Table of Contents
As filed with the Securities and Exchange Commission on September 23, 2013
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form F-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
Evogene Ltd.
(Exact Name of Registrant as Specified in its Charter)
| State of Israel | 2870 | Not Applicable | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
Evogene Ltd.
13 Gad Feinstein Street
Park Rehovot P.O.B 2100
Rehovot 76121
Israel
+972-8-931-1900
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Joshua G. Kiernan, Esq. Colin J. Diamond, Esq. White & Case LLP 1155 Avenue of the Americas New York, New York 10036 Tel: 212-819-8200 Fax: 212-354-8113 |
Dan Shamgar, Adv. Mike Rimon, Adv. Meitar Liquornik Geva Leshem Tal 16 Abba Hillel Road Ramat Gan 52506, Israel Tel: +972-3-610-3100 Fax: +972-3-610-3111 |
Phyllis G. Korff, Esq. Yossi Vebman, Esq. Skadden, Arps, Slate, Meagher 4 Times Square New York, New York 10036 Tel: 212-735-3000 Fax: 212-735-2000 |
Aaron M. Lampert, Adv. Goldfarb Seligman & Co. 98 Yigal Alon Street Tel Aviv 67891, Israel Tel: +972-3-608-9999 Fax: +972-3-608-9909 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after effectiveness of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee | ||
| Ordinary shares, par value NIS 0.02 |
$60,000,000 | $8,185 | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933. |
| (2) | Includes shares granted pursuant to the underwriters’ option to purchase additional shares. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED SEPTEMBER 23, 2013
PRELIMINARY PROSPECTUS

Shares
Evogene Ltd.
Ordinary Shares
$ per share
We are selling ordinary shares. This is our initial public offering in the United States. We have applied to have the ordinary shares listed on the New York Stock Exchange under the symbol “EVGN.” Our ordinary shares are listed on the Tel Aviv Stock Exchange, or TASE, under the symbol “EVGN.” On September 18, 2013, the last reported sale price of our ordinary shares on the TASE was NIS 51.18, or $14.47, per share (based on the exchange rate reported by the Bank of Israel on such date, which was NIS 3.54 = $1.00), giving prospective effect to the 1-for-2 reverse share split of our ordinary shares, which is to be effected immediately prior to the completion of this offering. The estimated initial public offering price is between $ and $ per share.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act and will therefore be subject to reduced reporting requirements.
Investing in our ordinary shares involves risks. See “Risk Factors” beginning on page 9.
None of the Securities and Exchange Commission, the Israeli Securities Authority or any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share |
Total | |||
| Public offering price |
$ | $ | ||
| Underwriting discounts and commissions(1) |
$ | $ | ||
| Proceeds to us (before expenses) |
$ | $ |
| (1) | See “Underwriting” for a description of the compensation payable to the underwriters. |
The underwriters expect to deliver the ordinary shares to purchasers on or about , 2013. We have granted the underwriters an option to purchase up to additional ordinary shares to cover over-allotments.
| Credit Suisse |
Deutsche Bank Securities |
| Oppenheimer |
Piper Jaffray |
, 2013
Table of Contents

Table of Contents
| Page | ||||
| 1 | ||||
| 9 | ||||
| 31 | ||||
| 32 | ||||
| 33 | ||||
| 34 | ||||
| 35 | ||||
| 36 | ||||
| 38 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
40 | |||
| 59 | ||||
| 69 | ||||
| Page | ||||
| 106 | ||||
| 123 | ||||
| 125 | ||||
| 126 | ||||
| 132 | ||||
| 133 | ||||
| 144 | ||||
| 148 | ||||
| 150 | ||||
| 150 | ||||
| 150 | ||||
| 151 | ||||
| F-1 | ||||
Neither we nor the underwriters have authorized anyone to provide information different from that contained in this prospectus, any amendment or supplement to this prospectus or in any free writing prospectus prepared by us or on our behalf. Neither we nor the underwriters take any responsibility for, and can provide no assurance as to the reliability of, any information other than the information in this prospectus and any free writing prospectus prepared by us or on our behalf. Neither the delivery of this prospectus nor the sale of our ordinary shares means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or the solicitation of an offer to buy these ordinary shares in any circumstances under which such offer or solicitation is unlawful.
Unless derived from our financial statements or otherwise noted, New Israeli Shekel, or NIS, amounts presented in this prospectus are translated at the rate of $1.00 = NIS 3.54, the exchange rate reported by the Bank of Israel as of September 18, 2013.
Until , 2013 (25 days after the date of this prospectus), all dealers that buy, sell or trade our ordinary shares, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
i
Table of Contents
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that you should consider before deciding to invest in our ordinary shares. You should read the entire prospectus carefully, including “Risk Factors” and our consolidated financial statements and notes to those consolidated financial statements, before making an investment decision. In this prospectus, the terms “Evogene,” “we,” “us,” “our” and “the company” refer to Evogene Ltd. and its subsidiary, Evofuel Ltd.
Our Business
We are a plant genomics company that uses a comprehensive and integrated technology infrastructure to enhance seed traits underlying crop performance and productivity through biotechnology and advanced breeding methods. We have strategic collaborations with world-leading agricultural companies, primarily to develop traits for improved yield and abiotic stress tolerance (such as improved tolerance to drought, heat and salinity) and biotic stress resistance (such as resistance to disease, pests and insects). Our products are focused on essential crops, including corn, soybean, wheat, rice and cotton, which, according to Phillips McDougall’s 2011 Seed Industry Overview report, account for over 70% of the value of the global seeds market. We also apply our technology infrastructure to two additional fields: (i) agriculture chemicals (“ag-chemical”) and (ii) seeds focusing on second generation feedstock for biodiesel, both of which have not yet generated revenue and are in the development stage.
The field of plant genomics, or the science of understanding and analyzing plant genomes to identify and impact biological elements affecting trait performance, continues to evolve. Agricultural product innovation is increasingly driven by the ability to analyze data, in order to make direct discoveries and gain insights into key underlying biological phenomena of such products. The seed and ag-chemical industry has witnessed a dramatic increase in the availability of genomic data. This is primarily as a result of the introduction of new technologies that facilitate rapid generation of such data at a significantly lower cost. As a result, the key opportunity, and challenge, for plant trait improvement has shifted from data generation to data integration and analysis of large volumes of data.
We believe that our competitive advantage is based on our continuously enhanced proprietary discovery and development infrastructure. This infrastructure is capable of integrating and analyzing vast amounts of data, through the use of proprietary computational technologies comprised of advanced algorithms and predictive methodologies. Our computational technologies are a key part of our broad technology infrastructure that also integrates extensive scientific expertise, public and proprietary genomic data and plant validation systems. Our proprietary gene identification capabilities, which are scalable and adaptable to a large variety of crops and traits, together with our highly educated and experienced multidisciplinary team of scientists, are, we believe, unique in the industry.
We currently generate revenues primarily through research and development and milestone payments as traits move through development phases, and in the future we expect to receive royalty revenues upon commercialization of products containing traits that we help our collaborators to develop. To date, we have identified and filed patents for over 4,000 novel genes and genomic components for the improvement of key traits, hundreds of which are under development in our collaborators’ pipelines. We believe that the extension and renewal of some of our main collaboration agreements highlight the value ascribed to our performance, our capabilities and our proprietary technology infrastructure.
We have collaboration agreements with most of the world’s leading seed and ag-chemical companies, including subsidiaries or affiliates of Monsanto Company, or Monsanto, Bayer AG, or Bayer, E.I. du Pont de Nemours and Company, or DuPont, and Syngenta AG, or Syngenta. Our collaborations with these companies are
1
Table of Contents
aimed at introducing improved seed traits into key commercial crops. Among our collaborators, Monsanto and Bayer are also key shareholders in our company, holding approximately 8.7% and 4.6% of our ordinary shares as of June 30, 2013, respectively.
We also aim to apply our technology infrastructure to two additional fields: ag-chemical and seeds focusing on second generation feedstock for biodiesel. For the ag-chemical market, we are focusing on the discovery of new biologically significant proteins called “targets” based on genes we identify that enable the development of herbicides with novel mechanisms to mitigate weed resistance. We also intend to use our proprietary discovery and development infrastructure to develop chemical molecules as crop enhancers that, when applied to crops, would improve yield and abiotic stress tolerance. To date, we have not yet entered into any collaboration agreements in our ag-chemical operations. For the biodiesel market, we are focusing on the development of improved, high-yielding, non-edible castor bean seeds as an economically viable alternative feedstock for the production of biodiesel with a reduced environmental footprint.
As of June 30, 2013, we employed 188 employees, of which approximately 78% are involved in research and development and 48 hold a Ph.D. degree. Our multi-disciplinary team includes experts in biology, chemistry, plant genetics, agronomics, mathematics, computer science and other related fields.
We have been listed for trading on the Tel Aviv Stock Exchange, or TASE, since 2007, are headquartered in the agricultural and biotech hub of Rehovot, Israel, and have field testing activities in Israel, Brazil and Argentina.
Our Strengths
We believe we are strategically positioned to capitalize on the fundamental need to increase agricultural yields in highly attractive end-markets such as food, feed and biofuel. Our competitive strengths include:
A leading position in the field of plant genomics. Since our company’s formation in 2002, we have established a powerful integrated infrastructure that we believe is unique in our industry for understanding plant genomics, addressing some of the key challenges in agriculture productivity. The infrastructure combines our know-how in plant genomics with our proprietary technologies and processes that span data integration and analysis, field experiments and plant validation. This infrastructure allows us to identify, prioritize and validate over 1,000 genes per year.
Innovative proprietary computational technologies, capable of efficiently integrating and analyzing vast amounts of complex genomic data. Our proprietary computational technologies have demonstrated the capability of addressing the current focus of the seed industry – data analysis – by integrating and comprehensively analysing vast amounts of public and proprietary data, covering more than 200 plant species, enabling us to identify and prioritize genes and genomic components aimed at improving key plant traits.
A partner of choice for industry leaders. We have over ten collaborations with five of the seven leading global seed and ag-chemical companies, focusing on the development of traits involving yield and abiotic stress and biotic stress for key crops in their product portfolios such as corn, soybean and wheat. Under these existing collaborations, hundreds of genes we identified are currently undergoing early testing in our collaborators’ pipelines. Two of our key collaborators, Monsanto and Bayer, have made significant equity investments in our company and have entered into more than one collaboration agreement with us.
A balanced and scalable business model. We currently generate revenues primarily through research and development and milestone payments; in the long term, we expect to also receive significant revenues from sales royalties generated by our collaborators upon commercialization of products. However, because the development
2
Table of Contents
cycle of products is lengthy, any revenue from royalty payments may not be earned before six years, if at all. We benefit from a business model in which, pursuant to most of our existing collaboration agreements, a significant part of our operating costs are borne by our collaborators. We self-fund our initial research and development costs in selected projects, with the goal of capturing a larger share of our collaborators’ future revenues.
Diversified product portfolio and multiple paths of commercialization. Our innovative and adaptable technology infrastructure, scientific and computational expertise, and our close links to major companies in the seed and ag-chemical industry allow us to benefit from multiple business opportunities in the agriculture markets. Our more than ten different collaborations cover a portfolio of 20 products tailored to address specific traits and crops. In addition, our expertise in plant genomics and flexible computational technologies will allow us to address new commercial opportunities in the ag-chemical and biodiesel markets.
Broad intellectual property portfolio, claiming protection for thousands of key genes impacting valuable key traits. In the past ten years we have established a broad intellectual property portfolio that includes more than 20 patent families, over 180 national filings and over 30 granted patents covering thousands of key genes and genomic components impacting valuable key traits.
Industry Background
Improving Plant Performance
The seed and ag-chemical industry continues to seek sustainable and economically viable solutions to feed the world’s growing and increasingly prosperous population. While the demand for grain is increasing, production is constrained due to finite arable land and water resources, climate variability, increasingly resilient weeds and insects, depletion of soil nutrients and diseases that impair crop yields.
In order to address these growing challenges, farmers need solutions to increase and maintain crop productivity. A fundamental way to improve plant productivity and performance is through the use of plant genomics. Plant genomics is the science of understanding and analyzing plants to modify biological elements which are responsible for traits that govern plant yield, tolerance to abiotic stress factors (such as drought and heat) and biotic stress factors (such as pests and diseases). Through plant genomics, researchers can identify and influence target genes and other genomic components that affect trait performance, which are then used in the development of (i) enhanced seeds and (ii) novel crop protection ag-chemicals.
(i) Enhanced seeds
There are two primary methods to improve plant performance through the use of genomic technologies in the development of seeds:
Biotechnology or genetic modification. Use of genomic technologies for the development of seeds in which genes that impact specific traits are introduced to the plant through genetic insertion. Such seeds are referred to as genetically modified (“GM”), or biotech seeds.
Advanced breeding. Use of genomic technologies to identify specific DNA sequence variations (such as single-nucleotide polymorphisms, or SNPs), linked to a particular trait. This allows breeders to determine which parent plants with favorable characteristics should be crossed to enhance desired native traits in the next generation.
As of today, the two major traits commercially available for a limited number of crops in the biotech seeds market are herbicide tolerance and resistance to insects. Factors expected to drive future growth include the application of advanced breeding and biotechnology in additional crops, such as rice and wheat, introduction of new target traits, such as durability to abiotic stress, and adoption of biotechnology crops by additional countries.
3
Table of Contents
(ii) Crop protection ag-chemicals
The market for crop protection ag-chemicals has grown by approximately 40% since 2007 to an estimated $47 billion in 2012, according to Phillips McDougall’s 2013 Industry Presentation on the Global Agrochemical and Seed Markets. Crop protection ag-chemicals, which as a group are referred to as “pesticides” or “crop protection products,” include herbicides (to control weeds), fungicides (to prevent and treat fungal diseases) and insecticides (to minimize damage caused by insects). In recent years, the rate of introduction of novel ag-chemicals has been steadily declining largely due to higher development costs and strict regulation, which in turn has led to the further acceleration of pest resistance to existing ag-chemicals.
Farmers and ag-chemical companies have therefore been seeking new solutions to mitigate pest resistance. This includes the use of plant genomic data to identify and target essential biological processes and leveraging this data for the discovery and development of novel herbicides with new mechanisms to cope with the growing resistance of weeds.
The Market for Biofuels
Biofuel demand in ground, aviation and maritime transportation has been steadily growing in past years, representing a market opportunity of approximately $140 billion in 2011, according to Ken Research’s 2012 Global Biofuel Market Outlook. The market is mainly driven by a need for increasing energy independence, reducing susceptibility to fluctuating oil prices and environmental concerns. At present, production of biodiesel, an alternative to diesel fuel derived from plant oil, relies on edible crops, such as soybean and canola, considered first generation feedstocks. Rising commodity prices and concerns surrounding the long-term sustainability of diverting food feedstock for biodiesel use has hindered widespread adoption of these alternative fuels, setting the stage for the demand of second generation feedstock for biodiesel. There is an increasing need to identify feedstock sources which would be: (i) economically viable; (ii) scalable and (iii) sustainable.
Our Growth Strategy
Our goal is to extend our market experience in improving plant productivity and performance using plant genomics. To achieve that goal we intend to pursue the following strategies.
Expand our innovative technologies in plant genomics. We intend to enhance our competitive advantage by further investing in our technology infrastructure and research and development capabilities.
Continue to advance our existing collaborations in seed traits. We are focused on executing and advancing our existing collaborations in order to expedite our genes and genetic components towards commercialization with a view to generating significant milestone and royalty revenues.
Extend and expand our seed trait project portfolio. We plan to continue to leverage our scalable and adaptable infrastructure, plant genomics expertise and close relationships with agriculture industry leaders.
Capture an additional share of the value chain by increasing self-funded research and development. In the future, we intend to complement our revenue streams by selectively self-funding a larger portion of our direct initial research and development costs, with the goal of capturing a larger share of our collaborators’ future revenues.
Further develop and commercialize our ag-chemical operations. We are leveraging our know-how and computational technologies to position our company at the forefront of discovery and innovation in ag-chemicals.
4
Table of Contents
Further develop and commercialize the activities of our subsidiary Evofuel. We intend to further develop our castor bean seed operations as a second generation feedstock for biodiesel.
Risk Factors
Investing in our ordinary shares involves risks. You should carefully consider the risks described in “Risk Factors” beginning on page 9 before making a decision to invest in our ordinary shares. If any of these risks actually occurs, our business, financial condition or results of operations would likely be materially adversely affected. In such case, the trading price of our ordinary shares would likely decline, and you may lose all or part of your investment. The following is a summary of some of the principal risks we face:
| • | We have a history of losses, and incurred operating losses of $(3.1 million), $(3.2 million) and $(2.0 million) for the years ended December 31, 2012, 2011 and 2010, respectively, and $(2.1 million) and $(1.1 million) in the six months ended June 30, 2013 and 2012, respectively. We may never achieve or maintain profitability. |
| • | We may not be successful in developing commercial products. |
| • | We derive substantially all of our current revenues from our strategic collaborations, most significantly with Monsanto and Bayer, and the termination or non-renewal of these collaborations would have a material adverse effect on our results of operations. |
| • | Our product development cycle is lengthy and uncertain, and it may take at least six years, if at all, before the first seeds containing our traits complete the development process and become commercially available. |
| • | Consumer and government resistance to genetically modified organisms may negatively affect our public image and reduce sales of plants containing our traits. |
Corporate Information
Our principal executive offices are located at 13 Gad Feinstein Street, Park Rehovot P.O.B. 2100, Rehovot 76121 Israel, and our telephone number is +972 (8) 931-1900. Our website address is www.evogene.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus and is not incorporated by reference herein. We have included our website address in this prospectus solely for informational purposes. We are a limited liability corporation, and we operate under the Israeli Companies Law 5759-1999. Our agent for service of process in the United States is .
Throughout this prospectus, we refer to various trademarks, service marks and trade names that we use in our business. The “Evogene” design logo, “Evogene” and other trademarks or service marks of Evogene Ltd. appearing in this prospectus are the property of Evogene Ltd. We have several other registered trademarks, service marks and pending applications relating to our computational technologies. Other trademarks and service marks appearing in this prospectus are the property of their respective holders.
5
Table of Contents
THE OFFERING
| Ordinary shares offered by us |
ordinary shares (or if the underwriters exercise their option to purchase additional shares in full). |
| Ordinary shares to be outstanding after this offering |
ordinary shares (or if the underwriters exercise their option to purchase additional shares in full). |
| Use of proceeds |
We intend to allocate the net proceeds from this offering to our different areas of activity as follows: |
1. Seed Traits:
| We estimate the total investment in our seed traits operation will be approximately 50% of our net proceeds from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
2. Ag-Chemicals:
We estimate the total investment in our ag-chemical operation will be approximately 28% of our net proceeds from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| 3. Evofuel: |
We estimate the total investment in our Evofuel operation will be approximately 22% of our net proceeds from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| We will have broad discretion in the way that we use the net proceeds from this offering. See “Use of Proceeds” |
| Risk factors |
See “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our ordinary shares. |
| Tel Aviv Stock Exchange symbol and proposed NYSE symbol |
“EVGN.” |
The number of ordinary shares to be outstanding after this offering is based on 18,879,987 ordinary shares outstanding as of June 30, 2013. The number of ordinary shares to be outstanding after this offering excludes 3,885,861 ordinary shares that were reserved for issuance under our equity incentive plans as of June 30, 2013, of which 2,653,759 shares were issuable upon exercise of options that had been granted and remained outstanding at a weighted average exercise price of $6.79 per share. See “Management—Option Plans.”
Unless otherwise indicated, this prospectus:
| • | assumes an initial public offering price of $ per ordinary share, the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus; |
| • | gives effect to a 1-for-2 reverse share split of our ordinary shares that will be effected immediately prior to the completion of this offering; and |
| • | assumes no exercise of the underwriters’ option to purchase up to an additional ordinary shares from us. |
6
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
The following tables set forth our summary consolidated financial data. You should read the following summary consolidated financial and other data in conjunction with “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected in the future. Our financial statements have been prepared in accordance with International Financial Reporting Standards, or IFRS.
The summary consolidated statements of comprehensive income data for each of the years in the three-year period ended December 31, 2012 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The summary consolidated statements of comprehensive income data for the six months ended June 30, 2012 and 2013 and the consolidated balance sheet data as of June 30, 2013 have been derived from our unaudited interim condensed consolidated financial statements included elsewhere in this prospectus. Results from interim periods are not necessarily indicative of results that may be expected for the entire year.
| Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| (in thousands, except per share and share data) | ||||||||||||||||||||
| Consolidated Statements of Comprehensive Income: |
||||||||||||||||||||
| Revenues |
$ | 12,563 | $ | 14,901 | $ | 17,072 | $ | 8,287 | $ | 8,934 | ||||||||||
| Cost of revenues |
5,811 | 8,247 | 9,552 | 4,483 | 4,688 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
6,752 | 6,654 | 7,520 | 3,804 | 4,246 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Research and development |
5,544 | 6,384 | 7,252 | 3,308 | 4,666 | |||||||||||||||
| Business development |
1,062 | 1,136 | 1,159 | 544 | 532 | |||||||||||||||
| General and administrative |
2,123 | 2,317 | 2,235 | 1,069 | 1,197 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
8,729 | 9,837 | 10,646 | 4,921 | 6,395 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(1,977 | ) | (3,183 | ) | (3,126 | ) | (1,117 | ) | (2,149 | ) | ||||||||||
| Financial income |
724 | 5,023 | 972 | 510 | 776 | |||||||||||||||
| Financial expenses |
(5,717 | ) | (1,195 | ) | (294 | ) | (76 | ) | (989 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before taxes on income |
(6,970 | ) | 645 | (2,448 | ) | (683 | ) | (2,362 | ) | |||||||||||
| Taxes on income |
— | — | 74 | 52 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | (6,970 | ) | $ | 645 | $ | (2,522 | ) | $ | (735 | ) | $ | (2,362 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted net income (loss) per share |
$ | (0.48 | ) | $ | 0.04 | $ | (0.14 | ) | $ | (0.04 | ) | $ | (0.12 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of ordinary shares used in computing basic income (loss) per share(1) |
14,824,703 | 17,505,136 | 18,421,568 | 18,328,598 | 18,817,847 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of ordinary shares used in computing diluted income (loss) per share(1) |
14,824,703 | 18,731,118 | 18,421,568 | 18,328,598 | 18,817,847 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Basic net income (loss) per share is computed based on the weighted average number of ordinary shares outstanding during each period. Diluted net income (loss) per share is computed based on the weighted |
7
Table of Contents
| average number of ordinary shares outstanding during each period plus dilutive potential equivalent ordinary shares considered outstanding during the period, in accordance with IAS 33, “Earnings per Share.” |
| As of June 30, 2013 | ||||||||
| Actual | As Adjusted(1) | |||||||
| (unaudited) | ||||||||
| (in thousands) | ||||||||
| Consolidated Balance Sheet Data: |
||||||||
| Cash and cash equivalents |
$ | 16,884 | $ | |||||
| Marketable securities |
33,657 | |||||||
| Trade receivables |
1,853 | |||||||
| Total assets |
62,004 | |||||||
| Deferred revenues and other advances |
6,507 | |||||||
| Total liabilities |
14,705 | |||||||
| Working capital(2) |
44,921 | |||||||
| Shareholders’ equity |
47,299 | |||||||
| (1) | Adjusted amounts give effect to the issuance and sale of ordinary shares by us in this offering at an assumed initial public offering price of $ per ordinary share, the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
| (2) | Working capital is defined as total current assets minus total current liabilities. |
8
Table of Contents
This offering and an investment in our ordinary shares involve a high degree of risk. You should consider carefully the risks described below and all other information contained in this prospectus, before you decide to buy our ordinary shares. If any of the following risks actually occurs, our business, financial condition and results of operations could be materially and adversely affected. In that event, the trading price of our ordinary shares would likely decline and you might lose all or part of your investment.
Risks Related to Our Business and Our Industry
We may not be successful in developing commercial products.
Our success depends in part on our ability to identify genes that will improve selected crop traits. These genes are licensed to our collaborators to develop and commercialize seeds that contain the genes. It may take at least six years, if at all, before the first seeds complete the development process and become commercially available. Certain of our agreements entitle us to annual research and development payments and milestone payments in the event that specified milestones are met. While we currently do not earn royalties from the sale of seeds containing our seed traits, our long-term growth strategy is based in part on the expectation that such royalties will comprise a significant portion of our revenues in the future. Pursuant to our collaboration agreements, we are usually entitled to receive royalties on any product that integrates a trait over which we hold a patent for either an agreed upon duration of time or until our patent on the trait expires. If seeds that contain our traits are never commercialized, we will not receive revenues from royalties on the products and may not earn a profit on the traits we develop, which could materially and adversely affect our results of operations and our long-term growth strategy.
Seeds containing the traits that we develop may never become commercialized for any of the following reasons:
| • | our traits may not be successfully validated in the target plants; |
| • | our traits may not have the desired effect sought by our collaborators on the relevant crop; |
| • | we may fail to satisfy relevant milestones under the agreements with our collaborators; |
| • | our collaborators may be unable to obtain the requisite regulatory approvals for the seeds containing our traits; |
| • | our competitors may launch competing or more effective seed traits or seeds; |
| • | a market may not exist for seeds containing our traits or such seeds may not be commercially successful; |
| • | our collaborators may be unable to fully develop and commercialize products containing our seed traits or may decide, for whatever reason, not to commercialize such products; and |
| • | we may be unable to patent our traits in the necessary jurisdictions. |
We derive substantially all of our current revenues from our strategic collaborations, most significantly with Monsanto and Bayer, and the termination or non-renewal of these collaborations would have a material adverse effect on our results of operations.
We have entered into multiple collaboration agreements and related arrangements, most significantly with Monsanto and Bayer, under which we currently generate revenues through research and development payments, up-front payments, milestone payments and revenues from shares purchased at a premium. Monsanto and Bayer are expected to continue to account for a substantial amount of our revenues for the next few years. In particular, revenues from Monsanto accounted for 70.1%, 70.6% and 74.3% of our total revenues in the years ended December 31, 2012, 2011 and 2010, respectively, and 65.8% and 73.3% of our total revenues in the six months ended June 30, 2013 and 2012, respectively. Revenues from Bayer accounted for 24.1%, 23.4% and 4.2% of
9
Table of Contents
our total revenues in the years ended December 31, 2012, 2011 and 2010, respectively and 31.9% and 25.9% in the six months ended June 30, 2013 and 2012, respectively. Our current agreement with Monsanto was signed in 2008 and was extended in November 2011, and the collaboration period of the extended agreement (i.e., the period of active computational discovery efforts, separate from validation efforts that may follow) is scheduled to expire in August 2014. Monsanto has an option to extend the collaboration period of the agreement for two additional years at its sole discretion. This option expires on February 1, 2014. We entered into two separate multi-year collaboration agreements with Bayer, the first covering rice in April 2009, the collaboration period under which expired in April 2012, and the second covering wheat in December 2010, the collaboration period under which is scheduled to expire in January 2016. In addition, as of June 30, 2013, Monsanto and Bayer held approximately 8.7% and 4.6% of our outstanding equity, respectively. See “Business—Key Collaborations.” We are substantially dependent on Monsanto and, to a lesser extent, on Bayer and our other collaborators to pay us annual research and development fees and milestone fees upon the occurrence of certain milestone events. The termination or non-renewal of our agreements with Monsanto and Bayer, including if Monsanto decides not to exercise its option to extend the collaboration period under our agreement with them by February 1, 2014 (and if Monsanto does not otherwise enter into a new collaboration agreement with us) would have a material adverse effect on our business, financial condition, results of operations and prospects.
There are only a few companies in our seed and ag-chemical market, and we rely on a limited number of collaborators to develop and commercialize products containing our seed traits.
The seed and ag-chemical market is highly consolidated and dominated by a relatively small number of large companies. For example, according to Phillips McDougall’s 2012 Industry Presentation on the Global Seed Market, in 2012, only five agricultural and seed companies, Monsanto, DuPont, Syngenta, Bayer and Limagrain, controlled more than 60% of market value in the global seed market. We are currently undertaking collaborations with these companies to develop improved seeds. Due to the small number of companies in our market, there are limited opportunities for us to grow our business with new collaborators. In addition, if we fail to develop or maintain our relationships with any of our current collaborators, we could not only lose our opportunity to work with that collaborator, but we could also suffer a reputational risk that could impact our relationships with other collaborators in what is a relatively small industry community.
We are currently working either with collaborators or on independent projects to research and develop 20 different seed traits. While we seek to expand our portfolio of traits in the future, the research and development required to discover and develop new traits is costly, time-intensive and requires significant infrastructure resources. Therefore, in order to discover and develop new traits, we must either enter into new collaborations with seed and ag-chemical companies or develop the traits ourselves, independent of any collaborators. If we are unable to enter into new collaborations, or if we do not have the resources to develop the capabilities necessary to discover and develop new seed traits independently, we may not be able to expand our portfolio of traits, which could have a material adverse effect on our business prospects.
Our product development cycle is lengthy and uncertain, and we may never earn royalties on the sale of products containing our seed traits.
Research and development in the seed and ag-chemical and larger agriculture industries is expensive and prolonged and entails considerable uncertainty. We may spend many years and dedicate significant financial and other resources, including the proceeds of this offering, developing products that will never be commercialized. Our process of discovering, developing and commercializing a seed trait through either genetic modification or advanced breeding involves several phases, and we estimate that it will take seven to thirteen years from discovery to commercialization of a product containing our seed trait.
We currently have 20 seed traits under development with our collaborators, most of which are in Discovery and Phase I, with one product in Phase II. See “Business—Product Development Cycle—Seed Trait Product Development Cycle” for a description of these phases. It may take at least six years, if at all, before the first
10
Table of Contents
products containing our seed traits complete the development process and become commercially available, however we have little to no certainty as to which, if any, of these products will eventually reach commercialization. Because of the long product development cycle and the complexities and uncertainties associated with chemical and biotechnological research, there is significant uncertainty as to whether we will ever generate significant royalties from the products that we are developing.
We or our collaborators may fail to perform obligations under the collaboration agreements.
We are obligated under our collaboration agreements to perform research activities over a particular period of time. If we fail to perform our obligations under these agreements, in some cases our collaborators may terminate our agreements with them and in other cases our collaborators’ obligations may be reduced and, as a result, our anticipated revenues may decrease. In addition, any of our collaborators may fail to perform their obligations, which may hinder development and commercialization of products containing the traits we develop and materially and adversely affect our future results of operations. Furthermore, the various payments we receive from our collaborators are our primary source of revenues. If our collaborators do not make these payments, either due to financial hardship, disagreement under the relevant collaboration agreement or for any other reason, our results of operations and business could be materially and adversely affected. If disagreements with a collaborator arise, any dispute with such collaborator may negatively affect our relationship with one or more of our other collaborators and may hinder our ability to enter into future collaboration agreements, each of which could negatively impact our business and results of operations.
Our collaborators have significant resources and development capabilities and may develop their own products that compete with or negatively impact the advancement or sale of products utilizing the traits we develop and license to them.
While we protect the traits we develop and license to our collaborators through both legal and contractual provisions, any of our collaborators could develop or pursue competing products and traits that may ultimately prove more commercially viable than the traits we develop. Our collaborators are significantly larger than us and may have substantially greater resources and development capabilities. The development or launch of a competing product by a collaborator may adversely affect the advancement and commercialization of any competing traits we develop and any associated research and development payments, milestone and royalty payments.
We are working to develop novel ag-chemical products, and our efforts to enter this market may be unsuccessful.
In addition to our seed trait business, we are currently developing solutions for crop protection and enhancement through chemistry, or ag-chemistry. We may use a significant portion of the proceeds of this offering to invest in the infrastructure and ongoing research needed for this operation. Although our computational technology PoinTar is designed to develop such ag-chemical products, and although we continue to develop additional technologies, we still need to compile the data that our technologies need to analyze in order to discover and develop new products. We are developing these products through a novel approach, focused on biologically significant proteins called “targets,” which is similar to certain approaches pharmaceutical companies undertake to develop new drugs. We currently do not have any collaborators for our ag-chemical products, and therefore are currently funding our data-collection and research and development efforts relating to our ag-chemical products ourselves. Our efforts to develop novel ag-chemical products may fail for a variety of reasons, including:
| • | Our failure to compile a sufficient amount of data and to develop the technological tools necessary to discover and develop any ag-chemical products; |
| • | Our failure to enter into collaborations similar to those in our seed trait activity; |
| • | The failure of our relatively novel target-based approach to lead to an effective product; and |
| • | Our failure to obtain sufficient funding to fully execute our ag-chemical business plan. |
11
Table of Contents
Furthermore, even if we are able to discover and develop an effective product, it may not be successful if we are unable to find collaborators to undertake the advanced stages of product development and to market and commercialize the product. If our efforts to develop ag-chemical products are unsuccessful, our results of operations could be negatively impacted.
Evofuel, our wholly owned subsidiary that develops seeds for biodiesel, may not be successful for a number of reasons.
Our wholly owned subsidiary Evofuel is currently developing improved, high-yield castor bean seeds to be used as a source of non-edible feedstock for the biodiesel market and other industries. We may use a significant portion of the proceeds of this offering to invest in the infrastructure needed for this operation, which has not yet generated revenues from seed sales. The renewable energy market in general and the biodiesel market more specifically are not well established and are evolving. Furthermore, the biodiesel market faces continuing competition from traditional petroleum-based fuels, and demand for biodiesel fluctuates with changing oil and gas prices. Although our castor bean development and production is targeted to produce biodiesel, biodiesel has historically been produced from soybean, rapeseed and corn. Accordingly, in order for us to be successful, we will need to demonstrate on a commercial scale that castor beans can reliably be used as a cost-efficient feedstock for biodiesel production. We will also need to show that the production cost and sales price of castor bean-based biodiesel are competitive with those of traditional oil and gas.
The success of these operations will largely depend on our ability to address several unique challenges, including:
| • | the high cost of producing castor bean grains, requiring a potentially expensive investment across the entire castor bean value chain (e.g., sowing, cleaning and transporting); |
| • | the health and environmental risks posed by the castor bean seed, which contains a naturally occurring poison called ricin; |
| • | any regulatory concerns related to sales of castor beans, particularly related to the import of such beans and the potential effects of ricin; |
| • | the amount of suitable land available to grow the necessary quantity of castor bean plants; |
| • | the risk that farmers may decide not to grow “second season” replacement crops such as the castor bean; |
| • | the ability to produce castor bean in a high throughput mechanized manner; and |
| • | the sustainability of our production and the biodiesel end-product. |
In addition, we have no prior experience operating as a seed company. We will therefore be operating in a new industry, with little knowledge of the dynamics involved in producing and selling seeds.
We are working to design improved castor bean seeds and address each of these issues so that we are able to grow a sufficient and sustainable amount of castor bean plants at a low cost. We have entered into strategic collaborations with SLC Agricola S.A., or SLC Agricola, one of Brazil’s largest producers of soybean, cotton and corn, and T6 Industrial S.A., or T6, a leading Argentinian biodiesel producer, which we expect will eventually facilitate commercialization of the castor beans we are currently developing. We are unable to foresee when significant sales will commence, and we do not expect to start selling seeds for at least three years. Furthermore, there can be no assurance that our collaborations with SLC Agricola or T6 will ultimately result in a commercialized castor bean seed. If we are unable to adequately address any of these issues, we may not find a market for our castor bean seeds and our results of operations could be materially and adversely affected.
Even if we are entitled to royalties from our collaborators, we may not actually receive these royalties, or we may experience difficulties in collecting the royalties that we believe we are entitled to.
After our collaborators launch commercial products containing our licensed genes, we will need to rely on the good faith of our collaborators to report to us the sales they earn from these products and to accurately
12
Table of Contents
calculate the royalties we are entitled to, a process that will involve complicated and difficult calculations. Although we seek to address these concerns in our collaboration agreements, such provisions may not be effective. Additionally, we may not be able to achieve our long-term goal of generating revenues from royalties, and in the coming years our revenues will be entirely dependent on fees we earn for our research and development services and milestone payments from our collaborators.
We depend on our key personnel and, if we are not able to attract and retain qualified scientific and business personnel, we may not be able to grow our business or develop and commercialize our products.
The vast majority of our workforce is involved in research and development. Our business is therefore dependent on our ability to recruit and maintain a highly skilled and educated workforce with expertise in a range of disciplines, including biology, chemistry, plant genetics, agronomics, mathematics, computer science and other subjects relevant to our operations. For example, approximately 25% of our staff holds a Ph.D. The number of qualified and highly educated personnel in Israel, where all of our operations are located, is limited and competition for the services of such persons is intense. Although we have employment agreements with all of our employees, most of these agreements may be terminated upon short notice. The failure to hire and retain skilled and highly educated personnel could limit our growth and hinder our research and development efforts.
We have recently begun to develop certain traits independent of our collaborators, and we may need to finance the cost of the initial development phase of such trait discovery ourselves.
Currently, our business plan is based primarily on the development of traits in collaboration with our collaborators through all six phases of the discovery-development-commercialization process. We have, however, recently begun to develop certain traits independent of our collaborators and are developing such traits on our own during the discovery phase, and may also undertake such independent discovery efforts during the Phase I or “proof of concept” phase, with a goal of making such traits available to collaborators during later phases, once we have identified what we believe to be promising traits. While we believe that this will allow us to negotiate more favorable license terms with respect to such traits, the up-front cost to us of developing traits without a collaborator (and therefore without external funding for the research and development expenditures we incur) in these early phases involves higher risks, since we need to fund the research and development of such traits ourselves. We intend to use a portion of the proceeds from this offering to fund such independent research and trait discovery projects. If we are unsuccessful in discovering promising traits after having invested significant funds, or if we are unable to find collaborators who are interested in such traits and willing to fund subsequent phases of development and commercialization, such failures could have a material and adverse effect on our business, financial condition and results of operations.
Our business is subject to various government regulations and, if we or our collaborators are unable to obtain the necessary regulatory approvals, we may not be able to continue our operations.
Our business is generally subject to two types of regulations: regulations that apply to how we operate and regulations that apply to products containing our seed traits. We apply for and maintain the regulatory approvals necessary for our operations, particularly those covering our field trials, while our collaborators apply for and maintain regulatory approvals necessary for the commercialization of products containing our seed traits. More recently, regulators have implemented delays in approving genetically engineered crops due to environmental concerns and negative publicity. Since our operations and the field trials for our seed traits currently only occur in Israel, only Israeli regulations govern our operations. We believe that our current activities are compliant with all currently applicable Israeli regulations, however we may become subject to new or revised regulations or approvals in the future. Furthermore, any violation of these regulations could expose us to criminal penalties.
The large-scale field trials that our collaborators conduct during advanced stages of product development are subject to regulations similar to those we are subject to. Pursuant to our collaboration agreements, our collaborators also apply for the requisite regulatory approvals prior to commercialization of products containing our seed traits. In most of our key target markets, including the United States and the European Union, regulatory approvals must be received prior to the importation of transgenic products. These regulatory regimes may be
13
Table of Contents
particularly onerous; for example, the U.S. federal government’s regulation of biotechnology is divided among the United States Environmental Protection Agency, which regulates activity related to the invention of plant pesticides and herbicides, the United States Department of Agriculture, which regulates the import, field testing and interstate movement of specific technologies that may be used in the creation of transgenic plants, and the United States Food and Drug Administration, which regulates foods derived from new plant varieties. None of our seed traits is currently being tested in large-scale field trial or is in the regulatory approval development stage. Once products containing our seed traits reach these stages, however, if our collaborators are unable to obtain the requisite regulatory approvals or there is a delay in obtaining such approvals as a result of negative market perception or heightened regulatory standards, such products will not be commercialized, which would negatively impact our business and results of operations.
Disruption to our IT system could adversely affect our reputation and have a material adverse effect on our business and results of operations.
Our computational technologies rely on our IT system to collect and analyze the genomic data we discover. We store significant amounts of data, and as of June 30, 2013, we had compiled over 500 terabytes of data. Although we are developing back-up storage for our stored data, there can be no assurance that our back-up storage arrangements will be effective if it becomes necessary to rely on them. Furthermore, we can provide no assurance that our current IT system is fully protected against third-party intrusions, viruses, hacker attacks, information or data theft or other similar threats.
As we continue to develop our computational technologies and expand our genomic datasets, we may need to update our IT system and storage capabilities. However, if our existing or future IT system does not function properly, or if the IT system proves incompatible with our new technologies, we could experience interruptions in data transmissions and slow response times, preventing us from completing routine research and business activities. Furthermore, disruption or failure of our IT system due to technical reasons, natural disaster or other unanticipated catastrophic events, including power interruptions, storms, fires, floods, earthquakes, terrorist attacks and wars could significantly impair our ability to deliver data related to our projects to our collaborators on schedule and materially and adversely affect the outcome of our collaborations, our relationships with our collaborators, our business and our results of operations.
Development of our seed traits, particularly during our field trials, may be adversely affected by circumstances caused by us and those beyond our control.
The seed and ag-chemical industry is subject to various factors that make its operations relatively unpredictable from period to period. Our field tests may be adversely affected by circumstances both caused by us and those beyond our control. Factors caused by us include any failure by us or our collaborators to follow proper agronomic practice or suggested protocols for growing the model validation plants and crops for our field trials, and failure to identify and address diseases, insects and pests, such as birds that may eat the seeds we are evaluating. Factors beyond our control include weather and climatic variations, such as droughts or heat stress, or other factors we are unable to identify. For example, if there was prolonged or permanent disruption to the electricity, climate control or water supply operating systems in our greenhouses or laboratories, the plants on which we are testing our traits and the samples we store in freezers, both of which are essential to our research and development activities, would be severely damaged or destroyed, adversely affecting our research and development activities and thereby our business and results of operations. We have also experienced crop failures in the past for then-unknown reasons, causing delays in our achievement of milestones and delivery of results, and necessitating that we re-start the field trials. Any field test failure we may experience is not covered by our insurance policy, and therefore could result in increased cost of the field trials and development of our seed traits, which may negatively impact our business and results of operations.
14
Table of Contents
Consumer and government resistance to genetically modified organisms may negatively affect our public image and reduce sales of plants containing our traits.
We are active in the field of biotech research and development in seeds and crop protection, including genetically modified seeds. Foods made from such seeds are not accepted by many consumers and in certain countries production of certain GM crops is effectively prohibited, including throughout the European Union, due to concerns over such products’ effects on food safety and the environment. The high public profile of biotechnology in food production and lack of consumer acceptance of products to which we have devoted substantial resources could negatively affect our public image and results of operations. The prohibition on the production of certain GM crops in select countries and the current resistance from consumer groups, particularly in Europe, to GM crops not only limits our access to such markets but also has the potential to spread to and influence the acceptance of products developed through biotechnology in other regions of the world and may also influence regulators in other countries to limit or ban production of GM crops, which could limit the commercial opportunities to exploit biotechnology.
GM crops are grown principally in the United States, Brazil and Argentina where there are fewer restrictions on the production of GM crops. If these or other countries where GM crops are grown enact laws or regulations that ban the production of such crops or make regulations more stringent, we could experience a longer product development cycle for our products and may even have to abandon projects related to certain crops or geographies, both of which would negatively affect our business and results of operations. Furthermore, any changes in such laws and regulations or consumer acceptance of GM crops could negatively impact our collaborators, who in turn might terminate or reduce the scope of their collaborations with us or seek to alter the financial terms of our agreements with them.
We have a history of operating losses and negative cash flow, and we may never achieve or maintain profitability.
We have a history of losses, and incurred operating losses of $(3.1 million), $(3.2 million) and $(2.0 million) for the years ended December 31, 2012, 2011 and 2010, respectively, and $(2.1 million) and $(1.1 million) in the six months ended June 30, 2013 and 2012, respectively. Although we are currently developing 20 distinct seed traits, there can be no assurance that these traits will result in commercially successful products. We expect to continue to incur losses in future periods, until we begin earning royalties on the products we are currently developing and any new seed traits we develop in the future, which may not occur for at least six years, if at all. Because we will incur significant costs and expenses for these efforts before we obtain any incremental revenues from them, our losses in future periods could be significant. In addition, we may find that these efforts are more expensive than we anticipate or that they do not result in profitability in the time period we anticipate, which would further increase our losses. If we are unable to adequately control the costs associated with operating our business, including our costs of development and sales, our business, financial condition, operating results and prospects will suffer.
The licenses we grant to our collaborators to use the genes we discover for specified traits in certain crops are exclusive. This limits our opportunities to license the genes that target the same traits in the same crop to more than one collaborator.
The licenses we grant our collaborators to use the genes we discover and patent in certain crops are exclusive. That means that once genes are licensed to a collaborator in a specified crop or crops, we are generally prohibited from licensing those genes to any third party. For example, in the Bayer Wheat Agreement, as defined herein, we are broadly prohibited from collaborating on both GM trait discovery and advanced breeding for wheat, irrespective of the trait, with any party other than Bayer. The limitations imposed by these exclusive licenses could prevent us from expanding our business and increasing our exposure to new licensees, both of which could adversely affect our business and results of operations.
15
Table of Contents
Competition in seed traits and seeds is intense and requires continuous technological development. If we are unable to compete effectively, our financial results will suffer.
We currently face significant competition in the markets in which we operate. The markets for seed traits and ag-chemicals are intensely competitive and rapidly changing. Many companies engage in research and development of seed traits and ag-chemicals, and speed in getting a new product to market can be a significant competitive advantage. As an example, some of our competitors have enhanced research and development budgets allocated for seeds that are more significant than our budget. In most segments of the seed and ag-chemical market, the number of products available to the consumer is steadily increasing as new products are introduced. At the same time, an increasing number of products are coming off patent and are thus available to generic manufacturers for production. We may be unable to compete successfully against our current and future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for products containing our traits. In addition, many of our competitors have substantially greater financial, marketing, sales, distribution and technical resources than us and some of our collaborators have more experience in research and development, regulatory matters, manufacturing and marketing. We anticipate increased competition in the future as new companies enter the market and new technologies become available. Our technology may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenues from the commercialization of our technology.
We may be required to pay royalties to employees who develop inventions that have been or will be commercialized by us, even if the rights to such inventions have been assigned to us and the employees have waived their rights to royalties or other additional compensation.
Under the Israeli Patents Law, 5727-1967, if there is no agreement that prescribes whether, to what extent and on what conditions an employee is entitled to remuneration from commercialization of an invention developed by or with the contribution of such employee, then such matter is decided by a government-appointed compensation and royalties committee established under the Patents Law. In a decision issued in February 2010, the committee raised (but did not answer) the question whether the waiver by an employee of the right to receive remuneration from the commercialization of such invention is enforceable. The committee stated that such waiver is not necessarily enforceable, since the entitlement to royalties from future commercialization of such invention may be deemed a basic labor law protective right that may not be waived. A subsequent decision of the Israeli Supreme Court from August 2012 left this question unresolved. If such waiver is not enforceable, then an employee may be entitled to seek a determination by the committee that royalties from the commercialization of such invention are payable to the employee by the employer despite the waiver. A significant portion of our intellectual property (including our patents) has been developed by our employees in the course of their employment for us. All of our employees execute invention assignment agreements upon commencement of employment, in which they assign their rights to potential inventions and acknowledge that they will not be entitled to additional compensation or royalties from commercialization of inventions. However, given the foregoing uncertainty with respect to the enforceability of a waiver of the right to future royalties, we may be required to pay royalties to our employees who have invented intellectual property that we have commercialized, which in turn may have a material adverse effect on our results of operations.
Our success depends on our ability to protect our intellectual property and our proprietary technologies.
Our commercial success depends in part on our ability to obtain and maintain patent protection and trade secret protection for our proprietary computational technologies, our traits and their uses, as well as our ability to operate without infringing upon the proprietary rights of others. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
16
Table of Contents
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected and our business would be harmed.
We treat our proprietary computational technologies, including unpatented know-how and other proprietary information, as trade secrets. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with any third parties who have access to them, such as our consultants, independent contractors, advisors, corporate collaborators and outside scientific collaborators. We also enter into confidentiality and invention or patent assignment agreements with employees and certain consultants. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, or if we otherwise lose protection for our trade secrets or proprietary know-how, the value of this information may be greatly reduced and our business and competitive position could be harmed.
Changes in laws and regulations to which we are subject, or to which we may become subject in the future, may materially increase our costs of operation, decrease our operating revenues and disrupt our business.
Laws and regulatory standards and procedures that impact our business are continuously changing. Responding to these changes and meeting existing and new requirements may be costly and burdensome. Changes in laws and regulations may occur that could:
| • | impair or eliminate our ability to research and develop our products, including validating our products through field trials; |
| • | increase our compliance and other costs of doing business through increases in the cost to patent or otherwise protect our intellectual property or increases in the cost to our collaborators to obtain the necessary regulatory approvals to commercialize and market the products we develop with them; |
| • | require significant product redesign or systems redevelopment; |
| • | render our products less profitable, obsolete or less attractive compared to competing products; |
| • | affect our collaborators’ willingness to do business with us; |
| • | reduce the amount of revenues we receive from our collaborators through milestone payments or royalties; and |
| • | discourage our collaborators from offering, and consumers from purchasing, products that incorporate our traits. |
Any of these events could have a material adverse effect on our business, results of operations and financial condition. Legislators and regulators have increased their focus on plant biotechnology in recent years, with particular attention paid to GM crops. Because our current products are primarily in the initial discovery and proof of concept development phase, the only GM-related regulations that currently affect our business are related to our validation trials in Israel. We believe that we are currently in compliance with Israeli regulations related to growing GM crops in Israel; however, if these regulations change, our validation trials may become costly and burdensome and could require us to relocate our trials outside of Israel or even change our business model to have our collaborators perform validation trials.
While none of our products are currently available for sale, our future growth relies on the ability of our collaborators to commercialize and market our products, and any restrictions on such activities could materially and adversely impact our business and results of operations. Any changes in regulations in countries where GM crops are grown or exported into could result in our collaborators being unable or unwilling to develop,
17
Table of Contents
commercialize or sell products that incorporate our traits. In addition, we rely on patents and other forms of intellectual property protection. Legislation and jurisprudence on patent protection in the key target markets where we seek patent protection, such as the United States and the European Union, is evolving and changes in laws could affect our ability to obtain or maintain patent protection for our products. Any changes to these existing laws and regulations may materially increase our costs of operation, decrease our operating revenues and disrupt our business. See “Business—Regulation.”
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotech companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing biotech patents involves technological and legal complexity, and is costly, time consuming, and inherently uncertain. In addition, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, the laws and regulations governing patents could change in unpredictable ways that may weaken or undermine our ability to obtain new patents or to enforce our existing patents and patents we might obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, maintaining and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States are less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we are unable to prevent third parties from using our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the jurisdictions in which we do not have patent protection. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, and we may be unable to prevent such competitors from importing those infringing products into territories where we have patent protection but enforcement is not as strong as in the United States. These products may compete with our product candidates and our patents and other intellectual property rights may not be effective or sufficient to prevent them from competing in those jurisdictions. Moreover, farmers or others in the chain of commerce may raise legal challenges against our intellectual property rights or may infringe upon our intellectual property rights, including through means that may be difficult to prevent or detect. For example, the practice by some farmers of saving seeds from non-hybrid crops (such as soybeans, canola and cotton) containing our biotechnological traits has prevented and may continue to prevent us from realizing the full value of our intellectual property in countries outside of the United States.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions, including China, where we have filed patent applications. The legal systems of certain countries, including China, have not historically favored the enforcement of patents or other intellectual property rights, which could hinder us from preventing the infringement of our patents or other intellectual property rights and result in substantial risks to us. Proceedings to enforce our patent rights in the United States or foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert patent infringement or other claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license from third parties.
18
Table of Contents
If we or one of our collaborators are sued for infringing the intellectual property rights of a third party, such litigation could be costly and time consuming and could prevent us or our collaborators from developing or commercializing our products.
Our ability to generate significant revenues from our products depends on our and our collaborators’ ability to develop, market and sell our products and utilize our proprietary technology without infringing the intellectual property and other rights of any third parties. In the United States and abroad there are numerous third party patents and patent applications that may be applied toward our proprietary technology, business processes or developed traits, some of which may be construed as containing claims that cover the subject matter of our products or intellectual property. Because of the rapid pace of technological change, the confidentiality of patent applications in some jurisdictions, and the fact that patent applications can take many years to issue, there may be currently pending applications that are unknown to us that may later result in issued patents upon which our product candidates or proprietary technologies infringe. Similarly, there may be issued patents relevant to our product candidates of which we are not aware. These patents could reduce the value of the traits we develop or the genetically modified plants containing our traits or, to the extent they cover key technologies on which we have unknowingly relied, require that we seek to obtain licenses or cease using the technology, no matter how valuable to our business. We may not be able to obtain such a license on commercially reasonable terms. There is a substantial amount of litigation involving patent and other intellectual property rights in the biotech industry generally. If any third party patent or patent application covers our intellectual property or proprietary rights and we are not able to obtain a license to it, we and our collaborators may be prevented from commercializing products containing our traits.
As the agricultural biotech industry continues to develop, we may become party to, or threatened with, litigation or other adverse proceedings regarding intellectual property or proprietary rights in our technology, processes or developed traits. Third parties may assert claims based on existing or future intellectual property rights and the outcome of any proceedings is subject to uncertainties that cannot be adequately quantified in advance. Any litigation proceedings could be costly and time consuming and negative outcomes could result in liability for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. There is also no guarantee that we would be able to obtain a license under such infringed intellectual property on commercially reasonable terms or at all. A finding of infringement could prevent us or our collaborators from developing, marketing or selling a product or force us to cease some or all of our business operations. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel may be diverted as a result of these proceedings, which could have a material adverse effect on us. Claims that we have misappropriated the confidential information or trade secrets of third parties could similarly have a negative impact on our business.
We and our collaborators may disagree over our right to receive payments under our collaboration agreements, potentially resulting in costly litigation and loss of reputation.
Our ability to generate royalty payments from our collaboration agreements depends on our ability to clearly delineate our intellectual property rights under those agreements. We often license patented genes or other intellectual property to our collaborators, who use or will use such intellectual property to develop and commercialize seeds with improved traits. However, a collaborator may use our intellectual property without our permission, dispute our ownership of certain intellectual property rights or argue that our intellectual property does not cover their marketed product. If a dispute arises, it may result in costly litigation, and our collaborator may refuse to pay us royalty payments while the dispute is ongoing. Furthermore, regardless of any resort to legal action, a dispute with a collaborator over intellectual property rights may damage our relationship with that collaborator, and may also harm our reputation in the industry.
We have not yet registered our trademarks. Failure to secure those registrations could adversely affect our business.
We have not yet filed applications to register our trademarks and those applications may be rejected or not be allowed for registration, and registered trademarks may not be successfully obtained, maintained or enforced.
19
Table of Contents
We are given an opportunity to respond to those rejections, but we may not be able to overcome such rejections. In addition, in the U.S. Patent and Trademark Office and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would, which could adversely affect our business.
We may be required to pay substantial damages as a result of product liability claims for which insurance coverage is not available.
Once products integrating our seed traits reach commercialization, product liability claims will be a commercial risk for our business, particularly as we are involved in the supply of biotechnological products, some of which can be harmful to humans and the environment. Courts have levied substantial damages in the United States and elsewhere against a number of companies in the agriculture industry in past years based upon claims for injuries allegedly caused by the use of their products. Product liability claims against us or our collaborators selling products that contain our seed traits or allegations of product liability relating to seeds containing traits developed by us could damage our reputation, harm our relationships with our collaborators and materially and adversely affect our business, results of operations, financial condition and prospects. We do not have product liability insurance coverage. Furthermore, while our collaboration agreements typically require that our collaborators indemnify us for the cost of product liability claims brought against us, such indemnification provisions may not always be enforced, and we may receive no indemnification if our own misconduct led to the claims.
Our employment agreements with our employees and other agreements with our collaborators and third parties may not adequately prevent disclosure of trade secrets, know-how and other proprietary information.
A substantial portion of our technologies and intellectual property is protected by trade secret laws. We rely on a combination of patent and other intellectual property laws as well as our employment agreements with our employees and other agreements with our collaborators and third parties to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not prevent disclosure, infringement or misappropriation of our confidential information. Our confidentiality, nondisclosure and assignment agreements or covenants may be breached, and we may not have adequate remedies for such a breach that would effectively prevent the further dissemination of our confidential information. We have limited control over the protection of trade secrets used by our collaborators and could lose future trade secret protection if any unauthorized disclosure of such information occurs. In addition, others may independently discover our trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such parties. Laws regarding trade secret rights in certain markets where we operate may afford little or no protection of our trade secrets. Failure to obtain or maintain trade secret protection could adversely affect our business, sales and competitive position.
We may be adversely affected by the current economic environment.
Our ability to obtain financing, invest in and grow our business, and meet our financial obligations depends on our operating and financial performance, which in turn is subject to numerous factors. In addition to factors specific to our business, prevailing economic conditions and financial, business and other factors beyond our control can also affect our business and ability to raise capital. We cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
20
Table of Contents
We may not be able to fully enforce covenants not to compete with our key employees, and therefore we may be unable to prevent our competitors from benefiting from the expertise of such employees.
Our employment agreements with key employees, which include executive officers, contain non-compete provisions. These provisions prohibit our key employees, if they cease working for us, from competing directly with us or working for our competitors for one year. Under applicable U.S. and Israeli laws, we may be unable to enforce these provisions. If we cannot enforce the non-compete provisions with our key employees, we may be unable to prevent our competitors from benefiting from the expertise of such employees. Even if these provisions are enforceable, they may not adequately protect our interests. The defection of one or more of our employees to a competitor could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
Our operations are subject to various health and environmental risks associated with our use, handling and disposal of potentially toxic materials.
As part of our seed trait operations, we assist in the development of GM crops by inserting new genes into the genomes of certain plants. Though we introduce these genes in order to improve plant traits, we cannot always predict the effect that these genes may have on the plant. In some cases, the genes may render the plant poisonous or toxic, or they may cause the plant to develop other dangerous characteristics that could harm the plant’s surrounding environment. Furthermore, while we comply with relevant environmental laws and regulations, there is a risk that, when testing genetically modified plants, the seeds of these plants may escape the greenhouse or field in which they are being tested and contaminate nearby fields. Poisonous or toxic plants may therefore be inadvertently introduced into the wild, or possibly enter the food production system, harming the people and animals who come in contact with them.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business, particularly after we are no longer an “emerging growth company.”
Following the completion of this offering, we will be required to comply with various regulatory and reporting requirements, including those required by the Securities and Exchange Commission, or the SEC. Complying with these reporting and regulatory requirements will be time consuming, result in increased costs to us and could have a negative effect on our business, results of operations and financial condition.
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the requirements of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act. These requirements may place a strain on our systems and resources. The Exchange Act requires that we file annual and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting. To maintain and improve the effectiveness of our disclosure controls and procedures, we will need to commit significant resources, hire additional staff and provide additional management oversight. We will be implementing additional procedures and processes for the purpose of addressing the standards and requirements applicable to public companies. These activities may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
As an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act, or the “JOBS Act,” we may take advantage of certain temporary exemptions from various reporting requirements including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act (and the rules and regulations of the SEC thereunder). These exemptions will cease to apply by no later than the last day of our fiscal year following the fifth anniversary of the completion of this offering, and we expect to incur additional expenses and devote increased management effort toward ensuring compliance with them. We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs.
21
Table of Contents
Risks Related to Our Ordinary Shares and the Offering
An active, liquid and orderly trading market for our ordinary shares may not develop in the United States, the price of our ordinary shares may be volatile, and you could lose all or part of your investment.
Prior to this offering, there has been no public market in the United States for our ordinary shares. The initial public offering price of our ordinary shares in this offering will be based, in part, on the price of our ordinary shares on the Tel Aviv Stock Exchange, or the TASE, and determined by negotiation among us and the representatives of the underwriters. This price may not reflect the market price of our ordinary shares following this offering and the price of our ordinary shares may decline. In addition, the market price of our ordinary shares could be highly volatile and may fluctuate substantially as a result of many factors, including:
| • | actual or anticipated fluctuations in our results of operations; |
| • | variance in our financial performance from the expectations of market analysts; |
| • | announcements by us or our competitors of significant business developments, changes in relationships with our collaborators, acquisitions or expansion plans; |
| • | our involvement in litigation; |
| • | our sale, or the sale by our significant shareholders, of ordinary shares or other securities in the future; |
| • | failure to publish research or the publishing of inaccurate or unfavorable research; |
| • | market conditions in our industry and changes in estimates of the future size and growth rate of our markets; |
| • | changes in key personnel; |
| • | the trading volume of our ordinary shares; and |
| • | general economic and market conditions. |
An active trading market on the New York Stock Exchange, or NYSE, for our ordinary shares may never develop or may not be sustained following this offering. If an active market for our ordinary shares does not develop, it may be difficult to sell your ordinary shares in the United States.
In addition, the stock markets have recently experienced extreme price and volume fluctuations. Broad market and industry factors may materially harm the market price of our ordinary shares, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. If we were involved in any similar litigation we could incur substantial costs and our management’s attention and resources could be diverted.
As a foreign private issuer whose shares are listed on the Tel Aviv Stock Exchange, we intend to follow certain home country corporate governance practices instead of certain NYSE corporate governance requirements.
As a foreign private issuer, in reliance on NYSE’s corporate governance rules, which permits a foreign private issuer to follow the corporate governance practices of its home country, we will be permitted to follow certain Israeli corporate governance practices instead of those otherwise required under the NYSE corporate governance standards for U.S. domestic issuers. As of the consummation of this offering, we intend to follow the NYSE corporate governance standards for domestic issuers, except with respect to the matters described in this risk factor. Our articles of association provide that the quorum for any meeting of shareholders shall be the presence of at least two shareholders present in person, by proxy or by a voting instrument, who hold at least 25% of the voting power of our shares. As permitted under the Israeli Companies Law, or the Companies Law, for an adjourned meeting at which a quorum is not present at the end of half an hour following the time fixed for the
22
Table of Contents
meeting, the meeting may generally proceed so long as at least one shareholder is present, regardless of the voting power held by him, her or it. We also intend to follow certain home country practices in Israel in lieu of complying with certain other NYSE corporate governance requirements, including separate executive sessions of independent directors and non-management directors and the requirement to obtain shareholder approval for certain dilutive events (such as for the establishment or amendment of certain equity-based compensation plans, issuances that will result in a change of control of the company and issuances of more than 1% of our outstanding shares or voting power to our affiliates). Accordingly, our shareholders will not be afforded the same protection as provided under NYSE corporate governance rules. Following our home country governance practices as opposed to the requirements that would otherwise apply to a United States company listed on NYSE may provide less protection than is accorded to investors of domestic issuers. See “Management—NYSE Listed Company Manual and Home Country Practices.”
Our ordinary shares will be traded on more than one market and this may result in price variations.
Our ordinary shares have been traded on the TASE since 2007, and we intend to apply to have our ordinary shares listed on NYSE. Trading in our ordinary shares on these markets will take place in different currencies (U.S. dollars on NYSE and NIS on the TASE), and at different times (resulting from different time zones, trading days and public holidays in the United States and Israel). The trading prices of our ordinary shares on these two markets may differ due to these and other factors. Any decrease in the price of our ordinary shares on the TASE could cause a decrease in the trading price of our ordinary shares on NYSE.
As a foreign private issuer we will not be subject to U.S. proxy rules and will be exempt from filing certain Exchange Act reports.
As a foreign private issuer, we will be exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors, and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual and current reports and financial statements with the SEC as frequently or as promptly as domestic companies whose securities are registered under the Exchange Act, and we will generally be exempt from filing quarterly reports with the SEC under the Exchange Act.
In addition, we would lose our foreign private issuer status if a majority of our directors or executive officers are U.S. citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. Although we have elected to comply with certain U.S. regulatory provisions, our loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with good governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers.
We may be subject to parallel reporting obligations in Israel and the United States, which may impose on us more stringent disclosure obligations than is customary for foreign private issuers whose securities are listed only in the United States.
As an Israeli public company with ordinary shares that are traded on the TASE, we have filed Hebrew language periodic and immediate reports with, and have furnished information to, the TASE and the Israeli Securities Authority, or the ISA, since the consummation of the initial public offering of our shares on the TASE in 2007. Prior to the commencement of this offering, we intend to convene a special meeting of our shareholders to be held shortly prior to completion of this offering, in order to solicit, among other things, the approval of our
23
Table of Contents
shareholders for a transition solely to U.S. reporting standards upon listing on the NYSE in accordance with an applicable exemption under the Israeli Securities Law, 5728-1968, and the regulations promulgated thereunder, or the Israeli Securities Law.
To the extent that we do not receive such approval of our shareholders, upon consummation of this offering we will become subject to separate reporting schemes of the Israel Securities Law and the Exchange Act. While similar in many respects, certain differences between these reporting schemes may impose on us disclosure obligations that are more stringent than those generally applied to foreign private issuers whose securities are listed only in the United States. For example, while the execution of a term sheet for a material transaction is generally not reportable by a foreign private issuer under the Exchange Act, such an event must be immediately reported to the TASE. Such disclose requirements might hamper our ability to freely conduct our operations, compared to other foreign private issuers whose securities are listed only in the United States. In addition, if we wish to engage in a capital market or another material transaction in which we are required to disclose certain information in one jurisdiction (whether Israel or the United States) that we are not required or willing to disclose under the securities laws of the other jurisdiction at the same time or at all, we may need to change the timing or form of such capital raising or other material transaction. Because we will be listed in different jurisdictions and operate in different geographic markets, we may sometimes not present information regarding our operations on a uniform basis. Accordingly, we may not present certain data under one set of securities laws that is typically presented under the other set of securities laws, thereby leading to a disparity of information between investors and shareholders in the two jurisdictions, which could cause a disparity of share prices and thereby present the opportunity for arbitrage in trading in our ordinary shares. Finally, if we do not receive the shareholders’ approval described above, the requirement to comply with the separate reporting obligations under U.S. and Israeli securities laws will require management attention and will burden us with additional costs.
Our U.S. shareholders may suffer adverse tax consequences if we are characterized as a passive foreign investment company.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets (which may be determined in part by the market value of our ordinary shares, which is subject to change) are held for the production of, or produce, passive income, we could be characterized as a passive foreign investment company, or PFIC, for United States federal income tax purposes. According to these rules, a publicly traded non-U.S. corporation may treat the aggregate fair market value of its assets as being equal to the sum of the aggregate value of its outstanding shares (“Market Capitalization”) and the total amount of its liabilities. We intend to take the position that the excess of our Market Capitalization plus liabilities over the book value of all of our assets (“Goodwill”) may generally be treated as a non-passive asset to the extent attributable to our non-passive activities. Based on certain estimates of our gross income and gross assets, our intended use of proceeds of this offering, and the nature of our business, we do not expect that we will be classified as a PFIC for the taxable year ending December 31, 2013; however, because we currently hold, and expect to continue to hold, a substantial amount of cash and cash equivalents and other passive assets used in our business, and because the value of our gross assets is likely to be determined in large party by reference to the market prices of our securities, we could be classified as a PFIC for a given taxable year if our Market Capitalization were to decrease significantly.
If we are characterized as a PFIC, our United States shareholders may suffer adverse tax consequences, including having gains realized on the sale of our ordinary shares treated as ordinary income, rather than a capital gain, the loss of the preferential rate applicable to dividends received on our ordinary shares by individuals who are U.S. Holders (as defined in “Taxation and Government Programs—United States Federal Income Taxation”), and having interest charges apply to distributions by us and the proceeds of share sales. If we are characterized as a PFIC, certain elections may be available that would alleviate some of the adverse consequences of PFIC status and result in an alternative treatment (such as mark-to-market treatment) of our ordinary shares; however, we do not intend to provide the information necessary for U.S. holders to make qualified electing fund elections if we are classified as a PFIC. See “Taxation and Government Programs—United States Federal Income Taxation—Passive Foreign Investment Company Considerations.”
24
Table of Contents
We are an “emerging growth company” and we cannot be certain whether the reduced requirements applicable to emerging growth companies will make our ordinary shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various requirements that are applicable to other public companies that are not “emerging growth companies.” For so long as we remain an “emerging growth company,” we will not be subject to the provision of Section 404(b) of the Sarbanes-Oxley Act that requires that our independent registered public accounting firm to provide an attestation report on the effectiveness of our internal control over financial reporting. This may increase the risk that we will fail to detect and remedy any weaknesses or deficiencies in our internal control over financial reporting. We have also elected to include three years of audited financial statements and selected financial data. In general, these reduced reporting requirements may allow us to refrain from disclosing information that you may find important. We have irrevocably elected not to avail ourselves of the election to delay adopting new or revised accounting standards until such time as those standards apply to private companies. Nevertheless, as a foreign private issuer that is an emerging growth company, we will not be required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act for up to five fiscal years after the date of this offering. We will remain an emerging growth company until the earliest of: (a) the last day of our fiscal year during which we have total annual gross revenues of at least $1.0 billion; (b) the last day of our fiscal year following the fifth anniversary of the completion of this offering; (c) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; or (d) the date on which we are deemed to be a “large accelerated filer” under the Exchange Act. When we are no longer deemed to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act. We cannot predict if investors will find our ordinary shares less attractive as a result of our reliance on exemptions under the JOBS Act. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares and our share price may be more volatile.
The market price of our ordinary shares could be negatively affected by future sales of our ordinary shares.
After this offering, we will have ordinary shares outstanding. If we or our shareholders sell substantial amounts of our ordinary shares, either on the TASE or on NYSE, or if there is a public perception that these sales may occur in the future, the market price of our ordinary shares may decline. We, together with our directors, officers and our significant shareholders, in the aggregate beneficially owning % of our outstanding ordinary shares as of June 30, 2013, have agreed with the underwriters of this offering not to sell any ordinary shares, other than the shares offered through this prospectus, for a period of 180 days following the date of this prospectus.
The ordinary shares we are offering for sale in this offering and the 18,879,987 ordinary shares that are outstanding as of June 30, 2013, will be freely tradable in the United States immediately following this offering. As a result, except for the 3,813,113 ordinary shares that are the subject of lock-up agreements entered into by the holders thereof in connection with this offering, all of our outstanding shares are available for sale on the TASE and NYSE without restriction.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
We intend to allocate the net proceeds from this offering to our different areas of activity, including by implementing computational platforms that will shorten development time and time-to-market for particular traits in our seed traits operation, investing in chemical screening and handling capabilities and additional facilities under our ag-chemical operation, and establishing seed manufacturing facilities for worldwide commercialization under our Evofuel operation. Our management may not apply the net proceeds in ways that ultimately increase the value of your investment. They will have broad discretion in the application of the use of proceeds from this offering, and you will be relying on the judgment of our management regarding the
25
Table of Contents
application of these proceeds. If we do not invest or apply the net proceeds from this offering in ways that enhance shareholder value, we may fail to achieve expected financial results, which could cause the price of our ordinary shares to decline. See “Use of Proceeds.”
We have not yet determined whether our existing internal controls over financial reporting systems are compliant with Section 404 of the Sarbanes-Oxley Act, and we cannot provide any assurance that there are no material weaknesses or significant deficiencies in our existing internal controls.
Pursuant to Section 404 of the Sarbanes-Oxley Act and the related rules adopted by the SEC and the Public Company Accounting Oversight Board, starting with the second annual report that we file with the SEC after the consummation of this offering, our management will be required to report on the effectiveness of our internal control over financial reporting. In addition, once we no longer qualify as an “emerging growth company” under the JOBS Act and lose the ability to rely on the exemptions related thereto discussed above, our independent registered public accounting firm will also need to attest to the effectiveness of our internal control over financial reporting under Section 404. We have not yet commenced the process of determining whether our existing internal controls over financial reporting systems are compliant with Section 404 and whether there are any material weaknesses or significant deficiencies in our existing internal controls. This process will require the investment of substantial time and resources, including by our Chief Financial Officer and other members of our senior management. As a result, this process may divert internal resources and take a significant amount of time and effort to complete. In addition, we cannot predict the outcome of this determination and whether we will need to implement remedial actions in order to implement effective control over financial reporting. The determination and any remedial actions required could result in us incurring additional costs that we did not anticipate. Irrespective of compliance with Section 404, any failure of our internal controls could have a material adverse effect on our stated results of operations and harm our reputation. As a result, we may experience higher than anticipated operating expenses, as well as higher independent auditor fees during and after the implementation of these changes. If we are unable to implement any of the required changes to our internal control over financial reporting effectively or efficiently or are required to do so earlier than anticipated, it could adversely affect our operations, financial reporting and/or results of operations and could result in an adverse opinion on internal controls from our independent auditors.
We will incur increased costs as a result of registering our ordinary shares under the Exchange Act and our management will be required to devote substantial time to compliance and new compliance initiatives.
As a public company in the United States, we will incur additional significant accounting, legal and other expenses that we did not incur before this offering. We also anticipate that we will incur costs associated with the requirements under Section 404 and other provisions of the Sarbanes-Oxley Act. We expect these rules and regulations to increase our legal and financial compliance costs, introduce new costs, such as additional stock exchange listing fees and shareholder reporting, and to take a significant amount of management’s time. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs.
In addition, changing laws, regulations and standards, in the United States or Israel, relating to corporate governance and public disclosure and other matters, may be implemented in the future, which may increase our legal and financial compliance costs, make some activities more time consuming and divert management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed. We also expect that being a publicly traded company in the United States and being subject to U.S. rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee, and qualified executive officers.
26
Table of Contents
Risks Relating to Our Incorporation and Location in Israel
Conditions in Israel could adversely affect our business.
We are incorporated under Israeli law and our principal offices and research and development facilities are located in Israel. Accordingly, political, economic and military conditions in Israel directly affect our business. Since the State of Israel was established in 1948, a number of armed conflicts have occurred between Israel and its Arab neighbors. Although Israel has entered into various agreements with Egypt, Jordan and the Palestinian Authority, there has been an increase in unrest and terrorist activity, which began in September 2000 and has continued with varying levels of severity into 2013. In mid-2006, Israel was engaged in an armed conflict with Hezbollah in Lebanon, resulting in thousands of rockets being fired from Lebanon and disrupting most day-to-day civilian activity in northern Israel. Starting in December 2008, for approximately three weeks, Israel engaged in an armed conflict with Hamas in the Gaza Strip, which involved missile strikes against civilian targets in various parts of Israel and negatively affected business conditions in Israel. A similar conflict arose due to Hamas missile attacks against Israeli civilian targets in November 2012. Our principal place of business is located in Rehovot, Israel, which is approximately 30 miles from the nearest point of the border with the Gaza Strip. There can be no assurance that attacks launched from the Gaza Strip will not reach our facilities, or that hostilities will not otherwise cause a significant disruption to our operations, such as preventing our employees from reaching our facilities and limiting our ability to monitor and otherwise conduct the crop and other experiments we conduct at the facilities.
Several countries, principally in the Middle East, still restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel or political instability in the region continues or increases. These restrictions may limit materially our ability to sell our products to companies in these countries. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or significant downturn in the economic or financial condition of Israel, could adversely affect our operations and research and development, cause our revenues to decrease and adversely affect the share price of publicly traded companies having operations in Israel, such as us.
In addition, our business insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle East. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained. Any losses or damages incurred by us could have a material adverse effect on our business and financial condition.
Our operations may be disrupted by the obligations of personnel to perform military service.
As of June 30, 2013, we had 188 full-time and 73 part-time employees, all of whom were based in Israel. Our employees in Israel, including executive officers, may be called upon to perform up to 36 days (and in some cases more) of annual military reserve duty until they reach the age of 45 (and in some cases, up to 49) and, in emergency circumstances, could be called to active duty. In response to increased tension and hostilities, since September 2000 there have been occasional call-ups of military reservists, including in connection with the mid-2006 war in Lebanon and the December 2008 and November 2012 conflicts with Hamas, and it is possible that there will be additional call-ups in the future. Our operations could be disrupted by the absence of a significant number of our male employees related to military service or the absence for extended periods of one or more of our key employees for military service. Such disruption could materially adversely affect our operations, business and results of operations.
The tax benefits that are available to us require us to continue to meet various conditions and may be terminated or reduced in the future, which could increase our costs and taxes.
Our Israeli facilities have the status of an “Approved Enterprise” and “Beneficiary Enterprise” under the Israeli Law for the Encouragement of Capital Investments, 1959, or the Investment Law, which makes us eligible
27
Table of Contents
for certain tax benefits under that law. For example, we are exempt from corporate tax for a period of two years and are subject to a reduced corporate tax rate of between 10% to 25% for the remainder of the benefits period, depending on the level of foreign investment in the company in each year.
In order to remain eligible for the tax benefits of an Approved Enterprise and Beneficiary Enterprise, we must continue to meet certain conditions stipulated in the Investment Law and its regulations, as amended, and in a tax ruling we received in October 2010, or the Tax Ruling. If we do not meet these requirements, we may not be eligible to receive tax benefits and we could be required to refund any tax benefits that we may receive in the future, in whole or in part, with interest. Further, the tax benefits available under the Investment Law may be terminated or reduced in the future. If these tax benefits are terminated, our Israeli taxable income would be subject to regular Israeli corporate tax rates. The standard corporate tax rate for Israeli companies in 2012 and 2013 is 25% (was previously 24% in 2011 and 25% in 2010), rising to 26.5% for the 2014 tax year and thereafter. See “Taxation and Government Programs—Israeli Tax Considerations and Government Programs—General Corporate Tax Structure in Israel.”
Additionally, if we increase our activities outside of Israel (for example, through acquisitions) our expanded activities might not be eligible for inclusion in future Israeli tax benefit programs. Finally, in the event of a distribution of a dividend from the income that will be tax exempt under the Investment Law, in addition to withholding tax at a rate of 20% (or a reduced rate under an applicable double tax treaty), we will be subject to tax at the corporate tax rate applicable to our Approved Enterprise’s and Beneficiary Enterprise’s income on the amount distributed in accordance with the reduced corporate tax applicable to such profits. See “Taxation and Government Programs—Israeli Tax Considerations and Government Programs—Law for the Encouragement of Capital Investments, 5719-1959.”
Exchange rate fluctuations between the U.S. dollar and the NIS may negatively affect our earnings and we have not hedged against such fluctuations.
Most of our revenues are denominated in U.S. dollars. The percentage of our revenues denominated in U.S. dollars accounted for 72%, 71% and 78% of our revenues in 2012, 2011 and 2010, respectively, and 68% and 74% in the six months ended June 30, 2013 and 2012, respectively. We incur expenses primarily in NIS, however, and our expenses in NIS accounted for 90%, 90% and 89% of our expenses in 2012, 2011 and 2010, respectively, and 91% of our expenses in each of the six months ended June 30, 2013 and 2012. As a result, any appreciation of the NIS relative to the U.S. dollar would adversely impact our profitability due to the portion of our expenses that are incurred in NIS. As of June 30, 2013, we did not have any hedging arrangements in place to protect our exposure to foreign currency fluctuations. If we choose to do so in the future, we may be unsuccessful in protecting against currency exchange rate fluctuations. Future currency exchange rate fluctuations could adversely affect our profitability. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Quantitative and Qualitative Disclosure About Market Risk—Foreign Currency Risk.”
We have received Israeli government grants for certain of our research and development activities. The terms of these grants may require us to satisfy specified conditions in order to develop and transfer technologies supported by such grants outside of Israel. In addition, in some circumstances, we may be required to pay penalties in addition to repaying the grants.
Our research and development operations have been partly financed through certain governmental grants, which impose certain restrictions on the transfer outside of Israel of the underlying know-how and the manufacturing or manufacturing rights of the underlying products and technologies. As of June 30, 2013, we had received approximately $4.1 million (approximately NIS 14.8 million) of such grants, on which interest of approximately $0.8 million (approximately NIS 2.9 million) had accrued as of such date. We may not receive the required approvals should we wish to transfer this know-how, technology or manufacturing rights outside of Israel in the future or, if we receive such required approvals, they may be subject to certain conditions and payment obligations. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Government Grants.”
28
Table of Contents
It may be difficult to enforce a U.S. judgment against us, our officers and directors and the Israeli experts named in this prospectus in Israel or the United States, or to assert U.S. securities laws claims in Israel or serve process on our officers and directors and these experts.
We are incorporated in Israel. None of our directors and executive officers is a resident of the United States, and the Israeli experts named in this prospectus are located in Israel. The majority of our assets are located outside the United States. Therefore, it may be difficult for an investor, or any other person or entity, to enforce a U.S. court judgment based upon the civil liability provisions of the U.S. federal securities laws against us or any of these persons in a U.S. or Israeli court, or to effect service of process upon these persons in the United States. Additionally, it may be difficult for an investor, or any other person or entity, to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws on the grounds that Israel is not the most appropriate forum in which to bring such a claim. Even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel addressing the matters described above. See “Enforceability of Civil Liabilities.”
Your rights and responsibilities as our shareholder will be governed by Israeli law, which may differ in some respects from the rights and responsibilities of shareholders of U.S. corporations.
Since we are incorporated under Israeli law, the rights and responsibilities of our shareholders are governed by our articles of association to be effective following this offering, or our “articles of association,” and Israeli law. These rights and responsibilities differ in some respects from the rights and responsibilities of shareholders of U.S.-based corporations. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders and to refrain from abusing its power in the company, including, among other things, in voting at the general meeting of shareholders on certain matters, such as an amendment to the company’s articles of association, an increase of the company’s authorized share capital, a merger of the company and approval of related party transactions that require shareholder approval. A shareholder also has a general duty to refrain from discriminating against other shareholders. In addition, a controlling shareholder or a shareholder who knows that it possesses the power to determine the outcome of a shareholders’ vote or to appoint or prevent the appointment of an office holder in the company has a duty to act in fairness towards the company. However, Israeli law does not define the substance of this duty of fairness. See “Management—Approval of Related Party Transactions Under Israeli Law—Shareholder Duties.” Since Israeli corporate law underwent extensive revisions approximately 12 years ago, the parameters and implications of the provisions that govern shareholder behavior have not been clearly determined. These provisions may be interpreted to impose additional obligations and liabilities on our shareholders that are not typically imposed on shareholders of U.S. corporations.
Provisions of Israeli law may delay, prevent or make undesirable an acquisition of all or a significant portion of our shares or assets.
Israeli corporate law regulates mergers and requires that a tender offer be effected when more than a specified percentage of shares in a company are purchased. Further, Israeli tax considerations may make potential transactions undesirable to us or to some of our shareholders whose country of residence does not have a tax treaty with Israel granting tax relief to such shareholders from Israeli tax. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of numerous conditions, including a holding period of two years from the date of the transaction during which certain sales and dispositions of shares of the participating companies are restricted. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no actual disposition of the shares has occurred. See “Description of Share Capital—Acquisitions Under Israeli Law.”
29
Table of Contents
Under Israeli law, our two external directors have terms of office of three years. In addition, according to the provisions of our articles of association, any amendment to the number of members appointed to our board of directors will require at least 75% of the votes cast. Additionally, the election of members to our board of directors will be staggered, meaning that every year only a portion of the members of our board of directors are subject to election. See “Management—Board of Directors.”
Furthermore, under the Encouragement of Industrial, Research and Development Law, 5744-1984, to which we are subject due to our receipt of grants from the Office of the Chief Scientist of the Israeli Ministry of Industry, Trade and Labor, or OCS, a recipient of OCS grants such as us must report to the applicable authority of the OCS any change in the holding of the means of control of our company which transforms any non-Israeli citizen or resident into a direct interested party in our company. The OCS Guidelines interpretation issued by the OCS provides that prior OCS approval is required for such change in the holding of the means of control.
These provisions of Israeli law and our articles of association could have the effect of delaying or preventing a change in control and may make it more difficult for a third party to acquire us or our shareholders to elect different individuals to our board of directors, even if doing so would be beneficial to our shareholders, and may limit the price that investors may be willing to pay in the future for our ordinary shares.
30
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
We make forward-looking statements in this prospectus that are subject to risks and uncertainties. These forward-looking statements include information about possible or assumed future results of our business, financial condition, results of operations, liquidity, plans and objectives. In some cases, you can identify forward-looking statements by terminology such as “believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” “predict,” “potential,” or the negative of these terms or other similar expressions. The statements we make regarding the following matters are forward-looking by their nature:
| • | our expectation regarding the future growth of the seed and ag-chemical market and larger agriculture market; |
| • | our ability to adapt to continuous technological change in our industry; |
| • | our ability to maintain our collaboration agreements with our current collaborators; |
| • | our ability to enter into new collaboration agreements and expand our research and development to new traits and crops; |
| • | our ability to pursue a new business model in which we pay for our own research and development costs and enter into collaboration agreements only in the later stages of product development; |
| • | our expectation regarding the commercial trait value of our key products in yield and abiotic stress and biotic stress; |
| • | our expectation regarding regulatory approval of products developed by our collaborators; |
| • | our expectation that products containing our seed traits will be commercialized and we will earn royalties from the sales of such products; |
| • | our ability to successfully develop our ag-chemical operations, enter into collaboration agreements to develop ag-chemical products and eventually commercialize ag-chemical products; |
| • | our ability to successfully develop improved castor bean seed varieties that serve as a viable alternative second generation feedstock for biodiesel; |
| • | our ability to maintain and recruit knowledgeable or specialized personnel to perform our research and development work; |
| • | our ability to assemble, store, integrate, and analyze significant amounts of public and proprietary genomic data; |
| • | our ability to improve our existing computational technologies and plant validation systems and to develop and launch new computational technologies and validation systems, including PoinTar; and |
| • | our ability to patent the genes that we identify and to protect our trade secrets and proprietary know-how. |
The preceding list is not intended to be an exhaustive list of all of our forward-looking statements. The forward-looking statements are based on our beliefs, assumptions and expectations of future performance, taking into account the information currently available to us. These statements are only predictions based upon our current expectations and projections about future events. There are important factors that could cause our actual results, levels of activity, performance or achievements to differ materially from the results, levels of activity, performance or achievements expressed or implied by the forward-looking statements. In particular, you should consider the risks provided under “Risk Factors” in this prospectus.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be achieved or will occur. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus or to conform these statements to actual results or to changes in our expectations.
31
Table of Contents
PRICE RANGE OF OUR ORDINARY SHARES
Our ordinary shares have been trading on the TASE under the symbol “EVGN” since 2007. No trading market currently exists for our ordinary shares in the United States. We intend to apply to have our ordinary shares listed on the NYSE under the symbol “EVGN.”
The following table sets forth, for the periods indicated, the reported high and low closing sale prices of our ordinary shares on the TASE in NIS and U.S. dollars (giving prospective effect to the 1-for-2 reverse share split of our ordinary shares, which is to be effected immediately prior to the completion of this offering).
| NIS | $ | |||||||||||||||||||
| Price Per Ordinary Share | Price Per Ordinary Share | |||||||||||||||||||
| High | Low | High | Low | |||||||||||||||||
| Annual: |
||||||||||||||||||||
| 2013 (through September 18, 2013) |
51.18 | 36.34 | 14.47 | 9.73 | ||||||||||||||||
| 2012 |
39.00 | 29.58 | 10.31 | 7.43 | ||||||||||||||||
| 2011 |
42.40 | 25.20 | 12.02 | 7.10 | ||||||||||||||||
| 2010 |
40.80 | 25.40 | 11.46 | 6.55 | ||||||||||||||||
| 2009 |
31.40 | 12.86 | 8.45 | 3.31 | ||||||||||||||||
| 2008 |
24.38 | 7.85 | 6.73 | 2.06 | ||||||||||||||||
| Quarterly: |
||||||||||||||||||||
| Third Quarter (through September 18, 2013) |
51.18 | 41.44 | 14.47 | 11.41 | ||||||||||||||||
| Second Quarter 2013 |
46.24 | 37.52 | 12.54 | 10.51 | ||||||||||||||||
| First Quarter 2013 |
41.42 | 36.34 | 11.13 | 9.73 | ||||||||||||||||
| Fourth Quarter 2012 |
39.00 | 31.82 | 10.31 | 8.20 | ||||||||||||||||
| Third Quarter 2012 |
35.16 | 29.58 | 8.97 | 7.43 | ||||||||||||||||
| Second Quarter 2012 |
35.96 | 31.84 | 9.13 | 8.20 | ||||||||||||||||
| First Quarter 2012 |
34.48 | 29.60 | 9.28 | 7.82 | ||||||||||||||||
| Fourth Quarter 2011 |
33.68 | 27.66 | 8.88 | 7.28 | ||||||||||||||||
| Third Quarter 2011 |
33.62 | 25.20 | 9.86 | 7.10 | ||||||||||||||||
| Second Quarter 2011 |
38.90 | 31.56 | 11.23 | 9.06 | ||||||||||||||||
| First Quarter 2011 |
42.40 | 35.10 | 12.02 | 9.86 | ||||||||||||||||
| Fourth Quarter 2010 |
40.80 | 33.82 | 11.46 | 9.35 | ||||||||||||||||
| Third Quarter 2010 |
35.80 | 25.60 | 9.63 | 6.59 | ||||||||||||||||
| Second Quarter 2010 |
38.48 | 25.40 | 10.39 | 6.55 | ||||||||||||||||
| First Quarter 2010 |
40.38 | 27.94 | 10.66 | 7.40 | ||||||||||||||||
| Fourth Quarter 2009 |
31.40 | 24.50 | 8.38 | 6.48 | ||||||||||||||||
| Third Quarter 2009 |
28.84 | 23.08 | 7.41 | 6.06 | ||||||||||||||||
| Most Recent Six Months: |
||||||||||||||||||||
| September 2013 (through September 18, 2013) |
51.18 | 47.12 | 14.47 | 13.22 | ||||||||||||||||
| August 2013 |
47.86 | 42.22 | 13.18 | 11.86 | ||||||||||||||||
| July 2013 |
44.42 | 41.44 | 12.31 | 11.61 | ||||||||||||||||
| June 2013 |
46.24 | 42.44 | 12.54 | 11.78 | ||||||||||||||||
| May 2013 |
42.22 | 37.52 | 11.47 | 10.51 | ||||||||||||||||
| April 2013 |
40.10 | 38.06 | 11.07 | 10.51 | ||||||||||||||||
| March 2013 |
41.42 | 37.76 | 11.13 | 10.22 | ||||||||||||||||
On September 18, 2013, the last reported sale price of our ordinary shares on the TASE was NIS 51.18 per share, or $14.47 per share (based on the exchange rate reported by the Bank of Israel on such date, which was NIS 3.54= $1.00).
As of September 18, 2013, we had two holders of record of our ordinary shares in the United States. The number of record holders is not representative of the number of beneficial holders of our ordinary shares, as the shares of all shareholders who hold shares on the TASE are recorded in the name of our Israeli share registrar, Bank Leumi Le’Israel Registration Company Ltd.
32
Table of Contents
We estimate that our net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters exercise in full their option to purchase additional ordinary shares, based upon an assumed initial public offering price of $ per ordinary share, the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 increase (decrease) in the assumed public offering price would increase (decrease) the net proceeds we receive from this offering by $ million.
Proceeds are planned to be allocated to our different areas of activity as follows:
| 1. | Seed Traits: |
| a. | Expedite time to market: we intend to invest in the implementation of our computational platforms in order to shorten the development time for particular traits, and ultimately the time to market. |
| b. | Extend and expand our seed trait project portfolio and technological capabilities: we intend to expand our seed traits project portfolio into new traits and new crops and thus increase the potential for significant collaborations generating potentially significant revenues. In addition, we intend to establish new validation and data generation capabilities, primarily for biotic stress traits. |
| c. | Capture an additional share of the value chain by increasing self-funded research and development: we intend to complement our revenue streams by selectively self-funding a larger portion of our direct initial research and development costs. |
| d. | Establishing technology infrastructure in new territories, in addition to our existing infrastructure in Israel: we intend to diversify and broaden our data generation activities, research capabilities and personnel in new geographic sites. |
We estimate the total investment in our seed traits operation will be approximately 50% of our net proceeds from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| 2. | Ag-Chemicals: |
Develop and expand our ag-chemical operations: We intend to establish capabilities and facilities in order to be at the forefront of discovery and innovation in ag-chemicals, such as herbicides and crop enhancers. This includes investment in new computational discovery platforms, plant validation systems and infrastructure for chemical screening and handling. We estimate the total investment in our ag-chemical operation will be approximately 28% of our net proceeds from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| 3. | Evofuel: |
Further develop and commercialize our Evofuel activities: We intend to invest in up-scaling our field trial activities towards commercialization of castor seeds, including establishment of seed production and marketing capabilities. Under these efforts, we intend to pursue new territories and markets. In addition, we intend to expand our research and development relating to the next generation of castor seeds. We estimate the total investment in our Evofuel operation will be approximately 22% of our net proceeds from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
We may also use a portion of the net proceeds to make acquisitions or investments in complementary companies or technologies, although we do not have any agreement or understanding with respect to any such acquisition or investment at this time. We will have broad discretion in the way that we use the net proceeds from this offering.
33
Table of Contents
Since our inception, we have not declared or paid any cash or other form of dividends on our ordinary shares. We currently intend to retain any earnings for use in our business and do not currently intend to pay cash dividends on our ordinary shares. Dividends, if any, on our outstanding ordinary shares will be declared by and subject to the discretion of our board of directors. Even if our board of directors decides to distribute dividends, the form, frequency and amount of such dividends will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors our board of directors may deem relevant.
In addition, the distribution of dividends may be limited by Israeli law, which permits the distribution of dividends only out of distributable profits. See “Description of Share Capital—Articles of Association—Dividend and Liquidation Rights.” In addition, if we pay a dividend out of income derived during the tax exemption period from the portion of the Company’s facilities that have been granted Approved Enterprise status, we may be required to recapture the deferred corporate tax with respect to the amount distributed. See “Taxation and Government Programs—Israeli Tax Considerations and Government Programs—Law for the Encouragement of Capital Investments, 5719-1959.”
34
Table of Contents
The following table sets forth our total capitalization, together with our cash, cash equivalents and marketable securities, as of June 30, 2013, as follows:
| • | on an actual basis; and |
| • | on an as adjusted basis to reflect the issuance and sale of ordinary shares in this offering at an assumed initial public offering price of $ per ordinary share, the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and the estimated offering expenses payable by us and a 1-for-2 reverse share split of our ordinary shares that will be effected immediately prior to the completion of this offering. |
You should read this information in conjunction with our consolidated financial statements and the related notes appearing at the end of this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section and other financial information contained in this prospectus.
| As of June 30, 2013 | ||||||||
| Actual | As adjusted | |||||||
| (unaudited) | ||||||||
| (in thousands) | ||||||||
| Cash, cash equivalents and marketable securities |
$ | 50,541 | $ | |||||
|
|
|
|
|
|||||
| Liability to the Office of the Chief Scientist |
$ | 3,629 | $ | |||||
| Ordinary shares, par value NIS 0.02 per share; 150,000,000 shares authorized, actual and as adjusted; 18,879,987 shares issued and outstanding, actual; shares issued and outstanding, as adjusted |
103 | |||||||
| Additional paid-in capital |
92,147 | |||||||
| Put option |
(7,764 | ) | ||||||
| Reserve—transaction with a former controlling shareholder |
1,156 | |||||||
| Accumulated deficit |
(38,343 | ) | ||||||
|
|
|
|
|
|||||
| Total shareholders’ equity |
47,299 | |||||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 50,928 | $ | |||||
|
|
|
|
|
|||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per ordinary share, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, additional paid-in capital, total equity and total capitalization by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
35
Table of Contents
If you invest in our ordinary shares in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share and the net tangible book value per ordinary share after this offering. Our net tangible book value as of June 30, 2013 was $2.51 per ordinary share.
After giving effect to the sale of ordinary shares that we are offering at an assumed initial public offering price of $ per share, the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our net tangible book value on an adjusted basis as of June 30, 2013 would have been $ per ordinary share. This amount represents an immediate decrease in net tangible book value of $ per ordinary share to our existing shareholders and an immediate increase in net tangible book value of $ per ordinary share to new investors purchasing ordinary shares in this offering. We determine dilution by subtracting the as adjusted net tangible book value per share after this offering from the amount of cash that a new investor paid for an ordinary share.
The following table illustrates this dilution:
| Assumed initial public offering price per share |
$ | |||||||
| Net tangible book value per share as of June 30, 2013 |
$ | 2.51 | ||||||
| Increase per share attributable to this offering |
||||||||
|
|
|
|||||||
| As adjusted net tangible book value per share after this offering |
||||||||
|
|
|
|||||||
| Dilution per share to new investors in this offering. |
$ | |||||||
|
|
|
A $1.00 increase (decrease) in the assumed initial public offering price of $ per ordinary share, the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus, would increase (decrease) the as adjusted amount of each of cash, cash equivalents and short-term investments, share capital, share premium, additional paid-in capital, total equity and total capitalization by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise their option to purchase additional ordinary shares in full in this offering, the as adjusted net tangible book value after the offering would be $ per share, the decrease in net tangible book value per share to existing shareholders would be $ and the increase in net tangible book value per share to new investors would be $ per share, in each case assuming an initial public offering price of $ per ordinary share, the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus.
The following table summarizes, as of June 30, 2013, the differences between the number of shares purchased from us, the total consideration paid to us in cash and the average price per share that directors or senior management and their respective affiliates paid during the past five years, on the one hand, and new investors are paying in this offering, on the other hand. The calculation below is based on an assumed initial public offering price of $ per share before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||
| Directors and senior management and their respective affiliates |
% | $ | % | $ | ||||||||||||||
| New investors |
||||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
| Total |
100 | % | 100 | % | ||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
36
Table of Contents
The foregoing tables and calculations exclude 3,885,860 ordinary shares reserved for issuance under our equity incentive plans as of June 30, 2013 of which 2,653,759 exercisable and outstanding options to purchase ordinary shares had been granted at a weighted average exercise price of $6.79 per share.
To the extent any of these outstanding options is exercised, there will be further dilution to new investors. To the extent all of such outstanding options had been exercised as of June 30, 2013, the as adjusted net tangible book value per share after this offering would be $ , and total dilution per share to new investors would be $ .
If the underwriters exercise their option to purchase additional shares in full:
| • | the percentage of ordinary shares held by existing shareholders will decrease to approximately % of the total number of our ordinary shares outstanding after this offering; and |
| • | the number of shares held by new investors will increase to , or approximately % of the total number of our ordinary shares outstanding after this offering. |
37
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
The following tables set forth our selected consolidated financial data. You should read the following selected consolidated financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected in the future. Our financial statements have been prepared in accordance with IFRS, as issued by the International Accounting Standards Board.
The selected consolidated statements of comprehensive income data for each of the years in the three-year period ended December 31, 2012 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The selected consolidated statements of comprehensive income data for the six months ended June 30, 2012 and 2013 and the consolidated balance sheet data as of June 30, 2012 and 2013 have been derived from our unaudited interim condensed consolidated financial statements included elsewhere in this prospectus. Results from interim periods are not necessarily indicative of results that may be expected for the entire year.
| Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| (in thousands, except per share and share data) | ||||||||||||||||||||
| Consolidated Statements of Comprehensive Income: |
||||||||||||||||||||
| Revenues |
$ | 12,563 | $ | 14,901 | $ | 17,072 | $ | 8,287 | $ | 8,934 | ||||||||||
| Cost of revenues |
5,811 | 8,247 | 9,552 | 4,483 | 4,688 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
6,752 | 6,654 | 7,520 | 3,804 | 4,246 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Research and development |
5,544 | 6,384 | 7,252 | 3,308 | 4,666 | |||||||||||||||
| Business development |
1,062 | 1,136 | 1,159 | 544 | 532 | |||||||||||||||
| General and administrative |
2,123 | 2,317 | 2,235 | 1,069 | 1,197 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
8,729 | 9,837 | 10,646 | 4,921 | 6,395 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(1,977 | ) | (3,183 | ) | (3,126 | ) | (1,117 | ) | (2,149 | ) | ||||||||||
| Financial income |
724 | 5,023 | 972 | 510 | 776 | |||||||||||||||
| Financial expenses |
(5,717 | ) | (1,195 | ) | (294 | ) | (76 | ) | (989 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before taxes on income |
|
(6,970 |
) |
|
645 |
|
|
(2,448 |
) |
|
(683 |
) |
|
(2,362 |
) | |||||
| Taxes on income |
— | — | 74 | 52 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | (6,970 | ) | $ | 645 | $ | (2,522 | ) | $ | (735 | ) | $ | (2,362 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted net income (loss) per share |
$ |
(0.48 |
) |
$ | 0.04 | $ | (0.14 | ) | $ | (0.04 | ) | $ | (0.12 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of ordinary shares used in computing basic income (loss) per share(1) |
|
14,824,703 |
|
|
17,505,136 |
|
|
18,421,568 |
|
|
18,328,598 |
|
|
18,817,847 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of ordinary shares used in computing diluted income (loss) per share(1) |
14,824,703 | 18,731,118 | 18,421,568 | 18,328,598 | 18,817,847 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Basic net income (loss) per share is computed based on the weighted average number of ordinary shares outstanding during each period. Diluted net income (loss) per share is computed based on the weighted average number of ordinary shares outstanding during each period plus dilutive potential equivalent ordinary shares considered outstanding during the period, in accordance with IAS 33, “Earnings per Share.” |
38
Table of Contents
| As of June 30, | ||||||||
| 2012 | 2013 | |||||||
| (unaudited) | ||||||||
| (in thousands) | ||||||||
| Consolidated Balance Sheet Data: |
||||||||
| Cash and cash equivalents |
$ | 11,558 | $ | 16,884 | ||||
| Marketable securities |
30,643 | 33,657 | ||||||
| Short-term deposits |
10,600 | — | ||||||
| Trade receivables |
3,911 | 1,853 | ||||||
| Total current assets |
57,393 | 53,967 | ||||||
| Deferred revenues and other advances |
10,401 | 6,507 | ||||||
| Total liabilities |
17,015 | 14,705 | ||||||
| Working capital(1) |
49,361 | 44,921 | ||||||
| Shareholders’ equity |
48,361 | 47,299 | ||||||
| (1) | Working capital is defined as total current assets less total current liabilities. |
39
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that are subject to known and unknown risks and uncertainties. Actual results and the timing of events may differ significantly from those expressed or implied in such forward-looking statements due to a number of factors, including those set forth in the section entitled “Risk Factors” and elsewhere in this prospectus. You should read the following discussion in conjunction with “Special note regarding forward-looking statements” and “Risk Factors.” We have prepared our financial statements in accordance with IFRS, as issued by the International Accounting Standards Board.
Company Overview
We are a plant genomics company that uses a comprehensive and integrated technology infrastructure to enhance seed traits underlying crop performance and productivity through biotechnology and advanced breeding methods. We have strategic collaborations with world-leading agricultural companies, primarily to develop traits for improved yield and abiotic stress tolerance (such as improved tolerance to drought, heat and salinity) and biotic stress resistance (such as resistance to disease, pests and insects). Our products are focused on essential crops, including corn, soybean, wheat, rice and cotton, which, according to Phillips McDougall’s 2011 Seed Industry Overview report, account for over 70% of the value of the global seeds market. We also apply our technology infrastructure to two additional fields: (i) ag-chemical and (ii) seeds focusing on second generation feedstock for biodiesel, both of which have not yet generated revenues and are in the development stage.
We were founded in 2000 as a division of Compugen Ltd., or Compugen and spun-off as an independent company in January 2002. The following are the key milestones in our technological, business and financial development as a plant genomics company:
| • | In March 2003, we completed the first closing of our initial private placement, raising $2.0 million. |
| • | In 2006, we launched our initial computational technology for gene identification and prioritization, the ATHLETE™ version 1.0. |
| • | In 2006 and 2007, we started licensing the genes we identify to leading seed companies, which include: |
| • | Biogemma, with which we began a collaboration focused on developing drought tolerance corn in July 2006; |
| • | Bayer, which in June 2007 we entered into a collaboration agreement focused on increasing productivity in rice; |
| • | Monsanto, entering into a collaboration in September 2007 to improve nitrogen use efficiency in corn, soybean, cotton and canola; and |
| • | Pioneer Hi-Bred International, a subsidiary of DuPont, with which we commenced a collaboration in October 2007 to improve yield and tolerance to abiotic stress in corn and soybean. |
| • | In June 2007, we completed an initial public offering in Israel and listed our ordinary shares on the Tel Aviv Stock Exchange. |
| • | In August 2008, we entered into our first broad, multi-year collaboration agreement, with a five-year collaboration period, with Monsanto focusing on improving yield, nitrogen use efficiency and abiotic stress tolerance in corn, soybean, cotton and canola. |
| • | In June 2009, we entered into a collaboration agreement with Syngenta Biotechnology, Inc., or Syngenta, to address the key biotic trait of resistance to soybean cyst nematodes. |
40
Table of Contents
| • | In November 2009, we completed a substantial increase of our research and development and administrative facilities and personnel, adding 15 greenhouses for our validation systems and expanding our computational and laboratory facilities, supporting our growing cooperative activities. |
| • | In December 2010, we entered into our broad, multi-year collaboration agreement with Bayer to develop improved wheat varieties, focusing on increased yield, drought tolerance and fertilizer use efficiency. |
| • | In January 2011, our first genes were advanced to Phase II development as part of our collaboration with Biogemma, commenced in 2006, for improving yield and drought tolerance in corn. |
| • | In mid-2011, we launched EvoBreed™, our computational technology for discovery of certain DNA sequences (such as genetic markers and single-nucleotide polymorphisms or SNPs) to enhance plant breeding. |
| • | In November 2011, we entered into a collaboration with DuPont aimed at improving soybean rust resistance. This was our first collaboration that includes self-funding of our research and development activities, with higher potential royalties. |
| • | In November 2011, we extended our 2008 collaboration agreement with Monsanto one additional year and expanded it to add our Gene2Product™ computational technology to our research and development efforts. |
| • | In January 2012, we established Evofuel Ltd., our wholly owned subsidiary incorporated in Israel, to focus on developing improved castor bean seeds that can be used as advanced second generation feedstock for biodiesel. Evofuel has pre-commercial collaborations with SLC Agricola in Brazil and T6 in Argentina to develop its products for these markets. |
| • | In January 2013, we launched Gene2Product™, our computational technology for improving trait efficacy in developing biotech seed products. |
Our business focuses on three distinct operations: seed traits, ag-chemicals and seeds focusing on second generation feedstock for biodiesel. We currently generate all of our revenues from our seed trait business, and these revenues are principally derived from our collaboration agreements and related arrangements with our collaborators. Our products are currently in the development stage, and we may use a significant portion of the proceeds of this offering to invest in the infrastructure and ongoing research needed for these operations.
We currently participate in more than ten collaboration agreements that cover 20 different products in various stages of development. Through these collaborations with some of the world’s leading agricultural companies, such as Monsanto, Bayer, DuPont and Syngenta, we have developed a balanced and scalable business model. We currently generate revenues from our collaboration agreements through both research and development services payments covering the costs of our gene identification and validation efforts, and milestone payments received upon achieving certain specified results. Our collaboration agreements with these companies also usually provide that we are entitled to receive milestone payments and royalties on any seeds that our collaborators commercialize that integrate a trait we have licensed to our collaborators. While we currently do not earn royalties from the sale of any such seeds, we expect to begin earning revenues from royalties in at least six years, and our long-term growth strategy is based in part on the expectation that such royalties will comprise a significant portion of our future revenues. The termination or non-renewal of our agreements with Monsanto and Bayer, including if Monsanto decides not to exercise its option to extend the collaboration period under our agreement with them by February 1, 2014 (and if Monsanto does not otherwise enter into a new collaboration agreement with us), would have a material adverse effect on our business, financial condition, results of operations and prospects.
41
Table of Contents
Components of Statements of Comprehensive Income
Revenues
Our revenues are principally derived from our collaboration agreements and related arrangements with our collaborators. A substantial majority of our current collaboration agreements focus on developing traits to be integrated into seeds through genetic modification (“GM”); our GM projects therefore generate the majority of our revenues from research and development services. The other component of our seed trait operations, advanced breeding, generates only a small portion of our revenues from research and development services. Revenues from our major collaborators, Monsanto and Bayer, accounted together for 94% of our revenues for the year ended December 31, 2012, of which Monsanto accounted for 70% and Bayer accounted for 24% of our total revenues. Monsanto and Bayer accounted together for 98% of our revenues for the six months ended June 30, 2013 of which Monsanto accounted for 66% and Bayer accounted for 32% of our total revenues. See “Business—Key Collaborations.” Under our collaboration agreements and related arrangements, our revenues are paid to us in the following four different forms of payments:
Periodic Payments for Research and Development Services
Periodic payments for research and development services are payments we receive primarily on a quarterly basis from our collaborators as consideration for the research and development services we provide them. Revenues from periodic payments for research and development services performed under our collaboration agreements amounted to $12.6 million and accounted for 70.1% of our total revenues for the year ended December 31, 2012, and $6.8 million, or 76.2%, in the six months ended June 30, 2013. As we have not yet entered into any collaborations for our operations in ag-chemical or seeds focusing on second generation feedstock for biodiesel, we have yet to generate any revenues from these operations.
Up-front Payments
We also derive a portion of our revenues from up-front payments made under our agreements with Monsanto and Bayer. Up-front payments primarily represent payments we receive upon entering into collaboration agreements for research and development services. These up-front payments are recognized as revenues over the duration of the relevant contract based on the proportion of actual costs incurred for each reporting period to the estimated total costs of the collaboration. Revenues derived from up-front payments under our agreements with Monsanto and Bayer amounted to approximately $1.0 million and accounted for approximately 5.6% of our total revenues for the year ended December 31, 2012, and $0.5 million, or 5.7%, in the six months ended June 30, 2013.
Share Purchases
We also entered into share purchase agreements with Monsanto and Bayer, which were signed in contemplation of our collaboration agreements with them. We attribute the proceeds from arrangements under these agreements to the value of our ordinary shares issued to Monsanto and Bayer at the time of the investments as well as to the services we perform under the collaboration agreements. As a result, we recognize as revenues the excess payment, which is the consideration investors paid for our ordinary shares over the market value of our ordinary shares traded on the TASE at the time of the investment. This excess payment is recognized as revenues beginning on the date of the investment, for the duration of the contract based on the proportion of actual costs incurred for each reporting period to the estimated total costs of the collaboration. We also recorded revenues with respect to Monsanto’s put option. We recognized as revenues the fair value of the put option with Monsanto throughout the term of the agreement, based on the proportion of actual costs incurred for each reporting period to the estimated total costs of the collaboration. See “Business—Share Purchase Agreements.” Total revenues from the excess amounts on the purchase of our ordinary shares by Monsanto and Bayer as well as the Monsanto put option amounted to $3.2 million and accounted for approximately 18.5% of our total revenues for the year ended December 31, 2012, and $1.6 million, or 18.1%, in the six months ended June 30, 2013.
42
Table of Contents
Milestone Payments
We also derive, to a lesser extent at this stage of our business, a portion of our revenues from milestone payments paid by our collaborators upon the occurrence of certain specified events pursuant to the agreements with our collaborators. These milestone payments accounted for approximately 5.8% of our total revenues for the year ended December 31, 2012, but for none of our revenues for the six months ended June 30, 2013.
Our agreements with collaborators also provide for royalty payments based on the sales or transfer of products our collaborators develop that contain the traits we discover and license to them. The calculation of royalties varies by collaboration, and is typically based on the value that the trait we provide adds to the end product. For example, the royalties we expect to be entitled to receive pursuant to our collaborations with Monsanto and Bayer will be a percentage of the commercial value conferred by the trait we provide on the end product Monsanto or Bayer sells. Pursuant to our collaboration with Rasi Seeds, however, our royalties will be a percentage of the commercial value conferred by the trait we provide on the end product in addition to a percentage of the net revenues of the end product. We have not yet generated revenues from such royalties, and may not commence generating significant royalties for at least six years.
Cost of Revenues
Cost of revenues primarily consists of development costs incurred in conjunction with our collaborations, which include salaries and related personnel costs (including share-based compensation) for our research and development employees working on the collaborations, payments to third party suppliers that assist us in producing genomic data and the cost of disposable materials (such as seeds, laboratory supplies, fertilizer, water and soil). Cost of revenues also includes the depreciation of our plant, property and equipment, operational overhead costs (which include costs related to leasing and operating our office and laboratory facilities and greenhouses) and expenses related to retaining advisors, which primarily consist of biological experts.
Operating Expenses
Research and Development Expenses: Research and development expenses consist of costs related to our internal or independent research and development activities, as opposed to development costs incurred in connection with our collaborations (which are included in cost of revenues). These activities include developing and improving our computational, scientific and validation technologies, know-how and capabilities used by our various divisions as well as research and development focused on developing our ag-chemical and seeds focusing on second generation feedstock for biodiesel operations. Research and development costs include salaries and related personnel costs (including share-based compensation), operational overhead costs, which include costs related to leasing and operating our office, laboratory facilities and greenhouses, and depreciation of plant, property and equipment. Expenses related to our intellectual property, such as legal and other costs associated with patent applications, are also included as research and development expenses. We expect that our research and development expenses will continue to increase on an absolute basis as we develop our ag-chemical and seeds focusing on second generation feedstock for biodiesel operations and expand our independent research and development projects.
Business Development Expenses: Business development expenses consist of costs primarily related to maintaining our relationships with our collaborators and establishing new collaborations. These costs include salaries and related personnel costs (including share-based compensation), expenses incident to foreign business travel and legal expenses related to our collaborations. We expect our business development expenses will continue to increase on an absolute basis as we develop our ag-chemical and seeds focusing on second generation feedstock for biodiesel operations and seek collaboration opportunities for those projects.
General and Administrative Expenses: General and administrative expenses include salaries and related personnel costs (including share-based compensation) for our general and administrative employees, consulting,
43
Table of Contents
legal and professional services related to our general and administrative operations and expenses related to HR activities and employee benefits. We expect to incur additional general and administrative costs associated with being a public company in the United States, including compliance under the Sarbanes-Oxley Act and rules promulgated by the SEC.
Financial Income and Expenses
Financial income consists primarily of interest income on our cash bank deposits and securities, foreign currency exchange income and income related to a revaluation of the marketable securities we hold, which consist of corporate bonds and Israeli government treasury notes. Financial expenses consist primarily of expenses related to bank charges, foreign currency exchange expense and associated fees and expenses related to a revaluation of the marketable securities we hold. The interest due on grants received from the OCS is also considered a financial expense, and is recognized beginning on the date we receive the grant until the date on which the grant is expected to be repaid through royalties paid to the OCS. Financial income and expenses also include non-cash financial income and expenses related to revaluations of then-outstanding publicly traded warrants granted upon our initial public offering on the TASE in 2007 based on the change in market price of our ordinary shares. Substantially all of these warrants were exercised and the remainder expired in May 2011.
Taxes on Income
We do not generate taxable income in Israel, as we have historically incurred operating losses resulting in carryforward tax losses totaling $(15.3 million) as of June 30, 2013, to be carried forward indefinitely to future tax years. Accordingly, we do not expect to pay taxes in Israel until we have taxable income after the full utilization of our carryforward tax losses. However, in 2012, we recognized $74 thousand in taxes on income resulting from withholding taxes our collaborators were obligated to withhold from the research and development payments they paid us.
Segment Data
Starting January 1, 2012, following the establishment of our Evofuel subsidiary, we split our operations into two operating segments: Evogene and Evofuel. The two segments perform the following operations:
| • | Evogene: Our Evogene segment develops technologies to improve plant performance through seed traits and ag-chemicals. Our seed trait operation utilizes our expertise in plant genomics to address yield and abiotic stress and biotic stress traits through the genetic modification or advanced breeding of seeds. Our ag-chemical operation utilizes this expertise to develop novel crop protection and crop enhancement products, including herbicides. |
| • | Evofuel: Our Evofuel segment develops improved species of the castor bean plant for second generation feedstock intended for use in the alternative fuel industry, specifically biodiesel and biojet. |
The following table presents our revenues and operating loss by segment for the period presented:
| Evogene | Evofuel | Total | ||||||||||
| (in thousands) | ||||||||||||
| Year ended December 31, 2012: |
||||||||||||
| Revenues |
$ | 17,072 | $ | — | $ | 17,072 | ||||||
| Operating loss |
(1,993 | ) | (1,133 | ) | (3,126 | ) | ||||||
| Six months ended June 30, 2013 (unaudited): |
||||||||||||
| Revenues |
$ | 8,934 | $ | — | $ | 8,934 | ||||||
| Operating loss |
(1,588 | ) | (561 | ) | (2,149 | ) | ||||||
Our revenues for the year ended December 31, 2012 and the six months ended June 30, 2013 were generated entirely from the Evogene segment. Our Evogene segment includes revenues generated from our seed
44
Table of Contents
trait operations and costs associated with both our seed trait and ag-chemical operations. We do not currently generate revenues in the Evofuel segment, as Evofuel is still in the development stage. Both of these operating segments recorded losses in 2012 and the six months ended June 30, 2013. The primary drivers of loss for Evofuel in 2012 and the six months ended June 30, 2013 were research and development and business development expenses. We do not expect Evofuel to start generating significant revenues in the near term.
Comparison of Period to Period Results of Operations
The period to period discussions for the year ended December 31, 2012 and the six months ended June 30, 2013 present our results of operations for both operating segments. A period to period comparison is not presented for Evofuel for any period prior to 2012 due to the lack of comparative results.
The following tables set forth our results of operations in dollars and as a percentage of revenues for the periods indicated:
| Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Consolidated Statements of Comprehensive Income: |
||||||||||||||||||||
| Revenues |
$ | 12,563 | $ | 14,901 | $ | 17,072 | $ | 8,287 | $ | 8,934 | ||||||||||
| Cost of revenues |
5,811 | 8,247 | 9,552 | 4,483 | 4,688 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
6,752 | 6,654 | 7,520 | 3,804 | 4,246 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Research and development |
5,544 | 6,384 | 7,252 | 3,308 | 4,666 | |||||||||||||||
| Business development |
1,062 | 1,136 | 1,159 | 544 | 532 | |||||||||||||||
| General and administrative |
2,123 | 2,317 | 2,235 | 1,069 | 1,197 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
8,729 | 9,837 | 10,646 | 4,921 | 6,395 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(1,977 | ) | (3,183 | ) | (3,126 | ) | (1,117 | ) | (2,149 | ) | ||||||||||
| Financial income |
724 | 5,023 | 972 | 510 | 776 | |||||||||||||||
| Financial expenses |
(5,717 | ) | (1,195 | ) | (294 | ) | (76 | ) | (989 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before taxes on income |
(6,970 | ) | 645 | (2,448 | ) | (683 | ) | (2,362 | ) | |||||||||||
| Taxes on income |
— | — | 74 | 52 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | (6,970 | ) | $ | 645 | $ | (2,522 | ) | $ | (735 | ) | $ | (2,362 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
45
Table of Contents
| Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| (as a percentage of revenues) | ||||||||||||||||||||
| Consolidated Statements of Comprehensive Income: |
||||||||||||||||||||
| Cost of revenues |
46.3 | % | 55.3 | % | 56 | % | 54.1 | % | 52.5 | % | ||||||||||
| Gross profit |
53.7 | 44.7 | 44 | 45.9 | 47.5 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Research and development |
44.1 | 42.8 | 42.5 | 39.9 | 52.2 | |||||||||||||||
| Business development |
8.5 | 7.6 | 6.8 | 6.6 | 6 | |||||||||||||||
| General and administrative |
16.9 | 15.5 | 13.1 | 12.9 | 13.4 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
69.5 | 65.9 | 62.4 | 59.4 | 71.6 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(15.7 | ) | (21.2 | ) | (18.3 | ) | (13.5 | ) | (24.1 | ) | ||||||||||
| Financial income |
5.8 | 33.7 | 5.7 | 6.2 | 8.7 | |||||||||||||||
| Financial expenses |
(45.5 | ) | (8.0 | ) | (1.7 | ) | (0.9 | ) | (11.1 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before taxes on income |
(55.5 | ) | 4.5 | (14.3 | ) | 8.2 | (26.4 | ) | ||||||||||||
| Taxes on income |
0 | 0 | 0.4 | 0.6 | 0 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
(55.5 | )% | 4.5 | % | (14.7 | )% | (8.8 | )% | (26.4 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Six Months Ended June 30, 2012 Compared to Six Months Ended June 30, 2013
The following table shows our operating results for the six months ended June 30, 2012 compared to the six months ended June 30, 2013
| Six Months Ended June 30, |
||||||||
| 2012 | 2013 | |||||||
| (unaudited) | ||||||||
| (in thousands) | ||||||||
| Consolidated Statements of Comprehensive Income: |
||||||||
| Revenues |
$ | 8,287 | $ | 8,934 | ||||
| Cost of revenues |
4,483 | 4,688 | ||||||
|
|
|
|
|
|||||
| Gross profit |
3,804 | 4,246 | ||||||
|
|
|
|
|
|||||
| Research and development |
3,308 | 4,666 | ||||||
| Business development |
544 | 532 | ||||||
| General and administrative |
1,069 | 1,197 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
4,921 | 6,395 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(1,117 | ) | (2,149 | ) | ||||
|
|
|
|
|
|||||
| Financial income |
510 | 776 | ||||||
| Financial expenses |
(76 | ) | (989 | ) | ||||
|
|
|
|
|
|||||
| Income (loss) before taxes on income |
(683 | ) | (2,362 | ) | ||||
| Taxes on income |
52 | — | ||||||
|
|
|
|
|
|||||
| Net income (loss) |
$ | (735 | ) | $ | (2,362 | ) | ||
|
|
|
|
|
|||||
Revenues. Our revenues increased by $0.6 million, or 7.8%, to $8.9 million for the six months ended June 30, 2013 from $8.3 million for the six months ended June 30, 2012. The increase in revenues is mainly attributable to an increase in revenues under the Bayer Wheat Agreement in the six months ended June 30, 2013 as compared to the six months ended June 30, 2012.
46
Table of Contents
Cost of Revenues. Cost of revenues increased by $0.2 million, or 4.6%, to $4.7 million for the six months ended June 30, 2013 from $4.5 million for the six months ended June 30, 2012. This increase is primarily attributable to an increase in salaries and benefits as a result of additional personnel we hired to support the increase in revenues.
Gross Profit. Gross profit increased by $0.4 million, or 11.6%, to $4.2 million for the six months ended June 30, 2013 from $3.8 million for the six months ended June 30, 2012. This increase is primarily a result of the increase in revenues offset by the cost of revenues, each as described above.
Operating Expenses.
Research and Development Expenses. Research and development expenses increased by $1.4 million, or 41%, to $4.7 million for the six months ended June 30, 2013 from $3.3 million for the six months ended June 30, 2012. This increase is primarily attributable to an increase in salaries and benefits as a result of additional employees in the research and development department hired to support our additional independent research and development programs.
Business Development Expenses. Business development expenses decreased by $12 thousand, or 2.2%, to $532 thousand for the six months ended June 30, 2013 from $544 thousand for the six months ended June 30, 2012, primarily due to a decrease in the cost of share-based compensation.
General and Administrative Expenses. General and administrative expenses increased by $0.1 million, or 12%, to $1.2 million for the six months ended June 30, 2013 from $1.1 million for the six months ended June 30, 2012, primarily due to an increase in salaries and benefits and an increase in payments to consultants.
Financial (Income) Expenses, Net. Financial (income) expenses, net increased by $0.6 million, or 149%, to financial expenses, net, of $0.2 million for the six months ended June 30, 2013 from financial income, net, of $(0.4) million for the six months ended June 30, 2012.
Financial Income. Financial income increased by $0.3 million, or 52%, to $0.8 million for the six months ended June 30, 2013 from $0.5 million for the six months ended June 30, 2012. This increase resulted mainly from an increase in interest income on marketable securities.
Financial Expense. Financial expense increased by $0.9 million to $1 million for the six months ended June 30, 2013 from $0.1 million for the six months ended June 30, 2012. The increase resulted mainly from a decrease in the fair market value of marketable securities we hold.
47
Table of Contents
Year Ended December 31, 2011 Compared to Year Ended December 31, 2012
The following table shows our operating results for the year ended December 31, 2011 compared to the year ended December 31, 2012.
| Year Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| (in thousands) | ||||||||
| Consolidated Statements of Comprehensive Income: |
||||||||
| Revenues |
$ | 17,072 | $ | 14,901 | ||||
| Cost of revenues |
9,552 | 8,247 | ||||||
|
|
|
|
|
|||||
| Gross profit |
7,520 | 6,654 | ||||||
|
|
|
|
|
|||||
| Research and development |
7,252 | 6,384 | ||||||
| Business development |
1,159 | 1,136 | ||||||
| General and administrative |
2,235 | 2,317 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
10,646 | 9,837 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(3,126 | ) | (3,183 | ) | ||||
|
|
|
|
|
|||||
| Financial income |
972 | 5,023 | ||||||
| Financial expenses |
(294 | ) | (1,195 | ) | ||||
|
|
|
|
|
|||||
| Income (loss) before taxes on income |
(2,448 | ) | 645 | |||||
| Taxes on income |
74 | — | ||||||
|
|
|
|
|
|||||
| Net income (loss) |
$ | (2,522 | ) | $ | 645 | |||
|
|
|
|
|
|||||
Revenues. Our revenues increased by $2.2 million, or 14.6%, to $17.1 million for the year ended December 31, 2012 from $14.9 million for the year ended December 31, 2011. The increase in revenues resulted primarily from an increase in revenues from Monsanto, which increased by $1.5 million, or 13.7%, to $12.0 million for the year ended December 31, 2012 from $10.5 million for the year ended December 31, 2011, as a result of the extension of our collaboration agreement with Monsanto in November 2011. The increase in revenues was also attributable to an increase in revenues under the Bayer Wheat Agreement in 2012 as compared to 2011.
Cost of Revenues. Cost of revenues increased by $1.3 million, or 15.8%, to $9.5 million for the year ended December 31, 2012 from $8.2 million for the year ended December 31, 2011. This increase is primarily attributable to an increase in salaries and benefits resulting from the hiring of additional employees and payments to additional suppliers and consultants hired in 2012, both due to our increased staffing needs as a result of additional projects with Monsanto, Bayer and DuPont during 2012, and an increase in depreciation expenses, partially off-set by a decrease in the cost of share-based compensation.
Gross Profit. Gross profit increased by $0.9 million, or 13.0%, to $7.5 million for the year ended December 31, 2012 from $6.7 million for the year ended December 31, 2011. This increase is primarily a result of an increase in revenues partially off-set by an increase in the cost of revenues, each as described above.
Operating Expenses.
Research and Development Expenses. Research and development expenses increased by $0.9 million, or 13.6%, to $7.3 million for the year ended December 31, 2012 from $6.4 million for the year ended December 31, 2011. This increase is primarily attributable to an increase in salaries and benefits as a result of additional research and development employees hired in 2012 compared to 2011 to support our additional research and development programs in 2012, partially off-set by a decrease in the cost of share-based compensation.
48
Table of Contents
Business Development Expenses. Business development expenses increased by $23 thousand, or 2.0%, to $1.2 million for the year ended December 31, 2012 from $1.1 million for the year ended December 31, 2011. This increase is primarily attributable to an increase in salaries and benefits for two additional business development employees in 2012, partially off-set by a decrease in the cost of share-based compensation and a decrease in fees paid to legal consultations.
General and Administrative Expenses. General and administrative expenses decreased by less than $100,000, or 3.5%, to $2.2 million for the year ended December 31, 2012 from $2.3 million for the year ended December 31, 2011. General and administrative expenses decreased primarily due to a decrease in the cost of share-based compensation, partially off-set by an increase in salaries and benefits paid to additional employees hired during the course of 2012.
Financial Income, Net. Financial income, net decreased by $3.2 million, or 82.2%, to $0.7 million for the year ended December 31, 2012 from $3.8 million for the year ended December 31, 2011.
Financial Income. Financial income decreased by $4.0 million, or 80.6%, to $1.0 million for the year ended December 31, 2012 from $5.0 million for the year ended December 31, 2011. This decrease is primarily attributable to a decrease of $3.7 million in financial income on revaluation of warrants. For the year ended December 31, 2011, we recorded $3.7 million of non-cash financial income related to an accounting revaluation of outstanding publicly traded warrants granted upon our initial public offering on the TASE in 2007 based on the change in market price of our ordinary shares. For the year ended December 31, 2012, we did not record any financial income related to revaluation of warrants as substantially all of these warrants were exercised and the remainder expired in May 2011. The decrease in financial income also derives from a decrease in the net change in the fair value of marketable securities we hold, which consist of corporate bonds and Israeli government treasury notes, and a decrease in interest income due to a decrease in interest rates.
Financial Expense. Financial expense decreased by $0.9 million, or 75.4%, to $0.3 million for the year ended December 31, 2012 from $1.2 million for the year ended December 31, 2011. Financing expense decreased primarily as a result of a decrease in expenses in respect of a change in foreign currency exchange rates in 2011.
Taxes on Income. Although we did not pay any taxes in Israel on our income for the years ended December 31, 2012 and 2011 as a result of our loss carryforwards, we recorded taxes on income in the amount of $74 thousand for the year ended December 31, 2012 as a result of taxes our collaborators were obligated to withhold from the payments we received from them during 2012.
49
Table of Contents
Year Ended December 31, 2010 Compared to Year Ended December 31, 2011
The following table shows our operating results for the year ended December 31, 2010 compared to the year ended December 31, 2011.
| Year Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Consolidated Statements of Comprehensive Income: |
||||||||
| Revenues |
$ | 14,901 | $ | 12,563 | ||||
| Cost of revenues |
8,247 | 5,811 | ||||||
|
|
|
|
|
|||||
| Gross profit |
6,654 | 6,752 | ||||||
|
|
|
|
|
|||||
| Research and development |
6,384 | 5,544 | ||||||
| Business development |
1,136 | 1,062 | ||||||
| General and administrative |
2,317 | 2,123 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
9,837 | 8,729 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(3,183 | ) | (1,977 | ) | ||||
| Financial income |
5,023 | 724 | ||||||
| Financial expenses |
(1,195 | ) | (5,717 | ) | ||||
|
|
|
|
|
|||||
| Net income (loss) |
$ | 645 | $ | (6,970 | ) | |||
|
|
|
|
|
|||||
Revenues. Our revenues increased by $2.3 million, or 18.6%, to $14.9 million for the year ended December 31, 2011 from $12.6 million for the year ended December 31, 2010. The increase in revenues resulted mainly from an increase in payments from Bayer, due to the execution of the Bayer Wheat Agreement in December 2010, which increased by $3.0 million, or 587%, to $3.5 million for the year ended December 31, 2011 from $0.5 million for the year ended December 31, 2010. The increase also resulted from an increase in revenues from Monsanto, which increased by $1.2 million, or 12.8%, to $10.5 million for the year ended December 31, 2011 from $9.3 million for the year ended December 31, 2010. This increase resulted from an extension to our collaboration with Monsanto in November 2011. These increases are partially off-set by a decrease of $1.5 million in revenues from Biogemma derived from one-time research and development services payments received in 2010 to cover our prior research and development efforts, to $0.7 million for the year ended December 31, 2011 from $2.1 million for the year ended December 31, 2010.
Cost of Revenues. Cost of revenues increased by $2.4 million, or 41.9%, to $8.2 million for the year ended December 31, 2011 from $5.8 million for the year ended December 31, 2010. Cost of revenues primarily increased as a result of an increase in salaries and benefits due to the hiring of additional personnel and payments to suppliers and consultants relating to an increase in projects with collaborators. Excluding the one-time revenues from Biogemma in 2010 against which there were no corresponding costs, the increase in cost of revenues was in line with the increase in revenues for the same period.
Gross Profit. Gross profit decreased by $0.1 million, or 1.5%, to $6.7 million for the year ended December 31, 2011 from $6.8 million for the year ended December 31, 2010. This decrease is primarily a result of the increase in revenues and cost of revenues, as described above.
Operating Expenses.
Research and Development Expenses. Research and development expenses increased by $0.8 million, or 15.2%, to $6.4 million for the year ended December 31, 2011 from $5.6 million for the year ended December 31, 2010. This increase is primarily attributable to an increase in salaries and benefits for research and development employees due to the hiring of additional research and development personnel and an increase in depreciation
50
Table of Contents
expenses, partially off-set by a decrease in expenses paid for suppliers hired by our research and development department and the cost of share-based compensation related to research and development employees.
Business Development Expenses. Business development expenses increased by $74 thousand, or 7.0%, to $1.14 million for the year ended December 31, 2011 from $1.06 million for the year ended December 31, 2010. This increase is primarily attributable to an increase in salaries and benefits for business development employees due to the hiring of additional business development personnel, partially off-set by a decrease in the cost of share-based compensation related to business development employees.
General and Administrative Expenses. General and administrative expenses increased by $0.2 million, or 9.1%, to $2.3 million for the year ended December 31, 2011 from $2.1 million for the year ended December 31, 2010. This increase is primarily attributable to an increase in salaries and benefits paid to management and other general and administrative employees due to the hiring of additional general and administrative personnel, partially off-set by a decrease in the expenses related to share-based compensation for management and other general and administrative employees.
Financial Income and Expenses, Net. Financial income and expenses, net increased by $8.8 million, or 176.7%, to income of $3.8 million for the year ended December 31, 2011 from an expense of $5.0 million for the year ended December 31, 2010.
Financial Income. Financial income increased by $4.3 million, or 593.8%, to $5.0 million for the year ended December 31, 2011 from $0.7 million for the year ended December 31, 2010. This increase is primarily attributable to an increase of $3.7 million in financial income on revaluation of warrants. For the year ended December 31, 2011, we recorded $3.7 million of non-cash financial income related to revaluation of outstanding publicly traded warrants granted upon our initial public offering on the TASE in 2007 based on the change in market price of our ordinary shares. For the year ended December 31, 2010, we recorded $5.4 million of non-cash financial expense related to revaluation of these warrants. The change derived from the change of the market price of our ordinary shares on the TASE. The increase in financial income also derives from an increase in the net change in the fair value of marketable securities we hold, partially off-set by a decrease in income derived from currency revaluations.
Financial Expense. Financial expense decreased by $4.5 million to $1.2 million for the year ended December 31, 2011 from $5.7 million for the year ended December 31, 2010. Financial expense decreased primarily due to a decrease of $5.4 million in non-cash financial expense related to revaluation of outstanding publicly traded warrants granted upon our initial public offering on the TASE in 2007 based on the change in market price of our ordinary shares. For the year ended December 31, 2011, we recorded $3.7 million of non-cash financial income related to revaluation of these warrants, whereas for the year ended December 31, 2010, we recorded $5.4 million of non-cash financial expense related to revaluation of these warrants. The change derived from the change of the market price of our ordinary shares at TASE. The decrease was partially offset by an increase in expenses derived from currency revaluations.
Liquidity and Capital Resources
Our working capital has generally been provided by cash raised from our investors, payments from our collaborators and grants received from OCS and the Canada-Israel Industrial Research and Development Foundation, or CIIRDF. As of June 30, 2013, we had cash and cash equivalents and marketable securities of $50.5 million and working capital of $44.9 million, which is calculated by subtracting our current liabilities from our current assets. As of June 30, 2013, we had $3.6 million of outstanding indebtedness related to the grants received from the OCS. Our principal uses of cash are to fund our operations, the largest component of which is cost of revenues associated with the research and development expenses related to our collaborations. We believe that our existing cash and cash equivalents and the net proceeds to us from this offering will be sufficient to fund our operations for at least the next 12 months. We also expect to continue receiving grants from OCS and CIIRDF in the future.
51
Table of Contents
Cash Flows
The following table presents the major components of net cash flows used in and provided by operating, investing and financing activities for the periods presented:
| Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | (2,840 | ) | $ | 2,133 | $ | (1,888 | ) | $ | (3,721 | ) | $ | (3,302 | ) | ||||||
| Net cash provided by (used in) investing activities |
(14,497 | ) | (30,582 | ) | 18,485 | 8,745 | (4,271 | ) | ||||||||||||
| Net cash provided by (used in) financing activities |
1,353 | 25,576 | 1,111 | (1 | ) | 178 | ||||||||||||||
| Exchange rate differences—cash and cash equivalents |
(111 | ) | (782 | ) | 89 | 70 | 17 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net increase (decrease) in cash and cash equivalents |
$ | (16,095 | ) | $ | (3,655 | ) | $ | 17,797 | $ | 5,093 | $ | (7,378 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Cash Provided by (Used in) Operating Activities
Cash provided by (used in) operating activities consists primarily of net income or loss adjusted for certain non-cash items. Adjustments to net income or loss for non-cash items include depreciation and amortization, cost of share-based compensation and financing expenses. In addition, operating cash flows are impacted by changes in operating assets and liabilities, principally deferred revenues, prepayments, trade receivables, other receivables, trade payables and other payables.
Cash flows used in operating activities for the six months ended June 30, 2013 were $3.3 million and resulted primarily from ($2.4 million) in net loss, a decrease of $2.2 million in deferred revenues, decreases of $0.8 million in trade payables and other payables and an increase in trade receivables by $0.3 million, primarily offset by $1.0 million in depreciation and amortization expenses, $0.6 million in share based compensation expenses and by $0.6 million in interest received during the six months ended June 30, 2013.
Cash flows used in operating activities for the year ended December 31, 2012 were $1.9 million and resulted primarily from ($2.5 million) in net loss reduced by $1.8 million in depreciation and amortization expenses, $1.2 million of share-based compensation, $1.2 million in increases in trade payables and other payables and $0.9 million in interest received, and primarily offset by a decrease of $3.3 million in deferred revenues and by a $0.7 million increase in trade receivables.
Cash provided by operating activities decreased by $4.0 million from 2011 to 2012. Net cash provided by operating activities was $2.1 million for the year ended December 31, 2011 and were generated primarily from $0.6 million in net income adjusted up by $1.4 million in depreciation and amortization expenses, $2.1 million in expenses related to share-based compensation, a $1.3 million decrease in trade receivables and $1.1 million in interest received, and adjusted down by $3.8 million in net financing income and $0.9 million in deferred revenues.
Cash Provided by (Used in) Investing Activities
Cash provided by investing activities was $8.8 million for the six months ended June 30, 2012 and cash used in investing activities was $4.3 million for the six months ended June 30, 2013. The change from cash provided by investing activities in the six months ended June 30, 2012 to cash used in investing activities in the six months ended June 30, 2013 primarily resulted from a decrease in proceeds from bank deposits and an increase in net acquisitions of marketable securities.
The majority of our investment activities have historically been related to the acquisition of property, plant and equipment and the purchase and sale of marketable securities. Cash used in investing activities was
52
Table of Contents
$14.5 million and $30.6 million for the years ended December 31, 2010 and December 31, 2011, respectively, and cash provided by investing activities was $18.5 million for the year ended December 31, 2012. The change from cash used in investing activities in 2010 and 2011 to cash generated by investing activities in 2012 derives primarily from an increase in proceeds from bank deposits and an increase in net proceeds from the sale of marketable securities.
Cash Provided by Financing Activities
Cash provided by financing activities was $0.2 million for the six months ended June 30, 2013 and cash used in financing activities was $1.0 thousand for the six months ended June 30, 2012. Cash provided by financing activities derives primarily from payments related to the exercise of options.
Cash provided by financing activities was $1.1 million, $25.6 million and $1.4 million for the year ended December 31, 2012, 2011 and 2010, respectively, and primarily resulted from payments related to the issuance of shares in 2011 and exercise of options in 2012, 2011 and 2010.
Government Grants
Our research and development efforts are financed, in part, through grants from the OCS, the Israel-U.S. Binational Industrial Research and Development Foundation, or BIRD, and CIIRDF. From our inception through 2012, we received grants totaling $4.8 million from the OCS and repaid royalties on sales of products derived from the research financed by such grants of $1.4 million, grants totaling $0.7 million from BIRD, out of which we repaid $0.4 million in 2012 and grants totaling $0.2 million from CIIRDF, which we have not yet paid any royalties on. As of December 31, 2012, we had seven active research grants under which we were receiving funding: five from the OCS, one from BIRD and one from the CIIRDF. We have applied to receive additional grants to support our research and development activities in 2013, none of which has been approved yet.
Under the Israeli R&D Law, royalties on the revenues derived from sales of products or services developed in whole or in part using grants from the OCS are due to the Israeli government, generally at a rate between 3.0% and 5.0%. The rate of the royalties payable to the Israeli government varies by the length of time a product has generated sales revenues. During the first three years of sales of products developed as a result of the OCS grants, we are required to pay royalties of 3.0% of the sales of such products, and from the fourth year on, we are required to pay royalties of 3.5% of such sales, in all cases, up to 100% of the amount of grants received by us from the OCS plus interest at the London Interbank Offered Rate, or LIBOR. In addition to paying any royalty due, we must abide by other restrictions associated with receiving such grants under the R&D Law. These restrictions may impair our ability to outsource development of products containing our traits, engage in change of control transactions or otherwise transfer our know-how outside Israel and may require us to obtain the approval from the OCS for certain actions and transactions and pay additional royalties and other amounts to the OCS.
Our BIRD grant is a joint development with DuPont to research and develop improvements to soybean rust resistance. Under the BIRD program, the grant will be repaid either (a) within 66 months from the original grant date, provided we do not generate royalties from the project and DuPont decides to continue the project or (b) through royalties at a rate of 5% of revenues of the products developed through the project or 30% of the revenues generated by the advanced technology developed through the project, in an amount of up to 150% of the total grant received. Should we choose to abandon the project, we will not be obligated to repay the grant, however we will also not be permitted to use the intellectual property developed during the project.
The CIIRDF grant was also provided as part of a joint project with Saskatchewan Wheat Pool Inc., operating under the name of Viterra, to develop canola with improved yield and abiotic stress tolerance. This grant will be repaid from income resulting from the commercialization of a product developed pursuant to the grant project, at a rate of 2.5% of royalties on sales of such product, in an amount up to 100% of the total grant
53
Table of Contents
received. Alternatively, we may repay the grant as royalties of 2.5% of the income we receive from licensing the product developed pursuant to the grant. Payment of such royalties is not required if commercial revenues are not generated as a result of the project.
See “Risk Factors—Risks Relating to Our Incorporation and Location in Israel—We have received Israeli government grants for certain of our research and development activities. The terms of these grants may require us to satisfy specified conditions in order to manufacture products and transfer technologies supported by such grants outside of Israel. We may be required to pay penalties in addition to repayment of the grants.”
Contractual Commitments and Contingencies
Our significant contractual obligations and commitments as of December 31, 2012 are summarized in the following table:
| Payments Due by Period | ||||||||||||||||||||||||||||
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total | ||||||||||||||||||||||
| (in thousands, unaudited) | ||||||||||||||||||||||||||||
| Trade payables |
$ | 1,416 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 1,416 | ||||||||||||||
| Other payables(1) |
3,139 | — | — | — | — | — | 3,139 | |||||||||||||||||||||
| Liabilities in respect of grants from the Chief Scientist(2) (undiscounted) |
745 | 501 | 605 | 511 | 525 | 1,175 | 4,062 | |||||||||||||||||||||
| Non-cancellable operating leases(3) |
467 | 431 | 383 | 22 | — | — | 1,303 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total |
$ | 5,767 | $ | 932 | $ | 988 | $ | 533 | $ | 525 | $ | 1,175 | $ | 9,920 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (1) | Consists of liabilities to employees for salaries and related personnel costs and accrued expenses to suppliers. Approximately $2.5 million of this amount was paid at the beginning of 2013. |
| (2) | Consists of the projected royalty payments of 3-3.5% on revenues derived from research and development projects that were funded in part by grants received from the OCS. |
| (3) | Consists of non-cancelable operating leases for the Company’s office space and motor vehicles. |
Off-Balance Sheet Arrangements
We do not currently engage in off-balance sheet financing arrangements. In addition, we do not have any interest in entities referred to as variable interest entities, which includes special purpose entities and other structured finance entities.
Application of Critical Accounting Policies and Estimates
Our accounting policies affecting our financial condition and results of operations are more fully described in our consolidated financial statements included elsewhere in this prospectus. The preparation of our financial statements requires management to make judgments, estimates and assumptions that affect the amounts reflected in the consolidated financial statements and accompanying notes, and related disclosure of contingent assets and liabilities. We base our estimates upon various factors, including past experience, where applicable, external sources and on other assumptions that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions, and could have a material adverse effect on our reported results.
In many cases, the accounting treatment of a particular transaction, event or activity is specifically dictated by accounting principles and does not require management’s judgment in its application, while in other cases,
54
Table of Contents
management’s judgment is required in the selection of the most appropriate alternative among the available accounting principles, that allow different accounting treatment for similar transactions.
We believe that the accounting policies discussed below are critical to our financial results and to the understanding of our past and future performance as these policies relate to the more significant areas involving management’s estimates and assumptions. We consider an accounting estimate to be critical if: (1) it requires us to make assumptions because information was not available at the time or it included matters that were highly uncertain at the time we were making our estimate; and (2) changes in the estimate or different estimates that we could have selected may have had a material impact on our financial condition or results of operations.
Revenue Recognition
We recognize revenues when such revenues and the costs incurred or to be incurred in respect of the transaction can be measured reliably and when it is probable that the economic benefits associated with the transaction will flow to us.
We have entered into collaboration agreements under which we grant to our collaborators an exclusive license to intellectual property rights for the development and commercialization of our proprietary products. The agreements contain multiple elements, including funding from periodic payments for research and development services, up-front payments, payments based on achievement of specified milestones and royalties on sales of products sold by our collaborators that include the licensed traits.
We assess the criteria for recognition of revenue related to up-front payments and multiple components of revenue as outlined by IAS 18, Revenue. Judgment is necessary to determine the period over which we will satisfy our obligations related to up-front payments, when revenue components can be recognized separately and how to allocate the related consideration for each revenue component.
Revenues from periodic payments for research and development services are recognized throughout the services period based on the proportion of actual costs incurred for each reporting period to the estimated total costs of the collaboration, subject to the enforceable rights. Up-front payments received upon entering into the license and collaboration agreements, in exchange for the transfer of our patented genes to licensees, are also recognized as revenues over the duration of the relevant contract based on the proportion of actual costs incurred for each reporting period to the estimated total costs of the collaboration.
Revenues from milestone events, which are contingent upon the occurrence of certain events specified in the collaboration agreement, are recognized as revenues when the milestones, as defined in the particular agreement, are achieved.
Share-Based Compensation
We account for share-based compensation in accordance with the fair value recognition provision of IFRS guidance on share-based compensation. Under these provisions, share-based compensation is measured at the grant date based on the fair value of the award and is recognized as an expense, net of estimated forfeitures, over the requisite service period, which is generally the vesting period of the respective award.
We selected the binomial option pricing model as the most appropriate method for determining the estimated fair value of our share-based compensation. The resulting cost of an equity incentive award is recognized as an expense over the requisite service period of the award, which is usually the vesting period. We recognize compensation expense over the vesting period using the graded vesting attribution method, which results in an accelerated recognition of compensation costs, and we classify these amounts in the consolidated statements of comprehensive income based on the department to which the related employee reports.
55
Table of Contents
The determination of the grant date fair value of options using an option pricing model is affected by estimates and assumptions regarding a number of complex and subjective variables. These variables include the estimated period of time that we expect employees to hold their options, the expected volatility of our share price over the expected term of the options, share option exercise and cancellation behaviors, risk-free interest rates and expected dividend yields, which are estimated as follows:
| • | Expected term. The expected term was estimated using historical data from prior years, including historical forfeiture rates. |
| • | Share price volatility. The expected share price volatility was based on the average historical price volatility of our shares on the TASE. |
| • | Risk-free interest rate. The risk-free interest rate is based on the yields of non-index-linked Bank of Israel treasury bonds with maturities similar to the expected term of the options for each option group. |
| • | Dividend yield. We have historically not paid and do not intend to pay dividends on our ordinary shares. We have therefore assumed a dividend yield of zero. |
If any of the assumptions used in the binomial model changes significantly, share-based compensation for future awards may differ materially compared with the awards granted previously.
The following table presents the weighted-average assumptions used to estimate the fair value of options granted during the periods presented:
| Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||
| (unaudited) | ||||||||||||||||||
| Expected volatility of the share prices |
25-65% | 33-69% | 25-65% | 32-65% | — % | |||||||||||||
| Expected term of options (in years) |
4.12 | 4.27 | 4.12 | 4.27 | — | |||||||||||||
| Risk-free interest rate |
1.87-6.81% | 3.01-6.93% | 1.87-6.81% | 2.26-6.81% | — % | |||||||||||||
| Dividend yield |
0% | 0% | 0% | |
0% |
|
— % | |||||||||||
| Weighted average share price |
NIS 17.99 / $4.67 | NIS 15.82 / $4.14 | NIS 17.99 / $4.67 | NIS 17.41 / $4.44 | NIS — | |||||||||||||
Government Grants
Government grants are recognized when there is reasonable assurance that we will receive the grants and that we will comply with the attached conditions. Research and development grants received from the OCS, BIRD and CIIRDF are recognized upon receipt as a liability if future economic benefits are expected from the project that will result in royalty-bearing sales.
A liability for the grant is first measured at fair value using a discount rate that reflects a market rate of interest. The difference between the amount of the grant received and the fair value of the liability is accounted for as a government grant and recognized as a reduction of research and development expenses. After initial recognition, the liability is measured at amortized cost using the effective interest method. Royalty payments we make to repay the grant are treated as a reduction of the liability. If no economic benefits are expected from the research activity, the grant receipts are recognized as a reduction of the related research and development expenses, in which case, the royalty obligation is treated as a contingent liability.
There is uncertainty regarding the estimates of future cash flows and the estimate of the capitalization rate that is used for determining the amount of the liability recognized. At the end of each reporting period, we evaluate whether there is reasonable assurance that the liability recognized, in whole or in part, will not be repaid
56
Table of Contents
(since we will not be required to pay royalties) based on the best estimate of future sales, and if so, the appropriate amount of the liability is reduced with a corresponding reduction in research and development expenses.
Quantitative and Qualitative Disclosure About Market Risk
Foreign Currency Risk
Most of our revenues are denominated in U.S. dollars. The percentage of our revenues denominated in U.S. dollars accounted for 72.1%, 72.2% and 77.8%, of our revenues in the year ended December 31, 2012, 2011 and 2010, respectively, and 68.0% and 73.9% of our revenues in the six months ended June 30, 2013 and 2012, respectively. We incur expenses primarily in NIS, however, and our expenses in NIS accounted for 89.8%, 90.3%, and 89.0%, of our expenses in the year ended December 31, 2012, 2011 and 2010, respectively, and 91.1% and 91.5% of our expenses in the six months ended June 30, 2013 and 2012, respectively. As a result, any appreciation of the NIS relative to the U.S. dollar would adversely impact our profitability due to the portion of our expenses that are incurred in NIS. As of June 30, 2013, we did not have any hedge arrangements in place to protect our exposure to foreign currency fluctuations.
The following table presents information about the changes in the exchange rates of the NIS against the U.S. dollar:
| Period |
Change in Average Exchange Rate of the NIS against the U.S. dollar (%) |
|||
| Six months ended June 30, 2013 |
(3.5 | ) | ||
| 2012 |
7.8 | |||
| 2011 |
(4.1 | ) | ||
| 2010 |
(4.9 | ) | ||
Our exposure related to exchange rate changes on our net asset position denominated in currencies other than USD varies with changes in our net asset position. Net asset position refers to financial assets, such as trade receivables and cash, less financial liabilities, such as trade payable and other payables. The impact of any such transaction gains or losses is reflected in finance expenses. Our most significant exposure relates to a potential change in the exchange rates of the U.S. dollar and the NIS. Assuming a 10% decrease in the U.S. dollar relative to the NIS, and assuming no other change, our finance expenses would have increased by $0.3 million in each of 2010 and 2011, decreased by $37,000 in 2012, and increased by $61,000 in the six months ended June 30, 2013 due to our positive current net asset position denominated in U.S. dollars in 2010 and 2011, and the six months ended June 30, 2013 and our negative current net asset position denominated in U.S. dollars in 2012.
Our results of operations are also impacted by currency translation gains and losses on monetary assets and liabilities, primarily cash deposits, denominated in currencies other than the U.S. dollar. Such gains or losses only impact the dollar value of our non-dollar denominated cash deposits and result from changes in reported values due to exchange rate fluctuations between the beginning and the end of reporting periods. Due to the limited amount of non-dollar denominated cash deposits, the amount of finance expenses recorded has been relatively insignificant.
Commodity Price Risk
Operating in the agribusiness sector, changes in certain commodity prices may affect our reported operating results and cash flows. We currently are not exposed to commodity price risks, however the prospects of our wholly owned subsidiary Evofuel will depend on biofuel and oil and natural gas prices, and the royalties we may receive from our collaborators on the sales and transfers of GM seeds containing the traits we develop could be affected by fluctuations in seed commodity prices. As of June 30, 2013, we did not have any hedge arrangements in place to protect our exposure to commodity price fluctuations.
57
Table of Contents
New and Revised Financial Accounting Standards
The JOBS Act permits emerging growth companies such as us to delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
58
Table of Contents
We are a plant genomics company using proprietary computational tools to develop solutions that improve crop productivity. Our company operates principally in the seed and ag-chemical industry through the analysis of genomic data to identify and develop seed traits associated with increased productivity utilizing both biotechnology and advanced breeding methods. In addition, we intend to use these tools to enable the development of novel ag-chemicals for crop protection and enhancement, and we are also developing high-yielding castor bean seeds as a second generation feedstock for biodiesel. We believe that these markets represent large revenue opportunities, driven by a variety of macro trends and technological developments in the agriculture industry.
| • | The market for seeds was estimated to be $37.6 billion in 2012, of which biotech seeds represented 49%, or $18.5 billion, according to Phillips McDougall’s March 2013 Seed Insight newsletter. |
| • | The market for crop protection ag-chemicals was estimated to be $47.3 billion in 2012, according to Phillips McDougall’s 2013 Industry Presentation on the Global Agrochemical and Seed Markets. |
| • | Global biodiesel consumption was estimated at 422.4 thousand barrels per day in 2011, according to Ken Research’s 2012 Global Biofuel Market Outlook. |
We believe these market trends and the industry trends described below have enabled us to expand our relationships with our existing collaborators, which in turn has increased our revenues from $12.6 million in the year ended December 31, 2010 to $17.1 million in the year ended December 31, 2012. The growth in the market for seeds and crop protection has also enabled us to enter into new collaborations with companies in the seed and ag-chemical industry that are seeking to improve crop productivity. We have not yet entered into any collaboration agreements in our ag-chemical operations. Because we are in the developmental stages, we have incurred operating losses of $(3.1 million), $(3.2 million) and $(2.0 million) for the years ended December 31, 2012, 2011 and 2010, respectively, and $(2.1 million) and $(1.1 million) in the six months ended June 30, 2013 and 2012, respectively. While we expect to continue to incur operating losses in the near term, we expect that these market trends will continue to drive the growth of our business and increase revenue in the long term. We expect that the market and industry trends will enable our growth, both by potentially expanding our relationships with existing collaborators and by creating opportunities to enter into new collaborations. However, we cannot provide any assurance that these market trends will continue or that we will be successful in taking advantage of these trends. See “Risk Factors.”
Agriculture Industry Trends
The agriculture industry continues to seek sustainable and economically viable solutions to feed the world’s growing and increasingly prosperous population. Population growth and prosperity are fueling increased demand for grain, however production is constrained in meeting that demand due to finite arable land and water resources, climate variability, increasingly resilient weeds and insects, depletion of soil nutrients and diseases that impair crop yields.
In order to address these growing challenges, farmers need solutions to increase and maintain crop productivity. Seed and ag-chemical companies are therefore increasingly seeking innovative agricultural technologies such as development of high-yield seeds and more effective crop protection ag-chemicals. Such technologies are expected to continue to play a significant role in maintaining the supply-demand balance.
59
Table of Contents
Growing Global Demand for Grain
The global demand for major grains (corn, soybean, wheat, rice and barley) is expected to continue to rise steadily, driven by population growth and increasing demand for more protein-rich diets. According to a 2013 Global Commodities Forecast from Business Monitor International, global demand is estimated to have increased 23% from 2000 to 2011, reaching 2.3 billion tons, and is expected to reach approximately 2.7 billion tons in 2017 representing a further increase of 14%.
Global demand for major grains
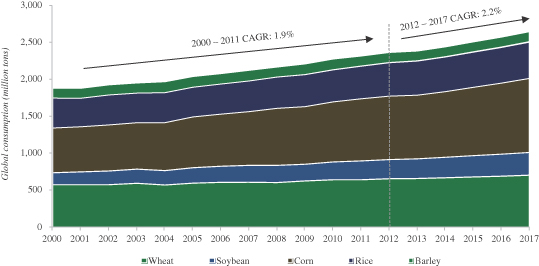
Source: Business Monitor International Global Commodities Forecast (April 2013)
Increasing population. The global population is projected to rise from approximately 7.0 billion people in 2012, to 9.4 billion people by 2050, according to the United States Census Bureau’s Demographic Overview Report. Most of this growth is expected to occur in developing countries, whose populations are expected to reach nearly 8.1 billion people by 2050. Population growth is the primary cause of increased grain demand.
Changing diets. In addition, rising levels of income, particularly in emerging markets, are expected to drive demand for higher protein diets, such as meat and dairy. For example, increased consumption of meat has a major effect on the demand for grains as the production of one kilogram of beef, pork or poultry requires multiple kilograms of grain feed. Global demand for meat is expected to increase by 1.1% per annum from 2005/2007 to 2050, according to the 2012 report, “World agriculture towards 2030/2050” by the Food and Agricultural Organisation of the United Nations (“FAO”), in comparison to approximately 0.8% of annual population growth, according to the United States Census Bureau.
60
Table of Contents
Limited Grain Supply
Finite land available for agriculture. Despite rising demand for grain, there is limited growth potential in arable land available for cultivation. According to the FAO’s 2012 report, “World agriculture towards 2030/2050”, within approximately 13.3 billion hectares of global dry land area, 4.5 billion hectares is suitable for agriculture. According to the same report, in 2005 to 2007 more than 1.5 billion hectares were cultivated. In addition, according to the 2011 Organisation for Economic Co-operation and Development (“OECD”)-FAO Agricultural Outlook, the cultivated land per capita declined at 1.5% p.a. from 1970 to 2008.
Hectares per capita, global
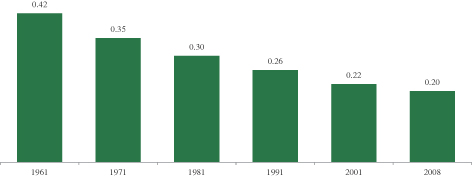
Source: OECD-FAO Agricultural Outlook (2011)
Climate variability and limited water supply. Farmers must also manage risks associated with climate variability and detrimental natural events. These include both cyclical weather fluctuations, such as mild to moderate changes in temperature and precipitation, and major natural events such as droughts and storms. For example, according to a 2012 news release by the United States Department of Agriculture, or USDA, in 2012 the United States experienced the most severe and extensive drought in at least 25 years with 80% of agricultural land affected.
Weeds, insects and disease. The presence of weeds, insects and diseases also harms crop yields. According to a 2009 Media Centre release by the FAO, $95 billion worth of global food production is lost annually to weeds, $46 billion to insects and $85 billion to diseases. Weeds reduce yield by competing with crops for water, light and nutrients, and can interfere with harvest operations and increase production costs, while insects and disease can severely affect crop yield and quality.
61
Table of Contents
The Supply—Demand Gap
Over the last five decades, demand for food has been largely met by increasing crop yield as a result of innovations in agricultural technology, such as ag-chemicals and enhanced seeds, as well as a gradual increase in cultivated land. However, along with the decline in arable land per capita, the rate of yield improvement provided by current agricultural technologies is slowing and may not be sufficient to provide future food security.
| Indexed area and production trends(a) |
10-year rolling average change in yield(b) | |
|
|
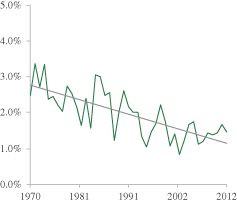
| |
|
(a) Reflects yield of corn, wheat, rice and barley crops. Yield defined as metric ton of crop produced per hectare. |
(b) Reflects 10 year rolling average yield of corn, wheat, rice and barley crops. | |
| Source: USDA, Foreign Agricultural Service’s Production, Supply and Distribution Database (2013) | Source: USDA, Foreign Agricultural Service’s Production, Supply and Distribution Database (2013) | |
Enhancing and accelerating agricultural productivity in a sustainable manner is a central component of achieving global food and nutrition security. According to the Global Harvest Initiative’s 2010 Global Agricultural Productivity Report, Total Factor Productivity (TFP), which represents the ratio of agricultural outputs (gross crop and livestock output) per input (land, labor, livestock, fertilizer and machinery) will need to grow at an annual rate of 1.75% per year to meet the global demand for agricultural output in 2050. This will need to be addressed by continued improvements in technology and productivity.
Improving Plant Productivity
In order to address the supply-demand gap, farmers need to rely on innovative technologies that are aimed at improving plant productivity and field performance. Plant performance is impacted by various plant characteristics and traits that can be divided into three major categories:
| • | Yield and abiotic stress tolerance traits govern plant yield per unit area, yield stability over varying environmental conditions and tolerance to environmental stress factors, such as drought and fertilizer utilization; |
| • | Biotic stress tolerance traits govern plant resistance to living organisms such as insects, diseases and fungi, as well as herbicides; |
| • | Quality traits govern the plant’s taste, shelf life, oil quality and nutrient content. |
A fundamental way to improve plant productivity and performance is through the use of plant genomics. Plant genomics is the science of understanding and analyzing plants to modify biological elements which are responsible for traits. Through plant genomics, researchers can identify and influence target genes and other genomic components that affect trait performance, which are then used in the development of (i) enhanced seeds and (ii) novel crop protection ag-chemicals.
62
Table of Contents
(i) Enhanced Seeds
There are two primary methods to improve plant performance through the use of genomic technologies in the development of seeds.
Biotechnology or genetic modification. Use of genomic technologies for the development of seeds in which genes that impact specific traits are introduced to the plant through genetic insertion. These genes are identified and sourced from either the same plant, other plants or another organism and are introduced to the plant through the gene insertion into the plant’s DNA. Such seeds are referred to as genetically modified (“GM”) or biotech seeds. Biotechnology provides for a wider range of traits in a plant and usually results in more substantial seed trait improvement compared to advanced breeding.
Advanced breeding. Breeding is the enhancement of desired native traits by pairing between two plant lines from the same plant species. Advanced breeding is the use of genomic technologies to identify specific genomic markers, such as single-nucleotide polymorphisms (or SNPs), linked to a particular trait. This allows breeders to determine which parent plants with favorable characteristics should be crossed to enhance desired traits in the next generation. Seeds produced from advanced breeding are referred to as conventional seeds.
The global seed market grew at 7.8% CAGR since 2001 to reach $37.6 billion in 2012, with the biotech seeds market the major driver, growing at an 18.8% CAGR in the same period to reach $18.5 billion in 2012, accounting for 49% of the total seeds market, according to Phillips McDougall’s March 2013 Seed Insight newsletter. As of today, the two major traits which are commercially available in the biotech seeds market are herbicide tolerance and resistance to insects. However, the seeds companies are investing substantial resources to develop additional traits. According to estimates from the 2012 Global Status of Commercialized Biotech/GM Crops report, prepared by the International Services for the Acquisition of Agri-Biotech Applications (“ISAAA”), the savings and added economic value for farmers globally since the initial adoption of biotech applications in plants between 1996 and 2011 was estimated at approximately $98 billion.
| Global value of seed market ($bn) & penetration of biotech seeds (%) |
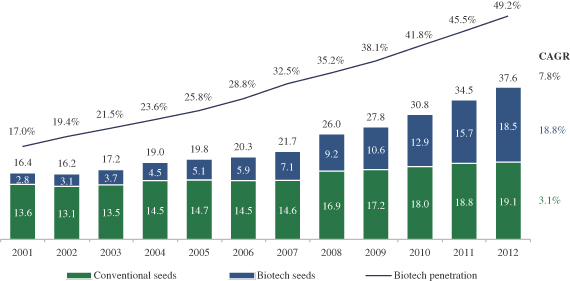
|
Source: Phillips McDougall, Seed Insight, March 2013
63
Table of Contents
To date, the two main commercial biotech crops are corn and soybean, which together accounted for 60% of the global seeds market in 2012.
| Total seed market, by crop (2011) |
Biotech seed market, by crop (2011) | |
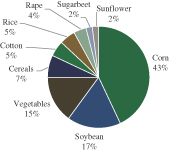
|
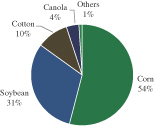
| |
| Source: Phillips McDougall 2011 Seed Industry Overview (June 2012) | Source: Phillips McDougall 2011 Seed Industry Overview (June 2012) | |
Biotech seeds comprise a global industry, with 28 countries growing biotech crops in 2012 and a further 31 allowing imports, according to the ISAAA’s 2012 Global Status of Commercialized Biotech/GM Crops report. To date, the United States, Brazil and Argentina have accounted for the majority of volume with 106.1m hectares out of 170.3m hectares utilized for biotech crops in 2012, while the cultivation of biotech crops in the European Union has not been widely accepted due to regulation and negative public market perception.
| Area of total crops, by country (2012) |
Area of biotech crops, by country (2012) | |
|
| |
| Source: USDA, Foreign Agricultural Service’s Production, Supply and Distribution Database (2013) | Source: ISAAA Global Status of Commercialized Biotech/GM Crops (2012) | |
Factors expected to drive future growth include the application of advanced breeding and biotechnology in additional crops such as rice and wheat, introduction of new target traits, such as durability to abiotic stress, and adoption of biotechnology crops by additional countries.
Product development for enhanced seeds
Biotech seeds. Producing biotech seeds requires trait development and commercial plant biotechnology, in addition to significant research and development capital, intellectual property protection and regulatory testing. The research and development process for biotech crops is complex and time consuming, requiring extensive application of technology in data analysis and field trials. The process of identification and development can take eight to 13 years before commercialization, depending on both the complexity of the trait and the type of crop involved. Total development costs on average typically reach approximately $140 million per gene addressing a specific trait in a specific crop, according to a Consultancy Study for Crop Life International conducted by Phillips McDougall in 2011.
64
Table of Contents
Conventional seeds. The product development process of enhancing desired native traits through advanced breeding includes identification of SNPs, validation in field trials and commercial launch of a seed product containing the trait. This process primarily differs from the development of biotech seeds in later phases of development wherein the process involved in receiving regulatory approval is far more comprehensive and lengthy in biotech seeds than in conventional seeds.
Major seed companies have declared their goal to significantly increase crop yield to meet the growing needs of the world population. For instance, Monsanto has indicated that it plans to double the yield in its core crops by 2030. These companies are increasingly focused on developing seeds with new target traits to improve yield and durability to abiotic stress, such as drought and heat. For example, Monsanto expects to commercially launch the first biotech drought tolerance trait in corn seed in 2013. In addition, these companies invest significant resources to broaden technological solutions for resistance to biotic stress such as nematodes, fungi and different plant diseases, in order to address the increasing resistance of pests to existing products, as reported by farmers. Such solutions include introducing seed traits with innovative mechanisms to cope with these pests, as well as new products to cope with different kinds of living organisms, such as nematodes, diseases and fungi.
According to Phillips McDougall’s 2013 Industry Presentation on the Global Agrochemical and Seed Markets, the total investment in seeds and traits by the major seed companies (Monsanto, DuPont, Syngenta, BASF, Dow and Bayer) was more than $3 billion in 2011. These companies often collaborate with specialized research and development companies that have complementary technological capabilities and expertise. Gene identification and early stage trials are often performed by such research and development companies on behalf of, and prior to, further development and commercialization by seed companies.
(ii) Crop Protection Ag-chemicals
The market for crop protection ag-chemicals has grown by approximately 40% since 2007 to an estimated $47 billion in 2012, according to Phillips McDougall’s 2013 Industry Presentation on the Global Agrochemical and Seed Markets. Ag-chemical products contain formulations of one or more chemicals designed to target an essential biological process within pests and prevent them from affecting agricultural crops. Crop protection ag-chemicals, which as a group are referred to as “pesticides” or “crop protection products,” include herbicides (to control weeds), fungicides (to prevent and treat fungal diseases) and insecticides (to minimize damage caused by insects).
| Crop protection ag-chemicals market size ($bn) and growth (%) |
Crop protection ag-chemical market breakdown (2011) | |
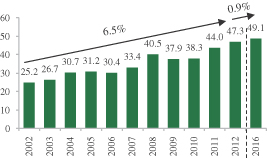
|

| |
| Source: Phillips McDougall Industry Presentation on the Global Agrochemical and Seed Markets (January 2013) | Source: Phillips McDougall Industry Presentation on the Global Agrochemical and Seed Markets (May 2013) | |
65
Table of Contents
Historically, the market was driven by the introduction of new chemicals which addressed many of the challenges faced by crop growers in handling pests. However, in recent years, the industry has faced a number of challenges including:
| • | The rate of introduction of novel ag-chemicals has been steadily declining due to high development costs and strict regulation, according to Phillips McDougall’s 2013 Industry Presentation on the Global Agrochemical and Seed Markets. The most extreme example of this is in the herbicides sector, in which there are only approximately 20 existing mechanisms to eradicate weeds, and the last product based on a new mechanism was launched over 20 years ago. |
| • | As a result, farmers are required to use the same product, or a combination of a number of products, repeatedly and in larger quantities and concentrations, against pests. This in turn leads to further acceleration in the development of pest resistance to existing chemicals and results in higher costs to the farmers, limiting the solutions available for the farmer as well in addition to having negative environmental impacts. |
We believe that the decline in new mechanisms for eradicating weeds combined with an increase in weed resistance will further drive the growth in the market for crop protection ag-chemicals.
Number of New Pesticide Products Launched Annually
Source: Phillips McDougall, The Global Agrochemical and Seed Markets Industry Developments, January 2013
Number of Species of Weed with Multiple Resistance

Source: Weed Science Society of America, Herbicide Resistance Management Lesson 2011
66
Table of Contents
Farmers and ag-chemical companies have therefore been seeking new solutions to mitigate pest resistance. Accordingly, the science of crop protection is expanding beyond traditional agriculture chemicals to include new technologies to develop more effective ag-chemicals, such as more effective herbicides.
Product development for crop protection ag-chemicals
The process of developing a new crop protection ag-chemical is lengthy and costly, and typically spans ten years and costs on average $260 million to bring a new product to market, according to a 2010 Consultancy Study for Crop Life America and the European Crop Protection Association conducted by Phillips McDougall. Most producers have traditionally relied on a process of trial-and-error which involves screening thousands of chemicals against weeds, insects or any other kind of pests until they find a chemical that is able to eradicate or severely damage the pest. The decrease in the rate of introduction of new crop protection products to the market indicates that this costly approach is becoming less effective to identify and develop new products.
An alternative approach, less employed by the major ag-chemical companies today, involves rational design similar to that utilized in the pharmaceutical industry, which combines elements of both chemistry and biology. This consists of the use of plant genomic data to identify and target essential biological processes and leveraging this data for the discovery and development of complementary crop protection ag-chemicals, thereby potentially increasing the probability of success in identifying and developing novel herbicides with new mechanisms to cope with the growing resistance of weeds.
While the majority of current crop protection ag-chemicals are focused on providing solutions to biotic stresses, as described above, the industry is increasingly expanding into additional application areas such as crop enhancement ag-chemicals. These solutions are focused on developing ag-chemicals which if sprayed on crops, would affect important biological processes and thereby enable those crops to improve yields and resistance to abiotic stresses.
The Market for Biofuels
Biofuel demand in ground, aviation and maritime transportation has been steadily growing in past years, representing a market opportunity of approximately $140 billion in 2011, according to Ken Research’s 2012 Global Biofuel Market Outlook, with new markets, such as biojet fuels for commercial airlines and the US military continuing to emerge and attract new investments in the industry. Similar to traditional fuels, the two main types of biofuels in broad use are bioethanols (based on sugars such as corn and sugar cane) and biodiesels (based on oil based feedstock such as soybean and canola). The market is mainly driven by a need for increasing energy independence, reducing susceptibility to fluctuating oil prices and environmental concerns. Global production of biodiesel reached over 368 thousand barrels per day in 2011.
Source: OECD-FAO Agricultural Outlook (2011)
67
Table of Contents
According to the 2007 McKinsey Quarterly (No. 2), feedstock accounts for the majority of biodiesel production costs. At present, production of biodiesel, an alternative to diesel fuel derived from plant oil, relies on edible crops, such as soybean and canola, considered first generation feedstocks. Rising commodity prices and concerns surrounding the long-term sustainability of diverting food feedstock for biodiesel use has hindered widespread adoption of these alternative fuels, setting the stage for the demand of second generation feedstock for biodiesel. Efforts are being undertaken worldwide to introduce second generation feedstock that will be: (i) economically viable, (ii) scalable and (iii) sustainable (e.g., achieving high productivity, with low input requirements and without competing with food crops). While there have been numerous attempts to develop a second generation feedstock (e.g., based on jatropha and algae), thus far the commercial success has been limited.
The castor bean plant has unique characteristics which make it a highly suitable potential second generation feedstock for biodiesels. Castor bean is a non-edible, high oil-yielding crop (oil comprises nearly 50% of the castor bean seed), and exhibits high tolerance to harsh environmental conditions, such heat and drought tolerance. Today castor beans are primarily grown in India, China and Brazil in a traditional manner and are mainly used in the biopolymer and lubricant markets. There is a need for advances in castor oil production as the castor market has exhibited unstable supply and price in the past, and has lacked an application of high-quality plant genetics and agronomics. While no commercial attempts have been made to develop castor bean seeds as a feedstock for biodiesel, by applying advanced seed technologies and modern agricultural practices, castor beans could be developed to bear the characteristics of an economically viable and sustainable second generation feedstock for biodiesel.
68
Table of Contents
Our Business
We are a plant genomics company that uses a comprehensive and integrated technology infrastructure to enhance seed traits underlying crop performance and productivity through biotechnology and advanced breeding methods. We have strategic collaborations with world-leading agricultural companies, primarily to develop traits for improved yield and abiotic stress tolerance (such as improved tolerance to drought, heat and salinity) and biotic stress resistance (such as resistance to disease, pests and insects). Our products are focused on essential crops, including corn, soybean, wheat, rice and cotton, which, according to Phillips McDougall’s 2011 Seed Industry Overview report, account for over 70% of the value of the global seeds market. We also apply our technology infrastructure to two additional fields: (i) ag-chemical and (ii) seeds focusing on second generation feedstock for biodiesel, both of which have not yet generated revenue and are in the development stage.
The field of plant genomics, or the science of understanding and analyzing plant genomes to identify and impact biological elements affecting trait performance, continues to evolve. Agricultural product innovation is increasingly driven by the ability to analyze data, in order to make direct discoveries and gain insights into key underlying biological phenomena of such products. The seed and ag-chemical industry has witnessed a dramatic increase in the availability of genomic data. This is primarily as a result of the introduction of new technologies that facilitate rapid generation of such data at a significantly lower cost. As a result, the key opportunity, and challenge, for plant trait improvement has shifted from data generation to data integration and analysis of large volumes of data.
We believe that our competitive advantage is based on our continuously enhanced proprietary discovery and development infrastructure. This infrastructure is capable of integrating and analyzing vast amounts of data, through the use of proprietary computational technologies comprised of advanced algorithms and predictive methodologies. Our computational technologies are a key part of our broad technology infrastructure that also integrates extensive scientific expertise, public and proprietary genomic data and plant validation systems. Our proprietary gene identification capabilities, which are scalable and adaptable to a large variety of crops and traits, together with our highly educated and experienced multidisciplinary team of scientists, are, we believe, unique in the industry.
We currently generate revenues primarily through research and development and milestone payments as traits move through development phases, and in the future we expect to receive royalty revenues upon commercialization of products containing traits that we help our collaborators to develop. To date, we have identified and filed patent applications for over 4,000 novel genes and genomic components for the improvement of key traits, hundreds of which are under development in our collaborators’ pipelines. We believe that the extension and renewal of some of our main collaboration agreements highlight the value ascribed to our performance, our capabilities and our proprietary technology infrastructure. However, we are still in the development stages, and it may take at least six years, if at all, before the first seeds containing our traits complete the development process and become commercially viable. We have a history of losses and incurred operating losses of $(3.1 million), $(3.2 million) and $(2.0 million) for the years ended December 31, 2012, 2011 and 2010, respectively, and $(2.1 million) and $(1.1 million) for the six months ended June 30, 2013 and 2012, respectively.
We have collaboration agreements with most of the world’s leading seed and ag-chemical companies, including subsidiaries or affiliates of Monsanto, Bayer, DuPont, and Syngenta. Our collaborations with these companies are aimed at introducing improved seed traits into key commercial crops. Among our collaborators, Monsanto and Bayer are also key shareholders in our company, holding approximately 8.7% and 4.6% of our ordinary shares as of June 30, 2013, respectively.
We also aim to apply our technology infrastructure to two additional fields: ag-chemical and seeds focusing on second generation feedstock for biodiesel. For the ag-chemical market, we are focusing on the discovery of
69
Table of Contents
new biologically significant proteins called “targets” based on genes we identify that enable the development of herbicides with novel mechanisms to mitigate weed resistance. We also intend to use our proprietary discovery and development plant infrastructure to develop chemical molecules as crop enhancers that, when applied to crops, would improve yield and abiotic stress tolerance. Although we have not yet entered into collaboration agreements in this area, we currently expect to complete the development of our computational technologies for ag-chemical operations in the next 12 to 18 months, which we believe will increase the likelihood of entering into new collaborations. However, we cannot provide any assurance that we will be successful in developing these computational technologies or in entering into collaborations during this timeframe, if at all.
For the biodiesel market, we are focusing on the development of improved, high-yielding, non-edible castor bean seeds as an economically viable alternative feedstock for the production of biodiesel with a reduced environmental footprint. The success of this operation will depend on our ability to address several unique challenges, including developing effective growth protocols for our castor bean varieties and achieving mechanized harvest capabilities. We do not expect, however, to start selling seeds under this operation for at least three years.
We currently employ 188 employees, of which approximately 78% are involved in research and development and 48 hold a Ph.D. degree. Our multi-disciplinary team includes experts in biology, chemistry, plant genetics, agronomics, mathematics, computer science and other related fields.
We have been listed for trading on the TASE since 2007, are headquartered in the agricultural and biotech hub of Rehovot, Israel, and have field testing activities in Israel, Brazil and Argentina.
Our Strengths
We believe we are strategically positioned to capitalize on the fundamental need to increase agricultural yields in highly attractive end-markets such as food, feed and biofuel. Our competitive strengths include.
A leading position in the field of plant genomics. Since our company’s formation in 2002, we have established a powerful integrated infrastructure that we believe is unique in our industry for understanding plant genomics, addressing some of the key challenges in agriculture productivity. The infrastructure combines our know-how in plant genomics with our proprietary technologies and processes that span data integration and analysis, field experiments and plant validation. This infrastructure allows us to identify, prioritize and validate over 1,000 genes per year in our in-house research center, labs, greenhouses and field locations. Our professional and skilled employee base includes experts in computer science, genetics, agriculture science, molecular biology and other fields. Approximately 78% of our 188 employees as of June 30, 2013 are involved in research and development, and 48 of our employees hold Ph.D. degrees.
Innovative proprietary computational technologies, capable of efficiently integrating and analyzing vast amounts of complex genomic data. Our core competency lies in our advanced proprietary computational tools, which are the cornerstone to our plant genomic capabilities. These proprietary computational technologies are based on advanced algorithms and predictive methodologies for data integration and data analysis. The focus in the seed industry has shifted from data generation to data analysis due to the significant increase in volume and complexity of genomic data. Our computational technologies have demonstrated capability to integrate and comprehensively analyze vast amounts of public and proprietary data, covering more than 200 plant species and in the scope of 500 terabytes, enabling us to identify and prioritize genes and genomic components aimed at improving key plant traits.
A partner of choice for industry leaders. We have collaborations with five of the seven leading global seed and ag-chemical companies, focusing on the development of traits for key crops in their product portfolios such as corn, soybean and wheat. We believe that our ability to identify and validate target genes and genomic components in an efficient and methodological manner is a driver of innovation in our collaborators’ product development pipelines. Two of our key collaborators, Monsanto and Bayer, have made significant equity
70
Table of Contents
investments in our company and have entered into more than one agreement with us, and we believe that our track record of renewing or entering into new collaboration agreements with our collaborators underscores the potential strategic nature of our relationships with most of the world’s leading seed and ag-chemical companies.
A balanced and scalable business model. We currently generate revenues primarily through research and development and milestone payments; in the long term, we expect to also receive significant revenues from sales royalties generated by our collaborators upon commercialization of seeds incorporating genes and genetic components we identify. However, because the development cycle of products is lengthy, any revenue from royalty payments may not be earned before six years, if at all. We benefit from a business model in which, pursuant to most of our existing collaboration agreements, a significant part of our operating costs are borne by our collaborators. We self-fund our initial research and development costs in selected projects, with a goal of capturing a larger share of our collaborators’ future revenues. For example, one of our collaboration agreements with DuPont provides for higher milestone and royalty payments as a consequence of fully self-funded initial costs.
Diversified product portfolio and multiple paths of commercialization. Our innovative and adaptable technology infrastructure, scientific and computational expertise, and our close links to major companies in the seed and ag-chemical industry allow us to benefit from multiple business opportunities in the agriculture markets. Our more than ten different collaborations with world-leading seed and ag-chemical companies cover a portfolio of 20 products tailored to address specific market needs across various traits, such as yield enhancement and disease resistance, in a variety of crops, such as corn, soybean and wheat. Under these collaborations, hundreds of genes we identified are currently undergoing early testing in our collaborators’ pipelines. In addition, our expertise in plant genomics and flexible computational technologies will allow us to address new commercial opportunities in agriculture markets, such as discovery of target proteins in the ag-chemical market; and the development of castor bean seeds for second generation feedstock for the biodiesel market. We believe that both activities represent substantial business opportunities in additional large markets.
Broad intellectual property portfolio, claiming protection for thousands of key genes impacting valuable key traits. In the past ten years we have established a broad intellectual property portfolio that includes patents covering key traits such as yield and drought tolerance. We have more than 20 patent families, over 180 national filings and over 30 granted patents covering thousands of key genes and genomic components impacting valuable key traits. We believe that our intellectual property portfolio will enable us to benefit from royalty payments in the long-term and maintain our competitive advantage.
Our Growth Strategy
Our goal is to extend our market experience in improving plant productivity and performance using plant genomics. To achieve that goal we intend to pursue the following strategies.
Expand our innovative technologies in plant genomics. We intend to enhance our competitive advantage by further investing in our technology infrastructure and research and development capabilities in order to improve the process of identification and validation of genes and genomic components. Specifically, with respect to our technology infrastructure, we intend to continue to (i) improve our existing computational technologies through the introduction of novel methodologies and more advanced algorithms, as well as the development of new computational technologies, such as our recently launched Gene2Product™; (ii) develop proprietary genomic data through field experiments utilizing advanced technologies, such as systems for collection and integration of field data; and (iii) improve our plant validation capabilities, such as the recent launch of our unique monocot validation system. In addition, we intend to continue investing resources in understanding the basic biological phenomena in plants at the genomic and molecular level.
Continue to advance our existing collaborations in seed traits. We are focused on executing and advancing our existing collaborations by continuing to provide innovative solutions, thereby maintaining high output quality
71
Table of Contents
standards. We strive to work closely with our collaborators throughout the development processes and support them with our technology infrastructure in order to expedite our genes and genetic components towards commercialization with a view to generating significant milestone and royalty revenues from our collaborations. Our efforts with collaborators has resulted in several of them expanding the scope of our existing collaborations over time, for example the extension and expansion of our collaboration with Monsanto in 2011.
Extend and expand our seed trait project portfolio. We plan to continue to leverage our scalable and adaptable infrastructure, plant genomics expertise and close relationships with agriculture industry leaders in order to (i) address new traits, such as insect resistance; (ii) add new crops to our portfolio, such as sugarcane or selected vegetables; and (iii) commercialize new offerings by extending our current collaborations and introducing new technologies into our existing collaborations. For example, in 2009 we commenced collaboration with Bayer to improve yield in rice, and in 2010 initiated a new collaboration with Bayer to improve the yield, nitrogen use efficiency and abiotic stress tolerance of wheat. Similarly, in 2011, Monsanto extended and expanded the collaboration we entered into with them in 2008 to include gene optimization approach using our Gene2Product™ computational technology in the collaboration projects.
Capture an additional share of the value chain by increasing self-funded research and development. In most of our current collaborations our collaborators fund our direct research and development and associated expenses in the early stages of the discovery process, prior to the delivery and license of genes to our collaborators. We intend to complement our revenue streams by selectively self-funding a larger portion of our direct research and development costs in the initial phases of selected projects and licensing genes to our collaborators at a later stage of the development process, with a goal of capturing a larger share of our collaborators’ future revenues.
Further develop and commercialize our ag-chemical operations. We are leveraging our know-how and computational technologies to position our company at the forefront of the discovery and innovation in ag-chemicals by (i) discovering new modes of action for the development of novel herbicides to mitigate growing weed resistance; (ii) identifying trait-specific chemical active ingredients displaying crop enhancing effects with respect to yield and abiotic stress tolerance; and (iii) leveraging our existing relationships with major seed and ag-chemical companies, such as Bayer and Syngenta, to enter into new collaborations in the ag-chemical sector.
Further develop and commercialize the activities of our subsidiary Evofuel. We intend to further develop our castor bean seed operations towards commercialization. Our key initiatives include: (i) commercializing advanced high yielding castor bean varieties, suitable for commercial production and mechanized harvest; (ii) introducing next generation traits to our castor bean varieties utilizing our genomic technologies; (iii) expanding our existing pre-commercial collaborations with SLC Agricola in Brazil and T6 in Argentina in order to be able to sell our castor beans on a commercial scale; and (iv) seeking new collaborations in additional target markets with key companies.
Our Products
We are a plant genomics company that uses a comprehensive and integrated technology infrastructure to enhance seed traits underlying crop performance and productivity through biotechnology and advanced breeding methods. Our business focuses on three distinct product-driven operations: (i) seed trait (divided into (a) yield and abiotic stress, and (b) biotic stress) (ii) ag-chemical and (iii) seeds focusing on second generation feedstock for biodiesel. We currently generate all of our revenues from our seed trait operations, and a significant majority of our employees are focused on these operations. Our operations in ag-chemicals and seeds focusing on second generation feedstock for biodiesel are in the pre-revenue stage and we have not yet entered into collaboration agreements in the ag-chemical area. We may use a significant portion of the proceeds of this offering to invest in and grow our ag-chemical and seeds focusing on second generation feedstock for biodiesel operations.
72
Table of Contents
Seed Traits
In this field, we use our expertise in plant genomics to improve plant performance through biotechnology and advanced breeding, helping address the global demand for food, feed and fuel. Our proprietary computational technologies, validation techniques and other capabilities enable us to identify promising “candidate” genes and genetic markers, or SNPs (i.e., a single-nucleotide polymorphism, which is essentially a DNA sequence variation that occurs when a single nucleotide, a basic gene “building block,” varies from one DNA sequence to another), that have the potential to improve traits of interest, such as yield, drought tolerance, and disease resistance in commercial (or “target”) crops, such as corn, soybean, wheat, rice and cotton. The most promising “candidate” genes will be used to develop improved biotechnological seeds; the SNPs will be used for conventional seeds improved through advanced breeding. We have entered into collaboration agreements with some of the world’s leading seed and ag-chemical companies, including Monsanto, Bayer, DuPont and Syngenta, which license the trait-improving genes that we generate with the goal of introducing these genes into the seeds of commercial crops.
Our seed traits programs specialize in improving plant yield and increasing plant tolerance to abiotic stress (such as drought, heat and salinity) and resistance to biotic stress (such as disease, pest and insect resistance) through both biotechnology and advanced breeding. The use of biotechnology, or the genetic modification of plants, involves the direct manipulation of a plant’s genome by inserting a gene into the plant’s DNA. This method of plant improvement provides for a wider range of traits in a plant and usually results in more substantial seed trait improvement compared to advanced breeding. Under the advanced breeding method, plants with favorable characteristics are selectively crossed through genomic-guided breeding schemes, with the goal of eventually improving seed traits.
Our product development cycle for seed traits is comprised of six phases. See “—Product Development Cycle—Seed Trait Product Development Cycle.” Currently, we specialize in the upstream portion of the development cycle, particularly in the Discovery phase (i.e., when candidate genes are identified and validated in model plants). In some cases, we also test candidate genes in target plants, as part of Phase I or “proof of concept.” We license our most promising candidate genes to our collaborators with the intent that they will further develop and commercialize the products.
We have over ten collaboration agreements with world leading seed and ag-chemical companies, covering a portfolio of 20 products tailored to address specific market needs across various traits. Under these agreements, hundreds of genes we have discovered are currently undergoing Phase I testing in our collaborators’ pipelines. In addition, under these collaboration agreements, our collaborators have committed to pay us more than $100 million in the form of research and development and related payments, as well as in the form of purchases of our ordinary shares at a premium. This does not include milestone payments, which we are entitled to when our products advance from phase to phase, or royalties, which we expect to be entitled to once our products are sold to farmers. A substantial majority of our collaborations currently focus on improving traits through biotechnology.
73
Table of Contents
The following table sets forth our products currently under development with our collaborators:
| Product # |
Trait |
Crop |
Collaborator | |||
| Yield and Abiotic Stress Tolerance* | ||||||
| 1 |
Yield | Corn | Monsanto, Biogemma (1), DuPont | |||
| 2 |
Yield | Soybean | Monsanto | |||
| 3 |
Yield | Wheat | Bayer (targeting both GM and non-GM products) | |||
| 4 |
Yield | Cotton | Monsanto | |||
| 5 |
Yield | Canola | Monsanto, Viterra | |||
| 6 |
Yield | Rice | Bayer, Rasi Seeds | |||
| 7 |
Abiotic Stress Tolerance | Corn | Monsanto, Biogemma, DuPont | |||
| 8 |
Abiotic Stress Tolerance | Soybean | Monsanto | |||
| 9 |
Abiotic Stress Tolerance | Wheat | Bayer (targeting both GM and non-GM products) | |||
| 10 |
Abiotic Stress Tolerance | Cotton | Monsanto, CIRAD | |||
| 11 |
Abiotic Stress Tolerance | Canola | Monsanto, Viterra | |||
| 12 |
Abiotic Stress Tolerance | Rice | Rasi Seeds, DBN | |||
| 13 |
Nitrogen Use Efficiency | Corn | Monsanto | |||
| 14 |
Nitrogen Use Efficiency | Wheat | Bayer (targeting both GM*** and non-GM products) | |||
| 15 |
Nitrogen Use Efficiency | Cotton | Monsanto | |||
| 16 |
Nitrogen Use Efficiency | Canola | Monsanto | |||
| 17 |
Nitrogen Use Efficiency | Rice | DBN | |||
| Biotic Stress Resistance** | ||||||
| 18 |
Soybean rust resistance | Soybean | DuPont | |||
| 19 |
Nematode resistance | Soybean | Syngenta | |||
| 20 |
Black sigatoka resistance | Banana | Rahan Meristem | |||
| (1) | Biogemma’s shareholders are Limagrain, RAGT, Euralis, Sofiproteol and Unigrain. |
| * | Yield and abiotic stress tolerance refers to a plant’s yield stability over varying environmental conditions and tolerance to environmental stress factors, such as drought and fertilizer utilization. |
| ** | Biotic stress resistance refers to a plant’s disease, pest and insect resistance. |
| *** | I.e., genetic modification. |
Based on a 2013 Phillips McDougall analysis, the cumulative annual future revenue potential for seed companies from key traits of significant biotechnology crops that we and our collaborators are developing is estimated at between $5-8 billion.
Yield and abiotic stress
Initiated in 2004, our yield and abiotic stress programs focus on important seed traits that have a direct impact on crop production and productivity. Pursuant to these programs, we seek to identify and prioritize genes capable of, among other things, increasing crop yield per acre of land, improving plants’ yield and abiotic stress tolerance (i.e., yield stability over varying environmental conditions and tolerance to environmental stress factors, such as drought and fertilizer utilization). Major seed companies have declared their goal to significantly increase crop yield to meet the growing needs of the world population. For instance, Monsanto has indicated that it plans to double the yield in its main crops by 2030. We believe that our advanced technologies, expertise and know-how position us to play an important role in assisting these companies to achieve the ambitious goal of substantially increasing crop yields in the future.
Since we initiated our yield and abiotic stress programs, we have assembled a substantial scientific knowledge center on plant mechanisms and biological pathways associated with yield and abiotic stress traits. Currently, we generate proprietary genomic data from 15 different plant species, and have two model validation plants that can validate over 1,000 genes annually under different greenhouse and tissue culture validation assays (i.e. tests designed to analyze plant performance under specific growth conditions, for instance, measuring a plant’s greenhouse seed yield under simulated drought conditions).
74
Table of Contents
Our most significant collaborations in yield and abiotic stress include two multi-year agreements with:
| • | Monsanto addressing yield, drought tolerance and fertilizer utilization in corn, soybean, cotton and canola through biotechnology. The collaboration period (i.e., the period of active discovery efforts, separate from continuing validation efforts or continuing diligence and development obligations) under this agreement is six years and entitles us to approximately $47.4 million in research and development and up-front payments. The collaboration, initiated in 2008, originally focused on gene discovery using our ATHLETE™ computational technology, but a 2011 expansion of the agreement added new research activities, including the use of our new proprietary computational technology, Gene2Product™, for increased trait efficacy; and |
| • | Bayer addressing yield, drought tolerance and fertilizer utilization in wheat through both biotechnology and advanced breeding. The collaboration period under this agreement is five years and entitles us to approximately €17 million in research and development and up-front payments. Our collaboration with Bayer calls for the use of our gene discovery computational technology, ATHLETE™, as well as our computational technology for advanced breeding, EvoBreed™. |
Our agreements with Monsanto and Bayer, through June 30, 2013, have accounted for approximately $48.4 million in revenues, and in the future could lead to substantial royalty payments if Monsanto or Bayer commercialize products that incorporate genes that we license to them. However, because the development cycle of products is lengthy, any revenue from royalty payments may not be earned before six years, if at all.
Additional collaborations in yield and abiotic stress include our agreement with Biogemma, currently our only agreement in Phase II of the product development cycle. Other agreements with Viterra, DuPont, Rasi Seeds and CIRAD are all in either Discovery or Phase I of the product development cycle.
The following table, based on a 2013 Phillips McDougall analysis, sets forth the estimated commercial value (the “Trait Commercial Value”) of key yield and abiotic stress traits in key crops that we and our collaborators are developing. Trait Commercial Value refers to the total revenue potential for seed companies generated from the premium charged on biotechnology seeds (a “Technology Fee”) due to the added value of the improved trait. Projected Trait Commercial Value was calculated by multiplying the estimated number of acres (per country) that could be planted with a particular biotechnology trait by the estimated Technology Fee that will be charged to growers by seed companies.
| Crop |
Trait | Estimated Trait Commercial Value ($M)1 |
Key growing countries | |||
| Corn |
Yield | 1,200-2,000 | U.S., Brazil, Argentina | |||
| Abiotic Stress Tolerance | 600-700 | |||||
| Soybean |
Yield | 1,000-1,200 | U.S., Brazil, Argentina | |||
| Abiotic Stress Tolerance | 400-500 | |||||
| Cotton |
Yield | 150-250 | U.S., India | |||
| Canola |
Yield | <200 | Canada, U.S. | |||
| Wheat |
Yield | 600-900 | U.S., Canada |
| 1 | The Trait Commercial Value reflects the estimated cumulative value of sales at maturity on an annual basis and is represented by a range derived from Phillips McDougall data. All values are expressed in constant U.S. dollar values in 2013 terms. |
75
Table of Contents
A number of factors could potentially reduce the estimated Trait Commercial Value in the future. These include the following factors as well as other factors described in “Risk Factors”:
| • | Traits may not achieve the desired effect in target plants; |
| • | The function of introduced traits may become less effective over time; |
| • | A market may not exist for certain traits or seeds containing the traits may not be commercially successful; |
| • | Increased consumer or government resistance to genetically modified organisms; |
| • | Failure to obtain regulatory approvals; |
| • | Declining crop prices, which may deter farmers from purchasing genetically modified seeds; |
| • | Adverse economic conditions in the major countries where genetically modified crops are grown; and |
| • | Competitors may launch competing or more effective products, including biological-or chemical-based products. |
In recent years, we have continued to expand our technological capabilities, introducing new computational technologies, such as EvoBreed™ for advanced breeding and Gene2Product™ for increasing trait efficacy, and launching more advanced versions of our computational technologies, including the launch of ATHLETE™ version 4 in 2012. Together with new validation technologies and new data-collection tools, we continue to enhance our ability to correctly link candidate genes with specific traits. These capabilities and assets continue to expand as we enter into additional collaborations and pursue new products. For example, we expect to enter into new collaborations focusing on yield and abiotic stress in the upcoming years for new crops, including rice, sugar beet and other vegetables, and new traits, such as cold tolerance.
Biotic stress
Initiated in 2007, our biotic stress programs focus on improving plant resistance to pests, insects and diseases, including nematodes, soil parasites that attack the roots of developing plants, and soybean rust, a severe fungal disease. To perform research activities relating to biotic stress, we leverage a significant amount of the expertise and know-how acquired in our yield and abiotic stress segment. At the same time, we seek to develop unique technological tools and capabilities aimed specifically at biotic stress traits. For example, we recently bolstered our comprehensive genomic database with data relating to plant-to-pathogen interaction, thus further enriching our candidate gene pool with pathogen genes (as distinct from plant genes). We have also adapted our computational technologies to better address biotic stress traits and developed tailored model validation systems to test and confirm candidate gene impact on model plants. As the resistance of pests, insects and diseases increases to existing products that address biotic stress, the seeds industry is seeking more advanced technological solutions to address these resistance issues. We believe that our cutting-edge technologies, expertise and know-how position us to play an important role in assisting the seed and ag-chemical industry in addressing these resistance issues.
To date, we have entered into three collaborations to develop biotic stress products. These include agreements with:
| • | Syngenta addressing resistance to soybean cyst nematode; |
| • | DuPont addressing resistance to Asian soybean rust; and |
| • | Rahan Meristem addressing resistance to black sigatoka in bananas. |
76
Table of Contents
The following table, based on a 2013 Phillips McDougall analysis, sets forth the estimated Trait Commercial Value of key biotic stress traits in key crops that we and our collaborators are developing.
| Crop |
Trait | Estimated Trait Commercial Value ($M)1 |
Key growing countries |
|||||
| Soybean |
Soybean rust resistance | 200-400 | U.S., Brazil, Argentina | |||||
| Nematode resistance | 400-600 | |||||||
| 1 | The Trait Commercial Value reflects the estimated cumulative value of sales at maturity on an annual basis and is represented by a range derived from Phillips McDougall data. All values are expressed in constant U.S. dollar values in 2013 terms. |
In the coming years, we plan to expand our presence in the field of disease and nematode resistance through new collaboration agreements. As we undertake new collaborations under our biotic stress program—some potentially involving new crops such as corn, wheat, rice and cotton—we expect to broaden our genomic datasets, improve our scientific know-how, update our computational technologies, and develop additional tailored validation assays. In addition, we plan on entering the field of insect resistance, and may also focus in the future on bacteria resistance, virus resistance and herbicide tolerance. We anticipate that this strategic expansion into new biotic stress segments will require a substantial investment of resources on our part, and may involve establishing research and testing infrastructure in the United States.
Ag-Chemical
Our ag-chemical operations utilize our core competency in plant genomics to develop novel crop protection and crop enhancement products. We currently focus on the early stages of the product development pipeline, specifically on discovering new herbicides and crop enhancement products. We initiated our ag-chemical operations in 2012 in order to leverage our existing plant genomics assets and capabilities and apply them to the discovery of biologically significant proteins, or “targets,” and chemical compounds. Our understanding of plant genomics and our capabilities in the area of gene discovery enable us to identify novel targets and, in turn, novel chemical compounds that can be used to develop more effective crop protection and crop enhancement products. Our ag-chemical operations are currently in the development stage and are pre-revenue; we have not yet entered into collaborations in this area. While we believe that our capabilities and assets in seed traits will facilitate our entry into the ag-chemical market, we are still developing the computational technologies, validation systems and tailored genomic datasets that will be necessary for ag-chemical operations.
Our current activities in ag-chemicals are dedicated to the discovery of new herbicides and crop enhancement products. Herbicides and crop enhancers share a similar underlying function: both are comprised of chemical compounds that affect target proteins in the plant. In the case of herbicides, the chemical compounds inhibit the target proteins, resulting in plant death; in the case of crop enhancers, however, the chemical compounds are designed to enhance plant performance. We intend to establish a “chemical library,” which we will use when performing high throughput testing of chemicals. These chemicals will be arranged according to the molecular characteristics of the targets that we discover, streamlining the process of identifying the chemicals with most potential to become ag-chemical products.
In the future, we may expand our ag-chemical operation to different products such as insecticides and fungicides. We may also potentially pursue ag-biologicals, which are products made from natural materials and applied as sprays or seed treatments, complementing or replacing ag-chemicals.
77
Table of Contents
The following is a summary of our current ag-chemical activities:
Herbicide discovery
Invasive plants such as weeds are a major cause of crop loss; they outgrow other plants and deprive crops of large amounts of water and nutrients. Extensive use of herbicides, however, has accelerated weed resistance, creating substantial weed-management problems for growers. We are working to develop new herbicides with new mechanisms that can mitigate the challenge of increasing weed resistance.
Most ag-chemical producers have traditionally relied on a process of trial-and-error in developing their herbicides, a process that involves screening thousands of chemicals against plants or weeds, in the hope that some of these chemicals may kill or damage the plant. This trial-and-error process is often referred to within the industry as “spray-and-pray.” Our herbicide discovery program is designed to assist ag-chemical producers in moving beyond the traditional spray-and-pray method of herbicide discovery by implementing a more targeted approach aimed at identifying and developing novel herbicides with new mechanisms to cope with the growing resistance of weeds. We will utilize our expertise in plant genomics, as well as our advanced technologies and know-how, to discover target proteins, ultimately leading to new herbicides that display new mechanisms or “modes of action.” In essence, we intend to follow the same development process we use in our seed traits operations, beginning with the production of large genomic datasets, moving to the analysis of data using our proprietary computational technologies, and ending with the validation of discovered targets in certain plants. Our PoinTar computational technology, which we are developing and aim to launch in 2013, will be our primary tool for novel target discovery. We have also begun to develop a second complementary computational technology capable of identifying chemical compounds that are likely to inhibit discovered targets, and that therefore may serve as the basis of new herbicides.
Crop enhancer discovery
Crop enhancers are ag-chemicals that are capable of improving crop traits such as yield, fertilizer use efficiency and drought tolerance. In this respect, crop enhancers are designed to achieve the same end goal as our yield and abiotic stress products, except through the use of chemistry rather than biotechnology or advanced breeding. We believe the market for crop enhancers is emerging and very promising. Major seed and ag-chemical companies have invested (and are continuing to invest) significant resources in the pursuit of effective crop enhancers. Similar to crop protection products, crop enhancers are comprised of chemicals that bind themselves to a target in the plant, and through the binding trigger can create an improvement in desired plant traits. As with our biotic stress activities, we plan on leveraging the significant expertise and know-how acquired in our yield and abiotic stress segment, including our innovative computational technologies, tailored proprietary data and established validation assays, to discover and develop novel crop enhancement products. We also plan on utilizing the over 3,500 genes that we have identified during our yield and abiotic stress research, focusing especially on key genes identified as high-impact for yield and abiotic stress traits.
Seeds Focusing on Second Generation Feedstock for Biodiesel
Our wholly owned subsidiary, Evofuel Ltd., or Evofuel, develops seeds for second generation feedstock intended for use in the alternative fuel industry. We initiated these operations in 2007, which were spun-off from Evogene in January 2012 to operate as a separate company. We currently focus on the development of advanced high-yielding castor bean varieties which are not genetically modified (“GM”) and that can serve as a feedstock source for biodiesel and biojet. Our target markets will initially be Latin America, particularly Brazil and Argentina, where there are already large and established markets for growing crops used as feedstock for alternative fuels. We have entered into two collaboration agreements with leading domestic companies in these markets, and expect to benefit from their established agriculture production models.
Castor bean is grown today for its high-quality oil, which is used for various products in the bio-polymers and lubricants industries. Though treated as a “low-tech” crop in its key production areas around the world (e.g., the castor bean is harvested using traditional techniques such as hand picking), the castor bean plant holds great
78
Table of Contents
promise as a source for the alternative fuel industry: oil comprises nearly 50% of the castor bean seed, and the plant itself contains innate characteristics of heat and drought tolerance. We believe that by leveraging our advanced breeding capabilities and methods to improve castor bean seeds, the improved castor bean and the oil it produces can serve as an economic, scalable, sustainable and suitable second generation feedstock for the biodiesel and biojet fuel industries. We believe that the benefits of using castor bean as second generation feedstock include:
| • | Economic. We are developing castor bean varieties that we believe will produce a high-yield-per-land (tons/Ha) with low input; that is, our castor bean crop is designed to be entirely rain fed (and not irrigated). We estimate that the improved yield-per-land ratio is aimed at enabling biodiesel production at an equivalent oil price of approximately $50 per barrel, making castor bean-based biodiesel an affordable and competitive commodity. In recent field trials in Brazil, we were able to demonstrate that our castor bean crop showed improved yields compared to crops grown by local farmers. |
| • | Scalable. We are developing castor bean varieties that can be harvested in a fully mechanized, efficient manner, and that are thus suitable for cultivation on a commercial scale. We have already engaged a leading agricultural machinery company to cooperate on development of mechanized harvest capabilities for castor bean crops. In addition, we have formulated a number of different key agronomic practices for the efficient growth and cultivation of the castor bean, including the use of castor bean as a rotation crop with soybean. |
| • | Sustainable and suitable. A 2010 life-cycle analysis we commissioned from Symbiotic Engineering found that, assuming the enhanced projected yield of our castor bean plant, castor bean biodiesel production and use in Brazil reduces net greenhouse emissions by more than 75% compared to conventional fossil fuel, indicating the castor bean’s potential suitability as a sustainable alternative fuel source. In addition, through a collaboration with the U.S. National Aeronautics and Space Administration, or NASA, and UOP LLC, a Honeywell Company, we successfully demonstrated that castor oil meets the international standards of biojet fuel and is therefore suitable for biojet fuel production. |
In addition to pursuing opportunities in the expanding biodiesel market, we anticipate that our improved castor bean varieties will also be useful for businesses in traditional castor oil markets, where the oil is relatively high priced and is used in a range of industrial products such as bio-polymers, lubricants, paints and cosmetics.
In 2010, we entered into a strategic, pre-commercial collaboration with SLC Agricola, a publicly traded Brazilian ag-business that grows 280,000 hectares of soybean, corn and cotton in Brazil. The collaboration, which involves rotating our castor bean seeds with SLC Agricola’s soybean inventory, focuses on developing improved, high-yielding castor bean varieties. In September 2013, we completed our third year of field trials in SLC Agricola’s plantations in northeast Brazil, and we expect to begin advanced product development and pre-commerical trials in 2014. Our agronomic model in Brazil is based on the practice of “crop rotation,” or the practice of sowing castor bean crops shortly after soybean harvests in order to replenish the soil and preserve its productivity. According to USDA’s Oilseeds and Products Annual Report estimates, in 2012, Brazil had over 27 million hectares dedicated to growing soybean, of which we estimate approximately seven million hectares may be suitable for castor bean growth under the rotation model.
In 2012, we also entered into a pre-commercial collaboration with T6, the leading biodiesel producer in Argentina. The collaboration with T6 aims to develop high-yielding castor bean varieties as a key source for biodiesel production, with Argentina as the target market. Our agronomic model in Argentina, however, is based on growing the castor bean crop in farmlands where food crops are marginally grown, an area that we estimate may be as large as five million hectares. We believe that we stand to benefit from T6’s position as a large-scale producer and exporter of biodiesel, as T6 has a strategic interest in diversifying and reducing the costs of its plant feedstock sources.
Our operations in seeds focusing on second generation feedstock for biodiesel are currently in the development stages and we have not yet sold seeds or generated revenues from these operations. We expect that
79
Table of Contents
sales will not commence for at least three years, however, we cannot guarantee that we will begin marketing and selling a product within this timeframe. Among other benchmarks, we believe that we will be able to begin commercializing our castor bean seeds once we achieve yields on a commercial scale in the target markets and once we have equipment in place for mechanized harvesting on a commercial scale.
Business Model
Our business model is primarily based on collaborations with seed and ag-chemical companies, where we mostly participate in the upstream innovative phases while downstream activities would be undertaken by our collaborators. We generate revenues primarily through annual research and development and milestone payments, and we expect to generate revenues from royalty payments once a commercial product containing our technology is sold.
Seed Traits
Under our current collaboration agreements for seed traits products, we usually conduct the upstream “Discovery” phase, which includes identification of candidate genes or SNPs that can improve the target traits, as well as validation of the candidate genes in our model plant systems. The genes and SNPs we identify are licensed to our collaborators, who continue to evaluate and further develop them, by, among other things: transforming the genes into the target crop and field trial testing of the transformed target crop; advanced testing of the genes or SNPs in elite genetic material; obtaining regulatory approval for a product containing the licensed genes or SNPs; and finally, commercializing a product with the improved traits. Our collaborators are usually required to reach certain development milestones within agreed-upon timeframes in order to retain their license to a gene.
Under our broad, multi-year agreements with our collaborators, we and our collaborators define the “field” on which we will collaborate on an exclusive basis during the collaboration period. The “field” includes specific trait(s), crop(s) and technology(s) (e.g., biotechnology or advanced breeding). For example, under our collaboration with Bayer, we granted Bayer an exclusive license in a “field” that consists of (i) the improvement of yield and abiotic stress tolerance, (ii) in wheat, (iii) using biotechnology and advanced breeding technologies, (iv) for a period of five years.
We currently generate revenues from our seed traits operations through research and development payments to cover the costs of our research and development activities and milestone payments received upon the achievement of certain specified results in our collaborator’s pipeline, for example when a candidate gene or SNP advances a phase in the product development cycle, or when a product containing the genes or SNPs is submitted for regulatory approval. We also expect to generate royalty payments once a commercial product containing our genes or SNPs is launched by a collaborator. Currently, in most of our existing collaboration agreements in which our collaborator funds the discovery phase, the standard royalty range is between 5% and 12%. Royalty payments are usually calculated as a percentage of the premium charged on the sale of the seeds containing our licensed genes or SNPs compared to the sale of similar seeds without the genes or SNPs. However, because the development cycle of products is lengthy, any revenue from royalty payments may not be earned before six years, if at all. These royalty payments are usually calculated as a percentage of the premium charged on the sale of the seeds containing our licensed genes or SNPs compared to the sale of similar seeds without the genes or SNPs.
With respect to selected traits, we intend to increase our share of our collaborators’ future revenues through larger future milestone and royalty payments from our collaborators by either (i) self-funding a larger portion of our direct research and development costs in the Discovery phase, or (ii) capturing an additional share of the product development value chain by sharing the development costs with our collaborators beyond the Discovery phase. Currently, in our existing collaboration agreements in which we fund the discovery phase, the standard royalty range is between 8% and 18%. Royalty payments are usually calculated as a percentage of the premium
80
Table of Contents
charged on the sale of the seeds containing our licensed genes or SNPs compared to the sale of similar seeds without the genes or SNPs. We expect the cost-sharing model to generate even greater royalty ranges than the self-funding model. For example, as part of our 2011 collaboration with DuPont, aimed at developing soybean varieties that are resistant to soybean rust, we agreed to finance our initial research and development activities in return for improved commercial terms in later stages. Additionally, we hold a contractual option with DuPont to co-invest in the development costs of a product up to the date of its commercial launch for even greater royalty percentages.
Ag-Chemical
We have not yet entered into any collaboration agreements in our ag-chemical operations. As with our seed trait activities, we anticipate that our activities in the ag-chemical market will focus on the initial research and development phases of the product development cycle. In essence, when developing a herbicide, we intend to discover and validate targets that, when inhibited, result in plant death; in a parallel process, we also intend to identify chemical compounds capable of inhibiting these targets. The chemical compounds that most effectively and consistently inhibit the targets may serve as a basis for new herbicides.
We expect to complete the development of our computational technologies for ag-chemical operations (including the PoinTar platform used for identifying novel targets) in 12 to 18 months, which we expect will facilitate our ability to enter into new collaboration agreements in this area. However, we cannot provide any assurance that we can complete these computational technologies or enter into new collaborations in this timeframe, if at all.
We anticipate that our business model for our ag-chemical operations will be similar to the business model in our seed traits operations.
Seeds Focusing on Second Generation Feedstock for Biodiesel
Through Evofuel, we expect to generate revenues from seed sales to various agriculture companies and large-scale seed growers. Although we will focus initially on the markets in Brazil and Argentina, we plan on eventually expanding our seed sales to other markets around the world.
Currently, as part of our research and development activities in seeds, we collaborate with companies that have the potential to become our future seeds customers, such as SLC Agricola and T6. We expect that these collaborations, which have not yet generated revenues and are currently in the early stages of developing a seed product, will facilitate eventual commercialization of improved seeds, once we have a product ready for launch. Furthermore, once we are able to commercialize our own seed product, we may decide to advance our activities further down the value chain. For example, we may enter into collaborations with leading agribusinesses to establish and operate large-scale production projects, covering thousands of hectares of farmland, for the commercialization of castor bean grain (i.e., castor bean seeds from which the oil has been extracted).
We currently do not expect to begin selling seeds under our Evofuel operation for at least three years.
Technology Infrastructure
We believe that we have achieved a unique position in the seed and ag-chemical industry through our ability to effectively integrate and analyze massive amounts of complex genomic data for the purpose of improving plant performance. Our technology infrastructure facilitates all of our product-driven operations: seed traits, ag-chemicals and seeds focusing on second generation feedstock for biodiesel. This infrastructure, which is highly flexible and synergistic, provides us with the means of integrating our plant genomics core competencies. Specifically, our technology infrastructure is comprised of four enablers that are key to our leading position in plant genomics: (i) plant science know-how and expertise, continuously enriched through advances in our
81
Table of Contents
discovery programs as we address new traits and crops; (ii) vast amounts of data generated in-house or collected from public sources, tailored to support hypotheses we develop based on our scientific know-how; (iii) computational technologies that integrate, assemble and mine the vast amount of genomic data; and (iv) validation systems and assays in various plants, used to validate the discoveries made through our computational technologies.
We continuously strive to improve and expand our technological capabilities. Since our initiation in 2002, we believe that we have developed valuable computational technologies containing unique features that cannot be found elsewhere in our industry. We intend to continue investing in our research capabilities in order to expand our technological capabilities in plant genomics and continue to provide innovative solutions to our collaborators.
Science and Know-how
Our research and development activities involve 147 employees as of June 30, 2013, amounting to approximately 78% of our total full-time workforce. Our staff possesses multidisciplinary and wide-ranging expertise, with employees specializing in biology, chemistry, plant genetics, agronomics, mathematics, computer science and other related fields. Additionally, 48 of our employees hold a Ph.D. Our physical research and development facilities are located near the agricultural and biotech hub in Rehovot, Israel, and we benefit from continuing professional relationships with members of the agriculture and plant-science academy. Furthermore, we employ a Scientific Advisory Board composed of representatives from the Faculty of Agriculture of the Hebrew University in Jerusalem, the Weizmann Institute of Science in Rehovot and other institutions of higher learning.
Our internal research and development activities are divided into three groups. Our first group develops our technological infrastructure to facilitate ongoing projects, including our computational technologies, our technologies to harvest genomic and phenotypic data and our validation systems. Our second group manages our trait discovery programs that focus on identifying genes, SNPs and other DNA fragments pursuant to our collaboration agreements or internal independent research projects. Researchers in this group develop the hypotheses that guide our trait discovery programs, design the type and scope of genomic data generation, determine data-mining queries run on our computational technologies and decide the type of model plant validation to be used. Our third group, upon the instruction of the second group, executes our trait discovery programs, which include genomic data generation and integration, computational discovery, gene cloning and insertion into plants and model system validation.
We are constantly improving our scientific skillset and know-how. As we enter into new collaborations involving new traits and new crops, we are able to leverage our existing know-how and enrich our genomic knowledge and capabilities. We also intend to leverage this know-how to expand our operations into new markets in which we currently do not operate, as we did when we commenced our ag-chemical operations in 2012.
Genomic Databases
The past decade has witnessed an explosion of genomic-related data, greatly expanding the reach of plant genomics. To successfully manage and data-mine the large and complex range of information presently available, we continue to develop increasingly sophisticated data-processing tools capable of synthesizing the information and enabling new discoveries and insights. While our genomic databases draw in part on the public domain (primarily from academic institutions and research publications), we are compiling increasing amounts of proprietary data, generated either in-house or received from our collaborators. Our database covers over 200 plant species, and accounts for various data types, including phenotypic (i.e., data related to a plant’s observable characteristics, morphology, development and physiological properties) and genotypic (i.e., data from the molecular level, derived from DNA, RNA or other sources).
82
Table of Contents
Our in-house data generation activities are able to support up to 40 experiments and field trials a year based on 15 different crops. In those trials, plants can undergo a dozen different treatments during the growing seasons, producing data that supports or refutes our trait researchers’ scientific hypotheses. For example, to support a discovery effort for drought-tolerant genes in corn, we may design specific experiments for a variety of crops, such as corn, wheat and sorghum, and test their resistance to different stress conditions. By pursuing parallel yet related experiments, we improve our ability to identify, in the computational discovery stage, key genes that are triggered in crops under stress. We expect to continue to expand the scale of our data-generation activities: for example, the large-scale wheat trials that we are conducting in Israel involve our most extensive and wide-ranging data-collection efforts to date, comprised of approximately 200 wheat lines, numerous testing conditions and hundreds of thousands of data points.
We also rely on our large seed bank with an inventory of approximately 60 species and sub-species of seeds and more than 4,000 imported seed lines. All together, we are able to record more than two million phenotypic data points directly from the field each year. For example, through the use of our Phenomix computational tool, we are able to collect, store and integrate phenotypic data directly from surveillance of field trials, evaluating crop behavior in controlled environments that closely resemble the growth conditions existing in commercial agriculture. This tool combines a number of modified sensors, digital imaging cameras and other devices to create a real-time and direct field-to-computer research channel.
To organize and process the vast amount of information available to us, we have developed a sophisticated internal IT system of central processing unit clusters and over 670 processing cores, capable of storing and analyzing the more than 500 terabytes of data that we have collected and generated to date. We also continue to pursue and develop innovative approaches to data transmission and storage.
Using field trials and advanced technologies for the collection and integration of different data types, we intend to continue developing and expanding our proprietary genomic database. We are focusing in particular on compiling the data for the “chemical library” we are designing to be used in conjunction with PoinTar for our ag-chemical operations.
Computational Technologies
We believe that the key to future innovation in the seed and ag-chemical industry has shifted from data creation to data integration and data analysis, focusing on the ability to harness and mine the vast amounts of genomic data that has become available over the last decade. Our computational technologies, utilized for data integration and analysis, are central to our operation as a plant genomics company, and we believe that we are positioned at the forefront of this shift in industry focus. We have developed advanced proprietary computational technologies comprised of novel algorithms and methodologies, which are designed to handle immense amounts of data. For example, our ATHLETE™ technology assembles and mines genomic data on millions of genes, resulting in tens to hundreds of genes that we are able to predict will be “key” genes for improving a desired trait. Each of our computational technologies includes a stage of database assembly, whereby any “junk” data is removed and a reliable, comprehensively detailed and integrated through building a structured gene-centric database. The next stage of each technology includes mining the database through hundreds of queries that utilize various methodologies and algorithms.
We believe the key features of our computational technologies are:
| • | Novel: Substantially all of the methodologies and tools utilized by our computational technologies were developed in-house and are proprietary and unique in the industry. |
| • | Reliable: We apply our methodologies and statistical tools to meaningfully sort the data we receive and have quality assurance processes to ensure the reliability of the outputs we generate. |
| • | Flexible: Our computational technologies are not restricted to a certain crop or trait, and thus permit us to continuously focus on new crops and traits and enter new fields in plant genomics that foster product innovation. |
83
Table of Contents
| • | Learning: As we generate new information related to our discovery efforts and validation results, our computational technologies are able to integrate this information and generate new computational solutions. We also continuously monitor and improve the performance of our existing tools and expand our capabilities. |
| • | Efficient: In our experience, in most cases, a period of only six to nine months is required to complete the discovery process for “key” genes, SNPs or other DNA fragments. |
We intend to continue to improve our existing computational technologies through novel methodologies and enhanced algorithms and to develop new technologies, both of which we expect will allow us to address new plant genomics fields and the arising needs of seed and ag-chemical industry pipelines. Where appropriate, we may also enter agreements with third parties to bolster our technological capabilities.
We today operate and develop the following computational technologies:
ATHLETE™
The ATHLETE™ computational technology was launched in 2006 and is our central computational technology for plant gene identification, comprised of unique algorithmic tools and novel data-mining concepts that allow generation of rapid and reliable lists of genes relevant to a target trait.
Using this technology, we are able to capture and dissect vast amounts of genomic data types from various plant species and other species and engage in the efficient discovery and prioritization of hundreds of genes linked to desired traits. Fundamentally, ATHLETE™ relies on statistical analysis and biological rationales to determine whether a certain gene is linked to a desired plant trait, facilitating the use of the gene to develop biotech-based traits. This technology is a gene-centric tool that links all available data relevant to a gene in a single assembled database. Such data includes available information on the gene’s biological activity, its molecular characteristics, and any available correlation between the gene’s phenotype and its activity on the molecular level. The data also includes the same type of information for similar genes in other plant species. Through hundreds of queries, the system is able to prioritize the genes linked to a desired trait. ATHLETE™ is one of our most versatile technological tools as well; we apply this tool to different traits and crops, all according to the needs of our various internal programs and collaboration agreements.
ATHLETE™ is the computational technology used in most of our collaborations, including our broad, multi-year collaborations with Monsanto and Bayer. Though our ATHLETE™ technology is already capable of cutting-edge data processing and analysis, we are continuing to make improvements and introduce new features to this technology, creating a faster and more efficient analytical tool. We have improved various aspects of ATHLETE™ since the launch of the technology in 2006; we launched version 4 in April 2012 and expect to launch version 5 later this year.
Gene2Product™
The Gene2Product™ technology was launched in 2013, although components of the tool have been used since 2010. Gene2Product™ is a unique integrated computational technology to develop biotechnology seed traits—by high throughput optimization of selected gene function in a target crop (which we refer to as “mode of use”). This technology complements our ATHLETE™ technology: efficacy of a gene depends not only on the presence or absence of the gene of interest, which is determined by ATHLETE™, but also on the optimization of the gene with other factors related to the mode of use of such gene, which is determined by Gene2Product™. Such factors include the choice of gene variant for the crop of interest, the interaction of the gene with other genes, the tissues in which the gene is expressed, the level and/or pattern of expression of the gene and the gene’s performance under changing environmental conditions. Gene2Product™ is designed to improve trait efficacy for certain genes identified (for example by ATHLETE™) through the following tools:
| • | PlaNet (Plant Network), which improves trait efficacy when approaching complex traits, such as yield, by predicting appropriate combinations of the identified gene with additional genes, designed to jointly |
84
Table of Contents
| impact the trait when combined, and prioritize possible combinations with respect to their ability to improve a given trait; |
| • | GeneSpec (Gene Spectrum), which selects the preferred variants for the selected gene of interest for the crop of interest by identifying and classifying, up to 1,000 possible variants per gene, through the use of novel algorithms, according to sequence-function and other relationships; |
| • | Repack (Regulation Package), which predicts the regulation mode for the selected gene that will provide the optimal expression pattern, including predicting where in the plant the expression would be beneficial and where it would be undesired in respect of tissue, organ, timing, level of expression and other aspects that can impact trait efficacy; and |
| • | GeneDex (Gene Index), currently under development, which predicts functional robustness of the selected gene across different genetic backgrounds and environmental conditions, providing multiple relative index scores for each gene predicting such gene’s contributions with respect to each trait of interest across different combinations of such variables. |
EvoBreed™
The EvoBreed™ computational technology was launched in 2010. EvoBreed™is our computational technology for discovery of SNPs, to enhance advanced plant breeding, designed to offer reliable correlations between genetic data and plant phenotype. Like ATHLETE™, EvoBreed™ specializes in comprehensive cross analysis, tapping into the extensive genomic datasets we have collected. The result is a prediction of SNP-to-trait association. The ultimate purpose of EvoBreed™ is to enable plant breeders to design optimal crosses between breeds, enhancing a desired trait or set of traits, allowing for logical and insightful breeding decisions, to accelerate and correct the breeding process from start to end.
PoinTar
We are currently developing a computational technology for our ag-chemical division, PoinTar, which we aim to launch this year. This tool will specialize in the identification of targets (proteins) for development of ag-chemicals such as herbicides. This technology is target-centric and integrates data aimed to predict the potential impact that a target, when inhibited, would have on a weed. In addition to integrating the tools available in ATHLETE™, PoinTar addresses the structural characteristics of a target in order to predict the target’s likelihood of binding to a small chemical molecule for use as a herbicide.
Validation Systems
Efforts to improve traits of interest in key crops require both the predictive tools of our computational technologies and the experimental tools of our validation systems, which allow us to test the actual performance of our predictions in plants. Such validation efforts typically begin with model plant systems, utilizing Arapidopsis and Brachypodium, which offer a faster and more efficient high throughput means of initially evaluating and prioritizing candidate genes, SNPs and other DNA fragments. Initial testing in model plants can usually provide a result within one year, whereas validation in target crops such as corn usually requires two to three years. Once the prioritized genes pass proof of concept in the model plants, they then undergo further validation in the target crops.
Our validation systems, which employ state-of-the-art facilities and techniques, allow high throughput and efficient plant validation of over 1,000 new genes per year. We primarily use our validation systems to evaluate genes for yield, drought tolerance and nitrogen use efficiency, however, we have also developed validation systems for other traits, such as our Arapidopsis-based validation for nematode resistance. The plant validation process is cross-disciplinary, requiring the use of molecular biology and plant transformation facilities, extensive greenhouse trials, and the application of advanced imaging and data-analysis techniques. Our candidate genes are first identified in silico (i.e., via computer) using our advanced computational technologies. These genes are then cloned and transformed (or transferred) into model plants, which, due to their short life cycles, well-known
85
Table of Contents
genomes and ease of cultivation, offer an efficient way of assessing gene impact. We use Arabidopsis as our model plant for dicots (i.e., a plant with two embryonic seed leaves such as soybean, canola, cotton and sunflower) and Brachypodium as our model plant for monocots (i.e., plants with a single embryonic seed leaf such as corn, rice and wheat). We are able to validate over 1,000 genes per year using the Arabidopsis model plant, with 16 types of experiments utilizing both our greenhouses and tissue-culture validation techniques. Through our recently launched Brachypodium system, we are able to additionally test over 100 genes per year through six types of experiments that take place in our greenhouses. We believe that we are the only company that has a high throughput Brachypodium model plant validation system.
Our molecular labs and tissue-culture facilities employ automated equipment and state-of-the-art technologies, greatly increasing our output of viable candidate genes. The bulk of the validation process, however, is conducted across our 34 cutting-edge GM greenhouses on 24,600 square meters (or approximately 264,800 square feet) of farmland. Our greenhouses have the capacity to accommodate over 15 established experiments at any given time. Each experiment involves dozens of genes and hundreds of model plants to allow for statistically meaningful results. In addition, each greenhouse is rigorously controlled for temperature, irrigation, light, nitrogen, salinity and other variables, allowing for controlled testing, monitoring and continuous year-round growth. Data from the plants are routinely acquired during the greenhouse trials and automatically fed into a statistical analysis system. The results are then evaluated by our trait researchers in order to prioritize the most promising genes.
In certain cases, certain prioritized genes are introduced into target crops and assessed in field trials following experiments in model plants. We operate field trial sites in various locations in Israel and collaborate with local partners to achieve more rapid and comprehensive field-test results.
We intend to continue to improve our plant validation capabilities by developing validation systems for new traits, improving the capacity and quality of our current validation systems and generally enhancing our facilities and quality assurance competencies. Where appropriate, we may also enter agreements with third parties to bolster our plant validation capabilities. For example, in 2013, we entered into an agreement with Plant Array for the use of Plant Array’s computer-based technology for high throughput screening. During the term of the agreement, we agreed to provide Plant Array with management support and access to a greenhouse as the technology is further tested.
Key Collaborations
Our seed trait projects are conducted through collaborations with leading seed and ag-chemical companies, with whom we share the development process of improving plant performance. In most cases, we generate revenue from our collaboration agreements at three different points: first, we usually receive research and development services payments to cover the costs of our research, including our gene discovery and validation efforts; second, we receive milestone payments when certain specified results are achieved, such as when a candidate gene progresses to a later phase in the product development cycle, or when a product containing our traits is submitted for regulatory approval; finally, we expect to receive royalty payments once a commercial product containing our traits is launched into the market. Royalty payments will generally be made for the longer of a specified number of years after product launch, or for the duration of our applicable patents in the United States.
Our principal collaborations are with Monsanto and Bayer which together accounted for 94% of our revenues for the year ended December 31, 2012, of which Monsanto accounted for 70% and Bayer accounted for 24% of our total revenues. Monsanto and Bayer, accounted together for 98% of our revenues for the six months ended June 30, 2013, of which Monsanto accounted for 66% and Bayer accounted for 32% of our total revenues. We also entered into share purchase agreements with Monsanto and Bayer, and a portion of the amounts paid under each of the share purchase agreements were considered to be advances under each of the collaboration agreements with Monsanto and Bayer. See “—Share Purchase Agreements.” We also have collaborations with other leading seed and ag-chemical companies, including DuPont, Syngenta and Biogemma, as well as companies such as Rahan Meristem, Rasi Seeds, CIRAD, Viterra, Zeraim Gedera and CDB Technologies. These additional collaborations do not currently account for a material portion of our revenues.
86
Table of Contents
Monsanto
2008 Collaboration Agreement, Amended and Restated in 2011
(i) Background and Duties
In August 2008, we entered into a Collaboration and License Agreement with Monsanto. This agreement was amended and restated in November 2011 to extend and expand the original agreement executed in 2008. This agreement, which we refer to as the “Monsanto Collaboration Agreement,” represents a significant portion of our current revenues. Pursuant to the terms of the Monsanto Collaboration Agreement, Monsanto funds a research program in which we apply two different proprietary computational technologies: (i) ATHLETE™, our computational gene discovery technology, used in this case to identify genes with the potential to improve yield, nitrogen use efficiency and abiotic stress tolerance in corn, soybean, cotton and canola, and (ii) Gene2Product™, our computational gene optimization technology, used to improve gene performance through recommendations on how to use the genes we identify in the target crop (e.g., corn). All of the genes that we discover are to be tested and validated by us in our model plants. The collaboration period under the Monsanto Collaboration Agreement (i.e., the period of active computational discovery efforts, separate from validation efforts that may follow) is six years, scheduled to expire in August 2014, and is followed by more than a year of validation activities. The collaboration period is subject to extension for two additional years if Monsanto exercises an extension option by February 1, 2014.
(ii) License Grants
Under the terms of the Monsanto Collaboration Agreement, we have granted Monsanto an exclusive, royalty-bearing, worldwide license under our patents and know-how to commercially exploit and conduct research on (i) the genes we discover and patent under the collaboration, and (ii) the recommendations stemming from our gene-optimization activity under the collaboration, each solely for transgenic applications in the specified crops. In addition, we have granted Monsanto certain ancillary research and development licenses, including, among others, a non-exclusive license to use the genes we generate and patent and the recommendations under our gene-optimization activity for research purposes in certain plant species specified under the agreement. As part of its consideration for these license grants, Monsanto agreed to provide us with research and development services payments, development milestone payments upon the occurrence of certain milestone events, and royalties based on the value added to each product as a result of either incorporating our licensed genes, or of applying our licensed recommendations under the gene-optimization activity. Royalty payments will generally be made for a specified number of years after product launch, or for the duration of our applicable patents in the United States.
In addition to the licenses we have granted to Monsanto, we have agreed for the duration of the research program under the Monsanto Collaboration Agreement (x) to not license or otherwise transfer to any third party the right to use in the specified crops for any transgenic application: (i) any gene we discover for the specified traits or (ii) any recommendation we make under our gene-optimization activity for the specified traits, and (y) to not engage or pursue any collaboration or other activity with a goal of (i) discovering genes that confer the specified traits in the specified crops for any transgenic application, or (ii) formulating recommendations with respect to gene-optimization for the specified traits in the specified crops for any transgenic application.
(iii) Diligence Obligations
We and Monsanto both have minimum diligence obligations under the Monsanto Collaboration Agreement: our diligence obligations surround (i) the discovery and research of candidate genes, and (ii) the discovery and research of recommendations using our gene-optimization technology, while Monsanto is obligated to test a specified number of these genes and recommendations for the purpose of ultimately developing and commercializing products containing the genes. For example, we have agreed to perform a minimum number of computational discovery efforts (or “generation rounds”) and deliver a minimum number of genes to Monsanto.
87
Table of Contents
A failure by us to meet our diligence obligations may have the effect of eliminating or reducing certain Monsanto diligence obligations, and diligence failures by Monsanto may result in the termination of certain of its licenses. While Monsanto has certain diligence obligations under the Agreement, there is no express requirement that it actually commercialize any products using the genes that we license to it.
The effects of failures by either party to perform other obligations under the Monsanto Collaboration Agreement are limited to impacts on particular rights and obligations under the agreement, and do not give rise to a general right to terminate the agreement entirely. For example, in the event of certain uncured breaches by Monsanto, some of the licenses we have granted to it would remain in effect, while others would terminate. In the event that we breach certain provisions of the Monsanto Collaboration Agreement and fail to cure these breaches, Monsanto’s licenses remain in effect but it may elect to cease further research activity, stop making annual research and data-generation payments for the relevant project, and have no further diligence obligations with respect to the project.
(iv) Change in Control
In the event that we experience a change of control, the majority of provisions under the Monsanto Collaboration Agreement would remain in full force and effect. However, if we come under the control of one of Monsanto’s competitors: (i) the research portion of the Monsanto Collaboration Agreement may be terminated either fully or in part by Monsanto, and if it is not terminated, we become subject to increased diligence obligations; and (ii) the timing of certain milestone payments and the duration of certain royalty payments due to us under the agreement may also be affected.
(v) Consideration and Costs
As of June 30, 2013, we had received approximately $37.6 million in research payments under the Monsanto Collaboration Agreement. This includes an up-front payment of $5 million paid upon entering into the agreement, as well as annual data generation and periodic research and development service payments. Between now and the completion of our research and development activities under the collaboration (i.e., our active discovery efforts, our continuing validation efforts and our continuing diligence obligations), which is scheduled to occur in 2015, and in the event that Monsanto does not exercise its extension option, we expect to receive an additional $12 million in research and development services payments from Monsanto. Although we have not yet begun to earn milestone payments or royalty payments, and may never earn such milestone payments or royalty payments, Monsanto also is obligated under the Monsanto Collaboration Agreement to provide us with royalty payments on any sales or other transfers of products it develops containing our licensed genes. These royalty payments are generally calculated as a percentage of the premium charged on the sale of the seeds containing our licensed genes compared to the sale of similar seeds without the genes. In addition, if Monsanto exercises its option to extend the initial research term for an additional two years, Monsanto will pay us a fee of $6 million for the extension of our exclusivity undertakings (described above) as well as an additional $26 million in research and development services payments during the extension period.
In August 2008, Monsanto purchased 1,636,364 of our ordinary shares at a price per share of $11.00, for an aggregate investment of $18.0 million. Prior to the completion of this offering, Monsanto held approximately 8.7% of our outstanding equity. In addition, we hold an irrevocable put option, exercisable beginning February 2014 until August 2014, pursuant to which we can require Monsanto to purchase 500,000 additional ordinary shares at a price per share of $24.00 for an additional aggregate investment of $12.0 million. However, in the event that Monsanto exercises its option to extend the Monsanto Collaboration Agreement for two additional years, then the put option allows us to require Monsanto to purchase only 214,286 additional ordinary shares at a price per share of $28.00 for an additional aggregate investment of $6.0 million.
88
Table of Contents
2007 Collaboration Agreement
We entered into our first collaboration agreement with Monsanto in September 2007. Under this agreement, structured according to an earlier business model, we granted Monsanto certain research licenses and an option to obtain an exclusive, royalty-bearing license to one or more of a group of genes with the potential to improve nitrogen use efficiency in corn, soybean, cotton and canola. While the period for Monsanto to evaluate the candidate genes in target crops was originally set to expire in September 2012, we and Monsanto mutually agreed to an extension of this period into 2013, and Monsanto has subsequently exercised its rights under the agreement to extend this period through January 2014. As of today, we have completed the performance of our obligations and are no longer performing any significant research under this agreement. This agreement accounts for only a very small percentage of our current revenues.
Bayer
2010 Collaboration Agreement
(i) Background and Duties
We currently have two research and development agreements with Bayer CropScience LP, an affiliate of Bayer CropScience AG, or Bayer. The later of the two agreements with Bayer is the collaboration agreement entered into in December 2010, which we refer to as the “Bayer Wheat Agreement.” This agreement focuses on the improvement of yield, nitrogen use efficiency, and abiotic stress tolerance of wheat. Pursuant to this agreement, and upon Bayer’s request, the parties may agree to expand the scope of research to any other trait in wheat. The initial term of the research period under the Bayer Wheat Agreement—i.e., the period of active computational gene and SNP discovery—is five years, expiring January 2016. The agreement also gives Bayer an option to extend the agreement for an additional year or, under certain conditions, to terminate the agreement in full or in part prior to the conclusion of the research period.
As in most of our collaboration efforts, the Bayer Wheat Agreement involves the use of our ATHLETE™ computational technology to identify and prioritize candidate genes with the potential to improve specified traits in wheat through the application of our proprietary technologies. More uniquely, however, the Bayer Wheat Agreement also requires the application of EvoBreed™, our computational technology for advanced breeding applications, which is used to identify SNPs capable of enhancing individual plant features and ultimately leading to enhanced traits. The genes that we discover undergo model plant validation.
(ii) License Grants
Under the Bayer Wheat Agreement, we granted Bayer an exclusive, worldwide, royalty-bearing license under certain of our patents and know-how to use, solely in wheat, certain genes and SNPs we identify during the collaboration in order to grow, commercialize and sell wheat products containing those genes or SNPs. We also agreed that during the collaboration period we would not: (i) license to any third party the right to use any of our patented genes or any of our SNPs in wheat for any transgenic or breeding application in wheat, regardless of trait; or (ii) undertake any discovery rounds that intentionally target genes or SNPs that may improve the specified traits specifically in wheat in collaboration with or for the benefit of any third party. However, we may freely license our genes to any third party for use in any crop other than wheat, and similarly may freely use our wheat datasets and any other Evogene datasets with respect to crops other than wheat for any and all purposes.
(iii) Diligence Obligations
Pursuant to the Bayer Wheat Agreement, both we and Bayer are subject to certain minimum diligence obligations, which relate to our respective responsibilities under the agreement: our own diligence obligations surround the discovery and research of candidate genes, as well as the discovery of candidate SNPs; Bayer’s diligence obligations require Bayer to evaluate a specified number of genes and SNPs for the purpose of
89
Table of Contents
ultimately developing and commercializing products containing the genes or SNPs. For example, by the conclusion of each year in which we undertake an ATHLETE™ discovery round, we are obligated to provide Bayer with a certain minimum number of reports reviewing the candidate genes that we have identified as having the potential to improve the target traits in wheat. Similarly, following each EvoBreed™ discovery round, we are obligated to provide Bayer with a certain number of reports reviewing the candidate SNPs that we have identified as having the potential to improve a target trait. For its part, Bayer is obliged under the Bayer Wheat Agreement to introduce a minimum number of our genes and SNPs into wheat plants and to perform field trials using the genes and SNPs that we deliver, with the goal of ultimately developing, launching and marketing commercial products containing our licensed genes and SNPs.
Various provisions under the Bayer Wheat Agreement lay out the consequences of either party’s failure to perform its diligence obligations. Absent certain extenuating circumstances, if we fail or are delayed in delivering the specified number of gene or SNP reports, we may have to complete and deliver the missing deliverables over an extended timeframe at no additional cost to Bayer, or reimburse Bayer for the applicable research fees paid in respect of such deliverables. Under certain circumstances, Bayer may also be able to terminate the research and development program under the agreement. If, on the other hand, Bayer does not fulfill its obligations, including its development obligations, within the relevant timeframes under the agreement, Bayer may lose its license to the relevant genes, or for SNPs, Bayer’s license to the relevant SNPs may become non-exclusive.
(iv) Change in Control
If we experience a change of control, the Bayer Wheat Agreement would remain in effect. However, if we come under the control of a Bayer competitor, Bayer may elect to terminate the research and development collaboration, including all of our activities under the collaboration. In such a case, Bayer would no longer have to provide us with annual research payments.
(v) Consideration and Costs
As of June 30, 2013, we had received approximately €7.6 million in research payments under the Bayer Wheat Agreement including an up-front payment upon entering the Bayer Wheat Agreement. Bayer also agreed to make certain annual research and development services payments during the collaboration period in addition to milestone payments upon the achievement of agreed-upon results. Between now and the completion of our research and development activities under the collaboration, which, subject to a right of earlier termination, is scheduled to occur in 2017, we are due to receive €10.7 million in research and development services payments. In the event that Bayer launches commercial wheat products containing one or more of our licensed genes or SNPs, Bayer will have an obligation to provide us with royalty payments based on the additional sales value conferred by the improved crop trait we identify for Bayer. Under our agreement, Bayer is required to use at least the specified levels of diligence in developing and commercializing the applicable products. Furthermore, Bayer’s royalty obligations run for periods specified based on patent coverage in the applicable country and/or a minimum period post-launch.
In connection with entering into the Bayer Wheat Agreement, in January 2011 Bayer purchased 863,310 of our ordinary shares at a price per share of $13.90, for an aggregate investment of approximately $12 million. Prior to the completion of this offering, Bayer held approximately 4.6% of our outstanding equity.
2009 Collaboration Agreement
Our first collaboration agreement with Bayer, entered into in April 2009, as amended and restated in June 2011, which we refer to as the “Bayer Rice Agreement,” required us to use our seed trait know-how and our computational technologies to identify genes that have the potential to improve the yield of rice under standard, abiotic, and sub-optimal nutrient conditions. The collaboration period under the Bayer Rice Agreement expired
90
Table of Contents
in April 2012. However, while this date marked the end of our own active research efforts with respect to rice-improving genes, Bayer remains responsible under the agreement to provide us with milestone and royalty payments should it decide to advance our candidate genes or related technology in its product development pipeline or license the genes or related technology in future rice products.
Other
DuPont
2007 Collaboration Agreement
In 2007, we signed an evaluation and commercial license agreement with Pioneer Hi-Bred International, a DuPont entity, focusing on the improvement of yield and tolerance to abiotic stress in corn and soybeans. In 2012, DuPont notified us of its election not to further evaluate the genes in soybeans. Pursuant to the agreement, DuPont must use reasonable efforts to develop or commercialize corn and soybean products containing our licensed genes. In the event that DuPont fails to achieve certain milestones within specific timeframes under the agreement, DuPont may forfeit its license to some or all of our genes.
Under this agreement, we granted DuPont: (i) an exclusive, research-only license to certain genes identified by our ATHLETE™ computational technology in corn and soybean, and a non-exclusive research only license in other plants; and (ii) an exclusive license to commercialize transgenic applications of such licensed genes in corn and soybeans. Pursuant to the agreement, DuPont agreed to pay us an up-front payment and annual licensing fees, to provide us with milestone payments upon achieving certain results, and to provide us with royalty payments once DuPont begins selling improved corn or soybean products containing our licensed genes. DuPont has the right to terminate the agreement at any time without cause, but if it exercises that right it loses the licenses we granted it under the agreement.
2011 Collaboration Agreement
(i) Background and Duties
In 2011, we entered a second multi-year research and development collaboration with DuPont to improve resistance to Asian Soybean Rust (“ASR”), a devastating fungal disease in soybean. Pursuant to this collaboration, we apply our proprietary ATHLETE™ computational discovery technology to identify relevant genes having the potential to improve in-plant resistance to ASR. The collaboration period under this agreement, including the stages of data generation, gene discovery, and preliminary testing by DuPont, is expected to last for approximately five years.
(ii) License Grants
Under the 2011 agreement, we granted DuPont a worldwide, royalty-bearing, exclusive license to develop and commercialize soybean products containing our licensed genes. We also granted DuPont an option, limited in time, to obtain an exclusive license to use the licensed genes for certain products other than soybean.
(iii) Diligence Obligations
The research program under this agreement is to be carried out in accordance with agreed timelines set forth in a project plan. In addition, we have diligence obligations that require us to identify a minimum number of genes intended to improve the target trait (i.e., ASR in-plant resistance), and to provide DuPont with a minimum number of reports describing such identified genes. DuPont’s diligence obligations, on the other hand, require it to test a specified number of genes before advancing any qualified genes through its product development pipeline. If DuPont fails to meet its obligations, some or all of the licenses it received under the agreement may terminate. In addition, under the 2011 agreement, DuPont is also obliged to use commercially reasonable efforts
91
Table of Contents
to advance, develop and commercialize products containing our licensed genes. However, at all times, DuPont retains the discretion to stop advancing or developing any products that it determines are not commercially viable, but only at the possible cost of losing some or all of the licenses it was granted.
(iv) Termination and Change in Control
Either party has the right to terminate the research project, with or without cause. The precise effects of such a termination depend on the point in time at which the right is exercised, but generally, the agreement allows the non-terminating party to continue with the project alone and at its own cost.
The 2011 agreement with DuPont does not automatically terminate upon our undergoing a change in control. However, if we experience a change in control to one of DuPont’s major competitors, DuPont may elect to terminate the agreement entirely, or terminate certain unexercised co-investment options (described below). If the agreement is terminated as a result of our change in control, DuPont’s licenses relating to genes that confer ASR-tolerance would terminate. Nevertheless, even following a change of control to a competitor, DuPont would retain a non-exclusive, royalty bearing, worldwide license to the genes discovered under the collaboration for traits other than ASR in certain specific crops.
(v) Consideration and Costs
As with our other research and development collaborations, our compensation under the 2011 agreement with DuPont is in the form of milestone payments and royalty payments based on the sales of resulting products. However, unlike our other collaborators, DuPont is not funding the costs of our research and development through annual research and development services payments; rather, we are funding the discovery phase using our own resources and a grant from BIRD, while DuPont is covering the cost of all downstream expenses. This arrangement likely provides us with higher milestone and royalty payments than in our other collaborations where our collaborators fund both our research and their own development costs. In addition, we hold a contractual option to co-invest in the development costs for greater royalty percentages downstream if a product is successfully commercialized.
Syngenta
(i) Background and Duties
Our multiyear collaboration with Syngenta, commenced in June 2009 and amended and restated in September 2013, focuses on another highly sought soybean trait: soybean cyst nematode, or SCN, resistance, along with resistance to other nematode species. The nematode is a soil parasite that attacks the roots of developing plants with significant yield-limiting results. The agreement is focused on identifying and developing genes targeting this trait in soybeans. However, Syngenta has a right of first negotiation to expand the scope of the agreement to cover additional crops. The research period under the agreement is expected to last until March 2017, and the collaboration is currently in Phase I testing by Syngenta.
Under this agreement, we use our ATHLETE™ computational technology to assemble and mine our genomic database in order to identify and prioritize genes with the potential to improve nematode tolerance. In addition, we are also to apply our PlaNet computational technology to make “gene stacking” recommendations, indicating whether certain identified genes can be combined with other genes to jointly impact SCN resistance. After we provide Syngenta with such genes, Syngenta is to validate the genes in the validation system generally used in Syngenta’s product development program, a process that is currently ongoing. Syngenta is obliged to test a specified number of genes and use commercially reasonable efforts to evaluate and advance the development of products containing our licensed genes, or alternately, to abandon them, in which case we will have the right to develop and commercialize the applicable genes in the target crops. If Syngenta fails to carry out its diligence obligations in regards to any relevant gene it may forfeit its rights and options to such gene in soybean.
92
Table of Contents
(ii) License Grants
Pursuant to the agreement, we granted Syngenta an exclusive license to develop and commercialize, solely for transgenic applications, soybean products containing certain genes identified as a result of the collaboration. Syngenta also holds an option to obtain a commercial license for non-transgenic application of the licensed genes in soybeans, although this option must be exercised within a certain period following final gene selection. Finally, we also granted Syngenta a limited in time, first right to negotiate commercial licenses to use the licensed genes in additional plant varieties. In return, Syngenta granted us an exclusive right to develop and commercialize products containing the licensed genes, with the payment of certain proceeds to Syngenta, but only using crops other than those already reserved for Syngenta.
(iii) Diligence Obligations
Syngenta is required to use commercially reasonable efforts to evaluate and progress the licensed genes in its product development programs, with the aim of developing and commercializing soybean products using our licensed genes. However, at all times, Syngenta retains the discretion to stop advancing or developing any products in its sole discretion, but only at the possible cost of losing its rights to such genes. Among other things, the diligence obligations require Syngenta to perform minimum numbers of validation experiments.
(iv) Termination and Change in Control
In addition to each party’s right to terminate the agreement upon a material breach by the other party, Syngenta may terminate the agreement (i) at any time without cause or (ii) if we experience a change of control to certain identified Syngenta competitors, provided, however, that the termination right in respect of a change of control is subject to certain exceptions. In both such cases, if Syngenta exercises its termination right, it must assign all of its rights in certain intellectual property to us, and we will then have the exclusive right to develop and commercialize the licensed genes in any crop, without any obligation to Syngenta.
(v) Consideration and Costs
In addition to milestone payments and royalty payments based on sales, Syngenta funds our research costs under the agreement.
Biogemma
(i) Background and Duties
In 2006, we entered a joint research and collaboration agreement with Biogemma SAS, a subsidiary of Limagrain, focusing on improving yield and abiotic stress tolerance in corn. In 2010, we signed a license agreement, replacing the commercialization provisions of the 2006 agreement, and enabling Biogemma and its shareholders (Limagrain, RAGT, Euralis, Sofiproteol and Unigrain) to pursue commercialization of corn products containing our licensed genes. This later agreement remains in effect. This marked the first time we entered into a license agreement with a collaborator for genes that were already tested in field trials involving the target crops. The license agreement with Biogemma is also our only current agreement that has entered Phase II of our product development cycle.
In the early stages of the 2006 agreement, we provided Biogemma with candidate genes that we identified using our ATHLETE™ computational technology, as well as data regarding those genes. We and Biogemma then jointly selected the most promising candidate genes for further validation and testing.
(ii) License Grants
Under the 2010 license agreement, we granted Biogemma an exclusive, worldwide, royalty-bearing license to (i) transgenically introduce specified genes into Biogemma corn products for research and development
93
Table of Contents
purposes, and (ii) commercialize and sell corn products containing our licensed genes. Pursuant to the license agreement, Biogemma will continue to test the impact of the licensed genes in its own research and development program.
(iii) Diligence Obligations
Under the 2010 license agreement, Biogemma is required to use its best efforts to develop, launch and market corn products containing our licensed genes and must achieve certain milestone events within specified timeframes. If it fails to meet those obligations, it may forfeit its license for the relevant gene.
(iv) Termination
In addition to each party’s right to terminate the 2010 agreement upon a material breach by the other party, or upon the commencement of bankruptcy proceedings against the other party, Biogemma has the right to terminate the license agreement at any point, if Biogemma determines that the licensed genes will not result in a commercially viable product. Should Biogemma exercise this right, Biogemma would forfeit its licenses.
(v) Consideration and Costs
The license agreement provides for several one-time research and development services payments to cover our prior research and development efforts, milestone payments, and royalty payments. In 2010, Biogemma accounted for 16.9% of our revenues. Since then, our revenues from Biogemma have decreased substantially.
Rahan Meristem
In 2007, we commenced a collaboration with Rahan Meristem, or Rahan, amended in 2009, focusing on the development of banana varieties showing increased tolerance to black sigatoka, a fungus that damages banana leaves. Rahan is an Israeli company dedicated to growing, cultivating and marketing banana plants. Under the agreement, following a field trial, both parties are to select up to a certain number of genes to be used by Rahan in developing and commercializing banana products. Pursuant to the terms of the agreement, we granted Rahan an exclusive license to use these genes in developing and commercializing banana plants or any other plants, except that Rahan is prohibited from using the genes in certain specified major crops. Rahan granted us, on the other hand, an exclusive license to develop and commercialize any product in the specified major crops using the genes identified as a result of the collaboration. Each party has agreed to pay the other royalty payments calculated as a percentage of net proceeds of the sale or licensing of products containing joint intellectual property. Each party otherwise bears its own costs under the agreement.
Rasi Seeds
We entered into an agreement in 2012 with Rasi Seeds Ltd., or Rasi, a leading developer and marketer of rice seeds and other crops in India. Under the agreement, we are to provide Rasi with a specified number of genes with the potential to improve yield and drought tolerance in rice. Rasi, in turn, agreed to meet certain diligence obligations, including integrating certain genes in rice and developing products containing those genes, and to use its best efforts to commercialize and market a rice product containing our licensed genes in India. To this end, we granted Rasi an exclusive, royalty-bearing license to develop and commercialize hybrid rice seeds containing our licensed genes in India. In addition, Rasi may seek to negotiate with us an expansion of the geographic scope of the agreement beyond India to cover certain other countries identified in the agreement. Rasi has covered our research costs through a one-time up-front fee, and is also committed to milestone payments and royalty payments.
94
Table of Contents
CIRAD
In 2004, we entered into a five-year collaboration agreement with the Centre de Coopération Internationale en Recherche Agronomique pour le Développement, or CIRAD, which is dedicated to research and development of agricultural technology in developing countries. In 2007, we entered into a separate agreement with CIRAD, called “Project 3,” and we also extended the term of our collaboration in 2011. The goal of our collaboration with CIRAD is to develop GM cotton showing improved abiotic stress resistance. Under the Project 3 agreement, CIRAD granted us (i) a royalty-bearing license to use the results of our cotton collaboration in any way we deem fit in cotton and (ii) a fully paid-up and royalty-free license to use the collaboration results in any plants other than cotton in any way we deem fit. We, in turn, granted CIRAD a royalty-bearing, non-exclusive license to use the collaboration results only with relation to the genetic modification of cotton, and only in certain specified territories. The Project 3 agreement also sets forth the royalty payments for the above licenses, pursuant to which either party will pay the other a fixed percentage of the sale proceeds of cotton products containing any jointly owned and licensed genes or know-how. Each party otherwise bears its own costs under the agreement.
Viterra
In 2008, we entered into a research and development agreement with Viterra Inc., a publicly traded Canadian agriculture company that develops and tests varieties of grains and oilseeds. Our goal of this collaboration is to develop transgenic canola and rapeseed plants that demonstrate improved yield and abiotic stress tolerance. Each party to the agreement pays for its own activities, and the collaboration is partially funded by a grant from the CIIRDF. Once the research phase of the collaboration agreement is complete, the agreement proposes three potential product commercialization pathways to be incorporated into new separate commercialization agreements each covering a different set of products. Throughout the agreement period, we maintain a full right to use our candidate genes in all crops other than canola and rapeseed. Viterra has recently completed a first field trial in Canada of our genes in canola and rapeseed. According to a draft report that Vittera provided to us for review, in Vittera’s opinion, the performance of the genes in the field trial does not support further testing and development toward commercialization.
Beijing Dabeinong Technology Group
In 2013, we entered into a licensing agreement with Beijing Dabeinong Technology Group Co., Ltd. (“Beijing Dabeinong Technology”), a publicly traded Chinese developer and marketer of seeds, particularly rice. Pursuant to the licensing agreement, we are to identify and deliver to Beijing Dabeinong Technology a specified number of genes with the potential to improve drought tolerance and the efficient use of fertilizer in rice. We have granted Beijing Dabeinong Technology an exclusive, royalty-bearing license to develop and commercialize hybrid rice seeds containing our licensed genes in China. In return, Beijing Dabeinong Technology will pay us a small upfront development fee and has committed to milestone and royalty payments.
We have also entered into several other collaboration agreements with other companies in the agriculture industry, such as Zeraim Gedera Ltd. and CBD Technologies Ltd., each of which is not material to our business.
Evofuel Collaboration Agreements
To further our goal of becoming a producer and seller of castor bean seeds, we have entered into two important collaboration agreements with leading biodiesel producers in Brazil and Argentina (described below). Both agreements involve pre-commercial evaluations and testing of our castor bean seeds. The agreements are in a pre-revenue stage as well, and do not provide for research, licensing, or royalty payments. If initial evaluations of the castor bean are positive, we may be able to extend these agreements or enter into new agreements at a commercial or semi-commercial level, with the aim of developing and producing castor bean seeds for the biodiesel market.
95
Table of Contents
SLC Agricola
In 2010, we entered into a collaboration agreement with SLC Agricola, a leading agribusiness and producer of soybean, corn and cotton in Brazil. We extended the term of the collaboration agreement in 2011, and again in 2012. In September 2013, we completed our third year of field trials in SLC Agricola’s plantations in northeast Brazil, and we expect to begin advanced product development and pre-commercial trials in 2014. Our goals under the agreement are to evaluate and identify castor bean varieties displaying economic yields under rain-fed conditions, and to develop more effective agronomic methods of growing castor bean crops. Eventually, we hope to develop improved castor bean varieties suitable for commercial scale production in rotation with soybean. The term of our collaboration with SLC Agricola is set to expire in December 2014.
Pursuant to the collaboration agreement, we are responsible for providing SLC Agricola with castor bean seeds that can be grown on SLC Agricola’s plantations in northeast Brazil. We have also committed to providing SLC Agricola with technical growth protocols and guidance for growing the castor bean crops. SLC Agricola has agreed to provide the land, employees, equipment, and other infrastructure needed to grow the castor bean crops. Our employees manage and supervise the execution of the collaboration agreement, visiting the SLC Agricola plantations and supervising the analysis and selection of the most promising castor bean varieties.
Under the agreement, SLC Agricola grows the castor bean strictly pursuant to the parameters of the collaboration. SLC Agricola is prohibited from using the castor bean varieties for any other purpose, including for commercializing castor bean products. Furthermore, all intellectual property that results from the collaboration—whether in the form of data, inventions, growth protocols, patents, or trade secrets—vests solely in us, and does not accrue to SLC Agricola.
Each party bears the costs and expenditures incurred in connection with its own activities under the collaboration agreement. We alone bear the cost, however, of obtaining government permits or licenses for importing and using the castor bean seeds in Brazil. SLC Agricola is responsible for obtaining all other secondary governmental or municipal licenses and permits. Both parties may terminate the agreement without cause, except that no termination is possible when any castor bean project has entered the growing season.
T6
In 2012, we entered into a collaboration agreement with T6, an Argentinian company that produces and supplies biodiesel to both domestic and foreign markets. The purpose of the agreement with T6 is to determine whether certain castor bean varieties can be used as a sustainable and cost-effective source for the production of biodiesel. The agreement is set to expire in May 2014.
Specifically, we are to provide T6 with castor bean seeds, and we are to guide and advise T6 with respect to castor bean growth protocols, storage and disposal. For its part, T6 is to provide us with field-trial infrastructure, including land, labor and equipment. While T6 is responsible for collecting data from the field trials, our agronomists and breeders analyze and select those castor bean varieties showing the greatest potential as second generation biodiesel feedstock.
T6 has agreed to refrain from growing, harvesting, or analyzing the castor bean varieties for any purpose other than that specified in the agreement. T6 is prohibited, for instance, from attempting to commercialize castor bean products resulting from the collaboration. While we hold all rights to the intellectual property created by and during the collaboration, T6retains ownership of any intellectual property relating specifically to the conversion of castor oil into biodiesel.
The agreement specifies that we and T6 will work jointly to obtain any permits or licenses required to import and use castor bean seeds in Argentina. If the requisite permits or licenses are not obtained, the performance of duties under the agreement will be postponed until the next planting season, or until any other time mutually agreed by us and T6.
96
Table of Contents
We and T6 have each agreed to fund our own costs expenditures under the agreement. Either party can terminate the agreement without cause, except that no termination is possible when any project castor bean has entered the growing season.
Share Purchase Agreements
Monsanto Share Purchase Agreement
In contemplation of entering into the Monsanto Collaboration Agreement in 2008, we also entered into a share purchase agreement with Monsanto, dated August 27, 2008, as subsequently amended, or the Monsanto Share Purchase Agreement. Under the Monsanto Share Purchase Agreement, Monsanto agreed to invest up to $30.0 million in our ordinary shares in two tranches: 1,636,364 ordinary shares were purchased at a price per share of $11.00 upon the closing of the Monsanto Share Purchase Agreement in August 2008, for an aggregate purchase price of $18.0 million, and an additional 500,000 ordinary shares are issuable pursuant to a put option whereby we can require Monsanto to purchase such additional 500,000 ordinary shares at a price per share of $24.00, for an aggregate purchase price of $12.0 million. We may only exercise this put option beginning February 1, 2014 until August 31, 2014. In the event that Monsanto extends the term of the Monsanto Collaboration Agreement prior to February 1, 2014, the put option will be reduced to 214,286 ordinary shares at a price per share of $28.00, for an aggregate purchase price of $6.0 million. Pursuant to the terms of the Monsanto Share Purchase Agreement, Monsanto has the right to appoint one observer to our board of directors, which they have not yet appointed. See “Management—Board of Directors.” Monsanto’s ownership has remained unchanged since its purchase of ordinary shares in 2008, and Monsanto does not have different or special voting rights under the Monsanto Share Purchase Agreement.
Bayer Share Purchase Agreement
On December 10, 2010, in contemplation of entering into the Bayer Wheat Agreement, we and Bayer entered into a share purchase agreement, or the Bayer Share Purchase Agreement. Pursuant to the Bayer Share Purchase Agreement, Bayer purchased 863,310 ordinary shares at a price per share of $13.90 for an aggregate investment of $12 million. Bayer also has the right under the Bayer Share Purchase Agreement to appoint one observer to our board of directors, which they have not yet appointed. Bayer’s right to appoint an observer to our board of directors will continue so long as Bayer holds at least 4% of our issued and outstanding shares.
Product Development Cycle
Seed Trait Product Development Cycle
Developing and integrating seed traits into commercial seeds using advanced breeding or biotechnology takes, on average, between six and thirteen years. The length of the process may vary depending on both the complexity of the trait and the type of crop involved. The length of the process of developing seed traits impacts the uncertainty of product development; for example, during the development process, the gene may fail to address the performance criteria required to advance to later development stages, changes in the competitive landscape may occur that could affect development and alternative methods of seed improvement may advance.
The development process for seed traits is divided into several discrete steps or “phases,” which generally include discovery, validation and development, and end with regulatory approval and commercial launch of a seed product containing the trait. The process for developing seed traits is relatively similar for both biotechnology and advanced breeding. However, the two differ significantly in later phases of development; for example, receiving regulatory approval for biotech seeds is a far more comprehensive and lengthy process than doing the same for advanced breeding seeds.
97
Table of Contents
The development process of biotech seed traits and their integration into commercial seeds is generally divided into six phases, which may vary depending on our collaborator and the specific crop and trait we are working on:
| • | Discovery: The first step in the seed trait development process is Discovery, or the identification of candidate genes, or SNPs in the case of advanced breeding, potentially capable of enhancing specified plant traits. These genes or SNPs are usually introduced into model plants, which serve as testing grounds to determine whether the gene or SNP will enhance the specified trait. We usually employ our own advanced greenhouse facilities in Israel to perform model plant validation utilizing Arabidopsis for dicots, such as soybean, canola, cotton and sunflower, and Brachypodium for monocots, such as corn and wheat. In our experience, using our technologies and methodologies, the Discovery phase typically lasts approximately 18 months. According to Monsanto’s 2011 Investor Toolkit, this phase has an average probability of success of approximately 5%. |
| • | Phase I, or “Proof of Concept”: Upon successful validation of the genes or SNPs, promising candidate genes or SNPs are advanced to Phase I, a process called “proof of concept.” In this phase, the genes or SNPs are inserted into target plants and their efficacy in improving plant performance is tested through greenhouse trials, field trials, or both. The goal of the proof of concept phase is to determine which candidate genes or SNPs have the greatest potential to improve plant performance. Phase I is typically conducted by our collaborators in their own facilities, although we are capable of conducting certain tests and actually do conduct them for some projects. In our experience, Phase I typically lasts between two to five years, and according to Monsanto’s 2011 Investor Toolkit, has an average probability of success of approximately 25%. |
| • | Phase II, or “Early Development”: In this phase, the field tests commenced in Phase I are expanded, and our collaborators evaluate various modes of use of the genes as well as other characteristics in order to optimize the performance in the plant on a large scale across various geographical locations and varieties. The goal of the “Early Development” phase is to identify the mode of use in which the genes or SNPs performed best and achieve the desired seed traits with commercially viable success rates. Based on our estimates, we expect Phase II to last between approximately two to four years, and according to Monsanto’s 2011 Investor Toolkit, has an average probability of success of approximately 50%. |
| • | Phase III, or “Advanced Development and Regulation”: In Phase III, extensive field tests are used to demonstrate the effectiveness of selected genes or SNPs in enhancing particular traits. The process of obtaining regulatory approvals from government authorities is also initiated during this phase, and tests are performed to evaluate the potential environmental impact of modified plants, including assessments of possible toxicity and allergenicity. According to Monsanto’s 2011 Investor Toolkit, Phase III typically lasts between one to two years, and has an average probability of success of approximately 75%. |
| • | Phase IV, or “Pre-Launch”: Phase IV involves finalizing the regulatory approval process and preparing for the launch and commercialization of new enhanced seeds. The range of activities here includes preparing the seeds for commercial sales, formulation of a marketing strategy and preparation of marketing materials. According to Monsanto’s 2011 Investor Toolkit, Phase IV typically lasts between one to three years, and has an average probability of success of approximately 90%. |
| • | Product Launch: We expect that our strategic collaborators will also perform the last step of the development process, the actual launch and commercialization of the seed containing the improved seed trait. Pursuant to our collaboration agreements, a successful product launch will trigger royalty payments from our collaborators, which are generally calculated as a percentage of the additional sales value conferred by the improved seed trait. |
As indicated, the estimated timeframes of phase duration and probability of success are based on our experience and estimates, as well as on the figures presented in Monsanto’s 2011 Investor Toolkit. The phases may overlap during the product development cycle, and the total development time for a particular product may be
98
Table of Contents
longer or shorter than the duration presented above depending on a range of factors, including the type of crop and trait involved, and the amount of resources available, or devoted to, particular research or collaboration projects.
All of our products are currently in either the Discovery, “Phase I” or “Phase II” stages.
Ag-Chemical Product Development Cycle
Our ag-chemical activities are still in the early stages, and we have not yet entered into collaboration agreements in this area. While we believe that our significant experience in seed traits will ease our entry into the ag-chemical market, we are still in the process of developing the technologies that will enable our success in ag-chemicals. Nevertheless, once we establish our technological technology, we plan to pursue a streamlined product development cycle that will improve upon the traditional development models used in the ag-chemical industry.
Similar to our seed trait product development cycle, the ag-chemical development cycle for herbicides begins with a discovery stage. We expect, based on our extensive plant genomics know-how and the strength of our technological infrastructure, that we will specialize in the early stage of the development cycle. Specifically, we will use our proprietary computational technology, PoinTar, to perform “Target Discovery,” which involves the identification of proteins that are important to plant function and performance. The targets we will be seeking are those that, when inhibited (for instance by a chemical), lead to plant or weed death. We will also utilize a new computational technology we are currently developing to assist us in the testing and analysis of chemicals that would inhibit these targets. Following the testing model commonly used in the pharmaceutical industry, we will perform high throughput screenings of chemicals to try and pinpoint those chemicals capable of influencing the targets that we have identified, and by extension, creating significant impact on plants.
While we have not yet entered into ag-chemical collaborations, we expect that, when we do, they will be structured similarly our seed trait collaborations. Our focus, accordingly, will lie in upstream discovery and development. We intend to either establish the screening and initial development capabilities ourselves, as we have done with PoinTar, or outsource them to collaborators or other third parties.
We expect that our collaborators will perform the remaining steps in the product development cycle. Screening will be followed by a “hit-to-lead” optimization process, in which the most promising chemical molecules are further assessed and optimized. If this process successfully indicates that certain chemical molecules have a desired effective impact on plants, these molecules may be developed and commercialized into herbicides. In the final phases, any new chemical product will be registered with the proper regulatory authorities and then launched for commercialization.
There is currently no common practice in the industry for developing crop enhancers. To the extent such a practice may emerge, we expect that it will be generally similar to the product development cycle used for herbicides. For both herbicides and crop enhancers, effective target identification is critical to commencing the development process, and we intend to use our PoinTar technology for this process in crop enhancers as well as herbicides. Our ability to combine our extensive knowledge of plant genomics with our tailored computational technologies and other tools will guide our effort to detect chemicals capable of positively impacting plant performance and enhancing targeted traits in plants. The development process for crop enhancers, however, poses at least one unique challenge not relevant to herbicides: the lack of an efficient “phenotypic screen.” Chemicals with the potential to be used in herbicides are relatively simple to identify in high throughput screens since, when applied, these chemicals lead to plant death. Chemicals that may be useful for crop enhancers, however, require a much more complex high throughput testing procedure, as their impact on plants performance may be subtle, gradual or otherwise more difficult to detect.
Seeds Focusing on Second Generation Feedstock for Biodiesel Product Development Cycle
The process for developing and commercializing seeds focusing on second generation feedstock for biodiesel varies from crop to crop, and also depends on the relative maturity of the particular seed market. In the
99
Table of Contents
case of our castor bean seeds, for example, in order to develop advanced seeds, we need to develop new growth protocols and design new mechanized harvest equipment in order to produce the seeds on a commercial scale (as current practices rely on very traditional methods). Such efforts, in contrast, are not required for traditional field crops such as corn or wheat, which have common and long-observed production practices. Our operations in seeds focusing on second generation feedstock for biodiesel were initiated in 2007, and while we expect to start selling seeds in at least three years, we are unable to foresee when significant sales will commence.
The product development cycle for our castor bean seeds includes the following elements:
| • | Variety Development: We collect different castor bean lines, cross them and select the varieties showing the most promise for economic and commercial development. The crossing trials are repeated and gradually increase in size until we can confidently evaluate whether a particular castor bean variety meets commercial targets. |
| • | Growth Protocol Development: Growth protocols allow growers to determine how to grow and cultivate their crop varieties, providing guidance on factors that include sowing time, sowing density, herbicide selection, fertilizer application and harvest timing. As with variety development, the process for formulating an appropriate and complete growth protocol that can enable commercial-scale seed production also involves repeating various crop trials under different growth conditions. |
| • | Mechanized Harvest Development: To the best of our knowledge, there is no current commercially viable solution for the mechanized harvest of castor beans. The process for developing new harvesting equipment requires testing existing machinery adaptations, designing a prototype, and eventually constructing a commercial prototype. We are currently collaborating with a leading agricultural machinery company to develop a mechanized castor bean harvester. |
| • | Seed Production: This step involves developing an efficient standardization protocol for the production of seeds, ensuring that all seeds meet a standard quality (e.g., variety uniformity, health, etc.). |
| • | Registration: To sell our castor bean seeds, we will need to receive certain government permits and licenses, which will vary from jurisdiction to jurisdiction. We believe that, in countries where the castor bean is already grown, such as Brazil, obtaining these permits and licenses will be a relatively rapid process, provided that the seeds are not developed using genetic modification. |
Intellectual Property
Our intellectual property rights are important to our business, as they generally determine our eligibility to receive royalties for seed traits. To date, we have identified and sought patent protection for over 4,000 plant genes linked to traits such as improved yield and drought tolerance. These genes are currently protected through more than 35 patents and 150 patent applications.
Our patenting process involves four stages, as follows:
| (i) | Provisional Filing. In most cases, soon after using ATHLETE™, our computational technology for gene discovery, we file a provisional application in the United States covering all the genes we have identified that we believe are likely to convey a specific trait in plant species. The number of genes covered in each provisional application ranges between 50 and 200 genes. |
| (ii) | PCT Filing. A PCT provisional is filed under the Patent Cooperation Treaty, or PCT, one year from the U.S. provisional filing. During this one year period, we insert the identified genes into model plants and test whether the genes actually improve target traits. This validation data is added to the PCT provisional and provides the “reduction to practice” required to advance the application. |
| (iii) | National Filing. National filing is conducted for most countries a year and a half after the PCT filing. For Argentina, which has not signed the PCT, a national filing is made at the same time as the PCT |
100
Table of Contents
| filing. Either we or our collaborators determine in which countries patent applications should be filed. If a collaborator requests that we file and prosecute a patent in a particular country, the collaborator will usually pay for the filing fee and any associated costs. The main countries in which we file are the United States, Brazil, Argentina, Canada, Australia, India, and certain other countries in Asia, South America and Europe. |
| (iv) | Prosecution. In the countries in which we file national phase applications, we prioritize the prosecution of genes identified as the most likely commercial candidates based on discussions with our collaborators. |
Our in-house know-how is another important element of our intellectual property. Our employment and consulting agreements include undertakings regarding confidentiality and assignment of inventions. Furthermore, the daily work at our various greenhouse sites, tissue culture facilities, and molecular labs involves the use of advanced mechanical tools, imaging devices, and computer hardware and software. We have established closed networks and physical security systems to prevent unapproved access.
In addition to seeking patent applications, we are in the process of obtaining trademark registrations in certain jurisdictions that we consider material to the marketing of our proprietary computational technologies, including ATHLETE™, Gene2Product™ and EvoBreed™. While we expect our patent applications to receive approval, and our trademark applications to mature into registrations, we cannot be certain that we will obtain such results. Despite our efforts to protect our proprietary rights, unauthorized third parties may attempt to use, copy or otherwise obtain and market or distribute our intellectual property rights or technology or otherwise develop products or solutions with the same functionality as our solutions. In addition, the laws of some foreign countries provide less protection for proprietary rights than U.S. law. We face the occasional risk, moreover, that third parties may assert copyright, trademark and other intellectual property rights against us. Such claims may result in direct or indirect liability as we have contractually agreed to indemnify certain parties for any damages suffered as a result of infringement by us of any third-party intellectual property rights.
Competition
Our market is characterized by intense commercial and technological change, and we face significant competition in many aspects of our business. The seed and ag-chemical market in particular is highly consolidated and dominated by a relatively small number of large companies. In order to provide their customers (mostly farmers) with cutting-edge products, these companies invest substantial resources in the development of seeds, traits and agronomic methods and products. Part of these companies’ research and development activity is conducted in-house and part of it is outsourced.
Generally, the competitors in our industry can be divided into three groups:
| • | Seed and Ag-Chemical Companies: Several seed and ag-chemical companies in our industry have internal research and development units dedicated to seed trait development, many of which also explore the development of ag-chemicals. Similar to our own operations, these internal research and development units engage in the discovery of genes, SNPs and other DNA fragments, and evaluate the application of these genes, SNPs and DNA fragments to model plants and target crops. These research and development units are also capable of pursuing post-discovery phases, and can develop their own improved seed traits. On the ag-chemical side, internal research and development units continue to test new chemicals using various approaches, the most common of which is large-scale screening operations in which thousands of chemicals may be applied to plants. Even though the internal research and development units at such companies often compete with our business activities, they may form part of our customer base as collaborators licensing our patented genes, SNPs and chemical molecules for their products. The most significant seed and ag-chemical companies in our industry are currently Monsanto, DuPont, Syngenta, Bayer, Biogemma, BASF, Dow, and KWS. These large seed and ag-chemical companies hold substantial brand recognition and wield greater financial, technical, marketing and other resources than we do. |
101
Table of Contents
| • | Small- to Mid-Size Biotech Companies Specializing in Plant Trait Enhancement: Companies with their own seed trait development programs are our most direct competitors. Like us, they engage in the discovery of genes, SNPS and other DNA fragments, and then license their discoveries to their customers. In addition, some of these companies also perform target discovery and identify ag-chemical molecules for crop protection and crop enhancement. In the past, several of these companies, such as Devgen, Athenix and Crop Design, were acquired by larger seed companies. We believe that there are currently dozens of small- to mid-size companies that specialize in plant trait enhancement, including Mendel Biotechnology, Targeted Growth, Inc., Hexima and Keygene. |
| • | Academic and Agricultural Research Institutions: Some academic and agricultural institutions in Israel and around the world are performing research into seed traits and the development of ag-chemical molecules. For the most part, the activities of these institutions include discovering and classifying genes, SNPs and other DNA fragments that may be relevant to specific target seed traits, as well as identifying targets and ag-chemical molecules. Many academic studies are primarily concerned with understanding the biological processes that are relevant to particular genes. Occasionally, however, academic and agricultural institutions grant licenses to third parties for the use of discovered genes, SNPs and other DNA fragments. Licensing and commercializing genes is less of a driver of academic research, however, and in most cases, academic research is limited to a small number of genes, SNPs and DNA fragments and is performed over a long period of time. |
We believe that our competitive advantage lies in our ability to assemble large amounts of genomic and phenotypic data and to analyze that data using our key computational technologies, enabling us to identify and prioritize genes, targets and chemical molecules more accurately, efficiently and quickly than is common in our industry. In addition, we continue to accumulate and expand our plant genomic database, drawing partly on publicly available data, partly on our plant experiments and field trials, and partly on research and experimentation results from our collaborators. Certain seed and ag-chemical companies specializing in seed traits may, however, have genes that are in more advanced stages of product development and commercialization, and these genes may relate to or directly compete with the same seed traits we are currently developing. To remain competitive in our industry, we pursue three main strategies: first, we invest in the improvement of our key technologies, focusing especially on our proprietary computational technologies. Second, we focus on increasing our gene discovery capabilities and improving our plant validation systems, thus enhancing our ability to discover and classify new genes in greater numbers. Finally, we seek to protect our intellectual property rights through proper patent application and prosecution in the jurisdictions where we operate, including the United States, India, China, Brazil, Argentina and Canada.
Government Regulation
Our business is subject to regulation related to agriculture, health and the environment. To operate, we must obtain various permits and licenses from government authorities and municipalities in our active jurisdictions, and we must maintain our compliance with the terms of those permits, licenses and other government standards as necessary. These laws and regulations, particularly in relation to biotechnology, are not fully settled, but continue to evolve in order to keep pace with technological advances.
While our expertise may contribute to the downstream commercialization of enhanced seeds, we are not in the process of obtaining regulatory approvals required for the commercialization of biotechnologically improved seeds. These approvals, when needed, will be obtained by our collaborators according to our collaboration agreements.
Regulations in Israel
As an Israeli company, our activities in the fields of biotechnology and plant genomics are regulated by the Israel Ministry of Agriculture and Rural Development, or ISARD, and more specifically by the ISARD’s Plants Protection and Inspection Services, or PPIS. Our activities are subject to various laws, regulations, orders and
102
Table of Contents
procedures, which require us, among other things, to obtain permits for conducting experiments on genetically enhanced plants and to satisfy special conditions determined by the ISARD regarding the growing procedures of such seeds and plants. Violation of these regulations may expose the company to criminal penalties.
Pursuant to these regulations, we are also obligated to obtain separate permits to own and operate our greenhouses and testing fields in Israel and we are routinely inspected by ISARD. Our activities, as well as those of our subsidiary, Evofuel, in the field of biodiesel, are regulated by the Ministry of Environmental Protection. Pursuant to these regulations, we are required, among other things, to (i) obtain toxins permits, which allow us to conduct experiments using “hazardous materials,” as such term is defined in the applicable regulations, and (ii) follow special rules regarding waste disposal. Violation of these regulations may expose the company to criminal penalties, administrative sanctions and responsibility to compensate the injured for any environmental damages.
As recently as September 2012, we engaged an outside third party specialist to conduct an environmental survey, at our own initiative, of all our facilities and operation sites. The survey found that our facilities and procedures fully complied with the directives of Israel’s environmental protection laws and were not causing environmental pollution.
In addition, some of our research activities involve exchanging seeds with our collaborators for research activity and not commercial use. In these cases, any import or export activity is subject to specific approvals in accordance with regulations promulgated by the PPIS or by the Ministry of Industry, Trade and Labor as well as certain target country regulation, which, among other requirements, impose on us the obligation to obtain import, or export, licenses.
In the future, we may become subject to regulation by additional Israeli governmental ministries as a result of changes in our operations or changes in regulation.
Regulations of Products Containing Our Traits
Regulatory approvals are required prior to the commercialization and importation of biotechnologically enhanced seeds in most countries. Most of the key target markets where we anticipate our collaborators will sell seeds containing our traits, including the United States, European Union, Brazil and Argentina, will require such regulatory approvals prior to the commercialization of such products. Additional regulatory approvals will be required for countries importing grain produced from seeds containing our traits, such as China, India and certain countries in the European Union. Pursuant to our collaboration agreements, our collaborators will apply for all requisite regulatory approvals prior to commercialization of the products we are developing with them.
Examples of regulations our collaborators may need to apply for include, in the United States, approvals required by the USDA prior to the commercial sale of genetically modified products. The USDA’s review and deregulation process for biotech products is costly and time-intensive, with no guarantee of success. In the United States, collaborators may also need to seek regulatory approval from the United States Environmental Protection Agency, or EPA, which regulates the marketing and use of new plant pesticides and herbicides. In addition, in Brazil, the commercialization of biotech products is regulated by the National Technical Commission of Biosafety, Comissão Técnica Nacional de Biossegurança, or CTNBio under the Ministry of Science and Technology. The approval process involves data collection and analysis, environmental impact assessments and public hearings on certain products, and is similarly costly and time-intensive.
Business Development
We are constantly evaluating business opportunities in our industry. When an opportunity is identified, we work with our scientific personnel to create a tailored strategy, including deciding which computational technology or other technology to use, for a unique seed trait, ag-chemical or other product. We market our
103
Table of Contents
services and capabilities to potential collaborators directly, often through meeting the management, business development or research and development staff of potential collaborators. Upon the commencement of a new collaboration, we typically jointly formulate a unique research and development work plan with our collaborator and then negotiate the final business terms of the collaboration. We also participate in professional and industry conferences and give presentations that discuss our in-house technologies and capabilities.
We are in the research and developments stages of our Evofuel operations. To date, we have entered into several collaboration agreements with companies in different areas of the castor bean development and marketing value chain. Once our castor bean seeds are ready for commercialization, we will expand our sales and marketing operations to include direct outreach to castor bean growers. We anticipate that the primary markets for our castor bean seeds will be Brazil and Argentina.
Employees
As of June 30, 2013, we had 188 employees, all of whom are based in Israel. For the year ended December 31, 2012, we employed an average of 57 part-time employees. The following table shows the breakdown of our workforce as of the dates indicated:
| Department |
As of December 31, | As of June 30, 2013 |
||||||||||||||
| 2010 | 2011 | 2012 | ||||||||||||||
| Management |
5 | 5 | 6 | 5 | ||||||||||||
| Project Management |
4 | 6 | 8 | 6 | ||||||||||||
| Research and development |
89 | 119 | 127 | 147 | ||||||||||||
| Intellectual property |
2 | 2 | 3 | 3 | ||||||||||||
| Business development |
3 | 3 | 4 | 4 | ||||||||||||
| General administrative |
12 | 17 | 20 | 23 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
115 | 152 | 168 | 188 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Israeli labor laws govern the length of the workday, minimum wages for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice of termination of employment, equal opportunity and anti-discrimination laws and other conditions of employment. Subject to certain exceptions, Israeli law generally requires severance pay upon the retirement, death or dismissal of an employee, and requires us and our employees to make payments to the National Insurance Institute, which is similar to the U.S. Social Security Administration. Our employees have pension plans that comply with the applicable Israeli legal requirements.
Facilities
Our principal facility is located in Rehovot, Israel and consists of 2,709 square meters (approximately 21,200 square feet) of leased office space. This facility accommodates our corporate offices and our molecular labs. The lease for this facility expires on December 31, 2015, and we have an option to renew the lease for an additional three year term.
We perform most of our research and plant validation work at our “Evogene Farm,” located on two adjacent lots we lease outside Rehovot. The first lot is subject to two leases: the first lease covers approximately 1,110 square meters (or approximately 11,950 square feet) of land, and expires on April 30, 2017. The second lease covers approximately 13,000 square meters (or approximately 140,000 square feet) of land, and expires on February 1, 2016. Pursuant to an extension option, we may extend the term of the second lease for an additional period of up to three years. The lease for the second lot covers 10,000 square meters (approximately 108,000 square feet) and, pursuant to an extension option, expires on October 1, 2016. The Evogene Farm contains 34
104
Table of Contents
greenhouses, which are used for gene validation in model and target plants, plant propagation, and plant nurseries. In addition, the Evogene Farm contains warehouses, office facilities and seed banks.
In June 2011, we entered into a lease for approximately 30 acres of agriculture land outside Rehovot. Pursuant to an amendment to this lease, beginning in November 2012, we exchanged the 30 acre lot for a 40 acre lot. We will occupy the 40 acre lot until September 1, 2013, at which time we will return to the original 30 acre lot. The lease expires on June 1, 2015, and we have an option to extend the lease for an additional period of three to five years at our discretion.
Legal Proceedings
From time to time, we may become party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business. We are not currently involved in any legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition or results of operations. We may become involved in material legal proceedings in the future.
105
Table of Contents
Executive Officers and Directors
The following table sets forth the name, age and position of each of our executive officers and directors:
| Name |
Age |
Position | ||
| Executive officers |
||||
| Ofer Haviv |
47 | President and Chief Executive Officer | ||
| Sigal Fattal |
42 | Chief Financial Officer | ||
| Assaf Kacen |
41 | Executive Vice President of Technology Infrastructure | ||
| Dr. Hagai Karchi |
51 | Chief Technology Officer and Executive Vice President of Development | ||
| Assaf Oron |
38 | Executive Vice President of Strategy and Business Development | ||
| Directors |
||||
| Martin S. Gerstel |
72 | Chairman of the Board | ||
| Leon Y. Recanati |
65 | Director | ||
| Dr. Michael Anghel(1)(2)(3) |
74 | Director | ||
| Dr. Kinneret Livnat Savitzky(1)(2)(3) |
46 | Director | ||
| Dr. Simcha Sadan(1)(2) |
71 | Director | ||
| Dr. Adina Makover |
62 | Director | ||
| (1) | Member of our Audit Committee |
| (2) | Member of our Compensation and Nominating Committee |
| (3) | External director. See “—External Directors.” |
Executive Officers
Ofer Haviv has served as our President and Chief Executive Officer since December 2004 and joined our company in January 2002 as Chief Financial Officer. Mr. Haviv serves as Chairman of the Board of Directors of each of Evogene Inc. and Evofuel Ltd., both subsidiaries of our company, and has held such positions since 2006 and 2012, respectively. From 2006 to 2007, Mr. Haviv served as a director of our company. Mr. Haviv is a Certified Public Accountant and holds a B.A. in Accounting and Economics from Tel Aviv University.
Sigal Fattal has served as our Chief Financial Officer since May 2013. Ms. Fattal originally joined our company in June 2012 as our Chief Executive Officer’s assistant and was appointed as our Vice President of Finance in October 2012. Prior to joining our company, Ms. Fattal served as the chief executive officer of wwCFO Ltd., from 2010 to 2012. Prior to that time, from 2008 to 2009 Ms. Fattal served as the chief financial officer at Cal International Ltd. and from 2006 to 2007 served as the Director of Finance and manager of the Economics Department at Cal Ltd. Ms. Fattal holds a B.A. in Accounting and Economics, with honors, and a MBA specializing in finance and marketing, both from Tel Aviv University.
Assaf Kacen has served as our Executive Vice President of Technology Infrastructure since March 2011 after having joined our company in July 2009 as Vice President of Technology Infrastructure. Mr. Kacen has also served as a director of our subsidiary, Evofuel Ltd., since January 2012. Prior to joining our company, Mr. Kacen served as a Solution Fit Product Manager at Nokia Siemens Network, from 2008 to 2009. Prior to that, Mr. Kacen served as a Manager of the Software Development Group at Nokia Siemens Network from 2004 to 2008. Mr. Kacen holds an MBA with a major in Finance from the Interdisciplinary Center Herzeliya, and a B.Sc in Communication Systems Engineering from Ben-Gurion University.
106
Table of Contents
Dr. Hagai Karchi joined our company from its origination in January 2002 as one of our founders, and has served as our Executive Vice President of Development and Chief Technology Officer since September 2008. Dr. Karchi also serves as a director of Evogene Inc., our U.S. subsidiary, and has held such position since September 2006. Prior to serving in his current position at our company, Dr. Karchi served as our Vice President of Development and Technologies from 2007 to 2008. Dr. Karchi holds a PhD in Plant Genetics and Genomics, earned jointly from the Weizmann Institute of Science and the Hebrew University in Jerusalem, and an MA and BA in Plant Genetics, both of which were received from the Hebrew University in Jerusalem.
Assaf Oron has served as our Executive Vice President of Strategy and Business Development since September 2008. After joining our company in March 2006, Mr. Oron served as Business Development Director and then as Vice President of Business Development prior to his current position. Mr. Oron also serves as a director and chief executive officer of Evofuel Ltd, our subsidiary, and has held those positions since January 2012. Prior to joining our company Mr. Oron served as CEO of ChondroSite Ltd., a biotechnology company that develops tissue engineered products in the field of orthopedics, from 2004 to 2006. Prior to that Mr. Oron served as Senior Project Manager and Strategic Consultant at POC Ltd., a leading Israeli management consulting company from 1999 to 2003. Mr. Oron holds an M.Sc in Biology (Bioinphormathic) and a B.Sc in Chemistry and Economics, both from Tel Aviv University.
Directors
Martin Gerstel has served as our chairman of the board of directors since December 2004 and as a director since February 2004. Mr. Gerstel has also served as the chairman of Compugen Ltd., a predictive drug discovery and development company, since 1997, other than from February 2009 to February 2010, during which time he served as either chief executive officer or co-chief executive officer and, in both cases, as a member of the board of directors. Prior to joining Compugen, Mr. Gerstel was co-chairman and chief executive officer of ALZA Corporation, a U.S. pharmaceutical company specializing in advanced drug delivery, which he helped to found in 1968. In addition, Mr. Gerstel has served as chairman of Keddem Bioscience Ltd., a drug discovery company, since 2004 and has been the co-founder and co-chairman of Itamar Medical Ltd., a medical device company, since 1997 and has been a board member of Yissum Ltd. and Yeda Ltd., two technology transfer companies, since 2003 and 1994, respectively, and the U.S. Foundation for the National Medals of Science and Technology since 1993. He is a member of the Board of Governors and the Executive Committee of the Weizmann Institute of Science and the Board of Governors of The Hebrew University of Jerusalem, and is an advisor to the Burrill Life Science Funds, the Life Science Foundation and the board of the Israel-U.S. Binational Industrial Research and Development Foundation. Mr. Gerstel holds a B.S. from Yale University and an MBA from Stanford University.
Leon Recanati has served as a director of our company since May 2005. Mr. Recanati has served as chairman and chief executive officer of GlenRock Israel Ltd. since 2003. Previously, Mr. Recanati was chief executive officer and/or chairman of IDB Holding Corporation; Clal Industries Ltd.; Azorim Investment Development and Construction Co Ltd.; Delek Israel Fuel Corporation; and Super-Sol Ltd. He also founded Clal Biotechnologies Industries Ltd., a biotechnology investment company operating in Israel. Mr. Recanati holds an MBA degree from the Hebrew University of Jerusalem and Honorary Doctorates from the Technion Institute of Technology and Tel Aviv University.
Dr. Michael Anghel has served as an external director of our company since September 2007. Dr. Anghel has served as the chairman of Matach—The Israeli Educational Technology Center since 2009 and as the chairman of Lahav—Executive Education Program in Tel Aviv University since 2005. Dr. Anghel also serves as a director in the following companies: BioLineRx Ltd., a drug development company, since 2010; Syneron Medical Ltd., a global aesthetic device company, since 2004; Partner Communications Company Ltd., a mobile network operator, since 2006; Strauss Group Ltd., an Israeli food and beverage company, since 2008; Orbotech Ltd., a developer of automated optical inspection systems, since 2008; and Dan Hotels Ltd., an Israeli luxury hotel chain, since 2008. Dr. Anghel earned a Ph.D. in Business Management with a major in Finance and an
107
Table of Contents
MBA each from Columbia University, New York and a BA in Economics from the Hebrew University in Jerusalem.
Dr. Kinneret Livnat Savitzky has served as an external director of our company since September 2010. Since 2010, Dr. Savitzky has served as the chief executive officer of BioLineRx Ltd., a drug development company. She also served on its board of directors from 2010 to 2011, and as its Vice President of Research and Development during 2004. From 2005 to 2009, she served as the General Manager of BioLine Innovations Jerusalem Ltd., after having been employed by Compugen Ltd. from 1997 to 2004, where she last served as Vice President of Biology. Dr. Savizky holds a B.Sc. in Biology from the Hebrew University in Jerusalem, as well as a Ms.C. in Biochemistry and a Ph.D. in Molecular Biology, both from Tel Aviv University, in Israel.
Dr. Simcha Sadan has served as a director of our company since February 2006. Dr. Sadan was a lecturer of the faculty of management at Tel Aviv University and the chairman of the accounting department from 1978 to 1981. Over the course of the last five years, Dr. Sadan has served as a consultant to various companies and organizations. He also serves as the chairman of the board of directors of Powerbrook Spain S.L., a holding company of a Greek hotel and casino and as a director of various companies, including: companies under the group of Club Hotel Eilat Ltd., a holding company of hotels and tourism companies; Elite Sports Center Ltd., a subsidiary of Tel Aviv University; SYS Ltd and SMB Ltd., economic and finance consulting firms; Maariv Holdings Ltd. (a holding company of a publishing house); Malrag Engineering and Construction Ltd., an infrastructure company; Ofakim Ltd., a holding company of a motorcar dealer; Siemens Israel Ltd., an electric company; and Rozen, Mintz Richter Ltd. (a cycles dealership). Dr. Sadan holds in a PhD in Business Administration from the University of California, at Berkeley, an MBA with a major in Finance and Accounting and a BA in Economics and Statistics, both from the Hebrew University in Jerusalem, and an LLB from Tel Aviv University.
Dr. Adina Makover has served as a director of our company since February 2003. Dr. Makover also serves as a director of the following companies: GeneGrafts Ltd., a biotechnology company, since 2006; Spine 21 Ltd., a medical device company, since 2008; EarlySense Ltd., a medical device company, since 2006; and PerfAction Technologies Ltd., a medical device company, since 2007. She has also served as a board observer at Argo Medical Ltd., a medical device company in the rehabilitation field, since 2011. From 2006 to present, Dr. Makover has served as the investment manager of the Life Sciences ventures at ProSeed Venture Capital Fund Ltd. Dr. Makover holds a PhD in Life Sciences earned jointly from the Weizmann Institute of Science and Columbia University, and an MBA from Bar-Ilan University.
NYSE Listed Company Manual and Home Country Practices
The Sarbanes Oxley Act, as well as related rules subsequently implemented by the SEC, requires foreign private issuers, such as us, to comply with various corporate governance practices. In addition, upon the contemplated listing of our ordinary shares on the NYSE, we will need to comply with the NYSE Listed Company Manual, to which we refer as the Listed Company Manual. Under the Listed Company Manual, we may elect to follow certain corporate governance practices permitted under the Companies Law in lieu of compliance with corresponding corporate governance requirements otherwise imposed by the Listed Company Manual.
In accordance with Israeli law and practice and subject to the foregoing exemption available to foreign private issuers, if we list on the NYSE we intend to follow the provisions of the Companies Law, rather than the Listed Company Manual, with respect to the following requirements:
| • | Quorum at shareholder meetings. Our amended and restated articles of association provide that the quorum for any meeting of shareholders shall be at least two shareholders present in person or by proxy, who hold at least 25% of the voting power of our shares. However, for an adjourned meeting at which a quorum is not present within half an hour from the time for such meeting, our articles of association |
108
Table of Contents
| generally allow the meeting to adjourn for one week following the date of the meeting. If no quorum is present at such adjourned meeting, our articles of association allow us to proceed so long as at least any one shareholder is present, irrespective of the voting power held by him, her or it. |
| • | Executive sessions of independent directors. Israeli law does not require executive sessions of independent directors. Although all of our current directors are “independent directors” under the applicable NYSE criteria, we do not intend to comply with this requirement if we have directors who are not independent. |
| • | Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the Companies Law, which include (i) transactions with directors concerning the terms of their service or indemnification, exemption and insurance for their service (or for any other position that they may hold at a company), (ii) transactions concerning the compensation, indemnification, exculpation and insurance of the chief executive officer; (iii) the compensation policy recommended by the compensation and nominating committee of our board of directors and approved by our board of directors (and any amendments thereto); (iv) extraordinary transactions with, and the terms of employment or other engagement of, a controlling shareholder (if and when this becomes relevant to our company), (v) amendments to our articles of association, and (vi) certain non-public issuances of securities. In addition, under the Companies Law, a merger requires approval of the shareholders of each of the merging companies. We will not, however, seek shareholder approval for any of the following events described in the Listed Company Manual: |
| • | issuance of more than 1% of our outstanding ordinary shares (or voting power) to our affiliates; |
| • | an issuance that will result in a change of control of our company; and |
| • | adoption of, or material changes to, our equity compensation plans. |
We otherwise intend to comply with the rules generally applicable to U.S. domestic companies listed on NYSE. We may in the future decide to use the foreign private issuer exemption with respect to some or all of the other NYSE corporate governance rules. We are also required to comply with Israeli corporate governance requirements under the Companies Law applicable to public companies such as us.
Board of Directors
Under the Companies Law and our articles of association, the supervision of the management of our business is vested in our board of directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to executive management. Our Chief Executive Officer (referred to as a “general manager” under the Companies Law) is responsible for our day-to-day management. Our Chief Executive Officer is appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with him. All other executive officers are appointed by the Chief Executive Officer and are subject to the terms of any applicable employment agreements that we may enter into with them.
Under our articles of association, our board of directors must consist of not less than three and no more than seven directors, not including two external directors as required by the Companies Law. See “—External Directors.” Our board of directors will consist of six directors upon the consummation of this offering, which will include two external directors. Other than external directors, for whom special election requirements apply under the Companies Law, our directors are divided into three classes such that one-third of the directors (or, if their number is not a multiple of three, the number closest to, but not exceeding, three), are elected at each general meeting of our shareholders, in a staggered fashion, and serve on the board of directors for three years and until their respective successor has been elected and qualified or until their earlier removal by our shareholders at a general meeting, or upon the occurrence of certain events, in accordance with the Companies Law and our articles of association.
109
Table of Contents
Upon the closing of this offering, the members of, and terms of, the classes will be as follows:
| • | Martin Gerstel and Leon Y. Recanati will serve for a term that expires at the annual meeting of shareholders to be held in 2014; |
| • | Dr. Adina Makover will serve for a term that expires at the annual meeting of shareholders to be held in 2015; and |
| • | Dr. Simcha Sadan will serve for a term that expires at the annual meeting of shareholders to be held in 2016. |
In addition to the directors referenced above, we have two external directors, who serve for terms of three years each and may be elected for an unlimited number of additional three-year terms (beyond their initial three year terms) under certain circumstances (as described under “External Directors” below). External directors may be removed from office only under the limited circumstances set forth in the Companies Law. See “—External Directors.” Pursuant to the terms of the Monsanto Share Purchase Agreement, Monsanto also has the right to appoint one observer to our board of directors as long as Monsanto holds at least 5% of the voting rights of the Company. The observer is entitled to receive notices convening board meetings, participate at such meetings and receive a copy of all information distributed in connection with such meetings. The observer will not have any voting rights. Monsanto has not yet appointed an observer. In addition, pursuant to the Bayer Share Purchase Agreement, Bayer also has the right to appoint one observer to our board of directors so long as Bayer holds at least 4% of our issued and outstanding shares. The observer is entitled to be advised reasonably in advance of board meetings, and is to receive copies of all material distributed in connection with such meetings. The observer will not have any voting rights. Bayer has not yet appointed an observer.
Each director, other than external directors, will be appointed by a simple majority vote of holders of our voting shares, participating and voting at an annual meeting of our shareholders.
In addition, our articles of association allow our board of directors to appoint directors to fill vacancies on our board of directors or as new directors, in each case for a term of office that lasts until the next annual meeting of our shareholders. In the event of a vacancy resulting in the board consisting of less than the minimum number of directors required by our articles of association, the board of directors may only act in order to convene a general meeting of our shareholders for the purpose of electing such additional number of directors.
The removal of any director, other than an external director, prior to the expiration of his or her term (regardless of how the director was appointed or elected), requires the vote of a special majority, consisting of at least three-quarters of the votes of our shareholders entitled to vote.
Chairman of the Board
Our articles of association provide that the chairman of the board is appointed by the members of the board of directors and serves as chairman of the board throughout his term as a director, unless resolved otherwise by the board of directors. Under the Companies Law, the general manager or a relative of the general manager may not serve as the chairman of the board of directors, and the chairman or a relative of the chairman may not be vested with authorities of the general manager without shareholder approval consisting of a majority vote of the shares present and voting at a shareholders meeting, provided that either:
| • | such majority includes at least 2/3 of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in such appointment, present and voting at such meeting; or |
| • | the total number of shares of non-controlling shareholders and shareholders who do not have a personal interest in such appointment voting against such appointment does not exceed two percent of the aggregate voting rights in the company. |
110
Table of Contents
In addition, a person subordinated, directly or indirectly, to the general manager may not serve as the chairman of the board of directors; the chairman of the board may not be vested with authorities that are granted to those subordinated to the general manager; and the chairman of the board may not serve in any other position in the company or a controlled company, except that he may serve as a director or chairman of a subsidiary.
External Directors
Under the Companies Law, the board of directors of a company whose shares are publicly traded is required to include at least two members who qualify as external directors. The qualifications of an “external director,” as detailed below, are mandated statutorily by the Companies Law, and are distinguishable in certain ways from the criteria for serving as an “independent director” under the Listed Company Manual. Although it is possible for a director to qualify as an “external director” under the Companies Law without qualifying as an “independent director” under the Listed Company Manual, or vice-versa, in many instances, directors will either qualify as both or neither. The terms of service of external directors, as characterized by the unique duration of their term of office, the special majority required for their election, the regulation of their compensation and their required service on board committees (in each case, as described below), are each mandated by the Companies Law. While these terms are paralleled in certain instances by corresponding service conditions applicable to independent directors under the Listed Company Manual and our articles of association, the details of those conditions often differ. Dr. Michael Anghel and Dr. Kinneret Livnat Savitzky qualify and currently serve as our external directors. Each of our external directors will be subject to re-election for an additional three-year term at a shareholders meeting that we intend to hold prior to the consummation of the offering. The principal requirements with respect to external directors are set forth below.
The Companies Law provides for special approval requirements for the election of external directors. External directors must be elected by a majority vote of the shares present and voting at a shareholders meeting, provided that either:
| • | such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in such election (other than a personal interest which is not derived from a relationship with a controlling shareholder), present and voting at such meeting; or |
| • | the total number of shares of non-controlling shareholders and shareholders who do not have a personal interest in such election (other than a personal interest which is not derived from a relationship with a controlling shareholder) voting against the election of an external director does not exceed two percent of the aggregate voting rights in the company. |
After an initial term of three years, external directors may be reelected to serve in that capacity for up to two additional terms of three years each, and for a company such as ours with shares listed on the NYSE, for an unlimited number of additional three-year terms, subject to certain requirements set forth in the Companies Law and the regulations promulgated thereunder.
External directors may be removed from office by the same shareholder vote percentage required for their election or by a court, but only under limited circumstances. If there are fewer than two external directors on the board of directors, the board of directors is required under the Companies Law to call a shareholders’ meeting as soon as practicable to appoint a replacement external director.
The audit committee and the compensation and nominating committee must each include all external directors then serving on the board of directors. Each other committee of the board of directors that exercises powers of the board of directors must include at least one external director. Under the Companies Law, external directors of a company are prohibited from receiving, directly or indirectly, any compensation from the company other than for their services as external directors, which compensation is determined prior to their appointment and in general may not be changed throughout the term of their service as external directors.
111
Table of Contents
The Companies Law provides that a person is not qualified to serve as an external director if, as of the appointment date or at any time during the two years preceding his or her appointment, that person or a relative, partner or employer of that person, any person to whom that person is subordinate (whether directly or indirectly), or any entity under that person’s control, had any affiliation or business relationship (which terms are defined in the Companies Law) with the company, any controlling shareholder or relative of a controlling shareholder or an entity that, as of the appointment date is, or at any time during the two years preceding that date was, controlled by the company or by any entity controlling the company.
The following additional qualifications apply to an external director:
| • | a person may not be elected as an external director if he or she is a relative of a controlling shareholder; |
| • | if a company does not have a controlling shareholder or a holder of 25% or more of the voting power, then a person may not be elected as an external director if he or she (or his or her relative, partner, employer or any entity under his or her control) has, as of the date of the person’s election to serve as an external director, any affiliation with the then chairman of the board of directors, Chief Executive Officer, a holder of 5% or more of the issued share capital or voting power, or the most senior financial officer of the company; |
| • | a person may not serve as an external director if he or she (or his or her relative, partner, employer, a person to whom he or she is subordinated or any entity under his or her control) has business or professional relations with anyone with whom affiliation is prohibited as described above, and even if these relations are not on a regular basis (other than de minimis relations); and |
| • | a person may not continue to serve as an external director if he or she accepts, during his or her tenure as an external director, direct or indirect compensation from the company for his or her role as a director, other than the amounts prescribed under the regulations promulgated under the Companies Law, indemnification, the company’s undertaking to indemnify such person and insurance coverage. |
The Companies Law provides additional requirements with respect to external directors as well as certain limitations on the relationship between external directors and their affiliates, on the one hand, and the companies, on the other hand, following termination of the external directors’ service. Among others, the Companies Law provides that if at the time an external director is appointed all members of the board of directors who are not controlling shareholders or their relatives are of the same gender, the external director must be of the other gender.
Pursuant to the regulations promulgated under the Companies Law, a person may be appointed as an external director only if he or she either has professional qualifications or has accounting and financial expertise (as defined in those regulations). In addition, at least one of the external directors must be determined by our board of directors to have accounting and financial expertise and the board is required to determine the minimum number of board members who are required to possess accounting and financial expertise. In determining the number of directors required to have such expertise, the members of our board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that at least one of our directors must possess accounting and financial expertise. In this regard, our board of directors has determined that two of our current directors, Dr. Michael Anghel and Dr. Simcha Sadan, each possess “accounting and financial” expertise, while another director, Dr. Kinneret Livnat Savitzky, has “professional qualifications”.
Audit Committee
Listing Requirements
Under the NYSE corporate governance rules, we are required to maintain an audit committee consisting of at least three independent directors, each of whom is financially literate and one of whom has accounting or related financial management expertise.
112
Table of Contents
Following the listing of our ordinary shares on NYSE, our audit committee will continue to consist of Dr. Michael Anghel, Dr. Kinneret Livnat Savitzky and Dr. Simcha Sadan. Dr. Michael Anghel will continue to serve as the Chairman of the audit committee. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and the NYSE corporate governance rules. Our board of directors has determined that that each of Dr. Michael Anghel and Dr. Simcha Sadan is an audit committee financial expert as defined by the SEC rules and has the requisite financial experience as defined by the NYSE corporate governance rules.
Each of the members of the audit committee is “independent” as such term is defined in Rule 10A-3(b)(1) under the Exchange Act, which is different from the general test for independence of board and committee members.
Companies Law Requirements
Under the Companies Law, the board of directors of a public company must appoint an audit committee. The audit committee must be comprised of at least three directors, including all of the external directors, and a majority of its members must be unaffiliated directors. An unaffiliated director is an external director or a director who is appointed or classified as such, and who meets the qualifications of an external director (other than the professional qualifications/accounting and financial expertise requirement), whom the audit committee has confirmed to meet the external director qualifications, and who has not served as a director of the company for more than nine consecutive years (with any period of up to two years during which such person does not serve as a director not being viewed as interrupting a nine-year period). For Israeli companies traded on certain foreign stock exchanges, including NYSE, a director who qualifies as an independent director for the purposes of such director’s membership in the audit committee in accordance with the rules of such stock exchange is also deemed to be an unaffiliated director under the Companies Law. Such person must meet the nine-year requirement described above. Following the nine-year period, a director of an Israeli company traded on such foreign stock exchange may continue to be considered an unaffiliated director for unlimited additional periods of three years each, subject to certain conditions set forth in the Companies Law. Our board of directors has determined that Dr. Michael Anghel, Dr. Simcha Sadan and Dr. Kinneret Livnat Savitzky are unaffiliated directors.
The audit committee may not include the chairman of the board, any director employed by the company or who regularly provides services to the company (other than as a board member), a controlling shareholder or any relative of the controlling shareholder, or any person who is affiliated with the controlling shareholder. The chairman of the audit committee must be an external director.
Audit Committee Role
Our board of directors (following the approval by our audit committee) has adopted an audit committee charter setting forth the responsibilities of the audit committee consistent with the rules of the SEC and the NYSE corporate governance rules, which include:
| • | retaining and terminating our independent auditors, subject to board of directors and shareholder approvals; |
| • | pre-approval of audit and non-audit services to be provided by the independent auditors; |
| • | reviewing with management and our independent directors our financial reports prior to their submission to the SEC; and |
| • | approval of certain transactions with office holders and other related-party transactions. |
Additionally, under the Companies Law, an audit committee is required, among other things, to (i) identify deficiencies in the administration of the company (including by consulting with the internal auditor), and
113
Table of Contents
recommend remedial actions with respect to such deficiencies, (ii) review and approve certain related party transactions, including determining whether or not such transactions are extraordinary transactions, and (iii) adopt procedures with respect to processing employee complaints in connection with deficiencies in the administration of the company, and the appropriate means of protection afforded to such employees. In addition, the audit committee is responsible for overseeing the internal control procedures of the company. Under the Companies Law, the approval of the audit committee is required for specified actions and transactions with office holders and controlling shareholders. See “—Approval of Related Party Transactions Under Israeli Law.” However, the audit committee may not approve an action or a transaction with a controlling shareholder or with an office holder unless at the time of approval the majority of the members of the audit committee are present, of whom a majority must be unaffiliated directors and at least one of whom must be an external director.
Compensation and Nominating Committee
Our compensation and nominating committee consists of Dr. Kinneret Livnat Savitzky (chairman of the committee), Dr. Michael Anghel and Dr. Simcha Sadan. Prior to this offering, our board of directors will adopt a compensation policy setting forth the responsibilities of the committee consistent with NYSE rules and the Companies Law, which include:
| • | reviewing and recommending an overall compensation policy with respect to our Chief Executive Officer and other executive officers, as described below under “Compensation Policy”; |
| • | reviewing and approving corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other executive officers, including evaluating their performance in light of such goals and objectives; |
| • | reviewing and approving the granting of options and other incentive awards; and |
| • | reviewing, evaluating and making recommendations regarding the compensation and benefits for our non-employee directors. |
Our board of directors (following approval by our compensation and nominating committee and our board of directors) has adopted a compensation and nominating committee charter. The charter governs the required composition, meeting procedures and other matters related to the terms of operation of the committee. The charter also describes the responsibilities of the committee with respect to its role as our nominating committee under the Listed Company Manual, including advising our board of directors in selecting individuals who are best able to fulfill the responsibilities of a director or executive officer of our Company.
Each of the members of our compensation and nominating committee is independent under the listing standards of NYSE. Under the Companies Law, all external directors must be members of, and constitute a majority of the members of, the compensation committee, and each remaining committee member must be a director whose compensation and nominating does not exceed an amount that may be paid to an external director.
Compensation Policy
Under an amendment to the Companies Law that was adopted in December 2012, in addition to appointing a compensation and nominating committee, we are required to establish a policy regarding the terms of engagement of office holders (which include directors and senior executive officers), or a compensation policy. Such policy will need to be set by our board, after considering the recommendation of our compensation and nominating committee, and will require shareholder approval.
The compensation policy serves as the basis for determining the financial terms of employment or engagement of office holders, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. The compensation policy must relate to certain
114
Table of Contents
factors specified in the Companies Law, including advancement of the company’s objectives, the company’s business and its long-term strategy, and creation of appropriate incentives for executives. It must also consider, among other things, the company’s risk management, size and the nature of its operations. The Companies Law describes what factors have to be considered by, and what principles must be included in, the compensation policy.
Under the Companies Law amendment, we have until September 12, 2013 to adopt our compensation policy.
Compensation of Directors and Officers
Under the Companies Law, the compensation of each of our directors and our Chief Executive Officer requires the approval of our compensation and nominating committee, the subsequent approval of the board of directors and, unless exempted under the regulations promulgated under the Companies Law, the approval of our shareholders at a general meeting (in the case of a company’s chief executive officer, the shareholder approval must include the special majority described under “—Exculpation, Insurance and Indemnification of Office Holders” below, which in our case (due to the fact that we have no controlling shareholder) is currently inapplicable).
The compensation of any other office holder (who is neither a director nor our Chief Executive Officer), if consistent with our then-effective compensation policy, requires the approval of our compensation and nominating committee, followed by our board of directors. Compensation of any such office holder that deviates from our then-effective compensation policy will also require shareholder approval.
External directors are entitled to remuneration subject to the provisions and limitations set forth in the regulations promulgated under the Companies Law.
For additional information, see “—Compensation of Officers and Directors.”
Finance Committee
Our board of directors has formed a finance committee, on which Mr. Martin Gerstel, Dr. Simcha Sadan and Dr. Michael Anghel serve as members. The finance committee assists our board of directors in fulfilling its oversight responsibilities across the principal areas of corporate finance for our company and its subsidiaries. This committee may also assist the board by reviewing such matters as capital structure, equity and debt financing, capital expenditures, cash management, banking activities and relationships, investments, risk management, insurance and securities repurchase activities and making recommendations for consideration by our board of directors.
Internal Auditor
Under the Companies Law, the board of directors of an Israeli public company must appoint an internal auditor recommended by the audit committee (subject to the limits imposed by the Companies Law on who may be appointed as an internal auditor). Under the Companies Law, the internal auditor may be an employee of the company but not an office holder, or an affiliate, or a relative of an office holder or affiliate, and he may not be the company’s independent accountant or its representative.
The role of the internal auditor is to examine, among other things, our compliance with applicable law and orderly business procedures. The audit committee is required to oversee the activities and to assess the performance of the internal auditor as well as to review the internal auditor’s work plan. Doron Cohen, CPA, has been appointed
115
Table of Contents
as our internal auditor and he has served in such role since November 19, 2009. Mr. Cohen is a certified internal auditor and a partner of Fahn Kanne Control Management Ltd, an affiliate of Grant Thornton LLP.
Approval of Related Party Transactions Under Israeli Law
Fiduciary Duties of Directors and Executive Officers
The Companies Law codifies the fiduciary duties that office holders owe to a company. Each person listed in the table under “Management—Executive Officers and Directors” is an office holder under the Companies Law.
An office holder’s fiduciary duties consist of a duty of care and a duty of loyalty. The duty of care requires an office holder to act with the level of care with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of loyalty requires that an office holder act in good faith and in the best interests of the company.
The duty of care includes a duty to use reasonable means to obtain:
| • | information on the appropriateness of a given action submitted for his or her approval or performed by virtue of his or her position; and |
| • | all other important information pertaining to these actions. |
The duty of loyalty includes a duty to:
| • | refrain from any conflict of interest between the performance of his or her duties in the company and his or her personal affairs; |
| • | refrain from any activity that is competitive with the business of the company; |
| • | refrain from exploiting any business opportunity of the company in order to receive a personal gain for himself or herself or others; and |
| • | disclose to the company any information or documents relating to the company’s affairs which the office holder received as a result of his or her position as an office holder. |
Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions
The Companies Law requires that an office holder promptly disclose to the board of directors any personal interest that he or she may have and all related material information known to him or her concerning any existing or proposed transaction with the company. If it is determined that an office holder has a personal interest in a transaction, approval by the board of directors is required for the transaction, unless the company’s articles of association provide for a different method of approval. Our articles of association provide that for non-extraordinary interested party transactions, the board of directors may delegate its approval, or may provide a general approval to certain types of non-extraordinary interested party transactions. No transaction that is adverse to the company’s interest may be approved by the board of directors. Approval first by the company’s audit committee and subsequently by the board of directors is required for an extraordinary transaction, meaning any transaction that is not in the ordinary course of business, not on market terms or that is likely to have a material impact on the company’s profitability, assets or liabilities. A director and any other office holder who has a personal interest in a transaction which is considered at a meeting of the board of directors or the audit committee may generally (unless it is with respect to a transaction which is not an extraordinary transaction) not be present at such a meeting or vote on that matter unless a majority of the directors or members of the audit committee, as applicable, have a personal interest in the matter. If a majority of the members of the audit committee or the board of directors has a personal interest in the approval of such a transaction then all of the directors may
116
Table of Contents
participate in deliberations of the audit committee or board of directors, as applicable, with respect to such transaction and vote on the approval thereof and, in such case, shareholder approval is also required.
Pursuant to the Companies Law, extraordinary transactions with our office holders who are not directors require audit committee approval and subsequent approval by our board of directors. Compensation, insurance, indemnification or exculpation arrangements for office holders who are not directors require approval by our compensation and nominating committee, followed by our board of directors and, if deviating from our then-effective compensation policy, our shareholders. Compensation arrangements with directors, including in their capacities as executive officers, or with or our Chief Executive Officer, as well as insurance (unless exempted under the applicable regulations), indemnification or exculpation of directors or our Chief Executive Officer, require the approval of the compensation and nominating committee, the board of directors and our shareholders, in that order. If the transaction or compensation arrangement of the office holder brought for approval amends an existing arrangement, then only the approval of the audit committee or compensation and nominating committee (as appropriate) is required if that committee determines that the amendment is not material in relation to the existing arrangement.
Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions
Pursuant to the Companies Law, the disclosure requirements regarding personal interests that apply to directors and executive officers also apply to a controlling shareholder of a public company. We currently do not have a controlling shareholder.
An extraordinary transaction between a public company and a controlling shareholder, or in which a controlling shareholder has a personal interest, require the approval of a company’s audit committee, board of directors and shareholders in that order. For the terms of compensation (or insurance, indemnification or exculpation) of a controlling shareholder who is an office holder, the approval by our compensation and nominating committee is required in lieu of audit committee approval. The shareholder approval for any such extraordinary transaction or compensatory arrangement must fulfill one of the following requirements:
| • | at least a majority of the voting rights in the company held by shareholders who have no personal interest in the transaction and who are present and voting at the general meeting, must be voted in favor of approving the transaction or arrangement (for this purpose, abstentions are disregarded); or |
| • | the voting rights held by shareholders who have no personal interest in the transaction or arrangement and who are present and voting at the general meeting, and who vote against the transaction, do not exceed two percent of the voting rights in the company. |
Shareholder Duties
Pursuant to the Companies Law, a shareholder has a duty to act in good faith and in an acceptable manner toward the company and other shareholders and to refrain from abusing his or her power with respect to the company, including, among other things, in voting at a general meeting and at shareholder class meetings with respect to the following matters:
| • | an amendment to the company’s articles of association; |
| • | an increase of the company’s authorized share capital; |
| • | a merger; or |
| • | interested party transactions that require shareholder approval. |
In addition, a shareholder has a general duty to refrain from discriminating against other shareholders.
Certain shareholders also have a duty of fairness toward the company. These shareholders include any controlling shareholder, any shareholder who knows that it has the power to determine the outcome of a
117
Table of Contents
shareholder vote and any shareholder who has the power to appoint or to prevent the appointment of an office holder of the company or exercise any other rights available to it under the company’s articles of association with respect to the company. The Companies Law does not define the substance of this duty of fairness, except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty of fairness.
Israeli courts have not yet interpreted the scope or nature of any of these duties.
Approval of Private Placements
Under the Companies Law, a significant private placement of securities requires approval by the board of directors and the shareholders by a simple majority. A private placement is considered a significant private placement if it results in a person becoming a controlling shareholder, or if all of the following conditions are met:
| • | the securities issued amount to 20% or more of the company’s outstanding voting rights before the issuance; |
| • | some or all of the consideration is other than cash or listed securities or the transaction is not on market terms; and |
| • | the transaction will increase the relative holdings of a shareholder who holds 5% or more of the company’s outstanding share capital or voting rights or that will cause any person to become, as a result of the issuance, a holder of more than 5% of the company’s outstanding share capital or voting rights. |
Exculpation, Insurance and Indemnification of Office Holders
Under the Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a provision authorizing such exculpation is included in its articles of association. Our articles of association include such a provision. An Israeli company may not exculpate a director from liability arising out of a prohibited dividend or distribution to shareholders.
An Israeli company may indemnify an office holder in respect of the following liabilities and expenses incurred for acts performed as an office holder, either in advance of an event or following an event, provided a provision authorizing such indemnification is contained in its articles of association:
| • | financial liability imposed on him or her in favor of another person pursuant to a judgment, settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned events and amount or criteria; |
| • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability, such as a criminal penalty, was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and |
| • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf or by a third party or in |
118
Table of Contents
| connection with criminal proceedings in which the office holder was acquitted or as a result of a conviction for an offense that does not require proof of criminal intent. |
An Israeli company may insure an office holder against the following liabilities incurred for acts performed as an office holder if and to the extent provided in the company’s articles of association:
| • | a breach of the duty of loyalty to the company, to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| • | a breach of the duty of care to the company or to a third party, including a breach arising out of the negligent conduct of the office holder; |
| • | a financial liability imposed on the office holder in favor of a third party; |
| • | a financial liability imposed on the office holder in favor of a third party harmed by a breach in an administrative proceeding; and |
| • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an administrative proceeding instituted against him or her. |
An Israeli company may not indemnify or insure an office holder against any of the following:
| • | a breach of the duty of loyalty, except to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| • | a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder; |
| • | an act or omission committed with intent to derive illegal personal benefit; or |
| • | a fine or forfeit levied against the office holder. |
Under the Companies Law, exculpation, indemnification and insurance of office holders must be approved by the compensation and nominating committee and the board of directors and, with respect to directors and our Chief Executive Officer, also by our shareholders (with a special majority among the shareholders who have no personal interest in such approval).
Our articles of association allow us to indemnify and insure our office holders for any liability imposed on them as a consequence of an act which was performed by virtue of being an office holder. We intend to ask our shareholders to approve, prior to consummation of this offering, an amendment to our articles of association that will extend such indemnification and insurance to cover omissions by our office holders (in their role as such) as well. Our office holders are currently covered by a directors’ and officers’ insurance policy.
We have entered into agreements with each of our directors and executive officers exculpating them, to the fullest extent permitted by law, from liability to us for damages caused to us as a result of a breach of duty of care, and undertaking to indemnify them to the fullest extent permitted by law. This indemnification is limited to events determined as foreseeable by the board of directors based on our activities, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances.
The maximum indemnification amount set forth in such agreements is limited to an amount equal to 25% of our shareholders’ equity as reflected in our most recent consolidated financial statements prior to the date on which the indemnity payment is made. We intend to ask our shareholders to approve, prior to the consummation of this offering, an amendment to such indemnification agreements that will provide that if the amount equal to 25% of our shareholders’ equity is insufficient to cover all indemnity amounts payable with respect to all indemnifiable directors and executive officers, such amount will be allocated among our directors and executive officers pro rata, in accordance with their relative culpabilities, as finally determined by a court with respect to a
119
Table of Contents
particular claim. The maximum amount set forth in such agreements is in addition to any amount paid (if paid) under insurance and/or by a third party pursuant to an indemnification arrangement. In the opinion of the SEC, indemnification of directors and office holders for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, is against public policy and therefore unenforceable.
Compensation of Officers and Directors
The compensation paid by us to our current executive officers, including salaries and share-based compensation, for the year ended December 31, 2012, was approximately $1,431,000. This amount includes $901,000 of gross salaries set aside or accrued to provide pension, severance, retirement, or similar benefits or expenses, but does not include any business travel, relocation, professional and business association dues and expenses reimbursed to office holders, and other expenses commonly reimbursed by companies in Israel.
Our compensation for our executive officers is derived from employment agreements and each executive officer’s personal contribution to our management, operations and our success, and will be determined consistently with our compensation policy (following its adoption by our compensation and nominating committee, board and shareholders). Each executive officer’s annual bonus is determined according to a formula that is consistent with the compensation policy and that links financial-target based goals and metrics determined at the beginning of each fiscal year to specific goals and objectives within the responsibility of the relevant executive officer. The financial target is uniform with respect to all of our executive officers, including our Chief Executive Officer; however, our Chief Executive Officer has discretion to determine its weight with respect to our other executive offers, taking into account the degree of influence such executive officer has on achieving our financial target. Our Chief Executive Officer’s goals and objectives are determined by the compensation and nominating committee and our board of directors, and our board of directors has authorized our Chief Executive Officer to set specific goals and objectives for each of our other executive officers. At the beginning of each fiscal year, our board of directors determines the maximum bonus for each of our executive officers, including our Chief Executive Officer. In the case of our executive officers other than the Chief Executive Officer, assuming that the bonus terms conform to the compensation policy, such terms only require approval by the compensation and nominating committee followed by the board of directors. For our Chief Executive Officer, the bonus terms will in general require approval by our shareholders as well.
Under Israeli law, as a public company with shares listed only on the TASE until the consummation of this offering, we have been required to disclose the compensation, on an individual basis, of our five most highly compensated executive officers. The following table presents information regarding compensation accrued in our financial statements for our executive officers with respect to whom specific disclosure has been made in Israel, namely our Chief Executive Officer (Ofer Haviv), our Chief Financial Officer (Sigal Fattal), our Chief Technology Officer and Executive Vice President of Development (Hagai Karchi), our Executive Vice President of Technology Infrastructure (Assaf Kacen) and our Executive Vice President of Strategy and Business Development, (Assaf Oron), as of December 31, 2012.
| Name and Position |
Salary | Bonus | Value of Options Granted |
Total | ||||||||||||
| Ofer Haviv, President and Chief Executive Officer |
$ | 278,123 | $ | 44,064 | $ | 151,374 | (2) | $ | 473,561 | |||||||
| Sigal Fattal, Chief Financial Officer(1) |
77,760 | 11,923 | 13,219 | 102,902 | ||||||||||||
| Hagai Karchi, Chief Technology Officer and Executive Vice President of Development , |
182,478 | 20,736 | 83,204 | 286,418 | ||||||||||||
| Assaf Kacen, Executive Vice President of Technology Infrastructure |
184,033 | 24,624 | 77,501 | 286,158 | ||||||||||||
| Assaf Oron, Executive Vice President of Strategy and Business Development |
178,330 | 20,736 | 83,204 | 282,270 | ||||||||||||
| (1) | Employment commenced June 2012. |
| (2) | In September 2013, our compensation and nominating committee and board of directors approved a revised grant to our President and Chief Executive Officer, Ofer Haviv, of 215,000 options to purchase 215,000 ordinary shares. The options shall be exercisable at a price of NIS 48.18 (approximately $13.62). The grant is subject to the approval of our shareholders at our general shareholders meeting. |
120
Table of Contents
We pay our external director Dr. Michael Anghel an annual fee of NIS 71,300 (approximately $20,158) as well as a fee of NIS 3,750 (approximately $1,060) for attendance at board meetings and committee meetings and our other external director, Dr. Kinneret Livnat Savitsky an annual fee of NIS 53,500 (approximately $15,126) as well as a fee of NIS 2,820 (approximately $797) for attendance at board meetings and committee meetings. Independent directors that are not external directors are also entitled to annual compensation and compensation for attendance at board meetings. Currently, our only independent director who is not also an external director is Dr. Simcha Sadan, to whom we pay an annual fee of NIS 71,300 (approximately $20,158) as well as a fee of NIS 3,750 (approximately $1,060) for attendance at board meetings and committee meetings. We also reimburse our directors for expenses arising from their board membership.
Employment and Consulting Agreements with Executive Officers
We have entered into written employment agreements with all of our executive officers. Each of these agreements contains provisions regarding non-competition, confidentiality of information and assignment of inventions. The non-competition provision applies for a period that is generally 12 months following termination of employment. The enforceability of covenants not to compete in Israel and in the United States is subject to limitations.
Directors’ Service Contracts
There are no arrangements or understandings between us, on the one hand, and any of our directors, on the other hand, providing for benefits upon termination of their service as directors of our company.
Option Plans
We maintain three share option and incentive plans which, as of June 30, 2013, are constituted as follows: (i) our Evogene Share Option Plan (2002), or the 2002 Plan, which provides for the grant of options to purchase up to 90,676 of our ordinary shares to our company’s and our subsidiaries’ respective directors, employees and consultants; (ii) our Evogene Ltd. Key Employee Share Incentive Plan, 2003, or the 2003 Plan, which provides for the grant of options to purchase up to 2,563,083 of our ordinary shares; and (iii) the Evogene Ltd. 2013 Share Option Plan, or the 2013 Plan, which provides for the grant of options to purchase up to 1,232,102 of our ordinary shares. As of June 30, 2013, a total of 1,232,102 ordinary shares remained available for issuance under our share option and incentive plans, all under our 2013 Plan, as all remaining ordinary shares under our 2002 Plan and 2003 Plan that are not subject to outstanding option grants had been transferred to our 2013 Plan. As of that date, 90,676 and 2,563,083 ordinary shares were issuable upon the exercise of outstanding options under our 2002 and 2003 Plans, respectively, while no options had yet been issued under our 2013 Plan. Of the foregoing outstanding options, options to purchase 1,972,417 ordinary shares, in the aggregate, had vested under the plans as of that date, with a weighted average exercise price of NIS 22.22, or $6.14 per share.
Among other option awards, the plans provide for granting options in compliance with Section 102 of the Israeli Income Tax Ordinance, 1961, or the Ordinance, which provides to employees, directors and officers, who are not controlling shareholders (i.e., hold less than 10% of a company’s share capital) and are Israeli residents, favorable tax treatment for compensation in the form of shares or options issued or granted, as applicable, to a trustee under the “capital gains track” for the benefit of the relevant employee, director or officer and are (or were) to be held by the trustee for at least two years after the date of grant or issuance. Under the “capital gains track”, we are not allowed to deduct an expense with respect to the grant or issuance of the options or shares.
The plans also permit us to grant options to U.S. residents, which options may qualify as “incentive stock options” within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986, as amended, or the Code, and to residents of other jurisdictions.
Options granted under our plans are subject to vesting schedules and generally expire 10 years from the grant date. The plans address the treatment of vested and unvested options upon the termination of employment
121
Table of Contents
of the option holder as well as upon consummation of a merger or consolidation of our company, or sale of all or substantially all of our shares or assets. The plans also provide for certain lock up arrangements (generally, for a 180-day period) to which option holders and holders of shares issued upon exercise of options will be subject upon consummation of a public offering, similarly to our directors and executive officers.
The plans are administered by our board of directors or by a committee appointed by our board of directors.
122
Table of Contents
The following table sets forth information with respect to the beneficial ownership of our shares as of September 10, 2013 and after this offering by:
| • | each person or entity known by us to own beneficially more than 5% of our outstanding shares; |
| • | each of our directors and executive officers individually; and |
| • | all of our executive officers and directors as a group. |
The beneficial ownership of ordinary shares is determined in accordance with the rules of the SEC and generally includes any ordinary shares over which a person exercises sole or shared voting or investment power, or the right to receive the economic benefit of ownership. For purposes of the table below, we deem shares subject to options or warrants that are currently exercisable or exercisable within 60 days of September 10, 2013, to be outstanding and to be beneficially owned by the person holding the options or warrants for the purposes of computing the percentage ownership of that person but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person. The percentage of shares beneficially owned prior to the offering is based on 18,879,987 ordinary shares outstanding as of September 10, 2013. We have also set forth below information known to us regarding any significant change in the percentage ownership of our ordinary shares by any major shareholders during the past three year.
As of September 10, 2013, we had two holders of record of our ordinary shares in the United States. Such holders of record currently hold 12.0% of our outstanding ordinary shares. The number of record holders is not representative of the number of beneficial holders of our ordinary shares, as the shares of all shareholders who hold shares on the TASE are recorded in the name of our Israeli share registrar, Bank Leumi Le’Israel Registration Company Ltd.
All of our shareholders, including the shareholders listed below, have the same voting rights attached to their ordinary shares. See “Description of Share Capital—Articles of Association—Voting.” Neither our principal shareholders nor our directors and executive officers have different or special voting rights. Unless otherwise noted below, each shareholder’s address is 13 Gad Feinstein Street, Park Rehovot P.O.B 2100, Rehovot 76121, Israel.
A description of any material relationship that our principal shareholders have had with us or any of our predecessors or affiliates within the past three years is included under “Certain Relationships and Related Party Transactions.”
| Name of Beneficial Owner |
Shares Beneficially Owned Prior to Offering |
Shares Beneficially Owned After Offering | ||||||||||
| Number | Percentage | Number | Percentage | |||||||||
| Directors and Executive Officers |
||||||||||||
| Ofer Haviv(1) |
512,500 | 2.7 | % | |||||||||
| Sigal Fattal(2) |
25,781 | * | ||||||||||
| Assaf Kacen(3) |
99,691 | * | ||||||||||
| Dr. Hagai Karchi(4) |
292,812 | 1.5 | % | |||||||||
| Assaf Oron(5) |
167,812 | * | ||||||||||
| Martin S. Gerstel(6) |
400,256 | 2.1 | % | |||||||||
| Leon Y. Recanati(7) |
856,984 | 4.5 | % | |||||||||
| Dr. Michael Anghel(8) |
15,000 | * | ||||||||||
| Dr. Kinneret Livnat Savitsky(9) |
7,500 | * | ||||||||||
| Dr. Simcha Sadan(10) |
52,275 | * | ||||||||||
| Dr. Adina Makover(11) |
12,674 | * | ||||||||||
| All executive officers and directors as a group (11 persons) |
2,443,285 | 12.9 | % | |||||||||
| Principal Shareholders |
||||||||||||
| Monsanto Company(12) |
1,636,364 | 8.7 | % | |||||||||
123
Table of Contents
| * | Less than 1%. |
| (1) | Consists of 512,500 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The weighted average exercise price of these options is NIS 19.11 or $5.40 per share and expire on June 19, 2020. Does not include 215,000 options to purchase 215,000 ordinary shares, which remain subject to shareholder approval at our general shareholders meeting. |
| (2) | Consists of 25,781 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The weighted average exercise price of these options is NIS 37.92 or $10.72 per share and expire on July 15, 2023. |
| (3) | Consists of 99,691 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 29.46 or $8.33 per share and expire on July 15, 2023. |
| (4) | Consists of 90,000 ordinary shares and 202,812 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 24.67 or $6.97 per share and expire on July 15, 2023. |
| (5) | Consists of 167,812 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 27.31 or $7.72 per share and expire on July 15, 2023. |
| (6) | Consists of 51,250 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 12.43 or $3.51 per share and expire on April 9, 2020. Also includes 349,006 ordinary shares, consisting of: (a) 133,815 shares ordinary shares held by Martin Gerstel and (b) 215,191 shares held by Shomar Corporation over which Martin Gerstel and his wife Mrs. Shoshana Gerstel possess voting and investment power. |
| (7) | Consists of 838,859 ordinary shares and 18,125 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 13.20 or $3.73 per share and expire on April 9, 2020. |
| (8) | Consists of 15,000 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 13.21 or $3.73 per share and expire on April 9, 2020. |
| (9) | Consists of 7,500 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 32.30 or $9.13 per share and expire on September 17, 2020. |
| (10) | Includes 18,125 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 13.20 or $3.73 per share and expire on April 9, 2020. Also includes 34,150 ordinary shares, held by S.M.B. Ltd., over which Dr. Sadan possesses voting and investment power. |
| (11) | Consists of 1,424 ordinary shares and 11,250 ordinary shares underlying options to purchase ordinary shares exercisable within 60 days of September 10, 2013. The exercise price of these options is NIS 15.18 or $4.29 per share and expire on April 9, 2020. |
| (12) | The address for Monsanto Company is 800 North Lindbergh Boulevard, St. Louis, Missouri 63167. The purchase price of the ordinary share was NIS 38.91 or $11.00 per share. Monsanto Company possesses voting and dispositive investment power. Monsanto Company is a Delaware corporation and is listed on the New York Stock Exchange. |
124
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Indemnification Agreements with Office Holders
Our articles of association to be effective following this offering permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted by law, subject to limited exceptions. We have entered into agreements with each of our current office holders exculpating them from a breach of their duty of care to us to the fullest extent permitted by law, subject to limited exceptions, and undertaking to indemnify them to the fullest extent permitted by law, including with respect to liabilities resulting from this offering to the extent that these liabilities are not covered by insurance. See “Management—Exculpation, Insurance and Indemnification of Office Holders.”
Investors’ Rights Agreement
On January 3, 2006, we entered into an Investors’ Rights Agreement (“IRA”) with our shareholders. Pursuant to the IRA, at any time upon or after an initial public offering of our securities, any shareholder or group of shareholders holding an aggregate of at least 12.5% of our ordinary shares may request in writing that (i) we use our best efforts to register such shareholder or shareholders’ ordinary shares, or any part thereof, for trading on a recognized European or U.S. securities exchange or quotation system (“demand rights”), provided that these demand rights may only be effected after six months from the consummation of the initial public offering and the aggregate anticipated offering price of such shares equals at least $4.0 million, or (ii) we effect a registration statement on Form F-3 for such shares (“F-3 rights”), provided that the aggregate anticipated offering price of such shares equals at least $2.5 million. The IRA also provides our shareholders with incidental or “piggy back” registration rights in the event that we determine to register any of our securities pursuant to or following an initial public offering, although no “piggy back” rights will be available in connection with an initial public offering of our securities in which security holders of ours do not participate as sellers.
Any rights to registration granted under the IRA, however, terminate upon the fifth anniversary of the closing of an initial public offering of our securities. Furthermore, we are not obligated to effect the demand rights or F-3 rights contemplated under the IRA when (i) effecting such rights would require us to execute a general consent to service of process in a particular jurisdiction, unless we are already subject to service in such jurisdiction and except as may be required by the Securities Act of 1933, (ii) we had already effected a registration of shares pursuant to the IRA within the previous 12 months, and such registration was declared or ordered effective, or (iii) we receive an opinion of counsel, satisfactory to us and the respective shareholder or shareholders, stating that the sale of shares may be made in a transaction exempted from the registration and prospectus delivery requirements of the Securities Act of 1933 and from the comparable requirements of the applicable state securities laws so that any transfer restrictions may be removed upon the consummation of such sale.
125
Table of Contents
Share Capital
Our authorized share capital consists of 150,000,000 ordinary shares, par value NIS 0.02 per share, of which 18,879,987 shares are issued and outstanding as of June 30, 2013.
All of our outstanding ordinary shares are validly issued, fully paid and non-assessable. Our ordinary shares are not redeemable and do not have any preemptive rights. The current position of the TASE is that a company whose shares are traded on the TASE may not issue or register for trading preferred shares. Under the Israeli Securities Law, all of our outstanding shares must be validly issued and fully paid, and must be registered for trading on the TASE. All of our ordinary shares have equal rights and are fully paid.
Our board of directors may determine the issue prices and terms for such shares or other securities, and may further determine any other provision relating to such issue of shares or securities. We may also issue and redeem redeemable securities on such terms and in such manner as our board of directors shall determine. Our board of directors may not make calls or assessments on our ordinary shares.
Our articles of association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Companies Law and must be approved by a resolution duly passed by our shareholders at an annual or extraordinary general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits and an issuance of shares for less than their nominal value, require the approval of both our board of directors and an Israeli court.
Articles of Association
All references to our articles of association in this section refer to our articles of association to be effective following this offering.
Objects and Purposes. Our registration number with the Israeli Registrar of Companies is 51-283872-3. Our purpose as set forth in article 4 of our articles of association is to engage in any legal business.
Voting. Holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote of shareholders at a shareholder meeting. Shareholders may vote at shareholder meetings either in person, by proxy or by written ballot. Israeli law does not allow public companies to adopt shareholder resolutions by means of written consent in lieu of a shareholder meeting. Shareholder voting rights may be affected by the grant of any special voting rights to the holders of a class of shares with preferential rights that may be authorized in the future. Except as otherwise disclosed herein, an amendment to our articles of association to change the rights of our shareholders requires the prior approval of a simple majority of our shares represented and voting at a general meeting and of the holders of a class of shares whose rights are being affected (or the consent in writing of all the holders of such class of shares).
Share Ownership Restrictions. The ownership or voting of ordinary shares by non-residents of Israel is not restricted in any way by our articles of association or the laws of the State of Israel, except that citizens of countries which are in a state of war with Israel may not be recognized as owners of ordinary shares.
Transfer of Shares. Fully paid ordinary shares are issued in registered form and may be freely transferred under our articles of association unless the transfer is restricted or prohibited by another instrument, Israeli law or the rules of a stock exchange on which the shares are traded.
Election of Directors. Our ordinary shares do not have cumulative voting rights for the election of directors. Rather, under our articles of association our directors are elected, upon expiration of the term of office of any
126
Table of Contents
director, by the holders of a simple majority of our ordinary shares at a general shareholder meeting (excluding abstentions). As a result, the holders of our ordinary shares that represent more than 50% of the voting power represented at a shareholder meeting and voting thereon (excluding abstentions) have the power to elect any or all of our directors whose positions are being filled at that meeting, subject to the special approval requirements for external directors described under “Board Practices—Board of Directors—External Directors.” Vacancies on our board of directors, resulting from a resignation or other termination of service by a then serving director, may be filled by a vote of a simple majority of the directors then in office as described under “Board Practices—Board of Directors—Directors and Officers.” For additional information regarding the election of and voting by directors, please refer to “Board Practices—Board of Directors.”
Dividend and Liquidation Rights. Under Israeli law, we may declare and pay a dividend only if, upon the reasonable determination of our board of directors, the distribution will not prevent us from being able to meet the terms of our existing and contingent obligations as they become due. Under the Companies Law, the distribution amount is further limited to the greater of retained earnings or earnings generated over the two most recent years according to our then last reviewed or audited financial statements, provided that the date of the financial statements is not more than six months prior to the date of distribution. In the event that we do not have retained earnings and earnings legally available for distribution, as defined in the Companies Law, we may seek the approval of the court in order to distribute a dividend. The court may approve our request if it is convinced that there is no reasonable concern that the payment of a dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of ordinary shares on a pro-rata basis. Dividend and liquidation rights may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future. See “Dividend Policy.”
Shareholder Meetings. Under the Companies Law, we are required to convene an annual general meeting of our shareholders once every calendar year, not more than 15 months following the preceding annual general meeting. Our board of directors may convene a special general meeting of our shareholders and is required to do so at the request of two directors or one quarter of the members of our board of directors, or at the request of one or more holders of 5% or more of our share capital and 1% of our voting power, or the holder or holders of 5% or more of our voting power. All shareholder meetings require prior notice of at least 14 days and, in certain cases, 35 days. The chairperson of our board of directors or another one of our directors authorized by our board of directors presides over our general meetings. If either of such persons is not present within 15 minutes from the appointed time for the commencement of the meeting, the directors present at such meeting shall appoint one of our directors as the chairperson for such meeting and if they fail to do so, then the shareholders present shall appoint one of our directors to act as chairperson and if no director is present, then one of the shareholders present at such meeting shall act as chairperson. Subject to the provisions of the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to the date of the meeting, depending on the type of meeting and whether written proxies are being used.
Quorum. Under our amended and restated articles of association, the quorum required for a meeting of shareholders consists of at least two shareholders present in person, by proxy or by written ballot, who hold or represent between them at least 25% of our voting power. A meeting adjourned for lack of a quorum generally is adjourned to the same day in the following week at the same time and place (without requirement of additional notification to the shareholders), or to a later time, if indicated in the notice to the meeting or to such other time and place as determined by the board of directors in a notice to our shareholders. At the reconvened meeting, if a quorum is not present within half an hour from the appointed time for the commencement of the meeting, the meeting will take place so long as at least one shareholder is present, unless the meeting was called pursuant to a request by our shareholders, in which case the quorum required is the number of shareholders required to call the meeting as described under “—Shareholder Meetings.”
127
Table of Contents
Resolutions. Under the Companies Law, unless otherwise provided in the articles of association or applicable law, all resolutions of the shareholders require a simple majority of the voting rights represented at the meeting, in person, by proxy or by written ballot, and voting on the resolution (excluding abstentions).
Access to Corporate Records. Under the Companies Law, all shareholders generally have the right to review minutes of our general meetings, our shareholder register, including with respect to material shareholders, our articles of association, our financial statements and any document we are required by law to file publicly with the Israeli Companies Registrar or the Israeli Securities Authority. Any shareholder who specifies the purpose of its request may request to review any document in our possession that relates to any action or transaction with a related party which requires shareholder approval under the Companies Law. We may deny a request to review a document if we determine that the request was not made in good faith, that the document contains a trade secret or patent or that the document’s disclosure may otherwise impair our interests.
Modification of Class Rights. The rights attached to any class of share (to the extent that we may have separate classes of shares in the future), such as voting, liquidation and dividend rights, may be amended by adoption of a resolution by the holders of a majority of our shares represented at the meeting and the holders or a majority of the shares of that class present at a separate class meeting, or otherwise in accordance with the rights attached to such class of shares, as set forth in our articles of association.
Acquisitions Under Israeli Law
Full Tender Offer. A person wishing to acquire shares of a public Israeli company and who could as a result hold over 90% of the target company’s voting rights or the target company’s issued and outstanding share capital (or of a class thereof), is required by the Companies Law to make a tender offer to all of the company’s shareholders for the purchase of all of the issued and outstanding shares of the company (or the applicable class). If (a) the shareholders who did not accept the offer hold less than 5% of the issued and outstanding share capital of the company (or the applicable class) and the shareholders who accept the offer constitute a majority of the offerees that do not have a personal interest in the acceptance of the tender offer or (b) the shareholders who did not accept the tender offer hold less than 2% of the issued and outstanding share capital of the company (or of the applicable class), all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. A shareholder who had its shares so transferred may petition the court within six months from the date of acceptance of the full tender offer, regardless of whether such shareholder agreed to the offer, to determine whether the tender offer was for less than fair value and whether the fair value should be paid as determined by the court. However, an offeror may provide in the offer documents that a shareholder who accepted the offer will not be entitled to appraisal rights as described in the preceding sentence, as long as the offeror and the company disclosed the information required by law in connection with the tender offer. If (a) the shareholders who did not accept the tender offer hold 5% or more of the issued and outstanding share capital of the company (or of the applicable class) or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in the acceptance of the tender offer, or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class) the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of the company’s issued and outstanding share capital (or of the applicable class) from shareholders who accepted the tender offer.
Special Tender Offer. The Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company. This rule does not apply if there is already another holder of 25% or more of the voting rights in the company. Similarly, the Companies Law provides that an acquisition of shares in a public company must be made by means of a tender offer if as a result of the acquisition the purchaser would become a holder of more than 45% of the voting rights in the company, if there is no other shareholder of the company who holds more than 45% of the voting rights in the company. These
128
Table of Contents
requirements do not apply if the acquisition (i) occurs in the context of a private placement by the company that received shareholder approval, (ii) was from a shareholder holding 25% or more of the voting rights in the company and resulted in the acquirer becoming a holder of 25% or more of the voting rights in the company, or (iii) was from a holder of more than 45% of the voting rights in the company and resulted in the acquirer becoming a holder of more than 45% of the voting rights in the company. A special tender offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding shares will be acquired by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer (excluding controlling shareholders, holders of 25% or more of the voting rights in the company and any person having a personal interest in the acceptance of the tender offer).
In the event that a special tender offer is made, a company’s board of directors is required to express its opinion on the advisability of the offer, or shall abstain from expressing any opinion if it is unable to do so, provided that it gives the reasons for its abstention. An office holder in a target company who, in his or her capacity as an office holder, performs an action the purpose of which is to cause the failure of an existing or foreseeable special tender offer or is to impair the chances of its acceptance, is liable to the potential purchaser and shareholders for damages, unless such office holder acted in good faith and had reasonable grounds to believe he or she was acting for the benefit of the company. However, office holders of the target company may negotiate with the potential purchaser in order to improve the terms of the special tender offer, and may further negotiate with third parties in order to obtain a competing offer.
If a special tender offer is accepted, then shareholders who did not respond to or that had objected the offer may accept the offer within four days of the last day set for the acceptance of the offer.
In the event that a special tender offer is accepted, then the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger. The Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain conditions described under the Companies Law are met, a majority of each party’s shareholders. The board of directors of a merging company is required pursuant to the Companies Law to discuss and determine whether in its opinion there exists a reasonable concern that as a result of a proposed merger, the surviving company will not be able to satisfy its obligations towards its creditors, such determination taking into account the financial status of the merging companies. If the board of directors determines that such a concern exists, it may not approve a proposed merger. Following the approval of the board of directors of each of the merging companies, the boards of directors must jointly prepare a merger proposal for submission to the Israeli Registrar of Companies.
For purposes of the shareholder vote, unless a court rules otherwise, if one of the merging companies (or any person who holds 25% or more of the outstanding shares or the right to appoint 25% or more of the directors of one of the merging companies) holds shares in the other merging company, the merger will not be deemed approved if a majority of the shares voted at the shareholders meeting by shareholders other than the other party to the merger, or by any person who holds 25% or more of the outstanding shares or the right to appoint 25% or more of the directors of the other party, vote against the merger. In addition, if the non-surviving entity of the merger has more than one class of shares, the merger must be approved by each class of shareholders. If the transaction would have been approved but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders. If a merger is with a company’s controlling shareholder or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same special majority approval that governs all extraordinary
129
Table of Contents
transactions with controlling shareholders (as described above under “Approval of Related Party Transactions Under Israeli Law—Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions”).
Under the Companies Law, each merging company must inform its secured creditors of the proposed merger plans. Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of any of the parties to the merger, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be completed unless at least 50 days have passed from the date that a proposal for approval of the merger is filed with the Israeli Registrar of Companies and 30 days from the date that shareholder approval of both merging companies is obtained.
Antitakeover Measures under Israeli Law
Besides the requirements described above with respect to tender offers and mergers, Israeli law and our articles of association enable the implementation of additional measures that may delay or prevent a takeover attempt and thereby preclude our shareholders from realizing a potential premium over the market value of our ordinary shares that they hold. Our articles of association allow our company to increase its registered share capital and provide that the increased capital will be divided into shares having ordinary, preferred or deferred rights or any other special rights, or may be subject to terms and restrictions in respect of dividend, repayment of capital, voting or other terms, in each case provided that the general meeting of our shareholders approves via a simple majority of shares present (in person or by proxy) and voting. Israeli law also permits the issuance of preferred stock. However, the TASE rules and regulations prohibit a listed company from having more than one class of shares listed, and the TASE’s current position is that a listed company may not issue or list preferred shares. Therefore, assuming that the TASE’s current position does not change, as long as our ordinary shares are listed on the TASE, we will be prohibited from issuing preferred stock.
To date, the legality of a poison pill as an additional antitakeover measure has not been examined in Israel.
Exchange Controls
Other than general anti-money laundering regulations, there are currently no Israeli currency control regulations in effect that restrict our import or export of capital to or from the State of Israel, or the availability of cash and cash equivalents for use by our affiliated companies. Under the Bank of Israel Law, 5770-2010, the Governor of the Bank of Israel, with the approval of the monetary policy committee of the Bank of Israel, is authorized to issue an administrative order restricting the transfer of funds to or from Israel. However, such an order is only likely to be issued under emergency circumstances and only for a temporary period, if necessary for the achievement of the goals of the Bank of Israel or the carrying out of its responsibilities under Israeli law. Furthermore, Israel has agreed, pursuant to international agreements to which it is a party (including incident to Israel’s having joined the International Monetary Fund) to allow for the free flow of capital to and from within its borders. Certain transactions nevertheless require the filing of reports with the Bank of Israel.
Similarly, there are no currently effective Israeli governmental laws, decrees, regulations or other legislation that restrict the payment of dividends or other distributions with respect to our ordinary shares or the proceeds from the sale of the shares, except for the obligation of Israeli residents to file reports with the Bank of Israel regarding some transactions. However, legislation remains in effect under which currency controls can be imposed by administrative action at any time.
Borrowing Powers
Pursuant to the Companies Law and our articles of association, our board of directors may exercise all powers and take all actions that are not required under law or under our articles of association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
130
Table of Contents
Transfer Agent and Registrar
Upon listing of our ordinary shares for trading on the NYSE, the transfer agent and registrar for our ordinary shares in the United States will be American Stock Transfer & Trust Company, LLC. Its address is 6201 15th Avenue, Brooklyn, New York 11219, and its telephone number is (800) 937-5449. The nominee company to the TASE in which name most of our shares are held of record is Bank Leumi Le’Israel Registration Company Ltd.
Listing
Our ordinary shares are listed on the Tel Aviv Stock Exchange under the symbol “EVGN.” We have applied to have our ordinary shares listed on the NYSE under the symbol “EVGN.”
131
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering our ordinary shares have been traded only on the Tel Aviv Stock Exchange. In connection with this offering, we intend to apply to have our ordinary shares listed on NYSE, under the symbol “EVGN.” Sales of substantial amounts of our ordinary shares in the public market would adversely affect prevailing market prices of our ordinary shares. Upon completion of this offering, we will have outstanding ordinary shares, assuming the underwriters do not exercise their option to purchase additional ordinary shares. All of the ordinary shares sold in this offering will be freely transferable without restriction or further registration under the Securities Act by persons other than by our affiliates. In addition, all of our ordinary shares outstanding before this offering will be freely transferable and may be resold in the United States without restriction or further registration under the Securities Act by persons other than shares held by our affiliates and those of our existing shareholders who have signed lock-up agreements. Under Rule 144 of the Securities Act, or Rule 144, an “affiliate” of a company is a person that directly or indirectly controls, is controlled by or is under common control with that company. Affiliates may sell only the volume of shares described below and their sales are subject to additional restrictions described below.
Lock-up Agreements
We and our executive officers, directors, and substantially all of our shareholders holding collectively 20.2% of our outstanding ordinary shares, have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any ordinary shares or any securities convertible into or exchangeable for ordinary shares except for the ordinary shares offered in this offering without the prior written consent of Credit Suisse Securities (USA) LLC and Deutsche Bank Securities Inc. for a period of 180 days after the date of this prospectus. After the expiration of the 180 day period, the ordinary shares held by our directors, executive officers or certain of our other existing shareholders may be sold subject to the restrictions under Rule 144 under the Securities Act or by means of registered public offerings.
Rule 144
In general, under Rule 144 of the Securities Act, a person (or persons whose shares are aggregated) who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months (including any period of consecutive ownership of preceding non-affiliated holders) would be entitled to sell those shares, subject only to the availability of current public information about us. A non-affiliated person who has beneficially owned restricted securities within the meaning of Rule 144 for at least one year would be entitled to sell those shares without regard to the provisions of Rule 144.
A person (or persons whose shares are aggregated) who is deemed to be an affiliate of ours and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months would be entitled to sell within any three-month period a number of shares that does not exceed the greater of one percent of the then outstanding shares of our ordinary shares or the average weekly trading volume of our ordinary shares on NYSE during the four calendar weeks preceding such sale. Such sales are also subject to certain manner of sale provisions, notice requirements and the availability of current public information about us.
Options
Following the completion of this offering, we intend to file a registration statement on Form S-8 under the Securities Act to register ordinary shares reserved for issuance under our share incentive plans. The registration statement on Form S-8 will become effective automatically upon filing.
Ordinary shares issued upon exercise of a share option and registered under the Form S-8 registration statement will, subject to vesting provisions and Rule 144 volume limitations applicable to our affiliates, be available for sale in the open market immediately after the 180 day lock-up agreements expire. See “Management—Option Plans.”
132
Table of Contents
TAXATION AND GOVERNMENT PROGRAMS
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our ordinary shares. You are encouraged to consult your tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Israeli Tax Considerations and Government Programs
The following is a brief summary of the material Israeli income tax laws applicable to us, and certain Israeli Government programs that benefit us. This section also contains a discussion of material Israeli income tax consequences concerning the ownership and disposition of our ordinary shares purchased by investors in this offering. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of such investors include residents of Israel or traders in securities who are subject to special tax regimes not covered in this discussion. Because parts of this discussion are based on new tax legislation that has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities or the courts will accept the views expressed in this discussion. The discussion below is subject to change, including due to amendments under Israeli law or changes to the applicable judicial or administrative interpretations of Israeli law, which change could affect the tax consequences described below.
General Corporate Tax Structure in Israel
Israeli companies are generally subject to corporate tax at the rate of 25% of their taxable income. However, the effective tax rate payable by a company that qualifies as an Industrial Company that derives income from an Approved Enterprise, a Preferred Enterprise or a Beneficiary Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli company are subject to the prevailing corporate tax rate. On July 29, 2013, an amendment to the Ordinance was adopted, or the Tax Amendment. Pursuant to the Tax Amendment, Israeli companies will be subject to corporate tax on their taxable income at the rate of 26.5% for the 2014 tax year and thereafter.
Law for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969, generally referred to as the Industry Encouragement Law, provides several tax benefits for “Industrial Companies.” We currently qualify as an Industrial Company within the meaning of the Industry Encouragement Law.
The Industry Encouragement Law defines an “Industrial Company” as a company resident in Israel, of which 90% or more of its income in any tax year, other than income from defense loans, is derived from an “Industrial Enterprise” owned by it. An “Industrial Enterprise” is defined as an enterprise whose principal activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are available to Industrial Companies:
| • | amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which are used for the development or advancement of the company; |
| • | under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies; and |
| • | expenses related to a public offering are deductible in equal amounts over a three-year period. |
Eligibility for benefits under the Industry Encouragement Law is not contingent upon the approval of any governmental authority.
133
Table of Contents
There can be no assurance that we will continue to qualify as an Industrial Company or that the benefits described above will be available in the future.
Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain incentives for capital investments in production facilities (or other eligible assets) by “Industrial Enterprises” (as defined under the Investment Law).
The Investment Law was significantly amended effective April 1, 2005 (the “2005 Amendment”), and further amended as of January 1, 2011 (the “2011 Amendment”). Pursuant to the 2005 Amendment, tax benefits granted in accordance with the provisions of the Investment Law prior to its revision by the 2005 Amendment remain in force but any benefits granted subsequently are subject to the provisions of the 2005 Amendment. Similarly, the 2011 Amendment introduced new benefits to replace those granted in accordance with the provisions of the Investment Law in effect prior to the 2011 Amendment. However, companies entitled to benefits under the Investment Law as in effect prior to January 1, 2011 were entitled to choose to continue to enjoy such benefits, provided that certain conditions are met, or elect instead irrevocably to forego such benefits and have the benefits of the 2011 Amendment apply.
Tax Benefits Prior to the 2005 Amendment
An investment program that is implemented in accordance with the provisions of the Investment Law prior to the 2005 Amendment, referred to as an “Approved Enterprise,” is entitled to certain benefits. A company that wished to receive benefits as an Approved Enterprise must have received approval from the Investment Center of the Israeli Ministry of Industry, Trade and Labor, or the Investment Center. Each certificate of approval for an Approved Enterprise relates to a specific investment program in the Approved Enterprise, delineated both by the financial scope of the investment and by the physical characteristics of the facility or the asset.
In general, an Approved Enterprise is entitled to receive a grant from the Government of Israel and certain tax benefits under the “Grant Track” or an alternative package of tax benefits under the Alternative Track. The tax benefits from any certificate of approval relate only to taxable profits attributable to the specific Approved Enterprise. Income derived from activity that is not approved by the Investment Center or not integral to the activity of the Approved Enterprise does not enjoy tax benefits.
The tax benefits include a tax exemption for at least the first two years of the benefit period (depending on the geographic location of the Approved Enterprise facility within Israel) and the taxation of income generated from an Approved Enterprise at a reduced corporate tax rate of up to 25% for the remainder of the benefit period. The benefit period is ordinarily seven years commencing with the year in which the Approved Enterprise first generates taxable income. The benefit period is limited to 12 years from the operational year as determined by the Investment Center or 14 years from the start of the tax year in which approval of the Approved Enterprise is obtained, whichever is earlier.
A company that has an Approved Enterprise program is eligible for further tax benefits if it qualifies as a Foreign Investors’ Company, or a FIC, which is a company with a level of foreign investment, as defined in the Investment Law, of more than 25%. The level of foreign investment is measured as the percentage of rights in the company (in terms of shares, rights to profits, voting and appointment of directors), and of combined share and loan capital, that are owned, directly or indirectly, by persons who are not residents of Israel. The determination as to whether a company qualifies as a FIC is made on an annual basis. A company that qualifies as a FIC and has an Approved Enterprise program is eligible for an extended ten-year benefit period. As specified above, depending on the geographic location of the Approved Enterprise within Israel, income derived from the Approved Enterprise program may be exempt from tax on its undistributed income for a period of between two to ten years, and will be subject to a reduced tax rate for the remainder of the benefit period. The tax rate for the
134
Table of Contents
remainder of the benefits period will be 25%, unless the level of foreign investment exceeds 49%, in which case the tax rate will be 20% if the foreign investment is more than 49% and less than 74%; 15% if more than 74% and less than 90%; and 10% if 90% or more.
If a company elects the Alternative Track and distributes a dividend, it may be required to recapture the deferred corporate income tax applicable to the gross amount of distributed dividend that is derived from the portion of the company’s facilities that has been granted Approved Enterprise status during the tax exemption period at the applicable rate of 10%-25%. In addition, dividends paid out of income attributed to an Approved Enterprise are generally subject to withholding tax at source at the rate of 20% or such lower rate as may be provided in an applicable tax treaty.
The Investment Law also provides that an Approved Enterprise is entitled to accelerated depreciation on its property and equipment that are included in an Approved Enterprise program during the first five years in which the equipment is used.
The benefits available to an Approved Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations and the criteria in the specific certificate of approval. If a company does not meet these conditions, it would be required to refund the amount of tax benefits, as adjusted by the Israeli consumer price index, and interest.
One of our facilities has Approved Enterprise status granted by the Investment Center, which made us eligible for certain tax benefits under the Alternative Track.
Tax Benefits Subsequent to the 2005 Amendment
The 2005 Amendment changed certain provisions of the Investment Law. As a result of the 2005 Amendment, a company was no longer obliged to obtain Approved Enterprise status in order to receive the tax benefits previously available under the Alternative Track, and therefore generally there was no need to apply to the Investment Center for this purpose (Approved Enterprise status remains mandatory for companies seeking cash grants). Rather, the Company may claim the tax benefits offered by the Investment Law directly in its tax returns by notifying the Israeli Tax Authority within 12 months of the end of that year, provided that its facilities meet the criteria for tax benefits set out by the 2005 Amendment. A company is also granted a right to approach the Israeli Tax Authority for a pre-ruling regarding its eligibility for benefits under the 2005 Amendment.
The 2005 Amendment applies to new investment programs and investment programs with an election year commencing after 2004, but does not apply to investment programs approved prior to April 1, 2005. The 2005 Amendment provides that terms and benefits included in any certificate of approval that was granted before the 2005 Amendment became effective (April 1, 2005) will remain subject to the provisions of the Investment Law as in effect on the date of such approval.
Tax benefits are available under the 2005 Amendment to production facilities (or other eligible facilities), which are generally required to derive more than 25% of their business income from export and meet additional criteria stipulate in the amendment (referred to as a “Beneficiary Enterprise”). In order to receive the tax benefits, the 2005 Amendment states that a company must make an investment which meets all of the conditions, including exceeding a minimum investment amount specified in the Investment Law. Such investment may be made over a period of no more than three years ending at the end of the year in which the company requested to have the tax benefits apply to its Beneficiary Enterprise.
The extent of the tax benefits available under the 2005 Amendment to qualifying income of a Beneficiary Enterprise depend on, among other things, the geographic location in Israel of the Beneficiary Enterprise. The geographic location of the company at the year of election will also determine the period for which tax benefits are available. Such tax benefits include an exemption from corporate tax on undistributed income for a period of
135
Table of Contents
between two to ten years, depending on the geographic location of the Beneficiary Enterprise in Israel, and a reduced corporate tax rate of between 10% to 25% for the remainder of the benefits period, depending on the level of foreign investment in the company in each year if it is a qualified FIC. A company qualifying for tax benefits under the 2005 Amendment which pays a dividend out of income derived by its Beneficiary Enterprise
during the tax exemption period will be subject to corporate tax in respect of the gross amount of the dividend at the otherwise applicable rate of 10%-25%. Dividends paid out of income attributed to a Beneficiary Enterprise are generally subject to withholding tax at source at the rate of 15% or such lower rate as may be provided in an applicable tax treaty.
The benefits available to a Beneficiary Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations. If a company does not meet these conditions, it may be required to refund the amount of tax benefits, as adjusted by the Israeli consumer price index, and interest, or other monetary penalties.
On October 24, 2010, we received a tax ruling from the Israeli Tax Authority, according to which, among other things, our activity has been qualified as an “industrial activity”, as defined in the Investment Law and is also eligible to tax benefits as a Beneficiary Enterprise, which will apply to the turnover attributed to such enterprise. The benefits available to us under this tax ruling are subject to the fulfillment of conditions stipulated in the ruling. If we do not meet these conditions, the ruling may be abolished which would result in adverse tax consequences to us.
Tax Benefits Under the 2011 Amendment
The 2011 Amendment canceled the availability of the benefits granted to companies under the Investment Law prior to 2011 and, instead, introduced new benefits for income generated by a “Preferred Company” through its “Preferred Enterprise” (as such terms are defined in the Investment Law) as of January 1, 2011. Pursuant to the 2011 Amendment, a Preferred Company is entitled to a reduced corporate tax rate of 15% with respect to its income derived by its Preferred Enterprise in 2011 and 2012, unless the Preferred Enterprise is located in a specified development zone (our company is not), in which case the rate will be 10%. Under the 2011 Amendment, such corporate tax rate will be reduced from 15% and 10%, respectively, to 12.5% and 7%, respectively, in 2013 and to 16% and 9% in 2014 and thereafter, respectively.
Dividends paid out of income attributed to a Preferred Enterprise are generally subject to withholding tax at the rate of 20% or such lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, no tax is required to be withheld (however, if afterward distributed to individuals or non-Israeli company a withholding of 20% or such lower rate as may be provided in an applicable tax treaty, will apply).
The 2011 Amendment also provided transitional provisions to address companies already enjoying existing tax benefits under the Investment Law. These transitional provisions provide, among other things, that unless an irrevocable request is made to apply the provisions of the Investment Law as amended in 2011 with respect to income to be derived as of January 1, 2011: (i) the terms and benefits included in any certificate of approval that was granted to an Approved Enterprise which chose to receive grants and certain tax benefits under the Grant Track before the 2011 Amendment became effective will remain subject to the provisions of the Investment Law as in effect on the date of such approval, and subject to certain conditions; and (ii) terms and benefits included in any certificate of approval that was granted to an Approved Enterprise under the Alternative Track before the 2011 Amendment became effective will remain subject to the provisions of the Investment Law as in effect on the date of such approval, provided that certain conditions are met; and (iii) a Beneficiary Enterprise can elect to continue to benefit from the benefits provided to it before the 2011 Amendment came into effect, provided that certain conditions are met.
We have reviewed and evaluated the implications and effect of the benefits under the 2011 Amendment, and, while potentially eligible for such benefits, we have not yet chosen to be subject to the tax benefits introduced by the 2011 Amendment.
136
Table of Contents
From time to time, the Israeli Government has discussed reducing the benefits available to companies under the Investment Law. The termination or substantial reduction of any of the benefits available under the Investment Law could materially increase our tax liabilities.
Taxation of our Shareholders
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. A non-Israeli resident who derives capital gains from the sale of shares in an Israeli resident company that were purchased after the company was listed for trading on a stock exchange outside of Israel will be exempt from Israeli tax so long as the shares were not held through a permanent establishment that the non-resident maintains in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents: (i) have a controlling interest of 25% or more in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly. Additionally, such exemption is not applicable to a person whose gains from selling or otherwise disposing of the shares are deemed to be business income.
Additionally, a sale of securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, under the United States-Israel Tax Treaty, the disposition of shares by a shareholder who is a United States resident (for purposes of the treaty) holding the shares as a capital asset is generally exempt from Israeli capital gains tax unless, among other things, (i) the capital gain arising from the disposition is attributed to business income derived by a permanent establishment of the shareholder in Israel; (ii) the shareholder holds, directly or indirectly, shares representing 10% or more of the voting capital during any part of the 12-month period preceding the disposition; or (iii) such U.S. resident is an individual and was present in Israel for 183 days or more during the relevant taxable year.
In some instances where our shareholders may be liable for Israeli tax on the sale of their ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source.
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-Israeli residents generally will be subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the rate of 25%, which tax will be withheld at source, unless relief is provided in a treaty between Israel and the shareholder’s country of residence. With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or on any time during the preceding twelve months, the applicable tax rate is 30%. A “substantial shareholder” is generally a person who alone or together with such person’s relative or another person who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an executive officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right. However, a distribution of dividends to non-Israeli residents is subject to withholding tax at source at a rate of 15% if the dividend is distributed from income attributed to an Approved Enterprise, a Preferred Enterprise or a Beneficiary Enterprise, unless a reduced tax rate is provided under an applicable tax treaty. Pursuant to the Tax Amendment, effective January 1, 2014, if the dividend is being paid out of certain income attributable to an Approved Enterprise, a Preferred Enterprise or a Beneficiary Enterprise, the dividend will be subject to tax at the rate of 20% (and not 15%). A different rate may be provided in a treaty between Israel and the shareholder’s country of residence, as mentioned below.
In this regard, under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our ordinary shares who is a United States resident (for purposes of the United States-Israel Tax Treaty) is 25%. Consequently, distributions to U.S. residents of income attributed to an Approved Enterprise, a Preferred Enterprise or a Beneficiary Enterprise will be subject to withholding tax at a rate of 15%. However, generally, the maximum rate of withholding tax on dividends, not generated by an Approved Enterprise, a Preferred Enterprise or a Beneficiary Enterprise, that are paid to a United States corporation holding 10% or more of the outstanding voting capital throughout the tax year in which the dividend
137
Table of Contents
is distributed as well as during the previous tax year, is 12.5%, provided that not more than 25% of the gross income for such preceding year consists of certain types of dividends and interest. We cannot assure you that we will designate the profits that we may distribute in a way that will reduce shareholders’ tax liability.
United States Federal Income Taxation
The following is a description of the material United States federal income tax consequences to U.S. Holders (as defined below) of the acquisition, ownership and disposition of our ordinary shares. This description addresses only the United States federal income tax consequences to holders that are initial purchasers of our ordinary shares pursuant to the offering and that will hold such ordinary shares as capital assets. This description does not address tax considerations applicable to holders that may be subject to special tax rules, including, without limitation:
| • | banks, financial institutions or insurance companies; |
| • | real estate investment trusts, regulated investment companies or grantor trusts; |
| • | dealers or traders in securities, commodities or currencies; |
| • | tax-exempt entities; |
| • | certain former citizens or long-term residents of the United States; |
| • | persons that received our shares as compensation for the performance of services; |
| • | persons that will hold our shares as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle” for United States federal income tax purposes; |
| • | partnerships (including entities classified as partnerships for United States federal income tax purposes) or other pass-through entities, or holders that will hold our shares through such an entity; |
| • | U.S. Holders (as defined below) whose “functional currency” is not the U.S. dollar; or |
| • | holders that own directly, indirectly or through attribution 10.0% or more of the voting power or value of our shares. |
Moreover, this description does not address the United States federal estate, gift or alternative minimum tax consequences, or any state, local or foreign tax consequences, of the acquisition, ownership and disposition of our ordinary shares.
This description is based on the United States Internal Revenue Code of 1986, as amended (the “Code”), existing, proposed and temporary United States Treasury Regulations and judicial and administrative interpretations thereof, in each case as in effect and available on the date hereof. Each of the foregoing is subject to change, which change could apply retroactively and could affect the tax consequences described below. There can be no assurances that the U.S. Internal Revenue Service will not take a different position concerning the tax consequences of the acquisition, ownership and disposition of our ordinary shares or that such a position would not be sustained.
For purposes of this description, a “U.S. Holder” is a beneficial owner of our ordinary shares that, for United States federal income tax purposes, is:
| • | a citizen or resident of the United States; |
| • | a corporation (or other entity treated as a corporation for United States federal income tax purposes) created or organized in or under the laws of the United States or any state thereof, including the District of Columbia; |
138
Table of Contents
| • | an estate the income of which is subject to United States federal income taxation regardless of its source; or |
| • | a trust if such trust has validly elected to be treated as a United States person for United States federal income tax purposes or if (1) a court within the United States is able to exercise primary supervision over its administration and (2) one or more United States persons have the authority to control all of the substantial decisions of such trust. |
A “Non-U.S. Holder” is a beneficial owner of our ordinary shares that is neither a U.S. Holder nor a partnership (or other entity treated as a partnership for United States federal income tax purposes).
If a partnership (or any other entity treated as a partnership for United States federal income tax purposes) holds our ordinary shares, the tax treatment of a partner in such partnership will generally depend on the status of the partner and the activities of the partnership. Such a partner or partnership is encouraged to consult its tax advisor as to its tax consequences.
Unless otherwise indicated, this description assumes that we are not, and will not become, a “passive foreign investment company,” or “PFIC,” for United States federal income tax purposes. See “—Passive Foreign Investment Company Considerations.”
You are encouraged to consult your advisor with respect to the United States federal, state, local and foreign tax consequences of acquiring, owning and disposing of our ordinary shares.
Distributions
If you are a U.S. Holder, the gross amount of any distribution that we pay you with respect to our ordinary shares before reduction for any Israeli taxes withheld therefrom generally will be includible in your income as dividend income to the extent such distribution is paid out of our current or accumulated earnings and profits as determined under United States federal income tax principles. To the extent that the amount of any cash distribution exceeds our current and accumulated earnings and profits as determined under United States federal income tax principles, it will be treated first as a tax-free return of your adjusted tax basis in our ordinary shares and thereafter as capital gain. We do not expect to maintain calculations of our earnings and profits under United States federal income tax principles. Therefore, if you are a U.S. Holder you should expect that the entire amount of any cash distribution generally will be reported as dividend income to you; provided, however, that distributions of ordinary shares to U.S. Holders that are part of a pro rata distribution to all of our shareholders generally will not be subject to United States federal income tax. Subject to the PFIC rules discussed below, non-corporate U.S. Holders may qualify for the lower rates of taxation with respect to dividends on ordinary shares applicable to long-term capital gains (i.e., gains from the sale of capital assets held for more than one year), provided that certain conditions are met, including certain holding period requirements and the absence of certain risk reduction transactions. Moreover, such reduced rate shall not apply if we are a PFIC for the taxable year in which we pay a dividend, or were a PFIC for the preceding taxable year. Dividends will not be eligible for the dividends received deduction generally allowed to corporate U.S. Holders.
If you are a U.S. Holder, dividends that we pay you with respect to our ordinary shares will be treated as foreign source income, which may be relevant in calculating your foreign tax credit limitation. Subject to certain conditions and limitations, Israeli tax withheld on dividends may be deducted from your taxable income or credited against your United States federal income tax liability. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends that we distribute generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income.” A foreign tax credit for foreign taxes imposed on distributions may be denied if you do not satisfy certain minimum holding period requirements. The rules relating to the determination of the foreign tax credit are complex, and you are encouraged to consult your tax advisor to determine whether and to what extent you will be entitled to this credit.
139
Table of Contents
Sale, Exchange or Other Disposition of Ordinary Shares
Subject to the discussion below under “Passive Foreign Investment Company Considerations,” if you are a U.S. Holder, you generally will recognize an amount of gain or loss on the sale, exchange or other disposition of our ordinary shares equal to the difference between the amount realized on such sale, exchange or other disposition and your tax basis in our ordinary shares, and such gain or loss will be capital gain or loss. The tax basis in an ordinary share generally will equal the cost of such ordinary share. If you are a non-corporate U.S. Holder, capital gain from the sale, exchange or other disposition of ordinary shares generally will be eligible for a preferential rate of taxation applicable to capital gains, if your holding period for such ordinary shares exceeds one year. The deductibility of capital losses for United States federal income tax purposes is subject to limitations under the Code. Any such gain or loss that a U.S. Holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes.
Passive Foreign Investment Company Considerations
If we were to be classified as a “passive foreign investment company,” or PFIC, in any taxable year, a U.S. Holder would be subject to special rules generally intended to reduce or eliminate any benefits from the deferral of U.S. federal income tax that a U.S. Holder could derive from investing in a non-U.S. company that does not distribute all of its earnings on a current basis.
A non-U.S. corporation will be classified as a PFIC for federal income tax purposes in any taxable year in which, after applying certain look-through rules, either
| • | at least 75% of its gross income is “passive income”; or |
| • | at least 50% of the average quarterly value of its gross assets (which may be determined in part by the market value of our ordinary shares, which is subject to change) is attributable to assets that produce “passive income” or are held for the production of passive income. |
Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income, and includes amounts derived by reason of the temporary investment of funds raised in offerings of our ordinary shares. If a non-U.S. corporation owns at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation’s income. For publicly traded corporations, the PFIC asset test described above is applied using the fair market value of the non-U.S. corporation’s assets. For purposes of a the PFIC asset test, a publicly traded non-U.S. corporation may treat the aggregate fair market value of its assets as being equal to the sum of the aggregate value of its outstanding stock (“Market Capitalization”) and the total amount of its liabilities. We intend to take the position that the excess of a non-U.S. corporation’s Market Capitalization plus liabilities over the book value of all of its assets (“Goodwill”) may generally be treated as a non-passive asset to the extent attributable to the non-passive activities of such corporation. If we are classified as a PFIC in any year with respect to which a U.S. Holder owns our ordinary shares, we will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding years during which the U.S. Holder owns our ordinary shares, regardless of whether we continue to meet the tests described above.
Based on certain estimates of our gross income and gross assets, our intended use of the proceeds of this offering, and the nature of our business, we do not expect that we will be classified as a PFIC for the taxable year ending December 31, 2013. However, because PFIC status is based on our income, assets and activities for the entire taxable year, and our Market Capitalization, it is not possible to determine whether we will be characterized as a PFIC for the 2013 taxable year until after the close of the year. Moreover, we must determine our PFIC status annually based on tests which are factual in nature, and our status in future years will depend on our income, assets, activities and Market Capitalization in those years. In addition, our status as a PFIC may
140
Table of Contents
depend on how quickly we utilize the cash proceeds from this offering in our business. Because we currently hold, and expect to continue to hold, a substantial amount of cash and cash equivalents and other passive assets used in our business, and because the value of our gross assets is likely to be determined in large part by reference to the market prices of our securities, we would likely become a PFIC for a given taxable year if our Market Capitalization were to decrease significantly. Thus, there can be no assurance that we will not be considered a PFIC for any taxable year.
If we were a PFIC, and you are a U.S. Holder, then unless you make one of the elections described below, a special tax regime will apply to both (a) any “excess distribution” by us to you (generally, your ratable portion of distributions in any year which are greater than 125% of the average annual distribution received by you in the shorter of the three preceding years or your holding period for our ordinary shares) and (b) any gain realized on the sale or other disposition of the ordinary shares. Under this regime, any excess distribution and realized gain will be treated as ordinary income and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over your holding period, (b) the amount deemed realized in each year had been subject to tax in each year of that holding period at the highest marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax at the U.S. Holder’s regular ordinary income rate for the current year and would not be subject to the interest change discussed below), and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable in those years.
If we are a PFIC for any taxable year during which a U.S. Holder holds our ordinary shares, then in lieu of being subject to the tax and interest charge rules discussed above, a U.S. Holder may make an election to include gain on the stock of a PFIC as ordinary income under a mark-to-market method, provided that such ordinary shares are “regularly traded” on a “qualified exchange.” In general, our ordinary shares will be treated as “regularly traded” for a given calendar year if more than a de minimis quantity of our ordinary shares are traded on a qualified exchange on at least 15 days during each calendar quarter of such calendar year. We intend to apply to have our ordinary shares listed on the New York Stock Exchange. However, no assurance can be given that our ordinary shares will be regularly traded on a “qualified exchange” for purposes of the mark-to-market election. In addition, because a mark-to-market election cannot be made for any lower-tier PFICs that we may own, a U.S. Holder may continue to be subject to the PFIC rules discussed above with respect to such holder’s indirect interest in any investments we hold that are treated as an equity interest in a PFIC for United States federal income tax purposes.
If a U.S. Holder makes an effective mark-to-market election, in each year that we are a PFIC, such U.S. Holder will include in each year that we are a PFIC as ordinary income the excess of the fair market value of such U.S. Holder’s ordinary shares at the end of the year over such U.S. Holder’s adjusted tax basis in the shares. Such U.S. Holder will be entitled to deduct as an ordinary loss in each such year the excess of such U.S. Holder’s adjusted tax basis in the ordinary shares over their fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. If a U.S. Holder makes an effective mark-to-market election, in each year that we are a PFIC, any gain such U.S. Holder recognizes upon the sale or other disposition of such U.S. Holder’s ordinary shares will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount of previously included income as a result of the mark-to-market election.
A U.S. Holder’s adjusted tax basis in the ordinary shares will be increased by the amount of any income inclusion and decreased by the amount of any deductions under the mark-to-market rules discussed above. If a U.S. Holder makes an effective mark-to-market election, it will be effective for the taxable year for which the election is made and all subsequent taxable years unless the ordinary shares are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. U.S. Holders are encouraged to consult their tax advisers about the availability of the mark-to-market election, and whether making the election would be advisable in their particular circumstances.
141
Table of Contents
In certain circumstances, a U.S. equityholder in a PFIC may avoid the adverse tax and interest-charge regime described above by making a “qualified electing fund” election to include in income its share of the corporation’s income on a current basis. However, a U.S. Holder may make a qualified electing fund election with respect to the ordinary shares only if we agree to furnish you annually with a PFIC annual information statement as specified in the applicable Treasury regulations.
We do not intend to provide the information necessary for U.S. Holders to make qualified electing fund elections if we are classified as a PFIC. U.S. Holders are encouraged to consult their tax advisors to determine whether any of these elections would be available and if so, what the consequences of the alternative treatments would be in their particular circumstances.
If we are determined to be a PFIC, the general tax treatment for U.S. Holders described in this paragraph would apply to indirect distributions and gains deemed to be realized by U.S. Holders in respect of any of our subsidiaries that also may be determined to be PFICs.
If a U.S. Holder owns ordinary shares during any year in which we are a PFIC and the U.S. Holder recognizes gain on a disposition of our ordinary shares or receives distributions with respect to our ordinary shares, the U.S. Holder generally will be required to file an IRS Form 8621 with respect to the company, generally with the U.S. Holder’s federal income tax return for that year. If our company were a PFIC for a given taxable year, then you are encouraged to consult your tax advisor concerning your annual filing requirements.
U.S. Holders are encouraged to their tax advisors regarding whether we are a PFIC and the potential application of the PFIC rules.
Backup Withholding Tax and Information Reporting Requirements
United States backup withholding tax and information reporting requirements may apply to certain payments to certain holders of stock. Information reporting generally will apply to payments of dividends on, and to proceeds from the sale or redemption of, our ordinary shares made within the United States, or by a United States payor or United States middleman, to a holder of our ordinary shares, other than an exempt recipient (including a payee that is not a United States person that provides an appropriate certification and certain other persons). A payor will be required to withhold backup withholding tax from any payments of dividends on, or the proceeds from the sale or redemption of, ordinary shares within the United States, or by a United States payor or United States middleman, to a holder, other than an exempt recipient, if such holder fails to furnish its correct taxpayer identification number or otherwise fails to comply with, or establish an exemption from, such backup withholding tax requirements. Any amounts withheld under the backup withholding rules will be allowed as a credit against the beneficial owner’s United States federal income tax liability, if any, and any excess amounts withheld under the backup withholding rules may be refunded, provided that the required information is timely furnished to the Internal Revenue Service.
Foreign Asset Reporting
Certain U.S. Holders who are individuals are required to report information relating to an interest in our ordinary shares, subject to certain exceptions (including an exception for shares held in accounts maintained by financial institutions). U.S. Holders are encouraged to consult their tax advisors regarding their information reporting obligations, if any, with respect to their ownership and disposition of our ordinary shares.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts are subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend income and net gains from the disposition of ordinary shares. Each U.S. Holder that is an individual, estate or trust is encouraged to consult
142
Table of Contents
its tax advisors regarding the applicability of the Medicare tax to its income and gains in respect of its investment in the ordinary shares.
The above description is not intended to constitute a complete analysis of all tax consequences relating to acquisition, ownership and disposition of our ordinary shares. You are encouraged to consult your tax advisor concerning the tax consequences of your particular situation.
143
Table of Contents
Under the terms and subject to the conditions contained in an underwriting agreement dated , 2013, we have agreed to sell to the underwriters named below, for whom Credit Suisse Securities (USA) LLC and Deutsche Bank Securities Inc. are acting as representatives, the following respective numbers of ordinary shares:
| Underwriter |
Number of Ordinary Shares | |
| Credit Suisse Securities (USA) LLC |
||
| Deutsche Bank Securities Inc. |
||
| Oppenheimer & Co. Inc. |
||
| Piper Jaffray & Co. |
||
|
| ||
| Total |
||
|
|
The underwriting agreement provides that the underwriters are obligated to purchase all the ordinary shares in the offering if any are purchased, other than those ordinary shares covered by the over-allotment option described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated.
We have granted to the underwriters a 30-day option to purchase on a pro rata basis up to additional ordinary shares at the initial public offering price less the underwriting discounts and commissions. The option may be exercised only to cover any over-allotments of ordinary shares.
The underwriters propose to offer the ordinary shares initially at the public offering price on the cover page of this prospectus. The underwriters and selling group members may allow a discount of $ per ordinary share on sales to other broker/dealers. After the initial public offering, the representatives may change the public offering price and discount to broker/dealers.
The following table summarizes the compensation and estimated expenses we will pay, which includes an amount not to exceed $ that we have agreed to reimburse the underwriters for certain expenses incurred by them in connection with this offering:
| Per Ordinary Share | Total | |||||||||||||||
| Without Over-allotment |
With Over-allotment |
Without Over-allotment |
With Over-allotment |
|||||||||||||
| Underwriting discounts and commissions paid by us |
$ | $ | $ | $ | ||||||||||||
| Expenses payable by us |
$ | $ | $ | $ | ||||||||||||
We estimate that our out-of-pocket expenses for this offering will be approximately $ , consisting of $ for SEC registration fees, $ for New York Stock Exchange listing fees, $ for printing expenses, $ for legal fees, $ for accounting fees, $ for FINRA filing fees and $ for miscellaneous expenses. The underwriters have informed us that they do not expect sales to accounts over which the underwriters have discretionary authority to exceed 5% of the ordinary shares being offered.
We have agreed that we will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any of our ordinary shares or securities convertible into or exchangeable or exercisable for any of our ordinary shares, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, without the prior written consent of Credit Suisse Securities (USA) LLC and Deutsche Bank Securities Inc. for a period of 180 days after the date of
144
Table of Contents
this prospectus, except issuances pursuant to the exercise of employee stock options outstanding on the date hereof or pursuant to our dividend reinvestment plan.
Our officers and directors and certain of our share and option holders have agreed that they will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, any of our ordinary shares or securities convertible into or exchangeable or exercisable for any of our ordinary shares, enter into a transaction that would have the same effect, or enter into any swap, hedge or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of our ordinary shares, whether any of these transactions are to be settled by delivery of our ordinary shares or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, without, in each case, the prior written consent of Credit Suisse Securities (USA) LLC and Deutsche Bank Securities Inc. for a period of 180 days after the date of this prospectus.
We agreed to indemnify the underwriters against liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in that respect.
Our ordinary shares are listed on the TASE under the symbol “EVGN.” We have applied to list the ordinary shares on the NYSE.
In connection with the listing of the ordinary shares on the NYSE, the underwriters will undertake to sell round lots of 100 shares or more to a minimum of 400 beneficial owners.
In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act.
| • | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| • | Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment option and/or purchasing shares in the open market. |
| • | Syndicate covering transactions involve purchases of the ordinary shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| • | Penalty bids permit the representatives to reclaim a selling concession from a syndicate member when the ordinary shares originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our ordinary shares or preventing or retarding a decline in the market price of the ordinary shares. As a result the price of our ordinary shares may be higher than the price that might
145
Table of Contents
otherwise exist in the open market. These transactions may be effected on the NYSE or otherwise and, if commenced, may be discontinued at any time.
A prospectus in electronic format may be made available on the web sites maintained by one or more of the underwriters, or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representatives may agree to allocate a number of ordinary shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations.
United Kingdom
This document is only being distributed to and is only directed at (i) persons who are outside the United Kingdom or (ii) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (iii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), from and including the date on which the European Union Prospectus Directive (the “EU Prospectus Directive”) was implemented in that Relevant Member State (the “Relevant Implementation Date”) an offer of securities described in this prospectus may not be made to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the EU Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities described in this prospectus may be made to the public in that Relevant Member State at any time:
| • | to any legal entity which is a qualified investor as defined under the EU Prospectus Directive; |
| • | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the EU Prospectus Directive); or |
| • | in any other circumstances falling within Article 3(2) of the EU Prospectus Directive, provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the EU Prospectus Directive. |
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the EU Prospectus Directive in that Member State. The expression “EU Prospectus Directive” means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
146
Table of Contents
Hong Kong
The shares may not be offered or sold by means of any document other than (i) in circumstances that do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances that do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), that is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted under the laws of Hong Kong) other than with respect to shares that are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 by a relevant person that is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and where each beneficiary of which is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that corporation or trust shall not be transferable for six months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Switzerland
This document, as well as any other material relating to the shares of our common stock, which are the subject of the offering contemplated by this prospectus, does not constitute an issue prospectus pursuant to Article 652a of the Swiss Code of Obligations. The shares will not be listed on the SIX Swiss Exchange and, therefore, the documents relating to the shares, including, but not limited to, this document, do not claim to comply with the disclosure standards of the listing rules of the SIX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SIX Swiss Exchange.
The shares are being offered in Switzerland by way of a private placement, i.e., to a small number of selected investors only, without any public offer and only to investors who do not purchase the shares with the intention to distribute them to the public. The investors will be individually approached by us from time to time.
Addresses of Representatives
The addresses of the representatives are as follows: Credit Suisse Securities (USA) LLC, Eleven Madison Avenue, New York, New York 10010 and Deutsche Bank Securities Inc., 60 Wall Street, New York, New York 10005.
147
Table of Contents
Resale Restrictions
The distribution of the ordinary shares in Canada is being made only in the provinces of Ontario, Quebec, Alberta, British Columbia and Manitoba on a private placement basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of ordinary shares are made. Any resale of the ordinary shares in Canada must be made under applicable securities laws which may vary depending on the relevant jurisdiction, and which may require resales to be made under available statutory exemptions or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of the ordinary shares.
Representations of Purchasers
By purchasing ordinary shares in Canada and accepting delivery of a purchase confirmation, a purchaser is representing to us and the dealer from whom the purchase confirmation is received that:
| • | the purchaser is entitled under applicable provincial securities laws to purchase the ordinary shares without the benefit of a prospectus qualified under those securities laws as it is an “accredited investor” as defined under National Instrument 45-106—Prospectus and Registration Exemptions, |
| • | the purchaser is a “Canadian permitted client” as defined in National Instrument 31-103—Registration Requirements and Exemptions, or as otherwise interpreted and applied by the Canadian Securities Administrators, |
| • | where required by law, the purchaser is purchasing as principal and not as agent, |
| • | the purchaser has reviewed the text above under Resale Restrictions, and |
| • | the purchaser acknowledges and consents to the provision of specified information concerning the purchase of the ordinary shares to the regulatory authority that by law is entitled to collect the information, including certain personal information. For purchasers in Ontario, questions about such indirect collection of personal information should be directed to Administrative Support Clerk, Ontario Securities Commission, Suite 1903, Box 55, 20 Queen Street West, Toronto, Ontario M5H 3S8 or on (416) 593-3684. |
Rights of Action—Ontario Purchasers
Under Ontario securities legislation, certain purchasers who purchase a security offered by this prospectus during the period of distribution will have a statutory right of action for damages, or while still the owner of the ordinary shares, for rescission against us in the event that this prospectus contains a misrepresentation without regard to whether the purchaser relied on the misrepresentation. The right of action for damages is exercisable not later than the earlier of 180 days from the date the purchaser first had knowledge of the facts giving rise to the cause of action and three years from the date on which payment is made for the ordinary shares. The right of action for rescission is exercisable not later than 180 days from the date on which payment is made for the ordinary shares. If a purchaser elects to exercise the right of action for rescission, the purchaser will have no right of action for damages against us. In no case will the amount recoverable in any action exceed the price at which the ordinary shares were offered to the purchaser and if the purchaser is shown to have purchased the securities with knowledge of the misrepresentation, we will have no liability. In the case of an action for damages, we will not be liable for all or any portion of the damages that are proven to not represent the depreciation in value of the ordinary shares as a result of the misrepresentation relied upon. These rights are in addition to, and without derogation from, any other rights or remedies available at law to an Ontario purchaser. The foregoing is a summary of the rights available to an Ontario purchaser. Ontario purchasers should refer to the complete text of the relevant statutory provisions.
148
Table of Contents
Enforcement of Legal Rights
All of our directors and officers as well as the experts named herein may be located outside of Canada and, as a result, it may not be possible for Canadian purchasers to effect service of process within Canada upon us or those persons. All or a substantial portion of our assets and the assets of those persons may be located outside of Canada and, as a result, it may not be possible to satisfy a judgment against us or those persons in Canada or to enforce a judgment obtained in Canadian courts against us or those persons outside of Canada.
Taxation and Eligibility for Investment
Canadian purchasers of ordinary shares should consult their own legal and tax advisors with respect to the tax consequences of an investment in the ordinary shares in their particular circumstances and about the eligibility of the ordinary shares for investment by the purchaser under relevant Canadian legislation.
149
Table of Contents
The validity of the ordinary shares being offered by this prospectus and other legal matters concerning this offering relating to Israeli law will be passed upon for us by Meitar Liquornik Geva Leshem Tal, Ramat Gan, Israel. Certain legal matters in connection with this offering relating to U.S. law will be passed upon for us by White & Case LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriters by Goldfarb Seligman & Co., Tel Aviv, Israel, with respect to Israeli law, and by Skadden, Arps, Slate, Meagher & Flom LLP, New York, New York, with respect to U.S. law.
The consolidated financial statements as of December 31, 2011 and 2012 and for each of the three years in the period ended December 31, 2012 included in this Prospectus have been so included in reliance on the reports of Kost Forer Gabbay & Kasierer, Certified Public Accountants, a member of Ernst & Young Global, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and any Israeli experts named in this registration statement, most of whom reside outside of the United States, may be difficult to obtain within the United States. Furthermore, because a majority of our assets and most of our directors and officers are located outside of the United States, any judgment obtained in the United States against us or certain of our directors and officers may be difficult to collect within the United States.
We have been informed by our legal counsel in Israel, Meitar Liquornik Geva Leshem Tal, that it may be difficult to assert U.S. securities laws claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws because Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact which can be a time-consuming and costly process. Matters of procedure will also be governed by Israeli law.
We have irrevocably appointed , as our agent to receive service of process in any action against us in any United States federal or state court arising out of this offering or any purchase or sale of securities in connection with this offering. Subject to specified time limitations and legal procedures, Israeli courts may enforce a United States judgment in a civil matter which is non-appealable, including a judgment based upon the civil liability provisions of the Securities Act or the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that, among other things:
| • | the judgment is obtained after due process before a court of competent jurisdiction, according to the laws of the state in which the judgment is given and the rules of private international law prevailing in Israel; |
| • | the prevailing law of the foreign state in which the judgment is rendered allows for the enforcement of judgments of Israeli courts; |
| • | adequate service of process has been effected and the defendant has had a reasonable opportunity to be heard and to present his or her evidence; |
| • | the judgment is not contrary to public policy of Israel, and the enforcement of the civil liabilities set forth in the judgment is not likely to impair the security or sovereignty of Israel; |
| • | the judgment was not obtained by fraud and does not conflict with any other valid judgment in the same matter between the same parties; |
150
Table of Contents
| • | an action between the same parties in the same matter was not pending in any Israeli court at the time at which the lawsuit was instituted in the foreign court; and |
| • | the judgment is enforceable according to the laws of Israel and according to the law of the foreign state in which the relief was granted. |
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rates.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act relating to this offering of our ordinary shares. This prospectus does not contain all of the information contained in the registration statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that is included in the registration statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document itself for a complete description of its terms.
You may read and copy the registration statement, including the related exhibits and schedules, and any document we file with the SEC without charge at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. The SEC also maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
We are not currently subject to the informational requirements of the Exchange Act. Upon completion of this offering, we will be subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under those requirements will file reports with the SEC. Those other reports or other information may be inspected without charge at the locations described above. As a foreign private issuer, we will be exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial information for the first three quarters of each fiscal year within 60 days after the end of each such quarter, or such applicable time as required by the SEC.
We maintain a corporate website at www.evogene.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely for informational purposes.
151
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
Consolidated Financial Statements
F-1
Table of Contents

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Evogene Ltd.
We have audited the accompanying consolidated balance sheets of Evogene Ltd. (the “Company”) and its subsidiaries as of December 31, 2012 and 2011 and the related consolidated statements of comprehensive income, changes in equity and cash flows for each of the three years in the period ended December 31, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, based on our audits, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company and its subsidiaries as of December 31, 2012 and 2011 and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2012, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
KOST FORER GABBAY & KASIERER
A Member of Ernst & Young Global
Tel-Aviv, Israel
, 2013
The foregoing report is the form that will be signed upon completion of the 1-for-2 reverse share split described in Note 16(a) to the financial statements.
/s/ Kost Forer Gabbay & Kasierer
KOST FORER GABBAY & KASIERER
A Member of Ernst & Young Global
Tel Aviv, Israel
September 23, 2013
F-2
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands
| December 31, | ||||||||||
| Note | 2011 | 2012 | ||||||||
| CURRENT ASSETS: |
||||||||||
| Cash and cash equivalents |
6 | $ | 6,465 | $ | 24,262 | |||||
| Marketable securities |
7 | 34,672 | 30,868 | |||||||
| Short-term bank deposits |
17,652 | — | ||||||||
| Trade receivables |
800 | 1,542 | ||||||||
| Other receivables |
8 | 981 | 650 | |||||||
|
|
|
|
|
|||||||
| 60,570 | 57,322 | |||||||||
|
|
|
|
|
|||||||
| LONG-TERM ASSETS: |
||||||||||
| Long term deposits |
48 | 43 | ||||||||
| Plant, property and equipment, net |
9 | 7,138 | 7,401 | |||||||
| Intangible assets, net |
134 | 89 | ||||||||
|
|
|
|
|
|||||||
| 7,320 | 7,533 | |||||||||
|
|
|
|
|
|||||||
| 67,890 | 64,855 | |||||||||
|
|
|
|
|
|||||||
| CURRENT LIABILITIES: |
||||||||||
| Trade payables |
2,059 | 1,416 | ||||||||
| Liabilities in respect of grants from the Chief Scientist |
11 | 905 | 733 | |||||||
| Deferred revenues |
5 | 4,037 | 4,211 | |||||||
| Other payables |
10 | 2,079 | 3,139 | |||||||
|
|
|
|
|
|||||||
| 9,080 | 9,499 | |||||||||
|
|
|
|
|
|||||||
| LONG-TERM LIABILITIES: |
||||||||||
| Liabilities in respect of grants from the Chief Scientist |
11 | 3,039 | 2,918 | |||||||
| Deferred revenues |
5a, b | 7,673 | 4,168 | |||||||
| Severance pay liability, net |
13 | 9 | 11 | |||||||
|
|
|
|
|
|||||||
| 10,721 | 7,097 | |||||||||
|
|
|
|
|
|||||||
| SHAREHOLDERS’ EQUITY: |
16 | |||||||||
| Ordinary shares of NIS 0.02 par value: Authorized—150,000,000 ordinary shares; Issued and outstanding—18,738,290 and 18,311,603 shares at December 31, 2012 and 2011, respectively |
100 | 102 | ||||||||
| Share premium |
88,056 | 90,746 | ||||||||
| Put option |
(7,764 | ) | (7,764 | ) | ||||||
| Reserve—transaction with a former controlling shareholder |
1,156 | 1,156 | ||||||||
| Accumulated deficit |
(33,459 | ) | (35,981 | ) | ||||||
|
|
|
|
|
|||||||
| 48,089 | 48,259 | |||||||||
|
|
|
|
|
|||||||
| $ | 67,890 | $ | 64,855 | |||||||
|
|
|
|
|
|||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-3
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
U.S. dollars in thousands (except share and per share data)
| Year Ended December 31, | ||||||||||||||
| Note | 2010 | 2011 | 2012 | |||||||||||
| Revenues |
20 | $ | 12,563 | $ | 14,901 | $ | 17,072 | |||||||
| Cost of revenues |
18a | 5,811 | 8,247 | 9,552 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Gross profit |
6,752 | 6,654 | 7,520 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Operating expenses: |
||||||||||||||
| Research and development |
18b | 5,544 | 6,384 | 7,252 | ||||||||||
| Business development |
18c | 1,062 | 1,136 | 1,159 | ||||||||||
| General and administrative |
18d | 2,123 | 2,317 | 2,235 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
8,729 | 9,837 | 10,646 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(1,977 | ) | (3,183 | ) | (3,126 | ) | ||||||||
| Financing income |
18e | 724 | 5,023 | 972 | ||||||||||
| Financing expenses |
18e | (5,717 | ) | (1,195 | ) | (294 | ) | |||||||
|
|
|
|
|
|
|
|||||||||
| Income (loss) before taxes on income |
(6,970 | ) | 645 | (2,448 | ) | |||||||||
| Taxes on income |
14 | — | — | 74 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Net income (loss) |
$ | (6,970 | ) | $ | 645 | $ | (2,522 | ) | ||||||
|
|
|
|
|
|
|
|||||||||
| Total comprehensive income (loss) |
$ | (6,970 | ) | $ | 645 | $ | (2,522 | ) | ||||||
|
|
|
|
|
|
|
|||||||||
| Basic and diluted net income (loss) per share |
19 | $ | (0.48 | ) | $ | 0.04 | $ | (0.14 | ) | |||||
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-4
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
U.S. dollars in thousands
| Share Capital |
Share Premium |
Put Option |
Reserve— Transaction With a Former Controlling Shareholder |
Accumulated Deficit |
Total | |||||||||||||||||||
| Balance as of January 1, 2010 |
$ | 80 | $ | 49,670 | $ | (4,433 | ) | $ | 1,156 | $ | (27,134 | ) | $ | 19,339 | ||||||||||
| Total comprehensive loss |
— | — | — | — | (6,970 | ) | (6,970 | ) | ||||||||||||||||
| Exercise of warrants and options |
2 | 1,798 | 1,800 | |||||||||||||||||||||
| Share-based compensation |
— | 2,562 | — | — | — | 2,562 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2010 |
82 | 54,030 | (4,433 | ) | 1,156 | (34,104 | ) | 16,731 | ||||||||||||||||
| Total comprehensive income |
— | — | — | — | 645 | 645 | ||||||||||||||||||
| Shares issued, net |
5 | 9,577 | — | — | — | 9,582 | ||||||||||||||||||
| Issuance of put option, net |
— | 727 | (3,331 | ) | — | — | (2,604 | ) | ||||||||||||||||
| Exercise of warrants and options |
13 | 21,593 | — | — | — | 21,606 | ||||||||||||||||||
| Share-based compensation |
— | 2,129 | — | — | — | 2,129 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2011 |
100 | 88,056 | (7,764 | ) | 1,156 | (33,459 | ) | 48,089 | ||||||||||||||||
| Total comprehensive loss |
— | — | — | — | (2,522 | ) | (2,522 | ) | ||||||||||||||||
| Exercise of options |
2 | 1,517 | — | — | — | 1,519 | ||||||||||||||||||
| Share-based compensation |
— | 1,173 | — | — | — | 1,173 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2012 |
$ | 102 | $ | 90,746 | $ | (7,764 | ) | $ | 1,156 | $ | (35,981 | ) | $ | 48,259 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net income (loss) |
$ | (6,970 | ) | $ | 645 | $ | (2,522 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
||||||||||||
| Adjustments to profit and loss items: |
||||||||||||
| Depreciation and amortization |
943 | 1,419 | 1,835 | |||||||||
| Share-based compensation |
2,562 | 2,129 | 1,173 | |||||||||
| Net financing expenses (income) |
4,993 | (3,828 | ) | (678 | ) | |||||||
| Taxes on income |
— | — | 74 | |||||||||
| Loss (gain) on disposal of property, plant and equipment |
54 | 4 | (33 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| 8,552 | (276 | ) | 2,371 | |||||||||
|
|
|
|
|
|
|
|||||||
| Changes in asset and liability items: |
||||||||||||
| Decrease (increase) in trade receivables |
(1,584 | ) | 1,341 | (742 | ) | |||||||
| Decrease (increase) in other receivables |
(208 | ) | (374 | ) | 331 | |||||||
| Decrease (increase) in prepaid expenses |
(12 | ) | (11 | ) | 5 | |||||||
| Increase in trade payables |
24 | 423 | 231 | |||||||||
| Increase in other payables |
950 | 220 | 974 | |||||||||
| Decrease in deferred revenues |
(3,779 | ) | (907 | ) | (3,331 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| (4,609 | ) | 692 | (2,532 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash received (paid) during the year for: |
||||||||||||
| Interest received |
187 | 1,072 | 869 | |||||||||
| Taxes paid (withheld by customers) |
— | — | (74 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) operating activities |
(2,840 | ) | 2,133 | (1,888 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Purchase of property, plant and equipment |
(1,403 | ) | (3,759 | ) | (2,963 | ) | ||||||
| Proceeds from disposal of property, plant and equipment |
— | 9 | 34 | |||||||||
| Proceeds from sale of marketable securities |
8,596 | 10,710 | 15,697 | |||||||||
| Purchase of marketable securities |
(24,261 | ) | (24,268 | ) | (11,935 | ) | ||||||
| Proceeds from (investment in) bank deposits, net |
2,571 | (13,274 | ) | 17,652 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
(14,497 | ) | (30,582 | ) | 18,485 | |||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-6
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands (except share and per share data)
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from issuance of shares, net |
— | 9,582 | — | |||||||||
| Proceeds from exercise of warrants and options |
1,313 | 16,136 | 1,519 | |||||||||
| Proceeds from the Chief Scientist grants (Note 11) |
320 | 260 | 484 | |||||||||
| Repayment of the Chief Scientist grants (Note 11) |
(280 | ) | (402 | ) | (892 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
1,353 | 25,576 | 1,111 | |||||||||
|
|
|
|
|
|
|
|||||||
| Exchange rate differences—cash and cash equivalent balances |
(111 | ) | (782 | ) | 89 | |||||||
|
|
|
|
|
|
|
|||||||
| Increase (decrease) in cash and cash equivalents |
(16,095 | ) | (3,655 | ) | 17,797 | |||||||
| Cash and cash equivalents, beginning of the year |
26,215 | 10,120 | 6,465 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of the year |
$ | 10,120 | $ | 6,465 | $ | 24,262 | ||||||
|
|
|
|
|
|
|
|||||||
| Significant non-cash activities: |
||||||||||||
| Acquisition of property, plant and equipment in credit |
$ | 355 | $ | 1,009 | $ | 101 | ||||||
|
|
|
|
|
|
|
|||||||
| Modification of put option |
$ | — | $ | 3,331 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Exercise of warrants |
$ | 487 | $ | 5,470 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-7
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 1:—GENERAL
a. Evogene Ltd. (“Company”) is a biotech company that is engaged in research and development in the field of plant improvement. The Company was founded in 2000 as a division of Compugen Ltd. (“Compugen”) and spun-off as an independent company in January 2002. The Company focuses on the development of technology to identify gene sequences (D.N.A. sequences that are responsible for producing proteins; hereafter – genes) and other D.N.A. sequences in plants (“D.N.A. sequences”), which are likely to improve one or more of the plant’s traits (“target plant” and “target trait”). These improvements are accomplished by either introducing the genes into the target plant through genetic modification, or through cultivating the genes in the target plant through advanced breeding, and accordingly, the seeds carry the target trait. In addition, the Company is involved in the improvement and breeding of designated non-edible crops that are likely to be used in the future for the production of oil for the biodiesel industry.
The Company is considering applying its technology to identify D.N.A. sequences, and to map chemical materials, with the goal to improve plant traits.
On January 1, 2012, Evogene established Evofuel Ltd., a wholly owned subsidiary (“Evofuel”), which is engaged in the development of improved species of the castor bean plant, which may serve as a source for extraction of oil for the biodiesel industry.
b. The Company principally derives its revenues from collaboration arrangement. Revenues from its major collaborators accounted together for 94%, 94% and 91% for the years ended December 31, 2012, 2011 and 2010, respectively. As to major customers, see Notes 20(c) and 21(e). If a major customer decides to terminate its collaboration agreement with the Company, the Company may not be able to make up the lost revenue and it may have a material adverse effect on its results of operations.
c. The Company’s securities are listed for trading on the Tel Aviv Stock Exchange (the “TASE”).
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES
The following accounting policies have been applied consistently in the financial statements for all periods presented, unless otherwise stated.
a. Basis of presentation of the financial statements:
These financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board.
The Company’s financial statements have been prepared on a cost basis, except for financial instruments measured at fair value through profit or loss.
The Company has elected to present profit or loss items using the function of expense method.
b. The Company’s operating cycle is one year.
c. The consolidated financial statements include the financial statements of companies that the Company controls (subsidiaries). Control exists when the Company has the power, directly or indirectly, to govern the financial and operating policies of an entity. The consolidation of the financial statements commences on the date on which control is obtained and ends when such control ceases.
F-8
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
The financial statements of the Company and its subsidiaries are prepared as of the same dates and periods. The consolidated financial statements are prepared using uniform accounting policies by all companies in the group. Significant intercompany balances and transactions and gains or losses resulting from intercompany transactions are eliminated in full in the consolidated financial statements.
d. Functional currency, reporting currency and foreign currency:
| 1. | Functional currency and reporting currency: |
The reporting currency of the financial statements is the U.S. dollar.
The Company and its subsidiaries determine the functional currency of each entity, based on the currency in which they primarily generate and expend cash. The Company determined that its functional currency is the U.S. dollar since most of its revenues are generated in U.S. dollars and despite having most of its expenses incurred in NIS.
The Company’s revenues were denominated mostly in U.S. dollars because of the Company’s collaboration agreement with Monsanto. This collaboration accounted for 70%, 71% and 74% of the Company’s revenues during 2012, 2011 and 2010, respectively. The Company’s costs were mostly denominated in NIS, mainly due to payroll and related costs. To further support the Company’s determination in light of these mixed indicators, the Company has analyzed the currency in which funds from financing activities are generated or held and the currency in which receipts from operating activities are usually retained.
Funds from financing activities were principally derived from significant funds raised in U.S. dollars in 2008 and 2011 from the Company’s major collaborators, Monsanto and Bayer. These funds were maintained mostly in U.S. dollars. A majority of the Company’s marketable securities are denominated in U.S. dollars as well.
The Company operates and plans its activities in U.S. dollars and accordingly its periodic budgets and internal management reports are prepared and monitored using the U.S. dollar as the primary currency and provides the basis for the determination of officers’ compensation. Further, the Company is mostly influenced by the U.S. regulatory environment.
This currency is used to separately measure each entity’s financial position and operating results.
| 2. | Transactions, assets and liabilities in foreign currency: |
Transactions denominated in a foreign currency are recorded upon initial recognition at the exchange rate on the date of the transaction. After initial recognition, monetary assets and liabilities denominated in a foreign currency are translated at the end of each reporting period into the functional currency at the exchange rate on that date. Non-monetary assets and liabilities measured at cost in foreign currency are translated at the exchange rate at the date of the transaction. Non-monetary assets and liabilities denominated in foreign currency and measured at fair value are translated into the functional currency using the exchange rate prevailing at the date when the fair value was determined.
e. Cash and cash equivalents:
Cash equivalents are highly liquid investments, including unrestricted short-term bank deposits with an original maturity of three months or less from the date of deposit.
F-9
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
f. Short-term deposits:
Short-term bank deposits are with a maturity of more than three months from the deposit day but less than one year.
g. Government grants:
Government grants are recognized when there is reasonable assurance that the grants will be received and the Company will comply with the attendant conditions.
Research and development grants received from the Office of the Chief Scientist in Israel (“OCS”), the Israel-U.S. Binational Industrial Research and Development Fund (“BIRD-F”) and the Canada-Israel Industrial Research and Development Foundation (“CIIRDF”) are recognized upon receipt as a liability if future economic benefits are expected from the project that will result in royalty-bearing sales.
A liability for the loan is first measured at fair value using a discount rate that reflects a market rate of interest. The difference between the amount of the grant received and the fair value of the liability is accounted for as a Government grant and recognized as a reduction of research and development expenses. After initial recognition, the liability is measured at amortized cost using the effective interest method. Royalty payments are treated as a reduction of the liability. If no economic benefits are expected from the research activity, the grant receipts are recognized as a reduction of the related research and development expenses. In that event, the royalty obligation is treated as a contingent liability in accordance with IAS 37, “Provisions, Contingent Liabilities and Contingent Assets” (“IAS 37”).
At the end of each reporting period, the Company evaluates whether there is reasonable assurance that the liability recognized, in whole or in part, will not be repaid (since the Company will not be required to pay royalties) based on the best estimate of future sales and if so, the appropriate amount of the liability is derecognized against a corresponding reduction in research and development expenses.
h. Leases:
The criteria for classifying leases as finance or operating leases depend on the substance of the agreements and are made at the inception of the lease in accordance with the following principles as set out in IAS 17, “Leases”.
The Company is only involved in operating lease transaction as a lessee.
Lease agreements are classified as an operating lease if they do not transfer substantially all the risks and benefits incidental to ownership of the leased asset. Lease payments are recognized as an expense in profit or loss on a straight-line basis over the lease term.
i. Property, plant and equipment:
Property, plant and equipment are measured at cost, including directly attributable costs, less accumulated depreciation, accumulated impairment losses and excluding day-to-day servicing expenses. Cost includes spare parts and auxiliary equipment that are used in connection with plant and equipment.
F-10
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
Depreciation is calculated on a straight-line basis over the useful life of the assets at annual rates as follows:
| % | Mainly % | |||
| Laboratory equipment |
15-33.33 | 15 | ||
| Computers and peripheral equipment |
33.33 | |||
| Office equipment and furniture |
6 | |||
| Motor vehicles |
15 | |||
| Leasehold improvements |
see below |
Leasehold improvements are depreciated on a straight-line basis over the shorter of the lease term (including the renewal options held by the Company or its subsidiaries which are expected to be exercised) and the expected life of the improvement.
The useful life, depreciation method and residual value of an asset are reviewed at least each year-end.
Depreciation of an asset ceases at the earlier of the date that the asset is classified as held for sale and the date that the asset is derecognized. An asset is derecognized on disposal or when no further economic benefits are expected from its use.
j. Intangible assets:
Separately acquired intangible assets with finite useful life are measured on initial recognition at cost.
Intangible assets are amortized over their useful life using the straight-line method and reviewed for impairment whenever there is an indication that the asset may be impaired.
Amortization expenses in respect of intangible assets in the statements of comprehensive income for 2012, 2011 and 2010 totaled $45, $37 and $46, respectively. The expenses were included in research and development expenses.
Research and development expenses:
Research and development expenses are recognized in profit or loss when incurred. An intangible asset arising from a development project or from the development phase of an internal project is recognized if the Company can demonstrate the technical feasibility of completing the intangible asset so that it will be available for use or sale; the Company’s intention to complete the intangible asset and use or sell it; the Company’s ability to use or sell the intangible asset; how the intangible asset will generate future economic benefits; the availability of adequate technical, financial and other resources to complete the intangible asset; and the Company’s ability to measure reliably the expenditure attributable to the intangible asset during its development. Since Company research and development projects are often subject to regulatory approval procedures and other uncertainties, the conditions for the capitalization of costs incurred before receipt of approvals are not normally satisfied and, therefore, research and development expenses are recognized in profit or loss when incurred.
k. Impairment of non-financial assets:
The Company evaluates the need to record an impairment of the carrying amount of non-financial assets whenever events or changes in circumstances indicate that the carrying amount is not recoverable. If the carrying
F-11
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
amount of non-financial assets exceeds their recoverable amount, the assets are reduced to their recoverable amount. The recoverable amount is the higher of fair value less costs of sale and value in use. The recoverable amount of an asset that does not generate independent cash flows is determined for the cash-generating unit to which the asset belongs.
An impairment loss of an asset, other than goodwill, is reversed only if there have been changes in the estimates used to determine the asset’s recoverable amount since the last impairment loss was recognized. Reversal of an impairment loss, as above, shall not be increased above the lower of the carrying amount that would have been determined (net of depreciation or amortization) had no impairment loss been recognized for the asset in prior years, and its recoverable amount.
l. Revenue recognition:
Revenues are recognized in profit or loss when the revenues can be measured reliably, it is probable that the economic benefits associated with the transaction will flow to the Company and the costs incurred or to be incurred in respect of the transaction can be measured reliably. Revenues are measured at the fair value of the consideration received.
The following are the specific revenue recognition criteria which must be met before revenue is recognized:
| • | Revenues from such agreements that do not contain a general right of return and are composed of multiple elements such as license, services, royalties and milestone events are allocated to the different elements and are recognized in respect of each element separately. An element constitutes a separate accounting unit if and only if it has a separate value to the customer. Revenue from the different element is recognized when the criteria for revenue recognition have been met and only to the extent of the consideration that is not contingent upon completion or performance of future services in the contract. |
| • | Revenues from the provision of research and development services as part of the Company’s collaboration agreements are recognized as service revenues. Recognition of the service is throughout the services period and is determined based on the proportion of actual costs incurred for each reporting period to the estimated total costs, subject to the enforceable rights. |
| • | Revenues from milestone events stipulated in the agreements are recognized upon the occurrence of a substantive element specified in the agreement. |
Deferred revenues:
Deferred revenues are unearned amounts including up-front payments received from customers not yet recognized as revenues. Up-front payments received upon entering into the collaboration agreements are initially deferred when received and then recognized as service revenues over the duration of the relevant contract based on the proportion of actual costs incurred for each reporting period to the estimated total costs of the collaboration.
m. Taxes on income:
Taxes on income in profit or loss comprise current and deferred taxes. Current or deferred taxes are recognized in profit or loss, except to the extent that the tax arises from items which are recognized directly in other comprehensive income or in equity.
F-12
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
| 1. | Current taxes: |
The current tax liability is measured using the tax rates and tax laws that have been enacted or substantively enacted by the end of reporting period as well as adjustments required in connection with the tax liability in respect of previous years.
| 2. | Deferred taxes: |
Deferred taxes are computed in respect of temporary differences between the carrying amounts in the financial statements and the amounts attributed for tax purposes.
Deferred taxes are measured at the tax rates that are expected to apply when the asset is realized or the liability is settled, based on tax laws that have been enacted or substantively enacted by the end of the reporting period.
Deferred tax assets are reviewed at the end of each reporting period and reduced to the extent that it is not probable that they will be utilized. Temporary differences (such as carryforward losses) for which deferred tax assets had not been recognized are reviewed at the end of each reporting period and a respective deferred tax asset is recognized to the extent that their utilization is probable.
n. Financial instruments:
| 1. | Financial assets: |
Financial assets within the scope of IAS 39, “Financial Instruments: Recognition and Measurement” (“IAS 39”) are initially recognized at fair value plus directly attributable transaction costs, except for financial assets measured at fair value through profit or loss in respect of which transaction costs are recorded in profit or loss.
After initial recognition, the accounting treatment of financial assets is based on their classification as follows:
Financial assets at fair value through profit or loss
This category includes financial assets designated upon initial recognition as at fair value through profit or loss.
Loans and receivables
The Company has receivables that are financial assets with fixed or determinable payments that are not quoted in an active market.
| 2. | Financial liabilities: |
Financial liabilities within the scope of IAS 39 are initially measured at fair value.
After initial recognition, the accounting treatment of financial liabilities is based on their classification as follows:
Financial liabilities measured at amortized cost
Loans and other liabilities are measured at amortized cost using the effective interest method taking into account directly attributable transaction costs.
F-13
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
Financial liabilities at fair value through profit or loss:
Financial liabilities at fair value through profit or loss include financial liabilities classified as held for trading and financial liabilities designated upon initial recognition as at fair value through profit or loss.
| 3. | Fair value: |
The fair value of financial instruments that are traded in an active market is determined by reference to market prices at the end of the reporting period. For financial instruments where there is no active market, fair value is determined using valuation techniques. Such techniques include using recent arm’s length market transactions; reference to the current market value of another instrument which is substantially the same; discounted cash flow or other valuation models.
| 4. | Offsetting financial instruments: |
Financial assets and financial liabilities are offset and the net amount is presented in the statement of financial position if there is a legally enforceable right to set off the recognized amounts and there is an intention either to settle on a net basis or to realize the asset and settle the liability simultaneously.
| 5. | Derecognition of financial instruments: |
| a) | Financial assets: |
A financial asset is derecognized when the contractual rights to the cash flows from the financial asset expire or the Company has transferred its contractual rights to receive cash flows from the financial asset or assumes an obligation to pay the cash flows in full without material delay to a third party and has transferred substantially all the risks and rewards of the asset, or has neither transferred nor retained substantially all the risks and rewards of the asset, but has transferred control of the asset.
| b) | Financial liabilities: |
A financial liability is derecognized when it is extinguished, that is when the obligation is discharged or cancelled or expires. A financial liability is extinguished when the debtor (the Company) discharges the liability by paying in cash, other financial assets, goods or services or is legally released from the liability.
When the financial liability is to a controlling shareholder and the debtor (the Company) is legally released from the liability, the derecognition is accounted as an equity contribution and presented in a separate line item within the shareholders’ equity.
o. Provisions:
A provision in accordance with IAS 37 is recognized when the Company has a present (legal or constructive) obligation as a result of a past event, it is expected to require the use of economic resources to clear the obligation and a reliable estimate can be made of it.
p. Employee benefit liabilities:
The Company and its subsidiaries have several employee benefit plans:
| 1. | Short-term employee benefits: |
Short-term employee benefits include salaries, paid annual leave, paid sick leave, recreation and social security contributions and are recognized as expenses as the services are rendered. A liability in respect
F-14
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
of a cash bonus or a profit-sharing plan is recognized when the Company and its subsidiaries have a legal or constructive obligation to make such payment as a result of past service rendered by an employee and a reliable estimate of the amount can be made.
| 2. | Post-employment benefits: |
The plans are normally financed by contributions to insurance companies and classified as defined contribution plans or as defined benefit plans.
The Company and its subsidiaries have defined contribution plans pursuant to Section 14 to the Severance Pay Law under which the Company and the subsidiaries pay fixed contributions and will have no legal or constructive obligation to pay further contributions if the fund does not hold sufficient amounts to pay all employee benefits relating to employee service in the current and prior periods. Contributions to the defined contribution plan in respect of severance or retirement pay are recognized as an expense when contributed concurrently with performance of the employee’s services.
q. Share-based compensation:
The Company’s employees and other service providers are entitled to remuneration in the form of equity-settled share-based compensation.
Equity-settled transactions:
The cost of equity-settled transactions with employees is measured at the fair value of the equity instruments granted at grant date. The fair value is determined using an acceptable option pricing model.
As for other service providers, the cost of the transactions is measured at the fair value of the goods or services received as consideration for equity instruments. In cases where the fair value of the goods or services received as consideration of equity instruments cannot be measured, they are measured by reference to the fair value of the equity instruments granted.
The cost of equity-settled transactions is recognized in profit or loss, together with a corresponding increase in equity, during the period which the performance and/or service conditions are to be satisfied, ending on the date on which the relevant employees become fully entitled to the award.
No expense is recognized for awards that do not ultimately vest, except for awards where vesting is conditional upon a market condition, which are treated as vesting irrespective of whether the market condition is satisfied, provided that all other vesting conditions (service and/or performance) are satisfied.
r. Income (loss) per share:
Income (loss) per share is calculated by dividing the net income (loss) attributable to the Company shareholders by the weighted number of ordinary shares outstanding during the period. Potential ordinary shares are only included when their conversion decreases income per share or increases loss per share. Furthermore, potential ordinary shares converted during the period are included in diluted income (loss) per share only until the conversion date and from that date in basic income (loss) per share.
F-15
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
s. Transactions with a controlling shareholder
Assets and liabilities derived from a transaction between the Company and a controlling shareholder are recognized at their fair value on the date of the transaction. The difference between the fair value and the consideration stipulated in the transaction is recorded within shareholders’ equity. A charge to equity essentially constitutes a dividend resulting in a reduction in retained earnings. A credit to equity essentially constitutes an investment by the shareholder which is presented as a separate component of shareholders’ equity, “Capital reserve from transactions with a controlling shareholder”.
The amount recorded in shareholders’ equity will not be transferred to the statement of income, even if in subsequent periods the items that were the subject of the transactions are derecognized from the financial statements.
NOTE 3:—SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUPMTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS
Judgments
Revenue:
The Company assesses the criteria for recognition of revenue related to up-front payments and multiple components as outlined by IAS 18, “Revenue”. Judgment is necessary to determine over which period the Company will satisfy its obligations related to up-front payments and when components can be recognized separately and the allocation of the related consideration to each component.
Estimates and assumptions
The preparation of the financial statements requires management to make estimates and assumptions that have an effect on the application of the accounting policies and on the reported amounts of assets, liabilities, revenues and expenses. Changes in accounting estimates are reported in the period of the change in estimate.
The key assumptions made in the financial statements concerning uncertainties at the end of the reporting period and the critical estimates computed by the Company and its subsidiaries that may result in a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below.
| • | Determining the fair value of share-based compensation: |
The fair value of share-based compensation is determined using an acceptable option pricing model.
The assumptions used in the model can include the expected volatility, expected life, expected dividend and risk-free interest rate.
| • | Government grants: |
Government grants received from the OCS, BIRD-F and CIIRDF are recognized as a liability if future economic benefits are expected from the research and development activity that will result in royalty-bearing sales. There is uncertainty regarding the estimated future cash flows and the estimated discount rate used to measure the amount of the liability.
| • | Determining the fair value of the put option |
The fair value of the put option is determined using an acceptable option pricing model and recorded as part of shareholders’ equity in accordance with IAS 32, “Financial Instruments: Presentation” (“IAS 32”).
F-16
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 3:—SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUPMTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS—(CONTINUED)
The assumptions used in the model include the expected volatility, expected life, expected dividend and risk-free interest rate (see also Note 5a).
NOTE 4:—DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION
IAS 32—Financial Instruments: Presentation and IFRS 7—Financial Instruments: Disclosure
The IASB issued certain amendments to IAS 32 (“the amendments to IAS 32”) regarding the offsetting of financial assets and liabilities. The IASB also issued amendments to IFRS 7 (“the amendments to IFRS 7”) regarding the offsetting of financial assets and liabilities.
The amendments to IAS 32 are to be applied retrospectively commencing from the financial statements for periods beginning on January 1, 2014, or thereafter. Earlier application is permitted. The amendments to IFRS 7 are to be applied retrospectively commencing from the financial statements for periods beginning on January 1, 2013, or thereafter.
The Company estimates that the amendments to IAS 32 are not expected to have a material impact on its financial statements. The required disclosures pursuant to the amendments to IFRS 7 will be included in the Company’s financial statements.
IFRS 9—Financial Instruments:
1. The IASB issued IFRS 9, “Financial Instruments”, the first part of Phase 1 of a project to replace IAS 39, “Financial Instruments: Recognition and Measurement”.
According to the IFRS 9, all financial assets should be measured at fair value upon initial recognition. In subsequent periods, debt instruments should be measured at amortized cost only if both of the following conditions are met:
| • | The asset is held within a business model whose objective is to hold assets in order to collect the contractual cash flows. |
| • | The contractual terms of the financial asset give rise on specified dates to cash flows that are solely payments of principal and interest on the principal amount outstanding. |
Subsequent measurement of all other debt instruments and financial assets should be at fair value.
Financial assets that are equity instruments should be measured in subsequent periods at fair value and the changes recognized in profit or loss or in other comprehensive income, in accordance with the election by the Company on an instrument-by-instrument basis. If equity instruments are held for trading, they should be measured at fair value through profit or loss.
IFRS 9 will be effective commencing from January 1, 2015. Earlier application is permitted.
2. The IASB issued certain amendments to IFRS 9 regarding derecognition and financial liabilities. According to those amendments, the provisions of IAS 39 will continue to apply to derecognition and to financial liabilities for which the fair value option has not been elected.
F-17
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 4:—DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION—(CONTINUED)
Pursuant to the amendments, the amount of the adjustment to the liability’s fair value that is attributable to changes in credit risk should be presented in other comprehensive income. All other fair value adjustments should be presented in profit or loss.
The amendments will be effective beginning January 1, 2015. Earlier application is permitted.
The Company believes that the application of IFRS 9 is not expected to have a material effect on the financial statements.
IFRS 10, IFRS 11, IFRS 12, IFRS 13—Consolidated Financial Statements, Joint Arrangements, Disclosure of Interests in Other Entities, Fair Value Measurement:
The IASB issued four new standards: IFRS 10, “Consolidated Financial Statements”, IFRS 11, “Joint Arrangements”, IFRS 12, “Disclosure of Interests in Other Entities” (“the new Standards”) and IFRS 13, “Fair Value Measurement”, and amended two existing standards, IAS 27R (Revised 2011), “Separate Financial Statements”, and IAS 28R (Revised 2011), “Investments in Associates and Joint Ventures”.
The new standards are to be applied retrospectively in financial statements for annual periods commencing on January 1, 2013 or thereafter. Earlier application is permitted.
The Company believes that the adoption of IFRS 10, IFRS 11, IFRS 12, IFRS 13 are not expected to have a material effect on the financial statements.
NOTE 5:—MAJOR COLLABORATION AGREEMENTS
a. On August 27, 2008, the Company signed share purchase and collaboration agreements with Monsanto. Under the share purchase agreement, which closed on September 8, 2008, Monsanto invested $18 million for the issuance of 1,636,364 ordinary shares, NIS 0.02 par value each, and a put option exercisable at a fixed price of $13.89 per share for the issuance of additional 863,637 shares.
The collaboration agreement also included payments for research and development services of up to $35 million. Pursuant to the agreement, the Company agreed to use its proprietary computational platform to discover genes with the potential to improve specified plant traits, and to validate those genes in the Company’s model plant system. Monsanto received an exclusive license to use these genes for the purpose of research and to possibly commercialize seeds with improved traits.
In addition, Monsanto agreed to provide the Company with milestone payments upon the achievement of certain agreed results in the product development process, as well as with royalty payments on future income generated by the sale of seeds containing licensed genes. Monsanto was given the right to either expand or reduce the scope of the collaboration activities.
As the share purchase agreement and the collaboration agreement were signed in contemplation of each other and the execution of the collaboration agreement was stipulated as a condition to the closing of the share purchase agreement, the agreements were treated as a multiple-elements arrangement. Following the closing of the agreements, the Company determined that the total consideration under the share purchase agreement, comprised of the $18,000 in cash and the fair value of the put option, should be allocated to two identifiable
F-18
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 5:—MAJOR COLLABORATION AGREEMENTS—(CONTINUED)
elements within this multiple-elements arrangement: (1) an equity investment in the Company’s ordinary shares and (2) research and development services performed under the collaboration agreement. Share capital and premium were recorded based on the market price of the shares on the TASE on the closing date. The put option was considered an equity instrument reduced from the Company’s equity, and its fair value was calculated using the Black & Scholes option pricing model at the issuance of the option. The cash consideration Monsanto paid for the ordinary shares exceeding their market value along with the fair value of the put option were recorded as deferred revenues from research and development services to be recognized over the term of the agreement. Accordingly, the total consideration received under the share purchase agreement was allocated as follows: share capital and premium of $9,529 based on the market price of the ordinary shares on the TASE at the closing date, a put option of $4,433 and deferred revenue of $12,882.
On November 28, 2011, the Company signed an agreement to amend and restate the 2008 collaboration with Monsanto. Accordingly, the collaboration period was extended by one year, and collaboration activities were expanded as well to include the use of the Company’s gene-optimization platform. Monsanto agreed to pay the Company an additional $12,000 for research and development services over the life of the extended agreement.
In addition, as part of the amended and restated 2011 agreement, the original put option (“original put option”) was replaced by a new put option (“new put option”).
Under the new put option, the Company may require Monsanto to invest $12,000 against an issue of 500,000 ordinary shares of the Company, at $24.00 per share. The new put option is exercisable between February 1, 2014 and August 31, 2014.
The fair value of the new option and the original put option at the amendment date were also calculated using the Black & Scholes option pricing model, and were determined to be $7,764 and $5,160, respectively. While the additional increase in the value of the original put option of $727 was recorded as share premium, reflecting the benefit derived from the revaluation of an equity instrument within the Company’s equity, the increase in value as a result of the modification of the put option amounting to $2,604 was also attributed to deferred revenue from research and development services to be recognized over the term of the agreement because the modification was performed in conjunction with the extension of the collaboration period and expansion of the collaboration activities under the amended agreement with Monsanto.
Under the amended and restated 2011 agreement, the Company also granted Monsanto an option to extend the collaboration period by an additional two years, up to August 31, 2016. If the option is exercised, Monsanto will provide the Company with a one-time payment of $6,000 at the time of exercise, and pay an additional $26,000 for research and development services over the term of the extended collaboration.
Furthermore, if Monsanto exercises the extension option by February 2014, the new put option will be amended, such that Monsanto may be required to purchase only 214,286 ordinary shares at $28.00 per share, for an aggregate investment of $6,000.
F-19
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 5:—MAJOR COLLABORATION AGREEMENTS—(CONTINUED)
The following table presents the various types of revenues recorded by the Company in connection with the agreements with Monsanto during all periods presented:
| Year ended December 31, | Three months ended March 31, |
|||||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Up-front payments |
$ | 964 | $ | 905 | $ | 699 | $ | 175 | $ | 175 | ||||||||||
| Allocated revenues from share purchase agreement and put option |
2,483 | 2,557 | 2,700 | 675 | 675 | |||||||||||||||
| Periodic research and development service payments |
5,885 | 7,061 | 8,569 | 2,187 | 2,100 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 9,332 | $ | 10,523 | $ | 11,968 | $ | 3,037 | $ | 2,950 | ||||||||||
b. On December 12, 2010, the Company signed share purchase and collaboration agreements with Bayer CropScience LP (“Bayer”). The collaboration agreement focuses on the improvement of yield, nitrogen use efficiency, and abiotic tolerance (or the increased resistance to conditions such as drought, heat and salinity) of wheat. Pursuant to the share purchase agreement, which closed on January 10, 2011, Bayer invested $12,000 in the Company in exchange for 863,310 ordinary shares at $13.90 per share, NIS 0.02 par value.
Over the course of the collaboration period, Bayer agreed to provide the Company with up-front payments and payments for research and development services amounting to €15,400 (or $21,000). In addition, Bayer agreed to provide the Company with milestone payments upon the achievement of agreed-upon results, as well as with royalty payments based on future revenue from the sale of improved wheat seeds. Under the agreement, the Company granted Bayer an exclusive license to research, develop and commercialize the sequences identified during the collaboration. The Company also agreed to not license any collaboration sequences to third parties during the course of the agreement.
As the share purchase agreement and the collaboration agreement were signed in contemplation of each other and the execution of the collaboration agreement was stipulated as a condition to the closing of the share purchase agreement, these agreements were treated as a multiple-elements arrangement. Following the closing of the agreements, the Company determined that the total consideration under the share purchase agreement of $12,000 should be allocated to two identifiable elements within this multiple-elements arrangement: (1) an equity investment in the Company’s ordinary shares and (2) research and development services performed under the collaboration agreement. Share capital and premium were recorded based on the market price of the shares on the TASE on the closing date. Accordingly, the consideration received under the share purchase agreement was allocated as follows: share capital and premium of $9,582 based on the market price of the ordinary shares on the Tel Aviv Stock Exchange on the closing date and the consideration Bayer paid for the ordinary shares exceeding their market value in the amount of $2,394 was recorded as deferred revenues from research and development services to be recognized over the term of the agreement.
F-20
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 5:—MAJOR COLLABORATION AGREEMENTS—(CONTINUED)
The following table presents the various types of revenues recorded by the Company in connection with the agreements with Bayer during all periods presented:
| Year ended December 31, | Three months ended March 31, |
|||||||||||||||||||
| 2010 | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Up-front payments |
$ | — | $ | 83 | $ | 265 | $ | 68 | $ | 77 | ||||||||||
| Allocated revenues from share purchase agreement |
— | 289 | 458 | 114 | 136 | |||||||||||||||
| Periodic research and development service payments |
— | 2,488 | 3,197 | 783 | 1,129 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | — | $ | 2,860 | $ | 3,920 | $ | 965 | $ | 1,342 | ||||||||||
NOTE 6:—CASH AND CASH EQUIVALENTS
| December 31, | ||||||||
| 2011 | 2012 | |||||||
| Cash for immediate withdrawal in NIS |
$ | 490 | $ | 217 | ||||
| Cash for immediate withdrawal in U.S.$ |
4,315 | 4,959 | ||||||
| Cash for immediate withdrawal in Euro and other currencies |
620 | 730 | ||||||
| Cash equivalents in NIS bank deposits(1) |
1,040 | 1,144 | ||||||
| Cash equivalents in U.S.$ bank deposits(1) |
— | 17,212 | ||||||
|
|
|
|
|
|||||
| $ | 6,465 | $ | 24,262 | |||||
|
|
|
|
|
|||||
| (1) | As of reporting date, the NIS deposits bear interest, ranging from 1.53% to 1.75%, while the U.S.$ deposits bear interest ranging from 0.3%-0.6%. The bank deposits are for periods ranging from one week to one month. |
NOTE 7:—MARKETABLE SECURITIES
| December 31, | ||||||||
| 2011 | 2012 | |||||||
| Financial assets measured at fair value through profit or loss: |
||||||||
| Mutual funds |
$ | 7,246 | $ | 1,072 | ||||
| Corporate bonds and government treasury notes |
27,426 | 29,796 | ||||||
|
|
|
|
|
|||||
| $ | 34,672 | $ | 30,868 | |||||
|
|
|
|
|
|||||
NOTE 8:—OTHER RECEIVABLES
| December 31, | ||||||||
| 2011 | 2012 | |||||||
| Prepaid expenses and other |
$ | 253 | $ | 313 | ||||
| Government authorities |
310 | 182 | ||||||
| Accrued reimbursement |
418 | 155 | ||||||
|
|
|
|
|
|||||
| $ | 981 | $ | 650 | |||||
|
|
|
|
|
|||||
F-21
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 9:—PROPERTY, PLANT AND EQUIPMENT
Balance at December 31, 2011:
| Laboratory Equipment |
Computers and Peripheral Equipment |
Office Equipment and Furniture |
Leasehold Improvements |
Total | ||||||||||||||||
| Cost: |
||||||||||||||||||||
| Balance at January 1, 2011 |
$ | 1,890 | $ | 1,135 | $ | 178 | $ | 3,611 | $ | 6,814 | ||||||||||
| Additions |
569 | 298 | 12 | 3,534 | 4,413 | |||||||||||||||
| Disposals |
(27 | ) | — | — | (199 | ) | (226 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2011 |
2,432 | 1,433 | 190 | 6,946 | 11,001 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Accumulated Depreciation: |
||||||||||||||||||||
| Balance at January 1, 2011 |
856 | 581 | 39 | 1,218 | 2,694 | |||||||||||||||
| Additions |
354 | 285 | 11 | 732 | 1,382 | |||||||||||||||
| Disposals |
(14 | ) | — | — | (199 | ) | (213 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2011 |
1,196 | 866 | 50 | 1,751 | 3,863 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Depreciated cost at December 31, 2011 |
$ | 1,236 | $ | 567 | $ | 140 | $ | 5,195 | $ | 7,138 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Balance at December 31, 2012:
| Laboratory Equipment |
Computers and Peripheral Equipment |
Office Equipment and Furniture |
Leasehold Improvements |
Vehicles | Total | |||||||||||||||||||
| Cost: |
||||||||||||||||||||||||
| Balance at January 1, 2012 |
$ | 2,432 | $ | 1,433 | $ | 190 | $ | 6,946 | $ | — | $ | 11,001 | ||||||||||||
| Additions |
250 | 296 | 9 | 1,450 | 49 | 2,054 | ||||||||||||||||||
| Disposals |
(43 | ) | — | — | (12 | ) | — | (55 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2012 |
2,639 | 1,729 | 199 | 8,384 | 49 | 13,000 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Accumulated Depreciation: |
||||||||||||||||||||||||
| Balance at January 1, 2012 |
1,196 | 866 | 50 | 1,751 | — | 3,863 | ||||||||||||||||||
| Additions |
407 | 326 | 12 | 1,040 | 5 | 1,790 | ||||||||||||||||||
| Disposals |
(43 | ) | — | — | (11 | ) | — | (54 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2012 |
1,560 | 1,192 | 62 | 2,780 | 5 | 5,599 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Depreciated cost at December 31, 2012 |
$ | 1,079 | $ | 537 | $ | 137 | $ | 5,604 | $ | 44 | $ | 7,401 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
NOTE 10:—OTHER PAYABLES
| December 31, | ||||||||
| 2011 | 2012 | |||||||
| Employees and payroll accruals |
$ | 1,381 | $ | 1,959 | ||||
| Government authorities |
433 | 672 | ||||||
| Accrued expenses |
265 | 508 | ||||||
|
|
|
|
|
|||||
| $ | 2,079 | $ | 3,139 | |||||
|
|
|
|
|
|||||
F-22
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 11:—GRANTS FROM THE CHIEF SCIENTIST
| December 31, | ||||||||
| 2011 | 2012 | |||||||
| Balance at January 1 |
$ | 3,899 | $ | 3,944 | ||||
| Grants received |
260 | 484 | ||||||
| Royalties paid |
(402 | ) | (892 | ) | ||||
| Amounts recorded in profit or loss |
187 | 115 | ||||||
|
|
|
|
|
|||||
| Balance at December 31 |
$ | 3,944 | $ | 3,651 | ||||
|
|
|
|
|
|||||
The Company received research and development grants from the OCS, and undertook to pay royalties of 3%-3.5% of revenues derived from research and development projects that were financed by the OCS, of up to 100% of the grants received. As of December 31, 2012, the Company received grants amounting to $4,760, (including interest), while total royalties paid as of that date amounted to $1,394.
The Company received research and development grants from BIRD-F, and undertook to pay royalties of 5% of revenues derived from research and the development projects that were financed by BIRD-F, of up to 150% of all grants received. As of December 31, 2012, the Company received grants in the amount of $ 432.
According to an agreement with BIRD-F, during January 2012 the Company repaid the full amount of grants received, and accordingly, the Company will not be required to make any royalty payments in the future with respect to those grants.
During 2012, the Company entered into another agreement with BIRD-F, according to which it undertook to pay royalties, of up to 150% of the grants received. As of December 31, 2012, the Company received under the new agreement grants in the amount of $236. No royalty payments have yet been paid.
The Company received research and development grants from CIIRDF, and undertook to pay royalties of 2.5% of revenues derived from research and the development projects that were financed by CIIRDF, of up to 100% of all grants received. As of December 31, 2012, the Company received grants amounting to $237. No royalties have yet been paid and the Company has not accrued any liability for such royalties.
NOTE 12:—FINANCIAL INSTRUMENTS
a. Classification of financial assets:
The classification of financial assets according to financial instrument groups, in accordance with IAS 39, is as follows:
| December 31, | ||||||||
| 2011 | 2012 | |||||||
| Financial Assets |
||||||||
| Financial assets at fair value through profit and loss Marketable securities |
$ | 34,672 | $ | 30,868 | ||||
|
|
|
|
|
|||||
All of the Company’s financial assets are classified as level 1 in the fair value hierarchy.
F-23
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 12:—FINANCIAL INSTRUMENTS—(CONTINUED)
b. Financial risk factors:
The Company and its subsidiaries’ operations are exposed to various financial risks, such as market risk (foreign currency risk, price risk), credit risk, and liquidity risk. The Company and its subsidiaries’ comprehensive risk management plan focuses on measures to minimize possible negative effects on the financial performance of the Company and its subsidiaries.
The Company’s Board of Directors has provided guidelines for risk management, and specific policies for various risk exposures, such as foreign currency risk, interest-rate risk, credit risk, and the use of derivative financial instruments, non-derivative financial instruments, and excess-liquidity investments.
| 1. | Market Risk: |
| a. | Foreign currency risk: |
The Company operates primarily in Israel, and has an exchange rate risk as it incurs fixed expenses in New Israel Shekels, which differs from its functional currency.
| b. | Price risk: |
The Company has investments in bonds, classified as financial instruments, which are measured at fair value through profit and loss. Accordingly, the Company and its subsidiaries are exposed to a risk from changes in fair value, determined by Stock Exchange prices.
| 2. | Credit Risk: |
The Company holds cash and cash equivalents, short-term investments and other financial instruments with various financial institutions. Its policy is to spread its investments among various institutions. In accordance with this policy, the Company invests its funds with stable financial institutions.
The Company has no trade receivables balances past due, and accordingly has not recognized any provision for doubtful accounts.
| 3. | Liquidity Risk: |
The following table presents the repayment dates of the Company and its subsidiaries’ financial liabilities, by contractual terms, in nominal amounts (including interest payments):
Balance at December 31, 2011:
| Up to 1 Year |
1 Year To 2 Years |
2 Years To 3 Years |
3 Years to 4 Years |
4 Years to 5 Years |
Over 5 Years |
Total | ||||||||||||||||||||||
| Trade payables |
$ | 2,059 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 2,059 | ||||||||||||||
| Other payables |
2,079 | — | — | — | — | — | 2,079 | |||||||||||||||||||||
| Liabilities in respect of grants from the Chief Scientist |
911 | 541 | 626 | 668 | 667 | 994 | 4,407 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| $ | 5,049 | $ | 541 | $ | 626 | $ | 668 | $ | 667 | $ | 994 | $ | 8,545 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
F-24
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 12:—FINANCIAL INSTRUMENTS—(CONTINUED)
Balance at December 31, 2012:
| Up to 1 Year |
1 Year To 2 Years |
2 Years To 3 Years |
3 Years to 4 Years |
4 Years to 5 Years |
Over 5 Years |
Total | ||||||||||||||||||||||
| Trade payables |
$ | 1,416 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 1,416 | ||||||||||||||
| Other payables |
3,139 | — | — | — | — | — | 3,139 | |||||||||||||||||||||
| Liabilities in respect of grants from the Chief Scientist |
745 | 501 | 605 | 511 | 525 | 1,175 | 4,062 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| $ | 5,300 | $ | 501 | $ | 605 | $ | 511 | $ | 525 | $ | 1,175 | $ | 8,617 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
c. Fair Value:
The carrying amounts of cash and cash equivalents, short-term investments, other receivables, trade payables and other payables approximate their fair values due to the short-term maturities of such instruments.
Liabilities in respect of grants from the Chief Scientist are capitalized using a discount rate that reflects the applicable market rate of interest at the date the grants are received which approximates the fair value at the respective balance sheet date.
d. Classification of financial instruments by fair value hierarchy
The financial instruments presented on the balance sheet at fair value are grouped into classes with similar characteristics using the following fair value hierarchy which is determined based on the source of input used in measuring fair value:
| Level 1 |
- | quoted prices (unadjusted) in active markets for identical assets or liabilities. | ||||
| Level 2 |
- | inputs other than quoted prices included within Level 1 that are observable either directly or indirectly. | ||||
| Level 3 |
- | inputs that are not based on observable market data (valuation techniques which use inputs that are not based on observable market data). | ||||
e. Sensitivity tests relating to changes in market factors:
| December 31, | ||||||||
| 2011 | 2012 | |||||||
| Sensitivity test to changes in the NIS exchange rate: |
||||||||
| Gain (loss) from the change: |
||||||||
| Increase of 5% in exchange rate |
$ | 231 | $ | (121 | ) | |||
| Decrease of 5% in exchange rate |
$ | (231 | ) | $ | 121 | |||
| Sensitivity test to changes in the market price of listed securities: |
||||||||
| Gain (loss) from the change: |
||||||||
| Increase of 5% in market price |
$ | 1,719 | $ | 1,544 | ||||
| Decrease of 5% in market price |
$ | (1,719 | ) | $ | (1,544 | ) | ||
| The change in equity: |
||||||||
| Increase of 5% in market price |
$ | 1,719 | $ | 1,544 | ||||
| Decrease of 5% in market price |
$ | (1,719 | ) | $ | (1,544 | ) | ||
F-25
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 12:—FINANCIAL INSTRUMENTS—(CONTINUED)
Sensitivity tests and principal work assumptions:
The selected changes in the relevant risk variables were determined based on management’s estimate as to reasonable possible changes in these risk variables.
The Company has performed sensitivity tests of principal market risk factors that are liable to affect its reported operating results or financial position. The sensitivity tests present the profit or loss and/or change in equity (before tax) in respect of each financial instrument for the relevant risk variable chosen for that instrument as of each reporting date. The test of risk factors was determined based on the materiality of the exposure of the operating results or financial condition of each risk with reference to the functional currency and assuming that all the other variables are constant.
NOTE 13:—SEVERANCE PAY LIABILITY
Labor laws and the Severance Pay Law in Israel (the “Severance Law”) require the Company to pay compensation to employees if they are dismissed or when they retire, or to make routine deposits with defined contribution plans under Section 14 of the Severance Pay Law, as described below. The Company’s liability for this is treated as a post-employment benefit. The Company’s liability for employee benefits is based on a valid labor agreement, the employee’s salary, and the applicable terms of employment, which together generate a right to severance compensation.
Post-employment employee benefits are financed by deposits with defined deposit plans, as detailed below.
Section 14 to the Severance Law applies to part of the compensation payments, pursuant to which the fixed contributions paid by the group into pension funds and/or policies of insurance companies release the group from any additional liability to employees for whom said contributions were made. These contributions and contributions for compensation represent defined contribution plans.
| Year ended December 31, | ||||||||||||||
|
|
2010 | 2011 | 2012 | |||||||||||
| Expenses—defined contribution plan |
$ | 309 | $ | 493 | $ | 556 | ||||||||
|
|
|
|
|
|
|
|
||||||||
NOTE 14:—TAXES ON INCOME
a. Corporate tax rates in Israel:
Taxable income of Israeli companies is subject to tax at the rate of 25% in 2010, 24% in 2011 and 25% in 2012 and onwards.
b. Tax benefits under the Israel Law for the Encouragement of Capital Investments, 1959 (the “Investment Law”):
Under the Investment Law, the Company has been granted “Approved Enterprise” and “Beneficiary Enterprise” status which provides certain benefits, including tax exemptions and reduced tax rates. Income not eligible for Approved Enterprise and Beneficiary Enterprise benefits is taxed at a regular rate.
F-26
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 14:—TAXES ON INCOME—(CONTINUED)
During the benefit period, the Company is tax exempt in the first two years of the benefit period and subject to tax at the reduced rate of 10%-25% for a period of five to eight years (depending on the level of foreign investments in the Company) of the benefit period.
The benefit entitlement period starts from the first year that the Approved/Beneficiary plant first earned taxable income, and is limited to 14 years from the year in which the approval was obtained, or 12 years from completion of the investment or commencement of production.
In the event of distribution of dividends from the said tax-exempt income, the amount distributed will be subject to corporate tax at the rate ordinarily applicable to the Approved and Beneficiary Enterprise’s income. The tax-exempt income attributable to the “Approved Enterprise” program mentioned above can be distributed to shareholders without subjecting the Company to taxes only upon the complete liquidation of the Company. Tax-exempt income generated under the Company’s “Beneficiary Enterprise” program will be subject to taxes upon dividend distribution or complete liquidation.
The entitlement to the above benefits is conditional upon the Company’s fulfilling the conditions stipulated by the Law and regulations published thereunder. Should the Company fail to meet such requirements in the future, income attributable to its Approved Enterprise and Privileged Enterprise programs could be subject to the statutory Israeli corporate tax rate and the Company could be required to refund a portion of the tax benefits already received, with respect to such programs.
In December 2010, the “Knesset” (Israeli Parliament) passed the Law for Economic Policy for 2011 and 2012 (Amended Legislation), 2011 (“the Amendment”), which prescribes, among others, amendments in the Law for the Encouragement of Capital Investments, 1959 (“the Law”). The Amendment became effective as of January 1, 2011. According to the Amendment, the benefit tracks in the Law were modified and a flat tax rate applies to the Company’s entire preferred income under its status as a preferred company with a preferred enterprise. Commencing from the 2011 tax year, the Company will be able to opt to apply (the waiver is non-recourse) the Amendment and from the elected tax year and onwards, it will be subject to the amended tax rates that are: 2011 and 2012—15%, 2013 and 2014—12.5% and in 2015 and thereafter—12%.
The Company has examined the effect of the adoption of the Amendment on its financial statements, and as of the date of the publication of the financial statements, the Company estimates that it will not apply the Amendment. The Company’s estimate may change in the future.
c. The Law for the Encouragement of Industry (Taxation), 1969:
The Company has the status of an “industrial company”, as implied by this law. According to this status and by virtue of regulations published thereunder, the Company is entitled to claim a deduction of accelerated depreciation on equipment used in industrial activities, as determined in the regulations issued under the Inflationary Law. The Company is also entitled to amortize a patent or a patent or know-how usage right that are used in the enterprise’s development or promotion, to deduct listed share issuance expenses and to file consolidated financial statements under certain conditions.
d. Tax assessments:
The Company received assessments that are considered final, up to and including the 2007 tax year.
F-27
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 14:—TAXES ON INCOME—(CONTINUED)
e. Net operating carry-forward losses for tax purposes and other temporary differences:
As of December 31, 2012, the Company and its Israeli subsidiary have carry-forward losses amounting to approximately $15 million, which can be carried forward for an indefinite period.
f. Deferred taxes:
The Company did not recognize deferred tax assets for carry-forward losses and other temporary differences, because their utilization in the foreseeable future is not probable.
g. Current taxes on income
The Company did not record any current taxes for years ended December 31, 2011 and 2010 as a result of its carry-forward losses.
The Company recorded current taxes in the amount of $74 for the year ended December 31, 2012, as a result of foreign withholding taxes.
h. Theoretical tax:
The reconciliation between the tax expense, assuming that all the income and expenses, gains and losses in the statement of income were taxed at the statutory tax rate and the taxes on income recorded in profit or loss does not provide significant information and therefore was not presented.
NOTE 15:—COMMITMENTS AND CONTINGENT LIABILITIES
The Company leases office space and motor vehicles under operating leases. Future minimum lease payments under non-cancelable operating leases for the years ended December 31, are as follows:
| 2013 |
$ | 467 | ||
| 2014 |
431 | |||
| 2015 |
383 | |||
| 2016 and after |
22 | |||
|
|
|
|||
| $ | 1,303 | |||
|
|
|
The Company has provided a NIS 842 thousand bank guarantee to secure compliance with office rental payment requirements.
As of December 31, 2012, the Company has deposited $66, in respect of vehicle operating leases.
NOTE 16:—SHAREHOLDERS’ EQUITY
a. General:
All ordinary shares, options, warrants, per share data and exercise prices included in these financial statements for all periods presented have been retroactively adjusted to reflect the 1-for-2 reverse share split to be effective immediately prior to the effectiveness of the registration statement.
b. Share capital:
| December 31, 2011 | December 31, 2012 | |||||||||||||||
| Authorized | Issued and Outstanding |
Authorized | Issued and Outstanding |
|||||||||||||
| Number of Shares | ||||||||||||||||
| Ordinary shares of NIS 0.02 par value each |
150,000,000 | 18,311,603 | 150,000,000 | 18,738,290 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-28
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 16:—SHAREHOLDERS’ EQUITY—(CONTINUED)
c. Changes in share capital:
Share capital issued and outstanding:
| Number of Shares |
NIS Par Value |
|||||||
| Outstanding at January 1, 2011 |
15,131,261 | 302,625 | ||||||
| Issuances of Share capital following the collaboration agreement |
863,310 | 17,266 | ||||||
| Exercise of Series 2 warrants |
2,147,877 | 42,958 | ||||||
| Exercise of options |
169,155 | 3,383 | ||||||
|
|
|
|
|
|||||
| Outstanding at December 31, 2011 |
18,311,603 | 366,232 | ||||||
| Exercise of options |
426,687 | 8,534 | ||||||
|
|
|
|
|
|||||
| Outstanding at December 31, 2012 |
18,738,290 | 374,766 | ||||||
|
|
|
|
|
|||||
d. Rights attached to shares:
| 1. | Voting rights at the general meeting, rights to dividends, rights upon liquidation of the Company and the right to appoint directors of the Company |
| 2. | The shares are traded on the TASE. |
e. Capital management in the Company:
The Company’s objectives in managing capital are as follows:
To maintain its ability to ensure the continuity of the business, and thus to generate a return to equity holders, investors and other parties.
The Company manages its capital structure and makes adjustments following changes in economic conditions and the risk-nature of its operations. In order to maintain or to adjust the necessary capital structure, the Company takes various steps, such as raising funds by capital issues.
f. Reserve—transaction with a former controlling shareholder
The capital reserve included as part of the Company’s shareholders’ equity is a result of prior years’ transactions with Compugen, which was formerly the controlling shareholder of the Company.
Those transactions included the receipt of a loan in the amount of $900 and a license to use Compugen’s technology. Eventually, the loan—including the accrued interest—was forgiven and the license was extended several times in consideration of the issuance of the Company’s ordinary shares with a total fair value of $185.
The terms of those transactions were a result of the relationship between the Company and Compugen and did not represent a transaction that may have been entered into between unrelated parties. Therefore, the benefit of these transactions for the Company resulting from both the forgiveness of the loan and the issuance of the ordinary shares for the license rights was recorded as a capital contribution to shareholders’ equity with a total amount of $1,156.
F-29
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 16:—SHAREHOLDERS’ EQUITY—(CONTINUED)
g. Capital issuances:
| 1. | On December 12, 2010, the Company signed with Bayer a collaboration agreement for the genetic improvement of wheat, combined with a share purchase agreement, as described in Note 5(b). On January 10, 2011, and in accordance with the collaboration agreement, the Company issued 863,310 ordinary shares, NIS 0.02 par value each. |
| 2. | During 2011, 2,147,877 Series 2 warrants were exercised for total consideration of $15,700. Upon the Series 2 warrants exercise, an additional amount of $5,500, which was previously recorded as a liability, was converted into equity. The Series 2 warrants were issued as part of the Company’s initial public offering on the TASE in 2007 and as part of the Company’s share purchase agreement with AquAgro venture fund in 2008. |
The exercise price of these warrants was NIS 11.05 linked to the Israeli consumer price index (“CPI”) (or $2.73 and $3.16 based on the exchange rate reported by the Bank of Israel on the issuance dates in 2007 and 2008, respectively). In total, 99.9% of all Series 2 warrants that were issued in the Company’s initial public offering on the TASE were exercised for an exercise price of NIS 11.05 linked to the Israeli CPI. The remaining unexercised warrants as of May 31, 2011 expired. The Company accounted for those warrants as financial liability as they were not considered instruments which can be settled by a fixed amount of cash. Accordingly, those warrants were accounted at fair value through profit and loss in accordance with IAS 39 provisions. Upon the exercise or expiration of those warrants, the financial liability was extinguished, and the revaluated carrying amount of the warrants at that date was contributed into the Company’s shareholders’ equity.
NOTE 17:—SHARE-BASED COMPENSATION
a. Expenses recognized in the financial statements:
The expense recognized in the Company’s financial statements for services provided by employees and service-providers is as follows:
| Year Ended December 31, | ||||||||||||||
|
|
2010 | 2011 | 2012 | |||||||||||
| Share-based compensation |
$ | 2,562 | $ | 2,129 | $ | 1,173 | ||||||||
|
|
|
|
|
|
|
|
||||||||
Share-based payment transactions that were granted by the Company to its employees are as described below.
b. Share-based payment plan for employees and service-providers:
| 1. | Under the Company’s 2002 option plan (“2002 Plan”) the Company may grant options to its directors and consultants. Options granted under the 2002 Plan may be exercised up to 10 years from the grant date, or until the expiry date (as defined by the Plan), whichever is earlier. The plan expired on March 13, 2012, however, on May 14, 2012 the Company’s Board of Directors approved an extension of the plan’s term in respect solely of consultants up to March 13, 2017. |
| 2. | In December 2003, an additional option plan was approved (“2003 Plan”), under which, the Company may grant options to its directors and employees. Options granted under the 2003 Plan may be exercised up to 10 years from the grant date, or until the expiry date (as defined by the Plan) whichever is earlier. |
F-30
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 17:—SHARE-BASED COMPENSATION—(CONTINUED)
| 3. | In January 2013, a third plan was approved (“2013 Plan”), under which the Company may grant options to its directors and employees. To date, no options have been granted under this plan. |
The vesting period of the options is four years, unless otherwise determined for a specific grant. Each option may be exchanged for 1 ordinary share, NIS 0.02 par value. Options forfeited or not exercised prior to expiry, may be granted in the future.
During 2010, the Company issued 1,262,400 options exercisable into 1,262,400 ordinary shares, NIS 0.02 par value each of the Company, for exercise prices ranging from NIS 28.66 to NIS 36.95 per share, to employees, officers and consultants (excluding options granted to the Company’s directors and the Chief Executive Officer). The fair value of the options determined upon grant date was $3,600.
During 2011, the Company issued 247,500 options, exercisable into 247,500 ordinary shares, NIS 0.02 par value each of the Company, for exercise prices ranging from NIS 29.19 to NIS 33.92 per share, to new employees. The fair value of the options determined upon grant date was $ 715.
During 2012, the Company issued 324,000 options exercisable into 324,000 ordinary shares, NIS 0.02 par value each of the Company, for exercise prices ranging from NIS 33.48 to NIS 35.84 per share, to various employees and consultants. The fair value of the options determined upon grant date was $1,085.
c. Option grants to key officers and directors:
During 2010, the Company issued 50,000 options exercisable into 50,000 ordinary shares, NIS 0.02 par value each of the Company, for exercise prices ranging from NIS 19.94 to NIS 33.86 per share. The fair value of the options determined upon grant date was $220.
On June 2, 2010, the Company’s board of directors approved a grant of 200,000 options to the Company’s CEO and President, exercisable into 200,000 ordinary shares, NIS 0.02 par value each of the Company, for an exercise price of NIS 28.66 per share. The fair value of the options determined upon grant date was $591.
On August 8, 2011, the Company issued 17,500 options, exercisable into 17,500 ordinary shares, NIS 0.02 par value each of the Company, for exercise prices ranging from NIS 28.70 to NIS 33.80 per share, to serving directors. The fair value of the options determined upon grant date was $58.
On October 24, 2012, the Company issued 17,500 options, exercisable into 17,500 ordinary shares, NIS 0.02 par value each of the Company, for exercise prices ranging from NIS 31.52 to NIS 33.41 per share, to serving directors. The fair value of the options determined upon grant date was $76.
d. Options exercised:
During 2010, 2011, and 2012 employees and consultants exercised 282,940, 169,155 and 426,687 options, respectively, into 878,782 ordinary shares, NIS 0.02 par value each of the Company, for a total consideration of $458, $378 and $1,519, respectively.
F-31
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 17:—SHARE-BASED COMPENSATION—(CONTINUED)
e. Share options activity:
The following table summarizes the number of share options, the weighted average exercise price, and the changes that were made in the option plans to employees, consultants and directors:
| 2010 | 2011 | 2012 | ||||||||||||||||||||||||
| Number of Options |
Weighted Average Exercise Prices ($) |
Number of Options |
Weighted Average Exercise Prices ($) |
Number of Options |
Weighted Average Exercise Prices ($) |
| ||||||||||||||||||||
| Outstanding at January 1 |
1,892,261 | 3.12 | 3,013,304 | 4.18 | 3,015,900 | 5.78 | ||||||||||||||||||||
| Grants |
1,512,400 | 8.20 | 265,000 | 8.28 | 341,500 | 9.22 | ||||||||||||||||||||
| Exercised |
(282,940) | 1.62 | (169,155 | ) | 2.10 | (426,687 | ) | 3.70 | ||||||||||||||||||
| Forfeited |
(108,417 | ) | 6.10 | (93,249 | ) | 7.24 | (118,772 | ) | 8.20 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||
| Outstanding at December 31 |
3,013,304 | 4.18 | 3,015,900 | 5.78 | 2,811,941 | 6.56 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||
| Exercisable at December 31 |
1,244,868 | 3.38 | 1,696,739 | 4.46 | 1,825,445 | 5.64 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||
The following table summarizes information about share options outstanding at December 31, 2012:
| Options outstanding | ||||||||||||
| Range of exercise prices ($) | Number outstanding |
Average remaining contractual life |
Weighted average exercise price |
|||||||||
| 0.40-1.72 |
147,805 | 2.62 | 0.56 | |||||||||
| 2.26-4.18 |
510,124 | 4.78 | 2.64 | |||||||||
| 6.76-7.80 |
1,640,012 | 7.36 | 7.46 | |||||||||
| 8.44-9.90 |
514,000 | 9.1 | 9.24 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
2,811,941 | 6.97 | 6.56 | |||||||||
|
|
|
|
|
|
|
|||||||
f. The weighted average outstanding remaining contractual term of the options as of December 31, 2012 is 6.97 years (as of December 31, 2011, it is 7.41 years).
g. The weighted average fair value of options granted during 2012 was NIS 12.70 ($3.40) (for options granted during 2011, the fair value was NIS 12.98 ($3.40)).
h. The fair value of the Company’s share options granted to employees and directors for the years ended December 31, 2012, 2011 and 2010 was estimated using binomial model using the following assumptions:
| 2010 | 2011 | 2012 | ||||||||||
| Dividend yield (%) |
— | — | — | |||||||||
| Expected volatility of the share prices (%) |
41-76 | 33-69 | 25-65 | |||||||||
| Risk-free interest rate (%) |
2.17-7.14 | 3.01-6.93 | 1.87-6.81 | |||||||||
| Expected life of the options (years) |
3.95 | 4.27 | 4.12 | |||||||||
| Share price (U.S.$/NIS) |
8.18/29.10 | 8.28/31.64 | 9.34/35.98 | |||||||||
The expected life of the options is based on historical data and is not necessarily indicative of the future exercise patterns of the options.
F-32
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 17:—SHARE-BASED COMPENSATION—(CONTINUED)
The expected volatility of the share prices reflects the assumption that the historical volatility of the share prices is reasonably indicative of expected future trends.
NOTE 18:—STATEMENTS OF COMPREHENSIVE INCOME—ADDITIONAL INFORMATION
a. Cost of revenues:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Salaries and benefits |
$ | 3,188 | $ | 4,586 | $ | 5,443 | ||||||
| Share-based compensation |
785 | 681 | 364 | |||||||||
| Sub-contractors and consultants |
419 | 842 | 1,307 | |||||||||
| Materials |
681 | 902 | 951 | |||||||||
| Depreciation |
284 | 528 | 912 | |||||||||
| Patents and related costs |
140 | 249 | 80 | |||||||||
| Rentals and maintenance |
314 | 459 | 495 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 5,811 | $ | 8,247 | $ | 9,552 | |||||||
|
|
|
|
|
|
|
|||||||
b. Research and development:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Salaries and benefits |
$ | 2,535 | $ | 3,265 | $ | 3,915 | ||||||
| Share-based compensation |
784 | 682 | 364 | |||||||||
| Materials and subcontractors |
944 | 518 | 675 | |||||||||
| Plant growth and greenhouse maintenance |
204 | 238 | 275 | |||||||||
| Rentals and office maintenance |
369 | 472 | 638 | |||||||||
| Patents |
180 | 73 | 72 | |||||||||
| Depreciation |
659 | 891 | 923 | |||||||||
| Consulting |
279 | 249 | 279 | |||||||||
| Other |
133 | 253 | 229 | |||||||||
| Participation by the Chief Scientist and other parties |
(543 | ) | (257 | ) | (118 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| $ | 5,544 | $ | 6,384 | $ | 7,252 | |||||||
|
|
|
|
|
|
|
|||||||
c. Business development:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Salaries and benefits |
$ | 556 | $ | 645 | $ | 732 | ||||||
| Share-based compensation |
307 | 230 | 164 | |||||||||
| Travel |
59 | 98 | 103 | |||||||||
| Legal |
86 | 103 | 50 | |||||||||
| Other |
54 | 60 | 110 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,062 | $ | 1,136 | $ | 1,159 | |||||||
|
|
|
|
|
|
|
|||||||
F-33
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 18:—STATEMENTS OF COMPREHENSIVE INCOME—ADDITIONAL INFORMATION—(CONTINUED)
d. General and administrative:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Salaries and benefits |
$ | 845 | $ | 1,160 | $ | 1,354 | ||||||
| Share-based compensation |
686 | 536 | 281 | |||||||||
| Consultation |
62 | 59 | 96 | |||||||||
| Professional fees |
231 | 218 | 239 | |||||||||
| Other |
299 | 344 | 265 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 2,123 | $ | 2,317 | $ | 2,235 | |||||||
|
|
|
|
|
|
|
|||||||
e. Financing income and expenses
Financing income:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Exchange differences, net |
$ | 386 | $ | — | $ | 61 | ||||||
| Change in the fair value of marketable securities |
159 | 911 | 42 | |||||||||
| Interest income |
179 | 383 | 869 | |||||||||
| Revaluation of Series 2 warrants |
— | 3,729 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 724 | $ | 5,023 | $ | 972 | |||||||
|
|
|
|
|
|
|
|||||||
Financing expenses:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Bank expenses and commissions |
$ | 10 | $ | 7 | $ | 89 | ||||||
| Exchange differences, net |
— | 1,032 | — | |||||||||
| Revaluation of Series 2 warrants |
5,393 | — | — | |||||||||
| Revaluation of liabilities to the Chief Scientist |
314 | 156 | 205 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 5,717 | $ | 1,195 | $ | 294 | |||||||
|
|
|
|
|
|
|
|||||||
F-34
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 19:—NET INCOME (LOSS) PER SHARE
Details of the number of shares and income (loss) used in the computation of net income (loss) per share:
| Year ended December 31, | ||||||||||||||||||||||||
| 2010 | 2011 | 2012 | ||||||||||||||||||||||
| Weighted number of shares *) |
Loss | Weighted number of shares *) |
Net income |
Weighted number of shares *) |
Loss | |||||||||||||||||||
| Number of shares and net income (loss) for the computation of basic net income (loss) per share |
14,824,703 | (6,970 | ) | 17,505,136 | 645 | 18,421,568 | (2,522 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Number of shares and net income (loss) for the computation of diluted net income (loss) per share |
14,824,703 | (6,970 | ) | 18,731,118 | 645 | 18,421,568 | (2,522 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| *) | To compute diluted net income (loss) per share, potential ordinary shares, detailed below, have not been taken into account due to their anti-dilutive effect: |
| 2010 | 2011 | 2012 | ||||||||||
| Options to employees and consultants under share-based payment plans |
3,013,304 | — | 2,811,941 | |||||||||
| Warrants |
2,150,599 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| 5,163,903 | — | 2,811,941 | ||||||||||
|
|
|
|
|
|
|
|||||||
Reconciliation of the weighted average shares used to compute basic and diluted earnings per share Year ended December 31, 2011:
| Number of shares for the computation of basic net income per share |
17,505,136 | |||
| Effect of dilutive securities: |
||||
| Options to employees and consultants under share-based payment plans |
1,225,727 | |||
| Warrants |
255 | |||
|
|
|
|||
| Number of shares for the computation of basic net income per share |
18,731,118 | |||
|
|
|
NOTE 20:—OPERATING SEGMENTS
a. General:
Commencing January 1, 2012, the Company operates in two segments. The segments were determined on the basis of information considered by the Chief Operating Decision-Maker (“CODM”) for purposes of decision-making on the allocation of resources and evaluation of performance. The following Company’s segments are engaged in business activities for which they earn revenues and incur expenses, their results are reviewed by the CODM and discrete financial information is available:
| Evogene segment |
— | Services for the development of gene sequence technology (D.N.A. sequences responsible for the production of proteins—“genes”) and other D.N.A. sequences in plants (“D.N.A. sequences”). | ||
| Evofuel segment |
— | Services for the development of improved species of the castor oil plant, which may serve as a source for extracting oil for the bio diesel fuel industry. | ||
F-35
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 20:—OPERATING SEGMENTS—(CONTINUED)
Segments performance (segment income (loss)) is determined based on operating income (loss) reported in the financial statements. The results of a segment reported to the CODM include items attributed directly to a segment, as well as other items, which are indirectly attributed using reasonable assumptions.
b. The following table presents our revenues and operating loss by segments:
| Evogene | Evofuel | Adjustments | Total | |||||||||||||
| For the Year Ended December 31, 2012 |
||||||||||||||||
| Revenues |
$ | 17,072 | $ | — | $ | — | $ | 17,072 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
$ | (1,993 | ) | $ | (1,133 | ) | $ | — | $ | (3,126 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net financing income |
678 | |||||||||||||||
|
|
|
|||||||||||||||
| Loss before taxes on income |
$ | (2,448 | ) | |||||||||||||
|
|
|
|||||||||||||||
c. Major customers:
Revenues from major customers each of whom amounts to 10% or more, of total revenues:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Customer A (shareholder) |
74 | % | 71 | % | 70 | % | ||||||
| Customer B (shareholder) |
— | *) | 23 | % | 24 | % | ||||||
| Customer C |
17 | % | — | *) | — | *) | ||||||
| *) | Represents revenues that are lower than 10% of total revenues |
d. Geographical information:
Revenues based on the location of the customers, are as follows:
| Year Ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Israel |
1 | % | 1 | % | — | |||||||
| United States |
76 | % | 71 | % | 72 | % | ||||||
| Germany |
4 | % | 23 | % | 24 | % | ||||||
| France |
17 | % | 5 | % | 4 | % | ||||||
| Others |
2 | % | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| 100 | % | 100 | % | 100 | % | |||||||
|
|
|
|
|
|
|
|||||||
F-36
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 21:—BALANCES AND TRANSACTIONS WITH KEY OFFICERS AND CERTAIN SHAREHOLDERS
a. The certain shareholders refer to Monsanto and Bayer, which hold 8.7% and 4.6%, respectively, of the Company’s ordinary shares, as of December 31, 2012, and are also major customers (see also Notes 5 and 20(c)).
b. Balances:
Balance at December 31, 2011:
| Key Officers |
Certain Shareholders |
|||||||
| Receivables |
$ | — | $ | 1,094 | ||||
|
|
|
|
|
|||||
| Other payables |
$ | 278 | $ | 14 | ||||
|
|
|
|
|
|||||
c. Benefits to directors:
Balance at December 31, 2012:
| Key Officers |
Certain Shareholders |
|||||||
| Receivables |
$ | — | $ | 1,698 | ||||
|
|
|
|
|
|||||
| Other payables |
$ | 296 | $ | 20 | ||||
|
|
|
|
|
|||||
| Year ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Remuneration to directors who are not employed by the Company or on its behalf |
$ | 59 | $ | 51 | $ | 71 | ||||||
|
|
|
|
|
|
|
|||||||
| Number of directors not employed by the Company |
3 | 3 | 3 | |||||||||
|
|
|
|
|
|
|
|||||||
d. Benefits to key officers:
| Year ended December 31, | ||||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| Short term benefits |
$ | 982 | $ | 1,225 | $ | 1,188 | ||||||
| Share-based compensation |
1,248 | 986 | 441 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 2,230 | $ | 2,211 | $ | 1,629 | |||||||
|
|
|
|
|
|
|
|||||||
| Number of people that received salary and benefits |
5 | 5 | 5 | |||||||||
|
|
|
|
|
|
|
|||||||
e. Transactions:
For the year ended December 31, 2010
| Key Officers |
Certain Shareholders |
|||||||
| Revenues |
$ | — | $ | (9,856 | ) | |||
| Cost of revenue |
721 | (392 | ) | |||||
| Research and development expenses |
110 | — | ||||||
| Business development expenses |
688 | — | ||||||
| General and administrative expenses |
711 | — | ||||||
|
|
|
|
|
|||||
| $ | 2,230 | $ | (10,248 | ) | ||||
|
|
|
|
|
|||||
F-37
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 21:—BALANCES AND TRANSACTIONS WITH KEY OFFICERS AND CERTAIN SHAREHOLDERS—(CONTINUED)
For the year ended December 31, 2011
| Key Officers |
Certain Shareholders |
|||||||
| Revenues |
$ | — | $ | (14,006 | ) | |||
| Cost of revenue |
677 | (575 | ) | |||||
| Research and development expenses |
315 | — | ||||||
| Business development expenses |
623 | — | ||||||
| General and administrative expenses |
596 | — | ||||||
|
|
|
|
|
|||||
| $ | 2,211 | $ | (14,581 | ) | ||||
|
|
|
|
|
|||||
For the year ended December 31, 2012
| Key Officers |
Certain Shareholders |
|||||||
| Revenues |
$ | — | $ | (16,083 | ) | |||
| Cost of revenue |
302 | (225 | ) | |||||
| Research and development expenses |
390 | — | ||||||
| Business development expenses |
440 | — | ||||||
| General and administrative expenses |
497 | — | ||||||
|
|
|
|
|
|||||
| $ | 1,629 | $ | (16,308 | ) | ||||
|
|
|
|
|
|||||
NOTE 22:—SUBSEQUENT EVENT
| a. | On February 4, 2013, the Company signed an agreement with a private Israeli company (the “Private Company”), according to which the Company undertook to provide the Private Company with rights to use its greenhouses and facilities, including support for the Private Company’s development process for the following consideration: |
| i. | 15% of the Private Company’s shares on an outstanding basis. The shares are subject to reverse vesting over a period of 36 months. |
| ii. | The Company also was granted with anti-dilution option up to an aggregate investment of $4 million in the Private Company. |
| iii. | A three years access to the system being developed by the Private Company, including an option to purchase the system which is exercisable over the term of the agreement. |
| b. | During the six months ended June 30, 2013, 141,697 options were exercised by employees into 141,697 ordinary shares of NIS 0.02 par value each for a total consideration of $828. |
| c. | On May 29, 2013, the Company signed a cooperation agreement with Beijing Dabeinong Technology Co. (“DBN”), a leading Chinese company in the field of development and commercialization of rice seeds and other agricultural products. According to the agreement, the Company will receive payments based on milestones stated in the agreement. In addition, the Company will receive sales-based royalties based on the stated terms in the agreement. |
F-38
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 22:—SUBSEQUENT EVENT—(CONTINUED)
| d. | On July 17, 2013, the board of directors of the Company approved an issuance to its officers and employees of 1,000,000 options exercisable into 1,000,000 ordinary shares of the Company, NIS 0.02 par value each, for an exercise price of NIS 44.28 ($12.52) per share. The estimated fair value of the options at their grant date using the binomial model was approximately $3,825. The main assumptions used by the Company in determining the fair value were: dividend yield, 0%; expected volatility of the share price, 26-60%; risk free interest rate, 1.14-6.34%; and share price of NIS 43.62 ($12.20). |
On September 22, 2013, the board of directors of the Company approved an issuance to the Company’s President and Chief Executive Officer of 215,000 options exercisable into 215,000 ordinary shares of the Company, NIS 0.02 par value each, for an exercise price of NIS 48.18 ($13.62) per share. The grant is subject to shareholder approval at a general meeting of the Company. The current estimate of the fair value of these options using the binomial model is approximately $1,108. The main assumptions used by the Company in determining the fair value were: dividend yield, 0%; expected volatility of the share price, 26.70-58.51%; risk free interest rate, 1.22-6.25%; and share price of NIS 51.18 ($14.46).
| e. | On July 30, 2013, the Israeli Parliament (the “Knesset”) approved the second and third readings of the Economic Plan for 2013-2014 (“Amended Budget Law”) which consists, among others, of fiscal changes whose main aim is to enhance long-term collection of taxes. |
These changes include, among others, raising the Israeli corporate tax rate from 25% to 26.5%, cancelling the lowering of the tax rates applicable to preferred enterprises (9% in development area A and 16% in other areas), taxing revaluation gains and increasing the tax rates on dividends within the scope of the Law for the Encouragement of Capital Investments to 20%, and are effective from January 1, 2014.
The Company estimates that the effect of the change in tax rates will not have a material effect on the Company’s financial statements.
F-39
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
U.S. dollars in thousands
| As of December 31, 2012 |
As of June 30, | |||||||||||
| 2012 | 2013 | |||||||||||
| Audited | Unaudited | |||||||||||
| CURRENT ASSETS: |
||||||||||||
| Cash and cash equivalents |
$ | 24,262 | $ | 11,558 | $ | 16,884 | ||||||
| Marketable securities |
30,868 | 30,643 | 33,657 | |||||||||
| Short-term bank deposits |
— | 10,600 | — | |||||||||
| Trade receivables |
1,542 | 3,911 | 1,853 | |||||||||
| Other receivables |
650 | 681 | 1,573 | |||||||||
|
|
|
|
|
|
|
|||||||
| 57,322 | 57,393 | 53,967 | ||||||||||
|
|
|
|
|
|
|
|||||||
| LONG-TERM ASSETS: |
||||||||||||
| Long term deposits |
43 | 47 | 33 | |||||||||
| Plant, property and equipment, net |
7,401 | 7,825 | 7,572 | |||||||||
| Other investment |
— | — | 365 | |||||||||
| Intangible assets, net |
89 | 111 | 67 | |||||||||
|
|
|
|
|
|
|
|||||||
| 7,533 | 7,983 | 8,037 | ||||||||||
|
|
|
|
|
|
|
|||||||
| $ | 64,855 | $ | 65,376 | $ | 62,004 | |||||||
|
|
|
|
|
|
|
|||||||
| CURRENT LIABILITIES: |
||||||||||||
| Trade payables |
$ | 1,416 | $ | 1,299 | $ | 1,873 | ||||||
| Liabilities in respect of grants from the Chief Scientist |
733 | 493 | 528 | |||||||||
| Deferred revenues and other advances |
4,211 | 4,374 | 3,968 | |||||||||
| Other payables |
3,139 | 1,866 | 2,677 | |||||||||
|
|
|
|
|
|
|
|||||||
| 9,499 | 8,032 | 9,046 | ||||||||||
|
|
|
|
|
|
|
|||||||
| LONG-TERM LIABILITIES: |
||||||||||||
| Liabilities in respect of grants from the Chief Scientist |
2,918 | 2,947 | 3,101 | |||||||||
| Deferred revenues and other advances |
4,168 | 6,027 | 2,539 | |||||||||
| Severance pay liability, net |
11 | 9 | 19 | |||||||||
|
|
|
|
|
|
|
|||||||
| 7,097 | 8,983 | 5,659 | ||||||||||
|
|
|
|
|
|
|
|||||||
| SHAREHOLDERS’ EQUITY: |
||||||||||||
| Ordinary shares of NIS 0.02 par value: authorized—150,000,000 ordinary shares; issued and outstanding—18,879,987, 18,403,376 and 18,738,290 shares at June 30, 2013 and 2012 and December 31, 2012, respectively |
102 | 100 | 103 | |||||||||
| Share premium |
90,746 | 89,063 | 92,147 | |||||||||
| Put option |
(7,764 | ) | (7,764 | ) | (7,764 | ) | ||||||
| Capital reserve—transaction with a former controlling shareholder |
1,156 | 1,156 | 1,156 | |||||||||
| Accumulated deficit |
(35,981 | ) | (34,194 | ) | (38,343 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| 48,259 | 48,361 | 47,299 | ||||||||||
|
|
|
|
|
|
|
|||||||
| $ | 64,855 | $ | 65,376 | $ | 62,004 | |||||||
|
|
|
|
|
|
|
|||||||
The accompanying Notes are an integral part of the Consolidated Financial Statements.
F-40
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
U.S. dollars in thousands (except share and per share data)
| Year
ended December 31, 2012 |
Six months ended June 30, |
|||||||||||
| 2012 | 2013 | |||||||||||
| Audited | Unaudited | |||||||||||
| Revenues |
$ | 17,072 | $ | 8,287 | $ | 8,934 | ||||||
| Cost of revenues |
9,552 | 4,483 | 4,688 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
7,520 | 3,804 | 4,246 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Research and development, net |
7,252 | 3,308 | 4,666 | |||||||||
| Business development |
1,159 | 544 | 532 | |||||||||
| General and administrative |
2,235 | 1,069 | 1,197 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
10,646 | 4,921 | 6,395 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(3,126 | ) | (1,117 | ) | (2,149 | ) | ||||||
| Financing income |
972 | 510 | 776 | |||||||||
| Financing expenses |
(294 | ) | (76 | ) | (989 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before taxes on income |
(2,448 | ) | (683 | ) | (2,362 | ) | ||||||
| Taxes on income |
74 | 52 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss |
$ | (2,522 | ) | $ | (735 | ) | $ | (2,362 | ) | |||
|
|
|
|
|
|
|
|||||||
| Total comprehensive loss |
$ | (2,522 | ) | $ | (735 | ) | $ | (2,362 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted loss per share |
$ | (0.14 | ) | $ | (0.04 | ) | $ | (0.12 | ) | |||
|
|
|
|
|
|
|
|||||||
The accompanying Notes are an integral part of the Consolidated Financial Statements
F-41
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
U.S. dollars in thousands
| Share Capital |
Share Premium |
Put Option |
Capital reserve— transaction with a former controlling shareholder |
Accumulated Deficit |
Total | |||||||||||||||||||
| Balance as of January 1, 2012 (audited) |
$ | 100 | $ | 88,056 | $ | (7,764 | ) | $ | 1,156 | $ | (33,459 | ) | $ | 48,089 | ||||||||||
| Total comprehensive loss |
— | — | — | — | (2,522 | ) | (2,522 | ) | ||||||||||||||||
| Exercise of options |
2 | 1,517 | — | — | — | 1,519 | ||||||||||||||||||
| Share-based compensation |
— | 1,173 | — | — | — | 1,173 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2012 (audited) |
$ | 102 | $ | 90,746 | $ | (7,764 | ) | $ | 1,156 | $ | (35,981 | ) | $ | 48,259 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Share Capital |
Share Premium |
Put Option |
Capital reserve— transaction with a former controlling shareholder |
Accumulated Deficit |
Total | |||||||||||||||||||
| Unaudited | ||||||||||||||||||||||||
| Balance as of January 1, 2012 (audited) |
$ | 100 | $ | 88,056 | $ | (7,764 | ) | $ | 1,156 | $ | (33,459 | ) | $ | 48,089 | ||||||||||
| Total comprehensive loss |
— | — | — | — | (735 | ) | (735 | ) | ||||||||||||||||
| Exercise of options |
* | ) | 433 | — | — | — | 433 | |||||||||||||||||
| Share-based compensation |
— | 574 | — | — | — | 574 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of June 30, 2012 |
$ | 100 | $ | 89,063 | $ | (7,764 | ) | $ | 1,156 | $ | (34,194 | ) | $ | 48,361 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Share Capital |
Share Premium |
Put Option |
Capital reserve— transaction with a former controlling shareholder |
Accumulated Deficit |
Total | |||||||||||||||||||
| Unaudited | ||||||||||||||||||||||||
| Balance as of January 1, 2013 (audited) |
$ | 102 | $ | 90,746 | $ | (7,764 | ) | $ | 1,156 | $ | (35,981 | ) | $ | 48,259 | ||||||||||
| Total comprehensive loss |
— | — | — | — | (2,362 | ) | (2,362 | ) | ||||||||||||||||
| Exercise of options |
1 | 827 | — | — | — | 828 | ||||||||||||||||||
| Share-based compensation |
— | 574 | — | — | — | 574 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of June 30, 2013 |
$ | 103 | $ | 92,147 | $ | (7,764 | ) | $ | 1,156 | $ | (38,343 | ) | $ | 47,299 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| *) | Represent amount lower than $1 |
The accompanying Notes are an integral part of the Consolidated Financial Statements.
F-42
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year
ended December 31, 2012 |
Six months ended June 30, |
|||||||||||
| 2012 | 2013 | |||||||||||
| Audited | Unaudited | |||||||||||
| Cash Flows from Operating Activities |
||||||||||||
| Loss |
$ | (2,522 | ) | $ | (735 | ) | $ | (2,362 | ) | |||
| Adjustments to reconcile loss to net cash used in operating activities: |
||||||||||||
| Adjustments to the profit or loss items: |
||||||||||||
| Depreciation and amortization |
1,835 | 874 | 1,007 | |||||||||
| Share-based compensation |
1,173 | 574 | 574 | |||||||||
| Net financing expenses (income) |
(678 | ) | (434 | ) | 213 | |||||||
| Taxes on income |
74 | — | — | |||||||||
| Gain on disposal of property, plant and equipment |
(33 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| 2,371 | 1,014 | 1,794 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Changes in asset and liability items: |
||||||||||||
| Decrease (increase) in trade receivables |
(742 | ) | (3,096 | ) | (323 | ) | ||||||
| Decrease (increase) in other receivables |
336 | 251 | (94 | ) | ||||||||
| Increase (decrease) in trade payables |
231 | (138 | ) | (255 | ) | |||||||
| Increase (decrease) in other payables |
974 | (192 | ) | (502 | ) | |||||||
| Decrease in deferred revenues and other advances |
(3,331 | ) | (1,309 | ) | (2,202 | ) | ||||||
| Increase in severance pay liability, net |
— | — | 8 | |||||||||
| Increase in liabilities in respect of grants from the Chief Scientist |
— | (90 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| (2,532 | ) | (4,574 | ) | (3,368 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash received (paid) during the period for: |
||||||||||||
| Interest received |
869 | 626 | 634 | |||||||||
| Taxes paid (withheld by customers) |
(74 | ) | (52 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
$ | 1,888 | ) | $ | (3,721 | ) | $ | (3,302 | ) | |||
|
|
|
|
|
|
|
|||||||
The accompanying Notes are an integral part of the Consolidated Financial Statements.
F-43
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year
ended December 31, 2012 |
Six months ended June 30, |
|||||||||||
| 2012 | 2013 | |||||||||||
| Audited | Unaudited | |||||||||||
| Cash Flows from Investing Activities |
||||||||||||
| Purchase of property, plant and equipment |
$ | (2,963 | ) | $ | (2,127 | ) | $ | (731 | ) | |||
| Proceeds from disposal of property, plant and equipment |
34 | — | — | |||||||||
| Proceeds from sale of marketable securities |
15,697 | 8,917 | 13,291 | |||||||||
| Purchase of marketable securities |
(11,935 | ) | (5,097 | ) | (16,831 | ) | ||||||
| Proceeds from bank deposits, net |
17,652 | 7,052 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
18,485 | 8,745 | (4,271 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash Flows from Financing Activities |
||||||||||||
| Proceeds from exercise of options |
1,519 | 433 | 828 | |||||||||
| Proceeds from the Chief Scientist grants |
484 | 230 | 155 | |||||||||
| Repayment of the Chief Scientist grants |
(892 | ) | (664 | ) | (255 | ) | ||||||
| Issuance expenses |
— | — | (550 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
1,111 | (1 | ) | 178 | ||||||||
|
|
|
|
|
|
|
|||||||
| Exchange rate differences—cash and cash equivalent balances |
89 | 70 | 17 | |||||||||
|
|
|
|
|
|
|
|||||||
| Increase (decrease) in cash and cash equivalents |
17,797 | 5,093 | (7,378 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, beginning of the period |
6,465 | 6,465 | 24,262 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of the period |
$ | 24,262 | $ | 11,558 | $ | 16,884 | ||||||
|
|
|
|
|
|
|
|||||||
| Significant non-cash transactions |
||||||||||||
| Acquisition of property, plant and equipment in credit |
$ | 101 | $ | 420 | $ | 526 | ||||||
|
|
|
|
|
|
|
|||||||
| Other investment |
$ | — | $ | — | $ | 365 | ||||||
|
|
|
|
|
|
|
|||||||
| Issuance expenses |
$ | — | $ | — | $ | 259 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying Notes are an integral part of the Consolidated Financial Statements.
F-44
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 1:—GENERAL
a. These Financial Statements have been prepared in a condensed format as of June 30, 2013 and for the six months then ended (“interim consolidated financial statements”).
These financial statements should be read in conjunction with the Company’s annual financial statements as of December 31, 2012 and for the year then ended and the accompanying notes (“annual consolidated financial statements”) as issued on September 23, 2013.
b. The Company principally derives its revenues from collaboration arrangements. Revenues from its major collaborators accounted together for 97.7% and 99.3% for the six months ended June 30, 2013 and 2012, respectively. Revenues from its major collaborators accounted together for 94.2% for the year ended December 31, 2012. As to major customers, see Note 4(c). If a major customer decides to terminate its collaboration agreement with the Company, the Company may not be able to make up the lost revenue and it may have a material adverse effect on its results of operations.
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES
a. Basis of preparation of the interim consolidated financial statements:
The interim consolidated financial statements have been prepared in accordance with generally accepted accounting principles for the preparation of financial statements for interim periods, as prescribed in IAS 34, “Interim Financial Reporting.”
b. New standards, interpretations and corrections first applied by the Company
The accounting policies applied in preparing the interim consolidated financial statements consistent to those applied in the preparation of the annual consolidated financial statements, except for the following:
IAS 39 - Financial Assets
Financial assets within the scope of IAS 39 are initially recognized at fair value plus directly attributable transaction costs, except for financial assets measured at fair value through profit or loss in respect of which transaction costs are recorded in profit or loss.
After initial recognition, the accounting treatment of financial assets is based on their classification as follows:
| 1. | This category includes financial assets held for trading and financial assets designated upon initial recognition as at fair value through profit or loss. |
Embedded derivatives are separated from the host contract and accounted for separately if: (a) the economic characteristics and risks of the embedded derivatives are not closely related to those of the host contract; (b) a separate instrument with the same terms as the embedded derivative would meet the definition of a derivative; and (c) the combined instrument is not measured at fair value through profit or loss.
The Company assesses whether embedded derivatives are required to be separated from host contracts when the Company first becomes party to the contract. Reassessment only occurs if there is a change in the terms of the contract that significantly modifies the cash flows that would otherwise be required.
F-45
Table of Contents
Derivatives, including separated embedded derivatives, are classified as held for trading unless they are designated as effective hedging instruments. In the event of a financial instrument that contains one or more embedded derivatives, the entire combined instrument may be designated as a financial asset / liability at fair value through profit or loss only upon initial recognition
| 2. | Available-for-sale financial assets: |
Available-for-sale financial assets are (non-derivative) financial assets that are designated as available for sale or are not classified in any of the three preceding categories. After initial recognition, available-for-sale financial assets are measured at fair value. Gains or losses from fair value adjustments, except for interest and exchange rate differences that relate to debt instruments, are recognized in other comprehensive income. When the investment is disposed of or in case of impairment, the other comprehensive income (loss) is recognized in profit or loss. Revenues from dividends from investments in equity instruments are recognized when the right to receive the dividends is established.
IAS 19 (Revised)—Employee Benefits
In June 2011, the IASB issued IAS 19 (Revised) to be applied from January 1, 2013 which included a number of amendments to the accounting for defined benefit plans.
The adoption of IAS 19 (Revised) did not have a material effect on the Company’s financial statements.
IFRS 13—Fair value measurement
IFRS 13 establishes guidance for the measurement of fair value, to the extent that such measurement is required according to IFRS. IFRS 13 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. IFRS 13 also specifies the characteristics of market participants and determines that fair value is based on the assumptions that would have been used by market participants. According to IFRS 13, fair value measurement is based on the assumption that the transaction will take place in the asset’s or the liability’s principal market, or in the absence of a principal market, in the most advantageous market. The new disclosures are to be applied prospectively beginning on January 1, 2013, and they do not apply to comparative figures.
The adoption of IFRS 13 did not have a material effect on the Company’s financial statements.
c. Functional currency and reporting currency
The reporting currency of the financial statements is the U.S. dollar.
The Company and its subsidiaries determine the functional currency of each entity based on the currency in which they primarily generate and expend cash. The Company determined that its functional currency is the U.S. dollar since most of its sales are generated in U.S. dollar and its funds are mostly held in U.S. dollar.
This currency is used to separately measure each entity’s financial position and operating results.
d. Transactions, assets and liabilities in foreign currency
Transactions denominated in a foreign currency are recorded upon initial recognition at the exchange rate on the date of the transaction. After initial recognition, monetary assets and liabilities denominated in a foreign currency are translated at the end of each reporting period into the functional currency at the exchange rate at that date. Non-monetary assets and liabilities measured at cost in foreign currency are translated at the exchange rate
F-46
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 2:—SIGNIFICANT ACCOUNTING POLICIES—(CONTINUED)
on the date of the transaction. Non-monetary assets and liabilities denominated in foreign currency and measured at fair value are translated into the functional currency using the exchange rate prevailing at the date when the fair value was determined.
NOTE 3:—SIGNIFICANT EVENT DURING THE PERIOD
a. During the six months ended June 30, 2013, 141,697 options were exercised by employees into 141,697 ordinary shares of NIS 0.02 par value each for a total consideration of $828.
b. On May 29, 2013, the Company signed a cooperation agreement with Beijing Dabeinong Technology Co. (“DBN”), a leading Chinese company in the field of development and commercialization of rice seeds and other agricultural products. According to the agreement the Company will receive payments based on milestones stated in the agreement. In addition, the Company shall receive sales based royalties based on the terms stated in the agreement.
NOTE 4:—OPERATING SEGMENTS
a. General:
Commencing January 1, 2012, the Company operates in two segments. The segments were determined on the basis of information considered by the Chief Operating Decision-Maker (“CODM”) for purposes of decision-making on the allocation of resources and evaluation of performance. The following Company’s segments are engaged in business activities for which they earn revenues and incur expenses, their results are reviewed by the CODM and discrete financial information is available:
| Evogene segment | – | Services for the development of gene sequence technology (D.N.A. sequences responsible for the production of proteins—“genes”) and other D.N.A. sequences in plants (“D.N.A. sequences”). | ||
| Evofuel segment | – | Services for the development of improved species of the castor oil plant, which may serve as a source for extracting oil for the bio diesel fuel industry. | ||
Segments performance (segment income (loss)) is determined based on operating income (loss) reported in the financial statements. The results of a segment reported to the CODM include items attributed directly to a segment, as well as other items, which are indirectly attributed using reasonable assumptions.
F-47
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 4:—OPERATING SEGMENTS—(CONTINUED)
b. The following table presents our revenues and operating loss by segments:
| Evogene | Evofuel | Total | ||||||||||
| Audited | ||||||||||||
| For the Year Ended December 31, 2012 |
||||||||||||
| Revenues |
$ | 17,072 | $ | — | $ | 17,072 | ||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
$ | (1,993 | ) | $ | (1,133 | ) | $ | (3,126 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net financing income |
678 | |||||||||||
|
|
|
|||||||||||
| Loss before taxes on income |
$ | (2,448 | ) | |||||||||
|
|
|
|||||||||||
| Evogene | Evofuel | Total | ||||||||||
| Unaudited | ||||||||||||
| For the six months period ended June 30, 2012 |
||||||||||||
| Revenues |
$ | 8,287 | $ | — | $ | 8,287 | ||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
$ | (596 | ) | $ | (521 | ) | $ | (1,117 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net financing income |
434 | |||||||||||
|
|
|
|||||||||||
| Loss before taxes on income |
$ | (683 | ) | |||||||||
|
|
|
|||||||||||
| Evogene | Evofuel | Total | ||||||||||
| Unaudited | ||||||||||||
| For the six months period ended June 30, 2013 |
||||||||||||
| Revenues |
$ | 8,934 | $ | — | $ | 8,934 | ||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
$ | (1,588 | ) | $ | (561 | ) | $ | (2,149 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net financing expenses |
(213 | ) | ||||||||||
|
|
|
|||||||||||
| Loss before taxes on income |
$ | (2,362 | ) | |||||||||
|
|
|
|||||||||||
c. Major customers:
Revenues from major customers each of whom amounts to 10% or more, of total revenues:
| Year
ended December 31, |
Six months
ended June 30, |
|||||||||||
| 2012 | 2012 | 2013 | ||||||||||
| Customer A (shareholder) |
70 | % | 74 | % | 66 | % | ||||||
| Customer B (shareholder) |
24 | % | 26 | % | 32 | % | ||||||
F-48
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
NOTE 5:—OTHER INVESTMENT
On February 4, 2013, the Company signed an agreement with a private Israeli company, according to which the Company undertook to provide the private Israeli company with rights to use its greenhouses and facilities, including support for the private Israeli company’s development process for the following consideration:
| 1. | 15% of the private Israeli company’s shares on an outstanding basis. The shares are subject to reverse vesting over a period of 36 months. |
| 2. | The Company also was granted with anti-dilution option up to an aggregate investment of $4,000 in the private Israeli company. |
| 3. | A three years access to the system being developed by the private Israeli company including an option to purchase the system for $200 which is exercisable over the term of the agreement. |
The Company recorded as other investment the total value of the above three elements of the consideration amounting to $365, which was determined based on a third party valuation. The total consideration of $365 was deferred and will be recognized over a period of 36 months. The investment in the private Israeli company will be accounted for as an available for sale investment under the provisions of IAS 39 and accordingly changes in fair value will be recorded in other comprehensive income (loss). Gains or losses from changes in fair value of the anti-dilution option and the option to purchase the system will be recorded in profit or loss. The three elements are financial assets classified as level 3 in the fair value hierarchy in IFRS 13.
NOTE 6:—SHAREHOLDERS’ EQUITY
All ordinary shares, options, warrants, per share data and exercise prices included in these financial statements for all periods presented have been retroactively adjusted to reflect the 1-for-2 reverse share split to be effective immediately prior to the effectiveness of the registration statement.
NOTE 7:—SUBSEQUENT EVENT
| a. | On July 17, 2013, the board of directors of the Company approved an issuance to its officers and employees of 1,000,000 options exercisable into 1,000,000 ordinary shares of the Company, NIS 0.02 par value each, for an exercise price of NIS 44.28 ($12.52) per share. The estimated fair value of the options at their grant date using the binomial model was approximately $3,825. The main assumptions used by the Company in determining the fair value were: dividend yield, 0%; expected volatility of the share price, 26-60%; risk free interest rate, 1.14-6.34%; and share price of NIS 43.62 ($12.20). |
On September 22, 2013, the board of directors approved an issuance to the Company’s President and Chief Executive Officer of 215,000 options exercisable into 215,000 ordinary shares of the Company, NIS 0.02 par value each, for an exercise price of NIS 48.18 ($13.62) per share. The grant is subject to shareholder approval at a general meeting of the Company. The current estimate of the fair value of these options using the binomial model is approximately $1,108. The main assumptions used by the Company in determining the fair value were: dividend yield, 0%; expected volatility of the share price, 26.70-58.51%; risk free interest rate, 1.22-6.25%; and share price of NIS 51.18 ($14.46).
| b. | On July 30, 2013, the Israeli Parliament (the “Knesset”) approved the second and third readings of the Economic Plan for 2013-2014 (“Amended Budget Law”) which consists, among others, of fiscal changes whose main aim is to enhance long-term collection of taxes. |
F-49
Table of Contents
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(CONTINUED)
U.S. dollars in thousands (except share and per share data)
These changes include, among others, raising the Israeli corporate tax rate from 25% to 26.5%, cancelling the lowering of the tax rates applicable to preferred enterprises (9% in development area A and 16% in other areas), taxing revaluation gains and increasing the tax rates on dividends within the scope of the Law for the Encouragement of Capital Investments to 20% and are effective from January 1, 2014.
The Company estimates that the effect of the change in tax rates will not have a material effect on the Company’s financial statements.
F-50
Table of Contents

Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers.
Under the Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a provision authorizing such exculpation is included in its articles of association. Our articles of association to be effective following this offering include such a provision. The company may not exculpate a director from liability arising out of a prohibited dividend or distribution to shareholders.
Under the Companies Law and the Israeli Securities Law, a company may indemnify an office holder in respect of the following liabilities and expenses incurred for acts performed as an office holder, either in advance of an event or following an event, provided a provision authorizing such indemnification is contained in its articles of association:
| • | a financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned events and amount or criteria; |
| • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability, such as a criminal penalty, was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and |
| • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf or by a third party or in connection with criminal proceedings in which the office holder was acquitted or as a result of a conviction for an offense that does not require proof of criminal intent. |
Under the Companies Law and the Israeli Securities Law, a company may insure an office holder against the following liabilities incurred for acts performed as an office holder if and to the extent provided in the company’s articles of association:
| • | a breach of the duty of loyalty to the company, to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| • | a breach of the duty of care to the company or to a third party, including a breach arising out of the negligent conduct of the office holder; |
| • | a financial liability imposed on the office holder in favor of a third party; |
| • | a financial liability imposed on the office holder in favor of a third party harmed by a breach in an administrative proceeding; and |
| • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an administrative proceeding instituted against him or her. |
Table of Contents
Under the Companies Law, a company may not indemnify, exculpate or insure an office holder against any of the following:
| • | a breach of the duty of loyalty, except to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| • | a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder; |
| • | an act or omission committed with intent to derive illegal personal benefit; or |
| • | a fine or forfeit levied against the office holder. |
Under the Companies Law, exculpation, indemnification and insurance of office holders must be approved by the compensation committee and the board of directors and, with respect to directors and the chief executive officer (subject to certain exemptions), also by the shareholders.
Our articles of association permit us to exculpate, indemnify and insure our office holders for any liability imposed on them as a consequence of an act which was performed by virtue of being an office holder. We intend to ask our shareholders to approve, prior to consummation of this offering, an amendment to our articles of association that will extend such exculpation, indemnification and insurance to cover omissions by our office holders (in their role as such) as well. Our office holders are currently covered by a directors’ and officers’ liability insurance policy.
We have entered into agreements with each of our directors and executive officers exculpating them, to the fullest extent permitted by law, from liability to us for damages caused to us as a result of a breach of duty of care, and undertaking to indemnify them to the fullest extent permitted by law. This indemnification is limited to events determined as foreseeable by the board of directors based on our activities, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances.
Effective as of the date of this offering, the maximum indemnification amount set forth in such agreements is limited to an amount equal to 25% of our shareholders’ equity as reflected in our most recent consolidated financial statements prior to the date on which the indemnity payment is made. We intend to ask our shareholders to approve, prior to consummation of this offering, an amendment to such indemnification agreements pursuant to which, to the extent that the amount equal to equal to 25% of our shareholders’ equity is insufficient to cover all indemnity amounts payable with respect to all indemnifiable directors and executive officers, such amount will be allocated among our directors and executive officers pro rata, in accordance with their relative culpabilities, as finally determined by a court with respect to a particular claim. The maximum amount set forth in such agreements is in addition to any amount paid (if paid) under insurance and/or by a third party pursuant to an indemnification arrangement.
In the opinion of the SEC, indemnification of directors and office holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable.
There is no pending litigation or proceeding against any of our office holders as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any office holder.
Item 7. Recent Sales of Unregistered Securities.
During the past three years (that is, from the start of 2010 through the date of this registration statement), we have issued securities in transactions that have not been registered under the Securities Act as set forth below. We believe that each such issuance has been exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act, Rule 701 and/or Regulation S under the Securities Act.
Table of Contents
We have granted 2,118,900 share options, in the aggregate, to employees, directors and consultants under our 2002 Plan and 2003 Plan during the foregoing period. The following table sets forth information with respect to the option and share awards from the start of 2010 through September 18, 2013. The U.S. dollar exercise price reflects a convenience translation at the rate of $1.00 = NIS 3.54, the representative exchange rate published by the Bank of Israel as of September 18, 2013.
| Date of grant |
Number
of Options/Awards(1) |
Exercise Price | ||||||||||
| NIS | $ | |||||||||||
| April 11, 2010 |
15,000 | 25.20 | 7.12 | |||||||||
| April 11, 2010 |
2,500 | 19.94 | 5.64 | |||||||||
| April 11, 2010 |
5,000 | 25.58 | 7.23 | |||||||||
| April 11, 2010 |
25,000 | 31.50 | 8.91 | |||||||||
| June 20, 2010 |
1,361,150 | 28.66 | 8.10 | |||||||||
| September 19, 2010 |
12,500 | 32.26 | 9.12 | |||||||||
| September 19, 2010 |
12,500 | 28.02 | 7.92 | |||||||||
| September 19, 2010 |
2,500 | 33.86 | 9.57 | |||||||||
| November 17, 2010 |
76,250 | 36.96 | 10.45 | |||||||||
| May 25, 2011 |
127,500 | 33.92 | 9.59 | |||||||||
| September 18, 2011 |
12,500 | 33.80 | 9.56 | |||||||||
| September 18, 2011 |
5,000 | 28.70 | 8.11 | |||||||||
| September 22, 2011 |
120,000 | 29.12 | 8.23 | |||||||||
| June 25, 2012 |
184,000 | 33.48 | 9.47 | |||||||||
| November 11, 2012 |
12,500 | 33.42 | 9.45 | |||||||||
| November 11, 2012 |
5,000 | 31.52 | 8.91 | |||||||||
| December 6, 2012 |
140,000 | 35.84 | 10.13 | |||||||||
| July 17, 2013 (2) |
1,000,000 | 44.28 | 12.52 | |||||||||
| (1) | Represents option awards unless otherwise indicated. |
| (2) | Does not include 215,000 options to our President and Chief Executive Officer, Ofer Haviv, which were approved in September 2013 by our compensation and nominating committee and board of directors but are subject to shareholder approval at our general shareholders meeting. |
We have issued an aggregate of 968,473 ordinary shares pursuant to the exercise of share options by our employees and directors from the start of 2010 through the date of this registration statement.
Item 8. Exhibits and Financial Statement Schedules.
(a) The Exhibit Index is hereby incorporated herein by reference.
(b) Financial Statement Schedules.
All schedules have been omitted because they are not required, are not applicable or the information is otherwise set forth in the Consolidated Financial Statements and related notes thereto.
Item 9. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the provisions described in Item 6 hereof, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
Table of Contents
The undersigned Registrant hereby undertakes:
| 1. | To provide the underwriters specified in the Underwriting Agreement, at the closing, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser. |
| 2. | That for purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| 3. | That for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Rehovot, Israel on this 23rd day of September, 2013.
| EVOGENE LTD. | ||||
| By: | /s/ Ofer Haviv | |||
| Name: | Ofer Haviv | |||
| Title: | President and Chief Executive Officer | |||
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints Ofer Haviv and Sigal Fattal, and each of them, as attorney-in-fact with full power of substitution, for him or her in any and all capacities, to do any and all acts and all things and to execute any and all instruments which said attorney and agent may deem necessary or desirable to enable the registrant to comply with the Securities Act, and any rules, regulations and requirements of the SEC thereunder, in connection with the registration under the Securities Act of ordinary shares of the registrant (the “Shares”), including, without limitation, the power and authority to sign the name of each of the undersigned in the capacities indicated below to the Registration Statement on Form F-1 (the “Registration Statement”) to be filed with the SEC with respect to such Shares, to any and all amendments or supplements to such Registration Statement, whether such amendments or supplements are filed before or after the effective date of such Registration Statement, to any related Registration Statement filed pursuant to Rule 462(b) under the Securities Act, and to any and all instruments or documents filed as part of or in connection with such Registration Statement or any and all amendments thereto, whether such amendments are filed before or after the effective date of such Registration Statement, and each of the undersigned hereby ratifies and confirms all that such attorney and agent shall do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signatures |
Title |
Date | ||
| /s/ Ofer Haviv Ofer Haviv |
President and Chief Executive Officer (Principal Executive Officer) |
September 23, 2013 | ||
| /s/ Sigal Fattal Sigal Fattal |
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
September 23, 2013 | ||
| /s/ Martin Gerstel Martin Gerstel |
Chairman of the Board | September 23, 2013 | ||
| /s/ Michael Anghel Dr. Michael Anghel |
Director | September 23, 2013 | ||
| /s/ Adina Makover Dr. Adina Makover |
Director | September 23, 2013 | ||
Table of Contents
| Signatures |
Title |
Date | ||
| /s/ Leon Recanati Leon Recanati |
Director | September 23, 2013 | ||
| /s/ Simcha Sadan Dr. Simcha Sadan |
Director | September 23, 2013 | ||
| /s/ Kinneret Livnat Savitzky Dr. Kinneret Livnat Savitzky |
Director | September 23, 2013 | ||
Table of Contents
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant’s duly authorized representative in the United States has signed this registration statement on Form F-1 in Newark, Delaware on September 23, 2013.
| By: |
/s/ Donald J. Puglisi | |
| Name: Donald J. Puglisi | ||
| Title: Managing Director, Puglisi & Associates | ||
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description | |
| 1.1 | Form of Underwriting Agreement* | |
| 3.1 | Articles of Association of the Registrant** | |
| 3.2 | Form of Amended and Restated Articles of Association of the Registrant to become effective upon closing of this offering** | |
| 5.1 | Opinion of Meitar Liquornik Geva Leshem Tal, Israeli counsel to the Registrant, as to the validity of the ordinary shares (including consent)* | |
| 10.1 | Amended & Restated 2008 Collaboration and License Agreement, by and between Monsanto Company and the Registrant, dated November 28, 2011 † | |
| 10.2 | Share Purchase Agreement, by and between Monsanto Company and the Registrant, dated August 27, 2008 | |
| 10.3 | Amendment to Share Purchase Agreement, by and between Monsanto Company and the Registrant, dated September 21, 2011** | |
| 10.4 | Second Amendment to Share Purchase Agreement, by and between Monsanto Company and the Registrant, dated November 14, 2011** | |
| 10.5 | 3rd Amendment to Share Purchase Agreement, by and between Monsanto Company and the Registrant, dated November 28, 2011** | |
| 10.6 | Wheat Collaboration and License Agreement, by and between Bayer CropScience AG and the Registrant, dated December 10, 2010 † | |
| 10.7 | Amendment to Wheat Collaboration and License Agreement, by and between Bayer CropScience AG and the Registrant, dated October 14, 2012 † | |
| 10.8 | Share Purchase Agreement, by and between Bayer CropScience AG and the Registrant, dated December 10, 2010 | |
| 10.9 | Form of Indemnification Agreement** | |
| 10.10 | Evogene Share Option Plan (2002)** | |
| 10.11 | Evogene Ltd. Key Employee Share Incentive Plan, 2003** | |
| 10.12 | The Evogene Ltd. 2013 Share Option Plan** | |
| 10.13 | English summary of lease agreements dated May 9, 2010, and July 22, 2010, respectively, by and between Reuven Zahari and the Registrant** | |
| 10.14 | English summary of the lease agreement dated May 14, 2008, by and between Nachum Levi and the Registrant** | |
| 10.15 | English summary of the lease agreement dated March 12, 2001, by and among Africa Israel Properties Ltd., as successor by assignment to Kiryat Weizmann Science Park Ltd., Ilot Investments (Ramat Vered) 1994 Ltd., Sardeh Ltd. and the Registrant, as successor by assignment to Compugen Ltd. via an assignment agreement dated January 1, 2002, by and between Compugen Ltd. and the Registrant, as supplemented by various addendums and supplements** | |
| 10.16 | English summary of the Memorandum of Understanding, dated as of June 1, 2011, by and between Asifey Bar (AGSH) and the Registrant, as amended by Amendments dated June 14, 2012 and October 27, 2012, with respect to land and services provided to the Registrant for its scientific and agricultural experiments** | |
| 10.17 | Investors’ Rights Agreement, by and among the Registrant and the investors named therein, dated January 3, 2006** | |
| 21.1 | List of subsidiaries of the Registrant** | |
| 23.1 | Consent of Kost Forer Gabbay and Kasierer, a member of Ernst & Young | |
Table of Contents
| Exhibit |
Description | |
| 23.2 | Consent of Meitar Liquornik Geva Leshem Tal (included in Exhibit 5.1)* | |
| 23.3 | Consent of Symbiotic Engineering, LLC | |
| 23.4 | Consent of Phillips McDougall | |
| 24.1 | Power of Attorney (included in signature page to Registration Statement) | |
| * | To be filed by amendment. |
| ** | Included with prior confidential submission. |
| † | Confidential treatment has been requested for portions of this document. The omitted portions of this document have been filed with the Securities and Exchange Commission. |

