Use these Links to rapidly review the document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One) | ||
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
For the fiscal year ended | ||
OR | ||
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
For the transition period from to | ||
Commission file number: 001-35113
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of Incorporation or organization) | (I.R.S. Employer Identification No.) | |
(Zip Code) | ||
(Address of principal executive offices) | ||
Registrant's telephone number, including area code: (412 ) 288-4600
Securities registered pursuant to section 12(b) of the Act:
Title of each class | Trading Symbol | Name of each exchange on which registered | ||
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definition of "large accelerated filer," "accelerated filer," "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | ☒ | Non-accelerated filer | ☐ | Smaller reporting company | Emerging growth company | |||
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of all common stock (based upon the closing price of the New York Stock Exchange) of the registrant held by non-affiliates of the registrant as of June 30, 2019 was approximately $123.0 million.
As of March 20, 2020, the number of outstanding shares of Class A common stock, par value $0.001 per share (the "common stock"), of GNC Holdings, Inc. was 84,608,976 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information in the Company's definitive Proxy Statement for the 2020 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission pursuant to Regulation 14A, not later than 120 days after the end of the fiscal year, is incorporated by reference in Part III of this Form 10-K.
1
TABLE OF CONTENTS
Page | |||
2
FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K (this "Annual Report") contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 with respect to our financial condition, results of operations and business, which could cause actual results to differ materially from projected results. Forward-looking statements include statements that may relate to our plans, objectives, goals, strategies, future events, future revenues or performance, capital expenditures, financing needs and other information that is not historical information. Forward-looking statements can often be identified by the use of terminology such as "subject to," "believe," "anticipate," "plan," "expect," "intend," "estimate," "project," "may," "will," "should," "would," "could," "can," the negatives thereof, variations thereon and similar expressions, or by discussions of strategy.
All forward-looking statements, including, without limitation, our examination of historical operating trends, are based upon our current expectations and various assumptions. We believe there is a reasonable basis for our expectations and beliefs, but they are inherently uncertain and subject to significant business, economic, competitive, regulatory and other risks, contingencies and uncertainties, most of which are difficult to predict and many of which are beyond our control. These risks, contingencies and uncertainties relate to, among other things: the highly competitive industry in which we operate; unfavorable publicity or consumer perception of our products; product innovation; our exploration of new strategic initiatives; our manufacturing operations; relationships with our vendors; our distribution network and inventory management; our ability to develop and maintain a relevant omni-channel experience for our customers; the performance of, and our relationships with, our franchisees; the location of our stores; availability of raw materials; risks related to COVID-19 (novel coronavirus) and its impacts on our markets (including decreased customer traffic at malls and other places our stores are located); general economic conditions; the risk of delays, interruptions and disruptions in our global supply chain, including disruptions in supply due to COVID-19 (novel coronavirus) or other disease outbreaks; material claims or product recalls; regulatory compliance; the value of our brand name; privacy protection and cyber-security; our current debt profile and risks related to our capital structure; possible joint ventures; our key executives and employees; insurance; and tax rate risks. A detailed discussion of risk and uncertainties that could cause actual results and events to differ materially from such forward-looking statements is included in the section titled "Risk Factors" (Item 1A of this Annual Report).
In addition, we operate in a highly competitive and rapidly changing environment; therefore, new risk factors can arise, and it is not possible for management to predict all such risk factors, nor to assess the impact of all such risk factors on our business or the extent to which any individual risk factor, or combination of risk factors, may cause results to differ materially from those contained in any forward-looking statement. Consequently, forward-looking statements should be regarded solely as our current plans, estimates and beliefs. You should not place undue reliance on forward-looking statements as a prediction of actual results. We cannot guarantee future results, events, levels of activity, performance or achievements. The forward-looking statements included in this Annual Report are made as of the date of this filing. We do not undertake and specifically decline any obligation to update, republish or revise forward-looking statements to reflect future events or circumstances or to reflect the occurrence of unanticipated events.
3
PART I
Item 1. BUSINESS.
GNC Holdings, Inc. (together with its subsidiaries, referred to as "Holdings", "GNC", "the Company", "we", "us" and "our" unless specified otherwise) connects customers to their best selves by offering a premium assortment of health, wellness and performance products, including protein, performance supplements, weight management supplements, vitamins, herbs and greens, wellness supplements, health and beauty, food and drink and other general merchandise, featuring both proprietary GNC and nationally recognized third-party brands. Our diversified, omni-channel business model generates revenue from product sales through company-owned retail stores, domestic and international franchise activities, third-party contract manufacturing, e-commerce and wholesale partnerships. We are headquartered in Pittsburgh, Pennsylvania and our common stock trades on the New York Stock Exchange (the "NYSE") under the symbol "GNC." Our business was founded in 1935 by David Shakarian who opened our first health food store in Pittsburgh, Pennsylvania.
Our principal executive office is located at 300 Sixth Avenue, Pittsburgh, Pennsylvania 15222, and our telephone number is (412) 288-4600. We maintain and make our Annual Reports on the Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports available on our website, GNC.com, free of charge, as soon as reasonably practical after we electronically file or furnish them to the United States Securities and Exchange Commission (the "SEC").
Business Strategy
Key elements of our business strategy are detailed below:
Leading brand of nutritional supplements. GNC has been in business for more than 80 years. The Company is built on a core foundation as a brand builder of high-quality nutritional supplements. We are a leading global brand of health, wellness and performance products, with a worldwide network of approximately 7,500 locations and our online channels.
Our objective is to offer a broad and deep mix of products for consumers interested in living well, whether they are looking to treat a health-related issue, maintain their overall wellness, or improve their performance. Our premium, value-added offerings include both proprietary GNC-branded products and other nationally recognized third-party brands.
We believe our depth of brands, exclusive products and range of merchandise, combined with the customer support and service we offer, differentiates us from competitors and allows us to effectively compete against food, drug and mass channel players, specialty stores, independent vitamin, supplement and natural food shops and online retailers.
Product development and innovation. We develop high-quality, innovative nutritional supplement products that can be purchased only through our company-owned and franchise store locations, GNC.com, our Amazon.com and other marketplaces and our select wholesale partners. Our high quality ingredients are rigorously tested before they are added to our products, undergoing multiple quality checks to ensure that they meet our high standards for identity, strength, purity, composition and limits in contaminants.
We believe our sector-leading innovation capability is a significant competitive advantage. We entered into a strategic partnership with International Vitamin Corporation ("IVC") in March 2019, which allows us to further focus on innovation while IVC drives increased efficiencies in manufacturing. GNC has demonstrated strength in developing unique, branded, and scientifically verified products and has a long history of delivering new ingredients, new flavors and convenient solutions. We directly employ scientists, nutritionists, formulators, and quality control experts and have access to a wide range of world-class research facilities and consultants. Refer to Item 8, “Financial Statements and Supplementary Data,” Note 9, “ Equity Method Investments” for more information.
A differentiated retail customer experience. Our retail strategy is to deliver a compelling experience at every customer touch point. We operate in a highly personalized, aspirational sector and believe that the nutritional supplement consumer often desires and seeks out product expertise and knowledgeable customer service.
We further differentiate ourselves from competitors through development of our well-trained "coaches" with regular training that focuses on solution-based selling, and through in-store technology such as tablets, which allow associates to view customers’ purchase history and preferences. With that knowledge, and help from sales tools built into the tablet platform, associates can engage customers in conversation, share product information, testimonials and before and after pictures, recommend solutions and help customers add complimentary products and build wellness regimens.
Our loyalty programs allow us to develop and maintain a large and loyal customer base, provide targeted offers and information, and connect with our customers on a regular basis. We harness data generated by these programs to better understand
4
customers’ buying behaviors and needs, so we can deliver a stronger experience, bring like-minded consumers into the channel and make well-informed decisions about the business.
Omni-channel development. We believe our diversified, omni-channel model, which includes company-owned stores, domestic and international franchise locations, wholesale locations and e-commerce channels, can differentiate us from online only competitors. Our strategy is to give consumers a seamless, integrated experience across digital, mobile and in-store channels and in every interaction they have with GNC and our products.
Through GNC.com, our Amazon.com storefront and other marketplaces, customers can research and purchase our products online. We believe our physical store base provides a competitive advantage, allowing customers to experience our products and get expert advice from our coaches.
Our omni-channel model can enhance the customer experience and increase the lifetime value of a GNC customer, and we are implementing strategies over the next 12-18 months to blend our digital, online and in-store platforms. These initiatives include increased cross-channel marketing, online and in-store subscription services, giving customers the option of picking up online purchases in GNC stores, shipping products purchased via e-commerce directly from stores, and additional educational content, information and advice on GNC.com.
International growth. We continue to see opportunity to expand internationally within the large global supplement market, through online channels and store locations, which is expected to continue to grow. In particular, our partnership with Harbin Pharmaceutical Group Co., Ltd ("Harbin") allows us to further expand our business in China. Harbin’s expertise in distribution and regulation in China is the ideal match for our highly valued brand and assortment of products in the China market. Refer to Item 8, “Financial Statements and Supplementary Data,” Note 9, “Equity Method Investments” for more information.
Driving constructive industry dialogue. We remain focused on continuously raising the bar on transparency and quality throughout the dietary supplement industry. We believe that over time the implementation of higher standards and more stringent industry self-regulation regarding manufacturing practices, ingredient traceability and product transparency will prove beneficial for the industry and lead to improved dialogue with regulators, stronger consumer trust and greater confidence in our industry.
Segments
We generate revenues from our three segments, U.S. and Canada; International; and Manufacturing / Wholesale. The following table outlines our total revenue by segments. For a description of operating income (loss) by segment, our total assets by segment and our total revenues by geographic area, see Item 8, “Financial Statements and Supplementary Data," Note 19, "Segments."
Year ended December 31, | ||||||||||||||||||||
2019 | 2018 | 2017 | ||||||||||||||||||
($ in millions) | ||||||||||||||||||||
U.S. and Canada | $ | 1,822.3 | 88.1 | % | $ | 1,951.2 | 82.9 | % | $ | 2,018.9 | 81.4 | % | ||||||||
International (1) | 158.2 | 7.6 | % | 191.4 | 8.1 | % | 177.8 | 7.2 | % | |||||||||||
Manufacturing / Wholesale (2) | 87.7 | 4.3 | % | 210.9 | 9.0 | % | 218.1 | 8.8 | % | |||||||||||
Other (3) | — | — | % | — | — | % | 66.2 | 2.6 | % | |||||||||||
Total revenue | $ | 2,068.2 | 100.0 | % | $ | 2,353.5 | 100.0 | % | $ | 2,481.0 | 100.0 | % | ||||||||
(1) Includes revenue related to China operations prior to the transfer of the China business to the joint ventures (the "HK JV" and "China JV") formed in February 2019 of $2.4 million, $42.9 million and $33.3 million in 2019, 2018 and 2017, respectively.
(2) Excludes intersegment sales; includes revenue related to Nutra manufacturing operation prior to the transfer of the Nutra manufacturing business to the manufacturing joint venture ("Manufacturing JV") formed in March 2019 of $15.8 million, $123.3 million and $128.9 million in 2019, 2018 and 2017, respectively.
(3) Relates to Lucky Vitamin which was sold in September 2017.
Although we believe that none of our segment operations experience significant seasonal fluctuations, historically we have experienced, and expect to continue to experience, the lowest amount of revenue in our fourth quarter compared with the first three quarters of the year.
U.S. and Canada
5
Our U.S. and Canada segment generates revenues primarily from the sale of products to customers at our company-owned stores in the United States, Canada and Puerto Rico, through product sales to domestic franchisees, royalties on domestic franchise retail sales, franchise fees and through GNC.com and our marketplace on Amazon.
Company-Owned Retail Stores in the U.S. and Canada
As of December 31, 2019, we operated 2,902 company-owned stores across all 50 states and the District of Columbia in the United States, in Canada and Puerto Rico. Most of our company-owned stores in the United States are between 1,000 and 2,000 square feet and are located primarily in strip shopping centers and shopping malls.
Domestic Franchise Stores
As of December 31, 2019, there were 956 domestic franchise stores operated by 408 franchisees. Our domestic franchise stores are typically between 1,000 and 2,000 square feet, and approximately 90% are located in strip shopping centers. We believe we have good relationships with our franchisees, as evidenced by our domestic franchisee renewal rate of approximately 87% between 2014 and 2019. We do not rely heavily on any single franchise operator in the United States, with our largest franchisee owning and/or operating 79 store locations.
All of our franchise stores in the United States offer both our proprietary products and third-party products, with a product selection similar to that of our company-owned stores.
Revenues from our franchisees in the United States accounted for approximately 16% of our total U.S. and Canada segment revenues for the year ended December 31, 2019. New franchisees in the United States are required to pay an initial fee of $40,000 for a franchise license and existing GNC franchise operators may purchase an additional franchise license for a $30,000 fee. Once a franchise store begins operations, franchisees are required to pay us a continuing royalty of 6% of sales and contribute 3% of sales to a national advertising fund. Our standard franchise agreements for the United States are effective for an initial ten-year period with unlimited five-year renewal options. At the end of the initial term and each of the renewal periods, the renewal fee is generally 33% of the franchise fee that is then in effect. The franchisee renewal option is generally at our election. Franchisees must meet certain conditions to exercise the franchisee renewal option. Our franchisees in the United States receive limited geographical exclusivity and are required to utilize the standard GNC store format.
Generally, we negotiate lease terms to secure locations at cost-effective rates, which we typically sublease to our franchisees at cost. Franchisees must meet certain minimum standards and duties prescribed by our franchise operations manual, and we conduct periodic field visit reports to ensure our minimum standards are maintained. If a franchisee does not meet specified performance and appearance criteria, we are permitted to terminate the franchise agreement. In these situations, we may take possession of the location, inventory and equipment, and operate the store as a company-owned store or refranchise the location.
Websites
GNC.com continues to represent a significant and growing part of our business. The ability to purchase our products through the internet also offers a convenient method for repeat customers to evaluate and purchase new and existing products. This additional sales channel has enabled us to market and sell our products in regions where we have limited or no retail operations. We may offer products on our website that are not available at our retail locations, enabling us to broaden the assortment of products available to our customers. We also offer a product assortment on our market place on Amazon, the revenue of which is included in our GNC.com business unit.
International
Our International segment generates revenue primarily from our international franchisees through product sales, royalties and franchise fees and also includes our Ireland operations, and prior to the formation of the HK JV and China JV effective February 13, 2019, China operations. Refer to Item 8, “Financial Statements and Supplementary Data,” Note 9, “ Equity Method Investments” for more information.
International Franchise Stores
As of December 31, 2019, there were 1,904 international franchise locations operating in approximately 50 international countries (including distribution centers where retail sales are made). The international franchise locations are typically smaller than our domestic locations and, depending upon the country and cultural preferences, are located in malls, strip shopping centers, streets or store-within-a-store locations. In addition, some international franchisees sell on the internet and distribute to other retail outlets in their respective countries. Typically, our international stores have a store format and signage similar to our United States
6
franchise stores. We believe that our franchise program enhances our brand awareness and market presence and will enable us to continue to expand our store base internationally with limited capital expenditures.
Our international franchise stores generally offer a more limited product selection than our franchise stores in the United States, primarily due to regulatory constraints.
Revenues from our international franchisees accounted for approximately 82% of our total international segment revenues for the year ended December 31, 2019. New international franchisees are required to pay an initial fee of approximately $25,000 for a franchise license for each full size store, $12,500 for a franchise license for a store-within-a-store and continuing royalty fees. Our international franchise program has enabled us to expand into international markets with limited investment.
We enter into development agreements with international franchisees which grants the right to develop a specific number of stores, for either full-size stores or store-within-a-store locations, in a territory, often the entire country. We enter into distribution agreements with international franchisees which grants the right to distribute product through the store locations, wholesale distribution centers and, in some cases, limited internet distribution. The franchisee then enters into a franchise agreement for each location. The full-size store franchise agreement has an initial ten-year term with two five-year renewal options. The franchisee typically has the option to renew the agreement at 33% of the current initial franchise fee that is then being charged to new franchisees. Franchise agreements for international store-within-a-store locations have an initial term of five years, with two five-year renewal options. At the end of the initial term and each of the renewal periods, the franchisee has the option to renew the store-within-a-store agreement for up to a maximum of 50% of the franchise fee that is then in effect. Our international franchisees often receive exclusive franchising rights to the entire country, generally excluding United States military bases. Our international franchisees must meet minimum standards and duties similar to our United States franchisees.
Manufacturing / Wholesale
Our Manufacturing / Wholesale segment is comprised of our manufacturing operations in South Carolina prior to the formation of the manufacturing joint venture (the "Manufacturing JV") in March 2019, and our wholesale partner relationships. The manufacturing facility supplies our U.S. and Canada segment, International segment and wholesale partner business with proprietary product and also manufactures products for other third parties. Our wholesale partner business includes the sale of products to wholesale customers, the largest of which include Rite Aid, Sam's Club and PetSmart.
In March 2019, we established the Manufacturing JV with IVC, which enables GNC quality and R&D teams to continue to support product development and increase focus on product innovation, while IVC manages manufacturing and integrates with GNC's supply chain thereby driving more efficient usage of capital. Under the terms of the agreement, GNC received $99.2 million, net of a working capital purchase price adjustment, in 2019 and contributed its Nutra manufacturing and Anderson facility net assets in exchange for an initial 43% interest in the manufacturing joint venture. Over the next three years, GNC expects to receive an additional $75 million from IVC, adjusted up or down based on the Manufacturing JV's future performance, as IVC’s ownership of the joint venture increases to 100%. Refer to Item 8, “Financial Statements and Supplementary Data,” Note 9, “Equity Method Investments” for more information.
To increase brand awareness and promote access to customers who may not frequent specialty nutrition stores, we entered into a strategic alliance with Rite Aid in December 1998 to open GNC franchise "store-within-a-store" locations. As of December 31, 2019, we had 1,759 Rite Aid store-within-a-store locations. Through this strategic alliance, we generate revenues from sales to Rite Aid of our products at wholesale prices, sales of Rite Aid private label products, retail sales of certain consigned inventory and license fees. We are Rite Aid's sole supplier for a number of Rite Aid private label supplements, pursuant to a supply agreement with Rite Aid that extends through 2021. The operating license that comprises our store-within-a store alliance with Rite Aid was extended through 2021. We terminated the consignment agreement with Rite Aid in the fourth quarter of 2018.
Other
Revenue prior to 2018 also included the results of an additional website, LuckyVitamin.com, beginning in August 2011 and through September 30, 2017. We sold substantially all of the assets of our Lucky Vitamin subsidiary effective September 30, 2017.
Brands and Products
We are a global health and wellness brand with a diversified omni-channel business. Our product assortment includes health, wellness and performance products, including protein, performance supplements, weight management supplements, vitamins, herbs and greens, wellness supplements, health and beauty, food and drink and other general merchandise. Refer to Item 8, "Financial Statements and Supplementary Data," Note 3, "Revenue" of this Annual Report for a breakdown of revenue by
7
product category. We offer an extensive mix of both GNC and third-party brands across multiple categories and products. This variety is designed to provide our customers with a wide selection of products to fit their specific needs and to generate a high number of transactions with purchases from multiple product categories.
We offer a wide range of high-quality nutritional supplements sold under our GNC proprietary brand names. Sales of our proprietary brands at our U.S. company-owned and franchise stores, GNC.com and wholesale partners including Rite Aid, PetSmart and Sam's Club represented 52% and 51% of total system-wide retail product sales in 2019 and 2018, or $1,015 million and $1,072 million, respectively. We also offer products through nationally recognized third-party brand names. Sales of our third-party products at our U.S. company-owned and franchise stores, GNC.com and wholesale partners represented approximately 48% and 49% of total system-wide retail product sales in 2019 and 2018, or $923 million and $1,011 million, respectively, and together with proprietary sales yielded total U.S. system-wide sales of $1,938 and $2,083 million. In 2019 and 2018, we did not have a material concentration of sales from any single product or product line. Our largest vendor, excluding the Nutra manufacturing facility, supplied approximately 14% and 17% of our products in 2019 and 2018, respectively.
We offer a free points-based loyalty program, which enables customers to earn points based on their purchases. Points earned by members are valid for one year and may be redeemed for cash discounts on any product we sell at both company-owned or franchise locations. In addition, we offer a paid membership program, "PRO Access," which provides members with the delivery of sample boxes throughout the membership year, as well as the offering of certain other benefits including the opportunity to earn multiple points on a periodic basis. The boxes include sample merchandise and other materials.
Product Distribution
Products are delivered to retail stores, wholesale distributors, international franchisees and directly to customers who make purchases online, via a third party transportation network, primarily through our distribution centers located in: Leetsdale, Pennsylvania; Whitestown, Indiana; Anderson, South Carolina, and Phoenix, Arizona. Our distribution centers support our company-owned stores as well as franchise stores and Rite Aid locations. Each of our distribution centers has a quality control department that monitors products received from our vendors to ensure they meet our quality standards. Internet purchases are fulfilled and shipped directly from our distribution centers to our consumers using a third-party transportation service, or directly by Amazon for certain marketplace orders. In connection with the Manufacturing JV agreement with IVC, the Company has transitioned out of the Anderson, South Carolina distribution center in the first quarter of 2020.
Employees
As of December 31, 2019, we had approximately 12,400 employees, including approximately 4,400 full-time and 8,000 part-time employees. None of our employees belong to a union or are party to any collective bargaining or similar agreement. We consider our relationship with our employees to be good.
Competition
The United States nutritional supplements retail and packaged goods industry is a large, highly fragmented and growing industry, with no single industry participant accounting for a majority of total industry retail sales. Competition is based on multiple factors, including price, quality and assortment of products, customer service, convenience of store locations and online platform, marketing support and availability of new products. In addition, the market is highly sensitive to the introduction of new products.
We compete with both publicly and privately owned companies, which are highly fragmented in terms of geographical market coverage and product categories. We also compete with other specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, mail-order companies, online-only retailers and a variety of other smaller participants. In the United States, many of our competitors have national brands that are heavily advertised and are manufactured by large pharmaceutical and food companies and other retailers. Most supermarkets, drugstores and mass merchants have narrow product offerings limited primarily to simple vitamins, herbs and popular third-party sports and diet products. Our international competitors also include large international pharmacy chains and major international supermarket chains, as well as other large U.S.-based companies with international operations. Our wholesale and manufacturing operations compete with other wholesalers and manufacturers of third-party nutritional supplements.
8
Trademarks and Other Intellectual Property
We believe trademark protection is particularly important to the maintenance of the recognized brand names under which we market our products. We own or have rights to material trademarks or trade names that we use in conjunction with the sale of our products, including the GNC brand name. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. We protect our intellectual property rights through a variety of methods, including trademark, patent and trade secret laws, as well as confidentiality agreements and proprietary information agreements with vendors, employees, consultants and others who have access to our proprietary information. Protection of our intellectual property often affords us the opportunity to enhance our position in the marketplace by precluding our competitors from using or otherwise exploiting our technology and brands. We are also a party to several intellectual property license agreements relating to certain of our products. The duration of our trademark registrations is generally 10, 15 or 20 years, depending on the country in which the marks are registered, and we can renew the registrations. The scope and duration of our intellectual property protection varies throughout the world by jurisdiction and by individual product. Our global trademark portfolio, with the aforementioned registration durations, consists of our core marks for our business and our proprietary product brands which drive significant brand awareness for all of our reportable segments. Our proprietary product formulas and recipes, maintained as trade secrets, are significant to our growth and success as they form the foundation for our production and sales of effective, high quality products.
Insurance and Risk Management
We are self-insured for certain losses related to workers' compensation and general liability insurance and maintain stop-loss coverage with third-party insurers to limit our liability exposure. We face an inherent risk of exposure to potential product liability claims in the event that, among other things, the use of products sold by us results in injury. We carry product liability insurance with a deductible/retention of $4.0 million per claim with an aggregate cap on retained losses of $10.0 million per policy year. For product liability claims stemming from third party products sold in our stores, we generally have the ability to refer such claims directly to our vendors and their insurers. In most cases, our insurance covers such claims that are not adequately covered by a vendor's insurance and provides for excess secondary coverage above the limits provided by our product vendors. We are fully insured for health insurance.
We also purchase insurance to cover auto liability, network and cyber security, privacy liability, employment practice, and other casualty and property risks. We self-insure certain property and casualty risks, such as property damage due to our analysis of the risk, the frequency and severity of a loss and the cost of insurance for the risk.
Government Regulation
Product Regulation
Domestic
The processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to regulation by one or more federal agencies, including the U.S. Food and Drug Administration (the "FDA"), the Federal Trade Commission (the "FTC"), the Consumer Product Safety Commission (the "CPSC"), the United States Department of Agriculture (the "USDA") and the Environmental Protection Agency (the "EPA"), and by various agencies of the states and localities in which our products are sold.
Food and Drug Administration
Dietary Supplements
The Dietary Supplement Health and Education Act of 1994 ("DSHEA") amended the Federal Food, Drug, and Cosmetic Act (the "FDC Act") to establish a new framework governing the composition, safety, labeling, manufacturing and marketing of dietary supplements. Generally, under the FDC Act, dietary ingredients (i.e., vitamins; minerals; herb or other botanical; amino acids; or dietary substances for use by humans to supplement diet by increasing total dietary intake; or any concentrate, metabolite, constituent, extract or combination of any of the above) that were marketed in the United States prior to October 15, 1994 may be used in dietary supplements without notifying the FDA. "New" dietary ingredients (i.e., dietary ingredients that were "not marketed in the United States before October 15, 1994") must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been "present in the food supply as an article used for food" without being "chemically altered." A new dietary ingredient notification must provide the FDA evidence of a "history of use or other evidence of safety" establishing that use of the dietary ingredient "will reasonably be expected to be safe." A new dietary ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. The FDA may determine that a new dietary ingredient notification does not provide an adequate basis to conclude that a dietary ingredient is reasonably expected to be safe.
9
Such a determination could prevent the marketing of such dietary ingredient. In 2011 and 2016, the FDA issued draft guidances setting forth recommendations for complying with the new dietary ingredient notification requirement. Although FDA guidance is non-binding and does not establish legally enforceable responsibilities, and companies are free to use an alternative approach if the approach satisfies the requirements of applicable laws and regulations, FDA guidance is a strong indication of the FDA's "current thinking" on the topic discussed in the guidance, including its position on enforcement. At this time, it is difficult to determine whether the 2016 draft guidance (which replaced the 2011 draft guidance), if finalized, would have a material impact on our operations. However, if the FDA were to enforce the applicable statutes and regulations in accordance with the draft guidance as written, such enforcement could require us to incur additional expenses, which could be significant, and negatively impact our business in several ways, including, but not limited to, enjoining the manufacturing of our products until the FDA determines that we are in compliance and can resume manufacturing, increasing our liability and reducing our growth prospects.
The FDA or other agencies could take actions against products or product ingredients that, in their determination, present an unreasonable health risk to consumers that would make it illegal for us to sell such products. In addition, the FDA could issue consumer warnings with respect to the products or ingredients in such products that are sold in our stores. Such actions or warnings could be based on information received through FDC Act-mandated reporting of serious adverse events.
We take a number of actions to ensure the products we sell comply with the FDC Act. Some of these actions include maintaining and continuously updating a list of restricted ingredients that will be prohibited from inclusion in any products that are sold in our stores or on our websites. Vendors selling product to us for the sale of such products by us will be required to warrant to us that the products sold to us do not contain any of these restricted ingredients. In addition, we have developed and maintain a list of ingredients that we believe comply with the applicable provisions of the FDC Act. As is common in our industry, we rely on our third-party vendors to ensure that the products they manufacture and sell to us comply with all applicable regulatory and legislative requirements. In general, we seek representations and warranties, indemnification and/or insurance from our vendors. However, even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our products. In addition, the failure of such products to comply with applicable regulatory and legislative requirements could prevent us from marketing the products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial condition and results of operations. A removal or recall could also result in negative publicity and damage to our reputation that could reduce future demand for our products. In the past, we have attempted to offset any losses related to recalls and removals with reformulated or alternative products; however, there can be no assurance that we would be able to offset all or any portion of losses related to any future removal or recall.
The FDC Act permits structure/function claims to be included in labels and labeling for dietary supplements without FDA pre-market approval. However, companies must have substantiation that the claims are “truthful and not misleading”, and must submit a notification with the text of the claims to the FDA no later than 30 days after marketing the dietary supplement with the claims. Permissible structure/function claims may describe how a particular nutrient or dietary ingredient affects the structure, function or general well-being of the body, or characterize the documented mechanism of action by which a nutrient or dietary ingredient acts to maintain such structure or function. The label or labeling of a product marketed as a dietary supplement may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat or prevent a disease (i.e. a disease claim). If the FDA determines that a particular structure/function claim is an unacceptable disease claim that causes the product to be regulated as a drug, a conventional food claim or an unauthorized version of a "health claim," or, if the FDA determines that a particular claim is not adequately supported by existing scientific data or is false or misleading in any particular, we would be prevented from using the claim and would have to update our product labels and labeling accordingly.
In addition, DSHEA provides that so-called "third-party literature," e.g., “a publication, including an article, a chapter in a book, or an official abstract of a peer-reviewed scientific publication that appears in an article and was prepared by the author or the editors of the publication” supplements, when reprinted in its entirety, may be used "in connection with the sale of a dietary supplement to consumers" without the literature being subject to regulation as labeling. Such literature: (1) must not be false or misleading; (2) may not "promote" a particular manufacturer or brand of dietary supplement; (3) must present a balanced view or is displayed or presented with other such items on the same subject matter so as to present a balanced view of the available scientific information; (4) if displayed in an establishment, must be physically separate from the dietary supplements; and (5) should not have appended to it any information by sticker or any other method. If the literature fails to satisfy each of these requirements, we may be prevented from disseminating such literature with our products, and any continued dissemination could subject our product to regulatory action as an illegal drug.
In June 2007, pursuant to the authority granted by the FDC Act as amended by DSHEA, the FDA published detailed current Good Manufacturing Practice ("cGMP") regulations that govern the manufacturing, packaging, labeling and holding operations of dietary supplement manufacturers. The cGMP regulations, among other things, impose significant recordkeeping requirements on manufacturers. The cGMP requirements are in effect for all dietary supplement manufacturers, and the FDA
10
conducts inspections of dietary supplement manufacturers pursuant to these requirements. There remains considerable uncertainty with respect to the FDA's interpretation of the regulations and their actual implementation in manufacturing facilities.
In addition, the FDA's interpretation of the regulations governing dietary supplements will likely change over time as the agency becomes more familiar with the industry and the regulations. The failure of a manufacturing facility to comply with the cGMP regulations renders products manufactured in such facility "adulterated," and subjects such products and the manufacturer to a variety of potential FDA enforcement actions. In addition, under the Food Safety Modernization Act ("FSMA"), which was enacted in January 2011, the manufacturing of dietary ingredients contained in dietary supplements will be subject to similar or even more burdensome manufacturing requirements, which will likely increase the costs of dietary ingredients and will subject suppliers of such ingredients to more rigorous inspections and enforcement. The FSMA will also require importers of food, including dietary supplements and dietary ingredients, to conduct verification activities to ensure that the food they might import meets applicable domestic requirements.
The FDA has broad authority to enforce the provisions of federal law applicable to dietary supplements, including powers to issue a public warning or notice of violation letter to a company, publicize information about illegal products, detain products intended for import, require the reporting of serious adverse events, require a recall of illegal or unsafe products from the market, and request the Department of Justice to initiate a seizure action, an injunction action or a criminal prosecution in the United States courts.
The FSMA expands the reach and regulatory powers of the FDA with respect to the production and importation of food, including dietary supplements. The expanded reach and regulatory powers include the FDA's ability to order mandatory recalls, administratively detain domestic products, and require certification of compliance with domestic requirements for imported foods associated with safety issues. FMSA also gave FDA the authority to administratively revoke manufacturing facility registrations, effectively enjoining manufacturing of dietary ingredients and dietary supplements without judicial process. The regulation of dietary supplements may increase or become more restrictive in the future.
Cosmetics
We recently began to market cosmetics, which is defined to include articles to be rubbed, introduced into or otherwise applied to the body, containing CBD. FDA’s regulatory approach for products containing cannabis and cannabis-derived compounds like CBD continues to evolve. FDA has taken the position that CBD cannot be marketed as dietary supplements, and recently determined that CBD is not generally recognized as safe (GRAS) for use in human or animal food. However, its position on use of CBD in cosmetics is more nebulous. The FDA has noted that cannabis and cannabis-derived ingredients are not prohibited or restricted by regulation from use in cosmetics, and reiterated its historic position that ingredients not specifically addressed by regulation must comply with all applicable requirements, and further noted that if a product claims or is intended to affect the structure of function of the body, or to diagnose, cure, mitigate, treat or prevent disease, it will be deemed a drug, subject to FDA’s drug approval requirements, even if it also affects the appearance of the user. At the state level, the rules regarding marketing of CBD-containing products varies from state to state. We have identified the states in which it is permissible to sell cosmetic products containing CBD, and our sales of CBD cosmetics is limited to these states. However, as with FDA, state laws regarding cannabis and cannabis-derived products is still evolving, and it is possible that states that currently allow marketing of products containing CBD may change their position in the future.
Federal Trade Commission
The FTC exercises jurisdiction over the advertising of dietary supplements and cosmetics and requires that all advertising to consumers be truthful and non-misleading. The FTC actively monitors the dietary supplement space and has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims.
We continue to be subject to a consent decree issued by the FTC in 1994 that limits our ability to make certain claims with respect to our hair care products. With respect to products containing CBD, FTC has stated that it is illegal to advertise that a product can prevent, treat, or cure human diseases without competent and reliable scientific evidence to support such claims.
The FTC continues to monitor our advertising and, from time to time, requests substantiation with respect to such advertising to assess compliance with the outstanding consent decree and with the Federal Trade Commission Act. Our policy is to use advertising that complies with the consent decree and applicable regulations. Nevertheless, there can be no assurance that inadvertent failures to comply with the consent decree and applicable regulations will not occur.
Some of the products sold by franchise stores are purchased by franchisees directly from other vendors and these products do not flow through our distribution centers. Although franchise contracts contain strict requirements for store operations, including
11
compliance with federal, state and local laws and regulations, we cannot exercise the same degree of control over franchisees as we do over our company-owned stores. Failure by us or our franchisees to comply with the consent decree and applicable regulations could result in substantial monetary penalties, which could have a material adverse effect on our financial condition or results of operations.
As a result of our efforts to comply with applicable statutes and regulations, we have from time to time reformulated, eliminated or relabeled certain of our products and revised certain provisions of our sales and marketing program.
Foreign
Our products sold in foreign countries are also subject to regulation under various national, local and international laws that include provisions governing, among other things, the formulation, manufacturing, packaging, labeling, advertising and distribution of dietary supplements and over-the-counter drugs. Government regulations in foreign countries may prevent or delay the introduction, or require the reformulation, of certain of our products.
New Legislation or Regulation
Legislation may be introduced which, if passed, would impose substantial new regulatory requirements on dietary supplements. We cannot determine what effect additional domestic or international governmental legislation, regulations, or administrative orders, when and if promulgated, would have on our business in the future. New legislation or regulations may require the reformulation of certain products to meet new standards, require the recall or discontinuance of certain products not capable of reformulation, impose additional record keeping or require expanded documentation of the properties of certain products, expanded or different labeling or scientific substantiation.
Franchise Regulation
We must comply with regulations adopted by the FTC and with the laws of several states that regulate the offer and sale of franchises. The FTC's Trade Regulation Rule on Franchising (the "FTC Franchise Rule") and certain state laws require that we furnish prospective franchisees with a franchise offering circular containing information prescribed by the FTC Franchise Rule and applicable state laws and regulations.
We also must comply with a number of state laws that regulate some substantive aspects of the franchisor-franchisee relationship. These laws may limit a franchisor's business practices in a number of ways, including limiting the ability to:
•terminate or not renew a franchise without good cause;
•interfere with the right of free association among franchisees;
•disapprove the transfer of a franchise;
•discriminate among franchisees with regard to franchise terms and charges, royalties and other fees;
•place new stores near existing franchises; and
•limit franchisees from hiring the employees of other franchisees or the employees who work in our company-owned stores.
To date, these laws have not precluded us from seeking franchisees in any given area and have not had a material adverse effect on our operations. Bills concerning the regulation of certain aspects of franchise relationships have been introduced into Congress on several occasions during the last decade, but none have been enacted. Revisions to the FTC Franchise Rule have also been proposed by the FTC and currently are in the comment stage of the rulemaking process.
Our international franchise agreements and franchise operations are regulated by various foreign laws, rules and regulations. These laws may limit a franchisor's business practices in a number of ways. To date, these laws have not precluded us from seeking franchisees in any given area and have not had a material adverse effect on our operations.
Environmental Compliance
As part of soil and groundwater remediation conducted at the Greenville, South Carolina manufacturing facility pursuant to an investigation conducted in partnership with the South Carolina Department of Health and Environmental Control (the "DHEC"), we previously completed additional investigations with the DHEC's approval, including the installation and operation of a pilot vapor extraction system under a portion of the facility in the second half of 2016, which was an immaterial cost to the Company. After an initial monitoring period, in October of 2017 the DHEC approved a work plan for extended monitoring of such
12
system and the contamination into 2021. As discussed elsewhere in this Annual Report on Form 10-K, in March 2019, we entered into a joint venture arrangement regarding the Company's manufacturing business, wherein we assigned all of our interests in the Greenville, South Carolina manufacturing facility to the joint venture. The joint venture will continue to consult with the DHEC on the next steps in the work after their review of the results of the extended monitoring is complete. At this stage of the investigation, however, it is not possible to estimate the timing and extent of any additional remedial action that may be required, the ultimate cost of remediation, or the amount of our potential liability. Therefore, no liability has been recorded in the Company's Consolidated Financial Statements.
In addition to the foregoing, we are subject to numerous federal, state, local and foreign environmental and health and safety laws and regulations governing our operations, including the handling, transportation and disposal of our non-hazardous and hazardous substances and wastes, as well as emissions and discharges from its operations into the environment, including discharges to air, surface water and groundwater. New laws, changes in existing laws or the interpretation thereof, or the development of new facts or changes in their processes could cause us, directly or indirectly through a joint venture entity, to incur additional capital and operating expenditures to maintain compliance with environmental laws and regulations and environmental permits. We are also subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties, or for properties to which substances or wastes that were sent in connection with current or former operations.
From time to time, we historically have incurred costs and obligations for correcting environmental and health and safety noncompliance matters and for remediation at or relating to certain of our properties or properties at which our waste has been disposed. However, compliance with the provisions of national, state and local environmental laws and regulations has not had a material effect upon our capital expenditures, earnings, financial position, liquidity or competitive position. We believe we have complied with, and are currently complying with, our environmental obligations pursuant to environmental and health and safety laws and regulations and that any liabilities for noncompliance will not have a material adverse effect on our business, financial performance or cash flows. However, it is difficult to predict future liabilities and obligations, which could be material.
13
Item 1A. RISK FACTORS.
An investment in our securities is subject to various risks, including risks and uncertainties inherent in our business. In addition to the other information contained in this Annual Report on Form 10-K, the following risk factors could cause our operational and/or financial performance to differ significantly from the goals, plans, objectives, intentions and expectations expressed in this Annual Report. If any of the following risks and uncertainties actually occur, our business, financial condition, results of operations or cash flows could be materially and adversely affected.
Risks Relating to Our Business and Industry
Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19”
Our business could be materially and adversely affected by the outbreak of a widespread epidemic or pandemic or other public health crisis, including arising from the novel strain of the coronavirus known as “COVID-19”, particularly when such health epidemic or pandemic has an adverse effect on the countries in which we or our suppliers operate or on the shopping habits of the people in the countries in which we operate. The occurrence of such an outbreak or other adverse public health developments could affect us in various ways, including disrupting our operations, supply chains and distribution systems and increasing our expenses, including as a result of impacts associated with preventive and precautionary measures that we, governments and other businesses may take. Such events could also significantly impact our industry and cause us and our franchisees to close some or all of our stores, which would severely disrupt our or our franchisees' operations and have a material adverse effect on our business, financial condition and results of operations.
In late 2019, a novel strain of coronavirus was first detected in Wuhan, China. Since then, the virus has spread to over 100 countries. During March 2020, many state governments ordered all but certain essential businesses closed and imposed significant limitations on the circulation of the populace. In the context of malls and other similar buildings, we have temporarily closed approximately 25% of our U.S. and Canada company-owned and franchise stores as of March 23, 2020. During this temporary closure, we will continue to serve our customers through our e-commerce sites. We will work with government and health officials to assess when we will reopen our stores.
In addition, many people limited their visits to stores in the months of February and March 2020 due to concerns about the coronavirus, which we believe negatively impacted footfall in most if not all of our corporate and our franchisees’ stores. We are unable to accurately predict the impact that the coronavirus will have on our results of operations, due to uncertainties including the ultimate geographic spread of the virus, the severity of the disease, the duration of the outbreak, the duration of the closure of our stores, the ultimate medium- and long-term impact of the outbreak on the global economy and any other actions that may be taken by governments to contain the coronavirus or to treat its impact. However, while it is premature to accurately predict the ultimate impact of these developments, we expect our results to be significantly impacted with potential continuing, adverse impacts beyond March 31, 2020. As a precautionary measure, given the current macro environment, we recently drew $30 million under our Revolving Credit Facility resulting in over $130 million in cash as of March 24, 2020.
Furthermore, certain illnesses may be transmitted through human or surface contact, and the risk of contracting such illnesses could cause employees and customers to avoid gathering in public places, as was the case in many places during February and March 2020 due to concerns about the coronavirus. This could not only adversely affect store traffic, but also our ability to adequately staff and supply our and our franchisees’ stores. We could be adversely affected if governments in the countries in which we or our suppliers operate impose mandatory closures, seeks voluntary closures, imposes restrictions on operations of stores or restricts the import or export of products, or if suppliers are unable to provide us with timely delivery or issue mass recalls of products. Even if such measures are not implemented and a communicable illness does not spread significantly, the perceived risk of infection or health risk may adversely affect our business, financial condition and results of operations.
We operate in a highly competitive industry, and our failure to compete effectively could adversely affect our market share, revenues and growth prospects.
The market for health, wellness and performance products is large, highly fragmented and intensely competitive. Current and prospective participants include specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, online merchants, mail-order companies and a variety of other smaller participants. We believe that the market is also highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. In the United States, we compete for sales with heavily advertised national brands manufactured by large pharmaceutical and food companies, as well as other brands, some of which have greater market presence, both brick and mortar and online, name recognition and financial, marketing and other resources, including some competitors that may spend more aggressively on advertising and
14
promotional activities than we do. In addition, as some products become more mainstream and achieve broader distribution, we may experience increased price competition and adverse impacts to category share and growth for those products as more participants enter the market or we otherwise fail to retain market share. Further, if we fail to build out our e-commerce platform or fail to provide our customers with a desired omni-channel experience, we may lose business to online retailers with a more robust and engaging e-commerce platform.
Our international competitors include large international pharmacy chains, major international supermarket chains and other large U.S.-based companies with international operations. Our wholesale and manufacturing partnerships compete with other wholesalers and manufacturers of third-party nutritional supplements. We may not be able to compete effectively and our attempts to do so may require us to reduce our prices, which may result in lower margins. Failure to effectively compete could adversely affect our market share, revenues and growth prospects.
Further, the ability of consumers to compare prices on a real-time basis through the use of smartphones and digital technology puts additional pressure on us to maintain competitive pricing. We compete in multiple product categories and sales channels, including traditional large store and specialty store formats, mass merchants, and catalog; and increasingly internet-based and direct-sell retailers and vendors. Many factors affect the extent to which competition could affect our results, including as it relates to pricing, quality, assortment, marketing, promotions and advertising, service, locations, capital expenditures, category share and reputation, and prolonged competitive pressures, any of which could have a material effect on our results of operations.
We continue to explore new strategic initiatives but we may not be able to successfully execute on, or realize the expected benefit from the implementation of, our strategic initiatives, and our pursuit of new strategic initiatives may pose significant costs and risks.
The continued success of our business is contingent on, among other things, the acquisition of new customers and the retention of existing customers. This success depends on our adoption of strategic alternatives, including those focused on improving the customer omni-channel experience, increasing customer engagement and personalization, providing a relevant and inspiring product assortment and improving customer loyalty and retention. Our future operating results are dependent, in part, on our management’s success in implementing strategic initiatives. Also, our short-term operating results could be unfavorably impacted by the opportunity and financial costs associated with the implementation of strategic plans, and we may not realize the expected benefits from such strategies. In addition, we may not be successful in achieving the intended objectives of strategic initiatives in a timely manner or at all.
Resources devoted to product innovation may not yield new products that achieve commercial success.
Our ability to develop new and innovative GNC-branded products, or identify and acquire new and innovative products from third-party vendors, depends on, among other factors, our ability to understand evolving customer and market trends and our ability to translate these insights into identifying, and then manufacturing or otherwise obtaining, commercially successful new products. If we are unable to do so, our customer relationships and product sales could be harmed significantly. Furthermore, the nutritional supplements industry is characterized by rapid and frequent changes in demand for products and new product introductions. Our failure to accurately predict these trends could negatively impact consumer opinion of our stores as a source for the latest products. This could harm our customer relationships and cause losses to our market share. The development of new and innovative products also requires significant investment in research and development and testing of new ingredients, formulas and possibly new production processes. The research and development process can be expensive and prolonged and entails considerable uncertainty. Products may appear promising in development but fail to reach market within the expected time frame, or at all. We may face significant challenges with regard to a key product launch. Further, products also may fail to achieve commercial viability due to pricing competitiveness with other retailers, including online retailers, failure to timely bring the product to market, failure to differentiate the product with our competitors and other reasons. Finally, there is no guarantee that our development teams will be able to successfully respond to competitive products that could render some of our offerings obsolete. Development of a new product, from discovery through testing to the store shelf, typically takes between four to seven months, but may require an even longer timeline if clinical trials are involved. Each of these time periods can vary considerably from product to product and therefore the costs and risks of producing a commercially viable product can increase significantly as time passes.
We have substantial indebtedness due within the next twelve months. If we are unable to refinance the indebtedness, we may not be able to continue as a going concern.
We have substantial indebtedness at December 31, 2019, including $154.7 million of outstanding indebtedness
under the Notes, maturing on August 15, 2020, and $441.5 million of outstanding indebtedness under the Tranche B-2 Term Loan. This loan becomes due on the earlier to occur of (i) the maturity date of March 4, 2021 or (ii) the acceleration of such debt in the event repayment of all but $50 million of our outstanding convertible notes before May 2020. Prior to the outbreak of the COVID-19
under the Notes, maturing on August 15, 2020, and $441.5 million of outstanding indebtedness under the Tranche B-2 Term Loan. This loan becomes due on the earlier to occur of (i) the maturity date of March 4, 2021 or (ii) the acceleration of such debt in the event repayment of all but $50 million of our outstanding convertible notes before May 2020. Prior to the outbreak of the COVID-19
15
pandemic in the United States, we believed that we had the ability to reduce the outstanding balance on the convertible notes below $50 million with projected cash on hand and new borrowings under the revolving credit facility, assuming such borrowing remain available subject to the covenant and reporting requirements. Given current circumstances around the COVID-19 pandemic as discussed further in Item 8, "Financial Statement and Supplementary Data,"Note 21, "Subsequent Events", there can be no assurances as our ability to do so. As a precautionary measure, given the current macro environment, we recently drew $30 million under our Revolving Credit Facility resulting in over $130 million in cash as of March 24, 2020. See “Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19.”
Our ability to continue as a going concern is substantially dependent upon our ability to refinance or restructure this debt. Since the Company has not refinanced the Tranche B-2 Term Loan and it will mature less than twelve months after the issuance date of these consolidated financial statements, we have concluded there is substantial doubt regarding the Company's ability to continue as a going concern within one year from the issuance date of the Company’s consolidated financial statements. We make no assurance regarding the likelihood, certainty or exact timing of any refinancing, restructuring or other strategic option with respect to the same. If we are unable to refinance the debt, we may need to consider additional actions including the following:
• | Raising additional capital through short-term loans |
• | Implementing additional restructuring and cost reductions |
• | Raising additional capital through a private placement or other transactions |
• | Disposing of assets |
• | Selling or licensing intellectual property |
Failure to obtain a waiver, complete a refinancing or other restructuring of our outstanding indebtedness or to reach an agreement with the Company's stakeholders on the terms of a restructuring would have a material adverse effect on the liquidity, financial condition and results of operations and may result in filing a voluntary petition for relief under Chapter 11 of the United States Bankruptcy Code in order to implement a restructuring plan.
We are currently are exploring several refinancing options in Asia and the United States. We had been in discussions with certain lenders in Asia with respect to refinancing options. We became aware on March 24, 2020, by those lenders, that they are no longer pursuing a refinancing with us. We will continue to explore all options to refinance and restructure our indebtedness. While we continue to work through a number of refinancing alternatives to address our upcoming debt maturities, we cannot make any assurances regarding the likelihood, certainty or exact timing of any alternatives.
Should our going concern assumption not be appropriate or should we become unable to continue in the normal course of operations, adjustments would be required to the amounts and classifications of assets and liabilities within our consolidated financial statements, and these adjustments could be significant. Our consolidated financial statements do not reflect the adjustments or reclassifications of assets and liabilities that would be necessary if we were to become unable to continue as a going concern.
Natural disasters (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks, terrorist acts and global political events could cause permanent or temporary distribution center or store closures, impair our ability to purchase, receive or replenish inventory or cause customer traffic to decline, all of which could result in lost sales and otherwise adversely affect our financial performance.
The occurrence of one or more natural disasters, such as hurricanes, fires, floods and earthquakes (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks (including the recent outbreak of the coronavirus, or COVID-19), terrorist acts or disruptive global political events, such as civil unrest in countries in which our suppliers are located, or similar disruptions could adversely affect our operations and financial performance. To the extent these events result in the closure of one or more of our distribution centers, a significant number of stores, a manufacturing facility or our corporate headquarters, or impact one or more of our key suppliers, our operations and financial performance could be materially adversely affected through an inability to make deliveries to our stores and through lost sales. In addition, these events could result in increases in fuel (or other energy) prices or a fuel shortage, delays in opening new stores, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products from some local and overseas suppliers, the temporary disruption in the transport of goods from overseas, delay in the delivery of goods to our distribution centers or stores, the temporary reduction in the availability of products in our stores, expiration of inventory, future long-lived asset impairment charges and disruption to our information systems. These events also could have indirect consequences, such as increases in the cost of insurance, if they were to result in significant loss of property or other insurable damage.
As of March 23, 2020, we have temporarily closed approximately 25% of our U.S. and Canada company-owned and franchise stores as a result of the COVID-19 pandemic. The full extent and duration of such temporary closures and their impacts
16
over the longer term remain uncertain and dependent on future developments that cannot be accurately predicted at this time, such as the severity and transmission rate of COVID-19 and the extent and effectiveness of containment actions taken.
Our current debt profile and obligations under our debt instruments could adversely affect our results of operations and financial condition and otherwise adversely impact our operating income and growth prospects.
As of December 31, 2019, our total consolidated long-term debt (including current portion) was $862.6 million, including $159.1 million principal amount of 1.5% convertible senior notes due 2020 that the Company issued in a private offering in August 2015 (the "Notes") (net of $4.4 million related to the conversion feature and discount), $448.5 million on our Tranche B-2 Term Loan due in 2021, and $275 million on our asset-based Term Loan due in 2022. Provided that all outstanding amounts under the convertible senior notes exceeding $50.0 million have not been repaid, refinanced, converted or effectively discharged prior May 2020, the maturity date of the Tranche B-2 becomes May 2020, subject to certain adjustments. For additional detail regarding our indebtedness and each of these facilities, see Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations - Liquidity" and Item 8, "Financial Statement and Supplementary Data," Note 8, "Long-Term Debt / Interest Expense."
This level of debt and their respective payment obligations could materially adversely affect our financial condition. We may be unable to generate sufficient cash flow from operations or other sources, or to obtain future borrowings under our credit facilities or otherwise in an amount sufficient to enable us to pay our debt or to fund our other liquidity needs. If we do not have sufficient liquidity, we may need to refinance or restructure all or a portion of our debt on or before maturity, sell assets or borrow more money, which we may not be able to do on terms satisfactory to us or at all. Further, we may be required to use all or a large portion of our cash flow from operations to pay principal and interest on our debt, thereby reducing the availability of our cash flow to fund working capital, capital expenditures and other business activities.
In addition, the agreements governing our existing indebtedness contain, and the agreements governing our future indebtedness will likely contain, customary restrictions on us or our subsidiaries, including covenants that restrict us or our subsidiaries, as the case may be, from:
• | incurring additional indebtedness and issuing preferred stock; |
• | granting liens on our assets; |
• | making investments; |
• | consolidating or merging with, or acquiring, another business; |
• | selling or otherwise disposing of our assets; |
• | paying dividends and making other distributions to our stockholders; |
• | entering into transactions with our affiliates; and |
• | incurring capital expenditures in excess of limitations set within the agreement. |
Our asset-based Revolving Credit Facility requires that, for so long as availability under the Revolving Credit Facility is below a certain level, we meet a Fixed Charge Coverage Ratio of (a) consolidated earnings before interest, taxes, depreciation and amortization, or EBITDA, less certain capital expenditures and taxes, to (b) the sum of cash interest expense, scheduled amortization and certain dividends and distributions. If we fail to satisfy such ratio, then we will be restricted from drawing available borrowings under the asset-based Revolving Credit Facility and any amount outstanding may become due and payable subject to defined rights to cure, which may impair our liquidity.
Our ability to comply with these covenants and other provisions of our Senior Credit Facility may be affected by changes in our operating and financial performance, changes in general business and economic conditions, adverse regulatory developments or other events beyond our control. The breach of any of these covenants could result in a default under our debt obligations, which could cause those and other obligations to become due and payable subject to defined rights to cure. A default on any of our debt obligations could trigger certain acceleration clauses and cause those and our other obligations to become due and payable subject to defined rights to cure. Upon an acceleration of any of our debt, we may not be able to make payments under our other outstanding debt.
In addition, the size of our current indebtedness, and the restrictions imposed under our current debt documents may increase our vulnerability as a potential acquisition target, as well as to general adverse economic and industry conditions, limit our flexibility in planning for and reacting to changes in our business and industry, and restrict us from making strategic acquisitions or capitalizing on business opportunities and generally place us at a competitive disadvantage compared to our competitors.
17
We operate a portion of our business with joint venture partners, which may restrict our operational and corporate flexibility; actions taken by the other partner may materially impact our financial position and results of operations; and we may not realize the benefits we expect to realize from a joint venture.
We are currently a partner in joint venture arrangements, as described in Item 8, "Financial Statements and Supplementary Data," Note 9, "Equity Method Investments." These relationships, which involve our e-commerce and retail business in China and our Nutra manufacturing business, require us to share or cede operational control with respect to a critical portion of our market or product supply source, such that we may no longer have the flexibility to control completely the long-term manufacturing strategy. If we do not timely meet our commitments in such circumstances, our rights may be adversely affected. If our joint venture partners are unable or fail to uphold their obligations or do not operate in accordance with our expectations, our costs of operations could be increased, our revenue could decrease, and our reputation and brand could be adversely impacted. We could also incur liability as a result of actions taken by our joint venture partners. Disputes between us and our joint venture partners may result in litigation or arbitration that would increase our expenses, delay or terminate product development and distract our officers and directors from focusing their time and effort on our business.
Difficulties with our vendors may adversely impact our business.
Our performance depends in material part on our ability to purchase products at sufficient levels and at competitive prices from vendors who can deliver said products in a timely and efficient manner, and in compliance with our vendor standards and all applicable laws and regulations. We currently have a large number of vendor relationships. Generally, we do not enter into committed, long-term purchase agreements with third-party vendors or provide other contractual assurances of continued supply, pricing or access to new products, and historically we have not relied on any single vendor for a larger percentage of our products and have not had difficulties replacing vendors for various products we sell. However, there is no assurance that we will continue to be able to acquire desired products in sufficient quantities or on terms acceptable to us, or be able to develop relationships with new vendors to replace any discontinued vendors. Our inability to acquire suitable products in the future or our failure to replace any one or more vendors may have a material adverse effect on our business, results of operations and financial condition. In addition, any significant change in the payment terms that we have with our suppliers could adversely affect our liquidity. Given recent circumstances and uncertainties around the duration of the COVID-19 pandemic, we cannot assure you that our vendors will continue to maintain our current payment terms. See “Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19.”
Many of our suppliers are small firms that produce a limited number of items. These smaller vendors generally have limited resources, production capacities and operating histories, and some of our vendors have limited the distribution of their products in the past. Accordingly, these smaller vendors may be more susceptible to cash flow issues, downturns in economic conditions, production difficulties, force majeure events and quality control issues than larger vendors. If a vendor fails to deliver on its commitments for any reason, we could experience product out-of-stocks that could lead to lost sales. In addition, although we generally have charge-back privileges in our vendor agreements, there is no assurance that we would be able, if necessary, to return product to these vendors, obtain refunds of our purchase price or obtain reimbursement or indemnification from any of our vendors should we so desire, or obtain the same in a timely manner. Many of these suppliers include extensive advance notice requirements in order to supply products in the quantities we need. This long lead time requires us to place orders far in advance of the time when certain products will be offered for sale, exposing us to shifts in consumer demand and discretionary spending.
Other supplier problems that we could face include product shortages due to ingredient or raw material shortages, excess supply, risks related to the terms of our contracts with suppliers, risks associated with contingent workers, supplier financial weaknesses, inability of suppliers to borrow funds in the credit markets, disputes with suppliers and risks related to our relationships with single source suppliers, as described above. Given the importance of third-party suppliers to our business, if any of these risks materializes, our ability to obtain raw materials and products and our results of operations may suffer.
We must successfully maintain and/or upgrade our information technology systems, including electronic payments systems, and our failure to do so, or other problems with these systems could have a material adverse effect on our business, financial condition or results of operations.
We rely on various third-party information technology systems to manage our operations and the core system needs of our business. These systems, if not functioning properly, could disrupt our operations, including our ability to track, record and analyze the merchandise that we sell, process shipments of goods, process financial information or credit card transactions, deliver products or engage in similar normal business activities. We continue to modify and undertake upgrades to such systems, including changes to legacy systems, replacing legacy systems with successor systems with new functionality, and acquiring new systems with new functionality. These types of activities subject us to inherent costs and risks associated with replacing and changing these systems, including impairment of our ability to fulfill customer orders, potential disruption of our internal control structure,
18
substantial capital expenditures, additional administration and operating expenses, retention of sufficiently skilled personnel to implement and operate the new systems, demands on management time and other risks and costs of delays or difficulties in transitioning to or integrating new systems into our current systems. Further, our information systems, including our back-up systems, are subject to damage or interruption from power outages, computer and telecommunications failures, computer viruses, worms, sabotage, ransomware other malicious computer programs, denial-of-service attacks, security breaches (through cyber-attacks from cyber-attackers or sophisticated organizations), catastrophic events such as fires, tornadoes, earthquakes and hurricanes, acts of war or terrorism and usage errors by our associates. Any of these events could lead to system interruptions, including the nonavailability or nonfunctionality of our website, processing and order fulfillment delays and loss of critical data for us, our suppliers or our internet service providers, and could prevent us from processing customer purchases. Because we are dependent on third-party service providers for the implementation and maintenance of certain aspects of our systems and operations, which may be outside of our control, we may not be able to remedy such interruptions in a timely manner, if at all. Accordingly, any computer, internet, network or system disruptions could have a material adverse effect on our business, financial condition or results of operations.
If we do not successfully develop and maintain a relevant omni-channel experience for our customers, our business and results of operations could be materially and adversely affected.
Omni-channel retailing is rapidly evolving, and we must keep pace with changing customer expectations and new developments by our competitors. Our customers are increasingly shopping for products online instead of in traditional brick-and-mortar shopping centers and retail locations. As part of our omni-channel strategy, we anticipate the need to continue making investments in technology. If we are unable to make, improve, or develop relevant customer-facing technology in a timely manner, our ability to compete and our business and results of operations could be materially and adversely affected. In addition, if our e-commerce businesses or our other customer-facing technology systems do not function as designed or we are unable to effectively blend our digital, online and in-store platforms, we may experience a loss of customer confidence, lost sales, or data security breaches, any of which could materially and adversely affect our business and results of operations.
Privacy protection is increasingly demanding, and we may be exposed to risks and costs associated with security breaches, data loss, credit card fraud and identity theft that could cause us to incur unexpected expenses and loss of revenue, suffer reputational harm with our customers, as well as other risks.
The protection of customer, employee, vendor, franchisee and other business data is critical to us. We and our franchisees receive confidential customer data, including payment card and personally identifiable information, in the normal course of customer transactions. In order for our sales channel to function, we and other parties involved in processing customer transactions must be able to transmit confidential information, including credit card information, securely over public networks. While we have taken significant steps to protect customer and confidential information, the intentional or negligent actions of employees, business associates or third parties may undermine our security measures and result in unauthorized parties obtaining access to our data systems and misappropriating confidential data. There can be no assurance that advances in computer capabilities, new discoveries in the field of cryptography or other developments will prevent a compromise of our customer transaction processing capabilities and personal data. Because the techniques used to obtain unauthorized access to, disable, degrade, or sabotage systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures. Any compromise of our data security could result in a violation of applicable privacy and other laws or standards, significant legal and financial exposure beyond the scope or limits of our insurance coverage, interruption of our operations, increased operating costs associated with remediation, equipment acquisitions or disposal, added personnel, and a loss of confidence in our security measures, which could harm our business or investor confidence. Any security breach involving the misappropriation, loss or other unauthorized disclosure of sensitive or confidential information could attract a substantial amount of media attention, damage our reputation, expose us to risk of litigation and material liability, disrupt our operations and harm our business.
Federal, state, provincial and international laws and regulations govern the collection, retention, sharing and security of data that we receive from and about our employees, customers, vendors and franchisees. The regulatory environment surrounding information security and privacy has been increasingly demanding in recent years, including General Data Protection Regulation (GDPR) in the European Union, and the recent implementation of the California Consumer Privacy Act, and may see the imposition of new and additional requirements by states and the federal government as well as foreign jurisdictions in which we do business. Compliance with these laws and regulations result in cost increases related to the development of new processes to meet these requirements by us and our franchisees, which may negatively impact our overall financial performance.
Economic, political and other risks associated with our international operations could adversely affect our revenues and international growth prospects.
19
As of December 31, 2019, we had 175 company-owned Canadian stores, 11 company-owned The Health Store stores located in Ireland, and 1,904 international franchise locations in approximately 50 international countries (including distribution centers where retail sales are made).
We intend to expand our international presence as part of our business strategy, including through the expansion of franchise locations. Our international operations are subject to a number of risks inherent to operating in foreign countries, and any expansion of our international operations will amplify the effects of these risks, which include, among others:
•political and economic instability of foreign markets;
•foreign governments' restrictive trade policies or the impact of trade tensions amongst nations;
•inconsistent product regulation or sudden policy changes by foreign agencies or governments;
•the imposition of, or increase in, duties, taxes, government royalties or non-tariff trade barriers;
•difficulty in collecting international accounts receivable and potentially longer payment cycles;
•difficulty of enforcing contractual obligations of foreign franchisees;
•increased costs in maintaining international franchise and marketing efforts;
•problems entering international markets with different cultural bases and consumer preferences;
•compliance with foreign regulatory requirements such as the GDPR, and domestic laws and regulations applicable to international operations, such as the Foreign Corrupt Practices Act and regulations promulgated by the Office of Foreign Asset Control;
•fluctuations in foreign currency exchange rates; and
•operating in new, developing or other markets in which there are significant uncertainties regarding the interpretation, application and enforceability of laws and regulations relating to contract and intellectual property rights.
Any of these risks could have a material adverse effect on our international operations and our growth strategy.
Additionally, if the opportunity arises, we may expand our operations into new and high-growth international markets. However, there is no assurance that we will expand our operations in such markets in our desired time frame. To expand our operations into new international markets, we may enter into business combination transactions, make acquisitions or enter into strategic partnerships, joint ventures or alliances, any of which may be material. We may enter into these transactions to acquire other businesses or products to expand our products or take advantage of new developments and potential changes in the industry. Our lack of experience operating in new international markets and our lack of familiarity with local economic, political and regulatory systems could prevent us from achieving the results that we expect on our anticipated time frame or at all. If we are unsuccessful in expanding into new or high-growth international markets, it could adversely affect our operating results and financial condition.
Additionally, our business is increasingly exposed to operational risks in China. These include, among others, changes in economic conditions (including consumer spending, unemployment levels and wage and commodity inflation), consumer preferences, the regulatory environment, and tax laws and regulations, as well as increased media scrutiny, fluctuations in foreign exchange rates, increased restrictions or tariffs on imported supplies as a result of trade disputes and increased competition. Any significant or prolonged deterioration in U.S.-China relations could adversely affect our operations in China if Chinese consumers reduce the frequency of their purchases of our products. Chinese law regulates our business conducted within China. In addition, if we are unable to enforce our intellectual property or contract rights in China, it could result in an interruption in the operation of our brands, which could negatively impact our financial results. If our business is harmed or development of our Chinese operations is slowed in China due to any of these factors, it could negatively impact our overall financial results or our growth prospects. For example, we are assessing the potential impact of the coronavirus outbreak that originated in China. The outbreak could substantially interfere with general commercial activity related to, among other things, our supply chain, logistics providers and customer base. Due to the recent outbreak, there has been a substantial curtailment of travel and business activities. China has also limited the shipment of products in and out of its borders, which could negatively impact our ability to ship products to customers in that region. If not resolved quickly, the impact of the outbreak could have a material adverse effect on our operations. See “Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19.”
20
Our brick-and-mortar retail operations are dependent on securing suitable store locations. Our operations require us to maintain significant lease obligations, which may require us to continue paying rent for store locations that we no longer operate.
Our sales are impacted, in part, by our store locations, especially in the United States. Our business is contingent on consumer traffic being driven to our store locations, which may be adversely affected by, among other factors, economic downturns, the closing or continued decline of anchor department stores and/or specialty stores in malls or other developments where our stores are located, and a general decline in the popularity of traditional retail shopping among our target customers. Further, any act of violence, natural disaster, public health or safety concern that decreases the level of shopping traffic generally, or that affects our ability to open and operate stores in such locations, could have a material adverse effect on our business. To take advantage of customer traffic and the shopping preferences of our customers, we need to maintain or acquire stores in desirable locations such as in regional and neighborhood malls, as well as high-traffic urban retail areas and streets. We cannot be certain that desirable locations will continue to be available at favorable rates. Some traditional enclosed malls are experiencing significantly lower levels of customer traffic, driven by economic conditions, the rise in popularity of e-commerce and online shopping, as well as the closure of certain mall anchor tenants.
Substantially all of our retail stores are leased, including stores that we lease and sublease to franchisees in the United States. We are subject to various risks associated with our current and future real estate leases. Our costs could increase because of changes in the real estate markets and supply or demand for real estate sites. We generally cannot cancel our leases, so if we decide to close or relocate a location (or a franchisee fails), we may nonetheless be committed to perform our obligations under the applicable lease including paying the base rent for the remaining lease term. As each lease expires, we may fail to negotiate renewals, either on commercially acceptable terms or any terms at all and may not be able to find replacement locations that will provide for the same success as current store locations.
Failure to effectively anticipate consumer preferences, unfavorable publicity or consumer perception of our products, the ingredients they contain and any similar products distributed by other companies could cause fluctuations in our operating results and could have a material adverse effect on our reputation, the demand for our products and our ability to generate revenues and the market price of our common stock.
We are highly dependent upon consumer perception of the safety and quality of our products and the ingredients they contain, as well as that of similar products distributed by other companies. Consumer perception of products and the ingredients they contain can be significantly influenced by scientific research or findings, national media attention and other publicity, including that generated via social media, about product use. A future research report or publicity related to our products and the ingredients they contain that is perceived by our consumers as less favorable or that questions earlier research or publicity could have a material adverse effect on our ability to generate revenues. As such, period-to-period comparisons of our results may not be a reliable indicator of our future performance. Adverse publicity in the form of published scientific research or otherwise, whether or not accurate, that associates consumption of our products or the ingredients they contain or any other similar products distributed by other companies with illness or other adverse effects, that questions the benefits of our or similar products, or that claims that such products are ineffective could have a material adverse effect on our reputation, the demand for our products, our ability to generate revenues and the market price of our common stock.
Our success also depends in part on our ability to anticipate and respond in a timely manner to changing consumer demand, consumer preferences, and shopping patterns regarding nutritional supplements. Consumer preferences cannot be predicted with certainty and are subject to continual change and evolution. Additionally, our customers may also have expectations about how they shop in stores or through e-Commerce or more generally engage with businesses across different channels or media (through online and other digital or mobile channels or particular forms of social media), which may vary across demographics and may evolve rapidly. We often make commitments to purchase products from our vendors several months in advance of the proposed delivery which may make it more difficult for us to adapt to rapid changes in consumer preferences.
Our sales may decline significantly if we misjudge the market for our new products, which may result in significant inventory markdowns and lower margins, missed opportunities for other products, or inventory write-downs, and could have a negative impact on our reputation and profitability.
Because we rely on the Nutra manufacturing joint venture to produce a significant amount of the products we sell, disruptions in our manufacturing system or losses of manufacturing certifications could adversely affect our sales and customer relationships.
21
Our Nutra manufacturing joint venture, which was wholly-owned by us until March 2019, produced approximately 28% of the products we sold in each of the years ended December 31, 2019 and 2018. Other than powders, chewables and liquids, nearly all of our proprietary products are produced at the Nutra manufacturing facility located in Greenville, South Carolina. Any significant disruption in the operations at Nutra’s Greenville, South Carolina facility for any reason, including as a result of regulatory requirements, an FDA determination that the facility is not in compliance with the cGMP regulations, the loss of certifications, power interruptions, fires, hurricanes, war or other force of nature, could disrupt our supply of products, adversely affecting our sales and customer relationships.
A significant disruption to our distribution network, inventory management system, or to the timely receipt of inventory could adversely impact sales and operations or increase our transportation costs, which would decrease our profits.
We rely on our ability to replenish depleted inventory in our stores through deliveries to our distribution centers from vendors and then from the distribution centers or direct ship vendors to our stores by various means of transportation, including shipments by sea and truck. Unexpected delays in those deliveries or increases in transportation costs (including through increased fuel costs) could significantly decrease our ability to make sales and earn profits. In addition, labor shortages in the transportation industry or long-term disruptions to the national and international transportation infrastructure that lead to delays or interruptions of deliveries could negatively affect our business. Further, we may not be able to maintain our existing distribution centers if the cost of the facilities increase or the location of a facility is no longer desirable. In those cases, we may not be able to locate suitable alternative sites or modify or enter into new leases on acceptable terms, which would force us to rely more heavily on our store network, third-party operated fulfillment centers and vendors to help meet our fulfillment needs. An inability to optimize our distribution and fulfillment network, including the expiration of a lease or an unexpected lease termination at one of our facilities (without timely replacement of the applicable facility) or serious disruptions (including natural disasters) at any of these facilities might impair our ability to adequately deliver products to our stores, franchisees and customers, process returns of products to vendors, increase costs associated with shipping and delivery, damage a material portion of our inventory, or otherwise negatively affect our operations, sales, profitability, and reputation.
We must maintain sufficient inventory levels to operate our business successfully. However, we also must guard against accumulating excess inventory. If we fail to anticipate accurately either the market for the merchandise in our stores or our customers’ purchasing habits, we may be forced to rely on markdowns or promotional sales to dispose of excess or slow moving inventory, which could have a material adverse effect on our business, financial condition, and results of operations.
A substantial amount of our revenue is generated from our franchisees, and our revenues could decrease significantly if our franchisees do not conduct their operations profitably or if we fail to attract new franchisees.
Our franchise operations generated approximately 20% and 18%, respectively, of our consolidated revenues in the years ended December 31, 2019 and 2018. Our revenues from franchise stores depend on the franchisees' ability to operate their stores profitably and adhere to our franchise standards. The closing of franchise stores or the failure of franchisees to comply with our policies could adversely affect our reputation and could reduce the amount of our franchise revenues. These factors could have a material adverse effect on our revenues and operating income.
If we are unable to attract new franchisees or to convince existing franchisees to open additional stores, any growth in royalties from franchise stores will depend solely upon increases in revenues at existing franchise stores. In addition, our ability to open additional franchise locations is limited by the territorial restrictions in our existing franchise agreements as well as our ability to identify additional markets in the United States and other countries. If we are unable to open additional franchise locations, we will have to sustain additional growth internally by attracting new and repeat customers to our existing locations. See “Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19.”
We or our vendors may incur material product liability claims, or experience product recalls, which could increase our costs and adversely affect our sales and margin, reputation, revenues and operating income.
As a retailer, distributor and historical manufacturer of products designed for human consumption, we are subject to product liability claims if the use of our products is alleged to have resulted in injury. The products that we sell consist of vitamins, minerals, herbs and other ingredients that are classified as foods or dietary supplements and are not subject to pre-market regulatory approval in the United States. The products that we sell could contain contaminated substances, and some of the products we sell contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur.
In addition, third-party manufacturers produce many of the products we sell. We rely on these manufacturers to ensure the integrity of their ingredients and formulations. As a distributor of products manufactured by third parties, we may also be liable for various product liability claims for products we do not manufacture. Our ultimate liability for these products that are
22
manufactured by third parties depends on a number of factors, including our contractual relationship with the vendor, the creditworthiness of the vendor and any insurance that we have. Therefore, we may be unable to adequately protect ourselves against claims with respect to products manufactured by a third-party.
We have been and may be subject to various product liability claims, including, among others, that our products include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. See Item 3, "Legal Proceedings." Even with adequate insurance and indemnification, product liability claims could significantly damage our reputation and consumer confidence in our products, regardless of the merits or outcomes of such claims. Our litigation expenses could increase as well, which also could have a material adverse effect on our results of operations even if a product liability claim is unsuccessful or is not fully pursued.
In addition, we may be subject to product recalls, withdrawals or seizures if any of the products we sell are believed to cause injury or illness or if we are alleged to have violated governmental regulations in the manufacturing, labeling, promotion, sale or distribution of such products. A significant recall, withdrawal or seizure of any of the products we sell may require significant management attention, would likely result in substantial and unexpected costs and may materially and adversely affect our business, financial condition or results of operations. Furthermore, a recall, withdrawal or seizure of any of the products that we sell may adversely affect consumer confidence in our brands and thus decrease consumer demand for such products.
In the past, due to frequently changing consumer preferences in the dietary supplement space, we have offset losses related to recalls and removals with reformulated or alternative products; however, there can be no assurance that we would be able to offset all or any portion of losses related to any future removal or recall. As a result of the indeterminable level of product substitution and reformulated product sales, we cannot reliably determine the potential impact of any such recall or removal on our business, financial condition or results of operation.
An increase in the price and shortage of supply of key raw materials could adversely affect our business.
Our products are composed of certain key raw materials. If the prices of these raw materials were to increase significantly, our costs to manufacture the product could increase, the prices our contract manufacturers and third-party manufacturers charge us for our GNC-branded products and third-party products could increase significantly and we may not be able to pass on such increases to our customers. Additionally, in the event any of our, or our contract manufacturer’s, third-party suppliers or vendors become unable or unwilling to continue to provide raw materials in the required volumes and quality levels or in a timely manner, we, or our contract manufacturers, would be required to identify and obtain acceptable replacement supply sources. If we, or they, are unable to identify and obtain alternative supply sources in a timely manner or at all, our business could be adversely affected. A significant increase in the price of raw materials that cannot be passed on to customers could have a material adverse effect on our results of operations and financial condition. Events such as COVID-19, the threat of political or social unrest, or the perceived threat thereof, may also have a significant impact on raw material prices and transportation costs for our products. In addition, the interruption in supply of certain key raw materials essential to the manufacturing of our products may have an adverse impact on our suppliers' ability to provide us with the necessary products needed to maintain our customer relationships and an adequate level of sales. See “Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19.”
General trade tensions between the U.S. and China have been escalating since 2018, with multiple rounds of U.S. tariffs on Chinese goods taking effect, with some subsequently being de-escalated. Furthermore, China or other countries may institute retaliatory trade measures in response to existing or future tariffs imposed by the U.S. that could have a negative impact on our business. If any of these events continue as described, we may need to seek alternative suppliers or vendors, raise prices, or make changes to our operations, any of which could have a material adverse effect on our sales and profitability, results of operations and financial condition.
General economic conditions, including a prolonged macroeconomic downturn, may negatively affect consumer purchases, which could adversely affect our sales and the sales of our business partners, as well as our ability to access credit on terms previously obtained.
Our results, and those of our business partners to whom we sell, are dependent on a number of factors impacting consumer spending, including general economic and business conditions; consumer confidence; wages and employment levels; the housing market; consumer debt levels; availability of consumer credit; credit and interest rates; fuel and energy costs; energy shortages; taxes; general political conditions, both domestic and abroad; and the level of customer traffic within department stores, malls and other shopping and selling environments. Consumer product purchases, including purchases of our products, may decline during recessionary periods. Further, historically, credit markets and the financial services industry have experienced disruption characterized by the bankruptcy, failure, collapse or sale of various financial institutions, increased volatility in securities prices, diminished liquidity and credit availability and intervention from the United States and other governments.
23
Continued concerns about the systemic impact of potential long-term or widespread downturn, energy costs and climate concerns, political and geopolitical issues, the availability and cost of credit, the global commercial and residential real estate markets and related mortgage markets and reduced consumer confidence have contributed to increased market volatility. The cost and availability of credit has been and may continue to be adversely affected by these conditions. A prolonged downturn or an uncertain outlook in the economy may materially adversely affect our business, revenues and profits and the market price of our common stock, and we cannot be certain that funding for our capital needs will be available from our existing financial institutions and the credit markets if needed, and if available, to the extent required and on acceptable terms. If we cannot obtain funding when needed, in each case on acceptable terms, we may be unable to adequately fund our operating expenses and fund required capital expenditures, which may have an adverse effect on our revenues and results of operations.
Harbin may exercise significant influence over us, including through its ability to elect up to five members of our Board of Directors.
Based on the number of shares of our common stock outstanding as of December 31, 2019, the shares of Convertible Preferred Stock owned by Harbin plus accumulated and unpaid dividends on such shares represent approximately 41% of the voting rights of our common stock, on an as-converted basis, so Harbin will have the ability to significantly influence the outcome of any matter submitted for the vote of our stockholders. Pursuant to the Stockholders Agreement between the Company and Harbin, Harbin has the right to designate up to five directors (each an “Investor Designee”) to the Board, at least two of whom must be Independent Investor Designees. The number of Investor Designees may be adjusted from time to time, not to exceed five, in accordance with the terms of the Stockholders Agreement, to equal Harbin or its assigns’ proportionate ownership of the Company, rounded up to the nearest whole number of directors. When Harbin’s ownership of the Company falls below 15% of the outstanding common stock on as-converted basis, Harbin will no longer have the right to designate directors. Each of the Investor Designees is required to be reasonably satisfactory to the Company’s Nominating and Corporate Governance Committee. Harbin and its affiliates may have interests that diverge from, or even conflict with, those of our other stockholders. For example, Harbin and its affiliates may have an interest in directly or indirectly pursuing acquisitions, divestitures, financings or other transactions that, in their judgment, could enhance their other equity investments, even though such transactions might involve risks to us.
We depend on the services of key executives and other skilled professionals and our ability to attract, train and retain highly qualified associates. Any failure to attract or retain such individuals could affect our business strategy and adversely impact our performance and results of operations.
Our senior executives are instrumental in setting our strategic direction, operating our business, identifying, recruiting and training key personnel, identifying opportunities and arranging necessary financing. In addition, other key employees below the executive level, who are skilled professionals with deep knowledge of our business, are critical to the execution and success of our strategy. Our success also depends on the continued contributions of our store and field associates. We must attract, train and retain a large and growing number of qualified associates.
Losing the services of any of these groups of individuals could adversely affect our business and we may be unable to identify candidates of sufficient experience and capabilities in a timely fashion or at all, which could negatively impact our business and operations. Further, our ability to control labor and benefit costs is subject to numerous external factors, including regulatory changes, prevailing wage rates, and healthcare and other insurance costs. We compete with other retail and non-retail businesses for these store and field associates and invest significant resources in training and motivating them. There is no assurance that we will be able to attract or retain qualified store and field associates in the future, which could have a material adverse effect on our business, financial condition and results of operations.
We are not insured for a significant portion of our claims exposure, which could materially and adversely affect our operating income and profitability.
We have procured insurance independently for the following areas: (1) general liability; (2) product liability; (3) directors and officers liability; (4) network security and privacy liability; (5) property losses; (6) workers' compensation; (7) employment practice; and (8) various other areas. In addition, although we believe that we will continue to be able to obtain insurance in these areas in the future, because of increased selectivity by insurance providers, we may only be able to obtain such insurance at increased rates and/or with reduced coverage levels. Furthermore, we are self-insured for other areas, including: (1) physical damage to our vehicles for field personnel use; and (2) physical damages that may occur at company-owned stores. We are not insured for some property and casualty risks due to the frequency and severity of a loss, the cost of insurance and the overall risk analysis. In addition, we carry product liability insurance coverage that requires us to pay deductibles/retentions with primary and excess liability coverage above the retention amount. Because of our deductibles and self-insured retention amounts, we have significant exposure to fluctuations in the number and severity of claims. We currently maintain product liability insurance with
24
a retention of $4.0 million per claim with an aggregate cap on retained loss of $10.0 million. We could raise our deductibles/retentions, which would increase our already significant exposure to expense from claims. If any claim exceeds our coverage, we would bear the excess expense, in addition to our other self-insured amounts. If the frequency or severity of claims or our expenses increase, our operating income and profitability could be materially and adversely affected.
Our franchisees are independent operators and we have limited influence over their activities, including their implementation of strategic marketing and advertising programs.
Our revenues substantially depend upon our franchisees' sales volumes, profitability and financial viability. The support of our franchisees is critical for the success of our marketing programs and other strategic initiatives we seek to undertake, and the successful execution of these initiatives will depend on our ability to maintain alignment with our franchisees. However, our franchisees are independent operators and we cannot control many factors that impact the profitability of their stores. Pursuant to the franchise agreements, we can, among other things, mandate signage, equipment and hours of operation, establish operating procedures and approve suppliers, distributors and products, as well as certain strategic initiatives. However, the quality of franchise store operations may be diminished by any number of factors beyond our control, and we need the active support of our franchisees if the implementation of our strategic initiatives is to be successful. Consequently, franchisees may not successfully operate stores in a manner consistent with our standards and requirements or standards set by federal, state and local governmental laws and regulations. In addition, franchisees may not hire and train qualified managers and other personnel. Our efforts to build alignment with franchisees may result in a delay in the implementation of our marketing and advertising programs and other key initiatives. Although we believe that our current relationships with our franchisees are generally good, there can be no assurance that our franchisees will continue to support our marketing programs and strategic initiatives.
While we ultimately can take action to terminate franchisees that do not comply with the standards contained in our franchise agreements, any delay in identifying and addressing problems could harm our image and reputation, and our franchise revenues and results of operations could decline.
Additionally, we have limited influence over their ability to invest in other businesses or incur excessive indebtedness. In some cases, these franchisees have used the cash generated by their stores to expand their other businesses or to subsidize losses incurred by such businesses. Additionally, as independent operators, franchisees do not require our consent to incur indebtedness. Consequently, our franchisees have in the past, and may in the future, experience financial distress as a result of over leveraging. To the extent that our franchisees use the cash from their GNC stores to subsidize their other businesses or experience financial distress, due to over leverage or otherwise, it could negatively affect (1) our operating results as a result of delayed or reduced payments of royalties, advertising fund contributions and rents for properties we lease to them, (2) our future revenue, earnings and cash flow growth (3) our financial condition and (4) our reputation. In addition, lenders that are adversely affected by franchisees who default on their indebtedness may be less likely to provide current or prospective franchisees necessary financing on favorable terms or at all.
Our use of derivative instruments for hedging purposes may result in financial losses.
We may from time to time utilize derivative instruments to manage our exposure to fluctuations in fuel and certain other commodity prices, interest rates and foreign currency exchange rates. We could recognize losses on these contracts or fail to recognize the benefits intended by these contracts as a result of volatility in the market values of the underlying commodities or to the extent that a counterparty fails to perform. In the absence of actively-quoted market prices and pricing information from external sources, the valuation of these instruments involves judgment or use of estimates. Furthermore, changes in the value of derivatives designated under hedge accounting to the extent not fully offset by changes in the value of the hedged transaction can result in ineffectiveness losses that may have an adverse effect on our results of operations.
25
We have recognized impairment charges in the past and may recognize additional such charges in the future, which could adversely affect our results of operations and financial condition.
We evaluate goodwill and our indefinite-lived brand intangible asset for impairment on at least an annual basis. We evaluate property and equipment and definite-lived intangible assets for recoverability when indicators of impairment exist. We will recognize an impairment charge if: our indefinite-lived brand intangible asset has a carrying value that exceeds its estimated fair value; our goodwill has a carrying value for an applicable reporting unit that exceeds its fair value; or our property and equipment and definite-lived intangible assets have estimated future undiscounted cash flows that are less than the applicable carrying values. In assessing fair value, we rely primarily on a discounted cash flow analysis, as well as other generally accepted valuation methodologies. These analyses rely on the judgments and estimates of management, which involve inherent uncertainties. Impairment losses are significantly affected by estimates of future operating cash flows and estimates of fair value as well as the Company's total market capitalization. Estimates of future operating cash flows are identified from strategic long-term plans, which are based upon experience, knowledge, and expectations; however, these estimates can be affected by such factors as future operating results, future store profitability, future volumes, revenue and expense growth rates and asset disposal values and future economic conditions, all of which can be difficult to predict accurately. Any significant deterioration in macroeconomic conditions could affect the fair value of our long-lived assets and could result in future impairment charges, which would adversely affect our results of operations. For example, we recorded long-lived asset impairment charges of $38.2 million during the year ended December 31, 2018. While we currently believe that the fair values of our long-lived assets exceed their respective carrying values, changes in our estimates and assumptions regarding the future performance of our business could result in further impairment charges, which may have a material adverse effect on our results of operations and overall financial condition.
Our holding company structure makes us dependent on our subsidiaries for our cash flow and subordinates the rights of our stockholders to the rights of creditors of our subsidiaries in the event of an insolvency or liquidation of any of our subsidiaries.
Holdings is a holding company and, accordingly, substantially all of our operations are conducted through its subsidiaries. Holdings' subsidiaries are separate and distinct legal entities. As a result, Holdings' cash flow depends upon the earnings of its subsidiaries. In addition, Holdings depends on the distribution of earnings, loans or other payments by its subsidiaries. Holdings' subsidiaries have no obligation to provide it with funds for its payment obligations. If there is an insolvency, liquidation or other reorganization of any of Holdings' subsidiaries, Holdings' stockholders will have no right to proceed against their assets. Creditors of those subsidiaries will be entitled to payment in full from the sale or other disposal of the assets of those subsidiaries before Holdings, as a stockholder, would be entitled to receive any distribution from that sale or disposal.
The price of our common stock historically has been volatile.
The market price for our common stock has varied during the twelve-month period ended December 31, 2019 between a high of $3.34 on November 11, 2019 and a low of $1.35 on June 6, 2019. Our stock price is likely to continue to be volatile and subject to significant price and volume fluctuations in response to market and other factors, including those additional factors discussed under the heading “Risk Factors” in this Annual Report, as well as: variations in our quarterly operating results from our expectations or those of securities analysts or other investors; revisions in analyst estimates or announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; or the sale of substantial amounts of our common stock. In addition, a substantial decline in the price of our securities that persists for a significant period of time could cause our securities to be delisted from the New York Stock Exchange, further reducing market liquidity. If an active market for our securities does not continue, the liquidity of an investor's investment may be limited, and the price of our securities may decline. If an active market does not exist, investors may lose their entire investment. As a result of any of these factors, the market price of our securities at any given point in time may not accurately reflect our long-term value.
Our current and historical effective tax rate may not be indicative of future rates.
On December 22, 2017, United States tax reform legislation known as The Tax Cuts and Jobs Act of 2017 (“2017 Tax Act”) was enacted. The 2017 Tax Act made significant changes to the Internal Revenue Code, including a reduction in the corporate tax rate from 35% to 21%. This rate reduction, subject to certain new limitations on deductions such as the business interest expense deduction, is effective for tax years beginning after December 31, 2017. As a result, our current and future effective tax rate may differ from historical rates due to the 2017 Tax Act. Furthermore, in light of our global earnings mix, future changes in domestic and international tax laws in the various jurisdictions in which we operate as well as changes to our tax positions could also impact the effective tax rate on a prospective basis.
Risks Related to Our Capital Structure
26
The issuance of Series A Convertible Preferred Stock to Harbin Pharmaceutical Group Holdings Co., Ltd. pursuant to a Securities Purchase Agreement, as previously disclosed, reduces the relative voting power of holders of our common stock, may further dilute the ownership of such holders, and may adversely affect the market price of our common stock.
As previously disclosed, in 2018 and 2019 the Company issued and sold, in three tranches, 299,950 shares of a newly created series of convertible preferred stock of the Company, designated the “Series A Convertible Preferred Stock” (the “Convertible Preferred Stock”), for a purchase price of $1,000 per share, or an aggregate of approximately $300 million to Harbin (the “Securities Purchase”). The Convertible Preferred Stock is convertible into shares of our common stock at an initial conversion price of $5.35 per share, subject to customary anti-dilution adjustments.
Harbin currently owns all of the outstanding shares of Convertible Preferred Stock, and based on the number of shares of our common stock outstanding as of December 31, 2019, the shares of Convertible Preferred Stock owned by Harbin plus accumulated and unpaid dividends on such shares represents 41% of our common stock. Holders of the Convertible Preferred Stock are entitled to receive cumulative preferential dividends, payable quarterly in arrears, at an annual rate of 6.5% of the stated value of the Convertible Preferred Stock, and are entitled to vote together with the holders of the Company’s common stock as a single class, in each case, on an as-converted basis. Holders of the Convertible Preferred Stock also have certain limited special approval rights, including over amendments to the Company’s articles of incorporation, bylaws or other charter documents, including the Certificate of Designations in any manner that adversely affects any rights, preferences, privileges or voting powers of the Convertible Preferred Stock or holders of shares of Convertible Preferred Stock.
Conversion of the Convertible Preferred Stock to common stock would dilute the ownership interest of existing holders of our common stock, and any sales in the public market of the common stock issuable upon conversion of the Convertible Preferred Stock could adversely affect prevailing market prices of our common stock. We have granted Harbin registration rights in respect of the shares of common stock underlying the conversion of the Convertible Preferred Stock. These registration rights would facilitate the resale of these shares of our common stock into the public market, and any such resale would increase the number of shares of our common stock available for public trading. Sales by Harbin of a substantial number of shares of our common stock in the public market, or the perception that such sales might occur, could have a material adverse effect on the price of our common stock.
We are required to pay dividends on the Convertible Preferred Stock, which ranks senior to our common stock, and we may be required under certain circumstances to repurchase the outstanding shares of Convertible Preferred Stock; such obligations could adversely affect our liquidity and financial condition.
The holder of shares of Convertible Preferred Stock is entitled to receive cumulative preferential dividends, payable quarterly in arrears, at an annual rate of 6.5% of the stated value of $1,000 per share, subject to increase in connection with the payment of dividends in kind. Dividends are payable, at the Company’s option, in cash from legally available funds or in kind by issuing additional shares of Convertible Preferred Stock with such stated value equal to the amount of payment being made or by increasing the stated value of the outstanding Convertible Preferred Stock by the amount per share of the dividend or in a combination thereof. In addition, the holders of our Convertible Preferred Stock have certain redemption rights, including upon certain change in control events involving us, which, if exercised, could require us to repurchase all of the outstanding shares of Convertible Preferred Stock, prior to any distributions to holders of our common stock or other capital stock of the Company ranking junior to the Convertible Preferred Stock. Our obligations to pay dividends to the holders of our Convertible Preferred Stock or any required repurchase of the outstanding shares of Convertible Preferred Stock could impact our liquidity and reduce the amount of cash flows available for distribution to holders of our common stock, or for working capital, capital expenditures, growth opportunities, acquisitions, and other general corporate purposes. Our obligations to the holders of Convertible Preferred Stock could also limit our ability to obtain additional financing or increase our borrowing costs, which could have an adverse effect on our business and financial results.
The terms and features of our current Notes may have a negative impact on our liquidity, dilution or reported financial results.
In the event the conditional conversion feature of the Notes is triggered, holders of Notes will be entitled to convert the Notes at any time during specified periods at their option. Further, upon the occurrence of certain “fundamental changes”, holders of our Notes will have the right to require us to repurchase their notes at a repurchase price equal to 100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest, if any. Unless we elect to satisfy our conversion obligation by delivering solely shares of our common stock (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation, as well as our repurchase obligations, through the payment of cash, which could adversely affect our liquidity. We may not have enough available cash or be able to obtain financing at the time we are required to make repurchases of notes surrendered therefor or pay cash upon conversions of Notes being converted. In addition,
27
our ability to repurchase the Notes or to pay cash upon conversions of the Notes may be limited by law, by regulatory authority or by agreements governing our existing or future indebtedness. Our failure to repurchase the Notes at a time when the repurchase is required by the Indenture or to pay any cash payable on future conversions of the Notes as required by the Indenture would constitute a default under the Indenture. A default under the indenture or the fundamental change itself could also lead to a default under agreements governing our future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Notes or make cash payments upon conversions thereof.
In addition, the effect of Accounting Standards Codification ("ASC") 470-20, Debt with Conversion and Other Options on the accounting for the Notes is that the equity component is required to be included in the additional paid-in capital section of stockholders’ equity on our Consolidated Balance Sheet, and the value of the equity component would be treated as original issue discount for purposes of accounting for the debt component of the Notes. As a result, we are required to record a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discounted carrying value of the Notes to their face amount over the term of the Notes. We will report lower net income in our financial results because ASC 470-20 will require interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which could adversely affect our reported or future financial results, the trading price of our common stock and the trading price of the Notes.
Legal and Regulatory Risks
Compliance with new and existing laws and governmental regulations could increase our costs significantly and adversely affect our results of operations.
The processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to federal laws and regulation by one or more federal agencies, including the FDA, the FTC, the CPSC, the USDA, and the EPA. These activities are also regulated by various state, local and international laws and agencies of the states and localities in which our products are sold. Government regulations may prevent or delay the introduction, or require the reformulation, of our products, which could result in lost revenues and increased costs to us. For instance, the FDA regulates, among other things, the composition, safety, manufacture, labeling and marketing of dietary ingredients and dietary supplements (including vitamins, minerals, herbs, and other dietary ingredients for human use). Dietary supplements and dietary ingredients that do not comply with FDA’s regulations and/or the DSHEA will be deemed adulterated or misbranded. Manufacturers and distributors of dietary supplements and dietary ingredients are prohibited from marketing products that are adulterated or misbranded, and the FDA may take enforcement action against any adulterated or misbranded dietary supplement on the market. The FDA has broad enforcement powers. If we violate applicable regulatory requirements, the FDA may bring enforcement actions against us, which could have a material adverse effect on our business, prospects, financial condition, and results of operations. The FDA may not accept the evidence of safety for any new dietary ingredient that we may wish to market, may determine that a particular dietary supplement or ingredient presents an unacceptable health risk based on the required submission of serious adverse events or other information, and may determine that a particular claim or statement of nutritional value that we use to support the marketing of a dietary supplement is an impermissible drug claim, is not substantiated, or is an unauthorized version of a "health claim." See Item 1, "Business-Government Regulation-Product Regulation" for additional information. Any of these actions could prevent us from marketing particular dietary supplement products or making certain claims or statements with respect to those products. The FDA could also require us to remove a particular product from the market. Any future recall or removal would result in additional costs to us, including lost revenues from any products that we are required to remove from the market, any of which could be material. Any product recalls or removals could also lead to an increased risk of litigation and liability, substantial costs, and reduced growth prospects.
Additional or more stringent laws and regulations of dietary supplements and other products have been considered from time to time. These developments could require reformulation of some products to meet new standards, recalls or discontinuance of some products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of some products, additional or different labeling, additional scientific substantiation, or other new requirements. Any of these developments could increase our costs significantly. In addition, regulators' evolving interpretation of existing laws could have similar effects.
Further, our franchise activities are subject to federal, state and international laws regulating the offer and sale of franchises and the governance of our franchise relationships. These laws impose registration, extensive disclosure requirements and bonding requirements on the offer and sale of franchises. In some jurisdictions, the laws relating to the governance of our franchise relationship impose fair dealing standards during the term of the franchise relationship and limitations on our ability to terminate or refuse to renew a franchise. We may, therefore, be required to retain an underperforming franchise and may be unable to replace
28
the franchisee, which could adversely impact franchise revenues. In addition, we cannot predict the nature and effect of any future legislation or regulation on our franchise operations.
Additionally, due to our significant international operations we are subject to The U.S. Foreign Corrupt Practices Act (“FCPA”) and similar worldwide anti-corruption laws, including the U.K. Bribery Act of 2010, which is broader in scope than the FCPA, that generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Despite our training and compliance programs, we cannot be assured that our internal control policies and procedures will always protect us from reckless or criminal acts committed by our employees or agents. Our continued expansion outside the United States, including in developing countries, could increase the risk of FCPA violations in the future. Violations of these laws, or allegations of such violations, could result in a material adverse effect on our results of operations or financial condition.
Our failure to comply with FTC regulations and the consent decree imposed on us by the FTC could result in substantial monetary penalties and could adversely affect our operating results.
The FTC exercises jurisdiction over the advertising of dietary supplements and requires that all advertising to consumers be truthful and non-misleading. The FTC actively monitors the dietary supplement space and has instituted numerous enforcement actions against dietary supplement companies, including us, for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. As a result of these enforcement actions, we are currently subject to a consent decree that limits our ability to make certain claims with respect to our hair care products. See Item 1, "Business-Government Regulation-Product Regulation" for more information. Failure by us or our franchisees to comply with the consent decree and applicable regulations could result in substantial monetary penalties, which could have a material adverse effect on our financial condition or results of operations.
If we fail to protect our brand name, competitors may adopt trade names that dilute the value of our brand name, and prosecuting or defending infringement claims could cause us to incur significant expenses or prevent us from manufacturing, selling or using some aspect of our products, which could adversely affect our revenues and market share.
We have invested significant resources to promote our GNC brand name in order to obtain the public recognition that we have today. Because of the differences in foreign trademark laws concerning proprietary rights, our trademarks may not receive the same degree of protection in foreign countries as they do in the United States. Also, we may not always be able to successfully enforce our trademarks against competitors or against challenges by others. For example, we are currently engaged in trademark disputes in foreign jurisdictions over "GNC", "LIVE WELL" and other similar trademarks and trademark applications. Our failure to successfully protect our trademarks could diminish the value and effectiveness of our past and future marketing efforts and could cause customer confusion. This could in turn adversely affect our revenues, profitability and the market price of our common stock.
We are currently and may in the future be subject to intellectual property litigation and infringement claims, which could cause us to incur significant expenses or prevent us from manufacturing, selling or using some aspect of our products. Claims of intellectual property infringement also may require us to enter into costly royalty or license agreements. However, we may be unable to obtain royalty or license agreements on terms acceptable to us or at all. Claims that our technology or products infringe on intellectual property rights could be costly and would divert the attention of management and key personnel, which in turn could adversely affect our revenues and profitability.
Our operations are subject to environmental and health and safety laws and regulations that may increase our cost of operations or expose us to environmental liabilities.
We are subject, directly or indirectly through joint ventures, to numerous federal, state, local and foreign environmental and health and safety laws and regulations governing our operations, including the handling, transportation and disposal of our non-hazardous and hazardous substances and wastes, as well as emissions and discharges from our operations into the environment, including discharges to air, surface water and groundwater. Failure to comply with such laws and regulations could result in costs for remedial actions, penalties or the imposition of other liabilities. New laws, changes in existing laws or the interpretation thereof, or the development of new facts or changes in their processes could also cause us to incur additional capital and operating expenditures to maintain compliance with environmental laws and regulations and environmental permits. Any failure by us to comply with environmental, health and safety requirements could result in the limitation or suspension of our operations, including operations at our manufacturing facility. We also could incur monetary fines, civil or criminal sanctions, third-party claims or cleanup or other costs as a result of violations of or liabilities under such requirements.
We also are subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under
29
certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties, or for properties to which substances or wastes that were sent in connection with current or former operations at its facilities. The presence of contamination from such substances or wastes could also adversely affect our ability to sell or lease our properties, or to use them as collateral for financing.
Item 1B. UNRESOLVED STAFF COMMENTS.
None.
30
Item 2. PROPERTIES.
As of December 31, 2019, there were 7,532 GNC store locations globally (including distribution centers where retail sales are made). In our U.S. and Canada segment substantially all of our stores are located on leased premises that typically range in size from 1,000 to 2,000 square feet. Most all of our domestic franchisees are located on premises GNC leases and then subleases to our respective franchisees. All of our franchise locations in the international markets are owned or leased directly by our franchisees. No single store is material to our operations. The table below presents our consolidated stores by location in the U.S. and international countries as of December 31, 2019.
Location | Company-Owned | Domestic Franchise | International Franchise* | ||||||||
Alabama | 34 | 14 | Argentina | 3 | |||||||
Alaska | 14 | — | Bangladesh | 1 | |||||||
Arizona | 69 | 2 | Bahrain | 7 | |||||||
Arkansas | 21 | 4 | Bolivia | 28 | |||||||
California | 237 | 113 | Brunei | 3 | |||||||
Colorado | 62 | 3 | Bulgaria | 7 | |||||||
Connecticut | 40 | 1 | Cayman Islands | 2 | |||||||
Delaware | 16 | 3 | Chile | 177 | |||||||
District of Columbia | 6 | 1 | Costa Rica | 26 | |||||||
Florida | 231 | 96 | Dominican Republic | 1 | |||||||
Georgia | 100 | 34 | Ecuador | 1 | |||||||
Hawaii | 26 | — | El Salvador | 15 | |||||||
Idaho | 12 | 3 | Guam | 3 | |||||||
Illinois | 96 | 39 | Guatemala | 61 | |||||||
Indiana | 53 | 19 | Honduras | 7 | |||||||
Iowa | 29 | 2 | Hong Kong | 92 | |||||||
Kansas | 25 | 7 | India | 41 | |||||||
Kentucky | 34 | 7 | Indonesia | 58 | |||||||
Louisiana | 45 | 11 | Japan | 3 | |||||||
Maine | 8 | — | Latvia | 1 | |||||||
Maryland | 60 | 13 | Lebanon | 9 | |||||||
Massachusetts | 62 | 3 | Lithuania | 1 | |||||||
Michigan | 76 | 31 | Malaysia | 83 | |||||||
Minnesota | 45 | 17 | Mexico | 606 | |||||||
Mississippi | 24 | 16 | Mongolia | 6 | |||||||
Missouri | 45 | 7 | Myanmar | 1 | |||||||
Montana | 8 | 2 | Nigeria | 10 | |||||||
Nebraska | 7 | 11 | Oman | 7 | |||||||
Nevada | 30 | 6 | Pakistan | 7 | |||||||
New Hampshire | 18 | 3 | Panama | 16 | |||||||
New Jersey | 85 | 38 | Paraguay | 6 | |||||||
New Mexico | 20 | 2 | Peru | 34 | |||||||
New York | 180 | 34 | Philippines | 40 | |||||||
North Carolina | 111 | 21 | Qatar | 7 | |||||||
North Dakota | 9 | — | Romania | 8 | |||||||
Ohio | 103 | 37 | Saudi Arabia | 51 | |||||||
Oklahoma | 24 | 15 | Singapore | 64 | |||||||
Oregon | 30 | 3 | South Africa | 172 | |||||||
Pennsylvania | 125 | 33 | South Korea | 127 | |||||||
Rhode Island | 11 | — | Sri Lanka | 1 | |||||||
South Carolina | 43 | 19 | Taiwan | 51 | |||||||
South Dakota | 4 | 4 | Thailand | 31 | |||||||
Tennessee | 46 | 23 | Trinidad | 8 | |||||||
Texas | 126 | 213 | Turks & Caicos | 2 | |||||||
Utah | 29 | 6 | Turkey | 1 | |||||||
Vermont | 2 | — | UAE | 17 | |||||||
Virginia | 80 | 25 | Ukraine | 1 | |||||||
Washington | 57 | 8 | |||||||||
West Virginia | 19 | 6 | |||||||||
Wisconsin | 54 | 1 | |||||||||
Wyoming | 8 | — | |||||||||
Puerto Rico | 28 | — | |||||||||
U.S. Subtotal | 2,727 | 956 | |||||||||
Canada | 175 | — | |||||||||
Ireland | 11 | — | |||||||||
Total | 2,913 | 956 | Total | 1,904 | |||||||
* Includes distribution centers where retail sales are made. | |||||||||||
31
In our Manufacturing / Wholesale segment, there are 1,759 GNC franchise "store-within-a-store" locations under our strategic alliance with Rite Aid.
In addition to the above, we own and lease the following locations to support our store operations:
Location | Approximate Square Footage (in 000s) | Own or Lease | |||
Corporate Headquarters: | |||||
Pittsburgh, PA | 253 | Own | |||
Distribution Centers: | |||||
Anderson, SC | 146 | Lease | |||
Indianapolis, IN | 343 | Lease | |||
Leetsdale, PA | 217 | Lease | |||
Phoenix, AZ | 112 | Lease | |||
Other Locations / Offices: | |||||
Boston, MA | 2 | Own | |||
Tustin, CA | 4 | Lease | |||
Mississauga, Ontario | 5 | Lease | |||
Dublin, Ireland | <7 | Lease | |||
Greenville, SC | 6 | Lease | |||
Distribution centers are used by all of our segments. Retail stores are used by the U.S. and Canada and International segments depending upon location.
Item 3. LEGAL PROCEEDINGS.
We are engaged in various legal actions, claims and proceedings arising in the normal course of business, some of which are covered by insurance for which we have rights of indemnification. These actions, claims and proceedings are of the sort that are commonly encountered in the nutritional supplement retail industry, including claims related to breach of contracts, products liabilities, intellectual property matters and employment-related matters resulting from our business activities. Although the impact of the final resolution of these matters on the Company's financial condition, results of operations or cash flows is not known, management does not believe that the resolution of these lawsuits will have a material adverse effect on the financial condition, results of operations or liquidity of the Company.
DMAA/Aegeline Claims. As disclosed in prior Annual Reports on Form 10-K and Quarterly Reports on Forms 10-Q, prior to December 2013, we sold products manufactured by third parties that contained derivatives from geranium known as 1.3-dimethylpentylamine/ dimethylamylamine/ 13-dimethylamylamine, or "DMAA," which were recalled from our stores in November 2013, and/or Aegeline, a compound extracted from bael trees. As of December 31, 2019, individuals (on their own behalf or on behalf of minors or estates) have filed 27 personal injury lawsuits involving products containing DMAA and/or Aegeline, where we (or one of our wholly-owned subsidiaries) along with the third-party vendor, have been named as parties:
• | Case No. 140502403, filed May 20, 2014 in Common Pleas Court of Philadelphia County, Pennsylvania |
• | Case No. 15-1-0847-05, filed May 1, 2015, in the first Circuit Court, State of Hawaii |
• | Cases filed in the District Court for the District of Hawaii as follows: |
- Case No. 3-00639 DMK, filed November 21, 2013 | - Case No. CV 14-00029, filed January 23, 2014 |
- Case No. CV 14-00030, filed January 23, 2013 | - Case No. CV 14-00031, filed January 23, 2014 |
- Case No. CV 14-00032, filed January 23, 2014 | - Case No. CV14-00029, filed January 23, 2014 |
- Case No. 14-cv-00364 filed October 24, 2014 | - Case No. CV14-00365 filed October 24, 2014 |
- Case No. CV14-00366 filed August 15, 2014 | - Case No. 14-cv-00367 filed October 24, 2014 |
- Case No. CV-15-00228, filed June 17, 2016 | |
• | Cases filed in the Superior Court of California as follows: |
32
Orange County:
- Case No. 2014-00740258 filed August 18, 2014 | - Case No. 30-2015-00776749, filed March 12, 2015 |
- Case No. 30-2015-00783256-CU-PL-CXC, filed April 16, 2015 | |
San Diego County:
- Case No. 37-2015-00008404, filed March 13, 2015 | - Case No. 37-2014-110924, filed September 8, 2014 |
- Case No. 37-2013-00074052-CU-PL-CTL, filed November 1, 2013 | |
Los Angeles County:
- Case No. BC559542, filed October 6, 2014 | - Case No. BC575264, filed March 13, 2015 |
- Case No. BC575262, filed March 13, 2015 | - Case No. BC534065, filed January 23, 2014 |
Monterey County:
- Case No. M131321, filed March 13, 2015 | - Case No. M131322, filed March 13, 2015 |
Santa Clara County:
- Case No. 115CV78045, filed March 13, 2015 | - Case No. CV-14-0037, filed January 24, 2014 |
These matters are currently stayed pending final resolution.
We are contractually entitled to indemnification by our third-party vendor with regard to these matters, although our ability to obtain full recovery in respect of any such claims against us is dependent upon the creditworthiness of our vendor and/or its insurance coverage and the absence of any significant defenses available to its insurer.
Other Legal Proceedings. For additional information regarding certain legal proceedings to which we are a party, see Item 8, "Financial Statements and Supplementary Data," Note 13, "Commitments and Contingencies."
Item 4. MINE SAFETY DISCLOSURES
Item 4 is not applicable.
33
PART II
Item 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF SECURITIES. |
Market Information
Since March 31, 2011, our common stock has been traded on the NYSE under the symbol "GNC." As of March 20, 2020, there were 84,608,976 shares of common stock outstanding, the closing price of our common stock was $0.46 per share, and we had 12 stockholders of record (including 7 holders of restricted stock).
Dividends
In February 2017, the Board of Directors approved our recommendation to suspend the quarterly dividend. The dividend suspension is part of a broader plan to utilize a greater portion of our free cash to reduce debt.
Issuer Purchases of Equity Securities
We made no purchases of shares of Class A common stock for the quarter ended December 31, 2019.
In August 2015, the Board approved a $500.0 million multi-year repurchase program in addition to the $500.0 million multi-year program approved in August 2014, bringing the aggregate share repurchase program to $1.0 billion of Holdings' common stock. As of December 31, 2019, $197.8 million remains available for purchase under the program.
34
Stock Performance Graph
The graph below matches GNC Holdings, Inc.'s cumulative 5-Year total shareholder return on common stock with the cumulative total returns of the S&P 500 index and the S&P 500 Retail index. The graph tracks the performance of a $100 investment in our common stock and in each index (with the reinvestment of all dividends) from December 31, 2014 to December 31, 2019.
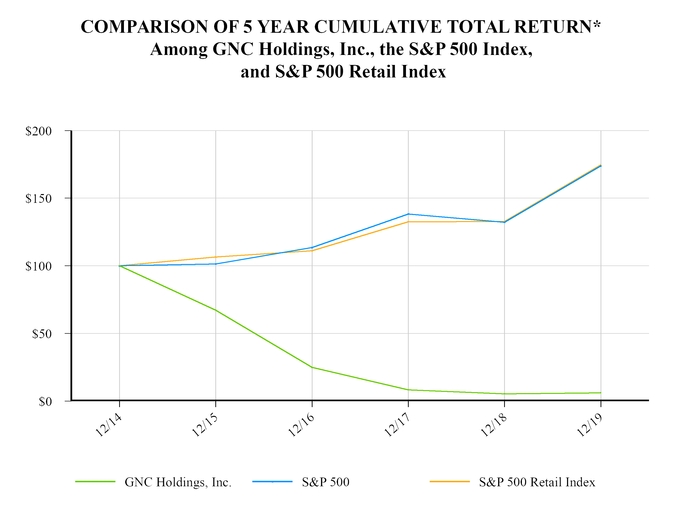
*$100 invested on December 31, 2014 in stock or index, including reinvestment of dividends.
Fiscal year ending December 31.
Copyright© 2020 Standard & Poor's, a division of S&P Global. All rights reserved
Fiscal year ending December 31.
Copyright© 2020 Standard & Poor's, a division of S&P Global. All rights reserved
35
Item 6. SELECTED FINANCIAL DATA.
The selected consolidated financial data presented below as of December 31, 2019 and 2018 and for the years ended December 31, 2019, 2018 and 2017 are derived from our audited Consolidated Financial Statements and Footnotes included in this Annual Report. The selected consolidated financial data presented below as of December 31, 2017, 2016 and 2015 and for the years ended December 31, 2016 and 2015 are derived from our Consolidated Financial Statements and Footnotes not included in this Annual Report.
You should read the following financial information together with the information under Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our audited Consolidated Financial Statements and related notes in Item 8, "Financial Statements and Supplementary Data."
As of and for the Year ended December 31, | |||||||||||||||||||
(in millions, except per share data) | 2019(2) | 2018 | 2017 (1) | 2016 (1) | 2015 | ||||||||||||||
Statement of Operations Data: (3) | |||||||||||||||||||
Revenue | $ | 2,068.2 | $ | 2,353.5 | $ | 2,481.0 | $ | 2,570.0 | $ | 2,683.3 | |||||||||
Cost of sales, including warehousing, distribution and occupancy | 1,353.8 | 1,581.8 | 1,656.5 | 1,683.4 | 1,698.7 | ||||||||||||||
Gross profit | 714.4 | 771.7 | 824.5 | 886.6 | 984.6 | ||||||||||||||
Selling, general and administrative | 566.5 | 620.9 | 624.3 | 597.0 | 567.3 | ||||||||||||||
Long-lived asset impairments | — | 38.2 | 457.8 | 476.6 | 28.3 | ||||||||||||||
Loss on net asset exchange for the formation of the joint ventures | 21.3 | — | — | — | — | ||||||||||||||
Other loss (income) net (4) | 1.9 | 0.3 | (0.8 | ) | (15.6 | ) | (4.1 | ) | |||||||||||
Operating income (loss) | 124.7 | 112.4 | (256.8 | ) | (171.3 | ) | 393.1 | ||||||||||||
Interest expense, net | 106.7 | 127.1 | 64.2 | 60.4 | 50.9 | ||||||||||||||
Gain on convertible debt and debt refinancing costs | (3.2 | ) | — | (11.0 | ) | — | — | ||||||||||||
Loss on debt refinancing | — | 16.7 | — | — | — | ||||||||||||||
Loss (gain) on forward contracts for the issuance of convertible preferred stock | 16.8 | (88.9 | ) | — | — | — | |||||||||||||
Income (loss) before income taxes | 4.5 | 57.5 | (310.0 | ) | (231.8 | ) | 342.2 | ||||||||||||
Income tax (benefit) expense | 44.9 | (12.3 | ) | (159.8 | ) | 53.5 | 122.9 | ||||||||||||
Net (loss) income before income from equity method investments | (40.4 | ) | 69.8 | (150.3 | ) | (285.2 | ) | 219.3 | |||||||||||
Income from equity method investments | 5.3 | — | — | — | — | ||||||||||||||
Net (loss) income | (35.1 | ) | 69.8 | (150.3 | ) | (285.2 | ) | 219.3 | |||||||||||
Weighted average shares outstanding: | |||||||||||||||||||
Basic | 83.7 | 83.4 | 68.8 | 69.4 | 83.9 | ||||||||||||||
Diluted | 83.7 | 86.2 | 68.8 | 69.4 | 84.2 | ||||||||||||||
(Loss) earnings per share: | |||||||||||||||||||
Basic | $ | (0.64 | ) | $ | 0.83 | $ | (2.18 | ) | $ | (4.11 | ) | $ | 2.61 | ||||||
Diluted | $ | (0.64 | ) | $ | 0.81 | $ | (2.18 | ) | $ | (4.11 | ) | $ | 2.60 | ||||||
Dividends declared per share | $ | — | $ | — | $ | — | $ | 0.80 | $ | 0.72 | |||||||||
Balance Sheet Data: | |||||||||||||||||||
Cash and cash equivalents | $ | 117.0 | $ | 67.2 | $ | 64.0 | $ | 34.5 | $ | 56.5 | |||||||||
Working capital (5)(6) | 79.0 | 376.5 | 475.9 | 475.4 | 504.3 | ||||||||||||||
Total assets (6) | 1,650.6 | 1,527.9 | 1,519.8 | 2,058.8 | 2,543.5 | ||||||||||||||
Total current and non-current long-term debt | 862.6 | 1,152.3 | 1,297.0 | 1,540.5 | 1,449.2 | ||||||||||||||
Mezzanine Equity | 211.4 | 98.8 | — | — | — | ||||||||||||||
Stockholders' (deficit) equity | (207.3 | ) | (114.3 | ) | (185.9 | ) | (117.6 | ) | 468.6 | ||||||||||
Statement of Cash Flows: | |||||||||||||||||||
36
Net cash provided by operating activities | $ | 96.5 | $ | 95.9 | $ | 220.5 | $ | 208.2 | $ | 354.5 | |||||||||
Net cash provided by (used in) investing activities | 73.4 | (16.5 | ) | (23.8 | ) | (22.4 | ) | (45.6 | ) | ||||||||||
Net cash used in financing activities | (119.3 | ) | (75.8 | ) | (168.1 | ) | (207.5 | ) | (384.5 | ) | |||||||||
Capital expenditures | 15.1 | 19.0 | 32.1 | 59.6 | 45.8 | ||||||||||||||
(1) | 2017 and 2016 Statement of Operations and Balance Sheet data have been updated to reflect the impact of the adoption of ASC 606. More information about the update can be found in Item 8, "Financial Statements and Supplementary Data," Note 2, "Basis of Presentation and Summary of Significant Accounting Policies" of the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2018. |
(2) | 2019 Balance Sheet data included the impact of the adoption of ASC 842. Refer to Item 8, "Financial Statements and Supplementary Data," Note 2, "Basis of Presentation and Summary of Significant Accounting Policies" for more information. |
(3) | Figures may not sum due to rounding. |
(4) | In 2019, other loss primarily related to $3.1 million loss on the termination of the corporate plane lease, partially offset by $0.6 million foreign currency gains and $0.6 million gains on refranchising. |
In 2018, other loss primarily related to $0.8 million of foreign currency loss, partially offset by $0.5 million gains on refranchising.
In 2017, other income primarily related to $1.2 million of foreign currency gains, $1.0 million related to insurance and lease settlements and $0.3 million gains on refranchising, partially offset by a $1.7 million loss attributed to the sale of substantially all of the assets of the Lucky Vitamin e-commerce business.
In 2016, other income loss primarily related to $16.0 million gains on refranchising, partially offset by $0.4 million of foreign currency losses.
In 2015, other income primarily related to $7.6 million gains on refranchising, partially offset by a $2.7 million loss on sale of Discount Supplements, which used to be a business unit within "International" segment, and $0.8 million of foreign currency losses.
(5) | Defined as current assets less current liabilities. |
(6) | Included the impact of the adoption of ASU 2015-17 in the first quarter of fiscal 2017 relating to the presentation of deferred tax assets and liabilities as non-current on the balance sheet. The Company reclassified current deferred income tax assets formerly presented within total current assets as a reduction to deferred income taxes presented within total long-term liabilities on the Consolidated Balance Sheet for 2016 and 2015 of $12.8 million and $10.9 million, respectively. |
37
The following table summarizes our stores for the periods indicated:
Year Ended December 31, | ||||||||||||||
2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||
U.S. & Canada | ||||||||||||||
Company-owned (a): | ||||||||||||||
Beginning of period balance | 3,206 | 3,423 | 3,513 | 3,584 | 3,487 | |||||||||
Store openings | 25 | 24 | 59 | 69 | 115 | |||||||||
Acquired franchise stores (b) | 34 | 25 | 60 | 21 | 44 | |||||||||
Franchise conversions (c) | (7 | ) | (9 | ) | (2 | ) | (102 | ) | (33 | ) | ||||
Store closings | (356 | ) | (257 | ) | (207 | ) | (59 | ) | (29 | ) | ||||
End of period balance | 2,902 | 3,206 | 3,423 | 3,513 | 3,584 | |||||||||
Domestic Franchise: | ||||||||||||||
Beginning of period balance | 1,037 | 1,099 | 1,178 | 1,084 | 1,070 | |||||||||
Store openings | 6 | 12 | 29 | 33 | 32 | |||||||||
Acquired franchise stores (b) | (34 | ) | (25 | ) | (60 | ) | (21 | ) | (44 | ) | ||||
Franchise conversions (c) | 7 | 9 | 2 | 102 | 33 | |||||||||
Store closings | (60 | ) | (58 | ) | (50 | ) | (20 | ) | (7 | ) | ||||
End of period balance | 956 | 1,037 | 1,099 | 1,178 | 1,084 | |||||||||
International (d) | ||||||||||||||
Beginning of period balance | 1,957 | 2,015 | 1,973 | 2,095 | 2,150 | |||||||||
Store openings (e) | 78 | 61 | 243 | 108 | 144 | |||||||||
Store closings (f) | (115 | ) | (119 | ) | (201 | ) | (230 | ) | (199 | ) | ||||
China locations contributed to the China joint venture | (5 | ) | — | — | — | — | ||||||||
End of period balance | 1,915 | 1,957 | 2,015 | 1,973 | 2,095 | |||||||||
Store-within-a-store (Rite Aid): | ||||||||||||||
Beginning of period balance | 2,183 | 2,418 | 2,358 | 2,327 | 2,269 | |||||||||
Store openings | 74 | 62 | 70 | 41 | 59 | |||||||||
Store closings (g) | (498 | ) | (297 | ) | (10 | ) | (10 | ) | (1 | ) | ||||
End of period balance | 1,759 | 2,183 | 2,418 | 2,358 | 2,327 | |||||||||
Total Stores | 7,532 | 8,383 | 8,955 | 9,022 | 9,090 | |||||||||
_______________________________________________________________________________
(a) | Includes Canada. |
(b) | Stores that were acquired from franchisees and subsequently converted into company-owned stores. |
(c) | Company-owned store locations sold to franchisees. |
(d) | Includes franchise locations in approximately 50 countries (including distribution centers where sales are made) and company-owned stores located in Ireland (branded as The Health Store). Prior year also includes company-owned locations in China. |
(e) | In 2017, store openings included 145 store-within-a-store locations in South Africa not formerly included in the store count. Effective at the end of the third quarter of 2017, these stores were subject to royalties on retail sales and as a result, have been included in the store count. |
(f) | In 2017, store closings included 68 store-within-a-store locations in Peru which did not contribute significantly to revenue. |
(g) | In 2019 and 2018, store closings primarily related to Walgreens acquisition of certain Rite Aid locations. |
38
Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
You should read the following discussion in conjunction with Item 6, "Selected Financial Data" and our audited Consolidated Financial Statements and the related notes included in Item 8, "Financial Statements and Supplementary Data." The discussion in this section contains forward-looking statements that involve risks and uncertainties. See Part I, Item 1A, "Risk Factors" in this Annual Report for a discussion of important factors that could cause actual results to differ materially from those described or implied by the forward-looking statements contained herein.
Overview
We are a global health and wellness brand with a diversified, omni-channel business. Our assortment of performance and nutritional supplements, vitamins, herbs and greens, health and beauty, food and drink and other general merchandise features innovative private-label products as well as nationally recognized third-party brands, some of which are exclusive to GNC. We derive our revenues principally from: product sales through our company-owned stores; on-line primarily through our website, GNC.com as well as third-party websites; domestic and international franchise activities; and prior to the formation of the Manufacturing JV, sales of products manufactured in our facility to third parties. We sell products through a worldwide network of approximately 7,500 locations operating under the GNC brand name.
In February 2019, we completed the formation of a commercial joint venture in Hong Kong with respect to our e-commerce business in China (the "HK JV") with Harbin Pharmaceutical Group Co., Ltd. (“Harbin”). The Hong Kong-based China e-commerce joint venture includes the operations of the existing profitable, growing cross border China e-commerce business. We anticipate completing the formation of the second, retail-focused joint venture located in China in the second quarter of 2020 following the completion of certain routine regulatory and legal requirements.
In March 2019, we completed the formation of a strategic joint venture with International Vitamin Corporation ("IVC"). The joint venture enables GNC quality and R&D teams to continue to support product development and increase focus on product innovation, while IVC will manage manufacturing and integrate with GNC's supply chain thereby driving more efficient usage of capital.
We believe the competitive strengths that position us as a leader in the specialty nutritional supplement space include our: well-recognized brand; stable base of long-term customers; geographically diverse store base; proprietary product and innovation capabilities; and differentiated service model designed to enhance the customer experience.
Our Current Strategy
Key elements of our strategy and areas of internal focus for the Company are as follows:
• | Leading brand of nutritional supplements. GNC has been in business for more than 80 years and the Company is built on a core foundation as a brand builder of high-quality nutritional supplements. Based on our worldwide network of approximately 7,500 locations and our online channels, we are a leading global brand of health, wellness and performance products. |
Our objective is to offer a broad and deep mix of products for consumers interested in living well, whether they are looking to treat a health-related issue, maintain their overall wellness, or improve their performance. Our premium, value-added offerings include both proprietary GNC-branded products and other nationally recognized third-party brands.
We believe our depth of brands, selection of exclusive products and overall range of merchandise, combined with the customer support and service we offer, differentiate us and allow us to effectively compete against food, drug and mass channel players, specialty stores, independent vitamin, supplement and natural food shops and online retailers.
• | Product development and innovation. We develop high-quality, innovative nutritional supplement products that can be purchased only through our store locations, online at GNC.com, our Amazon.com and other marketplaces or through our selected wholesale partners. Our high quality ingredients are rigorously tested before going into GNC products, undergoing multiple quality checks to ensure that they meet our high standards for identity, strength, purity, composition and limits in contaminants. |
We believe our sector-leading innovation capability is a significant competitive advantage. Our strategic partnership with IVC allows us to further focus on innovation while IVC drives increased efficiencies in manufacturing and supply chain. Refer to Item 8, "Financial Statements and Supplementary Data" Note 9, "Equity Method Investments" for more information. GNC has demonstrated strength in developing unique, branded, and scientifically verified products and has
39
a long history of delivering new ingredients, new flavors and convenient solutions. We directly employ scientists, nutritionists, formulators, and quality control experts and have access to a wide range of world-class research facilities and consultants.
• | A differentiated retail customer experience. Our retail strategy is to deliver a compelling experience at every customer touch point. We operate in a highly personalized, aspirational sector and believe that the nutritional supplement consumer often desires and seeks out product expertise and knowledgeable customer service. |
We further differentiate ourselves from competitors through development of our well-trained "coaches" with regular training that focuses on solution-based selling, and through in-store technology such as tablets, which allow associates to view customers’ purchase history and preferences in real time. With that knowledge, and help from sales tools built into the tablet platform, associates can engage customers in conversation, share product information and testimonials before and after pictures, recommend solutions and help customers add complimentary products and build wellness regimens.
Our loyalty programs allow us to develop and maintain a large and loyal customer base, provide targeted offers and information, and connect with our customers on a regular basis. We harness data generated by these programs to better understand customers’ buying behaviors and needs, so we can deliver a stronger experience, bring like-minded consumers into the channel and make well-informed decisions about the business.
• | Omni-channel development. We believe our diversified, omni-channel model, which includes company-owned stores, domestic and international franchise locations, wholesale locations and e-commerce channels, differentiates us from online-only competitors. Our strategy is to give consumers a seamless, integrated experience across digital, mobile and store channels and in every interaction they have with GNC. |
Through GNC.com, our Amazon.com storefront and other marketplaces, customers can research and purchase our products online. We believe our brick and mortar store base is a competitive advantage with respect to our online presence and platform, allowing customers to experience our products and get expert advice from a coach.
Our omni-channel model can enhance the customer experience and increase the lifetime value of a GNC customer, and we are implementing strategies over the next 12-18 months to blend our digital, online and in-store platforms. These initiatives include increased cross-channel marketing, online and in-store subscription services, giving customers the option of picking up online purchases in GNC stores, shipping products purchased via e-commerce directly from stores, and providing additional educational content, information and advice on GNC.com.
• | International growth. We see opportunity to expand internationally within the large global supplement market, through online channels and store locations, which is expected to continue to grow. In particular, our joint venture with Harbin Pharmaceutical Group Co., Ltd. ("Harbin") allows us to further expand our business in China. Harbin’s expertise in distribution and regulation is the ideal match for our highly valued brand and assortment of products in the China market. Refer to Item 8. " Financial Statements and Supplementary Data" Note 9, "Equity Method Investments" for more information. |
• | Driving constructive industry dialogue. We remain focused on continuously raising the bar on transparency and quality throughout the dietary supplement industry. We believe that over time the implementation of higher standards and more stringent industry self-regulation regarding manufacturing practices, ingredient traceability and product transparency will prove beneficial for the industry and lead to improved dialogue with regulators, stronger consumer trust and greater confidence in our industry. |
Key Performance Indicators
The primary key performance indicators that senior management focuses on include revenue and operating income for each segment, which are discussed in detail within "Results of Operations", as well as same store sales growth.
The table below presents the key components of same store sales.
U.S. Company-Owned Same Store Sales, including GNC.com | Q1 | Q2 | Q3 | Q4 | YTD 12/31 | ||||
2019 total same store sales | (1.6)% | (4.6)% | (2.8)% | (2.4)% | (2.9)% | ||||
2018 total same store sales | 0.5% | (0.4)% | (2.1)% | (0.6)% | (0.6)% | ||||
40
Same store sales include point-of-sale retail sales from all Company-owned domestic stores that have been operating for twelve full months following the opening day and retail sales from GNC.com. We are an omni-channel retailer with capabilities that allow a customer to use more than one channel when making a purchase, including in-store and e-commerce channels. Our e-commerce channels include our wholly-owned website GNC.com and third-party websites, including Amazon (the sales from which are included in the GNC.com business unit), where product assortment and price are controlled by us and purchases are fulfilled by direct shipment to the customer from one of our distribution facilities or from third-party e-commerce vendors. In-store sales are reduced by sales originally consummated online or through mobile devices and subsequently returned in-store. Sales of membership programs, including the PRO Access loyalty program and the net change in the deferred points liability associated with the myGNC Rewards program, are excluded from same store sales.
Same store sales are calculated on a daily basis for each store and exclude the net sales of a store for any period if the store was not open during the same period of the prior year. When a store’s square footage has been changed as a result of reconfiguration or relocation in the same mall or shopping center, the store continues to be treated as a same store. If, during the period presented, a store was closed, relocated to a different mall or shopping center, or converted to a franchise store or a company-owned store, sales from that store up to and including the closing day or the day immediately preceding the relocation or conversion are included as same store sales as long as the store was open during the same period of the prior year. Corporate stores are included in same store sales after the thirteenth month following a relocation or conversion to a company-owned store.
We also provide retail comparable same stores sales of our franchisees as well as our Canada business if meaningful to current results. While retail sales of franchisees are not included in the Consolidated Financial Statements, the metric serves as a key performance indicator of our franchisees, which ultimately impacts wholesale sales and royalties and fees received from franchisees. We compute same store sales for our franchisees and Canada business consistent with the description of corporate same store sales above. Same store sales for international franchisees and Canada exclude the impact of foreign exchange rate changes relative to the U.S. dollar.
41
Results of Operations
The following information presented was derived from our audited Consolidated Financial Statements and accompanying notes included in Item 8, "Financial Statements and Supplementary Data."
(Expressed as a percentage of total consolidated revenue unless indicated otherwise)
Year ended December 31, | ||||||||
2019(1) | 2018(1) | 2017 (1) | ||||||
Revenues: | ||||||||
U.S. and Canada | 88.1 | % | 82.9 | % | 81.4 | % | ||
International | 7.6 | % | 8.1 | % | 7.2 | % | ||
Manufacturing / Wholesale: | ||||||||
Intersegment revenues | 1.7 | % | 11.2 | % | 9.3 | % | ||
Third Party | 4.3 | % | 9.0 | % | 8.8 | % | ||
Subtotal Manufacturing / Wholesale | 6.0 | % | 20.2 | % | 18.1 | % | ||
Other | — | % | — | % | 2.6 | % | ||
Elimination of intersegment revenue | (1.7 | )% | (11.2 | )% | (9.3 | )% | ||
Total net revenues | 100.0 | % | 100.0 | % | 100.0 | % | ||
Operating expenses: | ||||||||
Cost of sales, including warehousing, distribution and occupancy | 65.5 | % | 67.2 | % | 66.8 | % | ||
Gross Profit | 34.5 | % | 32.8 | % | 33.2 | % | ||
Selling, general and administrative expenses | 27.4 | % | 26.4 | % | 25.2 | % | ||
Long-lived asset impairments | 0.0 | % | 1.6 | % | 18.5 | % | ||
Loss on net asset exchange or sale | (1.0 | )% | — | % | 0.0 | % | ||
Other loss (income), net | (0.1 | )% | — | % | (0.0 | )% | ||
Total operating expenses | 91.8 | % | 95.2 | % | 110.4 | % | ||
Operating income (loss): | ||||||||
U.S. and Canada (2) | 8.3 | % | 4.9 | % | (12.1 | )% | ||
International (2) | 35.0 | % | 31.5 | % | 34.3 | % | ||
Manufacturing / Wholesale (2) | 33.4 | % | 13.2 | % | 10.9 | % | ||
Unallocated corporate costs and other: | ||||||||
Corporate costs | (4.7 | )% | (4.5 | )% | (4.1 | )% | ||
Loss on net asset exchange for the formation of the joint ventures | (1.0 | )% | — | % | — | % | ||
Other loss, net | (0.2 | )% | — | % | (0.8 | )% | ||
Subtotal unallocated corporate, loss on net asset exchange and other loss, net | (5.9 | )% | (4.5 | )% | (5.0 | )% | ||
Total operating income (loss) | 6.0 | % | 4.8 | % | (10.4 | )% | ||
Interest expense, net | 5.2 | % | 5.4 | % | 2.6 | % | ||
Gain on convertible debt and debt refinancing costs | (0.2 | )% | — | % | (0.4 | )% | ||
Loss on debt refinancing | — | % | 0.7 | % | — | % | ||
Loss (gain) on forward contracts for the issuance of convertible preferred stock | 0.8 | % | (3.8 | )% | — | % | ||
Income (loss) before income taxes and income from equity method investments | 0.2 | % | 2.4 | % | (12.5 | )% | ||
Income tax (benefit) expense | 2.2 | % | (0.5 | )% | (6.4 | )% | ||
Net (loss) income before income from equity method investments | (2.0 | )% | 3.0 | % | (6.1 | )% | ||
Income from equity method investments | 0.3 | % | — | % | — | % | ||
Net (loss) income | (1.7 | )% | 3.0 | % | (6.1 | )% | ||
(1) Figures may not sum due to rounding
(2) Calculated as a percentage of segment revenue
42
Non-GAAP Measures
We have included non-GAAP financial measures below, which have been adjusted to exclude the impact of certain transactions, because we believe such measures represent an effective supplemental means by which to measure our operating performance. We believe that (i) net (loss) income, (ii) diluted earnings per share ("EPS") and (iii) EBITDA, each on an as adjusted basis to exclude certain items, and (iv) free cash flow are useful metrics to investors and enable management and our investors to evaluate and compare our results from operations in a more meaningful and consistent manner by excluding specific items that are not reflective of ongoing operating results. However, these metrics are not a measurement of our operating performance under GAAP and should not be considered as an alternative to net (loss) income, EPS, or any other performance measures derived in accordance with GAAP, or as an alternative to GAAP cash flow from operating activities, or as a measure of our profitability or liquidity.
Reconciliation of Net (Loss) Income and Diluted EPS to Adjusted Net Income and Adjusted EPS
(in thousands, except per share data)
Year ended December 31, | ||||||||||||||||||||||||
2019 | 2018 | 2017 | ||||||||||||||||||||||
Net (Loss) Income | Diluted EPS | Net Income | Diluted EPS | Net (Loss) Income | Diluted EPS | |||||||||||||||||||
Reported | $ | (35,112 | ) | $ | (0.64 | ) | $ | 69,780 | $ | 0.81 | $ | (150,262 | ) | $ | (2.18 | ) | ||||||||
Retention (1) | 2,064 | 0.02 | 6,971 | 0.08 | — | — | ||||||||||||||||||
Loss on net asset exchange for the formation of the joint ventures | 21,293 | 0.25 | — | — | — | — | ||||||||||||||||||
Loss on sale of Lucky Vitamin | — | — | — | — | 1,696 | 0.02 | ||||||||||||||||||
Gain on convertible debt and debt refinancing costs | (3,214 | ) | (0.04 | ) | — | — | (10,996 | ) | (0.16 | ) | ||||||||||||||
Loss on debt refinancing | — | — | 16,740 | 0.19 | — | — | ||||||||||||||||||
Loss (gain) on forward contracts for the issuance of convertible preferred stock (2) | 16,787 | 0.20 | (88,942 | ) | (1.03 | ) | — | — | ||||||||||||||||
Long-lived asset impairments | — | — | 38,236 | 0.44 | 457,794 | 6.64 | ||||||||||||||||||
Amortization of discount in connection with early debt payment | 3,119 | 0.04 | 3,542 | 0.04 | — | — | — | |||||||||||||||||
Other (3) | 3,005 | 0.04 | 3,273 | 0.04 | 7,416 | 0.11 | ||||||||||||||||||
Tax effect of items above (4) | 4,941 | 0.06 | (16,954 | ) | (0.20 | ) | (119,819 | ) | (1.73 | ) | ||||||||||||||
Adjustment to valuation allowance on DTA (5) | 27,117 | 0.32 | — | — | (3,860 | ) | (0.06 | ) | ||||||||||||||||
Revaluation of net deferred tax liabilities associated with tax reform | — | — | — | — | (86,786 | ) | (1.26 | ) | ||||||||||||||||
Discrete tax benefit (6) | — | — | (3,583 | ) | (0.04 | ) | — | — | ||||||||||||||||
Adjusted | $ | 40,000 | $ | 0.25 | $ | 29,063 | $ | 0.34 | $ | 95,183 | $ | 1.38 | ||||||||||||
Weighted average diluted common shares outstanding | 84,123 | 86,171 | 68,923 | |||||||||||||||||||||
(1) Related to an incentive program to retain senior executives and certain other key personnel below the executive level who are critical to the execution and success of the Company's strategy. The total amount awarded was approximately $10 million, of which approximately $1 million has been forfeited as of December 31, 2019, which vests in four installments of 25% each. Vesting dates are on the earlier of February 2019 or the closing of the Harbin transaction, February 2019, August 2019 and February 2020.
(2) Related to the change in fair value of the forward contracts related to the issuance of convertible preferred stock.
(3) The year ended December 31, 2019 included loss on the termination of the corporate plane lease of $3.1 million, severance expense of $0.5 million and gains on refranchising of $0.6 million. The year ended December 31, 2018 included $1.6 million start-up costs incurred in connection with the formation of commercial joint ventures in China with Harbin, $1.3 million of legal-related charge, $0.9 million of severance expense associated with the organizational realignment to more effectively align the structure in support of the key growth areas of the Company and $0.5 million gains on refranchising. The year ended December 31, 2017
43
included $3.3 million of executive placement costs primarily related to make-whole stock-based compensation awards including the impact of accelerated vesting associated with a Section 83(b) tax election, $4.4 million of legal-related charges and $0.3 million gains on refranchising.
(4) The 2019 and 2018 tax rate was calculated using a federal rate plus a net state rate that excluded the impact of certain state net operating losses, state credits and valuation allowance. The 2017 tax rates were calculated using the Company's annual effective tax rate, adjusted to exclude discrete items and the tax impact of certain significant transactions including goodwill and indefinite-lived assets impairment, gains on convertible debt, and reduction in valuation allowance.
(5) For the year ended December 31, 2019, the adjustment related to an increase in the valuation allowance against certain deferred tax assets that may not be realizable. For the year ended December 31, 2017, the adjustment related to a reduction to a valuation allowance based on a change in circumstances, which caused a change in judgment about the realizability of a deferred tax asset related to net operating losses.
(6) Related to discrete tax benefits associated with finalization of the Company’s 2017 federal income tax return.
Reconciliation of Net (Loss) Income to Adjusted EBITDA
(in thousands)
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Net (loss) income | $ | (35,112 | ) | $ | 69,780 | $ | (150,262 | ) | |||
Income tax expense (benefit) | 44,869 | (12,305 | ) | (159,779 | ) | ||||||
Interest expense, net | 106,709 | 127,080 | 64,221 | ||||||||
Depreciation and amortization (1) | 35,422 | 47,105 | 56,809 | ||||||||
Retention (2) | 2,064 | 6,971 | — | ||||||||
Loss on net asset exchange for the formation of the joint ventures | 21,293 | — | 1,696 | ||||||||
Gain on convertible debt transactions (3) | (3,214 | ) | — | (10,996 | ) | ||||||
Loss on debt refinancing | — | 16,740 | — | ||||||||
Loss (gain) on forward contracts for the issuance of convertible preferred stock (4) | 16,787 | (88,942 | ) | — | |||||||
Long-lived asset impairments | — | 38,236 | 457,794 | ||||||||
Other (5) | 3,005 | 3,273 | 7,416 | ||||||||
Adjusted EBITDA | $ | 191,823 | $ | 207,938 | $ | 266,899 | |||||
(1) The decrease in the current year compared with the prior year was primarily due to the transfer of the Nutra net assets to the Manufacturing JV effective March 1, 2019 and the long-lived asset impairments recorded in the third quarter of 2018. The decrease in the year ended December 31, 2018 compared with the year ended December 31, 2017 was primarily due to 2017 accelerated depreciation associated with the re-platforming of the GNC.com website from a third-party to a cloud-based solution, as well as long-lived asset impairments recorded within the U.S. and Canada segment for certain of our underperforming stores in the third and fourth quarter of 2017.
(2) Related to an incentive program to retain senior executives and certain other key personnel below the executive level who are critical to the execution and success of the Company's strategy. The total amount awarded was approximately $10 million, of which approximately $1 million has been forfeited as of December 31, 2019, which vests in four installments of 25% each. Vesting dates are on the earlier of February 2019 or the closing of the Harbin transaction, February 2019, August 2019 and February 2020.
(3) During the second quarter of 2019, the Company repurchased $29.5 million in aggregate principal amount of the Convertible Debt for $24.7 million in cash, which resulted in a gain of $3.2 million. During the fourth quarter of 2017, the Company exchanged in privately negotiated transactions $98.9 million in aggregate principal amount of the Convertible debt for an aggregate of 14.6 million newly issued shares of the Company’s Class A common stock, which had a value of $71.7 million at the time of the exchange. The exchange resulted in a gain, net of unamortized discount and third party fees, of $11.0 million.
(4) Related to the change in fair value of the forward contracts related to the issuance of convertible preferred stock.
(5) The year ended December 31, 2019 included loss on the termination of the corporate plane lease of $3.1 million, severance expense of $0.5 million and gains on refranchising of $0.6 million. The year ended December 31, 2018 included $1.6 million start-up costs incurred in connection with the formation of commercial joint ventures in China with Harbin, $1.3 million of legal-related charge, $0.9 million of severance expense associated with the organizational realignment to more effectively align the structure in support of the key growth areas of the Company and $0.5 million gains on refranchising. The year ended December 31, 2017 included $3.3 million of executive placement costs primarily related to make-whole stock-based compensation awards including the impact of accelerated vesting associated with a Section 83(b) tax election, $4.4 million of legal-related charges and immaterial gains on refranchising.
44
GNC HOLDINGS, INC. AND SUBSIDIARIES
Reconciliation of Net Cash Provided by Operating Activities to Free Cash Flow
(in thousands)
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(unaudited) | |||||||||||
Net cash provided by operating activities | $ | 96,520 | $ | 95,868 | 220,508 | ||||||
Capital expenditures | (15,151 | ) | (18,981 | ) | (32,123 | ) | |||||
Free cash flow | $ | 81,369 | $ | 76,887 | $ | 188,385 | |||||
45
Comparison of the Years Ended December 31, 2019 (current year) and 2018 (prior year)
Revenues
Our consolidated net revenues decreased $285.3 million, or 12.1%, to $2,068.2 million in the current year compared with $2,353.5 million in 2018. The decrease in revenue was largely due to the transfer of the Nutra manufacturing and China business to the joint ventures formed in the first quarter of 2019, the closure of company-owned stores under our store portfolio optimization strategy, a decrease in U.S. company-owned and Canada same store sales, and lower sales to our wholesale partners primarily due to the termination of the consignment agreement with Rite Aid in the fourth quarter of 2018. Additional detail by segment as follows:
U.S. and Canada. Revenues in our U.S. and Canada segment decreased $128.9 million, or 6.6%, to $1,822.3 million in the current year compared with $1,951.2 million in 2018. The decrease in revenue in the current year as compared with the prior year was primarily due to the following:
• | The decrease in the number of corporate stores from 3,206 at December 31, 2018 to 2,902 at December 31, 2019 as part of our store portfolio optimization strategy contributed a $66.7 million decrease to revenue; |
• | A decrease in U.S. company-owned same store sales of 2.9%, which includes e-commerce sales, resulted in a $41.2 million decrease to revenue. E-commerce sales were 8.8% of U.S. and Canada revenue in the current year compared with 7.9% in the prior year; |
• | A decrease in domestic franchise revenue of $11.6 million to $290.0 million in the current year compared with $301.6 million in 2018 resulting from a retail same store sales decrease of 1.2% and a decrease in the number of domestic franchise stores from 1,037 at December 31, 2018 to 956 at December 31, 2019; and |
• | A decrease in Canada same store sales of 5.2% resulted in a $5.2 million decrease to revenue. |
International. Revenues in our International segment decreased $33.2 million, or 17.4%, to $158.2 million in 2019 compared with $191.4 million in 2018. Revenues from our China business decreased by $27.4 million in the current year compared with the prior year due to the transfer of the China business to the HK JV and the China JV effective February 13, 2019 (the "HK JV" and the "China JV"). Revenue from our international franchisees decreased $4.6 million in the current year compared with the prior year primarily due to lower sales in Hong Kong and other temporary challenges in Saudi Arabia and South Korea.
Manufacturing / Wholesale. Revenues in our Manufacturing / Wholesale segment, excluding intersegment revenues, decreased $123.2 million in the current year compared with the prior year primarily due to the transfer of the Nutra manufacturing business to the Manufacturing JV with IVC, effective March 1, 2019. As a result, third-party contract manufacturing sales decreased $107.5 million from $123.3 million in the prior year to $15.8 million in the current year. Sales to our wholesale partners decreased $15.7 million, or 17.9% to $71.9 million for the year ended December 31, 2019 compared with $87.6 million in 2018 largely due to the termination of the consignment agreement with Rite Aid in the fourth quarter of 2018. Intersegment sales were $264.2 million in the prior year compared with $35.5 million in the current year as a result of the formation of the Manufacturing JV.
Cost of Sales and Gross Profit
Cost of sales, which includes product costs, warehousing, distribution and occupancy costs, decreased $228.0 million, or 14.4%, to $1,353.8 million in the current year compared with $1,581.8 million in 2018. Gross profit decreased $57.3 million from $771.7 million in the prior year to $714.4 million in the current year, and as a percentage of revenue, increased from 32.8% in the prior year to 34.5% in the current year. The increase in gross margin rate was primarily due to the transfer of the Nutra manufacturing business to the Manufacturing JV and lower occupancy expense as a result of the adoption of the new lease standard, store closures and rent reductions associated with our store portfolio optimization strategy.
Selling, General and Administrative ("SG&A") Expense
SG&A expense, including compensation and related benefits, advertising and other expenses, decreased $54.4 million, or 8.8%, to $566.5 million in the current year compared with $620.9 million in 2018. As a percentage of revenue, SG&A expense was 27.4% in the current year compared with 26.4% in 2018.
During the year ended December 31, 2019 and 2018, we recognized $2.1 million and $7.0 million, respectively, in expense related to a retention program adopted in the first quarter of 2018 to retain senior executives and certain other key personnel below the executive level who are critical to the execution and success of our strategy. The total amount awarded was approximately $10 million, of which approximately $1 million had been forfeited as of December 31, 2019, which vests in four installments of 25% each on the earlier of February 2019 or the closing of the Harbin transaction, February 2019, August 2019 and February 2020. We incurred severance expense in the current year and prior year of $0.5 million and $0.9 million, respectively, associated with our
46
organizational realignment to more effectively align the structure in support of the key growth areas of the Company. In the prior year, we also incurred $1.6 million related to our China joint ventures start-up costs and $1.3 million legal-related charges.
Excluding the impact of these items, SG&A decreased by $46.3 million, or 7.6%, and was 27.3% and 25.9% as a percentage of revenue in the current year and prior year, respectively. The decrease in SG&A expense was primarily due to lower salaries and benefits associated with the store portfolio optimization and cost saving initiatives, cost reduction realized in connection with the formations of the strategic joint ventures, lower marketing and lower consignment commissions as a result of the termination of the consignment agreement with Rite Aid in the fourth quarter of 2018, partially offset by higher consulting expenses in the current year. The increase in SG&A expense as a percentage of revenue was primarily driven by deleverage in salaries and benefits associated with a decrease in sales and to a lesser extent an increase in consulting fees.
Long-Lived Asset Impairments
There was no long-lived asset impairment for the year ended December 31, 2019. In the prior year, we recorded $38.2 million in non-cash long-lived asset impairments consisting of $23.7 million related to brand name (of which $21.6 million was allocated to U.S. and Canada segment and $2.1 million was allocated to the International segment) and the remaining related to property and equipment and other store closing charges associated with the store portfolio optimization strategy.
Refer to Item 8, “Financial Statements and Supplementary Data,” Note 6, “Goodwill and Intangible Assets” and Note 7, “Property, Plant and Equipment, Net” for more information.
Loss on net asset exchange for the formation of the joint ventures
In the current year we contributed our Nutra manufacturing and Anderson facility net assets to the Manufacturing JV in exchange for net $99.2 million and an initial 43% equity interest in the Manufacturing JV. In addition, we contributed our China business in exchange for 35% equity interest in the HK JV and China JV. As a result of the joint venture transactions, we recognized a net pre-tax loss of $21.3 million for the year ended December 31, 2019.
Other Loss (Income), Net
Other loss, net, in the current year of $1.9 million included $3.1 million loss related to the termination of the corporate plane lease, offset by foreign currency gains of $0.6 million and refranchising gains of $0.6 million. Other loss, net, in the prior year of $0.3 million consisted of foreign currency losses of $0.8 million, offset by refranchising gains of $0.5 million.
Operating Income (Loss)
As a result of the foregoing, consolidated operating income was $124.7 million in the current year compared with $112.4 million in 2018. Operating income was significantly impacted by the loss on net asset exchange for the formation of the joint ventures of $21.3 million in the current year and by non-cash long-lived asset impairment charges and other store closing costs of $38.2 million in the prior year.
U.S and Canada. Operating income was $151.0 million, or 8.3% of segment revenue in the current year compared with $94.7 million, or 4.9% of segment revenue in 2018. In the prior year, the U.S. and Canada operating income was significantly impacted by long-lived asset impairment charges and other store closing costs of $36.1 million. Excluding the long-lived asset impairment and other store closing costs in the prior year and immaterial gains on refranchising in the current year and prior year, operating income was $150.4 million, or 8.3% of segment revenue, in the current year compared with $130.2 million, or 6.7% of segment revenue in the prior year. The increase in operating income percentage in the current year compared to the prior year was primarily due to lower occupancy expense as a result of the adoption of the new lease standard, store closures and rent reductions associated with our store portfolio optimization strategy.
International. Operating income was $55.4 million, or 35.0% of segment revenue in the current year compared with $60.4 million, or 31.5% segment revenue in 2018. The prior year included China joint ventures start-up costs of $1.6 million and non-cash long-lived asset impairment charges of $2.1 million. Excluding these items, operating income was $64.1 million, or 33.5% of segment revenue in the prior year. The increase in operating income percentage was primarily a result of the transfer of the China business to the HK JV and China JV.
Manufacturing / Wholesale. Operating income was $41.2 million, or 33.4% of segment revenue in the current year compared with operating income of $62.9 million, or 13.2% of segment revenue in 2018. Revenue decreased as a result of the transfer of the Nutra manufacturing business to the Manufacturing JV, however, operating income margins were positively impacted as the Manufacturing / Wholesale segment recognized profit margin that resulted from maintaining consistent pricing to what was charged to our other operating segments prior to the inception of the Manufacturing JV, and recorded profit on the sales associated with inventory produced prior to the transfer of the Nutra manufacturing business to the joint venture.
47
Corporate costs. Corporate costs decreased $7.2 million to $98.2 million in the current year compared with $105.4 million in 2018. Excluding the retention and the severance expense associated with the organizational realignment in the current year and prior year, and a legal related charge in the prior year, corporate costs decreased by $0.6 million compared to the prior year.
Loss on net asset exchange for the formation of the joint ventures. As a result of the joint venture transactions, as described above, we recognized a pre-tax loss of $21.3 million for the year ended December 31, 2019.
Other. Operating loss was $3.3 million in the current year which included a $3.1 million loss on the termination of the corporate plane lease. Operating loss was $0.2 million in the prior year.
Interest Expense
Interest expense was $106.7 million for the year ended December 31, 2019 compared with $127.1 million in 2018 primarily as a result of the reduction in long-term debt of approximately $298 million during the first half of 2019.
Gain on Convertible Debt Repurchase
In the second quarter of 2019, the Company repurchased $29.5 million in aggregate principle amount of the Notes for $24.7 million in cash. The convertible debt repurchase resulted in a gain of $3.2 million in the year ended December 31, 2019, which included the unamortized conversion feature of $1.3 million and unamortized discount of $0.2 million. Refer to Item 8, "Financial Statements and Supplementary Data," Note 8, "Long-Term Debt /Interest Expense" for more information.
Loss on Debt Refinancing
The refinancing of the Senior Credit Facility in 2018 resulted in a loss of $16.7 million in the prior year, which primarily included third-party fees relating to the Tranche B-2 Term Loan and the FILO Term Loan. Refer to Item 8, "Financial Statements and Supplementary Data," Note 8, "Long-Term Debt /Interest Expense" for more information.
Loss (Gain) on Forward Contracts for the Issuance of Convertible Preferred Stock
A loss of $16.8 million was recorded in the current year for the change in fair value of the forward contracts related to the issuance of convertible preferred stock compared with a gain of $88.9 million in the prior year. Refer to Item 8, "Financial Statements and Supplementary Data," Note 14, "Mezzanine Equity" for more information.
Income Tax Expense (Benefit)
We recognized income tax expense of $44.9 million in the current year. The current year effective tax rate was significantly impacted by an increase to tax expense of $27.1 million relating to an increase in valuation allowance against certain deferred tax assets that may not be realizable and an increase to tax expense of $7.6 million resulting from the transfer of the Nutra manufacturing net assets to the Manufacturing JV. The current year effective tax rate was also impacted by a $4.8 million increase in the Company’s liability for uncertain tax positions and a $3.5 million increase related to a loss on forward contracts for the issuance of convertible preferred stock that was not recognizable for tax purposes.
In the prior year, we recognized a tax benefit of $12.3 million. The effective tax rate in 2018 was significantly impacted by an $88.9 million gain on forward contracts for the issuance of convertible preferred stock which was not included for income tax purposes and a discrete tax benefit of $3.6 million associated with the tax reform related impact of the finalization of the Company's 2017 federal income tax return.
The Company regularly evaluates the need for a valuation allowance for its deferred income tax benefits and attributes by assessing whether it is more likely than not it will realize these benefits in future periods. In assessing the need for a valuation allowance, the Company considers all available positive and negative evidence, including the Company’s operating results, reversals of deferred tax liabilities, and forecasts of future taxable income on a jurisdiction-by-jurisdiction basis.
During the fourth quarter of the year ended December 31, 2019, as further discussed in Item 8, "Financial Statements and Supplementary Data," Note 1, "Nature of Business," management concluded that there is substantial doubt regarding the Company’s ability to continue as a going concern. Management considered this in concluding that certain deferred tax assets were no longer more likely than not realizable. As a result, an increase in valuation allowance of $27.1 million on the Company’s deferred tax assets was recorded as of December 31, 2019 which related principally to deferred tax assets for state NOL carryforwards and other state tax attributes and deferred tax assets related to the Company’s interest expense deductions as determined under Section 163(j) of the Internal Revenue Code. This increase was partially offset by a valuation allowance decrease of $4.8 million of which $3.7 million related to the write off of deferred tax assets associated with certain China NOLs which are no longer available to the Company as a result of the China joint venture transaction and $1.1 million related to utilization of Puerto Rico
48
NOLs. Management will continue to assess its valuation allowance in forthcoming periods. This may result in a different conclusion as to the realizability of the Company's deferred tax assets in the future.
Our ability to use deferred tax assets is subject to volatility and could be adversely affected by earnings differing materially from projections, changes in the valuation of our deferred tax assets and liabilities, expiration of or lapses in tax credits, changes in ownership as defined by Section 382 of the Internal Revenue Code and outcomes as a result of tax examinations or by changes in tax laws, regulations, and accounting principles. As a result, our income tax provisions are also subject to volatility from these changes, as well as from changes in accounting for uncertain tax positions or interpretations thereof.
Income from Equity Method Investments
We recognized $5.3 million income from equity method investments during the year ended December 31, 2019 in connection with the joint ventures formed in the first quarter of 2019. Refer to Item 8, "Financial Statements and Supplementary Data," Note 9, "Equity Method Investments" for more information.
Net (Loss) Income
As a result of the foregoing, consolidated net loss was $35.1 million in the current year compared with net income of $69.8 million in the prior year.
Diluted (Loss) Earnings Per Share
Diluted loss per share was $0.64 in the current year compared with diluted earnings per share of $0.81 in the prior year.
Comparison of the Years Ended December 31, 2018 and 2017
Revenues
Our consolidated net revenues decreased $127.5 million, or 5.1%, to $2,353.5 million for the year ended December 31, 2018 compared with $2,481.0 million in 2017. The decrease was primarily due to the sale of Lucky Vitamin on September 30, 2017, which resulted in a $66.2 million reduction to revenue, and the closure of company-owned stores under our store portfolio optimization strategy.
U.S. and Canada. Revenues in our U.S. and Canada segment decreased $67.7 million, or 3.4%, to $1,951.2 million for the year ended December 31, 2018 compared with $2,018.9 million for the year ended December 31, 2017. The decrease in revenue in 2018 as compared with 2017 was primarily due to the following:
• | The decrease in the number of corporate stores from 3,423 at December 31, 2017 to 3,206 at December 31, 2018 contributed a decrease of approximately $34 million to revenue; |
• | A decrease of $23.0 million relating to the termination of the U.S. Gold Card Member Pricing program, which resulted in the recognition of domestic Gold Card deferred revenue of $24.4 million, net of $1.4 million of applicable coupon redemptions in 2017; |
• | A decrease in U.S. company-owned same store sales of 0.6%, which includes e-commerce sales, resulted in a $9.2 million decrease to revenue. E-commerce sales were 7.9% of U.S. and Canada revenue in 2018 compared with 6.3% in 2017; |
• | A decrease in domestic franchise revenue of $24.4 million to $301.6 million in 2018 compared with $326.0 million in 2017 due to the impact of a decrease in retail same store sales of 2.9% and a decrease in the number of franchise stores from 1,099 at December 31, 2017 to 1,037 at December 31, 2018; |
• | A decrease in Canada company-owned stores revenue of $8.3 million primarily due to negative same store sales of 7.6%; and |
• | Partially offsetting the above decreases in revenue was an increase of $32.9 million related to our loyalty program; PRO Access paid membership fees and the myGNC Rewards change in deferred points liability. |
International. Revenues in our International segment increased $13.6 million, or 7.7%, to $191.4 million for the year ended December 31, 2018 compared with $177.8 million in 2017. Revenues from our China business increased by $9.6 million in 2018 compared with the 2017 largely due to higher cross-border e-commerce sales. Revenue from our international franchisees increased $3.6 million in 2018 compared with 2017 despite reporting a decrease in retail same store sales of 1.3%.
Manufacturing / Wholesale. Revenues in our Manufacturing / Wholesale segment, excluding intersegment revenues, decreased $7.2 million in the year ended December 31, 2018 compared with the year ended December 31, 2017. Third-party contract manufacturing sales decreased by $5.6 million, or 4.3%, to $123.3 million for the year ended December 31, 2018 compared
49
with $128.9 million in 2017 primarily due to lower demand associated with deceased sales with certain customers. Sales to our wholesale partners decreased $1.6 million, or 1.8% to $87.6 million for the year ended December 31, 2018 compared with $89.2 million in 2017. Intersegment sales increased $32.7 million from $231.5 million for the year ended December 31, 2017 to $264.2 million in 2018 reflecting our increasing focus on proprietary products.
Other. In connection with the sale of Lucky Vitamin on September 30, 2017, revenue in 2018 decreased by $66.2 million compared with 2017.
Cost of Sales and Gross Profit
Cost of sales decreased $74.7 million, or 4.5%, to $1,581.8 million for the year ended December 31, 2018 compared with $1,656.5 million in 2017. Gross profit decreased $52.7 million from $824.4 million for the year ended December 31, 2017 to$771.7 million in 2018, and as a percentage of revenue, decreased from 33.2% in 2017 to 32.8% for the year ended December 31, 2018. The decrease in gross margin rate was primarily due to impacts from the new loyalty program and reserve related to third-party vendor risk in 2018, partially offset by a higher sales mix of proprietary product which contribute higher margins relative to third-party sales.
SG&A Expense
SG&A expense decreased $3.4 million, or 0.5%, to $620.9 million, for the year ended December 31, 2018 compared with $624.3 million in 2017. As a percentage of revenue, SG&A expense was 26.4% for the year ended December 31, 2018 compared with 25.2% in 2017.
During the year ended December 31, 2018, we recognized $7.0 million in expense related to a retention program as mentioned above. We also incurred $1.6 million related to our China joint ventures start-up costs, $1.3 million legal-related charges and $0.9 million severance expense associated with the organizational realignment to more effectively align the structure in support of the key growth areas of the Company in 2018. During the year ended December 31, 2017, we incurred $3.3 million executive placement costs primarily related to make-whole stock-based compensation awards including the impact of accelerated vesting associated with a Section 83(b) tax election and $4.4 million of legal-related charges.
Excluding the impact of these items, SG&A decreased by $6.4 million, or 1.0%, and was 25.9% and 24.9% as a percentage of revenue in 2018 and 2017, respectively. The decrease in SG&A expense was primarily due to the sale of our Lucky Vitamin e-commerce business effective September 30, 2017 and lower marketing expense, partially offset by an increase in store commissions associated with a higher sales mix of proprietary product, higher incentives and higher commissions to support e-commerce sales.
Long-Lived Asset Impairments
We recorded $38.2 million in non-cash long-lived asset impairments for the year ended December 31, 2018, consisting of $23.7 million related to brand name (of which $21.6 million was allocated to U.S. and Canada segment and $2.1 million was allocated to the International segment) and the remaining related to property and equipment and other store closing charges associated with the store portfolio optimization strategy.
We recorded $457.8 million in non-cash long-lived asset impairments for the year ended December 31, 2017, consisting of $395.6 million related to brand name (of which $394.0 million was allocated to U.S. and Canada segment and $1.6 million was allocated to the International segment), $24.3 million related to goodwill in our Wholesale reporting unit, $19.4 million related to Lucky Vitamin and the remaining related to property and equipment for certain of our underperforming stores.
Refer to Item 8, “Financial Statements and Supplementary Data,” Note 6, “Goodwill and Intangible Assets” and Note 7, “Property, Plant and Equipment, Net” for more information.
Other Loss (Income), Net
Other loss, net, in the year ended December 31, 2018, of $0.3 million related to $0.8 million foreign currency losses, partially offset by $0.5 million refranchising gains. Other income, net, in the year ended December 31, 2017, of $0.8 million consisted of $1.2 million in foreign currency gains, gains of $1.0 million related to insurance and lease settlements and $0.3 million refranchising gains, partially offset by a $1.7 million loss attributed to the sale of substantially all of the assets of the Lucky Vitamin e-commerce business.
Operating Income (Loss)
As a result of the foregoing, consolidated operating income was $112.4 million for the year ended December 31, 2018 compared with operating loss of $256.8 million in 2017. Operating income in the year ended December 31, 2018 and operating
50
loss in the year ended December 31, 2017 were impacted significantly by non-cash long-lived asset impairment charges of $38.2 million and $457.8 million, respectively, as described above.
U.S. and Canada. Operating income was $94.7 million for the year ended December 31, 2018 compared with a loss of $244.1 million in 2017. In 2018, we recorded long-lived asset impairments and other store closing costs totaling $36.1 million and $0.5 million refranchising gains, and in 2017, we recorded long-lived asset impairments of $412.5 million and $0.3 million refranchising gains. Excluding these items and the comparative prior year impact of the recognition of deferred Gold Card revenue as described above, operating income was $130.2 million, or 6.7% of segment revenue in 2018 compared with $145.0 million, or 7.3% of segment revenue in 2017. The decrease in operating income as a percentage of segment revenue was primarily due to an increase in store commissions associated with a higher sales mix of proprietary product.
International. Operating income was $60.4 million, or 31.5% of segment revenue for the year ended December 31, 2018 compared with $61.0 million, or 34.3% segment revenue in 2017. The year ended December 31, 2018 included $1.6 million related to China joint ventures start-up costs and $2.1 million non-cash long-lived asset impairment charges and the year ended December 31, 2017 included $1.6 million non-cash long-lived assets impairment. Excluding these items, operating income was $64.1 million, or 33.5% of segment revenue in the year ended December 31, 2018 compared with $62.6 million, or 35.2% of segment revenue in 2017. The decrease in operating income percentage was primarily due to a higher mix of China sales, which contribute lower margins relative to franchise sales, and increased marketing expense in our China business as we invest to grow the brand in China.
Manufacturing / Wholesale. Operating income was $62.9 million, or 13.2% of segment revenue for the year ended December 31, 2018 compared with loss of $49.2 million, or 10.9% of segment revenue in 2017. Operating income in 2017 was significantly impacted by goodwill impairment charges of $24.3 million. Excluding the non-cash impairment charges, operating income was $73.5 million, or 16.3% of segment revenue in 2017. The decrease in operating income percentage was primarily due to lower margin rate from third-party contract manufacturing, partially offset by higher intersegment sales, which contributed higher margin.
Corporate costs. Corporate costs increased by $3.3 million to $105.4 million in the year ended December 31, 2018 compared with $102.1 million in 2017. Excluding the retention, a legal-related charge and the severance expense associated with the organizational realignment in 2018, and the executive placement costs and legal-related charges in 2017 as explained above, corporate costs increased $1.8 million in 2018 compared with 2017.
Other. Operating loss was $0.2 million in the year ended December 31, 2018 compared with a loss of $20.8 million in 2017, which was primarily due to $19.4 million of non-cash long-lived asset impairments recorded in the second quarter of 2017 and $1.7 million of a loss on sale relating to the Lucky Vitamin e-commerce business.
Interest Expense
Interest expense was $127.1 million for the year ended December 31, 2018 compared with $64.2 million in 2017 primarily due to a higher interest rate on the Tranche B-2 Term Loan and the FILO Term Loan in connection with the debt refinancing.
Loss on Debt Refinancing
The refinancing of the Senior Credit Facility resulted in a loss of $16.7 million in the year ended December 31, 2018, which primarily included third-party fees relating to the Tranche B-2 Term Loan and the FILO Term Loan. Refer to Item 8, "Financial Statements and Supplementary Data," Note 8, "Long-Term Debt /Interest Expense" for more information.
Gain on Forward Contracts for the Issuance of Convertible Preferred Stock
A gain of $88.9 million was recorded in the year ended December 31, 2018 for the change in fair value of the forward contracts related to the issuance of convertible preferred stock. Refer to Item 8, "Financial Statements and Supplementary Data," Note 14, "Mezzanine Equity" for more information.
Income Tax (Benefit) Expense
We recognized an income tax benefit of $12.3 million for the year ended December 31, 2018. The effective tax rate was significantly impacted by an $88.9 million gain on forward contracts for the issuance of convertible preferred stock which was not included for income tax purposes and a discrete tax benefit of $3.6 million associated with the finalization of the Company's 2017 federal income tax return.
In the year ended December 31, 2017, we recognized tax expense of $159.8 million. The effective tax rate in 2017 was significantly impacted by an $86.8 million reduction to net deferred tax liabilities, which were revalued using a lower corporate
51
tax rate associated with the Tax Cuts and Jobs Act of 2017 and, a $24.3 million goodwill impairment charge, the majority of which was not deductible for tax purposes. The tax rate was also impacted by a reduction to a valuation allowance of $3.8 million.
The valuation allowance in each period was adjusted based on a change in circumstances, including anticipated future earnings, which caused a change in judgment about the realizability of certain deferred tax assets related to net operating losses.
Net Income (Loss)
As a result of the foregoing, we recorded a net income of $69.8 million for the year ended December 31, 2018, compared with a net loss of $150.3 million in 2017.
Diluted Earnings (Loss) Per Share
Diluted earnings per share was $0.81 for the year ended December 31, 2018 compared with diluted loss per share of $2.18 for the year ended December 31, 2017.
52
Liquidity and Capital Resources
At December 31, 2019, the Company had $66.2 million available under the Revolving Credit Facility, after giving effect to $4.9 million utilized to secure letters of credit and a $9.9 million reduction to borrowing ability as a result of decrease in net collateral. As a precautionary measure, given the current macro environment, we recently drew $30 million under our Revolving Credit Facility resulting in over $130 million in cash as of March 24, 2020. Our ability to make scheduled payments of principal on, to pay interest on or to refinance our debt and to satisfy our other debt obligations will depend on our future operating performance, which will be affected by general economic, financial and other factors beyond our control.
The Company has continued to experience negative same store sales and declining gross profit. The Company has closed underperforming stores under its store optimization strategy and implemented cost reduction measures to help mitigate the effect of these declines and improve its financial position and liquidity. At December 31, 2019, the Company has substantial indebtedness including $154.7 million of outstanding indebtedness under the Notes issued under that certain Indenture dated as of August 10, 2015, among the Company, certain of its subsidiaries, and The Bank of New York Mellon Trust Company, N.A, maturing on August 15, 2020 (the "Notes") and $441.5 million of outstanding indebtedness under the Amended and Restated Term Loan Credit Agreement, dated as of February 28, 2018, among GNC Corporation, GNC Nutrition Centers, Inc., as Borrower, the lenders and agents parties thereto, and JPMorgan Chase Bank, N.A., as administrative agent (the "Tranche B-2 Term Loan Credit Agreement” and the term loan thereunder, the “Tranche B-2 Term Loan"). The Company also has an excess cash flow payment of $25.9 million due in April 2020 (which will reduce the outstanding amount of the Tranche B-2 Term Loan). The Tranche B-2 Term Loan becomes due on the earlier to occur of (i) the maturity date of March 4, 2021 or (ii) May 16, 2020 if more than $50 million of the Notes are outstanding on such date. Each of the revolving credit facility (the "Revolving Credit Facility") under the Credit Agreement, dated as of February 28, 2018, among GNC Corporation, GNC Nutritional Centers, Inc., as Administrative Borrower, certain of its subsidiaries, as subsidiary borrowers, the lenders and agents parties thereto, and JPMorgan Chase Bank, N.A., as administrative agent (the “ABL Credit Agreement”) and the FILO term loan facility under the ABL Credit Agreement, which otherwise mature in August 2022 and December 2022 respectively, also include an accelerated maturity date of May 16, 2020 if more than $50 million of the Notes are outstanding on such date.
Prior to the outbreak of the COVID-19 pandemic in the United States, management believed that the Company had the ability to pay the excess cash flow payment of $25.9 million and reduce the outstanding balance on the Notes from $154.7 million to below $50 million with projected cash on hand and new borrowings under the Revolving Credit Facility, assuming such borrowings remain available subject to the covenant and reporting requirements discussed below. Given current circumstances around the COVID-19 pandemic as discussed in further in Item 8, "Financial Statements and Supplementary Data, " Note 21, "Subsequent Events", there can be no assurances as to our ability to do so. See “Risk Factors - Risks Related to Our Business and Industry - Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19." However, management does not expect to have sufficient cash flows from operations to repay the indebtedness under the Notes or the Tranche B-2 Term Loan when they become due. Since the Company has not refinanced the Tranche B-2 Term Loan and it will mature less than twelve months after the issuance date of these consolidated financial statements, management has concluded there is substantial doubt regarding the Company's ability to continue as a going concern within one year from the issuance date of the Company’s consolidated financial statements.
We were in compliance with our debt covenant reporting and compliance obligations under our Credit Facilities as of December 31, 2019. Prior to the outbreak of the COVID-19 pandemic in the United States, management believed that the Company had the ability to comply with the financial covenants under the Senior Credit Facility Agreements (as described further in Item 8, "Financial Statements and Supplementary Data," Note 8, "Long-Term Debt / Interest Expense") over the next twelve months; however, given the current circumstances around the COVID-19 pandemic as discussed further in Item 8, "Financial Statements and Supplementary Data, " Note 21, "Subsequent Events", there can be no assurances as to our ability to do so. See “Risk Factors - Risks Relating to Our Business and Industry - Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19.”
The Company is in the process of reviewing a range of refinancing options to refinance all of the Company’s outstanding indebtedness. The Company has been working with an independent committee of the Board supported by independent financial and legal advisors to conduct its review and has had a series of discussions with financing sources in the United States and Asia. We became aware on March 24, 2020, by the potential financing sources in Asia, that they are no longer pursuing a refinancing with us. We will continue to explore all options to refinance and restructure our indebtedness. While we continue to work through a number of refinancing alternatives to address our upcoming debt maturities, we cannot make any assurances regarding the likelihood, certainty or exact timing of any alternatives.
53
Reporting requirements under both the Tranche B-2 Term Loan and the Credit Agreement, dated as of February 28, 2018, among GNC Corporation, GNC Nutrition Centers, Inc., as Administrative Borrower, certain of its subsidiaries, as subsidiary borrowers, the lenders and agents parties thereto, and JPMorgan Chase Bank, N.A., as administrative agent (the “ABL Credit Agreement,” together with the Tranche B-2 Term Loan, the “Senior Credit Agreements”) require the Company to provide annual audited financial statements accompanied by an opinion of an independent public accountant without a "going concern" or like qualification or exception, or qualification arising out of the scope of the audit (other than a “going concern” statement, explanatory note or like qualification or exception resulting solely from an upcoming maturity date under the Tranche B-2 Term Loan or the Notes). Management believes the Company will satisfy this requirement. If the lenders take a contrary position, (a) they could decide to instruct the administrative agent under the Senior Credit Agreements to deliver a written notice thereof to the borrower, and if the alleged default continued uncured for 30 days thereafter it would become an alleged event of default (unless waived by the lenders) and (b) the Company intends to contest such position and any action the lenders may attempt to take as a result thereof. If the lenders were to prevail in any such dispute, the required lenders could instruct the administrative agent to exercise remedies under the Senior Credit Agreements (the "Revolving Credit Facility"), including accelerating the maturity of the loans, terminating commitments under the revolving credit facility under the ABL Credit Agreement and requiring the posting of cash collateral in respect of outstanding letters of credit issued under the Revolving Credit Facility ($4.9 million at December 31, 2019). If this were to occur, management would enter into discussions with the lenders to waive the default or forebear from the exercise of remedies. Failure to obtain such a waiver, complete the refinancing or other restructuring or to reach an agreement with the Company's stakeholders on the terms of a restructuring would have a material adverse effect on the liquidity, financial condition and results of operations and may result in filing a voluntary petition for relief under Chapter 11 of the United States Bankruptcy Code in order to implement a restructuring plan.
The Company’s Consolidated Financial Statements as of December 31, 2019 are being prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business.
Cash Provided by Operating Activities
Cash provided by operating activities was $96.5 million, $95.9 million and $220.5 million during the years ended December 31, 2019, 2018 and 2017 respectively. The increase in cash flow from operations in the current year compared with the prior year was driven primarily by an increase in accounts payable as a result of the Company's cash management efforts as well as the establishment of payables associated with the manufacturing joint venture and a decrease in inventory, partially offset by an increase in prepaid and other current assets. The decrease in cash flow from operations in 2018 compared with the 2017 was primarily due to reduced operating performance and comparative effect of an inventory reduction in the prior year as part of the supply chain optimization which was launched at the end of 2016. The remaining decrease was primarily due to higher interest payments and the refinancing of our long-term debt, which resulted in $16.3 million in fees paid to third-parties, partially offset by lower tax payments and a $12.4 million tax refund received in the fourth quarter of 2018.
Cash Provided by (Used in) Investing Activities
Cash provided by investing activities was $73.4 million during the year ended December 31, 2019 and cash used in investing activities was $16.5 million and $23.8 million for the years ended December 31, 2018 and 2017, respectively. Capital expenditures were $15.2 million, $19.0 million and $32.1 million during the year ended December 31, 2019, 2018 and 2017, respectively. The decrease in capital expenditure in 2019 compared with 2018 is primarily due to comparative effect of lower capital spend on the Nutra manufacturing facility in the current year as a result of the Manufacturing JV transaction in 2019. The decrease in capital expenditure in 2018 compared with 2017 primarily relates to decreased spend in new store construction and IT infrastructure at corporate. In 2019, we received cash proceeds from IVC of $99.2 million in exchange for their 57% ownership in the Manufacturing JV. In addition, we made a capital contribution of $10.7 million to the Manufacturing JV for our share of short-term working capital needs and contributed cash of $2.4 million from our China business to the HK JV and China JV. During 2017, we completed an asset sale of Lucky Vitamin on September 30, 2017 for a purchase price of $6.4 million, net of closing fees, the proceeds of which were received in October 2017.
In 2020, we expect our capital expenditures to be approximately $23 million, which includes investments for IT infrastructure, store development and maintenance. We anticipate funding our 2020 capital requirements with cash flows from operations.
Cash Used in Financing Activities
For the year ended December 31, 2019, cash used in financing activities was $119.3 million, primarily consisting of $147.3 million in payments on the Tranche B-1 Term Loan, $123.8 million in payments on the Tranche B-2 Term Loan, $24.7
54
million payments for the repurchase of Notes, $12.8 million in fees paid for the issuance of the Convertible Preferred Stock and a $10.4 million original issuance discount (“OID”) paid to the Tranche B-2 Term Loan lender at 2% of the outstanding balance, partially offset by approximately $200 million of proceeds from the issuance of the Convertible Preferred Stock.
For the year ended December 31, 2018, cash used in financing activities was $75.8 million, primarily consisting of $136.7 million payments on the Tranche B-1 and B-2 Term Loan and $35.2 million in an OID paid to lenders and fees associated with our new Revolving Credit Facility in connection with the debt refinancing, partially offset by the receipt of $100 million from the issuance of convertible preferred stock. The OID on the Tranche B-2 Term Loan included $11.4 million, the amount of which is subject to change based on the timing and the amount of outstanding balance and was included in Item 8, "Financial Statement and Supplementary Data," as a non-cash financing activity within the "Supplemental Cash Flow Information" of the Consolidated Statements of Cash Flows.
For the year ended December 31, 2017, cash used in financing activities was $168.1 million, primarily consisting of net payments of $127.0 million under our Revolving Credit Facility. In addition, based on the results of the year ended December 31, 2016, our Consolidated Net Senior Secured Leverage Ratio required us to make an excess cash flow payment on our outstanding Term Loan Facility. On April 10, 2017, we made a payment of $39.7 million, of which $28.2 million was paid with borrowings from the Revolving Credit Facility and $11.5 million was paid with cash on hand.
Indebtedness
Senior Credit Facility. On February 28, 2018, General Nutrition Centers, Inc. ("Centers") amended and restated (the “Amendment”) its Senior Credit Facility (the "Term Loan Agreement"), which at the time consisted of a $1,131.2 million term loan facility due in March 2019 and a $225.0 million revolving credit facility that was scheduled to mature in September 2018. The Amendment included an extension of the maturity date for $704.3 million of the $1,131.2 million term loan facility from March 2019 to March 2021 (the “Tranche B-2 Term Loan"). In the event that all outstanding amounts under the Notes in excess of $50.0 million have not been repaid, refinanced, converted or effectively discharged prior to the Springing Maturity Date, the maturity date of the Tranche B-2 Term Loan becomes the Springing Maturity Date, subject to certain adjustments. The Amendment also terminated the prior $225.0 million revolving credit facility.
After the effectiveness of the Amendment, the remaining term loan of $151.9 million as of February 28, 2018 continued to have a maturity date of March 2019 (the "Tranche B-1 Term Loan"). The Tranche B-2 Term Loan requires annual aggregate principal payments of at least $43 million and bears interest at a rate of, at our option, LIBOR plus a margin of 8.75% per annum subject to change under certain circumstances (with a minimum and maximum margin of 8.25% and 9.25%, respectively, per annum), or prime plus a margin of 8.25% per annum subject to change under certain circumstances (with a minimum and maximum of 7.25% and 8.25%, respectively, per annum). Any mandatory repayments as defined in the credit agreement shall be applied to the remaining annual aggregate principle payments in direct order of maturity. In November 2018, we paid $100 million on the Tranche B-2 Term Loan and elected to use the payment to satisfy the scheduled amortization payments on the Term Loan Facility through December 2020. The interest rate under the Tranche B-1 Term Loan was at a rate of, at our option, LIBOR plus a margin of 2.5% or prime plus a margin of 1.5%. The Term Loan Agreement is secured by a (i) first lien on certain assets of the Company primarily consisting of capital stock issued by General Nutrition Centers, Inc. ("Centers") and its subsidiaries, intellectual property and equipment (“Term Priority Collateral”) and (ii) second lien on certain assets of the Company primarily consisting of inventory and accounts receivable (“ABL Priority Collateral”). The Term Loan Agreement is guaranteed by all material, wholly-owned domestic subsidiaries of the Company (the “U.S. Guarantors”) and by General Nutrition Centres Company, an unlimited liability company organized under the laws of Nova Scotia (together with the U.S. Guarantors, the “Guarantors”).
On February 28 2018, we also entered into a new asset-based credit agreement (the "ABL Credit Agreement"), consisting of:
•a new $100 million asset-based Revolving Credit Facility (the "Revolving Credit Facility") with a maturity date of August 2022 (which maturity date will become May 2020, subject to certain adjustments, should the Springing Maturity Date be triggered). In connection with the transfer of the Nutra manufacturing and Anderson facility net assets to the Manufacturing JV, the Revolving Credit Facility commitment was reduced from $100 million to $81 million effective March 2019; and
•a $275.0 million asset-based Term Loan Facility advanced on a “first-in, last-out” basis (the "FILO Term Loan") with a maturity date of December 2022 (which maturity date will become May 2020, subject to certain adjustments, should the Springing Maturity Date be triggered).
There are no scheduled amortization payments associated with the FILO Term Loan, which bears interest at a rate of LIBOR plus a margin of 7.00% per annum subject to decrease under certain circumstances (with a minimum possible interest rate
55
of LIBOR plus a margin of 6.50% per annum). Outstanding borrowings under the Revolving Credit Facility bear interest at a rate of LIBOR plus 1.50% or prime plus 0.50% (both subject to an increase of 0.25% to 0.50% based on the amount available to be drawn under the Revolving Credit Facility). The Company is also required to pay an annual fronting fee of 0.125% to the applicable Issuing Bank and a fee to revolving lenders equal to a maximum of 2.0% (subject to adjustment based on the amount available to be drawn under the Revolving Credit Facility with a minimum of 1.5%) on outstanding letters of credit and an annual commitment fee of 0.375% on the undrawn portion of the Revolving Credit Facility subject to an increase to 0.5% based on the amount available to draw under the Revolving Credit Facility. The FILO Term Loan and Revolving Credit Facility are secured by a (i) first lien on ABL Priority Collateral and (ii) second lien on Term Priority Collateral. The FILO Term Loan and Revolving Credit Facility are guaranteed by the Guarantors.
As of December 31, 2019, our contractual interest rates under the Tranche B-2 Term Loan and the FILO Term Loan were 10.6% and 8.8%, respectively, which consist of LIBOR plus the applicable margin rate. At December 31, 2018, the Company's contractual interest rates under the Tranche B-1 Term Loan, Tranche B-2 Term Loan, and the FILO Term Loan were 5.7%, 11.8% and 9.5%, respectively. At December 31, 2019, the Company had $66.2 million available under the Revolving Credit Facility, after giving effect to $4.9 million utilized to secure letters of credit and a $9.9 million reduction to borrowing ability as a result of decrease in net collateral.
Convertible Senior Notes. On August 10, 2015, we issued $287.5 million principal amount of Notes. The Notes will mature on August 15, 2020, unless earlier repaid, discharged, refinanced or converted by the holders subject to restrictions through May 15, 2020. The Notes bear interest at a rate of 1.5% per annum.
On December 20, 2017, we exchanged in privately negotiated transactions $98.9 million in aggregate principal amount of the Notes for an aggregate of 14.6 million newly issued shares of the Company’s Class A common stock, which had a value of $71.7 million at the time of the exchange.
During the second quarter of 2019, the Company repurchased $29.5 million in aggregate principal amount of the Notes for $24.7 million in cash. The convertible debt repurchase resulted in a gain of $3.2 million, which included the unamortized conversion feature of $1.3 million and unamortized discount of $0.2 million. At December 31, 2019, we had $159.1 million of principal outstanding on the Notes.
Contractual Obligations
The following table summarizes our future minimum non-cancelable contractual obligations at December 31, 2019:
Payments due by period | |||||||||||||||||||
(in millions) | Total | 2020 | 2021-2022 | 2023-2024 | After 2024 | ||||||||||||||
Long-term debt obligations (1) | $ | 882.6 | $ | 185.0 | $ | 697.6 | $ | — | $ | — | |||||||||
Scheduled interest payments (2) | 140.4 | 78.5 | 61.9 | — | — | ||||||||||||||
Operating lease obligations (3) | 456.5 | 121.8 | 165.2 | 90.4 | 79.1 | ||||||||||||||
Purchase commitments (4) | 31.4 | 21.3 | 10.1 | — | — | ||||||||||||||
Total | $ | 1,510.9 | $ | 406.6 | $ | 934.8 | $ | 90.4 | $ | 79.1 | |||||||||
(1) | These balances consist of the following debt obligations: (a) $159.1 million of outstanding borrowings under the Notes including the unamortized conversion feature of $3.9 million and original issuance discount of $0.5 million due in August 2020; (d) $448.5 million of outstanding borrowing under the Tranche B-2 Term Loan, of which $25.9 million is due in the second quarter of 2020, including $7.0 million of unamortized original issuance discount due in March 2021; and (e) $275.0 million of outstanding borrowing under the FILO Term Loan including $8.2 million of unamortized original issuance discount due in December 2022, subject to certain adjustments, should the Springing Maturity Date be triggered ; |
(2) | The interest that will accrue on the long-term obligations includes variable rate payments, which are estimated using the associated LIBOR index as of December 31, 2019. Interest under the Term Loan Facility currently accrues based on a one-month LIBOR. Interest rate swaps accrue based on the fixed rates. |
(3) | Consists of the following contractual payments excluding optional renewals: (a) $547.1 million for company-owned retail and franchise stores; (b) $109.0 million of sublease income from franchisees; and (c) $18.4 million relating to various leases for warehouses, vehicles, and various equipment at our facility. Operating lease obligations exclude insurance, taxes, maintenance, percentage rent and other costs. These amounts are subject to fluctuation from year to year and collectively represented 39%, 36% and 35% of the aggregate costs associated with our company-owned retail store operating leases for years ended December 31, 2019, 2018 and 2017, respectively. |
(4) | These balances represent amounts owed under advertising, technology-related and inventory purchase agreements. |
56
COVID-19
The recent outbreak of the coronavirus, or COVID-19, has caused business disruption in our International segment beginning in January 2020. In late February 2020, the situation escalated as the scope of COVID-19 worsened to outside of the Asia-Pacific region, with Europe and the United States being impacted by outbreaks of COVID-19. As of March 23, 2020, we have temporarily closed approximately 25% of our U.S. and Canada company-owned and franchise stores as a result of the COVID-19 pandemic. There is significant uncertainty relating to the potential impacts of COVID-19 on the Company’s business going forward due to various global macroeconomic, operational and supply chain risks as a result of COVID-19. See “Risk Factors - Risks Relating to Our Business and Industry - Recent developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19.”
We have taken measures to protect our associates, customers and business partners by conforming to local government and global health organizations guidance and have implemented global travel restrictions. We are monitoring the impacts COVID-19 has had, and continues to have, on our supply chain and are collaborating with our third-party partners to mitigate significant delays in delivery of our products.
Impact of Inflation
Refer to Item 7A. "Quantitative and Qualitative Disclosures About Market Risk" for more information.
Off Balance Sheet Arrangements
We have not created, and are not a party to, any off-balance sheet entities for the purpose of raising capital, incurring debt or operating our business. We do not have any off-balance sheet arrangements or relationships with entities that are not consolidated into our financial statements that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Estimates
Use of Estimates
Certain amounts in our audited Consolidated Financial Statements require the use of estimates, judgments, and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the periods presented. Our accounting policies are described in Note 2, "Basis of Presentation and Summary of Significant Accounting Policies" to our audited Consolidated Financial Statements included in Item 8, "Financial Statements and Supplementary Data." Our critical accounting policies and estimates are described in this section. An accounting estimate is considered critical if:
•the estimate requires us to make assumptions about matters that were uncertain at the time the estimate was made;
•different estimates reasonably could have been used; or
•changes in the estimate that would have a material impact on our financial condition or our results of operations are likely to occur from period to period.
We believe that the accounting estimates used are appropriate and the resulting balances are reasonable. However, actual results could differ from the original estimates, requiring adjustments to these balances in future periods.
Inventory
Prior to the formation of the Manufacturing JV, our inventory components consisted of raw materials, work-in-process, packaging supplies and finished product. Inventory included costs associated with distribution and transportation costs, as well as manufacturing overhead, which were capitalized and expensed as merchandise is sold. After the transfer of the manufacturing facility to the Manufacturing JV, inventory only consists of finished product. Inventory is recorded on a first in/first out basis ("FIFO") at the lower of cost or net realizable value, net of obsolescence, shrinkage and vendor allowances. We regularly review our inventory levels in order to identify slow moving and short dated products, using factors such as amount of inventory on hand, the remaining shelf life, current and expected market conditions, historical trends and the likelihood of recovering the inventory costs based on anticipated demand.
Impairment of Definite-Long-Lived Assets
Property, plant and equipment and definite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset group may not be recoverable. If the sum of the undiscounted future cash flows is less than the carrying value of the asset group, we recognize an impairment loss, measured as the amount by which the carrying value exceeds the fair value of the long-lived asset. Estimates of cash flows require significant judgment and certain assumptions about future volume, revenue and expense growth rates and asset disposal values. While we make estimates based on historical experience, current expectations and assumptions that we believe are reasonable, actual results, including future cash
57
flows, could differ from our estimates resulting in required impairment charges. During the year ended December 31, 2019, no impairment of definite-long-lived assets was recognized. In 2018, we recorded $14.6 million of definite-long-lived asset impairment charges of which $9.5 million related to property, plant and equipment for certain underperforming stores and the remaining amount related to other store closing costs. Refer to Item 8 "Financial Statements and Supplementary Data," Note 7 "Property, Plant and Equipment, Net" for more information.
Goodwill and Indefinite-Lived Intangible Assets
As described in Item 8 "Financial Statements and Supplementary Data," Note 6 "Goodwill and Intangible Assets," we performed our annual impairment test of our goodwill in the fourth quarter of 2019 and concluded all of our reporting units' fair values were significantly in excess of their respective carrying values. We also performed the annual impairment test of our indefinite-lived brand name intangible asset and concluded that the fair value was in excess of the carrying value.
The fair value of the indefinite-lived brand intangible asset was estimated using a relief from royalty method (an income approach). Key assumptions included in the determination of the estimated fair value include projected future revenues, the royalty rate and the discount rate. The following table represents a sensitivity analysis on the indefinite-lived brand intangible asset depicting the percentage excess (deficit) of fair value compared with carrying value with a 1.0% increase in the discount rate used or a 0.5% decrease in the royalty rate used at December 31, 2019.
Assumption Change | % Excess (Deficit) | |
1.0% increase in discount rate | 8.1% | |
0.5% decrease in royalty rate | (7.6)% | |
For the goodwill impairment test, we determined the fair values of our reporting units using a discounted cash flow method (income approach) weighted 50% and a guideline company method (market approach) weighted 50%. The key assumptions under the income approach include future cash flow assumptions and the discount rate. The guideline company method involves analyzing transaction and financial data of publicly-traded companies to develop multiples, which are adjusted to account for differences in growth prospects and risk profiles of the reporting unit and the comparable entities. Based on the results of the impairment test, our reporting units with goodwill would all require a 50% or greater reduction in their respective fair values at December 31, 2019 to result in any goodwill impairment.
Although we believe we have used reasonable estimates and assumptions to calculate the fair values of the indefinite-lived brand intangible asset and the reporting units with goodwill balances, these estimates and assumptions could be materially different from actual results. If actual market conditions are less favorable than those projected, or if certain events occur, such as the potential negative impacts of COVID-19 discussed further in Item 8, "Financial Statements and Supplementary Data," Note 21, "Subsequent Events," or circumstances change, such as a significant decline in our market capitalization, that would reduce the fair values below the respective carrying values, we may be required to conduct an interim test and possibly recognize impairment charges, which may be material, in future periods. Certain events or circumstances that could reasonably be expected to negatively affect the underlying assumptions and ultimately impact the estimated fair values of our reporting units may include such items as: (i) a decrease in expected future cash flows, specifically a further decrease in same store sales or other adverse impacts on sales trends (ii) inability to successfully execute on, or realize the expected benefit from, the development of our omni-channel strategy and other strategic initiatives and (iii) inability to achieve the sales from our International growth initiatives.
Leases
We determine if a contract contains a lease at inception. The lease liabilities are recognized based on the present value of the future minimum lease payments over the term at the commencement date for leases exceeding 12 months. The lease agreements generally contain lease and non-lease components. Non-lease components primarily include payments for maintenance and utilities. The minimum lease payments include only fixed lease components, as well as any variable rate payments that depend on an index, initially measured using the index at the lease commencement date. Lease terms may include options to renew when it is reasonably certain that we will exercise an option. We estimate its incremental borrowing rate, which approximates the interest rate on a collateralized basis with similar terms and payments for each lease, using a portfolio approach. The right-of-use assets recognized are initially equal to the lease liability, adjusted for any lease payments made on or before the commencement dates and lease incentives.
The lease liabilities for the operating leases are amortized using the effective interest method. The right-of-use asset is amortized by taking the difference between total rent expense recorded on straight-line basis and the lease liability amortization. When the right-of-use asset for an operating lease is impaired, lease expense is no longer recognized on a straight-line basis. For
58
impaired leases, we continues to amortize the lease liability using the same effective interest method as before the impairment charge and the right-of-use asset is amortized on a straight-line basis.
Self-Insurance
We are self-insured for certain losses related to workers' compensation and general liability insurance and we maintain stop-loss coverage with third-party insurers to limit our liability exposure. Liabilities associated with these losses are estimated by considering historical claims experience, estimated lag time to report and pay claims, average cost per claim and other actuarial factors.
Income Taxes
We compute our annual tax rate based on the statutory tax rates and tax planning opportunities available to us in the various jurisdictions in which we earn income. Significant judgment is required in determining our annual tax rate and in evaluating uncertainty in our tax positions. We recognize a benefit for tax positions that we believe will more likely than not be sustained upon examination. The amount of benefit recognized is the largest amount of benefit that we believe has more than a 50% probability of being realized upon settlement. We regularly monitor our tax positions and adjust the amount of recognized tax benefit based on our evaluation of information that has become available since the end of our last financial reporting period.
We record valuation allowances to reduce deferred tax assets to the amount that is more likely than not to be realized. In assessing the need for a valuation allowance, the Company considers all available positive and negative evidence, including the Company’s operating results, reversals of deferred tax liabilities, and forecasts of future taxable income on a jurisdiction-by-jurisdiction basis. Should a change in circumstances lead to a change in judgment about the realizability of deferred tax assets in future years, we would adjust related valuation allowances in the period that the change in circumstances occurs. During the fourth quarter of the year ended December 31,2019, as further discussed in Item 8, "Financial Statements and Supplementary Data," Note 1, "Nature of Business", management concluded that there is substantial doubt regarding the Company’s ability to continue as a going concern. Management considered this in concluding that certain deferred tax assets were no longer more likely than not realizable. As a result, an increase in valuation allowance of $27.1 million on the Company’s deferred tax assets was recorded as of December 31, 2019. As of December 31, 2019 and 2018, we had a valuation allowance of $42.3 million and $20.0 million, respectively.
Recently Issued Accounting Pronouncements
Refer to Item 8, "Financial Statements and Supplementary Data," Note 2, "Basis of Presentation and Summary of Significant Accounting Policies."
59
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Market risk represents the risk of changes in the value of market risk sensitive instruments caused by fluctuations in interest rates, foreign exchange rates and commodity prices. Changes in these factors could cause fluctuations in the results of our operations and cash flows. In the ordinary course of business, we are primarily exposed to foreign currency, interest rate and fuel and certain other commodity risks.
Interest Rate Market Risk
The Term Loan Facility and the asset-based Revolving Credit Facility are subject to changing interest rates. Although changes in interest rates do not impact our operating income, the changes could affect the fair value of such debt and related interest payments. An increase of 1% on our variable rate debt balance at December 31, 2019 would result in an increase to interest expense, net of $8.8 million for the year ended December 31, 2019. As part of our strategy to limit exposure to interest rate risk, we entered into two interest rate swaps in the second quarter of 2018 with notional amount of $275 million and $225 million, which decreased to $175 million on June 30, 2019 as scheduled, to convert the variable interest rate to a fixed interest rate. Refer to Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations - Liquidity" and Item 8, "Financial Statement and Supplementary Data," Note 8, "Long-Term Debt/ Interest Expense" for more information. A decrease in the current interest rates would have no impact on interest expense due to an interest rate floor that exists under the Term Loan Facility.
Foreign Currency Exchange Rate Market Risk
We are subject to the risk of foreign currency exchange rate changes in the conversion from local currencies to the U.S. dollar of the reported financial position and operating results of our non-U.S. based subsidiaries. The primary currencies to which we are exposed to fluctuations are the Canadian Dollar, the Chinese Renminbi, Hong Kong Dollar, the British Pound, and the Euro. The impact of foreign exchange rate changes to our revenue in 2019 compared with 2018 was approximately $2.7 million. In addition, since our international franchisees pay for product sales and royalties to us in the U.S. dollar, any strengthening of the U.S. dollar relative to our franchisees' local currency could adversely impact our revenue.
We have intercompany balances with foreign entities that are routinely settled primarily relating to product sales and management fees. Gains or losses resulting from these foreign currency transactions were not material for the fiscal years ended December 31, 2019, 2018 and 2017.
Fuel Price Market Risk
We rely on our ability to replenish depleted inventory through deliveries to our distribution centers and stores by various means of transportation, including shipments by sea and truck. We are exposed to fluctuations in fuel prices in these arrangements which may have a favorable or unfavorable impact to our Consolidated Financial Statements.
Impact of Inflation
Inflationary factors such as increases in the cost of our products and overhead costs may adversely affect our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of margins and SG&A expenses as a percentage of revenue if the selling prices of our products do not increase with inflation.
60
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
TABLE OF CONTENTS
Page | |
61
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of GNC Holdings, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of GNC Holdings, Inc. and its subsidiaries (the “Company”) as of December 31, 2019 and 2018, and the related consolidated statements of operations, comprehensive (loss) income, stockholders’ (deficit) equity and cash flows for each of the three years in the period ended December 31, 2019, including the related notes and financial statement schedules of (i) condensed financial information of GNC Holdings, Inc. as of December 31, 2019 and 2018 and for each of the three years in the period ended December 31, 2019, and (ii) valuation and qualifying accounts for each of the three years in the period ended December 31, 2019 appearing under Item 15(a)(2) (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Substantial Doubt About the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has significant debt (specifically the Convertible Notes and the Tranche B-2 Term Loan) maturing at the latest in March 2021. The Company has insufficient cash flows from operations to repay these debt obligations as they come due, which raises substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for leases in 2019.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness
62
exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Pittsburgh, Pennsylvania
March 25, 2020
We have served as the Company’s auditor since 2003.
63
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
December 31, | |||||||
2019 | 2018 | ||||||
Current assets: | (in thousands) | ||||||
Cash and cash equivalents | $ | $ | |||||
Receivables, net | |||||||
Receivables due from related parties (Note 9) | |||||||
Inventory (Note 4) | |||||||
Forward contracts for the issuance of convertible preferred stock (Note 14) | |||||||
Prepaid and other current assets | |||||||
Total current assets | |||||||
Long-term assets: | |||||||
Goodwill (Note 6) | |||||||
Brand name (Note 6) | |||||||
Other intangible assets, net (Note 6) | |||||||
Property, plant and equipment, net (Note 7) | |||||||
Right-of-use assets (Note 12) | — | ||||||
Equity method investments (Note 9) | |||||||
Deferred income taxes (Note 5) | |||||||
Other long-term assets | |||||||
Total long-term assets | |||||||
Total assets | $ | $ | |||||
Current liabilities: | |||||||
Accounts payable | $ | $ | |||||
Accounts payable due to related parties (Note 9) | |||||||
Current portion of long-term debt (Note 8) | |||||||
Current lease liabilities (Note 12) | — | ||||||
Deferred revenue and other current liabilities (Note 10) | |||||||
Total current liabilities | |||||||
Long-term liabilities: | |||||||
Long-term debt (Note 8) | |||||||
Deferred income taxes (Note 5) | |||||||
Lease liabilities (Note 12) | — | ||||||
Other long-term liabilities | |||||||
Total long-term liabilities | |||||||
Total liabilities | |||||||
Commitments and contingencies (Note 13) | |||||||
Mezzanine equity: | |||||||
Preferred stock, $0.001 par value, 60,000 shares authorized: | |||||||
Series A convertible preferred stock - 300 shares issued and outstanding at December 31, 2019 and 100 shares issued and outstanding at December 31, 2018. (Note 14) | |||||||
Stockholders' deficit: | |||||||
Common stock, $0.001 par value, 300,000 shares authorized: | |||||||
Class A, 130,555 shares issued, 84,564 shares outstanding and 45,991 shares held in treasury at December 31, 2019 and 129,925 shares issued, 83,886 shares outstanding and 45,991 shares held in treasury at December 31, 2018 | |||||||
Additional paid-in capital | |||||||
Retained earnings | |||||||
Treasury stock, at cost (Note 15) | ( | ) | ( | ) | |||
Accumulated other comprehensive loss | ( | ) | ( | ) | |||
Total stockholders' deficit | ( | ) | ( | ) | |||
Total liabilities, mezzanine equity and stockholders' deficit | $ | $ | |||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
64
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands, except per share amounts) | |||||||||||
Revenue (Note 3) | $ | $ | $ | ||||||||
Cost of sales, including warehousing, distribution and occupancy | |||||||||||
Gross profit | |||||||||||
Selling, general, and administrative | |||||||||||
Long-lived asset impairments (Note 6) | |||||||||||
Loss on net asset exchange for the formation of the joint ventures | |||||||||||
Other loss (income), net | ( | ) | |||||||||
Operating income (loss) | ( | ) | |||||||||
Interest expense, net (Note 8) | |||||||||||
Gain on convertible debt and debt refinancing costs (Note 8) | ( | ) | ( | ) | |||||||
Loss on debt refinancing | |||||||||||
Loss (gain) on forward contracts for the issuance of convertible preferred stock (Note 14) | ( | ) | |||||||||
Income (loss) before income taxes and income from equity method investments | ( | ) | |||||||||
Income tax expense (benefit) (Note 5) | ( | ) | ( | ) | |||||||
Net (loss) income before income from equity method investments | $ | ( | ) | $ | $ | ( | ) | ||||
Income from equity method investments | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
(Loss) earnings per share (Note 16): | |||||||||||
Basic | $ | ( | ) | $ | $ | ( | ) | ||||
Diluted | $ | ( | ) | $ | $ | ( | ) | ||||
Weighted average common shares outstanding (Note 16): | |||||||||||
Basic | |||||||||||
Diluted | |||||||||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
65
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Comprehensive (Loss) Income
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
Other comprehensive (loss) income: | |||||||||||
Net change in interest rate swaps: | |||||||||||
Periodic revaluation of interest rate swap, net of tax benefit of $2.0 million and $1.5 million, respectively | ( | ) | ( | ) | |||||||
Reclassification adjustment for interest recognized in the Consolidated Statement of Operations, net of tax expense of $0.8 million and $0.5 million, respectively | |||||||||||
Net change in unrecognized loss on interest rate swaps, net of tax | ( | ) | ( | ) | |||||||
Foreign currency translation gain (loss) | ( | ) | |||||||||
Other comprehensive (loss) income | ( | ) | ( | ) | |||||||
Comprehensive (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
66
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders' (Deficit) Equity
(in thousands, except per share amounts)
Common Stock | |||||||||||||||||||||||||||
Class A | Additional Paid-In Capital | Retained Earnings | Accumulated Other Comprehensive (Loss) Income | Total Stockholders' (Deficit) Equity | |||||||||||||||||||||||
Shares | Dollars | Treasury Stock | |||||||||||||||||||||||||
Balance at December 31, 2016 | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||
Comprehensive (loss) income | — | — | — | — | ( | ) | ( | ) | |||||||||||||||||||
Dividends forfeitures on restricted stock | — | — | — | — | — | — | |||||||||||||||||||||
Restricted stock awards | — | — | — | — | |||||||||||||||||||||||
Minimum tax withholding requirements | ( | ) | — | — | ( | ) | — | — | ( | ) | |||||||||||||||||
Stock-based compensation | — | — | — | — | — | ||||||||||||||||||||||
Exchange of convertible senior notes (Note 8) | — | — | — | ||||||||||||||||||||||||
Balance at December 31, 2017 | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||||
Comprehensive income (loss) | — | — | — | — | ( | ) | |||||||||||||||||||||
Dividends forfeitures on restricted stock | — | — | — | — | — | ||||||||||||||||||||||
Restricted stock awards | — | — | — | — | |||||||||||||||||||||||
Minimum tax withholding requirements | ( | ) | — | — | ( | ) | — | — | ( | ) | |||||||||||||||||
Stock-based compensation | — | — | — | — | — | ||||||||||||||||||||||
Balance at December 31, 2018 | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||||
Impact of the adoption of ASC 842 | — | — | — | — | ( | ) | — | ( | ) | ||||||||||||||||||
Comprehensive (loss) income | — | — | — | — | ( | ) | ( | ) | ( | ) | |||||||||||||||||
Dividend forfeitures on restricted stock | — | — | — | — | — | ||||||||||||||||||||||
Restricted stock awards | — | ( | ) | — | — | ||||||||||||||||||||||
Minimum tax withholding requirements | ( | ) | — | — | ( | ) | — | — | ( | ) | |||||||||||||||||
Stock-based compensation | — | — | — | — | — | ||||||||||||||||||||||
Repurchase of convertible senior notes | — | — | — | ( | ) | — | — | ( | ) | ||||||||||||||||||
Balance at December 31, 2019 | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
67
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Cash flows from operating activities: | (in thousands) | ||||||||||
Net (loss) income | ( | ) | $ | $ | ( | ) | |||||
Adjustments to reconcile net (loss) income to net cash provided by operating activities: | |||||||||||
Depreciation and amortization expense | |||||||||||
Income from equity method investments | ( | ) | |||||||||
Amortization of debt costs | |||||||||||
Stock-based compensation | |||||||||||
Long-lived asset impairments | |||||||||||
Gain on convertible debt and debt refinancing costs | ( | ) | ( | ) | |||||||
Loss on debt refinancing | |||||||||||
Loss on net asset exchange for the formation of the joint ventures | |||||||||||
Loss (gain) on forward contracts for the issuance of convertible preferred stock | ( | ) | |||||||||
Third-party fees associated with refinancing | ( | ) | |||||||||
Distributions received from equity method investments | |||||||||||
Deferred income tax expense (benefit) | ( | ) | ( | ) | |||||||
Other | ( | ) | ( | ) | |||||||
Changes in assets and liabilities: | |||||||||||
Increase in receivables | ( | ) | ( | ) | ( | ) | |||||
Decrease in inventory | |||||||||||
(Increase) decrease in prepaid and other current assets | ( | ) | ( | ) | |||||||
Increase (decrease) in accounts payable | ( | ) | ( | ) | |||||||
(Decrease) increase in deferred revenue and accrued liabilities | ( | ) | |||||||||
Decrease in net lease liabilities | ( | ) | — | — | |||||||
Other changes in assets and liabilities | ( | ) | ( | ) | |||||||
Net cash provided by operating activities | |||||||||||
Cash flows from investing activities: | |||||||||||
Capital expenditures | ( | ) | ( | ) | ( | ) | |||||
Refranchising proceeds, net of store acquisition costs | |||||||||||
Capital contribution to the joint ventures | ( | ) | |||||||||
Proceeds from the assets exchange for the formation of the joint ventures | |||||||||||
Proceeds from the sale of Lucky Vitamin | |||||||||||
Net cash provided by (used in) investing activities | ( | ) | ( | ) | |||||||
Cash flows from financing activities: | |||||||||||
Borrowings under Revolving Credit Facility | |||||||||||
Payments on Revolving Credit Facility | ( | ) | ( | ) | ( | ) | |||||
Proceeds from the issuance of convertible preferred stock | |||||||||||
Payments on Tranche B-1 Term Loan | ( | ) | ( | ) | ( | ) | |||||
Payments on Tranche B-2 Term Loan | ( | ) | ( | ) | |||||||
Convertible notes repurchase | ( | ) | |||||||||
Original issuance discount and revolving credit facility fees | ( | ) | ( | ) | |||||||
Fees associated with the issuance of convertible preferred stock | ( | ) | ( | ) | |||||||
Minimum tax withholding requirements | ( | ) | ( | ) | ( | ) | |||||
Net cash used in financing activities | ( | ) | ( | ) | ( | ) | |||||
Effect of exchange rate changes on cash and cash equivalents | ( | ) | ( | ) | |||||||
Net increase in cash and cash equivalents | |||||||||||
Beginning balance, cash and cash equivalents | |||||||||||
Ending balance, cash and cash equivalents | $ | $ | $ | ||||||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
68
GNC HOLDINGS, INC. AND SUBSIDIARIES
Supplemental Cash Flow Information
(in thousands)
Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Cash (received) paid during the period for: | (in thousands) | ||||||||||
Income taxes * | $ | $ | ( | ) | $ | ||||||
Interest | |||||||||||
* Includes a $12.4 million tax refund received in the fourth quarter of 2018.
As of December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Non-cash investing activities: | (in thousands) | ||||||||||
Capital expenditures in current liabilities | $ | $ | $ | ||||||||
Net assets contributed to the joint ventures (Note 9) | |||||||||||
Non-cash financing activities: | |||||||||||
Issuance of shares associated with exchange of convertible senior notes | |||||||||||
Original issuance discount (Note 8) | |||||||||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
69
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. NATURE OF BUSINESS
GNC Holdings, Inc., a Delaware corporation (“Holdings,” and collectively with its subsidiaries and, unless the context requires otherwise, its and their respective predecessors, the “Company”), is a global health and wellness brand with a diversified, omni-channel business. The Company's assortment of performance and nutritional supplements, vitamins, herbs and greens, health and beauty, food and drink and other general merchandise features innovative private-label products as well as nationally recognized third-party brands, many of which are exclusive to GNC.
The Company's operations consist of purchasing raw materials, formulating and manufacturing products prior to the formation of the manufacturing joint venture with IVC (the "Manufacturing JV") in March 2019, and selling the finished products through its three reportable segments, U.S. and Canada, International, and Manufacturing / Wholesale (refer to Note 19, "Segments" for more information). Corporate retail store operations are located in the United States, Canada, Puerto Rico, Ireland and prior to the joint venture transaction with Harbin Pharmaceutical Group Co., Ltd ("Harbin") in February 2019, China. In addition, the Company offers products on the internet through GNC.com and third-party websites. Franchise locations exist in the United States and approximately 50 other countries. Additionally, the Company licenses the use of its trademarks and trade names.
In February 2019, the Company entered into two joint ventures with Harbin to operate its e-commerce business (the "HK JV") and retail business in China (the "China JV"), which will accelerate its presence and maximize the Company's opportunities for growth in the Chinese supplement market. Under the terms of the agreement, the Company contributed its China business and retained 35 % equity interest in the HK JV and China JV.
In March 2019, the Company entered into a strategic joint venture with International Vitamin Corporation ("IVC") regarding the Company's manufacturing business, which enables the Company to increase its focus on product innovation while IVC manages manufacturing and integrates with the Company's supply chain thereby driving more efficient usage of capital. Under the terms of the agreement, the Company received $99.2 million, net of a working capital adjustment, and contributed its Nutra manufacturing and Anderson facility net assets in exchange for an initial 43 % equity interest in the Manufacturing JV. IVC is expected to pay an additional $75.0 million over a four year period from the effective date of the transaction as IVC’s ownership of the joint venture increases to 100 %. The subsequent purchase price for each year is $18.8 million, adjusted up or down based on the Manufacturing JV's future performance.
Going Concern
The Company has continued to experience negative same store sales and declining gross profit. The Company has closed underperforming stores under its store optimization strategy and implemented cost reduction measures to help mitigate the effect of these declines and improve its financial position and liquidity. At December 31, 2019, the Company has substantial indebtedness including $154.7 million of outstanding indebtedness under the Notes issued under that certain Indenture dated as of August 10, 2015, among the Company, certain of its subsidiaries, and The Bank of New York Mellon Trust Company, N.A, maturing on August 15, 2020 (the "Notes") and $441.5 million of outstanding indebtedness under the Amended and Restated Term Loan Credit Agreement, dated as of February 28, 2018, among GNC Corporation, GNC Nutrition Centers, Inc., as Borrower, the lenders and agents parties thereto, and JPMorgan Chase Bank, N.A., as administrative agent (the “ Tranche B-2 Term Loan Credit Agreement" and the term loan thereunder, the "Tranche B-2 Term Loan"). The Company also has an excess cash flow payment of $25.9 million due in April 2020 (which will reduce the outstanding amount of the Tranche B-2 Term Loan). The Tranche B-2 Term Loan becomes due on the earlier to occur of (i) the maturity date of March 4, 2021 or (ii) May 16, 2020 if more than $50 million of the Notes are outstanding on such date. Each of the revolving credit facility (the "Revolving Credit Facility") under the Credit Agreement, dated as of February 28, 2018, among GNC Corporation, GNC Nutritional Centers, Inc., as Administrative Borrower, certain of its subsidiaries, as subsidiary borrowers, the lenders and agents parties thereto, and JPMorgan Chase Bank, N.A., as administrative agent (the “ABL Credit Agreement”) and the FILO term loan facility under the ABL Credit Agreement, which otherwise mature in August 2022 and December 2022 respectively, also include an accelerated maturity date of May 16, 2020 if more than $50 million of the Notes are outstanding on such date.
Prior to the outbreak of the COVID-19 pandemic in the United States, management believed that the Company had the ability to pay the excess cash flow payment of $25.9 million and reduce the outstanding balance on the Notes from $154.7 million to below $50 million with projected cash on hand and new borrowings under the Revolving Credit Facility, assuming such borrowings remain available subject to the covenant and reporting requirements discussed below. Given current circumstances around the COVID-19 pandemic as discussed further in Note 21, "Subsequent Events", there can be no assurances as to our ability
70
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
to do so. As a precautionary measure, given the current macro environment, we recently drew $30 million under our Revolving Credit Facility in March 2020. However, management does not expect to have sufficient cash flows from operations to repay the indebtedness under the Notes or the Tranche B-2 Term Loan when they become due. Since the Company has not refinanced the Tranche B-2 Term Loan and it will mature less than twelve months after the issuance date of these consolidated financial statements, management has concluded there is substantial doubt regarding the Company's ability to continue as a going concern within one year from the issuance date of the Company’s consolidated financial statements.
The Company was in compliance with the debt covenant reporting and compliance obligations under the Credit Facilities as of December 31, 2019. Prior to the outbreak of the COVID-19 pandemic in the United States, management believed that the Company had the ability to comply with the financial covenants under the Senior Credit Facility Agreements over the next twelve months; however, given the current circumstances around the COVID-19 pandemic as discussed further in Note 21, "Subsequent Events", there can be no assurances as to our ability to do so.
The Company is in the process of reviewing a range of refinancing options to refinance all of the Company’s outstanding indebtedness. The Company has been working with an independent committee of the Board supported by independent financial and legal advisors to conduct its review and has had a series of discussions with financing sources in the United States and Asia. We became aware on March 24, 2020, by the potential financing sources in Asia, that they are no longer pursuing a refinancing with us. We will continue to explore all options to refinance and restructure our indebtedness. While we continue to work through a number of refinancing alternatives to address our upcoming debt maturities, we cannot make any assurances regarding the likelihood, certainty or exact timing of any alternatives.
Reporting requirements under both the Tranche B-2 Term Loan and the Credit Agreement, dated as of February 28, 2018, among GNC Corporation, GNC Nutrition Centers, Inc., as Administrative Borrower, certain of its subsidiaries, as subsidiary borrowers, the lenders and agents parties thereto, and JPMorgan Chase Bank, N.A., as administrative agent (the “ABL Credit Agreement,” together with the Tranche B-2 Term Loan, the “Senior Credit Agreements”) require the Company to provide annual audited financial statements accompanied by an opinion of an independent public accountant without a "going concern" or like qualification or exception, or qualification arising out of the scope of the audit (other than a “going concern” statement, explanatory note or like qualification or exception resulting solely from an upcoming maturity date under the Tranche B-2 Term Loan or the Notes). Management believes the Company will satisfy this requirement. If the lenders take a contrary position, (a) they could decide to instruct the administrative agent under the Senior Credit Agreements to deliver a written notice thereof to the borrower, and if the alleged default continued uncured for 30 days thereafter it would become an alleged event of default (unless waived by the lenders) and (b) the Company intends to contest such position and any action the lenders may attempt to take as a result thereof. If the lenders were to prevail in any such dispute, the required lenders could instruct the administrative agent to exercise remedies under the Senior Credit Agreements (the "Revolving Credit Facility"), including accelerating the maturity of the loans, terminating commitments under the revolving credit facility under the ABL Credit Agreement and requiring the posting of cash collateral in respect of outstanding letters of credit issued under the Revolving Credit Facility ($4.9 million at December 31, 2019). If this were to occur, management would enter into discussions with the lenders to waive the default or forebear from the exercise of remedies. Failure to obtain such a waiver, complete the refinancing or other restructuring prior to August 2020 or to reach an agreement with the Company's stakeholders on the terms of a restructuring would have a material adverse effect on the liquidity, financial condition and results of operations and may result in filing a voluntary petition for relief under Chapter 11 of the United States Bankruptcy Code in order to implement a restructuring plan.
NOTE 2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying Consolidated Financial Statements and Footnotes have been prepared by the Company in accordance with accounting principles generally accepted in the United States of America ("GAAP") and Regulation S-X. The Company's annual reporting period is based on a calendar year.
Summary of Significant Accounting Policies
71
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Use of Estimates. The preparation of financial statements in conformity with GAAP requires management to make estimates that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases these estimates on assumptions that it believes to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
Cash and Cash Equivalents. The Company considers cash and cash equivalents to include all cash and liquid deposits and investments with an original maturity of three months or less. Payments due from banks for third-party credit and debit cards generally process within 24 to 72 hours, and are classified as cash equivalents.
Receivables, net. The Company extends credit terms for sales of product to its franchisees and wholesale partners. Receivables consist principally of unpaid invoices for product sales, franchisee royalties and sublease payments. Franchisees secure financing from lending institutions, which include but are not limited to the small business administration and national banks with franchise programs. These loans generally require the Company to subordinate its first lien position on inventory and furniture and fixtures at predetermined amounts.
Inventory. Prior to the formation of the Manufacturing JV, inventory components consisted of raw materials, work-in-process, packaging supplies and finished product. Inventory included costs associated with distribution and transportation, as well as manufacturing overhead, which were capitalized and expensed as merchandise is sold. After the transfer of the manufacturing facility to the Manufacturing JV, inventory only consists of finished product. Inventories are stated at the lower of cost or net realizable value on a first in/first out basis ("FIFO"). Inventory is recorded net of obsolescence, shrinkage and vendor allowances for product costs. The Company regularly reviews its inventory levels in order to identify slow moving and short dated products, using factors such as amount of inventory on hand, remaining shelf life, current and expected market conditions, historical trends and the likelihood of recovering the inventory costs based on anticipated demand.
Property, Plant and Equipment. Property, plant and equipment expenditures are recorded at cost. Depreciation and amortization are recognized using the straight-line method over the estimated useful life of the assets. The estimated useful lives are as follows:
Building | 30 yrs |
Machinery and equipment | 3-7 yrs |
Building and leasehold improvements | 3-15 yrs |
Furniture and fixtures | 5-8 yrs |
Software | 3-5 yrs |
Building improvements are depreciated over their estimated useful life or the remaining useful life of the related building, whichever period is shorter. Improvements to leased premises are depreciated over the estimated useful life of the improvements or the related leases including renewals that are reasonably assured, whichever period is shorter. Expenditures that materially increase the value or clearly extend the useful life of property, plant and equipment are capitalized while repair and maintenance costs incurred in the normal course of operations are expensed as incurred.
Goodwill and Indefinite-Lived Intangible Asset. The Company was acquired by Ares Corporate Opportunities Fund II L.P. and Ontario Teachers’ Pension Plan Board in March 2007 and subsequently completed an initial public offering in 2011 of its common stock. In connection with this acquisition, the Company recorded approximately $600 million of goodwill and a $720 million indefinite-lived intangible asset related to its brand name.
72
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Goodwill is allocated to the Company's reporting units, which are at or below the level of an operating segment as defined by Accounting Standards Codification ("ASC") 280 "Segment Reporting." The Company evaluates the carrying amount of goodwill for each of its reporting units annually in the fourth quarter. In addition, the Company performs an evaluation on an interim basis if it determines that recent events or prevailing conditions indicate a potential impairment of goodwill. A significant amount of judgment is involved in determining whether an indicator of impairment has occurred between annual impairment tests. These indicators include, but are not limited to, overall financial performance such as adverse changes in recent forecasts of operating results, industry and market considerations, a sustained decrease in the share price of the Company's common stock, updated business plans and regulatory and legal developments.
When the carrying value of a reporting unit exceeds its fair value, an impairment charge is recorded for the difference as an operating expense in the period incurred. For the year ended December 31, 2019 and 2018, no goodwill impairment was recorded. For the year ended December 31, 2017, the Company recorded a goodwill impairment charge of $24.3 million related to the Wholesale reporting unit as a result of a triggering event based on a decline in the Company's share price and previous challenges associated with the Company's efforts to refinance its long-term debt.
Revenue Recognition. Within the U.S. and Canada segment, retail sales in company-owned stores are recognized at the point of sale, net of sales tax. Revenue related to e-commerce sales is recognized upon shipment based on meeting the transfer of control criteria. The Company has made a policy election to treat shipping and handling as costs to fulfill the contract, and as a result, any fees from customers are included in the transaction price allocated to the performance obligation of providing goods with a corresponding amount accrued within cost of sales for amounts paid to applicable carriers. Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from revenue. A provision for anticipated returns is recorded through a reduction of sales and cost of sales (for product that can be resold or returned to vendors) in the period that the related sales are recorded.
Revenue was deferred on sales of the Company's Gold Cards and subsequently recognized over the one year membership period. The Gold Card Member Pricing program, which provided members product discounts, was discontinued in all domestic company-owned and franchise stores on December 28, 2016 in connection with the introduction of the One New GNC program. As a part of this launch, the Company provided former Gold Card customers that were within the membership period of generally one year with a coupon equivalent to a reimbursement of the unexpired portion of their Gold Card membership fee. As of December 31, 2016, the Company had $24.4 million of deferred Gold Card revenue which was recognized in the first quarter of 2017 over the coupon redemption period which expired in March 2017, net of $1.4 million of applicable redemptions.
Effective with the launch of the One New GNC program on December 29, 2016, the Company introduced myGNC Rewards, a free points-based loyalty program system-wide in the U.S. The program enables customers to earn points based on their purchases. Points earned by members are valid for one year and may be redeemed for cash discounts on any product the Company sells at both company-owned or franchise locations. The Company defers the estimated standalone selling price of points related to this program as a reduction to revenue as points are earned by allocating a portion of the transaction price the customer pays to a loyalty program liability within deferred revenue and other current liabilities on the Consolidated Balance Sheet. The estimated selling price of each point is based on the estimated value of product for which the point is expected to be redeemed,
73
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
net of points not expected to be redeemed, based on historical redemption. When a customer redeems earned points, revenue is recognized with a corresponding reduction to the program liability.
Also effective with the launch of the One New GNC program, the Company introduced a paid membership program, PRO Access, which provides members with the delivery of sample boxes throughout the membership year, as well as the offering of certain other benefits including the opportunity to earn triple points on a periodic basis. The boxes include sample merchandise and other materials. The Company allocates the transaction price of the membership to the sample boxes and other benefits based on the estimated stand-alone prices. The membership price paid is recorded within deferred revenue and other current liabilities on the Consolidated Balance Sheet and subsequently recognized revenue as the underlying performance obligations are satisfied.
Revenue from gift cards is recognized when the gift card is redeemed. Gift cards do not have expiration dates and are not required to be escheated to government authorities. Utilizing historical redemption rates, the Company recognizes revenue for amounts not expected to be redeemed proportionately as other gift card balances are redeemed.
Revenues from domestic and international franchisees include wholesale product sales, franchise fees and royalties, as well as cooperative advertising and other franchise support fees specific to domestic franchisees. Revenues are recorded within the U.S. and Canada segment for domestic franchisees and the International segment for international franchisees. The Company's franchisees purchase a significant amount of the products they sell in their retail stores from the Company at wholesale prices. Revenue on product sales to franchisees and other franchise support fees (including construction, equipment and other administrative fees) are recognized upon transfer of control to the franchisee, net of estimated returns and allowances. Franchise license fees, royalties and continuing services, such as cooperative advertising, are not separate and distinct performance obligations as they are highly dependent on each other in supporting the overall brand. Franchise fees for the license are paid in advance, and are deferred and recognized over the applicable license term as the Company satisfies the performance obligation of granting the customer access to the rights of its intellectual property. Franchise royalties and cooperative advertising contributions are variable consideration based on a percentage of the franchisees' retail sales, which are recognized in the period the franchisees' underlying sales occur, and are not included in the upfront transaction price for the overall performance obligation relating to providing access to the Company's intellectual property.
The Manufacturing / Wholesale segment sells product to the Company's other segments, which is eliminated in consolidation, and third-party customers. Revenue is recognized over time, net of estimated returns and allowances, as manufacturing occurs if the customized goods have no alternative use (specially made for the end customer) and the Company has an enforceable right to payment for performance completed to date. The selection of the method to measure progress towards completion requires judgment and is based on the nature of the products or services to be provided. The Company uses the cost-to-cost measure of progress for its contracts because it best depicts the transfer of control to the customer which occurs as the Company incurs costs on its contracts. Under the cost-to-cost measure of progress, the extent of progress towards completion is measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation. Revenues, including estimated fees or profits, are recorded proportionally as costs are incurred. Costs to fulfill include labor, materials, other direct costs and an allocation of indirect costs, which are recognized as cost of sales as revenue is recognized. Services for specialty manufacturing contracts typically have an expected duration of less than one year. In March 2019, the Company entered into a strategic joint venture with IVC regarding the Company's manufacturing business. The Company received $99.2 million and contributed its Nutra manufacturing and Anderson facility net assets in exchange for an initial 43 % equity interest in the Manufacturing JV. GNC expects to receive an additional $75 million from IVC, adjusted up or down based on the Manufacturing JV's future performance, over a four year period from the effective date of the transaction as IVC’s ownership of the joint venture increases to 100 %. The Company's interest in the joint venture is accounted for as an equity method investment. Refer to Note 9, "Equity Method Investments" for more information. We generate revenue from sales to our wholesale business partners on products at wholesale prices, retail sales of certain consigned inventory (prior to the termination of the consignment agreement with Rite Aid in December 2018) and license fees for the store-within-a-store alliance with Rite Aid. Wholesale sales are recognized upon transfer of control, net of estimated returns and allowances. License fees are paid in advance and are deferred and recognized over the applicable license term as the Company satisfies the performance obligation of granting the customer access to the rights of its intellectual property.
Cost of Sales. The Company purchases products directly from third-party vendors, the Manufacturing JV, and prior to the Manufacturing JV transaction, manufactured its own products. Cost of sales includes product costs, vendor allowances, inventory obsolescence, shrinkage, manufacturing overhead, warehousing, distribution, shipping and store occupancy costs. Store occupancy costs include rent, common area maintenance charges, real estate and other asset-based taxes, general maintenance, utilities, depreciation, lease incentives and certain insurance expenses.
74
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Leases. The Company leases substantially all of its retail stores in the U.S. and Canada segment, including most of the domestic franchise stores that are leased and subleased to franchisees, its distribution centers in the United States and retail stores in Ireland. In addition, the Company has leased office locations and vehicle and equipment leases to support our store and supply chain operations. All of the Company's leases are classified as operating leases.
The Company determines if a contract contains a lease at inception. The lease liabilities are recognized based on the present value of the future minimum lease payments over the term at the commencement date for leases exceeding 12 months. The lease agreements generally contain lease and non-lease components. Non-lease components primarily include payments for maintenance and utilities. The minimum lease payments include only fixed lease components, as well as any variable rate payments that depend on an index, initially measured using the index at the lease commencement date. Lease terms may include options to renew when it is reasonably certain that the Company will exercise an option. The Company estimates its incremental borrowing rate, which was estimated to approximate the interest rate on a collateralized basis with similar terms and payments for each lease, using a portfolio approach. The right-of-use assets recognized are initially equal to the lease liability, adjusted for any lease payments made on or before the commencement dates and lease incentives.
The lease liabilities for the operating leases are amortized using the effective interest method. The right-of-use asset is amortized by taking the difference between total rent expense recorded on straight-line basis and the lease liability amortization. When the right-of-use asset for an operating lease is impaired, lease expense is no longer recognized on a straight-line basis. For impaired leases, the Company continues to amortize the lease liability using the same effective interest method as before the impairment charge and the right-of-use asset is amortized on a straight-line basis.
Pre-Opening Expenditures. The Company recognizes the cost associated with the opening of new stores, which consist primarily of rent, marketing, payroll and recruiting costs, as incurred.
Income Taxes. The Company accounts for income taxes using the asset and liability method. Under this method, deferred tax assets and liabilities result from (i) the future tax impact of temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and (ii) differences between the recorded value of assets acquired in business combinations accounted for as purchases for financial reporting purposes and their corresponding tax bases. The company regularly reviews the components of the deferred tax assets. This review is to ascertain that, based upon all the information available at the time of the preparation of the financial statements, it is more likely than not that the Company expects to utilize these deferred tax assets in the future. If the Company determines that it is more likely than not that these deferred tax assets will not be utilized, a valuation allowance is recorded, reducing the deferred tax asset to the amount expected to be realized.
75
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Many factors are considered in the determination that the deferred tax assets are more likely than not to be realized, including recent cumulative earnings, the Company's ability to continue as a going concern, expectations regarding future taxable income, length of carryforward periods, and other relevant quantitative and qualitative factors. The recoverability of the deferred tax assets is determined by assessing the adequacy of future expected taxable income from all sources, including the reversal of taxable temporary differences, forecasted operating earnings, and tax planning strategies. The Company classifies interest and penalties accrued in connection with unrecognized tax benefits as income tax expense in its Consolidated Statements of Operations.
Refer to Note 5, "Income Taxes," for more information.
Stock-Based Compensation. The Company utilizes the Black-Scholes model to calculate the fair value of time-based stock option awards. The Company utilizes a Monte Carlo simulation for its performance awards with a market condition, which requires various inputs and assumptions, including the Company's own stock price. The grant-date fair value of all other stock-based compensation, including time-based and performance-based restricted stock awards, is based on the closing price for a share of the Company's common stock on the New York Stock Exchange (the "NYSE") on the grant date.
Compensation expense for time-based stock options and restricted stock awards is recognized over the applicable vesting period, net of expected forfeitures. Compensation expense for performance-based shares with a market condition is recognized over the applicable vesting period, net of expected forfeitures, regardless of whether the market condition is achieved. Compensation expense related to the performance-based units is recognized over the applicable vesting period, net of expected forfeitures, and adjusted as necessary to reflect changes in the probability that the vesting criteria will be achieved. The Company regularly reviews the probability of achieving the performance condition on these awards.
Refer to Note 17, "Stock-Based Compensation" for more information.
Refer to Note 16, "Earnings Per Share" for information on the Company's underlying shares of its convertible debt and convertible preferred stock in the computation of EPS.
Adoption of New Lease Standard
In February 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2016-02, which requires lessees to recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments for all leases with a term greater than 12 months. This standard is effective for annual reporting periods, and interim periods therein, beginning after December 15, 2018 and is required to be applied using a modified retrospective approach. In July 2018, the FASB issued ASU 2018-11, which provides companies with the option to apply the new lease standard either at the beginning of the earliest comparative period presented or in the period of adoption. The Company adopted ASU 2016-02 and its
76
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
related amendments (collectively known as "ASC 842") during the first quarter of fiscal 2019 electing the optional transition relief amendment that allows for a cumulative-effect adjustment in the period of adoption and did not restate prior periods. In transitioning to ASC 842, the Company elected to use the practical expedient package available under the guidance for leases that commenced before the effective date and did not elect to use hindsight. The Company has implemented a new lease management and accounting system and updated its processes and internal controls to comply with the new standard.
The Company leases substantially all of its retail stores in the U.S. and Canada segment, including most of the domestic franchise stores that are leased and subleased to franchisees, its distribution centers in the United States and retail stores in Ireland. In addition, the Company has leased office locations and vehicle and equipment leases to support our store and supply chain operations. All of the Company's leases are classified as operating leases.
The Company determines if a contract contains a lease at inception. The lease liabilities are recognized based on the present value of the future minimum lease payments over the term at the commencement date for leases exceeding 12 months. The lease agreements generally contain lease and non-lease components. Non-lease components primarily include payments for maintenance and utilities. The minimum lease payments include only fixed lease components, as well as any variable rate payments that depend on an index, initially measured using the index at the lease commencement date. Lease terms may include options to renew when it is reasonably certain that the Company will exercise an option. The Company estimates its incremental borrowing rate, which was estimated to approximate the interest rate on a collateralized basis with similar terms and payments for each lease, using a portfolio approach. The right-of-use assets recognized are initially equal to the lease liability, adjusted for any lease payments made on or before the commencement dates and lease incentives.
The Company recognized lease liabilities of $550.2 million on January 1, 2019. A right-of-use asset of $504.2 million was recognized based on the lease liability, adjusted for the reclassification of deferred rent of $53.3 million and prepaid rent of $7.3 million. Additionally, the Company recognized $79.8 million of right-of-use asset impairment charges related to certain of the Company's stores for which it was previously determined that the carrying value of the stores' assets were not recoverable. The right-of-use asset impairment charges were recorded as a reduction to January 1, 2019 (opening day) retained earnings, net of tax of $19.8 million. The new lease standard has no impact on the timing or classification of the Company's cash flows as reported in the Consolidated Statement of Cash Flows.
The lease liabilities for the operating leases are amortized using the effective interest method. The right-of-use asset is amortized by taking the difference between total rent expense recorded on straight-line basis and the lease liability amortization. When the right-of-use asset for an operating lease is impaired, lease expense is no longer recognized on a straight-line basis. For impaired leases, the Company continues to amortize the lease liability using the same effective interest method as before the impairment charge and the right-of-use asset is amortized on a straight-line basis.
Refer to Note 12 "Leases" for additional information.
Recently Issued Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, Income Taxes: Simplifying the Accounting for Income Taxes. This ASU simplifies accounting for income taxes by eliminating certain exceptions to ASC 740 related to the general approach for intraperiod tax allocation, methodology for calculating income taxes in an interim period and recognition of deferred taxes when there are investment ownership changes. The new guidance also simplifies aspects of accounting for franchise taxes and interim period effects of enacted changes in tax laws or rates. The new guidance provides clarification on accounting for transactions that result in a step-up in the tax basis of goodwill and allocation of consolidated income tax expense to separate financial statements of entities not subject to income tax. This ASU is effective for fiscal years beginning after December 15, 2020, including interim periods within those fiscal years, and early adoption is permitted. The Company is evaluating the impact this standard will have on its Consolidated Financial Statements and related disclosures.
NOTE 3. REVENUE
Revenue is recognized when obligations under the terms of a contract with the customer are satisfied; generally, this occurs with the transfer of control of products or services. The Company satisfies performance obligations either over time or at a point in time as discussed in further detail below. Revenue is measured as the amount of consideration expected to be received in exchange for transferring goods or providing services. Applicable sales tax collected concurrent with revenue-producing activities are excluded from revenue.
U.S. and Canada Revenue
The following is a summary of revenue disaggregated by major source in the U.S. and Canada segment:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
U.S. company-owned product sales: (1) | (in thousands) | ||||||||||
Protein | $ | $ | $ | ||||||||
Performance supplements | |||||||||||
Weight management | |||||||||||
Vitamins | |||||||||||
Herbs / Greens | |||||||||||
Wellness | |||||||||||
Health / Beauty | |||||||||||
Food / Drink | |||||||||||
General merchandise | |||||||||||
Total U.S. company-owned product sales | $ | $ | $ | ||||||||
Wholesale sales to franchisees | |||||||||||
Royalties and franchise fees | |||||||||||
Sublease income | |||||||||||
Cooperative advertising and other franchise support fees | |||||||||||
Gold Card revenue recognized in U.S.(2) | |||||||||||
Other (3) | |||||||||||
Total U.S. and Canada revenue | $ | $ | $ | ||||||||
(1) | Includes GNC.com sales. |
(2) | The Gold Card Member Pricing program in the U.S. was discontinued in December 2016 in connection with the launch of the One New GNC program which resulted in $ |
(3) | Includes revenue primarily related to Canada operations and loyalty programs, myGNC Rewards and PRO Access. |
77
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
International Revenue
The following is a summary of the revenue disaggregated by major source in the International reportable segment:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Wholesale sales to franchisees | $ | $ | $ | ||||||||
Royalties and franchise fees | |||||||||||
Other (1) | |||||||||||
Total International revenue | $ | $ | $ | ||||||||
(1) Includes revenue related to China operations prior to the transfer of the China business to the HK JV and China JV, which was effective February 13, 2019, wholesale sales to the HK JV and China JV, and revenue from company-owned locations in Ireland.
Manufacturing / Wholesale Revenue
The following is a summary of the revenue disaggregated by major source in the Manufacturing / Wholesale reportable segment:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Third-party contract manufacturing (1) | $ | $ | $ | ||||||||
Intersegment sales (1) | |||||||||||
Wholesale partner sales | |||||||||||
Total Manufacturing / Wholesale revenue | $ | $ | $ | ||||||||
(1) The decrease in third-party contract manufacturing and intersegment sales for the year ended December 31, 2019 compared to the prior year period is due to the transfer of the Nutra manufacturing business to the Manufacturing JV effective March 1, 2019.
Revenue by Geography
The following is a summary of the revenue by geography:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Total revenues by geographic areas(1): | (in thousands) | ||||||||||
United States | $ | $ | $ | ||||||||
Foreign | |||||||||||
Total revenues | $ | $ | $ | ||||||||
(1) Geographic areas are defined based on legal entity jurisdiction.
Contract assets represent amounts related to the Company's contractual right to consideration for completed performance obligations not yet invoiced. As of December 31, 2018, the Company had contract assets of $25.5 million for specialty manufacturing recorded within prepaid and other current assets on the Consolidated Balance Sheet (with a corresponding reduction to inventory at cost). Due to the transfer of the Nutra manufacturing net assets to the Manufacturing JV on March 1, 2019, the Company had no contract assets on the Consolidated Balance Sheet as of December 31, 2019.
78
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Contract liabilities include payments received in advance of performance under the contract. The Company's PRO Access and loyalty program points are recorded within deferred revenue and other current liabilities on the Consolidated Balance Sheets. Deferred franchise and license fees are recorded within deferred revenue and other current liabilities and other long-term liabilities on the Consolidated Balance Sheets.
The following table presents changes in the Company’s contract liabilities:
Year ended December 31, 2019 | |||||||||||||||
Balance at beginning of period | Recognition of revenue included in beginning balance | Contract liability, net of revenue, recognized during the period | Balance at end of period | ||||||||||||
(in thousands) | |||||||||||||||
Deferred franchise and license fees | $ | $ | ( | ) | $ | $ | |||||||||
PRO Access and loyalty program points (*) | ( | ) | |||||||||||||
Gift card liability (*) | ( | ) | |||||||||||||
Year ended December 31, 2018 | |||||||||||||||
Balance at beginning of period | Recognition of revenue included in beginning balance | Contract liability, net of revenue, recognized during the period | Balance at end of period | ||||||||||||
(in thousands) | |||||||||||||||
Deferred franchise and license fees | $ | $ | ( | ) | $ | $ | |||||||||
PRO Access and loyalty program points (*) | ( | ) | |||||||||||||
Gift card liability (*) | ( | ) | |||||||||||||
(*) Net of estimated breakage
NOTE 4. INVENTORY
The net realizable value of inventory consisted of the following:
December 31, | |||||||
2019 | 2018 | ||||||
(in thousands) | |||||||
Finished product ready for sale | $ | $ | |||||
Work-in-process, bulk product and raw materials (1) | |||||||
Packaging supplies (1) | |||||||
Inventory | $ | $ | |||||
(1) | The decrease in work-in-process, bulk product and raw materials and packaging supplies as of December 31, 2019 compared with December 31, 2018 is due to the transfer of the Nutra manufacturing net assets to the Manufacturing JV effective March 1, 2019. |
79
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 5. INCOME TAXES
Income (loss) before income taxes, including income from equity method investments, consisted of the following components:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Domestic | $ | $ | $ | ( | ) | ||||||
Foreign | ( | ) | ( | ) | |||||||
Income (loss) before income taxes (*) | $ | $ | $ | ( | ) | ||||||
(*) Includes income from equity method investments
Income tax expense (benefit) consisted of the following components:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Current: | |||||||||||
Federal | $ | $ | $ | ||||||||
State | |||||||||||
Foreign | |||||||||||
Total current income tax expense | |||||||||||
Deferred: | |||||||||||
Federal | ( | ) | ( | ) | |||||||
State | ( | ) | ( | ) | |||||||
Foreign | ( | ) | ( | ) | ( | ) | |||||
Total deferred income tax expense (benefit) | ( | ) | ( | ) | |||||||
Total income tax expense (benefit) | $ | $ | ( | ) | $ | ( | ) | ||||
80
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Income tax expense (benefit) reflected in the accompanying Consolidated Statements of Operations varies from the amounts that would have been provided by applying the United States federal statutory income tax rate of 21% to income (loss) before income taxes as shown below:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
U.S. federal statutory income tax | $ | $ | $ | ( | ) | ||||||
Increase (reduction) resulting from: | |||||||||||
State income tax, net of federal tax benefit | ( | ) | ( | ) | |||||||
International operations | ( | ) | |||||||||
Foreign derived intangible income | ( | ) | ( | ) | |||||||
Global intangible low taxed income | |||||||||||
Premiums paid to wholly owned subsidiary company | ( | ) | ( | ) | ( | ) | |||||
Nondeductible goodwill | |||||||||||
Brand impairment | |||||||||||
Exchange of convertible senior notes | ( | ) | |||||||||
Loss (gain) on forward contracts for the issuance of the convertible preferred stock | ( | ) | |||||||||
Formation of the joint ventures | |||||||||||
Change in valuation allowance | ( | ) | |||||||||
Stock based compensation | |||||||||||
Federal tax credits and income deductions | ( | ) | ( | ) | ( | ) | |||||
Tax impact of uncertain tax positions | |||||||||||
Return to provision adjustment | ( | ) | ( | ) | ( | ) | |||||
Impact of 2017 Tax Act | ( | ) | ( | ) | |||||||
Other permanent differences | |||||||||||
Income tax expense (benefit) | $ | $ | ( | ) | $ | ( | ) | ||||
During the year ended December 31, 2019, we recognized an income tax expense of $44.9 million. The current year effective tax rate was significantly impacted by an increase to income tax expense of $27.1 million relating to an increase in valuation allowance against certain deferred tax assets that may not be realizable and an increase to tax expense of $7.6 million resulting from the transfer of the Nutra manufacturing net assets to the Manufacturing JV. The current year tax expense was also impacted by a $4.8 million increase in the Company’s liability for uncertain tax positions and a $3.5 million increase related to a loss on forward contracts for the issuance of convertible preferred stock that was not recognizable for tax purposes.
During the year ended December 31, 2018, the Company finalized estimates of income tax impacts of the 2017 Tax Act based upon the regulations and other relevant guidance issued through December 31, 2018. The Company's 2018 income tax provision includes a discrete tax benefit of $3.6 million relating to the finalization of the remeasurement of its deferred tax assets and liabilities upon filing of the Company's 2017 federal income tax return.
On December 22, 2017, tax reform legislation known as The Tax Cuts and Jobs Act of 2017 (“2017 Tax Act”) was enacted. The 2017 Tax Act made significant changes to the Internal Revenue Code. During the year ended December 31, 2017, the Company recorded a non-cash income tax benefit of $86.8 million, related to the remeasurement of its deferred tax assets and liabilities to reflect the effects of these temporary differences at enacted tax rates expected to be in effect when taxes are actually paid or recovered.
81
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Deferred tax assets and liabilities consisted of the following at December 31:
Year ended December 31, | |||||||
2019 | 2018 | ||||||
(in thousands) | |||||||
Deferred tax assets: | |||||||
Operating reserves | $ | $ | |||||
Deferred revenue | |||||||
Net operating loss and credit carryforwards | |||||||
Lease liabilities | |||||||
Fixed assets | |||||||
Stock-based compensation | |||||||
Interest limitation | |||||||
Other | |||||||
Valuation allowance | ( | ) | ( | ) | |||
Total deferred tax assets | |||||||
Deferred tax liabilities: | |||||||
Prepaid expenses | ( | ) | ( | ) | |||
Right-of-use assets | ( | ) | — | ||||
Intangible assets | ( | ) | ( | ) | |||
Convertible senior notes | ( | ) | ( | ) | |||
Total deferred tax liabilities | ( | ) | ( | ) | |||
Net deferred tax liability | $ | ( | ) | $ | ( | ) | |
The Company regularly reviews deferred tax assets. The review is to ascertain that, based upon all the information available at the time of the preparation of the financial statements, it is more likely than not that the Company expects to utilize these deferred tax assets in the future. If the Company determines that it is more likely than not that these deferred tax assets will not be utilized, a valuation allowance is recorded, reducing the deferred tax asset to the amount expected to be realized. In assessing the need for a valuation allowance, the Company considers all available positive and negative evidence, including the Company’s operating results, reversals of deferred tax liabilities, and forecasts of future taxable income on a jurisdiction-by-jurisdiction basis.
During the fourth quarter of the year ended December 31, 2019, as further discussed in Note 1, "Nature of Business," management concluded that there is substantial doubt regarding the Company’s ability to continue as a going concern. Management considered this in concluding that certain deferred tax assets were no longer more likely than not realizable. As a result, an increase in valuation allowance of $27.1 million on the Company’s deferred tax assets was recorded as of December 31, 2019 which related principally to deferred tax assets for state NOL carryforwards and other state tax attributes and deferred tax assets related to the Company’s interest expense deductions as determined under Section 163(j) of the Internal Revenue Code. This increase was partially offset by a valuation allowance decrease of $4.8 million.
As of December 31, 2019 and 2018, a valuation allowance was provided for certain NOLs, as the Company currently believes that these NOLs may not be realizable prior to their expiration. As of December 31, 2019, the Company recorded an additional $18.6 million valuation allowance related to state net operating losses and other state attributes no longer determined to be realizable. In the current year, the Company also recorded a $7.4 million partial valuation allowance related to the deferred tax asset resulting from the limitation of the Company’s interest expense deduction as determined by Section 163(j) of the Internal
82
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Revenue Code and a $1.1 million valuation allowance related to federal foreign tax credit carryforwards. The Company reduced its foreign deferred tax assets and related valuation allowance by $4.8 million respectively, because it determined that $3.7 million of China NOLs would not be available to the Company as a result of the China joint venture transaction and $1.1 million of Puerto Rico NOLs were realized at the filing of the 2018 Puerto Rico statutory income tax returns.
As of December 31, 2019 and 2018, the Company has valuation allowances for deferred tax assets in the amount of $42.3 million and $20.0 million, respectively. Management will continue to assess the valuation allowance in forthcoming periods. This may result in a different conclusion as to the realizability of the Company's deferred tax assets in the future.
The Company’s foreign subsidiaries generate earnings that are not subject to U.S. income taxes so long as they are permanently reinvested in its operations outside of the U.S. Pursuant to ASC Topic No 740-30, undistributed earnings of foreign subsidiaries that are no longer permanently reinvested would become subject to deferred income taxes. The Company does not have any material undistributed earnings of international subsidiaries at December 31, 2019 as these subsidiaries are considered to be branches for United States tax purposes, to have incurred cumulative NOLs, or to have only minimal undistributed earnings.
GNC Holdings, Inc. files a consolidated federal tax return and various consolidated and separate tax returns as prescribed by the tax laws of the state, local and international jurisdictions in which it and its subsidiaries operate. The statutes of limitation for the Company’s U.S. federal income tax returns are closed for years through 2013. The Company has various state and local jurisdiction tax years open to possible examination (the earliest open period is generally 2011), and the Company also has certain state and local tax filings currently under audit.
A reconciliation of the beginning and ending amount of unrecognized tax benefits, excluding penalties and interest, is as follows:
December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Balance of unrecognized tax benefits at beginning of period | $ | $ | $ | ||||||||
Additions for tax positions taken during current period | |||||||||||
Additions for tax positions taken during prior periods | |||||||||||
Reductions for tax positions taken during prior periods | ( | ) | ( | ) | ( | ) | |||||
Settlements | ( | ) | ( | ) | |||||||
Balance of unrecognized tax benefits at end of period | $ | $ | $ | ||||||||
The Company's liability for uncertain tax positions, excluding penalties and interest, increased by a net $3.8 million during the current year.
As of December 31, 2019, the Company is not aware of any positions for which it is reasonably possible that the total amount of unrecognized tax benefits will significantly increase or decrease within the next 12 months. Accrued interest and penalties were $2.4 million and $2.0 million at December 31, 2019 and 2018, respectively. At December 31, 2019, the amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate was $13.1 million, including the impact of accrued interest and penalties. While it is often difficult to predict the final outcome or the timing of resolution of any particular uncertain tax position, the Company believes that its unrecognized tax benefits reflect the most likely outcome. The Company adjusts these unrecognized tax benefits, as well as the related interest, in light of changing facts and circumstances. Settlement of any particular position could require the use of cash. Favorable resolution would be recognized as a reduction to the effective income tax rate in the period of resolution.
83
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 6. GOODWILL AND INTANGIBLE ASSETS
Impairment Charges
The Company recorded the following impairment charges:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Brand name | $ | $ | $ | ||||||||
Goodwill | |||||||||||
Property and equipment(1) | |||||||||||
Lucky Vitamin (2) | |||||||||||
Other store closing costs | |||||||||||
Total long-lived asset impairment charges | $ | $ | $ | ||||||||
(1) Refer to Note 7, "Property, Plant and Equipment, Net" for more information on the property and equipment charges.
(2) Includes goodwill, intangible assets and property and equipment as explained below.
Brand Name
During the fourth quarter of 2019, management performed its annual impairment test of the indefinite-lived brand intangible asset. The brand name impairment test was performed in totality as it represents a single unit of account and the Company concluded that the estimated fair value under the relief from royalty method (income approach) exceeded its carrying value. The methodology utilized for the impairment test of the indefinite-lived brand intangible asset has not changed materially from the prior year. Key assumptions included in the estimation of the fair value include the following:
• | Future cash flow assumptions - Future cash flow assumptions include retail sales from the Company’s corporate retail store operations, GNC.com retail sales, wholesale partner sales, China JV and HK JV retail sales, and domestic and international franchise retail sales. Sales were based on organic growth and were derived from historical experience and assumptions regarding future growth. The Company's analysis incorporated an assumed period of cash flows of |
• | Royalty rate - The royalty rates utilized consider external market evidence and internal financial metrics including a review of available returns after the consideration of property, plant and equipment, working capital and other intangible assets. |
• | Discount rate - The discount rate was based on an estimated weighted average cost of capital ("WACC") for each business supported by the GNC brand name. The components of WACC are the cost of equity and the cost of debt, each of which requires judgment by management to estimate. The Company developed its cost of equity estimate based on perceived risks and predictability of future cash flows. The WACC used to estimate the fair values of the Company's reporting units was within a range of |
During the year ended December 31, 2018, the Company recognized an impairment charge of $23.7 million, which was allocated to the U.S. and Canada and International segments for $21.6 million and $2.1 million, respectively. During the year ended December 31, 2017, the Company recognized a $395.6 million impairment charge on its $720.0 million indefinite-lived brand intangible asset, which was allocated to the U.S. and Canada and International segments for $394.0 million and $1.6 million, respectively.
Goodwill
Management performed its annual impairment test of goodwill during the fourth quarter of 2019. Results of the impairment test indicated that all of the reporting units had fair values which were in excess of their respective carrying values and therefore there was no impairment for the year ended December 31, 2019.
84
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The Company estimated the fair values of its reporting units in the fourth quarter of 2019 using a discounted cash flow method (income approach) weighted 50 % and a guideline company method (market approach) weighted 50 %. The methodology utilized for the goodwill impairment test has not changed materially from the prior year. The key assumptions used under the income approach include the following:
• | Future cash flow assumptions - The Company's projections for its reporting units were based on organic growth and were derived from historical experience and assumptions regarding future growth and profitability trends. The Company's analysis incorporated an assumed period of cash flows of |
• | Discount rate - The discount rate was based on an estimated weighted average cost of capital ("WACC") for each reporting unit. The components of WACC are the cost of equity and the cost of debt, each of which requires judgment by management to estimate. The Company developed its cost of equity estimate based on perceived risks and predictability of future cash flows. The WACC used to estimate the fair values of the Company's reporting units was within a range of |
The guideline company method involves analyzing transaction and financial data of publicly-traded companies to develop multiples, which are adjusted to account for differences in growth prospects and risk profiles of the reporting unit and the comparable.
For the year ended December 31, 2018, no impairment was indicated as a result of the annual impairment test during the fourth quarter. For the year ended December 31, 2017, the Company recorded a goodwill impairment charge of $24.3 million related to the Wholesale reporting unit in the fourth quarter as a result of a triggering event based on a decline in the Company's share price and previous challenges associated with the Company's efforts to refinance its long-term debt.
Lucky Vitamin
During the second quarter of 2017, in order for the Company to focus on strategic changes around the One New GNC program, the Company considered strategic alternatives for the Lucky Vitamin e-commerce business, which was considered a triggering event requiring an interim goodwill impairment review of the Lucky Vitamin reporting unit as of June 30, 2017. The Company estimated the fair value of the Lucky Vitamin reporting unit using a discounted cash flow method (income approach) and a guideline company method (market approach), each of which took into account the expectations regarding the potential strategic alternatives for the Lucky Vitamin business being explored in the second quarter of 2017. As a result of the review, the Company concluded that the carrying value of the Lucky Vitamin reporting unit exceeded its fair value, which resulted in a goodwill impairment charge of $11.5 million being recorded in the second quarter of 2017. There was no remaining goodwill balance on the Lucky Vitamin reporting unit after the impact of this charge.
As a result of the impairment indicator described above, the Company also performed an impairment analysis with respect to its definite-lived intangible assets and other long-lived assets on the Lucky Vitamin reporting unit, consisting of a trade name and property and equipment. The fair value of the trade name was determined using a relief from royalty method (income approach) and the fair value of the property and equipment was determined using an income approach. Based on the results of the analyses, the Company concluded that the carrying value of the Lucky Vitamin trade name and property and equipment exceeded their fair values resulting in an impairment charge of $4.2 million and $3.7 million, respectively. All of the aforementioned non-cash charges totaling $19.4 million were recorded in long-lived asset impairments in the Consolidated Statement of Operations within the U.S. and Canada segment during the year ended December 31, 2017.
The Company completed an asset sale of Lucky Vitamin on September 30, 2017, resulting in a loss of $1.7 million recorded within other (income) loss, net on the Consolidated Statement of Operations consisting of the net assets sold subtracted from the purchase price of $6.4 million, which includes fees paid to a third-party. The proceeds were received in October 2017.
85
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Goodwill Roll-Forward
The following table summarizes the Company's goodwill activity by reportable segment:
U.S. and Canada | International | Manufacturing / Wholesale | Total | ||||||||||||
(in thousands) | |||||||||||||||
Goodwill at December 31, 2017 | $ | $ | $ | $ | |||||||||||
2018 Activity: | |||||||||||||||
Translation effect of exchange rates | ( | ) | ( | ) | |||||||||||
Total 2018 activity | ( | ) | ( | ) | |||||||||||
Balance at December 31, 2018: | |||||||||||||||
Gross | |||||||||||||||
Accumulated impairments | ( | ) | ( | ) | ( | ) | |||||||||
Goodwill | $ | $ | $ | $ | |||||||||||
2019 Activity: | |||||||||||||||
Translation effect of exchange rates | ( | ) | ( | ) | |||||||||||
Nutra manufacturing net assets exchange | ( | ) | ( | ) | |||||||||||
Total 2019 activity | ( | ) | ( | ) | ( | ) | |||||||||
Balance at December 31, 2019: | |||||||||||||||
Gross | |||||||||||||||
Accumulated impairments | ( | ) | ( | ) | ( | ) | |||||||||
Goodwill | $ | $ | $ | $ | |||||||||||
Intangible Assets
The following table reflects the gross carrying amount and accumulated amortization for each major intangible asset:
December 31, 2019 | December 31, 2018 | ||||||||||||||||||||||||
Weighted- Average Life | Gross | Accumulated Amortization/ Impairment | Carrying Amount | Gross | Accumulated Amortization/ Impairment | Carrying Amount | |||||||||||||||||||
(in thousands) | |||||||||||||||||||||||||
Brand name | Indefinite | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||||||
Retail agreements | ( | ) | ( | ) | |||||||||||||||||||||
Franchise agreements | ( | ) | ( | ) | |||||||||||||||||||||
Manufacturing agreements (1) | ( | ) | ( | ) | |||||||||||||||||||||
Other intangibles | ( | ) | ( | ) | |||||||||||||||||||||
Franchise rights | ( | ) | ( | ) | |||||||||||||||||||||
Total | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | |||||||||||||||
(1) In the first quarter of 2019, the Company transferred the Nutra manufacturing business net assets to the Manufacturing JV
Amortization expense during the years ended December 31, 2019, 2018 and 2017 was $5.9 million, $7.0 million and $7.4 million, respectively.
86
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The following table represents future amortization expense of definite-lived intangible assets at December 31, 2019:
Years ending December 31, | Amortization expense | ||
(in thousands) | |||
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
Thereafter | |||
Total future amortization expense | $ | ||
NOTE 7. PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment, net, consisted of the following:
December 31, | |||||||
2019 | 2018 | ||||||
(in thousands) | |||||||
Land, buildings and improvements | $ | $ | |||||
Machinery and equipment | |||||||
Leasehold improvements | |||||||
Furniture and fixtures | |||||||
Software | |||||||
Construction in progress | |||||||
Total property, plant and equipment | |||||||
Less: accumulated depreciation | ( | ) | ( | ) | |||
Less: accumulated impairment | ( | ) | ( | ) | |||
Net property, plant and equipment (1) | $ | $ | |||||
(1) In the first quarter of 2019, the Company transferred the Nutra manufacturing business net assets to the Manufacturing JV and transferred the China net assets to the HK JV and China JV.
The Company recognized depreciation expense on property, plant and equipment of $29.6 million, $40.1 million, and $49.4 million for the years ended December 31, 2019, 2018 and 2017, respectively, which is included in manufacturing overhead expense as part of cost of sales and SG&A expense on the Consolidated Statements of Operations.
Impairments and Other Store Closing Costs
During the third quarter of 2018, the Company performed a detailed review of its store portfolio and identified stores in the U.S. and Canada that will be closed within the next three years at the end of their lease terms. This review also identified other stores in which the Company is considering alternatives such as seeking lower rent or a shorter term. In connection with the review of the store portfolio, the Company recorded $14.6 million of impairment charges within the U.S. and Canada segment, of which $9.5 million related to its property, plant and equipment for certain underperforming stores and $5.1 million related to other store closing costs, presented as long-lived asset impairments in the accompanying Consolidated Statement of Operations.
87
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
During the year ended December 31, 2017, the Company recorded $18.6 million of impairment charges within the U.S. and Canada segment primarily related to certain of the Company's underperforming stores and the impact of Hurricane Maria on the Company's stores located in Puerto Rico. Refer to Note 6, "Goodwill and Intangible Assets" for fixed asset impairments related to Lucky Vitamin in 2017.
NOTE 8. LONG-TERM DEBT / INTEREST EXPENSE
Long-term debt consisted of the following:
December 31, | |||||||
2019 | 2018 | ||||||
(in thousands) | |||||||
Tranche B-1 Term Loan (net of $0.0 million discount) | $ | $ | |||||
Tranche B-2 Term Loan (net of $7.0 million and $17.5 million discount) | |||||||
FILO Term Loan (net of $8.2 million and $10.9 million discount) | |||||||
Unpaid original issuance discount | |||||||
Notes (net of $3.9 million and $11.5 million conversion feature and $0.5 million and $1.6 million discount) | |||||||
Debt issuance costs | ( | ) | ( | ) | |||
Total debt | $ | $ | |||||
Less: current maturities | ( | ) | ( | ) | |||
Long-term debt | $ | $ | |||||
At December 31, 2019, the Company's future annual contractual obligations on long-term debt are detailed below:
Year Ending December 31, | Tranche B-2 Term Loan (1) | FILO Term Loan (2) | Convertible Notes (3) | Total | |||||||||||
2020 | $ | $ | $ | $ | |||||||||||
2021 | |||||||||||||||
2022 | |||||||||||||||
Total | $ | $ | $ | $ | |||||||||||
(1) Includes the unamortized original issuance discount of $7.0 million
(2) Includes the unamortized original issuance discount of $8.2 million
88
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Senior Credit Facility
Issuance
In March 2011, General Nutrition Centers, Inc. ("Centers"), a wholly owned subsidiary of Holdings, entered into the Senior Credit Facility, consisting of the Term Loan Facility and the Revolving Credit Facility. The Senior Credit Facility permits the Company to prepay a portion or all of the outstanding balance without incurring penalties (except London Interbank Offering Rate ("LIBOR") breakage costs). GNC Corporation, the Company's indirect wholly owned subsidiary, and Centers' existing and future domestic subsidiaries have guaranteed Centers' obligations under the Senior Credit Facility. In addition, the Senior Credit Facility is collateralized by first priority pledges (subject to permitted liens) of substantially all of Centers' assets, including its equity interests and the equity interests of its domestic subsidiaries.
The Company amended the Revolving Credit Facility on March 4, 2016, to extend its maturity from March 2017 to September 2018 and increase total availability from $130.0 million to $300.0 million. In December 2017, the Company reduced the amount available under the Revolving Credit Facility from $300.0 million to $225.0 million.
2018 Refinancing
On February 28, 2018, the Company amended and restated its Senior Credit Facility which at the time consisted of a $1,131.2 million term loan facility due in March 2019 and a $225.0 million revolving credit facility that was scheduled to mature in September 2018 (the “Amendment”, and the Senior Credit Facility as so amended, the "Term Loan Agreement"). The Amendment extended the maturity date for $704.3 million of the $1,131.2 million term loan facility from March 2019 to March 2021 (the “Tranche B-2 Term Loan"). In the event that all outstanding amounts under the convertible senior notes in excess of $50.0 million have not been repaid, refinanced, converted or effectively discharged prior to May 2020 ("Springing Maturity Date"), the maturity date for the Tranche B-2 Term Loan becomes the Springing Maturity Date, subject to certain adjustments. The Amendment also terminated the $225.0 million revolving credit facility.
After the effectiveness of the Amendment, the remaining term loan of $151.9 million as of February 28, 2018 continued to have a maturity date of March 2019 (the "Tranche B-1 Term Loan"). The Tranche B-2 Term Loan requires annual aggregate principal payments of at least $43 million and bears interest at a rate of, at the Company's option, LIBOR plus a margin of 8.75 % per annum subject to change under certain circumstances (with a minimum and maximum margin of 8.25 % and 9.25 %, respectively, per annum), or prime plus a margin of 7.75 % per annum subject to change under certain circumstances (with a minimum and maximum of 7.25 % and 8.25 %, respectively, per annum). Any mandatory repayments as defined in the credit agreement shall be applied to the remaining annual aggregate principle payments in direct order of maturity. As discussed in further detail below, in November 2018, the Company paid $100 million on the Tranche B-2 Term Loan and elected to use the payment to satisfy the scheduled amortization payments on the Term Loan Facility through December 2020. The interest rate under the Tranche B-1 Term Loan is at a rate of, at the Company's option, LIBOR plus a margin of 2.5 % or prime plus a margin of 1.5 %. The Term Loan Agreement is secured by a (i) first lien on certain assets of the Company primarily consisting of capital stock issued by General Nutrition Centers, Inc. ("Centers") and its subsidiaries, intellectual property and equipment (“Term Priority Collateral”) and (ii) second lien on certain assets of the Company primarily consisting of inventory and accounts receivable (“ABL Priority Collateral”). The Term Loan Agreement is guaranteed by all material, wholly-owned domestic subsidiaries of the Company (the “U.S. Guarantors”) and by General Nutrition Centres Company, an unlimited liability company organized under the laws of Nova Scotia (together with the U.S. Guarantors, the “Guarantors”).
On February 28 2018, the Company also entered into a new asset-based credit agreement (the "ABL Credit Agreement"), consisting of:
•a new $100 million asset-based Revolving Credit Facility (the "Revolving Credit Facility") with a maturity date of August 2022 (which maturity date will become May 2020, subject to certain adjustments, should the Springing Maturity Date be triggered); In connection with the transfer of the Nutra manufacturing and Anderson facility net assets to the manufacturing JV with IVC, the Revolving Credit Facility commitment was reduced from $100 million to $81 million effective March 2019; and
•a $275.0 million asset-based Term Loan Facility advanced on a “first-in, last-out” basis (the "FILO Term Loan") with a maturity date of December 2022 (which maturity date will become May 2020, subject to certain adjustments, should the Springing Maturity Date be triggered).
89
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
There are no scheduled amortization payments associated with the FILO Term Loan, which bears interest at a rate of LIBOR plus a margin of 7.00 % per annum subject to decrease under certain circumstances (with a minimum possible interest rate of LIBOR plus a margin of 6.50 % per annum). Outstanding borrowings under the Revolving Credit Facility bear interest at a rate of LIBOR plus 1.50 % or prime plus 0.50 % (both subject to an increase or decrease of 0.25 % to 0.50 % based on the amount available to be drawn under the Revolving Credit Facility). The Company is also required to pay an annual fee of 0.125 % to the applicable Issuing Bank and a fee to revolving lenders equal to a maximum of 2.0 % (subject to adjustment based on the amount available to be drawn under the Revolving Credit Facility with a minimum of 1.5 %) on outstanding letters of credit and an annual commitment fee of 0.375 % on the undrawn portion of the Revolving Credit Facility subject to an increase to 0.5 % based on the amount available to draw under the Revolving Credit Facility. The FILO Term Loan and Revolving Credit Facility are secured by a (i) first lien on ABL Priority Collateral and (ii) second lien on Term Priority Collateral. The FILO Term Loan and Revolving Credit Facility are guaranteed by the Guarantors.
In connection with the debt refinancing, the Company recognized a loss of $16.7 million in the first quarter of 2018, which primarily includes third-party fees relating to the Tranche B-2 Term Loan and the FILO Term Loan, and is presented as an operating outflow on the accompanying Consolidated Statement of Cash Flows. The refinancing of our term debt was accounted for as a debt modification and therefore the fees paid to third parties associated with the term debt restructuring were expensed. In addition, the Company paid $30.2 million consisting of an original issuance discount (“OID”) to the Tranche B-2 Term Loan and the FILO Term Loan lenders. The remaining unpaid OID of $10.4 million, which was subject to change based on the timing and amount of the outstanding balance, was paid to the Tranche B-2 Term Loan lenders at 2 % of the outstanding balance during the first quarter of 2019. The OID together with $5.1 million in fees incurred relating to the Revolving Credit Facility (included within other long-term assets on the Consolidated Balance Sheet) are amortized through the applicable maturity dates as an increase to interest expense.
Under the Company’s Term Loan Agreement and ABL Credit Agreement (collectively, the "Credit Facilities"), the Company is required to make certain mandatory prepayments, including a requirement to prepay first the Tranche B-2 Term Loan (until repaid in full), second the FILO Term Loan (until repaid in full, but only if such prepayment is permitted under the ABL Credit Agreement), and third the Tranche B-1 Term Loan, in each case annually with amounts based on excess cash flow, as defined in the Company’s Credit Facilities, based on the results of the Company for the prior fiscal year. The payment will be 75 % of excess cash flow for each such fiscal year, subject to a reduction to 50 % based on the attainment of a certain Consolidated Net First Lien Leverage Ratio, and will be reduced by certain scheduled debt payment amounts. Based on the Company's results for the year ended December 31, 2018, the Company's required excess cash flow payment was $49.8 million, which was reduced to $9.8 million by the scheduled debt payments made in the first quarter of 2019 defined in the Company's Credit Facilities. The Company made the excess cash flow payment in April 2019. Based on the Company's results for the year ended December 31, 2019, the Company will be required to make an excess cash flow payment of $25.9 million in April 2020.
At December 31, 2019, the Company's contractual interest rates under the Tranche B-2 Term Loan and the FILO Term Loan were 10.6 % and 8.8 %, respectively, which consist of LIBOR plus the applicable margin rate. At December 31, 2018, the Company's contractual interest rates under the Tranche B-1 Term Loan, Tranche B-2 Term Loan, and the FILO Term Loan were 5.7 %, 11.8 % and 9.5 %, respectively. At December 31, 2019, the Company had $66.2 million available under the Revolving Credit Facility, after giving effect to $4.9 million utilized to secure letters of credit and a $9.9 million reduction to borrowing ability as a result of decrease in net collateral.
The Company’s Credit Facilities contain customary covenants, including limitations on the ability of GNC Corporation, Centers, and Centers' subsidiaries to, among other things, incur debt, grant liens on their assets, enter into mergers or liquidations, sell assets, make investments or acquisitions, make optional payments in respect of, or modify, certain other debt instruments, pay dividends or other payments on capital stock, or enter into arrangements that restrict their ability to pay dividends or grant liens. In addition, the Term Loan Agreement requires compliance, as of the end of each fiscal quarter of the Company, with a maximum Consolidated Net First Lien Leverage Ratio initially set at 5.50 to 1.00 through December 31, 2018 and decreasing to 5.00 to 1.00 from March 31, 2019 to December 31, 2019 and 4.25 to 1.00 thereafter. Depending on the amount available to be drawn under the Revolving Credit Facility, the ABL Credit Agreement requires compliance as of the end of each fiscal quarter of the Company with a minimum Fixed Charge Coverage Ratio of 1.00 to 1.00. The Company was in compliance with the terms of its Credit Facilities as of December 31, 2019.
Investment from Harbin and IVC
On November 7, 2018, The Company entered into an Amendment to the Securities Purchase Agreement with Harbin Pharmaceutical Group Holdings Co., Ltd. (the "Investor") for the purchase of 299,950 shares of convertible preferred stock.
90
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Pursuant to the terms of the Securities Purchase Agreement, the Investor assigned its interest in the Securities Purchase Agreement to Harbin Pharmaceutical Group Co., Ltd. ("Harbin"). Harbin's $300 million investment was funded in three separate tranches. On November 8, 2018, the Company received the initial $100 million investment for the purchase of 100,000 shares of convertible preferred stock. The Company utilized the $100 million to pay a portion of the Tranche B-2 Term Loan due in March 2021 pursuant to the Amendment to its Senior Credit Facility and elected to use the payment to satisfy the scheduled amortization payments on the Term Loan Facility through December 2020. On January 2, 2019, the Company received $50 million investment for the second purchase of 50,000 shares of convertible preferred stock, and on February 13, 2019, the Company received the remaining $150 million for the final purchase of 149,950 shares of convertible preferred stock.
In March 2019, the Company announced the formation of a strategic partnership with IVC. Under the terms of the agreement, GNC received $101 million from IVC in the first quarter of 2019 and contributed its Nutra manufacturing and Anderson facility net assets in exchange for an initial 43 % ownership in the joint venture.
In connection with the receipt of the investments in 2019 as mentioned above, the Company paid down the remaining balance of the Tranche B-1 Term Loan of $147.3 million. The remaining proceeds together with cash generated from operating activities were utilized to pay a portion of the Tranche B-2 of $114.0 million and the original issuance discount due to the Tranche B-2 Term Loan lenders at 2 % of the outstanding balance.
Convertible Debt
Issuance and Terms
On August 10, 2015, the Company issued $287.5 million principal amount of 1.5 % convertible senior notes due 2020 in a private offering (the "Notes"). The Notes are governed by the terms of an indenture between the Company and BNY Mellon Trust Company, N.A., as the Trustee (the "Indenture"). The Notes mature on August 15, 2020, unless earlier repaid, discharged, refinanced or converted by the holders subject to restrictions through May 15, 2020. The Notes bear interest at a rate of 1.5 % per annum, and additionally are subject to special interest in connection with any failure of the Company to perform certain of its obligations under the Indenture.
The Notes are unsecured obligations and do not contain any financial covenants or restrictions on the payments of dividends, the incurrence of indebtedness or the issuance or repurchase of securities by the Company or any of its subsidiaries. Certain events are considered “events of default” under the Notes, which may result in the acceleration of the maturity of the Notes, as described in the indenture governing the Notes. The Notes are fully and unconditionally guaranteed by certain operating subsidiaries of the Company (“Subsidiary Guarantors”) and are subordinated to the Subsidiary Guarantors obligations from time to time with respect to the Senior Credit Facility and ranks equal in right of payment with respect to the Subsidiary Guarantor’s other obligations.
The initial conversion rate applicable to the Notes is 15.1156 shares of common stock per $1,000 principal amount of Notes, which is equivalent to an initial conversion price of $66.16 per share. The conversion rate is subject to adjustment upon the occurrence of certain specified events, but will not be adjusted for any accrued and unpaid special interest. In addition, upon the occurrence of a “make-whole fundamental change" as defined in the Indenture, the Company will, in certain circumstances, increase the conversion rate by a number of additional shares for a holder that elects to convert its Notes in connection with such make-whole fundamental change.
Prior to May 15, 2020, the Notes are convertible only under the following circumstances: (1) during any calendar quarter commencing after September 30, 2015, if, for at least 20 trading days (whether or not consecutive) during the 30 consecutive trading day period ending on the last trading day of the immediately preceding calendar quarter, the last reported sale price of the Company’s common stock on such trading day is greater than or equal to 130 % of the applicable conversion price on such trading day; (2) during the 5 consecutive business day period after any ten consecutive trading day period in which, for each day of that period, the trading price per $1,000 principal amount of Notes for such trading day was less than 98 % of the product of the last reported sale price of the Company’s common stock and the applicable conversion rate on such trading day; or (3) upon the occurrence of specified corporate transactions. On and after May 15, 2020, until the close of business on the second scheduled trading day immediately preceding the maturity date, holders may convert all or a portion of their Notes at any time, regardless of the foregoing circumstances. Upon conversion, the Notes will be settled, at the Company’s election, in cash, shares of the Company’s common stock, or a combination of cash and shares of the Company’s common stock. If the Company has not delivered a notice of its election of settlement method prior to the final conversion period, it will be deemed to have elected combination settlement with a dollar amount per note to be received upon conversion of $1,000 . None of these circumstances was met during the year ended December 31, 2019 and 2018.
91
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Exchange
On December 20, 2017, the Company exchanged in privately negotiated transactions $98.9 million in aggregate principal amount of the Notes for an aggregate of 14.6 million newly issued shares of the Company’s Class A common stock, which had a value of $71.7 million at the time of the exchange. The Company accounted for the transaction as a troubled debt restructuring as a result of satisfying the below criteria.
• | Previous challenges associated with the Company’s refinancing efforts of its long term debt at the time of the convertible debt exchange. |
• | The holders of the convertible debt completed the exchange for a value lower than the face amount of the notes. As a result, management concluded a concession was granted to the Company. |
The convertible debt exchange resulted in a gain of $15.0 million, which includes the unamortized conversion feature of $9.6 million, unamortized discount of $1.4 million and other third party fees of $1.2 million and together with legal, investment banking and rating agency fees associated with the Company’s refinancing efforts, the Company recorded a net gain of $11.0 million in the fourth quarter of 2017.
Repurchase
During the second quarter of 2019, the Company repurchased $29.5 million in aggregate principal amount of the Notes for $24.7 million in cash. The convertible debt repurchase resulted in a gain of $3.2 million, which included the unamortized conversion feature of $1.3 million and unamortized discount of $0.2 million.
Notes by Component
The Notes consist of the following components:
As of December 31, | |||||||
2019 | 2018 | ||||||
(in thousands) | |||||||
Liability component | |||||||
Principal | $ | $ | |||||
Conversion feature | ( | ) | ( | ) | |||
Discount related to debt issuance costs | ( | ) | ( | ) | |||
Net carrying amount | $ | $ | |||||
Equity component | |||||||
Conversion feature | $ | $ | |||||
Debt issuance costs | ( | ) | ( | ) | |||
Deferred taxes (*) | ( | ) | ( | ) | |||
Net amount recorded in additional paid-in capital | $ | $ | |||||
(*) The balance at December 31, 2019 includes $0.1 million related to the tax provision that was allocated to additional paid in capital associated with the convertible debt repurchase.
Interest Rate Swaps
On June 13, 2018, the Company entered into two interest rate swaps with notional amounts of $275 million and $225 million to limit the exposure to its variable interest rate debt by effectively converting it to a fixed interest rate. The Company receives payments based on the one-month LIBOR and makes payments based on a fixed rate. The Company receives payments with a floor of 0.00 % and 0.75 %, respectively, on the $275 million and $225 million interest rate swaps, which aligns with the
92
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
related debt instruments, and makes payments on a fixed rate of 2.82 % and 2.74 %, respectively. The interest rate swap agreements had an effective date of June 29, 2018. The $225 million interest rate swap expires on February 28, 2021, and the $275 million interest rate swap expires on June 30, 2021. The notional amount of the $225 million interest rate swap has scheduled decreases to $175 million on June 30, 2019, $125 million on June 30, 2020 and $75 million on December 31, 2020. The Company designated these instruments as cash flow hedges and deemed effective upon initiation. The interest rate swaps are recognized on the balance sheet at fair value. Changes in fair value are recorded within other comprehensive income (loss) on the Consolidated Balance Sheet and reclassified into the Consolidated Statement of Operations as interest expense in the period in which the underlying transaction affects earnings.
The fair values of the derivative financial instruments included in the Consolidated Balance Sheets consisted of the following:
Fair Value at | ||||||||
Balance Sheet Classification | December 31, 2019 | December 31, 2018 | ||||||
(in thousands) | ||||||||
Other current liabilities | $ | $ | ||||||
Other long-term liabilities | ||||||||
Total liabilities | $ | $ | ||||||
At December 31, 2019, there was a cumulative unrealized loss of $4.8 million, net of tax, related to these interest rate swaps included in accumulated other comprehensive income (loss). This loss would be immediately recognized in the Consolidated Statement of Operations if these instruments fail to meet certain cash flow hedge requirements. As of December 31, 2019, the amount included in accumulated other comprehensive loss related to the interest rate swaps to be reclassified into earnings during the next 12 months is approximately $4 million. Refer to Note 11, "Fair Value Measurements of Financial Instruments" for more information on how the interest rate swaps are valued.
At December 31, 2018, there was a cumulative unrealized loss of $2.2 million, net of tax, related to these interest rate swaps included in accumulated other comprehensive income (loss). This loss would be immediately recognized in the Consolidated Statement of Operations if these instruments fail to meet certain cash flow hedge requirements. As of December 31, 2018, the amount included in accumulated other comprehensive loss related to the interest rate swaps to be reclassified into earnings during the next 12 months is not material.
93
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Interest Expense
For the year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Senior Credit Facility: | |||||||||||
Tranche B-1 Term Loan coupon | $ | $ | $ | ||||||||
Tranche B-2 Term Loan coupon | |||||||||||
FILO Term Loan coupon | |||||||||||
Revolving Credit Facility | |||||||||||
Terminated revolving credit facility | |||||||||||
Amortization of discount and debt issuance costs | |||||||||||
Total Senior Credit Facility | |||||||||||
Notes: | |||||||||||
Coupon | |||||||||||
Amortization of conversion feature | |||||||||||
Amortization of discount and debt issuance costs | |||||||||||
Total Notes | |||||||||||
Interest income and other | ( | ) | ( | ) | |||||||
Interest expense, net | $ | $ | $ | ||||||||
NOTE 9. EQUITY METHOD INVESTMENTS
In February 2019, the Company contributed its China business in exchange for 35 % ownership of each of the joint ventures with Harbin, the HK JV and China JV. The HK JV includes the operation of the cross-border China e-commerce business, and has an exclusive right to use the Company’s trademarks to manufacture and distribute the Company’s products in China (excluding Hong Kong, Taiwan and Macau) via e-commerce channels. The China JV is a retail-focused joint venture to operate GNC's brick-and-mortar retail business in China and it will have an exclusive right to use the Company's trademarks to manufacture and distribute the Company's products in China (excluding Hong Kong, Taiwan and Macau) via retail stores and pharmacies. The HK JV closed in February 2019 and the China JV agreement is expected to be completed in the second quarter of 2020, following the satisfaction of certain routine regulatory and legal requirements.
In March 2019, the Company entered into a strategic joint venture with IVC regarding the Company's manufacturing business. The Manufacturing JV is responsible for the manufacturing of the products previously produced by the Company at the Nutra manufacturing facility. The Company received $99.2 million from IVC and contributed the net assets of the Nutra manufacturing and Anderson facilities in exchange for an initial 43 % equity interest in the Manufacturing JV. In addition, the Company made a capital contribution of $10.7 million to the Manufacturing JV to fund its share of short-term working capital needs. IVC is expected to pay an additional $75.0 million over a four year period from the effective date of the transaction as IVC’s ownership of the joint venture increases to 100 %. The subsequent purchase price for each year is $18.8 million, adjusted up or down based on the Manufacturing JV's future performance. IVC's subsequent purchase price in the first quarter of 2020 is expected to be between approximately $16 million and $17 million based on the Manufacturing JV's performance in 2019.
Gain (loss) from the net asset exchange
In connection with the formation of the joint ventures effective in the first quarter of 2019, the Company deconsolidated its China business and its Nutra manufacturing business which resulted in a pre-tax gain of $5.8 million and loss of $27.1 million, respectively, recorded within loss on net asset exchange for the formation of the joint ventures on the Consolidated Statements of Operations. The $5.8 million gain from the Harbin transaction was calculated based on the difference between the fair value of the 35 % equity interest in the HK JV and China JV, less the carrying value of the contributed China business, including $2.4 million
94
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
of cash, and third-party closing fees. The $27.1 million loss from the Manufacturing JV transaction was calculated based on the fair value of the 43 % equity interest retained in the Manufacturing JV and the $101 million in cash received, net of a $1.8 million working capital purchase price adjustment in the second quarter of 2019, less the carrying value of the contributed Nutra and Anderson facilities and third-party closing fees.
The Company's interests in the joint ventures are accounted for as equity method investments due to the Company’s ability to exercise significant influence over management decisions of the joint ventures. Under the equity method, the Company's share of profits and losses from the joint ventures is recorded within income from equity method investments on the Consolidated Statement of Operations. The following table provides a reconciliation of equity method investments on the Company’s Consolidated Balance Sheets:
December 31, 2019 | |||
(in thousands) | |||
Manufacturing JV | $ | ||
Manufacturing JV capital contribution | |||
HK JV and China JV | |||
Income from equity method investments | |||
Distributions received from equity method investments | ( | ) | |
Total Equity method investments | $ | ||
NOTE 10. DEFERRED REVENUE AND OTHER CURRENT LIABILITIES
Deferred revenue and other current liabilities consisted of the following:
December 31, | |||||||
2019 | 2018 | ||||||
(in thousands) | |||||||
Deferred revenue | $ | $ | |||||
Accrued compensation and related benefits | |||||||
Accrued occupancy (*) | |||||||
Accrued sales tax | |||||||
Accrued interest | |||||||
Interest rate swap | |||||||
Other current liabilities | |||||||
Total deferred revenue and other current liabilities | $ | $ | |||||
(*) In connection with the the adoption of ASC 842, as further described in Note 2, "Basis of Presentation and Summary of Significant accounting policies", minimum lease payments are included in lease liabilities on the Consolidated Balance Sheet as of December 31, 2019.
NOTE 11. FAIR VALUE MEASUREMENTS AND FINANCIAL INSTRUMENTS
ASC 820, "Fair Value Measurements and Disclosures" defines fair value as a market-based measurement that should be determined based on the assumptions that marketplace participants would use in pricing an asset or liability. As a basis for considering such assumptions, the standard establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value as follows:
95
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Level 1 — observable inputs such as quoted prices in active markets for identical assets and liabilities; |
Level 2 — observable inputs such as quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, other inputs that are observable, or can be corroborated by observable market data; and |
Level 3 — unobservable inputs for which there are little or no market data, which require the reporting entity to develop its own assumptions. |
The carrying amounts of cash and cash equivalents, receivables, accounts payable, accrued liabilities and the Revolving Credit Facility approximate their respective fair values. Based on the interest rates currently available and their underlying risk, the carrying value of franchise notes receivable recorded primarily in Other long-term assets approximates its fair value.
The carrying value and estimated fair value of the forward contracts for the issuance of convertible preferred stock, the Term Loan Facility, net of discount, Notes (net of the equity component classified in stockholders' equity and discount) and the interest rate swaps were as follows:
December 31, 2019 | December 31, 2018 | ||||||||||||||
Carrying Amount | Fair Value | Carrying Amount | Fair Value | ||||||||||||
(in thousands) | |||||||||||||||
Assets: | |||||||||||||||
Forward contracts for the issuance of convertible preferred stock | $ | $ | $ | $ | |||||||||||
Liabilities: | |||||||||||||||
Tranche B-1 Term Loan | $ | $ | $ | $ | |||||||||||
Tranche B-2 Term Loan | |||||||||||||||
FILO Term Loan | |||||||||||||||
Notes | |||||||||||||||
Interest rate swaps | |||||||||||||||
The forward contracts for the issuance of convertible preferred stock were measured at fair value, as of the valuation date, using a single factor binomial lattice model ("Lattice Model") which incorporates the terms and conditions of the convertible preferred stock and is based on changes in the prices of the underlying common share price over successive periods of time. Key assumptions of the Lattice Model include the current price of the underlying stock and its historical and expected volatility, risk-neutral interest rates and the instruments remaining term. These assumptions require significant management judgment and are considered Level 3 inputs. The forward contract was revalued at each reporting period and changes in fair value are recognized in the Consolidated Statements of Operations. The forward contracts settled upon issuance on January 2, 2019 and February 13, 2019. Refer to Note 14, "Mezzanine Equity" for discussion of the Securities Purchase Agreement.
The fair values of the term loans were determined using the instrument’s trading value in markets that are not active, which are considered Level 2 inputs. The fair value of the Notes was determined based on quoted market prices and bond terms and conditions, which are considered Level 2 inputs. The Company's interest rate swaps are carried at fair value, which is based primarily on Level 2 inputs utilizing readily observable market data, such as LIBOR forward rates, for all substantial terms of the interest rate swap contracts and the assessment of nonperformance risk.
As described in Note 6, "Goodwill and Intangible Assets, Net," and Note 7, "Property, Plant and Equipment, Net," the Company recorded long-lived asset impairments in the years ended December 31, 2018 and 2017. This resulted in the following assets being measured at fair value on a non-recurring basis using Level 3 inputs:
•the indefinite-lived brand name intangible asset at December 31, 2018 and 2017;
•goodwill at December 31, 2017 for the Wholesale reporting unit;
•property and equipment at certain of the Company's stores at December 31, 2018 and 2017.
96
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 12. LEASES
The Company has operating leases for retail stores, distribution centers, other leased office locations, vehicles and certain equipment with remaining lease terms of one to 15 years, some of which include options to extend the leases for up to 10 years. As of December 31, 2019, the weighted average remaining lease term was 5.1 years and the weighted average discount rate was 10 %. On the Company’s Consolidated Balance Sheets as of December 31, 2019, the Company had lease liabilities of $442.5 million, of which $112 million are classified as current, and right-of-use assets of $350.6 million.
The components of the Company's lease costs, which are recorded within cost of sales on the Consolidated Statements of Operations, were as follows:
Year ended December 31, 2019 | |||
(in thousands) | |||
Operating lease costs | $ | ||
Variable lease costs | |||
Total lease costs | |||
Sublease income (1) | ( | ) | |
Lease costs, net | $ | ||
The Company has elected to apply the short-term lease exemption for all asset classes and excluded them from the balance sheet. Lease payments for short-term leases are recognized on a straight-line basis over the lease term. The short-term lease expense recognized during the year ended December 31, 2019 is immaterial.
Supplemental cash flow information related to leases was as follows:
Year ended December 31, 2019 | |||
(in thousands) | |||
Operating cash flow information: | |||
Cash paid for amounts included in the measurement of operating lease liabilities | $ | ||
Right-of-use assets obtained in exchange for operating lease liabilities | $ | ||
97
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Maturities of the lease liabilities (undiscounted lease payments, as defined in Note 2 "Basis of Presentation") as of December 31, 2019 were as follows:
Operating Leases for Company-Owned and Franchise Stores | Operating Leases for Other (1) | Total Operating Leases | Sublease Income from Franchisees | Rent on Operating Leases, net of Sublease Revenue | |||||||||||||||
(in thousands) | |||||||||||||||||||
2020 | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
2021 | ( | ) | |||||||||||||||||
2022 | ( | ) | |||||||||||||||||
2023 | ( | ) | |||||||||||||||||
2024 | ( | ) | |||||||||||||||||
Thereafter | ( | ) | |||||||||||||||||
Total future obligations | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Less amounts representing interest | ( | ) | |||||||||||||||||
Present value of lease obligations | $ | ||||||||||||||||||
(1) Includes various leases for warehouses, vehicles, and various equipment at the Company's facilities.
As of December 31, 2019, leases that the Company has entered into but have not yet commenced are immaterial.
In connection with the Manufacturing JV transaction effective March 1, 2019, the Company leased warehouse space within the Anderson facility from the Manufacturing JV for a term of one year . The lease was accounted for as a sale leaseback transaction and classified as an operating lease included in the current lease liabilities on the Consolidated Balance Sheet.
Disclosures related to periods prior to adoption of ASU 2016-02
The Company adopted ASU 2016-02 using a modified retrospective adoption method at January 1, 2019 as noted in Note 2. "Basis of Presentation." As required, the following disclosure is provided for periods prior to the adoption. The Company's rent expense, which is recorded within cost of sales on the Consolidated Statements of Operations, was as follows:
Year ended December 31, | |||||||
2018 | 2017 | ||||||
(in thousands) | |||||||
Company-owned and franchise stores: | |||||||
Rent on operating leases | $ | $ | |||||
Landlord related taxes | |||||||
Common operating expenses | |||||||
Percent and contingent rent | |||||||
Total company-owned and franchise stores | |||||||
Other | |||||||
Total rent expense | $ | $ | |||||
The Company recorded total sublease revenue relating to subleases with its franchisees, which includes rental income and other occupancy related items, within revenue on the Consolidated Statements of Operations, of $45.5 million and $49.0 million, respectively, in the year ended December 31, 2018 and 2017.
Minimum future rent obligations for non-cancelable operating leases, excluding optional renewal periods, were as follows for the period ended December 31, 2018 and exclude landlord related taxes, common operating expenses, and percent and contingent rent.
98
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Operating Leases for Company-Owned and Franchise Stores | Operating Leases for Other (1) | Total Operating Leases | Sublease Income from Franchisees | Rent on Operating Leases, net of Sublease Revenue | |||||||||||||||
(in thousands) | |||||||||||||||||||
2019 | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
2020 | ( | ) | |||||||||||||||||
2021 | ( | ) | |||||||||||||||||
2022 | ( | ) | |||||||||||||||||
2023 | ( | ) | |||||||||||||||||
Thereafter | ( | ) | |||||||||||||||||
Total future obligations | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
(1) Includes various leases for warehouses, vehicles, and various equipment at the Company's facilities.
99
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 13. COMMITMENTS AND CONTINGENCIES
The Company is engaged in various legal actions, claims and proceedings arising in the normal course of business, including claims related to breach of contracts, products liabilities, intellectual property matters and employment-related matters resulting from the Company's business activities.
The Company's contingencies are subject to substantial uncertainties, including for each such contingency the following, among other factors: (i) the procedural status of the case; (ii) whether the case has or may be certified as a class action suit; (iii) the outcome of preliminary motions; (iv) the impact of discovery; (v) whether there are significant factual issues to be determined or resolved; (vi) whether the proceedings involve a large number of parties and/or parties and claims in multiple jurisdictions or jurisdictions in which the relevant laws are complex or unclear; (vii) the extent of potential damages, which are often unspecified or indeterminate; and (viii) the status of settlement discussions, if any, and the settlement posture of the parties. Consequently, except as otherwise noted below with regard to a particular matter, the Company cannot predict with any reasonable certainty the timing or outcome of the legal matters described below, and the Company is unable to estimate a possible loss or range of loss. If the Company ultimately is required to make a payment in connection with an adverse outcome in any of the matters discussed below, it is possible that it could have a material adverse effect on the Company's business, financial condition, results of operations or cash flows.
As a retailer of nutritional supplements and other consumer products that are ingested by consumers or applied to their bodies, the Company has been and is currently subjected to various product liability claims. Although the effects of these claims to date have not been material to the Company, it is possible that current and future product liability claims could have a material adverse effect on its business or financial condition, results of operations or cash flows. The Company currently maintains product liability insurance with a deductible/retention of $4.0 million per claim with an aggregate cap on retained loss of $10.0 million per policy year. The Company typically seeks and has obtained contractual indemnification from most parties that supply raw materials for its products or that manufacture or market products it sells. The Company also typically seeks to be added, and has been added, as an additional insured under most of such parties' insurance policies. However, any such indemnification or insurance is limited by its terms and any such indemnification, as a practical matter, is limited to the creditworthiness of the indemnifying party and its insurer, and the absence of significant defenses by the insurers. Consequently, the Company may incur material product liability claims, which could increase its costs and adversely affect its reputation, revenue and operating income.
Litigation
DMAA / Aegeline Claims. Prior to December 2013, the Company sold products manufactured by third parties that contained derivatives from geranium known as 1.3-dimethylpentylamine/ dimethylamylamine/ 13-dimethylamylamine, or "DMAA," which were recalled from the Company's stores in November 2013, and/or Aegeline, a compound extracted from bael trees. As of December 31, 2019, the Company was named in 27 personal injury lawsuits involving products containing DMAA and/or Aegeline.
These matters are currently stayed pending final resolution.
The Company is contractually entitled to indemnification by its third-party vendors with regard to these matters, although the Company’s ability to obtain full recovery in respect of any such claims against it is dependent upon the creditworthiness of the vendors and/or their insurance coverage and the absence of any significant defenses available to their insurers.
California Wage and Break Claims. On February 29, 2012, former Senior Store Manager, Elizabeth Naranjo, individually and on behalf of all others similarly situated, sued General Nutrition Corporation in the Superior Court of the State of California for the County of Alameda. The complaint contains eight causes of action, alleging, among other matters, meal, rest break and overtime violations for which indeterminate money damages for wages, penalties, interest, and legal fees are sought. In June 2018, the Court granted in part and denied in part the Company's Motion for Decertification. In August 2018, the plaintiff voluntarily dismissed the class action claims alleging overtime violations. In November 2019, GNC filed a renewed Motion for Decertification, which was denied by the Court in January 2020. Trial is currently scheduled for July 2020. As of December 31, 2019, an immaterial liability has been accrued in the accompanying financial statements. The Company intends to vigorously defend against the remaining class action claims asserted in this action.
Pennsylvania Fluctuating Workweek. On September 18, 2013, Tawny Chevalier and Andrew Hiller commenced a class action in the Court of Common Pleas of Allegheny County, Pennsylvania. Plaintiff asserted a claim against the Company for a purported violation of the Pennsylvania Minimum Wage Act ("PMWA"), challenging the Company's utilization of the "fluctuating workweek" method to calculate overtime compensation, on behalf of all employees who worked for the Company in Pennsylvania
100
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
and who were paid according to the fluctuating workweek method. In October 2014, the Court entered an order holding that the use of the fluctuating workweek method violated the PMWA. In September 2016, the Court entered judgment in favor of Plaintiffs and the class in an immaterial amount, which has been recorded as a charge in the accompanying Consolidated Financial Statements. Plaintiffs subsequently filed a petition for an award of attorney's fees, costs and incentive payment. The court awarded an immaterial amount in legal fees. The Company appealed the adverse judgment and the award of attorney's fees. On December 22, 2017, the Pennsylvania Superior Court held that the Company correctly determined the "regular rate" by dividing weekly compensation by all hours worked (rather than 40), but held that the regular rate must be multiplied by 1.5 (rather than 0.5) to determine the amount of overtime owed. Taking accumulated interest into account, the net result of the Superior Court's decision was to reduce the Company's liability by an immaterial amount, which has been reflected in the accompanying Consolidated Financial Statements. The Company filed a petition for appeal to the Pennsylvania Supreme Court on January 22, 2018. The Pennsylvania Supreme Court accepted the Company's petition for appeal and the Company filed its appellant’s brief on August 27, 2018. The Pennsylvania Supreme Court ruled in favor of Plaintiffs.
Jason Olive v. General Nutrition Corp. In April 2012, Jason Olive filed a complaint in the Superior Court of California, County of Los Angeles, for misappropriation of likeness in which he alleges that the Company continued to use his image in stores after the expiration of the license to do so in violation of common law and California statutes. Mr. Olive is seeking compensatory, punitive and statutory damages and attorneys’ fees and costs. The trial in this matter began on July 20, 2016 and concluded on August 8, 2016. The jury awarded plaintiff immaterial amounts for actual damages and emotional distress damages, which are accrued in the accompanying Consolidated Financial Statements. The jury refused to award plaintiff any of the profits he sought to disgorge, or punitive damages. The court entered judgment in the case on October 14, 2016. In addition to the verdict, the Company and Mr. Olive sought attorneys' fees and other costs from the Court. The Court refused to award attorney's fees to either side but awarded plaintiff an immaterial amount for costs. Plaintiff has appealed the judgment, and separately, the order denying attorney's fees. The Company has cross-appealed the judgment and the Court's denial of attorney fees. Argument occurred in October 2018. On November 2, 2018, the Court affirmed the trial court's decision in part and reversed in part, reversing the denial of Mr. Olive's motion for attorneys' fees and remanding the matter to the trial court for further proceedings regarding his attorneys' fees and costs. On November 16, 2018, the Company filed a motion for reconsideration of the Court’s decision. On December 27, 2018, the Court reversed, in part, its November 2, 2018 ruling and held that there was no prevailing party for the purposes of the attorneys’ fee award. Olive has filed a petition for review with the Supreme Court of the State of California and the Company has opposed that petition. On April 17, 2019, the California Supreme Court denied Olive’s petition for review.
Oregon Attorney General. On October 22, 2015, the Attorney General for the State of Oregon sued the Company in Multnomah County Circuit Court for alleged violations of Oregon’s Unlawful Trade Practices Act, in connection with its sale in Oregon of certain third-party products. The Company is vigorously defending itself against these allegations. Along with its Amended Answer and Affirmative Defenses, the Company filed a counterclaim for declaratory relief, asking the court to make certain rulings in favor of the Company, and adding USPlabs, LLC and SK Laboratories as counterclaim defendants. In March 2018, the Oregon Attorney General filed a motion for summary judgment relating to its first claim for relief, which the Company contested. The Company filed a cross motion for summary judgment on the first claim for relief, which the Oregon Attorney General contested. Following oral argument in August 2018, the Court denied the State’s motion for summary judgment and granted in part and denied in part the Company’s motion for summary judgment. The parties are in the process of exchanging discovery. Trial is currently scheduled to begin in September 2020.
As any losses that may arise from this matter are not probable or reasonably estimable at this time, no liability has been accrued in the accompanying Consolidated Financial Statements. Moreover, the Company does not anticipate that any such losses are likely to have a material impact on the Company, its business or results of operations. The Company is contractually entitled to indemnification and defense by its third-party vendors. Ultimately, however, the Company's ability to obtain full recovery in respect of any such claims against it is dependent upon the creditworthiness of its vendors and/or their insurance coverage and the absence of any significant defenses available to their insurers.
E-Commerce Pricing Matters. In April 2016, Jenna Kaskorkis, et al. filed a complaint against General Nutrition Centers, Inc. followed by similar cases brought forth by Ashley Gennock in May 2016 and Kenneth Harrison in December 2016. Plaintiffs allege that the Company's promotional pricing on its website was misleading and did not fairly represent promotions based on average retail prices over a trended period of time being consistent with prices advertised as promotional. A tentative agreement was reached in the third quarter of 2017 on many of the key terms of a settlement. In December 2019, the Court approved the settlement agreement. The Company currently expects any settlement to be in a form that does not require the recording of a contingent liability.
Government Regulation
101
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In November 2013, the Company received a subpoena from the U.S. Department of Justice ("DOJ") for information related to its investigation of a third party product vendor, USPlabs, LLC. The Company fully cooperated with the investigation of the vendor and the related products, all of which were discontinued in 2013. In December 2016, the Company reached agreement with the DOJ in connection with the Company's cooperation, which agreement acknowledges the Company relied on the representations and written guarantees of USPlabs and the Company's representation that it did not knowingly sell products not in compliance with the Federal Food, Drug and Cosmetic Act (the "FDCA"). Under the agreement, which included an immaterial payment to the federal government, the Company will take a number of actions to broaden industry-wide knowledge of prohibited ingredients and improve compliance by vendors of third party products. These actions are in keeping with the leadership role the Company has taken in setting industry quality and compliance standards, and the Company's commitment over the course of the agreement (60 months) to support a combination of its own and the industry's initiatives. Some of these actions include maintaining and continuously updating a list of restricted ingredients that will be prohibited from inclusion in any products that are sold by the Company. Vendors selling products to the Company for the sale of such products by the Company will be required to warrant that the products sold do not contain any of these restricted ingredients. In addition, the Company will develop and maintain a list of ingredients that the Company believes comply with the applicable provisions of the FDCA.
Environmental Compliance
As part of soil and groundwater remediation conducted at the Nutra manufacturing facility pursuant to an investigation conducted in partnership with the South Carolina Department of Health and Environmental Control (the "DHEC"), the Company completed additional investigations with the DHEC's approval, including the installation and operation of a pilot vapor extraction system under a portion of the facility in the second half of 2016, which was an immaterial cost to the Company. After an initial monitoring period, in October of 2017 the DHEC approved a work plan for extended monitoring of such system and the contamination into 2021. While the Company contributed the net assets of the Nutra manufacturing and Anderson facilities to the Manufacturing JV in March of 2019 (refer to Note 9 “Equity Method Investments” for additional information), we retained certain liabilities, including historical environmental liabilities, related to the facilities. As such, the Company and the Manufacturing Joint Venture will continue to consult with the DHEC on the next steps in the work after their review of the results of the extended monitoring is complete. At this stage of the investigation, however, it is not possible to estimate the timing and extent of any additional remedial action that may be required, the ultimate cost of remediation, or the amount of our potential liability. Therefore, no liability has been recorded in the Company's Consolidated Financial Statements.
In addition to the foregoing, the Company is subject to numerous federal, state, local and foreign environmental and health and safety laws and regulations governing its operations, including the handling, transportation and disposal of non-hazardous and hazardous substances and wastes, as well as emissions and discharges from its operations into the environment, including discharges to air, surface water and groundwater. Failure to comply with such laws and regulations could result in costs for remedial actions, penalties or the imposition of other liabilities, including certain historic liabilities retained by the Company pursuant to the terms of the Manufacturing JV. New laws, changes in existing laws or the interpretation thereof, or the development of new facts or changes in their processes could also cause the Company to incur additional capital and operating expenditures to maintain compliance with environmental laws and regulations and environmental permits. The Company is also subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties, or for properties to which substances or wastes that were sent in connection with current or former operations at its facilities.
From time to time, the Company has incurred costs and obligations for correcting environmental and health and safety noncompliance matters and for remediation at or relating to certain of the Company's current or former properties or properties at which the Company's waste has been disposed. However, compliance with the provisions of national, state and local environmental laws and regulations has not had a material effect upon the Company's capital expenditures, earnings, financial position, liquidity or competitive position. The Company believes it has complied with, and is currently complying with, its environmental obligations pursuant to environmental and health and safety laws and regulations and that any liabilities for noncompliance will not have a material adverse effect on its business, financial performance or cash flows. However, it is difficult to predict future liabilities and obligations, which could be material.
Commitments
In addition to operating leases obtained in the normal course of business, the Company maintains certain purchase commitments with various vendors to ensure its operational needs are fulfilled. As of December 31, 2019, such future purchase commitments were $31.4 million. Other commitments related to the Company's business operations cover varying periods of time
102
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
and are not significant. All of these commitments are expected to be fulfilled with no adverse consequences to the Company's operations or financial condition.
NOTE 14. MEZZANINE EQUITY
Holdings is authorized to issue up to 60.0 million shares of preferred stock, par value $0.001 per share. On February 13, 2018, the Company entered into a Securities Purchase Agreement (as amended from time to time, the “Securities Purchase Agreement”) by and between the Company and Harbin Pharmaceutical Group Holdings Co., Ltd. (the “Investor”), pursuant to which the Company agreed to issue and sell to the Investor, and the Investor agreed to purchase from the Company, 299,950 shares of a newly created series of convertible preferred stock of the Company, designed the “Series A Convertible Preferred Stock” (the “Convertible Preferred Stock”), for a purchase price of $1,000 per share, or an aggregate of approximately $300 million (the “Securities Purchase”). The Convertible Preferred Stock is convertible into 56.1 million shares of the Company's Common Stock at an initial conversion price of $5.35 per share, subject to customary anti-dilution adjustments. Pursuant to the terms of the Securities Purchase Agreement, Investor assigned its interest in the Securities Purchase Agreement to Harbin Pharmaceutical Group Co., Ltd. ("Harbin").
On November 7, 2018, the Company and the Investor entered into an Amendment to the Securities Purchase Agreement (the “SPA Amendment”) for the funding of the Convertible Preferred Stock purchase and entered into definitive documentation (the "JV Framework Agreement") with respect to joint ventures in Hong Kong and China.
Pursuant to the SPA Amendment, the Company and the Investor agreed to complete the securities purchase as follows: (i) 100,000 shares of Convertible Preferred Stock issued on November 8, 2018 for a total purchase price of $100 million (the "Initial Issuance"), (ii) 50,000 shares of Convertible Preferred Stock issued on January 2, 2019 for a total purchase price of $50 million (the "Second Issuance") and (iii) 149,950 shares of Convertible Preferred Stock issued on February 13, 2019 for a total purchase price of approximately $150 million (the “Third Issuance”). Holders of shares of Convertible Preferred Stock are entitled to receive cumulative preferential dividends, payable quarterly in arrears, at an annual rate of 6.5 % of the stated value of $1,000 per share, subject to increase in connection with the payment of dividends in kind. Dividends are payable, at the Company's option, in cash from legally available funds or in kind by issuing additional shares of Convertible Preferred Stock with such stated value equal to the amount of payment being made or by increasing the stated value of the outstanding Convertible Preferred Stock by the amount per share of the dividend or in a combination thereof.
As of December 31, 2019 and 2018, the Company had issued a total of 299,950 shares and 100,000 shares, respectively, of Convertible Preferred Stock. The Convertible Preferred Stock was recorded as Mezzanine Equity, net of issuance cost, on the Consolidated Balance Sheets because the shares are redeemable at the option of the holder if a fundamental change occurs, which includes change in control or delisting. The guaranteed Second Issuance and Third Issuance were considered forward contracts that represented an obligation to both parties until the shares were issued. The forward contracts were recorded at fair value on the Consolidated Balance Sheets as of December 31, 2018, with any changes in fair value recorded in earnings in the Consolidated Statements of Operations. The Company recorded a $16.8 million loss on forward contracts for the issuance of Convertible Preferred Stock during the year ended December 31, 2019 and a $88.9 million gain for the year ended December 31, 2018. Upon issuance of the shares associated with the forward contracts, the carrying value of the forward contracts were recorded to Mezzanine Equity. Refer to Note 11, "Fair Value Measurement and Financial Instruments" for more information. The following table presents changes in the Company’s Mezzanine Equity:
Mezzanine Equity | ||||
(in thousands) | ||||
Balance at December 31, 2017 | $ | |||
Convertible Preferred Stock, net of issuance cost | ||||
Balance at December 31, 2018 | $ | |||
Convertible Preferred Stock, net of issuance cost | ||||
Change in fair value of the forward contracts | ( | ) | ||
Balance at December 31, 2019 | $ | |||
The Convertible Preferred Stock is not currently redeemable and is only redeemable upon a Fundamental Change at the Stated Value plus any accumulated and unpaid dividends on such shares on the Fundamental Change date. The Company does not believe a fundamental change is considered probable until it occurs. Subsequent adjustment of the amount presented in temporary
103
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 15. TREASURY STOCK
NOTE 16. EARNINGS PER SHARE
The following table represents the Company's basic and dilutive weighted average shares:
Year ended December 31, | ||||||||
2019 | 2018 | 2017 | ||||||
(in thousands) | ||||||||
Basic weighted average common shares outstanding | ||||||||
Effect of dilutive stock-based compensation awards | ||||||||
Effect of dilutive underlying shares of the convertible preferred stock | ||||||||
Diluted weighted averages common shares outstanding | ||||||||
For the year ended December 31, 2019 and 2017, all 3.9 million and 4.0 million outstanding stock-based awards, respectively, were excluded from the computation of diluted EPS because the Company was in a net loss position and as a result, inclusion of the awards would have been anti-dilutive. For the year ended December 31, 2018, the following awards were not included in the computation of diluted EPS because the impact of applying the treasury stock method was anti-dilutive or because certain conditions have not been met with respect to the Company's performance awards.
Anti-dilutive: | |||
Time-based options and restricted stock awards | |||
Performance-based restricted stock units | |||
Contingently issuable: | |||
Performance-based restricted stock awards with a market condition | |||
Total stock-based awards excluded from diluted EPS | |||
In connection with the issuance of the Convertible Preferred Stock as described in Note 14, "Mezzanine Equity", the Company had 300,000 and 100,000 convertible preferred shares outstanding as of December 31, 2019 and 2018, respectively. The Company applied the if-converted method to calculate dilution on the Convertible Preferred Stock, which resulted in all 54.0 million underlying weighted average convertible shares being anti-dilutive for the year ended December 31, 2019 and 2.7 million underlying weighted average convertible shares being dilutive for the year ended December 31, 2018.
In connection with the exchange of the Company's Notes as described in Note 8, "Long-Term Debt / Interest Expense," the Company issued 14.6 million shares, which are included in basic and diluted earnings per share for the weighted average days
104
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
they were outstanding in 2017. The remaining underlying convertible shares were anti-dilutive in 2017. The Company applied the if-converted method to calculate dilution on the Notes in 2019 and 2018, which has resulted in all 2.4 million and 2.9 million underlying convertible shares, respectively, being anti-dilutive.
The computations for basic and diluted earnings per common share are as follows:
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands, except per share data) | |||||||||||
(Loss) earnings per common share - Basic | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
Cumulative undeclared convertible preferred stock dividend | |||||||||||
Net (loss) income attributable to common shareholders | ( | ) | ( | ) | |||||||
Weighted average common shares outstanding - basic | |||||||||||
(Loss) income per common share - basic | $ | ( | ) | $ | $ | ( | ) | ||||
(Loss) income per common share - Diluted | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
Cumulative undeclared convertible preferred stock dividend | |||||||||||
Net (loss) income attributable to common shareholders | ( | ) | ( | ) | |||||||
Weighted average common shares outstanding - diluted | |||||||||||
(Loss) income per common share - diluted | $ | ( | ) | $ | $ | ( | ) | ||||
NOTE 17. STOCK-BASED COMPENSATION
Stock and Incentive Plans
The Company has outstanding stock-based compensation awards that were granted by the compensation committee of Holdings' Board of Directors (the "Compensation Committee") under the following three stock-based employee compensation plans:
•the GNC Holdings, Inc. 2018 Stock and Incentive Plan (the "2018 Stock Plan") amended adopted in May 2018, formerly the GNC Holdings, Inc. 2015 Stock and Incentive Plan adopted in May 2015;
•the GNC Holdings, Inc. 2015 Stock and Incentive Plan (the "2015 Stock Plan") amended and adopted in May 2015, formerly the GNC Holdings, Inc. 2011 Stock and Incentive Plan adopted in March 2011; and
•the GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan adopted in March 2007 (as amended, the "2007 Stock Plan").
All plans have provisions that allow for the granting of stock options, restricted stock and other stock-based awards and are available to eligible employees, directors, consultants or advisors as determined by the Compensation Committee. The Company will not grant any additional awards under either the 2007 Stock Plan or 2015 Stock Plan. Up to 20.2 million shares of common stock may be issued under the 2018 Stock Plan (subject to adjustment to reflect certain transactions and events specified in the 2018 Stock Plan for any award grant), of which 6.6 million and 11.1 million shares remain available for issuance as of December 31, 2019 and 2018, respectively, which has been reduced by 4.5 million shares and 2.2 million shares (which includes the allocation factor and performance multiplier), respectively, performance-based restricted stock units committed but not granted. See below "restricted stock awards" for more information.
Non-Plan Inducement Awards
On September 11, 2017, in connection with the appointment of the Company's new Chief Executive Officer, the Company made the following non-plan inducement awards:
• | "make-whole" restricted stock awards consisting of the following: |
105
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
◦ | $ |
◦ | $ |
◦ | $ |
• | time-vested awards consisting of |
The Company recognized $1.5 million in stock-based compensation in both 2019 and 2018 for the non-plan inducement awards.
Stock-Based Compensation Activity
The following table sets forth a summary of all stock-based compensation awards outstanding under all plans:
December 31, 2019 | December 31, 2018 | ||||
Time-based stock options | |||||
Time-based restricted stock awards | |||||
Performance-based restricted stock units | |||||
Performance-based restricted stock awards with a market condition | |||||
Total share awards outstanding | |||||
The Company recognized $4.6 million, $6.8 million and $8.4 million of total non-cash stock-based compensation expense for the years ended December 31, 2019, 2018 and 2017, respectively, net of estimated forfeitures based on the Company's historical experience and future expectations. At December 31, 2019, there was $9.9 million of total unrecognized compensation cost related to non-vested stock-based compensation, net of expected forfeitures, for all awards previously made that are expected to be recognized over a weighted-average period of 1.4 years. In 2019, 2018 and 2017, there were no stock options exercised.
Stock Options
Time-based stock options were valued using the Black-Scholes model with exercise prices at the Company's stock price on the date of grant which typically vest at 25 % per year over a four-year period except for the non-plan inducement awards as explained above. No stock options were granted during the year ended December 31, 2019 and 2018. The following table sets forth a summary of stock options under all plans.
Total Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | |||||||||
Outstanding at December 31, 2018 | $ | $ | ||||||||||
Granted | $ | |||||||||||
Exercised | $ | $ | ||||||||||
Forfeited and expired | ( | ) | $ | |||||||||
Outstanding at December 31, 2019 | $ | $ | ||||||||||
Exercisable at December 31, 2019 | $ | $ | ||||||||||
106
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The assumptions used in the Company's Black Scholes valuation during the year ended December 31, 2017 were as follows:
Year ended December 31, 2017 | |
Dividend yield | 0% |
Expected term | 6 - 6.3 years |
Volatility | 38.2% - 40.8% |
Risk free rate | 1.8% - 2.1% |
The option term has been estimated by considering both the vesting period and the contractual term. Volatility was estimated giving consideration to a peer group and the Company's own volatility. The Black Scholes valuation resulted in a weighted average grant date fair value in 2017 of $3.50 .
Restricted Stock Awards
Under the 2018 Stock Plan, the Company granted time-based and performance-based restricted stock and restricted stock units as well as, performance restricted shares with a market condition. Time-based awards vest in equal annual installments over a period of three years .
Performance-based restricted stock units vest after a period of three years and the achievement of performance targets; based on the extent to which the targets are achieved, vested shares may range from 0 % to 150 % of the original share amount. Performance targets are not determined until the beginning of each of the three fiscal years. Therefore, although the shares related to the second and third tranches are committed, they are not granted until performance targets are communicated to the participants. At December 31, 2019 and 2018, the Company had 4.5 million shares and 2.2 million shares, respectively, (which includes the allocation factor and performance multiplier) performance-based restricted stock units committed to be granted over the next two years.
Performance restricted shares with a market condition vest after a period of three years and the achievement of total shareholder return compared with that of a selected group of peer companies. Total shareholder return is defined as share price appreciation plus the value of dividends paid during the three year vesting period. Vested shares may range from 0 % to 200 % of the original target. Key assumptions used in the Monte Carlo simulation for the performance restricted shares with a market condition granted during the year ended December 31, 2017 includes a volatility of 34.6 % for the applicable peer group and a risk-free rate of 1.46 %. At December 31, 2019, all remaining outstanding performance restricted shares with a market condition were voluntarily forfeited by optionees.
The following table sets forth a summary of restricted stock awards granted under all plans:
Time-Based | Performance-Based | Performance Restricted Shares with a Market Condition | ||||||||||||||||||
Shares | Wtd Avg Grant Date Fair Value | Shares | Wtd Avg Grant Date Fair Value | Shares | Wtd Avg Grant Date Fair Value | |||||||||||||||
Outstanding at December 31, 2018 | $ | $ | $ | |||||||||||||||||
Granted | $ | $ | $ | |||||||||||||||||
Vested | ( | ) | $ | $ | $ | |||||||||||||||
Forfeited | ( | ) | $ | ( | ) | $ | ( | ) | $ | |||||||||||
Outstanding at December 31, 2019 | $ | $ | $ | |||||||||||||||||
The total intrinsic value of time-based restricted stock awards vested was $1.0 million, $1.3 million and $3.0 million for the years ended December 31, 2019, 2018 and 2017, respectively. The total intrinsic value of time-based restricted stock awards outstanding at December 31, 2019 was $2.8 million. The total intrinsic value of performance-based stock awards outstanding at December 31, 2019 was $2.6 million. In 2017, the weighted average grant date fair value of time-based and performance restricted shares with a market condition granted was $10.01 and $15.42 , respectively.
NOTE 18. RETIREMENT PLANS
The Company sponsors a 401(k) defined contribution savings plan covering substantially all employees who have attained age 21. Full time employees who have completed 30 days of service and part time employees who have completed 1,000 hours of service are eligible to participate in the plan. The plan provides for employee contributions of 1 % to 80 % of individual compensation into deferred savings, subject to IRS limitations. The plan provides for Company contributions upon the employee meeting the eligibility requirements. The Company match consists of both a fixed and a discretionary match. The fixed match is 50 % on the first 3 % of employee contributions and the discretionary match could be up to an additional 50 % match on the 3 % deferral. A discretionary match can be approved at any time by the Company.
An employee becomes vested in the Company match portion as follows:
Years of Service | Percent Vested | |
0-1 | % | |
1-2 | % | |
2-3 | % | |
3+ | % | |
The Company made cash contributions to the 401(k) plan of $1.5 million, $1.9 million and $2.1 million for the years ended December 31, 2019, 2018 and 2017, respectively.
107
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 19. SEGMENTS
The Company aggregates its operating segments into three reportable segments, which include U.S. and Canada, International and Manufacturing / Wholesale. Warehousing and distribution costs have been allocated to each reportable segment based on estimated utilization and benefit. The Company's chief operating decision maker (its chief executive officer) evaluates segment operating results based primarily on operating income. Operating income of each reportable segment excludes certain items that are managed at the consolidated level, such as corporate costs. The Manufacturing / Wholesale segment, prior to the formation of the Manufacturing JV, manufactured and sold product to the U.S. and Canada and International segments at cost with a markup, which was eliminated at consolidation. In connection with the asset sales of Lucky Vitamin as described in Note 6, "Goodwill and Intangible Assets," its results were included within Other for applicable prior periods.
The following table presents key financial information for each of the Company's reportable segments. During the year ended December 31, 2019, the Company entered into the China JV and HK JV with Harbin to operate its e-commerce and retail business in China and a strategic joint venture with IVC regarding the Company's manufacturing business which significantly impacted the operating results within the International and Manufacturing / Wholesale segments. During the year ended December 31, 2018, the Company recorded long-lived asset impairments of $38.2 million which significantly impacted the U.S. and Canada segment by $36.1 million and the International segment by $2.1 million. During the year ended December 31, 2017, the Company recorded long-lived asset impairments of $457.8 million which significantly impacted the U.S. and Canada segment by $412.5 million, the Manufacturing / Wholesale segment by $24.3 million, the International segment by $1.6 million and Lucky Vitamin within Other by $19.4 million. Refer to Note 6, "Goodwill and Intangible Assets" and Note 7, "Property, Plant and Equipment, Net" for more information.
108
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Revenue: | |||||||||||
U.S. and Canada | $ | $ | $ | ||||||||
International | |||||||||||
Manufacturing / Wholesale | |||||||||||
Intersegment revenues | |||||||||||
Third party | |||||||||||
Subtotal Manufacturing / Wholesale | |||||||||||
Total reportable segment revenues | |||||||||||
Other | |||||||||||
Elimination of intersegment revenues | ( | ) | ( | ) | ( | ) | |||||
Total revenue | $ | $ | $ | ||||||||
Operating income (loss): | |||||||||||
U.S. and Canada | $ | $ | $ | ( | ) | ||||||
International | |||||||||||
Manufacturing / Wholesale | |||||||||||
Total reportable segment operating income (loss) | ( | ) | |||||||||
Corporate costs | ( | ) | ( | ) | ( | ) | |||||
Loss on net asset exchange for the formation of the joint ventures | ( | ) | |||||||||
Other loss, net | ( | ) | ( | ) | ( | ) | |||||
Unallocated corporate costs, loss on net asset exchange or sale and other loss, net | ( | ) | ( | ) | ( | ) | |||||
Total operating income (loss) | ( | ) | |||||||||
Interest expense, net | |||||||||||
Gain on convertible debt and debt refinancing costs | ( | ) | ( | ) | |||||||
Loss on debt refinancing | |||||||||||
Loss (gain) on forward contracts for the issuance of convertible preferred stock | ( | ) | |||||||||
Income (loss) before income taxes | $ | $ | $ | ( | ) | ||||||
109
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Depreciation and amortization: | (in thousands) | ||||||||||
U.S. and Canada | $ | $ | $ | ||||||||
International | |||||||||||
Manufacturing / Wholesale | |||||||||||
Corporate and other | |||||||||||
Total depreciation and amortization | $ | $ | $ | ||||||||
Capital expenditures: | |||||||||||
U.S. and Canada | $ | $ | $ | ||||||||
International | |||||||||||
Manufacturing / Wholesale | |||||||||||
Corporate and Other | |||||||||||
Total capital expenditures | $ | $ | $ | ||||||||
As of December 31 | |||||||
2019 | 2018 | ||||||
Total assets: | (in thousands) | ||||||
U.S. and Canada | $ | $ | |||||
International | |||||||
Manufacturing / Wholesale | |||||||
Corporate and other | |||||||
Total assets (1) | $ | $ | |||||
Property, plant, and equipment, net: | |||||||
United States | $ | $ | |||||
Foreign | |||||||
Total property, plant and equipment, net | $ | $ | |||||
(1) Total assets as of December 31, 2019 included $350.6 million of right-of-use asset in connection with the adoption of ASC 842
110
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 20. UNAUDITED QUARTERLY FINANCIAL INFORMATION
The results of operations for the three months ended December 31, 2019 were impacted by a $27.1 million tax increase in valuation allowance against certain deferred tax assets that may not be realizable. The results of operations for the three months ended June 30, 2019 included $1.8 million loss on net asset exchange for the formation of joint venture and $3.2 million gain on convertible debt repurchase. The results of operations for the three months ended March 31, 2019 included $19.5 million loss on net asset exchange for the formation of joint ventures and $16.8 million loss on forward contracts for the issuance of convertible preferred stock. For more information on these items, refer to Note 5, "Income Taxes", Note 8, "Long-Term Debt / Interest Expense" and Note 9, "Equity Method Investments."
The results of operations for the three month ended December 31, 2018 were impacted significantly by the gain related to the forward contracts for the issuance of convertible preferred stock of $88.9 million and long-lived asset impairment charges of $23.7 million. Additionally, during the fourth quarter of 2018, the Company recorded an out-of-period adjustment to correct previously recorded specialty manufacturing revenue in the amount of $2.5 million to reduce contract manufacturing sales to third parties recorded in the Manufacturing/Wholesale segment as well as the corresponding contract asset included in Prepaid and other current assets. The impacts to the previously reported revenue and contract asset amounts were immaterial to the previously issued interim financial statements, and the adjustment was not material to the three months ended December 31, 2018. The results of operations for the three months ended September 30, 2018 included long-lived asset impairment charges and other store closing costs of $14.6 million. The results of operation for the three months ended March 31, 2018 included $16.7 million loss on debt refinancing. For more information on these items, refer to Note 6, "Goodwill and Intangible Assets", Note 8, "Long-Term Debt / Interest Expense" and Note 14, "Mezzanine Equity."
The following table summarizes the Company's 2019 and 2018 quarterly results:
Three months ended (unaudited) | Year ended | ||||||||||||||||||
March 31, | June 30, | September 30, | December 31, | December 31, | |||||||||||||||
2019 | 2019 | 2019 | 2019 | 2019 | |||||||||||||||
(In thousands, except per share amounts) | |||||||||||||||||||
Total revenue | $ | $ | $ | $ | $ | ||||||||||||||
Gross profit | |||||||||||||||||||
Operating income (loss) | |||||||||||||||||||
Net (loss) income | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||
Weighted average shares outstanding: | |||||||||||||||||||
Basic | |||||||||||||||||||
Diluted | |||||||||||||||||||
(Loss) earnings per share: | |||||||||||||||||||
Basic (1) | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
Diluted (1) | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
111
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Three months ended (unaudited) | Year ended | ||||||||||||||||||
March 31, | June 30, | September 30, | December 31, | December 31, | |||||||||||||||
2018 | 2018 | 2018 | 2018 | 2018 | |||||||||||||||
(In thousands, except per share amounts) | |||||||||||||||||||
Total revenue | $ | $ | $ | $ | $ | ||||||||||||||
Gross profit | |||||||||||||||||||
Operating income (loss) | ( | ) | |||||||||||||||||
Net income (loss) | ( | ) | |||||||||||||||||
Weighted average shares outstanding: | |||||||||||||||||||
Basic | |||||||||||||||||||
Diluted | |||||||||||||||||||
Earnings per share: | |||||||||||||||||||
Basic (1) | $ | $ | $ | ( | ) | $ | $ | ||||||||||||
Diluted (1) | $ | $ | $ | ( | ) | $ | $ | ||||||||||||
(1) Quarterly results for earnings per share may not add to full year results due to rounding or dilution impact.
112
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 21. SUBSEQUENT EVENTS
COVID-19
The recent outbreak of the coronavirus, or COVID-19, has caused business disruption in the International segment beginning in January 2020. In late February 2020, the situation escalated as the scope of COVID-19 worsened to outside of the Asia-Pacific region, with Europe and the United States recognizing outbreaks of COVID-19. As of March 23, 2020, the Company has temporarily closed approximately 25 % of the U.S. and Canada company-owned and franchise stores as a result of the COVID-19 pandemic. There is significant uncertainty relating to the potential impacts of COVID-19 on the Company’s business going forward due to various global macroeconomic, operational and supply chain risks as a result of COVID-19.
The Company could experience other potential impacts as a result of COVID-19, including, but not limited to, charges from potential adjustments to the carrying amount of inventory, goodwill, indefinite-lived intangibles and long-lived asset impairment charges. Actual results may differ materially from the Company’s current estimates as the scope of COVID-19 evolves or if the duration of business disruptions is longer than initially anticipated.
113
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
Item 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer ("CEO") and Chief Financial Officer ("CFO"), has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report. Disclosure controls and procedures are designed to provide reasonable assurance that the information required to be disclosed in the reports that we file or submit under the Exchange Act has been appropriately recorded, processed, summarized and reported on a timely basis and are effective in ensuring that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure. Based on such evaluation, our CEO and CFO have concluded that, as of December 31, 2019, our disclosure controls and procedures are effective at the reasonable assurance level.
Management's Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements.
Our management, with the participation of our CEO and CFO, has assessed the effectiveness of our internal control over financial reporting based on the framework and criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013). Based on this assessment, our management has concluded that, as of December 31, 2019, our internal control over financial reporting was effective based on that framework.
Our independent registered public accounting firm, PricewaterhouseCoopers LLP, has audited the effectiveness of our internal control over financial reporting as of December 31, 2019, as stated in their report, which is included in Item 8, "Financial Statements and Supplementary Data" of this Annual Report.
Changes in Internal Control Over Financial Reporting
There have not been any changes in our internal controls over financial reporting that occurred during the last fiscal quarter, which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. OTHER INFORMATION.
None.
114
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2020 Annual Meeting to be held on May 19, 2020, which is incorporated herein by reference, under the captions "Election of Directors," "Executive Officers," "Other Board Information," and "Section 16(a) Beneficial Ownership Reporting Compliance."
Item 11. EXECUTIVE COMPENSATION
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2020 Annual Meeting to be held on May 19, 2020, which is incorporated herein by reference, under the captions "Other Board Information", "Director Compensation," "Named Executive Officer Compensation" and "Compensation Discussion and Analysis;" provided, however, that the subsection entitled "Compensation Discussion and Analysis—Compensation Committee Report" shall be deemed to be furnished hereunder, but shall not be deemed to be incorporated by reference.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plans
The following table sets forth information regarding outstanding stock options and shares remaining available for future issuance under our equity compensation plans as of December 31, 2019:
Plan Category | Number of Securities to Be Issued upon Exercise of Outstanding Options, Warrants and Rights | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans | |||||||
Equity compensation plans approved by security holders (1) | ||||||||||
2007 Stock Plan | 50,700 | $ | 13.46 | — | ||||||
2015 Stock Plan | 1,846,409 | $ | 5.63 | — | ||||||
2018 Stock Plan | — | $ | — | 6,661,274 | (2)(3) | |||||
Subtotal | 1,897,109 | $ | 5.84 | 6,661,274 | ||||||
Equity compensation plans not approved by security holders (4) | 519,126 | $ | 8.95 | — | ||||||
Total | 2,416,235 | $ | 6.51 | 6,661,274 | ||||||
(1)Effective May 2018, our GNC Holdings, Inc. 2015 Stock and Incentive Plan was amended and restated as GNC Holdings Inc. 2018 Stock and Incentive Plan (the "2018 Stock Plan"). Effective May 21, 2015, our GNC Holdings, Inc. 2011 Stock and Incentive Plan was amended and restated as the GNC Holdings, Inc. 2015 Stock and Incentive Plan (the “2015 Stock Plan”). The GNC Holdings, Inc. 2007 Stock Incentive Plan (the “2007 Stock Plan”), the 2015 Stock Plan and the 2018 Stock Plan are the only equity compensation plans that we have adopted, and each of the 2007 Stock Plan,2015 Stock Plan and 2018 Stock Plan has been approved by our stockholders.
(2)Excludes 1,327,283 outstanding stock options as set forth in the first column, 591,234 shares of outstanding time vested restricted stock, 954,937 shares of outstanding performance vesting restricted stock units, 1,678,736 shares of outstanding performance vesting restricted stock units committed but not granted, 355,258 shares of outstanding time vesting restricted stock units.
(3)Up to 20,220,000 shares of our common stock may be issued under the 2018 Stock Plan, respectively (subject to adjustment to reflect certain transactions and events specified in the 2018 Stock Plan for any award grant). If any award granted under the 2018 Stock Plan expires, terminates or is canceled without having been exercised in full, the number of shares underlying such unexercised award will again become available for issuance under the 2018 Stock Plan. The total number of shares of our common stock available for awards under the 2018 Stock Plan will be reduced by (i) the total number of stock options or stock appreciation rights exercised, regardless of whether any of the shares of our common stock underlying such awards are not actually issued to the participant as the result of a net settlement and (ii) any shares of our common stock used to pay any exercise price or tax withholding obligation. In addition, the number of shares of our common stock that are subject to restricted stock, performance shares or other stock-based awards that are not subject
115
to the appreciation of the value of a share of our common stock ("Full Share Awards") is limited by counting shares granted pursuant to such Full Share Awards against the aggregate share reserve as 1.8 shares for every share granted. If any stock option, stock appreciation right or other stock-based award that is not a Full Share Award is canceled, expires or terminates unexercised for any reason, the shares covered by such awards will again be available for issuance. If any shares of our common stock that are subject to Full Share Awards are forfeited for any reason, 1.8 shares of our common stock will again be available for issuance under the 2018 Stock Plan.
(4)The Company's non-shareholder approved plan is the inducement exception plan pursuant to NYSE rules for awards granted to Kenneth A. Martindale pursuant to his employment agreement ("Inducement Exception Awards"), under which no further grants may be made. The Inducement Exception Awards were made pursuant to the inducement award exception under the NYSE rules to induce an executive officer to join the Company. These awards were granted to Kenneth A. Martindale pursuant to his employment agreement and were made in order to attract and retain an executive of his unique caliber and experience. Refer to Item 8, "Financial Statements and Supplementary Data," Note17, "Stock-Based Compensation" for details of the non-plan inducement awards.
Additional information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2020 Annual Meeting to be held on May 19, 2020, which is incorporated herein by reference, under the caption "Security Ownership of Certain Beneficial Owners and Management."
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE.
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2020 Annual Meeting to be held on May 19, 2020, which is incorporated herein by reference, under the captions "Certain Relationships and Related Transactions," and "Director Independence."
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2020 Annual Meeting to be held on May 19, 2020, which is incorporated herein by reference, under the caption "Ratification of Appointment of Auditors."
116
PART IV
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
(a) | Documents filed as part of this Annual Report: |
(1) | Financial statements filed in Part II, Item 8 of this Annual Report: |
• | Report of Independent Registered Public Accounting Firm |
• | Consolidated Balance Sheets |
As of December 31, 2019 and December 31, 2018
• | Consolidated Statements of Operations |
For the years ended December 31, 2019, 2018 and 2017
• | Consolidated Statements of Comprehensive (Loss) Income |
For the years ended December 31, 2019, 2018 and 2017
• | Consolidated Statements of Stockholders' (Deficit) Equity |
For the years ended December 31, 2019, 2018 and 2017
• | Consolidated Statements of Cash Flows |
For the years ended December 31, 2019, 2018 and 2017
• | Notes to Consolidated Financial Statements |
117
(2) | Financial statement schedules: |
SCHEDULE I—CONDENSED FINANCIAL INFORMATION OF GNC HOLDINGS, INC.
GNC HOLDINGS, INC.
(Parent Company Only)
Balance Sheets
December 31, | |||||||
2019 | 2018 | ||||||
Current assets: | (in thousands) | ||||||
Cash and cash equivalents | $ | $ | |||||
Intercompany receivable | |||||||
Forward contracts for the issuance of convertible preferred stock | |||||||
Prepaid and other current assets | |||||||
Total current assets | |||||||
Long-term assets: | |||||||
Deferred tax assets | |||||||
Intercompany receivable | |||||||
Investment in subsidiaries | ( | ) | |||||
Total long-term assets | |||||||
Total assets | $ | $ | |||||
Current liabilities: | |||||||
Intercompany payable | |||||||
Convertible senior notes | |||||||
Deferred revenue and other current liabilities | |||||||
Total current liabilities | |||||||
Long-term liabilities: | |||||||
Deferred tax liabilities | |||||||
Convertible senior notes | |||||||
Intercompany loan | |||||||
Total long term liabilities | |||||||
Total liabilities | |||||||
Mezzanine equity: | |||||||
Series A convertible preferred stock | |||||||
Stockholders' deficit: | |||||||
Class A common stock | |||||||
Additional paid-in capital | |||||||
Retained earnings | |||||||
Treasury stock, at cost | ( | ) | ( | ) | |||
Accumulated other comprehensive loss | ( | ) | ( | ) | |||
Total stockholders' deficit | ( | ) | ( | ) | |||
Total liabilities, mezzanine equity and stockholders' deficit | $ | $ | |||||
See the accompanying note to the condensed parent-only financial statements.
118
SCHEDULE I—CONDENSED FINANCIAL INFORMATION OF GNC HOLDINGS, INC.
GNC HOLDINGS, INC.
(Parent Company Only)
Statements of Operations and Comprehensive (Loss) Income
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands, except per share data) | |||||||||||
Selling, general and administrative | $ | $ | $ | ||||||||
Subsidiary loss | |||||||||||
Operating loss | ( | ) | ( | ) | ( | ) | |||||
Interest expense, net | |||||||||||
Gains on convertible notes transactions | ( | ) | ( | ) | |||||||
Loss (gain) on forward contracts for the issuance of convertible stock | ( | ) | |||||||||
(Loss) income before income taxes | ( | ) | ( | ) | |||||||
Income tax expense (benefit) | ( | ) | ( | ) | |||||||
Net (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
Other comprehensive (loss) income: | |||||||||||
Net change in unrecognized loss on interest rate swaps, net of tax | $ | ( | ) | $ | ( | ) | $ | ||||
Foreign currency translation gain (loss) | ( | ) | |||||||||
Other comprehensive (loss) gain | ( | ) | ( | ) | |||||||
Comprehensive (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
(Loss) earnings per share: | |||||||||||
Basic | $ | ( | ) | $ | $ | ( | ) | ||||
Diluted | $ | ( | ) | $ | $ | ( | ) | ||||
Weighted average common shares outstanding: | |||||||||||
Basic | |||||||||||
Diluted | |||||||||||
See the accompanying note to the condensed parent-only financial statements.
119
SCHEDULE I—CONDENSED FINANCIAL INFORMATION OF GNC HOLDINGS, INC.
GNC HOLDINGS, INC.
(Parent Company Only)
Statements of Cash Flows
Year ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in thousands) | |||||||||||
Cash flows from operating activities: | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ( | ) | ||||
Deficit in (income) loss of subsidiaries | |||||||||||
Interests received from intercompany loan | |||||||||||
(Loss) gain on forward contracts for the issuance of convertible preferred stock | ( | ) | |||||||||
Gains on convertible notes transactions | ( | ) | ( | ) | |||||||
Other operating activities | |||||||||||
Net cash provided by operating activities | |||||||||||
Cash flows from investing activities: | |||||||||||
Capital contribution to subsidiaries | ( | ) | |||||||||
Net cash used in investing activities | ( | ) | |||||||||
Cash flows from financing activities: | |||||||||||
Proceeds from the issuance of convertible preferred stock | |||||||||||
Proceeds from intercompany receivables | |||||||||||
Payments on intercompany loan | ( | ) | ( | ) | |||||||
Convertible notes repurchase | ( | ) | |||||||||
Minimum tax withholding requirements | ( | ) | ( | ) | ( | ) | |||||
Net cash provided by (used in) financing activities | ( | ) | ( | ) | |||||||
Net (decrease) increase in cash and cash equivalents | ( | ) | |||||||||
Beginning balance, cash and cash equivalents | |||||||||||
Ending balance, cash and cash equivalents | $ | $ | $ | ||||||||
See the accompanying note to the condensed parent-only financial statements.
120
GNC HOLDINGS, INC.
SCHEDULE I—NOTES TO THE CONDENSED FINANCIAL STATEMENTS (PARENT ONLY)
NOTE 1. BACKGROUND
These condensed parent company financial statements should be read in conjunction with the Consolidated Financial Statements of GNC Holdings, Inc. and subsidiaries. The Senior Credit Facility of General Nutrition Centers, Inc. ("Centers"), a wholly owned subsidiary of GNC Holdings, Inc., contains customary covenants, including incurrence covenants and certain other limitations on the ability of GNC Corporation, Centers, and Centers' subsidiaries to, among other things, make optional payments in respect of other debt instruments, pay dividends or other payments on capital stock, and enter into arrangements that restrict their ability to pay dividends or grant liens.
121
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
GNC Holdings, Inc. and Subsidiaries
Valuation and Qualifying Accounts
(in thousands)
Balance at Beginning of Period | Charged to Costs and Expenses | Deductions | Balance at End of Period | ||||||||||||
2017 | |||||||||||||||
Allowance for doubtful accounts | $ | $ | $ | ( | ) | $ | |||||||||
Reserve for sales returns | ( | ) | |||||||||||||
Tax valuation allowances | ( | ) | |||||||||||||
2018 | |||||||||||||||
Allowance for doubtful accounts | $ | $ | $ | ( | ) | $ | |||||||||
Reserve for sales returns | ( | ) | |||||||||||||
Tax valuation allowances | |||||||||||||||
2019 | |||||||||||||||
Allowance for doubtful accounts | $ | $ | $ | ( | ) | $ | |||||||||
Reserve for sales returns | ( | ) | |||||||||||||
Tax valuation allowances | ( | ) | |||||||||||||
122
(1) | Exhibits: |
Listed below are all exhibits filed as part of this Annual Report. Certain exhibits are incorporated by reference from statements and reports previously filed by Holdings or Centers with the SEC pursuant to Rule 12b-32 under the Exchange Act:
3.1 | |
3.2 | |
3.3 | |
4.1 | |
4.2 | |
4.3 | |
10.1 | |
10.2 | |
10.3 | |
10.4 | |
10.5 | |
10.6 | |
10.7 | |
10.8 | |
10.9 | |
10.10 | |
123
10.11 | |
10.12 | |
10.13 | |
10.14 | |
10.15 | |
10.16 | |
10.17 | |
10.18 | |
10.19 | |
10.20 | |
10.21 | |
10.22 | |
10.23 | |
124
10.24 | |
10.25 | |
10.26 | |
10.27 | |
10.28 | |
10.29 | |
10.30 | |
10.31 | |
10.32 | |
10.33 | |
10.34 | |
10.35 | |
10.36 | |
10.37 | |
10.38 | |
10.39 | Amended and Restated Stockholders Agreement, dated as of February 13, 2019, by and between GNC Holdings, Inc. and Harbin Pharmaceutical Group Co., Ltd. (Incorporated by reference to Exhibit 10.1 to Holdings’ Quarterly Report on Form 10-Q (File No. 001-35113), filed October 25, 2019.) |
10.40 | |
125
10.41 | |
10.42 | |
10.43 | |
21.1 | |
23.1 | |
31.1 | |
31.2 | |
32.1 | |
101.INS | XBRL Instance Document [The instance document does not appear in the interactive data file because its XBRL tags are embedded within the inline XBRL document.] |
101.SCH | XBRL Taxonomy Extension Schema |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase |
101.LAB | XBRL Taxonomy Extension Label Linkbase |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase |
101.DEF | XBRL Taxonomy Extension Definition Linkbase |
104 | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101). |
_______________________________________________________________________________
* Filed herewith
** Management contract or compensatory plan or arrangement of the Company required to be filed as an exhibit.
† Portions of this exhibit have been omitted pursuant to a request for confidential treatment. The omitted portions have been separately filed with the SEC.
126
Item 16. FORM 10-K SUMMARY.
We may voluntarily include a summary of information required by Form 10-K under this Item 16. We have elected not to include such summary information.
127
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
GNC HOLDINGS, INC. | ||
By: | /s/ KENNETH A. MARTINDALE | |
Kenneth A. Martindale Director, Chief Executive Officer Dated: Dated: March 25, 2020 | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
By: | /s/ KENNETH A. MARTINDALE | |
Kenneth A. Martindale Chairman, Chief Executive Officer (principal executive officer) Dated: March 25, 2020 | ||
By: | /s/ TRICIA K. TOLIVAR | |
Tricia K. Tolivar Chief Financial Officer (principal financial officer) Dated: March 25, 2020 | ||
By: | /s/ CAMERON W. LAWRENCE | |
Cameron W. Lawrence Chief Accounting Officer (principal accounting officer) Dated: March 25, 2020 | ||
By: | /s/ ALAN FELDMAN | |
Alan D. Feldman Director Dated: March 25, 2020 | ||
By: | /s/ MICHAEL F. HINES | |
Michael F. Hines Director Dated: March 25, 2020 | ||
By: | /s/ AMY B. LANE | |
Amy B. Lane Director Dated: March 25, 2020 | ||
By: | /s/ RACHEL LAU | |
Rachel Lau Director Dated: March 25, 2020 | ||
128
By: | /s/ PHILIP E. MALLOTT | |
Philip E. Mallott Director Dated: March 25, 2020 | ||
By: | /s/ MICHELE S. MEYER | |
Michele S. Meyer Director Dated: March 25, 2020 | ||
By: | /s/ ROBERT F. MORAN | |
Robert F. Moran Director Dated: March 25, 2020 | ||
129