UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D. C. 20549
FORM 10-K
(MARK ONE) | ||
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
For the fiscal year ended December 31, 2019
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
For the transition period from to
Commission file number 001-35565

(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (I.R.S. employer identification number) | |
(847 ) 932-7900
(Address, including zip code, and telephone number of principal executive offices)
Securities Registered Pursuant to Section 12(b) of the Act:
Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered | ||
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
☒ | Accelerated Filer | ☐ | |
Non-Accelerated Filer | ☐ | Smaller reporting company | |
Emerging growth company | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ☐ No ☒
The aggregate market value of the 1,462,630,048 shares of voting stock held by non-affiliates of the registrant, computed by reference to the closing price as reported on the New York Stock Exchange, as of the last business day of AbbVie Inc.'s most recently completed second fiscal quarter (June 30, 2019), was $106,362,457,090 . AbbVie has no non-voting common equity.
Number of common shares outstanding as of January 31, 2020: 1,479,156,683
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the 2020 AbbVie Inc. Proxy Statement are incorporated by reference into Part III. The Definitive Proxy Statement will be filed on or about March 19, 2020.
ABBVIE INC.
FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2019
TABLE OF CONTENTS
Page No. | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Item 15. | ||
Item 16. | ||
PART I
ITEM 1. BUSINESS
Overview
AbbVie(1) is a global, research-based biopharmaceutical company. AbbVie develops and markets advanced therapies that address some of the world's most complex and serious diseases. AbbVie's products are focused on treating conditions such as chronic autoimmune diseases in rheumatology, gastroenterology and dermatology; oncology, including blood cancers; virology, including hepatitis C virus (HCV) and human immunodeficiency virus (HIV); neurological disorders, such as Parkinson's disease; metabolic diseases, including thyroid disease and complications associated with cystic fibrosis; pain associated with endometriosis; as well as other serious health conditions. AbbVie also has a pipeline of promising new medicines in clinical development across such important medical specialties as immunology, oncology and neuroscience, with additional targeted investment in cystic fibrosis and women's health.
In June 2019, AbbVie announced that it entered into a definitive transaction agreement under which AbbVie will acquire Allergan plc (Allergan). Allergan is a global pharmaceutical leader focused on developing, manufacturing and commercializing branded pharmaceutical, device, biologic, surgical and regenerative medicine products for patients around the world. Allergan markets a portfolio of brands and products primarily focused on key therapeutic areas including aesthetics, eye care, neuroscience, gastroenterology and women's health. See Note 5 to the Consolidated Financial Statements for additional information regarding the proposed acquisition.
AbbVie was incorporated in Delaware on April 10, 2012. On January 1, 2013, AbbVie became an independent, publicly-traded company as a result of the distribution by Abbott Laboratories (Abbott) of 100% of the outstanding common stock of AbbVie to Abbott's shareholders.
Segments
AbbVie operates in one business segment—pharmaceutical products. See Note 16 to the Consolidated Financial Statements and the sales information related to HUMIRA, IMBRUVICA and MAVYRET included under Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations."
Products
AbbVie's portfolio of products includes a broad line of therapies that address some of the world's most complex and serious diseases.
Immunology products. AbbVie maintains an extensive immunology portfolio across rheumatology, dermatology and gastroenterology. AbbVie's immunology products address unmet needs for patients with autoimmune diseases. These products are:
HUMIRA. HUMIRA (adalimumab) is a biologic therapy administered as a subcutaneous injection. It is approved to treat the following autoimmune diseases in the United States, Canada and Mexico (collectively, North America) and in the European Union:
Condition | Principal Markets | |
Rheumatoid arthritis (moderate to severe) | North America, European Union | |
Psoriatic arthritis | North America, European Union | |
Ankylosing spondylitis | North America, European Union | |
Adult Crohn's disease (moderate to severe) | North America, European Union | |
Plaque psoriasis (moderate to severe chronic) | North America, European Union | |
Juvenile idiopathic arthritis (moderate to severe polyarticular) | North America, European Union | |
Ulcerative colitis (moderate to severe) | North America, European Union | |
Axial spondyloarthropathy | European Union | |
Pediatric Crohn's disease (moderate to severe) | North America, European Union | |
Hidradenitis Suppurativa (moderate to severe) | North America, European Union | |
Pediatric enthesitis-related arthritis | European Union | |
Non-infectious intermediate, posterior and panuveitis | North America, European Union | |
_______________________________________________________________________________
(1) | As used throughout the text of this report on Form 10-K, the terms "AbbVie" or "the company" refer to AbbVie Inc., a Delaware corporation, or AbbVie Inc. and its consolidated subsidiaries, as the context requires. |
2019 Form 10-K |  1 1 | ||
HUMIRA is also approved in Japan for the treatment of intestinal Behçet's disease.
HUMIRA is sold in numerous other markets worldwide, including Japan, China, Brazil and Australia, and accounted for approximately 58% of AbbVie's total net revenues in 2019.
SKYRIZI. SKYRIZI (risankizumab) is an interleukin-23 (IL-23) inhibitor that selectively blocks IL-23 by binding to its p19 subunit. It is a biologic therapy administered as a quarterly subcutaneous injection following an induction dose. SKYRIZI is approved in the United States, Canada and the European Union and is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. In Japan, SKYRIZI is approved for the treatment of plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adult patients who have an inadequate response to conventional therapies.
RINVOQ. RINVOQ (upadacitinib) is a once-daily oral selective and reversible JAK inhibitor and is approved in the United States, Canada and the European Union. RINVOQ is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate.
Oncology products. AbbVie’s oncology products target some of the most complex and difficult-to-treat cancers. These products are:
IMBRUVICA. IMBRUVICA (ibrutinib) is an oral, once-daily therapy that inhibits a protein called Bruton's tyrosine kinase (BTK). IMBRUVICA was one of the first medicines to receive a United States Food and Drug Administration (FDA) approval after being granted a Breakthrough Therapy Designation and is one of the few therapies to receive four separate designations. IMBRUVICA currently is approved for the treatment of adult patients with:
• | Chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL) and CLL/SLL with 17p deletion; |
• | Mantle cell lymphoma (MCL) who have received at least one prior therapy*; |
• | Waldenström’s macroglobulinemia (WM); |
• | Marginal zone lymphoma (MZL) who require systemic therapy and have received at least one prior anti-CD20-based therapy*; and |
• | Chronic graft versus host disease (cGVHD) after failure of one or more lines of systemic therapy. |
_______________________________________________________________________________
* Accelerated approval was granted for this indication based on overall response rate. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trials.
VENCLEXTA/VENCLYXTO. VENCLEXTA (venetoclax) is a BCL-2 inhibitor used to treat hematological malignancies. VENCLEXTA is approved by the FDA for adults with CLL or SLL. In addition, VENCLEXTA is approved in combination with azacitidine, or decitabine, or low-dose cytarabine to treat adults with newly-diagnosed acute myeloid leukemia (AML) who are 75 years of age or older or have other medical conditions that prevent the use of standard chemotherapy. VENCLYXTO is approved in Europe for CLL in combination with rituximab in patients who have received at least one previous treatment.
Virology Products. AbbVie's virology products address unmet needs for patients living with HCV and HIV.
HCV products. AbbVie's HCV products are:
MAVYRET/MAVIRET. MAVYRET (glecaprevir/pibrentasvir) is approved in the United States and European Union (MAVIRET) for the treatment of patients with chronic HCV genotype 1-6 infection without cirrhosis and with compensated cirrhosis (Child-Pugh A). It is also indicated for the treatment of adult patients with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor, but not both. It is an 8-week, pan-genotypic treatment for patients without cirrhosis and following the EXPEDITION-8 study, also in patients with compensated cirrhosis who are new to treatment. MAVIRET is now also indicated for the treatment of HCV genotypes 1-6 in children between 12-18 years.
VIEKIRA PAK AND TECHNIVIE. VIEKIRA PAK (ombitasvir, paritaprevir and ritonavir tablets; dasabuvir tablets) is an all-oral, short-course, interferon-free therapy, with or without ribavirin, for the treatment of adult patients with genotype 1 chronic HCV, including those with compensated cirrhosis. In Europe, VIEKIRA PAK is marketed as VIEKIRAX + EXVIERA and is approved for use in patients with genotype 1 and genotype 4 HCV. AbbVie's TECHNIVIE (ombitasvir, paritaprevir and
2  | 2019 Form 10-K | 2019 Form 10-K | ||
ritonavir) is FDA-approved for use in combination with ribavirin for the treatment of adults with genotype 4 HCV infection in the United States. The use of VIEKIRA in the United States, Europe and Japan is currently limited given the significant use of pangenotypic regimens, including MAVIRET.
Additional Virology products. AbbVie's additional virology products include:
SYNAGIS. SYNAGIS (palivizumab) is a product marketed by AbbVie outside of the United States that protects at-risk infants from severe respiratory disease caused by respiratory syncytial virus (RSV).
KALETRA. KALETRA (lopinavir/ritonavir), which is also marketed as ALUVIA in emerging markets, is a prescription anti-HIV-1 medicine that contains two protease inhibitors: lopinavir and ritonavir. KALETRA is used with other anti-HIV-1 medications as a treatment that maintains viral suppression in people with HIV-1.
Metabolics/Hormones products. Metabolic and hormone products target a number of conditions, including testosterone deficiency due to certain underlying conditions, exocrine pancreatic insufficiency and hypothyroidism. These products include:
CREON. CREON (pancrelipase) is a pancreatic enzyme therapy for exocrine pancreatic insufficiency, a condition that occurs in patients with cystic fibrosis, chronic pancreatitis and several other conditions.
Synthroid. Synthroid (levothyroxine sodium tablets, USP) is used in the treatment of hypothyroidism.
AndroGel. AndroGel (testosterone gel) is a testosterone replacement therapy for males diagnosed with symptomatic low testosterone due to certain underlying conditions.
AbbVie has the rights to sell AndroGel, CREON and Synthroid only in the United States.
Endocrinology products. Lupron (leuprolide acetate), which is also marketed as Lucrin and LUPRON DEPOT, is a product for the palliative treatment of advanced prostate cancer, treatment of endometriosis and central precocious puberty and for the preoperative treatment of patients with anemia caused by uterine fibroids. Lupron is approved for daily subcutaneous injection and one-month, three-month, four-month and six-month intramuscular injection.
Other products. AbbVie's other products include:
ORILISSA. ORILISSA (elagolix) is the first and only orally-administered, nonpeptide small molecule gonadotropin-releasing hormone (GnRH) antagonist specifically developed for women with moderate to severe endometriosis pain. The FDA approved ORILISSA under priority review. It represents the first FDA-approved oral treatment for the management of moderate to severe pain associated with endometriosis in over a decade. ORILISSA inhibits endogenous GnRH signaling by binding competitively to GnRH receptors in the pituitary gland. Administration results in dose-dependent suppression of luteinizing hormone and follicle-stimulating hormone, leading to decreased blood concentrations of ovarian sex hormones, estradiol and progesterone. Outside the United States, ORILISSA is also launched in Canada and Puerto Rico.
Duopa and Duodopa (carbidopa and levodopa). AbbVie's levodopa-carbidopa intestinal gel for the treatment of advanced Parkinson's disease is marketed as Duopa in the United States and as Duodopa outside of the United States.
Sevoflurane. Sevoflurane (sold under the trademarks Ultane and Sevorane) is an anesthesia product that AbbVie sells worldwide for human use.
Marketing, Sales and Distribution Capabilities
AbbVie utilizes a combination of dedicated commercial resources, regional commercial resources and distributorships to market, sell and distribute its products worldwide. AbbVie directs its primary marketing efforts toward securing the prescription, or recommendation, of its brand of products by physicians, key opinion leaders and other health care providers. Managed care providers (for example, health maintenance organizations and pharmacy benefit managers), hospitals and state and federal government agencies (for example, the United States Department of Veterans Affairs and the United States Department of Defense) are also important customers. AbbVie also markets directly to consumers themselves, although in the United States all of the company's products must be sold pursuant to a prescription. Outside of the United States, AbbVie focuses its marketing efforts on key opinion leaders, payers, physicians and country regulatory bodies. AbbVie also provides patient support programs closely related to its products.
AbbVie's products are generally sold worldwide directly to wholesalers, distributors, government agencies, health care facilities, specialty pharmacies and independent retailers from AbbVie-owned distribution centers and public warehouses. Although AbbVie's business does not have significant seasonality, AbbVie's product revenues may be affected by end customer and retail buying patterns, fluctuations in wholesaler inventory levels and other factors.
2019 Form 10-K |  3 3 | ||
In the United States, AbbVie distributes pharmaceutical products principally through independent wholesale distributors, with some sales directly to pharmacies and patients. In 2019, three wholesale distributors (McKesson Corporation, Cardinal Health, Inc. and AmerisourceBergen Corporation) accounted for substantially all of AbbVie's sales in the United States. No individual wholesaler accounted for greater than 42% of AbbVie's 2019 gross revenues in the United States. Outside the United States, AbbVie sells products primarily to customers or through distributors, depending on the market served. These wholesalers purchase product from AbbVie under standard terms and conditions of sale.
Certain products are co-marketed or co-promoted with other companies. AbbVie has no single customer that, if the customer were lost, would have a material adverse effect on the company's business. No material portion of AbbVie's business is subject to renegotiation of profits or termination of contracts at the election of the government. Orders are generally filled on a current basis and order backlog is not material to AbbVie's business.
Competition
The markets for AbbVie's products are highly competitive. AbbVie competes with other research-based pharmaceuticals and biotechnology companies that discover, manufacture, market and sell proprietary pharmaceutical products and biologics. For example, HUMIRA competes with anti-TNF products and other competitive products intended to treat a number of disease states and AbbVie's virology products compete with other available HCV treatment options. The search for technological innovations in pharmaceutical products is a significant aspect of competition. The introduction of new products by competitors and changes in medical practices and procedures can result in product obsolescence. Price is also a competitive factor. In addition, the substitution of generic pharmaceutical products for branded pharmaceutical products creates competitive pressures on AbbVie's products that do not have patent protection. New products or treatments brought to market by AbbVie’s competitors could cause revenues for AbbVie’s products to decrease due to price reductions and sales volume decreases.
Biosimilars. Competition for AbbVie’s biologic products is affected by the approval of follow-on biologics, also known as “biosimilars.” Biologics have added major therapeutic options for the treatment of many diseases, including some for which therapies were unavailable or inadequate. The cost of developing and producing biologic therapies is typically dramatically higher than for conventional (small molecule) medications, and many biologic medications are used for ongoing treatment of chronic diseases, such as rheumatoid arthritis or inflammatory bowel disease, or for the treatment of previously untreatable cancer. Significant investments in biologics infrastructure and manufacturing are necessary to produce biologic products.
HUMIRA is now facing direct biosimilar competition in Europe and other countries, and AbbVie will continue to face competitive pressure from these biologics and from orally administered products.
In the United States, the FDA regulates biologics under the Federal Food, Drug and Cosmetic Act, the Public Health Service Act and implementing regulations. The enactment of federal health care reform legislation in March 2010 provided a pathway for approval of biosimilars under the Public Health Service Act, but the approval process for, and science behind, biosimilars is complex. Approval by the FDA is dependent upon many factors, including a showing that the biosimilar is "highly similar" to the original product and has no clinically meaningful differences from the original product in terms of safety, purity and potency. The types of data that could ordinarily be required in an application to show similarity may include analytical data, bioequivalence studies and studies to demonstrate chemical similarity, animal studies (including toxicity studies) and clinical studies.
Furthermore, the law provides that only a biosimilar product that is determined to be "interchangeable" will be considered substitutable for the original biologic product without the intervention of the health care provider who prescribed the original biologic product. To prove that a biosimilar product is interchangeable, the applicant must demonstrate that the product can be expected to produce the same clinical results as the original biologic product in any given patient, and if the product is administered more than once in a patient, that safety risks and potential for diminished efficacy of alternating or switching between the use of the interchangeable biosimilar biologic product and the original biologic product is no greater than the risk of using the original biologic product without switching. The law continues to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning remains subject to substantial uncertainty.
Intellectual Property Protection and Regulatory Exclusivity
Generally, upon approval, products may be entitled to certain kinds of exclusivity under applicable intellectual property and regulatory regimes. AbbVie’s intellectual property is materially valuable to the company, and AbbVie seeks patent protection, where available, in all significant markets and/or countries for each product in development. In the United States, the expiration date for patents is 20 years after the filing date. Given that patents relating to pharmaceutical products are often obtained early in the development process and given the amount of time needed to complete clinical trials and other development activities required for regulatory approval, the length of time between product launch and patent expiration is significantly less than 20 years. The Drug Price Competition and Patent Term Restoration Act of 1984 (commonly known as the Hatch-Waxman Act) permits a patent holder to seek a patent extension, commonly called a “patent term restoration,” for
4  | 2019 Form 10-K | 2019 Form 10-K | ||
patents on products (or processes for making the product) regulated by the Federal Food, Drug, and Cosmetic Act. The length of the patent extension is roughly based on 50 percent of the period of time from the filing of an Investigational New Drug Application (NDA) for a compound to the submission of the NDA for such compound, plus 100 percent of the time period from NDA submission to regulatory approval. The extension, however, cannot exceed five years and the patent term remaining after regulatory approval cannot exceed 14 years. Biological products licensed under the Public Health Service Act are similarly eligible for terms of patent restoration.
Pharmaceutical products may be entitled to other forms of legal or regulatory exclusivity upon approval. The scope, length, and requirements for each of these exclusivities vary both in the United States and in other jurisdictions. In the United States, if the FDA approves a drug product that contains an active ingredient not previously approved, the product is typically entitled to five years of non-patent regulatory exclusivity. Other products may be entitled to three years of exclusivity if approval was based on the FDA’s reliance on new clinical studies essential to approval submitted by the NDA applicant. If the NDA applicant studies the product for use by children, the FDA may grant pediatric exclusivity, which extends by 180 days all existing exclusivities (patent and regulatory) related to the product. For products that are either used to treat conditions that afflict a relatively small population or for which there is not a reasonable expectation that the research and development costs will be recovered, the FDA may designate the pharmaceutical as an orphan drug and grant it seven years of market exclusivity.
Applicable laws and regulations dictate the scope of any exclusivity to which a product or particular characteristics of a product is entitled upon approval in any particular country. In certain instances, regulatory exclusivity may offer protection where patent protection is no longer available or for a period of time in excess of patent protection. It is not possible to estimate for each product in development the total period and scope of exclusivity to which it may become entitled until regulatory approval is obtained. However, given the length of time required to complete clinical development of a pharmaceutical product, the periods of exclusivity that might be achieved in any individual case would not be expected to exceed a minimum of three years and a maximum of 14 years. These estimates do not consider other factors, such as the difficulty of recreating the manufacturing process for a particular product or other proprietary knowledge that may delay the introduction of a generic or other follow-on product after the expiration of applicable patent and other regulatory exclusivity periods.
Biologics may be entitled to exclusivity under the Biologics Price Competition and Innovation Act, which was passed on March 23, 2010 as Title VII to the Patient Protection and Affordable Care Act. The law provides a pathway for approval of biosimilars following the expiration of 12 years of regulatory exclusivity for the innovator biologic and a potential additional 180 day-extension term for conducting pediatric studies. Biologics are also eligible for orphan drug exclusivity, as discussed above. The law also includes an extensive process for the innovator biologic and biosimilar manufacturer to litigate patent infringement, validity, and enforceability. The European Union has also created a pathway for approval of biosimilars and has published guidelines for approval of certain biosimilar products. The more complex nature of biologics and biosimilar products has led to close regulatory scrutiny over follow-on biosimilar products, which can reduce the effect of biosimilars on sales of the innovator biologic as compared to the sales erosion caused by generic versions of small molecule pharmaceutical products.
AbbVie owns or has licensed rights to a substantial number of patents and patent applications. AbbVie licenses or owns a patent portfolio of thousands of patent families, each of which includes United States patent applications and/or issued patents and may also contain the non-United States counterparts to these patents and applications.
These patents and applications, including various patents that expire during the period 2020 to the late 2030s, in aggregate are believed to be of material importance in the operation of AbbVie’s business. However, AbbVie believes that no single patent, license, trademark (or related group of patents, licenses, or trademarks), except for those related to adalimumab (which is sold under the trademark HUMIRA), are material in relation to the company’s business as a whole. The United States composition of matter (that is, compound) patent covering adalimumab expired in December 2016, and the equivalent European Union patent expired in October 2018 in the majority of European Union countries. In the United States, non-composition of matter patents covering adalimumab expire no earlier than 2022. AbbVie has entered into settlement and license agreements with several adalimumab biosimilar manufactures. Under the agreements, the license in the United States will begin in 2023 and the license in Europe began in 2018.
In addition, the following patents, licenses, and trademarks are significant: those related to ibrutinib (which is sold under the trademark IMBRUVICA) and those related to glecaprevir and pibrentasvir (which are sold under the trademarks MAVYRET and MAVIRET). The United States composition of matter patent covering ibrutinib is expected to expire in 2027. The United States composition of matter patents covering glecaprevir and pibrentasvir are expected to expire in 2032.
AbbVie may rely, in some circumstances, on trade secrets to protect its technology. However, trade secrets are difficult to protect. AbbVie seeks to protect its technology and product candidates, in part, by confidentiality agreements with its employees, consultants, advisors, contractors, and collaborators. These agreements may be breached and AbbVie may not have adequate remedies for any breach. In addition, AbbVie’s trade secrets may otherwise become known or be independently
2019 Form 10-K |  5 5 | ||
discovered by competitors. To the extent that AbbVie’s employees, consultants, advisors, contractors, and collaborators use intellectual property owned by others in their work for the company, disputes may arise as to the rights in related or resulting know-how and inventions.
Licensing, Acquisitions and Other Arrangements
In addition to its independent efforts to develop and market products, AbbVie enters into arrangements such as acquisitions, option-to-acquire agreements, licensing arrangements, option-to-license arrangements, strategic alliances, co-promotion arrangements, co-development and co-marketing agreements, and joint ventures. The acquisitions and option-to-acquire agreements typically include, among other terms and conditions, non-refundable purchase price payments or option fees, option exercise payments, milestones or earn-outs, and other customary terms and obligations. The licensing and other arrangements typically include, among other terms and conditions, non-refundable upfront license fees, option fees and option exercise payments, milestone payments and royalty and/or profit sharing obligations. See Note 5, "Licensing, Acquisitions and Other Arrangements—Other Licensing & Acquisitions Activity," to the Consolidated Financial Statements included under Item 8, "Financial Statements and Supplementary Data."
Third Party Agreements
AbbVie has agreements with third parties for process development, product distribution, analytical services and manufacturing of certain products. AbbVie procures certain products and services from a limited number of suppliers and, in some cases, a single supply source. In addition, AbbVie has agreements with third parties for active pharmaceutical ingredient and product manufacturing, formulation and development services, fill, finish and packaging services, transportation and distribution and logistics services for certain products. AbbVie does not believe that these manufacturing related agreements are material because AbbVie's business is not substantially dependent on any individual agreement. In most cases, AbbVie maintains alternate supply relationships that it can utilize without undue disruption of its manufacturing processes if a third party fails to perform its contractual obligations. AbbVie also maintains sufficient inventory of product to minimize the impact of any supply disruption.
AbbVie is also party to certain collaborations and other arrangements, as discussed in Note 5, "Licensing, Acquisitions and Other Arrangements—Other Licensing & Acquisitions Activity," to the Consolidated Financial Statements included under Item 8, "Financial Statements and Supplementary Data."
Sources and Availability of Raw Materials
AbbVie purchases, in the ordinary course of business, raw materials and supplies essential to its operations from numerous suppliers around the world. In addition, certain medical devices and components necessary for the manufacture of AbbVie products are provided by unaffiliated third party suppliers. AbbVie has not experienced any recent significant availability problems or supply shortages that impacted fulfillment of product demand.
Research and Development Activities
AbbVie makes a significant investment in research and development and has numerous compounds in clinical development, including potential treatments for complex, life-threatening diseases. AbbVie's ability to discover and develop new compounds is enhanced by the company's use of integrated discovery and development project teams, which include chemists, biologists, physicians and pharmacologists who work on the same compounds as a team. AbbVie also partners with third parties, such as biotechnology companies, other pharmaceutical companies and academic institutions to identify and prioritize promising new treatments that complement and enhance AbbVie’s existing portfolio.
The research and development process generally begins with discovery research which focuses on the identification of a molecule that has a desired effect against a given disease. If preclinical testing of an identified compound proves successful, the compound moves into clinical development which generally includes the following phases:
• | Phase 1—involves the first human tests in a small number of healthy volunteers or patients to assess safety, tolerability and potential dosing. |
• | Phase 2—tests the drug's efficacy against the disease in a relatively small group of patients. |
• | Phase 3—tests a drug that demonstrates favorable results in the earlier phases in a significantly larger patient population to further demonstrate efficacy and safety based on regulatory criteria. |
The clinical trials from all of the development phases provide the data required to prepare and submit an NDA, a Biological License Application (BLA) or other submission for regulatory approval to the FDA or similar government agencies outside the United States. The specific requirements (e.g., scope of clinical trials) for obtaining regulatory approval vary across different countries and geographic regions.
6  | 2019 Form 10-K | 2019 Form 10-K | ||
The research and development process from discovery through a new drug launch typically takes 8 to 12 years and can be even longer. The research and development of new pharmaceutical products has a significant amount of inherent uncertainty. There is no guarantee when, or if, a molecule will receive the regulatory approval required to launch a new drug or indication.
In addition to the development of new products and new formulations, research and development projects also may include Phase 4 trials, sometimes called post-marketing studies. For such projects, clinical trials are designed and conducted to collect additional data regarding, among other parameters, the benefits and risks of an approved drug.
Regulation—Discovery and Clinical Development
United States. Securing approval to market a new pharmaceutical product in the United States requires substantial effort and financial resources and takes several years to complete. The applicant must complete preclinical tests and submit protocols to the FDA before commencing clinical trials. Clinical trials are intended to establish the safety and efficacy of the pharmaceutical product and typically are conducted in sequential phases, although the phases may overlap or be combined. If the required clinical testing is successful, the results are submitted to the FDA in the form of an NDA or BLA requesting approval to market the product for one or more indications. The FDA reviews an NDA or BLA to determine whether a product is safe and effective for its intended use and whether its manufacturing is compliant with current Good Manufacturing Practices (cGMP).
Even if an NDA or a BLA receives approval, the applicant must comply with post-approval requirements. For example, holders of an approval must report adverse reactions, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional materials and activities. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval, and certain changes to the manufacturing procedures and finished product must be included in the NDA or BLA and approved by the FDA prior to implementation. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural and record keeping requirements. In addition, as a condition of approval, the FDA may require post-marketing testing and surveillance to further assess and monitor the product's safety or efficacy after commercialization, which may require additional clinical trials, patient registries, observational data or additional work on chemistry, manufacturing and controls. Any post-approval regulatory obligations, and the cost of complying with such obligations, could expand in the future.
Outside the United States. AbbVie is subject to similar regulatory requirements outside the United States for approval and marketing of pharmaceutical products. AbbVie must obtain approval of a clinical trial application or product from the applicable regulatory authorities before it can commence clinical trials or marketing of the product. The approval requirements and process for each country can vary, and the time required to obtain approval may be longer or shorter than that required for FDA approval in the United States. For example, AbbVie may submit marketing authorizations in the European Union under either a centralized or decentralized procedure. The centralized procedure is mandatory for the approval of biotechnology products and many pharmaceutical products and provides for a single marketing authorization that is valid for all European Union member states. Under the centralized procedure, a single marketing authorization application is submitted to the European Medicines Agency (EMA). After the agency evaluates the application, it makes a recommendation to the European Commission, which then makes the final determination on whether to approve the application. The decentralized procedure provides for mutual recognition of individual national approval decisions and is available for products that are not subject to the centralized procedure.
In Japan, applications for approval of a new product are made through the Pharmaceutical and Medical Devices Agency (PMDA). Bridging studies to demonstrate that the non-Japanese clinical data applies to Japanese patients may be required. After completing a comprehensive review, the PMDA reports to the Ministry of Health, Labour and Welfare, which then approves or denies the application.
The regulatory process in many emerging markets continues to evolve. Many emerging markets, including those in Asia, generally require regulatory approval to have been obtained in a large developed market (such as the United States or Europe) before the country will begin or complete its regulatory review process. Some countries also require that local clinical studies be conducted in order to obtain regulatory approval in the country.
The requirements governing the conduct of clinical trials and product licensing also vary. In addition, post-approval regulatory obligations such as adverse event reporting and cGMP compliance generally apply and may vary by country. For example, after a marketing authorization has been granted in the European Union, periodic safety reports must be submitted and other pharmacovigilance measures may be required (such as Risk Management Plans).
Regulation—Commercialization, Distribution and Manufacturing
The manufacture, marketing, sale, promotion and distribution of AbbVie's products are subject to comprehensive government regulation. Government regulation by various national, regional, federal, state and local agencies, both in the United States and other countries, addresses (among other matters) inspection of, and controls over, research and laboratory
2019 Form 10-K |  7 7 | ||
procedures, clinical investigations, product approvals and manufacturing, labeling, packaging, marketing and promotion, pricing and reimbursement, sampling, distribution, quality control, post-marketing surveillance, record keeping, storage and disposal practices. AbbVie's operations are also affected by trade regulations in many countries that limit the import of raw materials and finished products and by laws and regulations that seek to prevent corruption and bribery in the marketplace (including the United States Foreign Corrupt Practices Act and the United Kingdom Bribery Act, which provide guidance on corporate interactions with government officials) and require safeguards for the protection of personal data. In addition, AbbVie is subject to laws and regulations pertaining to health care fraud and abuse, including state and federal anti-kickback and false claims laws in the United States. Prescription drug manufacturers such as AbbVie are also subject to taxes, as well as application, product, user and other fees.
Compliance with these laws and regulations is costly and materially affects AbbVie's business. Among other effects, health care regulations substantially increase the time, difficulty and costs incurred in obtaining and maintaining approval to market newly developed and existing products. AbbVie expects compliance with these regulations to continue to require significant technical expertise and capital investment to ensure compliance. Failure to comply can delay the release of a new product or result in regulatory and enforcement actions, the seizure or recall of a product, the suspension or revocation of the authority necessary for a product's production and sale and other civil or criminal sanctions, including fines and penalties.
In addition to regulatory initiatives, AbbVie's business can be affected by ongoing studies of the utilization, safety, efficacy and outcomes of health care products and their components that are regularly conducted by industry participants, government agencies and others. These studies can call into question the utilization, safety and efficacy of previously marketed products. In some cases, these studies have resulted, and may in the future result, in the discontinuance of, or limitations on, marketing of such products domestically or worldwide, and may give rise to claims for damages from persons who believe they have been injured as a result of their use.
Access to human health care products continues to be a subject of oversight, investigation and action by governmental agencies, legislative bodies and private organizations in the United States and other countries. A major focus is cost containment. Efforts to reduce health care costs are also being made in the private sector, notably by health care payers and providers, which have instituted various cost reduction and containment measures. AbbVie expects insurers and providers to continue attempts to reduce the cost of health care products. Outside the United States, many countries control the price of health care products directly or indirectly, through reimbursement, payment, pricing, coverage limitations, or compulsory licensing. Political and budgetary pressures in the United States and in other countries may also heighten the scope and severity of pricing pressures on AbbVie's products for the foreseeable future.
United States. Specifically, U.S. federal laws require pharmaceutical manufacturers to pay certain statutorily-prescribed rebates to state Medicaid programs on prescription drugs reimbursed under state Medicaid plans, and the efforts by states to seek additional rebates affect AbbVie's business. Similarly, the Veterans Health Care Act of 1992, as a prerequisite to participation in Medicaid and other federal health care programs, requires that manufacturers extend additional discounts on pharmaceutical products to various federal agencies, including the United States Department of Veterans Affairs, Department of Defense and Public Health Service entities and institutions. In addition, recent legislative changes would require similarly discounted prices to be offered to TRICARE program beneficiaries. The Veterans Health Care Act of 1992 also established the 340B drug discount program, which requires pharmaceutical manufacturers to provide products at reduced prices to various designated health care entities and facilities.
In the United States, most states also have generic substitution legislation requiring or permitting a dispensing pharmacist to substitute a different manufacturer's generic version of a pharmaceutical product for the one prescribed. In addition, the federal government follows a diagnosis-related group (DRG) payment system for certain institutional services provided under Medicare or Medicaid and has implemented a prospective payment system (PPS) for services delivered in hospital outpatient, nursing home and home health settings. DRG and PPS entitle a health care facility to a fixed reimbursement based on the diagnosis and/or procedure rather than actual costs incurred in patient treatment, thereby increasing the incentive for the facility to limit or control expenditures for many health care products. Medicare reimburses Part B drugs based on average sales price plus a certain percentage to account for physician administration costs, which have been reduced in the hospital outpatient setting. Medicare enters into contracts with private plans to negotiate prices for most patient-administered medicine delivered under Part D.
Under the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act (together, the Affordable Care Act), AbbVie pays a fee related to its pharmaceuticals sales to government programs. In addition, AbbVie provides a discount of 50% for branded prescription drugs sold to patients who fall into the Medicare Part D coverage gap, or "donut hole."
The Affordable Care Act also includes provisions known as the Physician Payments Sunshine Act, which require manufacturers of drugs and biologics covered under Medicare and Medicaid to record any transfers of value to physicians and
8  | 2019 Form 10-K | 2019 Form 10-K | ||
teaching hospitals and to report this data to the Centers for Medicare and Medicaid Services for subsequent public disclosure. Similar reporting requirements have also been enacted on the state level in the United States, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring disclosure of interactions with health care professionals. Failure to report appropriate data may result in civil or criminal fines and/or penalties.
AbbVie expects debate to continue during 2020 at all government levels worldwide over the marketing, availability, method of delivery and payment for health care products and services. AbbVie believes that future legislation and regulation in the markets it serves could affect access to health care products and services, increase rebates, reduce prices or the rate of price increases for health care products and services, change health care delivery systems, create new fees and obligations for the pharmaceuticals industry, or require additional reporting and disclosure. It is not possible to predict the extent to which AbbVie or the health care industry in general might be affected by the matters discussed above.
European Union. The European Union has adopted directives and other legislation governing labeling, advertising, distribution, supply, pharmacovigilance and marketing of pharmaceutical products. Such legislation provides mandatory standards throughout the European Union and permits member states to supplement these standards with additional regulations. European governments also regulate pharmaceutical product prices through their control of national health care systems that fund a large part of the cost of such products to consumers. As a result, patients are unlikely to use a pharmaceutical product that is not reimbursed by the government. In many European countries, the government either regulates the pricing of a new product at launch or subsequent to launch through direct price controls or reference pricing. In recent years, many countries have also imposed new or additional cost containment measures on pharmaceutical products. Differences between national pricing regimes create price differentials within the European Union that can lead to significant parallel trade in pharmaceutical products.
Most governments also promote generic substitution by mandating or permitting a pharmacist to substitute a different manufacturer's generic version of a pharmaceutical product for the one prescribed and by permitting or mandating that health care professionals prescribe generic versions in certain circumstances. Many governments are also following a similar path for biosimilar therapies. In addition, governments use reimbursement lists to limit the pharmaceutical products that are eligible for reimbursement by national health care systems.
Japan. In Japan, the National Health Insurance system maintains a Drug Price List specifying which pharmaceutical products are eligible for reimbursement, and the Ministry of Health, Labour and Welfare sets the prices of the products on this list. The government generally introduces price cut rounds every other year and also mandates price decreases for specific products. New products judged innovative or useful, that are indicated for pediatric use, or that target orphan or small population diseases, however, may be eligible for a pricing premium. The government has also promoted the use of generics, where available.
Emerging Markets. Many emerging markets take steps to reduce pharmaceutical product prices, in some cases through direct price controls and in others through the promotion of generic/biosimilar alternatives to branded pharmaceuticals.
Since AbbVie markets its products worldwide, certain products of a local nature and variations of product lines must also meet other local regulatory requirements. Certain additional risks are inherent in conducting business outside the United States, including price and currency exchange controls, changes in currency exchange rates, limitations on participation in local enterprises, expropriation, nationalization and other governmental action.
Environmental Matters
AbbVie believes that its operations comply in all material respects with applicable laws and regulations concerning environmental protection. Regulations under federal and state environmental laws impose stringent limitations on emissions and discharges to the environment from various manufacturing operations. AbbVie's capital expenditures for pollution control in 2019 were approximately $29 million and operating expenditures were approximately $34 million. In 2020, capital expenditures for pollution control are estimated to be approximately $5 million and operating expenditures are estimated to be approximately $35 million.
Abbott was identified as one of many potentially responsible parties in investigations and/or remediations at several locations in the United States, including Puerto Rico, under the Comprehensive Environmental Response, Compensation and Liability Act, commonly known as Superfund. Some of these locations were transferred to AbbVie in connection with the separation and distribution, and AbbVie has become a party to these investigations and remediations. Abbott was also engaged in remediation at several other sites, some of which have been transferred to AbbVie in connection with the separation and distribution, in cooperation with the Environmental Protection Agency or similar agencies. While it is not feasible to predict with certainty the final costs related to those investigations and remediation activities, AbbVie believes that such costs, together with other expenditures to maintain compliance with applicable laws and regulations concerning environmental protection, should not have a material adverse effect on the company's financial position, cash flows, or results of operations.
2019 Form 10-K |  9 9 | ||
Employees
AbbVie employed approximately 30,000 persons as of January 31, 2020. Outside the United States, some of AbbVie's employees are represented by unions or works councils. AbbVie believes that it has good relations with its employees.
Internet Information
Copies of AbbVie's Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 are available free of charge through AbbVie's investor relations website (www.abbvieinvestor.com) as soon as reasonably practicable after AbbVie electronically files the material with, or furnishes it to, the Securities and Exchange Commission (SEC).
AbbVie's corporate governance guidelines, outline of directorship qualifications, code of business conduct and the charters of AbbVie's audit committee, compensation committee, nominations and governance committee and public policy committee are all available on AbbVie's investor relations website (www.abbvieinvestor.com).
ITEM 1A. RISK FACTORS
You should carefully consider the following risks and other information in this Form 10-K in evaluating AbbVie and AbbVie's common stock. Any of the following risks could materially and adversely affect AbbVie's results of operations, financial condition or cash flows. The risk factors generally have been separated into three groups: risks related to AbbVie's business, risks related to AbbVie's proposed acquisition of Allergan (the "Acquisition") and the combined company upon completion of the Acquisition, and risks related to AbbVie's common stock. Based on the information currently known to it, AbbVie believes that the following information identifies the most significant risk factors affecting it in each of these categories of risks. However, the risks and uncertainties AbbVie faces are not limited to those set forth in the risk factors described below and may not be in order of importance or probability of occurrence. Additional risks and uncertainties not presently known to AbbVie or that AbbVie currently believes to be immaterial may also adversely affect its business. In addition, past financial performance may not be a reliable indicator of future performance and historical trends should not be used to anticipate results or trends in future periods.
If any of the following risks and uncertainties develops into actual events, these events could have a material adverse effect on AbbVie's business, results of operations, financial condition or cash flows. In such case, the trading price of AbbVie's common stock could decline.
Risks Related to AbbVie's Business
The expiration or loss of patent protection and licenses may adversely affect AbbVie's future revenues and operating earnings.
AbbVie relies on patent, trademark and other intellectual property protection in the discovery, development, manufacturing and sale of its products. In particular, patent protection is, in the aggregate, important in AbbVie's marketing of pharmaceutical products in the United States and most major markets outside of the United States. Patents covering AbbVie products normally provide market exclusivity, which is important for the profitability of many of AbbVie's products.
As patents for certain of its products expire, AbbVie will or could face competition from lower priced generic or biosimilar products. The expiration or loss of patent protection for a product typically is followed promptly by substitutes that may significantly reduce sales for that product in a short amount of time. If AbbVie's competitive position is compromised because of generics, biosimilars or otherwise, it could have a material adverse effect on AbbVie's business and results of operations. In addition, proposals emerge from time to time for legislation to further encourage the early and rapid approval of generic drugs or biosimilars. Any such proposals that are enacted into law could increase the impact of generic competition.
AbbVie's principal patents and trademarks are described in greater detail in Item 1, "Business—Intellectual Property Protection and Regulatory Exclusivity" and Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations," and litigation regarding these patents is described in Item 3, "Legal Proceedings." The United States composition of matter patent for HUMIRA, which is AbbVie's largest product and had worldwide net revenues of approximately $19.2 billion in 2019, expired in December 2016, and the equivalent European Union patent expired in the majority of European Union countries in October 2018.
10  | 2019 Form 10-K | 2019 Form 10-K | ||
AbbVie's major products could lose patent protection earlier than expected, which could adversely affect AbbVie's future revenues and operating earnings.
Third parties or government authorities may challenge or seek to invalidate or circumvent AbbVie's patents and patent applications. For example, manufacturers of generic pharmaceutical products file, and may continue to file, Abbreviated New Drug Applications with the FDA seeking to market generic forms of AbbVie's products prior to the expiration of relevant patents owned or licensed by AbbVie by asserting that the patents are invalid, unenforceable and/or not infringed. In addition, petitioners have filed, and may continue to file, challenges to the validity of AbbVie patents under the 2011 Leahy-Smith America Invents Act, which created inter partes review and post grant review procedures for challenging patent validity in administrative proceedings at the United States Patent and Trademark Office.
Although most of the challenges to AbbVie's intellectual property have come from other businesses, governments may also challenge intellectual property rights. For example, court decisions and potential legislation relating to patents, such as legislation regarding biosimilars, and other regulatory initiatives may result in further erosion of intellectual property protection. In addition, certain governments outside the United States have indicated that compulsory licenses to patents may be sought to further their domestic policies or on the basis of national emergencies, such as HIV/AIDS. If triggered, compulsory licenses could diminish or eliminate sales and profits from those jurisdictions and negatively affect AbbVie's results of operations.
AbbVie normally responds to challenges by vigorously defending its patents, including by filing patent infringement lawsuits. Patent litigation, administrative proceedings and other challenges to AbbVie's patents are costly and unpredictable and may deprive AbbVie of market exclusivity for a patented product. To the extent AbbVie's intellectual property is successfully challenged, circumvented or weakened, or to the extent such intellectual property does not allow AbbVie to compete effectively, AbbVie's business will suffer. To the extent that countries do not enforce AbbVie's intellectual property rights or require compulsory licensing of AbbVie's intellectual property, AbbVie's future revenues and operating earnings will be reduced.
A third party's intellectual property may prevent AbbVie from selling its products or have a material adverse effect on AbbVie's future profitability and financial condition.
Third parties may claim that an AbbVie product infringes upon their intellectual property. Resolving an intellectual property infringement claim can be costly and time consuming and may require AbbVie to enter into license agreements. AbbVie cannot guarantee that it would be able to obtain license agreements on commercially reasonable terms. A successful claim of patent or other intellectual property infringement could subject AbbVie to significant damages or an injunction preventing the manufacture, sale, or use of the affected AbbVie product or products. Any of these events could have a material adverse effect on AbbVie's profitability and financial condition.
Any significant event that adversely affects HUMIRA revenues could have a material and negative impact on AbbVie's results of operations and cash flows.
HUMIRA accounted for approximately 58% of AbbVie's total net revenues in 2019. Any significant event that adversely affects HUMIRA's revenues could have a material adverse impact on AbbVie's results of operations and cash flows. These events could include loss of patent protection for HUMIRA (as described further in “—The expiration or loss of patent protection and licenses may adversely affect AbbVie’s future revenues and operating earnings” above), the commercialization of biosimilars of HUMIRA, the discovery of previously unknown side effects or impaired efficacy, increased competition from the introduction of new, more effective or less expensive treatments and discontinuation or removal from the market of HUMIRA for any reason.
AbbVie's research and development efforts may not succeed in developing and marketing commercially successful products and technologies, which may cause its revenues and profitability to decline.
To remain competitive, AbbVie must continue to launch new products and new indications and/or brand extensions for existing products, and such launches must generate revenue sufficient both to cover its substantial research and development costs and to replace revenues of profitable products that are lost to or displaced by competing products or therapies. Failure to do so would have a material adverse effect on AbbVie's revenue and profitability. Accordingly, AbbVie commits substantial effort, funds, and other resources to research and development and must make ongoing substantial expenditures without any assurance that its efforts will be commercially successful. A high rate of failure in the biopharmaceutical industry is inherent in the research and development of new products, and failure can occur at any point in the research and development process,
2019 Form 10-K |  11 11 | ||
including after significant funds have been invested. Products that appear promising in development may fail to reach the market for numerous reasons, including failure to demonstrate effectiveness, safety concerns, superior safety or efficacy of competing therapies, failure to achieve positive clinical or pre-clinical outcomes beyond the current standards of care, inability to obtain necessary regulatory approvals or delays in the approval of new products and new indications, limited scope of approved uses, excessive costs to manufacture, the failure to obtain or maintain intellectual property rights, or infringement of the intellectual property rights of others.
Decisions about research studies made early in the development process of a pharmaceutical product candidate can affect the marketing strategy once such candidate receives approval. More detailed studies may demonstrate additional benefits that can help in the marketing, but they also consume time and resources and may delay submitting the pharmaceutical product candidate for approval. AbbVie cannot guarantee that a proper balance of speed and testing will be made with respect to each pharmaceutical product candidate or that decisions in this area would not adversely affect AbbVie's future results of operations.
Even if AbbVie successfully develops and markets new products or enhancements to its existing products, they may be quickly rendered obsolete by changing clinical preferences, changing industry standards, or competitors' innovations. AbbVie's innovations may not be accepted quickly in the marketplace because of existing clinical practices or uncertainty over third-party reimbursement. AbbVie cannot state with certainty when or whether any of its products under development will be launched, whether it will be able to develop, license, or otherwise acquire compounds or products, or whether any products will be commercially successful. Failure to launch successful new products or new indications for existing products may cause AbbVie's products to become obsolete, causing AbbVie's revenues and operating results to suffer.
A portion of AbbVie's near-term pharmaceutical pipeline relies on collaborations with third parties, which may adversely affect the development and sale of its products.
AbbVie depends on alliances with pharmaceutical and biotechnology companies for a portion of the products in its near-term pharmaceutical pipeline. Failures by these parties to meet their contractual, regulatory, or other obligations to AbbVie, or any disruption in the relationships between AbbVie and these third parties, could have an adverse effect on AbbVie's pharmaceutical pipeline and business. In addition, AbbVie's collaborative relationships for research and development extend for many years and may give rise to disputes regarding the relative rights, obligations and revenues of AbbVie and its collaboration partners, including the ownership of intellectual property and associated rights and obligations. This could result in the loss of intellectual property rights or protection, delay the development and sale of potential pharmaceutical products and lead to lengthy and expensive litigation, administrative proceedings or arbitration.
Biologics carry unique risks and uncertainties, which could have a negative impact on future results of operations.
The successful discovery, development, manufacturing and sale of biologics is a long, expensive and uncertain process. There are unique risks and uncertainties with biologics. For example, access to and supply of necessary biological materials, such as cell lines, may be limited and governmental regulations restrict access to and regulate the transport and use of such materials. In addition, the development, manufacturing and sale of biologics is subject to regulations that are often more complex and extensive than the regulations applicable to other pharmaceutical products. Manufacturing biologics, especially in large quantities, is often complex and may require the use of innovative technologies. Such manufacturing also requires facilities specifically designed and validated for this purpose and sophisticated quality assurance and quality control procedures. Biologics are also frequently costly to manufacture because production inputs are derived from living animal or plant material, and some biologics cannot be made synthetically. Failure to successfully discover, develop, manufacture and sell biologics—including HUMIRA—could adversely impact AbbVie's business and results of operations.
AbbVie's biologic products are subject to competition from biosimilars.
The Biologics Price Competition and Innovation Act creates a framework for the approval of biosimilars in the United States and could allow competitors to reference data from biologic products already approved. In Europe, the European Commission has granted marketing authorizations for several biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued over the past few years. In addition, companies are developing biosimilars in other countries that could and do compete with AbbVie’s biologic products, including HUMIRA. As competitors obtain marketing approval for biosimilars referencing AbbVie’s biologic products, AbbVie’s products may become subject to competition from such biosimilars, with the attendant competitive pressure and consequences. Expiration or successful challenge of AbbVie’s applicable patent rights could also trigger competition from other products, assuming any relevant
12  | 2019 Form 10-K | 2019 Form 10-K | ||
exclusivity period has expired. As a result, AbbVie could face more litigation and administrative proceedings with respect to the validity and/or scope of patents relating to its biologic products.
New products and technological advances by AbbVie's competitors may negatively affect AbbVie's results of operations.
AbbVie competes with other research-based pharmaceutical and biotechnology companies that discover, manufacture, market and sell proprietary pharmaceutical products and biologics. For example, HUMIRA competes with anti-TNF products and other competitive products intended to treat a number of disease states and AbbVie’s virology products compete with other available hepatitis C treatment options. These competitors may introduce new products or develop technological advances that compete with AbbVie’s products in therapeutic areas such as immunology, virology/liver disease, oncology and neuroscience. AbbVie cannot predict with certainty the timing or impact of the introduction by competitors of new products or technological advances. Such competing products may be safer, more effective, more effectively marketed or sold, or have lower prices or superior performance features than AbbVie’s products, and this could negatively impact AbbVie’s business and results of operations.
The manufacture of many of AbbVie's products is a highly exacting and complex process, and if AbbVie or one of its suppliers encounters problems manufacturing AbbVie's products, AbbVie's business could suffer.
The manufacture of many of AbbVie's products is a highly exacting and complex process, due in part to strict regulatory requirements. Problems may arise during manufacturing for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with raw materials, delays related to the construction of new facilities or the expansion of existing facilities, including those intended to support future demand for AbbVie's products, changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in the types of products produced, physical limitations that could inhibit continuous supply, man-made or natural disasters and environmental factors. If problems arise during the production of a batch of product, that batch of product may have to be discarded and AbbVie may experience product shortages or incur added expenses. This could, among other things, lead to increased costs, lost revenue, damage to customer relations, time and expense spent investigating the cause and, depending on the cause, similar losses with respect to other batches or products. If problems are not discovered before the product is released to the market, recall and product liability costs may also be incurred.
AbbVie uses a number of products in its pharmaceutical and biologic manufacturing processes that are sourced from single suppliers, and an interruption in the supply of those products could adversely affect AbbVie's business and results of operations.
AbbVie uses a number of products in its pharmaceutical and biologic manufacturing processes that are sourced from single suppliers. The failure of these single-source suppliers to fulfill their contractual obligations in a timely manner or as a result of regulatory noncompliance or physical disruption at a manufacturing site may impair AbbVie's ability to deliver its products to customers on a timely and competitive basis, which could adversely affect AbbVie's business and results of operations. Finding an alternative supplier could take a significant amount of time and involve significant expense due to the nature of the products and the need to obtain regulatory approvals. AbbVie cannot guarantee that it will be able to reach agreement with alternative providers or that regulatory authorities would approve AbbVie's use of such alternatives. AbbVie does, however, carry business interruption insurance, which provides a degree of protection in the case of a failure by a single-source supplier.
Significant safety or efficacy issues could arise for AbbVie's products, which could have a material adverse effect on AbbVie's revenues and financial condition.
Pharmaceutical products receive regulatory approval based on data obtained in controlled clinical trials of limited duration. Following regulatory approval, these products will be used over longer periods of time in many patients. Investigators may also conduct additional, and perhaps more extensive, studies. If new safety or efficacy issues are reported or if new scientific information becomes available (including results of post-marketing Phase 4 trials), or if governments change standards regarding safety, efficacy or labeling, AbbVie may be required to amend the conditions of use for a product. For example, AbbVie may voluntarily provide or be required to provide updated information on a product's label or narrow its approved indication, either of which could reduce the product's market acceptance. If safety or efficacy issues with an AbbVie product arise, sales of the product could be halted by AbbVie or by regulatory authorities and regulatory action could be taken
2019 Form 10-K |  13 13 | ||
by such regulatory authorities. Safety or efficacy issues affecting suppliers' or competitors' products also may reduce the market acceptance of AbbVie's products.
New data about AbbVie's products, or products similar to its products, could negatively impact demand for AbbVie's products due to real or perceived safety issues or uncertainty regarding efficacy and, in some cases, could result in product withdrawal. Furthermore, new data and information, including information about product misuse, may lead government agencies, professional societies, practice management groups or organizations involved with various diseases to publish guidelines or recommendations related to the use of AbbVie's products or the use of related therapies or place restrictions on sales. Such guidelines or recommendations may lead to lower sales of AbbVie's products.
AbbVie is subject to product liability claims and other lawsuits that may adversely affect its business and results of operations.
In the ordinary course of business, AbbVie is the subject of product liability claims and lawsuits alleging that AbbVie's products or the products of other companies that it promotes have resulted or could result in an unsafe condition for or injury to patients. Product liability claims and lawsuits and safety alerts or product recalls, regardless of their ultimate outcome, may have a material adverse effect on AbbVie's business, results of operations and reputation and on its ability to attract and retain customers. Consequences may also include additional costs, a decrease in market share for the product in question, lower income and exposure to other claims. Product liability losses are self-insured.
AbbVie is also the subject of other claims, legal proceedings and investigations in the ordinary course of business, which relate to the intellectual property, commercial, securities and other matters. Adverse outcomes in such claims, legal proceedings and investigations may also adversely affect AbbVie’s business and results of operations.
AbbVie is subject to cost-containment efforts and pricing pressures that could cause a reduction in future revenues and operating earnings, and changes in the terms of rebate and chargeback programs, which are common in the pharmaceuticals industry, could have a material adverse effect on AbbVie's operations.
Cost-containment efforts by governments and private organizations are described in greater detail in Item 1, "Business—Regulation—Commercialization, Distribution and Manufacturing." To the extent these cost containment efforts are not offset by greater demand, increased patient access to health care, or other factors, AbbVie's future revenues and operating earnings will be reduced. In the United States, the European Union and other countries, AbbVie's business has experienced downward pressure on product pricing, and this pressure could increase in the future.
AbbVie is subject to increasing public and legislative pressure with respect to pharmaceutical pricing. In the United States, practices of managed care groups, and institutional and governmental purchasers, and United States federal laws and regulations related to Medicare and Medicaid, including the Medicare Prescription Drug Improvement and Modernization Act of 2003 and the Patient Protection and Affordable Care Act, contribute to pricing pressures. The potential for continuing changes to the health care system in the United States and the increased purchasing power of entities that negotiate on behalf of Medicare, Medicaid and private sector beneficiaries could result in additional pricing pressures.
In numerous major markets worldwide, the government plays a significant role in funding health care services and determining the pricing and reimbursement of pharmaceutical products. Consequently, in those markets, AbbVie is subject to government decision-making and budgetary actions with respect to its products. In particular, many European countries have ongoing government-mandated price reductions for many pharmaceutical products, and AbbVie anticipates continuing pricing pressures in Europe. Differences between countries in pricing regulations could lead to third-party cross-border trading in AbbVie's products that results in a reduction in future revenues and operating earnings.
Rebates related to government programs, such as fee-for-service Medicaid or Medicaid managed care programs, arise from laws and regulations. AbbVie cannot predict if additional government initiatives to contain health care costs or other factors could lead to new or modified regulatory requirements that include higher or incremental rebates or discounts. Other rebate and discount programs arise from contractual agreements with private payers. Various factors, including market factors and the ability of private payers to control patient access to products, may provide payers the leverage to negotiate higher or additional rebates or discounts that could have a material adverse effect on AbbVie's operations.
14  | 2019 Form 10-K | 2019 Form 10-K | ||
AbbVie is subject to numerous governmental regulations, and it can be costly to comply with these regulations and to develop compliant products and processes.
AbbVie's products are subject to rigorous regulation by numerous international, supranational, federal and state authorities, as described in Item 1, "Business—Regulation—Discovery and Clinical Development." The process of obtaining regulatory approvals to market a pharmaceutical product can be costly and time consuming, and approvals might not be granted for future products, or additional indications or uses of existing products, on a timely basis, if at all. Delays in the receipt of, or failure to obtain approvals for, future products, or new indications and uses, could result in delayed realization of product revenues, reduction in revenues and substantial additional costs.
In addition, AbbVie cannot guarantee that it will remain compliant with applicable regulatory requirements once approval has been obtained for a product. These requirements include, among other things, regulations regarding manufacturing practices, product labeling and advertising and post-marketing reporting, including adverse event reports and field alerts due to manufacturing quality concerns. AbbVie must incur expense and spend time and effort to ensure compliance with these complex regulations.
Possible regulatory actions could result in substantial modifications to AbbVie's business practices and operations; refunds, recalls or seizures of AbbVie's products; a total or partial shutdown of production in one or more of AbbVie's or its suppliers' facilities while AbbVie or its supplier remedies the alleged violation; the inability to obtain future approvals; and withdrawals or suspensions of current products from the market. Any of these events could disrupt AbbVie's business and have a material adverse effect on its business and results of operations.
Laws and regulations affecting government benefit programs could impose new obligations on AbbVie, require it to change its business practices, and restrict its operations in the future.
The health care industry is subject to various federal, state and international laws and regulations pertaining to government benefit programs reimbursement, rebates, price reporting and regulation and health care fraud and abuse. In the United States, these laws include anti-kickback and false claims laws, the Medicaid Rebate Statute, the Veterans Health Care Act and individual state laws relating to pricing and sales and marketing practices. Violations of these laws may be punishable by criminal and/or civil sanctions, including, in some instances, substantial fines, imprisonment and exclusion from participation in federal and state health care programs, including Medicare, Medicaid and Veterans Administration health programs. These laws and regulations are broad in scope and they are subject to change and evolving interpretations, which could require AbbVie to incur substantial costs associated with compliance or to alter one or more of its sales or marketing practices. In addition, violations of these laws, or allegations of such violations, could disrupt AbbVie's business and result in a material adverse effect on its business and results of operations.
The international nature of AbbVie's business subjects it to additional business risks that may cause its revenue and profitability to decline.
AbbVie's business is subject to risks associated with doing business internationally, including in emerging markets. Net revenues outside of the United States made up approximately 28% of AbbVie's total net revenues in 2019. The risks associated with AbbVie's operations outside the United States include:
• | fluctuations in currency exchange rates; |
• | changes in medical reimbursement policies and programs; |
• | multiple legal and regulatory requirements that are subject to change and that could restrict AbbVie's ability to manufacture, market and sell its products; |
• | differing local product preferences and product requirements; |
• | trade protection measures and import or export licensing requirements; |
• | international trade disruptions or disputes, including in connection with the ongoing trade negotiations between the United States and China; |
• | difficulty in establishing, staffing and managing operations; |
• | differing labor regulations; |
• | potentially negative consequences from changes in or interpretations of tax laws; |
2019 Form 10-K |  15 15 | ||
• | political and economic instability, including the United Kingdom’s exit from the European Union; |
• | sovereign debt issues; |
• | price and currency exchange controls, limitations on participation in local enterprises, expropriation, nationalization and other governmental action; |
• | inflation, recession and fluctuations in interest rates; |
• | potential deterioration in the economic position and credit quality of certain non-U.S. countries, including in Europe and Latin America; and |
• | potential penalties or other adverse consequences for violations of anti-corruption, anti-bribery and other similar laws and regulations, including the United States Foreign Corrupt Practices Act and the United Kingdom Bribery Act. |
Events contemplated by these risks may, individually or in the aggregate, have a material adverse effect on AbbVie's revenues and profitability.
If AbbVie does not effectively and profitably commercialize its products, AbbVie's revenues and financial condition could be adversely affected.
AbbVie must effectively and profitably commercialize its principal products by creating and meeting continued market demand; achieving market acceptance and generating product sales; ensuring that the active pharmaceutical ingredient(s) for a product and the finished product are manufactured in sufficient quantities and in compliance with requirements of the FDA and similar foreign regulatory agencies and with acceptable quality and pricing to meet commercial demand; and ensuring that the entire supply chain efficiently and consistently delivers AbbVie's products to its customers. The commercialization of AbbVie products may not be successful due to, among other things, unexpected challenges from competitors, new safety issues or concerns being reported that may impact or narrow approved indications, the relative price of AbbVie's product as compared to alternative treatment options and changes to a product's label that further restrict its marketing. If the commercialization of AbbVie's principal products is unsuccessful, AbbVie's ability to generate revenue from product sales will be adversely affected.
AbbVie may acquire other businesses, license rights to technologies or products, form alliances, or dispose of assets, which could cause it to incur significant expenses and could negatively affect profitability.
AbbVie may pursue acquisitions (such as the pending acquisition of Allergan), technology licensing arrangements, and strategic alliances, or dispose of some of its assets, as part of its business strategy. AbbVie may not complete these transactions in a timely manner, on a cost-effective basis, or at all, and may not realize the expected benefits. If AbbVie is successful in making an acquisition, the products and technologies that are acquired may not be successful or may require significantly greater resources and investments than originally anticipated. AbbVie may not be able to integrate acquisitions successfully into its existing business and could incur or assume significant debt and unknown or contingent liabilities. AbbVie could also experience negative effects on its reported results of operations from acquisition or disposition-related charges, amortization of expenses related to intangibles and charges for impairment of long-term assets. These effects could cause a deterioration of AbbVie's credit rating and result in increased borrowing costs and interest expense.
Additionally, changes in AbbVie's structure, operations, revenues, costs, or efficiency resulting from major transactions such as acquisitions, divestitures, mergers, alliances, restructurings or other strategic initiatives, may result in greater than expected costs, may take longer than expected to complete or encounter other difficulties, including the need for regulatory approval where appropriate.
AbbVie is dependent on wholesale distributors for distribution of its products in the United States and, accordingly, its results of operations could be adversely affected if they encounter financial difficulties.
In 2019, three wholesale distributors (McKesson Corporation, Cardinal Health, Inc. and AmerisourceBergen Corporation) accounted for substantially all of AbbVie's sales in the United States. If one of its significant wholesale distributors encounters financial or other difficulties, such distributor may decrease the amount of business that it does with AbbVie, and AbbVie may be unable to collect all the amounts that the distributor owes it on a timely basis or at all, which could negatively impact AbbVie's business and results of operations.
16  | 2019 Form 10-K | 2019 Form 10-K | ||
AbbVie has debt obligations that could adversely affect its business and its ability to meet its obligations.
The amount of debt that AbbVie has incurred and intends to incur could have important consequences to AbbVie and its investors. These consequences include, among other things, requiring a portion of AbbVie's cash flow from operations to make interest payments on this debt and reducing the cash flow available to fund capital expenditures and other corporate purposes and to grow AbbVie's business. To the extent AbbVie incurs additional indebtedness or interest rates increase, these risks could increase. In addition, AbbVie's cash flow from operations may not be sufficient to repay all of the outstanding debt as it becomes due, and AbbVie may not be able to borrow money, sell assets, or otherwise raise funds on acceptable terms, or at all, to refinance its debt.
AbbVie may need additional financing in the future to meet its capital needs or to make opportunistic acquisitions, and such financing may not be available on favorable terms, if at all.
AbbVie may need to seek additional financing for its general corporate purposes. For example, it may need to increase its investment in research and development activities or need funds to make acquisitions. AbbVie may be unable to obtain any desired additional financing on terms favorable to it, if at all. If AbbVie loses its investment grade credit rating or adequate funds are not available on acceptable terms, AbbVie may be unable to fund its expansion, successfully develop or enhance products, or respond to competitive pressures, any of which could negatively affect AbbVie's business. If AbbVie raises additional funds by issuing debt or entering into credit facilities, it may be subject to limitations on its operations due to restrictive covenants. Failure to comply with these covenants could adversely affect AbbVie's business.
AbbVie depends on information technology and a failure of those systems could adversely affect AbbVie's business.
AbbVie relies on sophisticated software applications and complex information technology systems to operate its business. These systems are potentially vulnerable to malicious intrusion, random attack, loss of data privacy, disruption, degradation or breakdown. Data privacy or security breaches by employees or others may result in the failure of critical business operations or may cause sensitive data, including intellectual property, trade secrets or personal information belonging to AbbVie, its patients, customers or business partners, to be exposed to unauthorized persons or to the public. Although AbbVie has invested in the protection of its data and information technology and also monitors its systems on an ongoing basis, there can be no assurance that these efforts will prevent breakdowns or breaches in AbbVie's information technology systems that could adversely affect AbbVie's business. Such adverse consequences could include loss of revenue, or the loss of critical or sensitive information from AbbVie’s or third-party providers’ databases or IT systems and could also result in legal, financial, reputational or business harm to AbbVie and potentially substantial remediation costs.
Failure to attract and retain highly qualified personnel could affect AbbVie’s ability to successfully develop and commercialize products.
AbbVie’s success is largely dependent on its continued ability to attract and retain highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical R&D, governmental regulation and commercialization. Competition for qualified personnel in the biopharmaceutical field is intense. AbbVie cannot be sure that it will be able to attract and retain quality personnel or that the costs of doing so will not materially increase.
Other factors can have a material adverse effect on AbbVie's profitability and financial condition.
Many other factors can affect AbbVie's results of operations, cash flows and financial condition, including:
• | changes in or interpretations of laws and regulations, including changes in accounting standards, taxation requirements, product marketing application standards, data privacy laws and environmental laws; |
• | differences between the fair value measurement of assets and liabilities and their actual value, particularly for pension and post-employment benefits, stock-based compensation, intangibles and goodwill; and for contingent liabilities such as litigation and contingent consideration, the absence of a recorded amount, or an amount recorded at the minimum, compared to the actual amount; |
• | changes in the rate of inflation (including the cost of raw materials, commodities and supplies), interest rates, market value of AbbVie's equity investments and the performance of investments held by it or its employee benefit trusts; |
2019 Form 10-K |  17 17 | ||
• | changes in the creditworthiness of counterparties that transact business with or provide services to AbbVie or its employee benefit trusts; |
• | changes in the ability of third parties that provide information technology, accounting, human resources, payroll and other outsourced services to AbbVie to meet their contractual obligations to AbbVie; and |
• | changes in business, economic and political conditions, including: war, political instability, terrorist attacks, the threat of future terrorist activity and related military action; natural disasters; the cost and availability of insurance due to any of the foregoing events; labor disputes, strikes, slow-downs, or other forms of labor or union activity; and pressure from third-party interest groups. |
Risks Related to the Acquisition and the Combined Company Upon Completion of the Acquisition
The pending acquisition of Allergan may not be completed on the currently contemplated timeline or terms, or at all, and may not achieve the intended benefits.
Consummation of the Acquisition is conditioned on, among other things, obtaining necessary governmental and regulatory approvals. If any of the conditions to the Acquisition is not satisfied, it could delay or prevent the Acquisition from occurring, which could negatively impact AbbVie’s share price and future business and financial results. Further, as a condition to their approval of the Acquisition, agencies may impose requirements, limitations or costs or require divestitures or place restrictions on the conduct of the AbbVie’s business after the closing. These requirements, limitations, costs, divestitures or restrictions could jeopardize or delay the consummation of the Acquisition or may reduce the anticipated benefits of the transaction. In addition, changes in laws and regulations, including Irish legislation implementing a tax increase payable upon completion of the Acquisition, could adversely impact AbbVie’s post-Acquisition profitability and financial results. Following the Acquisition, AbbVie may not realize the Acquisition’s intended benefits within the expected timeframe or at all.
The indebtedness of the combined company following the consummation of the Acquisition will be substantially greater than AbbVie’s indebtedness on a standalone basis and greater than the combined indebtedness of AbbVie and Allergan prior to the announcement of the acquisition. This increased level of indebtedness could adversely affect the combined company’s business flexibility and increase its borrowing costs.
AbbVie expects that the cash consideration due to Allergan’s shareholders under the transaction agreement and related fees and expenses will be approximately $41 billion. In addition to using cash on hand, AbbVie has incurred significant Acquisition-related debt financing, including unsecured term loans and senior notes. For more information, see Note 10“Debt, Credit Facilities and Commitments and Contingencies,” to the Consolidated Financial Statements included under Item 8, “Financial Statements and Supplementary Data.” AbbVie also intends to assume all the existing indebtedness of Allergan and its subsidiaries. AbbVie’s substantially increased indebtedness and higher debt to equity ratio following the consummation of the Acquisition may have the effect of, among other things, reducing its flexibility to respond to changing business and economic conditions, lowering its credit ratings, increasing its borrowing costs and/or requiring it to reduce or delay investments, strategic acquisitions and capital expenditures or to seek additional capital or restructure or refinance its indebtedness.
Risks Related to AbbVie's Common Stock
AbbVie cannot guarantee the timing, amount, or payment of dividends on its common stock.
Although AbbVie expects to pay regular cash dividends, the timing, declaration, amount and payment of future dividends to stockholders will fall within the discretion of AbbVie's board of directors. The board's decisions regarding the payment of dividends will depend on many factors, such as AbbVie's financial condition, earnings, capital requirements, debt service obligations, industry practice, legal requirements, regulatory constraints and other factors that the board deems relevant. For more information, see Item 5, "Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities." AbbVie's ability to pay dividends will depend on its ongoing ability to generate cash from operations and access capital markets. AbbVie cannot guarantee that it will continue to pay a dividend in the future.
An AbbVie stockholder's percentage of ownership in AbbVie may be diluted in the future.
In the future, a stockholder's percentage ownership in AbbVie may be diluted because of equity issuances for capital market transactions, equity awards that AbbVie will be granting to AbbVie's directors, officers and employees, acquisitions
18  | 2019 Form 10-K | 2019 Form 10-K | ||
(including AbbVie's pending acquisition of Allergan), or other purposes. AbbVie's employees have options to purchase shares of its common stock as a result of conversion of their Abbott stock options (in whole or in part) to AbbVie stock options. AbbVie anticipates its compensation committee will grant additional stock options or other stock-based awards to its employees. Such awards will have a dilutive effect on AbbVie's earnings per share, which could adversely affect the market price of AbbVie's common stock. From time to time, AbbVie will issue additional options or other stock-based awards to its employees under AbbVie's employee benefits plans.
In addition, AbbVie's amended and restated certificate of incorporation authorizes AbbVie to issue, without the approval of AbbVie's stockholders, one or more classes or series of preferred stock having such designation, powers, preferences and relative, participating, optional and other special rights, including preferences over AbbVie's common stock respecting dividends and distributions, as AbbVie's board of directors generally may determine. The terms of one or more classes or series of preferred stock could dilute the voting power or reduce the value of AbbVie's common stock. For example, AbbVie could grant the holders of preferred stock the right to elect some number of AbbVie's directors in all events or on the happening of specified events or the right to veto specified transactions. Similarly, the repurchase or redemption rights or liquidation preferences AbbVie could assign to holders of preferred stock could affect the residual value of the common stock.
Certain provisions in AbbVie's amended and restated certificate of incorporation and amended and restated by-laws, and of Delaware law, may prevent or delay an acquisition of AbbVie, which could decrease the trading price of AbbVie's common stock.
AbbVie's amended and restated certificate of incorporation and amended and restated by-laws contain, and Delaware law contains, provisions that are intended to deter coercive takeover practices and inadequate takeover bids by encouraging prospective acquirors to negotiate with AbbVie's board of directors rather than to attempt a hostile takeover. These provisions include, among others:
• | the inability of AbbVie's stockholders to call a special meeting; |
• | the division of AbbVie's board of directors into three classes of directors, with each class serving a staggered three-year term; |
• | a provision that stockholders may only remove directors for cause; |
• | the ability of AbbVie's directors, and not stockholders, to fill vacancies on AbbVie's board of directors; and |
• | the requirement that the affirmative vote of stockholders holding at least 80% of AbbVie's voting stock is required to amend certain provisions in AbbVie's amended and restated certificate of incorporation and AbbVie's amended and restated by-laws relating to the number, term and election of AbbVie's directors, the filling of board vacancies, the calling of special meetings of stockholders and director and officer indemnification provisions. |
In addition, Section 203 of the Delaware General Corporation Law provides that, subject to limited exceptions, persons that acquire, or are affiliated with a person that acquires, more than 15% of the outstanding voting stock of a Delaware corporation shall not engage in any business combination with that corporation, including by merger, consolidation or acquisitions of additional shares, for a three-year period following the date on which that person or its affiliates becomes the holder of more than 15% of the corporation's outstanding voting stock.
AbbVie believes these provisions protect its stockholders from coercive or otherwise unfair takeover tactics by requiring potential acquirors to negotiate with AbbVie's board of directors and by providing AbbVie's board of directors with more time to assess any acquisition proposal. These provisions are not intended to make the company immune from takeovers. However, these provisions apply even if the offer may be considered beneficial by some stockholders and could delay or prevent an acquisition that AbbVie's board of directors determines is not in the best interests of AbbVie and AbbVie's stockholders. These provisions may also prevent or discourage attempts to remove and replace incumbent directors.
2019 Form 10-K |  19 19 | ||
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains certain forward looking statements regarding business strategies, market potential, future financial performance and other matters. The words "believe," "expect," "anticipate," "project" and similar expressions, among others, generally identify "forward looking statements," which speak only as of the date the statements were made. The matters discussed in these forward looking statements are subject to risks, uncertainties and other factors that could cause actual results to differ materially from those projected, anticipated or implied in the forward looking statements. In particular, information included under Item 1, "Business," Item 1A, "Risk Factors," and Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" contain forward looking statements. Where, in any forward looking statement, an expectation or belief as to future results or events is expressed, such expectation or belief is based on the current plans and expectations of AbbVie management and expressed in good faith and believed to have a reasonable basis, but there can be no assurance that the expectation or belief will result or be achieved or accomplished. Factors that could cause actual results or events to differ materially from those anticipated include the matters described under Item 1A, "Risk Factors" and Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations." AbbVie does not undertake any obligation to update the forward-looking statements included in this Annual Report on Form 10-K to reflect events or circumstances after the date hereof, unless AbbVie is required by applicable securities law to do so.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
AbbVie's corporate offices are located at 1 North Waukegan Road, North Chicago, Illinois 60064-6400. AbbVie's manufacturing facilities are in the following locations:
United States | Outside the United States | |
Abbott Park, Illinois* | Campoverde di Aprilia, Italy | |
Barceloneta, Puerto Rico | Cork, Ireland | |
North Chicago, Illinois | Ludwigshafen, Germany | |
Worcester, Massachusetts* | Singapore* | |
Wyandotte, Michigan* | Sligo, Ireland | |
_______________________________________________________________________________
* | Leased property. |
In addition to the above, AbbVie has other manufacturing facilities worldwide. AbbVie believes its facilities are suitable and provide adequate production capacity. There are no material encumbrances on AbbVie's owned properties.
In the United States, including Puerto Rico, AbbVie has one distribution center. AbbVie also has research and development facilities in the United States located at: Abbott Park, Illinois; North Chicago, Illinois; Redwood City, California; South San Francisco, California; Sunnyvale, California; Cambridge, Massachusetts; and Worcester, Massachusetts. Outside the United States, AbbVie's principal research and development facilities are located in Ludwigshafen, Germany.
ITEM 3. LEGAL PROCEEDINGS
Information pertaining to legal proceedings is provided in Note 15, "Legal Proceedings and Contingencies" to the Consolidated Financial Statements included under Item 8, "Financial Statements and Supplementary Data," and is incorporated by reference herein.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
20  | 2019 Form 10-K | 2019 Form 10-K | ||
INFORMATION ABOUT OUR EXECUTIVE OFFICERS
The following table lists AbbVie's executive officers, each of whom was first appointed as an AbbVie corporate officer in December 2012, except as otherwise indicated:
Name | Age | Position | ||
Richard A. Gonzalez | 66 | Chairman of the Board and Chief Executive Officer | ||
Michael E. Severino, M.D.* | 54 | Vice Chairman and President | ||
Laura J. Schumacher | 56 | Vice Chairman, External Affairs and Chief Legal Officer | ||
Carlos Alban | 57 | Vice Chairman, Chief Commercial Officer | ||
Henry O. Gosebruch* | 47 | Executive Vice President and Chief Strategy Officer | ||
Robert A. Michael* | 49 | Executive Vice President, Chief Financial Officer | ||
Timothy J. Richmond | 53 | Executive Vice President, Chief Human Resources Officer | ||
Azita Saleki-Gerhardt, Ph.D. | 56 | Executive Vice President, Operations | ||
Nicholas Donoghoe, M.D.* | 39 | Senior Vice President, Enterprise Innovation | ||
Thomas J. Hudson, M.D.* | 58 | Senior Vice President, Research & Development and Chief Scientific Officer | ||
Jeffrey R. Stewart* | 51 | Senior Vice President, U.S. Commercial Operations | ||
Brian L. Durkin* | 59 | Vice President, Controller | ||
_______________________________________________________________________________
* | Dr. Severino was first appointed as a corporate officer in June 2014; Mr. Gosebruch was first appointed as a corporate officer in December 2015; Dr. Donoghoe was first appointed as a corporate officer in January 2019; Mr. Michael was first appointed as a corporate officer in December 2015; Dr. Hudson was first appointed as a corporate officer in July 2019; Mr. Stewart was first appointed as a corporate officer in December 2018; and Mr. Durkin was first appointed as a corporate officer in October 2018. |
Mr. Gonzalez is the Chairman and Chief Executive Officer of AbbVie. He served as Abbott’s Executive Vice President of the Pharmaceutical Products Group from July 2010 to December 2012, and was responsible for Abbott’s worldwide pharmaceutical business, including commercial operations, research and development, and manufacturing. He also served as President, Abbott Ventures Inc., Abbott’s medical technology investment arm, from 2009 to 2011. Mr. Gonzalez joined Abbott in 1977 and held various management positions.
Dr. Severino is AbbVie’s Vice Chairman and President, responsible for research and development, human resources, operations, and the corporate strategy office. He served as Executive Vice President, Research and Development and Chief Scientific Officer from 2014 to 2018. Dr. Severino served at Amgen Inc. as Senior Vice President, Global Development and Corporate Chief Medical Officer from 2012 to 2014, as Vice President, Global Development from 2010 to 2012 and as Vice President, Therapeutic Area Head, General Medicine and Inflammation Global Clinical Development from 2007 to 2012. He joined AbbVie in 2014.
Ms. Schumacher is AbbVie’s Vice Chairman, External Affairs and Chief Legal Officer, responsible for global legal, health economics outcomes research, corporate responsibility, brand and communications and government affairs. Prior to her current appointment in 2018, she served as AbbVie’s Executive Vice President, External Affairs, General Counsel and Corporate Secretary. Prior to AbbVie’s separation from Abbott, Ms. Schumacher served as Executive Vice President, General Counsel from 2007 to 2012. Both at Abbott and AbbVie, Ms. Schumacher also led Business Development and Ventures and Early Stage Collaborations. Ms. Schumacher joined Abbott in 1990. She serves on the board of General Dynamics Corporation.
Mr. Alban is AbbVie’s Vice Chairman, Chief Commercial Officer, responsible for global commercial operations of the company, including the Pharmacyclics commercial functions. He previously served as Executive Vice President, Commercial Operations from 2013 to 2018. He served as Abbott’s Senior Vice President, Proprietary Pharmaceutical Products, Global Commercial Operations from 2011 to 2012, as Senior Vice President, International Pharmaceuticals from 2009 to 2011, as Vice President, Western Europe and Canada from 2007 to 2009, and as Vice President, European Operations from 2006 to 2007. Mr. Alban joined Abbott in 1986.
Mr. Gosebruch is AbbVie's Executive Vice President and Chief Strategy Officer. He worked for more than 20 years in the Mergers & Acquisitions Group at J.P. Morgan Securities LLC, serving as Managing Director since 2007 and as Co-Head of M&A North America during 2015. Mr. Gosebruch joined AbbVie in 2015. He serves on the board of Aptinyx Inc.
2019 Form 10-K |  21 21 | ||
Mr. Michael is AbbVie’s Executive Vice President, Chief Financial Officer. Mr. Michael previously served as Senior Vice President, Chief Financial Officer from October 2018 to July 2019, and as Vice President, Controller from March 2017 to October 2018. He served as AbbVie’s Vice President, Treasurer from 2015 to 2016, as Vice President, Controller, Commercial Operations from 2013 to 2015 and Vice President, Financial Planning and Analysis from 2012 to 2013. At Abbott, Mr. Michael served as Division Controller, Nutrition Supply Chain from 2010 to 2012. Mr. Michael joined Abbott in 1993.
Mr. Richmond is AbbVie’s Executive Vice President, Chief Human Resources Officer. He served as Senior Vice President, Human Resources from 2013 to 2018. Mr. Richmond served as Abbott’s Divisional Vice President of Compensation & Benefits from 2008 to 2012, as Group Vice President of Talent and Rewards from 2007 to 2008, and as Divisional Vice President of Talent Acquisition from 2006 to 2007. Mr. Richmond joined Abbott in 2006.
Dr. Saleki-Gerhardt is AbbVie’s Executive Vice President, Operations. She served as Senior Vice President, Operations from 2013 to 2018. Dr. Saleki-Gerhardt served as Abbott’s Vice President, Pharmaceuticals Manufacturing and Supply from 2011 to 2012, and as Divisional Vice President, Quality Assurance, Global Pharmaceutical Operations from 2008 to 2011. Dr. Saleki-Gerhardt joined Abbott in 1993. She serves on the board of Entegris Inc.
Dr. Donoghoe is AbbVie's Senior Vice President, Enterprise Innovation. He previously served as a Partner at McKinsey & Company, leading the firm's West Coast pharma and biotechnology practice. Dr. Donoghoe joined the firm in 2007 and supported multiple successful launches in therapeutic areas such as oncology, immunology, and primary care. He joined AbbVie in 2019.
Dr. Hudson is AbbVie's Senior Vice President, Research & Development and Chief Scientific Officer. He previously served as Vice President, Head of Oncology Discovery and Early Development from 2016 to 2019. Prior to joining AbbVie, Dr. Hudson served at the Ontario Institute for Cancer Research as President and Scientific Director. He also previously served as Founder and Director of the McGill University and Genome Quebec Innovation Centre and Assistant Director of the Whitehead/MIT Center for Genome Research.
Mr. Stewart is AbbVie’s Senior Vice President, U.S. Commercial Operations. Mr. Stewart previously served as AbbVie’s President, Commercial Operations from 2013 to 2018. Prior to AbbVie’s separation from Abbott, he served as Vice President, Abbott Proprietary Pharmaceutical Division, United States. Mr. Stewart joined Abbott in 1992.
Mr. Durkin is AbbVie’s Vice President, Controller. Mr. Durkin previously served as Vice President, Internal Audit from 2016 to 2018. Prior to joining AbbVie, he served as Vice President of Finance and Division Controller for Abbott’s Vision Care business from 2009 to 2016 and Controller Pharmaceutical Research and Development from 2005 to 2009. Mr. Durkin joined Abbott in 1986.
The executive officers of AbbVie are elected annually by the board of directors. All other officers are elected by the board or appointed by the Chairman of the Board. All officers are either elected at the first meeting of the board of directors held after the annual stockholder meeting or appointed by the Chairman of the Board after that board meeting. Each officer holds office until a successor has been duly elected or appointed and qualified or until the officer's death, resignation, or removal. There are no family relationships between any of the executive officers listed above.
22  | 2019 Form 10-K | 2019 Form 10-K | ||
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Principal Market
The principal market for AbbVie's common stock is the New York Stock Exchange (Symbol: ABBV). AbbVie's common stock is also listed on the Chicago Stock Exchange and traded on various regional and electronic exchanges.
Stockholders
There were 46,544 stockholders of record of AbbVie common stock as of January 31, 2020.
Performance Graph
The following graph compares the cumulative total returns of AbbVie, the S&P 500 Index and the NYSE Arca Pharmaceuticals Index for the period from December 31, 2014 through December 31, 2019. This graph assumes $100 was invested in AbbVie common stock and each index on December 31, 2014 and also assumes the reinvestment of dividends. The stock price performance on the following graph is not necessarily indicative of future stock price performance.
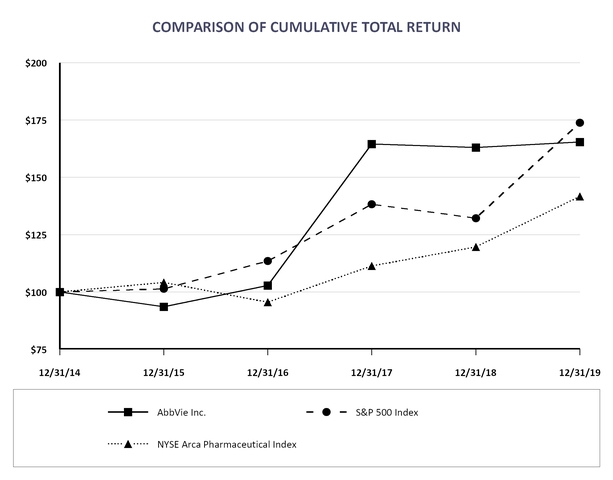
This performance graph is furnished and shall not be deemed "filed" with the SEC or subject to Section 18 of the Securities Exchange Act of 1934, nor shall it be deemed incorporated by reference in any of AbbVie's filings under the Securities Act of 1933, as amended.
2019 Form 10-K |  23 23 | ||
Dividends
On November 1, 2019, AbbVie's board of directors declared an increase in the quarterly cash dividend from $1.07 per share to $1.18 per share, payable on February 14, 2020 to stockholders of record as of January 15, 2020. The timing, declaration, amount of and payment of any dividends by AbbVie in the future is within the discretion of its board of directors and will depend upon many factors, including AbbVie's financial condition, earnings, capital requirements of its operating subsidiaries, covenants associated with certain of AbbVie's debt service obligations, legal requirements, regulatory constraints, industry practice, ability to access capital markets and other factors deemed relevant by its board of directors. Moreover, if AbbVie determines to pay any dividend in the future, there can be no assurance that it will continue to pay such dividends or the amount of such dividends.
Issuer Purchases of Equity Securities
Period | (a) Total Number of Shares (or Units) Purchased | (b) Average Price Paid per Share (or Unit) | (c) Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs | (d) Maximum Number (or Approximate Dollar Value) of Shares (or Units) that May Yet Be Purchased Under the Plans or Programs | ||||||||||
October 1, 2019 - October 31, 2019 | 4,293 | (1) | $ | 77.19 | (1) | — | $ | 3,950,021,071 | ||||||
November 1, 2019 - November 30, 2019 | 1,086 | (1) | $ | 80.53 | (1) | — | $ | 3,950,021,071 | ||||||
December 1, 2019 - December 31, 2019 | 1,016 | (1) | $ | 87.39 | (1) | — | $ | 3,950,021,071 | ||||||
Total | 6,395 | (1) | $ | 79.38 | (1) | — | $ | 3,950,021,071 | ||||||
1. | In addition to AbbVie shares repurchased on the open market under a publicly announced program, if any, these shares also included the shares purchased on the open market for the benefit of participants in the AbbVie Employee Stock Purchase Plan – 4,293 in October; 1,086 in November; and 1,016 in December. |
These shares do not include the shares surrendered to AbbVie to satisfy minimum tax withholding obligations in connection with the vesting or exercise of stock-based awards.
24  | 2019 Form 10-K | 2019 Form 10-K | ||
ITEM 6. SELECTED FINANCIAL DATA
The selected financial information should be read in conjunction with the financial statements and accompanying notes included under Item 8, "Financial Statements and Supplementary Data" and Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations."
as of and for the years ended December 31 (in millions, except per share data) | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||
Statement of earnings data | |||||||||||||||||||
Net revenues | $ | 33,266 | $ | 32,753 | $ | 28,216 | $ | 25,638 | $ | 22,859 | |||||||||
Net earnings | 7,882 | 5,687 | 5,309 | 5,953 | 5,144 | ||||||||||||||
Basic earnings per share | $ | 5.30 | $ | 3.67 | $ | 3.31 | $ | 3.65 | $ | 3.15 | |||||||||
Diluted earnings per share | $ | 5.28 | $ | 3.66 | $ | 3.30 | $ | 3.63 | $ | 3.13 | |||||||||
Cash dividends declared per common share | $ | 4.39 | $ | 3.95 | $ | 2.63 | $ | 2.35 | $ | 2.10 | |||||||||
Weighted-average basic shares outstanding | 1,481 | 1,541 | 1,596 | 1,622 | 1,625 | ||||||||||||||
Weighted-average diluted shares outstanding | 1,484 | 1,546 | 1,603 | 1,631 | 1,637 | ||||||||||||||
Balance sheet data | |||||||||||||||||||
Total assets (a) | $ | 89,115 | $ | 59,352 | $ | 70,786 | $ | 66,099 | $ | 53,050 | |||||||||
Long-term debt and finance lease obligations (a)(b) | 66,728 | 36,611 | 36,968 | 36,465 | 31,265 | ||||||||||||||
(a) | In November 2019, AbbVie issued $30.0 billion aggregate principal amount of floating rate and fixed rate unsecured senior notes at maturities ranging from 18 months to 30 years. AbbVie expects to use the net proceeds to fund a portion of the aggregate cash consideration due to Allergan shareholders in connection with the proposed acquisition and to pay related fees and expenses. See Note 5 to the Consolidated Financial Statements for information regarding the proposed acquisition and Note 10 for information on the senior notes. |
(b) | Includes current portion of both long-term debt and finance lease obligations. |
2019 Form 10-K |  25 25 | ||
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following is a discussion and analysis of the financial condition of AbbVie Inc. (AbbVie or the company) as of December 31, 2019 and 2018 and results of operations for each of the three years in the period ended December 31, 2019. This commentary should be read in conjunction with the consolidated financial statements and accompanying notes appearing in Item 8, "Financial Statements and Supplementary Data."
EXECUTIVE OVERVIEW
Company Overview
AbbVie is a global, research-based biopharmaceutical company formed in 2013 following separation from Abbott Laboratories (Abbott). AbbVie uses its expertise, dedicated people and unique approach to innovation to develop and market advanced therapies that address some of the world's most complex and serious diseases. AbbVie's products are focused on treating conditions such as chronic autoimmune diseases in rheumatology, gastroenterology and dermatology; oncology, including blood cancers; virology, including hepatitis C virus (HCV) and human immunodeficiency virus (HIV); neurological disorders, such as Parkinson's disease; metabolic diseases, including thyroid disease and complications associated with cystic fibrosis; pain associated with endometriosis; as well as other serious health conditions. AbbVie also has a pipeline of promising new medicines in clinical development across such important medical specialties as immunology, oncology and neuroscience, with additional targeted investment in cystic fibrosis and women's health.
AbbVie's products are generally sold worldwide directly to wholesalers, distributors, government agencies, health care facilities, specialty pharmacies and independent retailers from AbbVie-owned distribution centers and public warehouses. In the United States, AbbVie distributes pharmaceutical products principally through independent wholesale distributors, with some sales directly to pharmacies and patients. Outside the United States, AbbVie sells products primarily to customers or through distributors, depending on the market served. Certain products are co-marketed or co-promoted with other companies. AbbVie has approximately 30,000 employees. AbbVie operates in one business segment—pharmaceutical products.
On June 25, 2019, AbbVie announced that it entered into a definitive transaction agreement under which AbbVie will acquire Allergan plc (Allergan). See Note 5 to the Consolidated Financial Statements for additional information regarding the proposed acquisition.
2019 Financial Results
AbbVie's strategy has focused on delivering strong financial results, advancing and investing in its pipeline and returning value to shareholders while ensuring a strong, sustainable growth business over the long term. The company's financial performance in 2019 included delivering worldwide net revenues of $33.3 billion, operating earnings of $13.0 billion, diluted earnings per share of $5.28 and cash flows from operations of $13.3 billion. Worldwide net revenues grew by 3% on a constant currency basis, primarily driven by revenue growth related to IMBRUVICA and VENCLEXTA as well as the continued strength of HUMIRA in the U.S. and newly launched immunology assets SKYRIZI and RINVOQ, offset by international HUMIRA biosimilar competition.
Diluted earnings per share in 2019 was $5.28 and included the following after-tax costs: (i) $3.2 billion for the change in fair value of contingent consideration liabilities; (ii) $1.3 billion related to the amortization of intangible assets; (iii) a Stemcentrx-related impairment charge of $823 million net of the related fair value adjustment to contingent consideration liabilities; (iv) $364 million for acquired in-process research and development (IPR&D); and (v) $338 million of expenses related to the proposed Allergan acquisition. These costs were partially offset by the following after-tax benefits: (i) $414 million from litigation matters primarily due to the settlement of an intellectual property dispute with a third party; (ii) $400 million due to the favorable resolution of various tax positions; and (iii) $297 million from an amended and restated license agreement between AbbVie and Reata Pharmaceuticals, Inc. (Reata). Additionally, financial results reflected continued funding to support all stages of AbbVie’s emerging pipeline assets and continued investment in AbbVie’s on-market brands.
In November 2019, AbbVie's board of directors declared a quarterly cash dividend of $1.18 per share of common stock payable in February 2020. This reflects an increase of approximately 10.3% over the previous quarterly dividend of $1.07 per share of common stock.
26  | 2019 Form 10-K | 2019 Form 10-K | ||
2020 Strategic Objectives
AbbVie's mission is to be an innovation-driven, patient-focused specialty biopharmaceutical company capable of achieving top-tier financial performance through outstanding execution and a consistent stream of innovative new medicines. AbbVie intends to continue to advance its mission in a number of ways, including: (i) growing revenues by diversifying revenue streams, ensuring strong commercial execution of new product launches and driving late-stage pipeline assets to the market; (ii) continuing to invest and expand its pipeline in support of opportunities in immunology, oncology and neuroscience, with additional targeted investment in cystic fibrosis and women's health as well as continued investment in key on-market products; (iii) expanding operating margins; and (iv) returning cash to shareholders via a strong and growing dividend while also reducing incremental debt. In addition, AbbVie anticipates several regulatory submissions and key data readouts from key clinical trials in the next 12 months.
AbbVie expects to achieve its strategic objectives through:
• | Completion and successful integration of the proposed Allergan acquisition. |
• | Hematologic oncology revenue growth from both IMBRUVICA and VENCLEXTA. |
• | Immunology revenue growth driven by successful commercial launches of SKYRIZI and RINVOQ, as well as HUMIRA U.S. sales growth. |
• | Effective management of HUMIRA international biosimilar erosion. |
• | The favorable impact of pipeline products and indications recently approved or currently under regulatory review where approval is expected in 2020. These products are described in greater detail in the section labeled "Research and Development" included as part of this Item 7. |
AbbVie remains committed to driving continued expansion of operating margins and expects to achieve this objective through continued leverage from revenue growth, productivity initiatives in supply chain and ongoing efficiency programs to optimize manufacturing, commercial infrastructure, administrative costs and general corporate expenses.
The combination of AbbVie and Allergan will create a diverse entity with leadership positions across immunology, hematologic oncology, aesthetics, neuroscience, women's health, eye care and virology. AbbVie's existing product portfolio and pipeline will be enhanced with numerous Allergan assets and Allergan's product portfolio will benefit from AbbVie's commercial strength, expertise and international infrastructure.
Research and Development
Research and innovation are the cornerstones of AbbVie's business as a global biopharmaceutical company. AbbVie's long-term success depends to a great extent on its ability to continue to discover and develop innovative pharmaceutical products and acquire or collaborate on compounds currently in development by other biotechnology or pharmaceutical companies.
AbbVie's pipeline currently includes approximately 60 compounds or indications in clinical development individually or under collaboration or license agreements and is focused on such important medical specialties as immunology, oncology and neuroscience along with targeted investments in cystic fibrosis and women's health. Of these programs, approximately 30 are in mid- and late-stage development.
The following sections summarize transitions of significant programs from Phase 2 development to Phase 3 development as well as developments in significant Phase 3 and registration programs. AbbVie expects multiple Phase 2 programs to transition into Phase 3 programs in the next 12 months.
Significant Programs and Developments
Immunology
RINVOQ
• | In February 2019, the U.S. Food and Drug Administration (FDA) accepted for priority review AbbVie's New Drug Application (NDA) for upadacitinib, an investigational oral JAK1-selective inhibitor, for the treatment of adult patients with moderate to severe rheumatoid arthritis (RA). |
• | In February 2019, AbbVie initiated a Phase 3 clinical trial to evaluate the efficacy and safety of upadacitinib in subjects with giant cell arteritis. |
2019 Form 10-K |  27 27 | ||
• | In August 2019, the FDA approved RINVOQ (upadacitinib) for the treatment of adults with moderately to severely active RA who have had an inadequate response or intolerance to methotrexate. |
• | In October 2019, AbbVie announced top-line results from its first Phase 3 clinical trial of RINVOQ in adult patients with active psoriatic arthritis (PsA). Results from the SELECT-PsA 2 study, which evaluated RINVOQ versus placebo in patients who did not adequately respond to treatment with one or more biologic DMARDs, showed that both doses of RINVOQ (15 mg and 30 mg) met the primary and key secondary endpoints at week 12. The safety profile was consistent with that of previous studies across indications, with no new safety risks detected. |
• | In November 2019, AbbVie announced data from the Phase 2/3 SELECT-AXIS 1 trial in which twice as many adult patients with ankylosing spondylitis treated with RINVOQ achieved the primary endpoint at week 14 versus placebo. The safety profile was consistent with that of previous studies across indications, with no new safety risks detected. |
• | In November 2019, AbbVie initiated a Phase 3 clinical trial to evaluate the efficacy and safety of RINVOQ in adult patients with axial spondyloarthritis. |
• | In December 2019, the European Commission (EC) granted marketing authorization for RINVOQ for the treatment of adult patients with moderate to severe active rheumatoid arthritis who have had an inadequate response or intolerance to one or more DMARDs. |
• | In February 2020, AbbVie announced top-line results from its second Phase 3 clinical trial of RINVOQ in adult patients with active PsA. Results from the SELECT-PsA 1 study, which evaluated RINVOQ versus placebo in patients who did not adequately respond to treatment with one or more non-biologic DMARDs, showed that both doses of RINVOQ (15 mg and 30 mg) met the primary and key secondary endpoints. The safety profile was consistent with that of previous studies across indications, with no new safety risks detected. |
SKYRIZI
• | In March 2019, AbbVie initiated two Phase 3 clinical trials to evaluate the efficacy and safety of risankizumab, an investigational interleukin-23 (IL-23) inhibitor, in subjects with psoriatic arthritis. |
• | In April 2019, the FDA approved SKYRIZI (risankizumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. |
• | In April 2019, the EC granted marketing authorization for SKYRIZI for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy. |
Oncology
IMBRUVICA
• | In January 2019, the FDA approved IMBRUVICA, in combination with GAZYVA (obinutuzumab), for adult patients with previously untreated chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). |
• | In June 2019, AbbVie announced results from the Phase 3 CLL12 trial, evaluating IMBRUVICA in patients with previously untreated CLL, which demonstrated that IMBRUVICA significantly improved event- and progression-free survival. |
• | In November 2019, AbbVie submitted a supplemental New Drug Application (sNDA) to the FDA for IMBRUVICA in combination with rituximab for the first-line treatment of younger patients with CLL or SLL. |
VENCLEXTA
• | In March 2019, AbbVie announced that the FDA placed a partial clinical hold on all clinical trials evaluating VENCLEXTA for the investigational treatment of multiple myeloma (MM). The partial clinical hold followed a review of data from the ongoing Phase 3 BELLINI trial, a study in relapsed/refractory MM, in which a higher proportion of deaths was observed in the VENCLEXTA arm compared to the control arm of the trial. In June 2019, AbbVie announced that the FDA lifted the partial clinical hold placed on the Phase 3 CANOVA trial, evaluating VENCLEXTA for the investigational treatment of relapsed/refractory MM positive for the translocation (11;14) abnormality, based upon agreement on revisions to the CANOVA study protocol, including new risk mitigation measures, protocol-specified guidelines and updated futility criteria. This action does not impact any of the approved indications for VENCLEXTA, such as CLL or acute myeloid leukemia (AML). |
28  | 2019 Form 10-K | 2019 Form 10-K | ||
• | In May 2019, the FDA approved VENCLEXTA, in combination with obinutuzumab, for adult patients with previously untreated CLL/SLL. The approval was based on data from the Phase 3 CLL14 trial, evaluating the efficacy and safety of VENCLEXTA plus obinutuzumab versus obinutuzumab plus chlorambucil in previously untreated patients with CLL, which demonstrated that VENCLEXTA plus obinutuzumab prolonged progression-free survival and achieved higher rates of complete response and minimal residual disease-negativity compared to commonly used standard of care obinutuzumab plus chlorambucil. |
• | In January 2020, AbbVie announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) granted a positive opinion for VENCLYXTO in combination with obinutuzumab for patients with previously untreated CLL. |
Depatux-M
• | In May 2019, AbbVie announced the decision to discontinue the Phase 3 INTELLANCE-1 study of depatuxizumab mafodotin (Depatux-M, previously known as ABT-414) in patients with newly diagnosed glioblastoma, whose tumors have EGFR (epidermal growth factor receptor) amplification, at an interim analysis. An Independent Data Monitoring Committee recommended stopping enrollment in INTELLANCE-1 due to lack of survival benefit for patients receiving Depatux-M compared with placebo when added to the standard regimen of radiation and temozolomide. Enrollment has been halted in all ongoing Depatux-M studies. |
Veliparib
• | In July 2019, AbbVie announced that top-line results from the Phase 3 BROCADE3 study evaluating veliparib, an investigational, oral poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitor, in combination with carboplatin and paclitaxel met its primary endpoint of progression-free survival in patients with HER2 negative germline BRCA-mutated advanced breast cancer. |
• | In July 2019, AbbVie announced that top-line results from the Phase 3 VELIA study, conducted in collaboration with the GOG Foundation, Inc., evaluating veliparib with carboplatin and paclitaxel followed by veliparib maintenance therapy met its primary endpoint of progression-free survival in patients with newly diagnosed ovarian cancer, regardless of biomarker status. |
Rova-T
• | In August 2019, AbbVie announced the decision to terminate the MERU trial, a Phase 3 study evaluating rovalpituzumab tesirine (Rova-T) as a first-line maintenance therapy for advanced small-cell lung cancer (SCLC). An Independent Data Monitoring Committee recommended terminating the study after results demonstrated no survival benefit at a pre-planned interim analysis for patients receiving Rova-T as compared with placebo. With the closing of the MERU trial, AbbVie announced the termination of the Rova-T research and development program. |
Virology/Liver Disease
• | In August 2019, the EC granted marketing authorization for MAVIRET (glecaprevir/pibrentasvir) to shorten the once-daily treatment duration from 12 to 8 weeks in treatment-naïve, compensated cirrhotic, chronic HCV patients with genotype (GT)1, 2, 4, 5 and 6 infection. |
• | In September 2019, the FDA approved MAVYRET (glecaprevir/pibrentasvir) to shorten the once-daily treatment duration from 12 to 8 weeks in treatment-naïve, compensated cirrhotic, chronic HCV patients across all genotypes (GT1-6). |
• | In January 2020, AbbVie announced that the CHMP of the EMA has recommended a change to the marketing authorization for MAVIRET to shorten once-daily treatment duration from 12 to 8 weeks in treatment-naïve, compensated cirrhotic, chronic HCV patients with GT 3 infection. |
Neuroscience
• | In May 2019, AbbVie initiated a Phase 3 clinical trial to evaluate the safety and tolerability of ABBV-951, a subcutaneous levodopa/carbidopa delivery system, in subjects with Parkinson's disease. |
• | In July 2019, AbbVie announced the decision to discontinue the Phase 2 ARISE study evaluating ABBV-8E12, an investigational anti-tau antibody, in patients with progressive supranuclear palsy, after an Independent Data |
2019 Form 10-K |  29 29 | ||
Monitoring Committee recommended stopping the trial for futility after the trial showed that ABBV-8E12 did not provide efficacy.
Other
• | In July 2019, AbbVie submitted an NDA to the FDA for elagolix in combination with estradiol/norethindrone acetate (E2/NETA) daily add-back therapy for the management of heavy menstrual bleeding associated with uterine fibroids. |
RESULTS OF OPERATIONS
Net Revenues
The comparisons presented at constant currency rates reflect comparative local currency net revenues at the prior year's foreign exchange rates. This measure provides information on the change in net revenues assuming that foreign currency exchange rates had not changed between the prior and the current periods. AbbVie believes that the non-GAAP measure of change in net revenues at constant currency rates, when used in conjunction with the GAAP measure of change in net revenues at actual currency rates, may provide a more complete understanding of the company's operations and can facilitate analysis of the company's results of operations, particularly in evaluating performance from one period to another.
Percent change | |||||||||||||||||||||||
At actual currency rates | At constant currency rates | ||||||||||||||||||||||
years ended (dollars in millions) | 2019 | 2018 | 2017 | 2019 | 2018 | 2019 | 2018 | ||||||||||||||||
United States | $ | 23,907 | $ | 21,524 | $ | 18,251 | 11.1 | % | 17.9 | % | 11.1 | % | 17.9 | % | |||||||||
International | 9,359 | 11,229 | 9,965 | (16.7 | )% | 12.8 | % | (13.6 | )% | 10.4 | % | ||||||||||||
Net revenues | $ | 33,266 | $ | 32,753 | $ | 28,216 | 1.6 | % | 16.1 | % | 2.6 | % | 15.2 | % | |||||||||
30  | 2019 Form 10-K | 2019 Form 10-K | ||
The following table details AbbVie's worldwide net revenues:
Percent change | |||||||||||||||||||||||||
At actual currency rates | At constant currency rates | ||||||||||||||||||||||||
years ended December 31 (dollars in millions) | 2019 | 2018 | 2017 | 2019 | 2018 | 2019 | 2018 | ||||||||||||||||||
Immunology | |||||||||||||||||||||||||
HUMIRA | United States | $ | 14,864 | $ | 13,685 | $ | 12,361 | 8.6 | % | 10.7 | % | 8.6 | % | 10.7 | % | ||||||||||
International | 4,305 | 6,251 | 6,066 | (31.1 | )% | 3.1 | % | (27.8 | )% | 0.6 | % | ||||||||||||||
Total | $ | 19,169 | $ | 19,936 | $ | 18,427 | (3.9 | )% | 8.2 | % | (2.9 | )% | 7.4 | % | |||||||||||
SKYRIZI | United States | $ | 311 | $ | — | $ | — | n/m | n/m | n/m | n/m | ||||||||||||||
International | 44 | — | — | n/m | n/m | n/m | n/m | ||||||||||||||||||
Total | $ | 355 | $ | — | $ | — | n/m | n/m | n/m | n/m | |||||||||||||||
RINVOQ | United States | $ | 47 | $ | — | $ | — | n/m | n/m | n/m | n/m | ||||||||||||||
International | — | — | — | n/m | n/m | n/m | n/m | ||||||||||||||||||
Total | $ | 47 | $ | — | $ | — | n/m | n/m | n/m | n/m | |||||||||||||||
Hematologic Oncology | |||||||||||||||||||||||||
IMBRUVICA | United States | $ | 3,830 | $ | 2,968 | $ | 2,144 | 29.1 | % | 38.4 | % | 29.1 | % | 38.4 | % | ||||||||||
Collaboration revenues | 844 | 622 | 429 | 35.8 | % | 45.0 | % | 35.8 | % | 45.0 | % | ||||||||||||||
Total | $ | 4,674 | $ | 3,590 | $ | 2,573 | 30.2 | % | 39.5 | % | 30.2 | % | 39.5 | % | |||||||||||
VENCLEXTA | United States | $ | 521 | $ | 247 | $ | 89 | >100.0% | >100.0% | >100.0% | >100.0% | ||||||||||||||
International | 271 | 97 | 33 | >100.0% | >100.0% | >100.0% | >100.0% | ||||||||||||||||||
Total | $ | 792 | $ | 344 | $ | 122 | >100.0% | >100.0% | >100.0% | >100.0% | |||||||||||||||
HCV | |||||||||||||||||||||||||
MAVYRET | United States | $ | 1,473 | $ | 1,614 | $ | 277 | (8.8 | )% | >100.0% | (8.8 | )% | >100.0% | ||||||||||||
International | 1,420 | 1,824 | 213 | (22.1 | )% | >100.0% | (19.6 | )% | >100.0% | ||||||||||||||||
Total | $ | 2,893 | $ | 3,438 | $ | 490 | (15.9 | )% | >100.0% | (14.6 | )% | >100.0% | |||||||||||||
VIEKIRA | United States | $ | — | $ | 3 | $ | 61 | (100.0 | )% | (96.7 | )% | (100.0 | )% | (96.7 | )% | ||||||||||
International | 36 | 175 | 723 | (79.2 | )% | (75.6 | )% | (77.2 | )% | (74.8 | )% | ||||||||||||||
Total | $ | 36 | $ | 178 | $ | 784 | (79.6 | )% | (77.2 | )% | (77.6 | )% | (76.5 | )% | |||||||||||
Other Key Products | |||||||||||||||||||||||||
Creon | United States | $ | 1,041 | $ | 928 | $ | 831 | 12.2 | % | 11.7 | % | 12.2 | % | 11.7 | % | ||||||||||
Lupron | United States | $ | 720 | $ | 726 | $ | 669 | (0.8 | )% | 8.6 | % | (0.8 | )% | 8.6 | % | ||||||||||
International | 167 | 166 | 160 | 0.8 | % | 3.4 | % | 6.0 | % | 4.7 | % | ||||||||||||||
Total | $ | 887 | $ | 892 | $ | 829 | (0.5 | )% | 7.6 | % | 0.5 | % | 7.9 | % | |||||||||||
Synthroid | United States | $ | 786 | $ | 776 | $ | 781 | 1.3 | % | (0.6 | )% | 1.3 | % | (0.6 | )% | ||||||||||
Synagis | International | $ | 718 | $ | 726 | $ | 738 | (1.2 | )% | (1.6 | )% | 0.9 | % | (2.8 | )% | ||||||||||
Duodopa | United States | $ | 97 | $ | 80 | $ | 61 | 20.4 | % | 31.4 | % | 20.4 | % | 31.4 | % | ||||||||||
International | 364 | 350 | 294 | 4.2 | % | 19.1 | % | 9.8 | % | 14.8 | % | ||||||||||||||
Total | $ | 461 | $ | 430 | $ | 355 | 7.2 | % | 21.2 | % | 11.7 | % | 17.7 | % | |||||||||||
Sevoflurane | United States | $ | 74 | $ | 74 | $ | 78 | 2.0 | % | (6.2 | )% | 2.0 | % | (6.2 | )% | ||||||||||
International | 274 | 317 | 332 | (13.8 | )% | (4.4 | )% | (9.5 | )% | (4.3 | )% | ||||||||||||||
Total | $ | 348 | $ | 391 | $ | 410 | (10.9 | )% | (4.7 | )% | (7.4 | )% | (4.6 | )% | |||||||||||
Kaletra | United States | $ | 38 | $ | 55 | $ | 71 | (31.0 | )% | (22.1 | )% | (31.0 | )% | (22.1 | )% | ||||||||||
International | 245 | 281 | 352 | (12.9 | )% | (20.2 | )% | (9.5 | )% | (20.1 | )% | ||||||||||||||
Total | $ | 283 | $ | 336 | $ | 423 | (15.8 | )% | (20.5 | )% | (12.9 | )% | (20.4 | )% | |||||||||||
AndroGel | United States | $ | 172 | $ | 469 | $ | 577 | (63.3 | )% | (18.8 | )% | (63.3 | )% | (18.8 | )% | ||||||||||
ORILISSA | United States | $ | 91 | $ | 11 | $ | — | >100.0% | n/m | >100.0% | n/m | ||||||||||||||
International | 2 | — | — | n/m | n/m | n/m | n/m | ||||||||||||||||||
Total | $ | 93 | $ | 11 | $ | — | >100.0% | n/m | >100.0% | n/m | |||||||||||||||
All other | $ | 511 | $ | 308 | $ | 876 | 66.1 | % | (64.9 | )% | 73.0 | % | (73.2 | )% | |||||||||||
Total net revenues | $ | 33,266 | $ | 32,753 | $ | 28,216 | 1.6 | % | 16.1 | % | 2.6 | % | 15.2 | % | |||||||||||
n/m – Not meaningful
2019 Form 10-K |  31 31 | ||
The following discussion and analysis of AbbVie's net revenues by product is presented on a constant currency basis.
Global HUMIRA sales decreased 3% in 2019 and increased 7% in 2018. The sales decrease in 2019 was primarily driven by direct biosimilar competition in certain international markets, partially offset by market growth across therapeutic categories. The sales increase in 2018 was primarily driven by market growth across therapeutic categories and geographies as well as favorable pricing in certain geographies. In the United States, HUMIRA sales increased 9% in 2019 and 11% in 2018. The sales increases in 2019 and 2018 were primarily driven by market growth across all indications and favorable pricing. Internationally, HUMIRA revenues decreased 28% in 2019 and increased 1% in 2018. The sales decrease in 2019 was primarily driven by direct biosimilar competition in Europe following the expiration of the European Union composition of matter patent for adalimumab in October 2018. The sales increase in 2018 was primarily driven by market growth across indications partially offset by direct biosimilar competition. Biosimilar competition for HUMIRA is not expected in the United States until 2023. AbbVie continues to pursue strategies intended to further differentiate HUMIRA from competing products and add to the sustainability of HUMIRA.
Net revenues for SKYRIZI were $355 million in 2019 following the April 2019 regulatory approvals for the treatment of moderate to severe plaque psoriasis.
Net revenues for RINVOQ were $47 million in 2019 following the August 2019 FDA approval for the treatment of moderate to severe rheumatoid arthritis.
Net revenues for IMBRUVICA represent product revenues in the United States and collaboration revenues outside of the United States related to AbbVie's 50% share of IMBRUVICA profit. AbbVie's global IMBRUVICA revenues increased 30% in 2019 and 39% in 2018 as a result of continued penetration of IMBRUVICA for patients with CLL as well as favorable pricing.
Net revenues for VENCLEXTA increased by more than 100% in 2019 and 2018 primarily due to market share gains following additional regulatory approvals of VENCLEXTA for the treatment of patients with relapsed/refractory CLL and first-line AML in 2018 and first-line CLL in 2019.
Global MAVYRET sales decreased by 15% in 2019 primarily driven by lower patient volumes in certain international markets and competitive dynamics in the U.S. Global MAVYRET sales increased more than 100% in 2018 as a result of market share gains following the FDA and EMA approvals of MAVYRET in the second half of 2017 as well as further geographic expansion. Global VIEKIRA sales decreased by 78% in 2019 and 76% in 2018 primarily due to lower market share following the launch of MAVYRET.
Net revenues for Creon increased 12% in 2019 and 12% in 2018, primarily driven by continued market growth and favorable pricing. Creon maintains market leadership in the pancreatic enzyme market.
Net revenues for Duodopa increased 12% in 2019 and 18% in 2018, primarily driven by increased market penetration.
Gross Margin
Percent change | |||||||||||||||||
years ended December 31 (dollars in millions) | 2019 | 2018 | 2017 | 2019 | 2018 | ||||||||||||
Gross margin | $ | 25,827 | $ | 25,035 | $ | 21,174 | 3 | % | 18 | % | |||||||
as a percent of net revenues | 78 | % | 76 | % | 75 | % | |||||||||||
Gross margin as a percentage of net revenues in 2019 increased from 2018 primarily due to the full year effect of the expiration of HUMIRA royalties, partially offset by the IMBRUVICA profit sharing arrangement and unfavorable impact from higher intangible asset amortization.
Gross margin as a percentage of net revenues in 2018 increased from 2017 primarily due to the expiration of HUMIRA royalties and a 2017 intangible asset impairment charge of $354 million partially offset by the IMBRUVICA profit sharing arrangement.
32  | 2019 Form 10-K | 2019 Form 10-K | ||
Selling, General and Administrative
Percent change | |||||||||||||||||
years ended December 31 (dollars in millions) | 2019 | 2018 | 2017 | 2019 | 2018 | ||||||||||||
Selling, general and administrative | $ | 6,942 | $ | 7,399 | $ | 6,295 | (6 | )% | 18 | % | |||||||
as a percent of net revenues | 21 | % | 23 | % | 22 | % | |||||||||||
Selling, general and administrative (SG&A) expenses as a percentage of net revenues in 2019 decreased from 2018 primarily due to the favorable impacts of international HUMIRA expense reductions and lower litigation reserve charges that decreased by $326 million. This favorability was partially offset by new product launch expenses, higher restructuring charges and $103 million of transaction expenses associated with the proposed Allergan transaction. Additionally, SG&A expenses in 2018 included non-recurring philanthropic contributions of $350 million to certain U.S. not-for-profit organizations.
SG&A expenses as a percentage of net revenues in 2018 increased from 2017 primarily due to new product launch expenses and non-recurring philanthropic contributions to certain U.S. not-for-profit organizations partially offset by continued leverage from revenue growth.
Research and Development and Acquired In-Process Research and Development
Percent change | |||||||||||||||||
years ended December 31 (dollars in millions) | 2019 | 2018 | 2017 | 2019 | 2018 | ||||||||||||
Research and development | $ | 6,407 | $ | 10,329 | $ | 5,007 | (38 | )% | >100% | ||||||||
as a percent of net revenues | 19 | % | 32 | % | 18 | % | |||||||||||
Acquired in-process research and development | $ | 385 | $ | 424 | $ | 327 | (9 | )% | 30 | % | |||||||
Research and Development (R&D) expenses decreased in 2019 and increased in 2018 principally due to impairment charges related to IPR&D acquired as part of the 2016 Stemcentrx acquisition. In 2019, the company recorded a $1.0 billion intangible asset impairment charge which represented the remaining value of the IPR&D acquired following the decision to terminate the Rova-T R&D program. In 2018, the company recorded a $5.1 billion intangible asset impairment charge following the decision to stop enrollment in the TAHOE trial, which lowered the probabilities of success of achieving regulatory approval across Rova-T and other early-stage assets obtained in the acquisition. See Note 7 to the Consolidated Financial Statements for additional information regarding these impairment charges.
Acquired IPR&D expenses reflect upfront payments related to various collaborations. There were no individually significant transactions or cash flows during 2019 or 2018. Acquired IPR&D expense in 2017 included a charge of $205 million as a result of entering into a global strategic collaboration with Alector, Inc. (Alector) to develop and commercialize medicines to treat Alzheimer’s disease and other neurodegenerative disorders. See Note 5 to the Consolidated Financial Statements for additional information regarding the Alector agreement.
Other Operating Expenses and Income
Other operating income in 2019 included $550 million of income from a legal settlement related to an intellectual property dispute with a third party and $330 million of income related to an amended and restated license agreement between AbbVie and Reata. See Note 5 to the Consolidated Financial Statements for additional information on the Reata agreement.
Other operating expenses in 2018 included a $500 million charge related to the extension of the previously announced Calico collaboration to discover, develop and bring to market new therapies for patients with age-related diseases, including neurodegeneration and cancer. See Note 5 to the Consolidated Financial Statements for additional information regarding the Calico agreement.
2019 Form 10-K |  33 33 | ||
Other Non-Operating Expenses
years ended December 31 (in millions) | 2019 | 2018 | 2017 | |||||||||
Interest expense | $ | 1,784 | $ | 1,348 | $ | 1,150 | ||||||
Interest income | (275 | ) | (204 | ) | (146 | ) | ||||||
Interest expense, net | $ | 1,509 | $ | 1,144 | $ | 1,004 | ||||||
Net foreign exchange loss | $ | 42 | $ | 24 | $ | 348 | ||||||
Other expense, net | 3,006 | 18 | 466 | |||||||||
Interest expense in 2019 increased compared to 2018 primarily due to $363 million of incremental interest and debt issuance costs associated with financing the proposed acquisition of Allergan, as well as the unfavorable impact of higher interest rates on the company's debt obligations. Interest expense in 2018 increased compared to 2017 primarily due to the unfavorable impact of higher interest rates on the company's debt obligations and a higher average outstanding debt balance during 2018.
Interest income in 2019 increased compared to 2018 primarily due to a higher average cash and cash equivalents balance during 2019, partially offset by decreased investments in debt securities. Interest income in 2018 increased compared to 2017 primarily due to higher interest rates.
Net foreign exchange loss in 2017 included $316 million of historical currency translation losses that were reclassified from accumulated other comprehensive income (AOCI) related to the liquidation of certain foreign entities following the enactment of U.S. tax reform.
Other expense, net included charges related to the change in fair value of the contingent consideration liabilities of $3.1 billion in 2019, $49 million in 2018 and $626 million in 2017. The fair value of contingent consideration liabilities is impacted by the passage of time and multiple other inputs, including the probability of success of achieving regulatory/commercial milestones, discount rates, the estimated amount of future sales of the acquired products still in development and other market-based factors. In 2019, the Boehringer Ingelheim (BI) contingent consideration liability increased due to higher probabilities of success, higher estimated future sales, declining interest rates and passage of time. The higher probabilities of success primarily resulted from the April 2019 regulatory approvals of SKYRIZI for the treatment of moderate to severe plaque psoriasis. These changes were partially offset by a $91 million decrease in the Stemcentrx contingent consideration liability due to the termination of the Rova-T R&D program during the third quarter of 2019. In 2018, the BI contingent consideration liability increased due to the passage of time and higher estimated future sales partially offset by the effect of rising interest rates. This increase in the BI contingent consideration liability was primarily offset by a $428 million decrease in the Stemcentrx contingent consideration liability recorded during the fourth quarter of 2018 due to a reduction in probabilities of success of achieving regulatory approval across Rova-T and other early-stage Stemcentrx assets. In 2017, the change in fair value represented mainly higher probabilities of success, the passage of time and declining interest rates. Other expense, net for 2017 also included realized gains on available-for-sale investment securities of $90 million.
Income Tax Expense
The effective income tax rate was 6% in 2019, negative 9% in 2018 and 31% in 2017. The effective tax rate in each period differed from the statutory tax rate principally due to the allocation of the company's taxable earnings among jurisdictions, the benefit from foreign operations which reflects the impact of lower income tax rates in locations outside the United States, tax incentives in Puerto Rico and other foreign tax jurisdictions and business development activities. The increase in the effective tax rate for 2019 over the prior year was principally due to the timing of provisions of the Tax Cuts and Jobs Act (the Act) related to the earnings from certain foreign subsidiaries. The increase is also attributable to changes in the jurisdictional mix of earnings, including a change in fair value of contingent consideration liabilities. These increases were partially offset by the favorable resolution of various tax positions in the current year.
The effective tax rate for 2018 also included the effects of Stemcentrx intangible impairment related expenses.
The effective tax rate in 2017 included tax expense of $4.5 billion on the one-time mandatory repatriation of previously untaxed earnings of foreign subsidiaries, partially offset by a $3.6 billion net tax benefit for the remeasurement of deferred taxes related to the Act and foreign tax law changes.
34  | 2019 Form 10-K | 2019 Form 10-K | ||
The Act significantly changed the U.S. corporate tax system. The Act reduced the U.S. federal corporate tax rate from 35% to 21% and created a territorial tax system that included new taxes on certain foreign sourced earnings. See Note 14 to the Consolidated Financial Statements for additional information regarding the Act.
FINANCIAL POSITION, LIQUIDITY AND CAPITAL RESOURCES
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Cash flows from: | |||||||||||
Operating activities | $ | 13,324 | $ | 13,427 | $ | 9,960 | |||||
Investing activities | 596 | (1,006 | ) | (274 | ) | ||||||
Financing activities | 18,708 | (14,396 | ) | (5,512 | ) | ||||||
Operating cash flows in 2019 decreased slightly from 2018 primarily due to higher payments for income taxes offset by improved results of operations resulting from an increase in operating earnings. Operating cash flows in 2018 increased from 2017 primarily due to improved results of operations from revenue growth and a decrease in income tax payments. Operating cash flows also reflected AbbVie’s contributions to its defined benefit plans of $727 million in 2019, $873 million in 2018 and $246 million in 2017.
Investing cash flows in 2019 included net sales and maturities of investments totaling $2.1 billion resulting from the sale of substantially all of the company's investments in debt securities, payments made for other acquisitions and investments of $1.1 billion and capital expenditures of $552 million. Investing cash flows in 2018 included payments made for other acquisitions and investments of $736 million and capital expenditures of $638 million, partially offset by net sales and maturities of investment securities totaling $368 million. Investing cash flows in 2017 included capital expenditures of $529 million and payments made for other acquisitions and investments of $308 million, partially offset by net sales and maturities of investment securities totaling $563 million.
Financing cash flows in 2019 included the issuance of $30.0 billion aggregate principal amount of floating rate and fixed rate unsecured senior notes at maturities ranging from 18 months to 30 years. AbbVie expects to use the net proceeds of $29.8 billion to fund a portion of the aggregate cash consideration due to Allergan shareholders in connection with the proposed acquisition and to pay related fees and expenses. Pending the consummation of the proposed Allergan acquisition, the net proceeds from the offering are permitted to be invested temporarily in short-term investments. All of the notes are subject to special mandatory redemption at a redemption price equal to 101% of the aggregate principal amount of the notes plus accrued and unpaid interest if the proposed acquisition of Allergan is not completed by January 30, 2021 or the company notifies the trustee in respect of the notes that it will not pursue the consummation of the proposed Allergan acquisition.
Additionally, financing cash flows in 2019 included the issuance of €1.4 billion aggregate principal amount of unsecured senior Euro notes which the company used to redeem €1.4 billion aggregate principal amount of 0.38% senior Euro notes that were due to mature in November 2019, as well as the repayment of a $3.0 billion 364-day term loan credit agreement that was scheduled to mature in June 2019.
Financing cash flows in 2018 included proceeds from the issuance of $3.0 billion drawn under the term loan in June 2018. In September 2018, the company issued $6.0 billion aggregate principal amount of unsecured senior notes. Of the $5.9 billion net proceeds, $2.0 billion was used to repay the company's outstanding three-year term loan credit agreement in September 2018 and $1.0 billion was used to repay the aggregate principal amount of 2.00% senior notes at maturity in November 2018. Financing cash flows in 2018 also included the May 2018 repayment of $3.0 billion aggregate principal amount of the company's 1.80% senior notes at maturity.
In 2019, 2018 and 2017, the company issued and redeemed commercial paper. There were no commercial paper borrowings outstanding as of December 31, 2019 and there was $699 million outstanding as of December 31, 2018. AbbVie may issue additional commercial paper or retire commercial paper to meet liquidity requirements as needed.
Cash dividend payments totaled $6.4 billion in 2019, $5.6 billion in 2018 and $4.1 billion in 2017. The increase in cash dividend payments was primarily driven by an increase in the dividend rate. On November 1, 2019, AbbVie announced that its board of directors declared an increase in the quarterly cash dividend from $1.07 per share to $1.18 per share beginning with the dividend payable on February 14, 2020 to stockholders of record as of January 15, 2020. This reflects an increase of approximately 10.3% over the previous quarterly rate. The timing, declaration, amount of and payment of any dividends by AbbVie in the future is within the discretion of its board of directors and will depend upon many factors, including AbbVie's financial condition, earnings, capital requirements of its operating subsidiaries, covenants associated with certain of AbbVie's
2019 Form 10-K |  35 35 | ||
debt service obligations, legal requirements, regulatory constraints, industry practice, ability to access capital markets and other factors deemed relevant by its board of directors.
On February 15, 2018, AbbVie's board of directors authorized a new $10.0 billion stock repurchase program, which superseded AbbVie's previous stock repurchase program. On December 13, 2018, AbbVie's board of directors authorized a $5.0 billion increase to the existing $10.0 billion stock repurchase program. The company's stock repurchase authorization permits purchases of AbbVie shares from time to time in open-market or private transactions at management’s discretion. The program has no time limit and can be discontinued at any time. Under this authorization, AbbVie repurchased 4 million shares for $300 million in 2019 and 109 million shares for $10.7 billion in 2018. AbbVie cash-settled $201 million of its December 2018 open market purchases in January 2019. AbbVie's remaining stock repurchase authorization was $4.0 billion as of December 31, 2019.
Under previous stock repurchase programs, AbbVie made open market share repurchases of 11 million shares for $1.3 billion in 2018 and 13 million shares for $1.0 billion in 2017. AbbVie cash-settled $285 million of its December 2016 open market purchases in January 2017.
In 2019, AbbVie made contingent consideration milestone and royalty payments to BI totaling $234 million following the commercial launch of SKYRIZI in certain geographies. $163 million of these payments were included in financing cash flows and $71 million of the payments were included in operating cash flows. In 2018, AbbVie paid $100 million of contingent consideration to BI related to BLA and MAA acceptance milestones. $78 million of these payments were included in financing cash flows and $22 million of the payments were included in operating cash flows. In 2017, AbbVie paid $305 million of contingent consideration to BI related to a Phase 3 enrollment milestone. $268 million of this milestone was included in financing cash flows and $37 million was included in operating cash flows.
In connection with the proposed acquisition of Allergan, on June 25, 2019, AbbVie entered into a $38.0 billion 364-day bridge credit agreement and on July 12, 2019, AbbVie entered into a $6.0 billion term loan credit agreement. The company incurred a total of $242 million of debt issuance costs related to the two agreements. On October 25, 2019, AbbVie commenced offers to exchange any and all outstanding notes of certain series issued by Allergan for up to $15.5 billion aggregate principal amount and €3.7 billion aggregate principal amount of new notes to be issued by AbbVie and cash, subject to conditions including the closing of the proposed acquisition. See Note 10 to the Consolidated Financial Statements for additional information. In February 2020, the remaining commitments under the bridge credit agreement were reduced to $0 as a result of cash on hand at AbbVie. AbbVie subsequently terminated the bridge credit agreement in its entirety as permitted under its terms.
Credit Risk
AbbVie monitors economic conditions, the creditworthiness of customers and government regulations and funding, both domestically and abroad. AbbVie regularly communicates with its customers regarding the status of receivable balances, including their payment plans and obtains positive confirmation of the validity of the receivables. AbbVie establishes an allowance against accounts receivable when it is probable they will not be collected. AbbVie may also utilize factoring arrangements to mitigate credit risk, although the receivables included in such arrangements have historically not been a significant amount of total outstanding receivables.
Credit Facility, Access to Capital and Credit Ratings
Credit Facility
In August 2019, AbbVie entered into an amended and restated $4.0 billion five-year revolving credit facility that matures in August 2024. This amended facility enables the company to borrow funds on an unsecured basis at variable interest rates and contains various covenants. At December 31, 2019, the company was in compliance with all its credit facility covenants. Commitment fees under the credit facility were insignificant. No amounts were outstanding under the company's credit facilities as of December 31, 2019 and 2018.
Access to Capital
The company intends to fund short-term and long-term financial obligations as they mature through cash on hand, future cash flows from operations, or by issuing additional debt. The company's ability to generate cash flows from operations, issue debt or enter into financing arrangements on acceptable terms could be adversely affected if there is a material decline in the demand for the company's products or in the solvency of its customers or suppliers, deterioration in the company's key financial ratios or credit ratings, or other material unfavorable changes in business conditions. At the current time, the company believes it has sufficient financial flexibility to issue debt, enter into other financing arrangements and attract long-term capital on acceptable terms to support the company's growth objectives.
36  | 2019 Form 10-K | 2019 Form 10-K | ||
Credit Ratings
Following the announcement of the proposed acquisition of Allergan and the $30.0 billion senior notes issuance, Moody's Investor Service affirmed its Baa2 senior unsecured long-term rating and Prime-2 short-term rating with a stable outlook. S&P Global Ratings revised its ratings outlook to negative from stable and expects to lower the issuer credit rating by one notch to BBB+ from A- and the short-term rating to A-2 from A-1 when the acquisition is complete.
Unfavorable changes to the ratings may have an adverse impact on future financing arrangements; however, they would not affect the company's ability to draw on its credit facility and would not result in an acceleration of scheduled maturities of any of the company's outstanding debt.
Contractual Obligations
The following table summarizes AbbVie's estimated contractual obligations as of December 31, 2019:
(in millions) | Total | Less than one year | One to three years | Three to five years | More than five years | ||||||||||||||
Long-term debt, including current portion | $ | 67,233 | $ | 3,750 | $ | 14,150 | $ | 7,625 | $ | 41,708 | |||||||||
Interest on long-term debt(a) | 30,494 | 2,146 | 4,087 | 3,479 | 20,782 | ||||||||||||||
Non-cancelable operating and finance lease payments(f) | 774 | 129 | 224 | 125 | 296 | ||||||||||||||
Purchase obligations and other(b) | 3,532 | 3,295 | 186 | 45 | 6 | ||||||||||||||
Other long-term liabilities (c) (d) (e) | 11,544 | 166 | 1,395 | 2,123 | 7,860 | ||||||||||||||
Total | $ | 113,577 | $ | 9,486 | $ | 20,042 | $ | 13,397 | $ | 70,652 | |||||||||
(a) | Includes estimated future interest payments on long-term debt. Interest payments on debt are calculated for future periods using forecasted interest rates in effect at the end of 2019. Projected interest payments include the related effects of interest rate swap agreements. Certain of these projected interest payments may differ in the future based on changes in floating interest rates or other factors or events. The projected interest payments only pertain to obligations and agreements outstanding at December 31, 2019. See Note 10 to the Consolidated Financial Statements for additional information regarding the company's debt instruments and Note 11 for additional information on the interest rate swap agreements outstanding at December 31, 2019. |
(b) | Includes the company's significant unconditional purchase obligations. These commitments do not exceed the company's projected requirements and are made in the normal course of business. |
(c) | Excludes liabilities associated with the company's unrecognized tax benefits as it is not possible to reliably estimate the timing of the future cash outflows related to these liabilities. See Note 14 to the Consolidated Financial Statements for additional information on these unrecognized tax benefits. |
(d) | Includes $7.3 billion of contingent consideration liabilities which are recorded at fair value on the consolidated balance sheet. Potential contingent consideration payments that exceed the fair value recorded on the consolidated balance sheet are not included in the table of contractual obligations. See Note 11 to the Consolidated Financial Statements for additional information regarding these liabilities. |
(e) | Includes a one-time transition tax liability on a mandatory deemed repatriation of previously untaxed earnings of foreign subsidiaries resulting from U.S. tax reform enacted in 2017. The one-time transition tax is generally payable in eight annual installments. See Note 14 to the Consolidated Financial Statements for additional information regarding these tax liabilities. |
(f) | Lease payments include approximately $350 million of contractual minimum lease payments for leases executed but not yet commenced. These leases will commence in 2020 with lease terms of approximately 11 years. |
AbbVie enters into R&D collaboration arrangements with third parties that may require future milestone payments to third parties contingent upon the achievement of certain development, regulatory, or commercial milestones. Individually, these arrangements are insignificant in any one annual reporting period. However, if milestones for multiple products covered by these arrangements would happen to be reached in the same reporting period, the aggregate charge to expense could be material to the results of operations in that period. From a business perspective, the payments are viewed as positive because they signify that the product is successfully moving through development and is now generating or is more likely to generate future cash flows from product sales. It is not possible to predict with reasonable certainty whether these milestones will be achieved or the timing for achievement. As a result, these potential payments are not included in the table of contractual
2019 Form 10-K |  37 37 | ||
obligations. See Note 5 to the Consolidated Financial Statements for additional information on these collaboration arrangements.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of financial statements in accordance with generally accepted accounting principles in the United States requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities and the reported amounts of revenue and expenses. A summary of the company's significant accounting policies is included in Note 2 to the Consolidated Financial Statements. Certain of these policies are considered critical as these most significantly impact the company's financial condition and results of operations and require the most difficult, subjective, or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Actual results may vary from these estimates.
Revenue Recognition
AbbVie recognizes revenue when control of promised goods or services is transferred to the company’s customers, in an amount that reflects the consideration AbbVie expects to be entitled to in exchange for those goods or services. Sales, value add and other taxes collected concurrent with revenue-producing activities are excluded from revenue. AbbVie generates revenue primarily from product sales. For the majority of sales, the company transfers control, invoices the customer and recognizes revenue upon shipment to the customer.
Rebates
AbbVie provides rebates to pharmacy benefit managers, state government Medicaid programs, insurance companies that administer Medicare drug plans, wholesalers, group purchasing organizations and other government agencies and private entities.
Rebate and chargeback accruals are accounted for as variable consideration and are recorded as a reduction to revenue in the period the related product is sold. Rebates and chargebacks totaled $18.8 billion in 2019, $16.4 billion in 2018 and $12.9 billion in 2017. Rebate amounts are typically based upon the volume of purchases using contractual or statutory prices, which may vary by product and by payer. For each type of rebate, the factors used in the calculations of the accrual for that rebate include the identification of the products subject to the rebate, the applicable price terms and the estimated lag time between sale and payment of the rebate, which can be significant.
In order to establish its rebate and chargeback accruals, the company uses both internal and external data to estimate the level of inventory in the distribution channel and the rebate claims processing lag time for each type of rebate. To estimate the rebate percentage or net price, the company tracks sales by product and by customer or payer. The company evaluates inventory data reported by wholesalers, available prescription volume information, product pricing, historical experience and other factors in order to determine the adequacy of its reserves. AbbVie regularly monitors its reserves and records adjustments when rebate trends, rebate programs and contract terms, legislative changes, or other significant events indicate that a change in the reserve is appropriate. Historically, adjustments to rebate accruals have not been material to net earnings.
38  | 2019 Form 10-K | 2019 Form 10-K | ||
The following table is an analysis of the three largest rebate accruals and chargeback allowances, which comprise approximately 94% of the total consolidated rebate and chargebacks recorded as reductions to revenues in 2019. Remaining rebate provisions charged against gross revenues are not significant in the determination of operating earnings.
(in millions) | Medicaid and Medicare Rebates | Managed Care Rebates | Wholesaler Chargebacks | ||||||||
Balance at December 31, 2016 | $ | 1,167 | $ | 1,167 | $ | 383 | |||||
Provisions | 2,909 | 3,990 | 5,026 | ||||||||
Payments | (2,736 | ) | (3,962 | ) | (4,887 | ) | |||||
Balance at December 31, 2017 | 1,340 | 1,195 | 522 | ||||||||
Provisions | 3,493 | 4,729 | 6,659 | ||||||||
Payments | (3,188 | ) | (4,485 | ) | (6,525 | ) | |||||
Balance at December 31, 2018 | 1,645 | 1,439 | 656 | ||||||||
Provisions | 4,035 | 5,772 | 7,947 | ||||||||
Payments | (3,915 | ) | (5,275 | ) | (7,917 | ) | |||||
Balance at December 31, 2019 | $ | 1,765 | $ | 1,936 | $ | 686 | |||||
Cash Discounts and Product Returns
Cash discounts and product returns, which totaled $1.6 billion in 2019, $1.6 billion in 2018 and $1.3 billion in 2017, are accounted for as variable consideration and are recorded as a reduction to revenue in the same period the related product is sold. The reserve for cash discounts is readily determinable because the company's experience of payment history is fairly consistent. Product returns can be reliably estimated based on the company's historical return experience.
Pension and Other Post-Employment Benefits
AbbVie engages outside actuaries to assist in the determination of the obligations and costs under the pension and other post-employment benefit plans that are direct obligations of AbbVie. The valuation of the funded status and the net periodic benefit cost for these plans are calculated using actuarial assumptions. The significant assumptions, which are reviewed annually, include the discount rate, the expected long-term rate of return on plan assets and the health care cost trend rates, and are disclosed in Note 12 to the Consolidated Financial Statements.
The discount rate is selected based on current market rates on high-quality, fixed-income investments at December 31 each year. AbbVie employs a yield-curve approach for countries where a robust bond market exists. The yield curve is developed using high-quality bonds. The yield-curve approach reflects the plans' specific cash flows (i.e. duration) in calculating the benefit obligations by applying the corresponding individual spot rates along the yield curve. AbbVie reflects the plans' specific cash flows and applies them to the corresponding individual spot rates along the yield curve in calculating the service cost and interest cost portions of expense. For other countries, AbbVie reviews various indices such as corporate bond and government bond benchmarks to estimate the discount rate. AbbVie's assumed discount rates have a significant effect on the amounts reported for defined benefit pension and other post-employment plans as of December 31, 2019. A 50 basis point change in the assumed discount rate would have had the following effects on AbbVie's calculation of net periodic benefit costs in 2020 and projected benefit obligations as of December 31, 2019:
50 basis point | |||||||
(in millions) (brackets denote a reduction) | Increase | Decrease | |||||
Defined benefit plans | |||||||
Service and interest cost | $ | (76 | ) | $ | 92 | ||
Projected benefit obligation | (723 | ) | 825 | ||||
Other post-employment plans | |||||||
Service and interest cost | $ | (11 | ) | $ | 14 | ||
Projected benefit obligation | (101 | ) | 117 | ||||
The expected long-term rate of return is based on the asset allocation, historical performance and the current view of expected future returns. AbbVie considers these inputs with a long-term focus to avoid short-term market influences. The
2019 Form 10-K |  39 39 | ||
current long-term rate of return on plan assets for each plan is supported by the historical performance of the trust's actual and target asset allocation. AbbVie's assumed expected long-term rate of return has a significant effect on the amounts reported for defined benefit pension plans as of December 31, 2019 and will be used in the calculation of net periodic benefit cost in 2020. A one percentage point change in assumed expected long-term rate of return on plan assets would increase or decrease the net period benefit cost of these plans in 2020 by $71 million.
The health care cost trend rate is selected by reviewing historical trends and current views on projected future health care cost increases. The current health care cost trend rate is supported by the historical trend experience of each plan. Assumed health care cost trend rates have a significant effect on the amounts reported for health care plans as of December 31, 2019 and will be used in the calculation of net periodic benefit cost in 2020. A one percentage point change in assumed health care cost trend rates would have the following effects on AbbVie's calculation of net periodic benefit costs in 2020 and the projected benefit obligation as of December 31, 2019:
One percentage point | |||||||
(in millions) (brackets denote a reduction) | Increase | Decrease | |||||
Service and interest cost | $ | 40 | $ | (28 | ) | ||
Projected benefit obligation | 244 | (186 | ) | ||||
Income Taxes
AbbVie accounts for income taxes under the asset and liability method. Provisions for federal, state and foreign income taxes are calculated on reported pretax earnings based on current tax laws. Deferred taxes are provided using enacted tax rates on the future tax consequences of temporary differences, which are the differences between the financial statement carrying amount of assets and liabilities and their respective tax bases and the tax benefits of carryforwards. A valuation allowance is established or maintained when, based on currently available information, it is more likely than not that all or a portion of a deferred tax asset will not be realized.
Litigation
The company is subject to contingencies, such as various claims, legal proceedings and investigations regarding product liability, intellectual property, commercial, securities and other matters that arise in the normal course of business. See Note 15 to the Consolidated Financial Statements for additional information. Loss contingency provisions are recorded for probable losses at management's best estimate of a loss, or when a best estimate cannot be made, a minimum loss contingency amount within a probable range is recorded. Accordingly, AbbVie is often initially unable to develop a best estimate of loss and therefore, the minimum amount, which could be zero, is recorded. As information becomes known, either the minimum loss amount is increased, resulting in additional loss provisions, or a best estimate can be made, also resulting in additional loss provisions. Occasionally, a best estimate amount is changed to a lower amount when events result in an expectation of a more favorable outcome than previously expected.
Valuation of Goodwill and Intangible Assets
AbbVie has acquired and may continue to acquire significant intangible assets in connection with business combinations that AbbVie records at fair value. Transactions involving the purchase or sale of intangible assets occur with some frequency between companies in the pharmaceuticals industry and valuations are usually based on a discounted cash flow analysis incorporating the stage of completion. The discounted cash flow model requires assumptions about the timing and amount of future net cash flows, risk, cost of capital, terminal values and market participants. Each of these factors can significantly affect the value of the intangible asset. IPR&D acquired in a business combination is capitalized as an indefinite-lived intangible asset until regulatory approval is obtained, at which time it is accounted for as a definite-lived asset and amortized over its estimated useful life, or discontinuation, at which point the intangible asset will be written off. IPR&D acquired in transactions that are not business combinations is expensed immediately, unless deemed to have an alternative future use. Payments made to third parties subsequent to regulatory approval are capitalized and amortized over the remaining useful life.
AbbVie reviews the recoverability of definite-lived intangible assets whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable. Goodwill and indefinite-lived intangible assets are reviewed for impairment annually or when an event occurs that could result in an impairment. See Note 2 to the Consolidated Financial Statements for further information.
Annually, the company tests its goodwill for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. Some of the factors considered in the assessment include general macro-economic conditions, conditions specific to the industry and market, cost factors, the overall financial
40  | 2019 Form 10-K | 2019 Form 10-K | ||
performance and whether there have been sustained declines in the company's share price. If the company concludes it is more likely than not that the fair value of the reporting unit is less than its carrying amount, a quantitative impairment test is performed. AbbVie tests indefinite-lived intangible assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If the company concludes it is more likely than not that the fair value is less than its carrying amount, a quantitative impairment test is performed.
For its quantitative impairment tests, the company uses an estimated future cash flow approach that requires significant judgment with respect to future volume, revenue and expense growth rates, changes in working capital use, the selection of an appropriate discount rate, asset groupings and other assumptions and estimates. The estimates and assumptions used are consistent with the company's business plans and a market participant's views. The use of alternative estimates and assumptions could increase or decrease the estimated fair value of the assets and could potentially impact the company's results of operations. Actual results may differ from the company's estimates.
Contingent Consideration
The fair value measurements of contingent consideration liabilities are determined as of the acquisition date based on significant unobservable inputs, including the discount rate, estimated probabilities and timing of achieving specified development, regulatory and commercial milestones and the estimated amount of future sales of the acquired products. Contingent consideration liabilities are revalued to fair value at each subsequent reporting date until the related contingency is resolved. The potential contingent consideration payments are estimated by applying a probability-weighted expected payment model for contingent milestone payments and a Monte Carlo simulation model for contingent royalty payments, which are then discounted to present value. Changes to the fair value of the contingent consideration liabilities can result from changes to one or a number of inputs, including discount rates, the probabilities of achieving the milestones, the time required to achieve the milestones and estimated future sales. Significant judgment is employed in determining the appropriateness of certain of these inputs. Changes to the inputs described above could have a material impact on the company's financial position and results of operations in any given period. At December 31, 2019, a 50 basis point increase/decrease in the assumed discount rate would have decreased/increased the value of the contingent consideration liabilities by approximately $280 million. Additionally, at December 31, 2019, a five percentage point increase/decrease in the assumed probability of success across all potential indications would have increased/decreased the value of the contingent consideration liabilities by approximately $150 million.
Recent Accounting Pronouncements
See Note 2 to the Consolidated Financial Statements for additional information on recent accounting pronouncements.
2019 Form 10-K |  41 41 | ||
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The company is exposed to risk that its earnings, cash flows and equity could be adversely impacted by changes in foreign exchange rates and interest rates. Certain derivative instruments are used when available on a cost-effective basis to hedge the company's underlying economic exposures. See Note 11 to the Consolidated Financial Statements for additional information regarding the company's financial instruments and hedging strategies.
Foreign Currency Risk
AbbVie's primary net foreign currency exposures are the Euro, Japanese yen, Canadian dollar and British pound. The following table reflects the total foreign currency forward exchange contracts outstanding at December 31, 2019 and 2018:
2019 | 2018 | ||||||||||||||||||||
(in millions) | Contract amount | Weighted average exchange rate | Fair and carrying value receivable/(payable) | Contract amount | Weighted average exchange rate | Fair and carrying value receivable/(payable) | |||||||||||||||
Receive primarily U.S. dollars in exchange for the following currencies: | |||||||||||||||||||||
Euro | $ | 6,217 | 1.116 | $ | (12 | ) | $ | 6,660 | 1.157 | $ | 68 | ||||||||||
Japanese yen | 820 | 108.7 | — | 1,076 | 111.5 | (12 | ) | ||||||||||||||
Canadian dollar | 504 | 1.324 | (6 | ) | 406 | 1.314 | 14 | ||||||||||||||
British pound | 427 | 1.305 | (6 | ) | 499 | 1.328 | 21 | ||||||||||||||
All other currencies | 1,508 | n/a | (10 | ) | 1,370 | n/a | 15 | ||||||||||||||
Total | $ | 9,476 | $ | (34 | ) | $ | 10,011 | $ | 106 | ||||||||||||
The company estimates that a 10% appreciation in the underlying currencies being hedged from their levels against the U.S. dollar, with all other variables held constant, would decrease the fair value of foreign exchange forward contracts by $942 million at December 31, 2019. If realized, this appreciation would negatively affect earnings over the remaining life of the contracts. However, gains and losses on the hedging instruments offset losses and gains on the hedged transactions and reduce the earnings and stockholders' equity volatility relating to foreign exchange. A 10% appreciation is believed to be a reasonably possible near-term change in foreign currencies.
As of December 31, 2019, the company has €3.6 billion aggregate principal amount of unsecured senior Euro notes outstanding, which are exposed to foreign currency risk. The company designated these foreign currency denominated notes as hedges of its net investments in certain foreign subsidiaries and affiliates. As a result, any foreign currency translation gains or losses related to the Euro notes will be included in accumulated other comprehensive income. See Note 10 to the Consolidated Financial Statements for additional information regarding to the senior Euro notes and Note 11 to the Consolidated Financial Statements for additional information regarding to the net investment hedging program.
Interest Rate Risk
The company estimates that an increase in interest rates of 100 basis points would adversely impact the fair value of AbbVie's interest rate swap contracts by approximately $280 million at December 31, 2019. If realized, the fair value reduction would affect earnings over the remaining life of the contracts. The company estimates that an increase of 100 basis points in long-term interest rates would decrease the fair value of long-term debt by $5.0 billion at December 31, 2019. A 100 basis point change is believed to be a reasonably possible near-term change in interest rates.
42  | 2019 Form 10-K | 2019 Form 10-K | ||
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Page | |
Consolidated Financial Statements | |
2019 Form 10-K |  43 43 | ||
AbbVie Inc. and Subsidiaries
Consolidated Statements of Earnings
years ended December 31 (in millions, except per share data) | 2019 | 2018 | 2017 | ||||||||
Net revenues | $ | $ | $ | ||||||||
Cost of products sold | |||||||||||
Selling, general and administrative | |||||||||||
Research and development | |||||||||||
Acquired in-process research and development | |||||||||||
Other operating expense (income) | ( | ) | |||||||||
Total operating costs and expenses | |||||||||||
Operating earnings | |||||||||||
Interest expense, net | |||||||||||
Net foreign exchange loss | |||||||||||
Other expense, net | |||||||||||
Earnings before income tax | |||||||||||
Income tax expense (benefit) | ( | ) | |||||||||
Net earnings | $ | $ | $ | ||||||||
Per share data | |||||||||||
Basic earnings per share | $ | $ | $ | ||||||||
Diluted earnings per share | $ | $ | $ | ||||||||
Weighted-average basic shares outstanding | |||||||||||
Weighted-average diluted shares outstanding | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
44  | 2019 Form 10-K | 2019 Form 10-K | ||
AbbVie Inc. and Subsidiaries
Consolidated Statements of Comprehensive Income
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Net earnings | $ | $ | $ | ||||||||
Foreign currency translation adjustments, net of tax expense (benefit) of $(4) in 2019, $(18) in 2018 and $34 in 2017 | ( | ) | ( | ) | |||||||
Net investment hedging activities, net of tax expense (benefit) of $22 in 2019, $40 in 2018 and $(194) in 2017 | ( | ) | |||||||||
Pension and post-employment benefits, net of tax expense (benefit) of $(323) in 2019, $35 in 2018 and $(94) in 2017 | ( | ) | ( | ) | |||||||
Marketable security activities, net of tax expense (benefit) of $— in 2019, $— in 2018 and $(8) in 2017 | ( | ) | ( | ) | |||||||
Cash flow hedging activities, net of tax expense (benefit) of $70 in 2019, $23 in 2018 and $(26) in 2017 | ( | ) | |||||||||
Other comprehensive income (loss) | ( | ) | ( | ) | |||||||
Comprehensive income | $ | $ | $ | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
2019 Form 10-K |  45 45 | ||
AbbVie Inc. and Subsidiaries
Consolidated Balance Sheets
as of December 31 (in millions, except share data) | 2019 | 2018 | |||||
Assets | |||||||
Current assets | |||||||
Cash and equivalents | $ | $ | |||||
Short-term investments | |||||||
Accounts receivable, net | |||||||
Inventories | |||||||
Prepaid expenses and other | |||||||
Total current assets | |||||||
Investments | |||||||
Property and equipment, net | |||||||
Intangible assets, net | |||||||
Goodwill | |||||||
Other assets | |||||||
Total assets | $ | $ | |||||
Liabilities and Equity | |||||||
Current liabilities | |||||||
Short-term borrowings | $ | $ | |||||
Current portion of long-term debt and finance lease obligations | |||||||
Accounts payable and accrued liabilities | |||||||
Total current liabilities | |||||||
Long-term debt and finance lease obligations | |||||||
Deferred income taxes | |||||||
Other long-term liabilities | |||||||
Commitments and contingencies | |||||||
Stockholders’ equity (deficit) | |||||||
Common stock, $0.01 par value, 4,000,000,000 shares authorized, 1,781,582,608 shares issued as of December 31, 2019 and 1,776,510,871 as of December 31, 2018 | |||||||
Common stock held in treasury, at cost, 302,671,146 shares as of December 31, 2019 and 297,686,473 as of December 31, 2018 | ( | ) | ( | ) | |||
Additional paid-in capital | |||||||
Retained earnings | |||||||
Accumulated other comprehensive loss | ( | ) | ( | ) | |||
Total stockholders’ equity (deficit) | ( | ) | ( | ) | |||
Total liabilities and equity | $ | $ | |||||
The accompanying notes are an integral part of these consolidated financial statements.
46  | 2019 Form 10-K | 2019 Form 10-K | ||
AbbVie Inc. and Subsidiaries
Consolidated Statements of Equity
years ended December 31 (in millions) | Common shares outstanding | Common stock | Treasury stock | Additional paid-in capital | Retained earnings | Accumulated other comprehensive loss | Total | |||||||||||||||||||
Balance at December 31, 2016 | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||||||||
Net earnings | — | |||||||||||||||||||||||||
Other comprehensive loss, net of tax | — | ( | ) | ( | ) | |||||||||||||||||||||
Dividends declared | — | ( | ) | ( | ) | |||||||||||||||||||||
Purchases of treasury stock | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
Stock-based compensation plans and other | ( | ) | ||||||||||||||||||||||||
Balance at December 31, 2017 | ( | ) | ( | ) | ||||||||||||||||||||||
Adoption of new accounting standards(a) | — | ( | ) | ( | ) | |||||||||||||||||||||
Net earnings | — | |||||||||||||||||||||||||
Other comprehensive income, net of tax | — | |||||||||||||||||||||||||
Dividends declared | — | ( | ) | ( | ) | |||||||||||||||||||||
Purchases of treasury stock | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
Stock-based compensation plans and other | ||||||||||||||||||||||||||
Balance at December 31, 2018 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
Net earnings | — | |||||||||||||||||||||||||
Other comprehensive loss, net of tax | — | ( | ) | ( | ) | |||||||||||||||||||||
Dividends declared | — | ( | ) | ( | ) | |||||||||||||||||||||
Purchases of treasury stock | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
Stock-based compensation plans and other | ||||||||||||||||||||||||||
Balance at December 31, 2019 | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||
(a) | Adoption of new accounting standards primarily includes the cumulative-effect adjustment of Accounting Standards Update (ASU) No. 2016-16, Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other Than Inventory. |
The accompanying notes are an integral part of these consolidated financial statements.
2019 Form 10-K |  47 47 | ||
AbbVie Inc. and Subsidiaries
Consolidated Statements of Cash Flows
years ended December 31 (in millions) (brackets denote cash outflows) | 2019 | 2018 | 2017 | ||||||||
Cash flows from operating activities | |||||||||||
Net earnings | $ | $ | $ | ||||||||
Adjustments to reconcile net earnings to net cash from operating activities: | |||||||||||
Depreciation | |||||||||||
Amortization of intangible assets | |||||||||||
Change in fair value of contingent consideration liabilities | |||||||||||
Stock-based compensation | |||||||||||
Upfront costs and milestones related to collaborations | |||||||||||
Gain on divestitures | ( | ) | |||||||||
Intangible asset impairment | |||||||||||
Impacts related to U.S. tax reform | |||||||||||
Other, net | |||||||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts receivable | ( | ) | ( | ) | ( | ) | |||||
Inventories | ( | ) | ( | ) | |||||||
Prepaid expenses and other assets | ( | ) | ( | ) | |||||||
Accounts payable and other liabilities | ( | ) | |||||||||
Cash flows from operating activities | |||||||||||
Cash flows from investing activities | |||||||||||
Acquisitions and investments | ( | ) | ( | ) | ( | ) | |||||
Acquisitions of property and equipment | ( | ) | ( | ) | ( | ) | |||||
Purchases of investment securities | ( | ) | ( | ) | ( | ) | |||||
Sales and maturities of investment securities | |||||||||||
Other | |||||||||||
Cash flows from investing activities | ( | ) | ( | ) | |||||||
Cash flows from financing activities | |||||||||||
Net change in commercial paper borrowings | ( | ) | |||||||||
Proceeds from issuance of other short-term borrowings | |||||||||||
Repayments of other short-term borrowings | ( | ) | |||||||||
Proceeds from issuance of long-term debt | — | ||||||||||
Repayments of long-term debt and finance lease obligations | ( | ) | ( | ) | ( | ) | |||||
Debt issuance costs | ( | ) | ( | ) | |||||||
Dividends paid | ( | ) | ( | ) | ( | ) | |||||
Purchases of treasury stock | ( | ) | ( | ) | ( | ) | |||||
Proceeds from the exercise of stock options | |||||||||||
Payments of contingent consideration liabilities | ( | ) | ( | ) | ( | ) | |||||
Other, net | |||||||||||
Cash flows from financing activities | ( | ) | ( | ) | |||||||
Effect of exchange rate changes on cash and equivalents | ( | ) | |||||||||
Net change in cash and equivalents | ( | ) | |||||||||
Cash and equivalents, beginning of year | |||||||||||
Cash and equivalents, end of year | $ | $ | $ | ||||||||
Other supplemental information | |||||||||||
Interest paid, net of portion capitalized | $ | $ | $ | ||||||||
Income taxes paid (received) | ( | ) | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
48  | 2019 Form 10-K | 2019 Form 10-K | ||
AbbVie Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Note 1 Background
Background
The principal business of AbbVie Inc. (AbbVie or the company) is the discovery, development, manufacture and sale of a broad line of pharmaceutical products. AbbVie's products are generally sold worldwide directly to wholesalers, distributors, government agencies, health care facilities, specialty pharmacies and independent retailers from AbbVie-owned distribution centers and public warehouses. In the United States, AbbVie distributes pharmaceutical products principally through independent wholesale distributors, with some sales directly to pharmacies and patients. Outside the United States, AbbVie sells products primarily to customers or through distributors, depending on the market served.
AbbVie was incorporated in Delaware on April 10, 2012. On January 1, 2013, AbbVie became an independent, publicly-traded company as a result of the distribution by Abbott Laboratories (Abbott) of 100 % of the outstanding common stock of AbbVie to Abbott's shareholders.
On June 25, 2019, AbbVie announced that it entered into a definitive transaction agreement under which AbbVie will acquire Allergan plc (Allergan). See Note 5 for additional information regarding the proposed acquisition.
Note 2 Summary of Significant Accounting Policies
Use of Estimates
The consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (GAAP) and necessarily include amounts based on estimates and assumptions by management. Actual results could differ from those amounts. Significant estimates include amounts for rebates, pension and other post-employment benefits, income taxes, litigation, valuation of goodwill and intangible assets, contingent consideration liabilities, financial instruments and inventory and accounts receivable exposures.
Basis of Consolidation
The consolidated financial statements include the accounts of AbbVie and all of its subsidiaries in which a controlling interest is maintained. Controlling interest is determined by majority ownership interest and the absence of substantive third-party participating rights or, in the case of variable interest entities, where AbbVie is determined to be the primary beneficiary. Investments in companies over which AbbVie has a significant influence but not a controlling interest are accounted for using the equity method with AbbVie's share of earnings or losses reported in other expense, net in the consolidated statements of earnings. Intercompany balances and transactions are eliminated.
Certain reclassifications have been made to conform the prior period consolidated financial statements to the current period presentation.
Revenue Recognition
AbbVie recognizes revenue when control of promised goods or services is transferred to the company’s customers, in an amount that reflects the consideration AbbVie expects to be entitled to in exchange for those goods or services. Sales, value add and other taxes collected concurrent with revenue-producing activities are excluded from revenue. AbbVie generates revenue primarily from product sales. For the majority of sales, the company transfers control, invoices the customer and recognizes revenue upon shipment to the customer. The company recognizes shipping and handling costs as an expense in cost of products sold when the company transfers control to the customer. Payment terms vary depending on the type and location of the customer, are based on customary commercial terms and are generally less than one year. AbbVie does not adjust revenue for the effects of a significant financing component for contracts where AbbVie expects the period between the transfer of the good or service and collection to be one year or less.
Discounts, rebates, sales incentives to customers, returns and certain other adjustments are accounted for as variable consideration. Provisions for variable consideration are based on current pricing, executed contracts, government pricing legislation and historical data and are provided for in the period the related revenues are recorded. Rebate amounts are typically based upon the volume of purchases using contractual or statutory prices, which may vary by product and by payer.
2019 Form 10-K |  49 49 | ||
For each type of rebate, factors used in the calculation of the accrual include the identification of the products subject to the rebate, the applicable price terms and the estimated lag time between sale and payment of the rebate, which can be significant. Sales incentives to customers are insignificant.
In addition to revenue from contracts with customers, the company also recognizes certain collaboration revenues. See Note 6 for additional information related to the collaboration with Janssen Biotech, Inc. Additionally, see Note 16 for disaggregation of revenue by product and geography.
Research and Development Expenses
Internal research and development (R&D) costs are expensed as incurred. Clinical trial costs incurred by third parties are expensed as the contracted work is performed. Where contingent milestone payments are due to third parties under research and development collaborations, prior to regulatory approval, the payment obligations are expensed when the milestone results are achieved. Payments made to third parties subsequent to regulatory approval are capitalized as intangible assets and amortized to cost of products sold over the remaining useful life of the related product.
Collaborations and Other Arrangements
The company enters into collaborative agreements with third parties to develop and commercialize drug candidates. Collaborative activities may include joint research and development and commercialization of new products. AbbVie generally receives certain licensing rights under these arrangements. These collaborations often require upfront payments and may include additional milestone, research and development cost sharing, royalty or profit share payments, contingent upon the occurrence of certain future events linked to the success of the asset in development and commercialization. Upfront payments associated with collaborative arrangements during the development stage are expensed to acquired in-process research and development (IPR&D) expenses in the consolidated statements of earnings. Subsequent payments made to the partner for the achievement of milestones during the development stage are expensed to R&D expense in the consolidated statements of earnings when the milestone is achieved. Milestone payments made to the partner subsequent to regulatory approval are capitalized as intangible assets and amortized to cost of products sold over the estimated useful life of the related asset. Royalties are expensed to cost of products sold in the consolidated statements of earnings when incurred.
Advertising
Pension and Other Post-Employment Benefits
AbbVie records annual expenses relating to its defined benefit pension and other post-employment benefit plans based on calculations which utilize various actuarial assumptions, including discount rates, rates of return on assets, compensation increases, turnover rates and health care cost trend rates. AbbVie reviews its actuarial assumptions on an annual basis and makes modifications to the assumptions based on current rates and trends. Actuarial gains and losses are deferred in accumulated other comprehensive income (AOCI), net of tax and are amortized over the remaining service attribution periods of the employees under the corridor method. Differences between the expected long-term return on plan assets and the actual annual return are amortized to net periodic benefit cost over a five-year period.
Income Taxes
Income taxes are accounted for under the asset and liability method. Provisions for federal, state and foreign income taxes are calculated on reported pretax earnings based on current tax laws. Deferred taxes are provided using enacted tax rates on the future tax consequences of temporary differences, which are the differences between the financial statement carrying amounts of assets and liabilities and their respective tax bases and the tax benefits of carryforwards. A valuation allowance is established or maintained when, based on currently available information, it is more likely than not that all or a portion of a deferred tax asset will not be realized.
Cash and Equivalents
Cash and equivalents include money market funds and time deposits with original maturities of three months or less.
Investments
Investments consist primarily of time deposits, marketable debt securities, held-to-maturity debt securities and equity securities. Investments in marketable debt securities are classified as available-for-sale and are recorded at fair value with any
50  | 2019 Form 10-K | 2019 Form 10-K | ||
unrealized holding gains or losses, net of tax, included in AOCI on the consolidated balance sheets until realized, at which time the gains or losses are recognized in earnings. Investments in equity securities that have readily determinable fair values are recorded at fair value. Investments in equity securities that do not have readily determinable fair values are recorded at cost and are remeasured to fair value based on certain observable price changes or impairment events as they occur. Held-to-maturity debt securities are recorded at cost. Gains or losses on investments are included in other expense, net in the consolidated statements of earnings.
AbbVie periodically assesses its marketable debt securities for other-than-temporary impairment losses. This evaluation is based on a number of factors, including the length of time and the extent to which the fair value has been below the cost basis and adverse conditions related specifically to the security, including any changes to the credit rating of the security, intent to sell, or whether AbbVie will more likely than not be required to sell the security before recovery of its amortized cost basis. AbbVie also considers industry factors and general market trends. When AbbVie determines that an other-than-temporary decline has occurred, the cost basis of the investment is written down with a charge to other expense, net in the consolidated statements of earnings and an available-for-sale investment's unrealized loss is reclassified from AOCI to other expense, net in the consolidated statements of earnings. Realized gains and losses on sales of investments are computed using the first-in, first-out method adjusted for any other-than-temporary declines in fair value that were recorded in net earnings.
Accounts Receivable
Inventories
as of December 31 (in millions) | 2019 | 2018 | |||||
Finished goods | $ | $ | |||||
Work-in-process | |||||||
Raw materials | |||||||
Inventories | $ | $ | |||||
Property and Equipment
as of December 31 (in millions) | 2019 | 2018 | |||||
Land | $ | $ | |||||
Buildings | |||||||
Equipment | |||||||
Construction in progress | |||||||
Property and equipment, gross | |||||||
Less accumulated depreciation | ( | ) | ( | ) | |||
Property and equipment, net | $ | $ | |||||
2019 Form 10-K |  51 51 | ||
Leases
The company records lease liabilities based on the present value of lease payments over the lease term. AbbVie generally uses an incremental borrowing rate to discount its lease liabilities, as the rate implicit in the lease is typically not readily determinable. Certain lease agreements include renewal options that are under the company's control. AbbVie includes optional renewal periods in the lease term only when it is reasonably certain that AbbVie will exercise its option.
Variable lease payments include payments to lessors for taxes, maintenance, insurance and other operating costs as well as payments that are adjusted based on an index or rate. The company's lease agreements do not contain any significant residual value guarantees or restrictive covenants.
Litigation and Contingencies
Loss contingency provisions are recorded when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated based on existing information. When a best estimate cannot be made, the minimum loss contingency amount in a probable range is recorded. Legal fees are expensed as incurred. AbbVie accrues for product liability claims on an undiscounted basis. The liabilities are evaluated quarterly and adjusted if necessary as additional information becomes available. Receivables for insurance recoveries for product liability claims, if any, are recorded as assets on an undiscounted basis when it is probable that a recovery will be realized.
Business Combinations
AbbVie utilizes the acquisition method of accounting for business combinations. This method requires, among other things, that results of operations of acquired companies are included in AbbVie's results of operations beginning on the respective acquisition dates and that assets acquired and liabilities assumed are recognized at fair value as of the acquisition date. Any excess of the fair value of consideration transferred over the fair values of the net assets acquired is recognized as goodwill. Contingent consideration liabilities are recognized at the estimated fair value on the acquisition date. Subsequent changes to the fair value of contingent consideration liabilities are recognized in other expense, net in the consolidated statements of earnings. The fair value of assets acquired and liabilities assumed in certain cases may be subject to revision based on the final determination of fair value during a period of time not to exceed 12 months from the acquisition date. Legal costs, due diligence costs, business valuation costs and all other business acquisition costs are expensed when incurred.
Goodwill and Intangible Assets
Intangible assets acquired in a business combination are recorded at fair value using a discounted cash flow model. The discounted cash flow model requires assumptions about the timing and amount of future net cash flows, risk, the cost of capital and terminal values of market participants. Definite-lived intangibles are amortized over their estimated useful lives using the estimated pattern of economic benefit. AbbVie reviews the recoverability of definite-lived intangible assets whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable. AbbVie first compares the projected undiscounted cash flows to be generated by the asset to its carrying value. If the undiscounted cash flows of an intangible asset are less than the carrying value, the intangible asset is written down to its fair value. Where cash flows cannot be identified for an individual asset, the review is applied at the lowest level for which cash flows are largely independent of the cash flows of other assets and liabilities.
Goodwill and indefinite-lived assets are not amortized, but are subject to an impairment review annually and more frequently when indicators of impairment exist. An impairment of goodwill could occur if the carrying amount of a reporting unit exceeded the fair value of that reporting unit. An impairment of indefinite-lived intangible assets would occur if the fair value of the intangible asset is less than the carrying value.
The company tests its goodwill for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If the company concludes it is more likely than not that the fair value of the reporting unit is less than its carrying amount, a quantitative impairment test is performed. AbbVie tests indefinite-lived intangible assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If the company concludes it is more likely than not that the fair value is less than its carrying amount, a quantitative impairment test is performed. For its quantitative impairment tests, the company uses an estimated future cash flow approach that requires significant judgment with respect to future volume, revenue and expense growth rates, changes in working capital use, the selection of an appropriate discount rate, asset groupings and other assumptions and estimates. The estimates and assumptions used are consistent with the company's business plans and a market participant's views. The use of alternative estimates and assumptions could increase or decrease
52  | 2019 Form 10-K | 2019 Form 10-K | ||
the estimated fair value of the assets and potentially result in different impacts to the company's results of operations. Actual results may differ from the company's estimates.
Acquired In-Process Research and Development
In an asset acquisition, the initial costs of rights to IPR&D projects acquired are expensed as IPR&D in the consolidated statements of earnings unless the project has an alternative future use. These costs include initial payments incurred prior to regulatory approval in connection with research and development collaboration agreements that provide rights to develop, manufacture, market and/or sell pharmaceutical products. In a business combination, the fair value of IPR&D projects acquired are capitalized and accounted for as indefinite-lived intangible assets until the underlying project receives regulatory approval, at which point the intangible asset will be accounted for as a definite-lived intangible asset, or discontinuation, at which point the intangible asset will be written off. R&D costs incurred after the acquisition are expensed as incurred.
Foreign Currency Translation
Foreign subsidiary earnings are translated into U.S. dollars using average exchange rates. The net assets of foreign subsidiaries are translated into U.S. dollars using period-end exchange rates. The U.S. dollar effects that arise from translating the net assets of these subsidiaries at changing rates are recognized in other comprehensive income (loss) (OCI) in the consolidated statements of comprehensive income. The net assets of subsidiaries in highly inflationary economies are remeasured as if the functional currency were the reporting currency. The remeasurement is recognized in net foreign exchange loss in the consolidated statements of earnings.
Derivatives
All derivative instruments are recognized as either assets or liabilities at fair value on the consolidated balance sheets and are classified as current or long-term based on the scheduled maturity of the instrument.
For derivatives formally designated as hedges, the company assesses at inception and quarterly thereafter whether the hedging derivatives are highly effective in offsetting changes in the fair value or cash flows of the hedged item. The changes in fair value of a derivative designated as a fair value hedge and of the hedged item attributable to the hedged risk are recognized in earnings immediately. The effective portions of changes in the fair value of a derivative designated as a cash flow hedge are reported in AOCI and are subsequently recognized in earnings consistent with the underlying hedged item. If it is determined that a derivative is no longer highly effective as a hedge, the company discontinues hedge accounting prospectively. If a hedged forecasted transaction becomes probable of not occurring, any gains or losses are reclassified from AOCI to earnings. Derivatives that are not designated as hedges are adjusted to fair value through current earnings.
The company also uses derivative instruments or foreign currency denominated debt to hedge its net investments in certain foreign subsidiaries and affiliates. Realized and unrealized gains and losses from these hedges are included in AOCI.
Derivative cash flows, with the exception of net investment hedges, are principally classified in the operating section of the consolidated statements of cash flows, consistent with the underlying hedged item. Cash flows related to net investment hedges are classified in the investing section of the consolidated statements of cash flows.
Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
ASU No. 2016-02
In February 2016, the Financial Accounting Standards Board (FASB) issued ASU No. 2016-02, Leases (Topic 842). The standard outlined a comprehensive lease accounting model that superseded the previous lease guidance and required lessees to recognize lease liabilities and corresponding right-of-use assets for all leases with lease terms greater than 12 months. The guidance also changed the definition of a lease and expanded the disclosure requirements of lease arrangements. AbbVie adopted the standard in the first quarter of 2019 using the modified retrospective method. Results for reporting periods beginning after December 31, 2018 have been presented in accordance with the standard, while results for prior periods have not been adjusted and continue to be reported in accordance with AbbVie's historical accounting. The cumulative effect of initially applying the new leases standard was recognized as an adjustment to the opening consolidated balance sheet as of January 1, 2019.
The company elected a package of practical expedients for leases that commenced prior to January 1, 2019 and did not reassess historical conclusions on: (i) whether any expired or existing contracts are or contain leases; (ii) lease classification for any expired or existing leases; and (iii) initial direct costs capitalization for any existing leases.
2019 Form 10-K |  53 53 | ||
Under the new standard, on January 1, 2019, the company recognized a cumulative-effect adjustment to its consolidated balance sheet primarily related to the recognition of liabilities and corresponding right-of-use assets for operating leases. The adjustment to the consolidated balance sheet included: (i) a $405 million increase to other assets; (ii) a $115 million increase to accounts payable and accrued liabilities; and (iii) a $290 million increase to other long-term liabilities. Other cumulative-effect adjustments to the consolidated balance sheet were insignificant.
Adoption of the standard did not have a significant impact on AbbVie's consolidated statement of earnings in 2019.
ASU No. 2018-02
In February 2018, the FASB issued ASU No. 2018-02, Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income, which allowed a reclassification from AOCI to retained earnings for stranded tax effects related to adjustments to deferred taxes resulting from the December 2017 enactment of the Tax Cuts and Jobs Act (the Act). AbbVie adopted the standard in the first quarter of 2019. Upon adoption, the company made an election to not reclassify the income tax effects of the Act from AOCI to retained earnings. Therefore, the adoption of the standard had no impact on AbbVie's consolidated financial statements.
Recent Accounting Pronouncements Not Yet Adopted
ASU No. 2016-13
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments - Credit Losses (Topic 326). The standard changes how credit losses are measured for most financial assets and certain other instruments. For trade and other receivables, held-to-maturity debt securities, loans and other financial instruments, the standard requires the use of a new forward-looking "expected credit loss" model that generally will result in the earlier recognition of allowances for losses. For available-for-sale debt securities with unrealized losses, the standard now requires allowances to be recorded instead of reducing the amortized cost of the investment. Additionally, the standard requires new disclosures and will be effective for AbbVie starting with the first quarter of 2020. With certain exceptions, adjustments are to be applied using a modified-retrospective approach by reflecting adjustments through a cumulative-effect impact to retained earnings as of the beginning of the fiscal year of adoption. AbbVie has completed its assessment of the new standard as of December 31, 2019 and concluded that the adoption will not have a material impact on its consolidated financial statements based on the company's current portfolio of financial assets.
ASU No. 2019-12
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740). The standard includes simplifications related to accounting for income taxes including removing certain exceptions related to the approach for intraperiod tax allocation and the recognition of deferred tax liabilities for outside basis differences. The standard also clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. The standard will be effective for AbbVie starting with the first quarter of 2021, with early adoption permitted. AbbVie is currently assessing the impact and timing of adopting this guidance on its consolidated financial statements.
54  | 2019 Form 10-K | 2019 Form 10-K | ||
Note 3 Supplemental Financial Information
Interest Expense, Net
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Interest expense | $ | $ | $ | ||||||||
Interest income | ( | ) | ( | ) | ( | ) | |||||
Interest expense, net | $ | $ | $ | ||||||||
Accounts Payable and Accrued Liabilities
as of December 31 (in millions) | 2019 | 2018 | |||||
Sales rebates | $ | $ | |||||
Dividends payable | |||||||
Accounts payable | |||||||
Salaries, wages and commissions | |||||||
Royalty and license arrangements | |||||||
Other | |||||||
Accounts payable and accrued liabilities | $ | $ | |||||
Other Long-Term Liabilities
as of December 31 (in millions) | 2019 | 2018 | |||||
Contingent consideration liabilities | $ | $ | |||||
Income taxes payable | |||||||
Pension and other post-employment benefits | |||||||
Liabilities for unrecognized tax benefits | |||||||
Other | |||||||
Other long-term liabilities | $ | $ | |||||
Note 4 Earnings Per Share
AbbVie grants certain restricted stock units (RSUs) that are considered to be participating securities. Due to the presence of participating securities, AbbVie calculates earnings per share (EPS) using the more dilutive of the treasury stock or the two-class method. For all periods presented, the two-class method was more dilutive.
2019 Form 10-K |  55 55 | ||
The following table summarizes the impact of the two-class method:
Years ended December 31, | |||||||||||
(in millions, except per share data) | 2019 | 2018 | 2017 | ||||||||
Basic EPS | |||||||||||
Net earnings | $ | $ | $ | ||||||||
Earnings allocated to participating securities | |||||||||||
Earnings available to common shareholders | $ | $ | $ | ||||||||
Weighted-average basic shares outstanding | |||||||||||
Basic earnings per share | $ | $ | $ | ||||||||
Diluted EPS | |||||||||||
Net earnings | $ | $ | $ | ||||||||
Earnings allocated to participating securities | |||||||||||
Earnings available to common shareholders | $ | $ | $ | ||||||||
Weighted-average shares of common stock outstanding | |||||||||||
Effect of dilutive securities | |||||||||||
Weighted-average diluted shares outstanding | |||||||||||
Diluted earnings per share | $ | $ | $ | ||||||||
Certain shares issuable under stock-based compensation plans were excluded from the computation of EPS because the effect would have been antidilutive. The number of common shares excluded was insignificant for all periods presented.
Note 5 Licensing, Acquisitions and Other Arrangements
Proposed Acquisition of Allergan plc
On June 25, 2019, AbbVie announced that it entered into a definitive transaction agreement under which AbbVie will acquire Allergan plc (Allergan) in a cash and stock transaction for a transaction equity value of approximately $63 billion, based on the closing price of AbbVie’s common stock of $78.45 on June 24, 2019. Under the terms of the transaction agreement, Allergan shareholders will receive 0.8660 AbbVie shares and $120.30 in cash for each Allergan share. On October 14, 2019, Allergan shareholders approved the proposed transaction.
Allergan is a global pharmaceutical leader focused on developing, manufacturing and commercializing branded pharmaceutical, device, biologic, surgical and regenerative medicine products for patients around the world. Allergan markets a portfolio of brands and products primarily focused on key therapeutic areas including aesthetics, eye care, neuroscience, gastroenterology and women's health.
The transaction is subject to customary closing conditions and regulatory approvals. In September 2019, AbbVie and Allergan each received a Request for Additional Information (Second Request) from the Federal Trade Commission (FTC) in connection with the transaction. AbbVie and Allergan are cooperating fully with the FTC. In January 2020, the European Commission approved the proposed acquisition of Allergan by AbbVie conditional upon the divestiture of brazikumab, Allergan's IL-23 inhibitor pipeline product. In January 2020, Allergan entered into a definitive agreement to divest brazikumab contingent upon regulatory approvals and closing of AbbVie's acquisition of Allergan.
In anticipation of the proposed acquisition, AbbVie entered into several debt and financing arrangements in 2019. See Note 10 for additional information.
Other Licensing & Acquisitions Activity
Cash outflows related to other acquisitions and investments totaled $1.1 billion in 2019, $736 million in 2018 and $308 million in 2017. AbbVie recorded acquired IPR&D charges of $385 million in 2019, $424 million in 2018 and $327 million in 2017. Significant arrangements impacting 2019, 2018 and 2017, some of which require contingent milestone payments, are summarized below.
56  | 2019 Form 10-K | 2019 Form 10-K | ||
Reata Pharmaceuticals, Inc.
In October 2019, AbbVie and Reata Pharmaceuticals, Inc. (Reata) entered into an amended and restated license agreement. Under the terms of the agreement, Reata reacquired exclusive development, manufacturing and commercialization rights concerning its proprietary Nrf2 activator product platform originally licensed to AbbVie for territories outside of the United States with respect to bardoxolone methyl and worldwide with respect to omaveloxolone and other next-generation Nrf2 activators. As consideration for the rights reacquired by Reata, AbbVie will receive a total of $330 million in cash payable in three installments through 2021, which was recognized in other operating expense (income) in the fourth quarter of 2019. In addition, AbbVie will receive low single-digit, tiered royalties from worldwide sales of omaveloxolone and certain next-generation Nrf2 activators.
Calico Life Sciences LLC
In June 2018, AbbVie and Calico Life Sciences LLC (Calico) entered into an extension of a collaboration to discover, develop and bring to market new therapies for patients with age-related diseases, including neurodegeneration and cancer. Under the terms of the agreement, AbbVie and Calico will each contribute an additional $500 million to the collaboration and the term is extended for an additional three years . Calico will be responsible for research and early development until 2022 and will advance collaboration projects through Phase 2a through 2027. Following completion of Phase 2a, AbbVie will have the option to exclusively license collaboration compounds. AbbVie will support Calico in its early research and development efforts and, upon exercise, would be responsible for late-stage development and commercial activities. Collaboration costs and profits will be shared equally by both parties post option exercise. During 2018, AbbVie recorded $500 million in other operating expense (income) in the consolidated statement of earnings related to its commitments under the agreement.
Alector, Inc.
In October 2017, AbbVie entered into a global strategic collaboration with Alector, Inc. (Alector) to develop and commercialize medicines to treat Alzheimer’s disease and other neurodegenerative disorders. AbbVie and Alector have agreed to research a portfolio of antibody targets, and AbbVie has an option to global development and commercial rights to two targets. The terms of the arrangement included an initial upfront payment of $205 million, which was expensed to IPR&D in the fourth quarter of 2017. Alector will conduct exploratory research, drug discovery and development for lead programs up to the conclusion of the proof of concept studies. If the option is exercised, AbbVie will lead development and commercialization activities and could make additional payments to Alector of up to $986 million upon achievement of certain development and regulatory milestones. Alector and AbbVie will co-fund development and commercialization and will share global profits equally.
Other Arrangements
In addition to the significant arrangements described above, AbbVie entered into several other arrangements resulting in charges to IPR&D of $385 million in 2019, $424 million in 2018 and $122 million in 2017. In connection with the other individually insignificant early-stage arrangements entered into in 2019, AbbVie could make additional payments of up to $5.8 billion upon the achievement of certain development, regulatory and commercial milestones.
Note 6 Collaboration with Janssen Biotech, Inc.
In December 2011, Pharmacyclics, a wholly-owned subsidiary of AbbVie, entered into a worldwide collaboration and license agreement with Janssen Biotech, Inc. and its affiliates (Janssen), one of the Janssen Pharmaceutical companies of Johnson & Johnson, for the joint development and commercialization of IMBRUVICA, a novel, orally active, selective covalent inhibitor of Bruton's tyrosine kinase (BTK) and certain compounds structurally related to IMBRUVICA, for oncology and other indications, excluding all immune and inflammatory mediated diseases or conditions and all psychiatric or psychological diseases or conditions, in the United States and outside the United States.
The collaboration provides Janssen with an exclusive license to commercialize IMBRUVICA outside of the United States and co-exclusively with AbbVie in the United States. Both parties are responsible for the development, manufacturing and marketing of any products generated as a result of the collaboration. The collaboration has no set duration or specific expiration date and provides for potential future development, regulatory and approval milestone payments of up to $200 million to AbbVie. The collaboration also includes a cost sharing arrangement for associated collaboration activities. Except in certain cases, Janssen is responsible for approximately 60 % of collaboration development costs and AbbVie is responsible for the remaining 40 % of collaboration development costs.
2019 Form 10-K |  57 57 | ||
In the United States, both parties have co-exclusive rights to commercialize the products; however, AbbVie is the principal in the end-customer product sales. AbbVie and Janssen share pre-tax profits and losses equally from the commercialization of products. Sales of IMBRUVICA are included in AbbVie's net revenues. Janssen's share of profits is included in AbbVie's cost of products sold. Other costs incurred under the collaboration are reported in their respective expense line items, net of Janssen's share.
Outside the United States, Janssen is responsible for and has exclusive rights to commercialize IMBRUVICA. AbbVie and Janssen share pre-tax profits and losses equally from the commercialization of products. AbbVie's share of profits is included in AbbVie's net revenues. Other costs incurred under the collaboration are reported in their respective expense line items, net of Janssen's share.
The following table shows the profit and cost sharing relationship between Janssen and AbbVie:
years ended December 31 (in millions) | 2019 | 2018 | 2017 | |||||||||
United States - Janssen's share of profits (included in cost of products sold) | $ | $ | $ | |||||||||
International - AbbVie's share of profits (included in net revenues) | ||||||||||||
Global - AbbVie's share of other costs (included in respective line items) | ||||||||||||
AbbVie’s receivable from Janssen, included in accounts receivable, net, was $235 million at December 31, 2019 and $177 million at December 31, 2018. AbbVie’s payable to Janssen, included in accounts payable and accrued liabilities, was $455 million at December 31, 2019 and $376 million at December 31, 2018.
Note 7 Goodwill and Intangible Assets
Goodwill
The following table summarizes the changes in the carrying amount of goodwill:
(in millions) | |||
Balance as of December 31, 2017 | $ | ||
Foreign currency translation | ( | ) | |
Balance as of December 31, 2018 | |||
Foreign currency translation | ( | ) | |
Balance as of December 31, 2019 | $ | ||
The company performs its annual goodwill impairment assessment in the third quarter, or earlier if impairment indicators exist. As of December 31, 2019, there were no accumulated goodwill impairment losses.
Intangible Assets, Net
The following table summarizes intangible assets:
2019 | 2018 | ||||||||||||||||||||||
as of December 31 (in millions) | Gross carrying amount | Accumulated amortization | Net carrying amount | Gross carrying amount | Accumulated amortization | Net carrying amount | |||||||||||||||||
Definite-lived intangible assets | |||||||||||||||||||||||
Developed product rights | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | |||||||||||||
License agreements | ( | ) | ( | ) | |||||||||||||||||||
Total definite-lived intangible assets | ( | ) | ( | ) | |||||||||||||||||||
Indefinite-lived research and development | — | — | |||||||||||||||||||||
Total intangible assets, net | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | |||||||||||||
58  | 2019 Form 10-K | 2019 Form 10-K | ||
Indefinite-Lived Intangible Assets
Indefinite-lived intangible assets represent acquired IPR&D associated with products that have not yet received regulatory approval. The company performs its annual impairment assessment of indefinite-lived intangible assets in the third quarter, or earlier if impairment indicators exist.
In April 2019, the U.S. Food and Drug Administration (FDA) and the European Commission approved SKYRIZI (risankizumab) for the treatment of moderate to severe plaque psoriasis. As a result, AbbVie reclassified $3.9 billion of indefinite-lived intangible assets related to SKYRIZI to developed product rights definite-lived intangible assets. This amount will be amortized over its estimated useful life using the estimated pattern of economic benefit.
During the fourth quarter of 2018, the company made a decision to stop enrollment for the TAHOE trial, a Phase 3 study evaluating rovalpituzumab tesirine (Rova-T) as a second-line therapy for advanced small-cell lung cancer following a recommendation from an Independent Data Monitoring Committee. This decision lowered the probabilities of success of achieving regulatory approval across Rova-T and other early-stage assets and represented a triggering event which required the company to evaluate for impairment the IPR&D assets associated with the Stemcentrx acquisition. The company utilized multi-period excess earnings models of the “income approach” and determined that the fair value was $1.0 billion as of December 31, 2018, which was lower than the carrying value of $6.1 billion and resulted in an impairment charge of $5.1 billion. This impairment charge was recorded to R&D expense in the consolidated statement of earnings for the year ended December 31, 2018. In the third quarter of 2019, following the announcement of the decision to terminate the Rova-T research and development program, the company recorded an impairment charge of $1.0 billion which represented the remaining value of the IPR&D acquired as part of the 2016 Stemcentrx acquisition. This impairment charge was recorded to R&D expense in the consolidated statement of earnings for the year ended December 31, 2019.
Definite-Lived Intangible Assets
Definite-lived intangible assets are amortized over their estimated useful lives, which range between 2 to 16 years with an average of 11 years for both developed product rights and license agreements. Amortization expense was $1.6 billion in 2019, $1.3 billion in 2018 and $1.1 billion in 2017 and was included in cost of products sold in the consolidated statements of earnings. The anticipated annual amortization expense for definite-lived intangible assets recorded as of December 31, 2019 is as follows:
(in billions) | 2020 | 2021 | 2022 | 2023 | 2024 | ||||||||||||||
Anticipated annual amortization expense | $ | $ | $ | $ | $ | ||||||||||||||
Note 8 Restructuring Plans
AbbVie continuously evaluates its operations to identify opportunities to optimize its manufacturing and R&D operations, commercial infrastructure and administrative costs and to respond to changes in its business environment. As a result, AbbVie management periodically approves individual restructuring plans to achieve these objectives. In 2019, 2018 and 2017, no such plans were individually significant. Restructuring charges recorded were $234 million in 2019, $70 million in 2018 and $86 million in 2017 and were primarily related to employee severance and contractual obligations. These charges were recorded in cost of products sold, R&D expense and SG&A expenses in the consolidated statements of earnings based on the classification of the affected employees or operations.
2019 Form 10-K |  59 59 | ||
The following table summarizes the cash activity in the restructuring reserve for 2019, 2018 and 2017:
(in millions) | |||
Accrued balance as of December 31, 2016 | $ | ||
2017 restructuring charges | |||
Payments and other adjustments | ( | ) | |
Accrued balance as of December 31, 2017 | |||
2018 restructuring charges | |||
Payments and other adjustments | ( | ) | |
Accrued balance as of December 31, 2018 | |||
2019 restructuring charges | |||
Payments and other adjustments | ( | ) | |
Accrued balance as of December 31, 2019 | $ | ||
Note 9 Leases
AbbVie's lease portfolio primarily consists of real estate properties, vehicles and equipment. The following table summarizes the amounts and location of operating and finance leases on the consolidated balance sheet:
(in millions) | Balance sheet caption | December 31, 2019 | ||
Assets | ||||
Operating | Other assets | $ | ||
Finance | Property and equipment, net | |||
Total lease assets | $ | |||
Liabilities | ||||
Operating | ||||
Current | Accounts payable and accrued liabilities | $ | ||
Noncurrent | Other long-term liabilities | |||
Finance | ||||
Current | Current portion of long-term debt and finance lease obligations | |||
Noncurrent | Long-term debt and finance lease obligations | |||
Total lease liabilities | $ | |||
The following table summarizes the lease costs recognized in the consolidated statement of earnings:
year ended December 31 (in millions) | 2019 | |||
Operating lease cost | $ | |||
Short-term lease cost | ||||
Variable lease cost | ||||
Total lease cost | $ | |||
Sublease income and finance lease costs were insignificant in 2019. Lease expense prior to the adoption of ASU No. 2016-02 was $161 million in 2018 and $169 million in 2017.
60  | 2019 Form 10-K | 2019 Form 10-K | ||
The following table presents the weighted-average remaining lease term and weighted-average discount rate for operating and finance leases:
December 31, 2019 | ||
Weighted-average remaining lease term (years) | ||
Operating | ||
Finance | ||
Weighted-average discount rate | ||
Operating | % | |
Finance | % | |
The following table presents supplementary cash flow information regarding the company's leases:
year ended December 31 (in millions) | 2019 | ||
Cash paid for amounts included in the measurement of lease liabilities | |||
Operating cash flows from operating leases | $ | ||
Right-of-use assets obtained in exchange for new operating lease liabilities | |||
Finance lease cash flows were insignificant in 2019.
The following table summarizes the future maturities of AbbVie's operating and finance lease liabilities as of December 31, 2019:
(in millions) | Operating leases | Finance leases | Total (a)(b) | ||||||||
2020 | $ | $ | $ | ||||||||
2021 | |||||||||||
2022 | |||||||||||
2023 | |||||||||||
2024 | |||||||||||
Thereafter | |||||||||||
Total lease payments | |||||||||||
Less: Interest | |||||||||||
Present value of lease liabilities | $ | $ | $ | ||||||||
(a) | Total lease payments exclude approximately $ |
(b) | Lease payments recognized as part of lease liabilities for optional renewal periods are insignificant. |
Future minimum lease payments for non-cancelable operating leases and capital leases as of December 31, 2018 prior to the adoption of ASU No. 2016-02 did not differ materially from future lease payments, inclusive of payments for leases executed but not yet commenced, under the new standard.
2019 Form 10-K |  61 61 | ||
Note 10 Debt, Credit Facilities and Commitments and Contingencies
The following table summarizes long-term debt:
as of December 31 (dollars in millions) | Effective interest rate in 2019(a) | 2019 | Effective interest rate in 2018(a) | 2018 | |||||||||
Senior notes issued in 2012 | |||||||||||||
2.90% notes due 2022 | % | $ | % | $ | |||||||||
4.40% notes due 2042 | % | % | |||||||||||
Senior notes issued in 2015 | |||||||||||||
2.50% notes due 2020 | % | % | |||||||||||
3.20% notes due 2022 | % | % | |||||||||||
3.60% notes due 2025 | % | % | |||||||||||
4.50% notes due 2035 | % | % | |||||||||||
4.70% notes due 2045 | % | % | |||||||||||
Senior notes issued in 2016 | |||||||||||||
2.30% notes due 2021 | % | % | |||||||||||
2.85% notes due 2023 | % | % | |||||||||||
3.20% notes due 2026 | % | % | |||||||||||
4.30% notes due 2036 | % | % | |||||||||||
4.45% notes due 2046 | % | % | |||||||||||
Senior Euro notes issued in 2016 | |||||||||||||
0.375% notes due 2019 (€1,400 principal) | % | % | |||||||||||
1.375% notes due 2024 (€1,450 principal) | % | % | |||||||||||
2.125% notes due 2028 (€750 principal) | % | % | |||||||||||
Senior notes issued in 2018 | |||||||||||||
3.375% notes due 2021 | % | % | |||||||||||
3.75% notes due 2023 | % | % | |||||||||||
4.25% notes due 2028 | % | % | |||||||||||
4.875% notes due 2048 | % | % | |||||||||||
Senior Euro notes issued in 2019 | |||||||||||||
0.75% notes due 2027 (€750 principal) | % | ||||||||||||
1.25% notes due 2031 (€650 principal) | % | ||||||||||||
Senior notes issued in 2019 | |||||||||||||
Floating rate notes due May 2021 | % | ||||||||||||
Floating rate notes due November 2021 | % | ||||||||||||
Floating rate notes due 2022 | % | ||||||||||||
2.15% notes due 2021 | % | ||||||||||||
2.30% notes due 2022 | % | ||||||||||||
2.60% notes due 2024 | % | ||||||||||||
2.95% notes due 2026 | % | ||||||||||||
3.20% notes due 2029 | % | ||||||||||||
4.05% notes due 2039 | % | ||||||||||||
4.25% notes due 2049 | % | ||||||||||||
Other | |||||||||||||
Fair value hedges | ( | ) | ( | ) | |||||||||
Unamortized bond discounts | ( | ) | ( | ) | |||||||||
Unamortized deferred financing costs | ( | ) | ( | ) | |||||||||
Total long-term debt and finance lease obligations | |||||||||||||
Current portion | |||||||||||||
Noncurrent portion | $ | $ | |||||||||||
(a) | Excludes the effect of any related interest rate swaps. |
62  | 2019 Form 10-K | 2019 Form 10-K | ||
Allergan-Related Financing
In connection with the proposed acquisition of Allergan, in November 2019, the company issued $30.0 billion aggregate principal amount of unsecured senior notes, consisting of $750 million aggregate principal amount of floating rate senior notes due May 2021, $750 million aggregate principal amount of floating rate senior notes due November 2021, $750 million aggregate principal amount of floating rate senior notes due 2022, $1.75 billion aggregate principal amount of 2.15 % senior notes due 2021, $3.0 billion aggregate principal amount of 2.30 % senior notes due 2022, $3.75 billion aggregate principal amount of 2.60 % senior notes due 2024, $4.0 billion aggregate principal amount of 2.95 % senior notes due 2026, $5.5 billion aggregate principal amount of 3.20 % senior notes due 2029, $4.0 billion aggregate principal amount of 4.05 % senior notes due 2039 and $5.75 billion aggregate principal amount of 4.25 % senior notes due 2049. These senior notes rank equally with all other unsecured and unsubordinated indebtedness of the company. AbbVie may redeem the fixed-rate senior notes prior to maturity at a redemption price equal to the greater of the principal amount or the sum of present values of the remaining scheduled payments of principal and interest on the fixed-rate senior notes to be redeemed plus a make-whole premium. With exception of the fixed-rate notes due 2021 and 2022, AbbVie may also redeem the fixed-rate senior notes at par between one and six months prior to maturity. In connection with the offering, debt issuance costs incurred totaled $173 million and debt discounts totaled $52 million, which are being amortized over the respective terms of the notes to interest expense, net in the consolidated statements of earnings. AbbVie expects to use the net proceeds to fund a portion of the aggregate cash consideration due to Allergan shareholders in connection with the proposed acquisition described in Note 5 and to pay related fees and expenses. Pending the consummation of the proposed Allergan acquisition, the net proceeds from the offering are permitted to be invested temporarily in short-term investments. All of the notes are subject to special mandatory redemption at a redemption price equal to 101 % of the aggregate principal amount of the notes plus accrued and unpaid interest if the proposed acquisition of Allergan is not completed by January 30, 2021 or the company notifies the trustee in respect of the notes that it will not pursue the consummation of the proposed Allergan acquisition.
On June 25, 2019, AbbVie entered into a $38.0 billion 364 -day bridge credit agreement. On July 12, 2019, AbbVie entered into a term loan credit agreement with an aggregate principal amount of $6.0 billion consisting of a $1.5 billion 364 -day term loan tranche, a $2.5 billion three-year term loan tranche and a $2.0 billion five-year term loan tranche. In connection with the agreements, debt issuance costs incurred totaled $242 million and were recorded to interest expense, net in the consolidated statements of earnings. Upon commencement of the $6.0 billion term loan credit agreement and upon issuance of the $30.0 billion aggregate principal amount of senior notes, commitments under the bridge credit agreement were reduced to $2.0 billion. No amounts were drawn under the bridge credit agreement or term loan credit agreement at December 31, 2019. In February 2020, the remaining commitments under the bridge credit agreement were reduced to $0 as a result of cash on hand at AbbVie. AbbVie subsequently terminated the bridge credit agreement in its entirety as permitted under its terms.
On October 25, 2019, AbbVie commenced offers to exchange any and all outstanding notes of certain series issued by Allergan for up to $15.5 billion aggregate principal amount and €3.7 billion aggregate principal amount of new notes to be issued by AbbVie and cash, subject to conditions including the closing of the pending acquisition of Allergan. Concurrently with the offers to exchange the Allergan notes for AbbVie notes, the company solicited consents to adopt certain proposed amendments to each of the indentures governing the Allergan notes to, among other things, eliminate substantially all of the restrictive covenants in such indentures. In November 2019, the company announced that the requisite number of consents had been received to adopt the proposed amendments with respect to all Allergan notes and that Allergan executed a supplemental indenture with respect to each Allergan indenture implementing the amendments, which will become operative only upon settlement of the exchange offers. The expiration of the exchange offers is expected to occur on or about the closing date of AbbVie’s acquisition of Allergan.
Other Long-Term Debt
In September 2019, the company issued €1.4 billion aggregate principal amount of unsecured senior Euro notes, consisting of €750 million aggregate principal amount of 0.75 % senior notes due 2027 and €650 million aggregate principal amount of 1.25 % senior notes due 2031. These senior notes rank equally with all other unsecured and unsubordinated indebtedness of the company. AbbVie may redeem the senior notes prior to maturity at a redemption price equal to the principal amount of the senior notes redeemed plus a make-whole premium and may redeem the senior notes at par between one and three months prior to maturity. In connection with the offering, debt issuance costs incurred totaled $9 million and debt discounts totaled $5 million and are being amortized over the respective terms of the notes to interest expense, net in the consolidated statements of earnings. In October 2019, the company used the proceeds to redeem €1.4 billion aggregate principal amount of 0.375 % senior Euro notes that were due to mature in November 2019.
In September 2018, the company issued $6.0 billion aggregate principal amount of unsecured senior notes, consisting of $1.25 billion aggregate principal amount of 3.375 % senior notes due 2021, $1.25 billion aggregate principal amount of 3.75 %
2019 Form 10-K |  63 63 | ||
senior notes due 2023, $1.75 billion aggregate principal amount of 4.25 % senior notes due 2028 and $1.75 billion aggregate principal amount of 4.875 % senior notes due 2048. These senior notes rank equally with all other unsecured and unsubordinated indebtedness of the company. AbbVie may redeem the senior notes prior to maturity at a redemption price equal to the principal amount of the senior notes redeemed plus a make-whole premium, and except for the 3.375 % notes due 2021, AbbVie may redeem the senior notes at par between one and six months prior to maturity. In connection with the offering, debt issuance costs incurred totaled $37 million and debt discounts totaled $37 million and are being amortized over the respective terms of the senior notes to interest expense, net in the consolidated statements of earnings. Of the $5.9 billion net proceeds, $2.0 billion was used to repay the company's outstanding three-year term loan credit agreement in September 2018 and $1.0 billion was used to repay the aggregate principal amount of 2.00 % senior notes at maturity in November 2018. The company used the remaining proceeds to repay term loan obligations in 2019 as they became due.
In May 2018, the company also repaid $3.0 billion aggregate principal amount of 1.80 % senior notes at maturity.
AbbVie has outstanding €2.2 billion aggregate principal amount of unsecured senior Euro notes which were issued in 2016. AbbVie may redeem the senior notes prior to maturity at a redemption price equal to the principal amount of the senior notes redeemed plus a make-whole premium and AbbVie may redeem the senior notes at par between one and three months prior to maturity.
AbbVie has outstanding $7.8 billion aggregate principal amount of unsecured senior notes which were issued in 2016 and $13.7 billion aggregate principal amount of unsecured senior notes which were issued in 2015. AbbVie may redeem the senior notes, at any time, prior to maturity at a redemption price equal to the principal amount of the senior notes redeemed plus a make-whole premium and AbbVie may redeem the senior notes at par between one and six months prior to maturity.
AbbVie has outstanding $5.7 billion aggregate principal amount of unsecured senior notes which were issued in 2012. AbbVie may redeem all of the senior notes of each series, at any time, or some of the senior notes of each series, from time to time, at a redemption price equal to the principal amount of the senior notes redeemed plus a make-whole premium.
At December 31, 2019, the company was in compliance with its senior note covenants and term loan covenants.
Short-Term Borrowings
Short-term borrowings included commercial paper borrowings of $699 million as of December 31, 2018. There were no commercial paper borrowings as of December 31, 2019. The weighted-average interest rate on commercial paper borrowings was 2.5 % in 2019, 2.0 % in 2018 and 1.3 % in 2017.
In August 2019, AbbVie entered into an amended and restated $4.0 billion five-year revolving credit facility that matures in August 2024. This amended facility enables the company to borrow funds on an unsecured basis at variable interest rates and contains various covenants, all of which the company was in compliance with as of December 31, 2019. Commitment fees under AbbVie's revolving credit facilities were insignificant in 2019, 2018 and 2017. No amounts were outstanding under the company's credit facilities as of December 31, 2019 and December 31, 2018.
In March 2019, AbbVie repaid a $3.0 billion 364 -day term loan credit agreement that was drawn on in June 2018 and was scheduled to mature in June 2019.
Maturities of Long-Term Debt
The following table summarizes AbbVie's debt maturities as of December 31, 2019:
as of and for the years ending December 31 (in millions) | |||
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
Thereafter | |||
Total obligations and commitments | |||
Fair value hedges, unamortized bond discounts, deferred financing costs and finance lease obligations | ( | ) | |
Total long-term debt and finance lease obligations | $ | ||
64  | 2019 Form 10-K | 2019 Form 10-K | ||
Contingencies and Guarantees
In connection with the separation, AbbVie has indemnified Abbott for all liabilities resulting from the operation of AbbVie's business other than income tax liabilities with respect to periods prior to the distribution date and other liabilities as agreed to by AbbVie and Abbott. AbbVie has no material exposures to off-balance sheet arrangements and no special-purpose entities. In the ordinary course of business, AbbVie has periodically entered into third-party agreements, such as the assignment of product rights, which have resulted in AbbVie becoming secondarily liable for obligations for which AbbVie had previously been primarily liable. Based upon past experience, the likelihood of payments under these agreements is remote.
Note 11 Financial Instruments and Fair Value Measures
Risk Management Policy
The company is exposed to foreign currency exchange rate and interest rate risks related to its business operations. AbbVie's hedging policy attempts to manage these risks to an acceptable level based on the company's judgment of the appropriate trade-off between risk, opportunity and costs. The company uses derivative and nonderivative instruments to reduce its exposure to foreign currency exchange rates. AbbVie also periodically enters into interest rate swaps in which the company agrees to exchange, at specified intervals, the difference between fixed and floating interest amounts calculated by reference to an agreed-upon notional amount. Derivative instruments are not used for trading purposes or to manage exposure to changes in interest rates for investment securities, and none of the company's outstanding derivative instruments contain credit risk related contingent features; collateral is generally not required.
Financial Instruments
Various AbbVie foreign subsidiaries enter into foreign currency forward exchange contracts to manage exposures to changes in foreign exchange rates for anticipated intercompany transactions denominated in a currency other than the functional currency of the local entity. These contracts, with notional amounts totaling $957 million at December 31, 2019 and $1.4 billion at December 31, 2018, are designated as cash flow hedges and are recorded at fair value. The durations of these forward exchange contracts were generally less than eighteen months . Accumulated gains and losses as of December 31, 2019 will be reclassified from AOCI and included in cost of products sold at the time the products are sold, generally not exceeding six months from the date of settlement.
In the third quarter of 2019, the company entered into treasury rate lock agreements with notional amounts totaling $10.0 billion to hedge exposure to variability in future cash flows resulting from changes in interest rates related to the issuance of long-term debt in connection with the proposed acquisition of Allergan. The treasury rate lock agreements were designated as cash flow hedges and recorded at fair value. The agreements were net settled upon issuance of the senior notes in November 2019 resulting in a gain of $383 million recognized in other comprehensive income (loss). This gain will be reclassified to interest expense, net over the lives of the related debt.
In the fourth quarter of 2019, the company entered into interest rate swap contracts with notional amounts totaling $2.3 billion at December 31, 2019. The effect of the hedge contracts is to change a floating-rate interest obligation to a fixed rate for that portion of the floating-rate debt. The contracts were designated as cash flow hedges and are recorded at fair value. Realized and unrealized gains or losses are included in AOCI and will be reclassified to interest expense, net over the lives of the floating-rate debt.
The company also enters into foreign currency forward exchange contracts to manage its exposure to foreign currency denominated trade payables and receivables and intercompany loans. These contracts are not designated as hedges and are recorded at fair value. Resulting gains or losses are reflected in net foreign exchange loss in the consolidated statements of earnings and are generally offset by losses or gains on the foreign currency exposure being managed. These contracts had notional amounts totaling $7.1 billion at December 31, 2019 and $8.6 billion at December 31, 2018.
The company also uses foreign currency forward exchange contracts or foreign currency denominated debt to hedge its net investments in certain foreign subsidiaries and affiliates. The company had €3.6 billion aggregate principal amount of senior Euro notes designated as net investment hedges at December 31, 2019 and December 31, 2018. In the third quarter of 2019, the company issued €1.4 billion aggregate principal amount of senior Euro notes and designated the principal amounts of this foreign denominated debt as net investment hedges. Concurrently, the company elected to de-designate hedge accounting for €1.4 billion aggregate principal amount of existing senior Euro notes which were subsequently repaid in October 2019. In addition, in 2019, the company entered into foreign currency forward exchange contracts and designated the instruments as net investment hedges. These contracts had notional amounts totaling €971 million, £204 million and CHF62 million at December 31, 2019. The company uses the spot method of assessing hedge effectiveness for derivative
2019 Form 10-K |  65 65 | ||
instruments designated as net investment hedges. Realized and unrealized gains and losses from these hedges are included in AOCI and the initial fair value of hedge components excluded from the assessment of effectiveness is recognized in interest expense, net over the life of the hedging instrument.
AbbVie is a party to interest rate swap contracts designated as fair value hedges with notional amounts totaling $10.8 billion at December 31, 2019 and December 31, 2018. The effect of the hedge contracts is to change a fixed-rate interest obligation to a floating rate for that portion of the debt. AbbVie records the contracts at fair value and adjusts the carrying amount of the fixed-rate debt by an offsetting amount.
No amounts are excluded from the assessment of effectiveness for cash flow hedges or fair value hedges.
The following table summarizes the amounts and location of AbbVie's derivative instruments on the consolidated balance sheets:
Fair value - Derivatives in asset position | Fair value - Derivatives in liability position | ||||||||||||||
as of December 31 (in millions) | Balance sheet caption | 2019 | 2018 | Balance sheet caption | 2019 | 2018 | |||||||||
Foreign currency forward exchange contracts | |||||||||||||||
Designated as cash flow hedges | Prepaid expenses and other | $ | $ | Accounts payable and accrued liabilities | $ | $ | |||||||||
Designated as net investment hedges | Prepaid expenses and other | Accounts payable and accrued liabilities | |||||||||||||
Not designated as hedges | Prepaid expenses and other | Accounts payable and accrued liabilities | |||||||||||||
Interest rate swap contracts | |||||||||||||||
Designated as cash flow hedges | Other assets | Other long-term liabilities | |||||||||||||
Designated as fair value hedges | Prepaid expenses and other | Accounts payable and accrued liabilities | |||||||||||||
Designated as fair value hedges | Other assets | Other long-term liabilities | |||||||||||||
Total derivatives | $ | $ | $ | $ | |||||||||||
While certain derivatives are subject to netting arrangements with the company's counterparties, the company does not offset derivative assets and liabilities within the consolidated balance sheets.
The following table presents the pre-tax amounts of gains (losses) from derivative instruments recognized in other comprehensive income (loss):
years ended in December 31 (in millions) | 2019 | 2018 | 2017 | |||||||||
Foreign currency forward exchange contracts | ||||||||||||
Designated as cash flow hedges | $ | ( | ) | $ | $ | ( | ) | |||||
Designated as net investment hedges | ||||||||||||
Interest rate swap contracts designated as cash flow hedges | ||||||||||||
Treasury rate lock agreements designated as cash flow hedges | ||||||||||||
Related to AbbVie’s non-derivative, foreign currency denominated debt designated as net investment hedges, the company recognized in other comprehensive income (loss) pre-tax gains of $90 million in 2019, pre-tax gains of $178 million in 2018 and pre-tax losses of $537 million in 2017.
66  | 2019 Form 10-K | 2019 Form 10-K | ||
The following table summarizes the pre-tax amounts and location of derivative instrument net gains (losses) recognized in the consolidated statements of earnings, including the net gains (losses) reclassified out of AOCI into net earnings. See Note 13 for the amount of net gains (losses) reclassified out of AOCI.
years ended December 31 (in millions) | Statement of earnings caption | 2019 | 2018 | 2017 | ||||||||
Foreign currency forward exchange contracts | ||||||||||||
Designated as cash flow hedges | Cost of products sold | $ | $ | ( | ) | $ | ||||||
Designated as net investment hedges | Interest expense, net | |||||||||||
Not designated as hedges | Net foreign exchange loss | ( | ) | ( | ) | |||||||
Treasury rate lock agreements designated as cash flow hedges | Interest expense, net | |||||||||||
Interest rate swap contracts | ||||||||||||
Designated as cash flow hedges | Interest expense, net | |||||||||||
Designated as fair value hedges | Interest expense, net | ( | ) | ( | ) | |||||||
Debt designated as hedged item in fair value hedges | Interest expense, net | ( | ) | |||||||||
Fair Value Measures
The fair value hierarchy consists of the following three levels:
• | Level 1—Valuations based on unadjusted quoted prices in active markets for identical assets that the company has the ability to access; |
• | Level 2—Valuations based on quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuations in which all significant inputs are observable in the market; and |
• | Level 3—Valuations using significant inputs that are unobservable in the market and include the use of judgment by the company's management about the assumptions market participants would use in pricing the asset or liability. |
The following table summarizes the bases used to measure certain assets and liabilities carried at fair value on a recurring basis on the consolidated balance sheet as of December 31, 2019:
Basis of fair value measurement | |||||||||||||||
(in millions) | Total | Quoted prices in active markets for identical assets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable Inputs (Level 3) | |||||||||||
Assets | |||||||||||||||
Cash and equivalents | $ | $ | $ | $ | |||||||||||
Debt securities | |||||||||||||||
Equity securities | |||||||||||||||
Interest rate swap contracts | |||||||||||||||
Foreign currency contracts | |||||||||||||||
Total assets | $ | $ | $ | $ | |||||||||||
Liabilities | |||||||||||||||
Interest rate swap contracts | $ | $ | $ | $ | |||||||||||
Foreign currency contracts | |||||||||||||||
Contingent consideration | |||||||||||||||
Total liabilities | $ | $ | $ | $ | |||||||||||
2019 Form 10-K |  67 67 | ||
The following table summarizes the bases used to measure certain assets and liabilities carried at fair value on a recurring basis on the consolidated balance sheet as of December 31, 2018:
Basis of fair value measurement | |||||||||||||||
(in millions) | Total | Quoted prices in active markets for identical assets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable Inputs (Level 3) | |||||||||||
Assets | |||||||||||||||
Cash and equivalents | $ | $ | $ | $ | |||||||||||
Time deposits | |||||||||||||||
Debt securities | |||||||||||||||
Equity securities | |||||||||||||||
Foreign currency contracts | |||||||||||||||
Total assets | $ | $ | $ | $ | |||||||||||
Liabilities | |||||||||||||||
Interest rate swap contracts | $ | $ | $ | $ | |||||||||||
Foreign currency contracts | |||||||||||||||
Contingent consideration | |||||||||||||||
Total liabilities | $ | $ | $ | $ | |||||||||||
The fair values of time deposits approximate their amortized cost due to the short maturities of these instruments. The fair values of available-for-sale debt securities were determined based on prices obtained from commercial pricing services. The derivatives entered into by the company were valued using observable market inputs including published interest rate curves and both forward and spot prices for foreign currencies. The fair value measurements of the contingent consideration liabilities were determined based on significant unobservable inputs, including the discount rate, estimated probabilities and timing of achieving specified development, regulatory and commercial milestones and the estimated amount of future sales of the acquired products. The potential contingent consideration payments are estimated by applying a probability-weighted expected payment model for contingent milestone payments and a Monte Carlo simulation model for contingent royalty payments, which are then discounted to present value. Changes to the fair value of the contingent consideration liabilities can result from changes to one or a number of inputs, including discount rates, the probabilities of achieving the milestones, the time required to achieve the milestones and estimated future sales. Significant judgment is employed in determining the appropriateness of certain of these inputs. Changes to the inputs described above could have a material impact on the company's financial position and results of operations in any given period. At December 31, 2019, a 50 basis point increase/decrease in the assumed discount rate would have decreased/increased the value of the contingent consideration liabilities by approximately $280 million. Additionally, at December 31, 2019, a five percentage point increase/decrease in the assumed probability of success across all potential indications would have increased/decreased the value of the contingent consideration liabilities by approximately $150 million.
There have been no transfers of assets or liabilities between the fair value measurement levels. The following table presents the changes in fair value of contingent consideration liabilities which are measured using Level 3 inputs:
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Beginning balance | $ | $ | $ | ||||||||
Change in fair value recognized in net earnings | |||||||||||
Payments | ( | ) | ( | ) | ( | ) | |||||
Ending balance | $ | $ | $ | ||||||||
The change in fair value recognized in net earnings is recorded in other expense, net in the consolidated statements of earnings. During the second quarter of 2019, the company recorded a $2.3 billion increase in the SKYRIZI contingent consideration liability due to higher probabilities of success, higher estimated future sales and declining interest rates. The higher probabilities of success resulted from the April 2019 regulatory approvals of SKYRIZI for the treatment of moderate to severe plaque psoriasis. During the third quarter of 2019, the company recorded a $91 million decrease in the Stemcentrx contingent consideration liability due to the termination of the Rova-T research and development program. During the fourth quarter of 2018, the company recorded a $428 million decrease in the Stemcentrx contingent consideration liability due to a reduction in probabilities of success of achieving regulatory approval.
68  | 2019 Form 10-K | 2019 Form 10-K | ||
Certain financial instruments are carried at historical cost or some basis other than fair value. The book values, approximate fair values and bases used to measure the approximate fair values of certain financial instruments as of December 31, 2019 are shown in the table below:
Basis of fair value measurement | ||||||||||||||||||
(in millions) | Book value | Approximate fair values | Quoted prices in active markets for identical assets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable Inputs (Level 3) | |||||||||||||
Liabilities | ||||||||||||||||||
Current portion of long-term debt and finance lease obligations, excluding fair value hedges | $ | $ | $ | $ | $ | |||||||||||||
Long-term debt and finance lease obligations, excluding fair value hedges | ||||||||||||||||||
Total liabilities | $ | $ | $ | $ | $ | |||||||||||||
The book values, approximate fair values and bases used to measure the approximate fair values of certain financial instruments as of December 31, 2018 are shown in the table below:
Basis of fair value measurement | ||||||||||||||||||
(in millions) | Book value | Approximate fair values | Quoted prices in active markets for identical assets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable Inputs (Level 3) | |||||||||||||
Liabilities | ||||||||||||||||||
Short-term borrowings | $ | $ | $ | $ | $ | |||||||||||||
Current portion of long-term debt and finance lease obligations, excluding fair value hedges | ||||||||||||||||||
Long-term debt and finance lease obligations, excluding fair value hedges | ||||||||||||||||||
Total liabilities | $ | $ | $ | $ | $ | |||||||||||||
AbbVie also holds investments in equity securities that do not have readily determinable fair values. The company records these investments at cost and remeasures them to fair value based on certain observable price changes or impairment events as they occur. The carrying amount of these investments was $66 million as of December 31, 2019 and $84 million as of December 31, 2018. No significant cumulative upward or downward adjustments have been recorded for these investments as of December 31, 2019.
Available-for-sale Securities
Substantially all of the company’s investments in debt securities were classified as available-for-sale with changes in fair value recognized in other comprehensive income. In the third quarter of 2019, the company sold substantially all of its investments in debt securities. There were no debt securities classified as short-term as of December 31, 2019 and $204 million as of December 31, 2018. Long-term debt securities mature primarily within five years . Estimated fair values of available-for-sale debt securities were based on prices obtained from commercial pricing services.
The following table summarizes available-for-sale securities by type as of December 31, 2018:
Amortized cost | Gross unrealized | Fair value | |||||||||||||
(in millions) | Gains | Losses | |||||||||||||
Asset backed securities | $ | $ | $ | ( | ) | $ | |||||||||
Corporate debt securities | ( | ) | |||||||||||||
Other debt securities | |||||||||||||||
Total | $ | $ | $ | ( | ) | $ | |||||||||
AbbVie had no other-than-temporary impairments as of December 31, 2019. Net realized gains and losses were insignificant in 2019 and 2018. Net realized gains were $90 million in 2017.
2019 Form 10-K |  69 69 | ||
Concentrations of Risk
The company invests excess cash in time deposits, money market funds and debt securities to diversify the concentration of cash among different financial institutions. The company has established credit exposure limits and monitors concentrations of credit risk associated with financial institution deposits.
Of total net accounts receivable, three U.S. wholesalers accounted for 68 % as of December 31, 2019 and 63 % as of December 31, 2018, and substantially all of AbbVie's net revenues in the United States were to these three wholesalers.
HUMIRA (adalimumab) is AbbVie's single largest product and accounted for approximately 58 % of AbbVie's total net revenues in 2019, 61 % in 2018 and 65 % in 2017.
Note 12 Post-Employment Benefits
AbbVie sponsors various pension and other post-employment benefit plans, including defined benefit, defined contribution and termination indemnity plans, which cover most employees worldwide. In addition, AbbVie provides medical benefits, primarily to eligible retirees in the United States and Puerto Rico, through other post-retirement benefit plans. Net obligations for these plans have been reflected on the consolidated balance sheets as of December 31, 2019 and 2018.
The following table summarizes benefit plan information for the global AbbVie-sponsored defined benefit and other post-employment plans:
Defined benefit plans | Other post-employment plans | ||||||||||||||
as of and for the years ended December 31 (in millions) | 2019 | 2018 | 2019 | 2018 | |||||||||||
Projected benefit obligations | |||||||||||||||
Beginning of period | $ | $ | $ | $ | |||||||||||
Service cost | |||||||||||||||
Interest cost | |||||||||||||||
Employee contributions | — | — | |||||||||||||
Actuarial (gain) loss | ( | ) | ( | ) | |||||||||||
Benefits paid | ( | ) | ( | ) | ( | ) | ( | ) | |||||||
Other, primarily foreign currency translation adjustments | ( | ) | |||||||||||||
End of period | |||||||||||||||
Fair value of plan assets | |||||||||||||||
Beginning of period | — | — | |||||||||||||
Actual return on plan assets | ( | ) | — | — | |||||||||||
Company contributions | |||||||||||||||
Employee contributions | — | — | |||||||||||||
Benefits paid | ( | ) | ( | ) | ( | ) | ( | ) | |||||||
Other, primarily foreign currency translation adjustments | ( | ) | — | — | |||||||||||
End of period | — | — | |||||||||||||
Funded status, end of period | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
Amounts recognized on the consolidated balance sheets | |||||||||||||||
Other assets | $ | $ | $ | — | $ | — | |||||||||
Accounts payable and accrued liabilities | ( | ) | ( | ) | ( | ) | ( | ) | |||||||
Other long-term liabilities | ( | ) | ( | ) | ( | ) | ( | ) | |||||||
Net obligation | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
Actuarial loss, net | $ | $ | $ | $ | |||||||||||
Prior service cost (credit) | ( | ) | ( | ) | |||||||||||
Accumulated other comprehensive loss | $ | $ | $ | $ | |||||||||||
Actuarial losses for 2019 in the table above were primarily driven by lower discount rates.
70  | 2019 Form 10-K | 2019 Form 10-K | ||
The projected benefit obligations (PBO) in the table above included $2.3 billion at December 31, 2019 and $1.9 billion at December 31, 2018, related to international defined benefit plans.
For plans reflected in the table above, the accumulated benefit obligations (ABO) were $7.6 billion at December 31, 2019 and $6.0 billion at December 31, 2018. For those plans reflected in the table above in which the ABO exceeded plan assets at December 31, 2019, the ABO was $5.8 billion, the PBO was $6.7 billion and aggregate plan assets were $4.8 billion.
Amounts Recognized in Other Comprehensive Income (Loss)
The following table summarizes the pre-tax losses (gains) included in other comprehensive income (loss):
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Defined benefit plans | |||||||||||
Actuarial loss | $ | $ | $ | ||||||||
Amortization of actuarial loss and prior service cost | ( | ) | ( | ) | ( | ) | |||||
Foreign exchange loss (gain) and other | ( | ) | ( | ) | |||||||
Total loss | $ | $ | $ | ||||||||
Other post-employment plans | |||||||||||
Actuarial loss (gain) | $ | $ | ( | ) | $ | ||||||
Amortization of actuarial loss and prior service credit | ( | ) | ( | ) | — | ||||||
Total loss (gain) | $ | $ | ( | ) | $ | ||||||
The pre-tax amounts included in AOCI at December 31, 2019 expected to be recognized in net periodic benefit cost in 2020 consisted of $219 million of expense related to actuarial losses and prior service costs for defined benefit plans and $25 million of income related to actuarial losses and prior service credits for other post-employment plans.
Net Periodic Benefit Cost
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Defined benefit plans | |||||||||||
Service cost | $ | $ | $ | ||||||||
Interest cost | |||||||||||
Expected return on plan assets | ( | ) | ( | ) | ( | ) | |||||
Amortization of actuarial loss and prior service cost | |||||||||||
Net periodic benefit cost | $ | $ | $ | ||||||||
Other post-employment plans | |||||||||||
Service cost | $ | $ | $ | ||||||||
Interest cost | |||||||||||
Amortization of actuarial loss and prior service credit | |||||||||||
Net periodic benefit cost | $ | $ | $ | ||||||||
The components of net periodic benefit cost other than service cost are included in other expense, net in the consolidated statements of earnings.
Weighted-Average Assumptions Used in Determining Benefit Obligations at the Measurement Date
as of December 31 | 2019 | 2018 | |||
Defined benefit plans | |||||
Discount rate | % | % | |||
Rate of compensation increases | % | % | |||
Other post-employment plans | |||||
Discount rate | % | % | |||
The assumptions used in calculating the December 31, 2019 measurement date benefit obligations will be used in the calculation of net periodic benefit cost in 2020.
2019 Form 10-K |  71 71 | ||
Weighted-Average Assumptions Used in Determining Net Periodic Benefit Cost
years ended December 31 | 2019 | 2018 | 2017 | |||||
Defined benefit plans | ||||||||
Discount rate for determining service cost | % | % | % | |||||
Discount rate for determining interest cost | % | % | % | |||||
Expected long-term rate of return on plan assets | % | % | % | |||||
Expected rate of change in compensation | % | % | % | |||||
Other post-employment plans | ||||||||
Discount rate for determining service cost | % | % | % | |||||
Discount rate for determining interest cost | % | % | % | |||||
For the December 31, 2019 post-retirement health care obligations remeasurement, the company assumed a 6.4 % pre-65 (7.0 % post-65) annual rate of increase in the per capita cost of covered health care benefits. The rate was assumed to decrease gradually to 4.5 % in 2050 and remain at that level thereafter. For purposes of measuring the 2019 post-retirement health care costs, the company assumed a 6.6 % pre-65 (7.3 % post-65) annual rate of increase in the per capita cost of covered health care benefits. The rate was assumed to decrease gradually to 4.5 % for 2050 and remain at that level thereafter.
Assumed health care cost trend rates have a significant effect on the amounts reported for health care plans. As of December 31, 2019, a one percentage point change in assumed health care cost trend rates would have the following effects:
One percentage point | |||||||
year ended December 31, 2019 (in millions) (brackets denote a reduction) | Increase | Decrease | |||||
Service cost and interest cost | $ | $ | ( | ) | |||
Projected benefit obligation | ( | ) | |||||
Defined Benefit Pension Plan Assets
Basis of fair value measurement | |||||||||||||||
as of December 31 (in millions) | 2019 | Quoted prices in active markets for identical assets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | |||||||||||
Equities | |||||||||||||||
U.S. large cap(a) | $ | $ | $ | $ | |||||||||||
U.S. mid cap(b) | |||||||||||||||
International(c) | |||||||||||||||
Fixed income securities | |||||||||||||||
U.S. government securities(d) | |||||||||||||||
Corporate debt instruments(d) | |||||||||||||||
Non-U.S. government securities(d) | |||||||||||||||
Other(d) | |||||||||||||||
Absolute return funds(e) | |||||||||||||||
Real assets | |||||||||||||||
Other(f) | |||||||||||||||
Total | $ | $ | $ | $ | |||||||||||
Total assets measured at NAV | |||||||||||||||
Fair value of plan assets | $ | ||||||||||||||
72  | 2019 Form 10-K | 2019 Form 10-K | ||
Basis of fair value measurement | |||||||||||||||
as of December 31 (in millions) | 2018 | Quoted prices in active markets for identical assets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | |||||||||||
Equities | |||||||||||||||
U.S. large cap(a) | $ | $ | $ | $ | |||||||||||
U.S. mid cap(b) | |||||||||||||||
International(c) | |||||||||||||||
Fixed income securities | |||||||||||||||
U.S. government securities(d) | |||||||||||||||
Corporate debt instruments(d) | |||||||||||||||
Non-U.S. government securities(d) | |||||||||||||||
Other(d) | |||||||||||||||
Absolute return funds(e) | |||||||||||||||
Real assets | |||||||||||||||
Other(f) | |||||||||||||||
Total | $ | $ | $ | $ | |||||||||||
Total assets measured at NAV | |||||||||||||||
Fair value of plan assets | $ | ||||||||||||||
(a) | A mix of index funds and actively managed equity accounts that are benchmarked to various large cap indices. |
(b) | A mix of index funds and actively managed equity accounts that are benchmarked to various mid cap indices. |
(c) | A mix of index funds and actively managed equity accounts that are benchmarked to various non-U.S. equity indices in both developed and emerging markets. |
(d) | Securities held by actively managed accounts, index funds and mutual funds. |
(e) | Primarily funds having global mandates with the flexibility to allocate capital broadly across a wide range of asset classes and strategies, including but not limited to equities, fixed income, commodities, financial futures, currencies and other securities, with objectives to outperform agreed upon benchmarks of specific return and volatility targets. |
(f) | Investments in cash and cash equivalents. |
Equities and registered investment companies having quoted prices are valued at the published market prices. Fixed income securities that are valued using significant other observable inputs are quoted at prices obtained from independent financial service industry-recognized vendors. Investments held in pooled investment funds, common collective trusts or limited partnerships are valued at the net asset value (NAV) practical expedient to estimate fair value. The NAV is provided by the fund administrator and is based on the value of the underlying assets owned by the fund minus its liabilities.
The investment mix of equity securities, fixed income and other asset allocation strategies is based upon achieving a desired return, balancing higher return, more volatile equity securities and lower return, less volatile fixed income securities. Investment allocations are established for each plan and are generally made across a range of markets, industry sectors, capitalization sizes and in the case of fixed income securities, maturities and credit quality. The 2019 target investment allocation for the AbbVie Pension Plan was 35 % in equity securities, 20 % in fixed income securities and 45 % in asset allocation strategies and other holdings. There are no known significant concentrations of risk in the plan assets of the AbbVie Pension Plan or of any other plans.
The expected return on plan assets assumption for each plan is based on management's expectations of long-term average rates of return to be achieved by the underlying investment portfolio. In establishing this assumption, management considers historical and expected returns for the asset classes in which the plans are invested, as well as current economic and capital market conditions.
2019 Form 10-K |  73 73 | ||
Expected Benefit Payments
The following table summarizes total benefit payments expected to be paid to plan participants including payments funded from both plan and company assets:
years ending December 31 (in millions) | Defined benefit plans | Other post-employment plans | |||||
2020 | $ | $ | |||||
2021 | |||||||
2022 | |||||||
2023 | |||||||
2024 | |||||||
2025 to 2029 | |||||||
Defined Contribution Plan
AbbVie's principal defined contribution plan is the AbbVie Savings Plan. AbbVie recorded expense of $102 million in 2019, $89 million in 2018 and $82 million in 2017 related to this plan. AbbVie provides certain other post-employment benefits, primarily salary continuation arrangements, to qualifying employees and accrues for the related cost over the service lives of the employees.
Note 13 Equity
Stock-Based Compensation
AbbVie grants stock-based awards to eligible employees pursuant to the AbbVie 2013 Incentive Stock Program (2013 ISP), which provides for several different forms of benefits, including nonqualified stock options, RSUs and various performance-based awards. Under the 2013 ISP, 100 million shares of AbbVie common stock were reserved for issuance as awards to AbbVie employees. The 2013 ISP also facilitated the assumption of certain awards granted under Abbott’s incentive stock program, which were adjusted and converted into Abbott and AbbVie stock-based awards as a result of AbbVie's separation from Abbott.
AbbVie measures compensation expense for stock-based awards based on the grant date fair value of the awards and the estimated number of awards that are expected to vest. Forfeitures are estimated based on historical experience at the time of grant and are revised in subsequent periods if actual forfeitures differ from those estimates. Compensation cost for stock-based awards is amortized over the service period, which could be shorter than the vesting period if an employee is retirement eligible. Retirement eligible employees generally are those who are age 55 or older and have at least 10 years of service.
Stock-based compensation expense is principally related to awards issued pursuant to the 2013 ISP and is summarized as follows:
Years ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Cost of products sold | $ | $ | $ | ||||||||
Research and development | |||||||||||
Selling, general and administrative | |||||||||||
Pre-tax compensation expense | |||||||||||
Tax benefit | |||||||||||
After-tax compensation expense | $ | $ | $ | ||||||||
Realized excess tax benefits associated with stock-based compensation totaled $15 million in 2019, $78 million in 2018 and $71 million in 2017.
74  | 2019 Form 10-K | 2019 Form 10-K | ||
Stock Options
Stock options awarded to employees typically have a contractual term of 10 years and generally vest in one-third increments over a three-year period. The exercise price is equal to at least 100 % of the market value on the date of grant. The fair value is determined using the Black-Scholes model. The weighted-average grant-date fair values of stock options granted were $12.54 in 2019, $21.63 in 2018 and $9.80 in 2017.
The following table summarizes AbbVie stock option activity in 2019:
(options in thousands, aggregate intrinsic value in millions) | Options | Weighted- average exercise price | Weighted- average remaining life (in years) | Aggregate intrinsic value | ||||||||
Outstanding at December 31, 2018 | $ | $ | ||||||||||
Granted | ||||||||||||
Exercised | ( | ) | ||||||||||
Lapsed | ( | ) | ||||||||||
Outstanding at December 31, 2019 | $ | $ | ||||||||||
Exercisable at December 31, 2019 | $ | $ | ||||||||||
The total intrinsic value of options exercised was $22 million in 2019, $215 million in 2018 and $371 million in 2017. The total fair value of options vested during 2019 was $13 million. As of December 31, 2019, $6 million of unrecognized compensation cost related to stock options is expected to be recognized as expense over approximately the next two years .
RSUs and Performance Shares
RSUs awarded to employees other than senior executives and other key employees generally vest in one-third increments over a three year period. Recipients of these RSUs are entitled to receive dividend equivalents as dividends are declared and paid during the RSU vesting period.
The majority of the equity awards AbbVie grants to its senior executives and other key employees are performance-based. Equity awards granted to senior executives and other key employees consist of a combination of performance-vested RSUs and performance shares as well as non-qualified stock options described above. The performance-vested RSUs have the potential to vest in one-third increments during a three-year performance period based on AbbVie’s ROE relative to a defined peer group of pharmaceutical, biotech and life sciences companies. The recipient may receive one share of AbbVie common stock for each vested award. The performance shares have the potential to vest over a three-year performance period and may be earned based on AbbVie’s EPS achievement and AbbVie’s total stockholder return (TSR) (a market condition) relative to a defined peer group of pharmaceutical, biotech and life sciences companies. Dividend equivalents on performance-vested RSUs and performance shares accrue during the performance period and are payable at vesting only to the extent that shares are earned.
The weighted-average grant-date fair value of RSUs and performance shares generally is determined based on the number of shares/units granted and the quoted price of AbbVie’s common stock on the date of grant. The weighted-average grant-date fair values of performance shares with a TSR market condition are determined using the Monte Carlo simulation model.
The following table summarizes AbbVie RSU and performance share activity for 2019:
(share units in thousands) | Share units | Weighted-average grant date fair value | ||||
Outstanding at December 31, 2018 | $ | |||||
Granted | ||||||
Vested | ( | ) | ||||
Forfeited | ( | ) | ||||
Outstanding at December 31, 2019 | $ | |||||
The fair market value of RSUs and performance shares (as applicable) vested was $371 million in 2019, $583 million in 2018 and $348 million in 2017.
2019 Form 10-K |  75 75 | ||
As of December 31, 2019, $327 million of unrecognized compensation cost related to RSUs and performance shares is expected to be recognized as expense over approximately the next two years .
Cash Dividends
Cash dividends declared per common share totaled $4.39 in 2019, $3.95 in 2018 and $2.63 in 2017. The following table summarizes quarterly cash dividends declared during 2019, 2018 and 2017:
2019 | 2018 | 2017 | ||||||||||||||
Date Declared | Payment Date | Dividend Per Share | Date Declared | Payment Date | Dividend Per Share | Date Declared | Payment Date | Dividend Per Share | ||||||||
11/01/19 | 02/14/20 | $ | 11/02/18 | 02/15/19 | $ | 10/27/17 | 02/15/18 | $ | ||||||||
09/06/19 | 11/15/19 | $ | 09/07/18 | 11/15/18 | $ | 09/08/17 | 11/15/17 | $ | ||||||||
06/20/19 | 08/15/19 | $ | 06/14/18 | 08/15/18 | $ | 06/22/17 | 08/15/17 | $ | ||||||||
02/21/19 | 05/15/19 | $ | 02/15/18 | 05/15/18 | $ | 02/16/17 | 05/15/17 | $ | ||||||||
Stock Repurchase Program
The company's stock repurchase authorization permits purchases of AbbVie shares from time to time in open-market or private transactions at management’s discretion. The program has no time limit and can be discontinued at any time. Shares repurchased under these programs are recorded at acquisition cost, including related expenses and are available for general corporate purposes.
AbbVie repurchased 4 million shares for $300 million in 2019. AbbVie's remaining stock repurchase authorization was approximately $4.0 billion as of December 31, 2019.
On February 15, 2018, AbbVie's board of directors authorized a new $10.0 billion stock repurchase program, which superseded AbbVie's previous stock repurchase program. On December 13, 2018, AbbVie's board of directors authorized a $5.0 billion increase to the existing $10.0 billion stock repurchase program. Under this authorization, AbbVie repurchased approximately 109 million shares for $10.7 billion in 2018.
Under previous stock repurchase programs, AbbVie made open-market share repurchases of approximately 11 million shares for $1.3 billion in 2018 and approximately 13 million shares for $1.0 billion in 2017.
76  | 2019 Form 10-K | 2019 Form 10-K | ||
Accumulated Other Comprehensive Loss
The following table summarizes the changes in each component of accumulated other comprehensive loss, net of tax, for 2019, 2018 and 2017:
(in millions) (brackets denote losses) | Foreign currency translation adjustments | Net investment hedging activities | Pension and post- employment benefits | Marketable security activities | Cash flow hedging activities | Total | |||||||||||||||||
Balance as of December 31, 2016 | $ | ( | ) | $ | $ | ( | ) | $ | $ | $ | ( | ) | |||||||||||
Other comprehensive income (loss) before reclassifications | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||
Net losses (gains) reclassified from accumulated other comprehensive loss | ( | ) | ( | ) | |||||||||||||||||||
Net current-period other comprehensive income (loss) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||
Balance as of December 31, 2017 | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||
Other comprehensive income (loss) before reclassifications | ( | ) | ( | ) | ( | ) | |||||||||||||||||
Net losses reclassified from accumulated other comprehensive loss | — | ||||||||||||||||||||||
Net current-period other comprehensive income (loss) | ( | ) | ( | ) | |||||||||||||||||||
Balance as of December 31, 2018 | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||
Other comprehensive income (loss) before reclassifications | ( | ) | ( | ) | ( | ) | |||||||||||||||||
Net losses (gains) reclassified from accumulated other comprehensive loss | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||
Net current-period other comprehensive income (loss) | ( | ) | ( | ) | ( | ) | |||||||||||||||||
Balance as of December 31, 2019 | $ | ( | ) | $ | $ | ( | ) | $ | $ | $ | ( | ) | |||||||||||
Other comprehensive loss included foreign currency translation adjustments totaling losses of $98 million in 2019 and $391 million in 2018 which were principally due to the impact of the weakening of the Euro on the translation of the company’s Euro-denominated assets.
In 2017, AbbVie reclassified $316 million of historical currency translation losses from AOCI related to the liquidation of certain foreign entities following the enactment of U.S. tax reform. These losses were included in net foreign exchange loss in the consolidated statement of earnings and had no related income tax impacts. Other comprehensive loss in 2017 also included foreign currency translation adjustments totaling a gain of $680 million, which was principally due to the impact of the strengthening of the Euro on the translation of the company’s Euro-denominated assets.
Other comprehensive loss for 2019 included pension and post-employment benefit plan losses of $1.2 billion primarily due to an actuarial loss driven by lower discount rates. See Note 12 for additional information.
2019 Form 10-K |  77 77 | ||
The table below presents the impact on AbbVie's consolidated statements of earnings for significant amounts reclassified out of each component of accumulated other comprehensive loss:
years ended December 31 (in millions) (brackets denote gains) | 2019 | 2018 | 2017 | ||||||||
Net investment hedging activities | |||||||||||
Gains on derivative amount excluded from effectiveness testing(a) | $ | ( | ) | $ | $ | ||||||
Tax expense | |||||||||||
Total reclassifications, net of tax | $ | ( | ) | $ | $ | ||||||
Pension and post-employment benefits | |||||||||||
Amortization of actuarial losses and other(b) | $ | $ | $ | ||||||||
Tax benefit | ( | ) | ( | ) | ( | ) | |||||
Total reclassifications, net of tax | $ | $ | $ | ||||||||
Cash flow hedging activities | |||||||||||
Losses (gains) on foreign currency forward exchange contracts(c) | $ | ( | ) | $ | $ | ( | ) | ||||
Gains on treasury rate lock agreements and interest rate swap contracts(a) | ( | ) | |||||||||
Tax expense (benefit) | ( | ) | |||||||||
Total reclassifications, net of tax | $ | ( | ) | $ | $ | ( | ) | ||||
(a) | Amounts are included in interest expense, net (see Note 11). |
(b) | Amounts are included in the computation of net periodic benefit cost (see Note 12). |
(c) | Amounts are included in cost of products sold (see Note 11). |
Other
In addition to common stock, AbbVie's authorized capital includes 200 million shares of preferred stock, par value $0.01 . As of December 31, 2019, no shares of preferred stock were issued or outstanding.
Note 14 Income Taxes
Earnings Before Income Tax Expense
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Domestic | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Foreign | |||||||||||
Total earnings before income tax expense | $ | $ | $ | ||||||||
Income Tax Expense
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Current | |||||||||||
Domestic | $ | $ | $ | ||||||||
Foreign | |||||||||||
Total current taxes | $ | $ | $ | ||||||||
Deferred | |||||||||||
Domestic | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||
Foreign | ( | ) | |||||||||
Total deferred taxes | $ | $ | ( | ) | $ | ( | ) | ||||
Total income tax expense (benefit) | $ | $ | ( | ) | $ | ||||||
78  | 2019 Form 10-K | 2019 Form 10-K | ||
Impacts Related to U.S. Tax Reform
The Tax Cuts and Jobs Act (the Act) was signed into law in December 2017, resulting in significant changes to the U.S. corporate tax system. The Act reduced the U.S. federal corporate tax rate from 35 % to 21 % and required companies to pay a one-time transition tax on a mandatory deemed repatriation of earnings of certain foreign subsidiaries that were previously untaxed. These changes were generally effective for tax years beginning in 2018.
The Act also created a minimum tax on certain foreign sourced earnings. The company’s accounting policy for the minimum tax on foreign sourced earnings is to report the tax effects on the basis that the minimum tax will be recognized in tax expense in the year it is incurred as a period expense.
Additionally, the Act significantly changed the timing and manner in which earnings of foreign subsidiaries are subject to U.S. tax. Therefore, unremitted foreign earnings previously considered indefinitely reinvested that were subject to the Act’s transition tax are no longer considered indefinitely reinvested. Post-2017 earnings subject to the U.S. minimum tax on foreign sourced earnings and the 100 percent foreign dividends received deduction are also not considered indefinitely reinvested earnings. As such, the company records foreign withholding tax liabilities related to the future cash repatriation of such earnings. However, the company considers instances of outside basis differences in foreign subsidiaries that would incur additional U.S. tax upon reversal (e.g., capital gain distribution) to be permanent in duration. The unrecognized tax liability is not practicable to determine.
Effective Tax Rate Reconciliation
years ended December 31 | 2019 | 2018 | 2017 | |||||
Statutory tax rate | % | % | % | |||||
Effect of foreign operations | ( | ) | ( | ) | ( | ) | ||
U.S. tax credits | ( | ) | ( | ) | ( | ) | ||
Impacts related to U.S. tax reform | ( | ) | ||||||
Stock-based compensation excess tax benefit | ( | ) | ( | ) | ( | ) | ||
Tax audit settlements | ( | ) | ( | ) | ( | ) | ||
Deferred tax remeasurements due to change in tax rate | ||||||||
All other, net | ||||||||
Effective tax rate | % | ( | )% | % | ||||
The effective income tax rate fluctuates year to year due to the allocation of the company's taxable earnings among jurisdictions, as well as certain discrete factors and events in each year, including changes in tax law, acquisitions and collaborations. The effective income tax rates in 2019, 2018 and 2017 differed from the statutory tax rate principally due to changes in enacted tax rates and laws, the benefit from foreign operations which reflects the impact of lower income tax rates in locations outside the United States, tax incentives in Puerto Rico and other foreign tax jurisdictions, business development activities, the cost of repatriation decisions, Boehringer Ingelheim accretion on contingent consideration and Stemcentrx impairment related expenses. The effective tax rates for these periods also reflected the benefit from U.S. tax credits principally related to research and development credits, the orphan drug tax credit and Puerto Rico excise tax credits. The Puerto Rico excise tax credits relate to legislation enacted by Puerto Rico that assesses an excise tax on certain products manufactured in Puerto Rico. The tax is levied on gross inventory purchases from entities in Puerto Rico and is included in cost of products sold in the consolidated statements of earnings. The majority of the tax is creditable for U.S. income tax purposes.
The effective income tax rate in 2019, 2018 and 2017 included impacts related to U.S. tax reform. In 2018, there was a favorable impact of the effective date of provisions of the Act related to the earnings from certain foreign subsidiaries. For 2019, the impact of the Act affected the full year earnings of these subsidiaries, resulting in additional tax expense compared to prior year. The 2019 effective income tax rate also reflects the effects of deferred tax remeasurement due to a change in foreign tax law, accretion for contingent consideration and impairment related expenses. In addition, the company recognized a net tax benefit of $400 million in 2019, $131 million in 2018 and $91 million in 2017 related to the resolution of various tax positions pertaining to prior years.
2019 Form 10-K |  79 79 | ||
Deferred Tax Assets and Liabilities
as of December 31 (in millions) | 2019 | 2018 | |||||
Deferred tax assets | |||||||
Compensation and employee benefits | $ | $ | |||||
Accruals and reserves | |||||||
Chargebacks and rebates | |||||||
Advance payments | |||||||
Net operating losses and other credit carryforwards | |||||||
Other | |||||||
Total deferred tax assets | |||||||
Valuation allowances | ( | ) | ( | ) | |||
Total net deferred tax assets | |||||||
Deferred tax liabilities | |||||||
Excess of book basis over tax basis of intangible assets | ( | ) | ( | ) | |||
Excess of book basis over tax basis in investments | ( | ) | ( | ) | |||
Other | ( | ) | ( | ) | |||
Total deferred tax liabilities | ( | ) | ( | ) | |||
Net deferred tax liabilities | $ | ( | ) | $ | ( | ) | |
As of December 31, 2019, gross state net operating losses were $1.0 billion and tax credit carryforwards were $188 million. The state tax carryforwards expire between 2020 and 2039. As of December 31, 2019, foreign net operating loss carryforwards were $2.9 billion. Foreign net operating loss carryforwards of $2.8 billion expire between 2020 and 2036 and the remaining do not have an expiration period.
The company had valuation allowances of $731 million as of December 31, 2019 and $103 million as of December 31, 2018. These were principally related to foreign and state net operating losses and credit carryforwards that are not expected to be realized.
Unrecognized Tax Benefits
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
Beginning balance | $ | $ | $ | ||||||||
Increase due to current year tax positions | |||||||||||
Increase due to prior year tax positions | |||||||||||
Decrease due to prior year tax positions | ( | ) | ( | ) | ( | ) | |||||
Settlements | ( | ) | ( | ) | ( | ) | |||||
Lapse of statutes of limitations | ( | ) | ( | ) | ( | ) | |||||
Ending balance | $ | $ | $ | ||||||||
AbbVie and Abbott entered into a tax sharing agreement, effective on the date of separation, which provides that Abbott is liable for and has indemnified AbbVie against all income tax liabilities for periods prior to the separation. AbbVie will be responsible for unrecognized tax benefits and related interest and penalties for periods after separation or in instances where an existing entity was transferred to AbbVie upon separation.
If recognized, the net amount of potential tax benefits that would impact the company's effective tax rate is $2.4 billion in 2019 and $2.7 billion in 2018. Of the unrecognized tax benefits recorded in the table above as of December 31, 2019, AbbVie would be indemnified for approximately $83 million. The “Increase due to current year tax positions” and "Increase due to prior year tax positions" in the table above include amounts related to federal, state and international tax items.
AbbVie recognizes interest and penalties related to income tax matters in income tax expense in the consolidated statements of earnings. AbbVie recognized gross income tax expense of $51 million in 2019, $73 million in 2018 and $24 million in 2017, for interest and penalties related to income tax matters. AbbVie had an accrual for the payment of gross
80  | 2019 Form 10-K | 2019 Form 10-K | ||
interest and penalties of $191 million at December 31, 2019, $190 million at December 31, 2018 and $120 million at December 31, 2017.
The company is routinely audited by the tax authorities in significant jurisdictions and a number of audits are currently underway. It is reasonably possible during the next 12 months that uncertain tax positions may be settled, which could result in a decrease in the gross amount of unrecognized tax benefits. Due to the potential for resolution of federal, state and foreign examinations and the expiration of various statutes of limitation, the company's gross unrecognized tax benefits balance may change within the next 12 months up to $54 million. All significant federal, state, local and international matters have been concluded for years through 2012. The company believes adequate provision has been made for all income tax uncertainties.
Note 15 Legal Proceedings and Contingencies
AbbVie is subject to contingencies, such as various claims, legal proceedings and investigations regarding product liability, intellectual property, commercial, securities and other matters that arise in the normal course of business. Loss contingency provisions are recorded for probable losses at management’s best estimate of a loss, or when a best estimate cannot be made, a minimum loss contingency amount within a probable range is recorded. The recorded accrual balance for litigation was approximately $290 million as of December 31, 2019 and approximately $350 million as of December 31, 2018. Initiation of new legal proceedings or a change in the status of existing proceedings may result in a change in the estimated loss accrued by AbbVie. In addition, other operating income in 2019 included $550 million of income from a legal settlement related to an intellectual property dispute with a third party. While it is not feasible to predict the outcome of all proceedings and exposures with certainty, management believes that their ultimate disposition should not have a material adverse effect on AbbVie’s consolidated financial position, results of operations or cash flows.
Subject to certain exceptions specified in the separation agreement by and between Abbott and AbbVie, AbbVie assumed the liability for, and control of, all pending and threatened legal matters related to its business, including liabilities for any claims or legal proceedings related to products that had been part of its business, but were discontinued prior to the distribution, as well as assumed or retained liabilities, and will indemnify Abbott for any liability arising out of or resulting from such assumed legal matters.
Four lawsuits against Unimed Pharmaceuticals, LLC, Solvay Pharmaceuticals, Inc. (a company Abbott acquired in February 2010 and now known as AbbVie Products LLC) and others remained consolidated for pre-trial purposes in the United States District Court for the Northern District of Georgia under the Multi-District Litigation (MDL) Rules as In re: AndroGel Antitrust Litigation, MDL No. 2084. These cases, brought by direct AndroGel purchasers, generally allege Solvay's 2006 patent litigation settlement agreements and related agreements with three generic companies violate federal antitrust laws. Plaintiffs seek monetary damages and attorneys' fees. Three of those lawsuits were settled in December 2019 and will be dismissed.
In September 2014, the FTC filed a lawsuit, FTC v. AbbVie Inc., et al., against AbbVie and others in the United States District Court for the Eastern District of Pennsylvania, alleging that the 2011 patent litigation with two generic companies regarding AndroGel was sham litigation and the settlements of that litigation violated federal antitrust law. In May 2015, the court dismissed the FTC’s settlement-related claim. In June 2018, following a bench trial, the court found for the FTC on its sham litigation claim and ordered a disgorgement remedy of $448 million, plus prejudgment interest. The court denied the FTC’s request for injunctive relief. AbbVie is appealing the court’s liability and disgorgement rulings and, based on an assessment of the merits of that appeal, no liability has been accrued for this matter. The FTC is also appealing aspects of the court’s trial ruling and the dismissal of its settlement-related claim. In July 2018, a purported class action was filed in the United States District Court for the Eastern District of Pennsylvania on behalf of direct AndroGel purchasers based on the trial court’s ruling in the FTC’s case. In September 2019, two individual direct AndroGel purchasers substituted in as the plaintiffs in that lawsuit and withdrew the class allegations. That case, which was pending as Rochester Drug Co-Operative, Inc., et al. v. AbbVie Inc., et al., was settled in December 2019 and will be dismissed.
In August 2019, direct purchasers of AndroGel filed a lawsuit, King Drug Co. of Florence, Inc., et al. v. AbbVie Inc., et al., against AbbVie and others in the United States District Court for the Eastern District of Pennsylvania, making allegations similar to those in In re: AndroGel Antitrust Litigation (No. II), MDL No. 2084 (above) and FTC v. AbbVie Inc. (above).
Lawsuits are pending against AbbVie and others generally alleging that the 2005 patent litigation settlement involving Niaspan entered into between Kos Pharmaceuticals, Inc. (a company acquired by Abbott in 2006 and presently a subsidiary of AbbVie) and a generic company violates federal and state antitrust laws and state unfair and deceptive trade practices and unjust enrichment laws. Plaintiffs generally seek monetary damages and/or injunctive relief and attorneys' fees. The lawsuits consist of four individual plaintiff lawsuits and two consolidated purported class actions: one brought by Niaspan direct purchasers and one brought by Niaspan end-payers. The cases are pending in the United States District Court for the Eastern
2019 Form 10-K |  81 81 | ||
District of Pennsylvania for coordinated or consolidated pre-trial proceedings under the MDL Rules as In re: Niaspan Antitrust Litigation, MDL No. 2460. In August 2019, the court certified a class of direct purchasers of Niaspan. In October 2016, the Orange County, California District Attorney’s Office filed a lawsuit on behalf of the State of California regarding the Niaspan patent litigation settlement in Orange County Superior Court, asserting a claim under the unfair competition provision of the California Business and Professions Code seeking injunctive relief, restitution, civil penalties and attorneys’ fees. In May 2018, the California Court of Appeal ruled that the District Attorney’s Office may not bring monetary claims beyond the scope of Orange County, which the District Attorney’s Office is appealing.
Between March and May 2019, 12 putative class action lawsuits were filed in the United States District Court for the Northern District of Illinois by indirect HUMIRA purchasers, alleging that AbbVie’s settlements with biosimilar manufacturers and AbbVie’s HUMIRA patent portfolio violate state and federal antitrust laws. The court consolidated these lawsuits as In re: Humira (Adalimumab) Antitrust Litigation.
In November 2014, a putative class action lawsuit, Medical Mutual of Ohio v. AbbVie Inc., et al., was filed against several manufacturers of testosterone replacement therapies (TRTs), including AbbVie, in the United States District Court for the Northern District of Illinois on behalf of all insurance companies, health benefit providers, and other third party payers who paid for TRTs, including AndroGel. The claims asserted included violations of the federal RICO Act and state consumer fraud and deceptive trade practices laws. The complaint sought monetary damages and injunctive relief. In July 2018, the court denied the plaintiff’s motion for class certification. In November 2019, the United States Court of Appeals for the Seventh Circuit affirmed the district court’s grant of the defendants’ summary judgment motion.
In July 2019, the New Mexico Attorney General filed a lawsuit, State of New Mexico ex rel. Balderas v. AbbVie Inc., et al., in New Mexico District Court for Santa Fe County against AbbVie and other companies alleging their marketing of AndroGel violated New Mexico’s Unfair Practices Act.
In September 2018, the Commissioner of the California Department of Insurance intervened in a qui tam lawsuit, State of California and Lazaro Suarez v. AbbVie Inc., et al., brought under the California Insurance Frauds Prevention Act, in California Superior Court for Alameda County. The Department of Insurance’s complaint alleges that, through patient and reimbursement support services and other services and items of value provided in connection with HUMIRA, AbbVie caused the submission of fraudulent commercial insurance claims for HUMIRA in violation of the California statute. The complaint seeks injunctive relief, an assessment of up to three times the amount of the claims at issue, and civil penalties. In addition, a federal securities lawsuit (Holwill v. AbbVie Inc., et al.) is pending in the United States District Court for the Northern District of Illinois) against AbbVie, its chief executive officer and former chief financial officer, alleging that reasons stated for HUMIRA sales growth in financial filings between 2013 and 2017 were misleading because they omitted the conduct alleged in the Department of Insurance’s complaint.
In November 2014, five individuals filed a putative class action lawsuit, Rubinstein, et al. v Gonzalez, et al., on behalf of purchasers and sellers of certain Shire plc (Shire) securities between June 20 and October 14, 2014, against AbbVie and its chief executive officer in the United States District Court for the Northern District of Illinois alleging that the defendants made and/or are responsible for material misstatements in violation of federal securities laws in connection with AbbVie's proposed transaction with Shire. In October 2019, the court granted final approval to the parties’ class settlement agreement.
In June 2016, a lawsuit, Elliott Associates, L.P., et al. v. AbbVie Inc., was filed by five investment funds against AbbVie in the Cook County, Illinois Circuit Court alleging that AbbVie made misrepresentations and omissions in connection with its proposed transaction with Shire. Similar lawsuits were filed between July 2017 and October 2019 against AbbVie and in some instances its chief executive officer in the same court by additional investment funds. Plaintiffs seek compensatory and punitive damages.
Product liability cases were filed in which plaintiffs generally allege that AbbVie and other manufacturers of TRTs did not adequately warn about risks of certain injuries, primarily heart attacks, strokes and blood clots. Approximately 3,500 claims against AbbVie are consolidated for pre-trial purposes in the United States District Court for the Northern District of Illinois under the MDL Rules as In re: Testosterone Replacement Therapy Products Liability Litigation, MDL No. 2545. Approximately 175 claims against AbbVie are pending in various state courts. Plaintiffs generally seek compensatory and punitive damages. In November 2018, AbbVie entered into a Master Settlement Agreement with the Plaintiffs’ Steering Committee in the MDL encompassing existing claims in all courts. All proceedings in pending cases are effectively stayed during the settlement administration process.
Product liability cases are pending in which plaintiffs generally allege that AbbVie did not adequately warn about risk of certain injuries, primarily various birth defects, arising from use of Depakote. Approximately 120 cases are pending in the United States District Court for the Southern District of Illinois, and approximately 14 others are pending in various federal and state courts. Plaintiffs generally seek compensatory and punitive damages. Approximately eighty percent of these pending
82  | 2019 Form 10-K | 2019 Form 10-K | ||
cases, plus other unfiled claims, are subject to confidential settlement agreements and are expected to be dismissed with prejudice.
Beginning in May 2016, the Patent Trial & Appeal Board of the U.S. Patent & Trademark Office (PTO) instituted five inter partes review proceedings brought by Coherus Biosciences and Boehringer Ingelheim related to three AbbVie patents covering methods of treatment of rheumatoid arthritis using adalimumab. In these proceedings, the PTO reviewed the validity of the patents and issued decisions of invalidity in May, June and July of 2017. In January 2020, the Court of Appeals for the Federal Circuit affirmed the decisions.
In March 2017, AbbVie filed a lawsuit, AbbVie Inc. v. Novartis Vaccines and Diagnostics, Inc. and Grifols Worldwide Operations Ltd., in the United States District Court for the Northern District of California against Novartis Vaccines and Grifols Worldwide seeking a declaratory judgment that 11 HCV-related patents licensed to AbbVie in 2002 are invalid.
Pharmacyclics LLC, a wholly owned subsidiary of AbbVie, is seeking to enforce its patent rights relating to ibrutinib capsules (a drug Pharmacyclics sells under the trademark IMBRUVICA®). In February 2018, cases were filed in the United States District Court for the District of Delaware against the following defendants: Fresenius Kabi USA, LLC, Fresenius Kabi USA, Inc., and Fresenius Kabi Oncology Limited; Sun Pharma Global FZE and Sun Pharmaceutical Industries Ltd.; Cipla Limited and Cipla USA Inc.; and Zydus Worldwide DMCC, Cadila Healthcare Limited, Sandoz Inc., and Lek Pharmaceuticals D.D. In each case, Pharmacyclics alleges the defendant’s proposed generic ibrutinib product infringes certain Pharmacyclics patents and seeks declaratory and injunctive relief. Janssen Biotech, Inc. which is in a global collaboration with Pharmacyclics concerning the development and marketing of IMBRUVICA, is the co-plaintiff in these suits.
Pharmacyclics LLC, a wholly owned subsidiary of AbbVie, is seeking to enforce its patent rights relating to ibrutinib tablets (a drug Pharmacyclics sells under the trademark IMBRUVICA®). In a case filed in the United States District Court for the District of Delaware in March 2019, Pharmacyclics alleges that Alvogen Pine Brook LLC’s and Natco Pharma Ltd.’s proposed generic ibrutinib tablet product infringes certain Pharmacyclics patents. Pharmacyclics seeks declaratory and injunctive relief. Janssen Biotech, Inc. which is in a global collaboration with Pharmacyclics concerning the development and marketing of IMBRUVICA, is the co-plaintiff in this suit.
2019 Form 10-K |  83 83 | ||
Note 16 Segment and Geographic Area Information
AbbVie operates in one business segment—pharmaceutical products. Substantially all of AbbVie's net revenues in the United States are to three wholesalers. Outside the United States, products are sold primarily to health care providers or through distributors, depending on the market served. The following tables detail AbbVie's worldwide net revenues:
years ended December 31 (in millions) | 2019 | 2018 | 2017 | |||||||||
Immunology | ||||||||||||
HUMIRA | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
SKYRIZI | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
RINVOQ | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
Hematologic Oncology | ||||||||||||
IMBRUVICA | United States | $ | $ | $ | ||||||||
Collaboration revenues | ||||||||||||
Total | $ | $ | $ | |||||||||
VENCLEXTA | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
HCV | ||||||||||||
MAVYRET | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
VIEKIRA | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
Other Key Products | ||||||||||||
Creon | United States | $ | $ | $ | ||||||||
Lupron | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
Synthroid | United States | $ | $ | $ | ||||||||
Synagis | International | $ | $ | $ | ||||||||
Duodopa | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
Sevoflurane | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
Kaletra | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
AndroGel | United States | $ | $ | $ | ||||||||
ORILISSA | United States | $ | $ | $ | ||||||||
International | ||||||||||||
Total | $ | $ | $ | |||||||||
All other | $ | $ | $ | |||||||||
Total net revenues | $ | $ | $ | |||||||||
84  | 2019 Form 10-K | 2019 Form 10-K | ||
Net revenues to external customers by geographic area, based on product shipment destination, were as follows:
years ended December 31 (in millions) | 2019 | 2018 | 2017 | ||||||||
United States | $ | $ | $ | ||||||||
Japan | |||||||||||
Germany | |||||||||||
Canada | |||||||||||
France | |||||||||||
Spain | |||||||||||
United Kingdom | |||||||||||
Italy | |||||||||||
Brazil | |||||||||||
The Netherlands | |||||||||||
All other countries | |||||||||||
Total net revenues | $ | $ | $ | ||||||||
Long-lived assets, primarily net property and equipment, by geographic area were as follows:
as of December 31 (in millions) | 2019 | 2018 | |||||
United States and Puerto Rico | $ | $ | |||||
Europe | |||||||
All other | |||||||
Total long-lived assets | $ | $ | |||||
2019 Form 10-K |  85 85 | ||
Note 17 Quarterly Financial Data (unaudited)
(in millions except per share data) | 2019 | 2018 | |||||
First Quarter | |||||||
Net revenues | $ | $ | |||||
Gross margin | |||||||
Net earnings(a) | |||||||
Basic earnings per share | $ | $ | |||||
Diluted earnings per share | $ | $ | |||||
Cash dividends declared per common share | $ | $ | |||||
Second Quarter | |||||||
Net revenues | $ | $ | |||||
Gross margin | |||||||
Net earnings(b) | |||||||
Basic earnings per share | $ | $ | |||||
Diluted earnings per share | $ | $ | |||||
Cash dividends declared per common share | $ | $ | |||||
Third Quarter | |||||||
Net revenues | $ | $ | |||||
Gross margin | |||||||
Net earnings(c) | |||||||
Basic earnings per share | $ | $ | |||||
Diluted earnings per share | $ | $ | |||||
Cash dividends declared per common share | $ | $ | |||||
Fourth Quarter | |||||||
Net revenues | $ | $ | |||||
Gross margin | |||||||
Net earnings (loss)(d) | ( | ) | |||||
Basic earnings (loss) per share | $ | $ | ( | ) | |||
Diluted earnings (loss) per share | $ | $ | ( | ) | |||
Cash dividends declared per common share | $ | $ | |||||
(a) | First quarter results in 2019 included after-tax charges of $ |
(b) | Second quarter results in 2019 included an after-tax charge of $ |
(c) | Third quarter results in 2019 included after-tax charges of $ |
86  | 2019 Form 10-K | 2019 Form 10-K | ||
(d) | Fourth quarter results in 2019 included an after-tax charge of $ |
2019 Form 10-K |  87 87 | ||
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of AbbVie Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of AbbVie Inc. and subsidiaries (the Company) as of December 31, 2019 and 2018, and the related consolidated statements of earnings, comprehensive income, equity and cash flows for each of the three years in the period ended December 31, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 21, 2020 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
88  | 2019 Form 10-K | 2019 Form 10-K | ||
Sales rebate accruals for Medicaid, Medicare and managed care programs | ||
Description of the Matter | As discussed in Note 2 to the consolidated financial statements under the caption “Revenue Recognition,” the Company established provisions for sales rebates in the same period as the related product is sold. At December 31, 2019, the Company had $4,484 million in sales rebate accruals, a large portion of which were for rebates provided to pharmacy benefit managers, state government Medicaid programs, insurance companies that administer Medicare drug plans and private entities for Medicaid, Medicare and managed care programs. In order to establish these sales rebate accruals, the Company estimated its rebates based upon the identification of the products subject to a rebate, the applicable price and rebate terms and the estimated lag time between the sale and payment of the rebate. Auditing the Medicaid, Medicare and managed care sales rebate accruals was complex and required significant auditor judgment because the accruals consider multiple subjective and complex estimates and assumptions. These estimates and assumptions included the estimated inventory in the distribution channel, which impacts the lag time between the sale to the customer and payment of the rebate and the final payer related to product sales, which impacts the applicable price and rebate terms. In deriving these estimates and assumptions, the Company used both internal and external sources of information to estimate product in the distribution channels, payer mix, prescription volumes and historical experience. Management supplemented its historical data analysis with qualitative adjustments based upon changes in rebate trends, rebate programs and contract terms, legislative changes, or other significant events which indicate a change in the reserve is appropriate. | |
How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s sales rebate accruals for Medicaid, Medicare and managed care programs. This included testing controls over management’s review of the significant assumptions and other inputs used in the estimation of Medicaid, Medicare and managed care rebates, among others, including the significant assumptions discussed above. The testing was inclusive of management’s controls to evaluate the accuracy of its reserve judgments to actual rebates paid, rebate validation and processing, and controls to ensure that the data used to evaluate and support the significant assumptions was complete, accurate and, where applicable, verified to external data sources. To test the sales rebate accruals for Medicaid, Medicare, and managed care programs, our audit procedures included, among others, understanding and evaluating the significant assumptions and underlying data used in management’s calculations. Our testing of significant assumptions included corroboration to external data sources. We evaluated the reasonableness of assumptions in light of industry and economic trends, product profiles, and other regulatory factors. We assessed the historical accuracy of management’s estimates by comparing actual activity to previous estimates and performed analytical procedures, based on internal and external data sources, to evaluate the completeness of the reserves. For Medicaid, we involved a specialist with an understanding of statutory reimbursement requirements to assess the consistency of the Company’s calculation methodologies with applicable government regulations and policy. | |
Valuation of contingent consideration | ||
Description of the Matter | As discussed in Note 2 to the consolidated financial statements under the caption “Business Combinations” and in Note 11 under the caption “Financial Instruments and Fair Value Measures,” the Company recognized contingent consideration liabilities at the estimated fair value on the acquisition date in connection with applying the acquisition method of accounting for business combinations. Subsequent changes to the fair value of the contingent consideration liabilities were recorded within the consolidated statement of earnings in the period of change. At December 31, 2019, the Company had $7,340 million in contingent consideration liabilities, which represented a ‘Level 3’ fair value measurement in the fair value hierarchy due to the significant unobservable inputs used in determining the fair value and the use of management judgment about the assumptions market participants would use in pricing the liabilities. Auditing the valuation of contingent consideration liabilities was complex and required significant auditor judgment due to the use of a Monte Carlo simulation model and the high degree of subjectivity in evaluating certain assumptions required to estimate the fair value of contingent royalty payments. In particular, the fair value measurement was sensitive to the significant assumptions underlying the estimated amount of future sales of the acquired products. Management utilized its expertise within the industry and knowledge of clinical development and regulatory approval processes to determine certain of these assumptions. | |
2019 Form 10-K |  89 89 | ||
How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s contingent consideration liabilities process including, among others, management’s process to establish the significant assumptions and measure the liability. This included testing controls over management’s review of the significant assumptions and other inputs used in the determination of fair value. The testing was inclusive of key management review controls to monitor and evaluate clinical development of the acquired products and estimated future sales, and controls to ensure that the data used to evaluate and support the significant assumptions was complete, accurate and, where applicable, verified to external data sources. To test the estimated fair value of contingent consideration liabilities, our audit procedures included, among others, inspecting the terms of the executed agreement, assessing the Monte Carlo simulation model used and testing the key contractual inputs and significant assumptions discussed above. We evaluated the assumptions and judgments in light of observable industry and economic trends and standards, external data sources and regulatory factors. Estimated amounts of future sales were evaluated for reasonableness in relation to internal and external analyses, clinical development progress and timelines, probability of success benchmarks, and regulatory notices. Our procedures included evaluating the data sources used by management in determining its assumptions and, where necessary, included an evaluation of available information that either corroborated or contradicted management’s conclusions. We involved a valuation specialist to assess the Company’s Monte Carlo simulation model and to perform corroborative fair value calculations. | |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2013.
Chicago, Illinois
February 21, 2020
90  | 2019 Form 10-K | 2019 Form 10-K | ||
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures; Internal Control Over Financial Reporting
Evaluation of disclosure controls and procedures. The Chief Executive Officer, Richard A. Gonzalez, and the Chief Financial Officer, Robert A. Michael, evaluated the effectiveness of AbbVie's disclosure controls and procedures as of the end of the period covered by this report, and concluded that AbbVie's disclosure controls and procedures were effective to ensure that information AbbVie is required to disclose in the reports that it files or submits with the Securities and Exchange Commission under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported, within the time periods specified in the Commission's rules and forms, and to ensure that information required to be disclosed by AbbVie in the reports that it files or submits under the Securities Exchange Act of 1934 is accumulated and communicated to AbbVie's management, including its principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in internal control over financial reporting. There were no changes in AbbVie's internal control over financial reporting (as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934) that have materially affected, or are reasonably likely to materially affect, AbbVie's internal control over financial reporting during the quarter ended December 31, 2019.
Inherent limitations on effectiveness of controls. AbbVie's management, including its Chief Executive Officer and its Chief Financial Officer, do not expect that AbbVie's disclosure controls or internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls.
The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Management's annual report on internal control over financial reporting. Management of AbbVie is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. AbbVie's internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States. However, all internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and reporting.
Management assessed the effectiveness of AbbVie's internal control over financial reporting as of December 31, 2019. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013 framework). Based on that assessment, management concluded that AbbVie maintained effective internal control over financial reporting as of December 31, 2019, based on the COSO criteria.
The effectiveness of AbbVie's internal control over financial reporting as of December 31, 2019 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their attestation report below, which expresses an unqualified opinion on the effectiveness of AbbVie's internal control over financial reporting as of December 31, 2019.
2019 Form 10-K |  91 91 | ||
Report of independent registered public accounting firm. The report of AbbVie's independent registered public accounting firm related to its assessment of the effectiveness of internal control over financial reporting is included below.
92  | 2019 Form 10-K | 2019 Form 10-K | ||
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of AbbVie Inc.
Opinion on Internal Control over Financial Reporting
We have audited AbbVie Inc. and subsidiaries' internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, AbbVie Inc. and subsidiaries (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of AbbVie Inc. and subsidiaries as of December 31, 2019 and 2018, and the related consolidated statements of earnings, comprehensive income, equity and cash flows for each of the three years in the period ended December 31, 2019, and the related notes and our report dated February 21, 2020 expressed an unqualified opinion thereon.
Basis for Opinion
The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations on Internal Control Over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Chicago, Illinois
February 21, 2020
2019 Form 10-K |  93 93 | ||
ITEM 9B. OTHER INFORMATION
None.
94  | 2019 Form 10-K | 2019 Form 10-K | ||
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Incorporated herein by reference are "Information Concerning Director Nominees," "The Board of Directors and its Committees—Committees of the Board of Directors," and "Procedure for Recommendation and Nomination of Directors and Transaction of Business at Annual Meeting" to be included in the 2020 AbbVie Inc. Proxy Statement. The 2020 Definitive Proxy Statement will be filed on or about March 19, 2020. Also incorporated herein by reference is the text found in this Form 10-K under the caption, "Information about Our Executive Officers."
AbbVie's code of business conduct requires all its business activities to be conducted in compliance with all applicable laws, regulations and ethical principles and values. All directors, officers and employees of AbbVie are required to read, understand and abide by the requirements of the code of business conduct applicable to them. AbbVie's code of business conduct is available in the corporate governance section of AbbVie's investor relations website at www.abbvieinvestor.com.
Any waiver of the code of business conduct for directors or executive officers may be made only by AbbVie's audit committee. AbbVie will disclose any amendment to, or waiver from, a provision of the code of conduct for the principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, on its website within four business days following the date of the amendment or waiver. In addition, AbbVie will disclose any waiver from the code of business conduct for the other executive officers and for directors on the website.
AbbVie has a chief ethics and compliance officer who reports to the Vice Chairman, External Affairs and Chief Legal Officer and to the public policy committee. The chief ethics and compliance officer is responsible for overseeing, administering and monitoring AbbVie's compliance program.
ITEM 11. EXECUTIVE COMPENSATION
The material to be included in the 2020 AbbVie Inc. Proxy Statement under the headings "Director Compensation," "Executive Compensation," and "Compensation Committee Report" is incorporated herein by reference. The 2020 Definitive Proxy Statement will be filed on or about March 19, 2020.
2019 Form 10-K |  95 95 | ||
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
(a) Equity Compensation Plan Information.
The following table presents information as of December 31, 2019 about AbbVie's equity compensation plans under which AbbVie common stock has been authorized for issuance:
Plan Category | (a) Number of securities to be issued upon exercise of outstanding options, warrants and rights (1) | (b) Weighted- average exercise price of outstanding options, warrants and rights (2) | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (3) | ||||||
Equity compensation plans approved by security holders | 16,991,269 | $ | 60.39 | 62,161,107 | |||||
Equity compensation plans not approved by security holders | — | — | — | ||||||
Total | 16,991,269 | $ | 60.39 | 62,161,107 | |||||
(1) | Includes 837,960 shares issuable under AbbVie's Incentive Stock Program pursuant to awards granted by Abbott and adjusted into AbbVie awards in connection with AbbVie's separation from Abbott. |
(2) | The weighted-average exercise price does not include outstanding restricted stock units, restricted stock awards and performance shares that have no exercise price. |
(3) | Excludes shares issuable upon the exercise of stock options and pursuant to other rights granted under the Stemcentrx 2011 Equity Incentive Plan, which was assumed by AbbVie upon the consummation of its acquisition of Stemcentrx, Inc. As of December 31, 2019, 103,874 options remained outstanding under this plan. The options have a weighted-average exercise price of $16.36. No further awards will be granted under this plan. |
(b) | Information Concerning Security Ownership. Incorporated herein by reference is the material under the heading "Securities Ownership—Securities Ownership of Executive Officers and Directors" in the 2020 AbbVie Inc. Proxy Statement. The 2020 Definitive Proxy Statement will be filed on or about March 19, 2020. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The material to be included in the 2020 AbbVie Inc. Proxy Statement under the headings "The Board of Directors and its Committees," "Corporate Governance Materials," and "Procedures for Approval of Related Person Transactions" is incorporated herein by reference. The 2020 Definitive Proxy Statement will be filed on or about March 19, 2020.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The material to be included in the 2020 AbbVie Inc. Proxy Statement under the headings "Audit Fees and Non-Audit Fees" and "Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of the Independent Registered Public Accounting Firm" is incorporated herein by reference. The 2020 Definitive Proxy Statement will be filed on or about March 19, 2020.
96  | 2019 Form 10-K | 2019 Form 10-K | ||
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) | Documents filed as part of this Form 10-K. |
(1) | Financial Statements: See Item 8, "Financial Statements and Supplementary Data," on page 43 hereof, for a list of financial statements. |
(2) | Financial Statement Schedules: All schedules omitted are inapplicable or the information required is shown in the consolidated financial statements or notes thereto. |
(3) | Exhibits Required by Item 601 of Regulation S-K: The information called for by this paragraph is set forth in Item 15(b) below. |
(b) | Exhibits: |
Exhibit Number | Exhibit Description | ||
2019 Form 10-K |  97 97 | ||
Exhibit Number | Exhibit Description | ||
98  | 2019 Form 10-K | 2019 Form 10-K | ||
Exhibit Number | Exhibit Description | ||
2019 Form 10-K |  99 99 | ||
Exhibit Number | Exhibit Description | ||
101 | The following financial statements and notes from the AbbVie Inc. Annual Report on Form 10-K for the year ended December 31, 2019 filed on February 21, 2020, formatted in Inline XBRL (eXtensible Business Reporting Language): (i) Consolidated Statements of Earnings; (ii) Consolidated Statements of Comprehensive Income; (iii) Consolidated Balance Sheets; (iv) Consolidated Statements of Equity; (v) Consolidated Statements of Cash Flows; and (vi) the Notes to Consolidated Financial Statements. | ||
104 | Cover Page Interactive Data File (the cover page from the AbbVie Inc. Annual Report on Form 10-K formatted as Inline XBRL and contained in Exhibit 101). | ||
The AbbVie Inc. 2020 Definitive Proxy Statement will be filed with the Securities and Exchange Commission under separate cover on or about March 19, 2020. | |||
_______________________________________________________________________________
* | Incorporated herein by reference. Commission file number 001-35565. |
** | Denotes management contract or compensatory plan or arrangement required to be filed as an exhibit hereto. |
Exhibits 32.1 and 32.2, above, are furnished herewith and should not be deemed to be "filed" under the Securities Exchange Act of 1934. AbbVie will furnish copies of any of the above exhibits to a stockholder upon written request to the Secretary, AbbVie Inc., 1 North Waukegan Road, North Chicago, Illinois 60064.
100  | 2019 Form 10-K | 2019 Form 10-K | ||
ITEM 16. FORM 10-K SUMMARY
None.
2019 Form 10-K |  101 101 | ||
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, AbbVie Inc. has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
AbbVie Inc. | ||||
By: | /s/ RICHARD A. GONZALEZ | |||
Name: | Richard A. Gonzalez | |||
Title: | Chairman of the Board and Chief Executive Officer | |||
Date: | February 21, 2020 | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of AbbVie Inc. on February 21, 2020 in the capacities indicated below.
/s/ RICHARD A. GONZALEZ | /s/ ROBERT A. MICHAEL | |
Richard A. Gonzalez Chairman of the Board and Chief Executive Officer (Principal Executive Officer) | Robert A. Michael Executive Vice President, Chief Financial Officer (Principal Financial Officer) | |
/s/ BRIAN L. DURKIN | ||
Brian L. Durkin Vice President, Controller (Principal Accounting Officer) | ||
/s/ ROBERT J. ALPERN, M.D. | /s/ ROXANNE S. AUSTIN | |
Robert J. Alpern, M.D. Director of AbbVie Inc. | Roxanne S. Austin Director of AbbVie Inc. | |
/s/ WILLIAM H.L. BURNSIDE | /s/ BRETT J. HART | |
William H.L. Burnside Director of AbbVie Inc. | Brett J. Hart Director of AbbVie Inc. | |
/s/ EDWARD M. LIDDY | /s/ MELODY B. MEYER | |
Edward M. Liddy Director of AbbVie Inc. | Melody B. Meyer Director of AbbVie Inc. | |
/s/ EDWARD J. RAPP | /s/ REBECCA B. ROBERTS | |
Edward J. Rapp Director of AbbVie Inc. | Rebecca B. Roberts Director of AbbVie Inc. | |
/s/ GLENN F. TILTON | /s/ FREDERICK H. WADDELL | |
Glenn F. Tilton Director of AbbVie Inc. | Frederick H. Waddell Director of AbbVie Inc. | |
102  | 2019 Form 10-K | 2019 Form 10-K | ||