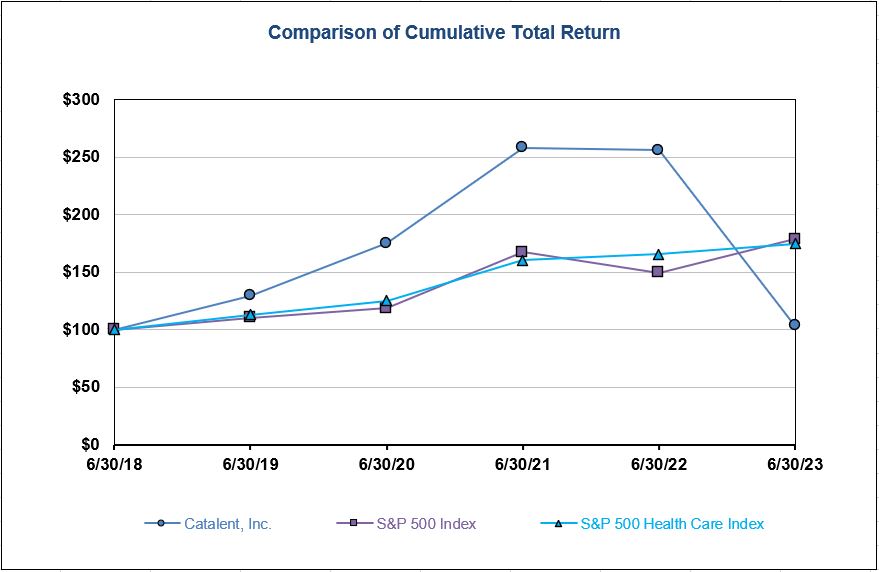
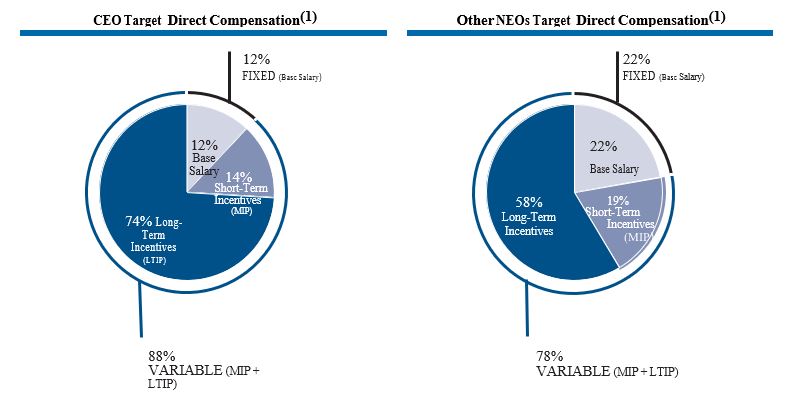
false2023FY180641272.0008873--06-300001596783False0.010.011,000,000,0001,000,000,000180,000,000179,000,0000.010.01100,000,000100,000,000————0.01———4629http://fasb.org/us-gaap/2023#SellingGeneralAndAdministrativeExpensehttp://fasb.org/us-gaap/2023#SellingGeneralAndAdministrativeExpenseP20YP3Yhttp://fasb.org/us-gaap/2023#DefinedBenefitPensionPlanLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#DefinedBenefitPensionPlanLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#DefinedBenefitPensionPlanLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#DefinedBenefitPensionPlanLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#DefinedBenefitPensionPlanLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#DefinedBenefitPensionPlanLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#OtherLiabilitieshttp://fasb.org/us-gaap/2023#OtherLiabilitieshttp://fasb.org/us-gaap/2023#PropertyPlantAndEquipmentNethttp://fasb.org/us-gaap/2023#PropertyPlantAndEquipmentNethttp://fasb.org/us-gaap/2023#OtherAssetsNoncurrent00015967832022-07-012023-06-3000015967832021-12-31iso4217:USD00015967832023-08-18xbrli:shares00015967832023-06-3000015967832022-06-300001596783us-gaap:FairValueInputsLevel1Member2023-06-30iso4217:USDxbrli:shares00015967832021-07-012022-06-3000015967832020-07-012021-06-3000015967832019-07-012020-06-300001596783us-gaap:RetainedEarningsMember2022-07-012023-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-07-012023-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2022-07-012023-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2021-07-012022-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2020-07-012021-06-300001596783us-gaap:CommonStockMember2020-06-300001596783us-gaap:AdditionalPaidInCapitalMember2020-06-300001596783us-gaap:RetainedEarningsMember2020-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-06-3000015967832020-06-300001596783us-gaap:CommonStockMember2022-07-012023-06-300001596783us-gaap:CommonStockMember2020-07-012021-06-300001596783us-gaap:AdditionalPaidInCapitalMember2020-07-012021-06-300001596783us-gaap:RetainedEarningsMember2020-07-012021-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-07-012021-06-300001596783us-gaap:CommonStockMember2021-06-300001596783us-gaap:AdditionalPaidInCapitalMember2021-06-300001596783us-gaap:RetainedEarningsMember2021-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-06-3000015967832021-06-300001596783us-gaap:CommonStockMember2021-07-012022-06-300001596783us-gaap:AdditionalPaidInCapitalMember2021-07-012022-06-300001596783us-gaap:RetainedEarningsMember2021-07-012022-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-07-012022-06-300001596783us-gaap:CommonStockMember2022-06-300001596783us-gaap:AdditionalPaidInCapitalMember2022-06-300001596783us-gaap:RetainedEarningsMember2022-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-300001596783us-gaap:AdditionalPaidInCapitalMember2022-07-012023-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-07-012023-06-300001596783us-gaap:CommonStockMember2023-06-300001596783us-gaap:AdditionalPaidInCapitalMember2023-06-300001596783us-gaap:RetainedEarningsMember2023-06-300001596783us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-30utr:Rate0001596783srt:MinimumMemberctlt:BuildingAndImprovementsMember2023-06-300001596783srt:MaximumMemberctlt:BuildingAndImprovementsMember2023-06-300001596783us-gaap:MachineryAndEquipmentMembersrt:MinimumMember2023-06-300001596783us-gaap:MachineryAndEquipmentMembersrt:MaximumMember2023-06-300001596783srt:MinimumMemberus-gaap:FurnitureAndFixturesMember2023-06-300001596783srt:MaximumMemberus-gaap:FurnitureAndFixturesMember2023-06-300001596783ctlt:BiologicsMemberctlt:ManufacturingCommercialProductSupplyMember2022-07-012023-06-300001596783ctlt:PharmaConsumerHealthMemberctlt:ManufacturingCommercialProductSupplyMember2022-07-012023-06-300001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2022-07-012023-06-300001596783ctlt:BiologicsMemberctlt:DevelopmentServicesMember2022-07-012023-06-300001596783ctlt:PharmaConsumerHealthMemberctlt:DevelopmentServicesMember2022-07-012023-06-300001596783ctlt:DevelopmentServicesMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2022-07-012023-06-300001596783ctlt:BiologicsMemberus-gaap:OperatingSegmentsMember2022-07-012023-06-300001596783us-gaap:OperatingSegmentsMemberctlt:PharmaConsumerHealthMember2022-07-012023-06-300001596783ctlt:OperatingSegmentsExcludingIntersegmentEliminationMember2022-07-012023-06-300001596783ctlt:TotalCatalentSegmentMemberus-gaap:IntersegmentEliminationMember2022-07-012023-06-300001596783us-gaap:OperatingSegmentsMember2022-07-012023-06-300001596783ctlt:BiologicsMemberctlt:ManufacturingCommercialProductSupplyMember2021-07-012022-06-300001596783ctlt:PharmaConsumerHealthMemberctlt:ManufacturingCommercialProductSupplyMember2021-07-012022-06-300001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2021-07-012022-06-300001596783ctlt:BiologicsMemberctlt:DevelopmentServicesMember2021-07-012022-06-300001596783ctlt:PharmaConsumerHealthMemberctlt:DevelopmentServicesMember2021-07-012022-06-300001596783ctlt:DevelopmentServicesMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2021-07-012022-06-300001596783ctlt:BiologicsMemberus-gaap:OperatingSegmentsMember2021-07-012022-06-300001596783us-gaap:OperatingSegmentsMemberctlt:PharmaConsumerHealthMember2021-07-012022-06-300001596783ctlt:OperatingSegmentsExcludingIntersegmentEliminationMember2021-07-012022-06-300001596783ctlt:TotalCatalentSegmentMemberus-gaap:IntersegmentEliminationMember2021-07-012022-06-300001596783us-gaap:OperatingSegmentsMember2021-07-012022-06-300001596783ctlt:BiologicsMemberctlt:ManufacturingCommercialProductSupplyMember2020-07-012021-06-300001596783ctlt:PharmaConsumerHealthMemberctlt:ManufacturingCommercialProductSupplyMember2020-07-012021-06-300001596783ctlt:ManufacturingCommercialProductSupplyMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2020-07-012021-06-300001596783ctlt:BiologicsMemberctlt:DevelopmentServicesMember2020-07-012021-06-300001596783ctlt:PharmaConsumerHealthMemberctlt:DevelopmentServicesMember2020-07-012021-06-300001596783ctlt:DevelopmentServicesMemberctlt:TotalCatalentbeforeintersegmentrevenueeliminationMember2020-07-012021-06-300001596783ctlt:BiologicsMemberus-gaap:OperatingSegmentsMember2020-07-012021-06-300001596783us-gaap:OperatingSegmentsMemberctlt:PharmaConsumerHealthMember2020-07-012021-06-300001596783ctlt:OperatingSegmentsExcludingIntersegmentEliminationMember2020-07-012021-06-300001596783ctlt:TotalCatalentSegmentMemberus-gaap:IntersegmentEliminationMember2020-07-012021-06-300001596783us-gaap:OperatingSegmentsMember2020-07-012021-06-300001596783ctlt:geographicalMember2022-07-012023-06-300001596783country:US2022-07-012023-06-300001596783country:US2021-07-012022-06-300001596783country:US2020-07-012021-06-300001596783srt:EuropeMember2022-07-012023-06-300001596783srt:EuropeMember2021-07-012022-06-300001596783srt:EuropeMember2020-07-012021-06-300001596783ctlt:InternationalOtherMember2022-07-012023-06-300001596783ctlt:InternationalOtherMember2021-07-012022-06-300001596783ctlt:InternationalOtherMember2020-07-012021-06-300001596783ctlt:GreaterThanOneYearMemberMember2022-06-30xbrli:pure0001596783ctlt:SkeletalCellTherapySupportSAMember2020-11-162020-11-160001596783ctlt:AcordaTherapeuticsIncMember2021-02-112021-02-110001596783ctlt:AcordaTherapeuticsIncMember2021-02-110001596783ctlt:DelphiGeneticsSAMember2021-02-232021-02-230001596783ctlt:DelphiGeneticsSAMember2021-02-230001596783ctlt:HepaticCellTherapySupportSA2021-02-232021-02-230001596783ctlt:HepaticCellTherapySupportSAMember2021-03-310001596783ctlt:RheinCellTherapeuticsMember2021-08-012021-08-010001596783ctlt:RheinCellTherapeuticsMember2021-08-010001596783ctlt:BetteraHoldingsLLCMember2021-10-012021-10-010001596783us-gaap:OtherIntangibleAssetsMemberctlt:BetteraHoldingsLLCMember2021-10-010001596783ctlt:BetteraHoldingsLLCMember2021-10-010001596783ctlt:BetteraHoldingsLLCMember2022-10-010001596783ctlt:BetteraHoldingsLLCMember2021-10-012021-10-310001596783ctlt:CoreTechnologyBetteraMemberctlt:BetteraHoldingsLLCMember2021-10-010001596783ctlt:CoreTechnologyMemberctlt:BetteraHoldingsLLCMember2022-10-012022-10-010001596783ctlt:VaccineManufacturingAndInnovationCentreMember2022-04-012022-04-300001596783ctlt:VaccineManufacturingAndInnovationCentreMember2022-04-300001596783ctlt:PrincetonCellTherapyMember2022-04-012022-04-300001596783ctlt:PrincetonCellTherapyMember2022-04-300001596783ctlt:MetricsMember2021-02-232021-02-230001596783ctlt:MetricsMember2022-10-010001596783ctlt:MetricsMember2022-10-012022-10-010001596783us-gaap:CustomerRelationshipsMemberctlt:MetricsMember2022-10-010001596783us-gaap:CustomerRelationshipsMemberctlt:MetricsMember2022-10-012022-10-010001596783ctlt:BlowFillSealBusinessWoodstockMember2022-06-300001596783ctlt:BlowFillSealBusinessWoodstockMember2021-07-012022-06-300001596783ctlt:BlowFillSealBusinessWoodstockMember2023-06-300001596783ctlt:BlowFillSealBusinessWoodstockMember2022-07-012023-06-300001596783ctlt:BlowFillSealBusinessWoodstockMember2022-01-012022-03-310001596783ctlt:BlowFillSealBusinessWoodstockMember2020-07-012021-06-300001596783ctlt:BlowFillSealBusinessWoodstockMember2021-06-300001596783ctlt:BiologicsMember2021-06-300001596783ctlt:SoftgelAndOralTechnologiesMember2021-06-300001596783ctlt:BiologicsMember2021-07-012022-06-300001596783ctlt:SoftgelAndOralTechnologiesMember2021-07-012022-06-300001596783ctlt:BiologicsMember2022-06-300001596783ctlt:SoftgelAndOralTechnologiesMember2022-06-300001596783ctlt:BiologicsMember2022-07-012023-06-300001596783ctlt:SoftgelAndOralTechnologiesMember2022-07-012023-06-300001596783ctlt:PharmaConsumerHealthMember2022-07-012023-06-300001596783ctlt:BiologicsMember2023-06-300001596783ctlt:SoftgelAndOralTechnologiesMember2023-06-300001596783ctlt:CoreTechnologyMember2022-07-012023-06-300001596783ctlt:CoreTechnologyMember2023-06-300001596783us-gaap:CustomerRelationshipsMember2022-07-012023-06-300001596783us-gaap:CustomerRelationshipsMember2023-06-300001596783ctlt:ProductRelationshipsMember2022-07-012023-06-300001596783ctlt:ProductRelationshipsMember2023-06-300001596783us-gaap:OtherIntangibleAssetsMember2022-07-012023-06-300001596783us-gaap:OtherIntangibleAssetsMember2023-06-300001596783ctlt:CoreTechnologyMember2021-07-012022-06-300001596783ctlt:CoreTechnologyMember2022-06-300001596783us-gaap:CustomerRelationshipsMember2021-07-012022-06-300001596783us-gaap:CustomerRelationshipsMember2022-06-300001596783ctlt:ProductRelationshipsMember2021-07-012022-06-300001596783ctlt:ProductRelationshipsMember2022-06-300001596783us-gaap:OtherIntangibleAssetsMember2021-07-012022-06-300001596783us-gaap:OtherIntangibleAssetsMember2022-06-30ctlt:employees0001596783ctlt:BiologicsMember2020-07-012021-06-300001596783ctlt:PharmaConsumerHealthMember2021-07-012022-06-300001596783ctlt:PharmaConsumerHealthMember2020-07-012021-06-300001596783ctlt:CorporateAndEliminationsMember2022-07-012023-06-300001596783ctlt:CorporateAndEliminationsMember2021-07-012022-06-300001596783ctlt:CorporateAndEliminationsMember2020-07-012021-06-300001596783ctlt:TermLoanThreeFacilityDollarDenominatedMember2023-06-300001596783ctlt:TermLoanThreeFacilityDollarDenominatedMember2022-06-300001596783ctlt:RevolvingCreditFacilityTwoMember2023-06-300001596783ctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2023-06-300001596783ctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2022-06-300001596783us-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMemberctlt:A2375SeniorEuroDenominatedNotesMember2023-06-300001596783us-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMemberctlt:A2375SeniorEuroDenominatedNotesMember2022-06-300001596783ctlt:A3125SeniorUSDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2023-06-300001596783ctlt:A3125SeniorUSDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2022-06-300001596783ctlt:A3500SeniorUSDenominatedNotesMemberMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2023-06-300001596783ctlt:A3500SeniorUSDenominatedNotesMemberMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2022-06-300001596783us-gaap:CapitalLeaseObligationsMember2023-06-300001596783us-gaap:CapitalLeaseObligationsMember2022-06-300001596783ctlt:OtherObligationsMember2023-06-300001596783ctlt:OtherObligationsMember2022-06-300001596783ctlt:DebtIssuanceCostsMemberus-gaap:CarryingReportedAmountFairValueDisclosureMember2023-06-300001596783ctlt:DebtIssuanceCostsMemberus-gaap:CarryingReportedAmountFairValueDisclosureMember2022-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMember2023-06-300001596783us-gaap:CarryingReportedAmountFairValueDisclosureMember2022-06-3000015967832023-04-012023-06-300001596783ctlt:RevolvingCreditCommitmentsMember2023-06-300001596783ctlt:RevolvingCreditCommitmentsMembersrt:MinimumMember2022-07-012023-06-300001596783ctlt:RevolvingCreditCommitmentsMembersrt:MaximumMember2022-07-012023-06-300001596783ctlt:RevolvingCreditCommitmentsMemberctlt:SecuredOvernightFinancingRateSOFRMember2022-07-012023-06-300001596783ctlt:TermLoanThreeFacilityDollarDenominatedMembersrt:MinimumMember2022-07-012023-06-300001596783ctlt:TermLoanThreeFacilityDollarDenominatedMembersrt:MaximumMember2022-07-012023-06-300001596783ctlt:USDollarDenominated500SeniorNotesMember2023-06-300001596783ctlt:IncrementalTermLoanMemberctlt:OneMonthMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMember2022-07-012023-06-300001596783ctlt:IncrementalTermLoanMemberctlt:ThreeMonthMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMember2022-07-012023-06-300001596783ctlt:IncrementalTermLoanMemberctlt:SixMonthMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMember2022-07-012023-06-300001596783ctlt:A2375SeniorEuroDenominatedNotesMember2023-06-30iso4217:EUR0001596783ctlt:FourPointSevenFivePercentSeniorEuroDenominatedNotesMember2023-06-300001596783ctlt:A3125SeniorUSDenominatedNotesMember2023-06-300001596783ctlt:U.S.Dollardenominated4.875SeniorNotesMember2017-10-180001596783ctlt:A3500SeniorUSDenominatedNotesMemberMember2023-06-300001596783us-gaap:AccruedLiabilitiesMember2017-10-012017-10-2300015967832017-10-012017-10-230001596783ctlt:SeniorSecuredCreditFacilityMember2022-07-012023-06-300001596783ctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783ctlt:USDollarDenominated500SeniorNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2022-06-300001596783us-gaap:FairValueInputsLevel2Memberctlt:A2375SeniorEuroDenominatedNotesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783us-gaap:FairValueInputsLevel2Memberctlt:A2375SeniorEuroDenominatedNotesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2022-06-300001596783ctlt:A3125SeniorUSDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783ctlt:A3125SeniorUSDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2022-06-300001596783ctlt:A3500SeniorUSDenominatedNotesMemberMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783ctlt:A3500SeniorUSDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2022-06-300001596783ctlt:A3500SeniorUSDenominatedNotesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2022-06-300001596783ctlt:SeniorSecuredCreditFacilitiesOtherMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2023-06-300001596783ctlt:SeniorSecuredCreditFacilitiesOtherMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783ctlt:SeniorSecuredCreditFacilitiesOtherMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CarryingReportedAmountFairValueDisclosureMember2022-06-300001596783ctlt:SeniorSecuredCreditFacilitiesOtherMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2022-06-300001596783us-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783us-gaap:EstimateOfFairValueFairValueDisclosureMember2022-06-300001596783ctlt:DebtIssuanceCostsMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2023-06-300001596783ctlt:DebtIssuanceCostsMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2022-06-3000015967832020-11-232020-11-2300015967832021-11-182021-11-180001596783us-gaap:SeriesAPreferredStockMember2022-07-012023-06-300001596783us-gaap:SeriesAPreferredStockMember2021-07-012022-06-300001596783us-gaap:SeriesAPreferredStockMember2020-07-012021-06-300001596783ctlt:USDenominatedTermLoanMember2023-06-300001596783ctlt:USDenominatedTermLoanMember2021-06-300001596783us-gaap:FairValueInputsLevel2Member2023-06-300001596783us-gaap:FairValueInputsLevel3Member2023-06-300001596783us-gaap:FairValueInputsLevel1Member2022-06-300001596783us-gaap:FairValueInputsLevel2Member2022-06-300001596783us-gaap:FairValueInputsLevel3Member2022-06-300001596783us-gaap:DomesticCountryMember2023-06-300001596783us-gaap:StateAndLocalJurisdictionMember2023-06-300001596783us-gaap:StateAndLocalJurisdictionMember2022-07-012023-06-300001596783us-gaap:ForeignCountryMember2023-06-300001596783us-gaap:ForeignCountryMember2022-07-012023-06-300001596783us-gaap:PensionPlansDefinedBenefitMember2022-07-012023-06-300001596783us-gaap:PensionPlansDefinedBenefitMember2023-06-300001596783us-gaap:PensionPlansDefinedBenefitMember2022-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-06-300001596783us-gaap:PensionPlansDefinedBenefitMember2021-06-300001596783us-gaap:PensionPlansDefinedBenefitMember2021-07-012022-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-07-012023-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-07-012022-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CashMember2023-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:PensionPlansDefinedBenefitMember2023-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:PensionPlansDefinedBenefitMember2022-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2023-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2022-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2023-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2022-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberus-gaap:RealEstateMember2023-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberus-gaap:RealEstateMember2022-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberus-gaap:RealEstateMember2023-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberus-gaap:RealEstateMember2022-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberctlt:InsuranceContractsMember2023-06-300001596783us-gaap:PensionPlansDefinedBenefitMemberctlt:InsuranceContractsMember2022-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberctlt:InsuranceContractsMember2023-06-300001596783us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMemberctlt:InsuranceContractsMember2022-06-300001596783us-gaap:OtherAssetsMemberus-gaap:PensionPlansDefinedBenefitMember2023-06-300001596783us-gaap:OtherAssetsMemberus-gaap:PensionPlansDefinedBenefitMember2022-06-300001596783us-gaap:OtherAssetsMemberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-06-300001596783us-gaap:OtherAssetsMemberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel1Member2023-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:EquitySecuritiesMember2023-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2023-06-300001596783us-gaap:EquitySecuritiesMember2023-06-300001596783us-gaap:FairValueInputsLevel1Memberus-gaap:DebtSecuritiesMember2023-06-300001596783us-gaap:FairValueInputsLevel2Memberus-gaap:DebtSecuritiesMember2023-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:DebtSecuritiesMember2023-06-300001596783us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:DebtSecuritiesMember2023-06-300001596783us-gaap:DebtSecuritiesMember2023-06-300001596783us-gaap:FairValueInputsLevel1Memberus-gaap:RealEstateMember2023-06-300001596783us-gaap:FairValueInputsLevel2Memberus-gaap:RealEstateMember2023-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:RealEstateMember2023-06-300001596783us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:RealEstateMember2023-06-300001596783us-gaap:RealEstateMember2023-06-300001596783us-gaap:FairValueInputsLevel1Memberus-gaap:OtherAssetsMember2023-06-300001596783us-gaap:OtherAssetsMemberus-gaap:FairValueInputsLevel2Member2023-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:OtherAssetsMember2023-06-300001596783us-gaap:OtherAssetsMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2023-06-300001596783us-gaap:OtherAssetsMember2023-06-300001596783us-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2023-06-300001596783us-gaap:HedgeFundsMemberus-gaap:FairValueInputsLevel2Member2023-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel1Member2022-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:EquitySecuritiesMember2022-06-300001596783us-gaap:EquitySecuritiesMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2022-06-300001596783us-gaap:EquitySecuritiesMember2022-06-300001596783us-gaap:FairValueInputsLevel1Memberus-gaap:DebtSecuritiesMember2022-06-300001596783us-gaap:FairValueInputsLevel2Memberus-gaap:DebtSecuritiesMember2022-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:DebtSecuritiesMember2022-06-300001596783us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:DebtSecuritiesMember2022-06-300001596783us-gaap:DebtSecuritiesMember2022-06-300001596783us-gaap:FairValueInputsLevel1Memberus-gaap:RealEstateMember2022-06-300001596783us-gaap:FairValueInputsLevel2Memberus-gaap:RealEstateMember2022-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:RealEstateMember2022-06-300001596783us-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberus-gaap:RealEstateMember2022-06-300001596783us-gaap:RealEstateMember2022-06-300001596783us-gaap:FairValueInputsLevel1Memberus-gaap:OtherAssetsMember2022-06-300001596783us-gaap:OtherAssetsMemberus-gaap:FairValueInputsLevel2Member2022-06-300001596783us-gaap:FairValueInputsLevel3Memberus-gaap:OtherAssetsMember2022-06-300001596783us-gaap:OtherAssetsMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2022-06-300001596783us-gaap:OtherAssetsMember2022-06-300001596783us-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2022-06-300001596783us-gaap:HedgeFundsMemberus-gaap:FairValueInputsLevel2Member2022-06-300001596783ctlt:EberbachPensionPromissoryNoteOrLoanMember2023-06-300001596783us-gaap:FairValueInputsLevel3Memberctlt:InsuranceContractsMember2022-06-300001596783us-gaap:FairValueInputsLevel3Memberctlt:OtherUnobservableAssetsMember2022-06-300001596783us-gaap:FairValueInputsLevel3Member2022-07-012023-06-300001596783us-gaap:FairValueInputsLevel3Memberctlt:InsuranceContractsMember2022-07-012023-06-300001596783us-gaap:FairValueInputsLevel3Memberctlt:OtherUnobservableAssetsMember2022-07-012023-06-300001596783us-gaap:FairValueInputsLevel3Memberctlt:InsuranceContractsMember2023-06-300001596783us-gaap:FairValueInputsLevel3Memberctlt:OtherUnobservableAssetsMember2023-06-300001596783ctlt:Post65Memberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-06-300001596783ctlt:Post65Memberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-06-300001596783ctlt:Post65Memberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-07-012023-06-300001596783ctlt:Post65Memberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-07-012022-06-3000015967832020-06-152020-06-1500015967832020-06-1500015967832020-02-062020-02-0600015967832020-02-060001596783ctlt:DesignatedSharesMember2021-06-3000015967832019-05-1700015967832020-11-230001596783us-gaap:AdditionalPaidInCapitalMember2020-11-232020-11-2300015967832021-11-180001596783us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-3100015967832022-07-012023-03-310001596783us-gaap:AccumulatedTranslationAdjustmentMember2020-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2020-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2020-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-06-300001596783us-gaap:AccumulatedTranslationAdjustmentMember2020-07-012021-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-07-012021-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2020-07-012021-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-07-012021-06-300001596783us-gaap:AccumulatedTranslationAdjustmentMember2021-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2021-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2021-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-06-300001596783us-gaap:AccumulatedTranslationAdjustmentMember2021-07-012022-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-07-012022-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2021-07-012022-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-07-012022-06-300001596783us-gaap:AccumulatedTranslationAdjustmentMember2022-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2022-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2022-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-07-012023-06-300001596783us-gaap:AccumulatedTranslationAdjustmentMember2023-06-300001596783us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-06-300001596783us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMember2023-06-300001596783ctlt:ACOIAccumulatedGainLossMarketableSecuritiesMember2023-06-300001596783us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-06-300001596783ctlt:StockCompensationPlanOmnibusMember2022-07-012023-06-300001596783ctlt:StockCompensationPlanOmnibusMember2021-07-012022-06-300001596783ctlt:StockCompensationPlanOmnibusMember2020-07-012021-06-300001596783us-gaap:EmployeeStockOptionMembersrt:MinimumMember2022-07-012023-06-300001596783us-gaap:EmployeeStockOptionMembersrt:MinimumMember2021-07-012022-06-300001596783us-gaap:EmployeeStockOptionMembersrt:MinimumMember2020-07-012021-06-300001596783us-gaap:EmployeeStockOptionMembersrt:MaximumMember2022-07-012023-06-300001596783us-gaap:EmployeeStockOptionMembersrt:MaximumMember2021-07-012022-06-300001596783us-gaap:EmployeeStockOptionMembersrt:MaximumMember2020-07-012021-06-300001596783ctlt:TimeMember2022-06-300001596783ctlt:TimeMember2021-07-012022-06-300001596783ctlt:TimeMember2022-07-012023-06-300001596783ctlt:TimeMember2023-06-300001596783ctlt:PerformanceMember2023-06-300001596783ctlt:TimeBasedRestrictedStockUnitsMember2022-06-300001596783ctlt:TimeBasedRestrictedStockUnitsMember2022-07-012023-06-300001596783ctlt:TimeBasedRestrictedStockUnitsMember2023-06-300001596783us-gaap:PerformanceSharesMember2022-06-300001596783us-gaap:PerformanceSharesMember2022-07-012023-06-300001596783us-gaap:PerformanceSharesMember2023-06-300001596783srt:MinimumMemberus-gaap:PerformanceSharesMember2022-07-012023-06-300001596783srt:MaximumMemberus-gaap:PerformanceSharesMember2022-07-012023-06-300001596783srt:MinimumMemberus-gaap:PerformanceSharesMember2021-07-012022-06-300001596783srt:MaximumMemberus-gaap:PerformanceSharesMember2021-07-012022-06-300001596783ctlt:RTSRPerformanceShareUnitsMember2022-06-300001596783ctlt:RTSRPerformanceShareUnitsMember2022-07-012023-06-300001596783ctlt:RTSRPerformanceShareUnitsMember2023-06-300001596783us-gaap:RestrictedStockUnitsRSUMember2023-06-300001596783us-gaap:RestrictedStockUnitsRSUMember2022-07-012023-06-300001596783us-gaap:RestrictedStockUnitsRSUMember2021-07-012022-06-300001596783us-gaap:RestrictedStockUnitsRSUMember2020-07-012021-06-300001596783ctlt:SoftgelAndOralTechnologiesMember2020-07-012021-06-300001596783ctlt:TotalCatalentSubTotalOfSegmentReportingMember2022-07-012023-06-300001596783ctlt:TotalCatalentSubTotalOfSegmentReportingMember2021-07-012022-06-300001596783ctlt:TotalCatalentSubTotalOfSegmentReportingMember2020-07-012021-06-300001596783ctlt:OxfordMemberctlt:BiologicsMember2022-07-012023-06-300001596783ctlt:BiologicsMemberctlt:SwindonMember2022-07-012023-06-300001596783ctlt:PharmaConsumerHealthMember2023-06-300001596783ctlt:PharmaConsumerHealthMember2022-06-300001596783ctlt:CorporateAndEliminationsMember2023-06-300001596783ctlt:CorporateAndEliminationsMember2022-06-300001596783us-gaap:CorporateMember2022-07-012023-06-300001596783us-gaap:CorporateMember2021-07-012022-06-300001596783us-gaap:CorporateMember2020-07-012021-06-300001596783country:US2023-06-300001596783country:US2022-06-300001596783srt:EuropeMember2023-06-300001596783srt:EuropeMember2022-06-300001596783ctlt:RestofWorldMember2023-06-300001596783ctlt:RestofWorldMember2022-06-3000015967832023-10-012023-12-3100015967832023-07-012023-09-3000015967832023-09-3000015967832023-10-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
| | | | | | | | |
| ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2023
or | | | | | | | | |
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-36587
| | | | | | | | |
| CATALENT, INC. | |
| (Exact name of registrant as specified in its charter) |
| | |
| | | | | | | | |
| Delaware | | 20-8737688 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| | |
14 Schoolhouse Road | 08873 |
| Somerset, | New Jersey |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (732) 537-6200
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, $0.01 par value per share | CTLT | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes o No ☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. | | | | | | | | | | | | | | | | | |
| Large accelerated filer | ý | | Accelerated filer | ¨ | |
| Non-accelerated filer | ¨ | | Smaller reporting company | ¨ | |
| | | Emerging growth company | ¨ | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). o Yes ☒ No
As of December 31, 2022, the aggregate market value of the registrant’s voting and non-voting common equity held by non-affiliates was $7.90 billion. On November 30, 2023, there were 180,641,272 shares of the Registrant’s Common Stock, par value $0.01 per share, issued and outstanding.
CATALENT, INC.
INDEX TO ANNUAL REPORT ON FORM 10-K
For the Fiscal Year Ended June 30, 2023
| | | | | | | | |
| Item | | Page |
| PART I | |
| | |
| | |
| | |
| Item 1. | | |
| | |
| Item 1A. | | |
| | |
| Item 1B. | | |
| | |
| Item 2. | | |
| | |
| Item 3. | | |
| | |
| Item 4. | | |
| | |
| PART II | |
| | |
| Item 5. | | |
| | |
| Item 6. | | |
| | |
| Item 7. | | |
| | |
| Item 7A. | | |
| | |
| Item 8. | | |
| | |
| Item 9. | | |
| | |
| Item 9A. | | |
| | |
| Item 9B. | | |
| | |
| Item 9C. | | |
| | |
| PART III | |
| | |
| Item 10. | | |
| | |
| Item 11. | | |
| | |
| Item 12. | | |
| | |
| Item 13. | | |
| | |
| Item 14. | | |
| | |
| PART IV | |
| | |
| Item 15. | | |
| | |
| Item 16. | | |
| | |
| |
PART I
Special Note Regarding Forward-Looking Statements
In addition to historical information, this Annual Report on Form 10-K for the fiscal year ended June 30, 2023 (this “Annual Report”) of Catalent, Inc. (“Catalent” or the “Company”) contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are subject to the “safe harbor” created by those sections. All statements, other than statements of historical facts, included in this Annual Report are forward-looking statements. In some cases, you can identify these forward-looking statements by the use of words such as “outlook,” “believes,” “expects,” “potential,” “continues,” “may,” “will,” “should,” “could,” “seeks,” “predicts,” “intends,” “plans,” “estimates,” “anticipates,” “future,” “forward,” “sustain,” or the negative version of these words or other comparable words.
These statements are based on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments, and other factors they believe to be appropriate. Any forward-looking statement is subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements.
Some of the factors that may cause actual results, developments, and business decisions to differ materially from those contemplated by such forward-looking statements include, but are not limited to, those described under the section entitled “Risk Factors” in this Annual Report, which are summarized below:
Summary of Principal Risk Factors
Any investment, including an investment in our common stock, par value $0.01 (the “Common Stock”), involves risk. The following summary highlights certain risks that an investor in our Common Stock should consider. The following should be read in conjunction with the fuller discussion of risk factors we face set forth in “Item 1A. - Risk Factors.”
Risks Relating to Our Business and the Industry in Which We Operate
•Actions of activist shareholders could impact the pursuit of our business strategies and adversely affect our results of operations, financial condition, or share price.
•We anticipate being subject to increasing focus by our investors, regulators, customers, and other stakeholders on environmental, social, and governance (“ESG”) matters.
•We are a part of the highly regulated healthcare industry, subject to stringent regulatory standards and other applicable laws and regulations, which can change unexpectedly or be the subject of unexpected changes in interpretation or enforcement, any of which may adversely impact our business.
•Any failure to implement fully, monitor, and continuously improve our quality management strategy could lead to quality or safety issues and expose us to significant costs, potential liability, and adverse publicity.
•We have experienced, and may continue to experience, productivity issues and higher-than-expected costs at certain of our facilities, which have resulted in, and may continue to result in, material and adverse impacts on our financial condition and results of operations.
•The declining demand for various COVID-19 vaccines and treatments from both patients and governments around the world has affected and may continue to affect sales of the COVID-19 products we manufacture and our financial condition.
•The demand for our offerings depends in part on our customers’ research and development and the clinical and market success of their products.
•Our results of operations are subject to fluctuations in the costs, availability, and suitability of the components of the products we manufacture, including active pharmaceutical ingredients, excipients, purchased components, and raw materials, and other supplies or equipment we need to run our business.
•Our goodwill has been subject to impairment and may be subject to further impairment in the future, which could have a material adverse effect on our results of operations, financial condition, or future operating results.
•Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
•We may acquire businesses and offerings that complement or expand our business or divest non-strategic businesses or assets. We may not be able to complete desired transactions, and such transactions, if executed, pose significant risks, including risks relating to our ability to successfully and efficiently integrate acquisitions or
execute on dispositions and realize anticipated benefits therefrom. The failure to execute or realize the full benefits from any such transaction could have a negative effect on our operations and profitability.
•We may become subject to litigation, other proceedings, and government investigations relating to us or our operations, and the ultimate outcome of any such matter may have an impact on our business, prospects, financial condition, and results of operations.
•Our global operations are subject to economic and political risks, including risks resulting from continuing inflation, disruptions to global supply chains, destabilization of a regional or national banking system, or from the Ukrainian-Russian war or the effect of the evolving nature of the recent war in Gaza between Israel and Hamas, which could affect the profitability of our operations or require costly changes to our procedures
•We use advanced information and communication systems to run our operations, compile and analyze financial and operational data, and communicate among our employees, customers, and counterparties, and the risks generally associated with information and communications systems could adversely affect our results of operations. We continuously work to install new, and upgrade existing, systems and provide employee awareness training around phishing, malware, and other cybersecurity risks to enhance the protections available to us, but such protections may be inadequate to address malicious attacks or inadvertent compromises affecting data security or the operability of such systems.
•Artificial intelligence-based platforms present new risks and challenges to our business.
•Our cash, cash equivalents, and financial investments could be adversely affected if the financial institutions in which we hold our cash, cash equivalents, and financial investments fail.
Risks Relating to Our Indebtedness
•The size of our indebtedness and the obligations associated with it could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or in our industry or to deploy capital to grow our business, expose us to interest-rate risk to the extent of our variable-rate debt, or prevent us from meeting our obligations under our indebtedness. These risks may be increased in a recessionary environment, particularly as sources of capital may become less available or more expensive.
•Despite our high indebtedness level, we and our subsidiaries are still capable of incurring significant additional debt, which could further exacerbate the risks associated with our substantial indebtedness.
•Our interest expense on our variable-rate debt may continue to increase if and to the extent that policymakers combat inflation through interest-rate increases on benchmark financial products.
•Despite the limitations in our debt agreements, we retain the ability to take certain actions that may interfere with our ability to timely pay our substantial indebtedness.
•We may not be able to pay our indebtedness when it becomes due.
•We are currently using and may in the future use derivative financial instruments to reduce our exposure to market risks from changes in interest rates on our variable-rate indebtedness or changes in currency exchange rates, and any such instrument may expose us to risks related to counterparty credit worthiness or non-performance of these instruments.
Risks Relating to Ownership of Our Common Stock
•We do not presently maintain effective disclosure controls and procedures due to material weaknesses we have identified in our internal control over financial reporting. Failure to remediate these material weaknesses or any other material weakness or significant deficiencies have resulted in a revision of our financial statements, in the future could result in material misstatements in our financial statements and have caused, and in the future could cause us to fail to timely meet our periodic reporting obligations.
•Our stock price has historically been and may continue to be volatile, and a holder of shares of our Common Stock may not be able to resell such shares at or above the price such stockholder paid, or at all, and could lose all or part of such investment as a result.
•Future sales, or the perception of future sales, of our Common Stock, by us or our existing stockholders could cause the market price for our Common Stock to decline.
•We are no longer eligible to use the Form S-3 registration statement, which could impair our capital-raising activities.
•Provisions in our organizational documents could delay or prevent a change of control.
We caution you that the risks, uncertainties, and other factors referenced above may not contain all of the risks, uncertainties, and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits, or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. There can be no assurance that (i) we have correctly measured or identified all of the factors affecting our business or the extent of these factors’ likely impact, (ii) the available information with respect to these factors on which such analysis is based is complete or accurate, (iii) such analysis is correct, or (iv) our strategy, which is based in part on this analysis, will be successful. All forward-looking statements in this report apply only as of the date of this report or as of the date they were made, and we undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments, or otherwise, except as required by law.
We file annual, quarterly, and current reports and other information with and furnish additional information to the U.S. Securities and Exchange Commission (the “SEC”). Our filings with the SEC are available to the public on the SEC’s website at www.sec.gov. Those filings are also available to the public on, or accessible through, our website (catalent.com) for free via the “Investors” section as soon as reasonably practicable after we file such material, or furnish it to, the SEC. We also use our website, Facebook page (facebook.com/CatalentPharmaSolutions), LinkedIn page (linkedin.com/company/catalent-pharma-solutions/) and Twitter account (@catalentpharma) as channels of distribution of information concerning our activities, our offerings, our various businesses, and other related matters. The information we post through these channels may be deemed material. Accordingly, investors should monitor these channels, in addition to following our press releases, SEC filings, and public conference calls and webcasts. The information we file with or furnish to the SEC (other than the information set forth or incorporated in this Annual Report) or contained on or accessible through our website, our social media channels, or any other website that we may maintain is not a part of this Annual Report.
Catalent References and Fiscal Year
Unless the context otherwise requires, in this Annual Report, the terms “Catalent,” “the company,” “we,” “us,” and “our” refer to Catalent, Inc. and its subsidiaries. All references to years in this Annual Report, unless otherwise stated, refer to fiscal years beginning July 1 and ending June 30. All references to quarters, unless otherwise stated, refer to fiscal quarters. Fiscal years are referred to by the calendar year in which they end. For example, “fiscal 2023” refers to the fiscal year ending June 30, 2023.
Trademarks and Service Marks
We have U.S. or foreign registrations for the following marks, among others: Bettera®, Catalent®, Clinicopia®, CosmoPod®, Easyburst®, FastChain®, FlexDirect®, Follow the Molecule®, Galacorin®, GPEx®, GPEx® Boost, GPEx® Lightning, Graphicaps®, Liqui-Gels®, Manufacturing Miracles®, Micron Technologies®, OmegaZero®, OneBio®, OneXpress Solution®, OptiDose®, OptiForm®, OptiGel®, OptiGel® Bio, OptiGel® DR, OptiMelt®, OptiShell®, PEEL-ID®, Pharmatek®, RP Scherer®, Savorgel®, Scherer®, SMARTag®, Softdrop®, Staby®, StabyExpress®, SupplyFlex®, Vegicaps®, Zydis®, and Zydis Ultra®. This Annual Report also includes trademarks and trade names owned by other parties, and these trademarks and trade names are the property of their respective owners. We use certain other trademarks and service marks, some on an unregistered basis and some have been applied for, but remain pending examination in trademark agencies in the U.S. and abroad, including, FlexDoseSM, Catalent Xpress PharmaceuticsSM, OptiPact™, ProteoSuiteSM, StartScoreSM, and VirtuosoSM.
Solely for convenience, the trademarks, service marks, and trade names identified in this Annual Report may appear without the ®, SM, and ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks, and trade names.
ITEM 1. BUSINESS
Overview
We provide differentiated development and manufacturing solutions for drugs, protein-based biologics, cell and gene therapies, vaccines, and consumer health products at over fifty facilities across four continents under rigorous quality and operational standards. Our oral, injectable, and respiratory delivery technologies, along with our state-of-the-art protein, plasmid, viral, and cell and gene therapy manufacturing capacity, address a wide and growing range of modalities and therapeutic and other categories across the biopharmaceutical, pharmaceutical, and consumer health industries. Through our extensive capabilities, growth-enabling capacity, and deep expertise in product development, regulatory compliance, and clinical trial and commercial supply, we can help our customers take products to market faster, including more than half of new drug products approved by the U.S. Food and Drug Administration (the “FDA”) in the last decade. Our development and manufacturing platforms, our proven formulation, supply, and regulatory expertise, and our broad and deep development and manufacturing know-how enable our customers to advance and then bring to market more products and better treatments for patients and consumers. Our commitment to reliably supply our customers’ and their patients’ needs is the foundation for the value we provide; annually, we produce approximately 70 billion unit doses for nearly 8,000 customer prescription and consumer health products, or approximately 1 in every 26 unit doses of such products taken each year by patients and consumers around the world. We believe that, through our investments in state-of-the-art facilities and capacity expansion, including investments in facilities focused on new treatment modalities and other attractive market segments, our continuous improvement activities devoted to operational and quality excellence, the sales of existing and introduction of new customer products, and, in some cases, our innovation activities and patents, we will continue to attract premium opportunities and realize the growth potential from these areas.
We continue to focus on enhancing both our product and service offerings and our sales and marketing activities in order to grow the number of active commercial manufacturing and development programs for our customers. This sustains our extensive, long-duration relationships and long-term contracts with a broad and diverse range of industry-leading customers. In fiscal 2023, we conducted business with 87 of the top 100 branded drug and consumer health marketers and 82 of the top 100 biologics marketers, measured on a global basis. Selected key customers include Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Haleon, Johnson & Johnson, Moderna, Pfizer, and Sarepta Therapeutics.
We have many long-standing relationships with our customers, particularly those with commercial products, as we provide support and reliable supply through each stage of a product's lifecycle. Our relationship with an innovator of a prescription pharmaceutical product will often last many years—in several cases, two decades or more—extending from pre-clinical development through more mature commercial stages of the product's life cycle. We serve customers requiring some combination of innovative product development, superior quality, state-of-the-art manufacturing, and skilled technical services to support their development and marketed product needs. Our broad and diverse range of technologies closely integrates with all aspects of our customers’ final formulations and dose forms, and this generally results in the inclusion of our facilities as manufacturing and testing sites in our customers’ prescription product regulatory filings. Both factors frequently translate to long-duration supply relationships at an individual product level.
We believe our customers value us because our depth of development solutions and state-of-the-art manufacturing technologies, continuous innovations and improvements, consistent and reliable supply, geographic reach, and substantial expertise enable us to create a broad range of business and product solutions that can be customized to fit their individual needs. Today we employ more than 9,000 highly trained direct manufacturing associates, as well as more than 3,000 formulation, analytical development, and process scientists and technicians. Our customers can also benefit from more than 1,800 patents and patent applications in advanced delivery platforms, drug and biologics formulation, and manufacturing. The aim of our offerings is to reliably supply our customers' commercial needs and also allow them to bring more products to market faster and develop and market differentiated products that improve patient outcomes. We believe our leading market position and diversity of customers, offerings, regulatory categories, products, and geographies reduce our exposure to potential strategic and product shifts within our industries.
We provide a wide variety of proprietary and non-proprietary, differentiated technologies, products, and service offerings to our customers across our development and manufacturing platforms, which we have advanced and grown over more than 90 years through internal development, strategic alliances, in-licensing, and acquisitions. We initially introduced our softgel capsule technologies in the 1930s and have continuously expanded our range of offerings. In recent years, we have launched more than a dozen internally developed new technology platform offerings. We have also augmented our portfolio through acquisitions. Among the technologies we currently offer are softgel capsules, including both gelatin and non-gelatin formulations, our Zydis orally disintegrating tablets, gummy and soft chew oral forms, protein production using advanced mammalian cell lines, adeno-associated virus (“AAV”) and other viral vectors, induced pluripotent stem cells (“iPSCs”) and
other cell types, plasmid DNA (“pDNA”), and a range of other oral, injectable, and respiratory delivery technologies. The technologies and service offerings within our development solution platforms span the full drug development process, ranging from our OptiForm Solution Suite for enhancement of bioavailability and other characteristics of early-stage small molecules, Gene Product Expression (“GPEx”), GPEx Boost, and GPEx Lightning for advanced cell line development, pDNA development and manufacturing and SMARTag platforms for development of biologics and antibody-drug conjugates (“ADCs”), to formulation, analytical, and bioanalytical services, early-stage clinical development, drug-device combination development and supply, fill and finish operations for injectable products, and clinical trials supply, including our unique FlexDirect direct-to-patient and FastChain demand-led clinical supply solutions. Our offerings serve a critical need in the development and manufacture of products across a broad range of product types. We focus on serving as an accelerator for new therapeutic modalities and formulation, delivery, and manufacturing technologies. Our expertise enables us to bring advanced products to market at scale, faster.
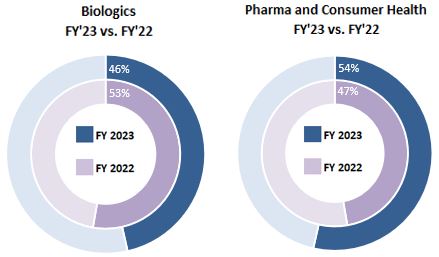
In large part due to acquisitions and investments, their subsequent organic growth, the revenue contribution from our Biologics segment has grown from approximately 17% in fiscal 2016 to 46% in fiscal 2023. We believe our own internal innovation and investments, supplemented by current and future external partnerships and acquisitions, will continue to extend our leadership positions in the development, reliable supply, and delivery of drugs, protein-based biologics, cell and gene therapies, and consumer health products.
History
We trace our history to the 1933 founding of the R.P. Scherer Corporation, which developed the first rotary die machine for the manufacture of soft gelatin capsules, and we assumed our current form in April 2007. We regularly review our portfolio of offerings and operations in the context of our strategic growth plan, and, where appropriate, have added to or divested from our portfolio of offering and sites, which has led to significant growth of the overall business. In July 2014, we completed the initial public offering of our Common Stock, which is listed on the New York Stock Exchange (the “NYSE”) under the symbol “CTLT.”
We are a holding company that indirectly owns Catalent Pharma Solutions, Inc. (“Operating Company”), which owns, directly or indirectly, all of our operating assets.
Our Competitive Strengths
Available, State-of-the-art Manufacturing Capacity in Attractive Market Segments
We have invested several billion dollars over the last few years to broaden our portfolio of offerings and expand our capacity with state-of-the-art manufacturing and development capabilities that focus on anticipating and meeting the needs of the evolving biopharmaceutical, pharmaceutical, and consumer health industries. In addition, we have hired and trained thousands of new direct manufacturing associates in our quality-focused culture of operational excellence. The capacity and capabilities we have built and purchased, along with our continuing efforts to assure operational and quality excellence, have and will continue to enable us to secure attractive new business opportunities in the expanding market for outsourced product development and supply.
Vibrant, Patient First-Driven Culture
From the manufacturing line to the executive suite, for all our critical decisions, we ask the question, “What would the impact be to the patient?”, and our culture is built on our cornerstone value of Patient First. We believe this mindset, which aligns closely with our customers’ values, enables a pervasive focus on patient safety, impact, and outcomes, and an uncompromising approach to product quality and compliance, by reminding us of those who depend upon our vigilance concerning the safety, quality, reliability, and sustainability of our product supply. Along with other key cultural strengths, including our commitments to diversity and inclusion and to science-based environmental sustainability, we believe our culture brings us both a unique reputation and an operating capability that is difficult to replicate.
Diversified Operating Platform
We are diversified by virtue of our broad range of product and service offerings, our geographic scope, our large customer portfolio, the extensive range of products we produce, and our ability to provide solutions at every stage of a product’s lifecycle. In fiscal 2023, we produced nearly 8,000 distinct products across multiple categories. Our fiscal 2023 net revenue was distributed by relevant product regulatory/marketing status as follows: biologics 51%, branded drugs 30%, generic prescription drugs 2%, over-the-counter drugs 7%, and consumer health and other 10% combined. In fiscal 2023, our top 20
products represented 37% of our total net revenue, with one customer accounting for approximately 10% of net revenue whose largest individual product accounted for approximately 9% of our net revenue. We serve more than 1,200 customers in more than 80 countries, with 35% of our fiscal 2023 net revenue coming from outside the U.S. This diversity, combined with long product lifecycles and close customer relationships, has contributed to the long-term stability of our business. It has also allowed us to reduce our exposure to the risks associated with potential strategic, customer, and product shifts as well as to payer-driven pricing pressures experienced by our drug and biologic customers.
Longstanding, Extensive Relationships with a Diverse Customer Portfolio
We have longstanding, extensive relationships with leading pharmaceutical, biotechnology, and consumer health customers. In fiscal 2023, we did business with 87 of the top 100 branded drug and consumer health marketers and 82 of the top 100 biologics marketers, measured on a global basis, as well as with more than 1,200 other customers, including emerging and specialty biotech and pharmaceutical companies, which are often more reliant on outside partners as a result of their more virtual business models. Regardless of size, our customers seek innovative product development, superior quality, advanced manufacturing, and skilled technical services to support their development and marketed product needs.
We believe our customers value us because our broad range of product and service offerings, recently expanded capacity in state-of-the-art manufacturing facilities, including facilities offering new treatment modalities, reliable supply, geographic reach, commitment to operational and quality excellence, and substantial expertise that enable us to create a broad range of tailored solutions, many of which are unavailable from other individual providers.
Deep, Broad, and Growing Advanced Technology Foundation
Our breadth of offerings employing advanced technologies and state-of-the-art manufacturing systems and long track record of innovation substantially differentiate us from other industry participants. Our leading softgel platforms, including Liqui-Gels, OptiShell, OptiGel DR, and Vegicaps capsules, our gummy and soft chew oral forms, and our modified release technologies, including the Zydis family of orally disintegrating tablets, our spray drying capabilities, and our OptiPact and OptiMelt technologies, provide formulation expertise to solve complex delivery challenges for our customers. We offer advanced technologies for delivery of small molecules and biologics via oral, respiratory, and injectable routes and also provide advanced biologics formulation options, including GPEx, GPEx Boost, and GPEx Lightning mammalian cell lines for protein production, SMARTag ADC technology, AAV vectors for cell and gene therapies, iPSC development and manufacturing, and pDNA development and manufacturing. We have a leadership position within respiratory delivery, including dry powder inhalers and intra-nasal forms. We have reinforced our leadership position in advanced technologies over the last three years, as we have launched more than a dozen new technology platforms and applications, and recently purchased or expanded our businesses developing and manufacturing consumer health products, protein-based biologics, fill and finish for injectable drugs and biologics, cell and gene therapies, and other new therapeutic modalities. Our culture of creativity, problem-solving, and innovation is grounded in our advanced technologies, the substantial expertise and experience of our scientists and engineers, and, in some cases, our patents and proprietary manufacturing processes. Our global product development and innovation teams drive a focused application of resources to opportunities for both new customer product introductions and platform technology development. As of June 30, 2023, we had more than 1,500 product development programs in active development across our businesses.
Long-Duration Relationships Provide Sustainability
Our broad and diverse range of technologies closely integrates with our customers’ molecules to yield safe and effective final formulations and dose forms, and this generally results in the inclusion of Catalent in our customers’ prescription product regulatory filings. Both factors translate to long-duration supply relationships at an individual product level, to which we apply our expertise in contracting to produce long-duration commercial supply agreements. These agreements typically have initial terms of two to seven years with regular renewals of one to three years (see “—Contractual Arrangements” for more detail). Approximately three-quarters of our fiscal 2023 net revenue from our product development and delivery offerings and related services were covered by such long-term contractual arrangements. We believe this base provides us with a sustainable competitive advantage.
Significant Recent Growth Investments
We have made over time, and expect to continue to make, significant investments in our manufacturing network, which is capable of serving customers and patients worldwide, and today employ approximately 8 million square feet of manufacturing, laboratory, and related space across four continents. We have deployed approximately $2.61 billion in the last five fiscal years in gross capital expenditures, not including approximately $3.58 billion spent acquiring new facilities and businesses. Growth-related investments in facilities, capacity, and capabilities across our businesses have positioned us for future growth in areas
aligned with anticipated future demand, including in pDNA, cell and gene therapies, fill and finish for injectable drugs and biologics, and other new therapeutic modalities. Through our continuing commitment to operational, quality, and regulatory excellence, we drive continuous improvements in safety, productivity, sustainability and reliable supply, which we believe further differentiates us. Our manufacturing network and capabilities allow us the flexibility to reliably supply the changing needs of our customers while consistently meeting their quality, delivery, sustainability, and regulatory compliance expectations.
High Standards of Regulatory Compliance and Operational and Quality Excellence
We operate our plants in accordance with current good manufacturing practices (“cGMP”) or other applicable requirements, following our own high standards that are consistent with those of many of our large global pharmaceutical and biotechnology customers. We have approximately 1,900 employees around the globe focused on quality and regulatory compliance. All of our facilities are registered where required with the FDA or other applicable regulatory agencies, such as the European Medicines Agency (the “EMA”). In many cases, our facilities are registered with multiple food, drug, or biologics regulatory agencies around the world. In fiscal 2023, we were subject to 58 regulatory audits, and, over the last five fiscal years, we successfully completed approximately 300 regulatory audits. We also undergo more than 700 customer and internal audits annually. We believe our quality and regulatory track record to be a favorable competitive differentiator.
Strong and Experienced Management Team
Our executive leadership team collectively has approximately 550 years of combined and diverse experience within the pharmaceutical and healthcare industries. With an average of approximately 28 years of functional experience, this team possesses deep knowledge and a wide network of industry relationships.
Our Strategy
Our strategic ambition, guided by and operationalized through our values, is to power the innovation and growth of the life science industry by becoming its leading development and commercial partner in reliable supply, conventional and advanced technologies, first-to-scale innovation, and therapeutic modalities, and integrated solutions. To achieve this, we continue to pursue the following key growth initiatives:
Capabilities & Capacity — Continued Expansion in Biologics and Other Attractive Markets
Recognizing the strategic importance of protein-based biologics, cell and gene therapies, pDNA, and other new biopharmaceutical modalities, we began to build a differentiated biologics platform in 2002. Since 2019, we have invested over $3.42 billion in our biologics business, including capital investments and approximately $1.83 billion for acquisitions of biologics-focused businesses and sites. Today, we are a recognized leader in biologics, including AAV vectors for gene therapies; development and supply for cell therapies; advanced cell-line development; formulation and fill-finish into vials, pre-filled syringes, and cartridges; specialized manufacturing of biologic drug substances; and bioanalytical analysis. We have partnered with customers from around the world to develop advanced cell expression for more than 1,100 cell lines, many using our advanced GPEx, GPEx Boost, and GPEx Lightning technologies, and have actively collaborated on developing and scaling up more than 125 cell and gene therapies. In the recent fiscal years, we expanded our existing cell therapy development and manufacturing capabilities, began offering pDNA production services, and acquired several facilities including a commercial-scale cell therapy manufacturing facility in Princeton, New Jersey (“Princeton”) and a developer and manufacturer of iPSCs located near Dusseldorf, Germany. We have also invested in a second-generation ADC technology, SMARTag, and see continued progress in this technology’s capabilities and our customers’ SMARTag product-development activities.
In addition to our expansion in biologics, we have invested additional capital in our facilities in order to expand in attractive markets, including significant expansion of our oral solid controlled release production capacity in Winchester, Kentucky, and the addition of specialized capabilities and capacity in early development. We acquired a leading position in consumer-preferred gummy and soft-chew formats for consumer health products with our acquisition of Bettera Holdings, LLC (“Bettera Wellness”) in fiscal 2022. We expanded our capacity for oral and injectable products via our fiscal 2020 acquisition of a facility in Anagni, Italy, and our capacity for spray dried dispersion and dry powder inhaler manufacturing via our fiscal 2021 acquisition of a facility located near Boston, Massachusetts.
Use Our Proprietary Technologies and Substantial Expertise to Help Our Customers Develop New Products
We have broad and diverse technology platforms that are supported by deep scientific and technical expertise, extensive know-how, and more than 1,800 patents and patent applications in approximately 170 families across advanced delivery platforms, drug and biologics formulation, and manufacturing. For example, we have significant softgel fill and formulation know-how, databases of formulated products, and substantial softgel regulatory approval expertise. As a result, nearly 90% of
approvals by the FDA over the last 25 years of new chemical entities presented in a softgel format have been developed and supplied by us.
In addition to resolving delivery challenges for our customers’ products, we have applied our technology platforms and development expertise to proactively develop proof-of-concept products, whether improved versions of existing drugs, new generic formulations, or innovative consumer health products. In the consumer health area, we file product dossiers with regulators in relevant jurisdictions for self-created products, which help contribute sustainable growth to our consumer health business. We expect to continue to seek proactive development opportunities and other non-traditional relationships to increase demand for and value realized from our technology platforms. These activities have provided us with opportunities to capture an increased share of end-market value through out-licensing, profit-sharing, and other arrangements.
Operational Leverage — Deploy Existing Infrastructure and Operational Discipline to Drive Profitable Growth
Through our existing infrastructure, including our global network of operating locations and programs, we promote operational discipline and drive margin expansion. With our active focus on continuous improvement and sustainability enhancement, global procurement function, and conversion cost productivity metrics in place, we continuously seek to enhance our culture of functional excellence and cost accountability. Along with the ongoing increase in the share of revenues from higher margin biologics offerings, we expect this discipline to further leverage our operational network for profitable growth.
Strategic Acquisitions and Licensing — Build on our Existing Platform
We operate in the markets for outsourced development solutions and commercial supply, generally provided by contract development and manufacturing organizations (“CDMO”), where we estimate current industry spending at more than $70 billion globally. Our broad platform, global infrastructure, and diversified customer portfolio provide us with a strong foundation from which to consolidate within these markets, to enter new markets, and generate operating leverage through acquisitions. Since fiscal 2013, we have executed 22 transactions, investing approximately $4.91 billion, and have demonstrated an ability to efficiently and effectively integrate these acquisitions.
While we are rigorously focused on driving our organic growth, we have in recent years substantially increased our participation in biologics, including protein-based biologics, cell and gene therapies, pDNA development and production, and drug product fill and finish, via strategy-driven inorganic transactions. We intend to identify and execute strategic transactions to optimize our portfolio of offerings and businesses, within the context of our long-term capital allocation strategy. We have a dedicated corporate development team in place to pursue these transactions, enabled by a rigorous and financially disciplined process for evaluating and executing these transactions.
“Follow the Molecule”® by Providing Solutions to our Customers across all Phases of the Product Lifecycle
We intend to continue to use our development and manufacturing solutions across the entire lifecycle of our customers’ products to drive future growth. Our development solutions span the drug development process, starting with our platforms for early pre-clinical development of small molecules, protein-based biologics, and cell and gene therapies; through formulation and analytical services, development and manufacturing of clinical trial supplies, and fill and finish of injectable products; to regulatory consulting. Once a molecule is ready for clinical trials and subsequent commercialization, we provide our customers with a range of advanced technologies and expert, state-of-the-art manufacturing solutions that allow them to deliver their molecules to the end-users in safe, effective, and, in some cases, patient-preferred dosage forms, to produce biologic drug substances needed for protein-based biologics and cell and gene therapies, and to provide primary and secondary packaging solutions and cold-storage distribution services. Our relationship with a molecule typically starts with developing and manufacturing the innovator product and can extend throughout the molecule’s commercial life. For prescription products, we are often the sole or primary outsourced provider and are frequently reflected in customers’ product approval applications. Our revenue from our development and manufacturing activities are primarily driven by volumes, and, as a result, the loss of an innovator drug’s market exclusivity may be mitigated if we supply customers offering generic or biosimilar equivalents.
An example of the long and mutually productive relationships we foster can be found in a leading over-the-counter anti-allergy brand, which today uses both our proprietary Zydis orally disintegrating tablets and Liqui-Gels softgel technology. We originally began development of the prescription format of this product for our multinational pharmaceutical company partner in 1992 to address specific patient sub-segment needs. After four years of development, we then commercially supplied the prescription product in our Zydis format for six years, and we have continued to provide the Zydis form since the switch to over-the-counter status in the U.S. and other markets in the early 2000s. Subsequently, we proactively brought a softgel product concept for the brand to the customer, which the customer elected to develop and launch as well. By following this molecule, we have built a strong, 3 decade-long relationship across multiple formats and markets.
Customer Product Pipeline — Continuing to Grow Through New Projects and Product Launches
We intend to continue to supplement our existing diverse base of commercialized customer products with new development programs. As of June 30, 2023, our product development teams were working on more than 1,500 customer development programs in active development across our business. Our base of active development programs has expanded in recent years from growing market demand, as well as from our expanded capabilities and technology platforms. Although there are many complex factors that affect the development and commercialization of pharmaceutical, protein-based biologic, cell and gene therapy, and consumer health products, we expect that a portion of these programs will reach full development and market approval in the future and thereby add to our long-duration commercial revenues under long-term contracts and grow our existing product base. In fiscal 2023, we introduced 216 new products for our customers.
Catalent continues to be a leader in providing chemistry, manufacturing, and controls-based product development services to the global pharmaceutical, biotechnology, and consumer health industries, driven by thousands of projects annually. In fiscal 2023, we recognized $1.95 billion of net revenue related to the development of products, down 16% from the prior year, principally driven by the substantial decrease in net revenue from the development of COVID-19 related products. In addition, substantially all of the revenue associated with the Clinical Supply Services business relates to our support of customer products in development.
Our Reportable Segments
At the beginning of fiscal 2023, in connection with the appointment of a new President and Chief Executive Officer, who also serves as the Company’s Chief Operating Decision Maker, the Company changed its operating structure and reorganized its executive leadership team. This new organizational structure includes operating and reporting in two segments: (i) Biologics and (ii) Pharma and Consumer Health.
Biologics
Our Biologics segment provides formulation, development, and manufacturing for biologic proteins, cell gene, and other nucleic acid therapies; pDNA, iPSCs, oncolytic viruses, and vaccines; formulation, development, and manufacturing for parenteral dose forms, including vials, prefilled syringes, and cartridges; and analytical development and testing services for large molecules. The business has extensive expertise in development, scale up, and commercial manufacturing. Representative customers of our Biologics segment include Bristol-Myers Squibb, Johnson & Johnson, Moderna, and Sarepta Therapeutics, along with a broad range of innovative small and mid-tier biopharmaceutical customers.
Our biologics offering includes cell-line development based on our advanced, patented GPEx suite of technologies, which are used to develop stable, high-yielding mammalian cell lines for both innovator and biosimilar biologic compounds. GPEx technology can provide rapid cell-line development, high biologics production yields, flexibility, and versatility. Our development and manufacturing facility in Madison, Wisconsin has the capability and capacity to produce cGMP quality biologics drug substance from 250L to 4000L scale using single-use technology across five suites to provide maximum efficiency, redundancy, and flexibility. Additionally, our Madison, Wisconsin facility features two flexible cGMP suites that are used to manufacture mRNA or other small-scale biomolecules. Our Bloomington, Indiana facility brings additional biologics development, clinical, and commercial drug substance manufacturing, and formulation development capabilities and capacity. Both Bloomington and our Anagni, Italy facility provide substantial capacity for finished-dose drug product manufacturing and packaging. Our SMARTag next-generation ADC technology, based in Emeryville, California, is a clinical-stage technology that enables development of ADCs and other protein conjugates with improved efficacy, safety, and manufacturability.
At our pDNA, cell therapy, and gene therapy global centers of excellence in Belgium, Maryland, and New Jersey, we develop and manufacture advanced therapeutics, including AAV, lentivirus, oncolytic virus, CAR-T, and other cell or virus modalities together with critical pDNA biological starting material for cell, mRNA, viral-based therapies and next-generation vaccines. In fiscal 2022, we acquired a fully operational, commercial-scale cell therapy campus in Princeton with 16 suites available for both autologous and allogeneic clinical and commercial manufacturing. The Princeton campus works in conjunction with our Gosselies, Belgium cell therapy center of excellence and our iPSC manufacturing center of excellence in Dusseldorf, Germany, to support our customers’ global cell therapy needs. Additionally, we have expanded our gene therapy flagship manufacturing campus in Harmans, Maryland, creating a total of 18 penthouse-style viral-vector suites, and added our Virtuoso AAV platform that reduces AAV development time by half, enabling our customers to reach first-in-human studies faster. Our specialized expertise in AAV vectors, the most commonly used delivery system for gene therapies, and iPSCs for next-generation allogeneic cell therapy manufacturing, together with our substantial global cell therapy manufacturing, capacity for clinical- through commercial-scale batches, and our capabilities in mRNA and pDNA manufacturing, position us to capitalize on strong industry demand and expansions in the use of newer modalities in the cell and gene therapy market.
Our range of injectable manufacturing offerings includes manufacturing drug substances and filling small molecules or biologics into vials, syringes, and cartridges, with flexibility to accommodate other formats within our existing network. In addition to primary packaging, our network provides secondary packaging capabilities, including auto-injector and safety device assembly for commercial launch and life-cycle management. Our clinical supply services business provides a global network for clinical distribution, as well as labeling, packaging, and cold-chain storage for clinical trials and commercial supply of biotherapeutics and cell and gene therapies. Our fill and finish services are largely focused on complex pharmaceuticals and biologics. With our range of technologies, we are able to meet a wide range of specifications, timelines, and budgets. We believe that the complexity of the manufacturing process, the importance of experience and know-how, a proven history of regulatory compliance, and substantial state-of-the-art capacity provide us with a meaningful competitive advantage in the market.
We also offer analytical development and testing services for proteins, gene and cell therapies, and other biologic modalities, including bioassay, biophysical characterization, and cGMP release and stability testing. Our OneBio Suite provides customers with the potential to seamlessly integrate drug substance, drug product, and clinical supply management for products in development, and for integrated commercial supply across both drug substance and drug product. We provide a broad range of technologies and services supporting the development and launch of new biologic entities, biosimilars, biobetters, and cell and gene therapies to bring a product from gene to commercialization, faster.
Our Biologics segment represented 46%, 53%, and 48% of our aggregate net revenue before inter-segment eliminations for fiscal 2023, 2022, and 2021, respectively.
Pharma and Consumer Health
Through our Pharma and Consumer Health segment, we provide market-leading capabilities for complex oral solids, softgel formulations, Zydis fast-dissolve technologies, and gummy, soft chew, and lozenge dosage forms; formulation, development, and manufacturing platforms for oral, nasal, inhaled, and topical dose forms; and clinical trial development and supply services.
Representative customers of our Pharma and Consumer Health segment include Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Haleon, Pfizer, and Procter & Gamble.
Our Pharma and Consumer Health segment represented 54%, 47%, and 52% of our aggregate net revenue before inter-segment eliminations for fiscal 2023, 2022, and 2021, respectively.
Formulation and development
Through our comprehensive pharmaceutical formulation and development platform, we provide pre-clinical screening, formulation, and analytical development, and cGMP manufacturing at both clinical and commercial scale for our market-leading softgel capsule and Zydis fast-dissolve tablet platforms, traditional and advanced complex oral solid-dose formats, dry powder inhalers, and nasal delivery devices. We have substantial, proven experience in developing and scaling up orphan and rare disease products, especially those requiring accelerated development timelines, solubility enhancement, specialized handling (e.g., potent or controlled substance materials), complex technology transfer and specialized manufacturing processes. We provide fluid bed coating, spray drying, hot melt extrusion, micronization, and lipid formulation capabilities, all of which are used to enhance a drug’s administration and release profile and its clinical performance. We offer comprehensive analytical method development and scientific capabilities, including stability testing and global regulatory services to support both fully integrated development programs or standalone fee-for-service work. We have a network of early development sites focused on earlier phase compounds (i.e., pre-clinical and Phase I) to engage with more customer molecules earlier in their development, with the intent to also support these molecules downstream as they progress towards commercial approval and supply. Demand for our offerings is driven by the need for scientific expertise, the depth and breadth of integrated services offered, as well as the reliability of our supply performance across quality and operational parameters.
Manufacturing
Our large-scale cGMP pharmaceutical manufacturing solutions typically include clinical trial supplies, registration batches, and commercial production across a broad range of formats, and may also involve finished dose packaging or advanced processing of intermediates to achieve the desired clinical performance of the prescription or over-the-counter pharmaceutical product. Finished dose forms include softgel capsules, our Zydis fast-dissolve tablets, and traditional and advanced complex oral solid-doses, including coated and uncoated tablets, pellet/bead/powder-filled two-piece hard capsules, granulated powders, and other immediate and modified release forms. Advanced intermediate processing may include coating, extrusion, or spheronization to achieve specific functional outcomes, including site- or time-specific drug release, taste
masking, or enhanced bioavailability. We have deep experience at managing complex technical transfers of clinical or commercial programs, whether from Catalent’s early development network, other contract development sites, or from customers directly.
Softgel technology platform
We provide formulation, development, and manufacturing services for soft capsules, or “softgels,” as well as large-scale manufacturing of oral solid dose forms for pharmaceutical and consumer health markets, along with supporting ancillary services. Our softgel manufacturing technology was first commercialized by our predecessor in the 1930s, and we have continually enhanced the platform since then. We are the market leader in overall softgel development and manufacturing and hold the leading market position in innovator drug softgels. Our principal softgel technologies include traditional softgel capsules, in which the shell is made of animal-derived gelatin, and Vegicaps and OptiShell capsules, in which the shell is made from plant-derived materials. Softgel capsules are used in a broad range of customer products, including prescription drugs, over-the-counter medications, dietary supplements, unit-dose cosmetics, and animal health medicinal preparations. Softgel capsules encapsulate liquid, paste, or oil-based formulations of active compounds in solution or suspension within an outer shell. In the manufacturing process, the capsules are formed, filled, and sealed simultaneously. We typically perform encapsulation for a product within one of our softgel facilities, with active ingredients provided by customers or sourced directly by us. Softgels have historically been used to solve formulation challenges or technical issues for a specific drug, to help improve the clinical performance of compounds, to provide important market differentiation, particularly for over-the-counter medications, and to provide safe handling of hormonal, highly potent, and cytotoxic drugs. We also participate in the softgel vitamin, mineral, and supplement business in selected regions around the world. Our plant-derived softgel shells, available as Vegicaps and OptiShell capsules, allow innovators and consumer health customers to extend the softgel dose form to a broader range of active ingredients and serve patient and consumer populations that were previously inaccessible due to religious, dietary, or cultural preferences. Our Vegicaps and OptiShell capsules are protected by patents in most major global markets. Physician and patient studies we have conducted have demonstrated a preference for softgels versus traditional tablet and hard capsule dose forms in terms of ease of swallowing, real or perceived speed of delivery, ability to remove or eliminate unpleasant odor or taste, and, for physicians, perceived improved patient adherence with dosing regimens.
In addition to softgel capsules, following our fiscal 2022 acquisition of Bettera Wellness, we also conduct formulation, development, and manufacturing of gummies, soft chews, and lozenges in a variety of sizes and shapes serving the dietary supplements market at three facilities in the United States. We use dietary and food ingredients provided by our customers or sourced directly by us, and we also provide ancillary services such as analytical testing and packaging.
Clinical Supply Services
Our Pharma and Consumer Health segment also provides clinical supply services through manufacturing, packaging, storage, distribution, and inventory management for small-molecule drugs, protein-based biologics, and cell and gene therapies in clinical trials. We offer customers flexible solutions for clinical supplies production and provide distribution and inventory management support for both simple and complex clinical trials. This includes over-encapsulation where needed; supplying placebos, comparator drug procurement, and clinical packages and kits for physicians and patients; inventory management; investigator kit ordering and fulfillment; cold-chain storage and distribution; and return supply reconciliation and reporting. We support trials in all regions of the world through our facilities and distribution network. In recent years, we have extended our network, with significant expansions at our Philadelphia, Pennsylvania and Shanghai, China free trade zone locations and facilities in California, China, and Japan. We also continue to develop new solutions for the evolving clinical trial environment, including FlexDirect direct-to-patient, CT Success clinical supply planning, and extensive cold-chain investments. We are the leading provider of integrated development solutions and one of the leading providers of clinical trial supplies.
We have partnered with companies who focus on the development of cannabis-based prescription medicines and high-value cannabinoid drug therapies whose goal is to achieve full regulatory approval under the strictest legal standards in effect in any jurisdiction affected, including cannabidiol and tetrahydrocannabinol pharmaceutical products using our Zydis technology in clinical trials across a range of indications, including multiple sclerosis spasticity, chemotherapy-induced nausea and vomiting, chronic pain for cancer, and epilepsy. Our total net revenue related to such development programs was less than 1% of total revenue generated in fiscal 2023. We do not provide any services for or otherwise partner with any company that does not comply with all applicable laws, including the U.S. federal controlled substances laws (or non-U.S. equivalent laws), relating to cannabis products.
Integrated Development and Product Supply Chain Solutions
In addition to our proprietary offerings, we are also differentiated in the market by our ability to bring together our development solutions and state-of-the-art product manufacturing to offer integrated development and product supply solutions that can be combined or tailored in many ways to enable our customers to take their drugs, biologics, and consumer health products from laboratory to market, faster. Once a product is on the market, we can provide comprehensive, integrated product supply, from the sourcing or supply of the bulk active ingredient to comprehensive manufacturing and packaging, to the testing required for release, and to cold-chain or ambient temperature distribution. The customer- and product-specific solutions we develop are flexible, scalable, and creative, so that they meet the unique needs of both large and emerging biopharmaceutical, pharmaceutical, and consumer health companies and are appropriate for products of all sizes. We believe that our development and product supply solutions, such as OptiForm Solution Suite and OneBio Suite, will continue to contribute to our future growth.
Sales and Marketing
Our target customers include large pharmaceutical and biotechnology companies, mid-size, emerging, and specialty pharmaceutical and biotechnology companies, and consumer health companies, along with companies in other selected healthcare market segments such as animal health and medical devices, and companies in adjacent industries, such as cosmetics. We have longstanding, extensive relationships with leading pharmaceutical, biotechnology, and consumer health customers. In fiscal 2023, we did business with 87 of the top 100 branded drug and consumer health marketers and 82 of the top 100 biologics marketers, measured on a global basis, as well as with more than 1,200 other customers. Faced with access, pricing, and reimbursement pressures as well as other market challenges, large pharmaceutical and biotechnology companies have increasingly sought partners to enhance the clinical competitiveness of their drugs and biologics and improve the productivity of their research and development activities, while reducing their fixed cost bases. Many mid-size, emerging, and specialty pharmaceutical and biotechnology companies, while facing the same pricing and market pressures, have chosen not to build a full infrastructure, but rather to partner with other companies through licensing agreements or outsourcing to access the critical skills, technologies, and services required to bring their products to market. Consumer health companies require rapidly developed, innovative dose forms and formulations to keep up with the fast-paced over-the-counter medication, dietary supplement, and personal care markets. These market segments are all important to our growth, but require distinct solutions, marketing and sales approaches, and market strategy.
We follow a hybrid demand-generation organization model, with strategic account teams offering the full breadth of Catalent’s solutions, and technical specialist teams providing the in-depth technical knowledge and practical experience essential for each individual offering, both supported by a dedicated team of deeply experienced scientific advisors. Our sales organization currently consists of more than 200 full-time, experienced sales professionals, supported by inside sales and sales operations. We also have built a dedicated strategic marketing team, providing strategic market and product planning and management for our offerings. As part of our marketing efforts, we participate in major trade shows relevant to our offerings globally and ensure adequate visibility to our offerings and solutions through a comprehensive advertising and publicity program. We believe that Catalent is a strong brand with high overall awareness in our established markets and universe of target customers, and that our brand identity is a competitive advantage for us.
Global Accounts
We manage select accounts globally due to their substantial current business or growth potential. We recorded approximately one-third of our total net revenue in fiscal 2023 from these global accounts. Each global account is assigned a lead business development professional with substantial industry experience. These account leaders, along with other members of the sales and executive leadership teams, are responsible for managing and extending the overall account relationship. Account leaders work closely with the rest of the sales organization as well as operational, quality, and project management personnel to ensure alignment around critical priorities for the accounts.
Emerging, Specialty, and Virtual Accounts
Emerging, specialty, and virtual pharmaceutical and biotechnology companies are expected to be critical drivers of industry growth globally and account for more than three-quarters of the active drug and biologic development pipeline. Historically, many of these companies have chosen not to build a full infrastructure, but rather partner with other companies to formulate, develop, analyze, test, and manufacture their products. We expect them to continue to do so in the future, providing a critical source for future integrated solutions demand. We expect to continue to increase our penetration of geographic clusters of emerging companies in North America, Europe, Central and South America, and Asia. We regularly use active pipeline and product screening and customer targeting to identify the optimal candidates for partnering based on product profiles, funding status, and relationships, to ensure that our technical sales specialists and field sales representatives develop custom solutions
designed to address the specific needs of these customers. In order to reach these emerging, specialty, and virtual companies, we actively partner with leading venture capital investors and biotech incubators.
Seasonality; Fluctuations in Operation Results
Our annual financial reporting period ends on June 30. As discussed further in “Item 7. - Management's Discussion and Analysis of Financial Condition and Results of Operations - Factors Affecting our Performance,” our revenue and net earnings are generally higher in the third and fourth quarters of each fiscal year, with our first fiscal quarter typically generating our lowest revenue of any quarter, and our last fiscal quarter typically generating our highest revenue. These fluctuations are primarily the result of the timing of our, and our customers’ annual operational maintenance periods at locations in the U.S. and Europe, the seasonality associated with pharmaceutical and biotechnology budgetary spending decisions, clinical trial and research and development schedules, the timing of new product launches and length of time needed to obtain full market penetration, and, to a lesser extent, the time of the year some of our customers’ products are in higher demand, or are being produced to support future seasonal demand.
Contractual Arrangements
We generally enter into a broad range of contractual arrangements with our customers, including agreements with respect to feasibility, development, supply, licenses, quality, and confidentiality. The terms of these contracts vary significantly depending on the offering and customer requirements. Some of our agreements may include a variety of revenue arrangements, such as fee-for-service, unit pricing in one or more tiers, minimum volume commitments, royalties, manufacturing preparation services, profit-sharing, and fixed fees. We generally secure pricing and other contract mechanisms in our supply agreements to allow for periodic resetting of pricing terms, and, in some cases, these agreements permit us to raise or renegotiate pricing in the event of certain price increases for the raw materials or other inputs we use to make products. Our typical supply agreements include indemnification from our customers for product liability and intellectual property matters and caps on our contractual liabilities, subject in each case to negotiated exclusions. The terms of our manufacturing supply agreements range from two to seven years with regular renewals of one to three years, although some of our agreements are terminable upon much shorter notice periods, such as 30 or 45 days. For our development solutions offerings, we may enter into master service agreements, which provide for standardized terms and conditions and make it easier and faster for customers with multiple development needs to access our offerings.
Backlog
While we generally have long-term supply agreements that provide for a revenue stream over a period of years, our backlog represents, as of a point in time, future service revenues from work not yet completed. For our Biologics segment and a majority of our Pharma and Consumer Health segment, backlog represents firm orders for manufacturing services and includes minimum volumes, where applicable. Manufacturing businesses backlog represents firm orders for manufacturing services and includes minimum volumes, where applicable. For the clinical supply services offered through our Pharma and Consumer Health segment, backlog represents estimated future service revenue from work not yet completed under signed contracts. Using these methods of reporting backlog, as of June 30, 2023, our backlog was $2.53 billion, compared to $2.85 billion as of June 30, 2022, including $557 million and $549 million, respectively, related to our scientific and clinical services offerings in our Pharma and Consumer Health segment. We expect to recognize as revenue by the end of fiscal 2024 approximately 83% of the value of the backlog in existence as of June 30, 2023.
To the extent projects are delayed, the timing of our revenue could be affected. If a customer cancels an order, we may be reimbursed for the costs we have incurred. For orders that are placed inside a contractual firm period or that involve minimum volume commitments, we generally have a contractual right to payment in the event of cancellation. Fluctuations in our reported backlog levels also result from the timing and order pattern of our customers, which often seek to manage their level of inventory on hand. Because of customer ordering patterns, the matters discussed in this paragraph, and other factors, our backlog reported for certain periods may fluctuate and may not be indicative of future results.
Manufacturing Capabilities
We operate manufacturing facilities, development centers, and sales offices throughout the world. As of June 30, 2023, we had 52 facilities (3 geographical locations operate as multiple facilities because they support more than one reporting segment, with one location including both a manufacturing facility and our corporate headquarters) on four continents with approximately 8 million square feet of manufacturing, laboratory, office, and related space. Our manufacturing capabilities generally include the full suite of competencies relevant to the support of each site’s activities, including regulatory, quality assurance, and in-house validation.
We operate our manufacturing facilities and development centers in accordance with cGMP or other applicable requirements. All of these sites are registered where required with the FDA or other applicable regulatory agencies, such as the EMA. In some cases, our sites are registered with multiple regulatory agencies.
We have invested $1.93 billion in our manufacturing and development facilities since fiscal 2021 for improvements and expansions, including $583 million in capital expenditures during fiscal 2023. We believe that our sites and equipment are in good condition, are well maintained, and are able to operate at or above present levels for the foreseeable future, in all material respects.
Our manufacturing operations are focused on employee health and safety, regulatory compliance, operational excellence, continuous improvement, and process standardization across the organization. In fiscal 2023, we achieved approximately 95% on-time shipment delivery versus customer request date across our network as a result of this focus. Our manufacturing operations are structured around an enterprise management philosophy and methodology that utilizes principles and tools common to a number of quality management programs, including Lean Six Sigma and Lean Manufacturing, which we brought together in a system that we refer to as “The Catalent Way.”
Raw Materials
We use a broad and diverse range of raw materials and other supplies in the design, development, and manufacture of our products. This includes, but is not limited to, key materials such as gelatin, starch, and iota carrageenan; packaging films; single-use production components for drug substance production, and glass vials and syringes for drug product. The raw materials and other supplies that we use are sourced externally on a global basis. Globally, our supplier relationships could be interrupted due to natural disasters and international supply disruptions, including those caused by pandemics or geopolitical and other issues. For example, commercially usable gelatin is available from a limited number of sources. In addition, much of the gelatin we use is bovine-derived. Past concerns of contamination from bovine spongiform encephalopathy have narrowed the number of possible sources of particular types of gelatin. If there were a future disruption in the supply of gelatin or any other key material from any one or more of our current principal suppliers, there can be no assurance that we could obtain an adequate alternative supply from our other suppliers. Any future restriction that were to emerge on the use of a key raw material used in our products from certain geographic sources or due to regulatory or consumer concerns could hinder our ability to timely supply our customers with products, and the use of alternative raw materials could be subject to lengthy formulation, testing and regulatory approval periods.
We work very closely with our suppliers to assure continuity of supply while maintaining excellence in material quality and reliability. We continually evaluate alternate sources of supply, although we do not frequently pursue regulatory qualification of alternative sources for key raw materials due to the strength of our existing supplier relationships, the reliability of our current supplier base, and the time and expense associated with the regulatory process, since regulators usually must approve changes to prescription product ingredient sources. Although a change in suppliers could require significant effort or investment by us in circumstances where the items supplied are integral to the performance of our products or incorporate specialized material such as gelatin, we do not believe that the loss of any existing supply arrangement would have a material adverse effect on our business. See “Item 1A. - Risk Factors—Risks Relating to Our Business and the Industry in Which We Operate—Our results of operations are subject to fluctuations in the costs, availability, and suitability of the components of the products we manufacture, including active pharmaceutical ingredients, excipients, purchased components, and raw materials, and other supplies or equipment we need to run our business."
Competition
We compete with multiple companies as to each of our offerings and in every region of the globe in which we operate, including with CDMOs and other companies that offer conventional and advanced technologies for the development, supply, and delivery of medicinal products, clinical trials support, outsourced dose form, protein-based biologics or cell or gene therapy manufacturing, or development services to pharmaceutical, biotechnology, and consumer health companies based in North America, Central and South America, Europe, and the Asia-Pacific region. We also compete in some cases with the internal operations of those pharmaceutical, biotechnology, and consumer health customers that also have manufacturing capabilities and choose to source these services internally. Some of our competitors are substantially larger than we are and have access to more substantial resources, which could be deployed to expand their range of offerings or capacity.
Competition is driven by proprietary technologies and know-how (where relevant), capabilities, consistency of operational performance, availability of equipment, quality, regulatory track record, price, value, responsiveness, and speed. While we have competitors that compete with us in our individual offerings, and a few competitors that compete across many of our offerings, we do not believe we have competition from any directly comparable company.
Research and Development Costs
Our research activities are primarily directed toward the development of new offerings and manufacturing process improvements. Research and development costs amounted to $18 million, $23 million, and $21 million for fiscal 2023, 2022, and 2021, respectively.
Employees
As of June 30, 2023, we had approximately 17,800 individuals providing services for us at 52 facilities on 4 continents, of which certain employees at two of our 24 U.S. facilities are represented by labor unions, with their terms and conditions of employment being subject to collective bargaining agreements. Various combinations of national works councils, labor unions, and other labor organizations are active at all of our European and many of our other ex-U.S. facilities consistent with labor environments and laws in those countries. Our management believes that our relations with our workforce are satisfactory. Most of our individual service providers are full-time employees, while approximately 700 of our workers as of June 30, 2023 are contingent workers who are either self-employed or employed by external services organizations.
| | | | | | | | | | | | | | | | | |
| North America | Europe | South America | Asia Pacific | Total |
| Approximate number of workers as of June 30, 2023 | 10,500 | 5,700 | 1,000 | 600 | 17,800 |
Human Capital Management
Our employees share common goals: to put patients first and to help people around the world live better, healthier lives. Our global workforce is united by our values: Patient First, commitment to our people, customer dedication, innovation, integrity, and excellence. Together, our values provide the foundation for our culture. We believe that an engaged, diverse workforce, empowered by inclusive leaders, will unlock our full potential as a company and as a leader in our sector. Our employees’ success is Catalent’s success.
We focus on employee development, engagement, and diversity and inclusion (“D&I”) to hire, develop, and retain the best talent. As of June 30, 2023, we had approximately 17,800 individuals providing services to us globally, with women representing 44% of our employees and holding 40% of roles at the manager level or higher. In fiscal 2023, ethnically diverse talent represented 35% of our U.S. employees.
Our turnover rate increased to 22% as of June 30, 2023, including 13% voluntary turnover, substantially driven by voluntary turnover in the U.S. and by a few reorganizations at some of our larger sites and within our corporate functions.
Reducing voluntary attrition and retaining our talent remains one of our top priorities. We continue to implement initiatives to build upon our values-based and inclusive culture, improve our employees’ experiences at Catalent, and better develop and engage internal talent. We continuously monitor local talent markets and provide differentiated pay arrangements and benefits to attract and retain talent. Additionally, we provide flexible work arrangements where possible, broader leadership development programs, an employee wellness program, and access to employee recognition programs at all levels.
We continue to take steps to ensure that Catalent is a company where all employees can develop a fulfilling career with support from our leadership team. We believe that our diverse pool of internal talent and our employees’ passion for excellence make a difference in the way we grow and deliver results.
Talent Acquisition
We have strong human resources processes and practices in place to support our employees through their careers at Catalent. This starts with a robust recruiting strategy and a strong employer brand. We attracted over 4,000 new employees in fiscal 2023, continuously working to reduce the time it takes to fill open positions and reduce our cost per hire, while striving for a best-in-class candidate experience.
We offer competitive compensation and a comprehensive suite of benefits, which, in the U.S., range from medical, dental, and vision coverage to retirement, disability, employee stock purchase, and life insurance programs. We also provide health promotion and wellness programs, remote work flexibility, tuition assistance, and employee assistance programs in several countries.
Our recruitment strategy aims to attract talent representing diverse backgrounds, perspectives, and ideas. This approach includes:
• engaging with potential top talent early in the career path through our university internship program;
• developing future leaders and enhancing their skills through programs, including various mentoring programs, our Global Organization Leadership Development (“GOLD”), Next Generation Global Leaders, and General Manager Excellence programs, as discussed further below;
• providing competitive compensation and benefits;
• continuously improving recruitment processes and platforms;
• working with several recruitment partners to attract diverse profiles and advertise open positions; and
•implementing unconscious bias workshops for hiring managers.
Catalent has been recognized as a TOP EMPLOYER USA since 2020 and as a TOP EMPLOYER in the United Kingdom ("U.K.") since 2022. We differentiate ourselves as a preferred employer to candidates through our reputation as a great place to work, offering a fast-paced and rewarding work environment.
Talent Development
We are also committed to the growth, development, and engagement of our people once they have joined our family. Through a strong learning and development culture, we provide opportunities for specialized technical training, leadership development, and high-potential growth opportunities to endow our employees with the knowledge and expertise needed to grow their careers at Catalent.
Our primary goal is to develop our people from within, thereby establishing a strong successor bench to help support company growth. In fiscal 2023, over 2,400 employees moved to a new role within the organization (of which 49% were women), whether as a developmental move or a promotion to a more senior position. Our senior leaders are committed to talent development and dedicate time each fiscal quarter to perform formalized talent reviews to discuss the development of key talent and to update succession plans for critical roles.
We strongly believe that the combination of experience (70%), exposure (20%), and education (10%) is the best recipe for personal development and career progression here. We have a library of tools and resources available for our employees within that framework, including access to a variety of tools and resources to learn new or expand existing skills.
Given our high volume of new hires, we continue to redesign our employee experience. We have upgraded our on-boarding experience to span employees’ first twelve months with Catalent.
We also offer four formal development programs to employees. All programs aim to prepare our talent to fill critical internal leadership roles. Through these programs, we have created a bench of leaders who model our values and are ready to take on more responsibility.
(1) Entry-level GOLD program. The GOLD program is a two-year rotational program for recent graduates from universities around the world in which the employee participates in three rotations at different sites in our network to learn about different aspects of our business and our varied offerings. GOLD employees receive assignments to perform strategic roles in key business initiatives. We provide them with coaching and opportunities to interact with senior executives, which both develop the skills and experience of our GOLD employees and provide a platform through which they contribute fresh ideas that challenge the status quo. The GOLD program operates in the U.S. and has been relaunched in Europe following a pause in the program during the U.K.'s withdrawal from the European Union (“E.U.”).
(2) Manager-level Next Generation Global Leader program. Our Next Generation Global Leader program, for employees at the manager level, is a 15-month on-the-job program focused on preparing high-potential managers for director-level roles. Since fiscal 2022, 86 employees graduated from this program, and a new group of 56 employees has been selected to join this program in fiscal 2024.
(3) Senior leader General Manager Excellence program. Our general managers run our operating sites and have substantial and wide-ranging responsibilities. This program enhances the skills of our general managers by giving them exposure to industry best practices and opportunities to network internally and receive personalized career coaching, including a 3-day business simulation. In fiscal 2023 and 2022, 24 and 35 general managers, respectively, participated in this program.
(4) Front-line leader level Lead Now program. In fiscal 2023, we launched “Lead Now,” a Catalent-wide leadership offering targeted for those who are new to people leadership. During the first 3 months of a new leadership role, this program teaches employees the fundamentals of leadership and identifies tools to inspire their teams while role-modeling Catalent values. We have already enrolled over 250 new leaders into this program.
Diversity and Inclusion
At Catalent, we cultivate a workplace that respects and welcomes all people; celebrates the unique backgrounds and experiences of our workforce; encourages all employees to bring their true, authentic selves to work; and leverages our diversity to drive innovation, inclusion, and excellence in every aspect of our business. By closing diversity and inclusion gaps, we energize our people to do their best work.
Our commitment to D&I starts at the top with our board of directors (referred to herein as the “board of directors” or “Board”) and executive leadership team that bring a broad spectrum of backgrounds and perspectives. Led by our Diversity and Inclusion Office, and effectuated at all levels of the Company, we focus on attracting. retaining, and developing our diverse talent and creating an inclusive work environment globally.
We are committed to identifying and acknowledging gaps in our D&I mission and taking action to address them. To drive progress within Catalent, we focus on four strategic initiatives:
• Strengthening our culture of inclusion, supported by our nine employee resource groups;
• Promoting inclusive leadership;
• Accelerating talent acquisition and development, including with support from external partners; and
• Activating a data- and accountability-driven strategy.
One key example of our global D&I initiatives are our nine global employee resource groups (“ERGs”), providing support, resources, and a forum for topical discussion and engagement for employees in the following categories: people with disabilities, people of Asian and Pacific Islander descent, women, indigenous peoples, people of Hispanic and/or LatinX descent, our LGBTQ+ community, people of African descent, Gen Y and Z, and people with military and/or first responder service. Our ERG network includes 127 global, virtual, and site-based chapters.
Key D&I performance highlights are captured in our Corporate Responsibility and Environmental, Social, and Governance Strategy section below.
Engagement
Our employee-focused practices make a clear impact on our employee engagement. Through increased engagement, we can grow our business by relying on strong, engaged leaders and professionals willing to ensure we can overcome and thrive during any challenge.
We periodically administer a company-wide engagement survey to garner direct feedback from our employees regarding how we can more deeply and meaningfully engage them, enabling us to focus on improving specific areas where we can support our people.
In fiscal 2023 we changed our approach to employee engagement surveys and launched our first “pulse survey,” using an online platform to deliver an updated engagement score and immediate access to results for all our people leaders on a more frequent basis. This first pulse survey, in November 2022, led to a companywide score of 6.9 out of a possible 10 for Catalent (using a rating scale from 1 to 10). The platform highlighted key strengths, including our inclusive culture, peer relationships, goal setting, and meaningful work. The survey also highlighted areas requiring further focus, including workload, strategy communication, and rewards.
Corporate Responsibility (“CR”) and ESG Strategy
Our CR strategy, which includes our ESG strategy, is integrated into our company-wide strategic plan, ensuring that we operate in alignment with our values, meet our commitments to all our stakeholders, and contribute to the long-term success of the broader biopharmaceutical, pharmaceutical, and consumer health industries and the communities where we operate. Our approach to ESG focuses on three areas of society relevant to our business, prioritizing our impact on (i) people, (ii) the environment, and (iii) our communities. We focus on ESG areas that are the most significant to our business, and our strategy is
informed by our employees, customers, investors, communities, and other key stakeholders. Our fiscal 2023 ESG performance, described below, demonstrates our contribution to the long-term success of the industries we serve and the communities where we operate, as we continue to invest in a corporate culture that understands and prioritizes our impact on people in our operations and employee-related decision-making.
Fiscal 2023 brought new and continued challenges for our operations, including the on-going response to the Ukrainian-Russian war, significant reductions in the volume of vaccines produced for the COVID-19 pandemic, and the on-going supply chain challenges amid rising global inflation. Through it all, our mission and values continued to provide steady, critical orientation and focus. Amid these fiscal 2023 challenges, our business delivered approximately 70 billion unit doses, across more than 50 sites, where our workforce of over 17,000 worked hard, with our Patient First value guiding the way, to ensure that we met our commitments to our customers and their patients.
Governance
We are committed to ensuring strong corporate governance practices on behalf of our shareholders and other stakeholders. We believe strong corporate governance and an independent board of directors provide the foundation for financial integrity and shareholder confidence. More information about our corporate governance features can be found in Item 10. — Directors, Executive Officers, and Corporate Governance.
Our CR council, which reports to the CEO and is composed of senior leaders from various parts of our business, guides our CR efforts and sets our overall CR strategy. Management, including members of the CR council, provide regular CR updates to our board of directors, which regularly reviews material aspects of our CR strategy and performance as a full board and through its several committees, including a formal annual review of our overall CR strategy and performance.
Business Benefits
Beyond being the right thing to do, our focus on CR strengthens our business by reducing risks, meeting customer and investor expectations, and positioning us to attract top talent. CR performance is an important contributor to our business success. It informs our risk management process, protects our reputation, and alerts us to regulatory, environmental, and societal threats to our business. Our CR activities also align with many of our customers’ CR programs and strengthen our relationships.
Our future success depends on our highly skilled and dedicated global team of employees, who are passionate about improving health outcomes. We compete for top talent in our industry and recognize that our culture and reputation as a responsible company can be a differentiator for attracting job candidates and keeping and motivating our existing employees.
ESG progress in fiscal 2023
We made significant progress in several ESG focus areas in fiscal 2023:
•In February 2023, we published our fourth annual Corporate Responsibility report (covering fiscal 2022), which includes an evaluation of our performance against the standards set by the Sustainability Accounting Standards Board (SASB) for Biotechnology and Pharmaceuticals and the recommendations of the Task Force on Climate-Related Financial Disclosures (TCFD). Some highlights of our progress include:
◦purchasing renewable electricity for our operating sites, with energy attribution certificates (EACs) accounting for 81% of our electricity use by the end of fiscal 2022, compared to 23% at the end of fiscal 2021, thus reducing our total Scope 1 and 2 emissions by 38%, from a fiscal 2020 baseline;
◦implementing 153 sustainability projects, resulting in annual energy savings of more than 4%, compared to our fiscal 2018 baseline;
◦achieving our water efficiency target, of <500m3/$1M revenue, a year early, reducing our water intensity by 26%, from a fiscal 2020 baseline;
◦converting 1,400 metric tons of uncontaminated by-product (24% of the total volume) diverted from landfill, converting it to alternative uses, such as adhesive;
◦philanthropic giving that exceeded $1 million, driven significantly by philanthropic efforts related to the humanitarian response to the war in Ukraine and, our on-going commitment to science, technology, engineering, and math (STEM) education, and nonprofits that serve patients, with a focus on underserved communities; and
◦2,200 employees participated in the Catalent Cares Matching Gift Program, a 46% increase from fiscal 2021, raising $1 million for 625 non-profits in combined employee donations and Company matches.
•In fiscal 2023, we continued to drive D&I by (1) investing in the inclusive capabilities of our leaders, (2) working with partners who share our values and help enable our strategy, (3) accelerating diverse talent acquisition and development, and (4) curating an even more inclusive culture. Some highlights of our progress include:
◦a 17% increase (from 23% to 27%) in our U.S.-based leaders who are racially or ethnically diverse;
◦a 9% increase (from 35% to 38%) in women in leadership roles globally;
◦confirmation that we closed the U.S. gender pay gap in fiscal 2021 through EDGE certification;
◦publication of a Supplier Diversity Policy and setting the ambition to increase diverse supplier spend;
◦designation as a 2022 Best Place to Work for people with disabilities, based on the Disabilities Equality Index;
◦rollout of our inclusive leadership workshops for site and functional leadership teams and ongoing conversations hosted by our leaders following challenging current events in the U.S.; and
◦expansion of our ERGs, with a 21% increase in the number of ERG chapters, and a record 43 global ERG events with 7,500 attendees.
Looking ahead
We are determined to play an integral role in moving our industry toward more responsible and sustainable business practices as we continue to be at the cutting edge of developing and reliably supplying drugs, biologics, and consumer health products.
We continue to reduce our carbon emissions and have committed to Science Based Targets (SBT). The SBT report we submitted for assessment details a target reduction for our Scope 1 and 2 emissions, based on a fiscal 2022 baseline, and a goal for engaging our Scope 3 footprint by aligning emission reduction targets to SBT goals. We have achieved our water intensity goal, to decrease water intensity to 500 cubic meters per $1 million in revenue and will now focus on sites in water-deprived or sensitive areas, to reduce our water usage further. At the end of fiscal 2023, 65% of our sites were able to eliminate waste sent to landfill; in fiscal 2024, we expect to achieve this at all of our facilities. We have performed a risk assessment and identified process and infrastructure changes needed for us to achieve our bold goal to ensure no residual active pharmaceutical ingredient (API) above Predicted No Effect Concentration (PNEC) in wastewater. Activities to implement this goal will occur throughout fiscal 2024.
We continue to strengthen our supply chain by expanding our supplier assessment and auditing program, including continued use of our third-party vetting and due diligence platform in alignment with the Pharmaceutical Supply Chain Initiative (PSCI) principles and the U.N. Guiding Principles on Business and Human Rights. Our diverse supplier network and spend will continue to increase, as outlined in our new Diverse Supplier Policy.
Measuring against our baseline D&I statistics, we will work to progress our goal of recruiting, developing, and retaining more diverse talent, including in leadership roles. In fiscal 2024, we will further implement our D&I action plans, which outline localized strategies and goals to help us meet our company-wide targets. We continue to participate in external benchmarks, including the Corporate Equality Index (LGBTQ+ inclusion) and the Disability Equality Index to guide our goals and progress. Through training, forums, and internal performance metrics, we will continue to combat unconscious biases that can impact the hiring and promotion of diverse talent. Our employee surveys reveal that our employees are energized and engaged by our CR and D&I initiatives. In fiscal 2024, we will continue to assess our employees’ overall engagement and inclusion through our next corporate engagement survey.
Further information on our CR program is available at catalent.com/about-us/corporate-responsibility/, but this website is not part of our public disclosures and is not incorporated by reference into this Annual Report.
Intellectual Property
We use a combination of know-how, trade secrets, patents, copyrights, trademarks, and other intellectual property, nondisclosure and other contractual provisions, and technical measures to protect certain innovative aspects of our offerings, services, and intangible assets that we have developed. These proprietary rights can be important to aspects of our ongoing operations. Many of our operations and products are covered by intellectual property licenses from third parties, particularly our customers that provide licenses to their proprietary active ingredients or formulations as part of our development or supply agreements with them, and in certain instances we license our technology to third parties.
We also have a long track record of innovation across our lines of business, and, to further encourage active innovation, we have developed incentive compensation systems linked to patent filings and other recognition and reward programs for
scientists and non-scientists alike. We have applied in the U.S. and certain other countries for registration of a number of trademarks, service marks, and patents, some of which have been registered and issued, and also hold common law rights in various trademarks and service marks. We hold more than 1,800 patents and patent applications worldwide relating to advanced drug delivery platforms, biologics formulations and technologies, and manufacturing.
We hold patents and license rights relating to certain aspects of our formulations, pharmaceutical and nutritional dosage forms, mammalian cell engineering, antibody-drug conjugation, iPSCs, and plasmid DNA manufacturing. We also hold patents relating to certain processes and products. We have pending patent applications in the U.S. and certain other countries and intend to pursue additional patents as appropriate. We have enforced and will continue to enforce our intellectual property rights in the U.S. and worldwide in appropriate circumstances.
We do not consider any particular patent, trademark, license, franchise, or concession to be material to our overall business.
Regulatory Matters
The manufacture, distribution, and marketing of healthcare products and the provision of certain services for development-stage pharmaceutical and biotechnology products are subject to extensive ongoing regulation by the FDA, other U.S. governmental authorities, and similar regulatory authorities in other countries. Certain of our subsidiaries are required to register for permits or licenses with, and must comply with the operating, cGMP, quality, and security standards of, applicable domestic and foreign healthcare regulators, including the FDA, the U.S. Drug Enforcement Agency (the “DEA”), the U.S. Department of Health and Human Services (the “DHHS”), the equivalent agencies of the E.U. and its member states, and various state boards of pharmacy, state health departments, and comparable agencies in other jurisdictions, as well as various accrediting bodies, each depending upon the type of operations and the locations of distribution and sale of the products manufactured or services provided by those subsidiaries.
In addition, various aspects of our business are subject to other healthcare laws, including the U.S. Federal Food, Drug, and Cosmetic Act, the Public Health Service Act, the Controlled Substances Act, and comparable state and foreign laws and regulations relevant to their activities.
We are also subject to various federal, state, local, national, and transnational laws, regulations, and requirements, both in the U.S. and other countries, relating to safe working conditions, laboratory and distribution practices, and the use, transportation, and disposal of hazardous or potentially hazardous substances. In addition, applicable import and export laws and regulations require us to abide by certain standards relating to the cross-border transit of finished goods, raw materials, and supplies and the handling of information. We are also subject to various other laws and regulations concerning the conduct of our non-U.S. operations, including the U.S. Foreign Corrupt Practices Act, the U.K. Anti-Bribery Act, and other anti-bribery laws and laws pertaining to the accuracy of our internal books and records. Furthermore, we are subject, in various jurisdictions, including the E.U. and certain U.S. states, to various privacy laws protecting data we may collect or process from employees, our customers' patients, or others.
The costs associated with complying with the various applicable federal, state, local, national, and transnational regulations could be significant, and the failure to comply with such legal requirements could have an adverse effect on our results of operations and financial condition. See “Item 1A. - Risk Factors—Risks Relating to Our Business and the Industry in Which We Operate—We are a part of the highly regulated healthcare industry, subject to stringent regulatory standards and other applicable laws and regulations, which can change unexpectedly or be the subject of unexpected changes in interpretation or enforcement, any of which may adversely impact our business,” for additional discussion of the costs associated with complying with the various regulations.
In fiscal 2023, we were subject to 51 regulatory audits, and, over the last five fiscal years, we completed approximately 300 regulatory audits.
Quality Assurance
We are committed to ensuring and maintaining the highest standard of regulatory compliance while providing high quality products to our customers, supported by our core value of Patient First. To meet these commitments, we have developed and implemented a Catalent-wide quality management system. We have employees around the globe focusing on quality and regulatory compliance. Our senior management team is actively involved in setting quality policies, standards, and internal guidance as well as managing internal and external quality performance. Our quality assurance department provides quality leadership and supervises our quality systems programs. An internal audit program monitors compliance with applicable regulations, standards, and internal policies. In addition, our facilities are subject to periodic inspection by the FDA, the DEA, and other equivalent local, state, and foreign regulatory authorities as well as our customers. All FDA, DEA, and other
regulatory inspection observations have been resolved or are on track to be completed at the prescribed timeframe provided in commitments to the applicable agency in all material respects. We believe that our operations are in compliance in all material respects with the regulations under which our facilities are governed.
Environmental, Health & Safety Matters
Our operations are subject to a variety of environmental, health, and safety laws and regulations, including those of the U.S. Environmental Protection Agency (the “EPA”), the U.S. Occupational Safety & Health Administration (“OSHA”), and equivalent state, local, and national regulatory agencies in each of the jurisdictions in which we operate. These laws and regulations govern, among other things, air emissions, wastewater discharges, the use, handling, and disposal of hazardous substances and wastes, soil and groundwater contamination, and employee health and safety. Our manufacturing facilities use, in varying degrees, hazardous substances in their processes. These substances include, among others, chlorinated solvents, and in the past chlorinated solvents were used at one or more of our facilities, including a number we no longer own or operate. As at our current facilities, contamination at such formerly owned or operated properties can result and has resulted in liability to us, for which we have recorded appropriate reserves as needed. We believe that our operations are in compliance in all material respects with the environment, health, and safety regulations applicable to our facilities.
ITEM 1A. RISK FACTORS
If any of the following risks actually occur, our business, financial condition, operating results, or cash flow could be materially and adversely affected. Additional risks or uncertainties not presently known to us, or that we currently believe are immaterial, may also impair our business operations.
Risks Relating to Our Business and the Industry in Which We Operate
Actions of activist shareholders could impact the pursuit of our business strategies and adversely affect our results of operations, financial condition, or share price.
We value constructive input from investors and regularly engage in dialogue with our shareholders regarding strategy and performance. Our board of directors and management team are committed to acting in the best interests of all shareholders. The actions taken by our board of directors and management in seeking to maintain constructive engagement with certain shareholders, however, may not be successful.
We have been, and may in the future be, subject to activities initiated by activist shareholders. In August 2023, we entered into a Cooperation Agreement (the “Cooperation Agreement”) with Elliott Investment Management L.P. (“Elliott”). Pursuant to the Cooperation Agreement, we appointed Steven Barg, Frank D’Amelio, Michelle Ryan, and Stephanie Okey as members of the Board, with an initial term expiring at the Company’s 2023 Annual Meeting of Shareholders.
We strive to maintain constructive, ongoing communications with all shareholders, including Elliott, and we welcome constructive input from all shareholders toward the shared goal of enhancing stakeholder value. Nonetheless, we may not be successful in engaging constructively with one or more shareholders, and any resulting activist campaign that contests, or seeks to change, our strategic direction or business mix could have an adverse effect on us because: (i) responding to actions by activist shareholders could disrupt our business and operations, be costly or time-consuming, or divert the attention of our board of directors or senior management from the pursuit of business strategies, which could adversely affect our results of operations or financial condition; (ii) perceived uncertainties as to our future direction may lead to the perception of a change in the direction of the business, instability, or lack of continuity, any of which may be exploited by our competitors, cause concern to our current or potential customers, cause concern in the minds of our employees and lead to the departure of critical employees, result in the loss of potential business opportunities, or make it more difficult to attract and retain qualified personnel and business partners; and (iii) these types of actions could cause significant fluctuations in our share price based on temporary or speculative market perceptions or other factors that do not necessarily reflect the underlying fundamentals and prospects of our business.
We anticipate being subject to increasing focus by our investors, regulators, customers, and other stakeholders on ESG matters.
Our investors, regulators, customers, and other stakeholders are increasingly focused on ESG matters. Certain investors, particularly institutional investors, and certain of our customers may use third-party benchmarks or scores to measure our ESG practices, and to decide whether to invest in our shares, engage with us regarding our practices, or engage or continue to use our services. If our ESG scores or practices do not meet desired standards, we may face reputational challenges. There can be no assurance that we will be able to accomplish any particular ESG goal or commitment, including any additional or revised commitment that we may announce in the future, as statements regarding such goals and commitments reflect our plans and aspirations at the time of announcement and do not guarantee achievement of such plans and aspirations within the timelines we announce or at all.
Different stakeholder groups have divergent views on ESG matters, which increases the risk that any action or lack thereof with respect to ESG matters will be perceived negatively by at least some stakeholders and adversely impact our reputation and business. Anti-ESG sentiment has gained some momentum across the United States, with several states having enacted or proposed “anti-ESG” policies or legislation, or issued related legal opinions. If we do not successfully manage ESG-related expectations across these varied stakeholder interests, it could erode stakeholder trust, impact our reputation, and constrain our business. Globally, a lack of harmonization in relation to ESG legal and regulatory reform across the jurisdictions in which we may operate may affect our future implementation of, and compliance with, rapidly developing ESG standards and requirements. Generally, we expect stakeholder demands and the prevailing legal environment to require us to devote additional resources to ESG matters in our review of prospective acquisitions. Additionally, collecting, measuring, and reporting ESG information and metrics can be costly, difficult, and time-consuming, are subject to evolving reporting standards, and can present numerous operational, reputational, financial, legal, and other risks. Compliance with ESG-related rules and efforts to meet investor expectations on ESG matters may place strain on our personnel, systems, and resources, and we may incur
significant compliance costs. Additionally, failure to comply with such rules or meet investor expectations may have a material adverse impact on our business, prospects, financial condition, or results of operations.
We are a part of the highly regulated healthcare industry, subject to stringent regulatory standards and other applicable laws and regulations, which can change unexpectedly or be the subject of unexpected changes in interpretation or enforcement, any of which may adversely impact our business.
The healthcare industry is highly regulated. We, and our customers, are subject to various local, state, federal, national, and transnational laws and regulations, which include the operating, quality, and security standards of the FDA, the DEA, various state boards of pharmacy, state health departments, the DHHS, similar bodies of the U.K., the E.U. and its member states, and other comparable agencies around the world, and, in the future, any change to such laws and regulations or the interpretation or application thereof could adversely affect us. Among other rules affecting us, we are subject to laws and regulations concerning cGMP and drug safety. New public health orders or best practice guidelines may increase our costs to operate or reduce our productivity, thereby affecting our business, financial condition, or results of operations.
We cannot ensure that our compliance controls, policies, and procedures will in every instance protect us from acts of our employees, agents, contractors, or collaborators that turn out to violate the laws or regulations of the jurisdictions in which we operate, including, without limitation, healthcare, employment, foreign corrupt practices, trade restrictions and sanctions, environmental, competition, and privacy laws and regulations. Failure by us or by our customers to comply with the requirements of applicable laws and regulations or requests from regulatory authorities could result in warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture or distribution, restrictions on our operations, civil or criminal sanctions, or withdrawal of existing or denial of pending approvals, permits, or registrations, including those relating to products or facilities. In addition, any such failure relating to the products or services we provide could expose us to contractual or product liability claims as well as claims from our customers, including claims for reimbursement for lost or damaged active pharmaceutical ingredients, which cost could be significant. Our business activities outside the U.S. are subject to the U.S. Foreign Corrupt Practices Act, the U.K. Anti-Bribery Act, and other anti-bribery or anti-corruption laws, regulations, or rules. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. There is no certainty that all of our employees, agents, contractors, or collaborators, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations may have a material adverse impact on our business, prospects, financial condition, or results of operations.
In addition, any new offering or product classified as a pharmaceutical or medical device must undergo lengthy and rigorous clinical testing and other extensive, costly, and time-consuming procedures mandated by the FDA, the EMA, and other equivalent local, state, federal, national, and transnational regulatory authorities in the jurisdictions that regulate our offerings and products.
Although we believe that we comply in all material respects with applicable laws and regulations, there can be no assurance that a regulatory agency or tribunal would not reach a different conclusion concerning the compliance of our operations with applicable laws and regulations. In addition, there can be no assurance that we will be able to maintain or renew existing permits, licenses, or other regulatory approvals or obtain, without significant delay, future permits, licenses, or other approvals needed for the operation of our businesses. Any noncompliance by us or our customers with applicable law or regulation or the failure to maintain, renew, or obtain necessary permits and licenses could have an adverse effect on our results of operations and financial condition. Furthermore, loss of a permit, license, or other approval in any one portion of our business may have indirect consequences in another portion of our business if regulators or customers adjust their reviews of such other portion as a result or customers cease business with such other portion due to fears that such loss is a sign of broader concerns about our ability to deliver products or services of sufficient quality.
Any failure to implement fully, monitor, and continuously improve our quality management strategy could lead to quality or safety issues and expose us to significant costs, potential liability, and adverse publicity.
Our results depend on our ability to execute and improve when necessary our quality management strategy and systems, and effectively train and maintain our workforce with respect to quality management. Quality management plays an essential role in determining and meeting customer requirements, preventing defects, and improving our offerings, and, despite our network of quality systems, a quality or safety issue, including with respect to a high-revenue product, could have an adverse effect on our business, financial condition, stock price, or results of operations and may subject us to regulatory action, including a product recall, product seizure, injunction to halt manufacture or distribution, or restriction on our operations; monetary fines; or other civil or criminal sanctions. In addition, such an issue could subject us to adverse publicity and costly
litigation, including claims from our customers for reimbursement for the cost of lost or damaged active pharmaceutical ingredients or other related losses, the cost of which could be significant.
We have experienced, and may continue to experience, productivity issues and higher-than-expected costs at certain of our facilities, which have resulted in, and may continue to result in, material and adverse impacts on our financial condition and results of operations.
In the fourth quarter of fiscal 2023, we announced that we experienced productivity issues at three of our facilities, including two of our largest manufacturing facilities in fiscal 2023, relating to, among other things, deployment of a new enterprise resource planning (ERP) system and continued need to implement enhancements to operational and engineering controls following regulatory inspections, which led to reductions in revenues and increases in costs at these sites in fiscal 2023. Our plans to increase capacity for a customer’s product at one of these sites did not move forward on schedule, and, due to manufacturing capacity constraints, revenue from the unproduced batches was not made up in fiscal 2023. There can be no assurance that such revenue will be recovered on expected timeframes or at all. In addition, we have experienced higher-than-expected costs at the three facilities. Although we have taken several measures at these facilities, including management and operational changes, there can be no assurance that such measures will successfully address the root causes of the issues identified at each site, that our costs will return to anticipated levels, or that productivity levels at these sites will return to normal in the expected timeframes or at all. If we are unsuccessful in remedying the productivity issues at our facilities, if we are unable to recover revenue from unproduced batches when expected or at all, or if our costs at our facilities remain elevated, we may continue to experience material and adverse impacts on our financial condition and results of operations. Furthermore, there can be no assurance that additional operational and productivity issues will not arise at these three sites, or that similar operational and productivity issues will not materialize in our other manufacturing facilities, which may result in material and adverse impacts on our financial condition and results of operations.
The declining demand for various COVID-19 vaccines and treatments from both patients and governments around the world has affected and may continue to affect sales of the COVID-19 products we manufacture and our financial condition.
We manufacture or provide services for a variety of products intended for the prevention or treatment of COVID-19 and its symptoms and effects, including both vaccines and treatments. Due to the substantially decreased demand for these products since the height of the COVID-19 pandemic, no single one of these products is currently material to our business. The duration and extent of future revenues from our development, testing, manufacturing, and packaging of COVID-19-related products is uncertain and dependent upon customer demand. As the COVID-19 pandemic evolved into an endemic phase, we anticipated greater seasonality for demand and a decreased patient population, which may result in overall lower demand for the COVID-19-related products we develop, test, manufacture, or package. The market for the COVID-19 vaccines we develop, test, manufacture, or package depends on several evolving factors that are outside of our control, including public health authority recommendations and consumer motivation to vaccinate.
Certain of the COVID-19-related products we develop and manufacture have not yet received full marketing approval from relevant regulatory authorities around the world or for certain patient populations. Should any of these COVID-19-related products be denied any necessary regulatory approval, the demand for such product could decrease significantly and therefore decrease customer orders for additional development, manufacturing, or packaging of those products. Additionally, the need for continued manufacture and supply of vaccines (including “booster” doses) and therapies to address COVID-19, including new and developing variants of COVID-19, is highly uncertain and subject to various political, economic, and regulatory factors that are outside of our control. In addition, highly public political and social debate relating to the need for, efficacy of, or side effects related to one or more specific COVID-19 vaccines could contribute to changes in public perception of one or more COVID-19 vaccines manufactured by us, which could decrease demand for a COVID-19 related products we develop, manufacture, or package. Any of these factors, or others, could lead to decreased demand for the COVID-19 related products we develop, manufacture, or package and, as a result, have an adverse effect on our financial results or financial condition.
The demand for our offerings depends in part on our customers’ research and development and the clinical and market success of their products. Our business, financial condition, and results of operations may be harmed if our customers spend less on, or are less successful in, these activities. In addition, customer spending may be affected by, among other things, recessionary economic conditions caused in whole or in part by lingering effects of the COVID-19 pandemic, the Ukrainian-Russian war, the war in Gaza between Israel and Hamas, higher interest rates, or the rise in inflation worldwide.
Our customers are engaged in research, development, production, and marketing of pharmaceutical, biotechnology, and consumer health products. The amount of customer spending on research, development, production, and marketing, as well as the outcomes of such research, development, and marketing activities, have a large impact on our sales and profitability,
particularly the amount our customers choose to spend on our offerings. Available resources, including funding for our biotechnology and other customers, the need to develop new products, and consolidation in the industries in which our customers operate may have an impact on such spending. Our customers and potential customers finance their research and development spending from private and public sources. A reduction in available financing for and spending by our customers, for these reasons or because of the direct or indirect lingering effects of the COVID-19 pandemic, inflation, higher interest rates, the Ukrainian-Russian war or other regional or global conflicts such as the war in Gaza, could have a material adverse effect on our business, financial condition, and results of operations. If our customers are not successful in attaining or retaining product sales due to market conditions, reimbursement issues, or other factors, our results of operations may be materially adversely affected.
We participate in a highly competitive market, and increased competition may adversely affect our business.
We operate in a market that is highly competitive. We compete with multiple companies as to each of our offerings and in every region of the globe in which we operate, including competing with other companies that offer advanced delivery technologies, outsourced dose form or biologics manufacturing, clinical trials support services, or development services to pharmaceutical, biotechnology, and consumer health companies globally. We also compete in some cases with the internal operations of those pharmaceutical, biotechnology, and consumer health customers that also have manufacturing capabilities and choose to source these services internally.
We face substantial competition in each of our markets. Competition is driven by proprietary technologies and know-how, capabilities, consistency of operational performance, quality, price, value, responsiveness, and speed. Some competitors have greater financial, research and development, operational, and marketing resources than we do. Competition may also increase as additional companies enter our markets or use their existing resources to compete directly with ours. Expanded competition from companies in low-cost jurisdictions, such as India and China, may in the future adversely affect our results of operations or limit our growth. Greater financial, research and development, operational, and marketing resources may allow our competitors to respond more quickly with strategic acquisitions, or with new, alternative, or emerging technologies. Changes in the nature or extent of our customers’ requirements may render our offerings obsolete or non-competitive and could adversely affect our results of operations and financial condition.
We are subject to product and other liability risks that could exceed our anticipated costs or adversely affect our results of operations, financial condition, liquidity, and cash flows.
We are subject to potentially significant product liability and other liability risks that are inherent in the design, development, manufacture, and marketing of our offerings. We may be named as a defendant in product liability lawsuits, which may allege that our offerings have resulted or could result in an unsafe condition or injury to consumers. Such lawsuits, even those without merit, could be costly to defend and could result in reduced sales, significant liabilities, adverse publicity, and diversion of management’s time, attention, and resources.
Furthermore, product liability claims and lawsuits, regardless of their ultimate outcome, could have a material adverse effect on our business operations, financial condition, and reputation and on our ability to attract and retain customers. The availability of product liability insurance for companies in the pharmaceutical industry is generally more limited than insurance available to companies in other industries. We maintain product liability insurance with annual aggregate limits in excess of $25 million. There can be no assurance that a successful product liability or other claim would be adequately covered by our applicable insurance policies or by any applicable contractual indemnity or liability limitations.
Our business, financial condition, and results of operations may be adversely affected by global health epidemics.
Any public health epidemic, such as the COVID-19 pandemic, may affect our operations and those of third parties on which we rely, including our customers and suppliers. Our business, financial condition, and results of operations may be affected by: disruptions in our customers’ abilities to fund, develop, or bring to market products as anticipated; delays in or disruptions to the conduct of clinical trials; cancellations of contracts or confirmed orders from our customers; decreased demand for categories of products in certain affected regions; governmental restrictions imposed to respond to the risks posed by any such epidemic; and inability, difficulty, or additional cost or delays in obtaining key raw materials, components, and other supplies from our existing supply chain; among other factors caused by a public health epidemic.
In addition, the impact of a public health epidemic could exacerbate other risks we face, including those described elsewhere in “Risk Factors.”
The services and offerings we provide are highly exacting and complex, and, if we encounter problems providing the services or support required, our business could suffer.
The offerings we provide are highly exacting and complex, due in part to complex and exacting manufacturing processes and strict regulatory requirements. From time to time, problems may arise in connection with facility operations or during preparation or provision of an offering, in both cases for a variety of reasons including, but not limited to, equipment malfunction, sterility variances or failures, failure to follow specific protocols and procedures, problems with raw materials, environmental factors, and damage to, or loss of, manufacturing operations due to fire, flood, or similar causes. Such problems could affect production of a particular batch or series of batches, require the destruction of or otherwise result in the loss of product or materials used in the production of product, or could halt facility production altogether. This could, among other things, lead to increased costs, lost revenue, damage to customer relations, reimbursement to customers for lost active pharmaceutical ingredients or other related losses, time and expense spent investigating the cause, lost production time, and, depending on the cause, similar losses with respect to other batches or products. Production problems in our biologic manufacturing operations could be particularly significant because the cost of raw materials is often appreciably higher than in our other businesses. If problems are not discovered before the product is released to the market, recall and product liability costs may also be incurred. In addition, such risks may be greater at facilities that are new or going through significant expansion or renovation. The risks associated with running a highly complex facility doing exacting work with substantial regulatory oversight are enhanced for our larger sites, like our Bloomington, Indiana, Harmans, Maryland, St. Petersburg, Florida, or Swindon, U.K. sites, which generally generate much more revenue.
If we cannot keep pace with rapid technological advances, our services may become uncompetitive or obsolete, and our revenue and profitability may decline.
The healthcare industry is characterized by rapid technological change. Demand for our offerings may change in ways we may not anticipate because of evolving industry standards as well as a result of evolving customer needs that are increasingly sophisticated and varied and the introduction by others of new offerings and technologies that provide alternatives to our offerings. Several of our higher margin offerings are based on proprietary technologies. To the extent that such technologies are protected by patents, their related offerings may become subject to competition as the patents expire. Without the timely introduction of enhanced or new offerings and technologies, our offerings may become obsolete or uncompetitive over time, in which case our revenue and operating results would suffer. For example, if we are unable to respond to changes in the nature or extent of the technological or other needs of our pharmaceutical customers through enhancing our offerings, our competition may develop offerings that are more competitive than ours and we could find it more difficult to renew or expand existing agreements or obtain new agreements. Potential innovations intended to facilitate enhanced or new offerings generally will require a substantial investment before we can determine their commercial viability, and we may not obtain access to the innovations or have financial resources sufficient to fund all desired innovations.
Even if we succeed in creating or acquiring enhanced or new offerings from these innovations, they may still fail to result in commercially successful offerings or may not produce revenue in excess of the costs of development, and they may be rendered obsolete by changing customer preferences or the introduction by our competitors of offerings embodying new technologies or features. Finally, innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice, the need for regulatory clearance, and uncertainty over market access or government or third-party reimbursement.
Any failure to protect or maintain our intellectual property may adversely affect our competitive edge that we hold and result in loss of revenue or reputation.
We rely on a combination of know-how, trade secrets, patents, copyrights, trademarks, and other intellectual property laws, nondisclosure and other contractual provisions, and technical measures to protect many of our offerings and intangible assets. These proprietary rights are important to our ongoing operations. There can be no assurance that these protections will provide uniqueness or meaningful competitive differentiation in our offerings or otherwise be commercially valuable or that we will be successful in obtaining additional intellectual property or enforcing our intellectual property rights against unauthorized users. The exclusive rights underlying certain of our offerings are protected by patents, some of which will expire in the near term. When patents covering an offering expire, loss of exclusivity may occur, which may force us to compete with third parties, thereby negatively affecting our revenue and profitability.
The proprietary rights that we or our customers may hold in these offerings may be invalidated, circumvented, or challenged. We may in the future be subject to proceedings seeking to oppose or limit the scope of our patent applications or issued patents. In addition, in the future, we may need to take legal actions to enforce our intellectual property rights, to protect our trade secrets, or to determine the validity or scope of the proprietary rights of others. Legal proceedings are inherently uncertain, and the outcome of such proceedings may be unfavorable to us. Any legal action regardless of outcome might result in substantial costs and diversion of resources and management attention.
There can be no assurance that our confidentiality agreements will not be breached, our trade secrets will not otherwise become known by competitors, or that we will have adequate remedies in the event of unauthorized use or disclosure of proprietary information. Even if the validity and enforceability of our intellectual property is upheld, an adjudicator might construe our intellectual property not to cover the alleged infringement. In addition, intellectual property enforcement may be unavailable or practically ineffective in some countries. There can be no assurance that our competitors will not independently develop technologies that are substantially equivalent or superior to our proprietary technology or that third parties will not design around our intellectual property claims to produce competitive offerings. The use of our technology or similar technology by others could reduce or eliminate any competitive advantage we have developed, cause us to lose sales, or otherwise harm our business.
While we continue to apply in the U.S. and certain other countries for registration of a number of trademarks, service marks, and patents, and also claim common law rights in various trademarks and service marks, there can be no assurance that third parties will not oppose our applications in the future. In addition, it is possible that in some cases we may be unable to obtain the registrations for trademarks, service marks, and patents for which we have applied, and a failure to obtain trademark and patent registrations in the U.S. or other countries could limit our ability to protect our trademarks and proprietary technologies and impede our marketing efforts in those jurisdictions.
License agreements with third parties control our rights to use certain patents, software, and information technology systems and proprietary technologies owned by third parties, some of which are important to our business. Termination of these license agreements for any reason could result in the loss of our rights to this intellectual property, causing an adverse change in our operations or the inability to commercialize certain offerings.
In addition, many of our branded pharmaceutical customers rely on patents to protect their products from generic competition. Because incentives exist in some countries, including the U.S., for generic pharmaceutical companies to challenge these patents, pharmaceutical and biotechnology companies are under the ongoing threat of challenges to their patents. If the patents on which our customers rely were successfully challenged and, as a result, the affected products become subject to generic competition, the market for our customers’ products could be significantly adversely affected, which could have an adverse effect on our results of operations and financial condition. We attempt to mitigate these risks by making our offerings available to generic as well as branded manufacturers and distributors, but there can be no assurance that we will be successful in marketing these offerings.
Our offerings or our customers’ products may infringe on the intellectual property rights of third parties.
From time to time, third parties have asserted intellectual property infringement claims against us and our customers, and there can be no assurance that third parties will not assert infringement claims against either us or our customers in the future. While we believe that our offerings do not infringe in any material respect upon proprietary rights of other parties, and that meritorious defenses would exist with respect to any assertion to the contrary, there can be no assurance that we could successfully avoid being found to infringe on the proprietary rights of others. Patent applications in the United States and certain other countries are generally not publicly disclosed until the patent is issued or published, and we and our customers may not be aware of currently filed patent applications that relate to our or their products, offerings, or processes. If patents later issue on these applications, we or they may be found liable for subsequent infringement. There has been substantial litigation in the pharmaceutical and biotechnology industries with respect to the manufacture, use, and sale of products that are the subject of conflicting patent rights.
Any claim that our offerings or processes infringe third-party intellectual property rights (including claims arising through our contractual indemnification of our customers), regardless of the claim’s merit or resolution, could be costly and may divert the efforts and attention of our management and technical personnel. We may not prevail against any such claim given the complex technical issues and inherent uncertainties in intellectual property matters. If any such claim results in an adverse outcome, we could, among other things, be required to: pay substantial damages (potentially including treble damages in the U.S.); cease the manufacture, use, or sale of the infringing offerings or processes; discontinue the use of the infringing technology; expend significant resources to develop non-infringing technology; license technology from the third party claiming infringement, which license may not be available on commercially reasonable terms or at all; and lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property against others.
In addition, our customers’ products may be subject to claims of intellectual property infringement and such claims could materially affect our business if their products cease to be manufactured or they have to discontinue the use of the infringing technology.
Any of the foregoing could affect our ability to compete or have a material adverse effect on our business, financial condition, and results of operations.
Events that diminish, tarnish, or otherwise damage our brand may have an adverse effect on our future financial condition and results of operations.
We have built a strong brand in “Catalent,” with high overall and generally favorable awareness of the brand in our established markets and with target customers. Our brand identity is a competitive advantage for us in sales and marketing, which is evidenced by our customer mix among top branded drug, generics, biologics, and consumer health marketers. We have spent and continue to spend substantial time, money, and other resources to establish both our brand awareness and a favorable perception of our brand in relevant markets. Among other strategies, we participate in major international trade shows in our established markets and ensure visibility into our offerings through a comprehensive print and on-line advertising and publicity program. It is possible that a single event, or aggregation of several events, may diminish, tarnish, or otherwise damage our brand and adversely affect our future financial condition and results of operations.
For example, meaningful interruptions to our ability to reliably supply one or more customers with products on time, whether as a result of supply chain disruptions, manufacturing delays or defects, or the need to address regulatory requirements at our facilities, may diminish our customers’ confidence in our ability to timely meet our commitments, thereby damaging our brand. In addition, we are subject to various local, state, federal, national, and transnational laws and regulations, including the operating, quality, and security standards of the FDA, the DEA, and similar bodies of the U.K., the E.U., and other comparable agencies around the world. Highly public or significant negative reports or findings from a regulatory agency with respect to one or more manufacturing or quality defects in our operations, inspections of our facilities, or other routine reviews could cause negative public perception of our operations, negatively impacting our brand, and adversely affecting our financial condition and results of operations. In addition, many of the other risks we face, including those described elsewhere in “Risk Factors” could diminish, tarnish, or otherwise damage our brand.
Our results of operations are subject to fluctuations in the costs, availability, and suitability of the components of the products we manufacture, including active pharmaceutical ingredients, excipients, purchased components, and raw materials, and other supplies or equipment we need to run our business.
We depend on various active pharmaceutical ingredients, components, compounds, raw materials, and energy supplied primarily by third parties for our offerings. Our customers also frequently provide us with their active pharmaceutical or biologic ingredient for formulation or incorporation in the finished product and may supply other raw materials as well. It is possible that any of our or our customers’ supplier relationships could be interrupted due to changing regulatory requirements, import or export restrictions, natural disasters, international supply disruptions, including those caused by public health emergencies, wars, geopolitical issues, operational or quality issues at the suppliers’ facilities, and other events, or could be terminated in the future.
For example, gelatin, a critical component for manufacturing many of our softgel formats is only available from a limited number of sources. In addition, much of the gelatin we use is bovine-derived. Past concerns of contamination from bovine spongiform encephalopathy have narrowed the number of possible sources of particular types of gelatin. If there were a future disruption in the supply of gelatin or any other key raw material used to manufacture our products, we may not be able to obtain an adequate alternative supply. If future restrictions or other developments limit our ability to obtain a key material, any such restriction or development could hinder our ability to timely supply our customers with products, and the use of alternative material could be subject to lengthy and uncertain formulation, testing, and regulatory approval.
In addition, certain of our inputs are currently sole-sourced, so any disruption related to such a supplier is more likely to have an impact on our operations. Replacing a sole-source supplier of a production input to a medicine requiring marketing approval may be impossible or time-consuming, due to the rigorous standards we are obliged to apply to any new supplier.
Any sustained interruption in our receipt of adequate supplies could have an adverse effect on our business and results of operations. In addition, while we have processes intended to reduce volatility in component and material pricing, we may not be able to successfully manage price fluctuations, and future price fluctuations or shortages may have an adverse effect on our results of operations.
Our goodwill has been subject to impairment and may be subject to further impairment in the future, which could have a material adverse effect on our results of operations, financial condition, or future operating results.
We perform an annual goodwill impairment test for each reporting unit on April 1, or more frequently if indicators for potential impairment exist. Indicators that are considered include significant changes in performance relative to expected operating results, significant negative industry or economic trends, or a significant decline in our stock price and/or market capitalization for a sustained period of time. In addition, we assess the current and future economic outlook for our reporting units in our Pharma and Consumer Health and Biologics segments during the fiscal year. While we believe the assumptions used in determining whether there was impairment and the amount of any resulting impairment were reasonable and commensurate with the views of a market participant, changes in key assumptions in the future, including increasing the discount rate, lowering forecasts for revenue and operating margin, or lowering the long-term growth rate, could result in additional charges; similarly, one or more changes in these assumptions in future periods due to changes in circumstances could result in future impairments in this reporting unit or other reporting units. We have incurred impairment charges in the past, and we cannot predict if or when additional future goodwill impairments may occur. For example, for the three months ended March 31, 2023, we recorded a goodwill impairment charge of $210 million in the Consumer Health reporting units within our Pharma and Consumer Health segment. In addition, for the three months ended September 30, 2023, we recorded goodwill impairment charges of $689 million associated with the Consumer Health and Biomodalities reporting units in our Pharma and Consumer Health and Biologics segments, respectively. Any goodwill impairments could have material adverse effects on our operating income, net assets, or our cost of, or access to, capital, which could harm our business. See Note 4, Goodwill and Note 20, Subsequent Events, “Impairment of Goodwill” to our consolidated financial statements as of and for the fiscal year ended June 30, 2023 (our “Consolidated Financial Statements”) for more details.
Changes in market access or healthcare reimbursement for, or public sentiment towards our customers’ products in the U. S. or internationally, or other changes in applicable policies regarding the healthcare industry, could adversely affect our results of operations and financial condition by affecting demand for our offerings.
The healthcare industry has changed significantly over time, and we expect the industry to continue to evolve. Some of these changes, such as ongoing healthcare reform, including with respect to reforming drug pricing, adverse changes in governmental or private funding of healthcare products and services, legislation or regulations governing patient access to care and privacy, or the delivery, pricing, or reimbursement approval of pharmaceuticals and healthcare services or mandated benefits, may cause healthcare industry participants to change the amount of our offerings that they purchase or the price they are willing to pay for these offerings. In particular, it is possible that future legislation in the U.S. may affect or put a cap on future pricing of pharmaceutical and biotechnology products. While we are unable to predict the likelihood of changes to U.S. and other international laws affecting pharmaceutical and biotechnology products, any substantial revision of applicable healthcare legislation could have a material adverse effect on the demand for our customers’ products, which in turn could have a negative impact on our results of operations, financial condition, or business. Changes in the healthcare industry’s pricing, selling, inventory, distribution, or supply policies or practices, or in public or government sentiment for the industry as a whole, could also significantly reduce our revenue and results of operations. In particular, volatility in individual product demand may result from changes in public or private payer reimbursement or coverage.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have generated net operating losses (“NOLs”) (and acquired affiliates with pre-existing NOLs) or certain other tax attributes that have been, and continue to be, used to reduce taxable income. In the case of our NOL carryforwards (and new NOLs that may arise), they may be subject to a substantial limitation under Section 382 of the Internal Revenue Code of 1986, as amended (the “Internal Revenue Code”), and comparable provisions of state, local, and foreign tax laws due to changes in ownership of our company that may occur in the future. Under Section 382 of the Internal Revenue Code and comparable provisions of state, local, and foreign tax laws, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership over a three-year period, the corporation’s ability to carry forward its pre-change NOLs to reduce its post-change income may be limited. In addition, we acquired companies that generated pre-acquisition NOLs for tax purposes that will also be subject to limitation under Section 382 and comparable provisions of state, local, and foreign tax laws. We may experience ownership changes in the future because of future changes in our stock ownership. As a result, our ability to use NOL carryforwards to reduce U.S. federal, state, local, and foreign taxable income we produce in the future years may be subject to limitations, which could result in increased future tax liability to us.
Changes to the estimated future profitability of the business may require that we establish an additional valuation allowance against all or some portion of our net deferred tax assets.
We have deferred tax assets for NOL carryforwards, certain other tax attributes, and other temporary differences. We currently maintain a valuation allowance for a portion of our U.S. net deferred tax assets and certain foreign net deferred tax assets. It is possible we may experience a decline in U.S. and foreign taxable income resulting from a decline in profitability of our relevant operations, an increased level of debt in the U.S., or other factors. In assessing our ability to realize our deferred
tax assets, we may conclude that it is more likely than not that some additional portion or all our deferred tax assets will not be realized. As a result, we may be required to record an additional valuation allowance against our deferred tax assets, which could adversely affect our effective income tax rate and therefore our financial results.
We depend on key personnel, and, if we are unable to attract, retain and motivate well-qualified employees, our business could be harmed.
We depend on our executive officers and other key personnel, including our technical personnel, to operate and grow our business and to develop new and enhanced offerings and technologies. The loss of any of these officers or other key personnel or a failure to attract and retain suitably skilled technical personnel could adversely affect our operations. In addition to our executive officers, we rely on approximately 170 senior employees to lead and direct our business. Our senior leadership team is comprised of our subsidiaries’ executive officers and other vice presidents and directors who hold critical positions and possess specialized talents and capabilities that give us a competitive advantage in the market. Any change in our senior leadership team in particular, even in the ordinary course of business, may be disruptive to our business. While we seek to manage these transitions carefully, such changes may result in a loss of institutional knowledge and cause disruptions to our business and new executive hires may fail to achieve any anticipated benefits. If our senior leadership team fails to work together effectively or execute our plans and strategies on a timely basis as a result of management turnover or otherwise, our business could be harmed. In addition, we employ more than 3,000 scientists and technicians whose areas of expertise and specialization cover subjects such as advanced delivery, biologics and gene and cell therapy formulation and manufacturing. Many of our sites and laboratories are located in competitive labor markets; therefore, global and regional competitors and, in some cases, customers and suppliers compete for the same skills and talent as we do. If we are unable to hire and retain sufficient qualified employees, our ability to conduct and expand our business could be meaningfully reduced.
We may acquire businesses and offerings that complement or expand our business or divest non-strategic businesses or assets. We may not be able to complete desired transactions, and such transactions, if executed, pose significant risks, including risks relating to our ability to successfully and efficiently integrate acquisitions or execute on dispositions and realize anticipated benefits therefrom. The failure to execute or realize the full benefits from any such transaction could have a negative effect on our operations and profitability.
Our future success may depend in part on opportunities to buy or otherwise acquire rights to other businesses or technologies, enter into joint ventures or otherwise enter into strategic arrangements with business partners that could complement, enhance, or expand our current business or offerings and services or that might otherwise offer us growth opportunities, or divest assets or an ongoing business. We face competition from other companies in pursuing acquisitions and similar transactions in the pharmaceutical and biotechnology industry. Our ability to complete transactions may also be limited by applicable antitrust and trade laws and regulations in the U.S. and other jurisdictions in which we or the operations or assets we seek to acquire carry on business. To the extent that we are successful in making acquisitions, we expend substantial amounts of cash, incur debt, or assume loss-making divisions as consideration. We or the purchaser of a divested asset or business may not be able to complete a desired transaction for any number of reasons, including a failure to secure financing.
Any acquisition that we are able to identify and complete may involve a number of risks, including, but not limited to, the diversion of management’s attention to integrate the acquired businesses or joint ventures, the possible adverse effects on our operating results during the integration process, the potential loss of customers or employees in connection with the acquisition, delays or reduction in realizing expected synergies, unexpected liabilities, and our potential inability to achieve our intended objectives for the transaction. In addition, we may be unable to maintain uniform standards, controls, procedures, and policies, which may lead to operational inefficiencies.
To the extent that we are not successful in completing desired divestitures, we may have to expend cash, incur debt, or continue to absorb the costs of loss-making or under-performing divisions. Any divestiture, whether we complete it or not, may involve numerous risks, including diversion of management’s attention, a negative impact on our customer relationships, costs associated with maintaining its business during the disposition process, and the costs of closing and disposing of the affected business or transferring remaining portions of the operations of the business to other facilities.
We provide services incorporating various advanced modalities, including protein and plasmid production and cell and gene therapies, and these modalities relate to relatively new modes of treatment that may be subject to changing public opinion, continuing research, and increased regulatory scrutiny, each of which may affect our customers’ abilities to conduct their businesses or obtain regulatory approvals for their therapies, and thereby adversely affect these offerings.
Cell and gene therapy, with or without the use of iPSCs or plasmids, remain relatively new means for treating disease and other medical conditions, with only a few cell and gene therapies approved to date in the U.S., the E.U., or elsewhere. Public
perception may be influenced by claims that cell or gene therapies are unsafe, and cell or gene therapy may not gain the acceptance of the public or the medical community. In addition, ethical, social, legal, and cost-benefit concerns about cell or gene therapy, genetic testing, genetic research, and the use of stem cells or materials derived from viruses could result in additional regulations or limitations or even outright prohibitions on certain cell or gene therapies or related products. Various regulatory and legislative bodies have expressed an interest in, or have taken steps towards, further regulation of various biotechnologies, including cell and gene therapies. More restrictive regulations or claims that certain cell or gene therapies are unsafe or pose a hazard could reduce our customers’ use of our services. We can provide no assurance whether legislative changes will be enacted, regulations, policies, or guidance changed, or interpretations of existing strictures by agencies or courts changed, or what the impact of such changes, if any, may be.
We may become subject to litigation, other proceedings, and government investigations relating to us or our operations, and the ultimate outcome of any such matter may have an impact on our business, prospects, financial condition, and results of operations.
We may become subject to litigation or government investigations in the U.S and foreign jurisdictions that may arise from the conduct of our business. We generally intend to defend ourselves vigorously against any litigation proceeding or government investigation; however, we cannot be certain of the ultimate outcomes of any legal proceedings or investigations that may arise in the future. Resolution of these types of matters against us may result in, among other things, the payment of significant fines, judgments, penalties or settlements, the imposition of administrative remedies, changes and additional costs to our business operations to avoid risks associated with such litigation or investigations, reputational damage and decreased demand for our products, and the expenditure of significant time and resources that would otherwise be available for operating our business, all of which may have an impact on our business, prospects, financial condition, or results of operations.
We are subject to environmental, health, and safety laws and regulations, which could increase our costs or restrict our operations in the future.
Our operations are subject to a variety of environmental, health, and safety laws and regulations, including those of the EPA, OSHA, and equivalent local, state, and national regulatory agencies in the jurisdictions in which we operate. Any failure by us to comply with environmental, health, and safety requirements could result in the limitation or suspension of production or subject us to monetary fines, civil or criminal sanctions, or other future liabilities in excess of our reserves. In particular, we are subject to laws and regulations governing the destruction and disposal of raw materials, byproducts of our manufacturing operations, and non-compliant products, the handling of regulated material included in our offerings, and the disposal of our products or their components at the end of their useful lives. In addition, compliance with environmental, health, and safety requirements could restrict our ability to expand our facilities or require us to acquire costly environmental or safety control equipment, incur other significant expenses, or modify our manufacturing processes. Our manufacturing facilities may use, in varying degrees, hazardous substances in their processes. These substances include, among others, chlorinated solvents, and in the past chlorinated solvents were used at one or more of our facilities, including a number we no longer own or operate. As at our current facilities, contamination at such formerly owned or operated properties can result and has resulted in liability to us. In the event of the discovery of new or previously unknown contamination either at our facilities, facilities we acquire in the future, or at third-party locations, including facilities we formerly owned or operated, the issuance of additional requirements with respect to existing contamination, or the imposition of other cleanup obligations for which we are responsible, we may be required to take additional, unplanned remedial measures for which we have not recorded reserves. We are conducting monitoring and cleanup of contamination at certain facilities currently or formerly owned or operated by us, and such activities may result in unanticipated costs or management distraction.
We are subject to labor and employment laws and regulations, which could increase our costs and restrict our operations in the future.
We have nearly 17,800 individuals providing services for us worldwide, including approximately 10,500 service providers in North America, 5,700 in Europe, 1,000 in South America, and 600 in the Asia-Pacific region. Certain employees at one of our North American facilities are represented by a labor organization, and national works councils or labor organizations are active at our European facilities and certain of our other facilities consistent with local labor environments and laws. Our management believes that our employee relations are satisfactory. However, further organizing activities, collective bargaining, or changes in the regulatory framework for employment may increase our employment-related costs or may result in work stoppages or other labor disruptions. Moreover, as employers are subject to various employment-related claims, such as individual and class actions relating to alleged employment discrimination and wage-hour and labor standards issues, such actions, if brought against us and successful in whole or in part, may affect our ability to compete or have a material adverse effect on our business, financial condition, and results of operations.
We have partnered with, and may continue to partner with, companies that focus on the development of cannabis-based prescription medicines and cannabinoid drug therapies solely to the extent such companies’ programs comply with all U.S. and non-U.S. equivalent laws, which is a business that attracts a high-level of public and media interest and an industry in which laws and regulations are constantly evolving.
We have partnered with, and may continue to partner with, companies that focus on the development of cannabis-based prescription medicines and high-value cannabinoid drug therapies, which may attract a high-level of public and media interest, and in the event of any resultant adverse publicity, our reputation may be harmed. In addition, the constant evolution of laws and regulations affecting the research and development of cannabinoid-based pharmaceutical products and treatments could detrimentally affect our business. Laws and regulations related to the therapeutic uses of cannabinoids are subject to changing interpretations. These changes may require us to incur costs associated with legal and compliance fees and ultimately require us to alter our business plan. Furthermore, violations or alleged violation of these laws could disrupt our business and result in a material adverse effect on our operations. We cannot predict the nature of any future laws, regulations, interpretations or applications of laws and regulations and it is possible that new laws and regulations may be enacted in the future that will be directly applicable to our business. In addition, regulatory approval of product candidates that contain controlled substances may generate public controversy or scrutiny. Adverse publicity from misuse or adverse side effects of cannabis-based prescription medicines may adversely affect the commercial success or market penetration achievable by such product candidates which could result in an adverse effect on our operations.
Certain of our pension plans are underfunded, and additional cash contributions we may make to increase the funding level will reduce the cash available for our business, such as the payment of our interest expense.
Certain of our current and former employees in the U.S., the U.K., Germany, France, Japan, Belgium, and Switzerland are participants in defined benefit pension plans that we sponsor. As of June 30, 2023, the underfunded amount of our pension plans on a worldwide basis was $44 million, primarily related to our pension plans in the U.K. and Germany. In addition, we have an estimated obligation of $38 million, as of June 30, 2023, related to our withdrawal from a multiemployer pension plan in which we formerly participated. In general, the amount of future contributions to the underfunded plans will depend upon asset returns, applicable actuarial assumptions, prevailing and expected interest rates, and other factors, and, as a result, the amount we may be required to contribute in the future to fund the obligations associated with such plans may vary. Such cash contributions to the plans will reduce the cash available for our business, including the funds available to pursue strategic growth initiatives or the payment of interest expense on our indebtedness.
Our global operations are subject to economic and political risks, including risks resulting from continuing inflation, disruptions to global supply chains, destabilization of a regional or national banking system, from the Ukrainian-Russian war, or the effect of the evolving nature of the recent war in Gaza between Israel and Hamas, which could affect the profitability of our operations or require costly changes to our procedures.
We conduct our operations in various regions of the world, including North America, South America, Europe, and the Asia-Pacific region. Global and regional economic and political developments affect businesses such as ours in many ways. Our operations are subject to the effects of global and regional competition. Our global operations are also affected by local economic environments, including inflation, recession, and changes to the availability of capital our customers may need to continue or expand their business with us. Political changes, some of which may be disruptive, and related hostilities can interfere with our supply chain, our customers, and some or all of our activities in a particular location. While some of these risks can be hedged using derivatives or other financial instruments and some are insurable, such mitigating measures may be unavailable, costly, or unsuccessful.
Beginning in fiscal 2022, much of the world, including the U.S. and the E.U., began to experience inflation levels not seen in more than 30 years. As a result, prices for many of our inputs have risen, in some cases dramatically. If inflation stays at elevated levels or increases, we may not be able to mitigate the impact of the increased costs we will bear through corresponding price increases to our customers, which could have an impact on our results of operations and financial condition.
The outbreak of hostilities between Israel and Hamas has the potential for further disruption of economic markets, particularly if the war expands to include other state actors. The Company has no operations in the Middle East at the current time. However, events there could result in political turmoil in Europe, which could directly affect our operations there, and could also adversely affect the business that we conduct with customers in the Middle East and other parts of the world. Also, the turmoil in the Middle East could have global economic effects that are the same as or more severe than those of the war in the Ukraine, with similar consequences for our business.
As a global enterprise, fluctuations in the exchange rates of the U.S. dollar, our reporting currency, against other currencies could have a material adverse effect on our financial performance and results of operations.
As a company with significant operations outside of the U.S., certain revenues, costs, assets, and liabilities, including our euro-denominated 2.375% Senior Notes due 2028 (the “2028 Notes”), are denominated in currencies other than the U.S. dollar, which is the currency that we use to report our financial results. As a result, changes in the exchange rates of these or any other applicable currency to the U.S. dollar will affect our revenues, earnings, and cash flows. There has been, and may continue to be, volatility in currency exchange rates affecting the various currencies in which we do business. Such volatility and other changes in exchange rates could result in unrealized and realized exchange losses, despite any effort we may undertake to manage or mitigate our exposure to fluctuations in the values of various currencies.
Tax legislative or regulatory initiatives, new interpretations or developments concerning existing tax laws, or challenges to our tax positions could adversely affect our results of operations and financial condition.
We are a large multinational enterprise with operations in the U.S. and more than a dozen other countries across North and South America, Europe, and the Asia-Pacific region, and we do business with suppliers and customers in many additional regions. As such, we are subject to the tax laws and regulations of the U.S. federal, state, and local governments and of many jurisdictions outside of the U.S. From time to time, various legislative initiatives may be proposed that could adversely affect our tax positions, and existing legislation may be subject to additional regulatory changes or new interpretations. There can be no assurance that our effective tax rate or tax payments will not be adversely affected by these initiatives.
In addition, U.S. federal, state, local, and foreign tax laws and regulations are extremely complex and subject to varying interpretations. We are subject to regular examination of our income tax returns by various tax authorities. Examinations or changes in laws, rules, regulations, or interpretations by taxing authorities could result in adverse impacts to tax years open under statute or to our operating structures currently in place. It is possible that the outcomes from these examinations or changes in laws, rules, regulations, or interpretations by taxing authorities will have a material adverse effect on our financial condition or results of operations.
We use advanced information and communication systems to run our operations, compile and analyze financial and operational data, and communicate among our employees, customers, and counterparties, and the risks generally associated with information and communications systems could adversely affect our results of operations. We continuously work to install new, and upgrade existing, systems and provide employee awareness training around phishing, malware, and other cyber security risks to enhance the protections available to us, but such protections may be inadequate to address malicious attacks or inadvertent compromises affecting data security or the operability of such systems.
We rely on information systems in our business to obtain, process, analyze, and manage data to:
•facilitate the manufacture and distribution of thousands of inventory items in, to, and from our facilities;
•receive, process, and ship orders on a timely basis;
•manage the accurate billing and collections for more than one thousand customers;
•create, compile, and retain testing and other product-, manufacturing-, or facility-related data necessary for meeting our and our customers’ regulatory obligations.
•manage the accurate accounting and payment for thousands of vendors and our employees;
•schedule and operate our global network of development, manufacturing, and packaging facilities;
•document various aspects of our activities, including the agreements we make with suppliers and customers;
•compile financial and other operational data into reports necessary to manage our business and comply with various regulatory or contractual obligations, including obligations under our bank loans and other indebtedness, the federal securities laws, the Internal Revenue Code, and other applicable state, local, and ex-U.S. tax laws; and communicate among our nearly 19,000 workers spread across dozens of facilities over four continents.
We face various security threats on a regular basis, including ongoing cyber security threats to and attacks on our information technology infrastructure. We deploy defenses against such threats and attacks and work to secure the integrity of our data systems using techniques, hardware, and software typical of companies of our size and scope. Despite our security measures, however, our information technology and infrastructure may be vulnerable to attacks by increasingly sophisticated intruders or others who try to cause harm to or interfere with our normal use of our systems. They are also susceptible to breach due to employee error, malfeasance, or other disruptions. Our suppliers, contractors, service providers, and other third parties with whom we do business also experience cyber threats and attacks that are similar in frequency and sophistication. In many cases, we have to rely on the controls and safeguards put in place by our suppliers, contractors, service providers, and other third parties to defend against, respond to, and report these attacks. We cannot know the potential impact of future cyber incidents, which vary widely in severity and scale. There can be no assurance that the various procedures and controls we
utilize to mitigate these threats will be sufficient to prevent disruptions to our systems, in part because (i) cyber-attack techniques change frequently and, at times, new techniques are not recognized until launched, and (ii) cyber-attacks can originate from a wide variety of sources. Our results of operations could be adversely affected if these systems are interrupted or damaged or fail for any extended period.
Efforts by governments around the world or our customers to secure or promote the benefits of locally produced supplies, as well as other risks associated with foreign operations, may render the locations of certain of our facilities less desirable, affecting their utilization rates and therefore our profitability, financial condition, or results of operations.
We serve more than 1,200 customers in more than 80 countries, with 35% of our fiscal 2023 net revenue coming from outside the U.S., and we operate facilities in more than a dozen U.S. states and more than a dozen countries outside the U.S. The global nature of our sales and operations subjects us to risks, including risks arising from efforts by governments around the world or our customers to secure or promote the benefits of locally produced supplies, higher import duties in some countries that may favor locally produced supplies, the differing impacts of varying economic conditions in different jurisdictions, changes in tariffs and trade relations, unexpected changes in regulatory requirements, certification requirements, environmental regulations, reduced protection for intellectual property rights in some countries, potentially adverse tax consequences, and political and economic instability. If one or more of these risks is realized, it could have a material adverse impact on our utilization rates for certain of our facilities, and therefore our profitability, financial condition, or results of operations.
Artificial intelligence-based platforms present new risks and challenges to our business.
Artificial intelligence, or AI, based platforms are increasingly being used in the biopharmaceutical, pharmaceutical, and consumer health industries. We are committed to providing a safe and secure environment for our personnel, our business partners, and our customers, including the responsible use of AI chatbots and generative AI data processor products (“AI Systems”). We have developed policies governing the use of AI Systems to help reasonably ensure that such AI Systems are used in a trustworthy manner by our employees, contractors, and authorized agents and that our assets, including intellectual property, competitive information, personal information we may collect or process, and customer information, are protected. Any failure by our personnel, contractors, or other agents to adhere to our established policies could violate confidentiality obligations or applicable laws and regulations, jeopardize our intellectual property rights, cause or contribute to unlawful discrimination, or result in the misuse of personally identifiable information or the injection of malware into our systems, any of which could have a material adverse effect on our business, results of operations, and financial condition.
The use of AI Systems by our business partners with access to our confidential information, including trade secrets, may continue to increase and could lead to the release of such information, which could negatively impact us, including our ability to realize the benefits of our intellectual property. The use of AI Systems by our business partners may lead to novel and urgent cybersecurity risks, which could have a material adverse effect on our operations and reputation as well as the operations of any of our business partners. We may also face increased competition from other companies that are using AI Systems, some of whom may develop more effective methods than we and any of our business partners have, which could have a material adverse effect on our business, results of operations, or financial condition. In addition, uncertainties regarding developing legal and regulatory requirements and standards may require significant resources to modify and maintain business practices to comply with U.S. and non-U.S. laws concerning the use of AI and AI Systems, the nature of which cannot be determined at this time.
Our cash, cash equivalents, and financial investments could be adversely affected if the financial institutions in which we hold our cash, cash equivalents, and financial investments fail.
We regularly maintain cash balances at third-party financial institutions in excess of the insurance limit of the Federal Deposit Insurance Corporation (the “FDIC”) and other countries’ deposit insurance systems.
The FDIC took control and was appointed receiver of Silicon Valley Bank and New York Signature Bank (collectively, the “Failed Banks”) on March 10, 2023 and March 12, 2023, respectively. We do not have any direct exposure to either of the Failed Banks. However, if banks and financial institutions where we maintain large cash balances, cash equivalents, or financial investments enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our existing cash, cash equivalents, and financial investments could be threatened and may have a material adverse impact on our business, prospects, financial condition, or results of operations. Moreover, events such as the closure of large regional or national banks like the Failed Banks, in addition to other global macroeconomic conditions, may cause further turbulence and uncertainty in the capital markets.
Risks Relating to Our Indebtedness
The size of our indebtedness and the obligations associated with it could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or in our industry or to deploy capital to grow our business, expose us to interest-rate risk to the extent of our variable-rate debt, or prevent us from meeting our obligations under our indebtedness. These risks may be increased in a recessionary environment, particularly as sources of capital may become less available or more expensive.
As of June 30, 2023, on a consolidated basis, we had $4.85 billion (U.S. dollar equivalent) of total indebtedness outstanding, consisting of $1.92 billion of secured indebtedness under our senior secured credit facilities and $2.93 billion of senior unsecured indebtedness, including $500 million aggregate principal amount of 5.000% U.S. dollar-denominated Senior Notes due 2027 (the “2027 Notes”), €825 million aggregate principal amount of the 2028 Notes, $550 million aggregate principal amount of U.S. dollar-denominated 3.125% Senior Notes due 2029 (the “2029 Notes”), and $650 million aggregate principal amount of U.S. dollar-denominated 3.500% Senior Notes due 2030 (the “2030 Notes” and, together with the 2027 Notes, the 2028 Notes, and the 2029 Notes, the “Senior Notes”). As of June 30, 2023, we also held $341 million in finance lease obligations. We also had the ability to incur significant additional indebtedness, including via $594 million of unutilized capacity under our $1.10 billion secured revolving credit facility, which part of our senior secured credit facilities (the “Revolving Credit Facility”) following borrowings of $500 million and $6 million of outstanding letters of credit.
The multi-billion-dollar size of our indebtedness could have important consequences for us, including:
•increasing our vulnerability to adverse economic, industry, or competitive developments;
•exposing us to the risk of increased interest rates because certain of our borrowings, including borrowings under our senior secured credit facilities, are at variable rates of interest;
•exposing us to the risk of fluctuations in exchange rates because of our euro-denominated notes;
•making it more difficult for us to satisfy our obligations with respect to our indebtedness, and any failure to comply with the obligations of any of our debt instruments, including restrictive covenants and borrowing conditions, could result in one or more events of default under the agreements governing such indebtedness or, through cross-defaults, in agreements governing other indebtedness;
•restricting us from making strategic acquisitions or capital investments or causing us to make non-strategic divestitures;
•limiting our ability to obtain additional financing for working capital, capital expenditures, product development, debt service requirements, acquisitions, and general corporate or other purposes;
•limiting our flexibility in planning for, or reacting to, changes in our business or market conditions and placing us at a competitive disadvantage compared to our competitors who have less indebtedness relative to their size and who, therefore, may be able to take advantage of opportunities that our higher level of indebtedness prevents us from exploiting; and
•limiting the types of investors who are willing to invest in our Common Stock, as certain investors prefer to invest in companies with lower levels of indebtedness relative to other financial metrics.
Our total interest expense, net was $186 million, $123 million, and $110 million for fiscal 2023, 2022, and 2021, respectively. After taking into consideration our ratio of fixed-to-floating-rate debt, including as a result of our June 2023 amendment to our interest-rate swap agreement with Bank of America N.A., and assuming that the Secured Overnight Financing Rate (“SOFR”) is above any applicable minimum floor, each change of 50 basis points in interest rates would result in a change of $7 million in annual interest expense on the indebtedness under our senior secured credit facilities.
Our interest expense may continue to increase as policymakers combat the inflation that has taken hold since fiscal 2022 through interest-rate increases on benchmark financial products that can affect the interest rates on our variable-rate debt.
The size of our indebtedness, alone or combined with volatility in our reported financial results, may cause suppliers or customers to opt not to do business with us or to do so under less attractive terms, or render it more costly or time-consuming to secure supplies or attract customers, which could affect our financial condition and results of operations.
There can be no assurance as to the effect that the size of our indebtedness, alone or combined with volatility in our reported financial results, will have on our relationships with our suppliers or customers. To the extent that the size of our indebtedness, alone or combined with volatility in our reported financial results, results in the tightening of payment or credit terms, increases in the price of supplied goods, or the loss of one or more major suppliers or customers, it could have a material adverse effect on our business, financial condition, liquidity, or results of operations.
Despite our high indebtedness level, we and our subsidiaries are still capable of incurring significant additional debt, which could further exacerbate the risks associated with our substantial indebtedness.
We and our subsidiaries may be able to incur substantial additional indebtedness in the future. Although the agreements governing our indebtedness contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of significant qualifications and exceptions, and, under certain circumstances, the amount of indebtedness that we may incur while remaining in compliance with these restrictions could be substantial. In addition, as of June 30, 2023, we had approximately $594 million available to us for borrowing, subject to certain conditions, under our Revolving Credit Facility. If new debt is added to the current debt levels for which we or our subsidiaries are responsible, the risks associated with debt we currently face would increase.
Our interest expense on our variable-rate debt may continue to increase if and to the extent that policymakers combat inflation through interest-rate increases on benchmark financial products.
Borrowings under our variable-rate debt are at variable rates of interest and are based upon benchmarks that are subject to potential change or elimination, and therefore expose us to interest-rate risk. If interest rates increase, our debt service obligations on our variable-rate debt will increase even though the amount borrowed remains the same, and our net income and cash flows, including cash available for servicing our indebtedness, will correspondingly decrease.
Our debt agreements contain restrictions that limit our flexibility in operating our business.
The agreements governing our outstanding indebtedness contain various covenants that limit our ability to engage in specified types of transactions. These covenants limit the ability of Operating Company and those of its subsidiaries to which these covenants apply (which Operating Company’s Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended, the “Credit Agreement”) calls “restricted subsidiaries”) to, among other things:
•incur additional indebtedness and issue certain preferred stock;
•pay certain dividends on, repurchase, or make distributions in respect of capital stock or make other restricted payments;
•pay distributions from restricted subsidiaries;
•issue or sell capital stock of restricted subsidiaries;
•guarantee certain indebtedness;
•make certain investments;
•sell or exchange certain assets;
•enter into transactions with affiliates;
•create certain liens; and
•consolidate, merge, or transfer all or substantially all of our assets and the assets of our subsidiaries on a consolidated basis.
A breach of any of these covenants could result in a default under one or more of these agreements, including as a result of cross-default provisions, and, in the case of our Revolving Credit Facility, permit the lenders to cease making loans to us.
Despite the limitations in our debt agreements, we retain the ability to take certain actions that may interfere with our ability to timely pay our substantial indebtedness.
The covenants in the Credit Agreement and in the several indentures governing our Senior Notes (collectively, the “Indentures”) contain various exceptions to the limitations they otherwise impose on our ability and the ability of our restricted subsidiaries to take the various actions described in the prior risk factor. For example, if the Senior Notes have investment-grade ratings and we are not in default under these agreements, certain of these covenants will not apply, including the covenants restricting certain dividends and other payments, the covenants concerning the incurrence of indebtedness, and the covenants limiting guarantees of indebtedness by our restricted subsidiaries. In addition, the covenants restricting dividends and other distributions by us, purchases or redemption of certain equity securities, and prepayment, redemption, or repurchase of any subordinated indebtedness are subject to various exceptions.
We may not be able to pay our indebtedness when it becomes due.
Our ability to pay principal and interest on our variable-rate debt and to satisfy our other debt obligations will depend upon, among other things:
•our future financial and operating performance, which will be affected by prevailing economic, industry, and competitive conditions and financial, business, legislative, regulatory, and other factors, many of which are beyond our control; and
•our future ability to borrow under the Revolving Credit Facility, the availability of which depends on, among other things, our complying with applicable covenants in our Credit Agreement.
We cannot assure you that our business will generate cash flow from operations, or that we will be able to draw under the Revolving Credit Facility or otherwise, in an amount sufficient to fund our liquidity needs, including the payment of principal and interest on the Senior Notes, our term loans, our existing borrowings under our Revolving Credit Facility, and our other debt obligations. If our cash flows and other capital resources are insufficient to service our indebtedness, we may be forced to reduce or delay capital expenditures, sell assets, seek additional capital, or restructure or refinance our indebtedness. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. In addition, the terms of existing or future debt agreements may restrict us from adopting some of these alternatives. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. We may not be able to consummate those dispositions for fair market value, on a timely basis to meet our needs, or at all. Furthermore, any proceeds that we could realize from any or all such dispositions may not be adequate to meet our debt service obligations then due. Our inability to generate sufficient cash flow to satisfy our debt obligations, or to refinance our indebtedness on commercially reasonable terms or at all, could result in a material adverse effect on our business, results of operations, or financial condition. If we cannot make scheduled payments on our indebtedness, we will be in default, and, as a result of existing “cross-default” terms in our indebtedness or otherwise, all outstanding principal and interest may be declared to be due and payable, the lenders under our variable-rate debt could terminate their commitments to loan money, our secured lenders (including the lenders under our senior secured credit facilities or the holders of the Senior Notes) could foreclose against the assets securing their loans and the Senior Notes, and we could be forced into bankruptcy or liquidation.
We are currently using and may in the future use derivative financial instruments to reduce our exposure to market risks from changes in interest rates on our variable-rate indebtedness or changes in currency exchange rates, and any such instrument may expose us to risks related to counterparty credit worthiness or non-performance of these instruments.
We have executed and may enter into additional or new interest-rate swap agreements, currency swap agreements, or other hedging transactions in an attempt to limit our exposure to adverse changes in variable interest rates and currency exchange rates. Such instruments may result in economic losses if, for example, prevailing interest rates decline to a point lower than any applicable fixed-rate commitment. Any such swap will expose us to credit-related risks that, if realized, could adversely affect our results of operations or financial condition.
Risks Relating to Ownership of Our Common Stock
We do not presently maintain effective disclosure controls and procedures due to material weaknesses we have identified in our internal control over financial reporting. Failure to remediate these material weaknesses or any other material weakness or significant deficiencies has resulted in a revision of our financial statements, in the future could result in material misstatements in our financial statements and has caused, and in the future could cause, us to fail to timely meet our periodic reporting obligations.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, our management is required to report on, and our independent registered public accounting firm is required to attest to, the effectiveness of our internal control over financial reporting. The rules governing the standards that must be met for management to determine the adequacy of our internal control over financial reporting are complex and require significant documentation, testing, and possible remediation if a weakness or deficiency is identified. Annually, we perform activities that include reviewing, documenting, and testing our internal control over financial reporting. Our failure to achieve and maintain effective disclosure controls and procedures and internal control has resulted in, and in the future could result in, misstated consolidated financial statements and restatements of previously issued financial statements related to prior periods and delays or a failure to meet our reporting obligations, which could cause investors to lose confidence in our reported financial information and could lead to a decline in our stock price. Additionally, ineffective or inadequate disclosures and internal control could expose us to increased risk of misuse of corporate assets or fraud, or subject us to litigation, regulatory investigations, or civil or criminal sanctions, including by the SEC or other regulatory authorities, or potential delisting from the NYSE or any other stock exchange on which we may list our Common Stock in the future.
As discussed below in “Item 9A. – Controls and Procedures,” due to certain inadequacies of our internal control over financial reporting, we have not been able to conclude on an ongoing basis that we have effective disclosure controls and procedures and internal control over financial reporting in accordance with legal requirements. For example, in the third quarter of fiscal 2023, management identified a material weakness in internal control related to revenue recognition at our Bloomington, Indiana facility during fiscal 2022. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Due to this material weakness in internal control over financial reporting, we concluded that, as of June 30, 2022, our disclosure controls and procedures were not effective and that we did not maintain effective internal control over financial reporting.
In addition, in preparing our consolidated financial statements for the three and nine months ended March 31, 2023, management identified a separate material weakness in internal control over financial reporting resulting from ineffective information technology general controls in the areas of user access management, application change management, operating system and database logical access controls, and segregation of duties for key information technology systems that support our financial reporting process. As a result, we identified this ineffectiveness as an additional material weakness in our internal control over financial reporting as of March 31, 2023, and concluded that our disclosure control and procedures were not effective as of March 31, 2023. During the fourth quarter of fiscal 2023, we successfully completed the testing necessary to conclude that this material weakness has been remedied.
In preparing our audited financial statements for the fiscal year ended June 30, 2023, management identified (i) a material weakness in internal control over financial reporting related to the consolidated financial statement close process, and (ii) a material weakness in internal control over financial reporting related to inventory reconciliation at our Baltimore, Maryland facility. Due to these material weaknesses in internal control over financial reporting, we concluded that, as of June 30, 2023, our disclosure controls and procedures were not effective and that we did not maintain effective internal control over financial reporting.
The failure to maintain effective disclosure control and procedures and internal control as a result of the material weaknesses described above has resulted in significant expenses to remediate the disclosure and internal control deficiencies. In addition, as a result of the material weaknesses described above, we failed to timely file our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, and this Annual Report, and filed an Amendment to our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 to revise our consolidated financial statements as a result of the error in the periods impacted.
Management is actively engaged in the implementation of remediation efforts to address our remaining material weaknesses and control deficiencies. However, we may not be successful in promptly remediating these material weaknesses or be able to identify and remediate any additional control deficiency, including any material weakness, that may arise in the future. Management is currently unable to conclude, and may not be able to conclude in future periods, that our disclosure controls and procedures are effective due to the effects of various factors, which may, in part, include unremediated material weaknesses in internal control over financial reporting. If not remediated, any failure to establish and maintain effective disclosure control and procedures and internal control over financial reporting could result in material misstatements in our consolidated financial statements or cause us to fail to meet our reporting and financial obligations, each of which could have a material adverse effect on the confidence that stockholders, customers, or suppliers have in our financial reporting, which could materially harm our business, our financial condition, or the trading price of our Common Stock.
For further discussion of our material weaknesses, see “Item 9A. – Controls and Procedures.”
Our stock price has historically been and may continue to be volatile, and a holder of shares of our Common Stock may not be able to resell such shares at or above the price such stockholder paid, or at all, and could lose all or part of such investment as a result.
The trading price of our Common Stock has been and continues to be volatile. For the three years ended June 30, 2023, our Common Stock price as quoted on the NYSE traded at a high of $142.64 on September 9, 2021 and a low of $31.45 on May 15, 2023. The trading price of our Common Stock may be adversely affected by any one or more of several factors, such as those listed above in “—Risks Relating to Our Business and Industry in Which We Operate” and the following:
•results of operations that vary from the expectations of securities analysts or investors;
•results of operations that vary from those of our competitors;
•changes in expectations as to our future financial performance, including financial estimates and investment recommendations by securities analysts or investors;
•declines in the market prices of stocks generally, or those of pharmaceutical or other healthcare companies;
•strategic actions by us or our competitors;
•announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships, or capital commitments;
•changes in general economic or market conditions or trends in our industry or markets, such as increased inflation;
•changes in business or regulatory conditions or regulatory actions taken with respect to our business or the business of any of our competitors or customers;
•future sales of our Common Stock or other securities we may issue in the future;
•investor perceptions of the investment opportunity associated with our Common Stock relative to other investment alternatives;
•any decision by securities analysts to not publish research or reports about our business or to downgrade our stock or our sector;
•additions or departures of key personnel;
•the public response to press releases or other public announcements by us or third parties, including our filings with or information furnished to the SEC;
•announcements relating to or developments in litigation, including shareholder lawsuits;
•guidance, if any, that we provide to the public, any change in this guidance, or any failure to meet this guidance;
•the availability of an active trading market for our Common Stock;
•public response to changes in the COVID-19 pandemic and public perceptions as to the need for manufacture of certain COVID-19-related products and our role in the successful manufacture of such products;
•changes in the accounting principles we use to record our results or our application of these principles to our business; and
•other events or factors, including those resulting from natural disasters, hostilities, the war in Ukraine, acts of terrorism, geopolitical activity, public health crises, including pandemics, or responses to these events.
Broad market and industry fluctuations may adversely affect the market price of our Common Stock, regardless of our actual operating performance. In addition, price volatility may be greater if the public float or trading volume of our Common Stock is low, and the amount of public float on any given day can vary depending on the individual actions of our stockholders.
Following periods of market volatility, stockholders have been known to institute securities class action litigation in an attempt to recover any resulting loss. In February 2023, a complaint styled City of Warwick Retirement System v. Catalent, Inc., et al., No. 23-cv-01108, was filed in New Jersey federal court against us and three of our then-officers purportedly on behalf of a putative “class” consisting of persons who purchased or otherwise acquired our securities between August 30, 2021 and October 31, 2022, inclusive, and on September 15, 2023, the Warwick complaint was amended (together with the original complaint, the “Warwick Complaint”), which expanded the class period to between August 30, 2021 and May 7, 2023, inclusive. The complaint purports to assert claims under Sections 10(b) and 20(a) of the Exchange Act, alleging that, unbeknownst to investors, the defendants purportedly engaged in accounting and channel stuffing schemes to pad our revenue and failed to disclose adverse facts that purportedly were known to or recklessly disregarded by defendants. Further, in August 2023, an alleged shareholder filed a derivative complaint styled Husty, et al. v. Carroll, et al., No. 23-cv-00891, in Delaware federal court against the current members of our board of directors, two former members of our board, and nominally against Catalent, Inc. The complaint mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action and claims that the alleged activities described there led to, and will continue to expose us to, costs and damages. Finally, in September 2023, an alleged shareholder filed a derivative complaint styled Brown, et al. v. Chiminski, et al., Case 3:23-cv-15722, in New Jersey federal court against certain current members of our board of directors, two former members of our board, and nominally against Catalent, Inc. The complaint also mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action and claims that the alleged activities described there led to, and will continue to expose us to, costs and damages. See “Item 3 - Legal Proceedings” and Note 17, Commitments and Contingencies to the Consolidated Financial Statements for additional information. These litigations, and any additional securities litigation, could have a substantial cost and divert resources and the attention of senior management from our business regardless of the outcomes of such litigations.
Because we have no plan to pay cash dividends on our Common Stock for the foreseeable future, receiving a return on an investment in our Common Stock may require a sale for a net price greater than what was paid for it.
We currently intend to retain future earnings, if any, for future operations, expansion, and debt repayment and have no current plan to pay any cash dividend on our Common Stock for the foreseeable future. Any future decision to pay a dividend in respect of our Common Stock, and the amount and timing of any such dividend, will be at the sole discretion of our board of
directors. Our board of directors may take into account, when deciding whether or how to pay a dividend, such factors as they may deem relevant, including general economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, possible future alternative deployments of our cash, our future capital requirements, and contractual, legal, tax, and regulatory restrictions and implications on the payment of dividends by us to our holders of shares of our Common Stock or by our subsidiaries to us. In addition, our ability to pay dividends is limited by covenants in the agreements governing our outstanding indebtedness and may be limited by covenants of any future indebtedness we or our subsidiaries incur. As a result, a holder of a share of our Common Stock may not receive any return on such investment unless it is sold for a price greater than that which was paid for it, taking into account any applicable commission or other costs of acquisition or sale.
Future sales, or the perception of future sales, of our Common Stock, by us or our existing stockholders could cause the market price for our Common Stock to decline.
The sale of shares of our Common Stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our Common Stock. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
The market price of shares of our Common Stock could drop significantly if the holders of our Common Stock sell their shares or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of shares of our equity securities that we wish to issue. In the future, we may also issue our securities in connection with investments or acquisitions or to pay down debt. The number of shares of our Common Stock issued or issuable as a result could constitute a material portion of then-outstanding shares of our Common Stock, subject to limitations on issuance of new shares imposed by the NYSE (including any applicable requirement for stockholder approval) or restrictions set forth in the agreements governing our indebtedness. Any issuance of additional securities in connection with investments, acquisitions, or otherwise may result in dilution to the holders of shares of our Common Stock.
We are no longer eligible to use the Form S-3 registration statement, which could impair our capital-raising activities.
As a result of our failure to timely file our periodic reports with the SEC, we are no longer eligible to use a Form S-3 registration statement. As a result of our late 10-Q filing, we are also no longer a “well-known seasoned issuer,” as such term is used in the SEC's regulations, which otherwise would allow us to, among other things, file automatically effective shelf registration statements. Our eligibility to use a Form S-3 registration statement may not be restored until December 1, 2024, and then only if we have not had any other filing delinquency that would preclude Form S-3 eligibility and satisfy all other requirements for Form S-3 eligibility. During any period when we are not eligible to use Form S-3 or qualify as a “well-known seasoned issuer,” our capital raising ability may be impaired. Under these circumstances, we will be required to use a registration statement on Form S-1 to register securities with the SEC, which could hinder our ability to act quickly in raising capital to take advantage of market conditions in our capital-raising activities and may increase our cost of raising capital. Further, the expenses associated with raising capital using Form S-1 are generally greater than those associated with using Form S-3.
Provisions in our organizational documents could delay or prevent a change of control.
Certain provisions of our current certificate of incorporation and bylaws may have an anti-takeover effect and may delay, defer, or prevent a merger, acquisition, tender offer, takeover attempt, or other change of control transaction that may otherwise be in the best interests of our stockholders, including transactions that might otherwise result in the payment of a premium over the market price for the shares held by our stockholders.
These provisions provide for, among other things:
•the ability of our board of directors to issue one or more series of preferred stock;
•advance notice for nominations of directors by stockholders and for stockholders to include matters to be considered at our annual meetings (though our board of directors has implemented shareholder proxy access); and
•certain limitations on convening special stockholder meetings.
Provisions such as those just described, to the extent that they remain in effect, could make it more difficult for a third party to acquire us, even if the third party’s offer may be considered beneficial by many of our stockholders. As a result, our stockholders may be limited in their ability to obtain a premium for their shares.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our principal executive offices are located at 14 Schoolhouse Road, Somerset, New Jersey. As of June 30, 2023, we had 52 facilities (3 geographical locations operate as multiple facilities because they support more than one reporting segment, with our Somerset location including both a manufacturing facility and our principal executive offices), comprising manufacturing operations, development centers, and sales offices contained in approximately 8 million square feet of manufacturing, laboratory, office and related space. Our manufacturing capabilities include all required regulatory, quality assurance and in-house validation space. The following table sets forth our facilities containing manufacturing, laboratory, office, and related space by reporting segment and geographic location as of June 30, 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Geographic Region | | Biologics | | Pharma and Consumer Health | | Corporate | | Total (1) |
| North America | | 10 | | 15 | | 1 | | 26 |
| South America | | — | | 3 | | 1 | | 4 |
| Europe | | 6 | | 10 | | 1 | | 17 |
| Asia-Pacific | | — | | 5 | | — | | 5 |
| Total | | 16 | | 33 | | 3 | | 52 |
(1) Sites that are used by multiple segments are included once for each segment in this table.
Two of our manufacturing facilities, located in Bloomington, Indiana and Harmans, Maryland, together generate a material portion of our net revenue. We believe these facilities are suitable for their intended purposes, with adequate capacity for current and projected demand for their contracted products.
We generally seek to own, rather than lease, our manufacturing facilities, although some facilities are leased. Our office space and warehouse facilities are often leased.
Additional information with respect to our leases and property, plant, and equipment is contained in Notes 16 and 19, respectively, to our Consolidated Financial Statements.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we may be involved in legal proceedings arising in the ordinary course of business, including, without limitation, inquiries and claims concerning environmental contamination as well as litigation and allegations in connection with acquisitions, product liability, manufacturing or packaging defects, and claims for reimbursement for the cost of lost or damaged active pharmaceutical ingredients, the cost of any of which could be significant. We intend to vigorously defend ourselves against any such litigation and do not currently believe that the outcome of any such litigation will have a material adverse effect on our financial statements. In addition, the healthcare industry is highly regulated and government agencies continue to scrutinize certain practices affecting government programs and otherwise.
In February 2023, an alleged shareholder filed a complaint styled City of Warwick Retirement System v. Catalent, Inc., et al., No. 23-cv-01108 in New Jersey federal court against us and three of our then-officers (collectively, the “Warwick Defendants”) purportedly on behalf of a putative “class” consisting of persons who purchased or otherwise acquired our securities between August 30, 2021 and October 31, 2022, inclusive. On September 15, 2023, the Warwick complaint was amended (together with the original complaint, the “Warwick Complaint”), which amended complaint expanded the class period to between August 30, 2021 and May 7, 2023, inclusive (the “Class Period”). The Warwick Complaint purports to assert claims under Sections 10(b) and 20(a) of the Exchange Act and the related regulations, alleging that, unbeknownst to investors, the Warwick Defendants purportedly engaged in accounting and channel stuffing schemes to pad our revenues and failed to disclose adverse facts that purportedly were known to or recklessly disregarded by the Warwick Defendants. Specifically, the Warwick Complaint alleges that the Warwick Defendants (i) overstated revenue and earnings by prematurely recognizing revenue in violation of accounting principles generally accepted in the U.S. (“U.S. GAAP”); (ii) suffered material weaknesses in our internal control over financial reporting related to revenue recognition; (iii) falsely represented demand for our products while we knowingly selling more product to our direct customers than could be sold to healthcare providers and end consumers; (iv) cut corners on safety and control procedures at key production facilities; (v) disregarded regulatory rules at key production facilities in order to rapidly produce excess inventory that was used to pad our financial results through premature revenue recognition in violation of U.S. GAAP or stuffing our direct customers with this excess inventory; and (vi) lacked a reasonable basis for their positive statements about our financial performance, outlook, and regulatory compliance during the Class Period.
We believe that the Warwick Defendants have defenses to the allegations and claims set forth in the complaint and filed a motion to dismiss the Warwick Complaint on November 15, 2023.
In June 2023, we received a demand from a company stockholder pursuant to 8 Del. C. § 220 to inspect books and records of the Company relating to, among other things, the allegations raised in the Warwick Complaint. We have responded to the demand and cannot determine at this time if the books and records demand will lead to litigation.
In August 2023, an alleged shareholder filed a derivative complaint styled Husty, et al. v. Carroll, et al., No. 23-cv-00891, in Delaware federal court against certain current and former members of our board of directors (the “Husty Defendants”) and nominally against Catalent, Inc. The complaint mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action described above and claims that the alleged activities described there led to, and will continue to expose us to, costs and damages. We believe that the Husty Defendants have defenses to the allegations and claims set forth in the complaint and once all Husty Defendants are properly served with the complaint, intend to vigorously defend the Husty Defendants against such allegations.
In September 2023, an alleged shareholder filed a derivative complaint styled Brown, et al. v. Chiminski, et al., Case 3:23-cv-15722, in New Jersey federal court against certain current and former officers and members of our board of directors (the "Brown Defendants") and nominally against Catalent, Inc. The complaint mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action described above and claims that the alleged activities described there led to, and will continue to expose us to, costs and damages. On November 8, 2023, the Court entered a stipulation between the parties extending the Brown Defendants' time to respond to the complaint until January 8, 2024. We believe that the Brown Defendants have defenses to the allegations and claims set forth in the complaint and intend to vigorously defend the Brown Defendants against such allegations.
From time to time, we receive subpoenas or requests for information from various governmental agencies or private parties, including from state attorneys general, the U.S. Department of Justice, and private parties. We generally respond to such subpoenas and requests in a timely and thorough manner, which responses sometimes require considerable time and effort and can result in considerable costs being incurred.
For additional information, see Note 17, Commitments and Contingencies, to the Consolidated Financial Statements.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
The principal market for trading of our Common Stock is the NYSE. Our Common Stock trades under the symbol “CTLT.”
As of November 30, 2023, we had 17 holders of record of outstanding shares of our Common Stock. This number does not include beneficial owners whose shares were held in street name.
We did not declare or pay any dividend on our Common Stock in fiscal 2023 or fiscal 2022. We have no current plan to pay any dividend on our Common Stock. Any decision to declare and pay dividends in the future will be made at the sole discretion of our board of directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restriction, and other factors that our board of directors may deem relevant. Because we are a holding company and have no direct operations, we will only be able to pay dividends from funds we receive from our subsidiaries. In addition, our ability to pay dividends will be limited by covenants in our existing indebtedness and may be limited by the agreements governing other indebtedness we or our subsidiaries incur in the future. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Debt and Financing Arrangements—Debt Covenants.”
Recent Sales of Unregistered Equity Securities
We did not sell any unregistered equity securities during the period covered by this Annual Report.
Purchases of Equity Securities
We did not purchase any of our equity securities during the period covered by this Annual Report.
Performance Graph
Set forth below is a line graph comparing the cumulative total shareholder return on our Common Stock from June 30, 2018 through June 30, 2023, based on the market price of our Common Stock and assuming reinvestment of dividends, with the cumulative total shareholder return of companies on the S&P 500 Index and S&P 500 Health Care Index. The graph assumes that $100 was invested in our Common Stock and in each index at the market close on June 30, 2018. The stock price performance of the following graph is not necessarily indicative of future stock performance.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our Consolidated Financial Statements and related notes, which appear elsewhere in this Annual Report. This section of the Annual Report generally discusses the fiscal years ended June 30, 2023 and 2022 and year-to-year comparisons between the fiscal years ended June 30, 2023 and 2022. The discussion of our results of operations for the fiscal year ended June 30, 2021 and a comparison of our results for the fiscal years ended June 30, 2022 and 2021 is included in Item 7. – Management’s Discussion and Analysis of Financial Condition and Results of Operations of our Annual Report on Form 10-K for the fiscal year ended June 30, 2022, filed with the SEC on August 29, 2022, as amended by Amendment No.1 to Annual Report on Form 10-K/A filed with the SEC on June 12, 2023 and is incorporated herein by reference. In addition to historical consolidated financial information, the following discussion contains forward-looking statements that reflect our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. You should carefully read “Special Note Regarding Forward-Looking Statements” in this Annual Report. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Annual Report, particularly in “Item 1A. - Risk Factors.”
Overview
We provide differentiated development and manufacturing solutions for drugs, protein-based biologics, cell and gene therapies, vaccines, and consumer health products at over fifty facilities across four continents under rigorous quality and operational standards. Our oral, injectable, and respiratory delivery technologies, along with our state-of-the-art protein and cell and gene therapy manufacturing capacity, address a wide and growing range of modalities and therapeutic and other categories across the biopharmaceutical, pharmaceutical, and consumer health industries. Through our extensive capabilities, growth-enabling capacity, and deep expertise in product development, regulatory compliance, and clinical trial supply, we can help our customers take products to market faster, and have done so for nearly half of new drug products approved by the FDA in the last decade. Our development and manufacturing platforms, our proven formulation, supply, and regulatory expertise, and our broad and deep development and manufacturing know-how enable our customers to advance and then bring to market more products and better treatments for patients and consumers. Our commitment to reliably supply our customers’ and their patients’ needs is the foundation for the value we provide; annually, we produce approximately 70 billion unit doses for nearly 8,000 customer prescription and consumer health products, or approximately 1 in every 26 unit doses of such products taken each year by patients and consumers around the world. We believe that, through our investments in state-of-the-art facilities and capacity expansion, including investments in facilities focused on new treatment modalities and other attractive market segments, our continuous improvement activities devoted to operational and quality excellence, the sales of existing and introduction of new customer products, and, in some cases, our innovation activities and patents, we will continue to attract premium opportunities and realize the growth potential from these areas.
At the commencement of fiscal 2023, in connection with our change in Chief Executive Officer and Chief Operating Decision Maker, we adopted a new operating structure with two operating and reportable segments: (i) Biologics and (ii) Pharma and Consumer Health. The Biologics segment provides development and manufacturing for biologic proteins; cell, gene, and other nucleic acid therapies; pDNA; iPSCs, and oncolytic viruses; and vaccines. It also provides formulation, development, and manufacturing for parenteral dose forms, including vials, prefilled syringes, and cartridges; and, as noted above, analytical development and testing services for large molecules. Our Pharma and Consumer Health segment, comprises the Company’s market-leading capabilities for complex oral solids, softgel formulations, Zydis fast-dissolve technologies, and gummy, soft chew, and lozenge dosage forms; formulation, development, and manufacturing platforms for oral, nasal, inhaled, and topical dose forms; cold-chain storage and distribution, and clinical trial development and supply services. Prior-period segment results were reclassified to conform to the current period presentation.
Critical Accounting Policies and Estimates
The following disclosure supplements the descriptions of our accounting policies contained in Note 1 to our Consolidated Financial Statements regarding significant areas of judgment. Management made certain estimates and assumptions during the preparation of the Consolidated Financial Statements in accordance with U.S. GAAP. These estimates and assumptions affect the reported amount of assets and liabilities and disclosures of contingent assets and liabilities in the Consolidated Financial Statements. These estimates also affect the reported amount of net earnings during the reporting periods. Actual results could differ from those estimates. Because of the size of the financial statement elements to which they relate, some of our accounting policies and estimates have a more significant impact on the Consolidated Financial Statements than others.
Management has discussed the development and selection of these critical accounting policies and estimates with the audit committee of our board of directors. A discussion of some of our more significant accounting policies and estimates follows.
Revenue Recognition
We sell products and services directly to our biopharmaceutical, pharmaceutical, and consumer health customers. The majority of our business is conducted through manufacturing and commercial product supply, development services, and clinical supply services.
Our contracts with customers often include promises to transfer multiple products and services to a customer. Determining whether products and services are considered distinct performance obligations that should be accounted for separately versus together may require judgment. For our manufacturing and commercial product supply revenue, the contract generally includes the terms of the manufacturing services and related product quality assurance procedures to comply with regulatory requirements. Due to the regulated nature of our business, these contract terms are highly interdependent and, therefore, are considered to be a single combined performance obligation. For our development services and clinical supply services revenue, our performance obligations vary per contract and are accounted for as separate performance obligations. If a contract contains a single performance obligation, we allocate the entire transaction price to the single performance obligation. If a contract contains multiple performance obligations, we allocate consideration to each performance obligation using the “relative standalone selling price” as defined under Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers. Generally, we utilize observable standalone selling prices in our allocations of consideration. If observable standalone selling prices are not available, we estimate the applicable standalone selling price using an adjusted market assessment approach, representing the amount that we believe the market is willing to pay for the applicable service. Revenue is recognized over time using an appropriate method of measuring progress towards fulfilling our performance obligation for the respective arrangement. Determining the measure of progress that consistently depicts our satisfaction of performance obligations within each of our revenue streams across similar arrangements requires judgment.
Our customer contracts generally include provisions entitling us to a termination penalty when the customer terminates prior to the contract’s nominal end date. The termination penalties in these customer contracts vary but are generally considered substantive for accounting purposes and create enforceable rights and obligations throughout the stated duration of the contract. We account for a contract termination as a contract modification in the period in which the customer gives notice of termination. The determination of the contract termination penalty is based on the terms stated in the relevant customer agreement. As of the modification date, we update our estimate of the transaction price using the expected value method, subject to constraints, and recognize the amount over the remaining performance period under the contract. In the event of a contract termination, revenues are recognized to the extent that it is probable that a significant reversal will not occur when any uncertainty is subsequently resolved.
Long-lived and Other Definite-Lived Intangible Assets
We allocate the cost of an acquired company to the tangible and identifiable intangible assets and liabilities acquired, with any remaining cost recorded as goodwill. Intangible assets primarily include customer relationships, technology, and trademarks. Valuing the identifiable intangible assets requires judgment. Intangible assets are generally amortized on a straight-line basis, reflecting the pattern in which the economic benefits are consumed, and are amortized over their estimated useful lives.
We assess the impairment of identifiable intangibles if events or changes in circumstances indicate that the carrying values of the assets may not be recoverable. Factors that could trigger an impairment review include the following:
•significant under-performance relative to historical or projected future operating results;
•significant changes in the manner of use of the acquired assets or the strategy of the overall business;
•significant negative industry or economic trends; and
•recognition of goodwill impairment charges.
If we determine that the carrying value of identifiable intangibles and/or long-lived assets may not be recoverable based on the existence of one or more of the above indicators of impairment, we measure recoverability of assets by comparing the respective carrying value of the assets to the current and expected future cash flows, on an un-discounted basis, to be generated from such assets. If such analysis indicates that the carrying value of these assets is not recoverable, we measure an impairment based on the amount by which the net carrying amount of the assets exceeds the fair values of the assets. See Notes 3, Business Combinations and Divestitures and 5, Other Intangibles, net to the Consolidated Financial Statements.
Goodwill and Indefinite-Lived Intangible Assets
We account for purchased goodwill and intangible assets with indefinite lives in accordance with ASC 350, Intangibles –– Goodwill and Other. Under ASC 350, goodwill and intangible assets with indefinite lives are not amortized, but instead are
tested for impairment at least annually. We perform an impairment evaluation of goodwill annually during the fourth quarter of our fiscal year or when circumstances otherwise indicate an evaluation should be performed. The evaluation may begin with a qualitative assessment for each reporting unit to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying value. If the qualitative assessment does not generate a positive response, or if no qualitative assessment is performed, a quantitative assessment, based upon discounted cash flows, is performed and requires management to estimate future cash flows, growth rates, and economic and market conditions.
We perform an annual goodwill impairment test for each reporting unit on April 1, the measurement date and more frequently if indicators of impairment exist.
Due to our underperformance of operating results relative to expectations and the decline in our stock price, we assessed the current and future economic outlook as of March 31, 2023. The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in each of its six reporting units as of March 31, 2023 which led to a quantitative assessment for each of our reporting units. The evaluation performed as of March 31, 2023 resulted in a goodwill impairment charge of $210 million in our Consumer Health reporting unit within the Pharma and Consumer Health segment. A 50 basis point increase in the discount rate would increase the goodwill impairment $70 million and a 50 basis point decrease in the long-term growth rate would increase the goodwill impairment by $40 million.
Subsequent to the quantitative assessment performed as of March 31, 2023, we performed a qualitative assessment as of April 1, 2023, which yielded no indicators of impairment.
In fiscal 2022, we proceeded immediately to the quantitative assessment, but in fiscal 2021 we began with the qualitative assessment. Accordingly, no sensitivity analysis was performed for fiscal 2021.
Subsequent to June 30, 2023, as a result of Consumer Health's underperformance of recent operating results relative to expectations, as well as current macroeconomic conditions impacting the consumer health and biotechnology industries, and higher interest rates, we assessed the current and future economic outlook as of September 30, 2023 for our Consumer Health and Biomodalities reporting units in our Pharma and Consumer Health and Biologics segments, respectively, and identified indicators for impairment of goodwill.
The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in our Consumer Health and Biomodalities reporting units, which led to a quantitative assessment for the corresponding reporting units. The evaluation performed as of September 30, 2023 resulted in a goodwill impairment charge of $689 million in our Consumer Health and Biomodalities reporting units within the Pharma and Consumer Health and Biologics segments, respectively. For further details on the impairment charges for the three months ended September 30, 2023, see Note 20, Subsequent Events, “Impairment of Goodwill.”
Income Taxes
In accordance with ASC 740, Income Taxes, we account for income taxes using the asset and liability method. The asset and liability method requires recognition of deferred tax assets and liabilities for expected future tax consequences of temporary differences that currently exist between tax bases and the corresponding financial reporting bases of our assets and liabilities. Deferred tax assets and liabilities are measured using enacted tax rates in the respective jurisdictions in which we operate. Deferred taxes are not provided on the undistributed earnings of subsidiaries outside of the U.S. when it is expected that these earnings will be permanently reinvested. In fiscal 2018, we recorded a provision for U.S. income taxes and foreign withholding taxes in relation to expected repatriations as a result of the 2017 U.S. Tax Cuts and Jobs Act (the “2017 Tax Act”), but we have not made any provision for U.S. income taxes on the remaining undistributed earnings of foreign subsidiaries as those earnings are considered permanently reinvested in the operations of those foreign subsidiaries in the years after 2018.
The 2017 Tax Act imposed taxes on so-called “global intangible low-taxed income” (“GILTI”) earned by certain foreign subsidiaries of a U.S. company. In accordance with ASC 740, we made an accounting policy election to treat taxes due on future U.S. inclusions in taxable income related to GILTI as a current-period expense when incurred.
We assess the realizability of deferred tax assets by considering all available evidence, both positive and negative. We evaluate four possible sources of taxable income when assessing the realizability of deferred tax assets:
•carrybacks of existing NOLs (if and to the extent permitted by tax law);
•future reversals of existing taxable temporary differences;
•tax planning strategies; and
•future taxable income exclusive of reversing temporary differences and carryforwards.
We consider the need to maintain a valuation allowance on deferred tax assets based on management’s assessment of whether it is more likely than not that we would realize those deferred tax assets as a result of future reversals of existing taxable temporary differences and the ability to generate sufficient taxable income within the carryforward period available under the applicable tax law.
Unrecognized tax benefits are generated when there are differences between tax positions taken in a tax return and amounts recognized in the Consolidated Financial Statements. Tax benefits are recognized in the Consolidated Financial Statements when it is more likely than not that a tax position will be sustained upon examination. To the extent we prevail in matters for which liabilities have been established or are required to pay amounts in excess of our liabilities, our effective income tax rate in a given period could be materially affected. An unfavorable income tax settlement may require the use of cash and result in an increase in our effective income tax rate in the year it is resolved. A favorable income tax settlement would be recognized as a reduction in the effective income tax rate in the year of resolution.
Our accounting for income taxes involves the application of complex tax regulations in the U.S. and in each of the non-U.S. jurisdictions in which we operate, particularly European tax jurisdictions. The determination of income subject to taxation in each tax-paying jurisdiction requires us to review reported book income and the events occurring during the year in each jurisdiction in which we operate. In addition, the application of deferred tax assets and liabilities will have an effect on the tax expense in each jurisdiction. For those entities engaging in transactions with affiliates, we apply transfer-pricing guidelines relevant in many jurisdictions in which we operate and make certain informed and reasonable assumptions and estimates about the relative value of contributions by affiliates when assessing the allocation of income and deductions between consolidated entities in different jurisdictions. The estimates and assumptions used in these allocations can result in uncertainty in the measured tax benefit.
Factors Affecting our Performance
Fluctuations in Operating Results
Our annual financial reporting period ends on June 30. Excluding the impact from COVID-19, our revenue and net earnings are generally higher in the third and fourth quarters of each fiscal year, with our first fiscal quarter typically generating our lowest revenue of any quarter, and our last fiscal quarter typically generating our highest revenue. These fluctuations are primarily the result of the timing of our, and our customers’, annual operational maintenance periods at locations in Europe and the U.S., the seasonality associated with pharmaceutical and biotechnology budgetary spending decisions, clinical trial and research and development schedules, the timing of new product launches and length of time needed to obtain full market penetration, and, to a lesser extent, the time of the year some of our customers’ products are in higher demand.
Acquisition and Related Integration Efforts
Our growth and profitability are affected by the acquisitions we complete and the speed at which we integrate those acquisitions into our existing operating platforms. In fiscal 2021, we expanded our capacity and capabilities through five acquisitions for our Biologics segment and through the acquisition of a dry powder inhaler and spray dry manufacturing business from Acorda Therapeutics, Inc. (“Acorda”). In fiscal 2022, we acquired each of Bettera Wellness, a manufacturer of a consumer-preferred gummy and other formats for consumer health products, a commercial-scale cell therapy manufacturing facility in Princeton, and a manufacturing facility for biologic therapies and vaccines near Oxford, U.K. In fiscal 2023, we acquired Metrics Contract Services (“Metrics”), an oral solids development and manufacturing business specializing in the manufacture of drugs containing highly potent active pharmaceutical ingredients.
Foreign Exchange Rates
Our operating network is global, and, as a result, we have substantial revenues and operating expenses that are denominated in currencies other than the U.S. dollar, the currency in which we report our financial results. Our results of operations and financial performance are therefore influenced by changes in currency exchange rates. In fiscal 2023, 35% of our net revenue was generated from our operations outside the U.S. Foreign currencies for our operations include the British pound, European euro, Brazilian real, Argentine peso, Japanese yen, and the Canadian dollar.
Inflation
In fiscal 2023, we began to experience the effects of increased global inflation, which has risen to levels not seen in more than 30 years. In response, we implemented various mitigation strategies, including in some cases increasing prices to
customers or reducing other costs of operation, including through price renegotiations with suppliers. The effects of inflation are likely to continue to affect us for most or all of fiscal 2024, at least, and there can be no assurance that our mitigating strategies will continue to enjoy the same degree of success.
Trends Affecting Our Business
Industry
We participate in nearly every sector of the global pharmaceutical and biotechnology industry, which has been estimated to generate more than $1 trillion in annual revenue, including, but not limited to, the prescription drug and biologic sectors as well as consumer health, which includes the over-the-counter and vitamins and nutritional supplement sectors. Innovative pharmaceuticals, and biologics in particular, continue to play a critical role in the global market, while the share of revenue due to generic drugs and biosimilars is increasing in both developed and developing markets. Sustained developed market demand and rapid growth in emerging economies is driving consumer health product growth. Payors, both public and private, have sought to limit the economic impact of pharmaceutical and biologics product demand through greater use of generic and biosimilar drugs, access and spending controls, and health technology assessment techniques, favoring products that deliver truly differentiated outcomes.
New Molecule Development and R&D Sourcing
Continued strengthening in early-stage development pipelines for drugs and biologics, compounded by increasing clinical trial breadth and complexity, support our belief in the attractive growth prospects for development solutions. Large companies are in many cases reconfiguring their R&D resources, increasingly involving the use of strategic partners for important outsourced functions and new treatment modalities. Additionally, an increasing portion of compounds in development are from companies that do not have a full research and development infrastructure, and thus are more likely to need strategic development solutions partners.
Demographics
Aging population demographics in developed countries, combined with the global COVID-19 pandemic and health care reforms in many global markets that are expanding access to treatments to a greater proportion of the global population, will continue to drive increases in demand for pharmaceuticals, biologics, and consumer health products. Increasing economic affluence in developing regions will further increase demand for healthcare treatments, and we are taking active steps to allow us to participate effectively in these growth regions and product categories.
Finally, we believe the market access and payor pressures our customers face, global supply chain complexity, and the increasing demand for improved and new modality treatments will continue to escalate the need for advanced formulation and manufacturing, product differentiation, improved outcomes, and treatment cost reduction, all of which can often be addressed using our advanced delivery technologies.
Non-GAAP Metrics
As described in this section, management uses various financial metrics, including certain metrics that are not based on concepts defined in U.S. GAAP, to measure and assess the performance of our business, to make critical business decisions, and to assess our compliance with certain financial obligations. We therefore believe that presentation of certain of these non-GAAP metrics in this Annual Report will aid investors in understanding our business.
EBITDA from operations
Management measures operating performance based on consolidated earnings from operations before interest expense, expense for income taxes, and depreciation and amortization, adjusted for the income attributable to non-controlling interests (“EBITDA from operations”). EBITDA from operations is not defined under U.S. GAAP, is not a measure of operating income, operating performance, or liquidity presented in accordance with U.S. GAAP, and is subject to important limitations.
We believe that the presentation of EBITDA from operations enhances an investor’s understanding of our financial performance. We believe this measure is a useful financial metric to assess our operating performance across periods and use this measure for business planning purposes. In addition, given the significant investments that we have made in the past in property, plant, and equipment, depreciation and amortization expenses represent a meaningful portion of our cost structure. We believe that disclosing EBITDA from operations provides investors with a useful tool for assessing the comparability between periods of our ability to generate cash from operations sufficient to pay taxes, service debt, and undertake capital expenditures without consideration of non-cash depreciation and amortization expense. We present EBITDA from operations in order to provide supplemental information that we consider relevant for readers of the Consolidated Financial Statements, and such
information is not meant to replace or supersede U.S. GAAP measures. Our definition of EBITDA from operations may not be the same as similarly titled measures used by other companies. The most directly comparable measure to EBITDA from operations defined under U.S. GAAP is net earnings. Included in this Management’s Discussion and Analysis is a reconciliation of net earnings to EBITDA from operations.
In addition, we evaluate the performance of our segments based on segment earnings before non-controlling interest, other (income) expense, impairments, restructuring costs, interest expense, income tax expense, stock-based compensation, gain (loss) on sale of subsidiary, and depreciation and amortization (“Segment EBITDA”).
Adjusted EBITDA
Under the Credit Agreement and in the Indentures, the ability of Operating Company to engage in certain activities, such as incurring certain additional indebtedness, making certain investments, and paying certain dividends, is tied to ratios based on Adjusted EBITDA (which is defined as “Consolidated EBITDA” in the Credit Agreement and “EBITDA” in the Indentures). Adjusted EBITDA is a covenant compliance measure in our Credit Agreement and Indentures, particularly those covenants governing debt incurrence and restricted payments. Adjusted EBITDA is based on the definitions in the Credit Agreement, is not defined under U.S. GAAP, is not a measure of operating income, operating performance, or liquidity presented in accordance with U.S. GAAP, and is subject to important limitations. Because not all companies use identical calculations, our presentation of Adjusted EBITDA may not be comparable to other similarly titled measures of other companies.
In addition, we use Adjusted EBITDA as a performance metric that guides management in its operation of and planning for the future of the business and drives certain management compensation programs. Management believes that Adjusted EBITDA provides a useful measure of our operating performance from period to period by excluding certain items that are not representative of our core business, including interest expense and non-cash charges like depreciation and amortization.
The measure under U.S. GAAP most directly comparable to Adjusted EBITDA is net earnings. In calculating Adjusted EBITDA, we add back certain non-cash, non-recurring, and other items that are deducted when calculating EBITDA from operations and net earnings, consistent with the requirements of the Credit Agreement. Adjusted EBITDA, among other things:
•does not include non-cash stock-based employee compensation expense and certain other non-cash charges;
•does not include cash and non-cash restructuring, severance, and relocation costs incurred to realize future cost savings and enhance operations;
•adds back any non-controlling interest expense, which represents minority investors’ ownership of non-wholly owned consolidated subsidiaries and is, therefore, not available; and
•includes estimated cost savings that have not yet been fully reflected in our results.
Adjusted Net Income and Adjusted Net Income per Share
We use Adjusted Net Income and Adjusted Net Income per share (which we sometimes refer to as “Adjusted EPS”) as performance metrics. Adjusted Net Income is not defined under U.S. GAAP, is not a measure of operating income, operating performance, or liquidity presented in accordance with U.S. GAAP, and is subject to important limitations. We believe that providing information concerning Adjusted Net Income and Adjusted Net Income per share enhances an investor’s understanding of our financial performance. We believe that these measures are useful financial metrics to assess our operating performance from period to period by excluding certain items that we believe are not representative of our core business, and we use these measures for business planning and executive compensation purposes. We define Adjusted Net Income as net earnings adjusted for (1) earnings or loss from discontinued operations, net of tax, (2) amortization attributable to purchase accounting, and (3) income or loss from non-controlling interest in majority-owned operations. We also make adjustments for other cash and non-cash items (as shown above, in “—Adjusted EBITDA”), partially offset by our estimate of the tax effect of such cash and non-cash items. Our definition of Adjusted Net Income may not be the same as similarly titled measures used by other companies. Adjusted Net Income per share is computed by dividing Adjusted Net Income by the weighted average diluted shares outstanding.
Use of Constant Currency
As exchange rates are an important factor in understanding period-to-period comparisons, we believe the presentation of results on a constant currency basis in addition to reported results helps improve investors’ ability to understand our operating results and evaluate our performance in comparison to prior periods. Constant currency information compares results between periods as if exchange rates had remained constant period-over-period. We use results on a constant currency basis as one measure to evaluate our performance. In this Annual Report, we calculate constant currency by calculating current-year results using prior-year foreign currency exchange rates. We generally refer to such amounts calculated on a constant currency basis as
excluding the impact of foreign exchange. These results should be considered in addition to, not as a substitute for, results reported in accordance with U.S. GAAP. Results on a constant currency basis, as we present them, may not be comparable to similarly titled measures used by other companies and are not measures of performance presented in accordance with U.S. GAAP.
Restructuring Programs
The contract manufacturing industry is labor intensive. As a result, we incur significant fixed costs to operate our facilities and maintain additional infrastructure required to obtain, develop, manufacture, transport, store, and test our products. Therefore, relatively small changes in demand can have a significant impact on short-term profitability.
In the second quarter of fiscal 2023, we engaged in a restructuring effort, which reduced our cost structure, consolidated facilities, and optimized our infrastructure across the organization. In the fourth quarter of fiscal 2023, we extended our restructuring effort, with further headcount reduction primarily in our Biologics segment and in our corporate functions. In connection with these restructuring plans, during the fiscal year ended June 30, 2023, we reduced our headcount by approximately 1,100 employees and incurred $66 million of cumulative pre-tax employee separation and other restructuring related costs. As a result of our restructuring plans, we expect to deliver an annualized run-rate savings of $150 to $160 million.
For further details regarding restructuring costs, see Note 6, Restructuring Costs to our Consolidated Financial Statements.
Summary Two-Year Key Financial Performance Metrics
Discussion of the year-over-year changes for the fiscal year ended June 30, 2022 compared to the fiscal year ended June 30, 2021 and the results of operations and cash flows for the fiscal year ended June 30, 2021, is included in Item 7. — Management's Discussion and Analysis of Financial Condition and Result of Operations of our Amendment No. 1 to Annual Report on Form 10-K/A for the fiscal year ended June 30, 2022, filed with the SEC on June 12, 2023, and is incorporated herein by reference.
The below tables summarize our results in fiscal 2023 and 2022 with respect to several financial metrics we use to measure performance. Refer to the discussions below regarding performance and the use of key financial metrics and “—Non-GAAP Metrics—Use of Constant Currency” concerning the measurement of revenue at “constant currency.”
Fiscal Year Ended June 30, 2023 compared to the Fiscal Year Ended June 30, 2022
Results for the fiscal year ended June 30, 2023 compared to the fiscal year ended June 30, 2022 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Fiscal Year Ended
June 30, | | FX Impact | | Constant Currency
Increase (Decrease) |
| 2023 | | 2022 | | | | Change $ | | Change %(1) |
| Net revenue | $ | 4,263 | | | $ | 4,802 | | | $ | (108) | | | $ | (431) | | | (9) | % |
| Cost of sales | 3,223 | | | 3,188 | | | (78) | | | 113 | | | 4 | % |
| Gross margin | 1,040 | | | 1,614 | | (30) | | | (544) | | | (34) | % |
| Selling, general, and administrative expenses | 829 | | | 844 | | | (11) | | | (4) | | | — | % |
| Gain on sale of subsidiary | — | | | (1) | | | — | | | 1 | | | * |
| Goodwill impairment charges | 210 | | | — | | | — | | | 210 | | | * |
| Other operating expense | 164 | | | 41 | | | 2 | | | 121 | | | 296 | % |
| Operating (loss) earnings | (163) | | | 730 | | | (21) | | | (872) | | | (120) | % |
| Interest expense, net | 186 | | | 123 | | | (2) | | | 65 | | | 53 | % |
| Other (income) expense, net | (7) | | | 28 | | | (11) | | | (24) | | | (85) | % |
| (Loss) earnings before income taxes | (342) | | | 579 | | | (8) | | | (913) | | | (158) | % |
| Income tax (benefit) expense | (86) | | | 80 | | | (5) | | | (161) | | | (201) | % |
| | | | | | | | | |
| | | | | | | | | |
| Net (loss) earnings | $ | (256) | | | $ | 499 | | | $ | (3) | | | $ | (752) | | | (151) | % |
| | | | | | | | | |
| | | | | | | | | |
* Not meaningful
(1) Change % calculations are based on amounts prior to rounding.
Net Revenue
| | | | | | | | | | | | | | | |
| | 2023 vs. 2022 | |
| Year-Over-Year Change | | Fiscal Year Ended
June 30, | |
| | Net Revenue | |
| Organic | | (12) | % | |
| Impact of acquisitions | | 3 | % | |
| | | |
| Constant currency change | | (9) | % | |
| Foreign currency translation impact on reporting | | (2) | % | |
| Total % change | | (11) | % | |
| | | | | |
Net revenue decreased by $431 million, or 9%, compared to the fiscal year ended June 30, 2022, excluding the impact of foreign exchange. Net revenue decreased 12% organically on a constant-currency basis, primarily related to a significant decline in demand for COVID-19 related programs and a decline in revenue from the manufacture of prescription products in the Pharma and Consumer Health segment, partially offset by growth in our gene therapy offerings, as well as growth in our clinical supply services.
Net revenue increased 3% inorganically as a result of our acquisitions of RheinCell Therapeutics GmbH (“RheinCell”) in August 2021, Bettera Wellness in October 2021, our Princeton facility and operations in April 2022, and Metrics in October 2022.
Gross Margin
Gross margin decreased by $544 million, or 34%, in fiscal 2023 compared to fiscal 2022, excluding the impact of foreign exchange, primarily due to an unfavorable shift in product mix, lower levels of utilization across the network, inventory write-offs, operational challenges that meaningfully reduced productivity, and higher costs from increased spending on operational and engineering enhancements in our Biologics segment.
On a constant-currency basis, gross margin, as a percentage of net revenue, decreased 910 basis points to 24.5% in the fiscal year ended June 30, 2023, compared to 33.6% in the prior year, primarily due to the factors described in the previous paragraph.
Selling, General, and Administrative Expense
Selling, general, and administrative expense decreased by $4 million in fiscal 2023, a negligible change compared to fiscal 2022, excluding the impact of foreign exchange. The year-over-year increase of $33 million in net incremental expenses from businesses acquired in the last 12 months were offset by a $6 million decrease in credit losses, an $11 million decline in acquisition, transaction and integration costs, and a $19 million decrease in stock-based compensation costs.
Goodwill Impairment Charges
Goodwill impairment charges during fiscal 2023 were associated with our Consumer Health reporting unit, which is part of our Pharma and Consumer Health segment. For further details, see Note 4, Goodwill to our Consolidated Financial Statements.
Other Operating Expense
Other operating expense for the fiscal years ended June 30, 2023 and 2022 was $164 million and $41 million, respectively. The year-over-year increase was primarily due to a $56 million increase in restructuring charges and a $71 million increase in fixed-asset impairment charges, partially offset by a $4 million gain from the sale of our facility in Bolton, U.K. The increase in fixed-asset impairment charges was primarily driven an idle facility in our Biologics segment and obsolete equipment that could not be sold or repurposed in the Pharma and Consumer Health segment.
Interest Expense, net
Interest expense, net, of $186 million in fiscal 2023 increased by $65 million, or 53%, compared to fiscal 2022, excluding the impact of foreign exchange. The increase was primarily attributable to incremental interest expense on our most recent tranche of term loans, increased borrowings on our Revolving Credit Facility, and the issuance of our 2030 Notes.
Other (Income) Expense, net
Other income, net of $7 million for fiscal 2023 was primarily driven by foreign currency gains of $8 million.
Other expense, net of $28 million for fiscal 2022 was primarily driven by $33 million of foreign currency losses and $4 million of financing charges related to our outstanding term loans, partially offset by a $2 million gain related to the change in fair value of the derivative liability arising from the dividend-adjustment mechanism of our formerly outstanding Series A convertible preferred stock, par value $0.01 (the “Series A Preferred Stock”).
Benefit for Income Taxes
Our benefit for income taxes for the fiscal year ended June 30, 2023 was $86 million relative to loss before income taxes of $342 million. Our provision for income taxes for the fiscal year ended June 30, 2022 was $80 million relative to earnings before income taxes of $579 million. The difference in the income tax provision/benefit in the current-year over the prior-year was largely the result of losses in the U.S., research and development credits in the U.S., the recognition of deferred tax assets on the book impairment of tax-deductible goodwill, and decreased earnings in non-U.S. jurisdictions with lower tax rates. This benefit was partially offset by fixed permanent tax adjustments in the U.S. on lower income. In comparison, the lower effective tax rate on the prior year global income was partially the result of a net deferred benefit of $21 million related to tax reform in Switzerland and related transition rules (collectively, “Swiss Tax Reform”) and additional foreign tax credits in the U.S. due to amended prior-year returns. The benefit for income taxes in each of fiscal 2023 and 2022 was also affected by the geographic distribution of our pretax income, the tax impact of permanent differences, restructuring, special items, and other discrete tax items that may have unique tax implications depending on the nature of the item.
Segment Review
The below charts depict the percentage of net revenue from each of our two reportable segments for the previous two years. Refer below for discussions regarding the segments’ net revenue and EBITDA performance and to “—Non-GAAP Metrics” for a discussion of our use of Segment EBITDA, a measure that is not defined under U.S. GAAP.
Our results on a segment basis for the fiscal year ended June 30, 2023 compared to the fiscal year ended June 30, 2022 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Fiscal Year Ended
June 30, | | FX Impact | | Constant Currency
Increase (Decrease) |
| 2023 | | 2022 | | | | Change $ | | Change % (1) |
| Biologics | | | | | | | | | |
| Net revenue | $ | 1,978 | | | $ | 2,534 | | | $ | (31) | | | $ | (525) | | | (21) | % |
| Segment EBITDA | 277 | | | 777 | | | (4) | | | (496) | | | (64) | % |
| Pharma and Consumer Health | | | | | | | | | |
| Net revenue | 2,287 | | | 2,271 | | | (77) | | | 93 | | | 4 | % |
| Segment EBITDA | 548 | | | 589 | | | (23) | | | (18) | | | (3) | % |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Inter-segment revenue elimination | (2) | | | (3) | | | — | | | 1 | | | * |
Unallocated Costs(2) | (559) | | | (286) | | | 10 | | | (283) | | | 99 | % |
| Combined totals | | | | | | | | | |
| Net revenue | $ | 4,263 | | | $ | 4,802 | | | $ | (108) | | | $ | (431) | | | (9) | % |
| | | | | | | | | |
| EBITDA from operations | $ | 266 | | | $ | 1,080 | | | $ | (17) | | | $ | (797) | | | (74) | % |
(1) Change % calculations are based on amounts prior to rounding.
* Not meaningful
(2) Unallocated costs include restructuring and special items, stock-based compensation, gain (loss) on sale of subsidiary, impairment charges, certain other corporate-directed costs, and other costs that are not allocated to the segments as follows:
| | | | | | | | | | | |
| | Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 |
Impairment charges and gain/loss on sale of assets (a) | $ | (98) | | | $ | (31) | |
| Stock-based compensation | (35) | | | (54) | |
Restructuring and other special items (b) | (98) | | | (55) | |
| | | |
| | | |
| | | |
Gain on sale of subsidiary (c) | — | | | 1 | |
Goodwill impairment charges (d) | (210) | | | — | |
Other income (expense), net (e) | 7 | | | (28) | |
| Non-allocated corporate costs, net | (125) | | | (119) | |
| Total unallocated costs | $ | (559) | | | $ | (286) | |
(a) For the fiscal year ended June 30, 2023, impairment charges and gain/loss on sale of assets include fixed-asset impairment charges that are primarily associated with an idle facility in the Biologics segment and obsolete equipment that could not be sold or repurposed in the Pharma and Consumer Health segment.
In the three months ended June 30, 2023, we identified an indicator of impairment related to one of our facilities in the Biologics segment given our plan to pause any additional spend on site development due to a lack of demand, leading to a partial impairment charge of $54 million. We primarily utilized a market and income approach for real property and a cost approach for personal property to record the partial impairment on its idle facility. Impairment charges are recorded in Other operating expense in the consolidated statements of operations.
Also, in the three months ended June 30, 2023, we identified an indicator of impairment related to obsolete equipment from a terminated project in the Pharma and Consumer Health segment, leading to a full impairment charge of $18 million.
For the fiscal year ended June 30, 2022, impairment charges and gain/loss on sale of assets include fixed-asset impairment charges associated with a product we no longer manufacture in our Pharma and Consumer Health segment.
(b) Restructuring and other special items for the fiscal year ended June 30, 2023 include (i) restructuring charges associated with the implementation of our restructuring efforts that reduced costs, consolidated facilities, and optimized infrastructure across the organization, (ii) transaction and integration costs associated with the Metrics acquisition, and (iii) warehouse exit costs for a product we no longer manufacture in our Pharma and Consumer Health segment.
Restructuring and other special items for the fiscal year ended June 30, 2022 include (i) transaction and integration costs primarily associated with the acquisition of our facility and operations in Princeton, and the Bettera Wellness, Delphi Genetics SA, Hepatic Cell Therapy Support SA, Acorda and RheinCell transactions and (ii) unrealized losses on venture capital investments.
(c) For the fiscal year ended June 30, 2022, gain on sale of subsidiary was due to the divestiture of our facility and related business in Woodstock, Illinois.
(d) Goodwill impairment charges during the fiscal year ended June 30, 2023 were associated with our Consumer Health reporting unit, which is part of our Pharma and Consumer Health segment. For further details, see Note 4, Goodwill to our Consolidated Financial Statements.
(e) Refer to Note 15, Other (Income) Expense, net for details of financing charges and foreign currency adjustments recorded within Other (Income) Expense, net in our Consolidated Financial Statements.
Provided below is a reconciliation of net earnings to EBITDA from operations:
| | | | | | | | | | | |
| | Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 |
| Net (loss) earnings | $ | (256) | | | $ | 499 | |
| Depreciation and amortization | 422 | | | 378 | |
| Interest expense, net | 186 | | | 123 | |
| Income tax (benefit) expense | (86) | | | 80 | |
| | | |
| EBITDA from operations | $ | 266 | | | $ | 1,080 | |
Biologics segment | | | | | | | | | | | |
| 2023 vs. 2022 |
| Year-Over-Year Change | Fiscal Year Ended
June 30, |
| Net Revenue | | Segment EBITDA |
| Organic | (21) | % | | (62) | % |
| Impact of acquisitions | — | % | | (2) | % |
| | | |
| Constant currency change | (21) | % | | (64) | % |
| Foreign exchange translation impact on reporting | (1) | % | | — | % |
| Total % change | (22) | % | | (64) | % |
Net revenue in our Biologics segment decreased by $525 million, or 21%, excluding the impact of foreign exchange, compared to the fiscal year ended June 30, 2022. The decrease was primarily driven by a significant decline in demand for COVID-19 related programs, partially offset by strong growth in our gene therapy offerings.
Biologics Segment EBITDA decreased by $496 million, or 64%, excluding the impacts of foreign exchange and acquisitions, compared to the fiscal year ended June 30, 2022. Segment EBITDA decreased 62%, compared to the fiscal year end June 30, 2022, excluding the impact of acquisitions. The decrease was primarily driven by a significant decline in demand for COVID-19 related programs, inventory write-offs, and lower levels of utilization across the Biologics segment, as well as an unfavorable impact from remediation-related activities at our Bloomington and Brussels facilities, which were partially offset by strong growth in our gene therapy offerings.
We completed the acquisitions of RheinCell in August 2021 and our Princeton facility and operations in April 2022. For the fiscal year ended June 30, 2023, these acquisitions decreased Segment EBITDA on an inorganic basis by 2% compared to the corresponding prior-year period and had a negligible impact on the segment's net revenue.
Pharma and Consumer Health segment
| | | | | | | | | | | |
| 2023 vs. 2022 |
| Year-Over-Year Change | Fiscal Year Ended
June 30, |
| Net Revenue | | Segment EBITDA |
| Organic | (1) | % | | (9) | % |
| Impact of acquisitions | 5 | % | | 6 | % |
| | | |
| Constant currency change | 4 | % | | (3) | % |
| Foreign exchange translation impact on reporting | (3) | % | | (4) | % |
| Total % change | 1 | % | | (7) | % |
Net revenue in our Pharma and Consumer Health segment increased by $93 million, or 4%, excluding the impact of foreign exchange, compared to the fiscal year ended June 30, 2022. Net revenue decreased 1%, compared to the fiscal year ended June 30, 2022, excluding the impact of acquisitions. The decrease in organic revenue was primarily driven by a decline in revenue from the manufacture of prescription products and a decline in demand for our consumer health products, primarily our wellness products, partially offset by growth in our clinical supply services.
Pharma and Consumer Health Segment EBITDA decreased by $18 million, or 3%, excluding the impact of foreign exchange, compared to the fiscal year ended June 30, 2022. Segment EBITDA decreased 9%, compared to the fiscal year ended June 30, 2022, excluding the impact of acquisitions. The decrease in organic Segment EBITDA was primarily driven by inflationary pressures, a decline in demand for our consumer health products, and a decline in revenue from the manufacture of prescription products, which were partially offset by growth in our clinical supply services.
We completed the Bettera Wellness acquisition in October 2021 and the Metrics acquisition in October 2022, which increased net revenue and Segment EBITDA on an inorganic basis by 5% and 6%, respectively, during the fiscal year ended June 30, 2023, compared to the corresponding prior-year period.
Liquidity and Capital Resources
Sources and Uses of Cash
Our principal source of liquidity has been cash flow generated from operations and the net proceeds of capital market activities. The principal uses of cash are to fund operating and capital expenditures, business or asset acquisitions, interest payments on debt, and any mandatory or discretionary principal payment on our debt. As of June 30, 2023, and following Operating Company's November 2022 execution of Amendment No. 7 (the “Seventh Amendment”) to the Credit Agreement, which increased the capacity of our revolving credit facility to $1.10 billion and extended its maturity to November 2027, we had available $594 million in borrowing capacity under our Revolving Credit Facility. The capacity of our Revolving Credit Facility is reduced by the amount of all outstanding letters of credit issued under the senior secured credit facilities and short-term borrowings referred to as swing-line borrowings. As of June 30, 2023, we had $6 million of outstanding letters of credit and $500 million in outstanding borrowing under our Revolving Credit Facility.
As of December 1, 2023, we have increased our borrowings under our revolving credit facility to $670 million. We believe that our cash on hand, cash from operations, and available borrowings under our Revolving Credit Facility will be adequate to meet our future liquidity needs for at least the next twelve months, including the amounts expected to become due with respect to our pending capital projects. We have no significant maturity under any of our bank or note debt until the July 2027 maturity of our 2027 Notes.
Cash Flows
Fiscal Year Ended June 30, 2023 Compared to the Fiscal Year Ended June 30, 2022
The following table summarizes our consolidated statements of cash flows for the fiscal year ended June 30, 2023 compared with the fiscal year ended June 30, 2022:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year Ended
June 30, | | |
| (Dollars in millions) | 2023 | | 2022 | | Change $ |
| Net cash provided by (used in): | | | | | |
| Operating activities | $ | 254 | | | $ | 439 | | | $ | (185) | |
| Investing activities | $ | (955) | | | $ | (1,884) | | | $ | 929 | |
| Financing activities | $ | 521 | | | $ | 1,031 | | | $ | (510) | |
Operating Activities
For the fiscal year ended June 30, 2023, cash provided by operating activities was $254 million, a decrease of $185 million compared to $439 million for the prior year. The year-over-year change was primarily due to a decrease in operating earnings, an increase in severance payments related to our restructuring plans, an increase in income taxes paid, and an increase in interest payments due to higher outstanding debt balances.
Investing Activities
For the fiscal year ended June 30, 2023, cash used in investing activities was $955 million, compared to $1.88 billion during fiscal 2022. The decrease in cash used in investing activities was primarily driven by the decrease in payment for acquisitions and an increase in proceeds from the maturity of marketable securities.
Financing Activities
For the fiscal year ended June 30, 2023, cash provided by financing activities was $521 million, which decreased $510 million compared to cash provided by financing activities of $1.03 billion during the fiscal year ended June 30, 2022. The decrease in cash provided by financing activities was primarily driven by $385 million less in borrowings and a $152 million increase in payments related to long-term obligations.
Debt and Financing Arrangements
Senior Secured Credit Facilities and Seventh and Eighth Amendments to the Credit Agreement
In November 2022, Operating Company entered into the Seventh Amendment to the Credit Agreement. Pursuant to the Seventh Amendment, Operating Company (i) terminated its existing revolving credit commitments (and the related outstanding revolving borrowings) under the Revolving Credit Facility, and (ii) obtained $1.10 billion aggregate amount of new revolving credit commitments, borrowing thereunder an amount equal to the previously outstanding borrowings under the terminated commitments so that they could be repaid. The new commitments have an interest rate margin, at Operating Company’s option, based on a (1) prime rate, plus a margin ranging from 0.750% to 1.250% based on Operating Company’s consolidated leverage ratio or (2) SOFR, plus 0.100%, plus a margin ranging from 1.750% to 2.250% based on Operating Company’s consolidated leverage ratio. The Revolving Credit Facility has a maturity date that is the earlier of (A) five years after November 22, 2022, and (B) the 91st day prior to the maturity of Operating Company’s 2027 Notes or any permitted refinancing thereof, if on such 91st day, any of the 2027 Notes remains outstanding. Otherwise, the Revolving Credit Facility under the Seventh Amendment has the same principal terms as the previously existing revolving credit commitments under the Credit Agreement.
In June 2023, Operating Company entered into Amendment No. 8 (the “Eighth Amendment”) to the Credit Agreement. Pursuant to the Eighth Amendment, effective as of July 1, 2023, the interest rate benchmark applicable to term loans under the Credit Agreement was updated, with the SOFR benchmark replacing the LIBOR benchmark. The Eighth Amendment includes a 0.1148% credit spread adjustment to the SOFR benchmark for loans with a 1-month interest period, a 0.26161% credit spread adjustment to the SOFR benchmark for loans with a 3-month interest period, and a 0.42826% credit spread adjustment to the SOFR benchmark for loans with a 6-month interest period. Other than the foregoing, the material terms of the Credit Agreement remain unchanged.
The availability of capacity under the Revolving Credit Facility is reduced by the aggregate value of all outstanding letters of credit under the Credit Agreement and outstanding borrowings under the Revolving Credit Facility. As of June 30, 2023, we had $594 million of unutilized capacity under the Revolving Credit Facility, due to $6 million of outstanding letters of credit and $500 million of outstanding borrowings on our Revolving Credit Facility.
Further information concerning the senior secured credit facilities, including our U.S. dollar-denominated term loans and the Revolving Credit Facility, can be found in Note 7, Long-Term Obligations and Short-Term Borrowings to the Consolidated Financial Statements
5.000% Senior Notes due 2027
In June 2019, Operating Company completed a private offering of the 2027 Notes. The 2027 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2027 Notes were offered in the U.S. to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the U.S. only to non-U.S. investors pursuant to Regulation S under the Securities Act. The 2027 Notes will mature on July 15, 2027 and bear interest at the rate of 5.000% per annum. Interest is payable semi-annually in arrears on January 15 and July 15 of each year. The proceeds of the 2027 Notes, after payment of the offering fees and expenses, were used to repay in full the outstanding borrowings under Operating Company's then-outstanding term loans, which would otherwise have matured in May 2024.
2.375% Euro-denominated Senior Notes due 2028
In March 2020, Operating Company completed a private offering of the 2028 Notes. The 2028 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2028 Notes were offered in the U.S. to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the U.S. only to non-U.S. investors pursuant to Regulation S under the Securities Act. The 2028 Notes will mature on March 1, 2028 and bear interest at the rate of 2.375% per annum. Interest is payable semi-annually in arrears on March 1 and September 1 of each year. The proceeds of the 2028 Notes, after payment of the offering fees and expenses, were used to repay in full the outstanding borrowings under Operating Company's euro-denominated term loans, which would otherwise have matured in May 2024, and to repay in full our Euro-denominated 4.75% Senior Notes due 2024, which would otherwise have matured in December 2024, plus accrued and unpaid interest thereon, with the remainder available for general corporate purposes.
3.125% Senior Notes due 2029
In February 2021, Operating Company completed a private offering of the 2029 Notes. The 2029 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2029 Notes were offered in the U.S. to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the U.S. only to non-U.S. investors pursuant to Regulation S under the Securities Act. The 2029 Notes will mature on February 15, 2029 and bear interest at the rate of 3.125% per annum payable semi-annually in arrears on February 15 and August 15 of each year. The proceeds of the 2029 Notes, after payment of the
offering fees and expenses were used to repay in full the outstanding borrowings under an earlier issue of unsecured notes, which would have otherwise matured in 2026, plus accrued and unpaid interest thereon, with the remainder available for general corporate purposes.
3.500% Senior Notes due 2030
In September 2021, Operating Company completed a private offering of the 2030 Notes. The 2030 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2030 Notes were offered in the U.S. to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the U.S. only to non-U.S. investors pursuant to Regulation S under the Securities Act. The 2030 Notes will mature on April 1, 2030 and bear interest at the rate of 3.500% per annum payable semi-annually in arrears on April 1 and October 1 of each year. The proceeds of the 2030 Notes, after payment of the offering fees and expenses, were used to fund a portion of the consideration paid at the closing of the Bettera Wellness acquisition.
Debt Covenants
Senior Secured Credit Facilities
The Credit Agreement contains covenants that, among other things, restrict, subject to certain exceptions, Operating Company’s (and Operating Company’s restricted subsidiaries’) ability to incur additional indebtedness or issue certain preferred shares; create liens on assets; engage in mergers and consolidations; sell assets; pay dividends and distributions or repurchase capital stock; repay subordinated indebtedness; engage in certain transactions with affiliates; make investments, loans, or advances; make certain acquisitions; enter into sale and leaseback transactions; amend material agreements governing Operating Company’s subordinated indebtedness; and change Operating Company’s lines of business.
The Credit Agreement also contains change-of-control provisions and certain customary affirmative covenants and events of default. The Revolving Credit Facility requires compliance with a net leverage covenant when there is a 30% or more draw outstanding at a period end. As of June 30, 2023, Operating Company was in compliance with all material covenants under the Credit Agreement.
Subject to certain exceptions, the Credit Agreement permits Operating Company and its restricted subsidiaries to incur certain additional indebtedness, including secured indebtedness. None of Operating Company’s non-U.S. subsidiaries nor its dormant Puerto Rico subsidiary is a guarantor of the loans.
Under the Credit Agreement, Operating Company’s ability to engage in certain activities such as incurring certain additional indebtedness, making certain investments, and paying certain dividends is tied to ratios based on Adjusted EBITDA (which is defined as “Consolidated EBITDA” in the Credit Agreement). Adjusted EBITDA is based on the definitions in the Credit Agreement, is not defined under U.S. GAAP, and is subject to important limitations. See “Non-GAAP Metrics” for further details on Adjusted EBITDA.
As market conditions warrant, we may from time to time seek to purchase our outstanding debt in privately negotiated or open-market transactions, by tender offer or otherwise. Subject to any limitation contained in the Credit Agreement, any purchase made by us may be funded by the use of cash on hand or the incurrence of new secured or unsecured debt. The amount involved in any such purchase transaction, individually or in the aggregate, may be material. Any such purchase may involve a substantial amount of one particular class or series of debt, with the attendant reduction in the trading liquidity of such class or series.
The Senior Notes
The Indentures govern the terms of our outstanding senior notes and contain certain covenants that, among other things, limit our ability to incur or guarantee more debt or issue certain preferred shares; pay dividends on, repurchase, or make distributions in respect of their capital stock or make other restricted payments; make certain investments; sell certain assets; create liens; consolidate, merge, sell; or otherwise dispose of all or substantially all of their assets; enter into certain transactions with their affiliates, and designate their subsidiaries as unrestricted subsidiaries. These covenants are subject to a number of exceptions, limitations, and qualifications as set forth in the Indentures. The Indentures also contain customary events of default, including, but not limited to, nonpayment, breach of covenants, and payment or acceleration defaults in certain other indebtedness of Operating Company or certain of its subsidiaries. Upon an event of default, either the holders of at least 30% in principal amount of each of the then-outstanding series of Senior Notes, or the applicable trustee under the Indentures, may declare the applicable senior notes immediately due and payable; or in certain circumstances, the applicable senior notes will become automatically immediately due and payable. As of June 30, 2023, Operating Company was in compliance with all material covenants under the Indentures.
Liquidity in Foreign Subsidiaries
As of June 30, 2023 and 2022, the amounts of cash and cash equivalents held by foreign subsidiaries were $181 million and $377 million, respectively, out of total consolidated cash and cash equivalents of $280 million and $449 million, respectively. These balances are dispersed across many international locations around the world.
Adjusted EBITDA and Adjusted Net Income per Share
The below tables summarize our fiscal 2023 and 2022 results with respect to certain financial metrics we use to measure performance throughout the fiscal year. Refer to “Non-GAAP Metrics” for further details regarding Adjusted EBITDA and Adjusted net income per share.
A reconciliation between Adjusted EBITDA and net earnings, the most directly comparable measure under U.S. GAAP, which also shows the adjustments from EBITDA from operations, follows:
| | | | | | | | | | | |
| Fiscal Year Ended |
| (In millions) | June 30, 2023 | | June 30, 2022 |
| Net (loss) earnings | $ | (256) | | | $ | 499 | |
| Interest expense, net | 186 | | | 123 | |
Income tax (benefit) expense | (86) | | | 80 | |
| Depreciation and amortization | 422 | | | 378 | |
| | | |
| EBITDA from operations | 266 | | | 1,080 | |
| Goodwill impairment charges | 210 | | | — | |
| Stock-based compensation | 35 | | | 54 | |
| Impairment charges and gain/loss on sale of assets | 98 | | | 31 | |
| Financing-related expenses and other | — | | | 4 | |
| Restructuring costs | 66 | | | 10 | |
| Acquisition, integration, and other special items | 31 | | | 46 | |
| Gain on sale of subsidiary | — | | | (1) | |
| | | |
Foreign exchange (gain) loss (included in other, net) (1) | (11) | | | 31 | |
| Inventory fair value step-up charges | — | | | 7 | |
| Other adjustments | 2 | | | (3) | |
| | | |
| | | |
| | | |
| Adjusted EBITDA | $ | 697 | | | $ | 1,259 | |
| Favorable (unfavorable) FX impact | (17) | | | |
| Adjusted EBITDA - constant currency | $ | 714 | | | |
(1) Foreign exchange gain of $11 million for the fiscal year ended June 30, 2023 includes $10 million of unrealized gains related to foreign trade receivables and payables and intercompany transactions.
Foreign exchange loss of $31 million for the fiscal year ended June 30, 2022 includes: (a) $12 million of unrealized gains related to foreign trade receivables and payables, (b) $11 million of unrealized losses on the unhedged portion of our euro-denominated debt, and (c) $34 million of unrealized losses on inter-company loans. The foreign exchange adjustment was also affected by the exclusion of realized foreign currency exchange rate gains from the settlement of inter-company loans of $2 million. Inter-company loans exist between our subsidiaries and do not reflect the ongoing results of our trade operations.
A reconciliation between Adjusted Net Income and net earnings, the most directly comparable measure under U.S. GAAP, follows.
| | | | | | | | | | | |
| Fiscal Year Ended |
| (In millions, except per share data) | June 30, 2023 | | June 30, 2022 |
| Net (loss) earnings | (256) | | | $ | 499 | |
| | | |
| | | |
Amortization (1) | 136 | | | 123 | |
| | | |
| Goodwill impairment charges | 210 | | | — | |
| Stock-based compensation | 35 | | | 54 | |
| Impairment charges and gain/loss on sale of assets | 98 | | | 31 | |
| Financing-related expenses | — | | | 4 | |
| Restructuring costs | 66 | | | 10 | |
| Acquisition, integration, and other special items | 31 | | | 46 | |
| | | |
| (Gain) on sale of subsidiary | — | | | (1) | |
Foreign exchange (gain) loss (included in other expense, net) (2) | (11) | | | 31 | |
| Inventory fair value step-up charges | — | | | 7 | |
| Other adjustments | 2 | | | (4) | |
Estimated tax effect of adjustments (3) | (126) | | | (72) | |
Discrete income tax benefit items (4) | (18) | | | (54) | |
| | | |
| Adjusted net income (ANI) | $ | 167 | | | $ | 674 | |
| | | |
| | | |
| | | |
| | | |
| ANI per share: | | | |
ANI per share - basic (5) | $ | 0.92 | | | $ | 3.82 | |
ANI per share - diluted (6) | $ | 0.92 | | | $ | 3.73 | |
(1) Represents the amortization attributable to purchase accounting for previously completed business combinations.
(2) Foreign exchange gain of $11 million for the fiscal year ended June 30, 2023 includes $10 million of unrealized gains related to foreign trade receivables and payables intercompany transactions.
Foreign exchange loss of $31 million for the fiscal year ended June 30, 2022 includes: (a) $12 million of unrealized gains related to foreign trade receivables and payables, (b) $11 million of unrealized losses on the unhedged portion of the euro-denominated debt, and (c) $34 million of unrealized losses on inter-company loans. The foreign exchange adjustment was also affected by the exclusion of realized foreign currency exchange rate gains from the settlement of inter-company loans of $2 million. Inter-company loans exist between our subsidiaries and do not reflect the ongoing results of our trade operations.
(3) We computed the tax effect of adjustments to net earnings by applying the statutory tax rate in the relevant jurisdictions to the income or expense items that are adjusted in the period presented. If a valuation allowance exists, the rate applied is zero.
(4) Discrete period income tax expense (benefit) items are unusual or infrequently occurring items, primarily including: changes in judgment related to the realizability of deferred tax assets in future years, changes in measurement of a prior-year tax position, deferred tax impact of changes in tax law, and purchase accounting.
(5) Represents Adjusted Net Income divided by the weighted average number of shares of Common Stock outstanding. For the fiscal year ended June 30, 2023 and 2022, the weighted average was 181 million and 176 million, respectively.
(6) Represents Adjusted Net Income divided by the weighted average sum of (a) the number of shares of Common Stock outstanding, plus (b) the number of shares of Common Stock that would be issued assuming exercise or vesting of all potentially dilutive instruments, plus, in fiscal 2022, (c) the number of shares of Common Stock equivalent to the shares of Series A Preferred Stock outstanding under the “if-converted” method. For the fiscal years ended June 30, 2023 and 2022, the weighted average was 181 million.
Interest Rate Risk Management
We have historically used interest-rate swaps to manage the economic effect of variable-rate interest obligations associated with our floating-rate term loans so that the interest payable on at least a portion of the term loans effectively becomes fixed at a certain rate, thereby reducing the impact of future interest-rate changes on our future interest expense.
A portion of our bank and note debt is exposed to interest-rate fluctuations. We have in the past used and may continue to use various hedging strategies and derivative financial instruments to create an appropriate mix of fixed- and floating-rate assets and liabilities. In February 2021, we entered into an interest-rate swap agreement with Bank of America N.A. that acted as a hedge against the economic effect of a portion of the variable-interest obligation associated with our U.S. dollar-denominated term loans under our senior secured credit facilities, so that the interest payable on that portion of the debt became fixed at a certain rate, thereby reducing the impact of future interest-rate increases on future interest expense (the “2021 Rate Swap”). From June 30, 2021 until the effective date of the Eighth Amendment, the applicable rate for the U.S. dollar-denominated term loan under the Credit Agreement was one-month LIBOR (subject to a floor of 0.50%) plus 2.00%; however, as a result of the 2021 Rate Swap, the variable portion of the applicable rate on $500 million of the U.S. dollar-denominated term loans was effectively fixed during this period at 0.9985%.
To conform with the adoption of ASC 848, Reference Rate Reform and the Eighth Amendment, the Company amended the 2021 Rate Swap in June 2023 (the “2023 Rate Swap”). The 2023 Rate Swap continues to effectively fix the rate of interest payable on the same portion of our U.S dollar-denominated term loans under our secured credit facilities. The applicable rate for the U.S. dollar-denominated term loan under the Credit Agreement was SOFR (subject to a floor of 0.39%) plus 2.00% as of June 30, 2023. As a result of the 2023 Rate Swap, the variable portion of the applicable interest rate on $500 million of the U.S. dollar-denominated term loans is now effectively fixed at 0.9431%.
Currency Risk Management
We are exposed to fluctuations in the euro-U.S. dollar exchange rate on our investments in our foreign operations in Europe. While we do not actively hedge against changes in foreign currency, we have mitigated the exposure of our investments in our European operations by denominating a portion of our bank and note debt in euros. As of June 30, 2023, we had $904 million of euro-denominated debt outstanding that qualifies as a hedge on a net investment in foreign operations. Refer to Note 9, Derivative Instruments and Hedging Activities, to our Consolidated Financial Statements for further discussion of net investment hedge activity in the period.
From time to time, we may use forward currency exchange contracts to manage our exposure to the variability of cash flows primarily related to the foreign exchange rate changes of future foreign currency transaction costs. In addition, we may use foreign currency forward contracts to protect the value of existing foreign currency assets and liabilities. Currently, we do not use foreign currency exchange contracts. We expect to continue to evaluate hedging opportunities for foreign currency in the future.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to cash flow and earnings fluctuations as a result of certain market risks. These market risks primarily relate to changes in interest rates associated with our bank and note debt obligations and foreign exchange rate changes.
Interest Rate Risk
A portion of the debt used to finance our operations is exposed to interest-rate fluctuations. We may use various hedging strategies and derivative financial instruments to create an appropriate mix of fixed- and floating-rate assets and liabilities. We entered into the 2021 Rate Swap with Bank of America N.A., which acted as a hedge against the economic effect of a portion of the variable-interest obligation associated with our U.S. dollar-denominated term loans under our senior secured credit facilities, so that the interest payable on that portion of the debt became fixed at a certain rate, thereby reducing the impact of future interest-rate changes on future interest expense. From June 30, 2021 until the effective date of the Eighth Amendment, the applicable rate for the U.S. dollar-denominated term loan under the Credit Agreement was one-month LIBOR (subject to a floor of 0.50%) plus 2.00%; however, as a result of the 2021 Rate Swap, the variable portion of the applicable rate on $500 million of the U.S. dollar-denominated term loans was effectively fixed during this period at 0.9985%.
To conform with the adoption of ASC 848 and the Eighth Amendment, the Company amended the 2021 Rate Swap as the 2023 Rate Swap. The 2023 Rate Swap continues to effectively fix the rate of interest payable on the same portion of our U.S dollar-denominated term loans under our secured credit facilities. The applicable rate for the U.S. dollar-denominated term loan under the Credit Agreement was SOFR (subject to a floor of 0.39%) plus 2.00% as of June 30, 2023. As a result of the 2023 Rate Swap, the variable portion of the applicable interest rate on $500 million of the U.S. dollar-denominated term loans is now effectively fixed at 0.9431%.
A hypothetical 50 basis change to the variable rate component of our variable rate indebtedness would change our annual interest expense by $7 million.
Foreign Currency Exchange Risk
By the nature of our global operations, we are exposed to cash flow and earnings fluctuations resulting from foreign exchange-rate variation. These exposures are transactional and translational in nature. Since we manufacture and sell our products globally, our foreign-currency risk is diversified. Principal drivers of this diversified foreign-exchange exposure include the European euro, British pound, Argentinean peso, and Brazilian real. Our transactional exposure arises from the purchase and sale of goods and services in currencies other than the functional currency of our operational units. We also have exposure related to the translation of financial statements of our foreign divisions into U.S. dollars, our functional currency. The financial statements of our operations outside the U.S. are measured using the local currency as the functional currency, except in Argentina, a hyper-inflationary economy, where our results are measured in U.S. dollars. Adjustments to translate the assets and liabilities of these foreign operations in U.S. dollars are accumulated as a component of other comprehensive income utilizing period-end exchange rates. Foreign-currency transaction gains and losses calculated by utilizing weighted average exchange rates for the period are included in the statements of operations in other expense, net. Such foreign currency transaction gains and losses include inter-company loans denominated in non-U.S. dollar currencies.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO FINANCIAL STATEMENTS
Consolidated Financial Statements as of June 30, 2023 and 2022 and for the years ended June 30, 2023, 2022 and 2021.
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Catalent, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Catalent, Inc. (the Company) as of June 30, 2023 and 2022, the related consolidated statements of operations, comprehensive (loss) income, changes in shareholders’ equity and cash flows for each of the three years in the period ended June 30, 2023, and the related notes and financial statement schedule listed in the Index at Item 15(a)(2) (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at June 30, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2023, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of June 30, 2023, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated December 8, 2023, expressed an adverse opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the account or disclosure to which it relates.
| | | | | |
| Goodwill impairment assessment for the Consumer Health reporting unit |
| |
| Description of the Matter | As of June 30, 2023, the Company’s consolidated goodwill balance was $3,039 million. As discussed in Notes 1 and 4 of the consolidated financial statements, the Company performs an impairment evaluation of goodwill annually during the fourth quarter of its fiscal year or when circumstances otherwise indicate an evaluation should be performed. As of March 31, 2023, the Company identified an indicator of impairment requiring an interim impairment assessment. The evaluation resulted in the Company recording a goodwill impairment loss of $210 million in its Consumer Health reporting unit in the third quarter of fiscal 2023. The Company estimates the fair value of its reporting units using a combination of income and market approaches. |
| |
| | | | | |
| Auditing management's goodwill impairment assessment for the Consumer Health reporting unit was complex and judgmental due to the significant estimation required in determining the fair value of the reporting unit. In particular, the fair value estimate was sensitive to significant assumptions such as revenue growth rates, EBITDA margin and discount rate, which are affected by expectations about business, market and overall economic conditions. Further, the identified material weakness relating to management not adequately preparing and maintaining evidence of their review of significant assumptions relating to the interim goodwill impairment assessment affected our audit procedures in this area. |
| |
| How We Addressed the Matter in Our Audit | To test the estimated fair value of the Consumer Health reporting unit, our audit procedures included, among others, involving an internal valuation specialist to assist in our evaluation of the methodologies and assumptions used by the Company. We evaluated whether management’s methodology for determining the discount rate reflected the risk associated with the forecasted cash flows of the reporting unit. We assessed the reasonableness of the Company’s assumptions of forecasted revenue growth rates and EBITDA margin by comparing to recent historical performance, current economic and industry trends, and other relevant factors, and performing sensitivity analyses. We also evaluated management’s historical accuracy of forecasting financial results by comparing past forecasts to subsequent actual activity. The nature and extent of our audit procedures considered the inability to rely on controls over management’s goodwill impairment review process as a result of the material weakness described above. |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
|
|
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2007.
Iselin, New Jersey
December 8, 2023
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Catalent, Inc.
Opinion on Internal Control Over Financial Reporting
We have audited Catalent, Inc.’s internal control over financial reporting as of June 30, 2023, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, because of the effect of the material weaknesses described below on the achievement of the objectives of the control criteria, Catalent, Inc. (the Company) has not maintained effective internal control over financial reporting as of June 30, 2023, based on the COSO criteria.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in management’s assessment. Management has identified material weaknesses in controls related to modifications to arrangements accounted for under ASC 606, Revenue from Contracts with Customers, management’s review of certain complex and non-routine transactions and controls over inventory.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of the Company as of June 30, 2023 and 2022, the related consolidated statements of operations, comprehensive (loss) income, changes in shareholders’ equity and cash flows for each of the three years in the period ended June 30, 2023, and the related notes and financial statement schedule listed in the Index at Item 15(a)(2). These material weaknesses were considered in determining the nature, timing and extent of audit tests applied in our audit of the 2023 consolidated financial statements, and this report does not affect our report dated December 8, 2023, which expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Iselin, New Jersey
December 8, 2023
Catalent, Inc.
Consolidated Balance Sheets
(Dollars in millions, except share and per share data) | | | | | | | | | | | |
| June 30,
2023 | | June 30,
2022 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 280 | | | $ | 449 | |
Trade receivables, net of allowance for credit losses of $46 and $29, respectively | 1,002 | | | 1,051 | |
| Inventories | 777 | | | 702 | |
| Prepaid expenses and other | 633 | | | 626 | |
| Marketable securities | — | | | 89 | |
| Total current assets | 2,692 | | | 2,917 | |
| Property, plant, and equipment, net | 3,682 | | | 3,127 | |
| Other assets: | | | |
| Goodwill | 3,039 | | | 3,006 | |
| Other intangibles, net | 980 | | | 1,060 | |
| Deferred income taxes | 55 | | | 49 | |
| Other long-term assets | 329 | | | 349 | |
| Total assets | $ | 10,777 | | | $ | 10,508 | |
| | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY |
| Current liabilities: | | | |
| Current portion of long-term obligations and other short-term borrowings | $ | 536 | | | $ | 31 | |
| Accounts payable | 424 | | | 421 | |
| Other accrued liabilities | 570 | | | 646 | |
| Total current liabilities | 1,530 | | | 1,098 | |
| Long-term obligations, less current portion | 4,313 | | | 4,171 | |
| Pension liability | 100 | | | 103 | |
| Deferred income taxes | 76 | | | 197 | |
| Other liabilities | 147 | | | 164 | |
| Commitment and contingencies (see Note 17) | | | |
| Total liabilities | 6,166 | | | 5,733 | |
Redeemable preferred stock, $0.01 par value; 0 shares authorized at June 30, 2023 and 2022; 0 shares issued and outstanding at June 30, 2023 and 2022 | — | | | — | |
| | | |
| | | |
| Shareholders’ equity: | | | |
Common stock, $0.01 par value; 1 billion shares authorized at June 30, 2023 and 2022; 180 million and 179 million shares issued and outstanding at June 30, 2023 and 2022, respectively | 2 | | | 2 | |
| | | |
Preferred stock, $0.01 par value; 100 million shares authorized at June 30, 2023 and 2022; 0 shares issued and outstanding at June 30, 2023 and 2022 | — | | | — | |
| Additional paid in capital | 4,701 | | | 4,649 | |
| Retained earnings | 262 | | | 518 | |
| Accumulated other comprehensive loss | (354) | | | (394) | |
| | | |
| | | |
| Total shareholders’ equity | 4,611 | | | 4,775 | |
| Total liabilities and shareholders’ equity | $ | 10,777 | | | $ | 10,508 | |
The accompanying notes are an integral part of these consolidated financial statements.
Catalent, Inc.
Consolidated Statements of Operations
(Dollars in millions, except per share data)
| | | | | | | | | | | | | | | | | |
| | Fiscal Year Ended June 30, |
| | 2023 | | 2022 | | 2021 |
| Net revenue | $ | 4,263 | | | $ | 4,802 | | | $ | 3,998 | |
| Cost of sales | 3,223 | | | 3,188 | | | 2,646 | |
| Gross margin | 1,040 | | | 1,614 | | | 1,352 | |
| Selling, general, and administrative expenses | 829 | | | 844 | | | 687 | |
| Gain on sale of subsidiary | — | | | (1) | | | (182) | |
| Goodwill impairment charges | 210 | | | — | | | — | |
| Other operating expense | 164 | | | 41 | | | 19 | |
| | | | | |
| Operating (loss) earnings | (163) | | | 730 | | | 828 | |
| Interest expense, net | 186 | | | 123 | | | 110 | |
| Other (income) expense, net | (7) | | | 28 | | | 3 | |
| (Loss) earnings before income taxes | (342) | | | 579 | | | 715 | |
| Income tax (benefit) expense | (86) | | | 80 | | | 130 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Net (loss) earnings | (256) | | | 499 | | | 585 | |
| Less: Net earnings attributable to preferred shareholders | — | | | (16) | | | (56) | |
| Net (loss) earnings attributable to common shareholders | $ | (256) | | | $ | 483 | | | $ | 529 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | |
| Earnings (loss) per share: | | | | | |
| Basic | | | | | |
| | | | | |
| Net (loss) earnings | $ | (1.42) | | | $ | 2.74 | | | $ | 3.15 | |
| Diluted | | | | | |
| | | | | |
| Net (loss) earnings | $ | (1.42) | | | $ | 2.73 | | | $ | 3.11 | |
The accompanying notes are an integral part of these consolidated financial statements.
Catalent, Inc.
Consolidated Statements of Comprehensive (Loss) Income
(Dollars in millions)
| | | | | | | | | | | | | | | | | |
| Fiscal year ended June 30, |
| 2023 | | 2022 | | 2021 |
| Net (loss) earnings | $ | (256) | | | $ | 499 | | | $ | 585 | |
| Other comprehensive (loss) income, net of tax | | | | | |
| Foreign currency translation adjustments | 32 | | | (110) | | | 67 | |
| Defined benefit pension plan | (14) | | | 9 | | | — | |
| Net change in marketable securities | 4 | | | (3) | | | (1) | |
| | | | | |
| Derivatives and hedges | 18 | | | 27 | | | 3 | |
| | | | | |
| Other comprehensive income (loss), net of tax | 40 | | | (77) | | | 69 | |
| Comprehensive (loss) income | $ | (216) | | | $ | 422 | | | $ | 654 | |
| | | | | |
| | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
Catalent, Inc.
Consolidated Statement of Changes in Shareholders’ Equity
(Dollars in millions, except share data in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Columns may not foot due to rounding | Shares of Common Stock | | Common Stock | | Additional Paid in Capital | | Accumulated Earnings (Deficit) | | Accumulated Other Comprehensive Loss | | | | Total Shareholders’ Equity | | Redeemable Preferred Stock | |
| Balance at June 30, 2020 | 162,788 | | | $ | 2 | | | $ | 3,818 | | | $ | (535) | | | $ | (386) | | | | | $ | 2,899 | | | $ | 607 | | |
| Equity offering, sale of common stock | 1,163 | | | — | | | 82 | | | — | | | — | | | | | 82 | | | — | | |
| Share issuances related to stock-based compensation | 1,206 | | | — | | | — | | | — | | | — | | | | | — | | | — | | |
| Conversion of redeemable preferred stock | 5,392 | | | — | | | 253 | | | — | | | — | | | | | 253 | | | (248) | | |
| Stock-based compensation | — | | | — | | | 51 | | | — | | | — | | | | | 51 | | | — | | |
| Cash paid, in lieu of equity, for tax withholding | — | | | — | | | (46) | | | — | | | — | | | | | (46) | | | — | | |
| Exercise of stock options | — | | | — | | | 38 | | | — | | | — | | | | | 38 | | | — | | |
| Employee stock purchase plan | — | | | — | | | 9 | | | — | | | — | | | | | 9 | | | — | | |
| Preferred dividend ($12.50 per share of redeemable preferred stock) | — | | | — | | | — | | | (25) | | | — | | | | | (25) | | | — | | |
| Net earnings | — | | | — | | | — | | | 585 | | | — | | | | | 585 | | | — | | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | — | | | 69 | | | | | 69 | | | — | | |
| Balance at June 30, 2021 | 170,549 | | | 2 | | | 4,205 | | | 25 | | | (317) | | | | | 3,915 | | | 359 | | |
| Share issuances related to stock-based compensation | 935 | | | — | | | — | | | — | | | — | | | | | — | | | — | | |
| Conversion of redeemable preferred stock | 7,818 | | | — | | | 362 | | | — | | | — | | | | | 362 | | | (359) | | |
| Stock-based compensation | — | | | — | | | 54 | | | — | | | — | | | | | 54 | | | — | | |
| Cash paid, in lieu of equity, for tax withholding | — | | | — | | | (10) | | | — | | | — | | | | | (10) | | | — | | |
| Exercise of stock options | — | | | — | | | 26 | | | — | | | — | | | | | 26 | | | — | | |
| Employee stock purchase plan | — | | | — | | | 12 | | | — | | | — | | | | | 12 | | | — | | |
| Preferred dividend ($12.50 per share of redeemable preferred stock) | — | | | — | | | — | | | (6) | | | — | | | | | (6) | | | — | | |
| Net earnings | — | | | — | | | — | | | 499 | | | — | | | | | 499 | | | — | | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | — | | | (77) | | | | | (77) | | | — | | |
| Balance at June 30, 2022 | 179,302 | | | 2 | | | 4,649 | | | 518 | | | (394) | | | | | 4,775 | | | — | | |
| | | | | | | | | | | | | | | | |
| Share issuances related to stock-based compensation | 971 | | | — | | | — | | | — | | | — | | | | | — | | | — | | |
| Stock-based compensation | — | | | — | | | 35 | | | — | | | — | | | | | 35 | | | — | | |
| Exercise of stock options | — | | | — | | | 4 | | | — | | | — | | | | | 4 | | | — | | |
| Employee stock purchase plan | — | | | — | | | 13 | | | — | | | — | | | | | 13 | | | — | | |
| Net loss | — | | | — | | | — | | | (256) | | | — | | | | | (256) | | | — | | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | — | | | 40 | | | | | 40 | | | — | | |
| Balance at June 30, 2023 | 180,273 | | | 2 | | | 4,701 | | | 262 | | | (354) | | | | | 4,611 | | | — | | |
The accompanying notes are an integral part of these consolidated financial statements.
Catalent, Inc.
Consolidated Statements of Cash Flows
(Dollars in millions)
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, |
| 2023 | | 2022 | | 2021 |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | |
Net (loss) earnings | $ | (256) | | | $ | 499 | | | $ | 585 | |
| | | | | |
| | | | | |
Adjustments to reconcile net earnings to net cash from operations: | | | | | |
| Depreciation and amortization | 422 | | | 378 | | | 289 | |
| Goodwill impairment charges | 210 | | | — | | | — | |
| Non-cash foreign currency transaction (gain) loss, net | (9) | | | 30 | | | (4) | |
Amortization of debt financing costs | 8 | | | 7 | | | 11 | |
| Non-cash restructuring charges | 18 | | | — | | | — | |
Impairment charges and loss/gain on sale of assets, net | 98 | | | 31 | | | 9 | |
| Gain on sale of subsidiary | — | | | (1) | | | (182) | |
Financing-related charges | — | | | 4 | | | 18 | |
| Gain on derivative instrument | — | | | (2) | | | (17) | |
| Stock-based compensation | 35 | | | 54 | | | 51 | |
| (Benefit) provision for deferred income taxes | (127) | | | 9 | | | 64 | |
| Provision for bad debts and inventory | 143 | | | 17 | | | 41 | |
| Change in operating assets and liabilities: | | | | | |
Decrease (increase) in trade receivables | 53 | | | (73) | | | (186) | |
Increase in inventories | (192) | | | (128) | | | (260) | |
| (Decrease) increase in accounts payable | (21) | | | 37 | | | 50 | |
Other assets/accrued liabilities, net - current and non-current | (128) | | | (423) | | | (36) | |
| | | | | |
| | | | | |
Net cash provided by operating activities | 254 | | | 439 | | | 433 | |
| CASH FLOWS USED IN INVESTING ACTIVITIES: | | | | | |
| Acquisition of property and equipment | (576) | | | (660) | | | (686) | |
| Proceeds from maturity (purchases) of marketable securities | 89 | | | (20) | | | (72) | |
| Proceeds from sale of property and equipment | 8 | | | — | | | — | |
| (Settlement on) proceeds from sale of subsidiaries | — | | | (3) | | | 287 | |
Payment for acquisitions, net of cash acquired | (474) | | | (1,199) | | | (147) | |
| Payment made for investments | (2) | | | (2) | | | (31) | |
| | | | | |
| | | | | |
Net cash used in investing activities | (955) | | | (1,884) | | | (649) | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | |
| | | | | |
Proceeds from borrowings | 715 | | | 1,100 | | | 166 | |
| Payments related to financing obligations | (230) | | | (78) | | | (67) | |
| | | | | |
Financing fees paid | (4) | | | (15) | | | (19) | |
| Dividends paid | — | | | (4) | | | (22) | |
| | | | | |
Proceeds from sale of common stock, net | — | | | — | | | 82 | |
| | | | | |
Exercise of stock options | 4 | | | 26 | | | 38 | |
| Cash paid, in lieu of equity, for tax withholding obligation | — | | | (10) | | | (46) | |
| Other financing activities | 36 | | | 12 | | | 10 | |
| | | | | |
| | | | | |
Net cash provided by financing activities | 521 | | | 1,031 | | | 142 | |
| | | | | |
| Effect of foreign currency on cash | 11 | | | (33) | | | 17 | |
| NET DECREASE IN CASH AND EQUIVALENTS | (169) | | | (447) | | | (57) | |
| CASH AND EQUIVALENTS AT BEGINNING OF PERIOD | 449 | | | 896 | | | 953 | |
| CASH AND EQUIVALENTS AT END OF PERIOD | $ | 280 | | | $ | 449 | | | $ | 896 | |
| SUPPLEMENTARY CASH FLOW INFORMATION: | | | | | |
| Interest paid | $ | 188 | | | $ | 116 | | | $ | 105 | |
| Income taxes paid, net | $ | 99 | | | $ | 53 | | | $ | 47 | |
| Non-cash purchase of property, equipment | $ | 18 | | | $ | 6 | | | $ | — | |
| SUPPLEMENTARY DISCLOSURE OF NON-CASH INVESTING AND FINANCING ACTIVITY: | | | | | |
| Issuance of Common Stock from partial conversion of redeemable preferred stock | $ | — | | | $ | 362 | | | $ | 253 | |
| Note receivable from sale of Blow-Fill-Seal Business | $ | — | | | $ | — | | | $ | 47 | |
The accompanying notes are an integral part of these consolidated financial statements.
Catalent, Inc.
Notes to Consolidated Financial Statements
1. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business
Catalent, Inc. (“Catalent” or the “Company”) directly and wholly owns PTS Intermediate Holdings LLC (“PTS Intermediate”). PTS Intermediate directly and wholly owns Catalent Pharma Solutions, Inc. (“Operating Company”). The financial results of Catalent are primarily comprised of the financial results of Operating Company and its subsidiaries on a consolidated basis.
The Company’s common stock, par value $0.01 (the “Common Stock”) trades on the New York Stock Exchange under the symbol “CTLT”.
The Company provides differentiated development and manufacturing solutions for drugs, protein-based biologics, cell, and gene therapies, vaccines, and consumer health products at over fifty facilities across four continents under rigorous quality and operational standards. Its oral, injectable, and respiratory delivery technologies, along with its state-of-the-art protein, plasmid, viral, and cell and gene therapy manufacturing capacity address a wide and growing range of modalities and therapeutic and other categories across the biopharmaceutical, pharmaceutical, and consumer health industries.
Reportable Segments
Effective July 1, 2022, in connection with the appointment of a new President and Chief Executive Officer, the Company changed its operating structure and reorganized its executive leadership team accordingly. The current organizational structure includes two operating and reportable segments—(i) Biologics and (ii) Pharma and Consumer Health—which are summarized below.
•The Biologics segment provides development and manufacturing for biologic proteins; cell, gene, and other nucleic acid therapies; plasmid DNA (“pDNA”); induced pluripotent stem cells (“iPSCs”), and oncolytic viruses; and vaccines. It also provides formulation, development, and manufacturing for parenteral dose forms, including vials, prefilled syringes, and cartridges; analytical development and testing services for large molecules.
•The Pharma and Consumer Health segment comprises the Company’s market-leading capabilities for complex oral solids, softgel formulations, Zydis® fast-dissolve technologies, and gummy, soft chew, and lozenge dosage forms; formulation, development, and manufacturing platforms for oral, nasal, inhaled, and topical dose forms; and clinical trial development and supply services.
Each segment reports through a separate management team and ultimately reports to the Company's President and Chief Executive Officer, who is designated as the Chief Operating Decision Maker for segment reporting purposes. The Company's operating segments are the same as its reportable segments. All prior-period comparative segment information has been recast retrospectively to reflect the current reportable segments in accordance with Accounting Standards Codification (“ASC”) 280, Segment Reporting, promulgated by the Financial Accounting Standards Board (the “FASB”).
Basis of Presentation
These financial statements include all of the Company’s subsidiaries, including those operating outside the United States (“U.S.”), and are prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). All significant transactions among the Company’s subsidiaries and reporting segments have been eliminated, other than as noted.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires the Company's management to make estimates and assumptions that affect amounts reported in the financial statements and accompanying notes. Such estimates include, but are not limited to, revenue recognition, including determining the transaction price and any associated constraint on variable consideration, allowance for credit losses, inventory and long-lived asset valuation, goodwill and other intangible asset valuation and impairment, equity-based compensation, income taxes, derivative valuation, and pension plan asset and liability valuation. Actual amounts may differ from these estimated amounts.
Reclassification
Certain prior-period amounts were reclassified to conform to the current period presentation. These reclassifications did not have a material impact on the consolidated statements of operations, consolidated balance sheets, consolidated statements of cash flows, or notes to the consolidated financial statements.
Foreign Currency Translation
The financial statements of the Company’s operations outside the U.S. are generally determined using the local currency as the functional currency. Adjustments to translate the assets and liabilities of the foreign operations into U.S. dollars are accumulated as a component of other comprehensive income utilizing period-end exchange rates. Since July 2018, the Company has accounted for its Argentine operations as highly inflationary, but this status has not had a material effect on the consolidated financial statements.
The currency fluctuation related to certain long-term inter-company loans where settlement is not planned or anticipated in the foreseeable future have been recorded within the cumulative translation adjustment, a component of other comprehensive income. In addition, the currency fluctuation associated with the portion of the Company’s euro-denominated debt designated as a net investment hedge is included as a component of other comprehensive income. Foreign currency transaction gains and losses are calculated using weighted average exchange rates for the period and are included in the consolidated statements of operations in “other expense, net.” Such foreign currency transaction gains and losses include inter-company loans that are repayable in the foreseeable future.
Cash and Cash Equivalents
All liquid investments purchased with original maturities of three months or less are considered cash equivalents. The carrying value of these cash equivalents approximates fair value.
Allowance for Credit Losses
Trade receivables, contract assets, and other amounts owed to the Company are presented net of an allowance that includes an assessment of expected credit losses. The Company determines its allowance methodology by considering various factors, including the Company’s previous loss history, aging of customer receivable balances, significant aspects of a geographic location's economic conditions, the current and anticipated future condition of the general economy and the industries in which the Company's primary customers operate. To the extent that the Company identifies that any individual customer's credit quality has deteriorated, the Company establishes allowances based on the individual risk characteristics of that customer. The Company makes concerted efforts to collect all outstanding balances due from customers; however, trade receivables and contract assets are written off against the allowance when the related balances are no longer deemed collectible.
Concentrations of Credit Risk and Major Customers
Concentration of credit risk, with respect to accounts receivable, is limited due to the large number of customers and their dispersion across different geographic areas. The customers are primarily concentrated in the biopharmaceutical, pharmaceutical, and consumer products industries. The Company does not normally require collateral or any other security to support credit sales. The Company performs ongoing credit evaluations of its customers’ financial conditions and maintains reserves for credit losses. Such losses historically have been within the Company’s expectations.
For the fiscal years ended June 30, 2023 and 2022, the Company had one customer that accounted for 10% of its net revenue, which was primarily recorded in the Biologics segment. No single customer exceeded 10% of revenue during the fiscal year ended June 30, 2021.
As of June 30, 2023 and 2022, the Company had one customer that accounted for 20% and 14%, respectively, of its aggregate net trade receivables and contract asset values, primarily associated with the Biologics segment. After performing a risk assessment of this customer, the Company has determined that a reserve is not warranted as of June 30, 2023.
Inventories
Inventory is stated at the lower of cost or net realizable value, using the first-in, first-out method. The Company provides for cost adjustments for excess, obsolete, or slow-moving inventory based on changes in customer demand, technology developments or other economic factors. Inventory consists of costs associated with raw material, labor, and overhead.
Goodwill
The Company accounts for purchased goodwill and intangible assets with indefinite lives in accordance with ASC 350, Intangibles - Goodwill and Other. Under ASC 350, goodwill and intangible assets with indefinite lives are not amortized, but instead are tested for impairment at least annually. The Company performs an impairment evaluation of goodwill annually during the fourth quarter of its fiscal year or when circumstances otherwise indicate an evaluation should be performed.
The evaluation may begin with a qualitative assessment for each reporting unit to determine whether it is more-likely-than-not that the fair value of the reporting unit is less than its carrying value. Factors considered in a qualitative assessment include, among other things, macroeconomic conditions, industry and market considerations, financial performance of the respective reporting unit, and other relevant entity and reporting-unit specific considerations. If the qualitative assessment does not generate a positive response, or if no qualitative assessment is performed, a quantitative assessment, based upon discounted cash flows, is performed and requires management to estimate future cash flows, growth rates, and macroeconomic, industry, and market conditions.
The Company performs an annual goodwill impairment test for each reporting unit on April 1, the measurement date. The evaluation begins with a qualitative assessment of each reporting unit to determine if it is more likely than not that the fair value of the reporting unit is less than its carrying value.
Due to the Company's underperformance of operating results relative to expectations and decline in the Company's stock price, the Company assessed the current and future economic outlook as of March 31, 2023. The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in each of its six reporting units as of March 31, 2023 which led to a quantitative assessment for each of the Company's reporting units. The evaluation performed as of March 31, 2023 resulted in a goodwill impairment charge in the Company's Consumer Health reporting unit within the Pharma and Consumer Health segment.
Subsequent to the quantitative assessment performed as of March 31, 2023, the Company performed a qualitative assessment as of April 1, 2023 which yielded no indicators of impairment.
In fiscal 2022, the Company proceeded directly to a quantitative assessment, but, in fiscal 2021, the Company performed its impairment evaluation with a qualitative assessment. The evaluations performed in fiscal 2022 and 2021 resulted in no impairment charge.
For more information regarding goodwill activity during fiscal 2022 and 2023 and the related balances at June 30, 2023, see Note 4, Goodwill.
Property and Equipment and Other Definite-Lived Intangible Assets
Property and equipment are stated at cost. Depreciation expense is computed using the straight-line method over the estimated useful lives of the assets, including leasehold improvements and finance lease right-of-use assets that are amortized over the shorter of their useful lives or the terms of the respective leases. The Company generally uses the following range of useful lives for its property and equipment categories: buildings and improvements—5 to 50 years; machinery and equipment—3 to 10 years; and furniture and fixtures—3 to 7 years. Depreciation expense was $286 million, $255 million, and $196 million for fiscal 2023, 2022, and 2021, respectively. Depreciation expense includes amortization of assets related to financing leases. The Company charges repairs and maintenance costs to expense as incurred. The amount of capitalized interest for fiscal 2023, 2022, and 2021 was $24 million, $15 million, and $17 million, respectively.
Intangible assets with finite lives, including customer relationships, core technology, patents, and trademarks, are amortized over their useful lives. The Company also capitalizes certain computer software and development costs in other intangibles, net, when incurred in connection with developing or obtaining computer software for internal use. Capitalized software costs are amortized over the estimated useful lives of the software, which generally range from 3 to 5 years. The Company evaluates the recoverability of its other long-lived assets, including amortizing intangible assets, if circumstances indicate impairment may have occurred pursuant to ASC 360, Property, Plant and Equipment. This analysis is performed by comparing the respective carrying values of the assets to the current and expected future cash flows, on an un-discounted basis, to be generated from such assets. If such analysis indicates that the carrying value of these assets is not recoverable, the carrying value of such assets is reduced to fair value through a charge to the consolidated statements of operations. Fair value is determined based on assumptions the Company believes marketplace participants would utilize and comparable marketplace information in similar arm’s length transactions.
In conjunction with the goodwill impairment test performed as of March 31, 2023, the Company identified indicators of impairment related to its definite-lived intangibles. The results of the analysis did not result in an impairment charge.
The Company recorded impairment charges related to definite-lived intangible assets and property, plant, and equipment of $98 million, $31 million, and $9 million for the fiscal years ended June 30, 2023, 2022, and 2021, respectively.
Post-Retirement and Pension Plans
The Company sponsors various retirement and pension plans, including defined benefit and defined contribution retirement plans. The measurement of the related benefit obligations and the net periodic benefit costs recorded each year are based upon actuarial computations, which require management’s judgment as to certain assumptions. These assumptions include the discount rates used in computing the present value of the benefit obligations and the net periodic benefit costs, the expected future rate of salary increases (for pay-related plans) and the expected long-term rate of return on plan assets (for funded plans). The Company uses the corridor approach to amortize actuarial gains and losses.
The Company has elected to utilize an approach to estimate the service and interest components of net periodic benefit cost for benefit plans that discounts the individual expected cash flows using the applicable spot rates derived from the yield curve over the projected cash flow period. The expected long-term rate of return on plan assets is based on the target asset allocation and the average expected rate of growth for the asset classes invested. The average expected rate of growth is derived from a combination of historic returns, current market indicators, and the expected risk premium for each asset class. The Company uses a measurement date of June 30 for all its retirement and postretirement benefit plans.
Derivative Instruments, Hedging Activities, and Fair Value
Derivative Instruments and Hedging Activities
The Company is exposed to certain risks arising from both its business operations and economic conditions. The Company principally manages its exposures to a wide variety of business and operational risks through management of its core business activities. The Company manages economic risks, including interest-rate, liquidity, and credit risk primarily by managing the amount, sources, and duration of its debt funding and the use of derivative financial instruments. Specifically, the Company enters into derivative financial instruments from time to time to manage exposures that arise from business activities that result in the receipt or payment of future known and uncertain cash amounts, the values of which are determined by interest rates. The Company’s derivative financial instruments are used to manage differences in the amount, timing, and duration of the Company’s known or expected cash receipts and its known or expected cash payments principally related to the Company’s borrowings. The Company does not net any of its derivative positions under master netting arrangements.
Primarily, the Company is exposed to fluctuations in the euro-U.S. dollar exchange rate on its investments in foreign operations in Europe. While the Company does not actively hedge against changes in foreign currency, it has mitigated the exposure of investments in its European operations through a net-investment hedge by denominating a portion of its debt in euros. In addition, a portion of Operating Company's interest payment obligation on its U.S dollar-denominated term loans is exposed to interest rate variability. The Company has mitigated its exposure to this risk by entering into interest-rate swap agreements, which qualify for and are designated as cash-flow hedges.
Fair Value
The Company is required to measure certain assets and liabilities at fair value, either upon initial measurement or for subsequent accounting or reporting. The Company uses fair value extensively, including in the initial measurement of net assets acquired in a business combination and when accounting for and reporting on certain financial instruments. The Company estimates fair value using an exit price approach, which requires, among other things, that it determine the price that would be received to sell an asset or paid to transfer a liability in an orderly market. The determination of an exit price is considered from the perspective of market participants, considering the highest and best use of assets and, for liabilities, assuming the risk of non-performance will be the same before and after the transfer. A single estimate of fair value results from a complex series of judgments about future events and uncertainties and relies heavily on estimates and assumptions. When estimating fair value, depending on the nature and complexity of the assets or liability, the Company may use one or all of the following approaches:
•Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities.
•Cost approach, which is based on the cost to acquire or construct comparable assets less an allowance for functional and/or economic obsolescence.
•Income approach, which is based on the present value of the future stream of net cash flows.
Certain investments that are measured at fair value using the net asset value per share (or its equivalent) practical expedient have not been classified in the fair value hierarchy.
Marketable Securities
The Company classifies its liquid debt investments with original maturities greater than ninety days as marketable securities. The Company invests in highly rated corporate debt securities, with the primary objective of minimizing the potential risk of principal loss. The Company’s investment policy generally requires securities to be investment grade and limits the amount of credit exposure to any single issuer. The Company regularly reviews its investments and utilizes quantitative and qualitative evidence to evaluate potential impairments. For available-for-sale debt securities in an unrealized loss position, the Company assesses whether it intends to sell or if it is more likely than not that the Company will be required to sell the security before recovery of its amortized cost basis. If either of the criteria regarding intent or requirement to sell is met, the security’s amortized cost basis is written down to fair value. If the criteria are not met, the Company evaluates whether the decline in fair value has resulted from a credit loss or other factors. In making this assessment, management considers, among other factors, the extent to which fair value is less than amortized cost, any change to the rating of the security by a rating agency, and adverse conditions specifically related to the security. If this assessment indicates that a credit loss exists, the present value of cash flows expected to be collected from the security are compared to the amortized cost basis of the security. If the present value of the cash flows expected to be collected is less than the amortized cost basis, a credit loss exists and an allowance for credit losses is recorded for the credit loss, limited by the amount that the fair value is less than the amortized costs basis.
The Company classifies its marketable securities as available-for-sale, because it may sell certain of its marketable securities prior to the stated maturity for various reasons, including management of liquidity, credit risk, duration, relative return, and asset allocation. The Company determines the fair value of each marketable security in its portfolio at each period end and recognizes gains and losses in the portfolio in other comprehensive income. As of June 30, 2023, all of the Company's outstanding marketable securities had matured. The amortized cost basis of all previously owned marketable securities approximated fair value and all previously outstanding marketable securities matured within one year.
Self-Insurance
The Company is partially self-insured for certain employee health benefits and partially self-insured for property losses and casualty claims. The Company accrues for losses based upon experience and actuarial assumptions, including provisions for losses incurred but not reported.
Accumulated Other Comprehensive Loss
Accumulated other comprehensive loss, which is reported in the accompanying consolidated statements of changes in shareholders’ equity, consists of foreign currency translation, net change in marketable securities, and defined benefit pension plan changes.
Research and Development Costs
The Company expenses research and development costs as incurred. Research and development costs amounted to $18 million, $23 million, and $21 million for the fiscal years ended June 30, 2023, 2022, and 2021, respectively.
Earnings Per Share
The Company reports net earnings per share in accordance with ASC 260, Earnings per Share. Effective as of the first quarter of fiscal 2023, the Company computed earnings per share (“EPS”) of the Company’s common stock, par value $0.01 (the “Common Stock”) using the treasury stock method. Prior to fiscal 2023, the Company computed earnings (loss) per share of the Common Stock using the two-class method required due to the participating nature of the previously outstanding Series A Preferred Stock (as defined and discussed in Note 13, Equity and Accumulated Other Comprehensive Loss).
Diluted earnings per common share measures the performance of the Company over the reporting period while giving effect to all potential shares of Common Stock that were dilutive and outstanding during the period. The denominator includes the weighted average over the measurement period of the sum of the number of shares of Common Stock outstanding and the
number of additional such shares that would have been outstanding if the shares of Common Stock that were both potentially issuable and dilutive had been issued.
Income Taxes
In accordance with ASC 740, Income Taxes, the Company accounts for income taxes using the asset and liability method. The asset and liability method requires recognition of deferred tax assets and liabilities for expected future tax consequences of temporary differences that currently exist between tax bases and financial reporting bases of the Company’s assets and liabilities. The Company measures deferred tax assets and liabilities using enacted tax rates in the respective jurisdictions in which it operates. In assessing the ability to realize deferred tax assets, the Company considers whether it is more likely than not that the Company will be able to realize some or all of the deferred tax assets. The calculation of the Company’s tax liabilities involves dealing with uncertainties in the application of complex tax regulations in each of its tax jurisdictions. The number of years with open tax audits varies by tax jurisdiction. A number of years may lapse before a particular matter is audited and finally resolved. The Company applies ASC 740 to determine the accounting for uncertain tax positions. This standard prescribes a minimum recognition threshold a tax position is required to meet before the Company may recognize the position in its financial statements. The standard also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, and disclosure. The Company previously elected not to reclassify the income tax effects stranded in accumulated other comprehensive income to retained earnings.
Stock-Based Compensation
The Company accounts for its stock-based compensation in accordance with ASC 718, Compensation—Stock Compensation. Under ASC 718, companies recognize compensation expense using a fair-value-based method for costs related to share-based payments, including stock options and restricted stock units. The expense is measured based on the grant date fair value of the awards, and the expense is recorded over the applicable requisite service period. Forfeitures are recognized as and when they occur. In the absence of an observable market price for a share-based award, the fair value is based upon a valuation methodology that takes into consideration various factors, including the exercise price of the award, the expected term of the award, the current price of the underlying shares, the expected volatility of the underlying share price, the expected dividends on the underlying shares and the risk-free interest rate.
The terms of the Company’s stock-based compensation plans permit an employee holding vested stock options or restricted stock units to elect to have the Company use a portion of the shares otherwise issuable upon the employee’s exercise of the option or grant, a so-called “net settlement transaction,” as a means of paying the exercise price, meeting tax withholding requirements, or both.
Recent Financial Accounting Standards
In March 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting, which provides optional guidance to ease the potential burden in accounting for the discontinuation of a reference rate such as LIBOR, formerly known as the London Interbank Offered Rate, because of reference rate reform. In December 2022, the FASB issued ASU 2022-06, Reference Rate Reform (Topic 848): Deferral of the Sunset Date of Topic 848, which delayed the effective date from December 31, 2022 to December 31, 2024. The Company adopted the guidance on June 30, 2023. The adoption of this standard did not have a material impact on the Company’s consolidated financial statements.
2. REVENUE RECOGNITION
The Company recognizes revenue in accordance with ASC 606, Revenue from Contracts with Customers. The Company generally earns its revenue by supplying goods or providing services under contracts with its customers in three primary revenue streams: manufacturing and commercial product supply, development services, and clinical supply services. The Company measures the revenue from customers based on the consideration specified in its contracts, excluding any sales incentive or amount collected on behalf of a third party, that the Company expects to be entitled to receive in exchange for transferring the promised goods to and/or performing services for the customer (the “Transaction Price”). To the extent the Transaction Price includes variable consideration, the Company estimates the amount of variable consideration that should be included in the Transaction Price utilizing either the expected value method or the most likely amount method, depending on which method is expected to better predict the amount of consideration to which the Company will be entitled. The value of variable consideration is included in the Transaction Price if, and to the extent, it is probable that a significant reversal of the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. These estimates are re-assessed each reporting period, as required, and any adjustment required is recorded on a cumulative catch-up basis, which would affect revenue and net income in the period of adjustment.
The Company’s customer contracts generally include provisions entitling the Company to a termination penalty when the customer terminates prior to the contract’s nominal end date. The termination penalties in customer contracts vary but are generally considered substantive for accounting purposes and create enforceable rights and obligations throughout the stated durations of the contracts. The Company accounts for a contract termination as a contract modification in the period in which the customer gives notice of termination. The determination of the contract termination penalty is based on the terms stated in the relevant customer agreement. As of the modification date, the Company updates its estimate of the Transaction Price using the expected value method, subject to constraints, and to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. These estimates are re-assessed each reporting period, as required, and any adjustment required is recorded on a cumulative catch-up basis, which would affect revenue and net income in the period of adjustment.
Where multiple performance obligations exist in a single contract, the Company allocates consideration to each performance obligation using the “relative standalone selling price” as defined under ASC 606. Generally, the Company utilizes observable standalone selling prices in its allocations of consideration. If observable standalone selling prices are not available, the Company estimates the applicable standalone selling price using a cost-plus-margin approach or an adjusted market assessment approach, in each case, representing the amount that the Company believes the market is willing to pay for the applicable service. Payment is typically due 30 to 45 days following the invoice date, based on the payment terms set forth in the applicable customer agreement.
The Company generally expenses sales commissions as incurred because either the amortization period is one year or less, or the balance with an amortization period greater than one year is not material.
Customer contracts that include commitments by the Company to make facility space or equipment available may be deemed to include lease components, which are evaluated under ASC 842, Leases. For arrangements that contain both lease and non-lease components, consideration in the contract is allocated on a relative standalone selling-price basis. Determining the lease term and contract term of non-lease components, as well as the variable and fixed consideration in these arrangements, including when variability is resolved, often requires management judgment in order to determine the allocation to the lease and non-lease components.
Manufacturing & Commercial Product Supply Revenue
Manufacturing and commercial product supply revenue consists of revenue earned by manufacturing products supplied to customers under long-term commercial supply arrangements. In these arrangements, the customer typically owns and supplies the active pharmaceutical ingredient (“API”) or other proprietary materials used in the manufacturing process. The contract generally includes the terms of the manufacturing services and related product quality assurance procedures to comply with regulatory requirements. Due to the regulated nature of the Company’s business, these contract terms are highly interdependent and, therefore, are considered to be a single combined performance obligation. The transaction price is generally stated in the agreement as a fixed price per unit, with no contractual provision for a refund or price concession. In most circumstances, control is transferred to the customer over time, creating a corresponding right to recognize the related revenue, because there is no alternative use to the Company for the asset created and the Company has an enforceable right to payment for performance completed as of that date. The selection of the method for measuring progress towards the completion of the Company’s performance obligation requires judgment and is based on the nature of the products to be manufactured. For the majority of the Company’s arrangements, progress is measured based on the units of product that have successfully completed the contractually required product quality assurance process, because the conclusion of that process defines the time when the applicable contract and the related regulatory requirements permit the customer to exercise control over the product’s disposition. The customer is typically responsible for arranging the shipping and handling of product following completion of the quality assurance process. Payment is typically due 30 to 45 days after invoice date, based on the payment terms set forth in the applicable customer agreement.
Beginning in the third quarter of fiscal 2023, the Company began recognizing commercial revenue for certain contracts in its Biologics segment that have a notably long manufacturing cycle, and for which the customer exercises control over the product throughout the manufacturing process. For these contracts, revenue is recognized over time and progress is measured using an input method based on effort expended, which provides an appropriate depiction of the Company’s progress toward fulfilling its performance obligation.
Development Services and Clinical Supply Revenue
Development services contracts generally take the form of short-term, fee-for-service arrangements. Performance obligations vary, but frequently include biologic cell-line development, performing formulation, analytical stability, or other services related to product development, and providing manufacturing services for products that are under development or
otherwise not intended for commercial sale. They can also include a combination of the following services: the manufacturing, packaging, storage, distribution, destruction, and inventory management of customer clinical trial material, as well as the sourcing of comparator drug products on behalf of customers to be used in clinical trials to compare performance with the drug under clinical investigation. The transaction prices for these arrangements are fixed and include amounts stated in the contracts for each promised service, and each service is generally considered to be a separate performance obligation. In most instances, the Company recognizes revenue over time because there is no alternative use to the Company for the asset created and the Company has an enforceable right to payment for performance completed as of that date.
The Company measures progress toward the completion of its performance obligations satisfied over time based on the nature of the services to be performed. For certain types of arrangements, revenue is recognized over time and measured using an output method based on the completion of tasks and activities that are performed to satisfy a performance obligation. For certain types of arrangements, revenue is recognized over time and measured using an input method based on effort expended. Each of these methods provides an appropriate depiction of the Company’s progress toward fulfilling its performance obligations for its respective arrangement. In certain development services arrangements that require a portion of the contract consideration to be received in advance at the commencement of the contract, such advance payment is initially recorded as a contract liability. In certain clinical supply arrangements, revenue is recognized at the point in time when control transfers, which occurs upon either the delivery of the related output of the service to the customer or the completion of quality testing with respect to the product, and the Company has an enforceable right to payment based on the terms of the arrangement.
The Company records revenue for comparator sourcing arrangements on a net basis because it is acting as an agent that does not control the product or service before it is transferred to the customer. Payment for comparator sourcing activity is typically received in advance at the commencement of the contract and is initially recorded as a contract liability.
The Company generally expenses sales commissions as incurred because either the amortization period is one year or less, or the balance with an amortization period greater than one year is not material.
The following tables reflect net revenue for the fiscal years ended June 30, 2023, 2022, and 2021 by type of activity and reporting segment (in millions):
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, 2023 | Biologics | | Pharma and Consumer Health | | Total |
| Manufacturing & commercial product supply | $ | 450 | | | $ | 1,452 | | | $ | 1,902 | |
| Development services & clinical supply | 1,528 | | | 835 | | | 2,363 | |
| Total | $ | 1,978 | | | $ | 2,287 | | | $ | 4,265 | |
| Inter-segment revenue elimination | | (2) | |
| Combined net revenue | | $ | 4,263 | |
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, 2022 | Biologics | | Pharma and Consumer Health | | Total |
| Manufacturing & commercial product supply | $ | 608 | | | $ | 1,474 | | | $ | 2,082 | |
| Development services & clinical supply | 1,926 | | | 797 | | | 2,723 | |
| Total | $ | 2,534 | | | $ | 2,271 | | | $ | 4,805 | |
| Inter-segment revenue elimination | (3) | |
| Combined net revenue | | $ | 4,802 | |
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, 2021 | Biologics | | Pharma and Consumer Health | | Total |
| Manufacturing & commercial product supply | $ | 532 | | | $ | 1,321 | | | $ | 1,853 | |
| Development services & clinical supply | 1,406 | | | 742 | | | 2,148 | |
| Total | $ | 1,938 | | | $ | 2,063 | | | $ | 4,001 | |
| Inter-segment revenue elimination | | (3) | |
| Combined net revenue | | $ | 3,998 | |
The following table reflects net revenue by the location where the goods were made or the service performed:
| | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | | Fiscal Year Ended
June 30, 2023 | | Fiscal Year Ended
June 30, 2022 | | Fiscal Year Ended
June 30, 2021 |
| | | | | | |
| United States | | $ | 2,768 | | | $ | 3,084 | | | $ | 2,462 | |
| Europe | | 1,257 | | | 1,506 | | | 1,343 | |
| Other | | 355 | | | 327 | | | 288 | |
| Elimination of revenue attributable to multiple locations | | (117) | | | (115) | | | (95) | |
| Total | | $ | 4,263 | | | $ | 4,802 | | | $ | 3,998 | |
Contract Liabilities
Contract liabilities relate to cash consideration that the Company receives in advance of satisfying the related performance obligations. The contract liabilities balances (current and non-current) as of June 30, 2023 and June 30, 2022 were as follows:
| | | | | | | | |
| (Dollars in millions) | | |
| | |
| Balance at June 30, 2022 | | $ | 220 | |
| | |
| | |
| | |
| | |
| Balance at June 30, 2023 | | $ | 180 | |
| Revenue recognized in the period from amounts included in contracts liability at the beginning of the period: | | $ | 126 | |
| | |
Contract liabilities that will be recognized within 12 months of June 30, 2023 are accounted for in Other accrued liabilities and those that will be recognized longer than 12 months after June 30, 2023 are accounted for within Other liabilities.
Contract Assets
Contract assets primarily relate to the Company's conditional right to receive consideration for services that have been performed for customers but had not yet been invoiced as of June 30, 2023. Contract assets are transferred to trade receivables, net when the Company’s right to receive the consideration becomes unconditional. Contract assets totaled $417 million and $441 million as of June 30, 2023 and 2022, respectively. Contract assets expected to transfer to trade receivables within 12 months are accounted for within Prepaid expenses and other. Contract assets expected to transfer to trade receivables after 12 months are accounted for within Other long-term assets.
As of June 30, 2023, the Company recorded no reserve against any of its contract asset balances.
Performance Obligations
Remaining performance obligations represent firm orders for future development services as well as manufacturing and commercial product supply, including minimum volume commitments, for which there are incomplete performance obligations for work not yet completed under executed contracts. Remaining performance obligations as of June 30, 2023 were $335 million. The Company expects to recognize approximately 42% of the remaining performance obligations in existence as of June 30, 2023 after June 30, 2024.
3. BUSINESS COMBINATIONS AND DIVESTITURES
Skeletal Cell Therapy Support SA Acquisition
In November 2020, the Company acquired 100% of the equity interest in Skeletal Cell Therapy Support SA (“Skeletal”) for $15 million and entered into related supply agreements with the seller. Skeletal operated a cell therapy manufacturing facility in Gosselies, Belgium. The operations were assigned to the Company’s Biologics segment, expanding the Company’s cell therapy capacity for clinical and commercial supply. The acquisition, combined with the Company's other European-based facilities and capabilities in cell therapy, has resulted in an integrated European center of excellence in cell therapy.
The Company accounted for the Skeletal acquisition using the acquisition method in accordance with ASC 805, Business Combinations. The Company funded the entire purchase price with cash on hand and allocated the purchase price among the acquired assets, recognizing $9 million of goodwill. The Company allocated the remainder of the purchase price to trade receivables, property, plant, and equipment, and other current and non-current assets and liabilities assumed in the acquisition. Results for the fiscal years ended June 30, 2023 and 2022 were not material to the Company’s statement of operations, financial position, or cash flows.
Acorda Therapeutics, Inc. Transaction
In February 2021, the Company acquired the manufacturing and packaging operations of Acorda Therapeutics, Inc.'s dry powder inhaler and spray dry manufacturing business, including its manufacturing facility located near Boston, Massachusetts, (“Acorda”) for $83 million. The facility and operations became part of the Company’s Pharma and Consumer Health segment.
The Company accounted for the Acorda transaction using the acquisition method in accordance with ASC 805, Business Combinations. The Company funded the entire purchase price with cash on hand and allocated the purchase price among the acquired assets, recognizing property, plant, and equipment of $79 million, inventory of $2 million, and goodwill of $2 million. The remainder of the purchase price was allocated to other current and non-current assets and liabilities assumed in the acquisition. Results of this business were not material to the Company's statement of operations, financial position, or cash flows for the fiscal years ended June 30, 2023 and 2022.
Delphi Genetics SA Acquisition
In February 2021, the Company acquired 100% of the equity interest in Delphi Genetics SA (“Delphi”) for $50 million. Delphi was a pDNA cell and gene therapy contract development and manufacturing organization based in Gosselies, Belgium. The facility and operations acquired became part of the Company’s Biologics segment.
The Company accounted for the Delphi transaction using the acquisition method in accordance with ASC 805, Business Combinations. The Company funded the entire purchase price with cash on hand and allocated the purchase price among the acquired assets, recognizing property, plant, and equipment of $4 million, intangible assets of $7 million, other current assets of $3 million, assumed debt of $6 million, other current liabilities of $1 million and goodwill of $43 million. Results of this business were not material to the Company's statement of operations, financial position, or cash flows for the fiscal years ended June 30, 2023 and 2022.
Hepatic Cell Therapy Support SA Asset Acquisition
In April 2021, the Company acquired 100% of the equity interest in Hepatic Cell Therapy Support SA (“Hepatic”) for $15 million, net of cash acquired and debt assumed. Hepatic operated a manufacturing facility at the same location where Skeletal operated its cell therapy manufacturing facility in Gosselies, Belgium. The facility acquired expanded the Company’s cell therapy capacity for clinical and commercial supply in its Biologics segment.
The Company accounted for the Hepatic transaction as an asset acquisition in accordance with ASC 805, Business Combinations. The Company funded the entire purchase price with cash on hand and allocated the purchase price to the assets acquired and liabilities assumed, recognizing property, plant, and equipment of $13 million, other current and non-current assets of $3 million, and assumed debt of $1 million. Results of this business were not material to the Company's statement of operations, financial position, or cash flows for the fiscal years ended June 30, 2023 and 2022.
RheinCell Therapeutics GmbH Acquisition
In August 2021, the Company acquired 100% of the equity interest in RheinCell Therapeutics GmbH (“RheinCell”) for $26 million, net of cash acquired. RheinCell was a developer and manufacturer of clinical- and cGMP-grade iPSCs based in Lagenfeld, Germany. The operations became part of the Company’s Biologics segment and built on its existing custom cell therapy process development and manufacturing capabilities with proprietary cGMP cell lines for iPSC-based therapies.
The Company accounted for the RheinCell transaction using the acquisition method in accordance with ASC 805, Business Combinations. The Company funded the entire purchase price with cash on hand and allocated the purchase price among the assets acquired, recognizing $4 million of current liabilities, $1 million of other liabilities, $14 million of intangible assets, and goodwill of $17 million. Results of this business were not material to the Company's statement of operations, financial position, or cash flows for the fiscal years ended June 30, 2023 and 2022.
Bettera Holdings, LLC Acquisition
In October 2021, the Company acquired 100% of the equity interest in Bettera Holdings, LLC (“Bettera Wellness”) for $1.00 billion. Bettera Wellness was a manufacturer of nutraceuticals and nutritional supplements in gummy, soft chew, and lozenge delivery formats and became part of the Company's Pharma and Consumer Health segment.
The Company accounted for the Bettera Wellness transaction using the acquisition method in accordance with ASC 805, Business Combinations. The Company estimated fair values at the date of acquisition for the allocation of consideration to the net tangible and intangible assets acquired and liabilities assumed and allocated the purchase price among the assets acquired, recognizing $361 million of other intangibles, net, $72 million of property, plant, and equipment, $31 million of inventories, $23 million of cash and cash equivalents, $16 million of trade receivables, $17 million of net other liabilities, and goodwill of $531 million. Results of this business were not material to the Company's statement of operations, financial position, or cash flows for the fiscal years ended June 30, 2023 and 2022.
The carrying value of trade receivables, inventory, and trade payables, as well as certain other current and non-current assets and liabilities, generally represented the fair value at the date of acquisition.
Property, plant, and equipment assets were valued using the cost approach, which is based on current replacement and/or reproduction cost of the related asset as new, less depreciation attributable to physical, functional, and economic factors. The Company then determined the remaining useful life based on the anticipated life of the asset and Company policy for similar assets.
Core technology intangible assets of $338 million were valued using the multi-period, excess-earnings method, a method that values the intangible asset using the present value of the after-tax cash flows attributable to the intangible asset only. The significant assumptions used in developing the valuation included the estimated annual net cash flows (including application of an appropriate margin to forecasted revenue, revenue obsolescence rate, selling and marketing costs, return on working capital, contributory asset charges, and other factors), the discount rate that appropriately reflects the risk inherent in each future cash flow stream, and an assessment of the asset’s life cycle, as well as other factors. The assumptions used in the financial forecasts were based on historical data, supplemented by current and anticipated growth rates, management plans, and market-comparable information. Fair-value determinations require considerable judgment and are sensitive to changes in underlying assumptions and factors. The core technology intangible asset has a weighted average useful life of 10 years.
Goodwill has been allocated to the Pharma and Consumer Health segment as shown in Note 4, Goodwill. Goodwill is mainly comprised of growth from expected increases in capacity utilization and new customers. The goodwill resulting from the Bettera Wellness acquisition is deductible for tax purposes.
Vaccine Manufacturing and Innovation Centre UK Limited Asset Acquisition
In April 2022, the Company, through its wholly owned subsidiary, Catalent Oxford Limited, acquired a development and manufacturing facility near Oxford, United Kingdom (“U.K.”) and certain related assets and liabilities from The Vaccine Manufacturing and Innovation Centre UK Limited for $134 million in cash, including $9 million of closing costs. The facility and related assets and liabilities became part of the Company’s Biologics segment.
The Company accounted for this transaction as an acquisition of assets in accordance with ASC 805, Business Combinations. The Company funded this acquisition with cash on hand and allocated the purchase price among the net assets acquired, recognizing $1 million of current assets, $165 million of property, plant, and equipment, $18 million of current liabilities, and $14 million of other liabilities. Results of this business were not material to the Company's statement of operations, financial position, or cash flows for the fiscal years ended June 30, 2023 and 2022.
Princeton Cell Therapy Development and Manufacturing Acquisition
In April 2022, the Company acquired a cell therapy commercial manufacturing facility and operations near Princeton, New Jersey (“Princeton”) from Erytech Pharma S.A. (“Erytech”) for $45 million in cash, subject to customary adjustments. In connection with the purchase, Erytech and the Company entered into a long-term supply agreement, under which the Company agreed to continue to manufacture and package an Erytech product at the Princeton facility. The operations and facility acquired became part of the Company’s Biologics segment.
The Company accounted for this transaction using the acquisition method in accordance with ASC 805, Business Combinations. The Company funded this acquisition with cash on hand and allocated the purchase price among the assets acquired, recognizing $22 million of property, plant, and equipment, $10 million of other assets, $1 million of current liabilities, $10 million of other liabilities, and goodwill of $24 million. Results of this business were not material to the Company's statement of operations, financial position, or cash flows for the fiscal years ended June 30,2023 and 2022.
Metrics Contract Services Acquisition
In October 2022, the Company acquired 100% of Metrics Contract Services (“Metrics”) from Mayne Pharma Group Limited for $474 million in cash. Metrics, based in Greenville, North Carolina, was an oral solids development and manufacturing business specializing in the manufacture of drugs containing highly potent active pharmaceutical ingredients. The operations and facility acquired became part of the Company’s Pharma and Consumer Health segment.
The Company accounted for the Metrics transaction using the acquisition method in accordance with ASC 805, Business Combinations. The Company funded this acquisition with a portion of the proceeds of an October 2022 drawdown from its senior secured revolving credit facility. The Company estimated fair values at the date of acquisition for the allocation of consideration to the net tangible and intangible assets acquired and liabilities assumed. The Company has not completed its analysis regarding the assets acquired and liabilities assumed. Therefore, the allocation to income taxes are preliminary and subject to finalization.
The preliminary purchase price was allocated to assets acquired and liabilities assumed in the acquisition as follows:
| | | | | |
| (Dollars in millions) | Preliminary Purchase Price Allocation |
| Trade receivables, net | 15 | |
| Inventories | 5 | |
| Property, plant, and equipment | 195 | |
| Other intangibles, net | 52 | |
| Other, net | (12) | |
| Goodwill | 219 | |
| Total assets acquired and liabilities assumed | $ | 474 | |
The carrying value of trade receivables, inventory, and trade payables, as well as certain other current and non-current assets and liabilities generally represented the fair value at the date of acquisition.
Other intangibles, net consists of customer relationships of $52 million, which were valued using the multi-period, excess-earnings method, a method that values the intangible asset using the present value of the after-tax cash flows attributable to the intangible asset only. The significant assumptions used in developing the valuation included the estimated annual net cash flows (including application of an appropriate margin to forecasted revenue, selling and marketing costs, return on working capital, contributory asset charges, and other factors), the discount rate that appropriately reflects the risk inherent in each future cash flow stream, and an assessment of the asset’s life cycle, as well as other factors. The assumptions used in the financial forecasts were based on historical data, supplemented by current and anticipated growth rates, management plans, and market-comparable information. Fair-value determinations require judgment and are sensitive to changes in underlying assumptions and factors. The customer relationship intangible asset has a weighted average useful life of 12 years.
Property, plant, and equipment was valued using the cost approach, which is based on current replacement and/or reproduction cost of the asset as new, less depreciation attributable to physical, functional, and economic factors. The Company then determined the remaining useful life based on the anticipated life of the asset and Company policy for similar assets.
Goodwill has been allocated to the Pharma and Consumer Health segment as shown in Note 4, Goodwill. Goodwill is mainly comprised of the growth from an expected increase in capacity utilization and potential new customers. The goodwill resulting from the Metrics acquisition is not deductible for U.S. income tax purposes.
Results of the business acquired were not material to the Company's consolidated statement of operations, financial position, or cash flows for the fiscal year ended June 30, 2023.
Blow-Fill-Seal Divestiture
In March 2021, the Company sold 100% of the shares of Catalent USA Woodstock, Inc. and certain related assets (collectively, the “Blow-Fill-Seal Business”) for $331 million in total consideration ($300 million in cash). The Blow-Fill-Seal Business was part of the Pharma and Consumer Health segment.
The carrying value of the net assets sold was $149 million, which included goodwill of $54 million. As a result of the sale, the Company realized a gain from divestiture of $182 million, net of transaction costs, for the fiscal year ended June 30, 2021.
During the fiscal year ended June 30, 2022, the Company settled a post-closing purchase price adjustment, which resulted in a gain on sale of subsidiary of $1 million.
All consideration received was measured at its divestiture date fair value. The Company valued the total consideration received from divestiture of the Blow-Fill-Seal Business as follows:
| | | | | |
| (Dollars in millions) | Fair value of consideration received |
| Cash, gross | $ | 300 | |
Note receivable (1) | 47 | |
Contingent consideration (2) | — | |
Other (3) | (16) | |
| Total | $ | 331 | |
(1) The note receivable, which provides for interest at a rate of 5.0% paid in kind, had an estimated fair value of $47 million and $51 million at June 30, 2021 and June 30, 2022, respectively. The fair value at divestiture date consisted of a $50 million aggregate principal amount less a $3 million discount determined using a discounted cash flow model.
(2) The Company determined that the estimated fair value of the contingent consideration from the sale of the Blow-Fill-Seal Business at June 30, 2022 was zero, and therefore no contingent consideration was recorded at divestiture. If any contingent consideration is subsequently received, it will be recorded in the period in which it is received. The Company has elected an accounting policy to recognize increases in the carrying amount of the contingent consideration asset using the gain contingency guidance in ASC 450, Contingencies.
(3) Other includes $8 million of transaction expenses, a working capital adjustment of $6 million, and a $2 million assumption of liabilities, resulting in net cash proceeds of $284 million.
4. GOODWILL
The following table summarizes the changes from June 30, 2021 to June 30, 2022 and then to June 30, 2023 in the carrying amount of goodwill in total and by reportable segment:
| | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Biologics | | Pharma and Consumer Health | | Total |
| Balance at June 30, 2021 | $ | 1,562 | | | $ | 957 | | | $ | 2,519 | |
Additions (1) | 41 | | | 531 | | | 572 | |
| | | | | |
| | | | | |
| | | | | |
| Foreign currency translation adjustments | (37) | | | (48) | | | (85) | |
| Balance at June 30, 2022 | 1,566 | | | 1,440 | | | 3,006 | |
Additions (2) | — | | | 219 | | | 219 | |
| | | | | |
| | | | | |
| | | | | |
| Reallocation | (15) | | | 15 | | | — | |
| Foreign currency translation adjustments | 12 | | | 12 | | | 24 | |
Impairment (3) | — | | | (210) | | | (210) | |
| Balance at June 30, 2023 | $ | 1,563 | | | $ | 1,476 | | | $ | 3,039 | |
(1) The additions to goodwill arise from the Bettera Wellness (Pharma and Consumer Health), Princeton (Biologics), RheinCell (Biologics), and Delphi (Biologics) acquisitions. For further details, see Note 3, Business Combinations and Divestitures.
(2) The addition to goodwill is a result of the Metrics acquisition. For further details, see Note 3, Business Combinations and Divestitures.
(3) Represents gross and accumulated impairment charges.
As part of the business reorganization discussed in Note 1, Basis of Presentation and Summary of Significant Accounting Policies, the goodwill from the previous Biologics, Softgel and Oral Technologies, Oral and Specialty Delivery, and Clinical Supply Services segments was reallocated between the current Biologics and Pharma and Consumer Health segments.
Goodwill Impairment Charges
The Company performs an annual goodwill impairment test for each reporting unit on April 1, the measurement date and more frequently if indicators for potential impairment exist.
Due to the Company's underperformance of operating results relative to expectations and decline in the Company's stock price, the Company assessed the current and future economic outlook as of March 31, 2023. The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in each of its six reporting units as of March 31, 2023 which led to a quantitative assessment for each of the Company's reporting units. The evaluation performed as of March 31, 2023 resulted in a goodwill impairment charge in the Company's Consumer Health reporting unit within the Pharma and Consumer Health segment.
For the impairment recorded in the three months ended March 31, 2023, the Company estimated the fair value of its reporting units using a combination of the income and market approaches. In performing the goodwill impairment test, the Company used a terminal revenue growth rate of 3.0% and discount rates ranging from 9.0% to 10.5% in its estimation of fair value. The evaluations performed resulted in cumulative year to date impairment charges of $210 million with respect to the Consumer Health reporting unit.
Subsequent to the quantitative assessment performed as of March 31, 2023, the Company performed qualitative assessments as of April 1, 2023, which yielded no indicators of impairment.
While the Company believes the assumptions it used were reasonable and commensurate with the views of a market participant, changes in key assumptions, including increasing the discount rate and, lowering forecasts for revenue and EBITDA margin, could lead to the conclusion that an additional impairment would be required.
Subsequent to June 30, 2023, as a result of the Consumer Health reporting unit's underperformance of recent operating results relative to expectations, the current macroeconomic conditions impacting the consumer health and biotechnology industries, and increased interest rates, the Company assessed the current and future economic outlook as of September 30, 2023 for its Consumer Health and Biomodalities reporting units in its Pharma and Consumer Health and Biologics segments, respectively, and identified indicators for impairment of goodwill.
The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in its Consumer Health and Biomodalities reporting units, which led to a quantitative assessment for the corresponding reporting units.
For further details, see Note 20, Subsequent Events, “Impairment of Goodwill.”
5. OTHER INTANGIBLES, NET
The details of other intangible assets subject to amortization as of June 30, 2023 and June 30, 2022 are as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| June 30, 2023 | Weighted Average Life | | Gross Carrying Value | | Accumulated Amortization | | Net Carrying Value |
| Amortized intangibles: | | | | | | | |
| Core technology | 11 years | | $ | 482 | | | $ | (164) | | | $ | 318 | |
| Customer relationships | 13 years | | 1,079 | | | (451) | | | 628 | |
| Product relationships | 8 years | | 243 | | | (216) | | | 27 | |
| Other | 5 years | | 24 | | | (17) | | | 7 | |
| Total other intangibles | | | $ | 1,828 | | | $ | (848) | | | $ | 980 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| June 30, 2022 | Weighted Average Life | | Gross Carrying Value | | Accumulated Amortization | | Net Carrying Value |
| Amortized intangibles: | | | | | | | |
| Core technology | 11 years | | $ | 480 | | | $ | (121) | | | $ | 359 | |
| Customer relationships | 13 years | | 1,020 | | | (366) | | | 654 | |
| Product relationships | 8 years | | 239 | | | (204) | | | 35 | |
| Other | 4 years | | 24 | | | (12) | | | 12 | |
| Total other intangibles | | | $ | 1,763 | | | $ | (703) | | | $ | 1,060 | |
Amortization expense was $136 million, $123 million, and $93 million for the fiscal years ended June 30, 2023, 2022, and 2021, respectively. Future amortization expense is estimated to be:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | 2024 | | 2025 | | 2026 | | 2027 | | 2028 | | Thereafter | | Total |
| Amortization | $ | 137 | | | $ | 134 | | | $ | 126 | | | $ | 109 | | | $ | 101 | | | $ | 373 | | | $ | 980 | |
6. RESTRUCTURING COSTS
From time to time, the Company implements plans to restructure certain operations, both domestically and internationally. The restructuring plans focus on various aspects of operations, including, among others, closing and consolidating certain manufacturing operations, rationalizing headcount and aligning operations in a strategic and more cost-efficient structure. In addition, the Company may incur restructuring charges in the future in cases where a material change in the scope of operation with its business occurs. Employee-related restructuring costs consist primarily of severance costs and also include outplacement services provided to employees who have been involuntarily terminated and duplicate payroll costs during transition periods. Facility exit and other such restructuring costs consist of equipment relocation costs and costs associated with planned facility expansions and closures to streamline Company operations.
During the three months ended December 31, 2022, the Company adopted plans to reduce costs, consolidate facilities, and optimize its infrastructure across the organization. During the three months ended June 30, 2023, the Company expanded its restructuring efforts by adopting an incremental plan to reduce costs and headcount, primarily in its Biologics segment and corporate functions. In connection with these restructuring plans, the Company reduced its headcount by approximately 1,100 employees and incurred cumulative employee-related charges of $38 million, primarily associated with cash severance programs through June 30, 2023.
Restructuring costs for the fiscal years ended June 30, 2023, 2022, and 2021 were recorded in Other operating (income) expense in the consolidated statement of operations.
The following tables summarize the restructuring costs by type of cost and reportable segment:
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, |
(Dollars in millions) | 2023 | | 2022 | | 2021 |
| Restructuring costs: | | | | | |
| Employee-related reorganization | $ | 38 | | | $ | 9 | | | $ | 8 | |
| Facility exit and other costs | 28 | | | 1 | | | 2 | |
| Total restructuring costs | $ | 66 | | | $ | 10 | | | $ | 10 | |
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, |
(Dollars in millions) | 2023 | | 2022 | | 2021 |
| Restructuring costs: | | | | | |
| Biologics | $ | 41 | | | $ | 1 | | | $ | — | |
| Pharma and Consumer Health | 8 | | | 5 | | | 9 | |
| Non-segment (Corporate) | 17 | | | 4 | | | 1 | |
| Total restructuring costs | $ | 66 | | | $ | 10 | | | $ | 10 | |
The following tables illustrates the change in the employee separation-related liability associated with the plans.
| | | | | |
(Dollars in millions) | Employee-related restructuring |
| Balance, June 30, 2022 | $ | 1 | |
| Charges to income | 38 | |
| Payments | (20) | |
| Balance, June 30, 2023 | $ | 19 | |
7. LONG-TERM OBLIGATIONS AND SHORT-TERM BORROWINGS
Long-term obligations and short-term borrowings consist of the following at June 30, 2023 and June 30, 2022:
| | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Maturity as of June 30, 2023 | | June 30, 2023 | | June 30, 2022 |
| Senior secured credit facilities | | | | | |
| | | | | |
| U.S dollar-denominated term loans (7.188% as of June 30, 2023) | February 2028 | | 1,418 | | | 1,433 | |
| | | | | |
| | | | | |
| | | | | |
Revolving credit facility(1) (7.427% as of June 30, 2023) | November 2027 | | 500 | | | — | |
| 5.000% senior notes due 2027 | July 2027 | | 500 | | | 500 | |
2.375% euro senior notes due 2028(2) | March 2028 | | 904 | | | 874 | |
| 3.125% senior notes due 2029 | February 2029 | | 550 | | | 550 | |
| 3.500% senior notes due 2030 | April 2030 | | 650 | | | 650 | |
| | | | | |
| | | | | |
| Financing lease obligations | 2024 to 2038 | | 341 | | | 234 | |
Other obligations(3) | 2024 to 2028 | | 25 | | | 2 | |
| Unamortized discount and debt issuance costs | | | (39) | | | (41) | |
| Total debt | | | 4,849 | | | 4,202 | |
Less: current portion of long-term obligations and other short-term
borrowings | | | 536 | | | 31 | |
| Long-term obligations, less current portion | | | $ | 4,313 | | | $ | 4,171 | |
(1) During the fiscal year ended June 30, 2023, the Company drew down $715 million on its revolving credit facility to supplement operating cash flows and fund the Metrics acquisition, of which $215 million was repaid during fiscal year ended June 30, 2023. The Company has elected to classify the borrowing on its revolving credit facility as current as it intends to repay a portion of the borrowing using cash flows from operations and/or refinance the borrowing within the next twelve months.
(2) The change in the carrying value of this euro-denominated debt was due to fluctuations in foreign currency exchange rates.
(3) The increase in other obligations is associated with $24 million in proceeds from a failed sale-leaseback transaction that occurred in fiscal year ended June 30, 2023.
Senior Secured Credit Facilities and the Seventh and Eighth Amendments to the Credit Agreement
Operating Company is the borrower under an Amended and Restated Credit Agreement, dated May 20, 2014 (as subsequently amended, the “Credit Agreement”), which provides for senior secured credit facilities consisting of a term loan facility, a revolving credit facility (as amended, the “Revolving Credit Facility”), and facilities for letters of credit and swing-line short-term borrowings. All of the term loans are U.S. dollar-denominated as of June 30, 2023, and borrowings under the Revolving Credit Facility must be in dollars. The Credit Agreement includes customary representations by Operating Company, affirmative and negative covenants, events of default, and change of control provisions. The Credit Agreement also provides in customary fashion for (a) amortization payments with respect to, and events that may require partial or complete prepayment of, amounts borrowed under the term loan facility and (b) customary commitment fees on the unused commitments under the Revolving Credit Facility and customary letter of credit fees.
In November 2022, Operating Company entered into Amendment No. 7 (the “Seventh Amendment”) to the Credit Agreement, pursuant to which Operating Company (i) terminated its existing revolving credit commitments (and the related outstanding revolving borrowings) and (ii) obtained $1.10 billion aggregate amount of new revolving credit commitments, borrowing thereunder an amount equal to the previously outstanding borrowings under the terminated commitments in order to repay those commitments. The new commitments have an interest rate margin, at Operating Company’s option, based on (1) the prime rate, plus a margin ranging from 0.75%to 1.2500% based on Operating Company’s consolidated leverage ratio or (2) the Secured Overnight Financing Rate (“SOFR”), plus 0.100%, plus a margin ranging from 1.75% to 2.25% based on Operating Company’s consolidated leverage ratio. The Revolving Credit Facility has a maturity date that is the earlier of (A) five years after November 22, 2022, and (B) the 91st day prior to the maturity of Operating Company’s 5.000% senior unsecured notes due 2027 (the “2027 Notes”) or any permitted refinancing thereof, if on such 91st day, any of the 2027 Notes remains outstanding.
In June 2023, Operating Company entered into Amendment No. 8 (the “Eighth Amendment”) to the Credit Agreement, pursuant to which the SOFR benchmark replaced the LIBOR benchmark for determining the interest rate applicable to outstanding term loans. The Eighth Amendment includes a 0.1148% credit spread adjustment to the SOFR benchmark for loans with a 1-month interest period, a 0.26161% credit spread adjustment to the SOFR benchmark for loans with a 3-month interest period and a 0.42826% credit spread adjustment to the SOFR benchmark for loans with a 6-month interest period.
The Revolving Credit Facility requires compliance with a net leverage covenant when there is a 30% or more draw outstanding at a period end. As of June 30, 2023, the Company was in compliance with all material covenants under the Credit Agreement.
In addition to outstanding borrowings under the Revolving Credit facility, the available capacity under the Revolving Credit Facility is further reduced by the aggregate value of all outstanding letters of credit under the Credit Agreement. As of June 30, 2023, Operating Company had $594 million of unutilized capacity under the Revolving Credit Facility, due to $500 million in short-term borrowings outstanding and $6 million of outstanding letters of credit.
5.000% Senior Notes due 2027
In June 2019, Operating Company completed a private offering of $500 million aggregate principal amount of the 2027 Notes. The 2027 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2027 Notes were offered in the U.S to qualified institutional buyers in reliance on Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”) and outside the U.S only to non-U.S. investors pursuant to Regulation S under the Securities Act. The 2027 Notes will mature on July 15, 2027 and bear interest at the rate of 5.000% per annum. Interest is payable semi-annually in arrears on January 15 and July 15 of each year. The proceeds of the 2027 Notes, after payment of the offering fees and expenses, were used to repay in full the borrowings under Operating Company’s then-outstanding term loans, which would otherwise have matured in May 2024.
2.375% Euro-denominated Senior Notes due 2028
In March 2020, Operating Company completed a private offering of €825 million aggregate principal amount of 2.375% Senior Notes due 2028 (the "2028 Notes"). The 2028 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2028 Notes were offered in the U.S. to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the U.S. only to non-U.S. investors pursuant to Regulation S under the Securities Act. The 2028 Notes will mature on March 1, 2028 and bear interest at the rate of 2.375% per annum. Interest is payable semi-annually in arrears on March 1 and September 1 of each year, with the first payment on September 1, 2020. The proceeds of the 2028 Notes, after payment of the offering fees and expenses, were used to repay in full the borrowings then outstanding under Operating Company's euro-denominated term loans, which would have matured in May 2024, and repay in full Operating Company's euro-denominated 4.75% Senior Notes due 2024, which would otherwise have matured in December 2024, plus accrued and unpaid interest thereon, with the remainder available for general corporate purposes.
3.125% Senior Notes due 2029
In February 2021, Operating Company completed a private offering of $550 million aggregate principal amount of 3.125% Senior Notes due 2029 (the “2029 Notes”). The 2029 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2029 Notes will mature on February 15, 2029 and bear interest at the rate of 3.125% per annum payable semi-annually in arrears on February 15 and August 15 of each year, with the first payment on August 15, 2021. The proceeds of the 2029 Notes, after payment of the offering fees and expenses, were used to repay in full the outstanding borrowings under Operating Company's 4.875% Senior Notes due 2026 (the “2026 Notes”), which would have otherwise matured in January 2026, plus accrued and unpaid interest thereon, with the remainder available for general corporate purposes.
3.500% Senior Notes due 2030
In September 2021, Operating Company completed a private offering of $650 million aggregate principal amount of 3.500% Senior Notes due 2030 (the “2030 Notes”) and together with the 2027 Notes, the 2028 Notes, and the 2029 Notes, the “Senior Notes”). The 2030 Notes are fully and unconditionally guaranteed, jointly and severally, by all of the wholly owned U.S. subsidiaries of Operating Company that guarantee its senior secured credit facilities. The 2030 Notes were offered in the U.S. to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the U.S. only to non-U.S. investors pursuant to Regulation S under the Securities Act. The 2030 Notes will mature on April 1, 2030 and bear interest at
the rate of 3.500% per annum payable semi-annually in arrears on April 1 and October 1 of each year, with the first payment on April 1, 2022. The proceeds of the 2030 Notes, after payment of the offering fees and expenses, were used to fund a portion of the consideration paid at the closing of the Bettera Wellness acquisition.
Deferred Purchase Consideration
In connection with the acquisition of Cook Pharmica LLC (now Catalent Indiana, LLC) in October 2017, $200 million of the $950 million aggregate nominal purchase price was payable in $50 million installments, on each of the first four anniversaries of the closing date. The Company made installment payments in October 2018, October 2019, and October 2020, and the final payment was made in October 2021.
Long-Term and Other Obligations
Other obligations consist primarily of finance leases for buildings and other loans for business and working capital needs. Maturities of long-term obligations, including finance leases of $341 million, and other short-term borrowings for future fiscal years are:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | | | 2024 | | 2025 | | 2026 | | 2027 | | 2028 | | Thereafter | | Total |
| Maturities of long-term and other obligations | | | $ | 536 | | | $ | 48 | | | $ | 31 | | | $ | 32 | | | $ | 1,429 | | | $ | 2,812 | | | $ | 4,888 | |
Debt Issuance Costs
Debt issuance costs associated with the Credit Agreement (other than its Revolving Credit Facility component) and the Senior Notes are presented as a reduction to the carrying value of the related debt, while debt issuance costs associated with the Revolving Credit Facility are capitalized within other long-term assets on the consolidated balance sheets. All debt issuance costs are amortized over the life of the related obligation through charges to interest expense in the consolidated statements of operations. The unamortized total debt issuance costs, including the costs associated with the Revolving Credit Facility capitalized within Other long-term assets, were $39 million and $42 million as of June 30, 2023 and 2022, respectively. Amortization of debt issuance costs totaled $8 million and $7 million for the fiscal years ended June 30, 2023 and 2022, respectively.
Guarantees and Security
Senior Secured Credit Facilities
All obligations under the Credit Agreement, and the guarantees of those obligations, are secured by substantially all of the following assets of Operating Company and each guarantor (Operating Company's parent entity, PTS Intermediate, and each of Operating Company's material domestic subsidiaries), subject to certain exceptions:
•a pledge of 100% of the capital stock of Operating Company and 100% of the equity interests directly held by Operating Company and each guarantor in any wholly owned material subsidiary of Operating Company or any guarantor (which pledge, in the case of any non-U.S. subsidiary of a U.S. subsidiary, will not include more than 65% of the voting stock of such non-U.S. subsidiary); and
•a security interest in, and mortgages on, substantially all tangible and intangible assets of Operating Company and of each guarantor, subject to certain limited exceptions.
The Senior Notes
All obligations under the Senior Notes are general, unsecured, and subordinated to all existing and future secured indebtedness of the guarantors to the extent of the value of the assets securing such indebtedness. Each of the Senior Notes is separately guaranteed by all of Operating Company's wholly owned U.S. subsidiaries that guarantee the senior secured credit facilities. None of the Senior Notes is guaranteed by either PTS Intermediate or the Company.
Debt Covenants
Senior Secured Credit Facilities
The Credit Agreement contains covenants that, among other things, restrict, subject to certain exceptions, Operating Company’s (and Operating Company’s restricted subsidiaries’) ability to incur additional indebtedness or issue certain preferred shares; create liens on assets; engage in mergers and consolidations; sell assets; pay dividends and distributions or repurchase capital stock; repay subordinated indebtedness; engage in certain transactions with affiliates; make investments,
loans or advances; make certain acquisitions; enter into sale and leaseback transactions; amend material agreements governing Operating Company's subordinated indebtedness and change Operating Company's lines of business.
Subject to certain exceptions, the Credit Agreement permits Operating Company and its restricted subsidiaries to incur certain additional indebtedness, including secured indebtedness. None of Operating Company’s non-U.S. subsidiaries nor its dormant Puerto Rico subsidiary is a guarantor of the loans.
Under the Credit Agreement, Operating Company’s ability to engage in certain activities such as incurring certain additional indebtedness, making certain investments, and paying certain dividends is tied to ratios based on Adjusted EBITDA (which is defined as “Consolidated EBITDA” in the Credit Agreement). Adjusted EBITDA is based on the definitions in the Credit Agreement, is not defined under U.S. GAAP, and is subject to important limitations.
The Senior Notes
The various indentures governing the Senior Notes (collectively, the “Indentures”) contain covenants that, among other things, limit the ability of Operating Company and its restricted subsidiaries to incur or guarantee more debt or issue certain preferred shares; pay dividends on, repurchase, or make distributions in respect of their capital stock or make other restricted payments; make certain investments; sell certain assets; create liens; consolidate, merge, sell; or otherwise dispose of all or substantially all of their assets; enter into certain transactions with their affiliates, and designate their subsidiaries as unrestricted subsidiaries. These covenants are subject to a number of exceptions, limitations, and qualifications as set forth in the Indentures. The Indentures also contain customary events of default, including, but not limited to, nonpayment, breach of covenants, and payment or acceleration defaults in certain other indebtedness of Operating Company or certain of its subsidiaries. Upon an event of default, either the holders of at least 30% in principal amount of each of the then-outstanding Senior Notes or the applicable trustee under the Indentures may declare the applicable notes immediately due and payable; or in certain circumstances, the applicable notes will automatically become immediately due and payable. As of June 30, 2023, Operating Company was in compliance with all material covenants under the Indentures.
Measurement of the Estimated Fair Value of Debt
The estimated fair values of the senior secured credit facilities and Senior Notes are classified as Level 2 (see Note 10, Fair Value Measurements for a description of the method by which fair value classifications are determined) in the fair value hierarchy and are calculated by using a discounted cash flow model with market interest rate as a significant input. The carrying amounts and the estimated fair values of financial instruments as of June 30, 2023 and June 30, 2022 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | June 30, 2023 | | June 30, 2022 |
| (Dollars in millions) | Fair Value Measurement | | Carrying Value | | Estimated Fair Value | | Carrying Value | | Estimated Fair Value |
| | | | | | | | | |
| | | | | | | | | |
| 5.000% Senior Notes due 2027 | Level 2 | | $ | 500 | | | $ | 482 | | | $ | 500 | | | $ | 483 | |
| 2.375% euro Senior Notes due 2028 | Level 2 | | 904 | | | 784 | | | 874 | | | 744 | |
| 3.125% Senior Notes due 2029 | Level 2 | | 550 | | | 481 | | | 550 | | | 476 | |
| 3.500% senior notes due 2030 | Level 2 | | 650 | | | 566 | | | 650 | | | 561 | |
| Senior secured credit facilities & other | Level 2 | | 2,284 | | | 2,141 | | | 1,669 | | | 1,575 | |
| Subtotal | | | $ | 4,888 | | | $ | 4,454 | | | $ | 4,243 | | | $ | 3,839 | |
Unamortized discount and debt issuance
costs | | | (39) | | | — | | | (41) | | | — | |
| Total debt | | | $ | 4,849 | | | $ | 4,454 | | | $ | 4,202 | | | $ | 3,839 | |
8. (LOSS) EARNINGS PER SHARE
Effective as of the first quarter of fiscal 2023, the Company computes EPS using the treasury stock method. Prior to fiscal 2023, the Company computed EPS using the two-class method required due to the participating nature of the previously outstanding Series A Preferred Stock (as defined and discussed in Note 13, Equity and Accumulated Other Comprehensive Loss).
The weighted-average number of shares outstanding utilized in diluted EPS is computed using the weighted-average number of shares of Common Stock outstanding plus the number of shares of Common Stock that would be issued assuming exercise or conversion of all potentially dilutive instruments. Dilutive securities having an anti-dilutive effect on diluted EPS are excluded from the calculation. The dilutive effect of the securities that are issuable under the Company’s equity incentive plans (see Note 14, Stock-Based Compensation) are reflected in diluted EPS by application of the treasury stock method. Prior to fiscal 2023, the Company applied the if-converted method to compute the potentially dilutive effect of the Series A Preferred
Stock. The reconciliations between basic and diluted EPS attributable to Catalent common shareholders for the fiscal years ended June 30, 2023, 2022, and 2021 are as follows:
| | | | | | | | | | | | | | | | | |
| | Fiscal year ended June 30, |
| (In millions, except per share data) | 2023 | | 2022 | | 2021 |
| Net earnings | $ | (256) | | | $ | 499 | | | $ | 585 | |
| Less: Net earnings attributable to preferred shareholders | — | | | (16) | | | (56) | |
| Net earnings attributable to common shareholders | $ | (256) | | | $ | 483 | | | $ | 529 | |
| | | | | |
| Weighted average shares outstanding - basic | 181 | | | 176 | | | 168 | |
Weighted average dilutive securities issuable - stock plans | — | | | 2 | | | 2 | |
| Total weighted average shares outstanding - diluted | 181 | | | 178 | | | 170 | |
| | | | | |
| (Loss) earnings per share: | | | | | |
| Basic | $ | (1.42) | | | $ | 2.74 | | | $ | 3.15 | |
| Diluted | $ | (1.42) | | | $ | 2.73 | | | $ | 3.11 | |
| | | | | |
| | | | | |
The Series A Preferred Stock was deemed a participating security, meaning that it had the right to participate in undistributed earnings with the Common Stock. On November 23, 2020 (the “Partial Conversion Date”), the holders of Series A Preferred Stock converted 265,223 shares and $2 million of unpaid accrued dividends into shares of Common Stock. On November 18, 2021 (the “Final Conversion Date”), the holders of Series A Preferred Stock converted the remaining 384,777 shares of Series A Preferred Stock and $2 million of unpaid accrued dividends into shares of Common Stock.
The diluted weighted average number of shares outstanding for the fiscal years ended June 30, 2023, 2022, and 2021 did not include the following weighted average number of shares of Common Stock associated with the formerly outstanding Series A Preferred Stock or the weighted average number of shares of Common Stock associated with outstanding equity grants due to their antidilutive effect:
| | | | | | | | | | | | | | | | | |
| Fiscal year ended June 30, |
| (share counts in millions) | 2023 | | 2022 | | 2021 |
| | | | | |
| | | | | |
| | | | | |
| Series A Preferred Stock | — | | | 3 | | | 10 | |
| | | | | |
| | | | | |
| | | | | |
9. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
Risk Management Objective of Using Derivatives
The Company is exposed to fluctuations in the currency exchange rates applicable to its investments in foreign operations. While the Company does not actively hedge against changes in foreign currency, the Company has mitigated the exposure arising from its investments in its European operations by denominating a portion of its Senior Notes in euros. At June 30, 2023, the Company had euro-denominated debt outstanding of $904 million (U.S. dollar equivalent), which qualifies as a hedge of a net investment in foreign operations. For non-derivatives designated and qualifying as net investment hedges, the effective portion of the translation gains or losses are reported in accumulated other comprehensive income (loss) as part of the cumulative translation adjustment. The unhedged portions of the translation gains or losses are reported in the consolidated statements of operations. The following table includes net investment hedge activity during the fiscal years ended June 30, 2023 and 2022, respectively:
| | | | | | | | | | | |
| (Dollars in millions) | June 30, 2023 | | June 30, 2022 |
| Unrealized foreign exchange gain (loss) within other comprehensive income | $ | (30) | | | $ | 121 | |
| Unrealized foreign exchange loss within statement of operations | $ | — | | | $ | (11) | |
The net accumulated gain of this net investment hedge within accumulated other comprehensive loss was $97 million as of June 30, 2023. Amounts are reclassified out of accumulated other comprehensive loss into earnings when the entity in which the gains and losses reside is either sold or substantially liquidated.
Interest-Rate Swap
In April 2020, pursuant to its interest rate and risk management strategy, the Company entered into an interest-rate swap agreement with Bank of America N.A. (the “2020 Rate Swap”) as a hedge against the economic effect of a portion of the variable interest obligation associated with its U.S. dollar-denominated term loans under its senior secured credit facilities.
In February 2021, in connection with an amendment to the Credit Agreement, the Company paid $2 million in cash to Bank of America N.A to settle the 2020 Rate Swap. This loss is deferred in stockholders’ equity, net of income taxes, as a component of accumulated other comprehensive loss, and amortized as an adjustment to interest expense, net over the original term of the formerly outstanding term loans. The net amount of deferred losses on cash flow hedges that is expected to be reclassified from accumulated other comprehensive loss into interest expense, net within the next twelve months is not material.
In February 2021, the Company entered into a new interest-rate swap agreement with Bank of America N.A. (the “2021 Rate Swap”) that acted as a hedge against the economic effect of a portion of the variable-interest obligation associated with the Company's U.S. dollar-denominated term loans under its senior secured credit facilities. The 2021 Rate Swap effectively fixed the rate of interest payable on that portion of the term loans, thereby reducing the impact of future interest rate changes on future interest expense. As a result of the 2021 Rate Swap, the variable portion of the applicable interest rate on $500 million of the U.S. dollar-denominated term loans was effectively fixed at 0.9985%.
To conform with the adoption of Topic 848, Reference Rate Reform and the Eighth Amendment, the Company amended the 2021 Rate Swap in June 2023 (the “2023 Rate Swap”). The 2023 Rate Swap continues to effectively fix the rate of interest payable on the same portion of our U.S. dollar-denominated term loans under our senior secured credit facilities. As a result of the 2023 Rate Swap, the variable portion of the applicable interest rate on $500 million of the U.S. dollar-denominated term loans is now effectively fixed at 0.9431%.
The 2023 Rate Swap continues to qualify for a cash-flow hedge. The Company evaluates hedge effectiveness at the inception of the hedge and on an ongoing basis. The cash flows associated with the 2023 Rate Swap amendment is reported in cash provided by operating activities in the consolidated statements of cash flows. The unrealized gain recorded in stockholder's equity from marking the 2021 Rate Swap to market during the fiscal year ended June 30, 2023 was $25 million.
A summary of the estimated fair value of the interest-rate swap reported in the consolidated balance sheets is stated in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | June 30, 2023 | | June 30, 2022 |
| (in millions) | | Balance Sheet Classification | | Estimated Fair Value | | Balance Sheet Classification | | Estimated Fair Value |
| Interest-rate swap | | Other long-term assets | | $ | 62 | | | Other long-term assets | | $ | 36 | |
10. FAIR VALUE MEASUREMENTS
ASC 820, Fair Value Measurement defines fair value as the exit price that would be received to sell an asset or paid to transfer a liability. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. Valuation techniques used to measure fair value should maximize the use of observable inputs and minimize the use of unobservable inputs. To measure fair value, the Company uses the following fair value hierarchy based on three levels of inputs, of which Level 1 and Level 2 are considered observable and Level 3 is considered unobservable:
Level 1 – Quoted prices in active markets for identical assets or liabilities.
Level 2 – Inputs other than Level 1 that are observable for the asset or liability, either directly or indirectly, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data by correlation or other means.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Value is determined using pricing models, discounted cash flow methodologies, or similar techniques and also includes instruments for which the determination of fair value requires significant judgment or estimation.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable and accrued expenses of the Company approximate fair value based on the short maturities of these instruments.
The Company evaluates its financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level of classification as of the end of each reporting period. The following table sets forth the Company’s financial assets and liabilities that were measured at fair value on a recurring basis and the fair value measurement for such assets and liabilities at June 30, 2023 and 2022, respectively:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | | Basis of Fair Value Measurement |
| June 30, 2023 | | Total | | Level 1 | | Level 2 | | Level 3 |
| Assets: | | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Interest-rate swap | | $ | 62 | | | $ | — | | | $ | 62 | | | $ | — | |
| Trading securities | | $ | 1 | | | $ | 1 | | | $ | — | | | $ | — | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| June 30, 2022 | | | | | | | | |
| Assets: | | | | | | | | |
| Marketable securities | | $ | 89 | | | $ | 89 | | | $ | — | | | $ | — | |
| Interest-rate swap | | 36 | | | — | | | 36 | | | — | |
| Trading securities | | $ | 2 | | | $ | 2 | | | $ | — | | | $ | — | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The fair value of the 2021 Rate Swap was determined, and the fair value of the 2023 Rate Swap will be determined, at the end of each reporting period based on valuation models that use interest rate yield curves and discount rates as inputs. The discount rates are based on U.S. deposit or U.S. Treasury rates. The significant inputs used in the valuation models are readily available in public markets or can be derived from observable market transactions, and the valuation is therefore classified as Level 2 in the fair-value hierarchy.
Assets and Liabilities Measured at Fair Value on a Non-Recurring Basis
Long-lived assets, goodwill, and other intangible assets are subject to non-recurring fair value measurement for the evaluation of potential impairment. The carrying value of the Consumer Health reporting unit approximates its fair value as of June 30, 2023 following the impairment charge. Other than the valuation of the Consumer Health reporting unit, there was no non-recurring fair value measurement during the fiscal years ended June 30, 2023 and 2022.
11. INCOME TAXES
Earnings before income taxes are as follows for fiscal 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2021 |
| U.S. operations | $ | (410) | | | $ | 224 | | | $ | 457 | |
| Non-U.S. operations | 68 | | | 355 | | | 258 | |
| Total | $ | (342) | | | $ | 579 | | | $ | 715 | |
The provision for income taxes consists of the following for fiscal 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| | Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2021 |
| Current: | | | | | |
| Federal | $ | (1) | | | $ | (8) | | | $ | 8 | |
| State and local | (1) | | | 14 | | | 20 | |
| Non-U.S. | 43 | | | 66 | | | 38 | |
| Total current expense | $ | 41 | | | $ | 72 | | | $ | 66 | |
| Deferred: | | | | | |
| Federal | $ | (99) | | | $ | 6 | | | $ | 62 | |
| State and local | (4) | | | (5) | | | 7 | |
| Non-U.S. | (24) | | | 7 | | | (5) | |
| Total deferred (benefit) expense | $ | (127) | | | $ | 8 | | | $ | 64 | |
| | | | | |
| Total (benefit) provision | $ | (86) | | | $ | 80 | | | $ | 130 | |
A reconciliation of the provision starting from the tax computed at the federal statutory income tax rate to the tax computed at the Company’s effective income tax rate is as follows for the fiscal years ended 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2021 |
| Provision at U.S. federal statutory tax rate | $ | (72) | | | $ | 122 | | | $ | 150 | |
| State and local income taxes | (13) | | | 10 | | | 26 | |
| Foreign tax rate differential | (5) | | | (28) | | | (14) | |
| Global intangible low tax income | 2 | | | 6 | | | 3 | |
| Other permanent items | 13 | | | 2 | | | (5) | |
| Unrecognized tax positions | (1) | | | 1 | | | 3 | |
| Tax valuation allowance | 5 | | | 94 | | | (7) | |
| Foreign tax credit | (30) | | | (43) | | | (24) | |
| Withholding tax and other foreign taxes | 1 | | | 1 | | | 1 | |
| Change in tax rate | 18 | | | 1 | | | 2 | |
| | | | | |
| R&D tax credit | (3) | | | (2) | | | (5) | |
| Swiss tax reform | — | | | (83) | | | — | |
| Other | (1) | | | (1) | | | — | |
| Total provision | $ | (86) | | | $ | 80 | | | $ | 130 | |
The income tax provision for the fiscal year ended June 30, 2023 is not comparable to the provision in the prior year due to changes in the geographic mix of pretax income and losses, changes in the tax impact of permanent differences and credits,
and the tax impact of discrete items. The higher effective tax rate on the global loss is primarily due to (i) research and development credits in the U.S., the recognition of deferred tax assets on the book impairment of tax deductible goodwill, and decreased earnings in non-U.S. jurisdictions with lower tax rates. This was partially offset by fixed permanent tax adjustments in the U.S. on lower income. In comparison, the lower effective tax rate on the prior year global income was partially the result of a net deferred benefit of $21 million related to tax reform in Switzerland and related transition rules (collectively, “Swiss Tax Reform”) and additional foreign tax credits in the U.S. due to amended prior-year returns.
The Company intends to repatriate foreign earnings taxed in prior fiscal years as a result of the changes imposed by the 2017 U.S. Tax Cuts and Jobs Act. In addition to these foreign earnings previously taxed, as of June 30, 2023, for purposes of ASC 740-10-25-3, the Company had $421 million of undistributed earnings from non-U.S. subsidiaries that it intends to reinvest permanently in its non-U.S. operations. As these ASC 740-10-25-3 earnings are considered permanently reinvested, no tax provision has been accrued. It is not feasible to estimate the amount of tax that might be payable on the eventual remittance of such earnings.
Deferred income taxes arise from temporary differences between the financial reporting and tax reporting bases of assets and liabilities, and operating loss and tax credit carryforwards for tax purposes. The components of the Company's deferred income tax assets and liabilities are as follows at June 30, 2023 and 2022:
| | | | | | | | | | | |
| Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 |
| Deferred income tax assets: | | | |
| Accrued liabilities | $ | 57 | | | $ | 33 | |
| Equity compensation | 13 | | | 14 | |
| Loss and tax credit carryforwards | 287 | | | 230 | |
| Foreign currency | 3 | | | 19 | |
| Pension | 19 | | | 17 | |
| Interest-related | 50 | | | 14 | |
| Deferred revenue | — | | | 1 | |
| | | |
Lease liabilities | 38 | | | 39 | |
| Euro-denominated debt | 1 | | | — | |
| Other | 1 | | | 2 | |
| Total deferred income tax assets | $ | 469 | | | $ | 369 | |
| Valuation allowance | (159) | | | (149) | |
| Net deferred income tax assets | $ | 310 | | | $ | 220 | |
| | | |
| | | |
| Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 |
| Deferred income tax liabilities: | | | |
| | | |
| | | |
| Euro-denominated debt | $ | — | | | $ | (6) | |
| Property-related | (247) | | | (227) | |
| Goodwill and other intangibles | (71) | | | (113) | |
| Right-of-use assets | (13) | | | (21) | |
| Other | — | | | (1) | |
| | | |
| Total deferred income tax liabilities | $ | (331) | | | $ | (368) | |
| | | |
| Net deferred tax liability | $ | (21) | | | $ | (148) | |
Deferred tax assets and liabilities in the preceding table are in the following captions in the consolidated balance sheets at June 30, 2023 and 2022: | | | | | | | | | | | |
| Fiscal Year Ended
June 30, |
| (Dollars in millions) | 2023 | | 2022 |
| Non-current deferred tax asset | $ | 55 | | | $ | 49 | |
| Non-current deferred tax liability | (76) | | | (197) | |
| Net deferred tax liability | $ | (21) | | | $ | (148) | |
At June 30, 2023, the Company had federal net operating loss (“NOL”) carryforwards of $609 million, $286 million of which are subject to limitations under Section 382 of the Internal Revenue Code of 1986, as amended (the “Internal Revenue Code”). The majority of the $286 million federal NOL carryforwards subject to Section 382 of the Internal Revenue Code are attributed to the Company's acquisitions of Pharmatek Laboratories, Inc., Juniper Pharmaceuticals, Inc., Paragon Bioservices, Inc., MastherCell Global Inc. (“MaSTherCell”), and Metrics. As of June 30, 2023, $564 million of the Company's federal NOL carryforwards have an indefinite life and the remaining NOL carryforwards will expire in fiscal years 2037 through 2038.
At June 30, 2023, the Company had state tax NOL carryforwards of $378 million. Substantially all state NOL carryforwards have a twenty-year carryforward period. At June 30, 2023, the Company had non-U.S. tax NOL carryforwards of $319 million, a majority of which are available for at least three years or have an indefinite carryforward period.
The Company had valuation allowances of $159 million and $149 million as of June 30, 2023 and 2022, respectively, against its deferred tax assets. The Company considered all available evidence, both positive and negative, in assessing the need for a valuation allowance against tax assets. Four possible sources of taxable income were evaluated when assessing the realizability of deferred tax assets:
•carrybacks of existing NOLs (if and to the extent permitted under the tax law);
•future reversals of existing taxable temporary differences;
•tax planning strategies; and
•future taxable income exclusive of reversing temporary differences and carryforwards.
While the valuation allowance related to certain U.S. combined states was released during the fiscal year ended June 30, 2019, there remained as of June 30, 2023 a valuation allowance for the NOLs and deductible temporary differences in the remaining combined and separate states of $23 million. The state valuation allowance as of June 30, 2023 is due to the Company’s history of tax losses and anticipated loss utilization rates in separate filing status states as well as the difference in the rules related to allocated and apportioned income for separate filing status states versus combined filing status states.
The Company considered the need to maintain a valuation allowance on deferred tax assets based on management’s assessment of whether it is more likely than not that the Company would realize the value of its deferred tax assets based on future reversals of existing taxable temporary differences and the ability to generate sufficient taxable income within the carryforward period available under the applicable tax laws. During the fiscal year ended June 30, 2023, the Company established valuation allowances on NOLs and temporary differences related to certain Belgian and Japanese operations in the aggregate amount of $7 million.
In the normal course of business, the Company's income taxes are subject to audits by federal, state, and foreign tax authorities, some of which are ongoing and may result in proposed assessments. Germany and the U.K. are among the jurisdictions where the Company has substantial tax positions. The Company is no longer subject to examinations by the relevant tax authorities for years prior to fiscal 2009. The Company’s estimate for the potential outcome for any uncertain tax issue is highly judgmental. The Company assesses its income tax positions and records benefits for all years subject to examination based upon management’s evaluation of the facts, circumstances, and information available at the reporting date. For those tax positions for which it is more likely than not that a tax benefit will be sustained, the Company records the amount that has a greater than 50% likelihood of being realized upon resolution with the taxing authority that has full knowledge of all relevant information based on the technical merit. Interest and penalties are accrued, where applicable.
As of June 30, 2023, the Company had a total of $4 million of unrecognized tax benefits. A reconciliation of unrecognized tax benefits, excluding accrued interest, as of June 30, 2023, 2022, and 2021 is as follows:
| | | | | |
| (Dollars in millions) |
| Balance at June 30, 2020 | $ | 4 | |
| |
| Additions for tax positions of prior years | 3 | |
| |
| |
| Lapse of the applicable statute of limitations | (2) | |
| Balance at June 30, 2021 | $ | 5 | |
| |
| Additions for tax positions related to the current year | 1 | |
| Additions for tax positions of prior years | 1 | |
| |
| |
| Settlements | (1) | |
| Lapse of the applicable statute of limitations | (1) | |
| Balance at June 30, 2022 | $ | 5 | |
| |
| Additions for tax positions of prior years | 2 | |
| Reductions for tax positions of prior years | (1) | |
| Settlements | (1) | |
| Lapse of the applicable statute of limitations | (1) | |
| Balance at June 30, 2023 | $ | 4 | |
All of the unrecognized tax benefits as of June 30, 2023 and 2022 would, if subsequently recognized, favorably affect the effective income tax rate.
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. As of June 30, 2023, the Company has $0 million of accrued interest related to uncertain tax positions, with a reduction from the prior year, as a result of statute of limitations lapses and settlements. The Company had $1 million of accrued interest related to uncertain tax positions as of both June 30, 2022 and 2021.
12. EMPLOYEE RETIREMENT BENEFIT PLANS
The Company sponsors various retirement plans, including defined benefit pension plans and defined contribution plans. Substantially all of the Company’s domestic non-union employees are eligible to participate in employer-sponsored retirement savings plans, which include plans created under Section 401(k) of the Internal Revenue Code that provide for the Company to match a portion of contributions by participating U.S. employees. The Company’s contributions to the plans are discretionary but are subject to certain minimum requirements as specified in the plans. The Company uses a measurement date of June 30 for all of its retirement and postretirement benefit plans.
The Company records obligations related to its withdrawal from one multi-employer pension plan that covered former employees at three former sites. This withdrawal was classified as a mass withdrawal under the Multiemployer Pension Plan Amendments Act of 1980, as amended, and the Pension Protection Act of 2006 and resulted in the recognition of liabilities associated with the Company’s long-term obligations in prior years not presented, which were primarily recorded as an expense within discontinued operations. The estimated discounted value of the projected contributions related to these plans is $38 million as of June 30, 2023 and 2022. The annual cash impact associated with the Company’s long-term obligation arising from this plan is $2 million per year.
The following table provides a reconciliation of the change in projected benefit obligation and fair value of plan assets for the defined benefit retirement and other retirement plans, excluding the multi-employer pension plan liability:
| | | | | | | | | | | | | | | | | | | | | | | |
| Retirement Benefits | | Other Post-Retirement Benefits |
| June 30, | | June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2023 | | 2022 |
| Accumulated Benefit Obligation | $ | 242 | | | $ | 262 | | | $ | 2 | | | $ | 2 | |
| | | | | | | |
| Change in Benefit Obligation | | | | | | | |
| Benefit obligation at beginning of year | 268 | | | 372 | | | 2 | | | 2 | |
| Company service cost | 3 | | | 4 | | | — | | | — | |
| Interest cost | 9 | | | 5 | | | — | | | — | |
| Employee contributions | 1 | | | — | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| Settlements | (4) | | | (1) | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Benefits paid | (10) | | | (9) | | | — | | | — | |
| | | | | | | |
Actuarial gain (1) | (28) | | | (71) | | | — | | | — | |
| Exchange rate gain (loss) | 7 | | | (32) | | | — | | | — | |
| Benefit obligation at end of year | $ | 246 | | | $ | 268 | | | $ | 2 | | | $ | 2 | |
| | | | | | | |
| Change in Plan Assets | | | | | | | |
| Fair value of plan assets at beginning of year | 240 | | | 318 | | | — | | | — | |
| Actual return on plan assets | (38) | | | (50) | | | — | | | — | |
| Company contributions | 7 | | | 10 | | | — | | | — | |
| Employee contributions | 1 | | | — | | | — | | | — | |
| Settlements | (4) | | | (1) | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Benefits paid | (10) | | | (9) | | | — | | | — | |
| | | | | | | |
| Exchange rate gain (loss) | 6 | | | (28) | | | — | | | — | |
| Fair value of plan assets at end of year | $ | 202 | | | $ | 240 | | | $ | — | | | $ | — | |
| | | | | | | |
| Funded Status | | | | | | | |
| Funded status at end of year | (44) | | | (28) | | | (2) | | | (2) | |
| | | | | | | |
| Net pension liability | $ | (44) | | | $ | (28) | | | $ | (2) | | | $ | (2) | |
(1) For the fiscal year ended June 30, 2022, the actuarial gain is driven by a large increase in the aggregate discount rate.
The following table provides a reconciliation of the net amount recognized in the consolidated balance sheets:
| | | | | | | | | | | | | | | | | | | | | | | |
| Retirement Benefits | | Other Post-Retirement Benefits |
| June 30, | | June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2023 | | 2022 |
| Amounts Recognized in Statement of Financial Position | | | | | | | |
| Noncurrent assets | $ | 18 | | | $ | 37 | | | $ | — | | | $ | — | |
| Current liabilities | (1) | | | (1) | | | — | | | — | |
| Noncurrent liabilities | (61) | | | (64) | | | (2) | | | (2) | |
| Total liability | (44) | | | (28) | | | (2) | | | (2) | |
| | | | | | | |
| Amounts Recognized in Accumulated Other Comprehensive Loss | | | | | | | |
| | | | | | | |
| Prior service cost | (1) | | | (1) | | | — | | | — | |
| Net loss (gain) | 66 | | | 49 | | | (1) | | | (1) | |
| Total accumulated other comprehensive loss (income) at the end of the fiscal year | 65 | | | 48 | | | (1) | | | (1) | |
| | | | | | | |
| Additional Information for Plan with ABO or PBO in Excess of Plan Assets | | | | | | | |
| Projected benefit obligation | 129 | | | 132 | | | 2 | | | 2 | |
| Accumulated benefit obligation | 126 | | | 128 | | | 2 | | | 2 | |
| Fair value of plan assets | 68 | | | 67 | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Components of Net Periodic Benefit Cost | | | | | | | |
| Service cost | 3 | | | 4 | | | — | | | — | |
| Interest cost | 9 | | | 5 | | | — | | | — | |
| Expected return on plan assets | (9) | | | (10) | | | — | | | — | |
| Amortization of unrecognized: | | | | | | | |
| | | | | | | |
| | | | | | | |
| Net loss | 1 | | | 2 | | | — | | | — | |
| | | | | | | |
| Settlement/curtailment expense | 1 | | | — | | | — | | | — | |
| Net periodic benefit cost | $ | 5 | | | $ | 1 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Retirement Benefits | | Other Post-Retirement Benefits |
| June 30, | | June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2023 | | 2022 |
| Other Changes in Plan Assets and Benefit Obligations Recognized in Other Comprehensive Income | | | | | | | |
| Net loss (gain) arising during the year | $ | 17 | | | $ | (14) | | | $ | — | | | $ | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Exchange rate loss recognized during the year | — | | | 1 | | | — | | | — | |
| Total recognized in other comprehensive income | $ | 17 | | | $ | (13) | | | $ | — | | | $ | — | |
| Total Recognized in Net Periodic Benefit Cost and Other Comprehensive Income | | | | | | | |
| Total recognized in net periodic benefit cost and other comprehensive income | $ | 22 | | | $ | (12) | | | $ | — | | | $ | — | |
| Estimated Amounts to be Amortized from Accumulated Other Comprehensive Income into Net Periodic Benefit Cost | | | | | | | |
| Amortization of: | | | | | | | |
| | | | | | | |
| | | | | | | |
| Net loss | $ | 2 | | | $ | 1 | | | $ | — | | | $ | — | |
| Financial Assumptions Used to Determine Benefit Obligations at the Balance Sheet Date | | | | | | | |
| Discount rate (%) | 4.3 | % | | 3.6 | % | | 4.7 | % | | 4.0 | % |
| Rate of compensation increases (%) | 2.7 | % | | 2.7 | % | | n/a | | n/a |
| Financial Assumptions Used to Determine Net Periodic Benefit Cost for Financial Year | | | | | | | |
| Discount rate (%) | 3.6 | % | | 1.6 | % | | 4.0 | % | | 2.0 | % |
| Rate of compensation increases (%) | 2.7 | % | | 2.0 | % | | n/a | | n/a |
| Expected long-term rate of return (%) | 3.9 | % | | 3.4 | % | | n/a | | n/a |
| Expected Future Contributions | | | | | | | |
| Fiscal year 2024 | $ | 8 | | | $ | 7 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Retirement Benefits | | Other Post-Retirement Benefits |
| June 30, | | June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2023 | | 2022 |
| Expected Future Benefit Payments | | | | | | | |
| Financial year | | | | | | | |
| 2024 | $ | 14 | | | $ | 14 | | | $ | — | | | $ | — | |
| 2025 | 15 | | | 13 | | | — | | | — | |
| 2026 | 16 | | | 14 | | | — | | | — | |
| 2027 | 15 | | | 16 | | | — | | | — | |
| 2028 | 15 | | | 14 | | | — | | | — | |
| 2029-2033 | 84 | | | 80 | | | 1 | | | 1 | |
| | | | | | | |
| Actual Asset Allocation (%) | | | | | | | |
| Cash | 4.5 | % | | — | % | | — | % | | — | % |
| Equities | 2.9 | % | | 4.1 | % | | — | % | | — | % |
| Government bonds | 36.8 | % | | 35.6 | % | | — | % | | — | % |
| Corporate bonds | 16.2 | % | | 18.3 | % | | — | % | | — | % |
| Property | 3.3 | % | | 4.9 | % | | — | % | | — | % |
| Insurance contracts | 14.9 | % | | 12.0 | % | | — | % | | — | % |
| Other | 21.4 | % | | 25.1 | % | | — | % | | — | % |
| Total | 100.0 | % | | 100.0 | % | | — | % | | — | % |
| | | | | | | |
| Actual Asset Allocation (Amount) | | | | | | | |
| Cash | $ | 9 | | | $ | — | | | $ | — | | | $ | — | |
| Equities | 6 | | | 10 | | | — | | | — | |
| Government bonds | 74 | | | 85 | | | — | | | — | |
| Corporate bonds | 33 | | | 44 | | | — | | | — | |
| Property | 7 | | | 12 | | | — | | | — | |
| Insurance contracts | 30 | | | 29 | | | — | | | — | |
| Other | 43 | | | 60 | | | — | | | — | |
| Total | $ | 202 | | | $ | 240 | | | $ | — | | | $ | — | |
| | | | | | | |
| Target Asset Allocation (%) | | | | | | | |
| Cash | 4.5 | % | | — | % | | — | % | | — | % |
| Equities | 2.9 | % | | 4.1 | % | | — | % | | — | % |
| Government bonds | 36.8 | % | | 35.6 | % | | — | % | | — | % |
| Corporate bonds | 16.2 | % | | 18.3 | % | | — | % | | — | % |
| Property | 3.3 | % | | 4.9 | % | | — | % | | — | % |
| Insurance contracts | 14.9 | % | | 12.0 | % | | — | % | | — | % |
| Other | 21.4 | % | | 25.1 | % | | — | % | | — | % |
| Total | 100.0 | % | | 100.0 | % | | — | % | | — | % |
The Company's Investment Committee employs a building-block approach in determining the long-term rate of return for plan assets, with proper consideration of diversification and rebalancing. Historical markets are studied and long-term historical relationships between equities and fixed income are preserved consistent with the widely accepted capital market principle that assets with higher volatility generate a greater return over the long run. Current market factors such as inflation and interest rates are evaluated before long-term capital market assumptions are determined. Peer data are reviewed to check for reasonability and appropriateness.
Plan assets are recognized and measured at fair value in accordance with the accounting standards regarding fair value measurements. The following are valuation techniques used to determine the fair value of each major category of assets:
•Short-term investments, equity securities, fixed-income securities, and real estate are valued using quoted market prices or other valuation methods, and thus are classified within Level 1 or Level 2.
•Insurance contracts and other types of investments include investments with some observable and unobservable prices that are adjusted by cash contributions and distributions, and thus are classified within Level 2 or Level 3.
The following tables provide a summary of plan assets that are measured at fair value as of June 30, 2023 and 2022, aggregated by the level in the fair value hierarchy within which those measurements fall:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2023 (dollars in millions) | Level 1 | | Level 2 | | Level 3 | | Investments Measured at Net Asset Value | | Total Assets |
|
| Equity securities | $ | — | | | $ | 6 | | | $ | — | | | $ | — | | | $ | 6 | |
| Debt securities | — | | | 107 | | | — | | | — | | | 107 | |
| Real estate | — | | | 5 | | | 2 | | | — | | | 7 | |
Other (1) | — | | | 57 | | | 25 | | | — | | | 82 | |
| Total | $ | — | | | $ | 175 | | | $ | 27 | | | $ | — | | | $ | 202 | |
(1) Other, as of June 30, 2023, included $20 million of investments in hedge funds related to the Company's U.K. pension plan, which were classified as Level 2.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2022 (dollars in millions) | Level 1 | | Level 2 | | Level 3 | | Investments Measured at Net Asset Value | | Total Assets |
|
| Equity securities | $ | — | | | $ | 10 | | | $ | — | | | $ | — | | | $ | 10 | |
| Debt securities | — | | | 129 | | | — | | | — | | | 129 | |
| Real estate | — | | | 10 | | | 2 | | | — | | | 12 | |
Other (1) | — | | | 64 | | | 25 | | | — | | | 89 | |
| Total | $ | — | | | $ | 213 | | | $ | 27 | | | $ | — | | | $ | 240 | |
(1) Other, as of June 30, 2022, included $35 million of investments in hedge funds related to the Company's U.K. pension plan, which were classified as Level 2.
Level 3 other assets as of June 30, 2023 and 2022 consist of an insurance contract in the U.K. to fulfill the benefit obligations for a portion of the participant benefits. The value of this commitment is determined using the same assumptions and methods used to value the pension liability of the associated plan. Level 3 other assets for the same periods also include the partial funding of a pension liability relating to current and former employees of the Company’s Eberbach, Germany facility through a Company promissory note with an annual rate of interest of 5%. The value of this commitment fluctuates due to contributions and benefit payments in addition to loan interest.
The following table provides a reconciliation of the beginning and ending balances of Level 3 assets as well as the changes during the period attributable to assets held and those purchases, sales, settlements, contributions, and benefits that were paid:
| | | | | | | | | | | | | | | | | |
| Fair Value Measurement Using Significant Unobservable Inputs Total (Level 3) | | Fair Value Measurement Using Significant Unobservable Inputs Insurance Contracts | | Fair Value Measurement Using Significant Unobservable Inputs Other |
| Total (Level 3) | | |
| (Dollars in millions) | | |
| Beginning Balance at June 30, 2022 | $ | 27 | | | $ | 9 | | | $ | 18 | |
| Actual return on plan assets: | | | | | |
| Relating to assets still held at the reporting date | 2 | | | — | | | 2 | |
| | | | | |
| Purchases, sales, settlements, contributions and benefits paid | (3) | | | (1) | | | (2) | |
| Transfers in or out of Level 3, net | 1 | | | — | | | 1 | |
| Ending Balance at June 30, 2023 | $ | 27 | | | $ | 8 | | | $ | 19 | |
The Company's investment policy reflects the long-term nature of the plans’ funding obligations. The assets are invested to provide the opportunity for both income and growth of principal. This objective is pursued as a long-term goal designed to provide required benefits for participants without undue risk. It is expected that this objective can be achieved through a well-diversified asset portfolio. All equity investments are made within the guidelines of quality, marketability, and diversification mandated by the Employee Retirement Income Security Act of 1974, as amended (for plans subject to the act) and other relevant legal requirements. Investment managers are directed to maintain equity portfolios at a risk level approximately equivalent to that of the specific benchmark established for that portfolio. Assets invested in fixed income securities and pooled fixed-income portfolios are managed actively to pursue opportunities presented by changes in interest rates, credit ratings, or maturity premiums.
| | | | | | | | | | | |
| Other Post-Retirement Benefits |
| Assumed Healthcare Cost Trend Rates at the Balance Sheet Date | 2023 | | 2022 |
| | | |
| | | |
| Healthcare cost trend rate – initial (%) | | | |
| Pre-65 | n/a | | n/a |
| Post-65 | 4.8 | % | | 4.6 | % |
| Healthcare cost trend rate – ultimate (%) | | | |
| Pre-65 | n/a | | n/a |
| Post-65 | 4.1 | % | | 4.1 | % |
| Year in which ultimate rates are reached | | | |
| Pre-65 | n/a | | n/a |
| Post-65 | 2040 | | | 2040 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
13. EQUITY AND ACCUMULATED OTHER COMPREHENSIVE LOSS
Description of Capital Stock
The Company is authorized to issue 1.00 billion shares of its Common Stock and 100 million shares of preferred stock, par value $0.01 per share. In accordance with the Company’s amended and restated certificate of incorporation, each share of Common Stock has one vote, and the Common Stock votes together as a single class.
Public Offerings of Common Stock
On June 15, 2020, the Company completed a public offering (the “June 2020 Equity Offering”), in which the Company sold 8 million shares of Common Stock at a price of $70.72 per share, net of underwriting discounts and commissions. The Company obtained total net proceeds from the June 2020 Equity Offering of $548 million after the payment of associated offering expenses, which were used to repay $200 million of prophylactic borrowings from the third quarter of fiscal 2020 under the Revolving Credit Facility, with the remainder available for general corporate purposes. On July 10, 2020, the underwriter for the June 2020 Equity Offering exercised its over-allotment option on 1 million additional shares, resulting in
additional net proceeds of $82 million from the June 2020 Equity Offering, which were recorded in the fiscal year ended June 30, 2021.
On February 6, 2020, the Company completed a public offering (the “February 2020 Equity Offering”), in which the Company sold 8 million shares of Common Stock at a price of $58.58 per share, net of underwriting discounts and commissions. The Company obtained total net proceeds from the February 2020 Equity Offering of $494 million after the payment of associated offering expenses, which were used to repay $100 million of borrowings earlier in the quarter under the Revolving Credit Facility and the consideration for the MaSTherCell acquisition due at its closing, with the remainder available for general corporate purposes.
Formerly Outstanding Redeemable Preferred Stock
In May 2019, the Company designated 1 million shares of its preferred stock, par value $0.01, as its “Series A Convertible Preferred Stock” (the “Series A Preferred Stock”), pursuant to a certificate of designation of preferences, rights, and limitations and issued and sold 650,000 shares of the Series A Preferred Stock for an aggregate price of $650 million, to affiliates of Leonard Green & Partners, L.P., each share having an stated value of $1,000.
Proceeds from the offering of the Series A Preferred Stock, net of stock issuance costs, were $646 million, of which $40 million was allocated to the dividend-adjustment feature at its issuance and separately accounted for as a derivative liability. Each change in the fair value of derivative liability during a fiscal quarter was recorded as a non-operating expense in the consolidated statement of operations.
As described in Note 8, (Loss) Earnings per Share, on the Partial Conversion Date, holders of Series A Preferred Stock converted 265,223 shares (approximately 41% of their holdings) and $2 million of unpaid accrued dividends into shares of Common Stock. The holders received 20.33 shares of Common Stock for each converted preferred share, resulting in the issuance of 5,392,280 shares of Common Stock. The Company recognized no gain or loss upon the Partial Conversion.
As a result of the Partial Conversion, additional paid in capital increased $253 million, which included $4 million related to the fair value of the portion of the derivative liability that was settled upon the Partial Conversion and $2 million related to the unpaid accrued dividend.
On the Final Conversion Date, holders of Series A Preferred Stock converted the remaining 384,777 shares and $2 million of unpaid accrued dividends into shares of Common Stock. These holders received 20.32 shares of Common Stock for each converted share of Series A Preferred Stock, resulting in the issuance of 7,817,554 shares of Common Stock. The Company recognized no gain or loss upon the Final Conversion.
As a result of the Final Conversion, additional paid in capital increased $362 million, which included $1 million related to the fair value of the portion of the derivative liability that was settled upon the Final Conversion and $2 million related to the unpaid accrued dividend.
Following the Final Conversion Date, no share of the Series A Preferred Stock remained outstanding, and the Company re-assigned all of the authorized shares of Series A Preferred Stock as undesignated shares of preferred stock.
Accumulated Other Comprehensive Loss
The components of the changes in the cumulative translation adjustment, derivatives and hedges, minimum pension liability, and marketable securities for the fiscal years ended June 30, 2023, 2022, and 2021 are presented below:
| | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2021 |
| Foreign currency translation adjustments: | | | | | |
| Net investment hedge | $ | (30) | | | $ | 121 | | | $ | (56) | |
| Long-term inter-company loans | 12 | | | (37) | | | 39 | |
| Translation adjustments | 43 | | | (169) | | | 72 | |
| Total foreign currency translation adjustments, pretax | 25 | | | (85) | | | 55 | |
| Tax (benefit) expense | (7) | | | 25 | | | (12) | |
| Total foreign currency translation adjustments, net of tax | $ | 32 | | | $ | (110) | | | $ | 67 | |
| | | | | |
| Net change in derivatives and hedges: | | | | | |
| Net gain recognized during the year, pretax | $ | 25 | | | $ | 36 | | | $ | 4 | |
| | | | | |
| | | | | |
| | | | | |
| Tax expense | 7 | | | 9 | | | 1 | |
| Net change in derivatives and hedges, net of tax | $ | 18 | | | $ | 27 | | | $ | 3 | |
| | | | | |
| Net change in minimum pension liability: | | | | | |
| Net (loss) gain recognized during the year, pretax | $ | (17) | | | $ | 13 | | | $ | — | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Tax (benefit) expense | (3) | | | 4 | | | — | |
| Net change in minimum pension liability, net of tax | $ | (14) | | | $ | 9 | | | $ | — | |
| | | | | |
| Net change in marketable securities: | | | | | |
| Net gain (loss) recognized during the year, pretax | $ | 5 | | | $ | (3) | | | $ | (1) | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Tax expense | 1 | | | — | | | — | |
| Net change in marketable securities, net of tax | $ | 4 | | | $ | (3) | | | $ | (1) | |
For the fiscal years ended June 30, 2023, 2022, and 2021, the changes in accumulated other comprehensive loss, net of tax by component are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Foreign Currency Translation Adjustment | | Pension Liability Adjustments | | Derivatives and Hedges | | Marketable Securities | | Other | | Total |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Balance at June 30, 2020 | $ | (335) | | | $ | (47) | | | $ | (3) | | | $ | — | | | $ | (1) | | | $ | (386) | |
| Other comprehensive loss before reclassifications | 67 | | | — | | | 3 | | | (1) | | | — | | | 69 | |
| | | | | | | | | | | |
| Balance at June 30, 2021 | (268) | | | (47) | | | — | | | (1) | | | (1) | | | (317) | |
| Other comprehensive income (loss) before reclassifications | (110) | | | 8 | | | 27 | | | (3) | | | — | | | (78) | |
| Amounts reclassified from other comprehensive loss | — | | | 1 | | | — | | | — | | | — | | | 1 | |
| Balance at June 30, 2022 | (378) | | | (38) | | | 27 | | | (4) | | | (1) | | | (394) | |
| Other comprehensive income (loss) before reclassifications | 32 | | | (16) | | | 18 | | | — | | | — | | | 34 | |
| Amounts reclassified from other comprehensive loss | — | | | 2 | | | — | | | 4 | | | — | | | 6 | |
| Balance at June 30, 2023 | $ | (346) | | | $ | (52) | | | $ | 45 | | | $ | — | | | $ | (1) | | | $ | (354) | |
14. STOCK-BASED COMPENSATION
The Company’s stock-based compensation is comprised of stock options, restricted stock units, performance-based restricted stock units, and restricted stock.
2014 and 2018 Omnibus Incentive Plans
In 2014, the Company’s board of directors adopted, and the holder of a majority of the shares approved, the 2014 Omnibus Incentive Plan (the “2014 Plan”). The 2014 Plan provided certain members of management, employees, and directors of the Company and its subsidiaries with the opportunity to obtain various incentives, including grants of stock options, restricted stock units (defined below), and restricted stock. In October 2018, the Company’s shareholders approved the 2018 Omnibus Incentive Plan (the “2018 Plan”), and, as a result, new awards may no longer be issued under the 2014 Plan, although it remains in effect as to any previously granted award. The 2018 Plan is substantially similar to the 2014 Plan, except that (a) a total of 15,600,000 shares of Common Stock (subject to adjustment) may be issued under the 2018 Plan, (b) each share of Common Stock issuable under the 2018 Plan pursuant to a restricted stock or restricted stock unit award will reduce the number of reserved shares by 2.25 shares, and (c) the 2018 Plan imposes a limit on the aggregate value of awards that may be made in a single year to a non-employee director. Both the 2014 Plan and the 2018 Plan permit “net settlement” of vested awards, pursuant to which the award holder forfeits a portion of the vested award to satisfy the purchase price (in the case of options), the holder’s withholding tax obligation, if any (in all cases), or both. Where the holder net-settles the tax obligation, the Company pays the amount of the withholding tax to the U.S. government in cash, which is accounted for as an adjustment to Additional paid in capital.
Stock Compensation Expense
Stock compensation expense recognized in the consolidated statements of operations was $35 million, $54 million, and $51 million in fiscal 2023, 2022, and 2021, respectively. Stock compensation expense is classified in selling, general, and administrative expenses as well as cost of sales. The Company has elected to account for forfeitures as they occur.
Stock Options
Stock options granted under the 2018 Plan, during fiscal 2023, 2022, and 2021 represent approximately 151,000, 183,000, and 231,000 shares of Common Stock, respectively. Each stock option granted under the 2018 Plan vests in equal annual installments over a four-year period from the date of grant, contingent upon the participant’s continued employment with the Company.
Methodology and Assumptions
All outstanding stock options have an exercise price per share equal to the fair market value of one share of Common Stock on the date of grant. All outstanding stock options have a contractual term of 10 years, subject to forfeiture under certain conditions upon separation of employment. The grant-date fair value is recognized as expense on a graded-vesting basis over the vesting period. The fair value of stock options is determined using the Black-Scholes-Merton option pricing model for service and performance-based awards, and an adaptation of the Black-Scholes-Merton option valuation model, which takes into consideration the internal rate of return thresholds, for market-based awards. This model adaptation is essentially equivalent to the use of a path dependent-lattice model.
The weighted average of assumptions used in estimating the fair value of stock options granted during each year were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, |
| 2023 | | 2022 | | 2021 |
| Expected volatility | 37% | | 37% | | 27% |
| Expected life (in years) | 4.3 | | 3.7 | | 6.25 |
| Risk-free interest rate | 3.2% | | 0.7% | | 0.3% |
| Dividend yield | None | | None | | None |
Public trading of the Common Stock commenced only in July 2014, and, as a result, there was only limited relevant historical volatility experience available; therefore, the expected volatility assumptions for fiscal year 2021 were based on the historical volatility of the closing share prices of a comparable peer group. The Company selected peer companies from the pharmaceutical industry with similar characteristics, including market capitalization, number of employees and product focus. In addition, since the Company did not have a pattern of exercise behavior of option holders, for fiscal year 2021, the Company used the simplified method to determine the expected life of each option, which is the mid-point between the vesting
date and the end of the contractual term. Effective in fiscal year 2022, the expected volatility and expected holding period were based on the historical volatility and historical holding period of the Common Stock of the Company. The risk-free interest rate for the expected life of the option is based on the comparable U.S. Treasury yield curve in effect at the time of the grant. The weighted-average grant-date fair value of stock options in fiscal 2023, 2022, and 2021 was $37.14 per share, $32.07 per share, and $24.36 per share, respectively.
The following table summarizes stock option activity and shares subject to outstanding options for the fiscal year ended June 30, 2023:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | |
| Weighted Average Exercise Price | Number of Shares | Weighted Average Contractual Term | Aggregate Intrinsic Value | | | | | | | |
| |
| |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Outstanding as of June 30, 2022 | $ | 63.74 | | 1,055,511 | | 6.91 | $ | 47,013,454 | | | | | | | | |
| Granted | 104.84 | | 151,454 | | — | | — | | | | | | | | |
| Exercised | 35.17 | | 114,922 | | — | | 3,293,593 | | | | | | | | |
| Forfeited | 94.34 | | 62,729 | | — | | — | | | | | | | | |
| Expired / Canceled | 67.37 | | 20,567 | | — | | — | | | | | | | | |
| Outstanding as of June 30, 2023 | 71.19 | | 1,008,747 | | 6.22 | 2,225,819 | | | | | | | | |
| Vested/expected to vest as of June 30, 2023 | 71.19 | | 1,008,747 | | 6.22 | 2,225,819 | | | | | | | | |
| Vested and exercisable as of June 30, 2023 | $ | 54.62 | | 596,627 | | 5.23 | $ | 2,225,819 | | | | | | | | |
The intrinsic value of the options exercised in fiscal 2023 was $3 million. The total fair value of options vested during the period was $9 million.
The intrinsic value of the options exercised in fiscal 2022 was $29 million. The total fair value of options vested during the period was $6 million.
As of June 30, 2023, $2 million of unrecognized compensation cost related to granted and not forfeited stock options is expected to be recognized as expense over a weighted-average period of approximately 2.6 years.
Restricted Stock and Restricted Stock Units
The Company may grant to employees and members of its board of directors under the 2018 Plan (and formerly granted under the 2014 Plan) shares of restricted stock and units each representing the right to one share of Common Stock (“restricted stock units”). Since the IPO, the Company has granted to employees and directors restricted stock units and restricted stock that vest over specified periods as well as restricted stock units and restricted stock that have certain performance-related vesting requirements (“performance share units” and “performance shares,” respectively). The restricted stock and restricted stock units granted during fiscal 2023 and 2022 had grant date fair values aggregating $76 million and $57 million, respectively, which represent approximately 1,124,000 and 535,000 shares of Common Stock, respectively. Under the 2014 Plan or 2018 Plan, as appropriate, the performance shares and performance share units vest upon achieving Company financial performance metrics established at the outset of the three-year performance period associated with each grant. The metrics for the fiscal 2021, 2022, and 2023 performance share unit grants were based on performance against a mix of adjusted EPS targets and relative total shareholder return (“RTSR”) targets. Note that adjusted EPS is calculated as a quotient of tax-effected Adjusted EBITDA by the weighted average number of fully diluted shares, a financial measure that is not defined under U.S. GAAP and is subject to important limitations. The performance share units vest following the end of their respective three-year performance periods upon a determination of achievement relative to the targets. Each quarter during the period in which the performance share units are outstanding, the Company estimates the likelihood of such achievement by the end of the performance period in order to determine the probability of vesting. The number of shares actually earned at the end of the three-year period for the fiscal 2021, 2022, and 2023 grants will vary, based only on actual performance, from 0% to 200%, or from 0% to 150%, of the target number of performance share units specified on the date of grant, in the case of the adjusted EPS and RTSR grants, respectively. Time-based restricted stock units and restricted stock generally vest on the second or third anniversary of the date of grant, subject to the participant’s continued employment with the Company.
Methodology and Assumptions - Expense Recognition and Grant Date Fair Value
The fair values of (a) time-based restricted stock units and restricted stock are recognized as straight-line expense on a cliff-vesting schedule over the applicable vesting period and (b) performance shares and performance share units are re-assessed quarterly as discussed above.
The grant date fair values of both time-based stock and units and performance-based shares and units are determined based on the number of shares of Common Stock subject to the grants and the fair value of the Common Stock on the dates of the grants, as determined by the closing market prices.
Time-Based Restricted Stock Units and Restricted Stock
The following table summarizes activity in unvested time-based restricted stock units and restricted stock for the fiscal year ended June 30, 2023:
| | | | | | | | | | | |
| Time-Based Units and Shares | | Weighted Average Grant-Date Fair Value |
| | | |
| | | |
| | | |
| | | |
| Unvested as of June 30, 2022 | 722,438 | | | $ | 91.42 | |
| Granted | 719,028 | | | 72.56 | |
| Vested | 251,943 | | | 66.84 | |
| Cancelled/forfeited | 162,794 | | | 96.53 | |
| Unvested as of June 30, 2023 | 1,026,729 | | | 83.07 | |
Adjusted EPS and RTSR-Based Performance Share Units and Performance Shares
The following table summarizes activity in unvested performance share units and performance shares for the fiscal year ended June 30, 2023: | | | | | | | | | | | |
| Performance-Based Units and Shares | | Weighted Average Grant-Date Fair Value |
| | | |
| | | |
| | | |
| | | |
| Target Number Unvested as of June 30, 2022 | 305,746 | | | $ | 83.75 | |
| Target Number Granted | 250,232 | | | 98.49 | |
| Target Number Vested | 228,129 | | | 55.03 | |
| Target Number Cancelled/forfeited | 53,864 | | | 102.28 | |
| Target Number Unvested as of June 30, 2023 | 273,985 | | | $ | 99.58 | |
Valuation of RTSR Performance Shares and Performance Share Units
The fair value of each RTSR performance share unit is determined using the Monte Carlo pricing model because the number of shares to be awarded is subject to a market condition. The Monte Carlo simulation is a generally accepted statistical technique used to simulate a range of possible future outcomes. Because the valuation model considers a range of possible outcomes, compensation cost is recognized regardless of whether the market condition is actually satisfied.
The assumptions used in estimating the fair value of the RTSR performance share units granted during each year were as follows: | | | | | | | | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, |
| 2023 | | 2022 |
| Expected volatility | 41 | % | - | 47% | | 39 | % | - | 41% |
| Expected life (in years) | 2.4 | - | 2.9 | | 2.4 | - | 2.9 |
| Risk-free interest rates | 3.0 | % | - | 4.6% | | 0.3 | % | - | 1.5% |
| Dividend yield | None | | None |
The following table summarizes activity in unvested RTSR performance share units and performance shares for the fiscal year ended June 30, 2023.
| | | | | | | | | | | |
| RTSR Units and Shares | | Weighted Average Grant-Date Fair Value |
| | | |
| | | |
| | | |
| | | |
| Target Number Unvested as of June 30, 2022 | 281,315 | | | $ | 91.04 | |
| Target Number Granted | 271,538 | | | 65.69 | |
| Target Number Vested | 149,786 | | | 62.89 | |
| Target Number Cancelled/forfeited | 60,521 | | | 93.64 | |
| Target Number Unvested as of June 30, 2023 | 342,546 | | | $ | 82.36 | |
As of June 30, 2023, $52 million of unrecognized compensation cost related to restricted stock and restricted stock units (including performance shares and performance share units, respectively) is expected to be recognized as expense over a weighted-average period of approximately 2 years. The weighted-average grant-date fair value of restricted stock and restricted stock units in fiscal 2023, 2022, and 2021 was $75.62 per share, $109.63 per share, and $94.19 per share, respectively. The fair value of restricted stock units vested in fiscal 2023, 2022, and 2021 was $39 million, $33 million, and $39 million, respectively.
15. OTHER (INCOME) EXPENSE, NET
The components of other expense, net for the fiscal years ended June 30, 2023, 2022, and 2021 are as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Fiscal Year Ended
June 30, |
| (Dollars in millions) | | | | | 2023 | | 2022 | | 2021 |
| Other (income) expense, net | | | | | | | | | |
Debt refinancing costs (1) | | | | | $ | — | | | $ | 4 | | | $ | 18 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
Foreign currency (gains) losses (2) | | | | | (8) | | | 33 | | | 5 | |
Other (3) | | | | | 1 | | | (9) | | | (20) | |
| Total other (income) expense, net | | | | | $ | (7) | | | $ | 28 | | | $ | 3 | |
(1) Debt financing costs for the fiscal year ended June 30, 2022 consists of $4 million of financing charges related to a tranche of U.S. dollar-denominated term loans under its senior secured credit facilities.
Debt financing costs for the fiscal year ended June 30, 2021 includes (a) a write-off of $4 million of previously capitalized financing charges related to the Company’s repayment of U.S. dollar-denominated term loans and the 2026 Notes in February 2021, (b) $3 million of financing charges related to the issuance of an earlier tranche of the Company's U.S dollar-denominated term loans, and (c) an $11 million premium on early redemption of the 2026 Notes.
(2) Foreign currency losses (gains) include both cash and non-cash transactions.
(3) Other, for the fiscal years ended June 30, 2022 and 2021 includes, in part, total realized and unrealized gain of $2 million, and $17 million, respectively, related to the fair value of the derivative liability associated with the formerly outstanding Series A Preferred Stock.
16. LEASES
The Company leases certain manufacturing and office facilities, land, vehicles, and equipment. The terms of these leases vary widely, although most have terms between 3 and 10 years.
In accordance with ASC 842, Leases, the Company recognizes a “right-of-use” asset and related lease liability at the commencement date of each lease based on the present value of the fixed lease payments over the expected lease term inclusive of any rent escalation provisions or incentives received. The lease term for this purpose will include any renewal period where the Company determines that it is reasonably certain that it will exercise the option to renew. While certain leases also permit the Company to terminate the lease in advance of the nominal term upon payment of an associated penalty, the Company generally does not take into account potential early termination dates in its determination of the lease term as it is reasonably certain not to exercise an early-termination option as of the lease commencement date.
The Company uses its incremental borrowing rate, which represents the interest rate the Company would expect to pay on a collateralized basis to borrow an amount equal to the lease payments under similar terms, in order to calculate the present value of a lease, when the implicit discount rate for its leases is not readily determinable.
For operating leases, fixed lease payments are recognized as operating lease expense on straight-line basis over the lease term. For finance leases, the Company recognizes depreciation expense associated with the leased asset acquired and interest expense related to the financing portion. Variable payments are recognized in the period incurred. As permitted by ASC 842, the Company has elected not to separate those components of a lease agreement not related to the leasing of an asset from those components that are related.
The Company does not record leases with an initial lease term of 12 months or less on its consolidated balance sheets. The Company recognizes lease expense for these short-term leases on a straight-line basis over the lease term.
Supplemental information concerning the leases recorded in the Company's consolidated balance sheet as of June 30, 2023 is detailed in the following table:
| | | | | | | | |
| (Dollars in millions) | Line item in the consolidated balance sheet | Balance at
June 30, 2023 |
| Right-of-use assets: | | |
| Finance leases | Property, plant, and equipment, net | $ | 274 | |
| Operating leases | Other long-term assets | 59 | |
| | |
| Current lease liabilities: | | |
| Finance leases | Current portion of long-term obligations and other short-term borrowings | 18 | |
| Operating leases | Other accrued liabilities | 11 | |
| | |
| Non-current lease liabilities: | | |
| Finance leases | Long-term obligations, less current portion | 323 | |
| Operating leases | Other liabilities | $ | 55 | |
The components of the net lease costs for the fiscal year ended June 30, 2023 reflected in the Company's consolidated statement of operations were as follows:
| | | | | | | | |
| (Dollars in millions) | | Fiscal Year Ended
June 30, 2023 |
| Financing lease costs: | | |
| Amortization of right-of-use assets | | $ | 21 | |
| Interest on lease liabilities | | 17 | |
| Total | | 38 | |
| | |
| Operating lease costs | | 35 | |
| Variable lease costs | | 10 | |
| Total lease costs | | $ | 83 | |
The short-term lease cost amounted to $10 million during the fiscal year ended June 30, 2023.
The weighted average remaining lease term and weighted average discount rate related to the Company's right-of-use assets and lease liabilities as of June 30, 2023 are as follows:
| | | | | |
| Weighted average remaining lease term (years): | |
| Finance leases | 17.4 |
| Operating leases | 10.7 |
| Weighted average discount rate: | |
| Finance leases | 6.4 | % |
| Operating leases | 4.3 | % |
Supplemental information concerning the cash-flow impact arising from the Company's leases for the fiscal year ended June 30, 2023 recorded in the Company's unaudited consolidated statement of cash flows is detailed in the following table (in millions):
| | | | | | | | | |
| | | Fiscal Year Ended
June 30, 2023 |
| Cash paid for amounts included in lease liabilities: | | | |
| Financing cash flows used for finance leases | | | $ | 19 | |
| Operating cash flows used for finance leases | | | 15 | |
| Operating cash flows used for operating leases | | | 16 | |
| | | |
| Non-cash transactions: | | | |
| Right-of-use assets obtained in exchange for new finance lease liabilities | | | 133 | |
| Right-of-use assets obtained in exchange for new operating lease liabilities | | | $ | 1 | |
As of June 30, 2023, the Company expects that its future minimum lease payments will become due and payable as follows:
| | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Financing Leases | | Operating Leases | | Total |
| 2024 | $ | 35 | | | $ | 13 | | | $ | 48 | |
| 2025 | 34 | | | 10 | | | 44 | |
| 2026 | 35 | | | 9 | | | 44 | |
| 2027 | 35 | | | 9 | | | 44 | |
| 2028 | 33 | | | 7 | | | 40 | |
| Thereafter | 380 | | | 38 | | | 418 | |
| Total minimum lease payments | 552 | | | 86 | | | 638 | |
| Less: interest | 211 | | | 20 | | | 231 | |
| Total lease liabilities | $ | 341 | | | $ | 66 | | | $ | 407 | |
17. COMMITMENTS AND CONTINGENCIES
Litigation
From time to time, the Company may be involved in legal proceedings arising in the ordinary course of business, including, without limitation, inquiries and claims concerning environmental contamination as well as litigation and allegations in connection with acquisitions, product liability, manufacturing or packaging defects, and claims for reimbursement for the cost of lost or damaged active pharmaceutical ingredients, the cost of any of which could be significant. Such matters are inherently uncertain, and there can be no guarantee that the outcome of any such matter will be decided favorably to the Company or that the resolution of any such matter will not have a material adverse effect upon the Company’s consolidated financial statements. The Company records a liability in its consolidated financial statements for these matters when a loss is known or considered probable and the amount can be reasonably estimated. The Company reviews these estimates each accounting period as additional information is known and adjusts the loss provision when appropriate. If a matter is both probable to result in a liability and the amount of loss can be reasonably estimated, the Company estimates and discloses the
possible loss or range of loss to the extent necessary for its consolidated financial statements not to be misleading. If the loss is not probable or cannot be reasonably estimated, a liability is not recorded in the Company's consolidated financial statements. Any legal or other expenses associated with the litigation are accrued for as the expenses are incurred. The Company intends to vigorously defend itself against any such litigation and does not currently believe that the outcome of any such litigation will have a material adverse effect on the Company’s consolidated financial statements. In addition, the healthcare industry is highly regulated and government agencies continue to scrutinize certain practices affecting government programs and otherwise.
City of Warwick Retirement System Class Action
In February 2023, an alleged shareholder filed a complaint styled City of Warwick Retirement System v. Catalent, Inc., et al., No. 23-cv-01108, in New Jersey federal court against the Company and three of its then-officers (collectively, “the Warwick Defendants”) purportedly on behalf of a putative “class” consisting of persons who purchased or otherwise acquired Company securities between August 30, 2021 and October 31, 2022, inclusive. On September 15, 2023, the Warwick complaint was amended (together with the original complaint, the “Warwick Complaint”), which amended complaint expanded the class period to between August 30, 2021 and May 7, 2023, inclusive (the “Class Period”). The Complaint purports to assert claims under Section 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended and the related regulations, alleging that, unbeknownst to investors, the Warwick Defendants purportedly engaged in accounting and channel stuffing schemes to pad Catalent’s revenues and failed to disclose adverse facts that purportedly were known to or recklessly disregarded by the Warwick Defendants. Specifically, the Warwick Complaint alleges that the Warwick Defendants (i) overstated revenue and earnings by prematurely recognizing revenue in violation of U.S. GAAP; (ii) suffered material weaknesses in its internal control over financial reporting related to revenue recognition; (iii) falsely represented demand for its products while knowingly selling more product to its direct customers than could be sold to healthcare providers and end consumers; (iv) cut corners on safety and control procedures at key production facilities; (v) disregarded regulatory rules at key production facilities in order to rapidly produce excess inventory that was used to pad the Company’s financial results through premature revenue recognition in violation of U.S. GAAP or stuffing its direct customers with this excess inventory; and (vi) lacked a reasonable basis for their positive statements about the Company’s financial performance, outlook, and regulatory compliance during the Class Period. The Company believes that the Warwick Defendants have defenses to the allegations and claims set forth in the complaint and filed a motion to dismiss the Warwick Complaint on November 15, 2023.
Subpoenas and Requests for Information
From time to time, the Company receives subpoenas or requests for information from various governmental agencies or private parties, including from state attorneys general, the U.S. Department of Justice, and private parties. The Company generally responds to such subpoenas and requests in a timely and thorough manner, which responses sometimes require considerable time and effort and can result in considerable costs being incurred.
In June 2023, the Company received a demand from a company stockholder pursuant to 8 Del. C. § 220 to inspect books and records of the Company relating to, among other things, the allegations raised in the Warwick Complaint. The Company has responded to the demand and cannot determine at this time if the books and records demand will lead to litigation.
18. SEGMENT AND GEOGRAPHIC INFORMATION
The Company evaluates the performance of its segments based on segment earnings before other (income) expense, impairments, restructuring costs, interest expense, income tax expense, and depreciation and amortization (“Segment EBITDA”).
Segment EBITDA is subject to important limitations. These consolidated financial statements include information concerning Segment EBITDA (a) because Segment EBITDA is an operational measure used by management in the assessment of the operating segments, the allocation of resources to the segments, and the setting of strategic goals and annual goals for the segments, and (b) in order to provide supplemental information that the Company considers relevant for the readers of the consolidated financial statements. The Company’s presentation of Segment EBITDA may not be comparable to similarly titled measures used by other companies.
The following table includes Segment EBITDA for each of the Company's current reporting segments during the fiscal years ended June 30, 2023, 2022, and 2021:
| | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Fiscal Year Ended June 30, |
| 2023 | | 2022 | | 2021 |
| Segment EBITDA reconciled to net (loss) earnings: | | | | | |
| Biologics | $ | 277 | | | $ | 777 | | | $ | 615 | |
| Pharma and Consumer Health | 548 | | | 589 | | | 498 | |
| | | | | |
| | | | | |
| Subtotal | $ | 825 | | | $ | 1,366 | | | $ | 1,113 | |
| Reconciling items to net (loss) earnings | | | | | |
Unallocated costs (1) | (559) | | | (286) | | | 1 | |
| Depreciation and amortization | (422) | | | (378) | | | (289) | |
| Interest expense, net | (186) | | | (123) | | | (110) | |
| Income tax benefit (expense) | 86 | | | (80) | | | (130) | |
| | | | | |
| Net (loss) earnings | $ | (256) | | | $ | 499 | | | $ | 585 | |
(1) Unallocated costs include restructuring and special items, stock-based compensation, gain (loss) on sale of subsidiary, impairment charges, certain other corporate directed costs, and other costs that are not allocated to the segments as follows:
| | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Fiscal Year Ended June 30, |
| 2023 | | 2022 | | 2021 |
Impairment charges and gain/loss on sale of assets (a) | $ | (98) | | | $ | (31) | | | $ | (9) | |
| Stock-based compensation | (35) | | | (54) | | | (51) | |
Restructuring and other special items (b) | (98) | | | (55) | | | (31) | |
Goodwill impairment charges(c) | (210) | | | — | | | — | |
Gain on sale of subsidiary (d) | — | | | 1 | | | 182 | |
| | | | | |
| | | | | |
| | | | | |
Other income (expense), net (e) | 7 | | | (28) | | | (3) | |
| Non-allocated corporate costs, net | (125) | | | (119) | | | (87) | |
| Total unallocated costs | $ | (559) | | | $ | (286) | | | $ | 1 | |
(a) For the fiscal year ended June 30, 2023, impairment charges are primarily associated with an idle facility in the Biologics segment and obsolete equipment that could not be sold or repurposed in the Pharma and Consumer Health segment.
In the three months ended June 30, 2023, the Company identified an indicator of impairment related to one of its facilities in the Biologics segment given the plans to pause any additional spend on site development due to a lack of demand, leading to a partial impairment charge of $54 million. The Company primarily utilized a market and income approach for real property and a cost approach for personal property to record the partial impairment on its idle facility. Impairment charges are recorded in Other operating expense in the consolidated statements of operations.
Also, in the three months ended June 30, 2023, the Company identified an indicator of impairment related to obsolete equipment from a terminated project in the Pharma and Consumer Health segment, leading to a full impairment charge of $18 million.
For the fiscal year ended June 30, 2022, impairment charges are primarily due to fixed asset impairment charges associated with dedicated equipment for a product the Company no longer manufactures in its Pharma and Consumer Health segment and obsolete equipment in its Biologics segment.
(b) Restructuring and other special items for the fiscal year ended June 30, 2023 includes (i) restructuring charges associated with plans to reduce costs, consolidate facilities, and optimize our infrastructure across the organization and (ii) transaction and integration costs associated with the Metrics acquisition
Restructuring and other special items for the fiscal year ended June 30, 2022 include (i) transaction and integration costs primarily associated with the Princeton, Bettera Wellness, Delphi, Hepatic, Acorda, and RheinCell transactions and (ii) unrealized losses on venture capital investments.
Restructuring and other special items for the fiscal year ended June 30, 2021 include transaction and integration costs associated with the Anagni, Italy facility acquisition and the MaSTherCell Global, Inc., Skeletal, Delphi, and Acorda transactions, in addition to restructuring costs associated with the closure of the Company's Pharma and Consumer Health facility in Bolton, U.K.
(c) The goodwill impairment charges for the fiscal year ended June 30, 2023 was associated with the Company’s Consumer Health reporting unit. For further details, see Note 4, Goodwill.
(d) Gain on sale of subsidiary for the fiscal year ended June 30, 2022 was due to the sale of the Company’s facility and Blow-Fill-Seal business in Woodstock, Illinois.
(e) Refer to Note 15, Other (income) expense, net for details of financing charges and foreign currency translation adjustments recorded within other (income) expense, net.
The following table includes total assets for each segment, as well as reconciling items necessary to total the amounts reported in the consolidated balance sheets.
Total Assets | | | | | | | | | | | |
| (Dollars in millions) | June 30, 2023 | | June 30, 2022 |
| Biologics | $ | 5,746 | | | $ | 5,770 | |
| Pharma and Consumer Health | 4,867 | | | 4,356 | |
| Corporate and eliminations | 164 | | | 382 | |
| Total assets | $ | 10,777 | | | $ | 10,508 | |
Capital Expenditures | | | | | | | | | | | | | | | | | |
| Fiscal Year Ended June 30, |
| (Dollars in millions) | 2023 | | 2022 | | 2021 |
| Biologics | $ | 346 | | | $ | 453 | | | $ | 516 | |
| Pharma and Consumer Health | 214 | | | 183 | | | 151 | |
| Corporate | 34 | | | 30 | | | 19 | |
| Total capital expenditures | $ | 594 | | | $ | 666 | | | $ | 686 | |
Long Lived Assets
The following table presents long-lived assets—consisting of property, plant, and equipment, net of accumulated depreciation—by geographic area:
| | | | | | | | | | | | | | | | | |
| | | |
| | | | | |
| (Dollars in millions) | | | | | | | June 30, 2023 | | June 30, 2022 |
| United States | | | | | | | $ | 2,758 | | | $ | 2,267 | |
| Europe | | | | | | | 765 | | | 747 | |
| Other | | | | | | | 159 | | | 113 | |
| | | | | | | | | |
| Total | | | | | | | $ | 3,682 | | | $ | 3,127 | |
For further details on segment and geographic information, see Note 2, Revenue Recognition.
19. SUPPLEMENTAL BALANCE SHEET INFORMATION
Supplemental balance sheet information at June 30, 2023 and June 30, 2022 is detailed in the following tables.
Inventories
Work-in-process and inventories include raw materials, labor, and overhead. Total inventories consist of the following: | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2023 | | June 30,
2022 |
| Raw materials and supplies | $ | 781 | | | $ | 651 | |
| Work-in-process | 186 | | | 109 | |
| | | |
| Total inventories, gross | 967 | | | 760 | |
Inventory cost adjustment(1) | (190) | | | (58) | |
| Total inventories | $ | 777 | | | $ | 702 | |
(1) Increase in inventory cost adjustment is primarily associated with inventory write-offs resulting from the terminations of certain take-or-pay arrangements.
Prepaid expenses and other
Prepaid expenses and other current assets consist of the following: | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2023 | | June 30,
2022 |
| Prepaid expenses | $ | 53 | | | $ | 61 | |
| Short-term contract assets | 399 | | | 398 | |
| Spare parts supplies | 24 | | | 22 | |
| Prepaid income tax | 77 | | | 27 | |
| Non-U.S. value-added tax | 38 | | | 48 | |
| | | |
| | | |
| | | |
| Other current assets | 42 | | | 70 | |
| Total prepaid expenses and other | $ | 633 | | | $ | 626 | |
Property, plant, and equipment, net
Property, plant, and equipment, net consist of the following: | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2023 | | June 30,
2022 |
| Land, buildings, and improvements | $ | 1,887 | | | $ | 1,687 | |
| Machinery and equipment | 2,287 | | | 1,891 | |
| Furniture and fixtures | 61 | | | 48 | |
| Construction in progress | 1,043 | | | 848 | |
| Property, plant, and equipment, at cost | 5,278 | | | 4,474 | |
| Accumulated depreciation | (1,596) | | | (1,347) | |
| Property, plant, and equipment, net | $ | 3,682 | | | $ | 3,127 | |
Other long-term assets
Other long-term assets consist of the following: | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2023 | | June 30,
2022 |
| Operating lease right-of-use-assets | $ | 59 | | | $ | 93 | |
| Note receivable | 53 | | | 51 | |
| | | |
| Pension assets | 18 | | | 37 | |
| Corporate-owned life insurance policies | 41 | | | 35 | |
| | | |
| Venture capital investments | 36 | | | 33 | |
| Interest-rate swap | 62 | | | 36 | |
| Long-term contract assets | 18 | | | 43 | |
| Other | 42 | | | 21 | |
| Total other long-term assets | $ | 329 | | | $ | 349 | |
Other accrued liabilities
Other accrued liabilities consist of the following: | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2023 | | June 30,
2022 |
| Contract liability | $ | 167 | | | $ | 211 | |
| Accrued employee-related expenses | 160 | | | 198 | |
| Accrued expenses | 134 | | | 140 | |
| Operating lease liabilities | 11 | | | 14 | |
| Restructuring accrual | 19 | | | 1 | |
| | | |
| Accrued interest | 35 | | | 32 | |
| Accrued income tax | 44 | | | 50 | |
| Total other accrued liabilities | $ | 570 | | | $ | 646 | |
20. SUBSEQUENT EVENTS
Husty Derivative Claim
In August 2023, an alleged shareholder filed a derivative complaint styled Husty et al. v. Carroll, et al., No. 23-cv-00891, in Delaware federal court against certain current and former members of the Company's board of directors, (the “Husty Defendants”), and nominally against Catalent, Inc. The complaint mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action described in Note 17, Commitments and Contingencies and claims that the alleged activities described there led to, and will continue to expose the Company to, costs and damages. The Company believes that the Husty Defendants have defenses to the allegations and claims set forth in the complaint and, once all Husty Defendants are properly served with the complaint, intends to vigorously defend the Husty Defendants against such allegations.
Brown Derivative Claim
In September 2023, an alleged shareholder filed a derivative complaint styled Brown, et al. v. Chiminski, et al., Case 3:23-cv-15722, in New Jersey federal court against certain current and former officers and members of the Company’s board of directors (the “Brown Defendants”) and nominally against Catalent, Inc. The complaint mimics the allegations set out in the original complaint filed in the City of Warwick Retirement System action described in Note 17, Commitments and Contingencies and claims that the alleged activities described there led to, and will continue to expose the Company to, costs and damages. On November 8, 2023, the Court entered a stipulation between the parties extending the Brown Defendants time to respond to the Complaint until January 8, 2024. The Company believes that the Brown Defendants have defenses to the allegations and claims set forth in the complaint and intends to vigorously defend the Brown Defendants against such allegations.
Impairment of Goodwill
The Company assessed the current and future economic outlook as of September 30, 2023 for its Consumer Health and Biomodalities reporting units in its Pharma and Consumer Health and Biologics segments, respectively, and identified indicators for impairment of goodwill. The evaluation began with a qualitative assessment of each reporting unit to determine if it was more likely than not that the fair value of the reporting unit was less than its carrying value. The qualitative assessment did not indicate that it was more likely than not that the fair value exceeded the carrying value in its Consumer Health and Biomodalities reporting units, which led to a quantitative assessment for the corresponding reporting units. Interim goodwill tests were not performed on the Company's remaining reporting units as there was no indication of a possible goodwill impairment.
For the three months ended September 30, 2023, the Company recorded goodwill impairment charges of $689 million associated with the Company's Consumer Health and Biomodalities reporting units in its Pharma and Consumer Health and Biologics segments, respectively. The impairment charges also generated additional deferred tax assets to the extent of the tax goodwill remaining in the reporting units being impaired. The additional deferred tax assets measured against the deferred tax liabilities at the Company’s domestic sites, along with the Company’s forecast losses triggered in part by the impairment charge itself, resulted in a $53 million valuation allowance to be recorded against the U.S. Federal and state net deferred tax assets for the three months ended September 30, 2023.
Amendment No. 9 to Credit Agreement
On September 27, 2023, Operating Company entered into Amendment No. 9 to its Amended and Restated Credit Agreement (“Amendment No. 9”) by and among Operating Company, PTS Intermediate, the subsidiaries of Operating Company party thereto, JPMorgan Chase Bank, N.A., as the administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto, which Amendment No. 9 amends the Credit Agreement to extend the deadlines by which the Operating Company is required to deliver to the administrative agent (i) its audited financial statements as at the end of and for the fiscal year ended June 30, 2023, together with the auditor’s report and opinion on such audited financial statements, to November 27, 2023, and (ii) its unaudited financial statements as at the end of and for the fiscal quarter ending September 30, 2023 to January 13, 2024.
Amendment No. 10 to Credit Agreement
On November 22, 2023, Operating Company, entered into Amendment No. 10 to its Amended and Restated Credit Agreement (“Amendment No. 10”) by and among Operating Company, PTS Intermediate, the subsidiaries of Operating Company party thereto, JPMorgan Chase Bank, N.A., as the administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto, which Amendment No. 10 further extends the deadlines by which the Operating Company is required to deliver to the Administrative Agent (i) its audited financial statements as at the end of and for the fiscal year ended June 30, 2023, together with the auditor’s report and opinion on such audited financial statements, to January 26, 2024, and (ii) its unaudited financial statements as at the end of and for the fiscal quarter ending September 30, 2023 to March 13, 2024.
Restructuring
In October 2023, and in connection with the Company's restructuring plans, the Company committed to a plan to close operations at its San Francisco facility and to transfer those operations to other sites within its network. The costs associated with this site closure are under evaluation, which may affect the amount and expected timing of costs and associated payments. The Company expects to incur cash and non-cash charges of at least $25 million in connection with the site closure, primarily related to accelerated depreciation of the facility in the second half of fiscal 2024. The estimated charges are subject to a number of assumptions, and actual results may differ materially from this initial estimate.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) that are designed to ensure that information required to be disclosed in our periodic reports under the Exchange Act, such as this Annual Report, is recorded, processed, summarized, and reported within the periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures. In designing and evaluating the disclosure controls and procedures, our management recognizes that any set of controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. As required by Rule 13a-15(b) under the Exchange Act, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this Annual Report. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of June 30, 2023, our disclosure controls and procedures were not effective, due to the material weaknesses in internal control over financial reporting described below.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting form a set of policies and procedures designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our consolidated financial statements in accordance with U.S. GAAP.
Our internal control over financial reporting include policies and procedures that:
•pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions relating to our business and dispositions of our assets;
•provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
•provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Because of inherent limitations regarding the nature of controls, no set of internal control over financial reporting can guarantee that it will prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because either conditions change or the degree of compliance with our policies and procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2023. In making this assessment, management used the framework set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013). Based on this assessment, our management concluded that, as of June 30, 2023, our internal control over financial reporting were not effective because of the unremediated material weaknesses described below.
The effectiveness of our internal control over financial reporting as of June 30, 2023 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in its report, which is included in "Item 8. – Financial Statements and Supplementary Data."
Material Weaknesses in Internal Control over Financial Reporting
A material weakness (as defined in Rule 12b-2 under the Exchange Act) is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
Previously disclosed Material Weakness in Internal Control over Financial Reporting – Revenue recognition
As previously disclosed, management identified during the preparation of our unaudited consolidated financial statements for the three and nine months ended March 31, 2023 a material weakness in our internal control over financial reporting relating to the year ended June 30, 2022, which remained unremediated as of June 30, 2023.
We did not maintain effective controls over the appropriateness of revenue recognition related to modifications of customer agreements at our Bloomington, Indiana facility. Specifically, we did not maintain effective controls to properly identify and assess the accounting treatment of modifications to arrangements that were accounted for under ASC 606, Revenue from Contracts with Customers. The reviewer had insufficient knowledge of the requirements of the ASC 606 revenue recognition accounting model, and therefore the review procedures were not performed with the necessary level of competence to prevent or detect a material misstatement on a timely basis.
Furthermore, the compensating control to review the accounting assessments for contract modifications was not sufficiently designed to detect accounting misstatements.
Material Weakness in Internal Control over Financial Reporting – Consolidated financial statement close process
We did not maintain effective internal control over the evaluation and accounting of certain complex and non-routine transactions. Due to an insufficient complement of technical resources within its corporate accounting function, management was unable to complete its evaluation of certain complex non-routine transactions in a timely manner. Specifically, management did not adequately prepare and maintain sufficient evidence of management’s review of (i) significant assumptions, relating to the interim goodwill and long-lived assets impairment assessments as of March 31, 2023, (ii) the evaluation of indicators and assessment of impairment of goodwill and long-lived assets as of June 30, 2023, and (iii) the evaluation of the accounting, measurement and disclosure of events occurring subsequent to the balance sheet date, specifically management’s evaluation of disclosure and the related measurement of a goodwill impairment charge disclosed in the subsequent events footnote.
Material Weakness in Internal Control over Financial Reporting – Inventory reconciliation
We did not maintain effective internal controls over inventory reconciliation at our Baltimore, Maryland facility. Specifically, we did not implement and design controls at an appropriate level of precision to (i) properly recognize certain third party costs on the balance sheet separately from the inventory balance, (ii) properly and timely update our perpetual inventory subledger to value inventory at lower of cost or market, and (iii) reconcile our perpetual inventory subledger to the related general ledger accounts.
Plan to Remediate Material Weakness in Internal Control Over Financial Reporting – Revenue recognition
The Company, with oversight by the Audit Committee of the Board, is devoting significant time, attention, and resources to remediating the revenue modification material weakness in our internal control over financing reporting described above. As of June 30, 2023, we had initiated the following steps intended to remediate this material weakness and strengthen our internal controls over financial reporting:
•Hiring additional technical accounting resources within our Bloomington, Indiana site and within the corporate controllership group.
•Enhancing the design of our management review controls relating to the accounting for contract modifications, including offered concessions.
•Additional training for our executive leadership team, and other critical customer-facing personnel, on revenue recognition principles, including contract modifications relating to offered concessions.
We plan to continue to devote significant time and attention to remediate this material weakness as soon as reasonably practicable. We believe these actions will be sufficient to remediate the identified material weakness and strengthen our internal control over financial reporting; however, there can be no guarantee that such remediation will be sufficient. We will continue to monitor the design and effectiveness of these and other processes, procedures, and controls and make any further change
management determines appropriate. We expect to complete the remediation of this material weakness by the third quarter of fiscal 2024, although no assurance can be given regarding the time and effort needed to complete the remediation.
Plan to Remediate Material Weakness in Internal Control over Financial Reporting – Consolidated financial statement close process
The Company, with oversight by the Audit Committee of the Board, is actively developing and implementing a comprehensive remediation plan that will include the following key initiatives:
•We have already engaged temporary third-party resources with the appropriate level of technical knowledge and experience in accounting related to complex non-routine transactions and the related internal control activities to complement the existing corporate accounting resources.
•We plan to hire, develop and retain incremental full-time personnel with appropriate accounting and internal control expertise.
•We will review and update (as appropriate) our methodologies, policies and procedures designed to ensure we are able to more timely address our evaluation of complex non-routine transactions, including the related evidence of management’s review of the significant assumptions used in those evaluations.
•We will review and update (as appropriate) our training programs related to the relevant internal control over financial reporting matters pertaining to complex non-routine transactions.
Plan to Remediate Material Weakness in Internal Control Over Financial Reporting – Inventory reconciliation
Management, with oversight by the Audit Committee of the Board of Directors of the Company, has updated our design of controls for the valuation of inventory at our Baltimore location.
Remediation of Previously Reported Material Weakness in Internal Control Over Financial Reporting
As previously disclosed, during the third quarter of fiscal 2023, we identified and disclosed in our Quarterly Report on Form 10-Q filed with the SEC on June 12, 2023 a material weakness related to our internal control over financial reporting resulting from ineffective information technology general controls (“ITGCs”) in the areas of user access management (privileged and regular users), application change management, operating system/database logical access controls, and segregation of duties for key information technology (“IT”) systems that support our financial reporting processes. As a result, the related process-level IT dependent controls and application controls were also ineffective.
As previously disclosed, we immediately terminated the privileged and regular user access related to the individuals who no longer required such access and developed the following plans to complete the remediation of this IT material weakness:
•increased automation of manual controls;
•developed and implemented additional training and awareness programs addressing ITGCs and policies, including regarding educating control owners concerning the principles and requirements of each control, with a focus on understanding user access and program changes;
•strengthened the review process by adding additional reviews and approvals; and
•provided regular updates to the Audit Committee on the remediation efforts.
During the fourth quarter of fiscal 2023, we successfully implemented the actions necessary to remediate the control deficiency, and completed the testing necessary to conclude that this material weakness has been remediated.
Changes in Internal Control over Financial Reporting
We are taking actions to complete the remediation of the remaining material weaknesses relating to our internal control over financial reporting, as described above. Except as otherwise described herein, there was no change in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
New Severance Arrangements
On December 8, 2023, the Company entered into new severance agreements with each of Matti Masanovich, Aris Gennadios, and Ricky Hopson (each a “Severance Agreement”). The new Severance Agreements provide that in the event of a termination by the Company without cause or by the executive for good reason within 18 months following a change in control, the executive would be entitled to increased cash severance equal to two times the sum of annual base salary plus target annual bonus, payable in equal installments over the one-year period following the date of termination, subject to entering into a release of claims and certain other terms and conditions. In addition, the new Severance Agreements provide that if any of the payments provided for under such Severance Agreement or otherwise payable to the individual would constitute “parachute payments” within the meaning of Section 280G of the Internal Revenue Code and would be subject to the related excise tax under Section 4999 of the Internal Revenue Code, then such individual will be entitled to receive either full payment of benefits or such lesser amount that would result in no portion of the benefits being subject to the excise tax, whichever results in the greater amount of after-tax benefits to such individual. The new Severance Agreements also include certain technical changes to the prior severance agreements with the executives, but otherwise are substantially the same as prior severance agreements. The foregoing description of the Severance Agreements does not purport to be complete and is subject to, and qualified in its entirety by, the full text of the Severance Agreements, a form of which is attached hereto as Exhibit 10.9 and is incorporated herein by reference.
Trading Arrangements
During the fiscal quarter ended June 30, 2023, our directors and officers (as defined in Rule 16a-1(f) under the Exchange Act adopted or terminated the contracts, instructions or written plans for the purchase or sale of our securities as set forth in the table below:
| | | | | | | | | | | | | | | | | | | | |
| | | Trading Arrangement | | |
Name and Title | Action | Date | Rule10b5-1* | Non-Rule 10b5-1** | Total Shares of Common Stock to be Sold | Expiration Date |
Scott Gunther, Senior Vice President of Quality & Regulatory Affairs | Adopt | June 16, 2023 | X | | Up to 1,286 shares(1) | October 3, 2023 |
* Intended to satisfy the affirmative defense of Rule 10b5-1(c)
** “None-Rule 10b5-1 trading arrangement” as defined in Item 408(c) of Regulation S-K under the Exchange Act.
(1) Represents up to 50% of the shares scheduled to vest under a restricted stock unit award and two performance share unit awards (the “PSUs”) granted to the executive officer (after the sale of shares to cover any applicable taxes). The shares included under the PSUs are an estimate, and the actual number of shares to be sold will depend on the value of the stock on the day the PSUs vests.
None of the trades anticipated under this trading plan were executed.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information Regarding Directors
Set forth below is certain information with respect to our current directors as of December 8, 2023.
| | | | | |
Madhavan “Madhu” Balachandran
Director since 2017
Age: 72
| Madhu Balachandran has been a member of the Board since May 2017. Mr. Balachandran was Chief Operating Officer of Nutcracker Therapeutics, a developer of mRNA therapeutics, from September 2020 until March 2022. He previously served as the Chief Executive Officer of ADRx Inc., a preclinical stage biotechnology company, from August 2019 until October 2020. Prior to that he was Executive Vice President, Operations of Amgen Inc., a global biotechnology company, from August 2012 until July 2016 and retired as an Executive Vice President in January 2017. Mr. Balachandran joined Amgen in 1997 as Associate Director, Engineering. He became Director, Engineering in 1998, and, from 1999 to 2001, he held the position of Senior Director, Engineering and Operations Services before moving to the position of Vice President, Information Systems from 2001 to 2002. Thereafter, Mr. Balachandran was Vice President, Puerto Rico Operations from May 2002 to February 2007. From February 2007 to October 2007, Mr. Balachandran was Vice President, Site Operations, and, from October 2007 to August 2012, he held the position of Senior Vice President, Manufacturing. Prior to his tenure at Amgen, Mr. Balachandran held leadership positions at Copley Pharmaceuticals, now a part of Teva Pharmaceuticals Industries Ltd., and Burroughs Welcome Company, a predecessor through mergers of GlaxoSmithKline plc. He has served as a director of A2 Biotherapeutics since September 2019, as a director of Stevanato Group since September 2018, and as a director of uniQure NV since September 2017. He is on the Audit Committee at A2, on the Compensation Committee at uniQure, and on the Remuneration, Strategy, and Nominating and Governance Committees at Stevanato Group. Mr. Balachandran holds a Master of Science degree in Chemical Engineering from The State University of New York at Buffalo and an MBA from East Carolina University. |
Michael J. Barber Director since 2021 Age: 63 Committees: • Compensation and Leadership • Nominating and Corporate Governance
| Michael J. Barber has been a member of the Board since April 2021. He retired as the Chief Diversity Officer for General Electric Company in January 2022. During his forty-year career at GE, Mr. Barber held a variety of progressively senior roles in engineering, operations, and product management, including service as President and CEO of GE Molecular Imaging and Computed Tomography from 2016 until 2020; as Chief Engineer, GE Healthcare and Chief Operating Officer, GE Healthcare Systems from 2013 until 2015; as VP and General Manager, Molecular Imaging, GE Healthcare in 2012; as Vice President, healthymagination (GE Corporate) from 2009 until 2011; and as Vice President and CTO, GE Healthcare from 2007 until 2008. Among other prestigious awards, he was named a “Master of Innovation” by Black Enterprise in 2009 and elected a Fellow of the American Institute of Medical and Biological Engineering in 2014. He served as a director of Talix, Inc. from 2017 until it was acquired by Edifecs in 2021, and served as a director of Healthline, Inc. from 2009 until its acquisition by Summit Partners in 2016. He also served as a board member of the National Action Council for Minorities in Engineering (NACME) from 2009 until 2022. He also serves on the Board of the Green Bay Packers Football Club and Chairs the Foundation Committee. Mr. Barber received a B.S. in electrical engineering and an honorary doctorate in engineering from the Milwaukee School of Engineering, where he also serves as a Regent. |
Steven K. Barg Director since 2023 Age: 61 Committees: • Quality & Regulatory Compliance • Strategic and Operational Review
| Steven Barg has been a member of the Board since September 2023. Mr. Barg is Global Head of Engagement at Elliott. Prior to joining Elliott in February 2020, Mr. Barg spent 30 years in investment banking, most recently as a Participating Managing Director at Goldman Sachs. During his time at Goldman Sachs, Mr. Barg established and led what became the firm's Global Activism and Shareholder Advisory practice; founded and led the M&A Capital Markets practice; and ran Asian Equity Capital Markets in Hong Kong. In addition, Mr. Barg served on both the Asian and Global Equity Commitments Committees and was Global Head of Diversity for the Investment Banking Division. Prior to joining Goldman Sachs, Mr. Barg served as a Managing Director in Equity Capital Markets at UBS and Credit Suisse, with postings in New York, Hong Kong, and London. Mr. Barg has served on the Board of Directors of Cardinal Health since September 2022. Mr. Barg holds an M.B.A. from the Stanford University Graduate School of Business and a B.A. from Wesleyan University. In addition, Mr. Barg was a Henry Luce Scholar in Hong Kong and a Coro Fellow in Public Affairs in New York. |
J. Martin Carroll Director since 2015 Age: 74 Committees: • Compensation and Leadership • Nominating and Corporate Governance • Quality and Regulatory Compliance (chair
| J. Martin Carroll has been a member of the Board since July 2015 and served as our lead independent director from October 2021 to June 2023 and our non-executive Chair from July 2023 to August 2023. He served as President and Chief Executive Officer of Boehringer Ingelheim Corporation and of Boehringer Pharmaceuticals, Inc. from 2003 until 2011 and as Head, Corporate Strategy and Development of Boehringer Ingelheim GmbH from 2012 until his retirement in 2013. He served as a director of Boehringer Ingelheim Corporation from 2003 until December 2012. Mr. Carroll joined the Boehringer Ingelheim organization in 2002 as President of Boehringer Pharmaceuticals, Inc. Mr. Carroll worked at Merck & Company, Inc. from 1976 to 2001. From 1972 to 1976, he served in the United States Air Force where he attained the rank of Captain. Mr. Carroll has been chairperson of the board of directors of Esperion Therapeutics since June 2022. He served as a director of Durata Therapeutics, Inc. from August 2014 until November 2014 when it was acquired by Actavis, as a director of Vivus, Inc. from May 2013 until September 2014, as a director of Therapeutics MD from March 2015 until December 2021, and as a director of Mallinckrodt plc from June 2013 until May 2022. He also served as a director of Inotek from April 2016 to June 2016 and as Chairman of its Board from June 2016 until January 2018 when Inotek was sold to Rocket Pharmaceutical. Mr. Carroll received a B.A. in accounting and economics from the College of the Holy Cross and an M.B.A. from Babson College. |
| | | | | |
Rolf Classon Director since 2014 Age: 78 Committees: • Audit • Compensation and Leadership • Nominating and Corporate Governance (chair)
| Rolf Classon has been a member of the Board since August 2014. From October 2002 until his retirement in July 2004, Mr. Classon was Chairman of the Executive Committee of Bayer HealthCare AG, a subsidiary of Bayer AG. He served as President of Bayer Diagnostics from 1995 to 2002 and as Executive Vice President of Bayer Diagnostics from 1991 to 1995. Prior to 1991, Mr. Classon held various management positions with Pharmacia Corporation. Mr. Classon currently serves as Vice Chairman of the Supervisory Board of Fresenius Medical Care AG & Co. KGaA. He was previously Chairman of the Board of Directors of Perrigo Company plc from 2018 to 2022, having joined that board as a director in 2017, and Chairman of the Board of Directors of Tecan Group Ltd., serving from 2009 until April 2018. Mr. Classon served as Chairman of the Board of Directors of Hill-Rom Corporation from 2006 until March 2018, also serving as Vice Chairman of the Board from 2003 through May 2005 and as interim chief executive officer from May 2005 until March 2006. From 2005 to 2015, Mr. Classon served as Chairman of the Board of Directors of Auxilium Pharmaceuticals, Inc., and as Vice Chairman from March 2005 to April 2005. He also previously served as a director of Sequanna Medical AG from 2016 to 2017; of Aerocrine AB, Stockholm from 2013 to 2015; of Millipore Corporation from 2005 to 2010; of Prometheus Laboratories Inc. from 2004 to 2010; and of Enzon Pharmaceuticals Inc. from 1997 to 2011. Mr. Classon received his Chemical Engineering Certificate from the Gothenburg School of Engineering and a Business Degree from the Gothenburg University. Mr. Classon was granted a waiver, which will end at our 2024 Annual Meeting of Shareholders, from the resignation obligation imposed by our Corporate Governance Guidelines on directors over the age of 75 |
Rosemary A. Crane
Director since 2018
Age: 64
| Rosemary Crane has been a member of the Board since February 2018. Ms. Crane is currently a member of the boards of Teva Pharmaceutical Industries Limited, where she serves as chair of the Human Resources Compensation Committee; of Tarsus Pharmaceuticals, where she serves as chair of the Science and Technology Committee; of Certara, Inc.; and of Hackensack Meridian Health Center for Discovery and Innovation. She previously served as a director of Edge Therapeutics, Inc., Unilife Corporation, Cipher Pharmaceuticals, MELA Sciences, Inc., Epocrates Inc., Targanta Therapeutics, and Zealand Pharma A/S. Ms. Crane retired in 2014 from MELA Sciences, Inc., where she served as President and Chief Executive Officer beginning in 2013. From 2011 to 2013, she was a Partner and Head of Commercialization at Appletree Partners and, from 2008 to 2011, served as Chief Executive Officer and President of Epocrates Inc. From 2002 to 2008, Ms. Crane served in several senior executive positions at the Johnson & Johnson Group of Companies, ending as Company Group Chairman, OTC and Nutritional Group. From 1982 to 2002, she was at Bristol-Myers Squibb Company, ending her tenure there as President, U.S. Primary Care. Ms. Crane received her M.B.A. from Kent State University and her B.A. in Communications and English from the State University of New York at Oswego. |
Frank D’Amelio Director since 2023 Age: 66 Committees: • Compensation & Leadership • Quality & Regulatory Compliance
| Frank D’Amelio has been a member of the Board since August 2023. Mr. D’Amelio is the former Chief Financial Officer and Executive Vice President, Global Supply, of Pfizer Inc. where he was responsible for all corporate finance functions including audit, controllers, tax, and treasury, as well as global supply. Prior to joining Pfizer, Mr. D’Amelio served as Senior Executive Vice President of Integration and Chief Administrative Officer of Alcatel-Lucent, responsible for the 2006 Alcatel-Lucent merger as well as procurement, real estate, IT, and supply chain. Prior to that, Mr. D’Amelio was the Chief Operating Officer of Lucent Technologies, responsible for leading business operations, including sales, the product groups, the services business, the supply chain, information technology operations, human resources, and labor relations. In 2001, he was appointed Executive Vice President and Chief Financial Officer of Lucent. In addition, Mr. D’Amelio held a number of roles while at Lucent Technologies, and before that, served in a variety of positions while at AT&T, including CFO, Transmission Systems and Controller, Network Systems. Mr. D’Amelio has served on the Board of Directors of Humana since September 2003, where he currently serves as Chair of the Audit Committee, on the Board of Directors of Zoetis, Inc. since July 2012, and on the Board of Directors of Hewlett Packard Enterprise since January 2023. He currently serves as a CFO in residence at the Deloitte CFO Academy. Mr. D’Amelio holds an M.B.A. in Finance from St. John’s University and a bachelor’s degree in Accounting from St. Peter’s College. |
Karen Flynn
Director since 2022
Age: 60
| Karen Flynn has been a member of the Board since September 2022. Karen Flynn served as Interim President, Division Head for BioModalities from April 2023 until the end of September 2023. Ms. Flynn retired in July 2022 from her position as Catalent’s Senior Vice President & Chief Commercial Officer, a position she had held since 2021. She joined the Company as President, Biologics and Chief Commercial Officer in 2020. Prior to joining Catalent, she served as the Senior Vice President and Chief Commercial Officer of West Pharmaceutical Services, Inc. from 2016 to 2019, having previously served as that company’s President of Pharmaceutical Packaging Systems since 2014. Ms. Flynn is also a director of Quanterix Corporation and Sotera Health Company and was a member of the board of directors for Recro Pharmaceuticals. She serves with the University of Notre Dame’s Hesburgh Women of Impact mentorship program and also has been on the Chester County Economic Development Council Board of Directors and the Downingtown STEM Academy Advisory Board. Ms. Flynn holds a Master of Science in Business Administration from Boston University, a Master of Science in Engineering from the University of Pennsylvania, and a Bachelor of Science in Pre-Professional Studies (Pre-Med) from the University of Notre Dame. |
| | | | | |
John J. Greisch Director since 2018 Executive Chair since August 2023 Age: 68 Committees: • Strategic and Operational Review (chair)
| John Greisch has been a member of the Board since February 2018 and was appointed as Executive Chair on August 28, 2023. Mr. Greisch retired in May 2018 from his position as President and Chief Executive Officer of Hill-Rom Holdings, Inc., a position that he had held since 2010. Prior to that, Mr. Greisch was President International Operations for Baxter International, Inc., a position he held beginning in 2006. During his seven-year tenure with Baxter, he also served as Baxter’s Chief Financial Officer and as President of Baxter’s BioScience division. Before his time with Baxter, Mr. Greisch was President and Chief Executive Officer for FleetPride Corporation in Deerfield, Illinois, an independent after-market distribution company serving the transportation industry. Prior to his tenure at FleetPride, he held various positions at The Interlake Corporation, including serving as President of its Materials Handling Group. Mr. Greisch currently serves as chairman of the board of Viant Medical LLC and as lead independent director on the board of Carrier Corporation. He previously served on the boards of Cerner Corporation, Idorsia Pharmaceuticals Ltd., Hill-Rom Holdings, Inc., Actelion Ltd, and TomoTherapy, Inc. Additionally, he serves as a senior advisor to TPG Capital and is on the board of directors for Ann & Robert H. Lurie Children’s Hospital of Chicago. He received a Masters in Management from the Kellogg School of Management at Northwestern University and a B.S. degree from Miami University. |
Christa Kreuzburg, PH.D.
Director since 2018
Age: 63
| Dr. Christa Kreuzburg has been a member of the Board since February 2018. Dr. Kreuzburg has been consulting in the healthcare sector since retiring from Bayer AG in 2009 after 19 years of service in a variety of roles, including service as Head of the Bayer Schering Pharma Europe/Canada unit of Bayer Healthcare from 2007 to 2008 and as Head of the Pharma Primary Care/International Operations unit of Bayer Healthcare from 2006 to 2007. She also held roles in the Strategic Planning and Central Research groups. Dr. Kreuzburg is currently a member of the board of directors of Tecan Trading AG of Switzerland and has previously served as a director of Freedom Innovations LLC. She received her Ph.D. and Bachelor’s degrees in Physical Chemistry from Duisburg University in Germany. |
Gregory T. Lucier Director since 2015 Age: 59 Committees: • Audit • Compensation and Leadership (chair) • Strategic and Operational Review
| Gregory T. Lucier has been a member of the Board since April 2015. Mr. Lucier has served as the chief executive officer of Corza Health, Inc., a company focused on acquiring companies and assets as part of a strategy to build a market-leading healthcare business since 2019. Prior to that, he served as Chief Executive Officer of NuVasive, Inc., a medical device company, from 2015 to 2018. Before joining NuVasive, Mr. Lucier was Chairman and Chief Executive Officer of Life Technologies Corporation (formerly Invitrogen Corporation), a global biotechnology company, from May 2003 until it was acquired by Thermo Fisher Scientific Inc. in February 2014. Prior to that, Mr. Lucier was a corporate officer at General Electric Company, where he served in a variety of leadership roles. Mr. Lucier is chairman of the board of Berkeley Lights and serves as a director of Dentsply Sirona and Maravai LifeSciences. He previously served as a director of Life Technologies Corporation from May 2003 to February 2014, of Carefusion Corporation from August 2009 until its sale to Becton Dickinson in March 2015, of Invuity, Inc. from October 2014 until its sale to Stryker in October 2018, and of Nuvasive from December 2013 to May 2021. Mr. Lucier received an M.B.A. from Harvard Business School and a B.S. in industrial engineering from Pennsylvania State University. |
Alessandro Maselli
Director since 2022
Age: 51
| Alessandro Maselli was appointed Catalent’s President and Chief Executive Officer and joined the Board of Directors in July 2022. He previously served as the company’s President & Chief Operating Officer from February 2019 until July 2022. Mr. Maselli joined Catalent in 2010 as Director of Operations at Catalent’s pharmaceutical, nutritional and cosmetics plant in Aprilia, Italy. In 2013, he was appointed General Manager of Zydis® operations at Catalent’s facility in Swindon, U.K., in 2015 he became Vice President of Operations, Europe, for Catalent’s Drug Delivery Solutions business unit, and in 2016 he was named Catalent’s Senior Vice President, Global Operations. Prior to Catalent, Mr. Maselli held operational and business leadership roles at Alstom and SGS. From 1998 to 2006, he held roles of increasing responsibility from process engineer to operations director at ABB. Mr. Maselli began his career as an automation systems engineer in the food industry. A native of Italy, Mr. Maselli earned bachelor and master degrees in electronic engineering from La Sapienza University of Rome. |
Donald E. Morel, Jr., Ph.D. Director since 2015 Age: 66 Committees: • Quality & Regulatory Compliance
| Dr. Donald E. Morel has been a member of the Board since November 2015. Dr. Morel retired in June 2015 as Chairman of West Pharmaceutical Services, Inc., a leading manufacturer of packaging components and delivery systems for injectable drugs and healthcare products, a position he had held since March 2003. He also served as West’s Chief Executive Officer from April 2002 until April 2015 and as its President from April 2002 until June 2005. Currently, Dr. Morel serves as Chairman of the Board of Directors of the American Oncologic Hospital of the Fox Chase Cancer Center. He also serves as Chairman of the Board of Trustees of the Franklin Institute, a trustee of the University of Virginia Darden School Foundation, and an Emeritus Trustee of Lafayette College. Additionally, Dr. Morel has been a Director of Stevanato Group since September 2018 and of Integra LifeSciences Holdings Corporation since August 2013. Prior to that, he served as a Director of Kensey Nash Corporation from 2010 until 2012. Dr. Morel obtained a Master of Science degree and a Ph.D. in Materials Science from Cornell University and a Bachelor of Science degree in Engineering from Lafayette College. |
| | | | | |
Stephanie Okey Director since 2023 Age: 62 Committees: • Compensation & Leadership • Nominating & Corporate Governance
| Stephanie Okey has been a member of the Board since August 2023. Ms. Okey is the former Senior Vice President, Head of North America, Rare Diseases, and U.S. General Manager, Rare Diseases at Genzyme, a Sanofi company, where she worked for 19 years in various executive management roles. By the time of her retirement in July 2015, Ms. Okey had acquired launch and commercialization experience with nine rare disease therapeutics and 4 large market therapeutics during her career. Prior to joining Genzyme, Ms. Okey served in various positions of increasing responsibility in the biopharmaceutical industry, having held roles in field sales and marketing at Bristol Myers Squibb and later Genentech, Inc. Ms. Okey is currently a member of the board of directors of PTC Therapeutics, Inc. and Crinetics Pharmaceuticals, Inc., both publicly traded biopharmaceutical companies. In addition, Ms. Okey previously served as a member of the board of directors of the California Life Sciences Association from October 2014 to January 2016, and on the board of directors of Albireo Pharma, Inc. from 2018 until its acquisition by Ipsen in March 2023. Ms. Okey holds a B.S. in Zoology from The Ohio State University and an M.S. in Immunology and Medical Microbiology from Wright State University. She has also completed executive training and education in manufacturing resource planning and organizational leadership. |
Michelle R. Ryan Director since 2023 Age: 57 Committees: • Audit • Strategic and Operational Review
| Michelle Ryan has been a member of the Board since August 2023. Ms. Ryan is the former Treasurer of Johnson & Johnson, where she worked for almost 30 years. As Treasurer, Ms. Ryan was responsible for providing financial oversight and insights to Johnson & Johnson’s M&A activities. Additionally, she was responsible for managing Johnson & Johnson’s global retirement assets, capital market transactions, and risk management activities. Prior to her role as Treasurer, Ms. Ryan worked in various financial leadership roles across Johnson & Johnson’s businesses, including as Chief Financial Officer of its Global Consumer Business and Chief Financial Officer of its Pharmaceutical Business of the Americas. Ms. Ryan has served on the board of directors of Aledade, Inc., a public benefit corporation helping independent practices, health centers, and clinics deliver better care to their patients and thrive in value-based care, since December 2021. Ms. Ryan received a B.S. in Accounting and an M.B.A. in Finance from the Wharton School of the University of Pennsylvania and is a Certified Public Accountant (inactive) and Certified Management Accountant (inactive). |
Jack Stahl Director since 2014 Lead Independent Director since August 2023 Age: 70 Committees: • Audit (chair) • Strategic and Operational Review
| Jack Stahl has been a member of the Board since August 2014 and was appointed as Lead Independent Director on August 28, 2023. Mr. Stahl was the President and Chief Executive Officer of Revlon Inc. from 2002 until his retirement in 2006. Prior to joining Revlon, Mr. Stahl served as President and Chief Operating Officer of The Coca-Cola Company from 2000 to 2001, having previously served in various management positions at that company, including Executive Vice President of The Americas Group and earlier as Chief Financial Officer, since joining it in 1979. Mr. Stahl is the chair of the board of directors of United Natural Food, Inc. and serves on the U.S. board of advisors of CVC Capital Partners. Additionally, he formerly served on the boards of Schering-Plough Corporation, Dr Pepper Snapple Group, Saks, Inc., Coty Inc., Ahold Delhaize, and Advantage Solutions LLC, Coca-Cola Enterprises, Coca-Cola Amatil Limited, and was chairman of the board of managers of New Avon LLC. Mr. Stahl holds a bachelor’s degree in economics from Emory University and a master’s degree from the Wharton School of Business at the University of Pennsylvania. |
Information Regarding Executive Officers
Set forth below is certain information with respect to our current executive officers as of December 8, 2023.
| | | | | |
Alessandro Maselli
President and Chief Executive Officer
Age: 51
| Mr. Maselli’s biography is set forth above. |
| |
Matti Masanovich
Senior Vice President
and Chief Financial Officer
Age: 51
| Matti Masanovich was named Senior Vice President & Chief Financial Officer in July 2023. Prior to joining Catalent, Mr. Masanovich served as Executive Vice President & Chief Financial Officer of Tenneco Automotive from August 2020 until it was acquired by Apollo in November 2023. Previously he was Chief Financial Officer at Superior Industries International from September 2018 until August 2020 and General Cable Corporation from November 2016 until July 2018. Earlier in his career, Mr. Masanovich held finance leadership roles of increasing responsibility in a number of companies in the automotive industry, where he demonstrated a strong history of improvement and profitability and operating efficiency. Mr. Masanovich began his career with PricewaterhouseCoopers LLP. He has Bachelor of Commerce, Finance & Accounting and M.B.A. degrees from the University of Windsor and is a Chartered Accountant in Canada. |
| |
| | | | | |
Lisa Evoli
Senior Vice President and Chief Human Resources Officer
Age: 54 | Lisa Evoli was named Senior Vice President and Chief Human Resources Officer in August 2023. Prior to joining Catalent, Ms. Evoli served as Executive Vice President and Chief Human Resources Officer of Integra LifeSciences Holdings Corporation from January 2016 until July 2023. Prior to that, she served for over two decades in a number of senior HR leadership roles, including at TE Connectivity Ltd., Johnson & Johnson and Motorola. Ms. Evoli holds a bachelor’s degree in business administration from the California University of Pennsylvania and a master’s degree in human resources development from Villanova University. |
Joseph A. Ferraro
Senior Vice President, General Counsel, Chief Compliance Officer, and Secretary
Age: 46
| Joseph A. Ferraro was named Senior Vice President, General Counsel, Chief Compliance Officer, and Secretary in February 2023 and is responsible for managing Catalent’s global legal and compliance operations. Prior to joining Catalent, Mr. Ferraro served as Chief Legal Officer and Secretary for Innovate Corp. from September 2017 until October 2022, where he managed global legal and compliance operations. Prior to Innovate, Mr. Ferraro served as General Counsel and Deputy Chief Compliance Officer at Prospect Capital from October 2008 until August 2017, having previously spent his early career at two leading law firms, Sullivan & Cromwell and Boies, Schiller & Flexner, where he focused on corporate and securities law. Mr. Ferraro holds a Juris Doctor degree with honors from The Law School at the University of Chicago, where he was a managing editor of The University of Chicago Law Review, and a Bachelor of Arts cum laude in public and international affairs from Princeton University. He is NACD Directorship Certified®. |
Aristippos Gennadios, Ph.D.
Group President, Pharma & Consumer Health
Age: 58
| Aris Gennadios was named Group President, Pharma and Consumer Health in July 2022. Prior to that, he served as President of our Softgel & Oral Technologies business since September 2013 and, earlier, as Vice President and General Manager of Softgel Technologies. Dr. Gennadios has worked in the pharmaceutical industry since 1996 in roles including R&D, field sales, business development, operations, and leadership. He joined Catalent’s predecessor company, Cardinal Health, in 2002 and held several leadership posts within the softgel technologies business, including Global Vice President of Business Development for Softgel Technologies, General Manager of the Oral Development Center in Somerset, NJ, and Vice President and General Manager for Rx Softgel and Consumer Health products. Dr. Gennadios earned his bachelor’s degree in chemical engineering from the National Technical University of Athens, Greece and his master’s degree in agricultural engineering from Clemson University. Dr. Gennadios holds a doctorate in engineering from the University of Nebraska and an MBA from Wake Forest University. |
Scott Gunther
Senior Vice President, Quality & Regulatory Affairs
Age: 56
| Scott Gunther was named Senior Vice President of Quality & Regulatory Affairs in May 2017. Mr. Gunther joined Catalent in 2012 as Vice President, Quality and was responsible for overseeing the quality function for the United States and other countries with sites in the Drug Delivery Solutions business unit. He previously also concurrently served as an interim Vice President of Product Development for the DDS business unit. Prior to joining Catalent, Scott spent 22 years with Bristol-Myers-Squibb in various roles of increasing responsibility. In his last role at BMS, he held the position of Executive Director Quality Operations Americas, where he was responsible for quality operations at its manufacturing sites in the U.S., Puerto Rico, and Latin America. Scott holds a Bachelor of Science degree from the State University of New York at Buffalo and an MBA from Canisius College. |
| |
Ricky Hopson
President, Division Head for BioProduct Delivery and Chief of Staff
Age: 48
| Ricky Hopson was named President, Division Head for BioProduct Delivery and Chief of Staff in August 2023. From July 2022 until August 2023, he was President, Division Head for Clinical Development and Supply. Concurrently, from April 2023 through July 2023, he served as Interim Chief Financial Officer. Prior to that, he served as Vice President & Chief Accounting Officer since June 2021. Mr. Hopson has been with Catalent for more than 20 years, serving in a variety of finance roles, including Vice President & Corporate Controller, Global Vice President, Operational Finance, and Vice President of Finance for two different business units. Mr. Hopson graduated from the University of Portsmouth and is a chartered management accountant in the U.K. |
David McErlane
Group President, Biologics
Age: 50 | David McErlane joined Catalent as Group President of Biologics in September 2023. Prior to joining Catalent, Mr. McErlane served as senior vice president and business unit head for Lonza's Bioscience business from July 2021 to September 2023 - where he led strategy, sales, marketing, innovation, digital, and operations functions for this division while developing transformational business strategies, executing major investments to digitize the customer journey, and acquiring and integrating new technologies. Earlier in his career, Mr. McErlane held several leadership positions at MilliporeSigma from 2016 to 2021 and BioReliance from 2006 to 2016 after beginning his career as an engineering manager at Zeneca from 1990 to 1999. Mr. McErlane holds a bachelor's degree in electronic systems engineering from Glasgow Caledonian University. |
Karen Santiago
Vice President & Chief Accounting Officer
Age: 52
| Karen Santiago was named Vice President & Chief Accounting Officer in September 2022. Prior to joining Catalent, she spent 19 years with Bristol-Myers Squibb Company in various roles of increasing responsibility, including Senior Vice President & Corporate Controller, Principal Accounting Officer from 2018 to 2022 and Lead Enabling Functions and Finance Transformation from 2016 to 2018. Since 2013 Ms. Santiago has served on the board of The Arc of New Jersey, the state’s largest organization advocating for and serving children and adults with intellectual and developmental disabilities and their families. She holds a Masters in Business Administration and a Bachelor of Science in Accounting from Rutgers University. |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors, executive officers, and beneficial owners of 10% or more of our shares of common stock to file reports with the SEC about their ownership of and transactions in our common stock. Based solely on our review of reports filed with the SEC and written representations from our executive officers and directors, we believe that all reports required to be filed under Section 16(a) during fiscal 2023 were timely filed.
Standards of Business Conduct
Our Board and all of our employees, including our CEO, principal financial officer, principal accounting officer, and all other executive officers are required to abide by our Standards of Business Conduct to ensure that our business is conducted in a consistently legal and ethical manner. A copy of our Standards of Business Conduct can be found on our website at investor.catalent.com/corporate-governance. We will disclose on our website any future amendment to, or waiver from, provisions of our Standards of Business Conduct affecting our directors or executive officers as and to the extent required under applicable SEC and NYSE rules.
Director Nomination Process
The Nominating and Corporate Governance Committee (the “Nominating Committee”) considers and recommends the annual slate of director nominees for approval by our Board. The Nominating Committee considers a number of factors and principles in making its recommendations, including the following:
•individual qualifications, including strength of character, mature judgment, familiarity with our business and industry, independence of thought, an ability to work collegially, and all other factors it considers appropriate, which may include age, gender, and ethnic and racial background
•existing commitments to other businesses or any other board of directors (or similar body)
•potential conflicts of interest with other pursuits
•legal considerations, such as antitrust issues
•corporate governance background
•varied and relevant career experience
•relevant technical skills and education
•relevant business or government acumen
•financial and accounting background
•executive compensation background
•the size, composition, and combined expertise of the existing Board
Although our Board and Nominating Committee consider diversity of viewpoints, background, and experiences when identifying and reviewing candidates for our Board, our Board does not have a separate diversity policy. In identifying and evaluating prospective director candidates, the Nominating Committee may seek referrals and assistance from other members of our Board, management, shareholders, and other sources, including third-party search consultants. The Nominating Committee uses the same criteria for evaluating candidates regardless of the source of the referral. When considering director candidates, the Nominating Committee seeks individuals with backgrounds and qualities that, when combined with those of our incumbent directors, provide a blend of skills and experiences to further enhance our Board’s effectiveness.
Elliott initially identified Mr. Barg and Ms. Ryan as potential candidates for consideration by the Nominating Committee, which interviewed and discussed the candidates before recommending them to the Board. The Nominating Committee also identified Mr. D’Amelio and Ms. Okey as candidates for our Board due to their background and leadership experience and recommended each of them to the Board for consideration.
Shareholders may nominate directors for election by following the provisions set forth in our bylaws concerning such matters. The Nominating Committee, in accordance with our Governance Guidelines, will consider the qualifications of any nominee proposed by one or more shareholders in the same manner in which it evaluates any other candidate.
Audit Committee and Audit Committee Financial Expert
The members of the Audit Committee are Jack Stahl (Chair), Rolf Classon, Gregory T. Lucier and Michelle R. Ryan. All members of the Audit Committee are “independent” in accordance with the NYSE listing standards and SEC rules applicable to
boards of directors in general and audit committee members in particular. Our Board has determined that each member of the Audit Committee qualifies as an “audit committee financial expert” as defined in SEC rules.
ITEM 11. EXECUTIVE COMPENSATION
Executive Summary
Our executive compensation program is intended to attract, motivate, retain, and reward our leadership in a manner that will align their interests with those of our shareholders on an annual and long-term basis and promote sustainable shareholder value creation. We believe attracting, motivating, retaining, and rewarding superior talent is needed to maintain and improve our performance and shareholder returns. We therefore seek to maintain a competitive program that ties a significant portion of executive pay to our financial and stock price performance.
The following is a summary of important aspects of our executive compensation program.
| | |
|
Balanced mix of pay components and incentives. Our compensation program targets a market-based mix of cash and equity compensation, and of short- and long-term incentives. The principal elements of our program are base salary; performance-based annual bonus; and long-term equity awards, split 80/20 between performance-contingent (PSUs and stock options) and time-vested (RSUs). |
Pay for Performance. We emphasize pay-for-performance to align executive compensation with our business strategy. Approximately 88% of the target total direct compensation of our CEO in 2023 was variable or performance-based. |
Share Retention. Our Compensation Committee has established stock ownership guidelines directing our executive officers to hold a multiple of annual salary in the form of shares of common stock in order to align management and shareholder interests. |
Pledging and Hedging. Our executives are prohibited from pledging our shares (absent our General Counsel’s permission, which has never been granted) or hedging against the economic risk of such ownership. |
Use of Independent Consultant. The Compensation Committee has engaged an independent, third-party consultant, Frederic W. Cook & Co., Inc. (“FW Cook”), to assist it in designing our compensation program and making compensation decisions. |
Clawback/Forfeiture Provisions. The terms of our long-term, equity-based awards and our short-term, cash-based award plan allow us in certain circumstances to “claw back” shares and cash received pursuant to such awards or, in the case of the equity-based awards, to require the repayment of all gains realized on the vesting or exercise of such awards. In connection with the revisions to our audited consolidated financial statements for fiscal 2022, under the clawback provisions of the management incentive plan (“MIP”), the Compensation Committee approved a clawback of a portion of the amounts paid under the MIP for fiscal 2022 for all named executive officers (“NEOs”) who received a MIP payment for such fiscal year. The amount of the clawback was determined in the same manner as the original MIP payment by calculating the value of the appropriate business factor in light of the revision to the audited consolidated financial statements and was equal to approximately 3% of the value of the original fiscal 2022 MIP payout to each NEO for fiscal 2022 performance. For NEOs who are active employees, each NEO’s 2023 MIP payment or other amounts due to them are being reduced by the amount of the clawback. |
Compensation Peer Group. The Compensation Committee uses a group of peer companies, selected with the assistance of FW Cook to be aligned with corporate governance best practices, to benchmark target total direct compensation levels, other executive compensation-related programs and policies, and benefit packages. |
Shareholder Say-on-Pay. At the 2022 Annual Meeting of Shareholders, our shareholders voted 95.3% in favor of our say-on-pay proposal, demonstrating their concurrence that our executive compensation program reflects a strong pay-for-performance orientation. In fiscal 2023, the Compensation Committee considered the outcome of the shareholder advisory vote when making decisions relating to the compensation of our NEOs and our executive compensation program and policies. Based in part on the demonstrated level of support reflected in this vote and the success of the program in retaining our talent and incentivizing superior performance, the Compensation Committee determined that no substantive change to our compensation program was necessary for fiscal 2023. |
Overview of 2023 Financial Performance and Executive Compensation
2023 FINANCIAL PERFORMANCE
•Net revenue declined 11%, from $4.8 billion to $4.3 billion on a reported basis and from $4.8 billion to $4.4 billion on a constant-currency basis.
•Net earnings decreased 151%, from earnings of $499 million to a loss of $256 million.
•Adjusted EBITDA declined 43%, from $1.3 billion to $0.7 billion on a constant-currency basis.
•Our net leverage ratio increased from 2.9x at the end of fiscal 2022 to 6.6x at the end of fiscal 2023.
2023 COMPENSATION HIGHLIGHTS
In fiscal 2023, our financial performance was muted, with Budget-Based Revenue and Budget-Based EBITDA (both as defined below) missing target by 24% and 50%, respectively. As a result, our performance-based compensation awarded in fiscal 2023 and paid out in fiscal 2023 from prior awards was meaningfully below target. The fiscal 2023 MIP, comprised of 70% business performance and 30% personal goals performance, for our executive officers other than the CEO who remained in service at the payout date, including each of our NEOs besides Mr. Castellano (who departed Catalent prior to payout and therefore received nothing from the MIP), and Dr. Boerman and Mr. Maselli (who received no payout under the MIP), was awarded materially below target, as the business performance factor was 0% for the fiscal year. In addition, our financial performance in fiscal 2023 resulted in materially lower payouts under the long-term incentive program compared to fiscal 2022 payouts, with our Relative Return PSUs, as described below, for the fiscal 2021-2023 performance period having 0% payout, and our Adjusted EPS PSUs, as described below, for the fiscal 2021-2023 performance period vesting at 106%, modestly above target due to our strong performance in fiscal 2021 and 2022 relative to fiscal 2020. Similarly, the values of time-vested RSUs at their vesting in early fiscal 2024 were materially lower than their stated values at the time of their grant due to the decline in our stock price compared to our stock price in early fiscal 2021 when awarded. As of the end of fiscal 2023, outstanding PSUs aligned to both the fiscal 2022-2024 and fiscal 2023-2025 performance periods were also tracking meaningfully below targets.
EXECUTIVE PAY MIX FOR 2023
The majority of target total direct compensation for our NEOs during fiscal 2023 consisted of variable pay elements. The Compensation Committee believes this allocation aligns with our pay-for-performance compensation philosophy of motivating our NEOs to achieve our performance objectives in the short-term and to grow the business to create sustainable value for our shareholders in the long term.
(1) Does not include other compensation, pension values, and nonqualified deferred compensation earnings, which are shown in the Summary Compensation Table below. Mr. Maselli actually received zero short-term compensation for fiscal 2023 due to the performance level of the business for that year.
CEO 2023 TARGET DIRECT COMPENSATION OVERVIEW
| | | | | |
| |
BASE SALARY | • $925,000 |
| |
MANAGEMENT INCENTIVE PLAN (MIP) | • $1,018,000 at target, equal to 110% of base salary |
| |
LONG-TERM INCENTIVE AWARD | • $5,500,000 in grant-date fair value awarded under our long-term incentive plan |
Our Executive Compensation Program
OUR COMPENSATION PHILOSOPHY AND PRINCIPLES
Our executive compensation program ties pay delivery to the successful execution of our overall business goals and adherence to our core values, which we believe best serves the interests of our shareholders. We believe that attracting, motivating, retaining, and rewarding superior executive talent is a key to delivering attractive shareholder returns, and that an appropriately structured executive compensation program is critical to that end, with each element supporting the achievement of our compensation philosophy.
Our executives must be of a caliber and level of experience necessary to manage our complex, global business effectively. Given the long-cycle nature of most of our businesses, the complexity and highly regulated nature of our operations, and the competitive nature of our industry, it is especially important for us to retain our executive talent to ensure continuity of management. We seek to implement this philosophy by following three key principles:
| | | | | |
• Competitive compensation. Providing a competitive compensation opportunity that enables us to attract, motivate, retain, and reward superior executive talent. | • Alignment with shareholder interests. Aligning our executives’ interests with our shareholders’ through equity compensation, short- and long-term absolute and relative performance metrics and share retention guidelines. |
• Linking compensation to performance. Fostering a pay-for-performance philosophy by tying a significant portion of pay to financial and stock-price performance as well as other goals that support the creation of sustainable long-term shareholder value. |
EXECUTIVE COMPENSATION PROGRAM ELEMENTS
| | | | | | | | |
COMPONENT | DESCRIPTION | OBJECTIVES AND COMMENTS |
| | |
Cash Compensation | | |
Base Salary | Fixed cash compensation that is based on scope of responsibilities, experience, prior performance, and the pay practices of key competitors for executive-level talent. | • Attract, motivate, and retain superior talent.
• Provide a fixed, baseline level of compensation.
• Annual increase, if any, based on market positioning and individual performance.
|
| | |
Annual Bonus Opportunity: Management Incentive Plan (MIP) | Annual cash payment tied to our financial results and a set of individually tailored financial and strategic performance objectives. | • Variable pay for short-term achievement of financial results and individual goals.
• For fiscal 2023, 70% based on financial performance (Budget-Based EBITDA and Budget-Based Revenue, each as defined below) and 30% based on individual goals.1
|
Note that “Budget-Based Revenue” and “Budget-Based EBITDA” are non-GAAP financial measures and subject to important limitations. For a discussion of these measures and how they reconcile to our results reported under U.S. GAAP, please see the heading “Non-GAAP Metrics” under Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations and at the end of Item 11. Executive Compensation of this Annual Report.
| | | | | | | | |
COMPONENT | DESCRIPTION | OBJECTIVES AND COMMENTS |
Long-Term Incentive | | |
| | | | | | | | |
Awards under our Long-Term Incentive Plan (“LTIP”) | Annual grants of equity-based awards under our 2018 Omnibus Incentive Plan (the “2018 Omnibus Plan”) intended to drive (1) absolute and relative long-term performance relative to pre-established objectives and (2) robust, continuous executive retention. Includes grants of Nonqualified Stock Options, RSUs, and PSUs. | • Align compensation with the creation of shareholder value and achievement of long-term performance objectives.
• Increase equity ownership by executives.
• Promote executive retention.
• Reward absolute and relative stock price performance over a multi-year period. |
COMPONENT | DESCRIPTION | OBJECTIVES AND COMMENTS |
| Retirement Benefits | | |
| U.S Savings Plan | A tax-qualified 401(k) defined contribution plan that allows U.S. participants to defer a portion of their compensation, subject to Internal Revenue Code limits, and receive a partial employer matching contribution. | • Attract, motivate, and retain superior talent. |
| U.K. Retirement Plan | A defined contribution retirement plan open to U.K. participants, which also permits a partial employer match on contributions. | • Attract, motivate, and retain superior talent. |
| Deferred Compensation Plan | A nonqualified deferred compensation plan for qualifying U.S. and U.K. employees that provides opportunities to defer income taxation of a portion of compensation beyond what is permitted under our Savings Plan.
The plan allows NEOs and certain other executives to defer up to 80% of total cash compensation, to receive matching contributions equal to 50% of the first 6% of compensation deferred, and to invest cash amounts deferred in a variety of investment options. In addition, the plan allows for U.S.-based executives to defer certain grants received under our 2018 Omnibus Plan.
| • Attract, motivate, and retain superior talent. |
| Severance Benefits | | |
| Executive Severance and Change-in-Control Benefits | Severance benefits provided to NEOs and certain other senior executives upon company-initiated involuntary termination of employment without cause, or upon a “good reason” termination by the executive.
Equity grants provide for vesting if employment is terminated involuntarily without cause following a change in control.
| • Attract, motivate, and retain superior talent.
• Facilitate recruitment and retention of executives by providing income security in the event of involuntary job loss. |
The Compensation Process
THE ROLE OF THE COMPENSATION COMMITTEE, ITS CONSULTANT, AND MANAGEMENT
The Compensation Committee oversees the compensation program for our CEO and our other executive officers, including our other NEOs. Management typically formulates new proposals concerning executive compensation, including, but not limited to
salary levels, the form and content of various incentive or other compensation programs, and benefits such as healthcare and retirement programs (though management does not propose or otherwise participate in the setting of our CEO’s compensation). All management proposals as they relate to our NEOs are subject to Compensation Committee review and approval. The Compensation Committee has retained an independent consultant, FW Cook, to help it fulfill its responsibilities, including its review of management proposals. Among other things, FW Cook benchmarks compensation levels using available market data and trends and the Comparison Group approved by the Compensation Committee (see discussion of Comparison Group below). In compliance with the NYSE’s listing standards and SEC rules, the Compensation Committee in April 2023 conducted its annual independence assessment of FW Cook and concluded that it remains independent of management and that its work did not raise any conflict of interest.
THE COMPENSATION COMMITTEE’S PROCESS
In accordance with its charter, the Compensation Committee is responsible for, among other duties:
• reviewing and approving our overall executive compensation philosophy;
• overseeing the administration of compensation and benefit programs, policies, and practices;
• reviewing and approving the identification of our peer companies with respect to various benchmarking activities and data sources used in evaluating our compensation competitiveness;
• evaluating the performance of the CEO against performance goals and objectives approved by our Board; and
• approving the performance metric and corresponding goals, evaluating the performance, and approving the compensation of our executive officers.
THE USE OF MARKET DATA IN DETERMINING COMPENSATION
The Compensation Committee considers numerous factors as it formulates, reviews, and approves pay components and the overall structure of our executive compensation program. Among these factors are survey data, scoped to focus on companies with revenue comparable to ours, and the compensation practices of select peer companies, which we refer to as the “Comparison Group.” In January 2022, the Compensation Committee reviewed the Comparison Group that would be used to inform pay decisions for fiscal 2023. One of the peers, Varian Medical, was acquired, leaving 15 companies in the Comparison Group. Catalent's trailing four quarter revenue and market cap at the time the peers in the Comparison Group were reviewed were both near the median of the remaining 15 peers. Additionally, all remaining peers were found to be within a reasonable range of Catalent’s revenue and market cap at the time, and the Compensation Committee determined not to make any change to the Comparison Group (other than removing Varian Medical due to its acquisition).
Six of the 15 peer companies are pharmaceutical / biotechnology companies, and the remaining peer companies are health care equipment/supplies or life sciences tools/services companies. Other factors that are reviewed during the annual Comparison Group selection process include business similarity, profitability, enterprise value, and number of employees. The Compensation Committee further believes that reference to the Comparison Group is appropriate when reviewing our compensation program for fiscal 2023 because of the potential likelihood that this group competed with us for executive talent. The 15 companies in the Comparison Group that informed compensation decisions for fiscal 2023 were:

The Compensation Committee also considers other factors in addition to the market benchmarks, including individual executives’ tenure, proficiency in role, and criticality to our performance. The Compensation Committee concluded that our pay strategy is appropriate to assure the attraction and retention of top talent in a competitive market, particularly as we continue to move into areas where the competition for top talent is particularly fierce, including cell and gene therapy, and demands on our
senior executives have increased as the business has grown and become more complex. While the Compensation Committee considers peer and market data, it does not target a specific market position when determining executive target compensation levels. In addition to referencing market data, as described above, the Compensation Committee considered prior year compensation history and compensation levels of other Company executives to provide context for fiscal 2023 executive compensation.
Details of Total Direct Compensation Elements
For fiscal 2023, compensation paid to our NEOs consisted of base salary; short-term incentive pay in the form of participation in the MIP; equity-based, long-term incentive awards subject to multi-year time- and performance-vesting criteria; and the opportunity to participate in certain benefit programs and other perquisites.
We generally review the base salary and other incentive compensation target amounts of our executive officers, including our NEOs, annually, consistent with the process for our employees generally.
BASE SALARY
Base salary is the principal fixed component of target total direct compensation for NEOs, and is determined by considering the executive’s job responsibilities, market data, and the individual’s performance and contributions.
MANAGEMENT INCENTIVE PLAN
SUMMARY
The MIP is an annual cash incentive program that rewards performance against annual individual and overall business goals. We extend MIP participation to a broad group of our executives, including our NEOs. For fiscal 2023, 70% of a participant’s MIP target payout was based on business goals applicable to that participant and 30% was based on the participant’s individual goals. The Compensation Committee selects the overall business goals applicable to the NEOs participating in the MIP from among the corporate financial and strategic growth objectives set each year by our Board. The individual goals for each of our NEOs other than our CEO and former Executive Chair are set jointly by that NEO and the CEO. The individual goals for our CEO and former Executive Chair are set jointly by each of these individuals and the Compensation Committee. These individual goals relate generally to the following categories but are not assigned numerical weightings or measuring criteria: quality and compliance, operational excellence, customer innovation/growth, organizational vitality/leadership, and financial accountability.
2023 PERFORMANCE TARGETS
For fiscal 2023, the Compensation Committee based the business goals portion of our MIP on achievement of our Budget-Based EBITDA goal (as defined under “Non-GAAP Financial Measures” below in this Annual Report) and our Budget-Based Revenue goal (also as defined under “Non-GAAP Financial Measures” below in this Annual Report). The Compensation Committee uses Budget-Based EBITDA and Budget-Based Revenue because:
(a) it believes that they are important indicators of our increasing value and growth,
(b) they are the primary measures by which we set and measure performance for the fiscal year,
(c) they exclude certain items that would normally be part of a calculation of net earnings but that we believe are not representative of our core business, and
(d) they are widely used measures of overall financial performance.
The Compensation Committee concluded for fiscal 2023 that (1) using a combination of these two measures would provide a balanced set of business performance targets that focus on growth, profitability, and the most efficient conversion of revenue to profit, (2) at the time the goals are set, the performance targets provide a reasonably achievable, but challenging, set of goals for our NEOs and other MIP participants, and (3) linking our NEOs’ bonuses to company-wide performance goals encourages collaboration across the executive leadership team. These goals are intended to incentivize all participants to maximize their performance for the benefit of our shareholders.
CALCULATING 2023 MIP AWARDS
When determining MIP awards, the Compensation Committee used a matrix approach that simultaneously evaluates performance of the two components that comprise the business-goal portion of the MIP. Performance at target for each of the metrics results in achievement of the business-goal portion of the MIP award at 100% of a participant’s target amount.
Performance below or above the targets, subject to a range of 80% to 125% and a minimum 80% achievement of Budget-Based EBITDA target, results in an achievement of the business-goal portion of the MIP award in the manner set forth in the following table (at 0-200% of target), with linear interpolation applied for results that fall between two consecutive revenue or EBITDA achievement levels:
Achievement by each participant, including each of our NEOs, against individual goals can result in payment of the individual performance portion of the MIP award between 0% and 200% of the target amount. Payout of the MIP requires achievement of the minimum thresholds of both the business-goal portion and the individual-performance portion. The target amount for each participant in our MIP, including each of our NEOs, is a fixed sum and is reviewed annually by the Compensation Committee, consistent with the process for our employees generally.
For fiscal 2023, the business goals were collectively weighted at 70% of the total payout, the individual goals were weighted at 30%, and the maximum payout under our MIP was 200% of each executive target opportunity (200% x 70%, plus 200% x 30%).
CLAWBACK/FORFEITURE
Participation in the MIP may be cancelled or forfeited and repaid to us if the participant engages in any “Detrimental Activity,” including but not limited to fraud, breaches of restrictive covenants, and disparagement of the company, as defined in the 2018 Omnibus Plan. In addition, if a participant receives any amount in excess of what the participant should have received for any reason (including by reason of a financial restatement, mistake in calculation, or other administrative error), the participant must repay the excess. Without limiting the foregoing, all MIP awards are subject to reduction, cancellation, forfeiture, or recoupment to the extent necessary to comply with applicable law.
In connection with the revisions to our audited consolidated financial statements for fiscal 2022, under the clawback provisions of the MIP, the Compensation Committee approved a clawback of a portion of the amounts paid under the MIP for fiscal 2022 for all NEOs who received a MIP payment for such fiscal year. The amount of the clawback was determined in the same manner as the original MIP payment by calculating the value of the appropriate business factor in light of the revision to the audited consolidated financial statements and was equal to approximately 3% of the value of the original fiscal 2022 MIP payout to each NEO for fiscal 2022 performance. For NEOs who are active employees, each NEO’s 2023 MIP payment or other amounts due to them are being reduced by the amount of the clawback.
In October 2023, the Company adopted a Clawback Policy in accordance with Section 10D of the Exchange Act and Rule 10D-1 promulgated thereunder and intended to comply with the NYSE listing standards.
2023 MIP AWARDS
The business performance goals and achievement levels for fiscal 2023, which collectively represented 70% of the overall target MIP award, are as follows (in millions of U.S. dollars, using our internal budget-based currency exchange rates, or percentages):
| | | | | | | | | | | |
Performance Measure | Threshold / Target / Maximum Performance (1) | Actual Performance | Business
Performance
Factor
Payout
Percentage |
| Budget-Based Revenue | $4,232 / 5,290 / 6,612 | $ | 4,263 | | 0% |
| Budget-Based EBITDA | $1,126 / 1,407/ 1,759 | $ | 698 | |
(1) When calculating Budget-Based EBITDA and Budget-Based Revenue performance, the target, threshold, and maximum are adjusted by the Compensation Committee for the projected pro forma performance from completed acquisitions over the measurement period.
The CEO, together with the Senior Vice President and Chief Human Resources Officer during fiscal 2023, evaluated the individual performance of each of our executive officers, including the NEOs other than the CEO, based on each individual’s fiscal 2023 goals and objectives. After combining the individual performance metric with the business performance metrics, management determined a recommended MIP award for each such executive officer, which they presented to the Compensation Committee. In approving MIP awards for the NEOs other than the CEO, the Compensation Committee considered our financial performance in fiscal 2023 and the individual assessment of performance and accomplishments relative to their respective goals and objectives.
The CEO also presented to the Compensation Committee an assessment of his own individual performance, which the Compensation Committee evaluated in determining the CEO’s MIP award. The CEO’s MIP award was based on his fiscal 2023 goals and objectives in the areas of: (1) continuing to progress Catalent’s strategic ambition to build on its broad base of offerings and to expand its position as the preferred strategic CDMO partner in core and advanced technologies, integrated solutions, and first-to-scale innovation, including solidifying and accelerating growth in Catalent’s base of product offerings, maximizing growth in Catalent’s biologics division, and continuing to invest in growth enablers; (2) strengthening Catalent’s foundation, including addressing variabilities in performance and On Time Delivery and driving continuous improvement centrally, including by continuing to enhance the IACP audit program and improving IT; (3) offsetting inflationary pressures with total cost excellence ("TCE") and other cost control and restructuring programs; (4) leveraging a strong financial position to increase M&A selectivity, pursuing portfolio management, delivering fiscal year 2023 financials and making working capital improvements; and (5) improving on our culture and organizational vitality, including executing on cultural assimilation, continuing to find ways to deepen Catalent’s Patient First culture, and achieving measurable progress against stated D&I and ESG goals and targets. The Compensation Committee did not assign weights in considering these areas, but took account of the differing levels of focus in each area as the year progressed. After consideration of all of these factors, the Compensation Committee decided not to award the CEO any MIP bonus for fiscal 2023.
The former Executive Chair also presented to the Compensation Committee an assessment of his own individual performance, which the Compensation Committee evaluated in determining the former Executive Chair’s MIP award, based on his fiscal 2023 goals and objectives in the areas of: (1) advancing our culture and values through acceleration of our ESG goals and targets, focused on sustainability (waste, water usage, and emissions), and acceleration of our TCE goals, including reactivating a LEAN culture; (2) executive coaching to advance our CEO’s transition and to activate CEO succession planning; (3) enhancing Board processes to create better interaction among directors; (4) driving key executive and senior leadership talent acquisition, with a primary focus on senior leadership positions within the finance, operations and quality functions in our Biologics segment and development a bench of industry leaders poised for executive leadership team roles; (5) providing active counsel and support to the company on matters of M&A strategy and execution, transformational projects, and emerging biotech customer development, among others; and (6) leading the Board through setting meeting agendas, working closely with the Lead Independent Director and CEO to set content and manage Board logistics. The Compensation Committee did not assign weights in considering these areas but took account of the differing levels of focus in each area as the year progressed. After consideration of all of these factors, the Compensation Committee decided not to award the former Executive Chair any MIP bonus for fiscal 2023.
LONG-TERM INCENTIVE AWARDS
Our long-term incentive compensation program is potentially available to all our employees, including our NEOs, and includes one or some combination of three types of equity-based awards:
• time-based stock options, in which there is a fixed grant to the recipient subject only to a time- and service-based vesting requirement;
• time-based RSUs, in which there is a fixed grant to the recipient subject only to a time- and service-based vesting requirement; and
• performance-based PSUs, in which vesting is based on the achievement of pre-established performance criteria over a multi-year performance period, subject to continuing service through the date of certification of final performance by the Compensation Committee.
By awarding grants with multi-year performance or vesting periods, we appropriately align program participants with the long-term best interests of our shareholders. Those interests are also protected by restrictive covenants that are imposed on our participants, including a confidentiality obligation, a limitation on competing with us for the greater of one year post-departure and the final vesting of outstanding equity-based awards, and an agreement not to solicit our employees for one year after leaving our employ.
Awards to our NEOs for the fiscal 2023-25 performance period, awarded in early fiscal 2023, were divided into PSUs (with the target number of shares providing 50% of the target value awarded), stock options (30% of the target value awarded), and RSUs (20% of the target value awarded). In turn, the target value awarded as PSUs was divided evenly between PSUs that use Adjusted EPS as their performance metric and those that use relative total shareholder return (“Relative Return”), as described below in this section. The target size for our NEOs’ LTIP awards was set by the Compensation Committee using a market-based determination of LTIP grant value, individual performance, and other factors.
Awards to our NEOs for the fiscal 2023-25 performance period, awarded in early fiscal 2023 under our LTIP were generally determined and approved by the Compensation Committee on a dollar-value basis, which is then translated into a fixed or target number of options, RSUs, or PSUs by dividing the award by the per-instrument price, using a Black-Scholes valuation for options, grant date share price for RSUs and Adjusted EPS PSUs, and the value derived from a Monte Carlo pricing model for Relative Return PSUs, and then rounding up to the nearest whole number of shares. Subject to the recipient’s continued service with us through each applicable vesting date, options vest in equal installments over the first four anniversaries of the grant date, RSUs vest on the third anniversary of the grant date, and PSUs vest when and if we determine that the performance criteria are met at the end of the three-year performance period. The continued service requirement is waived in the event of a participant’s disability or retirement in accordance with the “Rule of 65,” which applies if a participant retires on or after the date on which the sum of the participant’s age and period of service with us equals sixty-five (65) years, so long as they are at least the age of fifty-five (55) and give at least six-months’ notice and, beginning with grants awarded in fiscal 2021, have completed at least five years of service with us.
The performance criteria for the PSUs granted during fiscal 2023 are as follows:
• Adjusted EPS is separately calculated for each fiscal year in the 3-year performance period and then totaled and compared to the 3-year, cumulative target set by the Compensation Committee at the beginning of the performance period.
• Achievement of the target Adjusted EPS will earn the participant the number of shares equal to 100% of the target number of Adjusted EPS PSUs. At 75% achievement, 50% of the target will be earned, with no shares earned for achievement below that threshold. At the maximum achievement level of the greater of (i) 150% of target Adjusted EPS and (ii) the amount determined using the financial goals set forth for the performance period portion of the most recent strategic planning period, the resulting earnout is 200% of the target. Earnouts are interpolated for levels of performance between threshold and target, and between target and maximum.
• Relative Return is the percentile rank of our total shareholder return during the 3-year performance period relative to the total shareholder return of each of the companies comprising the S&P 500 Healthcare Index (with total shareholder return being the change in the price per share over the performance period, assuming reinvestment of dividends, if any, paid during the performance period). There were 63 other companies in the comparison group at the start of the fiscal year 2023-2025 three-year performance period.
• Achievement of the median Relative Return will earn the participant the number of shares equal to 100% of the target number of Relative Return PSUs. At the 25th percentile, 50% of target will be earned, with no shares earned for achievement below that threshold. At the maximum achievement level of the 75th percentile, the resulting earnout is at 150% of target. Earnouts are interpolated for levels of achievement between threshold and target, and between target and maximum. In addition, earnouts on our Relative Return PSUs are subject to an additional cap so that the total value of the shares earned at payout cannot exceed 300% of the grant date value of such incentive awards.
The Compensation Committee believes that the performance targets for both the Adjusted EPS PSUs and the Relative Return PSUs represent reasonably achievable but challenging goals and are intended to incentivize all participants to maximize their performance for the long-term benefit of our shareholders.
*Note that Adjusted Net Income is a non-GAAP financial measure, is not a measure of operating income, operating performance, or liquidity presented in accordance with U.S. GAAP and is subject to important limitations. For a discussion of these measures and how they reconcile to our results reported under U.S. GAAP, please see the heading “Non-GAAP Metrics” under Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations and at the end of Item 11. Executive Compensation of this Annual Report.
FISCAL 2021-2023 PSU PERFORMANCE
In fiscal 2021, the Compensation Committee granted PSUs representing 50% of the total long-term incentives to executives for the fiscal 2021-23 performance period, awarded one-half as Adjusted EPS PSUs and one-half as Relative Return PSUs. These PSUs issued in respect of the fiscal 2021-23 performance period vested in fiscal 2024 at a performance level of 106% of target for the Adjusted EPS PSUs and 0% of target for the Relative Return PSUs earned by our NEOs.
| | | | | | | | | | | | | | | | | | | | | | | |
Fiscal 2021-2023 Performance Targets |
| Performance Schedule | | Corresponding Earnout Range (% of Target) |
| |
| Threshold | Goal | Maximum | | Thresh. | Goal | Max. |
| Adjusted EPS PSUs and Performance Shares | $5.69 | $7.58 | $9.48 | | 50% | 100% | 200% |
| Relative Return PSUs and Performance Shares | 25th Percentile | 50th Percentile | 75th Percentile | | 50% | 100% | 150% |
| | | | | | | | | | | |
Fiscal 2021-2023 Performance Achievement |
| Actual Performance | |
| Achievement Level | % of
Goal | Earnout as
% of Target |
| Adjusted EPS PSUs | $7.69 | 101 | % | 106 | % |
| Relative Return PSUs | 15th Percentile | N/A | 0% |
Other Benefits Under Our Executive Compensation Program
BENEFITS AND PERQUISITES
We provide to all our employees, including our NEOs, broad-based benefits that are intended to attract and retain employees while providing them with retirement and health and welfare security. Broad-based employee benefits available to our NEOs include:
• a 401(k) savings plan for U.S. NEOs, and an equivalent plan under U.K. law for our U.K.-domiciled NEO, both of which provide for a partial employer match of employee contributions;
• an employee stock purchase plan, allowing the purchase of shares of our common stock at a 10% discount;
• medical, dental, vision, life and accident insurance, disability coverage, and health savings, dependent care, and healthcare flexible spending accounts; and
• employee assistance program benefits.
Under our 401(k) savings plan and the equivalent U.K. plan, we match a portion of the funds set aside by the employee. In the U.S., we match 100% of up to 4% of eligible annual compensation contributed, up to federal tax law limits on both eligible compensation that may be considered for contribution and the amount employees may contribute. In the U.K., the plan provides for an employer matching contribution of 5.5-8% of eligible base salary compensation dependent on the participant contributing 3.5-6% of eligible base salary compensation.
Our employee stock purchase plan (the "Employee Stock Purchase Plan") is designed to allow our eligible employees to purchase shares of our common stock at designated intervals at a discounted price of 10% through their accumulated payroll deductions or other contributions. Employees who are United States tax residents may benefit from favorable tax treatment as the Employee Stock Purchase Plan is intended to qualify as an employee stock purchase plan under Section 423 of the Internal Revenue Code.
We provide basic life and accident insurance coverage valued at two times the employee’s annual base salary at no cost to our employees. The employee may also select supplemental life and accident insurance, for a premium to be paid by the employee.
We also provide our NEOs with limited perquisites and personal benefits that are not generally available to all employees, such as financial counseling services. We provide these limited perquisites and personal benefits in order to further our goal of attracting and retaining our executive talent and to avoid unnecessary personal distractions that may impede maximum personal performance. These benefits and perquisites are reflected in the “All Other Compensation” column of the Summary Compensation Table and the accompanying footnotes in accordance with SEC rules. Other than with respect to tax equalization and related tax gross-up payments made in respect of two of our NEOs, Mr. Maselli and Dr. Boerman, who lived and worked, at our request, in a jurisdiction other than his or her primary tax domicile, as described below in note 6(C) to our Fiscal 2023 Summary Compensation Table, during fiscal 2023 we did not “gross up” for the income tax consequences of any benefit or perquisite.
DEFERRED COMPENSATION PLAN
Our deferred compensation programs (collectively, the “Deferred Compensation Plan”) permit a broad group of U.S.- and U.K.-based executives, including all of our NEOs (other than Dr. Boerman), to defer up to 80% of base salary, commissions (not applicable to NEOs), and MIP bonus. We credit the first 6% of cash compensation deferred with a matching contribution equal to 50% of the amount deferred. Participants are immediately vested in all amounts they contribute and the related investment gains, but matching contributions and their related investment gains vest ratably over the participant’s first four years of service. Participants may choose from a variety of investment options for the cash amounts deferred.
Under the Deferred Compensation Plan, we also credit each participant’s deferral account with notional earnings and/or losses based on the deemed investment of the accounts in one or more of a variety of investment alternatives selected by such participant. Participants may elect from a variety of forms of payout, including lump-sum payment and various types of annual installments, with the timing depending on the form selected.
In addition, our Deferred Compensation Plan permits U.S. participants to defer unvested incentive compensation grants (other than options) in order to delay recognition of income on these awards upon vesting.
Cash and equity deferrals, company contributions, and applicable gains are held in a “rabbi” trust. “Rabbi” trust assets are ultimately controlled by us. Operating the Deferred Compensation Plan this way permits participants to defer recognition of income for tax purposes on the amounts deferred until they are paid to the participants.
Our U.S.- and U.K.-based directors can also participate in the Deferred Compensation Plan on the same terms as our executives, though they are not provided a matching contribution on their cash deferrals.
We believe that providing the NEOs and other eligible participants with deferred compensation opportunities is a market-based benefit plan necessary for us to deliver competitive benefit packages. Additional details of the Deferred Compensation Plan follow the table entitled “Fiscal 2023 Nonqualified Deferred Compensation Table,” following this Compensation Discussion & Analysis ("CD&A") section.
SEVERANCE AND PAYMENTS ON A CHANGE OF CONTROL
Our NEOs are eligible for severance benefits in connection with a termination of employment and/or a change of control in certain circumstances. The amounts of such benefits and the conditions for their payment are described in the Fiscal 2023 Potential Payments upon Employment Termination or Change of Control Tables, including the accompanying notes.
Compensation Determinations for 2023
We generally review the base salary and other incentive compensation target amounts of our executive officers, including our NEOs, annually, consistent with the process for our employees generally. For fiscal 2023, compensation paid to our NEOs consisted of base salary, short-term incentive pay in the form of participation in the MIP, equity-based, long-term incentive awards subject to multi-year time- and performance-vesting criteria, and the opportunity to participate in certain benefit programs and other perquisites.
The Compensation Committee observed at the beginning of fiscal 2022 that executive compensation opportunities were meaningfully low versus peer and market data overall and the Compensation Committee determined to move targeted pay levels over a multi-year period which resulted in larger pay increases than in the past for certain individuals, particularly in their long-term incentive award grant values. It continued with this strategy when setting fiscal 2023 target pay opportunities in July 2022. Despite the target total direct compensation increases, and due to continued market movement, Catalent’s fiscal 2023 target total direct compensation levels generally remained below the market median (except for two NEOs who were provided one-time promotion awards in fiscal 2023 to recognize their increased responsibilities and incentivize continued performance). In line with the above, the Compensation Committee does not target a specific market position when determining executive target compensation levels.
| | | | | |
| Alessandro Maselli | The following determinations reflect Mr. Maselli’s transition to President and Chief Executive Officer in fiscal 2023 • Base Salary: Increased to $925,000 from $654,183 as President and Chief Operating Officer in fiscal 2022 • MIP: Zero bonus, equal to 0% of target opportunity of $1,018,000, or 0% of salary (target increased from 80% of salary as President and Chief Operating Officer in fiscal 2022) • LTIP: Award with a grant date fair value of $5,500,235 (increased from $1,700,177 as President and Chief Operating Officer in fiscal 2022) |
| Thomas Castellano | • Base Salary: Increased by $50,000 to $ 550,000 • MIP: Target opportunity of $450,000, or 82% of salary (target increased from 80% of salary in fiscal 2022) (forfeited upon his departure from Catalent) • LTIP: Award with a grant date fair value of $1,250,101 (increased from $600,166 in fiscal 2022 in his prior role); all fiscal 2023 awards were forfeited upon his departure from Catalent |
| Ricky Hopson | • Base Salary: $380,000 • MIP: $139,500 bonus, equal to 45% of target opportunity of $310,000, or 37% of base salary • LTIP: Award with a grant date fair value of $350,182 (increased from $280,160 in fiscal 2022) • Monthly stipend in the amount of $10,000 for April 2023, $20,000 for May 2023 and $20,000 for June 2023 ($50,000 paid in fiscal 2023), for his service as Interim Chief Financial Officer |
| Steven L. Fasman | The following determinations reflect Mr. Fasman’s transition to Executive Vice President and Chief Administrative Officer in fiscal 2023 • Base Salary: Increased to $625,000 from $600,000 as Senior Vice President, General Counsel, and Corporate Secretary in fiscal 2022 • MIP: $135,000 bonus, equal to 27% of target opportunity of $500,000, or 22% of base salary (target increased from 77% of base salary as Senior Vice President, General Counsel, and Corporate Secretary in fiscal 2022) • LTIP: Award with a grant date fair value of $1,500,246 (increased from $1,000,193 in fiscal 2022) |
| Aristippos Gennadios | The following determinations reflect Mr. Gennadios’ transition to Group President, Pharma and Consumer Health in fiscal 2023 • Base Salary: Increased by $100,000 to $600,000 • MIP: $135,000 bonus, equal to 27% of target opportunity of $500,000, 23% of salary (target increased from 80% of salary as President, Softgel & Oral Technologies in fiscal 2022) • LTIP: Award with a grant date fair value of $1,000,197 (increased from $500,207 in fiscal 2022) • Award of RSUs with a grant date fair value of $2,000,097 granted in July 2022 in connection with his promotion and additional responsibilities |
| John Chiminski | The following determinations reflect Mr. Chiminski’s transition to Executive Chair of the Board in fiscal 2023 • Base Salary: Decreased to $700,000 from $1,075,000 as Chair and CEO in fiscal 2022 • MIP: Zero bonus, equal to 0% of target opportunity of $700,000, or 0% of salary (target decreased from 126% of salary as Chair and CEO in fiscal 2022) • LTIP: Award with a grant date fair value of $4,000,069 (decreased from $9,300,340 in fiscal 2022) |
| Manja Boerman | • Base Salary: Increased by $75,000 to $500,000 • MIP: Zero bonus, equal to 0% of target opportunity of $400,000, or 0% of base salary • LTIP: Award with a grant date fair value of $650,151 (increased from $500,207 in fiscal 2022) • Award of Performance Restricted Stock Units ("PRSUs") with a grant date fair value of $2,000,088 in connection with her expanded responsibilities, which would vest from 0-200% of target based upon the achievement against pre-determined revenue of the BioModalities Division during fiscal 2026 (all outstanding unvested equity-based awards, including the PRSUs, will be cancelled based on the existing terms of the awards, in connection with her termination by mutual consent when such negotiations are complete) |
1 Converted from pounds sterling to U.S. dollars at an exchange rate of 1.3325:1, which represents the average of the monthly rates during fiscal 2022.
Other Compensation Practices and Policies
EXECUTIVE AGREEMENTS
The following is a description of the provisions of employment agreements and offer letters with our NEOs, as in effect during fiscal 2023. In addition, our NEOs have entered into agreements with respect to the long-term incentive grants they have received, the terms of which are described elsewhere in this Annual Report. Severance agreements and arrangements affecting our NEOs are further described in the table entitled "Fiscal 2023 Potential Payments upon Employment Termination or Change of Control Tables" and accompanying notes.
EMPLOYMENT AGREEMENT FOR ALESSANDRO MASELLI
On January 4, 2022, we entered into an employment agreement with Mr. Maselli in connection with his transition to his current position as President and Chief Executive Officer. Effective July 1, 2022, (1) his base salary increased to $925,000, (2) his target cash incentive opportunity under the MIP for fiscal 2023 increased to $1,018,000, and (3) his LTIP grant in respect of fiscal 2023 increased to $5,500,000. The terms also include (a) a one-year employment term commencing July 1, 2022, which automatically extends for successive one-year periods unless either party gives notice of non-renewal at least 60 days before the end of the then-current term, and (b) participation in all group health, life, disability, and other employee benefit and perquisite plans and programs in which our other senior executives generally participate.
Mr. Maselli is subject to a covenant not to (x) compete with us or solicit the business of any client or prospective client while employed and for one year following his termination of employment for any reason or (y) solicit our employees or consultants while employed and for two years following his termination of employment for any reason, in each case subject to certain specified exclusions. The agreement also contains customary confidential information, assignment of intellectual property rights, and indemnification provisions, as well as the severance terms described below under “Fiscal 2023 Potential Payments upon Employment Termination or Change of Control Tables—Severance and Payments on a Change of Control.”
OFFER LETTER FOR THOMAS CASTELLANO
On May 10, 2021, we provided a letter to Mr. Castellano, with an effective date of June 1, 2021, in connection with his appointment as our senior vice president and chief financial officer. The letter set his base salary and MIP target at $500,000 and $400,000, respectively, and provided that he be recommended to receive an LTIP grant for fiscal 2022 of $600,000. On July 27, 2022, we provided a letter to Mr. Castellano that increased his base pay to $550,000, effective July 21, 2022, increased his MIP target to $450,000, and increased his LTIP target for the fiscal 2023-2025 performance period to $1,250,000 for fiscal 2023.
Mr. Castellano ceased serving as Chief Financial Officer effective April 13, 2023 and separated from the Company effective April 21, 2023. For a description of the severance benefits that Mr. Castellano is entitled to receive in connection with his involuntary termination without cause under his pre-existing severance agreement, please see the discussion below under the heading “Severance and Termination Benefits – Mr. Castellano.”
OFFER LETTER FOR RICKY HOPSON
On July 1, 2022, we provided a letter to Mr. Hopson in connection with his promotion to President, Division Head for Clinical Development & Supply. The letter set his base salary and MIP target at $380,000 and $310,000, respectively, and provided that he be recommended to receive an LTIP grant for fiscal 2023 of $350,000.
On May 1, 2023, we provided a letter to Mr. Hopson, with an effective date of April 14, 2023, in connection with his appointment as our Interim Chief Financial Officer. The letter provided that he would be entitled to receive an additional cash stipend of $20,000 per month for the duration of his assignment until such time as the Company hired a permanent Chief Financial Officer, and that all other elements of his existing compensation would remain unchanged.
OFFER LETTER FOR STEVEN L. FASMAN
On March 13, 2018, we provided a letter to Mr. Fasman setting forth certain terms of his employment, with immediate effect. The letter set his base salary and MIP target at $550,000 and $412,500, respectively, and provided that he be recommended to receive an LTIP grant for fiscal 2019 of $650,000. We increased Mr. Fasman’s base salary, effective July 2020, to $600,000. On July 7, 2022, we provided an updated letter to Mr. Fasman in connection with his transition to Executive Vice President and Chief Administrative Officer. Effective July 1, 2022, (1) his base salary increased to $625,000, (2) his target cash incentive opportunity under the MIP for fiscal 2023 increased to $500,000, and (3) his LTIP grant in respect of fiscal 2023 increased to $1,500,000. Mr. Fasman left our company in September 2023 to take another opportunity.
OFFER LETTER FOR ARISTIPPOS GENNADIOS
On March 15, 2018, we provided a letter to Dr. Gennadios setting forth certain terms of his employment, with immediate effect. The letter set his base salary and MIP target at $420,000 and $315,000, respectively, and provided that he be recommended to receive an LTIP grant for fiscal 2019 of $450,000. We increased Dr. Gennadios’s base salary, effective July 2021, to $500,000. On July 7, 2022, we provided an updated letter to Dr. Gennadios in connection with his transition to Group President, Pharma and Consumer Health. Effective July 1, 2022, (1) his base salary increased to $600,000, (2) his target cash incentive opportunity under the MIP for fiscal 2023 increased to $500,000, and (3) his LTIP grant in respect of fiscal 2023 increased to $1,000,000. In addition, Dr. Gennadios received a one-time grant of RSUs vesting three years from the grant date with a grant-date value of $2,000,000.
EMPLOYMENT AGREEMENT OF JOHN CHIMINSKI
As in effect at the beginning of fiscal 2022, Mr. Chiminski’s employment agreement, as amended, provided for a three-year employment term commencing August 23, 2017, which automatically extended for successive one-year periods unless either party gave notice of non-renewal at least 60 days before the end of the then-current term. The terms included (1) an annual base salary of $1,075,000, subject to discretionary increases from time to time, (2) continued participation in our MIP, with a minimum annual target amount of $1,350,000, (3) continued participation in our annual LTIP with a minimum annual target grant value of $9,075,000, and (4) participation in all group health, life, disability, and other employee benefit and perquisite plans and programs in which our other senior executives generally participate. He also received annual reimbursements for the reasonable cost of (1) premiums for an executive life insurance policy (not to exceed $15,000) and (2) financial services/planning (not to exceed $15,000). On January 4, 2022, we entered into an amended and restated one-year employment agreement with Mr. Chiminski in connection with his transition to Executive Chair. Effective July 1, 2022, (1) his annual base salary decreased to $700,000, (2) his target cash incentive opportunity under the MIP for fiscal 2023 decreased to $700,000, and (3) his LTIP grant in respect of fiscal 2023 decreased to $4,000,000 (granted entirely in the form of RSUs vesting one year from the grant date).
Mr. Chiminski is subject to a covenant not to (x) compete with us or solicit the business of any client or prospective client while employed and for one year following his termination of employment for any reason or (y) solicit our employees or consultants while employed and for two years following his termination of employment for any reason, in each case subject to certain specified exclusions. The agreement also contains customary confidential information, assignment of intellectual property rights, and indemnification provisions.
Effective June 30, 2023, Mr. Chiminski retired from the Company. In connection with Mr. Chiminski’s retirement from the Company, all of his then-outstanding equity awards will continue to vest in accordance with the terms of his outstanding award agreements and he continues to be eligible to receive financial planning reimbursements up to $15,000 (per calendar year) for one-year following his departure in accordance with the policy approved by the Compensation Committee for all members of the executive leadership team following their retirement from the Company.
EMPLOYMENT AGREEMENT AND LONG-TERM ASSIGNMENT LETTER FOR MANJA BOERMAN
On October 8, 2019, Dr. Boerman entered into an employment agreement with Catalent Pharma Solutions GmbH, for employment in the Netherlands as Region President, Biologics – EU to commence on January 2, 2020. Effective June 1, 2020, Dr. Boerman was promoted to President, Cell & Gene Therapy, her annual base salary increased to $425,000, her MIP target increased to $340,000, and her LTIP target increased to $500,000 for the fiscal 2021-2023 performance period. She was also granted RSUs valued at $200,000 that would vest 100% on the third anniversary of the grant date. Effective July 21, 2022, Dr. Boerman’s base salary increased to $500,000, her MIP target increased to $400,000 for fiscal year 2023, and her LTIP target for the fiscal 2023-2025 performance period increased to $650,000. In addition, Dr. Boerman received a PRSU incentive grant with a target value of $2,000,000. The actual number of PRSUs that would ultimately vest would range from 0-200% of the target number of shares. The vesting of the PRSU grant and distribution of shares under the grant, if any, would be based on revenue targets for fiscal year 2026 and would occur after the Board approves the Company’s audited consolidated financial statements for that fiscal year. If Dr. Boerman’s employment was terminated before the completion of such revenue determination for any reason other than death or disability, the PRSUs would cease vesting and would be forfeited. On October 10, 2022, we provided Dr. Boerman a long-term international assignment letter setting forth certain terms of her long-term assignment from the Netherlands to the United States. Dr. Boerman was provided a car allowance of €24,000 per year, a cost of living differential of $3,455 per month, a lodging stipend of $6,360 net per month, and was enrolled in an international benefit plan. Dr. Boerman’s assignment-related allowances and benefits are consistent with our standard practices and polices applicable, by location, to employees on long-term assignments. Dr. Boerman’s base salary, MIP and LTIP targets, and other conditions of employment remained unchanged.
Dr. Boerman was removed from her position as President, Division Head for Biomodalities effective as of April 25, 2023, and upon her removal was offered “garden leave” for the entirety of the six months’ notice period under her employment agreement. Dr. Boerman continued to receive her salary through the end of fiscal 2023 while we continued to negotiate the terms of her separation during her period of garden leave. All outstanding unvested equity-based awards granted to Dr. Boerman, including the PRSUs, will be cancelled based upon the existing terms of the awards, in connection with her termination by mutual consent when such negotiations are complete.
EXECUTIVE STOCK OWNERSHIP GUIDELINES
Our executive stock ownership guidelines for our CEO and certain of our executives, including the other NEOs, set a multiple of each executive’s base salary as the amount of qualifying equity to be acquired and held by each executive. In assessing compliance with the guidelines, we count shares held outright, 50% of the value of unvested RSUs (or restricted stock issued in lieu thereof), and 100% of shares held in benefit plans, if any. Shares underlying stock options (vested or unvested) or unearned PSUs do not count toward achievement of the guidelines. Our guidelines by executive level are as follows:
If, on the date of any exercise of an option to purchase our common stock or the delivery of our common stock underlying any vested RSU or PSU, an executive has not reached the minimum ownership level under the guidelines, then the executive should retain and not sell that portion of the delivered shares whose market value is equal to at least 50% of the after-tax market value of all shares delivered on that date. For purposes of complying with this provision of the guidelines, the market value is equal to the average closing price per share of our common stock as reported on the NYSE for all trading days in the last month of the prior fiscal year.
All of our NEOs complied with these guidelines during fiscal 2023 and have remained in compliance through the date of this Annual Report.
HEDGING AND PLEDGING
Our Insider Trading Policy prohibits directors and all of our employees, including our executive officers, from engaging in any transactions that are designed to hedge or offset any decrease in the market value of our securities, including, but not limited to, through the use of financial instruments such as exchange funds, variable forward contracts, equity swaps, puts, calls, and other derivative instruments, or through the establishment of a short position in our securities. Though our Insider Trading Policy allows the pledging by our directors and employees, including our executive officers, of our securities in situations approved by our General Counsel, our current policy and practice is that no such pledging is allowed.
RISK ASSESSMENT OF COMPENSATION PRACTICES AND POLICIES
With the assistance of its independent consultant, the Compensation Committee annually reviews our compensation program from a risk perspective. Based on that review, the Compensation Committee believes that our program is not reasonably likely to have a material adverse effect on us and our shareholders. Our compensation program achieves this by striking an appropriate balance between short-term and long-term incentives, using a diversity of metrics to assess performance and payout under our incentive programs, placing caps on our incentive award payout opportunities, and having stock ownership and retention requirements. For example, our current long-term equity incentive program incorporates our financial performance and stock price into its performance measures and generally magnifies the impact of changes in our stock price as well as Relative Return performance.
Compensation Committee Report
The Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis contained in this Annual Report on Form 10-K. Based on its review and discussions, the Compensation Committee recommended to our Board that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K as filed with the SEC.
Submitted by the Compensation Committee:
Gregory T. Lucier, Chair
Michael J. Barber
J. Martin Carroll
Rolf Classon
Frank D’Amelio
Stephanie Okey
Date: December 6, 2023
Executive Compensation Tables
Fiscal 2023 Summary Compensation Table
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Name and Principal position(1)
| Year
| Salary ($)(2)
| Bonus ($)
| Stock Awards ($)(3)
| Option Awards ($)(4)
| Non-Equity Incentive Plan Compensation ($)(5)
| All Other Compensation ($)(6)
| Total ($)(7)
|
| | | | | | | | |
Alessandro Maselli
| 2023
| 925,000 | -
| 3,850,216 | 1,650,019 | - | 158,437 | 6,583,672 |
President and Chief Executive Officer
| 2022
| 654,183
| -
| 1,190,169
| 510,008
| 733,000
| 146,670
| 3,234,030
|
2021
| 639,689
| -
| 908,287
| 375,022
| 770,144
| 1,866,588
| 4,559,730
|
Thomas Castellano(8)
| 2023
| 443,654 | -
| 875,098 | 375,003 | - | 1,036,228 | 2,729,983 |
Former Senior Vice President and Chief Financial Officer
| 2022
| 500,000
| -
| 420,150
| 180,016
| 548,240
| 22,661
| 1,671,067
|
2021 | 372,949 | - | 964,123 | 82,507 | 337,003 | 21,964 | 1,778,546 |
Ricky Hopson(9)
| 2023
| 380,000 | -
| 245,150 | 105,032 | 139,500 | 102,625 | 972,307 |
President, Division Head for Clinical Development & Supply and Former Interim Chief Financial Officer | | | | | | | | |
| | | | | | | |
| | | | | | | | |
Steven L. Fasman(10)
| 2023
| 625,000 | -
| 1,050,221 | 450,025 | 135,000 | 50,053 | 2,310,299 |
Former Executive Vice President & Chief Administrative Officer | 2022
| 600,000
| -
| 1,200,253
| 300,016
| 644,276
| 54,978
| 2,799,523
|
2021
| 591,313
| -
| 791,744
| 210,008
| 670,036
| 54,504
| 2,317,605
|
Aristippos Gennadios(9)
| 2023
| 600,000 | -
| 2,700,277 | 300,017 | 135,000 | 64,263 | 3,799,557 |
Group President, Pharma and Consumer Health
| 2022
| 485,769
| -
| 850,275
| 150,008
| 572,240
| 67,212
| 2,125,504
|
| | | | | | | |
| | | | | | | | |
John Chiminski
| 2023
| 700,000 | -
| 4,000,069 | - | - | 107,698 | 4,807,767 |
Former Executive Chair
| 2022
| 1,075,000
| -
| 6,510,335
| 2,790,005
| 1,890,810
| 141,367
| 12,407,517
|
2021
| 1,052,569
| -
| 6,689,674
| 2,722,522
| 2,000,000
| 116,374
| 12,581,139
|
Manja Boerman(9) (11)
| 2023
| 511,387 | -
| 2,455,217 | 195,022 | - | 818,262 | 3,979,888 |
Former President, Division Head for Biomodalities | | | | | | | | |
| | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
(1) As of June 30, 2023. Mr. Castellano ceased serving as Chief Financial Officer on April 13, 2023 and separated from the Company on April 21, 2023. Mr. Hopson assumed the additional role of Interim Chief Financial Officer effective as of April 14, 2023 until Matti Masanovich was appointed as Senior Vice President and Chief Financial Officer effective as of July 5, 2023. Following Mr. Masanovich’s appointment, Mr. Hopson returned to his previous role as President, Division Head of Clinical Development and Supply. Effective June 30, 2023, Mr. Chiminski retired from the Company. Dr. Boerman served as President, Division Head for Biomodalities until April 24, 2023, and, upon her removal from that position, was offered “garden leave” for the entirety of the six months’ notice period under her employment agreement. Compensation changes related to these transitions are described in the CD&A in the section entitled “Executive Agreements.”
(2) Values reflect the amounts paid to the NEOs for each fiscal year reported. Amounts reported include the portion, if any, of base salary each NEO elected to defer under the Deferred Compensation Plan, as applicable. The values reported for Mr. Maselli during fiscal years 2022 and 2021 include a portion of his annual base salary rate expressed in U.K. pounds sterling that was converted to and paid in U.S. dollars, based on average monthly currency exchange rates applicable at the time of payment, in connection with his relocation to the United States. The value reported for Dr. Boerman reflects her U.S. dollar denominated salary, a portion of which is allocated to a statutorily-required holiday allowance in the Netherlands, expressed in Euros based on average monthly currency exchange rates. Dr. Boerman continued to receive her salary through the end of fiscal 2023 while we continued to negotiate the terms of her separation, during her period of garden leave. Please see the CD&A for additional details of changes to the salaries of the NEOs during fiscal year 2023, as applicable.
(3) Represents the aggregate grant date fair value of stock awards for fiscal years 2023, 2022, and 2021 computed in accordance with FASB ASC Topic 718, using the assumptions discussed in Note 14, “Stock-Based Compensation,” to the consolidated financial statements included in this Annual Report. The amounts reported in this column assume, in accordance with FASB ASC Topic 718, that the NEOs will receive or retain the target number of PSUs awarded to them in each such fiscal year. All of our NEOs, other than Mr. Chiminski, received PSUs during fiscal year 2023. If, instead, the performance during the 2023-25 performance period is such that the NEOs receive or retain the maximum number capable of being awarded (200% of target for Adjusted EPS and 150% of target for Relative Return PSUs), the value of the PSU grants for 2023 would be as follows:
| | | | | | | | | | | | | | |
| Name | | | | ASC Topic 718 Value at Maximum ($) |
| Alessandro Maselli | | | | 4,812,785 | |
| Thomas Castellano | | | | 1,093,889 | |
| Ricky Hopson | | | | 306,425 | |
| Steven L. Fasman | | | | 1,312,769 | |
| Aristippos Gennadios | | | | 875,206 | |
| Manja Boerman | | | | 568,979 | |
Relative Return PSUs are subject to market conditions, as opposed to performance conditions, and therefore do not have maximum grant date fair values that differ from the grant date fair values under FASB ASC Topic 718. The actual value of the PSUs, if any, that ultimately convert to shares of our common stock or are no longer subject to forfeiture, respectively, on the vesting dates will depend on (x) our share price on such dates and (y) our performance according to the applicable performance criteria.
The amount reported for Dr. Gennadios for fiscal 2023 include RSUs with a grant date fair value of $2,000,097 granted on July 1, 2022 in connection with his promotion to Group President, Pharma and Consumer Health. The amount reported for Mr. Chiminski for fiscal 2023 represents RSUs granted in connection with his role as Executive Chair. The amount reported for Dr. Boerman for fiscal 2023 includes PRSUs with a grant date target value of $2,000,088 granted on July 26, 2022. Under the terms of the award to Dr. Boerman, the actual number of PRSUs that will become payable can range from 0% to a maximum 200% of target ($4,000,176 at maximum), based on the future net revenue achievement of the BioModalities division during fiscal 2026.
(4) Reflects nonqualified stock options to acquire shares of our common stock. Amounts reported reflect the aggregate grant date fair value computed in accordance with FASB ASC Topic 718 using the assumptions discussed in Note 14, “Stock-Based Compensation,” to the consolidated financial statements included in this Annual Report.
(5) Amounts reported reflect the MIP awards earned by our NEOs, which includes the portion of the MIP award, if any, each NEO elected to defer under the Deferred Compensation Plan, as applicable. Amounts reported for Mr. Maselli in fiscal 2021 and 2022 were denominated in U.K. pounds sterling and converted to U.S. dollars (as well as paid in U.S. dollars for fiscal 2021 and 2022) based on the average monthly currency exchange rates applicable to annual bonus payments in each period.
(6) The amounts set forth as “All Other Compensation” for fiscal 2023 are further detailed below:
| | | | | | | | | | | | | | | | | | | | | | | |
Name | Employer 401(k) Matching Contributions ($)(A) | Employer Non - Qualified Deferred Compensation Matching Contributions ($)(B) | Assignment- Related Allowances & Benefits ($)(C) | Financial Services Reimbursement ($)(D) | Severance Benefits ($)(E) | Other ($)(F) | Total ($) |
| | | | | | | |
Alessandro Maselli | - | - | 139,104 | 19,333 | - | - | 158,437 |
Thomas Castellano | 10,831 | - | - | - | 1,025,397 | - | 1,036,228 |
Ricky Hopson | 12,800 | 23,386 | - | 16,439 | - | 50,000 | 102,625 |
| | | | | | | |
Steven L. Fasman | 12,700 | 18,724 | - | 16,629 | - | 2,000 | 50,053 |
| | | | | | | |
Aristippos Gennadios | 14,200 | 35,063 | - | 15,000 | - | - | 64,263 |
John Chiminski | 10,044 | 78,114 | - | 10,765 | - | 8,775 | 107,698 |
| | | | | | | |
Manja Boerman | - | - | 818,262 | - | - | - | 818,262 |
(A) Under our 401(k) Savings Plan, we match up to a maximum 4% of annual compensation contributed by participants, up to federal tax law limits on both eligible compensation and individual contributions.
(B) Represents company contributions under our Deferred Compensation Plans, representing 50% of each participant’s contribution up to the first 6% of eligible pay that such participant contributes to the plan, up to any applicable limit.
(C) Mr. Maselli received certain tax equalization benefits during fiscal 2023 in connection with his relocation from the United Kingdom and long-term assignment in the United States prior to fiscal 2023, resulting primarily from timing differences between the determination and payment of U.S. and U.K. taxes across multiple tax years. Such benefits are consistent with our standard policies and practices applicable, by location, to employees on long-term assignments.
Dr. Boerman received certain benefits, including tax equalization, during fiscal year 2023 in connection with the start of her long-term assignment from the Netherlands to the U.S. in October 2022. Such benefits are consistent with our standard policies and practices applicable, by location, to employees on long-term assignments. The amount reported in this column for Dr. Boerman comprises the following: allowances through April 2023 for housing, car and cost of living in the amounts of $32,778, $20,958, and $21,643, respectively; a $74,219 pension allowance – Dr. Boerman did not participate in any formal pension scheme in fiscal 2023; $40,896 for relocation expenses; and aggregate tax equalization benefits and accompanying tax gross-ups paid by us of $562,616. The amounts reported in this column for Dr. Boerman also include allowances paid during May 2023 and June 2023 while we were continuing to negotiate the terms of her separation for car, cost of living, housing and pension in the amounts of $4,192, $6,378, $11,742 and $15,105, respectively. Dr. Boerman became eligible for health care coverage in the U.S. effective September 1, 2022. The amount reported includes the U.S. employer health benefit cost during fiscal 2023 in the amount of $27,735, including costs paid prior to the start of her assignment in October 2022 and while negotiating the terms of her separation (during May 2023 and June 2023) in the amount of $8,367. Amounts reported in this column include certain benefits that were paid in Euros for Dr. Boerman converted to U.S. dollars using an exchange rate of 1.0479:1, which represents the average of the monthly rates during fiscal 2023.
(D) Each of the NEOs, pursuant to the terms of an employment agreement or otherwise, is entitled to services, which may be submitted in the form of a reimbursement, for the reasonable cost of financial services/planning, subject to an aggregate cap of $15,000 during each calendar year. The amounts reported in each fiscal year may differ from this cap due to timing differences between each fiscal year and calendar year. During fiscal 2023, Messrs. Maselli, Hopson, and Fasman received financial services/planning services in the amounts of $19,333, $16,439, and $16,629, respectively, applicable to calendar years 2022 and 2023. During fiscal 2023, Dr. Gennadios and Mr. Chiminski received financial services/planning reimbursements totaling $15,000 and $10,765, respectively, applicable to calendar years 2022 and 2023. The amount reported in this column for Mr. Maselli includes $2,704 for tax preparation services paid in connection with his long-term assignment in the U.S. prior to fiscal 2023.
(E) The amount reported for Mr. Castellano includes a severance benefit in the amount of $1,000,012 that will be paid over a one-year period following his separation from Catalent on April 21, 2023 and a one-time payment of $25,385 representing unused paid-time-off for fiscal 2023.
(F) The amount reported for Mr. Hopson includes an aggregate stipend of $50,000 paid in connection with Mr. Hopson’s services as Interim Chief Financial Officer from April through June 2023. The amount reported for Mr. Fasman represents contributions we made under our Catalent Cares matching gift program. Mr. Chiminski’s employment agreement entitled him each calendar year during the employment term to be reimbursed for the reasonable cost of premiums for an executive life insurance policy, subject to an aggregate cap of $15,000 each such year. For fiscal 2023, Mr. Chiminski received a reimbursement in the amount of $8,775. From time to time, family members of executives may accompany them on a business-related flight aboard a private aircraft. There is no incremental cost to the Company, and therefore no incremental costs are reflected in the amounts above, for the use of such flights by family members of executives.
(7) We have not included columns reporting any amount as “Change in Pension Value and Nonqualified Deferred Compensation Earnings” because none of our NEOs received or earned any above-market or preferential earnings during fiscal 2021 to 2023.
(8) The grants awarded to Mr. Castellano in fiscal 2023 were cancelled in accordance with their terms when his employment ended on April 21, 2023.
(9) Mr. Hopson, Dr. Gennadios, and Dr. Boerman did not qualify as NEOs in one or more previous years. Accordingly, disclosure of their compensation for such prior years is not required.
(10) The grants awarded to Mr. Fasman in fiscal 2023 were cancelled in accordance with their terms when his employment ended on September 13, 2023.
(11) All outstanding unvested equity-based awards granted to Dr. Boerman, including the awards granted during fiscal 2023 and shown in the table above, will be cancelled in accordance with their terms upon her termination by mutual consent when such negotiations are complete.
Fiscal 2023 Grants of Plan-Based Awards Table
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Estimated Possible Payouts Under Non-Equity Incentive Plan Awards (1) | | Estimated Future Payments Under Non-Equity Incentive Plan Awards (2) | All Other Stock Awards: Number of Shares of Stock or Units (3) (#) | All Other Option Awards: Number of Securities Underlying Options (4) (#) | Exercise or Base
Price of Option Awards
($/Sh) | Grant Date Fair Value of Stock and Option Awards (5) ($) |
| Name | Grant Date | Threshold ($) | Target
($) | Max
($) | | Threshold (#) | Target
(#) | Max
(#) |
| Alessandro Maselli | | 228,032 | | 1,018,000 | | 2,036,000 | | | — | | — | | — | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | — | | 44,427 | | 107.63 | | 1,650,019 | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | 10,221 | | — | | — | | 1,100,086 | |
| 7/26/2022 | — | | — | | — | | | 6,388 | | 12,776 | | 25,552 | | — | | — | | — | | 1,375,081 | |
| 7/26/2022 | — | | — | | — | | | 6,948 | | 13,895 | | 20,843 | | — | | — | | — | | 1,375,049 | |
Thomas Castellano (6) | | 100,800 | | 450,000 | | 900,000 | | | — | | — | | — | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | — | | 10,097 | | 107.63 | | 375,003 | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | 2,323 | | — | | — | | 250,024 | |
| 7/26/2022 | — | | — | | — | | | 1,452 | | 2,904 | | 5,808 | | — | | — | | — | | 312,558 | |
| 7/26/2022 | — | | — | | — | | | 1,579 | | 3,158 | | 4,737 | | — | | — | | — | | 312,516 | |
| Ricky Hopson | | 69,440 | | 310,000 | | 620,000 | | | — | | — | | — | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | — | | 2,828 | | 107.63 | | 105,032 | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | 651 | | — | | — | | 70,067 | |
| 7/26/2022 | — | | — | | — | | | 407 | | 813 | | 1,626 | | — | | — | | — | | 87,503 | |
| 7/26/2022 | — | | — | | — | | | 443 | | 885 | | 1,328 | | — | | — | | — | | 87,580 | |
Steven L. Fasman (7) | | 112,000 | | 500,000 | | 1,000,000 | | | — | | — | | — | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | — | | 12,117 | | 107.63 | | 450,025 | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | 2,788 | | — | | — | | 300,072 | |
| 7/26/2022 | — | | — | | — | | | 1,743 | | 3,485 | | 6,970 | | — | | — | | — | | 375,091 | |
| 7/26/2022 | — | | — | | — | | | 1,895 | | 3,790 | | 5,685 | | — | | — | | — | | 375,058 | |
| Aristippos Gennadios | | 112,000 | | 500,000 | | 1,000,000 | | | — | | — | | — | | — | | — | | — | | — | |
| 7/01/2022 | — | | — | | — | | | — | | — | | — | | 18,689 | | — | | — | | 2,000,097 | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | — | | 8,078 | | 107.63 | | 300,017 | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | 1,859 | | — | | — | | 200,084 | |
| 7/26/2022 | — | | — | | — | | | 1,162 | | 2,323 | | 4,646 | | — | | — | | — | | 250,024 | |
| 7/26/2022 | — | | — | | — | | | 1,264 | | 2,527 | | 3,791 | | — | | — | | — | | 250,072 | |
| John Chiminski | | 156,800 | | 700,000 | | 1,400,000 | | | — | | — | | — | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | 37,165 | | — | | — | | 4,000,069 | |
Manja Boerman (8) | | 89,600 | | 400,000 | | 800,000 | | | — | | — | | — | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | — | | 5,251 | | 107.63 | | 195,022 | |
| 7/26/2022 | — | | — | | — | | | — | | — | | — | | 1,208 | | — | | — | | 130,017 | |
| 7/26/2022 | — | | — | | — | | | 755 | | 1,510 | | 3,020 | | — | | — | | — | | 162,521 | |
| 7/26/2022 | — | | — | | — | | | 822 | | 1,643 | | 2,465 | | — | | — | | — | | 162,591 | |
| 7/26/2022 | — | | — | | — | | | 9,292 | | 18,583 | | 37,166 | | — | | — | | — | | 2,000,088 | |
(1) For each NEO, represents potential cash payments for fiscal 2023 under our MIP. See the section in our CD&A entitled “Details of Total Direct Compensation Elements—Management Incentive Plan” for a further description of our MIP.
(2) The amounts shown reflect PSUs, and for Dr. Boerman, PRSUs, granted to the NEOs during fiscal 2023. In fiscal 2023, the Compensation Committee continued to set the performance metrics for the PSUs awarded under our LTIP using Adjusted EPS and Relative Return, each of which will apply to 50% of the total PSU value awarded, as reflected in the table above. The final number of PSUs can range from 0-200% of the target number of Adjusted EPS PSUs and 0-150% of the target number of Relative Return PSUs, depending on our achievement against each relevant performance metric established by the Compensation Committee at the beginning of the performance period. The final number of PRSUs granted to Dr. Boerman can range from 0 to 200% of the target based on the future Net Revenue achievement of the BioModalities Division during fiscal 2026. See the section in our CD&A entitled “Details of Total Direct Compensation Elements—Long-Term Incentive Awards” for a further description of our long-term incentive compensation program.
(3) Represents RSUs granted to the NEOs during fiscal 2023. Each NEO received RSUs on July 26, 2022 under our LTIP as their fiscal 2023 annual grant. Dr. Gennadios received an additional RSU award on July 1, 2022 in connection with his promotion to Group President, Pharma & Consumer Health. The vesting and settlement terms of the RSUs are described in more detail in the section in our CD&A entitled “Details of Total Direct Compensation Elements—Long-Term Incentive Awards.”
(4) Represents nonqualified stock options granted during fiscal 2023 under our LTIP. Stock options have an exercise price based on the closing price per share of our common stock on the date of grant, as reported on the NYSE. Each NEO, except for Mr. Chiminski, was granted stock options on July 26, 2022 under our LTIP as their fiscal 2023 annual grant. See the section in our CD&A entitled “Details of Total Direct Compensation Elements—Long-Term Incentive Awards” for a further description of our stock option grants.
(5) The values of equity-based grants presented in this table were calculated in accordance with FASB ASC Topic 718 using the assumptions discussed in Note 14, “Stock-Based Compensation,” to the consolidated financial statements included in this Annual Report. The stock price used in each calculation is the closing price per share of our common stock on each respective grant date, as reported on the NYSE. The values of the Adjusted EPS PSU grants reported in this column assume that the awards will vest at their target amounts.
(6) The grants awarded to Mr. Castellano in fiscal 2023 were cancelled in accordance with their terms when his employment ended on April 21, 2023, which also made him ineligible for a bonus under our MIP for fiscal 2023.
(7) The grants awarded to Mr. Fasman in fiscal 2023 were cancelled in accordance with their terms when his employment ended on September 13, 2023.
(8) All outstanding unvested equity-based awards granted to Dr. Boerman, including the awards granted during fiscal 2023 and shown in the table above, will be cancelled in accordance with their terms upon her termination by mutual consent when such negotiations are complete.
Fiscal 2023 Outstanding Equity Awards at Year-End Table
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Option Awards (1) | | Stock Awards |
| Name | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | Option Exercise Price ($)
(e) | Option Expiration Date (2) (f) | | Number of Shares or Units of Stock That Have Not Vested (#) (3) (g) | Market Value of Shares or Units of Stock That Have Not Vested ($) (4) (h) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (5) (i) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) (4) (5) (J) |
| Alessandro Maselli | 7/26/2022 | — | | 44,427 | | 107.63 | | 7/26/2032 | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | 10,221 | | 443,183 | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 6,388 | | 276,984 | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 6,948 | | 301,265 | |
| 7/26/2021 | 3,892 | | 11,676 | | 113.00 | | 7/26/2031 | | — | | — | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | 3,009 | | 130,470 | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 1,881 | | 81,560 | |
| 7/26/2021 | — | | — | | — | | | | — | | — | | 1,949 | | 84,509 | |
| 7/30/2020 | 7,696 | | 7,699 | | 88.10 | | 7/30/2030 | | — | | — | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 2,838 | | 123,056 | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 3,761 | | 163,077 | | — | | — | |
| 7/22/2019 | 10,299 | | 3,436 | | 54.94 | | 7/22/2029 | | — | | — | | — | | — | |
| 7/23/2018 | 10,523 | | — | | 43.88 | | 7/23/2028 | | — | | — | | — | | — | |
| 7/24/2017 | 10,375 | | — | | 36.02 | | 7/24/2027 | | — | | — | | — | | — | |
| 9/08/2016 | 11,093 | | — | | 23.89 | | 9/8/2026 | | — | | — | | — | | — | |
Thomas Castellano (6) | 7/26/2021 | 1,373 | | — | | 113.00 | | 7/26/2031 | | — | | — | | — | | — | |
| 7/30/2020 | 1,692 | | — | | 88.10 | | 7/30/2030 | | — | | — | | — | | — | |
| 7/22/2019 | 2,698 | | — | | 54.94 | | 7/22/2029 | | — | | — | | — | | — | |
| 7/23/2018 | 2,806 | | — | | 43.88 | | 7/23/2028 | | — | | — | | — | | — | |
| 7/24/2017 | 1,730 | | — | | 36.02 | | 7/24/2027 | | — | | — | | — | | — | |
| Ricky Hopson | 7/26/2022 | — | | 2,828 | | 107.63 | | 7/26/2032 | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | 651 | | 28,227 | | — | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 407 | | 17,648 | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 443 | | 19,208 | |
| 7/26/2021 | 641 | | 1,924 | | 113.00 | | 7/26/2031 | | — | | — | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | 496 | | 21,507 | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 310 | | 13,442 | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 321 | | 13,919 | |
| 6/01/2021 | — | | — | | — | | — | | | 3,432 | | 148,812 | | — | | — | |
| 7/30/2020 | 1,526 | | 1,529 | | 88.10 | | 7/30/2020 | | — | | — | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 563 | | 24,412 | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 747 | | 32,390 | | — | | — | |
| 7/22/2019 | 2,432 | | 1,218 | | 54.94 | | 7/22/2029 | | — | | — | | — | | — | |
| 7/23/2018 | 2,622 | | — | | 43.88 | | 7/23/2028 | | — | | — | | — | | — | |
| 7/24/2017 | 1,550 | | — | | 36.02 | | 7/24/2027 | | — | | — | | — | | — | |
Steven L. Fasman (7) | 7/26/2022 | — | | 12,117 | | 107.63 | | 7/26/2032 | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | 2,788 | | 120,888 | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 1,743 | | 75,576 | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 1,895 | | 82,167 | |
| 1/03/2022 | — | | — | | — | | — | | | 4,017 | | 174,177 | | — | | — | |
| 7/26/2021 | 2,289 | | 6,869 | | 113.00 | | 7/26/2031 | | — | | — | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | 1,770 | | 76,747 | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 1,107 | | 48,000 | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 1,147 | | 49,734 | |
| 7/30/2020 | 2,155 | | 4,311 | | 88.10 | | 7/30/2030 | | — | | — | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 1,590 | | 68,942 | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 2,838 | | 123,056 | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 2,107 | | 91,360 | | — | | — | |
| 7/22/2019 | 3,311 | | 3,311 | | 54.94 | | 7/22/2029 | | — | | — | | — | | — | |
| 7/23/2018 | 3,802 | | — | | 43.88 | | 7/23/2028 | | — | | — | | — | | — | |
| Aristippos Gennadios | 7/26/2022 | — | | 8,078 | | 107.63 | | 7/26/2032 | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | 1,859 | | 80,606 | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 1,162 | | 50,384 | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 1,264 | | 54,807 | |
| 7/01/2022 | — | | — | | — | | — | | | 18,689 | | 810,355 | | — | | — | |
| 1/03/2022 | — | | — | | — | | — | | | 4,017 | | 174,177 | | — | | — | |
| 7/26/2021 | 1,144 | | 3,435 | | 113.00 | | 7/26/2031 | | — | | — | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | 885 | | 38,374 | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 554 | | 24,021 | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 574 | | 24,889 | |
| 7/30/2020 | 3,078 | | 3,080 | | 88.10 | | 7/30/2030 | | — | | — | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 1,136 | | 49,257 | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 1,505 | | 65,257 | | — | | — | |
| 7/22/2019 | 6,621 | | 2,209 | | 54.94 | | 7/22/2029 | | — | | — | | — | | — | |
| 7/23/2018 | 10,523 | | — | | 43.88 | | 7/23/2028 | | — | | — | | — | | — | |
| 7/24/2017 | 3,243 | | — | | 36.02 | | 7/24/2027 | | — | | — | | — | | — | |
| John Chiminski | 7/26/2022 | — | | — | | — | | — | | | 37,165 | | 1,611,474 | | — | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 7/26/2021 | 21,291 | | 63,874 | | 113.00 | | 7/26/2031 | | — | | — | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | 16,461 | | 713,749 | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 6,859 | | 297,406 | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 7,107 | | 308,160 | |
| 7/30/2020 | 55,880 | | 55,882 | | 88.10 | | 7/30/2030 | | — | | — | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 20,602 | | 893,303 | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 27,298 | | 1,183,641 | | — | | — | |
| 7/22/2019 | 64,748 | | 32,375 | | 54.94 | | 7/22/2029 | | — | | — | | — | | — | |
| 7/23/2018 | 34,638 | | — | | 43.88 | | 7/23/2028 | | — | | — | | — | | — | |
Manja Boerman (8) | 7/26/2022 | — | | 5,251 | | 107.63 | | 7/26/2032 | | — | | — | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | 1,208 | | 52,379 | | — | | — | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 755 | | 32,737 | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 822 | | 35,642 | |
| 7/26/2022 | — | | — | | — | | — | | | — | | — | | 18,583 | | 805,759 | |
| 1/03/2022 | — | | — | | — | | — | | | 4,017 | | 174,177 | | — | | — | |
| 7/26/2021 | 1,144 | | 3,435 | | 113.00 | | 7/26/2031 | | — | | — | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | 885 | | 38,374 | | — | | — | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 554 | | 24,021 | |
| 7/26/2021 | — | | — | | — | | — | | | — | | — | | 574 | | 24,889 | |
| 7/30/2020 | 3,078 | | 3,080 | | 88.10 | | 7/30/2030 | | — | | — | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 1,136 | | 49,257 | | — | | — | |
| 7/30/2020 | — | | — | | — | | — | | | 1,505 | | 67,257 | | — | | — | |
| 12/02/2019 | 6,333 | | 2,112 | | 51.43 | | 12/2/2029 | | — | | — | | — | | — | |
(1) Unvested outstanding time-based options are scheduled to vest on the applicable anniversaries of the respective grant dates. Options granted prior to fiscal 2023 for which a portion vested during fiscal 2023 are as follows: options granted July 23, 2018 – 25% on each of July 23, 2019, 2020, 2021 and 2022; options granted July 22, 2019 – 25% on each of July 22, 2020, 2021 and 2022; options granted July 30, 2020 – 25% on each of July 30, 2021 and 2022; options granted July 26, 2021 – 25% on July 26, 2022. All other options shown above were fully vested prior to the start of fiscal 2023. As described in the section of this Annual Report entitled “Fiscal 2023 Potential Payments Upon Employment Termination or Change in Control Tables,” the vesting of all or a portion of each option grant may potentially differ from the normal vesting schedule due to a change of control of our company or certain terminations of employment.
(2) The expiration dates shown represent the 10-year anniversary of each respective grant date. Options may terminate earlier under certain circumstances, such as in connection with an NEO’s termination of employment or in connection with certain corporate transactions, including a change of control of our company.
(3) The amounts shown for all of our NEOs include RSUs scheduled to vest on July 30, 2023, July 26, 2024 and July 26, 2025, with additional RSUs granted to the following NEOs as follows: Mr. Hopson – June 1, 2024; Mr. Fasman – July 30, 2023 and January 3, 2025; Dr. Gennadios – January 3, 2025 and July 1, 2025; Dr. Boerman – January 3, 2025. Unvested RSUs are scheduled to vest on the third anniversary of each respective grant date. The amounts shown also include PSUs granted on July 30, 2020 that were earned as of the end of the three-year performance period ending on June 30, 2023 and vested on December 8, 2023, the date the Compensation Committee certified the attainment of actual performance levels achieved relative to the pre-determined Adjusted EPS performance targets. No portion of the PSUs granted on July 30, 2020 were earned based on performance relative to pre-determined Relative Return performance targets. As described in the section of this Annual Report entitled “Fiscal 2023 Potential Payments Upon Employment Termination or Change in Control,” all or a portion of the RSUs or PSUs may vest earlier in connection with a change of control of our company or certain terminations of employment.
(4) Shares/units are valued based on the $43.36 closing price per share of our common stock on June 30, 2023, as reported on the NYSE.
(5) The number of shares and payout values reported include PSUs based on achieving the threshold payout percentages, Adjusted EPS PSUs and Relative Return PSUs that vest at the end of the respective three-year performance periods ending on June 30, 2024 and June 30, 2025. Due to Mr. Chiminski’s retirement on June 30, 2023, the number and payout values reported for his July 26, 2021 PSUs represent the pro-rated number of units outstanding for the time he was an active employee during the three-year performance period. The number and payout values reported for Dr. Boerman also include PRSUs granted on July 26, 2022 based on achieving the target payout percentage. Actual PSU payout levels will be determined by the Compensation Committee following the end of each applicable three-year performance period beginning with the fiscal year in which such grant is made, based on actual performance levels achieved relative to the pre-determined performance targets. The actual payout level of PRSUs granted to Dr. Boerman are designed to reflect actual performance of the BioModalities Division during fiscal 2026 relative to the pre-determined performance target. However, all outstanding unvested equity-based awards granted to Dr. Boerman, including the awards granted during fiscal 2023, will be cancelled in accordance with their terms upon her termination by mutual consent when such negotiations are complete.
(6) Mr. Castellano’s employment ended on April 21, 2023. As a result of his departure, all of his outstanding unvested awards were immediately forfeited. In addition, Mr. Castellano had the right to exercise all of his 10,299 vested stock options within 90 days of his departure.
(7) Mr. Fasman’s employment ended on September 13, 2023. As a result of his departure, all of his outstanding unvested awards were immediately forfeited. In addition, Mr. Fasman has the right to exercise all vested stock options within 90 days of his departure.
(8) Dr. Boerman was offered “garden leave” for the entirety of the six months’ notice period under her employment agreement while we continue to negotiate the terms of her separation. All of her outstanding unvested awards will be forfeited based on the existing terms of the awards, in connection with her termination by mutual consent when such negotiations are complete. Dr. Boerman will have the right to exercise all vested stock options within 90 days of her separation.
Fiscal 2023 Option Exercises and Stock Vested Table
| | | | | | | | | | | | | | |
| Option Awards | Stock Awards |
| Name | Number of
Shares
Acquired on
Exercise
(#) | Value Realized
on Exercise
($) | Number of Shares Acquired on Vesting (#) (1) | Value Realized on Vesting ($) (2) |
| Alessandro Maselli | — | | — | | 13,081 | | 1,355,829 | |
| Thomas Castellano | — | | — | | 5,141 | | 532,859 | |
| Ricky Hopson | — | | — | | 6,256 | | 560,623 | |
| Steven L. Fasman | — | | — | | 17,164 | | 1,797,055 | |
| Aristippos Gennadios | — | | — | | 8,410 | | 871,687 | |
| John Chiminski | — | | — | | 123,302 | | 12,780,095 | |
| Manja Boerman | — | | — | | 11,136 | | 912,408 | |
(1) Represents the vesting during fiscal 2023 of RSU and PSU grants awarded for the fiscal 2020-22 LTIP performance period. In addition to this vesting, the following awards also vested: Mr. Hopson’s January 22, 2020 retention grant of 1,620 RSUs, which vested on January 22, 2023; Mr. Fasman’s July 22, 2019 recognition-related award of 4,551 RSUs which vested on July 22, 2022; and Dr. Boerman’s April 29, 2020 promotion-related grant of 2,904 RSUs which vested on April 29, 2023.
(2) Value realized reflects (i) the closing price per share of our common stock on the vesting date, multiplied by (ii) the number of RSUs or PSUs, as applicable, that vested.
Fiscal 2023 Nonqualified Deferred Compensation Table
| | | | | | | | | | | | | | | | | |
| Name | Executive Contributions in Last FY ($) (1) | Registrant Contributions in Last FY ($) (2) | Aggregate Earnings in Last FY ($) (3) | Aggregate Withdrawals/ Distributions ($) | Aggregate Balance at Last FYE ($) (4) |
Alessandro Maselli (5) | — | | — | | — | | — | | — | |
| Thomas Castellano | — | | — | | — | | — | | — | |
| Ricky Hopson | 123,700 | | 23,386 | | 60,360 | | — | | 593,774 | |
| Steven L. Fasman | 37,448 | | 18,724 | | 28,532 | | — | | 327,706 | |
| Aristippos Gennadios | 70,127 | | 35,063 | | 151,802 | | — | | 1,271,889 | |
| John Chiminski | 911,327 | | 78,114 | | 731,608 | | — | | 7,999,846 | |
| Manja Boerman | — | | — | | — | | — | | — | |
(1) Represents (a) salary deferrals during fiscal 2023, included in the amounts reported, as applicable, for fiscal 2023 under “Salary” in the Summary Compensation Table and (b) fiscal 2022 bonus deferrals that otherwise would have been payable during fiscal 2023, included in the amounts reported in the Summary Compensation Table for fiscal 2022, as applicable, under “Non-Equity Incentive Plan Compensation.” Each NEO’s deferral amount during fiscal 2023 is summarized below.
| | | | | | | | | | | | | | |
| Name | | Fiscal 2022 Bonus Deferral
($) | Fiscal 2023 Salary Deferral
($) |
| Alessandro Masselli | | — | | — | |
| Thomas Castellano | | — | | — | |
| Ricky Hopson | | 80,114 | | 43,586 | |
| Steven L. Fasman | | — | | 37,448 | |
| Aristippos Gennadios | | 34,334 | | 35,793 | |
| John Chiminski | | 661,784 | | 249,543 | |
| Manja Boerman | | — | | — | |
(2) The amounts reported for Messrs. Hopson, Fasman, Chiminski and Dr. Gennadios are reported as compensation for fiscal 2023 under “All Other Compensation” in the Summary Compensation Table.
(3) The amounts reported in this column are not above-market or preferential earnings thus not reportable in the Summary Compensation Table.
(4) Includes amounts previously reported as compensation in the “Salary,” “Non-Equity Incentive Plan Compensation,” and “All Other Compensation” columns in the Summary Compensation Table in prior years.
(5) Messrs. Maselli and Castellano did not participate in the Deferred Compensation Plan during fiscal 2023. Dr. Boerman was ineligible to participate in our U.S.-based plan as she was an expatriate employee during fiscal 2023.
Deferred Compensation
We provide certain of our U.S.- and U.K.-based executives, including our U.S.- and U.K.-based NEOs, with the opportunity to participate in the Deferred Compensation Plan, which allows participating executives to defer receipt of a portion of their compensation. Deferrals occur and may be invested notionally on a pre-tax basis, in addition to the amounts that the executive is allowed to contribute to our tax-qualified 401(k) and U.K. pension plans.
Deferred Compensation Plan participants may elect to defer up to 80% of base salary, commissions (not applicable to NEOs), and MIP bonus. In addition, U.S.-based executives may elect to defer their PSU and RSU grants. We credit the first 6% of cash compensation deferred with a matching contribution equal to 50% of the amount deferred. Participants are immediately vested in all amounts they contribute and the related investment gains, but matching contributions and their related investment gains vest ratably over the participant’s first four years of service to the Company. Participants in the Deferred Compensation Plan may elect from a variety of payout options under the plan, including lump-sum or installment payments, with the timing depending on the form selected at the time of the deferral election.
Under the Deferred Compensation Plan, we also credit each participant’s deferral account with notional earnings and/or losses based on the deemed investment of the accounts in one or more of a variety of investment alternatives available under the plan. Participants are able to make changes to their investment elections on a daily basis.
The accounts of U.S.-based participants in the prior version of the Deferred Compensation Plan that are paid out in a lump-sum cash payment are paid on the 15th day of the month immediately following the month that includes the six-month anniversary of the participant’s separation from our service (other than due to death) (“separation” as defined by Section 409A of the Internal Revenue Code). In the event of the death of a participant prior to the commencement of the distribution of benefits under the plan, such benefits will be paid no later than the later of (x) December 31 of the year in which the participant’s death occurs and (y) the 90th day following the date of the participant’s death. The accounts for U.K.-based participants are paid in a lump sum cash payment in the next available paycheck following the elected distribution date.
A U.S.-based participant in the Deferred Compensation Plan may also elect to receive a payout in annual installments over a period of five or ten years after the participant’s separation from service (including death), although, notwithstanding any such election, the participant’s account will be paid in a lump-sum cash payment in connection with a participant’s separation from service within two years following a change of control. The Deferred Compensation Plan also permits participants to receive a distribution in connection with an unforeseeable emergency, in accordance with the requirements of Section 409A of the Internal Revenue Code.
A U.K.-based participant receives a lump sum payout of all outstanding cash deferrals six months after the participant’s separation from service.
Cash and equity deferrals, employer contributions, and applicable gains are held in a “rabbi trust.” Rabbi trust assets are ultimately controlled by us, permitting participants to defer recognition of income for tax purposes on the amounts deferred until they are paid in accordance with their elections.
Our U.S.- and U.K.-based directors can also participate in the Deferred Compensation Plan by deferring receipt of their cash retainers, though they are not provided a matching contribution.
Fiscal 2023 Potential Payments upon Employment Termination or Change of Control Tables
Except in the case of Messrs. Castellano and Chiminski, the tables below set out what the specified NEOs would have received assuming a termination of employment effective as of June 30, 2023. With respect to Mr. Castellano, the table below sets out the actual payments that Mr. Castellano was contractually entitled to receive, which includes a severance payment equal to the sum of his annual base salary and target annual bonus, payment for any unused paid-time-off days accrued in fiscal 2023, and the right to exercise all vested stock options within 90 days of his departure, in each case, as a result of his termination without “cause” effective April 21, 2023. With respect to Mr. Chiminski, all of his outstanding equity awards will continue to vest and he continues to be eligible to receive financial planning reimbursements for one-year following his departure as a result of his retirement on June 30, 2023 in accordance with the policy approved by the Compensation Committee for all members of the executive leadership team following their retirement from the Company.
Alessandro Maselli (CEO)
| | | | | | | | | | | | | | |
| Triggering Event | Value of Option/RSU/PSU/Restricted Stock/Performance Share Acceleration ($) (1) | Value of Base Salary and Bonus Payments ($) (2) | Value of Continued Benefits Participation ($) (3) | Total ($) |
Death or Disability (4) | 2,472,214 | | 1,018,000 | | — | | 3,490,214 | |
| Termination by Us without Cause or By Mr. Maselli for Good Reason | — | | 4,904,000 | | 36,670 | | 4,940,670 | |
| Terminated by Us without Cause Within 2 Years Following a Change of Control (assuming awards have been assumed, continued, or substituted) | 1,604,103 | | 4,904,000 | | 36,670 | | 6,544,773 | |
| Termination by Us For Cause or By Mr. Maselli without Good Reason | — | | — | | — | | — | |
(1) Amounts reported for a termination by us without cause within 18 months following a change of control (assuming awards have been assumed, continued, or substituted) represent accelerated vesting of unvested equity-based awards and reflect (a) the “spread” value of the options, equal to $0 per share for 44,427 options (same in the case of death) granted on July 26, 2022 (award is underwater as of June 30, 2023 and has no value), $0 per share for 11,676 options (same in the case of death) granted on July 26, 2021 (award is underwater as of June 30, 2023 and has no value), $0 per share for 7,699 options (same in the case of death) granted on July 30, 2020 (award is underwater as of June 30, 2023 and has no value), and $0 per share for 3,436 options (same in the case of death), granted on July 22, 2019 (award is underwater as of June 30, 2023 and has no value), in each case representing the difference between the $43.36 closing price per share of our common stock on June 30, 2023, as reported on the NYSE (the “Fiscal 2023 Closing Price”), and the exercise price of the option; and (b) 10,221 RSUs (same in the case of death), granted on July 26, 2022, 3,009 RSUs granted (same in the case of death) on July 26, 2021 2,838 RSUs (same in the case of death), granted on July 30, 2020, 3,761 PSUs (Adjusted EPS) (3,548 in the case of death) and 0 PSUs (Relative Return) (3,070 in the case of death) granted on July 30, 2020, 1,881 PSUs (Adjusted EPS) (3,762 in the case of death) and 1,949 PSUs (Relative Return) (3,897 in the case of death) granted on July 26, 2021, and 6,388 PSUs (Adjusted EPS) (12,776 in the case of death) and 6,948 PSUs (Relative Return) (13,895 in the case of death) granted on July 26, 2022, multiplied by the Fiscal 2023 Closing Price.
The amount reported for Mr. Maselli for (i) termination by us without cause within 18 months following a change of control (assuming awards have been assumed, continued, or substituted), take into account future performance as disclosed in the “Fiscal 2023 Outstanding Equity Awards at Year-End” and accompanying footnote in this Annual Report; however, the number of Relative Return PSUs that vest in connection with a change of control may vary based on when a change of control occurs during a performance period.
Distribution of shares underlying PSUs are accelerated upon termination due to death. In the event Mr. Maselli meets the requirements of disability under the terms of the PSU awards, the shares underlying the PSUs remain subject to adjustment and will be distributed following the end of each relevant performance period based on final performance measured against the relevant pre-determined metrics for each award. The amounts shown above in the “Option/RSU/PSU/Restricted Stock/Performance Shares Acceleration” column in the event of termination due to death or disability assume that the PSUs vest at target. The amount would equal $1,604,103 when taking into account assumptions for future performance as disclosed in the “Fiscal 2023 Outstanding Equity Awards at Year-End” and accompanying footnote in this Annual Report.
(2) Upon termination due to death or disability, Mr. Maselli or his estate is entitled to receive a pro-rata portion of the annual bonus that he would have been entitled to for the bonus year in which the termination occurs, based on our actual performance (the “Annual Bonus”). The amount reported above for death or disability represents his target annual bonus for fiscal 2023 and assumes (a) he would have served for the entire year and (b) on-target business and individual performance results. The amounts reported for "Termination by Us Without Cause" or "By Mr. Maselli for Good Reason and Termination by Us Without Cause Within 2 Years Following a Change of Control" (assuming awards have been assumed, continued, or substituted) are comprised of (a) the Annual Bonus plus (b) two (2) times the sum of (i) his annual base salary and (ii) his target annual bonus.
(3) The amount reported represents income attributable to the health care premiums paid by us with respect to Mr. Maselli’s participation in our employee benefit plans for a two-year period. Mr. Maselli would also be entitled to be paid for any unused paid-time-off days accrued during 2023.
(4) Receipt of shares in the event of disability occurs when the relevant vesting period for each grant ends rather than being accelerated to the date of disability.
MESSR. HOPSON, FASMAN, CASTELLANO, CHIMINSKI AND DRS. GENNADIOS AND BOERMAN
| | | | | | | | | | | | | | |
| Triggering Event | Value of Option/RSU/PSU/Restricted Stock/Performance Shares Acceleration ($) (1) | Value of Base Salary and Target Bonus Payments ($) (2) | Value of Continued Benefits Participation/Unused Paid-Time-Off Accrued (3) | Total ($) |
Death or Disability (4) | | | | |
| Ricky Hopson | 408,278 | | 690,000 | | 18,204 | | 1,116,482 | |
| Steven L. Fasman | 1,235,370 | | 1,125,000 | | 12,762 | | 2,373,132 | |
| Aristippos Gennadios | 1,575,572 | | 1,100,000 | | 6,155 | | 2,681,727 | |
| Termination by US Without Cause or By the Executive Officer for Good Reason | | | | |
| Ricky Hopson | — | | 690,000 | | 18,204 | | 708,204 | |
| Steven L. Fasman | — | | 1,125,000 | | 12,762 | | 1,137,762 | |
| Thomas Castellano | — | | 1,000,012 | | 25,385 | | 1,025,397 | |
| Aristippos Gennadios | — | | 1,100,000 | | 6,155 | | 1,106,155 | |
Manja Boerman (6) | — | | 900,000 | | 35,083 | | 935,083 | |
| Termination by US Without Cause Within 18 Months Following a Change of Control | | | | |
| Ricky Hopson | 319,563 | | 690,000 | | 18,204 | | 1,027,767 | |
| Steven L. Fasman | 910,647 | | 1,125,000 | | 12,762 | | 2,048,409 | |
| Aristippos Gennadios | 1,372,127 | | 1,100,000 | | 6,155 | | 2,478,282 | |
Retirement (5) | | | | |
Steven L. Fasman (7) | 475,703 | | — | | — | | 475,703 | |
| Aristippos Gennadios | 301,222 | | — | | — | | 301,222 | |
| John Chiminski | 5,007,733 | | — | | — | | 5,007,733 | |
(1) For Mr. Hopson, the amounts reported for a termination by us without cause within 18 months following a change of control (assuming awards have been
assumed, continued, or substituted) reflects (a) the “spread” value of $0 per share for the 2,828 options (same in the case of death) granted on July 26, 2022 (award is underwater as of June 30, 2023 and has no value), $0 per share for the 1,924 options (same in the case of death) granted on July 26, 2021 (award is underwater as of June 30, 2023 and has no value), $0 per share for the 1,529 options (same in the case of death) granted on July 30, 2020 (award is underwater as of June 30, 2023 and has no value) and $0 per share for the 1,218 options (same in the case of death) granted on July 22, 2019 (award is underwater as of June 30, 2023 and has no value), representing the difference between the Fiscal 2023 Closing Price and the exercise of the option, and (b) 651 RSUs (same in the case of death) granted on July 26, 2022, 496 RSUs (same in the case as death) granted on July 26, 2021, 3,432 RSUs (same in the case as death) granted on June 1, 2021, 563 RSUs (same in the case as death), granted on July 30, 2020, 747 PSUs (Adjusted EPS) (704 in the case of death) and 0 PSUs (Relative Return) granted on July 30, 2020 (610 in the case of death), 310 PSUs (Adjusted EPS) (620 in the case of death) and 321 PSUs (Relative Return) (642 in the case of death) granted on July 26, 2021 and 407 PSUs (Adjusted EPS) (813 in the case of death) and 443 PSUs (Relative Return) (885 in the case of death) granted on July 26, 2022, multiplied by the Fiscal 2023 Closing Price.
For Mr. Fasman, the amounts reported for a termination by us without cause within 18 months following a change of control (assuming awards have been assumed, continued, or substituted) reflects (a) the “spread” value of $0 per share for the 12,117 options (same in the case of death and retirement) granted on July 26, 2022 (award is underwater as of June 30, 2023 and has no value), $0 per share for the 6,869 options (same in the case of death and retirement) granted on July 26, 2021 (award is underwater as of June 30, 2023 and has no value), $0 per share for the 4,311 options (same in the case of death and retirement) granted on July 30, 2020 (award is underwater as of June 30, 2023 and has no value), and $0 per share for the 3,311 options (same in the case of death and retirement) granted on July 22, 2019 (award is underwater as of June 30, 2023 and has no value), representing the difference between the Fiscal 2023 Closing Price and the exercise price of the option, and (b) 2,788 RSUs (same in the case of death and retirement) granted on July 26, 2022, 4,017 RSUs (same in the case of death) granted on January 3, 2022 (as to which the retirement provisions do not apply), 1,770 RSUs (same in the case of death and retirement) granted on July 26, 2021, 1,590 RSUs (same in the case of death and retirement) granted on July 30, 2020, 2,838 RSUs (same in the case of death) granted on July 30, 2020 (as to which the retirement provisions do not apply), 2,107 PSUs (Adjusted EPS) (same in the case of retirement and 1,987 in the case of death) and 0 PSUs (Relative Return) (1,720 in the case of death and 0 in the case of retirement) granted on July 30, 2020, 1,107 PSUs (Adjusted EPS) (2,213 in the case of death and 738 in the case of retirement) and 1,147 PSUs (Relative Return) (2,293 in the case of death and 765 in the case of retirement) granted on July 26, 2021, and 1,743 PSUs (Adjusted EPS) (3,485 in the case of death and 581 in the case of retirement) and 1,895 PSUs (Relative Return) (3,790 in the case of death and 632 in the case of retirement) granted on July 26, 2022, multiplied by the Fiscal 2023 Closing Price. In the event of retirement, the number of PSUs that vest is pro-rated based on the portion of the relevant performance period during which Mr. Fasman is actively employed.
Mr. Castellano’s employment ended on April 21, 2023. As a result of his departure, all of his outstanding unvested awards were immediately forfeited. In addition, Mr. Castellano had the right to exercise all of his 10,299 vested stock options within 90 days of his departure.
For Dr. Gennadios, the amount reported for termination by us without cause within 18 months following a change of control (assuming awards have been assumed, continued, or substituted) reflects (a) the “spread” value of $0 per share for 8,078 options (same in the case of death and retirement) granted on July 26, 2022 (award is underwater as of June 30, 2023 and has no value), $0 per share for 3,435 options (same in the case of death and retirement) granted on July 26, 2021 (award is underwater as of June 30, 2022 and has no value), $0 per share for 3,080 options (same in the case of death and retirement) granted on July 30, 2020 (award is underwater as of June 30, 2023 and has no value), and $0 per share for 2,209 options (same in the case of
death and retirement) granted on July 22, 2019 (award is underwater as of June 30, 2023 and has no value), representing the difference between the Fiscal 2023 Closing Price and the exercise price of the option, and (b) 1,859 RSUs (same in the case of death and retirement) granted on July 26, 2022, 18,689 RSUs (same in the case of death) granted on July 1, 2022 (as to which the retirement provisions do not apply), 4,017 RSUs (same in the case of death) granted on January 3, 2022 (as to which the retirement provisions do not apply), 885 RSUs (same in the case of death and retirement) granted on July 26, 2021, 1,136 RSUs (same in the case of death and retirement) granted on July 30, 2020, 1,505 PSUs (Adjusted EPS) (same in the case of retirement and 1,419 in the case of death) and 0 PSUs (Relative Return) (1,228 in the case of death and 0 in the case of retirement) granted on July 30, 2020, 554 PSUs (Adjusted EPS) (1,107 in the case of death and 369 in the case of retirement) and 574 PSUs (Relative Return) (1,147 in the case of death and 383 in the case of retirement) granted on July 26, 2021, and 1,162 PSUs (Adjusted EPS) (2,323 in the case of death and 388 in the case of retirement) and 1,264 PSUs (Relative Return) (2,527 in the case of death and 422 in the case of retirement), multiplied by the Fiscal 2023 Closing Price. In the event of retirement, the number of PSUs that vest is pro-rated based on the portion of the relevant performance period during which Dr. Gennadios is actively employed.
For Mr. Chiminski, the amounts reported represent accelerated vesting of unvested equity-based awards and reflect (a) the “spread” value of the options, equal to $0 per share for the 63,874 options granted on July 26, 2021 (award is underwater as of June 30, 2023 and has no value), $0 per share for 55,882 options granted on July 30, 2020 (award is underwater as of June 30, 2023 and has no value), and $0 per share for 32,375 options granted on July 22, 2019 (award is underwater as of June 30, 2023 and has no value), in each case representing the difference between the Fiscal 2023 Closing Price, and the exercise price of the option; and (b) 37,165 RSUs granted on July 26, 2022, 16,461 RSUs granted on July 26, 2021, 20,602 RSUs granted on July 30, 2020, 27,298 PSUs (Adjusted EPS) and 0 PSUs (Relative Return) granted on July 30, 2020, and 6,859 PSUs (Adjusted EPS) and 7,107 PSUs (Relative Return) granted on July 26, 2021, multiplied by the Fiscal 2023 Closing Price.
Distribution of shares underlying PSUs are accelerated upon termination due to death. In the event an NEO meets the requirements of disability under the terms of the PSU awards, the shares underlying the PSUs remain subject to adjustment and will be distributed following the end of each relevant performance period based on final performance measured against the relevant pre-determined metrics for each award. The amounts shown above in the “Option/RSU/PSU/Restricted Stock/Performance Shares Acceleration” column in the event of termination due to death or disability assume that the PSUs vest at target. The amounts would differ, as follows, when taking into account assumptions for future performance as disclosed in the “Fiscal 2023 Outstanding Equity Awards at Year-End” and accompanying footnote in this Annual Report: Mr. Hopson - $319,563; Mr. Fasman - $910,647; Dr. Gennadios - $1,372,127.
The amounts reported for Messrs. Hopson and Fasman and Dr. Gennadios for (i) termination by us without cause within 18 months following a change of control (assuming awards have been assumed, continued, or substituted) and (ii) for Messrs. Fasman and Chiminski and Dr. Gennadios under retirement, take into account future performance as disclosed in the “Fiscal 2023 Outstanding Equity Awards at Year-End” and accompanying footnote in this Annual Report; however, the number of Relative Return PSUs may vary based on when a change of control occurs during a performance period.
(2) The amounts reported represent, for each executive, the sum of that executive’s annual base salary and target annual bonus.
(3) The amounts reported for Messrs. Hopson and Fasman, Dr. Gennadios, and Dr. Boerman represent income attributable to the health care premiums paid by us with respect to their continued participation in our employee benefit plans for a one-year period. Each executive would also be entitled to be paid for any unused paid-time-off days accrued during 2023. The amount reported for Mr. Castellano represents payment for unused paid-time-off accrued in fiscal 2023 as a result of his departure on April 21, 2023.
(4) Receipt of shares in the event of disability occurs when the relevant vesting period for each grant ends rather than being accelerated to the date of disability.
(5) Messrs. Chiminski and Fasman and Dr. Gennadios were the only NEOs eligible for retirement as of June 30, 2023. Receipt of shares occurs when the relevant vesting period for each grant ends rather than being accelerated to the date of retirement.
(6) Dr. Boerman was removed as President, Division Head for Biomodalities effective April 25, 2023 and was offered “garden leave” for the entirety of the six months’ notice period under her employment agreement. As of the date of this Annual Report, the terms of Dr. Boerman’s separation payments and benefits from the Company are still being negotiated and were not finalized. Accordingly, the figures included in the table above are not necessarily representative of actual payments to be received by Dr. Boerman.
(7) Mr. Fasman left our employ on September 13, 2023 to take another opportunity. The figures included in the table above set out what Mr. Fasman would
have received assuming one of the enumerated termination of employment events occurred effective as of June 30, 2023.
Payments that would be made under our Deferred Compensation Plan are described above in the Fiscal 2023 Nonqualified
Deferred Compensation Table.
SEVERANCE AND PAYMENTS ON A CHANGE OF CONTROL
MR. MASELLI’S SEVERANCE, TERMINATION, AND CHANGE OF CONTROL BENEFITS
Mr. Maselli’s employment agreement, the Omnibus Plans, and the grant agreements thereunder each provide for certain benefits to be paid to him upon termination.
Upon disability or death, a pro-rata portion of any annual bonus he would have earned for the year of termination, based on our actual performance in respect of the full fiscal year in which the date of termination occurs, and the prior fiscal year’s annual cash bonus if earned but not then paid, payable as if Mr. Maselli’s employment had not been terminated.
Should Mr. Maselli’s employment terminate due to death, his beneficiaries (i) will receive a death benefit equal to 1.5 times his base salary under a group life insurance program we provide that covers all eligible active U.S.-based employees, and (ii) will be entitled to accelerated vesting of all unvested grants under the Omnibus Plans. If his employment is terminated due to disability, all unvested grants under the Omnibus Plans will continue to vest as if he had continued employment through each applicable anniversary of the grant date.
Under his employment agreement, upon any termination for good reason or due to his election not to extend the term, Mr. Maselli receives certain accrued amounts and benefits and a pro-rata portion of any annual bonus he would have earned for the year of termination, and the prior fiscal year’s annual cash bonus if earned but not then paid, payable as if Mr. Maselli’s employment had not been terminated.
The employment agreement further provides that upon termination by us without cause, or by Mr. Maselli for good reason, or due to our election not to extend the term, subject to a release of claims, he will also be entitled to receive an amount equal to two times the sum of (x) his annualized base salary and (y) his annual target bonus, payable in equal monthly installments over a two-year period; provided, however, that, if such termination occurs within the two-year period following a change in control, such payment will instead be made in a single lump-sum payment within thirty days following termination. Notwithstanding the foregoing, our obligation to make such payments will cease in the event of an uncured material breach by Mr. Maselli of the restrictive covenants contained in the employment agreement.
In addition to the payments described above, if Mr. Maselli’s employment is terminated by us without cause, by Mr. Maselli for good reason, or due to our election not to extend the term, Mr. Maselli (and his spouse and eligible dependents, to the extent covered prior to such termination) will also be entitled to continued participation in our group health plans for up to two years.
For grants under the Omnibus Plans, if Mr. Maselli incurred a termination, other than for death, disability, or a change of control that occurs during the period commencing on the date of the consummation of a change of control and ending on the date that is eighteen months following the consummation of such change of control, we could cancel any unvested option, RSU, or PSU. Any vested option will remain outstanding and exercisable generally for 90 days, and vested options will terminate immediately if we terminate Mr. Maselli’s employment for cause. Any vested option that he does not exercise within the applicable post-termination exercise period will terminate.
SEVERANCE, TERMINATION, AND CHANGE OF CONTROL BENEFITS FOR MESSRS. HOPSON AND FASMAN AND DR. GENNADIOS
The severance and equity grant agreements with each of Messrs. Hopson, Fasman and Dr. Gennadios, as well as the Omnibus Plans and the grant agreements thereunder, provide (or in the case of Mr. Fasman, provided) for benefits in the event of certain events of termination.
Under the Omnibus Plans, any unvested equity-based grant would become fully vested and exercisable in the event of termination due to death; however, if termination was due to disability, unvested equity-based awards would continue to vest as if the executive had continued employment through each applicable anniversary of the date of grant. Under the Omnibus Plans, in the event of a change in control, to the extent the acquiring or successor entity does assume, continue, or substitute for a grants option, if the NEO were to incur a termination without cause during the eighteen months following the consummation of such change in control, the then-outstanding equity awards thereunder would become fully vested and exercisable. Other than in the cases of change of control, death, or disability, a termination will result in the cancellation of unvested equity-based awards under the Omnibus Plans held by any of the NEOs.
Our group life insurance program, which covers all eligible active U.S.-based employees, provides for a death benefit equal to 1.5 times current base salary (currently, the benefit would pay a total of $637,500 (with respect to Mr. Hopson), $937,500 (with respect to Mr. Fasman), and $900,000 (with respect to Dr. Gennadios)).
Under our standard severance arrangement, in the event of death, disability, or termination by us without cause or by the executive for good reason, the executive would be entitled to severance equal to annual base salary plus target annual bonus, payable in equal installments over the one-year period following the date of termination. The NEOs would also be entitled to continued participation in our group health plans (to the extent receiving such coverage as of immediately prior to the termination date), at the premium rates charged to our employees generally, until the earlier of (1) one year after termination and (2) the date the executive becomes eligible for coverage under at least one group health plan of another employer. Each NEO must enter into a release of claims as a condition of receiving most severance payments and benefits.
On December 8, 2023, the Company entered into new Severance Agreements with each of Messrs. Masanovich and Hopson and Dr. Gennadios. The new Severance Agreements provide that in the event of a termination by the Company without cause or by the executive for good reason within 18 months following a change in control, the executive would be entitled to increased cash severance equal to two times the sum of annual base salary plus target annual bonus, payable in equal installments over the one-year period following the date of termination, subject to entering into a release of claims and certain other terms and conditions. In addition, the new Severance Agreements provide that if any of the payments provided for under such Severance Agreement or otherwise payable to the individual would constitute "parachute payments" within the meaning of Section 280G of the Internal Revenue Code and would be subject to the related excise tax under Section 4999 of the Internal Revenue Code, then such individual will be entitled to receive either full payment of benefits or such lesser amount that would result in no portion of the benefits being subject to the excise tax, whichever results in the greater amount of after-tax benefits to such
individual. The new Severance Agreements also include certain technical changes to the prior severance agreements with the executives, but otherwise are substantially the same as the prior severance agreements.
SEVERANCE, TERMINATION, AND CHANGE OF CONTROL BENEFITS FOR DR. BOERMAN
Dr. Boerman was removed from her position as President, Division Head for Biomodalities effective as of April 25, 2023, and upon her removal was offered “garden leave” for the entirety of the six months’ notice period under her employment agreement. Under Dr. Boerman’s employment agreement with the Company, if each of Dr. Boerman and the Company agree to termination by mutual consent and enter into a written settlement agreement in that regard, she is entitled to six months’ notice of such termination and a severance payment equal to her base salary and target MIP following a termination without cause (as such term is defined under Dutch law). As of the date of this Annual Report, the terms of Dr. Boerman’s separation payments and benefits from the Company are still being negotiated and were not finalized. Accordingly, the figures included in the table above are not necessarily representative of actual payments to be received by Dr. Boerman.
SEVERANCE AND TERMINATION BENEFITS FOR MR. CASTELLANO
In connection with Mr. Castellano’s separation from the Company effective April 21, 2023, he is contractually entitled to receive a severance payment equal to the sum of his annual base salary and target annual bonus equivalent to $1,000,012, payment for any unused paid-time-off days accrued in fiscal 2023, and the right to exercise all vested stock options within 90 days of his departure. In fiscal 2023, Mr. Castellano received $153,848 of severance pay and $25,385 representing unused paid-time-off days he accrued in fiscal 2023.
SEVERANCE AND TERMINATION BENEFITS FOR MR. CHIMINSKI
In connection with Mr. Chiminski’s retirement from the Company effective June 30, 2023, all outstanding equity awards will continue to vest in accordance with the terms of his outstanding award agreements and he continues to be eligible to receive financial planning reimbursements up to $15,000 (per calendar year) for one-year following his departure in accordance with the policy approved by the Compensation Committee for all members of the executive leadership team following their retirement from the Company.
Pay Ratio
Presented below is the ratio of annual total compensation in fiscal 2023 of our CEO to the annual total compensation of our median employee (excluding our CEO). We believe the ratio presented below is a reasonable estimate calculated in a manner consistent with the rules set forth in Item 402(u) of Regulation S-K promulgated under the Exchange Act (the “Pay Ratio Rules”).
In identifying our median employee, we calculated the annual total cash compensation for fiscal 2023 of each employee as of June 30, 2023. For these purposes, annual total cash compensation included base salary or hourly wages, cash incentives, commissions, and comparable cash elements of compensation in non-U.S. jurisdictions and was calculated using internal human resources records. All amounts were annualized for permanent employees who did not work for the entire year, such as new hires, employees on paid or unpaid leave of absence and employees called for active military duty. We did not apply any cost-of-living adjustment as part of the calculation.
We selected the median employee from among 17,219 full-time and part-time workers who were employed as of June 30, 2023. We did not exclude any employee (whether pursuant to the de minimis exemption for foreign employees or any other permitted exclusion).
In accordance with the Pay Ratio Rules, we calculated the median employee’s annual total compensation in the same manner as the CEO’s annual total compensation was calculated in the Fiscal 2023 Summary Compensation Table. The median employee’s annual total compensation was $59,026. The CEO’s annual total compensation was $6,583,672, the amount reported in the “Total” column of the Summary Compensation Table. Accordingly, the ratio of our CEO’s total compensation to our median employee’s total compensation for fiscal 2023 was 112 to 1.
In considering this pay ratio, please note that the Pay Ratio Rules permit companies to calculate pay ratios using a variety of methods, both in determining the median employee and in determining the compensation to be used in calculating the ratio. Thus, our ratio may not be comparable to the ratio determined by any other company.
Director Compensation
We provide competitive compensation to our non-employee directors to attract and retain qualified individuals. The principal elements of our non-employee director compensation are an annual cash retainer; an annual equity award of RSUs, each of which represents the right to receive one share of our common stock; and additional cash fees for our Lead Independent
Director, Committee Chairs (other than the Strategic and Operational Review Committee (the “Strategic Committee”)), Audit Committee members, and Strategic Committee members. In addition, non-employee directors are reimbursed for reasonable out-of-pocket expenses. Mr. Maselli, our CEO, and Mr. Chiminski, our former Executive Chair, did not receive additional compensation for their service as directors during fiscal 2023. Mr. Greisch received compensation as a non-employee director in fiscal 2023 and in fiscal 2024 until his election as Executive Chair, at which point he ceased being eligible for such compensation. In addition, Ms. Flynn, our former Interim President, Division Head for Biomodalities did not receive any additional compensation for her service as a director during her appointment to such position during fiscal 2023 and for the portion of fiscal 2024 in which she served in that role.
The Compensation Committee biennially reviews and considers information from its independent compensation consultant regarding the amounts and type of compensation paid to our non-employee directors at companies within the same Comparison Group (as defined in the CD&A above under the heading “The Use of Market Data in Determining Compensation”) used by the committee to assess executive compensation.
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| Cash Retainer | | Equity Award | | Committee Fees | | Deferred Compensation |
| Annual $100,000 cash retainer, with an additional $45,000 retainer for the Lead Independent Director. | | Annual SU grant with a grant date fair value of $275,000, vesting on the first anniversary of the grant date (subject to continued service) or upon a change of control. | | Annual cash fees to the Chair and each member of the Audit Committee of $25,000 and $10,000, respectively. Annual cash fees to the Chair and each member of the Finance & Capital Markets Committee (prior to its dissolution) of $15,000 and $2,500, respectively, and annual cash fees to each member of the Strategic Committee of $5,000.
Annual cash fees to the Chair of the Compensation Committee of $12,500 and $10,000 to the Chairs of each of the Nominating and Quality and Regulatory Compliance Committees, as well as the M&A Committee (prior to its dissolution).
| | Directors may elect to defer any portion of their cash fees or RSUs on a pre-tax basis under our Deferred Compensation Plan.
The terms of the plan are described in the executive compensation section above.
|
Matching Gift Program
Our directors may also participate in the Catalent Cares matching gift program, which matches on a 1-to-1 basis gifts made by our employees and non-employee directors to eligible nonprofit organizations, subject to a yearly maximum of $2,000. In addition, gifts of up to $1,000 made during fiscal 2023 to support humanitarian efforts in Ukraine were matched on a 2-to-1 basis.
Director Stock Ownership Policy
Each of our non-employee directors is required to own stock in an amount equal to five times the annual cash retainer. For purposes of this requirement, a director’s holdings include shares held directly or indirectly, individually or jointly, and shares held under a deferral or similar plan. Each non-employee director is required to retain 100% of the shares received upon settlement of vested RSUs (net of shares used to satisfy applicable tax withholding obligation, if any) until the ownership level is met. All of our non-employee directors complied with the retention provisions of this policy throughout fiscal 2023 and through the date of this Annual Report.
Director Compensation for Fiscal 2023
For fiscal 2023, our non-employee directors received the amounts shown in the schedule below. All cash fees were paid on a quarterly basis, in arrears.
| | | | | | | | | | | |
Name (1) | Fees Earned or
Paid in Cash
($) | Stock Awards ($) (2) | Total ($) |
Madhavan Balachandran(3)(4) | 100,000 | | 274,954 | | 374,954 | |
Michael J. Barber (3) | 100,000 | | 274,954 | | 374,954 | |
| J. Martin Carroll | 151,401 | | 274,954 | | 426,355 | |
Rolf Classon (3) (4) | 110,000 | | 274,954 | | 384,954 | |
Rosemary A. Crane (3) | 110,000 | | 274,954 | | 384,954 | |
Karen Flynn (1) | 60,440 | | 296,818 | | 357,258 | |
John J. Greisch (3) (4) (6) | 125,907 | | 274,954 | | 400,861 | |
| Christa Kreuzburg | 100,151 | | 274,954 | | 375,105 | |
Gregory T. Lucier (3) | 112,651 | | 274,954 | | 387,605 | |
| Donald E. Morel, Jr. | 110,000 | | 274,954 | | 384,954 | |
Jack Stahl (3) | 120,151 | | 274,954 | | 395,105 | |
Peter Zippelius (5) | 58,611 | | 274,954 | | 333,565 | |
(1) Neither Mr. Chiminski nor Mr. Maselli received any compensation as a director during fiscal 2023, though they received compensation as our employees, as reported in the
executive compensation tables in this Annual Report. In addition, Ms. Flynn only received director compensation during fiscal 2023 between September 15, 2022, when she joined the Board and April 23, 2023, the day before she was named Interim President, Division Head for BioModalities.
(2) Represents the aggregate grant date fair value of stock awards for fiscal 2023, computed in accordance with the Financial Accounting Standards Board’s Accounting Standards
Codification (“FASB ASC”) Topic 718, using the assumptions discussed in Note 14, “Stock-Based Compensation,” to the consolidated financial statements included in this Annual Report. Each non-employee director had 4,149 unvested RSUs as of June 30, 2023, except for Ms. Flynn who had 4,394 unvested RSUs as of the date she was named Interim President, Division Head for BioModalities and as of June 30, 2023 due to a pro-rated RSU grant received on September 15, 2022 when she joined the Board.
(3) Messrs. Balachandran, Barber, and Classon, Ms. Crane, Messrs. Greisch and Lucier and Mr. Stahl elected to defer their annual RSU award under the Deferred Compensation
Plan, as defined and described below under “Other Benefits Under Our Executive Compensation Program—Deferred Compensation Plan.”
(4) Messrs. Balachandran and Greisch elected to defer 100% of their annual cash retainers for calendar 2022 and 2023 under the Deferred Compensation Plan. Mr. Classon elected
to defer 50% of his annual cash retainer for calendar 2022 and 2023 under the Deferred Compensation Plan.
(5) Mr. Zippelius instructed that his cash retainer should be paid to his employer, Leonard Green. He also disclaimed beneficial ownership of his stock award and is holding it on
behalf of Leonard Green. Mr. Zippelius retired from the Board, effective as of the end of January 2023 in accordance with the terms and conditions of the Stockholders’ Agreement, and forfeited the RSUs granted through his stock award for fiscal 2023 pursuant to the terms of the award agreement.
(6) The annual RSU award made to Mr. Greisch was reduced by $45,284 through a cancellation/forfeiture of awarded RSUs on August 27, 2023 due to his appointment as Executive
Chair of the Company on August 28, 2023, at which time he became ineligible to receive compensation pursuant to the Company’s non-employee director compensation plan.
Compensation Committee Interlocks and Insider Participation
The individuals that served as a member of the Compensation Committee during fiscal 2023 were Gregory T. Lucier, Michael J. Barber, Rolf Classon and John J. Greisch. During fiscal 2023, no member of our Compensation Committee was an employee or officer or former officer of Catalent or had any relationship requiring disclosure under Item 404 of Regulation S-K. None of our executive officers has served on the board of directors or compensation committee of any other entity that has or has had one or more executive officers who served as a member of our Board or our Compensation Committee during fiscal 2023.
Non-GAAP Financial Measures
For a discussion of these measures and how they reconcile to our results reported under U.S. GAAP, please see the heading “Non-GAAP Metrics” under Item 7. Management’s Discussion and Analysis of Financial Condition of this Annual Report, as well as the following:
Use of Constant Currency, Budget-Based Revenue, and Budget-Based EBITDA
When we set the financial goals that we use to operate the business, including the goals that our executives must meet to qualify for our fiscal 2021 performance-based incentive compensation, and when we determine whether those goals have been met, we use, among other metrics, revenue and Adjusted EBITDA computed using the currency exchange rates that we use internally in budgeting and in measuring performance against budget, in part because we believe that the compensation of our executives should not be affected, to the extent practicable, by factors beyond those executives’ control. We refer in this Annual Report to revenue and Adjusted EBITDA computed on this type of constant-currency basis as “Budget-Based Revenue” and “Budget-Based EBITDA,” respectively.
Results on a constant-currency basis, Budget-Based Revenue, and Budget-Based EBITDA should be considered in addition to, not as a substitute for, results reported in accordance with U.S. GAAP. Results on a constant-currency basis, Budget-Based Revenue, and Budget-Based EBITDA, as we present them, may not be comparable to similarly titled measures used by other companies and are not measures of performance presented in accordance with U.S. GAAP.
The reconciliation of net earnings to Adjusted EBITDA also includes a reconciliation to Budget-Based EBITDA. The reconciliation of fiscal 2023 consolidated net revenue reported in accordance with U.S. GAAP to net revenue at budgeted foreign exchange rates is as follows (in millions of U.S. dollars):
| | | | | |
Revenue (GAAP) | $ | 4,263 | |
Foreign exchange impact | (108) | |
Budget-Based Revenue | 4,155 | |
Catalent, Inc.
Reconciliation of Net Earnings to EBITDA from operations,
Adjusted EBITDA and Budget-Based EBITDA
| | | | | | | | |
| Fiscal Year Ended June, 30 |
| (In million of U.S. dollars) | 2023 | 2022 |
| Net (loss) earnings | (256) | | 499 | |
| Depreciation and Amortization | 422 | | 378 | |
| Interest expense, net | 186 | | 123 | |
| Income tax (benefit) expense | (86) | | 80 | |
| EBITDA from operations | 266 | | 1,080 | |
| Goodwill impairment charges | 210 | | — | |
| Stock-based compensation | 35 | | 54 | |
| Impairment charges and gain/loss on sale of assets | 98 | | 31 | |
| Financing-related expenses and other | — | | 4 | |
| Restructuring costs | 66 | | 10 | |
| Acquisition, integration, and other special items | 31 | | 46 | |
| Gain on sale of subsidiary | — | | (1) | |
Foreign exchange loss (gain) included in other, net) (1) | (11) | | 31 | |
| Inventory fair value step-up charges | — | | 7 | |
| Other adjustments | 2 | | (3) | |
| Adjusted EBITDA | 697 | | 1,259 | |
| Favorable (unfavorable) FX impact | (17) | | (23) | |
| Adjusted EBITDA at constant currency | 714 | | 1,282 | |
| Adjusted EBITDA | 697 | | 1,259 | |
| Foreign exchange impact | (1) | | (30) | |
| | |
| Budget-Based EBITDA | 698 | | 1,289 | |
(1) Foreign exchange gain of $11 million for the fiscal year ended June 30, 2023, includes $10 million of unrealized gains related to foreign trade receivables and payables
and intercompany transactions.
Foreign exchange gain of $31 million for the fiscal year ended June 30, 2022, includes: (a) $12 million of unrealized gains related to foreign trade receivables and payables, (b) $11 million of unrealized losses on the unhedged portion of our euro-denominated debt, and (c) $34 million of unrealized losses on inter-company loans. The foreign exchange adjustment was also affected by the exclusion of realized foreign currency exchange rate gains from the settlement of inter-company loans of $2 million. Inter-company loans exist between our subsidiaries and do not reflect the ongoing results of our trade operations.
Catalent, Inc.
Reconciliation of Net Earnings to Adjusted Net Income and
Adjusted Net Income per share
| | | | | | | | |
| Fiscal Year Ended June 30, |
| (In million of U.S. dollars, except per share data) | 2023 | 2022 |
| Net (Loss) Earnings | (256) | | 499 | |
Amortization (1) | 136 | | 123 | |
| Goodwill impairment charges | 210 | | — | |
| Stock-based compensation | 35 | | 54 | |
| Impairment charges and gain/loss on sale of assets | 98 | | 31 | |
| Financing-related expenses | — | | 4 | |
| Restructuring Costs | 66 | | 10 | |
| Acquisition, integration, and other special items | 31 | | 46 | |
| Gain on sale of subsidiary | — | | (1) | |
Foreign exchange (gain) loss (included in other, net) (2) | (11) | | 31 | |
| Inventory fair value step-up charges | — | | 7 | |
| Other adjustments | 2 | | (4) | |
Estimated tax effect of adjustments (3) | (126) | | (72) | |
Discrete income tax benefit items (4) | (18) | | (54) | |
| Adjusted net income (ANI) | 167 | | 674 | |
| | |
| ANI per share: | | |
ANI per basic share (5) | $0.92 | $3.82 |
ANI per diluted share (6) | $0.92 | $3.73 |
(1) Represents the amortization attributable to purchase accounting for previously completed business combinations.
(2) Foreign exchange loss of $11 million for the fiscal year ended June 30, 2023, includes $10 million of unrealized gains related to foreign trade receivables and payable intercompany transactions.
Foreign exchange loss of $31 million for the fiscal year ended June 30, 2022, includes: (a) $12 million of unrealized gains related to foreign trade receivables and payables, (b) $11 million of unrealized losses on the unhedged portion of the euro-denominated debt, and (c) $34 million of unrealized losses on inter-company loans. The foreign exchange adjustment was also affected by the exclusion of realized foreign currency exchange rate gains from the settlement of inter-company loans of $2 million. Inter-company loans exist between our subsidiaries and do not reflect the ongoing results of our trade operations.
(3) We computed the tax effect of adjustments to net earnings by applying the statutory tax rate in the relevant jurisdictions to the income or expense items that are adjusted in the period presented. If a valuation allowance exists, the rate applied is zero.
(4) Discrete period income tax expense (benefit) items are unusual or infrequently occurring items, primarily including: changes in judgment related to the realizability of deferred tax assets in future years, changes in measurement of a prior-year tax position, deferred tax impact of changes in tax law, and purchase accounting.
(5) Represents Adjusted Net Income divided by the weighted average number of shares of Common Stock outstanding. For the fiscal year ended June 30, 2023 and 2022, the weighted average was 181 million and 176 million, respectively.
(6) Represents Adjusted Net Income divided by the weighted average sum of (a) the number of shares of Common Stock outstanding, plus (b) the number of shares of Common Stock that would be issued assuming exercise or vesting of all potentially dilutive instruments, plus, in fiscal 2022, (c) the number of shares of Common Stock equivalent to the shares of Series A Preferred Stock outstanding under the “if-converted” method. For the fiscal years ended June 30, 2023 and 2022, the weighted average was 181 million.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Securities Owned by Certain Beneficial Owners, Directors, and Management
The table below shows how many shares of our common stock were beneficially owned as of October 31, 2023 by (1) owners of more than 5% of the outstanding shares of our common stock, (2) our current directors, (3) our NEOs, and (4) all current directors and executive officers as a group. A person has beneficial ownership of shares if the person has voting or investment power over the shares or the right to acquire such power within 60 days. Investment power means the power to direct the sale or other disposition of the shares. Each person has (a) an address at 14 Schoolhouse Road, Somerset, NJ 08873 and (b) sole voting
and investment power over the shares, in each case except as described below. The percent of class is based upon 180,633,402 shares of common stock outstanding as of October 31, 2023.
| | | | | | | | |
Name of Beneficial Owner
| Common Stock
|
| Shares Owned | Percent of Class |
| | |
The Vanguard Group(1) | 19,588,103 | 10.8% |
Capital World Investors(2) | 18,029,128 | | 10.0 | % |
T. Rowe Price Investment Management, Inc.(3) | 16,578,946 | 9.2% |
BlackRock, Inc.(4) | 13,788,094 | 7.6% |
Veritas Asset Management LLP(5) | 11,169,815 | 6.2% |
Alessandro Maselli(6) | 112,029 | | * |
| Thomas Castellano | 12,545 | | * |
Ricky Hopson(6) | 20,544 | | * |
Steven L. Fasman(6) | 76,467 | | * |
Aristippos Gennadios(6) | 87,088 | | * |
John Chiminski(6) | 548,321 | | * |
Manja Boerman(6) | 25,897 | | * |
Madhavan Balachandran(7) | 20,448 | | * |
Michael J. Barber(7) | 6,396 | | * |
Steven K. Barg(9) | 0 | * |
| J. Martin Carroll | 32,186 | | * |
Rolf Classon(7) | 36,328 | | * |
Rosemary A. Crane(7) | 16,880 | | * |
Frank A. D’Amelio(9) | 0 | * |
| Karen Flynn | 17,346 | | * |
John J. Greisch(7) | 29,196 | | * |
| Christa Kreuzburg | 11,813 | | * |
Gregory T. Lucier(7) | 25,258 | | * |
Donald E. Morel, Jr.(7) | 58,462 | | * |
Stephanie Okey(9) | 0 | * |
Michelle R. Ryan(9) | 1,000 | | * |
Jack Stahl(7) | 36,328 | | * |
Current directors and executive officers as a group (24 persons)(8) | 554,265 | | * |
* Represents less than 1%
(1) Information shown is based on information reported by the filer on a Schedule 13G/A filed with the SEC on February 9, 2023, in which The Vanguard Group reported that it and its affiliates have shared voting power over 252,698 shares, sole dispositive power over 18,859,037 shares, and shared dispositive power over 729,066 shares. Filer’s address is 100 Vanguard Boulevard, Malvern, PA 19355.
(2) Information shown is based on information reported by the filer on a Schedule 13G/A filed with the SEC on October 10, 2023, in which Capital World Investors reported that it and its affiliates have sole voting power over 18,012,132 shares and sole dispositive power over 18,029,128 shares. Filer’s address is 333 South Hope Street, 55th Floor, Los Angeles, CA 90071.
(3) Information shown is based on information reported by the filer on a Schedule 13G/A filed with the SEC on February 14, 2023, in which T. Rowe Price Investment Management, Inc. (Price Investment Management) reported that it has sole voting power over 5,925,659 shares and sole dispositive power over 16,578,946 shares. Filer’s address is 101 E. Pratt Street, Baltimore, MD 21201.
(4) Information shown is based on information reported by the filer on a Schedule 13G/A filed with the SEC on February 7, 2023, in which Blackrock, Inc. reported that it has sole voting power over 12,670,195 shares and sole dispositive power over 13,788,094 shares. Filer’s address is 55 East 52nd Street, New York, NY 10055.
(5) Information shown is based on information reported by the filer on a Schedule 13G filed with the SEC on January 27, 2023, in which Veritas Asset Management LLP reported that it and its affiliates have shared voting power over 11,169,815 shares and shared dispositive power over 11,169,815 shares. Filer’s address is 1 Smart’s Place, London, WC2B 5LW, United Kingdom.
(6) The number of shares beneficially owned includes shares of common stock issuable upon (a) vesting of restricted stock units within 60 days after October 31, 2023, (b) vesting of performance share units within 60 days after October 31, 2023, or (c) exercise of options that are currently exercisable and/or will be exercisable within 60 days after October 31, 2023, as follows: Mr. Chiminski 285,461, Mr. Fasman 24,448, Dr. Gennadios 33,025, Mr. Maselli 79,921, Mr. Hopson 12,847 and Dr. Boerman 18,167.
(7) Includes vested restricted stock units that that have been deferred under our Deferred Compensation Plan (described below), as follows: Mr. Balachandran 15,687, Mr. Barber 4,149, Mr. Classon 25,258, Ms. Crane 8,337, Mr. Greisch 6,632, Mr. Lucier 23,856, Dr. Morel 11,548, and Mr. Stahl 8,337.
(8) Includes 438,964 shares of common stock issuable upon (a) vesting of restricted stock units within 60 days after October 31, 2023 or (b) exercise of options that are currently exercisable and/or will be exercisable within 60 days after October 31, 2023.
(9) Mr. D’Amelio and Mses. Ryan and Okey joined the Board on August 28, 2023, and Mr. Barg joined the Board on September 10, 2023.
Equity Compensation Plan Information
The following table provides certain information as of June 30, 2023 regarding our equity compensation plans.
| | | | | | | | | | | |
Plan category
| (a) Number of securities to be issued upon exercise of outstanding options, warrants and rights
| (b) Weighted-average exercise price of outstanding options, warrants and rights
| (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
|
| | | |
Equity compensation plans approved by security holders(1)
| 2,772,082(2)
| $71.19(3)
| 7,882,520 |
| | | |
Employee Stock Purchase Plan approved by security holders(4) | - | - | 3,180,009 |
| | | |
(1) The amounts set forth in this row relate to grants under (a) our 2014 Omnibus Incentive Plan (the “2014 Omnibus Plan”), which was approved by a majority shareholder prior to our IPO, and (b) our 2018 Omnibus Plan together with the 2014 Omnibus Plan, the “Omnibus Plans”), which was approved by our shareholders at the 2018 Annual Meeting of Shareholders. No additional award will be issued under the 2014 Omnibus Plan, but the shares that otherwise would have been available for issuance thereunder are available for issuance under the 2018 Omnibus Plan. Under the terms of the 2018 Omnibus Plan, each issued RSU and PSU reduces the amount remaining available by 2.25 shares, which is reflected in the amount reported in column c above, as well as incremental shares underlying PSUs representing performance at maximum above the respective targets.
(2) The amount shown includes 120,075 vested RSUs and PSUs that have been deferred under our Deferred Compensation Plan (described below), and (b) Adjusted EPS PSUs and Relative Return PSUs at target (each as described in the CD&A), which may increase by up to an additional 445,596 shares (not included in the number above) representing the number of shares above target if the maximum performance thresholds are met.
(3) The weighted-average exercise price shown above reflects stock options only and does not take into account outstanding RSUs or PSUs as these forms of equity securities by their nature have no exercise price.
(4) The amount set forth in this row relates to our 2019 Employee Stock Purchase Plan and reflects shares purchased through the end of the purchase period that ended on June 30, 2023.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
Our Board has adopted a written policy regarding the review, approval, and ratification of transactions with related persons. This policy provides that a related person must promptly disclose to our Board any related person transaction. No related person transaction will be executed without the approval or ratification of our Board or the Audit Committee. It is our policy that directors interested in a related person transaction will recuse themselves from any vote on a related person transaction in which they have an interest if the amount involved exceeds $120,000 and a “related person” has a direct or indirect material interest. In general, “related persons” are our directors and executive officers, shareholders beneficially owning more than 5% of our outstanding stock, and their immediate family members. We refer to such a transaction as a “related person transaction.”
Except as set forth below with respect to the Stockholders’ Agreement and Registration Rights Agreement (each as defined below), during fiscal 2023 we did not enter into or have outstanding any reportable related person transaction, nor is any related person transaction currently proposed, in which any of our directors, CEO, or executive officers has a direct or indirect material interest.
Stockholders’ Agreement
In connection with our sale of our formerly outstanding Series A Preferred Stock in May 2019, we entered into a stockholders’ agreement (the “Stockholders’ Agreement”) with certain affiliates of Leonard Green & Partners, L.P. that purchased those securities (the “Leonard Green Investors”). Pursuant to the Stockholders’ Agreement, as long as the holders of common stock issued upon conversion of Series A Preferred Stock (the “Relevant Holders”) beneficially owned shares of common stock having an aggregate value of at least $250 million (measured in accordance with the Stockholders’ Agreement), they had the right to designate one nominee for election to our Board and certain customary access and information rights. Peter Zippelius was the designated director of the Relevant Holders; however, on December 14, 2022, Mr. Zippelius notified the Board of his intent to retire, which was made effective as of the end of January 2023 in accordance with the terms and conditions of the Stockholders’ Agreement. The Relevant Holders no longer hold shares of common stock converted from the Series A Preferred Stock having an aggregate value in excess of $250 million, and, therefore, the right to designate a nominee has lapsed.
For so long as the Relevant Holders were entitled to designate a nominee, they were generally required to vote in the manner recommended by our Board in connection with director elections, our “say-on-pay” and other equity compensation proposals, ratification of the appointment of our independent registered public accounting firm, and with respect to any proposed merger or other similar transaction between us and another party. The Relevant Holders were also subject to standstill restrictions that, subject to certain exceptions, prohibited them from purchasing our common stock, publicly proposing any merger or other
extraordinary corporate transaction, initiating any shareholder proposal, or soliciting proxies until the date on which they were no longer entitled to designate a nominee to our Board. Restrictions on the ability of the Relevant Holders to transfer the shares of common stock they hold that were issued upon conversion of the Series A Preferred Stock expired on November 17, 2021.
REGISTRATION RIGHTS AGREEMENT
We also entered into a registration rights agreement (the “Registration Rights Agreement”) with the Leonard Green Investors, pursuant to which we must provide to the Leonard Green Investors certain customary registration rights with respect to the shares of common stock they hold that were issued upon conversion of the Series A Preferred Stock. The Registration Rights Agreement contains customary terms and conditions, including certain customary indemnification obligations.
The foregoing descriptions of the Stockholders’ Agreement and the Registration Rights Agreement do not purport to be complete and are subject to, and qualified in their entirety by, the full text of the Stockholders’ Agreement and Registration Rights Agreement, which are filed with the SEC as exhibits to this Annual Report.
Director Independence
Our Governance Guidelines define an “independent” director in accordance with Section 303A.02 of the NYSE’s Listed Company Manual. In addition, members of the Audit Committee and Compensation Committee are subject to the additional independence requirements of applicable SEC rules and NYSE listing standards. Under our Governance Guidelines and the NYSE listing standards, a director is not independent if the director has or had certain specified relationships with us. As part of its process to approve for nomination the current slate of nominees, our Board determined that each of our director nominees is independent for purposes of our Governance Guidelines, applicable NYSE standards, and applicable SEC rules, including with respect to committee service, other than Mr. Maselli, who is also our CEO, and Mr. Greisch, who is our Executive Chair. The Board previously determined that each of our directors who is not seeking re-election at the Annual Meeting was independent, other than Ms. Flynn, due to her prior service as our Chief Commercial Officer and Interim President, Division Head for BioModalities. In addition, the Board had previously determined that Peter Zippelius, who retired from the Board effective January 31, 2023, was independent.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table presents the fees for professional services rendered by Ernst & Young for the audit of our annual financial statements for the fiscal years that ended on June 30, 2023 and June 30, 2022, and the fees billed for other services rendered by Ernst & Young during those same periods.
| | | | | | | | |
SERVICES
| 2023 | 2022 |
| | |
Audit Fees(1)
| $ | 12,967,500 | | $ | 6,229,100 | |
| | |
Audit-Related Fees(2)
| $ | 7,200 | | $ | 366,200 | |
| | |
Tax Fees(3)
| $ | 597,500 | | $ | 1,283,700 | |
All Other Fees(4)
| $ | — | | $ | — | |
| | |
Total
| $ | 13,572,200 | | $ | 7,879,000 | |
(1) Includes fees associated with the integrated audit of our annual consolidated financial statements and internal control over financial reporting, review of our quarterly reports on Form 10-Q, and other services that are normally provided by the independent registered public accounting firm in connection with statutory and regulatory filings or engagements.
(2) Includes the aggregate fees recognized in each of the last two fiscal years for professional services rendered by Ernst & Young that are reasonably related to the performance of the audit of our financial statements. Specifically, these costs include fees for accounting and audit consultation and other attest services.
(3) Includes the aggregate fees recognized in each of the last two fiscal years for professional services rendered by Ernst & Young for tax compliance, tax advice, and tax planning.
(4) Ernst & Young did not provide any “other services” during the last two fiscal years.
All of the services covered under the captions “Audit Fees,” “Audit-Related Fees,” and “Tax Fees” were pre-approved by the Audit Committee.
Pre-Approval of Audit and Non-Audit Services
Consistent with requirements of the SEC and the Public Company Accounting Oversight Board regarding auditor independence, the Audit Committee charter provides that the Audit Committee has responsibility for appointing, setting compensation for, and overseeing the work of the independent auditor. Accordingly, all audit and permitted non-audit services for which Ernst & Young was engaged were pre-approved by the Audit Committee.
Prior to engagement of the independent auditor for 2024, management will submit for Audit Committee approval a list of services and related fees expected to be rendered in 2024 within each of the following categories of services:
• Audit services include audit work performed on the financial statements and internal control over financial reporting, as well as work that generally only the independent auditor can reasonably be expected to provide, including comfort letters, statutory audits, and discussions surrounding the proper application of financial accounting and/or reporting standards.
• Audit-Related services are for assurance and related services that are traditionally performed by the independent auditor, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements.
• Tax services include all services, except those services specifically related to the financial statements, performed by the independent auditor’s tax personnel, including tax analysis; assisting with coordination of execution of tax-related activities, primarily in the area of corporate development; supporting other tax-related regulatory requirements; tax planning; and tax compliance and reporting.
• All Other services are those services not captured in the audit, audit-related or tax categories.
PART IV
ITEM 15. EXHIBIT AND FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements. The Financial Statements listed in the Index to Financial Statements are filed under Item 8 Financial Statements and Supplementary Data of this Annual Report.
(a)(2) Financial Statements Schedule. The valuation allowance for credit losses is not material to the Company's consolidated balance sheets.
Deferred Tax Assets - Valuation Allowance | | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | Beginning Balance | | Current Period (Charge) Benefit | | Deductions and Other | | Ending Balance |
| Fiscal year ended June 30, 2021 | | | | | | | |
| Tax valuation allowance | $ | (53) | | | $ | 6 | | | $ | (18) | | | $ | (65) | |
| | | | | | | |
| Fiscal year ended June 30, 2022 | | | | | | | |
| Tax valuation allowance | $ | (65) | | | $ | (94) | | | $ | 10 | | | $ | (149) | |
| | | | | | | |
| Fiscal year ended June 30, 2023 | | | | | | | |
| Tax valuation allowance | $ | (149) | | | $ | (5) | | | $ | (5) | | | $ | (159) | |
(b) Exhibits.
The agreements and other documents filed as exhibits to this report are not intended to provide factual information or other disclosure other than with respect to the terms of the agreements or other documents themselves and you should not rely on them for that purpose. In particular, any representation or warranty made by us in these agreements or other documents were made solely within the specific context of the relevant agreement or document and may not describe the actual state of affairs as of the date they were made or at any other time.
| | | | | | | | | | |
| Exhibit No. | Description | | |
| | Membership Interest Purchase Agreement, dated August 29, 2021, by and among Catalent Pharma Solutions, Inc., Bettera Holdings, LLC, the members of Bettera Holdings, LLC, and Highlander Partners Candy, LLC (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on August 30, 2021). | | |
| | | | |
| | Fourth Amended and Restated Certificate of Incorporation of Catalent, Inc., as filed with the Secretary of State of the State of Delaware on October 28, 2021 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on November 2, 2021). | | |
| | | |
| | Bylaws of Catalent, Inc., effective February 2, 2023 (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed on February 7, 2023). | | |
| | | | |
| | Indenture, dated June 27, 2019, by and among Catalent Pharma Solutions, Inc., the subsidiary guarantors named therein, and Deutsche Bank Trust Company Americas, as trustee (incorporated by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K filed on June 27, 2019). | | |
| | | | |
| | Form of 5.00% Senior Notes due 2027 (included as part of Exhibit 4.1 above). | | |
| | | | |
| | Indenture, dated March 2, 2020, by and among Catalent Pharma Solutions, Inc., the subsidiary guarantors named therein, Deutsche Trustee Company Limited, as trustee, Deutsche Bank AG, London Branch, as principal paying agent, and Deutsche Bank Luxembourg S.A., as transfer agent and registrar (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 3, 2020). | | |
| | | | |
| | Form of 2.375% Senior Notes due 2028 (included as part of Exhibit 4.2 above). | | |
| | | | |
| | Indenture, dated February 22, 2021, by and among Catalent Pharma Solutions, Inc., the subsidiary guarantors named therein, and Deutsche Bank Trust Company Americas, as trustee (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on February 21, 2021). | | |
| | | | |
| | Form of 3.125% Senior Notes due 2029 (included as part of Exhibit 4.3 above). | | |
| | | | |
| | | | | | | | | | |
| | Indenture, dated September 29, 2021, by and among Catalent Pharma Solutions, Inc., the subsidiary guarantors named therein, and Deutsche Bank Trust Company Americas, as trustee (incorporated by reference to exhibit 4.1 to the Company’s Current Report on Form 8-K filed on September 29, 2021). | | |
| | | | |
| | Form of 3.500% Senior Notes due 2030 (included as part of Exhibit 4.4 above). | | |
| | | | |
| | Description of the Company’s Capital Stock (incorporated by reference to Exhibit 4.1 to the Company’s Quarterly Report on Form 10-Q filed on November 1, 2022). | | |
| | | | |
| | Amended and Restated Credit Agreement, dated as of May 20, 2014, relating to the Credit Agreement, dated as of April 10, 2007, as amended, among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc., as the administrative agent, collateral agent and swing line lender and other lenders as parties thereto (incorporated by reference to Exhibit 10.1 to Catalent Pharma Solutions, Inc.’s Current Report on Form 8-K filed on May 27, 2014). | | |
| | | | |
| | Amendment No. 1, dated December 1, 2014 to Amended and Restated Credit Agreement, dated as of May 20, 2014 among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc., as the administrative agent, collateral agent and swing line lender and other lenders as parties thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 2, 2014). | | |
| | | | |
| | Amendment No. 2 to Amended and Restated Credit Agreement, dated as of December 9, 2016, by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc. as administrative agent, collateral agent and swing line lender and the lenders party thereto, which amends that certain Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended), by and among Catalent Pharma Solutions, Inc. PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc. and JP Morgan Chase Bank, N.A. as L/C Issuers, the other lenders party thereto and the other agents party thereto (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on December 12, 2016). | | |
| | | | |
| | Amendment No. 3 to Amended and Restated Credit Agreement, dated as of October 18, 2017, by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc., as administrative agent, collateral agent and swing line lender and the lenders party thereto, which amends that certain Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended), by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc. and JPMorgan Chase Bank, N.A., as L/C Issuers, the other lenders party thereto and the other agents party thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 18, 2017). | | |
| | | | |
| | Amendment No. 4 to Amended and Restated Credit Agreement, dated as of May 17, 2019, by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc., as administrative agent, collateral agent and swing line lender and the lenders party thereto, which amends that certain Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended), by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc. and JPMorgan Chase Bank, N.A., as L/C Issuers, the other lenders party thereto and the other agents party thereto (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on May 22, 2019). | | |
| | | | |
| | Amendment No. 5 to Amended and Restated Credit Agreement, dated as of February 22, 2021, by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, JP Morgan Chase Bank, N.A., as the administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto, which amends that certain Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended), by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, JP Morgan Chase Bank, N.A., as the successor administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 22, 2021). | | |
| | | | |
| | Amendment No. 6 to Amended and Restated Credit Agreement, dated as of September 29, 2021, by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, JP Morgan Chase Bank, N.A., as the administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto, which amends that certain Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended), by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, JP Morgan Chase Bank, N.A., as the successor administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 29, 2021). | | |
| | | | |
| | | | | | | | | | |
| | Amendment No. 7 to Amended and Restated Credit Agreement, dated as of November 22, 2022, by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, JP Morgan Chase Bank, N.A., as the administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto, which amends that certain Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended), by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, JP Morgan Chase Bank, N.A., as the successor administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 22, 2022). | | |
| | | | |
| | Amendment No. 8 to Amended and Restated Credit Agreement, dated as of June 27, 2023, by JP Morgan Chase Bank, N.A., as the administrative agent, which amends that certain Amended and Restated Credit Agreement, dated as of May 20, 2014 (as amended), by and among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, JP Morgan Chase Bank, N.A., as the administrative agent, collateral agent, swing line lender, and letter of credit issuer, and the lenders and other parties thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 27, 2023). | | |
| | | | |
| | Intellectual Property Security Agreement, dated as of April 10, 2007, among PTS Acquisition Corp., Cardinal Health 409, Inc., PTS Intermediate Holdings LLC, Certain Subsidiaries of Holdings Identified Therein and Morgan Stanley Senior Funding, Inc. (incorporated by reference to Exhibit 10.21 to Catalent Pharma Solutions, Inc.’s Registration Statement on Form S-4 filed on December 6, 2007). | | |
| | | | |
| | Intellectual Property Security Agreement Supplement, dated as of July 1, 2008, to the Intellectual Property Security Agreement, dated as of April 10, 2007, among PTS Acquisition Corp., Cardinal Health 409, Inc., PTS Intermediate Holdings LLC, Certain Subsidiaries of Holdings Identified Therein and Morgan Stanley Senior Funding, Inc. (incorporated by reference to Exhibit 10.28 to Catalent Pharma Solutions, Inc.’s Annual Report on Form 10-K filed on September 29, 2008). | | |
| | | | |
| | Stockholders’ Agreement, dated as of May 17, 2019, by and among Catalent, Inc., Green Equity Investors VII, L.P., Green Equity Investors Side VII, L.P., LGP Associates VII-A LLC and LGP Associates VII-B LLC (incorporated by reference to exhibit 10.1 to the Company's Current Report on Form 8-K filed on May 22, 2019). | | |
| | | | |
| | Registration Rights Agreement, dated as of May 17, 2019, by and among Catalent, Inc., Green Equity Investors VII, L.P., Green Equity Investors Side VII, L.P., LGP Associates VII-A LLC and LGP Associates VII-B LLC (incorporated by reference to exhibit 10.2 to the Company's Current Report on Form 8-K filed on May 22, 2019). | | |
| | | | |
| | Catalent, Inc. 2014 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on August 5, 2014). † | | |
| | | | |
| | Catalent, Inc. 2018 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 6, 2018). † | | |
| | | | |
| | Form of Restricted Stock Unit Agreement for Non-Employee Directors †* | | |
| | | | |
| | Form of 2018 Omnibus Incentive Plan Restricted Stock Unit Agreement for Employees †* | | |
| | | | |
| | Form of 2018 Omnibus Incentive Plan Option Agreement for Employees †* | | |
| | | | |
| | Form of 2018 Omnibus Incentive Plan Performance Share Unit Agreement for Employees †* | | |
| | | | |
| | Catalent Pharma Solutions, Inc. Deferred Compensation Plan, as amended and restated effective October 1, 2022 (incorporated by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q filed on November 1, 2022). † | | |
| | | | |
| | Management Incentive Plan Summary for the fiscal year ending June 30, 2023 (incorporated by reference to Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q filed on November 1, 2022). † | | |
| | | | |
| | | | |
| | | | |
| | Form of Severance Agreement between US-based executive officers and Catalent Pharma Solutions, Inc. †* | | |
| | | | |
| | Form of Severance Agreement between non-US-based executive officers and Catalent Pharma Solutions, Inc. †* | | |
| | | | |
| | Employment Agreement, dated January 4, 2022, by and between Catalent, Inc. and Alessandro Maselli (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 5, 2022). | | |
| | | | |
| | Offer letter, dated June 15, 2023, between Matti Masanovich and Catalent, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 20, 2023) † | | |
| | | | |
| | Offer letter, dated July 7, 2022, between Steven Fasman and Catalent, Inc. (incorporated by reference to Exhibit 10.12.1 to the Company’s Annual Report on Form 10-K filed on August 29, 2022) † | | |
| | | | |
| | Offer letter, dated July 7, 2022, between Aristippos Gennadios and Catalent, Inc. (incorporated by reference to Exhibit 10.13.1 to the Company’s Annual Report on Form 10-K filed on August 29, 2022) † | | |
| | | | | | | | | | |
| | | | |
| | Offer letter, dated August 29, 2022, between Karen Santiago and Catalent, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 19, 2022). † | | |
| | | | |
| | Amended and Restated Employment Agreement, dated January 4, 2022, by and between Catalent, Inc. and John R. Chiminski (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on January 5, 2022). † | | |
| | | | |
| | Offer letter, dated May 10, 2021, between Thomas Castellano and Catalent Pharma Solutions, Inc. (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on May 11, 2021). † | | |
| | | | |
| | Offer letter, dated July 27, 2022, by and between Catalent, Inc. and Thomas Castellano (incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on November 1, 2022). † | | |
| | | | |
| | Offer letter, dated July 1, 2022, by and between Catalent, Inc. and Ricky Hopson †* | | |
| | | | |
| | Offer letter, dated May 1, 2023, by and between Catalent, Inc. and Ricky Hopson †* | | |
| | | | |
| | Amendment to Offer letter, dated August 14, 2023, by and between Catalent, Inc. and Ricky Hopson †* | | |
| | | | |
| | Employment Agreement, dated October 8, 2019, by and between Catalent Pharma Solutions GmbH and Manja Boerman †* | | |
| | | | |
| | Long Term International Assignment Letter, dated October 10, 2022, by and between Catalent Pharma Solutions LLC and Manja Boerman †* | | |
| | | | |
| | Subsidiaries of the Registrant. * | | |
| | | | |
| | Consent of Ernst & Young LLP. * | | |
| | | | |
| | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended. * | | |
| | | | |
| | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended. * | | |
| | | | |
| | Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. ** | | |
| | | | |
| | Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. ** | | |
| | | | |
| | Catalent, Inc. Executive Compensation Recoupment Policy* | | |
| | | | |
| 101.1 | | The following materials are formatted in inline XBRL (inline eXtensible Business Reporting Language): (i) the Consolidated Statements of Operations, (ii) the Consolidated Statements of Comprehensive Income, (iii) the Consolidated Balance Sheets, (iv) the Consolidated Statement of Changes in Shareholders’ Equity, (v) the Consolidated Statements of Cash Flows and (vi) Notes to Consolidated Financial Statements. * | | |
| | | | |
| 104 | | The cover page of this Annual Report on Form 10-K, formatted as Inline XBRL and contained in Exhibit 101.1. | | |
* Filed herewith
** Furnished herewith
† Represents a management contract, compensatory plan or arrangement in which directors and/or executive officers are eligible to participate.
ITEM 16. FORM 10-K SUMMARY
Registrants may voluntarily include a summary of information required by Form 10-K under this Item 16. We elect not to include such summary information.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | |
| | CATALENT, INC. |
| | | |
| Date: | December 8, 2023 | By: | | /s/ KAREN SANTIAGO |
| | | | Karen Santiago |
| | | | Vice President and Chief Accounting Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. | | | | | | | | | | | |
| Signature | | Title | Date |
| | | |
| /s/ ALESSANDRO MASELLI | | President and Chief Executive Officer (Principal Executive Officer) and Director | 12/8/2023 |
| Alessandro Maselli | | |
| | | |
| /s/ MATTI MASANOVICH | | Senior Vice President & Chief Financial Officer | 12/8/2023 |
| Matti Masanovich | | (Principal Financial Officer) | |
| | | |
| /s/ KAREN SANTIAGO | | Vice President and Chief Accounting Officer | 12/8/2023 |
| Karen Santiago | | | |
| | | |
| /s/ MADHAVAN BALACHANDRAN | | Director | 12/8/2023 |
| Madhavan Balachandran | | | |
| | | |
| /s/ MICHAEL J. BARBER | | Director | 12/8/2023 |
| Michael J. Barber | | | |
| | | |
| /s/ STEVEN BARG | | Director | 12/8/2023 |
| Steven Barg | | | |
| | | |
| /s/ J. MARTIN CARROLL | | Director | 12/8/2023 |
| J. Martin Carroll | | | |
| | | |
| /s/ ROLF CLASSON | | Director | 12/8/2023 |
| Rolf Classon | | | |
| | | |
| /s/ ROSEMARY A. CRANE | | Director | 12/8/2023 |
| Rosemary A. Crane | | | |
| | | |
| /s/ FRANK D'AMELIO | | Director | 12/8/2023 |
| Frank A. D'Amelio | | | |
| | | |
| /s/ KAREN FLYNN | | Director | 12/8/2023 |
| Karen Flynn | | | |
| | | |
| | | | | | | | | | | |
| /s/ JOHN J. GREISCH | | Executive Chair | 12/8/2023 |
| John J. Greisch | | | |
| | | |
| /s/ CHRISTA KREUZBURG | | Director | 12/8/2023 |
| Christa Kreuzburg | | | |
| | | |
| /s/ GREGORY T. LUCIER | | Director | 12/8/2023 |
| Gregory T. Lucier | | | |
| | | |
| /s/ DONALD E. MOREL, JR. | | Director | 12/8/2023 |
| Donald E. Morel, Jr. | | | |
| | | |
| /s/ STEPHANIE OKEY | | Director | 12/8/2023 |
| Stephanie Okey | | | |
| | | |
| /s/ MICHELLE RYAN | | Director | 12/8/2023 |
| Michelle R. Ryan | | | |
| | | |
| /s/ JACK STAHL | | Director | 12/8/2023 |
| Jack Stahl | | | |