false--12-31Q22019000158536400640000063000000.190.190.190.210.0010.00110000000000100000000008684000000.00010.0001100000001000000000
0001585364
2019-01-01
2019-06-29
0001585364
2019-08-02
0001585364
2018-04-01
2018-06-30
0001585364
2018-01-01
2018-06-30
0001585364
2019-03-31
2019-06-29
0001585364
2019-06-29
0001585364
2018-12-31
0001585364
us-gaap:CommonStockMember
2018-12-31
0001585364
us-gaap:CommonStockMember
2019-03-31
2019-06-29
0001585364
us-gaap:CommonStockMember
2019-01-01
2019-03-30
0001585364
us-gaap:RetainedEarningsMember
2019-01-01
2019-03-30
0001585364
us-gaap:RetainedEarningsMember
2019-03-30
0001585364
2019-03-30
0001585364
2019-01-01
2019-03-30
0001585364
us-gaap:CommonStockMember
2019-03-30
0001585364
us-gaap:RetainedEarningsMember
2018-12-31
0001585364
us-gaap:RetainedEarningsMember
2019-06-29
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2019-06-29
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-12-31
0001585364
us-gaap:CommonStockMember
2019-06-29
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2019-03-30
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2019-01-01
2019-03-30
0001585364
us-gaap:RetainedEarningsMember
2019-03-31
2019-06-29
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2019-03-31
2019-06-29
0001585364
us-gaap:NewAccountingPronouncementMember
2018-12-31
0001585364
us-gaap:NewAccountingPronouncementMember
us-gaap:RetainedEarningsMember
2018-12-31
0001585364
us-gaap:CommonStockMember
2018-04-01
2018-06-30
0001585364
us-gaap:CommonStockMember
2018-01-01
2018-03-31
0001585364
2018-01-01
2018-03-31
0001585364
us-gaap:RetainedEarningsMember
2017-12-31
0001585364
2018-06-30
0001585364
us-gaap:RetainedEarningsMember
2018-04-01
2018-06-30
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2017-12-31
0001585364
us-gaap:RetainedEarningsMember
2018-06-30
0001585364
us-gaap:CommonStockMember
2018-06-30
0001585364
2017-12-31
0001585364
us-gaap:NewAccountingPronouncementMember
2017-12-31
0001585364
us-gaap:CommonStockMember
2018-03-31
0001585364
us-gaap:RetainedEarningsMember
2018-03-31
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-03-31
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-01-01
2018-03-31
0001585364
us-gaap:CommonStockMember
2017-12-31
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-06-30
0001585364
us-gaap:NewAccountingPronouncementMember
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2017-12-31
0001585364
us-gaap:RetainedEarningsMember
2018-01-01
2018-03-31
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2018-04-01
2018-06-30
0001585364
2018-03-31
0001585364
us-gaap:NewAccountingPronouncementMember
us-gaap:RetainedEarningsMember
2017-12-31
0001585364
country:IE
2019-01-01
2019-06-29
0001585364
country:IE
2019-03-31
2019-06-29
0001585364
prgo:ContractManufacturingMember
2018-01-01
2018-06-30
0001585364
prgo:ContractManufacturingMember
2019-03-31
2019-06-29
0001585364
country:IE
2018-04-01
2018-06-30
0001585364
prgo:ContractManufacturingMember
2018-04-01
2018-06-30
0001585364
country:IE
2018-01-01
2018-06-30
0001585364
prgo:ContractManufacturingMember
2019-01-01
2019-06-29
0001585364
prgo:RXPharmaceuticalsMember
2019-01-01
2019-06-29
0001585364
prgo:AnimalHealthMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:ConsumerSelfCareInternationalMember
2019-01-01
2019-06-29
0001585364
prgo:LifestyleMember
prgo:ConsumerSelfCareInternationalMember
2019-03-31
2019-06-29
0001585364
prgo:ConsumerSelfCareInternationalMember
2018-04-01
2018-06-30
0001585364
prgo:InfantNutritionalsMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:AnimalHealthMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:AnalgesicsMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:InfantNutritionalsMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:VitaminsMineralsAndDietarySupplementsMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:AnalgesicsMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:InfantNutritionalsMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:AnimalHealthMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:PersonalCareandDermaTherapeuticsMember
prgo:ConsumerSelfCareInternationalMember
2019-01-01
2019-06-29
0001585364
prgo:OtherCSCAMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:OtherCSCIMember
prgo:ConsumerSelfCareInternationalMember
2019-03-31
2019-06-29
0001585364
prgo:AnimalHealthMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:NaturalHealthandVMSMember
prgo:ConsumerSelfCareInternationalMember
2019-03-31
2019-06-29
0001585364
prgo:SmokingCessationMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:AnalgesicsMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:NaturalHealthandVMSMember
prgo:ConsumerSelfCareInternationalMember
2019-01-01
2019-06-29
0001585364
prgo:CoughColdAllergyandSinusMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:GastrointestinalMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:GastrointestinalMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:InfantNutritionalsMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:GastrointestinalMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:CoughColdAllergyandSinusMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:CoughColdandAllergyMember
prgo:ConsumerSelfCareInternationalMember
2019-01-01
2019-06-29
0001585364
prgo:OtherCSCIMember
prgo:ConsumerSelfCareInternationalMember
2019-01-01
2019-06-29
0001585364
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:LifestyleMember
prgo:ConsumerSelfCareInternationalMember
2018-04-01
2018-06-30
0001585364
prgo:AntiParasiteMember
prgo:ConsumerSelfCareInternationalMember
2019-03-31
2019-06-29
0001585364
prgo:CoughColdAllergyandSinusMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:LifestyleMember
prgo:ConsumerSelfCareInternationalMember
2019-01-01
2019-06-29
0001585364
prgo:RXPharmaceuticalsMember
2019-03-31
2019-06-29
0001585364
prgo:CoughColdandAllergyMember
prgo:ConsumerSelfCareInternationalMember
2018-01-01
2018-06-30
0001585364
prgo:AntiParasiteMember
prgo:ConsumerSelfCareInternationalMember
2018-04-01
2018-06-30
0001585364
prgo:LifestyleMember
prgo:ConsumerSelfCareInternationalMember
2018-01-01
2018-06-30
0001585364
prgo:RXPharmaceuticalsMember
2018-04-01
2018-06-30
0001585364
prgo:OtherCSCAMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:PersonalCareandDermaTherapeuticsMember
prgo:ConsumerSelfCareInternationalMember
2018-04-01
2018-06-30
0001585364
prgo:SmokingCessationMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:ConsumerSelfCareInternationalMember
2019-03-31
2019-06-29
0001585364
prgo:NaturalHealthandVMSMember
prgo:ConsumerSelfCareInternationalMember
2018-04-01
2018-06-30
0001585364
prgo:CoughColdAllergyandSinusMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:PersonalCareandDermaTherapeuticsMember
prgo:ConsumerSelfCareInternationalMember
2019-03-31
2019-06-29
0001585364
prgo:CoughColdandAllergyMember
prgo:ConsumerSelfCareInternationalMember
2018-04-01
2018-06-30
0001585364
prgo:OtherCSCIMember
prgo:ConsumerSelfCareInternationalMember
2018-01-01
2018-06-30
0001585364
prgo:GastrointestinalMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:VitaminsMineralsAndDietarySupplementsMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:ConsumerSelfCareInternationalMember
2018-01-01
2018-06-30
0001585364
prgo:OtherCSCAMember
prgo:ConsumerSelfCareAmericasMember
2018-04-01
2018-06-30
0001585364
prgo:OtherCSCIMember
prgo:ConsumerSelfCareInternationalMember
2018-04-01
2018-06-30
0001585364
prgo:VitaminsMineralsAndDietarySupplementsMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:VitaminsMineralsAndDietarySupplementsMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:AnalgesicsMember
prgo:ConsumerSelfCareAmericasMember
2019-03-31
2019-06-29
0001585364
prgo:SmokingCessationMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-06-30
0001585364
prgo:OtherCSCAMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:AntiParasiteMember
prgo:ConsumerSelfCareInternationalMember
2019-01-01
2019-06-29
0001585364
prgo:PersonalCareandDermaTherapeuticsMember
prgo:ConsumerSelfCareInternationalMember
2018-01-01
2018-06-30
0001585364
prgo:SmokingCessationMember
prgo:ConsumerSelfCareAmericasMember
2019-01-01
2019-06-29
0001585364
prgo:CoughColdandAllergyMember
prgo:ConsumerSelfCareInternationalMember
2019-03-31
2019-06-29
0001585364
prgo:RXPharmaceuticalsMember
2018-01-01
2018-06-30
0001585364
prgo:NaturalHealthandVMSMember
prgo:ConsumerSelfCareInternationalMember
2018-01-01
2018-06-30
0001585364
prgo:AntiParasiteMember
prgo:ConsumerSelfCareInternationalMember
2018-01-01
2018-06-30
0001585364
prgo:OtherGeographicalAreasMember
2018-04-01
2018-06-30
0001585364
country:US
2018-01-01
2018-06-30
0001585364
srt:EuropeMember
2019-03-31
2019-06-29
0001585364
country:US
2018-04-01
2018-06-30
0001585364
srt:EuropeMember
2018-01-01
2018-06-30
0001585364
country:US
2019-01-01
2019-06-29
0001585364
prgo:OtherGeographicalAreasMember
2019-01-01
2019-06-29
0001585364
prgo:OtherGeographicalAreasMember
2019-03-31
2019-06-29
0001585364
prgo:OtherGeographicalAreasMember
2018-01-01
2018-06-30
0001585364
srt:EuropeMember
2019-01-01
2019-06-29
0001585364
srt:EuropeMember
2018-04-01
2018-06-30
0001585364
country:US
2019-03-31
2019-06-29
0001585364
prgo:BudesonideNasalSprayandTriamcinoloneNasalSprayMember
us-gaap:DevelopedTechnologyRightsMember
2019-04-01
2019-04-01
0001585364
prgo:ConsumerHealthcareAmericasMember
prgo:MerckSharpDohmeLicenseAgreementMember
2018-05-29
2018-05-29
0001585364
prgo:GenericProductAcquisitionMember
us-gaap:DevelopedTechnologyRightsMember
2019-05-17
2019-05-17
0001585364
prgo:RXPharmaceuticalsMember
2019-06-29
0001585364
prgo:ConsumerSelfCareAmericasMember
2019-06-29
0001585364
prgo:ConsumerSelfCareAmericasMember
2018-06-30
0001585364
prgo:GenericProductAcquisitionMember
prgo:RXPharmaceuticalsMember
2019-03-31
2019-06-29
0001585364
us-gaap:InProcessResearchAndDevelopmentMember
2019-01-01
2019-03-30
0001585364
prgo:RXPharmaceuticalsMember
2018-10-01
0001585364
prgo:ConsumerSelfCareInternationalMember
2019-06-29
0001585364
prgo:TrademarksTradeNamesandBrandsMember
2019-06-29
0001585364
us-gaap:LicensingAgreementsMember
2018-12-31
0001585364
us-gaap:DevelopedTechnologyRightsMember
2019-06-29
0001585364
us-gaap:CustomerRelationshipsMember
2018-12-31
0001585364
us-gaap:TrademarksAndTradeNamesMember
2018-12-31
0001585364
us-gaap:InProcessResearchAndDevelopmentMember
2018-12-31
0001585364
us-gaap:NoncompeteAgreementsMember
2019-06-29
0001585364
us-gaap:TrademarksAndTradeNamesMember
2019-06-29
0001585364
us-gaap:DevelopedTechnologyRightsMember
2018-12-31
0001585364
prgo:TrademarksTradeNamesandBrandsMember
2018-12-31
0001585364
us-gaap:NoncompeteAgreementsMember
2018-12-31
0001585364
us-gaap:CustomerRelationshipsMember
2019-06-29
0001585364
us-gaap:LicensingAgreementsMember
2019-06-29
0001585364
us-gaap:InProcessResearchAndDevelopmentMember
2019-06-29
0001585364
prgo:RXPharmaceuticalsMember
2018-12-31
0001585364
prgo:ConsumerSelfCareAmericasMember
2018-12-31
0001585364
prgo:ConsumerSelfCareInternationalMember
2018-12-31
0001585364
prgo:ConsumerSelfCareInternationalMember
2018-06-30
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-04-01
2018-06-30
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-01-01
2018-06-30
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-03-31
2019-06-29
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-06-30
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-03-30
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-06-29
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-03-31
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-01-01
2019-06-29
0001585364
prgo:PublicBondsandPrivatePlacementMember
us-gaap:FairValueInputsLevel1Member
us-gaap:CarryingReportedAmountFairValueDisclosureMember
2019-06-29
0001585364
prgo:RetailBondsMember
us-gaap:FairValueInputsLevel2Member
us-gaap:CarryingReportedAmountFairValueDisclosureMember
2019-06-29
0001585364
prgo:RetailBondsMember
us-gaap:FairValueInputsLevel2Member
2018-12-31
0001585364
prgo:RetailBondsMember
us-gaap:FairValueInputsLevel2Member
2019-06-29
0001585364
prgo:PublicBondsandPrivatePlacementMember
us-gaap:FairValueInputsLevel1Member
us-gaap:CarryingReportedAmountFairValueDisclosureMember
2018-12-31
0001585364
prgo:PublicBondsandPrivatePlacementMember
us-gaap:FairValueInputsLevel1Member
2018-12-31
0001585364
prgo:RetailBondsMember
us-gaap:FairValueInputsLevel2Member
us-gaap:CarryingReportedAmountFairValueDisclosureMember
2018-12-31
0001585364
prgo:PublicBondsandPrivatePlacementMember
us-gaap:FairValueInputsLevel1Member
2019-06-29
0001585364
prgo:RoyaltyPharmaMember
2019-01-01
2019-06-29
0001585364
prgo:RoyaltyPharmaMember
2018-01-01
2018-06-30
0001585364
prgo:RoyaltyPharmaMember
us-gaap:MeasurementInputPriceVolatilityMember
2018-06-30
0001585364
prgo:RoyaltyPharmaMember
us-gaap:MeasurementInputPriceVolatilityMember
2019-06-29
0001585364
srt:ScenarioForecastMember
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
2020-01-01
2020-12-31
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsNonrecurringMember
2018-12-31
0001585364
us-gaap:MeasurementInputDiscountRateMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-12-31
0001585364
prgo:RoyaltyPharma2018ContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-04-01
2018-06-30
0001585364
prgo:MeasurementInputTaxRateMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-12-31
0001585364
prgo:RoyaltyPharma2020ContingentMilestonePaymentsMember
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-04-01
2018-06-30
0001585364
us-gaap:FairValueMeasurementsNonrecurringMember
2018-12-31
0001585364
srt:ScenarioForecastMember
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
2020-12-31
0001585364
us-gaap:MeasurementInputLongTermRevenueGrowthRateMember
prgo:ConsumerSelfCareAmericasMember
2018-01-01
2018-12-31
0001585364
us-gaap:FairValueMeasurementsNonrecurringMember
prgo:ConsumerSelfCareAmericasMember
2018-12-31
0001585364
srt:ScenarioForecastMember
2020-12-31
0001585364
prgo:RoyaltyPharmaContingentMilestonePaymentsMember
2018-01-01
2018-12-31
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-06-30
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-01-01
2018-06-30
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-03-31
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-03-31
2019-06-29
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-01-01
2019-06-29
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-03-30
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2019-06-29
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2018-04-01
2018-06-30
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2017-12-31
0001585364
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsNonrecurringMember
2018-12-31
0001585364
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsNonrecurringMember
2019-06-29
0001585364
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001585364
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2019-06-29
0001585364
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsNonrecurringMember
2019-06-29
0001585364
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2018-12-31
0001585364
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2019-06-29
0001585364
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsNonrecurringMember
2018-12-31
0001585364
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsNonrecurringMember
2019-06-29
0001585364
prgo:ZiboXinhuaMember
2018-04-01
2018-06-30
0001585364
prgo:OtherExpenseIncomeNetMember
2018-04-01
2018-06-30
0001585364
prgo:OtherExpenseIncomeNetMember
2018-01-01
2018-06-30
0001585364
prgo:OtherExpenseIncomeNetMember
2019-01-01
2019-06-29
0001585364
prgo:OtherExpenseIncomeNetMember
2019-03-31
2019-06-29
0001585364
us-gaap:OtherNoncurrentAssetsMember
2019-06-29
0001585364
us-gaap:PrepaidExpensesAndOtherCurrentAssetsMember
2018-12-31
0001585364
us-gaap:OtherNoncurrentAssetsMember
2018-12-31
0001585364
us-gaap:PrepaidExpensesAndOtherCurrentAssetsMember
2019-06-29
0001585364
us-gaap:AccruedLiabilitiesMember
2019-06-29
0001585364
us-gaap:InterestExpenseMember
2019-03-31
2019-06-29
0001585364
us-gaap:CostOfSalesMember
2019-03-31
2019-06-29
0001585364
us-gaap:ForeignExchangeForwardMember
2019-03-31
2019-06-29
0001585364
us-gaap:SalesRevenueNetMember
2019-03-31
2019-06-29
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:OtherNonoperatingIncomeExpenseMember
2018-01-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
2018-01-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:SalesMember
2018-04-01
2018-06-30
0001585364
us-gaap:DesignatedAsHedgingInstrumentMember
2018-01-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:CostOfSalesMember
2018-01-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:InterestExpenseMember
2018-01-01
2018-06-30
0001585364
us-gaap:InterestRateSwapMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:InterestExpenseMember
2018-01-01
2018-06-30
0001585364
us-gaap:CostOfSalesMember
2019-01-01
2019-06-29
0001585364
us-gaap:ForeignExchangeForwardMember
2019-01-01
2019-06-29
0001585364
us-gaap:SalesRevenueNetMember
2019-01-01
2019-06-29
0001585364
us-gaap:InterestExpenseMember
2019-01-01
2019-06-29
0001585364
currency:CNY
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:PLN
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:EUR
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:USD
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:GBP
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:RON
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:CAD
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:EUR
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:RON
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:DKK
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:CAD
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:XXX
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:SEK
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:XXX
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:NOK
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:DKK
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:MXN
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:ILS
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:GBP
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:MXN
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:NOK
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:USD
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:CNY
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
currency:ILS
us-gaap:ForeignExchangeForwardMember
2018-12-31
0001585364
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:SEK
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
currency:PLN
us-gaap:ForeignExchangeForwardMember
2019-06-29
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:OtherNonoperatingIncomeExpenseMember
2018-01-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:InterestExpenseMember
2019-01-01
2019-06-29
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:OtherNonoperatingIncomeExpenseMember
2018-04-01
2018-06-30
0001585364
us-gaap:NondesignatedMember
2018-04-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:InterestExpenseMember
2018-01-01
2018-06-30
0001585364
us-gaap:NondesignatedMember
2019-01-01
2019-06-29
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:InterestExpenseMember
2019-03-31
2019-06-29
0001585364
us-gaap:NondesignatedMember
2018-01-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:OtherNonoperatingIncomeExpenseMember
2019-03-31
2019-06-29
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:InterestExpenseMember
2018-04-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
us-gaap:OtherNonoperatingIncomeExpenseMember
2019-01-01
2019-06-29
0001585364
us-gaap:NondesignatedMember
2019-03-31
2019-06-29
0001585364
us-gaap:DesignatedAsHedgingInstrumentMember
2018-04-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:CostOfSalesMember
2018-04-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:OtherNonoperatingIncomeExpenseMember
2018-04-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:InterestExpenseMember
2018-04-01
2018-06-30
0001585364
us-gaap:ForeignExchangeForwardMember
2018-04-01
2018-06-30
0001585364
us-gaap:InterestRateSwapMember
us-gaap:DesignatedAsHedgingInstrumentMember
us-gaap:InterestExpenseMember
2018-04-01
2018-06-30
0001585364
us-gaap:PrepaidExpensesAndOtherCurrentAssetsMember
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
2019-06-29
0001585364
us-gaap:PrepaidExpensesAndOtherCurrentAssetsMember
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
2019-06-29
0001585364
us-gaap:PrepaidExpensesAndOtherCurrentAssetsMember
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
2018-12-31
0001585364
us-gaap:PrepaidExpensesAndOtherCurrentAssetsMember
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
2018-12-31
0001585364
us-gaap:OtherLiabilitiesMember
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
2018-12-31
0001585364
us-gaap:OtherLiabilitiesMember
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
2019-06-29
0001585364
us-gaap:OtherLiabilitiesMember
us-gaap:ForeignExchangeForwardMember
us-gaap:DesignatedAsHedgingInstrumentMember
2018-12-31
0001585364
us-gaap:OtherLiabilitiesMember
us-gaap:ForeignExchangeForwardMember
us-gaap:NondesignatedMember
2019-06-29
0001585364
prgo:OperatingLeasesMember
2019-06-29
0001585364
prgo:LongtermDebtLessCurrentPortionMember
2019-06-29
0001585364
us-gaap:OtherNoncurrentLiabilitiesMember
2019-06-29
0001585364
prgo:CurrentIndebtednessMember
2019-06-29
0001585364
2019-01-01
0001585364
us-gaap:MaterialReconcilingItemsMember
2019-06-29
0001585364
prgo:A2018EuroDenominatedTermLoandueMarch82020Member
2018-03-08
0001585364
prgo:A2018RevolverMember
2019-06-29
0001585364
prgo:A2014EuroDenominatedTermLoandueDecember52019Member
2014-12-05
0001585364
prgo:A2018RevolverMember
2018-03-08
0001585364
prgo:A2014EuroDenominatedTermLoandueDecember52019Member
2018-03-08
0001585364
2014-12-05
2014-12-05
0001585364
prgo:A2018RevolverMember
2019-03-31
2019-06-29
0001585364
us-gaap:CorporateDebtSecuritiesMember
2019-05-23
0001585364
prgo:A2018RevolverMember
2018-12-31
0001585364
us-gaap:CorporateDebtSecuritiesMember
2019-05-23
2019-05-23
0001585364
prgo:A2014EuroDenominatedTermLoandueDecember52019Member
2018-04-01
2018-06-30
0001585364
prgo:A4.375seniornotedueMarch152026Member
2019-06-29
0001585364
prgo:A4.9SeniorLoandue2044Member
2019-06-29
0001585364
prgo:A3.5SeniornotedueDecember152021Member
2019-01-01
2019-06-29
0001585364
prgo:A5.105SeniornotedueJuly282023Member
2018-12-31
0001585364
prgo:A5.105SeniornotedueJuly282023Member
2019-06-29
0001585364
prgo:A4.375seniornotedueMarch152026Member
2019-01-01
2019-06-29
0001585364
prgo:A5.30UnsecuredSeniorNotesdueNovember152043Member
2019-01-01
2019-06-29
0001585364
prgo:A2018EuroDenominatedTermLoandueMarch82020Member
2019-01-01
2019-06-29
0001585364
prgo:A4.00UnsecuredSeniorNotesdueNovember152023Member
2019-01-01
2019-06-29
0001585364
prgo:A4.00UnsecuredSeniorNotesdueNovember152023Member
2019-06-29
0001585364
prgo:A5.000UnsecuredSeniornotesdueMay232019Member
2019-06-29
0001585364
prgo:A5.105SeniornotedueJuly282023Member
2019-01-01
2019-06-29
0001585364
prgo:A3.5SeniornotedueDecember152021Member
2019-06-29
0001585364
prgo:A2018EuroDenominatedTermLoandueMarch82020Member
2019-06-29
0001585364
prgo:A3.500UnsecuredSeniornotesdueMarch152021Member
2019-06-29
0001585364
prgo:A4.9SeniorLoandue2044Member
2018-12-31
0001585364
prgo:A5.30UnsecuredSeniorNotesdueNovember152043Member
2019-06-29
0001585364
prgo:A5.000UnsecuredSeniornotesdueMay232019Member
2019-01-01
2019-06-29
0001585364
prgo:A2018EuroDenominatedTermLoandueMarch82020Member
2018-12-31
0001585364
prgo:A3.9seniornotedue2024Member
2019-01-01
2019-06-29
0001585364
prgo:A3.9seniornotedue2024Member
2019-06-29
0001585364
prgo:A3.5SeniornotedueDecember152021Member
2018-12-31
0001585364
prgo:A3.500UnsecuredSeniornotesdueMarch152021Member
2018-12-31
0001585364
prgo:A4.375seniornotedueMarch152026Member
2018-12-31
0001585364
prgo:A4.9SeniorLoandue2044Member
2019-01-01
2019-06-29
0001585364
prgo:A3.500UnsecuredSeniornotesdueMarch152021Member
2019-01-01
2019-06-29
0001585364
prgo:A5.30UnsecuredSeniorNotesdueNovember152043Member
2018-12-31
0001585364
prgo:A4.00UnsecuredSeniorNotesdueNovember152023Member
2018-12-31
0001585364
prgo:A5.000UnsecuredSeniornotesdueMay232019Member
2018-12-31
0001585364
prgo:A3.9seniornotedue2024Member
2018-12-31
0001585364
us-gaap:CommonStockMember
2018-01-01
2018-06-30
0001585364
2018-10-31
0001585364
us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember
2019-01-01
2019-06-29
0001585364
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2019-01-01
2019-06-29
0001585364
us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember
2019-01-01
2019-06-29
0001585364
us-gaap:AccumulatedTranslationAdjustmentMember
2019-01-01
2019-06-29
0001585364
us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember
2018-12-31
0001585364
us-gaap:AccumulatedTranslationAdjustmentMember
2019-06-29
0001585364
us-gaap:AccumulatedTranslationAdjustmentMember
2018-12-31
0001585364
us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember
2018-12-31
0001585364
us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember
2019-06-29
0001585364
us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember
2019-06-29
0001585364
2017-08-15
2017-08-15
0001585364
2012-01-01
2012-12-31
0001585364
2019-04-26
0001585364
us-gaap:RevenueCommissionersIrelandMember
2018-11-29
0001585364
2010-01-01
2010-12-31
0001585364
2009-01-01
2009-12-31
0001585364
2011-01-01
2011-12-31
0001585364
prgo:HarelInsuranceCompanyLTD.etal.v.PerrigoCompanyplcetal.Member
2018-02-13
0001585364
prgo:PricefixingLawsuitManagedCareOrganizationMember
2018-08-03
0001585364
prgo:NationwideMutualFundsetal.v.PerrigoCompanyplcetal.Member
2018-10-29
0001585364
2018-01-26
0001585364
prgo:HudsonBayMasterFundLtd.etal.v.PerrigoCo.plcetal.Member
2018-11-15
0001585364
prgo:OZMasterFundLtd.etal.v.PerrigoCompanyplcetal.Member
2019-02-06
0001585364
prgo:FirstManhattanCo.v.PerrigoCompanyplcetal.Member
2018-02-16
0001585364
prgo:PricefixingLawsuitHealthInsuranceCarrierMember
2019-01-16
2019-01-16
0001585364
prgo:AberdeenCanadaFundsGlobalEquityFundetal.v.PerrigoCompanyplcetal.Member
2019-02-22
0001585364
prgo:TIAACREFInvestmentManagementLLC.etal.v.PerrigoCompanyplcetal.Member
2018-04-20
0001585364
prgo:HighfieldsCapitalILPetal.v.PerrigoCompanyplcetal.Member
2019-02-14
0001585364
2011-12-31
0001585364
prgo:SchwabCapitalTrustetal.v.PerrigoCompanyplcetal.Member
2019-01-31
0001585364
prgo:IsraelElec.Corp.EmployeesEduc.Fundv.PerrigoCompanyplcetal.Member
2017-06-28
2017-06-28
0001585364
prgo:CasesFiledinIsraelMember
2017-12-31
0001585364
prgo:CasesFiledinIsraelMember
2018-01-01
2018-12-31
0001585364
2017-06-21
0001585364
prgo:WCMAlternativeEventDriveFundetal.v.PerrigoCo.plcetal.Member
2018-11-15
0001585364
prgo:IsraelElec.Corp.EmployeesEduc.Fundv.PerrigoCompanyplcetal.Member
2019-06-29
0001585364
prgo:PricefixingLawsuitHealthInsuranceCarrierMember
2019-01-16
0001585364
prgo:InrePerrigoCompanyplcSec.Litig.Member
2019-05-31
2019-05-31
0001585364
prgo:CarmignacGestionS.A.v.PerrigoCompanyplcetal.Member
2017-11-01
0001585364
prgo:ManningNapierAdvisorsLLCv.PerrigoCompanyplcetal.Member
2018-01-16
0001585364
prgo:PriceFixingLawsuitSupermarketChainsMember
2013-12-28
0001585364
prgo:IsraelElec.Corp.EmployeesEduc.Fundv.PerrigoCompanyplcetal.Member
2017-06-28
0001585364
us-gaap:MaterialReconcilingItemsMember
2019-03-31
2019-06-29
0001585364
us-gaap:MaterialReconcilingItemsMember
2018-04-01
2018-06-30
0001585364
us-gaap:MaterialReconcilingItemsMember
2019-01-01
2019-06-29
0001585364
us-gaap:MaterialReconcilingItemsMember
2018-01-01
2018-06-30
0001585364
prgo:GenericProductAcquisitionMember
us-gaap:DevelopedTechnologyRightsMember
us-gaap:SubsequentEventMember
2019-07-02
2019-07-02
0001585364
srt:MaximumMember
us-gaap:SubsequentEventMember
2019-07-08
2019-07-08
0001585364
srt:MinimumMember
us-gaap:SubsequentEventMember
2019-07-08
2019-07-08
0001585364
prgo:RanirGlobalHoldingsLLCMember
us-gaap:SubsequentEventMember
2019-07-01
0001585364
prgo:RanirGlobalHoldingsLLCMember
us-gaap:SubsequentEventMember
2019-07-01
2019-07-01
0001585364
us-gaap:SubsequentEventMember
2019-07-08
2019-07-08
0001585364
prgo:RanirGlobalHoldingsLLCMember
2019-01-01
2019-06-29
xbrli:pure
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
iso4217:EUR
xbrli:shares
prgo:product
iso4217:ILS
prgo:supermarket
prgo:manufacturer
prgo:individual
prgo:complaint
prgo:plaintiff
prgo:case
iso4217:USD
iso4217:ILS
iso4217:EUR
prgo:dozens
prgo:defendant
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
_______________________________________________
FORM 10-Q
_______________________________________________
|
| |
☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
|
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 001-36353
_______________________________________________
Perrigo Company plc
(Exact name of registrant as specified in its charter)
_______________________________________________
|
| | |
Ireland | | Not Applicable |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
The Sharp Building, Hogan Place, Dublin 2, Ireland D02 TY74
+353 1 7094000
(Address, including zip code, and telephone number, including
area code, of registrant’s principal executive offices)
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
|
| | |
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Ordinary shares | PRGO | New York Stock Exchange |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
| | | | | | | | | | |
Large accelerated filer | ☒ | | Accelerated filer | ☐ | | Non-accelerated filer | ☐ | | Smaller reporting company | ☐ |
Emerging growth company | ☐ | | | | | | | | | |
| | | | | | | | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. | ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☒ No
As of August 2, 2019, there were 136,054,652 ordinary shares outstanding.
PERRIGO COMPANY PLC
FORM 10-Q
INDEX
|
| | |
| PAGE NUMBER |
| |
| |
PART I. FINANCIAL INFORMATION | |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
| | |
1 | | |
| | |
2 | | |
| | |
3 | | |
| | |
4 | | |
| | |
5 | | |
| | |
6 | | |
| | |
7 | | |
| | |
8 | | |
| | |
9 | | |
| | |
10 | | |
| | |
11 | | |
| | |
12 | | |
| | |
13 | | |
| | |
14 | | |
| | |
15 | | |
| | |
16 | | |
| | |
17 | | |
| | |
18 | | |
| | |
| |
| | |
| |
| | |
| |
| | |
PART II. OTHER INFORMATION | |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this report are “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and are subject to the safe harbor created thereby. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our, or our industry’s actual results, levels of activity, performance or achievements to be materially different from those expressed or implied by any forward-looking statements. In particular, statements about our expectations, beliefs, plans, objectives, assumptions, future events or future performance contained in this report, including certain statements contained in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” are forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” "forecast," “predict,” “potential” or the negative of those terms or other comparable terminology.
We have based these forward-looking statements on our current expectations, assumptions, estimates and projections. While we believe these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond our control, including: the timing, amount and cost of any share repurchases; future impairment charges; the success of management transition; customer acceptance of new products; competition from other industry participants, some of whom have greater marketing resources or larger market shares in certain product categories than we do; pricing pressures from customers and consumers; resolution of uncertain tax positions, including the Company's appeal of the Notice of Assessment ("NoA") issued by the Irish Office of the Revenue Commissioners (“Irish Revenue”) and the Notice of Proposed Adjustment ("NOPA") issued by the U.S. Internal Revenue Service and the impact that an adverse result in such proceedings would have on operating results, cash flows and liquidity; potential third-party claims and litigation, including litigation relating to our restatement of previously-filed financial information and litigation relating to uncertain tax positions, including the NoA and the NOPA; potential impacts of ongoing or future government investigations and regulatory initiatives; the impact of tax reform legislation and healthcare policy; general economic conditions; fluctuations in currency exchange rates and interest rates; the consummation of announced acquisitions or dispositions and the success of such transactions, and our ability to realize the desired benefits thereof; and our ability to execute and achieve the desired benefits of announced cost-reduction efforts, and strategic and other initiatives. Statements regarding the separation of the RX business, including the expected benefits, anticipated timing, form of any such separation and whether the separation ultimately occurs, are all subject to various risks and uncertainties, including future financial and operating results, our ability to separate the business, the effect of existing interdependencies with our manufacturing and shared service operations, and the tax consequences of the planned separation to us or our shareholders. Furthermore, we may incur additional tax liabilities in respect of 2016 and prior years or be found to have breached certain provisions of Irish company law in connection with our restatement of our previously-filed financial statements, which may result in additional expenses and penalties. These and other important factors, including those discussed in our form 10-K for the year-ended December 31, 2018, this report under “Risk Factors” and in any subsequent filings with the United States Securities and Exchange Commission, may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. The forward-looking statements in this report are made only as of the date hereof, and unless otherwise required by applicable securities laws, we disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
This report contains trademarks, trade names and service marks that are the property of Perrigo Company plc, as well as, for informational purposes, trademarks, trade names, and service marks that are the property of other organizations. Solely for convenience, certain trademarks, trade names, and service marks referred to in this report appear without the ®, ™ and SM symbols, but those references are not intended to indicate that we or the applicable owner, as the case may be, will not assert, to the fullest extent under applicable law, our or their rights to such trademarks, trade names, and service marks.
Perrigo Company plc - Item 1
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (UNAUDITED)
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in millions, except per share amounts)
(unaudited)
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 1,149.0 |
| | $ | 1,186.4 |
| | $ | 2,323.5 |
| | $ | 2,403.4 |
|
Cost of sales | 718.2 |
| | 715.4 |
| | 1,443.9 |
| | 1,439.7 |
|
Gross profit | 430.8 |
| | 471.0 |
| | 879.6 |
| | 963.7 |
|
| | | | | | | |
Operating expenses | | | | | | | |
Distribution | 23.7 |
| | 23.8 |
| | 47.1 |
| | 48.5 |
|
Research and development | 43.9 |
| | 91.9 |
| | 84.0 |
| | 130.3 |
|
Selling | 140.1 |
| | 155.2 |
| | 288.7 |
| | 316.5 |
|
Administration | 127.2 |
| | 96.8 |
| | 252.3 |
| | 204.5 |
|
Impairment charges | 27.8 |
| | 1.7 |
| | 31.9 |
| | 1.7 |
|
Restructuring | 12.2 |
| | 3.7 |
| | 21.5 |
| | 5.2 |
|
Other operating expense (income) | 0.9 |
| | 3.2 |
| | (3.2 | ) | | 6.1 |
|
Total operating expenses | 375.8 |
| | 376.3 |
| | 722.3 |
| | 712.8 |
|
| | | | | | | |
Operating income | 55.0 |
| | 94.7 |
| | 157.3 |
| | 250.9 |
|
| | | | | | | |
Change in financial assets | (5.5 | ) | | (0.6 | ) | | (15.9 | ) | | 9.0 |
|
Interest expense, net | 31.2 |
| | 32.1 |
| | 59.8 |
| | 63.5 |
|
Other (income) expense, net | 2.3 |
| | 7.9 |
| | 5.5 |
| | 12.1 |
|
Loss on extinguishment of debt | — |
| | — |
| | — |
| | 0.5 |
|
Income before income taxes | 27.0 |
| | 55.3 |
| | 107.9 |
| | 165.8 |
|
Income tax expense | 18.0 |
| | 19.1 |
| | 35.0 |
| | 48.8 |
|
Net income | $ | 9.0 |
| | $ | 36.2 |
| | $ | 72.9 |
| | $ | 117.0 |
|
| | | | | | | |
Earnings per share | | | | | | | |
Basic | $ | 0.07 |
| | $ | 0.26 |
| | $ | 0.54 |
| | $ | 0.84 |
|
Diluted | $ | 0.07 |
| | $ | 0.26 |
| | $ | 0.54 |
| | $ | 0.84 |
|
| | | | | | | |
Weighted-average shares outstanding | | | | | | | |
Basic | 136.0 |
| | 138.1 |
| | 136.0 |
| | 139.5 |
|
Diluted | 136.5 |
| | 138.7 |
| | 136.3 |
| | 140.0 |
|
See accompanying Notes to the Condensed Consolidated Financial Statements.
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in millions)
(unaudited)
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Net income | $ | 9.0 |
| | $ | 36.2 |
| | $ | 72.9 |
| | $ | 117.0 |
|
Other comprehensive income (loss): | | | | | | | |
Foreign currency translation adjustments | 27.8 |
| | (165.6 | ) | | 11.0 |
| | (92.6 | ) |
Change in fair value of derivative financial instruments, net of tax | 2.7 |
| | (3.5 | ) | | 4.4 |
| | (4.1 | ) |
Change in post-retirement and pension liability, net of tax | — |
| | (0.2 | ) | | (0.5 | ) | | (0.4 | ) |
Other comprehensive income (loss), net of tax | 30.5 |
| | (169.3 | ) | | 14.9 |
| | (97.1 | ) |
Comprehensive income (loss) | $ | 39.5 |
| | $ | (133.1 | ) | | $ | 87.8 |
| | $ | 19.9 |
|
See accompanying Notes to the Condensed Consolidated Financial Statements.
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED BALANCE SHEETS
(in millions, except per share amounts)
(unaudited)
|
| | | | | | | |
| June 29,
2019 | | December 31,
2018 |
Assets | | | |
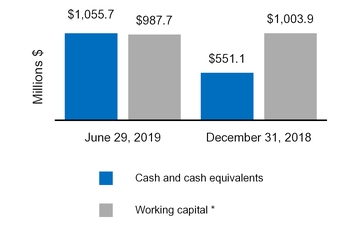
Cash and cash equivalents | $ | 1,055.7 |
| | $ | 551.1 |
|
Accounts receivable, net of allowance for doubtful accounts of $6.3 and $6.4, respectively | 1,117.1 |
| | 1,073.1 |
|
Inventories | 940.5 |
| | 878.0 |
|
Prepaid expenses and other current assets | 322.1 |
| | 400.0 |
|
Total current assets | 3,435.4 |
| | 2,902.2 |
|
Property, plant and equipment, net | 824.3 |
| | 829.1 |
|
Operating lease assets | 135.4 |
| | — |
|
Goodwill and indefinite-lived intangible assets | 3,967.8 |
| | 4,029.1 |
|
Definite-lived intangible assets, net | 2,675.2 |
| | 2,858.9 |
|
Deferred income taxes | 6.8 |
| | 1.2 |
|
Other non-current assets | 383.8 |
| | 362.9 |
|
Total non-current assets | 7,993.3 |
| | 8,081.2 |
|
Total assets | $ | 11,428.7 |
| | $ | 10,983.4 |
|
Liabilities and Shareholders’ Equity | | | |
Accounts payable | $ | 513.7 |
| | $ | 474.9 |
|
Payroll and related taxes | 126.3 |
| | 132.1 |
|
Accrued customer programs | 385.2 |
| | 442.4 |
|
Accrued liabilities | 262.7 |
| | 201.3 |
|
Accrued income taxes | 104.1 |
| | 96.5 |
|
Current indebtedness | 398.8 |
| | 190.2 |
|
Total current liabilities | 1,790.8 |
| | 1,537.4 |
|
Long-term debt, less current portion | 3,084.4 |
| | 3,052.2 |
|
Deferred income taxes | 280.2 |
| | 282.3 |
|
Other non-current liabilities | 546.9 |
| | 443.4 |
|
Total non-current liabilities | 3,911.5 |
| | 3,777.9 |
|
Total liabilities | 5,702.3 |
| | 5,315.3 |
|
Commitments and contingencies - Refer to Note 15 |
| |
|
Shareholders’ equity | | | |
Controlling interests: | | | |
Preferred shares, $0.0001 par value per share, 10 shares authorized | — |
| | — |
|
Ordinary shares, €0.001 par value per share, 10,000 shares authorized | 7,395.5 |
| | 7,421.7 |
|
Accumulated other comprehensive income | 99.5 |
| | 84.6 |
|
Retained earnings (accumulated deficit) | (1,768.8 | ) | | (1,838.3 | ) |
Total controlling interest | 5,726.2 |
| | 5,668.0 |
|
Noncontrolling interest | 0.2 |
| | 0.1 |
|
Total shareholders’ equity | 5,726.4 |
| | 5,668.1 |
|
Total liabilities and shareholders' equity | $ | 11,428.7 |
| | $ | 10,983.4 |
|
| | | |
Supplemental Disclosures of Balance Sheet Information | | | |
Preferred shares, issued and outstanding | — |
| | — |
|
Ordinary shares, issued and outstanding | 136.0 |
| | 135.9 |
|
See accompanying Notes to the Condensed Consolidated Financial Statements.
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(in millions, except per share amounts)
(unaudited)
|
| | | | | | | | | | | | | | | | | | |
| Ordinary Shares
Issued | | Accumulated
Other
Comprehensive
Income | | Retained
Earnings
(Accumulated Deficit) | | Total |
| Shares | | Amount |
Balance at December 31, 2017 | 140.8 |
| | $ | 7,892.9 |
| | $ | 253.1 |
| | $ | (1,975.5 | ) | | $ | 6,170.5 |
|
Adoption of new accounting standards | — |
| | — |
| | (1.0 | ) | | 6.3 |
| | 5.3 |
|
Net income | — |
| | — |
| | — |
| | 80.8 |
| | 80.8 |
|
Other comprehensive income | — |
| | — |
| | 72.2 |
| | — |
| | 72.2 |
|
Stock options exercised | — |
| | 0.2 |
| | — |
| | — |
| | 0.2 |
|
Restricted stock plan | 0.2 |
| | — |
| | — |
| | — |
| | — |
|
Compensation for stock options | — |
| | 2.7 |
| | — |
| | — |
| | 2.7 |
|
Compensation for restricted stock | — |
| | 10.0 |
| | — |
| | — |
| | 10.0 |
|
Cash dividends, $0.19 per share | — |
| | (26.7 | ) | | — |
| | — |
| | (26.7 | ) |
Shares withheld for payment of employees' withholding tax liability | — |
| | (1.5 | ) | | — |
| | — |
| | (1.5 | ) |
Repurchases of ordinary shares | (1.3 | ) | | (108.1 | ) | | — |
| | — |
| | (108.1 | ) |
Balance at March 31, 2018 | 139.7 |
| | $ | 7,769.5 |
| | $ | 324.3 |
| | $ | (1,888.4 | ) | | $ | 6,205.4 |
|
| | | | | | | | | |
Net income | — |
| | — |
| | — |
| | 36.2 |
| | 36.2 |
|
Other comprehensive income | — |
| | — |
| | (169.3 | ) | | — |
| | (169.3 | ) |
Stock options exercised | — |
| | 0.1 |
| | — |
| | — |
| | 0.1 |
|
Restricted stock plan | 0.1 |
| | — |
| | — |
| | — |
| | — |
|
Compensation for stock options | — |
| | 2.7 |
| | — |
| | — |
| | 2.7 |
|
Compensation for restricted stock | — |
| | 6.9 |
| | — |
| | — |
| | 6.9 |
|
Cash dividends, $0.19 per share | — |
| | (26.1 | ) | | — |
| | — |
| | (26.1 | ) |
Shares withheld for payment of employees' withholding tax liability | (0.1 | ) | | (1.9 | ) | | — |
| | — |
| | (1.9 | ) |
Repurchases of ordinary shares | (2.0 | ) | | (156.9 | ) | | — |
| | — |
| | (156.9 | ) |
Balance at June 30, 2018 | 137.7 |
| | $ | 7,594.3 |
| | $ | 155.0 |
| | $ | (1,852.2 | ) | | $ | 5,897.1 |
|
|
| | | | | | | | | | | | | | | | | | |
| Ordinary Shares
Issued | | Accumulated
Other
Comprehensive
Income | | Retained
Earnings
(Accumulated Deficit) | | Total |
| Shares | | Amount |
Balance at December 31, 2018 | 135.9 |
| | $ | 7,421.7 |
| | $ | 84.6 |
| | $ | (1,838.3 | ) | | $ | 5,668.0 |
|
Adoption of new accounting standards | — |
| | — |
| | — |
| | (3.4 | ) | | (3.4 | ) |
Net income | — |
| | — |
| | — |
| | 63.9 |
| | 63.9 |
|
Other comprehensive loss | — |
| | — |
| | (15.6 | ) | | — |
| | (15.6 | ) |
Restricted stock plan | 0.2 |
| | — |
| | — |
| | — |
| | — |
|
Compensation for stock options | — |
| | 1.8 |
| | — |
| | — |
| | 1.8 |
|
Compensation for restricted stock | — |
| | 14.2 |
| | — |
| | — |
| | 14.2 |
|
Cash dividends, $0.19 per share | — |
| | (25.9 | ) | | — |
| | — |
| | (25.9 | ) |
Shares withheld for payment of employees' withholding tax liability | (0.1 | ) | | (2.4 | ) | | — |
| | — |
| | (2.4 | ) |
Balance at March 30, 2019 | 136.0 |
| | $ | 7,409.4 |
| | $ | 69.0 |
| | $ | (1,777.8 | ) | | $ | 5,700.6 |
|
| | | | | | | | | |
Net income | — |
| | — |
| | — |
| | 9.0 |
| | 9.0 |
|
Other comprehensive income | — |
| | — |
| | 30.5 |
| | — |
| | 30.5 |
|
Stock options exercised | — |
| | 0.3 |
| | — |
| | — |
| | 0.3 |
|
Compensation for stock options | — |
| | 1.3 |
| | — |
| | — |
| | 1.3 |
|
Compensation for restricted stock | — |
| | 14.2 |
| | — |
| | — |
| | 14.2 |
|
Cash dividends, $0.21 per share | — |
| | (28.9 | ) | | — |
| | — |
| | (28.9 | ) |
Shares withheld for payment of employees' withholding tax liability | — |
| | (0.8 | ) | | — |
| | — |
| | (0.8 | ) |
Balance at June 29, 2019 | 136.0 |
| | $ | 7,395.5 |
| | $ | 99.5 |
| | $ | (1,768.8 | ) | | $ | 5,726.2 |
|
See accompanying Notes to the Condensed Consolidated Financial Statements.
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in millions)
(unaudited)
|
| | | | | | | |
| Six Months Ended |
| June 29,
2019 | | June 30,
2018 |
Cash Flows From (For) Operating Activities | | | |
Net income | $ | 72.9 |
| | $ | 117.0 |
|
Adjustments to derive cash flows: | | | |
Depreciation and amortization | 191.5 |
| | 217.8 |
|
Share-based compensation | 28.0 |
| | 22.3 |
|
Impairment charges | 31.9 |
| | 1.7 |
|
Change in financial assets | (15.9 | ) | | 9.0 |
|
Loss on extinguishment of debt | — |
| | 0.5 |
|
Restructuring charges | 21.5 |
| | 5.2 |
|
Deferred income taxes | 9.2 |
| | (14.2 | ) |
Amortization of debt premium | (3.0 | ) | | (3.7 | ) |
Other non-cash adjustments, net | 26.4 |
| | 5.1 |
|
Subtotal | 362.5 |
| | 360.7 |
|
Increase (decrease) in cash due to: | | | |
Accounts receivable | (55.3 | ) | | (24.3 | ) |
Inventories | (78.3 | ) | | (99.3 | ) |
Accounts payable | 41.2 |
| | 89.2 |
|
Payroll and related taxes | (23.0 | ) | | (48.4 | ) |
Accrued customer programs | (52.8 | ) | | 33.9 |
|
Accrued liabilities | (19.2 | ) | | (30.4 | ) |
Accrued income taxes | (36.7 | ) | | (20.8 | ) |
Other, net | 19.9 |
| | (5.9 | ) |
Subtotal | (204.2 | ) | | (106.0 | ) |
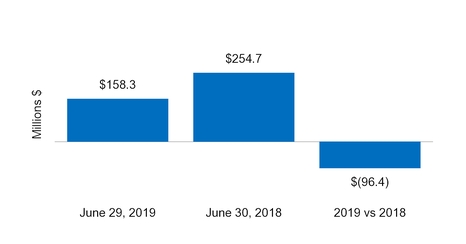
Net cash from (for) operating activities | 158.3 |
| | 254.7 |
|
Cash Flows From (For) Investing Activities | | | |
Proceeds from royalty rights | 1.7 |
| | 10.3 |
|
Purchase of investment securities | — |
| | (7.5 | ) |
Royalty Pharma contingent milestone payment | 250.0 |
| | — |
|
Asset acquisitions | (35.0 | ) | | — |
|
Additions to property, plant and equipment | (54.7 | ) | | (33.3 | ) |
Net proceeds from sale of business and other assets | — |
| | 1.3 |
|
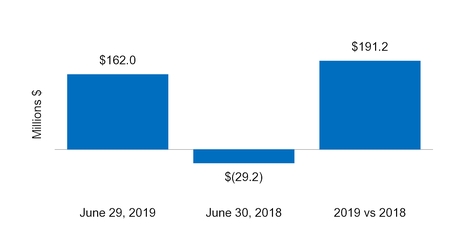
Net cash from (for) investing activities | 162.0 |
| | (29.2 | ) |
Cash Flows From (For) Financing Activities | | | |
Issuances of long-term debt | — |
| | 431.0 |
|
Payments on long-term debt | (158.9 | ) | | (457.3 | ) |
Borrowings (repayments) of revolving credit agreements and other financing, net | 397.5 |
| | (8.2 | ) |
Deferred financing fees | — |
| | (2.4 | ) |
Issuance of ordinary shares | 0.3 |
| | — |
|
Repurchase of ordinary shares | — |
| | (265.0 | ) |
Cash dividends | (54.8 | ) | | (52.8 | ) |
Other financing, net | (5.9 | ) | | (7.5 | ) |
Net cash from (for) financing activities | 178.2 |
| | (362.2 | ) |
Effect of exchange rate changes on cash and cash equivalents | 6.1 |
| | (15.5 | ) |
Net increase (decrease) in cash and cash equivalents | 504.6 |
| | (152.2 | ) |
Cash and cash equivalents, beginning of period | 551.1 |
| | 678.7 |
|
Cash and cash equivalents, end of period | $ | 1,055.7 |
| | $ | 526.5 |
|
See accompanying Notes to the Condensed Consolidated Financial Statements.
Perrigo Company plc - Item 1
Note 1
NOTE 1 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
General Information
The Company
Perrigo Company plc was incorporated under the laws of Ireland on June 28, 2013 and became the successor registrant of Perrigo Company, a Michigan corporation, on December 18, 2013 in connection with the acquisition of Elan Corporation, plc ("Elan"). Unless the context requires otherwise, the terms "Perrigo," the "Company," "we," "our," "us," and similar pronouns used herein refer to Perrigo Company plc, its subsidiaries, and all predecessors of Perrigo Company plc and its subsidiaries.
We are dedicated to making lives better by bringing “Quality, Affordable Self-Care Products™” that consumers trust everywhere they are sold. We are a leading provider of over-the-counter ("OTC") health and wellness solutions that enhance individual well-being by empowering consumers to proactively prevent or treat conditions that can be self-managed. We are also a leading producer of generic prescription pharmaceutical topical products such as creams, lotions, gels, and nasal sprays.
Basis of Presentation
The accompanying unaudited Condensed Consolidated Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles ("GAAP") for interim financial information and with the instructions to Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. The unaudited Condensed Consolidated Financial Statements should be read in conjunction with the Consolidated Financial Statements and footnotes included in our Annual Report on Form 10-K for the year ended December 31, 2018. In the opinion of management, all adjustments (consisting of normal recurring accruals and other adjustments) considered necessary for a fair presentation of the unaudited Condensed Consolidated Financial Statements have been included and include our accounts and the accounts of all majority-owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Segment Reporting Change
During the three months ended March 30, 2019, we changed the composition of our operating and reporting segments. We moved our Israeli diagnostic business from the Consumer Self-Care International segment to the Prescription Pharmaceuticals segment and we made certain adjustments to our allocations between segments. These changes were made to reflect changes in the way in which management makes operating decisions, allocates resources, and manages the growth and profitability of the Company. Financial information related to our business segments and geographic locations can be found in Note 2 and Note 17.
Our new reporting and operating segments are as follows:
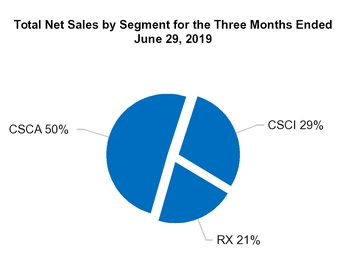
| |
• | Consumer Self-Care Americas ("CSCA"), formerly Consumer Healthcare Americas, comprises our consumer self-care business (OTC, contract manufacturing, infant formula and animal health categories) in the U.S., Mexico and Canada. |
| |
• | Consumer Self-Care International ("CSCI"), formerly Consumer Healthcare International, comprises our branded consumer self-care business primarily in Europe, our consumer-focused business in the United Kingdom and Australia, and our liquid licensed products business in the United Kingdom. |
| |
• | Prescription Pharmaceuticals ("RX") comprises our Prescription Pharmaceuticals business in the U.S. and our diagnostic business in Israel, which was previously in our CSCI segment. |
Perrigo Company plc - Item 1
Note 1
Recent Accounting Standard Pronouncements
Below are recent Accounting Standard Updates ("ASU") that we are still assessing to determine the effect on our Condensed Consolidated Financial Statements. We do not believe that any other recently issued accounting standards could have a material effect on our Condensed Consolidated Financial Statements. As new accounting pronouncements are issued, we will adopt those that are applicable under the circumstances.
|
| | | | | | |
Recently Issued Accounting Standards Not Yet Adopted |
Standard | | Description | | Effective Date | | Effect on the Financial Statements or Other Significant Matters |
ASU 2018-15: Intangibles-Goodwill and Other-Internal-Use Software (Subtopic 350-40): Customer's Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract | | This guidance requires a customer in a cloud computing arrangement that is a service contract to follow the internal-use software guidance in ASC 350-40 to determine which implementation costs to capitalize as assets or expense as incurred. | | January 1, 2020 | | We currently plan to adopt the standard prospectively on the effective date. Upon adoption, no impact is currently expected, however, future hosting arrangements treated as service contracts will need to be evaluated for capitalizable costs during implementation. The Consolidated Financial Statement impact will align with the presentation of the underlying hosting contracts, which will be included within Operating expenses. |
ASU 2018-13: Fair Value Measurement (Topic 820): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement | | This guidance amends ASC 820 to add, remove, and modify certain disclosure requirements for fair value measurements. | | January 1, 2020 | | We currently plan to adopt the standard on the effective date. Upon adoption, we will be required to disclose the range and weighted average used to develop significant unobservable inputs for Level 3 fair value measurement. We will no longer be required to disclose the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy. |
ASU 2016-13: Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments
ASU 2018-19: Codification Improvements for Topic 326: Measurement of Credit Losses on Financial Instruments
ASU 2019-05: Financial Instruments-Credit Losses: Targeted Transition Relief | | This guidance changes the impairment model for most financial assets and certain other instruments, replacing the current "incurred loss" approach with an "expected loss" credit impairment model, which will apply to most financial assets measured at amortized cost, and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities and off-balance sheet credit exposures such as letters of credit. | | January 1, 2020 | | We are in the process of completing our evaluation. Upon adoption, we are not expecting a material impact on the financial statements.
|
ASU 2018-18: Collaborative Arrangements (Topic 808): Clarifying the Interaction between Topic 808 and Topic 606 | | This guidance amends ASC 808 to clarify that certain transactions between participants in a collaborative arrangement should be accounted for under ASC 606 when the counterparty is a customer. The proposed guidance would be applied retrospectively to the date of initial adoption of Topic 606. | | January 1, 2020 | | We are in the process of completing our evaluation. Upon adoption, we are not expecting a material impact on the financial statements. |
ASU 2018-14: Compensation-Retirement Benefits-Defined Benefit Plans-General (Subtopic 715-20): Disclosure Framework-Changes to the Disclosure Requirements for Defined Benefit Plans | | This guidance amends ASC 715 to add, remove, and clarify disclosure requirements related to defined benefit pension and other post-retirement plans. | | December 31, 2020 | | We are currently evaluating the implications of adoption on our Consolidated Financial Statements.
|
Perrigo Company plc - Item 1
Note 2
NOTE 2 – REVENUE RECOGNITION
Revenue is recognized when or as a customer obtains control of promised products. The amount of revenue recognized reflects the consideration we expect to be entitled to receive in exchange for these products.
Disaggregation of Revenue
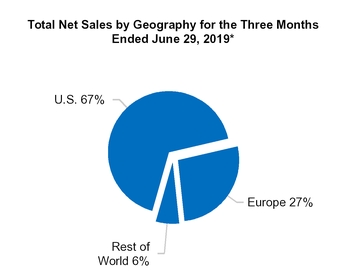
We generated net sales in the following geographic locations(1) (in millions):
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
U.S. | $ | 768.6 |
| | $ | 772.3 |
| | $ | 1,537.4 |
| | $ | 1,558.7 |
|
Europe(2) | 315.8 |
| | 344.3 |
| | 656.7 |
| | 706.2 |
|
All other countries(3) | 64.6 |
| | 69.8 |
| | 129.4 |
| | 138.5 |
|
| $ | 1,149.0 |
| | $ | 1,186.4 |
| | $ | 2,323.5 |
| | $ | 2,403.4 |
|
(1)
(2) $6.9 million and $12.1 million for the three and six months ended June 29, 2019, respectively, and $5.0 million and $10.4 million for the three and six months ended June 30, 2018, respectively.
(3)
The following is a summary of our net sales by category (in millions):
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
CSCA(1) | | | | | | | |
Cough/cold/allergy/sinus | $ | 120.9 |
| | $ | 109.3 |
| | $ | 253.5 |
| | $ | 250.8 |
|
Gastrointestinal | 106.2 |
| | 103.0 |
| | 206.5 |
| | 195.2 |
|
Infant nutritionals | 85.5 |
| | 109.2 |
| | 181.5 |
| | 212.6 |
|
Analgesics | 88.2 |
| | 92.2 |
| | 177.0 |
| | 185.9 |
|
Smoking cessation | 77.0 |
| | 71.2 |
| | 143.8 |
| | 137.1 |
|
Animal health | 22.4 |
| | 31.9 |
| | 42.0 |
| | 58.2 |
|
Vitamins, minerals and dietary supplements | 3.9 |
| | 4.3 |
| | 7.3 |
| | 7.3 |
|
Other CSCA(2) | 78.0 |
| | 75.8 |
| | 152.3 |
| | 151.4 |
|
Total CSCA | 582.1 |
| | 596.9 |
| | 1,163.9 |
| | 1,198.5 |
|
CSCI | | | | | | | |
Cough/cold/allergy/sinus | 74.1 |
| | 84.7 |
| | 169.8 |
| | 183.4 |
|
Lifestyle | 81.2 |
| | 86.1 |
| | 162.7 |
| | 175.8 |
|
Personal care and derma-therapeutics | 69.2 |
| | 79.7 |
| | 135.2 |
| | 155.3 |
|
Natural health and vitamins, minerals and dietary supplements | 25.6 |
| | 27.9 |
| | 55.4 |
| | 61.1 |
|
Anti-parasites | 26.0 |
| | 30.4 |
| | 52.7 |
| | 58.5 |
|
Other CSCI(3) | 51.4 |
| | 49.1 |
| | 102.5 |
| | 101.6 |
|
Total CSCI | 327.5 |
| | 357.9 |
| | 678.3 |
| | 735.7 |
|
Total RX | 239.4 |
| | 231.6 |
| | 481.3 |
| | 469.2 |
|
Total net sales | $ | 1,149.0 |
| | $ | 1,186.4 |
| | $ | 2,323.5 |
| | $ | 2,403.4 |
|
(1)
Perrigo Company plc - Item 1
Note 2
While the majority of revenue is recognized at a point in time, certain of our product revenue is recognized on an over time basis. Predominately, over time customer contracts exist in contract manufacturing arrangements, which occur in both the CSCA and CSCI segments. Contract manufacturing revenue was $65.8 million and $133.1 million for the three and six months ended June 29, 2019, respectively, and $77.1 million and $146.5 million for the three and six months ended June 30, 2018, respectively.
We also recognize a portion of the store brand OTC product revenues in the CSCA segment on an over time basis; however, the timing difference between over time and point in time revenue recognition for store brand contracts is not significant due to the short time period between the customization of the product and shipment or delivery.
Contract Balances
The following table provides information about contract assets from contracts with customers (in millions):
|
| | | | | | | | | |
| Balance Sheet Location | | June 29,
2019 | | December 31,
2018 |
Short-term contract assets | Prepaid expenses and other current assets | | $ | 16.6 |
| | $ | 25.5 |
|
NOTE 3 – ACQUISITIONS
Acquisitions Completed During the Six Months Ended June 29, 2019
Generic Product Acquisition
On May 17, 2019, we purchased the Abbreviated New Drug Application ("ANDA") for a generic product used to relieve pain from osteoarthritis, for $15.7 million in cash, which we capitalized as a developed product technology intangible asset. We plan to launch the product during the third quarter of 2019 and begin amortizing it over a 20-year useful life. Operating results attributable to the product are included within our RX segment.
Budesonide Nasal Spray and Triamcinolone Nasal Spray
On April 1, 2019, we purchased product ANDAs and other records and registrations of Budesonide Nasal Spray, a generic equivalent of Rhinocort Allergy®, and Triamcinolone Nasal Spray, a generic equivalent of Nasacort Allergy®, from Barr Laboratories, Inc. ("Barr"), a subsidiary of Teva Pharmaceuticals, for $14.0 million in cash. We previously developed and marketed the products in collaboration with Barr under a development, marketing and commercialization agreement that originated in August 2003. Under this prior agreement, we paid Barr a percentage of net income from products sold by Perrigo in the U.S. By purchasing the assets from Barr and terminating the original development, marketing and commercialization agreement, we are now entitled to 100% of the income from sales of the product. Operating results attributable to these products are included within our CSCA segment. The intangible assets acquired are classified as developed product technology with a 10-year useful life.
Acquisitions Completed During the Year Ended December 31, 2018
Nasonex-branded products
On May 29, 2018, we entered into a license agreement with Merck Sharp & Dohme Corp. ("Merck"), which allows us to develop and commercialize an OTC version of Nasonex-branded products containing the compound, mometasone furoate monohydrate. The acquisition was accounted for as an asset acquisition based on our assessment that substantially all of the fair value of the gross assets acquired was concentrated in a single identifiable asset to be used for R&D. In accordance with Accounting Standards Codification Topic 730 Research and Development, the non-refundable upfront license fee of $50.0 million was recorded in R&D expense in our CHCA segment because the intangible research and development asset acquired has no alternative use. The agreement requires us to make contingent payments if we obtain regulatory approval and achieve certain sales milestones. We will also be obligated to make royalty payments on potential future sales. The contingent consideration will be included in the measurement of the cost of the asset when the contingency is resolved and the consideration is paid or becomes payable. Consideration paid after U.S. Food and Drug Administration approval will be capitalized and amortized to cost of goods sold over the economic life of each product.
NOTE 4 – GOODWILL AND INTANGIBLE ASSETS
Goodwill
Changes in the carrying amount of goodwill, by reportable segment, were as follows (in millions): |
| | | | | | | | | | | | | | | | |
| | December 31,
2018 | | Transfer to assets held-for-sale | | Currency Translation Adjustments | | June 29,
2019 |
CSCA(1) | | $ | 1,713.7 |
| | $ | (42.2 | ) | | $ | 2.1 |
| | $ | 1,673.6 |
|
CSCI(2) | | 1,151.3 |
| | — |
| | (9.2 | ) | | 1,142.1 |
|
RX | | 1,114.8 |
| | — |
| | 4.8 |
| | 1,119.6 |
|
Total goodwill | | $ | 3,979.8 |
| | $ | (42.2 | ) | | $ | (2.3 | ) | | $ | 3,935.3 |
|
| |
(1) | We had accumulated impairments of $24.5 million and $161.2 million as of June 30, 2018 and June 29, 2019, respectively. |
| |
(2) | We had accumulated impairments of $868.4 million as of June 30, 2018 and June 29, 2019. |
During the three months ended June 29, 2019, our RX U.S. reporting unit had an indication of potential impairment which was driven by a combination of industry and market factors and uncertainty related to the timing
Perrigo Company plc - Item 1
Note 4
and associated cash flows of the projected ProAir launch. Goodwill remaining in this reporting unit was $1,119.6 million as of June 29, 2019. We prepared an impairment test as of June 29, 2019 and determined that the fair value of the RX U.S. reporting unit continued to exceed net book value by approximately 10%. The excess was lower than our annual impairment test as of October 1, 2018, in which fair value exceeded carrying value by more than 25%. While no impairment was recorded as of June 29, 2019, future developments such as deterioration in business performance or market multiples could reduce the fair value of this reporting unit and lead to impairment in a future period.
In conjunction with the test performed during the three months ended June 29, 2019, we early adopted ASU 2017-04 which removes the Step 2 requirement in instances when the carrying value of a reporting unit exceeds its fair value. Prospectively, if a reporting unit’s carrying value exceeds its fair value, we will record an impairment charge in the amount of the difference, limited to the amount of goodwill attributed to that reporting unit.
Intangible Assets
Intangible assets and related accumulated amortization consisted of the following (in millions):
|
| | | | | | | | | | | | | | | |
| June 29, 2019 | | December 31, 2018 |
| Gross | | Accumulated Amortization | | Gross | | Accumulated Amortization |
Indefinite-lived intangibles: | | | | | | | |
Trademarks, trade names, and brands | $ | 18.0 |
| | $ | — |
| | $ | 18.1 |
| | $ | — |
|
In-process research and development | 14.5 |
| | — |
| | 31.2 |
| | — |
|
Total indefinite-lived intangibles | $ | 32.5 |
| | $ | — |
| | $ | 49.3 |
| | $ | — |
|
Definite-lived intangibles: | | | | | | | |
Distribution and license agreements and supply agreements | $ | 173.7 |
| | $ | 105.2 |
| | $ | 178.6 |
| | $ | 99.0 |
|
Developed product technology, formulations, and product rights | 1,321.0 |
| | 706.1 |
| | 1,318.8 |
| | 654.6 |
|
Customer relationships and distribution networks | 1,558.5 |
| | 613.9 |
| | 1,586.6 |
| | 566.5 |
|
Trademarks, trade names, and brands | 1,266.0 |
| | 219.5 |
| | 1,282.4 |
| | 188.5 |
|
Non-compete agreements | 8.3 |
| | 7.6 |
| | 12.9 |
| | 11.8 |
|
Total definite-lived intangibles | $ | 4,327.5 |
| | $ | 1,652.3 |
| | $ | 4,379.3 |
| | $ | 1,520.4 |
|
Total intangible assets | $ | 4,360.0 |
| | $ | 1,652.3 |
| | $ | 4,428.6 |
| | $ | 1,520.4 |
|
We recorded amortization expense of $73.6 million and $149.0 million for the three and six months ended June 29, 2019, respectively, and $85.3 million and $172.4 million for the three and six months ended June 30, 2018, respectively.
During the three months ended June 29, 2019, we identified impairment indicators for a certain definite-lived asset related to changes in pricing and competition in the market, which lowered the projected cash flows we expect to generate from the asset. We determined the asset was impaired by $27.8 million in our RX segment.
We recorded an impairment charge of $4.1 million on an in process R&D asset in the CSCA segment during the three months ended March 30, 2019 due to changes in projected development and regulatory timelines.
NOTE 5 – INVENTORIES
Major components of inventory were as follows (in millions):
|
| | | | | | | |
| June 29,
2019 | | December 31,
2018 |
Finished goods | $ | 501.2 |
| | $ | 444.9 |
|
Work in process | 192.8 |
| | 197.5 |
|
Raw materials | 246.5 |
| | 235.6 |
|
Total inventories | $ | 940.5 |
| | $ | 878.0 |
|
NOTE 6 – FAIR VALUE MEASUREMENTS
The table below summarizes the valuation of our financial instruments carried at fair value by the applicable pricing categories (in millions):
|
| | | | | | | | | | | | | | | | | | | | | | | | |
| | June 29, 2019 | | December 31, 2018 |
| | Level 1 | | Level 2 | | Level 3 | | Level 1 | | Level 2 | | Level 3 |
Measured at fair value on a recurring basis: | | | | | | | | | | | | |
Assets: | | | | | | | | | | | | |
Investment securities | | $ | 2.8 |
| | $ | — |
| | $ | — |
| | $ | 9.4 |
| | $ | — |
| | $ | — |
|
Foreign currency forward contracts | | — |
| | 4.0 |
| | — |
| | — |
| | 3.8 |
| | — |
|
Funds associated with Israeli severance liability | | — |
| | 14.0 |
| | — |
| | — |
| | 13.0 |
| | — |
|
Royalty Pharma contingent milestone payments | | — |
| | — |
| | 89.1 |
| | — |
| | — |
| | 323.2 |
|
Total assets | | $ | 2.8 |
| | $ | 18.0 |
| | $ | 89.1 |
| | $ | 9.4 |
| | $ | 16.8 |
| | $ | 323.2 |
|
| | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | |
Foreign currency forward contracts | | $ | — |
| | $ | 3.6 |
| | $ | — |
| | $ | — |
| | $ | 9.2 |
| | $ | — |
|
Contingent consideration | | — |
| | — |
| | 12.1 |
| | — |
| | — |
| | 15.3 |
|
Total liabilities | | $ | — |
| | $ | 3.6 |
| | $ | 12.1 |
| | $ | — |
| | $ | 9.2 |
| | $ | 15.3 |
|
| | | | | | | | | | | | |
Measured at fair value on a non-recurring basis: | | | | | | | | | | | | |
Assets: | | | | | | | | | | | | |
Goodwill(1) | | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | 42.2 |
|
Indefinite-lived intangible assets(2) | | — |
| | — |
| | — |
| | — |
| | — |
| | 10.5 |
|
Definite-lived intangible assets(3) | | — |
| | — |
| | — |
| | — |
| | — |
| | 22.4 |
|
Total assets | | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | 75.1 |
|
| |
| As of December 31, 2018, goodwill with a carrying amount of $178.9 million was written down to a fair value of $42.2 million. |
| |
| As of December 31, 2018, indefinite-lived intangible assets with a carrying amount of $46.9 million were written down to a fair value of $10.5 million. |
| |
| As of December 31, 2018, definite-lived intangible assets with a carrying amount of $72.0 million were written down to a fair value of $22.4 million. |
There were no transfers among Level 1, 2, and 3 during the three and six months ended June 29, 2019 or the year ended December 31, 2018.
Perrigo Company plc - Item 1
Note 6
Royalty Pharma Contingent Milestone Payments
The table below summarizes the change in fair value of the Royalty Pharma contingent milestone payments (in millions):
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Beginning balance | $ | 83.6 |
| | $ | 124.9 |
| | $ | 323.2 |
| | $ | 134.5 |
|
Payments received | — |
| | — |
| | (250.0 | ) | | — |
|
Change in fair value | 5.5 |
| | 0.6 |
| | 15.9 |
| | (9.0 | ) |
Ending balance | $ | 89.1 |
| | $ | 125.5 |
| | $ | 89.1 |
| | $ | 125.5 |
|
We value our contingent milestone payments from Royalty Pharma using a modified Black-Scholes Option Pricing Model ("BSOPM"). Key inputs in the BSOPM are the estimated volatility and rate of return of royalties on global net sales of Tysabri® that are received by Royalty Pharma until the contingent milestones are resolved. Volatility and the estimated fair value of the milestones have a positive relationship such that higher volatility translates to a higher estimated fair value of the contingent milestone payments. We assess volatility and rate of return inputs quarterly by analyzing certain market volatility benchmarks and the risk associated with Royalty Pharma achieving the underlying projected royalties. The table below represents the volatility and rate of return:
|
| | | | | |
| Three Months Ended |
| June 29,
2019 | | June 30,
2018 |
Volatility | 30.0 | % | | 30.0 | % |
Rate of return | 7.99 | % | | 8.09 | % |
The fair value of the Royalty Pharma contingent milestone payment increased by $5.5 million and $15.9 million during the three and six months ended June 29, 2019, respectively. These adjustments were driven by higher projected global net sales of Tysabri® and the estimated probability of achieving the earn-out. The fair value of the Royalty Pharma contingent milestone payments increased by $0.6 million during the three months ended June 30, 2018. This increase included a $2.9 million decrease in the fair value of the 2018 contingent milestone payment, more than offset by a $3.5 million increase in the fair value of the 2020 contingent milestone payment. During the six months ended June 30, 2018, the fair value of the Royalty Pharma contingent milestone payments decreased $9.0 million. The net changes in the fair value of the contingent milestone payments were due to the fluctuation of the projected global net sales of Tysabri®, which were impacted by competition, namely the launch of Ocrevus® in the U.S. and European markets.
In order for us to receive the 2020 milestone payment of $400.0 million, Royalty Pharma contingent payments for Tysabri® sales in 2020 must exceed $351.0 million. In 2018, the Royalty Pharma contingent payments for Tysabri® were $337.5 million, which exceeded the threshold. If Royalty Pharma contingent payments for Tysabri® sales do not meet the prescribed threshold in 2020, we will write off the $89.1 million asset as an expense. If the prescribed threshold is exceeded, we will increase the asset to $400.0 million and recognize income of $310.9 million in Change in financial assets on the Condensed Consolidated Statements of Operations.
Perrigo Company plc - Item 1
Note 6
Contingent Consideration
The table below summarizes the change in fair value of contingent consideration (in millions):
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Beginning balance | $ | 12.4 |
| | $ | 18.1 |
| | $ | 15.3 |
| | $ | 22.0 |
|
Changes in value | 0.9 |
| | (1.8 | ) | | (2.0 | ) | | (1.4 | ) |
Currency translation adjustments | — |
| | (0.1 | ) | | — |
| | — |
|
Settlements and other adjustments | (1.2 | ) | | — |
| | (1.2 | ) | | (4.4 | ) |
Ending balance | $ | 12.1 |
| | $ | 16.2 |
| | $ | 12.1 |
| | $ | 16.2 |
|
Goodwill and Intangible Assets
Animal Health
When determining the fair value of our animal health reporting unit for the year ended December 31, 2018, we utilized a combination of comparable company market and discounted cash flow techniques. In our comparable company market approach, we considered observable market information and transactions for companies that we deemed to be of a comparable nature, scope, and size of animal health (Level 2 inputs). Our cash flow projections included revenue assumptions related to new products, product line extensions, and existing products, plus gross margin, advertising and promotion, and other operating expenses based on the growth plans (Level 3 inputs). In our discounted cash flow analysis, we utilized projected sales growth rate and discount rate assumptions of 2.5% and 9.8%, respectively. The discount rate correlates with the required investment return and risk that we believe market participants would apply to the projected growth rate. In addition, we burdened projected free cash flows with the capital spending deemed necessary to support the cash flows and applied the jurisdictional tax rate of 22.8%. We weighted indications of fair value resulting from the market approach and present value techniques, considering the reasonableness of the range of measurements and the point within the range that we determined was most representative of fair market conditions.
When assessing our animal health indefinite-lived intangible asset for the year ended December 31, 2018, we utilized a multi-period excess earnings method ("MPEEM") to determine the fair value of the intangible asset. Our cash flow projections included revenue assumptions related to new products, product line extensions, and existing products. We utilized long-term growth rate and discount rate assumptions of (0.3)% and 9.8%, respectively, and we applied a jurisdictional tax rate of 22.8%.
When assessing our animal health definite-lived assets for impairment for the year ended December 31, 2018, we utilized a combination of MPEEM and relief from royalty methods to determine the fair values of definite-lived assets within the asset group. The projected financial information, inputs, and assumptions utilized were consistent with those utilized in the goodwill discounted cash flow analysis described above.
Generic product
When measuring the impairment of a certain definite-lived asset during the three months ended June 29, 2019, we utilized a discounted cash flow technique to estimate the fair value of the asset. Significant valuation inputs and assumptions relate to our projected future cash flows, including the total market size, our estimated market share, and our average selling price.
Perrigo Company plc - Item 1
Note 6
Fixed Rate Long-term Debt
Our fixed rate long-term debt consisted of the following (in millions):
|
| | | | | | | | | | | | | | | |
| June 29,
2019 | | December 31,
2018 |
| Level 1 | | Level 2 | | Level 1 | | Level 2 |
Public Bonds | | | | | | | |
Carrying Value (excluding discount) | $ | 2,600.0 |
| | | | $ | 2,600.0 |
| | |
Fair value | $ | 2,533.0 |
| | | | $ | 2,316.6 |
| | |
| | | | | | | |
Retail bond and private placement note | | | | | | | |
Carrying value (excluding premium) | | | $ | 153.5 |
| | | | $ | 292.5 |
|
Fair value | | | $ | 167.8 |
| | | | $ | 307.9 |
|
The fair values of our public bonds for all periods were based on quoted market prices. The fair value of our private placement note for all periods was based on interest rates offered for borrowings of a similar nature and remaining maturities. The fair value of our retail bond for the year ended December 31, 2018 was based on interest rates offered for borrowings of a similar nature and remaining maturities. On May 23, 2019, we repaid the retail bond in full (refer to Note 11).
The carrying amounts of our other financial instruments, consisting of cash and cash equivalents, accounts receivable, accounts payable, short-term debt, revolving credit agreements, and variable rate long-term debt, approximate their fair value.
NOTE 7 – INVESTMENTS
The following table summarizes the measurement category, balance sheet location, and balances of our equity securities (in millions):
|
| | | | | | | | | | |
Measurement Category | | Balance Sheet Location | | June 29,
2019 | | December 31, 2018 |
Fair value method | | Prepaid expenses and other current assets | | $ | 2.8 |
| | $ | 9.4 |
|
Fair value method(1) | | Other non-current assets | | $ | 2.8 |
| | $ | 4.4 |
|
Equity method | | Other non-current assets | | $ | 16.8 |
| | $ | 15.1 |
|
| |
| Measured at fair value using the Net Asset Value practical expedient. |
The following table summarizes the expense (income) recognized in earnings of our equity securities (in millions):
|
| | | | | | | | | | | | | | | | | | |
| | | | Three Months Ended | | Six Months Ended |
Measurement Category | | Income Statement Location | | June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Fair value method | | Other (income) expense, net | | $ | 1.8 |
| | $ | 6.3 |
| | $ | 7.9 |
| | $ | 10.6 |
|
Equity method | | Other (income) expense, net | | $ | (1.0 | ) | | $ | (0.9 | ) | | $ | (1.7 | ) | | $ | (0.7 | ) |
During the three months ended June 30, 2018, Perrigo Asia Holding Company Limited increased its investment in Zibo Xinhua - Perrigo Pharmaceutical Company Limited by $7.5 million.
Perrigo Company plc - Item 1
Note 8
NOTE 8 – ASSETS HELD FOR SALE
We classify assets as "held for sale" when, among other factors, management approves and commits to a formal plan of sale with the expectation the sale will be completed within one year. The net assets of the business held for sale are then recorded at the lower of their current carrying value and the fair market value, less costs to sell.
During the three months ended June 29, 2019, management committed to a plan to sell our animal health business; as a result, such assets were classified as held-for-sale. On July 8, 2019, we completed the sale of our animal health business (refer to Note 18). The assets associated with our animal health business were reported in our CSCA segment.
The assets held-for-sale were reported within Prepaid expenses and other current assets and liabilities held-for-sale were reported in Accrued liabilities. The amounts consisted of the following (in millions):
|
| | | |
| June 29,
2019 |
Assets held for sale | |
Current assets | $ | 29.3 |
|
Property, plant and equipment, net | 10.8 |
|
Goodwill and indefinite-lived intangible assets | 42.2 |
|
Definite-lived intangible assets, net | 36.4 |
|
Other assets | 25.7 |
|
Total assets held for sale | $ | 144.4 |
|
Liabilities held for sale | |
Current liabilities | $ | 11.3 |
|
Other liabilities | 38.3 |
|
Total liabilities held for sale | $ | 49.6 |
|
NOTE 9 – DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
On January 1, 2019, we adopted Accounting Standards Update No. 2017-12 Targeted Improvements to Accounting for Hedge Activities ("ASU 2017-12") using a modified retrospective approach. Among other provisions, the new standard required modifications to existing presentation and disclosure requirements on a prospective basis. As such, certain disclosures for the three and six months ended June 30, 2018 below conform to the disclosure requirements prior to the adoption of ASU 2017-12.
Prior to the adoption of ASU 2017-12, we were required to separately measure and reflect the amount by which the hedging instrument did not offset the changes in the fair value or cash flows of hedged items, which was referred to as the ineffective amount. We assessed hedge effectiveness on a quarterly basis and recorded the gain or loss related to the ineffective portion of derivative instruments, if any, in Other (income) expense, net on the Condensed Consolidated Statements of Operations. Pursuant to the provisions of ASU 2017-12, we are no longer required to separately measure and recognize hedge ineffectiveness. Therefore, we no longer recognize hedge ineffectiveness separately on our Condensed Consolidated Statements of Income, but instead recognize the entire change in the fair value of:
| |
• | Cash flow hedges included in the assessment of hedge effectiveness in OCI. The amounts recorded in OCI will subsequently be reclassified to earnings in the same line item on the Condensed Consolidated Statements of Operations as impacted by the hedged item when the hedged item affects earnings; and |
| |
• | Fair value hedges included in the assessment of hedge effectiveness in the same line item on the Condensed Consolidated Statements of Operations that is used to present the earnings effect of the hedged item. |
Prior to the adoption of ASU 2017-12, we excluded option premiums and forward points (excluded components) from our assessment of hedge effectiveness for our foreign exchange cash flow hedges. We
Perrigo Company plc - Item 1
Note 9
recognized all changes in fair value of the excluded components in Other (income) expense, net, on the Condensed Consolidated Statements of Operations. The amendments in ASU 2017-12 continue to allow those components to be excluded from the assessment of hedge effectiveness and add cross-currency basis spread as an allowable excluded component. The provisions of ASU 2017-12 allow a policy election to either continue to recognize changes in the fair value of the excluded components currently in earnings or to recognize the initial value of the excluded component using an amortization approach. We have elected to recognize the initial value of the excluded component on a straight-line basis over the life of the derivative instrument, within the same line item on the Condensed Consolidated Statements of Operations that is used to present the earnings effect of the hedged item. The cumulative effect adjustment between Accumulated Other Comprehensive Income ("AOCI") and Retained earnings (accumulated deficit) from applying this policy on existing hedges at the date of adoption was immaterial.
We record derivative instruments on the balance sheet on a gross basis as either an asset or liability measured at fair value (refer to Note 6). Additionally, changes in a derivative's fair value, which are measured at the end of each period, are recognized in earnings unless a derivative can be designated in a qualifying hedging relationship.
Designated derivatives meet hedge accounting criteria, which means the fair value of the hedge is recorded in shareholders’ equity as a component of OCI, net of tax. The deferred gains and losses are recognized in income in the period in which the hedged item affects earnings. We have elected to recognize the fair value of the excluded component in OCI and amortize on a straight-line basis over the life of the derivative instrument, within the same line item on the Condensed Consolidated Statements of Operations that is used to present the earnings effect of the hedged item. All of our designated derivatives are assessed for hedge effectiveness quarterly. All of our designated derivatives were classified as cash flow hedges as of June 29, 2019 and December 31, 2018.
We also have economic non-designated derivatives that do not meet hedge accounting criteria. These derivative instruments are adjusted to current market value at the end of each period through earnings. Gains or losses on these instruments are offset substantially by the remeasurement adjustment on the hedged item.
We are exposed to credit loss in the event of nonperformance by the counterparties on derivative contracts. It is our policy to manage our credit risk on these transactions by dealing only with financial institutions having a long-term credit rating of "Aa3" or better and by distributing the contracts among several financial institutions to diversify credit concentration risk. Should a counterparty default, our maximum exposure to loss is the asset balance of the instrument. The maximum term of our forward currency exchange contracts is 18 months.
We enter into certain derivative financial instruments, when available on a cost-effective basis, to mitigate our risk associated with changes in interest rates and foreign currency exchange rates as follows:
Interest rate risk management - We are exposed to the impact of interest rate changes through our cash investments and borrowings. We utilize a variety of strategies to manage the impact of changes in interest rates including using a mix of debt maturities along with both fixed-rate and variable-rate debt. In addition, we may enter into treasury-lock agreements and interest rate swap agreements on certain investing and borrowing transactions to manage our exposure to interest rate changes and our overall cost of borrowing.
Foreign currency exchange risk management - We conduct business in several major currencies other than the U.S. dollar and are subject to risks associated with changing foreign exchange rates. Our objective is to reduce cash flow volatility associated with foreign exchange rate changes on a consolidated basis to allow management to focus its attention on business operations. Accordingly, we enter into various contracts that change in value as foreign exchange rates change to protect the value of existing foreign currency assets and liabilities, commitments, and anticipated foreign currency sales and expenses.
All derivative instruments are managed on a consolidated basis to efficiently net exposures and thus take advantage of any natural offsets. Gains and losses related to the derivative instruments are expected to be offset largely by gains and losses on the original underlying asset or liability. We do not use derivative financial instruments for speculative purposes.
Perrigo Company plc - Item 1
Note 9
Interest Rate Swaps
Interest rate swap agreements are contracts to exchange floating rate for fixed rate payments (or vice versa) over the life of the agreement without the exchange of the underlying notional amounts. The notional amounts of the interest rate swap agreements are used to measure interest to be paid or received and do not represent the amount of exposure to credit loss. The differential paid or received on the interest rate swap agreements is recognized as an adjustment to interest expense. There were no active designated or non-designated interest rate swaps as of June 29, 2019 and December 31, 2018.
Foreign Currency Derivatives
Foreign currency forward contracts entered into to hedge forecasted revenue and expenses were as follows (in millions):
|
| | | | | | | | |
| | Notional Amount |
| | June 29,
2019 | | December 31,
2018 |
Israeli Shekel (ILS) | | $ | 340.6 |
| | $ | 232.6 |
|
British Pound (GBP) | | 111.0 |
| | 90.2 |
|
European Euro (EUR) | | 108.0 |
| | 134.2 |
|
Swedish (SEK) | | 42.2 |
| | 38.7 |
|
Danish Krone (DKK) | | 58.2 |
| | 56.5 |
|
United States Dollar (USD) | | 46.2 |
| | 39.3 |
|
Canadian Dollar (CAD) | | 41.9 |
| | 31.7 |
|
Polish Zloty (PLZ) | | 33.5 |
| | 18.2 |
|
Chinese Yuan (CNY) | | 20.0 |
| | — |
|
Norwegian Krone (NOK) | | 6.8 |
| | 6.2 |
|
Romanian New Leu (RON) | | 5.9 |
| | 4.4 |
|
Mexican Peso (MPX) | | 4.2 |
| | 25.9 |
|
Other | | 12.6 |
| | 8.7 |
|
Total | | $ | 831.1 |
| | $ | 686.6 |
|
Effects of Derivatives on the Financial Statements
The below tables indicate the effects of all derivative instruments on the Condensed Consolidated Financial Statements. All amounts exclude income tax effects.
The balance sheet location and gross fair value of our outstanding derivative instruments were as follows (in millions):
|
| | | | | | | | | |
| | | Asset Derivatives |
| | | Fair Value |
| Balance Sheet Location | | June 29,
2019 | | December 31,
2018 |
Designated derivatives: | | | | | |
Foreign currency forward contracts | Prepaid expenses and other current assets | | $ | 1.0 |
| | $ | 2.0 |
|
Non-designated derivatives: | | | | | |
Foreign currency forward contracts | Prepaid expenses and other current assets | | $ | 3.0 |
| | $ | 1.8 |
|
Perrigo Company plc - Item 1
Note 9
|
| | | | | | | | | |
| | | Liability Derivatives |
| | | Fair Value |
| Balance Sheet Location | | June 29,
2019 | | December 31,
2018 |
Designated derivatives: | | | | | |
Foreign currency forward contracts | Accrued liabilities | | $ | 2.6 |
| | $ | 6.4 |
|
Non-designated derivatives: | | | | | |
Foreign currency forward contracts | Accrued liabilities | | $ | 1.0 |
| | $ | 2.8 |
|
The following tables summarize the effect of derivative instruments designated as cash-flow hedging instruments in AOCI (in millions):
|
| | | | | | | | | | | | | | | | |
| | Three Months Ended |
| | June 29, 2019 |
Instrument | | Amount of Gain/(Loss) Recorded in OCI | | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | | Amount of Gain/(Loss) Reclassified from AOCI into Earnings | | Classification of Gain/(Loss) Recognized into Earnings Related to Amounts Excluded from Effectiveness Testing | | Amount of Gain/(Loss) Recognized in Earnings on Derivatives Related to Amounts Excluded from Effectiveness Testing |
Interest rate swap agreements | | | | Interest expense, net | | $ | (0.4 | ) | | Interest expense, net | | $ | — |
|
Foreign currency forward contracts | | $ | 1.1 |
| | Net sales | | 0.1 |
| | Net sales | | 0.1 |
|
| | | | Cost of sales | | (0.2 | ) | | Cost of sales | | (1.1 | ) |
| | | | | | $ | (0.5 | ) | | | | $ | (1.0 | ) |
|
| | | | | | | | | | | | | | | | |
| | Six Months Ended |
| | June 29, 2019 |
Instrument | | Amount of Gain/(Loss) Recorded in OCI(1) | | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | | Amount of Gain/(Loss) Reclassified from AOCI into Earnings | | Classification of Gain/(Loss) Recognized into Earnings Related to Amounts Excluded from Effectiveness Testing | | Amount of Gain/(Loss) Recognized in Earnings on Derivatives Related to Amounts Excluded from Effectiveness Testing |
Interest rate swap agreements | | | | Interest expense, net | | $ | (1.0 | ) | | Interest expense, net | | $ | — |
|
Foreign currency forward contracts | | $ | — |
| | Net sales | | 0.3 |
| | Net sales | | — |
|
| | | | Cost of sales | | (1.4 | ) | | Cost of sales | | (2.2 | ) |
| | | | | | $ | (2.1 | ) | | | | $ | (2.2 | ) |
(1) Net loss of $1.6 million is expected to be reclassified out of AOCI into earnings during the next 12 months.
Perrigo Company plc - Item 1
Note 9
|
| | | | | | | | | | |
| | Three Months Ended |
| | June 30, 2018 |
| | Effective Portion |
Instrument | | Amount of Gain/(Loss) Recorded in OCI | | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | | Amount of Gain/(Loss) Reclassified from AOCI into Earnings |
Interest rate swap agreements | | | | Interest expense, net | | $ | (0.4 | ) |
Foreign currency forward contracts | | $ | (4.3 | ) | | Net sales | | — |
|
| | | | Cost of sales | | 1.6 |
|
| | | | Interest expense, net | | (1.1 | ) |
| | | | Other (income) expense, net | | (0.1 | ) |
| | | | | | $ | — |
|
|
| | | | | | | | | | |
| | Six Months Ended |
| | June 30, 2018 |
| | Effective Portion |
Instrument | | Amount of Gain/(Loss) Recorded in OCI | | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | | Amount of Gain/(Loss) Reclassified from AOCI into Earnings |
Interest rate swap agreements | | | | Interest expense, net | | $ | (0.9 | ) |
Foreign currency forward contracts | | $ | (4.3 | ) | | Net sales | | — |
|
| | | | Cost of sales | | 3.9 |
|
| | | | Interest expense, net | | (2.0 | ) |
| | | | Other (income) expense, net | | (0.5 | ) |
| | | | | | $ | 0.5 |
|
The amounts of gain/(loss) recognized in earnings related to our non-designated derivatives on the Condensed Consolidated Statements of Operations were as follows (in millions):
|
| | | | | | | | | | | | | | | | | | |
| | | | Three Months Ended | | Six Months Ended |
Non-Designated Derivatives | | Income Statement Location | | June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Foreign currency forward contracts | | Other (income) expense, net | | $ | (4.3 | ) | | $ | 11.8 |
| | $ | (13.1 | ) | | $ | 8.6 |
|
| | Interest expense, net | | 1.4 |
| | 0.2 |
| | 1.8 |
| | (0.7 | ) |
Total | | | | $ | (2.9 | ) | | $ | 12.0 |
| | $ | (11.3 | ) | | $ | 7.9 |
|
The impact of gains and losses on foreign exchange contracts not designated as hedging instruments related to changes in the fair value of assets and liabilities denominated in foreign currencies are generally offset by net foreign exchange gains and losses, which are also included on the Condensed Consolidated Statements of Operations in Other (income) expense, net for all periods presented. When we enter into foreign exchange contracts not designated as hedging instruments to mitigate the impact of exchange rate volatility in the translation of foreign earnings, gains and losses will generally be offset by fluctuations in the U.S. Dollar-translated amounts of each Income Statement account in current and/or future periods.
Perrigo Company plc - Item 1
Note 9
The classification and amount of gain/(loss) recognized in earnings on fair value and cash flow hedging relationships are as follows (in millions):
|
| | | | | | | | | | | | | | | | |
| | Three Months Ended |
| | June 29, 2019 |
| | Net Sales | | Cost of Sales | | Interest Expense, net | | Other (Income) Expense, net |
Total amounts of income and expense line items presented on the Condensed Consolidated Statements of Operations in which the effects of fair value or cash flow hedges are recorded | | $ | 1,149.0 |
| | $ | 718.2 |
| | $ | 31.2 |
| | $ | 2.3 |
|
| | | | | | | | |
The effects of cash flow hedging: | | | | | | | | |
Gain (loss) on cash flow hedging relationships | | | | | | | | |
Foreign currency forward contracts | | | | | | | | |
Amount of gain or (loss) reclassified from AOCI into earnings | | $ | 0.1 |
| | $ | (0.2 | ) | | $ | — |
| | $ | — |
|
Amount excluded from effectiveness testing recognized using a systematic and rational amortization approach | | $ | 0.1 |
| | $ | (1.1 | ) | | $ | — |
| | $ | — |
|
Interest rate swap agreements | | | | | | | | |
Amount of gain or (loss) reclassified from AOCI into earnings | | $ | — |
| | $ | — |
| | $ | (0.4 | ) | | $ | — |
|
|
| | | | | | | | | | | | | | | | |
| | Six Months Ended |
| | June 29, 2019 |
| | Net Sales | | Cost of Sales | | Interest Expense, net | | Other (Income) Expense, net |
Total amounts of income and expense line items presented on the Condensed Consolidated Statements of Operations in which the effects of fair value or cash flow hedges are recorded | | $ | 2,323.5 |
| | $ | 1,443.9 |
| | $ | 59.8 |
| | $ | 5.5 |
|
| | | | | | | | |
The effects of cash flow hedging: | | | | | | | | |
Gain (loss) on cash flow hedging relationships | | | | | | | | |
Foreign currency forward contracts | | | | | | | | |
Amount of gain or (loss) reclassified from AOCI into earnings | | $ | 0.3 |
| | $ | (1.4 | ) | | $ | — |
| | $ | — |
|
Amount excluded from effectiveness testing recognized using a systematic and rational amortization approach | | $ | — |
| | $ | (2.2 | ) | | $ | — |
| | $ | — |
|
Interest rate swap agreements | | | | | | | | |
Amount of gain or (loss) reclassified from AOCI into earnings | | $ | — |
| | $ | — |
| | $ | (1.0 | ) | | $ | — |
|
NOTE 10 – LEASES
We adopted ASU 2016-02, Leases, as of January 1, 2019, using the modified retrospective transition approach, with a cumulative-effect adjustment to the opening balance of retained earnings as of the effective date. The financial results reported in periods prior to 2019 are unchanged. In addition, we elected the package of practical expedients permitted under the transition guidance within the new standard, which among other things, allowed us to carry forward the historical lease classification.
Adoption of the new standard resulted in additional operating lease liabilities and lease assets, including the transition of existing capital lease liabilities and lease assets to finance classification, of approximately $166.5 million and $164.0 million, respectively, as of January 1, 2019. Upon adoption, the difference between the lease assets and lease liabilities partially related to existing deferred lease liabilities reclassified to lease assets and the transition of capital lease assets and liabilities at their carrying values. In addition, historical build-to-suit assets and liabilities were removed on transition and recorded as an adjustment to retained earnings, net of deferred tax impact. The standard did not materially impact our consolidated net income or cash flow classification.
Perrigo Company plc - Item 1
Note 10
We lease certain office buildings, warehouse facilities, vehicles, and plant, office, and computer equipment. Lease assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease.
We evaluate arrangements at inception to determine if lease components are included. An arrangement includes a lease component if it identifies an asset and we have control over the asset. For new leases beginning January 1, 2019 or later, we have elected for all asset classes not to separate lease components from the non-lease components included in an arrangement when measuring the leased asset and leased liability.
Lease assets and liabilities are recognized at the lease commencement date based on the estimated present value of lease payments over the lease term. Leases with an initial term of 12 months or less are not recorded on the balance sheet. We recognize lease expense for leases on a straight-line basis over the lease term. We apply the portfolio approach to certain groups of computer equipment and vehicle leases when the term, classification, and asset type are identical. The discount rate selected is the incremental borrowing rate we would obtain for a secured financing of the lease asset over a similar term.
Many of our leases include one or more options to extend the lease term. Certain leases also include options to terminate early or purchase the leased property, all of which are executed at our sole discretion. Optional periods may be included in the lease term and measured as part of the lease asset and lease liability if we are reasonably certain to exercise our right to use the leased asset during the optional periods. We generally consider renewal options to be reasonably certain of execution and included in the lease term when significant leasehold improvements have been made by us to the leased assets. The depreciable lives of assets and leasehold improvements are limited by the expected lease term, unless there is a transfer of title or purchase option reasonably certain of exercise.
Certain of our lease agreements include contingent rental payments based on per unit usage over contractual levels (e.g., miles driven or machine hours used) and others include rental payments adjusted periodically for market reviews or inflationary indexes. Our lease agreements do not contain any material residual value guarantees or material restrictive covenants.
The balance sheet location of our lease assets and liabilities were as follows (in millions):
|
| | | | | | |
Assets | | Balance Sheet Location | | June 29,
2019 |
Operating | | Operating lease assets | | $ | 135.4 |
|
Finance | | Other non-current assets | | 17.1 |
|
Total | | | | $ | 152.5 |
|
|
| | | | | | |
Liabilities | | Balance Sheet Location | | June 29,
2019 |
Current | | | | |
Operating | | Accrued liabilities | | $ | 32.9 |
|
Finance | | Current indebtedness | | 1.9 |
|
Non-Current | | | | |
Operating | | Other non-current liabilities | | 107.4 |
|
Finance | | Long-term debt, less current portion | | 11.8 |
|
Total | | | | $ | 154.0 |
|
Perrigo Company plc - Item 1
Note 10
The below table shows our lease assets and liabilities by reporting segment (in millions):
|
| | | | | | | | | | | | | | | | |
| | Assets | | Liabilities |
| | Operating | | Financing | | Operating | | Financing |
| | June 29,
2019 | | June 29,
2019 | | June 29,
2019 | | June 29,
2019 |
CSCA | | $ | 22.7 |
| | $ | 8.9 |
| | $ | 22.9 |
| | $ | 8.4 |
|
CSCI | | 42.0 |
| | 6.1 |
| | 42.9 |
| | 3.2 |
|
RX | | 37.5 |
| | 0.3 |
| | 38.9 |
| | 0.3 |
|
Unallocated | | 33.2 |
| | 1.8 |
| | 35.6 |
| | 1.8 |
|
Total | | $ | 135.4 |
| | $ | 17.1 |
| | $ | 140.3 |
| | $ | 13.7 |
|
Lease expense was as follows (in millions):
|
| | | | | | | | |
| | Three Months Ended | | Six Months Ended |
| | June 29,
2019 | | June 29,
2019 |
Operating leases(a) | | $ | 11.2 |
| | $ | 23.2 |
|
| | | | |
Finance leases | | | | |
Amortization | | $ | 0.7 |
| | $ | 1.3 |
|
Interest | | 0.1 |
| | 0.2 |
|
Total finance leases | | $ | 0.8 |
| | $ | 1.5 |
|
Total operating lease expense for the three and six months ended June 30, 2018 was $14.2 million and $26.1 million, respectively.
The annual future maturities of our leases as of June 29, 2019 are as follows (in millions):
|
| | | | | | | | | | | | |
| | Operating Leases | | Finance Leases | | Total |
2019 | | $ | 19.4 |
| | $ | 1.2 |
| | $ | 20.6 |
|
2020 | | 34.6 |
| | 2.3 |
| | 36.9 |
|
2021 | | 24.9 |
| | 3.6 |
| | 28.5 |
|
2022 | | 18.3 |
| | 1.2 |
| | 19.5 |
|
2023 | | 14.0 |
| | 1.0 |
| | 15.0 |
|
After 2023 | | 51.7 |
| | 7.8 |
| | 59.5 |
|
Total lease payments | | 162.9 |
| | 17.1 |
| | 180.0 |
|
Less: Interest | | 22.6 |
| | 3.4 |
| | 26.0 |
|
Present value of lease liabilities | | $ | 140.3 |
| | $ | 13.7 |
| | $ | 154.0 |
|
Our weighted average lease terms and discount rates are as follows:
|
| | | |
| | June 29,
2019 |
Weighted-average remaining lease term (in years) | | |
Operating leases | | 6.59 |
|
Finance leases | | 9.43 |
|
Weighted-average discount rate | | |
Operating leases | | 4.19 | % |
Finance leases | | 4.35 | % |
Perrigo Company plc - Item 1
Note 10
Our lease cash flow classifications are as follows (in millions):
|
| | | | |
| | Six Months Ended |
| | June 29,
2019 |
Cash paid for amounts included in the measurement of lease liabilities | | |
Operating cash flows for operating leases | | $ | 23.0 |
|
Operating cash flows for finance leases | | $ | 0.2 |
|
Financing cash flows for finance leases | | $ | 1.4 |
|
| | |
Leased assets obtained in exchange for new finance lease liabilities | | $ | 7.8 |
|
Leased assets obtained in exchange for new operating lease liabilities | | $ | 12.8 |
|
NOTE 11 – INDEBTEDNESS
Total borrowings outstanding are summarized as follows (in millions):
|
| | | | | | | | | | | |
| | | | | June 29,
2019 | | December 31,
2018 |
Revolving Credit Agreement | | | | | |
| 2018 Revolver due March 8, 2023 | | | $ | 325.0 |
| | $ | — |
|
Term loan | | | | | |
| 2018 Term loan due March 8, 2020(1) | | | 323.4 |
| | 351.3 |
|
Notes and Bonds | | | | | |
| Coupon | Due | | | | | |
| 5.000% | May 23, 2019 | | | — |
| | 137.6 |
|
| 3.500% | March 15, 2021 | | | 280.4 |
| | 280.4 |
|
| 3.500% | December 15, 2021 | | | 309.6 |
| | 309.6 |
|
| 5.105% | July 28, 2023(1) | | | 153.5 |
| | 154.9 |
|
| 4.000% | November 15, 2023 | | | 215.6 |
| | 215.6 |
|
| 3.900% | December 15, 2024 | | | 700.0 |
| | 700.0 |
|
| 4.375% | March 15, 2026 | | | 700.0 |
| | 700.0 |
|
| 5.300% | November 15, 2043 | | | 90.5 |
| | 90.5 |
|
| 4.900% | December 15, 2044 | | | 303.9 |
| | 303.9 |
|
| Total notes and bonds | | | 2,753.5 |
| | 2,892.5 |
|
Other financing | 87.2 |
| | 2.8 |
|
Unamortized premium (discount), net | 8.9 |
| | 12.2 |
|
Deferred financing fees | (14.8 | ) | | (16.4 | ) |
Total borrowings outstanding | 3,483.2 |
| | 3,242.4 |
|
| Current indebtedness | (398.8 | ) | | (190.2 | ) |
Total long-term debt less current portion | $ | 3,084.4 |
| | $ | 3,052.2 |
|
(1)
We are in compliance with all covenants under our debt agreements as of June 29, 2019.
Revolving Credit Agreements
On March 8, 2018, we terminated the revolving credit agreement entered into on December 5, 2014 and entered into a $1.0 billion revolving credit agreement maturing on March 8, 2023 (the "2018 Revolver"). During the three months ended June 29, 2019, in anticipation of the Ranir Global Holdings, LLC (“Ranir”) acquisition (refer to Note 18), we borrowed $375.0 million under this facility. There were $325.0 million of borrowings outstanding under the 2018 Revolver as of June 29, 2019. There were no borrowings outstanding under the 2018 Revolver as of December 31, 2018.
Term Loans
On December 5, 2014, Perrigo Finance entered into a term loan agreement consisting of a €500.0 million ($614.3 million) tranche, maturing on December 5, 2019. On March 8, 2018, we refinanced the €350.0 million outstanding under the term loan with the proceeds of a new €350.0 million ($431.0 million) term loan, maturing on March 8, 2020 (the "2018 Term Loan"). In addition, as a result of the refinancing during the three months ended March 31, 2018, we recorded a loss of $0.5 million, consisting of the write-off of deferred financing fees in Loss on extinguishment of debt on the Condensed Consolidated Statements of Operations. During the six months ended June 29, 2019, we made $24.7 million in scheduled principal payments on the 2018 Term Loan.
Perrigo Company plc - Item 1
Note 11
Notes and Bonds
In connection with the Omega acquisition, on March 30, 2015, we assumed a 5.000% retail bond due in 2019 in the amount of €120.0 million ($130.7 million). On May 23, 2019 we repaid the bond in full.
Other Financing
We have overdraft facilities available that we use to support our cash management operations. We report any balances outstanding in the above table under "Other financing". The balance outstanding under the facilities was $73.9 million at June 29, 2019. There were no borrowings outstanding under the facilities as of December 31, 2018.
We have financing leases that are reported in the above table under "Other financing" (refer to Note 10).
NOTE 12 – EARNINGS PER SHARE AND SHAREHOLDERS' EQUITY
Earnings per Share
A reconciliation of the numerators and denominators used in the basic and diluted earnings per share ("EPS") calculation is as follows (in millions):
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Numerator: | | | | | | | |
Net income | $ | 9.0 |
| | $ | 36.2 |
| | $ | 72.9 |
| | $ | 117.0 |
|
| | | | | | | |
Denominator: | | | | | | | |
Weighted average shares outstanding for basic EPS | 136.0 |
| | 138.1 |
| | 136.0 |
| | 139.5 |
|
Dilutive effect of share-based awards | 0.5 |
| | 0.6 |
| | 0.3 |
| | 0.5 |
|
Weighted average shares outstanding for diluted EPS | 136.5 |
| | 138.7 |
| | 136.3 |
| | 140.0 |
|
| | | | | | | |
Anti-dilutive share-based awards excluded from computation of diluted EPS | 1.7 |
| | 1.7 |
| | 1.8 |
| | 0.9 |
|
Shareholders' Equity
Share Repurchases
Following the expiration of our 2015 share repurchase plan authorization, in October 2018, our Board of Directors authorized up to $1.0 billion of share repurchases with no expiration date, subject to the Board of Directors’ approval of the pricing parameters and amount that may be repurchased under each specific share repurchase program. We did not repurchase any shares during the three and six months ended June 29, 2019. During the three and six months ended June 30, 2018, we repurchased 2.0 million and 3.3 million ordinary shares
Perrigo Company plc - Item 1
Note 12
at an average repurchase price of $79.42 and $80.42 per share, for a total of $156.9 million and $265.0 million, respectively.
NOTE 13 – ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS)
Changes in our AOCI balances, net of tax were as follows (in millions):
|
| | | | | | | | | | | | | | | |
| Fair Value of Derivative Financial Instruments, net of tax | | Foreign Currency Translation Adjustments | | Post-Retirement and Pension Liability Adjustments, net of tax | | Total AOCI |
Balance at December 31, 2018 | $ | (15.5 | ) | | $ | 104.5 |
| | $ | (4.4 | ) | | $ | 84.6 |
|
OCI before reclassifications | 0.2 |
| | 11.0 |
| | — |
| | 11.2 |
|
Amounts reclassified from AOCI | 4.2 |
| | — |
| | (0.5 | ) | | 3.7 |
|
Other comprehensive loss | $ | 4.4 |
| | $ | 11.0 |
| | $ | (0.5 | ) | | $ | 14.9 |
|
Balance at June 29, 2019 | $ | (11.1 | ) | | $ | 115.5 |
| | $ | (4.9 | ) | | $ | 99.5 |
|
NOTE 14 – INCOME TAXES
The effective tax rates were as follows:
|
| | | | | | | | | | |
Three Months Ended | | Six Months Ended |
June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
66.6 | % | | 34.5 | % | | 32.5 | % | | 29.4 | % |
The effective tax rate for the three months ended June 29, 2019 increased compared to the prior year period due primarily to changes in the jurisdictional mix of pre-tax book income and a $27.8 million reduction in pre-tax income due to an impairment charge related to a definite-lived intangible asset.
The effective tax rate for the six months ended June 29, 2019 increased compared to the prior year period due primarily to changes in the jurisdictional mix of pre-tax book income.
On October 31, 2018, we received an audit finding letter from the Irish Office of the Revenue Commissioners (“Irish Revenue”) for the years ended December 31, 2012 and December 31, 2013. The audit finding letter relates to the tax treatment of the 2013 sale of the Tysabri® intellectual property and other assets related to Tysabri® to Biogen Idec from Elan Pharma. The consideration paid by Biogen to Elan Pharma took the form of an upfront payment and future contingent royalty payments. Irish Revenue issued a Notice of Amended Assessment (“NoA”) on November 29, 2018 which assesses an Irish corporation tax liability against Elan Pharma in the amount of €1,636 million, not including interest or any applicable penalties. We disagree with this assessment and believe that the NoA is without merit and incorrect as a matter of law. We filed an appeal of the NoA on December 27, 2018 and will pursue all available administrative and judicial avenues as may be necessary or appropriate. As part of this strategy to pursue all available administrative and judicial avenues, Elan Pharma was, on February 25, 2019, granted leave by the Irish High Court to seek judicial review of the issuance of the NoA by Irish Revenue. The judicial review filing is based on our belief that Elan Pharma's legitimate expectations as a taxpayer have been breached, not on the merits of the NoA itself. The High Court has scheduled a hearing in this judicial review proceeding in April 2020, and we would expect a decision in this matter in the second half of 2020. If we are ultimately successful in the judicial review proceedings the NoA will be invalidated and Irish Revenue will not be able to re-issue the NoA. The proceedings before the Tax Appeals Commission have been stayed until a decision on the judicial review application has been made.
On August 15, 2017, we filed a complaint in the United States District Court for the Western District of Michigan to recover $163.6 million of Federal income tax, penalties, and interest assessed and collected by the IRS, plus statutory interest thereon from the dates of payment, for the fiscal years ended June 27, 2009, June 26, 2010, June 25, 2011, and June 30, 2012 (the “2009 tax year,” “2010 tax year,” “2011 tax year,” and “2012 tax year,” respectively). The IRS audits of those years culminated in the issuances of two statutory notices of deficiency: (1) on August 27, 2014 for the 2009 and 2010 tax years and (2) on April 20, 2017 for the 2011 and 2012 tax years. The statutory notices of deficiency both included un-agreed income adjustments related principally to transfer pricing adjustments regarding the purchase, distribution, and sale of store-brand OTC pharmaceutical products in the
Perrigo Company plc - Item 1
Note 14
United States. In addition, the statutory notice of deficiency for the 2011 and 2012 tax years included the capitalization of certain expenses that were deducted when paid or incurred in defending against certain patent infringement lawsuits. We fully paid the assessed amounts of tax, interest, and penalties set forth in the statutory notices and filed timely claims for refund on June 11, 2015 and June 7, 2017 for the 2009-2010 tax years and 2011-2012 tax years, respectively. Our claims for refund were disallowed by certified letters dated August 18, 2015 and July 11, 2017, for the 2009-2010 tax years and 2011-2012 tax years, respectively. The complaint was timely, based upon the refund claim denials, and seeks refunds of tax, interest, and penalties of $37.2 million for the 2009 tax year, $61.5 million for the 2010 tax year, $40.2 million for the 2011 tax year, and $24.7 million for the 2012 tax year. The amounts sought in the complaint for the 2009 and 2010 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended March 28, 2015, and the amounts sought in the complaint for the 2011 and 2012 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended July 1, 2017. The cumulative deferred charge as recorded on the balance sheet is $29.7 million lower than the amounts reflected above due to overpayments credited to succeeding years, such that the actual refund the company is seeking to receive will be reduced by that amount. In addition, we recently conceded a royalty due to Perrigo U.S. on all omeprazole sales that equates to a 24% of our refund claims and any omeprazole adjustments that may be asserted by the IRS for future years.
On July 11, 2017, we received a draft Notice of Proposed Adjustment (“NOPA”) associated with transfer pricing positions for the IRS audit of Athena Neurosciences, Inc. (“Athena”), a subsidiary of Elan acquired in 1996, for the years ended December 31, 2011, December 31, 2012, and December 31, 2013. Athena was the originator of the patents associated with Tysabri® prior to the acquisition of Athena by Elan in 1996. The draft NOPA asserted that when Elan took over the future funding of Athena’s in-process R&D in 1996, after it acquired Athena in 1996, it should have paid a substantially higher royalty rate for the right to exploit Athena’s intellectual property, rather than rates based on transfer pricing documentation prepared by Elan's external tax advisors. In response to the draft NOPA, we provided the IRS with substantial additional documentation supporting our position. On April 26, 2019, we received a revised NOPA from the IRS regarding transfer pricing positions related to the IRS audit of Athena for the years ended December 31, 2011, 2012 and 2013. The NOPA carries forward the theory from a 2017 draft NOPA. The revised NOPA proposes a payment of $843.0 million, which represents additional tax and a 40.0% penalty. This amount excludes consideration of offsetting tax attributes and potentially material interest. We strongly disagree with the IRS position and will pursue all available administrative and judicial remedies, including potentially those available under the U.S. - Ireland Income Tax Treaty to alleviate double taxation. No payment of the additional amounts is required until the matter is resolved administratively, judicially, or through treaty negotiation.
On December 22, 2016, we received a notice of proposed adjustment for the IRS audit of Athena for the years ended December 31, 2011, December 31, 2012, and December 31, 2013. Perrigo acquired Elan in December 2013. This proposed adjustment relates to the deductibility of litigation costs. We disagree with the IRS’s position asserted in the notice of proposed adjustment and intend to contest it.
We have ongoing audits in multiple other jurisdictions the resolution of which remains uncertain. These jurisdictions include, but are not limited to, the United States, Ireland and other jurisdictions in Europe. In addition to the matters discussed above, the IRS is currently auditing our fiscal years ended June 29, 2013, June 28, 2014, and June 27, 2015 (which covers the period of the Elan transaction). The Israel Tax Authority's audit of our fiscal years ended June 29, 2013 and June 28, 2014 concluded with no material impact to the financial statements and the Israel Tax Authority is now auditing our fiscal years ended December 31, 2015, December 31, 2016 and December 31, 2017.
Perrigo Company plc - Item 1
Note 15
NOTE 15 – CONTINGENCIES
In view of the inherent difficulties of predicting the outcome of various types of legal proceedings, we cannot determine the ultimate resolution of the matters described below. We establish reserves for litigation and regulatory matters when losses associated with the claims become probable and the amounts can be reasonably estimated. The actual costs of resolving legal matters may be substantially higher or lower than the amounts reserved for those matters. For matters where the likelihood or extent of a loss is not probable or cannot be reasonably estimated as of June 29, 2019, we have not recorded a loss reserve. If certain of these matters are determined against us, there could be a material adverse effect on our financial condition, results of operations, or cash flows. We currently believe we have valid defenses to the claims in these lawsuits and intend to defend these lawsuits vigorously regardless of whether or not we have a loss reserve. Other than what is disclosed below, we do not expect the outcome of the litigation matters to which we are currently subject to, individually or in the aggregate, have a material adverse effect on our financial condition, results of operations, or cash flows.
Price-Fixing Lawsuits
We have been named as a co-defendant with certain other generic pharmaceutical manufacturers in a number of class actions alleging that we and other manufacturers of the same product engaged in anti-competitive behavior to fix or raise the prices of certain drugs and/or allocate customers starting, in some instances, as early as June 2013. The class actions were filed on behalf of putative classes of (a) direct purchasers, (b) end payors, and (c) indirect resellers. The products in question are Clobetasol gel, Desonide, and Econazole. The same class plaintiffs have filed complaints naming us as a co-defendant, along with 27 other manufacturers, alleging an overarching conspiracy to fix or raise the prices of 15 generic prescription pharmaceutical products starting in 2011. Perrigo manufactures only two of the products at issue, Nystatin cream and Nystatin ointment.
We have also been named a co-defendant along with 35 other manufacturers in a complaint filed by three supermarket chains alleging that defendants conspired to fix prices of 31 generic prescription pharmaceutical products starting in 2013. The only allegations specific to us relate to Clobetasol, Desonide, Econazole, Nystatin cream, and Nystatin ointment.
On August 3, 2018, a large managed care organization filed a complaint against us alleging price-fixing and customer allocation concerning 17 different products among 27 manufacturers including Perrigo. The only allegations specific to us concern Clobetasol gel, Desonide, Econazole, Nystatin cream, and Nystatin ointment.
Most recently, on January 16, 2019, a similar suit was brought by a health insurance carrier in the U.S. District Court for the District of Minnesota alleging a conspiracy to fix prices of 30 products among 30 defendants. The only allegations specific to us concern Clobetasol gel, Desonide, Econazole, Nystatin cream, and Nystatin ointment.
Certain complaints listed above were amended in December 2017, January 2018, and April 2019. All of the above complaints have been consolidated for pretrial proceedings, along with complaints filed against other companies alleging price fixing with respect to more than two dozen other drugs, as part of a case captioned In re Generic Pharmaceuticals Pricing Antitrust Litigation, MDL No. 2724 in the U.S. District Court for the Eastern District of Pennsylvania.
Pursuant to the court’s schedule staging various cases in phases, we moved to dismiss the complaints relating to Clobetasol and Econazole. The court issued a decision denying the motions in part in October 2018 and issued a second decision in February 2019 dismissing various state law claims, but allowing other state law claims to proceed. We filed answers to the Clobetasol gel complaints on December 31, 2018. We filed answers to the Desonide and Econazole complaints on March 15, 2019.
Motions to dismiss certain other complaints listed above were filed on February 21, 2019. Plaintiffs’ oppositions were due on May 2, 2019 and defendants’ replies were filed on June 13, 2019. Certain deposition discovery is allowed to proceed as of the court’s July 15, 2019 order in all cases and documentary discovery is also proceeding. At this stage, we cannot reasonably predict the outcome of the liability, if any, associated with these claims.
Perrigo Company plc - Item 1
Note 15
Securities Litigation
In the United States (cases related to events in 2015-2017)
On May 18, 2016, a shareholder filed a securities case against us and our former CEO, Joseph Papa, in the U.S. District Court for the District of New Jersey (Roofers’ Pension Fund v. Papa, et al.). The plaintiff purported to represent a class of shareholders for the period from April 21, 2015 through May 11, 2016, inclusive. The original complaint alleged violations of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against both defendants and 20(a) control person liability against Mr. Papa. In general, the allegations concerned the actions taken by us and the former executive to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015. The plaintiff also alleged that the defendants provided inadequate disclosure concerning alleged integration problems related to the Omega acquisition in the period from April 21, 2015 through May 11, 2016. On July 19, 2016, a different shareholder filed a securities class action against us and our former CEO, Joseph Papa, also in the District of New Jersey (Wilson v. Papa, et al.). The plaintiff purported to represent a class of persons who sold put options on our shares between April 21, 2015 and May 11, 2016. In general, the allegations and the claims were the same as those made in the original complaint filed in the Roofers' Pension Fund case described above. On December 8, 2016, the court consolidated the Roofers' Pension Fund case and the Wilson case under the Roofers' Pension Fund case number. In February 2017, the court selected the lead plaintiffs for the consolidated case and the lead counsel to the putative class. In March 2017, the court entered a scheduling order.
On June 21, 2017, the court-appointed lead plaintiffs filed an amended complaint that superseded the original complaints in the Roofers’ Pension Fund case and the Wilson case. In the amended complaint, the lead plaintiffs seek to represent three classes of shareholders - shareholders who purchased shares during the period April 21, 2015 through May 3, 2017 on the U.S. exchanges; shareholders who purchased shares during the same period on the Tel Aviv exchange; and shareholders who owned shares on November 12, 2015 and held such stock through at least 8:00 a.m. on November 13, 2015 (the final day of the Mylan tender offer) regardless of whether the shareholders tendered their shares. The amended complaint names as defendants us and 11 current or former directors and officers of Perrigo (Mses. Judy Brown, Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, and Messrs. Joe Papa, Marc Coucke, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, and Donal O’Connor). The amended complaint alleges violations of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against all defendants and 20(a) control person liability against the 11 individuals. In general, the allegations concern the actions taken by us and the former executives to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015 and the allegedly inadequate disclosure throughout the entire class period related to purported integration problems related to the Omega acquisition, alleges incorrect reporting of organic growth at the Company and at Omega, alleges price fixing activities with respect to six generic prescription pharmaceuticals, and alleges improper accounting for the Tysabri® royalty stream. The amended complaint does not include an estimate of damages. During 2017, the defendants filed motions to dismiss, which the plaintiffs opposed. On July 27, 2018, the court issued an opinion and order granting the defendants’ motions to dismiss in part and denying the motions to dismiss in part. The court dismissed without prejudice defendants Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, Donal O’Connor, and Marc Coucke. The court also dismissed without prejudice claims arising from the Tysabri® accounting issue described above and claims alleging incorrect disclosure of organic growth described above. The defendants who were not dismissed are Perrigo Company plc, Joe Papa, and Judy Brown. The claims (described above) that were not dismissed relate to the integration issues regarding the Omega acquisition and the alleged price fixing activities with respect to six generic prescription pharmaceuticals. The defendants who remain in the case (the Company, Mr. Papa, and Ms. Brown) have filed answers denying liability, and the discovery stage of litigation has begun. We intend to defend the lawsuit vigorously.
On November 1, 2017, Carmignac Gestion, S.A., filed a securities lawsuit against us and three individuals (former Chairman and CEO Joseph Papa, former CFO Judy Brown, and former Executive Vice President and Board member Marc Coucke). This lawsuit is not a securities class action. The case is styled Carmignac Gestion, S.A. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b-5), 14(e), and 18 against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiff’s allegations focus on events during the period from April 2015 through April 2016. Plaintiff contends that the defendants provided
Perrigo Company plc - Item 1
Note 15
inadequate disclosure throughout the period concerning the valuation and integration of Omega, the financial guidance provided by us during that period, our reporting about the generic prescription pharmaceutical business and its prospects, and the activities surrounding the efforts to defeat the Mylan tender offer during 2015. Many of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers’ Pension Fund case described above. The plaintiff does not provide an estimate of damages. After the court issued its July 2018 opinion in the Roofers’ Pension Fund case (described above) the parties to this case conferred about how this case should proceed. Because this plaintiff made some factual allegations that were not asserted in the Roofers’ Pension Fund case, the parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in this case and the remaining defendants (the Company, Mr. Papa, and Ms. Brown) filed a motion to dismiss addressing the additional allegations in this case. On July 31, 2019, the court granted the motion to dismiss in part and denied it in part. The case is now in the discovery phase. We intend to defend the lawsuit vigorously.
On January 16, 2018, Manning & Napier Advisors, LLC filed a securities lawsuit against us and three individuals (former Chairman and CEO Joseph Papa, former CFO Judy Brown, and former Executive Vice President and Board member Marc Coucke). This lawsuit is not a securities class action. The case is styled Manning & Napier Advisors, LLC v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b-5) and 18 against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiff’s allegations focus on events during the period from April 2015 through May 2017. Plaintiff contends that the defendants provided inadequate disclosure at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset. Many of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above. The plaintiff does not provide an estimate of damages. After the court issued its July 2018 opinion in the Roofers’ Pension Fund case (described above) the parties to this case conferred about how this case should proceed. Because this plaintiff made some factual allegations that were not asserted in the Roofers’ Pension Fund case, the parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in this case and the remaining defendants (the Company, Mr. Papa, and Ms. Brown) filed a motion to dismiss addressing the additional allegations in this case. On July 31, 2019, the court granted the motion to dismiss in part and denied it in part. The case is now in the discovery phase. We intend to defend the lawsuit vigorously.
On January 26, 2018, two different plaintiff groups (the Mason Capital group and the Pentwater group) each filed a lawsuit against us and the same individuals who are defendants in the amended complaint in the securities class action case described above (Roofers’ Pension Fund case). The same law firm represents these two plaintiff groups, and the two complaints are substantially similar. These two cases are not securities class actions. One case is styled Mason Capital L.P., et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The other case is styled Pentwater Equity Opportunities Master Fund Ltd., et al. v. Perrigo Company plc, et al., and also was filed in the U.S. District Court for the District of New Jersey. Both cases are assigned to the same federal judge that is hearing the class action case and the other individual cases described above (Carmignac and Manning & Napier). Each complaint asserts claims under Securities Exchange Act sections 14(e) (related to tender offer disclosures) against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiffs' allegations describe events during the period from April 2015 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer period in 2015 and point to disclosures at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset. Many of the factual allegations in these two cases overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above and the allegations in the Carmignac case described above. The plaintiffs do not provide an estimate of damages. After the court issued its July 2018 opinion in the Roofers’ Pension Fund case (described above), the parties to these cases conferred about how these cases should proceed. The parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in these cases. The defendants (the Company, Mr. Papa, and Ms. Brown) filed answers denying liability, and the discovery stage of the cases has begun. We intend to defend the lawsuits vigorously.
Perrigo Company plc - Item 1
Note 15
On February 13, 2018, a group of plaintiff investors affiliated with Harel Insurance Investments & Financial Services, Ltd. filed a lawsuit against us and the same individuals who are defendants in the amended complaint in the securities class action case described above (Roofers’ Pension Fund case). This lawsuit is not a securities class action. The new complaint is substantially similar to the amended complaint in the Roofers' Pension Fund case. The relevant period in the new complaint stretches from February 2014 to May 2, 2017. The complaint adds as defendants two individuals who served on our Board prior to 2016. The case is styled Harel Insurance Company, Ltd., et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey and is assigned to the same federal judge that is hearing the class action cases and the four other individual cases described above (Carmignac, Manning & Napier, Mason Capital, and Pentwater). The Harel Insurance Company complaint asserts claims under Securities Exchange Act section 10(b) (and related SEC Rule 10b‑5) and section 14(e) (related to tender offer disclosures) against all defendants as well as 20(a) control person liability against the individual defendants. The complaint also asserts claims based on Israeli securities laws. In general, the plaintiffs' allegations describe events during the period from February 2014 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer events in 2015 and point to disclosures at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset from February 2014 until the withdrawal of past financial statements in April 2017. Many of the factual allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above and the allegations in the four opt out cases also described above. The plaintiffs do not provide an estimate of damages. After the court issued its July 2018 opinion in the Roofers’ Pension Fund case (described above), the parties to this case conferred about how this case should proceed. The parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in this case and the remaining defendants (the Company, Mr. Papa, and Ms. Brown) filed answers denying liability, and the discovery stage of the litigation has begun. We intend to defend the lawsuit vigorously.
On February 16, 2018, First Manhattan Company filed a securities lawsuit against us and three individuals (former Chairman and CEO Joseph Papa, former CFO Judy Brown, and former Executive Vice President and Board member Marc Coucke). This lawsuit is not a securities class action. The case is styled First Manhattan Co. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The case was assigned to the same judge hearing the class action case and the five other opt out cases. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b-5), 14(e), and 18 against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiff’s allegations focus on events during the period from April 2015 through May 2017. Plaintiff contends that the defendants provided inadequate disclosure at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset. This lawsuit was filed by the same law firm that filed the Manning & Napier Advisors case and the Carmignac case described above and generally makes the same factual assertions as in the Manning & Napier Advisors case. Many of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above. The plaintiff does not provide an estimate of damages. On April 20, 2018, the plaintiff filed an amended complaint that did not materially change the factual allegations of the original complaint. After the court issued its July 2018 opinion in the Roofers’ Pension Fund case (described above), the parties to this case conferred about how this case should proceed. Because this plaintiff made some factual allegations that were not asserted in the Roofers’ Pension Fund case, the parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in this case and the remaining defendants filed a motion to dismiss addressing the additional allegations in this case. On July 31, 2019, the court granted the motion to dismiss in part and denied it in part. The case is now in the discovery phase. We intend to defend the lawsuit vigorously.
On April 20, 2018, a group of plaintiff investors affiliated with TIAA-CREF filed a lawsuit against us and the same individuals who are the defendants in the Harel Insurance case complaint. This lawsuit is not a securities class action. The law firm representing the plaintiffs in the Harel Insurance case also represents the TIAA-CREF plaintiff entities in this case, and the new complaint is substantially similar to the Harel Insurance complaint. The relevant period in the new complaint is August 14, 2014 to May 2, 2017 inclusive. The case is styled TIAA-CREF Investment Management, LLC., et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey and is assigned to the same federal judge that is hearing the class action case and the six other individual cases described above (Carmignac, Manning & Napier, Mason Capital, Pentwater, Harel Insurance,
Perrigo Company plc - Item 1
Note 15
and First Manhattan). The TIAA-CREF Investment Management complaint asserts claims under Securities Exchange Act section 10(b) (and related SEC Rule l0b-5), section 14(e) (related to tender offer disclosures) against all defendants as well as section 20(a) control person liability against the individual defendants. In general, plaintiffs' allegations describe events during the period from August 2014 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer events in 2015 and point to disclosures at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset from August 2014 until the withdrawal of past financial statements in April 2017. Many of the factual allegations in this case also overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above. The plaintiffs do not provide an estimate of damages. After the court issued its July 2018 opinion in the Roofers’ Pension Fund case (described above) the parties to this case conferred about how this case should proceed. The parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to this case and the remaining defendants (the Company, Mr. Papa, and Ms. Brown) filed answers denying liability, and the discovery stage of the litigation has begun. We intend to defend the lawsuit vigorously.
On October 29, 2018, Nationwide Mutual Funds and Nationwide Variable Insurance Trust (both on behalf of several fund series) filed a securities lawsuit against us and two individuals (former Chairman and CEO Joseph Papa and former CFO Judy Brown). This lawsuit is not a securities class action. The case is styled Nationwide Mutual Funds, et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The case was assigned to the same judge hearing the class action case and the seven other opt out cases. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b-5), and 14(e) against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiffs' allegations focus on events during the period from April 2015 through May 2017 (including the period of the Mylan tender offer). Plaintiffs contend that the defendants provided inadequate disclosure at various times during the period concerning the valuation and integration of Omega, the financial guidance provided by us during that period, and alleged price fixing activities with respect to six generic prescription pharmaceuticals. This lawsuit was filed by the same law firm that filed the First Manhattan case, the Manning & Napier Advisors case, and the Carmignac case described above and generally makes the same factual assertions as in the Manning & Napier case. The complaint does not include factual allegations that the Court dismissed in the July 2018 ruling in the Roofer’s Pension Fund case also described above. Many of the allegations in this case also overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above. The plaintiff does not provide an estimate of damages. The defendants (the Company, Mr. Papa, and Ms. Brown) filed a motion to dismiss addressing the additional allegations in this case. On July 31, 2019, the court granted the motion to dismiss in part and denied it in part. The case is now in the discovery phase. We intend to defend the lawsuit vigorously.
On November 15, 2018, a group of plaintiff investors affiliated with Westchester Capital Funds filed a lawsuit against us, our former Chairman and CEO Joseph Papa and our former CFO Judy Brown. This lawsuit is not a securities class action. The same law firm that represents the plaintiffs in the Mason Capital L.P. case and the Pentwater Equity Opportunities Master Fund Ltd. case (described above) represents the affiliates of the Westchester Funds in this lawsuit. The factual allegations of the complaint are substantially similar to the factual allegations of the complaints in the Mason Capital and in the Pentwater cases described above. The case is styled WCM Alternative: Event-Drive Fund, et al. v. Perrigo Co., plc, et al., and is filed in the U.S. District Court for the District of New Jersey. The WCM case is assigned to the same federal judge that is hearing the Roofer’s Fund class action case and the eight other individual cases described above. The complaint asserts claims under Securities Exchange Act sections 10(b) (and SEC Rule 10b‑5) and 14(e) against all defendants as well as 20(a) control person claims against the individual defendants. In general, the plaintiffs’ allegations describe events during the period from April 2015 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer period in 2015 as well us up through May 3, 2017. Plaintiffs identify disclosures concerning the valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset. Many of the factual allegations in this complaint overlap with the allegations of the June 2017 amended complaint in the Roofers’ Pension Fund case described above. The plaintiffs do not provide an estimate of damages. In view of the court’s July 2018 opinion in the Roofers’ Pension Fund case (described above), the parties to this case conferred about how this case should proceed. The parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in this case. The defendants (the Company, Mr.
Perrigo Company plc - Item 1
Note 15
Papa, and Ms. Brown) filed answers denying liability, and the discovery stage of the cases has begun. We intend to defend the lawsuit vigorously.
On November 15, 2018, a group of plaintiff investors affiliated with Hudson Bay Capital Management LP filed a lawsuit against us, our former Chairman and CEO Joseph Papa and our former CFO Judy Brown. This lawsuit is not a securities class action. The same law firm that represents the plaintiffs in the Mason Capital L.P., the Pentwater Equity Opportunities Master Fund Ltd., and the WCM cases (described above) represents the affiliates of Hudson Bay Capital Management in this lawsuit. The factual allegations of the complaint are substantially similar to the factual allegations of the complaints in the Mason Capital, in the Pentwater, and in the WCM cases described above. The case is styled Hudson Bay Master Fund Ltd., et al. v. Perrigo Co., plc, et al., and is filed in the U.S. District Court for the District of New Jersey. The Hudson Bay Fund case is assigned to the same federal judge that is hearing the Roofer’s Fund class action case and the nine other individual cases described above. The complaint asserts claims under Securities Exchange Act section 14(e) against all defendants and section 20(a) control person claims against the individual defendants. In general, the plaintiffs’ allegations describe events during the period from April 2015 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer period in 2015 and point to disclosures at various times during the period concerning the valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset. Many of the factual allegations in this complaint overlap with the allegations of the June 2017 amended complaint in the Roofers’ Pension Fund case described above. The plaintiffs do not provide an estimate of damages. In view of the court’s July 2018 opinion in the Roofers’ Pension Fund case (described above), the parties to this case conferred about how this case should proceed. The parties agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in this case. The defendants (the Company, Mr. Papa, and Ms. Brown) filed answers denying liability, and the discovery stage of the cases has begun. We intend to defend the lawsuit vigorously.
On January 31, 2019, Schwab Capital Trust and a variety of other Schwab entities filed a securities lawsuit against us and two individuals (former Chairman and CEO Joseph Papa and former CFO Judy Brown). This lawsuit is not a securities class action. The case is styled Schwab Capital Trust, et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The case was assigned to the same judge hearing the class action case and the ten other opt out cases. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b‑5), and 14(e) against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiffs’ allegations focus on events during the period from April 2015 through May 2017 (including the period of the Mylan tender offer). Plaintiffs contend that the defendants provided inadequate disclosure at various times during the period concerning the valuation and integration of Omega, the financial guidance provided by us during that period, and alleged price fixing activities with respect to six generic prescription pharmaceuticals. This lawsuit was filed by the same law firm that filed the Carmignac case, the Manning & Napier case, the First Manhattan case, and the Nationwide Mutual Funds case described above and generally makes the same factual assertions as in the Nationwide Mutual Funds case. The complaint does not include factual allegations that the court dismissed in the July 2018 ruling in the Roofer’s Pension Fund case also described above. Many of the allegations in this case also overlap with the allegations of the June 2017 amended complaint in the Roofer’s Pension Fund case described above. The plaintiff does not provide an estimate of damages. The parties have agreed that the defendants will not have to respond to the complaint until 45 days after the court decides the motion to dismiss pending in the Carmignac, Manning & Napier, First Manhattan, and Nationwide Mutual cases described above. On July 31, 2019, the court granted in part and denied in part the motion to dismiss in the Carmignac and related cases; therefore this case has now also moved into the discovery phase. We intend to defend the lawsuit vigorously.
On February 6, 2019, OZ Master Fund, Ltd. and a related entity filed a securities lawsuit against us and two individuals (former Chairman and CEO Joseph Papa and former CFO Judy Brown). This lawsuit is not a securities class action. The case is styled OZ Master Fund, Ltd., et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The case was assigned to the same judge hearing the class action case and the eleven other opt out cases described above. The complaint asserts claims under Securities Exchange Act sections 10(b) (and SEC Rule 10b‑5), and 14(e) against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiffs’ allegations focus on events during the period from April 2015 through May 2017 (including the period of the Mylan tender offer). Plaintiffs contend that the defendants provided inadequate disclosure at various times during the period concerning the valuation and integration of
Perrigo Company plc - Item 1
Note 15
Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset. Many of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofer’s Pension Fund case described above. The plaintiff does not provide an estimate of damages. The parties agreed that the court's rulings in July 2018 in the Roofer's case (discussed above) and in July 2019 in the Carmignac and other cases (discussed above) will apply to this case as well.The parties agreed to a proposed schedule, which the court approved in July 2019, by which the plaintiffs are participating in the discovery proceedings in the Roofer's Pension Fund case described above and the various individual cases also described above. We intend to defend the lawsuit vigorously.
On February 14, 2019, Highfields Capital I LP and related entities filed a securities lawsuit against the Company and two individuals (former Chairman and CEO Joseph Papa and former CFO Judy Brown). This lawsuit is not a securities class action. The case is styled Highfields Capital I LP, et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of Massachusetts. The complaint asserts claims under Securities Exchange Act sections 14(e) and 18 against all defendants, as well as 20(a) control person liability against the individual defendants. The complaint also asserts Massachusetts state law claims under Massachusetts Unfair Business Methods Law (chapter 93A § 11), and Massachusetts common law claims of tortious interference with prospective economic advantage, common law fraud, negligent misrepresentation, and unjust enrichment. In general, the plaintiffs’ allegations focus on events during the period from April 2015 through May 2017 (including the period of the Mylan tender offer). Plaintiffs contend that the defendants provided inadequate disclosure at various times during the period concerning the valuation and integration of Omega, the financial guidance provided by the Company during that period, and alleged improper accounting for the Tysabri® asset. Some of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofer’s Pension Fund case described above and with allegations in one or more of the opt out cases described above. Plaintiffs do not provide a clear calculation of how they estimated damages and seek treble damages, punitive damages, and attorney's fees. On May 7, 2019, defendants filed a motion to transfer this case to the U.S. District Court for the District of New Jersey so that the proceedings in this case can be coordinated with the other cases (discussed above) pending in that court. The transfer motion has been fully briefed. We intend to defend the lawsuit vigorously.
On February 22, 2019, Aberdeen Canada Funds -- Global Equity Funds (and 30 other entities, some unrelated to Aberdeen) filed a securities lawsuit against the Company and two individuals (former Chairman and CEO Joseph Papa and former CFO Judy Brown). This lawsuit is not a securities class action. The case is styled Aberdeen Canada Funds -- Global Equity Fund, et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The case was assigned to the same judge hearing the class action case and the twelve other opt-out cases pending in that Court. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b‑5) against all defendants and 20(a) control person liability against the individual defendants. In general, the plaintiffs’ allegations focus on events during the period from April 2015 through May 2017 (including the period of the Mylan tender offer). Plaintiffs contend that the defendants provided inadequate disclosure at various times during the period concerning the valuation and integration of Omega, the financial guidance provided by the Company during that period, and alleged undisclosed pricing pressure for generic prescription pharmaceuticals, and alleged price fixing activities with respect to six generic prescription pharmaceuticals. This lawsuit was filed by the same law firm that filed the Carmignac case, the Manning & Napier case, the First Manhattan case, the Nationwide Mutual Funds case, and the Schwab Capital Trust case described above, and generally makes the same factual assertions as in the Nationwide Mutual Funds case. The complaint does not include factual allegations that the Court dismissed in the July 2018 ruling in the Roofer’s Pension Fund case also described above. Many of the allegations in this case also overlap with the allegations of the June 2017 amended complaint in the Roofer’s Pension Fund case described above. The parties have agreed that the defendants will not have to respond to the complaint until 45 days after the court decides the motion to dismiss pending the Carmignac, Manning & Napier, First Manhattan, and Nationwide Mutual Funds cases described above. The plaintiff does not provide an estimate of damages. On July 31, 2019, the court granted in part and denied in part the motion to dismiss in the Carmignac and related cases; therefore this case has now also moved into the discovery phase. We intend to defend the lawsuit vigorously.
Perrigo Company plc - Item 1
Note 15
In Israel (cases related to events in 2015-2017)
Because our shares are traded on the Tel Aviv exchange under a dual trading arrangement, we are potentially subject to securities litigation in Israel. Three cases were filed; one was voluntarily dismissed in each of 2017 and 2018 and one was stayed in 2018. We are consulting Israeli counsel about our response to these allegations and we intend to defend this case vigorously.
On June 28, 2017, a plaintiff filed a complaint in Tel Aviv District Court styled Israel Elec. Corp. Employees’ Educ. Fund v. Perrigo Company plc, et al. The lead plaintiff seeks to represent a class of shareholders who purchased Perrigo stock on the Tel Aviv exchange during the period April 24, 2015 through May 3, 2017 and also a claim for those that owned shares on the final day of the Mylan tender offer (November 13, 2015). The amended complaint names as defendants the Company, Ernst & Young LLP (the Company’s auditor), and 11 current or former directors and officers of Perrigo (Mses. Judy Brown, Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, and Messrs. Joe Papa, Marc Coucke, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, and Donal O’Connor). The complaint alleges violations under U.S. securities laws of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against all defendants and 20(a) control person liability against the 11 individuals or, in the alternative, under Israeli securities laws. In general, the allegations concern the actions taken by us and our former executives to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015 and the allegedly inadequate disclosure concerning purported integration problems related to the Omega acquisition, alleges incorrect reporting of organic growth at the Company, alleges price fixing activities with respect to six generic prescription pharmaceuticals, and alleges improper accounting for the Tysabri® royalty stream. The plaintiff indicates an initial, preliminary class damages estimate of 2.7 billion NIS (approximately $760.0 million at 1 NIS = 0.28 cents). After the other two cases filed in Israel were voluntarily dismissed, the plaintiff in this case agreed to stay this case pending the outcome of the Roofers’ Pension Fund case in the U.S. (described above). The Israeli court approved the stay, and this case is now stayed. We intend to defend the lawsuit vigorously.
In the United States (cases related to Irish Tax events)
On January 3, 2019, a shareholder filed a complaint against the Company, our CEO Murray Kessler, and our former CFO Ronald Winowiecki in the U.S. District Court for the Southern District of New York (Masih v. Perrigo Company, et al.). Plaintiff purports to represent a class of shareholders for the period November 8, 2018 through December 20, 2018, inclusive. The complaint alleges violations of Securities Exchange Act section 10(b) (and Rule 10b‑5) against all defendants and section 20(a) control person liability against the individual defendants. In general the allegations contend that the Company, in its Form 10‑Q filed November 8, 2018, disclosed information about an October 31, 2018 audit finding letter received from Irish tax authorities but failed to disclose enough material information about that letter until December 20, 2018, when we filed a current report on Form 8‑K about Irish tax matters. The plaintiff does not provide an estimate of class damages. The Court selected lead plaintiffs and changed the name of the case to In re Perrigo Company plc Sec. Litig. The lead plaintiffs filed an amended complaint on April 12, 2019, which named the same defendants, asserted the same class period, and invoked the same Exchange Act sections. The amended complaint generally repeated the allegations of the original complaint with a few additional details and adds that the defendants also failed to timely disclose the Irish tax authorities’ Notice of Amended Assessment received on November 29, 2018. Defendants filed a motion to dismiss on May 3, 2019. On May 31, 2019, the plaintiffs filed a second amended complaint, which asserted a longer class period (March 1, 2018 through December 20, 2018) and added one additional individual defendant, former CEO Uwe Roehrhoff. In general, the second amended complaint contends that Perrigo’s disclosures about the Irish tax audit were inadequate beginning with Perrigo’s 10-K filed on March 1, 2018 through December 20, 2018 and repeats many of the allegations of the April 2019 amended complaint. The second amended complaint alleges violations of Securities Exchange Act section 10(b) (and SEC Rule 10b-5) against all defendants and section 20(a) control person liability against the three individual defendants. All defendants filed a joint motion to dismiss, and the plaintiffs filed an opposition. The defendants' reply is due in mid-August 2019. At some time thereafter the court will decide the motion. We intend to defend the lawsuit vigorously.
Perrigo Company plc - Item 1
Note 15
In Israel (cases related to Irish Tax events)
On December 31, 2018, a shareholder filed an action against the Company, our CEO Murray Kessler, and our former CFO Ronald Winowiecki in Tel Aviv District Court (Baton v. Perrigo Company plc, et. al.). The case is a securities class action brought in Israel making similar factual allegations for the same period as those asserted in the In re Perrigo Company plc Sec. Litig case in New York federal court. This case alleges that persons who invested through the Tel Aviv stock exchange can assert claims under Israeli securities law that will follow the liability principles of Sections 10(b) and 20(a) of the U.S. Securities Exchange Act. The plaintiff does not provide an estimate of class damages. We filed a request for a stay, the plaintiff agreed in part, and the court approved a stay and required the parties to update the court about the U.S. proceedings by September 1, 2019. We intend to defend the lawsuit vigorously.
Claim Arising from the Omega Acquisition
On December 16, 2016, we and Perrigo Ireland 2 brought an arbitral claim ("Claim") against Alychlo NV ("Alychlo") and Holdco I BE NV (together the "Sellers") in accordance with clause 26.2 of the Share Purchase Agreement dated November 6, 2014 ("SPA") and the rules of the Belgian Centre for Arbitration and Mediation ("CEPANI"). Our Claim relates to the accuracy and completeness of information about Omega provided by the Sellers as part of the sale process, the withholding of information by the Sellers during that process and breaches of Sellers’ warranties. We are seeking monetary damages from the Sellers. The Sellers served their respective responses to the Claim on February 20, 2017. In its response, Alychlo has asserted a counterclaim for monetary damages contending that we breached a warranty in the SPA and breached the duty of good faith in performing the SPA. Alychlo has recently filed papers seeking permission to introduce an additional counterclaim theory of recovery related to the Irish tax issue recently disclosed by the Company such that if the position of the Irish tax authorities prevails, Alychlo would have a further basis for its counterclaim against Perrigo. The proposed additional counterclaim theory does not appear to increase any damages sought. In June 2019, the Tribunal denied permission for Alychlo to introduce the additional counterclaim and dismissed certain aspects of the original Alychlo counterclaim. There can be no assurance that our Claim will be successful, and the Sellers deny liability for the Claim. To the extent that aspects of Alychlo’s counterclaim survived the Tribunal’s ruling in June 2019, we deny that Alychlo is entitled to any relief (including monetary relief). The arbitration proceedings are confidential as required by the SPA and the rules of the CEPANI.
Other Matters
Our Board of Directors received a shareholder demand letter dated October 30, 2018 relating to the allegations in the securities cases and price fixing lawsuits described above. The letter demands that the Board of Directors initiate an action against certain current and former executives and Board members to recover damages allegedly caused to the Company. In response, the Company reminded the shareholder that any derivative claim can only proceed in accordance with Irish law, the law that governs the Company’s internal affairs. The shareholder has responded that he intends to file a lawsuit asserting derivative claims but has not yet filed a lawsuit.
NOTE 16 – RESTRUCTURING CHARGES
We periodically take action to reduce redundant expenses and improve operating efficiencies. Restructuring activity includes severance, lease exit costs, and asset impairments. The following reflects our restructuring activity (in millions):
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
| June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Beginning balance | $ | 20.5 |
| | $ | 12.4 |
| | $ | 24.0 |
| | $ | 21.4 |
|
Additional charges | 12.2 |
| | 3.7 |
| | 18.1 |
| | 5.2 |
|
Payments | (9.0 | ) | | (3.1 | ) | | (18.0 | ) | | (13.8 | ) |
Non-cash adjustments | 0.3 |
| | (0.3 | ) | | (0.1 | ) | | (0.1 | ) |
Ending balance | $ | 24.0 |
| | $ | 12.7 |
| | $ | 24.0 |
| | $ | 12.7 |
|
Perrigo Company plc - Item 1
Note 16
The charges incurred during the three and six months ended June 29, 2019 were primarily associated with the reorganization of our executive management team and other actions taken to streamline the organization. Of the amount recorded during the six months ended June 29, 2019, $9.8 million related to the CSCI segment due primarily to the sales force reorganization in France. The charges incurred during the six months ended June 30, 2018 were primarily associated with continued costs from actions we took to streamline our organization as announced on February 21, 2017, as well as additional lease exit costs. All charges are recorded in Restructuring expense on the Condensed Consolidated Financial Statements. The remaining $24.0 million liability for employee severance benefits is expected to be paid within the next year.
NOTE 17 – SEGMENT INFORMATION
Our segments reflect the way in which our management makes operating decisions, allocates resources, and manages the growth and profitability of the Company.
The below tables show select financial measures by reporting segment (in millions):
|
| | | | | | | | |
| | Total Assets |
| | June 29,
2019 | | December 31,
2018 |
CSCA | | $ | 4,041.9 |
| | $ | 3,571.7 |
|
CSCI | | 4,593.5 |
| | 4,613.0 |
|
RX | | 2,793.3 |
| | 2,798.7 |
|
Total | | $ | 11,428.7 |
| | $ | 10,983.4 |
|
|
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended |
| June 29, 2019 | | June 30, 2018 |
| Net Sales | | Operating Income (Loss) | | Intangible Asset Amortization | | Net Sales | | Operating Income (Loss) | | Intangible Asset Amortization |
CSCA | $ | 582.1 |
| | $ | 107.8 |
| | $ | 9.3 |
| | $ | 596.9 |
| | $ | 64.6 |
| | $ | 15.3 |
|
CSCI | 327.5 |
| | (2.9 | ) | | 43.0 |
| | 357.9 |
| | 4.0 |
| | 49.2 |
|
RX | 239.4 |
| | 14.7 |
| | 21.3 |
| | 231.6 |
| | 53.6 |
| | 20.8 |
|
Unallocated | — |
| | (64.6 | ) | | — |
| | — |
| | (27.5 | ) | | — |
|
Total | $ | 1,149.0 |
| | $ | 55.0 |
| | $ | 73.6 |
| | $ | 1,186.4 |
| | $ | 94.7 |
| | $ | 85.3 |
|
|
| | | | | | | | | | | | | | | | | | | | | | | |
| Six Months Ended |
| June 29, 2019 | | June 30, 2018 |
| Net Sales | | Operating Income (Loss) | | Intangible Asset Amortization | | Net Sales | | Operating Income (Loss) | | Intangible Asset Amortization |
CSCA | $ | 1,163.9 |
| | $ | 202.0 |
| | $ | 19.3 |
| | $ | 1,198.5 |
| | $ | 183.2 |
| | $ | 30.5 |
|
CSCI | 678.3 |
| | 5.1 |
| | 87.1 |
| | 735.7 |
| | 16.3 |
| | 100.3 |
|
RX | 481.3 |
| | 75.3 |
| | 42.6 |
| | 469.2 |
| | 114.8 |
| | 41.6 |
|
Unallocated | — |
| | (125.1 | ) | | — |
| | — |
| | (63.4 | ) | | — |
|
Total | $ | 2,323.5 |
| | $ | 157.3 |
| | $ | 149.0 |
| | $ | 2,403.4 |
| | $ | 250.9 |
| | $ | 172.4 |
|
NOTE 18 – SUBSEQUENT EVENTS
Animal health business
On July 8, 2019, we completed the sale of our animal health business to PetIQ for base consideration of$185.0 million, which we estimate will result in a pre-tax gain of $80.0 million to $90.0 million. The final purchase price and gain is subject to customary post-closing adjustments for changes to working capital compared to the target working capital on the closing date and is expected to be finalized by the fourth quarter of 2019.
Generic product acquisition
On July 2, 2019, we purchased the ANDA for a generic gel product used for the treatment of Hemophilus vaginitis for $49.0 million in cash, which we capitalized as a developed product technology intangible asset. We plan to launch the product during the three months ended September 28, 2019 and begin amortizing it over a 20-year useful life. Operating results attributable to the product will be included within our RX segment.
Ranir Global Holdings, LLC
Perrigo Company plc - Item 1
Note 18
On July 1, 2019, we acquired 100% of the outstanding equity interest in Ranir, a privately-held company, for total base consideration of $750.0 million in a debt-free, cash-free transaction. We funded the transaction with cash on hand and borrowings under the 2018 Revolver (refer to Note 11).
Ranir is headquartered in Grand Rapids, Michigan, and is a leading global supplier of private label and branded oral care products. This transaction advances our transformation to a consumer-focused, self-care company while enhancing our position as a global leader in consumer self-care solutions. Ranir operations will be reported in our CSCA segment.
During the three and six months ended June 29, 2019, in connection with the acquisition, we incurred $2.2 million of general transaction costs (legal, banking and other professional fees). The amounts were recorded in Administration expenses and were not allocated to the CSCA segment.
We are in the process of gathering significant relevant information needed to complete the valuation for the assets acquired and liabilities assumed. As a result, the initial accounting for the acquisition accounting is incomplete. The provisional acquisition amounts recognized for assets acquired and liabilities assumed and the supplemental pro-forma information will be included in our quarterly Report on Form 10-Q for the third quarter of 2019.
ITEM 2. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
EXECUTIVE OVERVIEW
This Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the financial statements included in this Form 10-Q and our Form 10-K for the year ended December 31, 2018 (the “2018 Form 10-K”). These historical financial statements may not be indicative of our future performance. This discussion contains a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks referred to under “Risk Factors” in Item 1A of our 2018 Form 10-K and Part II. Item 1A of this Form 10-Q.
Perrigo Company plc was incorporated under the laws of Ireland on June 28, 2013 and became the successor registrant of Perrigo Company, a Michigan corporation, on December 18, 2013 in connection with the acquisition of Elan Corporation, plc ("Elan"). Unless the context requires otherwise, the terms "Perrigo," the "Company," "we," "our," "us," and similar pronouns used herein refer to Perrigo Company plc, its subsidiaries, and all predecessors of Perrigo Company plc and its subsidiaries.
We are dedicated to making lives better by bringing “Quality, Affordable Self-Care Products™” that consumers trust everywhere they are sold. We are a leading provider of over-the-counter ("OTC") health and wellness solutions that enhance individual well-being by empowering consumers to proactively prevent or treat conditions that can be self-managed. We are also a leading producer of generic prescription pharmaceutical topical products such as creams, lotions, gels, and nasal sprays.
Segment Reporting Change
During the three months ended March 30, 2019, we changed the composition of our operating and reporting segments. We moved our Israeli diagnostic business from the Consumer Self-Care International segment to the Prescription Pharmaceuticals segment and we made certain adjustments to our allocations between
Perrigo Company plc - Item 2
Executive Overview
segments. These changes were made to reflect changes in the way in which management makes operating decisions, allocates resources, and manages the growth and profitability of the Company. Financial information related to our business segments and geographic locations can be found in Item 1. Note 2 and Note 17. For results by segment, see "Segment Results" below.
Our new reporting and operating segments are as follows:
| |
• | Consumer Self-Care Americas ("CSCA"), formerly Consumer Healthcare Americas, comprises our consumer self-care business (OTC, contract manufacturing, infant formula and animal health categories) in the U.S., Mexico and Canada. |
| |
• | Consumer Self-Care International ("CSCI"), formerly Consumer Healthcare International, comprises our branded consumer self-care business primarily in Europe, our consumer-focused business in the United Kingdom and Australia, and our liquid licensed products business in the United Kingdom. |
| |
• | Prescription Pharmaceuticals ("RX") comprises our Prescription Pharmaceuticals business in the U.S. and our diagnostic business in Israel, which was previously in our CSCI segment. |
Highlights
| |
• | On August 9, 2018, we announced a plan to separate our RX business, which, when completed, will enable us to focus on expanding our consumer-facing businesses. We have made significant progress related to the preparations for separation, which may include a possible sale, spin-off, merger or other form of separation. While we remain committed to transforming to a consumer-focused business, we cannot commit to a specific date for the separation. In connection with the proposed separation, we anticipate incurring significant preparation costs, excluding restructuring expenses and transaction costs, in the range of $45.0 million to $80.0 million depending on the final structure of the transaction. |
| |
• | On July 1, 2019, we acquired 100% of the outstanding equity interest in Ranir Global Holdings, LLC (“Ranir”), a privately-held company, for total base consideration of $750.0 million in a debt-free, cash-free transaction, subject to post-closing adjustments for changes to working capital compared to the target working capital on the closing date. This transaction advances our transformation to a consumer-focused, self-care company while enhancing our position as a global leader in consumer self-care solutions (refer to Item 1. Note 18). |
RESULTS OF OPERATIONS
CONSOLIDATED
Recent Developments
Notice of Proposed Adjustment
On April 26, 2019, we received a revised Notice of Proposed Adjustment (“NOPA”) from the IRS regarding transfer pricing positions related to the IRS audit of Athena for the years ended December 31, 2011, 2012 and 2013. The NOPA carries forward the theory from a 2017 draft NOPA that when Elan took over the future funding of Athena’s in-process Research & Development ("R&D") in 1996, after it acquired Athena in 1996, it should have paid a substantially higher royalty rate for the right to exploit Athena’s intellectual property, rather than rates based on transfer pricing documentation prepared by Elan's external tax advisors. The NOPA proposes a payment of $843.0 million, which represents additional tax and a 40.0% penalty. This amount excludes consideration of offsetting tax attributes and potentially material interest. We strongly disagree with the IRS income position and will pursue all available administrative and judicial remedies, including potentially those available under the U.S. - Ireland Income Tax Treaty to alleviate double taxation. No payment of the additional amounts is required until the matter is resolved administratively, judicially, or through treaty negotiation. While we believe our position to be correct, there can be no assurance of an ultimate favorable outcome, and if the matter is resolved unfavorably it could have a material adverse impact on our liquidity and capital resources (refer to Item 1. Note 14).
Perrigo Company plc - Item 2
Consolidated
Consolidated Results
Three Month Comparison
|
| | | | | | | |
| Three Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 1,149.0 |
| | $ | 1,186.4 |
|
Gross profit | $ | 430.8 |
| | $ | 471.0 |
|
Gross profit % | 37.5 | % | | 39.7 | % |
Operating income | $ | 55.0 |
| | $ | 94.7 |
|
Operating income % | 4.8 | % | | 8.0 | % |
* Total net sales by geography is derived from the location of the entity that sells to a third party.
Operating income decreased $39.7 million, or 42%, over the prior year period due to:
| |
• | $37.4 million, or 3%, decrease in net sales over the prior year period due to: |
| |
• | $51.9 million decrease in sales of existing products due primarily to pricing pressure and decreased sales volumes of certain products; |
| |
• | $26.6 million decrease due to discontinued products; and |
| |
• | $23.8 million decrease due primarily to unfavorable European Euro foreign currency translation; partially offset by |
| |
• | $64.9 million increase due to new product sales. |
| |
• | $40.2 million decrease in gross profit, or a 220 basis point decrease in gross profit as a percentage of net sales, due primarily to the net sales decreases discussed above. |
| |
• | $0.5 million decrease in operating expenses due primarily to: |
| |
• | $50.0 million decrease in R&D expense due to the absence of an upfront license fee payment to enter into a license agreement with Merck Sharp & Dohme Corp ("Merck") in the prior year period; |
| |
• | $15.3 million decrease in selling expenses due primarily to the effect of European Euro favorable foreign currency translation; partially offset by |
| |
• | $30.4 million increase in administrative expenses due primarily to increased legal and consulting fees; |
| |
• | $27.8 million impairment charge related to a definite-lived intangible asset; |
| |
• | $8.4 million increase in restructuring expense due primarily to the reorganization of our sales force in France. |
Perrigo Company plc - Item 2
Consolidated
Six Month Comparison
|
| | | | | | | |
| Six Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 2,323.5 |
| | $ | 2,403.4 |
|
Gross profit | $ | 879.6 |
| | $ | 963.7 |
|
Gross profit % | 37.9 | % | | 40.1 | % |
Operating income | $ | 157.3 |
| | $ | 250.9 |
|
Operating income % | 6.8 | % | | 10.4 | % |
* Total net sales by geography is derived from the location of the entity that sells to a third party.
Operating income decreased $93.6 million, or 37%, over the prior year period due to:
| |
• | $79.9 million, or 3%, decrease in net sales over the prior year period due to: |
| |
• | $90.3 million decrease in sales of existing products due primarily to pricing pressure; |
| |
• | $59.6 million decrease due primarily to unfavorable European Euro foreign currency translation; and |
| |
• | $49.7 million decrease due to discontinued products; partially offset by |
| |
• | $119.7 million increase due to new product sales. |
| |
• | $84.1 million decrease in gross profit, or a 220 basis point decrease in gross profit as a percentage of net sales, due primarily to the decrease in net sales discussed above, increased commodity costs related to certain products and operating inefficiencies in the CSCA segment. |
| |
• | $9.5 million increase in operating expenses due primarily to: |
| |
• | $47.8 million increase in administrative expenses due primarily to increased legal and consulting fees; |
| |
• | $31.9 million of impairment charges related to a definite-lived intangible asset and an in-process R&D asset; and |
| |
• | $16.3 million increase in restructuring expense due primarily to the reorganization of our sales force in France; partially offset by |
| |
• | $50.0 million decrease in R&D expense due to the absence of an upfront license fee payment to enter into a license agreement with Merck in the prior year period; |
| |
• | $27.8 million decrease in selling expenses due primarily to the effect of European Euro favorable foreign currency translation; and |
| |
• | $9.4 million decrease in acquisition and integration-related charges and contingent consideration adjustments. |
Perrigo Company plc - Item 2
Consolidated
CONSUMER SELF-CARE AMERICAS
Recent Developments
| |
• | On July 8, 2019, we completed the sale of our animal health business to PetIQ for base consideration of$185.0 million, which we estimate will result in a pre-tax gain of $80.0 million to $90.0 million. The final purchase price and gain is subject to customary post-closing adjustments for changes to working capital compared to the target working capital on the closing date and is expected to be finalized by the fourth quarter of 2019 (refer to Item 1. Note 18). |
| |
• | On July 1, 2019, we acquired 100% of the outstanding equity interest in Ranir, a privately-held company, for total base consideration of $750.0 million in a debt-free, cash-free transaction, subject to post-closing adjustments for changes to working capital compared to the target working capital on the closing date. This transaction advances our transformation to a consumer-focused, self-care company while enhancing our position as a global leader in consumer self-care solutions (refer to Item 1. Note 18). |
| |
• | On April 1, 2019, we purchased product Abbreviated New Drug Application ("ANDA"s) and other records and registrations of Budesonide Nasal Spray, a generic equivalent of Rhinocort Allergy® and Triamcinolone Nasal Spray, a generic equivalent of Nasacort Allergy®, from Barr Laboratories, Inc., a subsidiary of Teva Pharmaceuticals, for $14.0 million in cash (refer to Item 1. Note 3). |
Segment Results
Three Month Comparison
|
| | | | | | | |
| Three Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 582.1 |
| | $ | 596.9 |
|
Gross profit | $ | 196.8 |
| | $ | 202.5 |
|
Gross profit % | 33.8 | % | | 33.9 | % |
Operating income | $ | 107.8 |
| | $ | 64.6 |
|
Operating income % | 18.5 | % | | 10.8 | % |
Three Months Ended June 29, 2019 vs. Three Months Ended June 30, 2018
Operating income increased $43.2 million, or 67%, over the prior year period due to:
| |
• | $14.8 million, or 3%, decrease in net sales over the prior year period due primarily to: |
| |
• | $11.8 million decrease in sales of existing products due primarily to: |
| |
▪ | Pricing pressure across all categories; and |
| |
▪ | Lower sales volume in our infant nutritionals and animal health categories; partially offset by |
| |
▪ | Higher sales volume in our cough/cold/allergy/sinus, gastrointestinal, and smoking cessation categories; and |
| |
• | $11.2 million decrease due to discontinued products; partially offset by |
| |
• | $8.0 million increase due primarily to the launches of various new antacids and smoking cessation products. |
| |
• | $5.7 million decrease in gross profit due primarily to the net sales decrease discussed above and certain operational inefficiencies. |
| |
• | $48.9 million decrease in operating expenses due primarily to the absence of a $50.0 million upfront license fee payment to enter into a license agreement with Merck in the prior year period in R&D. |
Perrigo Company plc - Item 2
CSCA
Six Month Comparison
|
| | | | | | | |
| Six Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 1,163.9 |
| | $ | 1,198.5 |
|
Gross profit | $ | 380.8 |
| | $ | 408.4 |
|
Gross profit % | 32.7 | % | | 34.1 | % |
Operating income | $ | 202.0 |
| | $ | 183.2 |
|
Operating income % | 17.4 | % | | 15.3 | % |
Six Months Ended June 29, 2019 vs. Six Months Ended June 30, 2018
Operating income increased $18.8 million, or 10%, over the prior year period as a result of:
| |
• | $34.6 million, or 3%, decrease in net sales over the prior year period due primarily to: |
| |
• | $27.2 million decrease in sales of existing products due primarily to: |
| |
▪ | Pricing pressure across all categories; and |
| |
▪ | Lower sales volume in our infant nutritionals and animal health categories; partially offset by |
| |
▪ | Higher sales volume in our cough/cold/allergy/sinus, gastrointestinal, and smoking cessation categories; and |
| |
• | $21.9 million decrease due to discontinued products; partially offset by |
| |
• | $15.0 million increase due primarily to the launches of various new antacids, cough/cold/allergy/sinus, and smoking cessation products. |
| |
• | $27.6 million decrease in gross profit, or a 140 basis point decrease in gross profit as a percentage of net sales, due primarily to the decrease in net sales discussed above, and increased commodity costs related to certain products in the cough/cold/allergy/sinus categories and operating inefficiencies related to infant formula production. |
| |
• | $46.4 million decrease in operating expenses due primarily to the absence of a $50.0 million upfront license fee payment to enter into a license agreement with Merck in R&D in the prior year period. |
CONSUMER SELF-CARE INTERNATIONAL
Recent Trends and Developments
| |
• | Management continues to implement its previously disclosed strategy for brand prioritization, sales force restructuring, and manufacturing insourcing, which is expected to reduce selling costs, improve operating margins and focus on higher value OTC products. As part of this strategy, we are making progress on the previously reported CSCI restructuring plan that we expect to improve our cost structure. |
Segment Results
Three Month Comparison
|
| | | | | | | |
| Three Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 327.5 |
| | $ | 357.9 |
|
Gross profit | $ | 155.4 |
| | $ | 173.2 |
|
Gross profit % | 47.4 | % | | 48.4 | % |
Operating income (loss) | $ | (2.9 | ) | | $ | 4.0 |
|
Operating income (loss) % | (0.9 | )% | | 1.1 | % |
Perrigo Company plc - Item 2
CSCI
Three Months Ended June 29, 2019 vs. Three Months Ended June 30, 2018
Operating income decreased $6.9 million, or 173%, over the prior year period due to:
| |
• | $30.4 million, or 9%, decrease in net sales over the prior year period due to: |
| |
• | $30.6 million decrease in sales of existing products due primarily to: |
| |
▪ | Lower net sales in France due to the reorganization of our sales force, which disrupted sales effectiveness and customer outreach; and |
| |
▪ | Lower net sales in the cough/cold/allergy/sinus and anti-parasites categories; partially offset by |
| |
▪ | Higher net sales in the distribution business and in analgesic products ; |
| |
• | $24.0 million decrease due to unfavorable European Euro foreign currency translation; and |
| |
• | $6.0 million decrease due to discontinued products; partially offset by |
| |
• | $30.2 million increase due primarily to the launches of XLS Forte 5 and ACO brands in the lifestyle and personal care and derma-therapeutic categories, respectively. |
| |
• | $17.8 million decrease in gross profit due primarily to the net sales decrease discussed above. |
| |
• | $10.9 million decrease in operating expenses due primarily to: |
| |
• | $18.5 million decrease in selling and administrative expense due to the effect of European Euro favorable foreign currency translation; partially offset by |
| |
• | $8.6 million increase in restructuring expense due primarily to the reorganization of our sales force in France. |
Six Month Comparison
|
| | | | | | | |
| Six Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 678.3 |
| | $ | 735.7 |
|
Gross profit | $ | 323.7 |
| | $ | 359.1 |
|
Gross profit % | 47.7 | % | | 48.8 | % |
Operating income | $ | 5.1 |
| | $ | 16.3 |
|
Operating income % | 0.8 | % | | 2.2 | % |
Six Months Ended June 29, 2019 vs. Six Months Ended June 30, 2018
Operating income decreased $11.2 million, or 69%, over the prior year period as a result of:
| |
• | $57.4 million, or 8%, decrease in net sales over the prior year period as a result of: |
| |
• | $57.7 million decrease due to unfavorable European Euro foreign currency translation; |
| |
• | $48.0 million decrease in sales of existing products due primarily to: |
| |
▪ | Lower net sales in the personal care and derma-therapeutics and lifestyle categories; partially offset by |
| |
▪ | Higher net sales in the distribution business; and |
| |
• | $8.0 million decrease due to discontinued products; partially offset by |
| |
• | $56.3 million increase due primarily to the launches of XLS Forte 5 and ACO brands in the lifestyle and personal care and derma-therapeutic categories, respectively. |
| |
• | $35.4 million decrease in gross profit, due primarily to the net sales decrease discussed above. |
| |
• | $24.2 million decrease in operating expenses due primarily to: |
| |
• | $31.6 million decrease in selling and administrative expense due to the effect of European Euro favorable foreign currency translation; partially offset by |
| |
• | $8.7 million increase in restructuring expense due primarily to the reorganization of our sales force in France. |
Perrigo Company plc - Item 2
CSCI
PRESCRIPTION PHARMACEUTICALS
Recent Trends and Developments
| |
• | Although pricing pressure is beginning to moderate, we continue to experience a year-over-year reduction in pricing in our RX segment due to competitive pressures. Similar to the first quarter of 2019, we experienced a year-over-year decrease in pricing pressure in the second quarter and expect softness in pricing to continue to impact the segment for the foreseeable future. |
| |
• | On May 17, 2019, we purchased the ANDA for a generic product used to relieve pain from osteoarthritis, for $15.7 million in cash, which we capitalized as a developed product technology intangible asset. We plan to launch the the product during the third quarter of 2019 (refer to Item 1. Note 3). |
| |
• | During the three months ended June 29, 2019, we identified impairment indicators for a certain definite-lived asset related to changes in pricing and competition in the market, which lowered the projected cash flows we expect to generate from the asset. We determined the asset was impaired by $27.8 million. Competition and other industry and market factors may contribute to future impairment charges or indications of impairment in the segment (refer to Item 1. Note 4). |
| |
• | On July 2, 2019, we purchased the ANDA for a generic gel product used for the treatment of Hemophilus vaginitis for $49.0 million in cash, which we capitalized as a developed product technology intangible asset. We plan to launch the product during the third quarter of 2019. |
Segment Results
Three Month Comparison
|
| | | | | | | |
| Three Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 239.4 |
| | $ | 231.6 |
|
Gross profit | $ | 78.6 |
| | $ | 95.3 |
|
Gross profit % | 32.8 | % | | 41.1 | % |
Operating income | $ | 14.7 |
| | $ | 53.6 |
|
Operating income % | 6.1 | % | | 23.1 | % |
Three Months Ended June 29, 2019 vs. Three Months Ended June 30, 2018
Operating income decreased $38.9 million, or 73%, over the prior year period due to:
| |
• | $7.8 million, or 3%, increase in net sales over the prior year period due primarily to: |
| |
• | $26.7 million increase due to new product sales; partially offset by |
| |
• | $9.4 million decrease in sales of existing products due primarily to pricing pressure and decreased sales volumes of certain existing products; and |
| |
• | $9.4 million decrease due to discontinued products. |
| |
• | $16.7 million decrease in gross profit, or a 830 basis point decrease in gross profit as a percentage of net sales, due primarily to pricing pressure and less favorable product mix. |
| |
• | $22.2 million increase in operating expenses due primarily to an impairment charge related to a definite-lived intangible assets of $27.8 million; partially offset by the absence of $4.7 million of acquisition and integration-related charges and contingent consideration adjustments. |
Perrigo Company plc - Item 2
RX
Six Month Comparison
|
| | | | | | | |
| Six Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 |
Net sales | $ | 481.3 |
| | $ | 469.2 |
|
Gross profit | $ | 175.1 |
| | $ | 196.2 |
|
Gross profit % | 36.4 | % | | 41.8 | % |
Operating income | $ | 75.3 |
| | $ | 114.8 |
|
Operating income % | 15.7 | % | | 24.5 | % |
Six Months Ended June 29, 2019 vs. Six Months Ended June 30, 2018
Operating income decreased $39.5 million, or 34%, over the prior year period as a result of:
| |
• | $12.1 million, or 3%, increase in net sales over the prior year period due to: |
| |
• | $48.4 million increase due to new product sales; partially offset by |
| |
• | $19.8 million decrease due to discontinued products; |
| |
• | $15.1 million decrease in sales of existing products due to pricing pressure; partially offset by increased sales volumes of certain products; and |
| |
• | $1.4 million decrease due to unfavorable Israeli Shekel foreign currency translation. |
| |
• | $21.1 million decrease in gross profit, or 540 basis points decrease of gross profit as a percentage of net sales, due primarily to pricing pressure and less favorable product mix. |
| |
• | $18.4 million increase in operating expenses due primarily to an impairment charge related to a definite-lived intangible asset of $27.8 million; partially offset by the absence of $8.7 million of acquisition and integration-related charges and contingent consideration adjustments. |
Unallocated Expenses
Unallocated expenses are comprised of certain corporate services not allocated to our reporting segments and are recorded in Operating income on the Condensed Consolidated Statements of Operations. Unallocated expenses were as follows (in millions):
|
| | | | | | | | | | | | | | |
Three Months Ended | | Six Months Ended |
June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
$ | 64.6 |
| | $ | 27.5 |
| | $ | 125.1 |
| | $ | 63.4 |
|
The increase of $37.1 million in unallocated expenses during the three months ended June 29, 2019 compared to the prior year period was due to a $23.5 million increase in legal and consulting fees and a $4.0 million increase in share-based compensation expense driven primarily by the on-boarding of new executives.
The increase of $61.7 million in unallocated expenses during the six months ended June 29, 2019 compared to the prior year period was due to a $45.9 million increase in legal and consulting fees, a $7.4 million increase due to the reorganization of our executive management team, and an increase in share-based compensation expense of $3.2 million driven by the on-boarding of new executives.
Perrigo Company plc - Item 2
Unallocated, Interest, Other, and Taxes
Change in Financial Assets, Interest expense, net, and Other (income) expense, net (Consolidated)
|
| | | | | | | | | | | | | | | |
| Three Months Ended | | Six Months Ended |
(in millions) | June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
Change in financial assets | $ | (5.5 | ) | | $ | (0.6 | ) | | $ | (15.9 | ) | | $ | 9.0 |
|
Interest expense, net | $ | 31.2 |
| | $ | 32.1 |
| | $ | 59.8 |
| | $ | 63.5 |
|
Other (income) expense, net | $ | 2.3 |
| | $ | 7.9 |
| | $ | 5.5 |
| | $ | 12.1 |
|
Change in Financial Assets
During the three and six months ended June 29, 2019, the fair value of the Royalty Pharma contingent milestone payments increased $5.5 million and $15.9 million, respectively. These increases were driven by higher projected global net sales of Tysabri® and the estimated probability of achieving the earn-out. The fair value of the Royalty Pharma contingent milestone payments increased by $0.6 million during the three months ended June 30, 2018. This increase included a $2.9 million decrease in the fair value of the 2018 contingent milestone payment, more than offset by a $3.5 million increase in the fair value of the 2020 contingent milestone payment. During the six months ended June 30, 2018, the fair value of the Royalty Pharma contingent milestone payments decreased $9.0 million. The net changes in the fair value of the contingent milestone payments were due to the fluctuation of the projected global net sales of Tysabri®, which were impacted by competition, namely the launch of Ocrevus® in the U.S. and European markets.
In order for us to receive the 2020 milestone payment of $400.0 million, Royalty Pharma contingent payments for Tysabri® sales in 2020 must exceed $351.0 million. In 2018, the Royalty Pharma contingent payments for Tysabri® were $337.5 million, which exceeded the threshold. If Royalty Pharma contingent payments for Tysabri® sales do not meet the prescribed threshold in 2020, we will write off the $89.1 million asset as an expense. If the prescribed threshold is exceeded, we will increase the asset to $400.0 million and recognize income of $310.9 million in Change in financial assets on the Condensed Consolidated Statements of Operations (refer to Item 1. Note 6).
Interest Expense, Net
Interest expense, net was $31.2 million and $59.8 million during the three and six months ended June 29, 2019, respectively, compared to $32.1 million and $63.5 million for the three and six months ended June 30, 2018, respectively. The $0.9 million and $3.7 million decreases for the three and six months ended June 29, 2019, respectively, were due primarily to changes in our underlying hedge exposure (refer to Item 1. Note 9).
Other (Income) Expense, Net
Other (income) expense, net was $2.3 million expense for the three months ended June 29, 2019 compared to $7.9 million expense for the three months ended June 30, 2018. The $5.6 million decrease was due primarily to the absence of a $6.3 million loss on investment securities in the prior year period (refer to Item 1. Note 7); partially offset by $1.0 million of favorable changes in revaluation of monetary assets and liabilities held in foreign currencies.
Other (income) expense, net was $5.5 million expense during the six months ended June 29, 2019 compared to $12.1 million expense for the six months ended June 30, 2018. The $6.6 million change was due primarily to the decrease of $3.7 million in losses on investment securities (refer to Item 1. Note 7) and $1.9 million of favorable changes in revaluation of monetary assets and liabilities held in foreign currencies.
Perrigo Company plc - Item 2
Unallocated, Interest, Other, and Taxes
Income Taxes (Consolidated)
The effective tax rates were as follows:
|
| | | | | | | | | | |
Three Months Ended | | Six Months Ended |
June 29,
2019 | | June 30,
2018 | | June 29,
2019 | | June 30,
2018 |
66.6 | % | | 34.5 | % | | 32.5 | % | | 29.4 | % |
The effective tax rate for the three months ended June 29, 2019 increased compared to the prior year period due primarily to changes in the jurisdictional mix of pre-tax book income and a $27.8 million reduction in pre-tax income due to an impairment charge related to a definite-lived intangible asset.
The effective tax rate for the six months ended June 29, 2019 increased compared to the prior year period due primarily to changes in the jurisdictional mix of pre-tax book income (refer to Item 1. Note 14 for more information on income taxes).
FINANCIAL CONDITION, LIQUIDITY, AND CAPITAL RESOURCES
We finance our operations with internally generated funds, supplemented by credit arrangements with third parties and capital market financing. We routinely monitor current and expected operational requirements and financial market conditions to evaluate other available financing sources including revolving bank credit and securities offerings. In determining our future capital requirements we regularly consider, among other factors, known trends and uncertainties, such as the Notice of Assessment ("NoA") and the Notice of Proposed Adjustment ("NOPA") and other contingencies. We note that no payment of the additional amounts assessed by Irish Revenue pursuant to the NoA or proposed by the IRS in the NOPA is currently required, and no such payment is expected to be required, unless and until a final determination of the matter is reached that is adverse to us, which could take several years in either case. Based on the foregoing, management believes that our operations and borrowing resources are sufficient to provide for our short-term and long-term capital requirements, as described below. However, we continue to evaluate the impact of the above factors on liquidity and may determine that modifications to our capital structure are appropriate if market conditions deteriorate, favorable capital market opportunities become available, or any change in conditions relating to the NoA, the NOPA or other contingencies has a material impact on our capital requirements.
Cash and Cash Equivalents
* Working capital represents current assets less current liabilities, excluding cash and cash equivalents, and current indebtedness.
Cash, cash equivalents, cash flows from operations, and borrowings available under our credit facilities are expected to be sufficient to finance our liquidity and capital expenditures in both the short and long term. Although our lenders have made commitments to make funds available to us in a timely fashion under our revolving credit agreements and overdraft facilities, if economic conditions worsen or new information becomes publicly available
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
impacting the institutions’ credit rating or capital ratios, these lenders may be unable or unwilling to lend money pursuant to our existing credit facilities. Should our outlook on liquidity requirements change substantially from current projections, we may seek additional sources of liquidity in the future.
Cash Generated by (Used in) Operating Activities
The $96.4 million decrease in operating cash inflow was due primarily to:
| |
• | Decreased net earnings after adjustments for items such as deferred income taxes, impairment charges, restructuring charges, changes in our financial assets, share-based compensation, amortization of debt premium, and depreciation and amortization; |
| |
• | Changes in accrued customer programs due primarily to pricing dynamics in our RX segment, as well as timing of rebate and chargeback payments; |
| |
• | Changes in accounts payable due primarily to the timing of payments, and mix of payment terms; and |
| |
• | Changes in accounts receivable due primarily to timing of shipments and receipt of payments in our CSCA and RX segments; partially offset by |
| |
• | Changes in payroll and related taxes due primarily to increase in employee-related expenses; |
| |
• | Changes in inventory due primarily to the build-up of inventory levels to support customer demands; and |
| |
• | Changes in accrued liabilities due primarily to the change in royalty and profit sharing accruals, and changes in legal, consulting and litigation accruals, partially offset by deferred revenue associated with BCH-Belgium distribution contracts and changes in our underlying hedge exposure. |
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
Cash Generated by (Used in) Investing Activities
The $191.2 million increase in investing cash inflow was due primarily to:
| |
• | $250.0 million increase from the receipt of the Royalty Pharma contingent milestone payment; |
| |
• | $7.5 million increase due to the absence of the investment in Zibo Xinhua - Perrigo Pharmaceutical Company Limited in the prior year period (refer to Item 1. Note 7); partially offset by |
| |
• | $35.0 million decrease due primarily to the acquisition of an ANDA for a generic product used to relieve pain from osteoartheritis for $15.7 million, and Budesonide Nasal Spray and Triamcinolone Nasal Spray for $14.0 million (refer to Item 1. Note 3); |
| |
• | $21.4 million increase in capital spending due primarily to increased tablet and infant formula capacity and quality/regulation projects; and |
| |
• | $8.6 million decrease in proceeds from royalty rights. |
Cash Generated by (Used in) Financing Activities
The $540.4 million decrease in financing cash outflow was due primarily to:
| |
• | $405.7 million increase in net borrowings of revolving credit agreements and other financing; |
| |
• | $298.4 million net decrease in payments on long-term debt; and |
| |
• | $265.0 million decrease in share repurchases; partially offset by |
| |
• | $431.0 million decrease in issuances of long-term debt. |
The declaration and payment of dividends, if any, is subject to the discretion of our Board of Directors and will depend on our earnings, financial condition, availability of distributable reserves, capital and surplus requirements, and other factors our Board of Directors may consider relevant.
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
In October 2018, our Board of Directors authorized up to $1.0 billion of share repurchases with no expiration date, subject to the Board of Directors’ approval of the pricing parameters and amount that may be repurchased under each specific share repurchase program.
Borrowings and Capital Resources
Revolving Credit Agreements
On March 8, 2018, we terminated the revolving credit agreement entered into in December 2014 and entered into a $1.0 billion revolving credit agreement maturing on March 8, 2023 (the "2018 Revolver"). There were $325.0 million of borrowings outstanding under the 2018 Revolver as of June 29, 2019. There were no borrowings outstanding under the 2018 Revolver as of December 31, 2018.
Term Loans and Notes
We had $2.8 billion outstanding under our notes and bonds as of both June 29, 2019 and December 31, 2018. We had $323.4 million and $351.3 million outstanding under our 2018 Term Loan as of June 29, 2019 and December 31, 2018, respectively.
On December 5, 2014, Perrigo Finance entered into a term loan agreement consisting of a €500.0 million ($614.3 million) tranche, maturing December 5, 2019. On March 8, 2018, we refinanced the €350.0 million outstanding under the term loan with the proceeds of a new €350.0 million ($431.0 million) term loan, maturing March 8, 2020 (the "2018 Term Loan"). During the six months ended June 29, 2019, we made $24.7 million in scheduled principal payments on the 2018 Term Loan.
In connection with the Omega acquisition, on March 30, 2015, we assumed a 5.000% retail bond due 2019 in the amount of €120.0 million ($130.7 million). On May 23, 2019 we repaid the bond in full.
Overdraft Facilities
We have overdraft facilities available that we use to support our cash management operations. The balance outstanding under the facilities was $73.9 million at June 29, 2019. There were no borrowings outstanding under the facilities as of December 31, 2018.
Leases
We had $154.0 million of lease liabilities and $152.5 million of lease assets as of June 29, 2019.
Accounts Receivable Factoring
The total amount factored on a non-recourse basis and excluded from accounts receivable was $12.8 million and $24.3 million at June 29, 2019 and December 31, 2018, respectively.
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
We are in compliance with all covenants under our debt agreements as of June 29, 2019 (refer to Item 1. Note 10 and Note 11 for more information on all of the above lease activity and debt facilities, respectively).
Credit Ratings
Our credit ratings on June 29, 2019 were Baa3 (stable) and BBB- (stable) by Moody's Investors Service and Standard and Poor's Rating Services, respectively.
Credit rating agencies review their ratings periodically and, therefore, the credit rating assigned to us by each agency may be subject to revision at any time. Accordingly, we are not able to predict whether current credit ratings will remain as disclosed above. Factors that can affect our credit ratings include changes in operating performance, the economic environment, our financial position, and changes in business strategy. If changes in our credit ratings were to occur, they could impact, among other things, future borrowing costs, access to capital markets, and vendor financing terms.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have a material current effect or that are reasonably likely to have a material future effect on our financial condition, changes in financial condition, net sales or expenses, results of operations, liquidity, capital expenditures, or capital resources. We acquire and collaborate on potential products still in development and enter into R&D arrangements with third parties that often require milestone payments to the third-party contingent upon the occurrence of certain future events linked to the success of the asset in development. Milestone payments may be required upon the successful achievement of an important point in the development life cycle of the product. Because of the contingent nature of these payments, they are not included in the table of contractual obligations included in our 2018 Form 10-K and referred to below.
Contractual Obligations and Commitments
There were no material changes in contractual obligations as of June 29, 2019 from those provided in our 2018 Form 10-K.
Critical Accounting Policies
The determination of certain amounts in our financial statements requires the use of estimates. These estimates are based upon our historical experiences combined with management’s understanding of current facts and circumstances. Although the estimates are considered reasonable based on the currently available information, actual results could differ from the estimates we have used. There have been no material changes to the critical accounting policies disclosed in our 2018 Form 10-K other than the hedging policies that we updated upon adoption of ASU 2017-12 (refer to Item 1. Note 9) and our leasing polices that we updated upon adoption of ASU 2016-02 (refer to Item 1. Note 10).
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
There have been no material changes to our quantitative or qualitative disclosures found in Item 7A, "Quantitative and Qualitative Disclosures about Market Risk," of our 2018 Form 10-K.
ITEM 4. CONTROLS AND PROCEDURES
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) or 15d-15(e) of the Exchange Act) as of June 29, 2019. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective in ensuring that all material information relating to us and our consolidated subsidiaries required to be included in our periodic SEC filings would be made known to them by others within those entities in a timely manner and that no changes are required at this time.
Perrigo Company plc - Item 4
Controls and Procedures
Evaluation of the Effectiveness of Internal Control over Financial Reporting
Our management assessed the effectiveness of our internal control over financial reporting as of June 29, 2019. The framework used in carrying out our evaluation was the 2013 Internal Control - Integrated Framework published by the Committee of Sponsoring Organizations ("COSO") of the Treadway Commission. In evaluating our information technology controls, we also used components of the framework contained in the Control Objectives for Information and related Technology, which was developed by the Information Systems Audit and Control Association’s IT Governance Institute, as a complement to the COSO internal control framework. Management has concluded that our internal control over financial reporting was effective as of June 29, 2019. The results of management’s assessment have been reviewed with our Audit Committee.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during the three months ended June 29, 2019 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
ITEM 1A. RISK FACTORS
Our Annual Report on Form 10-K for the year ended December 31, 2018 includes a detailed discussion of our risk factors. At the time of this filing, there have been no material changes to the risk factors that were included in the Form 10-K, other than as described below.
Management transition creates uncertainties, and any difficulties we experience in managing such transitions may negatively impact our business.
Over the last several years, we have experienced a number of changes in our executive leadership. Most recently, on March 20, 2019, we announced the appointment of Raymond P. Silcock as Chief Financial Officer and principal accounting officer. Mr. Silcock’s appointment followed the resignation of Ronald L. Winowiecki, who had held those roles since his appointment in February 2017. Changes in executive management create uncertainty. Moreover, changes in our company as a result of management transition could have a disruptive impact on our ability to implement, or result in changes to, our strategy and could negatively impact our business, financial condition and results of operations.
The resolution of uncertain tax positions could be unfavorable, which could have an adverse effect on our business.
Although we believe that our tax estimates are reasonable and that our tax filings are prepared in accordance with all applicable tax laws, the final determination with respect to any tax audit or any related litigation could be materially different from our estimates or from our historical income tax provisions and accruals. The results of an audit or litigation could have a material effect on operating results or cash flows in the periods for which that determination is made and in future periods after the determination. In addition, future period earnings may be adversely impacted by litigation costs, settlements, penalties or interest assessments.
We are currently involved in several audits and adjustment-related disputes, including litigation, with the IRS. These include litigation regarding our 2009, 2010, 2011, 2012, and 2013 tax years, as well as proposed audit adjustments related to litigation costs related to Athena Neurosciences, Inc. (“Athena”), a subsidiary of Elan acquired in 1996, for the 2011, 2012 and 2013 tax years.
In addition, on October 31, 2018, we received an audit finding letter from Irish Revenue for the years under audit 2012-2013. The audit finding letter relates to the tax treatment of the 2013 sale of the Tysabri® intellectual
Perrigo Company plc - Item 1A
Risk Factors
property and other assets related to Tysabri® to Biogen Idec from Elan Pharma. The consideration paid by Biogen to Elan Pharma took the form of an upfront payment and future contingent royalty payments. Irish Revenue issued a Notice of Assessment ("NoA") on November 29, 2018, which assesses an Irish corporation tax liability against Elan Pharma in the amount of €1,636.0 million, not including interest or any applicable penalties. We disagree with this assessment and believe that the NoA is without merit and incorrect as a matter of law. We filed an appeal of the NoA on December 27, 2018 and will pursue all available administrative and judicial avenues as may be necessary or appropriate. As part of this strategy to pursue all available administrative and judicial avenues, Elan Pharma was, on February 25, 2019, granted leave by the Irish High Court to seek judicial review of the issuance of the NoA. The judicial review filing is based on our belief that Elan Pharma's legitimate expectations as a taxpayer have been breached, not on the merits of the NoA itself. The High Court has scheduled a hearing in this judicial review proceeding in April 2020, and we would expect a decision in this matter in the second half of 2020. If Perrigo is ultimately successful in the judicial review proceedings, the NoA will be invalidated and Irish Revenue will not be able to re-issue the NoA. The proceedings before the Tax Appeals Commission have been stayed until a decision on the judicial review application has been made, which could take up to, or more than, a year. No payment of any amount related to this assessment is required to be made, if at all, until all applicable proceedings have been completed, which could take a number of years. However, while we believe our position to be correct, there can be no assurance of an ultimate favorable outcome, and if the matter is ultimately resolved unfavorably it would have a material adverse impact on us, including on liquidity and capital resources.
On April 26, 2019, we received a revised Notice of Proposed Adjustment (“NOPA”) from the IRS regarding transfer pricing positions related to the IRS audit of Athena for the years ended December 31, 2011, 2012 and 2013. The NOPA carries forward the theory from a 2017 draft NOPA that when Elan took over the future funding of Athena’s in-process R&D in 1996, after it acquired Athena in 1996, it should have paid a substantially higher royalty rate for the right to exploit Athena’s intellectual property, rather than rates based on transfer pricing documentation prepared by Elan's external tax advisors. The NOPA proposes a payment of $843.0 million, which represents additional tax and a 40.0% penalty. This amount excludes consideration of offsetting tax attributes and potentially material interest. We strongly disagree with the IRS income position and will pursue all available administrative and judicial remedies, including potentially those available under the U.S. - Ireland Income Tax Treaty to alleviate double taxation. No payment of the additional amounts is required until the matter is resolved administratively, judicially, or through treaty negotiation. While we believe our position to be correct, there can be no assurance of an ultimate favorable outcome, and if the matter is resolved unfavorably it could have a material adverse impact on our liquidity and capital resources.
In addition, going forward, uncertainty regarding the future outcome of tax disputes such as the NoA or NOPA may have an adverse impact on our financial condition and strategy, including our plan to separate our RX business.
At this time, we cannot predict the outcome of any audit or related litigation. Unfavorable developments in or resolutions of matters such as those discussed above could, individually or in the aggregate, have a material impact on our Condensed Consolidated Financial Statements in future periods (refer to Item 1, Note 14 for further information related to uncertain tax positions and ongoing tax audits and Item 1. Note 15 for further information related to legal proceedings).
Perrigo Company plc - Item 6
Exhibits
ITEM 6. EXHIBITS
|
| | |
Exhibit Number | | Description |
| | |
3.1 | | |
| | |
3.2 | | |
| | |
10.1 | |
|
| | |
10.2 | |
|
| | |
10.3 | | Stock Purchase Agreement and Agreement and Plan of Merger by and among Perrigo Oral Health Care Holdings, Inc., Perrigo Ireland 6 DAC, Big Mouth Merger Sub, LLC, Ranir Global Holdings, LLC, Camden Partners III SPV, L.P., RGH SELLER REP, LLC and Perrigo Company plc, effective as of May 8, 2019 (filed herewith).
|
| | |
10.4 | | |
| | |
31.1 | | |
| | |
31.2 | | |
| | |
32 | | |
| | |
101. INS | | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| | |
101.SCH | | XBRL Taxonomy Extension Schema Document. |
| | |
101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document. |
| | |
101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document. |
| | |
101.LAB | | XBRL Taxonomy Extension Label Linkbase Document. |
| | |
101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document. |
| | |
104 | | Cover Page Interactive Date File, formatted in Inline XBRL (contained in Exhibit 101). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
| | | |
| | | PERRIGO COMPANY PLC |
| | | (Registrant) |
| | | |
Date: | August 8, 2019 | | /s/ Murray S. Kessler |
| | | Murray S. Kessler |
| | | Chief Executive Officer and President |
| | | (Principal Executive Officer) |
| | | |
Date: | August 8, 2019 | | /s/ Raymond P. Silcock |
| | | Raymond P. Silcock |
| | | Chief Financial Officer |
| | | (Principal Accounting and Financial Officer) |