UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
_________________________________________
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
|
o
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
OR
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2013
|
OR
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from __________ to __________
|
OR
|
o
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report _____________
|
Commission file number [ ]
PACIFIC THERAPEUTICS LTD.
(Exact name of registrant as specified in its charter)
(Exact name of registrant as specified in its charter)
N/A
(Translation of registrant’s name into English)
(Translation of registrant’s name into English)
British Columbia, Canada
(Jurisdiction of incorporation or organization)
(Jurisdiction of incorporation or organization)
409 Granville Street, Suite #1500
Vancouver, BC, V6C-1T2 Canada
(Address of principal executive offices)
(Address of principal executive offices)
Tel: 604-738-1049
Fax: 604-738-1094
(Name, Telephone, E-Mail and/or Facsimile Number and Address of Company Contact Person)
(Name, Telephone, E-Mail and/or Facsimile Number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
Title of each class:
|
Name of each exchange on which registered:
|
|
|
Not Applicable
|
Not Applicable
|
i
Securities registered or to be registered pursuant to Section 12(g) of the Act:
37,456,825 CLASS A COMMON SHARES, NO PAR VALUE
Title of Class
Title of Class
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
NONE
Indicate the number of outstanding shares of each of the Company’s classes of capital or common stock as of the close of the period covered by the annual report: 37,456,825 Class A Common Shares, no par value, as of December 31, 2013.
Indicate by check mark if the registrant is a well-known seasoned Company, as defined in Rule 405 of the Securities Act.
YES o NO x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
YES o NO x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
YES x NO o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
YES o NO x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 in the Exchange Act. (Check one):
Large Accelerated Filer o Accelerated Filer o Non-Accelerated Filer x
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
o
|
U.S. GAAP
|
|
x
|
International Financial Reporting Standards as issued by the International Accounting Standards Board
|
|
o
|
Other
|
If "Other" has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow:
o Item 17 o Item 18
If this is an annual report indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act:
YES o NO x
ii
PACIFIC THERAPEUTICS LTD.
Table of Contents
|
Page
|
||
|
PART I
|
1
|
|
|
Item 1. Identity of Directors, Senior Management and Advisors
|
1
|
|
|
Item 2. Offer Statistics and Expected Timetable
|
1
|
|
|
Item 3. Key Information
|
1
|
|
|
Item 4. Information on the Company
|
15
|
|
|
Item 5. Operating and Financial Review
|
36
|
|
|
Item 6. Directors, Senior Management and Employees
|
42
|
|
|
Item 7. Major Shareholders and Related Party Transactions
|
51
|
|
|
Item 8. Financial Information
|
52
|
|
|
Item 9. The Offer and Listing
|
52
|
|
|
Item 10. Additional Information
|
54
|
|
|
Item 11. Quantitative and Qualitative Disclosures about Market Risk
|
63
|
|
|
Item 12. Description of Securities Other Than Equity Securities
|
63
|
|
|
PART II
|
63
|
|
|
Item 13. Defaults, Dividend Arrearages and Delinquencies
|
63
|
|
|
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
|
63
|
|
|
Item 15. Controls and Procedures
|
63
|
|
|
Item 16A. Audit Committee Financial Experts
|
63
|
|
|
Item 16B. Code of Ethics
|
63
|
|
|
Item 16C. Principal Accountant Fees and Services
|
63
|
|
|
Item 16D. Exemptions from the Listing Standards for Audit Committees
|
63
|
|
|
Item 16E. Purchases of Equity Securities by the Company and Affiliated Purchasers
|
64
|
|
|
Item 16F. Change in Registrant’s Certifying Accountant
|
64
|
|
|
Item 16G. Corporate Governance
|
64
|
|
|
PART III
|
64
|
|
|
Item 17. Financial Statements
|
64
|
|
|
Item 18. Financial Statements
|
64
|
|
|
Item 19. Exhibits
|
64
|
|
|
SIGNATURES
|
65
|
|
iii
Part I
Brief Introduction
Pacific Therapeutics Ltd. (the “Company”, “Issuer” or “PTL”) is a British Columbia, Canada corporation, incorporated on September 12, 2005. It is a reporting Company in British Columbia and Ontario, and its shares are listed for trading on the Canadian Securities Exchange (“CSE”). The shares are also quoted on the OTC QB Market beginning on April 16, 2014 and the Frankfurt Stock Exchange on September 16, 2013.
|
Item 1.
|
Identity of Directors, Senior Management and Advisers
|
|
A.
|
Directors and Senior Management.
|
The term of office of the Company’s directors expires annually at the time of the Company’s annual general meeting. The term of the office of the officers expires at the discretion of the Company’s directors. Below is a list of the Company’s directors and senior management and their business addresses:
|
Name
|
Title in the Company
|
Date of Appointment to Office
|
|
Douglas H. Unwin, B.Sc., MBA
1500 – 409 Granville Street
Vancouver, BC V7G 2S2
|
President, CEO, Director
|
September 12, 2005
|
|
Douglas Wallis, CA
7178 Quatsino Drive
Vancouver, BC V5S 4C3
|
Director
|
May 10, 2011
|
|
M. Greg Beniston, LLB
1802 – 1000 each Ave
Vancouver, BC V6E 4M2
|
Chairman
|
Chairman since October 31, 2007;
Corporate Secretary: from September 2005 to October 31, 2007
|
|
Wendi Rodrigueza, PhD
46701 Commerce Drive Rd
Plymouth, MI 48170
|
Director
|
November 5, 2009
|
|
Derick Sinclair, CA
1550 Ostler Court
North Vancouver, BC V7G 2P1
|
CFO and Corporate Secretary
|
Chief Financial Officer since September 1, 2007;
Corporate Secretary since October 31, 2007
|
Please also see Item 6 of this Form 20-F for more information about the directors and senior management.
|
B.
|
Advisers.
|
The Company’s outside legal counsel with regard to U.S. legal matters, exclusive of tax matters, is Hunter Taubman Weiss LLP, at 130 w. 42nd Street, Suite 1050, New York, NY 10036; Tel: (212) 732-7184.
|
C.
|
Auditors.
|
On February 3, 2014, the Board of Directors of the Company approved the termination of Leed Advisors Inc., Chartered Accountants (“Leed Advisors”) as auditors of the Company, effective February 11, 2014. Effective on the same date, the Company has appointed James Stafford, Chartered Accountants (“James Stafford”), as auditors of the Company.
The Audit Committee of the Board of Directors made the recommendation for the change of auditors and therefore has terminated Leed Advisors as auditor for the Company. Leed Advisors has not expressed any reservation in its reports for the two most recently completed fiscal years of the Company, nor for the period from the most recently completed period for which Leed Advisors issued an audit report in respect of the Company and the date of the termination. The resignation of Leed Advisors and appointment of James Stafford as auditors of the Company were both considered by the Audit Committee and approved by the Board of Directors of the Company. In the opinion of the Company, and the Board of Directors of the Company, there have been no “Reportable Events” as defined in NI 51-102 in connection with the audits of the two most recently completed financial years of the Company, nor any period from the most recently completed period for which Leed Advisors issued an audit report in respect of the Company and the date of the termination. The notice of change of auditors filed on Form 6-K on February 18, 2014, termination of appointment of Leed Advisors, and letters of the auditors have been reviewed by the Audit Committee and the Board of Directors of the Company.
James Stafford’s address is Suite 350, 1111 Melville Street, Vancouver, BC V6E 3V6; Tel (604) 669-0711.
|
Item 2.
|
Offer Statistics and Expected Timetable
|
Not Applicable
|
Item 3.
|
Key Information
|
|
A.
|
Selected financial data For the Years Ended December 31, 2013, 2012 and 2011
|
The following selected information should be read in conjunction with the Company’s financial statements, and notes, filed with this Form 20-F. This information, and all other financial information in this Form 20-F, is stated in Canadian dollars unless otherwise noted.
1
|
Item 3.
|
Key Information - continued
|
The financial information for the fiscal years ended (“FYE”) December 31, 2013, 2012 and 2011 are presented on the basis of International Financial Reporting Standards (“IFRS”). With respect to the Company’s financial statements, there are no material differences from applying these principles compared to applying United States Generally Accepted Accounting Principles (“US GAAP”).
|
Year ended
|
FYE 2013
(IFRS)
|
FYE 2012
(IFRS)
|
FYE 2011
(IFRS)
|
|||||||||
|
Net Sales
|
$ | Nil | $ | Nil | $ | Nil | ||||||
|
Net Loss and Comprehensive Loss
|
$ | (740,846 | ) | $ | (605,468 | ) | $ | (463,768 | ) | |||
|
Basic and diluted loss per share
|
$ | (0.03 | ) | $ | (0.03 | ) | $ | (0.03 | ) | |||
|
Weighted average shares
|
27,561,948 | 21,637,193 | 18,172,472 | |||||||||
|
Year ended
|
FYE 2013
(IFRS)
|
FYE 2012
(IFRS)
|
FYE 2011
(IFRS)
|
|||||||||
|
Total assets
|
$ | 287,044 | $ | 206,533 | $ | 422,178 | ||||||
|
Net assets
|
$ | (440,144 | ) | $ | (430,990 | ) | $ | (166,309 | ) | |||
|
Share capital and Subscriptions received
|
$ | 2,699,210 | $ | 2,025,716 | $ | 1,765,754 | ||||||
|
Contributed surplus
|
$ | 123,704 | $ | 206,212 | $ | 162,052 | ||||||
|
Deficit accumulated during the development stage
|
$ |
(3,263,058
|
) | $ | (2,662,918 | ) | $ | (2,094,115 | ) | |||
|
Common shares, issued
|
37,456,825 | 22,586,825 | 20,989,157 | |||||||||
|
Dividends
|
$ | Nil | $ | Nil | $ | Nil | ||||||
|
B.
|
Capitalization and indebtedness.
|
The following table sets forth our capitalization as of December 31, 2013 and December 31, 2012. This table should be read in conjunction with “Item 5. Operating and Financial Review and Prospects” and our financial statements and related notes included elsewhere in this Registration Statement on Form 20-F.
|
As of December 31, 2013
|
As of December 31, 2012
|
|||||||
|
Current Liabilities:
|
||||||||
|
Accounts payable, accruals and loans from shareholders
|
$ | 727,188 | $ | 637,523 | ||||
|
Non-Current Liabilities:
|
$ | Nil | $ | Nil | ||||
|
Equity:
|
||||||||
|
Common shares
|
$ | 2,699,210 | $ | 1,995,716 | ||||
|
Subscriptions received
|
$ | Nil | $ | 30,000 | ||||
|
Contributed surplus
|
$ |
123,704
|
$ | 206,212 | ||||
|
Accumulated deficit
|
$ | (3,263,058 | ) | $ | (2,662,918 | ) | ||
|
Net asset
|
$ | (440,144 | ) | $ | (430,990 | ) | ||
| $ | 287,044 | $ | 206,533 | |||||
|
C.
|
Reasons for the offer and use of proceeds.
|
Not Applicable.
2
|
Item 3.
|
Key Information - continued
|
|
D.
|
Forward-Looking Statement and Risk factors.
|
Forward-Looking Statements
This Form 20-F and the documents to which we refer you contain forward-looking statements. In addition, from time to time, we or our representatives may make forward-looking statements orally or in writing. We base these forward-looking statements on our expectations and projections about future events, which we derive from the information currently available to us. Such forward-looking statements relate to future events or our future performance. You can identify forward-looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “will,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential” or “continue” or the negative of these or similar terms. In evaluating these forward-looking statements, you should consider various factors, including those described in “Risk Factors” below. These and other factors may cause our actual results to differ materially from any forward-looking statement. Forward-looking statements are only predictions. The forward-looking events discussed in this Form 20-F, the documents to which we refer you and other statements made from time to time by us or our representatives, may not occur, and actual events and results may differ materially and are subject to risks, uncertainties and assumptions about us.
Risk Factors
An investment in the Common Shares of the Company must be considered highly speculative due to the nature of the Company’s business. The risk and uncertainties below are not the only risks and uncertainties the Company may have. Additional risks and uncertainties not presently known to the Company or that the Company currently considers immaterial may also impair the business, operations and future prospects of the Company and cause the price of the Common Shares to decline. If any of the following risks actually occur, the business of the Company may be harmed and its financial condition and results of operations may suffer significantly. In addition to the risks described elsewhere and the other information in this Form 20-F, the Company notes the following risk factors:
Issuer Risk - Risks that are specific to the Company
Insufficient Funds to Accomplish the Company's Business Objectives
The Company remains under constant working capital pressures. As of December 31, 2013, the Company had a working capital deficit of $502,500. As of December 31, 2013 the Company had cash and cash equivalents of $180,692, which is not sufficient to allow us to continue our operations through the next 12 months without additional financing. The Company is currently attempting to raise additional capital to continue operations. In the near future, potential revenues cannot support existing and upcoming expenses or other capital requirements. When the current funding has been expended, the Company will require and is planning for additional funding. There is no assurance that this funding will be available when required by the Company and/or available on suitable terms. Furthermore, the Company expects negative operating cash flows for the immediately foreseeable future.
Substantial Capital Requirements for Research and Development
The Company anticipates that it may make substantial research and development expenditures for clinical trials in the future. As the Company has no operating revenue being generated from its research and development activities, the Company does not expect to generate any revenue in the near future and may have limited ability to expend the capital necessary to undertake or complete future research and development work. There can be no assurance that debt or equity financing will be available or sufficient to meet these requirements or for other corporate purposes or, if debt or equity financing is available, that it will be on terms acceptable to the Company. Moreover, future activities may require the Company to alter its capitalization significantly. If the Company is unable to obtain additional financing, it may be unable to complete the development and commercialization of PTL-202, PTL-2015 and PTL-303 or continue its research and development programs.
Unanticipated Costs and Delays
The Company may be subject to unanticipated costs or delays that would accelerate its need for additional capital or increase the costs of individual clinical trials. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale back or discontinue the development and/or commercialization of one or more of its product candidates. The Company may also be required to:
3
|
Item 3.
|
Key Information - continued
|
|
•
|
seek collaborators for our product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; or
|
|
•
|
relinquish or license on unfavorable terms its rights to technologies or product candidates that it otherwise would seek to develop or commercialize itself.
|
Uncertainty of Additional Financing
The Company does not have the existing capital resources to fund operations to complete a pivotal bioequivalency study of PTL-202 providing information for future development studies or complete a pivotal bioequivalency study of PTL-2015 providing information for filing with a regulator for marketing authorization.
The United States Food and Drug Administration (the “FDA”) has defined bioequivalence as, "the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same dose under similar conditions in an appropriately designed study. In determining bioequivalence, for example, between two products such as a commercially-available Brand product and a potential to-be-marketed Generic product, studies are conducted whereby each of the preparations are administered in a study to volunteer subjects, generally healthy individuals but occasionally in patients. Serum/plasma samples are obtained at regular intervals and assayed for parent drug (or occasionally metabolite) concentration. For a pharmacokinetic comparison, the plasma concentration data are used to assess key pharmacokinetic parameters such as area under the curve (AUC), peak concentration (C max ), time to peak concentration (T max ), and absorption lag time (t lag ). The FDA considers two products bioequivalent if the 90% confidence interval of the relative mean C max , AUC (0-t) and AUC (0-∞) of the test (e.g. generic formulation) to reference (e.g. innovator brand formulation) should be within 80.00% to 125.00% in the fasting state.
At this time the Company has only nominal funds available and requires immediate funds to continue meaningful operations and conduct bio-equivalency studies of PTL-202 and PTL-2015. The Company anticipates that it will need to raise additional capital, through private placements or public offerings of its equity or debt securities, in addition to the capital on hand, to complete the long-term development and commercialization of its current product candidates. The inability of the Company to access sufficient additional capital for its operations could have a material adverse effect on the Company’s financial condition, results of operations or prospects. In particular, failure to obtain such financing on a timely basis could cause the Company to miss certain acquisition opportunities and reduce or terminate its business.
Dilution
To date the Company’s sources of cash have been limited primarily to proceeds from the founders, friends and retail investors. It is likely that the Company will enter into more agreements to issue Common Shares and warrants and options to purchase Common Shares.
The impact of the issuance of a significant amount of Common Shares from the exercise of the Company’s outstanding warrants and options could place downward pressure on the market price of the Common Shares.
The Company cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that the Company raises additional funds by issuing equity securities, its shareholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants, such as limitations on the Company’s ability to incur additional indebtedness, limitations on its ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact its ability to conduct business.
No History of Sales or Profits
The Company does not have a history of earnings or profit, has never had any products available for commercial sale and has not generated any revenue from product sales. The Company does not anticipate that it will generate revenue from the sale of products for the foreseeable future and has not yet submitted any products for approval by regulatory authorities. The Company continues to incur research and development and general and administrative expenses related to its operations. There is no assurance that in the future the Company will develop revenues, operate profitably or provide a return on investment. Therefore, investors should not invest on the expectation of receiving dividends or any guaranteed return on their investment of any nature. The Company is expected to continue to incur losses for the foreseeable future and expects these losses to increase as it continues research activities and development of its product candidates, seeks regulatory approvals for its product candidates, and acquires rights to additional products for development. If the Company’s product candidates fail in clinical trials or do not gain regulatory approval, or if its product candidates do not achieve market acceptance, the Company may never become profitable. Even if the Company achieves profitability in the future, it may not be able to sustain profitability in subsequent periods.
4
|
Item 3.
|
Key Information - continued
|
No History of Paying Dividends
An increase in the market price of the Company’s Common Shares, which is uncertain and unpredictable, may be an investor’s sole source of gain from an investment in the Company’s Common Shares. An investment in the Company’s Common Shares may not be appropriate for investors who require dividend income.
No dividends have been paid on the Company's Common Shares since inception and there is no assurance that such dividends will be earned or paid in the future. For the foreseeable future, the Company expects to re-invest in its operations all cash flow that might otherwise be available for distribution to shareholders in the form of cash dividends. While the payment of stock dividends is an alternative, there is no assurance that these will be paid in the foreseeable future. The Company does not anticipate paying any dividends on the Shares in the foreseeable future. As a result, capital appreciation, if any, of the Company’s Common Shares will be the shareholder’s sole source of gain for the foreseeable future.
Influence of Principal Shareholders
As at the date of this filing, Directors and Officers will own approximately 15.6% of the issued and outstanding Common Shares of the Company. As a result, these shareholders, together or individually will have the ability to control or influence the outcome of most corporate actions requiring shareholder approval, including the election of directors of the Company and the approval of certain corporate transactions. The concentration of ownership of the Company may also have the effect of delaying or preventing a change in control of the Company.
Commercializing of Drug Candidates
In order to successfully commercialize drugs, the Company must enter into collaborations with partners to develop a capable sales, marketing and distribution infrastructure. The Company intends to enter into partnering, co-promotion and other distribution arrangements to commercialize products in most markets. However, the Company may not be able to enter into collaborations on acceptable terms, if at all, and may face competition in its search for partners with whom to collaborate. If the Company is unable to develop collaborations with one or more partners to perform these functions, it may not be able to successfully commercialize its products, which could cause the Company to cease operations.
Dependence on the Success of PTL-202 and PTL-2015
PTL-202, the Company’s lead product candidate for fibrosis, has been tested in pre-clinical models of lung Fibrosis. These tests indicate that PTL-202 may be an effective drug to treat Pulmonary Fibrosis. PTL-202 was cleared by regulators to begin Phase 1 clinical trials during 2012. This drug/drug interaction trial was concluded in September 2012.
Potential barriers to the success of PTL-202 include the physical size of an ingestible tablet combining Pentoxifylline (“PTX”) and NAC (“N-acetylcysteine”) and the high water-solubility of both PTX and NAC.
If the PTL-202 pill were to contain the maximum daily dose of both PTX and NAC it would be very large and difficult to swallow. The dosage will likely be divided into multiple tablets to overcome this issue. The high water solubility may be overcome through the use of the VersaTab formulation in collaboration with Intelgenx. The technology used in this formulation is a trilayer tablet. The release of the pentoxifylline and NAC are both controlled by limitation of the surface area available for release and by using a mixture of insoluble and high viscous polymers, controlling the water solubility.
In order to market PTL-202 for a new indication such as fibrosis, the Company, in conjunction with its collaborators, will have to conduct additional clinical trials, including pivotal bioequivelency, Phase 2 proof-of-concept clinical trials as well as Phase 3 clinical trials, to demonstrate safety and efficacy. The Company has not initiated any Phase 2 or Phase 3 clinical trials with any of its product candidates. If the proposed clinical trials generate safety concerns or demonstrate a lack of efficacy, or competitive products developed by third parties show significant benefit in the indications in which the Company is developing product candidates, any planned clinical trial may be delayed, altered or not initiated and PTL-202 may never receive regulatory approval or be successfully commercialized.
5
|
Item 3.
|
Key Information - continued
|
The Company’s candidate for the treatment of erectile dysfunction is PTL-2015. The company has licensed this product from Globe Labs Inc. in Vancouver, BC. Pilot bioequivalence studies have been conducted by a previous licensee and the Company is looking to conduct a larger bioequivalency study to supply data for a potential marketing application with a regulatory agency.
The Company’s other product candidate, PTL-303, has only been tested in cellular assays to determine a signal as a possible drug candidate. PTL-303 has not been tested in animals or humans.
Even if the Company’s product candidates receive regulatory approval, the Company or its collaborators may not be successful in marketing them for a number of reasons, including the introduction by competitors of more clinically-effective or cost-effective alternatives or failure in the Company or collaborator’s sales and marketing efforts.
Any failure to obtain approval of PTL-202, PTL-2015 or PTL-303, and successfully commercialize them, would have a material and adverse impact on the Company’s business, which could cause the Company to cease operations.
Reliance on the Company’s management
Investors will in large part entrust their funds to the directors, management, and other professional advisors in whose judgment investors must depend with only limited information about their specific evaluation of the “sound business reasons” on which any reallocation of funds would be based. The Company's financing and enterprise acquisition/development policies and practices may be changed at the discretion of the Board of Directors. Persons who are not willing to rely on the Company’s management and/or Directors should not purchase the Company’s Shares.
Attraction and Retention of the Company’s Management
The Company will need to expand and effectively manage its managerial, operational, financial, development and other resources in order to successfully pursue its research, development and commercialization efforts of existing and future product candidates. The Company's success depends on its continued ability to attract, retain and motivate highly qualified management, pre-clinical and clinical personnel. The loss of the services of any of the Company’s senior management could delay or prevent the commercialization of its product candidates. Although the Company has entered into an employment agreement with Douglas H. Unwin, its Chief Executive Officer ("CEO"), the agreement permits Mr. Unwin to terminate his employment with the Company at any time, subject to providing the Company with advance written notice.
The Company may not be able to attract or retain qualified management and scientific personnel in the future due to the intense competition for qualified personnel among specialty pharmaceutical, biotechnology, pharmaceutical and other businesses. If the Company is not able to attract and retain the necessary personnel to accomplish its business objectives, the achievement of its development objectives, its ability to raise additional capital and its ability to implement its business strategy may be significantly reduced. In particular, if the Company loses any members of its senior management team, it may not be able to find suitable replacements in a timely fashion, or at all, and the business may be harmed as a result.
Use of Contract Personnel
From time to time the Company will need to contract additional personnel to continue its expansion. The Company uses scientific, clinical and regulatory advisors extensively to assist in formulating its development and clinical strategies. These advisors are not the Company’s employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to the Company. In addition, these advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with the Company’s. If the Company is unable to contract the correct personnel, it may be unable to implement or complete its product development programs, resulting in the inability to commercialize its product candidates or generate sufficient revenue to continue in business.
Dependence on key employees, suppliers or agreements
Executive management of the Company's business is primarily provided by the Company's CEO, Chief Financial Officer (“CFO”), and Board of Directors. At this stage of its corporate development, the Company has necessarily limited the establishment of extensive administrative and operating infrastructure. Instead, the Company may rely, for necessary skills, on external adviser/consultants with extensive senior level management experience in such fields as formulation, drug development, pharmaceutical regulations, finance, manufacturing, marketing, law, and investment. The future success of the Company is very dependent upon the ongoing availability and commitment of its directors, officers and advisor consultants, not all of whom are or will be bound by formal contractual employment agreements. The absence of these formal contractual relationships may be considered to represent an area of risk.
6
|
Item 3.
|
Key Information - continued
|
Dependence on Third Parties to Conduct Clinical Trials
The Company will hire third parties to conduct clinical trials. If these third parties do not perform as contracted or expected, the Company may not be able to obtain regulatory approval for its drug candidates, preventing the Company from becoming profitable.
The Company relies on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct its pre-clinical research and clinical trials. Although the Company relies on these third parties to conduct its clinical trials, it is responsible for ensuring that each of its clinical trials is conducted in accordance with its investigational plan and protocol, as approved by the FDA and non-U.S. regulatory authorities. Moreover, the FDA and non-U.S. regulatory authorities require the Company to comply with regulations and standards, commonly referred to as Good Clinical Practices (“GCPs”), for conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate and that the clinical trial subjects are adequately informed of the potential risks of participating in clinical trials.
The Company’s reliance on third parties does not relieve it of the above responsibilities and regulatory requirements. If the third parties conducting the Company’s clinical trials do not perform their contractual duties or obligations, do not meet expected deadlines or need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to GCPs or for any other reason, the Company may need to enter into new arrangements with alternative third parties, and its clinical trials may be extended, delayed or terminated. In addition, a failure by third parties to perform their obligations in compliance with GCPs may cause the Company’s clinical trials to fail to meet regulatory requirements, which may require the Company to repeat its clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, the Company may be unable to obtain regulatory approval for or commercialize its current and future product candidates.
Marketing and Distribution Risk
If the Company is unable to develop its sales and marketing and distribution capability on its own or through collaborations with marketing partners, it will not be successful in commercializing its product candidates. The Company currently does not have a marketing staff nor a sales or distribution organization. The Company does not intend to develop a sales or distribution organization internally.
The Company currently does not have marketing, sales or distribution capabilities. The Company has decided to collaborate with third parties that have direct sales forces and established distribution systems, either to augment or substitute in lieu of its own sales force and distribution systems. To the extent that the Company enters into co-promotion or other licensing arrangements, its product revenue is likely to be lower than if the Company directly marketed or sold its products, when and if it has any. In addition, any revenue received will depend in whole or in part upon the efforts of such third parties, which may not be successful and will generally not be within the Company’s control. If the Company is unable to enter into such arrangements on acceptable terms or at all, it may not be able to successfully commercialize its existing and future product candidates. If the Company is not successful in commercializing its existing and future product candidates, either on its own or through collaborations with one or more third parties, future product revenue will suffer and the Company may incur significant additional losses.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are and will remain through December 31, 2014, an “emerging growth company” within the meaning under the Jumpstart Our Business Startups Act of 2012 ("JOBS Act"), and until we cease to be an emerging growth company we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act (“SOA”), reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. Investors may find our common stock less attractive because we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
7
|
Item 3.
|
Key Information - continued
|
We will remain an emerging growth company until we lose such status. We could lose the status of emerging growth company at the earliest of (i) the last day of the first fiscal year in which we have total annual gross revenue of $1 billion or more, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act (which would occur if the market value of our common stock held by non-affiliates exceeds $700 million, measured as of the last business day of our most recently completed second fiscal quarter), or (iii) the date on which we have, during the preceding three year period, issued more than $1 billion in non-convertible debt.
Industry Risk - Risks faced by the Company because of the industry in which it operates
Research and Development
The Company is developing new, proprietary substances, methods and processes intended to enhance the therapeutic effects of existing drugs in the treatment of diseases characterized by progressive Fibrosis. The existing drugs that form the basis of the Company’s efforts to develop new substances, methods and processes are well known, yet any scientific evidence that may exist to support the feasibility of the Company’s goals is not conclusive. If the Company is not successful in developing and marketing any new drugs, combinations of existing drugs or reformulation and repurposing of existing approved drugs it may never generate revenues and the business may fail.
Clinical Trial Design
The Company’s business strategy is to combine, reformulate and repurpose existing drugs for the treatment of new indications, and these new drug combinations or formulations may have the ability to treat many indications. The Company may incorrectly assess the market opportunities of an indication or may incorrectly estimate or fail to fully appreciate the scientific and technological difficulties associated with treating a specific indication. Furthermore, the quality and robustness of the results and data of any clinical study the Company conducts will depend upon the selection of a patient population for clinical testing. If the selected population is not representative of the intended population, further clinical testing of product candidates or termination of research and development activities related to the selected indication may be required. The Company’s ability to commence clinical testing or the choice of clinical development path could compromise business prospects and prevent the achievement of revenue.
Product Failure in Clinical Trials
Clinical trials may fail to adequately demonstrate the safety and efficacy of product candidates. The Company will be required to demonstrate with substantial evidence through well-controlled clinical trials that its product candidates are safe and effective for use in a diverse population before the Company can seek regulatory approvals for their commercial sale. Negative results from clinical trials will prevent the commercialization of a drug candidate. If the Company cannot show that its product candidates are both safe and effective in clinical trials, it will need to re-evaluate its strategic plans.
Positive results from pre-clinical studies and early clinical trials should not be relied upon as evidence that later-stage or large-scale clinical trials will succeed. Success in early clinical trials does not mean that future clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate sufficient safety and efficacy to the satisfaction of the FDA and other non-U.S. regulatory authorities, despite having progressed through initial clinical trials.
Even after the completion of Phase 3 clinical trials, the FDA or other non-U.S. regulatory authorities may disagree with the Company’s clinical trial design and its interpretation of data, and may require the Company to conduct additional clinical trials to demonstrate the efficacy of its product candidates.
Regulatory Risk and Market Approval
Any products that the Company develops will be subject to extensive government regulations relating to development, clinical trials, manufacturing and commercialization. In the United States, for example, the drug combinations that the Company intends to develop and market are regulated by the FDA under its new drug development and review process. Before any therapeutic products can be marketed, the sponsor company must obtain clearance from the FDA by submitting an investigational new drug application, then by successfully completing human testing under three phases of clinical trials, and finally by submitting a new drug application.
The time required to obtain approvals for drug combinations or reformulations from the FDA and other agencies in foreign locales with similar processes is unpredictable. There is no assurance that the Company will ever receive regulatory approval to use its proprietary drug combinations or reformulations as human therapeutics. If such regulatory approval is not obtained, the Company may never become profitable.
8
|
Item 3.
|
Key Information - continued
|
Failure to Receive Regulatory Approval f or Clinical Trials
The Company’s clinical development programs for PTL-202, PTL-2015 and PTL-303 may not receive regulatory approval for clinical trials if the Company fails to demonstrate that they are safe and effective in pre-clinical trials. Consequently, failure to obtain necessary approvals from the FDA or similar non-U.S. regulatory agencies to operate clinical trials for the Company’s product candidates could result in delays to the Company’s product development efforts.
Manufacture and Supply of Drug Candidates
The Company does not own or operate manufacturing facilities, and it depends on third-party contract manufacturers for production of its product candidates. The Company has no experience in drug formulation or manufacturing, and it lacks the resources and the capability to manufacture any of its product candidates. Product candidates have been purchased in limited quantities for pre-clinical studies from scientific supply houses. For the Phase 1 clinical trial of PTL-202 supplies of existing approved products were purchased from distributors. For phase 2 clinical trials of PTL-202, the Company will need to obtain additional quantities of active pharmaceutical ingredients and have clinical supplies of the PTL-202 formulation manufactured. For the pilot trial of PTL-2015 a manufacturer was contracted to make both 25mg and 50mg oral dissolving tablets of sildenafil citrate. The Company will need to contract a manufacturer to supply the PTL-2015 for any additional trials. The Company will need to contract with a manufacturer for a supply of PTL-303 for pre-clinical, and Investigational New Drug-enabling toxicology studies and initial clinical trials (Phase 1 and 2). If, in the future, one of the Company’s product candidates is approved for commercial sale, the Company or its collaborator will need to manufacture that product candidate in commercial quantities. The Company cannot guarantee that the third-party manufacturers with which it has previously contracted will have sufficient capacity to satisfy future manufacturing needs of PTL-202, PTL-2015 or PTL-303, or that the Company will be able to negotiate additional purchases of active pharmaceutical ingredients or drug products from these or alternative manufacturers on terms favourable to the Company, or at all.
Third party manufacturers may fail to perform under their contractual obligations, or may fail to deliver the required commercial quantities of bulk active ingredients or finished product on a timely basis and at commercially reasonable prices. Any performance failure on the part of the Company’s contract manufacturers could delay clinical development or regulatory approval of the Company’s product candidates or commercialization of its future product candidates, depriving the Company of potential product revenue and resulting in additional losses.
If the Company is required to identify and qualify an alternate manufacturer, it may be forced to delay or suspend its clinical trials, regulatory submissions, required approvals or commercialization of its product candidates, which may cause it to incur higher costs and could prevent the successfully commercializing its product candidates. If the Company is unable to find one or more replacement manufacturers capable of production at a reasonably favourable cost, in enough volume, of adequate quality, and on a timely basis, the Company would likely be unable to meet demand for its product candidates and its clinical trials could be delayed or it could lose potential revenue. The Company’s ability to replace an existing active pharmaceutical ingredient manufacturer may be difficult because the number of potential manufacturers may be limited and the FDA or non-US regulator must approve any replacement manufacturer before it can begin manufacturing the Company’s product candidates. Such approval would require new testing and compliance inspections. It may be difficult or impossible for the Company to identify and engage a replacement manufacturer on acceptable terms in a timely manner, or at all.
The Company expects to continue to depend on third-party contract manufacturers for the foreseeable future. The Company’s product candidates require precise, high quality manufacturing. Any of the Company’s contract manufacturers will be subject to ongoing periodic unannounced inspection by the FDA and non-U.S. regulatory authorities to ensure strict compliance with current Good Manufacturing Practice (“CGMP”), and other applicable government regulations and corresponding standards. If the Company’s contract manufacturers fail to achieve and maintain high manufacturing standards in compliance with CGMP regulations, the Company may experience manufacturing errors resulting in patient injury or death, product recalls or withdrawals, delays or interruptions of production or failures in product testing or delivery, delay or prevention of filing or approval of marketing applications for the Company’s product candidates, cost overruns or other problems that could seriously harm its business.
Significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. Additionally, any third party manufacturers the Company retains to manufacture its product candidates on a commercial scale must pass an FDA or non-US regulator pre-approval inspection for conformance to the CGMPs before the Company can obtain approval of its product candidates. If the Company is unable to successfully increase the manufacturing capacity for a product candidate in conformance with CGMPs, the regulatory approval or commercial launch of any related products may be delayed or there may be a shortage in supply.
9
|
Item 3.
|
Key Information - continued
|
Market Acceptance of the Company’s Products
Even if the Company receives the necessary regulatory approvals to commercially sell its drug candidates, the success of these candidates will depend on their acceptance by physicians and patients, and reimbursement among other things.
In the United States and elsewhere, the Company’s product revenues will depend principally upon the reimbursement rates established by third-party payors, including government health administration authorities, managed-care providers, public health insurers, private health insurers and other organizations. These third-party payors are increasingly challenging the price, and examining the cost effectiveness, of medical products and services. In addition, significant uncertainty exists as to the reimbursement status, if any, of newly approved drugs, pharmaceutical products or product indications. The Company may need to conduct post-marketing clinical trials in order to demonstrate the cost-effectiveness of products. Such clinical trials may require the Company to commit a significant amount of management time, financial and other resources. If reimbursement of the Company’s product candidates is unavailable or limited in scope or amount or if pricing is set at unsatisfactory levels, the Company’s revenues could be reduced.
In some countries other than the United States, particularly the countries of the European Union and Canada, the pricing of prescription pharmaceuticals is subject to government controls. In these countries, obtaining pricing approval from government authorities can take six to twelve months or longer after the receipt of regulatory marketing approval of a product for an indication. To obtain reimbursement or pricing approval in some countries, the Company may be required to conduct a clinical trial that compares the cost-effectiveness of its product candidate to other available therapies. The Company’s revenues could be reduced if reimbursement of a product candidate is unavailable or limited in scope or amount or if pricing is set at unsatisfactory levels.
Canadian, US, European and other foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare, including drugs. In the United States, there have been, and the Company expects that there will continue to be, federal and state proposals to implement similar government control. In addition, increasing emphasis on managed care in the United States will continue to put pressure on the pricing of pharmaceutical products. For example, the Medicare Prescription Drug Improvement and Modernization Act (“MPDI & MA) of 2003 reforms the way Medicare will cover and reimburse for pharmaceutical products. The legislation expands Medicare coverage for drug purchases by the elderly and eventually will introduce a new reimbursement methodology based on average sales prices for certain drugs. In addition, the new legislation provides authority for limiting the number of outpatient drugs that will be covered in any therapeutic class. As a result of the new legislation and the expansion of federal coverage of drug products, the Company expects that there will be additional pressure to contain and reduce costs.
The Medicaid program and state healthcare laws and regulations in the US may also be modified to change the scope of covered products and/or reimbursement methodology. Cost control initiatives could decrease the established reimbursement rates that the Company receives for any products in the future, which would limit its revenues and profitability. Legislation and regulations affecting the pricing of pharmaceutical products, including PTL-202, PTL-2015 and PTL-303, may change at any time, which could further limit or eliminate reimbursement rates for the Company’s product candidates.
If the Company’s product candidates fail to gain market acceptance, it may be unable to generate sufficient revenue to continue in business.
Failure to Obtain Regulatory Approval Outside the United States
The Company intends to market certain of its existing and future product candidates in non-North American markets. In order to market its existing and future product candidates in the European Union and many other non-North American jurisdictions, the Company must obtain separate regulatory approvals. The Company has had no interactions with non-North American regulatory authorities, and the approval procedures vary among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA approval.
Approval by the FDA or other regulatory authorities does not ensure approval by regulatory authorities in other countries, and approval by one or more non-North American regulatory authorities does not ensure approval by regulatory authorities in other countries or by the FDA. The non-North American regulatory approval process may include all of the risks associated with obtaining FDA approval. The Company may not obtain non-North American regulatory approvals on a timely basis, if at all. In addition, the Company may not be able to file for non-North American regulatory approvals and may not receive necessary approvals to commercialize its existing and future product candidates in any market. If such regulatory approval is not obtained, the Company may never become profitable.
10
|
Item 3.
|
Key Information - continued
|
Product Liability
The use of the Company’s drug candidates in clinical trials and the sale of any products for which regulatory approval is obtained may expose the Company to product liability claims from consumers, health care providers, pharmaceutical companies or other entities. Any claim brought against the Company may cause the diversion of resources from normal operations or cause the Company to cease the sale, distribution and marketing of its products that have received regulatory approval. This may cause the Company to cease operations.
Intellectual Property Rights
The Company’s commercial success will depend, in part, on obtaining and maintaining patent protection, trade secret protection and regulatory protection of its proprietary technology and information as well as successfully defending third-party challenges to its proprietary technology and information. The Company will be able to protect its proprietary technology and information from use by third parties only to the extent that valid and enforceable patents, trade secrets or regulatory protection cover them and the Company has exclusive rights to utilize them. The ability of the Company’s licensors, collaborators and suppliers to maintain their patent rights against third-party challenges to their validity, scope or enforceability will also play an important role in determining the Company’s future.
Reliance on Licensors to Maintain Patent Rights
The Company’s commercial success may also depend, in part, on maintaining patent rights that have been licensed related to products that the Company may market in the future. Since the Company will not fully control the patent prosecution of any licensed patent applications, it is possible that the licensors will not devote the same resources or attention to the prosecution of the licensed patent applications as the Company would if it controlled the prosecution of the applications. The licensor may not pursue and successfully prosecute any potential patent infringement claim, may fail to maintain their patent applications, or may pursue any litigation less aggressively than the Company would. Consequently, the resulting patent protection, if any, may not be as strong or comprehensive.
Under the license agreement with Intelgenx, the Company has acquired from IntelGenx the intellectual property solely relating to PTL-202 and US Patent Application 2007/0190144 and all corresponding know-how, patents, patent applications, continuation applications, continuation-in-part applications, divisional applications, provisional applications, supplementary protection certificates, inventors’ certificates, any corresponding foreign patent applications to any of the foregoing, and all patents that may be granted or that may have been granted on any of the foregoing, including reissues, re-examination and extensions worldwide.
Under the agreement with Intelgenx, each party shall promptly notify the other party upon learning of any infringement allegation or licensed patent dispute. If IntelGenx or the Company determines that any licensed patent or patent right under the agreement is being infringed by a third party’s activities and that such infringement could affect the exercise of the license under the agreement, it will promptly notify the other party in writing. In addition, if IntelGenx or the Company determines that any licensed know-how is being misappropriated by a third party’s activities and that such misappropriation could affect the exercise of the license under this agreement, it will promptly notify the other party in writing.
If the Company decides to continue any further development of the product within 90 days of the completion of a pilot biostudy and delivery of the final report on the results of the pilot biostudy, the Company will have the sole, exclusive and first right but not the obligation to remove such infringement and/or misappropriation and to control all litigation to remove such infringement and/or misappropriation relating to the product in the field of use (as defined in the agreement), all as the Company shall deem appropriate in its sole discretion. IntelGenx shall provide notice to the Company of its decision to co-defend (at the Company’s cost) within sixty (60) calendar days from the date the relevant proceeding becomes known to IntelGenx. In the event the Company does, at its discretion, undertake any infringement or misappropriation action and IntelGenx does not co-defend, the Company will provide IntelGenx with copies of all relevant documentation so that IntelGenx will be informed of the continuing action and may comment upon such documentation. The Company shall not be permitted to settle any threatened, pending or completed or any claim, issue or matter therein, on behalf of IntelGenx, without the prior written consent of IntelGenx, such consent not to be unreasonably withheld, delayed or conditioned.
According to the agreement, IntelGenx has no obligation nor is it responsible, financially and/or otherwise, for the enforcement of the patent rights related to PTL-202.
11
|
Item 3.
|
Key Information - continued
|
Under the license agreement with Globe Labs Inc. (“Globe”), the Company has acquired from Globe the intellectual property solely relating to Globe’s oral dissolving technology, all corresponding know-how, patents, patent applications, continuation applications, continuation-in-part applications, divisional applications, provisional applications, supplementary protection certificates, inventors’ certificates, any corresponding foreign patent applications to any of the foregoing, and all patents that may be granted or that may have been granted on any of the foregoing, including reissues, re-examination and extensions worldwide.
Under the agreement with Globe, each party shall promptly notify the other party upon learning of any infringement allegation or licensed patent dispute. If Globe or the Company determines that any licensed patent or patent right under the agreement is being infringed by a third party’s activities and that such infringement could affect the exercise of the license under the agreement, it will promptly notify the other party in writing. In addition, if Globe or the Company determines that any licensed know-how is being misappropriated by a third party’s activities and that such misappropriation could affect the exercise of the license under this agreement, it will promptly notify the other party in writing.
If the Company decides to continue any further development of the product within 90 days of the completion of a pilot biostudy and delivery of the final report on the results of the pilot biostudy, the Company will have the sole, exclusive and first right but not the obligation to remove such infringement and/or misappropriation and to control all litigation to remove such infringement and/or misappropriation relating to the product in the field of use (as defined in the agreement), all as the Company shall deem appropriate in its sole discretion. Globe shall provide notice to the Company of its decision to co-defend (at the Company’s cost) within sixty (60) calendar days from the date the relevant proceeding becomes known to Globe. In the event the Company does, at its discretion, undertake any infringement or misappropriation action and Globe does not co-defend, the Company will provide Globe with copies of all relevant documentation so that Globe will be informed of the continuing action and may comment upon such documentation. The Company shall not be permitted to settle any threatened, pending or completed or any claim, issue or matter therein, on behalf of Globe, without the prior written consent of Globe, such consent not to be unreasonably withheld, delayed or conditioned.
According to the agreement, Globe has no obligation nor is it responsible, financially and/or otherwise, for the enforcement of the patent rights related to the oral dissolving technology.
Uncertainty of Patent Protection
The patent positions of life science companies including specialty pharmaceutical companies can be highly uncertain and involve complex legal and factual questions that include unresolved principles and issues. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States, and the patent situation outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of the Company’s intellectual property rights. Therefore, the Company cannot predict with any certainty the range of claims that may be allowed or enforced in its patents or in-licensed patents.
Reliance on Trade Secrets
The Company also relies on trade secrets to protect its technology, especially where the Company does not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. While the Company seeks to protect confidential information, in part, through confidentiality agreements with employees, consultants, contractors, or scientific and other advisors, they may unintentionally or willfully disclose the Company’s confidential information to competitors. Enforcing a claim against a third party related to the illegal acquisition and use of trade secrets can be expensive and time consuming, and the outcome is often unpredictable. If the Company is not able to maintain patent or trade secret protection on its technologies and product candidates, then the Company may not be able to exclude competitors from developing or marketing competing products, and the Company may not be able to operate profitability.
Intellectual Property Infringement Claims
There has been, and there will continue to be, significant litigation and demands for licenses in the life sciences industry regarding patent and other intellectual property rights. Although the Company anticipates having a valid defence to any allegation that its current product candidates, production methods and other activities infringe the valid and enforceable intellectual property rights of any third parties, the Company cannot be certain that a third party will not challenge this position in the future. Other parties may own patent rights that the Company might infringe with its drug candidates, products or other activities, and the Company’s competitors or other patent holders may assert that the Company’s products and the methods employed are covered by their patents. These parties could bring claims against the Company causing substantial litigation expenses and, if successful, may require payment of substantial damages. Some of the Company’s potential competitors may be better able to sustain the costs of complex patent litigation, and depending on the circumstances, the Company could be forced to stop or delay its research, development, manufacturing or sales activities. Any of these costs could cause the Company to go out of business.
12
|
Item 3.
|
Key Information - continued
|
Licensed Patent Rights
The Company has licensed patents and plans to license technologies and other patents if it believes it is necessary or useful to use third party intellectual property to develop its products, or if its product development threatens to infringe upon the intellectual property rights of third parties. The Company may be required to pay license fees or royalties or both to obtain such licenses, and there is no guarantee that such licenses will be available on acceptable terms, if at all. Even if the Company is able to successfully obtain a license, the rights may be non-exclusive, which would give the Company’s competitors’ access to the same intellectual property it has rights to, which could prevent the Company from commercializing a product.
Under the license agreement with Intelgenx the Company has acquired from IntelGenx the intellectual property solely relating to PTL-202 and US Patent Application 2007/0190144 and all corresponding know-how, patents, patent applications, continuation applications, continuation-in-part applications, divisional applications, provisional applications, supplementary protection certificates, inventors’ certificates, any corresponding foreign patent applications to any of the foregoing, and all patents that may be granted or that may have been granted on any of the foregoing, including reissues, re-examination and extensions worldwide.
The US Patent Application 2007/0190144 is a methods patent. This patent will protect the method of manufacturing the VersaTab formulation of PTL-202. Specifically, the Patent Application claims “A method of making a controlled release trilayer oral dosage form comprising: combining at least one pharmaceutically active compound and/or at least one nutritionally active compound with one or more polymers capable of forming a non-erodible core layer upon compression to form a core mixture, the resulting core combination optionally including at least one excipient and/or at least one adjuvant; preparing a release-regulating powder comprising at least one polymer selected from swellable erodible polymers, insoluble erodible polymers, and water permeable erodible polymers; and forming a compressed tablet having a core layer consisting of compressed core mixture and a release-regulating layer laminated to each side of the core layer, the release-regulating layer consisting of compressed release-regulating powder.”
The Company’s licensors may terminate the license. Without protection for the intellectual property that is licensed, other companies may be able to offer substantially similar products for sale and the Company may not be able to market or sell the planned products or generate any revenues.
Licenses and Permits to Operate
The operations of the Company may require licenses and permits from various governmental authorities, in both domestic and foreign jurisdictions. There can be no assurance that the Company will be able to obtain all necessary licenses and permits that may be required to carry out its research and development of its projects. Without these licenses and permits the Company may not be able to market or sell the planned products or generate any revenues.
Competition
The pharmaceutical industry is intensely competitive in all its phases, and the Company competes with other companies that have greater financial resource and technical facilities. Competition could adversely affect the Company’s ability to acquire suitable projects in the future.
Significant and increasing competition exists for pharmaceutical opportunities internationally. There are a number of large established pharmaceutical companies with substantial capabilities and far greater financial and technical resources than the Company. The Company may be unable to acquire additional attractive pharmaceutical development opportunities on terms it considers acceptable and there can be no assurance that the Company's research and development programs will yield any new drugs or result in any commercially viable compounds or technologies.
For more information, please refer to “Competitive Conditions” beginning at page 23.
13
|
Item 3.
|
Key Information - continued
|
Conflicts of Interest
Certain of the directors and officers of the Company will be engaged in, and will continue to engage in, other business activities on their own behalf and on behalf of other companies (including life science companies) and, as a result of these and other activities, such directors and officers may become subject to conflicts of interest. The British Columbia Business Corporation Act (“BCBCA”) provides that in the event that a director has a material interest in a contract or proposed contract or agreement that is material to the Company, the director shall disclose his interest in such contract or agreement and shall refrain from voting on any matter in respect of such contract or agreement, subject to and in accordance with the BCBCA. To the extent that conflicts of interest arise, such conflicts will be resolved in accordance with the provisions of the BCBCA. To the knowledge of the management of the Company, there are no existing or potential material conflicts of interest between the Company and a proposed director or officer of the Company except as otherwise disclosed herein.
Foreign Currency Risk
A substantial portion of the Company’s expenses and future revenues may be incurred in foreign currencies. The Company’s business will be subject to risks typical of an international business including, but not limited to, differing tax structures, regulations and restrictions and general foreign exchange rate volatility. Fluctuations in the exchange rate between the Canadian dollar and such other currencies may have a material effect on the Company’s business, financial condition and results of operations and could result in downward pressure for the Company’s products or in losses from currency exchange rate fluctuations. The Company does not actively hedge against foreign currency fluctuations.
Public Company Risk - Risks related to the Company’s shares being listed on a stock exchange
Price Volatility of Publicly Traded Securities
In recent years, the securities markets in the United States and Canada have experienced a high level of price and volume volatility, and the market prices of securities of many companies have experiences wide fluctuations in price which have not necessarily been related to the operating performance, underlying asset values or prospects of such companies. It may be anticipated that any quoted market for the Common Shares will be subject to market trends generally, notwithstanding any potential success of the Company in creating revenues, cash flows or earnings. The value of the Company’s Common Shares will be affected by such volatility.
An active public market for the Common Shares might not develop or be sustained. If an active public market for the Common Shares does not develop, the liquidity of a shareholder’s investment may be limited and the share price may decline below the initial price shareholders paid for their shares.
Certain Canadian Laws Could Delay or Deter a Change of Control
Limitations on the ability to acquire and hold the Company’s Common Shares may be imposed by the Competition Act (Canada). This legislation permits the Commissioner of Competition (Canada) to review any acquisition of a significant interest in the Company. This legislation grants the Commissioner jurisdiction to challenge such an acquisition before the Canadian Competition Tribunal if the Commissioner believes that it would, or would be likely to, result in a substantial lessening or prevention of competition in any market in Canada.
The Investment Canada Act (Canada) subjects an acquisition of control of a company by a non-Canadian to government review if the value of the Company’s assets as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to be a net benefit to Canada. The threshold amount for review if the buyer is a member of World Trade Organization (“WTO”) is $ 344 million for the calendar year 2013. For non-WTO investors, the threshold is $5 million for a direct acquisition and over $50 million for an indirect acquisition; the $5 million threshold will apply however for an indirect acquisition if the asset value of the Canadian business being acquired exceeds 50% of the asset value of the global transaction.
Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities for the Company’s shareholders to sell their Common Shares.
The Company is at Risk of Securities Class Action Litigation
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for the Company because biotechnology, specialty pharmaceutical and biopharmaceutical companies have experienced significant stock price volatility in recent years. If the Company faces such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm the Company’s business.
14
|
Item 4.
|
Information on the Company
|
|
A.
|
History and development of the Company.
|
Name and Incorporation
The Company was incorporated under the BCBCA on September 12, 2005 as “Pacific Therapeutics Ltd.”.
The head office of the Company is located at Suite 1500, 409 Granville Street, Vancouver, British Columbia, V6C 1T2, and the registered and records office of the Company is located at Suite 1500, 409 Granville Street, Vancouver, British Columbia, V6C 1T2. The Company’s phone number is (604) 738-1049 and its web site is www.pacifictherapeutics.com. The information on our website does not form a part of this Form 20-F.
The Company is a reporting Company in British Columbia and Ontario and its shares were listed for trading on the Canadian Securities Exchange on November 16, 2011. The Company’s shares were approved for quotation on the OTC QB Market on April 16, 2014 and Frankfurt Stock Exchange on September 16, 2013.
Emerging Growth Company
We are and will remain through December 31, 2014, an “emerging growth company” within the meaning under the JOBS Act. For as long as we are an emerging growth company, we will not be required to comply with certain requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the SOA, the reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and the exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We intend to take advantage of these reporting exemptions until we are no longer an emerging growth company. For a description of the qualifications and other requirements applicable to emerging growth companies and certain elections that we have made due to our status as an emerging growth company, see “Risk Factors—Risks Related to this Offering and our Common Stock—We are an ‘emerging growth company’ and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors” in this Form 20-F.
Inter-Corporate Relationships
The Company does not have any inter-corporate relationships.
Significant Acquisitions and Dispositions
Other than the licensing of a technology for oral disintegration, the Company has not completed any acquisitions or dispositions since its date of incorporation and is not currently in negotiations with respect to any potential material acquisitions or dispositions.
Operating and Financial Review and Prospects
From inception through to December 31, 2013, the Company incurred total expenses in the development of its intellectual property of $1,715,075, which includes $554,712 of research and development expenses (research and development expenses on the financial statements have been offset by $53,277 in funding from the Industrial Research Assistance Program (“IRAP”) and $193,935 in Scientific Research and Experimental Development ("SR&ED") tax credits), $122,677 of professional fees and $1,047,686 of wages and benefits.
|
B.
|
Business overview
|
Business Strategy
The Company is focused on combing, reformulating and repurposing approved drugs. It is initially developing drugs for erectile dysfunction and diseases of progressive excessive scarring, including Idiopathic Pulmonary Fibrosis (“IPF”), Liver Cirrhosis, Pulmonary Fibrosis associated with Scleroderma and Post Lung Transplant Bronchiolitis Obliterans. The Company assumes the clinical, regulatory and commercial development activities of its product candidates and advances them through the regulatory and clinical pathways toward commercial approval. This strategy reduces the risk, time and cost of developing therapies for erectile dysfunction and fibrosis by avoiding the risks associated with basic research and using compounds with unknown safety and toxicity. The Company leverages its expertise to manage and perform critical steps in drug development including the design and conduct of clinical trials, the development and execution of intellectual property strategies, the recruitment and selection of development partners and the interaction with drug regulatory authorities.
15
|
Item 4.
|
Information on the Company - continued
|
The Company is focused on combing, reformulating and repurposing approved drugs. It is initially developing drugs for erectile dysfunction and diseases of progressive excessive scarring, including Idiopathic Pulmonary Fibrosis (“IPF”), Liver Cirrhosis, Pulmonary Fibrosis associated with Scleroderma and Post Lung Transplant Bronchiolitis Obliterans. The Company assumes the clinical, regulatory and commercial development activities of its product candidates and advances them through the regulatory and clinical pathways toward commercial approval. This strategy reduces the risk, time and cost of developing therapies for erectile dysfunction and fibrosis by avoiding the risks associated with basic research and using compounds with unknown safety and toxicity. The Company leverages its expertise to manage and perform critical steps in drug development including the design and conduct of clinical trials, the development and execution of intellectual property strategies, the recruitment and selection of development partners and the interaction with drug regulatory authorities.
The main elements of the Company’s strategy are as follows:
1) Identification of Product Candidates
The Company performs scientific evaluations and market assessments of drugs and drug combinations and research from academics and other drug development companies. As part of this process, the Company will evaluate the clinical and pre-clinical research and the intellectual property rights associated with the potential products and research to determine the commercial potential of the product candidate.
The Company intends to mitigate the risks associated with development and commercialization of drug candidates by targeting drug candidates that:
• are combinations of already approved compounds;
• have well established safety records;
• have potential to be reformulated to a once a day oral dose;
• have potential to be reformulated to an oral dissolving technology;
• are already marketed in countries other than the United States or Europe; and
• have pre-clinical animal data or clinical data of potential efficacy in Fibrosis additional indications.
The Company has initially focused on fibrosis indications as it believes there is a large unmet medical need in this area. As an example, IPF affects more than 130,000 Americans (IPF Summit 2011). An estimated 48,000 cases of IPF are diagnosed annually (Am J Respir Crit Care Med. 2006;174(7):810-816). In addition, patients with IPF have poor prognosis; an estimated 40,000 people die each year from IPF.
The Company has developed the Scientific and Advisory Board (“SAB”) to support this strategy. The members of both of this group are very experienced in the clinical development of drug candidates for Pulmonary Fibrosis, lung transplant, airway disease and Scleroderma. In the future, the Company may develop product candidates for other indications but the current strategy is to leverage the expertise and skills the Company has in Fibrosis, particularly IPF.
The Company has continued its development with the in-licensing of an oral dissolving tablet (sublingual delivery) technology. The first compound to be incorporated into this technology is sildenafil citrate to treat erectile dysfunction. In 2011 the total market for drugs to treat erectile dysfunction exceeded $5 billion.
2) In-licensing
In identifying a promising product candidate, the Company seeks to negotiate a license to the rights for the candidate from the holder of those rights. Typically the goal is to secure licenses that permit the Company to conduct further research, development and clinical trials as well as engage in additional intellectual property protection. The Company will also seek terms that provide it with the rights to further licensing of manufacturing and marketing rights to any resulting products. This process is known as in-licensing.
3) Product Development
Upon securing the appropriate rights to the product candidate, the Company will advance the candidate through the regulatory and commercialization pathways for marketing approval in major markets. This process includes implementing intellectual property strategies, formulation and reformulation strategies, making regulatory submissions, conducting or managing clinical trials, and performing or managing the collection, collation and interpretation of clinical and field data and the submission of this data to relevant regulatory authorities.
16
|
Item 4.
|
Information on the Company - continued
|
4) Partnering
To enhance its capabilities to develop and market its product candidates, the Company may enter into agreements or partnerships with companies that have formulation, drug development, sales or marketing expertise, or all of the above. Entering into such an agreement may provide cash to develop other products or advance other products in the Company’s portfolio. In addition, entering into a partnership with a company that has complementary skills and using that company’s expertise to further accelerate development of its product candidates, may enhance the returns to the Company from the product candidate.
5) Outsourcing
In order to optimize return on investment and the development of product candidates, the Company uses a virtual company business model which includes outsourcing all non-core business activities. Factors that the Company considers to determine core and non-core activities include:
• Infrastructure cost
• Operating cost
• Frequency of use
• Regulatory protocol
• Requirement for third party verification
• Capacity
• Quality control
Management has determined that having its own laboratory and staff for conducting infrequent pre-clinical studies is not a core capacity that is required and, therefore, it has to develop relationships with labs that it may outsource this work to. In order to maintain quality control, these projects are managed very closely by the Company’s staff and the Company develops all protocols for the completion of this work. Other functions the Company has decided to outsource include analytical assay development, formulation, clinical trials and manufacturing. It is currently more cost-effective to outsource these tasks due to the Company’s sporadic requirements. As these requirements become less sporadic the Company may develop internal capabilities to complete currently outsourced tasks.
6) Principal Products
|
•
|
PTL-202
|
The Company’s lead product candidate for fibrosis, PTL-202, is a combination of drugs that have been separately approved for marketing by the FDA for sale in the United States. In animal trials, the combination was more effective than either of its components at reducing indicators of Fibrosis. The Company is planning to complete development of a once a day pill of the combination using proprietary in licensed technologies. The Company conducted a drug/drug interaction study in 2012 and plans to conduct a bioequivalence study in 2013 with PTL-202 in humans.
The Company found a combination of two compounds developed in France that demonstrated an ability to improve radiation induced fibrosis. The potential efficacy of this combination of Pentoxifylline and Vitamin E to improve further Fibrosis in humans was demonstrated in two separate independent proofs of concept Phase 2 clinical trials in Radiation Induced Fibrosis. The following table describes the clinical trials and results.
|
Location
|
France
|
Tehran, Iran
|
|
Dates
|
December 1998 – April 2000
|
June 2002 to
April 2003
|
|
Trial Sponsor
|
De'le'gation a' la Recherche Clinique of
Assistance Publique des Hoˆpitaux de Paris, Paris, France.
|
Tehran University of Medical Science
|
|
Number of Patients Treated
|
24
|
37
|
|
End Points
|
Relative regression of measurable
RIF surface after 6 months of treatment
|
Mean surface area of fibrotic lesion
|
|
Statistical Results
|
Mean RIF surface regression was
significant with combined PTX/Vit E versus double placebo
(60% +-10% v 43% +- 17%; P=.038).
|
Mean surface area of the lesions decreased from 112 to 65 cm2 after treatment (P<0.001)
|
17
|
Item 4.
|
Information on the Company - continued
|
To the Company’s knowledge, neither the FDA nor any comparable foreign regulatory body has approved the combined compounds of Pentoxifylline and Vitamin E for sale as a treatment for a specific disease.
The Company took one of the compounds, Pentoxifylline, and combined it with a powerful antioxidant N-Acetylcysteine and then conducted experiments in a mouse. These experiments showed that the new combination was effective at reducing the progression of the Fibrosis in the mouse lung. This new combination is being developed as the Company’s lead drug candidate, PTL-202. A provisional patent was filed in the United States by the Company in October 2007 to cover the composition of matter and method of use of this combination. In October 2008, the Company filed an application under the Patent Cooperation Treaty (“PCT”) based on the above provisional application. The Company received a positive preliminary examination of the PCT application in the spring of 2009. During 2012 the patent prosecution continued and the Company filed Reponses to patent examiners in Europe and the US.
The combination has now been formulated into a once a day tablet and the drugs included in PTL-202 have been successfully tested in a drug/drug interaction study in humans in 2012.
PTL-2015
In November 2012 the Company executed a letter of intent with Globe Labs Inc. of Vancouver, BC for the potential in-license of an oral dissolving tablet technology. The technology may be used for the delivery of up to 3 different compounds. The initial use of the technology may be for the delivery of sildenafil citrate for the treatment of erectile dysfunction (“ED”).
In 2011, the total market for drugs to treat ED exceeded $5 billion. The Company has finalized a license to an oral dissolving technology (“sublingual formulation”) of an approved drug to treat ED.
Sales of the market leader alone exceeded $2 billion in 2012. The sublingual formulation improves on existing drugs for ED potentially acting faster and with fewer side effects. As large pharmaceutical companies lose their patents on these drugs, a massive opportunity has developed for innovative formulations of drugs for ED. This is a very exciting development for the Company as it shortens the time to market for the Company’s first product and may add significantly to future revenues.
Working from the most conservative projections the British Journal of Urology expects the number of men with ED to more than double from 152 million in 1995 to 322 million by 2025. It is estimated that up to 20 million Europeans and 30 million North Americans experience recurring ED at some point in their lives (Life Science Intelligence). Total sales of ED drugs by the top 3 producers was $3.1 billion in 2006 growing rapidly to a total market of $5 billion in 2011. In addition, it is estimated that up to 90% of all ED goes undiagnosed, which is another driving force for market growth.
Tablets using oral dissolve technology (ODT) are the most preferred and accepted solid dosing forms by patients. This dosage form is also considered by consumers to be of higher quality, having faster onset and being longer lasting than conventional tablets. In addition, 70% of consumers will ask their doctor for the ODT version of a drug if it is available. The Company is currently reviewing all the necessary protocols’ for the rapid development of additional ODT drugs, which have the potential to reach the market in less than 2 years.
|
•
|
PTL-303
|
The Company’s other product candidate, PTL-303, is a combination of drugs including one that has been approved for use in Japan and other jurisdictions. This combination may have a wide range of uses, including treating, preventing and reducing disorders of progressive scarring in humans such as liver cirrhosis.
The Company does not have any funds allocated for the further development of PTL-303 at this time.
18
|
Item 4.
|
Information on the Company - continued
|
The composition, including a cytokine modifier and N-Acetlycysteine which is a precursor of Glutathione, was investigated for its antifibrotic activity by employing two in vitro collagen synthesis assays. The Company discovered that the combination PTL-303 brings about substantial synergistic anti-fibrotic effects in a TGF-Beta1 mediated collagen synthesis assay, when compared to the individual drugs
The composition can be administered in any convenient manner, such as a pill, or inhalation, and may be formulated for injection.
A provisional patent titled “Composition and Method for Treating Fibrosis” was filed by the Company with the United States patent office on October 29, 2008. The application number is 61/109,446. A PCT application covering the technology of PTL-303 was filed by the Company in October 2009.
Principal Products
1) PTL-202
Once a Day Formulation Combination of Pan-phosphodiesterase inhibitor, PTX, with NAC IPF is a long term, progressive form of lung disease characterized by progressive buildup of scar tissue on the supporting framework (interstitium) of the lungs. The term Idiopathic is used only when the cause of the Fibrosis is unknown. Despite extensive investigation, the cause of IPF remains unknown. The disease involves abnormal and excessive deposition of scar tissue (Fibrosis) in the Pulmonary Interstitium (mainly the walls of the Alveoli) with minimal associated inflammation (Figure 1). The symptoms initially develop slowly. The most common symptom is progressive difficulty in breathing, but also includes dry cough.
Figure 1 - Human Airways
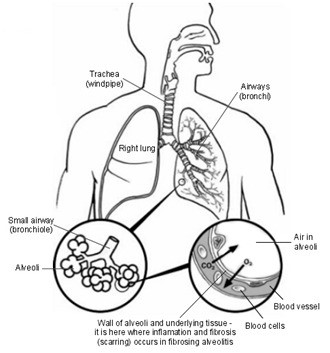
The Company’s lead product, PTL-202, is a combination of two compounds designed to treat IPF: PTX and NAC. The Company has completed two pre-clinical studies in mice models on PTL-202, development of a pill that can be taken once a day and completed a drug/drug interaction study in humans in 2012. A pivotal bio-equivalency study is planned for 2013 and maybe followed by a Phase 2 Proof-of-Concept clinical trial beginning in 2014.
Therapeutic Approach
The combination of drugs in PTL-202 is intended to stop the progression of IPF by reducing the amount of several messenger molecules that are known to be associated with scarring. In addition, the combination has anti-oxidant properties that protect the lung cells from further damage caused by the Fibrosis.
19
|
Item 4.
|
Information on the Company - continued
|
PTX increases the diameter of blood vessels and enhances blood flow. PTX has been successfully and safely used for many years for treatment of vascular diseases such as cramping in the leg.
There is growing evidence that PTX is an anti-inflammatory and may inhibit scarring in the lung.
NAC, the second compound in the PTL-202 combination, has been shown in animal models of pulmonary fibrosis to prevent some of the effects of pulmonary fibrosis, including reducing the progressive scarring of the lung tissue, and reducing the amount of tumor necrosis factor – alpha (“TNF-alpha”) in the lung fluid. In humans NAC has been shown to reduce symptoms of progressive deterioration which include increased difficulty breathing, decreased vital capacity and a decrease in oxygen levels in the blood. In addition, NAC will supplement the amount of glutathione in the lungs, an essential amino acid that is reduced in IPF patients. Also NAC acts as an anti-oxidant reducing the number of reactive oxygen species in the lung which are elevated in IPF patients lungs when compared to a healthy lung.
Pre-clinical Studies
The Company has conducted two pre-clinical studies using PTL-202 for the treatment of lung Fibrosis in a mouse. The Company believes that these studies show that PTL-202 has the potential to be a safe and effective treatment for IPF.
The Company conducted proof-of-principal animal studies to evaluate the relative efficacy of stand-alone or combination treatments of PTX and NAC in lung Fibrosis. In the initial experiment, wet lung weight was measured under various treatments. From this early experiment, it was determined that PTL-202 may be more effective than its separate components.
In further pre-clinical experiments, PTL-202 treatment was more effective than either PTX or NAC alone on lung Fibrosis in mice. In addition, treatment with PTL-202 caused a significant reduction in TNF-alpha in the lung fluid. This finding indicates that PTL-202 may act by reducing the amount of TNF-alpha. TNF-alpha is thought to stimulate the formation of scar tissue. Moreover, there were no deaths or abnormal reactions with a daily administration of PTL-202 during the experiments, indicating a lack of side effects which is consistent with the data from earlier clinical trials in humans for PTX and NAC separately.
The results of these extensive pre-clinical studies suggest that PTL-202 may be safe and effective agent for the treatment of Pulmonary Fibrosis.
PTL-2015 – Erectile Dysfunction
ED (impotence) occurs when a man can no longer get or keep an erection firm enough for sexual intercourse. Problems getting or keeping an erection can be a sign of a health condition that needs treatment, such as heart disease or poorly controlled diabetes. Treating an underlying problem may be enough to reverse ED. ED symptoms may include persistent:
|
|
·
|
Trouble getting an erection
|
|
|
·
|
Trouble keeping an erection
|
|
|
·
|
Reduced sexual desire
|
Male sexual arousal is a complex process that involves the brain, hormones, emotions, nerves, muscles and blood vessels. ED can result from a problem with any of these. Likewise, stress and mental health problems can cause or worsen erectile dysfunction. Sometimes a combination of physical and psychological issues causes ED. For instance, a minor physical problem that slows your sexual response may cause anxiety about maintaining an erection. The resulting anxiety can lead to or worsen erectile dysfunction.
Physical causes of ED
In most cases, ED is caused by something physical. Common causes include:
|
|
·
|
Heart disease
|
|
|
·
|
Clogged blood vessels (atherosclerosis)
|
|
|
·
|
High cholesterol
|
|
|
·
|
High blood pressure
|
|
|
·
|
Diabetes
|
20
|
Item 4.
|
Information on the Company - continued
|
|
|
·
|
Obesity
|
|
|
·
|
Metabolic syndrome, a condition involving increased blood pressure, high insulin levels, body fat around the waist and high cholesterol
|
|
|
·
|
Parkinson's disease
|
|
|
·
|
Multiple sclerosis
|
|
|
·
|
Low testosterone
|
|
|
·
|
Peyronie's disease, development of scar tissue inside the penis
|
|
|
·
|
Certain prescription medications
|
|
|
·
|
Tobacco use
|
|
|
·
|
Alcoholism and other forms of substance abuse
|
|
|
·
|
Treatments for prostate cancer or enlarged prostate
|
|
|
·
|
Surgeries or injuries that affect the pelvic area or spinal cord
|
Psychological causes of ED
The brain plays a key role in triggering the series of physical events that cause an erection, starting with feelings of sexual excitement. A number of things can interfere with sexual feelings and cause or worsen ED. These include:
|
|
·
|
Depression, anxiety or other mental health conditions
|
|
|
·
|
Stress
|
|
|
·
|
Relationship problems due to stress, poor communication or other concerns
|
Therapeutic Approach
Current oral medications are a successful ED treatment for many men. These drugs including sildenafil citrate, the active ingredient in PTL-2015 enhance the effects of nitric oxide, a natural chemical the body produces that relaxes muscles in the penis. This enhancement is the result of the drugs ability to inhibit a cytokine PDE5. This increases blood flow and allows for an erection in response to sexual stimulation. Oral medications for ED vary in dosage, how long they work and their side effects.
DEVELOPMENT PLANS
PTL-202 – Lung Fibrosis
Formulation Development
The goal of the Company is to develop a pill containing both drugs that only needs to be taken once per day. Existing, marketed, modified release versions of the drugs will be evaluated as a simple daily or twice daily fixed dose combination formulation. Given, the preliminary dose ranges/strengths of 600-1200mg of PTX combination with 600mg-1200mg NAC once a day, the physical size for an ingestible tablet will be a barrier to success. Current formulation of PTX, with Vitamin E rather than NAC, in a Phase 2 study has shown inhibition of messenger molecules and a reduction in scar tissue. Both PTX and NAC are water soluble molecules, with short resident time in the bloodstream. This high water solubility presents a challenge to once-a-day administration as both molecules are rapidly absorbed and metabolized quickly by the body. The goal from a development perspective is to deliver an appropriately formulated controlled release product, reducing the absolute amount of drug per tablet needed to achieve a clinically effective blood level. To accomplish this goal formulation development prototypes will target release of the drugs to provide sustained levels of the drug in the blood.
A controlled release formulation of PTL-202, a fixed dose combination of PTX and NAC, for the potential treatment of IPF, Liver Cirrhosis and other fibrotic diseases was completed in 2012.
The Company has completed the initial clinical study of PTL-202. The study was a drug/drug interaction study. This study, conducted in humans in India, was intended to determine if any new by products are created by the combination of the drugs in PTL-202 and to determine if the combination is bio-equivalent to its generic counter parts. The study included 12 healthy males. The bio-analytical portion (PK assay development and good laboratory practice validation) of the above-mentioned study was completed in India by a lab contracted by the Company. Therefore, following the Company’s stated business strategy, the Company acted as a sponsor of the study and the Contract Research Organizations (“CROs”) were hired to execute the objectives of the study. Successful completion of this Phase 1 study of PTL-202 is a major milestone because as many as 30% of Phase 1 drug trials are failures. The following table describes the Phase 1 study.
21
|
Item 4.
|
Information on the Company - continued
|
|
Location
|
Hyderabad, India
|
|
Trial Dates
|
August 22, 2012 – September 28, 2012
|
|
Number of Patients
|
12
|
|
Regulatory Approval sought
|
To conduct drug drug interation study
|
|
Regulatory Approval Received
|
To conduct drug drug interation study
|
|
Clinical Endpoints
|
Is the plasma levels of PTX equivalent +- 20% when dose alone and in combination with NAC
|
The trial was considered a success as the administration of the combination showed a definite synergistic effect increasing the plasma concentrations of PTX and the measured metabolites in the blood outside the range that would be considered equivalent.
The statistics from the trial show that there was a significant difference in the pharmacokinetic responses of PTX and its metabolites alone compared with in combination with NAC. The PTX Cmax was over 40% greater in the combination compared with when administered alone. There was a significant difference in the pharmacokinetic response of NAC alone and in combination with PTX. The Cmax of the NAC in the combination was less than 85% of the Cmax when NAC was dosed alone.
These increased concentrations may be one reason for the effectiveness of the combination in the animal models.
A further pivotal bio-equivalency study is planned for 2014. This study would include up to 20 individuals and take about 2 months to complete.
Phase 2: Proof- of-Concept in Humans
The proposed Phase 2 study is a proof-of-concept or proof-of-relevance trial. The proposed study utilizes principles of adaptive design approach and has two interconnected parts. The proof-of-concept trial will be designed to assess the safety and efficacy of PTL-202 in patients with IPF. Study patients will receive formulated PTL-202 or individual components of PTL-202. Neither the patient or the physician will know if they are getting PTL-202 or a component.
The objectives of the study are:
|
•
|
To evaluate the safety and tolerability of 12 months of treatment with PTL-202 in patients with IPF versus placebo and individual components of PTL-202 (PTX and NAC);
|
|
•
|
To compare changes in forced vital volume capacity in IPF patients treated with PTL-202 versus treatment with PTX and NAC alone and placebo;
|
|
•
|
To compare changes in the following parameters in IPF patients treated with PTL-202;
|
|
•
|
Diffusion capacity for carbon monoxide;
|
|
•
|
Extent and nature of IPF-related abnormalities on high resolution CT; and
|
|
•
|
To compare quality of life evaluations in IPF patients treated with PTL-202 and individual components of PTL-202 versus placebo.
|
The proposed study will include 65 patients, and will be an international study conducted in the USA, Canada and Europe. It’s subject to approval by regulators by filling and IND or its equivalent in the countries where we conduct the trial.
The Company has not filled any applications with any regulators to conduct the phase 2 study and as such cannot make an estimate as to the start date of the trial.
The cost to complete the Phase 2 study is estimated at $8 million and will be borne 100% by the Company. Additional funds will be required to complete this phase of the development of PTL-202. On completion of this proof-of-concept study the Company will look to out-license PTL-202 to a larger company capable of completing the development and commercialization of PTL-202. Such additional funds would likely be raised through a private placement of securities. There is no assurance that such funding will be available. See also “Risk Factors” in this filing.
22
|
Item 4.
|
Information on the Company - continued
|
2) PTL-2015 Erectile Dysfunction
Prior to the Company licensing the ODT technology the licensor, Globe Labs Inc., had completed the following steps in the development of PTL-2015 sublingual sildenafil citrate:
|
|
·
|
ODT Formulation
|
|
|
·
|
Pilot Pharmacokinetic Study
|
|
|
·
|
ODT Formula Optimization
|
|
|
·
|
ODT Manufacturing and Scale up
|
The Company plans to continue the development of PTL-2015 to gain marketing approval in the European Union. Working with a regulatory consultant in Europe, the Company plans further studies in humans which may include a Pivotal Bioequivalency Study. The pivotal bioequivalence study may include up to 40 individuals and take about 4 months to complete. The study would compare PTL-2015 to an ODT formulation of sildenafil citrate already approved in Europe. The cost to take PTL-2015 to market is estimated at $1,000,000.
3) PIPELINE PRODUCT: PTL-303
Fixed Dose Combination of TGF-β Inhibitor and NAC - Pre-clinical
The Company has completed In Vitro studies of this combination that have confirmed the compounds method of action. This data has been included in the provisional patent application for PTL-303 to support the composition of matter and methods claims.
The Company does not have any funds allocated for the further development of PTL-303 at this time.
In the future, as funds are available, the Company will initiate animal studies using Liver Fibrosis in mice to generate additional data to assess the efficacy of PTL-303. The pre-clinical work will also focus on the delivery of the compound to the liver and the efficacy of the compound in a liver Fibrosis model.
Efficacy in the liver Fibrosis model will lead to further Investigational New Drug application (“IND”) enabling studies when funds are available.
Manufacturing
The Company has limited experience in, and does not own facilities for, manufacturing any products or product candidates. The Company, in partnership with IntelGenx Corp. (“IntelGenx”), will utilize contract manufacturers to produce clinical supplies of any components of PTL-202that are not commercially available. Although the Company intends to continue to rely on contract manufacturers to produce certain of its products for both clinical and commercial supplies, the Company and IntelGenx will oversee the production of those products related to PTL-202.
Similarly for PTL-2015 the Company will utilize contract manufacturers to produce clinical supplies of any components of PTL-2015 that are not commercially available. Although the Company intends to continue to rely on contract manufacturers to produce certain of its products for both clinical and commercial supplies, the Company will oversee the production of those products related to PTL-2015.
Sales, Marketing and Distribution
The Company currently has no sales or distribution capabilities and limited marketing capabilities. In order to commercialize its products, the Company must develop sales, marketing and distribution capabilities or make arrangements with other parties to perform these services for us. The Company’s intention is to out-license its products once Phase 2 testing has been completed. It is anticipated the licensee will have the capability to market, sell and distribute the Company’s products.
23
|
Item 4.
|
Information on the Company - continued
|
Licenses and Collaboration Agreements
Integenx Collaboration Agreement
The Company has a development and commercialization agreement with Intelgenx of Montreal. The agreement covers the formulation of PTL-202 utilizing the VersaTab multilayer oral delivery formulation owned by Intelgenx. Under the terms of the agreement, Intelgenx is to complete the formulation of PTL-202 as a multilayer sustained release oral tablet that the patient will only need to take once per day. The initial formulation work is substantially complete. The Company is responsible for any all clinical trials related to the development of PTL-202. To date the Company has completed the pilot bio-equivalency study as required under the agreement. To date the Company has paid to intelgenx $10,315 and has expended $10,315 on clinical development of PTL-202 under the agreement. Under the terms of the agreement, the Company will pay a royalty of an amount equal to seven percent (7%) on the first $5,000,000 of net sales received annually and 5% of net sales above the first $5,000,000.
The term of the agreement will continue until the occurrence of the following:
|
1)
|
A party fails to comply with its material obligations under the agreement and fails to cure the material breach within 90 days of written notice;
|
|
2)
|
Either party may terminate the agreement if:
|
|
(a)
|
The other party fails to perform any of its obligations hereunder or makes any material misrepresentation in the agreement, which has not been cured within ninety days of written notice;
|
|
(b)
|
The other party enters into any compromise with creditors or a general agreement for referral of payment with its creditor;
|
|
(c)
|
The other party makes or suffers to be made any transfer to any person, trustee, receiver, liquidator, or referee for the benefit of creditors;
|
|
(d)
|
The other party files a voluntary petition in bankruptcy; and
|
|
(e)
|
An involuntary petition in bankruptcy is filed against the other party and not dismissed within sixty (60) days of filing.
|
|
3)
|
Intelgenx is entitled, in its sole discretion, to terminate the agreement at any time on thirty days written notice to the Company, without the need to pay the Company any compensation in respect of such termination, in which case the license granted under the agreement shall immediately terminate.
|
Globe Labs Inc. License Agreement
The Company has a development and commercialization agreement with Globe Labs Inc. (“Globe”), a Vancouver BC company. The agreement covers the formulation of ODT technology owned by Globe. Under the terms of the agreement, the Company is to complete the required studies and regulatory tasks to commercialize products using the oral dissolving technology. The Company is responsible for any all clinical trials related to the development of products using ODT technology. Under the terms of the agreement, the Company will pay a royalty of an amount equal to seven percent (7%) on the first $5,000,000 of net sales received annually and 5% of net sales above the first $5,000,000.
The term of the agreement will continue until the occurrence of the following:
|
1)
|
A party fails to comply with its material obligations under the agreement and fails to cure the material breach within 90 days of written notice;
|
|
2)
|
Either party may terminate the agreement if:
|
|
(a)
|
The other party fails to perform any of its obligations hereunder or makes any material misrepresentation in the agreement, which has not been cured within ninety days of written notice;
|
|
(b)
|
The other party enters into any compromise with creditors or a general agreement for referral of payment with its creditor;
|
24
|
Item 4.
|
Information on the Company - continued
|
|
(c)
|
The other party makes or suffers to be made any transfer to any person, trustee, receiver, liquidator, or referee for the benefit of creditors;
|
|
(d)
|
The other party files a voluntary petition in bankruptcy; and
|
|
(e)
|
An involuntary petition in bankruptcy is filed against the other party and not dismissed within sixty (60) days of filing.
|
|
3)
|
Globe is entitled, in its sole discretion, to terminate the agreement at any time on thirty days written notice to the Company, without the need to pay the Company any compensation in respect of such termination, in which case the license granted under the agreement shall immediately terminate.
|
Dalhousie License Agreement
The Company entered into a license agreement with Dalhousie University of Halifax Nova Scotia (“Dalhousie”) on April 20, 2007 and subsequently amended the agreement on May 6, 2008, July 9, 2008, March 24, 2009, January 25, 2010 and February 2, 2011. The agreement covers PTX and Functional Derivatives/Metabolites and its applications. The license allows the Company to use PTX to develop a pill for diseases including diseases of the lung and radiation induced fibrosis.
Under this agreement, the Company was required to make annual maintenance payments of $7,500 which are credited towards future royalties. In addition, the Company was to make milestone payments of up to $825,000 to Dalhousie based on patient enrolment, clinical studies, and regulatory approval for sale of the product as well as a $25,000 payment into the patent fund maintained by Dalhousie. As further consideration under the license agreement with Dalhousie, the Company would have been required to pay to Dalhousie a royalty on revenue earned from marketing, manufacturing, licensing, sale or distribution of the technology, improvements relating to the technology or products. Under the terms of the agreement, the Company was required to a) secure $2,000,000 in capital or debt financing by December 31, 2010, b) complete enrolment of a first patient in a Phase II clinical study, and c) expend $200,000 per year in research and development related activities. As at December 31, 2010, the Company had not met any of the requirements of the agreement outlined above. On February 2, 2011, the Company received a waiver from Dalhousie for the requirements (a) and (b) above, and requirement (c) was amended to also include: the parties will dose the first human subject by December 31, 2012; and the parties will initiate a phase 2 study by December 12, 2015.
This license agreement was terminated on January 9, 2013 as the Company had not paid the maintenance fees required under the agreement and Dalhousie elected to enact the termination provision available to it. The Company was unable to pay the maintenance fee due to a lack of cash and a difficult fund raising environment. The loss of the license is not expected to have an impact on the Company’s future as the Company’s own patents will replace the intellectual property protection that was provided by the Dalhousie Patents. In addition the Dalhousie Patents will begin to expire prior to PTL-202 reaching market. Pursuant to the termination of the agreement, there are no outstanding contractual obligations between the parties under this agreement other than outstanding maintenance fees of $12,750.
Intellectual Property
We believe we are developing a valuable portfolio of intellectual property rights to protect the technologies, inventions and improvements that we believe are significant to our business, which includes patent applications in the United States, Europe and Canada.
Our success in the specialty pharmaceutical industry depends in substantial part on effective management of both intellectual property assets and infringement risks. In particular, we must be able to protect our own intellectual property as well as minimize the risk that any of our products infringes on the intellectual property rights of others.
We enter into agreements with all our employees involved in research and development, under which all intellectual property during their employment belongs to us, and they waive all relevant rights or claims to such intellectual property. All our employees involved in research and development are also bound by a confidentiality obligation, and have agreed to disclose and assign to us all inventions conceived by them during their term of employment. Despite measures we take to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or our proprietary technology or to obtain and use information that we regard as proprietary. If we fail to protect our intellectual property rights, it could harm our business and competitive position. For more information, see “Risk Factors” in this Form 20-F.
25
|
Item 4.
|
Information on the Company - continued
|
The Company has filed the below patent:
Patent Cooperation Treaty Patent Application No. PCT/CA2008/001880
Filed 23 October 2008
COMPOSITIONS AND METHODS FOR TREATING FIBROPROLIFERATIVE DISORDERS
A provisional patent was filed in the United States by the Company in October 2007 to cover the composition of matter and method of use of this combination. In October 2008, the Company filed an application under the Patent Cooperation Treaty (“PCT”) based on the above provisional application. The Company received a positive preliminary examination of the PCT application in the spring of 2009. During 2012 the patent prosecution continued and the Company filed Reponses to patent examiners in Europe and the US.
The Company has licensed from IntelGenx intellectual property covered by the US patent application 2007/0190144 and all corresponding know-how, patents, patent applications, continuation applications, continuation in part applications, divisional applications, supplementary protection certificates, inventor’s certificates and all patents that may be granted or that may have been granted on any of the foregoing, including reissues, re-examination and extensions worldwide. This license is not exclusive. This patent is related to the formulation of PTL-202. The patent covers the VersaTab technology for the released delivery of active ingredients using a layered tablet technology to deliver separate active ingredients in separate layers. The patent application is a method patent and is currently pending, the patent has not been issued.
Specialized Skills and Knowledge
All aspects of the Company’s business require specialized skills and knowledge. Such skills and knowledge include pre-clinical research, clinical drug development, regulatory, intellectual property management, business development, licensing, legal, corporate finance and accounting. Below is a list of the Company’s consultants and advisors.
Scientific Advisory Board (“SAB”)
The members of the Company’s strategic advisory board, none of whom are officers or employees, provide advice, assistance and consultation in the fields of drug development, clinical trials and Fibrosis. The SAB consists of clinical advisors considered to be known opinion leaders in their respective fields, and they offer the Company advice and feedback regarding the following:
• the Company’s drug development programs;
• the opportunities provided by unmet needs and market opportunities; and
• the existence of new products and technologies among other things.
The SAB does not meet on a regular basis but is available for consultation with the CEO as required. The SAB members are not compensated in cash and receive stock options as their only compensation. We have no agreements with the SAB members other than stock option agreements (filed as Exhibit 2.6 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference).
Two SAB members, Daryl Knight and James Seibold, have each been granted options as of July 3, 2012 to purchase 100,000 shares at an exercise price of CAD $0.10 per share. The options are all fully vested, and have an expiry date of July 3, 2017. Other SAB members have declined to receive stock options.
The following is a brief biography of each of the Company’s Pulmonary Fibrosis and Bronchiolitis Obliterans clinical advisors, which includes a description of each individual’s credentials and recent professional experience.
Daryl Knight, Ph.D. Dr. Darryl Knight is a professor at University of Newcastle, Australia. Dr. Knight obtained his PhD at the University of Western Australia in 1993 and did post-doctoral training at the University of British Columbia. From 1997 to 2001 he was a Senior Research Officer in the Asthma & Allergy Research Institute of the University of Western Australia and was Head of the Experimental Biology division of the Institute from 2002 to 2004. He was also an Adjunct Senior Lecturer in the Department of Medicine at the University of Western Australia. Dr. Daryl Knight was the Canada Research Chair in Airway Disease and Associate Director of the James Hogg iCAPTURE Centre for Cardiovascular and Pulmonary Research. He was a tenured Professor of Pharmacology and Therapeutics at the University of British Columbia, Vancouver. Dr Knight sits on the Respiratory Science Grant Review panel at the CIHR, the Basic Science Review Panel of the Canadian Lung Association and is on the editorial board of 5 Respiratory Journals.
26
|
Item 4.
|
Information on the Company - continued
|
Ganesh Raghu MD, FCCP, FACP Dr. Ganesh Raghu MD, FCCP, FACP is a specialist in Idiopathic Pulmonary Fibrosis (IPF). He is a professor of Pulmonary Medicine at the University of Washington Medical Centre and a Director of the Lung transplant program there. He has conducted several clinical trials for the treatment of IPF with antifibrotic drugs. His current research interests include quality of life measures in IPF and lung transplantation, as well as the treatment of rejection and infection in lung transplants. Dr. Raghu received his M.D. in 1973 from Mysore Medical College, University of Mysore, Mysore, India. He Interned at University Hospitals, University of Mysore in 1974 and was a resident in General Medicine and Chest Medicine, Hartlepool General Hospital and Postgraduate Medical Center (University of Newcastle Upon Tyne), Hartlepool, England in 1977. In 1980 he conducted his residency in Internal Medicine at State University of New York in Buffalo. He was the Chief Medical Resident at the State University of New York, Buffalo from 1980 to 1981. Dr. Raghu moved to Seattle in 1983 to complete fellowships in Pulmonary and Critical Care Medicine as well as Lung Cell Biology at the University of Washington.
Dr. Andreas Zuckermann, MD Dr. Zuckermann’s specialties include heart and lung transplantation. He is a Staff Surgeon in the Department of Cardiothoracic Surgery at University of Vienna in Austria and Co-Director of the Cardiac Transplantation Program there. He is also a Director the International Society for Heart and Lung Transplantation. He has been involved in over 170 thoracic transplantations and has conducted clinical research in post-transplant patients. His current interests are focused on heart lung transplantation and beating heart transplants. Dr. Zuckermann received his MD from Vienna Medical School, University of Vienna in 1991. From 1991 – 1993 he was the transplant coordinator in the Department of Cardiothoracic Surgery at the University of Vienna. From 1993 to 2000 prior to his appointment as a Staff Surgeon he trained in Cardio-thoracic surgery at St. Polten Hospital in Vienna where he assisted in over 30 lung transplants.
James R. Seibold, MD, FACP, FACR Dr. Seibold is the past Director of the Scleroderma Program University of Michigan. He had been on the faculty of UMDNJ from 1980-2004 where he had served as Chief of the Division of Rheumatology, Director of the Clinical Research Center and as the W.H. Conzen Chair of Clinical Pharmacology. Author of more than 300 scientific publications, his medical practice specializes in Scleroderma, Raynaud’s phenomenon and interventional research in the rheumatologic diseases. He has received multiple awards from arthritis and Scleroderma patient organizations. Dr. Seibold is currently the President of the Scleroderma Clinical Trials Consortium.
Competitive Conditions
PTL-202
Current Therapies for Idiopathic Pulmonary Fibrosis
In North America the current therapy for IPF is based on the premise that recruitment and activation of inflammatory cells leads to the progressive development of IPF. However, massive doses of immunosuppressive agents meant to decrease the number or activity of inflammatory cells do not alter the development of IPF. Instead, patients develop serious side effects leading to a shortened lifespan. Since the current therapy is not successful, there is an urgent need to develop alternative potent, non-toxic, long lasting therapy for these unmet medical needs. PTL-202 works by inhibiting messenger molecules that are active in the development of IPF, specifically TNF-alpha.
Until recently treatment for IPF in North America consists of using immunosuppressants and anti-oxidants. In an attempt to minimize side effects many patients are prescribed drugs to prevent GI side effects, osteoporosis and infection. A clinical study during 2012 concluded that this treatment using immunosuppressants was actually harmful to IPF patients. Supplemental oxygen is prescribed to patients who are unable to maintain a satisfactory amount of oxygen in the blood. This treatment remains unsatisfactory as the median survival is 2 – 4 years once diagnosis is made and the 5 year survival rate ranges from 20% – 40% (Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. “Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003”. Am J Respir Crit Care Med. 2007;176(3):277-284).
InterMune Inc.’s (“InterMune”) Pirfenidone represents the most advanced therapy being developed to treat IPF. Pirfenidone is a synthetic small molecule that is orally available for the prevention of fibrotic lesions in general. Pirfenidone has gastrointestinal side effects and will darken the skin and cover from the sun is required with its use. In contrast to PTL-202, Pirfenidone works by reducing the activity of an enzyme.
Both InterMune and Shinogi & Co., Ltd. (“Shinogi”) have conducted Phase 3 trials of Pirfenidone to treat IPF. InterMune has recently received approval to market Pirfenidone for IPF in the European Union and Canada, Shinogi recently received approval to market Pirfenidone for IPF in Japan. Pirfenidone is currently undergoing clinical trials for uterine fibrosis (PhII), scleroderma (PhII), proliferative vitreoretinopathy (PhII), multiple sclerosis (PhII), liver fibrosis (PhII), wound healing (PhI), and benign prostatic hyperplasia (PhI). Pirfenidone is not approved for use in either the United States. There are no therapies for IPF approved in the United States.
27
|
Item 4.
|
Information on the Company - continued
|
Current Therapies for Scleroderma
Treatment of Scleroderma is directed toward the individual feature(s) affecting different areas of the body:
|
•
|
Aggressive treatment of elevations in blood pressure have been extremely important in preventing kidney failure;
|
|
•
|
Blood-pressure medications, such as captopril, are frequently used; and
|
|
•
|
Serious inflammation of the lungs (alveolitis) can require immune suppression with cyclophosphamide (Cytoxan) along with prednisone.
|
Additionally, medications are used to suppress the overly active immune system that seems to be spontaneously causing the disease in organs affected. Medications used for this purpose include penicillamine, azathioprine, and methotrexate. The optimal treatment of Scleroderma lung disease is an area of active research. Stem-cell transplantation is being explored as a possible option. There are no effective treatments for lung fibrosis associated with Scleroderma.
No medication has been found to be universally effective for all patients with scleroderma. In an individual patient, the illness may be mild and not require treatments. In some, the disease is ravaging and relentless. Lung fibrosis in Scleroderma may be fatal. PTL-202’s main focus in Scleroderma is treating patients with lung fibrosis.
Current Therapies for Post Lung Transplant Bronchiolitis Obliterans
The current therapy for Post Lung Transplant Bronchiolitis Obliterans (“PLT-BO”) is based on the same premise as in IPF, recruitment and activation of inflammatory cells leads to the development and progression of PLT-BO. However, massive doses of immunosuppressive agents meant to decrease the number or activity of inflammatory cells do not alter the course of IPF or PLT-BO. Instead, patients develop serious side effects leading to a shortened lifespan. There are no FDA-approved treatments for IPF. Despite these advances, optimal therapy for IPF remains elusive and has yet to be identified (http://www.ipfsummit.org/pdf/Needs-Assessment.pdf)
Competing IPF Drugs Currently Under Development
ACTIVE PHASE III CLINICAL TRIALS
|
•
|
BIBF 1120 - Manufactured by Boehringer Ingelheim: A Phase III, double-blind trial in patients with PF to investigate the effect of Oral BIBF 1120, 150 mg Twice Daily, on Annual Forced Vital Capacity Decline. A primary outcome measure will be the annual rate of decline in in FVC over 32 weeks;
|
|
•
|
Cyclosporine Inhalation Solution (CIS) - Manufactured by APT Pharmaceuticals - (For lung transplant recipients only): A Phase III multi-center, randomized, controlled study to demonstrate the efficacy and safety of cyclosporine inhalation solution (CIS) in Improving Bronchiolitis Obliterans Syndrome-Free Survival Following Lung Transplantation. The study seeks to demonstrate the efficacy and safety of CIS in improving survival, and preventing BOS when given prophylactically to lung transplant recipients in addition to their standard immunosuppressive regimen. The study is no longer recruiting patients; and
|
|
|
•
|
Thalomid (Thalidomide) - Manufactured by Celgene: A Phase III, double-blind, placebo-controlled safety and efficacy study investigating the Treatment of Chronic Cough in Idiopathic Pulmonary Fibrosis with Thalidomide. Please visit www.clinicaltrials.gov and search "IPF" for a complete description of this trial, including inclusion/exclusion criteria. The study is sponsored by Johns Hopkins Medical Center (Baltimore, MD).
|
ACTIVE PHASE II CLINICAL TRIALS
|
•
|
CNTO 888 (Manufactured by Centocor, Inc.): A Phase 2, Multicenter, Multinational, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose-Ranging Study Evaluating the Efficacy and Safety of CNTO 888 Administered Intravenously. The primary objective is to determine the efficacy (as measured by pulmonary function) and safety of CNTO 888 in patients with IPF. The secondary outcome measures are to assess the effect of CNTO 888 on measures of disease progression, patient reported outcomes, functional capacity and health-related quality of life, and to assess the pharmacokinetics and pharmacodynamics of CNTO 888 in IPF. The study began recruiting patients in October, 2008, with a goal of recruiting 120 patients. Patients will be in the study for about 74 weeks;
|
28
|
Item 4.
|
Information on the Company - continued
|
|
•
|
FG-3019 (Manufactured by FibroGen, Inc.): A Phase II study of FG-3019 (therapeutic antibody against connective tissue growth factor) for patients with IPF. The study is a Phase 2a, Open-Label, Single Arm Study to Evaluate the Safety, Tolerability, and Efficacy of FG-3019 in Subjects With Idiopathic Pulmonary Fibrosis;
|
|
•
|
Interferon alpha (Amarillo Biosciences): A Phase II, randomized, double-blind, placebo-controlled trial to determine whether interferon-alpha, delivered in low doses via orally dissolving lozenges given 3 times per day for 4 weeks, can reduce the frequency and severity of chronic cough in patients with COPD or IPF. Cough frequency will be assessed via 24-hour digital audio recordings made prior to entry, and at weeks 2 and 4 of treatment. Patients will also complete questionnaires regarding cough frequency, duration and intensity, QOL, dyspnea, and antitussive medication usage weekly during treatment. The study is seeking 80 patients however it is only recruiting patients at Texas Tech University (Lubbock, TX). THIS IS TEMPORARILY ON HOLD; and
|
|
•
|
STX-100 (Stromedix, Inc.): STX-100 is a monoclonal antibody being developed for the treatment of idiopathic pulmonary fibrosis (IPF). This multi-center, randomized, double-blind, placebo-controlled study will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of STX-100 in patients with IPF. The study began recruitment in Q4 11 and will enroll patients at sites across the United States.
|
ACTIVE PHASE I CLINICAL TRIALS
|
•
|
GS 6624 (formerly AB0024) - Developed by Gilead Sciences, Inc. Phase 1 drug candidate (humanized monoclonal antibody targeting the human LOXL2 protein) for patients with IPF. An ongoing Phase 1 study is designed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of GS 6624 in adult patients with IPF;
|
|
•
|
IW001 - Manufactured by Immuneworks. A Phase I study of the safety and tolerability of 3 doses of IW001 per day in patients with IPF over a 24 week treatment period. To explore the biologic effects of IW001 on T-cell and B-cell reactivity. This study will also explore relationships between Collagen V reactivity and clinical measures of lung function in patients with IPF. This study is now recruiting; and
|
|
•
|
PRM-151 - Manufactured by Promedior, Inc. A Phase 1b study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of IV PRM-151 in patients with idiopathic pulmonary fibrosis has initiated in the US and the Netherlands. This study is now recruiting patients. PRM-151 is being developed for potential therapeutic uses to prevent, treat, and reduce fibrosis in a number of disease settings.
|
PTL-2015
Oral Dissolving Technology
The Oral Dissolving Technology is exclusively available to the Company through the License Agreement with Globe dated October 22, 2013. The first product the Company will be developed with this technology is sublingual sildenafil.
It is the Company’s goal to develop additional products utilizing this technology.
Competitive Technologies
Current technologies used by other specialty pharmaceutical companies are deficient in several ways. Overall, current technologies add significant manufacturing expenses beyond the inherent cost of the chemically active ingredient (i.e. drug) and standard costs associated with making more classical dosage forms such as tablets and capsules. In addition, most of the technology platforms fall short of being able to deliver the performance required for acceptance of the product. For example, taste-masked systems usually do not mask the taste of the drug in a sufficient manner to render it palatable. Also, such systems can inhibit the drug from being readily absorbed, which would prevent it from being classified as an immediate release system. PTL’s formulation has overcome these problems. The combination of taste masking, rapid dissociation and adhesiveness make this product rapidly absorbable through the pores and provide superior performance activity, thereby reducing dosage and side effects.
29
|
Item 4.
|
Information on the Company - continued
|
The current formulations used by other companies consist of coating beads or large particles that are typically over 300 microns with an aqueous-based coating. These systems pose the following problems:
|
1.
|
Particles are too large and leave a gritty feel in the mouth;
|
|
2.
|
Tablets have a drug content that is about 50% of their total weight (“w/w”);
|
|
3.
|
Pharmaceutical products are restricted to lower dosage products;
|
|
4.
|
Coatings are non-elastic and break quickly;
|
|
5.
|
Coatings inhibit the release of drugs into the mouth and do not meet strict requirements for immediate release; and
|
|
6.
|
Processes are lengthy, costly and require strict handling.
|
The leaders in the field that promote these services include Catalent Pharma Solutions, Coating Place, Inc., Contract PharmaCal, Eurand and EthyPharm.
The Company’s ODT products that require taste masking have many advantages over competing technologies and, in most cases, offer the only means known to produce the desired benefits. The Company believes that the ODT formulation systems are state-of-the art and are not known to be in practice anywhere in the pharmaceutical field. These technologies are very robust and consist of taste-masking small drug particles that are typically less than 250 microns with an adhesive component. These systems pose the following advantages:
|
1.
|
Particles are very small and do not leave a gritty feel in the mouth;
|
|
2.
|
Particles are small enough to be placed in thin films, similar to Listerine Thin Strips; and
|
|
3.
|
Particles have drug content in excess of 50% w/w and typically greater than 80% w/w enabling products to be smaller and contain a higher dosage – some products may be capable of delivering up to a 1,500 mg oral equivalent in a single tablet.
|
The Company’s ODT products contain rapidly disintegrating compositions that are coated by taste masking chemicals.
Other companies’ technologies enable drugs to be absorbed in the gastrointestinal tract, but produce unwanted side effects. Additionally, orally absorbed drugs break down in the liver upon first absorption by the body. It is known that a large number of side effects associated with drugs are due to the liver breakdown metabolites. According to Pfizer filings with FDA, sildenafil breaks down in the liver, which may involve some side effects such as headaches, flushing, etc.
Technology Assessment
The technologies of sublingual drug delivery are specific to our projects and business. The Company will rely on its review of product development results from clinical trials as one means to measure the effectiveness of the technologies available and assess their commercial viability.
It is understood that the sublingual formulation is a platform which may apply to many products. In the future the Company may use this formulation to develop new drugs. The Company anticipates that these drugs will be patentable; however, it cannot guarantee that any patent applications will ultimately result in a patent being issued by the relevant authority.
Economic Dependence
The Company’s business is substantially dependent on its own patent applications to use intellectual property protected by patent, trade secret and know-how owned by the Company. It is not expected that the Company’s business will be affected in the current financial year by the termination of the Dalhousie license.
The Company’s business is substantially dependent on contracts to purchase the major part of its requirements for research and development services for the development of assays, formulation, pre-clinical research, clinical research and/or raw materials and manufactured product upon which its business depends. The Company expects that its business will be affected in the current financial year by the negotiation of new contracts and renegotiation or termination of contracts or sub-contracts.
30
|
Item 4.
|
Information on the Company - continued
|
Seasonality
Not Applicable
Insurance
We maintain commercial general liability insurance coverage to cover product liability claims arising from the use of our products. Each claim under this policy has a limit of USD $1,000,000 and the aggregate limit is USD $1,000,000. We also maintain liability insurance to cover specific clinical trials risks. The Company had a policy in place to cover the pilot clinical trial for the combination of drugs in PTL-202. Each claim under this policy has a limit of USD $1,000,000 and the aggregate limit is USD $1,000,000. Our insurance coverage, however, may not be sufficient to cover any claim for product liability.
We are subject to product liability exposure and have limited insurance coverage. Any product liability claims or potential safety-related regulatory actions could damage our reputation and materially and adversely affect our business, financial condition and results of operations.
We also provide directors’ and officers’ liability and company reimbursement insurance to cover all of our directors and officers against losses arising from claims we indemnify for. Our current officers and directors insurance coverage expires on November 29, 2014. We plan to renew the insurance upon its expiration.
Facilities
See Item 4D, “Information on the Company — Property, Plant and Equipment.”
Legal Proceedings
We are not currently a party to any material legal proceeding. From time to time, we may bring against others or be subject to various claims and legal actions arising in the ordinary course of business.
Regulation
Government Regulations
The current and future operations and research and development activities of the Company are or will be subject to various laws and regulations in the countries in which the Company conducts or plans to conduct activities, including but not limited to the United States, Canada and the European Union. These laws and regulations govern the research, development, sale and marketing of pharmaceuticals, taxes, labor standards, occupational health and safety, toxic substances, chemical products and materials, waste management and other matters relating to the pharmaceutical industry. Permits, registrations or other authorizations may also be required to maintain operations and to carry out the Company’s future research and development activities, and these permits, registrations or authorizations will be subject to revocation, modification and renewal.
Governmental authorities have the power to enforce compliance with lease conditions, regulatory requirements and the provisions of required permits, registrations or other authorizations, and violators may be subject to civil and criminal penalties including fines, injunctions, or both. The failure to obtain or maintain a required permit may also result in the imposition of civil and criminal penalties, and third parties may have the right to sue to enforce compliance.
The Company expects to be able to comply with all applicable laws and regulations and does not believe that such compliance will have a material adverse effect on its competitive position. The Company has obtained and intends to obtain all permits, licenses and approvals required by all applicable regulatory agencies to maintain current operations and to carry out future research and development activities.
U.S. Pharmaceutical Regulatory Agency
Regulation by governmental authorities in the US and other countries is a significant factor in the development, manufacture and marketing of pharmaceuticals. All of the Company’s product candidates will require regulatory approval by government agencies prior to commercialization. In particular, product candidates are subject to rigorous pre-clinical testing and clinical trials and other premarketing approval requirements of the FDA and regulatory authorities in other countries. Various federal, state and foreign statutes and regulations govern the manufacturing, safety, labeling, storage, record-keeping and marketing of pharmaceutical products. The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources. When and if regulatory approval is obtained for any of the Company’s product candidates, the approval may be limited in scope, which may significantly limit the indicated uses for which the product candidates may be marketed, promoted and advertised. In addition, approved pharmaceuticals and manufacturers are subject to ongoing review and discovery of previously unknown problems that may result in restrictions on the manufacture, sale or use of approved pharmaceuticals or in their withdrawal from the market.
31
|
Item 4.
|
Information on the Company - continued
|
Pre-clinical Studies
Before testing any compounds with potential therapeutic value in human subjects in the US, stringent governmental requirements for pre-clinical data must be satisfied. Pre-clinical testing includes both in vitro and in vivo laboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. Pre-clinical testing results obtained from these studies, including tests in several animal species, are submitted to the FDA as part of an investigational new drug application, or IND, and are reviewed by the FDA prior to the commencement of human clinical trials. These pre-clinical data must provide an adequate basis for evaluating both the safety and the scientific rationale for the initial trials in human volunteers.
Clinical Trials
If a company wants to conduct clinical trials in the United States to test a new drug in humans, an IND must be prepared and submitted to the FDA. The IND becomes effective if not rejected or put on clinical hold by the FDA within 30 days of filing the application. The IND process can result in substantial delay and expense.
Clinical Trial Phases
Clinical trials typically are conducted in three sequential phases, Phases 1, 2 and 3, with Phase 4 trials potentially conducted after marketing approval. These phases may be compressed, may overlap or may be omitted in some circumstances.
|
•
|
Phase 1 clinical trials: After an IND becomes effective, Phase 1 human clinical trials can begin. These trials evaluate a drug’s safety profile and the range of safe dosages that can be administered to healthy volunteers or patients, including the maximum tolerated dose that can be given to a trial subject. Phase 1 trials also determine how a drug is absorbed, distributed, metabolized and excreted by the body, and duration of its action.
|
|
•
|
Phase 2 clinical trials: Phase 2 clinical trials are generally designed to establish the optimal dose, to evaluate the potential effectiveness of the drug in patients who have the target disease or condition and to further ascertain the safety of the drug at the dosage given in a larger patient population.
|
|
•
|
Phase 3 clinical trials: In Phase 3 clinical trials, the drug is usually tested in a controlled, randomized trial comparing the investigational new drug to a control (which may be an approved form of therapy) in an expanded and well-defined patient population and at multiple clinical sites. The goal of these trials is to obtain definitive statistical evidence of safety and effectiveness of the investigational new drug regime as compared to control in defined patient populations with a given disease and stage of illness.
|
New Drug Application (“NDA”)
After completion of clinical trials, if there is substantial evidence that the drug is both safe and effective, a NDA, is prepared and submitted for the FDA to review. The NDA must contain all of the essential information on the drug gathered to that date, including data from pre-clinical studies and clinical trials, and the content and format of a NDA must conform with all FDA regulations and guidelines. Accordingly, the preparation and submission of a NDA is an expensive and major undertaking for a company.
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information from the sponsor rather than accepting a NDA for filing. In such an event, the NDA must be submitted with the additional information and, again, is subject to review before filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. By law, the FDA has 180 days in which to review the NDA and respond to the applicant. The review process is often significantly extended by the FDA through requests for additional information and clarification. The FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation as to whether the application should be approved and the scope of any approval. The FDA is not bound by the recommendation, but gives great weight to it. If the FDA evaluations of both the NDA and the manufacturing facilities are favorable, the FDA may issue either an approval letter or an approvable letter, which usually contains a number of conditions that must be satisfied in order to secure final approval. If the FDA’s evaluation of the NDA submission or manufacturing facility is not favorable, the FDA may refuse to approve the NDA or issue a not approvable letter.
32
|
Item 4.
|
Information on the Company - continued
|
Fast Track Designation and Priority Review
The FDA’s fast track program is intended to facilitate the development and expedite the review of drugs that are intended for the treatment of a serious or life-threatening condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for their condition. Under the fast track program, the sponsor of a new drug may request the FDA to designate the drug for a specific indication as a fast track product at any time during the clinical development process.
In some cases, the FDA may designate a product for priority review. A product is eligible for priority review, or review within a targeted six-month time frame from the time a NDA is accepted for filing, if the product provides a significant improvement compared to marketed products in the treatment, diagnosis or prevention of a disease. The Company regularly assesses its products for fast track potential but cannot guarantee any of its products will receive a priority review designation, or if a priority designation is received, that review or approval will be faster than conventional FDA procedures.
Orphan Drug Designation
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States, such as IPF. If the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. If a product that has orphan drug designation subsequently receives FDA approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, which means the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for up to seven years after receiving FDA approval.
When appropriate, the Company will seek orphan status for certain indications that may be treated with its products.
Other Regulatory Requirements
Any products manufactured or distributed under FDA approvals are subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the products. Drug manufacturers and their subcontractors are required to register with the FDA and, where appropriate, state agencies, and are subject to periodic unannounced inspections by the FDA and state agencies for compliance with current good manufacturing practices and regulations which impose procedural and documentation requirements upon drug developers and each third party manufacturer they utilize.
The FDA closely regulates the marketing and promotion of drugs. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturers from communicating on the subject of off-label use.
European Union
Clinical Trials
In common with the US, the various phases of pre-clinical and clinical research in the European Union are subject to significant regulatory controls. The regulatory controls on clinical research in the European Union are now largely harmonized following the implementation of the Clinical Trials Directive 2001/20/EC (“CTD”). Compliance with the national implementations of the CTD has been mandatory from May 1, 2004. However, variations in the member state review agencies continue to exist, particularly in the small number of member states that have yet to implement the CTD fully.
33
|
Item 4.
|
Information on the Company - continued
|
All member states currently require regulatory and independent ethics committee approval of interventional clinical trials. European regulators and ethics committees also require the submission of adverse event reports during a study and a copy of the final study report.
Marketing Authorization
In the European Union, approval of new medicinal products can be obtained through the mutual recognition procedure or the centralized procedure. The mutual recognition procedure entails initial assessment by the national authorities of a single member state and subsequent review by national authorities in other member states based on the initial assessment. The centralized procedure entails submission of a single Marketing Authorization Application (“MAA”) to the European Medicines Agency (“EMEA”) leading to an approval that is valid in all European Union member states. EMEA approval is required for certain medicinal products, such as biotechnology products and certain new chemical entities, and optional, or available at the EMEA’s discretion for other new chemical entities or innovative medicinal products with novel characteristics.
Under the centralized procedure, an MAA is submitted to the EMEA. Two European Union member states are appointed to conduct an initial evaluation of each MAA. These countries each prepare an assessment report, which are then used as the basis of a scientific opinion of the Committee for Medicinal Products for Human Use (“CHMP”). If this opinion is favorable, it is sent to the European Commission which drafts a decision. After consulting with the member states, the European Commission adopts a decision and grants a marketing authorization, which is valid throughout the European Union and confers the same rights and obligations in each of the member states as a marketing authorization granted by that member state.
The European Union expanded its membership by ten in May 2004. Two more countries joined on January 1, 2007. Several other European countries outside of the European Union, particularly those intending to accede to the European Union, accept European Union review and approval as a basis for their own national approval.
Advertising
In the European Union, the promotion of prescription medicines is subject to intense regulation and control, including a prohibition on direct-to-consumer advertising. Some jurisdictions require that all promotional materials for prescription medicines be subjected to either prior internal or regulatory review and approval.
Data Exclusivity
For applications filed after October 30, 2005, European Union regulators offer eight years data exclusivity during which generic drug manufacturers cannot file abridged applications. This is followed by two years market exclusivity during which generic applications may be reviewed and approved but during which generic drug manufacturers cannot launch products.
Other Regulatory Requirements
If a marketing authorization is granted for the Company’s products in the European Union, the holder of the marketing authorization will be subject to ongoing regulatory obligations. A holder of a marketing authorization for the Company’s products is legally obliged to fulfill a number of obligations by virtue of its status as a Marketing Authorization Holder (“MAH”). While the associated legal responsibility and liability cannot be delegated, the MAH can delegate the performance of related tasks to third parties, provided that this delegation is appropriately documented. A MAH can therefore either ensure that it has adequate resources, policies and procedures to fulfill its responsibilities, or can delegate the performance of some or all of its obligations to others, such as distributors or marketing partners.
34
|
Item 4.
|
Information on the Company - continued
|
The obligations of a MAH include:
|
•
|
Manufacturing and Batch Release: MAHs should guarantee that all manufacturing operations comply with relevant laws and regulations, applicable good manufacturing practices, with the product specifications and manufacturing conditions set out in the marketing authorization and that each batch of product is subject to appropriate release formalities;
|
|
•
|
Pharmaco-vigilance: MAHs are obliged to monitor the safety of products post-approval and to submit to the regulators safety reports on an expedited and periodic basis. There is an obligation to notify regulators of any other information that may affect the risk benefit ratio for the product;
|
|
•
|
Advertising and Promotion: MAHs remain responsible for all advertising and promotion of its products in the relevant jurisdiction, including promotional activities by other companies or individuals on their behalf. Some jurisdictions require that a MAH subject all promotional materials to either internal or prior regulatory review and approval;
|
|
•
|
Medical Affairs/Scientific Service: MAHs are required to have a function responsible for disseminating scientific and medical information on its medicinal products, predominantly to healthcare professionals, but also to regulators and patients;
|
|
•
|
Legal Representation and Distributor Issues: MAHs are responsible for regulatory actions or inactions of their distributors and agents, including the failure of distributors to provide a MAH with safety data within a timeframe that allows the MAH to fulfill its reporting obligations; and
|
|
•
|
Preparation, Filing and Maintenance of the Application and Subsequent Marketing Authorization: MAHs have general obligations to maintain appropriate records, to comply with the marketing authorization’s terms and conditions, to submit renewal applications and to pay all appropriate fees to the authorities. There are also general reporting obligations, such as an obligation to inform regulators of any information that may lead to the modification of the marketing authorization dossier or product labeling, and of any action to suspend, revoke or withdraw an approval or to prohibit or suspend the marketing of a product.
|
The Company may hold marketing authorizations for products in its own name, or appoint an affiliate or a collaboration partner to hold the marketing authorization on its behalf. Any failure by a MAH to comply with these obligations may result in regulatory action against the MAH and its approvals and ultimately threaten our ability to commercialize our products.
Canada
In Canada, applications for a marketing authorization, known as a notice of compliance, are submitted to the Health Canada Therapeutic Products Directorate (“HCTPD”), which is the federal regulatory body that oversees the approval of pharmaceutical products for human use. Under the Canada FDA and the regulations there under, a manufacturer must present substantive scientific evidence of a product’s safety, efficacy and quality. At present, Health Canada targets 355 days for application review and approvals. Once the application is approved and the applicant receives a notice of compliance, the applicant has the right to sell the product in Canada.
In addition to regulations in the US, Europe and Canada, the Company is subject to a variety of foreign regulations governing clinical trials and commercial distribution of our future product candidates in other jurisdictions.
Approvals Outside of the United States, Canada and the European Union
The Company and its products will also be subject to a wide variety of foreign regulations governing development, manufacture and marketing. Whether or not FDA approval or European marketing authorization has been obtained, approval of a product by the comparable regulatory authorities of other foreign countries must still be obtained prior to manufacturing or marketing the product in those countries. The approval process varies from country to country and the time needed to secure approval may be longer or shorter than that required for FDA approval or a European marketing authorization. The Company cannot assure investors that clinical trials conducted in one country will be accepted by other countries or that approval in one country will result in approval in any other country.
35
|
Item 4.
|
Information on the Company - continued
|
C. Organizational structure
Not Applicable.
D. Property, plant and equipment.
As we operate as a virtual company and we have no products approved for marketing, and no research and development facilities or manufacturing plant, we therefore have no property, plant and equipment relating to manufacturing equipment at this time. However, we do have minimal amounts of lab equipment, computer equipment, furniture and fixtures and leasehold improvements relating to our head office. See the financial statements for the fiscal year ended December 31, 2013 included in this Form 20-F. Our head office is located at 1500 – 409 Granville St. in Vancouver BC, Canada.
|
Item 5.
|
Operating and Financial Review and Prospects
|
A. Operating Results
The following discussion of our financial condition and results of operations is based upon and should be read in conjunction with our financial statements for the fiscal years ended December 31, 2013, December 31, 2012 and December 31, 2011, together with the notes thereto. The Company’s financial statements for the years ended December 31, 2013, December 31, 2012 and the opening statement of financial position as at January 1, 2011, have been prepared in accordance with IFRS. This discussion contains forward-looking statements that involve certain risks and uncertainties. We caution you that our businesses and financial performance are subject to substantial risks and uncertainties. See Item 3.D, “Key Information — Risk Factors”.
The Company’s net loss for the year ended December 31, 2013, totaled $740,846 or $0.03 loss per share (compared with $605,468 or $0.03 loss per share for 2012 and $463,768 or $0.03 loss per share for 2011). The main contributors to these increases in losses in 2013 compared with 2012 were the following increases: advertising and promotion $143,874 (compared with an increase of $35,842 for 2012), Bank Charges and Interest $17,328 (compared with a decrease of $2,636 for 2012) Investor Relations $9,300 (compared to an increase of $51,950 in 2012), Professional Fees $98,024 (compared to a decrease of $31,886 in 2012), wages and benefits $57,032 (compared to a decrease of $14,590). And reductions in Research and Development $50,941 (compared to an increase of $50,941 in 2012) and decrease in share-based payments of $32,843 (compared to an increase of $69,162 for 2012).
Revenues
The Company has not generated any revenue from the sale of drug therapies. The Company has not recognized any revenue since inception through December 31, 2013. The Company does not expect to receive any revenues until after PTL-2015 is register for sale or the completion of the Phase 2 trial of PTL-202. The Company expects to complete this trial by the end of 2015.
The Company’s revenues will be earned through upfront payments from licenses, milestone payments included in-licenses and royalty income from licenses. The Company’s revenues will depend on out licensing the Company’s drug candidates to suitable development and commercialization partners and its partners’ abilities to successfully complete clinical trials and commercialize the Company’s drug candidates worldwide.
General and Administrative Expenses
General and administrative costs consist primarily of personnel related costs, non-intellectual property related legal costs, accounting costs and other professional and administrative costs associated with general corporate activities.
During the year ended December 31, 2013 total general and administrative costs were $711,453 (compared with $483,813 for 2012) an increase of $227,640. The increased expense for the year ended 2013 was mainly due to increased advertising and promotion, $143,874 (compared with an increase of $35,842 for 2012), bank charges and interest of $17,328 (compared with a decrease of $2,636 for 2012), investor relations $9,300 (compared to an increase of $51,950 in 2012), professional Fees $98,024 (compared to a decrease of $31,886 in 2012), wages and benefits $57,032 (compared to a decrease of $14,590)
From 2013 forward, as PTL-202 begins clinical development and as operations are developed to move PTL-202 and other drug candidates through the clinical trial process, general and administrative expenses will increase. Increases in personnel costs, professional fees and contract services will make up a significant portion of these planned expenditures.
36
|
Item 5.
|
Operating and Financial Review and Prospects - continued
|
Intellectual Property and Intangible Assets
All license and option fees paid to licensors for intellectual property licenses are accrued to intangible assets on the Company’s financial statements. In addition, any expenses for intellectual property protection including patent lawyers services fees and any filing fees with government agencies or the World Intellectual Property Organization (“WIPO”) are accrued to intangible assets. This cost will increase in the twelve months following the date of this filing.
Interest Income
Interest expense for the year ended December 31, 2013, was $16,861 (compared with $104,378 for 2012, and $122,503 for 2011).The decrease in 2013 was due to elimination of the interest expense related to the elimination of the interest charges related to the Irrevocable Subscription Agreements (“ISA”).
Profits
At this time, the Company is not anticipating profit from operations. Until such time as the Company is able to realize profits from the out licensing of products under development, the Company will report an annual deficit and will rely on its ability to obtain equity and/or debt financing to fund on-going operations. For information concerning the business of the Company, please see “Item 4. Information on the Company”.
Warrants & options reserve, which arises from the recognition of the estimated fair value of stock options and warrants. As of December 31, 2013, contributed surplus was $123,704, compared with $206,212 as of December 31, 2012 and $162,052 as of December 31, 2011. The decrease in warrants & options reserve of $82,508 was due to the conversion of Class B Series II Preference Shares and expiry of options and finders warrants valued at $140,706 partly offset issuance of 450,000 new options valued at $42,192 and 480,000 finders’ warrants valued at $16,006. The fair value of these share based awards are determined using the Black-Scholes Option Pricing Model which utilizes certain subjective assumptions including the expected life of the option or warrant and expected future stock price volatility.
The accumulated deficit as of December 31, 2013 increased to $3,263,058 from $2,662,918 as of December 31, 2012, for an increase of $600,140 which consisted of the loss for 2013 of $740,846 partially offset by a $140,706 for the conversion of Class B Series II Preference Shares and expiry of options and finders warrants. The deficit as of December 31, 2012 increased to $2,662,918 from $2,094,115 as of December 31, 2011, for an increase of $568,803 which consisted of the loss for 2012 $605,468 and partially offset by previously issued warrants valued at $36,665 which expired during 2012.
During the fiscal year 2013, the Company’s net cash provided by financing activities increased by $415,755, compared with $315,518 for fiscal year 2012 and $282,578 for fiscal year 2011.
At present, the Company’s operations do not generate cash inflows and its financial success after 2013 is dependent on management’s ability to continue to obtain sufficient funding to sustain operations through the development stage and successfully bring the Company’s technologies to the point that they may be out licensed so that the Company achieves profitable operations. The research and development process can take many years and is subject to factors that are beyond the Company’s control.
In order to finance the Company’s future research and development and to cover administrative and overhead expenses in the coming years the Company may raise money through equity sales. Many factors influence the Company’s ability to raise funds, including the Company’s track record, and the experience and caliber of its management. Actual funding requirements may vary from those planned due to a number of factors, including the progress of research activities. Management believes it will be able to raise equity capital as required in the long term, but recognizes there will be risks involved that may be beyond their control. Should those risks fully materialize, it may not be able to raise adequate funds to continue its operations.
37
|
Item 5.
|
Operating and Financial Review and Prospects - continued
|
Comparison of Years Ended December 31, 2013 and 2012
|
2013
|
2012
|
Change
|
Change
|
|||||||||||||
| $ | $ | $ | % | |||||||||||||
|
Revenue
|
Nil
|
Nil
|
N/A | N/A | ||||||||||||
|
Research and Development
|
Nil
|
50,941 | (50,941 | ) | -100 | % | ||||||||||
|
Wages and Benefits
|
157,916 | 100,843 | 57,073 | 57 | % | |||||||||||
|
Professional Fees
|
178,947 | 80,923 | 98,024 | 121 | % | |||||||||||
|
Advertising and Promotion
|
187,511 | 43,637 | 143,874 | 330 | % | |||||||||||
|
Investor Relations
|
61,250 | 51,950 | 9,300 | 18 | % | |||||||||||
|
General and Administrative
|
90,084 | 112,828 | (22,744 | ) | -20 | % | ||||||||||
|
Insurance
|
22,461 | 24,948 | (2,487 | ) | -10 | % | ||||||||||
|
Rent and Occupancy Cost
|
13,284 | 17,743 | (4,459 | ) | -25 | % | ||||||||||
|
Interest Expense
|
16,861 | 104,378 | (87,517 | ) | -84 | % | ||||||||||
|
Other Expense
|
12,532 | 17,277 | (4,745 | ) | -27 | % | ||||||||||
|
Net and Comprehensive Loss
|
740,846 | 605,468 | 135,378 | 22 | % | |||||||||||
|
* The Research and Development expense for 2011 is $Nil because all research and development during the year was carried out by our partner on the development of PTL-202, IntelGenx Research and Development expense for 2012 was a total of $57,449, including $41,475 for the clinical trial paid to RA Chem Pharma, $5,659 for insurance for the clinical trial and $10,315 for costs paid to Intelgenx for materials and outside contractors. The $50,941 for Research and Development in 2012 is the net of the $57,449 paid for Research and Development less the Scientific Research Tax Credit of $6,508. No research and development was carried on in 2013.
|
Selected Quarterly Information (1)
|
December 31, 2013
$
|
September 31, 2013
$
|
June
30, 2013
$
|
March
31, 2013
$
|
December 31, 2012
$
|
September 31, 2012
$
|
June
30, 2012
$
|
March
31, 2012
$
|
|||||||||||||||||||||||||
|
Total Revenues
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
||||||||||||||||||||||||
|
Net Loss
|
(308,768 | ) | (104,895 | ) | (152,648 | ) | (174,535 | ) | (205,919 | ) | (163,356 | ) | (89,056 | ) | (147,137 | ) | ||||||||||||||||
|
Loss per Share basic and diluted
|
(0.01 | ) | (0.00 | ) | (0.01 | ) | (0.01 | ) | (0.01 | ) | (0.01 | ) | (0.01 | ) | (0.01 | ) | ||||||||||||||||
|
Cash
|
180,692 | 7,523 | 1,927 | 7,220 | 9,854 | 36,004 | 2,486 | 7,221 | ||||||||||||||||||||||||
|
Restricted Cash
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
||||||||||||||||||||||||
|
Total Assets
|
287,044 | 136,900 | 78,413 | 121,075 | 206,533 | 280,629 | 197,091 | 119,505 | ||||||||||||||||||||||||
|
Non-Current Liabilities
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
||||||||||||||||||||||||
|
(1)
|
Financial statements for the three month periods are prepared by management and are not audited.
|
Critical Accounting Estimates
The Company’s accounting policies are presented in Note 3 of the December 31, 2013 audited financial statements. The preparation of financial statements in accordance with IFRSs requires management to select accounting policies and make estimates. Such estimates may have a significant impact on the financial statements. Actual amounts could differ materially from the estimates used and, accordingly, affect the results of the operations. These include:
|
•
|
the assumptions used for the determinations of the timing of future income tax events; and
|
|
•
|
the carrying values of property, plant and equipment, intangible assets such as technology licenses and patents, derivative liability, convertible note, and the valuation of stock-based payment.
|
38
|
Item 5.
|
Operating and Financial Review and Prospects - continued
|
Changes in Accounting Policies including Initial Adoption
The Company has adopted IFRS, as of January 1, 2010, as discussed in Note 2 of the December 31, 2013 Financial Statements.
Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, accounts payable and accrued liabilities, due to related parties, the liability portion of the convertible note and the derivative liability. Cash and cash equivalents are classified as financial assets. Unless otherwise noted, it is management’s opinion that the Company is not exposed to significant interest, currency or credit risks arising from financial instruments. The fair value of cash and cash equivalents and accounts payable and accrued liabilities approximates their carrying values due to their short-term maturity or capacity for prompt liquidation.
The balance due to related parties have no default provisions. Therefore, we believe that the default on the loans payable will not impact our future ability to obtain funding.
Accounts payable and accrued liabilities, amounts due to related parties, the liability portion of the convertible note and the derivative liability are classified as financial liabilities. Accounts payable and accrued liabilities, amounts due to related parties and the liability portion of the convertible note are recognized initially at fair value, and subsequently stated at amortized cost. The derivative financial liability is initially measured at fair value, with subsequent measurement to fair value at the end of each reporting period.
Foreign exchange risk is the risk arising from changes in foreign currency fluctuations. The Company does not use any derivative instruments to reduce its exposure to fluctuations in foreign currency rates. It is the opinion of management that the foreign exchange risk to which the Company is exposed is minimal.
Limitations of Controls and Procedures
The Company’s management, including the CEO and CFO, believe that any disclosure controls and procedures or internal controls over financial reporting, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, they cannot provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been prevented or detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by unauthorized override of the control. The design of any systems of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Accordingly, because of the inherent limitations in a cost effective control system, misstatements due to error or fraud may occur and not be detected.
B. Liquidity and Capital Resources.
Overview
The Company is a development stage company and therefore has no regular cash inflows. Selected financial data pertaining to liquidity and capital resources for the fiscal years ended December 31, 2013 and December 31, 2012 is presented below.
39
|
Item 5.
|
Operating and Financial Review and Prospects - continued
|
Comparison of Years Ended December 31, 2013 and 2012
|
Years ended
|
2013
|
2012
|
Change between two years
|
Change between two years
|
||||||||||||
| $ | $ | $ | % | |||||||||||||
|
Cash and Cash Equivalents
|
180,692 | 9,854 | 170,838 | 1734 | % | |||||||||||
|
Current Assets
|
224,688 | 108,107 | 116,581 | 108 | % | |||||||||||
|
Current Liabilities
|
727,188 | 637,523 | 89,665 | 14 | % | |||||||||||
|
Working Capital Deficit
|
(502,500 | ) | (529,416 | ) | 26,916 | 5 | % | |||||||||
|
Accumulated Deficit
|
3,263,058 | 2,662,918 | 600,140 | 23 | % | |||||||||||
|
Cash used in Operations
|
546,866 | 304,983 | 241,883 | 79 | % | |||||||||||
|
Cash Flows from Financing Activities
|
731,273 | 315,518 | 415,755 | 132 | % | |||||||||||
|
Interest Income
|
0 | 0 | N/A | N/A | ||||||||||||
As of December 31, 2013, the Company had cash and cash equivalents of $180,692 (compared with $9,854 for fiscal 2012 and $6,094 for fiscal year 2011) and working capital deficiency of $502,500 (compared with working capital deficiency of $529,416 for fiscal year 2012 and working capital of $143,118 for fiscal year 2011). Working capital deficiency is calculated as current assets less current liabilities.
Cash and cash equivalents increased by $170,838 between fiscal year 2013 and fiscal year 2012. The increase was the result of increase in financing activities of $415,755 including issuing shares, partially offset by cash used in operations in the amount of $546,866.
Working capital deficiency increased by $26,916 from fiscal year end 2012 primarily due to an increase in financing activities. Working Capital decreased by $672,534 from fiscal year 2011 to fiscal year 2012, which is primarily due to the decrease in restricted cash of $300,000 from the ISA, upon the cancellation of those agreements and a reclassification of amounts due to shareholders of $175,935 from non-current to current liabilities. Total liabilities increased by $89,665 for the fiscal year ended 2013 when compared to the total liabilities for the Fiscal year ended 2012. Total liabilities only increased by $49,036 for the fiscal year ended December 31, 2012 when compared to the total liabilities for the fiscal year ended December 31, 2011. Accounts payable increased significantly from the fiscal year 2011 to the fiscal year ended 2012 primarily due to the accrual of salaries and expenses owed to the CEO in the amount of $91,666, consulting fees owed to the CFO in the amount of $19,140 and fees owed to the Company’s auditor $43,044 related to the Company being public. The increase in prepaid expenses for the year ended 2012 as compared to the year ended 2011 was due primarily to five months remaining (i.e. $35,155) on the a full year contract in the total amount of $85,002 for media and advertising and eight months remaining (i.e. $60,000) on a full year investor relations contract in the total amount of $90,000.
Operating Activities
Cash utilized in operating activities during fiscal year 2013 was $546,866 (compared with $304,983 for fiscal 2012 and $284,361 for fiscal year 2011). The net increase in cash utilized in operations between 2013 and 2012 was $241,883. The change was primarily due to increased expenses with the increases in Advertising and promotion, Bank Charges and interest, Wages and benefits, Investor relations and Professional fees.
Investing Activities
Investing activities primarily include additions to fixed assets and intangible assets. Net cash used in investing activities was $13,569, $6,775 and $22,580 in fiscal years ended December 31, 2013, 2012 and 2011, respectively. The investing activities were mainly investment in patents and the development of intellectual property.
Financing Activities
The Company’s cash inflows from financing activities comprised proceeds from common share issuances, warrants and increases of loan payable during fiscal year ended December 31, 2013 totaling $731,273 (compared with $315,518 for 2012 and $282,578 for 2011). Cash from financing activities increased by $415,755 between fiscal year 2013 and fiscal year 2012 and increased by $32,940 between fiscal year 2012 and fiscal year 2011.
As part of the CSE listing requirements no more than 20% of the issued and outstanding shares of a company listed on the exchange may be “Builders Shares”. Builders Shares include any share issued at a price of less than $0.02 per share. In order to meet this listing requirement, the founders of the Company contributed $Nil in fiscal year 2012 (compared with $41,600 for fiscal year 2011 and $57,000 for fiscal year 2010) to re-price common shares to $0.02 per share. The founders originally purchased the shares that were repriced for $0.001 per share. $41,600 for fiscal year ended 2011 (compared with $57,000 for 2010) is included in the Company’s Financing Activities in its financial statements.
40
|
Item 5.
|
Operating and Financial Review and Prospects - continued
|
Capital Expenditures
Capital expenditures were $Nil in 2013, $6,200 in 2012 and $Nil in 2011.
C. Research and Development
Research and development expense consists primarily of salaries for management of research contracts and research contracts for pre-clinical studies, clinical studies and assay development as well as the development of clinical trial protocols and application to government agencies to conduct clinical trials, including consulting services fees related to regulatory issues and business development expenses related to the identification and evaluation of new drug candidates. Research and development costs are expensed as they are incurred.
Comparison of Years Ended December 31, 2013, 2012 and 2011
From inception through to December 31, 2013, the Company incurred total expenses in the development of its intellectual property of $1,867,913, which includes $554,712 of research and development expenses (research and development expenses on the financial statements have been offset by $53,277 in Industrial Research Assistance Program (“IRAP”) funding and $193,935 in SR&ED tax credits), $423,431 of professional fees and $889,770 of wages and benefits.
The Company does not separately account for each research project. Prior to the 2010 fiscal year all research was pre clinical.
|
Year ended December 31, 2013
|
Year ended December 31, 2012
|
Year ended December 31, 2011
|
||||||||||
|
Research and Development Expenses
|
||||||||||||
|
Personnel, Consulting, and Stock-based Payment
|
$ | Nil | $ | Nil | $ | Nil | ||||||
|
License Fees and Subcontract research
|
$ | Nil | $ | 51,790 | $ | Nil | ||||||
|
Facilities and Operations
|
$ | Nil | $ | 5,659 | $ | Nil | ||||||
|
Less: Government contributions
|
$ | Nil | $ | (6,508 | ) | $ | Nil | |||||
|
Total
|
$ | Nil | $ | 50,941 | $ | Nil | ||||||
The decrease in research expense in 2013 was due to a lack of cash. The increase in research expense in 2012 compared to fiscal year 2011 is due to the initiation of clinical trials of PTL-202. The fees paid to the contract research operation for the drug/drug interaction trial in India was $41,475. An additional $5,659 was expended on Insurance for the trial. Moreover, during 2012 $10,315 was paid to Intelgenx for materials and outside consulting related to the formulation of PTL-202. There is no research and development expense for 2011 as all research and development was conducted by IntelGenx Corp. under the agreement the Company has with them.
An additional $250,000 will be required for the pivotal clinical trial of the formulated product. The results of this work may provide the information required for a regulatory submission to move PTL-202 into a phase 2 study. The cost of the regulatory submission is budgeted at $125,000. All of these costs are to be borne by the Company.
Additional financing will be required to complete the development and commercialize PTL-202 as well as the Company’s other products. There is no assurance that such financing will be available or that the Company will have the capital to complete this proposed development and commercialization.
41
|
Item 5.
|
Operating and Financial Review and Prospects - continued
|
The Company has substantially completed the formulation, drug/drug interaction study of PTL-202, analyzing the blood samples and analyzing the data in 2012. The Company’s clinical development studies and regulatory considerations relating to PTL-202 are subject to risks and uncertainties that may significantly impact its expense estimates and development schedules, including:
|
|
·
|
the scope, rate of progress and cost of the development of PTL-202;
|
|
|
·
|
uncertainties as to future results of the drug/drug interaction study of PTL-202;
|
|
|
·
|
uncertainties as to future results of the formulation development and pilot study of PTL-202;
|
|
|
·
|
the Company’s ability to enroll subjects in clinical trials for current and future studies;
|
|
|
·
|
the Company’s ability to raise additional capital; and
|
|
|
·
|
the expense and timing of the receipt of regulatory approvals.
|
In addition to the formulation and clinical development plans for PTL-202 and PTL-2015 the Company may begin development of PTL-303 for the treatment of Liver Cirrhosis. The Company will only be able to begin development of PTL-303 if additional funds are available. There is no guarantee that these funds will be available to the Company and, if they are available, they may not be available on acceptable terms. Development of PTL-303 may significantly impact the Company’s expense projections and development timelines.
D. Trend Information
Other than as disclosed elsewhere in this Form 20-F, we are not aware of any trends, uncertainties, demands, commitments or events for the period from January 1, 2011 to December 31, 2013 that are reasonably likely to have a material adverse effect on our revenues, income, profitability, liquidity or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Off Balance Sheet Arrangements
The Company is not a party to any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future material effect on the Company’s financial condition, changes in financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources.
F. Tabular Disclosure of Contractual Obligations
The Company has no known contractual obligations specified in Item 5.F.1 as of the latest fiscal year end balance sheet date, other than the license agreement with Dalhousie which was cancelled on January 9, 2013.
|
Item 6.
|
Directors, Senior Management and Employees
|
Directors and Senior Management
The following table sets forth certain information relating to our directors and executive officers as of the date of this filing:
|
Name and Position
|
Principal Occupation for Past Five Years
|
Date of Appointment to Office
|
Common Shares Held
|
Percentage of Common Shares Outstanding (1)
|
|
Douglas H. Unwin, B.Sc., BA
President, CEO, Director
1500 – 409 Granville Street Vancouver, BC
|
President & CEO of the Company since September 2005
|
September 12, 2005
|
4,539,667
|
12.1%
|
42
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
|
Name and Position
|
Principal Occupation for Past Five Years
|
Date of Appointment to Office
|
Common Shares Held
|
Percentage of Common Shares Outstanding (1)
|
|
Douglas Wallis
Director, CA
7178 Quatsino Drive
Vancouver, BC V5S 4C3
|
Independent Consultant July 2013 to present,
Partner, Smyth Ratcliffe Chartered Accountants to July 2013
|
May 10, 2011
|
350,510
|
0.9%
|
|
M. Greg Beniston, BA, LLB
Chairman
1802 – 1000 each Ave
Vancouver, BC
V6E 4M2
|
Senior Legal Counsel, CHC Helicopter May 2006 to present, Sole Practitioner , January 2004 to present
|
Chairman since October 31, 2007;
Corporate Secretary: from September 2005 to October 31, 2007
|
400,000
|
1.1%
|
|
Wendi Rodrigueza, PhD
Director
46701 Commerce Drive Rd
Plymouth, MI 48170
|
VP Product Development, ProNAi Therapeutics, Inc . September 2006 to present, Director Project Management, Novartis September 2005, to September 2008, Sr.
|
November 5, 2009
|
100,000
|
0.27%
|
|
Derick Sinclair, CA
CFO and Corporate Secretary
1500 – 409 Granville Street Vancouver, BC
|
CFO, Cadan Resource Corporation, May 2007 to present, CFO Madeira Minerals Ltd 2009 to present, CFO Viscount Mining Corp 2010 to present.
|
Chief Financial Officer since September 1, 2007;
Corporate Secretary
Since October 31, 2007
|
435,510
|
1.2%
|
|
|
(1)
|
The percentages are calculated based on 37,456,825 shares of issued and outstanding stock of the Company as of the date of this filing.
|
The Company’s Audit Committee consists of Doug Unwin, Greg Beniston and Douglas Wallis.
The Company’s Compensation Committee consists of Doug Unwin, Greg Beniston and Douglas Wallis.
The following is a brief description of the background of the key management, directors and the promoters of the Company:
Douglas H. Unwin, B.Sc., MBA President and Chief Executive Officer & Director - Mr. Unwin is the Company’s founder and has served as President and CEO since the Company’s inception in September 2005. Mr. Unwin graduated from the University of British Columbia with a B.Sc. in Biology in 1981. In 1985 he graduated from the University of Saskatchewan with a Master’s in Business Administration. He is a full time employee of the Company and devotes the majority of his working hours to the Company’s business. Mr. Unwin is responsible for the Company’s overall strategic direction and the implementation of that strategy. He is based at the Company’s head office in Vancouver, British Columbia. Mr. Unwin is an experienced executive with 27 years of diverse experience including 22 years as an entrepreneur in life sciences, aquaculture and telecommunications. He has spent his last 12 years focused on life science start-ups, technology commercialization and venture capital financing. Mr. Unwin was an associate with Neuro Discovery Inc. a venture capital company focused on investing in therapies for neurological disorders. During his tenure Mr. Unwin reviewed numerous business plans and assisted in the structuring of investments. Prior to founding the Company, Mr. Unwin was the CEO of Med BioGene Inc. a start-up medical device company.
Derick Sinclair, B.Comm., CA Chief Financial Officer - Mr. Sinclair, is an experienced CFO having worked with US and Canadian public and private companies for over 20 years. He is a contractor and devotes approximately 15% of his time to the Company. His duties with the Company include, bookkeeping, financial management and reporting, assisting the CEO where necessary and liaising between the board and the Company’s auditors. Mr. Sinclair began his accounting career in 1982 as an auditor with KPMG. He received his CA designation in 1985 and his Bachelor of Commerce (Honours) University of Windsor in 1982. From 1985 to 2003, Mr. Sinclair was employed by BC Rail and its subsidiaries and their successors. He began at BC Rail as a Manager in General Accounting rising in 1998 to the role of CFO & VP Administration Westel Telecommunications Ltd. Mr. Sinclair currently operates DR Financial Services Limited focused on providing CFO and controller services to small and medium size public companies. He is also CFO of Cadan Resources Corporation, Madeira Minerals Ltd, and Viscount Mining Corp publicly traded exploration companies on the TSX Venture Exchange.
43
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
M. Greg Beniston, BA, LLB Chairman Of the Board & Director - Mr. Beniston, is an experienced counsel with expertise in technology, corporate/commercial, securities, corporate governance and aviation. Mr. Beniston received his BA (Honours) with a major in Commerce from Simon Fraser University in 1979. Greg received his LLB from the University of British Columbia in 1987. Mr. Beniston devotes less than 10% of his time to the affairs of the Company. He was Legal Counsel and Corporate Secretary for Xillix Technologies Corp. (TSX) a cancer imaging company from 1993 until 2000 and was Vice President Legal and Corporate Secretary of MDSI Mobile Data Solutions Inc. (TSX, NASDQ) from 1996 to 2003. From 2007 through 2013 Mr. Beniston has been employed by The CHC Helicopter Group Of Companies as Senior Legal Counsel. Mr. Beniston also served as the Company’s Corporate Secretary from inception through October 2007.
Douglas Wallis, CA Director - A Chartered Accountant for over 30 years, Doug Wallis specializes in work with Canadian and US public companies. Doug received his CA after completing a five-year post-secondary education articling program. His work involved everything from assisting in the structure of initial public offerings to comprehensive audit services. Doug retired from partnership with Smythe Ratcliffe LLP. in June 2013 and now provides consultancy services to public companies or to companies in the process of going public. Doug's extensive experience in accounting and the rules of professional conduct are highly valued in his consultancy assignments. While a partner with Smythe he was heavily involved in Professional Standards, he brings a commitment to integrity, professionalism and quality that permeates throughout the entire leadership team. Previously, Doug was the Director of Professional Advisory Services, Institute of Chartered Accountants of BC. Mr. Wallis devotes less than 10% of his time to the affairs of the Company. Doug was the Past Chairman of the Board for the Canadian Network for International Surgery (CNIS).
Wendi Rodrigueza, PhD. Director – Dr. Rodrigueza brings over 16 years of drug development experience to the Company’s Board of Directors. Ms. Rodrigueza devotes less than 10% of her time to the affairs of the Company. From 1994 – 1998 she conducted post doctorate fellow studies at Thomas Jefferson University and The Medical College of Pennsylvania. Wendi received her Ph.D. from the University of British Columbia in 1994. From 1998 to 2003, she was employed by Esperion Therapeutics Inc. culminating in the position of Director, Product Development. Dr. Rodrigueza was a co-inventor of the technology Esperion was founded on. Esperion was sold to Pfizer Global Research and Development for $1.3 billion in 2003. She is currently VP of Drug Development for ProNAi Therapeutics and since 2003 has been a consultant to several companies including CuraGen Corporation and Novartis Institute of Biomedical Research.
Other Reporting Company Experience
The following table sets out the directors, officers and promoters of the Company that are, or have been within the last five years, directors, officers or promoters of other companies that are or were reporting companies in any Canadian jurisdiction:
|
Name of Director, Officer or Promoter
|
Name of Reporting Company
|
Exchange
|
Position
|
Period
|
|
Derick Sinclair, CA
|
Cadan Resources Corporation
|
TSX Venture
|
CFO
|
May 2007 - Present
|
|
Derick Sinclair, CA
|
Madeira Minerals Ltd.
|
NEX
|
CFO
|
May 2009 - Present
|
|
Derick Sinclair, CA
|
Viscount Mining Corp
|
TSX Venture
|
CFO
|
December 2010 - Present
|
B. Compensation.
Remuneration and Borrowing
The Board of Directors may determine remuneration to be paid to the directors. The Compensation Committee assists the Board of Directors in reviewing and approving the compensation structure for the directors. The Board of Directors may exercise all the powers of the Company to borrow money and to mortgage or charge its undertaking, property and uncalled capital, and to issue debentures or other securities whether outright or as security for any debt obligations of the Company or of any third party.
Compensation of Directors and Executive Officers
In 2013, we paid or accrued aggregate cash compensation of approximately $157,916 to our directors and executive officers as a group. We do not pay or set aside any amounts for pension, retirement or other benefits for our officers and directors.
44
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
We provide directors and officer’s liability and company reimbursement insurance to cover all of our directors and officers against losses arising from claims we indemnify for. Our current insurance coverage will expire on November 29, 2014. We plan to renew the insurance upon its expiration.
2013 Stock Option Plan
The 2013 Stock Option Plan was approved by shareholders on August 14, 2013 at the Company’s Annual General Meeting. A copy of this stock option plan is filed herewith as Exhibit 2.7. The purpose of this option plan is to provide incentives to attract, retain and motivate executive officers, directors and employees whose present and future contributions are important to the Company. Subject to regulatory approval, the maximum number of the Company’s Common Shares reserved for issuance pursuant to stock options granted under the stock option plan will, at any time, be 10% of the number of Common Shares then outstanding. The number of the Company’s Common Shares that may be issued to any one person shall not exceed 5% of the Common Shares issued and outstanding on a non-diluted basis. The price at which the Company’s Common Shares may be issued under the stock option plan will be determined from time to time by the Company’s Board of Directors in compliance with the rules and policies of any stock exchange upon which the Company’s Common Shares are listed. The vesting of options granted under the stock option plan will be determined by the Board of Directors at the time of the grant. Options granted under the stock option plan may be exercisable over a maximum period of 5 years. They will generally have a term of 5 years and vest over four years, 25% on each of the first four anniversaries of the date of grant, provided the optionee is in continuous service to the Company. The Board of Directors may amend the terms of the stock option plan from time to time, to the extent permitted by the stock option plan and any rules and policies of any stock exchange on which the Common Shares are listed, or terminate it at any time. If the Company accepts any offer to amalgamate, merge or consolidate with any other company (other than a wholly-owned subsidiary) or if holders of greater than 50% of the Company’s Common Shares accept an offer made to all or substantially all of the holders of the Company’s Common Shares to purchase in excess of 50% of our current issued and outstanding Common Shares, any then-unvested options will automatically vest in full.
This stock option plan was approved by shareholders on August 14, 2013 at the Company’s Annual General Meeting.
Equity Compensation Plan Information as of December 31, 2013
|
Plan Category
|
Column (a)
Number of securities to be issued upon exercise of outstanding options
|
Column (b)
Weighted-average exercise price of outstanding options
|
Column (c)
Number of securities remaining available for future issuance under equity compensation plans
|
|||||||||
|
Equity compensation plans approved by security holders
|
1,900,000 | 0.17 | 1,845,683 | |||||||||
|
Equity compensation plans not approved by security holders
|
Nil
|
N/A |
Nil
|
|||||||||
|
Total
|
1,900,000 | 0.17 | 1,845,683 | |||||||||
Outstanding Options as of April 14, 2014
|
Holders (current and former positions)
|
No. of Shares Under Option
|
Exercise Price
|
Expiry Date
|
|
Directors (including directors (which are also officers)
|
|||
|
Douglas H. Unwin
(CEO & President, Director)
|
375,000
75,000
150,000
100,000
100,000
|
$0.27
$0.10
$0.10
$0.10
$0.10
|
March 5, 2015
July 3, 2017
December 21, 2017
April 4, 2018
March 2, 2019
|
45
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
|
Holders (current and former positions)
|
No. of Shares Under Option
|
Exercise Price
|
Expiry Date
|
|
Directors
|
|||
|
M. Greg Beniston
(Chairman of the Board)
|
75,000
150,000
50,000
200,000
|
$0.10
$0.10
$0.10
$0.10
|
July 3, 2017
December 21, 2017
April 4, 2018
March 2, 2019
|
|
Doug Wallis
(Director)
|
100,000
50,000
50,000
|
$0.10
$0.10
$0.10
|
July 3, 2017
April 4, 2018
March 2, 2019
|
|
Wendi Rodrigueza
(Director)
|
150,000
50,000
50,000
|
$0.27
$0.10
$0.10
|
November 4, 2014
April 4, 2018
March 2, 2018
|
|
Officers (who are not also directors)
|
|||
|
Derick Sinclair
(CFO)
|
150,000
100,000
100,000
|
$0.10
$0.10
$0.10
|
December 21, 2017
April 4, 2018
March 2, 2019
|
|
Consultants
|
|||
|
Monita Farris
(Consultant)
|
25,000
25,000
|
$0.10
$0.10
|
July 3, 2017
March 2, 2019
|
|
Darryl Knight
(Consultant)
|
100,000
|
$0.10
|
July 3, 2017
|
|
James Siebold
(Consultant)
|
100,000
|
$0.10
|
July 3, 2017
|
|
Gale Caital Cop.
|
300,000
|
$0.10
|
January 10, 2017
|
|
(Consultant)
|
|||
|
Kyran Consulring Services
|
100,000
|
$.10
|
January 10, 217
|
|
(Consultant)
|
|||
|
Wendy Chan
(Consultant)
|
100,000
|
$0.10
|
September 1, 2018
|
|
Total Options
|
2,825,000
|
Employment Agreements
The Company entered into an employment agreement with Mr. Unwin effective as of January 1, 2010. This is the only employment agreement the Company has entered into. Under the agreement, Mr. Unwin is to receive an annual base salary of $160,000, subject to increases at the discretion of the Company’s Board of Directors. Mr. Unwin is also eligible for a discretionary performance bonus as determined by the Company’s Board of Directors. Under the agreement, other than in the event of a change in control of the Company, Mr. Unwin may terminate his employment at any time by giving three months prior written notice of the effective date of his resignation. If the Company terminates Mr. Unwin’s employment without cause, the Company is obligated to pay to him a lump sum of up to 12 months of his then current base salary plus such other sums owed for arrears of salary, vacation pay and any performance bonus. The Company is also obligated to maintain Mr. Unwin’s benefits during the notice period. If Mr. Unwin obtains a new source of remuneration for personal services, the payment of benefits will cease six months from the date of termination of his employment, excluding the notice period.
46
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
As of March 1, 2011 Mr. Unwin voluntarily reduced his annual base salary to $120,000 to remain in place until January 31, 2013. On June 1, 2011, Mr. Unwin took a further annual base salary reduction to $100,000. On February 1, 2013, Mr. Unwin’s salary was returned to $160,000 per year.
As of March 1, 2011 Mr. Sinclair voluntarily reduced his base annual fee to $18,000 to remain in place until January 31, 2013. On February 1, 2013 Mr. Sinclair’s base annual fee was returned to $36,000.
Change in Control Agreements
As part of his Employment Agreement, the Company entered into a change of control agreement with Mr. Unwin effective as of January 1, 2010. This is the only change of control agreement the Company has entered into. In the event of a potential change in control and until 12 months after a change in control, unless Mr. Unwin terminates his employment with the Company for good reason, Mr. Unwin will continue to diligently carry out his duties and obligations under his employment agreement. If within 12 months following a change of control of the Company, Mr. Unwin terminates his employment for good reason, or the Company terminates his employment other than for cause, the Company is obligated to pay to Mr. Unwin a lump sum equal to 12 months of his then current base salary plus other sums owed for arrears of salary, vacation pay and any performance bonus. In such case, The Company is also obligated to maintain Mr. Unwin’s benefits for the 12-month period and his unvested stock options will immediately vest.
Board Practices
Duties of Directors
Under British Columbia law, our directors have a duty of loyalty to act honestly in good faith with a view to our best interest. Our directors also have a duty to exercise the care, diligence and skills that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our amended and restated memorandum and articles of association. A shareholder has the right to seek damages if a duty owed by our directors is breached.
The functions and powers of our Board of Directors include, among others:
|
•
|
convening shareholders’ annual general meetings and reporting its work to shareholders at such meetings;
|
|
•
|
issuing authorized but unissued shares and redeem or purchase outstanding shares of our company;
|
|
•
|
declaring dividends and distributions;
|
|
•
|
appointing officers and determining the term of office and compensation of officers;
|
|
•
|
exercising the borrowing powers of our company and mortgaging the property of our company; and
|
|
•
|
approving the transfer of shares of our company, including the registering of such shares in our share register.
|
Qualification
There is no shareholding qualification for directors.
Board Committees
Our Board of Directors has established an Audit Committee and a Compensation Committee.
AUDIT COMMITTEE
The Audit Committee has various responsibilities as set forth in Multilateral Instrument 52-110 (“MI 52-110”). The Audit Committee oversees the accounting and financial reporting practices and procedures of the Company and the audits of the Company’s financial statements. The principal responsibilities of the Audit Committee include: (i) overseeing the quality, integrity and appropriateness of the internal controls and accounting procedures of the Company, including reviewing the Company’s procedures for internal control with the Company’s auditors and Chief Financial Officer; (ii) reviewing and assessing the quality and integrity of the Company’s internal and external reporting processes, its annual and quarterly financial statements and related management discussion and analysis, and all other material continuous disclosure documents; (iii) establishing separate reviews with management and external auditors of significant changes in procedures or financial and accounting practices, difficulties encountered during auditing, and significant judgments made in management's preparation of financial statements; (iv) monitoring compliance with legal and regulatory requirements related to financial reporting; (v) reviewing and pre-approving the engagement of the auditor of the Company and independent audit fees; and (vi) assessing the Company’s accounting policies, and considering, approving, and monitoring significant changes in accounting principles and practices recommended by management and the auditor.
47
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
Audit Committee Charter
A copy of the Charter of the Audit Committee was filed as Exhibit 11.2 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference.
Composition of the Audit Committee
As noted above, the members of the Audit Committee are Douglas Unwin, Greg Beniston and Douglas Wallis, all of whom are considered independent pursuant to NI 52-110, except Mr. Unwin who is also an officer of the Company. All members of the Audit Committee are considered to be financially literate.
A member of the Audit Committee is independent if the member has no direct or indirect material relationship with the Company. A material relationship means a relationship which could, in the view of the Board, reasonably interfere with the exercise of a member’s independent judgment.
A member of the Audit Committee is considered financially literate if he or she has the ability to read and understand a set of financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by the Company.
Relevant Education and Experience
Please see above for the biographies of Douglas Unwin, Greg Beniston and Douglas Wallis.
Audit Committee Oversight
The Audit Committee has not made any recommendations to the Board to nominate or compensate any external auditor.
Reliance of Certain Exemptions
The Company’s auditors have not provided any material non-audit services.
The Company is relying on the exemptions provided for in Section 6.1 of NI 52-110 in respect of the composition of its Audit Committee and in respect of certain of its reporting obligations under NI 52-110.
Pre-Approval Policies on Certain Exemptions
The Audit Committee has not adopted specific policies and procedures for the engagement of non-audit services.
Compensation Committee
Our Compensation Committee consists of Mr. Beniston, Mr. Wallis and Mr. Unwin. Mr. Beniston is the chairman of our Compensation Committee. Our Board of Directors has determined that Mr. Wallis and Mr. Beniston are “independent directors” within the meaning of NYSE Manual Section 303A.
Our Compensation Committee is responsible for, among other things:
|
•
|
reviewing and approving corporate goals and objectives relevant to the compensation of our co-chief executive officers, evaluating the performance of our co-chief executive officers in light of those goals and objectives, and setting the compensation level of our co-chief executive officers based on this evaluation;
|
|
•
|
reviewing and making recommendations to our Board of Directors regarding our compensation policies and forms of compensation provided to our directors and officers;
|
48
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
|
•
|
reviewing and making recommendations to our co-chief executive regarding the compensation level, share-based compensation and bonuses for our officers other than our co-chief executive officers;
|
|
•
|
reviewing and determining cash and share-based compensation for our directors;
|
|
•
|
administering our equity incentive plans in accordance with the terms thereof; and
|
|
•
|
such other matters that are specifically delegated to the Compensation Committee by our Board of Directors from time to time.
|
Corporate Governance
General
Effective June 30, 2005, NI 58-101 and NP 58-201 were adopted in each of the provinces and territories of Canada. NI 58-101 requires companies to disclose the corporate governance practices that they have adopted. NP 58-201 provides guidance on corporate governance practices.
The Board believes that good corporate governance improves corporate performances and benefits all shareholders. The Canadian Securities Administrators (“CSA”) have adopted NP 58-201, which provides non-prescriptive guidelines on corporate governance practices for reporting companies such as the Company. In addition, the CSA have implemented NI 58-101, which prescribes certain disclosure by the Company of its corporate governance practices. This section sets out the Company’s approach to corporate governance and addresses the Company’s compliance with NI 58-101.
Composition of the Board
The Board of Directors facilitates its exercise of independent supervision over management by ensuring that the Board is composed of a majority of independent directors. Directors are considered to be independent if they have no direct or indirect material relationship with the Company. A “material relationship” is a relationship which could, in the view of the Board, be reasonably expected to interfere with the exercise of a director’s independent judgment. The Board has four directors, three of which are considered to be independent. Mr. Beniston, Mr. Wallis, and Ms. Rodrigueza are considered to be independent directors for the purposes of NI 58-101, and Mr. Unwin is not considered to be independent as he is also a senior officer of the Company.
The mandate of the Board is to act in the best interests of the Company and to supervise management. The Board is responsible for approving long-term strategic plans and annual operating budgets recommended by management. Board consideration and approval is also required for material contracts and business transactions, and all debt and equity financing transactions. Any responsibility which is not delegated to management or to the committees of the Board remains with the Board. The Board meets on a regular basis consistent with the state of the Company’s affairs and also from time to time as deemed necessary to enable it to fulfill its responsibilities.
The Chairman of the Board is Mr. Greg Beniston, LLB, who is an independent director.
Directorship
None of the directors of the Company is also a director of other reporting companies (or equivalent) in a Canadian or foreign jurisdiction as of the date of this listing statement.
Position Descriptions
The Board has not developed written position descriptions for the chair or the chair of any board committees or for the CEO. Given the size of the Company’s infrastructure and the existence of only a small number of officers, the Board does not feel that it is necessary at this time to formalize position descriptions in order to delineate their respective responsibilities.
Meetings of Independent Directors
The Board has appointed two committees, the Audit Committee and the Compensation Committee. The Audit committee is comprised of a majority of independent directors and meets regularly. Additional information concerning the committee is found in ‘ Audit Committee ’ above and in the disclosure below in this ‘ Corporate Governance ’ section.
49
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
The Compensation Committee is comprised of two independent directors plus the CEO. This committee meets as required. The members of the Compensation Committee are Mr. Beniston, Mr. Wallis and Mr. Unwin.
Orientation and Continuing Education
When new directors are appointed, they receive orientation, commensurate with their previous experience, on the Company’s technologies, product candidates, business and industry and on the responsibilities of directors. New directors also receive historical public information about the Company and the mandates of the committees of the Board. Board meetings may also include presentations by the Company’s management and employees to give the directors additional insight into the Company’s business. In addition, new directors are encouraged to visit and meet with management on a regular basis and to pursue continuing education opportunities where appropriate.
Ethical Business Conduct
The Board has approved a Code of Business Conduct and Ethics (the “Code”, filed as Exhibit 11.1 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference) to be followed by the Company’s directors, officers, employees and principal consultants and those of its subsidiaries. The Code is also to be followed, where appropriate, by the Company’s agents and representatives, including consultants where specifically required. The purpose of the Code is to, among other things, promote honest and ethical conduct, avoid conflicts of interest, protect confidential or proprietary information and comply with the applicable government laws and securities rules and regulations. In the event that a director, officer or employee departs from the Code, the Company is authorized to file a material change report. The board does not actively monitor compliance with the Code, but requires prompt notification of apparent or actual breaches so that it may investigate and take action. The Code has been circulated to all employees.
When proposed transactions or agreements in which directors or officers may have an interest, material or not, are presented to the Board, such interest is disclosed and the persons who have such an interest are excluded from all discussion on the matter and are not allowed to vote on the proposal.
Nomination of Directors
The Company does not have a formal process or committee for proposing new nominees for election to the Board of Directors. The nominees are generally the result of recruitment efforts by the Board members, including both formal and informal discussions among Board members.
Compensation
The Board has established a Compensation Committee. The Compensation Committee is responsible for reviewing the adequacy and form of compensation paid to the Company’s executives and key employees, and ensuring that such compensation realistically reflects the responsibilities and risks of such positions. In fulfilling its responsibilities, the Board evaluates the performance of the chief executive officer and other senior management in light of corporate goals and objectives, and makes recommendations with respect to compensation levels based on such evaluations.
Other Board Committees
Other than the Audit Committee and Compensation Committee described in this Form 20-F, the Board has no other committees.
Assessments
The Board regularly assesses its own effectiveness and the effectiveness and contribution of each Board committee member and Director.
Interested Transactions
A director may vote with respect to any contract or transaction in which he or she is interested, provided that the nature of the interest of any director in such contract or transaction is disclosed by him or her at or prior to its consideration and any vote in that matter.
50
|
Item 6.
|
Directors, Senior Management and Employees - continued
|
D. Employees
As of December 31, 2013 the Company had the following number of employees and contractors:
|
Location
|
Full Time Employees
|
Contractors
|
|
Vancouver, British Columbia
|
1
|
2
|
The Company utilizes consultants and contractors to carry on many of its activities and, in particular, to supervise and conduct pre-clinical scientific experiments, assay development and validation. In addition, the Company’s Chief Financial Officer is a contractor not a full time employee. Other functions the Company has decided to outsource include assay development, formulation, clinical trials and manufacturing. It is currently more cost-effective to outsource these functions due to the Company’s sporadic requirements. As the Company expands its activities, it is probable that it will hire additional employees. In addition, contractors and employees may move between locations from time to time as conditions and business opportunities warrant.
E. Share Ownership.
As of December 31, 2013, the Company has 37,456,825 shares of Common stock outstanding.
The following table sets forth, as of December 31, 2013: (a) the names of each beneficial owner of more than five percent (5%) of our Common Stock known to us, the number of shares of Common Stock beneficially owned by each such person, and the percent of our Common Stock so owned before and after the Share Exchange; and (b) the names of each director, executive officer and significant employee, the number of shares our Common Stock beneficially owned, and the percentage of our Common Stock so owned, by each such person, and by all of our directors and executive officers as a group. Each person has sole voting and investment power with respect to the shares of our Common Stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of Common Stock, except as otherwise indicated. Individual beneficial ownership also includes shares of Common Stock that a person has the right to acquire within 60 days from December 31.
|
Name and Municipality of Residence and Position
|
Common Shares Held
|
Percentage of Common Shares Outstanding (2)
|
Percentage of Votes Held
|
|
|
Douglas H. Unwin
North Vancouver, BC
President, CEO, Director (1)
|
4,539,667
|
(3)(4)
|
12.1%
|
12.1%
|
|
Douglas Wallis Vancouver, BC
Director (1)
|
350,510
|
0.9%
|
0.9%
|
|
|
M. Greg Beniston, BA, LLB
Vancouver, BC
Chairman (1)
|
400,000
|
1.1%
|
1.1%
|
|
|
Wendi Rodrigueza Boston, Mass
Director
|
100,000
|
0.34%
|
0.34%
|
|
|
Derick Sinclair, CA
North Vancouver, BC
CFO and Corp. Secretary
|
435,510
|
1.2%
|
1.2%
|
|
|
Directors and Officers as a Group
|
15.6%
|
15.6%
|
||
|
(1)
|
Members of the Audit and Compensation Committee.
|
|
(2)
|
The calculations are based on 37,456,825 shares of Common Stock issued and outstanding as of December 31, 2013.
|
|
(3)
|
549,800 shares of these are held by Donna Armstrong, Mr. Unwin’s spouse.
|
|
(4)
|
1,660,500 shares of these are held by Douglas Cove Capital Corp., a company owned jointly between Douglas H. Unwin and his spouse Donna Armstrong.
|
|
Item 7.
|
Major Shareholders and Related Party Transactions
|
A . Major Shareholders.
51
|
Item 7.
|
Major Shareholders and Related Party Transactions - continued
|
There are 2 shareholders of record in the United States holding 108,504 common shares or 0.4%.
For more information, please refer to Item 6.E, “Directors, Senior Management and Employees — Share Ownership”.
B. Related Party Transactions.
Transactions with related parties are in the normal course of operations and are measured at the exchange amount, which is the consideration agreed to by the parties. During the years ended December 31, 2013, December 31, 2012, December 31, 2011, the Company entered into the following transactions with related parties:
|
|
·
|
During the year ended December 31, 2013, the CEO of the Company exercised Nil common share purchase warrants, [FYE 2012 – 66,666, FYE 2011 - 7,500];
|
|
|
·
|
During the year ended December 31, 2013 the Company received $Nil from two founders to re-price common shares to $0.02 per share [FYE 2012 - $Nil, FYE 2011 - $30,000);
|
|
|
·
|
During the year ended December 31, 2013, a company controlled by the CEO of the Company paid $Nil and re-priced Nil common shares owned by it to $0.02 per share [FYE 2012 - $Nil, FYE 2011 – $41,600];
|
|
|
·
|
The Company incurred accounting fees for the year ended December 31, 2013, to a company controlled by its CFO, in the amount of $34,500 [FYE 2012 - $Nil , FYE 2011 – $11,600];
|
|
·
|
The Company incurred legal fees from a consultant and director of the Issuer in the amount of $8,575 for the year ended December 31, 2013, [FYE 2012 -$3,200, FYE 2011 – $7,934];
|
|
·
|
The Company incurred salaries, directors fees and other benefits relating to directors and officers of the company in the amount of $187,824 for the year ended December 31, 2013 [FYE 2012 – $142,788, FYE 2011 - $121,297]; and
|
|
·
|
During the year ended December 31, 2013, the Company issued 480,000 shares to settle $24,000 outstanding debt owing to a shareholder of the Company [FYE 2012 - $7,500, FYE 2011 - $7,500].
|
There are no amounts due to the Company from companies that have directors in common with the Issuer or have a partner who is a director of the Issuer.
There were no amounts due to the Company from shareholders in either fiscal year.
C . Interests of Experts and Counsel.
Not applicable.
|
Item 8.
|
Financial Information
|
A . Financial statements and other financial information.
We have appended financial statements filed as part of this Form 20-F. See Item 18, “Financial Statements.”
|
Item 9.
|
The Offer and Listing
|
A. Offer and Listing Details
The Company’s shares were listed for trading on the Canadian Securities Exchange under the symbol of “PT” on November 16, 2011. The Company’s shares were approved for quotation on the OTC QB Market on April 16, 2014 and Frankfurt Stock Exchange on September 16, 2013
The annual high and low market prices most recent full financial years since the company’s shares were listed for trading are:
|
Year Ended
|
High Sales Price
|
Low Sales Price
|
||||||
|
2011
|
$
|
0.25
|
$
|
0.15
|
||||
|
2012
|
$
|
0.17
|
$
|
0.03
|
||||
|
2013
|
$
|
0.16 |
$
|
0.04 | ||||
52
|
Item 9.
|
The Offer and Listing - continued
|
The quarterly high and low sale prices for our ordinary shares for the two most recent full financial years and any subsequent quarters are:
|
Quarters Ended
|
High Sales Price
|
Low Sales Price
|
||||||
|
March 31, 2012
|
$ | 0.20 | $ | 0.10 | ||||
|
June 30, 2012
|
$ | 0.19 | $ | 0.07 | ||||
|
September 30, 2012
|
$ | 0.17 | $ | 0.09 | ||||
|
December 31, 2012
|
$ | 0.17 | $ | 0.03 | ||||
|
March 31, 2013
|
$ | 0.08 | $ | 0.04 | ||||
|
June 30, 2013
|
$ | 0.12 | $ | 0.05 | ||||
|
September 30, 2013
|
$ | 0.10 | $ | 0.05 | ||||
|
December 31, 2013
|
$ | 0.16 | $ | 0.05 | ||||
The monthly high and low sale prices for our ordinary shares for the 12 most recent months are:
|
Month
|
High Sales Price
|
Low Sales Price
|
||||||
|
January 2013
|
0.04
|
0.07
|
||||||
|
February 2013
|
$
|
0.06
|
$
|
0.06
|
||||
|
March 2013
|
$
|
0.08
|
$
|
0.05
|
||||
|
April 2013
|
$
|
0.115
|
$
|
0.10
|
||||
|
May 2013
|
$
|
0.11
|
$
|
0.09
|
||||
|
June 2013
|
$
|
0.085
|
$
|
0.06
|
||||
|
July 2013
|
$
|
0.05
|
$
|
0.05
|
||||
|
August 2013
|
$
|
0.10
|
$
|
0.05
|
||||
|
September 2013
|
$
|
0.10
|
$
|
0.05
|
||||
|
October 2013
|
$
|
0.15
|
$
|
0.05
|
||||
|
November 2013
|
$
|
0.10
|
$
|
0.05
|
||||
|
December 2013
|
$
|
0.10
|
$
|
0.06
|
||||
There are no pre-emptive rights in the articles of Pacific Therapeutics Ltd. and we have never done any rights offerings.
On April 28, 2014, the closing price of our stock was $0.08 per share.
The company’s shares do not trade regularly and are illiquid.
B. Plan of Distribution
Not applicable.
C. Markets
Please see “Offer and Listing Details” above in this Item 9.
D. Selling Shareholders
Not applicable.
E. Dilution
Not Applicable.
F. Expense of the Issue
Not Applicable.
53
|
Item 10.
|
Additional Information
|
A. Share Capital
As of December 31, 2013, the Company has the following shares authorized and issued:
|
Class of Share
|
Number of Authorized Shares
|
Number of Issued Shares
|
|
Class A common shares without par value
|
Unlimited
|
37,456,825
|
|
Class B Series I preferred shares without par value
|
1,500,000
|
NIL
|
|
Class B Series II preferred shares without par value
|
1,000,000
|
NIL
|
Class A Common Shares
The holders of Common Shares are entitled to receive notice of and to attend and vote at all meetings of shareholders of the Company and each Common Share shall confer the right to one vote in person or by proxy at all meetings of the shareholders of the Company. The holders of the Common Shares, are entitled to receive dividends as and when declared by the directors and, subject to the rights of holders of any shares ranking in priority to or on a parity with the Common Shares, to participate ratably in any distribution of property or assets upon the liquidation, winding-up or other dissolution of the Company.
Class B Series I Preferred Shares
Each Series I Class B Preferred Share automatically converted into one (1) Common Share when the Common Shares of the Company were listed for trading on the CSE.
In the event of a change in control of the Company involving greater than fifty percent (50%) of the issued and outstanding Common Shares of the Company at a valuation of less than $0.40 per share, or the liquidation, dissolution or wind-up of the Company or any other distribution of the assets of the Company among its shareholders for the purpose of winding up its affairs, the holders of the Series I Preferred Shares shall be entitled to receive, in preference and priority to any payment or distribution to the holders of the Common Shares or any other class of shares ranking junior to the Series I Preferred Shares, an amount equal to $0.20 per share, together with all accrued and unpaid dividends thereon. After payment to the holders of the Series I Preferred Shares of the amounts so payable to them, they shall be entitled to share in any further distribution of the property or assets of the Company. There are no Series I Preferred shares issued.
Subject to the rights, privileges, restrictions and conditions attaching to any other class or series of shares of the Company, the holders of the Series I Preferred Shares shall be entitled to receive any dividends declared and payable by the Company on the Series I Preferred Shares. No dividend shall be declared or paid or set apart for the Common Shares then issued and outstanding until an equal or greater dividend on all Series I Preferred Shares then issued and outstanding shall have been declared or paid or provided for at the date of such declaration or payment or setting apart. No dividend has been declared on the Series I Preferred Shares.
Class B Series 2 Preferred Shares
Subject to the rights, privileges, restrictions and conditions attaching to any other class or series of shares of the Company, the holders of the Series II Preferred Shares shall be entitled to receive any dividends declared and payable by the Company on the Series II Preferred Shares. No dividend shall be declared or paid or set apart for the Common Shares then issued and outstanding until an equal or greater dividend on all Series I Preferred Shares and all Series II Preferred Shares then issued and outstanding shall have been declared or paid or provided for at the date of such declaration or payment or setting apart. A 12% annual cumulative dividend shall be paid on the Series II Preferred Shares. This dividend shall be paid “in-kind” to the holders in the form of Common Shares of the Company converted at the Transaction Price at the time of a Transaction. For greater certainty, any unpaid cumulative dividend(s) due to the holders of Series II Preferred Shares shall be paid to the holders at the time of the Transaction in that number Common Shares equal to the amount of any unpaid cumulative dividend(s) due to the holders divided by the Transaction Price. No fractional shares shall be issued upon the granting of any dividend in-kind of Common Shares. There are no Series 2 Preferred Shares issued.
Each Series II Preferred Share automatically converted upon the listing of the Company’s Common Shares on the CSE.
Each Series II Preferred Share converted into Common Shares at the Conversion Rate plus one-half (1/2) of a purchase warrant in the capital of the Company where one (1) full Series II Purchase Warrant may be exercised at the Transaction Price for a period of two (2) years from its date of issue to purchase one (1) Common Share. No fractional shares shall be issued under any conversion into Common Shares.
54
|
Item 10.
|
Additional Information - continued
|
Stock Options:
As of December 31, 2013 and 2012, the following stock options of the Company were outstanding:
|
Expiry Date
|
Exercise Price $
|
2013
|
2012
|
|||||||||
|
14-Aug-13
|
0.27 | - | 225,000 | |||||||||
|
4-Nov-14
|
0.27 | 150,000 | 150,000 | |||||||||
|
5-Mar-15
|
0.27 | 375,000 | 375,000 | |||||||||
|
3-Jul-17
|
0.10 | 475,000 | 475,000 | |||||||||
|
21-Dec-17
|
0.10 | 450,000 | 450,000 | |||||||||
|
04-Apr-18
|
0.10 | 350,000 | - | |||||||||
|
16-Sep-18
|
0.10 | 100,000 | - | |||||||||
|
Balance
|
0.15 | 1,900,000 | 1,675,000 | |||||||||
Warrants
As at December 31, 2013, the following share purchase warrants were issued and outstanding:
|
Expiry Date
|
Exercise Price $
|
2013
|
2012
|
||||||||||
|
15-Nov-13
|
$ | 0.15 | - | 602,223 | |||||||||
|
31-Jan-14
|
$ | 0.15 | (1 | ) | 2,473,334 | 2,473,334 | |||||||
|
28-Feb-14
|
$ | 0.25 | (2 | ) | 60,000 | 60,000 | |||||||
|
16-May-14
|
$ | 0.15 | (3 | ) | 600,000 | 600,000 | |||||||
|
19-Jun-14
|
$ | 0.22 | 56,666 | 56,666 | |||||||||
|
20-Jun-14
|
$ | 0.22 | 732,670 | 732,670 | |||||||||
|
21-Sep-14
|
$ | 0.22 | 747,166 | 747,166 | |||||||||
|
24-Sep-14
|
$ | 0.22 | 200,000 | - | |||||||||
|
12-Feb-15
|
$ | 0.22 | 1,000,000 | - | |||||||||
|
01-May-15
|
$ | 0.22 | 1,300,000 | - | |||||||||
|
08-Oct-16
|
$ | 0.10 | 2,250,000 | - | |||||||||
|
18-Oct-16
|
$ | 0.10 | 2,020,000 | - | |||||||||
|
05-Nov-16
|
$ | 0.10 | 6,780,000 | - | |||||||||
|
BALANCE AT DECEMBER 31
|
18,219,836 | 5,272,059 | |||||||||||
* On January 18, 2013 the warrants with expiration dates of January 31, 2013, February 28, 2013 and May 16, 2013 were all extended by one year to expire on the same days in 2014.
|
(1)
|
The content of the warrant was filed as Exhibit 2.2 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference;
|
|
(2)
|
The content of the warrant was filed as Exhibit 2.3 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference; and
|
|
(3)
|
The content of the warrant was filed as Exhibit 2.4 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference.
|
55
|
Item 10.
|
Additional Information - continued
|
B. Memorandum and Articles of Association
According to our Articles of Incorporation (the “Articles”), the directors must, subject to the Business Corporations Act and our Articles, manage or supervise the management of the business and affairs of the Company and have the authority to exercise all such powers of the Company as are not, by the Business Corporations Act or by these Articles, required to be exercised by the shareholders of the Company. The directors may from time to time appoint any person to be the attorney of the Company for such purposes, and with such powers, authorities and discretions (not exceeding those vested in or exercisable by the directors under these Articles and excepting the power to fill vacancies in the board of directors, to remove a director, to change the membership of, or fill vacancies in, any committee of the directors, to appoint or remove officers appointed by the directors and to declare dividends) and for such period, and with such remuneration and subject to such conditions as the directors may think fit. Any such power of attorney may contain such provisions for the protection or convenience of persons dealing with such attorney as the directors think fit. Any such attorney may be authorized by the directors to sub-delegate all or any of the powers, authorities and discretions for the time being vested in him or her.
A director who holds a disclosable interest in a contract or transaction into which the Company has entered or proposes to enter is not entitled to vote on any directors’ resolution to approve that contract or transaction, unless all the directors have a disclosable interest in that contract or transaction, in which case any or all of those directors may vote on such resolution. A director who holds a disclosable interest in a contract or transaction into which the Company has entered or proposes to enter and who is present at the meeting of directors at which the contract or transaction is considered for approval may be counted in the quorum at the meeting whether or not the director votes on any or all of the resolutions considered at the meeting. A director or senior officer who holds any office or possesses any property, right or interest that could result, directly or indirectly, in the creation of a duty or interest that materially conflicts with that individual’s duty or interest as a director or senior officer, must disclose the nature and extent of the conflict as required by the Business Corporations Act.
The directors may meet together for the conduct of business, adjourn and otherwise regulate their meetings as they think fit, and meetings of the directors held at regular intervals may be held at the place, at the time and on the notice, if any, as the directors may from time to time determine. Questions arising at any meeting of directors are to be decided by a majority of votes and, in the case of an equality of votes, the chair of the meeting shall have a second or casting vote. The quorum necessary for the transaction of the business of the directors may be set by the directors and, if not so set, is deemed to be a majority or, if the number of directors is set at one, is deemed to be set at one director, and that director may constitute a meeting.
The Company is authorized to issue an unlimited number of Class A Common Shares and an unlimited number of Class B Preferred Shares. The holders of the Common Shares shall be entitled to receive notice of and to attend all meetings of shareholders of the Company and shall have one vote for each Common Share held. The holders of the Common Shares shall be entitled to receive any dividends declared and payable by the Company on the Common Shares, and entitled to receive the remaining property of the Company upon the liquidation, dissolution or winding-up of the Company. The Class B Preferred Share may at any time and from time to time be issued in one or more series. The Class B Preferred Shares of each series shall, with respect to the payment of dividends and the distribution of assets in the event of liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, rank on a parity with the Class B Preferred Shares of every other series and be entitled to preference over the Class A Common Shares and over any other shares of the Company ranking junior to the Class B Preferred Shares. If any amount of cumulative dividends (whether or not declared) or declared non-cumulative dividends or any amount payable on any such distribution of assets constituting a return of capital in respect of the preferred shares of any series is not paid in full, the Class B Preferred Shares of that series shall participate rateably with the Class B Preferred Shares of every other series in respect of such dividends and amounts.
The Company must hold an annual general meeting at least once in each calendar year and not more than 15 months after the last annual reference date at such time and place as may be determined by the directors. If all the shareholders who are entitled to vote at an annual general meeting consent by a unanimous resolution under the Business Corporations Act to all of the business that is required to be transacted at that annual general meeting, the annual general meeting is deemed to have been held on the date of the unanimous resolution. The shareholders must, in any unanimous resolution passed, select as the Company’s annual reference date a date that would be appropriate for the holding of the applicable annual general meeting. The directors may, whenever they think fit, call a meeting of shareholders. The quorum for the transaction of business at a meeting of shareholders is two persons who are, or who represent by proxy, shareholders who, in the aggregate, hold at least 5% of the issued shares entitled to be voted at the meeting. The majority of votes required for the Company to pass a special resolution (“special business” is defined under Article 11.1) at a meeting of shareholders is two-thirds of the votes cast on the resolution.
The Company’s Articles of Incorporation were filed as Exhibit 1.1 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference.
56
|
Item 10.
|
Additional Information - continued
|
C. Material Contracts
Except as otherwise disclosed in this Form 20-F, the Company has no other material contracts. The following are the material contracts of the Company entered into since September 12, 2005 and still in effect:
|
(a)
|
Employment Agreement with the CEO, dated January 1, 2010 (filed as Exhibit 4.1 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference);
|
|
(b)
|
Directors and Officers Insurance with an effective date of November 29, 2013;
|
|
(c)
|
Co-development and Licensing Agreement with IntelGenx, dated February 28, 2010 (filed as Exhibit 4.3 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference). This agreement supersedes the Letter of Intent between the parties dated November 23, 2010; and
|
|
(d)
|
Licensing Agreement with Globe. This agreement supersedes the Letter of Intent with Globe, dated November 22, 2012 (filed herewith as Exhibit 4.4).
|
Escrow Agreements
The Company is classified as an “emerging issuer” under National Policy 46-201. An “emerging issuer” is one that does not meet the “established issuer” criteria based on the Company being an “emerging issuer”, the Escrowed Securities (as hereinafter defined) will be subject to a three year escrow.
If the Company achieves “established issuer” status during the term of the 46-201 Escrow Agreement (as hereinafter defined), it will ‘graduate’, resulting in a catch-up release and an accelerated release of any securities remaining in escrow under the 18 month schedule applicable to established issuers as if the Company had originally been classified as an established issuer.
The Principals of the Company and holders of Shares having an issuance price of less than $0.02 per share have entered into an escrow agreement dated August 30, 2011 (the “46-201 Escrow Agreement”) among the Company, the Transfer Agent, the Principals of the Company and holders of shares having an issuance price of less than $0.02 per share (collectively with the Principals, the “Escrow Holders”), as required pursuant to the policies of the CSE. The Escrow Holders will agree to deposit in escrow their shares (the “Escrowed Securities”) with the Transfer Agent. Under the 46-201 Escrow Agreement, 10% of the Escrowed Securities will be released from escrow on the Listing Date (the “Initial Release”) and an additional 15% will be released on the dates which are 6 months, 12 months, 18 months, 24 months, 30 months and 36 months following the Initial Release.
Pursuant to the terms of the Escrow Agreement, the Escrowed Securities may not be transferred or otherwise dealt with during the term of the 46-201 Escrow Agreement unless the transfers or dealings within escrow are:
|
(1)
|
transfers to continuing or, upon their appointment, incoming directors and senior officers of the Company or of a material operating subsidiary, with approval of the Company’s Board;
|
|
(2)
|
transfers to an RRSP or similar trustee plan provided that the only beneficiaries are the transferor or the transferor’s spouse, children or parents;
|
|
(3)
|
transfers upon bankruptcy to the trustee in bankruptcy; and
|
|
(4)
|
pledges to a financial institution as collateral for a bona fide loan, provided that upon a realization the securities remain subject to escrow.
|
Tenders of Escrowed Securities to a take-over bid are permitted provided that, if the tenderer is a Principal of the successor corporation upon completion of the take-over bid, securities received in exchange for tendered Escrow securities are substitute in escrow on the basis of the successor corporation’s escrow classification.
Where the Common Shares of the Company which are required to be held in escrow are held by a non-individual (a “holding company”), each holding company pursuant to the 46-201 Escrow Agreement, has agreed, or will agree, not to carry out any transactions during the currency of the 46-201 Escrow Agreement which would result in a change of control of the holding company, without the consent of the Exchange. Any holding company must sign an undertaking to the Exchange that, to the extent reasonably possible, it will not permit or authorize any issuance of securities or transfer of securities could reasonably result in a change of control of the holding company. In addition, the Exchange may require an undertaking from any control person of the holding company not to transfer the shares of that company.
57
|
Item 10.
|
Additional Information - continued
|
D. Exchange Controls
There are no laws, decrees or regulations in Canada relating to restrictions on the export or import of capital, or affecting the remittance of interest, dividends or other payments to non-resident holders of our shares of common stock.
E. Taxation
Canada
Canadian Federal Income Tax Information for United States Residents
The following is a discussion of material Canadian federal income tax considerations generally applicable to holders of our common shares who, for purposes of the Income Tax Act (Canada) and the regulations thereunder, or the Canadian Tax Act:
|
·
|
deal at arm’s length and are not affiliated with us;
|
|
·
|
hold such shares as capital property;
|
|
·
|
do not use or hold (and will not use or hold) and are not deemed to use or hold our common shares, in or in the course of carrying on business in Canada;
|
|
·
|
have not been at any time residents of Canada; and
|
|
·
|
are, at all relevant times, residents of the United States, or U.S. Residents, under the Canada-United States Income Tax Convention (1980), or the Convention.
|
TAX MATTERS ARE VERY COMPLICATED AND THE CANADIAN FEDERAL INCOME TAX CONSEQUENCES OF PURCHASING, OWNING AND DISPOSING OF OUR COMMON SHARES WILL DEPEND UPON THE STOCKHOLDER’S PARTICULAR SITUATION. THE SUMMARY OF MATERIAL CANADIAN FEDERAL INCOME TAX CONSEQUENCES SET FORTH BELOW IS INTENDED TO PROVIDE ONLY A GENERAL SUMMARY AND IS NOT INTENDED TO BE A COMPLETE ANALYSIS OR DESCRIPTION OF ALL POTENTIAL CANADIAN FEDERAL INCOME TAX CONSEQUENCES.
THIS DISCUSSION DOES NOT INCLUDE A DESCRIPTION OF THE TAX LAWS OF ANY PROVINCE OR TERRITORY WITHIN CANADA. ACCORDINGLY, HOLDERS AND PROSPECTIVE HOLDERS OF OUR COMMON SHARES ARE ENCOURAGED TO CONSULT WITH THEIR OWN TAX ADVISERS ABOUT THE TAX CONSEQUENCES TO THEM HAVING REGARD TO THEIR OWN PARTICULAR CIRCUMSTANCES, INCLUDING ANY CONSEQUENCES OF PURCHASING, OWNING OR DISPOSING OF OUR COMMON SHARES ARISING UNDER CANADIAN FEDERAL, CANADIAN PROVINCIAL OR TERRITORIAL, U.S. FEDERAL, U.S. STATE OR LOCAL TAX LAWS OR TAX LAWS OF JURISDICTIONS OUTSIDE THE UNITED STATES OR CANADA.
This summary is based on the current provisions of the Canadian Income Tax Act, proposed amendments to the Canadian Income Tax Act publicly announced by the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”), and the provisions of the Convention as in effect on the date hereof. No assurance can be given that the Proposed Amendments will be entered into law in the manner proposed, or at all. No advance income tax ruling has been requested or obtained from the Canada Revenue Agency (“CRA”) to confirm the tax consequences of any of the transactions described herein.
This summary is not exhaustive of all possible Canadian federal income tax consequences for U.S. Residents, and other than the Proposed Amendments, does not take into account or anticipate any changes in law, whether by legislative, administrative, governmental or judicial decision or action, nor does it take into account Canadian provincial, U.S. or foreign tax considerations which may differ significantly from those discussed herein. No assurances can be given that subsequent changes in law or administrative policy will not affect or modify the opinions expressed herein.
A U.S. Resident will not be subject to tax under the Canadian Tax Act in respect of any capital gain on a disposition of our common shares unless such shares constitute “taxable Canadian property”, as defined in the Canadian Tax Act, of the U.S. Resident and the U.S. Resident is not eligible for relief pursuant to the Convention. Our common shares will not constitute “taxable Canadian property” if, at any time during the 60-month period immediately preceding the disposition of the common shares, the U.S. Resident, persons with whom the U.S. Resident did not deal at arm’s length, or the U.S. Resident together with all such persons, did not own 25% or more of the issued shares of any class or series of shares of our capital stock. In addition, the Convention generally will exempt a U.S. Resident who would otherwise be liable to pay Canadian income tax in respect of any capital gain realized by the U.S. Resident on the disposition of our common shares, from such liability provided that the value of our common shares is not derived principally from real property situated in Canada, Canadian Resource Property and Canadian Timber Resource Property. However, where the US resident and purchaser are related the purchaser must generally report the transaction to the CRA within 30 days of the transaction date to benefit from the Convention. The Convention may not be available to a U.S. Resident that is a U.S. LLC which is not subject to tax in the U.S.
58
|
Item 10.
|
Additional Information - continued
|
Amounts in respect of our common shares paid or credited or deemed to be paid or credited as, on account or in lieu of payment of, or in satisfaction of, dividends to a U.S. Resident will generally be subject to Canadian non-resident withholding tax at the rate of 25%. Currently, under the Convention the rate of Canadian non-resident withholding tax will generally be reduced to:
|
·
|
5% of the gross amount of dividends if the beneficial owner is a company that is resident in the U.S. and that owns at least 10% of our voting shares; or
|
|
·
|
15% of the gross amount of dividends if the beneficial owner is some other resident of the U.S.
|
Generally, the Convention does not apply to US resident LLC’s that are fiscally transparent. However, the Convention may apply to afford reduced withholding tax rates on dividends attributed to a US resident member of a US resident fiscally transparent LLC to the extent of the dividend being considered to have been received by that member.
United States Federal Income Tax Information for United States Holders.
The following is a general discussion of material U.S. federal income tax consequences of the ownership and disposition of our common shares by U.S. Holders (as defined below). This discussion is based on the United States Internal Revenue Code of 1986, as amended, Treasury regulations promulgated thereunder, and judicial and administrative interpretations thereof, all as in effect at the date hereof and all of which are subject to change, possibly with retroactive effect. This discussion only addresses the tax consequences for U.S. Holders that will hold their common shares as a “capital asset” and does not address U.S. federal income tax consequences that may be relevant to particular U.S. Holders in light of their individual circumstances or U.S. Holders that are subject to special treatment under certain U.S. federal income tax laws, such as:
|
·
|
tax-exempt organizations and pension plans;
|
|
·
|
persons subject to alternative minimum tax;
|
|
·
|
banks and other financial institutions;
|
|
·
|
insurance companies;
|
|
·
|
partnerships and other pass-through entities (as determined for United States federal income tax purposes);
|
|
·
|
broker-dealers;
|
|
·
|
persons who hold their common shares as a hedge or as part of a straddle, constructive sale, conversion transaction, and other risk management transaction; and
|
|
·
|
persons who acquired their common shares through the exercise of employee stock options or otherwise as compensation.
|
As used herein, the term “U.S. Holder” means a beneficial owner of our common shares that is:
|
·
|
an individual citizen or resident of the United States;
|
|
·
|
a corporation, a partnership or entity treated as a corporation or partnership for U.S. federal income tax purposes, that is created or organized in or under the laws of the United States or any political subdivision thereof;
|
|
·
|
an estate the income of which is subject to U.S. federal income taxation regardless of its source; and
|
59
|
Item 10.
|
Additional Information - continued
|
|
·
|
a trust if both: a United States court is able to exercise primary supervision over the administration of the trust; and one or more United States persons have the authority to control all substantial decisions of the trust.
|
TAX MATTERS ARE VERY COMPLICATED AND THE UNITED STATES FEDERAL INCOME TAX CONSEQUENCES OF PURCHASING, OWNING AND DISPOSING OF OUR COMMON SHARES WILL DEPEND UPON THE STOCKHOLDER’S PARTICULAR SITUATION. THE SUMMARY OF MATERIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES SET FORTH BELOW IS INTENDED TO PROVIDE ONLY A GENERAL SUMMARY AND IS NOT INTENDED TO BE A COMPLETE ANALYSIS OR DESCRIPTION OF ALL POTENTIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES.
NOTE THAT THIS DISCUSSION DOES NOT INCLUDE A DESCRIPTION OF THE TAX LAWS OF ANY STATE OR LOCAL GOVERNMENT WITHIN THE UNITED STATES. ACCORDINGLY, HOLDERS AND PROSPECTIVE HOLDERS OF OUR COMMON SHARES ARE ENCOURAGED TO CONSULT THEIR TAX ADVISORS ABOUT THE U.S. FEDERAL, STATE, LOCAL, AND FOREIGN TAX CONSEQUENCES OF PURCHASING, OWNING AND DISPOSING OF OUR COMMON SHARES.
Ownership of Shares
The gross amount of any distribution received by a U.S. Holder with respect to our common shares generally will be included in the U.S. Holder’s gross income as a dividend to the extent attributable to our current and accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s shares. To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s shares, the remainder will be taxed as capital gain (the taxation of capital gain is discussed under the heading “Sale of Shares” below).
For taxable years beginning before January 1, 2009, dividends received by non-corporate U.S. Holders from a qualified foreign corporation are taxed at the same preferential rates that apply to long-term capital gains. A foreign corporation is a “qualified foreign corporation” if it is eligible for the benefits of a comprehensive income tax treaty with the United States (the income tax treaty between Canada and the United States is such a treaty) or the shares with respect to which such dividend is paid is readily tradable on an established securities market in the United States (such as the Nasdaq Capital Market). Notwithstanding satisfaction of one or both of these conditions, a foreign corporation is not a qualified foreign corporation if it is a passive foreign investment company (“PFIC”) for the taxable year of the corporation in which the dividend is paid or the preceding taxable year. (Whether a foreign corporation is a PFIC is discussed below under the heading “Passive Foreign Investment Companies”). A foreign corporation that is a PFIC for any taxable year within a U.S. person’s holding period generally is treated as a PFIC for all subsequent years in the U.S. person’s holding period. Although we have not been, are not now, and do not expect to be a PFIC, and we don’t expect to pay dividends, you should be aware of the following matters in the event that we do become a PFIC and do pay dividends.
If we were to become a PFIC, then U.S. Holders who acquire our common shares may be treated as holding shares of a PFIC throughout their holding period for the purpose of determining whether dividends received from us are dividends from a qualified foreign corporation. As a consequence, dividends received by U.S. Holders may not be eligible for taxation at the preferential rates applicable to long-term capital gains.
If a distribution is paid in Canadian dollars, the U.S. dollar value of such distribution on the date of receipt is used to determine the amount of the distribution received by a U.S. Holder. A U.S. Holder who continues to hold such Canadian dollars after the date on which they are received, may recognize gain or loss upon their disposition due to exchange rate fluctuations. Generally such gains and losses will be ordinary income or loss from U.S. sources.
U.S. Holders may deduct Canadian tax withheld from distributions they receive for the purpose of computing their U.S. federal taxable income (or alternatively a credit may be claimed against the U.S. Holder’s U.S. federal income tax liability as discussed below under the heading “Foreign Tax Credit”). Corporate U.S. Holders generally will not be allowed a dividend received deduction with respect to dividends they receive from us.
Foreign Tax Credit
Generally, the dividend portion of a distribution received by a U.S. Holder will be treated as income in the passive income category for foreign tax credit purposes. Subject to a number of limitations, a U.S. Holder may elect to claim a credit against its U.S. federal income tax liability (in lieu of a deduction) for Canadian withholding tax deducted from its distributions. The credit may be claimed only against U.S. federal income tax attributable to a U.S. Holder’s passive income that is from foreign sources.
60
|
Item 10.
|
Additional Information - continued
|
If we were to become a qualified foreign corporation with respect to a non-corporate U.S. Holder, dividends received by such U.S. Holder will qualify for taxation at the same preferential rates that apply to long-term capital gains. In such case, the dividend amount that would otherwise be from foreign sources is reduced by multiplying the dividend amount by a fraction, the numerator of which is the U.S. Holder’s preferential capital gains tax rate and the denominator of which is the U.S. Holder’s ordinary income tax rate. The effect is to reduce the dividend amount from foreign sources, thereby reducing the U.S. federal income tax attributable to foreign source income against which the credit may be claimed. Canadian withholding taxes that cannot be claimed as a credit in the year paid may be carried back to the preceding year and then forward 10 years and claimed as a credit in those years, subject to the same limitations referred to above.
The rules relating to the determination of the foreign tax credit are very complex. U.S. Holders and prospective U.S. Holders should consult their own tax advisors to determine whether and to what extent they would be entitled to claim a foreign tax credit.
Sale of Shares
Subject to the discussion of the “passive foreign investment company” rules below, a U.S. Holder generally will recognize capital gain or loss upon the sale of our shares equal to the difference between: (a) the amount of cash plus the fair market value of any property received; and (b) the U.S. Holder’s adjusted tax basis in such shares. This gain or loss generally will be capital gain or loss from U.S. sources, and will be long-term capital gain or loss if the U.S. Holder held its shares for more than 12 months. Generally, the net long-term capital gain of a non-corporate U.S. Holder from the sale of shares is subject to taxation at a top marginal rate of 15%. A Capital gain that is not long-term capital gain is taxed at ordinary income rates. The deductibility of capital losses is subject to certain limitations.
Passive Foreign Investment Companies
We will be a PFIC if, in any taxable year either: (a) 75% or more of our gross income consists of passive income; or (b) 50% or more of the value of our assets is attributable to assets that produce, or are held for the production of, passive income. We believe that we are not a PFIC because we have no revenue and we do not own any investments that provide revenue in the form of rent, interest or dividends or any other passive source. However, the determination whether the Company is a PFIC is a factual determination that is made annually and thus may be subject to change. Subject to certain limited exceptions, if we meet the gross income test or the asset test for a particular taxable year, our shares held by a U.S. Holder in that year will be treated as shares of a PFIC for that year and all subsequent years in the U.S. Holder’s holding period, even if we fail to meet either test in a subsequent year.
If we were a PFIC in the future, gain realized by a U.S. Holder from the sale of PFIC Shares and certain dividends received on such shares would be subject to tax under the excess distribution regime, unless the U.S. Holder made one of the elections discussed below. Under the excess distribution regime, federal income tax on a U.S. Holder’s gain from the sale of PFIC Shares would be calculated by allocating the gain ratably to each day the U.S. Holder held its shares. Gain allocated to years preceding the first year in which we were a PFIC in the U.S. Holder’s holding period, if any, and gain allocated to the year of disposition would be treated as gain arising in the year of disposition and taxed as ordinary income. Gain allocated to all other years would be taxed at the highest tax rate in effect for each of those years. Interest for the late payment of tax would be calculated and added to the tax due for each of the PFIC Years, as if the tax was due and payable with the tax return filed for that year. A distribution that exceeds 125% of the average distributions received on PFIC Shares by a U.S. Holder during the 3 preceding taxable years (or, if shorter, the portion of the U.S. Holder’s holding period before the taxable year) would be taxed in a similar manner.
A U.S. Holder may avoid taxation under the excess distribution regime by making a qualified electing fund (“QEF”) election. For each year that we would meet the PFIC gross income test or asset test, an electing U.S. Holder would be required to include in gross income, its pro rata share of our net ordinary income and net capital gains, if any. The U.S. Holder’s adjusted tax basis in our shares would be increased by the amount of such income inclusions. An actual distribution to the U.S. Holder out of such income generally would not be treated as a dividend and would decrease the U.S. Holder’s adjusted tax basis in our shares. Gain realized from the sale of our shares covered by a QEF election would be taxed as a capital gain. U.S. Holders will be eligible to make QEF elections, only if we agree to provide to the U.S. Holders, which we do, the information they will need to comply with the QEF rules. Generally, a QEF election should be made by the due date of the U.S. Holder’s tax return for the first taxable year in which the U.S. Holder held our shares that includes the close of our taxable year for which we met the PFIC gross income test or asset test. A QEF election is made on IRS Form 8621.
A U.S. Holder may also avoid taxation under the excess distribution regime by timely making a mark-to-market election. An electing U.S. Holder would include in gross income the increase in the value of its PFIC Shares during each of its taxable years and deduct from gross income the decrease in the value of its PFIC Shares during each of its taxable years. Amounts included in gross income or deducted from gross income by an electing U.S. Holder are treated as ordinary income and ordinary deductions from U.S. sources. Deductions for any year are limited to the amount by which the income inclusions of prior years’ exceed the income deductions of prior years. Gain from the sale of PFIC Shares covered by an election is treated as ordinary income from U.S. sources while a loss is treated as an ordinary deduction from U.S. sources only to the extent of prior income inclusions. Losses in excess of such prior income inclusions are treated as capital losses from U.S. sources. A mark-to-market election is timely if it is made by the due date of the U.S. Holder’s tax return for the first taxable year in which the U.S. Holder held our shares that includes the close of our taxable year for which we met the PFIC gross income test or asset test. A mark-to-market election is also made on IRS Form 8621.
61
|
Item 10.
|
Additional Information - continued
|
As noted above, a PFIC is not a qualified foreign corporation and hence dividends received from a PFIC are not eligible for taxation at preferential long-term capital gain tax rates. Similarly, ordinary income included in the gross income of a U.S. Holder who has made a QEF election or a market-to-market election, and dividends received from corporations subject to such election, are not eligible for taxation at preferential long-term capital gain rates. The PFIC rules are extremely complex and could, if they apply, have significant, adverse effects on the taxation of dividends received and gains realized by a U.S. Holder. Accordingly, prospective U.S. Holders are strongly urged to consult their tax adviser concerning the potential application of these rules to their particular circumstances.
Controlled Foreign Corporation
Special rules apply to certain U.S. Holders that own stock in a foreign corporation that is classified as a “controlled foreign corporation” (“CFC”). We do not expect to be classified as a CFC. However, future ownership changes could cause us to become a CFC. Prospective U.S. Holders are urged to consult their tax advisor concerning the potential application of the CFC rules to their particular circumstances.
Information Reporting and Backup Withholding
United States information reporting and backup withholding requirements may apply with respect to distributions to U.S. Holders, or the payment of proceeds from the sale of shares, unless the U.S. Holder: (a) is an exempt recipient (including a corporation); (b) complies with certain requirements, including applicable certification requirements; or (c) is described in certain other categories of persons. The backup withholding tax rate is currently 28%. Any amounts withheld from a payment to a U.S. Holder under the backup withholding rules may be credited against any U.S. federal income tax liability of the U.S. Holder and may entitle the U.S. Holder to a refund.
Foreign Account Tax Compliance
The Foreign Account Tax Compliance provisions of the Hiring Incentives to Restore Employment Act (“HIRE”), enacted in 2010, generally impose a new reporting regime and potentially a 30% withholding tax with respect to certain U.S. source income (including dividends and interest) and gross proceeds from the sale or other disposition of property that can produce U.S. source interest or dividends (“Withholdable Payments”). As a general matter, the new rules are designed to require U.S. persons’ direct and indirect ownership of Non-U.S. accounts and Non-U.S. entities to be reported to the IRS. The 30% withholding tax regime applies if there is a failure to provide required information regarding U.S. ownership.
The new rules will subject a Non-U.S. holder’s share of Withholdable Payments and a portion of other payments from Non-U.S. entities (who have entered into FFI Agreements (as defined below)) (“Passthru Payments”) to 30% withholding tax unless such holder provides information, representations and waivers of Non-U.S. law as may be required to comply with the provisions of the new rules. A Non-U.S. Holder that is treated as a “foreign financial institution” will generally be subject to withholding unless it enters into an agreement (a “FFI Agreement”) with the IRS with respect to the foregoing, including reporting certain information to the IRS regarding its U.S. accountholders and those of its affiliates. The new withholding rules generally apply to U.S. source payments made after June 30, 2014 and to other Passthru Payments and the disposition proceeds of U.S. securities after December 31, 2016.
Holders should consult their own advisors regarding the requirements under HIRE with respect to their own situation.
F. Dividends and Paying Agents
Not applicable.
62
|
Item 10.
|
Additional Information - continued
|
G. Statements by Experts
Not applicable.
H. Documents on Display
Not applicable.
I. Subsidiary Information
None.
|
Item 11.
|
Quantitative and Qualitative Disclosures about Market Risk
|
Not applicable.
|
Item 12.
|
Description of Securities Other Than Equity Securities
|
Not applicable.
Part II
|
Item 13.
|
Defaults, Dividend Arrearages and Delinquencies
|
Not applicable.
|
Item 14.
|
Material Modifications to the Rights of Security Holders and Use of Proceeds
|
Not applicable.
|
Item 15.
|
Controls and Procedures
|
(a) Evaluation of Disclosure Controls and Procedures
Pursuant to Rule 13a-15(b) under the Securities Exchange Act of 1934 (“Exchange Act”), the Company carried out an evaluation, with the participation of the Company’s management, including the Company’s Chief Executive Officer and Chief Financial Officer (the Company’s principal financial and accounting officer), of the effectiveness of the Company’s disclosure controls and procedures (as defined under Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report. Based on that evaluation, the President and Chief Executive Officer and Chief Financial Officer concluded that, as of the end of the period covered by this report, the Company’s disclosure controls and procedures were not effective to ensure that information required to be included in our periodic SEC filings is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms, due to a material weakness resulting from limited staffing as the CEO is the Company’s only full time position.
(b) Management’s Annual Report on Internal Control over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Our internal control system was designed to, in general, provide reasonable assurance to the Company’s management and board regarding the preparation and fair presentation of published financial statements, but because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2013. The framework used by management in making that assessment was the criteria set forth in the document entitled “Internal Control – Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that assessment, our management has determined that as of December 31, 2013, the Company’s internal control over financial reporting was ineffective for the purposes for which it is intended.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. In assessment of the effectiveness of internal control over financial reporting as of December 31, 2013, our management determined that limited staffing as the CEO is the Company’s only full time position constituted a material weakness.
At this time the Company is not in a position to remediate the material weakness identified above. We are not able to estimate with reasonable certainty the costs that we will incur to improve our internal control over financial reporting. If we fail to establish an effective system of internal controls, we may be unable to accurately report our financial results or prevent fraud, and investor confidence and the market value of our equity and/or debt may be adversely impacted.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting occurred during the fourth fiscal quarter of the year ended December 31, 2013 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
63
|
Item 16A.
|
Audit Committee Financial Experts
|
A Chartered Accountant for over 30 years, Doug Wallis specializes in work with Canadian and US public companies. Doug received his CA after completing a five-year post-secondary education articling program. His work involved everything from assisting in the structure of initial public offerings to comprehensive audit services. Doug retired from partnership with Smythe Ratcliffe LLP. in June 2013 and now provides consultancy services to public companies or to companies in the process of going public. Doug's extensive experience in accounting and the rules of professional conduct are highly valued in his consultancy assignments.
|
Item 16B.
|
Code of Ethics
|
Our Board of Directors has approved a Code of Business Conduct and Ethics, which was filed as Exhibit 11.1 to our Registration Statement on Form 20-F on November 13, 2013, incorporated herewith by reference.
|
Item 16C.
|
Principal Accountant Fees and Services
|
Audit fees for the year ended December 31, 2013 are estimated at $20,000.
|
Item 16D.
|
Exemptions from the Listing Standards for Audit Committees
|
Not applicable.
|
Item 16E.
|
Purchases of Equity Securities by the Company and Affiliated Purchasers
|
None.
|
Item 16F.
|
Change in Registrant’s Certifying Accountant
|
None.
|
Item 16G.
|
Corporate Governance
|
Not applicable.
For information regarding the Company’s corporate governance, please refer to “Item 6. Directors, Senior Management and Employees – Corporate Governance.”
Part III
|
Item 17.
|
Financial Statements
|
In lieu of responding to this item, we have responded to Item 18 of this annual report.
|
Item 18.
|
Financial Statements
|
The Company’s audited financial statements for fiscal year ended December 31, 2013 are filed as Exhibit 15.3 with this Form 20-F. All of the financial information is presented herein in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
|
Item 19.
|
Exhibits
|
|
Exhibit Number
|
Description of Exhibit
|
|
1.1
|
Articles of Incorporation (Incorporated by reference to Exhibit 1.1 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
2.1
|
Form of Promissory Note (Incorporated by reference to Exhibit 2.1 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
2.2
|
Form of Warrant (Incorporated by reference to Exhibit 2.2 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
2.3
|
Form of Warrant (Incorporated by reference to Exhibit 2.3 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
2.4
|
Form of Warrant (Incorporated by reference to Exhibit 2.4 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
2.5
|
2012 Stock Option Plan (Incorporated by reference to Exhibit 2.5 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
2.6
|
Form of Stock Option Agreement for Scientific Advisory Board Members (Incorporated by reference to Exhibit 2.6 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
4.1
|
Employment Agreement by and between the Company and Doug Unwin, dated January 1, 2010 (Incorporated by reference to Exhibit 4.1 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
4.2
|
Form of Agreement for Escrow Arrangements under National Policy 46-201 Escrow for Initial Public Offerings , dated August 30, 2011 (Incorporated by reference to Exhibit 4.2 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
4.3
|
Development and Commercialization Agreement by and between the Company and IntelGenx Corp, dated February 28, 2011 (Incorporated by reference to Exhibit 4.3 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
11.1
|
Code of Business Conduct and Ethnics (Incorporated by reference to Exhibit 11.1 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
|
11.2
|
Charter of Audit Committee (Incorporated by reference to Exhibit 12.2 to our Registration Statement on Form 20-F filed on November 13, 2013)
|
| 15.3 | |
| 15.4 | |
64
SIGNATURE
The Registrant hereby certifies that it meets all of the requirements for filing on this Form 20-F and that it has duly caused and authorized the undersigned to sign this registration statement on its behalf.
|
PACIFIC THERAPEUTICS LTD
|
|||
|
Date: April 30, 2014
|
By:
|
/s/ Douglas H. Unwin
|
|
|
Name: Douglas H. Unwin
|
|||
|
Title: Chief Executive Officer
|
|||
|
By:
|
/s/ Derick Sinclair
|
|
|
Name: Derick Sinclair
|
||
|
Title: Chief Financial Officer
|
||
65
