medovexs1a_june2014.htm
As filed with the Securities and Exchange Commission on July 7, 2014
Registration No. 333- ___________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
MEDOVEX CORP.
(Exact name of registrant as specified in its charter)
|
Nevada
|
3841
|
46-3312262
|
|
(State or other jurisdiction of incorporation or organization)
|
(Primary Standard Industrial Classification Code Number)
|
(I.R.S. Employer Identification No.)
|
3279 Hardee Avenue
Atlanta, Georgia 30341
(844) 633-6839
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
Charles Farrahar
Chief Financial Officer
MEDOVEX CORP.
1735 Buford Hwy., Ste 215-113
Cumming, Georgia 30041
(844) 633-6839
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
Harvey Kesner, Esq.
Arthur S. Marcus, Esq.
Sichenzia Ross Friedman Ference LLP
61 Broadway, 32nd Floor
New York, New York 10006
(212) 930-9700
|
Jarrett Gorlin
Chief Executive Officer
MEDOVEX CORP.
1735 Buford Hwy., Ste 215-113
Cumming, Georgia 30040
844-633-6839
|
Joel Mayersohn, Esq.
Roetzel & Andress, LPA
350 East Las Olas Blvd.
Las Olas Centre II, Suite 1150
Fort Lauderdale, Florida 33301
(954) 462-4150
|
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box [X]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. [ ]
Large accelerated filer [ ]
|
|
|
|
Non-accelerated filer [ ]
(Do not check if a smaller reporting company)
|
|
Smaller reporting company [X]
|
CALCULATION OF REGISTRATION FEE
| Title of each class of
securities to be registered
|
|
Amount
to be
registered
|
|
Proposed
maximum
offering price
per Share (1)
|
|
|
Proposed
maximum
aggregate
offering
price (1)
|
|
|
Amount of
registration fee (2)
|
|
| Units, Each Consisting of One Share of Common Stock, $0.001 Par Value Per Share and One Series A Warrant |
|
1,552,500 units |
|
$ |
6.50 |
|
|
$ |
10,091,250 |
|
|
$ |
1,299.75 |
|
| Shares of Common Stock Included as Part of the Units (3) |
|
1,350,000 shares |
|
|
(4 |
) |
|
|
(4 |
) |
|
|
- |
|
| Series A Warrants Included as Part of the Units |
|
1,350,000 warrants |
|
|
(4 |
) |
|
|
(4 |
) |
|
|
- |
|
| Shares of Common Stock Underlying the Series A Warrants Included in the Units (3)(5) |
|
1,350,000 shares |
|
$ |
7.80 |
|
|
$ |
10,530,000 |
|
|
$ |
1,356.26 |
|
| Series B Warrants |
|
1,350,000 warrants |
|
|
(4 |
) |
|
|
(4 |
) |
|
|
- |
|
| Shares of Common Stock Underlying the Series B Warrants (3)(5) |
|
1,350,000 shares |
|
$ |
7.80 |
|
|
$ |
10,530,000 |
|
|
$ |
1,356.26 |
|
| Units underlying the Representative’s Unit Warrant (“Representative’s Units”) (6) |
|
205,500 units |
|
|
(4 |
) |
|
|
(4 |
) |
|
|
- |
|
| Shares of Common Stock underlying the Representative’s Units (3) |
|
202,500 shares |
|
$ |
6.50 |
|
|
$ |
1,316,250 |
|
|
$ |
169.53 |
|
| Series A Warrants Included as Part of the Representative’s Units |
|
202,500 warrants |
|
|
(4 |
) |
|
|
(4 |
) |
|
|
- |
|
| Shares of Common Stock Underlying the Series A Warrants included in the Representative’s Units (3)(5) |
|
202,500 shares |
|
$ |
7.80 |
|
|
$ |
1,579,500 |
|
|
$ |
203.44 |
|
| Series B Warrants Included as Part of the Representative’s Units |
|
202,500 warrants |
|
|
(4 |
) |
|
|
(4 |
) |
|
|
- |
|
| Shares of Common Stock Underlying the Series B Warrants included in the Representative’s Units (3)(5) |
|
202,500 shares |
|
$ |
7.80 |
|
|
$ |
1,579,500 |
|
|
$ |
203.44 |
|
| Total |
|
|
|
|
|
|
|
$ |
36,949,000 |
|
|
$ |
4,588.68 |
|
| (1) |
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the "Securities Act"). Includes the offering price of shares from the units that the underwriters have the option to purchase to cover over-allotments, if any. |
| (2) |
Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
| (3) |
Pursuant to Rule 416 under the Securities Act, the shares of Common Stock registered hereby also include an indeterminate number of additional shares of Common Stock as may from time to time become issuable by reason of stock splits, stock dividends, recapitalizations or other similar transactions. |
| (4) |
No registration fee pursuant to Rule 457(g) under the Securities Act. |
| (5) |
Estimated solely for the purposes of calculating the registration fee pursuant to Rule 457(g) under the Securities Act. The warrants are exercisable at a per share exercise price equal to 120% of the public offering price per unit. |
| (6) |
Estimated solely for the purpose of recalculating the registration fee pursuant to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the Representative's Units is $3,213,000 (which is equal to 10% of $32,130,000 ). |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities or the solicitation of an offer to buy these securities in any state in which such offer, solicitation or sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT TO COMPLETION
|
DATED MAY 1, 2014
|
1,350,000 Units and 1,350,000 Series B Warrants
Each Unit Consisting of One Share of Common Stock and One Series A Warrant to Purchase One Share of Common Stock
We are offering 1,350,000 units and 1,350,000 Series B Warrants in an initial public offering. Each unit consists of one share of common stock and one Series A Warrant. In addition, the holder of each unit will receive one Series B Warrant to purchase one share of common stock per unit purchased, but such Series B Warrants will not be transferable. No public market currently exists for our common stock or warrants. We are offering all of the shares of common stock offered by this prospectus. We expect the public offering price to be between $5.50 and $6.50 per unit, including the Series B Warrant. The units will not be certificated and the shares of common stock and Series A Warrants will trade initially as a unit, and will trade separately not later than 90 days from the date hereof, or earlier with the underwriter’s consent.
Each Series A Warrant is exercisable for one share of common stock. The Series A Warrants are immediately exercisable upon issuance in this initial public offering at an initial exercise price of 120% of the initial public offering price of one unit in this offering. The Series A Warrants will expire on the third anniversary of the date of issuance.
Each Series B Warrant is exercisable for one share of common stock. The Series B Warrants are immediately exercisable after issuance at an initial exercise price of 120% of the initial public offering price of one unit in this offering. The Series B Warrants will expire on the fifth anniversary of the date of issuance, and will not be listed on any securities exchange.
The shares of common stock issuable from time to time upon the exercise of the Series A Warrants and the Series B Warrants are also being offered pursuant to this prospectus.
We intend to apply to list our common stock on The NASDAQ Capital Market under the symbol “MDVX,” the Series A Warrants will trade under the symbol “MDVXW,” and our units will trade under the symbol “MDVXU.” No assurance can be given that our application will be approved.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012, and, as such, we have elected to take advantage of certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page15 in this prospectus. You should carefully consider these risk factors, as well as the information contained in this prospectus, before you invest.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| |
|
Per Unit and Series B Warrant
|
|
|
Total
|
|
Initial public offering price
|
|
|
6.00 |
|
|
|
8,100,000 |
|
Underwriting discounts and commissions (1)
|
|
|
0.82 |
|
|
|
1,096,000 |
|
Proceeds to us, before expenses
|
|
|
5.18 |
|
|
|
7,004,000 |
|
| |
|
|
|
|
|
|
|
|
(1) The underwriters will receive compensation in addition to the underwriting discount and commissions. See "Underwriting" beginning on page 74 for a description of compensation payable to the underwriters.
We have granted a 45-day option to the representative of the underwriters to purchase up to (i) 202,500 additional units, and Series B Warrants solely to cover over-allotments, if any. The over-allotment option must be used to purchase an equal numbers of units and Series B Warrants, but such purchases cannot exceed an aggregate of 15% of the number of Units and Series B Warrants sold in the primary offering. In addition, the registration statement of which this prospectus forms a part relates to the registration of 135,000 Units and 135,000 Series B Warrants issuable upon exercise of the Representative’s unit warrant
The underwriters expect to deliver our securities to purchasers in the offering on or about , 2014.
.
ViewTrade Securities Inc.
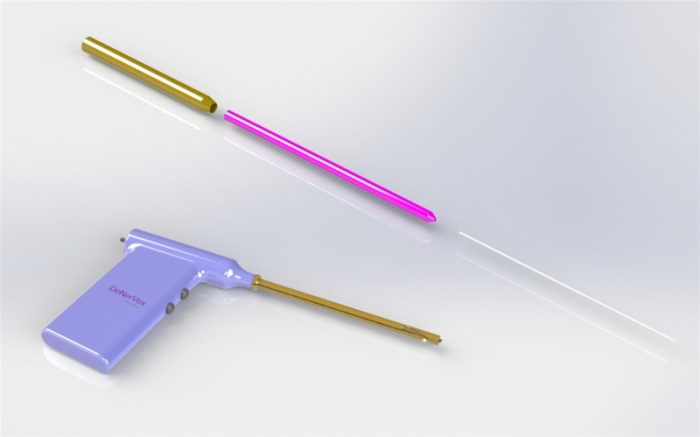
This is a conceptual prototype of the DenerVex device. MEDOVEX has not sold this device and has not applied to any regulatory agency to obtain clearance for this prototype. The final device used to obtain regulatory clearance may appear differently than the image above.
| |
Page
|
| |
|
|
|
1 |
|
|
10 |
|
|
30 |
|
|
30 |
|
|
31 |
|
|
31 |
|
|
33 |
|
|
34 |
|
|
41 |
|
|
57 |
|
|
65 |
|
|
66 |
|
|
69 |
|
|
70 |
|
|
74 |
|
|
76 |
|
|
79 |
|
|
79 |
|
|
79 |
|
|
|
________________________________________
Neither we nor the underwriters have authorized anyone to provide you with information that is different from that contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. When you make a decision about whether to invest in our common stock, you should not rely upon any information other than the information in this prospectus or in any free writing prospectus that we may authorize to be delivered or made available to you. This prospectus is not an offer to sell or the solicitation of an offer to buy the shares of common stock in any circumstances under which the offer or solicitation is unlawful.
________________________________________
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market share, is based on information from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. Our management estimates have not been verified by any independent source, and we have not independently verified any third-party information. In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Special Note Regarding Forward-Looking Statements.”
________________________________________
We have applied for trademarks on “MEDOVEX” and “DenerVex”. This prospectus may include trademarks, trade names and service marks that are the property of other organizations. Solely for convenience, trademarks and trade names referred to in this prospectus appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these trademarks and trade names.
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. You should read this entire prospectus carefully, especially the "Risk Factors" section of this prospectus and our financial statements and the related notes appearing at the end of this prospectus, before making an investment decision.
As used in this prospectus, unless the context otherwise requires, references to Medovex we, us, our, our company refer to MEDOVEX Corp. and its wholly-owned subsidiary, Debride, Inc. All share numbers give effect to a 2:1 reverse stock split effected in March, 2014.
Overview
MEDOVEX Corp.’s goal is to create better living through better medicine.
We intend to build a portfolio of medical device products in areas where our world-class management team believes the largest, most targetable market opportunities exist. We intend to build this portfolio primarily by acquiring companies or technologies that we believe have promising commercial potential. In September 2013, we acquired Debride, Inc. (Debride). Debride is a development stage company that has acquired a patent, patent applications and other intellectual property rights relating to the use, development, and commercialization of the DenerVex device (the DenerVex). Debride acquired from Scott M.W. Haufe, MD (Dr. Haufe) all right, title and ownership of U.S. Patent 8,167,879 B2, together with all of Dr. Haufe’s rights, title and interest in and to the DenerVex. Dr. Haufe is a director of the Company. In September 2013, we entered into a Co-Development Agreement with Dr. James Andrews, a renowned orthopedic surgeon who also is a director of the Company. Dr. Andrews has agreed to evaluate the DenerVex and to seek to make modifications and improvements to such technology, as well as to strategize in the rollout of the DenerVex. See “Transactions with Related Parties”.
We are a development stage enterprise with no revenues. In their opinion letter for the fiscal year ended December 31, 2013, our auditors included an explanatory paragraph that disclosed conditions that raise concerns about the Company's ability to continue as a going concern. Please refer to the audited financial statements and accompanying auditors report within this filing.
Initial Product
To date, our efforts have focused on the development of the patent-protected DenerVex device (U.S. Patent 8,167,879 B2). The DenerVex is designed to provide long lasting relief of pain associated with Facet Joint Syndrome (FJS), a condition in which the joints in the back of the spine degenerate and subsequently cause back pain. The concept is simple: remove the affected nerve endings sending pain signals to the brain in such a way that they won’t grow back. A current treatment for this pain removes the nerve endings, but the nerve endings regenerate and the pain returns. A patient is forced to return again and again to seek pain relief at a substantial cost over time.
In order to avoid having his patients suffering from chronic back pain associated with FJS have to return to him for repeated treatments, Dr. Haufe performed a 2-step procedure where he first scraped away synovial tissue using an already available deburring device around the facet joints in the spine so nerves would not have tissue that could be used for regrowth. Once this tissue was removed, he then used a standard electrocautery unit to cauterize the nerve endings, as is done with the temporary treatment to render the nerves incapable of sending pain signals to the brain. Due to the complexity and time required to complete this procedure, it is only employed by a small number of other surgeons besides Dr. Haufe.
In order to see if these patients were indeed enjoying pain relief and not experiencing nerve regrowth, Dr. Haufe followed 174 of his patients receiving this treatment over a three year period (temporary approaches generally do not last more than 2 years and often less). Over 70% of patients were still pain free or had greater than 50% reduction in pain. While this follow-up did not constitute a clinical study that could be used for FDA clearance, the results encouraged Dr. Haufe to think of ways to expand the treatment to other patients who did not have the resources to afford a relatively complex procedure (assuming they could even locate a surgeon capable of performing it). The key was simplifying the procedure to the point where doctors specializing in pain management and other areas besides spine surgery could perform the procedure quickly and safely, at a cost much less than the cost of temporary treatments over time. To simplify the procedure, Dr. Haufe conceptualized a hand held device that combined the tissue scraping and electrocautery procedures into one action. A simple solution that, based on our internal research on non-living tissue, produces the same result as the 2-step procedure, but requires significantly less surgical skill and takes much less time.
We believe the DenerVex is the first device of its kind that combines tissue scraping and electrocautery simultaneously in order to achieve a long lasting solution to FJS. The DenerVex is a single use device that will be provided to health care practitioners in a package that contains all of the items necessary in the sterile field of the operating room to carry out the surgery necessary to address FJS. The DenerVex does not require a special power source and can be connected to the cauterizing generator generally found in most operating rooms. While the device is still in the prototype phase, we estimate a sales price of approximately $695.00 per device, which we believe will be an attractive price point for end-users. We arrived at the sales price after conducting focus group meetings with potential end-users and obtaining their feedback on ranges of prices they would be willing to pay for the type of pain relief the DenerVex could provide. These end users included pain management physicians, an anesthesiologist, orthopedic surgeons who perform spinal surgery and neurosurgeons. As reimbursement is available for the treatment of Facet Joint Pain in the United States and in major countries in Europe, they could base their responses on reimbursement rates for currently available treatments.
Besides the potential cost savings from the use of the DenerVex device, we believe the procedure using the DenerVex is less evasive, faster and represents a lower risk of infection from surgery than its competitors, while offering a longer term solution to FJS than is currently available. In the procedure where the DenerVex is used, a tiny incision is made above the facet joint and the DenerVex is inserted through a cannula to the facet joint region. The cannula has a bullet-shaped tip that acts as a retractor system. The physician performing the procedure inserts a guidewire down to the facet joint region and the cannula/retractor is inserted over that wire. The inner bullet is removed, leaving a hollow tube for working space. The device is positioned and manipulated via fluoroscopy. No other devices or tools are required beyond standard surgical tools for closure. The DenerVex is designed to be powered by (and compatible with) currently marketed electrocautery consoles currently in most procedure rooms, so no additional specialized capital equipment is required. Activation of the device results in the combined effect of a tissue removal action with electrocautery to the facet joint surface resulting in removal of the nerves and synovial membrane tissues. The treatment of each joint takes a couple of minutes and the incision is closed with a single suture stitch.
The DenerVex is currently in the prototype development phase. In order to commercialize the device, we will:
|
1.
|
Conduct additional research to further refine the product.
|
|
2.
|
Submit the final version to regulatory agencies for their approval, including conducting additional studies as they may require.
|
|
3.
|
Finalize reimbursement, marketing and distribution strategies.
|
|
4.
|
Source vendor(s) for production and marketing/distribution partners (we currently do not plan to manufacture or distribute the product ourselves).
|
Market
Our first device, the DenerVex, is targeted at the $11.5 billion global spine surgery devices market, which is largely focused on the treatment of lower back pain. It is estimated that 10% of the adult U.S. population, suffer from chronic lower back pain. Chronic lower back pain is the second leading cause of disability in the U.S.
Approximately 31% of chronic lower back pain is attributed to FJS, a condition in which joints located at the back of the spine degenerate and subsequently cause pain. Each joint in the spine has a rich supply of tiny nerve fibers that provide a painful stimulus when the joint is injured or irritated. When such joints degenerate due to wearing or tearing, the cartilage separating the joints in the spine may become thin or disappear. The bone in the joints underneath the cartilage can then produce an overgrowth of bone spurs and an enlargement of the joints, which can repeatedly send pain signals through the nerve fibers in the spine. Such pain signals can result in considerable back pain, especially when a person is in motion.
Initial treatment of FJS includes a number of non-invasive, non-surgical techniques or therapies. Examples of such therapies are activity modification exercises, over-the-counter medication, chiropractic intervention and physical therapy. If those non-invasive, non-surgical approaches fail to provide long-term relief to a patient, there are currently six approaches used to remedy FJS: spinal injections, radiofrequency (“RF”) ablation (also known as radiofrequency rhizotomy or radiofrequency neurotomy), cryodenervation, pulsed RF, manual tissue scraping and electrocautery performed separately and spine fusion surgery. Management believes that the primary downsides of the current surgical approaches are as follows:
|
·
|
Temporary Relief. Spinal injection, cryodenervation, RF ablation, and pulsed RF therapies are temporary, such that patients must return for repeated treatment over the course of the patient’s lifetime. Spinal injections effectiveness only lasts a period measured in months, while cryodenervation, RF ablation, and pulsed RF have generally been shown to be effective for approximately 6 months to 2 years.
|
|
·
|
Difficult to learn and teach. Compared to the temporary relief treatments described above, the long term relief treatment of manual tissue scraping and electrocautery performed separately requires a spinal surgeon with excellent endoscopy skills and special training. Spinal fusion is a very complex procedure requiring hours to complete and a team of medical professionals to perform.
|
|
·
|
Surgical mechanisms are bulky and difficult to use. Spinal injections require large, painful needles. RF ablation requires the purchase of a special power source to power its ablation needles. Manual tissue scraping and electrocautery performed separately requires four separate devices to be used that cannot be deployed simultaneously. Spinal fusion surgery is a very invasive and significant surgical procedure.
|
|
·
|
High cost. Spinal injection, cryodenervation, RF ablation, and pulsed RF therapies must be repeatedly applied to be effective, and the cost of such repeated visits to healthcare facilities can aggregate significantly over the course of a lifetime, not to mention the cost of lost productivity due to lower back pain in between treatments.
|
|
·
|
Invasive with long recovery time. Spinal fusion surgeries are potentially risky, highly invasive surgeries that require significant recovery time with an average cost per patient of up to $108,000 , plus an estimated 3 to 6 months of lost productivity in the workplace.
|
We believe that the DenerVex represents a significant leap forward in FJS treatment, as it addresses the shortcomings listed above in the following ways:
|
·
|
Longer Lasting. The DenerVex is designed to provide long lasting relief from pain for patients that would otherwise be required to undergo repetitive therapies such as spinal injections and RF ablation.
|
|
·
|
Easy to teach and intuitive to use. The DenerVex combines two procedures that are currently carried out in the manual tissue scraping and electrocautery performed separately into one procedure that does not require a specialist to conduct. We intend to offer a simple one-day or weekend course featuring a cadaver lab to train physicians in usage of the DenerVex.
|
|
·
|
Compact, next generation design. The DenerVex is an all-in-one solution that comes in a sterile, single use package.
|
|
·
|
Cost efficient. The DenerVex is a single use device, so there are no sterilization or repair costs that are associated with many medical devices. Unlike RF ablation, the Denervex does not require a specially-purchased power source, and uses the already-existing cauterization power sources currently found in almost every operating room.
|
|
·
|
Less Invasive with minimal recovery time. Use of the DenerVex represents a less invasive solution to FJS than spinal fusion surgery and can be carried out through an out-patient procedure that does not require follow-up surgery to be effective.
|
By addressing the specific product and technology limitations outlined above (that have been raised by both patients and practitioners alike according to our market research), we believe DenerVex has the ability to greatly penetrate and expand the spine surgery device market. In order to begin our anticipated expansion into the spine surgery device market, the primary targets for the sale of the DenerVex device are pain management practitioners. There are an estimated 6,200 pain management specialists in the United States. We expect that the primary users of the DenerVex device will be these specialists, as well as clinicians who currently utilize interventional procedures to treat chronic back pain. Secondary targets for the DenerVex device are orthopedists and neurosurgeons, in addition to the 4,000 interventional radiologists, and 8,672 spine surgeons using one or a combination of the techniques now currently in use to treat FJS.
Our Strategy
Indeed, in order to provide solutions and devices that impact the quality of patient care while simultaneously lowering costs in a rapidly changing healthcare environment, we have assembled a seasoned management team composed of individuals with both medical expertise and experience in monetizing medical devices. Dr. Haufe, who co-developed the DenerVex, was a co-founder of Debride and is a director of the Company, not only has 11 years of experience in carrying out over 450 manual tissue scraping and electrocautery procedures performed separately in order to treat back pain related conditions, but also has eight (8) years of experience designing and developing innovative and proprietary spine technologies and techniques. Patrick Kullmann, who is President and Chief Operating Officer of the Company, has nearly 30 years of experience marketing and growing companies in the healthcare, scientific and technology industries and is the author of the book “The Inventor’s Guide to Medical Technology”. We believe that other members of our management team, particularly our Chief Executive Officer, Jarrett Gorlin, provide us with the management expertise necessary to effectively manage our anticipated growth. We intend to leverage this expertise to accelerate the growth of the businesses or assets we acquire. We believe that our management team’s diverse range of experience also provides us with the flexibility to review growth and turnaround situations for underleveraged or underperforming medical devices and their parent companies. We are pursuing the following strategies:
|
·
|
Promoting the differentiated features of our DenerVex exclusive technology to penetrate the $11.5 billion global spine surgery devices market and establish DenerVex as a standard of care for treating FJS, as well as broadening the clinical applications of the DenerVex;
|
|
·
|
Investing in our infrastructure to broaden our market presence first in Europe, then in the U.S. and eventually worldwide, expand our global distribution footprint, and to ensure regulatory approval internationally; and
|
|
·
|
Leveraging the strength and experience of our world-class management team to selectively pursue opportunities to expand our portfolio of medical device products and businesses.
|
Our marketing strategy will be supported by a solid core of clinical and cost effectiveness data that we intend to develop to support our portfolio of medical companies and assets. We intend to employ the same disciplined approach to all future opportunities that we employed prior to acquiring the assets for the DenerVex device, by careful research, reviewing of existing devices and examination of marketplace possibilities. A compelling value proposition and subsequent market positioning for our devices versus other treatment options will also be developed and tailored as appropriate for the key decision-makers along the course of the patient flow. We believe we are well positioned to create devices and tailor their value propositions to meet a rapidly changing marketplace and fluid regulatory environment, by creating and marketing devices that are designed to result in patients spending less time in the hospital, lower the chance of patient infection, simplify surgical procedures and diminish the potential incidence of hospital and surgery-related accidents. Please see “Management—Board of Directors” for more information regarding the background and experience of our management team.
Risks That We Face
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus beginning on page 15. These risks include, but are not limited to, the following:
|
·
|
our lack of operating history.
|
| · |
our liquidity and the net losses that we expect to incur as we develop our business. If such losses mean that we do not continue as a going concern, investors could lose their entire investment. |
|
·
|
we will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our product development or commercialization efforts.
|
|
·
|
obtaining FDA or other regulatory approvals or clearances for our technology. We are heavily dependent on the success of our first product, the DenerVex, which has not yet received regulatory approval. We will not be able to generate any revenue unless we receive regulatory approval. The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time-consuming, costly and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product, our business will be substantially harmed.
|
|
·
|
implementing and achieving successful outcomes for clinical trials of our products. Our initial product, the DenerVex, is based on a currently clinically unproven approach to back pain treatment.
|
|
·
|
convincing physicians, hospitals and patients of the benefits of our technology and to convert them from current technology.
|
|
·
|
we face substantial competition, which may result in others discovering, developing or commercializing products before, or more successfully, than we do. We expect any product that we commercialize will compete with products from other companies in the medical device industry. Many of our potential competitors have substantially greater commercial infrastructures and financial, technical and personnel resources than we have. If we are not able to compete effectively against our competitors, our business will not grow and our financial condition and operations will suffer.
|
|
·
|
the ability of users of our products (when and as developed) to obtain third-party reimbursement.
|
|
|
any failure to comply with rigorous FDA and other government regulations, which may change or be reformed.
|
|
|
we depend on key personnel.
|
|
|
securing, maintaining and defending patent or other intellectual property protections for our technology. Our inability to obtain adequate patent protection for our products or failure to successfully defend against any claims that our products infringe the rights of third parties could also adversely affect our business. Any challenges relating to our intellectual property may require us to spend a substantial amount of time and money to resolve, if at all possible.
|
Our Corporate Information
MEDOVEX is a development stage company that was incorporated in Nevada on July 30, 2013 as Spinez Corp. and changed its name to MEDOVEX Corp. on March 20, 2014. MEDOVEX is the parent company of Debride, which was incorporated under the laws of Florida on October 1, 2012. MEDOVEX acquired all of the issued and outstanding capital stock of Debride pursuant to an agreement and plan of merger, dated September 13, 2013.
Our principal executive offices are located at 3279 Hardee Avenue, Atlanta, Georgia 30341 and our telephone number is (844) MEDOVEX. Our website address is www.MEDOVEX.com. The information contained on, or that can be accessed through, our website is not incorporated into and is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe these industry publications and third party research, surveys and studies are reliable, we have not independently verified such data.
Implications of being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012. An “emerging growth company” may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
|
|
being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion & Analysis of Financial Condition and Results of Operations in this prospectus;
|
|
|
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act;
|
|
|
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
|
|
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
|
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended, (the “Securities Act”), which fifth anniversary will occur in 2019. However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.0 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
We have elected to take advantage of certain of the reduced disclosure obligations and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies is irrevocable.
The Offering
| |
Securities offered
|
|
1,350,000 units , consisting of one share of common stock and one Series A Warrant to purchase one share of common stock. In addition, the holder of each unit issued in the offering will receive one Series B Warrant to purchase one share of common stock per unit purchased, but such Series B Warrants will not be transferable. The units will not be certificated and the shares of common stock and Series A Warrants will trade initially as a unit, and will trade separately not later than 90 days from the date hereof, or earlier with the underwriter’s consent.
Each Series A Warrant is exercisable for one share of common stock. The Series A Warrants are immediately exercisable upon issuance in this initial public offering at an initial exercise price of 120% of the initial public offering price of one unit in this offering. The Series A Warrants will expire on the third anniversary of the date of issuance, and will not be listed on any securities exchange.
Each Series B Warrant is exercisable for one share of common stock. The Series B Warrants are exercisable immediately after issuance at an initial exercise price of 120% of the initial public offering price of one unit in this offering. The Series B Warrants will expire on the fifth anniversary of the date of issuance.
The shares of common stock issuable from time to time upon the exercise of the Series A Warrants and the Series B Warrants are also being offered pursuant to this prospectus.
|
| |
|
|
|
| |
Common Stock:
|
|
|
| |
Common stock offered by us
|
|
1,350,000 shares
|
| |
Common stock to be outstanding after this offering
|
|
9,131,175 shares
|
| |
|
|
|
| |
Offering price
|
|
|
| |
Over-allotment option
|
|
The underwriters have an option for a period of 45 days to purchase up to fifteen percent (15%) of the units and Series B Warrants being offered under this registration statement to cover over-allotments, as long as such purchases do not exceed an aggregate of fifteen (15%) of the number of units and Series B Warrants sold in the primary offering.
|
| |
|
|
|
| |
Use of proceeds
|
|
We expect to receive approximately $7,000,000 in net proceeds from the sale of our units offered by us in this offering (approximately $8,085,000 if the underwriters exercise their over-allotment option in full), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, assuming the units are offered at $6.00 per unit and Series B Warrant, the midpoint of the estimated price range set forth on the cover of this prospectus. We intend to use the net proceeds from this offering for:
|
| |
|
|
|
Regulatory approval, pre-clinical testing, clinical trials if required, product development and commercialization;
|
| |
|
|
|
continuing research and development related activities with respect to the DenerVex;
|
| |
|
|
|
maintenance and enforcement of existing and future patents, pursuit of existing patent applications and additional future patent applications;
|
| |
|
|
|
the remainder for working capital and general corporate purposes which may include the acquisition of medical products’ companies or their technologies or the development of new technologies.
|
| |
|
|
|
|
| |
|
|
See “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering.
|
| |
Risk factors
|
|
You should read the "Risk Factors" section starting on page 15 of this prospectus for a discussion of factors to consider carefully before deciding to invest in our securities.
|
| |
Proposed NASDAQ Capital Market Symbol
|
|
We intend to apply to list our common stock on The NASDAQ Capital Market under the symbol “MDVX,” the Series A Warrants will trade under the symbol “MDVXW,” and our units will trade under the symbol “MDVXU.” No assurance can be given that our application will be approved.
|
The number of shares of our common stock outstanding after this offering is based on 7,781,175 shares of our common stock outstanding as of December 31, 2013, assuming an initial public offering price of $6.00 per unit and Series B Warrant which is the midpoint of the price range set forth on the cover page of this prospectus.
The number of shares of our common stock outstanding after this offering excludes:
|
|
1,150,000 shares of our common stock available for future issuance as of March 31, 2014 under our 2013 Stock Incentive Plan of which options to purchase 60,000 shares of common stock have been granted at a price of $2.50 per share; and
|
|
|
up to shares of common stock issuable upon the full exercise of the Series A Warrants and the Series B Warrants offered hereby;
|
|
|
shares of common stock underlying the Representative’s Unit Warrant to be issued to the representative of the underwriters in connection with this offering, at an exercise price per share equal to 120% of the public offering price, plus up to 202,500 shares, 202,500 Series A Warrants and 202,500 Series B Warrants if the over-allotment unit purchase option is exercised in full.
|
Unless otherwise indicated, all information in this prospectus assumes:
|
|
no exercise of the Representative’s unit warrant included as part of the Representative’s Units described above; and
|
|
|
no exercise by the representative of the underwriters of its over-allotment purchase option.
|
Summary Financial Information
You should read the following summary financial data together with our financial statements and the related notes appearing at the end of this prospectus and the "Capitalization," and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and related notes included elsewhere in this prospectus. We have derived the statements of operations data for the year ended December 31, 2013 from our audited financial statements included in this prospectus. We have derived the balance sheet and statement of operations for March 31, 2014 from our unaudited internal statements. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period.
| |
|
Period from Inception at February 1, 2013 to December 31, 2013 |
|
|
Three Months
Ended March 31,
2014
(unaudited)
|
|
| |
|
(in thousands, except
per share data)
|
|
|
(in thousands,
except
per share data)
|
|
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
General and administrative
|
|
$ |
583 |
|
$ |
435 |
|
|
Research and development
|
|
|
90 |
|
|
122 |
|
|
Total operating expenses
|
|
|
673 |
|
|
557 |
|
|
Loss from operations
|
|
|
(673 |
) |
|
( 557 |
) |
|
Net loss attributable to common stockholders
|
|
$ |
(673 |
) |
$ |
( 557 |
) |
|
Net loss per share- basic and diluted (1)
|
|
$ |
(0.15 |
) |
$ |
(0.07 |
) |
|
Weighted average shares- basic and diluted
|
|
|
4,469 |
|
|
7,781 |
|
| |
|
As of March 31, 2014 |
|
|
(As
Adjusted(2))
|
|
| |
|
(in thousands)
|
|
|
(unaudited) |
|
|
Consolidated Balance Sheet Data:
|
|
(Actual) |
|
|
|
|
|
Cash
|
|
$ |
2,241 |
|
|
9,241 |
|
|
Working capital
|
|
|
2,047 |
|
|
9,047 |
|
|
Total assets
|
|
|
3,934 |
|
|
10,934 |
|
|
Total stockholders’ equity
|
|
|
3,127 |
|
|
10,127 |
|
| (1) |
See our consolidated financial statements and related notes for a description of the calculation of the weighted-average number of shares used in computing the per common share data. |
| (2) |
Gives effect to the sale of the Units offered hereby, and the receipt of $7,000,000 of net proceeds therefrom. |
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus, before deciding to invest in our securities. If any of the following risks actually occur, our business, prospects, operating results and financial condition could suffer materially, the trading price of our securities could decline and you could lose all or part of your investment.
Risks Related to Our Financial Position and Capital Requirements
We are a development stage company and face uncertainties associated with being an early stage venture.
Our operating subsidiary, Debride, was incorporated in October 2012. MEDOVEX was incorporated on July 30, 2013. We currently do not have any material assets, other than the intellectual property relating to the DenerVex obtained from Scott M. W. Haufe, M.D. in connection with our acquisition of Debride. We face all of the potential expenses, delays, uncertainties and complications typically encountered by development stage businesses, many of which may be beyond our control. These include, but are not limited to, lack of sufficient capital, unanticipated problems, delays or expenses relating to product development and licensing and marketing activities, competition, technological changes and uncertain market acceptance. In addition, if we are unable to manage growth effectively, our operating results could be materially and adversely affected.
We are in the early stage of product development and there can be no assurance that we will effectively and successfully develop products for commercialization.
We do not have any presently marketable products. The initial product we are developing has had only limited research and testing in the fields of use we are presently intending to explore and to commercialize. We will have to continue to go through extensive research and testing to develop the initial product and any additional products and to determine or demonstrate the safety and effectiveness of their proposed use. Our products and our proposed testing of those products will require various regulatory approvals and clearances. Accordingly, the products we intend to pursue are not presently marketable in the fields of use for which we hope to develop them, and it is possible that some or all of them may never become legally and commercially marketable. The development and testing of medical devices and related treatments and therapies is difficult, time-consuming and expensive, and the successful development of any products based on innovative technologies is subject to inherent uncertainties and risks of failure. These risks include the possibilities that any or all of the proposed products or procedures may be found to be ineffective, or may otherwise fail to receive necessary regulatory clearances; that the proposed products or procedures may be uneconomical to produce and market or may never achieve broad market acceptance; that third parties may hold proprietary rights that preclude the Company from marketing its intended products or procedures; or that third parties may develop and market superior or equivalent products and procedures. We are unable to predict whether our research and development or acquisition activities will result in any commercially viable products or procedures. Furthermore, due to the extended testing and regulatory review process required before marketing clearances can be obtained, the time frames for commercialization of any products or procedures are long and uncertain.
We expect to continue to incur losses for the immediate future.
We have incurred losses since our inception and have never generated any revenues. We expect to continue to incur losses for the foreseeable future. The principal causes of our losses are likely to be personnel costs, working capital costs, research and development costs, intellectual property protection costs, brand development costs, marketing and promotion costs, and the lack of any significant revenue stream for the foreseeable future. We may never achieve profitability.
Our independent registered public accounting firm has included an explanatory paragraph with respect to our ability to continue as a going concern in its report on our consolidated financial statements for the period ended December 31, 2013.
Our independent registered public accounting firm has included an explanatory paragraph with respect to our ability to continue as a going concern in its report on our consolidated financial statements for the period ended December 31, 2013. The presence of the going concern explanatory paragraph may have an adverse impact on our relationship with third parties with whom we do business, including our customers, vendors and employees and could make it challenging and difficult for us to raise additional debt or equity financing to the extent needed, all of which could have a material adverse impact on our business, results of operations, financial condition and prospects.
Raising additional capital and carrying out further acquisitions may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or products.
We will likely seek additional capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect stockholder rights. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise or expend additional funds through strategic partnerships, acquisitions, alliances and/or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies or products, or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts or grant rights to develop and market products that we would otherwise prefer to develop and market ourselves.
Our operating results may fluctuate significantly as a result of a variety of factors, many of which are outside of our control, which could cause fluctuations in the price of our securities.
We are subject to the following factors that may negatively affect our operating results:
| ● |
the announcement or introduction of new products by our competitors; |
| ● |
our ability to upgrade and develop our systems and infrastructure to accommodate growth; |
| ● |
our ability to attract and retain key personnel in a timely and cost effective manner; |
| ● |
technical difficulties; |
| ● |
the amount and timing of operating costs and capital expenditures relating to the expansion of our business, operations and infrastructure; |
| ● |
our ability to identify and enter into relationships with appropriate and qualified third-party providers such as Devicix, LLC for necessary testing, clinical trials and manufacturing services; |
| ● |
regulation by federal, state or local governments; and |
| ● |
general economic conditions, as well as economic conditions specific to the medical device and healthcare industries.
|
As a result of our lack of any operating history and the nature of the markets in which we compete, it is difficult for us to forecast our revenues or earnings accurately. As a strategic response to changes in the competitive environment, we may from time to time make certain decisions concerning expenditures, pricing, service or marketing that could have a material and adverse effect on our business, results of operations and financial condition. Due to the foregoing factors, our quarterly revenues and operating results are difficult to forecast.
We may be unable to manage growth effectively.
As we seek to advance our product candidates, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. We anticipate that a period of significant expansion will be required to address potential growth and to handle licensing and research activities. This expansion will place a significant strain on our management, operational and financial resources. To manage the expected growth of our operations and personnel, we must establish appropriate and scalable operational and financial systems, procedures and controls and must establish a qualified finance, administrative and operations staff. As a public company, we will have to implement internal controls to comply with government mandated regulations. Our management may be unable to hire, train, retain, motivate and manage the necessary personnel or to identify, manage and exploit potential strategic relationships and market opportunities. Our failure to manage growth effectively could have a material and adverse effect on our business, results of operations and financial condition.
Risks Related to Development, Clinical Testing and Regulatory Approval of Our Products
Government regulation of our business is extensive and regulatory approvals are uncertain, expensive and time-consuming.
Our research, development, testing and clinical trials, manufacturing and marketing of most of our intended products are subject to an extensive regulatory approval process by the FDA and other regulatory agencies in the U.S. and abroad. The process of obtaining FDA and other required regulatory approvals for medical device products, including the potential for being required to engage in pre-clinical and clinical testing, is lengthy, expensive and uncertain. There can be no assurance that, even after such time and expenditures, the Company will be able to obtain necessary regulatory approvals for clinical testing or for the manufacturing or marketing of any products. In addition, during the regulatory process, other companies may develop other technologies with the same intended use as our products. Even if regulatory clearance is obtained, a marketed product is subject to continual review, and later discovery of previously unknown safety issues or failure to comply with the applicable regulatory requirements may result in restrictions on a product’s marketing or withdrawal of the product from the market, as well as possible civil or criminal sanctions.
If the third-parties on which we may need to rely to conduct any clinical trials and to assist us with pre-clinical development or other key steps do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval for or commercialize our product.
We do not have (and do not expect to develop) the independent ability to independently conduct pre-clinical and clinical trials for our products and to the extent we will need to conduct such trials, we will likely need to rely on third-parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct such trials. We also do not have (and do not expect to develop) the independent ability to manufacture our proposed products, and will therefore need to rely on third parties such as contract manufacturing organizations. If these various third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, or if the quality or accuracy of the data they obtain or the quality of the products they produce for us is compromised due to the failure to adhere to our clinical or manufacturing protocols or regulatory requirements or for any other reasons, we may have difficulty replacing them with other qualified third-party providers of the necessary services or products and in the meantime, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance or approval for, or successfully commercialize, a product on a timely basis, if at all. As such, our business, operating results and prospects may be adversely affected and may even fail entirely. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their (or our) control.
The results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects.
Even if the clinical trials that we may need to undertake are completed as planned, we cannot be certain that their results will support our product claims or that the FDA or foreign authorities will agree with our conclusions regarding the results of the trials. The clinical trial process may fail to demonstrate that a product is safe and effective for the proposed indicated use, which could cause us to abandon a product and could delay development of other products. Any delay or termination of our clinical trials will delay the filing of our product submissions and, ultimately, our ability to commercialize a product and generate revenue. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product’s profile and not predicted or foreseen on the basis of prior experience. Even if clinical trials are otherwise successful, we may be unable to develop a commercially viable product, treatment or therapy based on those trials.
Risks Related to Our Business and Industry
If our products and products do not gain market acceptance among physicians, patients and the medical community, we may be unable to generate significant revenues, if any.
Even if we obtain regulatory approval for our products, they may not gain market acceptance among physicians, healthcare payers, patients and the medical community. In particular, the US government agency Center for Medicare/Medicaid Service or other private reimbursement agencies may decline to reimburse physicians and health care facilities whose patients are on Medicare or Medicaid or private insurance for use of our product, significantly reducing our potential market. Market acceptance will depend on our ability to demonstrate the benefits of our approved products in terms of safety, efficacy, convenience, ease of administration and cost effectiveness. In addition, we believe market acceptance depends on the effectiveness of our marketing strategy, the pricing of our approved products and the reimbursement policies of government and third party payers with respect to our products. Physicians may not utilize our approved products for a variety of reasons and patients may determine for any reason that our product is not useful to them. If any of our approved products fail to achieve market acceptance, our ability to generate revenues will be limited.
The industry in which we plan to operate is highly competitive and there can be no assurances that we will be able to compete effectively.
We are engaged in a rapidly evolving industry. Competition from other medical device companies and from other research and academic institutions is intense and expected to increase. Many of these companies have substantially greater financial and other resources and development capabilities than we do, have substantially greater experience in undertaking pre-clinical and clinical testing of products, and are commonly regarded in the medical device industries as very aggressive competitors. In addition to competing with universities and other research institutions in the development of products, technologies and processes, we compete with other companies in acquiring rights to products or technologies from universities. There can be no assurance that we can develop products that are more effective or achieve greater market acceptance than competitive products, or that our competitors will not succeed in developing products and technologies that are more effective than those being developed by us and that would therefore render our products and technologies less competitive or even obsolete.
Third parties may claim that we infringe on their proprietary rights and may prevent us from commercializing and selling our products.
There has been substantial litigation in the medical device industry with respect to the manufacture, use and sale of new products. These lawsuits often involve claims relating to the validity of patents supporting the new products and/or the validity and alleged infringement of patents or proprietary rights of third parties. We may be required to defend against challenges to the validity of our patents and against claims relating to the alleged infringement of patent or proprietary rights of third parties.
Litigation initiated by a third party claiming patent invalidity or patent infringement could:
|
|
require us to incur substantial litigation expense, even if we are successful in the litigation;
|
|
|
require us to divert significant time and effort of our management;
|
|
|
result in the loss of our rights to develop, make or market our products; and
|
|
|
require us to pay substantial monetary damages or royalties in order to license proprietary rights from third parties or to satisfy judgments or to settle actual or threatened litigation.
|
Although patent and intellectual property disputes within the medical device industry have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. Furthermore, the required licenses may not be made available to us on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing and selling our products or increase our costs to market our products.
Healthcare policy changes, including the recently enacted legislation to reform the United States healthcare system, may have a material adverse effect on us.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively the “PPACA”), which substantially changes the way healthcare is financed by both governmental and private insurers, encourages improvements in the quality of healthcare items and services, and significantly impacts the medical device industry. The PPACA includes, among other things, the following measures:
|
|
a 2.3% excise tax on any entity that manufactures or imports medical devices offered for sale in the United States, with limited exceptions;
|
|
|
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities and conduct comparative clinical effectiveness research;
|
|
|
new reporting and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to physicians and teaching hospitals, as well as reporting of certain physician ownership interests
|
|
|
payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and
|
|
|
an independent payment advisory board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate.
|
These provisions could meaningfully change the way healthcare is delivered and financed, and could have a material adverse impact on numerous aspects of our business.
In the future, there may continue to be additional proposals relating to the reform of the United States healthcare system. Certain of these proposals could limit the prices we are able to charge for our products or the amounts of reimbursement available for our products, and could limit the acceptance and availability of our products. The adoption of some or all of these proposals could have a material adverse effect on our business, results of operations and financial condition.
Additionally, initiatives sponsored by government agencies, legislative bodies and the private sector to limit the growth of healthcare costs, including price regulation and competitive pricing, are ongoing in markets where we do business. We could experience an adverse impact on our operating results due to increased pricing pressure in the United States and in other markets. Governments, hospitals and other third party payors could reduce the amount of approved reimbursement for our products or deny coverage altogether. Reductions in reimbursement levels or coverage or other cost-containment measures could adversely affect our future operating results.
We depend on key personnel.
We depend greatly on Dr. Scott M. W. Haufe, a member of the board of directors and the co-founder of Debride, Jarrett Gorlin, our Chief Executive Officer, and a member of the board of directors and Patrick Kullmann, our President and Chief Operating Officer, among others. Our success will depend, in part, upon our ability to attract and retain additional skilled personnel, which will require substantial additional funds. There can be no assurance that we will be able to find, attract and retain additional qualified employees, directors, and advisors having the skills necessary to operate, develop and grow our business. Our inability to hire qualified personnel, the loss of services of Dr. Haufe, Mr. Gorlin or Mr. Kullmann, or the loss of services of other executive officers, key employees, or advisors that may be hired in the future, may have a material and adverse effect on our business. We currently do not maintain “key man” insurance policies on the lives of these individuals or the lives of any of our other employees.
In the future, we could experience difficulties attracting and retaining qualified employees. Competition for qualified personnel in the medical products field is intense. We may need to hire additional personnel as we expand our clinical development and commercial activities. We may not be able to attract and retain quality personnel on acceptable terms or at all.
In addition, we may enter into arrangements with consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
If we are unable to hire qualified personnel, our business and financial condition may suffer.
Our success and achievement of our growth plans depend on our ability to recruit, hire, train and retain other highly qualified technical and managerial personnel. In this regard, we have limited resources and as such we may not able to provide an employee with the same amount of compensation that he or she would likely receive at a larger company and as a result we may face difficulty in finding qualified employees. The inability to attract, retain and motivate any additional highly skilled employees required for the expansion of our activities, could have a materially adverse effect on our ability to conduct our business and as such can impair our operations.
If we obtain approval to commercialize our products outside of the United States, a variety of risks associated with international operations could materially adversely affect our business.
If our products are approved for commercialization outside the United States, we will likely seek to enter into agreements with third parties to market our products outside the United States. We expect that we will be subject to additional risks related to entering into or maintaining international business relationships, including:
|
|
different regulatory requirements for medical devices or treatments in foreign countries;
|
|
|
lack of adequate reimbursement for the use of our product;
|
|
|
differing United States and foreign import and export rules;
|
|
|
reduced protection for intellectual property rights in foreign countries;
|
|
|
unexpected changes in tariffs, trade barriers and regulatory requirements;
|
|
|
economic weakness, including inflation, or political instability in particular foreign economies and markets;
|
|
|
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
|
|
|
foreign taxes, including withholding of payroll taxes;
|
|
|
foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
|
|
|
workforce uncertainty in countries where labor unrest is more common than in the United States;
|
|
|
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad;
|
|
|
potential liability resulting from development work conducted by these distributors; and
|
|
|
business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters.
|
Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenues from our products. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be adversely affected.
We face substantial competition, which may result in others discovering, developing or commercializing products before, or more successfully, than we do.
Our future success depends on our ability to demonstrate and maintain a competitive advantage with respect to the design, development and commercialization of new and novel products. Our competitors may succeed in developing competing products before we do for the same indications that we are pursuing, obtaining regulatory approval for products or gaining acceptance for the same markets that we are targeting. If we are not "first to market" with one of our products, our competitive position could be compromised because it may be more difficult for us to obtain marketing approval for that product and successfully market that product as a second competitor.
Many of our competitors have substantially greater commercial infrastructures and financial, technical and personnel resources than we have. We will not be able to compete successfully unless we successfully:
|
|
design and develop products that are superior to other products in the market;
|
|
|
attract qualified scientific, medical, sales and marketing and commercial personnel;
|
|
|
obtain patent and/or other proprietary protection for our processes and products;
|
|
|
obtain required regulatory approvals; and
|
|
|
collaborate with others in the design, development and commercialization of new products.
|
Established competitors may invest heavily to quickly discover and develop novel treatments that could make our products obsolete. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
If our future employees or third parties with whom we contract commit fraud or other misconduct, including noncompliance with regulatory standards and requirements, our business may experience serious adverse consequences.
We are exposed to the risk of employee or third party fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, to provide accurate information to the FDA, to comply with manufacturing standards we have established, to comply with federal and state health-care fraud and abuse laws and regulations, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a Code of Business Conduct and Ethics but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of any products.
We face an inherent risk of product liability as a result of any clinical testing of our products and will face an even greater risk if we commercialize any products. We may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
|
|
decreased demand for our products or products that we may develop;
|
|
|
injury to our reputation;
|
|
|
withdrawal of clinical trial participants;
|
|
|
costs to defend the related litigation;
|
|
|
a diversion of management's time and our resources;
|
|
|
substantial monetary awards to trial participants or patients;
|
|
|
product recalls, withdrawals or labeling, marketing or promotional restrictions;
|
|
|
the inability to commercialize our products; and
|
|
|
a decline in our stock price.
|
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. We currently do not maintain product liability insurance because it is generally expensive, and in light of our developmental stage we do not believe it is cost effective to obtain at this time. If we commence sales, we expect to secure product liability insurance; however, we may not be able to obtain or maintain product liability insurance in the future on acceptable terms or with adequate coverage against potential liabilities, if at all. If we are the subject of a successful product liability claim that exceeds the limits of any insurance coverage we obtain, we would incur substantial charges that would adversely affect our earnings and require the commitment of capital resources that might otherwise be available for the development and commercial launch of our products.
We may not be able to secure adequate clinical trial liability insurance for all of our products and a successful clinical trial liability claim against us could have an adverse effect on our financial condition even with such insurance coverage.
Our business will expose us to potential liability that results from risks associated with conducting clinical trials of our products. There is no guarantee that we will be able to procure clinical trial liability insurance at favorable rates, if at all, and even if procured that we will procure adequate coverage to satisfy any liability we may incur. A successful clinical trial liability claim, if any, brought against us could have a material adverse effect on our business, prospects, financial condition and results of operations even though clinical trial insurance is successfully maintained or obtained. The current and planned insurance coverages may only mitigate a small portion of a substantial claim against us.
Our relationships with customers and third-party payors in the United States and elsewhere will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors in the United States and elsewhere play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal, state and foreign healthcare laws and regulations include the following:
|
|
the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid;
|
|
|
the federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
|
the federal Health Insurance Portability and Accountability Act of 1996, or HIPPA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program. HIPAA and HITECH also regulate the use and disclosure of identifiable health information by health care providers, health plans and health care clearinghouses, and also impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of identifiable health information as well as requiring notification of regulatory breaches. HIPAA and HITECH violations may prompt civil and criminal enforcement actions as well as enforcement by state attorneys general;
|
|
|
the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
|
|
|
the federal transparency requirements under the Health Care Reform Law requires manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests;
|
|
|
analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; and
|
|
|
analogous anti-kickback, fraud and abuse and healthcare laws and regulations in foreign countries.
|
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Risks Related to Commercialization of Our Products
If, in the future, we are unable to establish our own sales, marketing and distribution capabilities or enter into licensing or collaboration agreements for these purposes, we may not be successful in commercializing our products.
We currently have a relatively small number of employees and do not have a sales or marketing infrastructure, and we, do not have any significant sales, marketing or distribution experience. We intend to be opportunistic in seeking to either build our own commercial infrastructure to commercialize our products if and when they are approved, or enter into licensing or collaboration agreements to assist in the future development and commercialization of such products.
If we choose to develop internal sales, distribution and marketing capabilities, we will likely have to invest significant amounts of financial and management resources, some of which will be committed prior to any confirmation that any product will be approved. For products for which we decide to perform sales, marketing and distribution functions ourselves, we could face a number of additional risks, including:
|
|
our inability to recruit and retain adequate numbers of effective sales and marketing personnel;
|
|
|
the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to utilize our procedures;
|
|
|
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and
|
|
|
unforeseen costs and expenses associated with creating an independent sales and marketing organization.
|
Where and when appropriate, we may elect to utilize contract sales forces or strategic partners to assist in the commercialization of our products. If we enter into arrangements with third parties to perform sales, marketing and distribution services for our products, the resulting revenues or the profitability from these revenues to us are likely to be lower than if we had sold, marketed and distributed our products ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute our products or may be unable to do so on terms that are favorable to us. We likely will have limited control over such third parties, and any of these third parties may fail to devote the necessary resources and attention to sell, market and distribute our products effectively.
If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our products.
Risks Related to Our Dependence on Third Parties
We are dependent on contract research organizations and other contractors to assist in our clinical testing and for certain research and development activities, thus, the timing and adequacy of our clinical trials and such research activities are, to a certain extent, beyond our control.
The nature of clinical trials and our business strategy will likely require us to rely on contract research organizations, independent clinical investigators and other third party service providers to assist us with clinical testing and certain research and development activities. Our success is dependent upon the success of these outside parties in performing their responsibilities. Although we believe our contractors are economically motivated to perform on their contractual obligations, we cannot directly control the adequacy and timeliness of the resources and expertise applied to these activities by our contractors. If our contractors do not perform their activities in an adequate or timely manner, the development and commercialization of our products could be delayed.
We rely on third parties to manufacture our products and as a result we may not be able to control our product development.
We do not currently own or operate any manufacturing facilities, and we lack sufficient internal staff to produce clinical and preclinical product supplies ourselves. As a result, we are working with a third-party contract manufacturer to produce sufficient quantities of our products for future clinical trials, preclinical testing and commercialization.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the third party for regulatory compliance and quality assurance, the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control (including a failure to manufacture our products in accordance with our product specifications) and the possibility of termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or damaging to us. We will be dependent on the ability of these third-party manufacturers to produce adequate supplies of medical products to support our clinical development programs and future commercialization of our products. In addition, the FDA and other regulatory authorities require that our products be manufactured according to cGMP and similar foreign standards. Any failure by our third-party manufacturer to comply with cGMP or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of products in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our products. In addition, such failure could be the basis for action by the FDA to withdraw approvals for products previously granted to us and for other regulatory action, including recall or seizure, fines, imposition of operating restrictions, total or partial suspension of production or injunctions.
We have limited staffing and rely on our third party manufacturer to purchase from third-party suppliers the materials necessary to produce our products. There are a limited number of suppliers for certain capital equipment and materials that we use to manufacture our products. Such suppliers may not sell these materials to our manufacturer at the times we need them or on commercially reasonable terms. We do not have any control over the process or timing of the acquisition of these materials by our third party manufacturer. If our manufacturer or we are unable to purchase these materials after regulatory approval has been obtained for our products, the commercial launch of our products would be delayed or there would be a shortage in supply, which would impair our ability to generate revenues from the sale of our products.
In addition, our manufacturer may not be able to manufacture our products at a cost or in quantities or in a timely manner necessary to develop and commercialize them. If we successfully commercialize the DenerVex or any of our products, we may be required to establish or access large-scale commercial manufacturing capabilities. In addition, as our development pipeline increases and matures, we may have a greater need for clinical trial and commercial manufacturing capacity. To meet our projected needs for commercial manufacturing the third party with whom we currently work will need to increase its scale of production or we will need to secure an alternate supplier.
We may not be successful in establishing and maintaining strategic partnerships, which could adversely affect our ability to develop and commercialize products.
We may seek to enter into strategic partnerships in the future, including alliances with other healthcare companies, to enhance and accelerate the development and commercialization of our products. We face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for any future products and programs because our research and development pipeline may be insufficient, our products and programs may be deemed to be at too early of a stage of development for collaborative effort and/or third parties may not view our products and programs as having the requisite potential to demonstrate safety and efficacy. Even if we are successful in our efforts to establish strategic partnerships, the terms that we agree upon may not be favorable to us and we may not be able to maintain such strategic partnerships if, for example, development or approval of a product is delayed or sales of an approved product are disappointing.
If we ultimately determine that entering into strategic partnerships is in our best interest but either fail to enter into, are delayed in entering into or fail to maintain such strategic partnerships:
|
|
the development of certain of our current or future products may be terminated or delayed;
|
|
|
our cash expenditures related to development of certain of our current or future products would increase significantly and we may need to seek additional financing;
|
|
|
we may be required to hire additional employees or otherwise develop expertise, such as sales and marketing expertise, for which we have not budgeted;
|
|
|
we will bear all of the risk related to the development of any such products; and
|
|
|
the competitiveness of any product that is commercialized could be reduced.
|
Risks Related to Our Intellectual Property Rights
We could be unsuccessful in obtaining adequate patent protection for one or more of our products.
We cannot be certain that our patents will not later be found to be invalid and/or unenforceable or that any new patents that we seek to obtain will be issued or granted. The patent position of medical products companies is generally uncertain because it involves complex legal and factual considerations. The standards applied by the United States Patent and Trademark Office and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in medical product patents. Consequently, patents may not issue from our pending patent applications. As such, we do not know the degree of future protection that we will have on our proprietary products and technology.
We have obtained a patent with respect to our technology both domestically and internationally and anticipate potentially filing multiple patent applications, in the future. While we believe that we will be able to secure adequate and enforceable patent protection for our products and technologies, there is no guarantee that patent protection can be obtained, and even if it is obtained that such patent protection will ultimately be deemed valid, sufficiently enforceable, sufficient to preclude competition or not infringe upon the rights of other parties.
Our commercial success may depend in part on our ability to obtain additional patents and protect our existing patent position as well as our ability to maintain adequate protection of other intellectual property for our technologies, products, and any future products in the United States and other countries. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any market exclusivity related competitive advantage we may have, which could harm our business and ability to achieve profitability. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries.
Issued patents covering one or more of our products could be found invalid or unenforceable if challenged in court.
If we were to initiate legal proceedings against a third party to enforce a patent covering one of our products, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. Patent and Trademark Office, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our products. Such a loss of patent protection could have a material adverse impact on our business.
Claims that our products or the sale or use of our products infringe the patent rights of third parties could result in costly litigation or could require substantial time and money to resolve, even if litigation is avoided.
We cannot guarantee that our products or, the use of our products does not infringe any third party patents. Third parties might allege that we are infringing their patent rights or that we have misappropriated their trade secrets. Such third parties might resort to litigation against us. The basis of such litigation could be existing patents or patents that issue in the future. Our failure to successfully defend against any claims that our products infringe the rights of third parties could also adversely affect our business.
It is also possible that we failed to identify relevant patents or applications. For example, applications filed before November 29, 2000 and certain applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our products could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our products or the use of our products.
In order to avoid or settle potential claims with respect to any patent rights of third parties, we may choose or be required to seek a license from a third party and be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we or any future strategic partners were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing one or more of our products, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. This could harm our business significantly.
Defending against claims of patent infringement or misappropriation of trade secrets could be costly and time consuming, regardless of the outcome. Thus, even if we were to ultimately prevail, or to settle at an early stage, such litigation could burden us with substantial unanticipated costs. In addition, litigation or threatened litigation could result in significant demands on the time and attention of our management team, distracting them from the pursuit of other Company business.
Unfavorable outcomes in intellectual property litigation could limit our research and development activities and/or our ability to commercialize certain products.
If third parties successfully assert intellectual property rights against us, we might be barred from using certain aspects of our product technology, or we may be barred from developing and commercializing certain products. Prohibitions against using certain technologies, or prohibitions against commercializing certain products, could be imposed by a court or by a settlement agreement between us and a plaintiff. In addition, if we are unsuccessful in defending against allegations of patent infringement or misappropriation of trade secrets, we may be forced to pay substantial damage awards to the plaintiff. There is inevitable uncertainty in any litigation, including intellectual property litigation. There can be no assurance that we would prevail in any intellectual property litigation, even if the case against us is weak or flawed. If litigation leads to an outcome unfavorable to us, we may be required to obtain a license from the patent owner, in order to continue our research and development programs or to market our product(s). It is possible that the necessary license will not be available to us on commercially acceptable terms, or at all. This could limit our research and development activities, our ability to commercialize certain products, or both.
Most of our competitors are larger than we are and have substantially greater resources. They are, therefore, likely to be able to sustain the costs of complex patent litigation longer than we could. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations, or enter into strategic partnerships that would help us bring our products to market.
In addition, any future patent litigation, interference or other administrative proceedings will result in additional expense and distraction of our personnel. An adverse outcome in such litigation or proceedings may expose us or any future strategic partners to loss of our proprietary position, expose us to significant liabilities, or require us to seek licenses that may not be available on commercially acceptable terms, if at all.
Confidentiality agreements with employees and third parties may not prevent unauthorized disclosure of trade secrets and other proprietary information.
In addition to patents, we rely on trade secrets, technical know-how, and proprietary information concerning our business strategy in order to protect our competitive position. In the course of our research and development activities and our business activities, we often rely on confidentiality agreements to protect our proprietary information. Such confidentiality agreements are used, for example, when we talk to manufacturers or clinical development services or potential strategic partners. In addition, each of our employees is required to sign a confidentiality agreement upon joining us. We take steps to protect our proprietary information, and our confidentiality agreements are carefully drafted to protect our proprietary interests. Nevertheless, there can be no guarantee that an employee or an outside party will not make an unauthorized disclosure of our proprietary confidential information. This might happen intentionally or inadvertently. It is possible that a competitor will make use of such information, and that our competitive position will be compromised, in spite of any legal action we might take against persons making such unauthorized disclosures.
Trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, or outside scientific collaborators might intentionally or inadvertently disclose our trade secret information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States sometimes are less willing than U.S. courts to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
Our research and development strategic partners may have rights to publish data and other information to which we have rights. In addition, we may engage individuals or entities to conduct research relevant to our business. The ability of these individuals or entities to publish or otherwise publicly disclose data and other information generated during the course of their research is subject to certain contractual limitations. These contractual provisions may be insufficient or inadequate to protect our confidential information. If we do not apply for patent protection prior to such publication, or if we cannot otherwise maintain the confidentiality of our proprietary technology and other confidential information, then our ability to obtain patent protection or to protect our trade secret information may be jeopardized.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
|
|
others may be able to make products that are the same as or similar to our products but that are not covered by the claims of the patents that we own or have exclusively licensed;
|
|
|
we or any future strategic partners might not have been the first to make the inventions covered by the issued patent or pending patent application that we own;
|
|
|
we or any future strategic partners might not have been the first to file patent applications covering certain of our inventions;
|
|
|
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
|
|
|
issued patents that we own may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors;
|
|
|
our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
|
|
|
we may not develop additional proprietary technologies that are patentable; and
|
|
|
the patents of others may have an adverse effect on our business.
|
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other medical products companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the medical products industry involve both technological complexity and legal complexity. Therefore, obtaining and enforcing medical industry patents is costly, time-consuming and inherently uncertain. In addition, Congress may pass patent reform legislation. The Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The U.S. Patent and Trademark Office and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
Internationally, we may apply for patent protection relating to certain existing and proposed products and processes. While we will generally apply for patents in those countries where we intend to make, use or sell patented products, we may not accurately predict all of the countries where patent protection will ultimately be desirable. If we fail to timely file a patent application in any such country, we may be precluded from doing so at a later date. Furthermore, we cannot assure you that any of our patent applications (domestic or international) will be approved. The rights granted to us under our patents, including prospective rights sought in our pending patent applications, may not be meaningful or provide us with any commercial advantage and they could be opposed, contested or circumvented by our competitors or be declared invalid or unenforceable in judicial or administrative proceedings. The failure of our patents to adequately protect our technology might make it easier for our competitors to offer the same or similar products or technologies. Competitors may be able to design around our patents or develop products that provide outcomes which are comparable to ours without infringing on our international intellectual property rights. In the event of unauthorized use or disclosure or other breaches of such agreements, we may not be provided with meaningful protection for our trade secrets or other proprietary information. Due to differences between foreign and U.S. patent laws, our patented intellectual property rights may not receive the same degree of protection in foreign countries as they would in the United States. Even if patents are granted outside the United States, effective enforcement in those countries may not be available. In countries where we do not have significant patent protection, we may not be able to stop a competitor from marketing products in such countries that are the same as or similar to our products. Moreover, we may not have sufficient resources or desire to defend our patents or trademarks against challenges or to enforce our intellectual property rights, especially if those rights are international in scope and venue.
Risks Related to Our Common Stock and this Offering
After this offering, our executive officers, directors and principal stockholders will maintain the ability to control all matters submitted to stockholders for approval.
Upon the closing of this offering, our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock before this offering will, in the aggregate, beneficially own 3,573,576 shares representing approximately 38.7% of our outstanding capital stock. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of us on terms that other stockholders may desire.
If you purchase shares of common stock in this offering, you will suffer immediate dilution in the book value of your shares.
The initial public offering price of our common stock is substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent outstanding options are exercised, you will incur further dilution. Based on an assumed initial public offering price of $6.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and attributing no value to the Series A and Series B Warrants, you will experience immediate dilution of $5.01 per share, representing the difference between our pro forma net tangible book value per share after giving effect to this offering and the assumed initial public offering price. In addition, purchasers of common stock in this offering will have contributed approximately 70 % of the aggregate price paid by all purchasers of our stock but will own only approximately 15 % of our common stock outstanding after this offering. For a further description of the dilution that you will experience immediately after this offering, see “Dilution.”
The issuance of warrants in this offering will cause you to experience additional dilution if those warrants are exercised.
In addition to the shares of common stock we are issuing in this offering, we are also issuing an equal number of Series A Warrants and Series B Warrants. The Series A Warrants and Series B Warrants being issued in conjunction with this offering are exercisable for an equal number of shares of our common stock. If the holders of the Series A Warrants and the Series B Warrants exercise their warrants, you will experience dilution at the time they exercise their warrants.
We are also offering a representative's Unit Warrant to the representative of the underwriters in this offering that is exercisable for 10% of the securities sold in this offering, excluding shares of common stock from units sold pursuant to the over-allotment option, if any. If the representative of the underwriters exercises this unit purchase option, you will experience additional dilution. If the representative of the underwriter exercises its unit purchase over-allotment option, you will experience additional dilution. For a further description of the dilution that you will experience immediately after this offering, see “Dilution.”
We do not know whether a market will develop for our common stock and warrants or what the market price of our common stock and warrants will be and as a result it may be difficult for you to sell your shares of our common stock and/or warrants.
Prior to this offering, there has been no public market for our common stock and we do not expect any active market to develop for our warrants. Although we intend to apply to list our common stock and our Series A Warrants on the NASDAQ Capital Market, an active trading market for our securities may never develop or be sustained following this offering. If an active market for our securities does not develop, it may be difficult for you to sell shares or Series A Warrants you purchase in this offering without depressing the market price for the shares or at all. The initial public offering price for our common stock will be determined through negotiations with the underwriters and the negotiated price may not be indicative of the market price for our common stock after this offering. The initial public offering price may vary from the market price of our common stock and Series A Warrants after the offering. As a result of these and other factors, you may not be able to sell your shares of our common stock and Series A Warrants at or above the initial public offering price or at all. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration.
The Series B Warrants are non-transferable and there will be no market for them which may negatively impact the secondary trading value of the Units and the underlying securities.
You may not sell, transfer or assign your Series B Warrants to anyone else. We do not intend to list the Series B Warrants on any securities exchange or any other trading market. Because the Series B Warrants are non-transferable, there is no market or other means for you to directly realize any value associated with them. You must exercise the Series B Warrants and purchase Common Stock underlying the Series B Warrants to realize any potential value from the Series B Warrants. Because the Series B Warrants are being sold with the Units and are not part of the Units, the secondary value of the Units and the underlying securities may be adversely affected.
If we do not keep this registration statement updated for the term of the warrants, the holders will not be able to exercise the warrants.
While we intend to keep this registration statement/prospectus updated until _______, 2019 (five years from the effective date of the Registration Statement), we may not be able to do so, nor will we necessarily be providing adequate public financial information to allow the holders to sell the Common Stock underlying the Series A and Series B Warrants. Accordingly, investors might not be able to exercise their Series A and Series B Warrants and sell the underlying Common Stock at a time when it is beneficial to do so.
In order to keep the prospectus effective, we will be required to, among other actions, file post-effective amendments to the registration statement containing current financial and other information. Each such registration statement will have to be filed with, and declared effective by the Securities and Exchange Commission. We have no assurance that such post-effective amendments will be declared effective.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. We will be subject to the reporting and other requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and the Dodd-Frank Wall Street Reform and Protection Act, as well as rules subsequently adopted by the SEC and the NASDAQ Stock Market. These rules and regulations will require, among other things, that we file annual, quarterly and current reports with respect to our business and financial condition and establish and maintain effective disclosure and financial controls and corporate governance practices. We expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly, particularly after we are no longer an "emerging growth company" as defined in the recently enacted Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance. We estimate that we will incur between $200,000 and $500,000 annually in expenses in response to these requirements.
If we take advantage of specified reduced disclosure requirements applicable to an "emerging growth company" under the JOBS Act, the information that we provide to stockholders may be different than they might receive from other public companies.
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" under the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
|
|
only two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly reduced "Management's Discussion and Analysis of Financial Condition and Results of Operations" disclosure;
|
|
|
reduced disclosure about our executive compensation arrangements;
|
|
|
no non-binding advisory votes on executive compensation or golden parachute arrangements;
|
|
|
exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting.
|
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1 billion in annual revenues, we have more than $700 million in market value of our stock held by non-affiliates, or we issue more than $1 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced burdens. We have not taken advantage of any of these reduced reporting burdens in this prospectus, although we may choose to do so in future filings. If we do, the information that we provide stockholders may be different than you might get from other public companies in which you hold stock.
If we fail to comply with the rules and regulations under the Sarbanes-Oxley Act, our operating results, our ability to operate our business and investors’ views of us may be harmed.
We will be required to comply with the rules and regulations under the Sarbanes-Oxley Act. Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls and attestations of the effectiveness of internal controls by independent auditors. Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Our failure to maintain the effectiveness of our internal controls in accordance with the requirements of the Sarbanes-Oxley Act could have a material adverse effect on our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our common stock. In addition, our efforts to comply with the rules and regulations under the Sarbanes-Oxley or new or changed laws, regulations, and standards may differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice. Regulatory authorities may investigate transactions disclosed in our “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and if legal proceedings are initiated against us, it may harm our business.
Our management has identified internal control deficiencies which we believe constitutes a material weakness. Any future material weaknesses or deficiencies in our internal control over financial reporting could harm stockholder and business confidence on our financial reporting, our ability to obtain financing and other aspects of our business.
In connection with the preparation of our audited financial statements for the period from February 1, 2013 (inception) through December 31, 2013, we concluded that a material weakness existed in internal control over financial reporting and our disclosure controls. Specifically, our Chief Financial Officer currently performs all accounting related functions. In order to obtain proper segregation of accounting related duties, another person will have to be hired and duties allocated so this material weakness can be corrected. Although we are committed to continuing to improve our internal control processes, and although we will continue to diligently and vigorously review our internal control over financial reporting, any control system, regardless of how well designed, operated and evaluated, can provide only reasonable, not absolute, assurance that its objectives will be met. Therefore, we cannot be certain that, in the future, additional material weaknesses or significant deficiencies will not exist or otherwise be discovered. If our efforts to address the weakness identified are not successful, or if other deficiencies occur, these weaknesses or deficiencies could result in misstatements of our results of operations, restatements of our financial statements, a decline in our stock price and investor confidence or other material effects on our business, reputation, results of operations, financial condition or liquidity.
If our stock price is volatile, our stockholders could incur substantial losses.
Our stock price following this offering is likely to be volatile. The stock market in general and the market for medical products companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your common stock at or above the initial public offering price, if at all. The market price for our common stock may be influenced by many factors, including:
|
|
our ability to commercialize our products, if approved;
|
|
|
results from or delays of clinical trials of our products, as well as results of regulatory reviews relating to the approval of our products;
|
|
|
our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial;
|
|
|
new products, products or new uses for existing products or technologies introduced or announced by our competitors and the timing of these introductions or announcements;
|
|
|
regulatory or legal developments in the United States and other countries;
|
|
|
developments or disputes concerning patent applications, issued patents or other proprietary rights;
|
|
|
the recruitment or departure of key scientific or management personnel;
|
|
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
|
sales of common stock by us or our stockholders in the future, as well as the overall trading volume of our common stock;
|
|
|
market conditions in the medical products sectors; and
|
|
|
general economic, industry and market conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors, including changes in market valuations of similar companies.
|
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our products. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
We do not anticipate paying any cash dividends on our capital stock in the foreseeable future.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business, and we do not anticipate paying any cash dividends on our capital stock in the foreseeable future. We believe it is likely that the Board of Directors will continue to conclude, that it is in the best interests of the Company and its shareholders to retain all earnings (if any) for the development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have 9,131,275 outstanding shares of common stock. This includes the 1,350,000 shares that we are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, 7,781,275 shares are currently restricted as a result of securities laws or lock-up agreements but will be able to be sold after the offering as described in the “Shares Eligible for Future Sale” section of this prospectus. We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the “Underwriting” section of this prospectus.
The rights of the holders of common stock may be impaired by the potential issuance of preferred stock.
The rights of the holders of common stock may be impaired by the potential issuance of preferred stock. Our board of directors has the right, without stockholder approval, to issue preferred stock with voting, dividend, conversion, liquidation or other rights which could adversely affect the voting power and equity interest of the holders of common stock, which could be issued with the right to more than one vote per share, and could be utilized as a method of discouraging, delaying or preventing a change of control. The possible negative impact on takeover attempts could adversely affect the price of our common stock. Although we have no present intention to issue any shares of preferred stock or to create any series of preferred stock, we may issue such shares in the future, as we have authorized (but not issued) 500,000 shares of preferred stock.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our Company. If no or too few securities or industry analysts commence coverage of our Company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts cease coverage of our Company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes statements that are, or may be deemed, "forward-looking statements." In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms "believes," "estimates," "anticipates," "expects," "plans," "intends," "may," "could," "might," "will," "should," "approximately" or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this prospectus and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of products, the strength and breadth of our intellectual property, our potential and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our products, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us.
Industry and Market Data
This prospectus contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this prospectus from our own research as well as from industry and general publications, surveys, and studies conducted by third parties, some of which may not be publicly available. Estimates, forecasts, and surveys are periodically updated by third parties and may materially impact projections in the future.
We estimate that the net proceeds from our issuance and sale of 1,350,000 units and Series B Warrants in this offering will be approximately $7,000,000 assuming an initial public offering price of $6.00 per unit and Series B Warrant which is the midpoint of the price range listed on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their over-allotment option in full, we estimate that the net proceeds from this offering will be approximately $8,085,000.
The largest single variable in our estimates of bringing the product to market is what requirements will be placed on us by the FDA for product clearance (these can vary greatly). We have assumed the most probable path and requirements necessary for clearance, but unexpected requirements or significantly larger patient counts required in a human clinical trial than we are assuming could increase these estimates. We have estimated the following costs to take the product to market and absorb losses until a cash breakeven point could be reached:
|
·
|
$2,300 ,000 for a human clinical trial
|
|
·
|
$1,100,000 for product development (including production of sample units)
|
|
·
|
$890,000 for regulatory, reimbursement and other experts to obtain regulatory approvals and be ready to sell to and be paid by our target customers
|
|
·
|
$726,000 for general and administrative expenses
|
|
·
|
$1,984,000 for working capital and other general corporate purposes
|
The amounts allocated for working capital and other general corporate purposes, may include the development, acquisition or in licensing of medical products’ companies or their technologies. We have no current commitments or binding agreements with respect to any material acquisition of or investment in any technologies, products or companies. Any amounts received from the exercise of the Series A and Series B Warrants will be allocated for working capital.
This expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development efforts, the status of and results from clinical trials, as well as any collaborations that we may enter into with third parties for our products, and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
We expect that the net proceeds from this offering, together with our existing cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements for at least 18 months. Until such time, if ever, as we can generate substantial product revenues, we may finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. We do not have any committed external source of funds. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We have never declared or paid cash dividends on our common stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. We do not intend to pay cash dividends to holders of our common stock in the foreseeable future.
We have two authorized classes of stock: Preferred Stock (500,000 shares authorized), and Common Stock (49,500,000) shares authorized).
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2014 :
|
|
on a pro forma as adjusted basis to give further effect to the issuance and sale of shares of our common stock in this offering at an assumed initial public offering price of $6.00 per unit and Series B Warrant, which is the midpoint of the price range listed on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
|
Our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table together with the section entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and the related notes included elsewhere in this prospectus.
| |
|
As of March 31, 2014 |
|
| |
|
Actual
|
|
|
Pro Forma as Adjusted (1)
|
|
| |
|
|
|
|
|
|
|
|
|
Stockholders’ Equity:
|
|
|
|
|
|
|
|
|
|
Preferred stock, $.001 par value, 500,000 shares authorized, none issued or outstanding actual, pro forma or pro forma as adjusted
|
|
|
- |
|
|
|
- |
|
|
Common stock, $.001 par value, 49,500,000 shares authorized, 7,781,175 issued and outstanding actual; 9,131,275 shares issued and outstanding pro forma as adjusted
|
|
|
7,782 |
|
|
|
9,131
|
|
|
Additional paid in capital
|
|
|
4,349,492 |
|
|
|
11,352,142 |
|
|
Accumulated deficit
|
|
|
(1,230,508 |
) |
|
|
(1,230,508 |
) |
|
Total stockholders’ equity
|
|
$ |
3,126,766 |
|
|
$ |
10,130,765 |
|
(1) 9,131,175 shares issued and outstanding, pro forma, as adjusted, includes 1,350,000 shares of common stock included in the units being sold in this offering and does not include 2,700,000 shares of common stock issuable upon the full exercise of Series A Warrants included in the units being sold in this offering and the full exercise of Series B Warrants included in this offering as well.
The number of shares of common stock to be outstanding after this offering is based on 7,781,175 shares outstanding as of March 31, 2014 and it does not include:
|
|
1,150,000 shares of our common stock available for future issuance as of March 31, 2014 under our 2013 Stock Incentive Plan of which options to purchase 60,000 shares of common stock have been granted at a price of $2.50 per share; and
|
|
|
up to 2,700,000 shares of common stock issuable upon the full exercise of the Series A Warrants and the Series B Warrants offered hereby;
|
|
|
shares of common stock underlying the representatives' unit warrants to be issued to the representative of the underwriters in connection with this offering, at an exercise price per share equal to 120% of the public offering price, plus up to 202,500 shares, 202,500 Series A Warrants and 202,500 Series B Warrants if the over-allotment unit purchase option is exercised in full.
|
The pro forma as adjusted data is illustrative only and will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.
If you purchase any of the units and Series B Warrants offered by this prospectus, your ownership interest will be diluted immediately to the extent of the difference between the initial public offering price per share of our common stock and the pro forma net tangible book value per share of our common stock after this initial public offering.
Our historical net tangible book value as of March 31, 2014 was $2.1 million , or $0. 27 per share of our common stock. Historical net tangible book value per share represents the amount of our total tangible assets less total liabilities, divided by the number of shares of our common stock outstanding.
After giving effect to the sale of 1,350,000 units and Series B Warrants in this offering at an assumed initial public offering price of $6.00 per unit and Series B Warrant, which is the midpoint of the price range listed on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma net tangible book value as of March 31, 2014 would have been $9.1 million, or $1.00 per share. This represents an immediate increase in pro forma net tangible book value per share of $0.73 to existing stockholders and immediate dilution of $5.00 in pro forma net tangible book value per share to new investors purchasing common stock in this offering.
Dilution per share to new investors is determined by subtracting pro forma net tangible book value per share after this offering from the initial public offering price per unit and Series B Warrant paid by new investors. The following table illustrates this dilution on a per share basis.
| Assumed Initial Public Offering Price Per Share |
|
|
$ |
6.00 |
|
| Historical Net Tangible Book Value per share as of March 31, 2014 |
|
|
$ |
0.26 |
|
| Pro Forma Net, Tangible Book Value per share as of March 31, 2014 |
|
|
$ |
0.99 |
|
| Increase in Net Tangible Book Value per share Attribute to New Investment |
|
|
$ |
0.73 |
|
| Pro Forma Net Tangible Book Value per share after this Offering |
|
|
$ |
0.99 |
|
| Dilution per share to New Investors |
|
|
$ |
5.01 |
|
At 1.00 increase (decrease) in the assumed initial public offering price of $6.00 per unit and Series B Warrant, which is the midpoint of the price range listed on the cover page of this prospectus, would increase (decrease) our pro forma net tangible book value by approximately $1,200,000, our pro forma net tangible book value per share by approximately $0.13 and dilution per share to new investors by approximately $0.13, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCE CONDITION AND RESULTS OF OPERATIONS
You should read the following plan of operations together with our financial statements and related notes appearing at the end of this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should read the “Risk Factors” section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
MEDOVEX was incorporated in Nevada in July 30, 2013 as Spinez Corp. MEDOVEX is the parent company of Debride, which was incorporated under the laws of Florida on October 1, 2012, but did not commence operations until February 1, 2013. MEDOVEX is a development stage company that has acquired a patent, patent applications and other intellectual property rights relating to the use, development, and commercialization of the DenerVex device (the “DenerVex”). The DenerVex is a unique device that combines tissue scraping and electrocautery designed to alleviate the back pain associated with facet joint syndrome.
The goal of the Company is to obtain, develop and commercialize various intellectual property rights (patents, patent applications, knowhow, etc.) in the medical technology area, with particular focus on the development of medical devices. Our first acquisition is the DenerVex device. The primary purpose of the registration of this offering is to fund the pursuit, with appropriate governmental approvals and clinical trials of, the commercial development of the DenerVex device. We believe that the DenerVex device can be developed to encompass a number of medical applications, including pain relief. Besides development of the DenerVex device, the balance of the net proceeds of this offering will be used for working capital including any potential acquisition of other companies or their technologies in areas where our experienced, world-class management team believes the largest, most targetable market opportunities exist.
The Company acquired the DenerVex patent on January 31, 2013 from Scott Haufe, M.D. (“Dr. Haufe”), a director of the Company, in exchange for 750,108 shares of common stock in the Company and a 1% royalty on all sales of any product sold based on the patent. In September 2013, we entered into a Co-Development Agreement with James Andrews, M.D. (“Dr. Andrews”), a director of the Company, whereby Dr. Andrews committed to further evaluate the DenerVex device and to seek to make modifications and improvements to such technology. In exchange for such services, the Company agreed to pay Dr. Andrews a royalty equal to two (2%) percent of the DenerVex net sales during the five (5) year term of the Co-Development Agreement. Upon the termination of the term of the Co-Development Agreement, which has a minimum term of five (5) years, then the royalty payable to Dr. Andrews shall be reduced to one (1%) percent of DenerVex net sales after such termination of products covered by any U.S. patent on which Dr. Andrews is listed as a co-inventor; if any such patents are obtained. Such one (1%) percent royalty shall continue during the effectiveness of such patent. Pursuant to the Co-Development Agreement, Dr. Andrews agreed to assign any modifications or improvements to the DenerVex to the Company subject to the royalty rights described above.
Our arrangement with Devicix, the third party design and development firm, which was entered into in November 2013, contemplates a five-phase development process with a targeted FDA submission for clearance on or around March 2015. The anticipated date of a response from the FDA to our submission cannot be predicted. Including materials, the Company estimates that just the product development process will cost approximately $1,000,000 and will take approximately 16 months. The product development plan does not budget for IP work, marketing work, regulatory, or training material or manufacturing development, including fixtures and validation. The project is currently in the latter stages of Phase 2 and the beginning of Phase 3, where the concept prototype is refined. Through March 31, 2014, the Company had paid Devicix $204,000, of which $51,000 was in payables at March 31, 2014.
Following is a brief description of the phases and their expected costs:
Phase I: Concept – This phase creates a preliminary set of requirements and early prototypes of the design. Estimated costs: $100,000
Phase II: Architecture and Planning – This phase focuses on defining the final system architecture, updating requirements and updating the project plan. Manufacturing, regulatory and safety/ risk management plans are created, as well as a clinical hazards assessment. Estimated costs: $50,000
Phase III: Design and Development – In this phase the detailed electrical and mechanical design and drawings are created and the first prototypes are fabricated. Ship packaging for the product will be designed and the manufacturing process finalized. Estimated costs: $400,000.
Phase IV: Test and Optimization – This phase will focus on completing the product design verification testing, design optimization as required, and the completion of manufacturing transfer. The labeling material including package inserts and label templates, Instructions for Use and Operator’s Manual (Surgical Technique Guide) are completed. Also, required regulatory testing will be performed. Estimated costs: $400,000
Phase V: Validation and Launch – In this final product development phase some of the units made as part of the build in Phase IV will be used for the validation testing as defined by regulatory requirements specific to the product. Estimated costs: $50,000
Our intention is to market the product as a disposable, single-use kit which will include all components of the DenerVex product. We intend to offer the DenerVex kit at a sales price of approximately $695.00 per device, which we believe will be an attractive price point for end-users. We believe that the “disposable single use” business model offers us the opportunity to achieve a profitable revenue stream.
We intend to seek out additional product candidates for licensing, acquisition or development. By utilizing management’s expertise in the medical industry, we believe that we are in a unique position to evaluate new technologies.
Financial operations overview
Revenue
We have not produced any revenues to date.
Operating Expenses
We classify our operating expenses into two categories: research and development and general and administrative.
Research and Development Costs and Expenses
Research and development costs and expenses consist primarily of fees paid to external service providers, laboratory supplies, costs for facilities and equipment, and other costs for research and development activities. Research and development expenses are recorded in operating expenses in the period in which they are incurred. Estimates have been used in determining the expense liability of certain costs where services have been performed but not yet invoiced. We monitor levels of performance under each significant contract for external service providers, including the extent of patient enrollment and other activities through communications with the service providers to reflect the actual amount expended.
General and Administrative Expenses
Beginning in October 2013, the Company hired full time management, began sourcing vendors, retained consultants, issued stock options to its Board of Directors and other activities consistent with a new operation. Prior to that time, most activity focused on raising money in a founders round and an offering of common stock under Regulation D of the Securities Act of 1933. During 2013, the Company paid approximately $172,000 in personnel costs, compared to $159,000 in the first quarter of 2014. Professional fees were $336,000 in 2013 and $218,000 during the first quarter of 2014. Travel expenses were $75,000 during 2013 and $26,000 the first quarter 2014. We anticipate that our general and administrative expenses will increase in the future to support continued research and development activities, commercialization of our product candidate and increased costs of operating as a public company. These increases will likely include increased costs related to the hiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we anticipate increased costs associated with being a public company including expenses related to services associated with maintaining compliance with exchange listing and Securities and Exchange Commission requirements, insurance, and investor relations costs.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which we have prepared in accordance with United States generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those described in greater detail below. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements included elsewhere in this prospectus, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our financial condition and results of operations.
Controls and Procedures
We recognize that having only one person performing all accounting functions and preparing the financial statements constitutes a material weakness in our internal controls over financial reporting. We have and will take certain steps in an effort to correct this material weakness, including, among other things, hiring a controller to perform certain accounting and reporting functions to further segregate duties within the Company. We also added John C. Thomas, Jr. to our Board of Directors in April 2014 as our financial expert and chair of the Audit Committee. Mr. Thomas is a certified public accountant with over 24 years of experience as a chief financial officer for public and private companies.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. Valuation allowances have been established as it is more likely than not that the deferred tax assets will not be realized.
We file income tax returns in the U.S. federal jurisdiction and certain state jurisdictions. The tax years subject to audit are 2013.
We have sustained losses since inception which has generally resulted in a zero percent effective tax rate; in addition we have not incurred any interest or penalties. Our policy is to recognize interest and penalties accrued on tax matters as a component of income tax expense.
The Company paid approximately $90,000 to Devicix LLC, third party contract manufacturer located in Minneapolis Minnesota during 2013 to commence development of the DenerVex. Actual development work began in December 2013. During the first quarter of 2014, the Company expensed $123,000 in Devicix invoices. The contractor bills on a time and materials basis and all time worked in the respective periods is reflected on the income statement. At various times since inception, the Company incurred costs associated with strengthening patent protection and obtaining patents outside the United States.
Stock-Based Compensation
A summary of significant assumptions used to estimate the fair value of the equity awards granted in 2013 follows:
Stock-based compensation expense for the period ended December 31, 2013 and the three months ended March 31, 2014 includes stock options granted to directors and has been recorded as general and administrative expense. We follow the provisions of the ASC Topic 718, Compensation- Stock Compensation which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees and non-employee directors, including employee stock options. Stock compensation expense based on the grant date fair value estimated in accordance with the provisions of ASC 718 is generally recognized as an expense over the requisite service period. No options were granted during the first quarter 2014 . For the period ended December 31, 2013, the following stock option grants were made, giving
effect to a 1 for 2 reverse stock split:
|
Grant Date
|
|
Options Granted
|
|
|
Exercise Price
|
|
|
Estimated Fair Value of Underlying Stock
|
|
Intrinsic Value
|
|
October 14, 2013
|
|
|
60,000 |
|
|
$ |
2.50 |
|
|
$ |
2.50 |
|
None
|
The option price was set at the estimated fair value of the common stock on the date of grant using the market approach. Under the market approach, the fair value of the common stock was determined to be the value of a private placement of common stock that began in September 2013 and concluded in December 2013, at $2.50 per share.
We utilize the Black-Scholes valuation method to recognize compensation expense over the vesting period. The expected life represents the period that our stock-based compensation awards are expected to be outstanding. We use a simplified method provided in Securities and Exchange Commission release, Staff Accounting Bulletin No. 110, which averages an award's weighted average vesting period and contractual term for "plain vanilla" share options. The expected volatility was estimated by analyzing the historic volatility of similar public biotech companies in an earlier stage of development. No dividend payouts were assumed as we have not historically paid, and do not anticipated to pay, dividends in the foreseeable future. The risk-free rate of return reflects the weighted average interest rate offered for US treasury rates over the expected term of the options.
The significant assumptions used to estimate the fair value of the equity awards granted in 2013 are:
| Weighted fair value of options granted |
|
$ |
2.50 |
|
| Expected term (years) |
|
|
6 |
|
| Risk-free interest rate |
|
|
2.75 |
% |
| Volatility |
|
|
81 |
% |
| Dividend yield |
|
None |
|
Results of Operations
We are a developmental stage company that started operations late in 2013, and as such have no earnings. We anticipate that our quarterly and annual results of operations will be impacted for the foreseeable future by several factors, including successful development of a prototype product, approval of the product by regulatory agencies in the United States and abroad, and the rate of adoption of our product by medical professionals. Due to these factors we believe that period to period comparisons of our results of operations are not a good indication of our future performance.
Three Months Ended March 31, 2014
The following table sets for our results of operation for the three months ended March 31, 2014:
|
Operating expenses:
|
|
|
|
General and administrative
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2013
The following table sets for our results of operation for the period of our inception on February 1, 2013 through December 31, 2013:
|
Operating expenses:
|
|
|
|
|
General and administrative
|
|
$ |
582,946 |
|
|
Research and development
|
|
|
90,387 |
|
|
Total operating expenses
|
|
|
673,333 |
|
| |
|
|
|
|
|
Net loss
|
|
$ |
(673,333 |
) |
Liquidity and Capital Resources
Since our inception, we have incurred losses and anticipate that we will continue to incur losses in the foreseeable future. We expect our research and development, general and administrative and eventually sales and marketing expenses will grow and, as a result, we will need to generate significant net sales to achieve profitability.
Our independent registered public accounting firm has included an explanatory paragraph with respect to our ability to continue as a going concern in its report on our consolidated financial statements for the year ended December 31, 2013. The presence of the going concern explanatory paragraph may suggest that we may not have sufficient liquidity or minimum cash levels to operate the business.
Sources of Liquidity
To date our operations have been funded with the private sale of common stock. Gross proceeds from the sale of stock during 2013 were approximately $3,522,000. We paid brokers approximately $91,000 and 10,000 shares of common stock in connection with their services. We did not raise any additional funds during the three months ended March 31, 2014. Since we believe that the likelihood of obtaining debt financing in a development stage is low, our source of funds in the foreseeable future will likely be from the sale of capital stock, including the proceeds of this offering. We believe that the net proceeds of this offering will be sufficient to fund our operating expenses and capital expenditures requirements for at least 5 months.
Cash Flows
Net cash used in operating activities was approximately $418,000 during the first three months ended March 31, 2014, compared to $574,000 in 2013. During the first quarter 2014,the Company invested another $1,000 in property in addition to the $4,000 invested in 2013. Net cash provided from the sale of common stock in 2013 was $3,214,000. The Company had $2,241,000 of cash on hand at March 31, 2014.
Funding Requirements
Presently, the Company incurs about $160,000 of cash expenditures per month. We anticipate this number will increase as product development moves into later phases, or if we are compelled to seek FDA approval following a de novo regulatory path, as opposed to 510-K approval. We also anticipate it will increase due to the additional costs associated with being a public entity. However, the Company believes its current cash reserves are sufficient for the next twelve months regardless of which regulatory path it will take.
Contractual Obligations
The Company does not have any long term contractual obligations. The Company currently reimburses its CEO, Jarrett Gorlin, for the lease of executive office space at a cost of $2,000 per month, which it believes is at fair market value. The agreement with the third party design and development firm, Devicix, is estimated to be $960,000 based on their contractual estimate; however it is billed as work is performed and can be cancelled by either party with a 30 day notice. We estimate that in addition to Devicix, there are additional development costs that will bring the total cost of development to $1,000,000, excluding regulatory and other costs not strictly associated with prototype development. The Company also has one consulting agreement with a monthly payment of $10,000 which either party can cancel with 30 days’ notice. We have employment agreements with each of our executive officers that commit us to a six month severance and benefits package if those employees separate under certain conditions, including a change in control of the Company.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any relationships with any organizations or financial partnerships, such as structured finance or special purpose entities, that would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Quantitative and Qualitative Disclosure about Market Risk
Our exposure to interest rate risk is minimal as the bank accounts we use pay little or no interest. After this offering, we anticipate being able to obtain more favorable terms for excess cash, but would still not anticipate any significant exposure to interest rate fluctuations.
Recently Adopted Accounting Standards
There are no recently issued accounting standards that have a material impact on us.
Jumpstart Our Business Startups Act of 2012
The JOBS Act permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to "opt out" of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
MEDOVEX Corp.’s goal is to create better living through better medicine.
We intend to build a portfolio of medical device products in areas where our world-class management team believes the largest, most targetable market opportunities exist. We intend to build this portfolio primarily by acquiring companies or technologies that we believe have promising commercial potential. In September 2013, we acquired Debride, Inc. (Debride). Debride is a development stage company that has acquired a patent, patent applications and other intellectual property rights relating to the use, development, and commercialization of the DenerVex Device (the DenerVex). Debride acquired from Scott M.W. Haufe, MD (Dr. Haufe) all right, title and ownership of U.S. Patent 8,167,879 B2, together with all of Dr. Haufe’s rights, title and interest in and to the DenerVex. Dr. Haufe is a director of the Company. In September 2013, we entered into a Co-Development Agreement with Dr. James Andrews, a renowned orthopedic surgeon who also is a director of the Company. Dr. Andrews has agreed to evaluate the DenerVex and to seek to make modifications and improvements to such technology, as well as to strategize in the rollout of the DenerVex. See “Transactions with Related Parties”.
Initial Product
To date, our efforts have focused on the development of the patent-protected DenerVex device (U.S. Patent 8,167,879 B2). The DenerVex is designed to provide long lasting relief of pain associated with Facet Joint Syndrome (FJS), a condition in which the joints in the back of the spine degenerate and subsequently cause back pain. The concept is simple: remove the affected nerve endings sending pain signals to the brain in such a way that they won’t grow back. A current treatment for this pain removes the nerve endings, but the nerve endings regenerate and the pain returns. A patient is forced to return again and again to seek pain relief at a substantial cost over time.
In order to avoid having his patients suffering from chronic back pain associated with FJS have to return to him for repeated treatments, Dr. Haufe performed a 2-step procedure where he first scraped away synovial tissue using an already available deburring device around the facet joints in the spine so nerves would not have tissue that could be used for regrowth. Once this tissue was removed, he then used a standard electrocautery unit to cauterize the nerve endings, as is done with the temporary treatment to render the nerves incapable of sending pain signals to the brain. Due to the complexity and time required to complete this procedure, it is only employed by a small number of other surgeons besides Dr. Haufe.
In order to see if these patients were indeed enjoying pain relief and not experiencing nerve regrowth, Dr. Haufe followed 174 of his patients receiving this treatment over a three year period (temporary approaches generally do not last more than 2 years and often less). The vast majority of patients were still pain free. While this follow-up did not constitute a clinical study that could be used for FDA clearance, the results encouraged Dr. Haufe to think of ways to expand the treatment to other patients who did not have the resources to afford a relatively complex procedure (assuming they could even locate a surgeon capable of performing it). The key was simplifying the procedure to the point where doctors specializing in pain management and other areas besides spine surgery could perform the procedure quickly and safely, at a cost much less than the cost of temporary treatments over time. To simplify the procedure, Dr. Haufe conceptualized a hand held device that combined the tissue scraping and electrocautery procedures into one action. A simple solution that, based on our internal research on non-living tissue, produces the same result as the 2-step procedure, but requires significantly less surgical skill and takes much less time.
We believe the DenerVex is the first device of its kind that combines tissue scraping and electrocautery simultaneously in order to achieve a long lasting solution to FJS. The DenerVex is a single use device that will be provided to health care practitioners in a package that contains all of the items necessary in the sterile field of the operating room to carry out the surgery necessary to address FJS. The DenerVex does not require a special power source and can be connected to the cauterizing generator generally found in most operating rooms. While the device is still in the prototype phase, we estimate a sales price of approximately $695.00 per device, which we believe will be an attractive price point for end-users. We arrived at the sales price after conducting focus group meetings with potential end-users and obtaining their feedback on ranges of prices they would be willing to pay for the type of pain relief the DenerVex could provide. These end users included pain management physicians, an anesthesiologist, orthopedic surgeons who perform spinal surgery and neurosurgeons. As reimbursement is available for the treatment of Facet Joint Pain in the United States and in major countries in Europe, they could base their responses on reimbursement rates for currently available treatments.
Besides the potential cost savings from the use of the DenerVex device, we believe the procedure using the DenerVex is less evasive, faster and represents a lower risk of infection from surgery than its competitors, while offering a longer term solution to FJS than is currently available. In the procedure where the DenerVex is used, a tiny incision is made above the facet joint and the DenerVex is inserted through a cannula to the facet joint region. The cannula has a bullet-shaped tip that acts as a retractor system. The physician performing the procedure inserts a guidewire down to the facet joint region and the cannula/retractor is inserted over that wire. The inner bullet is removed, leaving a hollow tube for working space. The device is positioned and manipulated via fluoroscopy. No other devices or tools are required beyond standard surgical tools for closure. The DenerVex is designed to be powered by (and compatible with) currently marketed electrocautery consoles currently in most procedure rooms, so no additional specialized capital equipment is required. Activation of the device results in the combined effect of a tissue removal action with electrocautery to the facet joint surface resulting in removal of the nerves and synovial membrane tissues. The treatment of each joint takes a couple of minutes and the incision is closed with a single suture stitch.
The DenerVex is currently in the prototype development phase. In order to commercialize the device, we will:
|
1.
|
Conduct additional research to further refine the product.
|
|
2.
|
Submit the final version to regulatory agencies for their approval, including conducting additional studies as they may require.
|
|
3.
|
Finalize reimbursement, marketing and distribution strategies.
|
|
4.
|
Source vendor(s) for production and marketing/distribution partners (we currently do not plan to manufacture or distribute the product ourselves).
|
Market
Our first device, the DenerVex is targeted at the $11.5 billion global spine surgery devices market, which is largely focused on the treatment of lower back pain. It is estimated that 10% of the adult U.S. population suffer from chronic lower back pain.
Approximately 31% of chronic lower back pain is attributed to FJS, a condition in which joints located at the back of the spine degenerate and subsequently cause pain. Each joint in the spine has a rich supply of tiny nerve fibers that provide a painful stimulus when the joint is injured or irritated. When such joints degenerate due to wearing or tearing, the cartilage separating the joints in the spine may become thin or disappear. The bone in the joints underneath the cartilage can then produce an overgrowth of bone spurs and an enlargement of the joints, which can repeatedly send pain signals through the nerve fibers in the spine. Such pain signals can result in considerable back pain, especially when a person is in motion.
Initial treatment of FJS includes a number of non-invasive, non-surgical techniques or therapies. Examples of such therapies are activity modification exercises, over-the-counter medication, chiropractic intervention and physical therapy. If those non-invasive, non-surgical approaches fail to provide long-term relief to a patient, there are currently six approaches used to remedy FJS: spinal injections, radiofrequency (“RF”) ablation (also known as radiofrequency rhizotomy or radiofrequency neurotomy), cryodenervation, pulsed RF, manual tissue scraping and electrocautery performed separately and spine fusion surgery. Management believes that the primary downsides of the current surgical approaches are as follows:
|
|
Temporary relief. Spinal injection, cryodenervation, RF ablation, and pulsed RF therapies are non-permanent, such that patients must return for repeated treatment over the course of the patient’s lifetime. Spinal injections and cryodenervation effectiveness only lasts a few months, while RF ablation, and pulsed RF have generally been shown to be effective for approximately 6 months to2 years.
|
|
|
Difficult to learn and teach. Manual tissue scraping and electrocautery performed separately and spine fusion procedures are specialized procedures that require a highly skilled specialist that is well-versed in endoscopy and surgery skills to administer.
|
|
●
|
Surgical mechanisms are bulky and difficult to use. Spinal injections require large, painful needles. RF ablation requires the purchase of a special power source to power its ablation needles. Manual tissue scraping and electrocautery requires four separate devices to be used that cannot be deployed simultaneously. Spinal fusion surgery is a very invasive and significant surgical procedure. |
|
|
High cost Spinal injection, cryodenervation, RF ablation, and pulsed RF therapies must be repeatedly applied to be effective, and the cost of such repeated visits to healthcare facilities can aggregate significantly over the course of a lifetime, not to mention the cost of lost productivity due to lower back pain in between treatments.
|
|
|
Invasive with long recovery time. Spinal fusion surgeries are potentially risky, highly invasive surgeries that require significant recovery time with an average cost per client of up to $ 108,000 per patient, plus an estimated 3 to 6 months of lost productivity in the workplace.
|
We believe that the DenerVex represents a significant leap forward in FJS treatment, as it addresses the shortcomings listed above in the following ways:
|
|
Longer lasting. The DenerVex is designed to provide long lasting relief to patients that would otherwise be required to undergo repetitive therapies such as spinal injection and RF ablation.
|
|
|
Easy to teach and intuitive to use. The DenerVex combines two procedures that are currently carried out in the manual tissue scraping and electrocautery performed separately into one procedure that does not require a specialist to conduct. We intend to offer a simple one-day or weekend course featuring a cadaver lab to train physicians in usage of the DenerVex.
|
|
|
Compact, next generation design. The DenerVex is an all-in-one solution that comes in a sterile, single use package.
|
|
|
Cost efficient. The DenerVex is a single use device, so there are no sterilization or repair costs that are associated with many medical devices. Unlike RF ablation, the Denervex does not require a specially-purchased power source, and uses the already-existing cauterization power sources currently found in most operating rooms.
|
|
|
Less Invasive with minimal recovery time. Use of the DenerVex represents a less invasive solution to FJS than spinal fusion surgery and can be carried out through an out-patient procedure that does not require follow-up surgery to be effective.
|
By addressing the specific product and technology limitations outlined above (that have been raised by both patients and practitioners alike according to our market research), we believe DenerVex has the opportunity to greatly penetrate and expand the spine surgery device market. In order to begin our anticipated expansion into the spine surgery device market, the primary targets for the sale of the DenerVex device are pain management practitioners. There are an estimated 6,200 pain management specialists in the United States. We expect that the primary users of the DenerVex device will be these specialists, as well as clinicians who currently utilize interventional procedures to treat chronic back pain. Secondary targets for the DenerVex device are orthopedists and neurosurgeons, in addition to the 4,000 interventional radiologists, and 8,672 spine surgeons using one or a combination of the techniques now currently in use to treat FJS.
Our Strategy
We believe we have significant opportunity to create better living through better medicine by assembling a portfolio of companies and exclusive medical device products. We believe that we can acquire technologies from inventors and under-funded companies at attractive prices and that we may be able to use shares of our common stock to effect such acquisitions or for a portion of the compensation paid in such acquisitions. We intend that such a portfolio will consist of a multi-device, multi-sector combination of intellectual properties and devices that our management team will acquire at a fair market value, but because the properties will be run through our development engine, we believe they will ultimately result in increased shareholder value through the increased value of these properties.
In order to provide these increased-value solutions and devices that impact the quality of patient care while simultaneously lowering costs in a rapidly changing healthcare environment, we have assembled a seasoned management team composed of individuals with both medical expertise and experience in monetizing medical devices. Dr. Haufe, who co-developed the first device to emerge from our product development engine, the DenerVex, was a co-founder of Debride and is a director of the Company, not only has 11 years of experience in carrying out over 450 manual tissue scraping and electrocautery performed separately procedures in order to treat back pain related conditions, but also has 8 years of experience designing, developing and commercializing innovative and proprietary spine technologies and techniques. Patrick Kullmann who is the President and Chief Operating Officer of the Company has nearly 30 years of experience marketing and growing companies in the healthcare, scientific and technology industries and is the author of the book “The Inventors Guide to Medical Technology”. We believe that other members of our management team, particularly our Chief Executive Officer, Jarrett Gorlin, provide us with the management expertise necessary to effectively manage our anticipated growth. We intend to leverage this expertise to accelerate the growth of the businesses or assets we acquire. We believe that our management team’s diverse range of experience also provides us with the flexibility to review growth and turnaround situations for underleveraged or underperforming medical devices and their parent companies. We are pursuing the following strategies:
|
|
Promoting the differentiated features of our DenerVex exclusive technology to penetrate the $11.5 billion global spine surgery devices market and establish DenerVex as a standard of care for treating FJS, as well as broadening the clinical applications of the DenerVex;
|
|
|
Investing in our infrastructure to broaden our market presence first in Europe (with particular focus on the European Union and the business and patient data that we may be able to gather there), then in the U.S., and eventually worldwide, expand our global distribution footprint, and to ensure regulatory approval internationally; and
|
|
|
Leveraging the strength and experience of our world-class management team to selectively pursue opportunities to expand our portfolio of medical device products and businesses.
|
Our marketing strategy will be supported by a solid core of clinical and cost effectiveness data that we will develop to support our portfolio of medical companies and assets. We intend to employ the same disciplined approach to all future opportunities that we have employed prior to acquiring the assets for the DenerVex device, by careful research, reviewing of existing devices and examination of marketplace possibilities. A compelling value proposition and subsequent market positioning for our devices versus other treatment options will also be developed and tailored as appropriate for the key decision-makers along the course of the patient flow. We believe we are well positioned to create devices and tailor their value propositions to meet a rapidly changing marketplace and fluid regulatory environment, by creating and marketing devices that are designed to result in patients spending less time in the hospital, lower the chance of patient infection, simplify surgical procedures and diminish the potential incidence of hospital and surgery-related accidents.
The spine is the central core of the human skeleton and provides structural support, alignment and flexibility to the body. It extends from the base of the skull to the pelvis and consists of approximately 24 interlocking bones, called vertebrae, which are stacked on top of one another. The spine encloses and protects the spinal cord, a column of nerve tracts that run from every area of the body to the brain, through openings between the vertebrae called foramen, and which deliver sensation and control to the entire body.
There are seven vertebrae in the cervical, or neck, region of the spine, 12 vertebrae in the thoracic, or central, region of the spine, and five vertebrae in the lumbar, or lower back, which is the primary load-bearing region of the spine. The bottom of the spine consists of the sacrum and finally the coccyx. Vertebrae are paired into motion segments, or levels, that move by means of two facet joints that provide stability and enable the spine to bend and twist, with the neck being the most mobile region of the spine. Facet joints also prevent each vertebra from slipping over the one below. A small capsule surrounds each facet joint, providing a nourishing lubricant for the joint. Also, each joint has a rich supply of tiny nerve fibers that provide a painful stimulus when the joint is injured or irritated. Inflamed facets can cause a powerful muscle spasm.
Facet joints are in almost constant motion with the spine and it is quite common for them to simply wear out in many patients. When facet joints become worn or torn, the cartilage may become thin or disappear. The bone in the joint underneath can produce an overgrowth of bone spurs and an enlargement of the joints. When that happens, the joint has arthritic changes (osteoarthritis – also known as spinal arthritis), which can produce considerable back pain when a person moves.
Overview of Spinal Disorders and Traditional Treatment Alternatives
Given the complexity of its structure, the spine is susceptible to various ailments that can lead to instability and significant pain for a patient. Back pain affects an estimated 10% of the adult population in the United States and is the second most common cause of disability in U.S. adults. Approximately 31% of chronic low back pain is attributed to FJS, which effects the facet joints in the spine, the small stabilizing joints located between and behind adjacent vertebrae. When FJS occurs, the facet joints in the back of the spine degenerate and subsequently cause pain. The facet joints are found at every level on both sides of the lumbar spine. They provide about 20% of the twisting stability in the low back. Each facet joint is positioned at each level of the spine to provide the needed support, especially with rotation. Current treatment options include non-surgical treatment options, injections, surgical treatment for temporary relief and surgical treatment for permanent relief. Virtually all FJS patients are initially treated initially with a combination of non-surgical methods including posture correction, activity modification, chiropractic intervention, exercise/physical therapy and over-the-counter medication.
Temporary options
The field for the treatment of FJS includes initial treatment using noninvasive techniques. If those noninvasive approaches fail to provide long-term relief, injections are typically performed. Injections temporarily deaden the nerves so pain is diminished, but the effect typically wears off after 3 to 4 months. After injections, the following surgical approaches are considered.
Temporary Surgical approaches
When non-surgical treatment and injections alone fail, a surgical procedure called RF rhizotomy is often prescribed as necessary. RF rhizotomy, also called RF neurotomy or RF ablation, is the surgical de-nerving (nerve block) of the facet joint. The doctor uses X-ray imaging to accurately place a needle with a small single tipped or bifurcated tip electrode into the facet joint. An electric current is used to destroy the sensory nerves of the joint, leading to temporary pain relief. The treatment is temporary because the nerve ending will grow back and continue to be irritated by bone spurs or enlarged joints. RF ablation devices consist of a specialized generator costing about $30,000 and disposable electrode probe sets costing about $120 each.,
Pulsed radiofrequency refers to a technique used for creating a carefully controlled electrical field around an electrode. For its use in treating pain, this electrode is usually built into the shape and size of a needle. It was first used as a pain treatment option in the mid 1990′s. This technique is similar to that used for RF ablation procedures. The word “pulsed” means this technique applies energy to the electrode intermittently. This keeps the temperature very low, unlike the higher temperatures required for an RF ablation.
Studies have shown that the application of pulsed RF to certain nerves can block some of their ability to transmit pain. It appears that when pulsed RF is applied to a nerve, it selectively affects only the portion of the nerve responsible for sending pain signals. Pulsed RF is most effective at treating difficult types of pain that typically originate from either nerve damage or irritated nerves and may be useful in offering additional pain relief when other treatment options have failed.
Pulsed RF is generally administered in an outpatient setting. An electrode is placed through the skin and the clinician uses fluoroscopy (x-ray) to position the electrode closely to the affected nerve or nerves as they exit the spine. Once the electrode is positioned, a small amount of pulsed RF is applied to ensure proper location of the electrode. When the electrode is properly positioned, the pulsed RF procedure is performed. Usually, after completion of the stimulation, a small amount of corticosteroid is injected along with local anesthetic to decrease any temporary irritation to the nerve. The electrode is removed and a small bandage is placed over the injection site.
Relief from the pulsed RF treatment may take up to several weeks to demonstrate a full effect, and the onset is usually subtle, becoming progressively better, until the effects of the treatment wear off and the patient has to return to the outpatient facility for another round of treatment. The cost of the equipment needed to administer the pulsed RF treatment is the same as that for RF ablation.
Another treatment option that was once used more extensively, but now rarely used, is cryodenervation. This procedure is similar to the pulsed RF procedure described above, except instead of using RF bursts to block the pain, bursts of freezing temperature are administered. While the costs of the procedure are about the same as an RF procedure, the effect does not seem to last nearly as long, typically 3 to 4 months, the same as spinal injections. As such, it is only used in special cases and we do not consider it a commercial comparable for treatment of FJS.
Long lasting Surgical approaches
One long lasting surgical treatment for FJS is spine fusion surgery, which stops motion at the painful joint by fusing two spinal vertebrae together. Spinal fusion may also be referred to as “arthrodesis.” The procedure is highly invasive and often involves bone tissue grafts and the use of screws to fuse the vertebrae together.
Another long lasting surgical treatment for FJS is manual tissue scraping and electrocautery performed separately. This procedure is only performed by a handful of highly-skilled surgeons, whom must have both orthopedic and endoscopic experience. Our director, Dr. Scott M. W. Haufe, is one of the very few surgeons performing this procedure. Manual tissue scraping and electrocautery performed separately is time consuming and requires the use of four separate instruments: a burr set, an electric cautery device, a dilating tube and a stimmin pin.
We believe that the primary downsides of the current surgical approaches are as follows:
|
|
Not permanent. Spinal injection, cryodenervation, RF ablation, and pulsed RF therapies are non-permanent, such that patients must return for repeated treatment over the course of the patient’s lifetime. The effectiveness of spinal injections and cryodenervation only last a few months. RF ablation and pulsed RF have generally been shown to be effective for only 6 months to 2 years.
|
|
|
Difficult to learn and teach. Manual tissue scraping and electrocautery performed separately and spine fusion procedures are specialized procedures that require a highly skilled specialist that is well-versed in endoscopy and surgery skills to administer.
|
|
|
Surgical mechanisms are bulky and difficult to use. Spinal injections require large, painful needles. RF ablation requires the purchase of a special power source to power its ablation needles. Manual tissue scraping and electrocautery performed separately requires four separate devices to be used that cannot be deployed simultaneously. Spinal fusion surgery is a very invasive and a significant surgical procedure.
|
|
|
High cost Spinal injection, cryodenervation, RF ablation, and pulsed RF therapies must be repeatedly applied to be effective, and the cost of such repeated visits to healthcare facilities can aggregate significantly over the course of a lifetime, not to mention the cost of lost productivity due to lower back pain in between treatments.
|
|
|
Invasive with long recovery time. Spinal fusion surgeries are potentially risky, highly invasive surgeries that require 3 to 6 months of recovery time.
|
We believe that in recent years, the trend in surgical procedures for the spine has been toward more minimally invasive alternatives. Historically, meaningful technology improvements to conventional therapies have resulted in surgical procedures being performed earlier in the patient continuum of care, such as minimally invasive knee arthroscopy in the sports medicine market, resulting in enhanced opportunities that we believe can be marketed to larger groups of practitioners with less need for specialized surgeons or facilities. We believe that, a minimally invasive spine surgery procedure could have the same impact on lower back surgery, especially given aging patient demographics, increased life expectancies and the growing demand for faster procedure times with minimized recovery periods afterward.
Our Product
Our product, the DenerVex device (U.S. Patent 8,167,879 B2), is intended to provide long lasting pain relief for persons suffering from a form of back pain associated with FJS. Rather than fuse the spine in a drawn-out and invasive procedure, the MEDOVEX DenerVex combines tissue scraping and electrocautery for long lasting relief from back pain. A tiny incision is made above the facet joint and the DenerVex is inserted through a cannula to the facet joint region. The cannula has a bullet-shaped tip that acts as a retractor system. The physician performing the procedure inserts a guidewire down to the facet joint region and the cannula/retractor is inserted over that wire. The inner bullet is removed, leaving a hollow tube for working space. The device is positioned and manipulated via fluoroscopy. No other devices or tools are required beyond standard surgical tools for closure. The DenerVex device is designed to be powered by (and compatible with) currently marketed electrocautery consoles currently in most procedure rooms. Activation of the device results in the combined effect of a tissue removal action with electrocautery to the facet joint surface resulting in removal of the nerves and synovial membrane tissues. The treatment of each joint takes a couple of minutes and the incision is closed with a single suture stitch. To illustrate the cost advantages of our device over current treatment options, our management has prepared the following chart:
| |
|
Injections
|
|
|
RF Ablation & Pulsed RF
|
|
|
Spinal Fusion
|
|
|
DenerVex Device
|
|
|
Total Cost Per Procedure
|
|
$ |
860 |
|
|
$ |
1,625 |
|
|
$108,985
|
|
|
$2,010
|
|
|
Procedures Over a 3 Year Period
|
|
15
|
|
|
6
|
|
|
|
(1 |
) |
|
|
(1 |
) |
|
Cost Over 3 Years
|
|
$ |
12,900 |
|
|
$ |
9,750 |
|
|
$ |
108,985 |
|
|
$ |
2,010 |
|
(1) While non-surgical interventions will likely remain the “front-line option” for initial treatment of FJS, as illustrated above, we believe that the DenerVex device will have distinct advantages versus other interventional techniques.
The clinical efficacy of the DenerVex concept has been confirmed with a study of 174 patients that Dr. Haufe conducted starting in in 2002, with a three year follow-up to determine efficacy of the surgery. In that non-FDA clinical study, patients were treated using a two-step procedure of tissue scraping and electrocautery. In November 2013, we entered into a development plan with Devicix, LLC, an FDA registered, ISO certified engineering firm, for the development of the DenerVex and assistance in the regulatory submission process.
Management believes that Devicix has a reputation for bringing the foremost concept and product development expertise to both early-stage and established medical device manufacturers, physicians and universities. In order to assist us in developing a strategy to compete in the global, medical device marketplace, Devicix helped us to design a five phase program at a cost of approximately $1,000,000 which will lead to submission for FDA clearance around March 2015. Devicix has finished Phase 1 of the plan, is finishing Phase 2 and has already started on Phase 3 of the plan, where we refine the prototype device developed in the first two phases.
We believe the DenerVex is the first device of its kind that combines tissue scraping and electrocautery simultaneously in order to achieve a long-lasting solution to FJS. The DenerVex is a single use device that will be provided to health care practitioners in a package that contains all of the items necessary in the sterile field of the operating room to carry out the surgery necessary to address FJS. The device does not require a special power source, and can be connected to the cauterizing generator generally found in most operating rooms. We estimate that we will market the product at a sales price of approximately $695.00 per device, which we believe will be an attractive price point for end-users.
Market
The global medical device market in 2012 was approximately $351 billion, and our strategy is to leverage the expertise of our management, Board of Directors and other advisors to build a portfolio of medical device companies and assets in order to take advantage of many of the largest market opportunity segments in the medical field including Osteoarthritis (“OA”), sports medicine, stroke, diabetes, infection control and cardiovascular applications.
Our first product focuses on OA and its manifestation as FJS. OA is the number one cause of disability in the U.S. and while the specific incidence of spinal OA is not known, close to 50,000,000 people in the U.S. have osteoarthritis in at least one joint in the body. OA is associated with aging and 50% of people age 65 or older have OA in at least one joint. Ten percent of the U.S. population, suffer from chronic lower back pain. Approximately 31% of chronic lower back pain is attributed to FJS, which effects the spine’s facet joints, small stabilizing joints located between and behind adjacent vertebrae.
The market to treat FJS is part of the global spine surgery market.
We believe that the DenerVex device represents a significant leap forward in FJS treatment, as it addresses what we see as the shortcomings listed above in the following ways:
|
|
Longer lasting. The DenerVex provides long lasting pain relief to patients that would otherwise be required to undergo repetitive therapies such as spinal injection and RF ablation.
|
|
|
Easy to teach and intuitive to use. The DenerVex combines two procedures carried out in the manual tissue scraping and electrocautry process into one procedure that does not require a specialist to conduct. We intend to offer a simple one-day or weekend course featuring a cadaver lab to train physicians in usage of the DenerVex device.
|
|
|
Compact, next generation design. The DenerVex is an all-in-one solution that comes in a sterile, single use package.
|
|
|
Cost efficient. The DenerVex is a single use device, so there are no sterilization or repair costs associated with the device. Unlike RF ablation, the Denervex does not require a specially-purchased power source, and uses the already-existing cauterization power sources generally found in most operating rooms.
|
|
|
Less Invasive with minimal recovery time. Use of the DenerVex device represents a less invasive solution to FJS that can be carried out through an out-patient procedure that does not require follow-up surgery to be effective.
|
According to a Markets and Markets research report, the global spine surgery device market was estimated at $11.6 billion in 2012, and in 2017, is estimated to reach $14.8 billion.
By addressing the specific product and technology limitations outlined above (that have been raised by both patients and practitioners alike according to our market research), we believe DenerVex has the ability to greatly penetrate and expand the spine surgery device market. In order to begin our anticipated expansion into the spine surgery device market, the primary targets for the sale of the DenerVex device are pain management practitioners. There are an estimated 6,200 pain management specialists in the United States. We expect that the primary users of the DenerVex device will be these specialists, as well as clinicians who utilize interventional procedures to treat chronic back pain. Secondary targets for the DenerVex device are orthopedists and neurosurgeons, in addition to the 4,000 interventional radiologists, and 8,672 spine surgeons using one or a combination of the techniques now currently in use to treat FJS.
Strategy
Marketing Strategy
We believe we have significant opportunity to create better living through better medicine by assembling a portfolio of companies and exclusive medical device products. In order to provide ground-breaking solutions and devices that impact the quality of patient care while simultaneously lowering costs in a rapidly changing healthcare environment, we are pursuing the following strategies:
|
|
Promoting the differentiated features of our DenerVex exclusive technology to patients, pain care providers, doctors and hospitals in order to penetrate the $11.5 billion global spine surgery devices market and establish DenerVex as a standard of care for treating FJS. We will seek to increase awareness of FJS and new treatment options for FJS by developing a brand identity, promotional literature and training support materials in both traditional and electronic forms. As the market launch unfolds, we will monitor feedback from clinicians and field personnel and adjust the value proposition, market positioning and promotional/training materials accordingly;
|
|
|
Capitalizing on our highly efficient product development process expertise to innovate new technologies and techniques, while continuing to broaden the clinical applications of the DenerVex device. We believe that the involvement of James R. Andrews M.D. and the other prominent members of the Company’s Board of Directors, including Scott M.W. Haufe, M.D. and Randall R. Betz, M.D. will aid us in gaining market acceptance from practitioners and if needed, procuring clinical sites;
|
|
|
Leveraging the strength and experience of our world-class management team to selectively pursue opportunities to expand our portfolio of medical device products and businesses; and
|
|
|
Investing in our U.S. and international marketing infrastructure to broaden our market presence, expand our global distribution footprint, and to ensure regulatory approval internationally. Based on what we believe will bring the best value for shareholders, we may elect in the future to utilize strategic partners, distributors, or contract sales forces to assist in the commercialization of our products.
|
Our marketing strategy will be supported by a solid core of clinical and cost effectiveness data supporting the DenerVex device and based on a thorough review of Strengths/Weaknesses, Opportunities/Threats (“SWOT”) to assess the advantages and disadvantages of the DenerVex device vs. other treatment options. A compelling value proposition and subsequent market positioning for the DenerVex device vs. other treatment options will also be developed and tailored as appropriate for the key decision-makers along the course of the patient flow.
Intellectual Property Strategy
A key element of our success depends on our ability to identify and create proprietary medical device technologies. In order to proactively protect those proprietary technologies, we intend to continue to develop and enforce our intellectual property rights in patents, trademarks and copyrights, as available through registration in the United States and internationally, as well as through the use of we trade secrets, domain names and contractual agreements such as confidentiality agreements and proprietary information agreements.
Currently, our intellectual property rights at present consist of the Contributed Intellectual Property, which includes the Patent. The Patent was originally filed in 2007and was issued on May 1, 2012. We intend to leverage the Patent to the fullest extent possible through market development and prosecution of our rights under the Patent.
In addition to filing and prosecuting patent applications in the United States, we intend to file counterpart patent applications in Europe, Canada, Japan, Australia and China in additional countries where we think such foreign filing is warranted.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent’s term may be shortened if a patent is terminally disclaimed over another patent or as a result of delays in patent prosecution by the patentee, and a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office in granting a patent.
We intend to continually re-assess and fine-tune our intellectual property strategy in order to fortify our position in our market space in the United States and internationally. To that end, we are prepared to file additional patent applications should our intellectual property strategy require such filings and/or where we seek to adapt to competition or seize business opportunities. Further, we are prepared to file patent applications relating to any other products that we develop or require soon after the experimental data necessary for a strong application become available and our cost-benefit analyses justifying filing such applications.
Many biotechnology companies and academic institutions are competing with us in the medical device field and filing patent applications potentially relevant to our business. Internally, we have established business procedures designed to maintain the confidentiality of our proprietary information, including the use of confidentiality agreements with employees, independent contractors, consultants and companies with which we conduct business. Also, we generally require employees to assign patents and other intellectual property to us as a condition of employment with us.
In order to contend with the inevitable possibility of third party intellectual property conflicts, from time to time, we will review and assess the third-party intellectual property landscape for competitive and other developments that may inform or impact our intellectual property development and commercialization strategies. We may find it necessary or prudent to obtain licenses from third party intellectual property holders. Where licenses are readily available at reasonable cost, such licenses are considered a normal cost of doing business. In other instances, however, where a third party holds relevant property and is a direct competitor, a license might not be available on commercially reasonable terms or available at all. We will attempt to manage the risk that such third party intellectual property may pose by conducting, among other measures, freedom-to-operate studies to guide our early-stage research away from areas where we are likely to encounter obstacles in the form of third party intellectual property.
For important additional information related to our intellectual property position, please review the information set forth in “Risk Factors — Risks Related to Our Intellectual Property.”
Competition
Developing and commercializing new products is highly competitive. The market is characterized by extensive research and clinical efforts and rapid technological change. We face intense competition worldwide from medical device, biomedical technology and medical products and combination products companies, including major medical products companies. We may be unable to respond to technological advances through the development and introduction of new products. Most of our existing and potential competitors have substantially greater financial, marketing, sales, distribution, manufacturing and technological resources. These competitors may also be in the process of seeking FDA or other regulatory approvals, or patent protection, for new products. Our competitors may commercialize new products in advance of our products. Our products also face competition from numerous existing products and procedures, which currently are considered part of the standard of care. We believe that the principal competitive factors in our markets are:
|
|
the quality of outcomes for medical conditions;
|
|
|
acceptance by surgeons and the medical device market generally;
|
|
|
ease of use and reliability;
|
|
|
technical leadership and superiority;
|
|
|
effective marketing and distribution;
|
|
|
product price and qualification for coverage and reimbursement.
|
We will also compete in the marketplace to recruit and retain qualified scientific, management and sales personnel, as well as in acquiring technologies and licenses complementary to our products or advantageous to our business.
We are aware of several companies that compete or are developing technologies in our current and future products areas. With regard to the DenerVex device, we believe that our principal competitors include RF ablation devices manufacturers Cosman and Stryker. We may also face competition from cryodenervation device manufacturers, such as Spembly Medical Systems. We may also face competition from developing but potentially untested technologies such as as Zyga’s GLYDER device. In order to compete effectively, our products will have to achieve widespread market acceptance, receive adequate insurance coverage and reimbursement, be cost effective and be simultaneously safe and effective. Please see “Business-Industry Market” for a detailed description of the currently available treatments for FJS.
Opportunities Created by Healthcare Legislation
We anticipate that recent healthcare legislation will create greater opportunities for cost-effective providers of healthcare. PPACA and other related healthcare reform activities reform seek to expand coverage to a broader range of patients. Since insurance coverage reduces the out-of-pocket expense for physician visits, patients will be better able to afford visits to the doctor and accordingly, the number of physician visits is projected to rebound, particularly in 2014, when health insurance exchanges and subsidization of insurance premiums will be established for individuals with income less than 400% of the poverty line.
Additionally, PPACA accomplishes its proposed expansion of coverage to previously uninsured individuals through a significant loosening of the eligibility criteria for enrollment in Medicaid. As Americans grow older and live longer, there will be greater public and private spending on healthcare, which is expected to help hospitals afford to upgrade their equipment in order to speed surgical processes while lowering associated risks, such as infection and recovery time. Consequently, areas of strongest growth in the hospital market will be in medical devices that treat age-related illnesses, such as those that are likely to seek reimbursement through Medicaid, and those devices that help hospitals achieve faster procedures while lowering the associated risks as discussed above.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we are developing. Any product that we develop must be approved by the FDA before they may be legally marketed in the United States and by the appropriate foreign regulatory agency before they may be legally marketed in foreign countries.
FDA Regulation
The DenerVex and any other product we may develop must be approved or cleared by the FDA before it is marketed in the United States. Before and after approval or clearance in the United States, our products are subject to extensive regulation by the FDA under the Federal Food, Drug, and Cosmetic Act and/or the Public Health Service Act, as well as by other regulatory bodies. FDA regulations govern, among other things, the development, testing, manufacturing, labeling, safety, storage, record-keeping, market clearance or approval, advertising and promotion, import and export, marketing and sales, and distribution of medical devices and products.
In the United States, the FDA subjects medical products to rigorous review. If we do not comply with applicable requirements, we may be fined, the government may refuse to approve our marketing applications or to allow us to manufacture or market our products, and we may be criminally prosecuted. Failure to comply with the law could result in, among other things, warning letters, civil penalties, delays in approving or refusal to approve a product, product recall, product seizure, interruption of production, operating restrictions, suspension or withdrawal of product approval, injunctions, or criminal prosecution.
FDA Approval or Clearance of Medical Devices
In the United States, medical devices are subject to varying degrees of regulatory control and are classified in one of three classes depending on the extent of controls the FDA determines are necessary to reasonably ensure their safety and efficacy:
|
|
Class I: general controls, such as labeling and adherence to quality system regulations;
|
|
|
Class II: special controls, pre-market notification(often referred to as a 510(k) application), specific controls such as performance standards, patient registries, postmarket surveillance, additional controls such as labeling and adherence to quality system regulations; and
|
|
|
Class III: special controls and approval of a pre-market approval (“PMA”) application.
|
In general, the higher the classification, the greater the time and cost to obtain approval to market. There are no “standardized” requirements for approval, even within each class. For example, the FDA could grant 510(k) status, but require a human clinical trial, a typical requirement of a PMA. They could also initially assign a device Class III status, but end up approving a device as a 510(k) device if certain requirements are met. The range of the number and expense of the various requirements is significant. The quickest and least expensive pathway would be 510(k) approval with a just a review of existing data. The longest and most expensive path would be a PMA with extensive randomized human clinical trials. We cannot predict how the FDA will classify the DenerVex device, nor predict what requirements will be placed upon us to obtain market approval, or even if they will approve the DenerVex device at all. However, we believe the pathway that will be required by the FDA will be somewhere between the two extremes described above.
We intend to apply to the FDA for 510(k) clearance. However, it is very possible the FDA will deny this request and require the more expensive PMA process. The Company has budgeted based on the assumption that the PMA process will be required.
To request marketing authorization by means of a 510(k) clearance, we must submit a pre-market notification demonstrating that the proposed device is substantially equivalent to another currently legally marketed medical device, has the same intended use, and is as safe and effective as a currently legally marketed device and does not raise different questions of safety and effectiveness than does a currently legally marketed device. 510(k) submissions generally include, among other things, a description of the device and its manufacturing, device labeling, medical devices to which the device is substantially equivalent, safety and biocompatibility information, and the results of performance testing. In some cases, a 510(k) submission must include data from human clinical studies. We believe that other medical devices which have been approved by the FDA have many aspects that are substantially similar to the DenerVex device, which may make obtaining 510(k) clearance possible. Marketing may commence only when the FDA issues a clearance letter finding substantial equivalence. After a device receives 510(k) clearance, any product modification that could significantly affect the safety or effectiveness of the product, or that would constitute a significant change in intended use, requires a new 510(k) clearance or, if the device would no longer be substantially equivalent, would require PMA. In addition, any additional claims the Company wished to make at a later date, such as the permanent relief of pain, may require a PMA. If the FDA determines that the product does not qualify for 510(k) clearance, they will issue a Not Substantially Equivalent letter, at which point the Company must submit and the FDA must approve a PMA before marketing can begin.
During the review of a 510(k) submission, the FDA may request more information or additional studies and may decide that the indications for which we seek approval or clearance should be limited. In addition, laws and regulations and the interpretation of those laws and regulations by the FDA may change in the future. We cannot foresee what effect, if any, such changes may have on us.
European Union Approvals
The EU will require a CE mark certification or approval in order to market the DenerVex device in the various countries of the European Union or other countries outside the United States. To obtain CE mark certification of the device, we will be required to work with an accredited European notified body organization to determine the appropriate documents required to support certification in accordance with existing medical device directive. The predictability of the length of time and cost associated with such a CE marketing may vary, or may include lengthy clinical trials to support such a marking. Once the CE mark is obtained, a company may market the device in the countries of the EU. We have targeted the submission of our application for CE marketing in March 2015. Management believes it will be able to obtain European CE mark approval to market the Denervex device before it will obtain US FDA approval. The Company has retained third party experts to assist with the European approval. Management will be interviewing potential European distributors and will likely retain a European sales manager to assist the distributor and promote the product in Europe.
Clinical Trials of Medical Devices
One or more clinical trials are becoming necessary to support an FDA submission. Clinical studies of unapproved or uncleared medical devices or devices being studied for uses for which they are not approved or cleared (investigational devices) must be conducted in compliance with FDA requirements. If an investigational device could pose a significant risk to patients, the sponsor company must submit an Investigational Device Exemption, or IDE application to the FDA prior to initiation of the clinical study. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device on humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies the company that the investigation may not begin. Clinical studies of investigational devices may not begin until an institutional review board (IRB) has approved the study.
During any study, the sponsor must comply with the FDA’s IDE requirements. These requirements include investigator selection, trial monitoring, adverse event reporting, and record keeping. The investigators must obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of investigational devices, and comply with reporting and record keeping requirements. We, the FDA, or the IRB at each institution at which a clinical trial is being conducted may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable risk. During the approval or clearance process, the FDA typically inspects the records relating to the conduct of one or more investigational sites participating in the study supporting the application.
Post-Approval Regulation of Medical Devices
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
|
|
the FDA Quality Systems Regulation (QSR), which governs, among other things, how manufacturers design, test, manufacture, exercise quality control over, and document manufacturing of their products;
|
|
|
labeling and claims regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and
|
|
|
the Medical Device Reporting regulation, which requires reporting to the FDA of certain adverse experiences associated with use of the product.
|
We will continue to be subject to inspection by the FDA to determine our compliance with regulatory requirements.
Manufacturing cGMP Requirements
Manufacturers of medical devices are required to comply with FDA manufacturing requirements contained in the FDA’s current Good Manufacturing Practices (cGMP) set forth in the quality system regulations promulgated under section 520 of the Food, Drug and Cosmetic Act. cGMP regulations require, among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations, and civil and criminal penalties. Adverse experiences with the product must be reported to the FDA and could result in the imposition of marketing restrictions through labeling changes or in product withdrawal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following the approval.
International Regulation
We are subject to regulations and product registration requirements in many foreign countries in which we may seek to sell our products, including in the areas of product standards, packaging requirements, labeling requirements, import and export restrictions and tariff regulations, duties and tax requirements. The time required to obtain clearance required by foreign countries may be longer or shorter than that required for FDA clearance, and requirements for licensing a product in a foreign country may differ significantly from FDA requirements.
European Good Manufacturing Practices
In the European Union, the manufacture of medical devices is subject to good manufacturing practice (GMP), as set forth in the relevant laws and guidelines of the European Union and its member states. Compliance with GMP is generally assessed by the competent regulatory authorities. Typically, quality system evaluation is performed by a Notified Body, which also recommends to the relevant competent authority for the European Community CE Marking of a device. The Competent Authority may conduct inspections of relevant facilities, and review manufacturing procedures, operating systems and personnel qualifications. In addition to obtaining approval for each product, in many cases each device manufacturing facility must be audited on a periodic basis by the Notified Body. Further inspections may occur over the life of the product. Our third party manufacturer has ISO certification which is generally one of the requirements for approval under the guidelines established in the European Union.
United States Anti-Kickback and False Claims Laws
In the United States, there are Federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in Federal healthcare programs. These laws are potentially applicable to manufacturers of products regulated by the FDA as medical devices, and hospitals, physicians and other potential purchasers of such products. Other provisions of Federal and state laws provide civil and criminal penalties for presenting, or causing to be presented, to third-party payers for reimbursement, claims that are false or fraudulent, or which are for items or services that were not provided as claimed. In addition, certain states have implemented regulations requiring medical device and pharmaceutical companies to report all gifts and payments over $50 to medical practitioners. This does not apply to instances involving clinical trials. Although we intend to structure our future business relationships with clinical investigators and purchasers of our products to comply with these and other applicable laws, it is possible that some of our business practices in the future could be subject to scrutiny and challenge by Federal or state enforcement officials under these laws.
Manufacturing
We do not currently own or operate any manufacturing facilities for the clinical or commercial production of our products. Our arrangement with Devicix LLC, the third party design and development firm contracted to develop the DenerVex device, which was entered into in November 2013, contemplates a five-phase development process with an FDA submission application for clearance on or around March 2015. Including materials, the Company estimates that the process will cost approximately $1,000,000 and will take approximately 16 months. The project plan does not budget for IP work, marketing work, regulatory, or training material or manufacturing development, including fixtures and validation. The project is currently in the latter stages of Phase 2 and the beginning stages of Phase 3, and we are currently evaluating a concept prototype of the DenerVex.
Facilities
The Company reimburses CEO Jarrett Gorlin for approximately 1,200 square feet of general office space he leases in Atlanta, Georgia as its corporate headquarters from a company owned by our President, Jarrett Gorlin. The Company pays Mr. Gorlin $2,000 per month for this space plus related utilities, the exact amount billed to him from the owner of the property which we believe to be at least as favorable as we would able to obtain from an unaffiliated third party. Mr. Gorlin rents this space on a month to month basis from the owner.
Employees
We currently have 4 full-time employees, of which three (3) are engaged in administration and one (1) is engaged in direct oversight of our research and development activities. None of our employees is subject to a collective bargaining agreement or represented by trade or labor union. We believe that we have a good relationship with our employees.
Legal Proceedings
We are not currently a party to any material legal proceedings.
Our Board of Directors consists of nine (9) members: Larry Papasan, Scott M. W. Haufe, M.D., James R. Andrews, M.D., Jarrett Gorlin, Randal R. Betz, M.D., Major General C.A. “Lou” Hennies (retired), Thomas E. Hill, Steve Gorlin and John C. Thomas, Jr. Steve Gorlin is the father of Jarrett Gorlin, the Company’s Chief Executive Officer.
Our current executive officers are Jarrett Gorlin, Chief Executive Officer, Patrick Kullmann, President and Chief Operating Officer, Charles Farrahar, Chief Financial Officer, Secretary, and Treasurer, and Dennis Moon, Senior Vice President.
Directors and Executive Officers
The following sets forth certain information about the directors and executive officers of the Company:
|
Name
|
Position(s)
|
Age
|
| Steve Gorlin |
Director |
77 |
| Major General C.A. “Lou” Hennies |
Director (2) (3) |
78 |
| James R. Andrews, M.D. |
Director |
72 |
| Scott M. W. Haufe, M.D. |
Director (2) |
48 |
| Thomas E. Hills |
Director (1) (3) |
38 |
| Randal R. Betz, M.D. |
Director |
62 |
| John C. Thomas, Jr. |
Director (1) |
60 |
| Larry Papasan |
Chairman of the Board (2) (3) |
73 |
| Jarrett Gorlin |
Chief Executive Officer and Director (1) |
39 |
| Patrick Kullmann |
President and Chief Operating Officer |
58 |
| Charles Farrahar |
Chief Financial Officer |
53 |
| Dennis Moon |
Senior Vice President |
38 |
|
(1)
|
Member of audit committee
|
|
(2)
|
Member of compensation committee
|
|
(3)
|
Member of nominating and corporate governance committee
|
BOARD OF DIRECTORS
Steve Gorlin
Steve Gorlin is the Co-founder of Debride Inc., the first company acquired by Medovex. Over the past 40 years, Mr. Gorlin has founded several biotechnology and pharmaceutical companies, including HycorBiomedical, Inc. (acquired by Agilent), Theragenics Corporation (NYSE: TGX) , CytRx Corporation (NASDAQ: CYTR), Medicis Pharmaceutical Corporation (sold to Valeant for approximately $2.6 billion), EntreMed, Inc. (NASDAQ: ENMD), MRI Interventions (MRIC), DARA BioSciences, Inc. (NASDAQ: DARA), MiMedx (NASDAQ: MDXG), and Medivation, Inc. (NASDAQ: MDVN). Mr. Gorlin served for many years on the Business Advisory Council to the Johns Hopkins School of Medicine and on The Johns Hopkins BioMedical Engineering Advisory Board. He presently serves on the board of directors of the Andrews Institute. Mr. Gorlin founded a number of non-medical related companies, including Perma-Fix, Inc., Pretty Good Privacy, Inc. (sold to Network Associates), and NTC China. He started The Touch Foundation, a nonprofit organization for the blind and was a principal financial contributor to the founding of Camp Kudzu for diabetic children. Presently, he serves as a member of the board of directors and of the executive committee of DemeRx, Inc., Conkwest, Inc., and is on the Board of NTC China, Inc.
Major General C.A. “Lou” Hennies
Mr. Hennies became a director of the Company in September 2013. Lou Hennies is a career soldier having served his country in uniform for 41 years where he rose through the ranks from enlisted status to that of a commissioned officer retiring in 2001 as a Major General.
He served a total of 37 months in combat in Republic of Vietnam as a Company/Troop commander of four units and as a battalion/squadron staff officer in the 4th Battalion, 23rd Infantry Regiment, 25th Infantry Division, Cu Chi, and the 7th Squadron, 17th Air Cavalry in II Corps. Stateside he commanded another Air Cavalry Troop followed by command of the 1st Squadron, 17th Air Cavalry in the 82nd Airborne Division.
Selected for Brigadier General in 1986, he subsequently served as the Army’s Deputy Chief of Public Affairs and Director of Army Safety and Commanding General of the U.S Army Safety Center. Initially retiring in 1991, he returned to service in 1995 as The Adjutant General (TAG) of the Alabama Army and Air National Guard and as a Cabinet Officer in the Administration of Governor Fob James Jr.
He is a graduate of the Army’s Command and General Staff College, The Army War College, and The Center for Creative Leadership. A graduate of the University of Nebraska-Omaha with a Bachelor Degree in Political Science, he also holds a Master of Arts Degree in Journalism from the University of Nebraska-Lincoln and a Master of Science in Public Administration from Shippensburg University, Pennsylvania.
His awards and decorations include the Army Distinguished Medal with Oak Leaf Cluster, the Silver Star, the Legion of Merit with Oak Leaf Cluster, the Distinguished Flying Cross, the Soldiers Medal, the Bronze Star with “V” device and 5 Oak Leaf Clusters, the Purple Heart, the Air Medal with “V” (2) and numeral 29, and the Alabama Distinguished Medal with Oak Leaf Cluster. He is also a recipient of numerous foreign decorations from the Republic of Vietnam and the Republic of Korea.
He has been awarded the Army Aviation Order of Saint Michael (Gold), the Infantry’s Order of Saint Maurice (Primercius) and the Army Aviation Hall of Fame Medallion and has been inducted into the Infantry Officer Candidate Hall of Fame, the Army Aviation Hall of Fame, and the Air Force Gathering of Eagles Class of 2000.
James R. Andrews, M.D.
James R. Andrews, M.D., has served as a Director of the Company since September 2013. Dr. Andrews is recognized throughout the world for his scientific and clinical research contributions in knee, shoulder and elbow injuries, and his skill as an orthopedic surgeon. Dr. Andrews is a founder and current Medical Director for the American Sports Medicine Institute, a non-profit organization dedicated to the prevention, education and research in orthopaedic and sports medicine, as well as the Andrews Research and Education Institute.
He is Clinical Professor of Orthopaedic Surgery at the University of Alabama Birmingham Medical School, the University of Virginia School of Medicine and the University of South Carolina Medical School. He is Adjunct Professor in the Department of Orthopaedic Surgery at the University of South Alabama and Clinical Professor of Orthopaedics at Tulane University School of Medicine.
He serves as Medical Director for Auburn University Intercollegiate Athletics and Team Orthopaedic Surgeon; Senior Orthopaedic Consultant at the University of Alabama; Orthopaedic Consultant for the college athletic teams at Troy University, University of West Alabama, Tuskegee University and Samford University. He serves on the Tulane School of Medicine Board of Governors.
Dr. Andrews serves on the Medical and Safety Advisory Committee of USA Baseball and on the Board of Little League Baseball, Inc. He has been a member of the Sports Medicine Committee of the United States Olympic Committee and served on the NCAA Competitive Safeguards in Medical Aspects of Sports Committee.
In the professional sports arena, Dr. Andrews is Senior Consultant for the Washington Redskins Football team; Medical Director for the Tampa Bay Rays Baseball team and Medical Director of the Ladies Professional Golf Association.
Dr. Andrews serves as the National Medical Director for Physiotherapy Associates, a national outpatient rehabilitation provider. He serves on the Board of Directors of Fast Health Corporation and Robins Morton Construction Company. He has a Doctor of Laws Degree from Livingston University and Doctor of Science Degrees from Troy and Louisiana State Universities.He has recently written a book, Any Given Monday, about sports injuries and how to prevent them for athletes, parents and coaches.
Scott M. W. Haufe, M.D.
Scott M. W. Haufe, M.D., is a co-founder of Debride and has been a Director of the Company since September 2013. Dr. Haufe is a board certified physician in the fields of Anesthesiology, Pain Medicine and Hospice /Palliative Medicine. He began his career in the field of Anesthesiology where he served as Chief of Anesthesiology and Pain Management with St. Lucie Anesthesia Associates until 1998 while continuing his passion for research. Beginning in 1993, Dr. Haufe was first published and has since authored numerous peer reviewed journal articles. Specifically, in 2005, he was recognized for his publication on the endoscopic treatment for sacroilitis. During 2006, he again authored the first paper on intradiscal stem cell therapy in an attempt to rejuvenate the human disc and in 2010 he developed a minimally invasive procedure for resolving spinal arthritis and subsequently published his findings in the Internal Journal of Med Sci. Additionally, he is named on multiple patents for treating pain related issues. Dr. Haufe earned his MD from the University of South Florida College of Medicine in 1992 with honors and completed his residency in Anesthesiology in 1996. He currently practices in Destin, FL with Anesthesia, Inc., and is affiliated with Sacred Heart Hospital, Destin Surgery Center, and Healthmark Medical Center. He is a member of the American Society of Anesthesiologists and the Florida Society of Anesthesiologists.
Larry Papasan
Larry Papasan has served as Chairman of the Board of Directors of the Company since September 2013. From July 1991 until his retirement in May 2002, Mr. Papasan served as President of Smith & Nephew Orthopedics. He has been a Director and Chairman of the Board of Directors of BioMimetic Therapeutics, Inc. [NasdaqGM:BMTI] since August 2005. BioMimetic Therapeutics is developing and commercializing bio-active recombinant protein-device combination products for the healing of musculoskeletal injuries and disease, including orthopedic, periodontal, spine and sports injury applications. Mr. Papasan has also served as a member of the Board of Directors of Reaves Utility Income Fund [NasdaqCM:UTG], a closed-end management investment company, since February 2003 and of Triumph Bancshares, Inc. (a bank holding company) since April 2005. Mr. Papasan also serves as a Director of SSR Engineering, Inc., AxioMed Spine Corporation, and MiMedx Group, Inc.
John C. Thomas, Jr.
John Thomas has been a director of the Company since September 2013 and currently serves as the CFO for Cormatrix Cardiovascular, Inc., a privately held medical device company which he joined in 2001. Over the past 24 years, Mr. Thomas has served as the CFO of numerous startup companies and managed their financing activities from the initial financing up to their initial public offering. Some of these companies are still private and some have become public entities. The companies in the health care industry that have gone public while Mr. Thomas was the CFO include CytRx Corporation (1986 – 1990), CytRx Biopool (1988 – 1991), Medicis Pharmaceutical Corporation (1988 –1991), EntreMed, Inc. (1991 – 1997), DARA BioSciences, Inc. (1998 – 2009) and, MiMedx, Inc. (2006 – 2009). He has also been the CFO of Surgi-Vision, Inc., a private research company involved in MRI technology (1998 – 2010).Mr. Thomas has also been the CFO of Motion Reality, Inc., a privately-held company with proprietary software that captures and analyzes motion data since 1991. Mr. Thomas is a certified public accountant and is the Chairman of the Finance Committee and a Trustee at The Walker School, a private pre-K through 12th grade school in Marietta, Georgia.
Thomas E. Hills
Thomas Hills has been a Director of the Company since September 2013. Mr. Hills is currently President and Chief Investment Officer of healthcare focused Hills Capital Management; the family office for the Paul F. Hills family in Barrington, IL which he joined in 2007. In addition to his experience in the asset management business and prior to founding Hills Capital Management, Tom was in sales and marketing at Sage Products, Inc. At Hills Capital, Tom leads the family’s public and private equity healthcare investment efforts. He is a board member of MedShape in Atlanta, Georgia and Chairman of Barrington Children’s Charities which he and his wife founded. Tom holds a BBA from St. Norbert College and an MBA from Loyola University of Chicago.
Randal R. Betz, M.D.
Dr. Randal Betz has been a director of the Company since September 2013. Dr. Betz is an orthopedic spine surgeon with a private practice in Princeton, New Jersey. Dr. Betz has held hospital positions as Chief of Staff at Shriners Hospitals for Children and Medical Director of Shriners’ Spinal Cord Injury Unit. Dr. Betz is also a Professor of Orthopedic Surgery at Temple University School of Medicine.
Dr. Betz earned a Medical Degree from Temple University School of Medicine and was awarded the Alpha Omega Alpha honor. His Internship in general surgery and Residency in Orthopedic Surgery were at Temple University Hospital. Dr. Betz’s Fellowship in Pediatric Orthopedics was at the Alfred I DuPont Institute. Since his graduate work, Dr. Betz has had postdoctoral fellowship experiences with ABC Traveling Fellowship, North American Traveling Fellowship, SRS Traveling fellowship and the Berg-Sloat Traveling Fellowship.
Many national and international professional societies count Dr. Betz as a member including: the Academic Orthopedic Society, American Academy for Cerebral Palsy and Developmental Medicine, American Academy of Orthopedic Surgeons, American Orthopedic Association, American Paraplegia Society, American Spinal Injury Association, British Scoliosis Society, International Functional Electrical Stimulation Society, North American Spine Society, Pediatric Orthopedic Society of North America, Scoliosis Research Society, and Spinal Deformity Education Group. For many of these organizations, Dr. Betz has fulfilled the roles of Board of Director Member and Committee Member and President of the Scoliosis Research Society in 2005.
In addition to an active hospital practice in pediatric spinal surgery, research is an important area of Dr. Betz’s career. He is a recipient of many research grants and he has ten patents, including several involving research in spinal deformities: fusionless treatment of spinal deformities. Dr. Betz is author of several medical texts. He has contributed 45 chapters to medical books and written 280 peer-reviewed or invited articles. Worldwide, Dr. Betz has delivered hundreds of paper presentations and invited lectures. Dr. Betz is on the Editorial Board of the Journal of Pediatric Orthopedics and a Reviewer for the Journal of Bone and Joint Surgery, Journal of Pediatric Orthopedics, and Spine.
Jarrett Gorlin
Jarrett Gorlin has served as the Chief Executive Officer, President, and a Director of the Company since November, 2013. Prior to joining the Company, Mr. Gorlin served as the President of Judicial Correction Services, Inc. (“JCS”), the largest provider of private probation services in the country, which he co-founded in 2001. In 2011, he successfully negotiated the sale of JCS to Correctional Healthcare Companies (“CHC”), after which he has continued to serve as the President of JCS. Under Mr. Gorlin’s leadership, JCS made INC. Magazine’s list of the Fastest Growing Companies in America in 2010, 2011, and 2012. Mr. Gorlin began his career by becoming the youngest rated commercial helicopter pilot at the age of 16, and becoming the chief pilot for the Fulton County Sheriff’s Office in Atlanta, Georgia. Mr. Gorlin has served a Captain and Commander at the Fulton County Sheriff’s Office where he has worked from 1996 to present. He continues to serve his community through law enforcement as the commander of a reserve unit overseeing 90 deputy sheriffs, who work in the courts, jail and warrant divisions. Mr. Gorlin also serves as a political advisor and consultant to many elected officials in the Atlanta area, including the current sitting Sheriff of Fulton and Clayton County, Georgia. He has also served on the campaign finance committee for the former Governor of Georgia Roy Barnes.
NON-DIRECTOR EXECUTIVE OFFICERS
President and Chief Operating Officer – Patrick Kullmann
Patrick Kullmann has been our Chief Operating Officer since September, 2013. Mr. Kullmann has served in a contract capacity as the Chief Executive Officer of Streamline, Inc., a medical technology company since 2012. He is also the Founder of CG3 Consulting, LLC, a global medical technology advisory firm in Minneapolis, Boston and San Diego which he founded in 2008. CG3 Consulting provides consulting services to clients in the healthcare, scientific and technology industries. Prior to establishing CG3 Consulting, Mr. Kullmann was a senior director at Medtronic in their $2.3 billion Cardiovascular Division. He started his career working as a surgical sales representative in the Texas Medical Center in Houston. Mr. Kullmann has served in senior marketing, market development and sales leadership positions at Boston Scientific, Baxter, Johnson & Johnson, and four start-up medical device companies – two of which had successful liquidity events for a combined value of $220m. He is a graduate of Northern Michigan University, and has an MBA from California Coastal University. The Board believes that Mr. Kullmann has the experience, qualifications, attributes and skills necessary to serve as President and Chief Operating Officer because of his years of experience in the medical technology field.
Chief Financial Officer, Secretary and Treasurer – Charles Farrahar
Charles “Charlie” Farrahar has served as our Chief Financial Officer, Secretary and Treasurer since September 2013. He is a certified public accountant with 30 years of managerial finance, administration, human resource and accounting experience in the public, private and non-profit sectors. He was CFO for publicly traded Credit Depot Corp (1997 -1998), but has since focused on privately held early stage companies, including three companies formed to develop various medical technologies. He served as CFO of Judicial Correction Services from 2003 – 2012, helping it grow from a 25 to 400+ employee organization. The Board believes that Mr. Farrahar has the experience, qualifications, attributes and skills necessary to serve as Chief Financial Officer, Secretary and Treasurer because of his years of experience in accounting and managerial finance.
Senior Vice President – Dennis Moon
Dennis Moon has served as our Senior Vice President since November, 2013. Prior to joining the Company, he was the Chief Operations Officer for Judicial Correction Services (2006 – 2013) supervising the day to day operations of the JCS community supervision division, which supervised over 50,000 active probationers throughout the southeast United States. He was responsible for supervision of over 400 employees and over 1.8 million financial transactions per year. Dennis is a graduate of the University of Central Florida and has a degree in Psychology with an emphasis on Drug and Alcohol addiction. After graduating high school, he joined the United States Army where he served for eight years as an Intelligence Analyst and received numerous awards and medals for various services. The Board believes that Mr. Moon has the experience, qualifications, attributes and skills necessary to serve as Senior Vice President because of his years of experience in the military and in management of employees.
Liability and Indemnification of Directors and Officers
Our Articles of Incorporation provide that to the fullest extent permitted under Nevada law, our directors will not be personally liable to the Company or its shareholders for monetary damages for breach of the duty of care, breach of fiduciary duty or breach of any other duties as directors. Our Articles of Incorporation also provide for indemnification of our directors and officers by the Company to the fullest extent permitted by law.
Board Composition and Election of Directors
Board Leadership Structure
Role of Board in Risk Oversight Process
Our board of directors has responsibility for the oversight of the Company’s risk management processes and, either as a whole or through its committees, regularly discusses with management our major risk exposures, their potential impact on our business and the steps we take to manage them. The risk oversight process includes receiving regular reports from board committees and members of senior management to enable our board to understand the company’s risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, finance, legal, regulatory, strategic and reputational risk.
The audit committee reviews information regarding liquidity and operations, and oversees our management of financial risks. Periodically, the audit committee reviews our policies with respect to risk assessment, risk management, loss prevention and regulatory compliance. Oversight by the audit committee includes direct communication with our external auditors, and discussions with management regarding significant risk exposures and the actions management has taken to limit, monitor or control such exposures. The compensation committee is responsible for assessing whether any of our compensation policies or programs has the potential to encourage excessive risk-taking. The nominating/corporate governance committee manages risks associated with the independence of the board, corporate disclosure practices, and potential conflicts of interest. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, the entire board is regularly informed through committee reports about such risks. Matters of significant strategic risk are considered by our board as a whole.
Board Committees and Independence
Our board of directors has established an audit committee, a nominating and corporate governance committee and a compensation committee, each of which operates under a charter that has been approved by our board and which goes into effect upon closing of this offering.
Our board has determined that all of the members of each of the board’s three standing committees are independent as defined under the rules of The NASDAQ Capital Market. In addition, all members of the audit committee meet the independence requirements contemplated by Rule 10A-3 under the Securities Exchange Act of 1934, or the Exchange Act.
Audit Committee
The members of our audit committee are John C. Thomas, Jr., Thomas Hills and Jarrett Gorlin. Mr. Thomas chairs the audit committee. The audit committee’s main function is to oversee our accounting and financial reporting processes, internal systems of control, independent registered public accounting firm relationships and the audits of our financial statements. Upon closing of this offering, this committee’s responsibilities will include, among other things:
|
●
|
appointing, approving the compensation of and assessing the independence of our registered public accounting firm;
|
|
●
|
overseeing the work of our independent registered public accounting firm, including through the receipt and consideration of reports from such firm;
|
|
●
|
reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures;
|
|
●
|
monitoring our internal control over financial reporting, disclosure controls and procedures and code of business conduct and ethics;
|
|
●
|
overseeing our internal audit function;
|
|
●
|
overseeing our risk assessment and risk management policies;
|
|
●
|
establishing policies regarding hiring employees from the independent registered public accounting firm and procedures for the receipt and retention of accounting related complaints and concerns;
|
|
●
|
meeting independently with our internal auditing staff, independent registered public accounting firm and management;
|
|
●
|
reviewing and approving or ratifying any related person transactions; and
|
|
●
|
preparing the audit committee report required by Securities and Exchange Commission, or SEC, rules.
|
All audit and non-audit services, other than de minimis non-audit services, to be provided to us by our independent registered public accounting firm must be approved in advance by our audit committee.
Our board of directors has determined that John C. Thomas, Jr. is an “audit committee financial expert” as defined in applicable SEC rules.
Nominating and Corporate Governance Committee
The members of our nominating and corporate governance committee are Major General C.A. “Lou” Hennies, Thomas Hills and Larry Papasan. Mr. Hennies chairs the nominating and corporate governance committee. Upon closing of this offering, this committee’s responsibilities will include, among other things:
|
●
|
identifying individuals qualified to become members of our board of directors;
|
|
●
|
recommending to our board of directors the persons to be nominated for election as directors and to each of our board’s committees;
|
|
●
|
developing, recommending to the board, and assessing corporate governance principles, codes of conduct and compliance mechanisms; and
|
|
●
|
overseeing the evaluation of our board of directors.
|
Compensation Committee
The members of our compensation committee are Larry Papasan, Major General C.A. “Lou” Hennies and Scott M. W. Haufe, M.D. Mr. Papasan chairs the compensation committee. Upon closing of this offering, this committee’s responsibilities will include, among other things:
|
●
|
reviewing and recommending corporate goals and objectives relevant to the compensation of our chief executive officer and other executive officers;
|
|
●
|
making recommendations to our board of directors with respect to, the compensation level of our executive officers;
|
|
●
|
reviewing and recommending to our board of directors employment agreements and significant arrangements or transactions with executive officers;
|
|
●
|
reviewing and recommending to our board of directors with respect to director compensation; and
|
|
●
|
overseeing and administering our equity-based incentive plans;
|
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the compensation committee, or other committee serving an equivalent function, of any other entity that has one or more of its executive officers serving as a member of our board of directors or our compensation committee. Mr. Gorlin, CEO and Director, will abstain on any Board vote involving executive compensation by the Board as a whole.
Board Diversity
Upon closing of this offering, our nominating and corporate governance committee will be responsible for reviewing with the board of directors, on an annual basis, the appropriate characteristics, skills and experience required for the board of directors as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the nominating and corporate governance committee, in recommending candidates for election, and the board of directors, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including the following:
|
|
personal and professional integrity, ethics and values;
|
|
|
experience in corporate management, such as serving as an officer or former officer of a publicly-held company;
|
|
|
development or commercialization experience in large medical products companies;
|
|
|
experience as a board member or executive officer of another publicly-held company;
|
|
|
strong finance experience;
|
|
|
diversity of expertise and experience in substantive matters pertaining to our business relative to other board members;
|
|
|
diversity of background and perspective, including with respect to age, gender, race, place of residence and specialized experience;
|
|
|
conflicts of interest; and
|
|
|
practical and mature business judgment.
|
Currently, our board of directors evaluates, and following the closing of this offering will evaluate, each individual in the context of the board of directors as a whole, with the objective of assembling a group that can best maximize the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Following this offering, a current copy of the code will be posted on the Corporate Governance section of our website, www.MEDOVEX.com. In addition, we intend to post on our website all disclosures that are required by law or the listing standards of The NASDAQ Capital Market concerning any amendments to, or waivers from, any provision of the code. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this prospectus.
Summary Compensation Table
The following table sets forth information concerning the compensation of our named executive officers during the fiscal years ended December 31, 2013:
|
Name & Position
|
|
Fiscal Year
|
|
Salary
($)
|
|
|
Bonus
($)
|
|
|
Stock Awards
($)
|
|
|
All Other Compensation ($)
|
|
|
Total
($)
|
|
|
Jarrett Gorlin, CEO
|
|
2013
|
|
|
37,500 |
|
|
|
0 |
|
|
|
0 |
|
|
|
0 |
|
|
|
37,500 |
|
|
Patrick Kullmann, COO
|
|
2013
|
|
|
25,000 |
|
|
|
0 |
|
|
|
0 |
|
|
|
0 |
|
|
|
25,000 |
|
|
Charles Farrahar, CFO
|
|
2013
|
|
|
22,917 |
|
|
|
0 |
|
|
|
0 |
|
|
|
0 |
|
|
|
22,917 |
|
|
Dennis Moon, VP
|
|
2013
|
|
|
25,000 |
|
|
|
0 |
|
|
|
0 |
|
|
|
0 |
|
|
|
25,000 |
|
Employment Agreements
From their first date of employment, the Company entered into Employment and Confidential Information and Inventions Assignment (“Confidentiality”) Agreements with each of its four officers. These agreements are identical with the exception of the salary amount in the Employment Agreement.
The Confidentiality Agreement, among other things, obligates each officer not to disclose Confidential Information (as defined in the Agreement) for a period of 5 years after their last date of employment. It commits the employee to assign any work product developed at Medovex to the Company and assist with obtaining patents for that work as necessary. It contains a provision prohibiting employees from soliciting clients or hiring Company personnel for a period of 2 years after their separation.
The Employment Agreements are for a term of three years and define the compensation and benefits each employee will receive when they start employment. They also define the circumstances for and the effect on compensation and benefits under the following scenarios:
|
a.
|
Termination without cause
|
|
b.
|
Termination upon death or disability
|
|
c.
|
Termination by the Company for cause
|
|
d.
|
Termination by the employee for good reason, including material diminishment of position, demands to move or change in control of the Company
|
|
e.
|
Termination by the Company without cause, upon disability or by employee with good reason
|
|
f.
|
Termination for other reasons
|
If the Company terminates without cause or the employee terminates with good reason, the employee continues to collect his salary and benefits for 6 months after termination. The Employment Agreement also contains a non-compete clause prohibiting the employee from competing with the Company for 1 year after their separation.
The current annualized salaries of our executive officers are as follows:
|
Name & Position
|
|
Annual Salary
|
|
|
Jarrett Gorlin, CEO
|
|
$ |
180,000 |
|
|
Patrick Kullmann, President & COO
|
|
$ |
170,000 |
|
|
Charles Farrahar, CFO
|
|
$ |
110,000 |
|
|
Dennis Moon, VP
|
|
$ |
120,000 |
|
Director Compensation
There was no compensation paid to directors in 2013, but $30,000 of directors’ fees were incurred. The Board established a policy of paying outside (non-employee) directors $5,000 per quarter for each full quarter of service. The Board also voted to grant each outside director on the Board at October 14, 2013 (6 persons at that time) a stock option for 10,000 shares of common stock at an exercise price of $2.50 per share.
Outstanding Equity Awards at 2013 Fiscal Year-End
There were no equity awards granted to the named executive officers in 2013.
TRANSACTIONS WITH RELATED PERSONS
On January 31, 2013, Debride entered into a Contribution and Royalty Agreement with Scott W. Haufe, M.D., a Director of the Company, pursuant to which agreement, Debride acquired all of Dr. Haufe’s right, title and ownership of U.S. Patent 8,167,879 B2, together with all of Dr. Haufe’s right, title and interest in and to the Debride intellectual property in exchange for shares of common stock of Debride. The agreement provides that Dr. Haufe shall receive a royalty of one (1%) percent of Debride’s net sales during the life of the patent.
In September 2013, we entered into a Co-Development Agreement with James Andrews, M.D., a director of the Company, whereby Dr. Andrews committed to further evaluate the Debride and to seek to make modifications and improvements to such technology. In exchange for such services, the Company agreed to pay Dr. Andrews a royalty equal to two (2%) percent of Debride’s net sales during the five (5) year term of the Co-Development Agreement. Upon the termination of the term of the Co-Development Agreement, which has a minimum term of five (5) years, then the royalty payable to Dr. Andrews shall be reduced to one (1%) percent of Debride’s net sales after such termination of products covered by any U.S. patent on which Dr. Andrews is listed as a co-inventor; if any such patents are obtained. Such one (1%) percent royalty shall continue during the effectiveness of such patent. Pursuant to the Co-Development Agreement, Dr. Andrews agreed to assign any modifications or improvements to the Debride to the Company subject to the royalty rights described above.
Several directors of the Company or their family members participated in the 2013 private placement of shares of common stock of the Company at $2.50 per share on the same terms as non-affiliated shareholders. In the aggregate, directors of the Company purchased 212,500 shares in the 2013 private placement. The resale of these shares are subject to the lock up. See “Shares Eligible Future Sale – Lock-Up”.
The Company reimburses CEO Jarrett Gorlin for approximately 1,200 square feet of office space he rents in Atlanta Georgia for executive office space at a rate of $2,000 per month plus related utilities, the exact amount billed to him by the owner of the property. The Company believes that such reimbursement is on terms no less favorable then it would receive from leasing space from a third party. The Company has also chartered aircraft from TAG Aviation, a privately-held aircraft leasing company owned by CEO Jarrett Gorlin. The total amount spent in 2013 with TAG Aviation was approximately $34,000. The Company believes that such aircraft charter is on terms no less favorable then it would receive from a third party.
Policies and Procedures for Related Person Transactions
Our board of directors has adopted written policies and procedures for the review of any transaction, arrangement or relationship in which we are a participant, the amount involved exceeds $120,000 and one of our executive officers, directors, director nominees or 5% stockholders, or their immediate family members, each of whom we refer to as a “related person,” has a direct or indirect material interest.
If a related person proposes to enter into such a transaction, arrangement or relationship, which we refer to as a “related person transaction,” the related person must report the proposed related person transaction to our Chief Executive Officer. The policy calls for the proposed related person transaction to be reviewed and, if deemed appropriate, approved by our audit committee. Whenever practicable, the reporting, review and approval will occur prior to entry into the transaction. If advance review and approval is not practicable, the committee will review, and, in its discretion, may ratify the related person transaction. The policy also permits the chairman of the committee to review and, if deemed appropriate, approve proposed related person transactions that arise between committee meetings, subject to ratification by the committee at its next meeting. Any related person transactions that are ongoing in nature will be reviewed annually.
A related person transaction reviewed under the policy will be considered approved or ratified if it is authorized by the committee after full disclosure of the related person’s interest in the transaction. As appropriate for the circumstances, the committee will review and consider:
|
|
●
|
the related person’s interest in the related person transaction;
|
|
|
●
|
the approximate dollar value of the amount involved in the related person transaction;
|
|
|
●
|
the approximate dollar value of the amount of the related person’s interest in the transaction without regard to the amount of any profit or loss;
|
|
|
|
●
|
whether the transaction was undertaken in the ordinary course of our business;
|
|
|
●
|
whether the terms of the transaction are no less favorable to us than terms that could have been reached with an unrelated third party;
|
|
|
|
●
|
the purpose of, and the potential benefits to us of, the transaction; and
|
|
|
●
|
any other information regarding the related person transaction or the related person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction.
|
|
|
The committee may approve or ratify the transaction only if the committee determines that, under all of the circumstances, the transaction is in our best interests. The committee may impose any conditions on the related person transaction that it deems appropriate.
In addition to the transactions that are excluded by the instructions to the SEC’s related person transaction disclosure rule, our board of directors has determined that the following transactions do not create a material direct or indirect interest on behalf of related persons and, therefore, are not related person transactions for purposes of this policy:
|
|
●
|
interests arising solely from the related person’s position as an executive officer of another entity (whether or not the person is also a director of such entity) that is a participant in the transaction, where (i) the related person and all other related persons own in the aggregate less than a 10% equity interest in such entity, (ii) the related person and his or her immediate family members are not involved in the negotiation of the terms of the transaction and do not receive any special benefits as a result of the transaction and (iii) the amount involved in the transaction is less than the greater of $200,000 or 5% of the annual gross revenues of the company receiving payment under the transaction; and
|
|
|
|
|
●
|
a transaction that is specifically contemplated by provisions of our charter or bylaws.
|
|
|
The policy provides that transactions involving compensation of executive officers shall be reviewed and approved by the compensation committee in the manner specified in its charter.
We did not have a written policy regarding the review and approval of related person transactions prior to this offering. Nevertheless, with respect to such transactions, it was our policy for our board of directors to consider the nature of and business reason for such transactions, how the terms of such transactions compared to those which might be obtained from unaffiliated third parties and whether such transactions were otherwise fair to and in the best interests of, or not contrary to, our best interests. In addition, all related person transactions required prior approval, or later ratification, by our board of directors.
Indemnification Agreements
Our certificate of incorporation provides that we will indemnify our directors and officers to the fullest extent permitted by Nevada law. In addition, we have entered into indemnification agreements with our directors.
Stock Option Grants to Executive Officers and Directors
In October 2013, the Company adopted the 2013 Stock Incentive Plan (the “Plan”). The Plan will also be submitted in due course for approval by our shareholders, to the extent required under federal tax laws or other applicable laws.
The Plan is intended to secure for us and our shareholders the benefits arising from ownership of our Common Stock by individuals we employ or retain who will be responsible for the future growth of the enterprise. The Plan is also designed to help attract and retain superior personnel for positions of substantial responsibility, including advisory relationships where appropriate, and to provide individuals with an additional incentive to contribute to our success. The “Administrator” of the Plan is the Compensation Committee of the Board; however, the Administrator may also delegate to one or more officers of the Company the authority to make most determinations otherwise reserved for decision by the Administrator. Under the Plan, the Administrator has the flexibility to determine eligible participants and the type and amount of awards to grant to eligible participants.
The Administrator may make the following types of grants under the Plan, each of which will be an “Award”:
|
|
qualified incentive stock options (“QISOs”);
|
|
|
nonqualified stock options; and
|
|
|
awards of restricted stock and/or restricted stock units.
|
Our officers, key employees, directors, consultants and other independent contractors or agents who are responsible for or contribute to our management, growth or profitability will be eligible for selection by the Administrator to participate in the Plan, provided, however, that QISOs may be granted only to our employees.
We authorized and reserved for issuance under the Plan an aggregate of 1,150,000 shares of our Common Stock. To date, we have granted an aggregate of 60,000 options to purchase common stock at $2.50 per share to our outside directors. If any of the Awards granted under the Plan expire, terminate or are forfeited for any reason before they have been exercised, vested or issued in full, the unused shares allocable to or subject to those expired, terminated or forfeited awards will become available for further grants under the Plan.
The following table sets forth as of the March 31, 2014 . The information is presented for each person we know to be a beneficial owner of 5% or more of our securities, each of our directors and executive officers, and our officers and directors as a group.
The column entitled “Shares beneficially owned prior to Offering-Percentage is based on a total of 7,781,175 shares of our common stock outstanding as of March 31, 2014 . The column entitled “Shares beneficially owned after offering-percentage” is based on 9,131,275 shares of our common stock to be outstanding after this offering, including the shares of common stock that we are selling in this offering.
The number of shares beneficially owned by each stockholder is determined under the rules issued by the Securities and Exchange Commission and includes voting or investment power with respect to such securities. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sale or shared voting power or investment power. Unless otherwise indicated, the address of all listed stockholders is c/o MEDOVEX, 3279 Hardee Avenue, Atlanta, Georgia 30041. Unless otherwise indicated each of the stockholders listed has sole voting and investment power with respect to the shares beneficially owned, subject to community property laws where applicable.
| |
|
|
|
|
Percentage of Shares
Beneficially Owned
|
| |
|
Number of Shares Beneficially Owned(1)
|
|
|
|
|
|
|
Scott M.W. Haufe, M.D., Director
|
|
|
772,608 |
(2) |
|
|
9.9 |
% |
8.5% |
|
Renee Honig
|
|
|
600,000 |
|
|
|
7.7 |
% |
6.6% |
|
Jarrett Gorlin, Director and Officer
|
|
|
559,478 |
(3) |
|
|
7.2 |
% |
6.1% |
|
Larry W. Papasan, Director
|
|
|
196,076 |
(4) |
|
|
2.5 |
% |
2.1% |
|
John C. Thomas, Jr.
|
|
|
50,000 |
(5) |
|
|
0.6 |
% |
0.5% |
|
Patrick Kullmann, Officer
|
|
|
193,576 |
(6) |
|
|
2.5 |
% |
2.1% |
|
Charles Farrahar, Officer
|
|
|
193,576 |
|
|
|
2.5 |
% |
2.1% |
|
Major General C.A. “Lou” Hennies, Chairman
|
|
|
99,288 |
(4) |
|
|
1.3 |
% |
1.1% |
|
James R. Andrews, M.D., Director
|
|
|
99,288 |
(4) |
|
|
1.3 |
% |
1.1% |
|
Thomas E. Hills, Director
|
|
|
99,288 |
(4) |
|
|
1.3 |
% |
1.1% |
|
Steve Gorlin, Director
|
|
|
417,474 |
(7) |
|
|
5.4 |
% |
4.6% |
|
Randal R. Betz, M.D., Director
|
|
|
99,288 |
(4) |
|
|
1.3 |
% |
1.1% |
|
Dennis Moon, Officer
|
|
|
193,576 |
(4) |
|
|
2.5 |
% |
2.1% |
|
Officers and Directors as a Group (12 persons)
|
|
|
2,973,576 |
|
|
|
38.2 |
% |
32.3% |
|
(1)
|
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and includes voting or investment power with respect to shares beneficially owned and options exercisable within 60 days. Beneficial ownership is based on information furnished by the individuals or entities.
|
|
(2)
|
Includes 532,335 shares held by Morgan Stanley Smith Barney custodian for Nicole Haufe Roth IRA and 237,773 shares held by Nicole Haufe. Mr. Haufe disclaims beneficial ownership of the shares.
|
|
(3)
|
Represents shares held by The Jarrett S. & Rebecca L. Gorlin Family Limited Partnership. Mr. Gorlin disclaims beneficial ownership of the shares.
|
|
(4)
|
Includes 2,500 shares pursuant to options exercisable within 60 days.
|
|
(5)
|
Includes 50,000 shares pursuant to options exercisable within 60 days. Does not include 150,000 shares issuable upon the exercise of options which are not exercisable within 60 days.
|
|
(6)
|
Includes 96,788 shares held by Pamela M.C. Kullmann. Mr. Kullmann disclaims beneficial ownership of Pamela M.C. Kullmann’s shares.
|
|
(7)
|
Includes 125,000 shares held by his spouse, Debbie Gorlin. Steve Gorlin disclaims beneficial ownership of Debbie Gorlin’s shares.
|
DESCRIPTION OF CAPITAL STOCK
General
The following description of our capital stock and provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to the certificate of incorporation and the bylaws that will be in effect upon the closing of this offering. We have filed copies of these documents with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The description of the capital stock reflects changes to our capital structure that will occur upon the closing of this offering.
We have two authorized classes of stock: Preferred Stock (500,000 shares authorized), and Common Stock (49,500,000 million shares authorized).
Common Stock
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. An election of directors by our stockholders shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of outstanding preferred stock.
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our certificate of incorporation, our board of directors is authorized to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our board of directors to issue preferred stock and determination its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance is preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock. There will be no shares of Preferred Stock outstanding upon the closing of this Offering.
Anti-Takeover Provisions
Because we are incorporated in Nevada, we are governed by the provisions of Nevada Revised Statutes 78.378 to 78.3793, which prohibits a person who owns in excess of 10% of our outstanding voting stock from merging, consolidating or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 10% of our outstanding voting stock, unless the merger, consolidation or combination is approved in a prescribed manner. Any provision in our corporate charter or our bylaws or Nevada law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Removal of Directors
A director may be removed only for cause and only by the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in an annual election of directors. Any vacancy on our board of directors, including a vacancy resulting from an enlargement of our board of directors, may be filled only by vote of a majority of our directors then in office.
Authorized but Unissued Shares
The authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of the NASDAQ Capital Market. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be Interwest Transfer Co. .
NASDAQ Capital Market
We intend to apply to list our common stock on The NASDAQ Capital Market under the symbol “MDVX,” the Series A Warrants will trade under the symbol “MDVXW,” and our units will trade under the symbol “MDVXU.” No assurance can be given that our application will be approved.
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering units, consisting of one share of common stock and one Series A Warrant. In addition, we are offering one Series B Warrant for each unit purchased. The units will not be certificated and the shares of common stock and Series A Warrants may be transferred separately upon issuance. The Series B Warrants may be transferred immediately after their issuance, but will not be listed securities. Each Series A Warrant is exercisable for one share of common stock, and each Series B Warrant is also exercisable for one share of common stock. The shares of common stock issuable from time to time upon exercise of the Series A Warrants and the Series B Warrants are also being offered pursuant to this prospectus.
Common Stock
The material terms and provisions of our common stock and each other class of securities which qualifies or limits our common stock are described under the caption “Description of Capital Stock” in this prospectus.
Warrants
Series A Warrants
Each purchaser of units in this offering will receive, for each unit purchased, a Series A Warrant representing the right to purchase one share of common stock at an exercise price of 120% of the public offering price per unit. The number of shares of common stock issued to each purchaser will be equal to the number of units purchased by such purchaser in this offering. The Series A Warrants will be exercisable on the date that the warrants are issued and will terminate on the third anniversary date the warrants are first exercisable. The exercise price and number of shares for which each Series A Warrant may be exercised is subject to adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock. The Series A Warrants may be called by us, for consideration equal to $0.0001 per warrant, on not less than 10 business days’ notice if the closing price of the common stock is above 150% of the public offering price per unit for any period of 20 consecutive business days ending not more than three business days prior to the call notice date.
Series B Warrants
The Series B Warrants will be exercisable immediately after the date the Series B Warrants are issued and will terminate on the fifth anniversary of the date the warrants are first issued at an exercise price of 120% of the public offering price per unit. The exercise price and number of shares for which each Series B Warrant may be exercised is subject to adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock. The Series B Warrants may be called (cancelled) by us, for consideration equal to $0.0001 per warrant, on not less than 10 business days’ notice if the closing price of the common stock is above 150% of the public offering price per unit for any period of 20 consecutive business days ending not more than three business days prior to the call notice date.
We do not intend to apply to list the Series B Warrants on any securities exchange. Without an active market, the liquidity of the Series B Warrants will be limited.
Provisions Applicable to Series A Warrants and Series B Warrants
There is no established public trading market for either series of warrants, and there can be no assurances that a market will develop. In the event our common stock price does not exceed the per share exercise price of the warrants during the period when the warrants are exercisable, the warrants will not have any value.
Holders of the warrants may exercise their warrants to purchase shares of our common stock on or before the termination date by delivering an exercise notice, appropriately completed and duly signed. Payment of the exercise price for the number of shares for which the warrant is being exercised must be made within two trading days following such exercise. In the event that the registration statement relating to the warrant shares is not effective, a holder of warrants may only exercise its warrants for a net number of warrant shares pursuant to the cashless exercise procedures specified in the warrants. Warrants may be exercised in whole or in part, and any portion of a warrant not exercised prior to the termination date shall be and become void and of no value. The absence of an effective registration statement or applicable exemption from registration does not alleviate our obligation to deliver common stock issuable upon exercise of a warrant.
Upon the holder’s exercise of a warrant, we will issue the shares of common stock issuable upon exercise of the warrant within three trading days of our receipt of notice of exercise, subject to timely payment of the aggregate exercise price therefor.
The shares of common stock issuable on exercise of the warrants will be, when issued in accordance with the warrants, duly and validly authorized, issued and fully paid and non-assessable. We will authorize and reserve at least that number of shares of common stock equal to the number of shares of common stock issuable upon exercise of all outstanding warrants.
If, at any time a warrant is outstanding, we consummate any fundamental transaction, as described in the warrants and generally including any consolidation or merger into another corporation, the consummation of a transaction whereby another entity acquires more than 50% of our outstanding common stock, or the sale of all or substantially all of our assets, or other transaction in which our common stock is converted into or exchanged for other securities or other consideration, the holder of any warrants will thereafter receive upon exercise of the warrants, the securities or other consideration to which a holder of the number of shares of common stock then deliverable upon the exercise or conversion of such warrants would have been entitled upon such consolidation or merger or other transaction.
In the event that we issue shares of Common Stock or securities convertible into or exercisable for Common Stock at below $6.00 per share, except in the event of an "exempt issuance", as defined in the applicable warrant agreements which are filed as an exhibit to the registration statement of which this prospectus forms a part, then the exercise price of the A and B Warrants shall be adjusted to such lower price.
The warrants are not exercisable by their holder to the extent (but only to the extent) that such holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock.
Amendments and waivers of the terms of the warrants require the written consent of the holder of such warrant and us. The warrants will be issued in book-entry form under a warrant agent agreement between InterWest Transfer Co. as warrant agent, and us, and shall initially be represented by one or more book-entry certificates deposited with The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
You should review a copy of the warrant agent agreement and the forms of each series of warrant, each of which are included as exhibits to the registration statement of which this prospectus forms a part.
Representatives’ Unit Warrant
In addition, we have agreed to issue to the representative of the underwriters a unit warrant to purchase up to an aggregate of 10% of the units sold in this offering, excluding the shares of common stock sold pursuant to the over-allotment option, if any. The shares of common stock issuable upon exercise of the warrants included as part of these units are identical to those offered by this prospectus. The representative’s warrants included as part of the representatives’ unit warrants are exercisable for cash or on a cashless basis at per share exercise price equal to 120% of the public offering price of one unit in this offering commencing on a date which is one year from the date of effectiveness of the registration statement of which this prospectus is a part and expiring on a date which is no more than five years from such effective date incompliance with FINRA Rule 5110(f)(2)(H)(i). These representative’s warrants do not have anti-dilution protections and are not transferable for 180 days from the date of the commencement of sales of the offering except as allowed by FINRA Rule 5110(g).
THE HOLDER OF A WARRANT WILL NOT POSSESS ANY RIGHTS AS A STOCKHOLDER UNDER THAT WARRANT UNTIL THE HOLDER EXERCISES THE WARRANT. THE WARRANTS MAY BE TRANSFERRED INDEPENDENT OF THE COMMON STOCK WITH WHICH THEY WERE ISSUED, SUBJECT TO APPLICABLE LAWS.
Prior to this offering, there has been no public market for our common stock, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options, or the anticipation of these sales, could materially and adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of equity or equity-related securities.
As described below, only a limited number of shares of our common stock will be available for sale in the public market for a period of several months after closing of this offering due to contractual and legal restrictions on resale described below. Nevertheless, sales of a substantial number of shares of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could materially and adversely affect the prevailing market price of our common stock. Although we intend to list our common stock on the NASDAQ Capital Market, we cannot assure you that there will be an active market for our common stock.
Upon the closing of this offering, we will have outstanding an aggregate of 9,131,175 shares of our common stock.
Of the shares to be outstanding immediately after the closing of this offering, we expect that the 1,350,000 shares to be sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act. The remaining 7,781,175 shares of our common stock outstanding after this offering will be “restricted securities” under Rule 144, and we expect that a substantial portion of these restricted securities will be subject to the one-year lock-up period under the lock-up agreements as described below. These restricted securities may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act.
Rule 144
Affiliate Resales of Restricted Securities
In general, subject to the lock-up restrictions described below, beginning 90 days after the effective date of the registration statement, of which this prospectus is a part, a person who is an affiliate of ours, or who was an affiliate at any time during the 90 days before a sale, who has beneficially owned shares of our common stock for at least six months would be entitled to sell in “broker’s transactions” or certain “riskless principal transactions” or to market makers, a number of shares within any three-month period that does not exceed the greater of:
|
|
1% of the number of shares of our common stock then outstanding, which will equal approximately 91,312 shares immediately after this offering; or
|
|
|
the average weekly trading volume in our common stock on the NASDAQ Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale.
|
Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the SEC and the NASDAQ Capital Market concurrently with either the placing of a sale order with the broker or the execution directly with a market maker.
Non-Affiliate Resales of Restricted Securities
In general, subject to the lock-up restrictions described above, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the three months preceding a sale, and who has beneficially owned shares of our common stock for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us.
If such person has held our shares for at least one year, such person can resell under Rule 144(b)(1) without regard to any Rule 144 restrictions, including the 90-day public company requirement and the current public information requirement.
Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
Lock-up
All of our shareholders will have agreed not to sell any shares of common stock owned by them for a period of 12 months from the date of this offering. In the event that our common stock trades at $9.00 per share for ten (10) consecutive trading days at anytime after 180 days from the effective date of this offering the lock-up shall terminate.
Upon expiration of the one year lock-up period, all of our shares of our common stock will be eligible for sale under Rule 144, including shares eligible for resale immediately upon the closing of this offering as described above. We cannot estimate the number of shares of our common stock that our existing stockholders will elect to sell under Rule 144.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of ours during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation, or notice provisions of Rule 144. Rule 701 also permits affiliates of ours to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701 and until expiration of the one year lock-up period described below.
ViewTrade Securities Inc. is acting as the representative of the underwriters of this offering (the “Representative”). We have entered into an underwriting agreement dated the date of this prospectus with the Representative. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of our units and Series B Warrants next to its name in the following table:
|
Underwriters
|
|
Number of Units and Series B Warrants
|
ViewTrade Securities Inc.
|
|
|
| |
|
|
| |
|
|
|
|
|
|
The underwriters are committed to purchase all the units and Series B Warrants offered by us if any units and Series B Warrants are purchased, other than those covered by the option to purchase additional units described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated.
The underwriters propose to offer the units and Series B Warrants offered by us to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriters may offer some of the units and Series B Warrants to other securities dealers at such price less a concession of $ per unit and Series B Warrant. If all of the units and Series B Warrants offered by us are not sold at the public offering price, the underwriters may change the offering price and other selling terms by means of a supplement to this prospectus.
The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties, such as receipt by the underwriters of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act of 1933, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters are offering the units and Series B Warrants, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 45 days after the date of this prospectus, permits the underwriters to purchase a maximum of 200,250 additional units and Series B Warrants from us to cover over-allotments. If the underwriters exercise all or part of this option, they will purchase units and Series B Warrants covered by the option at the public offering price less the underwriting discounts and commissions that appear on the cover page of this prospectus. If this option is exercised in full, the total price to the public will be approximately $ , and the total proceeds to us, before expenses, will be $ .
Underwriting Discounts and Commissions. We have agreed to pay underwriting discounts and commissions of 10% of the gross proceeds of the offering. The following table shows the public offering price, underwriting discounts and commissions and expenses to be paid by us to the underwriters and the proceeds of the public offering, before expenses, to us.
| |
|
Without over-allotment exercise
|
|
|
With full over-allotment exercise
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Underwriting discounts and commissions paid by us (per share)
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Underwriting discounts and commissions paid by us (total)
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Proceeds before other expenses (1)
|
|
|
|
|
|
|
|
|
|
(1)
|
We have agreed to pay the underwriters a non-accountable expense allowance in the amount of 2% of the total public offering price of the units sold (excluding the over-allotment option).
|
We have paid a $60,000 advance to the underwriters, which will be applied against the non- accountable expenses that will be paid by us to the underwriters in connection with this offering. The underwriting agreement provides that in the event the offering is terminated, the $60,000 advance paid to the underwriters will be returned to us to the extent that offering expenses are not actually incurred by the underwriters. In addition, the representative of the underwriter has the right to appoint one non-voting observer to attend all meetings of the Company's Board of Directors for a period of three years which commences in December 2013, subject to certain limitations relating to Regulation FD compliance and preservation of the attorney-client privilege . We have also agreed to pay or reimburse the underwriters, over and above the underwriting discount, for certain out of pocket expenses in connection with this offering, including (i) legal fees for the representative’s counsel, not to exceed $75,000, and (ii) miscellaneous expenses associated with the offering not to exceed $25,000. Such expenses can include filing costs with the SEC and FINRA, printing and reproduction of presentation materials, preparation and delivery of stock transfer agent fees, and the costs of informal and road show meetings.
The total estimated expenses of the offering, including the registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts, commissions and non-accountable expense allowances, are approximately $ and are payable by us.
Representative’s Unit Warrant. In addition, we have agreed to issue to the representative of the underwriters a unit warrant to purchase up to an aggregate of 10% of the units sold in this offering, excluding the shares of common stock sold pursuant to the over-allotment option, if any. The shares of common stock issuable upon exercise of the warrants included as part of the representative of the underwriters’ unit warrant are identical to those offered by this prospectus. The representative’s unit warrant is exercisable for cash or on a cashless basis at per share exercise price equal to 120% of the public offering price of one unit in this offering commencing on a date which is one year from the date of effectiveness of the registration statement of which this prospectus is a part and expiring on a date which is no more than five years from such effective date incompliance with FINRA Rule 5110(f)(2)(H)(i). These warrants do not have anti-dilution protections and are not transferable for 180 days from the date of the commencement of sales of the offering except as allowed by FINRA Rule 5110(g).
Discretionary Accounts. The underwriters do not intend to confirm sales of the units and Series B Warrants offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements. All of the holders of our common stock will have entered into lock-up agreements with the underwriters prior to the effective date of the Offering. Under these agreements, our shareholders have agreed, subject to specified exceptions, not to sell or transfer any common stock or securities convertible into, or exchangeable or exercisable for, our common stock, during a period ending 365 days after the date of this prospectus. In the event that our common stock trades at $9.00 per share for ten (10) consecutive trading days at anytime after 180 days from the effective date of this offering the lock-up shall terminate. In addition, certain holders of options to purchase our shares have entered into similar lock-up agreements.
Electronic Offer, Sale and Distribution of Units. A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The Representative may agree to allocate a number of units to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Other Relationships. Certain of the underwriters and their affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which may receive customary fees; however, except as disclosed in this prospectus, no such services were provided in the 180-day period preceding this filing and we have no present arrangements with any of the underwriters or their affiliates for any further services to be provided through the 90-day period following the effectiveness of this offering or thereafter.
Stabilization. In connection with this offering, the underwriters may engage in stabilizing transactions, overallotment transactions, syndicate covering transactions, penalty bids and purchases to cover positions created by short sales.
|
|
stabilizing transactions permit bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the shares while the offering is in progress.
|
|
|
overallotment transactions involve sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the overallotment option. In a naked short position, the number of shares involved is greater than the number of shares in the overallotment option. The underwriters may close out any short position by exercising their overallotment option and/or purchasing shares in the open market.
|
|
|
syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared with the price at which they may purchase shares through exercise of the overallotment option. If the underwriters sell more shares than could be covered by exercise of the overallotment option and, therefore, have a naked short position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering.
|
|
|
penalty bids permit the representative to reclaim a selling concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
|
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our shares of common stock or preventing or retarding a decline in the market price of our shares of common stock. As a result, the price of our shares of common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our shares of common stock. These transactions may be effected on Nasdaq, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Initial public offering of shares of common stock
Prior to this offering, there has been no public market for our shares of common stock. The initial public offering price will be determined by negotiations between us and the representatives of the underwriters. In determining the initial public offering price, we and the representatives of the underwriters expect to consider a number of factors including:
|
|
the information set forth in this prospectus and otherwise available to the representative;
|
|
|
our prospects and the history and prospects for the industry in which we compete;
|
|
|
an assessment of our management;
|
|
|
our prospects for future earnings;
|
|
|
the general condition of the securities markets at the time of this offering;
|
|
|
the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and
|
|
|
other factors deemed relevant by the underwriters and us.
|
Neither we nor the underwriters can assure investors that an active trading market will develop for our shares of common stock, or that the shares will trade in the public market at or above the initial public offering price.
The address of ViewTrade Securities Incorporated is 7280 West Palmetto Park Road, #105, Boca Raton, FL 33433.
The validity of the securities offered hereby is being passed upon for us by Sichenzia Ross Friedman Ference LLP. Certain legal matters related to the offering will be passed upon for the underwriters by Roetzel & Andress, LPA, Fort Lauderdale, Florida.
The audited consolidated financial statements of Medovex Corp and subsidiaries as of December 31, 2013 and the related statements of operations, changes in stockholder’s equity and cash flows for the period from February 1, 2013 (inception) through December 31, 2013, and related footnotes appearing in this registration statement, have been so included in reliance on the report of Marcum LLP, an independent registered public accounting firm, appearing elsewhere in this registration statement, given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities we are offering to sell. This prospectus, which constitutes part of the registration statement, does not include all of the information contained in the registration statement and the exhibits, schedules and amendments to the registration statement. For further information with respect to us and our securities, we refer you to the registration statement and to the exhibits and schedules to the registration statement. Statements contained in this prospectus about the contents of any contract, agreement or other document are not necessarily complete, and, in each instance, we refer you to the copy of the contract, agreement or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You may read and copy the registration statement of which this prospectus is a part at the SEC’s public reference room, which is located at 100 F Street, N.E., Room 1580, Washington, DC 20549. You can request copies of the registration statement by writing to the Securities and Exchange Commission and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s public reference room. In addition, the SEC maintains a website, which is located at www.sec.gov, that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access the registration statement of which this prospectus is a part at the SEC’s website.
Upon closing of this offering, we will be subject to the information reporting requirements of the Securities Exchange Act of 1934, and we will file reports, proxy statements and other information with the SEC. All documents filed with the SEC are available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at www.MEDOVEX.com. You may access our reports, proxy statements and other information free of charge at this website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information on such website is not incorporated by reference and is not a part of this prospectus.
MEDOVEX CORP. AND SUBSIDIARY
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE PERIOD FROM FEBRUARY 1, 2013 (INCEPTION)
TO DECEMBER 31, 2013 AND THE THREE MONTHS ENDED MARCH 31, 2014
CONTENTS
Independent Auditors’ Report
|
|
| |
|
Consolidated Financial Statements
|
|
Consolidated Balance Sheet
|
|
Consolidated Statements of Operations
|
|
Consolidated Statement of Changed in Stockholders’ Equity
|
|
Consolidated Statements of Cash Flows
|
|
| |
|
Notes to Consolidated Financial Statements
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Medovex Corporation and Subsidiary
We have audited the accompanying consolidated balance sheet of Medovex Corporation and Subsidiary (the “Company”) as of December 31, 2013, and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the period from February 1, 2013 (inception) to December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Medovex Corporation and Subsidiary, as of December 31, 2013, and the results of its operations and its cash flows for the period from February 1, 2013 (inception) to December 31, 2013 then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company is a development stage enterprise with no revenues, an inception period loss and limited capital resources. The Company, as a development stage enterprise, is subject to risks and uncertainties as to whether it will be able to the raise capital it needs to sustain its near term operations and pursue its longer term business strategy. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters also are described in Note 2. The consolidated financial statements do not include any adjustments relating to the recovery of assets or classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
/s/ Marcum LLP
New York, NY
May 1, 2014
MEDOVEX CORP. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
| |
|
March 31,
|
|
|
December 31,
|
|
| |
|
2014
|
|
|
2013
|
|
| |
|
(Unaudited)
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Property and Equipment, Net
|
|
|
|
|
|
|
|
Deferred initial public offering costs
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Liabilities and Stockholders' Equity
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Total Current Liabilities
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock - $.001 par value: 500,000 shares authorized, no shares outstanding
|
|
|
|
|
|
|
|
Common stock - $.001 par value: 49,500,000 shares authorized, 7,781,175 shares issued and outstanding
|
|
|
|
|
|
|
|
Common stock subscriptions
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
|
|
|
|
|
Deficit accumulated during the development stage
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Total Stockholders' Equity
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Total Liabilities and Stockholders' Equity
|
|
|
|
|
|
|
|
MEDOVEX CORP. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS
| |
|
For the three months ended March 31, 2014
|
|
|
Period of Inception (February 1, 2013) to December 31, 2013
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
$ |
- |
|
|
$ |
-- |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative
|
|
|
434,581 |
|
|
|
582,946 |
|
|
|
|
|
122,594 |
|
|
|
90,387 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
557,175 |
|
|
|
673,333 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
$ |
(557,175 |
) |
|
$ |
(673,333 |
) |
| |
|
|
|
|
|
|
|
|
Basic and diluted net loss per common share
|
|
$ |
(0.07 |
) |
|
$ |
(0.15 |
) |
| |
|
|
|
|
|
|
|
|
Basic and Diluted weighted average common shares outstanding
|
|
|
7,781,175 |
|
|
|
4,469,000 |
|
MEDOVEX CORP. AND SUBSIDIARY
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' EQUITY
FOR THE PERIOD FROM FEBRUARY 1, 2013 (INCEPTION) TO DECEMBER 31, 2013
AND THE THREE MONTHS ENDED MARCH 31, 2014
| |
Common Stock
|
|
|
Common
Stock
Subscription
|
|
|
Additional
Paid-in Capital
|
|
|
Accumulated Deficit
|
|
|
Total Stockholders' Equity
|
|
| |
Shares
|
|
Amount
|
|
|
|
|
|
|
|
|
|
Balance - February 1, 2013 (Inception)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock to founders, on February 1, 2013 at $0.01 per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock to founder in exchange for cash of $7,750 ($0.01 per share on February 1, 2013) and contribution of technology asset pursuant to a Contribution and Royalty Agreement dated January 31, 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock of the Company outstanding upon completion of the merger on September 13, 2013, originally issued on August 28, 2013 at $0.04 per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock in private placement, completed in December 2013, net of offering costs at $2.50 pursuant to a private placement memorandum dated September 16, 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance - December 31, 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Collection of subscription receivable
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MEDOVEX CORP. AND SUBSIDIARY
CONSOLIDATED STATEMENT OF CASH FLOWS
| |
|
For the three months ended March 31, 2014
|
|
|
Period of Inception (February 1, 2013) to December 31, 2013
|
|
| |
|
(Unaudited)
|
|
|
|
|
|
Cash Flows from Operating Activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Cash Used in Operating Activities
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Cash Flows from Investing Activities
|
|
|
|
|
|
|
|
Expenditures for property and equipment
|
|
|
|
|
|
|
|
Net Cash Used in Investing Activities
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Cash Flows from Financing Activities
|
|
|
|
|
|
|
|
Deferred initial public offering costs
|
|
|
|
|
|
|
|
Proceeds from issuances of stock to founders
|
|
|
|
|
|
|
|
Collection of common stock subscription receivable
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock in private placement, net of offering costs
|
|
|
|
|
|
|
|
Net Cash Provided by Financing Activities
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Cash - Beginning of period
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Supplemental Disclosures on Non-cash Investing and Financing Activities:
|
|
| |
|
|
|
|
|
|
|
Exchange of 750,108 shares of stock in exchange for technology asset
|
|
|
|
|
|
|
|
Increase in basis of technology asset due to accrual of deferred tax liability
|
|
|
|
|
|
|
|
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 1 - Organization and Significant Accounting Policies
Description of Business
Medovex Corp. (the “Company” or “Medovex”), was incorporated under the laws of the State of Florida on October 1 2012 under the name of Debride Inc. (“Debride”). The Company was inactive until it February 1, 2013, at which time it raised a limited amount of capital and acquired certain technology from one of its founding stockholders. There was no significant activity until August 2013, at which time the Company commenced merger discussions concurrent with launching its plans to pursue the development and eventual commercialization of its patented technology.
On September 13, 2013, Debride completed an Agreement and Plan of Merger with SpineZ Corp. (“SpineZ”), a privately owned company with no operations (the “SpineZ Merger”). The SpineZ Merger was effectuated as a share exchange transaction in which the former stockholders of Debride exchanged each share that they owned of Debride for 1.936 shares of SpineZ. As a result of the SpineZ Merger, the former owners of Debride became 53% majority owners of SpineZ. The Company accounted for this transaction as a reverse merger and recapitalization of Debride into SpineZ. The Company is a early stage enterprise that has acquired a patent, patent applications and other intellectual property rights relating to the use, development, and commercialization of the DenerVex Device (“DenerVex”). DenerVex is a device that is intended to be used in the treatment of conditions resulting from the degeneration of joints in the spine that cause back pain.
As described in Note 11, SpineZ changed its legal name to Medovex Corp. and effectuated a 1 for 2 reverse stock split on March 13, 2014. All share related amounts in the accompanying consolidated financial statements and notes thereto have been retroactively adjusted to reflect this reverse split.
Note 2 – Liquidity, Going Concern and Management’s plans
The Company incurred a net loss of approximately $673,000 and used approximately $574,000 of cash in its operating activities during its initial operating period of February 1, 2013 (date of inception) to December 31, 2013. During the three months ended March 31, 2014, the Company incurred a net loss of approximately $557,000 and used approximately $418,000 of cash in its operating activities.
The Company was initially capitalized by its founders with a limited contribution of funds and a contribution of the DenerVex technology that was developed and owned by one of its founding stockholders described in Note 5. This stockholder transferred the technology to the Company in exchange for approximately 22% of its then issued and outstanding common stock. Aggregate funds raised from the founders initially amounted to approximately $34,870 in a financing transaction completed at the Company’s inception of February 1, 2013. SpineZ was formed on August 28, 2013 with an aggregate capital contribution of $122,000 that became available to the Company for its use as general working capital upon completion of the SpineZ Merger on September 13, 2013. The Company also commenced an offering of its common stock under a Private Placement Memorandum dated September 16, 2013.
This offering closed in December 2013 upon the Company’s issuance of 1,356,175 shares of its common stock for proceeds of approximately $3,157,000, net of offering costs and a $100,000 subscription receivable that was paid on January 24, 2014. The Company is using the proceeds from this offering principally to fund its’ near term operations and pursue the potential commercialization of its patented technology.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 2 – Liquidity, Going Concern and Management’s plans (continued)
The Company’s ability to continue its operations and pursue the realization of its business plan is dependent upon its ability to raise additional capital. Management currently anticipates that the Company will need to raise additional funds through issuances of debt or equity securities until such time that it is able to generate revenue and operating cash flow through the execution of its business plan. Although Management believes that the Company has access to capital resources, the Company has not secured any commitments for new financing at this time. As described in Note 11, Company Management, with Board approval, engaged View Trade Securities, Inc. (“View Trade”) to act as an underwriter representative in a proposed initial public offering of the Company’s common stock (the “Proposed IPO”).
Management cannot provide any assurance that the Company will be successful in its efforts to obtain new financing through private placements of debt or equity securities or that the Company will in fact complete the Proposed IPO. If the Company is unable to raise sufficient financing, it could be required to undertake initiatives to conserve its capital resources which could include, but not necessarily be limited to, delaying or suspending plans to pursue the commercialization of its technology. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments to the carrying amounts of the Company’s assets or liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 3 – Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
These consolidated financial statements include the accounts of Medovex Corp. and its wholly-owned subsidiary, Debride. All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of the financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the amounts reported in financial statements and accompanying notes. The Company’s significant estimates currently include the fair value, useful life and carrying amount of its patented technology, the deferred income tax asset and the related valuation allowance, and the fair value of its share based payment arrangements. For those estimates that are sensitive to the outcome of future events, actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. The Company’s cash balance at December 31, 2013 and March 31, 2014 consists of funds deposited in checking accounts with commercial banks.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 3 – Summary of Significant Accounting Policies (continued)
Concentration of Credit Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist solely of cash. At times throughout the year, the Company may maintain certain bank account balances in excess of FDIC insured limits. At December 31, 2013 and March 31, 2014 the Company had cash deposits that exceeded federally insured deposit limits. The Company believes that its funds are deposited in high credit quality financial institutions. The Company has not experienced any losses in such accounts to date and believes it is not exposed to any significant credit risk associated with its cash deposits.
Intangible Asset
The Company’s intangible asset consists of a patent, patent applications and other intellectual property rights relating to the use, development, and commercialization of DenerVex (the “DenerVex Technology”). The intangible asset was recorded at fair value in accordance with the guidelines of ASC 845 “Non-Monetary Transactions’(“ASC 845”) when contributed to the Company at the time of its formation in exchange for shares of common stock (Note 5).
Intangible assets with finite lives, such as the Company’s technology, are amortized on a straight-line basis over the estimated useful lives of the assets. The Company will begin amortizing its technology asset when the commercial distribution of a product or products developed under the technology commences.
Intangible and other long lived assets with finite lives are periodically tested for impairment whenever changes in events or circumstances indicate their carrying amounts might not be recoverable. Impairment is evaluated by comparing the carrying amount of the long lived asset or asset group to the estimated undiscounted future cash flows expected to result from their use and eventual disposition. If such analysis indicates that the carrying amount of the asset or asset group exceeds the undiscounted cash flow, an impairment charge is recorded in an amount that is equal to the excess of the carrying amount of the asset or asset group and fair value, if less.
Property and Equipment
Property and equipment are stated at cost and are depreciated using the straight-line method over the estimated useful lives of the related assets, generally three to five years. Repairs and maintenance are expensed as incurred. Improvements and betterments, which extend the lives of the assets, are capitalized.
Research and Development
Research and development costs are expensed as incurred.
Stock-Based Compensation
The Company accounts for stock-based compensation in accordance with ASC 718 Compensation — Stock Compensation (“ASC 718”). ASC 718 addresses all forms of share-based payment (“SBP”) awards including shares issued under employee stock purchase plans and stock incentive shares. Under ASC 718 awards result in a cost that is measured at fair value on the awards’ grant date, based on the estimated number of awards that are expected to vest and will result in a charge to operations.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 3 – Summary of Significant Accounting Policies (continued)
Income Taxes
The Company accounts for income taxes under ASC 740, Income Taxes, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the asset will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
The standard addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the tax authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC 740 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. As of December 31, 2013, and March 31, 2014 the Company did not have a liability for unrecognized tax uncertainties.
The Company’s policy is to record interest and penalties on uncertain tax positions as a component of income tax expense. As of March 31, 2014, the Company has not incurred any interest or penalties relating to uncertain tax positions.
Loss per Share
Basic loss per share is computed on the basis of the weighted average number of shares outstanding for the reporting period. Diluted loss per share is computed on the basis of the weighted average number of common shares plus dilutive potential common shares outstanding using the treasury stock method. Any potentially dilutive securities are anti-dilutive due to the Company’s net losses. For the years presented, there is no difference between the basic and diluted net loss per share.
The Company excluded 60,000 common stock options from the computation of loss per share for all periods presented because they are anti-dilutive.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 3 – Summary of Significant Accounting Policies (continued)
Recent Accounting Standards
In June 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-10, “Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation.” This ASU removes the definition of a development stage entity from the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from GAAP. In addition, the ASU eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of operations, cash flows, and shareholders’ deficit, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. Early adoption is permitted. The Company has elected to adopt this ASU effective with these Annual Financial Statements and its adoption resulted in the removal of all development stage disclosures.
The Company has implemented all new accounting standards that are in effect and may impact its financial statements and does not believe that there are any other new accounting standards that have been issued that might have a material impact on its financial position or results of operations.
Note 4 – Property and Equipment
Property and equipment, net, consists of the following:
| |
Useful Life
|
|
March 31, 2014
|
|
|
December 31, 2013
|
|
|
|
|
|
$ |
2,760 |
|
|
$ |
2,760 |
|
|
|
|
|
|
2,545 |
|
|
|
1,175 |
|
| |
|
|
|
5,305 |
|
|
|
3,935 |
|
Less accumulated depreciation
|
|
|
|
(765 |
) |
|
|
(472 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
4,540 |
|
|
$ |
3,463 |
|
Depreciation expense amounted to $472 for the period from February 1, 2013 (inception) through December 31, 2013 and $293 for the three months ended March 31, 2014.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 5 – Patent Assignment and Contribution and Royalty Agreement
On February 1, 2013 Scott Haufe, M.D. (“Dr. Haufe”), who is one of our founding stockholders and the originator of the DenerVex Technology, contributed the technology to the Company pursuant to the terms of a Contribution and Royalty Agreement dated January 31, 2013 between the Company and Dr. Haufe. This agreement provides for the Company to pay Dr. Haufe royalties equal to 1% of revenues earned from sales of any and all products derived from the use of the DenerVex technology. Royalties are payable to Dr. Haufe within 30 days after the close of each calendar quarter based on actual cash collected from sales of applicable products. The royalty period expires on September 6, 2030.
The Company recorded the patents at their contribution date fair value of $1,000,000 in accordance with the guidelines of ASC 845. The Company determined the fair value of the asset using the relief from royalty method. This method requires management to estimate the net present value of the expected after tax cash flows that would be earned from licensing the use of the technology to another market participant based on sales of products derived from the use of the patented technology. Significant assumptions used in the development of this estimate include annual revenue growth ranging from 310% in the initial period following commercialization of the technology to 3% over an estimated economic life of the technology of 15 years commencing in 2015, a royalty rate of 2% based on average anticipated royalties for licensing of similar types of medical device products, income tax rate of 38%, and a discount rate of 50% based on the highest tier of a risk adjusted return that would be required on an early stage venture capital investment. Management based its estimates on data obtained from certain third party studies, published data regarding market rates of return for high risk/high yield venture capital investments, and management’s knowledge of the Company’s business and industry.
ASC 740-10-55 (“ASC 740”) addresses the accounting treatment for assets acquired outside of a business combination, and when the tax basis of such assets differs from the amount paid. As a result of this guidance, the Company has stepped-up the basis of its intangible assets by $611,000 and has recorded a deferred tax liability in the same amount, to account for the basis difference resulting from the acquisition of the asset in exchange for common stock.
Note 6 - Equity Transactions
Private Placements
Founders’ Shares
On February 1, 2013, the Company issued an aggregate of 2,624,892 shares of common stock to its founders in exchange for a contribution of $0.01 cent per share. Aggregate proceeds from this transaction amounted to $27,120. The Company concurrently issued 750,108 additional shares to another founding stockholder in exchange for $7,750 of cash and the transfer of patented technology to the Company pursuant to the terms of the Contribution and Royalty Agreement described in Note 5.
On August 28, 2013, the Company issued 3,050,000 shares of common stock to the initial SpineZ stockholders in exchange for a contribution of $.04 cents per share. Aggregate proceeds from this transaction amounted to $122,000, which became available to the Company for its use as general working capital upon the completion of the SpineZ Merger on September 13, 2013.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 6 - Equity Transactions (continued)
Private Placement
On September 16, 2013, the Company commenced a private placement of its common stock at an offering price of $2.50 per share. This financing transaction was completed in December 2013 with an aggregate of 1,346,175 shares issued for proceeds amounting to $3,056,651, net of issuance costs of $208,786, and a $100,000 subscription receivable that was paid on January 24, 2014. The Company also issued 10,000 shares of common stock as a partial fee paid to the placement agent who represented the Company in this financing transaction. The shares sold in this private placement were issued with certain rights that provide for such shares to be registered by the Company under the Securities Act of 1933 in the event that the Company files a registration statement with the Securities and Exchange Commission (“SEC”).
Stock-Based Compensation Plan
2013 Stock Option Incentive Plan
On October 14, 2013, the Medovex Corp. Board of Directors approved the Medovex Corp. 2013 Stock Incentive Plan (the “Plan”). The Company may grant incentive stock options to employees and non-statutory stock options to employees, consultants, and directors for up to 1,150,000 shares of common stock. The stock options are exercisable at a price equal to the market value on the date of the grant. The Plan gives full authority for granting options, determining the type of options granted, and determining the fair market value of the options to the Plan Administrator.
The Company has the right, but not obligation, to repurchase any shares obtained through exercise of an option from terminated Plan participants. The Company has 90 days from the date of termination to exercise its repurchase right. The Company must pay the Fair Market Value (“FMV”) of the shares if the termination was for any reason other than for cause, or the option price (if less than FMV of the shares) if the termination is for cause. The FMV is determined by the Plan Administrator on the date of termination.
On October 14, 2013, the Board of Directors authorized the Company to issue options to purchase an aggregate of up to 60,000 shares of common stock to its outside directors. The options vest as follows: 25% on date of grant and 25% on each of the next three anniversaries. The Company estimated the fair value of these options using the Black-Scholes option pricing model with the following assumptions: risk free interest rate of 2.75% (based on US Treasury note yield), expected term of 6 years (based on guidance provided by the SEC under Staff Accounting Bulletin 107, which allows issuers to use the simplified method for “plain vanilla” options for this calculation), expected volatility of 81% (based on the historical stock volatilities of several publicly traded companies in the medical device industry over a period equal to the expected term of the options (as the Company has no trading history to estimate volatility of its own common stock) and an exercise price equal to the grant date fair value of the stock of $2.50 per share. The fair value of the Company’s common stock was determined by reference to the private placement commenced by the Company in September 2013 and completed in December 2013, which was a cash transaction conducted at arm’s length with an unrelated group of investors. For the period ended December 31, 2013 and the three months ended March 31, 2014, the Company recognized $36,252 and $7,500, respectively, as compensation expense with respect to these options.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Stock-Based Compensation Plan (continued)
Stock Option Activity
The following is a summary of stock option activity for 2013 and the three months ended March 31, 2014:
| |
|
Shares
|
|
|
Weighted
Average
Exercise
Price
|
|
|
Weighted
Average
Remaining Term
(Years)
|
|
|
Aggregate
Intrinsic Value
|
|
Outstanding at 02/01/2013 (Inception)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at 12/31/2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at 12/31/2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrecognized compensation cost amounts to $83,748 and $76,248 as of December 31, 2013 and March 31, 2014, respectively, and will be recognized as an expense over a remaining service period of 3 years.
Note 7 - Commitments
Operating Leases
The Company reimburses its Chief Executive Officer, Jarret Gorlin (“Mr. Gorlin”) for his rental of certain office that is currently being used as the Company’s principal business location plus utilities cost (see “Related Party Transactions”). Lease payments under this arrangement amount to $2,000 per month. Rent expense and utilities cost paid to Mr. Gorlin amounted to approximately $7,000 and $8,000 in 2013 and the three months ended 2014, respectively.
Consulting Agreement
On December 2, 2013, the Company engaged one of its founding stockholders to provide business development consulting services over a one-year period at a fee of $10,000 per month. Either party can cancel this agreement upon 30 days’ notice, subject to a minimum fee of at least $60,000 should either party elect to cancel the agreement at any time on or prior to May 2, 2014 (Note 9).
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 7 – Commitments (continued)
Employment Agreements
The Company entered into Employment Agreements with each of its four executive officers for aggregate compensation amounting to approximately $530,000 per annum, plus customary benefits. These employment agreements are for terms of three years and provide for the Company to pay six months of severance in the event of (i) the Company’s termination of an executive’s employment without cause, (ii) the resignation by an executive for good reason, (iii) a change in control of the Company, (iv) a material reduction in an executive’s duties, or (v) a requirement that an executive move their primary work location more than 50 miles.
Co-Development Agreement
In September 2013, the Company executed a Co-Development Agreement with James R. Andrews, M.D. (“Dr. Andrews”) to further evaluate, test and advise on the development of products incorporating the use of the patented technology. In exchange for these services the Company is obligated to pay Dr. Andrews a royalty of 2% of revenues earned from applicable product sales over a period 5 years. If Dr. Andrews is listed as inventor of any Improvement Patent during the 5 year term, he would continue to receive a 1% royalty after the 2% royalty expires for the duration of the effectiveness of the Improvement Patent.
Note 8 - Income Taxes
For the period from February 1, 2013 (inception) to March 31, 2014, the Company has incurred net losses and, therefore, has no current income tax liability. The net deferred tax asset generated by these losses, which principally consist of start-up costs deferred for income tax purposes, is fully reserved as of December 31, 2013 and March 31, 2014, since it is currently more likely than not that the benefit will not be realized in future periods.
The provision for income tax consists of the following at:
| |
|
March 31, 2014
|
|
|
December 31, 2013
|
|
| |
|
(Unaudited)
|
|
|
(Audited)
|
|
|
Current Income Tax Expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Current Income Tax Expense
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Deferred Income Tax Benefit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Deferred Tax Benefit
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 8 - Income Taxes (continued)
A reconciliation of the statutory federal income tax expense (benefit) to the effective tax is as follows:
|
|
|
|
|
|
State taxes, net of federal benefit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax assets and liabilities consist of the following at:
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Deferred Tax Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Deferred Tax Liability – Difference on basis of intangible asset to tax value
|
|
|
|
|
|
|
|
As described in Note 3, the Company has stepped-up the basis of its intangible technology asset by $611,000 and has recorded a deferred tax liability for the same amount, to account for the difference between the financial reporting and income tax bases resulting from the acquisition of the technology asset in exchange for common stock.
The Company is required to file federal income tax returns and state income tax returns in the states of Florida, Georgia and Minnesota. There are no uncertain tax positions at December 31, 2013 or March 31, 2014. The Company has not undergone any tax examinations since inception and is therefore subject to examination by any applicable tax authorities.
Note 9 - Related-Party Transactions
Aviation Expense
The Company charters general aviation aircraft from TAG Aviation LLC (“TAG”), a company owned Mr. Gorlin. The total amount of general aviation expense paid to TAG amounted to approximately $34,000 and $11,000 in 2013 and the three months ended March 31, 2014, respectively.
Operating Lease
As described in Note 7 the Company reimburses Mr. Gorlin for his rental of office space that he leases at Dekalb-Peachtree Airport in Atlanta Georgia plus cost of utilities (see “Commitments”). The total amount paid to Mr. Gorlin as a reimbursement of his cost amounted to approximately $7,000 and $8,000 in 2013 and the three months ended March 31, 2014, respectively.
MEDOVEX CORP. AND SUBSIDIARY
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information for the three months ended March 31, 2014 is unaudited)
Note 9 - Related-Party Transactions (continued)
Consulting Expense
The Company utilized the services of CG3 Consulting LLC (“CGS”), a company founded by the Company’s Chief Operating Officer Patrick Kullmann (“Mr. Kullmann”). The Company paid approximately $2,000 of fees to CG3 in 2013 for consulting services relating to the potential commercialization the Company’s technology.
On December 2, 2013, the Company engaged a founding stockholder who owns 375,000 shares of its common stock to provide the Company with business development advisory services. Fees under this arrangement include a $45,000 up-front payment that is non-refundable and $10,000 per month for each month of services provided to the Company under this arrangement. This arrangement is cancelable by either party upon 30 days’ notice at any time after May 2, 2014. The Company paid $55,000 of fees under this arrangement in 2013 and $30,000 of fees during the three months ended March 31, 2014.
Note 10 - Research and Development
Devicix Design and Development Agreement
In November 2013, the Company accepted a proposal from Devicix, a Minneapolis Minnesota based FDA registered design and development contractor, to develop a commercially viable prototype of its product that could be used to receive regulatory approval from the FDA and other international agencies for use on humans to relieve pain associated with Facet Joint Syndrome. The development work commenced in December 2013. The total estimated cost of this work is $960,000; however, the terms of the proposal allow either the Company or the manufacturer to cancel the development work with 10-days’ notice. The Company incurred approximately $81,000 in research and development expense under the agreement in 2013 of which $16,000 is included in accounts payable in the accompanying consolidated balance sheet at December 31, 2013. During the three months ended March 31, 2014, the Company incurred approximately $123,000 of expense pursuant to this agreement, of which $51,000 is included in accounts payable at March 31, 2014.
Note 11 - Subsequent Events
In January 2014, Management, with Board approval, engaged View Trade Securities to act as underwriter representative in a public offering of its common stock. While most of the terms of that agreement are variable depending on the success of the offering, the Company paid View Trade a non-refundable retainers of $30,000 each in January 2014 and May 2014, and will be required to remit other expenses related to the filing and offering.
On March 13, 2014, a 1 for 2 reverse stock split was approved by the Company’s Board of Directors and a majority of its stockholders. Accordingly, the Company filed with the State of Nevada, an amendment to its Articles of Incorporation. All share related amounts in the accompanying consolidated financial statements and notes thereto have been retroactively adjusted to reflect this reverse split.
On May 2, 2014, the Company filed Form S-1 with SEC to register its securities for public trading. The Company intends a public offering of its common stock at such time as the registration statement may be declared effective
PROSPECTUS
ViewTrade Securities Inc.
Through and including , 2014 (the 25th day after the commencement of this offering) all dealers that effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II – INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the Securities and Exchange Commission registration fee, the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee and the NASDAQ Capital Market listing fee.
| |
|
Amount
|
|
Securities and Exchange Commission registration fee
|
|
|
|
|
|
|
|
|
|
|
NASDAQ Capital Stock Market listing fee
|
|
|
|
|
Accountants’ fees and expenses
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Transfer Agent’s fees and expenses
|
|
|
|
|
Printing and engraving expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*
|
To be provided by amendment.
|
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Nevada Law permits a corporation to eliminate the personal liability of its directors or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his or her duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Nevada corporate law or obtained an improper personal benefit. Our certificate of incorporation provides that no director shall be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the Nevada Law prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Nevada Law provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation and certain other persons serving at the request of the corporation in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlements actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he or she is party or is threatened to be made a party by reason of such position, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnification for such expenses which the court shall deem proper.
Our certificate of incorporation provides that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding whether civil, criminal, administrative or investigative (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful.
Our certificate of incorporation also provides that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee or, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred by him or her or on his or her behalf in connection therewith. If we don’t assume the defense, expenses must be advanced to an Indemnitee under certain circumstances.
We have entered into indemnification agreements with our directors. In general, these agreements provide that we will indemnify the director to the fullest extent permitted by law for claims arising in his or her capacity as a director of our Company or in connection with his or her service at our request for another corporation or entity. The indemnification agreements also provide for procedures that will apply in the event that a director makes a claim for indemnification and establish certain presumptions that are favorable to the director.
We maintain a general liability insurance policy which covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers.
The underwriting agreement we will enter into in connection with the offering of common stock being registered hereby provides that the underwriters will indemnify, under certain conditions, our directors and officers (as well as certain other persons) against certain liabilities arising in connection with such offering.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
Set forth below is information regarding shares of common stock issued by us within the past three years that were not registered under the Securities Act of 1933, as amended, or the Securities Act. Also included is the consideration, if any, received by us for such shares, notes, options and warrants and information relating to the section of the Securities Act, or rule of the Securities and Exchange Commission (“SEC”), under which exemption from registration was claimed.
We issued 3,050,000 shares of common stock in a founder’s round in August and September 2013 to 14 individuals. The offering was made in reliance on the Section 4(a)(2) exemption from the registration requirements of the Securities Act. There were no underwriters involved in such offering.
We issued 3,375,000 shares of the Company’s Common Stock to the 23 shareholders of Debride, representing 54% of the Company’s aggregate issued and outstanding common stock following the closing of the Merger Agreement with Debride. The offering was made in reliance on the Section 4(a)(2) exemption from the registration requirements of the Securities Act. There were no underwriters involved in such offering.
From November, 2013 to December, 2013, we conducted a private placement of 1,346,175 shares of the Common Stock of the Company pursuant to Rule 506 of Regulation D promulgated under the Securities Act. Palladium Capital Advisors, LLC acted as Placement Agent for this offering and received an aggregate commission of $75,000 for acting in such capacity. Palladium also received an additional 10,000 shares of Common Stock in partial consideration of their services. In connection, with the private placement we paid $15,931 to Dawson James in connection with their services in the private placement. The offering was made in reliance on the Section 4(a)(2) exemption from the registration requirements of the Securities Act.
The recipients of securities in the transactions described above represented that they were accredited investors and were acquiring the securities for their own account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time and appropriate legends were affixed to the instruments representing such securities issued in the foregoing transactions.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
The exhibits to the Registration Statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes to provide to the underwriter, at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned hereby undertakes that:
|
23
|
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
|
|
23
|
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
|
23
|
For the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
|
|
23
|
In a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
|
|
23
|
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
|
|
|
(ii)
|
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
|
|
|
(iii)
|
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
|
|
|
(iv)
|
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
|
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New York, State of New York, on this 7th day of July, 2014 .
| |
MEDOVEX CORP.
|
|
| |
|
|
|
| |
By:
|
/s/ Jarrett Gorlin
|
|
| |
|
Jarrett Gorlin
|
|
| |
|
Chief Executive Officer
|
|
| |
|
|
|
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
| |
|
|
|
|
|
/s/ Jarrett Gorlin
|
|
Chief Executive Officer, President and Director
|
|
|
|
Jarrett Gorlin
|
|
(principal executive officer)
|
|
|
| |
|
|
|
|
|
/s/ Charles Farrahr
|
|
Chief Financial Officer
|
|
|
|
Charles Farrahar
|
|
(principal financial and accounting officer)
|
|
|
| |
|
|
|
|
|
/s/ *
|
|
|
|
|
|
Larry Papasan
|
|
Chairman of the Board of Directors
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Clyde A. Hennies
|
|
Director
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Scott M.W. Haufe
|
|
Director
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
James R. Andrews
|
|
Director
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Thomas E. Hills
|
|
Director
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Randal Betz
|
|
Director
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Steve Gorlin
|
|
Director
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
John Thomas
|
|
Director
|
|
July 7, 2014
|
|
Exhibit Number
|
|
Description
|
| 1.1 |
|
Form of Underwriting Agreement |
| 1.2 |
|
Form of Underwriter's Warrant Agreement |
| 1.3 |
|
Form of Public A Warrant Agreeemnt |
| 1.4 |
|
Form of Public B Warrant Agreement |
|
|
|
Agreement and Plan of Merger, dated September 16, among MEDOVEX Corp. f/k/a SpineZ Corp. and Debrider Inc. **
|
|
|
|
Articles of Incorporation of Spinez Corp. **
|
|
|
|
Certificate of Amendment to the Articles of Incorporation of Spinez Corp. (changing the name of the company to MEDOVEX Corp. and effecting the reverse split of the outstanding shares of MEDOVEX Corp.’s common stock) **
|
|
|
|
Bylaws of MEDOVEX Corp. **
|
|
|
|
Specimen of certificate for MEDOVEX Corp. Common Stock. **
|
|
|
|
Opinion of Sichenzia Ross Friedman Ference LLP.*
|
|
|
|
2013 Stock Incentive Plan. **
|
|
|
|
Employment Agreement between MEDOVEX Corp. and Jarrett Gorlin dated October 14, 2013. **
|
|
|
|
Employment Agreement between MEDOVEX Corp. and Patrick Kullmann dated October 14, 2013. **
|
|
|
|
Employment Agreement between MEDOVEX Corp. and Charlie Farrahar dated October 14, 2013. **
|
|
|
|
Employment Agreement between MEDOVEX Corp. and Dennis Moon dated November 11, 2013. **
|
|
|
|
Contribution and Royalty Agreement between MEDOVEX and Scott W. Haufe, dated January 31, 2013. **
|
|
|
|
Co-Development Agreement between MEDOVEX Corp. and Dr. James Andrews dated September 30, 2013. **
|
|
|
|
Consulting Agreement between MEDOVEX Corp. and Robb Knie dated December 2, 2013. **
|
|
|
|
Engineering Services Agreement between MEDOVEX Corp. and Devicix, LLC dated November 25, 2013. **
|
|
|
|
Form of Indemnification Agreement.*
|
|
|
|
Business and Code of Ethics of MEDOVEX Corp.*
|
|
|
|
Subsidiaries of MEDOVEX Corp.
|
|
|
|
|
|
|
|
Consent of Sichenzia Ross Friedman Ference LLP (Included in Exhibit 5.1).*
|
|
|
|
Power of Attorney (included on signature page)
|
|
|
|
Audit Committee Charter.*
|
|
|
|
Nominating and Corporate Governance Charter.*
|
|
|
|
Compensation Committee Charter.*
|
* To be filed by amendment.
** Previously filed.