false2024Q3Catalent, Inc.6/30FALSEFALSE00015967830.010.011,000,000,0001,000,000,000181,000,000180,000,000180,000,000179,000,0000.010.01100,000,000100,000,000————32461,8421,596REVISIONS OF PREVIOUSLY ISSUED FINANCIAL STATEMENTSAs described in the Amended Fiscal 2022 10-K, in preparing the consolidated financial statements for the three and nine months ended March 31, 2023, the Company identified a $26 million error related to the over-recognition of revenue in the consolidated financial statements it issued with respect to its fiscal year ended June 30, 2022. This error resulted from the misapplication of the contract modification guidance in accordance with ASC 606, Revenue from Contracts with Customers, related to one of the Company’s customer arrangements. The Company assessed the materiality of the error both quantitatively and qualitatively and determined this error to be immaterial to those consolidated financial statements. However, the Company concluded that the effect of correcting the error in the quarter ended March 31, 2023 would materially misstate the Company’s unaudited consolidated financial statements for the three and nine months ended March 31, 2023 and, accordingly, determined that it was necessary to revise the consolidated financial statements it previously issued with respect to the fiscal year ended June 30, 2022.
The following tables reflect the impact of this revision on the Company’s consolidated balance sheet as of June 30, 2022:
| | | | | | | | | | | | | | | | | | | | |
| Consolidated Balance Sheet | | June 30, 2022 |
| (Dollars in millions) | | As Previously | | | | |
| | Reported | | Adjustment | | As Revised |
| Prepaid expenses and other | | $ | 625 | | | $ | 1 | | | $ | 626 | |
| Total current assets | | 2,916 | | | 1 | | | 2,917 | |
| Total assets | | 10,507 | | | 1 | | | 10,508 | |
| Other accrued liabilities | | 620 | | | 26 | | | 646 | |
| Total current liabilities | | 1,072 | | | 26 | | | 1,098 | |
| Deferred income taxes | | 202 | | | (5) | | | 197 | |
| Total liabilities | | 5,712 | | | 21 | | | 5,733 | |
| Retained earnings | | 538 | | | (20) | | | 518 | |
| Total shareholders' equity | | 4,795 | | | (20) | | | 4,775 | |
| Total liabilities and shareholders' equity | | $ | 10,507 | | | $ | 1 | | | 10,508 | |
| | | | | | |
17. SUBSEQUENT EVENTS
Entry Into an Agreement and Plan of Merger
On February 5, 2024, the Company entered into the Agreement and Plan of Merger (the “Merger Agreement”), with Creek Parent, Inc. (“Parent”), a Delaware corporation and a wholly owned subsidiary of Novo Holdings A/S (“Novo Holdings”), and Creek Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”). The Merger Agreement provides that, upon the terms and subject to the conditions set forth therein and in accordance with the General Corporation Law of the State of Delaware (the “DGCL”), Merger Sub will be merged with and into the Company (the “Merger”), with the Company surviving the Merger as a wholly owned subsidiary of Parent. Parent will acquire all the issued and outstanding shares of common stock of the Company.
At the effective time of the Merger (the “Effective Time”), each share of common stock, par value $0.01 per share, of the Company (such shares, collectively, the “Company Common Stock,” and each, a “Share”) that is issued and outstanding immediately prior to the Effective Time (other than any Shares held by (i) the Company, Parent or Merger Sub or any other direct or indirect wholly owned subsidiary of the Company or Parent immediately prior to the Effective Time, or (ii) a holder who has not voted in favor of the adoption of the Merger Agreement and is entitled to demand and properly demands appraisal of such Shares under the DGCL), will be converted automatically into the right to receive an amount in cash equal to $63.50 per Share, without interest (the “Merger Consideration”). The transaction values the Company at $16.5 billion on an enterprise value basis.
Consummation of the Merger is subject to customary closing conditions, including approval of the Merger by the Company’s stockholders (which has not been obtained at this stage). Further conditions include (i) receipt of certain governmental waivers, consents, clearances, decisions, declarations, approvals, and expirations of applicable waiting periods, including the expiration or early termination of the waiting period under the U.S. Hart-Scott-Rodino Antitrust Improvements Act of 1976, with respect to (A) the Merger and (B) the sale of three of the Company’s fill-finish sites (which are located in Anagni, Italy, Bloomington, Indiana USA, and Brussels, Belgium) and related assets from Novo Holdings to Novo Nordisk A/S (“Novo Nordisk”), of which Novo Holdings is the controlling shareholder (the “Carve-Out”), and (ii) the absence of any order, injunction or law prohibiting the Merger or the Carve-Out, in each case, without a Burdensome Condition (as defined in the Merger Agreement). Parent’s and Merger Sub’s obligations to close the Merger are also conditioned upon the absence of a Material Adverse Effect (as defined in the Merger Agreement) on the Company.
0.0163.5016.5345March 31, 2024xbrli:sharesiso4217:USDxbrli:sharesiso4217:USDutr:Ratexbrli:purectlt:employees00015967832023-07-012024-03-3100015967832024-04-2500015967832024-03-3100015967832024-01-012024-03-3100015967832023-01-012023-03-3100015967832022-07-012023-03-310001596783us-gaap:RetainedEarningsMember2024-01-012024-03-310001596783us-gaap:RetainedEarningsMember2023-01-012023-03-310001596783us-gaap:RetainedEarningsMember2023-07-012024-06-300001596783us-gaap:RetainedEarningsMember2022-07-012023-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2024-01-012024-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2023-07-012024-03-310001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2024-01-012024-03-310001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-07-012024-03-310001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2023-01-012023-03-310001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2023-07-012024-03-310001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2022-07-012023-03-3100015967832023-06-300001596783us-gaap:CommonStockMember2023-12-310001596783us-gaap:AdditionalPaidInCapitalMember2023-12-310001596783us-gaap:RetainedEarningsMember2023-12-310001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-3100015967832023-12-310001596783us-gaap:CommonStockMember2024-01-012024-03-310001596783us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-03-310001596783us-gaap:CommonStockMember2024-03-310001596783us-gaap:AdditionalPaidInCapitalMember2024-03-310001596783us-gaap:RetainedEarningsMember2024-03-310001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310001596783us-gaap:CommonStockMember2022-12-310001596783us-gaap:AdditionalPaidInCapitalMember2022-12-310001596783us-gaap:RetainedEarningsMember2022-12-310001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-3100015967832022-12-310001596783us-gaap:CommonStockMember2023-01-012023-03-310001596783us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-310001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-310001596783us-gaap:CommonStockMember2023-03-310001596783us-gaap:AdditionalPaidInCapitalMember2023-03-310001596783us-gaap:RetainedEarningsMember2023-03-310001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-3100015967832023-03-310001596783us-gaap:CommonStockMember2023-06-300001596783us-gaap:AdditionalPaidInCapitalMember2023-06-300001596783us-gaap:RetainedEarningsMember2023-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001596783us-gaap:CommonStockMember2023-07-012024-06-300001596783us-gaap:AdditionalPaidInCapitalMember2023-07-012024-06-3000015967832023-07-012024-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-07-012024-06-300001596783us-gaap:CommonStockMember2022-06-300001596783us-gaap:AdditionalPaidInCapitalMember2022-06-300001596783us-gaap:RetainedEarningsMember2022-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-3000015967832022-06-300001596783us-gaap:CommonStockMember2022-07-012023-03-310001596783us-gaap:AdditionalPaidInCapitalMember2022-07-012023-03-310001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-07-012023-03-310001596783srt:ScenarioPreviouslyReportedMember2024-06-300001596783ctlt:RevisionAdjustmentsMember2024-06-3000015967832024-06-300001596783srt:RestatementAdjustmentMember2024-06-300001596783srt:ScenarioPreviouslyReportedMember2023-06-300001596783ctlt:RevisionAdjustmentsMember2023-06-300001596783us-gaap:ProductAndServiceOtherMember2023-07-012024-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:BiologicsMember2024-01-012024-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:ManufacturingCommercialProductSupplyMember2024-01-012024-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2024-01-012024-03-310001596783ctlt:BiologicsMemberctlt:DevelopmentServicesMember2024-01-012024-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:DevelopmentServicesMember2024-01-012024-03-310001596783ctlt:TotalCatalentbeforeintersegmentrevenueeliminationMemberctlt:DevelopmentServicesMember2024-01-012024-03-310001596783us-gaap:OperatingSegmentsMemberctlt:BiologicsMember2024-01-012024-03-310001596783ctlt:PharmaConsumerHealthMemberus-gaap:OperatingSegmentsMember2024-01-012024-03-310001596783ctlt:OperatingSegmentsExcludingIntersegmentEliminationMember2024-01-012024-03-310001596783us-gaap:IntersegmentEliminationMemberctlt:TotalCatalentSegmentMember2024-01-012024-03-310001596783us-gaap:OperatingSegmentsMember2024-01-012024-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:BiologicsMember2023-01-012023-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:ManufacturingCommercialProductSupplyMember2023-01-012023-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2023-01-012023-03-310001596783ctlt:BiologicsMemberctlt:DevelopmentServicesMember2023-01-012023-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:DevelopmentServicesMember2023-01-012023-03-310001596783ctlt:TotalCatalentbeforeintersegmentrevenueeliminationMemberctlt:DevelopmentServicesMember2023-01-012023-03-310001596783us-gaap:OperatingSegmentsMemberctlt:BiologicsMember2023-01-012023-03-310001596783ctlt:PharmaConsumerHealthMemberus-gaap:OperatingSegmentsMember2023-01-012023-03-310001596783ctlt:OperatingSegmentsExcludingIntersegmentEliminationMember2023-01-012023-03-310001596783us-gaap:IntersegmentEliminationMemberctlt:TotalCatalentSegmentMember2023-01-012023-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:BiologicsMember2023-07-012024-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:ManufacturingCommercialProductSupplyMember2023-07-012024-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2023-07-012024-03-310001596783ctlt:BiologicsMemberctlt:DevelopmentServicesMember2023-07-012024-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:DevelopmentServicesMember2023-07-012024-03-310001596783ctlt:TotalCatalentbeforeintersegmentrevenueeliminationMemberctlt:DevelopmentServicesMember2023-07-012024-03-310001596783us-gaap:OperatingSegmentsMemberctlt:BiologicsMember2023-07-012024-03-310001596783ctlt:PharmaConsumerHealthMemberus-gaap:OperatingSegmentsMember2023-07-012024-03-310001596783ctlt:OperatingSegmentsExcludingIntersegmentEliminationMember2023-07-012024-03-310001596783us-gaap:IntersegmentEliminationMemberctlt:TotalCatalentSegmentMember2023-07-012024-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:BiologicsMember2022-07-012023-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:ManufacturingCommercialProductSupplyMember2022-07-012023-03-310001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2022-07-012023-03-310001596783ctlt:BiologicsMemberctlt:DevelopmentServicesMember2022-07-012023-03-310001596783ctlt:PharmaConsumerHealthMemberctlt:DevelopmentServicesMember2022-07-012023-03-310001596783ctlt:TotalCatalentbeforeintersegmentrevenueeliminationMemberctlt:DevelopmentServicesMember2022-07-012023-03-310001596783us-gaap:OperatingSegmentsMemberctlt:BiologicsMember2022-07-012023-03-310001596783ctlt:PharmaConsumerHealthMemberus-gaap:OperatingSegmentsMember2022-07-012023-03-310001596783ctlt:OperatingSegmentsExcludingIntersegmentEliminationMember2022-07-012023-03-310001596783us-gaap:IntersegmentEliminationMemberctlt:TotalCatalentSegmentMember2022-07-012023-03-310001596783ctlt:GeographicalMember2023-07-012024-03-310001596783srt:NorthAmericaMember2024-01-012024-03-310001596783srt:NorthAmericaMember2023-01-012023-03-310001596783srt:NorthAmericaMember2023-07-012024-03-310001596783srt:NorthAmericaMember2022-07-012023-03-310001596783srt:EuropeMember2024-01-012024-03-310001596783srt:EuropeMember2023-01-012023-03-310001596783srt:EuropeMember2023-07-012024-03-310001596783srt:EuropeMember2022-07-012023-03-310001596783ctlt:InternationalOtherMember2024-01-012024-03-310001596783ctlt:InternationalOtherMember2023-01-012023-03-310001596783ctlt:InternationalOtherMember2023-07-012024-03-310001596783ctlt:InternationalOtherMember2022-07-012023-03-310001596783ctlt:GreaterThanOneYearMemberMember2023-03-310001596783ctlt:MetricsMember2022-10-012022-10-010001596783ctlt:MetricsMember2022-10-010001596783ctlt:MetricsMemberus-gaap:CustomerRelationshipsMember2022-10-010001596783ctlt:MetricsMemberus-gaap:CustomerRelationshipsMember2022-10-012022-10-010001596783ctlt:BiologicsMember2023-06-300001596783ctlt:PharmaConsumerHealthMember2023-06-300001596783ctlt:BiologicsMember2023-07-012024-03-310001596783ctlt:PharmaConsumerHealthMember2023-07-012024-03-310001596783ctlt:PharmaConsumerHealthMember2024-01-012024-03-310001596783ctlt:BiologicsMember2024-03-310001596783ctlt:PharmaConsumerHealthMember2024-03-310001596783ctlt:TermLoanThreeFacilityDollarDenominatedMember2024-03-310001596783ctlt:TermLoanThreeFacilityDollarDenominatedMember2023-06-300001596783ctlt:TermLoanFourFacilityDollarDenominatedMember2024-03-310001596783ctlt:RevolvingCreditFacilityTwoMember2024-03-310001596783ctlt:RevolvingCreditFacilityTwoMember2023-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Member2024-03-310001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Member2023-06-300001596783ctlt:A2375SeniorEuroDenominatedNotesMemberus-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Member2024-03-310001596783ctlt:A2375SeniorEuroDenominatedNotesMemberus-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Member2023-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Memberctlt:A3125SeniorUSDenominatedNotesMember2024-03-310001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Memberctlt:A3125SeniorUSDenominatedNotesMember2023-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Memberctlt:A3500SeniorUSDenominatedNotesMember2024-03-310001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Memberctlt:A3500SeniorUSDenominatedNotesMember2023-06-300001596783us-gaap:CapitalLeaseObligationsMember2024-03-310001596783us-gaap:CapitalLeaseObligationsMember2023-06-300001596783ctlt:OtherObligationsMember2024-03-310001596783ctlt:OtherObligationsMember2023-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberctlt:DebtIssuanceCostsMember2024-03-310001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberctlt:DebtIssuanceCostsMember2023-06-300001596783ctlt:TermLoanFourFacilityDollarDenominatedMemberctlt:SecuredOvernightFinancingRateSOFRMember2024-03-310001596783ctlt:TermLoanFourFacilityDollarDenominatedMembersrt:MinimumMemberctlt:SecuredOvernightFinancingRateSOFRMember2024-03-310001596783us-gaap:RevolvingCreditFacilityMember2024-03-310001596783ctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2024-03-310001596783ctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783ctlt:A2375SeniorEuroDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2024-03-310001596783ctlt:A2375SeniorEuroDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783us-gaap:FairValueInputsLevel2Memberctlt:A3125SeniorUSDenominatedNotesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2024-03-310001596783us-gaap:FairValueInputsLevel2Memberctlt:A3125SeniorUSDenominatedNotesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783us-gaap:FairValueInputsLevel2Memberctlt:A3500SeniorUSDenominatedNotesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2024-03-310001596783us-gaap:FairValueInputsLevel2Memberctlt:A3500SeniorUSDenominatedNotesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Memberctlt:SeniorSecuredCreditFacilitiesOtherMember2024-03-310001596783us-gaap:FairValueInputsLevel2Memberctlt:SeniorSecuredCreditFacilitiesOtherMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2024-03-310001596783us-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Memberctlt:SeniorSecuredCreditFacilitiesOtherMember2023-06-300001596783us-gaap:FairValueInputsLevel2Memberctlt:SeniorSecuredCreditFacilitiesOtherMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMember2024-03-310001596783us-gaap:EstimateOfFairValueFairValueDisclosureMember2024-03-310001596783us-gaap:CarryingReportedAmountFairValueDisclosureMember2023-06-300001596783us-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783ctlt:DebtIssuanceCostsMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2024-03-310001596783ctlt:DebtIssuanceCostsMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-3000015967832022-01-012022-03-310001596783ctlt:BiologicsMember2024-01-012024-03-310001596783ctlt:BiologicsMember2023-01-012023-03-310001596783ctlt:BiologicsMember2022-07-012023-03-310001596783ctlt:PharmaConsumerHealthMember2023-01-012023-03-310001596783ctlt:PharmaConsumerHealthMember2022-07-012023-03-310001596783ctlt:CorporateAndEliminationsMember2024-01-012024-03-310001596783ctlt:CorporateAndEliminationsMember2023-01-012023-03-310001596783ctlt:CorporateAndEliminationsMember2023-07-012024-03-310001596783ctlt:CorporateAndEliminationsMember2022-07-012023-03-310001596783ctlt:EuroDenominatedDebtOutstandingMember2024-03-310001596783ctlt:USDenominatedTermLoanMember2024-03-310001596783ctlt:USDenominatedTermLoanMember2021-02-280001596783ctlt:USDenominatedTermLoanMember2023-06-300001596783us-gaap:FairValueInputsLevel1Member2024-03-310001596783us-gaap:FairValueInputsLevel2Member2024-03-310001596783us-gaap:FairValueInputsLevel3Member2024-03-310001596783us-gaap:FairValueInputsLevel1Member2023-06-300001596783us-gaap:FairValueInputsLevel2Member2023-06-300001596783us-gaap:FairValueInputsLevel3Member2023-06-3000015967832023-10-012023-12-310001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2024-01-012024-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2023-12-310001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-12-310001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2023-12-310001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-12-310001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2024-01-012024-03-310001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-01-012024-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2024-03-310001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2024-03-310001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2024-03-310001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-12-310001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2022-12-310001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2022-12-310001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-12-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2023-01-012023-03-310001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2023-01-012023-03-310001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-01-012023-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2023-03-310001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-03-310001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2023-03-310001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2023-03-310001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2023-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2023-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-06-300001596783us-gaap:AccumulatedTranslationAdjustmentMember2022-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2022-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2022-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2022-07-012023-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2022-07-012023-03-3100015967832024-02-050001596783ctlt:MergerAgreementMember2024-02-050001596783ctlt:TotalCatalentSubTotalOfSegmentReportingMember2024-01-012024-03-310001596783ctlt:TotalCatalentSubTotalOfSegmentReportingMember2023-01-012023-03-310001596783ctlt:TotalCatalentSubTotalOfSegmentReportingMember2023-07-012024-03-310001596783ctlt:TotalCatalentSubTotalOfSegmentReportingMember2022-07-012023-03-310001596783ctlt:CorporateAndEliminationsMember2024-03-310001596783ctlt:CorporateAndEliminationsMember2023-06-30 UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________
FORM 10-Q
______________________________ | | | | | | | | | | | | | | |
| ý | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | | |
For the quarterly period ended March 31, 2024
or | | | | | | | | | | | | | | |
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | | |
001-36587
(Commission File Number)
_____________________________
Catalent, Inc.
(Exact name of registrant as specified in its charter)
_____________________________ | | | | | | | | |
| Delaware | 20-8737688 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| | |
| 14 Schoolhouse Road | |
| Somerset, | New Jersey | 08873 |
(Address of principal executive offices)_______ | (Zip code) |
(732) 537-6200
Registrant’s telephone number, including area code
____________________________________
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, $0.01 par value per share | CTLT | New York Stock Exchange |
____________________________________
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ¨ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. | | | | | | | | | | | | | | | | | |
Large accelerated filer | ☒ | | Accelerated filer | | ¨ |
Non-accelerated filer | ¨ | | Smaller reporting company | ¨ |
| | | Emerging growth company | ¨ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨ Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes ☒ No
On April 25, 2024, there were 180,979,849 shares of the Registrant’s common stock, par value $0.01 per share, issued and outstanding.
CATALENT, INC.
Index to Form 10-Q
For the Three and Nine Months Ended March 31, 2024
| | | | | | | | |
| Item | | Page |
| | |
| Part I. | | |
| | |
| Item 1. | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| Item 2. | | |
| | |
| Item 3. | | |
| | |
| Item 4. | | |
| | |
| Part II. | | |
| | |
| Item 1. | | |
| | |
| Item 1A. | | |
| | |
| Item 2. | | |
| | |
| Item 3. | | |
| | |
| Item 4. | | |
| | |
| Item 5. | | |
| | |
| Item 6. | | |
| |
| |
Special Note Regarding Forward-Looking Statements
In addition to historical information, this Quarterly Report on Form 10-Q of Catalent, Inc. (“Catalent” or the “Company”) contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are subject to the “safe harbor” created by those sections. All statements, other than statements of historical facts, included in this Quarterly Report on Form 10-Q are forward-looking statements. In some cases, you can identify these forward-looking statements by the use of words such as “outlook,” “believes,” “expects,” “potential,” “continues,” “may,” “will,” “should,” “could,” “seeks,” “approximately,” “predicts,” “intends,” “plans,” “estimates,” “anticipates” or the negative version of these words or other comparable words.
These statements are based on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments, and other factors they believe to be appropriate. Any forward-looking statement is subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements.
Some of the factors that may cause actual results, developments and business decisions to differ materially from those contemplated by such forward-looking statements include, but are not limited to, those summarized below, in addition to those described more fully (i) from time to time in reports that we have filed or in the future may file with the Securities and Exchange Commission (the “SEC”), and (ii) under the section entitled “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended June 30, 2023 (the “Fiscal 2023 10-K”).
Risks Relating to Our Business and the Industry in Which We Operate
•We may not complete the pending merger with Novo Holdings within the timeframe anticipated, or at all, which could have a material adverse effect on our business, financial condition or results of operations, as well as negatively impact our share price.
•Actions of activist shareholders could impact the pursuit of our business strategies and adversely affect our results of operations, financial condition, or share price.
•We anticipate being subject to increasing focus by our investors, regulators, customers, and other stakeholders on environmental, social, and governance (“ESG”) matters.
•Any failure to implement fully, monitor, and continuously improve our quality management strategy could lead to quality or safety issues and expose us to significant costs, potential liability, and adverse publicity.
•We have experienced, and may continue to experience, productivity issues and higher-than-expected costs at certain of our facilities, which have resulted in, and may continue to result in, material and adverse impacts on our financial condition and results of operations.
•The declining demand for various COVID-19 vaccines and treatments from both patients and governments around the world has affected and may continue to affect sales of the COVID-19 products we manufacture and our financial condition.
•The demand for our offerings depends in part on our customers’ research and development and the clinical and market success of their products.
•Our results of operations are subject to fluctuations in the costs, availability, and suitability of the components of the products we manufacture, including active pharmaceutical ingredients, excipients, purchased components, and raw materials, and other supplies or equipment we need to run our business.
•Our goodwill has been subject to impairment and may be subject to further impairment in the future, which could have a material adverse effect on our results of operations, financial condition, or future operating results.
•Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
•We may acquire businesses and offerings that complement or expand our business or divest non-strategic businesses or assets. We may not be able to complete desired transactions, and such transactions, if executed, pose significant risks, including risks relating to our ability to successfully and efficiently integrate acquisitions or execute on dispositions and realize anticipated benefits therefrom. The failure to execute or realize the full benefits from any such transaction could have a negative effect on our operations and profitability.
•We may become subject to litigation, other proceedings, and government investigations relating to us or our operations, and the ultimate outcome of any such matter may have an impact on our business, prospects, financial condition, and results of operations.
•Our global operations are subject to economic and political risks, including risks resulting from continuing inflation, disruptions to global supply chains, destabilization of a regional or national banking system, or from the Ukrainian-Russian war or the effect of the evolving nature of the recent war in Gaza between Israel and Hamas and conflict in the Middle East, which could affect the profitability of our operations or require costly changes to our procedures.
•We use advanced information and communication systems to run our operations, compile and analyze financial and operational data, and communicate among our employees, customers, and counterparties, and the risks generally associated with information and communications systems could adversely affect our results of operations. We continuously work to install new, and upgrade existing, systems and provide employee awareness training around phishing, malware, and other cybersecurity risks to enhance the protections available to us, but such protections may be inadequate to address malicious attacks or inadvertent compromises affecting data security or the operability of such systems.
•Artificial intelligence-based platforms present new risks and challenges to our business.
•Our cash, cash equivalents, and financial investments could be adversely affected if the financial institutions in which we hold our cash, cash equivalents, and financial investments fail.
Risks Relating to Our Indebtedness
•The size of our indebtedness and the obligations associated with it could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or in our industry or to deploy capital to grow our business, expose us to interest-rate risk to the extent of our variable-rate debt, or prevent us from meeting our obligations under our indebtedness. These risks may be increased in a recessionary environment, particularly as sources of capital may become less available or more expensive.
•Despite our high indebtedness level, we and our subsidiaries are still capable of incurring significant additional debt, which could further exacerbate the risks associated with our substantial indebtedness.
•Our interest expense on our variable-rate debt may continue to increase if and to the extent that policymakers combat inflation through interest-rate increases on benchmark financial products.
•Despite the limitations in our debt agreements, we retain the ability to take certain actions that may interfere with our ability to timely pay our substantial indebtedness.
•We may not be able to pay our indebtedness when it becomes due.
•We are currently using and may in the future use derivative financial instruments to reduce our exposure to market risks from changes in interest rates on our variable-rate indebtedness or changes in currency exchange rates, and any such instrument may expose us to risks related to counterparty credit worthiness or non-performance of these instruments.
Risks Relating to Ownership of Our Common Stock
•We do not presently maintain effective disclosure controls and procedures due to material weaknesses we have identified in our internal controls over financial reporting. Failure to remediate these material weaknesses or any other material weakness or significant deficiencies have resulted in a revision of our financial statements, in the future could result in material misstatements in our financial statements and have caused, and in the future could cause us to fail to timely meet our periodic reporting obligations.
•Our stock price has historically been and may continue to be volatile, and a holder of shares of our Common Stock may not be able to resell such shares at or above the price such stockholder paid, or at all, and could lose all or part of such investment as a result.
•Future sales, or the perception of future sales, of our Common Stock, by us or our existing stockholders could cause the market price for our Common Stock to decline.
•We are no longer eligible to use the Form S-3 registration statement, which could impair our capital-raising activities.
•Provisions in our organizational documents could delay or prevent a change of control.
We caution that the risks, uncertainties, and other factors referenced above may not contain all of the risks, uncertainties, and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits, or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. There can be no assurance that (i) we have correctly measured or identified all of the factors affecting our business or the extent of these factors’ likely impact, (ii) the available information with respect to these factors on which such analysis is based is complete or accurate, (iii) such analysis is correct, or (iv) our strategy, which is based in part on this analysis, will be successful. All forward-looking statements in this report apply only as of the date of this report or as of the date they were made and we undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments, or otherwise, except as required by law.
Social Media
We use our website (catalent.com), Facebook page (facebook.com/CatalentPharmaSolutions), LinkedIn page (linkedin.com/company/catalent-pharma-solutions/) and Twitter account (@catalentpharma) as channels of distribution of information concerning our activities, our offerings, our various businesses, and other related matters. The information we post through these channels may be deemed material. Accordingly, investors should monitor these channels, in addition to following our press releases, SEC filings, and public conference calls and webcasts. The information contained on or accessible through our website, our social media channels, or any other website that we may maintain is not a part of this Quarterly Report.
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
Catalent, Inc.
Consolidated Statements of Operations
(Unaudited; dollars in millions, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, | | | | |
| 2024 | | 2023 | | 2024 | | 2023 | | | | |
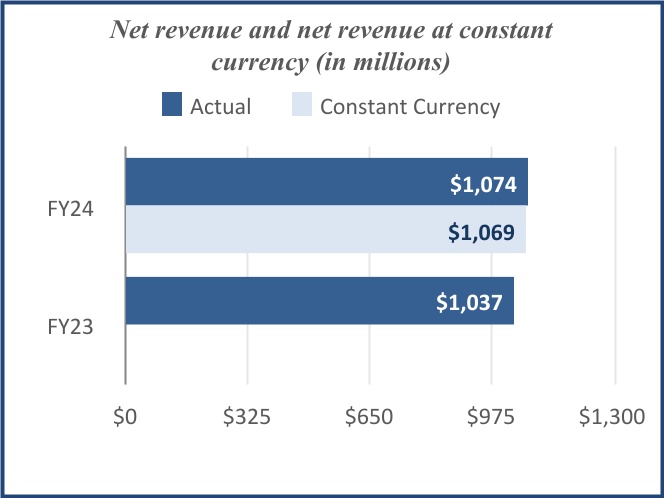
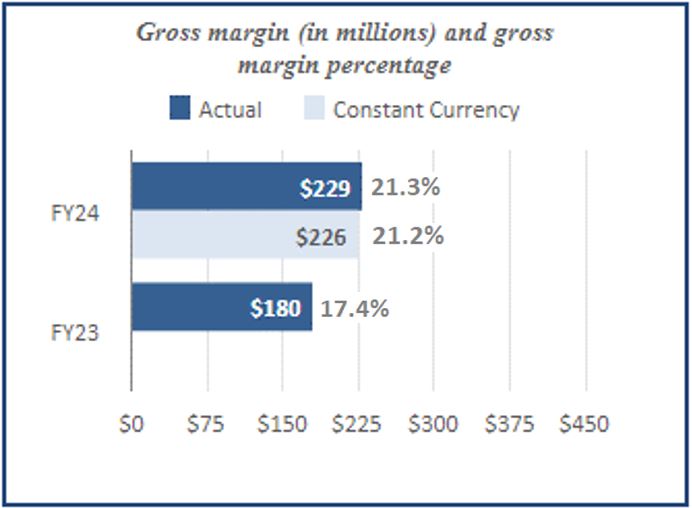
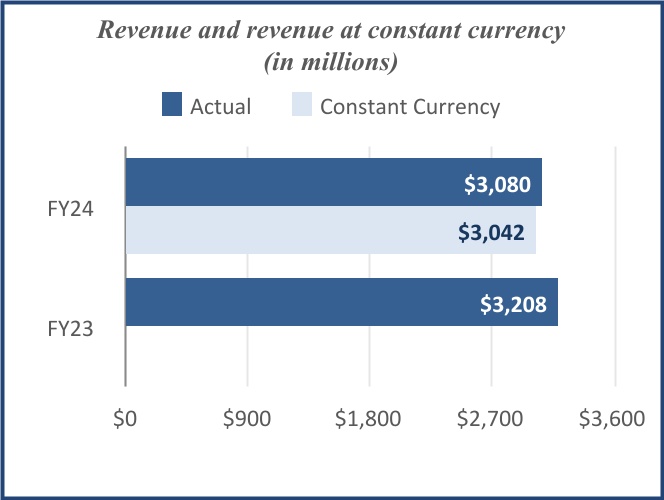
| Net revenue | $ | 1,074 | | | $ | 1,037 | | | $ | 3,080 | | | $ | 3,208 | | | | | |
| Cost of sales | 845 | | | 857 | | | 2,511 | | | 2,383 | | | | | |
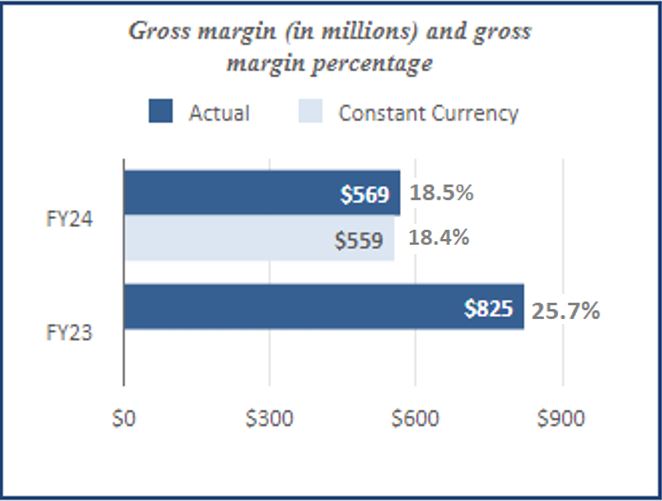
| Gross margin | 229 | | | 180 | | | 569 | | | 825 | | | | | |
| Selling, general, and administrative expenses | 214 | | | 190 | | | 669 | | | 612 | | | | | |
| | | | | | | | | | | |
| Goodwill impairment charges | — | | | 210 | | | 687 | | | 210 | | | | | |
| Other operating expense, net | 32 | | | 15 | | | 68 | | | 40 | | | | | |
| Operating loss | (17) | | | (235) | | | (855) | | | (37) | | | | | |
| Interest expense, net | 65 | | | 51 | | | 189 | | | 130 | | | | | |
| Other expense (income), net | 4 | | | (4) | | | 21 | | | (2) | | | | | |
| Loss before income taxes | (86) | | | (282) | | | (1,065) | | | (165) | | | | | |
| Income tax expense (benefit) | 15 | | | (55) | | | 1 | | | (19) | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Net loss | $ | (101) | | | $ | (227) | | | $ | (1,066) | | | $ | (146) | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Earnings (loss) per share: | | | | | | | | | | | |
| Basic | | | | | | | | | | | |
| | | | | | | | | | | |
| Net loss | $ | (0.56) | | | $ | (1.26) | | | $ | (5.87) | | | $ | (0.81) | | | | | |
| Diluted | | | | | | | | | | | |
| | | | | | | | | | | |
| Net loss | $ | (0.56) | | | $ | (1.26) | | | $ | (5.87) | | | $ | (0.81) | | | | | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
Catalent, Inc.
Consolidated Statements of Comprehensive Loss
(Unaudited; dollars in millions)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| 2024 | | 2023 | | 2024 | | 2023 |
| Net loss | $ | (101) | | | $ | (227) | | | $ | (1,066) | | | $ | (146) | |
| Other comprehensive (loss) income, net of tax | | | | | | | |
| Foreign currency translation adjustments | (14) | | | 27 | | | (16) | | | 10 | |
| Pension and other post-retirement adjustments | 7 | | | — | | | 11 | | | — | |
| Net change in marketable securities | — | | | 2 | | | — | | | 4 | |
| | | | | | | |
| | | | | | | |
| Derivatives and hedges | 5 | | | (2) | | | 3 | | | 12 | |
| Other comprehensive (loss) income, net of tax | (2) | | | 27 | | | (2) | | | 26 | |
| Comprehensive loss | $ | (103) | | | $ | (200) | | | $ | (1,068) | | | $ | (120) | |
| | | | | | | |
| | | | | | | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
Catalent, Inc.
Consolidated Balance Sheets
(Unaudited; dollars in millions, except share and per share data)
| | | | | | | | | | | |
| March 31,
2024 | | June 30,
2023 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 162 | | | $ | 280 | |
Trade receivables, net of allowance for credit losses of $32 and $46, respectively | 875 | | | 1,002 | |
| Inventories | 742 | | | 777 | |
| Prepaid expenses and other | 744 | | | 633 | |
| | | |
| Total current assets | 2,523 | | | 2,692 | |
Property, plant, and equipment, net of accumulated depreciation of $1,842 and $1,596, respectively | 3,735 | | | 3,682 | |
| Other assets: | | | |
| Goodwill | 2,339 | | | 3,039 | |
| Other intangibles, net | 875 | | | 980 | |
| Deferred income taxes | 66 | | | 55 | |
| Other long-term assets | 341 | | | 329 | |
| Total assets | $ | 9,879 | | | $ | 10,777 | |
| | | |
| LIABILITIES AND SHAREHOLDERS' EQUITY |
| Current liabilities: | | | |
| Current portion of long-term obligations and other short-term borrowings | $ | 47 | | | $ | 536 | |
| Accounts payable | 377 | | | 424 | |
| Other accrued liabilities | 583 | | | 570 | |
| Total current liabilities | 1,007 | | | 1,530 | |
| Long-term obligations, less current portion | 4,933 | | | 4,313 | |
| Pension liability | 97 | | | 100 | |
| Deferred income taxes | 64 | | | 76 | |
| Other liabilities | 167 | | | 147 | |
| | | |
| | | |
| Total liabilities | 6,268 | | | 6,166 | |
| | | |
| Commitments and contingencies (see Note 14) | | | |
| Shareholders' equity: | | | |
Common stock, $0.01 par value; 1.00 billion shares authorized at March 31, 2024 and June 30, 2023; 181 million and 180 million issued and outstanding at March 31, 2024 and June 30, 2023, respectively | 2 | | | 2 | |
Preferred stock, $0.01 par value; 100 million shares authorized at March 31, 2024 and June 30, 2023; 0 shares issued and outstanding at March 31, 2024 and June 30, 2023 | — | | | — | |
| | | |
| Additional paid in capital | 4,769 | | | 4,701 | |
| (Accumulated deficit) retained earnings | (804) | | | 262 | |
| Accumulated other comprehensive loss | (356) | | | (354) | |
| | | |
| | | |
| Total shareholders' equity | 3,611 | | | 4,611 | |
| Total liabilities and shareholders' equity | $ | 9,879 | | | $ | 10,777 | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
Catalent, Inc.
Consolidated Statements of Changes in Shareholders' Equity
(Unaudited; dollars in millions, except share data in thousands)
Three Months Ended March 31, 2024 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares of Common Stock | | Common Stock | | Additional Paid in Capital | | | | Accumulated Deficit | | Accumulated Other Comprehensive Loss | | | | Total Shareholders' Equity | | |
| Balance at December 31, 2023 | 180,668 | | | $ | 2 | | | $ | 4,742 | | | | | $ | (703) | | | $ | (354) | | | | | $ | 3,687 | | | |
| | | | | | | | | | | | | | | | | |
Share issuances related to stock- based compensation | 246 | | | — | | | — | | | | | — | | | — | | | | | — | | | |
| | | | | | | | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 17 | | | | | — | | | — | | | | | 17 | | | |
| | | | | | | | | | | | | | | | | |
| Exercise of stock options | — | | | — | | | 8 | | | | | — | | | — | | | | | 8 | | | |
| Employee stock purchase plan | — | | | — | | | 2 | | | | | — | | | — | | | | | 2 | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | | | (101) | | | — | | | | | (101) | | | |
Other comprehensive income, net
of tax | — | | | — | | | — | | | | | — | | | (2) | | | | | (2) | | | |
| Balance at March 31, 2024 | 180,914 | | | $ | 2 | | | $ | 4,769 | | | | | $ | (804) | | | $ | (356) | | | | | $ | 3,611 | | | |
Three Months Ended March 31, 2023 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares of Common Stock | | Common Stock | | Additional Paid in Capital | | | | Retained Earnings | | Accumulated Other Comprehensive Loss | | | | Total Shareholders' Equity | | |
| Balance at December 31, 2022 | 179,988 | | | $ | 2 | | | $ | 4,686 | | | | | $ | 599 | | | $ | (395) | | | | | $ | 4,892 | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Share issuances related to stock- based compensation | 169 | | | — | | | — | | | | | — | | | — | | | | | — | | | |
| | | | | | | | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 6 | | | | | — | | | — | | | | | 6 | | | |
| | | | | | | | | | | | | | | | | |
| Exercise of stock options | — | | | — | | | 3 | | | | | — | | | — | | | | | 3 | | | |
| Employee stock purchase plan | — | | | — | | | 2 | | | | | — | | | — | | | | | 2 | | | |
| | | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | | | (227) | | | — | | | | | (227) | | | |
Other comprehensive loss, net of tax | — | | | — | | | — | | | | | — | | | 27 | | | | | 27 | | | |
| Balance at March 31, 2023 | 180,157 | | | $ | 2 | | | $ | 4,697 | | | | | $ | 372 | | | $ | (368) | | | | | $ | 4,703 | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
Catalent, Inc.
Consolidated Statements of Changes in Shareholders' Equity
(Unaudited; dollars in millions, except share data in thousands)
Nine Months ended March 31, 2024 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares of Common Stock | | Common Stock | | Additional Paid in Capital | | | | Retained Earnings | | Accumulated Other Comprehensive Loss | | | | Total Shareholders' Equity | | |
| Balance at June 30, 2023 | 180,273 | | | $ | 2 | | | $ | 4,701 | | | | | $ | 262 | | | $ | (354) | | | | | $ | 4,611 | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Share issuances related to stock- based compensation | 641 | | | — | | | — | | | | | — | | | — | | | | | — | | | |
| | | | | | | | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 52 | | | | | — | | | — | | | | | 52 | | | |
| | | | | | | | | | | | | | | | | |
| Exercise of stock options | — | | | — | | | 9 | | | | | — | | | — | | | | | 9 | | | |
| Employee stock purchase plan | — | | | — | | | 7 | | | | | — | | | — | | | | | 7 | | | |
| | | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | | | (1,066) | | | — | | | | | (1,066) | | | |
Other comprehensive income,
net of tax | — | | | — | | | — | | | | | — | | | (2) | | | | | (2) | | | |
| Balance at March 31, 2024 | 180,914 | | | $ | 2 | | | $ | 4,769 | | | | | $ | (804) | | | $ | (356) | | | | | $ | 3,611 | | | |
Nine Months Ended March 31, 2023
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares of Common Stock | | Common Stock | | Additional Paid in Capital | | | | Accumulated Deficit | | Accumulated Other Comprehensive Loss | | | | Total Shareholders' Equity | |
| Balance at June 30, 2022 | 179,302 | | | $ | 2 | | | $ | 4,649 | | | | | $ | 518 | | | $ | (394) | | | | | $ | 4,775 | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Share issuances related to stock- based compensation | 855 | | | — | | | — | | | | | — | | | — | | | | | — | | |
| | | | | | | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 35 | | | | | — | | | — | | | | | 35 | | |
| | | | | | | | | | | | | | | | |
| Exercise of stock options | — | | | — | | | 4 | | | | | — | | | — | | | | | 4 | | |
| Employee stock purchase plan | — | | | — | | | 9 | | | | | — | | | — | | | | | 9 | | |
| | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | | | (146) | | | — | | | | | (146) | | |
Other comprehensive income,
net of tax | — | | | — | | | — | | | | | — | | | 26 | | | | | 26 | | |
| Balance at March 31, 2023 | 180,157 | | | $ | 2 | | | $ | 4,697 | | | | | $ | 372 | | | $ | (368) | | | | | $ | 4,703 | | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
Catalent, Inc.
Consolidated Statements of Cash Flows
(Unaudited; dollars in millions)
| | | | | | | | | | | |
| Nine Months Ended March 31, |
| 2024 | | 2023 |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | |
| Net loss | $ | (1,066) | | | $ | (146) | |
| | | |
| | | |
Adjustments to reconcile net loss to net cash provided by operating activities: | | | |
| Depreciation and amortization | 359 | | | 308 | |
| Goodwill impairment charges | 687 | | | 210 | |
| Non-cash foreign currency transaction loss (gain), net | 10 | | | (6) | |
| Non-cash restructuring charges | 7 | | | 18 | |
Amortization of debt issuance costs | 10 | | | 6 | |
Impairment charges and loss/gain on sale of assets, net | 27 | | | 4 | |
| | | |
| | | |
| | | |
Stock-based compensation | 52 | | | 35 | |
| Benefit from deferred income taxes | (24) | | | (69) | |
| Provision for bad debts and inventory | 87 | | | 99 | |
| Pension settlement charges | 12 | | | — | |
| Change in operating assets and liabilities: | | | |
| Decrease in trade receivables | 130 | | | 18 | |
| Increase in inventories | (61) | | | (135) | |
| Decrease in accounts payable | (58) | | | (39) | |
Other assets/accrued liabilities, net—current and non-current | (118) | | | (245) | |
| | | |
| | | |
| Net cash provided by operating activities | 54 | | | 58 | |
| CASH FLOWS USED IN INVESTING ACTIVITIES: | | | |
| Acquisition of property, equipment, and other productive assets | (252) | | | (455) | |
| Proceeds from maturity of marketable securities | — | | | 89 | |
| Proceeds from sale of property and equipment | 1 | | | 8 | |
| | | |
| | | |
| Payment for acquisitions, net of cash acquired | — | | | (474) | |
| Payment for investments | (2) | | | (2) | |
| | | |
| | | |
| | | |
| Net cash used in investing activities | (253) | | | (834) | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | |
| Proceeds from borrowings | 1,060 | | | 715 | |
| | | |
| | | |
| Payments related to long-term obligations | (971) | | | (176) | |
Financing fees paid | (16) | | | (4) | |
| | | |
| | | |
| | | |
| | | |
| Exercise of stock options | 9 | | | 4 | |
| Other financing activities | 2 | | | 33 | |
| | | |
| | | |
| Net cash provided by financing activities | 84 | | | 572 | |
| Effect of foreign currency exchange on cash and cash equivalents | (3) | | | 7 | |
| NET DECREASE IN CASH AND CASH EQUIVALENTS | (118) | | | (197) | |
| CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 280 | | | 449 | |
| CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 162 | | | $ | 252 | |
| SUPPLEMENTARY CASH FLOW INFORMATION: | | | |
| Interest paid | $ | 182 | | | $ | 145 | |
| Income taxes paid, net | $ | 64 | | | $ | 83 | |
| Non-cash purchase of property, equipment, and other productive assets | $ | 13 | | | $ | 8 | |
The accompanying notes are an integral part of these unaudited consolidated financial statements.
Catalent, Inc.
Notes to Unaudited Consolidated Financial Statements
1. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business
Catalent, Inc. (“Catalent” or the “Company”) directly and wholly owns PTS Intermediate Holdings LLC (“Intermediate Holdings”). Intermediate Holdings directly and wholly owns Catalent Pharma Solutions, Inc. (“Operating Company”). The financial results of Catalent are comprised of the financial results of Operating Company and its subsidiaries on a consolidated basis.
Basis of Presentation
The accompanying unaudited consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the three and nine months ended March 31, 2024 are not necessarily indicative of the results that may be expected for the year ending June 30, 2024. The consolidated balance sheet at June 30, 2023 has been derived from the audited consolidated financial statements at that date but does not include all of the information and footnotes required by U.S. GAAP for complete financial statements. For further information on the Company's accounting policies and footnotes, refer to the consolidated financial statements and notes thereto included in the Company’s Annual Report on Form 10-K for the year ended June 30, 2023 filed with the Securities and Exchange Commission (the “SEC”) on December 8, 2023.
Reportable Segments
Set forth below is a summary description of the Company's two current operating and reportable segments.
Biologics—The Biologics segment provides development and manufacturing for biologic proteins; cell, gene, and other nucleic acid therapies; plasmid DNA ("pDNA"); induced pluripotent stem cells ("iPSCs"), and oncolytic viruses; and vaccines. It also provides formulation, development, and manufacturing for parenteral dose forms, including vials, prefilled syringes, and cartridges; analytical development and testing services for large molecules.
Pharma and Consumer Health—The Pharma and Consumer Health segment comprises the Company’s market-leading capabilities for complex oral solids, softgel formulations, Zydis® fast-dissolve technologies, and gummy, soft chew, and lozenge dosage forms; formulation, development, and manufacturing platforms for oral, nasal, inhaled, and topical dose forms; and clinical trial development and supply services.
Each segment reports through a separate management team and ultimately reports to the Company's President and Chief Executive Officer, who is designated as the Chief Operating Decision Maker for segment reporting purposes. The Company's operating segments are the same as its reportable segments.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Foreign Currency Translation
The financial statements of the Company’s operations are generally measured using the local currency as the functional currency. Adjustments to translate the assets and liabilities of operations outside the United States (“U.S.”) into U.S. dollars are accumulated as a component of other comprehensive income/(loss) utilizing period-end exchange rates. Since July 1, 2018, the Company has accounted for its Argentine operations as highly inflationary.
Concentrations of Credit Risk and Major Customers
Concentration of credit risk, with respect to accounts receivable, is limited due to the large number of customers and their dispersion across different geographic areas. The customers are primarily concentrated in the pharmaceutical, biopharmaceutical and consumer products industries. The Company does not normally require collateral or any other security to support credit sales. The Company performs ongoing credit evaluations of its customers’ financial conditions and maintains reserves for credit losses. Such losses historically have been within the Company’s expectations.
As of March 31, 2024 and June 30, 2023, the Company had one customer that represented 31% and 20%, respectively, of its aggregate net trade receivables and current contract asset values, primarily associated with the Company's Biologics segment. Additionally, the Company had one customer in its Biologics segment that represented approximately 14% and 16% of consolidated net revenue during the three and nine months ended March 31, 2024, respectively. The Company had two customers in its Biologics segment that each represented approximately 11% and 10% of consolidated net revenue during the three and nine months ended March 31, 2023, respectively.
Depreciation
Depreciation expense was $92 million and $72 million for the three months ended March 31, 2024 and 2023, respectively. Depreciation expense was $258 million and $207 million for the nine months ended March 31, 2024 and 2023, respectively. Depreciation expense includes amortization of assets related to finance leases. The Company charges repairs and maintenance costs to expense as incurred.
Amortization
Amortization expense related to other intangible assets was $34 million and $34 million for the three months ended March 31, 2024 and 2023, respectively. Amortization expense related to other intangible assets was $101 million and $101 million for the nine months ended March 31, 2024 and 2023, respectively.
Research and Development Costs
The Company expenses research and development costs as incurred. Research and development costs amounted to $4 million for both the three months ended March 31, 2024 and 2023. Research and development costs amounted to $12 million and $13 million for the nine months ended March 31, 2024 and 2023, respectively. Research and development costs are recorded in selling, general and administrative expenses in the consolidated statement of operations.
2. REVENUE RECOGNITION
The Company recognizes revenue in accordance with ASC 606, Revenue from Contracts with Customers. The Company generally earns its revenue by supplying goods or providing services under contracts with its customers in three primary revenue streams: manufacturing and commercial product supply, development services, and clinical supply services. The Company measures the revenue from customers based on the consideration specified in its contracts, excluding any sales incentive or amount collected on behalf of a third party, that the Company expects to be entitled to receive in exchange for transferring the promised goods to and/or performing services for the customer (the “Transaction Price”). To the extent the Transaction Price includes variable consideration, the Company estimates the amount of variable consideration that should be included in the Transaction Price utilizing either the expected value method or the most likely amount method, depending on which method is expected to better predict the amount of consideration to which the Company will be entitled. The value of variable consideration is included in the Transaction Price if, and to the extent, it is probable that a significant reversal of the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. These estimates are re-assessed each reporting period, as required, and any adjustment required is recorded on a cumulative catch-up basis, which would affect revenue and net income in the period of adjustment.
The Company’s customer contracts generally include provisions entitling the Company to a termination penalty when the customer terminates prior to the contract’s nominal end date. The termination penalties in customer contracts vary but are generally considered substantive for accounting purposes and create enforceable rights and obligations throughout the stated durations of the contracts. The Company accounts for a contract termination as a contract modification in the period in which the customer gives notice of termination. The determination of the contract termination penalty is based on the terms stated in the relevant customer agreement. As of the modification date, the Company updates its estimate of the Transaction Price using the expected value method, subject to constraints, and to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. These estimates are re-assessed each reporting period, as required, and any adjustment required is recorded on a cumulative catch-up basis, which would affect revenue and net income in the period of adjustment.
Where multiple performance obligations exist in a single contract, the Company allocates consideration to each performance obligation using the “relative standalone selling price” as defined under ASC 606. Generally, the Company utilizes observable standalone selling prices in its allocations of consideration. If observable standalone selling prices are not available, the Company estimates the applicable standalone selling price using a cost-plus-margin approach or an adjusted market assessment approach, in each case, representing the amount that the Company believes the market is willing to pay for the applicable service. Payment is typically due 30 to 45 days following the invoice date, based on the payment terms set forth in the applicable customer agreement.
The Company generally expenses sales commissions as incurred because either the amortization period is one year or less, or the balance with an amortization period greater than one year is not material.
Customer contracts that include commitments by the Company to make facility space or equipment available may be deemed to include lease components, which are evaluated under ASC 842, Leases. For arrangements that contain both lease and non-lease components, consideration in the contract is allocated on a relative standalone selling-price basis. Determining the lease term and contract term of non-lease components, as well as the variable and fixed consideration in these arrangements, including when variability is resolved, often requires management judgment in order to determine the allocation to the lease and non-lease components.
Manufacturing & Commercial Product Supply Revenue
Manufacturing and commercial product supply revenue consists of revenue earned by manufacturing products supplied to customers under long-term commercial supply arrangements. In these arrangements, the customer typically owns and supplies the active pharmaceutical ingredient (“API”) or other proprietary materials used in the manufacturing process. The contract generally includes the terms of the manufacturing services and related product quality assurance procedures to comply with regulatory requirements. Due to the regulated nature of the Company’s business, these contract terms are highly interdependent and, therefore, are considered to be a single combined performance obligation. The transaction price is generally stated in the agreement as a fixed price per unit, with no contractual provision for a refund or price concession. In most circumstances, control is transferred to the customer over time, creating a corresponding right to recognize the related revenue, because there is no alternative use to the Company for the asset created and the Company has an enforceable right to payment for performance completed as of that date. The selection of the method for measuring progress towards the completion of the Company’s performance obligation requires judgment and is based on the nature of the products to be manufactured. For the majority of the Company’s arrangements, progress is measured based on the units of product that have successfully completed the contractually required product quality assurance process, because the conclusion of that process defines the time when the applicable contract and the related regulatory requirements permit the customer to exercise control over the product’s disposition. The customer is typically responsible for arranging the shipping and handling of product following completion of the quality assurance process. Payment is typically due 30 to 45 days after invoice date, based on the payment terms set forth in the applicable customer agreement.
Beginning in the third quarter of fiscal 2023, the Company began recognizing commercial revenue for certain contracts in its Biologics segment that have a notably long manufacturing cycle, and for which the customer exercises control over the product throughout the manufacturing process. For these contracts, revenue is recognized over time and progress is measured using an input method based on effort expended, which provides an appropriate depiction of the Company’s progress toward fulfilling its performance obligation.
Development Services and Clinical Supply Revenue
Development services contracts generally take the form of short-term, fee-for-service arrangements. Performance obligations vary, but frequently include biologic cell-line development, performing formulation, analytical stability, or other services related to product development, and providing manufacturing services for products that are under development or otherwise not intended for commercial sale. They can also include a combination of the following services: the manufacturing, packaging, storage, distribution, destruction, and inventory management of customer clinical trial material, as well as the sourcing of comparator drug products on behalf of customers to be used in clinical trials to compare performance with the drug under clinical investigation. The transaction prices for these arrangements are fixed and include amounts stated in the contracts for each promised service, and each service is generally considered to be a separate performance obligation. In most instances, the Company recognizes revenue over time because there is no alternative use to the Company for the asset created and the Company has an enforceable right to payment for performance completed as of that date.
The Company measures progress toward the completion of its performance obligations satisfied over time based on the nature of the services to be performed. For certain types of arrangements, revenue is recognized over time and measured using an output method based on the completion of tasks and activities that are performed to satisfy a performance obligation. For
certain types of arrangements, revenue is recognized over time and measured using an input method based on effort expended. Each of these methods provides an appropriate depiction of the Company’s progress toward fulfilling its performance obligations for its respective arrangement. In certain development services arrangements that require a portion of the contract consideration to be received in advance at the commencement of the contract, such advance payment is initially recorded as a contract liability. In certain clinical supply arrangements, revenue is recognized at the point in time when control transfers, which occurs upon either the delivery of the related output of the service to the customer or the completion of quality testing with respect to the product, and the Company has an enforceable right to payment based on the terms of the arrangement.
The Company records revenue for comparator sourcing arrangements on a net basis because it is acting as an agent that does not control the product or service before it is transferred to the customer. Payment for comparator sourcing activity is typically received in advance at the commencement of the contract and is initially recorded as a contract liability.
The following tables reflect net revenue for the three and nine months ended March 31, 2024 and 2023, by type of activity and reportable segment (in millions):
| | | | | | | | | | | | | | | | | |
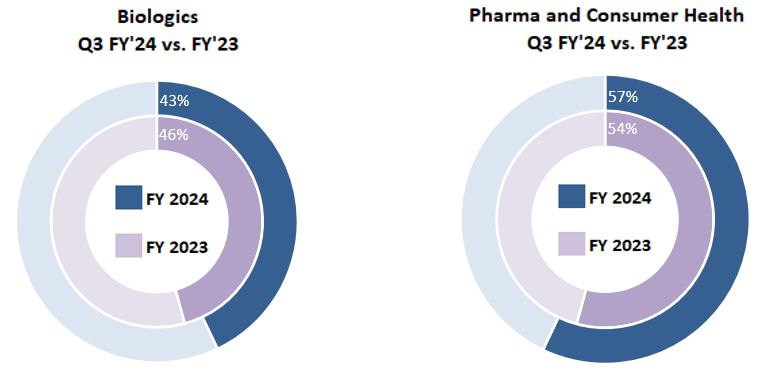
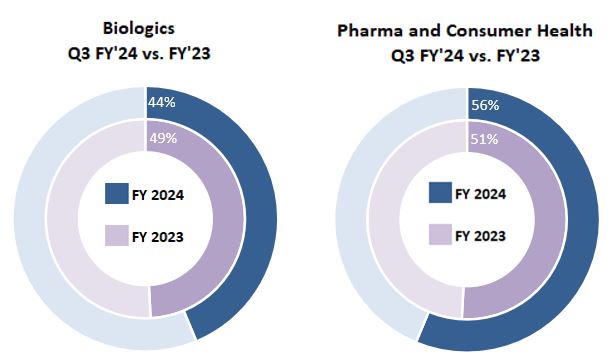
| Three Months Ended March 31, 2024 | Biologics | | Pharma and Consumer Health | | Total |
| Manufacturing & commercial product supply | $ | 261 | | | $ | 402 | | | $ | 663 | |
| Development services & clinical supply | 200 | | | 211 | | | 411 | |
| Total | $ | 461 | | | $ | 613 | | | $ | 1,074 | |
| Inter-segment revenue elimination | | — | |
| Combined net revenue | | $ | 1,074 | |
| | | | | | | | | | | | | | | | | |
| Three Months Ended March 31, 2023 | Biologics | | Pharma and Consumer Health | | Total |
| Manufacturing & commercial product supply | $ | 133 | | | $ | 349 | | | $ | 482 | |
| Development services & clinical supply | 342 | | | 214 | | | 556 | |
| Total | $ | 475 | | | $ | 563 | | | $ | 1,038 | |
| Inter-segment revenue elimination | (1) | |
| Combined net revenue | | $ | 1,037 | |
| | | | | | | | | | | | | | | | | |
Nine Months Ended March 31, 2024 | Biologics | | Pharma and Consumer Health | | Total |
| Manufacturing & commercial product supply | $ | 821 | | | $ | 1,109 | | | $ | 1,930 | |
| Development services & clinical supply | 526 | | | 625 | | | 1,151 | |
| Total | $ | 1,347 | | | $ | 1,734 | | | $ | 3,081 | |
| Inter-segment revenue elimination | | (1) | |
| Combined net revenue | | $ | 3,080 | |
| | | | | | | | | | | | | | | | | |
Nine Months Ended March 31, 2023 | Biologics | | Pharma and Consumer Health | | Total |
| Manufacturing & commercial product supply | $ | 304 | | | $ | 1,027 | | | $ | 1,331 | |
| Development services & clinical supply | 1,274 | | | 605 | | | 1,879 | |
| Total | $ | 1,578 | | | $ | 1,632 | | | $ | 3,210 | |
| Inter-segment revenue elimination | (2) | |
| Combined net revenue | | $ | 3,208 | |
The following table allocates revenue by the location where the goods were made or the service performed:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| (Dollars in millions) | | 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | | |
| United States | | $ | 690 | | | $ | 685 | | | $ | 1,990 | | | $ | 2,117 | |
| Europe | | 345 | | | 306 | | | 942 | | | 936 | |
| Other | | 88 | | | 79 | | | 261 | | | 249 | |
| Elimination of revenue attributable to multiple locations | | (49) | | | (33) | | | (113) | | | (94) | |
| Total | | $ | 1,074 | | | $ | 1,037 | | | $ | 3,080 | | | $ | 3,208 | |
Contract Liabilities
Contract liabilities relate to cash consideration that the Company receives in advance of satisfying the related performance obligations. The contract liabilities balances (current and non-current) as of March 31, 2024 and June 30, 2023 are as follows:
| | | | | | | | |
| (Dollars in millions) | | |
| | |
| Balance at June 30, 2023 | | $ | 180 | |
| | |
| | |
| | |
| | |
| Balance at March 31, 2024 | | $ | 255 | |
| Revenue recognized in the period from amounts included in contracts liability at the beginning of the period: | | $ | (122) | |
Contract liabilities that will be recognized within 12 months of March 31, 2024 are accounted for in Other accrued liabilities and those that will be recognized longer than 12 months after March 31, 2024 are accounted for in Other liabilities.
Contract Assets
Contract assets primarily relate to the Company's conditional right to receive consideration for services that have been performed for customers as of March 31, 2024 relating to the Company's development and commercial services but had not yet been invoiced as of March 31, 2024. Contract assets are transferred to trade receivables, net when the Company’s right to receive the consideration becomes unconditional. Contract assets totaled $490 million and $417 million as of March 31, 2024 and June 30, 2023, respectively. Contract assets expected to transfer to trade receivables within 12 months are accounted for within Prepaid expenses and other. Contract assets expected to transfer to trade receivables longer than 12 months are accounted for within Other long-term assets.
As of March 31, 2024, the Company's aggregate contract asset balance increased $73 million or 18% compared to June 30, 2023. The majority of this increase is related to large development and commercial programs in the Biologics segment, such as manufacturing and development services for gene therapy offerings, where revenue is recorded over time and the ability to invoice customers is dictated by contractual terms.
Performance Obligations
Remaining performance obligations represent firm orders for future development services as well as manufacturing and commercial product supply, including minimum volume commitments, for which there are incomplete performance obligations for work not yet completed under executed contracts. Remaining performance obligations as of March 31, 2024 were $585 million. The Company expects to recognize approximately 26% of the remaining performance obligations in existence as of March 31, 2024 after June 30, 2025.
3. BUSINESS COMBINATIONS
Metrics Contract Services Acquisition
In October 2022, the Company acquired 100% of Metrics Contract Services (“Metrics”) from Mayne Pharma Group Limited for $474 million in cash. Metrics, based in Greenville, North Carolina, is an oral solids development and manufacturing business specializing in the manufacture of drugs containing highly potent active pharmaceutical ingredients. The operations and facility acquired have become part of the Company’s Pharma and Consumer Health segment.
The Company accounted for the Metrics transaction using the acquisition method in accordance with ASC 805, Business Combinations. The Company funded this acquisition with a portion of the proceeds of an October 2022 drawdown from its senior secured revolving credit facility. The Company estimated fair values at the date of acquisition for the allocation of consideration to the net tangible and intangible assets acquired and liabilities assumed.
The purchase price allocation to assets acquired and liabilities assumed in the transaction is as follows:
| | | | | |
| (Dollars in millions) | Final Purchase Price Allocation |
| Trade receivables, net | $ | 15 | |
| Inventories | 5 | |
| Property, plant, and equipment | 195 | |
| Other intangibles, net | 52 | |
| |
| Other, net | (12) | |
| Goodwill | 219 | |
| Total assets acquired and liabilities assumed | $ | 474 | |
The carrying value of trade receivables, inventory, and trade payables, as well as certain other current and non-current assets and liabilities generally represented the fair value at the date of acquisition.
Other intangibles, net consists of customer relationships of $52 million, which were valued using the multi-period, excess-earnings method, a method that values the intangible asset using the present value of the after-tax cash flows attributable to the intangible asset only. The significant assumptions used in developing the valuation included the estimated annual net cash flows (including application of an appropriate margin to forecasted revenue, selling and marketing costs, return on working capital, contributory asset charges, and other factors), the discount rate that appropriately reflects the risk inherent in each future cash flow stream, and an assessment of the asset’s life cycle, as well as other factors. The assumptions used in the financial forecasts were based on historical data, supplemented by current and anticipated growth rates, management plans, and market-comparable information. Fair-value determinations require considerable judgment and are sensitive to changes in underlying assumptions and factors. The customer relationship intangible asset has a weighted average useful life of 12 years.
Property, plant, and equipment was valued using the cost approach, which is based on current replacement and/or reproduction cost of the asset as new, less depreciation attributable to physical, functional, and economic factors. The Company then determined the remaining useful life based on the anticipated life of the asset and Company policy for similar assets.
Goodwill was allocated to the Pharma and Consumer Health segment. Goodwill is mainly comprised of the growth from an expected increase in capacity utilization and potential new customers. The goodwill resulting from the Metrics acquisition is not deductible for tax purposes.
4. GOODWILL
The following table summarizes the changes between June 30, 2023 and March 31, 2024 in the carrying amount of goodwill in total and by segment:
| | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Biologics | | Pharma and Consumer Health | | | | | | Total |
| Balance at June 30, 2023 | $ | 1,563 | | | $ | 1,476 | | | | | | | $ | 3,039 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Foreign currency translation adjustments | (4) | | | (9) | | | | | | | (13) | |
Impairment (1) | (392) | | | (295) | | | | | | | (687) | |
| Balance at March 31, 2024 | $ | 1,167 | | | $ | 1,172 | | | | | | | $ | 2,339 | |
(1) Represents gross impairment charges in the period. Accumulated goodwill impairment charges amount to $897 million as of March 31, 2024.
Goodwill Impairment Charges
As a result of the Consumer Health reporting unit's underperformance of recent operating results relative to expectations, the current macroeconomic conditions impacting the consumer health and biotechnology industries, and increased interest rates, the Company assessed the current and future economic outlook as of September 30, 2023 for its reporting units in its Pharma and Consumer Health and Biologics segments and identified indicators for impairment of the goodwill previously recorded for two of its reporting units. The evaluation began with a qualitative assessment of the Company's Consumer Health and Biomodalities reporting units to determine if it was more likely than not that the fair value of the reporting units was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in its Consumer Health and Biomodalities reporting units, which led to a quantitative assessment for the corresponding reporting units.
The Company estimated the fair value of its reporting units using a combination of the income and market approaches. In performing the goodwill impairment test, the Company used a terminal revenue growth rate of 3.5% and discount rates ranging from 9% to 10% in its estimation of fair value. The evaluation performed resulted in impairment charges of $687 million with respect to the Consumer Health and Biomodalities reporting units.
While the Company believes the assumptions it used were reasonable and commensurate with the views of a market participant, changes in key assumptions, including increasing the discount rate, lowering forecasts for revenue and operating margin or lowering the long-term growth rate could lead to the conclusion that an additional impairment was appropriate.
A qualitative assessment was performed as of March 31, 2024, which yielded no indicators of impairment.
The Company assessed the current and future economic outlook as of March 31, 2023 for its reporting units in its Pharma and Consumer Health and Biologics segments and identified an indicator for impairment of the goodwill previously recorded for one of the reporting units in its Pharma and Consumer Health segment. The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in its Consumer Health reporting unit, which led to a quantitative assessment for each of the Company's reporting units.
The Company estimated the fair value of its reporting units using a combination of the income and market approaches. In performing the goodwill impairment test, the Company used a long-term revenue growth rate of 3% and discount rates ranging from 9% to 10.50% in its estimation of fair value. The evaluation performed resulted in an impairment charge of $210 million with respect to the Consumer Health reporting unit.
5. LONG-TERM OBLIGATIONS AND SHORT-TERM BORROWINGS
Long-term obligations and short-term borrowings consisted of the following at March 31, 2024 and June 30, 2023:
| | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Maturity | | March 31, 2024 | | June 30, 2023 |
| Senior secured credit facilities | | | | | |
| Term loan facility B-3 (7.443% as of March 31, 2024) | February 2028 | | $ | 1,408 | | | $ | 1,418 | |
| Term loan facility B-4 (8.329% as of March 31, 2024) | February 2028 | | 600 | | | — | |
| Revolving credit facility | November 2027 | | — | | | 500 | |
| 5.000% senior notes due 2027 | July 2027 | | 500 | | | 500 | |
2.375% Euro senior notes due 2028(1) | March 2028 | | 893 | | | 904 | |
| 3.125% senior notes due 2029 | February 2029 | | 550 | | | 550 | |
| 3.500% senior notes due 2030 | April 2030 | | 650 | | | 650 | |
| Financing lease obligations | 2024 to 2038 | | 392 | | | 341 | |
Other obligations(2) | 2024 to 2028 | | 34 | | | 25 | |
| Unamortized discount and debt issuance costs | | | (47) | | | (39) | |
| Total debt | | | $ | 4,980 | | | $ | 4,849 | |
Less: current portion of long-term obligations and other short-term
borrowings | | | 47 | | | 536 | |
| Long-term obligations, less current portion | | | $ | 4,933 | | | $ | 4,313 | |
(1) The change in the carrying value of this euro-denominated debt was due to fluctuations in foreign currency exchange rates.
(2) The increase in other obligations is primarily associated with $15 million in proceeds from a failed sale-leaseback transaction that occurred in the three months ended September 30, 2023.
On November 22, 2023, Operating Company, entered into Amendment No. 10 (the “Tenth Amendment”) to its Amended and Restated Credit Agreement dated May 20, 2014 (as amended, the “Credit Agreement”), which Tenth Amendment further extends the deadlines by which the Operating Company is required to deliver to the administrative agent (i) its audited financial statements as at the end of and for the fiscal year ended June 30, 2023, together with the auditor’s report and opinion on such audited financial statements, to January 26, 2024, and (ii) its unaudited financial statements as at the end of and for the fiscal quarter ending September 30, 2023 to March 13, 2024.
On December 19, 2023, Operating Company entered into Amendment No. 11 (the “Eleventh Amendment”) to the Credit Agreement. Pursuant to the Eleventh Amendment, the Operating Company incurred $600 million aggregate principal amount of U.S. dollar-denominated term B-4 loans (the “Term B-4 Loans”).The Term B-4 Loans are a new class of term loans under the Credit Agreement, with an interest rate, at Operating Company’s option, of either (i) the term SOFR rate plus 3.00% or (ii) the base rate plus 2.00%; provided, that the term SOFR rate shall not be less than 0.50%. The Term B-4 Loans have a maturity date of February 2028, quarterly amortization of principal equal to 1.00% with payments on the last business day of March, June, September, and December. The proceeds of the Term B-4 Loans, after payment of fees and expenses, were used to repay the existing Revolving Credit Facility under the Credit Agreement, plus accrued and unpaid interest thereon.
The Credit Agreement requires compliance with a net leverage covenant when there is a 30% or more draw outstanding at a period end. As of March 31, 2024, we were in compliance with all covenants under the Credit Agreement.
In addition to outstanding borrowings under the Revolving Credit facility, the available capacity under the Revolving Credit Facility is further reduced by the aggregate value of all outstanding letters of credit under the Credit Agreement. As of March 31, 2024, Operating Company had $1.10 billion of available capacity under the Revolving Credit Facility, due to $4 million of outstanding letters of credit.
Measurement of the Estimated Fair Value of Debt
The estimated fair value of the Company’s senior secured credit facilities and other senior indebtedness is classified as a Level 2 determination (see Note 10, Fair Value Measurements to our consolidated financial statements, for a description of the method by which fair value classifications are determined) in the fair-value hierarchy and is calculated by using a discounted cash flow model with a market interest rate as a significant input. The carrying amounts and the estimated fair values of the Company’s principal categories of debt as of March 31, 2024 and June 30, 2023 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | March 31, 2024 | | June 30, 2023 |
| (Dollars in millions) | Fair Value Measurement | | Carrying Value | | Estimated Fair Value | | Carrying Value | | Estimated Fair Value |
| | | | | | | | | |
| | | | | | | | | |
| 5.000% senior notes due 2027 | Level 2 | | $ | 500 | | | $ | 495 | | | $ | 500 | | | $ | 482 | |
| 2.375% Euro senior notes due 2028 | Level 2 | | 893 | | | 849 | | | 904 | | | 784 | |
| 3.125% senior notes due 2029 | Level 2 | | 550 | | | 528 | | | 550 | | | 481 | |
| 3.500% senior notes due 2030 | Level 2 | | 650 | | | 631 | | | 650 | | | 566 | |
| Senior secured credit facilities & other | Level 2 | | 2,434 | | | 2,161 | | | 2,284 | | | 2,141 | |
| Subtotal | | | $ | 5,027 | | | $ | 4,664 | | | $ | 4,888 | | | $ | 4,454 | |
Unamortized discount and debt issuance
costs | | | (47) | | | — | | | (39) | | | — | |
| Total debt | | | $ | 4,980 | | | $ | 4,664 | | | $ | 4,849 | | | $ | 4,454 | |
6. LOSS PER SHARE
The Company computes (loss) earnings per share of the Company’s common stock, par value $0.01 (the “Common Stock”) using the treasury stock method. Diluted net (loss) earnings per share is computed using the weighted average number of shares of Common Stock outstanding plus the weighted average number of shares of Common Stock that would be issued assuming exercise or conversion of all potentially dilutive instruments. Dilutive securities having an anti-dilutive effect on diluted net earnings per share are excluded from the calculation. The dilutive effect of the securities that are issuable under the Company’s equity incentive plans are reflected in diluted earnings per share by application of the treasury stock method. The reconciliations between basic and diluted earnings per share attributable to Catalent common shareholders for the three and nine months ended March 31, 2024 and 2023, respectively, are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| (In millions except per share data) | 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| | | | | | | |
| Net loss | $ | (101) | | | $ | (227) | | | $ | (1,066) | | | $ | (146) | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Weighted average shares outstanding - basic | 182 | | | 181 | | | 181 | | | 180 | |
| Weighted average dilutive securities issuable - stock plans | — | | | — | | | — | | | — | |
| Weighted average shares outstanding - diluted | 182 | | | 181 | | | 181 | | | 180 | |
| | | | | | | |
| Loss per share: | | | | | | | |
| | | | | | | |
| | | | | | | |
| Basic | $ | (0.56) | | | $ | (1.26) | | | $ | (5.87) | | | $ | (0.81) | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Diluted | $ | (0.56) | | | $ | (1.26) | | | $ | (5.87) | | | $ | (0.81) | |
Shares with an antidilutive effect on the weighted average shares outstanding for the three and nine months ended March 31, 2024 and 2023 were not material.
7. OTHER EXPENSE (INCOME), NET
The components of other expense (income), net for the three and nine months ended March 31, 2024 and 2023 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Foreign currency losses (gains) (1) | $ | 2 | | | $ | (4) | | | 17 | | | (5) | |
| Other | 2 | | | — | | | 4 | | | 3 | |
| Total other expense (income), net | $ | 4 | | | $ | (4) | | | $ | 21 | | | $ | (2) | |
(1) Foreign currency remeasurement gains/losses include both cash and non-cash transactions.
8. RESTRUCTURING COSTS
From time to time, the Company implements plans to restructure certain operations, both domestically and internationally. The restructuring plans focused on various aspects of operations, including, among others, closing and consolidating certain manufacturing operations, rationalizing headcount and aligning operations in a strategic and more cost-efficient structure. In addition, the Company may incur restructuring charges in the future in cases where a material change in the scope of operation with its business occurs. Employee-related restructuring costs consist primarily of severance costs and also include outplacement services provided to employees who have been involuntarily terminated and duplicate payroll costs during transition periods. Facility exit and other such restructuring costs consist of equipment relocation costs and costs associated with planned facility expansions and closures to streamline Company operations.
During the fiscal year ended June 30, 2023, the Company adopted plans to reduce costs, consolidate facilities, and optimize its infrastructure across the organization. During the three months ended March 31, 2024, the Company extended its restructuring efforts to reduce costs and headcount in both its Biologics and Pharma and Consumer Health segments.
In October 2023, and in connection with the Company's restructuring plans, the Company committed to a plan to close operations at its San Francisco facility and to transfer those operations to other sites within its network. The Company expects to incur cash and non-cash charges of at least $25 million in connection with the site closure, primarily related to a pension liability from a multi-employer pension plan and accelerated depreciation of property, plant and equipment in the second half of fiscal 2024. Results for the three and nine months ended March 31, 2024 are reflected in the tables below under the Pharma and Consumer Health segment.
In connection with these restructuring plans, the Company reduced its headcount by approximately 550 employees and incurred cumulative employee-related charges of approximately $22 million, primarily associated with cash severance programs through March 31, 2024.
Restructuring costs for the three and nine months ended March 31, 2024 and 2023 were recorded in Other Operating Expense in the Consolidated Statement of Operations.
The following table summarizes the charges recorded within restructuring costs:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
(Dollars in millions) | 2024 | | 2023 | | 2024 | | 2023 |
| Restructuring costs: | | | | | | | |
| Employee-related reorganization | $ | 10 | | | $ | 4 | | | $ | 22 | | | $ | 18 | |
| Facility exit and other costs | 1 | | | 5 | | | 8 | | | 18 | |
| Total restructuring costs | $ | 11 | | | $ | 9 | | | $ | 30 | | | $ | 36 | |
The following table summarizes the charges recorded within restructuring costs by segment. These amounts are excluded from Segment EBITDA as described in Note 15, Segment Information.
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
(Dollars in millions) | 2024 | | 2023 | | 2024 | | 2023 |
| Restructuring costs: | | | | | | | |
| Biologics | $ | 3 | | | $ | 7 | | | $ | 10 | | | $ | 25 | |
| Pharma and Consumer Health | 2 | | | 1 | | | 13 | | | 5 | |
| Non-segment (Corporate) | 6 | | | 1 | | | 7 | | | 6 | |
| Total restructuring costs | $ | 11 | | | $ | 9 | | | $ | 30 | | | $ | 36 | |
The following tables summarizes the change in the employee separation-related liability associated with the restructuring plans.
| | | | | |
| Employee-related restructuring |
(Dollars in millions) | |
| Balance, June 30, 2023 | $ | 19 | |
| Charges | 22 | |
| Payments | (25) | |
| Balance, March 31, 2024 | $ | 16 | |
9. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
Risk Management Objective of Using Derivatives
The Company is exposed to fluctuations in the currency exchange rates applicable to its investments in operations outside the U.S. While the Company does not actively hedge against changes in foreign currency, the Company has mitigated exposure from its investments in its European operations by denominating a portion of its debt in euros. At March 31, 2024, the Company had euro-denominated debt outstanding of $893 million (U.S. dollar equivalent), which is designated and qualifies as a hedge against its net investment in its European operations. For non-derivatives designated and qualifying as net investment hedges, the effective portion of translation gains or losses are reported in accumulated other comprehensive loss as part of the cumulative translation adjustment. The following table summarizes net investment hedge activity during the three and nine months ended March 31, 2024 and 2023.
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 | | 2024 | | 2023 |
Unrealized foreign exchange gain (loss) within other comprehensive income | $ | 17 | | | $ | (16) | | | $ | 11 | | | $ | (20) | |
| | | | | | | |
The net accumulated gain on the instrument designated as a hedge as of March 31, 2024 within other comprehensive loss was approximately $108 million. Amounts are reclassified out of accumulated other comprehensive loss into earnings when the entity to which the gains and losses relate is either sold or substantially liquidated.
Interest-Rate Swap
In February 2021, the Company entered into a seven year interest-rate swap agreement with Bank of America N.A. (the “2021 Rate Swap”) as a hedge against the economic effect of a portion of the variable interest obligation associated with its Term B-3 Loans. The 2021 Rate Swap effectively fixed the rate of interest payable on that portion of the Term B-3 Loans, thereby reducing the impact of future interest rate changes on future interest expense. As a result of the 2021 Rate Swap, the variable portion of the applicable interest rate on $500 million of the Term B-3 Loans was fixed at 0.9985%.
To conform with the adoption of Topic 848, Reference Rate Reform and the Eighth Amendment, the Company amended the 2021 Rate Swap in June 2023 (the “2023 Rate Swap”). The 2023 Rate Swap continues to effectively fix the rate of interest payable on the same portion of our U.S. dollar-denominated term loans under our senior secured credit facilities. As a result of
the 2023 Rate Swap, the variable portion of the applicable interest rate on $500 million of the U.S. dollar-denominated term loans is now effectively fixed at 0.9431%.
The 2023 Rate Swap continues to qualify for a cash-flow hedge. The Company evaluates hedge effectiveness at the inception of the hedge and on an ongoing basis. The cash flows associated with the 2023 Rate Swap amendment is reported in cash provided by operating activities in the consolidated statements of cash flows. The unrealized gain recorded in stockholder's equity related to the mark-to-market change in the 2023 Rate Swap during the nine months ended March 31, 2024 was $5 million.
A summary of the estimated fair value of the 2023 Rate Swap reported in the consolidated balance sheets is stated in the table below:
| | | | | | | | | | | | | | | | | | | | | | | |
| March 31, 2024 | | June 30, 2023 |
| (Dollars in millions) | Balance Sheet Classification | | Estimated Fair Value | | Balance Sheet Classification | | Estimated Fair Value |
| Interest-rate swap | Other long-term assets | | $ | 67 | | | Other long-term assets | | $ | 62 | |
10. FAIR VALUE MEASUREMENTS
ASC 820, Fair Value Measurement, defines fair value as the exit price that would be received to sell an asset or paid to transfer a liability. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. Valuation techniques used to measure fair value should maximize the use of observable inputs and minimize the use of unobservable inputs. To measure fair value, the Company uses the following fair value hierarchy based on three levels of inputs, of which Level 1 and Level 2 are considered observable and Level 3 is considered unobservable:
Level 1 – Quoted prices in active markets for identical assets or liabilities.
Level 2 – Inputs other than Level 1 that are observable for the asset or liability, either directly or indirectly, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data by correlation or other means.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Value is determined using pricing models, discounted cash flow methodologies, or similar techniques and also includes instruments for which the determination of fair value requires significant judgment or estimation.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable and accrued expenses of the Company approximate fair value based on the short maturities of these instruments.
The Company evaluates its financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level of classification as of the end of each reporting period. The following table sets forth the Company’s financial assets and liabilities that were measured at fair value on a recurring basis and the fair value measurement for such assets and liabilities at March 31, 2024 and June 30, 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | | Basis of Fair Value Measurement |
| March 31, 2024 | | Total | | Level 1 | | Level 2 | | Level 3 |
| Assets: | | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Interest-rate swap | | $ | 67 | | | $ | — | | | $ | 67 | | | $ | — | |
| Trading securities | | 1 | | | 1 | | | — | | | — | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| June 30, 2023 | | | | | | | | |
| Assets: | | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Interest-rate swap | | $ | 62 | | | $ | — | | | $ | 62 | | | $ | — | |
| Trading securities | | 1 | | | 1 | | | — | | | — | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The fair value of the 2021 Rate Swap was determined, and the fair value of the 2023 Rate Swap is determined, at the end of each reporting period based on valuation models that use interest rate yield curves and discount rates as inputs. The discount rates are based on U.S. deposit or U.S. Treasury rates. The significant inputs used in the valuation models are readily available in public markets or can be derived from observable market transactions, and the valuation is therefore classified as Level 2 in the fair-value hierarchy.
Assets and Liabilities Measured at Fair Value on a Non-Recurring Basis
Long-lived assets, goodwill, and other intangible assets are subject to non-recurring fair value measurement for the evaluation of potential impairment. Except as noted in Note 4, Goodwill, there was no non-recurring fair value measurement during the nine months ended March 31, 2024.
11. INCOME TAXES
The Company accounts for income taxes in accordance with ASC 740, Income Taxes. Generally, fluctuations in the effective tax rate are due to changes in relative amounts of U.S. and non-U.S. pretax income, the tax impact of special items, and other discrete tax items. Discrete items include, but are not limited to, changes in non-U.S. statutory tax rates, amortization of certain assets, changes in the Company’s reserve for uncertain tax positions, and tax impact of certain equity compensation.
In the normal course of business, the Company is subject to examination by taxing authorities around the world. The Company is presently under audit in select jurisdictions in the United States and in Europe, but no material impact is expected to the financial results once these audits are completed.
ASC 740 provides guidance for the accounting of uncertain income tax positions recognized in the Company's tax filings. This guidance provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that, based on technical merits, the position will be sustained upon examination, including resolution of any related appeal or litigation process. As of March 31, 2024 and June 30, 2023, the Company’s reserve against uncertain income tax positions was $3 million and $4 million, respectively. Interest and penalties related to uncertain tax positions are recognized as a component of income tax expense.
The Company recorded a provision for income taxes for the three months ended March 31, 2024 of $15 million relative to loss before income taxes of $86 million. The Company recorded a benefit for income taxes for the three months ended March 31, 2023 of $55 million relative to loss before income taxes of $282 million. The income tax expense for the quarter is primarily the result of income tax expense in select international jurisdictions and the inability to recognize income tax benefit on incremental domestic losses. The quarterly provision was also impacted by the geographic distribution of the Company's pretax income resulting from our business mix, changes in the tax impact of permanent differences, restructuring, special items, certain equity related compensation, and other discrete tax items that may have unique tax implications depending on the nature of the item.
The Company recorded a provision for income taxes for the nine months ended March 31, 2024 of $1 million relative to loss before income taxes of $1.065 billion. The Company recorded a benefit for income taxes for the nine months ended March 31, 2023 of $19 million relative to loss before income taxes of $165 million. The income tax expense for the period is primarily the result of income tax expense in select international jurisdictions and the inability to recognize income tax benefit on incremental domestic losses.
The year to date provision was also impacted by the geographic distribution of the Company's pretax loss resulting from our business mix, changes in the tax impact of permanent differences, restructuring, special items, certain equity related compensation, and other discrete tax items that may have unique tax implications depending on the nature of the item.
12. EMPLOYEE RETIREMENT BENEFIT PLANS
Components of the Company’s net periodic benefit costs are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 | | 2024 | | 2023 |
| Components of net periodic benefit cost: | | | | | | | |
| Selling, general, and administrative expenses: | | | | | | | |
| Service cost | $ | 1 | | | $ | 1 | | | $ | 3 | | | $ | 3 | |
| Other operating expense: | | | | | | | |
| Settlement charges | 9 | | | — | | | 12 | | | — | |
| Other expense, net: | | | | | | | |
| Interest cost | 3 | | | 3 | | | 8 | | | 7 | |
| Expected return on plan assets | (2) | | | (2) | | | (6) | | | (6) | |
Amortization (1) | — | | | — | | | 1 | | | — | |
| Net amount recognized | $ | 11 | | | $ | 2 | | | $ | 18 | | | $ | 4 | |
(1) Amount represents the amortization of unrecognized actuarial losses.
As previously disclosed, the Company notified the trustees of a multi-employer pension plan of its withdrawal from participation in such plan in fiscal 2012. The actuarial review process administered by the plan trustees ended in fiscal 2015. For the nine months ended December 31, 2023, the Company decided to withdraw its participation from a multi-employer pension plan as a result of recent restructuring activities in its Pharma and Consumer Health segment. The liabilities reported reflect the present value of the Company’s expected future long-term obligations. The estimated discounted value of the projected contributions related to such plans was $44 million as of March 31, 2024 and $38 million as of June 30, 2023, and is included within pension liability on the consolidated balance sheets. The annual cash impact associated with the Company’s obligations in such plan is approximately $2 million.
The Company terminated its U.S. pension plan and settled with its participants by a lump sum payout or through the purchase of an annuity contract, which was dependent upon the participant’s selection of payment. The Company purchased nonparticipating annuities for participants who did not elect lump sum payouts.
Participants who elected a lump sum payout were settled in the nine months ended March 31, 2024 and resulted in $7 million of lump sum payments made with cash from the assets of the qualified pension plan. Participants who elected an annuity contract were settled in the three and nine months ended March 31, 2024 and resulted in $24 million of payments with cash from the assets of the qualified pension plan and $3 million of Company cash.
13. EQUITY AND ACCUMULATED OTHER COMPREHENSIVE LOSS
Description of Capital Stock
The Company is authorized to issue 1.00 billion shares of its Common Stock and 100 million shares of preferred stock, par value $0.01 per share. In accordance with the Company’s amended and restated certificate of incorporation, each share of Common Stock has one vote, and the Common Stock votes together as a single class.
Accumulated Other Comprehensive Loss
The components of the changes in the cumulative translation adjustment, derivatives and hedges, minimum pension liability, and marketable securities for the three and nine months ended March 31, 2024 and 2023 are presented below.
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 | | 2024 | | 2023 |
| Foreign currency translation adjustments: | | | | | | | |
| Net investment hedge | $ | 17 | | | $ | (16) | | | $ | 11 | | | $ | (20) | |
| Long-term intercompany loans | — | | | 9 | | | (2) | | | (1) | |
| Translation adjustments | (36) | | | 31 | | | (28) | | | 26 | |
| Total foreign currency translation adjustment, pretax | (19) | | | 24 | | | (19) | | | 5 | |
| Tax benefit | (5) | | | (3) | | | (3) | | | (5) | |
| Total foreign currency translation adjustment, net of tax | $ | (14) | | | $ | 27 | | | $ | (16) | | | $ | 10 | |
| | | | | | | |
| Net change in derivatives and hedges: | | | | | | | |
| Net gain (loss) recognized during the period | $ | 8 | | | $ | (3) | | | $ | 6 | | | $ | 15 | |
| Total derivatives and hedges, pretax | 8 | | | (3) | | | 6 | | | 15 | |
| Tax expense (benefit) | 3 | | | (1) | | | 3 | | | 3 | |
| Net change in derivatives and hedges, net of tax | $ | 5 | | | $ | (2) | | | $ | 3 | | | $ | 12 | |
| | | | | | | |
| Net change in minimum pension liability: | | | | | | | |
| | | | | | | |
| Net gain recognized during the period | $ | 9 | | | $ | — | | | $ | 12 | | | $ | — | |
| | | | | | | |
| Total pension liability, pretax | 9 | | | — | | | 12 | | | — | |
| Tax expense | 2 | | | — | | | 1 | | | — | |
| Net change in minimum pension liability, net of tax | $ | 7 | | | $ | — | | | $ | 11 | | | $ | — | |
| | | | | | | |
| Net change in marketable securities: | | | | | | | |
| Net gain recognized during the period | $ | — | | | $ | — | | | $ | — | | | $ | 1 | |
Amounts reclassified from accumulated other
comprehensive loss | — | | | 2 | | | — | | | 4 | |
| Net change in marketable securities, pretax | — | | | 2 | | | — | | | 5 | |
| Tax expense | — | | | — | | | — | | | 1 | |
| Net change in marketable securities, net of tax | $ | — | | | $ | 2 | | | $ | — | | | $ | 4 | |
| | | | | | | |
For the three months ended March 31, 2024 and 2023, the changes in accumulated other comprehensive loss, net of tax by component are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Foreign Exchange Translation Adjustments | | Pension and Liability Adjustments | | Derivatives and Hedges | | | | | | | Other | | Total |
| Balance at December 31, 2023 | $ | (348) | | | $ | (48) | | | $ | 43 | | | | | | | | $ | (1) | | | $ | (354) | |
Other comprehensive (loss) income before
reclassifications | (14) | | | — | | | 5 | | | | | | | | — | | | (9) | |
Amounts reclassified from accumulated other
comprehensive loss | — | | | 7 | | | — | | | | | | | | — | | | 7 | |
Net current period other comprehensive (loss) income | (14) | | | 7 | | | 5 | | | | | | | | — | | | (2) | |
| Balance at March 31, 2024 | $ | (362) | | | $ | (41) | | | $ | 48 | | | | | | | | $ | (1) | | | $ | (356) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Foreign Exchange Translation Adjustments | | Pension and Liability Adjustments | | Derivatives and Hedges | | | | | Marketable Securities | | Other | | Total |
| Balance at December 31, 2022 | $ | (395) | | | $ | (38) | | | $ | 41 | | | | | | $ | (2) | | | $ | (1) | | | $ | (395) | |
Other comprehensive income (loss) before
reclassifications | 27 | | | — | | | (2) | | | | | | — | | | — | | | 25 | |
Amounts reclassified from accumulated other
comprehensive loss | — | | | — | | | — | | | | | | 2 | | | — | | | 2 | |
Net current period other comprehensive income (loss) | 27 | | | — | | | (2) | | | | | | 2 | | | — | | | 27 | |
| Balance at March 31, 2023 | $ | (368) | | | $ | (38) | | | $ | 39 | | | | | | $ | — | | | $ | (1) | | | $ | (368) | |
For the nine months ended March 31, 2024 and 2023, the changes in accumulated other comprehensive loss, net of tax by component are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Foreign Exchange Translation Adjustments | | Pension and Liability Adjustments | | Derivatives and Hedges | | | | | | | Other | | Total |
| Balance at June 30, 2023 | $ | (346) | | | $ | (52) | | | $ | 45 | | | | | | | | $ | (1) | | | $ | (354) | |
Other comprehensive income (loss) before
reclassifications | (16) | | | — | | | 3 | | | | | | | | — | | | (13) | |
Amounts reclassified from accumulated other
comprehensive loss | — | | | 11 | | | — | | | | | | | | — | | | 11 | |
Net current period other comprehensive income (loss) | (16) | | | 11 | | | 3 | | | | | | | | — | | | (2) | |
| Balance at March 31, 2024 | $ | (362) | | | $ | (41) | | | $ | 48 | | | | | | | | $ | (1) | | | $ | (356) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Foreign Exchange Translation Adjustments | | Pension and Liability Adjustments | | Derivatives and Hedges | | | | | Marketable Securities | | Other | | Total |
| Balance at June 30, 2022 | $ | (378) | | | $ | (38) | | | $ | 27 | | | | | | $ | (4) | | | $ | (1) | | | $ | (394) | |
Other comprehensive income before
reclassifications | 10 | | | — | | | 12 | | | | | | — | | | — | | | 22 | |
Amounts reclassified from accumulated other
comprehensive loss | — | | | — | | | — | | | | | | 4 | | | — | | | 4 | |
Net current period other comprehensive income | 10 | | | — | | | 12 | | | | | | 4 | | | — | | | 26 | |
| Balance at March 31, 2023 | $ | (368) | | | $ | (38) | | | $ | 39 | | | | | | $ | — | | | $ | (1) | | | $ | (368) | |
14. COMMITMENTS AND CONTINGENCIES
Litigation
From time to time, the Company may be involved in legal proceedings arising in the ordinary course of business, including, without limitation, inquiries and claims concerning environmental contamination as well as litigation and allegations in connection with acquisitions, product liability, manufacturing or packaging defects, and claims for reimbursement for the cost of lost or damaged active pharmaceutical ingredients, the cost of any of which could be significant. Such matters are inherently uncertain, and there can be no guarantee that the outcome of any such matter will be decided favorably to the Company or that the resolution of any such matter will not have a material adverse effect upon the Company’s consolidated financial statements. The Company records a liability in its consolidated financial statements for these matters when a loss is known or considered probable and the amount can be reasonably estimated. The Company reviews these estimates each accounting period as additional information is known and adjusts the loss provision when appropriate. If a matter is both probable to result in a liability and the amount of loss can be reasonably estimated, the Company estimates and discloses the
possible loss or range of loss to the extent necessary for its consolidated financial statements not to be misleading. If the loss is not probable or cannot be reasonably estimated, a liability is not recorded in the Company's consolidated financial statements. Any legal or other expenses associated with the litigation are accrued for as the expenses are incurred. The Company intends to vigorously defend itself against any such litigation and does not currently believe that the outcome of any such litigation will have a material adverse effect on the Company’s consolidated financial statements. In addition, the healthcare industry is highly regulated and government agencies continue to scrutinize certain practices affecting government programs and otherwise.
City of Warwick Retirement System Class Action
In February 2023, an alleged shareholder filed a complaint styled City of Warwick Retirement System v. Catalent, Inc., et al., No. 23-cv-01108, in New Jersey federal court against the Company and three of its then-officers (collectively, “the Warwick Defendants”) purportedly on behalf of a putative “class” consisting of persons who purchased or otherwise acquired Company securities between August 30, 2021 and October 31, 2022, inclusive. On September 15, 2023, the Warwick complaint was amended (together with the original complaint, the “Warwick Complaint”), which amended complaint expanded the class period to between August 30, 2021 and May 7, 2023, inclusive (the “Class Period”). The Warwick Complaint purports to assert claims under Section 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended and the related regulations, alleging that, unbeknownst to investors, the Warwick Defendants purportedly engaged in accounting and channel stuffing schemes to pad the Company’s revenues and failed to disclose adverse facts that purportedly were known to or recklessly disregarded by the Warwick Defendants. Specifically, the Warwick Complaint alleges that the Warwick Defendants (i) overstated revenue and earnings by prematurely recognizing revenue in violation of U.S. GAAP; (ii) suffered material weaknesses in its internal controls over financial reporting related to revenue recognition; (iii) falsely represented demand for its products while knowingly selling more product to its direct customers than could be sold to healthcare providers and end consumers; (iv) cut corners on safety and control procedures at key production facilities; (v) disregarded regulatory rules at key production facilities in order to rapidly produce excess inventory that was used to pad the Company’s financial results through premature revenue recognition in violation of U.S. GAAP or stuffing its direct customers with this excess inventory; and (vi) lacked a reasonable basis for their positive statements about the Company’s financial performance, outlook, and regulatory compliance during the Class Period. The Company believes that the Warwick Defendants have defenses to the allegations and claims set forth in the complaint and filed a motion to dismiss the Warwick Complaint on November 15, 2023. The plaintiffs filed their opposition to the Company's motion to dismiss on January 12, 2024, and the Company filed its reply to the plaintiffs' opposition on February 15, 2024. The parties are awaiting the court's decision.
Husty Derivative Claim
In August 2023, an alleged shareholder filed a derivative complaint styled Husty et al. v. Carroll, et al., No. 23-cv-00891, in Delaware federal court against certain current and former members of the Company's board of directors, (the “Husty Defendants”), and nominally against Catalent, Inc. The complaint mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action described above and claims that the alleged activities described there led to, and will continue to expose the Company to, costs and damages. On February 20, 2024, the court entered a stipulation staying the case pending the outcome of motion to dismiss that was filed in the City of Warwick Retirement System action. On April 23, 2024, the plaintiff voluntarily dismissed the action without prejudice.
Brown Derivative Claim
In September 2023, an alleged shareholder filed a derivative complaint styled Brown, et al. v. Chiminski, et al., Case 3:23-cv-15722, in New Jersey federal court against certain current and former officers and members of the Company's board of directors (the “Brown Defendants”) and nominally against Catalent, Inc. The complaint mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action described above and claims that the alleged activities described there led to, and will continue to expose the Company to, costs and damages. On January 8, 2024, the court entered a stipulation staying the case pending the outcome of motion to dismiss that was filed in the City of Warwick Retirement System action. On April 19, 2024, the plaintiff voluntarily dismissed the action without prejudice. On May 2, 2024, the Court dismissed the action without prejudice.
Subpoenas and Requests for Information
From time to time, the Company receives subpoenas or requests for information from various governmental agencies or private parties, including from state attorneys general, the U.S. Department of Justice, and private parties. The Company generally responds to such subpoenas and requests in a timely and thorough manner, which responses sometimes require considerable time and effort and can result in considerable costs being incurred.
In June 2023, the Company received a demand from a company stockholder pursuant to 8 Del. C. § 220 to inspect books and records of the Company relating to, among other things, the allegations raised in the Warwick Complaint. The Company has responded to the demand and cannot determine at this time if the books and records demand will lead to litigation.
Fire at Biologics Facility
During the three months ended December 31, 2023, the Company had a small fire at a facility in its Biologics segment. The fire activated the sprinkler systems, which then caused minor flooding in certain parts of the facility. The Company accrued $9 million for estimated damages, repairs, and lost inventory. The Company is insured for such incidents and has submitted claims for reimbursement. Proceeds from potential reimbursement are not included in the financial statements for the three and nine months ended March 31, 2024.
Entry Into an Agreement and Plan of Merger
On February 5, 2024, the Company entered into the Agreement and Plan of Merger (the “Merger Agreement”), with Creek Parent, Inc. (“Parent”), a Delaware corporation and a wholly owned subsidiary of Novo Holdings A/S (“Novo Holdings”), and Creek Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”). The Merger Agreement provides that, upon the terms and subject to the conditions set forth therein and in accordance with the General Corporation Law of the State of Delaware (the “DGCL”), Merger Sub will be merged with and into the Company (the “Merger”), with the Company surviving the Merger as a wholly owned subsidiary of Parent. Parent will acquire all the issued and outstanding shares of common stock of the Company.
At the effective time of the Merger (the “Effective Time”), each share of common stock, par value $0.01 per share, of the Company (such shares, collectively, the “Company Common Stock,” and each, a “Share”) that is issued and outstanding immediately prior to the Effective Time (other than any Shares held by (i) the Company, Parent or Merger Sub or any other direct or indirect wholly owned subsidiary of the Company or Parent immediately prior to the Effective Time, or (ii) a holder who has not voted in favor of the adoption of the Merger Agreement and is entitled to demand and properly demands appraisal of such Shares under the DGCL), will be converted automatically into the right to receive an amount in cash equal to $63.50 per Share, without interest (the “Merger Consideration”). The transaction values the Company at $16.5 billion on an enterprise value basis.
Consummation of the Merger is subject to customary closing conditions, including approval of the Merger by the Company’s stockholders (which has not been obtained at this stage). Further conditions include (i) receipt of certain governmental waivers, consents, clearances, decisions, declarations, approvals, and expirations of applicable waiting periods, including the expiration or early termination of the waiting period under the U.S. Hart-Scott-Rodino Antitrust Improvements Act of 1976, with respect to (A) the Merger and (B) the sale of three of the Company’s fill-finish sites (which are located in Anagni, Italy, Bloomington, Indiana USA, and Brussels, Belgium) and related assets from Novo Holdings to Novo Nordisk A/S (“Novo Nordisk”), of which Novo Holdings is the controlling shareholder (the “Carve-Out”), and (ii) the absence of any order, injunction or law prohibiting the Merger or the Carve-Out, in each case, without a Burdensome Condition (as defined in the Merger Agreement). Parent’s and Merger Sub’s obligations to close the Merger are also conditioned upon the absence of a Material Adverse Effect (as defined in the Merger Agreement) on the Company.
Upon termination of the Merger Agreement with Parent, the Company, under specified circumstances and conditions set forth in the Merger Agreement, could be required to pay Parent a termination fee of approximately $345 million.
15. SEGMENT INFORMATION
The Company evaluates the performance of its segments based on segment earnings before other (expense) income, impairments, restructuring costs, interest expense, income tax expense, and depreciation and amortization (“Segment EBITDA”).
Segment EBITDA is subject to important limitations. These consolidated financial statements include information concerning Segment EBITDA (a) because Segment EBITDA is an operational measure used by management in the assessment of the operating segments, the allocation of resources to the segments, and the setting of strategic goals and annual goals for the segments, and (b) in order to provide supplemental information that the Company considers relevant for the readers of the consolidated financial statements. The Company’s presentation of Segment EBITDA may not be comparable to similarly titled measures used by other companies.
The following tables include Segment EBITDA for each of the Company's current reportable segments during the three and nine months ended March 31, 2024 and 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Three Months Ended
March 31, | | Nine Months Ended
March 31, |
| 2024 | | 2023 | | 2024 | | 2023 |
| Segment EBITDA reconciled to net (loss) earnings: | | | | | | | |
| Biologics | $ | 49 | | | $ | 6 | | | $ | 136 | | | $ | 299 | |
| Pharma and Consumer Health | 153 | | | 125 | | | 380 | | | 368 | |
| | | | | | | |
| | | | | | | |
| Sub-Total | $ | 202 | | | $ | 131 | | | $ | 516 | | | $ | 667 | |
| Reconciling items to net earnings | | | | | | | |
Unallocated costs (1) | (97) | | | (256) | | | (1,033) | | | (394) | |
| Depreciation and amortization | (126) | | | (106) | | | (359) | | | (308) | |
| Interest expense, net | (65) | | | (51) | | | (189) | | | (130) | |
| Income tax (expense) benefit | (15) | | | 55 | | | (1) | | | 19 | |
| | | | | | | |
| Net loss | $ | (101) | | | $ | (227) | | | $ | (1,066) | | | $ | (146) | |
(1) Unallocated costs include restructuring and special items, stock-based compensation, impairment charges, certain other corporate directed costs, and other costs that are not allocated to the segments as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Three Months Ended March 31, | | Nine Months Ended
March 31, |
| 2024 | | 2023 | | 2024 | | 2023 |
Impairment charges and gain/loss on sale of assets(a) | $ | (13) | | | $ | (6) | | | $ | (27) | | | $ | (4) | |
| Stock-based compensation | (17) | | | (6) | | | (52) | | | (35) | |
Restructuring and other special items(b) | (20) | | | (17) | | | (126) | | | (59) | |
Goodwill impairment charges(c) | — | | | (210) | | | (687) | | | (210) | |
| | | | | | | |
| Pension settlement charges | (9) | | | — | | | (12) | | | — | |
| | | | | | | |
| | | | | | | |
Other (expense) income, net(d) | (4) | | | 4 | | | (21) | | | 2 | |
| Unallocated corporate costs, net | (34) | | | (21) | | | (108) | | | (88) | |
| Total unallocated costs | $ | (97) | | | $ | (256) | | | $ | (1,033) | | | $ | (394) | |
(a) Impairment charges and gain/loss on sale of assets for the three and nine months ended March 31, 2024 includes right-of-use asset impairment charges associated with under utilized facilities in our Biologics segment.
Impairment charges and gain/loss on sale of assets for the three and nine months ended March 31, 2024 includes fixed asset impairment charges associated with equipment for a product with significant decline in demand in the Company's Biologics segment.
(b) Restructuring and other special items during the three and nine months ended March 31, 2024 include restructuring charges associated with plans to reduce costs, consolidate facilities, and optimize our infrastructure across the organization. For further details on restructuring charges, see Note 8, Restructuring Costs to the Consolidated Financial Statements.
Restructuring and other special items during the three months ended March 31, 2023 include (i) restructuring charges associated with plans to reduce costs, consolidate facilities, and optimize our infrastructure across the organization and (ii) transaction and integration costs associated with the Metrics acquisition. Restructuring and other special items for the nine months ended March 31, 2023 also includes warehouse exit costs for a product the Company no longer manufactures in its Pharma and Consumer Health segment. For further details on restructuring charges, see Note 8, Restructuring Costs to the Consolidated Financial Statements.
(c) Goodwill impairment charges during the nine months ended March 31, 2024 were associated with the Company's Consumer Health and Biomodalities reporting units, which are part of the Company's Pharma and Consumer Health and Biologics segments, respectively. For further details, see Note 4, Goodwill to the Consolidated Financial Statements.
Goodwill impairment charges during the three and nine months ended March 31, 2023 was associated with the Company’s Consumer Health reporting unit. For further details, see Note 4, Goodwill.
(d) Other expense, net during the three and nine months ended March 31, 2024 and 2023 primarily includes foreign currency remeasurement losses/gains.
The following table includes total assets for each segment, as well as reconciling items necessary to total the amounts reported in the consolidated financial statements.
| | | | | | | | | | | |
| (Dollars in millions) | March 31,
2024 | | June 30,
2023 |
| Assets: | | | |
| Biologics | $ | 5,263 | | | $ | 5,746 | |
| Pharma and Consumer Health | 4,588 | | | 4,867 | |
| | | |
| | | |
| Corporate and eliminations | 28 | | | 164 | |
| Total assets | $ | 9,879 | | | $ | 10,777 | |
16. SUPPLEMENTAL BALANCE SHEET INFORMATION
Supplemental balance sheet information at March 31, 2024 and June 30, 2023 is detailed in the following tables.
Inventories
Work-in-process and inventories include raw materials, labor, and overhead. Total inventories consist of the following:
| | | | | | | | | | | |
| (Dollars in millions) | March 31,
2024 | | June 30,
2023 |
| Raw materials and supplies | $ | 747 | | | $ | 781 | |
| Work-in-process | 211 | | | 186 | |
| | | |
| Total inventories, gross | 958 | | | 967 | |
| Inventory cost adjustment | (216) | | | (190) | |
| Total inventories | $ | 742 | | | $ | 777 | |
Prepaid expenses and other
Prepaid expenses and other consist of the following: | | | | | | | | | | | |
| (Dollars in millions) | March 31,
2024 | | June 30,
2023 |
| Prepaid expenses | $ | 55 | | | $ | 53 | |
| | | |
| Short-term contract assets | 472 | | | 399 | |
| | | |
| Spare parts supplies | 28 | | | 24 | |
| Prepaid income tax | 84 | | | 77 | |
| | | |
| Non-U.S. value-added tax | 59 | | | 38 | |
| | | |
| | | |
| | | |
| Other current assets | 46 | | | 42 | |
| Total prepaid expenses and other | $ | 744 | | | $ | 633 | |
Other accrued liabilities
Other accrued liabilities consist of the following: | | | | | | | | | | | |
| (Dollars in millions) | March 31,
2024 | | June 30,
2023 |
| Contract liabilities | $ | 249 | | | $ | 167 | |
| Accrued employee-related expenses | 146 | | | 160 | |
| Accrued expenses | 120 | | | 134 | |
| Operating lease liabilities | 10 | | | 11 | |
| Restructuring accrual | 16 | | | 19 | |
| Accrued interest | 28 | | | 35 | |
| Accrued income tax | 14 | | | 44 | |
| Total other accrued liabilities | $ | 583 | | | $ | 570 | |
Allowance for credit losses
The rollforward of allowance for credit losses for the nine months ended March 31, 2024 is as follows:
| | | | | |
| Allowance for credit losses |
(Dollars in millions) | |
| Balance, June 30, 2023 | $ | 46 | |
| Charges (Credits) | (6) | |
| Write-offs | (8) | |
| Balance, March 31, 2024 | $ | 32 | |
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The Company
We provide differentiated development and manufacturing solutions for drugs, protein-based biologics, cell and gene therapies, vaccines, and consumer health products at over fifty facilities across four continents under rigorous quality and operational standards. Our oral, injectable, and respiratory delivery technologies, along with our state-of-the-art protein, plasmid, viral, and cell and gene therapy manufacturing capacity, address a wide and growing range of modalities and therapeutic and other categories across the biopharmaceutical, pharmaceutical, and consumer health industries. Through our extensive capabilities, growth-enabling capacity, and deep expertise in product development, regulatory compliance, and clinical trial and commercial supply, we can help our customers take products to market faster, including more than half of new drug products approved by the U.S. Food and Drug Administration (the “FDA”) in the last decade. Our development and manufacturing platforms, our proven formulation, supply, and regulatory expertise, and our broad and deep development and manufacturing know-how enable our customers to advance and then bring to market more products and better treatments for patients and consumers. Our commitment to reliably supply our customers’ and their patients’ needs is the foundation for the value we provide; annually, we produce approximately 70 billion unit doses for nearly 8,000 customer prescription and consumer health products, or approximately 1 in every 26 unit doses of such products taken each year by patients and consumers around the world. We believe that, through our investments in state-of-the-art facilities and capacity expansion, including investments in facilities focused on new treatment modalities and other attractive market segments, our continuous improvement activities devoted to operational and quality excellence, the sales of existing and introduction of new customer products, and, in some cases, our innovation activities and patents, we will continue to attract premium opportunities and realize the growth potential from these areas.
Our operating structure consists of two operating and reportable segments: (i) Biologics and (ii) Pharma and Consumer Health. The Biologics segment provides formulation, development, and manufacturing for biologic proteins, cell gene, and other nucleic acid therapies; pDNA; iPSCs, oncolytic viruses, and vaccines; formulation, development, and manufacturing for parenteral dose forms, including vials, prefilled syringes, and cartridges; and analytical development and testing services for large molecules. Our Pharma and Consumer Health segment provides market-leading capabilities for complex oral solids, softgel formulations, Zydis fast-dissolve technologies, and gummy, soft chew, and lozenge dosage forms; formulation,
development, and manufacturing platforms for oral, nasal, inhaled, and topical dose forms; cold-chain storage and distribution, and clinical trial development and supply services.
Critical Accounting Policies and Estimates
We prepare our financial statements in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”). Management made certain estimates and assumptions during the preparation of the Consolidated Financial Statements in accordance with U.S. GAAP. These estimates and assumptions affect the reported amount of assets and liabilities and disclosures of contingent assets and liabilities in the consolidated financial statements. These estimates also affect the reported amount of net earnings during the reporting periods. Actual results could differ from those estimates. Because of the size of the financial statement elements to which they relate, some of our accounting policies and estimates have a more significant impact on the Consolidated Financial Statements than others.
Goodwill and Indefinite-Lived Intangible Assets
We account for purchased goodwill and intangible assets with indefinite lives in accordance with ASC 350, Intangibles –– Goodwill and Other. Under ASC 350, goodwill and intangible assets with indefinite lives are not amortized, but instead are tested for impairment at least annually. We perform an impairment evaluation of goodwill annually during the fourth quarter of our fiscal year or when circumstances otherwise indicate an evaluation should be performed. The evaluation may begin with a qualitative assessment for each reporting unit to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying value. If the qualitative assessment does not generate a positive response, or if no qualitative assessment is performed, a quantitative assessment, based upon discounted cash flows, is performed and requires management to estimate future cash flows, growth rates, and economic and market conditions.
As a result of Consumer Health's underperformance of recent operating results relative to expectations, as well as current macroeconomic conditions impacting the consumer health and biotechnology industries, and higher interest rates, we assessed the current and future economic outlook as of September 30, 2023 for our Consumer Health and Biomodalities reporting units in our Pharma and Consumer Health and Biologics segments, respectively, and identified indicators for impairment of goodwill.
The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in our Consumer Health and Biomodalities reporting units, which led to a quantitative assessment for the corresponding reporting units. The evaluation performed as of September 30, 2023 resulted in a combined goodwill impairment charge of $689 million for our Consumer Health and Biomodalities reporting units within the Pharma and Consumer Health and Biologics segments, respectively.
A 50 basis point increase in the discount rate would increase the goodwill impairment $220 million and $50 million for its Biomodalities and Consumer Health reporting units, respectively. A 50 basis point decrease in the long-term growth rate would increase the goodwill impairment by $120 million and $30 million for its Biomodalities and Consumer Health reporting units, respectively.
For further details on the impairment charges for the nine months ended March 31, 2024, see Note 4, Goodwill to our Consolidated Financial Statements.
Other than the above, there was no material change to our critical accounting policies or in the underlying accounting assumptions and estimates from those described in our Fiscal 2023 10-K.
Non-GAAP Metrics
EBITDA from operations
Management measures operating performance based on consolidated earnings from operations before interest expense, expense for income taxes, and depreciation and amortization, adjusted for the income or loss attributable to non-controlling interests (“EBITDA from operations”). EBITDA from operations is not defined under U.S. GAAP, is not a measure of operating income, operating performance, or liquidity presented in accordance with U.S. GAAP, and is subject to important limitations.
We believe that the presentation of EBITDA from operations enhances an investor’s understanding of our financial performance. We believe this measure is a useful financial metric to assess our operating performance from period to period by excluding certain items that we believe are not representative of our core business and use this measure for business planning purposes. In addition, given the significant historical investments that we have made in property, plant, and equipment, depreciation and amortization expenses represent a meaningful portion of our cost structure. We believe that EBITDA from
operations will provide investors with a useful tool for assessing the comparability between periods of our ability to generate cash from operations sufficient to pay taxes, to service debt, and to undertake capital expenditures because it eliminates depreciation and amortization expense. We present EBITDA from operations in order to provide supplemental information that we consider relevant for the readers of the Consolidated Financial Statements, and such information is not meant to replace or supersede U.S. GAAP measures. Our definition of EBITDA from operations may not be the same as similarly titled measures used by other companies. The most directly comparable measure to EBITDA from operations defined under U.S. GAAP is net earnings. Included in this Management’s Discussion and Analysis is a reconciliation of net earnings to EBITDA from operations.
In addition, we evaluate the performance of our segments based on segment earnings before non-controlling interests, other expense (income), impairments, restructuring costs, interest expense, income tax expense, and depreciation and amortization (“Segment EBITDA”). For a reconciliation of Segment EBITDA to net earnings, see Note 15, Segment Information to our Consolidated Financial Statements.
Use of Constant Currency
As exchange rates are an important factor in understanding period-to-period comparisons, we believe the presentation of results on a constant-currency basis in addition to reported results helps improve investors’ ability to understand our operating results and evaluate our performance in comparison to prior periods. Constant-currency information compares results between periods as if exchange rates had remained constant period-over-period. We use results on a constant-currency basis as one measure to evaluate our performance. In this Quarterly Report on Form 10-Q, we compute constant currency by calculating current period results using prior period foreign currency exchange rates. We generally refer to such amounts calculated on a constant-currency basis as excluding the impact of foreign currency exchange. These results should be considered in addition to, not as a substitute for, results reported in accordance with U.S. GAAP. Results on a constant-currency basis, as we present them, may not be comparable to similarly titled measures used by other companies and are not measures of performance presented in accordance with U.S. GAAP.
Other Non-GAAP Measures
Organic revenue growth and Segment EBITDA growth are measures we use to explain the underlying results and trends in the business. Organic revenue growth and Segment EBITDA growth are measures used to show current period sales and profitability from existing operations. Organic revenue growth and Segment EBITDA growth exclude the impact of foreign currency exchange, acquisitions of operating or legal entities, and divestitures within the applicable periods. These measures should be considered in addition to, not as a substitute for, performance measures reported in accordance with U.S. GAAP. These measures, as we present them, may not be comparable to similarly titled measures used by other companies and are not measures of performance presented in accordance with U.S. GAAP.
Three Months Ended March 31, 2024 Compared to the Three Months Ended March 31, 2023
The below tables summarize several financial metrics we use to measure performance for the three months ended March 31, 2024 and 2023. Refer to the discussions below regarding performance and use of key financial metrics.
Results for the three months ended March 31, 2024 compared to the three months ended March 31, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | FX Impact | | Constant Currency Increase (Decrease) |
| (Dollars in millions) | 2024 | | 2023 | | | | Change $ | | Change % |
| Net revenue | $ | 1,074 | | | $ | 1,037 | | | $ | 5 | | | $ | 32 | | | 3 | % |
| Cost of sales | 845 | | | 857 | | | 2 | | | (14) | | | (2) | % |
| Gross margin | 229 | | | 180 | | | 3 | | | 46 | | | 26 | % |
| Selling, general, and administrative expenses | 214 | | | 190 | | | — | | | 24 | | | 12 | % |
| | | | | | | | | |
| | | | | | | | | |
| Goodwill impairment charges | — | | | 210 | | | — | | | (210) | | | (100) | % |
| Other operating expense, net | 32 | | | 15 | | | (1) | | | 18 | | | 147 | % |
| | | | | | | | | |
| Operating loss | (17) | | | (235) | | | 4 | | | 214 | | | 91 | % |
| Interest expense, net | 65 | | | 51 | | | — | | | 14 | | | 28 | % |
| Other expense (income), net | 4 | | | (4) | | | 3 | | | 5 | | | 132 | % |
| Loss before income taxes | (86) | | | (282) | | | 1 | | | 195 | | | 69 | % |
| Income tax expense (benefit) | 15 | | | (55) | | | — | | | 70 | | | 126 | % |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Net loss | $ | (101) | | | $ | (227) | | | $ | 1 | | | $ | 125 | | | 55 | % |
* Not meaningful
Change % calculations are based on amounts prior to rounding
Net Revenue | | | | | | | |
| 2024 vs. 2023 | | |
| Year-Over-Year Change | Three Months Ended
March 31, |
| Net Revenue | | |
| Organic | 3 | % | | |
| | | |
| | | |
| Constant-currency change | 3 | % | | |
| Foreign currency translation impact on reporting | 1 | % | | |
| Total % change | 4 | % | | |
Net revenue increased $32 million, or 3%, excluding the impact of foreign exchange, compared to the three months ended March 31, 2023. Net revenue increased 3% organically primarily due to growth from the manufacture of prescription products, our gene therapy offerings, our orally delivered Zydis commercial products, and an increase in demand for our consumer health products, partially offset by a decline in demand for COVID-19 related programs.
Gross Margin
Gross margin increased $46 million, or 26%, compared to the three months ended March 31, 2023, excluding the impact of foreign exchange. On a constant-currency basis, gross margin, as a percentage of revenue, increased 370 basis points to 21.2% in the three months ended March 31, 2024, compared to 17.5% in the prior-year period, primarily due to a favorable shift in product mix, improved levels of utilization across the network, and a decrease in inventory write-offs, partially offset by increased spending on operational and engineering enhancements in our Biologics segment.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses increased $24 million, or 12%, compared to the three months ended March 31, 2023, excluding the impact of foreign exchange. The year-over-year increase was attributable to an $13 million increase in employee-related costs an $11 million increase in stock-based compensation, and a $7 million increase in depreciation and amortization, which were partially offset by a $7 million benefit from a decline in credit losses.
Other Operating Expense, net
Other operating expense, net for the three months ended March 31, 2024 increased $18 million or 147%, excluding the impact of foreign exchange, compared to the three months ended March 31, 2023. This increase was primarily driven by a $7 million increase in in fixed-asset impairment charges and $9 million of pension settlement charges.
Interest Expense, net
Interest expense, net of $65 million for the three months ended March 31, 2024 increased $14 million, or 28%, compared to the three months ended March 31, 2023, excluding the impact of foreign exchange. The increase was primarily attributable to higher interest rates on our variable rate debt.
For additional information concerning our debt and financing arrangements, including the changing mix of debt and equity in our capital structure, see “—Liquidity and Capital Resources” below and Note 5, Long-Term Obligations and Short-Term Borrowings to our Consolidated Financial Statements.
Other Expense (Income), net
Other expense, net of $4 million for the three months ended March 31, 2024 was primarily driven by $2 million of foreign currency losses.
Other income, net of $4 million for the three months ended March 31, 2023 was primarily driven by $4 million of foreign currency gains.
Income Tax Expense (Benefit)
Our provision for income taxes for the three months ended March 31, 2024 was $15 million relative to loss before taxes of $86 million. Our benefit for income taxes for the three months ended March 31, 2023 was $55 million relative to loss before income taxes of $282 million. The income tax expense for the quarter is primarily the result of income tax expense in select international jurisdictions and the inability to recognize income tax benefit on incremental domestic losses. The quarterly provision was also impacted by the geographic distribution of our pretax income resulting from our business mix, changes in the tax impact of permanent differences, restructuring, special items, certain equity related compensation, and other discrete tax items that may have unique tax implications depending on the nature of the item.
Segment Review
The following charts depict the percentages of net revenue from each of our two reportable segments for the three months ended March 31, 2024 compared to the three months ended March 31, 2023. Refer below for discussions regarding each segment’s net revenue and EBITDA performance and to “Non-GAAP Metrics” for a discussion of our use of Segment EBITDA, a measure that is not defined under U.S. GAAP.
Our results on a segment basis for the three months ended March 31, 2024 compared to the three months ended March 31, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | FX Impact | | Constant Currency Increase (Decrease) |
| (Dollars in millions) | 2024 | | 2023 | | | | Change $ | | Change % |
| Biologics | | | | | | | | | |
| Net revenue | $ | 461 | | | $ | 475 | | | $ | 1 | | | $ | (15) | | | (3) | % |
| Segment EBITDA | 49 | | | 6 | | | — | | | 43 | | | 876 | % |
| Pharma and Consumer Health | | | | | | | | | |
| Net revenue | 613 | | | 563 | | | 3 | | | 47 | | | 8 | % |
| Segment EBITDA | 153 | | | 125 | | | 2 | | | 26 | | | 21 | % |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Inter-segment revenue elimination | — | | | (1) | | | 1 | | | — | | | (75) | % |
Unallocated Costs (1) | (97) | | | (256) | | | — | | | 159 | | | 62 | % |
| Combined totals | | | | | | | | | |
| Net revenue | $ | 1,074 | | | $ | 1,037 | | | $ | 5 | | | $ | 32 | | | 3 | % |
| | | | | | | | | |
| EBITDA (loss) from operations | $ | 105 | | | $ | (125) | | | $ | 2 | | | $ | 228 | | | * |
Change % calculations are based on amounts prior to rounding.
(1) Unallocated costs include restructuring and special items, stock-based compensation, impairment charges, certain other corporate directed costs, and other costs that are not allocated to the segments as follows:
| | | | | | | | | | | |
| | Three Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 |
Impairment charges and gain/loss on sale of assets (a) | $ | (13) | | | $ | (6) | |
| Stock-based compensation | (17) | | | (6) | |
Restructuring and other special items (b) | (20) | | | (17) | |
| | | |
| | | |
| | | |
| Pension settlement charges | (9) | | | — | |
| Goodwill impairment charges | — | | | (210) | |
Other (expense) income, net (c) | (4) | | | 4 | |
| Unallocated corporate costs, net | (34) | | | (21) | |
| Total unallocated costs | $ | (97) | | | $ | (256) | |
(a) Impairment charges and gain/loss on sale of assets during the three months ended March 31, 2024 includes right-of-use asset impairment charges associated with underutilized facilities in our Biologics segment.
(b) Restructuring and other special items during the three months ended March 31, 2024 include restructuring charges associated with our plans to reduce costs, consolidate facilities, and optimize infrastructure across the organization.
Restructuring and other special items during the three months ended March 31, 2023 include (i) restructuring charges associated with our plans to reduce costs, consolidate facilities, and optimize infrastructure across the organization and (ii) transaction and integration costs associated with our Metrics acquisition.
(c) Refer to Note 7, Other Expense (Income), Net to our consolidated financial statements for details of amounts recorded within other expense, net in our Consolidated Financial Statements.
Provided below is a reconciliation of net loss to EBITDA from operations:
| | | | | | | | | | | |
| | Three Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 |
| Net loss | $ | (101) | | | $ | (227) | |
| Depreciation and amortization | 126 | | | 106 | |
| Interest expense, net | 65 | | | 51 | |
| Income tax expense (benefit) | 15 | | | (55) | |
| | | |
| EBITDA (loss) from operations | $ | 105 | | | $ | (125) | |
Biologics segment | | | | | | | | | | | |
| 2024 vs. 2023 |
| Year-Over-Year Change | Three Months Ended
March 31, |
| Net Revenue | | Segment EBITDA |
| Organic | (3) | % | | 876 | % |
| | | |
| Constant-currency change | (3) | % | | 876 | % |
| Foreign exchange translation impact on reporting | — | % | | 6 | % |
| Total % change | (3) | % | | 882 | % |
Biologics net revenue decreased by $15 million, or 3%, excluding the impact of foreign exchange, compared to the three months ended March 31, 2023. A decline in demand for COVID-19 related programs, was partially offset by growth in our gene therapy offerings.
Biologics Segment EBITDA increased by $43 million, or 876%, excluding the impact of foreign exchange, compared to the three months ended March 31, 2023. The increase was primarily driven by a decrease in inventory write-offs, partially offset by higher costs due to increased spending on operational and engineering enhancements.
Pharma and Consumer Health segment | | | | | | | | | | | |
| 2024 vs. 2023 |
| Year-Over-Year Change | Three Months Ended
March 31, |
| Net Revenue | | Segment EBITDA |
| Organic | 8 | % | | 21 | % |
| | | |
| Constant-currency change | 8 | % | | 21 | % |
| Foreign currency translation impact on reporting | 1 | % | | 1 | % |
| Total % change | 9 | % | | 22 | % |
Pharma and Consumer Health net revenue increased by $47 million, or 8%, excluding the impact of foreign exchange, compared to the three months ended March 31, 2023. Net revenue increased 8% organically primarily driven by revenue from the manufacture of prescription products, our orally delivered Zydis commercial products, and our consumer health products.
Pharma and Consumer Health Segment EBITDA increased $26 million, or 21%, excluding the impact of foreign exchange, compared to the three months ended March 31, 2023. The increase, similar to that of net revenue, was driven by an increase from the manufacture of prescription products, our orally delivered Zydis commercial products, and our consumer health products. Our year-over-year increase in Segment EBITDA was also driven by a beneficial shift in product mix, enhanced utilization and impacts from our cost cutting initiatives.
Nine Months Ended March 31, 2024 Compared to the Nine Months Ended March 31, 2023
The below tables summarize several financial metrics we use to measure performance for the nine months ended March 31, 2024 and nine months ended March 31, 2023. Refer to the discussions below regarding performance and use of key financial metrics.
Results for the nine months ended March 31, 2024 compared to the nine months ended March 31, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Nine Months Ended
March 31, | | FX Impact | | Constant Currency Increase (Decrease) |
| (Dollars in millions) | 2024 | | 2023 | | | | Change $ | | Change % |
| Net revenue | $ | 3,080 | | | $ | 3,208 | | | $ | 38 | | | $ | (166) | | | (5) | % |
| Cost of sales | 2,511 | | | 2,383 | | | 28 | | | 100 | | | 4 | % |
| Gross margin | 569 | | | 825 | | | 10 | | | (266) | | | (32) | % |
| Selling, general, and administrative expenses | 669 | | | 612 | | | 4 | | | 53 | | | 9 | % |
| | | | | | | | | |
| Goodwill impairment charges | 687 | | | 210 | | | — | | | 477 | | | * |
| Other operating expense | 68 | | | 40 | | | (1) | | | 29 | | | 73 | % |
| Operating loss | (855) | | | (37) | | | 7 | | | (825) | | | * |
| Interest expense, net | 189 | | | 130 | | | — | | | 59 | | | 45 | % |
| Other expense (income), net | 21 | | | (2) | | | 5 | | | 18 | | | * |
| Loss before income taxes | (1,065) | | | (165) | | | 2 | | | (902) | | | * |
| Income tax expense (benefit) | 1 | | | (19) | | | 2 | | | 18 | | | 96 | % |
| | | | | | | | | |
| | | | | | | | | |
| Net loss | $ | (1,066) | | | $ | (146) | | | $ | — | | | $ | (920) | | | * |
| | | | | | | | | |
| | | | | | | | | |
* Not meaningful
Change % calculations are based on amounts prior to rounding.
Net Revenue | | | | | | | |
| 2024 vs. 2023 |
| Year-Over-Year Change | Nine Months Ended
March 31, | | |
| Net Revenue | | |
| Organic | (6) | % | | |
| Impact of acquisitions | 1 | % | | |
| | | |
| Constant-currency change | (5) | % | | |
| Foreign currency translation impact on reporting | 1 | % | | |
| Total % change | (4) | % | | |
Net revenue decreased by $166 million, or 5%, excluding the impact of foreign exchange, compared to the nine months ended March 31, 2023. Net revenue decreased 6% organically on a constant-currency basis, primarily related to a significant decline in demand for COVID-19 related programs, partially offset by growth in our gene therapy offerings.
Net revenue increased 1% inorganically as a result of acquisitions. We acquired Metrics Contract Services (“Metrics”) in October 2022.
Gross Margin
Gross margin decreased by $266 million, or 32%, compared to the nine months ended March 31, 2023, excluding the impact of foreign exchange, primarily due to an unfavorable shift in product mix, lower levels of utilization across the network, reduced productivity, and higher costs from increased spending on operational and engineering enhancements in our Biologics segment, partially offset by a decrease in inventory write-offs. On a constant-currency basis, gross margin, as a percentage of revenue, decreased 740 basis points to 18.4% in the nine months ended March 31, 2024, compared to the corresponding prior-year period, primarily due to the factors described in the immediately preceding sentence.
Selling, General, and Administrative Expense
Selling, general, and administrative expense increased $53 million or 9%, compared to the nine months ended March 31, 2023, excluding the impact of foreign exchange. The year-over-year increase was primarily driven by increased spending on operational and engineering enhancements, a $16 million increase in employee related expenses, a $17 million increase in stock-based compensation, a $14 million increase in depreciation and amortization, a one-time insurance benefit of $10 million in the prior year, and $6 million in net incremental expenses from businesses acquired in the last 12 months, partially offset by a $6 million benefit from a decline in credit losses and a $5 million decline in integration costs.
Goodwill Impairment Charges
Goodwill impairment charges during the nine months ended March 31, 2024 were associated with our Consumer Health and Biomodalities reporting units, which are part of our Pharma and Consumer Health and Biologics segments, respectively. For further details, see Note 4, Goodwill to our Consolidated Financial Statements.
Other Operating Expense
Other operating expense of $68 million for the nine months ended March 31, 2024 increased by $29 million, or 73%, compared to the nine months ended March 31, 2023, excluding the impact of foreign exchange. The year-over-year increase was primarily due to a $23 million increase in fixed-asset impairment charges and $12 million of pension settlement charges, partially offset by a $6 million decrease in restructuring charges.
Interest Expense, net
Interest expense, net of $189 million for the nine months ended March 31, 2024 increased by $59 million, or 45%, compared to the nine months ended March 31, 2023, excluding the impact of foreign exchange. The increase was primarily attributable to higher interest rates on our variable rate debt and incremental borrowings.
For additional information concerning our debt and financing arrangements, including the changing mix of debt and equity in our capital structure, see “—Liquidity and Capital Resources” below and Note 5, Long-Term Obligations and Short-Term Borrowings to our Consolidated Financial Statements.
Other Expense (Income), net
Other expense, net of $21 million for the nine months ended March 31, 2024 was primarily driven by foreign currency losses of $17 million.
Other income, net of $2 million for the nine months ended March 31, 2023 was primarily driven by foreign currency gains of $5 million.
Income Tax Expense (Benefit)
Our provision for income taxes for the nine months ended March 31, 2024 was $1 million relative to loss before income taxes of $1.07 billion. Our benefit for income taxes for the nine months ended March 31, 2023 was $19 million relative to loss before income taxes of $165 million. The income tax expense for the period is primarily the result of income tax expense in select international jurisdictions and the inability to recognize income tax benefit on incremental domestic losses. The year to date benefit was also impacted by the geographic distribution of our pretax loss resulting from our business mix, changes in the tax impact of permanent differences, restructuring, special items, certain equity related compensation, and other discrete tax items that may have unique tax implications depending on the nature of the item.
Segment Review
The following charts depict the percentage of net revenue for each of our two reportable segments for the nine months ended March 31, 2024 compared to the nine months ended March 31, 2023. Refer below for discussions regarding each segment’s net revenue and EBITDA performance and to “Non-GAAP Metrics” for discussions of our use of Segment EBITDA and EBITDA from operations, measures that are not defined under U.S. GAAP.
Our results on a segment basis for the nine months ended March 31, 2024 compared to the nine months ended March 31, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Nine Months Ended
March 31, | | FX Impact | | Constant Currency Increase (Decrease) |
| (Dollars in millions) | 2024 | | 2023 | | | | Change $ | | Change % |
| Biologics | | | | | | | | | |
| Net revenue | $ | 1,347 | | | $ | 1,578 | | | $ | 11 | | | $ | (242) | | | (15) | % |
| Segment EBITDA | 136 | | | 299 | | | 1 | | | (164) | | | (55) | % |
| Pharma and Consumer Health | | | | | | | | | |
| Net revenue | 1,734 | | 1,632 | | 26 | | | 76 | | | 5 | % |
| Segment EBITDA | 380 | | | 368 | | 8 | | | 4 | | | 1 | % |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Inter-segment revenue elimination | (1) | | | (2) | | | 1 | | | — | | | * |
Unallocated Costs (1) | (1,033) | | | (394) | | | (4) | | | (635) | | | * |
| Combined totals | | | | | | | | | |
| Net revenue | $ | 3,080 | | | $ | 3,208 | | | $ | 38 | | | $ | (166) | | | (5) | % |
| | | | | | | | | |
| (Loss) EBITDA from operations | $ | (517) | | | $ | 273 | | | $ | 5 | | | $ | (795) | | | * |
Change % calculations are based on amounts prior to rounding.
*Percentage not meaningful
(1) Unallocated costs include restructuring and special items, stock-based compensation, goodwill impairment charges, fixed asset impairment charges, certain other corporate-directed costs, and other costs that are not allocated to the segments as follows:
| | | | | | | | | | | |
| | Nine Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 |
Impairment charges and gain/loss on sale of assets (a) | $ | (27) | | | $ | (4) | |
| Stock-based compensation | (52) | | | (35) | |
Restructuring and other special items (b) | (126) | | | (59) | |
| | | |
| | | |
| | | |
| Pension settlement charges | (12) | | | — | |
Goodwill impairment charges (c) | (687) | | | (210) | |
Other (expense) income, net (d) | (21) | | | 2 | |
| Non-allocated corporate costs, net | (108) | | | (88) | |
| Total unallocated costs | $ | (1,033) | | | $ | (394) | |
(a) Impairment charges and gain/loss on sale of assets during the nine months ended March 31, 2024 include fixed-asset impairment charges associated with equipment for a product with significant decline in demand in our Biologics segment and right-of-use asset impairment charges associated with underutilized facilities in our Biologics segment.
(b) Restructuring and other special items during the nine months ended March 31, 2024 include (i) restructuring charges associated with our plans that reduced costs, consolidated facilities, and optimized our infrastructure across the organization, most notably the announced closure of our San Francisco facility and (ii) transaction and integration costs associated with the Metrics acquisition.
Restructuring and other special items during the nine months ended March 31, 2023 include (i) restructuring charges associated with our plans that reduced costs, consolidated facilities, and optimized our infrastructure across the organization, (ii) transaction and integration costs associated with the Metrics acquisition, and (iii) warehouse exit costs for a product we no longer manufacture in our Pharma and Consumer Health segment.
(c) Goodwill impairment charges during the nine months ended March 31, 2024 were associated with our Consumer Health and Biomodalities reporting units, which are part of our Pharma and Consumer Health and Biologics segments, respectively. For further details, see Note 4, Goodwill to our Consolidated Financial Statements.
(d) Refer to Note 7, Other Expense (Income), Net to our consolidated financial statements for details.
Provided below is a reconciliation of net earnings to EBITDA from operations: | | | | | | | | | | | |
| | Nine Months Ended
March 31, |
| (Dollars in millions) | 2024 | | 2023 |
| Net loss | $ | (1,066) | | | $ | (146) | |
| Depreciation and amortization | 359 | | | 308 | |
| Interest expense, net | 189 | | | 130 | |
| Income tax expense (benefit) | 1 | | | (19) | |
| | | |
| (Loss) EBITDA from operations | $ | (517) | | | $ | 273 | |
Biologics segment | | | | | | | | | | | |
| 2024 vs. 2023 |
| Year-Over-Year Change | Nine Months Ended
March 31, |
| Net Revenue | | Segment EBITDA |
| Organic | (15) | % | | (55) | % |
| | | |
| Constant-currency change | (15) | % | | (55) | % |
| Foreign exchange translation impact on reporting | — | % | | — | % |
| Total % change | (15) | % | | (55) | % |
| | | |
Net revenue in our Biologics segment decreased by $242 million, or 15%, excluding the impact of foreign exchange, compared to the nine months ended March 31, 2023. The decrease was primarily driven by a significant decline in demand for COVID-19 related programs, partially offset by strong growth in our gene therapy offerings.
Biologics Segment EBITDA decreased by $164 million, or 55%, excluding the impact of foreign exchange, compared to the nine months ended March 31, 2023. Segment EBITDA decreased 55%, compared to the nine months ended March 31, 2023, excluding the impact of acquisitions. The decrease was primarily driven by a significant decline in demand for COVID-19 related programs, lower levels of utilization across the Biologics network, and higher costs due to increased spending on operational and engineering enhancements, which were partially offset by strong growth in our gene therapy offerings and a decrease in inventory write-offs.
Pharma and Consumer Health segment
| | | | | | | | | | | |
| 2024 vs. 2023 |
| Year-Over-Year Change | Nine Months Ended
March 31, |
| Net Revenue | | Segment EBITDA |
| Organic | 3 | % | | (2) | % |
| Impact of acquisitions | 2 | % | | 3 | % |
| | | |
| Constant-currency change | 5 | % | | 1 | % |
| Foreign exchange translation impact on reporting | 1 | % | | 2 | % |
| Total % change | 6 | % | | 3 | % |
Pharma and Consumer Health net revenue increased $76 million, or 5%, excluding the impact of foreign exchange, compared to the nine months ended March 31, 2023. Organic revenue increased 3% when compared to the nine months ended March 31, 2023. The increase in revenue from the manufacture of prescription products and growth in our clinical supply services, were partially offset by a decline in demand for our consumer health products, primarily our wellness products.
Pharma and Consumer Health Segment EBITDA increased $4 million, or 1%, excluding the impact of foreign exchange, compared to the nine months ended March 31, 2023. Segment EBITDA decreased 2%, compared to the nine months ended March 31, 2023, excluding the impact of acquisitions. The decrease in organic Segment EBITDA was primarily driven by a decline in demand for our consumer health products, partially offset by an increase in revenue from the manufacture of prescription products.
We acquired Metrics in October 2022, which increased the segment's net revenue and Segment EBITDA on an inorganic basis by 2% and 3%, respectively, in the nine months ended March 31, 2024, compared to the corresponding prior-year period.
Liquidity and Capital Resources
Sources and Uses of Cash
Our principal sources of liquidity have been cash flows generated from operations and occasional capital market activities. The principal uses of cash are to fund operating and capital expenditures, business or asset acquisitions, interest payments on our debt, income taxes, and any mandatory or discretionary principal payment on our debt. As of March 31, 2024, Catalent Pharma Solutions, Inc., our principal operating subsidiary (“Operating Company”), had available $1.10 billion in borrowing capacity under our revolving credit facility, net of $4 million in letters of credit outstanding as of March 31, 2024.
We believe that our cash on hand, cash from operations, and available borrowings under our revolving credit facility will be adequate to meet our liquidity needs for at least the next 12 months, as well as the amounts expected to become due with respect to our pending capital projects.
Cash Flows
The following table summarizes our consolidated statements of cash flows: | | | | | | | | | | | | | | | | | |
| | Nine Months Ended
March 31, | | |
| (Dollars in millions) | 2024 | | 2023 | | $ Change |
| Net cash provided by (used in): | | | | | |
| Operating activities | $ | 54 | | | $ | 58 | | | $ | (4) | |
| Investing activities | $ | (253) | | | $ | (834) | | | $ | 581 | |
| Financing activities | $ | 84 | | | $ | 572 | | | $ | (488) | |
Operating Activities
For the nine months ended March 31, 2024, cash provided by operations was $54 million compared to $58 million in cash provided by operations for the nine months ended March 31, 2023. The year-over-year change was primarily due to a benefit in working capital partially offset by an increase in interest payments due to higher outstanding debt balances and higher rates on our variable rate debt.
Investing Activities
For the nine months ended March 31, 2024, cash used in investing activities was $253 million, compared to $834 million for the nine months ended March 31, 2023. The decrease in cash used in investing activities was primarily driven by a decrease in mergers and acquisitions activity and a decrease in acquisition of property, equipment, and other productive assets.
Financing Activities
For the nine months ended March 31, 2024, cash provided by financing activities was $84 million, compared to cash provided by financing activities of $572 million for the nine months ended March 31, 2023. The decrease in cash provided by financing activities was primarily driven by a $450 million decrease in net borrowings.
Debt Covenants
Senior Secured Credit Facilities
The Credit Agreement contains a number of covenants that, among other things, restrict, subject to certain exceptions, our (and our restricted subsidiaries’) ability to incur additional indebtedness or issue certain preferred shares; create liens on assets; engage in mergers and consolidations; sell assets; pay dividends and distributions or repurchase capital stock; repay subordinated indebtedness; engage in certain transactions with affiliates; make investments, loans, or advances; make certain acquisitions; enter into sale and leaseback transactions; amend material agreements governing our subordinated indebtedness; and change our lines of business.
The Credit Agreement also contains change-of-control provisions and certain customary affirmative covenants and events of default. The revolving credit facility requires compliance with a net leverage covenant when there is a 30% or more draw
outstanding at a period end. As of March 31, 2024, we were in compliance with all material covenants under the Credit Agreement.
Subject to certain exceptions, the Credit Agreement permits us and our restricted subsidiaries to incur certain additional indebtedness, including secured indebtedness. None of our non-U.S. subsidiaries or our Puerto Rico subsidiary is a guarantor of the loans.
On November 22, 2023, Operating Company, entered into Amendment No. 10 to the Credit Agreement, which further extended the deadlines by which we are required to deliver to the administrative agent (i) our audited financial statements as at the end of and for the fiscal year ended June 30, 2023, together with the auditor’s report and opinion on such audited financial statements, to January 26, 2024, and (ii) our unaudited financial statements as at the end of and for the fiscal quarter ending September 30, 2023 to March 31, 2024.
On December 19, 2023, Operating Company entered into Amendment No. 11 to the Credit Agreement, pursuant to which Operating Company incurred $600 million aggregate principal amount of new incremental dollar term loans.
Under the Credit Agreement, our ability to engage in certain activities such as incurring certain additional indebtedness, making certain investments, and paying certain dividends is tied to ratios based on Adjusted EBITDA (which is defined as “Consolidated EBITDA” in the Credit Agreement). Adjusted EBITDA is based on the definitions in the Credit Agreement, is not defined under U.S. GAAP, and is subject to important limitations.
The Senior Notes
The several indentures governing each series of our outstanding senior notes (collectively, the “Indentures”) contain certain covenants that, among other things, limit our ability to incur or guarantee more debt or issue certain preferred shares; pay dividends on, repurchase, or make distributions in respect of their capital stock or make other restricted payments; make certain investments; sell certain assets; create liens; consolidate, merge, sell; or otherwise dispose of all or substantially all of their assets; enter into certain transactions with their affiliates, and designate their subsidiaries as unrestricted subsidiaries. These covenants are subject to a number of exceptions, limitations, and qualifications as set forth in the Indentures. The Indentures also contain customary events of default, including, but not limited to, nonpayment, breach of covenants, and payment or acceleration defaults in certain other indebtedness of Operating Company or certain of its subsidiaries. Upon an event of default, either the holders of at least 30% in principal amount of each of the then-outstanding series of Senior Notes, or the applicable Trustee under the Indentures, may declare the applicable senior notes immediately due and payable; or in certain circumstances, the applicable senior notes will become automatically immediately due and payable. As of March 31, 2024, Operating Company was in compliance with all material covenants under the Indentures.
Capital Resources
As market conditions warrant, we and our affiliates may from time to time seek to purchase our outstanding debt in privately negotiated or open-market transactions, by tender offer or otherwise. Subject to any applicable limitation contained in the Credit Agreement, any purchase made by us may be funded by the use of cash on hand or the incurrence of new secured or unsecured debt. The amounts involved in any such purchase transaction, individually or in the aggregate, may be material. Any such purchase may be with respect to a substantial amount of a particular class or series of debt, with the attendant reduction in the trading liquidity of such class or series. In addition, any such purchase made at prices below the “adjusted issue price” (as defined for U.S. federal income tax purposes) may result in taxable cancellation of indebtedness income to us, which amounts may be material, or in related adverse tax consequences to us.
Geographic Allocation of Cash
As of March 31, 2024 and June 30, 2023, our foreign subsidiaries held cash and cash equivalents of $138 million and $181 million, respectively, out of the total consolidated cash and cash equivalents of $162 million and $280 million, respectively. These balances are dispersed across many locations around the world.
Interest Rate Risk Management
A portion of the debt used to finance our operations is exposed to interest-rate fluctuations. We may use various hedging strategies and derivative financial instruments to create an appropriate mix of fixed-and floating-rate assets and liabilities. In February 2021, we entered into an interest-rate swap agreement with Bank of America N.A. that acts as a hedge against the economic effect of a portion of the variable-interest obligation associated with our U.S. dollar-denominated term loans under our senior secured credit facilities, so that the interest payable on that portion of the debt becomes fixed at a certain rate, thereby reducing the impact of future interest-rate changes on future interest expense. The applicable rate for the U.S. dollar-denominated term loan under the Credit Agreement was LIBOR (subject to a floor of 0.50%) plus 2.00% as of March 31, 2024;
however, as a result of this interest-rate swap agreement, the variable portion of the applicable rate on $500 million of the term loan was effectively fixed at 0.9985%.
To conform with the adoption of ASC 848, Reference Rate Reform and the Eighth Amendment, the Company amended the 2021 Rate Swap as the 2023 Rate Swap. The 2023 Rate Swap continues to effectively fix the rate of interest payable on the same portion of our U.S dollar-denominated term loans under our secured credit facilities. The applicable rate for the U.S. dollar-denominated term loan under the Credit Agreement was SOFR (subject to a floor of 0.39%) plus 2.00% as of March 31, 2024. As a result of the 2023 Rate Swap, the variable portion of the applicable interest rate on $500 million of the U.S. dollar-denominated term loans is now effectively fixed at 0.9431%.
Currency Risk Management
We are exposed to fluctuations in the euro-U.S. dollar exchange rate on our investments in our operations in Europe. While we do not actively hedge against changes in foreign currency, we have mitigated the exposure of our investments in our European operations by denominating a portion of our debt in euros. At March 31, 2024, we had $893 million of euro-denominated debt outstanding that qualifies as a hedge of a net investment in European operations. Refer to Note 9, Derivative Instruments and Hedging Activities, to our Consolidated Financial Statements for further discussion of net investment hedge activity in the period.
From time to time, we may use forward foreign currency exchange contracts to manage our exposure to the variability of cash flows primarily related to the foreign exchange rate changes of future foreign currency transaction costs. In addition, we may use such contracts to protect the value of existing foreign currency assets and liabilities. Currently, we do not use any forward foreign currency exchange contracts. We continue to evaluate hedging opportunities for foreign currency in the future.
Off-Balance Sheet Arrangements
Other than short-term operating leases and outstanding letters of credit as discussed above, we do not have any material off-balance sheet arrangement as of March 31, 2024.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
For a discussion of our quantitative and qualitative disclosures about market risks, see the section titled Item 7A, Quantitative and Qualitative Disclosures About Market Risks in our Fiscal 2023 10-K. As of March 31, 2024, there has been no material change in this information.
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and our Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures. Any control or procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Our management, including our Chief Executive Officer and our Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this Quarterly Report on Form 10-Q. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of March 31, 2024, our disclosure controls and procedures were not effective to accomplish their objectives at the reasonable assurance level, as a result of the material weaknesses in our internal control over financial reporting disclosed below.
Material Weaknesses in Internal Control over Financial Reporting
A material weakness (as defined in Rule 12b-2 under the Exchange Act) is a deficiency, or a combination of deficiencies, in internal controls over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
Previously Disclosed Material Weakness in Internal Controls over Financial Reporting – Contract Modifications
As previously disclosed, in connection with our evaluation for the three months ended December 31, 2023, we identified a material weakness in internal control over financial reporting as we did not maintain effective internal controls to properly identify and assess the accounting treatment of contract modification arrangements under ASC 606, Revenue from Contracts with Customers. The review of the accounting assessments for certain modified contracts during the quarter ended December 31, 2023, was not performed with the necessary level of technical competency to detect a material misstatement.
Previously Disclosed Material Weakness in Internal Controls over Financial Reporting – Income Tax Provision
As previously disclosed, in connection with our evaluation for the three months ended December 31, 2023, we identified a material weakness in internal control over financial reporting as we did not maintain effective internal control over the preparation and review of the interim income tax provision. Specifically, the Company did not design an appropriate interim review control over the completeness and accuracy of certain inputs to the quarterly income tax provision calculations. While the control deficiency did not result in a material misstatement to the Company’s interim consolidated financial statements, there is a reasonable possibility that the control deficiency could have resulted in a material misstatement of the income tax related accounts or disclosures in the Company’s interim consolidated financial statements that would not have been prevented or detected on a timely basis.
Previously Disclosed Material Weakness in Internal Controls over Financial Reporting – Revenue Recognition
As previously disclosed, management identified during the preparation of our unaudited consolidated financial statements for the fiscal year ended June 30, 2023 a material weakness in our internal controls over financial reporting relating to the year ended June 30, 2022, which remains unremediated as of March 31, 2024.
We did not maintain effective controls over the appropriateness of revenue recognition related to modifications of customer agreements at our Bloomington, Indiana facility. Specifically, we did not maintain effective controls to properly identify and assess the accounting treatment of modifications to arrangements that were accounted for under ASC 606, Revenue from Contracts with Customers. The reviewer had insufficient knowledge of the requirements of the ASC 606 revenue recognition accounting model, and therefore the review procedures were not performed with the necessary level of competence to prevent or detect a material misstatement on a timely basis.
Furthermore, the compensating control to review the accounting assessments for contract modifications was not sufficiently designed to detect accounting misstatements. As previously disclosed, this control deficiency resulted in an immaterial revision to our consolidated financial statements for the fiscal year ended June 30, 2022 to correct an overstatement of revenue of $26 million. While this control deficiency did not result in a material misstatement of our consolidated financial statements, there is a reasonable possibility that this deficiency could have resulted in a material misstatement of our annual or interim consolidated financial statements that would not be prevented or detected on a timely basis.
Previously Disclosed Material Weakness in Internal Controls over Financial Reporting – Consolidated Financial Statement Close Process
As previously disclosed, management identified during the preparation of our audited consolidated financial statements for the year ended June 30, 2023 a material weakness in our internal controls over financial which remains unremediated as of March 31, 2024.
We did not maintain effective internal control over the evaluation and accounting of certain complex and non-routine transactions. Due to an insufficient complement of technical resources within its corporate accounting function, management was unable to complete its evaluation of certain complex non-routine transactions in a timely manner. Specifically, management did not adequately prepare and maintain sufficient evidence of management’s review of (i) significant assumptions, relating to the interim goodwill and long-lived assets impairment assessments as of March 31, 2023, (ii) the evaluation of indicators and assessment of impairment of goodwill and long-lived assets as of June 30, 2023 and (iii) the evaluation of the accounting, measurement and disclosure of events occurring subsequent to the balance sheet date, specifically management’s evaluation of disclosure and the related measurement of a goodwill impairment charge disclosed in the subsequent events footnote.
Previously Disclosed Material Weakness in Internal Control over Financial Reporting – Inventory Reconciliation
As previously disclosed, management identified during the preparation of our audited consolidated financial statements for the year ended June 30, 2023 a material weakness in our internal controls over financial which remains unremediated as of March 31, 2024.
We did not maintain effective internal controls over inventory reconciliation at our Baltimore, Maryland facility. Specifically, we did not implement and design controls at an appropriate level of precision to (i) properly recognize certain third party costs on the balance sheet separately from the inventory balance, (ii) properly and timely update our perpetual inventory subledger to value inventory at lower of cost or market, and (iii) reconcile our perpetual inventory subledger to the related general ledger accounts.
Plan to Remediate Material Weakness in Internal Controls Over Financial Reporting – Contract Modifications
The Company, with oversight by the Audit Committee of the Board of Directors of the Company, is devoting significant time, attention, and resources to remediating the contract modification material weakness in our internal controls over financial reporting described above. We have initiated the following steps intended to remediate this material weakness and strengthen our internal controls over financial reporting:
•We hired additional technical accounting resources within the corporate controllership group.
•Ensuring that appropriate levels of our management, including our necessary technical accounting resources, are consulted before significant contract modifications are executed.
We plan to continue to devote significant time and attention to remediate this material weakness as soon as reasonably practicable. We believe these actions will be sufficient to remediate the identified material weakness and strengthen our internal controls over financial reporting; however, there can be no guarantee that such remediation will be sufficient. We will continue to monitor the design and effectiveness of these and other processes, procedures, and controls and make any further change management determines appropriate. We expect to complete the remediation of this material weakness by the fourth quarter of fiscal 2024, although no assurance can be given regarding the time and effort needed to complete the remediation.
Plan to Remediate Material Weakness in Internal Controls Over Financial Reporting – Income Tax Provision
Management, with the oversight of the Audit Committee of the Board, will update our design of controls over the completeness and accuracy of inputs to the quarterly income tax provision calculations.
We plan to continue to devote significant time and attention to remediate this material weakness as soon as reasonably practicable. We believe these actions will be sufficient to remediate the identified material weakness and strengthen our internal controls over financial reporting; however, there can be no guarantee that such remediation will be sufficient. We will continue to monitor the design and effectiveness of these and other processes, procedures, and controls and make any further change management determines appropriate. We expect to complete the remediation of this material weakness by the fourth quarter of fiscal 2024, although no assurance can be given regarding the time and effort needed to complete the remediation.
Plan to Remediate Material Weakness in Internal Controls Over Financial Reporting – Revenue Recognition
The Company, with oversight by the Audit Committee of the Board of Directors of the Company, is devoting significant time, attention, and resources to remediating the revenue modification material weakness in our internal controls over financial reporting described above. We are taking initiated the following steps intended to remediate this material weakness and strengthen our internal controls over financial reporting:
•We hired additional technical accounting resources within our Bloomington, Indiana site and within the corporate controllership group.
•Enhanced the design of our management review controls relating to the accounting for contract modifications, including offered concessions.
•Continue to provide additional training for our executive leadership team, and other critical customer-facing personnel, on revenue recognition principles, including contract modifications relating to offered concessions.
We plan to continue to devote significant time and attention to remediate this material weakness as soon as reasonably practicable. We believe these actions will be sufficient to remediate the identified material weakness and strengthen our internal controls over financial reporting; however, there can be no guarantee that such remediation will be sufficient. We will continue to monitor the design and effectiveness of these and other processes, procedures, and controls and make any further change management determines appropriate. We expect to complete the remediation of this material weakness by the fourth quarter of fiscal 2024, although no assurance can be given regarding the time and effort needed to complete the remediation.
Plan to Remediate Material Weakness in Internal Controls over Financial Reporting – Consolidated Financial Statement Close Process
The Company, with oversight by the Audit Committee of the Board of Directors of the Company, developed and implemented a comprehensive remediation plan which includes the following key initiatives:
•we engaged temporary third-party resources with the appropriate level of technical knowledge and experience in accounting related to complex non-routine transactions and the related internal control activities to complement the existing corporate accounting resources;
•we continue to hire, develop and retain incremental full-time personnel with appropriate accounting and internal controls expertise;
•we will review and update (as appropriate) our methodologies, policies and procedures designed to ensure we are able to more timely address our evaluation of complex non-routine transactions, including the related evidence of management’s review of the significant assumptions used in those evaluations; and
•review and update (as appropriate) our training programs related to the relevant internal control over financial reporting matters pertaining to complex non-routine transactions.
We plan to continue to devote significant time and attention to remediate this material weakness as soon as reasonably practicable. We believe these actions will be sufficient to remediate the identified material weakness and strengthen our internal controls over financial reporting; however, there can be no guarantee that such remediation will be sufficient. We will continue to monitor the design and effectiveness of these and other processes, procedures, and controls and make any further change management determines appropriate. We expect to complete the remediation of this material weakness by the fourth quarter of fiscal 2024, although no assurance can be given regarding the time and effort needed to complete the remediation.
Plan to Remediate Material Weakness in Internal Controls Over Financial Reporting – Inventory Reconciliation
Management, with oversight by the Audit Committee of the Board, has updated our design of controls for the valuation of inventory at our Baltimore location and we will continue to review and update our procedures as well as provide additional training to the control owners. We believe these actions will be sufficient to remediate the identified material weakness and strengthen our internal controls over financial reporting; however, there can be no guarantee that such remediation will be sufficient. We will continue to monitor the design and effectiveness of these and other processes, procedures, and controls and make any further change management determines appropriate. We expect to complete the remediation of this material weakness by the fourth quarter of fiscal 2024, although no assurance can be given regarding the time and effort needed to complete the remediation.
Changes in Internal Control over Financial Reporting
We are taking actions to complete the remediation of the remaining material weaknesses relating to our internal control over financial reporting, as described above. Except as otherwise described herein, there was no change in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
Information pertaining to legal proceedings can be found in Note 14, Commitments and Contingencies, to the Consolidated Financial Statements and is incorporated by reference herein.
ITEM 1A. RISK FACTORS
In addition to the other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the factors discussed in the section entitled “Risk Factors” in our Fiscal 2023 10-K, which could materially affect our business, financial condition, or future results. The risks described in our Fiscal 2023 10-K are not the only risks facing us. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition, or operating results.
We may not complete the pending Merger with Novo Holdings within the timeframe anticipated, or at all, which could have a material adverse effect on our business, financial condition or results of operations, as well as negatively impact our share price.
On February 5, 2024, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Creek Parent, Inc. (“Parent”), a Delaware corporation and a wholly owned subsidiary of Novo Holdings A/S (“Novo Holdings”), and Creek Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”). The Merger Agreement provides that, upon the terms and subject to the conditions set forth therein and in accordance with the General Corporation Law of the State of Delaware, Merger Sub will be merged with and into the Company, with the Company surviving the Merger as a wholly owned subsidiary of Parent (the “Merger”).
Consummation of the Merger is subject to customary closing conditions, including (i) approval of the Merger by the Company’s stockholders, (ii) receipt of certain governmental waivers, consents, clearances, decisions, declarations, approvals, and expirations of applicable waiting periods, including the expiration or early termination of the waiting period under the U.S. Hart-Scott-Rodino Antitrust Improvements Act of 1976, with respect to (A) the Merger and (B) the sale of three of the Company’s fill-finish sites (which are located in Anagni, Italy, Bloomington, Indiana USA, and Brussels, Belgium) and related assets from Novo Holdings to Novo Nordisk A/S, of which Novo Holdings has a controlling interest (the “Carve-Out”), (iii) the absence of any order, injunction or law prohibiting the Merger or the Carve-Out, in each case, without a Burdensome Condition (as defined in the Merger Agreement), and (iv) the absence of a Material Adverse Effect (as defined in the Merger Agreement) on the Company. Furthermore, the granting of regulatory approvals by antitrust authorities could involve the imposition of additional conditions on the closing of the Merger. The imposition of such conditions or the failure or delay to obtain regulatory approvals could have the effect of delaying completion of the Merger or of imposing additional costs or limitations on us or may result in the failure to close the Merger. We cannot provide any assurance that the conditions to the consummation of the Merger will be satisfied or waived or that, if the Merger is consummated, it will be on the terms specified in the Merger Agreement or within the anticipated timeframe.
Failure to complete the Merger within the timeframe anticipated could adversely affect our business and the market price of our Shares in a number of ways, including:
•The price of our common stock may decline to the extent that current market prices of our common stock reflect assumptions that the Merger will be completed on a timely basis.
•We could be required to pay Novo Holdings a termination fee of approximately $345 million if the Merger Agreement is terminated under specific circumstances described in the Merger Agreement.
•The failure to complete the Merger may result in negative publicity and negatively affect our relationship with our stockholders, employees, customers, suppliers and lenders.
•If the Merger is not completed, the time and resources committed by our management team could have been devoted to pursuing other opportunities.
•We have incurred, and will continue to incur, significant expenses for professional services in connection with the Merger for which we will have received little or no benefit if the Merger is not completed.
In addition, any litigation or enforcement proceeding commenced against us in connection with the Merger may require us to devote significant time and resources and could require us to incur significant costs. This also could result in the Merger being delayed and/or enjoined by a court of competent jurisdiction, which could prevent the Merger from becoming effective.
The occurrence of any of these events individually or in combination which could have a material adverse effect on our business, financial condition or results of operations, as well as negatively impact our share price.
The announcement and pendency of the Merger with Novo Holdings could materially adversely affect our business, financial condition or results of operations, as well as negatively impact our share price.
Our efforts to complete the Merger with Novo Holdings could cause substantial disruptions in, and create uncertainty surrounding, our business, which may materially adversely affect our business, financial condition or results of operations, or the price of our common stock. Uncertainty as to whether the Merger will be completed may affect our ability to recruit prospective employees or to retain and motivate existing employees. Employee retention may be particularly challenging while the transaction is pending because employees may experience uncertainty about their roles following consummation of the Merger. A substantial amount of our management’s and employees’ attention is being directed toward the completion of the transaction and thus is being diverted from our day-to-day operations. Uncertainty as to our future also could adversely affect our business and our relationship with collaborators, strategic partners, suppliers, existing or prospective customers or regulators. For example, collaborators, suppliers, existing or prospective customers and other counterparties may defer decisions concerning us, or seek to change existing business relationships with us, whether pursuant to the terms of their existing agreements with us or otherwise. Changes to or termination of existing business relationships could materially adversely affect our financial condition and results of operations, as well as negatively impact our share price. The adverse effects of the pendency of the transaction could be exacerbated by any delays in completion of the transaction, changes to the terms of the transaction or termination of the Merger Agreement.
In certain instances, the Merger Agreement requires us to pay a termination fee to Novo Holdings, which could require us to use available cash that would have otherwise been available for general corporate purposes and other uses.
The Merger Agreement contains certain termination rights for us and Novo Holdings. Subject to certain limitations, we or Novo Holdings may terminate the Merger Agreement if the Merger is not closed by February 5, 2025 (as may be extended as described in the following sentence, the “End Date”), either the Company or Parent may terminate the Merger Agreement. However, if as of such End Date all conditions set forth in the Merger Agreement have been satisfied or waived (other than those conditions that (i) by their nature are to be satisfied by actions to be taken at the closing, or (ii) relate to receipt of required regulatory approvals or legal restraints in connection with required regulatory approvals or applicable antitrust laws), then the End Date will automatically be extended by three (3) months on each of four (4) occasions. In addition, if the condition relating to receipt of the Company’s stockholders’ approval has not been satisfied solely due to the Company’s inability to timely file the Merger proxy statement in certain limited circumstances, then, subject to the satisfaction or waiver of all conditions other than the foregoing and those noted in the preceding sentence, the End Date will be extended by three (3) months on each of the first two (2) such occasions.
Upon termination of the Merger Agreement, the Company, under specified circumstances and conditions set forth in the Merger Agreement, including termination (1) by the Company in order to enter into an alternative acquisition agreement with respect to a Superior Offer (as defined in the Merger Agreement) or (2) by Parent upon a change in our board of directors’ stockholder recommendation of the Merger, will be required to pay Parent a termination fee equal to $345 million.
If the Merger Agreement is terminated under such circumstances, the termination fee we would be required to pay under the Merger Agreement may require us to use available cash that would have otherwise been available for general corporate purposes and other uses. Further, a failed transaction may result in negative publicity and a negative impression of us in the investment community. For these and other reasons, termination of the Merger Agreement could materially and adversely affect our business, results of operations and financial condition, which in turn would materially and adversely affect the price of our common stock.
We have incurred, and will continue to incur, direct and indirect costs as a result of the pending Merger with Novo Holdings.
We have incurred, and will continue to incur, significant costs and expenses, including legal, accounting and other advisory fees and other transaction costs, in connection with the pending Merger. We will be required to pay a substantial portion of these costs and expenses whether or not the Merger is completed. There are a number of factors beyond our control that could affect the total amount or the timing of these costs and expenses.
While the Merger Agreement is in effect, we are subject to restrictions on our business activities.
While the Merger Agreement is in effect, we are subject to restrictions on our business activities, generally requiring us to conduct our business in the ordinary course, consistent with past practice, in all material respects, and subjecting us to a variety of specified limitations absent Novo Holdings’ prior consent. These limitations include, among other things, restrictions on our ability to acquire other businesses and material assets (including certain governmental licenses and authorizations), dispose of material assets, make investments, enter into or amend certain material contracts, repurchase or issue securities, pay dividends,
make capital expenditures, take certain actions relating to intellectual property, amend our organizational documents, incur indebtedness, hire/terminate certain employees and provide increases to compensation and benefits to certain employees. These restrictions could prevent us from pursuing strategic business opportunities, taking actions with respect to our business that we may consider advantageous and responding effectively or on a timely basis to competitive pressures and industry developments, and may as a result materially adversely affect our business, financial condition and results of operations.
The Merger Agreement contains provisions that could deter or make it difficult for a third party from proposing an alternative transaction or acquire our Company prior to the consummation of the Merger.
The Merger Agreement contains provisions that limit our ability to entertain a third-party proposal for an acquisition of our company or an alternative transaction in lieu of the Merger. These provisions include our agreement not to, directly or indirectly, solicit, initiate, or knowingly facilitate, or knowingly encourage or negotiate with any person regarding other proposals for an acquisition of our Company, as well as restrictions on our ability to respond to such proposals, subject to certain exceptions including fulfillment of certain fiduciary requirements of our board of directors. In addition, we could be required to pay Novo Holdings a termination fee of approximately $344.8 million if the Merger Agreement is terminated under specific circumstances. These or other provisions in the Merger Agreement might discourage a third party with a potential interest in acquiring all or a significant part of the outstanding shares of our common stock from considering or proposing an acquisition, even one that may be deemed of greater value to our stockholders than the proposed Merger with Novo Holdings. Furthermore, even if a third party elects to propose an acquisition of us, the potential competing acquirer may propose to pay a lower amount as a result of the termination fee that will become payable by us.
We may be targets of securities class action and derivative lawsuits that could result in substantial costs and may delay or prevent the Merger from being completed.
Securities class action lawsuits and derivative lawsuits are often brought against public companies that have entered into merger agreements. The outcome of litigation is uncertain and we may not be successful in defending against future claims brought against us even if they are without merit. Regardless of the outcome of any lawsuits brought against us, such lawsuits could delay or prevent the Merger, divert the attention of our management and employees from our day-to-day business, result in substantial costs and otherwise adversely affect us financially. A potential adverse judgment could result in monetary damages, which could have a negative impact on our liquidity and financial condition. Additionally, if a plaintiff is successful in obtaining an injunction prohibiting completion of the Merger, that injunction may delay or prevent the Merger from being completed, or from being completed within the anticipated timeframe, which may adversely affect our business, financial condition or results of operations.
Our executive officers and directors may have interests in the proposed Merger that are different from, or in addition to, those of our stockholders generally.
Our executive officers and directors may have interests in the proposed Merger that are different from the interests of our stockholders generally, including, among others, the acceleration of the vesting of equity awards and receipt of change in control or other severance payments in connection with the proposed Merger, continued indemnification and insurance and potentially continued service to the combined company. These interests, among others, may influence, or appear to influence, our executive officers and directors and cause them to view the Merger differently from how our stockholders generally may view it.
Additional information regarding our executive officers and directors and their interests in the proposed Merger will be included in the proxy statement relating to the proposed Merger when it is filed with the Securities and Exchange Commission.
If the Merger occurs, our stockholders will not be able to participate in any further upside to our business.
If the Merger is consummated, our stockholders will receive the right to receive an amount in cash equal to $63.50 per Share, without interest, and will not receive any equity interests of Parent. As a result, if our business following the Merger performs well, our current stockholders will not receive any additional consideration and will therefore not receive any benefit from any such future performance of our business.
Other than the risk factors noted above, there has been no material change to the risk factors disclosed in our Fiscal 2023 10-K.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
Recent Sales of Unregistered Equity Securities
We did not sell any unregistered equity securities during the period covered by this Quarterly Report.
Issuer Purchases of Equity Securities
We did not purchase any of our equity securities during the period covered by this Quarterly Report.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
Trading Arrangements
During the fiscal quarter ended March 31, 2024, none of our directors or officers (as defined in Rule 16a-1(f) under the Exchange Act) adopted or terminated contracts, instructions or written plans for the purchase or sale of our securities.
ITEM 6. EXHIBITS
Exhibits: | | | | | | | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | Agreement and Plan of Merger, dated as of February 5, 2024, by and among the Company, Creek Parent, Inc., and Creek Merger Sub, Inc. (incorporated by reference to Exhibit 2.1 to the Current Report on Form 8-K filed on February 5, 2024). |
| | Amendment No.1 to Catalent, Inc. 2018 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on January 26, 2024). † |
| | |
| | Offer letter, dated January 11, 2024, between Michael J. Hatzfeld, Jr. and Catalent, Inc. *† |
| | |
| | Employment Agreement, dated August 28, 2023, between John Greisch and Catalent, Inc. *† |
| | |
| | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended. * |
| |
| | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended. * |
| |
| | Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. ** |
| |
| | Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. ** |
| | |
| 101 | | The following financial information from Catalent, Inc.’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2024 formatted in inline XBRL: (i) Consolidated Statements of Operations for the Three and Nine Months Ended March 31, 2024 and 2023; (ii) Consolidated Statements of Comprehensive Loss for the Three and Nine Months Ended March 31, 2024 and 2023; (iii) Consolidated Balance Sheets as of March 31, 2024 and June 30, 2023; (iv) Consolidated Statement of Changes in Shareholders’ Equity for the Three and Nine Months Ended March 31, 2024 and 2023; (v) Consolidated Statements of Cash Flows for the Nine Months Ended March, 2024 and 2023; and (vi) Notes to Unaudited Consolidated Financial Statements. |
| | |
| 104 | | The cover page of this Quarterly Report on Form 10-Q, formatted as Inline XBRL and contained in Exhibit 101. |
| | | | | | | | |
| * | | Filed herewith |
| ** | | Furnished herewith |
| † | | Represents a management contract, compensatory plan or arrangement in which directors and/or executive officers are eligible to participate. |
| | |
| | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | |
| | CATALENT, INC. (Registrant) |
| | | |
| Date: | May 8, 2024 | By: | | /s/ MICHAEL HATZFELD |
| | | | Michael Hatzfeld |
| | | | Vice President and Chief Accounting Officer |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |