dr. Dewan Fazlul Hoque Chowdhury, President, Chairman and Chief Executive Officer. The loss of the services of any member of our senior management or our scientific or technical staff may significantly delay or prevent the development of our product and other business objectives by diverting management’s attention to transition matters and identification of suitable replacements, if any, and could have a material adverse effect on our business, operating results and financial condition.
We also rely on consultants and advisors to assist us in formulating our research and development strategy. All of our consultants and advisors are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to contribute to us.
In addition, we believe that we will need to recruit additional executive management and scientific and technical personnel. There is currently intense competition for skilled executives and employees with relevant scientific and technical expertise, and this competition is likely to continue. The inability to attract and retain sufficient scientific, technical and managerial personnel could limit or delay our product development efforts, which would adversely affect the development of our product and commercialization of our potential product and growth of our business.
We expect to expand our marketing capabilities and, as a result of which we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to have growth in expenditures, the number of our employees and the scope of our operations, in particular with respect to those potential products that we elect to commercialize independently or together with others. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to train qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plan or disrupt our operations.
Fluctuations in foreign exchange rates may adversely affect our financial condition and results of operations.
Our functional currency is the Great Britain Pound Sterling (“GBP”). The reporting currency is the United States dollar (U.S.$). Income and expenditures are translated at the appropriate weighted average exchange rates prevailing during the reporting period. Assets and liabilities are translated at the exchange rates as of balance sheet date. Stockholders’ equity is translated into United States dollars from GBP at historical exchange rates. Currency fluctuations and restrictions on currency exchange may adversely affect our business, including limiting our ability to convert GBP into foreign currencies and, if the GBP were to decline in value, reducing our revenue in U.S. dollar terms. To the extent the U.S. dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions results in reduced revenue, operating expenses and net income for our international operations. Similarly, to the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions results in increased revenue, operating expenses and net income for our international operations. We are also exposed to foreign exchange rate fluctuations as we convert the financial statements of our foreign subsidiaries into U.S. dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign subsidiaries’ financial statements into U.S. dollars will lead to a translation gain or loss which is recorded as a component of other comprehensive income (loss). We have not entered into agreements or purchased instruments to hedge our exchange rate risks. The availability and effectiveness of any hedging transaction may be limited, and we may not be able to successfully hedge our exchange rate risks.
| 21 |
In addition, a number of events have occurred in recent years, including the UK’s Brexit vote to leave the EU, the impact of Covid-19, and the invasion of Ukraine by Russia, that have had significant and potentially lasting effect of both the global economic outlook as well as a weakening of GBP against many currencies. We expect to have to pay some of our service providers and vendors in U.S.$ which given the exchange rate impact and knock on inflationary pressure, will represent a significant increase in costs to the business compared to prior years. The currency exchange rate continues to be very unstable and therefore the future impact or further weakening of GBP is not known at this time.
Our business, financial condition and results of operations may be materially adversely affected by global health epidemics, including the COVID-19 pandemic.
A regional or global health pandemic, including COVID-19, could severely affect our business, results of operations and financial condition. A regional or global health pandemic, depending upon its duration and severity, could have a material adverse effect on our business. For example, the COVID-19 pandemic has had numerous effects on the global economy and governmental authorities around the world have implemented measures to reduce the spread of COVID-19. These measures, including shutdowns and “shelter-in-place” orders suggested or mandated by governmental authorities or otherwise elected by companies as a preventive measure, have adversely affected workforces, customers, consumer sentiment, economies and financial markets, and, along with decreased consumer spending, have led to an economic downturn in many of our markets.
As a result of the COVID-19 pandemic, we evaluated and executed the steps available to us to ensure we were able to provide protection of our employees and instigated remote working where possible combined with following all government advice and guidance regarding any engagement within the workplace that could not be completed remotely. To date this transition has had little impact on our employee productivity and has caused limited interruption to our business. Whilst restrictions associated with COVID-19 have largely been removed, we will continue to assess the situation, including abiding by any government-imposed restrictions, as and where relevant.
At this point in time, there remains some uncertainty relating to the potential effect of COVID-19 on our business. As infections may continue to become more widespread, we could experience a severe negative impact on our business, financial condition, and results of operations. To the extent the COVID-19 pandemic adversely affects our business and financial results, it may also have the effect of heightening many of the other risks described in this “Risk factors” section.
Risks Related to Our Industry
Our competitors may develop products that are less expensive, safer or more effective, which may diminish or eliminate the commercial success of any potential products that we may commercialize.
If our competitors market products that are less expensive, safer or more effective than our future products developed from our product candidates, or that reach the market before our products, we may not achieve commercial success. For example, if approved, the sugarBEAT® device’s primary competition in the glucose monitoring device setting will be companies such as Dexcom, Abbott, and Senseonics who produce glucose monitoring devices. The market may choose to continue utilizing the existing products for any number of reasons, including familiarity with or pricing of these existing products. The failure of our product to compete with products marketed by our competitors would impair our ability to generate revenue, which would have a material adverse effect on our future business, financial condition and results of operations.
We expect to compete with several companies including Dexcom, Abbott, and Senseonics, and our competitors may:
| – | develop and market products that are less expensive or more effective than our future product; |
| – | commercialize competing products before we can launch any products developed from our product candidate; |
| – | operate larger research and development programs or have substantially greater financial resources than we do; |
| – | initiate or withstand substantial price competition more successfully than we can; |
| – | have greater success in recruiting skilled technical and scientific workers from the limited pool of available talent; |
| – | more effectively negotiate third-party licenses and strategic relationships; and |
| – | take advantage of acquisition or other opportunities more readily than we can. |
We expect to compete for market share against large medical diagnostic device manufacturing companies, smaller companies that are collaborating with larger companies, new companies, and other public and private research organizations.
In addition, our industry is characterized by rapid technological change. Because our research approach integrates many technologies, it may be difficult for us to stay abreast of the rapid changes in each technology. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our product discovery process that we believe we derive from our research approach and proprietary technologies.
| 22 |
The use of hazardous materials in our operations may subject us to environmental claims or liabilities.
Our research and development activities involve the use of hazardous chemical materials. Injury or contamination from these materials may occur and we could be held liable for any damages, which could exceed our available financial resources. This liability could materially adversely affect our business, financial condition and results of operations.
We are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We may be required to incur significant costs to comply with environmental laws and regulations in the future that could materially adversely affect our business, financial condition and results of operations.
If we fail to comply with extensive regulations enforced by regulatory agencies with respect to diagnostic medical device products, the commercialization of our product could be prevented, delayed or halted.
Research, preclinical development, clinical trials, manufacturing and marketing of our product is subject to extensive regulation by various government authorities. We have not received marketing approval for the sugarBEAT® device in all of our target markets. The process of obtaining the required regulatory approvals is lengthy and expensive, and the time required for such approvals is uncertain. The approval process is affected by such factors as:
| – | the indication and claims of the diagnostic device; |
| – | the quality of submission relating to the product; |
| – | the product’s clinical efficacy and safety; |
| – | the manufacturing facility compliance; |
| – | the availability of alternative devices; |
| – | the risks and benefits demonstrated in clinical trials; and |
| – | the patent status and marketing exclusivity rights of certain innovative products. |
Any regulatory approvals that we or our partners receive for our product may also be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially costly post-marketing follow-up studies. The subsequent discovery of previously unknown problems with the product, including adverse events of unanticipated severity or frequency, may result in restrictions on the marketing of the product and withdrawal of the product from the market.
Manufacturing, labelling, storage and distribution activities also are subject to strict regulation and licensing by government authorities. The manufacturing facilities for our product will be subject to periodic inspection by the regulatory authorities and from time to time, these agencies may send notice of deficiencies as a result of such inspections. Our failure, or the failure of our manufacturing facilities, to continue to meet regulatory standards or to remedy any deficiencies could result in corrective action by the authorities, including the interruption or prevention of marketing, closure of our manufacturing facilities, and fines or penalties.
Regulatory authorities also will require post-marketing surveillance to monitor and report potential adverse effects of our product. If approved, any of our products’ subsequent failure to comply with applicable regulatory requirements could, among other things, result in warning letters, fines, suspension or revocation of regulatory approvals, product recalls or seizures, operating restrictions, injunctions and criminal prosecutions.
Government policies may change, and additional government regulations may be enacted that could prevent or delay regulatory approval of our product. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action. If we are not able to maintain regulatory compliance, we might not be permitted to market our product and our business could suffer.
In the future, we hope to distribute and sell our product outside of the United Kingdom and the European Union, which will subject us to further regulatory risk.
In addition to maintaining approval from the United Kingdom and the European Union for the sugarBEAT® device, we may seek regulatory approval from Saudi Arabia and the United Arab Emirates, Hong Kong, Australia, and the U.S., to market the sugarBEAT® device, however, there is no guarantee we will do so. We may in the future also seek approvals for additional countries. The regulatory review process varies from country to country, and approval by foreign government authorities is unpredictable, uncertain and generally expensive. The ability to market our product could be substantially limited due to delays in receipt of, or failure to receive, the necessary approvals or clearances. Marketing of our product in these countries, and in most other countries, is not permitted until we have obtained required approvals or exemptions in each individual country. Failure to obtain necessary regulatory approvals could impair our ability to generate revenue from international sources.
| 23 |
Market acceptance of our product will be limited if users are unable to obtain adequate reimbursement from third-party payers.
Government health administration authorities, private health insurers and other organizations generally provide reimbursement for products like our product and our commercial success will depend in part on these third-party payers agreeing to reimburse patients for the costs of our product. Even if we succeed in bringing our product to market, we cannot assure you that third-party payers will consider our product cost effective or provide reimbursement in whole or in part for its use.
Significant uncertainty exists as to the reimbursement status of newly approved health care products. Our product is intended to replace or alter existing therapies or procedures. These third-party payers may conclude that our product is less safe, effective or cost-effective than existing therapies or procedures. Therefore, third-party payers may not approve our product for reimbursement.
If third-party payers do not approve our product for reimbursement or fail to reimburse for them adequately, sales will suffer as some physicians, or their patients will opt for a competing product that is approved for reimbursement or is adequately reimbursed. Even if third-party payers make reimbursement available, these payers’ reimbursement policies may adversely affect our ability and the ability of our potential collaborators to sell our product on a profitable basis.
The trend toward managed healthcare, the growth of organizations such as health maintenance organizations and legislative proposals to reform healthcare and government insurance programs could significantly influence the purchase of healthcare services and products, resulting in lower prices and reduced demand for our product which could adversely affect our business, financial condition and results of operations.
In addition, legislation and regulations affecting the pricing of our product may change in ways adverse to us before or after the regulatory agencies approve our product for marketing. While we cannot predict the likelihood of any of these legislative or regulatory proposals, if any government or regulatory agencies adopt these proposals, they could materially adversely affect our business, financial condition and results of operations.
Product liability claims may damage our reputation and, if insurance proves inadequate, the product liability claims may harm our business.
As with other companies in our field, we may be exposed to the risk of product liability claims that is inherent in the diagnostic medical device sector. A product liability claim may damage our reputation by raising questions about our product’s safety and efficacy and could limit our ability to sell our product by preventing or interfering with commercialization of our product.
In addition, product liability insurance for our industry is generally expensive to the extent it is available at all. There can be no assurance that we will be able to maintain such insurance on acceptable terms or that we will be able to secure increased coverage as the commercialization of our product progresses, or that future claims against us will be covered by our product liability insurance. Moreover, there can be no assurance that any product liability coverage from any insurance policy and/or any rights of indemnification and contribution that we have in place currently will offset any / all future claims. A successful claim against us with respect to uninsured liabilities and not subject to any indemnification or contribution could have a material adverse effect on our business, financial condition, and results of operations.
We could be negatively impacted by the application or enforcement of fraud and abuse laws, including anti-kickback laws and other anti-referral laws.
We are not aware of any current business practice which is in violation of any fraud and abuse law. However, continued vigilance to assure compliance with all potentially applicable laws will be a necessary expense associated with product development. For example, all product marketing efforts must be strictly scrutinized to assure that they are not associated with improper remunerations to referral sources in violation of any anti-kickback statutes. Remunerations may include potential future activities for our product, including discounts, rebates and bundled sales, which must be appropriately structured to take advantage of statutory and regulatory “safe harbors”. From time to time we may engage physicians in consulting activities. In addition, we may decide to sponsor continuing medical education activities for physicians or other medical personnel. We may also award or sponsor study grants to physicians from time to time. All relationships with physicians, including consulting arrangements, continuing medical education and study grants, must be similarly reviewed for compliance with any anti-kickback statute to assure that remuneration is not provided in return for referrals. Patient inducements may also be unlawful. Inaccurate reports of product pricing, or a failure to provide a product at an appropriate price to various governmental entities, could also serve as a basis for an enforcement action under various theories.
Claims which are “tainted” by virtue of kickbacks or a violation of self-referral rules may be alleged as false claims if other elements of a violation are established. Because our potential customers may seek payments from healthcare programs for our product, even during the clinical trial stages, we must assure that we take no actions which could result in the submission of false claims. For example, free product samples which are knowingly or with reckless disregard billed to healthcare programs could constitute false claims. If the practice was facilitated or fostered by us, we could be liable. Moreover, inadequate accounting for or a misuse of grant funds used for product research and development could be alleged as a violation of relevant statutes.
| 24 |
The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations, and additional legal or regulatory change.
Risks Related to Our Common Stock
Our stock price may be volatile.
The stock market, particularly in recent years, has experienced significant volatility with respect to pharmaceutical, biotechnology and other diagnostic medical device company stocks. The volatility of pharmaceutical, biotechnology and other diagnostic medical device company stocks often does not relate to the operating performance of the companies represented by the stock. Factors that could cause this volatility in the market price of our common stock include:
| – | results from and any delays in our clinical trials; |
| – | failure or delays in entering our product into clinical trials; |
| – | failure or discontinuation of any of our research programs; |
| – | delays in establishing new strategic relationships; |
| – | delays in the development or commercialization of our product; |
| – | market conditions in the diagnostic medical device sectors and issuance of new or changed securities analysts’ reports or recommendations; |
| – | actual and anticipated fluctuations in our financial and operating results; |
| – | developments or disputes concerning our intellectual property or other proprietary rights; |
| – | introduction of technological innovations or new commercial products by us or our competitors; |
| – | issues in manufacturing our product; |
| – | market acceptance of our product; |
| – | third-party healthcare reimbursement policies; |
| – | regulatory actions affecting us or our industry; |
| – | litigation or public concern about the safety of our product; and |
| – | additions or departures of key personnel. |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
We have not paid and may not pay any dividends on our common stock.
We have paid no dividends on our common stock to date and may not pay dividends to holders of our common stock in the foreseeable future. While our future dividend policy will be based on the operating results and capital needs of the business, it is currently anticipated that any earnings will be retained to finance our future expansion and for the implementation of our business plan. As an investor, you should take note of the fact that a lack of a dividend can further affect the market value of our stock and could significantly affect the value of any investment in our Company.
We are subject to the reporting requirements of federal securities laws. This can be expensive and may divert resources from other projects, and thus impairing our ability to grow.
We are subject to the information and reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and other federal securities laws, including compliance with the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). The costs of preparing and filing annual and quarterly reports, proxy statements and other information with the Securities and Exchange Commission (“SEC”) (including reporting of any Merger that may occur in the future) and furnishing audited reports to stockholders will cause our expenses to be higher than they would have been if we had remained privately held.
If we fail to establish and maintain an effective system of internal control, we may not be able to report our financial results accurately or to prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely impact the trading price of our common stock.
We are subject to reporting obligations under the U.S. securities laws. The SEC, as required by Section 404 of the Sarbanes- Oxley Act (“SOX”), adopted rules requiring every public company to include a management report on such company’s internal control over financial reporting in its annual report, which contains management’s assessment of the effectiveness of the company’s internal control over financial reporting.
| 25 |
Public company compliance may make it more difficult to attract and retain officers and directors.
The Sarbanes-Oxley Act and rules subsequently implemented by the SEC have required changes in corporate governance practices of public companies. As a public company, we expect these rules and regulations to increase our compliance costs in 2021 and beyond and to make certain activities more time consuming and costly. As a public company, we also expect that these rules and regulations may make it more difficult and expensive for us to obtain director and officer liability insurance in the future and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors or as executive officers.
If our common stock is deemed a “penny stock,” it will make it more difficult for our investors to sell their shares.
Our common stock will be subject to the “penny stock” rules adopted under Section 15(g) of the Exchange Act. The penny stock rules generally apply to companies whose common stock is not listed on The Nasdaq Stock Market or other national securities exchange and trades at less than $5.00 per share, other than companies that have had average revenue of at least $6,000,000 for the last three years or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stock to persons other than “established customers” complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our stockholders sell substantial amounts of our common stock in the public market upon the expiration of any statutory holding period, under Rule 144, or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
The interests of Dr D.F.H. Chowdhury, or the controlling shareholders, may not always coincide with the interests of us and our other shareholders, and the controlling shareholders may exert significant control or substantial influence over us and may take actions that are not in, or may conflict with, public shareholders’ best interests.
The controlling shareholders control the exercise of voting rights of over 50% of the shares eligible to vote in any of our annual or special meetings. Therefore, these controlling shareholders will be able to exercise significant influence over all matters that require us to obtain shareholder approval, including the election of directors to our board and approval of significant corporate transactions that we may consider, such as a merger or other sale of our company or its assets. The controlling shareholders may cause us to take actions that are not in, or may conflict with, the interests of us or the public shareholders. In the case where the interests of the controlling shareholders conflict with those of our other shareholders, or if the controlling shareholders choose to cause us to pursue objectives that would conflict with the interests of our other shareholders, such other shareholders could be left in a disadvantageous position by such actions caused by the controlling shareholders and the price of our common stock could be adversely affected.
We are subject to the anti-takeover provisions of the Nevada Revised Statutes governing business combinations and control share acquisitions.
Applicability of the Nevada business combination statute would discourage parties interested in taking control of our company if they cannot obtain the approval of our board of directors. These provisions could prohibit or delay a merger or other takeover or change in control attempt and, accordingly, may discourage attempts to acquire our company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
The effect of the Nevada control share statute is that the acquiring person, and those acting in association with the acquiring person, will obtain only such voting rights in the control shares as are conferred by a resolution of the stockholders at an annual or special meeting of the stockholders. The Nevada control share law, if applicable, could have the effect of discouraging takeovers of our company based on our organizational structure.
We are subject to compliance with multiple tax jurisdictions.
As we transact out of both the UK and United States, we must comply with tax filing requirements in both jurisdictions.
| 26 |
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
ITEM 2. PROPERTIES.
We have registered corporate offices in the U.S. at 57 West 57th Street, Manhattan, NY 10019. We have offices and laboratories located across two locations on the Loughborough University Science and Enterprise Park (LUSEP), Loughborough, Leicestershire, United Kingdom. The aggregate monthly rent is approximately $34,000. All leases are currently operated on rolling 12-month terms. The terms of the different leases provide break options allowing both landlord and tenant to terminate on provision of not less than one month’s prior written notice.
ITEM 3. LEGAL PROCEEDINGS.
We do not know of any material, active, pending or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material proceeding or pending litigation.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable
| 27 |
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common stock is traded on the NASDAQ Capital Market under the trading symbol, “NMRD”. On June 28, 2022, the closing price for our common stock as reported on the NASDAQ Capital Market was $2.61.
As of June 28, 2022, we had 83 holders on record of our common stock. The number of record holders does not include beneficial owners of common stock whose shares are held in the names of banks, brokers, nominees or other fiduciaries.
Dividends
Since incorporation, we have not paid any dividend on any class of equity securities. We anticipate that for the foreseeable future all earnings will be retained for use in our business and no cash dividends will be paid to stockholders. Any payment of cash dividends in the future on the Company’s common stock or preferred stock, will be dependent upon our financial condition, results of operations, current and anticipated cash requirements, plans for expansion, as well as other factors that the Board of Directors deems relevant. The ability to pay dividends will be reliant on the ability of DDL, the UK trading entity, to pay dividends to the Company and satisfying the capital maintenance requirements of UK company’s legislation in line with statutory and company law.
Securities Authorized for Issuance Under Equity Compensation Plans
We approved the adoption of an employee equity compensation plan at our Annual General Meeting (“AGM”) on May 15, 2020. No awards have been made to date.
Unregistered Sales of Securities
None.
Purchases of Equity Securities by the Registrant and Affiliated Purchasers
We have not repurchased any shares of our common stock during the fiscal year ended March 31, 2022.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the notes to those statements included elsewhere in this Annual Report on Form 10-K. In addition to historical financial information, this discussion and analysis contains forward-looking statements that reflect our plans, estimates and beliefs. You should not place undue reliance on these forward-looking statements, which involve risks and uncertainties. As a result of many factors, including but not limited to those set forth under ‘‘Risk Factors,’’ our actual results may differ materially from those anticipated in these forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements.”.
Overview
Business Review and Outlook
It is management’s view that the Company has made good progress during the fiscal year ended March 31, 2022, with December 2021 marking a significant milestone in the Company’s evolutionary journey as the first commercial deliveries of our sugarBEAT® non-invasive glucose monitor (“CGM”) were made to the UK licensee, MySugarWatch Limited (“MSW”). It is expected that MSW will sell the CGM under the brand MySugarWatch® and MSW has developed a subscription-based diabetes coaching and management service that will be provided alongside the CGM, primarily targeting those with type 2 diabetes. A key priority of the Company’s being to provide ongoing support to MSW as it commences its own launch plan.
Furthermore, on September 24, 2021, the Company entered into a License, Supply and Distribution Agreement with ‘MySugarWatch DuoPack Limited’ (“MSW-DP”), a sister company of MSW, whereby MSW-DP will provide CGM sensors free of charge with certain medications that are widely prescribed to persons with Type 2 diabetes. These medications are due to come off patent in the fourth calendar quarter of 2022 in Europe and the UK, and 2023 in the USA. The agreed sale price of sensors to MSW-DP under the terms of the agreement is $20 per box of 5 sensors for the USA market, and in Europe and the UK 12.50 Euros in the first 12 months from product launch and 10 Euros thereafter per box of 5 sensors. Nemaura’s anticipated cost of goods per sensor on large-scale production is $1 per sensor. As of January 2022, there were over 2 million prescriptions written for these medications each month in the combined key EU and UK territories. The company believes this will provide an opportunity for rapid market penetration in the use of its CGM sensors, at a scale that can enable the targeted lower cost of goods to be achieved and thereby support both revenue and margin growth into the future.
| 28 |
Management is now focused on fulfilling the remainder of the UK licensees’ initial orders and supporting MSW’s UK launch, while also developing the capabilities of the Company to develop and service new channels of business across other geographic markets via the use of our BEAT platform. To this end the company is now actively planning product launch in other territories that accept the CE mark registration. In addition, the company is seeking to exploit its product platform in the consumer space. All of these avenues are expected to strengthen revenue generation in future periods.
In line with this view, the Company has taken the following actions during the fiscal year ended March 31, 2022
- Entered into a new leased facility to provide the additional space requirements for commercial product assembly.
- Increased headcount of production operatives; this will be phased in line with the volume forecasts currently available, however the Company has also factored in an ability to scale further and faster should this be required.
- Moved forward with placing phased orders for raw materials to ensure future product availability to support both our UK Licensee while also providing for capacity to flex up further as other routes to market materialize in line with management’s commercialization program.
- Engaged with external third party manufacturers with the ability to provide significant scale up services for product manufacture moving forward.
Affiliated Company Relationships
Pharma was incorporated in November 2005. Through October 2013, all technology development and related transactions were incurred by Pharma. As new technology platforms were invented and developed, additional companies were set up to contain these new technology platforms, and to aid in the process of raising further investments to progress the development of these subsequent technologies. However, due to the small size of the operations, low number of employees and laboratory and office space required, initially, certain costs were borne by Pharma and charged to DDL as required. On April 4, 2018, a service agreement was put into place between Pharma and DDL which covered the development of sugarBEAT® under Pharma’s ISO13485 Accreditation. In lieu of these services, Pharma invoices DDL on a periodic basis for said services. Services are provided at cost plus a service surcharge amounting to less than 10% of the total costs incurred. This agreement includes all aspects of the development, registration and manufacture of sugarBEAT®.
Full legal title and beneficial ownership of the CE mark and all related intellectual property remains with Nemaura Medical under the terms of the service contract.
Dr. D.F.H. Chowdhury and Mr. Bashir Timol are officers of Pharma. The current management at DDL, including Dr. D. F. H. Chowdhury allocate 15% - 20% of their time to oversee the current operations at Pharma and will in due course implement a new management team in Pharma, and provide ongoing support in an advisory role. Pharma is a drug delivery company, which means that its activities are entirely related to the administration of drugs to the body of a human or animal subject. DDL is a diagnostic company, which means it is entirely focused on extracting molecules from the human or animal subject and analyzing it to make a diagnosis or to monitor the level of a particular molecule such as glucose. These are two independent businesses engaged in different activities, therefore there is no conflict of interest between the two and management does not see any conflicts arising from the allocations of some of DDL management time to overseeing the operations of Pharma.
Payments made solely for work that Dr. D. F. H. Chowdhury performs for Pharma in his capacity as manager are not charged to Nemaura Medical Inc. and are not included in our consolidated financial statements.
RESULTS OF OPERATIONS
Management’s plans and basis of presentation
The Company has experienced recurring losses and negative cash flows from operations. On March 31, 2022, the Company had cash balances of $17,749,233, total stockholders’ equity of $466,804 and an accumulated deficit of $37,731,476. To date, the Company has in large part relied on equity financing to fund its operations. Initially additional funding also came from related party contributions. The Company expects to continue to incur losses from operations for the near-term and these losses could be significant as the Company implements and scales its product commercialization strategy.
Management’s strategic assessment includes the following potential options:
| – | support the UK and EU launch of sugarBEAT®; |
| – | obtaining further regulatory approval for the sugarBEAT® device in other countries such as the U.S.; |
| – | exploring licensing and partnership opportunities in other territories; |
| – | developing the sugarBEAT® device for commercialization for other applications; and |
| – | considering whether additional future capital raises can further enhance and accelerate the delivery of the Company’s strategic growth objectives. |
| 29 |
Results of Operations
Fiscal Year Ended March 31, 2022 Compared to Fiscal Year Ended March 31, 2021
Revenue
December 2021 marked a pivotal milestone for the Company as the Company commenced deliveries of sugarBEAT® to MSW pursuant to the initial order placed in April 2021. These deliveries continued in line with the schedule agreed with MSW during the remainder of the current fiscal year.
While the majority of the $503,906 revenue recognized related to the delivery of goods, a proportion also related to the recognition of the GBP 1 million (approximately $1.32 million), that was previously received and held within deferred revenue, relating to the exclusive Marketing Rights Agreement that was signed with MSW. We expect to record the remainder of the revenue over an approximately 10-year term from the date sales to MSW commenced.
There was no revenue recognized in the year ended March 31, 2021.
Research and Development Expenses
Research and development expenses were $1,556,988 and $1,554,603 for the fiscal years ended March 31, 2022 and 2021, respectively. The stabilization in costs here being established as the Company’s historically more significant research and development expenditure relating to clinical trials and improvements made to the sugarBEAT® device started to flatten out over the year. We expect that the sugarBEAT® related research and development expenses will reduce in future periods as the product is launched, however the Company expects to continue to incur research and development costs to both enhance, refine and extend the platform capabilities for alternative applications.
General and Administrative Expenses
General and administrative expenses were $6,173,049 and $3,032,138 for the fiscal years ended March 31, 2022 and 2021, respectively. These consisted of fees for legal, professional, consultancy, audit services, investor relations, insurance, advertising and general and operational wages. The increase in expenses being driven predominantly by increased wages, as additional headcount has been added to support the operational scale up process across both our UK and U.S. teams. Increases have also been seen in insurance, rent and depreciation and amortization, which are considered to be directly related to the commercialization steps undertaken during the period. In addition to this, a non-cash item charge of $440,196 was booked as a result of the mark-to-market impact from the revaluation of the foreign currency forward contracts in place as of the fiscal period end, along with a further non-cash charge of $133,529 in relation to the fair value of share options issued to directors.
As the Company continues to scale up to service its existing order book, it is expected that general and administrative expenses will continue to exhibit a similar higher cost profile moving forward, as the business continues to transition to an operationally focused base that is expected to result in increased functional expenses relating to production, sales, marketing, customer service, as well as enhancements to other existing functions.
Other Comprehensive Income
For the fiscal years ended March 31, 2022 and 2021 other comprehensive income saw a charge of $257,885 versus a credit of $472,559, respectively, arising from foreign currency translation adjustments.
Liquidity and Capital Resources
We have experienced net losses and negative cash flows from operations since our inception. We have sustained cumulative losses of $37,731,476 through March 31, 2022. We have historically financed our operations through a combination of debt and equity funding. During the fiscal year ended March 31, 2022, warrants to purchase 366,892 shares of common stock were exercised and provided $2,963,658 of additional funding. Additionally, 397,524 shares of common stock were issued under the ATM facility, which provided $1,568,027 of additional funds, net of costs of $50,765, and a further 375,000 shares of common stock were issued to generate further funding of $1,500,000.
At March 31, 2022, the Company had net working capital of ($494,444) which included cash balances of $17,749,233 and notes payable of $19,188,724. The Company reported a net loss of $13,886,805 for the fiscal year ended March 31, 2022, which included interest expense of $6,666,630.
| 30 |
Having reviewed the company’s forward looking cashflow requirements in relation to the cash balance held at March 31, 2022, management is aware of the need to raise additional funds in order to finance the ongoing commercialization of sugarBEAT®. The Company had $17,749,233 of cash at March 31, 2022, which management consider to be more than sufficient to fund the ongoing operational expenses of the business, however the terms of the existing debt held on balance sheet will fall due for repayment as of February 2023, which will trigger a requirement to either restructure the debt or obtain additional, new, funding.
In evaluating the going concern position of the company, management have considered the ability of the company to raise additional funding in combination with one or more of the different funding options available to it at this time. Based on current and ongoing engagement with potential funding providers, Management believe that there is a reasonable expectation that funding could be provided by one, or more, of the following options:
| · | Equity funding – the company has immediate access to funds through the ATM facility that is currently in place; in addition to this, there are various alternative mechanisms available to the company similar to those used previously e.g. direct sale of shares to interested third parties, similar to the stake sold to Tiger Trading Partners L.L.C. in February 2022, as well as other mechanisms to sell common stock via an underwritten agreement or the further exercise of warrants by the current warrant holders etc. |
| · | Debt funding – the company continues to be in ongoing discussions with third party debt providers, including the incumbent, to enable the existing debt facility to be restructured or renewed, should management feel that this route offers a more attractive option compared to the sale of equity that is dependent on the current market conditions. |
| · | Alternative funding as used in the past such as the sale of licenses. As product development is now at a significant more advanced stage then it was, it is management’s belief that the sufficient funding could be provided through the sale of licenses in a similar way to the UK license agreement sale that help provided early-stage development funding. |
As a consequence of this funding requirement being triggered without the funding bridge have been put in place by the date of filing these consolidated financial statements, ASC 205-40 requires that management recognize and disclose this point as an event which creates a substantial doubt as the Company’s ability to continue as a going concern for at least one year from the date of filing of these consolidate financial statements.
Cash Flows
Net cash used by our operating activities for the fiscal year ended March 31, 2022, was $6,504,041 which reflected the following key cashflow movements: a net loss of $13,886,805 which included non-cash items booked as an expense relating to the accretion of the debt discount ($6,666,630), mark-to-market valuation of the foreign currency forward contracts that were held at fiscal year-end ($440,196), stock-based compensation paid to an employee combined the fair value of options issued to directors ($220,917), and depreciation and amortization ($229,810).
Cashflows were also impacted by increases in inventory of $637,149, and accounts receivable – related parties of $250,092 relating to the acquisition of raw materials to support manufacture and delivery of product to our UK Licensee; the increase in inventory being largely offset by the decrease in prepaid expenses of $519,346 vs the prior year end which reflected upfront payments for inventory at that time.
Working capital cashflow was also impacted by a decrease in accounts payable of $117,384, due to timing of purchases and an increase in other liabilities and accrued expenses of $310,490.
Net cash used by our operating activities for the fiscal year ended March 31, 2021, was $5,998,097 which were driven by cashflow movements relating to the following: A net loss of $6,258,596 which was partially offset by non-cash items booked as an expense relating to the accretion of the debt discount ($2,007,687), stock-based compensation paid to third party suppliers ($113,171), and depreciation and amortization ($98,075). Cashflows were also impacted by an increase in inventory of $564,313, combined with and increase in prepaid expenses of $767,050, and an increase in Accounts receivable – related party, as the Company geared up towards commercialization.
Net cash used in investing activities was $956,482 for the fiscal year ended March 31, 2022, which reflected expenditure on property and equipment to support the commencement of manufacture of product for sale during the year of $481,718, combined with ongoing spend on software development ($391,073) and patent costs ($83,691), to enhance the businesses digital offering and protect the intellectual property developed.
Net cash used in investing activities was $836,440 for the fiscal year ended March 31, 2021, which reflected expenditure for software development costs of $663,758, combined with costs related to patent filings of $81,952, and the purchase of property and equipment of $90,730.
Net cash utilized in financing activities for the fiscal year ended March 31, 2022, was $6,368,315. This includes repayments made in relation to debt funding of $12,400,000, which was partially offset by the proceeds from the issuance of common stock in relation to equity funding was $3,118,792, with associated cash costs of $50,765, combined with the sale of warrants which provided a further $2,963,658 of cash funding.
Net cash provided by financing activities for the fiscal year ended March 31, 2021, was $37,986,392. Proceeds from the issuance of common stock in relation to equity funding was $15,750,672 with associated cash costs of $957,193; the sale of warrants providing a further $400,503. $25,000,000 was provided via the issuance of two notes payable during the year, with associated cash costs incurred of $1,525,035 while repayments made were $600,000. $82,555 of cash expense was incurred in relation to concluding the full repayment of the insurance financing arrangement.
| 31 |
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Contractual Obligations
None.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our audited consolidated financial statements, which have been prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”). The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported results of operations during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from these estimates under different assumptions or conditions. Our significant accounting policies are described in more detail in the notes to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
ITEM 7A. Quantitative and Qualitative Disclosures About Market Risk
Not required for smaller reporting companies.
| 32 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Nemaura Medical Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Nemaura Medical Inc. (the Company) as of March 31, 2022 and 2021, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the years in the two-year period ended March 31, 2022, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt about Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations, current debt due over current cash balances, and has accumulated deficits that raised substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there were no critical audit matters.
/s/
We have served as the Company’s auditor since 2018.
Denver,
June 29, 2022
| F-2 |
NEMAURA MEDICAL INC.
Consolidated Balance Sheets
| As of March 31, | As of March 31, | |||||||
2022 ($) | 2021 ($) | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | ||||||||
| Prepaid expenses and other receivables | ||||||||
| Accounts receivable – related party | ||||||||
| Inventory | ||||||||
| Total current assets | ||||||||
| Other assets: | ||||||||
| Property and equipment, net of accumulated depreciation | ||||||||
| Intangible assets, net of accumulated amortization | ||||||||
| Total other assets | ||||||||
| Total assets | ||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | ||||||||
| Liability due to related parties | ||||||||
| Other liabilities and accrued expenses | ||||||||
| Notes payable, current portion | ||||||||
| Deferred revenue | ||||||||
| Total current liabilities | ||||||||
| Non-current portion of notes payable | ||||||||
| Non-current portion of deferred revenue | ||||||||
| Total liabilities | ||||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity: | ||||||||
| Common stock, par value $ - authorized: shares; issued and outstanding: and as of March 31, 2022 and 2021, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive (loss) income | ( | ) | ||||||
| Total stockholders’ equity | ||||||||
| Total liabilities and stockholders’ equity | ||||||||
See notes to consolidated financial statements.
| F-3 |
NEMAURA MEDICAL INC.
Consolidated Statements of Operations
and Comprehensive Loss
| Years Ended March 31, | ||||||||
2022 | 2021 | |||||||
| Sales | ||||||||
| Cost of Sales | ( | ) | ||||||
| Gross Profit | ||||||||
| Operating expenses: | ||||||||
| Research and development | ||||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Interest expense | ( | ) | ( | ) | ||||
| Loss before income tax benefit | ( | ) | ( | ) | ||||
| Provision for income tax benefit | ||||||||
| Net loss | ( | ) | ( | ) | ||||
| Other comprehensive income: | ||||||||
| Foreign currency translation adjustment | ( | ) | ||||||
| Comprehensive loss | ( | ) | ( | ) | ||||
| Net loss per share, basic and diluted | $ | ( | ) | $ | ( | ) | ||
| Weighted average number of shares outstanding | ||||||||
See notes to consolidated financial statements.
| F-4 |
NEMAURA MEDICAL INC.
Consolidated Statements of Changes in
Stockholders’ Equity
| Common Stock | ||||||||||||||||||||||||
| Shares | Amount ($) | Additional Paid-in Capital ($) | Accumulated Deficit ($) | Accumulated Other Comprehensive Loss ($) | Total Stockholders’ Equity (Deficit) ($) | |||||||||||||||||||
| Balance at March 31, 2020 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||
| Issuance of common shares, net of costs of $ | ||||||||||||||||||||||||
| Exercise of warrants | ||||||||||||||||||||||||
| Restricted shares issued as stock-based compensation | ||||||||||||||||||||||||
| Foreign currency translation adjustment | — | |||||||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||
| Balance at March 31, 2021 | ( | ) | ||||||||||||||||||||||
| Issuance of common shares, net of costs of $ | ||||||||||||||||||||||||
| Exercise of warrants | ||||||||||||||||||||||||
| Restricted shares issued as stock-based compensation | ||||||||||||||||||||||||
| Options issued to directors | — | |||||||||||||||||||||||
| Foreign currency translation adjustment | — | ( | ) | ( | ) | |||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||
| Balance at March 31, 2022 | ( | ) | ( | ) | ||||||||||||||||||||
See notes to consolidated financial statements.
| F-5 |
NEMAURA MEDICAL INC.
Consolidated Statements of Cash Flows
| Year Ended March 31, | ||||||||
2022 | 2021 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | ( | ) | ( | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | ||||||||
| Accretion of debt discount | ||||||||
| Mark-to-market foreign exchange revaluation | ||||||||
| Stock-based compensation | ||||||||
| Changes in assets and liabilities: | ||||||||
| Prepaid expenses and other receivables | ( | ) | ||||||
| Inventory | ( | ) | ( | ) | ||||
| Accounts payable | ( | ) | ( | ) | ||||
| Accounts receivable – related party | ( | ) | ( | ) | ||||
| Other liabilities and accrued expenses | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Capitalized patent costs | ( | ) | ( | ) | ||||
| Purchase of property and equipment | ( | ) | ( | ) | ||||
| Capitalized software development costs | ( | ) | ( | ) | ||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from issuance of common stock | ||||||||
| Costs incurred in relation to equity financing | ( | ) | ( | ) | ||||
| Proceeds from warrant exercise | ||||||||
| Proceeds from issuance of notes payable | ||||||||
| Debt issuance costs paid | ( | ) | ||||||
| Repayments of notes payable | ( | ) | ( | ) | ||||
| Repayments of insurance financing | ( | ) | ||||||
| Net cash (used in) provided by financing activities | ( | ) | ||||||
| Net (decrease) increase in cash | ( | ) | ||||||
| Effect of exchange rate changes on cash | ( | ) | ||||||
| Cash at beginning of year | ||||||||
| Cash at end of year | ||||||||
Supplemental disclosure of non-cash financing activities:
| ||||||||
| Prepayment of equity compensation | $ | |||||||
| Licenses acquired through stock issuance | $ | |||||||
| Monitoring fees added to notes payable | $ | $ | ||||||
See notes to consolidated financial statements.
| F-6 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
NOTE 1 – ORGANIZATION, PRINCIPAL ACTIVITIES AND MANAGEMENT’S PLANS
Nemaura Medical Inc. (“Nemaura” or the “Company”), through its operating subsidiaries, performs medical device research and manufacturing of a continuous glucose monitoring system (“CGM”), named sugarBEAT®. The sugarBEAT® device is a non-invasive, wireless device for use by persons with Type I and Type II diabetes and may also be used to screen pre-diabetic patients. The sugarBEAT® device extracts analytes, such as glucose, to the surface of the skin in a non-invasive manner where it is measured using unique sensors and interpreted using a unique algorithm.
Nemaura is a Nevada holding company organized in 2013. Nemaura owns one hundred percent (100%) of Dermal Diagnostic (Holdings) Limited, an England and Wales corporation (“DDHL”) formed on December 11, 2013, which in turn owns one hundred percent (100%) of Dermal Diagnostics Limited, an England and Wales corporation formed on January 20, 2009 (“DDL”), and one hundred percent (100%) of Trial Clinic Limited, an England and Wales corporation formed on January 12, 2011 (“TCL”).
DDL is a diagnostic medical device company headquartered in Loughborough, Leicestershire, England, and is engaged in the discovery, development and commercialization of diagnostic medical devices. The Company’s initial focus has been on the development of the sugarBEAT® device, which consists of a disposable patch containing a sensor, and a non-disposable miniature transmitter device with a re-chargeable power source, which is designed to enable trending or tracking of blood glucose levels. All of the Company’s operations and assets are located in England.
The following diagram illustrates Nemaura’s corporate structure as of March 31, 2022:

During the fiscal year ended March 31, 2021, the Board of Directors assessed the adequacy of the group’s organizational structure
and concluded that an intermediary holding company, Region Green Limited, was no longer required, as the entity had been effectively dormant
since inception, and no longer represented a requirement to be maintained. It was therefore determined that Region Green Limited should
be unwound, with the assets held by Region Green Limited being transferred up to Nemaura Medical Inc. following which Region Green Limited
would be dissolved.
The transfer of assets took place on March 5, 2021 and Region Green Limited was formally dissolved as of April 23, 2021.
| F-7 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
The Company was incorporated in 2013 and has reported recurring losses from operations to date and an accumulated deficit of $37,731,476 as of March 31, 2022. These operations have resulted in the successful completion of clinical programs to support a CE mark (European Union approval of the product) approval, as well as a De Novo 510(k) medical device application to the U.S. Food and Drug Administration (“FDA”) submission. The Company expects to continue to incur losses from operations until revenues are generated through licensing fees or product sales. However, given the completion of the requisite clinical programs, these losses are expected to decrease over time. Management has entered into licensing, supply, or collaboration agreements with unrelated third parties relating to the United Kingdom (“UK”), Europe, Qatar, and all countries in the Gulf Cooperation Council.
Going Concern
As identified under Item 1A, management is aware of the need to raise additional funds in order to finance the ongoing commercialization of sugarBEAT®. The Company had $17,749,233 of cash at March 31, 2022, which management consider to be more than sufficient to fund the ongoing operational expenses of the business, however the terms of the existing debt held on balance sheet will fall due for repayment as of February 2023, which will trigger a requirement to either restructure the debt or obtain additional, new, funding.
In evaluating the going concern position of the company, management have considered the ability of the company to raise additional funding in combination with one or more of the different funding options available to it at this time. Based on current and ongoing engagement with potential funding providers, Management believe that there is a reasonable expectation that funding could be provided by one, or more, of the following options:
| · | Equity funding – the company has immediate access to funds through the ATM facility that is currently in place; in addition to this, there are various alternative mechanisms available to the company similar to those used previously e.g. direct sale of shares to interested third parties, similar to the stake sold to Tiger Trading Partners L.L.C. in February 2022, as well as other mechanisms to sell common stock via an underwritten agreement or the further exercise of warrants by the current warrant holders etc. |
| · | Debt funding – the company continues to be in ongoing discussions with third party debt providers, including the incumbent, to enable the existing debt facility to be restructured or renewed, should management feel that this route offers a more attractive option compared to the sale of equity that is dependent on the current market conditions. |
| · | Alternative funding as used in the past such as the sale of licenses. As product development is now at a significant more advanced stage then it was, it is management’s belief that the sufficient funding could be provided through the sale of licenses in a similar way to the UK license agreement sale that help provided early-stage development funding. |
However, as a consequence of this funding requirement being triggered without the funding bridge having been put in place by the filing date of these consolidated financial statements, ASC 205-40 requires that management recognise and disclose this point as an event which creates a substantial doubt as to the Company’s ability to continue as a going concern for at least one year from the date of filing of these consolidate financial statements.
Following the receipt of the CE mark approval in the EU, and in support of our plans for similar certification with the FDA in the U.S., our plan is to utilize the cash on hand to continue establishing commercial manufacturing operations for the commercial supply of the sugarBEAT® device and sensor patches in our target markets.
Management's strategic plans include the following:
| – | support the UK and EU launch of sugarBEAT®; |
| – | obtaining further regulatory approval for the sugarBEAT® device in other countries such as the U.S.; |
| – | exploring licensing and partnership opportunities in other territories; |
| – | developing the sugarBEAT® device platform for commercialization across other applications; and |
| – | pursue additional capital raising opportunities as and when required to further enhance our growth plans. |
NOTE 2 – BASIS OF PRESENTATION
The accompanying consolidated financial statements include the accounts of the Company and the Company’s subsidiaries, DDL, TCL, DDHL and RGL. The consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”), and all significant intercompany balances and transactions have been eliminated in consolidation.
The functional currency for the majority of the Company’s operations is the Great Britain Pound Sterling (“GBP”), and the reporting currency is the U.S. Dollar (“U.S.$”).
| F-8 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND RECENT ACCOUNTING PRONOUNCEMENTS
Revenue recognition
The Company has considered the guidelines within the Financial Accounting Standards Board’s (the “FASB”) Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers as a requirement of the revenue recognition that it commenced during the current fiscal year. This standard applies to all contracts with customers, except for contracts that are within the scope of other standards, such as leases, insurance, collaboration arrangements and financial instruments. Under ASC Topic 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services.
To determine revenue recognition for arrangements that an entity determines are within the scope of ASC Topic 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation.
The Company only applies the five-step model to contracts when it is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. At contract inception, once the contract is determined to be within the scope of ASC Topic 606, the Company assesses the goods or services promised within each contract and determines those that are performance obligations and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
The Company may enter into product development and other agreements with collaborative partners. The terms of the agreements may include non-refundable signing and licensing fees, milestone payments and royalties on any product sales derived from collaborations.
Deferred revenue
The Company has entered into license agreements and recognizes up front license payments as revenue upon delivery of the license only if the license has stand-alone value to the customer. However, where further performance criteria must be met, revenue is deferred and recognized on a basis that is considered to be appropriate to the conditions associated with the license and over the period the Company is expected to complete these performance obligations.
Royalty revenue will be recognized upon the sale of the related products provided the Company has no remaining performance obligations under the agreement.
Research and development expenses
The Company charges research and development expenses to operations as incurred. Research and development expenses primarily consist of salaries and related expenses for personnel and outside contractor and consulting services. Other research and development expenses include the costs of materials and supplies used in research and development, prototype manufacturing, clinical studies, related information technology and an allocation of facilities costs.
Cash
Cash consists primarily of cash deposits maintained in the UK.
Fair value of financial instruments
In accordance with ASC 820, “Fair Value Measurements and Disclosures,” the Company determines the fair value of financial instruments with the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1: Applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2: Applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3: Applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
| F-9 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
Property and equipment
Property and equipment is stated at cost and depreciated using the straight-line method over the estimated useful lives of the assets, generally four to five years. This is charged to operating expenses.
Intangible assets
Intangible assets consist of licenses and
patents associated primarily with the sugarBEAT® device and are amortized on a straight-line basis, generally over their legal lives
of up to 20 years and are reviewed for impairment. Costs capitalized relate to invoices received from third parties and not any internal
costs. The Company evaluates its intangible assets (all have finite lives) and other long-lived assets for impairment whenever events
or circumstances indicate that they may not be recoverable, or at least annually. Recoverability of finite and other long-lived assets
is measured by comparing the carrying amount of an asset group to the future undiscounted net cash flows expected to be generated by that
asset group. The Company groups assets for purposes of such review at the lowest level for which identifiable cash flows of the asset
group are largely independent of the cash flows of the other groups of assets and liabilities. The amount of impairment to be recognized
for finite and other long-lived assets is calculated as the difference between the carrying value and the fair value of the asset group,
generally measured by discounting estimated future cash flows. There were
Software development costs
Capitalization of software development costs incurred in the research and development of new software products and enhancements to existing software products for external use begins when a product’s technological feasibility has been established and ends when the resulting product is available for general market release. Amortization of the capitalized software is classified within product cost of goods sold in the consolidated statements of operations and comprehensive loss.
For each capitalized software product, the annual amortization is equal to the greater of:
1. The amount computed using the ratio of software product’s current fiscal year gross revenue bears to the total current fiscal year and anticipated future gross revenues for the product, or
2. The amount computed based on a straight-line method over the remaining estimated economic life of the product, which can be a range between 3 – 8 years.
Annually, or more frequently if required by triggering events, an analysis of the net realizable value of the capitalized software is completed and the amount by which unamortized software costs exceeds the net realisable value, if any, is recognized as a charge to income in the period it is determined.
Inventory
Inventory is stated at the lower of cost or net realizable value, with cost determined on a first-in first-out basis. At present all inventory relates to raw materials purchased from third parties and to be used in the Company’s product.
Income taxes
Income taxes are accounted for under the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating loss carry forwards. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided to reduce the carrying amount of deferred income tax assets if it is considered more likely than not that some portion, or all, of the deferred income tax assets will not be realized.
The Company recognizes the effect of income tax positions only
if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that
is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in
judgment occurs. The Company has elected to classify interest and penalties related to unrecognized tax benefits as part of income tax
expense in the consolidated statements of comprehensive loss. The Company does
In December 2017, the U.S. Tax Cuts and
Jobs Act was signed into law. Generally, this Act reduces corporate rates from a top rate of
| F-10 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
Basic earnings (loss) per share is computed by dividing income (loss) available to common stockholders by the weighted-average number of common shares outstanding during the period. For the fiscal year ended March 31, 2022, warrants to purchase shares of common stock, options to purchase shares of common stock and a unit purchase option to purchase shares of common stock as well as warrants were considered anti-dilutive and were excluded from the calculation of diluted loss per share. For the fiscal year ended March 31, 2021, warrants to purchase shares of common stock and a unit purchase option to purchase shares of common stock as well as warrants were considered anti-dilutive and were excluded from the calculation of diluted loss per share.
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Actual results may differ from those estimates.
Foreign currency translation
The functional currency of the Company is the GBP, while the reporting currency is the U.S.$. Assets and liabilities are translated at the exchange rates as of the balance sheet date with income and expenses being translated at the weighted-average exchange rates prevailing during the reporting period. Stockholders' equity is translated into U.S.$ from GBP at historical exchange rates.
Adjustments resulting from translating the consolidated financial statements into U.S.$ are recorded as a separate component of accumulated other comprehensive loss in stockholders’ equity.
Derivative Financial Instruments
Derivative financial instruments are used as part of the overall strategy to manage exposure to foreign currency primarily associated with fluctuations in foreign currency exchange rates. Derivative financial instruments are included in the consolidated balance sheets and are measured at fair value on a recurring basis.
The Company is exposed to the impact of foreign currency exchange
fluctuations as a significant proportion of our expenses are incurred within our UK subsidiary which is denominated GBP, with the remaining
portion denominated in USD and a small amount in Euros (“EUR”). In addition to this, we hold the majority of our cash in
USD, with amounts also held in GBP and, to a much smaller amount, in EURs. The Company’s objective is to reduce the volatility
associated with these foreign exchange rate changes to allow management to focus our attention on our core business strategy and objectives.
Accordingly, the Company entered into a target accrual redemption forward (“TARF”) agreement to sell USD and buy GBP across
25 defined monthly fixings in order to fix the costs associated with the foreign currency exchange fluctuations associated with our GBP
denominated expenses. These fixings allow for $
At March 31, 2022, the Company held a forward contract to sell
up to $ million, which when remeasured at fair value generated a non-cash item loss of $
The Company’s foreign currency forward contracts are measured at fair value on a recurring basis and are classified as Level 2 under our fair value of financial instruments policy.
Retirement benefit plan
The Company operates a retirement plan which covers most of our regular employees in the UK and allows them to make contributions. The Company also provides a matching contribution on a portion of the employee contributions. Total expenses incurred under this plan for the fiscal years ended March 31, 2022 and 2021, were approximately $ and $, respectively. The increase in the fiscal year ended March 31, 2022 was driven by the increase in our employee numbers.
| F-11 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
Stock-based compensation
The Company accounts for stock-based payments in accordance with stock-based payment accounting guidance which requires all stock-based payments to be recognized based upon their fair values. The fair value of stock-based awards is estimated at the grant date using the Black-Scholes Option Pricing Model and the portion that is ultimately expected to vest is recognized as compensation cost over the requisite service period. The determination of fair value using the Black-Scholes Option Pricing Model is affected by the Company’s stock price as well as assumptions regarding a number of complex and subjective variables, including expected stock price volatility, risk-free interest rate, expected dividends and projected employee stock option exercise behaviors. The Company accounts for forfeitures of unvested awards as they occur.
The Company accounts for share-based payments to non-employees by aligning it with the accounting for share-based payments to employees, with certain exceptions.
Direct costs incurred for equity financing
The Company includes all direct costs incurred in connection with successful equity financings as a component of additional paid-in capital. Direct costs incurred for equity financings that are unsuccessful are expensed.
Risks and Uncertainties
The Company is in the commercialization stage for its primary product, sugarBEAT®, following the receipt of the CE mark covering the EU, with the intention of entering into sales and marketing agreements for the product with prioritization having been initially set for the UK and Germany.
Aside from the UK and Germany, the Company considers the U.S.A. to be a primary market for its product offerings, and while uncertainties exist with regards to regulatory acceptance of the Company’s primary product, an FDA PMA application has been submitted and is currently being reviewed; some delays have been experienced as a direct consequence of COVID-19, whereby the application remained dormant with the FDA for a period of 6 months. In the interim, and further to discussions with the FDA, the Company has determined that it may sell an adapted version of the CGM device as a wellbeing device, whereby the Company will gather the data and provide feedback in the form of educational reports providing insights into factors that may be causing glucose fluctuations and therefore how lifestyle interventions may help improve control of the fluctuations.
The Company has taken steps to support the commercialization process by increasing raw material inventory over the year in order to support the transition to product manufacturing in relation to sale to the UK Licensee.
The Company is also in the process of establishing options to broaden the existing internal manufacturing capabilities with the expectation that it will leverage the manufacturing capacity and capabilities of one or more contract manufacturers as volume increases.
Recent accounting pronouncements
The Company continually assesses any new accounting pronouncements to determine their applicability. When it is determined that a new accounting pronouncement affects the Company's financial reporting, the Company undertakes a study to determine the consequences of the change to its consolidated financial statements and assures that there are proper controls in place to ascertain that the Company's consolidated financial statements properly reflect the change.
NOTE 4 – LICENSING AGREEMENTS
United Kingdom and the Republic of Ireland, the Channel Islands and the Isle of Man
In March 2014, the Company entered into an Exclusive Marketing
Rights Agreement with an unrelated third party that granted to the third party the exclusive right to market and promote the sugarBEAT®
device and related patches under its own brand in the United Kingdom and the Republic of Ireland, the Channel Islands and the Isle of
Man. The Company received a non-refundable, up-front cash payment of GBP 1,000,000 (approximately $
As the Company has continuing performance
obligations under the agreement, the up-front fees received from this agreement have been deferred with the expectation that this deferred
revenue would be treated income over the term of the commercial licensing agreement beginning from the date of clinical evaluation approval.
The Company received this confirmation during the current fiscal year, along with an initial order against which deliveries commenced
in December 2021. At March 31, 2022, approximately $
| F-12 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
NOTE 5 – PROPERTY AND EQUIPMENT
As of March 31, 2022 and 2021, property and equipment is summarized as follows:
| March 31, | ||||||||
2022 | 2021 | |||||||
| Property and equipment | ||||||||
| Less accumulated depreciation | ( | ) | ( | ) | ||||
Depreciation expensed within the consolidated statements of
operations and comprehensive loss relating to property and equipment for the years ended March 31, 2022 and 2021 was approximately $
NOTE 6 - INTANGIBLE ASSETS
The following table summarises our intangible assets and capitalized software development costs at March 31, 2022 and 2021:
| March 31, | ||||||||
2022 | 2021 ($) | |||||||
| Patents and licenses | ||||||||
| Less accumulated amortization | ( | ) | ( | ) | ||||
| Software development costs | ||||||||
Amortization expensed within the consolidated statements of operations
and comprehensive loss relating to intangible assets for the years ended March 31, 2022 and 2021 was approximately $
Assuming a constant currency, the following table represents the estimated amortization for intangible assets relating to patents and licenses for the years ending March 31; no amortization has been estimated for software development as this is considered to be work-in-progress and the final costs are yet to be determined:
| ($) | ||||||
| 2023 | ||||||
| 2024 | ||||||
| 2025 | ||||||
| 2026 | ||||||
| 2027 | ||||||
| Thereafter | ||||||
| Total future net intangible amortization expense | ||||||
NOTE 7 – PREPAID EXPENSES
| March 31, | ||||||||
2022 | 2021 | |||||||
| Prepaid expenses | ||||||||
| Prepaid inventory | ||||||||
| Other taxes | ||||||||
| F-13 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
NOTE 8 – NOTES PAYABLE
NOTE PURCHASE AGREEMENT 1
On April 15, 2020, the Company entered into a note purchase agreement (the “Note Purchase Agreement 1”) by and among the Company, DDL, TCL and a third-party investor (the “Investor”).
Pursuant to the terms of the Note Purchase Agreement, the Company
agreed to issue and sell to the Investor and the Investor agreed to purchase from the Company a secured promissory note (the “Secured
Note”) in the original principal amount of $
The Secured Note is secured by the Collateral (as hereinafter
defined). The Secured Note carries an original issue discount (“OID”) of $
The borrowing period is
Security Agreement
On April 15, 2020, the Company entered into the Security Agreement by the Company, DDL and TCL, in favor of the Investor (the “Security Agreement”). Pursuant to the terms of the Security Agreement, the Company granted the Investor a first-priority security interest in all rights, title, interest, claims and demands of the Company in and to all of the Company’s patents and all other proprietary rights, and all rights corresponding to the Company’s patents throughout the world, now owned and existing, and all replacements, proceeds, products, and accessions thereof.
NOTE PURCHASE AGREEMENT 2
On February 8, 2021, the Company
entered into an additional note purchase agreement (“Note Purchase Agreement 2”) with the Investor. Pursuant to the
terms of Note Purchase Agreement 2, the Company agreed to issue and sell to the Investor and the Investor agreed to purchase from the
Company, a secured promissory note (“Secured Note 2”) in the original principal amount of $
In consideration thereof, on
February 9, 2021 (the “closing date”), (i) the Investor paid $
The borrowing terms for Note
Purchase Agreement 2 are consistent with those of Note Purchase Agreement 1, with the borrowing period being
| F-14 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
Security Agreement
On February 8, 2021, the Security Agreement established in respect to Note Purchase Agreement 1 was extended to include Note Purchase Agreement 2, which is also secured against all of the Company’s assets owned as of the closing date and extends to any assets acquired at any time that the Company’s obligations under Secured Note 2 are outstanding.
As of March 31, 2022, all outstanding debt in relation to the Note Purchase Agreements is due for repayment within the next 12 months.
NOTE 9 – RELATED PARTY TRANSACTIONS
Nemaura Pharma Limited (“Pharma”), Black and White Health Care Limited (“B&W”) and NDM Technologies Limited (“NDM”) are entities controlled by the Company’s chief executive officer and majority shareholder, D.F.H. Chowdhury.
Pharma has a service agreement with DDL, to undertake development, manufacture, and regulatory approvals under Pharma’s ISO13485 Accreditation. In lieu of these services, Pharma invoices DDL on a periodic basis for said services. Services are provided at cost plus a service surcharge amounting to less than 10% of the total costs incurred.
The following is a summary of activity between the Company and Pharma, B&W and NDM for the years ended March 31, 2022 and 2021:
| March 31, | ||||||||
2022 | 2021 | |||||||
| Liability due to related parties at beginning of year | ||||||||
| Amounts invoiced by Pharma to DDL, NM and TCL (1) | ||||||||
| Amounts invoiced by DDL to Pharma | ( | ) | ( | ) | ||||
| Amounts repaid by DDL to Pharma | ( | ) | ( | ) | ||||
| Foreign exchange differences | ( | ) | ||||||
| (Receivable)/Liability due (from) to related parties at end of year | ( | ) | ||||||
| (1) |
All related party transactions relate to operating activities in the years ended March 31, 2022 and 2021.
NOTE 10 – INCOME TAXES
The Company and its subsidiaries file separate income tax returns.
United States of America
The Company is incorporated in the U.S. and is subject to a
U.S. federal corporate income tax rate of
British Virgin Islands
RGL was incorporated in the British Virgin Islands (“BVI”). Under the current laws of the BVI, RGL was not subject to tax on income or capital gains. In addition, upon payments of dividends by RGL, no BVI withholding tax was imposed. During the years ended March 31, 2022 and 2021, there were no income or expenses in the BVI; RGL was formally dissolved as of April 23, 2021.
UK
DDL, TCL and DDHL are all incorporated in
the UK and the applicable UK statutory income tax rate for these companies is
| F-15 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
For the fiscal years ended March 31, 2022 and 2021 loss before income tax benefit arose in the UK and U.S. as follows:
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| $ | $ | |||||||
| Loss before income taxes arising in UK | ( | ) | ( | ) | ||||
| Loss before income taxes arising in U.S. | ( | ) | ( | ) | ||||
| Total loss before income tax benefit | ( | ) | ( | ) | ||||
Reconciliation of our effective tax rate to the loss calculated at the statutory U.S. federal tax rate is as follows:
| March 31, | ||||||||||||||||
| 2022 | 2021 | |||||||||||||||
| $ | $ | |||||||||||||||
| Loss before income taxes | ( | ) | ( | ) | ||||||||||||
| Expected tax benefit | ( | ) | ( | ( | ) | ( | ||||||||||
| Foreign tax differential | ||||||||||||||||
| Enhanced research and development | ( | ) | ( | ( | ) | ( | ||||||||||
| Prior year true-up of NOL’s | ||||||||||||||||
| Other | ||||||||||||||||
| Change in valuation allowance | ||||||||||||||||
| R&D credit received | ||||||||||||||||
| Actual income tax benefit | ||||||||||||||||
The tax effects of the temporary differences that give rise to significant portions of deferred income tax assets are presented below:
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| $ | $ | |||||||
| Net operating tax loss carried forward | ||||||||
| Research and development enhancement | ||||||||
| Other items | ( | ) | ( | ) | ||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Net deferred tax assets | ||||||||
In the fiscal year ended March 31, 2022, the Company received
$
For each of the fiscal years ended March 31, 2022 and 2021, the Company did not have unrecognized tax benefits, and therefore no interest or penalties related to unrecognized tax benefits were accrued. Management does not expect that the amount of unrecognized tax benefits will change significantly within the next twelve months.
The Company mainly files income tax returns in the U.S. and the UK. The Company is subject to U.S. federal income tax examination by tax authorities for tax years beginning in 2017. The UK tax returns for the Company’s UK subsidiaries are open to examination by the UK tax authorities for the tax years beginning April 1, 2016.
As of March 31, 2022, the Company
has net operating losses (“NOLs”) of approximately $
| F-16 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
NOTE 11 – STOCKHOLDERS’ EQUITY
Shelf Registration Statement
The Company filed a shelf registration
statement on Form S-3 with the SEC, which was declared effective by the SEC on March 24, 2022 (the “2022 Shelf Registration Statement”).
The 2022 Shelf Registration Statement provides the Company with the ability to issue common stock and other securities as described in
the registration statement from time to time up to an aggregate amount of $
Other equity transactions
On July 23, 2021, the Company entered into an At The Market Offering Agreement (the “2021 ATM”) with H.C. Wainwright & Co., LLC (the “Agent”) pursuant to which the Company may offer and sell from time to time to, at its option, up to an aggregate of $100 million of shares of its common stock. Shares sold under the 2021 ATM are issued pursuant to the Company’s 2019 Shelf Registration Statement and a prospectus supplement dated July 23, 2021.
The Company is required to pay the Agent a commission of 3%
of the gross proceeds from the sale of shares and has also agreed to provide the Agent with customary indemnification rights. During
the year ended March 31, 2022, the Company issued and sold shares
of its common stock at an average price of $ per
share under the 2021 ATM for aggregate net proceeds of $
During the fourth quarter of the fiscal year ended March 31,
2022, the Company was approached by Tiger Management L.L.C. (a vehicle for the family office of Julian H. Robertson) with a view to acquiring
a direct stake in the Company. The Company agreed to sell shares to Tiger Trading Partners L.L.C. (an affiliate undertaking)
at a price of $ per share and gross proceeds of $
The Company commenced an offering of up to $
On December 18, 2018, the Company issued a unit purchase option,
to purchase shares and warrants, to Dawson James Securities, Inc. The Company has classified this option as equity. The unit
purchase option has an exercise price of $
On December 20, 2018, the Company sold units, with
each unit consisting of one share of common stock and one
On July 30, 2020, the Company sold shares of the
Company’s common stock and warrants to purchase up to 793,103 shares of common stock. Each share of common stock and accompanying
one-half of a warrant were sold for a combined purchase price of $7.25, for gross proceeds of $
| F-17 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
Stock options
On January 28, 2022, the Board of Directors granted to each of the directors, an option to purchase shares of common stock at an exercise price of $ per share, being the closing price of the Company’s common stock on the date of grant. The option was fully vested at grant and is exercisable for a period of five years from the date of grant.
The following table provides a summary of the Options Award activity is presented below:
| Number of Options | Weighted Average Exercise Price $ | Weight Average remaining Contractual Term (years) | ||||||||||
| Balance at April 1, 2021 | ||||||||||||
| Granted | ||||||||||||
| Exercised | ||||||||||||
| Forfeited | ||||||||||||
| Expired | ||||||||||||
| Balance at March 31, 2022 | ||||||||||||
| Vested and exercisable at March 31, 2022 | ||||||||||||
The fair value of stock options granted during the fiscal year ended March 31, 2022 was determined using a Black-Scholes Option Pricing Model (there were no options granted as at April 1, 2021). The key assumptions for which have been set-out below:
| Stock Price | $ | |||
| Exercise Price | $ | |||
| Term | years | |||
| Volatility | % | |||
| Expected dividend yield (%) | ||||
| Discount Rate (Bond Equivalent Yield) | % |
NOTE 12 – OTHER ITEMS
(a) COVID-19 Pandemic
The outbreak of COVID-19 originating in Wuhan, China, in December 2019 has since rapidly increased its exposure globally. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. We continue to monitor the global outbreak of COVID-19 and are working with our employees, suppliers and other stakeholders to mitigate the risks posed by its spread, COVID-19 is not expected to have any long-term detrimental effect on the Company’s success. While key suppliers have not been accessible throughout the whole period of the outbreak, we have, to date, been able to be flexible in our priorities and respond favorably to the challenges faced during the outbreak. We have also seen a surge in the uptake of technologies for remote and patient self-monitoring, which therefore potentially enhances the prospects for the likes of the Company and its CGM product and planned digital healthcare offering.
| F-18 |
NEMAURA MEDICAL INC.
Notes to Consolidated Financial Statements
Whilst restrictions associated with COVID-19 have largely been removed in our operational locations, we will continue to assess the situation, including abiding by any government-imposed restrictions, as and where relevant.
(b) Investor relations agreements
The Company has entered into contracts with several investor relations specialists to help support the ongoing financing activities of the business.
During the fiscal year ended March 31, 2022, the Company extended the contractual agreement that it had entered into in the year ended March 31, 2021, into a rolling monthly agreement, compensation for which was settled in cash. Stock-based compensation of $ was expensed during the current year end, all of which related to the previous agreement terms.
During the fiscal year ended March 31, 2021, the Company entered
into a contractual agreement with a new investor relations company, the term of which was set at 12 months with the related compensation
being paid via a mixture of cash and common stock. Total stock-based compensation expense for the year ended March 31, 2021, in relation
to this was $. In addition to this, $
NOTE 13 – Subsequent Events
At The Market Offering
The At The Market Offering Agreement, or the sales agreement,
that was entered into with H.C. Wainwright & Co., LLC, or the sales agent or Wainwright, dated as of July 23, 2021 was amended as
of April 1, 2022, relating to the offer and sale of shares of our common stock. In accordance with the terms of the sales agreement,
we may offer and sell up to a maximum aggregate amount of $
Note Purchase Agreement
On May 20, 2022, the Company entered into a new note purchase agreement (“Note Purchase Agreement 3”) by and among the Company, DDL, TCL and a third-party investor.
Pursuant to the terms of the Note Purchase Agreement 3, the
Company agreed to issue and sell to the Investor and the Investor agreed to purchase from the Company a secured promissory note (the
“Secured Note”) in the original principal amount of $
The Secured Note is secured by the Collateral (as hereinafter
defined). The Secured Note carries an original issue discount (“OID”) of $
The borrowing period is
Security Agreement
On May 20, 2022, the Company entered into the Security Agreement by the Company, DDL and TCL, in favor of the Investor (the “Security Agreement”). Pursuant to the terms of the Security Agreement, the Company granted the Investor a first-priority security interest in all rights, title, interest, claims and demands of the Company in and to all of the Company’s patents and all other proprietary rights, and all rights corresponding to the Company’s patents throughout the world, now owned and existing, and all replacements, proceeds, products, and accessions thereof.
| F-19 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
We have established disclosure controls and procedures to ensure that the information required to be disclosed by the Company in the reports that it files or submits under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission and that such information is accumulated and communicated to the officers who certify the Company's financial reports and to other members of senior management and the Board of Directors as appropriate to allow timely decisions regarding required disclosure.
The Company’s Chief Executive Officer and Chief Financial Officer have evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of March 31, 2022. Based on their evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were effective as of March 31, 2022.
Management's Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. Our internal control over financial reporting is a process designed under the supervision of the Chief Executive Officer and Chief Financial Officer to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our internal control over financial reporting includes those policies and procedures that:
1. Pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of our company;
2. Provide reasonable assurance that the transaction is recorded as necessary to permit preparation of consolidated financial
statements in accordance with accounting principles generally accepted in the United States of America, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
3. Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the consolidated financial statements.
Management, including our Chief Executive Officer and Chief Financial Officer conducted an evaluation, as of March 31, 2022, of the design and effectiveness of our internal control over financial reporting based on the criteria set forth in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation our management concluded that our internal control over financial reporting was effective as of March 31, 2022.
Since we are a smaller reporting company, this Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm on internal control over financial reporting.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Securities and Exchange Act of 1934, as amended) during the quarter ended March 31, 2022, that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| 33 |
ITEM 9B. OTHER INFORMATION.
On May 20, 2022, the Company entered into a new note purchase agreement (“note Purchase Agreement 3”) by and among the Company, DDL, TCL and a third-party investor.
Pursuant to the terms of the Note Purchase Agreement, the Company agreed to issue and sell to the Investor and the Investor agreed to purchase from the Company a secured promissory note (the “Secured Note”) in the original principal amount of $6,015,000. In consideration thereof, on May 20, 2023 (the closing date), (i) the Investor paid $5,000,000 in cash, and (ii) the Company delivered the Secured Note on behalf of the Company, to the Investor, against delivery of the Purchase Price. For these purposes, the “Purchase Price” means the Investor’s initial cash purchase price.
The Secured Note is secured by the Collateral (as hereinafter defined). The Secured Note carries an original issue discount (“OID”) of $1,000,000 (16.7%). In addition, the Company agreed to pay $15,000 to the Investor to cover the Investor’s legal fees, accounting costs, due diligence, monitoring and other transaction costs incurred in connection with the purchase and sale of the Secured Note (the “Transaction Expense Amount”). In addition to this, a payment of $300,000 was made to Ascendiant Capital Markets, LLC, (the “Commission”) for structuring the agreement between both parties. The Purchase Price for the Secured Note is $4,700,000, computed as follows: $6,015,000 original principal balance, less: OID, Transaction Expense Amount, and commission paid.
The borrowing period is 24 months, and the Company shall pay the outstanding balance and all fees on maturity. A monitoring fee equal to 0.833% of the outstanding balance will automatically be added to the outstanding balance on the first day of each month. The debt less the discount and transaction expenses will be accreted over the term of the Note using the effective interest method.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
| 34 |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The following persons are our executive officers and directors, and hold the positions set forth opposite their respective names as of the date hereof.
| Name | Age | Position | Date of Appointment | |||||||
| Dewan Fazlul Hoque Chowdhury | 49 | Chief Executive Officer, President and Director | December 24, 2013 | |||||||
| Justin Mclarney | 50 | Chief Financial Officer | September 15, 2020 | |||||||
| Arash Ghadar | 45 | Chief Operating Officer | January 5, 2022 | |||||||
| Bashir Timol | 47 | Director, Chief Business Officer | December 24, 2013 April 9, 2018 | |||||||
| Thomas Moore | 58 | Independent Director | August 3, 2017 | |||||||
| Dr. Salim Natha | 55 | Independent Director | July 26, 2017 | |||||||
| Timothy Johnson | 38 | Independent Director | July 17, 2017 | |||||||
Our directors hold office until the earlier of their death, resignation, or removal or until their successors have been qualified.
Dewan Fazlul Hoque Chowdhury. Dr. D.F.H. Chowdhury has been our President, Chief Executive Officer and a member of our board of directors since the incorporation of DDL on January 20, 2009. He is in charge of research and development of our core technologies, product development, innovation and commercialization. He also coordinates and oversees legal compliance; development of the company mission; policy and planning. Prior to establishing the Company, Dr. D.F.H. Chowdhury was the founder and CEO of Microneedle Technologies and Nemaura Pharma Limited. Dr. D.F.H. Chowdhury has been responsible for negotiating licensing deals for a transdermal patch to treat Alzheimer’s disease. Additionally, he is involved in commercial negotiations and global strategy development.
Dr. D.F.H. Chowdhury originally trained as a pharmaceutical scientist and has an MSc in Microsystems and Nanotechnology from Cranfield University, and a Doctorate from the University of Oxford on nano-drug delivery. His experience in the Pharmaceutical Industry includes product development; manufacturing; and technical and corporate management.
Justin Mclarney. Mr. Mclarney joined the business as Chief Financial Officer in September 2020, having over 20 years’ experience in corporate and international financial management, accounting, and process development and control. He has a strong track record of driving profitable growth across businesses encompassing ecommerce, retail, logistics and supply chain operations at an international level. Mr. Mclarney has held various Senior Finance & Operational roles, including most recently the position of Senior Director, International Finance at Lands’ End Inc. from January 2016 to June 2020 where he was responsible for all Finance teams across the European and Japanese business units. From February 2007 to September 2015, Mr. Mclarney worked for Office Depot in a range of increasingly senior roles culminating in the Senior Director of Finance for the European Contract business. Prior to this, he spent over 10 years in practice, the final 7 years of which was with Ernst & Young LLP. Before transitioning to become a qualified Chartered Accountant, Mr. Mclarney studied Law and obtained his Legal Practice Certificate.
Arash Ghadar. Dr Ghadar joined the business as Chief Operating Officer on January 5, 2022, prior to joining Nemaura, Dr. Ghadar spent a decade as the Technical Director of Datalink Electronics (Datalink) in Loughborough, England where he managed the design team as an autonomous entity within Datalink. He was responsible for management of the day-to-day operations, business planning, legal affairs, finance, sales, and business development of the design team. In this role, he also oversaw numerous technical projects for healthcare and industrial customers that included product development lifecycle, feasibility studies, design, development, prototyping, validation, certification, quality management, and volume manufacturing.
Dr. Ghadar is also currently a non-executive director at Medilink Midlands, the Midlands (England) Life Sciences industry association with a vision to stimulate the growth of the Midlands life science sector. He has a BSc Degree and Masters in Electronics and Control Systems Engineering, where he achieved a First Class degree and Distinction respectively, and he also has a Ph.D. in Biosensors from the University of Warwick (U.K.).
Bashir Timol. Mr. Timol has served as member of the board of Nemaura Medical since formation in December 2013. He has co-founded, managed, and funded several biotech and life science companies, and led the investment consortium that provided capital for the initial two funding rounds for Nemaura Medical. Mr. Timol obtained his Bachelor of Arts degree in Economics from the University of Central Lancashire, UK.
Timothy Johnson. Mr. Johnson was elected as a director in July 2017. He is currently serving in executive positions in several tax consultancy and accountancy businesses in the UK. He is a practicing Chartered Tax Adviser and holds a first-class Master of Science in Mathematics and Physics from the University of Manchester, UK. Mr Johnson’s work involves in depth review and analysis of financial statements on a daily basis, and he has significant experience in matters relating to financial accounts, tax, financial management, financial regulatory requirements and anti-money laundering requirements.
| 35 |
Thomas Moore. Mr. Moore was elected as a director in August 2017. He is currently working as a director, tax consultant and co-owner of a tax consultancy and pensions administration business (WestBridge), having built up three decades of experience in accounting and consulting fields at leading accounting firms including Grant Thornton, KPMG and PricewaterhouseCoopers. Throughout the last five years, Mr Moore has held his current role with WestBridge since May 2017 and before that was a Director with Grant Thornton UK PLC. He is a practicing Chartered Tax Adviser and earned his first-class Bachelor of Arts in French and Russian from the University of Northumbria, UK. The qualifications Mr Moore brings to the role include a wealth of experience in matters relating to accounts, financial management and financial regulatory requirements including his current experience as an MLRO in two companies.
Dr. Salim Natha. Dr. Natha was elected as a director in July 2017. He is currently practicing as an Eye Surgeon in the UK National Health Service (NHS), and is the clinical lead for a retinopathy screening program for over 20,000 diabetics in the Ashton, Wigan and Leigh region. He has published several articles in the medical literature and is a peer reviewer for the English National Diabetic Retinopathy Screening Program. Dr. Natha graduated with honours from the University of Liverpool Medical School.
Family Relationships
There are no family relationships between any of our directors or executive officers.
Involvement in Certain Legal Proceedings.
None.
Board of Directors
All directors hold office until the next Annual Meeting of shareholders and until their successors have been duly elected and qualified. Directors are elected at the annual meetings to serve for one-year terms. Officers are elected by, and serve at the discretion of, the Board of Directors. Our Board of Directors shall hold meetings on at least a quarterly basis.
The Board of Directors complies with the NASDAQ Listing Rules with respect to corporate governance matters. Under the NASDAQ rules we are required to maintain a board of directors comprised of at least 50% independent directors, and an audit committee of at least two members, comprised solely of independent directors who also meet the requirements of Rule 10A-3 under the Securities Exchange Act of 1934.
Director Independence
The board of directors has reviewed the independence of our directors, applying the NASDAQ independence standards. Based on this review, the board of directors determined that each of Thomas Moore, Dr. Salim Natha and Timothy Johnson are independent within the meaning of the NASDAQ rules. In making this determination, our board of directors considered the relationships that each of these non-employee directors has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence. As required under applicable NASDAQ rules, we anticipate that our independent directors will meet on a regular basis as often as necessary to fulfil their responsibilities, including at least annually in executive session without the presence of non-independent directors and management.
Board Committees
Our board of directors has established standing committees in connection with the discharge of its responsibilities. These committees include an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. Our board of directors has adopted written charters for each of these committees. Copies of the charters are available on our website. Our board of directors may establish other committees as it deems necessary or appropriate from time to time.
Audit Committee
Our Audit Committee is comprised of our independent directors: Thomas Moore, Dr. Salim Natha and Timothy Johnson. Mr. Johnson qualifies as the Audit Committee financial expert as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act.
According to its charter, the Audit Committee consists of at least three members, each of whom shall be a non-employee director who has been determined by the Board to meet the independence requirements of NASDAQ, and also Rule 10A-3(b)(1) of the SEC, subject to the exemptions provided in Rule 10A-3(c). The Audit Committee Charter describes the primary functions of the Audit Committee, including the following:
| – | Oversee the Company’s accounting and financial reporting processes; |
| – | Oversee audits of the Company’s consolidated financial statements; |
| – | Discuss policies with respect to risk assessment and risk management, and discuss the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures; |
| 36 |
| – | Review and discuss with management the Company’s audited consolidated financial statements and review with management and the Company’s independent registered public accounting firm the Company’s consolidated financial statements prior to the filing with the SEC of any report containing such consolidated financial statements. |
| – | Recommend to the board that the Company’s audited consolidated financial statements be included in its annual report on Form 10-K for the last fiscal year; |
| – | Meet separately, periodically, with management, with the Company’s internal auditors (or other personnel responsible for the internal audit function) and with the Company’s independent registered public accounting firm; |
| – | Be directly responsible for the appointment, compensation, retention and oversight of the work of any independent registered public accounting firm engaged to prepare or issue an audit report for the Company; |
| – | Take, or recommend that the board take, appropriate action to oversee and ensure the independence of the Company’s independent registered public accounting firm; and |
| – | Review major changes to the Company’s auditing and accounting principles and practices as suggested by the Company’s independent registered public accounting firm, internal auditors or management. |
Compensation Committee
The Compensation Committee is responsible for, among other matters:
| – | reviewing and approving, or recommending to the board of directors to approve the compensation of our CEO and other executive officers and directors reviewing key employee compensation goals, policies, plans and programs; |
| – | administering incentive and equity-based compensation; |
| – | reviewing and approving employment agreements and other similar arrangements between us and our executive officers; and |
| – | appointing and overseeing any compensation consultants or advisors. |
Our Compensation Committee consists of Thomas Moore, Dr. Salim Natha and Timothy Johnson. Dr. Salim Natha serves as chair of the Compensation Committee.
Corporate Governance and Nominating Committee
The Corporate Governance and Nominating Committee is responsible for, among other matters:
| – | selecting or recommending for selection candidates for directorships; |
| – | evaluating the independence of directors and director nominees; |
| – | reviewing and making recommendations regarding the structure and composition of our board and the board committees; |
| – | developing and recommending to the board corporate governance principles and practices; |
| – | reviewing and monitoring the Company’s Code of Ethics; and |
| – | overseeing the evaluation of the Company’s management. |
Our Corporate Governance and Nominating Committee consists of Thomas Moore, Dr. Salim Natha and Timothy Johnson. Mr. Johnson serves as chair of the Corporate Governance and Nominating Committee.
Material Changes to Procedures by which Security Holders May Recommend Board Nominees
We do not currently have a procedure by which security holders may recommend nominees to the Board. Prior to the listing of our common stock on NASDAQ, as a private company with a limited shareholder base, we did not believe that it was important to provide such a procedure. However, as a publicly traded NASDAQ company with the requirement to hold annual shareholder meetings, we will consider implementing such a policy in the future.
| 37 |
The Board does not have a formal policy on Board candidate qualifications. The Board may consider those factors it deems appropriate in evaluating director nominees made either by the Board or stockholders, including judgment, skill, strength of character, experience with businesses and organizations comparable in size or scope to the Company, experience and skill relative to other Board members, and specialized knowledge or experience. Depending upon the current needs of the Board, certain factors may be weighed more or less heavily. In considering candidates for the Board, the directors evaluate the entirety of each candidate’s credentials and do not have any specific minimum qualifications that must be met. The directors will consider candidates from any reasonable source, including current Board members, stockholders, professional search firms or other persons. The directors will not evaluate candidates differently based on who has made the recommendation.
Board Leadership Structure and Role in Risk Oversight
Dr. Chowdhury holds the positions of chief executive officer, and chairman of the board of the Company. Prior to the appointment of Mr. Justin Mclarney to the role of chief financial officer as of September 15, 2020, Dr. Chowdhury also acted as interim chief financial officer. The board believes that Dr. Chowdhury’s services as both chief executive officer and chairman of the board is in the best interest of the Company and its shareholders. Dr. Chowdhury possesses detailed and in-depth knowledge of the issues, opportunities and challenges facing the Company in its business and is thus best positioned to develop agendas that ensure that the Board’s time and attention are focused on the most critical matters relating to the business of the Company. His combined role enables decisive leadership, ensures clear accountability, and enhances the Company’s ability to communicate its message and strategy clearly and consistently to the Company’s shareholders, employees and customers.
The board has not designated a lead director. Given the limited number of directors comprising the Board, the independent directors call and plan their executive sessions collaboratively and, between meetings of the Board, communicate with management and one another directly. Under these circumstances, the directors believe designating a lead director to take on responsibility for functions in which they all currently participate might detract from rather than enhance performance of their responsibilities as directors.
Management is responsible for assessing and managing risk, subject to oversight by the board of directors. The board oversees our risk management policies and risk appetite, including operational risks and risks relating to our business strategy and transactions. Various committees of the board assist the board in this oversight responsibility in their respective areas of expertise.
| – | The Audit Committee assists the board with the oversight of our financial reporting, independent auditors, and internal controls. It is charged with identifying any flaws in business management and recommending remedies, detecting fraud risks and implementing anti-fraud measures. The audit committee further discusses Nemaura’s policies with respect to risk assessment and management with respect to financial reporting. |
| – | The Compensation Committee oversees compensation, retention, succession and other human resources-related issues and risks. |
| – | The Corporate Governance and Nominating Committee overviews risks relating to our governance policies and initiatives. |
Code of Ethics
We have adopted a Code of Ethics that applies to our principal executive officer, principal financial officer and other persons performing similar functions. A copy of our Code of Ethics is available on our website. We intend to post amendments to, or waivers from a provision of, our Code of Ethics that apply to our principal executive officer, principal financial officer or persons performing similar functions on our website.
ITEM 11. EXECUTIVE COMPENSATION.
2022 Summary Compensation Table
This table provides disclosure, for fiscal years 2022 and 2021, of the compensation paid to our named executive officers.
| Named Executive Officer and Principal Position | Year | Salary | Bonus | Stock Awards | Option Awards | All Other Compensation | Total | ||||||||||||||||||||
| $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||
| Dr. D.F.H. Chowdhury, Chief Executive Officer and President | 2022 | 109,416 | — | — | 26,706 | (1) | 3,849 | 139,971 | |||||||||||||||||||
| 2021 | 104,840 | — | — | — | 3.368 | 108.208 | |||||||||||||||||||||
| Justin McLarney Chief Financial Officer (2) | 2022 | 256,444 | (3) | — | — | — | 2,498 | 258,942 | |||||||||||||||||||
| 2021 | 67,107 | — | — | — | 1,162 | 68,269 | |||||||||||||||||||||
| 38 |
| (1) | On January 28, 2022, in compensation for Dr. Chowdhury’s service as a director, the Company’s Board of Directors granted to Dr. Chowdhury an option to purchase 8,000 shares of common stock at an exercise price of $3.98 per share, the closing price of the Company’s common stock on the date of grant. The option was fully vested at grant and is exercisable for a period of five years from the date of grant. The fair value attributed to the options has been calculated using a Black-Scholes Option Pricing Model. |
| (2) | Mr. Mclarney was appointed Chief Financial Officer of the Company on September 15, 2020. Prior to Mr. Mclarney’s appointment, Dr. Chowdhury acted as Interim Chief Financial Officer. |
| (3) | Of this amount, $169,055 was paid in cash, and $87,389 was paid in stock. At Mr. Mclarney’s election, a portion of his base salary was paid in stock. Accordingly, on January 31, 2022, Mr. Mclarney received 22,293 shares at the market price of $3.92. |
Dr. D.F.H. Chowdhury
We entered into an employment agreement with Dr. D.F.H. Chowdhury on November 2, 2013. Dr. D.F.H. Chowdhury’s contract is for an unspecified period. He may leave the Company with notice, or the Company may terminate his contract with notice. Termination may be with or without cause. Dr. D.F.H. Chowdhury receives an annual salary of £80,000 pounds sterling (approximately $109,000). Our contract with Dr. D.F.H. Chowdhury does not include any provision for stock options or equity incentives.
Under the executive employment agreement Dr. D.F.H. Chowdhury’s annual salary was adjusted on a pro rata basis to reflect only work that was performed for Nemaura Medical Inc. The disclosure set forth in the table reflects his pro rata compensation for the periods ending March 31, 2022 and March 31, 2021, respectively.
Mr. McLarney
We entered into an employment agreement with our Chief Financial Officer, Mr. Justin Mclarney on September 15, 2020. Mr Mclarney’s contract is for an unspecified period. He may leave the Company with notice, or the Company may terminate his contract with notice. Termination may be with or without cause. Mr Mclarney receives an annual base salary of £90,000 pounds sterling (approximately $123,000). Our contractual arrangements with Mr Mclarney allow for stock options, and equity or cash incentives to be provided upon certain conditions having been met.
Outstanding Equity Awards for fiscal year ended March 31, 2022.
The table below sets forth the outstanding option awards for the named executive officers, as of March 31, 2022; there were no outstanding stock awards as of this date:
Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date | |||||||||||
| Dr. D.F.H. Chowdhury | 8,000 | — | 3.98 | January 28, 2027 | ||||||||||
Potential payments upon termination or change-in-control.
None.
Director Compensation
Each of our independent directors received annual fees of £5,000 pounds sterling (approximately $6,838) for the year ended March 31, 2022, for their service on our board of directors and committees. In addition, on January 28, 2022, the Board of Directors granted to each of the directors, including Dr. Chowdhury, an option to purchase 8,000 shares of common stock at an exercise price of $3.98 per share, the closing price of the Company’s common stock on the date of grant. The option was fully vested at grant and is exercisable for a period of five years from the date of grant.
| Name | Fees Earned or Paid in Cash ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | ||||||||||
| Timothy Johnson | 6,838 | 26,706 (1) | — | 33,544 | ||||||||||
| Dr. Salim Natha | 6,838 | 26,706 (1) | — | 33,544 | ||||||||||
| Thomas Moore | 6,838 | 26,706 (1) | — | 33,544 | ||||||||||
| (1) | On January 28, 2022, the Board of Directors granted to each of the directors, including Dr. Chowdhury, an option to purchase 8,000 shares of common stock at an exercise price of $3.98 per share, the closing price of the Company’s common stock on the date of grant. The option was fully vested at grant and is exercisable for a period of five years from the date of grant. The fair value attributed to the options has been calculated using a Black-Scholes Option Pricing Model. |
| 39 |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following tables set forth certain information as of March 31, 2022, regarding the beneficial ownership of our common stock, by (i) each person or entity who, to our knowledge, owns more than 5% of our common stock; (ii) our named executive officers; (iii) each director; and (iv) all of our executive officers and directors as a group.
Unless otherwise indicated in the footnotes to the following table, each person named in the table has sole voting and investment power and that person’s address is c/o NEMAURA MEDICAL INC., Advanced Technology Innovation Centre, 5 Oakwood Drive, Loughborough, Leicestershire, United Kingdom LE11 3QF.
| Name of Beneficial Owner | Amount and Nature of Beneficial Ownership | Percentage (1) | ||||||
| Dr. D.F.H. Chowdhury | 8,761,700 | (2) | 36.4% | |||||
| Justin Mclarney | 22,293 | * | ||||||
| Bashir Timol | 2,798,310 | (3) | 11.6% | |||||
| Timothy Johnson | 8,000 | (4) | * | |||||
| Dr. Salim Natha | 427,390 | (2) | 1.8% | |||||
| Thomas Moore | 8,000 | (4) | * | |||||
| All Executive Officers and Directors as a Group (6 persons) | 12,025,693 | (5) | 49.9% | |||||
| Holders of 5% or more of our common stock | ||||||||
| Ismail, Sufyan (6) | 2,270,525 | 9.4% | ||||||
* Less than 1%.
(1) Based upon 24,102,866 shares of our common stock outstanding at March 31, 2022.
(2) Includes 8,000 shares the reporting person has the right to acquire within 60 days of March 31, 2022 upon exercise of a vested option to purchase 8,000 shares of common stock.
(3) Represents (i) 2,708,210 shares held directly by the reporting person, (ii) 82,100 shares held by the reporting person’s spouse, and (iii) 8,000 shares the reporting person has the right to acquire within 60 days of March 31, 2022 upon exercise of a vested option to purchase 8,000 shares of common stock.
(4) Represents 8,000 shares the reporting person has the right to acquire within 60 days of March 31, 2022 upon exercise of a vested option to purchase 8,000 shares of common stock.
(5) Includes 40,000 shares the Company’s executive officers and directors have the right to acquire within 60 days of March 31, 2022 upon exercise of vested options to purchase 40,000 shares of common stock.
(6) Mr. Ismail’s address is Hollybank High Bank Lane, Lostock, Bolton, Lancashire BL6 HDT United Kingdom.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Pharma and NDM are entities controlled by our Chief Executive Officer, President, Chairman of the Board and majority shareholder, Dr. D.F.H. Chowdhury.
Pharma has invoiced our subsidiaries, DDL and TCL for research and development services. In addition, certain operating expenses of DDL and TCL were incurred and paid by Pharma and NDM which have been invoiced to us. Certain costs incurred by Pharma and NDM are directly attributable to DDL and TCL and such costs were billed to us.
Total costs charged to us by Pharma and NDM were $3,245,985 for the year ended March 31, 2022.
| 40 |
The following is a summary of activity between the Company and Pharma and NDM for the years ended March 31, 2022 and 2021.
| March 31, | ||||||||
2022 | 2021 | |||||||
| Liability due to related parties at beginning of year | 148,795 | 830,093 | ||||||
| Amounts invoiced by Pharma to DDL, NM and TCL | 3,245,985 | 2,441,108 | ||||||
| Amounts invoiced by DDL to Pharma | (2,495 | ) | (17,213 | ) | ||||
| Amounts paid by DDL to Pharma | (3,492,962 | ) | (3,209,084 | ) | ||||
| Foreign exchange differences | (620 | ) | 103,891 | |||||
| (Receivable) / Liability due (from) to related parties at end of year | (101,297 | ) | 148,795 | |||||
REVIEW, APPROVAL OR RATIFICATION OF TRANSACTIONS WITH RELATED PERSONS
It is Company policy to not enter any transaction (other than compensation arrangements in the ordinary course) with any director, executive officer, employee, or principal stockholder or party related to them, unless authorized by a majority of the directors having no interest in the transaction, upon a favorable recommendation by the Audit Committee (or a majority of its disinterested members).
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees billed in the fiscal years ended March 31, 2022 and 2021 by Mayer Hoffman McCann P.C.
2022 | 2021 | |||||||
| Audit Fees | 87,500 | 123,385 | ||||||
| Audit Related Fees | 80,000 | 83,500 | ||||||
| Tax Fees | 10,000 | 10,000 | ||||||
| Other Fees | 10,000 | 28,250 | ||||||
| Totals | 187,500 | 245,135 | ||||||
Audit fees represent amounts billed for professional services rendered or expected to be rendered for the audit of our annual consolidated financial statements.
Audit-related fees represent professional services rendered or expected to be rendered for assurance and related services by the accounting firm that are reasonably related to the performance of the audit or review of our consolidated financial statements that are not reported under audit fees.
Tax fees represent professional services rendered by the accounting firm for tax compliance and this includes preparing our annual tax filings.
Other fees represent charges made for the provision of a comfort letter in relation to the ATM Offering made on July 23, 2021.
The Audit Committee approves all auditing services and the terms thereof and non-audit services (other than non-audit services published under Section 10A(g) of the Exchange Act or the applicable rules of the SEC or the Pubic Company Accounting Oversight Board) to be provided to us by the independent auditor; provided, however, the pre-approval requirement is waived with respect to the provisions of non-audit services for us if the “de minimus” provisions of Section 10A(i)(1)(B) of the Exchange Act are satisfied.
Audit Committee Pre-Approval Policy
Under provisions of the Sarbanes-Oxley Act of 2002, our principal accountant may not be engaged to provide non-audit services that are prohibited by law or regulation to be provided by it, and the Audit Committee must pre-approve the engagement of our independent accountant to provide audit and permissible non-audit services. The Audit Committee has not established any policies or procedures other than those required by applicable laws and regulations.
Our independent auditor, Mayer Hoffman McCann P.C., leases substantially all of its personnel who work under the control of Mayer Hoffman McCann P.C. shareholders, from wholly owned subsidiaries of CBIZ, Inc., in an alternative practice structure.
| 41 |
PART IV
| ITEM 15. |
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. | ||
| (a) | Exhibits: | ||
*Filed herewith.
** Furnished herewith.
+Portions of this Exhibit have been omitted pursuant to a request for confidential treatment.
† Management contract or compensatory plan or arrangement.
ITEM 16. FORM 10-K SUMMARY.
None
| 42 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf on June 29, 2022, by the undersigned thereunto duly authorized.
| NEMAURA MEDICAL INC. | |
| /s/ Dr. D.F.H. Chowdhury | |
| Dr. D.F.H. Chowdhury President and Chief Executive Officer (Principal Executive Officer) |
Pursuant to the requirements of the Securities and Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Dr. D.F.H. Chowdhury | President, Chief Executive Officer and Director | June 29, 2022 | ||
| Dr. D.F.H.Chowdhury | (Principal Executive Officer) | |||
|
|
||||
| /s/ Justin Mclarney | Chief Financial Officer | June 29, 2022 | ||
| Justin Mclarney | (Principal Financial Officer and Principal Accounting Officer) | |||
|
|
||||
| /s/ Bashir Timol | Director | June 29, 2022 | ||
| Bashir Timol | ||||
|
|
||||
| /s/ Timothy Johnson | Director | June 29, 2022 | ||
| Timothy Johnson | ||||
|
|
||||
| /s/ Salim Natha | Director | June 29, 2022 | ||
| Salim Natha | ||||
|
|
||||
| /s/ Thomas Moore | Director | June 29, 2022 | ||
| Thomas Moore |
| 43 |
