zk1618398.htm
|
UNITED STATES SECURITIES
|
|
AND EXCHANGE COMMISSION
|
|
WASHINGTON, DC 20549
|
FORM 20-F
|
o
|
Registration Statement Pursuant to Section 12(b) or (g) of the Securities Exchange Act of 1934
|
or
|
x
|
Annual Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
For the fiscal year ended December 31, 2015
or
|
o
|
Transition Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
or
|
o
|
Shell Company Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of event requiring this shell company report: __________
For the transition period from ________ to _______________
Commission file no.:
| |
|
MAZOR ROBOTICS LTD.
|
|
(Exact name of registrant as specified in its charter)
|
Translation of registrant’s name into English: Not applicable
| |
|
|
|
State of Israel
|
|
7 HaEshel Street
|
|
(Jurisdiction of incorporation or organization)
|
|
Caesarea Industrial Park South
|
| |
|
3088900 Israel
|
| |
|
(Address of principal executive offices)
|
Sharon Levita
Chief Financial Officer
+972-4-618-7101
Sharon.Levita@MazorRobotics.com
7 HaEshel Street
Caesarea Industrial Park South
3088900 Israel
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| |
|
|
|
Title of each class:
|
|
Name of each exchange on which registered or to be
|
|
American Depository Shares each representing 2
|
|
registered:
|
|
Ordinary Shares, par value NIS 0.01 per share(1)
|
|
NASDAQ Global Market
|
|
Ordinary Shares, par value NIS 0.01 per share(2)
|
|
|
|
(1)
|
Evidenced by American Depositary Receipts.
|
|
(2)
|
Not for trading, but only in connection with the listing of the American Depositary Shares.
|
Securities registered or to be registered pursuant to Section 12(g) of the Exchange Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Exchange Act: None
Number of outstanding shares of each of the issuer’s classes of capital or common stock as of April 28, 2016: 42,514,698 ordinary shares.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months.
Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer.
| |
|
|
|
Large accelerated filer o
|
Accelerated filer x
|
Non-accelerated filer o
|
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing.
U.S. GAAP o
International Financial Reporting Standards as issued by the International Accounting Standards Board x
Other o
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
o Item 17 o Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company.
o Yes x No
TABLE OF CONTENTS
| |
|
|
| |
|
Page
|
|
|
5
|
|
|
5
|
| |
|
|
|
|
6
|
| |
|
|
| |
PART I
|
7
|
| |
|
|
|
|
|
7
|
|
|
|
7
|
|
|
|
8
|
|
A.
|
Selected Financial Data.
|
8
|
|
B.
|
Capitalization and Indebtedness.
|
9
|
|
C.
|
Reasons for the Offer and Use of Proceeds.
|
9
|
|
D.
|
Risk Factors.
|
9
|
|
|
|
43
|
|
A.
|
History and Development of the Company.
|
43
|
|
B.
|
Business Overview.
|
44
|
|
C.
|
Organizational Structure.
|
69
|
|
D.
|
Property, Plants and Equipment.
|
69
|
|
|
|
70
|
|
|
|
70
|
|
A.
|
Operating Results.
|
70
|
|
B.
|
Liquidity and Capital Resources.
|
80
|
|
C.
|
Research and Development, Patents and Licenses, Etc.
|
82
|
|
D.
|
Trend Information.
|
82
|
|
E.
|
Off-Balance Sheet Arrangements.
|
83
|
|
F.
|
Tabular Disclosure of Contractual Obligations.
|
84
|
|
|
|
84
|
|
A.
|
Directors and Senior Management.
|
84
|
|
B.
|
Compensation.
|
87
|
|
C.
|
Board Practices.
|
91
|
|
D.
|
Employees.
|
104
|
|
E.
|
Share Ownership.
|
105
|
|
|
|
108
|
|
A.
|
Major Shareholders.
|
108
|
|
B.
|
Related Party Transactions.
|
109
|
|
C.
|
Interests of Experts and Counsel.
|
109
|
|
|
|
110
|
|
A.
|
Consolidated Statements and Other Financial Information.
|
110
|
|
B.
|
Significant Changes.
|
110
|
|
|
|
111
|
|
A.
|
Offer and Listing Details.
|
111
|
|
B.
|
Plan of Distribution.
|
112
|
|
C.
|
Markets.
|
|
|
D.
|
Selling Shareholders.
|
|
|
E.
|
Dilution.
|
|
|
F.
|
Expenses of the Issue.
|
|
|
|
|
113
|
|
A.
|
Share Capital.
|
|
|
B.
|
Memorandum and Articles of Association.
|
|
|
C.
|
Material Contracts.
|
|
|
D.
|
Exchange Controls.
|
|
|
E.
|
Taxation.
|
114
|
|
F.
|
Dividends and Paying Agents.
|
125
|
|
G.
|
Statement by Experts.
|
125
|
|
H.
|
Documents on Display.
|
126
|
|
I.
|
Subsidiary Information.
|
|
|
|
|
|
|
|
|
128
|
|
A.
|
Debt Securities.
|
|
|
B.
|
Warrants and Rights.
|
|
|
C.
|
Other Securities.
|
|
|
D.
|
American Depositary Shares.
|
|
| |
|
|
| |
PART II
|
129
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
130
|
|
|
|
|
|
|
|
|
|
|
|
131
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
133
|
| |
|
|
| |
PART III
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
134
|
| |
|
|
|
|
136
|

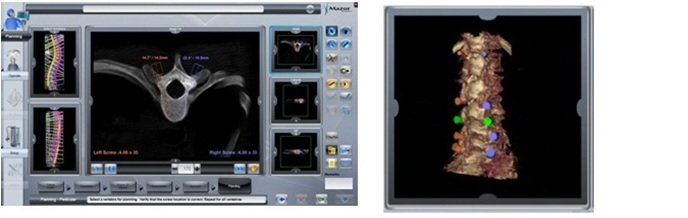
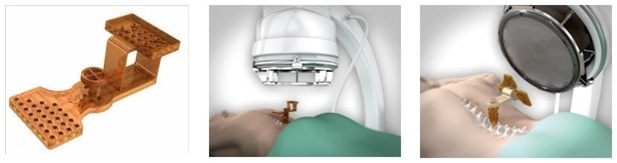
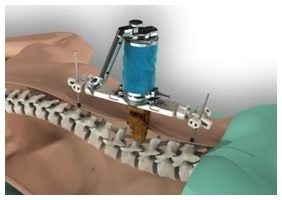
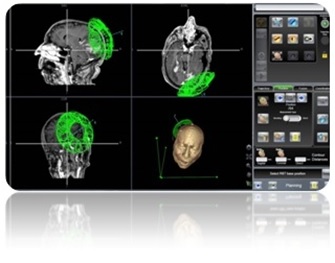
Mazor Robotics, an Israeli Company, is a leading innovator that has pioneered surgical guidance systems and complementary products in the spine surgical markets which we believe provide a safer surgical environment for patients, surgeons and operating room staff. We engage in the development, production and marketing of innovative medical devices for supporting surgical procedures in the fields of orthopedics and neurosurgery. We operate in the fields of image guided surgery and computer-assisted surgery enabling the use of surgical instruments with high precision and minimal invasiveness and aiming to simplify complex and minimally-invasive surgical procedures. We believe that our flagship product, the Renaissance® Surgical Guidance System, or Renaissance, is transforming spine surgery from freehand procedures to highly accurate, state-of-the-art, guided procedures that raise the standard of care with better clinical results. Our Renaissance and SpineAssist (our predecessor to the Renaissance) systems have been used to perform over 16,000 procedures worldwide (over 98,000 implants) in a wide variety of spinal procedures, many of which would not have been attempted without this technology. In 2014 we introduced the Renaissance for brain surgery and in 2015 we introduced the PRO (Predictable Renaissance Operation) product line, which currently includes three solutions designed to support brain procedures, as well as, trauma and lateral spine procedures. We are continuing the development of the Renaissance platform for additional spine and brain surgery procedures.
We were incorporated under the laws of the State of Israel on September 12, 2000. Our ordinary shares are listed on the Tel Aviv Stock Exchange, or TASE, under the symbol “MZOR”. In May 2013, our American Depositary Shares, or ADSs, representing our Ordinary Shares commenced trading on the NASDAQ Capital Market under the trading symbol “MZOR” and are currently traded on the NASDAQ Global Market. Each ADS represents two of our Ordinary Shares.
Unless the context otherwise indicates or requires, “Mazor Robotics”, “Mazor,” the Mazor Robotics logo and all product names and trade names used by us in this annual report, including Renaissance™, are our proprietary trademarks and service marks. These trademarks and service marks are important to our business. Although we have omitted the “®” and “TM” trademark designations for such marks in this annual report, all rights to such trademarks and service marks are nevertheless reserved.
Unless derived from our financial statements or otherwise indicated, U.S. dollar translations of NIS amounts presented in this annual report are translated using a rate of NIS 4.00 to USD 1.00.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain information included or incorporated by reference in this annual report may be deemed to be “forward-looking statements”. Forward-looking statements are often characterized by the use of forward-looking terminology such as “may,” “will,” “expect,” “anticipate,” “estimate,” “continue,” “believe,” “should,” “intend,” “project” or other similar words, but are not the only way these statements are identified.
These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition, statements relating to the research, development and use of our products, and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future.
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate.
Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things:
|
|
●
|
the overall global economic environment;
|
|
|
●
|
the impact of competition and new technologies;
|
|
|
●
|
general market, political and economic conditions in the countries in which we operate;
|
|
|
●
|
projected capital expenditures and liquidity;
|
|
|
●
|
changes in our strategy;
|
|
|
●
|
government regulations and approvals;
|
|
|
●
|
changes in customers’ budgeting priorities;
|
|
|
●
|
litigation and regulatory proceedings; and
|
|
|
●
|
those factors referred to in “Item 3. Key Information - D. Risk Factors”, “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects”, as well as in this annual report generally.
|
Readers are urged to carefully review and consider the various disclosures made throughout this annual report, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
In addition, the section of this annual report entitled “Item 4. Information on the Company” contains information obtained from independent industry and other sources that we have not independently verified. You should not put undue reliance on any forward-looking statements. Any forward-looking statements in this annual report are made as of the date hereof, and we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
In this Form 20-F, unless the context otherwise requires, references to:
|
|
●
|
“Mazor Robotics”, “Mazor,” the “Company”, the “registrant”, “us”, “we” and “our” refer to Mazor Robotics Ltd., an Israeli company, and, unless the context indicates otherwise, the Subsidiaries;
|
|
|
●
|
“ADSs” are to our American Depositary Shares, each representing two of our Ordinary Shares;
|
|
|
●
|
“Companies Law” are to Israel’s Companies Law, 5759-1999, as amended;
|
|
|
●
|
“Dollars”, “U.S. dollars”, “U.S. $” and “$” are to United States Dollars;
|
|
|
●
|
“Exchange Act” are to the United States Securities Exchange Act of 1934, as amended;
|
|
|
●
|
“FDA” are to the United States Food and Drug Administration;
|
|
|
●
|
“IRS” are to the United States Internal Revenue Service;
|
|
|
●
|
“NASDAQ” are to the NASDAQ Global Market;
|
|
|
●
|
“OCS” are to the Israel’s Office of the Chief Scientist of the Ministry of Economy;
|
|
|
●
|
“Ordinary Shares”, “our shares” and similar expressions refer to our ordinary shares, par value NIS 0.01 per share;
|
|
|
●
|
“SEC” are to the United States Securities and Exchange Commission;
|
|
|
●
|
“Securities Act” are to the United States Securities Act of 1933, as amended;
|
|
|
●
|
“Shekels” and “NIS” are to New Israel Shekels, the Israeli currency;
|
|
|
●
|
“Subsidiaries” are to the U.S. Subsidiary and to Mazor Robotics Pte Ltd., a Singapore company, and a wholly owned subsidiary of Mazor;
|
|
|
●
|
“TASE” are to the Tel Aviv Stock Exchange; and
|
|
|
●
|
“U.S. Subsidiary” are to Mazor Robotics, Inc., a Delaware corporation, and a wholly owned subsidiary of Mazor.
|
PART I
|
|
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
|
Not applicable.
|
|
OFFER STATISTICS AND EXPECTED TIMETABLE
|
Not applicable.
|
A.
|
Selected Financial Data
|
The selected consolidated financial data for the fiscal years set forth in the table below have been derived from our consolidated financial statements and notes thereto. The selected consolidated statement of profit or loss data for fiscal years 2015, 2014 and 2013, and the selected consolidated statement of financial position data as of December 31, 2015 and 2014, have been derived from our audited consolidated financial statements and notes thereto set forth elsewhere in this Form 20-F. The selected consolidated statement of profit or loss data for fiscal years 2012 and 2011, and the selected consolidated statement of financial position data as of December 31, 2013, 2012, and 2011, respectively, has been derived from other audited consolidated financial statements not included herein. The selected financial data should be read in conjunction with our consolidated financial statements, and are qualified entirely by reference to such consolidated financial statements. Additionally, and as explained in Note 2B to the December 31, 2015 consolidated financial statements, our functional currency is U.S. dollars.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands except net loss per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years Ended December 31,
|
|
| |
|
2015
|
|
|
2014
|
|
|
2013
|
|
|
2012
|
|
|
2011
|
|
|
Statement of Profit or Loss Data
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues
|
|
$ |
26,096 |
|
|
$ |
21,208 |
|
|
$ |
19,983 |
|
|
$ |
12,175 |
|
|
$ |
5,904 |
|
|
Cost of sales
|
|
$ |
5,827 |
|
|
$ |
4,396 |
|
|
$ |
4,280 |
|
|
$ |
2,893 |
|
|
$ |
1,879 |
|
|
Gross profit
|
|
$ |
20,269 |
|
|
$ |
16,812 |
|
|
$ |
15,703 |
|
|
$ |
9,282 |
|
|
$ |
4,025 |
|
|
Operating costs and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses, net
|
|
$ |
6,324 |
|
|
$ |
5,776 |
|
|
$ |
4,174 |
|
|
$ |
2,760 |
|
|
$ |
3,062 |
|
|
Selling and marketing expenses
|
|
$ |
24,947 |
|
|
$ |
21,352 |
|
|
$ |
15,692 |
|
|
$ |
8,887 |
|
|
$ |
6,990 |
|
|
General and administrative expenses
|
|
$ |
4,305 |
|
|
$ |
4,392 |
|
|
$ |
2,766 |
|
|
$ |
1,845 |
|
|
$ |
1,639 |
|
|
Total operating costs and expenses
|
|
$ |
35,576 |
|
|
$ |
31,520 |
|
|
$ |
22,632 |
|
|
$ |
13,492 |
|
|
$ |
11,691 |
|
|
Operating loss
|
|
$ |
(15,307 |
) |
|
$ |
(14,708 |
) |
|
$ |
(6,929 |
) |
|
$ |
(4,210 |
) |
|
$ |
(7,666 |
) |
|
Loss for the year
|
|
$ |
(15,385 |
) |
|
$ |
(15,272 |
) |
|
$ |
(20,529 |
) |
|
$ |
(7,064 |
) |
|
$ |
(7,782 |
) |
|
Loss per share – Basic and diluted
|
|
$ |
(0.36 |
) |
|
$ |
(0.37 |
) |
|
$ |
(0.57 |
) |
|
$ |
(0.29 |
) |
|
$ |
(0.36 |
) |
|
Weighted average number of ordinary shares used to calculate basic and diluted loss per share
|
|
|
42,284 |
|
|
|
41,808 |
|
|
|
35,781 |
|
|
|
24,011 |
|
|
|
21,815 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands)
|
|
As of December 31,
|
|
| |
|
2015
|
|
|
2014
|
|
|
2013
|
|
|
2012
|
|
|
2011
|
|
|
Statement of Financial Position Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$ |
13,519 |
|
|
$ |
22,255 |
|
|
$ |
19,803 |
|
|
$ |
12,797 |
|
|
$ |
1,655 |
|
|
Short-term investments
|
|
$ |
21,687 |
|
|
$ |
24,507 |
|
|
$ |
45,014 |
|
|
$ |
4,156 |
|
|
$ |
14,455 |
|
|
Long-term investments
|
|
$ |
5,023 |
|
|
$ |
5,473 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
Total assets
|
|
$ |
50,970 |
|
|
$ |
60,686 |
|
|
$ |
70,889 |
|
|
$ |
21,334 |
|
|
$ |
20,424 |
|
|
Total non-current liabilities
|
|
$ |
299 |
|
|
$ |
278 |
|
|
$ |
332 |
|
|
$ |
4,490 |
|
|
$ |
616 |
|
|
Accumulated loss
|
|
$ |
(103,192 |
) |
|
$ |
(87,807 |
) |
|
$ |
(72,535 |
) |
|
$ |
(52,006 |
) |
|
$ |
(44,942 |
) |
|
Total equity
|
|
$ |
42,400 |
|
|
$ |
54,267 |
|
|
$ |
64,093 |
|
|
$ |
12,820 |
|
|
$ |
13,484 |
|
|
B.
|
Capitalization and Indebtedness
|
Not applicable.
|
C.
|
Reasons for the Offer and Use of Proceeds
|
Not applicable.
You should carefully consider the risks described below, together with all of the other information in this Form 20-F. The risks described below are not the only risks facing us. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially and adversely affect our business operations. If any of these risks actually occurs, our business and financial condition could suffer and the price of our shares could decline.
Risks Related to Our Business
We are an emerging growth company, and we have incurred significant losses since our inception.
We are an emerging growth company. The future success of our business depends on our ability to continue to develop and obtain regulatory clearances or approvals for innovative and commercially successful products in our field, which we may be unable to do in a timely manner, or at all. Our success and ability to generate revenue or be profitable also depends on our ability to establish our sales and marketing force, generate product sales and control costs, all of which we may be unable to do. Our limited operating history also limits your ability to make a comparative evaluation of us, our products and our prospects.
We have sustained net losses in every fiscal year since our inception in 2000, including a net loss of $15.4 million for the year ended December 31, 2015. As of December 31, 2015, we had total shareholders’ equity of $42.4 million and cash and cash equivalents, short term investments and long term investments of approximately $40.2 million. Our accumulated deficit as of December 31, 2015 was $103.2 million. We anticipate that we will continue to incur substantial net losses for at least the next two years as we expand our sales and marketing capabilities in the spine and neurosurgery products market, continue our commercialization of Renaissance, expand its adoption and clinical implementation, and continue to develop the corporate infrastructure required to sell and market our products and invest in product development. Our losses have had and will continue to have an adverse effect on our shareholders’ equity and working capital. Any failure to achieve and maintain profitability would continue to have an adverse effect on our shareholders’ equity and working capital and could result in a decline in our share price or cause us to cease operations.
We cannot assure investors that our existing cash and investment balances will be sufficient to meet our future capital requirements.
We believe our existing cash, cash equivalents, investment balances, and interest income we earn on these balances, if any, will be sufficient to meet our anticipated cash requirements through at least the next 24 months. To the extent our available cash, cash equivalents and investment balances are insufficient to satisfy our operating requirements or other strategic needs, we will either need to seek additional sources of funds, including selling additional equity or debt securities or entering into a credit facility. However, we may be unable to obtain additional financing. As a result, we may be required to reduce the scope of, or delay or eliminate, some or all of our current and planned research, development and commercialization activities. We also may have to reduce marketing, customer service or other resources devoted to our products. Any of these actions could materially harm our business and results of operations. Even if we are able to continue to finance our business, the sale of additional equity and debt securities may result in dilution to our current shareholders or may require us to grant a security interest in our assets. If we raise additional funds through the issuance of debt securities, these securities may have rights senior to those of our Ordinary Shares and could contain covenants that could restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, or at all.
We depend on the success of one product for our revenue, which could impair our ability to achieve profitability.
We expect to derive most of our revenue from sales of Renaissance, recurring sales of disposable products required to use Renaissance in each surgical procedure, and service plans that are sold with Renaissance. Our future growth and success is dependent on successfully increasing the commercialization of Renaissance and the adoption of Renaissance by the end users. If we are unable to achieve increased commercial adoption of Renaissance, are unable to obtain regulatory clearances or approvals for future products, or experience a decrease in the utilization of our product line or procedure volume, our revenue would be adversely affected and we would not become profitable. If adverse economic, industry or regulatory events or changes occur, we may have to write off inventory as obsolete, which could negatively impact our business and revenue.
If surgeons and hospitals do not broadly adopt the concept of computer assisted spine surgeries and do not perceive such technology and related products as valuable and having significant advantages over the current “freehand” standard-of-care procedures, patients will be less likely to accept or be offered surgery with Renaissance, and we will fail to meet our business objectives.
Surgeons’ and hospitals’ perceptions of our technology having significant advantages are likely to be based on a determination that, among other factors, our products are safe, reliable, cost-effective and represent acceptable methods of treatment. Even if we can prove the clinical value of Renaissance through continued clinical use, surgeons may elect not to use our current and future Renaissance solutions for any number of other reasons. For example, surgeons may continue to operate freehand simply because such surgeries are already widely accepted. In addition, surgeons may be slow to adopt our current and future Renaissance solutions because of the perceived liability risks arising from the use of new products. Surgeons may not accept our current and future Renaissance solutions if we fail to maintain an acceptable level of product reliability or if we encounter regulatory approval or compliance issues. Hospitals may not accept Renaissance because of the capital expense, which may represent a significant portion of a hospital’s capital budget. Renaissance may not be cost-efficient if hospitals are not able to perform a significant volume of procedures using it.
If our current and future Renaissance solutions fail to achieve increased market acceptance for any of these or other reasons or if we are not successful in enforcing the contractual commitment to purchase disposable products exclusively from us, we will not be able to generate the revenue necessary to develop a sustainable, profitable business.
We depend on key employees, and if we fail to attract and retain employees with the expertise required for our business and to provide for the succession of senior management, we cannot grow or achieve profitability.
We are dependent on members of our senior management, in particular Ori Hadomi and Eliyahu Zehavi. Our future success will depend in part on our ability to retain our management and scientific teams, to identify, hire and retain additional qualified personnel with expertise in research and development and sales and marketing, and to effectively provide for the succession of senior management. Competition for qualified personnel in the medical device industry is intense and finding and retaining qualified personnel with experience in our industry is very difficult. We believe that there are only a limited number of individuals with the requisite skills to serve in many of our key positions, particularly in Israel, and we compete for key personnel with other medical equipment and software manufacturers and technology companies, as well as research institutions. It is often difficult to hire and retain these persons, and we may be unable to replace key persons if they leave or to fill new positions requiring key persons with appropriate experience. A significant portion of our compensation to our key employees is in the form of stock option grants. A prolonged depression in our stock price could make it difficult for us to retain our employees and recruit additional qualified personnel.
We do not maintain life insurance on any of our personnel. The loss of key employees, the failure of any key employee to perform or our inability to attract and retain skilled employees, as needed, or an inability to effectively plan for and implement a succession plan for key employees could harm our business.
Adverse changes in economic conditions and reduced spending on innovative medical technology may adversely impact our business.
The purchase of Renaissance is discretionary and requires our customers to make significant initial commitments of capital and other resources. In addition, purchase of Renaissance requires a commitment to purchase exclusively from us other products and services, including our single-use disposable components. Continuing weak economic conditions or reduction in healthcare technology spending, even if economic conditions improve, could adversely impact our business, operating results and financial condition in a number of ways, including by causing longer sales cycles, lower prices for our products and services and reduced unit sales.
Fluctuations in credit and financial market conditions could delay or prevent our customers from obtaining financing to purchase or lease a Renaissance system, which would adversely affect our business, financial condition and results of operations.
Due to the fluctuations in credit markets and currency exchange rates, our customers and overseas distributors may be delayed in obtaining, or may not be able to obtain, necessary financing for their purchases or leases of Renaissance. Shifts in the world economy that have systemic or local impact, the effects of EU sanctions on Russia and the drop in oil prices that affect its economy, or the continuing uncertainty regarding Greece’s solvency and its status as a member state in the European Union are examples of scenarios that might cause a significant effect on the European and even global markets. Such changes might in some instances lead to our customers or overseas distributors postponing ordering of a Renaissance system or the shipment and installation of previously ordered systems, cancelling their system orders, or cancelling their agreements with us. An increase in delays and order cancellations of this nature could adversely affect our product sales and revenues and, therefore, harm our business and results of operations.
Long lead times required by certain suppliers could prevent us from accurately forecasting demand for our products, which could adversely affect our operating results.
Market uncertainty makes it difficult for us, our customers, our overseas distributors and our suppliers to accurately forecast future product demand trends, which could cause us to order and/or produce excess products that can increase our inventory costs and result in obsolete inventory. Alternatively, this forecasting difficulty could cause a shortage of products, or materials used in our products, that could result in an inability to satisfy demand for our products and a resulting material loss of potential revenue.
In addition, some of our suppliers may require extensive advance notice of our requirements in order to produce products in the quantities we desire. This long lead time, which can be up to six months, may require us to place orders far in advance of the time when certain products will be offered for sale, thereby also making it difficult for us to accurately forecast demand for our products, exposing us to risks relating to shifts in consumer demand and trends and adversely affecting our operating results.
Because of the numerous risks and uncertainties associated with the development of medical devices, including future products, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to complete the development of our products and successfully deliver commercial products to the market.
Our future capital requirements will depend on many factors, including but not limited to the following:
|
|
●
|
the revenue generated by sales of our current and future products;
|
|
|
●
|
our ability to manage our inventory;
|
|
|
●
|
the expenses we incur in selling and marketing our products and supporting our growth;
|
|
|
●
|
the costs and timing of regulatory clearance or approvals for new products or upgrades or changes to our current products;
|
|
|
●
|
the rate of progress, cost, and success or failure of on-going development activities;
|
|
|
●
|
the emergence of competing or complementary technological developments;
|
|
|
●
|
the costs of filing, prosecuting, defending and enforcing any patent or license claims and other intellectual property rights, or participating in litigation related activities;
|
|
|
●
|
the terms and timing of any collaborative, licensing, or other arrangements that we may establish;
|
|
|
●
|
the acquisition of businesses, products and technologies; and
|
|
|
●
|
general economic conditions and interest rates, including the continuing weak conditions.
|
Our reliance on third-party suppliers, including single source suppliers, for most of the components of Renaissance could harm our ability to meet demand for our products in a timely and cost effective manner.
We rely on third-party suppliers to manufacture and supply almost all of the components used in Renaissance, including a number of single source suppliers to provide us with several of the major components of Renaissance. We currently do not have long-term contracts with most of our suppliers. As a result, some of our suppliers are not required to provide us with any guaranteed minimum production levels, and we cannot guarantee that we will be able to obtain sufficient quantities of key components in the future. In addition, our reliance on third-party suppliers involves a number of risks, including, among other things:
|
|
●
|
our suppliers may encounter financial hardships as a result of unfavorable economic and market conditions unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements;
|
|
|
●
|
suppliers may fail to comply with regulatory requirements, be subject to lengthy compliance, validation or qualification periods, or make errors in manufacturing components that could negatively affect the efficacy or safety of our products or cause delays in supplying of our products to our customers;
|
|
|
●
|
newly identified suppliers may not qualify under the stringent regulatory standards to which our business is subject;
|
|
|
●
|
we or our suppliers may not be able to respond to unanticipated changes in customer orders, and if orders do not match forecasts, we or our suppliers may have excess or inadequate inventory of materials and components;
|
|
|
●
|
we may be subject to price fluctuations due to a lack of long-term supply arrangements for key components;
|
|
|
●
|
we may experience delays in delivery by our suppliers due to changes in demand from us or their other customers;
|
|
|
●
|
we or our suppliers may lose access to critical services and components, resulting in an interruption in the manufacture, assembly and shipment of our systems;
|
|
|
●
|
our suppliers may be subject to allegations by other parties of misappropriation of proprietary information in connection with their supply of products to us, which could inhibit their ability to fulfill our orders and meet our requirements;
|
|
|
●
|
fluctuations in demand for products that our suppliers manufacture for others may affect their ability or willingness to deliver components to us in a timely manner;
|
|
|
●
|
our suppliers may wish to discontinue supplying components or services to us (e.g., for risk management reasons); and
|
|
|
●
|
we may not be able to find new or alternative components or reconfigure our system and manufacturing processes in a timely manner if the necessary components become unavailable.
|
If any of these risks materialize, costs could significantly increase and our ability to meet demand for our products could be impacted. If we are unable to satisfy commercial demand for Renaissance or for our single-use disposable components in a timely manner, our ability to generate revenue would be impaired, market acceptance of our products could be adversely affected, and customers may instead purchase or use alternative products. In addition, we could be forced to secure new or alternative components through a replacement supplier. Securing a replacement supplier could be difficult, especially for complex components such as Renaissance components that are manufactured in accordance with our custom specifications. The introduction of new or alternative components may require design changes to our system that may be subject to FDA and other regulatory clearances or approvals. We may also be required to assess the new manufacturer’s compliance with all applicable regulations and guidelines, which could further impede our ability to manufacture our products in a timely manner. As a result, we could incur increased production costs, experience delays in deliveries of our products, suffer damage to our reputation and experience an adverse effect on our business and financial results.
The overall size and risk of turnover in our sales and marketing teams might impair our ability to generate revenues.
To reach our revenue targets, we need to expand and strengthen our U.S. direct sales force and our foreign sales channels. Developing a sales and marketing infrastructure is expensive and time consuming and an inability to develop such an organization in a timely manner could delay the successful adoption of our products. Additionally, any sales and marketing organization that we develop may be competing against the experienced and well-funded sales and marketing infrastructure of some of our competitors. We will face significant challenges and risks in developing our sales and marketing organization, including, among others:
|
|
●
|
our ability to recruit, train and retain adequate numbers of qualified sales and marketing personnel;
|
|
|
●
|
the ability of sales personnel to obtain access to surgeons and persuade adequate numbers of hospitals to purchase our products;
|
|
|
●
|
costs associated with hiring, maintaining and expanding a sales and marketing organization; and
|
|
|
●
|
government scrutiny with respect to promotional activities in the healthcare industry both domestically and abroad.
|
We believe that to sell and market our products effectively, we must establish a compelling clinical and commercial offering with our products. However, potential customers (e.g., surgeons and hospitals) sometimes have long-standing relationships with large, better known companies that dominate the medical devices industry through collaborative research programs and other relationships. Because of these existing relationships, some of which may be contractually enforced, surgeons and hospitals may be reluctant to adopt Renaissance, particularly if it competes with or has the potential to compete with or diminish the need/utilization of products supported through their own collaborative research program or by these existing relationships. Even if these surgeons and hospitals purchase Renaissance, they may be unwilling to enter into collaborative relationships with us to promote joint marketing programs or to provide us with clinical and financial data.
We face competition from large, well-established medical device companies that are likely to launch new navigation or robotic–based products, as well as new techniques and devices for minimally invasive approaches in spine surgeries.
Large, well-established medical device companies, such as Medtronic Inc., Stryker Corporation, and Brainlab AG, have navigation-based products with applications for spine surgeries that have a relatively low market share but which could be further developed and marketed with greater success. These companies are actively innovating in the field and offer their navigation systems with intra-operative 3-dimensional imaging systems that are often valuable to hospitals and physicians beyond spine surgeries but also in fields such as trauma, ear, nose and throat, and brain surgeries. Other companies, including Intuitive Surgical Inc. and Stryker Corporation, have developed or acquired robotic devices for use in other parts of the human anatomy that could be modified or improved to better compete in the spine and brain surgery markets. Even if these companies currently do not have an established presence in our fields, they may attempt to enter our markets and to apply their technologies to compete directly with us. For example, Intuitive Surgical’s da Vinci® robot has been clinically applied in several medical centers to perform anterior-approach spine surgeries. Based on information published by Globus Medical Inc., or Globus, it expects to receive FDA clearance for its surgical robotic positioning platform for spine, brain and trauma markets in the first half of 2016 and generate significant revenues in 2017. Globus’s robot is described as a robotic surgical aid for navigating and facilitating surgical access, implant sizing, positioning and placement, designed to enable surgeons to perform procedures more quickly and with greater accuracy, safety and reproducibility. Medtech SA, or Medtech, announced on December 1, 2014 the first surgical intervention with its ROSA™ Spine robot since it received the CE mark earlier that year. Medtech announced that they received FDA clearance in January 2016. This means that a surgeon interested in computer assisted spine surgery in 2016 will have a choice between 3 different robots for spine surgery and at least 3 intra-operative imaging systems coupled with navigation systems. We cannot assure that the surgeon will prefer our product over the current and evolving competition.
While we are unaware of any other current computer-assisted product or robotic-device that could directly compete with Renaissance at this time in the spine surgery market, it is likely that at some point there will be new market entrants, such as KB Medical with their AQrate robot. Many medical device competitors enjoy competitive advantages over us, including:
|
|
●
|
significantly greater name recognition;
|
|
|
●
|
longer operating histories;
|
|
|
●
|
established exclusive relations with healthcare professionals, customers and third-party payors;
|
|
|
●
|
established distribution networks;
|
|
|
●
|
a more compelling technology with greater clinical value;
|
|
|
●
|
additional lines of products and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage;
|
|
|
●
|
greater experience in conducting research and development, manufacturing, clinical trials, obtaining regulatory clearance for products and marketing approved products; and
|
|
|
●
|
greater financial and human resources for product development, sales and marketing and patent litigation.
|
There can be no assurance that we will be able to compete successfully against current or future competitors or that competition will not have a material adverse effect on our future revenues and, consequently, on our business, operating results and financial condition.
Medical device development is costly and involves continual technological change, which may render our current or future products obsolete.
Innovation is rapid and continuous in the medical device industry, and our competitors in the medical device industry make significant investments in research and development. If new products or technologies emerge that provide the same or superior benefits as our products at equal or lower cost, they could render our products obsolete or unmarketable. Because our products can have long development and regulatory clearance or approval cycles, we must anticipate changes in the marketplace and the direction of technological innovation and customer demands. In addition, we face increasing competition from well-financed medical device companies in our attempts to acquire such new technologies, products and businesses. As a result, we cannot be certain that our products will be competitive with current or future products and technologies.
Our success depends, in part, on our ability to enter the brain-surgery market, and this market has significant barriers to entry.
Computer-assisted surgeries are the accepted standard-of-care in brain procedures, and stereotactic frames and frameless navigation devices have dominated this market for almost two decades. There are currently 2 other dominant robotic devices for brain surgeries, which are Medtech’s ROSA™ robot and Renishaw’s Neuromate©. These products compete directly with Renaissance. As a result, we cannot be certain that surgeons will use our products or that our products will be competitive with current or future products and technologies. If we are unable to penetrate the brain-surgery market, we may not be able to generate the revenue necessary to develop a sustainable, profitable business.
We may encounter problems or delays in the assembly of our products or fail to meet certain regulatory requirements.
The current and intended future versions of Renaissance are complex and require the integration of a number of separate components and processes. To become profitable, we must assemble and test Renaissance in commercial quantities in compliance with regulatory requirements and at an acceptable cost. Increasing our capacity to assemble and test our products on a commercial scale will require us to improve internal efficiencies. We may encounter a number of difficulties in increasing our assembly and testing capacity, including:
|
|
●
|
managing production yields;
|
|
|
●
|
maintaining quality control and assurance;
|
|
|
●
|
providing component and service availability;
|
|
|
●
|
managing subcontractors;
|
|
|
●
|
hiring and retaining qualified personnel; and
|
|
|
●
|
complying with state, federal and foreign regulations.
|
If we are unable to satisfy commercial demand for our Renaissance system due to our inability to assemble and test the system in compliance with applicable regulations, our business and financial results, including our ability to generate revenue, would be impaired, market acceptance of our products could be materially adversely affected and customers may instead purchase or use competing products.
Any failure in our efforts to train surgeons or hospital staff adequately could result in lower than expected product sales and potential liabilities.
A critical component of our sales and marketing efforts is the training of surgeons and operating room staff to properly use Renaissance. We rely on surgeons and hospital staff to devote adequate time to learn to use our products. Convincing surgeons and hospital staff to dedicate the time and energy necessary for adequate training in the use of our system is challenging, and we cannot assure that we will be successful in these efforts. If surgeons or hospital staffs are not properly trained, they may misuse or ineffectively use our products. If nurses or other members of the hospital staff are not adequately trained to assist in using our Renaissance system, surgeons may be unable to use our products. Insufficient training may result in reduced system use, unsatisfactory patient outcomes, patient injury and related liability or negative publicity, which could have an adverse effect on our product sales or create substantial potential liabilities.
We will likely continue to experience extended and variable sales cycles, which could cause significant variability in our results of operations for any given quarter.
Our Renaissance system has a lengthy sales cycle because it is a major piece of capital equipment, the purchase of which will generally require the approval of senior management at hospitals, inclusion in the hospitals’ budget process for capital expenditures and, in some instances, a certificate of need from the state or other regulatory clearance. As a result, a relatively small number of units are currently installed each quarter. We estimate that the sales cycle of Renaissance will continue to take an average of nine months from the point of initial identification and contact with a qualified surgeon until closing of the purchase with the hospital. In addition, the introduction of new products could adversely impact our sales cycle as customers take additional time to assess them. Because of the lengthy sales cycle, the unit price of Renaissance and the relatively small number of systems installed each quarter, each installation of a Renaissance system can represent a significant component of our revenue for a particular quarter, particularly in the near term and during any other periods in which our sales volume is relatively low.
Certain factors that may contribute to variability in our operating results may include:
|
|
●
|
delays in shipments due, for example, to natural disasters or labor disturbances;
|
|
|
●
|
delays or unexpected difficulties in the manufacturing processes of our suppliers or in our assembly process;
|
|
|
●
|
timing of the announcement, introduction and delivery of new products or product upgrades by us and by our competitors;
|
|
|
●
|
timing and level of expenditures associated with expansion of sales and marketing activities and our overall operations;
|
|
|
●
|
changes in third-party coverage and reimbursement, changes in government regulation, or a change in a customer’s financial condition or ability to obtain financing; and
|
|
|
●
|
hospitals’ tendency to group purchases at the beginning of their budgetary cycle, which is different among hospitals.
|
These factors are difficult to forecast and may contribute to substantial fluctuations in our quarterly revenue and substantial variation from our projections, particularly during the periods in which our sales volume is low. Moreover, many of our expenses, such as office leases and most personnel costs, are relatively fixed. We may be unable to adjust spending quickly enough to offset any unexpected revenue shortfall. Accordingly, any shortfall in revenue may cause significant variation in operating results in any quarter. Based on the above factors, we believe that quarter-to-quarter comparisons of our operating results are not a good indication of our future performance. These and other potential fluctuations also mean that you will not be able to rely upon our operating results in any particular period as an indication of future performance.
We may fail to respond to cost containment efforts by our customers, which could have an adverse impact on our sales, financial condition and results of operations.
Some of our customers and potential customers have joined group purchasing organizations in an effort to contain costs; these group purchasing organizations negotiate pricing arrangements with medical supply manufacturers and distributors and make these negotiated prices available to the group purchasing organization’s affiliated hospitals and other members. If we fail to respond to the cost containment efforts of our customers and potential customers, we may lose sales or face downward pricing pressure, which could result in an adverse impact on our financial condition and results of operations.
If we receive a significant number of warranty claims or our Renaissance system units require significant amounts of service after sale, our costs will increase and our business and financial results will be adversely affected.
Sales of the our Renaissance system generally include a warranty and maintenance obligation on our part for services for a period of twelve months from the date Renaissance is installed at a customer’s facility. We also provide technical and other services to customers beyond the warranty period pursuant to a supplemental service plan sold with each system. If product returns or warranty claims are significant or exceed our expectations, we could incur unanticipated reductions in sales or additional expenditures for parts and service. In addition, our reputation could be damaged and our products may not achieve market acceptance.
Software defects may be discovered in our products.
Our Renaissance system incorporates sophisticated computer software. Complex software frequently contains errors, especially when first introduced. Because our products are designed to be used to perform complex surgical procedures, we expect that physicians and hospitals will have an increased sensitivity to the potential for software defects. We cannot assure you that our software will not experience errors or performance problems in the future. If we experience software errors or performance problems, we would likely also experience:
|
|
●
|
delay in market acceptance of our products;
|
|
|
●
|
damage to our reputation;
|
|
|
●
|
additional regulatory filings;
|
|
|
●
|
increased service or warranty costs; and
|
|
|
●
|
product liability claims relating to the software defects.
|
We may be subject to product liability claims, product actions, including product recalls, and other field or regulatory actions that could be expensive, divert management’s attention and harm our business.
Our business exposes us to potential liability risks, product actions and other field or regulatory actions that are inherent in the manufacturing, marketing and sale of medical device products, particularly those used in surgery. We may be held liable if our products cause injury or death or are found otherwise unsuitable or defective during usage. Renaissance incorporates mechanical and electrical parts, complex computer software and other sophisticated components, any of which can contain errors or failures. Complex computer software is particularly vulnerable to errors and failures, especially when first introduced. In addition, new products or enhancements to our existing products may contain undetected errors or performance problems that, despite testing, are discovered only after installation.
If any of our products are defective, whether due to design or manufacturing defects, improper use of the product or other reasons, we may voluntarily or involuntarily undertake an action to remove, repair, or replace the product at our expense. In some circumstances we will be required to notify regulatory authorities of an action pursuant to a product failure. We are also required to submit a Medical Device Report, or MDR, to the FDA for any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury.
A required notification to a regulatory authority or a failure to make a timely required notification could result in an investigation by regulatory authorities of our products, which could in turn result in field corrective actions, restrictions on the sale of the products, and civil or criminal penalties. In addition, because our products are designed to perform complex surgical procedures, defects could result in a number of complications, some of which could be serious and could cause significant harm to the patient or even cause death. The adverse publicity resulting from any of these events could cause surgeons or hospitals to review and potentially terminate their relationships with us.
The medical device industry has historically been subject to extensive litigation over product liability claims. We anticipate that as part of our ordinary course of business we will be subject to product liability claims alleging defects in the design, manufacture or labeling of our products. A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs and high punitive damage payments. Although we maintain product liability insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims. Additionally, we may be unable to maintain our existing product liability insurance in the future at satisfactory rates or adequate amounts.
If coverage or reimbursement from third-party payors for procedures in which Renaissance is used, namely spinal fusions, is decreased or limited, hospitals may not purchase Renaissance and surgeons may perform fewer spinal fusions, which would harm our business and financial results.
Our ability to successfully commercialize Renaissance depends significantly on the availability of coverage and reimbursement for thoracic-lumbar spinal fusion procedures from third-party payors, including governmental programs such as Medicare and Medicaid, as well as private insurance and private health plans. Reimbursement is a significant factor considered by hospitals in determining whether to acquire new capital equipment such as our technology. Although our customers have been successful in obtaining coverage and reimbursement for procedures using our products, we cannot be assured that procedures using our technology will be covered or reimbursed by third-party payors in the future or that such reimbursements will not be reduced to the extent that they will adversely affect capital allocations for purchase of our Renaissance system.
As part of healthcare reform and other cost containment initiatives, the U.S. Congress, or the Congress, may pass legislation impacting coverage and reimbursement for healthcare services, including Medicare reimbursement to physicians and hospitals. Many private third-party payors look to Medicare’s coverage and reimbursement policies in setting their coverage policies and reimbursement amounts. If the Centers for Medicare & Medicaid Services, or CMS, the federal agency that administers the Medicare program, or Medicare contractors limit payments to hospitals or surgeons for thoracic-lumbar spinal fusion surgeries, private payors may similarly limit payments. In addition, state legislatures may enact laws limiting or otherwise affecting the level of Medicaid reimbursements. As a result, hospitals may not purchase Renaissance and surgeons may choose to decrease their volume of thoracic-lumbar spinal fusions, and, as a result, our business and financial results would be adversely affected.
Because hospitals receive a fixed reimbursement amount from Medicare for specified procedures or conditions, a hospital must absorb the cost of our products as part of the reimbursement payment it receives, which makes the hospital’s purchasing decisions more risky, particularly those related to expensive capital equipment.
Medicare pays acute care hospitals a prospectively determined amount for inpatient operating costs under the Medicare hospital inpatient prospective payment system, or PPS. Under the Medicare PPS, the prospective payment for a patient’s stay in an acute care hospital is determined by the patient’s condition and other patient data and procedures performed during the inpatient stay using a classification system known as diagnosis related groups, or DRGs. CMS implemented a revised version of the DRG system that uses Medicare Severity DRGs, or MS-DRGs, instead of the DRGs which Medicare used previously. The MS-DRGs are intended to more accurately account for the patient’s severity of illness when assigning each patient’s stay to a payment classification. Medicare pays a fixed amount to the hospital based on the MS-DRG into which the patient’s stay is assigned, regardless of the actual cost to the hospital of furnishing the procedures, items and services provided. Accordingly, acute care hospitals generally do not receive direct Medicare reimbursement under the PPS for the specific costs incurred in purchasing medical devices, except under limited circumstances. Rather, reimbursement for these costs is deemed to be included within the MS-DRG based payments made to hospitals for the services furnished to Medicare eligible inpatients in which the devices are utilized. Accordingly, a hospital must absorb the cost of our products as part of the payment it receives for the procedure in which the device is used. In addition, physicians that perform procedures in hospitals are paid a set amount by Medicare for performing such services under the Medicare Physician Fee Schedule. Medicare payment rates for both systems are established annually.
At this time, we do not know the extent to which hospitals and physicians would consider third-party reimbursement levels adequate to cover the cost of our products. Failure by hospitals and surgeons to receive an amount that they consider to be adequate reimbursement for procedures in which our products are used could deter them from purchasing or using our products and limit our sales growth. In addition, pre-determined MS-DRG payments or Medicare Physician Fee Schedule payments may decline over time, which could deter hospitals from purchasing our products or physicians from using them. If hospitals are unable to justify the costs of our products or physicians are not adequately compensated for procedures in which our products are utilized, they may refuse to purchase or use them, which would significantly harm our business.
Notwithstanding current or future FDA clearances, if granted, third-party payors may deny coverage and reimbursement if the payor determines that a therapeutic medical device is unnecessary, inappropriate, not cost-effective or experimental, or is used for a non-approved indication. Although we are not aware of any potential customer that has declined to purchase our Renaissance system based upon third-party payors’ coverage and reimbursement policies, cost control measures adopted by third-party payors may have a significant effect on surgeries performed using Renaissance or as to the levels of reimbursement.
Broad-based domestic and international government initiatives to reduce spending, particularly those related to healthcare costs, may reduce reimbursement rates for spinal surgery procedures, which will reduce the cost-effectiveness of our products.
Healthcare reforms, changes in healthcare policies and changes to third-party coverage and reimbursement, including recently enacted legislation reforming the U.S. healthcare system, may affect demand for our products and may have a material adverse effect on our financial condition and results of operations. There can be no assurance that current levels of reimbursement will not be decreased in the future, or that future legislation, regulation, or reimbursement policies of third-party payors will not adversely affect the demand for our products or our ability to sell products on a profitable basis.
The Patient Protection and Affordable Care Act, or PPACA, adopted in the United States in March 2010 and related regulations include new taxes impacting certain health-related industries, including medical device manufacturers. The legislation imposes significant new taxes on medical device makers in the form of a 2.3% excise tax on all U.S. medical device sales beginning in 2013. This excise tax applies to our medical devices. The Consolidated Appropriations Act, 2016, signed into law on December 18, 2015, includes a two-year suspension on the medical device excise tax. Thus, the medical device excise tax does not apply to the sale of a taxable medical device by the manufacturer, producer, or importer of the device during the period beginning on January 1, 2016, and ending on December 31, 2017. However, there is no guarantee that the excise tax will continue to be suspended by congressional action after this two-year period ends, and absent further congressional action, the excise tax will be reinstated for medical device sales beginning January 1, 2018.
Other significant measures contained in PPACA include initiatives to revise Medicare payment methodologies, initiatives to promote quality indicators in payment methodologies, initiatives related to the coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, and annual reporting requirements related to payments to physicians and teaching hospitals.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. On August 2, 2011, the President of the United States, or the President, signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year. On January 2, 2013, the President signed into law the American Taxpayer Relief Act of 2012, or the ATRA, which delayed for another two months the budget cuts mandated by these sequestration provisions of the Budget Control Act of 2011. On March 1, 2013, the President signed an executive order implementing sequestration, and on April 1, 2013, the 2% Medicare payment reductions went into effect. The ATRA also, among other things, reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure.
We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or internationally, or the effect any future legislation or regulation will have on us. The taxes imposed by PPACA and the expansion of government’s role in the U.S. healthcare industry may result in decreased profits to us, lower reimbursements by third-party payors for surgeries in which our products are used, and reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations, possibly materially.
Changing models for the provision of healthcare may affect the cost-effectiveness of Renaissance.
All third-party payors, whether governmental or private, whether within the United States or abroad, are developing increasingly sophisticated methods of controlling healthcare costs. These cost control methods include PPSs, capitated rates, benefit redesigns, pre-authorization or second opinion requirements prior to major surgery, an emphasis on wellness and healthier lifestyle interventions and an exploration of other cost-effective methods of delivering healthcare. These cost control methods also potentially limit the amount which healthcare providers may be willing to pay for medical technology which could, as a result, adversely affect our business and financial results. In addition, no uniform policy of coverage and reimbursement for medical technology exists among all these payors. Therefore, coverage and reimbursement for medical technology can differ significantly from payor to payor, and country to country.
We may attempt to acquire new products or technologies, and if we are unable to successfully complete these acquisitions or to integrate acquired businesses, products, technologies or employees, we may fail to realize expected growth.
Our success will depend, in part, on our ability to expand our product offerings and continue to offer the advanced computer assisted solutions for spine surgery and grow our business in response to changing technologies, customer demands and competitive pressures. In some circumstances, we may determine to do so through the acquisition of complementary businesses, products or technologies rather than through internal development. Successful acquisitions present a number of hurdles and risk, including:
|
|
●
|
the identification of suitable acquisition candidates can be difficult, time consuming and costly;
|
|
|
●
|
integrating any acquisitions that we make into our operations is difficult, time consuming, and expensive, and may involve new regulatory requirements; and
|
|
|
●
|
future acquisitions could result in potentially dilutive issuances of equity securities or the incurrence of debt, contingent liabilities or expenses, or other charges such as amortization of intangible assets, any of which could harm our business and materially adversely affect our financial results or cause a reduction in the price of our Ordinary Shares.
|
If we do not effectively manage our growth, we may be unable to successfully develop, market and sell our products.
In order to achieve our business objectives, we must continue to grow. Continued growth presents numerous challenges, including:
|
|
●
|
implementing appropriate operational and financial systems and controls;
|
|
|
●
|
expanding manufacturing and assembly capacity and increasing production;
|
|
|
●
|
developing our sales and marketing infrastructure and capabilities;
|
|
|
●
|
identifying, attracting and retaining qualified personnel in our areas of activity;
|
|
|
●
|
hiring, training, managing and supervising our personnel; and
|
|
|
●
|
continuous compliance with regulatory and quality assurance requirements.
|
We cannot be certain that our systems, controls, infrastructure and personnel will be adequate to support our future operations. Any failure to effectively manage our growth could impede our ability to successfully develop, market and sell our products and our business will be harmed.
If we are successful in our efforts to market and sell Renaissance outside of the United States, we will be subject to various risks relating to our international activities, which could adversely affect our business and financial results.
We are continuing to pursue international markets for the sale of our products and, as of December 31, 2015, there were 44 SpineAssist and Renaissance systems installed in Europe, Asia and Australia. As a result of these efforts and sales, we are exposed to risks separate and distinct from those we face in our U.S. operations. Our international business may be adversely affected by changing economic conditions in foreign countries. In addition, because international sales may be denominated in the functional currency of the country where the product is being shipped, increases or decreases in the value of the U.S. dollar relative to foreign currencies could affect our results of operations. Engaging in international business inherently involves a number of other difficulties and risks, including:
|
|
●
|
approval of product submissions with healthcare systems outside the United States;
|
|
|
●
|
gathering the clinical data that may be required for product submissions with healthcare systems outside the United States;
|
|
|
●
|
import restrictions and controls and other government regulation relating to technology;
|
|
|
●
|
pricing pressures that we may experience internationally;
|
|
|
●
|
the availability and level of reimbursement within prevailing foreign healthcare payment systems;
|
|
|
●
|
compliance with existing and changing applicable foreign regulatory laws and requirements, including but not limited to the European Medical Device Directive (Council Directive 93/42/EEC), the U.S. Foreign Corrupt Practices Act of 1977, or FCPA, OECD Convention on Combating Bribery of Foreign Public Officials in International Business Transactions;
|
|
|
●
|
foreign laws and business practices favoring local companies;
|
|
|
●
|
longer payment cycles; and
|
Our exposure to each of these risks may increase our costs, impair our ability to market and sell our products and require significant management attention, resulting in harm to our business and financial results.
Our Renaissance system is used mainly for vertebral fixation procedures during thoracic-lumbar spinal fusion surgeries. Should the standard of care change and these procedures be abandoned as the treatment of choice for the current indications, it might negatively affect our business.
According to the Orthopedic Network News report dated October 2015, an estimated 409,400 thoracic-lumbar fusion surgeries will be performed in the United States during 2015. These surgeries are the standard of care in several common spinal pathologies. However, new treatment methods continue to be innovated, such as motion preserving techniques and devices that might not be benefitted by the use of Renaissance during such surgical procedures. In such a case, the appeal to surgeons in using Renaissance could be diminished and have a negative effect on our business performance.
We may face both reputational and SEC enforcement risks with respect to conflict minerals obligations.
The SEC has adopted disclosure requirements under section 102 of the Dodd-Frank Wall Street Reform and Consumer Protection Act regarding the source of certain minerals for which such conflict minerals are necessary to the functionality or production of a product manufactured, or contracted to be manufactured which are mined from the Democratic Republic of Congo, and adjoining countries, including: Sudan, Uganda, Burundi, United Republic of Tanzania, Zambia, Angola, and Central African Republic. These rules require reporting companies to file a conflict minerals report as an exhibit to a Form SD report with the SEC. The conflict minerals report is required to set out the due diligence efforts and procedures exercised on the source and chain of custody of such conflict minerals, in accordance with internationally recognized due diligence framework, and a description of our products containing such conflict minerals. Although we expect that we will be able to comply with the requirements of any rules promulgated by the SEC and file our third report by May 31, 2016, as required, in preparing to do so we are dependent upon information supplied by certain suppliers of products that contain, or potentially contain, conflict minerals. Such preparation may be costly. To the extent that the information that we receive from our suppliers is inaccurate or inadequate or our processes in obtaining that information do not fulfill the SEC’s requirements, we could face both reputational and SEC enforcement risks.
Risks Related to Our Intellectual Property
If we, or the other parties from whom we license intellectual property, are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products, our ability to compete will be harmed.
Our commercial success depends, in part, on obtaining and maintaining patent and other intellectual property protection for the technologies used in our products. The patent positions of medical device companies, including ours, can be highly uncertain and involve complex and evolving legal and factual questions. Furthermore, we presently license intellectual property from other parties, and we might in the future opt to license additional intellectual property from other parties. If we, or the other parties from whom we license or would license intellectual property, fail to obtain and maintain adequate patent or other intellectual property protection for intellectual property used in our products, or if any protection is reduced or eliminated, others could use the intellectual property used in our products, resulting in harm to our competitive business position. In addition, patent and other intellectual property protection may not provide us with a competitive advantage against competitors that devise ways of making competitive products without infringing any patents that we own or have rights to.
U.S. patents and patent applications may be subject to interference proceedings, and U.S. patents may be subject to re-examination proceedings in the U.S. Patent and Trademark Office. Foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent offices. Any of these proceedings could result in loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of the patent or patent application. Changes in either patent laws or in interpretations of patent laws may also diminish the value of our intellectual property or narrow the scope of our protection. Interference, re-examination and opposition proceedings may be costly and time consuming, and we, or the other parties from whom we might potentially license intellectual property, may be unsuccessful in defending against such proceedings. Thus, any patents that we own or might license may provide limited or no protection against competitors. In addition, our pending patent applications and those we may file in the future may have claims narrowed during prosecution or may not result in patents being issued. Even if any of our pending or future applications are issued, they may not provide us with adequate protection or any competitive advantages. Our ability to develop additional patentable technology is also uncertain.
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may also result in the loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to other parties. In addition, many countries limit the enforceability of patents against other parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries have different rules of protecting intellectual property rights, particularly in the field of medical products and procedures. Among these countries is China where we have sales pursuant to a distribution agreement with a local distributor.
If we are unable to prevent unauthorized use or disclosure of our proprietary trade secrets and unpatented know-how, our ability to compete will be harmed.
Proprietary trade secrets, copyrights, trademarks and unpatented know-how are also very important to our business. We rely on a combination of trade secrets, copyrights, trademarks, confidentiality agreements and other contractual provisions and technical security measures to protect certain aspects of our technology, especially where we do not believe that patent protection is appropriate or obtainable. We require our employees and consultants to execute confidentiality agreements in connection with their employment or consulting relationships with us. We also require our employees and consultants to disclose and assign to us all inventions conceived during the term of their employment or engagement while using our property or which relate to our business. We also have taken precautions to initiate reasonable safeguards to protect our information technology systems. However, these measures may not be adequate to safeguard our proprietary intellectual property and conflicts may, nonetheless, arise regarding ownership of inventions. Such conflicts may lead to the loss or impairment of our intellectual property or to expensive litigation to defend our rights against competitors, who may be better funded and have superior resources. Our employees, consultants, contractors, outside clinical collaborators and other advisors may unintentionally or willfully disclose our confidential information to competitors. In addition, confidentiality agreements may be unenforceable or may not provide an adequate remedy in the event of unauthorized disclosure. Enforcing a claim, that a third party illegally obtained and is using our trade secrets, is expensive and time consuming, and the outcome is unpredictable. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our products that we consider proprietary. As a result, other parties may be able to use our proprietary technology or information, and our ability to compete in the market would be harmed.
We could become subject to patent and other intellectual property litigation that could be costly, result in the diversion of management’s attention, require us to pay damages and force us to discontinue selling our products.
The medical device industry is characterized by competing intellectual property and a substantial amount of litigation over patent and other intellectual property rights. In particular, the fields of orthopedic implants, computer-assisted surgery, or CAS, systems, and robotics are well established and crowded with the intellectual property of competitors and others. A number of companies in our market, as well as universities and research institutions, have been issued patents and have filed patent applications which relate to the use of CAS.
Determining whether a product infringes a patent involves complex legal and factual issues, and the outcome of a patent litigation action is often uncertain. We have not conducted an extensive search of patents issued or assigned to other parties, including our competitors, and no assurance can be given that patents containing claims covering our products, parts of our products, technology or methods do not exist, have not been filed or could not be filed or issued. Because of the number of patents issued and patent applications filed in our technical areas, our competitors or other parties may assert that our products and the methods we employ in the use of our products are covered by U.S. or foreign patents held by them. In addition, because patent applications can take many years to issue and because publication schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware and which may result in issued patents which our current or future products infringe. Also, because the claims of published patent applications can change between publication and patent grant, there may be published patent applications that may ultimately issue with claims that we infringe. There could also be existing patents that one or more of our products or parts may infringe and of which we are unaware. As the number of competitors in the market for computer and robotic-assisted surgery grows, and as the number of patents issued in this area grows, the possibility of patent infringement claims against us increases. In certain situations, we may determine that it is in our best interests or their best interests to voluntarily challenge a party’s products or patents in litigation or other proceedings, including patent interferences or re-examinations. As a result, we may become involved in litigation that could be costly, result in diversion of management’s attention, require us to pay damages and force us to discontinue selling our products.
Infringement actions and other intellectual property claims and proceedings brought against or by us, whether with or without merit, may cause us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management from our business and harm our reputation. Some of our competitors may be able to sustain the costs of complex patent or intellectual property litigation more effectively than we can because they have substantially greater resources.
We cannot be certain that we will successfully defend against allegations of infringement of patents and intellectual property rights of others. In the event that we become subject to a patent infringement or other intellectual property lawsuit and if the other party’s patents or other intellectual property were to be upheld as valid and enforceable and we were to be found to infringe the other party’s patents or violate the terms of a license to which we are a party, we could be required to pay damages. We could also be prevented from selling our products unless we could obtain a license to use technology or processes covered by such patents or were able to redesign the product to avoid infringement. A license may not be available at all or on commercially reasonable terms or we may not be able to redesign our products to avoid infringement. Modification of our products or development of new products could require us to conduct clinical trials and to revise our filings with the FDA and other regulatory bodies, which would be time consuming and expensive. In these circumstances, we may be unable to sell our products at competitive prices or at all, and our business and operating results could be harmed.
Our product development is limited by existing intellectual property owned by other companies. Our development of new generations of our products might depend on licensing of such intellectual property.
As we enhance our current product offerings and develop new ones, we may find it advisable or necessary to seek licenses from other parties who hold patents covering technology or methods necessary for the development of our products. If we cannot obtain these licenses, we could be forced to design around those patents at additional cost or abandon the product altogether. As a result, our ability to grow our business and compete in the market may be harmed.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other medical device companies, including our competitors or potential competitors. We could in the future be subject to claims that these employees, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending against such claims, a court could order us to pay substantial damages and prohibit us from using technologies or features that are essential to our products, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. An inability to incorporate technologies or features that are important or essential to our products would have a material adverse effect on our business, and may prevent us from selling our products. In addition, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, such litigation could result in substantial costs and be a distraction to management. Incurring such costs could have a material adverse effect on our financial condition, results of operations and cash flow.
We may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could result in litigation and adversely affect our business.
We enter into agreements with our employees pursuant to which such individuals grant us all rights to any inventions created in the scope of their employment or engagement with us. A significant portion of our intellectual property has been developed by our employees in the course of their employment for us. Under the Israel Patents Law, 5727-1967, or the Patent Law, inventions conceived by an employee during the scope of his or her employment with a company are regarded as “service inventions,” which belong to the employer, absent a specific agreement between employee and employer giving the employee service invention rights. The Patent Law also provides that in the absence of an agreement between an employer and an employee regarding compensation for service inventions, the Israeli Compensation and Royalties Committee, or the Committee, a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration for their inventions. Recent decisions by the Committee have created uncertainty in this area, as it held that employees may be entitled to remuneration for their service inventions despite having specifically waived any such rights. Further, the Committee has not yet determined the method for calculating this Committee-enforced remuneration. Although our employees have agreed to assign to us invention ownership rights, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be forced to litigate such claims, which could otherwise negatively affect our business.
Risks Related to Regulatory Compliance
If we fail to comply with the extensive government regulations relating to our business, we may be subject to fines, injunctions and other penalties that could harm our business.
Our medical device products and operations are subject to extensive regulation by the FDA, pursuant to the Federal Food, Drug, and Cosmetic Act, or FDCA, and various other federal, state and foreign governmental authorities. Government regulations and requirements specific to medical devices are wide ranging and govern, among other things:
|
|
●
|
design, development and manufacturing;
|
|
|
●
|
testing, labeling and storage;
|
|
|
●
|
marketing, sales and distribution;
|
|
|
●
|
premarket clearance or approval;
|
|
|
●
|
record keeping procedures;
|
|
|
●
|
advertising and promotions;
|
|
|
●
|
recalls and field corrective actions;
|
|
|
●
|
post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; and
|
|
|
●
|
product import and export.
|
In the United States, before we can market a new medical device, or a new use of, or claim for, or significant modification to, an existing product, we must first receive either premarket clearance under Section 510(k) of the FDCA, or Premarket Approval, or PMA, from the FDA, unless an exemption applies. In the 510(k) marketing clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a legally marketed device, known as a “predicate” device, with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. Bench tests, pre-clinical and/or clinical data are sometimes required to support substantial equivalence. The PMA approval pathway requires an applicant to demonstrate the safety and effectiveness of the device based, in part, on data obtained in clinical trials. Both of these processes can be expensive and lengthy and entail significant fees, unless exempt. The FDA’s 510(k) marketing clearance process usually takes from three to 12 months, but it can last longer. The process of obtaining PMA approval is much more costly and uncertain than the 510(k) marketing clearance process. It generally takes from one to three years, or even longer, from the time the PMA application is submitted to the FDA, until an approval is obtained. There is no assurance that we will be able to obtain FDA clearance or approval for any of our new products on a timely basis, or at all.
In the United States, our currently commercialized products have received pre-market clearance under Section 510(k) of the FDCA. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, our product introductions or modifications could be delayed or canceled, which could cause our sales to decline. In addition, the FDA may determine that future products will require the more costly, lengthy and uncertain PMA process. Although we do not currently market any devices under PMA, the FDA may demand that we obtain a PMA prior to marketing certain of our future products.
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions which may prevent or delay approval or clearance of our products under development or impact our ability to modify our currently cleared products on a timely basis. For example, in response to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) regulatory pathway, the FDA initiated an evaluation of the program, and in January 2011, announced several proposed actions intended to reform the review process governing the clearance of medical devices. The FDA intends these reform actions to improve the efficiency and transparency of the clearance process, as well as bolster patient safety. In addition, as part of the Food and Drug Administration Safety and Innovation Act, or FDASIA, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms which are further intended to clarify and improve medical device regulation both pre- and post-approval.
Even after we have obtained the proper regulatory clearance or approval to market a product, we have ongoing responsibilities under FDA regulations. The failure to comply with applicable regulations could jeopardize our ability to sell our products and result in enforcement actions such as:
|
|
●
|
termination of distribution;
|
|
|
●
|
recalls or seizures of products;
|
|
|
●
|
delays in the introduction of products into the market;
|
|
|
●
|
total or partial suspension of production;
|
| |
●
|
refusal to grant future clearances or approvals;
|
|
|
●
|
withdrawals or suspensions of current clearances or approvals, resulting in prohibitions on sales of our products; and
|
|
|
●
|
in the most serious cases, criminal penalties.
|
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, results of operations and financial condition.
Modifications to our currently FDA-cleared products or the introduction of new products may require new regulatory clearances or approvals or require us to recall or cease marketing our current products until clearances or approvals are obtained.
Our Renaissance system has received marketing clearance from the FDA based on 510(k) applications. See “Item 4. Information on the Company - B. Business Overview - Regulatory Requirements of the U.S. Food and Drug Administration.” We have not been required by the FDA to obtain PMA nor to conduct any clinical trials in support of these applications. Modifications to our products, however, may require new regulatory approvals or clearances or require us to recall or cease marketing the modified products until these clearances or approvals are obtained. Any modification to one of our 510(k) cleared products that would constitute a major change in its intended use, or any change that could significantly affect the safety or effectiveness of the device would require us to obtain a new 510(k) marketing clearance and may even, in some circumstances, require the submission of a PMA application, if the change raises complex or novel scientific issues or the product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer’s decision. The latest 510(k) marketing clearance expands the indication for use of Renaissance to brain surgeries. We may continue to make additional modifications in the future to Renaissance without seeking additional clearances or approvals if we believe such clearances or approvals are not necessary. It is expected that the FDA will introduce stringent new changes to existing policy and practices regarding the assessment of whether a new 510(k) is required for changes or modifications to existing devices. Most recently, on July 9, 2012, FDASIA was enacted which, among other requirements, obligates the FDA to prepare a report for Congress on the FDA’s approach for determining when a new 510(k) will be required for modifications or changes to a previously cleared device. After submitting this report, the FDA is expected to issue revised guidance to assist device manufacturers in making this determination. Until then, manufacturers may continue to adhere to the FDA’s 1997 guidance on this topic when making a determination as to whether or not a new 510(k) is required for a change or modification to a device, but the practical impact of the FDA’s continuing scrutiny of these issues remains unclear. If the FDA disagrees with our past or future decisions not to seek a new 510(k) for changes or modifications to existing devices and requires new clearances or approvals, we may be required to recall and stop marketing our products as modified, which could require us to redesign our products, conduct clinical trials to support any modifications, and pay significant regulatory fines or penalties. In addition, the FDA may not approve or clear our products for the indications that are necessary or desirable for successful commercialization or could require clinical trials to support any modifications. Any delay or failure in obtaining required clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth. Any of these actions would harm our operating results.
Moreover, clearances and approvals are subject to continual review, and the later discovery of previously unknown problems can result in product labeling restrictions or withdrawal of the product from the market. The loss of previously received approvals or clearances, or the failure to comply with existing or future regulatory requirements could reduce our sales, profitability and future growth prospects.
We are currently required by the FDA to refrain from using certain terms to label and market our products, which could harm our ability to market and commercialize our current or future products.
The FDA’s 510(k) clearances include a specification of a product’s indication for use, and also authorize specific labeling and marketing claims and language in promotional materials for the U.S. market. Failure to conform with the specific cleared labeling of our product or the use of the term “Robot” in our product or corporate promotional material would be considered mislabeling or off-label promotion which might lead to:
|
|
●
|
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
|
●
|
customer notifications, refunds, detention or seizure of our products;
|
|
|
●
|
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
|
●
|
withdrawing 510(k) marketing clearances that have already been granted, or PMA approvals which we may receive in the future;
|
|
|
●
|
refusing to provide Certificates for Foreign Government;
|
|
|
●
|
refusing to grant export approval for our products; or
|
|
|
●
|
pursuing criminal prosecution.
|
Any of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and financial condition.
We may inadvertently breach government and contractual privacy laws and obligations.
In the course of performing our business, we obtain certain confidential patient health information, such as patient names and dates of Renaissance procedures. In the event of an inadvertent disclosure, we could be subject to enforcement measures, including civil and criminal penalties and fines for violations of the privacy or security standards, such as the Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, including the final omnibus rule published on January 25, 2013, or subject to violation of contractual claims of customers.
Failure to obtain regulatory approval in additional foreign jurisdictions will prevent us from expanding the commercialization of our products abroad.
To be able to market and sell our products in most countries other than the United States, we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals and the time required for regulatory review, vary from country to country. Clearance or approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA clearance or approval. Obtaining and maintaining foreign regulatory approvals are expensive, and we cannot be certain that we will receive regulatory approvals in any foreign country in which we plan to market our products, on a timely basis, if at all. If we fail to obtain or maintain regulatory approval in any foreign country in which we plan to market our products on a timely basis, or at all, our ability to generate revenue will be harmed.
As we modify existing products or develop new products in the future, including new accessories, we apply for permission to affix to such products a European Union CE mark, which is a legal requirement for medical devices intended for sale in the European Union. In addition, we will be subject to annual regulatory audits in order to maintain those CE mark permissions. We do not know whether we will be able to continue to affix the CE mark for new or modified products or that we will continue to meet the quality and safety standards required to maintain the permissions we have already received. If we are unable to maintain permission to affix the CE mark to our products, we will no longer be able to sell our products in member countries of the European Union or other areas of the world that require CE marking for the marketing and distribution of medical devices.
If we or our third-party manufacturers or suppliers fail to comply with the FDA’s Quality System Regulation, our manufacturing operations could be interrupted and our product sales and operating results could suffer.
We and some of our third-party manufacturers and suppliers are required to comply with the FDA’s Quality System Regulation, or QSR, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. We and our manufacturers and suppliers are also subject to the regulations of foreign jurisdictions regarding the manufacturing process if we or our distributors market our products abroad. We continue to monitor our quality management in order to improve our overall level of compliance. Our facilities are subject to periodic and unannounced inspection by U.S. and foreign regulatory agencies, including notified bodies, to audit compliance with the QSR and comparable foreign regulations. If our facilities or those of our manufacturers or suppliers are found to be in violation of applicable laws and regulations, or if we or our manufacturers or suppliers fail to take satisfactory corrective action in response to an adverse inspection, the regulatory authority could take enforcement action, including any of the following sanctions:
|
|
●
|
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
|
●
|
customer notifications or repair, replacement, refunds, detention or seizure of our products;
|
|
|
●
|
operating restrictions or partial suspension or total shutdown of production;
|
|
|
●
|
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
|
●
|
withdrawing 510(k) marketing clearances that have already been granted, or PMA approvals that we may receive in the future;
|
|
|
●
|
refusing to provide Certificates for Foreign Government;
|
|
|
●
|
refusing to grant export approval for our products; or
|
|
|
●
|
pursuing criminal prosecution.
|
Any of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands, and could have a material adverse effect on our reputation, business, results of operations and financial condition. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
Our products may in the future be subject to product actions that could harm our reputation, business operations and financial results.
Manufacturers may, on their own initiative, initiate actions, including a non-reportable market withdrawal or a reportable product recall, for the purpose of correcting a material deficiency, improving device performance, or other reasons. Additionally, the FDA and similar foreign health or governmental authorities have the authority to require an involuntary recall of commercialized products in the event of material deficiencies or defects in design, manufacturing or labeling or in the event that a product poses an unacceptable risk to health. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that a device intended for human use would cause serious, adverse health consequences or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Product actions involving any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations.
Companies are required to maintain certain records of actions, even if they determine such actions are not reportable to the FDA. If we determine that certain actions do not require notification of the FDA, the FDA may disagree with our determinations and require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted or failing to timely report or initiate a reportable product action.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals or clearances for the device before we may market or distribute the corrected device. Seeking such approvals or clearances may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties and civil or criminal fines.
If our products, or malfunction of our products, cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under FDA regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. In addition, all manufacturers placing medical devices in European Union markets are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the relevant authority in whose jurisdiction the incident occurred. We anticipate that in the future it is likely that we may experience events that would require reporting to the FDA pursuant to the MDR regulations. Any adverse event involving our products could result in future voluntary corrective actions, such as product actions or customer notifications, or agency actions, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
In addition, as the frequency of use of Renaissance increases and our business continues to grow, we may experience an increase in the number of incidents that could lead to MDR reports which we might need to file. The decision to file an MDR involves a judgment by us as the manufacturer. We have made decisions that certain types of events are not reportable under the MDR regulations; however, there can be no assurance that the FDA will agree with our decisions. If we fail to report MDRs to the FDA within the required timeframes, or at all, or if the FDA disagrees with any of our determinations regarding the reportability of certain events, the FDA could take enforcement actions against us, which could have an adverse impact on our reputation and financial results.
We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses, resulting in damage to our reputation and business.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of a medical device for a use that has not been cleared or approved by FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. Physicians may use our products off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. If the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, which could have an adverse impact on our reputation and financial results. In addition, any FDA action could trigger scrutiny by other federal and state regulatory agencies. Such scrutiny could also occur, regardless of FDA action.
We may be subject to fines, penalties, or licensure requirements, or legal liability, if it is determined that our Renaissance clinical sales representatives and other employees are practicing medicine without a license.
State laws prohibit the practice of medicine without a license. Our clinical sales representatives, or CSRs, provide preoperative and intraoperative clinical and technical support to our customers, including assistance setting up the equipment, participation in the preoperative planning process, and facilitation of the surgeon’s use of Renaissance during surgery. Our CSRs are not engaged in the practice of medicine, but rather are assisting our customers in the safe and proper usage of our equipment and products. Nevertheless, a governmental authority or individual actor could allege the activities of our CSRs to constitute the practice of medicine. A state may seek to have us discontinue the services provided by our CSRs or subject us to fine, penalties or licensure requirements. Any determination that our CSRs are practicing medicine without a license may result in significant liability to us.
The application of state certificate of need regulations could substantially limit our ability to sell our products and grow our business.
Some states require healthcare providers to obtain a certificate of need or similar regulatory approval prior to the acquisition of high-cost capital equipment such as Renaissance. In some states, the process required of our customers to obtain this certificate is lengthy and could result in a longer sales cycle for Renaissance. Further, in many cases, only a limited number of these certificates are available. As a result, our customers may be unable to obtain a certificate of need for the purchase of our Renaissance system which could cause our sales to decline.
Federal regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of a medical device. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
Without limiting the generality of the foregoing, Congress has enacted, and the President signed into law, the Food and Drug Administration Amendments Act of 2007, or the Amendments. This law requires, among other things, that the FDA propose, and ultimately implement, regulations that will require manufacturers to label medical devices with unique identifiers unless a waiver is received from the FDA. Once implemented, compliance with those regulations may require us to take additional steps in the manufacture of our products and labeling. These steps may require additional resources and could be costly. In addition, the Amendments will require us to, among other things, pay annual establishment registration fees to the FDA for each of our FDA registered facilities and certify to the clinical trial reporting provisions contained in the Amendments.
We may be subject, directly or indirectly, to federal and state healthcare regulations and could face substantial penalties if we are unable to fully comply with such regulations and laws.
While we do not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, many healthcare laws and regulations apply to our business. For example, we could be subject to patient privacy regulation and enforcement by both the federal government and the states in which we conduct our business. There are multiple healthcare laws and regulations that may affect our ability to operate. New laws and regulations are being continually proposed and adopted. For example, the PPACA imposes new tracking reporting and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to physicians and teaching hospitals. Device manufacturers were required to begin collecting data on August 1, 2013, register with CMS by March 31, 2014 and are required to submit certain reports to CMS disclosing payments and transfers of value made to physicians and teaching hospitals in the preceding calendar year on or before the 90th day of each calendar year. Any failure to provide the required information may result in civil monetary penalties. There are also a number of states that require the establishment of healthcare compliance programs or reporting of certain compensation or benefits provided to healthcare professionals. See “Item 4. Information on the Company - B. Business Overview - Fraud and Abuse Laws - Anti-Kickback Statutes and Federal False Claims Act.”
The implementation of and compliance with the reporting and disclosure obligations of the Physician Payment Sunshine Act, which is part of the Affordable Care Act of 2010, could adversely affect our business.
The implementation of and compliance with the reporting and disclosure obligations of the Physician Payment Sunshine Act, or the Sunshine Act, which is part of the Affordable Care Act of 2010, could adversely affect our business.
The Sunshine Act has imposed new reporting and disclosure requirements for drug and device manufacturers with regard to payments or other transfers of value made to certain practitioners (including physicians, dentists and teaching hospitals), and for such manufacturers and for group purchasing organizations, with regard to certain ownership interests held by physicians in the reporting entity. CMS has been charged with implementing the Sunshine Act and has called it the Open Payment Program. Drug and device manufacturers are required to track and publicly report this information annually to CMS. Implementing regulations for these tracking and reporting obligations were finalized in 2013, and companies are now required to track and report payments made since August 1, 2013. The first disclosure reports were due by March 31, 2014 for the period August 1, 2013 through December 31, 2013. On or before the 90th day of each calendar year, manufacturers covered under the Sunshine Act will be required to submit a report disclosing payments and transfers of value made in the preceding calendar year, and CMS then will publish the reported data on or before June 30 of the reporting year. In addition, medical device companies are also required to report payments to the government on an annual basis. On September 30, 2014, CMS published the first set of data collected under the Sunshine Act.
The final rules implementing the Sunshine Act are complex, ambiguous, and broad in scope. Accordingly, we are required to collect and report detailed information regarding certain financial relationships we have with physicians, dentists and teaching hospitals. It is difficult to predict how the new requirements may impact existing relationships among manufacturers, distributors, physicians, dentists and teaching hospitals. The Sunshine Act preempts similar state reporting laws, although we or our Subsidiaries may be required to continue to report under certain of such state laws. While we believe we have substantially compliant programs, systems and controls in place to comply with the Sunshine Act requirements, and we have already completed our registration with CMS and submitted the 2013 and 2014 reports, our compliance with the new final rule imposes additional costs on us. If we fail to comply with the data collection and reporting obligations imposed by the Sunshine Act, we may be subject to severe penalties, including fines.
Additionally, we have implemented a series of policies and procedures for employees involved in the data collection process, and have systems in place to capture the necessary data. We have also established policies and procedures to ensure that data was reported completely, in the correct format, and on time. Despite these policies and procedures, we cannot assure you that we will collect and report all data accurately and in a timely manner. If we fail to accurately or timely report this information, we could suffer severe penalties, including fines.
If we fail to comply with federal or state anti-kickback laws, we could be subject to criminal and civil penalties, loss of licenses and exclusion from Medicare, Medicaid and other federal and state healthcare programs, which could have a material adverse effect on our business, financial condition and results of operations.
Section 1128B(b) of the Social Security Act, or the SSA, commonly referred to as the “Anti-Kickback Statute,” prohibits the offer, payment, solicitation or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing or arranging for or recommending the ordering, purchasing or leasing of items or services payable by the Medicare and Medicaid programs or any other federally funded healthcare program. The Anti-Kickback Statute is very broad in scope, and many of its provisions have not been uniformly or definitively interpreted by courts or regulations.
We have arrangements with surgeons, hospitals and other entities which may be subject to scrutiny. For example, we have consulting agreements with spine surgeons and neurosurgeons using or considering the use of our present and future Renaissance systems, for assistance in product development, and professional training and education, among other things. Payment for some of these consulting services has been in the form of stock options rather than per hour or per diem amounts that would require verification of time worked. We may continue in the future to make payment for these consulting services in the form of royalties or also possibly in the form of part-time employment. In addition, various agencies may view these arrangements with our customers, including the provision of marketing grants to customers for the purposes of training surgeons and the provision of accessories at no charge or discounted prices with the purchase of our Renaissance system, as not fully complying with federal and state fraud and abuse laws. To the extent we are found to not be in compliance, we could face potentially significant fines and penalties in addition to other more significant sanctions and we may be required to restructure our operations.
Violations of the Anti-Kickback Statute and similar state laws may result in significant fines, imprisonment and exclusion from the Medicare, Medicaid and other federal or state healthcare programs. Such fines and exclusion could have a material adverse effect on our business, financial condition and results of operations. While we believe that our arrangements with physician consultants in product development and product training and education do not violate the law, there can be no assurance that federal or state regulatory authorities will not challenge these arrangements under anti-kickback laws. See “Item 4. Information on the Company B. Business Overview – Fraud and Abuse Laws - Anti-Kickback Statutes and Federal False Claims Act.”
The orthopedic medical device industry is, and in recent years has been, under heightened scrutiny.
The orthopedic medical device industry is, and in recent years has been, under heightened scrutiny as the subject of government investigations and enforcement actions involving manufacturers who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business, specifically including arrangements with physician consultants.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, including the FCPA, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations. Any penalties, damages, fines, exclusions, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of these laws are broad and their provisions are open to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If the surgeons or other providers or entities with which we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on our business.
Risks Relating Primarily to Our Location in Israel
Our headquarters and other significant operations are located in Israel and, therefore, our results may be adversely affected by military instability in Israel.
Our executive offices are located in Israel. In addition, the majority of our officers and directors are residents of Israel. Accordingly, geopolitical and/or military conditions in Israel and its region may directly or indirectly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring countries. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of operations. During the summer of 2014 and in November 2012, Israel was engaged in armed conflicts with a militia group and political party, which controls the Gaza Strip, and during the summer of 2006, Israel was engaged in an armed conflict with Hezbollah, a Lebanese Islamist Shiite militia group and political party. These conflicts involved missile strikes against civilian targets in various parts of northern Israel, including areas in which our employees and consultants are located, and negatively affected business conditions in Israel. An escalation in tension and violence between Israel and the militant Hamas movement (which controls the Gaza Strip) and other Palestinian Arab groups, culminated with Israel’s military campaign in Gaza in December 2008, November 2012 and again in the summer of 2014 in an endeavor to prevent continued rocket attacks against Israel’s southern towns. In addition, Israel faces threats from more distant neighbors, in particular, Iran, an ally of Hezbollah and Hamas. The United States has threatened Syria, another ally of Iran, with military action and there is a risk that as a result of such military confrontation, Israel will be attacked.
Popular uprisings in various countries in the Middle East and North Africa are affecting the political stability of those countries. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and these countries. Furthermore, several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in the region continue or intensify. Such restrictions may seriously limit our ability to sell our products to customers in those countries. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or significant downturns in the economic or financial condition of Israel, could adversely affect our operations and product development, cause our revenues to decrease and adversely affect the share price of publicly traded companies having operations in Israel, such as us. Similarly, Israeli corporations are limited in conducting business with entities from several countries. For example, in 2008, the Israeli legislature provided a law forbidding any investments in entities that transact business with Iran.
Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions, could harm our results of operations and could make it more difficult for us to raise capital. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary in order to meet our business partners face to face.
Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Although the Israeli government is currently committed to covering the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business.
Our operations may be disrupted as a result of the obligation of management or key personnel to perform military service.
Our employees and consultants in Israel, including members of our senior management, may be obligated to perform up to one month, and in some cases longer periods, of annual military reserve duty until they reach the age of 40 (or older, for citizens who hold certain positions in the Israeli armed forces reserves), and, in the event of a military conflict, may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be similar large-scale military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant number of our officers, employees and consultants. Such disruption could materially adversely affect our business and operations.
Exchange rate fluctuations between the U.S. dollar and the NIS currencies may negatively affect our earnings.
We incur expenses both in U.S. dollars and NIS, but our financial statements are denominated in U.S. dollars. As a result, we are exposed to the risks that the NIS may appreciate relative to the U.S. dollar, or the NIS instead devalues relative to the U.S. dollar, and the inflation rate in Israel may exceed such rate of devaluation of the NIS, or that the timing of such devaluation may lag behind inflation in Israel. In any such event, the U.S. dollar cost of our operations in Israel would increase and our U.S. dollar-denominated results of operations would be adversely affected. We cannot predict any future trends in the rate of inflation in Israel or the rate of devaluation (if any) of the NIS against the U.S. dollar.
Our operations also could be adversely affected if we are unable to effectively protect ourselves against currency fluctuations in the future. We engage in short-term currency hedging activities. These measures, however, may not adequately protect us from material adverse effects due to the impact of inflation in Israel and United States or from fluctuations in the relative values of the dollar and foreign currencies in which we transact business, and may result in a financial loss. For further information, see Item 5 “Operating and Financial Review and Prospects” elsewhere in this annual report.
We receive significant tax benefits in Israel that may be reduced or eliminated in the future.
Our investment program in Israel has been granted “Beneficiary Enterprise” status and we are therefore eligible for significant tax benefits under the Israeli Law for the Encouragement of Capital Investments, 1959, or the Investment Law, which was significantly amended by an amendment effective April 1, 2005, or the 2005 Amendment, and further amended by an amendment effective January 1, 2011, or the 2011 Amendment.
For example, once we reach profitability for tax purposes, we will be exempt from corporate tax for a period of two years and will be subject to a reduced corporate tax rate of between 10% and 25% for the remainder of the benefits period, depending on the level of foreign investment in our Company in each year and on the period of when profitability is reached.
In order to remain eligible for the tax benefits of an investment program that is implemented in accordance with the provisions of the Investment Law, referred to as an “Approved Enterprise”, or a “Beneficiary Enterprise”, we must continue to meet certain conditions stipulated in the Investment Law and its regulations. If we do not meet these requirements, we may not be eligible to receive tax benefits and we could be required to refund any tax benefits that we may receive in the future, in whole or in part, with interest. Furthermore, the tax benefits available under the Investment Law may be terminated or reduced in the future. If these tax benefits are terminated, our Israeli taxable income would be subject to regular Israeli corporate tax rates. The standard corporate tax rate for Israeli companies in 2014 and 2015 was 26.5%. See “Item 10. Additional Information - E. Taxation.”
Additionally, if we increase our activities outside of Israel (for example, through acquisitions) our expanded activities might not be eligible for inclusion in future Israeli tax benefit programs. Finally, in the event of a distribution of a dividend from the income that will be tax exempt under the Investment Law, in addition to withholding tax at a rate of 15% (or a reduced rate under an applicable double tax treaty), we will be subject to tax at the corporate tax rate applicable to our Approved Enterprise’s and Beneficiary Enterprise’s income on the amount distributed in accordance with the reduced corporate tax applicable to such profits. See “Item 10. Additional Information - E. Taxation.”
Government authorities may question our tax positions or transfer pricing policies or change their laws in a manner that could increase our effective tax rate or otherwise harm our business.
We conduct operations with our Subsidiaries pursuant to transfer pricing arrangements. Transfer prices are prices that one company in a group of related companies charges to another member of the group for goods, services or the use of property. If two or more affiliated companies are located in different countries, the tax laws or regulations of each country generally will require that transfer prices be the same as those between unrelated companies dealing at arms’ length and that contemporaneous documentation is maintained to support the transfer prices. While we believe we have proper transfer pricing arrangements, our transfer pricing procedures are not binding on applicable tax authorities. Tax laws are continually changing and are subject to the interpretation of government agencies, which from time to time review and audit our business in the jurisdictions in which we conduct business throughout the world. If regulators challenge our tax positions, corporate structure, transfer pricing arrangements or intercompany transfers, we may be subject to fines and payment of back taxes, our effective tax rate may increase and our financial condition, results of operations and cash flow could be materially adversely affected.
In the past, we received Israeli government grants for certain of our research and development activities. The terms of those grants may require us, in addition to payment of royalties, to satisfy specified conditions in order to manufacture products and transfer technologies outside of Israel. We may be required to pay penalties in addition to repayment of the grants.
Our research and development efforts, during the period between 2003 through 2010 were financed in part through royalty-bearing grants, in an amount of $1.3 million that we received from the OCS. With respect to such grants we are committed to pay royalties at a rate of 3% to 3.5% on sales proceeds up to the total amount of grants received, linked to the dollar and bearing interest at an annual rate of LIBOR applicable to dollar deposits. Even though we have repaid in full of these amounts, we will still be required to comply with the requirements of the Israeli Encouragement of Industrial Research and Development Law, 1984, or the R&D Law, and related regulations, with respect to those past grants. When a company develops know-how, technology or products using OCS grants, the terms of these grants and the R&D Law restrict the transfer outside of Israel of such know-how, and the manufacturing or manufacturing rights of such products, technologies or know-how, without the prior approval of the OCS. Therefore, if aspects of our technologies are deemed to have been developed with OCS funding, the discretionary approval of an OCS committee would be required for any transfer to third parties outside of Israel of know how or manufacturing or manufacturing rights related to those aspects of such technologies. Furthermore, the OCS may impose certain conditions on any arrangement under which it permits us to transfer technology or development out of Israel or may not grant such approvals at all.
The transfer of OCS-supported technology or know-how outside of Israel may involve the payment of significant amounts, depending upon the value of the transferred technology or know-how, the amount of OCS support, the time of completion of the OCS-supported research project and other factors. These restrictions and requirements for payment may impair our ability to sell our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. Furthermore, the consideration available to our shareholders in a transaction involving the transfer outside of Israel of technology or know-how developed with OCS funding (such as a merger or similar transaction) may be reduced by any amounts that we are required to pay to the OCS.
Provisions of Israeli law may delay, prevent or otherwise impede a merger with, or an acquisition of, our company, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date on which a merger proposal is filed by each merging company with the Israel Registrar of Companies and at least 30 days have passed from the date on which the shareholders of both merging companies have approved the merger. In addition, a majority of each class of securities of the target company must approve a merger. Moreover, a tender offer for all of a company’s issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion of the tender offer also requires approval of a majority of the offerees that do not have a personal interest in the tender offer, unless, following consummation of the tender offer, the acquirer would hold at least 98% of the company’s outstanding shares. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completion of the tender offer, petition an Israeli court to alter the consideration for the acquisition, unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek such appraisal rights.
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to a shareholder whose country of residence does not have a tax treaty with Israel exempting such shareholder from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred.
These and other similar provisions could delay, prevent or impede an acquisition of us or our merger with another company, even if such an acquisition or merger would be beneficial to us or to our shareholders.
It may be difficult to enforce a judgment of a U.S. court against us and our officers and directors and the Israeli experts named in this annual report in Israel or the U.S., to assert United States securities laws claims in Israel or to serve process on our officers and directors and these experts.
We were incorporated in Israel. Substantially all of our executive officers and directors currently reside outside of the United States, and all of our assets and most of the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not necessarily be enforced by an Israeli court. It also may be difficult to affect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law often involves the testimony of expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, it may be impossible to collect any damages awarded by either a U.S. or foreign court.
The rights and responsibilities of a shareholder will be governed by Israeli law which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
The rights and responsibilities of the holders of our Ordinary Shares are governed by our articles of association and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders typical corporations incorporated in the United States. In particular, a shareholder of an Israeli company has certain duties to act in good faith and fairness towards the company and other shareholders, and to refrain from abusing its power in the company, including, among other things, in voting at the general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval. In addition, a shareholder who is aware that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company. There is limited case law available to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our Ordinary Shares that are not typically imposed on shareholders of U.S. corporations.
Risks Related to an Investment in Our Shares and ADSs
We may be a “passive foreign investment company”, or PFIC, for U.S. federal income tax purposes in the current taxable year or may become one in any subsequent taxable year. There generally would be negative tax consequences for U.S. taxpayers that are holders of our Ordinary Shares or ADSs if we are or were to become a PFIC.
We will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (1) at least 75% of our gross income is “passive income” or (2) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account. We believe that we will not be a PFIC for our current taxable year and do not expect to become a PFIC in the foreseeable future. The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our Ordinary Shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC in the future. If we are a PFIC in any taxable year during which a U.S. taxpayer holds our Ordinary Shares or ADSs, such U.S. taxpayer would be subject to certain adverse U.S. federal income tax rules. In particular, if the U.S. taxpayer did not make an election to treat us as a “qualified electing fund,” or QEF, or make a “mark-to-market” election, then “excess distributions” to the U.S. taxpayer, and any gain realized on the sale or other disposition of our Ordinary Shares or ADSs by the U.S. taxpayer: (1) would be allocated ratably over the U.S. taxpayer’s holding period for the Ordinary Shares (or ADSs, as the case may be); (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, if the IRS determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. taxpayer to make a timely QEF or mark-to-market election. U.S. taxpayers that have held our Ordinary Shares or ADSs during a period when we were a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to exceptions for U.S. taxpayer who made a timely QEF or mark-to-market election. A U.S. taxpayer can make a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. Although we have no obligation to do so, we intend to notify U.S. taxpayers that hold our Ordinary Shares or ADSs if we believe we will be treated as a PFIC for any taxable year in order to enable U.S. taxpayers to consider whether to make a QEF election. In addition, we intend to furnish such U.S. taxpayers annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our Subsidiaries are a PFIC. U.S. taxpayers that hold our Ordinary Shares or ADSs are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner, and consequences to them of making a QEF or mark-to-market election with respect to our Ordinary Shares or ADSs in the event that we are a PFIC. See “Item 10. Additional Information - E. Taxation - U.S. Federal Income Tax Considerations” for additional information.
The market prices of our Ordinary Shares and ADSs are subject to fluctuation, which could result in substantial losses by our investors.
The stock market in general and the market prices of our Ordinary Shares on the TASE and our ADSs on NASDAQ, in particular, are subject to fluctuation, and changes in these prices may be unrelated to our operating performance. The market price of our Ordinary Shares and ADSs are subject to a number of factors, including:
|
|
●
|
announcements of technological innovations or new products by us or others;
|
|
|
●
|
announcements by us of significant acquisitions, strategic partnerships, in-licensing, out-licensing, joint ventures or capital commitments;
|
|
|
●
|
expiration or terminations of licenses, research contracts or other collaboration agreements;
|
|
|
●
|
public concern as to the safety of our equipment we sell;
|
|
|
●
|
general market conditions;
|
|
|
●
|
the volatility of market prices for shares of medical devices companies generally;
|
|
|
●
|
success or failure of research and development projects;
|
|
|
●
|
departure of key personnel;
|
|
|
●
|
developments concerning intellectual property rights;
|
|
|
●
|
developments concerning regulatory approvals;
|
|
|
●
|
developments concerning standard-of-care in spine surgeries;
|
|
|
●
|
variations in our and our competitors’ results of operations;
|
|
|
●
|
changes in revenues, gross profits and earnings announced by the Company;
|
|
|
●
|
changes in estimates or recommendations by securities analysts, if our Ordinary Shares or the ADSs are covered by analysts;
|
|
|
●
|
changes in government regulations or patent decisions; and
|
|
|
●
|
general market conditions and other factors, including factors unrelated to our operating performance.
|
These factors may materially and adversely affect the market price of our Ordinary Shares and the ADSs and result in substantial losses by our investors.
We do not know whether a market for our ADSs will be sustained or what the trading price of our ADSs will be and as a result it may be difficult for you to sell your ADSs.
Although our ADSs trade on NASDAQ, an active trading market for our ADSs may not be sustained. It may be difficult for you to sell your ADSs without depressing the market price for the ADSs or at all. As a result of these and other factors, you may not be able to sell your ADSs at or above your purchase price or at all. Further, an inactive market may also impair our ability to raise capital by selling ADSs and Ordinary Shares and may impair our ability to enter into strategic partnerships or acquire companies or products by using our Ordinary Shares as consideration.
Future sales of our Ordinary Shares or ADSs could reduce the market price of our Ordinary Shares and ADSs.
Substantial sales of our Ordinary Shares or ADSs, either on the TASE or on NASDAQ may cause the market price of our Ordinary Shares or ADSs to decline. All of our outstanding Ordinary Shares are registered and available for sale in Israel. Sales by us or our security holders of substantial amounts of our Ordinary Shares or ADSs, or the perception that these sales may occur in the future, could cause a reduction in the market price of our Ordinary Shares or ADSs.
The issuance of any additional Ordinary Shares, any additional ADSs, or any securities that are exercisable for or convertible into our Ordinary Shares or ADSs, may have an adverse effect on the market price of our Ordinary Shares and ADSs and will have a dilutive effect on our existing shareholders and holders of ADSs.
We do not intend to pay any cash dividends on our Ordinary Shares in the foreseeable future and, therefore, any return on your investment in our Ordinary Shares or ADSs must come from increases in the value and trading price of our Ordinary Shares and ADSs.
We have never declared or paid cash dividends on our Ordinary Shares and do not anticipate that we will pay any cash dividends on our Ordinary Shares in the foreseeable future; therefore, any return on your investment in our Ordinary Shares or ADSs must come from increases in the value and trading price of our Ordinary Shares and ADSs.
We intend to retain our earnings to finance the development and expenses of our business. Any future determination relating to our dividend policy will be at the discretion of our board of directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, applicable Israeli law and other factors our board of directors may deem relevant.
You may not receive the same distributions or dividends as those we make to the holders of our Ordinary Shares, and, in some limited circumstances, you may not receive dividends or other distributions on our Ordinary Shares and you may not receive any value for them, if it is illegal or impractical to make them available to you.
The depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities underlying the ADSs, after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act, but that are not properly registered or distributed under an applicable exemption from registration. In addition, conversion into U.S. dollars from foreign currency that was part of a dividend made in respect of deposited Ordinary Shares may require the approval or license of, or a filing with, any government or agency thereof, which may be unobtainable. In these cases, the depositary may determine not to distribute such property and hold it as “deposited securities” or may seek to effect a substitute dividend or distribution, including net cash proceeds from the sale of the dividends that the depositary deems an equitable and practicable substitute. We have no obligation to register under U.S. securities laws any ADSs, Ordinary Shares, rights or other securities received through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, Ordinary Shares, rights or anything else to holders of ADSs. In addition, the depositary may withhold from such dividends or distributions its fees and an amount on account of taxes or other governmental charges to the extent the depositary believes it is required to make such withholding. This means that you may not receive the same distributions or dividends as those we make to the holders of our Ordinary Shares, and, in some limited circumstances, you may not receive any value for such distributions or dividends if it is illegal or impractical for us to make them available to you. These restrictions may cause a material decline in the value of the ADSs.
Holders of ADSs must act through the depositary to exercise their rights as shareholders of our company.
Holders of our ADSs do not have the same rights of our shareholders and may only exercise the voting rights with respect to the underlying Ordinary Shares in accordance with the provisions of the deposit agreement for the ADSs. Under Israeli law, the minimum notice period required to convene a shareholders meeting is no less than 35 or 21 calendar days, depending on the proposals on the agenda for the shareholders meeting. When a shareholder meeting is convened, holders of our ADSs may not receive sufficient notice of a shareholders’ meeting to permit them to withdraw their Ordinary Shares to allow them to cast their vote with respect to any specific matter. In addition, the depositary and its agents may not be able to send voting instructions to holders of our ADSs or carry out their voting instructions in a timely manner. We will make all reasonable efforts to cause the depositary to extend voting rights to holders of our ADSs in a timely manner, but we cannot assure holders that they will receive the voting materials in time to ensure that they can instruct the depositary to vote their ADSs. Furthermore, the depositary and its agents will not be responsible for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result, holders of our ADSs may not be able to exercise their right to vote and they may lack recourse if their ADSs are not voted as they requested. In addition, in the capacity as a holder of ADSs, they will not be able to call a shareholders’ meeting.
Raising additional capital by issuing securities may cause dilution to existing shareholders.
We may need to raise substantial future capital to continue to complete commercialization of our products and the research and development and clinical and regulatory activities necessary to develop new products. Our future capital requirements will depend on many factors, including:
|
|
●
|
the revenue generated by sales of our current and future products;
|
|
|
●
|
our ability to manage our inventory;
|
|
|
●
|
the expenses we incur in selling and marketing our products and supporting our growth;
|
|
|
●
|
the costs and timing of regulatory clearance or approvals for new products or upgrades or changes to our current products;
|
|
|
●
|
the rate of progress, cost, and success or failure of on-going development activities;
|
|
|
●
|
the emergence of competing or complementary technological developments;
|
|
|
●
|
the costs of filing, prosecuting, defending and enforcing any patent or license claims and other intellectual property rights, or participating in litigation related activities;
|
|
|
●
|
the terms and timing of any collaborative, licensing, or other arrangements that we may establish;
|
|
|
●
|
the acquisition of businesses, products and technologies; and
|
|
|
●
|
general economic conditions and interest rates, including the continuing weak conditions.
|
If we raise additional funds by issuing equity or convertible debt securities, we will reduce the percentage ownership of our then-existing shareholders, and these securities may have rights, preferences or privileges senior to those of our existing shareholders.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company for up to five years from our initial public offering in the United States which occurred in 2013. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved, to the extent applicable. In this annual report, we have included certain information about executive compensation related information that is not required by an emerging growth company. We cannot predict whether investors will find our Ordinary Shares or ADSs less attractive if we rely on these exemptions. If some investors find our Ordinary Shares or ADSs less attractive as a result, there may be a less active trading market for our Ordinary Shares or the ADSs and the price of our Ordinary Shares or the ADSs may be more volatile.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We chose to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period is irrevocable.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our Ordinary Shares and ADSs depends on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us, or provide favorable coverage. If one or more analysts downgrade our stock or change their opinion of our Ordinary Shares and ADSs, the price of our Ordinary Shares and ADSs would likely decline. In addition, if one or more analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Risks Associated with the NASDAQ Listing of the ADSs
Our Ordinary Shares and ADSs are traded on different markets and this may result in price variations.
Our Ordinary Shares have been traded on the TASE since August 2007. The ADSs have been traded on the NASDAQ Capital Market since May 2013 and are currently traded on the NASDAQ Global Market. Trading in those securities on those markets takes place in different currencies (dollars on NASDAQ and NIS on the TASE), and at different times (resulting from different time zones, different trading days and different public holidays in the United States and Israel). The trading prices of our securities on these two markets may differ due to these and other factors. Any decrease in the price of our securities on one of these markets could cause a decrease in the trading price of our securities on the other market.
We incur costs as a result of our ADSs trading on NASDAQ, and our management is required to devote substantial time to compliance initiatives and reporting requirements.
As a public company in the United States, we incur significant accounting, legal and other expenses as a result of the trading of the ADSs on NASDAQ. These include costs associated with corporate governance requirements of the SEC and NASDAQ rules, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act. These rules and regulations generate legal and financial compliance costs, investor relations costs, stock exchange listing fees and shareholder reporting costs, and made some activities more time consuming and costly. Any future changes in the laws and regulations affecting public companies in the United States and Israel, including Section 404 and other provisions of the Sarbanes-Oxley Act, the rules and regulations adopted by the SEC and the rules of NASDAQ, as well as applicable Israeli reporting requirements, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
As a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of applicable SEC and NASDAQ requirements, which may result in less protection than is accorded to investors under rules applicable to domestic issuers.
As a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under the rules of NASDAQ for domestic issuers. For instance, we follow home country practice in Israel with regard to, among other things, composition of the board of directors, director nomination procedures, approval of compensation of officers, and quorum at shareholders’ meetings. In addition, we follow our home country law, instead of the rules of NASDAQ which require that we obtain shareholder approval, for certain dilutive events, such as for the establishment or amendment of certain equity based compensation plans, an issuance that will result in a change of control of the Company, certain transactions other than a public offering involving issuances of a 20% or more interest in the Company and certain acquisitions of the stock or assets of another company. Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on NASDAQ may provide less protection than is accorded to investors under the rules of NASDAQ applicable to domestic issuers.
In addition, as a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act, related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as domestic companies whose securities are registered under the Exchange Act.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
We are a “foreign private issuer,” as such term is defined in Rule 405 under the Securities Act, and therefore, we are not required to comply with all the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. Under Rule 405, the determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter and, accordingly, the next determination will be made with respect to us on June 30, 2016.
In the future, we would lose our foreign private issuer status if a majority of our shareholders, directors or management is comprised of U.S. citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. Although we have elected to comply with certain U.S. regulatory provisions, our loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC which are more detailed, more extensive and are subject to more demanding deadlines than the forms available to a foreign private issuer. For example, the annual report on Form 10-K requires domestic issuers to disclose executive compensation information on an individual basis with specific disclosure regarding the domestic compensation philosophy, objectives, annual total compensation (base salary, bonus, equity compensation) and potential payments in connection with change in control, retirement, death or disability, while the SEC forms applicable to foreign private issuers permit them to disclose compensation information on an aggregate basis if executive compensation disclosure on an individual basis is not required or otherwise has not been provided in the issuer’s home jurisdiction. We disclose individual compensation information, but this disclosure is not as comprehensive as that required of U.S. domestic issuers since we are not required to disclose more detailed information in Israel. We intend to continue this practice as long as it is permitted under the SEC’s rules and Israel’s rules do not require more detailed disclosure. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. We may also be required to modify certain of our policies to comply with good governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on NASDAQ that are available to foreign private issuers.
If we are unable to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act, or our internal control over financial reporting is not effective, the reliability of our financial statements may be questioned and the price of our Ordinary Shares and the ADSs may suffer.
Section 404 of the Sarbanes-Oxley Act requires a company subject to the reporting requirements of the U.S. securities laws to do a comprehensive evaluation of its and its subsidiaries’ internal control over financial reporting. To comply with this statute, we will be required to document and test our internal control procedures; our management will be required to assess and issue a report concerning our internal control over financial reporting. In addition, our independent registered public accounting firm may be required to issue an opinion on the effectiveness of our internal control over financial reporting at a later date.
The continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. Furthermore, as our business continues to grow both domestically and internationally, our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of its testing, our management may identify material weaknesses or significant deficiencies, which may not be remedied in a timely manner. If our management cannot favorably assess the effectiveness of our internal control over financial reporting, or if our independent registered public accounting firm identifies material weaknesses in our internal control, investor confidence in our financial results may weaken, and the market price of our securities may suffer.
|
|
INFORMATION ON THE COMPANY
|
|
A.
|
History and Development of the Company
|
Our legal and commercial name is Mazor Robotics Ltd. We were incorporated in the State of Israel on September 12, 2000. In July 2003, we changed our name to Mazor Surgical Technologies Ltd., and in 2010 we changed our name back to Mazor Robotics Ltd. In August 2007, we completed our initial public offering in Israel, and our ordinary shares have since been traded on the TASE, under the symbol “MZOR.” In May 2013, ADSs representing our Ordinary Shares commenced trading on the NASDAQ Capital Market under the trading symbol “MZOR” and are currently traded on the NASDAQ Global Market. Each ADS represents two of our Ordinary Shares.
We are a public limited liability company and operate under the provisions of the Companies Law. Our registered office and principal place of business are located at 7 Haeshel St., Caesarea Industrial Park South 3088900, Israel. Our telephone number in Israel is +972-4-618-7100. Our website address is www.mazorrobotics.com. The information contained on our website or available through our website is not incorporated by reference into and should not be considered a part of this annual report and the reference to our website in this annual report is an inactive textual reference only.
In August 2004, we formed a wholly owned subsidiary in the State of Delaware under the name Mazor Surgical Technologies Inc. In October 2010, the U.S. Subsidiary changed its name to Mazor Robotics Inc. The U.S. Subsidiary has been appointed as our agent in the United States, and its registered office is located at 2711 Centerville Rd., Suite 400, Wilmington, New Castle, DE 19808.
In August 2014, we formed a wholly owned subsidiary in Singapore under the name Mazor Robotics Pte. Ltd and its registered office is located at 10 Anson Road, #26-04 International Plaza, Singapore 079903. This Singaporean subsidiary has been established to accommodate the clinical service activities we provide to Renaissance sites in the Asia-Pacific region.
We engage in the development, production, marketing and servicing of innovative medical devices for supporting surgical procedures in the field of orthopedics and neurosurgery. We are a leading innovator in spine and brain surgery and pioneered cutting-edge guidance systems and complementary products in the spine and brain surgery market. These products provide a safer surgical environment for patients, surgeons and operating room staff.
We operate in the field of computer assisted surgery (also known as CAS) that enables the use of surgical instruments with high precision and minimal invasiveness and that contributes to the safety of a wide range of surgical procedures. Our flagship product, the Renaissance system, is transforming spine surgery from freehand procedures to highly accurate, state-of-the-art, guided procedures that raise the standard of care with better clinical results. Our Renaissance system has been used to perform over 16,000 procedures worldwide (with over 98,000 implants placed in those procedures) in a wide variety of spinal procedures, many of which would not have been attempted without this technology. We are continuing the development of the Renaissance platform for additional spine and brain surgery applications.
Principal Capital Expenditures
We had capital expenditures of approximately $702,000 in 2015, $503,000 in 2014 and $343,000 in 2013. Our capital expenditures consisted mainly of machinery and equipment and computers. We have financed our capital expenditures from our available cash and short term investment and equity offerings, and expect to continue to finance our capital expenditures in a similar manner in 2016. We expect our capital expenditures in 2016 will be over $1.6 million, and will include amounts expended towards manufacturing infrastructure.
We are a medical device company developing and marketing innovative surgical guidance systems and complementary products. Our expertise is computerized and imaging-based systems, primarily in the field of spine surgery. Our Renaissance Surgical Guidance System, enables surgeons to advance from freehand surgical procedures to accurate, state-of-the-art, precision guided procedures. Our Renaissance system is used in multiple types of spine surgeries, whether open or minimally invasive, for a variety of clinical indications. Our Renaissance system and its predecessor have been used in over 16,000 spine surgeries, including fusion, correction of spinal deformities, biopsy collection, tumor excision and cement augmentations. Our Renaissance system has the ability to improve clinical outcomes for patients, provide a safer surgical environment for surgeons and operating room staff by possible reduction of exposure to radiation.
The key elements of the Renaissance system include our RBT Device, which is a portable, computer-controlled Stewart platform that spatially positions and orients surgical tools, our Renaissance Work Station, which is a mobile workstation that houses our proprietary software, and several mounting platforms we have designed to serve as an interface between the patient and the RBT Device. Our Renaissance system enables surgeons to perform procedures with a higher degree of accuracy and precision compared to the current freehand standard of care. A pre-operative plan for each patient is developed by the surgeon using our proprietary software based on a standard three-dimensional, or 3D, computed tomography, or CT, image. The surgeon performs the procedure using surgical tools attached to the RBT Device and is guided by the RBT Device to a precise location and trajectory along the spine or in the brain in accordance with the pre-operative plan. At the beginning of the surgical procedure, an automatic 3D synchronization process independently registers the location of the system relative to the position of the patient’s spine or in his brain and the pre-operative plan. Unlike conventional robotic surgery, where the robot performs the procedure guided by the surgeon, the Renaissance system guides the surgeon who performs the procedure in accordance with the pre-operative plan.
The Renaissance system is FDA–cleared, CE–marked and has regulatory clearances in several other markets, including China, Taiwan, South Korea, Canada, Russia, India, Singapore, Israel and Australia. Mazor Robotics’ products are currently active in 13 countries, with 13 distributors representing us in 21 countries. We recently received 510(k) clearance from the FDA for the Renaissance X system, a future product which is in a development stage. The Renaissance X system is one of several of our research and development programs. Prior to any commercialization efforts, the Renaissance X system will require additional technology and clinical development.
Industry Overview - Spine
Spine Disorder Market Overview
Spine disorders are a leading driver of healthcare costs worldwide. Spinal disorders also are a leading cause of disability among people aged between 19 and 45 in the United States, and are the most common cause of job-related disability. Spine disorders afflict women and men equally and are the second most common neurological ailment in the United States - only headaches are more common. In the United States, according to the Orthopedic Network News, there are approximately 1.34 million spinal operations performed annually.
We believe the spine disorder market will continue to grow as a result of a growing, aging and more active population and rising obesity rates, which all are expected to be key drivers in the continued growth of incidence of spine disorders. The U.S. Census Bureau projects that the 65 and older age group will double from 43.1 million in 2012 to 83.7 million in 2050. In addition, improvements in healthcare have led to increasing life expectancies worldwide and the opportunity to lead more active lifestyles at advanced ages. These trends are expected to generate increased demand for spine surgeries.
Overview of Spine Disorders
Spine disorders range in severity, causing symptoms ranging from mild pain and loss of feeling to extreme pain and paralysis. These disorders are primarily caused by degenerative disc diseases, stenosis, deformity, osteoporosis, tumors and trauma.
|
|
●
|
Degenerative disc disease describes the most common type of spine disorder which primarily results from repetitive stresses experienced during the normal aging process. Disc degeneration occurs as the outer layer starts to shear and the inner cores of intervertebral discs lose elasticity and shrink. Over time, these changes can cause the discs to lose their normal height and shock-absorbing characteristics, which leads to back pain and reduced flexibility. Herniated discs are a common form of degenerative disc disease.
|
|
|
●
|
Lumbar stenosis is a condition whereby either the spinal canal or vertebral foramen becomes narrowed in the lower back impinging the nerves in the lumbar spine. This condition is often caused by the degenerative processes in the spine and the resulting compression can lead to back and leg pain. If the narrowing is substantial, it causes compression of the nerves and the painful symptoms of lumbar spinal stenosis.
|
|
|
●
|
Spine deformity is a term used to describe any variation in the natural curvature of the spine. Natural curves help the upper body maintain proper balance and alignment over the pelvis. Common forms of deformity include scoliosis, which is a lateral or side-to-side curvature of the spine, and kyphosis, which is an abnormal concave curvature leading to a rounded (humped) back.
|
|
|
●
|
Vertebral compression fractures are fractures of the vertebrae that result in the collapse of the vertebral body. These fractures, which can be very painful to the patient, are often the result of osteoporosis, which causes the vertebrae to weaken and become brittle, or spine tumors, but can also result from trauma.
|
|
|
●
|
Primary spine tumors are relatively rare. Benign tumors are typically removed surgically while malignant tumors are more difficult to treat and are often metastases which originate from tumors in other organs.
|
Current Treatments for Spine Disorders
Treatment alternatives for spine disorders range from non-operative conservative therapies to surgical interventions. Conservative therapies include bed rest, medication and physical therapy. Surgical treatments for spine disorders can be instrumented, which include the use of implants, or non-instrumented, which forego the use of any such implants. The most common instrumented treatment is spinal fusion, where two or more adjacent vertebrae are fused together with implants to restore disc height and provide stability.
Introduction of Minimally Invasive Surgery
Over the past 30 years, minimally invasive surgical techniques have transformed many surgical procedures. Compared to traditional open surgical techniques, minimally invasive techniques potentially offer benefits for patients, surgeons and hospitals. For patients, these techniques can result in significantly reduced trauma, risk of infections, faster convalescence and better aesthetic outcomes. For the surgeon, these techniques can reduce procedure-related complications and have the potential to reduce risks associated with more invasive procedures. For the hospital, these procedures can result in reduced hospital stays due to faster recovery times, lower rates of complications and a higher level of patient satisfaction.
Despite the potential benefits of minimally invasive spinal surgery techniques, they can also present several notable limitations, including the need for additional training for the surgeon, increased intraoperative use of X-ray radiation, and longer operations, and have been shown in some studies to lower the accuracy of implant placement. As a result, while minimally invasive approaches have seen substantial adoption in various surgical fields where procedures can be performed within existing anatomical cavities, they are currently used in only 10-15% of spinal fusion procedures which are currently performed in a minimally invasive approach, according to the SRS database (Hamilton et al. Spine 2011) and the Orthopedic Network News report from October 2015.
Robot Assisted Surgery
We believe that the application of robotics technologies in minimally invasive surgical procedures represents the next generation in the evolution of the surgical technique. These technologies are being developed to provide surgeons with a more precise, repeatable and controlled ability to perform complex procedures. With the assistance of robotic technology, an increasing number of surgeons have been able to perform procedures previously limited to a small subset of highly-skilled surgeons. In addition, robotic technology has enabled these procedures to be performed in a more minimally invasive manner, requiring only small incisions, which result in reduced procedure related trauma, fewer infections and post-procedure complications, and reduced recovery and hospitalization times.
The Limitations of Current Spine Procedures
Although minimally invasive techniques have been widely adopted in many fields of surgery, they have had limited adoption in spine surgery. We believe that the principal barriers to the adoption of minimally invasive techniques for spine surgery are:
|
|
●
|
restricted or even no line-of-sight at the anatomical site;
|
|
|
●
|
cumbersome handling of surgical instruments, limiting the procedure;
|
|
|
●
|
dependence on two-dimensional imaging for three-dimensional surroundings; and
|
|
|
●
|
intra-operative exposure to radiation.
|
As a result, the majority of spine surgeries are performed freehand. According to a review of over 108,000 cases (Hamilton et al., Spine 2011) only 13.2% of spine surgeries are performed in a minimally invasive manner. This was echoed in a report by the Orthopedic Research Network reporting that cannulated pedicle screws (designed and used for minimally invasive spine surgeries) have hovered between 9-15% since 2008. Although freehand surgery allows for direct visualization of the anatomy, open freehand surgeries may result in:
|
|
●
|
increased procedure-related blood loss, pain and scarring at the incision site;
|
|
|
●
|
increased likelihood of complications, such as infections;
|
|
|
●
|
slower recovery times and longer post-operative hospital stays; and
|
|
|
●
|
undesirable aesthetic outcomes.
|
Industry Overview - Brain
Neurosurgical Market Overview
It is estimated that 50 million Americans suffer from neurological illnesses, at an annual cost of over $450 billion in direct and indirect costs. Only a fraction of them are candidates for neurosurgical treatments, and in 2010, 585,700 direct (open) intra-cranial neurosurgical procedures were performed, of which over half were shunt placements.
It is forecasted that the volume of intracranial neurosurgical procedures will continue to grow at about 1.2% per year, mainly dependent on demographic trends. This statistic does not take into account changes in indications for surgeries and new treatment options. New indications may increase the market potential, but new, less-invasive, treatment options may decrease the market potential of open neurosurgical treatments. Costs of procedures are expected to grow, driven by more sophisticated technologies and treatment options, and in 2011 it was forecasted that the market would grow at a 2.7% annual growth rate from the estimated $577 million spent in 2010.
Overview of Brain Biopsies
The incidence of primary brain tumors for 2013 is estimated by the American Brain Tumor Association at almost 70,000 cases. Of these cases, almost 25,000 cases are malignancies and over 45,000 are benign. The majority of brain tumors are metastases from malignancies in other organs (mainly lung and breast), but statistics for brain metastases are not readily available. Therefore, the incidence of primary and secondary brain tumors is estimated at more than 140,000 cases annually.
In some of the cases, the CT- or Magnetic Resonance Imaging (MRI) generated images are insufficient for the determination of the appropriate treatment option. In such cases a biopsy is usually indicated. It is estimated by MedTech Insight that in 2010, 19,700 biopsies were performed and that the incidence of this procedure is slightly declining at about 1.4% annual rate.
Overview of Deep Brain Stimulation (DBS) Electrode Placement Surgeries
The FDA approved DBS as a treatment for essential tremor in 1997, later adding further indications, including Parkinson’s Disease (2002), dystonia (2003) and Obsessive Compulsive Disorder (OCD) (2009). Several other indications are in various phases of research, like chronic pain, various affective disorders, including major depression, and other neurological disorders, mainly in severe cases and/or refractory to medication or other treatments. It was estimated by MedTech Insight, 2011, that 7,900 DBS procedures were performed with an expected 5.1% annual growth rate. The most common indication was movement disorders, mainly Parkinson’s disease which has an annual incidence estimated between 50,000-60,000 cases.
Current Neurosurgical Options
Treatment options for neurological illnesses range widely by diagnosis and disease state from “watchful waiting” to non-operative conservative therapies (e.g., medications), External Beam Radiation Therapy, and a number of surgical interventions.
When neurosurgical procedures are indicated, much care is taken to avoid damage to neighboring regions of the brain and the vascular system, as well as along the surgical pathway to the lesion. Careful planning of the surgical approach is based on advanced imaging modalities. Execution of the required precise spatial localization according to the surgical plan is performed using intra-operative guidance systems, which are generally categorized as either frame-based or frameless systems. Frame-based systems, or standard stereotaxy, are considered a more accurate option but are uncomfortable for the patient. Frameless trackable/fiducial marker-based systems use image guided navigation or patient-specific, custom-made mounts to improve accuracy.
The clinical benefits of Image Guided Surgery (IGS) include:
|
|
●
|
allowing for less invasive surgical approaches;
|
|
|
●
|
enhanced ability to execute the surgical plan;
|
|
|
●
|
precision in lesion localization; and
|
|
|
●
|
reduced risk of damage to adjacent vital structures.
|
According to MedTech Insight in 2011, the U.S. market for computer-assisted IGS intraoperative navigation systems (including hardware and software) was approximately $273.8 million in 2010, of which cranial/neurosurgery-attributable revenues were estimated at $76.7 million, with an estimated compound annual growth rate of 3.5%, reaching an estimated $91.0 million in 2015, reflecting the maturity and saturation of this market segment.
Of the 19,700 biopsies performed in 2010, about 17,000 were performed with a frame-based system and about 2,700 used a frameless system. It was estimated in 2011 that by 2015 frameless systems will be used more frequently in these procedures, from 13.7% of the cases to over 20%.
Of the 7,900 DBS procedures in 2010, about 5,200 were frameless procedures and 2,700 were frame-based. It was estimated in 2011 that by 2015 frameless systems will be used more frequently in these procedures, from 66% of the cases to over 75% by 2015.
A new market segment in brain surgery is robotic neurosurgical systems. While a few product options are available, these apparently account for a minor number of procedures.
The Limitations of Current Neurosurgical Procedures
Frame-based systems limit the surgeon’s movement and are difficult to redirect intra-operatively. The rigid frames are cumbersome for the patients and the complex set-up can make operating times longer.
Navigation based systems (e.g. Brainlab AG’s VectorVision) depend on direct line of sight between an infra-red camera and specialized, reflective markers. These systems are considered to be less accurate by surgeons than frame-based systems. Their online representation of spatial location in real-time does not represent the actual location of the surgical instruments but rather the system’s perception of the location of the instruments. The representation of the instruments in 3 planes can lengthen the learning curve of these systems as the surgeon needs to correct the position in a single plain in a three dimensional world.
Current robotic systems (e.g. Medtech’s ROSATM) depend on either frame-based or navigation-based systems.
The Mazor Robotics Solution
Our Renaissance system enables surgeons to advance from freehand surgical procedures to accurate, state-of-the-art, precision guided procedures. It has the ability to improve clinical outcomes for patients, provide a safer surgical environment for surgeons and operating room staff by possibly reducing exposure to radiation, and deliver economic value to hospitals and payors. We believe our Renaissance system offers the following benefits to patients, surgeons and hospitals:
Reproducible Precision and Accuracy. The Renaissance system significantly increases the level of instrument placement accuracy over freehand surgery. A 14-center study involving 635 patients published in Spine demonstrated 98.3% placement accuracy of the spinal implants guided with our technology. While the scientific literature on placement accuracy varies, by comparison a meta-analysis of 12,299 thoracolumbar screws, published in Spine, demonstrated 90.3% placement accuracy in freehand surgeries.
Use in a Variety of Procedures. While the Renaissance system is often used for conventional or routine spinal surgeries, the system is particularly advantageous in complex spinal procedures, such as the correction of scoliosis and other spinal deformities, long fusions and repeat/revision surgery. Precision and planning is of particular importance in complex procedures where accuracy and precision are a challenge for even the most experienced surgeons.
Possible Reduced Exposure to Radiation. Spine surgeries, particularly minimally invasive surgeries, require the use of high levels of X-ray imaging, and exposes surgeons and patients to harmful radiation. The use of our Renaissance system may significantly reduce the need for X-ray imaging during the surgery and provides for a safer overall surgical environment.
Ease of Use. The Renaissance system leverages and complements the surgical skills and techniques already familiar to the surgeon. This familiarity in approach combined with greater accuracy and precision accelerates the learning curve, making it usable by surgeons with a broad range of training and skills.
Reduced Costs. We believe the use of the Renaissance system results in shorter hospital stays due to faster recovery times, lower rates of complications and a higher level of patient satisfaction.
Clinical Differentiation. We believe the benefits mentioned above will help surgeons and hospitals differentiate themselves, attracting more patients to seek medical care from them, over competitors offering less innovative and precise alternatives.
Our Strategy
Our goal is to continue to drive sales of our Renaissance system and generate recurring revenues through sales of disposable products and service contracts by establishing our Renaissance system as the standard-of-care in the eyes of surgeons, patients and medical facilities. We believe that we can achieve this objective by working with hospitals to demonstrate the key benefits of our Renaissance system. Our strategy includes the following key elements:
|
|
●
|
Continue to commercialize our Renaissance system. We intend to continue to focus on commercializing our Renaissance system by expanding our sales and marketing infrastructure in the United States and our global distribution footprint. We currently maintain a presence in more than 100 hospitals in 15 countries, including over 50 Renaissance systems installed in the United States. Within the United States alone there are over 2,000 hospitals and surgical centers that we believe could benefit from our Renaissance system, creating significant opportunity for us to expand our presence and accelerate our revenue growth.
|
|
|
●
|
Drive utilization of our installed base of Renaissance systems. Following the initial installation of the Renaissance system at a given hospital, we take steps to expand the number of surgeons who use our system and work with the hospitals and their surgeons to promote patient education on the benefits of the Renaissance system. Increased usage of our installed Renaissance systems through surgeon education and training will accelerate our recurring revenues through increased sales of our disposable products. We also intend product offerings to provide end to end solutions that address more steps in Spine surgical procedures, to increase our portfolio of disposable and ancillary product offerings, and to promote the use of the Renaissance system for brain applications.
|
|
|
●
|
Demonstrate the clinical and financial value proposition of our Renaissance system. Following our overall penetration strategy, we installed systems at leading academic centers and entered new U.S. metropolitan area markets. We intend to collaborate with leading surgeons and early-adopting hospitals to build additional data that supports the clinical and financial benefits of our Renaissance system. We intend to demonstrate that using our Renaissance system promotes and differentiates surgeons and hospitals as leaders for the treatment of spine disorders, while demonstrating to hospitals the financial benefits of our Renaissance system.
|
|
|
●
|
Invest in research and development. We will continue to make significant investments in research and development including investments to upgrade the hardware and software components of our Renaissance system and to develop additional applications using our proprietary technologies and develop future products.
|
|
|
●
|
Explore new ways to accelerate adoption of our products. We intend to achieve this by striving for partnerships with strategic players and to gain the benefits associated with the synergy between Mazor and potential partners.
|
Our Products
Components of the Renaissance system
RBT Device. Our RBT Device is a portable, computer-controlled Stewart platform that spatially positions and orients surgical tools intra-operatively in accordance with the planned surgical blueprint. All RBT Device movements are a result of the pre-operative plan and are monitored by a closed-loop control process.
Renaissance Workstation. The RBT Device is housed in our Renaissance Workstation, a mobile workstation that houses our proprietary software which also contains the controllers for the RBT Device, image processing unit, electronics, computer and graphical user interface software. It is equipped with a control panel, including a multi-touch screen monitor. The Renaissance Workstation is used both for pre-operative planning of the procedure, as well as for intra-operative control of the system to implement the pre-operative plan.
Mounting platforms. There are several different mounting platforms that serve as an interface and reference frame between the patient and the RBT Device. All are rigidly attached to the patient’s spine or skull to maintain accuracy, despite breathing and other minor patient movements. The mounting platforms are selected by the surgeon for each procedure based on the surgical approach and surgeon’s preference.
Renaissance Spine Disposables. Renaissance disposable kits are designed to easily adapt the RBT Device to a multitude of surgical applications and for the different mounting platforms utilized by the surgeon.
Renaissance Spine Accessories. Renaissance accessories include trays of reusable surgical tools.
Surgical Workflow using Renaissance for Spine procedures
Surgical workflow using Renaissance involves four basic steps:
|
|
●
|
pre-operative planning;
|
|
|
●
|
attachment of hardware;
|
|
|
●
|
3D synchronization; and
|
Pre-operative planning. A CT scan of the patient’s spine is uploaded to Renaissance software to create a detailed 3D model of the patient’s spine. This stage enables accurate visualization of the patient’s spinal anatomy and condition, and enables the creation of a customized surgical plan in a virtual 3D environment. In addition, pre-operative planning provides better preparation for each surgery, identifies anatomical challenges, and predefines trajectories for the implants. The surgeon selects implant sizes optimized for achieving the best surgical outcome. All pre-operative planning can be performed on a personal computer with our proprietary software. To enhance safety, the pre-operative “blueprint” can be reviewed in a virtual video mode in our planning software, which provides a slice-by-slice image display in all three surgical planes, as well as a full 3D review of the surgical blueprint (Fig. 1).
Figure 1: Planning Software and 3D visualization of planning
Attachment of hardware. In the operating room, one of a few Renaissance mounting options is selected in accordance with the clinical indication and surgeon’s preference. The mounting platform is rigidly attached to the patient’s spine or skull to ensure that maximum accuracy is maintained throughout the surgical procedure, even if patient movement occurs.
Three-dimensional (3D) synchronization. To execute the surgical blueprint, it is necessary to match the CT-based plan with the patient’s spine and the mounting platform. The mounting platform’s spatial location is marked by a proprietary 3D Marker which is attached to it. Two fluoroscopic images of the 3D marker and the spine are taken (anterior-posterior and oblique views). Renaissance software then automatically matches the vertebrae seen in the fluoroscopic images to those in the pre-operative CT. This automatic registration process is critical for the software to identify the location of the mounting platform relative to the patient’s spine. It allows the software to calculate the motion necessary for the RBT Device. The accuracy of the 3D synchronization process is confirmed by the surgeon after visual verification for each vertebra.
Figure 2: 3D Marker; C-arm in 2 positions taking registration images for 3D Synchronization process
Surgical execution. Once the 3D Synchronization is completed, the RBT Device is attached to the mounting platform based on instructions that are defined by the software after processing registration data with the pre-operative planning. Upon activation by the surgeon, the RBT Device moves an arm that is attached to it, and positions it at the location so that its trajectory is precisely aligned with the pre-operative plan. A cannula which is passed through the arm, along the line of the trajectory, is used by the surgeon to guide the surgical tools and drills used in the surgery. This process is repeated for each vertebrae until the surgeon completes the instrumentation according to the pre-operative plan and intraoperative clinical judgment.
Figure 3: RBT Device (with an arm connected to it) on Clamp Mount ready to guide the surgeon
In October 2015, we announced the launch of ‘PRO’ (Predictable Renaissance® Operation) Solutions at the annual meeting of the North American Spine Society (NASS) which was held in Chicago, IL. The PRO Solutions that were launched included:
PROceph Brain Procedures
We have developed the Renaissance Brain Module, a new application of our Renaissance system intended to provide precise control over the insertion of surgical instruments (drills, cannulas, electrodes, needles, etc.) during brain surgery. The Renaissance Brain Module can be used for guiding high accuracy linear trajectory into the brain. Our head-mounted Renaissance Brain Module was cleared by the FDA in July 2012 and CE-marked in August 2012. We initially launched the Renaissance Brain Module at the annual meeting of the American Association of Neurological Surgeons in April 2014.
The Renaissance Brain Module utilizes a small, frameless platform with three points of fixation to the skull to provide highly accurate access to the areas of the brain where surgical intervention is needed. This helps to minimize incisions and scarring while providing surgeons with high versatility in their surgical approach as well as facilitating intra-operative changes of trajectories.
Surgical workflow using the Renaissance Brain Module for brain surgery involves four basic steps:
|
|
●
|
pre-operative planning;
|
|
|
●
|
attachment of hardware;
|
Pre-operative planning: A Renaissance brain procedure usually begins with a pre-operative MRI scan of the patient. The scan is uploaded into Renaissance’s pre-operative 3D software for surgeons to plan the optimal trajectories prior to the procedure. Sometimes other imaging studies are loaded and fused together to provide additional layers of information such as Magnetic Resonance Angiography, CT, Diffusion Tensor Imaging and Positron Emission Tomography.
Figure 4: Planning Software
Attachment of hardware: A small platform is mounted to the skull using local anesthesia. This can be a less-invasive and faster approach compared to the larger frames that are traditionally used during these procedures. The Renaissance Brain Module’s smaller platform may also improve patient comfort and increase freedom of movement. It also enables trajectories that are beyond the working volume of other guidance systems.
Figure 5: Mounting of the platform to the skull
Synchronization: A proprietary Star Marker is attached to the platform and a CT scan is taken. The CT scan is then fused with the pre-operative plan created by the surgeon with the Renaissance software. This correlates the virtual plan with the physical location of the patient and the attached mounting platform. Based on this information, the software controls the kinematics of the RBT Device to provide the surgeon with the desired trajectories above the patient’s skull.
Figure 6: Star Marker attached to the mounting platform on the patient’s head, while a CT scan is taken for the synchronization with the pre-operative plan.
Surgical execution: After the scans are synchronized, the RBT Device is attached to the mounting platform based on instructions that are defined by the software after processing registration data with the pre-operative planning. Upon activation by the surgeon, the RBT Device moves an arm that is attached to it, and positions it at the location so that its trajectory is precisely aligned with the pre-operative plan.
Figure 7: RBT Device (with an arm connected to it) mounted ready to guide the surgeon.
We collaborated with Alpha-Omega Ltd., a manufacturer of actuators that advance electrodes into the patient’s brain. The first in-vivo procedure using the Renaissance system was performed in August 2013.
PRO Scan & Plan
This application is designed to obviate the need for a pre-operative CT scan by using an available intra-operative 3D imaging system (fluoroscopy- or CT-based). This 3D scan is performed after attaching the selected Renaissance mounting system to the patient. When using this application, there is no need to correlate the plan with the patient’s location relative to the RBT Device, as the 3D synchronization process is inherent to the image acquisition process. Once the 3D images are acquired, the surgeon utilizes them to plan the operation based on these images in the operating room. While this application has been cleared by the FDA, it is currently used clinically in a selective manner.
PROlat Posterior guided instrumentation in the lateral position
PROlat™ enables placement of pedicle screws posteriorly while the patient is in the lateral decubitus position. By maintaining the patient in the lateral position, it is possible to shorten the surgery time and reduce the potential for unnecessary risks to the patient.
PROdef Software module for planning spinal deformity correction
PROdef™ fuses a pre-operative CT scan with standing X-rays, where the latter is used to adjust the intervertebral relationships on the prone CT, based on the weight-bearing position of the vertebras on the X-rays. This automated process is based on the software’s anatomical recognition abilities, which allows it to segment the CT and X-rays into individual vertebras, to account for physiological and anatomical considerations such as the axis of rotation, limitations on the inter-vertebral range of motion, maintenance of spinal cord integrity and collision avoidance between vertebras. These allow surgeons to create a detailed and realistic, personalized surgical plan of correction in three dimensions. We expect it to be commercially available in the third quarter of 2016.
Other Potential Applications
We believe that with further research and development of our technology, we can develop applications for other areas of the body or for additional applications within brain and spine surgeries. Should we elect to develop and commercialize additional potential applications of Renaissance within or outside of the brain and spine surgery markets, we will need to seek the appropriate marketing clearance from the FDA and any other required regulatory approvals for such applications.
Sales and Marketing
We are continuing to develop a sales and marketing organization that consists of a capital sales team, a clinical sales team and a marketing team. Our sales and marketing team is comprised of executives with experience from major surgical robotic technology companies including Intuitive Surgical Inc., Hansen Medical Inc., and Stereotaxis Inc. The capital sales team drives capital equipment sales of Renaissance and associated applications while the clinical sales team focuses on the further penetration of existing clients through education marketing activities and training.
As of December 31, 2015, our U.S. sales team had a total of 62 employees, 16 of whom handle capital sales, including the Vice President of Capital Sales of United States, and 3 senior sales directors. 7 employees out of the capital sales team are former sales executives from major surgical robotic technology companies. The U.S. clinical sales team includes 36 representatives, the Vice President of Clinical Sales of United States, 1 strategic account manager, 6 regional managers and 2 regional directors. The international sales team includes the Vice President for Sales, 2 capital sales managers, 1 clinical sales director and 2 clinical sales representatives. The international sales team sells mainly through distributors covering 9 countries in Europe and 12 countries in Asia-Pacific. Together with the marketing team, they are responsible for defining and executing our global commercialization strategy. Our marketing team includes 12 members and one part-time employee, including 8 members in the U.S. based team. We intend to continue to increase the number of sales and marketing personnel as we expand our business.
Our sales and marketing goals are to continue to drive capital equipment sales of the Renaissance system and associated applications and to generate recurring revenue through sales of disposable products and service contracts. To achieve these goals, we must continue to promote adoption of Renaissance by surgeons and hospitals and build demand for the procedure among patients through the following sales and marketing strategy:
|
|
●
|
Actively target hospitals with a significant spine practice. We believe that successful adoption depends on the routine implementation of Renaissance into the surgeon’s and facility’s routine operations. Such facilities also provide the potential to sell systems into additional practices.
|
|
|
●
|
Facilitate independent clinical research by surgeons. We collaborate with surgeons to conduct research on the implementation, clinical outcomes, radiation exposure and other variables which are inherent to, or derived from, the adoption and utilization of Renaissance in a surgical spine program.
|
|
|
●
|
Encourage medical facilities to embark on a marketing program that would promote and publicize their Renaissance spine program. We work with hospitals and help them to educate surgeons, referring physicians and patients regarding the clinical benefits that Renaissance provides. This increased patient awareness in the community regarding such benefits has the potential to improve quality of care for patients undergoing procedures using our products.
|
|
|
●
|
Target early adopters of cutting-edge technology. We work with surgeons who adopt the use of technology early to promote the widespread use of Renaissance within their facilities.
|
|
|
●
|
Training and Education. We train and educate through a variety of activities such as observing Renaissance cases, participating in bio-skills workshops to provide prospective customers with hands-on experience by operating on a cadaver using the Renaissance system and participating in peer-to-peer interactions. We also offer comprehensive and advanced training to our surgeons and operating room staff.
|
The generation of recurring revenues through sales of our disposable products and service contracts is an important part of the Renaissance business model. We anticipate that as we leverage each new installation of our Renaissance system to generate recurring sales of disposable products our recurring revenues will grow. Because of the technical design and programming of our Renaissance system, the system only works with Mazor’s proprietary disposable kits. We also offer annual service contracts that provide maintenance and support services related to Renaissance beyond the basic one-year warranty period.
We provide training to surgeons and hospital staff on the use of our Renaissance system. Through training we are increasing familiarity with the Renaissance system and helping ensure safe and proper usage of our equipment and products by surgeons and hospitals, which we hope enables seamless adoption of our Renaissance system. The presence of our representatives in the hospitals also provides us with immediate feedback and understanding of our customers’ preferences and requirements in clinical conditions.
Seasonality of Business
While our business is growing and changing rapidly, we believe it is subject to quarterly seasonal fluctuations because of customary capital expenditure trends by hospitals due to various hospital budget considerations which are not in our control. Hospitals tend to group purchases at the beginning of their budgetary cycle, which is different among hospitals. Therefore, it is hard to predict results of a certain quarter and some quarters may be weaker than others. For the year ended December 31, 2015, no single hospital customer accounted for more than ten percent of our total revenue and the loss of any single hospital customer is not expected to have a material adverse effect on us.
Intellectual Property
We seek patent protection for our products and technologies in the United States and internationally. Our policy is to pursue, maintain and defend patent rights developed internally and to protect the technology, inventions and improvements that are commercially important to the development of our business.
We own fourteen U.S. patents. In addition, we have filed in the United States ten additional patent applications and three provisional patent applications. A provisional patent application is a preliminary application that can be filed less formally than a non-provisional application, and establishes a priority date for the patenting process for the invention disclosed therein.
We have also licensed five U.S. patents. In addition, we own thirty-one patents, grouped in six families of separate inventions that were granted in other countries. We also have one further application in which claims were allowed and grant is awaited. We also have thirteen pending patent applications outside of United States, grouped in eight families of separate inventions. We also have three PCT (Patent Cooperation Treaty) international patent applications. A PCT application is an application made in multiple countries, which ultimately needs converting into National Phases in separate countries. All of our patents and patent applications are in the areas of computer-assisted surgery, robotics, imaging and implants. Our patents expire between the years 2021 and 2033. Certain of our in-licensed patents have royalty obligations.
We cannot be sure that any patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future. There is also a significant risk that any issued patents will have substantially narrower claims than those that are currently sought.
We cannot be sure that any of our patents will be commercially useful in protecting our technology. We also rely on trade secrets to protect our product candidates. Our commercial success also depends in part on our non-infringement of the patents or proprietary rights of third parties. For a more comprehensive discussion of the risks related to our intellectual property, please see “Item 3. Key Information – D. Risk Factors – Risks Related to Our Intellectual Property.”
We also protect our proprietary technology and processes, in part, by confidentiality and invention assignment agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. We also rely on trade secrets to protect our product candidates. However, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Research Agreement with Cleveland Clinic Foundation (CCF)
In April 2008, we entered into a set of strategic agreements to cooperate with the Cleveland Clinic Foundation, or CCF, pursuant to which the parties intended to jointly develop a group of products for the fixation of spinal vertebrae while using our guidance technology. The procedure developed by us under the CCF licensed technology was named GO-LIF (Guided Lumbar Inter-body Fusion) and was aimed at utilizing Renaissance to place implants percutaneously. Under these agreements, we received an exclusive worldwide license under two patents owned by CCF for the development of unique spinal fixation implants. We agreed to pay CCF an upfront payment and certain milestone payments that are not material. In addition, we undertook to pay royalties at a decreasing rate, in the low- to mid-single digits, on net sales (as defined in the agreement) of any licensed products. Our obligation to pay royalties continues on a country-by-country basis until expiration of the last to expire licensed patent, or, for licensed products covered by one of the licensed patents, 15 years after receipt of an FDA approval of such licensed products, if earlier. We must also pay to CCF an agreed portion of revenues derived from our sub-licensing of the CCF licensed technology, if any. Under the terms of the agreement, we must achieve certain diligence milestone events by specified dates. If we do not meet these targets, CCF may convert our exclusive license into a non-exclusive license or terminate the agreement. Either we or CCF may terminate the agreement for the other party’s uncured material breach of the agreement, and we may terminate the agreement, on a country-by-country and licensed product-by-licensed product basis, for our convenience. In 2011, we decided to discontinue our investment in developing products based on the patents licensed from CCF.
Competition
We believe that the principal competitive factors in our market include:
|
|
●
|
the safety and efficacy of the procedure and product offerings, as documented through published studies and other clinical reports;
|
|
|
●
|
product benefits, including the ability to offer spine surgeons a complete solution for posterior thoracic-lumbar procedures;
|
|
|
●
|
the cost of product offerings and the availability of product coverage and reimbursement from third-party payors, insurance companies and others parties;
|
|
|
●
|
the strength of acceptance and adoption by spine surgeons and hospitals;
|
|
|
●
|
the ability to deliver new product offerings and enhanced technology to expand or improve upon existing applications through continued research and development;
|
|
|
●
|
the quality of training, services and clinical support provided to surgeons and hospitals;
|
|
|
●
|
the ability to provide proprietary products protected by strong intellectual property rights; and
|
|
|
●
|
the ability to offer products that are intuitive and easy to learn and use.
|
Competitors
In the spine surgical market, there are several companies that are attempting to develop robotic guidance systems. Medtech has received a CE mark for its ROSA™ system and initially announced it received an FDA clearance for spine in January 2016. Excelsius Surgical LLC was purchased by spine-implants maker Globus Inc. during 2014. Globus unveiled its robotic surgical system on November 12, 2015, and stated that it expects to receive the FDA clearance in 2016. Both companies utilize “mechanical arms”, which are essentially serial robots utilizing stereotaxic navigation to control them. There are a few other robotic guidance systems for various indications in the field of spine surgeries that we believe are further behind on their development path. There are alternative CAS systems, but these are infrared-based optical navigation systems that do not have a robotic component. These CAS systems are sold by large companies that are well-known in the surgical market in general and in spine in particular, including Medtronic, Inc., Stryker Corporation and Brainlab AG. While these CAS systems have not seen wide adoption in spine surgeries, they are applicable in many other anatomies, increasing their utility to hospitals.
We believe that surgeons are likely to adopt robotic-based technologies for spine and brain surgeries and view this as the main competitive field for our products. Large, well-known companies, however, have the ability to acquire and/or develop robotic technologies that may compete with our products. We are aware of certain companies developing robotic applications in orthopedics and spine such as Stryker Corporation that purchased Mako Surgical Corp. in late 2013, Globus and Intuitive Surgical Inc. Even companies that currently do not have an established presence in the field of spine surgery (e.g., Intuitive Surgical Inc.) may attempt to apply their robotic technologies to this field and compete directly with us.
The use of three-dimensional imaging for image guided surgeries is a growing field comprised mainly of fluoroscopy-based systems, such as Medtronic’s O-arm and Siemens’s ARCADIS Orbic 3D, and intra-operative CT scanners, such as Samsung Electronics America’s BodyTom and Brainlab’s Airo. These systems can be integrated with navigation systems and are also generally compatible with the Renaissance system. These advanced imaging modalities, if integrated with a navigation system, can reduce the need for the Renaissance system.
CAS is more established in the brain market than in spine, with several actively marketed offerings of both robotic systems (e.g., Renishaw PLC’s NeuroMate or Medtech’s ROSA™, which was FDA-cleared in 2009 for brain procedures) and navigation systems (e.g., Brainlab AG’s VectorVision) which are mainly utilized in similar indications to those in which the Renaissance Brain Module competes.
We intend to compete and drive increased adoption of our Renaissance system based on the benefits of the Renaissance system, including the safety and efficacy of our procedure, the ability to expand or improve upon existing applications through continued research and development and the ability to offer products that are intuitive and easy to learn and use.
Many of our competitors and potential competitors have significantly greater financial, human and other resources than we do, and have established relationships with healthcare professionals, customers and third-party payors. In addition, many of our competitors are more established globally and better positioned with sales and distribution networks, greater resources for product development, additional lines of products and the ability to offer financial incentives such as rebates, bundled products or discounts on other product lines that we cannot provide. Our products could also be rendered obsolete or uneconomical by technological advances developed by one or more of our competitors.
Regulatory Requirements of the U.S. Food and Drug Administration
Our research, development and clinical programs, as well as our manufacturing and marketing operations, are subject to extensive regulation in the United States and other countries. Most notably, all of our products sold in the U.S. are subject to regulation as medical devices under the FDCA, as implemented and enforced by the FDA. The FDA governs the following activities that we perform or that are performed on our behalf, to ensure that medical products we manufacture, promote and distribute domestically or export internationally are safe and effective for their intended uses:
|
|
●
|
product design, preclinical and clinical development and manufacture;
|
|
|
●
|
product premarket clearance and approval;
|
|
|
●
|
product safety, testing, labeling, advertising, promotion and storage;
|
|
|
●
|
record keeping procedures;
|
|
|
●
|
product marketing, sales and distribution;
|
|
|
●
|
quality system requirements;
|
|
|
●
|
recalls and field safety corrective actions;
|
|
|
●
|
post-market approval studies;
|
|
|
●
|
product import and export; and
|
|
|
●
|
post-marketing surveillance, complaint handling, medical device reporting, reporting of deaths, serious injuries or device malfunctions and repair or recall of products.
|
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require either premarket notification (510(k)) marketing clearance or approval of a PMA from the FDA. The FDA classifies medical devices into one of three classes. Class I devices, considered to have the lowest risk, are those for which safety and effectiveness can be assured by adherence to the FDA’s general regulatory controls for medical devices, which include compliance with the applicable portions of the QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials (General Controls). Class II devices are subject to the FDA’s General Controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device (Special Controls). Manufacturers of most class II and some class I devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. This process is generally known as 510(k) marketing clearance. Devices deemed by the FDA to pose the greatest risks, such as life sustaining, life supporting or some implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in class III, requiring approval of a PMA.
510(k) Marketing Clearance Pathway
The 510(k) clearance process is the regulatory process applicable to our current, marketed products. To obtain 510(k) marketing clearance, we must submit a premarket notification demonstrating that the proposed device is “substantially equivalent” to a legally marketed “predicate device” that is either in class I or class II, or to a class III device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of a PMA. A Special 510(k) is an abbreviated 510(k) application which can be used to obtain clearance for certain types of device modification such as modifications that do not affect the intended use of the device or alter the device’s fundamental scientific technology. A Special 510(k) generally requires less information and data than a complete, or Traditional 510(k). In addition, a Special 510(k) application often takes a shorter period of time, which could be as short as 30 days, than a Traditional 510(k) marketing clearance application. An abbreviated 510(k) is another type of 510(k) that is intended to streamline the review of data in a 510(k) through the reliance on one or more FDA-recognized consensus standards, special controls established by regulation, or FDA guidance documents. In most cases, an abbreviated 510(k) includes one or more declarations of conformity to an FDA-recognized consensus standard. The FDA’s 510(k) marketing clearance pathway usually takes from three to twelve months, but may take significantly longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence. There is no guarantee that the FDA will grant 510(k) marketing clearance for our future products and failure to obtain necessary clearances for our future products would adversely affect our ability to grow our business.
The FDA is currently considering proposals to reform its 510(k) marketing clearance process and such proposals could include increased requirements for clinical data and a longer review period. In response to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) regulatory pathway, the FDA initiated an evaluation of the 510(k) program, and in January 2011, announced several proposed actions intended to reform the review process governing the clearance of medical devices. The FDA intends these reform actions to improve the efficiency and transparency of the clearance process, as well as bolster patient safety. For example, in July 2011, the FDA issued a draft guidance document entitled “510(k) Device Modifications: Deciding When to Submit a 510(k) for a Change to an Existing Device,” which was intended to assist manufacturers in deciding whether to submit a new 510(k) for changes or modifications made to the manufacturer’s previously cleared device. While this draft guidance was subsequently withdrawn, the FDA is expected to replace the 1997 guidance document on the same topic. As part of FDASIA, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms which are further intended to clarify and improve medical device regulation both pre- and post-approval. One of these provisions obligates the FDA to prepare a report for Congress on the FDA’s approach for determining when a new 510(k) will be required for modifications or changes to a previously cleared device. After submitting this report, the FDA is expected to issue revised guidance to assist device manufacturers in making this determination. Until then, manufacturers may continue to adhere to the FDA’s 1997 guidance on this topic when making a determination as to whether or not a new 510(k) is required for a change or modification to a device, but the practical impact of the FDA’s continuing scrutiny of these issues remains unclear. It is possible that any new guidance will make substantive changes to existing policy and practice regarding the assessment of whether a new 510(k) is required for changes or modifications to existing devices. Specifically, industry has interpreted the withdrawn draft guidance to take a more conservative approach in requiring a new 510(k) for certain changes or modifications to existing, cleared devices that might not have triggered a new 510(k) under the 1997 guidance. We cannot predict which of the 510(k) marketing clearance reforms currently being discussed and/or proposed might be enacted, finalized or implemented by the FDA and whether the FDA will propose additional modifications to the regulations governing medical devices in the future. Any such modification could have a material adverse effect on our ability to commercialize our products.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, PMA approval. The FDA requires each manufacturer to determine whether the proposed changes requires submission of a 510(k) or a PMA, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) marketing clearance or PMA approval is obtained. Also, in these circumstances, we may be subject to significant regulatory fines or penalties. We have made and plan to continue to make additional product enhancements to the Renaissance system and other products that we believe do not require new 510(k) marketing clearances. We cannot be assured that the FDA would agree with any of our decisions not to seek 510(k) marketing clearance or PMA approval.
For risks related to 510(k) marketing clearance, see “Item 3. Key Information – D. Risk Factors – Risks Related to Regulatory Compliance.”
PMA Approval Pathway
A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process or is class III (although the FDA has discretion to continue to allow certain pre-amendment class III devices to use the 510(k) process). A PMA must generally be supported by, among other things, extensive data, including, but not limited to, technical, preclinical and clinical data, and manufacturing and labeling information, to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. After a pre-market approval application is submitted and filed, the FDA begins an in-depth review of the submitted information, which typically takes between one and three years, but may take significantly longer. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide responses to specific questions that the FDA asks of them related to the supportive data and approvability of the device. The FDA may or may not accept the panel’s responses. In addition, the FDA will generally conduct a pre-approval inspection of the applicant or its third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR. The FDA may also conduct a Bioresearch Monitoring Program, or BIMO inspection of the Sponsor and/or Contract Research Organization, or CRO and/or the clinical facilities involved in the clinical study supporting the PMA.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel. None of our products are currently approved under a PMA approval. However, we may in the future develop devices which will require the approval of a PMA. There is no guarantee that the FDA will grant PMA approval of our future products and failure to obtain necessary approvals for our future products would adversely affect our ability to grow our business.
The Food and Drug Administration Modernization Act of 1997 added the “De Novo” classification option as an alternative pathway to classify new devices that had automatically been placed in Class III due to the lack of a predicate device. The De Novo process applies to low and moderate risk devices that have been classified as Class III, because they were found not substantially equivalent to existing devices. At this time, we do not intend to use the De Novo process but, if we do for any future modifications that might otherwise require a PMA, there is no guarantee it will be successful and we could be required to submit a PMA.
Clinical Trials
Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) marketing clearance. Such trials generally require submission of an investigational device exemption application, or IDE, to the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. If an IDE is required, the FDA will review the submission and it must be approved, and the appropriate institutional review boards, or IRBs, at the clinical sites must approve the study, before clinical trials may begin. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an IRB, for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, we are also required to obtain the patient’s informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product in the United States. Similarly, in Europe the clinical study must be approved by a local ethics committee and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
Post-Market Studies
To date, none of our submissions to the FDA has required the submission of clinical data and all of our clinical studies to date have been post-market studies.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
|
|
●
|
product listing and establishment registration;
|
|
|
●
|
QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all phases of the design and manufacturing process;
|
|
|
●
|
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication;
|
|
|
●
|
clearance or approval of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices;
|
|
|
●
|
post-approval restrictions or conditions, including post-approval study commitments;
|
|
|
●
|
post-market surveillance regulations, including MDR requirements, which require that we report to the FDA any incident in which our products may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury;
|
|
|
●
|
the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and
|
|
|
●
|
notices of corrections or removals.
|
We must also obtain all necessary state permits or licenses to operate our business. As a manufacturer, we are subject to announced and unannounced inspections by the FDA to determine our compliance with FDA’s QSR and other regulations.
Failure to comply with applicable regulatory requirements, including delays in or failures to report incidents to the FDA as required under the MDR regulations, can result in enforcement action by the FDA, which may include any of the following sanctions:
|
|
●
|
warning letters, untitled letters fines, injunctions, consent decrees and civil penalties;
|
|
|
●
|
customer notifications or repair, replacement, refunds, recall, administrative detention or seizure of our products;
|
|
|
●
|
operating restrictions or partial suspension or total shutdown of production;
|
|
|
●
|
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
|
●
|
withdrawing 510(k) marketing clearances or PMA approvals that have already been granted;
|
|
|
●
|
refusal to grant export approval for our products; or
|
We cannot be assured that we have adequately complied with all regulatory requirements or that one or more of the referenced sanctions will not be applied to us as a result of a failure to comply.
Marketing Approvals Outside the United States
Sales of medical devices outside the United States are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ.
In the European Economic Area, or the EEA (which is comprised of the 28 Member States of the EU plus Norway, Iceland and Liechtenstein), medical devices must comply with the essential requirements of the EU Medical Devices Directive (Council Directive 93/42/EEC). Other countries, such as Switzerland and Turkey, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices compliance with the essential requirements of the EU Medical Devices Directive, as a prerequisite to be able to affix the CE mark of conformity, without which medical devices cannot be marketed or sold in these countries. To demonstrate compliance with the essential requirements medical devices manufacturers must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low risk medical devices (class I with no measuring function and which are not sterile), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements, a conformity assessment procedure requires the intervention of a Notified Body, a third party organization designated by competent authorities of an EEA country to conduct conformity assessments. The Notified Body would typically audit and examine the products’ Technical File and the quality system for the manufacture, design and final inspection of the devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant essential requirements of the Medical Devices Directive. In addition, compliance with ISO 13845 on quality systems issued by the International Organization for Standards, among other standards, establishes the presumption of conformity with the quality management system requirements of the Medical Devices Directive. In addition, many countries apply requirements in their reimbursement, pricing or health care systems that affect companies’ ability to market products.
Health Care Laws and Regulations
Third-Party Coverage and Reimbursement
In the United States and elsewhere, health care providers that perform surgical procedures using medical devices such as ours generally rely on third-party payors, including governmental payors such as Medicare and Medicaid and private payors, to cover and reimburse the associated medical and surgical costs. Consequently, sales of medical devices are dependent in part on the availability of reimbursement to the customer from third-party payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. In general, third-party payors will provide coverage and reimbursement for medically reasonable and necessary procedures and tests that utilize medical devices and may provide separate payments for the implanted or disposable devices themselves. Most payors, however, will not pay separately for capital equipment, such as for the Renaissance system. Instead, payment for the cost of using the capital equipment is considered to be covered as part of payments received for performing the procedure. In determining payment rates, third-party payors are increasingly scrutinizing the prices charged for medical products and services in comparison to other therapies. The procedures in which our products are used may not be reimbursed by these third-party payors at rates sufficient to allow us to sell our products on a competitive and profitable basis.
In addition, in many foreign markets, including the countries in the European Union, pricing of medical devices is subject to governmental control. In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to limit payments by governmental payors for medical devices, and the procedures in which medical devices are used.
In March 2010, comprehensive health care reform legislation was enacted through the passage of PPACA. Significant measures contained in the PPACA include initiatives to revise Medicare payment methodologies, initiatives to promote quality indicators in payment methodologies (including the bundling of hospital and physician payments), initiatives related to the coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, and annual reporting requirements related to payments to physicians and teaching hospitals. At this time it is not possible to predict whether these initiatives will have a positive or negative impact on us. The PPACA also includes new taxes impacting certain health-related industries, including medical device manufacturers. Beginning in 2013, each medical device manufacturer or importer is required to pay an excise tax (or sales tax) in an amount equal to 2.3% of the price for which such manufacturer sells its medical devices. This excise tax applies to our medical devices. However, the Consolidated Appropriations Act, 2016, signed into law on December 18, 2015, includes a two year moratorium on the medical device excise tax. Thus, the medical device excise tax does not apply to the sale of a taxable medical device by the manufacturer, producer, or importer of the device during the period beginning on January 1, 2016, and ending on December 31, 2017. Absent further congressional action, the excise tax will be reinstated for medical device sales beginning January 1, 2018.
In addition to PPACA, various healthcare reform proposals have also emerged at the state level. We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or internationally, or the effect any future legislation or regulation will have on us. The taxes imposed by the PPACA and the expansion in government’s role in the U.S. healthcare industry may result in decreased profits to us, lower reimbursements by payors for our products, and reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations, possibly materially.
Medicare and Medicaid
The Medicare program is a federal health benefit program administered by the CMS, that covers and pays for certain medical care items and services for eligible elderly (age>65), blind and disabled individuals, and individuals with end stage renal disease. The Medicaid program is a federal-state partnership under which states receive matching federal payments to fund healthcare services for the poor. Because about 40% of patients undergoing spine surgery are Medicare beneficiaries, and because some private commercial health insurers and some state Medicaid programs may follow the coverage and payment policies for Medicare, Medicare’s coverage and payment policies are significant to our business.
Medicare coverage for procedures using our technology currently exists in the hospital inpatient setting, which falls under Part A of the Medicare program. Under Medicare Part A, Medicare reimburses acute care hospitals a flat prospectively determined payment amount for beneficiaries receiving covered inpatient services in an acute care hospital. This method of payment is known as the PPS. Under PPS, the prospective payment for a patient’s stay in an acute care hospital is determined by the patient’s condition and other patient data and procedures performed during the inpatient stay using a classification system known as DRGs. CMS has implemented a revised version of the DRG system that uses MS-DRGs, instead of the DRGs Medicare previously used. The MS-DRGs are intended to more accurately account for the patient’s severity of illness when assigning each patient’s stay to a payment classification. Medicare pays a fixed amount to the hospital based on the MS-DRG into which the patient’s stay is classified, regardless of the actual cost to the hospital of furnishing the procedures, items and services that the patient’s condition requires, except under limited circumstances. Accordingly, acute care hospitals generally do not receive direct Medicare reimbursement under PPS for the specific costs incurred in purchasing medical devices. Rather, reimbursement for these costs is deemed to be included within the MS-DRG based payments made to hospitals for the services furnished to Medicare eligible inpatients in which the devices are utilized. For cases involving unusually high costs, a hospital may receive additional “outlier” payments above the pre-determined amount. In addition, there is a mechanism by which new technology services can apply to Medicare for additional payments above the pre-determined amount, although such requests have not been granted frequently.
Because PPS payments are based on predetermined rates and may be less than a hospital’s actual costs in furnishing care, acute care hospitals have incentives to lower their inpatient operating costs by utilizing products, devices and supplies that will reduce the length of inpatient stays, decrease labor or otherwise lower their costs. For each MS-DRG, a relative weight is calculated representing the average resources required to care for cases grouped in that particular MS-DRG relative to the average resources used to treat cases in all MS-DRGs. MS-DRG relative weights are recalculated every year to reflect changes in technology and medical practice in a budget neutral manner. Under the MS-DRG payment system, there can be significant delays in obtaining adequate reimbursement amounts for hospitals for new technologies such that reimbursement may be insufficient to permit broad acceptance by hospitals.
The Renaissance system is usually used in the inpatient setting in spinal fusion procedures which are considered standard-of-care for several diseases. Renaissance is employed in several other spine surgeries (e.g., spinal biopsy, cement augmentations), all of which are well established treatments for specified spinal diseases. We anticipate that Medicare will continue to reimburse hospitals for spinal fusions using Renaissance, but CMS can revise MS-DRG assignments from year to year.
In addition to payments to hospitals for procedures using our technology, Medicare makes separate payments to physicians for their professional services. The American Medical Association, or AMA, has developed a coding system known as the Current Procedural Terminology, or CPT, codes, which have been adopted by the Medicare program to describe and develop payment amounts for certain physician services. The Medicare Physician Fee Schedule uses CPT codes (and other codes) as part of the determination of allowable payment amounts to physicians. In determining appropriate payment amounts for surgeons, CMS receives guidance from the AMA regarding the relative technical skill level, level of resources used, and complexity of a new surgical procedure. Generally, the FDA approval of a new product is necessary, but not necessarily sufficient, for the designation of a new procedure code for a new surgical procedure using that product. Codes are assigned by either the AMA (for CPT codes) or CMS (for Medicare specific codes) and new codes usually become effective on January 1st of each year. Physicians placing pedicle screws in posterior spinal fixation procedures submit bills under various CPT codes. These codes are separate from the arthrodesis codes (for the fusion procedure) and other intraoperative procedures such as bone grafting.
Commercial Insurers
In addition to the Medicare program, many private payors look to CMS policies as a guideline in setting their coverage policies and payment amounts. The current coverage policies of these private payors may differ from the Medicare program, and the payment rates they make may be higher, lower, or the same as the Medicare program. A decrease of, or limitation on, reimbursement payments for doctors and hospitals by CMS or other agencies may affect coverage and reimbursement determinations by many private payors. Additionally, some private payors do not follow the Medicare guidelines, and those payors may reimburse only a portion of the costs associated with the use of our products, or not at all.
Fraud and Abuse Laws
Because of the significant federal funding involved in Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws whose purpose is to eliminate fraud and abuse in federal health care programs. Our business is subject to compliance with these laws.
Anti-Kickback Statutes and Federal False Claims Act
The federal healthcare programs’ Anti-Kickback Statute prohibits persons from soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid. The definition of “remuneration” has been broadly interpreted to include anything of value, including for example gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payments of cash and waivers of payments. The PPACA amended the intent requirement of the Anti-Kickback Statute. A person or entity no longer needs to have actual knowledge of the Anti-Kickback Statute or specific intent in order to violate it. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act or federal civil money penalties statute, discussed in more detail below.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Office of Inspector General of the U.S. Department of Health and Human Services, or OIG, to issue a series of regulations, known as “safe harbors.” These safe harbors, issued by the OIG beginning in July 1991, set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
Government officials have focused their enforcement efforts on marketing of healthcare services and products, among other activities, and recently have brought cases against companies, and certain sales, marketing and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Another development affecting the healthcare industry is the increased use of the federal Civil False Claims Act and, in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. In addition, various states have enacted false claim laws analogous to the Civil False Claims Act, although many of these state laws apply where a claim is submitted to any third-party payor and not merely a federal healthcare program.
When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties from $5,500 to $11,000 for each separate false claim. There are many potential bases for liability under the False Claims Act. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The False Claims Act has been used to assert liability on the basis of inadequate care, kickbacks and other improper referrals, and improper use of Medicare numbers when detailing the provider of services, in addition to the more predictable allegations as to misrepresentations with respect to the services rendered. In addition, companies have been prosecuted under the False Claims Act in connection with alleged off-label promotion of products. Our future activities relating to the reporting of wholesale or estimated retail prices for our products, the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products, and the sale and marketing of our products, may be subject to scrutiny under these laws.
Additionally, several bills have been passed or are pending, at both the state and federal levels that expand the anti-kickback laws to require, among other things, extensive tracking and maintenance of databases regarding relationships to physicians and healthcare providers. The PPACA imposes new reporting and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to physicians and teaching hospitals, otherwise known as the Sunshine Act. Device manufacturers were required to begin collecting data on August 1, 2013, register with CMS by March 31, 2014 and are required to submit certain reports to CMS disclosing payments and transfers of value made to physicians and teaching hospitals in the preceding calendar year on or before the 90th day of each calendar year.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians. Some states, such as California, Massachusetts and Nevada, mandate implementation of commercial compliance programs, while certain states, such as Massachusetts and Vermont, impose restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to physicians. The implementation of the infrastructure to comply with these bills and regulations could be costly and any failure to provide the required information may result in civil monetary penalties.
We believe our current consulting agreements with physicians represent legitimate compensation for needed documented services actually furnished to us. However, engagement of physician consultants by orthopedic medical device manufacturers has recently been subject to heightened scrutiny, and has resulted in four of the major orthopedic medical device implant manufacturers entering deferred prosecution agreements with the federal government and agreeing to pay substantial amounts to the federal government in settlement of Anti-Kickback Statute allegations, and all such companies submitting to supervision by a court appointed monitor throughout the term of the eighteen month agreements. In this environment, our engagement of physician consultants in product development and product training and education could subject us to similar scrutiny. We are unable to predict whether we would be subject to actions under the Anti-Kickback Statute or False Claims Act or any similar state law, or the impact of such actions.
It is possible that regulatory agencies may view our physician and customer arrangements as prohibited arrangements that must be restructured, or discontinued, or for which we could be subject to other significant penalties. We would be materially and adversely affected if regulatory agencies interpret our financial relationships with spine surgeons, hospitals or other customers who order our products to be in violation of applicable laws. In addition, various agencies may view these arrangements with our customers, including the provision of marketing grants to customers for the purposes of training surgeons and the provision of accessories at no charge or discounted prices with the purchase of the Renaissance system as not fully complying with federal and state fraud and abuse laws. To the extent we are found to not be in compliance, we could face potentially significant fines and penalties in addition to other more significant sanctions and we may be required to restructure our operations. This could subject us to monetary penalties for non-compliance, the cost of which could be substantial. The costs of defending such claims, as well as any sanctions imposed or negative public perceptions resulting therefrom could have a material adverse effect on our financial performance.
HIPAA and Other Fraud and Privacy Regulations
Among other things, HIPAA created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The HIPAA health care fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment and/or exclusion from government sponsored programs. The HIPAA false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment.
In addition to creating the two new federal healthcare crimes, regulations implementing HIPAA also establish uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses, which are referred to as “covered entities.” Three standards have been promulgated under HIPAA’s regulations: the Standards for Privacy of Individually Identifiable Health Information, which restrict the use and disclosure of certain individually identifiable health information, the Standards for Electronic Transactions, which establish standards for common healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures, and the Security Standards, which require covered entities to implement and maintain certain security measures to safeguard certain electronic health information, including the adoption of administrative, physical and technical safeguards to protect such information.
In 2009, Congress passed the American Recovery and Reinvestment Act of 2009, or ARRA, which included sweeping changes to HIPAA, including an expansion of HIPAA’s privacy and security standards. ARRA includes HITECH, which, among other things, made HIPAA’s privacy and security standards directly applicable to “business associates” of covered entities effective February 17, 2010. A business associate is a person or entity that performs certain functions or activities on behalf of a covered entity that involve the use or disclosure of protected health information in connection with recognized health care operations activities. As a result, business associates are now subject to significant civil and criminal penalties for failure to comply with applicable standards. Moreover, HITECH creates a new requirement to report certain breaches of unsecured, individually identifiable health information and imposes penalties on entities that fail to do so. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney fees and costs associated with pursuing federal civil actions. The final omnibus rule modifies the breach reporting standard in a manner that will likely make more data security incidents qualify as reportable breaches. We believe that we are neither a covered entity nor, as of February 17, 2010, a business associate of our hospital customers. As such, we believe that we are not directly subject to these HIPAA standards; however, there is no guarantee that the government will agree with our determination. If the government determines that we are a business associate, we could be subject to enforcement measures, including civil and criminal penalties and fines for violations of the privacy or security standards. For the purpose of avoiding risk associated with our exposure to individually identifiable health information, we have voluntarily adopted and trained our personnel on an internal policy addressing the fundamentals of HIPAA compliance. While the government intended this legislation to reduce administrative expenses and burdens for the healthcare industry, our compliance with certain provisions of these standards entails significant costs for us.
In addition to federal regulations issued under HIPAA, some states have enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases, it may be necessary to modify our planned operations and procedures to comply with the more stringent state laws. If we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions.
Anti-Bribery Laws
Compliance with complex foreign and U.S. laws and regulations that apply to our international operations increases our cost of doing business in international jurisdictions and could expose us or our employees to fines and penalties in the U.S. and abroad. These numerous and sometimes conflicting laws and regulations include the FCPA. The FCPA prohibits U.S. companies, companies whose securities are listed for trading in the United States and other entities, and their officers, directors, employees, shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment. The FCPA also requires companies to maintain records that fairly and accurately reflect transactions and maintain internal accounting controls. In many countries, hospitals are government-owned and healthcare professionals employed by such hospitals, with whom we regularly interact, may meet the definition of a foreign official for purposes of the FCPA. Additionally, recently enacted U.S. legislation increases the monetary reward available to whistleblowers who report violations of federal securities laws, including the FCPA, which may result in increased scrutiny and allegations of violations of these laws and regulations. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers, or our employees, prohibitions on the conduct of our business, and damage to our reputation.
Manufacturing and Assembly
The Renaissance system includes off-the-shelf and custom made components produced to our specifications by various third parties. We purchase from a number of suppliers major components of the Renaissance system, including the computer hardware, the RBT Device and its controllers, the screen, system console, the molded fiberglass housing and machined metal parts, and the various electro-mechanical components that support the Renaissance system. We internally develop the software components and license certain software components that are generally available for commercial use as open source software.
We outsource manufacturing of the Renaissance system and most of the product’s sub-assemblies which are assembled by subcontractors according to work plans and designs prepared by us. We believe that outsourcing allows us to carry lower inventory levels and maintain fixed unit costs with minimal infrastructure and without incurring significant capital expenditures and rely on the economy of scale of the subcontractors. We have non-disclosure agreements with our subcontractors. The manufacturing plan is based on our sales forecast, and we believe that at this stage, by using our current subcontractors, we are able to meet demand level and increase production quantities if necessary.
We believe that our subcontractors’ manufacturing processes are in compliance with pertinent U.S. and/or international quality and safety standards, such as ISO 9001, ISO 13485, or the FDA’s QSR.
We conduct in-house prototype development and present detailed manufacturing documentation to our subcontractors, who then purchase most of the necessary components and manufacture the product or subassemblies. These manufacturing subcontractors provide us fully assembled, or “turn-key,” services.
We control and monitor the quality of our products by testing each product and through extensive involvement in the production process in house and at the facilities of our subcontractors. To the best of our knowledge, our subcontractors have no significant manufacturing limitation in reference to our manufacturing needs.
As of the date hereof, five of our subcontractors are single sources subcontractors. Replacement of three of these single source subcontractors may take between three to four months, while replacement of the other two single source subcontractors, who are key suppliers, may take between six to eight months. One of these key supplier single source subcontractors, MPS Micro Precision Systems AG, or MPS, manufactures the RBT Device. Due to their nature, certain components must be ordered up to six months in advance, resulting in substantial lead time for certain production runs. In the event that such limited source suppliers are unable to meet our requirements in a timely manner, we may experience an interruption in production until we can obtain an alternate source of supply. See “Item 3. Key Information - D. Risk Factors - Risks Related to Our Business - Our reliance on third-party suppliers, including single source suppliers, for most of the components of the Renaissance system could harm our ability to meet demand for our products in a timely and cost effective manner.” In order to mitigate this risk, we provide our suppliers with a purchasing plan and a three to nine month estimate of future orders. In addition, our agreements with such single source subcontractors provide, among other things, that should the subcontractor wish to terminate our agreement, it must provide us with a long prior notice with respect thereof. With respect to MPS, our agreement requires a prior notice of eighteen months for termination. We engaged with Sanmina - SCI Israel Medical Systems Ltd., or Sanmina, during 2014. We expect Sanmina to be our main turn-key supplier by the end of 2016. Furthermore, to mitigate the risk of loss of our suppliers, we constantly hold safety inventory stock of complete units of the Renaissance system.
|
C.
|
Organizational Structure
|
We currently have two wholly owned subsidiaries: Mazor Robotics, Inc., which is incorporated in Delaware, United States and Mazor Robotics Pte. Ltd., which is incorporated in Singapore.
|
D.
|
Property, Plant and Equipment
|
Our offices and research and development facility are located at 7 HaEshel St., Caesarea Park South 3088900, Israel, where we occupy approximately 1,143 square meters. We lease this facility and our lease ends on December 31, 2017. Our monthly rent payment pursuant to the lease for our offices and research and development facility as of December 2015 was NIS 46,745 partially linked to the Israeli CPI (approximately $11,976).
Our U.S. headquarters are located in Orlando, Florida, where we occupy approximately 6,445 square feet. We lease our U.S. headquarters, and this lease ends on July 1, 2016. We have a right to renew the lease for two twelve month terms. Our monthly rent payment pursuant to the lease for our U.S. headquarters as of December 31, 2015 is $12,535 and will escalate by 3% for 2016.
We are considering expanding our office space in Israel to better meet our anticipated needs for the foreseeable future and be better suited for the conduct of our business.
|
|
UNRESOLVED STAFF COMMENTS
|
Not applicable.
|
|
OPERATING AND FINANCIAL REVIEW AND PROSPECTS
|
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes included in this annual report. The discussion below contains forward-looking statements that are based upon our current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to inaccurate assumptions and known or unknown risks and uncertainties, including those identified in “Cautionary Note Regarding Forward-Looking Statements” and in “Item 3. Key Information - D. Risk Factors”.
Overview
We are a medical device company developing and marketing innovative surgical guidance systems and complementary products. Our expertise is in robotic, computerized and imaging-based systems, primarily in the field of spine surgery. Our Renaissance Surgical Guidance System enables surgeons to advance from freehand surgical procedures to accurate, state-of-the-art, precision guided procedures. Our FDA-cleared and CE-marked Renaissance system is used in multiple types of spine surgeries, whether open or minimally invasive, for a variety of clinical indications. Our Renaissance system and its predecessor have been used in over 16,000 spine surgeries, including fusion, correction of spinal deformities, biopsy collection, tumor excision and cement augmentations. Our Renaissance system has the ability to improve clinical outcomes for patients, provide a safer surgical environment for surgeons and operating room staff by reducing exposure to radiation, and deliver economic value to hospitals and payors.
We have incurred net losses in each year since our inception in 2000 and, as of December 31, 2015, we had an accumulated deficit of $103,192,000. We expect to continue to incur significant operating losses as we increase our sales and marketing activities associated with the growing commercialization of the Renaissance system in the United States, Europe, Asia and Australia, and otherwise continue to invest capital in the development and expansion of our products and our business generally. We also expect our research and development expenses to increase as we continue to expand our research and development activities, including the support of existing products and the research and development of potential future products. We also intend to continue to research and publish the clinical value proposition of the Renaissance system.
Recent business events and key milestones in the development of our business include the following:
|
|
●
|
a significant increase of our installed base globally, with 108 active systems, including 64 systems installed in the United States, as of December 31, 2015. During the year ended December 31, 2015, we received orders for 25 systems, including 16 systems and 2 placements in the United States and seven systems in the international market in China, Taiwan, Spain, Russia and Germany. In addition, we received an order for a system upgrade in Germany.
|
|
|
●
|
penetrating new metropolitan areas.
|
|
|
●
|
penetrating teaching and academic centers in the U.S.
|
|
|
●
|
increased utilization demonstrated by an increase in the annual average number of procedures for U.S. installed systems in use for more than 12 months to 80 procedures per system, compared to 72 procedures in 2014.
|
|
|
●
|
the introduction of the PRO (Predictable Renaissance® Operation) solutions line of products at the NASS conference in October 2015.
|
|
|
●
|
the 510(k) clearance from the FDA for the Renaissance X system, a future product which is in a development stage. The Renaissance X system is one of several of our research and development programs. Prior to any commercialization efforts, the Renaissance X system requires additional technology and clinical development.
|
We believe that the key to the continuing growth of our business is expanding the acceptance of the Renaissance system for both spinal and brain surgery, and introducing other potential future applications.
Critical Accounting Policies and Significant Judgments and Estimates
The preparation of financial statements in conformity with International Financial Reporting Standards, or IFRS, requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
The accounting estimates used in the preparation of our financial statements require management to make assumptions regarding circumstances and events that involve considerable uncertainty. Management prepares the estimates on the basis of past experience, various facts, external circumstances, and reasonable assumptions according to the pertinent circumstances of each estimate.
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any affected future periods.
Our significant accounting policies are more fully described in Note 3 to our consolidated financial statements as of December 31, 2015 included elsewhere in this annual report. However, certain of our accounting policies are particularly important to the description of our financial position and results of operations. In applying these critical accounting policies, our management uses its judgment to determine the appropriate assumptions to be used in making certain estimates. Those estimates are based on our historical experience, the terms of existing contracts, our observation of trends in the industry, information provided by our customers and information available from other outside sources, as appropriate. These estimates are subject to an inherent degree of uncertainty. Our critical accounting policies include:
Revenue Recognition
Revenue is generated from three main components: (1) sales of our Renaissance system, including installation services and training; (2) sales of disposable components and accessories; and (3) warranty and maintenance services related to the systems sold, which includes replacement parts, software updates, preventive maintenance and on-call support as detailed in the agreement.
The allocation of consideration from a revenue arrangement to its separate units of account is based on the relative fair values of each unit. If the fair value of the delivered item is not reliably measurable, then revenue is allocated based on the difference between the total arrangement consideration and the fair value of the undelivered item. We usually determine the fair value of the warranty and maintenance services component based on the renewal quote offered in the agreement. Warranties granted to customers are considered an additional element in the sale of the system, because they include training, service, the provision of spare parts, telephone support, on-site support and a positive representation that the product will either perform according to certain specifications or that we will repair or replace the product if it ceases to work properly. Therefore, we consider such warranty as a separate element of the system sale.
We recognize revenue from the above mentioned components in accordance with International Accounting Standards No.18, “Revenue”, including provisions related to recognition of revenue from multiple-component transactions, when the significant risks and rewards of ownership of the goods transferred to the customer; it is probable that the economic benefits associated with the transaction will flow to us; the costs incurred or to be incurred in respect of the transaction can be measured reliably; we retain neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold; and the amount of revenue can be measured reliably.
The revenue from sales of systems is recognized at the time of transfer of the significant risks and rewards of ownership as follows:
|
|
●
|
sales to end customers - Upon the completion of installation of the system, training of at least one surgeon, which typically occurs prior to or concurrent with the system installation, and customer acceptance, if required.
|
|
|
●
|
sales to distributors - Upon delivery to the distributor, provided that the significant risks and rewards of ownership of the system are transferred to the distributor upon delivery, the distributor has no right of return, receipt of the consideration is probable and not dependent on the distributor’s ability to collect from the end customer, the commitment to carry out installation and training for the end customer lies with the distributor and that the distributor has been authorized to perform the installation and training for the end customers. If the above conditions are not met, we recognize revenue at the time of fulfillment of the conditions for recognition of revenue from the end customer.
|
Revenue from the disposable components sales is recognized at the time of the transfer of the significant risks and rewards of ownership as follows:
|
|
●
|
in sales to end customers - Upon delivery.
|
|
|
●
|
in sales to distributors - Upon delivery to the distributor, provided that the significant risks and rewards of ownership of the components are transferred to the distributor upon delivery, the distributor has no right of return and that the receipt of the consideration is probable and not dependent on the distributor’s ability to collect from the end customer.
|
Revenue from warranty and maintenance services is recognized proportionately over the period of rendering of the service and subject to the other conditions for revenue recognition specified above.
Functional Currency
The consolidated financial statements are presented in U.S. dollars, which is the Company’s functional currency.
Share-Based Compensation
We account for share-based compensation arrangements in accordance with the provisions of International Financial Reporting Standard 2, or IFRS2. IFRS2 requires us to recognize share-based compensation expense for awards of equity instruments based on the grant-date fair value of those awards. The cost is recognized as compensation expense, based upon the grant-date fair value of the equity or liability instruments issued. The fair value of our option grants is computed as of the grant date based on the binominal model, using the standard parameters established in that model including estimates relating to the share price on the measurement date, exercise price of the instrument, expected volatility (based on the historical volatility), the expected life span of the options, and the risk-free interest rate (based on government debentures). As our stock is publicly traded on the TASE, we do not need to estimate the fair market value of our shares. Rather, we use the actual closing market price of our ordinary shares on the date of grant, as reported by the TASE. The value of the transactions, measured as described above, is recognized as an expense over the vesting period. When award grant have graded-vesting feature, each installment of the awards is separately measured and the expenses are recognized over the related vesting period.
Capitalization of Development Costs
We capitalize development expenditure in accordance with International Accounting Standard No. 38 “Intangible Assets”, or IAS 38, only if development costs can be measured reliably; the product or process is technically and commercially feasible; future economic benefits are probable and we intend to and have sufficient resources to complete development and to use or sell the asset.
We capitalize development costs based on our judgment regarding technological and economic feasibility, which generally exists when a product development project reaches a defined milestone, or when we enter into a transaction to sell the know-how that was derived from the development. In regards to our products, technological feasibility usually occur only when the clinical trials succeed and following receipt of approval from the FDA.
Inventory Valuation
Inventory is measured at the lower of cost and net realizable value. Inventory costs include direct materials and direct labor. We review our inventory periodically to determine net realizable value and the necessity of provisions for obsolescence, which may result from excess, slow-moving or obsolete inventories. We write down inventory, if required, based on forecasted demand and technological obsolescence. These factors are impacted by market and economic conditions, technology changes and new product introductions and require estimates that may include uncertain elements.
Accounting for Income Taxes
As part of the process of preparing our consolidated financial statements we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process requires us to estimate our actual current tax exposures and make an assessment of temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. Significant management judgment is required in determining our provision for income taxes, deferred tax assets and liabilities. Changes to these estimates may result in a significant increase or decrease to our tax provision in the current or subsequent period.
We recognize deferred tax assets for unused tax losses, tax benefits, and deductible temporary differences to the extent that it is probable that future taxable income will be available against which that can be utilized. Deferred tax assets are reviewed at each reporting date and are reduced to the extent that it is no longer probable that the related tax benefit will be realized. A provision for uncertain tax positions, including additional tax and interest expenses, is recognized when it is more probable than not that the Group will have to use its economic resources to pay the obligation.
The calculation of our tax liabilities or reduction in deferred tax asset involves dealing with uncertainties in the application of complex tax regulations and estimates of future taxable income in different geographical jurisdictions. It is inherently difficult and subjective to estimate such amounts, as we have to determine the probability of various possible outcomes. We re-evaluate these uncertain tax positions on a periodical basis. This evaluation is based on factors including, but not limited to, changes in facts or circumstances, changes in tax law, effective settlement of audit issues, and new audit activity. Such a change in recognition or measurement would result in the recognition of a tax benefit or an additional charge to the tax provision.
Warrants
In August 2012, we entered into an agreement, described in Note 22C(2) to our consolidated financial statements as of December 31, 2015, according to which a group of investors led by Oracle Associates, LLC, or the Oracle Investors, were issued on September 27, 2012 shares and warrants exercisable into variable number of our shares. See “Item 10. Additional Information - C. Material Contracts”, for more details. In accordance with IFRS, warrants for a variable number of shares represent a financial liability that is a derivative. We measured the fair value of the derivative instruments at each period end using standard valuation technique for this type of instrument (Monte Carlo model) on the basis of observable inputs (such as the price of our shares and the NIS/USD exchange rate) and the following unobservable inputs: expected volatility, correlation between the share price and the change in the exchange rate, risk-free interest rate, expected life and the probability that mandatory exercise will occur. We used the services of Financial Immunities Dealing Room Ltd., an independent valuation specialist, which has appropriate professional qualifications and experience in the valuation of financial derivatives to assist us with the fair value measurement of the derivative instrument.
On May 28, 2013, we provided the Oracle Investors with a notice of mandatory exercise, pursuant to which the Oracle Investors shall, within 30 days, exercise such warrants for total consideration of $7,500,000 into a fixed number of 4,996,251 Ordinary Shares as determined as of May 28, 2013. Due to this change in the effective terms of the derivative instrument, we chose as an accounting policy to reassess the terms of the derivative instrument as of May 28, 2013. Accordingly, we determined the derivative instrument to be an equity instrument, and as a result, we reclassified the derivative instrument according to its fair value as of May 28, 2013 to equity. All of such warrants have been exercised into 4,996,251 Ordinary Shares.
While we believe we have applied appropriate judgment in the assumptions and estimates, variations in judgment in applying assumptions and estimates used in the valuations could have had a material effect upon the valuation results, and thus, on our financial statements. As fully described in Note 22C(2) to our consolidated financial statements as of December 31, 2015, the derivative instrument was classified to equity on May 28, 2013 and is no longer measured at fair value.
Results of Operations
Comparison of year ended December 31, 2015 and year ended December 31, 2014
Revenue
The following table presents our total revenues by geographic area and by line of product for the fiscal years indicated (in thousands of U.S. dollars and as a percentage of total revenues):
| |
|
For the Year Ended December 31,
|
|
| |
|
2015
|
|
|
2014
|
|
|
United States
|
|
$ |
20,271 |
|
|
|
78 |
% |
|
$ |
15,486 |
|
|
|
73 |
% |
|
International
|
|
$ |
5,825 |
|
|
|
22 |
% |
|
$ |
5,722 |
|
|
|
27 |
% |
|
Total
|
|
$ |
26,096 |
|
|
|
100 |
% |
|
$ |
21,208 |
|
|
|
100 |
% |
| |
|
2015
|
|
|
2014
|
|
|
Systems
|
|
$ |
13,373 |
|
|
|
51 |
% |
|
$ |
12,040 |
|
|
|
57 |
% |
|
Sale of disposables
|
|
$ |
7,648 |
|
|
|
29 |
% |
|
$ |
4,916 |
|
|
|
23 |
% |
|
Services and other
|
|
$ |
5,075 |
|
|
|
20 |
% |
|
$ |
4,252 |
|
|
|
20 |
% |
|
Total
|
|
$ |
26,096 |
|
|
|
100 |
% |
|
$ |
21,208 |
|
|
|
100 |
% |
Total revenue was $26,096,000 for the year ended December 31, 2015, compared to $21,208,000 for the year ended December 31, 2014. The increase in revenue of $4,888,000, or 23%, was due to a $2,732,000, or 56%, increase in disposables revenue, a $1,333,000, or 11%, increase in Renaissance system revenue, and by an $823,000, or 19%, increase in service and other revenue.
The increase in sales of our Renaissance systems during the year ended December 31, 2015 compared to the year ended December 31, 2014 was due to the sales of 23 units of our Renaissance system, one system upgrade and 2 placements during the year ended December 31, 2015, compared to 20 units sold during the year ended December 31, 2014.
The increase in disposables revenue during the year ended December 31, 2015 compared to the year ended December 31, 2014 was primarily due to the continued adoption and usage of Renaissance, driven by the growth of our commercial installed base, mainly in the United States.
The increase in service and other revenue during the year ended December 31, 2015 compared to the year ended December 31, 2014 was attributable to an increase in the installed base of Renaissance systems covered under warranty and maintenance contracts, mainly in the United States.
The increase in revenue derived from the United States of $4,785,000, or 31%, was primarily due to the increase in revenues from commercial Renaissance system sales during the year ended December 31, 2015 compared to Renaissance system sales during the year ended December 31, 2014, as well as an increase in utilization of the installed base, reflected in revenues from sales of disposables, service and others.
Cost of Sales
Cost of sales was $5,827,000 for the year ended December 31, 2015, compared to $4,396,000 for the year ended December 31, 2014. The increase in cost of sales of $1,431,000, or 33%, was primarily due to an increase in recognition of the costs from sales. Additionally, increased costs were in respect to an increase in salaries and related expenses associated with the incremental number of employees added in the operations department to support growth in our activities.
Gross Profit
Gross profit was $20,269,000 for the year ended December 31, 2015, or 77.7% of revenues, compared to $16,812,000, or 79.3% of revenues, for the year ended December 31, 2014. The decrease in gross profit margin was 1.6% due to increase in costs. The increase in gross profit of $3,457,000, or 20.6%, was mainly due to the increase in sales volume in the period.
Operating Expenses
| |
|
For the Year Ended December 31,
|
|
|
|
|
| |
|
|
|
|
Increase (decrease) in
|
|
| |
|
2015
|
|
|
2014
|
|
|
Dollars
|
|
|
%
|
|
| |
|
(in thousands)
|
|
|
|
|
Research and development
|
|
$ |
6,324 |
|
|
$ |
5,776 |
|
|
|
548 |
|
|
|
9 |
|
|
Selling and marketing
|
|
$ |
24,947 |
|
|
$ |
21,352 |
|
|
|
3,595 |
|
|
|
17 |
|
|
General and administrative
|
|
$ |
4,305 |
|
|
$ |
4,392 |
|
|
|
(87 |
) |
|
|
(2 |
) |
|
Total operating expenses
|
|
$ |
35,576 |
|
|
$ |
31,520 |
|
|
|
4,056 |
|
|
|
13 |
|
Research and Development Cost
Research and development cost was $6,324,000 for the year ended December 31, 2015, compared to $5,776,000 for the year ended December 31, 2014. The increase of $548,000, or 9%, was primarily due to our continuous efforts in developing additional applications and new products, mainly resulting in additional purchasing for current R&D projects, as well as recruitment of R&D personnel.
Selling and Marketing Expenses
Selling and marketing expenses were $24,947,000 for the year ended December 31, 2015, compared to $21,352,000 for the year ended December 31, 2014. The increase of $3,595,000, or 17%, was primarily due to the expansion of efforts to further penetrate the U.S. and the Asian markets. Such efforts included, among other things, recruitment of additional sales personnel in the United States and continued introduction activities for our Renaissance system. Selling and marketing expenses for the year ended December 31, 2015 also included an excise tax fee in the amount of $308,000, this amount is a result of certain healthcare legislation. For further information see “Item 10. Additional Information - E. Taxation - Medical Devices Excise Tax.”
General and Administrative Expenses
General and administrative expenses remained generally at the same level as 2014, and were $4,305,000 for the year ended December 31, 2015, compared to $4,392,000 for the year ended December 31, 2014.
Financing income (expenses), net
Financing income, net was $135,000 for the year ended December 31, 2015, compared to financing expenses, net of $419,000 for the year ended December 31, 2014. The main difference between 2015 and 2014 financing expenses derives from high hedging transactions expenses of approximately $408,000 in the year ended December 31, 2014 and from an increase in the year ended December 31, 2015 of approximately $135,000 in interest income from bank deposits.
Taxes on Income
We recorded a tax expense of $213,000 for the year ended December 31, 2015, compared to an expense of $145,000 for the year ended December 31, 2014. We incur tax expense only in the United States, which recorded higher sales in 2015 that resulted in higher tax expenses.
Loss and Loss per Share
Loss was $15,385,000, or $0.36 per share, for the year ended December 31, 2015, compared to loss of $15,272,000, or $0.37 per share, for the year ended December 31, 2014.
Comparison of year ended December 31, 2014 and year ended December 31, 2013
Revenue
The following table presents our total revenues by geographic area and by line of product for the fiscal years indicated (in thousands of U.S. dollars and as a percentage of total revenues):
| |
|
For the Year Ended December 31,
|
|
| |
|
2014
|
|
|
2013
|
|
|
United States
|
|
$ |
15,486 |
|
|
|
73 |
% |
|
$ |
15,021 |
|
|
|
75 |
% |
|
International
|
|
$ |
5,722 |
|
|
|
27 |
% |
|
$ |
4,962 |
|
|
|
25 |
% |
|
Total
|
|
$ |
21,208 |
|
|
|
100 |
% |
|
$ |
19,983 |
|
|
|
100 |
% |
| |
|
2014
|
|
|
2013
|
|
|
Systems
|
|
$ |
12,040 |
|
|
|
57 |
% |
|
$ |
13,527 |
|
|
|
68 |
% |
|
Sale of disposables
|
|
$ |
4,916 |
|
|
|
23 |
% |
|
$ |
3,505 |
|
|
|
17 |
% |
|
Services and other
|
|
$ |
4,252 |
|
|
|
20 |
% |
|
$ |
2,951 |
|
|
|
15 |
% |
|
Total
|
|
$ |
21,208 |
|
|
|
100 |
% |
|
$ |
19,983 |
|
|
|
100 |
% |
Total revenue was $21,208,000 for the year ended December 31, 2014, compared to $19,983,000 for the year ended December 31, 2013. The increase in revenue of $1,225,000, or 6%, was due to a $1,411,000, or 40%, increase in disposables revenue, and a $1,301,000, or 44%, increase in service and other revenue, offset by a $1,487,000, or 11%, decrease in Renaissance system revenue.
The decrease in sales of our Renaissance systems during the year ended December 31, 2014 compared to the year ended December 31, 2013 was due to the sales of 20 units of our Renaissance system during the year ended December 31, 2014, compared to 23 units and one system upgrade sold during the year ended December 31, 2013.
The increase in disposables revenue during the year ended December 31, 2014 compared to the year ended December 31, 2013 was primarily due to the continued adoption and usage of Renaissance, driven by the growth of our commercial installed base worldwide.
The increase in service and other revenue during the year ended December 31, 2014 compared to the year ended December 31, 2013 was attributable to an increase in the installed base of Renaissance systems covered under warranty and maintenance contracts, mainly in the United States.
The increase in revenue derived from the United States of $465,000, or 3%, was primarily due to the increase in revenues from disposables, service and others, offset by a decrease in revenues from 12 commercial Renaissance system sales during the year ended December 31, 2014 compared to 15 commercial Renaissance system sales and one system upgrade during the year ended December 31, 2013. The increase in international revenue of $760,000, or 15%, was primarily due to the increase in revenues from disposables, service and others.
Cost of Sales
Cost of sales was $4,396,000 for the year ended December 31, 2014, compared to $4,280,000 for the year ended December 31, 2013. The increase in cost of sales of $116,000, or 3%, reflects an increase in recognition of the costs from sales. The increased costs were primarily attributed to an increase in salaries and related expenses associated with the incremental number of employees added in the operations department, offset by a decrease in intangible asset amortization.
Gross Profit
Gross profit was $16,812,000 for the year ended December 31, 2014, or 79.3% of revenues, compared to $15,703,000, or 78.6% of revenues, for the year ended December 31, 2013. The increase in gross profit of $1,109,000, or 7%, and the gross margin as a percentage of sales was primarily due to the increase in sales volume in the period. In addition, the volume of fixed expenses did not increase by the same rate as the 6% increase in sales in the year ended December 31, 2014 compared to the corresponding period of 2013.
Operating Expenses
| |
|
For the Year Ended December 31,
|
|
|
|
|
| |
|
|
|
|
Increase in
|
|
| |
|
2014
|
|
|
2013
|
|
|
Dollars
|
|
|
%
|
|
| |
|
(in thousands)
|
|
|
|
|
|
Research and development
|
|
$ |
5,776 |
|
|
$ |
4,174 |
|
|
|
1,602 |
|
|
|
38 |
|
|
Selling and marketing
|
|
$ |
21,352 |
|
|
$ |
15,692 |
|
|
|
5,660 |
|
|
|
36 |
|
|
General and administrative
|
|
$ |
4,392 |
|
|
$ |
2,766 |
|
|
|
1,626 |
|
|
|
59 |
|
|
Total operating expenses
|
|
$ |
31,520 |
|
|
$ |
22,632 |
|
|
|
8,888 |
|
|
|
39 |
|
Research and Development Cost
Research and development cost was $5,776,000 for the year ended December 31, 2014, compared to $4,174,000 for the year ended December 31, 2013. The increase of $1,602,000, or 38%, was primarily due to our continuous efforts in developing additional applications and new products.
Selling and Marketing Expenses
Selling and marketing expenses were $21,352,000 for the year ended December 31, 2014, compared to $15,692,000 for the year ended December 31, 2013. The increase of $5,660,000, or 36%, was primarily due to the expansion of efforts to further penetrate the U.S. and the Asian markets. Such efforts included, among other things, recruitment of additional sales personnel in the United States, and expansion of marketing activities, such as participation in exhibitions, holding cadaver labs and continued introduction activities for our Renaissance system. Selling and marketing expenses for the years ended December 31, 2014 and December 31, 2013 also included an excise tax fee in the amount of $238,000 and $241,000, respectively, this amounts were a result of certain healthcare legislation. For further information, see “Item 10. Additional Information - E. Taxation - Medical Devices Excise Tax.”
General and Administrative Expenses
General and administrative expenses were $4,392,000 for the year ended December 31, 2014, compared to $2,766,000 for the year ended December 31, 2013. The increase is mostly due to recruiting new employees and the increase in professional consulting needed to support the expansion of our activities and compliance related expense related to the trading of our ADSs on the NASDAQ Global Market.
Financing expenses, net
Financing expenses, net was $419,000 for the year ended December 31, 2014, compared to $13,433,000 for the year ended December 31, 2013. The main difference between 2014 and 2013 financing expenses derives from the fact that in the year ended December 31, 2013 our finance expense included an expense of $13,510,000 due to the change in the fair value of the warrants which were issued to investors, recorded as a derivative instrument. Whereas those warrants were exercised in 2013, no such impact exists in the year ended December 31, 2014.
Taxes on Income
We recorded a tax expense of $145,000 for the year ended December 31, 2014, compared to an expense of $167,000 for the year ended December 31, 2013. We incur tax expense only in the United States.
Loss and Loss per Share
Loss was $15,272,000, or $0.37 per share, for the year ended December 31, 2014, compared to loss of $20,529,000, or $0.57 per share, for the year ended December 31, 2013.
The decrease in loss in the year ended December 31, 2014 mainly derives from financing expenses in the amount of $13,510,000 recorded in the year ended December 31, 2013 due to the change in the fair value of a derivative instrument, as discussed above, offset by an increase of $7,779,000 in operating loss in the year ended December 31, 2014 compared to the corresponding period of 2013. In addition, the weighted average number of ordinary shares used to calculate loss per share increased in the year ended December 31, 2014, when compared to year ended December 31, 2013, due to sale of ADSs and exercises of warrants and share options.
Effective Corporate Tax Rate
Our effective consolidated tax rate in 2015, 2014 and 2013 was close to zero percent primarily due to the tax losses we accrued in Israel since our inception, for which deferred tax assets were not recognized. We expect to continue to accrue losses for tax purposes in Israel in the coming years and to increase our profits for tax purposes in our U.S. Subsidiary, derived from our expected revenues in the coming years in the United States, which would increase our effective consolidated tax rate in the coming years.
Impact of Inflation, Devaluation and Fluctuation in Currencies on Results of Operations, Liabilities and Assets
We generate a majority of our revenues in U.S. dollars, which is our functional currency while some of our revenues are generated in other currencies, such as the Euro and NIS. As a result, some of our financial assets are denominated in these currencies, and fluctuations in these currencies could adversely affect our financial results. A considerable amount of our expenses are generated in dollars, but a significant portion of our expenses such as salaries are generated in other currencies such as NIS. In addition to our operations in Israel, we are expanding our international operations. Accordingly, we incur and expect to continue to incur additional expenses in non-dollar currencies, such as the Euro. As a result, some of our financial liabilities are denominated in these non-dollar currencies. Usually, our non-dollar assets are not fully offset by our non-dollar liabilities. Due to the foregoing and the fact that our financial results are measured in dollars, our results could be adversely affected as a result of a strengthening or weakening of the dollar compared to these other currencies. During 2015, 2014 and 2013, we incurred net currency loss of $113,000, loss of $107,000 and a gain of $109,000, respectively. During 2015 and 2014, we incurred net gain of $3,000 and net loss of $408,000 from hedging transactions, respectively.
Some portions of our expenses, primarily expenses associated with employee compensation, are denominated in NIS unlinked to the U.S. dollar. A devaluation of the NIS in relation to the U.S. dollar has the effect of decreasing the U.S. dollar value of any asset of ours that consists of NIS or receivables payable in NIS, unless such receivables are linked to the U.S. dollar. Such devaluation also has the effect of reducing the U.S. dollar amount of any of our expenses or liabilities which are payable in NIS, unless such expenses or payables are linked to the U.S. dollar. Conversely, any increase in the value of the NIS in relation to the U.S. dollar has the effect of increasing the U.S. dollar value of any of our unlinked NIS assets and the U.S. dollar amounts of any of our unlinked NIS liabilities and expenses. In addition, some of our expenses are linked to some extent to the rate of inflation in Israel. An increase in the rate of inflation in Israel that is not offset by a devaluation of the NIS relative to the U.S. dollar can cause the dollar amount of our expenses to increase. We believe that inflation in Israel has not had a material effect on our results of operations.
We engage in currency hedging activities. These measures, however, may not adequately protect us from material adverse effects due to the impact of inflation in Israel or from fluctuations in the relative values of the dollar and foreign currencies in which we transact business, and may result in a financial loss. See further discussion under “Item 11 - Quantitative and Qualitative Disclosures about Market Risk” below.
|
B.
|
Liquidity and Capital Resources
|
We have incurred net losses and negative cash flow from operating activities for each year since our inception in September 2000. As of December 31, 2015, we had an accumulated loss of $103,192,000 and have financed our operations principally through the sale of our products, disposables and other services, sale of our equity securities (including ADSs, Ordinary Shares and warrants), issuance of convertible debentures and grants from the OCS.
As of December 31, 2015, we had $40,229,000 in cash, cash equivalents, short-term investments and long-term investments. Our cash and investment balances are held mainly in bank deposits, in accordance with directives of our board of directors as further described below.
As of December 31, 2015, more than 50% of our expenses are in U.S. dollars and the rest are mainly in NIS or Euros. We engage in currency hedging transactions, such as options and forward contracts, for the purposes of hedging our NIS payments to local suppliers and for salaries in Israel. Our currency hedging transactions are aimed to decrease a certain portion of the financial exposure risk of fluctuations in the exchange rates of our operating currency, which is the U.S. dollar against the NIS.
Our board of directors periodically examines the financial exposure of our balance sheet, as set forth above. As part of this approach, we carry out financial activities to reduce our exposure to risk. The Audit Committee and the board of directors held discussions concerning our exposure to risk from the currencies other than the U.S. dollar, and decided that we shall maintain sufficient cash for our activities, including those in other currencies.
Net Cash Used in Operating Activities
Net cash used in operating activities primarily reflects the net loss for those periods, which was adjusted, mainly by non-cash items, such as depreciation and amortization, stock-based compensation and non-cash finance income and expenses and is also affected by changes in operating assets and liabilities.
Net cash used in operating activities for the year ended December 31, 2015 was $11,572,000 compared to $14,290,000 for the year ended December 31, 2014. The decrease of $2,718,000, or 19%, in cash used in operating activities for the year ended December 31, 2015 was mainly due to an increase in accounts payable, realizing inventory and non-cash expenses. This is a result of higher investments in developing additional applications and new products and the expansion of efforts to further penetrate to the U.S. and the Asian markets. The decrease was offset by the increase in cash used in operating activities from trade and other account receivables of approximately $1,112,000, as a result of higher sales in 2015.
Net cash used in operating activities for the year ended December 31, 2014 was $14,290,000 compared to $5,050,000 for the year ended December 31, 2013. The increase of $9,240,000, or 183%, in cash used in operating activities for the year ended December 31, 2014 was mainly due to an increase in our operating expenses of $8,888,000. The increase in operating expenses is attributed to our efforts in developing additional applications and new products and the expansion of efforts to further penetrate the U.S. and the Asian markets and professional consulting costs, required to support the expansion of our activities and listing and trading of our ADSs on the NASDAQ Global Market.
Net Cash Provided by (Used in) Investing Activities
Net cash provided by investing activities for the year ended December 31, 2015, was $2,568,000, attributable to net proceeds from maturities of short-term investments of $9,816,000 and to proceeds from the sale of long-term investments in the amount of $992,000 offset by the purchase of $7,538,000 of long term investments and purchases of fixed assets of $702,000.
Net cash provided by investing activities for the year ended December 31, 2014 was $14,531,000, attributable to proceeds from short-term investments, net in the amount of $22,271,000, the purchase of $7,237,000 of long term investments and purchases of fixed assets of $503,000.
Net cash used in investing activities for the year ended December 31, 2013, was $41,092,000, attributable to payments of $40,820,000 for acquisition of deposits and investment in marketable securities and purchase of fixed assets, such as computers, machinery and leasing improvements of $272,000.
Net Cash provided by Financing Activities
Net cash flow from financing activities in the year ended December 31, 2015 was $370,000. The net cash from financing activities in 2015 is derived from the exercise of share options by employees and consultants in the amount of $370,000.
Net cash flow from financing activities in the year ended December 31, 2014 was $2,424,000. The net cash from financing activities in 2014 is mainly from the exercise of share options by employees and consultants in the amount of $3,042,000, offset by repayment of loans to the OCS of $324,000 and costs related to the ADSs offering from 2013 of $294,000, that were paid in 2014.
Net cash flow from financing activities in the year ended December 31, 2013 was $53,048,000. The net cash from financing activities in 2013 is mainly attributable to proceeds from the exercise of warrants and the sale of our ADSs in amount of $53,198,000 and proceeds from the exercise of share options by employees and service providers for the amount of $479,000. The proceeds were offset by repayment of loans to the OCS for the amount of $629,000.
Operating Capital and Capital Expenditure Requirements
To date, we have not achieved profitability and have sustained net losses in every fiscal year since our inception in 2000, including a net loss of $15.4 million for the year ended December 31, 2015. We anticipate that we will continue to incur substantial net losses for at least the next two years as we expand our sales and marketing capabilities in the spine and brain markets, continue to invest in marketing activities for our Renaissance system in the U.S. and Asian market, continue research and development of existing and future products, and continue development of the corporate infrastructure required to sell and market our products and support operations. We also expect to experience increased cash requirements for inventory to meet increased demand of our Renaissance system. We believe that our current cash, cash equivalents and investment balances, and interest income we earn on these balances will be sufficient to meet our anticipated cash requirements at least for the next 24 months. To the extent our available cash, cash equivalents and investment balances are insufficient to satisfy our operating requirements, we will need to seek additional sources of funds, including selling additional equity, debt or other securities or entering into a credit facility, or modify our current business plan. The sale of additional equity may result in dilution to our current shareholders. If we raise additional funds through the issuance of debt securities, these securities may have rights senior to those of our ordinary shares and could contain covenants that could restrict our operations and ability to issue dividends. We may also require additional capital beyond our currently forecasted amounts. Any required additional capital, whether forecasted or not, may not be available on reasonable terms, or at all. If we are unable to obtain additional financing, we may be required to reduce the scope of, delay or eliminate some or all of our planned research, development and commercialization activities, which could materially harm our business and results of operations.
Because of the numerous risks and uncertainties associated with the development of medical devices and the current economic situation, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to complete the development of our products and successfully deliver commercial products to the market. Our future capital requirements will depend on many factors, including but not limited to the following:
| |
●
|
the revenue generated by sales of our current and future products;
|
|
|
●
|
our ability to manage our inventory;
|
|
|
●
|
the expenses we incur in selling and marketing our products and supporting our growth;
|
|
|
●
|
the costs and timing of regulatory clearance or approvals for new products or upgrades or changes to our current products;
|
|
|
●
|
the rate of progress, cost, and success or failure of on-going development activities;
|
|
|
●
|
the emergence of competing or complementary technological developments;
|
|
|
●
|
the costs of filing, prosecuting, defending and enforcing any patent or license claims and other intellectual property rights, or participating in litigation related activities;
|
|
|
●
|
the terms and timing of any collaborative, licensing, or other arrangements that we may establish;
|
|
|
●
|
the acquisition of businesses, products and technologies; and
|
| |
●
|
general economic conditions and interest rates, including the continuing weak conditions
|
|
C.
|
Research and Development, Patents and Licenses, Etc.
|
Our research and development activities are focused on the development of surgical guidance systems and complementary products in the spine and brain surgical markets.
As of December 31, 2015, our research and development team consisted of 27 people, of which 26 full-time employees engaged in research and development, as well as one part time employee primarily working on our research and development program. In addition, we work with subcontractors for the development and design when needed. We have assembled an experienced team with recognized expertise in robotics, mechanical and electrical engineering, software, control algorithms and systems integration, as well as significant clinical knowledge and expertise.
Our research and development efforts are focused on continuous improvement of the Renaissance system including adding new applications for the spine market and the development of the brain application as well as investment in future products.
We invest resources in the protection of our intellectual property. For this purpose, we file from time to time applications for patent registration in the certain countries in which we are active and in other countries which we consider as potential markets.
From our inception, we have entered into research and clinical alliances in order to substantiate the knowledge which is at the basis of the products developed and marketed thereby, as well as for the innovative and ongoing development of such products. We use such research to gain recognition in the medical community and for scientific publications. We are currently involved in research activity conducted in several centers in Israel, the United States and Germany.
We finance our research and development activities mainly through sale of our products and capital raises.
To date, we have received total grants from the OCS of $1,326,000, which amount bore LIBOR interest in the amount of $249,000. In March 2014, we repaid all of our remaining obligations, including interest, to the OCS.
For a description of the amount spent during each of the last three fiscal years on company-sponsored research and development activities, see “Item 5. Operating and Financial Review and Prospects - A. Operating Results.”
The following is a description of factors that may influence our future results of operations, including significant trends and challenges that we believe are important to an understanding of our business and results of operations.
We generate revenues from: (1) Renaissance system sales; (2) sales of disposable accessories to the Renaissance system; and (3) sales of warranty and maintenance services on the Renaissance system and accessories. The level of our future revenues is hard to predict and depends on many factors which are not in our control. For instance, future revenues from the sale of Renaissance systems may be adversely affected by current general economic conditions and the resulting tightening of hospital budgets, which may cause purchasing decisions to be delayed or our customers to have difficulty securing adequate funding to buy our products. In addition, revenue growth depends on the acceptance of our technology in the market. Sales of our disposable accessories depend on the adoption of our technology by hospitals. We continue to encourage use of the Renaissance system by our U.S. clinical sales team and expect to see growth in our recurring revenues; however, we anticipate that quarterly results will be variable because our sales cycle generally averages nine months, and since we are dependent on hospital purchasing decisions which in turn are affected by quarterly and other variations in the hospital budgeting process.
We sell our products and services in the United States through our direct sales force, and in most other territories, we sell our products using third-party distributors. In 2014 we entered into a distribution agreement in Hong Kong, in 2015 we entered into distribution agreements in Thailand and in 2016 we entered into distribution agreements in Vietnam. Additionally, we currently have existing distribution agreements with distribution partners in China, Japan, South Korea, Taiwan, Italy, Russia, Turkey, Switzerland and Germany. While we plan to continue to expand our indirect sales efforts outside of the United States, we expect that most of our growth over the next two years will be driven by the U.S. market. Our sales in Europe, once the primary market of our sales, decreased materially in the last few years. Considering the current economic situation in Europe, we expect only moderate growth in our activities in this region.
Assuming that factors outside of our control will not adversely affect us, we believe that we will be able to continue to grow our business in the foreseeable future, which would result in an increase in our revenues. However, such increase will require our continued commitment of substantial resources toward our sales and marketing operations, mainly in the United States. We expect continuous growth of headcount of our direct sales force, to support both system and disposables sales. In addition, since the launch of our Renaissance system in June 2011, our business has benefitted from research and development expenses that have been comparatively low. However, this trend is beginning to change; we plan to increase our expenditures on research and development to improve existing products and accelerate the creation of new products for spine and brain surgery. In connection with the expansion of our research and development activities, we expect to retain additional personnel for our research and development team. We believe that our general and administrative expenses will increase as we grow our business and as a result of listing our securities for trading in the United States.
For at least the next two years, we expect that the potential increases in revenue will not necessarily reduce our losses due to the increase in our planned expenditures, most of which we expect to be related to sales and marketing and research and development activities. An additional item of expense is the cost of manufacturing the Renaissance system, accessories, spare parts and disposable accessories products. Such expense includes manufacturing overhead costs, freight, amortization of intangible assets, and the cost of service. We expect to increase our expenditures on manufacturing and service overhead to accommodate the anticipated growth of our installed base.
|
E.
|
Off-Balance Sheet Arrangements
|
We currently do not have any off-balance sheet arrangements.
|
F.
|
Tabular Disclosure of Contractual Obligations
|
The following table summarizes our known contractual obligations and commitments as of December 31, 2015:
|
(in thousands)
|
|
Payment Due by Period
|
|
| |
|
Total
|
|
|
Less than 1
year
|
|
|
1-3 years
|
|
|
3-5 years
|
|
|
more than 5
years
|
|
|
Contractual Obligations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Premises leasing obligations
|
|
$ |
563 |
|
|
$ |
356 |
|
|
$ |
207 |
|
|
$ |
- |
|
|
$ |
- |
|
|
Car leasing obligations
|
|
$ |
378 |
|
|
$ |
223 |
|
|
$ |
155 |
|
|
$ |
- |
|
|
$ |
- |
|
|
Purchase commitments and obligations
|
|
$ |
2,468
|
|
|
$ |
2,468
|
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
Total
|
|
$ |
3,409
|
|
|
$ |
3 ,047
|
|
|
$ |
362 |
|
|
$ |
- |
|
|
$ |
- |
|
* Undiscounted amounts.
In addition, as of December 31, 2015, our liabilities in respect of long-term employee benefits were approximately $299,000. These liabilities will be paid upon termination of employment of certain employees. Due to the difficulty in determining the timing of payments, these long-term employee benefits obligations are not included in the table above.
|
|
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
|
|
A.
|
Directors and Senior Management
|
The following table lists the names and ages of our directors as of April 28, 2016:
|
Name
|
|
Age
|
|
Position
|
| |
|
|
|
|
|
Jonathan Adereth
|
|
69
|
|
Chairman of the Board of Directors
|
| |
|
|
|
|
|
Ori Hadomi
|
|
48
|
|
Director and Chief Executive Officer
|
| |
|
|
|
|
|
Gil Bianco
|
|
64
|
|
External Director
|
| |
|
|
|
|
|
David Schlachet
|
|
70
|
|
External Director
|
| |
|
|
|
|
|
Sarit Soccary Ben-Yochanan
|
|
43
|
|
Director
|
| |
|
|
|
|
|
Michael Berman
|
|
58
|
|
Director
|
| |
|
|
|
|
The following table lists the names, ages and positions of our senior management as of April 28, 2016:
|
| |
|
|
|
|
|
Name
|
|
Age
|
|
Position
|
| |
|
|
|
|
|
Sharon Levita
|
|
48
|
|
Chief Financial Officer, Secretary
|
| |
|
|
|
|
|
Moshe Shoham
|
|
64
|
|
Chief Technology Officer
|
| |
|
|
|
|
|
Eliyahu Zehavi
|
|
61
|
|
Chief Operating Officer
|
| |
|
|
|
|
|
Sandra Adzic
|
|
37
|
|
Vice President of International Sales
|
| |
|
|
|
|
|
Doron Dinstein
|
|
45
|
|
Chief Medical Officer
|
| |
|
|
|
|
|
Anat Kaphan
|
|
46
|
|
Vice President of Product & International Marketing
|
| |
|
|
|
|
|
Christopher Prentice
|
|
45
|
|
Chief Executive Officer, Mazor Robotics Inc.
|
Jonathan Adereth, Chairman of the Board of Directors
Mr. Adereth has been serving as the chairman of our board of directors since December 2007. Since May 2009, Mr. Adereth has been serving as the chairman of Medic Vision Imaging Solutions Ltd., an Israeli company in the field of dose reduction in Computed Tomography. From October 2004, Mr. Adereth has been serving as a board member of UltraSPECT Ltd., an Israeli company in the field of dose reduction in Nuclear Medicine. From 1994 to 1998, Mr. Adereth served as the President and Chief Executive Officer of Elscint Ltd. (NYSE: ELT), a global developer and manufacturer of CT, MRI and NM systems. Mr. Adereth holds a B.Sc. degree in Physics from the Technion - Israel Institute of Technology.
Gil Bianco, External Director
Mr. Bianco has been serving as an external director since 2007. Mr. Bianco serves as a director of Fischer Pharmaceuticals Ltd., Intec Pharma Ltd., Clear Cut Ltd., Gil Bianco Ltd., and Turquoise GEI Ltd. From 2001 to 2003, he served as chief executive officer of pharmaceutical manufacturer, Agis Industries Ltd., most notably taking part in the company’s listing on the TASE and its global expansion. In the past five years Mr. Bianco had also served as a director of Healor Ltd., Solgel Technologies Ltd., BioCancell Inc., Top Spin Ltd., Kolbar Ltd., BioLineRx Ltd., DPharm Ltd., Optima Ltd., and the Tel Aviv Stock Exchange Ltd. Mr. Bianco holds a B.A. in Economics and Accounting from the Tel-Aviv University, and is a certified public accountant.
David Schlachet, External Director
Mr. Schlachet has been serving as an external director since November 2007. Since 2007, Mr. Schlachet has been serving as a director of Syneron Medical Ltd. (NASDAQ: ELOS), or Syneron, and since April 2014, as a director in BioTime Inc. (NYSEMKT: BTX). In addition, since May 2014, Mr. Schlachet has been serving as a chairman of Taya Investments Ltd., an Israeli public company, and since October 2010, as a director of BioCancell Therapeutics Inc., an Israeli public company and as chairman of CellCure Therapeutics, a privately-owned Israeli company. From 2005 to February 2016, Mr. Schalechet served as a director of Ezchip Semiconductor Ltd. (NASDAQ: EZCH), From November 2008 to November 2012, Mr. Schlachet served as a director of the Tel Aviv Stock Exchange Ltd., as the chairman of the audit committee of the Tel Aviv Stock Exchange Ltd., and as a director and audit committee member of the Tel Aviv Stock Exchange Clearing House Ltd. From November 2005 to May 2007, Mr. Schlachet served as the chief executive officer of Syneron, after having served as its chief financial officer from July 2004 to November 2005. From 2000 to June 2004, Mr. Schlachet served as Managing Partner of Biocom, a venture capital fund specializing in the life sciences area. From 1995 to 2000, Mr. Schlachet served as a senior Vice President and Chief Financial Officer of Strauss Elite Holdings, a packaged food group. From June 1997 to June 2000, Mr. Schlachet also served as an active chairman of Elite Industries, a coffee confectionary and salty snacks company which is a subsidiary of Strauss Group Ltd. (TASE: STRS). From 1990 to 1995, Mr. Schlachet served as Vice President of Finance and Administration of the Weizmann Institute of Science. Mr. Schlachet holds a B.Sc. degree in chemical engineering and an M.B.A. with specialization in finance from Tel-Aviv University.
Sarit Soccary Ben-Yochanan, Director
Mrs. Soccary has been serving as a director since October 2006. Since July 2013, Mrs. Soccary has been serving as the vice president of strategy and business development for Syneron. Until July 2013, Mrs. Soccary had been serving as the chief executive officer of Gefen Biomed Investments Ltd., an Israeli public company. Mrs. Soccary also served as a director of Proteologics Ltd., an Israeli public biotech company, and as a director of several private companies in the fields of technology and healthcare. Mrs. Soccary holds a B.A. and an M.A. in economics from Tel Aviv University.
Michael Berman, Director
Mr. Berman has been serving as a director since February 2014. Mr. Berman is a medical device entrepreneur and investor. He is a co-founder of eight medical device companies and is currently an active board member of several health care companies including Inspire-MD, InterValve Inc., BeneChill Inc., PharmaCentra LLC, Endospam Ltd. and Rebiotix Inc. Michael Berman was co-founder and Chairman of BridgePoint Medical from 2005 until 2012 and served as a Director of Lutonix and UltraShape Inc. until 2011. From 1995 to 2000 Mr. Berman was the president of the cardiology business of Boston Scientific. Mr. Berman received his B.Sc. and M.B.A. degrees from Cornell University.
Ori Hadomi, Director and Chief Executive Officer
Mr. Hadomi has been serving as our Chief Executive Officer and a member of our board of directors since January 2003. Prior to joining us, Mr. Hadomi served as the chief financial officer and vice president of business development of Image Navigation Ltd. (formerly known as DenX Medical Software Systems Ltd.). Mr. Hadomi holds a B.A. in chemistry with a minor in economics, as well as a M.Sc. in industrial chemistry and business administration from the Hebrew University, Jerusalem.
Sharon Levita, Chief Financial Officer
Mrs. Levita has served as our Chief Financial Officer since February 2008. Prior to joining Mazor, from 1999 to 2008, Mrs. Levita held various senior positions at Lumenis Ltd. (NASDAQ: LMNS), a medical lasers and light-based technology company, including Director of Business Development, Executive Vice President of Finance, and Corporate Controller. She holds an M.A. in Business Administration, specializing in finance from Bar-Ilan University, and received her B.A. in Economics and Accounting from Haifa University. Mrs. Levita is a certified public accountant.
Professor Moshe Shoham, Chief Technology Officer
Professor Shoham, one of our co-founders, has served as our Chief Technology Officer since 2003. From 2001 to 2011, Professor Shoham served as one of our directors. From 2005 to 2010, Professor Shoham served as the Head of the Center for Manufacturing Systems and Robotics of the Technion-Israel Institute of Technology, or Technion. Since 1990, Professor Shoham has been serving as a faculty member of the Technion, and since 2005, as an endowed Chaired Professor at the Department of Mechanical Engineering of the Technion and also serves as a director at Microbot Medical Ltd. Professor Shoham holds a B.Sc. degree in Aeronautical Engineering, an M.Sc. degree and a Ph.D. in Mechanical Engineering, all degrees from the Technion.
Eliyahu Zehavi, Chief Operating Officer
Mr. Zehavi has been serving as our Chief Operating Officer, responsible for our research and development department and operation since 2001. From June 1998 to January 2001, Mr. Zehavi served as the vice president of engineering of Elscint, Ltd. Mr. Zehavi holds a B.Sc. in Computer and Electrical Engineering from Ben-Gurion University, and an M.B.A. from the Interdisciplinary Center, Herzliya, Israel.
Sandra Adzic, Vice President of International Sales
Ms. Adzic has served as our Vice President of Sales since February 2015, following the retirement of Avi Posen who previously held this position. From 2011 to 2015, Ms. Adzic served as a Senior Area Sales Manager for our Asia Pacific market. Prior to joining Mazor in 2011, Ms. Adzic held several managerial positions in sales including positions at Mennen Medical Ltd. and Caesarea Medical Electronics.
Doron Dinstein, MD, Chief Medical Officer
Dr. Dinstein has served as our Chief Medical Officer since December 2012. From 2009 to 2012, Dr. Dinstein served as our Vice President of Marketing and Business Development. From 2004 to 2009, Dr. Dinstein served as the Manager of Cardiovascular Applications at Itamar Medical, Ltd., an Israeli public company. Dr. Dinstein holds a joint Executive M.B.A degree from Northwestern University and Tel Aviv University. Dr. Dinstein received a B.M.Sc. and an M.D. from Tel-Aviv University School of Medicine.
Anat Kaphan, Vice President of Product & International Marketing
Mrs. Kaphan has been serving as our Vice President of Product & International Marketing since July 2015. Ms. Kaphan has previously held several product, marketing and business development positions in leading medical device companies, such as from April 2011 to February 2014 at Philips Medical Systems and from December 2001 to April 2011 at Lumenis Ltd. Mrs. Kaphan holds a Bachelor’s degree in Economics and Accounting from Haifa University and an M.B.A in International Marketing from Tel-Aviv University.
Christopher Prentice, Chief Executive Officer, Mazor Robotics Inc.
Mr. Prentice has served as the Chief Executive Officer of our U.S. Subsidiary, Mazor Robotics Inc., since October 2014. From September 2013 until October 2014, Mr. Prentice served as our Senior Vice President of America & Global Marketing and as Vice President of Marketing from July 2012 until September 2013. From 2010 to July 2012, Mr. Prentice served as our sales director for the Southeast region in the United States. Prior to joining Mazor, Mr. Prentice served on the leadership team of Tampa General Hospital. Mr. Prentice graduated from the United States Military Academy at West Point, holds an M.B.A degree from Western New England University, and a Master of Health Administration from the University of South Florida.
Family Relationships
There are no family relationships between any members of our executive management and our directors.
Arrangements for Election of Directors and Members of Management
Except as required by the agreement with the Oracle Investors, there are no arrangements or understandings with major shareholders, customers, suppliers or others pursuant to which any of our executive management or our directors were selected. Pursuant to the agreement with the Oracle Investors, our board of directors was required to appoint one director on behalf of the Oracle Investors, pursuant to a written notice to be provided by the Oracle Investors within 60 days after the closing of the agreement with the Oracle Investors and subject to the exercise of the Oracle Warrants in full. The Oracle Investors did not exercise this right. In the event that at the time of appointment our board of directors consists of seven members or more, which is not currently the case, the Oracle Investors may appoint one additional director on their behalf to our board of directors. The appointment of any such director by the Oracle Investors shall be in effect only until the first general meeting of our shareholders following such appointment. Thereafter, the appointment of any such director nominated by the Oracle Investors shall be subject to election at the shareholders’ general meeting. If the appointment of such director nominee is not approved by the shareholders’ general meeting, then for as long as the Oracle Investors hold together 10% of our issued and outstanding share capital, they will have the right to appoint an observer to our board of directors. For a description of the agreement with the Oracle Investors, see “Item 10. Additional Information - C. Material Contracts.” To date, the Oracle Investors have not appointed any directors.
Director Compensation
Under the Companies Law and the rules and regulations promulgated thereunder, external directors are entitled to fixed annual compensation and to an additional payment for each meeting attended. We currently pay our external directors Gil Bianco and David Schlachet an annual fee of NIS 100,000 (approximately $25,000), and a per meeting fee of NIS 2,500 (approximately $625). In 2007, each of Gil Bianco and David Schlachet received a grant of 40,000 options to purchase our ordinary shares at an exercise price of NIS 12.40 (approximately $3.1) per share, which options were subject to shareholder approval which was duly obtained. These options were exercised in 2014. In addition, in November 2013, our shareholders approved a grant of 40,000 options to purchase our ordinary shares to each of Gil Bianco and David Schlachet at an exercise price of NIS 27.67 (approximately $6.92) per share. These options are subject to a 3 year vesting schedule, commencing on the date of grant, so that upon the lapse of 12 months from the date of grant, 34% of the shares underlying the options shall vest, and thereafter, upon the lapse of each calendar quarter, 8.25% of the shares underlying the options shall vest.
We currently pay our independent directors, Sarit Soccary Ben-Yochanan and Michael Berman, an annual fee of NIS 100,000 (approximately $25,000) and NIS 60,000 (approximately $15,000), respectively, and a per meeting fee of NIS 2,500 (approximately $625). In December 2012, Mrs. Soccary Ben-Yochanan received a grant of 40,000 options to purchase our ordinary shares at an exercise price of NIS 4.521 (approximately $1.30) per share, which options were subject to shareholder approval which was duly obtained. Of these options, 26,800 were exercised during 2014. In addition, in November 2013, our shareholders approved an additional grant of 40,000 options to purchase our ordinary shares to Sarit Soccary Ben-Yochanan, at an exercise price of NIS 27.67 (approximately $6.92) per share. These options are subject to a 3 year vesting schedule, commencing on the date of grant, so that upon the lapse of 12 months from the date of grant, 34% of the shares underlying the options shall vest, and thereafter, upon the lapse of each calendar quarter, 8.25% of the shares underlying the options shall vest. In July 2014, our shareholders approved a grant of 40,000 options to purchase our ordinary shares to Michael Berman, at an exercise price of NIS 27.67 (approximately $6.92) per share. These options are subject to a 3 year vesting schedule, commencing on the date of grant, so that upon the lapse of 12 months from the date of grant, 34% of the shares underlying the options shall vest, and thereafter, upon the lapse of each calendar quarter, 8.25% of the shares underlying the options shall vest.
Since December 2007, Jonathan Adereth has been the Chairman of our board of directors, or the Chairman. We currently pay the Chairman for providing us with management services a monthly fee of NIS 35,000 (approximately $8,750). This amount includes social benefits, as provided by law. In November 2007, the Chairman was granted options to purchase 40,000 of our shares at an exercise price of NIS 12.40 (approximately $3.10) per share. In addition, in November 2013 Mr. Jonathan Adereth received a grant of 80,000 options to purchase our ordinary shares at an exercise price of NIS 32.46 (approximately $8.12) per share, which options were subject to shareholder approval which was duly obtained.
The following table presents all compensation we incurred for the year ended December 31, 2015, to all persons who served as directors at any time during the year. The table does not include any amounts we paid to reimburse any of these persons for costs incurred in providing us with services during this period.
| |
|
Salary and
Related
Benefits
|
|
|
Directors
|
|
|
|
|
| |
|
|
|
|
|
Jonathan Adereth
|
|
$
|
108,167
|
|
| |
|
|
|
|
|
Compensation to directors not employed by us
|
|
$
|
136,525
|
|
All amounts are denominated in NIS and were translated using the rate of NIS 3.8869 to USD 1.00, the average exchange rate reported by the Bank of Israel for 2015.
Employment Agreements
We have entered into written employment agreements with each of our executive officers. All of these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, we have entered into agreements with each executive officer and director pursuant to which we have agreed to indemnify each of them to the fullest extent permitted by law to the extent that these liabilities are not covered by directors and officers insurance. Members of our senior management are eligible for bonuses each year. The bonuses are payable upon meeting objectives and targets that are set by our chief executive officer and approved annually by our board of directors that also set the bonus targets for our chief executive officer.
For a description of the terms of our options and option plans, see “Item 6. Directors, Senior Management and Employees - E. Share Ownership” below.
Employment Agreement with Ori Hadomi
In August 2010, our shareholders approved that as of August 2010, our chief executive officer, or the CEO, would relocate to the United States in his position as the chief executive officer of the U.S. Subsidiary. This agreement with the CEO was in effect until August 2013, and could have been extended by mutual consent, and could have been terminated at any time and for any reason (other than in the event of breach of trust) by the CEO or the U.S. Subsidiary with an advance notice of 60 days. Pursuant to his employment agreement and during fiscal year 2012, Mr. Hadomi’s gross annual salary was $150,000 and he was entitled to (1) monthly reimbursement of expenses in the amount of $8,300, and (2) an annual target bonus equivalent to $120,000, out of which $80,000 would be paid in cash and up to $40,000 would be paid in options at a value based on the Black-Scholes option pricing model.
In December 2012, our shareholders approved a grant to the CEO of options that are exercisable into 150,000 Ordinary Shares. The exercise price of these options is NIS 4.521 ($1.13) per share, and their vesting period is over 36 months, of which 50,000 options will become vested within a year following the date of grant, and the remaining 100,000 options will vest in quarterly installments of 12,500 options per quarter thereafter.
On March 31, 2013, our shareholders approved an amendment to the CEO agreement in connection with his relocation back to Israel, based on the recommendation of the compensation committee and the board of directors, as follows: (1) the CEO annual salary, effective January 1, 2013, has been updated to NIS 65,000 (approximately $16,700) per month and (2) a target bonus that will be equivalent to seven months’ base salary, out of which up to six months’ base salary will be paid in cash and one month base salary will be paid in options at a value based on the Black-Scholes option pricing model. The CEO is entitled to an allocation to a manager’s insurance policy equivalent to 13.33% of his gross monthly salary and 7.5% of his gross monthly salary for an education fund. 5% of his gross monthly salary is deducted for the manager’s insurance policy and 2.5% is deducted for the education fund. The CEO is also entitled to reimbursement for vehicle maintenance costs and reasonable expenses. Upon termination of the CEO’s employment (other than in the event of breach of trust), the CEO will be entitled to a readjustment payment equal to four months’ base salary from the date that he is no longer employed.
On January 22, 2014, our shareholders approved an amendment to the CEO’s employment agreement so that the re-adjustment payment shall be extended from four monthly payments to six monthly payments in the event that the CEO resigns subsequent to a change of control in the Company and it shall be extended from six monthly payments to nine monthly payments in the event that the CEO is terminated subsequent to a change of control in the Company.
On January 22, 2014, our shareholders approved a bonus to the CEO in the sum of NIS 600,000 (approximately $150,000) following the completion of offering of our ADSs in November 2013.
On July 22, 2014, our shareholders approved an update of the employment agreement of the CEO, so that as of April 1, 2014, the CEO’s monthly fixed salary will be increased to NIS 70,000 (approximately $18,000).
On October 22, 2014, our shareholders approved a grant to the CEO of options that are exercisable into 150,000 Ordinary Shares. The exercise price of these options is NIS 26.62 ($6.66) per share, and their vesting period is over 48 months, of which 75,000 options will become vested on July 31, 2016, and the remaining 75,000 options will vest in quarterly installments of 9,375 options per quarter thereafter.
On October 8, 2015, our shareholders approved a grant to the CEO of options that are exercisable into 60,000 Ordinary Shares. The exercise price of these options is NIS 26.99 ($6.75) per share, and their vesting period is over 48 months, of which 30,000 options will become vested on July 15, 2017, and the remaining 30,000 options will vest in quarterly installments of 3,750 options per quarter thereafter.
Employment Agreement with Eliyahu Zehavi
Mr. Zehavi’s current gross monthly salary is NIS 57,000 (approximately $14,250). During fiscal year 2015 Mr. Zehavi’s gross monthly salary was NIS 52,000 (approximately $13,000). In accordance with his employment agreement, Mr. Zehavi is entitled to an allocation to a manager’s insurance policy equivalent to 13.33% of his gross monthly salary and 7.5% of his gross monthly salary for an education fund. 5% of his gross monthly salary is deducted for the manager’s insurance policy and 2.5% is deducted for the education fund. Mr. Zehavi is also entitled to reimbursement for vehicle maintenance costs and reasonable expenses. In addition Mr. Zehavi is entitled to a bonus based on our performance, department performance and personal objectives (as are determined on an annual basis). His target bonus for 2015 was equal to the value of 4 times his gross monthly salary and his target bonus for 2016 is set equal to the value of 4 times his gross monthly salary. His target bonus is set annually based on our business objectives.
In addition, pursuant to his employment agreement, and in accordance with our stock option plans, Mr. Zehavi is also entitled to receive options exercisable into our Ordinary Shares from time to time. As of December 31, 2015, we have granted him options to purchase 674,426 Ordinary Shares in the aggregate, 187,044 of which have vested and are outstanding, and 353,782 of which have been exercised by him. In accordance with our stock option plans, Mr. Zehavi’s options vest over a period of three to four years from the applicable grant date. If we terminate the employment relationship with Mr. Zehavi for cause, all of Mr. Zehavi’s vested and unvested options shall terminate immediately. Upon termination of the employment for any reason (other than cause, death, or disability), vested options may be exercised within 90 days of termination of employment, unless otherwise determined by our compensation committee or the board of directors in accordance with the applicable stock option plan.
Employment Agreement with Sharon Levita
Mrs. Levita’s current gross monthly salary is NIS 55,000 (approximately $13,750). During fiscal year 2015, Mrs. Levita’s gross monthly salary was NIS 50,000 (approximately $12,500). In accordance with her employment agreement, Mrs. Levita is entitled to an allocation to a manager’s insurance policy equivalent to 13.33% of her gross monthly salary and 7.5% of her gross monthly salary for an education fund. 5% of her gross monthly salary is deducted for the manager’s insurance policy and 2.5% is deducted for the education fund. Mrs. Levita is also entitled to reimbursement for vehicle maintenance costs and reasonable expenses. In addition, Mrs. Levita is entitled to a bonus based on our performance and personal objectives. Her target bonus for 2015 was equal to the value of 4 times her monthly gross salary. Her target bonus for 2016 is set equal to the value of 4 times her monthly gross salary. Her target bonus is set annually based on our business objectives.
In addition, pursuant to her employment agreement, and in accordance with our stock option plans, Mrs. Levita is also entitled to receive options exercisable into our Ordinary Shares from time to time. As of December 31, 2015, we have granted her options to purchase 405,600 Ordinary Shares in the aggregate, 80,000 of which have vested and are outstanding, and 192,000 of which have been exercised by her. In accordance with our stock option plans, Mrs. Levita’s options vest over a period between three to four years from the applicable grant date. If we terminate the employment relationship with Mrs. Levita for cause, all of Mrs. Levita’s vested and unvested options shall terminate immediately. Upon termination of employment for any reason (other than cause, death, or disability), vested options may be exercised within 90 days of termination of employment, unless otherwise determined by our compensation committee or the board of directors in accordance with the applicable stock option plan.
Employment Offer with Christopher Prentice
As of September 17, 2014, and in accordance with the resolution of the board of directors of the U.S. Subsidiary, Mr. Prentice serves as the Chief Executive Officer of our U.S. Subsidiary, Mazor Robotics Inc. Mr. Prentice’s current annual salary is $275,000 and he is entitled to customary social benefits (e.g., health insurance, vacation days, and 401(k) plan participation). During fiscal year 2015, Mr. Prentice’s annual salary was $250,000. Mr. Prentice will be entitled to 2 months of salary, in case of resignation, and 3 months in case of termination by the Company (other than for cause or breach of fiduciary duties). In addition, Mr. Prentice is entitled to a bonus based on our performance and personal objectives. His target bonus for 2015 was set at $100,000 and the target bonus for 2016 is set at $100,000. His target bonus is set annually based on our business objectives.
In addition, pursuant to his employment agreement, and in accordance with our stock option plans, Mr. Prentice is also entitled to receive options exercisable into our Ordinary Shares from time to time. As of December 31, 2015, we have granted Mr. Prentice options to purchase 398,500 ordinary shares in the aggregate, 142,500 of which have vested and are outstanding. In accordance with our stock option plans, Mr. Prentice’ options vest over a period of three to four years from the applicable grant date. If we terminate the employment relationship with Mr. Prentice for cause, all of Mr. Prentice’ vested and unvested options shall terminate immediately. Upon termination of employment for any reason (other than cause, death, or disability), vested options may be exercised within 90 days of termination of employment, unless otherwise determined by our compensation committee or the board of directors in accordance with the applicable stock option plan.
Prior to September 2014, Mr. Prentice held a position of Senior Vice President, America & Global Marketing. His compensation package included an annual salary of $207,500, and he was entitled to a bonus based on our performance and personal objectives.
Employment Agreement with Sandra Adzic
Mrs. Adzic’s current gross monthly salary is NIS 39,100 (approximately $9,775). During fiscal year 2015, Mrs. Adzic’s gross monthly salary was NIS 34,000 (approximately $8,500). In accordance with her employment agreement, Mrs. Adzic is entitled to an allocation to a manager’s insurance policy equivalent to 13.33% of her gross monthly salary and 7.5% of her gross monthly salary for an education fund. 5% of her gross monthly salary is deducted for the manager’s insurance policy and 2.5% is deducted for the education fund. Mrs. Adzic is also entitled to reimbursement for vehicle maintenance costs and reasonable expenses. In addition Mrs. Adzic is entitled to a sales commission based on our sales in Europe and Asia-Pacific regions. The sales targets and commission is updated on an annual basis per our business objectives.
In addition, pursuant to her employment agreement, and in accordance with our stock option plans, Mrs. Adzic is also entitled to receive options exercisable into our Ordinary Shares from time to time. As of December 31, 2015, we have granted her options to purchase 168,500 Ordinary Shares in the aggregate, 15,000 of which have vested. In accordance with our stock option plans, Mrs. Adzic’s options vest over a period of three to four years from the applicable grant date. If we terminate the employment relationship with Mrs. Adzic for cause, all of Mrs. Adzic’s vested and unvested options shall terminate immediately. Upon termination of the employment for any reason (other than cause, death, or disability), vested options may be exercised within 90 days of termination of employment, unless otherwise determined by our compensation committee or the board of directors in accordance with the applicable stock option plan.
Annual compensation
The following table presents all compensation we incurred for the year ended December 31, 2015 to the five highest paid officers in U.S. dollars. The table does not include any amounts we paid to reimburse any of these persons for costs incurred in providing us with services during this period:
| |
|
Annual Compensation
|
|
|
Executive Officer
|
|
Salary and
Related
Benefits
|
|
|
Retirement
and Other
Similar
Benefits
|
|
|
Long Term
Compensation
Share Based
Compensation*
|
|
|
Total
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Christopher Prentice
|
|
$ |
362,786 |
(1)
|
|
$ |
8,610 |
|
|
$ |
389,864 |
|
|
$ |
761,260 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ori Hadomi
|
|
$ |
384,525 |
(1)
|
|
$ |
5,674 |
|
|
$ |
180,650 |
|
|
$ |
570,849
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eliyahu Zehavi
|
|
$ |
272,537 |
(1)
|
|
$ |
14,965 |
|
|
$ |
151,800 |
|
|
$ |
439,302 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sharon Levita
|
|
$ |
263,633 |
(1)
|
|
$ |
12,169 |
|
|
$ |
152,601 |
|
|
$ |
428,403 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sandra Adzic
|
|
$ |
174,042 |
(1)
|
|
$ |
3,873 |
|
|
$ |
134,279 |
|
|
$ |
312,194 |
|
Amounts denominated in NIS were translated using the rate of NIS 3.8869 to USD 1.00, the average exchange rate reported by the Bank of Israel for 2015.
|
(*)
|
The fair value of share options granted to employees, directors and service providers is measured using the binomial model. Measurement inputs include the share price on the measurement date, the exercise price of the instrument, expected volatility (based on the historical volatility), the expected life span of the options, and the risk-free interest rate (based on government debentures). Service and non-market performance conditions attached to the transactions are not taken into account in determining fair value.
|
|
(1)
|
Includes base salary, social benefits, bonus or commission, and car allowances.
|
C. Board Practices
Introduction
Our board of directors presently consists of six members, including two external directors, as required to be appointed under the Companies Law. Our articles of association provide that the number of board of directors’ members (including external directors) shall be set by the general meeting of the shareholders provided that it will consist of not less than five and not more than nine members. Pursuant to the Companies Law, the management of our business is vested in our board of directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our board of directors. Our Chief Executive Officer is appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with him. All other executive officers are appointed by our Chief Executive Officer. Their terms of employment are subject to the approval of the board of directors’ compensation committee and of the board of directors, and are subject to the terms of any applicable employment agreements that we may enter into with them; provided, however, that if the terms of employment are not in compliance with our compensation policy, the terms of employment may only be approved by the board of directors’ compensation committee and by the board of directors for special reasons to be noted, and with regards to our Chief Executive Officer the terms of employment shall also require the shareholders' approval. If the terms of employment are immaterially non-compliant with our Chief Executive Officer’s compensation arrangement and are in compliance with our compensation policy, the approval of the compensation committee is sufficient.
Each director, except external directors, will hold office until the annual general meeting of our shareholders for the year in which his or her term expires, unless he or she is removed by a majority vote of our shareholders at a general meeting of our shareholders or upon the occurrence of certain events, in accordance with the Companies Law and our articles of association.
In addition, our articles of association allow our board of directors to appoint directors to fill vacancies on our board of directors or in addition to the acting directors (subject to the limitation on the number of directors), until the next annual general meeting or special general meeting in which directors may be appointed or terminated. External directors may be elected for up to two additional three-year terms after their initial three-year term under the circumstances described below, with certain exceptions as described in “External Directors” below. External directors may be removed from office only under the limited circumstances set forth in the Companies Law. See “—External Directors” below.
Under the Companies Law nominations for directors may be made by any shareholder holding at least one percent of our outstanding voting power. However, any such shareholder may make such a nomination only if a written notice of such shareholder’s intent to make such nomination has been given to our board of directors. Any such notice must include certain information, the consent of the proposed director nominee(s) to serve as our director(s) if elected and a declaration signed by the nominee(s) declaring that there is no limitation under the Companies Law preventing their election and that all of the information that is required to be provided to us in connection with such election under the Companies Law has been provided.
Under the Companies Law, our board of directors must determine the minimum number of directors who are required to have accounting and financial expertise. In determining the number of directors required to have such expertise, our board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that the minimum number of directors of our company who are required to have accounting and financial expertise is one.
Pursuant to the Companies Law and our articles of association, a resolution proposed at any meeting of the board of directors, at which a quorum is present, is adopted if approved by a vote of at least a majority of the directors present at the meeting. A quorum of the board of directors (or any committee thereof, other than the audit committee) is at least a majority of the directors then in office who are lawfully entitled to participate in the meeting (until otherwise unanimously decided by the directors). Minutes of the meetings are recorded and kept at our offices.
The board of directors may elect one director to serve as the chairman of the board of directors to preside at the meetings of the board of directors, and may also remove that director as chairman. Pursuant to the Companies Law, neither the chief executive officer nor any of his or her relatives is permitted to serve as the chairman of the board of directors, and a company may not vest the chairman or any of his or her relatives with the chief executive officer’s authorities. In addition, a person who reports, directly or indirectly, to the chief executive officer may not serve as the chairman of the board of directors; the chairman may not be vested with authorities of a person who reports, directly or indirectly, to the chief executive officer; and the chairman may not serve in any other position in the company or a controlled company, but he or she may serve as a director or chairman of a controlled company. However, the Companies Law permits a company’s shareholders to determine, for a period not exceeding three years from each such determination, that the chairman or his or her relative may serve as chief executive officer or be vested with the chief executive officer’s authorities, and that the chief executive officer or his or her relative may serve as chairman or be vested with the chairman’s authorities. Such determination of a company’s shareholders requires either: (1) the approval of at least two-thirds of the shares of those shareholders present and voting on the matter (other than controlling shareholders and those having a personal interest in the determination); or (2) that the total number of shares opposing such determination does not exceed 2% of the total voting power in the company.
The board of directors may, subject to the provisions of the Companies Law, delegate any or all of its powers to committees of the board, and it may, from time to time, revoke such delegation or alter the composition of any such committees, subject to certain limitations. Unless otherwise expressly provided by the board of directors, the committees shall not be empowered to further delegate such powers. The composition and duties of our audit committee, financial statement examination committee and compensation committee are described below. Any committee exercising the powers of the board of directors must contain at least one external director.
The board of directors oversees how management monitors compliance with our risk management policies and procedures, and reviews the adequacy of the risk management framework in relation to the risks faced by us. The board of directors is assisted in its oversight role by an internal auditor. The internal auditor undertakes both regular and ad hoc reviews of risk management controls and procedures, the results of which are reported to our audit committee.
External Directors
Under the Companies Law, an Israeli company whose shares have been offered to the public or whose shares are listed for trading on a stock exchange in or outside of Israel is required to appoint at least two external directors to serve on its board of directors. Our external directors are Mr. Gil Bianco and Mr. David Schlachet. At least one of the external directors is required to have “financial and accounting expertise,” unless another member of the audit committee, who is an independent director under the NASDAQ Stock Market rules, has “financial and accounting expertise,” and the other external director or directors are required to have “professional expertise”. An external director may not be appointed to an additional term unless: (1) such director has “accounting and financial expertise;” or (2) he or she has “professional expertise,” and on the date of appointment for another term there is another external director who has “accounting and financial expertise” and the number of “accounting and financial experts” on the board of directors is at least equal to the minimum number determined appropriate by the board of directors.
A director has “professional expertise” if he or she satisfies one of the following:
|
|
●
|
the director holds an academic degree in one of these areas: economics, business administration, accounting, law or public administration;
|
|
|
●
|
the director holds an academic degree or has other higher education, all in the main business sector of the company or in a relevant area for the board position; or
|
|
|
●
|
the director has at least five years’ experience in one or more of the following (or a combined five years’ experience in at least two or more of these): (a) senior management position in a corporation of significant business scope; (b) senior public office or senior position in the public sector; or (c) senior position in the main business sector of the company.
|
A director with “financial and accounting expertise” is a person that due to his or her education, experience and skills has high skills and understanding of business-accounting issues and financial reports which allow him to deeply understand the financial reports of the company and hold a discussion relating to the presentation of financial information. The company’s board of directors will take into consideration in determining whether a director has “accounting and financial expertise”, among other things, his or her education, experience and knowledge in any of the following:
|
|
●
|
accounting issues and accounting control issues characteristic to the segment in which the company operates and to companies of the size and complexity of the company;
|
|
|
●
|
the functions of the external auditor and the obligations imposed on such auditor; and
|
|
|
●
|
preparation of financial reports and their approval in accordance with the Companies Law and the securities law.
|
A person may not serve as an external director if the person is a relative of a controlling shareholder or if at the date of the person’s appointment or within the prior two years the person, or his or her relatives, partners, employers or entities under the person’s control, or someone to whom he or she is subordinate, whether directly or indirectly, have or had any affiliation with any of: (1) us, (2) any entity controlling us, (3) a relative of the controlling shareholder on the date of such appointment, or (4) any entity controlled, on the date of such appointment or within the preceding two years, by us or by our controlling shareholder. If there is no controlling shareholder or no one shareholder holding 25% or more of voting rights in the company, a person may not serve as an external director if the person has any affiliation with any person who, as of the date of the person’s appointment, was the chairman of the board of directors, the general manager (chief executive officer), any shareholder holding 5% or more of the company’s shares or voting rights, or the senior financial officer. We refer to each of the relationships set forth in this paragraph as an Affiliated Party.
Under the Companies Law, “affiliation” includes:
|
|
●
|
an employment relationship;
|
|
|
●
|
a business or professional relationship maintained on a regular basis;
|
|
|
●
|
service as an office holder, excluding service as a director of a private company prior to the first offering of its shares to the public if such director was appointed as a director of the private company in order to serve as an external director following the initial public offering.
|
A “relative” is defined as a spouse, sibling, parent, grandparent, descendant, and a descendant, sibling or parent or the spouse of each of the foregoing.
An “office holder” is defined as a general manager, chief operating officer, executive vice president, vice president, director or manager directly subordinate to the general manager or any other person assuming the responsibilities of any of these positions regardless of that person’s title. Each person listed in the table under “Item 6. Directors, Senior Management and Employees – A. Directors and Senior Management” is an office holder.
A person may not serve as an external director if that person or that person’s relative, partner, employer, a person to whom such person is subordinate (directly or indirectly) or any entity under the person’s control has a business or professional relationship with any entity that has an affiliation with any Affiliated Party, even if such relationship is intermittent (excluding insignificant relationships). Additionally, any person who has received compensation in breach of the Companies Law (excluding compensation from insignificant relationships) other than compensation permitted under the Companies Law may not continue to serve as an external director.
A person may not serve as an external director if that person’s position or other business activities create, or may create, a conflict of interest with the person’s service as a director or may otherwise interfere with the person’s ability to serve as a director. If at the time any external director is appointed, all members of the board who are neither controlling shareholders nor relatives of controlling shareholders are the same gender, then the external director to be appointed must be of the other gender. A director of a company shall not be appointed as an external director of another company if at such time a director of the other company is acting as an external director of the first company.
Until the lapse of two years from the termination of office, none of the company in which such external director served, its controlling shareholder or any entity under the control of such controlling shareholder may, either directly or indirectly, grant such former external director, or his or her spouse or child, any benefit, including via (1) the appointment of such former director or his or her spouse or his child as an office holder in the company or in an entity controlled by the company’s controlling shareholder, (2) the employment of such former director and (3) the engagement, either directly or indirectly, of such former director as a provider of professional services for compensation, including through an entity under his or her control. The same restrictions above apply to relatives other than a spouse or a child, but such limitations shall only apply for one year from the date such external director ceased to be engaged in such capacity. External directors are elected by a majority vote at a shareholders’ meeting, so long as either:
|
|
●
|
at least a majority of the shares held by shareholders who are not controlling shareholders and do not have personal interest in the appointment (excluding a personal interest that did not result from the shareholder’s relationship with the controlling shareholder) have voted in favor of the proposal (shares held by abstaining shareholders shall not be considered); or
|
|
|
●
|
the total number of shares of such shareholders voted against the election of the external director does not exceed 2% of the aggregate voting rights of our company.
|
The Companies Law provides for an initial three-year term for an external director. Thereafter, an external director may be reelected by shareholders to serve in that capacity for up to two additional three-year terms, with certain exceptions as explained below, provided that either:
|
(i)
|
his or her service for each such additional term is recommended by one or more shareholders holding at least one percent of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds two percent of the aggregate voting rights in the company and such external director is not an interested shareholder or a competitor or relative of such shareholder, at the time of appointment, and is not affiliated with or related to an interested shareholder or competitor, at the time of appointment or the two years prior to the date of appointment.
|
“Interested shareholder or a competitor “ – a shareholder who recommended the appointment for each such additional term or a substantial shareholder, if at the time of appointment, it, its controlling shareholder or a company controlled by any of them, has business relations with the company or any of them are competitors of the company.; or
|
(ii)
|
his or her service for each such additional term is recommended by the board of directors and is approved at a shareholders meeting by the same disinterested majority required for the initial election of an external director (as described above).
|
|
(iii)
|
the external director offered his or her service for each such additional term and was approved in accordance with the provisions of section (i) above.
|
The term of office for external directors for Israeli companies traded on certain foreign stock exchanges, including the NASDAQ Global Market, may be extended indefinitely in increments of additional three-year terms, in each case provided that the audit committee and the board of directors of the company confirm that, in light of the external director’s expertise and special contribution to the work of the board of directors and its committees, the reelection for such additional period(s) is beneficial to the company, and provided that the external director is reelected subject to the same shareholder vote requirements as if elected for the first time (as described above). Prior to the approval of the reelection of the external director at a general shareholders meeting, the company’s shareholders must be informed of the term previously served by him or her and of the reasons why the board of directors and audit committee recommended the extension of his or her term.
External directors may be removed only by the same special majority of shareholders required for their election or by a court, and in both cases only if the external directors cease to meet the statutory qualifications for their appointment or if they violate their duty of loyalty to our company. In the event of a vacancy created by an external director which causes the company to have fewer than two external directors, the board of directors is required under the Companies Law to call a shareholders meeting as soon as possible to appoint such number of new external directors in order that the company thereafter has two external directors.
External directors may be compensated only in accordance with regulations adopted under the Companies Law.
Fiduciary Duties of Office Holders
The Companies Law imposes a duty of care and a duty of loyalty on all office holders of a company.
The duty of care requires an office holder to act with the level of skill with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care of an office holder includes a duty to use reasonable means to obtain:
|
|
●
|
information on the advisability of a given action brought for his approval or performed by him by virtue of his position; and
|
|
|
●
|
all other important information pertaining to these actions.
|
The duty of loyalty of an office holder requires an office holder to act in good faith and for the benefit of the company, and includes a duty to:
|
|
●
|
refrain from any conflict of interest between the performance of his duties in the company and his performance of his other duties or personal affairs;
|
|
|
●
|
refrain from any action that constitutes competition with the company’s business;
|
|
|
●
|
refrain from exploiting any business opportunity of the company to receive a personal gain for himself or others; and
|
|
|
●
|
disclose to the company any information or documents relating to the company’s affairs which the office holder has received due to his position as an office holder.
|
Approval of Related Party Transactions under Israeli Law
General. Under the Companies Law, we may approve an action by an office holder from which the office holder would otherwise have to refrain, as described above, if:
|
|
●
|
the office holder acts in good faith and the act or its approval does not cause harm to the company; and
|
|
|
●
|
the office holder disclosed the nature of his or her interest in the transaction (including any significant fact or document) to the company at a reasonable time before the company’s approval of such matter.
|
Disclosure of Personal Interests of an Office Holder
The Companies Law requires that an office holder disclose to the company, promptly, and, in any event, not later than the board meeting at which the transaction is first discussed, any direct or indirect personal interest that he or she may have and all related material information known to him or her relating to any existing or proposed transaction by the company. If the transaction is an extraordinary transaction, the office holder must also disclose any personal interest held by:
|
|
●
|
the office holder’s relatives; or
|
|
|
●
|
any corporation in which the office holder or his or her relatives holds 5% or more of the shares or voting rights, serves as a director or general manager or has the right to appoint at least one director or the general manager.
|
Under the Companies Law, an extraordinary transaction is a transaction:
|
|
●
|
not in the ordinary course of business;
|
|
|
●
|
not on market terms; or
|
|
|
●
|
that is likely to have a material effect on the company’s profitability, assets or liabilities.
|
The Companies Law does not specify to whom within us nor the manner in which required disclosures are to be made. We require our office holders to make such disclosures to our board of directors.
Under the Companies Law, once an office holder complies with the above disclosure requirement, the board of directors may approve a transaction between the company and an office holder, or a third party in which an office holder has a personal interest, unless the articles of association provide otherwise and provided that the transaction is not detrimental to the company’s interest. If the transaction is an extraordinary transaction, first the audit committee and then the board of directors, in that order, must approve the transaction. Under specific circumstances, shareholder approval may also be required. A director who has a personal interest in an extraordinary transaction, which is considered at a meeting of the board of directors or the audit committee, may not be present at this meeting or vote on this matter, unless a majority of the board of directors or the audit committee, as the case may be, has a personal interest. If a majority of the board of directors has a personal interest, then shareholder approval is generally also required.
Under the Companies Law, all arrangements as to compensation of office holders require approval of the compensation committee and board of directors, and compensation of office holders who are directors must be also approved, subject to certain exceptions, by the shareholders, in that order.
Disclosure of Personal Interests of a Controlling Shareholder
Under the Companies Law, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, as well as transactions for the provision of services whether directly or indirectly by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, and transactions concerning the terms of engagement of a controlling shareholder or a controlling shareholder’s relative, whether as an office holder or an employee, require the approval of the audit committee or the compensation committee, as the case may be, the board of directors and a majority of the shares voted by the shareholders of the company participating and voting on the matter in a shareholders’ meeting. In addition, the shareholder approval must fulfill one of the following requirements:
|
|
●
|
at least a majority of the shares held by shareholders who have no personal interest in the transaction and are voting at the meeting must be voted in favor of approving the transaction, excluding abstentions; or
|
|
|
●
|
the shares voted by shareholders who have no personal interest in the transaction who vote against the transaction represent no more than 2% of the voting rights in the company.
|
In addition, any extraordinary transaction with a controlling shareholder or in which a controlling shareholder has a personal interest with a term of more than three years requires the abovementioned approval every three years, however, such transactions not involving the receipt of services or compensation can be approved for a longer term, provided that the audit committee determines that such longer term is reasonable under the circumstances.
The Companies Law requires that every shareholder that participates, in person, by proxy or by voting instrument, in a vote regarding a transaction with a controlling shareholder, must indicate in advance or in the ballot whether or not that shareholder has a personal interest in the vote in question. Failure to so indicate will result in the invalidation of that shareholder’s vote.
Duties of Shareholders
Under the Companies Law, a shareholder has a duty to refrain from abusing its power in the company and to act in good faith and in an acceptable manner in exercising its rights and performing its obligations to the company and other shareholders, including, among other things, voting at general meetings of shareholders on the following matters:
|
|
●
|
amendment of the articles of association;
|
|
|
●
|
increase in the company’s authorized share capital;
|
|
|
●
|
the approval of related party transactions and acts of office holders that require shareholder approval.
|
A shareholder also has a general duty to refrain from oppressing other shareholders.
The remedies generally available upon a breach of contract will also apply to a breach of the above mentioned duties, and in the event of oppression of other shareholders, additional remedies are available to the injured shareholder.
In addition, any controlling shareholder, any shareholder that knows that its vote can determine the outcome of a shareholder vote and any shareholder that, under a company’s articles of association, has the power to appoint or prevent the appointment of an office holder, or has another power with respect to a company, is under a duty to act with fairness towards the company. The Companies Law does not describe the substance of this duty except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness, taking the shareholder’s position in the company into account.
Committees of the Board of Directors
Our board of directors has established three standing committees, the audit committee, the compensation committee and the Financial Statement Examination Committee.
Audit Committee
Under the Companies Law, the board of directors of any public company must establish an audit committee. The audit committee must consist of at least three directors and must include all of the external directors. Under the NASDAQ Stock Market rules, we are required to maintain an audit committee of at least three members, all of whom must be independent directors as defined therein. The NASDAQ Stock Market rules also require that at least one member of the audit committee be a financial expert.
Under the Companies Law, the majority of members of the audit committee, as well as a majority of members present at audit committee meetings, must be unaffiliated directors (as defined below), and the audit committee chairman shall be an external director. In addition, the following are disqualified from serving as members of the audit committee: the chairman of the board, a controlling shareholder and his relatives, any director employed by the company or by its controlling shareholder or by an entity controlled by the controlling shareholder, a director who regularly provides services to the company or to its controlling shareholder or to an entity controlled by the controlling shareholder, and any director who derives the majority of his or her income from the controlling shareholder. Any persons disqualified from serving as a member of the audit committee may not be present at the audit committee meetings, unless the chairman of the audit committee has determined that the presence of such person is required to present a matter to the meeting or if such person qualifies under an available exemption in the Companies Law.
An “unaffiliated director” is defined under the Companies Law as an external director or a director who meets the following conditions: (1) satisfies the conditions for appointment as an external director (as described above) except for (a) the requirement that the director be an Israeli resident (which does not apply to companies such as ours whose securities have been offered outside of Israel or are listed outside of Israel) and (b) the requirement for accounting and financial expertise or professional qualifications and the audit committee has determined that such conditions have been met and (2) he or she has not served as a director of the company for more than nine consecutive years, with any interruption of up to two years in his or her service not being deemed a disruption in the continuity of such service. In companies such as ours whose securities have been offered outside of Israel or are listed outside of Israel the term of nine years may be extended by the audit committee and by the board of directors in increments up to three years, provided that the audit committee and thereafter the board of directors have approved that in light of such director’s expertise and special contribution to the work of board of directors and its committees, the appointment to another term is for the good of the company. On September 3, 2015, our audit committee and board of directors approved that in light of Mrs. Soccary Ben-Yochanan’s knowledge and experience with financial reporting and the medical device industry, as well as her unique contribution to our board of directors and its committees, her appointment to another term as an unaffiliated director is s in the best interest of the Company, and proposed her re-election to the general meeting held on October 8, 2015.
Our audit committee, acting pursuant to a written charter, is comprised of Mr. Bianco, Mrs. Soccary Ben-Yochanan, and Mr. Schlachet.
Our audit committee acts as a committee for review of our financial statements as required under the Companies Law, and in such capacity oversees and monitors our accounting; financial reporting processes and controls; audits of the financial statements; compliance with legal and regulatory requirements as they relate to financial statements or accounting matters; the independent registered public accounting firm’s qualifications, independence and performance; and provide the board of directors with the results of the foregoing.
Under the Companies Law, our audit committee is responsible for:
|
(i)
|
determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices. If the audit committee determines that such a deficiency is material it shall hold at least one meeting concerning such deficiency in the presence of the internal auditor without the presence of office holders who are not members of the audit committee;
|
|
(ii)
|
determining whether certain actions of office holders in breach of their fiduciary duties are material or not and whether certain transactions with office holders or in which office holders have a personal interest are extraordinary or ordinary for their approval under the Companies Law.
|
|
(iii)
|
determining whether transaction with controlling shareholders, even if they are not extraordinary, the duty to employ a competitive process concerning thereto, to be supervised by the audit committee or whoever it will appoint in accordance with criteria determined by it. The audit committee may determine such criteria one a year in advance for the whole year.
|
|
(iv)
|
determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest and whether such transaction is extraordinary or material under Companies Law) (see “Approval of Related Party Transactions under Israeli law”);
|
|
(v)
|
determining the approval process of non-negligible transactions. For the matter “non-negligible transactions” shall mean transactions with controlling shareholders which the audit committee had defined as not extraordinary and not negligible. The audit committee may determine such criteria one a year in advance for the whole year.
|
|
(vi)
|
where the board of directors approves the work plan of the internal auditor, to examine such working plan before its submission to the board of directors and proposing amendments thereto;
|
|
(vii)
|
examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to dispose of its responsibilities;
|
|
(viii)
|
examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the appointment of our auditor; and
|
|
(ix)
|
establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
|
Our audit committee may not conduct any discussions or approve any actions requiring its approval (see “Approval of Related Party Transactions under Israeli law”), unless at the time of the approval a majority of the committee’s members are present, which majority consists of unaffiliated directors including at least one external director.
NASDAQ Stock Market Requirements for Audit Committee
Under the NASDAQ Stock Market rules, we are required to maintain an audit committee consisting of at least three members, all of whom are independent and are financially literate and one of whom has accounting or related financial management expertise.
In accordance with the Sarbanes-Oxley Act of 2002 and the NASDAQ Stock Market rules, the audit committee is directly responsible for the appointment, compensation and performance of our independent auditors. In addition, the audit committee is responsible for assisting the board of directors in reviewing our annual financial statements, the adequacy of our internal controls and our compliance with legal and regulatory requirements. The audit committee also oversees our major financial risk exposures and policies for managing such potential risks, discusses with management and our independent auditor significant risks or exposure and assesses the steps management has taken to minimize such risk.
As noted above, the members of our audit committee include Mr. Bianco, Mrs. Soccary Ben-Yochanan, and Mr. Schlachet, each of whom is “independent,” as such term is defined in under NASDAQ Stock Market rules. Mr. Bianco serves as the chairman of our audit committee. All members of our audit committee meet the requirements for financial literacy under the NASDAQ Stock Market rules. Our board of directors has determined that each member of our audit committee is an audit committee financial expert as defined by the SEC rules and has the requisite financial experience as defined by the NASDAQ Stock Market rules.
Financial Statement Examination Committee
Under the Companies Law, the board of directors of a public company in Israel must appoint a financial statement examination committee, which consists of members with accounting and financial expertise or the ability to read and understand financial statements. According to a resolution of our board of directors, the audit committee has been assigned the responsibilities and duties of a financial statements examination committee, as permitted under relevant regulations promulgated under the Companies Law. From time to time as necessary and required to approve our financial statements, the audit committee holds separate meetings, prior to the scheduled meetings of the entire board of directors regarding financial statement approval. The function of a financial statements examination committee is to discuss and provide recommendations to its board of directors (including the report of any deficiency found) with respect to the following issues: (1) estimations and assessments made in connection with the preparation of financial statements; (2) internal controls related to the financial statements; (3) completeness and propriety of the disclosure in the financial statements; (4) the accounting policies adopted and the accounting treatments implemented in material matters of the company; (5) value evaluations, including the assumptions and assessments on which evaluations are based and the supporting data in the financial statements. Our independent registered public accounting firm and our internal auditors are invited to attend all meetings of the audit committee when it is acting in the role of the financial statements examination committee.
Compensation Committee
Under the Companies Law, the board of directors of any public company must establish a compensation committee. The compensation committee must be comprised of at least three directors, including all of the external directors, who must constitute a majority of the members of the compensation committee. However, subject to certain exceptions, Israeli companies whose securities are traded on stock exchanges such as NASDAQ, and who do not have a shareholder holding 25% or more of the company’s share capital, do not have to meet this majority requirement; provided, however, that the compensation committee meets other Companies Law composition requirements, as well as the requirements of the jurisdiction where the company’s securities are traded. Each compensation committee member that is not an external director must be a director whose compensation does not exceed an amount that may be paid to an external director. The compensation committee is subject to the same Companies Law restrictions as the audit committee as to (a) who may not be a member of the committee and (b) who may not be present during committee deliberations as described above.
Our compensation committee is acting pursuant to a written charter, and consists of Mr. Bianco, Mrs. Ben-Yochanan and Mr. Schlachet, each of whom is “independent,” as such term is defined under the NASDAQ Stock Market rules. Our compensation committee complies with the provisions of the Companies Law, the regulations promulgated thereunder, and the Articles, on all aspects referring to its independence, authorities and practice. Our compensation committee follows home country practice as opposed to complying with the compensation committee membership and charter requirements prescribed under the NASDAQ Stock Market rules.
Our compensation committee reviews and recommends to our board of directors: (1) the annual base compensation of our executive officers and directors; (2) annual incentive bonus, including the specific goals and amount; (3) equity compensation; (4) employment agreements, severance arrangements, and change in control agreements/provisions; and (5) retirement grants and/or retirement bonuses (6) any other benefits, compensation, compensation policies or arrangements.
The duties of the compensation committee include the recommendation to the company’s board of directors of a policy regarding the terms of engagement of office holders, to which we refer as a compensation policy. That policy must be adopted by the company’s board of directors, after considering the recommendations of the compensation committee. The compensation policy is then brought for approval by our shareholders. On January 21, 2014, our shareholders approved our compensation policy.
The compensation policy must serve as the basis for decisions concerning the financial terms of employment or engagement of executive officers and directors, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. The compensation policy must relate to certain factors, including advancement of the company’s objectives, the company’s business and its long-term strategy, and creation of appropriate incentives for executives. It must also consider, among other things, the company’s risk management, size and the nature of its operations. The compensation policy must furthermore consider the following additional factors:
|
|
●
|
the knowledge, skills, expertise and accomplishments of the relevant director or executive;
|
|
|
●
|
the director’s or executive’s roles and responsibilities and prior compensation agreements with him or her;
|
|
|
●
|
the relationship between the terms offered and the average and median compensation of the other employees of the company, including those employed through manpower companies;
|
|
|
●
|
the impact of disparities in salary upon work relationships in the company;
|
|
|
●
|
the possibility of reducing variable compensation at the discretion of the board of directors; and the possibility of setting a limit on the exercise value of non-cash variable compensation; and
|
|
|
●
|
as to severance compensation, the period of service of the director or executive, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company.
|
The compensation policy must also include the following principles:
|
|
●
|
the link between variable compensation and long-term performance and measurable criteria;
|
|
|
●
|
the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation;
|
|
|
●
|
the conditions under which a director or executive would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements;
|
|
|
●
|
the minimum holding or vesting period for variable, equity-based compensation; and
|
|
|
●
|
maximum limits for severance compensation.
|
The compensation policy must also consider appropriate incentives from a long-term perspective and maximum limits for severance compensation.
The compensation committee is responsible for (a) recommending the compensation policy to a company’s board of directors for its approval (and subsequent approval by our shareholders) and (b) duties related to the compensation policy and to the compensation of a company’s office holders as well as functions previously fulfilled by a company’s audit committee with respect to matters related to approval of the terms of engagement of office holders, including:
|
|
●
|
recommending whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years);
|
|
|
●
|
recommending to the board of directors periodic updates to the compensation policy;
|
|
|
●
|
assessing implementation of the compensation policy; and
|
|
|
●
|
determining whether the compensation terms of the chief executive officer of the company need not be brought to approval of the shareholders.
|
Internal Auditor
Under the Companies Law, the board of directors must also appoint an internal auditor nominated by the audit committee. Our internal auditor is Doron Cohen, CPA (Israel), a partner at Fahn Kanne Control Management Ltd., Grant Thornton Israel. The role of the internal auditor is to examine whether a company’s actions comply with the law and proper business procedure. The internal auditor may not be an interested party or office holder, or a relative of any interested party or office holder, and may not be a member of the company’s independent accounting firm or its representative. The Companies Law defines an interested party as a holder of 5% or more of the shares or voting rights of a company, any person or entity that has the right to nominate or appoint at least one director or the general manager of the company or any person who serves as a director or as the general manager of a company. Our internal auditor is not our employee, but the managing partner of an accounting firm which specializes in internal auditing.
Remuneration of Directors
Under the Companies Law, remuneration of directors is subject to the approval of the compensation committee, thereafter by the board of directors and thereafter by the general meeting of the shareholders. In case the remuneration of the directors is in accordance with regulation applicable to remuneration of the external directors then such remuneration shall be exempt from the approval of the general meeting.
Insurance
Under the Companies Law, a company may obtain insurance for any of its office holders for:
|
|
●
|
a breach of his or her duty of care to the company or to another person;
|
|
|
●
|
a breach of his or her duty of loyalty to the company, provided that the office holder acted in good faith and had reasonable cause to assume that his or her act would not prejudice the company’s interests; and
|
|
|
●
|
a financial liability imposed upon him or her in favor of another person concerning an act performed by such office holder in his or her capacity as an officer holder.
|
We currently have directors’ and officers’ liability insurance providing total coverage of $30 million for the benefit of all of our directors and officers, in respect of which we paid a twelve-month premium of approximately $80,000, which expires July 26, 2016. This insurance policy was updated in July 2014 and our coverage was increased from $17.5 million following the listing of the ADSs on the NASDAQ Capital Market to include additional coverage for our officers and directors.
On November 26, 2013, our shareholders approved the extension of our current directors and officers liability insurance coverage and the engagement for future directors and officers liability insurance coverage with respect to all officers of us and our U.S. Subsidiary, for the period commencing on May 28, 2014 and ending upon the lapse of up to three annual insurance periods, in the aggregate, as shall be determined by the Board of Directors; provided, however, that the limit of liability under each such policy shall not exceed $35,000,000 and that the annual premium for each such policy shall not exceed $200,000.
Indemnification
The Companies Law provides that a company may indemnify an office holder against:
|
|
●
|
a financial liability imposed on him or her in favor of another person by any judgment concerning an act performed in his or her capacity as an office holder;
|
|
|
●
|
reasonable litigation expenses, including attorneys’ fees, expended by the office holder or charged to him or her by a court relating to an act performed in his or her capacity as an office holder, in connection with: (1) proceedings that the company institutes, or that another person institutes on the company’s behalf, against him or her; (2) a criminal charge of which he or she was acquitted; or (3) a criminal charge for which he or she was convicted for a criminal offense that does not require proof of criminal thought; and
|
|
|
●
|
reasonable litigation expenses, including attorneys’ fees, expended by the office holder as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (1) no indictment (as defined in the Companies Law) was filed against such office holder as a result of such investigation or proceeding; and (2) no financial liability as a substitute for the criminal proceeding (as defined in the Companies Law) was imposed upon him or her as a result of such investigation or proceeding, or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent.
|
Our articles of association allow us to indemnify our office holders to the fullest extent permitted by law. The Companies Law also permits a company to undertake in advance to indemnify an office holder, provided that if such indemnification relates to financial liability imposed on him or her, as described above, then the undertaking should be limited:
|
|
●
|
to categories of events that the board of directors determines are likely to occur in light of the operations of the company at the time that the undertaking to indemnify is made; and
|
|
|
●
|
in amount or criterion determined by the board of directors, at the time of the giving of such undertaking to indemnify, to be reasonable under the circumstances.
|
We have entered into indemnification agreements with all of our directors and with certain members of our senior management. Each such indemnification agreement provides the office holder with the maximum indemnification permitted under applicable.
Exculpation
Under the Companies Law, an Israeli company may not exculpate an office holder from liability for a breach of his or her duty of loyalty, but may exculpate in advance an office holder from his or her liability to the company, in whole or in part, for a breach of his or her duty of care (other than in relation to distributions). Our articles of association provide that we may exculpate any office holder from liability to us to the fullest extent permitted by law. Under the indemnification agreements, we exculpate and release our office holders from any and all liability to us related to any breach by them of their duty of care to us to the fullest extent permitted by law.
Limitations
The Companies Law provides that we may not exculpate or indemnify an office holder nor enter into an insurance contract that would provide coverage for any liability incurred as a result of any of the following: (a) a breach by the office holder of his or her duty of loyalty unless (in the case of indemnity or insurance only, but not exculpation) the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice us; (b) a breach by the office holder of his or her duty of care if the breach was carried out intentionally or recklessly (as opposed to merely negligently); (c) any action taken with the intent to derive an illegal personal benefit; or (d) any fine levied against the office holder.
The foregoing descriptions are general summaries only, and are qualified entirely by reference to the full text of the Companies Law, as well as of our articles of association and our form of indemnification agreement, which are exhibits to this annual report and are incorporated herein by reference.
There are no service contracts between us or our U.S. Subsidiary, on the one hand, and our directors in their capacity as directors, on the other hand, providing for benefits upon termination of service.
The following table sets forth certain data concerning our workforce (excluding temporary employees), as of the end of each of the last three fiscal years:
| |
|
As of December 31,
|
|
| |
|
2015
|
|
|
2014
|
|
|
2013
|
|
|
Numbers of employees by category of activity
|
|
|
|
|
|
|
|
|
|
|
Management and administrative
|
|
|
24 |
|
|
|
21 |
|
|
|
16 |
|
|
Research and development
|
|
|
27 |
|
|
|
26 |
|
|
|
22 |
|
|
Operations
|
|
|
17 |
|
|
|
16 |
|
|
|
12 |
|
|
Sales and marketing
|
|
|
86 |
|
|
|
78 |
|
|
|
63 |
|
|
Total workforce
|
|
|
154 |
|
|
|
141 |
|
|
|
113 |
|
|
Numbers of employees by geographic location
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Israel
|
|
|
75 |
|
|
|
67 |
|
|
|
57 |
|
|
United States
|
|
|
79 |
|
|
|
74 |
|
|
|
56 |
|
|
Total workforce
|
|
|
154 |
|
|
|
141 |
|
|
|
113 |
|
During the years covered by the above table, we did not employ a significant number of temporary employees.
The increase in the size of our workforce in 2015, 2014 and 2013 was primarily the result of the expansion of our sales, marketing and service activities in the United States, expansion of our Research and Development department and an increase in headcount in the finance department in Israel to support our growth.
We are subject to Israeli labor laws and regulations with respect to our employees located in Israel. These laws principally concern matters such as pensions, paid annual vacation, paid sick days, length of the workday and work week, minimum wages, overtime pay, insurance for work-related accidents, severance pay and other conditions of employment. Our employees are not represented by a labor union. We consider our relationship with our employees to be good. To date, we have not experienced any work stoppages.
The employees of our Subsidiaries are subject to local labor laws and regulations.
The following table lists as of April 28, 2016, the number of our shares owned, and stock options held, by each of our directors, our CEO and others members of our senior management as a group:
| |
|
Number of
Ordinary
Shares
Beneficially
Owned(1)
|
|
|
Percent of
Class(2)
|
|
| |
|
|
|
|
|
|
|
Directors
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Jonathan Adereth (3)
|
|
|
106,668 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Ori Hadomi (4)
|
|
|
248,286 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Gil Bianco (5)
|
|
|
23,500 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
David Schlachet (6)
|
|
|
23,500 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Sarit Soccary Ben-Yochanan (7)
|
|
|
36,700 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Michael Berman (8)
|
|
|
31,500 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Executive Officers
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Sharon Levita (9)
|
|
|
106,632 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Moshe Shoham (10)
|
|
|
506,689 |
|
|
|
1.19 |
% |
| |
|
|
|
|
|
|
|
|
|
Eliyahu Zehavi (11)
|
|
|
187,044 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Sandra Adzic (12)
|
|
|
15,000 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Doron Dinstein (13)
|
|
|
110,000 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
Anat Kaphan (14)
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
Christopher Prentice (15)
|
|
|
150,400 |
|
|
|
* |
|
| |
|
|
|
|
|
|
|
|
|
All directors and executive officers as a group (13 persons)
|
|
|
1,545,919
|
|
|
|
3.62 |
% |
|
|
(1)
|
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Ordinary shares relating to options currently exercisable or exercisable within 60 days of the date of this table are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person. Except as indicated by footnote, and subject to community property laws where applicable, the persons named in the table above have sole voting and investment power with respect to all shares shown as beneficially owned by them.
|
|
|
(2)
|
The percentages shown are based on 42,514,698 ordinary shares issued and outstanding as of April 28, 2016 plus ordinary shares relating to options currently exercisable or exercisable within 60 days of the date of this table, which are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person.
|
|
|
(3)
|
Consists of 106,668 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 12.4 ($3.10) and NIS 32.46 ($8.12) per share, and the options expire between January 2018 and November 2020. Does not include 13,332 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options is NIS 32.46 ($8.12) and the options expire in November 2020.
|
|
|
(4)
|
Includes 74,229 shares and 174,057 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 4.521 ($1.13) and NIS 17.16 ($4.29) per share. These options expire between March 2018 and May 2020. Does not include 210,000 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 26.62 ($6.66) and NIS 26.99 ($6.75) per share, and the options expire between July 2012 and August 2021.
|
|
|
(5)
|
Consists of 23,500 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options is NIS 27.67 ($6.92) and the options expire in July 2021. Does not include 16,500 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options is NIS 27.67 ($6.92) per share, and the options expire in July 2021.
|
|
|
(6)
|
Consists of 23,500 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options is NIS 27.67 ($6.92) and the options expire in July 2021. Does not include 16,500 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options is NIS 27.67 ($6.92) per share, and the options expire in July 2021.
|
|
|
(7)
|
Consists of 36,700 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 4.52 ($1.13) and NIS 27.67 ($6.92) and the options expire in July 2021. Does not include 16,500 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options is NIS 27.67 ($6.92) per share. These options expire between December 2019 and July 2021.
|
|
|
(8)
|
Includes 8,000 shares and 23,500 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options is NIS 27.67 ($6.92) and the options expire in July 2021. Does not include 16,500 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options is NIS 27.67 ($6.92) per share, and the options expire in July 2021.
|
|
|
(9)
|
Includes 26,632 shares and includes 80,000 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 4.521 ($1.13) and NIS 9.64 ($2.41) and the options expire between December 2017 and August 2019. Does not include 133,600 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 26.62 ($6.66) and NIS 26.99 ($6.75) and the options expire between July 2021 and July 2022.
|
| |
(10)
|
Includes 373,689 shares and 133,000 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016, of which: (a) 33,000 options have an exercise price of $2.73 per share and (b) 100,000 options have an exercise price of NIS 4.521 ($1.13) per share. The options expire between May 2017 and August 2019. Does not include 87,300 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 26.62 ($6.66) and NIS 26.99 ($6.75) and the options expire between July 2021 and July 2022.
|
| |
(11)
|
Includes 187,044 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 4.521 ($1.13) and NIS 10.00 ($2.50) per share. These options expire between December 2017 and August 2019. Does not include 133,600 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 26.62 ($6.66) and NIS 26.99 ($6.75) and the options expire between July 2021 and July 2022.
|
| |
(12)
|
Includes of 15,000 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016. The exercise price of these options is NIS 4.099 ($1.02) per share, and the options expire in July 2019. Does not include 153,500 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 22.90 ($5.73) and NIS 26.99 ($6.75) and the options expire between July 2021 and January 2022.
|
| |
(13)
|
Includes 110,000 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2016, these options have an exercise price that ranges between NIS 8.29 ($2.07) and 9.64 NIS ($2.41) per share. These options expire between December 2017 and February 2020. Does not include 81,200 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 26.62 ($6.66) and NIS 26.99 ($6.75) per share, and the options expire between December 2019 and July 2022
|
| |
(14)
|
Does not include 125,000 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options is NIS 26.99 ($6.75) and expire in July 2022.
|
| |
(15)
|
Consists of 2,900 shares and 147,500 ordinary shares issuable upon exercise of outstanding options within 60 days of April 28, 2015. The exercise price of these options ranges between NIS 4.521 ($1.13) and NIS 26.86 ($6.72) per share, and the options expire between August 2019 and October 2020. Does not include 251,000 ordinary shares issuable upon exercise of outstanding options that are not exercisable within 60 days of April 28, 2016. The exercise price of these options ranges between NIS 24.71 ($6.18) and NIS 26.99 ($6.75) per share, and the options expire between July 2020 and July 2022.
|
Stock Option Plans
The following sets forth certain information with respect to our current share option plans. The following description is only a summary of the plans and is qualified in its entirety by reference to the full text of the plans, which are exhibits to this annual report and are incorporated herein by reference.
All of our share option plans are administered by our board of directors. Upon the expiration of the plans, no further grants may be made thereunder, although any existing awards will continue in full force in accordance with the terms under which they were granted. Options granted under any of the plans may not expire later than ten years from the date of grant, although, in recent years, options grants have generally provided for an expiration date of seven years from the grant date. Unvested awards that are cancelled and/or forfeited go back into the respective plan.
2003 Stock Option Plan
In July 2003, we adopted our 2003 Share Option Plan, or the 2003 Plan, which expired in November 2010. Accordingly no further grants may be made under the 2003 Plan, although any existing awards continue in full force in accordance with the terms under which they were granted. Our directors, officers, employees and certain consultants and dealers were eligible to participate in this plan. As of December 31, 2015, there were 296,594 ordinary shares issuable upon the exercise of outstanding options under the 2003 Plan.
Israeli grantees who were our directors, officers and employees could be granted options under the 2003 Plan that would qualify for special tax treatment under the “capital gains route” provisions of Section 102(b)(2) of the Israeli Income Tax Ordinance, to which we refer as the Ordinance. Pursuant to such Section 102(b)(2), qualifying options and shares issued upon exercise of such options are held in trust and registered in the name of a trustee selected by the board of directors. The trustee may not release these options or shares to the holders thereof before the second anniversary of the registration of the options in the name of the trustee. The Israeli Tax Authority, or the ITA, approved this plan as required by applicable law. The 2003 Plan also permitted the grant to Israeli grantees of options that do not qualify under Section 102(b)(2). The 2003 Plan also provided for the grant of options to U.S. resident employees that are “qualified”, i.e., incentive stock options, under the U.S. Internal Revenue Code of 1986, as amended, and options that are not qualified. In addition to the grant of awards under the relevant tax regimes of the United States and Israel, the 2003 Plan allowed for the grant of awards to grantees in other jurisdictions.
2011 Share Option Plan
In May 2011, our board of directors approved and adopted our 2011 Share Option Plan, or the 2011 Plan, which expires in June 2021. As of December 31, 2015, the number of shares reserved for the exercise of options granted under the plan is 6,262,529. Our employees, directors, officer, consultants, advisors, suppliers and any other person or entity whose services are considered valuable to us are eligible to participate in this plan. The 2011 Plan provides for the grant of awards consisting of stock options. As of December 31, 2015, there were 5,235,029 ordinary shares issuable upon the exercise of outstanding options under the 2011 Plan.
The 2011 Plan provides for the grant to residents of Israel of options that qualify under the provisions of Section 102 of the Ordinance (see “2003 Stock Option Plan” above), as well as for the grant of options that do not qualify under such provisions. The 2011 Plan has been approved by the ITA. The 2011 Plan also provides for the grant of options to U.S. resident employees that are “qualified”, i.e., incentive stock options, under the U.S. Internal Revenue Code of 1986, as amended, or the Code, and options that are not qualified. In addition to the grant of awards under the relevant tax regimes of the United States and Israel, the 2011 Plan allows for the grant of awards to grantees in other jurisdictions, with respect to which our board of directors is empowered to make the requisite adjustments in the plan.
Both the 2003 Plan and the 2011 Plan are administered by our board of directors or a committee appointed thereby. Subject to the 2003 Plan, the 2011 Plan and applicable law, the board of directors has the authority to make all determinations deemed necessary or advisable for the administration of such plans, including to whom options may be granted, the time and the extent to which the options may be exercised, the exercise price of shares covered by each option, the type of options and how to interpret such plans.
The following table presents certain option data information for the above-described plans as of December 31, 2015:
|
Plan
|
|
Total
Ordinary
Shares
Reserved
for Option
Grants
|
|
|
Aggregate
Number of
Options
Exercised
|
|
|
Shares
Available
for Future
Grants
|
|
|
Aggregate
Number of
Options
Outstanding
|
|
|
Weighted
Average
Exercise
Price of
Options
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2003 plan
|
|
|
3,000,000 |
|
|
|
1,616,867 |
|
|
|
— |
|
|
|
296,594 |
|
|
$ |
2.32 |
|
|
2011 plan
|
|
|
6,262,529 |
|
|
|
612,080 |
|
|
|
410,422 |
|
|
|
5,235,029 |
|
|
$ |
5.61 |
|
| |
|
|
9,262,529 |
|
|
|
2,228,947 |
|
|
|
410,422 |
|
|
|
5,531,623 |
|
|
$ |
5.43 |
|
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
Ownership by Major Shareholders
The following table presents as of April 28, 2016 (unless otherwise noted below) the beneficial ownership of our ordinary shares by each person who is known by us to be the beneficial owner of 5% or more of our outstanding ordinary shares (to whom we refer as our Major Shareholders). The data presented is based on information provided to us by the holders or disclosed in public regulatory filings.
Except where otherwise indicated, and except pursuant to community property laws, we believe, based on information furnished by such owners, that the beneficial owners of the shares listed below have sole investment and voting power with respect to, and the sole right to receive the economic benefit of ownership of, such shares. The shareholders listed below do not have any different voting rights from any of our other shareholders. We know of no arrangements that would, at a subsequent date, result in a change of control of us.
|
Name
|
|
Number of
Ordinary
Shares
Beneficially
Owned(1)
|
|
|
Percent of Class(2)
|
|
|
Larry Feinberg (3)
|
|
|
5,721,672 |
|
|
|
13.46 |
% |
| |
|
|
|
|
|
|
|
|
|
Jack Schuler (4)
|
|
|
2,811,615 |
|
|
|
6.61 |
% |
| |
|
|
|
|
|
|
|
|
|
Migdal Insurance & Financial Holdings LTD (5)
|
|
|
3,030,413 |
|
|
|
7.13 |
% |
|
(1)
|
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Ordinary shares relating to options currently exercisable or exercisable within 60 days of the date of this table are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person.
|
|
(2)
|
The percentages shown are based on 42,514,698 ordinary shares issued and outstanding as of April 28, 2016.
|
|
(3)
|
Based solely on a Schedule 13D/A filed with the SEC on January 27, 2016, and which reflects holdings as of January 22, 2016. Mr. Feinberg may be deemed to beneficially own 5,721,672 ordinary shares due to his relationship with Oracle Associates, LLC, Oracle Partners, LP, Oracle Institutional Partners, LP, Oracle Ten Master, LP, Oracle Investment Management, Inc., Oracle Investment Management, Inc. Employees’ Retirement Plan and The Feinberg Family Foundation. See “Item 10. Additional Information – C. Material Contracts,” for more details on these ordinary shares.
|
|
(4)
|
Based solely on data held by the Company at the date of issuing Mr. Schuler ordinary shares.
|
|
(5)
|
Based solely on a Schedule 13G filed with the SEC on February 10, 2016, and which reflects holdings as of December 31, 2015.
|
Changes in Percentage Ownership by Major Shareholders
The Oracle Agreement
Under the agreement with the Oracle Investors, or the Oracle Agreement, the Oracle Investors initially invested an amount of $7.5 million, and agreed that, upon the fulfillment of certain conditions as specified in the Oracle Agreement, the Oracle Investors would invest an additional amount of up to $7.5 million. In connection with the Oracle Agreement we issued to the Oracle Investors an aggregate of 7,053,529 Ordinary Shares, or the Oracle Shares, for an aggregate amount of $7.5 million, or the Invested Amount, reflecting a price per Oracle Share of NIS 4.25 (based on the exchange rate of August 8, 2012, of NIS 3.997 to $1, or the Rate of Exchange). In addition, we issued to the Oracle Investors, for no additional consideration, warrants, to purchase an aggregate of up to 7,053,529 Ordinary Shares, or the Oracle Warrants and the Oracle Warrant Shares, respectively, and in total for all the Oracle Investors, Oracle Warrant Shares for an aggregate exercise price of up to $7.5 million, calculated pursuant to the Rate of Exchange, or the Total Warrant Consideration. The Oracle Warrants were exercisable for a period of 36 months from September 27, 2012. The exercise price per each Oracle Warrant Share was the lower of: (a) NIS 6; and (b) the average price of our Ordinary Shares on the TASE in the 10 trading days preceding exercise (according to the Rate of Exchange), provided, however, that if the price under (b) above was lower than NIS 4.25, each Oracle Investor would be entitled to exercise only up to 50% of its portion of the Total Warrant Consideration at NIS 4.25, and any exercise with respect the balance of the Oracle Warrant would be at an exercise price of NIS 6.00. The investment under the Oracle Agreement closed on September 27, 2012. On May 28, 2013, we provided the Oracle Investors with a notice of mandatory exercise, pursuant to which the Oracle Investors were required to exercise such warrants for total consideration of $7,500,000 ($6,966,000, net of issuance expenses in the amount of $534,000) into a fixed number of 4,996,251 Ordinary Shares as determined as of May 28, 2013. The proceeds were paid to us through July 2, 2013.
For a detailed description of the Oracle Agreement, see “Item 10. Additional Information – C. Material Contracts.”
Record Holders
Based upon a review of the information provided to us by TASE, as of February 29, 2016, there were 6,432 holders of record of our ordinary shares, of which 56 record holders holding 24,565,591 shares, or approximately 57.8%, of our outstanding shares had registered addresses in the United States and there was one holder of record of the ADSs. These numbers are not representative of the number of beneficial holders of our shares nor is it representative of where such beneficial holders reside, since many of these shares were held of record by brokers or other nominees.
We are not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and here are no arrangements known to us which would result in a change in control of us at a subsequent date.
|
B.
|
Related Party Transactions
|
In August 2012, we entered into the Oracle Agreement with the Oracle Investors. The Oracle Investors owned 22.8% of our outstanding ordinary shares as of that date. For a description of the Oracle Agreement, including the securities issued to the Oracle Investors and the rights conferred upon the Oracle Investors, see “Item 10. Additional Information - C. Material Contracts.”
|
C.
|
Interests of Experts and Counsel
|
Not applicable.
ITEM 8. FINANCIAL INFORMATION.
|
A.
|
Consolidated Statements and Other Financial Information.
|
See “Item 18. Financial Statements.”
Export Sales
The following table presents total export sales for each of the fiscal years indicated (in thousands):
| |
For the year ended December 31,
|
|
| |
2015
|
|
2014
|
|
2013
|
|
|
Total export sales*
|
|
$ |
25,859 |
|
|
$ |
20,766 |
|
|
$ |
19,597 |
|
|
as a percentage of total revenues
|
|
|
99 |
% |
|
|
98 |
% |
|
|
98 |
% |
* Export sales, as presented, are defined as sales to customers located outside of Israel.
Legal Proceedings
From time to time, we are involved in various routine legal proceedings incidental to the ordinary course of our business. We do not believe that the outcomes of these legal proceedings have had in the recent past, or will have (with respect to any pending proceedings), significant effects on our financial position or profitability.
Dividends
We have never declared or paid cash dividends on our Ordinary Shares and do not anticipate that we will pay any cash dividends on our Ordinary Shares or ADSs in the foreseeable future.
We intend to retain our earnings to finance the development and expenses of our business. Any future determination relating to our dividend policy will be at the discretion of our board of directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, applicable Israeli law and other factors our board of directors may deem relevant.
Pursuant to our articles of association, dividends may be declared by our board of directors. Dividends must be paid out of our profits and other surplus funds, as defined in the Companies Law, as of the end of the most recent year or as accrued over a period of the most recent two years, whichever amount is greater, provided that there is no reasonable concern that payment of a dividend will prevent us from satisfying our existing and foreseeable obligations as they become due. In addition, because we have received certain benefits under the Israeli law relating to approved enterprises and privileged enterprises, our payment of dividends may subject us to certain Israeli taxes to which we would not otherwise be subject. In the event that we declare cash dividends, we may pay those dividends in NIS. See “Item 3. Key Information – D. Risk Factors—We do not intend to pay any cash dividends on our Ordinary Shares in the foreseeable future and, therefore, any return on your investment in our Ordinary Shares or the ADSs must come from increases in the value and trading price of our Ordinary Shares and the ADSs.”
No significant change, other than as otherwise described in this annual report, has occurred in our operations since the date of our consolidated financial statements included in this annual report.
ITEM 9. THE OFFER AND LISTING
|
A.
|
Offer and Listing Details
|
PRICE RANGE OF OUR ORDINARY SHARES
Our Ordinary Shares have been trading on the TASE under the symbol “MZOR” since August 2007.
The following table sets forth, for the periods indicated, the reported high and low closing sale prices of our Ordinary Shares on the TASE in NIS and U.S. dollars. U.S. dollar per Ordinary Share amounts are calculated using the U.S. dollar representative rate of exchange on the date to which the high or low market price is applicable, as reported by the Bank of Israel.
| |
|
NIS
Price Per
Ordinary
Share
|
|
|
U.S.$
Price Per
Ordinary
Share
|
|
| |
|
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual:
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
2016 (through April 28, 2016)
|
|
|
23.69 |
|
|
|
16.88 |
|
|
|
6.26 |
|
|
|
4.34 |
|
|
2015
|
|
|
28.21 |
|
|
|
16.40 |
|
|
|
7.38 |
|
|
|
4.25 |
|
|
2014
|
|
|
44.92 |
|
|
|
18.66 |
|
|
|
12.87 |
|
|
|
4.83 |
|
|
2013
|
|
|
40.10 |
|
|
|
8.28 |
|
|
|
11.26 |
|
|
|
2.21 |
|
|
2012
|
|
|
9.00 |
|
|
|
3.46 |
|
|
|
2.38 |
|
|
|
0.88 |
|
|
2011
|
|
|
10.80 |
|
|
|
2.82 |
|
|
|
2.99 |
|
|
|
0.76 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarterly:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Second Quarter 2016 (through April 28, 2016)
|
|
|
23.69 |
|
|
|
21.57 |
|
|
|
6.26 |
|
|
|
5.73 |
|
|
First Quarter 2016
|
|
|
23.14 |
|
|
|
16.88 |
|
|
|
6.05 |
|
|
|
4.34 |
|
|
Fourth Quarter 2015
|
|
|
23.80 |
|
|
|
16.40 |
|
|
|
6.17 |
|
|
|
4.25 |
|
|
Third Quarter 2015
|
|
|
28.21 |
|
|
|
21.30 |
|
|
|
7.38 |
|
|
|
5.43 |
|
|
Second Quarter 2015
|
|
|
26.99 |
|
|
|
21.67 |
|
|
|
7.16 |
|
|
|
5.50 |
|
|
First Quarter 2015
|
|
|
25.48 |
|
|
|
20.06 |
|
|
|
6.40 |
|
|
|
5.06 |
|
|
Fourth Quarter 2014
|
|
|
29.40 |
|
|
|
18.66 |
|
|
|
7.53 |
|
|
|
4.83 |
|
|
Third Quarter 2014
|
|
|
30.95 |
|
|
|
21.22 |
|
|
|
9.03 |
|
|
|
5.74 |
|
|
Second Quarter 2014
|
|
|
37.91 |
|
|
|
28.20 |
|
|
|
10.91 |
|
|
|
8.19 |
|
|
First Quarter 2014
|
|
|
44.92 |
|
|
|
34.54 |
|
|
|
12.87 |
|
|
|
9.95 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Most Recent Six Months:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
April 2016 (through April 28, 2016)
|
|
|
23.69 |
|
|
|
21.57 |
|
|
|
6.26 |
|
|
|
5.73 |
|
|
March 2016
|
|
|
22.80 |
|
|
|
18.98 |
|
|
|
6.05 |
|
|
|
4.86 |
|
|
February 2016
|
|
|
20.35 |
|
|
|
16.88 |
|
|
|
5.13 |
|
|
|
4.34 |
|
|
January 2016
|
|
|
23.14 |
|
|
|
19.87 |
|
|
|
5.89 |
|
|
|
5.08 |
|
|
December 2015
|
|
|
20.26 |
|
|
|
16.40 |
|
|
|
5.21 |
|
|
|
4.25 |
|
|
November 2015
|
|
|
20.91 |
|
|
|
18.97 |
|
|
|
5.39 |
|
|
|
4.88 |
|
PRICE RANGE OF OUR ADSs
The ADSs have been trading under the symbol “MZOR” on the NASDAQ Capital Market since May 2013 and the NASDAQ Global Market since January 16, 2014.
The following table sets forth, for the periods indicated, the reported high and low closing sale prices of the ADSs on NASDAQ in U.S. dollars.
| |
|
U.S.$
Price Per
ADSs
|
|
| |
|
High
|
|
|
Low
|
|
| |
|
|
|
|
|
|
|
Annual:
|
|
|
|
|
|
|
|
2016 (through April 28, 2016)
|
|
|
13.19 |
|
|
|
8.79
|
|
|
2015
|
|
|
14.90 |
|
|
|
8.86 |
|
|
2014
|
|
|
25.34 |
|
|
|
9.90 |
|
|
2013 (since May 28, 2013)
|
|
|
21.85 |
|
|
|
10.40 |
|
| |
|
|
|
|
|
|
|
|
|
Quarterly:
|
|
|
|
|
|
|
|
|
|
Second Quarter 2016 (through April 28, 2016)
|
|
|
13.19 |
|
|
|
11.35 |
|
|
First Quarter 2016
|
|
|
11.42 |
|
|
|
8.79 |
|
|
Fourth Quarter 2015
|
|
|
12.08 |
|
|
|
8.86 |
|
|
Third Quarter 2015
|
|
|
14.90 |
|
|
|
10.66 |
|
|
Second Quarter 2015
|
|
|
14.72 |
|
|
|
11.23 |
|
|
First Quarter 2015
|
|
|
12.74 |
|
|
|
10.16 |
|
|
Fourth Quarter 2014
|
|
|
14.61 |
|
|
|
9.90 |
|
|
Third Quarter 2014
|
|
|
18.12 |
|
|
|
11.36 |
|
|
Second Quarter 2014
|
|
|
21.94 |
|
|
|
16.36 |
|
|
First Quarter 2014
|
|
|
25.34 |
|
|
|
20.44 |
|
| |
|
|
|
|
|
|
|
|
|
Most Recent Six Months:
|
|
|
|
|
|
|
|
|
|
April 2016 (through April 28, 2016)
|
|
|
13.19 |
|
|
|
11.35 |
|
|
March 2016
|
|
|
12.72 |
|
|
|
9.67 |
|
|
February 2016
|
|
|
10.35 |
|
|
|
8.79 |
|
|
January 2016
|
|
|
11.42 |
|
|
|
10.17 |
|
|
December 2015
|
|
|
10.42 |
|
|
|
8.86 |
|
|
November 2015
|
|
|
10.71 |
|
|
|
9.81 |
|
Not applicable.
Our Ordinary Shares are listed and traded on the TASE. Our ADSs are traded on the NASDAQ Global Market.
Not applicable.
Not applicable.
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
Not applicable.
|
B.
|
Memorandum and Articles of Association
|
This information is incorporated by reference into this annual report on Form 20-F from our registration statement on Form F-1 filed with the SEC on October 18, 2013.
Except as set forth below, we have not entered into any material contract within the two years prior to the date of this annual report, other than contracts entered into in the ordinary course of business, or as otherwise described herein in “Item 4. Information on the Company - A. History and Development of the Company” above, “Item 4. Information on the Company - B. Business Overview” above, or “Item 7. Major Shareholders and Related Party Transactions - A. Major Shareholders” above.
Agreement with the Oracle Investors
Under the Oracle Agreement entered into in August 2012, the Oracle Investors initially invested an amount of $7.5 million, and agreed that, upon the fulfillment of certain conditions as specified in the Oracle Agreement, the Oracle Investors would invest an additional amount of up to $7.5 million. In connection with the Oracle Agreement we issued to the Oracle Investors an aggregate of 7,053,529 Ordinary Shares, or the Oracle Shares, for an aggregate amount of $7.5 million, or the Invested Amount, reflecting a price per Oracle Share of NIS 4.25 (based on the exchange rate of August 8, 2012, of NIS 3.997 to $1, or the Rate of Exchange). In addition, we issued to the Oracle Investors, for no additional consideration, warrants, to purchase an aggregate of up to 7,053,529 Ordinary Shares, or the Oracle Warrants and the Oracle Warrant Shares, respectively, and in total for all the Oracle Investors, Oracle Warrant Shares for an aggregate exercise price of up to $7.5 million, calculated pursuant to the Rate of Exchange, or the Total Warrant Consideration. The Oracle Warrants are exercisable for a period of 36 months from September 27, 2012. The exercise price per each Oracle Warrant Share is the lower of: (a) NIS 6; and (b) the average price of our Ordinary Shares on the TASE in the 10 trading days preceding exercise (according to the Rate of Exchange), provided, however, that if the price under (b) above is lower than NIS 4.25, each Oracle Investor will be entitled to exercise only up to 50% of its portion of the Total Warrant Consideration at NIS 4.25, and any exercise with respect the balance of the Oracle Warrant shall be at an exercise price of NIS 6.00. The investment under the Oracle Agreement closed on September 27, 2012. On May 28, 2013, in accordance with the Oracle Agreement, we provided the Oracle Investors with a notice of mandatory exercise, pursuant to which the Oracle Investors were required within 30 days to exercise the Oracle Warrants. In connection with the exercise of the Oracle Warrants, we issued 4,996,251 Ordinary Shares to the Oracle Investors for a Total Warrant Consideration of $7,500,000.
In addition, the Oracle Agreement provided the Oracle Investors with certain rights, including, a right to have representation on our board of directors, certain demand registration rights, tag-along right and preemptive right in connection with an offer by us to sell its shares under certain circumstances.
See additional information regarding a right to have representation on our board of directors under “Item 6. Directors, Senior Management and Employees - A. Directors and Senior Management - Arrangements for Election of Directors and Members of Management.” The Oracle Agreement was filed as Exhibit 4.4 to our registration statement on Form 20-F filed with the SEC on May 10, 2013.
There are currently no Israeli currency control restrictions on payments of dividends or other distributions with respect to our ordinary shares or the proceeds from the sale of the shares, except for the obligation of Israeli residents to file reports with the Bank of Israel regarding certain transactions. However, legislation remains in effect pursuant to which currency controls can be imposed by administrative action at any time.
The ownership or voting of our ordinary shares by non-residents of Israel, except with respect to citizens of countries that are in a state of war with Israel, is not restricted in any way by our memorandum of association or articles of association or by the laws of the State of Israel.
Israeli Tax Considerations
The following is a description of the material Israeli income tax consequences of the ownership of our ordinary shares. The following also contains a description of material relevant provisions of the current Israeli income tax structure applicable to companies in Israel, with special reference to its effect on us. To the extent that the discussion is based on new tax legislation which has not been subject to judicial or administrative interpretation, there can be no assurance that the tax authorities will accept the views expressed in the discussion in question. The discussion is not intended, and should not be taken, as legal or professional tax advice and is not exhaustive of all possible tax considerations.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our Ordinary Shares and ADSs. Shareholders should consult their own tax advisors concerning the tax consequences of their particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Corporate tax rate
Israeli companies are generally subject to corporate tax. In 2015 and 2014, the corporate tax rate is 26.5% of their taxable income. However, the effective tax rate payable by a company that derives income from a “Beneficiary Enterprise”, as discussed further below, may be considerably less. Capital gains derived by an Israeli company are subject to tax rate of 26.5%.
On January 5, 2016, the Israeli Parliament officially published the Law for the Amendment of the Israeli Tax Ordinance (Amendment 216), that reduces the corporate tax rate and the capital gain tax rate from 26.5% to 25%.
Current taxes for the reported periods are calculated according to the tax rates presented above.
The Israeli Law for the Encouragement of Capital Investments, 1959
The Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain incentives for capital investments in production facilities (or other eligible assets) by “Industrial Enterprises” (as defined under the Investment Law).
The Investment Law was significantly amended effective April 1, 2005, or the 2005 Amendment, and further amended as of January 1, 2011, or the 2011 Amendment. Pursuant to the 2005 Amendment, tax benefits granted in accordance with the provisions of the Investment Law prior to its revision by the 2005 Amendment remain in force but any benefits granted subsequently are subject to the provisions of the 2005 Amendment. Similarly, the 2011 Amendment introduced new benefits to replace those granted in accordance with the provisions of the Investment Law in effect prior to the 2011 Amendment. However, companies entitled to benefits under the Investment Law as in effect prior to January 1, 2011 were entitled to choose to continue to enjoy such benefits, provided that certain conditions are met, or elect instead irrevocably to forego such benefits and have the benefits of the 2011 Amendment apply.
Tax Benefits Prior to the 2005 Amendment. An investment program that is implemented in accordance with the provisions of the Investment Law prior to the 2005 Amendment, referred to as an “Approved Enterprise,” is entitled to certain benefits. A company that wished to receive benefits as an Approved Enterprise must have received approval from the Investment Center of the Israeli Ministry of Economy, or the Investment Center. Each certificate of approval for an Approved Enterprise relates to a specific investment program in the Approved Enterprise, delineated both by the financial scope of the investment and by the physical characteristics of the facility or the asset.
In general, an Approved Enterprise is entitled to receive a grant from the Government of Israel and certain tax benefits under the “Grant Track” or an alternative package of tax benefits under the "Alternative Track". The tax benefits from any certificate of approval relate only to taxable income attributable to the specific Approved Enterprise. Income derived from activity that is not approved by the Investment Center or not integral to the activity of the Approved Enterprise does not enjoy tax benefits.
The tax benefits include a tax exemption for at least the first two years of the benefit period (depending on the geographic location of the Approved Enterprise facility within Israel) and the taxation of income generated from an Approved Enterprise at a reduced corporate tax rate of up to 25% for the remainder of the benefit period. The benefit period is ordinarily seven years commencing with the year in which the Approved Enterprise first generates taxable income. The benefit period is limited to 12 years from the operational year as determined by the Investment Center or 14 years from the start of the tax year in which approval of the Approved Enterprise is obtained, whichever is earlier.
A company that has an Approved Enterprise program is eligible for further tax benefits if it qualifies as a Foreign Investors’ Company, or a FIC, which is a company with a level of foreign investment, as defined in the Investment Law, of more than 25%. The level of foreign investment is measured as the percentage of rights in the company (in terms of shares, rights to profits, voting and appointment of directors), and of combined share and loan capital, that are owned, directly or indirectly, by persons who are not residents of Israel. The determination as to whether a company qualifies as a FIC is made on an annual basis. A company that qualifies as a FIC and has an Approved Enterprise program is eligible for an extended ten-year benefit period. As specified above, depending on the geographic location of the Approved Enterprise within Israel, income derived from the Approved Enterprise program may be exempt from tax on its undistributed income for a period of between two to ten years, and will be subject to a reduced tax rate for the remainder of the benefit period. The tax rate for the remainder of the benefits period will be 25%, unless the level of foreign investment exceeds 49%, in which case the tax rate will be 20% if the foreign investment is more than 49% and less than 74%; 15% if more than 74% and less than 90%; and 10% if 90% or more.
If a company elects the Alternative Track and distributes a dividend, it will be required to recapture the deferred corporate income tax applicable to the gross amount of distributed dividend that is derived from the portion of the company’s facilities that has been granted Approved Enterprise status during the tax exemption period at the applicable rate of 10%-25%. In addition, dividends paid out of income attributed to an Approved Enterprise are generally subject to withholding tax at source at the rate of 15% or such lower rate as may be provided in an applicable tax treaty.
The Investment Law also provides that an Approved Enterprise is entitled to accelerated depreciation on its property and equipment that are included in an Approved Enterprise program during the first five years in which the equipment is used.
The benefits available to an Approved Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations and the criteria in the specific certificate of approval. If a company does not meet these conditions, it may be required to refund the amount of tax benefits, as adjusted by the Israeli consumer price index, and interest. In April 2004 we were granted an Approved Enterprise status with respect to a plan to construct a plant in Caesarea for the manufacture of systems for assisting and guiding complex surgical procedures.
Tax Benefits Subsequent to the 2005 Amendment. The 2005 Amendment changed certain provisions of the Investment Law. As a result of the 2005 Amendment, a company was no longer obliged to obtain Approved Enterprise status in order to receive the tax benefits previously available under the Alternative Track, and therefore generally there was no need to apply to the Investment Center for this purpose (Approved Enterprise status remains mandatory for companies seeking cash grants). Rather, we may claim the tax benefits offered by the Investment Law directly in our tax returns by notifying the Israeli Tax Authority within 12 months after the end of that year, provided that its facilities meet the criteria for tax benefits set out by the 2005 Amendment. A company is also granted a right to approach the Israeli Tax Authority for a pre-ruling regarding its eligibility for benefits under the 2005 Amendment.
The 2005 Amendment applies to new investment programs and investment programs with an election year commencing after 2004, but does not apply to investment programs approved prior to April 1, 2005. The 2005 Amendment provides that terms and benefits included in any certificate of approval that was granted before the 2005 Amendment became effective (April 1, 2005) will remain subject to the provisions of the Investment Law as in effect on the date of such approval.
Tax benefits are available under the 2005 Amendment to production facilities (or other eligible facilities), which are generally required to derive more than 25% of their business income from sales to territories with over 14 million inhabitants and meet additional criteria stipulate in the amendment. This is referred to as a “Beneficiary Enterprise”. In order to receive the tax benefits, the 2005 Amendment states that a company must make an investment which meets all of the conditions, including exceeding a minimum investment amount specified in the Investment Law. Such investment may be made over a period of no more than three years ending at the end of the year in which the company requested to have the tax benefits apply to its Beneficiary Enterprise.
The extent of the tax benefits available under the 2005 Amendment to qualifying income of a Beneficiary Enterprise depend on, among other things, the geographic location in Israel of the Beneficiary Enterprise. The geographic location of the company at the year of election will also determine the period for which tax benefits are available. Such tax benefits include an exemption from corporate tax on undistributed income for a period of between two to ten years, depending on the geographic location of the Beneficiary Enterprise in Israel, and a reduced corporate tax rate of between 10% to 25% for the remainder of the benefits period, depending on the level of foreign investment in the company in each year if it is a qualified FIC. A company qualifying for tax benefits under the 2005 Amendment which pays a dividend out of income derived by its Beneficiary Enterprise during the tax exemption period will be subject to corporate tax in respect of the gross amount of the dividend at the otherwise applicable rate of 10%-25%. Dividends paid out of income attributed to a Beneficiary Enterprise are generally subject to withholding tax at source at the rate of 15% or such lower rate as may be provided in an applicable tax treaty.
The benefits available to a Beneficiary Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations. If a company does not meet these conditions, it may be required to refund the amount of tax benefits, as adjusted by the Israeli consumer price index, and interest, or other monetary penalties.
In February 2007, our aforementioned Approved Enterprise status was revoked at our request, and in respect of an expansion of our plant in the Caesarea industrial park it was granted a Beneficiary Enterprise status. In accordance with this status, we will be entitled to the tax benefits provided by the Encouragement Law with respect to income of the Beneficiary Enterprise from productive activity. Income of the Beneficiary Enterprise from productive activity will be exempt from tax for two years from the year in which we first has taxable income, and will be subject to tax of 25% in the following 5 years, providing that 12 years have not passed from the beginning of the year of election (i.e., 2005).
In July 2009, we submitted a declaration to the Israeli tax authority that 2008 shall be the “base” year for our beneficiary enterprise status, and hence the tax benefits described above will apply to the increase in revenues compared to that base year. In addition, in the event of a change in the field of activity and/or business model and/or a significant reduction in production levels or in product variety, the tax benefits will become void.
In 2013 we notified the tax authorities that 2012 tax year is the year of election.
Tax Benefits Under the 2011 Amendment
The 2011 Amendment canceled the availability of the benefits granted to companies under the Investment Law prior to 2011 and, instead, introduced new benefits for income generated by a “Preferred Company” through its “Preferred Enterprise” (as such terms are defined in the Investment Law) as of January 1, 2011. Pursuant to the 2011 Amendment, a Preferred Company is entitled to a reduced corporate tax rate of 15% with respect to its income derived by its Preferred Enterprise in 2011 and 2012, unless the Preferred Enterprise is located in a specified development zone, in which case the rate will be 10%. Under the 2011 Amendment, such corporate tax rate will be 12.5% and 7%, respectively, in 2013 and will increase to 16% and 9% in 2014 and thereafter, respectively.
Dividends paid out of income attributed to a Preferred Enterprise are generally subject to withholding tax at the rate of 15% or such lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, no tax is required to be withheld (however, if afterward distributed to individuals or non-Israeli companies a withholding of 20% or such lower rate as may be provided in an applicable tax treaty, will apply).
The 2011 Amendment also provided transitional provisions to address companies already enjoying existing tax benefits under the Investment Law. These transitional provisions provide, among other things, that unless an irrevocable request is made to apply the provisions of the Investment Law as amended in 2011 with respect to income to be derived as of January 1, 2011: (i) the terms and benefits included in any certificate of approval that was granted to an Approved Enterprise which chose to receive grants and certain tax benefits under the Grant Track before the 2011 Amendment became effective will remain subject to the provisions of the Investment Law as in effect on the date of such approval, and subject to certain conditions; and (ii) terms and benefits included in any certificate of approval that was granted to an Approved Enterprise under the Alternative Track before the 2011 Amendment became effective will remain subject to the provisions of the Investment Law as in effect on the date of such approval, provided that certain conditions are met; and (iii) a Beneficiary Enterprise can elect to continue to benefit from the benefits provided to it before the 2011 Amendment came into effect, provided that certain conditions are met.
We have reviewed and evaluated the implications and effect of the benefits under the 2011 Amendment, and, while potentially eligible for such benefits, we have not yet chosen to be subject to the tax benefits introduced by the 2011 Amendment. From time to time, the Israeli Government has discussed reducing the benefits available to companies under the Investment Law. The termination or substantial reduction of any of the benefits available under the Investment Law could materially increase our tax liabilities in the future.
Grants under the R&D Law
Under the R&D Law research and development programs which meet specified criteria and are approved by a governmental committee of the Office of the Chief Scientist, or OCS, are eligible for grants of up to 50% of the project’s expenditure, as determined by the research committee, in exchange for the payment of royalties from the revenues generated from the sale of products and related services developed, in whole or in part pursuant to, or as a result of, a research and development program funded by the OCS. The royalties are generally at a range of 3.0% to 5.0% of revenues until the entire OCS grant is repaid, together with an annual interest generally equal to the 12 month London Interbank Offered Rate applicable to dollar deposits that is published on the first business day of each calendar year.
The terms of the R&D Law also require that the manufacture of products developed with government grants be performed in Israel. The transfer of manufacturing activity outside Israel may be subject to the prior approval of the OCS. Under the regulations of the R&D Law, assuming we receive approval from the Chief Scientist to manufacture our OCS-funded products outside Israel, we may be required to pay increased royalties. The increase in royalties depends upon the manufacturing volume that is performed outside of Israel as follows:
|
Manufacturing Volume Outside of Israel
|
|
Royalties to
the Chief
Scientist as
a
Percentage
of Grant
|
|
| |
|
|
|
|
Up to 50%
|
|
|
120 |
% |
|
between 50% and 90%
|
|
|
150 |
% |
|
90% and more
|
|
|
300 |
% |
If the manufacturing is performed outside of Israel by us, the rate of royalties payable by us on revenues from the sale of products manufactured outside of Israel will increase by 1% over the regular rates. If the manufacturing is performed outside of Israel by a third party, the rate of royalties payable by us on those revenues will be equal to the ratio obtained by dividing the amount of the grants received from the Office of the Chief Scientist and our total investment in the project that was funded by these grants. The transfer of no more than 10% of the manufacturing capacity in the aggregate outside of Israel is exempt under the R&D Law from obtaining the prior approval of the OCS. A company requesting funds from the OCS also has the option of declaring in its OCS grant application an intention to perform part of its manufacturing outside Israel, thus avoiding the need to obtain additional approval. On January 6, 2011, the R&D Law was amended to clarify that the potential increased royalties specified in the table above will apply even in those cases where the OCS approval for transfer of manufacturing outside of Israel is not required, namely when the volume of the transferred manufacturing capacity is less than 10% of total capacity or when the company received an advance approval to manufacture abroad in the framework of its OCS grant application.
The know-how developed within the framework of the Chief Scientist plan may not be transferred to third parties outside Israel without the prior approval of a governmental committee charted under the R&D Law. The approval, however, is not required for the export of any products developed using grants received from the Chief Scientist. The OCS approval to transfer know-how created, in whole or in part, in connection with an OCS-funded project to third party outside Israel where the transferring company remains an operating Israeli entity is subject to payment of a redemption fee to the OCS calculated according to a formula provided under the R&D Law that is based, in general, on the ratio between the aggregate OCS grants to the company’s aggregate investments in the project that was funded by these OCS grants, multiplied by the transaction consideration. The transfer of such know-how to a party outside Israel where the transferring company ceases to exist as an Israeli entity is subject to a redemption fee formula that is based, in general, on the ratio between the aggregate OCS grants to the total financial investments in the company, multiplied by the transaction consideration. According to the January 2011 amendment, the redemption fee in case of transfer of know-how to a party outside Israel will be based on the ratio between the aggregate OCS grants received by the company and the company’s aggregate R&D expenses, multiplied by the transaction consideration. According to regulations promulgated following the 2011 amendment, the maximum amount payable to the OCS in case of transfer of know how outside Israel shall not exceed 6 times the value of the grants received plus interest, and in the event that the receiver of the grants ceases to be an Israeli corporation such payment shall not exceed 6 times the value of the grants received plus interest, with a possibility to reduce such payment to up to 3 times the value of the grants received plus interest if the R&D activity remains in Israel for a period of three years after payment to the OCS.
Transfer of know-how within Israel is subject to an undertaking of the recipient Israeli entity to comply with the provisions of the R&D Law and related regulations, including the restrictions on the transfer of know-how and the obligation to pay royalties, as further described in the R&D Law and related regulations.
These restrictions may impair our ability to outsource manufacturing, engage in change of control transactions or otherwise transfer our know-how outside Israel and may require us to obtain the approval or the OCS for certain actions and transactions and pay additional royalties to the OCS. In particular, any change of control and any change of ownership of our ordinary shares that would make a non-Israeli citizen or resident an “interested party,” as defined in the R&D Law, requires a prior written notice to the OCS in addition to any payment that may be required of us for transfer of manufacturing or know-how outside Israel. If we fail to comply with the R&D Law, we may be subject to criminal charges.
Tax Benefits under the Law for the Encouragement of Industry (Taxes), 1969
According to the Law for the Encouragement of Industry (Taxes), 1969, or the Industry Encouragement Law, an Industrial Company is a company resident in Israel, at least 90% of the income of which, in a given tax year, determined in Israeli currency (exclusive of income from some government loans, capital gains, interest and dividends), is derived from an Industrial Enterprise owned by it. An “Industrial Enterprise” is defined as an enterprise whose major activity in a given tax year is industrial production activity.
Under the Industry Encouragement Law, industrial companies are entitled to the following preferred corporate tax benefits:
|
|
●
|
amortization of purchases of know-how and patents over an eight-year period for tax purposes;
|
|
|
●
|
deductions over a three-year period of expenses involved with the issuance and listing of shares on a stock market;
|
|
|
●
|
the right to elect, under specified conditions, to file a consolidated tax return with additional related Israeli Industrial Companies; and
|
|
|
●
|
accelerated depreciation rates on equipment and buildings.
|
Eligibility for benefits under the Industry Encouragement Law is not subject to receipt of prior approval from any governmental authority.
We believe that we currently qualify as an Industrial Company within the definition of the Industry Encouragement Law. We cannot assure you that we will continue to qualify as an Industrial Company or that the benefits described above will be available to us in the future.
Israeli Transfer Pricing Regulations
On November 29, 2006, Income Tax Regulations (Determination of Market Terms), 2006, promulgated under Section 85A of the Tax Ordinance, came into force (the “TP Regulations”). Section 85A of the Tax Ordinance and the TP Regulations generally require that all cross-border transactions carried out between related parties will be conducted on an arm’s length principle basis and will be taxed accordingly.
Taxation of our Shareholders
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. A non-Israeli resident who derives capital gains from the sale of shares in an Israeli resident company that were purchased after the company was listed for trading on a stock exchange outside of Israel will be exempt from Israeli tax so long as the shares were not held through a permanent establishment that the non-resident maintains in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents: (i) have a controlling interest of 25% or more in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly. Additionally, such exemption is not applicable to a person whose gains from selling or otherwise disposing of the shares are deemed to be business income.
Additionally, a sale of securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, under the United States-Israel Tax Treaty, the disposition of shares by a shareholder who is a United States resident (for purposes of the treaty) holding the shares as a capital asset is generally exempt from Israeli capital gains tax unless, among other things, (i) the capital gain arising from the disposition is attributed to business income derived by a permanent establishment of the shareholder in Israel; or (ii) such U.S. resident is an individual and was present in Israel for 183 days or more during the relevant taxable year.
In some instances where our shareholders may be liable for Israeli tax on the sale of their Ordinary Shares, the payment of the consideration may be subject to the withholding of Israeli tax at source.
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-Israeli residents generally will be subject to Israeli income tax on the receipt of dividends paid on our Ordinary Shares at the rate of 25%, which tax will be withheld at source, unless relief is provided in a treaty between Israel and the shareholder’s country of residence. With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or on any time during the preceding twelve months, the applicable tax rate is 30%. A “substantial shareholder” is generally a person who alone or together with such person’s relative or another person who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an executive officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right. However, a distribution of dividends to non-Israeli residents is subject to withholding tax at source at a rate of 15% if the dividend is distributed from income attributed to an Approved Enterprise or a Preferred Enterprise. When the dividend is distributed by a Beneficiary Enterprise, the withholding rate is 20%, unless a reduced tax rate is provided under an applicable tax treaty. In this regard, under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our Ordinary Shares who is a United States resident (for purposes of the United States-Israel Tax Treaty) is 25%. Consequently, distributions to U.S. residents of income attributed to an Approved Enterprise, a Preferred Enterprise or a Beneficiary Enterprise will be subject to withholding tax at a rate of 15%, or 20%, as explained above. However, generally, the maximum rate of withholding tax on dividends, not generated by an Approved Enterprise, a Preferred Enterprise or a Beneficiary Enterprise, that are paid to a United States corporation holding 10% or more of the outstanding voting capital throughout the tax year in which the dividend is distributed as well as during the previous tax year, is 12.5%, provided that not more than 25% of the gross income for such preceding year consists of certain types of dividends and interest. We cannot assure you that we will designate the profits that we may distribute in a way that will reduce shareholders’ tax liability.
Taxation of the U.S. Subsidiary in the United States
The corporate tax applicable to the U.S. Subsidiary is at graduated rates of up to 34% plus state tax of 0.95% to 9.99% (according to the tax rates in the states in which the U.S. Subsidiary operates). Furthermore, certain states in which the U.S. Subsidiary operates have a minimum tax rate.
Israel and the United States have a double tax prevention treaty. According to the treaty, dividends and interest paid to us by our U.S. Subsidiary are generally subject to withholding tax of 12.5% and 17.5%, respectively.
Exchange Controls
There are currently no Israeli currency control restrictions on payments of dividends or other distributions with respect to our Ordinary Shares or ADSs, or the proceeds from the sale of the Ordinary Shares or ADSs, except for the obligation of Israeli residents to file reports with the Bank of Israel regarding certain transactions. However, legislation remains in effect pursuant to which currency controls can be imposed by administrative action at any time.
The ownership or voting of our Ordinary Shares or ADSs by non-residents of Israel, except with respect to citizens of countries that are in a state of war with Israel, is not restricted in any way by our articles of association or by the laws of the State of Israel.
U.S. Tax Considerations
U.S. Federal Income Tax Considerations
THE FOLLOWING SUMMARY IS INCLUDED HEREIN FOR GENERAL INFORMATION AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND SALE OF ORDINARY SHARES AND AMERICAN DEPOSITORY SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Subject to the limitations described in the next paragraph, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the ordinary shares and ADSs. For this purpose, a “U.S. Holder” is a holder of ordinary shares or ADSs that is: (1) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of source; (4) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; or (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our ordinary shares or ADSs. This summary generally considers only U.S. Holders that will own our ordinary shares or ADSs as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Internal Revenue Code of 1986, as amended, or the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, and the U.S./Israel Income Tax Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the U.S. Internal Revenue Service, or IRS with regard to the U.S. federal income tax treatment of an investment in our ordinary shares or ADSs by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. holder based on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping, transfer, state, local, excise or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity”; (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our ordinary shares or ADSs in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our ordinary shares or ADSs as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, ordinary shares or ADSs representing 10% or more of our voting power. Additionally, the U.S. federal income tax treatment of persons who hold ordinary shares or ADSs through a partnership or other pass-through entity are not considered.
Each prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our ordinary shares or ADSs, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
Taxation of Dividends Paid on Ordinary Shares or ADSs
We do not intend to pay dividends in the foreseeable future. In the event that we do pay dividends, and subject to the discussion under the heading “Passive Foreign Investment Companies” below, a U.S. Holder will be required to include in gross income as ordinary income the amount of any distribution paid on ordinary shares or ADSs (including the amount of any Israeli tax withheld on the date of the distribution), to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. The amount of a distribution which exceeds our earnings and profits will be treated first as a non-taxable return of capital, reducing the U.S. Holder’s tax basis for the ordinary shares to the extent thereof, and then capital gain. Corporate holders generally will not be allowed a deduction for dividends received.
In general, preferential tax rates for “qualified dividend income” and long-term capital gains are applicable for U.S. Holders that are individuals, estates or trusts. For this purpose, “qualified dividend income” means, inter alia, dividends received from a “qualified foreign corporation.” A “qualified foreign corporation” is a corporation that is entitled to the benefits of a comprehensive tax treaty with the United States which includes an exchange of information program. The IRS has stated that the Israel/U.S. Tax Treaty satisfies this requirement and we believe we are eligible for the benefits of that treaty.
In addition, our dividends will be qualified dividend income if our ordinary shares or ADSs are readily tradable on the NASDAQ or another established securities market in the United States. Dividends will not qualify for the preferential rate if we are treated, in the year the dividend is paid or in the prior year, as a passive foreign investment company, or PFIC, as described below under “Passive Foreign Investment Companies”. A U.S. Holder will not be entitled to the preferential rate: (1) if the U.S. Holder has not held our ordinary shares or ADSs for at least 61 days of the 121 day period beginning on the date which is 60 days before the ex-dividend date, or (2) to the extent the U.S. Holder is under an obligation to make related payments on substantially similar property. Any days during which the U.S. Holder has diminished its risk of loss on our ordinary shares or ADSs are not counted towards meeting the 61-day holding period. Finally, U.S. Holders who elect to treat the dividend income as “investment income” pursuant to Code section 163(d)(4) will not be eligible for the preferential rate of taxation.
The amount of a distribution with respect to our ordinary shares or ADSs will be measured by the amount of the fair market value of any property distributed, and for U.S. federal income tax purposes, the amount of any Israeli taxes withheld therefrom. Cash distributions paid by us in NIS will be included in the income of U.S. Holders at a U.S. dollar amount based upon the spot rate of exchange in effect on the date the dividend is includible in the income of the U.S. Holder, and U.S. Holders will have a tax basis in such NIS for U.S. federal income tax purposes equal to such U.S. dollar value. If the U.S. Holder subsequently converts the NIS into U.S. dollars or otherwise disposes of it, any subsequent gain or loss in respect of such NIS arising from exchange rate fluctuations will be U.S. source ordinary exchange gain or loss.
Distributions paid by us will generally be foreign source income for U.S. foreign tax credit purposes and will generally be considered passive category income for such purposes. Subject to the limitations set forth in the Code, U.S. Holders may elect to claim a foreign tax credit against their U.S. federal income tax liability for Israeli income tax withheld from distributions received in respect of the Ordinary Shares or ADSs. The rules relating to the determination of the U.S. foreign tax credit are complex, and U.S. Holders should consult with their own tax advisors to determine whether, and to what extent, they are entitled to such credit. U.S. Holders that do not elect to claim a foreign tax credit may instead claim a deduction for Israeli income taxes withheld, provided such U.S. Holders itemize their deductions.
Taxation of the Disposition of Ordinary Shares or ADSs
Except as provided under the PFIC rules described below under “Passive Foreign Investment Companies”, upon the sale, exchange or other disposition of our ordinary shares or ADSs, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the ordinary shares or ADSs in U.S. dollars and the amount realized on the disposition in U.S. dollar (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of ordinary shares or ADSs will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition.
Gain realized by a U.S. Holder on a sale, exchange or other disposition of ordinary shares or ADSs will generally be treated as U.S. source income for U.S. foreign tax credit purposes. A loss realized by a U.S. Holder on the sale, exchange or other disposition of ordinary shares or ADSs is generally allocated to U.S. source income. The deductibility of a loss realized on the sale, exchange or other disposition of ordinary shares or ADSs is subject to limitations.
Passive Foreign Investment Companies
Special U.S. federal income tax laws apply to U.S. taxpayers who own shares of a corporation that is a PFIC. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year that either:
|
|
●
|
75% or more of our gross income (including our pro rata share of gross income for any company, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or
|
|
|
●
|
At least 50% of our assets, averaged over the year and generally determined based upon fair market value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value) are held for the production of, or produce, passive income.
|
For this purpose, passive income generally consists of dividends, interest, rents, royalties, annuities and income from certain commodities transactions and from notional principal contracts. Cash is treated as generating passive income.
We believe that we will not be a PFIC for the current taxable year and do not expect to become a PFIC in the foreseeable future. The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our Ordinary Shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC.
If we currently are or become a PFIC, each U.S. Holder who has not elected to treat us as a qualified electing fund by making a “QEF election”, or who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain distributions by us and upon disposition of our ordinary shares or ADSs at a gain: (1) have such distribution or gain allocated ratably over the U.S. Holder’s holding period for the Ordinary Shares or ADSs, as the case may be; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, when shares of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
The PFIC rules described above would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the ordinary shares or ADSs while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. Although we have no obligation to do so, we intend to notify U.S. Holders if we believe we will be treated as a PFIC for any tax year in order to enable U.S. Holders to consider whether to make a QEF election. In addition, we intend to furnish U.S. Holders annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our Subsidiaries are a PFIC. U.S. Holders should consult with their own tax advisors regarding eligibility, manner and advisability of making a QEF election if we are treated as a PFIC.
In addition, the PFIC rules described above would not apply if we were a PFIC and a U.S. Holder made a mark-to-market election. A U.S. Holder of our Ordinary Shares or ADSs which are regularly traded on a qualifying exchange, including Nasdaq, can elect to mark the Ordinary Shares or ADSs to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the Ordinary Shares or ADSs and the U.S. Holder’s adjusted tax basis in the Ordinary Shares or ADSs. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years.
U.S. Holders who hold our Ordinary Shares or ADSs during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC. U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner, and consequences to them of making a QEF or mark-to-market election with respect to our Ordinary Shares or ADSs in the event that we are a PFIC.
New Tax on Investment Income
For taxable years beginning after December 31, 2013, U.S. Holders who are individuals, estates or trusts will generally be required to pay a new 3.8% Medicare tax on their net investment income (including dividends on and gains from the sale or other disposition of our ordinary shares or ADSs), or in the case of estates and trusts on their net investment income that is not distributed. In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Tax Consequences for Non-U.S. Holders of Ordinary Shares or ADSs
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder referred to below as a non-U.S. Holder, generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our Ordinary Shares or ADSs.
A non-U.S. Holder may be subject to U.S. federal income tax on a dividend paid on our Ordinary Shares or ADSs or gain from the disposition of our Ordinary Shares or ADSs if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States and, if required by an applicable income tax treaty is attributable to a permanent establishment or fixed place of business in the United States; (2) in the case of a disposition of our Ordinary Shares or ADSs, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the disposition and other specified conditions are met.
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our ordinary shares or ADSs if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides an applicable IRS Form W-8 (or a substantially similar form) certifying its foreign status, or otherwise establishes an exemption.
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding at a rate of 28% with respect to cash dividends and proceeds from a disposition of ordinary shares or ADSs. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Pursuant to recently enacted legislation, a U.S. Holder with interests in “specified foreign financial assets” (including, among other assets, our Ordinary Shares or ADSs, unless such Ordinary Shares or ADSs are held on such U.S. Holder’s behalf through a financial institution) may be required to file an information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000 at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance); and may be required to file an FBAR if the aggregate value of the foreign financial accounts exceeds $10,000 at any time during the calendar year. You should consult your own tax advisor as to the possible obligation to file such information report.
Medical Devices Excise Tax
The Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 imposes significant new taxes on medical device makers in the form of a 2.3% excise tax on U.S. medical device sales, with certain exemptions, beginning in January 2013. In the fiscal year 2015 we paid, or accrued an excise tax fee in the amount of $308,000, which is included within sales and marketing expense, net in our Consolidated Statement of Profit or Loss. The Consolidated Appropriations Act, 2016, signed into law on December 18, 2015, includes a two year suspension on the medical device excise tax. Thus, the medical device excise tax does not apply to the sale of a taxable medical device by the manufacturer, producer, or importer of the device during the period beginning on January 1, 2016, and ending on December 31, 2017. However, there is no guarantee that the excise tax will continue to be suspended by congressional action after this two-year period ends, and absent further congressional action, the excise tax will be reinstated for medical device sales beginning January 1, 2018.
|
F.
|
Dividends and Paying Agents
|
We are subject to the information reporting requirements of the Exchange Act, applicable to foreign private issuers and under those requirements will file reports with the SEC. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we file with the SEC, within 120 days after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and will submit to the SEC, on a Form 6-K, unaudited quarterly financial information.
You may read and copy any document we file with the SEC at its public reference facilities at 100 F Street, NE, Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, NE, Washington, D.C. 20549. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of this website is http://www.sec.gov. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
In addition, since our ordinary shares are traded on the TASE, we have filed Hebrew language periodic and immediate reports with, and furnish information to, the TASE and the Israel Securities Authority, or the ISA, as required under Chapter Six of the Israel Securities Law, 1968. Copies of our filings with the ISA can be retrieved electronically through the MAGNA distribution site of the ISA (www.magna.isa.gov.il) and the TASE website (www.maya.tase.co.il).
We maintain a corporate website at www.mazorrobotics.com. Information contained on, or that can be accessed through, our website does not constitute a part of this annual report. We have included our website address in this annual report solely as an inactive textual reference.
|
I.
|
Subsidiary Information.
|
Not applicable.
|
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
Market risk is the risk of loss related to changes in market prices, including interest rates and foreign exchange rates, of financial instruments that may adversely impact our consolidated financial position, results of operations or cash flows.
Risk of Interest Rate Fluctuation
We do not currently anticipate undertaking any significant long-term borrowings. We follow an investment policy that was set by our board of directors whose primary objectives are to preserve principal while maximizing the income that we receive from our investments without significantly increasing risk and loss. Our investments are exposed to market risk due to fluctuation in interest rates, which may affect our interest income and the fair market value of our investments, provided, however, that given the low levels of interest rates worldwide, our interest income is not material and a further reduction in interest rates would not cause us a significant reduction in the absolute amounts of interest income to us. We manage this exposure by performing ongoing evaluations of our investments. Our investment balances are comprised mainly of bank deposits. The carrying value of the investment balances usually approximates their fair value. It is be our current policy to hold investments to maturity in order to limit our exposure to interest rate fluctuations.
Foreign Currency Exchange Risk
Our functional and reporting currency from September 27, 2012 is the U.S. dollar. Although the U.S. dollar is our functional currency, a significant portion of our expenses are denominated in both NIS and Euros and currently most of our revenues are denominated in U.S. dollars. Therefore, our foreign currency exposures give rise to market risk associated with exchange rate movements of the U.S. dollar, mainly against the NIS and the Euro. Our NIS and Euro expenses consist principally of payroll to our employees in Israel, payments made to subcontractors for purchasing components to our products, research and development activities and marketing and sales activities. We anticipate that a sizable portion of our expenses will continue to be denominated in currencies other than the U.S. dollar. If the U.S. dollar fluctuates significantly against either the NIS or the Euro, it may have a negative impact on our results of operations. To date, fluctuations in the exchange rates have not materially affected our results of operations or financial condition.
Due to the fact that exchange rates between the U.S. dollar and the NIS (as well as between the U.S. dollar and other currencies) fluctuate continuously, such fluctuations have an impact on our results and period-to-period comparisons of our results. The effects of foreign currency re-measurements are reported in our consolidated statements of operations. We engage in short-term currency hedging activities in order to reduce some of this currency exposure. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
As of December 31, 2015, we have open currency hedging transactions in the amount of 4,000,000 NIS (approximately $1,029,000). All transactions were settled in the first quarter of 2016.We will continue to monitor exposure to currency fluctuations. Instruments that may be used to hedge future risks may include foreign currency forward, options and swap contracts. These instruments may be used to selectively manage risks, but there can be no assurance that we will be fully protected against foreign currency fluctuations.
In addition, we have balance sheet exposure arising from assets and liabilities denominated in currencies other than U.S. dollar, mainly in NIS and Euros. Any change of the conversion rates between the U.S. dollar and these currencies may create financial gain or loss.
The tables below provide information as of December 31, 2015 regarding our foreign currency-denominated monetary assets and liabilities.
Foreign currency denominated monetary assets and liabilities.
Position as of December 31, 2015:
| |
|
Total as of
December
31,
2015
|
|
| |
|
(U.S. $ in
thousands)
|
|
|
Current Assets:
|
|
|
|
|
Shekels
|
|
|
2,669 |
|
|
Euros
|
|
|
753 |
|
|
Total
|
|
|
3,422 |
|
| |
|
|
|
|
|
Long term Assets:
|
|
|
|
|
|
Shekels
|
|
|
73 |
|
|
Total
|
|
|
73 |
|
| |
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
Shekels
|
|
|
2,585 |
|
|
Euros
|
|
|
192 |
|
|
Total
|
|
|
2,777 |
|
| |
|
|
|
|
|
Long term Liabilities:
|
|
|
|
|
|
Shekels
|
|
|
- |
|
|
Total
|
|
|
- |
|
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
Not applicable.
Not applicable.
|
D.
|
American Depositary Shares
|
Fees and Expenses
The following table shows the fees and expenses that a holder of our ADSs may have to pay, either directly or indirectly:
|
Persons depositing or withdrawing shares or ADS holders must pay:
|
|
For:
|
|
| |
|
|
|
|
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs).
|
|
●
|
Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property.
|
| |
|
|
|
| |
|
●
|
Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates.
|
| |
|
|
|
|
$.05 (or less) per ADS.
|
|
●
|
Any cash distribution to ADS holders.
|
| |
|
|
|
|
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs.
|
|
●
|
Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to ADS holders.
|
| |
|
|
|
|
$.05 (or less) per ADSs per calendar year.
|
|
●
|
Depositary services.
|
| |
|
|
|
|
Registration or transfer fees.
|
|
●
|
Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares.
|
| |
|
|
|
|
Expenses of the depositary.
|
|
●
|
Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement).
|
| |
|
|
|
| |
|
●
|
Converting foreign currency to U.S. dollars.
|
| |
|
|
|
|
Taxes and other governmental charges the depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes.
|
|
●
|
As necessary.
|
| |
|
|
|
|
Any charges incurred by the depositary or its agents for servicing the deposited securities.
|
|
●
|
As necessary.
|
The Bank of New York Mellon, as depositary, collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid. The depositary may collect any of its fees by deduction from any cash distributions made to ADS holders that are obligated to pay those fees.
From time to time, the depositary may make payments to us to reimburse and/or share revenue from the fees collected from ADS holders, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS program. In performing its duties under the deposit agreement, the depositary may use brokers, dealers or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
PART II
|
|
DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
|
None.
|
|
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
|
None.
|
(a)
|
Evaluation of Disclosure Controls and Procedures
|
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report on Form 20-F.
Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, 2015, our disclosure controls and procedures are effective in recording, processing, summarizing and reporting, on a timely basis, information required to be included in periodic filings under the Exchange Act and that such information is accumulated and communicated to management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding required disclosure.
|
(b)
|
Management’s Annual Report on Internal Control over Financial Reporting
|
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting. Based principally on the framework in Internal Control - Integrated Framework (2013 framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management has concluded that our internal control over financial reporting was effective as of December 31, 2015. Management reviewed the results of its assessment with our Audit Committee.
|
(c)
|
Attestation Report of the Registered Public Accounting Firm
|
The effectiveness of our internal control over financial reporting as of December 31, 2015 has not been audited by our registered public accounting firm due to an exemption for emerging growth companies provided in the JOBS Act.
|
(d)
|
Changes in Internal Control over Financial Reporting
|
During the year ended December 31, 2015, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
|
(e)
|
Limitations on Effectiveness of Controls and Procedures
|
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
|
|
AUDIT COMMITTEE FINANCIAL EXPERT
|
The board of directors has determined that Gil Bianco, David Schlachet and Sarit Soccary Ben-Yochanan are the financial experts serving on our audit committee and that these individuals are independent as that term "audit committee financial expert" is defined under the rules under the Exchange Act, and are independent in accordance with applicable Exchange Act rules and the NASDAQ Stock Market rules.
We have adopted a code of business conduct and ethics applicable to our employees in all locations. The code of business conduct and ethics is available on our website, www.mazorrobotics.com.
|
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
The following fees were billed by Somekh Chaikin, a member firm of KPMG International, and affiliated firms, for professional services rendered thereby for the years ended December 31, 2015 and 2014:
| |
|
2015
|
|
|
2014
|
|
| |
|
(In Thousands)
|
|
| |
|
|
|
|
Audit Fees (1)
|
|
$ |
171 |
|
|
$ |
179 |
|
|
Audit-Related Fees (2)
|
|
$ |
5 |
|
|
$ |
25 |
|
|
Tax Fees (3)
|
|
$ |
13 |
|
|
$ |
8 |
|
|
All Other Fees
|
|
$ |
- |
|
|
$ |
- |
|
|
Total
|
|
$ |
189 |
|
|
$ |
212 |
|
|
(1)
|
Includes audit fee for registration statements and related prospectuses.
|
|
(2)
|
Includes fees for professional services rendered in connection with the audit of the Company's annual consolidated financial statements, reviews of interim financial statements and other services provided in connection with statutory or regulatory filings.
|
|
(3)
|
Includes fees for professional services rendered by our auditors for tax compliance and tax advice on actual or contemplated transactions.
|
Our audit committee pre-approves all audit and non-audit services provided to us and to our Subsidiaries during the periods listed above. Audit services must be pre-approved by the full audit committee. The authority to pre-approve non-audit services has been delegated to the Chairman of the audit committee. Any services pre-approved by the Chairman are reported to the full committee at its next scheduled meeting.
|
|
EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
|
Not applicable.
|
|
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
|
Not applicable.
|
|
CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
|
Not applicable.
The Sarbanes-Oxley Act, as well as related rules subsequently implemented by the SEC, require foreign private issuers, such as us, to comply with various corporate governance practices. In addition, following the listing of our ADSs on the NASDAQ Capital Market (and now the NASDAQ Global Market), we are required to comply with the Listing Rules of the NASDAQ Stock Market. Under those Listing Rules, we may elect to follow certain corporate governance practices permitted under the Companies Law in lieu of compliance with corresponding corporate governance requirements otherwise imposed by the Listing Rules of the NASDAQ Stock Market for U.S. domestic issuers.
In accordance with Israeli law and practice and subject to the exemption set forth in Rule 5615 of the Listing Rules of the NASDAQ Stock Market, we have elected to follow the provisions of the Companies Law, rather than the Listing Rules of the NASDAQ Stock Market, with respect to the following requirements:
|
|
●
|
Distribution of periodic reports to shareholders; proxy solicitation. As opposed to the Listing Rules of the NASDAQ Stock Market, which require listed issuers to make such reports available to shareholders in one of a number of specific manners, Israeli law does not require us to distribute periodic reports directly to shareholders, and the generally accepted business practice in Israel is not to distribute such reports to shareholders but to make such reports available through a public website. In addition to making such reports available on a public website, we currently make our audited financial statements available to our shareholders at our offices and will only mail such reports to shareholders upon request. As a foreign private issuer, we are generally exempt from the SEC’s proxy solicitation rules.
|
|
|
●
|
Quorum. While the Listing Rules of the NASDAQ Stock Market require that the quorum for purposes of any meeting of the holders of a listed company’s common voting stock, as specified in the company’s bylaws, be no less than 33% (1/3) of the company’s outstanding common voting stock, under Israeli law, a company is entitled to determine in its articles of association the number of shareholders and percentage of holdings required for a quorum at a shareholders meeting. Our Articles of Association provide that a quorum of two or more shareholders holding at least 25% of the voting rights in person or by proxy is required for commencement of business at a general meeting. However, the quorum set forth in our Articles of Association with respect to an adjourned meeting consists of any number of shareholders present in person or by proxy.
|
|
|
●
|
Nomination of our directors. With the exception of our external directors and directors elected by our board of directors, our directors are elected by an annual meeting of our shareholders to hold office until the next annual meeting following one year from his or her election. See “Management—Board Practices” The nominations for directors, which are presented to our shareholders by our board of directors, are generally made by the board of directors itself, in accordance with the provisions of our amended and restated articles of association and the Companies Law. Nominations need not be made by a nominating committee of our board of directors consisting solely of independent directors, as required under the Listing Rules of the NASDAQ Stock Market.
|
|
|
●
|
Compensation of officers. Israeli law and our amended and restated articles of association do not require that the independent members of our board of directors (or a compensation committee composed solely of independent members of our board of directors) determine an executive officer’s compensation, as is generally required under the Listing Rules of the NASDAQ Stock Market with respect to the CEO and all other executive officers.
|
Instead, compensation of executive officers is determined and approved by our compensation committee and our board of directors, and in certain circumstances by our shareholders, either in consistency with our office holder compensation policy or, in special circumstances in deviation therefrom, taking into account certain considerations stated in the Companies Law.
Shareholder approval is generally required for officer compensation in the event (i) approval by our board of directors and our compensation committee is not consistent with our office holders compensation policy, or (ii) compensation required to be approved is that of our chief executive officer who is not a director or an executive officer who is also the controlling shareholder of our company (including an affiliate thereof). Such shareholder approval shall require a majority vote of the shares present and voting at a shareholders meeting, provided either (i) such majority includes a majority of the shares held by non-controlling shareholders who do not otherwise have a personal interest in the compensation arrangement that are voted at the meeting, excluding for such purpose any abstentions disinterested majority, or (ii) the total shares held by non-controlling and disinterested shareholders voted against the arrangement does not exceed two percent (2%) of the voting rights in our company.
Additionally, approval of the compensation of an executive officer, who is also a director, shall generally require a simple majority vote of the shares present and voting at a shareholders meeting, if consistent with our office holders compensation policy. Our compensation committee and board of directors may, in special circumstances, approve the compensation of an executive officer (other than a director, a chief executive officer or a controlling shareholder) or approve the compensation policy despite shareholders’ objection, based on specified arguments and taking shareholders’ objection into account. Our compensation committee may further exempt an engagement with a nominee for the position of chief executive officer, who meets the non-affiliation requirements set forth for an external director, from requiring shareholders’ approval, if such engagement is consistent with our office holders compensation policy and our compensation committee determines based on specified arguments that presentation of such engagement to shareholders’ approval is likely to prevent such engagement. To the extent that any such transaction with a controlling shareholder is for a period exceeding three years, approval is required once every three years.
A director or executive officer may not be present when the board of directors of a company discusses or votes upon a transaction in which he or she has a personal interest, except in case of ordinary transactions, unless the chairman of the board of directors determines that he or she should be present to present the transaction that is subject to approval.
|
|
●
|
Independent directors. Israeli law does not require that a majority of the directors serving on our board of directors be “independent,” as defined under NASDAQ Listing Rule 5605(a)(2), and rather requires we have at least two external directors who meet the requirements of the Companies Law, as described above under “Management—Board Practices—External Directors.” We are required, however, to ensure that all members of our Audit Committee are “independent” under the applicable NASDAQ and SEC criteria for independence (as we cannot exempt ourselves from compliance with that SEC independence requirement, despite our status as a foreign private issuer), and we must also ensure that a majority of the members of our Audit Committee are “unaffiliated directors” as defined in the Companies Law. Furthermore, Israeli law does not require, nor do our independent directors conduct, regularly scheduled meetings at which only they are present, which the NASDAQ Listing Rules otherwise require.
|
|
|
●
|
Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Companies Law, rather than seeking approval for corporation actions in accordance with NASDAQ Listing Rule 5635. In particular, under this NASDAQ rule, shareholder approval is generally required for: (i) an acquisition of shares/assets of another company that involves the issuance of 20% or more of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest in the target company or the consideration to be received; (ii) the issuance of shares leading to a change of control; (iii) adoption/amendment of equity compensation arrangements (although under the provisions of the Companies Law there is no requirement for shareholders’ approval for the adoption/amendment of the equity compensation plan); and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (and/or via sales by directors/officers/5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. By contrast, under the Companies Law, shareholder approval is required for, among other things: (i) transactions with directors concerning the terms of their service or indemnification, exemption and insurance for their service (or for any other position that they may hold at a company), for which approvals of the compensation committee, board of directors and shareholders are all required, (ii) extraordinary transactions with controlling shareholders of publicly held companies, which require the special approval described below under “Approval of Related Party Transactions under Israeli Law - Disclosure of personal interests of controlling shareholders”, and (iii) terms of employment or other engagement of the controlling shareholder of us or such controlling shareholder’s relative, which require the special approval described above, under “Approval of Related Party Transactions under Israeli Law - Disclosure of personal interests of controlling shareholders”. In addition, under the Companies Law, a merger requires approval of the shareholders of each of the merging companies.
|
|
|
●
|
Approval of Related Party Transactions. All related party transactions are approved in accordance with the requirements and procedures for approval of interested party acts and transactions, set forth in sections 268 to 275 of the Companies Law, and the regulations promulgated thereunder, which require the approval of the audit committee, or the compensation committee, as the case may be, the board of directors and shareholders, as may be applicable, for specified transactions, rather than approval by the audit committee or other independent body of our board of directors as required under the Listing Rules of the NASDAQ Stock Market.
|
Not applicable.
PART III
We have elected to provide financial statements and related information pursuant to Item 18.
The consolidated financial statements and the related notes required by this Item are included in this annual report beginning on page F-1.
MAZOR ROBOTICS LTD.
CONSOLIDATED
FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2015
Mazor Robotics Ltd.
Consolidated Financial Statements as of December 31, 2015
|
Contents
|
|
| |
|
| |
|
| |
|
|
|
F-2
|
| |
|
|
|
F-3 - F-4
|
| |
|
|
|
F-5
|
| |
|
|
|
F-6 - F-7
|
| |
|
|
|
F-8
|
| |
|
|
|
F-9 - F-44
|
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Mazor Robotics Ltd.
We have audited the accompanying consolidated statements of financial position of Mazor Robotics Ltd. (hereinafter - “the Company”) and its subsidiaries as of December 31, 2015 and 2014 and the related consolidated statements of profit or loss, changes in equity and cash flows, for each of the years in the three-year period ended December 31, 2015. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company and its subsidiaries as of December 31, 2015 and 2014 and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2015, in conformity with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“IASB”).
/s/ Somekh Chaikin
Certified Public Accountants (Isr.)
Member firm of KPMG International
Tel-Aviv, Israel
Consolidated Statements of Financial Position as of December 31
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
4 |
|
|
|
13,519 |
|
|
|
22,255 |
|
|
Short-term investments
|
|
|
5 |
|
|
|
21,687 |
|
|
|
24,507 |
|
|
Trade receivables
|
|
|
|
|
|
|
5,002 |
|
|
|
2,797 |
|
|
Other current assets
|
|
|
6 |
|
|
|
1,420 |
|
|
|
1,110 |
|
|
Inventory
|
|
|
7 |
|
|
|
2,777 |
|
|
|
3,050 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
|
|
|
|
44,405 |
|
|
|
53,719 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid lease fees
|
|
|
13B |
|
|
|
73 |
|
|
|
79 |
|
|
Long-term investments
|
|
|
5 |
|
|
|
5,023 |
|
|
|
5,473 |
|
|
Property and equipment, net
|
|
|
8 |
|
|
|
1,432 |
|
|
|
1,257 |
|
|
Deferred tax asset
|
|
|
12 |
|
|
|
37 |
|
|
|
158 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
|
|
|
|
6,565 |
|
|
|
6,967 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
|
|
|
|
50,970 |
|
|
|
60,686 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
Mazor Robotics Ltd.
Consolidated Statements of Financial Position as of December 31
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Trade payables
|
|
|
|
|
|
2,219 |
|
|
|
1,689 |
|
|
Other current liabilities
|
|
|
10 |
|
|
|
6,052 |
|
|
|
4,452 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
|
|
|
|
8,271 |
|
|
|
6,141 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities - Employee benefits
|
|
|
11 |
|
|
|
299 |
|
|
|
278 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
|
|
|
|
8,570 |
|
|
|
6,419 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
22 |
|
|
|
|
|
|
|
|
|
|
Share capital
|
|
|
|
|
|
|
110 |
|
|
|
110 |
|
|
Share premium
|
|
|
|
|
|
|
136,107 |
|
|
|
135,182 |
|
|
Amounts allocated to share options
|
|
|
|
|
|
|
77 |
|
|
|
77 |
|
|
Capital reserve for share-based payment transactions
|
|
|
|
|
|
|
7,179 |
|
|
|
4,586 |
|
|
Foreign currency translation reserve
|
|
|
|
|
|
|
2,119 |
|
|
|
2,119 |
|
|
Accumulated loss
|
|
|
|
|
|
|
(103,192 |
) |
|
|
(87,807 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
|
|
|
|
42,400 |
|
|
|
54,267 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
|
|
|
|
|
50,970 |
|
|
|
60,686 |
|
|
|
|
|
|
|
|
Chairman of the Board of Directors
|
|
CEO
|
|
CFO
|
Date of approval of the financial statements: May 2, 2016.
The accompanying notes are an integral part of these consolidated financial statements.
Consolidated Statements of Profit or Loss
| |
|
|
|
|
Year ended December 31,
|
|
| |
|
|
|
|
2015
|
|
|
2014
|
|
|
2013
|
|
| |
|
Note
|
|
|
USD thousands
|
|
|
USD thousands
|
|
|
USD thousands
|
|
|
Revenues
|
|
|
14 |
|
|
|
26,096 |
|
|
|
21,208 |
|
|
|
19,983 |
|
|
Cost of sales
|
|
|
16 |
|
|
|
5,827 |
|
|
|
4,396 |
|
|
|
4,280 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
|
|
|
|
20,269 |
|
|
|
16,812 |
|
|
|
15,703 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses
|
|
|
17 |
|
|
|
6,324 |
|
|
|
5,776 |
|
|
|
4,174 |
|
|
Selling and marketing expenses
|
|
|
18 |
|
|
|
24,947 |
|
|
|
21,352 |
|
|
|
15,692 |
|
|
General and administrative expenses
|
|
|
19 |
|
|
|
4,305 |
|
|
|
4,392 |
|
|
|
2,766 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
|
|
|
|
(15,307 |
) |
|
|
(14,708 |
) |
|
|
(6,929 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing income
|
|
|
20 |
|
|
|
272 |
|
|
|
134 |
|
|
|
214 |
|
|
Financing expenses
|
|
|
20 |
|
|
|
(137 |
) |
|
|
(553 |
) |
|
|
(13,647 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing income (expenses), net
|
|
|
|
|
|
|
135 |
|
|
|
(419 |
) |
|
|
(13,433 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before taxes on income
|
|
|
|
|
|
|
(15,172 |
) |
|
|
(15,127 |
) |
|
|
(20,362 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Taxes on income
|
|
|
13 |
|
|
|
213 |
|
|
|
145 |
|
|
|
167 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss for the year
|
|
|
|
|
|
|
(15,385 |
) |
|
|
(15,272 |
) |
|
|
(20,529 |
) |
|
Loss per share
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share (in USD)
|
|
|
24 |
|
|
|
(0.36 |
) |
|
|
(0.37 |
) |
|
|
(0.57 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
Consolidated Statements of Changes in Equity
| |
|
|
|
|
|
|
|
|
|
|
Capital
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Amounts
|
|
|
reserve for
|
|
|
Foreign
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
allocated
|
|
|
share-based
|
|
|
currency
|
|
|
|
|
|
|
|
| |
|
Share
|
|
|
Share
|
|
|
to share
|
|
|
payment
|
|
|
translation
|
|
|
Accumulated
|
|
|
Total
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
For the year ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
January 1, 2015
|
|
|
110 |
|
|
|
135,182 |
|
|
|
77 |
|
|
|
4,586 |
|
|
|
2,119 |
|
|
|
(87,807 |
) |
|
|
54,267 |
|
|
Loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(15,385 |
) |
|
|
(15,385 |
) |
|
Exercise of share options
|
|
|
*) - |
|
|
|
904 |
|
|
|
- |
|
|
|
(477 |
) |
|
|
- |
|
|
|
- |
|
|
|
427 |
|
|
Expiration of share options
|
|
|
- |
|
|
|
21 |
|
|
|
- |
|
|
|
(21 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Share-based payments
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
3,091 |
|
|
|
- |
|
|
|
- |
|
|
|
3,091 |
|
|
Balance as of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2015
|
|
|
110 |
|
|
|
136,107 |
|
|
|
77 |
|
|
|
7,179 |
|
|
|
2,119 |
|
|
|
(103,192 |
) |
|
|
42,400 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
January 1, 2014
|
|
|
106 |
|
|
|
130,472 |
|
|
|
77 |
|
|
|
3,854 |
|
|
|
2,119 |
|
|
|
(72,535 |
) |
|
|
64,093 |
|
|
Loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(15,272 |
) |
|
|
(15,272 |
) |
|
Exercise of share options
|
|
|
4 |
|
|
|
4,635 |
|
|
|
- |
|
|
|
(1,350 |
) |
|
|
- |
|
|
|
- |
|
|
|
3,289 |
|
|
Expiration of share options
|
|
|
- |
|
|
|
75 |
|
|
|
- |
|
|
|
(75 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Share-based payments
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
2,157 |
|
|
|
- |
|
|
|
- |
|
|
|
2,157 |
|
|
Balance as of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2014
|
|
|
110 |
|
|
|
135,182 |
|
|
|
77 |
|
|
|
4,586 |
|
|
|
2,119 |
|
|
|
(87,807 |
) |
|
|
54,267 |
|
*) Represents an amount less than USD 1 thousand.
The accompanying notes are an integral part of these consolidated financial statements.
Mazor Robotics Ltd.
Consolidated Statements of Changes in Equity (cont'd)
| |
|
|
|
|
|
|
|
|
|
|
Capital
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Amounts
|
|
|
reserve for
|
|
|
Foreign
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
allocated
|
|
|
share-based
|
|
|
currency
|
|
|
|
|
|
|
|
| |
|
Share
|
|
|
Share
|
|
|
to share
|
|
|
payment
|
|
|
translation
|
|
|
Accumulated
|
|
|
Total
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
For the year ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
January 1, 2013
|
|
|
73 |
|
|
|
58,910 |
|
|
|
554 |
|
|
|
3,170 |
|
|
|
2,119 |
|
|
|
(52,006 |
) |
|
|
12,820 |
|
|
Loss for the year
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(20,529 |
) |
|
|
(20,529 |
) |
|
Issuance of shares(1)
|
|
|
16 |
|
|
|
42,604 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
42,620 |
|
|
Classification of derivative
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
liability on account of warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
to equity(2)
|
|
|
- |
|
|
|
- |
|
|
|
17,500 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
17,500 |
|
|
Exercise of share options
|
|
|
17 |
|
|
|
28,948 |
|
|
|
(17,977 |
) |
|
|
(325 |
) |
|
|
- |
|
|
|
- |
|
|
|
10,663 |
|
|
Expiration of share options
|
|
|
- |
|
|
|
10 |
|
|
|
- |
|
|
|
(10 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Share-based payments
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,019 |
|
|
|
- |
|
|
|
- |
|
|
|
1,019 |
|
|
Balance as of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2013
|
|
|
106 |
|
|
|
130,472 |
|
|
|
77 |
|
|
|
3,854 |
|
|
|
2,119 |
|
|
|
(72,535 |
) |
|
|
64,093 |
|
The accompanying notes are an integral part of these consolidated financial statements.
Consolidated Statements of Cash Flows for the Year Ended December 31
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
Loss for the year
|
|
|
(15,385 |
) |
|
|
(15,272 |
) |
|
|
(20,529 |
) |
|
Adjustments:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
527 |
|
|
|
540 |
|
|
|
611 |
|
|
Financing expenses (income), net
|
|
|
(207 |
) |
|
|
278 |
|
|
|
13,400 |
|
|
Share-based payments
|
|
|
3,091 |
|
|
|
2,157 |
|
|
|
1,019 |
|
|
Income tax expense
|
|
|
213 |
|
|
|
145 |
|
|
|
167 |
|
| |
|
|
3,624 |
|
|
|
3,120 |
|
|
|
15,197 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in inventory
|
|
|
273 |
|
|
|
(980 |
) |
|
|
(1,223 |
) |
|
Change in trade and other accounts receivable
|
|
|
(2,408 |
) |
|
|
(1,296 |
) |
|
|
(899 |
) |
|
Change in prepaid lease fees
|
|
|
6 |
|
|
|
(1 |
) |
|
|
(14 |
) |
|
Change in trade and other accounts payable
|
|
|
2,217 |
|
|
|
138 |
|
|
|
2,254 |
|
|
Change in employee benefits
|
|
|
21 |
|
|
|
(33 |
) |
|
|
112 |
|
| |
|
|
109 |
|
|
|
(2,172 |
) |
|
|
230 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest received
|
|
|
194 |
|
|
|
85 |
|
|
|
78 |
|
|
Income tax paid
|
|
|
(114 |
) |
|
|
(51 |
) |
|
|
(26 |
) |
| |
|
|
80 |
|
|
|
34 |
|
|
|
52 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(11,572 |
) |
|
|
(14,290 |
) |
|
|
(5,050 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from (investment in) short-term
|
|
|
|
|
|
|
|
|
|
|
|
|
|
investments, net
|
|
|
9,816 |
|
|
|
22,271 |
|
|
|
(40,820 |
) |
|
Investment in long-term investments
|
|
|
(7,538 |
) |
|
|
(7,237 |
) |
|
|
- |
|
|
Proceeds from sale of long-term investments
|
|
|
992 |
|
|
|
- |
|
|
|
- |
|
|
Acquisition of property and equipment
|
|
|
(702 |
) |
|
|
(503 |
) |
|
|
(272 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities
|
|
|
2,568 |
|
|
|
14,531 |
|
|
|
(41,092 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from warrants and shares issue, net
|
|
|
- |
|
|
|
- |
|
|
|
53,198 |
|
|
Issuance expenses
|
|
|
- |
|
|
|
(294 |
) |
|
|
- |
|
|
Proceeds from exercise of share options by
|
|
|
|
|
|
|
|
|
|
|
|
|
|
employees and service providers
|
|
|
370 |
|
|
|
3,042 |
|
|
|
479 |
|
|
Repayment of loans to the Chief Scientist
|
|
|
- |
|
|
|
(324 |
) |
|
|
(629 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
370 |
|
|
|
2,424 |
|
|
|
53,048 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (decrease) increase in cash and cash equivalents
|
|
|
(8,634 |
) |
|
|
2,665 |
|
|
|
6,906 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at the beginning of the year
|
|
|
22,255 |
|
|
|
19,803 |
|
|
|
12,797 |
|
|
Effect of exchange rate differences on
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cash and cash equivalents
|
|
|
(102 |
) |
|
|
(213 |
) |
|
|
100 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at the end of the year
|
|
|
13,519 |
|
|
|
22,255 |
|
|
|
19,803 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplementary cash flows information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transfer of inventory to property and equipment
|
|
|
- |
|
|
|
410 |
|
|
|
67 |
|
The accompanying notes are an integral part of these consolidated financial statements.
Notes to the Consolidated Financial Statements
Note 1 - Reporting Entity
|
A.
|
Mazor Robotics Ltd. (the “Company”) is an Israeli company incorporated in Israel. The address of the Company’s registered office is 7 HaEeshel St., Caesarea Industrial Park, Caesarea, Israel. These consolidated financial statements as of and for the year ended December 31, 2015 comprise the Company and its wholly owned subsidiaries incorporated in the United States (U.S.) and Singapore, Mazor Robotics Inc. and Mazor Robotics Pte Ltd., respectively (together referred to as the “Group”). The Group is a leading innovator in spine surgery and has pioneered surgical guidance systems and complementary products in the spine and brain surgical markets that provide a safer surgical environment for patients, surgeons and operating room staff. The Group engages in the development, production and marketing of innovative medical devices for supporting surgical procedures in the field of orthopedics and neurosurgery. The Group operates in the field of image guided surgery (also known as computer assisted surgery) that enables the use of surgical instruments with high precision and minimal invasiveness and that simplifies complex surgical procedures. Since August 2007, the ordinary shares of the Company have been registered for trade on the Tel Aviv Stock Exchange. On May 28, 2013, the Company’s American Depositary Shares ("ADSs"), each of which represents 2 ordinary shares of the Company, represented by American Depositary Receipts ("ADRs"), were registered for trade on the NASDAQ Capital Market and are currently traded on the NASDAQ Global Market (see Note 22(C)(3)).
|
In these financial statements -
|
|
(1)
|
International Financial Reporting Standards ("IFRS") - Standards and interpretations as issued by the International Accounting Standards Board ("IASB").
|
|
|
(2)
|
The Company - Mazor Robotics Ltd.
|
|
|
(3)
|
The Group - Mazor Robotics Ltd. and its subsidiaries.
|
|
|
(4)
|
Subsidiary - A company, the financial statements of which are fully consolidated, directly or indirectly, with the financial statements of the Company.
|
|
|
(5)
|
Related party - Within its meaning in IAS 24 (2009), “Related Party Disclosures”.
|
|
|
(6)
|
CPI - The Consumer Price Index as published by the Israeli Central Bureau of Statistics.
|
|
|
(7)
|
OCS - Office of the Chief Scientist of the Israeli Ministry of Economy.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 2 - Basis of Preparation
|
A.
|
Statement of compliance
|
|
|
The consolidated financial statements have been prepared in accordance with IFRS as issued by the IASB.
|
|
|
The consolidated financial statements were authorized for issue by the Company’s Board of Directors on May 2, 2016.
|
|
B.
|
Reporting and functional currency
|
|
|
These consolidated financial statements are presented in US dollars ("USD"), which is the Company’s functional currency as of the date of these consolidated financial statements.
|
The financial statements have been prepared on the historical cost basis except for certain investments and derivative instruments measured at fair value through profit or loss, inventory (measured at the lower of cost or net realizable value), deferred tax assets and liabilities and assets and liabilities for employee benefits. For further information regarding the measurement of these assets and liabilities see Note 3 regarding significant accounting policies.
|
D.
|
Use of estimates and judgments
|
|
|
The preparation of financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
|
|
|
The preparation of accounting estimates used in the preparation of the Company’s financial statements requires management to make assumptions regarding circumstances and events that involve considerable uncertainty. Management of the Company prepares the estimates on the basis of past experience, various facts, external circumstances, and reasonable assumptions according to the pertinent circumstances of each estimate.
|
|
|
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any affected future periods.
|
|
|
Material accounting estimates and judgments
|
Presented hereunder is information with respect to material assumptions and estimates, which were made by the Group’s management while implementing Group accounting policies:
|
|
Fair value measurement of Warrants convertible into a variable number of Company's shares
|
|
|
During 2012, the Company entered into an investment agreement as described in Note 22(C)(2), according to which investors were issued warrants convertible into a variable number of the Company's shares, thereby representing a financial liability that is a derivative instrument. This liability is measured at fair value using standard valuation technique for this type of instrument ("Monte Carlo model") on the basis of observable inputs (such as the price of the Company's shares and the NIS/USD exchange rate) and the following unobservable inputs: expected volatility, correlation between the share price and the change in the exchange rate, risk-free interest rate, expected life and the probability that mandatory exercise will occur.
|
Changes in the financial inputs underlying the model and/or in the valuation technique could have caused significant changes in the fair value of said liability and could have a material effect upon the valuation results, and thus, on the financial statements. As fully described in Note 22(C), as of May 28, 2013 the derivative instrument was reclassified to equity and is no longer measured at fair value.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 2 - Basis of Preparation (cont’d)
|
D.
|
Use of estimates and judgments (cont’d)
|
Capitalization of development costs - Development costs are capitalized and recognized as an intangible asset according to the accounting policy described in Note 3E. The capitalization of the costs is based among others on management’s judgment regarding technological and economic feasibility, which generally exists when a product development project reaches a defined milestone, or when the Company enters into a transaction to sell the know-how that was derived from the development. In determining the amount to be capitalized, management makes assumptions as to the future anticipated cash inflows from the assets.
Recognition of deferred tax assets in respect of tax losses - Management of the Company evaluates whether it is probable that in the foreseeable future there will be taxable profits against which losses can be utilized and quantify the portion of tax losses that are more likely than not to be allowable under applicable tax laws, and accordingly it recognizes (or does not recognize) deferred tax assets. For further information on losses for which a deferred tax asset was recognized, see Note 12 regarding taxes on income.
Fair value measurement of share-based payment transactions - The Group grants share based payment to directors, employees and consultants. The fair value of the share options is measured at grant date on the basis of accepted valuation models and assumptions regarding unobservable inputs used in the valuation models. The value of the transactions, measured as described above, is recognized as an expense over the vesting period. Concurrently with the periodic recognition of an expense, an increase is recognized in a capital reserve, within the Group’s equity.
|
E.
|
New standards and interpretations
|
|
|
(1)
|
IFRS 16, Leases ("IFRS 16")
|
IFRS 16 replaces International Accounting Standard 17 - Leases (IAS 17) and its related interpretations. IFRS 16 instructions annul the existing requirement from lessees to classify leases as operating or finance leases. Instead of this, for lessees, the new standard presents a unified model for the accounting treatment of all leases according to which the lessee has to recognize an asset and liability in respect of the lease in its financial statements. Similarly, IFRS 16 determines new and expanded disclosure requirements from those required at present.
IFRS 16 will become effective for annual periods as of January 1, 2019, with the possibility of early adoption, so long as the Company has also early adopted IFRS 15 - Revenue from contracts with customers. IFRS 16 includes a number of alternatives for the implementation of transitional provisions, so that companies can choose one of the following alternatives at the implementation date: full retrospective implementation or implementation from the effective date while adjusting the balance of retained earnings at that date.
The Company has not yet commenced examining the effects of adopting the amendments on the financial statements.
|
|
(2)
|
IFRS 15, Revenue from Contracts with Customers ("IFRS 15")
|
IFRS 15 replaces the current guidance regarding recognition of revenues and presents a new model for recognizing revenue from contracts with customers. IFRS 15 provides two approaches for recognizing revenue: at a point in time or over time. The model includes five steps for analyzing transactions so as to determine when to recognize revenue and at what amount. Furthermore, IFRS 15 provides new and more extensive disclosure requirements than those that exist under current guidance.
IFRS 15 is applicable for annual periods beginning on or after January 1, 2018 and earlier application is permitted .
The Company has not yet commenced examining the effects of adopting IFRS 15 on the financial statements.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 2 - Basis of Preparation (cont’d)
|
E.
|
Use of estimates and judgments (cont’d)
|
|
|
(3)
|
IFRS 9 (2014), Financial Instruments ("IFRS 9 (2014)")
|
A final version of the standard was released, which includes revised guidance on the classification and measurement of financial instruments, and a new model for measuring impairment of financial assets. This guidance has been added to the chapter dealing with general hedge accounting requirements issued in 2013.
Classification and measurement
In accordance with IFRS 9 (2014), there are three principal categories for measuring financial assets: amortized cost, fair value through profit and loss and fair value through other comprehensive income. The basis of classification for debt instruments is the entity’s business model for managing financial assets and the contractual cash flow characteristics of the financial asset. Investments in equity instruments will be measured at fair value through profit and loss (unless the entity elected at initial recognition to present fair value changes in other comprehensive income).
IFRS 9 (2014) requires that changes in fair value of financial liabilities designated at fair value through profit or loss that are attributable to changes in its credit risk, should usually be recognized in other comprehensive income (loss).
Hedge accounting - general
Under IFRS 9 (2014), additional hedging strategies that are used for risk management will qualify for hedge accounting. IFRS 9 (2014) replaces the present 80%-125% test for determining hedge effectiveness, with the requirement that there be an economic relationship between the hedged item and the hedging instrument, with no quantitative threshold. In addition, IFRS 9 (2014) introduces new models that are alternatives to hedge accounting as regards credit exposures and certain contracts outside the scope of IFRS 9 (2014) and sets new principles for accounting for hedging instruments. In addition, IFRS 9 (2014) provides new disclosure requirements.
Impairment of financial assets
IFRS 9 (2014) presents a new ‘expected credit loss’ model for calculating impairment. For most assets, the new model presents a dual measurement approach for impairment: if the credit risk of a financial asset has not increased significantly since its initial recognition, an impairment provision will be recorded in the amount of the expected credit losses that result from default events that are possible within the twelve months after the reporting date .
If the credit risk has increased significantly, in most cases the impairment provision will increase and be recorded at the level of lifetime expected credit losses of the financial asset.
IFRS 9 (2014) is effective for annual periods beginning on or after January 1, 2018 with early adoption being permitted. It will be applied retrospectively with some exemptions.
The Company has not yet commenced examining the effects of adopting IFRS 9 (2014) on the financial statements.
|
F.
|
Capital management - objectives, procedures and processes
|
|
|
Management’s policy is to maintain a solid capital base in order to preserve the ability of the Company to continue operating so that it may provide a return on capital to its shareholders, benefits to other holders of interests in the Company such as credit providers and employees of the Company and sustain future development of the business. Neither the Company nor its Subsidiaries are subject to externally imposed capital requirements.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies
The accounting policies set out below have been applied consistently for all periods presented in these consolidated financial statements, and have been applied consistently by Group entities.
|
A.
|
Basis of consolidation
|
Subsidiaries are entities controlled by the Group. The financial statements of subsidiaries are included in the consolidated financial statements from the date that control commences until the date that control ceases. The accounting policies of subsidiaries have been changed when necessary to align them with the policies adopted by the Group.
|
|
(2)
|
Transactions eliminated on consolidation
|
Intra-group balances and transactions, and any unrealized income and expenses arising from intra-group transactions, are eliminated in preparing the consolidated financial statements. Unrealized losses are eliminated in the same way as unrealized gains, but only to the extent that there is no evidence of impairment.
|
B.
|
Foreign currency transactions
|
Transactions in foreign currencies are translated to the respective functional currencies of Group entities at exchange rates at the dates of the transactions.
Monetary assets and liabilities denominated in foreign currencies at the reporting date are translated to the functional currency at the exchange rate at that date. The foreign currency gain or loss on monetary items is the difference between amortized cost in the functional currency at the beginning of the year, adjusted for effective interest and payments during the year, and the amortized cost in foreign currency translated at the exchange rate at the end of the reporting period. Foreign currency differences arising on translation are recognized in profit or loss.
Non-monetary items that are measured in terms of historical cost in a foreign currency are translated using the exchange rate at the date of the transaction.
|
|
(1)
|
Non-derivative financial assets
|
Initial recognition of financial assets
The Group initially recognizes loans and receivables and deposits on the date that they are originated. All other financial assets acquired in a regular way purchase are recognized initially on the trade date at which the Group becomes a party to the contractual provisions of the instrument (i.e., on the date the Group undertook to purchase or sell the asset). Non-derivative financial instruments comprise investments in marketable securities, deposits, trade and other receivables, and cash and cash equivalents.
Derecognition of financial assets
Financial assets are derecognized when the Group’s contractual rights to the cash flows from the asset expire, or when the Group transfers the rights to receive the contractual cash flows on the financial asset in a transaction in which substantially all of the risks and rewards of ownership of the financial asset are transferred. Any interest in transferred financial assets that is created or retained by the Group is recognized as a separate asset or liability.
Regular way sales of financial assets are recognized on the trade date, which is the date that the Company undertook to sell the asset.
See (2) hereunder regarding the offset of financial assets and financial liabilities.
The Group classifies its financial assets according to the following categories:
Financial assets at fair value through profit or loss
A financial asset is classified at fair value through profit or loss if it is classified as held for trading. Attributable transaction costs are recognized in profit or loss as incurred. Financial assets at fair value through profit or loss are measured at fair value, and changes therein are recognized in profit or loss.
Financial assets held for trading comprise investments in marketable securities.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies (cont’d)
|
C.
|
Financial instruments (cont’d)
|
|
|
(1)
|
Non-derivative financial assets (cont’d)
|
Loans and receivables
Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. Such assets are recognized initially at fair value plus any direct attributable transaction costs. Subsequent to initial recognition, loans and receivables are measured at amortized cost using the effective interest method, less any impairment losses. Loans and receivables comprise trade receivables, deposits, other accounts receivable and cash and cash equivalents.
Cash and cash equivalents comprise cash balances available for immediate use and call deposits. Cash equivalents comprise short-term highly liquid investments (with original maturities of three months or less) that are readily convertible into known amounts of cash and are not exposed to significant risks of change in value.
|
|
(2)
|
Non-derivative financial liabilities
|
The Group initially recognizes debt securities issued on the date that they are originated. All other financial liabilities are recognized initially on the trade date at which the Group becomes a party to the contractual provisions of the instrument. Financial liabilities are derecognized when the obligation of the Group, as specified in the agreement, expires or when it is discharged or cancelled.
Financial liabilities are recognized initially at fair value plus any directly attributable transaction costs. Subsequent to initial recognition these financial liabilities are measured at amortized cost using the effective interest method. Non-derivative financial liabilities comprise convertible debentures, liability to the OCS, trade and other accounts payable.
Financial assets and liabilities are offset and the net amount presented in the statement of financial position when, and only when, the Group currently has a legal right to offset the amounts and intends either to settle on a net basis or to realize the asset and settle the liability simultaneously.
|
|
(3)
|
CPI-linked assets and liabilities that are not measured at fair value
|
The value of CPI-linked financial assets and liabilities, which are not measured at fair value, is revalued every period in accordance with the actual increase/decrease in the CPI.
Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of ordinary shares and share options are recognized as a deduction from equity.
|
|
Receipts in respect of share options are classified as equity to the extent that they confer the right to purchase a fixed number of shares for a fixed exercise price.
|
|
|
(6)
|
Issuance of compound financial instruments
|
|
|
(a)
|
The consideration received from the issuance of compound financial instruments, which consist of equity components and liability-classified options, is attributed at first to financial liabilities that are measured each period at fair value through profit or loss, and then to financial liabilities that are measured only upon initial recognition at fair value. The remaining amount is the value of the equity component.
|
|
|
(b)
|
Direct issuance costs are attributed to the specific securities in respect of which they were incurred, whereas joint issuance costs are attributed to the securities on a proportionate basis according to the allocation of the consideration from the issuance of the compound financial instruments, as described in sub-paragraph (a) above.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies (cont’d)
|
D.
|
Property and Equipment
|
|
|
(1)
|
Recognition and measurement
|
|
|
Property and equipment are measured at cost less accumulated depreciation and accumulated impairment losses.
|
|
|
Cost includes expenditure that is directly attributable to the acquisition of the asset. The cost of self-constructed assets includes the cost of materials and direct labor, any other costs directly attributable to bringing the assets to a working condition for their intended use.
|
Purchased software that is integral to the functionality of the related equipment is capitalized as part of that equipment.
|
|
Gains and losses on disposal of property and equipment are determined by comparing the net proceeds from disposal with the carrying amount of the asset, and are recognized net within the relevant line item in profit or loss.
|
|
|
The cost of replacing part of a property and equipment asset item is recognized in the carrying amount of the item if it is probable that the future economic benefits embodied within the part will flow to the Group and its cost can be measured reliably. The carrying amount of the replaced part is derecognized. The costs of day-to-day servicing are recognized in profit or loss as incurred.
|
|
|
Depreciation is a systematic allocation of the depreciable amount of an asset over its useful life. The depreciable amount is the cost of the asset, or other amount substituted for cost.
|
|
|
Depreciation is recognized in profit or loss on a straight-line basis over the estimated useful lives of the property and equipment, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset.
|
|
|
The estimated useful lives for the current and comparative periods are as follows:
|
|
Computers
|
3 years
|
|
Motor vehicles, machinery and equipment
|
4-7 years
|
|
Office furniture and equipment
|
10-17 years
|
|
Leasehold improvements
|
4-6 years
|
Depreciation methods and useful lives are reviewed at each financial year-end and adjusted if appropriate.
|
|
(1)
|
Research and development
|
|
|
Expenditure on research activities, undertaken with the prospect of gaining new scientific or technical knowledge and understanding, is recognized in profit or loss when incurred.
|
|
|
Development activities involve plans or design for the production of new or substantially improved products and processes.
|
Development expenditure is capitalized only if:
|
|
·
|
development costs can be measured reliably;
|
|
|
·
|
the product or process is technically and commercially feasible;
|
|
|
·
|
future economic benefits are probable; and
|
|
|
·
|
the Group intends to and has sufficient resources to complete development and to use or sell the asset.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies (cont’d)
|
E.
|
Intangible assets (cont’d)
|
|
|
(1)
|
Research and development (cont’d)
|
As regard to some of the Company’s products, technological feasibility may occur only when the Company’s clinical trials succeed and following receipt of approval from the U.S. Food and Drug Administration (the FDA). Sometimes the costs incurred between the successful completion of the product’s development and successful clinical trials, and the time the product is ready for sale are immaterial, so that in reality all of the development costs will be recognized in profit or loss as incurred.
|
|
The capitalized expenditure includes the cost of materials, direct labor and overhead costs that are directly attributable to developing the asset for its intended use. Other development expenditure is recognized in profit or loss as incurred.
|
|
|
Capitalized development expenditure is measured at cost less accumulated amortization and accumulated impairment losses.
|
|
|
(2)
|
Subsequent expenditure
|
|
|
Subsequent expenditure is capitalized only when it increases the future economic benefits embodied in the specific asset to which it relates. All other expenditure, including expenditure on internally generated brands, is recognized in profit or loss as incurred.
|
|
|
Amortization is a systematic allocation of the amortizable amount of an intangible asset over its useful life. The amortizable amount is the cost of the asset.
|
|
|
Amortization is recognized in profit or loss on a straight-line basis over the estimated useful lives of the intangible assets, from the date they are available for use, since these methods most closely reflect the expected pattern of consumption of the future economic benefits embodied in each asset.
|
The estimated useful lives for comparative periods of the capitalized development costs is 4 years.
|
|
Amortization methods and useful lives are reviewed at each reporting date and adjusted if appropriate.
|
Inventory is measured at the lower of cost and net realizable value. The cost of inventory is based on the moving average method, and includes expenditure incurred in acquiring the inventory and the costs incurred in bringing it to its existing location and condition. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and selling expenses. Management regularly evaluates the necessity of provisions for obsolescence, which may result from excess, slow-moving or obsolete inventories.
|
|
(1)
|
Non-derivative financial assets
|
|
|
A financial asset not carried at fair value through profit or loss is tested for impairment when objective evidence indicates that one or more events had a negative effect on the estimated future cash flows of the asset.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies (cont’d)
|
|
(1)
|
Non-derivative financial assets (cont'd)
|
|
|
An impairment loss in respect of a financial asset measured at amortized cost is calculated as the difference between its carrying amount and the present value of the estimated future cash flows discounted at the asset’s original effective interest rate. All individually significant financial assets are assessed for specific impairment, and all impairment losses are recognized in profit or loss and reflected in a provision for loss against the balance of the financial asset measured at amortized cost.
|
|
|
An impairment loss is reversed if the reversal can be related objectively to an event occurring after the recognition of the impairment loss. For financial assets measured at amortized cost the reversal is recognized in profit or loss.
|
|
|
The carrying amounts of the Group’s non-financial assets, other than inventories and deferred tax assets, are reviewed at each reporting date to determine whether there is any indication of impairment. If any such indication exists, then the asset’s recoverable amount is estimated.
|
|
|
The recoverable amount of an asset or cash-generating unit is the greater of its value in use and its net selling price (fair value less costs to sell). In assessing value in use, the estimated future cash flows are discounted to their present value using a discount rate that reflects current market assessments of the time value of money and the risks specific to the asset. For the purpose of impairment testing, assets are grouped together into the smallest group of assets that generates cash inflows from continuing use that are largely independent of the cash inflows of other assets or groups of assets (the “cash-generating unit”).
|
|
|
An impairment loss is recognized if the carrying amount of an asset or its cash-generating unit exceeds its estimated recoverable amount. Impairment losses are recognized in profit or loss. Impairment losses recognized in respect of cash-generating units are allocated to reduce the carrying amounts of the assets in the cash-generating unit on a pro rata basis.
|
|
|
Impairment losses recognized in prior periods are assessed at each reporting date for any indications that the loss has decreased or no longer exists. An impairment loss is reversed if there has been a change in the estimates used to determine the recoverable amount. An impairment loss is reversed only to the extent that the asset’s carrying amount does not exceed the carrying amount that would have been determined, net of depreciation or amortization, if no impairment loss had been recognized.
|
|
|
(1)
|
Post-employment benefits
|
Most of the Group’s Israeli employees are subject to Section 14 of the Israeli Severance Pay Law - 1963 and therefore substantially all of the post-employment plans of the Group are classified as defined contribution plans.
Defined contribution plans
Obligations for contributions to defined contribution pension plans are recognized as an expense in profit or loss in the periods during which services are rendered by employees.
Short-term employee benefit obligations are measured on an undiscounted basis and are expensed as the related service is provided.
A liability is recognized for the amount expected to be paid under short-term cash bonus or profit-sharing plans if the Group has a present legal or constructive obligation to pay this amount as a result of past service provided by the employee and the obligation can be estimated reliably.
The employee benefits are classified as short-term benefits or as other long-term benefits depending on when the Company expects the benefits to be wholly settled.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies (cont’d)
|
H.
|
Employee benefits (cont'd)
|
|
|
(3)
|
Share-based payment transactions
|
The fair value of share-based payment, measured on the grant date, granted to employees is recognized as a salary expense, with a corresponding increase in equity, over the period that the employees become unconditionally entitled to the awards. The amount recognized as an expense in respect of share-based payment awards that are conditional upon meeting service and non-market performance conditions, is adjusted to reflect the number of awards that are expected to vest.
|
|
A provision is recognized if, as a result of a past event, the Group has a present legal or constructive obligation that can be estimated reliably, and it is probable that an outflow of economic benefits will be required to settle the obligation.
|
General
|
|
The Group recognizes revenue in accordance with IAS 18, Revenue Recognition, including provisions related to recognition of revenue from multiple-component transactions. Accordingly, the Group recognizes revenue from the sale of goods when:
|
|
|
·
|
The significant risks and rewards of ownership of the goods have been transferred to the customer;
|
|
|
·
|
It is probable that the economic benefits associated with the transaction will flow to the Group;
|
|
|
·
|
The costs incurred or to be incurred in respect of the transaction can be measured reliably;
|
|
|
·
|
The Group retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold; and
|
|
|
·
|
The amount of revenue can be measured reliably.
|
The revenue from sales in the ordinary course of business is measured according to the fair value of the consideration received or receivable, which is based on the selling price of each component, net of discounts.
|
|
In general, the Group's sales agreements include several components:
|
|
|
·
|
disposable components and accessories; and
|
|
|
·
|
warranty and maintenance services related to the systems sold, which includes replacement parts, software updates, preventive maintenance and on-call support as detailed in the agreement.
|
These components are split into separate accounting units if and only if each component has separate value for the customer and there is reliable evidence of the fair value of the components not yet supplied. Components not split into a separate accounting unit due to non-compliance with the above conditions, are grouped together as a single accounting unit. The revenue from each such accounting unit is recognized upon fulfillment of the conditions for recognition of revenue from the components included therein, according to their type. The allocation of consideration from a revenue arrangement to its separate units of account is based on the relative fair values of each unit. If the fair value of the delivered item is not reliably measurable, then revenue is allocated based on the difference between the total arrangement consideration and the fair value of the undelivered item. Usually, fair value of the warranty and maintenance services component is determined based on the renewal quote offered in the sales agreement.
The timing of revenue recognition from the various components is as follows:
Sales of systems - The revenue from sales of systems is recognized at the time of transfer of the significant risks and rewards of ownership as follows:
|
|
·
|
Sales to end customers - Upon the completion of installation of the system, training of at least one surgeon, which typically occurs prior to or concurrent with the system installation, and customer acceptance, if required.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies (cont’d)
|
J.
|
Revenue Recognition (cont’d)
|
|
|
·
|
Sales to distributors - Upon delivery to the distributor, provided that the significant risks and rewards of ownership of the system are transferred to the distributor upon delivery, the distributor has no right of return, receipt of the consideration is probable and not dependent on the distributor’s ability to collect from the end customer, the commitment to carry out installation and training for the end customer lies with the distributor and that the distributor has been authorized to perform the installation and training for the end customers. If the above conditions are not met, the Group recognizes revenue at the time of fulfillment of the conditions for recognition of revenue from the end customer.
|
Disposable components sales - Revenue from the disposable components sales is recognized at the time of the transfer of the significant risks and rewards of ownership as follows:
| |
·
|
In sales to end customers - Upon delivery.
|
| |
·
|
In sales to distributors - Upon delivery to the distributor, provided that the significant risks and rewards of ownership of the components are transferred to the distributor upon delivery, the distributor has no right of return and that the receipt of the consideration is probable and not dependent on the distributor’s ability to collect from the end customer.
|
Warranty and maintenance services (“services”) - Revenue from services is recognized proportionately over the period of rendering of the service and subject to the other conditions for revenue recognition specified above.
Grants from the OCS in respect of research and development projects are accounted for as forgivable loans according to IAS 20, Accounting for Government Grants and Disclosure of Government Assistance. Grants received from the OCS are recognized as a liability according to their fair value on the date of their receipt, unless on that date it is reasonably certain that the amount received will not be refunded. The amount of the liability is reexamined each period, and any changes in the present value of the cash flows discounted at the original interest rate of the grant are recognized in profit or loss. The difference between the actual grants received and the fair value of the liability on the date of receiving the grant is recognized as a deduction of development expenses. As of December 31, 2015 and 2014, the Company has no outstanding liability to the OCS.
|
L.
|
Financing income and expenses
|
Financing income comprises interest income on funds invested, changes in the fair value of financial assets at fair value through profit or loss, changes in fair value of derivative instruments and foreign currency gains. Interest income is recognized as it accrues using the effective interest method.
Financing expenses comprise changes in fair value of derivative instruments, interest expense on debentures, liability to the OCS as well as changes in the fair value of financial assets at fair value through profit or loss and foreign currency losses. Interest costs are recognized in profit or loss using the effective interest method.
Foreign currency gains and losses are reported on a net basis.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 3 - Significant Accounting Policies (cont’d)
Taxes on income are comprised of current and deferred tax. Current tax is the expected tax payable (or receivable) on the taxable income for the year, using tax rates enacted or substantively enacted at the reporting date.
Deferred tax is recognized in respect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes.
Deferred tax is measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the laws that have been enacted or substantively enacted by the reporting date.
A deferred tax asset is recognized for unused tax losses, tax benefits and deductible temporary differences, to the extent that it is probable that future taxable profits will be available against which that can be utilized. Deferred tax assets are reviewed at each reporting date and are reduced to the extent that it is no longer probable that the related tax benefit will be realized.
A provision for uncertain tax positions, including additional tax and interest expenses, is recognized when it is more probable than not that the Group will have to use its economic resources to pay the obligation.
Tax benefit arising from tax deduction on exercise of share options is recognized in statement of profit or loss to the extent of the cumulative remuneration expense recognized. Any excess benefit is recognized directly in equity.
Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset current tax liabilities and assets, and they relate to income taxes levied by the same tax authority on the same taxable entity.
The Group presents basic and diluted loss per share data for its ordinary shares. Basic loss per share is calculated by dividing the loss attributable to ordinary shareholders of the Group by the weighted average number of ordinary shares outstanding during the year. Diluted loss per share is determined by adjusting the loss attributable to ordinary shareholders and the weighted average number of ordinary shares outstanding, for the effects of all dilutive potential ordinary shares, which comprise convertible debentures, share options and share options granted to employees.
The Group has no comprehensive income components other than net income or loss.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 4 - Cash and Cash Equivalents
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Current balances in banks
|
|
|
10,972 |
|
|
|
19,073 |
|
|
Deposits held at financial institutions, with original
|
|
|
|
|
|
|
|
|
|
maturity periods of up to three months
|
|
|
2,547 |
|
|
|
3,182 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
13,519 |
|
|
|
22,255 |
|
The deposits outstanding, as of December 31, 2015, bear annual interest of 0.50%-0.88%.
The Group’s exposure to credit, interest rate and currency risks and a sensitivity analysis for financial assets are disclosed in Note 26.
Note 5 - Investments
Breakdown according to type of investment
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Short-term investments
|
|
|
|
|
|
|
|
Deposits held at financial institutions, in USD
|
|
|
21,674 |
|
|
|
24,494 |
|
| |
|
|
|
|
|
|
|
|
|
Investments in marketable securities
|
|
|
|
|
|
|
|
|
|
Mutual funds
|
|
|
13 |
|
|
|
13 |
|
| |
|
|
21,687 |
|
|
|
24,507 |
|
|
Long-term investments (*)
|
|
|
|
|
|
|
|
|
|
Deposits held at financial institutions, in USD
|
|
|
5,023 |
|
|
|
5,473 |
|
The deposits outstanding, as of December 31, 2015, bear annual interest of 0.33%-1.28%.
The Group’s exposure to credit, interest rate and currency risks, and a sensitivity analysis for financial assets are disclosed in Note 26.
(*) Including restricted cash in the amount of USD 60 thousand for bank guarantees.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 6 - Other Current Assets
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Institutions
|
|
|
656 |
|
|
|
317 |
|
|
Prepaid expenses
|
|
|
476 |
|
|
|
656 |
|
|
Advances to suppliers
|
|
|
102 |
|
|
|
48 |
|
|
Other receivables
|
|
|
186 |
|
|
|
89 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
1,420 |
|
|
|
1,110 |
|
The Group’s exposure to credit and currency risk is disclosed in Note 26.
Note 7 - Inventory
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Raw materials and spare parts
|
|
|
970 |
|
|
|
1,154 |
|
|
Work in progress
|
|
|
277 |
|
|
|
99 |
|
|
Finished goods
|
|
|
1,530 |
|
|
|
1,797 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
2,777 |
|
|
|
3,050 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 8 - Property and Equipment, net
| |
|
|
|
|
|
|
|
Office
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Machinery
|
|
|
furniture
|
|
|
|
|
|
|
|
|
|
|
| |
|
Motor
|
|
|
and
|
|
|
and
|
|
|
Leasehold
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Cost
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of January 1, 2015
|
|
|
33 |
|
|
|
1,366 |
|
|
|
178 |
|
|
|
261 |
|
|
|
1,146 |
|
|
|
2,984 |
|
|
Additions
|
|
|
- |
|
|
|
514 |
|
|
|
15 |
|
|
|
40 |
|
|
|
133 |
|
|
|
702 |
|
|
Disposals
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(43 |
) |
|
|
(43 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2015
|
|
|
33 |
|
|
|
1,880 |
|
|
|
193 |
|
|
|
301 |
|
|
|
1,236 |
|
|
|
3,643 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of January 1, 2014
|
|
|
33 |
|
|
|
743 |
|
|
|
142 |
|
|
|
206 |
|
|
|
993 |
|
|
|
2,117 |
|
|
Additions
|
|
|
- |
|
|
|
623 |
|
|
|
36 |
|
|
|
55 |
|
|
|
199 |
|
|
|
913 |
|
|
Disposals
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(46 |
) |
|
|
(46 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2014
|
|
|
33 |
|
|
|
1,366 |
|
|
|
178 |
|
|
|
261 |
|
|
|
1,146 |
|
|
|
2,984 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of January 1, 2015
|
|
|
21 |
|
|
|
673 |
|
|
|
52 |
|
|
|
149 |
|
|
|
832 |
|
|
|
1,727 |
|
|
Depreciation for the year
|
|
|
5 |
|
|
|
281 |
|
|
|
12 |
|
|
|
39 |
|
|
|
190 |
|
|
|
527 |
|
|
Disposals
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(43 |
) |
|
|
(43 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2015
|
|
|
26 |
|
|
|
954 |
|
|
|
64 |
|
|
|
188 |
|
|
|
979 |
|
|
|
2,211 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of January 1, 2014
|
|
|
16 |
|
|
|
485 |
|
|
|
41 |
|
|
|
119 |
|
|
|
664 |
|
|
|
1,325 |
|
|
Depreciation for the year
|
|
|
5 |
|
|
|
188 |
|
|
|
11 |
|
|
|
30 |
|
|
|
213 |
|
|
|
447 |
|
|
Disposals
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(45 |
) |
|
|
(45 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2014
|
|
|
21 |
|
|
|
673 |
|
|
|
52 |
|
|
|
149 |
|
|
|
832 |
|
|
|
1,727 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of January 1, 2014
|
|
|
17 |
|
|
|
258 |
|
|
|
101 |
|
|
|
87 |
|
|
|
329 |
|
|
|
792 |
|
|
Balance as of December 31, 2014
|
|
|
12 |
|
|
|
693 |
|
|
|
125 |
|
|
|
112 |
|
|
|
315 |
|
|
|
1,257 |
|
|
Balance as of December 31, 2015
|
|
|
7 |
|
|
|
926 |
|
|
|
129 |
|
|
|
113 |
|
|
|
257 |
|
|
|
1,432 |
|
Note 9 - Intangible Assets
Intangible assets include capitalized development costs relating to one of the Company’s products in accordance with the requirements of IAS 38, Intangible Assets, as described in Note 3E.
During the years 2015, 2014 and 2013 the Company did not capitalize development costs.
Presented hereunder is the movement in the balance of intangible assets during the years 2014 and 2015:
| |
|
Capitalized development
|
|
| |
|
|
|
| |
|
|
|
|
Cost
|
|
|
|
|
Balance as of January 1, 2014, December 31, 2014 and 2015
|
|
|
1,247 |
|
| |
|
|
|
|
|
Amortization
|
|
|
|
|
|
Balance as of January 1, 2014
|
|
|
1,154 |
|
|
Amortization for the year ended December 31, 2014
|
|
|
93 |
|
| |
|
|
|
|
|
Balance as of December 31, 2014 and 2015
|
|
|
1,247 |
|
| |
|
|
|
|
|
Carrying amount
|
|
|
|
|
|
December 31, 2014 and 2015
|
|
|
- |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 10 - Other Current Liabilities
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Salary and related liabilities
|
|
|
3,161 |
|
|
|
1,994 |
|
|
Deferred income
|
|
|
1,221 |
|
|
|
1,157 |
|
|
Accrued expenses
|
|
|
1,283 |
|
|
|
625 |
|
|
Institutions
|
|
|
155 |
|
|
|
41 |
|
|
Tax provision
|
|
|
111 |
|
|
|
101 |
|
|
Hedging transactions
|
|
|
4 |
|
|
|
120 |
|
|
Related parties*
|
|
|
35 |
|
|
|
60 |
|
|
Other
|
|
|
82 |
|
|
|
354 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
6,052 |
|
|
|
4,452 |
|
|
*
|
See Note 21 - Related Parties, for additional information regarding transactions and balances with related parties.
|
The Group’s exposure to currency and liquidity risks related to certain payables is disclosed in Note 26.
Note 11 - Employee Benefits
Employee benefits mostly include post-employment benefits for Israeli employees who are in the scope of Section 14 of the Israeli Severance Pay Law - 1963, that are accounted for as defined contribution plans. The Group also has immaterial defined benefit plans for which it deposits amounts in appropriate insurance policies.
Regarding short-term benefits see Note 10 - Other Current Liabilities.
Regarding share-based payments see Note 23 - Share-Based Payments.
Post-employment benefit plans - defined contribution plan
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Amount recognized as expense in respect of
|
|
|
|
|
|
|
|
|
|
|
defined contribution plan
|
|
|
243 |
|
|
|
229 |
|
|
|
173 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 12 - Taxes on Income
|
A.
|
Details regarding the tax environment of the Group
|
|
|
(a)
|
Presented hereunder are the Israeli tax rates in the years 2013-2015:
|
|
|
Capital gains derived by an Israeli company are subject to tax rate of 26.5%.
|
|
|
(b)
|
On January 5, 2016, the Knesset published the Law for the Amendment of the Israeli Tax Ordinance (Amendment 216), that reduces the corporate tax rate and the capital gain tax rate from 26.5% to 25%.
|
|
|
(2)
|
Benefits under the Israeli Law for the Encouragement of Capital Investments - 1959 (hereinafter - “the Law”)
|
|
|
(a)
|
In April 2004, the Company was granted “Approved Enterprise” status in accordance with the Law with respect to a plan to construct a plant in Caesarea, Israel for the manufacture of systems for assisting and guiding complex surgical procedures. In February 2007, the aforementioned approved enterprise status was revoked at the request of the Company, and in respect of an expansion of its plant in the Caesarea industrial park it was granted “Beneficiary Enterprise” status per the definition of this term in the Law. In accordance with this status, the Company will be entitled to the tax benefits provided by the Law with respect to income of the beneficiary enterprise from productive activity. Income of the beneficiary enterprise from productive activity will be exempt from tax for two years from the year in which the Company first has taxable income, and will be subject to tax of 10%-25% in the following 5 years, provided that 12 years have not passed from the beginning of the year of election. In the event of a dividend distribution from income that is exempt from company tax, as aforementioned, the Company will be required to pay tax of 25% on that income. In 2013, the Company notified the tax authorities that 2012 tax year is the year of election.
|
|
|
In addition, in the event of a change in the field of activity and/or business model and/or a significant reduction in production levels or in product variety, the tax ruling will become void. The Company will be controlled and managed in Israel throughout the benefit period.
|
|
|
(b)
|
On December 29, 2010, the Knesset approved the Economic Policy Law for 2011-2012, which includes an amendment to the Law for the Encouragement of Capital Investments - 1959 (hereinafter - “the Amendment to the Law”), effective from January 1, 2011. Companies can choose to not be included in the scope of the Amendment to the Law and to stay in the scope of the Law before its amendment until the end of the benefits period. The 2012 tax year is the last year companies can choose as the year of election, provided that the minimum qualifying investment began in 2010. As mentioned above, the Company chose to stay in the scope of the previous Law and elected 2012 tax year as the year of election.
|
|
|
The Amendment to the Law provides that the existing tax benefit tracks were eliminated and two new tax tracks were introduced in their place, a preferred enterprise and a special preferred enterprise, which mainly provide a uniform and reduced tax rate for all of the company’s income entitled to benefits, such as: in the tax years 2011-2012 - a tax rate of 15%, in the tax years 2013-2014 - a tax rate of 12.5%, and as from the tax year 2015 - a tax rate of 12%.
|
|
|
On August 5, 2013, the Knesset passed the Law for Changes in National Priorities (Legislative Amendments for Achieving Budget Objectives in the Years 2013 and 2014) - 2013, which cancelled the planned tax reduction so that as from the 2014 tax year the tax rate on preferred income will be 16%.
|
|
|
The Company meets the conditions provided in the Amendment to the Law for inclusion in the scope of the tax benefits track, but currently chose to stay in the scope of the Law before its amendment.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 12 - Taxes on Income (cont'd)
|
A.
|
Details regarding the tax environment of the Group (cont'd)
|
|
|
(3)
|
Tax benefits under the Law for the Encouragement of Industry (Taxes), 1969
|
|
|
The Company is an ‘industrial company’ as defined by this law and as such is entitled to certain tax benefits, consisting mainly of accelerated depreciation as prescribed by regulations published under the Inflationary Adjustments Law, recognition of share issuance costs as an expense over three years and amortization of patents and certain other intangible property.
|
|
|
(4)
|
Measurement of taxable income under the Income Tax (Inflationary Adjustments) Law, 1985
|
|
|
Through 2014, the Company has maintained its books and records for Israeli tax purposes in NIS. Calculation of the Company’s results in NIS differs from the results reported in the financial statements, which are based on the functional currency of the Company which is the U.S. Dollar.
|
|
|
The Company has elected, commencing January 1, 2015, to maintain its books and records in U.S. dollars for tax purposes, as permitted under the tax regulations, since the Company is considered a “foreign invested company”. The Company must continue to be taxed on this basis for at least three years.
|
|
|
(5)
|
Taxation of the subsidiary in the U.S.
|
|
|
The tax rates applicable to the subsidiary incorporated in the U.S. are federal tax rate of 34% plus state tax of 0.95% to 9.99%, depending on the state. Furthermore, certain states in which the subsidiary operates have a minimum tax.
|
|
|
Israel and the U.S. have a double tax avoidance treaty. According to the treaty, dividends and interest are subject to withholding tax of 12.5% and 17.5%, respectively.
|
|
B.
|
Composition of income tax expense
|
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Current tax expense
|
|
|
|
|
|
|
|
|
|
|
Current tax
|
|
|
97 |
|
|
|
194 |
|
|
|
66 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax expense (income)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in deferred tax assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(liabilities) in subsidiary
|
|
|
116 |
|
|
|
(49 |
) |
|
|
101 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total tax expense
|
|
|
213 |
|
|
|
145 |
|
|
|
167 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 12 - Taxes on Income (cont'd)
|
C.
|
Reconciliation between the theoretical tax on the pre-tax profit and the tax expense
|
| |
|
For the year ended December 31
|
|
| |
|
2015
|
|
|
2014
|
|
|
2013
|
|
| |
|
USD thousands
|
|
|
USD thousands
|
|
|
USD thousands
|
|
| |
|
|
|
|
|
|
|
|
|
|
Loss before taxes on income
|
|
|
(15,172 |
) |
|
|
(15,127 |
) |
|
|
(20,362 |
) |
|
Primary tax rate of the Company
|
|
|
26.5 |
% |
|
|
26.5 |
% |
|
|
25 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax calculated according to the Company’s
|
|
|
|
|
|
|
|
|
|
|
|
|
|
primary tax rate
|
|
|
(4,021 |
) |
|
|
(4,009 |
) |
|
|
(5,090 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional tax in respect of:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Different tax rate of foreign subsidiaries
|
|
|
54 |
|
|
|
20 |
|
|
|
19 |
|
|
Non-deductible expenses
|
|
|
875 |
|
|
|
615 |
|
|
|
3,825 |
|
|
Difference between the measurement basis of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
the Company’s results for tax purposes and the
|
|
|
|
|
|
|
|
|
|
|
|
|
|
measurement basis of the Company’s results in
|
|
|
|
|
|
|
|
|
|
|
|
|
|
the financial statements (see A(4) above)
|
|
|
- |
|
|
|
1,905 |
|
|
|
- |
|
|
Tax losses and benefits for which deferred
|
|
|
|
|
|
|
|
|
|
|
|
|
|
tax assets were not created
|
|
|
3,290 |
|
|
|
1,578 |
|
|
|
1,404 |
|
|
Other differences
|
|
|
15 |
|
|
|
36 |
|
|
|
9 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
213 |
|
|
|
145 |
|
|
|
167 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 12 - Taxes on Income (cont'd)
|
D.
|
Deferred tax assets and liabilities
|
|
|
(1)
|
The Company has recognized deferred tax assets and liabilities in respect of the following items:
|
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Property and equipment
|
|
|
(101 |
) |
|
|
(27 |
) |
|
Tax losses and other temporary differences
|
|
|
138 |
|
|
|
185 |
|
|
Deferred tax assets
|
|
|
37 |
|
|
|
158 |
|
Deferred taxes in respect of the losses of the U.S. subsidiary were recognized, following the profitability of the U.S. subsidiary in recent years and convincing evidence that the U.S. subsidiary will experience sufficient taxable income in the near future and following the evaluation of the losses that more likely than not will be allowable under applicable tax laws.
|
|
(2)
|
Unrecognized deferred tax assets
|
Deferred tax assets have not been recognized in respect of the following items:
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Deductible temporary differences, net
|
|
|
6,112 |
|
|
|
5,315 |
|
|
Tax losses
|
|
|
74,519 |
|
|
|
65,461 |
|
The deductible temporary differences and tax losses incurred by the Israeli company do not expire under current tax legislation in Israel.
In general, the losses of the subsidiary in the USA can be used for up to a period of 20 years according to the tax laws of its state of incorporation. The utilization of the subsidiary’s tax losses has been limited to USD 207 thousand per year, by an “ownership change” under Section 382 of the Internal Revenue Code (the “Code”), which occurred during July 2009. An “ownership change” generally is a 50% increase in ownership over a three-year period by stockholders who directly or indirectly own at least 5% of the Company’s stock. The limitation applies to all tax losses existing at the time of the ownership change.
The amount of benefits the Company may receive from the operating loss carry forwards for income tax purposes is further dependent, in part, upon the tax laws in effect, the future earnings of the Company, and other future events, such as additional changes in ownership, the effects of which cannot be determined.
The Group did not recognize deferred tax assets in respect of these items since it is not probable that future taxable income will be available against which the Group can use the benefits therefrom, other than a deferred tax asset in respect of losses of the U.S. subsidiary that will probably be utilized.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 12 - Taxes on Income (cont'd)
|
|
The Company has carry-forward operating tax losses and carry-forward capital tax losses of USD 64,640 thousand and USD 7,375 thousand, respectively, as of December 31, 2015.
|
|
|
The U.S. subsidiary has carry-forward operating tax losses of USD 2,808 thousand as of December 31, 2015.
|
Tax years up to and including the year ended 2011 are considered final for both the Company and the U.S. subsidiary.
Note 13 - Commitments
|
A.
|
The Company and the U.S. subsidiary have operating lease agreements with respect to the buildings they use. The agreements will end in December 2017 and in April 2017, respectively. The Company provided a promissory note in the amount of USD 15 thousand as security for the lease.
|
The rent payments for buildings in Israel are linked to the CPI and for those in the U.S. are stated at the U.S. dollar. The minimum annual lease payments under the agreements, including the extension period, are as follows:
The lease payments amounted to USD 341 thousand in 2015 and USD 327 thousand in 2014.
|
B.
|
The Company leases motor vehicles under operating lease agreements for a period of 32 to 36 months. With regards to these agreements, the Company has deposited amounts as security for the future rent payments. As of the reporting date the balance of prepaid expenses on account of the lease of motor vehicles is USD 73 thousand. The deposits are linked to the CPI and do not bear interest. The minimum annual payments according to the agreements are as follows:
|
| |
|
|
|
| |
|
|
|
|
2016
|
|
|
223 |
|
|
2017
|
|
|
122 |
|
|
2018
|
|
|
33 |
|
| |
|
|
378 |
|
The lease motor vehicles payments amounted to USD 280 thousand in 2015 and USD 263 thousand in 2014.
|
C.
|
In February 2007, the Company signed a software development agreement with a third party (hereinafter: “the Developer”). According to the agreement, the Developer will provide to the Company software research and development services that are essential to the development of one of the Company’s products. In consideration of the development services, the Company will also pay royalties at the rate of 6% of the sales of the future product, which will be gradually reduced to 1% in the tenth year of selling the product. These payments will commence only after the royalty commitment will exceed the agreed amount of USD 168 thousand.
|
Currently, no royalties are being paid to the Developer, because the product is not commercially sold.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 13 - Commitments (cont'd)
|
D.
|
In January 2012, the Company entered into a distribution agreement with Mazor Robotics GmbH (hereinafter: “Mazor Germany”). According to the agreement, the Company will grant to Mazor Germany exclusive distribution rights in Germany, Austria and Switzerland (the “Territory”) with respect to various products of the Company, and limited service also in other European countries according to the needs of the Company, and will also pay a monthly fee to support penetration cost to the Territory. The monthly fee will be agreed by both parties in advanced each calendar year. The monthly fee will be paid 3 months in advance each calendar month. The Company granted to Mazor Germany the right to use the name “Mazor”, and this right will expire on the last date of a binding agreement. The intellectual property will at all times continue to be the property of the Company. The agreement will continue until terminated by either party with 180 days written notice. During the 180 day advance notice the Company will continue to pay the monthly fee as agreed. An amount of 56 thousand Euro was agreed as the monthly fee for the year 2015.
|
|
E.
|
As of December 31, 2015, the Company has purchase obligations in the amount of USD 2,468 thousand (in 2014: USD 629 thousand) which mainly represent outstanding purchase commitments for inventory components and R&D materials ordered in the normal course of business.
|
|
|
On August 14, 2013, the Company received a letter from Neutar L.L.C. (“Neutar”), advising that Neutar believes that the Company's Spine Assist miniature robot infringes patents allegedly owned by Neutar. In January 2015, the parties entered into a written settlement agreement by which all claims asserted by Neutar were fully and finally settled and the Company obtained a non-assignable, non-exclusive license to use several patents for indefinite time.
|
Note 14 - Revenues
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Sales of systems
|
|
|
13,373 |
|
|
|
12,040 |
|
|
|
13,527 |
|
|
Sales of disposables
|
|
|
7,648 |
|
|
|
4,916 |
|
|
|
3,505 |
|
|
Services and other
|
|
|
5,075 |
|
|
|
4,252 |
|
|
|
2,951 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
26,096 |
|
|
|
21,208 |
|
|
|
19,983 |
|
Note 15 - Segment Reporting
|
A.
|
Information about reportable segments
|
The Group has one reportable segment.
|
B.
|
Entity level disclosures
|
In the years ended December 31, 2015, 2014 and 2013, there were no major customers.
|
|
(2)
|
Information on products and services
|
The Group’s revenues from external parties in respect of each category of similar products and services are presented in Note 14.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 15 - Segment Reporting (cont'd)
|
B.
|
Entity level disclosures (cont'd)
|
|
|
(3)
|
Information on geographical areas
|
| |
|
For the year ended December 31, 2015
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Total revenues
|
|
|
20,271 |
|
|
|
5,825 |
|
|
|
26,096 |
|
| |
|
For the year ended December 31, 2014
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Total revenues
|
|
|
15,486 |
|
|
|
5,722 |
|
|
|
21,208 |
|
| |
|
For the year ended December 31, 2013
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Total revenues
|
|
|
15,021 |
|
|
|
4,962 |
|
|
|
19,983 |
|
Virtually all of the Company’s long-lived assets are located in Israel.
Note 16 - Cost of Sales
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Materials and subcontractors
|
|
|
3,669 |
|
|
|
2,616 |
|
|
|
2,664 |
|
|
Salaries, wages and related expenses
|
|
|
1,264 |
|
|
|
1,061 |
|
|
|
838 |
|
|
Depreciation and amortization
|
|
|
193 |
|
|
|
189 |
|
|
|
316 |
|
|
Other manufacturing expenses
|
|
|
701 |
|
|
|
530 |
|
|
|
462 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of sales
|
|
|
5,827 |
|
|
|
4,396 |
|
|
|
4,280 |
|
Note 17 - Research and Development Expenses
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Materials and subcontractors
|
|
|
2,690 |
|
|
|
2,358 |
|
|
|
1,549 |
|
|
Salaries, wages and related expenses
|
|
|
2,875 |
|
|
|
2,630 |
|
|
|
2,052 |
|
|
Depreciation
|
|
|
45 |
|
|
|
94 |
|
|
|
97 |
|
|
Patent registration expenses
|
|
|
106 |
|
|
|
139 |
|
|
|
110 |
|
|
Other research and development expenses
|
|
|
608 |
|
|
|
555 |
|
|
|
366 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total research and development expenses
|
|
|
6,324 |
|
|
|
5,776 |
|
|
|
4,174 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 18 - Selling and Marketing Expenses
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Salaries, wages and related expenses
|
|
|
15,996 |
|
|
|
12,952 |
|
|
|
9,176 |
|
|
Consultation
|
|
|
2,093 |
|
|
|
1,659 |
|
|
|
1,239 |
|
|
Advertising, demonstrations and exhibitions
|
|
|
2,546 |
|
|
|
2,579 |
|
|
|
1,790 |
|
|
Travel expenses
|
|
|
2,627 |
|
|
|
2,412 |
|
|
|
1,828 |
|
|
Depreciation
|
|
|
213 |
|
|
|
199 |
|
|
|
146 |
|
|
Excise tax
|
|
|
308 |
|
|
|
238 |
|
|
|
241 |
|
|
Overhead
|
|
|
707 |
|
|
|
675 |
|
|
|
572 |
|
|
Other selling and marketing expenses
|
|
|
457 |
|
|
|
638 |
|
|
|
700 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total selling and marketing expenses
|
|
|
24,947 |
|
|
|
21,352 |
|
|
|
15,692 |
|
Note 19 - General and Administrative Expenses
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Salaries, wages and related expenses
|
|
|
2,395 |
|
|
|
2,183 |
|
|
|
1,781 |
|
|
Professional services
|
|
|
1,271 |
|
|
|
1,274 |
|
|
|
651 |
|
|
Travel expenses
|
|
|
214 |
|
|
|
155 |
|
|
|
185 |
|
|
Overhead
|
|
|
133 |
|
|
|
125 |
|
|
|
107 |
|
|
Other general and administrative expenses
|
|
|
292 |
|
|
|
655 |
|
|
|
42 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total general and administrative expenses
|
|
|
4,305 |
|
|
|
4,392 |
|
|
|
2,766 |
|
Note 20 - Financing Income and Expenses
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Interest income and net change in fair value of
|
|
|
|
|
|
|
|
|
|
|
financial assets held-for-trading and bank deposits
|
|
|
269 |
|
|
|
134 |
|
|
|
45 |
|
|
Net income from change in exchange rates
|
|
|
- |
|
|
|
- |
|
|
|
109 |
|
|
Hedging transactions
|
|
|
3 |
|
|
|
- |
|
|
|
60 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing income recognized in profit or loss
|
|
|
272 |
|
|
|
134 |
|
|
|
214 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net expenses from change in exchange rates
|
|
|
(113 |
) |
|
|
(107 |
) |
|
|
- |
|
|
Financing expenses on liabilities to the OCS
|
|
|
- |
|
|
|
(15 |
) |
|
|
(106 |
) |
|
Change in fair value of derivative liability on account
|
|
|
|
|
|
|
|
|
|
|
|
|
|
of warrants
|
|
|
- |
|
|
|
- |
|
|
|
(13,510 |
) |
|
Hedging transactions
|
|
|
- |
|
|
|
(408 |
) |
|
|
- |
|
|
Other financing expenses
|
|
|
(24 |
) |
|
|
(23 |
) |
|
|
(31 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing expenses recognized in profit or loss
|
|
|
(137 |
) |
|
|
(553 |
) |
|
|
(13,647 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net financing income (expenses)
|
|
|
135 |
|
|
|
(419 |
) |
|
|
(13,433 |
) |
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 21 - Related Parties
|
A.
|
Key management personnel compensation (including directors)
|
In addition to their salaries, the Group also provides non-cash benefits to directors and executive officers (such as a car, etc.), and contributes to post-employment plans on their behalf. Executive officers and directors also participate in the Company’s share option program (see Note 23 regarding share-based payments).
Compensation to key management personnel (including one director) that are employed by the Group:
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Number
|
|
|
USD
|
|
|
Number of
|
|
|
USD
|
|
|
Number
|
|
|
USD
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee benefits
|
|
|
9 |
|
|
|
2,092 |
|
|
|
7 |
|
|
|
2,020 |
|
|
|
7 |
|
|
|
2,398 |
|
|
Share-based payments
|
|
|
9 |
|
|
|
1,285 |
|
|
|
7 |
|
|
|
501 |
|
|
|
7 |
|
|
|
342 |
|
| |
|
|
|
|
|
|
3,377 |
|
|
|
|
|
|
|
2,521 |
|
|
|
|
|
|
|
2,740 |
|
Compensation to directors:
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Number
|
|
|
USD
|
|
|
Number of
|
|
|
USD
|
|
|
Number
|
|
|
USD
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total compensation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
to directors employed
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
by the Company*
|
|
|
1 |
|
|
|
187 |
|
|
|
1 |
|
|
|
314 |
|
|
|
1 |
|
|
|
141 |
|
|
Compensation to
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
independent directors**
|
|
|
4 |
|
|
|
423 |
|
|
|
4 |
|
|
|
305 |
|
|
|
3 |
|
|
|
106 |
|
|
|
*
|
Including share-based payments in the amount of USD 78 thousand, USD 196 thousand and USD 19 thousand in 2015, 2014 and 2013, respectively.
|
|
|
**
|
Including share-based payments in the amount of USD 287 thousand, USD 166 thousand and USD 36 thousand in 2015, 2014 and 2013, respectively.
|
|
B.
|
Engagements between the Company and related parties
|
|
|
(1)
|
On March 31, 2013, the Company’s shareholders approved, based on the compensation committee and the Board of Directors recommendation, an amendment to the employment terms with the CEO, in connection with the relocation of CEO back to Israel, as follows: (1) the CEO salary, effective January 1, 2013, has been updated to NIS 65 thousand (USD 16.7 thousand) per month, and (2) a target bonus that will be equivalent to seven months’ base salary, out of which up to six base salary will be paid in cash and one month base salary will be paid in options in a value based on Black-Scholes model. The CEO is entitled to an allocation to a manager's insurance policy equivalent to 13.33% of his gross monthly salary and 7.5% of his gross monthly salary for an education fund. 5% of his gross monthly salary is deducted for the manager's insurance policy and 2.5% is deducted for the education fund. The CEO is also entitled to reimbursement for vehicle maintenance costs and reasonable expenses. Upon termination of the CEO’s employment (other than in the event of breach of trust), the CEO will be entitled a readjustment payment equal to four months’ base salary from the date that he is no longer employed (the "Re-adjustment Payment").
|
|
|
(2)
|
On November 26, 2013, the Company’s general meeting of shareholders approved additional share-based compensation to one of the directors- see Note 23(C).
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 21 - Related Parties (cont'd)
|
B.
|
Engagements between the Company and related parties (cont'd)
|
|
|
(3)
|
On January 22, 2014, the Company’s shareholders approved, based on the recommendations of the Company’s compensation committee and board of directors, an amendment to the employment terms of the Company’s CEO, so that the Re-adjustment Payment shall be extended from four monthly payments to six monthly payments in the event that the CEO will resign subsequent to a change of control in the Company and it shall be extended from six monthly payments to nine monthly payments in the event that the CEO will be terminated subsequent to a change of control in the Company.
|
|
|
(4)
|
On July 22, 2014, the Company’s shareholders approved an amendment to the employment terms of the CEO, based on the compensation committee and the Board of Directors recommendation, such that the CEO salary, effective April 1, 2014, has been updated to NIS 70 thousand (USD 18 thousand) per month.
|
|
|
(5)
|
On July 22, 2014, the Company’s shareholders approved additional share-based compensation to one of the directors. On the same date, the terms for granting share-based compensation to three directors were met - see Note 23(C).
|
|
|
(6)
|
On October 22, 2014 and October 8, 2015, the Company’s general meeting of shareholders approved additional share-based compensation to the CEO - see Note 23(C).
|
Note 22 - Equity
| |
|
|
|
| |
|
Number of Ordinary shares
|
|
| |
|
|
|
|
|
|
| |
|
|
|
| |
|
|
|
|
Issued and paid-in share capital as of January 1
|
|
|
42,133 |
|
|
|
40,820 |
|
|
Exercise of share options by employees
|
|
|
219 |
|
|
|
1,313 |
|
| |
|
|
|
|
|
|
|
|
|
Issued and paid-in share capital as of December 31
|
|
|
42,352 |
|
|
|
42,133 |
|
|
Authorized share capital
|
|
|
75,000 |
|
|
|
75,000 |
|
The holders of ordinary shares are entitled to receive dividends, if declared, and are entitled to one vote per share at general meetings of the Company.
|
B.
|
Share Options held by investors
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
| |
|
|
|
|
Number of outstanding options as of January 1
|
|
|
134 |
|
|
|
134 |
|
|
Exercised during the period
|
|
|
- |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
Number of outstanding options as of December 31
|
|
|
134 |
|
|
|
134 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 22- Equity (cont'd)
|
C.
|
Issuances of share capital
|
|
|
(1)
|
Private placement - 2011
|
As part of private placement that took place in 2011, the Company allotted options, as to the following:
|
|
(1)
|
The Phoenix Insurance Company Ltd., for itself and for other companies of the Phoenix Group (together Phoenix), on the basis of an internal distribution agreed to by the parties, 2,000,000 ordinary shares of the Company with a par value of NIS 0.01, and 800,000 non-marketable options that will not be listed for trading and are exercisable into 800,000 ordinary shares of the Company with a par value of NIS 0.01 over a period of five years from the date of their allotment at an exercise price of NIS 14 (approximately USD 3.88) per option.
|
|
|
(2)
|
Leader Issuances (1993) Ltd. 421,053 ordinary shares of the Company with a par value of NIS 0.01, and 168,421 non-marketable options that will not be listed for trading and are exercisable into 168,421 ordinary shares of the Company with a par value of NIS 0.01 over a period of five years from the date of closing at an exercise price of NIS 14 (approximately USD 3.88) per option.
|
According to the binomial model, on the grant date the fair value of each one of the options is USD 1.02 and the fair value of all the options allotted to the offerees is USD 995 thousand.
The Company split the overall consideration from the issuance pro rata to the fair value of the equity instruments that were issued so that an amount of USD 825 thousand was recognized as proceeds from options and an amount of USD 5,561 thousand was included in share capital and premium.
On April 3, 2013, the Company issued an aggregate of 34,000 Ordinary Shares for total aggregate consideration of NIS 476 thousand (approximately USD 115 thousand).
On July 2, 2013, the Company issued an aggregate of 800,000 Ordinary Shares for total aggregate consideration of NIS 11,200 thousand (approximately USD 3,086 thousand).
|
|
(2)
|
Private placement - 2012
|
On August 8, 2012, the Company signed an investment agreement in which the Company issued to all the investors together an aggregate of 7,053,529 Ordinary Shares of the Company for an aggregate amount of USD 7,500 thousand (USD 7,298 thousand, net of issuance expenses in the amount of USD 202 thousand) and non-registered warrants to purchase up to 7,053,529 ordinary shares of the Company for an amount equal to the portion of the investment amount remitted by each investor, for no additional consideration.
The warrants issued to the investors are to purchase a variable number of the Company's shares, thereby representing a financial liability that is a derivative instrument.
On May 28, 2013, the Company provided the investors with a notice of mandatory exercise (“the Notice Date”), pursuant to which the investors shall, within 30 days, exercise such warrants for total consideration of USD 7,500 thousands (USD 6,966 thousand, net of issuance expenses in the amount of USD 534 thousand) into a fixed number of 4,996,251 ordinary shares as determined at the Notice Date. Due to this change in the effective terms of the derivative instrument, the Company chose as an accounting policy to reassess the terms of the derivative instrument as of the Notice Date. Accordingly, the Company determined the derivative instrument to be an equity instrument, and as a result, the derivative liability in the amount of USD 17,500 thousands was reclassified to equity, according to its fair value as of the Notice Date.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 22 - Equity (cont'd)
|
C.
|
Issuances of share capital (cont'd)
|
|
|
(3)
|
Public offering - 2013
|
On November 4, 2013, the Company completed the public offering of 2,760,000 ADSs, including ADSs issued pursuant to the underwriters' option to purchase additional shares, at a price of USD 17.00 per ADS, bringing total gross proceeds from the offering to USD 46,920 thousands before deducting underwriting discounts and commissions and other offering expenses payable by the Company. Each ADS represents two of the Company's ordinary shares, par value NIS 0.01.
The total issuance expenses amounted to approximately USD 4,300 thousands, of which USD 3,284 thousands were underwriting fees.
Note 23 - Share-Based Payments
|
A.
|
Grant of share options to employees and directors of the Company
|
|
|
The Company regularly compensates its employees, directors and consultants by means of options to purchase ordinary shares of the Company. As of December 31, 2015, the Company has outstanding options to purchase 5,531,623 ordinary shares of the Company with a par value of NIS 0.01. All of the grants are equity grants.
|
|
|
As of that date, options to purchase 1,949,459 ordinary shares are exercisable.
|
|
B.
|
As of December 31, 2015, the Company has 2 stock option plans for employees, directors, consultants and other service providers of the Group (the "2003 Plan" and the "2011 Plan"). No further grants may be made under the 2003 Plan.
|
|
|
On May 30, 2011, the Company’s Board of Directors approved the 2011 Plan which allows for grants to the Company's employees, directors, consultants and other service providers of the Group. The Company will be able to grant up to 6,262,529 options at any time throughout a period of 10 years from the date of approval of the 2011 Plan according to the terms of the plan.
|
|
|
As of December 31, 2015, there are 410,422 additional options available for grant under the 2011 Plan.
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 23 - Share-Based Payments (cont'd)
|
C.
|
The fair value of share options granted to employees, directors and service providers is measured using the binomial model. Measurement inputs include the share price on the measurement date, the exercise price of the instrument, expected volatility (based on the historical volatility), the expected life span of the options, and the risk-free interest rate (based on government debentures). Service and non-market performance conditions attached to the transactions are not taken into account in determining fair value.
|
The table below summarizes the grant terms and the parameters that were used to determine the fair value of the benefit for grants which are not fully vested as of the balance sheet date:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share price
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Contractual
|
|
|
|
|
|
|
|
|
|
|
|
that served
|
|
|
Total
|
|
| |
|
|
|
|
|
|
Vesting
|
|
|
life of the
|
|
|
|
|
|
|
|
|
Average
|
|
|
as a basis
|
|
|
fair value of
|
|
|
Grant date
|
|
|
|
Number of
|
|
|
period
|
|
|
options
|
|
|
Interest
|
|
|
Expected
|
|
|
exercise
|
|
|
for pricing
|
|
|
the benefit on
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
13/05/2013
|
|
Employees
|
|
|
177,000 |
|
|
|
1-3 |
|
|
|
7 |
|
|
|
1.5-5.5 |
|
|
|
47.91 |
|
|
|
4.87 |
|
|
|
4.87 |
|
|
|
378 |
|
|
13/05/2013
|
|
Consultant
|
|
|
10,000 |
|
|
|
1-3 |
|
|
|
7 |
|
|
|
1.5-5.5 |
|
|
|
47.91 |
|
|
|
4.87 |
|
|
|
4.87 |
|
|
|
26 |
|
|
28/05/2013
|
|
CEO
|
|
|
11,577 |
|
|
|
1-2 |
|
|
|
7 |
|
|
|
1.3-5.7 |
|
|
|
47.80 |
|
|
|
4.63 |
|
|
|
4.95 |
|
|
|
28 |
|
|
01/08/2013
|
|
Employees
|
|
|
184,750 |
|
|
|
1-3 |
|
|
|
7 |
|
|
|
1.1-6.1 |
|
|
|
47.99 |
|
|
|
6.91 |
|
|
|
6.69 |
|
|
|
575 |
|
|
02/09/2013
|
|
Officer
|
|
|
30,000 |
|
|
|
1-3 |
|
|
|
7 |
|
|
|
1.3-6.1 |
|
|
|
48.00 |
|
|
|
7.43 |
|
|
|
7.43 |
|
|
|
108 |
|
|
14/11/2013
|
|
Employees
|
|
|
50,000 |
|
|
|
1-3 |
|
|
|
7 |
|
|
|
0.9-5.8 |
|
|
|
48.58 |
|
|
|
9.60 |
|
|
|
9.49 |
|
|
|
211 |
|
|
26/11/2013
|
|
Director
|
|
|
80,000 |
|
|
|
1-3 |
|
|
|
7 |
|
|
|
0.9-5.8 |
|
|
|
48.60 |
|
|
|
9.16 |
|
|
|
8.47 |
|
|
|
317 |
|
|
02/02/2014
|
|
Employees
|
|
|
64,500 |
|
|
|
1-4 |
|
|
|
7 |
|
|
|
3.31 |
|
|
|
48.55 |
|
|
|
12.54 |
|
|
|
12.54 |
|
|
|
381 |
|
|
03/04/2014
|
|
Employees
|
|
|
89,200 |
|
|
|
1-4 |
|
|
|
7 |
|
|
|
3.09 |
|
|
|
48.26 |
|
|
|
11.98 |
|
|
|
10.92 |
|
|
|
431 |
|
|
22/07/2014
|
|
Directors
|
|
|
160,000 |
|
|
|
1-3 |
|
|
|
7 |
|
|
|
2.52 |
|
|
|
48.11 |
|
|
|
8.10 |
|
|
|
7.46 |
|
|
|
543 |
|
|
31/07/2014
|
|
Employees
|
|
|
1,363,750 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
2.48 |
|
|
|
48.03 |
|
|
|
7.76 |
|
|
|
6.97 |
|
|
|
4,116 |
|
|
31/07/2014
|
|
Officers
|
|
|
513,700 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
2.48 |
|
|
|
48.03 |
|
|
|
7.76 |
|
|
|
6.97 |
|
|
|
1,631 |
|
|
22/10/2014
|
|
CEO
|
|
|
150,000 |
|
|
|
2-4 |
|
|
|
6.78 |
|
|
|
1.80 |
|
|
|
48.50 |
|
|
|
7.11 |
|
|
|
5.59 |
|
|
|
345 |
|
|
29/10/2014
|
|
Employees
|
|
|
47,500 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
1.94 |
|
|
|
48.09 |
|
|
|
6.07 |
|
|
|
5.64 |
|
|
|
114 |
|
|
29/01/2015
|
|
Consultant
|
|
|
10,000 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
1.5 |
|
|
|
48.61 |
|
|
|
5.83 |
|
|
|
5.35 |
|
|
|
26 |
|
|
29/01/2015
|
|
Officer
|
|
|
100,000 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
1.5 |
|
|
|
48.61 |
|
|
|
5.83 |
|
|
|
5.35 |
|
|
|
242 |
|
|
15/02/2015
|
|
Employees
|
|
|
104,250 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
1.65 |
|
|
|
48.65 |
|
|
|
5.86 |
|
|
|
5.86 |
|
|
|
269 |
|
|
26/02/2015
|
|
Officer
|
|
|
100,000 |
|
|
|
1.5-3.5 |
|
|
|
7 |
|
|
|
1.44 |
|
|
|
48.69 |
|
|
|
6.28 |
|
|
|
5.82 |
|
|
|
261 |
|
|
04/05/2015
|
|
Employees
|
|
|
139,500 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
1.36 |
|
|
|
48.88 |
|
|
|
6.44 |
|
|
|
6.44 |
|
|
|
423 |
|
|
04/05/2015
|
|
Consultant
|
|
|
5,000 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
1.36 |
|
|
|
48.88 |
|
|
|
6.44 |
|
|
|
6.44 |
|
|
|
16 |
|
|
15/07/2015
|
|
Officers
|
|
|
331,000 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
2.03 |
|
|
|
48.84 |
|
|
|
7.17 |
|
|
|
7.17 |
|
|
|
1,171 |
|
|
15/07/2015
|
|
Employees
|
|
|
604,400 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
2.03 |
|
|
|
48.84 |
|
|
|
7.17 |
|
|
|
7.17 |
|
|
|
2,074 |
|
|
15/07/2015
|
|
Consultant
|
|
|
6,500 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
2.03 |
|
|
|
48.84 |
|
|
|
7.17 |
|
|
|
7.17 |
|
|
|
24 |
|
|
08/10/2015
|
|
CEO
|
|
|
60,000 |
|
|
|
2-4 |
|
|
|
6.77 |
|
|
|
1.7 |
|
|
|
49.37 |
|
|
|
7.01 |
|
|
|
5.75 |
|
|
|
153 |
|
|
29/10/2015
|
|
Employees
|
|
|
113,500 |
|
|
|
2-4 |
|
|
|
7 |
|
|
|
1.64 |
|
|
|
48.92 |
|
|
|
5.81 |
|
|
|
5.56 |
|
|
|
294 |
|
| |
*
|
The exercise price and share price are denominated in NIS and are re-measured using historic exchange rates.
|
Expected volatility is estimated by considering historic share price volatility of the Company. The risk-free interest rate was determined on the basis of non-interest bearing NIS-denominated Government debentures with a remaining life equal to the expected term of the options.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 23 - Share-Based Payments (cont'd)
|
D.
|
The number and weighted average exercise prices of share options are as follows:
|
| |
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
| |
|
average
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
average
|
|
|
|
|
| |
|
exercise
|
|
|
Number of
|
|
|
average
|
|
|
Number of
|
|
|
exercise
|
|
|
Number of
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1
|
|
|
5.43 |
|
|
|
4,540,158 |
|
|
|
2.77 |
|
|
|
3,715,613 |
|
|
|
2.06 |
|
|
|
3,627,808 |
|
|
Forfeited during the year
|
|
|
7.09 |
|
|
|
(364,408 |
) |
|
|
4.75 |
|
|
|
(250,935 |
) |
|
|
2.06 |
|
|
|
(220,957 |
) |
|
Exercised during the year
|
|
|
1.70 |
|
|
|
(218,277 |
) |
|
|
2.31 |
|
|
|
(1,313,170 |
) |
|
|
2.04 |
|
|
|
(234,565 |
) |
|
Granted during the year
|
|
|
6.76 |
|
|
|
1,574,150 |
|
|
|
7.96 |
|
|
|
2,388,650 |
|
|
|
6.67 |
|
|
|
543,327 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31
|
|
|
5.43 |
|
|
|
5,531,623 |
|
|
|
5.43 |
|
|
|
4,540,158 |
|
|
|
2.77 |
|
|
|
3,715,613 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31
|
|
|
2.91 |
|
|
|
1,949,459 |
|
|
|
2.65 |
|
|
|
1,640,803 |
|
|
|
2.24 |
|
|
|
2,318,958 |
|
| |
*
|
The exercise price is denominated in NIS.
|
With respect to options granted to related parties, see Note 21 on related parties.
Note 24 - Loss Per Share
The calculation of basic loss per share for the years ended December 31, 2015, 2014 and 2013 was based on the loss attributable to ordinary shareholders divided by a weighted average number of ordinary shares outstanding calculated as follows:
|
|
(1)
|
Loss attributable to ordinary shareholders
|
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Continuing
|
|
|
Continuing
|
|
|
Continuing
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Loss for the year
|
|
|
15,385 |
|
|
|
15,272 |
|
|
|
20,529 |
|
|
|
(2)
|
Weighted average number of ordinary shares
|
| |
|
For the year ended December 31
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Balance as of January 1
|
|
|
42,133 |
|
|
|
40,820 |
|
|
|
29,235 |
|
|
Effect of shares issued during the year
|
|
|
151 |
|
|
|
988 |
|
|
|
6,546 |
|
|
Weighted average number of ordinary shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
used to calculate basic loss per share
|
|
|
42,284 |
|
|
|
41,808 |
|
|
|
35,781 |
|
|
B.
|
Diluted loss per share
|
At December 31, 2015 5,532 thousand options (in 2014 and 2013: 4,540 thousand and 3,716 thousand, respectively) were excluded from the diluted weighted average number of ordinary shares calculation as their effect would have been anti-dilutive.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 25 - Financial Risk Management
The Group has exposure to the following risks from its use of financial instruments:
— Credit risk
— Liquidity risk
— Market risk (including currency, interest and other market price risks)
|
B.
|
Risk management framework
|
This note presents information about the Group’s exposure to each of the above risks, and the Group’s objectives, policies and processes for measuring and managing risk. Further quantitative disclosures are included throughout these consolidated financial statements.
The Board of Directors has overall responsibility for the establishment and oversight of the Group’s risk management framework.
The Group’s risk management policies are established to identify and analyze the risks faced by the Group, to set appropriate risk limits and controls, and to monitor risks and adherence to limits. Risk management policies and systems are reviewed regularly to reflect changes in market conditions and the Group’s activities. The Group, through its training and management standards and procedures, aims to develop a disciplined and constructive control environment in which all employees understand their roles and obligations.
The Board of Directors oversees how management monitors compliance with the Group’s risk management policies and procedures, and reviews the adequacy of the risk management framework in relation to the risks faced by the Group. The Board of Directors is assisted in its oversight role by Internal Audit. Internal Audit undertakes both regular and ad hoc reviews of risk management controls and procedures, the results of which are reported to the Audit Committee.
Credit risk is the risk of financial loss to the Group if a customer or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Group’s trade and other receivables, as well as from cash and cash equivalents and investment in marketable securities.
Trade and other receivables
The Group’s exposure to credit risk is influenced mainly by the individual characteristics of each customer. The demographics of the Group’s customer base, including the default risk of the industry and country in which customers operate have only a small effect on the credit risk.
The Group establishes a provision for doubtful debts that represents its estimate of incurred losses in respect of trade and other receivables. The main components of this provision are specific loss components that relate to individually significant exposures.
Cash and cash equivalents and Investments
The Group limits its exposure to credit risk by holding cash and investing only in bank deposits and debentures and only with counterparties that have a credit rating of at least A+ according to the rating accepted in Israel. Given these high credit ratings, management does not expect any counterparty to fail to meet its obligations.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 25 - Financial Risk Management (cont’d)
Liquidity risk is the risk that the Company will encounter difficulty in meeting its financial obligations when due. The Company’s approach to managing liquidity is to ensure, as far as possible, that it will always have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses.
Market risk is the risk that changes in market prices, such as foreign exchange rates, the CPI, interest rates and equity prices will affect the Group’s income or the value of its holdings of financial instruments. The objective of market risk management is to manage and control market risk exposures within acceptable parameters, while optimizing the return.
Currency risk
The Group is exposed to currency risk arising primarily from exposure to NIS given that the significant portion of the expenses in respect of consultants, contractors and Israel salary expenses is denominated in NIS. In respect of other monetary assets and liabilities denominated in currency other than the Group’s functional currency, the Group ensures that its net exposure is kept to an acceptable level by buying or selling foreign currencies at spot rates when necessary to address short-term imbalances.
The Company engages in derivative instruments transactions, such as options and forward contracts, for the purposes of hedging the Company's NIS payments to local suppliers and for salaries in Israel. The Company's hedging transactions are aimed to decrease a certain portion of the financial exposure risk of fluctuations in the exchange rates of the Company's operating currency, which is the U.S. dollar against the NIS.
Interest rate risk
The Group is exposed to changes in interest rates, primarily possible changes in the risk-free market interest rate which may have an effect on the fair value of the Group’s short and long term investments.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 26 - Financial Instruments
|
|
(1)
|
Exposure to credit risk
|
The maximum exposure to credit risk for cash and cash equivalents, deposits, short-term investments, trade receivables and long-term investments at the reporting date by type of counterparty was:
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
Carrying
|
|
|
Carrying
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
Cash and cash equivalents
|
|
|
13,519 |
|
|
|
22,255 |
|
|
Mutual funds
|
|
|
13 |
|
|
|
13 |
|
|
Short-term investments - bank deposits
|
|
|
21,674 |
|
|
|
24,494 |
|
|
Trade receivables
|
|
|
5,002 |
|
|
|
2,797 |
|
|
Other current assets
|
|
|
125 |
|
|
|
42 |
|
|
Long-term investments - bank deposits
|
|
|
5,023 |
|
|
|
5,473 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
45,356 |
|
|
|
55,074 |
|
The maximum exposure to credit risk for trade receivables at the reporting date by geographic region was as follows:
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
Israel
|
|
|
14 |
|
|
|
56 |
|
|
United States
|
|
|
3,884 |
|
|
|
1,560 |
|
|
Asia Pacific
|
|
|
870 |
|
|
|
1,178 |
|
|
Rest of the world
|
|
|
234 |
|
|
|
3 |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
5,002 |
|
|
|
2,797 |
|
|
|
(2)
|
Aging of debts and impairment losses
|
The aging of trade receivables at the reporting date was:
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Not past due
|
|
|
4,827 |
|
|
|
- |
|
|
|
2,321 |
|
|
|
- |
|
|
Past due 0-30 days
|
|
|
175 |
|
|
|
- |
|
|
|
441 |
|
|
|
- |
|
|
Past due 31-60 days
|
|
|
- |
|
|
|
- |
|
|
|
35 |
|
|
|
- |
|
| |
|
|
5,002 |
|
|
|
- |
|
|
|
2,797 |
|
|
|
- |
|
The movement in the provision for impairment in respect of trade receivables and other receivables was as follows:
| |
|
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Balance as of January 1
|
|
|
- |
|
|
|
(2 |
) |
|
|
(2 |
) |
|
Impairment loss recognized
|
|
|
- |
|
|
|
(6 |
) |
|
|
- |
|
|
Bad debt written off
|
|
|
- |
|
|
|
8 |
|
|
|
- |
|
|
Balance as of December 31
|
|
|
- |
|
|
|
- |
|
|
|
(2 |
) |
All outstanding liabilities at December 31, 2015 and 2014 are to be paid within 6 months.
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 26 - Financial Instruments (cont’d)
|
|
(1)
|
Linkage and foreign currency risks
|
The Group’s exposure to linkage and foreign currency risk was as follows based on notional amounts:
| |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
US dollar
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
2,114 |
|
|
|
- |
|
|
|
10,886 |
|
|
|
519 |
|
|
|
- |
|
|
|
13,519 |
|
|
Short-term investments
|
|
|
13 |
|
|
|
- |
|
|
|
21,674 |
|
|
|
- |
|
|
|
- |
|
|
|
21,687 |
|
|
Trade receivables
|
|
|
14 |
|
|
|
- |
|
|
|
4,754 |
|
|
|
234 |
|
|
|
- |
|
|
|
5,002 |
|
|
Other current assets
|
|
|
528 |
|
|
|
- |
|
|
|
253 |
|
|
|
- |
|
|
|
639 |
|
|
|
1,420 |
|
|
Inventory
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
2,777 |
|
|
|
2,777 |
|
|
Total current assets
|
|
|
2,669 |
|
|
|
- |
|
|
|
37,567 |
|
|
|
753 |
|
|
|
3,416 |
|
|
|
44,405 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid lease fees
|
|
|
- |
|
|
|
73 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
73 |
|
|
Property and equipment, net
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,432 |
|
|
|
1,432 |
|
|
Deferred tax asset
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
37 |
|
|
|
37 |
|
|
Long-term investments
|
|
|
- |
|
|
|
- |
|
|
|
5,023 |
|
|
|
- |
|
|
|
- |
|
|
|
5,023 |
|
|
Total assets
|
|
|
2,669 |
|
|
|
73 |
|
|
|
42,590 |
|
|
|
753 |
|
|
|
4,885 |
|
|
|
50,970 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade payables
|
|
|
958 |
|
|
|
- |
|
|
|
1,134 |
|
|
|
127 |
|
|
|
- |
|
|
|
2,219 |
|
|
Other current liabilities
|
|
|
1,627 |
|
|
|
- |
|
|
|
2,852 |
|
|
|
65 |
|
|
|
1,508 |
|
|
|
6,052 |
|
|
Total current liabilities
|
|
|
2,585 |
|
|
|
- |
|
|
|
3,986 |
|
|
|
192 |
|
|
|
1,508 |
|
|
|
8,271 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee benefits
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
299 |
|
|
|
299 |
|
|
Total liabilities
|
|
|
2,585 |
|
|
|
- |
|
|
|
3,986 |
|
|
|
192 |
|
|
|
1,807 |
|
|
|
8,570 |
|
|
Total balance, net
|
|
|
84 |
|
|
|
73 |
|
|
|
38,604 |
|
|
|
561 |
|
|
|
3,078 |
|
|
|
42,400 |
|
| |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
1,765 |
|
|
|
- |
|
|
|
19,790 |
|
|
|
700 |
|
|
|
- |
|
|
|
22,255 |
|
|
Short-term investments
|
|
|
13 |
|
|
|
- |
|
|
|
24,494 |
|
|
|
- |
|
|
|
- |
|
|
|
24,507 |
|
|
Trade receivables
|
|
|
56 |
|
|
|
- |
|
|
|
2,738 |
|
|
|
3 |
|
|
|
- |
|
|
|
2,797 |
|
|
Other current assets
|
|
|
237 |
|
|
|
- |
|
|
|
122 |
|
|
|
- |
|
|
|
751 |
|
|
|
1,110 |
|
|
Inventory
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
3,050 |
|
|
|
3,050 |
|
|
Total current assets
|
|
|
2,071 |
|
|
|
- |
|
|
|
47,144 |
|
|
|
703 |
|
|
|
3,801 |
|
|
|
53,719 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid lease fees
|
|
|
- |
|
|
|
79 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
79 |
|
|
Property and equipment, net
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,257 |
|
|
|
1,257 |
|
|
Deferred tax asset
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
158 |
|
|
|
158 |
|
|
Long-term investments
|
|
|
- |
|
|
|
- |
|
|
|
5,473 |
|
|
|
- |
|
|
|
- |
|
|
|
5,473 |
|
|
Total assets
|
|
|
2,071 |
|
|
|
79 |
|
|
|
52,617 |
|
|
|
703 |
|
|
|
5,216 |
|
|
|
60,686 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade payables
|
|
|
647 |
|
|
|
- |
|
|
|
604 |
|
|
|
438 |
|
|
|
- |
|
|
|
1,689 |
|
|
Other current liabilities
|
|
|
1,239 |
|
|
|
- |
|
|
|
1,848 |
|
|
|
46 |
|
|
|
1,319 |
|
|
|
4,452 |
|
|
Total current liabilities
|
|
|
1,886 |
|
|
|
- |
|
|
|
2,452 |
|
|
|
484 |
|
|
|
1,319 |
|
|
|
6,141 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee benefits
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
278 |
|
|
|
278 |
|
|
Total liabilities
|
|
|
1,886 |
|
|
|
- |
|
|
|
2,452 |
|
|
|
484 |
|
|
|
1,597 |
|
|
|
6,419 |
|
|
Total balance, net
|
|
|
185 |
|
|
|
79 |
|
|
|
50,165 |
|
|
|
219 |
|
|
|
3,619 |
|
|
|
54,267 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 26 - Financial Instruments (cont’d)
|
|
(1)
|
Linkage and foreign currency risks (cont'd)
|
Information regarding the CPI and significant exchange rates:
| |
|
For the year ended December 31,
|
|
|
For the year ended December 31,
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Spot price of 1 USD at the reporting date
|
|
|
1 NIS
|
|
|
(0.3 |
) |
|
|
(11 |
) |
|
|
7.5 |
|
|
|
0.2563 |
|
|
|
0.2571 |
|
|
|
0.2881 |
|
|
1 Euro
|
|
|
(10 |
) |
|
|
(12 |
) |
|
|
4.5 |
|
|
|
1.0884 |
|
|
|
1.2149 |
|
|
|
1.3777 |
|
|
CPI in points *
|
|
|
(1 |
) |
|
|
(0.2 |
) |
|
|
1.8 |
|
|
|
112.82 |
|
|
|
113.96 |
|
|
|
114.18 |
|
* According to an average basis of 2008=100.
| |
|
The Group’s exposure to linkage and foreign currency risk in respect of derivatives is as follows:
|
| |
Currency/
|
|
Currency/
|
|
|
|
|
|
|
|
|
| |
linkage
|
|
linkage
|
|
Amount
|
|
|
Amount
|
|
Date of
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Instruments not
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
accounted for as hedging:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forward
|
NIS
|
|
USD
|
|
|
4,000 |
|
|
|
(1,029 |
) |
Jan-March 2016
|
|
|
(4 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4 |
) |
|
December 31, 2014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Instruments not
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
accounted for as hedging:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Buy put options
|
NIS
|
|
USD
|
|
|
7,300 |
|
|
|
2,065 |
|
Jan-April 2015
|
|
|
1 |
|
|
Sell call options
|
NIS
|
|
USD
|
|
|
7,300 |
|
|
|
1,993 |
|
Jan-April 2015
|
|
|
(121 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(120 |
) |
At the reporting date the interest rate profile of the Group’s interest-bearing financial instruments was as follows:
| |
|
|
|
| |
|
|
|
|
|
|
| |
|
Carrying
|
|
|
Carrying
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Fixed rate instruments
|
|
|
|
|
|
|
|
Deposits held at financial institutions, with
|
|
|
|
|
|
|
|
original maturity periods of up to three months
|
|
|
2,547 |
|
|
|
3,182 |
|
|
Short-term investments
|
|
|
21,674 |
|
|
|
24,494 |
|
|
Long-term investments
|
|
|
5,023 |
|
|
|
5,473 |
|
| |
|
|
29,244 |
|
|
|
33,149 |
|
| |
|
|
|
|
|
|
|
|
|
Variable rate instruments
|
|
|
|
|
|
|
|
|
|
Mutual funds
|
|
|
13 |
|
|
|
13 |
|
| |
|
|
13 |
|
|
|
13 |
|
Mazor Robotics Ltd.
Notes to the Consolidated Financial Statements
Note 26 - Financial Instruments (cont’d)
Fair value hierarchy
|
|
1.
|
As of December 31, 2015 and 2014, the marketable securities in the amount of USD 13 thousand held for trading are presented at fair value through profit or loss. The fair value is determined on the basis of quoted prices (unadjusted) in active markets for identical instruments (level 1).
|
|
|
2.
|
As of December 31, 2015, derivative financial instruments classified as a liability in the amount of USD 4 thousand (As of December 31, 2014 - USD 120 thousand) are presented at fair value and changes recognized in the profit or loss statement. The fair value was valued utilizing market observable inputs (level 2). The fair value of these derivative financial instruments is measured based on observable market data, such as spot rate, yield curves and exchange rate volatility, as of the fair value calculation date.
|
Note 27 - Subsequent Events
On February 21, 2016, the Company issued 134,421 ordinary shares in connection with the exercise of warrants held by investors for a total consideration of NIS 1,882 thousand (approximately USD 482 thousand).
Through May 2, 2016, the Company issued an aggregate of 30,667 ordinary shares in connection with the exercise of options granted to employees under the 2003 Plan and 2011 Plan for a total consideration of NIS 332 thousand (approximately USD 85 thousand).
|
Exhibit
|
Description
|
| |
|
|
1.1*
|
Articles of Association of Mazor Robotics Ltd. (unofficial English translation from Hebrew).
|
| |
|
|
2.1*
|
Form of Deposit Agreement between Mazor Robotics Ltd., The Bank of New York Mellon as Depositary, and owners and holders from time to time of ADSs issued thereunder, including the Form of American Depositary Shares.
|
| |
|
|
2.2*
|
Form of Ordinary Shares Purchase Warrant issued to the Oracle Investors in August 2012.
|
| |
|
|
2.3*
|
Registration Rights Agreement dated September 27, 2012, among Mazor Robotics Ltd. and the Oracle Investors.
|
| |
|
|
4.1*
|
Mazor Robotics Ltd. 2003 Stock Option Plan.
|
| |
|
|
4.2*
|
Mazor Robotics Ltd. 2011 Share Option Plan.
|
| |
|
|
4.3*
|
Summary of Lease Agreement dated April 30, 2003, between Mazor Robotics Ltd. and Hayel Investments and Properties Ltd., as amended on March 27, 2007, September 16, 2009, and July 7, 2011.
|
| |
|
|
4.4*
|
Share Purchase Agreement dated August 8, 2012, among Mazor Robotics Ltd. and the Oracle Investors.
|
| |
|
|
4.5*
|
Form of Allocation Agreement used in February 2011 private placement with the Phoenix Insurance Company and Leader Underwriters (1993) Ltd. (unofficial English translation from Hebrew).
|
| |
|
|
4.6*
|
Employment Agreement dated December 26, 2007, between Mazor Robotics Ltd. and Jonathan Adereth (unofficial English translation from Hebrew).
|
| |
|
|
4.7*
|
Personal Employment Agreement dated April 9, 2013, between Mazor Robotics Ltd. and Ori Hadomi.
|
| |
|
|
4.8*
|
Employment Agreement dated December 12, 2007, between Mazor Robotics Ltd. and Sharon Levita (unofficial English translation from Hebrew).
|
| |
|
|
4.9*^
|
Sub-Contracting and Supply Agreement dated September 28, 2005, between Mazor Robotics Ltd. and MPS Micro Precision Systems AG.
|
| |
|
|
4.10*
|
Extension letter dated January 18, 2013, to the Sub-Contracting and Supply Agreement dated September 28, 2005, between Mazor Robotics Ltd. and MPS Micro Precision Systems AG.
|
| |
|
|
4.11*^
|
Manufacturing Agreement dated May 15, 2005, between Mazor Robotics Ltd. and Tamuz F.T.K Solutions Ltd. (formerly Yizrael Tamuz Ltd).
|
| |
|
|
4.12*
|
Extension letter dated January 10, 2013, to the Manufacturing Agreement dated May 15, 2005, between Mazor Robotics Ltd. and Tamuz F.T.K Solutions Ltd. (formerly Yizrael Tamuz Ltd.)
|
| |
|
|
4.13*
|
Form of Directors and Officers Indemnification Agreement.
|
| |
|
|
4.14*
|
Employment Agreement dated November 28, 2000, between Mazor Robotics Ltd. and Eliyahu Zehavi, including an amendment thereto dated January 2003 (unofficial English translation from Hebrew original).
|
|
4.15*
|
Commercial Lease Agreement dated March 7, 2013, between Mazor Robotics Inc. and ACM DT Properties, LLC.
|
| |
|
|
4.16**
|
Employment Offer dated September 17, 2014, between Mazor Robotics Inc. and Christopher Prentice.
|
| |
|
|
4.17**
|
Employment Agreement dated January 25, 2015, between Mazor Robotics Ltd. and Sandra Adzic (unofficial English translation from Hebrew original).
|
| |
|
| 4.18*** |
Amendment to Mazor Robotics 2011 Share Option Plan
|
| |
|
| 4.19 |
Conversion employment agreement dated May 1, 2014 between Mazor Robotics Ltd., Mr. Jonathan Adereth and J. Adereth Marketing and Strategic Consulting Ltd. (unofficial English translation from Hebrew original). |
| |
|
|
8.1**
|
List of Subsidiaries.
|
| |
|
|
12.(a).1
|
Certification of the Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
| |
|
|
12.(a).2
|
Certification of the Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
| |
|
|
13.(a).1
|
Certification of the Chief Executive Officer required by Rule 13a-14(b) and Section 1350 of Chapter 63 of Title 18 of the U.S. Code.
|
| |
|
|
13.(a).2
|
Certification of the Chief Financial Officer required by Rule 13a-14(b) and Section 1350 of Chapter 63 of Title 18 of the U.S. Code.
|
| |
|
|
15.1
|
Consent of Somekh Chaikin, a member firm of KPMG International, independent registered public accounting firm.
|
| |
|
|
15.2
|
Consent of Financial Immunities Dealing Room Ltd.
|
|
*
|
Previously filed with the Company’s registration statement on Form 20-F, filed with the SEC on May 10, 2013.
|
|
**
|
Previously filed with the Company’s annual report on Form 20-F, filed with the SEC on April 29, 2015.
|
|
***
|
Previously filed with the Company's registration statement on Form S-8, filed with the SEC on June 17, 2015.
|
|
^
|
Portions of this exhibit have been omitted pursuant to a grant of confidential treatment.
|
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report filed on its behalf.
| |
|
|
|
| |
MAZOR ROBOTICS LTD.
|
|
| |
|
|
|
| |
By:
|
/s/ Ori Hadomi
|
|
| |
|
Ori Hadomi
|
|
| |
|
Chief Executive Officer
|
|
| |
|
|
|
|
Date: May 2, 2016
|
|
|
|
136