
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For
the fiscal year ended
or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________________ to ________________
Commission
file number

(Exact Name of Registrant as Specified In Its Charter)
| (State of Incorporation) | (I.R.S. Employer Identification No.) |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s
Telephone Number, Including Area Code: +
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| None | N/A | N/A |
Securities registered pursuant to section 12(g) of the Act:
Common Stock, $0.0001 per share
(Title of class)
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days. ☒
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” or “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| ☐ | Smaller reporting company | |||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate
whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) ☐ Yes ☒
The
aggregate market value of the common stock held by non-affiliates of the registrant was $
The registrant had shares of common stock outstanding as of March 21, 2023.
CITRINE GLOBAL CORP
2022 FORM 10-K ANNUAL REPORT
TABLE OF CONTENTS
| 2 |
Cautionary Statement regarding Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, or the Exchange Act. These forward-looking statements relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” or “continue” or the negative of these terms or other comparable terminology. These forward-looking statements are only predictions and involve known and unknown risks, uncertainties and other factors, any of which may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Forward-looking statements are made based on management’s beliefs, estimates and opinions on the date the forward-looking statements are made, and we undertake no obligation to update forward-looking statements should these beliefs, estimates, and opinions or other circumstances change. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these forward-looking statements to actual results.
Our financial statements are stated in United States dollars, or US$, and are prepared in accordance with United States generally accepted accounting principles, or GAAP. In this Annual Report, unless otherwise specified, all dollar amounts are expressed in United States dollars and all references to “common stock” refer to the shares of our common stock. As used in this Annual Report, the terms “we,” “us,” “our,” “Citrine Global,” the “Company” and the “Registrant” mean Citrine Global, Corp. and its subsidiaries unless the context clearly requires otherwise.
PART I
ITEM 1. BUSINESS
This summary highlights selected information contained elsewhere in this report and does not contain all the information that you should consider before making your investment decision. Before investing in our common stock, you should carefully read this entire report, including the information set forth under the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this report and our consolidated financial statements and the accompanying notes included in this report. Except as otherwise indicated herein or as the context otherwise requires, references in this report to “Citrine Global,” the “Company,” “we,” “us,” and “our” refer to Citrine Global, Corp. and our consolidated subsidiaries, including our wholly-owned subsidiary, CTGL-Citrine Global Israel Ltd. and to our partially owned subsidiary Cannovation Center Israel Ltd.;
Description of our Business and Industry Background:
We are a plant-based wellness & pharma solutions company with a vision of becoming a leading company in these fields and improve people’s health and quality of life worldwide.
The global health and wellness market is expected to reach USD 7.6 trillion by 2030, growing at a CAGR of 5.5% from 2021 to 20301 with growing awareness of health and wellness solutions for improving people’s quality of life2.
We are witnessing a global movement of health and wellbeing becoming a priority for the public, further emphasized by the global COVID-19 pandemic. There is increasing recognition that people need to take charge of their own health, improve their quality of life, use natural products, and balance side effects caused by medicines and treatment3.
Our headquarters and executives are based in Israel, where we operate via our 100%-owned-subsidiary “CTGL Citrine Global Israel Ltd.” and 60%-owned “Cannovation Center Israel Ltd.”
1 Research, P., 2022. Health and Wellness Market Size to Hit USD 7,656.7 Bn by 2030. [online] GlobeNewswire Newsroom.
2 NielsenIQ. 2022. An inside look into the 2021 global consumer health and wellness revolution. [online]
3 Sullivan, F., 2022. Increasing Health Consciousness Among Consumers to Shift the Global Prebiotic Ingredients Market. [online] Prnewswire.com.
| 3 |
We believe the power of plant-based solutions from nature can help improve people’s health and quality of life. Our business activity is primarily composed of developing wellness and pharma solutions, focused on science backed plant-based products to improve quality of life and complementary solutions for balancing side effects caused by using medicines, treatments, or an unbalanced lifestyle.
We have built an end-to-end strategy to bring to market on a global scale innovative plant-based wellness and pharma solutions covering the whole spectrum from innovation, research and development, product development, infrastructure for production and manufacturing, distribution, marketing and sales.
We seek to bring to the market wellness and pharma innovative products, such as food supplements, healthy snacks, healthy beverages and natural cosmetics, to help improve people’s health and quality of life and complementary products that aim to balance selected side effects associated with medicines, treatments or an unbalanced lifestyle.
Leveraging technology and research, we are focused on developing a products portfolio based on rigorous scientific research ranging from synergistic botanicals, herbal extract, tinctures, medicinal mushrooms together with plant extracts, vitamins, minerals, botanical formulations from seeds, roots, bark, fruits, and a wide variety of plants that contain substances with health-supportive effects. Such supportive effects include, but are not limited to, enhancing oral care, anti-inflammatory properties, relaxation, sleep enhancement, energizing, mood and body balancing, alleviating side effects, and more.
We have more than 100 plant-based formulations and product lines under the brands GreenFeels™ and Green Side by Side™ targeting the nutritional supplements market that is expected to reach $625 billion by 20304.
The product lines categories include:
| ● | Personal Protection & Health Supportive Product Line | |
| ● | Balance & Calm Product Line | |
| ● | Digestion , Weight Management Product Line | |
| ● | Men Product Line | |
| ● | Women Product Line | |
| ● | Sports & Energy Product Line | |
| ● | Oral Cavity Care Product Line | |
| ● | Vitamins & Minerals Product Line | |
| ● | Medicinal Mushrooms Product Line |
We started beta-testing several products in the Israeli market with plans for an international network focusing on the U.S. market through local teams, distributors, online and physical shops and collaboration with partners worldwide.
Our presence in Israel combined with our close contacts with leading universities, researchers, companies, shareholders and governmental support, allows us to access the latest technologies, talent, and innovation to bring innovative solutions to the global market.
We have strategic alliance and manufacturing agreements with iBOT Israel Botanicals, an affiliated and nutritional supplements’ company and GMP-certified manufacturing facility approved by the Israeli Ministry of Health. As part of our activity with iBOT, we are developing and manufacturing our nutritional supplements product lines, including the GreenFeels™ & Green Side by Side™ product lines.
Acquisition of MyPlant Bio Ltd. - On December 30, 2022, we purchased a 10% equity interest (on a fully diluted basis) in MyPlant Bio Ltd and Citrine Global has an option to purchase an additional 45% of MyPlant equity. See Business- The MyPlant Transaction.
MyPlant Bio Ltd, specializes in botanical drug development and owns certain know-how and intellectual property rights that include a developed platform and cell-disease models to screen plant extracts to understand their biological effect, and has screening platforms using cell line models for certain diseases and conditions to detect effective plant materials and/or other substances for the treatment of these conditions. MyPlant was founded by Cannasoul Analytics, a leading botanical research and development company and Prof. Dedi Meiri from the Faculty of Biology at the Israeli Institute of Technology (Technion) and a member of the Technion Integrated Cancer Center. Citrine Global’s acquisition of MyPlant is in line with the Citrine Global’s strategy to be a leader in plant-based wellness and pharma solutions.
4 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire
| 4 |
We view the acquisition of MyPlant as an opportunity to advance its wellness and planned pharma and botanical drug products with MyPlant’s scientific research as to the effects of specific plant substances and compounds on different wellness and medical conditions. We believe that we can benefit from the collaboration with MyPlant and Professor Dedi Meiri to advance the company’s strategy for developing scientifically backed plant-based products composed of specific active plant substances proven by research to be effective for different medical conditions and possibly offer personally customized products for individual patients tailored for their healing process.
The collaboration with MyPlant is part of our strategy to add value to our product lines and position our company with a competitive edge in the Wellness market and the Nutritional Supplements.
As the worldwide use of botanical nutritional supplements and botanical drugs continues to grow, the need for scientific evaluation of the safety and efficacy of these products is becoming ever greater. We are targeting and positioning our product lines for the nutritional supplements market that is expected to reach $625 billion by 20305.
Our mission is to leverage the power of plant-based solutions from nature to help improve people’s health and quality of life.
We created multi-strategy solutions to realize our mission, the highlights of which include the following:
Developing & Bringing Plant-Based Wellness & Pharma Products to Market:
The plant-based products market is booming with health-conscious consumers spending more on natural products, ranging from nutraceuticals, natural superfoods, beverages, cosmetics, to legal cannabis and the evolving market of botanical and plant-derived drugs. The COVID-19 pandemic has left a lasting impression on consumer behavior, particularly in relation to plant-based nutrition and natural immunity boosters6.
Here are the various growing plant-based product global market segments
| ● | The nutritional supplements market is expected to reach USD 624.7 billion by 20307. | |
| ● | The superfoods market is expected to reach USD 287.7 billion by 20278. | |
| ● | The legal cannabis market is expected to reach USD 70.6 billion by 20289. | |
| ● | The botanical and plant-derived drug market is expected to reach USD 53 billion by 202610. | |
| ● | The natural cosmetics market is expected to reach USD 24.8 billion by 202711. |
We are basing our efforts on technologies to create research and innovation, developing plant-based solutions which include products for improving quality of life and complementary solutions for balancing selected side effects caused by using medicines, cannabis, treatments, or an unbalanced lifestyle.
5 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire
6 Sullivan, F., 2022. Increasing Health Consciousness Among Consumers to Shift the Global Prebiotic Ingredients Market. [online] Prnewswire.com
7 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire News Room
8 Research, I., 2022. Global Superfoods Market Size is Projected To Reach US$ 287.75 Billion by 2027 | Superfoods Market Store, Delivery Options, Emerging Trends 2022 | Segmentation by Product Type, Applications, Regions, & Key-Players (ADM, Ardent Mills, Bunge). [online] GlobeNewswire News Room
9 Grandviewresearch.com. 2022. Legal Marijuana Market Size Worth $70.6 Billion By 2028
10 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online] Inkwood Research.
11 Fortune Business Insights, The global vegan cosmetics market is projected to grow to $24.79 billion in 2028 Report ID FBI106594 [online]
| 5 |
Our IP Strategy and R&D Roadmap include:
| ● | Developing wellness plant-based product portfolio across the range from scientific and research-based plants, such as herbal extracts, medicinal mushrooms, and other natural ingredients. | |
| ● | Expanding our current product lines and registering the products for worldwide regulatory approvals. | |
| ● | Developing complementary products for balancing selected side effects caused by medicines, treatments, aging, stress, or an unbalanced lifestyle. | |
| ● | Researching and developing pharma solutions with the mission of developing plant-based medicines and botanical drugs. | |
| ● | Building our patent portfolio, conducting clinical trials, and advancing our products through innovation and technology. | |
| ● | We filed provisional patent applications in the field of Balancing Side Effects in the United States Patent and Trademark Office (USPTO): Patent Application No. 63/418,046, and Patent Application No. 63/388,361 for compositions and methods for balancing side effects associated with the use of medicines, treatments, aging and unbalanced or unhealthy lifestyle and balancing side effects related to symptoms in the oral cavity. |
About Side Effects Caused by Using Medicines, Treatments or an Unbalanced Lifestyle
Side effects are unexpected reactions which may result from using medicines, treatments and an unbalanced lifestyle. There are common side effects, such as dryness in the oral cavity (xerostomia), headaches, dizziness, drowsiness, fatigue, nausea, vomiting, lack of concentration, and impaired appetite that are associated with the use of medicines and treatments12.
The public health impact of harms associated with medicines and treatments is a growing area of investigation, given the expanding pharma industry and widespread availability of drugs and different medical treatments around the world. Current evidence suggests that use of medicines is associated with side effects. Exploring the relationship between drug side-effects and therapeutic indications demonstrates that 69% of drugs have between 10 and 100 different side effects; 22% of drugs have more than 100 side-effects; only 9% of drugs have less than 10 side-effects (Please see Figure 1 below)13.
12 U.S. Food and Drug Administration. 2022. Learning about Side Effects.
13 P. Zhang, F. Wang, J. Hu, and R. 2013, Exploring the Relationship Between Drug Side-Effects and Therapeutic Indications, PubMed Central, PMCID: PMC3900166; PMID: 24551427
| 6 |
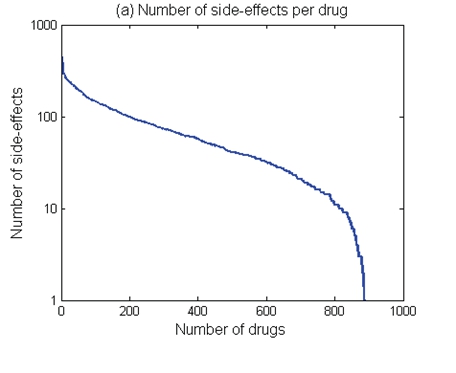
Figure 1: Exploring drug side-effects: 69% of drugs have between 10 and 100 different side effects
*Illustration Taken from: PubMed – Exploring the Relationship Between Drug Side-
Effects and Therapeutic Indications
P. Zhang, F. Wang, J. Hu, and R. 2013, Exploring the Relationship Between Drug Side-Effects and Therapeutic Indications, PubMed Central, PMCID: PMC3900166; PMID: 24551427
Treatment of side-effects, or adverse drug reactions, has become a healthcare concern. The new market of Pharmacovigilance, also known as drug safety – the pharmaceutical science relating to the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products – is developing and expected to reach $12.48 Billion in 2027. Driving this are increasing public awareness and demand for safer medications and increasing government initiatives to promote drug safety around the globe14.
We believe that natural plant-based products show great promise in improving quality of life and can be used as complementary products to balance side effects. Antibiotics and probiotics are an excellent use case. Antibiotics are important for treating bacterial infections; however, they can sometimes cause side effects such as diarrhea, liver disease and changes to the gut microbiota. Using probiotics during and after a treatment with antibiotics can help reduce the risk of diarrhea and restore the gut microbiota to a healthy state15.
Addressing a significant market need, we included in our strategy the development of plant based complementary solutions through wellness as well as clinically developing plant-based pharmaceutical products to address the need to balance selected effects and support people who experience side effects from using medicines, cannabis, and various treatments.
Oral Cavity Dry-Mouth -Side-Effect
Research shows that the overall estimated prevalence of dry mouth is over one in four people in the general population with higher prevalence rates observed in studies conducted with elderly people16 demonstrating that oral cavity-related symptoms are linked to different factors, such as using medicines, treatments, aging, an unbalanced or unhealthy lifestyle, various chronic diseases, psychological reasons, stress, and more17.
It is important to maintain the saliva level in the mouth to prevent problems and damage, as saliva plays a key role in maintaining health in the oral cavity. Saliva contains calcium and phosphorous which protects teeth, helps the digestive system, prevents bad smell through balancing the acidity that comes from food and bacteria, has enzymes that help break down food, washes food scraps and bacteria, and helps speech as pronunciation of movements and syllables is done with saliva and tongue.
The Dry Mouth Treatment Market is expected to reach $1.81 billion by 203218.
In July 2022, we filed a provisional patent application for “COMPOSITIONS AND METHODS FOR TREATING, AMELIORATING, ALLEVIATING, MITIGATING OR BALANCING SIDE-EFFECTS IN THE ORAL CAVITY ASSOCIATED WITH THE USE OF MEDICINES, TREATMENTS, AGING OR UNBALANCED/UNHEALTHY LIFESTYLE”, patent No: 63/388,361 in the U.S. Patent & Trademark Office. The patent application relates to compositions and methods for answering the need for treatment, amelioration, alleviation, mitigation, or balance of side effects in the Oral Cavity related to medications, treatments, using cannabis 24, smoking, adult population experiencing constant dry mouth and unhealthy lifestyle.
14 Pharmacovigilance Market Size to Reach 12.48 Billion in 2027 | Industry Trend - Rising Prevalence of Chronic Diseases Worldwide, Increasing Cases of Adverse Drug Reactions and Drug Toxicity and High Consumption of Drugs in Developed Economies, 2022, Bio Space Article [online]
15 Healthline. 2022. What You Should Eat During and After Antibiotics. [online]
16 How Common is Dry Mouth? Systematic Review and Meta-Regression Analysis of Prevalence Estimates Brazilian Dental Journal (2018) 29(6): 606-618
17 American Dental Association (ADA) Science & Research Institute, LLC Oral Health Topic: Xerostomia, Department of Scientific Information, Evidence Synthesis & Translation Research. Feb 2021
18 Dry Mouth Treatment Market Outlook (2022-2032), Persistence Market Research, 2021
| 7 |
We developed Oral Cavity Care products lines the SmokLyTM and DryLessTM series of sprays that target to balance the dry mouth side effect that may result from using medicines, cannabis, smoking, treatments and unhealthy lifestyle, and is also targeting adult population that tends to experience constant dry mouth.
The products contain plant extracts distilled from seeds, roots, bark, fruits with active anti-inflammatory substances that encourage saliva production and taste in the oral cavity.
Our Oral Cavity Care Product Lines include:
| ● | SmokLyTM line of sprays targeting the market of cannabis users and tobacco smokers. |
| ● | DryLessTM line of sprays targeting adult population experiencing constant dry mouth and certain cancer treatment patients that experience constant dry mouth as part of their cancer treatment regimen. |

Figure 2: Our Oral Cavity Care Product Lines SmokLyTM & DryLessTM
The SmokLyTM & DryLessTM lines of sprays were launched in the Israeli market and are manufactured in a GMP certified facility approved by the Israeli Ministry of Health.
Side Effects from Cannabis Use
Following thorough investigation of cannabis’ side effects, we filed a provisional patent application titled “PHARMACEUTICAL COMPOSITIONS AND METHODS FOR THE TREATMENT OF SIDE-EFFECTS ASSOCIATED WITH THE USE OF CANNABIS, CANNABINOIDS AND RELATED PRODUCTS”, patent No: 63/257,673 in the U.S. Patent & Trademark Office. In October 2022 we filed an extension to the provisional patent application No: 63/257,673 in the U.S. Patent & Trademark Office by filing a provisional patent application patent No: 63/418,046 for “COMPOSITIONS AND METHODS FOR TREATING, AMELIORATING, ALLEVIATING, MITIGATING OR BALANCING SIDE-EFFECTS ASSOCIATED WITH THE USE OF MEDICINES, TREATMENTS, AGING, CANNABIS, AND UNBALANCED OR UNHEALTHY LIFESTYLE”. The patent application describes common side effects associated with the use of medicines, treatments, aging, cannabis and cannabinoids, and unbalanced or unhealthy life style, such as headaches, dizziness, drowsiness, fatigue, nausea, vomiting, lack of concentration, impaired appetite, and more.
| 8 |
In July 2022, we filed a provisional patent application for “COMPOSITIONS AND METHODS FOR TREATING, AMELIORATING, ALLEVIATING, MITIGATING OR BALANCING SIDE-EFFECTS IN THE ORAL CAVITY ASSOCIATED WITH THE USE OF MEDICINES, TREATMENTS, AGING OR UNBALANCED/UNHEALTHY LIFESTYLE”, patent No: 63/388,361 in the U.S. Patent & Trademark Office. The patent application relates to compositions and methods for answering the need for treatment, amelioration, alleviation, mitigation, or balance of side effects in the Oral Cavity related to medications, treatments, using cannabis 24, smoking, adult population experiencing constant dry mouth and unhealthy lifestyle.
There are currently over 200 million cannabis users worldwide and an increased interest in cannabis as a medicine in recent years19. Cannabis was approved for medical use showing benefit in serious medical conditions including cancer, multiple sclerosis, Parkinson’s, epilepsy, chronic pain, and post trauma20. Research indicates that some medical cannabis users experience side effects during their cannabis treatment, which may cause them to discontinue treatment despite good clinical outcomes achieved with the cannabis treatment21.
According to the Mayo Clinic in the US these are the most reported side effects in association with cannabis use22:
| ● | Headaches | |
| ● | Dry mouth and dry eyes | |
| ● | Lightheadedness and dizziness | |
| ● | Drowsiness | |
| ● | Fatigue | |
| ● | Nausea and vomiting | |
| ● | Disorientation | |
| ● | Hallucinations | |
| ● | Increased heart rate | |
| ● | Increased appetite | |
| ● | Impaired attention, judgement, and coordination | |
| ● | Worsened manic symptoms in people who have bipolar disorder | |
| ● | Increased risk of depression or worsen depression symptoms | |
| ● | Increased risk of psychosis in people who have schizophrenia | |
| ● | Impaired memory and cognitive function | |
| ● | Harmful cardiovascular effects, such as high blood pressure | |
| ● | Worsened respiratory conditions | |
| ● | Adverse interactions including with alcohol and anticoagulants. |
19 Statista. 2022. Cannabis users worldwide number by region 2011-2019 | Statista. [online]
20 2017. The Health Effects of Cannabis and Cannabinoids
21 Kudahl, B., Berg, M., Posselt, C., Nordentoft, M. and Hjorthøj, C., 2021. Medical cannabis and cannabis-based medicine show both potential efficacy and potential harms: Cross-sectional comparison with controls on self-rated and interviewer-rated outcomes within the Danish pilot program on medical cannabis. Complementary Therapies in Clinical Practice, 45, p.101476
22 Mayo Clinic. 2022. What you can expect from medical marijuana. [online]
| 9 |
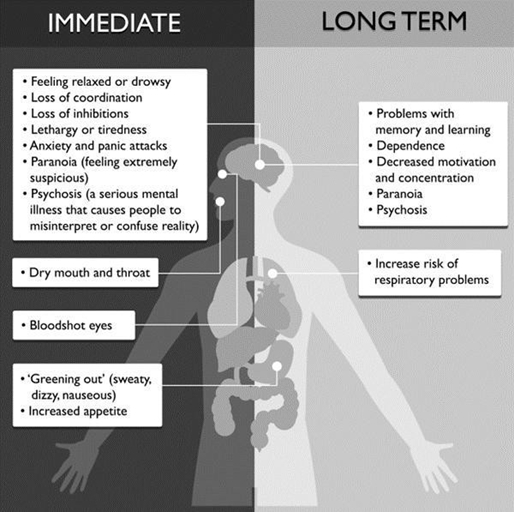
Figure 3: Schematic Representation of Side Effects Associated with the Use of Cannabis
*Illustration Taken from: Positive Choices Educational Program
Positive Choices. 2022. Cannabis: Factsheet. [online]
Our Plant-based product lines
We believe in the power of plant-based solutions from nature can improve people’s health and quality of life. We have developed our strategy to add value to our product lines and position our company with a competitive edge in the Wellness and the Nutritional Supplements market.
Leveraging technology and research, we are developing plant-based solutions targeting to improve quality of life and complementary products to balance selected side effects caused by medicines, cannabis, treatments, or an unbalanced lifestyle.
| 10 |
The GreenFeels™ & Green Side by Side™ Product Lines:

Figure 4: Our Product Lines
We have more than 100 formulations & products under the brand names of GreenFeels™ and Green Side by Side™ product lines that include wellness plant-based products and nutritional supplements for improving quality of life and complementary products for balancing selected side effects caused by medicines, cannabis, treatments, or an unbalanced lifestyle. We position our product lines to capture market share in the nutritional supplements market that is expected to reach $625 billion by 203023.
The products arrive in multiple form factors, such as sprays, powders, tablets, capsules, and tinctures.
We used innovative technologies and experience to create the products combining a variety of well researched plants including herbal extracts, medicinal mushrooms, vitamins, minerals and variety of researched plants known for their healing qualities that contain substances with different anti-inflammatory properties and a variety of health-supportive effects that are relaxing, sleep enhancing, energizing, mood and body balancing, enhancing oral care and alleviating side effects.
We started our beta testing and initial launch of Green Side by Side™ in the Israeli market with a local company, an Israeli medical cannabis company, focusing on the SmokLy series, a line of sprays for the oral cavity to support people with oral cavity dryness (xerostomia), a common side effect experienced by many cannabis users. The products are available in several pharmacies and medical cannabis pharmacies as complementary products to medical cannabis users.
23 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire
| 11 |
Following the initial trial period, we developed an additional brand under the name GreenFeels™ and we plan to expand our distribution efforts with retail, pharmacy chains and natural products’ distributors in Israel. We also plan to distribute the product lines in the United States and worldwide with local partners consistent with local regulations.
Our nutritional supplements products are manufactured in Israel in iBOT Israel Botanicals Ltd under a GMP-certified manufacturing facility approved by the Israeli Ministry of Health.
The GreenFeels™ and Green Side by Side™ product lines that include the following categories:
1. The Oral Cavity Care Family Line
The Oral Cavity Care Product Family Line targets to balance oral cavity dryness side effect. The Oral Cavity Care Family Line includes the SmokLy TM and DryLess TM series of sprays for the oral cavity targeting to balance the dry mouth side effect (xerostomia) that may result from using medicines, cannabis, smoking or treatments.
Benefits of the Oral Cavity Care Product series:
| ● | The SmokLyTM line of sprays targeting the market of cannabis users and tobacco smokers. | |
| ● | The DryLessTM line of sprays targeting adult population experiencing constant dry mouth and certain cancer treatment patients that experience constant dry mouth as part of their cancer treatment regimen | |
| ● | The products contain plant extracts distilled from seeds, roots, bark, fruits with active anti-inflammatory substances that encourage saliva production, taste and can promote saliva production and moisture in the oral cavity. | |
| ● | Convenient to use by spraying into the oral cavity | |
| ● | The products come in 7 different flavors: lemon, strawberry, passion fruit, aniseed, mango, maple and mint |
2. The Medicinal Mushrooms Family Line:
Medicinal Mushrooms Family Line targets to balance important body systems.
The Medicinal Mushrooms Family Line is based on researched medicinal mushrooms that have been used for thousands of years in traditional medicine and have been proven to be efficient for various medicinal uses. Medicinal Mushrooms were found to have a wide potential in the treatment and prevention of diseases, including protection of the heart, antioxidant activity, balancing and strengthening the immune and digestive systems, lowering cholesterol and balancing blood sugar. Furthermore, it has been shown to protect other important organs such as the liver, with anti-cancer activity.
The Medicinal Mushrooms Family Line includes synergistic combinations of research-based medicinal mushrooms and herbs composed in an herbalist method and made of purely natural ingredients.
The products contain dry form, concentrated powders and extracts of mushrooms and herbals researched and found to benefit headaches, changes in blood pressure, anxiety, fatigue, and sleep disorders. We are harnessing the power of medicinal mushrooms for restoring nutritional balance and strengthening the immune system and other body systems.
| 12 |
3. The Booster (Energy & Sports) Family Line
The Booster Family Line targets to provide energy and strength. The Booster Family Line contains unique research-based ingredients and herbal extracts with a high concentration of antioxidants composed in an herbalist method. The Booster Family Line products create a synergistic combination of researched plants and natural ingredients that have been shown to have health supportive anti-inflammatory properties, which strengthen the immune system and contribute to an improved overall feeling. Imbalance in the body, resulting from poor diet and unhealthy lifestyle, chronic diseases, weakness of the immune system, and side effects of medicines and treatments may lead to recurrent infections, chronic coughing, weakness, and gastrointestinal disorders. The Booster Family Line includes herbal syrups that are suitable for morning drinking preventively and target to support daily overload as energy and booster products. The Product Line for Sports & Energy contains research-based herbal formulas including powders and extracts of researched plants that have been demonstrated to have effects of enhancing exercise and athletic performance and include ingredients that improve strength or endurance, increase exercise efficiency, achieve a performance goal more quickly, and increase tolerance for more intense training. These products can be used to prepare the body for exercise, reduce the chance of injury during training, and enhance recovery from exercise. The products come in a variety of forms, including tablets, capsules, liquids, powders, and bars.
4. The Balance & Calm Family Line
Balance & Calm Family Line for mood and body balancing. The Balance & Calm Family Line contains research-based herbal formulas composed in an herbalist method including powders and extracts of researched plants that have been demonstrated to have health supporting effects of calming the digestive system, reducing anxiety and fatigue, and improving sleep quality.
The Balance & Calm Family Line serves as support for the digestive system, balancing and strengthening the body, calming and improving sleep quality. Modern lifestyles that include many tasks and heavy stress, with a non-optimal diet, can lead to fatigue, restlessness, pain, and a particularly sensitive digestive system. All of these can also be side effects of taking various medications and having an unbalanced lifestyle. Continuous stress releases toxic substances in the body, which over time can cause significant health problems. Studies showed that reducing stress improves sleep quality through affecting the nervous system. The Balance & Calm Family Line targets to restore and maintain emotional and body balance and calm the digestive and other systems of the body.
5. The Personal Protection & Health Supportive Family Line:
Personal Protection Family Line targets to support various health chronic and other conditions and prevent contagion with viruses and bacteria. The Personal Protection & Health Supportive Family Line contains research-based balanced combinations of plants, vitamins and minerals composed in an herbalist method, which together form a shell that supports the proper functioning of many body systems, giving an incentive to the immune system and preventing contagion with viruses and bacteria. In the current reality of pandemics, such as COVID-19, and the widespread use of medicines and treatments we believe it is important to balance and nurture different body systems and to strengthen the immune system.
6. The Digestion & Weight Management Family Line
The Product Line for Digestions & Weight Management contains research-based herbal formulas including powders and extracts of researched plants that have been demonstrated to have effects on the digestive system, metabolism and appetite and include ingredients that improve weight management and include ingredients with thermogenic, lipotropic, satiety, and other metabolic effects demonstrating improved markers of metabolic health, such as glucose, lipids, and blood pressure. These products can be used to support various digestive system health conditions and limited calory diet intake without suffering from nutritional deficiencies, successful weight management, which includes not only weight loss but also weight loss maintenance (i.e., limiting weight regain); control of appetite, and more.
7. The Men Family Line
The Product Line for Men contains research-based herbal formulas including powders and extracts of researched plants that have been demonstrated to have health supporting effects on Men’s Health issues in different areas, such as men’s sexual potency & fertility, prostate gland issues, fitness, weight loss, and more. The Product Line for Men targets to restore and maintain specific health issues common for men and is adapted to men’s physiology, nutritional needs, common health issues, and more.
| 13 |
8. The Women Family Line
The Product Line for Women contains research-based herbal formulas including powders and extracts of researched plants that have been demonstrated to have health supporting effects on Women’s Health issues in different areas, such as candidiasis, fertility issues, vaginal microflora, menstrual cycle normalization, PMS relief, weight loss, and more. The Product Line for Women targets to restore and maintain specific health issues common for women and is adapted to women’s physiology, nutritional needs, common health issues, and more.
9. The Vitamins & Minerals Family Line
Vitamins and minerals are essential organic compounds that are required in order to maintain good health and overcome various infections and retain a good health condition. They are involved in a variety of metabolic processes and many physiological systems and functions in the body.
The Vitamins & Minerals Product Line is based on researched substances that have been proven to be efficient for various medicinal uses. Thy contain research-based balanced combinations of vitamins and minerals composed in an herbalist method, which support the proper functioning of many body systems, and specifically the immune system to prevent contagion with viruses and bacteria, support a healthy digestive system, cognitive functions, and more.
Acquisition of MyPlant Bio Ltd.
In December 30, 2022, we purchased a 10% equity interest (on a fully diluted basis) in MyPlant Bio Ltd. under the terms of a Share Purchase and Option Agreement. The agreement provides Citrine Global with an option to purchase an additional 45% of MyPlant equity.
MyPlant Bio Ltd, a pioneering Israeli company that is specialized in botanical drug development and owns certain know-how and intellectual property rights that include a developed platform and cell-disease models to screen plant extracts to understand their biological effect and has screening platforms using cell line models for certain diseases and conditions to detect effective plant materials and/or other substances for the treatment of these conditions. MyPlant was founded by Cannasoul Analytics, a leading botanical research and development company and Prof. Dedi Meiri from the Faculty of Biology at the Israeli Institute of Technology (Technion) and a member of the Technion Integrated Cancer Center. Citrine Global’s acquisition of MyPlant is in line with the Citrine Global’s strategy to be a leader in plant-based wellness and pharma solutions.
Under the terms of the Share Purchase and Option Agreement, we purchased from the MyPlant shareholders an aggregate of 44,328 ordinary shares of MyPlant (the “MyPlant Shares”) representing, on a fully diluted basis, 10% of the outstanding MyPlant Shares, in consideration of $444,444 payable by the issuance by Citrine Global to the MyPlant shareholders of an aggregate of 9,259,250 shares of our common stock. In addition, under the Share Purchase and Option Agreement, we were granted an option by the MyPlant shareholders to purchase an additional 35% of MyPlant Shares, on a fully diluted basis (the “Shareholders Option”), in consideration of $1,555,556 payable by the issuance of up to 32,407,417 shares of our common stock to the MyPlant shareholders, and a separate option by MyPlant to purchase an additional 10% of the MyPlant Shares, on a fully diluted basis (the “MyPlant Option”), in consideration of $444,444, which is payable, in our sole discretion, in cash or in the issuance to MyPlant of up to 9,259,250 shares of our common stock. Said options are exercisable through September 30, 2023 (the “Option Expiry Date”). If both the Shareholders Option and the Company Options are exercised, we will hold 55% of MyPlant Shares, on a fully diluted basis. Under the Share Purchase and Option Agreement, are authorized to continue its due diligence through the Option Expiry Date.
The transactions under the Share Purchase and Option Agreement are based on a MyPlant company valuation of approximately $4.45 million. Under the agreement, we are authorized at any time on or before the Option Expiry Date to obtain an independent third-party valuation of MyPlant. If it is determined by such third-party valuation that the MyPlant valuation is less than $4.45 million then the consideration payable in respect of the exercise price of the options will be accordingly adjusted, provided however that in any case the Company’s valuation in the transaction shall not be below US$1,000,000.
| 14 |
Under the Share Purchase and Option Agreement, MyPlant granted to our company the exclusive right to utilize MyPlant’s activities as specified in the agreement, including without limitation, the screening platforms using cell line models for certain diseases and conditions to detect effective plant materials and/or other substances for the treatment of these conditions and a and a right of first opportunity to commercialize intellectual property developed by MyPlant that is in Citrine (or its subsidiaries’) field of business, provided that, if by December 31, 2023 we do not exercise either of the Shareholders Option or the Company Option and/or enter into a service agreement with MyPlant, then the exclusive rights shall terminate but the right of first opportunity to commercialize intellectual property developed by MyPlant shall continue thereafter until June 31, 2024, unless such rights have been extended beyond such date under the terms to be agreed in the service agreement entered into by MyPlant and out company. In addition, under the Share Purchase and Option Agreement, Cannasoul, MyPlant’s majority Shareholder, agreed to not that may compete with MyPlant’s activities.
We were also granted observer rights on the MyPlant board of Directors (the “MyPlant Board”). Following the exercise by us of the Shareholders Option, the MyPlant Board shall be comprised of four (4) directors of which MyPlant will be authorized to designate two of such directors.
The acquisition of MyPlant Bio is part of our strategy for developing scientifically backed plant-based products composed of specific active plant substances proven by research to advance our wellness and planned pharma product offerings as to the effects of specific plant substances and compounds on different wellness and medical conditions and possibly the option to offer personally customized products for individual patients tailored for their healing process. As the worldwide use of botanical nutritional supplements and botanical drugs continues to grow, the need for scientific evaluation of the safety and efficacy of these products is becoming ever greater and positions our company with a competitive edge in the Wellness market, the Nutritional Supplements market that is expected to reach USD 625 billion by 203024 and the botanical and plant-derived drug market that is expected to reach USD 53 billion by 202625.
Our IP Strategy and R&D Roadmap
Our IP strategy and R&D roadmap include developing plant-based wellness and pharma solutions, building our patent portfolio, conducting clinical trials, advancing products through regulatory approvals, and bringing innovative products to market.
Currently we have a provisional patent application, and as part of our IP strategy, we plan to build a patent portfolio. We are also considering purchasing patents and IP.
Our strategy includes developing plant-based wellness, and plant-based medicines products & solutions including:
| ● | Developing plant-based products portfolio based on rigorous scientific research ranging from synergistic botanicals, herbal extract tinctures, medicinal mushrooms together with plant extracts, botanical formulations from seeds, roots, bark, fruits and a wide variety of plants that contain substances with health-supportive effects. Such supportive effects include, but aren’t limited to, enhancing oral care, anti-inflammatory properties, relaxation, sleep enhancement, energizing, mood and body balancing and alleviating side effects. |
24 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire
25 201-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online]
| 15 |
| ● | Researching plant-based medicines and solutions for the botanical and plant-derived drug market. Botanical drugs are derivative of medicinal plants and may contain algae, plant-based substances and fungi. Botanical and plant derived drugs help in the treatment of various diseases, such as central nervous system disorders, infectious diseases, cardiovascular diseases, and respiratory diseases and are available in various forms, such as pills, tablets, and injections. The key factors driving this market are growing applications in diseases, growing FDA approvals and a dedicated Botanical Drugs Approval pathway, technological development in the manufacturing process, and rising demand for and focus on traditional and natural source medicines. | |
| ● | The acquisition of MyPlant is an opportunity to advance our wellness and planned pharma and botanical drug products with MyPlant’s scientific research as to the effects of specific plant substances and compounds on different wellness and medical conditions. As we have more than 100 researched plant-based formulations and product lines targeting to promote wellbeing and complementary products for balancing selected side effects caused by medicines, treatments, or an unbalanced lifestyle and we continue our research, we plan to benefit from the collaboration with MyPlant and Professor Dedi Meiri to advance our strategy for developing scientifically backed plant-based products composed of specific active plant substances proven by research to be effective for different medical conditions and possibly offer personally customized products for individual patients tailored for their healing process. We will also have access to MyPlant’s database of verified substances and their researched effects on different medical conditions to advance development our pharma products positioned to capture market share in the botanical and plant-derived drug market that is expected to reach USD 53 billion by 202626. |
Our research and development program includes:
| ● | Developing wellness plant-based product portfolio across the range from scientific and research-based plants, such as herbal extracts, medicinal mushrooms, and other natural ingredients |
| ● | Developing complementary products portfolio for balancing selected side effects caused by medicines, treatments, cannabis, aging, stress, and an unbalanced lifestyle |
| ● | Expanding our product lines and registering the products for worldwide regulatory approvals. |
| ● | Researching and developing plant-based medicines and pharma solutions with the mission of developing plant-based medicines and botanical drugs |
| ● | Building patent portfolio |
| ● | Building clinical trials program & portfolio |
| ● | Registering products for regulatory approval |
| ● | Building the infrastructure for production and innovation centers to leverage IP & competitive advantage in developing and manufacturing wellness to pharma plant-based products |
| ● | Currently our product line does not include any cannabis, cannabinoid, or cannabis related components. However, pending changes in the regulatory, market landscape and pending approval of the board of directors, we may consider developing cannabis, cannabinoid, and related products. |
Provisional Patent Applications
In October 2021 we filed a provisional patent application for “PHARMACEUTICAL COMPOSITIONS AND METHODS FOR THE TREATMENT OF SIDE-EFFECTS ASSOCIATED WITH THE USE OF CANNABIS, CANNABINOIDS AND RELATED PRODUCTS”, patent No: 63/257,673 in the U.S. Patent & Trademark Office. The patent application describes certain side effects of cannabis use, the needs, technologies and solutions to support medical cannabis users who experience side effects related to their cannabis treatment.
In October 2022 we filed an extension to the provisional patent application No: 63/257,673 in the U.S. Patent & Trademark Office by filing a provisional patent application patent No: 63/418,046 for “COMPOSITIONS AND METHODS FOR TREATING, AMELIORATING, ALLEVIATING, MITIGATING OR BALANCING SIDE-EFFECTS ASSOCIATED WITH THE USE OF MEDICINES, TREATMENTS, AGING, CANNABIS, AND UNBALANCED OR UNHEALTHY LIFESTYLE”. The patent application describes common side effects associated with the use of medicines, treatments, aging, cannabis and cannabinoids, and unbalanced or unhealthy life style, such as headaches, dizziness, drowsiness, fatigue, nausea, vomiting, lack of concentration, impaired appetite, and more.
26 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online]
| 16 |
In July 2022, we filed a provisional patent application patent No: 63/388,361 in the U.S. Patent & Trademark Office for “COMPOSITIONS AND METHODS FOR TREATING, AMELIORATING, ALLEVIATING, MITIGATING OR BALANCING SIDE-EFFECTS IN THE ORAL CAVITY ASSOCIATED WITH THE USE OF MEDICINES, TREATMENTS, AGING OR UNBALANCED/UNHEALTHY LIFESTYLE”. The patent application relates to compositions and methods for answering the need for treatment, amelioration, alleviation, mitigation, or balance of side effects in the Oral Cavity related to medications, treatments (such as chemotherapy), and more. It describes technologies and solutions to support people who experience side effects related to their treatment. Oral cavity side effects are common. The overall estimated prevalence of dry mouth is over one in four people in the general population with higher prevalence rates observed in studies conducted with elderly people27. Research shows that oral cavity-related symptoms are linked to different factors, such as using medicines, treatments, aging, an unbalanced or unhealthy lifestyle, various chronic diseases, psychological reasons, stress, and more28.
Green Vision Center Production and Innovation Center for Plant-based Wellness & Pharma Products
Green Vision Center is part of our strategy to create end-to-end plant-based solutions covering all the infrastructure, facilities, and activities required for developing, manufacturing, and bringing to market innovative plant-based wellness and pharma products.

Figure 5: Green Vision Center Israel Building Demonstration
**All image rights are reserved to the Company and are for illustration purposes only and do not bind the company
Green Vision Center Israel
In February of 2022, we completed the acquisition from the Israel Lands Authority (ILA) of 125,000 square feet (approximately 11,687 square meters) or approximately three acres of industrial land in Yerucham, a city in southern Israel, to build Green Vision Center Israel. Approximately 90% of the acquisition cost was provided by Israeli government programs that encourage industrial development and includes additional grants and tax incentives.
27 How Common is Dry Mouth? Systematic Review and Meta-Regression Analysis of Prevalence Estimates Brazilian Dental Journal (2018) 29(6): 606-618
28 American Dental Association (ADA) Science & Research Institute, LLC Oral Health Topic: Xerostomia, Department of Scientific Information, Evidence Synthesis & Translation Research. Feb 2021
| 17 |
It is currently anticipated that the Green Vision Center Israel will include approximately 65,000 sq. ft. (~ 5,800 sqm) a first-of-its-kind center’s infrastructure and facilities will be focused on the development and production of wellness and pharma plant-based products and planned to include:
| ● | Manufacturing facilities for botanicals and nutritional supplements | |
| ● | Manufacturing facilities for pharma plant-based products & botanical drugs | |
| ● | Manufacturing facilities for healthy snacks & beverages | |
| ● | Manufacturing facilities for plant-based cosmetics | |
| ● | Manufacturing facilities for medical cannabis and related products | |
| ● | R&D laboratories for development, clinical studies, and quality control testing | |
| ● | Distribution and global logistics center | |
| ● | Management and consultant offices | |
| ● | Conference, training & visitor center |
Green Vision Center Israel: Planned Divisions and Internal Design
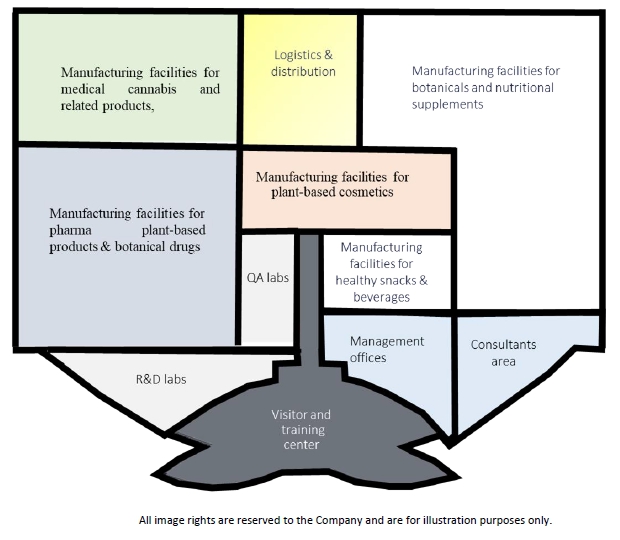
Figure 6: Green Vision Center Israel Planned Internal Design
**All image rights are reserved to the Company and are for illustration purposes only and do not bind the company
Green Vision Center Israel was designed by Avner Sher, one of Israel’s most highly regarded architects. Its design includes a unique roof in the shape of a lotus flower and will be built with solar panels and according to ecological green principles of saving energy.
The center will be constructed by a professional project construction company and sub-contractors that will oversee all aspects of the building including interfacing and obtaining all facilities and products relevant licenses and regulatory approvals, the requisite building permits and other required authorizations.
| 18 |
Our Business Model for the Green Vision Center includes:
| ● | Production & sales of our branded products; | |
| ● | Production & services to third parties; | |
| ● | Full turnkey solutions for all the services that the center can provide, including R&D, QA, production, market positioning, and sales; | |
| ● | Partnerships and collaborations with international companies in the wellness and pharma industries that are interested in establishing an innovation and production infrastructure in Israel; | |
| ● | Mergers & acquisitions and strategic partnership activities; and | |
| ● | Partnerships based on models of profit sharing. |
Our mission is to become a leading worldwide production and innovation center for natural plant-based products and health, wellness, and pharma solutions and to bring partners, market leaders, companies, technologies, and scientific collaborations from Israel and around the world.
Israel as a Source of Innovation
Our presence in Israel combined with our close contacts with leading universities, researchers and companies empowers us to access the latest technologies, talent, and innovations and bring them to the global market.
We chose to focus on Israel for the following reasons:
| ● | Israel is well positioned as a leader in technology with a critical mass of technology companies, researchers, and scientists29. |
| ● | Our headquarters, our executives and strategic partners are based in Israel, where we have been operating for years and have a strong network with Israeli companies, universities, labs, entrepreneurs, and businesses. |
| ● | Israel is considered a pharma powerhouse and a world leader in clinical trials due to its advanced regulatory environment and local experience30. |
| ● | The Israeli government views and supports technological innovation a major growth engine for the Israeli economy and supports it. The government support includes grants for the purchase of equipment, tax incentives, incentives for employing workers, and other benefits as part of a program of the Israeli government to encourages industrial development and benefits for the city of Yerucham. |
| ● | We acquired land in the south of Israel, backed by government support, to build the Green Vision Center, a first-of-its-kind production and innovation center for plant-based wellness & pharma products. |
Creating a Global Network & Growth Strategy
A core part of our strategy includes building a worldwide network with local teams, partners, subsidiaries, Green Vision Centers, strategic partnerships, collaborations, and mergers & acquisitions of technology and distribution companies.
Initially, we are planning to build infrastructure for business development and sales with local teams in North America and Europe.
Our strategy includes various business models that are intended to bring new products to market leveraging, and thereby, maximizing company’s value, building the company’s intellectual property and growth strategy that includes mergers & acquisitions of technology and distribution companies.
Go to Market Strategy and Prospective Revenue Sources
The plant-based wellness & pharma market is booming, with health-conscious consumers spending more on natural products ranging from nutraceuticals, natural superfoods, beverages, and cosmetics to legal cannabis and the evolving market of botanical and plant-derived drugs.
| ● | The nutritional supplements market is expected to reach USD 624.7 billion by 203031. | |
| ● | The superfoods market is expected to reach USD 287.7 billion by 202732. | |
| ● | The legal cannabis market is expected to reach USD 70.6 billion by 202833 | |
| ● | The botanical and plant-derived drug market is expected to reach USD 53 billion by 202634. | |
| ● | The natural cosmetics market is expected to reach USD 24.8 billion by 202735. |
29 PwC-Startup Nation Central Report Explores Israel’s Multinational Innovation Ecosystem
30 Portfolio of Israeli companies Life science and Clean-tech sectors October 2020
31 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire]
32 Research, I., 2022. Global Superfoods Market Size is Projected To Reach US$ 287.75 Billion by 2027 | Superfoods Market Store, Delivery Options, Emerging Trends 2022 | Segmentation by Product Type, Applications, Regions, & Key-Players (ADM, Ardent Mills, Bunge). [online] GlobeNewswire Newsroom
33 Grandviewresearch.com. 2022. Legal Marijuana Market Size Worth $70.6 Billion By 2028
34 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online]
35 52 Fortune Business Insights, The global vegan cosmetics market is projected to grow to $24.79 billion in 2028 Report ID FBI106594 [online]
| 19 |
The wellness products are sold through different distribution channels which include online digital direct sales, online retailer websites, physical shops and retailers including food, drug, and mass merchandise retail networks. We are currently focused on building a B2B distribution network worldwide with select local partners who will be handling import, distribution, marketing, and sales while adhering with local regulations.
Our strategy for generating revenue in the near term and future include:
| ● | Sales of our proprietary products including Green Feels & Green Side by Side product lines | |
| ● | Commercialization and licensing our future IP, products & brands. | |
| ● | Collaborations & acquisitions of distribution companies and strategic partnership activities |
Competition
The global health and wellness market is expected to reach USD 7.6 trillion by 203036 and is very crowded and competitive. Many companies, from startups to corporate giants, operate in these spaces.
Plant-based wellness sector: Nutritional supplements
The Nutritional supplements and OTC wellness products markets are growing thanks to increased attention to natural products, health and prevention by the consumers and increased health care costs and search for alternatives to cure specific problems.
Some of the key players in this market are Herbalife Nutrition Ltd., Amway Corp., Abbott, Arkopharma, Bayer AG, Glanbia PLC, Pfizer Inc., ADM, and Ayanda37.
Botanical Drugs
The botanical and plant-derived drug market is expected to reach $53 billion by 202638.
Botanical drugs are derivative of medicinal plants and may contain algae, plant-based substances and fungi. Botanical and plant derived drugs help in the treatment of various diseases, such as central nervous system disorders, infectious diseases, cardiovascular diseases, and respiratory diseases and are available in various forms, such as pills, tablets, and injections.
We believe that the key factors driving this market are:
| ● | Growing applications of Botanical drugs in diseases | |
| ● | Growing FDA approvals and a dedicated Botanical Drugs Approval pathway | |
| ● | Technological development in the manufacturing process, | |
| ● | Rising demand for and focus on traditional and natural source medicines39. |
Large companies are involved in this market with the major players including Bayer AG, Boehringer Ingelheim International GmbH, F. Hoffmann La Roche Ltd., Jazz Pharmaceuticals Plc, Johnson and Johnson Inc., Merck KGaA, Mitsubishi Chemical Corp., Novartis AG, Sanofi SA, and Tilray Inc.40.
36 Research, P., 2022. Health and Wellness Market Size to Hit USD 7,656.7 Bn by 2030. [online] GlobeNewswire News Room.
37 MarketView Research: Dietary Supplements Market Size, Share & Trends Analysis Report By Ingredient (Vitamins, Minerals), By Form, By Application, By End User, By Distribution Channel, By Region, And Segment Forecasts, 2022 - 2030
38 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online] Inkwood Research
39 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online] Inkwood Research.
40 Technavio Report: Botanical and Plant-Derived Drugs Market by Type and Geography - Forecast and Analysis 2022-2026
| 20 |
We have differentiated ourselves through our end-to-end strategy of bringing to market innovative plant-based wellness and pharma products covering the whole spectrum from research, product development, building the infrastructure, manufacturing, and marketing. We built the following strategy and unique business model that can support our ability to remain competitive:
| ● | We are leveraging technology and research and focus on developing plant-based wellness and pharma solutions to improve quality of life and complementary products for balancing selected side effects caused by medicines and treatments, cannabis, aging, stress, and an unbalanced lifestyle | |
| ● | We have the ability to develop innovative products and solutions that meet customer and market needs | |
| ● | We develop our IP strategy by building patent portfolio, conducting clinical studies, and obtaining regulatory approvals | |
| ● | We have a leading experienced team and partners with proven track record in technology, high-tech and biotech and proven experience in bringing companies to global success | |
| ● | Our presence in Israel combined with our close contacts with leading universities, researchers and companies powers us with the latest technologies, talent, and innovation and to offer innovative solutions to the global market. | |
| ● | Potential partnerships and other collaborations with international companies in the wellness and pharma industries |
The Health & Wellness Industries Global Market Size and Potential:
The global health and wellness market is expected to reach USD 7.6 trillion by 2030, growing at a CAGR of 5.5% from 2021 to 2030. The hectic, unbalanced lifestyle has resulted in the prevalence of lack of proper diet and sleep, stress, depression, anxiety, cancer, diabetes, and various other health related issues. Lack of proper diet has resulted in the reduced intake of essential nutrients and minerals required for the healthy and active functioning of the human body. Precedence research identifies growth opportunities to the health and wellness market players across the globe in the adoption of smart technologies and innovative ways in manufacturing various health and wellness products, nutritional supplements, healthy snacks and beverages, the growing biopharmaceutical industry and development of botanical drugs41.
Health and wellness have been found by Nielsen IQ researchers to be the most powerful consumer force of 2021. In contrast to the unpredictable nature of COVID-19, consumers are being very deliberate with their choices. A survey conducted discovered that consumers emphasize having meaningful and purposeful living, health management, strength and wellness, mental health and stability, happiness, social connections, environmental betterment, balance, and fulfillment. We are witnessing a global movement of health and wellbeing becoming a priority for the public, further emphasized by the global COVID-19 pandemic. There is increasing recognition that people need to take charge of their own health, improve their quality of life, use natural products, and balance side effects caused by medicines and treatment42.
41 Research, P., 2022. Health and Wellness Market Size to Hit USD 7,656.7 Bn by 2030. [online] GlobeNewswire News Room
42 NielsenIQ. 2022. An inside look into the 2021 global consumer health and wellness revolution. [online]
| 21 |
The Plant-Based Global Market Size and Potential:
The plant-based products market is booming with health-conscious consumers spending more on natural products, ranging from nutraceuticals, natural superfoods, beverages, cosmetics to legal cannabis and the evolving market for botanical and plant-derived drugs. For example:
| ● | The nutritional supplements market is expected to reach USD 624.7 billion by 203043. | |
| ● | The superfoods market is expected to reach USD 287.7 billion by 202744. | |
| ● | The legal cannabis market is expected to reach USD 70.6 billion by 202845. | |
| ● | The botanical and plant-derived drug market is expected to reach USD 53 billion by 202646. | |
| ● | The natural cosmetics market is expected to reach USD 24.8 billion by 202747 |
The Global Nutritional Supplements Market
The global nutritional supplements market is expected to reach USD 624.7 billion by 2030 and is expanding growth at a CAGR of 7.1% over the forecast period 2021 to 2030 with plant-based supplements containing natural ingredients and extracts of plants and mushrooms that have a beneficial biological effect48. The global superfoods market is expected to reach USD 214.95 billion by 2027 with superfoods being foods that have a very high nutritional density. This means they provide a substantial amount of nutrients and very few calories. They contain a high volume of minerals, vitamins, and antioxidants.
Growth in the nutritional supplements is driven by growing awareness of health and safety in the traditional pharma, food, and beverage industries as well as higher healthcare costs. Authentic consumption has become a major food and beverage trend as consumers increasingly seek natural ingredients. Products such as ginseng, echinacea, ginkgo biloba, and garlic, the top selling botanical products are considered natural remedies for inflammation and infections. This is further driven by the COVID-19 pandemic, with consumers looking to strengthen the natural immune system. This is also driving growth of vitamins and minerals and moving towards natural colorant-based plant juice products, since they provide better and long-lasting protection from viruses and bacteria. In addition, botanicals and nutritional supplements are widely used by people who suffer from diseases related to weight management, clinical nutrition, digestive health (gut health problems), immunity, diabetes, and cardio fitness, either as treatment or prevention49.
We believe that the market demand for Nutritional Supplements is driven by50:
| ● | Increasing attention to health and prevention by the consumers | |
| ● | Greater customization of needs for different segments of the population | |
| ● | Increased health care costs and search for alternatives to cure specific problems | |
| ● | The growth in demand for supplements is mainly driven by probiotic supplements, Fatty Acids (i.e. fish oils) and protein supplements | |
| ● | Herbal/Botanical Supplements usage has emerged as a popular complementary and alternative medicine or supplement to modern medicine | |
| ● | Rising consumer awareness regarding the severity of digestive disorders, stimulate the growth of the Enzymes segment. |
43 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire
44 Research, I., 2022. Global Superfoods Market Size is Projected To Reach US$ 287.75 Billion by 2027 | Superfoods Market Store, Delivery Options, Emerging Trends 2022 | Segmentation by Product Type, Applications, Regions, & Key-Players (ADM, Ardent Mills, Bunge). [online] GlobeNewswire News Room
45 Grandviewresearch.com. 2022. Legal Marijuana Market Size Worth $70.6 Billion By 2028. [online]
46 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online]
47 Fortune Business Insights, The global vegan cosmetics market is projected to grow to $24.79 billion in 2028 Report ID FBI106594 [online]
48 Research, P., 2022. Nutritional Supplements Market to Hit US$ 624.7 Billion by 2030. [online] GlobeNewswire
49 PwC “Vitamins and Dietary Supplements Market Overview Report, https://www.pwc.com/it/it/publications/assets/docs/Vitamins-Dietary-Supplements-Market-Overview.pdf
50 PwC “Vitamins and Dietary Supplements Market Overview Report, https://www.pwc.com/it/it/publications/assets/docs/Vitamins-Dietary-Supplements-Market-Overview.pdf
| 22 |
The Botanical and Plant-derived Drug Market
The global botanical and plant-derivative drug market is anticipated to grow to $53 billion by 2026 driven by growing applications in diseases, technological developments in manufacturing processes and a growing focus and demand for naturally sourced medicines51.
Botanical drugs are derived from natural sources, plants and mushrooms, and are considered to have fewer side-effects as compared to synthetic drugs while showing high efficacy in helping to treat different medical conditions and chronic diseases52.
The important driver for growth in the global botanical and plant-derivative drug market is its growing applications in diseases. Botanical drugs are derivative of medicinal plants and may contain algae and vegetable substances, along with macroscopic fungi. These may assist in the treatment of various diseases, such as central nervous system disorders, infectious diseases, cardiovascular diseases, and respiratory diseases. Botanical and plant derivative drugs are available in various forms, such as pills, tablets, and injections53.
The Botanical and plant-derivative drug market is primarily driven by the following factors 54
| ● | Growing applications in diseases |
| ● | Growing FDA approvals |
| ● | Technological development in the manufacturing process |
| ● | Rising demand for traditional medicines |
| ● | Growing focus on natural source medicines |
The Global Cannabis Market
The global legal cannabis market size is expected to reach USD 70.6 billion by 2028 driven mainly by increased legalization of cannabis for medical and adult-use and the growing adoption of these products for the treatment of chronic diseases55.
There are currently over 200 million cannabis users worldwide and an increased interest in cannabis as a medicine in recent years56. Cannabis was approved for medical use showing benefit in serious medical conditions including cancer, multiple sclerosis, Parkinson’s, epilepsy, chronic pain, and post trauma. Research indicates that some medical cannabis users experience side effects during their cannabis treatment, which may cause them to discontinue treatment despite good clinical outcomes achieved with the cannabis treatment57.
The Global Natural Cosmetics Market
The global natural cosmetics market is projected to reach USD 24.86 billion by 2028 driven mainly by increasing demand for harmful chemical-free cosmetics, rising awareness against the use of animal derivatives and growing social media movements endorsing naturally derived products58.
The cosmetic and personal care segment of botanicals is also on the rise with companies increasingly discovering novel herbal ingredients as consumers are seeking more natural products with ingredients that are of plant origin: extracts or oils obtained from raw plant materials. Natural cosmetics are cosmetics that have ingredients of plant origin. The absence of chemical compounds and animal-by products are specifically suited to sensitive skin people. The natural cosmetic products are biodegradable and environmentally friendly. Many companies in the field focus on the production of natural cosmetics that are cruelty-free as these products have increasing demand59.
51 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online] Inkwood Research
52 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online] Inkwood Research
53 Sciences, L. and Discovery, D., 2022. Global Botanical and Plant-Derived Drugs Market 2022-2026. [online]
54 2018-2026, G. and 2018-2026, G., 2022. Botanical and Plant Derivative Drug Market - Global Forecast 2018-2026. [online] Inkwood Research
55 Statista. 2022. Cannabis users worldwide number by region 2011-2019 | Statista
56 Statista. 2022. Cannabis users worldwide number by region 2011-2019 | Statista
57 Statista. 2022. Cannabis users worldwide number by region 2011-2019 | Statista
58 Fortune Business Insights, The global vegan cosmetics market is projected to grow to $24.79 billion in 2028 Report ID FBI106594 [online]
59 Fortune Business Insights, The global vegan cosmetics market is projected to grow to $24.79 billion in 2028 Report ID FBI106594 [online]
| 23 |
Side Effects and Drug Safety/Pharmacovigilance Evolving Market
The public health impact of harms associated with medicines and treatments is a growing area of investigation, given the expanding pharma industry and widespread availability of drugs, and different medical treatments around the world.
Current evidence suggests that use of medicines, and medical treatments are associated with a series of side effects. For example, exploring the relationship between drug side-effects and therapeutic indications demonstrate that 69% of drugs have between 10 and 100 different side effects; 22% of drugs have more than 100 side-effects; only 9% of drugs have fewer than 10 side-effects (Please see Figure 7 below)60
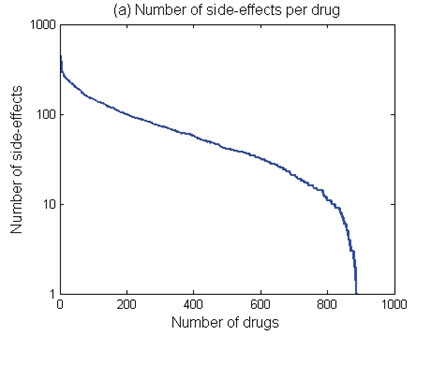
Figure 7: Exploring drug side-effects: 69% of drugs have between 10 and 100 different side effects
*Illustration Taken from: PubMed – Exploring the Relationship Between Drug Side-
Effects and Therapeutic Indications
P. Zhang, F. Wang, J. Hu, and R. 2013, Exploring the Relationship Between Drug Side-Effects and Therapeutic Indications, PubMed Central, PMCID: PMC3900166; PMID: 24551427
Drug side-effects, or adverse drug reactions, have become a healthcare concern. The new market of Pharmacovigilance, also known as drug safety—the pharmaceutical science relating to the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products—is developing and expected to reach $12.48 billion in 2027. Driving this are increasing public awareness and demand for safer medications and increasing government initiatives to promote drug safety around the globe61.
60 P. Zhang, F. Wang, J. Hu, and R. 2013, Exploring the Relationship Between Drug Side-Effects and Therapeutic Indications, PubMed Central, PMCID: PMC3900166; PMID: 24551427
61 BioSpace. 2022. Pharmacovigilance Market Size to Reach 12.48 Billion in 2027 | Industry Trend - Rising Prevalence of Chronic Diseases Worldwide, Increasing Cases of Adverse Drug Reactions and Drug Toxicity and High Consumption of Drugs in Developed Economies | BioSpace. [online]
| 24 |
Regulatory Environment
In every jurisdiction in which we plan to operate, we will be subject to extensive governmental regulations on the formulation, manufacturing, packaging, labeling, advertising, promoting, importing, distributing, shipping, and selling our products, may they be nutritional supplements, cosmetics, foods, or any other category.
Prior to commencing operations and/or permitting sales of our products in the market, we may be required to obtain an approval, license, or certification from the relevant country’s ministry of health or another responsible agency. Prior to entering a new market, we plan to work with local authorities, either directly or via our local partner, to obtain the requisite approvals. The approval process usually requires us to present each product and product ingredients and, in some cases, arrange for testing of products by local technicians for ingredient analysis.
We or our local partners would need to obtain various regulatory approvals and licenses for our different product lines and activities, including production of botanicals, nutritional supplements, natural snacks and beverages, and natural cosmetics. We intend to obtain all regulatory approvals required for different product categories in the different countries in which we will operate either directly or through our local partners.
We describe in this section primarily the material regulations that are currently applicable to our products.
Regulatory Environment for the Our Products
While the number of people using nutritional supplements and herbal medicine products continues to increase in many countries, the regulations for these products vary by territory. In some countries supplement use is limited to general health and well-being while in other countries they are permitted for use as medicinal products. To date, there is little consensus from country to country on the scope, requirements, definition, or even the terminology in which the nutritional supplement and herbal medicines categories could be classified62.
Our products are regulated in Israel as nutritional supplements and meet all regulatory compliance requirements for nutritional supplements in Israel. iBOT Israel Botanicals, our manufacturing facility for our products, is approved by the Israeli Ministry of Health and is GMP-certified.
Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. GMP covers all aspects of production from the starting materials, premises, and equipment to the training and personal hygiene of staff. Detailed written procedures are essential for each process that could affect the quality of the finished product. There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process - every time a product is made63. The National Food Service (NFS) is the regulatory body at the Israeli Ministry of Health, that is responsible for food and nutritional supplements approval. The NFS strictly examines the safety and quality of each nutritional supplement product that is about to be registered and marketed in Israel64.
We currently expect to launch our products in Europe in the first half of 2023. We will obtain all relevant regulatory approvals for the products before launching them in other territories, such as European countries and the U.S..
The Israeli Ministry of Health maintains a comprehensive list of authorized nutritional supplements for marketing. This list includes over a thousand different vitamins, minerals, amino acids, and herbs including their extracts. Items under this list can be legally marketed, however, no medical claims can be made without adequate supporting information. The final products can be in various forms such as powders, tablets, hard or soft capsules, liquids, including oils and tinctures. Each product must be manufactured under GMP conditions and be approved by the Ministry of Health prior to selling.
Regulatory Compliance for the Green Vision Center Israel
We acquired 125,000 sq ft (11,687 sqm), or approximately three acres, of industrial land in the south of Israel upon which a 65,000 sq. ft. (~5,800 sqm) facility will be built composed of manufacturing plants, laboratories, logistics, import and export, offices, training, conference center, and an international visitor complex. The center will be constructed by a real estate professional project construction company and regulatory consultants in the relevant fields that will obtain the required authorizations.
62 Thakkar, S., Anklam, E., Xu, A., Ulberth, F., Li, J., Li, B., Hugas, M., Sarma, N., Crerar, S., Swift, S., Hakamatsuka, T., Curtui, V., Yan, W., Geng, X., Slikker, W. and Tong, W., 2020. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regulatory Toxicology and Pharmacology, 114, p.104647
63 ISPE organization, Regulatory-Resources - GMP
64 Israeli Ministry of Health, National Food Services Department Website (NFS)
| 25 |
We intend to obtain all necessary regulatory approvals and licenses for the Green Vision Center’s production and operation facilities and products.
Corporate Overview
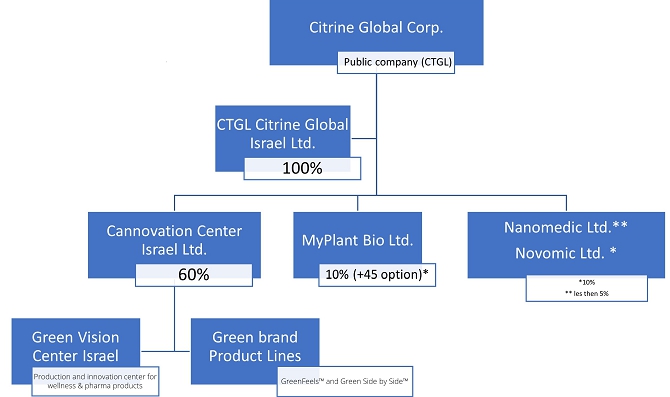
Figure 8: Corporate Overview
* Represents shares retained after selling 90% we held in Novomic Ltd. (“Novomic”)
**See above detailed description of the share purchase agreement with Nanomedic
**8-K Citrine Global Signs Share Purchase and Option agreement with MyPlant Bio
Our registered office address in the State of Delaware is c/o Business Filings Incorporated, 108 West 13th St., City of Wilmington, County of Newcastle, Delaware 19801, and the address of our primary executive office is 5 Golden Beach, Caesarea 3088900, Israel. Our website address is www.citrine-global.com.
Properties
Through our subsidiary Cannovation Israel Center, in February of 2022, we completed the acquisition of 125,000 sq ft (11,687 sq meters), or approximately three acres, of industrial land in Yerucham, a city in southern Israel, to build the Green Vision Center Israel with Israeli government’s support. Under the Development Agreement entered into with the Israel Lands Authority (“ILA”), Cannovation Ltd. will build and develop the Green Vision Center in accordance with by the time frames, terms and conditions of the agreement. Typically, the initial time frame for completing the development is four (4) years, subject to extensions that the ILA may approve. Upon completion of the development within the time frames and other requirements specified in the Development Agreement, then Cannovation Ltd. will be entitled subject to Israeli law to long term lease agreement (49 years) to the land (equivalent to ownership rights as most of the land in Israel is government owned and when marketed usually the developers are granted with development/long lease rights). Our subsidiary, Cannovation Ltd., holds title to the land under the Development Agreement. Cannovation Ltd. is developing its Green Vision Center as development and production of wellness & pharma plant-based products, including botanical solutions, nutritional supplements, vitamins, healthy snacks & beverages, natural cosmetics, medical cannabis & cannabinoid-based products, plant-based pharma products and botanical drugs, and it is planned to include manufacturing plants, laboratories, logistics, import and export, offices, training, conference center, and an international visitor complex.
| 26 |
Employees/Consultants
We currently engage 18consultants, including our officers, on a part- time basis, working in various fields of management, research and development, product management, marketing and regulatory advice. Most of our activities are done with external consultants and professional companies that provide us the required services.
Legal Proceedings
We are not currently subject to any material legal proceedings.
Our Corporate History
We were incorporated under the laws of the State of Delaware on May 26, 2010 under the name “TechCare Corp.”.
On January 6, 2020, our predecessor company, TechCare Corp., a Delaware corporation (“TechCare”), and Citrine S A L Investment & Holdings Ltd., an Israeli corporation and a major shareholder of our company (“Citrine S A L”), and a group of related persons and entities (the “Citrine S A L Group”) entered into a Common Stock Purchase Agreement (the “Citrine S A L Group Agreement”), which was later amended and restated on February 23, 2020 (the “AR Citrine S A L Group Agreement”). Pursuant to the AR Citrine Agreement, TechCare agreed to sell Citrine S A L Group and its group of business partners, up to an aggregate of 893,699,276 shares of TechCare’s common stock, representing approximately 95% of TechCare’s fully diluted capital, in two tranches, with the initial tranche of up to 452,063,196 shares of the TechCare’s common stock to be sold conditioned upon (i) the resignation of the then members of its board of directors, (ii) the appointment of the current members of the Board, and (iii) the transfer of the TechCare’s signatory rights to all Company bank accounts in the name of Citrine S A L Group’s nominee. In addition, the AR Citrine S A L Group Agreement provides for the second tranche of up to the remaining number of shares of common stock that will result in Citrine S A L Group, owning 95% of the TechCare’s fully diluted capital stock, to be sold conditioned upon the filing of the Company’s previously approved amendment to its First Amended and Restated Certificate of Incorporation to increase the Company’s authorized capital. Shares of the Company were issued and sold in accordance with this amended agreement to Citrine S A L Group on February 27, 2020, March 5, 2020, and, after the Company amended its Certificate of Incorporation to increase its authorized share capital, on November 11, 2020.
ITEM 1A. RISK FACTORS
You should consider carefully the risks and uncertainties described below, together with all of the other information in this Annual Report on Form 10-K. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. The risks described below are not the only risks facing the Company. Risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition, results of operations and prospects.
| 27 |
Risks Related to our Financial position
We have a limited operating history and if we are not successful in continuing to grow our business, then we may have to scale back or even cease our ongoing business operations.
We have been operating in our current business since March 2020. Accordingly, our operations are subject to all the risks inherent in the establishment of a developing enterprise and the uncertainties arising from the absence of a significant operating history. As of December 31, 2023, we have not generated revenues and there can be no assurance that we will ever be profitable. If our business plan is not successful, and we are not able to operate profitably, investors may lose some or all of their investment in our company.
We expect to incur losses for the foreseeable future as we continue the implementation of our business plan. If we fail to generate revenue and eventually become profitable, or if we are unable to fund our continuing losses, our shareholders could lose all or a substantial part of their investment.
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never achieve, we expect to finance our cash needs primarily through public or private equity offerings, debt financings or through the establishment of possible strategic alliances. We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are not able to secure additional equity funding when needed, we may have to delay, reduce the scope of, or eliminate, development programs or future commercialization initiatives.
In addition, any additional equity funding that we do obtain will dilute the ownership held by our existing security holders.. Any debt financing that we obtain in the future could involve substantial restrictions on activities and creditors could seek a pledge of some or all of our assets. We have not identified potential sources for such financing that we will require, and we do not have commitments from any third parties to provide any future debt financing. If we fail to obtain funding as needed, we may be forced to cease or scale back operations, and our results, financial condition and stock price would be adversely affected.
We may never achieve profitability.
We are unable to accurately predict the timing or amount of future revenue or expenses or when, or if, we will be able to achieve profitability. We have financed our operations primarily through issuance and sale of equity and equity linked securities. The size of our future net losses will depend, in part, on the rate of growth or contraction of our expenses and the level and rate of growth, if any, of our revenues. We expect to continue to expend substantial financial and other resources on, among other things:
| ● | sales and marketing, including expanding our indirect sales organization and marketing programs; |
| ● | planning and conducting clinical trials to obtain regulatory approval/clearance for the commercialization of our products; |
| ● | expansion of our operations and infrastructure, both domestically and internationally; and |
| ● | general administration, including legal, accounting and other expenses related to being a public company. |
If we are unable to successfully commercialize our products or if revenue from any of our products that receives marketing approval is insufficient, we will not achieve profitability. Furthermore, even if we successfully commercialize our products, our planned investments may not result in increased revenue or growth of our business. We may not be able to generate net revenues sufficient to offset our expected cost increases and planned investments in our business. As a result, we may incur significant losses for the foreseeable future, and may not be able to achieve and sustain profitability. If we fail to achieve and sustain profitability, then we may not be able to achieve our business plan, fund our business or continue as a going concern.
| 28 |
Currency exchange rate fluctuations affect our results of operations, as reported in our financial statements.
We incur expenses in U.S. Dollars and in NIS but our functional currency is the U.S. dollar. However, a significant portion of our headcount related expenses, consisting principally of personnel expenses as well as R&D consulting services, leases and certain other operating expenses, are denominated in NIS. This foreign currency exposure gives rise to market risk associated with exchange rate movements of the U.S. dollar against the NIS. Furthermore, we anticipate that a material portion of our expenses will continue to be denominated in NIS.
In addition, increased international sales in the future may result in greater foreign currency denominated sales, increasing our foreign currency risk. If we are not able to successfully hedge against the risks associated with currency fluctuations, our financial condition and results of operations could be adversely affected. which could adversely affect our financial condition and results of operations.
Risks Related to Our Business and Industry and Regulatory Process
Our failure to manage growth effectively could impair our business.
Our business strategy envisions a period of rapid growth that may put a strain on our administrative and operational resources and funding requirements. Our ability to effectively manage growth will require us to continue to expand the capabilities of our operational and management systems and to attract, train, manage, and retain qualified personnel. There can be no assurance that we will be able to do so, particularly if losses continue and we are unable to obtain sufficient financing. If we are unable to successfully manage growth, our business, prospects, financial condition, and results of operations could be adversely affected.
Our plans are dependent upon key individuals and the ability to attract qualified personnel.
In order to execute our business plan, we will be dependent on Ora Elharar Soffer, our Chief Executive Officer and Director. The loss of Ms. Elharar Soffer could have a material adverse effect upon our business prospects. Moreover, our success continues to depend to a significant extent on our ability to identify, attract, hire, train and retain qualified professional, creative, technical and managerial personnel.
Competition for such personnel is intense, and there can be no assurance that we will be successful in identifying, attracting, hiring, training, and retaining such personnel in the future. If we are unable to hire, assimilate and retain qualified personnel in the future, our business, operating results, and financial condition could be materially adversely effected. We may also depend on third party contractors and other partners to assist with the execution of our business plan. There can be no assurance that we will be successful in either attracting and retaining qualified personnel, or creating arrangements with such third parties. The failure to succeed in these endeavors would have a material adverse effect on our ability to consummate our business plans.
Failure in the Company’s information technology systems, including by cybersecurity attacks or other data security incidents, could significantly disrupt its operations.
Our operations depend, in part, on the continued performance of our information technology systems. Our information technology systems are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptions. Failure of our information technology systems could adversely affect our business, profitability, and financial condition. Although we have information technology security systems, a successful cybersecurity attack or other data security incident could result in the misappropriation and/or loss of confidential or personal information, create system interruptions, or deploy malicious software that attacks our systems. It is possible that we not notice a cybersecurity attack for some period. The occurrence of a cybersecurity attack or incident could result in business interruptions from the disruption of the Company’s information technology systems, or negative publicity resulting in reputational damage with its shareholders and other stakeholders and/or increased costs to prevent, respond to or mitigate cybersecurity events. In addition, the unauthorized dissemination of sensitive personal information or proprietary or confidential information could expose the Company or other third parties to regulatory fines or penalties, litigation, and potential liability, or otherwise harm its business.
| 29 |
We may grow through mergers or acquisitions, which strategy may not be successful or, if successful, may produce risks in successfully integrating and managing the merged companies or acquisition and may dilute our stockholders.
As part of our growth strategy, we may pursue mergers and acquisitions of entities and/or assets that we believe will have synergistic and/or other value to us. We currently have no agreements or understandings to merge with or acquire any entity and/or assets, and may not find suitable merger or acquisition opportunities. Mergers and acquisitions involve numerous risks, any of which could harm our business, including, without limitation:
● difficulties in integrating the operations, technologies, existing contracts, accounting processes and personnel of the target and realizing the anticipated synergies of the combined businesses;
● difficulties in supporting and transitioning customers of the target company;
● diversion of financial and management resources from existing operations;
● the price we pay or other resources that we devote may exceed the value we realize, or the value we could have realized if we had allocated the purchase price or other resources to another opportunity;
● entering new markets or areas in which we have limited or no experience;
● potential loss of key associates and customers from either our business or the target’s business;
● assumption of unanticipated problems or latent liabilities of the target; and
● the inability to generate sufficient revenue to offset acquisition costs.
Mergers and acquisitions also frequently result in the recording of goodwill and other intangible assets, which are subject to potential impairments in the future and that could harm our financial results. In addition, if we finance acquisitions by issuing convertible debt or equity securities, our existing stockholders may be diluted, which could affect the market price of our common shares. As a result, if we fail to properly evaluate mergers, acquisitions or investments, we may not achieve the anticipated benefits of any such merger or acquisition, and we may incur costs in excess of what we anticipate. The failure to successfully evaluate and execute mergers, acquisitions or investments or otherwise adequately address these risks could materially harm our business, financial condition and results of operations.
We may be subject to product liability claims which may have a material adverse effect on our business
Through our subsidiary Cannovation Center Israel, we developed the ‘Green Side by Side’ product line containing natural and herbal formulas based on researched and science-based plants, herbal extracts, mushrooms and other natural ingredients. The Green Side By side product lines are manufactured in Israel in iBOT Israel Botanicals Ltd, iBOT being a related party with which we have manufacturing and strategic cooperation agreements, under a GMP-certified manufacturing facility approved by the Israeli Ministry of Health. In the future we plan to develop additional products which may be subject to different regulations for manufacturing, depending on country and product. As these products are designed to be ingested by humans, we face an inherent risk of exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of cannabis products involve the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of cannabis products alone or in combination with other medications or substances could occur. We may be subject to various product liability claims, including, among others, that the products produced by us caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against us could result in increased costs, could adversely affect our reputation with our clients and consumers generally, and could have a material adverse effect on the business, financial condition and operating results of the Company. There can be no assurances that we will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of products.
| 30 |
Product recalls may harm our reputation.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If any of our products are recalled due to an alleged product defect or for any other reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. We may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention. Although we have detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if one of the products produced by the Company were subject to recall, the image of that product and the Company could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for products produced by the Company and could have a material adverse effect on the results of operations and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of the operations of the Company by any applicable regulatory agencies, requiring further management attention and potential legal fees and other expenses.
Our officers and directors may be subject to conflict of interest.
The Company may be subject to various potential conflicts of interest because of the fact that some of its officers and directors may be engaged in a range of business activities. In addition, the Company’s executive officers and directors may devote time to their outside business interests, so long as such activities do not materially or adversely interfere with their duties to the Company. In some cases, the Company’s executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to the Company’s business and affairs and that could adversely affect the Company’s operations. These business interests could require significant time and attention of the Company’s executive officers and directors.
In addition, the Company may also become involved in other transactions which conflict with the interests of certain directors and the officers who may from time-to-time deal with persons, firms, institutions or companies with which the Company may be dealing, or which may be seeking investments similar to those desired by it. The interests of these persons could conflict with those of the Company. In addition, from time to time, these persons may be competing with the Company for available investment opportunities. Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws. In particular, in the event that such a conflict of interest arises at a meeting of the Company’s directors, a director who has such a conflict will abstain from voting for or against the approval of such participation or such terms. In accordance with applicable laws, the directors of the Company are required to act honestly, in good faith and in the best interests of the Company.
We face significant competition in the market.
We face intense competition from other companies, some of which can be expected to have more financial resources and manufacturing and marketing experience than the Company. Increased competition by larger and better financed competitors could materially and adversely affect the business, financial condition and results of operations of the Company
We may not be able to obtain adequate insurance coverage and in the case of liability the lack of adequate insurance may have a material adverse effect on our business.
We have insurance to protect our assets, operations and employees. While we believe our insurance coverage addresses all material risks to which may be exposed and is adequate and customary in our current state of operations, such insurance is subject to coverage limits and exclusions and may not be available for the risks and hazards to which the Company is exposed. In addition, no assurance can be given that such insurance will be adequate to cover the Company’s liabilities or will be generally available in the future or, if available, that premiums will be commercially justifiable. If the Company were to incur substantial liability and such damages were not covered by insurance or were in excess of policy limits, or if the Company were to incur such liability at a time when it is not able to obtain liability insurance, its business, results of operations and financial condition could be materially adversely affected.
| 31 |
Research and development and product obsolescence may impair our ability to compete in our target market.
Rapidly changing markets, technology, emerging industry standards and frequent introduction of new products characterize our business. The introduction of new products embodying new technologies, including new manufacturing processes, and the emergence of new industry standards may render our planned product offerings obsolete, less competitive or less marketable. The process of developing our planned products is complex and requires significant continuing costs, development efforts and third-party commitments The Company’s failure to develop new technologies and products and the obsolescence of existing technologies could adversely affect our business, financial condition and operating results. The Company’s success will depend, in part, on its ability to continue to enhance its existing technologies, develop new technology that addresses the increasing sophistication and varied needs of the market, and respond to technological advances and emerging industry standards and practices on a timely and cost-effective basis. The development of the Company’s proprietary technology entails significant technical and business risks. The Company may not be successful in using its new technologies or exploiting its niche markets effectively or adapting its businesses to evolving customer or medical requirements or preferences or emerging industry standards.
It may be difficult to enforce a judgment of a U.S. court against us and our executive officers and directors in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our executive officers and directors.
While we were incorporated in Delaware, all of our executive officers and directors reside outside of the United States, and all of our assets and most of the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
The continuing prevalence of the COVID-19 pandemic may adversely affect our operations and our capital raising efforts.
In late 2019, a novel strain of Corona virus, also known as COVID-19, was reported in Wuhan, China. While initially the outbreak was largely concentrated in China, it has now spread globally. Many countries around the world, have significant governmental measures implemented to control the spread of the virus, including temporary closure of businesses, severe restrictions on travel and the movement of people, limited access to nursey homes, hospitals and other medical institutes and other material limitations on the conduct of business. These measures have resulted in work stoppages and other disruptions. Our research and development activities, sales and marketing efforts, as well as our ability to perform clinical trials (if needed) depend, in part, on attendance at in-person meetings, industry conferences and other events, facility visiting, and as a result some of our sales and marketing activities have been halted.
The extent to which the coronavirus impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration and severity of the outbreak, and the actions that may be required to contain the coronavirus or treat its impact. In particular, the continued spread of the coronavirus globally, could have a material adverse impact on our operations and workforce, including our marketing and sales activities and ability to raise additional capital, and our ability to perform clinical trials, which in turn could have a material adverse impact on our business, financial condition and results of operation.
| 32 |
We intend to rely on third parties to conduct clinical trials (if needed). If these third parties do not meet our deadlines or otherwise conduct the trials as required, our clinical trials programs could be delayed or unsuccessful and we may not be able to obtain regulatory approval for or commercialize our product candidates when expected or at all.
We may conduct clinical trials on our products. We do not have the ability to conduct all aspects of our clinical trials ourselves. We may rely upon medical institutions, clinical investigators and contract research organizations, or CROs, and consultants to conduct these trials in accordance with our clinical protocols. Our future CROs, investigators and other third parties play a significant role in the conduct of these trials and the subsequent collection and analysis of data from the clinical trials.
There is no guarantee that any CROs, investigators and other third parties upon which we rely for administration and conduct of clinical trials will devote adequate time and resources to such trials or perform as contractually required. If any of these third parties fail to meet expected deadlines, fail to adhere to our clinical protocols or otherwise perform in a substandard manner, our clinical trials may be extended, delayed or terminated. If any of these clinical trial sites terminate for any reason, we may experience the loss of follow-up information on patients enrolled in our ongoing clinical trials unless we are able to transfer the care of those patients to another qualified clinical trial site. In addition, principal investigators for any clinical trials we conduct may serve as scientific advisors or consultants to us from time to time and receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, the integrity of the data generated at the applicable clinical trial site may be jeopardized.
We will be subject to regulation of plant-based nutritional supplements, botanicals, cannabis, cosmetics and pharmaceuticals.
Our products, solutions and Green Vision Center activities are subject to rules and regulations pertaining to plant-based nutritional supplements, botanicals, cannabis, cosmetics, and pharmaceuticals, as applicable. The company cannot predict the time required to secure all appropriate regulatory approvals or the extent of testing and documentation that may be required by governmental authorities. Any delays in obtaining, or failure to obtain, regulatory approvals would significantly delay the development of markets and products and could have a material adverse effect on the business, results of operations and financial condition of the Company.
Cannabis remains illegal under U.S. federal law.
As of January 2023, cannabis is legal for recreational use in 18 states and legal for medical use in 37 states. On a federal level, all cannabis remains illegal. The federal government classifies cannabis, along with heroin and cocaine, as a Schedule I drug with a high potential for abuse and little to no medical benefit. Despite the development of a regulated cannabis industry under the laws of certain states, these state laws regulating medical and adult cannabis use are in conflict with the Federal Controlled Substances Act, which classifies cannabis as a Schedule I controlled substance and makes cannabis use and possession illegal on a national level. The United States Supreme Court has ruled that the Federal government has the right to regulate and criminalize cannabis, even for medical purposes, and thus Federal law criminalizing the use of cannabis preempts state laws that regulate its use. During President Biden’s first year in office, Attorney General Merrick Garland rescinded two key memos that were part of the Trump Administration’s stated regulatory reform agenda: the Sessions Memo, which prohibited Department of Justice (DOJ) components from issuing “guidance documents” that effectively bound the public without undergoing notice-and-comment rulemaking, and the Brand Memo, which prohibited the DOJ from using noncompliance with DOJ’s or other agencies’ nonbinding guidance documents as a basis for affirmative civil enforcement actions. Calling the procedures laid out in the Sessions and Brand memos “overly restrictive,” Attorney General Garland replaced these memos with the Garland memo, which largely makes it easier for the DOJ to issue guidance and to rely on its own or other agencies’ guidance documents in enforcement actions. The Sessions Memo rescinded the Cole Memo which was adopted by the Obama administration as a policy of noninterference with cannabis-friendly state laws. The Sessions Memo shifted federal policy from a hands-off approach adopted by the Obama administration to permitting federal prosecutors across the country to decide how to prioritize resources to regulate cannabis possession, distribution and cultivation in states where cannabis use is regulated. With these changes in policy with every administration, there can be no assurance that federal prosecutors will not prosecute and dedicate resources to regulate cannabis possession, distribution and cultivation in states where cannabis use is regulated which may cause states to reconsider their regulation of cannabis which would have a detrimental effect on the cannabis industry.
| 33 |
Risks Related to our Intellectual Property
If we are unable to obtain and maintain intellectual property protection for our product offerings, or if the scope of the intellectual property protection we obtain is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products may be impaired.
Our ability to compete successfully will depend in part on our ability to obtain and enforce patent protection for our products, preserve our trade secrets and operate without infringing the proprietary rights of third parties. Filing, prosecuting, and defending patents on our products and other technologies in all countries throughout the world would be prohibitively expensive and time-consuming, and the laws of some foreign countries may not protect our rights to the same extent as the laws of the United States. We may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patents or patent applications at a reasonable cost or in a timely manner, or in all jurisdictions, or at all, or may choose not to do any of the foregoing.
Moreover, while we have applied for a patent that protect aspects of our products in the United States but our products are not covered by any patent protection, and we cannot assure you that our intellectual property position, will not be challenged or that all patents for which we have applied will be issued on a timely basis or at all, or that such patents will protect our technology, in whole or in part, or be issued in a form that will provide us with meaningful protection, prevent competitors from competing with us, or otherwise provide us with any competitive advantage. Although patents are presumed valid and enforceable upon issuance, a patent may be challenged as to its inventorship, scope, validity, or enforceability.
Patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the invention claimed in our pending patent application, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are uncertain. Given the amount of time required for the development, testing, and regulatory review of new products, patents protecting such products might expire before or shortly after such products are commercialized. As a result, any patent portfolio we develop may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may be sued by third parties for alleged infringement of their proprietary rights, which could adversely affect our business, results of operations and financial condition.
There is often litigation between competing companies relying on their respective technologies based on allegations of infringement or other violations of intellectual property rights. Our future success depends, in part, on not infringing the intellectual property rights of others. We may be unaware of the intellectual property rights of others that may cover some or all of our technology. Any such claims or litigation could cause us to incur significant expenses and, if successfully asserted against us, could require that we pay substantial damages or ongoing royalty payments, prevent us from offering some portion of our products, or require that we comply with other unfavorable terms. We may also be obligated to indemnify our customers or channel partners in connection with any such litigation and to obtain licenses or modify our products, which could further exhaust our resources. Patent infringement, trademark infringement, trade secret misappropriation and other intellectual property claims and proceedings brought against us, whether successful or not, could harm our brand, business, results of operations and financial condition. Litigation is inherently uncertain, and any judgment or injunctive relief entered against us or any adverse settlement could negatively affect our business, results of operations and financial condition. In addition, litigation can involve significant management time and attention and be expensive, regardless of the outcome. During the course of litigation, there may be announcements of the results of hearings and motions and other interim developments related to the litigation. If securities analysts or investors regard these announcements as negative, the trading price of our common stock may decline.
| 34 |
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful.
If we attempt enforcement of our patents or other intellectual property rights, we may be subject or party to claims, negotiations or complex, protracted litigation. These claims and any resulting lawsuits, if resolved adversely to us, could subject us to significant liability for damages, impose temporary or permanent injunctions against our solutions or business operations, or invalidate or render unenforceable our intellectual property
Intellectual property disputes and litigation, regardless of merit, can be costly and disruptive to our business operations by diverting attention and energies of management and key technical personnel, and by increasing our costs of doing business. Such litigation, regardless of its success, could seriously harm our reputation with our channel partners, business partners and patients and in the industry at large. Some of our competitors may be able to sustain the costs of complex patent or intellectual property litigation more effectively than we can because they have substantially greater resources. Any of the foregoing could adversely affect our operating results.
Risks Relating to Our Israel Operations
Our development efforts are headquartered in Israel and, therefore, our results may be adversely affected by economic restrictions imposed on, and political and military instability in, Israel.
Our development headquarters, which houses substantially all of our research and development team, including engineers, machinists, researchers, and clinical and regulatory personnel as well as the facility of our contract manufacturer and final assembly are located in Israel. Our employees, service providers, directors and officers are residents of Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region may directly affect our business. Any hostilities involving Israel or the interruption or curtailment of trade within Israel or between Israel and its trading partners could materially and adversely affect our business, financial condition and results of operations and could make it more difficult for us to raise capital. Although we plan to maintain inventory in the United States and Germany, an extended interruption could materially and adversely affect our business, financial condition and results of operations.
Recent political uprisings, social unrest and violence in various countries in the Middle East and North Africa, including Israel’s neighbors Egypt and Syria, are affecting the political stability of those countries. This instability may lead to deterioration of the political relationships that exist between Israel and these countries and has raised concerns regarding security in the region and the potential for armed conflict. Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Any losses or damages incurred by us could have a material adverse effect on our business. In addition, Iran has threatened to attack Israel and is widely believed to be developing nuclear weapons. Iran is also believed to have a strong influence among parties hostile to Israel in areas that neighbor Israel, such as the Syrian government, Hamas in Gaza and Hezbollah in Lebanon. Any armed conflicts, terrorist activities or political instability in the region could materially and adversely affect our business, financial condition and results of operations.
| 35 |
Our operations and the operations of our contract manufacturer may be disrupted as a result of the obligation of Israeli citizens to perform military service.
Many Israeli citizens are obligated to perform one month, and in some cases more, of annual military reserve duty until they reach the age of 45 (or older, for reservists with certain occupations) and, in the event of a military conflict, may be called to active duty. In response to terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be additional military reserve duty call-ups in the future. Some of our employees, consultants and employees of the manufacturer of our products, are required to perform annual military reserve duty in Israel and may be called to active duty at any time under emergency circumstances. Our operations and the operations of our manufacturer could be disrupted by such call-ups.
Our sales may be adversely affected by boycotts of Israel.
Several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies whether as a result of hostilities in the region or otherwise. In addition, there have been increased efforts by activists to cause companies and consumers to boycott Israeli goods based on Israeli government policies. Such actions, particularly if they become more widespread, may adversely impact our ability to sell our products.
Risks Related Ownership of Our Securities
A certain group of the Company’s stockholders may exert significant influence over its affairs, including the outcome of matters requiring stockholder approval.
Currently, a certain group of stockholders, including our Chairperson and Chief Executive Officer, Ora Elharar Soffer (directly and through Beezz Home Technologies Ltd and Citrine S A L Investment & Holdings Ltd) and others, collectively own a majority of the issued and outstanding shares of the Company. As a result, such individuals will have the ability, acting together, to control the election of the Company’s directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of the Company, (ii) a sale of all or substantially all of its assets, and (iii) amendments to its certificate of incorporation. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to the Company’s other stockholders and be disadvantageous to the Company’s stockholders with interests different from those individuals. Certain of these individuals also have significant control over the Company’s business, policies and affairs as officers or directors of the Company. Therefore, you should not invest in reliance on your ability to have any control over the Company.
As of the date of this report, our executive officers and directors own, in the aggregate, beneficially own approximately 45.43% of our outstanding common stock as of the date of this filing. As a result, these persons, acting together, would be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors, any merger, consolidation, sale of all or substantially all of our assets, or other significant corporate transactions.
You may experience future dilution as a result of future equity offerings.
Our Amended and Restated Articles of Incorporation authorize the issuance of a maximum 1,500,000 shares of common stock. Any additional financings effected by us may result in the issuance of additional securities without stockholder approval and the substantial dilution in the percentage of common stock held by our then existing stockholders. In addition, we have reserved 180,000,000 shares of common stock for issuance under the 2018 Equity Incentive Plan. The issuance of such additional shares of common stock, or securities convertible or exchangeable into common stock, may cause the price of our common stock to decline. Additionally, if all or a substantial portion of these shares are resold into the public markets then the trading price of our common stock may decline.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
We currently do not have and may never obtain research coverage by securities analysts. If no securities analysts commence coverage of our company, or if industry analysts cease coverage of our company, the trading price for our stock could be materially and adversely impacted. In the event we obtain securities analyst coverage, if one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price may be materially and adversely impacted. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
| 36 |
If the price of our common stock fluctuates significantly, your investment could lose value.
Our common stock is quoted on the OTCQB, under the symbol “CTGL,” and, to date, has traded on a limited basis. We have applied to list our common stock on Nasdaq under the symbol “CTGL.” We cannot assure you that an active public market will continue for our common stock. If an active public market for our common stock does not continue, the trading price and liquidity of our common stock will be materially and adversely affected. If there is a thin trading market or “float” for our stock, the market price for our common stock may fluctuate significantly more than the stock market as a whole. Without a large float, our common stock would be less liquid than the stock of companies with broader public ownership and, as a result, the trading prices of our common stock may be more volatile. In addition, in the absence of an active public trading market, investors may be unable to liquidate their investment in us. Furthermore, the stock market is subject to significant price and volume fluctuations, and the price of our common stock could fluctuate widely in response to several factors, including, but not limited to:
| ● | our quarterly or annual operating results; | |
| ● | changes in our earnings estimates or the failure to accurately forecast and appropriately plan our expenses; | |
| ● | failure to achieve our growth expectations; | |
| ● | failure to attract new customers or retain existing customers; | |
| ● | the effect of increased or variable competition on our business; | |
| ● | additions or departures of key or qualified personnel; | |
| ● | failure to adequately protect our intellectual property; | |
| ● | costs associated with defending claims, including intellectual property infringement claims and related judgments or settlements; | |
| ● | changes in governmental or other regulations affecting our business; | |
| ● | our compliance with governmental or other regulations affecting our business; and | |
| ● | changes in global or regional industry, general market, or economic conditions. |
The stock market has experienced extreme price and volume fluctuations in recent years that have significantly affected the quoted prices of the securities of many companies, including companies in our industry. The changes may not be possible to predict and often appear to occur without regard to specific operating performance. The price of our common stock could fluctuate based upon factors that have little or nothing to do with our company and these fluctuations could materially reduce our stock price.
Delaware law contains provisions that could discourage, delay, or prevent a change in control of the Company, prevent attempts to replace or remove current management and reduce the market price of its common stock.
Provisions in the Company’s certificate of incorporation and bylaws may discourage, delay or prevent a merger or acquisition involving the Company that its stockholders may consider favorable. For example, the Company is subject to the anti-takeover provisions of the Delaware General Corporation Law (“DGCL”). Under these provisions, if anyone becomes an “interested stockholder,” the Company may not enter into a “business combination” with that person for three years without special approval, which could discourage a third party from making a takeover offer and could delay or prevent a change in control of the Company. An “interested stockholder” is, generally, a stockholder who owns 15% or more of the Company’s outstanding voting stock or an affiliate of the Company who has owned 15% or more of the Company’s outstanding voting stock during the past three years, subject to certain exceptions as described in the DGCL.
| 37 |
We do not intend to pay dividends for the foreseeable future.
We have never declared or paid cash dividends on our capital stock nor are we under any obligation to declare or pay such cash dividends. We currently intend to retain any future earnings to fund our operations and the development and growth of our business, and we do not expect to declare or pay any dividends in the foreseeable future. Our future ability to pay cash dividends on our capital stock may be limited by any future debt instruments or preferred securities. As a result, investors may only receive a return on their investment in our common stock if the market price of our common stock increases to a price above the price paid for them and then sell such shares.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
A description of the premises we utilize in several of our facilities is as follows:
| Entity | Property Description | |
| Citrine Global, Corp | Our principal office consists of leased premises and is located at 5 Golden Beach, Caesarea 3088900, Israel | |
| Cannovation Center Israel Ltd. | 125,000 square feet (approximately 11,687 square meters) or approximately three acres of industrial land in Yerucham, a city in southern Israel, where we intend to build Green Vision Center Israel |
We believe that our facilities are generally in good condition and suitable to carry on our business. We also believe that, if required, suitable alternative or additional space will be available to us on commercially reasonable terms
ITEM 3. LEGAL PROCEEDINGS
There are no active or pending material legal proceedings against the Company, nor of any proceedings that a governmental authority is contemplating against the Company.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
The Company’s common stock is quoted in the United States on the OTCQB market under the ticker symbol “CTGL.”
We have applied to list our common stock on the Nasdaq Capital Market. No assurance can be given that our application will be approved or that a trading market will develop.
Holders of our Common Stock
As of March 20, 2023, the Company had 120 registered stockholders holding 956,479,039 shares of common stock.
Dividends
Since the Company’s inception, it has not declared nor paid any cash dividends on its capital stock and the Company does not anticipate paying any cash dividends in the foreseeable future. Its current policy is to retain any earnings in order to finance its operations. Its Board of directors will determine future declarations and payments dividends, if any, in light of the then-current conditions it deems relevant and in accordance with applicable corporate law.
| 38 |
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides certain aggregate information with respect to the Company’s shares of common stock that as of December 31, 2022 were issuable under its equity compensation plans in effect as of December 31, 2022.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (1) | Weighted-average exercise price of outstanding options, warrants and rights (2) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in first column) (3) | |||||||||
| Equity compensation plans approved by security holders | 122,529,342 | $ | 0.043 | 57,470,658 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 122,529,342 | $ | 0.043 | 57,470,658 | ||||||||
| (1) | Represents shares of common stock issuable under our 2017 and 2018 Employee Incentive Plan and upon exercise of outstanding options to purchase 122,529,342 shares of common stock. |
| (2) | The weighted average remaining term for the expiration of remaining stock options is 2.53 years. |
| (3) | Represents shares of common stock available for future issuance under equity compensation plans. “Equity Compensation Plan” under Item 11 hereof contains a description of the material features of the 2017 Employee Incentive Plan and the 2018 Stock Incentive Plan. |
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
ITEM 6. RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand our audited consolidated financial statements for the fiscal years ended December 31, 2022 and December 31, 2021 and highlight certain other information which, in the opinion of management, will enhance a reader’s understanding of our financial condition, changes in financial condition and results of operations. In particular, the discussion is intended to provide an analysis of significant trends and material changes in our financial position and the operating results of our business during the year ended December 31, 2022, as compared to the fiscal year ended December 31, 2021. This discussion should be read in conjunction with our consolidated financial statements for the fiscal years ended December 31, 2022 and December 31, 2021 and related notes included elsewhere in this Annual Report on Form 10-K. These historical financial statements may not be indicative of our future performance. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains numerous forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks described throughout this filing, particularly in “Item 1A. Risk Factors.”
| 39 |
The full extent to which the COVID-19 pandemic may directly or indirectly impact our business, results of operations and financial condition, will depend on future developments that are uncertain, including as a result of new information that may emerge concerning COVID-19 and the actions taken to contain it or treat COVID-19, as well as the economic impact on local, regional, national and international customers and markets. We have made estimates of the impact of COVID-19 within our financial statements, and although there is currently no major impact, there may be changes to those estimates in future periods. Actual results may differ from these estimates.
Significant Recent Events
(i) On January 30, 2023 the Company and each of Citrine High Tech 7 LP (“LP 7”), Citrine 8 LP (“LP 8 “) and Citrine 9 LP (“LP 9”; together with LP 7 and LP 8, the “Lending LP”), the lending entities under and parties to the Convertible Note Purchase Agreement entered into by the Company and several related parties in April 2020, as subsequently amended (the “CL Agreement”), have entered into an agreement (the “Agreement”) pursuant to which they have agreed to extend the maturity date on all outstanding convertible loans in the principal amount of $1,800,000 under the CL Agreement to May 31, 2024. LP 7 also agreed to extend to May 31, 2024 the note in the principal amount of $80,000
In addition, under the Agreement the Company and the Lending LPs have also agreed that if the Company’s common stock is listed on the Nasdaq Stock Market, then the Company, in its sole discretion, shall determine to convert, in whole or in part, the outstanding amount of the above mentioned notes to shares of the Company’s common stock at a conversion price equal to the price paid by the public investors for the common stock in such offering.
(ii) On January 17, 2023, the Board of Citrine Global, Corp., a Delaware corporation ( the “Company”), appointed Ms. Ora Elharar Soffer to serve as president of the Company. Ms. Elharar Soffer has been continuously serving as the Company’s Chief Executive Officer since May 7, 2020 and as a Company director since February 21, 2020 and as Chairperson of the Board since March 3, 2020.
(iii) On March 5, 2023, the Board of the Company provided that in the event that the Company’s stock is listed on the Nasdaq Stock Exchange, then one half of the awarded and unvested option grants made in each of August 2021 and in August 2022 to our current officers, directors and specific service providers, will immediately vest at such time. In addition, the Board also determined to provide that following the termination of services by our current officers, directors and specific service providers, for any reason other than cause, they shall have a one year period from the date of termination to exercise any option that was vested at the time of the termination of services. Previously, on November 13, 2022, the board of directors ratified the Stock Option Agreements for the previously disclosed stock options grants that were awarded in each of August 2021 and in August 2022 to our current officers and directors and specific service providers, to provide that the exercise price of the options that were awarded to date, shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options and all other relevant terms of such options (other than the exercise price) shall continue in full force and effect following the implementation of such reverse stock split. Any and all tax implication of this decision shall rest solely with the optionee.
(iv) On March 6, 2023 Cannovation, the Company’s majority owned subsidiary and S.R. Accord Ltd., an Israeli public company (“Lender”), entered into an 18-month credit facility agreement (the “Credit Facility”) pursuant to which Lender has committed to fund Cannovation in an aggregate amount of 3,000,000 NIS (approximately $857,000) as needed. At the time of each draw down, Cannovation and Lender will determine the repayment of the loan. All amounts drawn under the Credit Facility will bear interest at a monthly rate of 1.7% and. Cannovation has the right to pre-pay the entire amount outstanding under the Credit Facility at any time. As security for any loans under the Credit Facility, Cannovation granted Lender a first priority lien on its rights to the 125,000 sq ft (11,687 sqm) of industrial land in Yerucham, a city in southern Israel which Cannovation acquired in February of 2022(the “Premises”) to build the Green Vision Center Israel with the support of the government of Israel. The lien will become effective only if Cannovation utilizes the Credit Facility. If the market value of the Premises is less than the amount outstanding under the Credit Facility, then Lender will be entitled to additional security including additional shares of Citrine Global common stock, on such terms and conditions as the parties may agree. As additional security for any payments due to Lender, CTGL Citrine Global Israel Ltd., a wholly owned subsidiary of Citrine Global, (ii) Beezzhome Technologies Ltd. an entity wholly owned by Ora Elharar Soffer, the Chief Executive Officer of Citrine Global and (iii) Netto Holdings, an unaffiliated entity under the partial control of Ilan Ben Ishay, a director on the board of Cannovation, as well as each of Ms. Elharar Soffer and Mr. Ben Ishay in their personal capacities, are providing guarantees for the repayment of any amounts that may be owing to Lender under the Credit Facility. Cannovation and the Company has agreed to indemnify Ms. Elharar Soffer and Mr. Ben Ishay for any losses they incur as a result of the guarantee.
On March 7, 2023, the Company issued to the Lender 2,154,677 shares of the Company’s common stock a commitment fee in respect of the provision of the Credit Facility. As of the date of this report, Cannovation utilized $50,000 out of the credit line. Ilan Ben Ishay and Ms. Elharar Soffer and Mr. Ben Ishay in their personal capacities in are provided guarantees to Lender for the repayment this amounts.
| 40 |
(v) On March 16, 2023, the consulting agreement originally entered into as of July 2020 with Ms. Ora Elharar Soffer, the Company’s Chairperson, CEO and President, was amended. The amendment provides for the following: (i) the monthly consulting to which Ms. Elharar Soffer is entitled will increase from $20,000 to $25,000 (in invoice plus VAT if applicable) upon a listing of the Company’s stock on the Nasdaq Stock Market, retroactive to January 1, 2023, (ii) the terms contained in her original agreement and all other terms and awards previously approved by the Company’s board relating to her, including payment of her monthly fee and reimbursement of social benefits payments made by Mr Elharar Soffer, shall continue in full force and effect so long as Ms. Elharar Soffer serves as either director and /or executive officer (iii) all previous awards and bonuses previously made to her were affirmed and (iv) Ms. Elharar Soffer has agreed to defer compensation due to her until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities, at which time all of the outstanding consulting fees from March 2020 and all reimbursement for related social benefits would be paid to her. In addition, The amendment also provides that the committee of the Board that will be responsible for setting the compensation terms of senior management shall prepare and present for approval a compensation program for the Consultant that takes into consideration Ms. Elharar Soffer’s role in founding and leading the Company and that such compensation package shall be competitive with compensation programs for top senior executives/founders generally available in the market and which will include, among other things, appropriate bonuses, severance payments and other amenities generally made available in the market to senior executive and that Ms. Elharar Soffer shall receive the most extensive of such compensation terms amongst senior management.
(vii) On March 16, 2023, the consulting agreement originally entered into as of July 2020 with Ms Ilanit Halperin, the Company’s director and CFO, was amended. The amendment provides for the following: (i) the monthly consulting to which Ms. Elharar Soffer is entitled will increase from $7,500 to $10,000 ( in invoice plus VAT if applicable) upon a listing of the Company’s stock on the Nasdaq Stock Market, retroactive to January 1, 2023, (ii) the terms contained in her original agreement and all other terms and awards previously approved by the Company’s board relating to her, including payment of her monthly fee and reimbursement of social benefits payments made by Mr Elharar Soffer ,shall continue in full force and effect so long as Ms. Halperin serves as either director and /or executive officer, (iii) all previous awards and bonuses previously made to her were affirmed and (iv) s. has agreed o defer compensation due to her until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities, at which time all of the outstanding consulting fees from March 2020 and all reimbursement for related social benefits would be paid to her. In addition, The Company undertakes that the committee of the Board that will be responsible for setting the compensation terms of senior management shall prepare and present for approval a compensation program for Ms. Halperin that shall be competitive with compensation programs for senior executives generally available in the market and which will include, among other things, appropriate bonuses, severance payments and other amenities generally made available in the market to senior executives.
Components of Operating Results
The following discussion summarizes the key factors our management believes are necessary for an understanding of our consolidated financial statements.
Revenues
We have not generated any revenues from product sales as of December 31, 2022.
Research and Development Expenses
The process of researching and developing our products is lengthy, unpredictable, and subject to many risks. We expect to continue incurring expenses for the next several years for research and development as we continue to develop products and innovative solutions. We are unable, with any certainty, to estimate either the costs or the timelines in which those expenses will be incurred. Our current development plans focus on the development of plant-based solutions including GreenFeels™ and Green Side by Side Products lines.
Our research and development costs include costs are composed of:
● internal recurring costs, such as personnel-related and consultants costs (salaries, employee benefits, equity compensation and other costs), materials and supplies, facilities and maintenance costs attributable to research and development functions; and
● fees paid to external parties who provide us with contract services, such as preclinical testing, manufacturing and related testing and activities.
| 41 |
Marketing
Marketing expenses consist primarily of salaries, employee benefits, equity compensation, and other personnel-related costs associated with executive and other support staff. Other significant marketing expenses include the costs associated with professional fees to develop our marketing strategy.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, employee benefits, equity compensation, and other personnel-related costs associated with executive, administrative and other support staff. Other significant general and administrative expenses include the costs associated with professional fees for accounting, auditing, insurance costs, consulting and legal services, along with facility and maintenance costs attributable to general and administrative functions.
Financial Expenses
Financial expenses consist primarily impact of exchange rate derived from re-measurement of monetary balance sheet items denominated in non-dollar currencies. Other financial expenses include bank’s fees and interest on long term loans.
Results of Operations
Year ended December 31, 2022 as compared to the year ended December 31, 2021
The following table presents our results of operations for the years ended December 31, 2022 and 2021
| Year Ended | ||||||||
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| U.S. Dollars in thousands | ||||||||
| Revenues | - | - | ||||||
| Cost of sales | - | - | ||||||
| Gross loss | - | |||||||
| Research and development expenses | (120 | ) | (96 | ) | ||||
| Marketing, general and administrative expenses | (1,866 | ) | (3,239 | ) | ||||
| Operating loss | (1,986 | ) | (3,335 | ) | ||||
| Expenses related to convertible loan terms | (635 | ) | (1,129 | ) | ||||
| Other financing expenses, net | (24 | ) | (52 | ) | ||||
| (659 | ) | (1,181 | ) | |||||
| Net loss | (2,645 | ) | (4,516 | ) | ||||
During the year ended December 31, 2022 and 2021, the Company did not record any revenue.
The Company’s research and development expenses increased to $120,000 during the year ended December 31, 2022, compared to approximately $96,000 during the prior year. The increase is mainly attributable to professional expenses related to the development of our Green Botanical product line.
The Company’s marketing, general and administrative expenses during the year ended December 31, 2022, were $1,866,000 compared to $3,239,000 during the year ended December 31, 2021. The decrease in our marketing, general and administrative expenses is mainly attributable to the decrease in our non-cash share-based compensation expenses.
| 42 |
During the year ended December 31, 2022, the Company incurred financial expenses, net of $659,000, as compared to financial expenses of $1,181,000 during the year ended December 31, 2021. The reason for the decrease in financial expense is attributable to changes in terms of our convertible loans.
As a result of the above, the Company incurred a net loss of approximately $2,636,000 during the twelve months ended December 31, 2022 as compared to a net loss of approximately $4,516,000 in 2021.
Liquidity and Capital Resources
At December 31, 2022, we had current assets of $185,000 compared to total current assets of $349,000 as of December 31, 2021. At December 31, 2022, we had current liabilities of $1,805,000 as compared to $1,064,000 as of December 31, 2021. At December 31, 2022, we had total liabilities of $3,780,000 as compared to $2,495,000 as of December 31, 2021. The increase is mainly attributed to the increase in the balance of accrued expenses and the balance of convertible component in convertible notes.
At December 31, 2022, we had a cash balance of $77,000 compared to the cash balance of $270,000 as of December 31, 2021.
At December 31, 2022, we had a working capital deficiency of $1,620,000 as compared with a working capital deficiency of $715,000 at December 31, 2021.
The following table provides a summary of operating, investing, and financing cash flows for the years ended December 31, 2022 and 2021 respectively (in thousands):
| Year Ended | ||||||||
| December 31, 2022 | December 31, 2021 | |||||||
| Net cash used in operating activities | (567,000 | ) | (582,000 | ) | ||||
| Net cash provided by investment activities | 11,000 | 286,000 | ||||||
| Net cash provided by Financing Activities | 360,000 | 350,000 | ||||||
On March 6, 2023 our majority owned subsidiary, Cannovation and S.R. Accord Ltd., an Israeli company (“Lender”), entered into an 18-month credit facility agreement (the “Credit Facility”) pursuant to which Lender has committed to fund Cannovation in an aggregate amount of 3,000,000 NIS (approximately $857,000) as needed. At the time of each draw down, Cannovation and Lender will determine the repayment of the loan. All amounts drawn under the Credit Facility will bear interest at a monthly rate of 1.7% and will be due by no later than September 2024. Cannovation has the right to pre-pay the entire amount outstanding under the Credit Facility at any time. As security for any loans under the Credit Facility, Cannovation granted Lender a first priority lien on its rights to the 125,000 sq ft (11,687 sqm) of industrial land in Yerucham, a city in southern Israel which Cannovation acquired in February of 2022 (the “Premises”) to build the Green Vision Center Israel with the support of the government of Israel. The lien will become effective only if Cannovation utilizes the Credit Facility. If the market value of the Premises is less than the amount outstanding under the Credit Facility, then Lender will be entitled to additional security on such terms and conditions as the parties may agree.
On July 15, 2022, Citrine 9 LP (hereinafter “Citrine 9”), one of the related entities who are the signatory lenders (hereinafter the “Buyers”) to the Convertible Note Purchase Agreement entered into by the Company and such Buyers in April 2020, as subsequently amended (the “CL Agreement”) agreed to honor a Draw Down Notice for, and has advanced to the Company, $100,000 on the same terms and conditions as are specified in the CL Agreement. The annual interest on the loan continues to be nine percent (9%). The principal and interest payment on the Note shall be made in New Israeli Shekels (NIS) at the conversion rate which was in effect on the date on which the loan was advanced. As provided for under the terms of the Convertible Note Agreement, Citrine 9 is entitled to 8,333,333 Series A warrants and 8,333,333 Series B warrants for shares of common stock, where each of the series are exercisable beginning January 15, 2023 through October 31, 2025, in each case at an exercise price of $0.05 per share. On August 9, 2022, the Company’s board of directors agreed to extend the exercise period of the warrants through August 9, 2027. On January 26, 2023, the CL Agreement was further amended to extend to May 31, 2024 the maturity date thereof. The amendment also provides that upon a public offering of securities that the Company may effect in connection with a listing of the Company’s stock on a U.S. National Securities Exchange, the note is automatically convertible into the securities that are the subject matter such offering, in whole or in part, as the Citrine Global Board may determine.
| 43 |
On September 30, 2022, Citrine Global received a loan from Citrine Hi Tech 7 LP, an Israeli limited partnership and an affiliated entity (the “Lender”), in the principal amount of $80,000. The loans bears interest at 12% per annum and was scheduled to mature on December 15, 2022. On January 29, 2023 the maturity date was extended to May 31, 2024. The holder also agreed that upon a public offering of securities that the Company may effect in connection with a listing of the Company’s stock on a U.S. National Securities Exchange, the note is automatically convertible into the securities that are the subject matter such offering, in whole or in part, as the Citrine Global Board may determine
Based on the Company’s current cash balances and the access to the Credit Facility described above, the Company believes that it has sufficient funds for its plans for the next twelve months from the issuance of these financial statements. As the Company is embarking on its activities as detailed herein, it is incurring losses. It cannot determine with reasonable certainty when and if it will have sustainable profits.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Recently issued accounting pronouncements
Recently issued accounting pronouncements are described in the notes to our financial statements for the years ended December 31, 2022 and 2021, which are included within Item 8 in this annual report.
Critical Accounting Policies and Estimates
Our significant accounting policies are described in the notes to our financial statements for the years ended December 31, 2022 and 2021 and which included within Item 8 in this annual report.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
| 44 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
CITRINE GLOBAL CORP.
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2022
| 45 |
CITRINE GLOBAL CORP.
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2022
IN U.S. DOLLARS IN THOUSANDS
TABLE OF CONTENTS
| Page | |
| REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (Firm Name: Somekh Chaikin / PCAOB ID No. |
F-2 |
| CONSOLIDATED FINANCIAL STATEMENTS: | |
| Consolidated Balance Sheets as of December 31, 2022 and 2021 | F-3 |
| Consolidated Statements of Operation and Comprehensive Loss for the years ended December 31, 2022 and 2021 | F-4 |
| Statements of Changes in Shareholders’ Deficit for the years ended December 31, 2022 and 2021 | F-5 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2022 and 2021 | F-6 |
| Notes to Consolidated Financial Statements | F-8 – F-36 |
| F-1 |

Somekh Chaikin
KPMG Millennium Tower
17 Ha’arba’a Street, PO Box 609
Tel Aviv 61006, Israel
+972 3 684 8000
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Citrine Global Corp
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Citrine Global Corp and subsidiaries (the Company) as of December 31, 2022 and 2021, the related consolidated statements of operations and comprehensive loss, changes in shareholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 2022, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2022, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/
Somekh Chaikin
Member Firm of KPMG International
We have served as the Company’s auditor since 2022.
March 22, 2023
KPMG Somekh Chaikin, an Israeli partnership and a member firm of the KPMG global organization of independent member firms affiliated with KPMG International Limited, a private English company limited by guarantee
| F-2 |
CITRINE GLOBAL CORP.
CONSOLIDATED BALANCE SHEETS
(U.S. dollars in thousands except share and per share data)
| December 31, | December 31, | |||||||
| 2022 | 2021 | |||||||
| A s s e t s | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | ||||||||
| Restricted cash | ||||||||
| Short-term loan to others (Note 8H) | ||||||||
| Prepaid expenses | ||||||||
| Other current assets | ||||||||
| T o t a l Current assets | ||||||||
| Non-current assets | ||||||||
| Investments valued under the measurement alternative (Note 3) | ||||||||
| Property and equipment, net (Note 4) | ||||||||
| Total non-current assets | ||||||||
| T o t a l assets | ||||||||
| Liabilities and Shareholders’ Deficit | ||||||||
| Current Liabilities | ||||||||
| Short term loan (Note 5I) | ||||||||
| Accounts payable and accrued expenses | ||||||||
| Accrued compensation | ||||||||
| T o t a l current liabilities | ||||||||
| Non-current liability | ||||||||
| Convertible component in convertible notes (Note 5) | ||||||||
| Convertible notes (Note 5) | ||||||||
| T o t a l liabilities | ||||||||
| Stockholders’ Deficit (Note 6) | ||||||||
| Common stock, par value $ per share, shares authorized at December 31, 2022 and December 31, 2021; and shares issued and outstanding at December 31, 2022 and December 31, 2021 | ||||||||
| Additional paid-in capital | ||||||||
| Stock to be issued | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive income | ||||||||
| T o t a l stockholders’ deficit | ( | ) | ( | ) | ||||
| T o t a l liabilities and stockholders’ deficit | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-3 |
CITRINE GLOBAL CORP.
CONSOLIDATED STATEMENTS OF OPERATION AND COMPREHENSIVE LOSS
(U.S. dollars in thousands except share and per share data)
| Years ended | ||||||||
| December 31 | ||||||||
| 2022 | 2021 | |||||||
| Research and development expenses | ( | ) | ( | ) | ||||
| Marketing, general and administrative expenses | ( | ) | ( | ) | ||||
| Operating loss | ( | ) | ( | ) | ||||
| Financing expenses, net: | ||||||||
| Expenses related to convertible loan terms | ( | ) | ( | ) | ||||
| Other financing expenses, net | ( | ) | ( | ) | ||||
| Financing expenses, net | ( | ) | ( | ) | ||||
| Net loss attributable to Common stockholders | ( | ) | ( | ) | ||||
| Loss per Common Stock (basic and diluted) | (0.00 | ) | (0.00 | ) | ||||
| Basic weighted average number of shares of Common Stock outstanding | ||||||||
| Comprehensive loss: | ||||||||
| Net loss | ( | ) | ( | ) | ||||
| Other comprehensive loss attributable to foreign currency translation | ||||||||
| Comprehensive loss | ( | ) | ( | ) | ||||
| (*) |
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
CITRINE GLOBAL CORP.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
(U.S. dollars in thousands, except share and per share data)
| Common Stock | Additional paid-in | Stock to be | Accumulated | Accumulated other comprehensive | Total stockholders’ equity | |||||||||||||||||||||||
| Stock | Amount | Capital | issued | deficit | Income | (deficit) | ||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2020 | ( | ) | ||||||||||||||||||||||||||
| CHANGES DURING THE YEAR ENDED DECEMBER 31, 2021: | ||||||||||||||||||||||||||||
| Modification of warrants in connection with convertible loan restructuring (Note 5) | - | |||||||||||||||||||||||||||
| Warrants issued in connection with convertible notes | - | |||||||||||||||||||||||||||
| Classification of embedded conversion feature from liability to equity (Note 5) | - | |||||||||||||||||||||||||||
| Commitment for issuance of fixed number of ordinary shares | - | |||||||||||||||||||||||||||
| Share based compensation | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2021 | ( | ) | ( | ) | ||||||||||||||||||||||||
| CHANGES DURING THE YEAR ENDED DECEMBER 31, 2022: | ||||||||||||||||||||||||||||
| Issuance of shares | * | ( | ) | |||||||||||||||||||||||||
| Stock based compensation to service providers | * | |||||||||||||||||||||||||||
| Share based compensation | - | |||||||||||||||||||||||||||
| Issuance of warrants | - | |||||||||||||||||||||||||||
| Warrants issued in connection with convertible notes | - | |||||||||||||||||||||||||||
| Modification of warrants in connection with convertible loan restructuring | - | |||||||||||||||||||||||||||
| Change in terms of convertible component in convertible notes | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Commitment for issuance of fixed number of ordinary shares (note 3b) | - | |||||||||||||||||||||||||||
| Other comprehensive income | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2022 | ( | ) | ( | ) | ||||||||||||||||||||||||
| (*) |
The accompanying notes are an integral part of the consolidated financial statements.
| F-5 |
CITRINE GLOBAL CORP.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars in thousands)
| Year ended | ||||||||
| December 31 | ||||||||
| 2022 | 2021 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | ||||||||
| Finance expenses, net | ( | ) | ||||||
| Financial expenses with respect to convertible notes and loans | ||||||||
| Interest and change in fair value of short-term loan measured at fair value | ||||||||
| Share-based compensation | ||||||||
| Change in fair value of marketable securities | ||||||||
| Fair value adjustment of liability in connection with stock exchange agreement | ( | ) | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid share based payment to a service provider | ||||||||
| Prepaid expenses and other current assets | ( | ) | ( | ) | ||||
| Accounts payable and accrued expenses | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | ( | ) | ( | ) | ||||
| Repayment (Grant) of short-term loan | ( | ) | ||||||
| Proceeds from sale of trading securities | ||||||||
| Proceeds from repayments of short-term loan | ||||||||
| Net cash provided by investing activities | ||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from short term loan | ||||||||
| Proceeds from issuance of convertible note | ||||||||
| Proceeds from the issuance of convertible notes and warrants | ||||||||
| Net cash provided by financing activities | ||||||||
| Effect of exchange rates on cash and cash equivalents | ( | ) | ||||||
| Net increase (decrease) in cash and cash equivalents and restricted cash | ( | ) | ||||||
| CASH AND CASH EQUIVALENTS AND RESTRICTED CASH AT BEGINNING OF THE YEAR | ||||||||
| CASH AND CASH EQUIVALENTS AND RESTRICTED CASH AT END OF THE YEAR | ||||||||
The accompanying notes are an integral part of the consolidated financial statement
| F-6 |
CITRINE GLOBAL CORP.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars in thousands)
| Year ended | ||||||||
| December 31 | ||||||||
| 2022 | 2021 | |||||||
| Supplemental disclosure of cash flow information: | ||||||||
| Non-cash transactions: | ||||||||
| Fair value of convertible component in convertible loan | ( | ) | ||||||
| Warrants issued in connection with convertible notes | ( | ) | ||||||
| Extinguishment of convertible notes | ( | ) | ||||||
| Investment in MyPlant for a fixed number of shares | ||||||||
| Classification of embedded conversion feature from liability to equity | ||||||||
| Commitment for issuance of fixed number of ordinary shares | ||||||||
| F-7 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – GENERAL
Citrine Global, Corp. (“Citrine Global” or the “Company”) was incorporated under the laws of the State of Delaware on May 26, 2010. The Company’s common stock is traded in the United States on the OTCQB market under the ticker symbol “CTGL.”
On June 3, 2020 the Company established a wholly owned new Israeli subsidiary: CTGL – Citrine Global Israel Ltd, (the “Israeli Subsidiary”).
On July 21, 2020, the Israeli Subsidiary began to work with certain Company shareholders, Beezz Home Technologies Ltd. (“Beezzhome”), in which Ora Elharar Soffer, the Company’s chairperson and CEO holds shares, and Golden Holdings Neto Ltd., in which Ilan Ben-Ishai, a former director of the Company, holds shares, have been working towards establishing an Operational Innovation Center focuses on plant based wellness and pharma products and solutions. The Company’s Board of Directors approved the Israeli Subsidiary to proceed with preparations for entering into an agreement to incorporate a new company, named Cannovation Center Israel Ltd. (“Cannovation”), with Beezz Home Technologies Ltd. and Golden Holdings Neto Ltd., and to accept limitations on the Israeli Subsidiary’s rights in the Cannovation Center if and as mandated under Israeli regulations on the involvement of foreign entities. On August 20, 2020, the Israeli Subsidiary, Beezz Home Technologies Ltd., and Golden Holdings Neto Ltd. incorporated Cannovation. Israeli Subsidiary holds of Cannovation’s shares, while each of Beezz Home Technologies Ltd. and Golden Holdings Neto Ltd. holds of its shares.
On
August 4, 2020, the Board of the Company approved for the Company and its Israeli Subsidiary to proceed with preparations for
investing in iBOT Israel Botanicals Ltd., (an affiliate) an Israeli nutritional supplements’ company developing and
manufacturing botanical formulas and nutritional supplements for custom & contract manufacturing for leading botanical companies
(“iBOT”). The principal shareholders and control persons of iBOT are the Company’s Chief Executive Officer,
President and Chairperson. iBOT has a manufacturing facility for a wide range of botanical formulations. iBOT’s
manufacturing facility is approved by the Israeli Ministry of Health and is GMP-certified, ISO 9001-certified and HACCP certified by
IQC. On August 4, 2020, the Board of Directors approved for the Company and Citrine Global Israel to proceed with preparations for
investing in iBOT. On August 9, 2021, through the
In
November 2021,
In
November 2022 the Company and iBOT agreed to extend to March 31, 2023 the pre-emption right previously granted to the Company with respect
to any equity or equity linked securities that iBOT proposes to issue to an unrelated third party with aggregate gross proceeds to iBOT
exceeding $
In March 2023, the Company and iBOT agreed
to extend to December 31, 2023 the pre-emption right previously granted to the Company with respect to any equity or equity linked securities
that iBOT proposes to issue to an unrelated third party with aggregate gross proceeds to iBOT exceeding $
| F-8 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – GENERAL (cont’d)
Stock split
Financial support
The Company has not yet to generate revenues and is dependent on raising funds from its current shareholders or from other sources. On April 13, 2021, Citrine S A L, on behalf of itself and its affiliates and related parties, has furnished the Company with an irrevocable letter of obligation to financially support the Company until June 30, 2022. On March 17, 2022, Citrine S A L Investment & Holding Ltd. extended this support through June 30, 2023. On August 14, 2022, Citrine S A L Investment & Holding Ltd. further extended this support through June 30, 2024.
In addition, on March 6, 2023 Cannovation and S.R. Accord Ltd., an Israeli company (“Lender”), entered into an -month credit facility agreement (the “Credit Facility”) pursuant to which Lender has committed to fund Cannovation in an aggregate amount of NIS (approximately $), as needed. At the time of each draw down, Cannovation and Lender will determine the maturity date of the loan. All amounts drawn under the Credit Facility will bear interest at an monthly rate of . Cannovation has the right to pre-pay the entire amount outstanding under the Credit Facility at any time.
On March 7, 2023, the Company issued to the Lender shares of the Company’s common stock a commitment fee in respect of the provision of the Credit Facility. As of the date of this report, Cannovation utilized $ out of the credit line.
The Company has no significant firm commitments that require it to remit cash and can control the level of expenses it incurs. Based on the Company’s current cash balances, and the access to the Credit Facility noted above, the Company believes it will have sufficient funds for its plans for the next twelve months from the issuance of these financial statements. As the Company is embarking on its business plan, it is incurring losses. It cannot determine with reasonable certainty when and if it will have sustainable profits.
COVID-19
On March 11, 2020, the World Health Organization declared the outbreak of a novel coronavirus (SARS-CoV-2) to be a global pandemic (COVID-19), which continues to spread throughout the world. The COVID-19 pandemic is having significant effects on global markets, supply chains, businesses, and communities. Specifically with respect to the Company, COVID-19 may impact various parts of its 2022 plans, operations and financial results, including but not limited to difficulties in obtaining additional financing. The Company considered the impact of COVID-19 on the estimates and assumptions and determined that there were no material adverse impacts on the consolidated financial statements for the year ended December 31, 2022. The Company believes it is taking appropriate actions to mitigate the negative impact, including by focusing its activities initially only within the country of Israel. However, the full impact of COVID-19 is unknown and cannot be reasonably estimated as these events are still developing.
| F-9 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND BASIS OF PRESENTATION
The financial statements were prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Citrine Global and its Israeli Subsidiaries, CTGL - Citrine Global Israel Ltd and Cannovation. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of the financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of expenses during the reporting periods. Significant estimates include share-based compensation and fair value measurements of the convertible notes. Actual results could differ from those estimates.
Functional Currency and Foreign Currency Translation and Transactions.
The currency of the primary economic environment in which the operations of the Company and its subsidiaries are conducted is the U.S. dollar.
Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Monetary assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. Non-monetary assets and liabilities are translated using the historical rate on the date of the transaction. All exchange gains or losses arising from translation of these foreign currency transactions are included in net loss for the year.
Cash, cash equivalents and restricted cash
Cash equivalents are short-term highly liquid investments which include short term bank deposits (up to three months from date of deposit), that are not restricted as to withdrawals or use that are readily convertible to cash with maturities of three months or less as of the date acquired.
Restricted
cash as of December 31, 2021 included a $
Property, plant and equipment, net
| 1. | Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. When an asset is retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition is reflected in the Statements of Operations and Comprehensive Loss. |
| 2. | Rates of depreciation: |
% | ||||
| Computers and office equipment |
| F-10 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND BASIS OF PRESENTATION (cont.)
Investments valued under the measurement alternative
The Company’s investments as described in Notes 3 are valued under the measurement alternative include equity securities in other proprietary investments for which the Company does not have significant influence and fair value is not readily determinable. Accounting Standard Update (“ASU”) 2016-01 requires equity securities to be recorded at cost and adjusted to fair value at each reporting period. However, the guidance allows for a measurement alternative, which is to record investments at cost, less impairment, if any, and subsequently adjust for observable price changes of identical or similar investments of the same issuer.
Due to the lack of readily determinable fair values for such investments, for which the Company does not have significant influence, the Company accounts for these investments under the measurement alternative at cost, less impairment.
The Company performs qualitative impairment assessments on its investments recorded under the measurement alternative.
Impairment of long-lived assets
The Group’s long-lived assets are reviewed for impairment in accordance with ASC Topic 360, “Property, Plant and Equipment”, whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. No indicators of impairment have been identified as of December 31, 2022 and 2021.
Derivatives
Derivative instruments are recognized on the balance sheet at their fair value, with changes in the fair value recognized as a component of financial expenses, net in the statements of operation.
Once determined, derivative liabilities and assets are adjusted to reflect fair value at each reporting period end, with any increase or decrease in the fair value being recorded in results of operations as an adjustment to fair value of derivatives.
Income taxes
The Company accounts for income taxes in accordance with ASC Topic 740, “Income Taxes”. Accordingly, deferred taxes assets and liabilities are determined utilizing the asset and liability method based on the estimated future tax effects of differences between the financial statement carrying amount and the tax bases of assets and liabilities under the applicable tax law. Deferred tax balances are measured using the enacted tax rates expected to be in effect when these differences reverse. Valuation allowances in respect of deferred tax assets are provided for, if necessary, to reduce deferred tax assets to amounts more likely than not to be realized.
The Company accounts for uncertainty in income tax in accordance with ASC
Topic 740, which prescribes detailed guidance for the financial statement recognition, measurement and disclosure of tax positions. According
to ASC Topic 740,
| F-11 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND BASIS OF PRESENTATION (cont.)
Research and development expenses
Research and development expenses are charged to operations as incurred.
Basic loss per share of Common Stock is computed by dividing the loss for the period applicable to holders of shares of Common Stock, by the weighted average number of shares of Common Stock outstanding during the period. However, in periods of net loss, only the convertible Preferred Stock are considered, since such shares have a contractual obligation to share in the losses of the Company.
In computing diluted loss per share, basic loss per share is adjusted to reflect the potential dilution that could occur upon the exercise of potential shares. Accordingly, in periods of net loss, no potential shares are considered.
The Company measures and recognizes the compensation expense for all equity-based payments based on their estimated fair values in accordance with ASC 718, “Compensation-Stock Compensation”. Share-based payments including grants of stock options are recognized in the statement of operation as an operating expense based on the fair value of the award at the date of grant. The fair value of stock options granted is estimated using the Black-Scholes option-pricing model. The Company has expensed compensation costs, net of estimated forfeitures, applying the accelerated vesting method, over the requisite service period or over the implicit service period.
Fair value
Fair value of certain of the Company’s financial instruments including cash, accounts receivable, accounts payable, accrued expenses, convertible notes and other accrued liabilities approximate cost because of their short maturities. The Company measures and reports fair value in accordance with Accounting Standards Codification (“ASC”) 820, “Fair Value Measurements and Disclosure,” which defines fair value, establishes a framework for measuring fair value in accordance with generally accepted accounting principles and expands disclosures about fair value measurements.
Fair value, as defined by ASC 820, is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value of an asset should reflect its highest and best use by market participants, principal (or most advantageous) markets, and an in-use or an in-exchange valuation premise. The fair value of a liability should reflect the risk of nonperformance, which includes, among other things, the Company’s credit risk.
Valuation techniques are generally classified into three categories: (i) the market approach; (ii) the income approach; and (iii) the cost approach. The selection and application of one or more of the techniques may require significant judgment and are primarily dependent upon the characteristics of the asset or liability, and the quality and availability of inputs. Valuation techniques used to measure fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 also provides fair value hierarchy for inputs and resulting measurement as follows:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities.
| F-12 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND BASIS OF PRESENTATION (cont.)
Level 2: Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; inputs other than quoted prices that are observable for the asset or liability; and inputs that are derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities; and
Level 3: Unobservable inputs for the asset or liability that are supported by little or no market activity, and that are significant to the fair values.
Fair value measurements are required to be disclosed by the level within the fair value hierarchy in which the fair value measurements in their entirety fall. Fair value measurements using significant unobservable inputs (in level 3 measurements) are subject to expanded disclosure requirements including a reconciliation of the beginning and ending balances, separately presenting changes during the period attributable to the following: (i) total gains or losses for the period (realized and unrealized), (ii) segregating those gains or losses included in earnings, and (iii) a description of where those gains or losses included in earning are reported in the statement of operations.
The Company’s financial assets and liabilities that are measured at fair value on a recurring basis by level within the fair value hierarchy are as follows:
| Balance as of December 31, 2022 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| US$ in thousands | ||||||||||||||||
| Liabilities: | ||||||||||||||||
| Fair value of convertible component in convertible notes | ||||||||||||||||
| Total liabilities | ||||||||||||||||
The following table presents the changes in fair value of the level 3 liabilities for the period ended December 31, 2022:
| Changes in Fair value | ||||
| US$ in thousands | ||||
| Liabilities: | ||||
| Outstanding at December 31, 2021 | ||||
| Initial recognition of convertible component as part of modification in note terms | ||||
| Initial recognition of convertible component as part of convertible notes issued | ||||
| Changes in fair value | ( | ) | ||
| Outstanding at December 31, 2022 | ||||
| F-13 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND BASIS OF PRESENTATION (cont.)
Concentrations of credit risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents as well as certain other current assets that do not amount to a significant amount. Cash and cash equivalents, which are primarily held in Dollars and New Israeli Shekels, are deposited with major banks in Israel and United States. Management believes that such financial institutions are financially sound and, accordingly, minimal credit risk exists with respect to these financial instruments. The Company does not have any significant off-balance-sheet concentration of credit risk, such as foreign exchange contracts, option contracts or other foreign hedging arrangements.
Contingencies
The Company records accruals for loss contingencies arising from claims, litigation and other sources when it is probable that a liability has been incurred and the amount can be reasonably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. Legal costs incurred in connection with loss contingencies are expensed as incurred.
Recent Accounting Pronouncements
On October 1, 2021, the Company early adopted ASU No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (ASU 2020-06), which simplifies the accounting for convertible instruments by reducing the number of accounting models available for convertible debt instruments. This guidance also eliminates the treasury stock method to calculate diluted earnings per share for convertible instruments and requires the use of the if-converted method. The adoption of this new standard did not have a material impact on the consolidated financial statements.
In May 2021, the FASB issued ASU 2021-04—Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options, which clarifies and reduces diversity in accounting for modifications or exchanges of freestanding equity-written call options that remain equity classified after modifications or exchanges based on the substance of the transactions. The amendments in this ASU are effective for all entities for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. The adoption of this new standard did not have a material impact on the consolidated financial statements.
In November 2021, the FASB issued ASU 2021-10, Government Assistance (ASC 832). This ASU increases transparency of government assistance including the disclosure of (1) the types of assistance, (2) an entity’s accounting for the assistance, and (3) the effect of the assistance on an entity’s financial statements. This ASU is expected to reduce diversity in the recognition, measurement, presentation, and disclosure of government assistance received by business entities because of the lack of specific authoritative guidance. The ASU was adopted by the Company on January 1, 2022. As to the grant of industrial parcel of land at a subsidized price (see Note 4), the grant was recorded when there is reasonable assurance the conditions of the subsidies will be met and the subsidies will be received.
Other new pronouncements issued but not effective as of December 31, 2022 are not expected to have a material impact on the Company’s consolidated financial statements.
| F-14 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 - INVESTMENT VALUED UNDER THE MEASUREMENT ALTERNATIVE
| A. | On June 22, 2020, the Company entered into a share purchase agreement with Nanomedic Technologies Ltd., an Israeli private company and a related party as further described below (“Nanomedic”) as part of an A-1 funding round open only to existing Nanomedic shareholders and their affiliates. Nanomedic developed SpinCare™, a system that integrates electrospinning technology into a portable, bedside device, offering immediate wound and burn care treatment. The Company paid $ thousand for A-1 preferred shares of Nanomedic and also received warrants to purchase A-1 preferred shares. Such investment represents a holding of approximately in Nanomedic. The round raised approximately $ million in total. Citrine S A L and certain of its partnerships, all affiliates of the Company, were already beneficial shareholders of Nanomedic immediately prior to the A-1 funding round. Ilan Ben-Ishay, a former director of the Company was already a beneficial shareholder of Nanomedic immediately prior to the A-1 funding round. Ora Elharar Soffer, chairperson and CEO of the Company, was already a director of both Nanomedic and its Israeli parent company Nicast Ltd. immediately prior to the A-1 funding round, and she was also already a beneficial shareholder of Nanomedic immediately prior to the A-1 funding round. |
The Company accounts for the investment in Nanomedic in accordance with the provisions of ASC 321, “Investments - Equity Securities”, and elected to use the measurement alternative therein. The investment will be re-measured upon future observable price change(s) in orderly transaction(s) or upon impairment, if any. No such observable price changes have occurred during 2022 and 2021.
| B. | On
December 30, 2022, the Company, MyPlant Bio Ltd., a company incorporated under the laws of
the State of Israel (“MyPlant”), Cannasoul Analytics Ltd., a company incorporated
under the laws of Israel (“Cannasoul”), and PurPlant Inc., a company duly incorporated
under the laws of Canada (“PurPlant”) (Cannasoul and PurPlant are collectively
referred to as the “Shareholders”), and Professor Dedi Meiri, an Israeli individual (“Prof
Meiri”) entered into the Share Purchase and Option Agreement (the “Share Purchase
and Option Agreement”) for the purchase by the Company of up to |
The Company purchased 10% of
the outstanding MyPlant Shares from the Shareholders an aggregate of ordinary
shares of MyPlant (the “MyPlant Shares”) representing, on a fully diluted basis, in consideration of $
The transactions under the Share Purchase and Option Agreement are based on a MyPlant company valuation of approximately $ million. The Company is authorized at any time on or before the Option Expiry Date to obtain an independent third-party valuation of MyPlant. If it is determined by such third party valuation that the MyPlant valuation is less than $ million then the consideration payable in respect of the exercise price of the options will be accordingly adjusted, provided however that in any case MyPlant’s valuation in the transaction shall not be below US$.
| F-15 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Under the Share Purchase and Option Agreement, MyPlant granted to the Company the exclusive right to utilize MyPlant’s activities as specified in the agreement, including without limitation, the screening platforms using cell line models for certain diseases and conditions to detect effective plant materials and/or other substances for the treatment of these conditions and a and a right of first opportunity to commercialize intellectual property developed by MyPlant that is in the Company’s (or its subsidiaries’) field of business, provided that, if by December 31, 2023 the Company does not exercise either of the Shareholders Option or the Company Option and/or enter into a service agreement with MyPlant, then the exclusive rights shall terminate but the right of first opportunity to commercialize intellectual property developed by MyPlant shall continue thereafter until June 31, 2024, unless such rights have been extended beyond such date under the terms to be agreed in the service agreement entered into by the Company and Citrine Global. In addition, under the Share Purchase and Option Agreement, Cannasoul, MyPlant’s majority Shareholder, agreed to not compete with MyPlant’s activities.
The Company was granted observer rights on the MyPlant board of Directors (the “MyPlant Board”). Following the exercise by Citrine Global of the Shareholders Option, the MyPlant Board shall be comprised of four (4) directors of which MyPlant will be authorized to designate two of such directors.
The Company accounts for the investment in MyPlant in accordance with the provisions of ASC 321, “Investments - Equity Securities”, and elected to use the measurement alternative therein. The investment will be re-measured upon future observable price change(s) in orderly transaction(s) or upon impairment, if any.
NOTE 4 - PROPERTY AND EQUIPMENT, NET
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| U.S. Dollars in thousands | ||||||||
| Computers and office equipment | ||||||||
| Payment on land lease | ||||||||
| Less - accumulated depreciation | ( | ) | ( | ) | ||||
| Total property and equipment, net | ||||||||
In
the years ended December 31, 2022 and 2021, depreciation expenses amounted to US$
| 1. | On
July 13, 2021, the Ministry of Economy of the Israeli government recommended to the Israel
Land Authority (“ILA”) that it approve a grant of 11,687 square meters of industrial
parcel of land in Yeruham, Israel (the “Land”) for Cannovation to build the Cannovation
Center, at a subsidized price and exempt from a tender procedures typically required under
Israeli law, to include factories, laboratories, logistics and a distribution center for
plant based wellness and pharma products and solutions. As noted, Citrine Global owns |
During
December 2021, Cannovation remitted to the Israeli Ministry of the Economy and the ILA the aggregate amount of NIS
| F-16 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 - PROPERTY AND EQUIPMENT, NET (continue)
The
Company has also classified $
On February 8, 2022, Cannovation Ltd received from the Israel Land Authority (“ILA”) a counter-signed development agreement to purchase rights for long term lease to 11,687 square meters of Land for purposes of building the Green Vision Center Israel, which is intended to include factories, laboratories, logistics and a distribution center for plant based wellness and pharma products and solutions.
NOTE 5 – CONVERTIBLE NOTES
| A. | On
April 1, 2020 the Company entered into a Convertible Note Purchase Agreement (the “CL
Agreement”) with Citrine S A L , WealthStone Private Equity Ltd, WealthStone Holdings
Ltd, Golden Holdings Neto Ltd, Beezz Home Technologies Ltd, Citrine Biotech 5 LP, Citrine
High Tech 6 LP, Citrine High Tech 7 LP, Citrine 8 LP, Citrine 9 LP and Citrine Biotech 10
LP (together, the “Buyer”), all of which are affiliated with the Company. Under
the CL Agreement, the Buyer agreed to purchase and the Company agreed to issue and sell,
for up to an aggregate principal amount of up to $ |
On
April 19, 2020 and June 12, 2020, the Company provided draw down notices under the CL Agreement for amounts of $
On
June 12, 2020, the CL Agreement was amended (hereafter “Amendment”) to provide that for each draw down made by
On
April 12, 2021, the parties to the Convertible Note Purchase Agreement (the “CL Agreement”) amended the CL Agreement to (i)
change the annual interest on the Notes to nine percent (9%), applicable from January 1, 2021, (ii) ensure that the Company shall repay
the loans at the time it consummates an investment in the amount of at least $
The Company concluded that the change in term does not constitute a trouble debt restructuring. Thereafter, the Company applied the guidance in ASC 470-50, Modifications and Extinguishments. The accounting treatment is determined by whether terms of the new debt and original debt are substantially different.
| F-17 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
The new debt and the old debt are considered “substantially different” pursuant to ASC 470-50 when the present value of the cash flows under the terms of the new debt instrument is at least 10% different from the present value of the remaining cash flows under the terms of the original instrument (including the incremental fair value resulting from the change in the terms of the warrants held by the lender). If the original and new debt instruments are substantially different, the original debt is derecognized and the new debt should be initially recorded at fair value, with the difference recognized as an extinguishment gain or loss. Based on the analysis, the Company concluded that the change in terms should be accounted for as an extinguishment.
The
extinguishment resulted in a loss of $
The fair value of the warrants was estimated using the Black-Scholes option pricing model. The assumptions used to perform the calculations are detailed below:
Fair value of the warrants immediately before the change:
| Fair value of the warrants | A Warrant | B Warrant | ||||||
| Expected volatility (%) | % | % | ||||||
| Risk-free interest rate (%) | % | % | ||||||
| Expected dividend yield | % | % | ||||||
| Contractual term (years) | ||||||||
| Conversion price | ||||||||
| Underlying share price (U.S. dollars) | ||||||||
| Fair value (U.S. dollars in thousands) | ||||||||
Fair value of the warrants immediately after the change:
| Fair value of the warrants | A Warrant | B Warrant | ||||||
| Expected volatility (%) | % | % | ||||||
| Risk-free interest rate (%) | % | % | ||||||
| Expected dividend yield | % | % | ||||||
| Contractual term (years) | ||||||||
| Conversion price | ||||||||
| Underlying share price (U.S. dollars) | ||||||||
| Fair value (U.S. dollars in thousands) | ||||||||
| B. | On
June 24, 2021, the Company received from Citrine 8 LP, a related party, a convertible loan of $ |
| F-18 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
Convertible Component of the Loan
The fair value of the conversion feature (hereafter “Convertible Component”) was estimated using the Monte Carlo Simulation Model to compute the Convertible Component’s fair value. The assumptions used to perform the Monte-Carlo simulation model on June 24, 2021 were consistent with those utilized in the Company’s Black-Scholes valuation for stock options are detailed below:
| June 24, 2021 | ||||
| Expected volatility (%) | % | |||
| Risk-free interest rate (%) | % | |||
| Expected dividend yield | % | |||
| Contractual term (years) | ||||
| Conversion price | (*) | |||
| Underlying share price (US dollars) | ||||
| Convertible notes amount | ||||
| Fair value of the conversion feature (US dollars in thousands) | ||||
| (*) |
Warrants
The
fair value of such warrants granted as part of the June 24 agreement was estimated at $
The assumptions used to perform the calculations are detailed below:
| A Warrant | B Warrant | |||||||
| Expected volatility (%) | % | % | ||||||
| Risk-free interest rate (%) | % | % | ||||||
| Expected dividend yield | % | % | ||||||
| Contractual term (years) | ||||||||
| Conversion price | ||||||||
| Underlying share price (U.S. dollars) | ||||||||
| Fair value (U.S. dollars in thousands) | ||||||||
Fair Value Proportional Allocation for the June 24 Agreement
The
fair value of the note was estimated at $
Based on the above, the fair value proportion allocation as of June 24, 2021 was as follows:
June 24, 2021 (US dollars in thousands) | ||||
| Conversion Component | $ | |||
| Warrants | ||||
| Convertible Notes | ||||
| Total | $ | |||
| F-19 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
| C. | On
August 13, 2021, the Company and the holders of $ |
| (i) | Extension
of the maturity date on the Outstanding CL Notes to | |
| (ii) | Amendment
of the conversion price on the Outstanding CL Notes to a fixed conversion price of $ | |
| (iii) | Confirming
the agreement of |
The Company concluded that the change in term constitutes a trouble debt restructuring, due to its financial condition and the relief that the abovementioned changes provided.
Therefore, the Company concluded that the change in terms should be accounted for as a modification. A new effective interest rate was established based on the carrying value of the debt and the revised cash flows.
Conversion feature In accordance with ASC 815-15-25, the conversion feature was considered embedded derivative instrument, and is to be recorded at its fair value separately from the convertible notes, within current liabilities in the Company’s balance sheet. The conversion component is then marked to market each reporting period with the resulting gains or losses shown in the statements of operations.
Loan #1 that was amended on August 13, 2021:
| August 13, 2021 | ||||
| Expected volatility (%) | % | |||
| Risk-free interest rate (%) | % | |||
| Expected dividend yield | % | |||
| Contractual term (years) | ||||
| Conversion price | (*) | |||
| Underlying share price (U.S. dollars) | ||||
| Convertible notes amount | ||||
| Fair value of the conversion feature (U.S. dollars in thousands) | ||||
| (*) |
| F-20 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
Loan #2 that was amended on August 13, 2021:
| August 13, 2021 | ||||
| Expected volatility (%) | % | |||
| Risk-free interest rate (%) | % | |||
| Expected dividend yield | % | |||
| Contractual term (years) | ||||
| Conversion price | (*) | |||
| Underlying share price (U.S. dollars) | ||||
| Convertible notes amount | ||||
| Fair value of the conversion feature (U.S. dollars in thousands) | ||||
| (*) |
Following
the abovementioned amendment on August 13, 2021, the conversion component is qualifying for the scope exception under ASC 815-10-15-74(a).
In accordance with ASC 815-15-35-4, since the embedded conversion option in the convertible debt no longer meets the bifurcation criteria,
the fair value of the conversion component, in the amount of $
| D. | On
January 5, 2022, Citrine 9 LP, one of the Buyer entities (hereinafter “Citrine 9”)
agreed to honor a Draw Down Notice (as defined in the Convertible Note Agreement) for, and
has advanced to the Company, $ |
As
provided for under the terms of the Convertible Note Agreement, Citrine 9 will be issued
The Company allocated the proceeds received to the freestanding components – the convertible loan, A Warrants and B Warrants, based on their relative fair values, since all three components will not be subsequently measured at fair value (see below).
Conversion feature
In accordance with ASC 815-15-25 the conversion feature was considered a liability classified embedded derivative instrument, and is to be recorded at its fair value separately from the convertible notes, within non-current liabilities in the Company’s balance sheet. The conversion component is then remeasured at fair value at each reporting period with the resulting gains or losses shown in the statements of operations.
The fair value of the convertible component was estimated by third party appraiser as weighted average of the two possible scenarios of the total convertible notes amount conversion (each, 50% probability):
| F-21 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
The scenario in which the convertible loan would be converted prior to its maturity (scenario 1) was estimated by the appraiser using the Black-Scholes option pricing model, to compute the fair value of the derivative and to market the fair value of the derivative at each balance sheet date. The following are the data and assumptions used as of issuance dates and as of the balance sheet date:
| January 5, 2022 | ||||
| Dividend yield (%) | % | |||
| Risk-free interest rate (%) | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the conversion feature (U.S. dollars in thousands) | ||||
The
scenario in which the Company would raise at least $
| January 5, 2022 | ||||
| Dividend yield (%) | % | |||
| Risk-free interest rate (%) | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the conversion feature U.S. dollars in thousands) | ||||
The
fair value of the convertible component was estimated by the third-party appraiser after giving effect to the weighted average of the
two possible scenarios as of issuance dates was $
Warrants
The
fair value of the warrants as of the drawdowns dates was estimated at $
The following are the data and assumptions used:
| Warrants A | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the warrants (U.S. dollars in thousands) | ||||
| Warrants B | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the warrants (U.S. dollars in thousands) | ||||
| F-22 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
Fair Value Proportional Allocation
The
fair value of the note was estimated at $
Based on the above, the fair value proportion allocation as of January 5, 2022 was as follows:
January 5, 2022 (US dollars in thousands) | ||||
| Conversion Component | $ | | ||
| Warrants | ||||
| Convertible Notes | ||||
| Total | $ | |||
| E. | On January 5, 2022, the Company and the related entities who are the signatory lenders (hereinafter the “Buyers”) under the Convertible Loan Agreement dated as of April 1, 2020 (the “CL Agreement”) with the Company entered into the Fourth Amendment to the CL Agreement pursuant to which the following was agreed to: |
| (i) | The
principal and accrued interest on all outstanding loans in the aggregate principal amount
of $ | |
| (ii) | The
conversion price on all outstanding notes under the CL Agreement was adjusted to a conversion
price of $ | |
| (iii) | The
exercise price on all outstanding warrants issued in connection with advances made under
the CL Agreement was adjusted to an exercise price of $ |
The Company concluded that the change in terms does not give rise to a trouble debt restructuring, as no concession was given to the Company.
Therefore, the Company went on to assess the whether the terms of the modified note are substantially different. The Company concluded that the change in terms should be accounted for as a debt extinguishment.
In accordance with ASC 815-15-35-4, since the embedded conversion option in the convertible debt meets the bifurcation criteria, the
fair value of the conversion component calculated as of January 5, 2022, in the amount of $
| F-23 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
| F. | On
July 15, 2022, Citrine 9 LP, (hereinafter “Citrine 9”), one of the related entities
and a signatory lender (to the Convertible Note Purchase Agreement entered into by the Company
and several related parties (hereinafter the “Buyers”) in April 2020, as subsequently
amended (the “CL Agreement”) agreed to honor a Draw Down Notice for, and has
advanced to the Company, $ |
The Company allocated the proceeds received to the freestanding components – the convertible loan, A Warrants and B Warrants, based on their relative fair values, since all three components will not be subsequently measured at fair value (see below).
Conversion feature
In accordance with ASC 815-15-25 the conversion feature was considered a liability classified embedded derivative instrument, and is to be recorded at its fair value separately from the convertible notes, within non-current liabilities in the Company’s balance sheet. The conversion component is then remeasured at fair value at each reporting period with the resulting gains or losses shown in the statements of operations.
The fair value of the convertible component was estimated by third party appraiser as weighted average of the two possible scenarios of the total convertible notes amount conversion (each, 50% probability):
The scenario in which the convertible loan would be converted prior to its maturity (scenario 1) was estimated by the appraiser using the Black-Scholes option pricing model, to compute the fair value of the derivative and to market the fair value of the derivative at each balance sheet date. The following are the data and assumptions used as of issuance dates
| July 15, 2022 | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the conversion feature (U.S. dollars in thousands) | ||||
| F-24 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
The
scenario in which the Company would raise at least $
| July 15, 2022 | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the conversion feature (U.S. dollars in thousands) | ||||
The
fair value of the convertible component was estimated by the third-party appraiser after giving effect to the weighted average of the
two possible scenarios as of issuance dates was $
Warrants
The
fair value of the warrants as of the drawdowns dates was estimated at $
The following are the data and assumptions used:
| Warrants A | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the warrants (U.S. dollars in thousands) | ||||
| Warrants B | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the warrants (U.S. dollars in thousands) | ||||
| F-25 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
Fair Value Proportional Allocation
The
fair value of the note was estimated at $
Based on the above, the fair value proportion allocation as of July 15, 2022 was as follows:
July 15, 2022 (US dollars in thousands) | ||||
| Conversion Component | $ | | ||
| Warrants | ||||
| Convertible Notes | ||||
| Total | $ | |||
| G. | On August 9, 2022, the board of directors of the Company agreed to the following: |
| 1. | The maturity date on all of the outstanding convertible loans under the CL Agreement was extended to October 31, 2023 (from July 31, 2023); and |
| 2. | The exercise period on all of the outstanding Series A and Series B warrants issued to date in connection with the convertible loans under the CL Agreement was extended to August 9, 2027 |
The Company concluded that the change in terms does not give rise to a trouble debt restructuring, as no concession
was given to the Company.
Therefore, the Company went on to assess whether the terms of the modified note are substantially different. The Company concluded that the change in terms of the loans should be accounted for as a debt extinguishment.
Following
the abovementioned amendment on August 9, 2022, the changes in the fair value of the conversion component and the warrants in the amount
of $
As of December 31, 2022, the fair value of the convertible component was estimated by third party appraiser as weighted average of the two possible scenarios of the total convertible notes amount conversion (20% probability for scenario 1 and 80% probability for scenario 2):
The scenario in which the convertible loans would be converted prior to its maturity (scenario 1) was estimated by the appraiser using the Black-Scholes option pricing model, to compute the fair value of the derivative and to market the fair value of the derivative at each balance sheet date. The following are the data and assumptions used as of the balance sheet date:
| December 31, 2022 | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the conversion feature (U.S. dollars in thousands) | ||||
| F-26 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – CONVERTIBLE NOTES (cont’d)
The
scenario in which the Company would raise at least $
| December 31, 2022 | ||||
| Dividend yield | % | |||
| Risk-free interest rate | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the conversion feature (U.S. dollars in thousands) | ||||
The
fair value of the convertible component was estimated by the third-party appraiser after giving effect to the weighted average of the
two possible scenarios as of December 31, 2022 was $
| H. | On
August 9, 2022, the Board agreed to issue to the related entities who advanced an aggregate
of $ |
The
fair value of the warrants as of the drawdowns dates was estimated at $
The following are the data and assumptions used:
| Warrants A | ||||
| Dividend yield (%) | % | |||
| Risk-free interest rate (%) | % | |||
| Expected term (years) | ||||
| Volatility | % | |||
| Share price (U.S. dollars) | ||||
| Exercise price (U.S. dollars) | ||||
| Fair value of the warrants (U.S. dollars in thousands) | ||||
| I. | On
September 30, 2022, the Company received a loan from Citrine S A L Hi Tech 7 LP, an Israeli limited partnership and an affiliated
entity (the “Lender”), in the principal amount of $ |
In the event that the Company agrees to such extension, the terms of this loan shall be adjusted on a pro-rata basis, to those terms applicable to the Company’s convertible notes then outstanding under the Convertible Note Agreement, date as of April 1, 2020, as subsequently amended, amongst the Company and the affiliated parties thereto (of which the Lender is a party).
| F-27 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – SHAREHOLDERS’ EQUITY
Description of the rights attached to the Shares in the Company:
Common Stock:
Each share of Common Stock entitles the holder to one vote, either in person or by proxy, at meetings of stockholders. The holders of Common Stock are not permitted to vote their shares cumulatively. Accordingly, the holders of the Company’s Common Stock who hold, in the aggregate, more than fifty percent of the total voting rights can elect all of the directors and, in such event, the holders of the remaining minority shares will not be able to elect any of such directors. The vote of the holders of a majority of the issued and outstanding shares of Common Stock entitled to vote thereon is sufficient to authorize, affirm, ratify or consent to such act or action, except as otherwise provided by law.
Transactions:
On
February 15, 2022, the Company signed an investor relations service agreement with a consultant pursuant to which the Company agreed
to pay the consultant a monthly retainer of $
On August 26, 2022 the Company issued shares to Intelicanna Ltd in respect to its strategic partnership agreement signed on May 31, 2020.
On August 15, 2021, the Company’s board of directors determined to increase the number of shares reserved for issuance under the 2018 Stock Incentive Plan (the “2018 Plan”) to shares of common stock thereunder and recommended to the Company shareholders to approve the increase in the pool. The Board also determined to grant to each of Ilanit Halperin and David Kretzmer, directors of the Company, a grant of options to purchase shares of common stock, and Doron Birger, a Company director, options to purchase shares, in each case at per share exercise price of $ per share. The options vest over a two year period, in eight (8) equal installments, with the first instalment vesting on the third month anniversary of each individual’s start date and each further instalment on each subsequent third month anniversary, where the start date is, in the case of Ilanit Halperin February 27, 2020, in the case of Doron Birger September 20, 2020 and in the case of David Kretzmer is March 1, 2021, subject to such individual’s continued service with the Company. These options expire after 5 years from the grant date. The total recognized tax benefit related to the share based payments, after valuation allowance, totaled to zero.
| F-28 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 – STOCK OPTIONS (continue)
On December 29, 2021 the Company’s board of directors approved the grants of the options.
The fair value at December 29, 2021 was determined using the Black-Scholes pricing model, assuming a risk free rate of %, a volatility factor of %, dividend yields of % and an expected life of years. The Company estimated the fair value of each option granted at December 29, 2021 at $, totaling $thousands.
In May 2022 the Company appointed Prof. Itamar Grotto, a world-renowned expert in Public Health as Director in Cannovation Center Israel Ltd. and President of Green Vision Center Israel. Upon his appointment, Prof. Itamar Gruto was granted options under the 2018 Plan to purchase shares of the Company’s common stock at per share exercise price of $. The options vest over a three year period, in annual instalments beginning on June 1, 2023 and thereafter on each subsequent anniversary, subject to his continued service to the Company.
On June 8, 2022, the Board also approved the issuance of options to two service providers under the 2018 Plan. The options are exercisable at a per share price of $. The options are scheduled to vest over a three year period, in twelve (12) equal installments, with the first installment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. options shall be accelerated upon uplisting to Nasdaq.
On August 9, 2022, the Company’s board of directors determined to increase the number of shares reserved for issuance under the 2018 Plan by million shares to a total of shares of common stock thereunder and on August 12, 2022 the Company shareholders approved the same.
On August 9, 2022, the Board also determined to grant to the directors and officers set forth below options under the 2018 Plan. The options are exercisable at a per share price of $ and through the seventh anniversary of the grant date, except in the case of Ora Elharar Soffer, the Company’s chief executive officer, the per share exercise price is and the exercise period is years from the date of grant. The options are scheduled to vest over a year period, in twelve (12) equal installments, with the first installment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. In the event of a change in control, the vesting schedule is accelerated and all unvested options vest.
| Director/Officer | Number of Options | |||
| Ora Elharar Soffer (Chairperson, CEO) | ||||
| Ilanit Halperin (Director, CFO) | ||||
| Ilan Ben Ishay (Director) | ||||
| Doron Birger (Director) | ||||
| David Kretzmer (Director) | ||||
The terms relating to the options grants are included in stock option agreements under the 2018 Plan. Amongst other things, the stock option agreements for selected service providers of Citrine Global, including the directors and officers, provide that the exercise price of the options that were awarded to date, shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options. All other relevant terms of such shall continue in full force and effect and are such reverse stock split. Any and all tax implications rest solely with the optionee and not the Company.
| F-29 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Number of Options | Weighted Average Exercise Price ($) | |||||||
| Outstanding at December 31, 2020 | ||||||||
| Granted | ||||||||
| Exercised | ||||||||
| Forfeited or expired | ||||||||
| Outstanding at December 31, 2021 | ||||||||
| Granted | ||||||||
| Exercised | ||||||||
| Forfeited or expired | ||||||||
| Outstanding at December 31, 2022 | ||||||||
| Number of options exercisable at December 31, 2022 | ||||||||
| Exercise price | Stock options outstanding | Weighted average remaining contractual life – years | Stock options vested | |||||||||||
| $ | As of December 31, 2022 | |||||||||||||
As of December 31, 2022, there was $ of total unrecognized compensation cost related to non-vested options. The cost is expected to be recognized over a weighted average period of years. Compensation expense recorded by the Company in respect of its options awards for the years ended December 31, 2022 and 2021, were $ thousands and $ thousands, respectively and are included in General and Administrative expenses in the Statements of Operations.
The aggregate intrinsic value of the awards outstanding as of December 31, 2022 is $. These amounts represent the total intrinsic value, based on the Company’s stock price of $ as of December 31, 2022, less the weighted exercise price.
| 2022 | 2021 | |||||||
| Dividend yield (%) | % | % | ||||||
| Risk-free interest rate (%) | % - % | % | ||||||
| Expected term (years) | - | |||||||
| Volatility | % - % | % | ||||||
| Share price (U.S. dollars) | - | |||||||
| Exercise price (U.S. dollars) | – | |||||||
| Estimated total fair value of options granted (U.S. dollars thousands) | ||||||||
The intrinsic value of options outstanding and exercisable at December 31, 2022 totaled $ thousand.
| F-30 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – RELATED PARTIES
| A. | Transactions and balances with related parties |
Year ended December 31 | ||||||||
| 2022 | 2021 | |||||||
| U.S. Dollars in thousands | ||||||||
| Research and development expenses: | ||||||||
| Directors compensation and fees to officers | ||||||||
| General and administrative expenses: | ||||||||
| Directors compensation and fees to officers (*) | ||||||||
| (*) Share based compensation | ||||||||
| Financing expenses, net: | ||||||||
| Financial expenses related to convertible loan | ||||||||
| Interest on loan | * | |||||||
| (*) |
| B. | Balances with related parties: |
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| U.S. Dollars in thousands | ||||||||
| Current Assets: | ||||||||
| Short term loan granted to others | ||||||||
| Current Liabilities: | ||||||||
| Short term loan | ||||||||
| Convertible notes | ||||||||
| Accounts payable | ||||||||
| Accrued compensation | ||||||||
| Non-current Liabilities: | ||||||||
| Convertible notes | ||||||||
| F-31 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| C. | Commencing
in February 2020, Ora Elharar Soffer, CEO and Chairperson of the Board, was entitled to a
monthly fee of $ |
In
addition, on August 15, 2021,
As
of December 31, 2022, and 2021, an amount of $
| D. | Commencing
in February 2020, Ilan Ben-Ishay, a director in Citrine Global, is entitled to a monthly fee of $ |
In
addition, on August 15, 2021, the board of directors of Cannovation determined to adjust the compensation of Ilan Ben Ishay, a director
at Cannovation, to $
As
of December, 31, 2022, and 2021, an amount of $
On January 18, 2023, Mr. Ilan Ben Ishay resigned from his position as a director on the Board of the Company
| E. | Commencing
in May 2020, Ms. Halperin, director & CFO of the Company, was entitled to a monthly fee of an additional $ |
In
addition, on August 15, 2021, the board of directors of Cannovation determined to adjust the compensation of Ilanit Halperin at Cannovation, to $
As
of December, 31, 2022, and 2021, an amount of $
| F. | Commencing
in March 2021, Adv. David Kretzmer, a director, is entitled to a monthly fee of $ |
In
addition, on August 15, 2021,
| F-32 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On
August 9, 2022, Mr. David Kretzmer’s fee in respect of services provided to the Company was reduced from $
As
of December, 31, 2022, and 2021, an amount of $
| G. | Commencing
in September 2020, Doron Birger, a director, is entitled to a monthly fee of $ |
As
of December, 31, 2022 an amount of $
| H. | On
August 15, 2021, |
| I. | On
September 29, 2021, the Company advanced to iBOT, a related party, a loan of NIS |
| J. | On October 19, 2022, Mr. Dror Shaked and Mr. David Freidenberg were appointed to serve as independent directors on the Board of Directors of Citrine Global, Corp., effective upon (and subject to) the listing of the Company’s stock on the Nasdaq Stock Market . |
| F-33 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – INCOME TAXES
| A. | United
States resident companies are taxed on their worldwide income at a statutory rate of |
Income
of the Israeli Subsidiaries is taxable from 2021 and onwards, at corporate tax rate of
The Company and its Israeli Subsidiaries have not received final tax assessments since the Israeli Subsidiary’s inception. tax years are open for assessment Company’s tax years, from 2018 onwards, are open for assessment and for the Israeli Subsidiaries all tax years from commencement are open for assessment.
As
of December 31, 2022 and 2021, the Company and the Israeli Subsidiaries have operating loss carryforwards of
approximately $
| B. | Composition of loss for the year: |
Year ended December 31 | ||||||||
| 2022 | 2021 | |||||||
| U.S. Dollars in thousands | ||||||||
| U.S. | ||||||||
| Israel | ||||||||
| C. | The following is a reconciliation between the theoretical tax on pre-tax loss, at the federal income tax rate applicable to the Company and the income tax expense reported in the financial statements: |
| Year ended December 31 | ||||||||
| 2022 | 2021 | |||||||
| U.S. Dollars in thousands | ||||||||
| Pretax loss | ||||||||
| U.S. federal income tax rate | % | % | ||||||
| Income tax benefit computed at the applicable tax rate | ( | ) | ( | ) | ||||
| Non-deductible expenses | ||||||||
| Effect of differences in corporate income tax rates | ( | ) | ( | ) | ||||
| Change in valuation allowance | ||||||||
| F-34 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| D. | Deferred taxes are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Significant components of the Company’s deferred tax assets and liabilities are as follows: |
| December 31 | ||||||||
| 2022 | 2021 | |||||||
| Composition of deferred tax assets: | U.S. Dollars in thousands | |||||||
| Operating loss carry forwards | ||||||||
| Share based compensation | ||||||||
| Convertible component in convertible notes | ||||||||
| Accrued compensation | ||||||||
| Total deferred tax assets | ||||||||
| Composition of deferred tax liabilities: | ||||||||
| Convertible notes | ( | ) | ( | ) | ||||
| Total deferred tax liabilities | ( | ) | ( | ) | ||||
| Valuation allowance | ( | ) | ( | ) | ||||
| E. | Roll forward of valuation allowance |
| US dollars in thousands | ||||
| Balance at January 1, 2021 | ||||
| Additional paid in capital | ( | ) | ||
| Income tax expense | ||||
| Balance at December 31, 2021 | ||||
| Additional paid in capital | ( | ) | ||
| Income tax expense | ||||
| Balance at December 31, 2022 | ||||
Basic loss per share is computed by dividing net loss by the weighted average number of shares outstanding during the year. The weighted average number of shares of Common Stock used in computing basic and diluted loss per ordinary share for the years ended December 31, 2021 and 2020, are as follows:
| Year ended December 31 | ||||||||
| 2022 | 2021 | |||||||
| Number of shares | ||||||||
| Weighted average number of shares of Common Stock outstanding attributable to ordinary shareholders | ||||||||
| Total weighted average number of shares of Common Stock related to outstanding options, excluded from the calculations of diluted loss per share (*) | ||||||||
| (*) |
| F-35 |
CITRINE GLOBAL CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – SUBSEQUENT EVENTS
| a. | On
January 30, 2023 the Company and each of Citrine High Tech 7 LP (“LP 7”), Citrine
8 LP (“LP 8 “) and Citrine 9 LP (“LP 9”; together with LP 7 and LP
8, the “Lending LP”), the lending entities under and parties to
the Convertible Note Purchase Agreement entered into by the Company and several related parties
in April 2020, as subsequently amended (the “CL Agreement”), have entered into
an agreement (the “Agreement”) pursuant to which they have agreed to extend the
maturity date on all outstanding convertible loans in the principal amount of $ | |
| In addition, under the Agreement the Company and the Lending LPs have also agreed that if the Company’s common stock is listed on the Nasdaq Stock Market, then, then the Company, in its sole discretion, shall determine to convert, in whole or in part, the outstanding amount of the above mentioned notes to shares of the Company’s common stock at a conversion price equal to the price paid by the public investors for the common stock in such offering. | ||
| b. | On January 17, 2023, the Board of Citrine Global, appointed Ms. Ora Elharar Soffer to serve as president of the Company. Ms. Elharar Soffer has been continuously serving as the Company’s Chief Executive Officer since May 7, 2020 and as a Company director since February 21, 2020 and as Chairperson of the Board since March 3, 2020. | |
| c. | On January 17, 2023, the Board of Citrine Global, appointed Ms. Ilanit Halperin to serve as treasurer and secretary of the Company. Ms. Halperin has been continuously serving as the Company’s Chief Financial Officer since May 7, 2020 and as a Company director since February 21, 2020. | |
| d. | On January 18, 2023, Mr. Ilan Ben Ishay resigned from his position as a director on the Board of the Company for personal reasons. Mr. Ben Ishay’s resignation did not result from any disagreement with the Company on any matter relating to the Company’s operations, policies and practices. | |
| e. | On March 5, 2023, the Board of the Company provided that in the event that the Company’s stock is listed on the Nasdaq Stock Exchange, then one half of the awarded and unvested option grants made in each of August 2021 and in August 2022 to service providers, including officers, directors and selected service providers, will immediately vest at such time. In addition, the Board also determined to provide that following the termination of services by an officer, director or a selected service provider for any reason other than cause, such person shall have a one year period from the date of termination to exercise any option that was vested at the time of the termination of services. | |
| f. | On
March 6, 2023 Cannovation, the Company’s majority owned subsidiary and S.R. Accord Ltd., an Israeli company
(“Lender”), entered into an 18-month credit facility agreement (the “Credit Facility”) pursuant to which
Lender has committed to fund Cannovation in an aggregate amount of | |
| . The lien will become effective only if Cannovation utilizes the Credit Facility. If the market value of the Premises is less than the amount outstanding under the Credit Facility, then Lender will be entitled to additional security, including shares of Citrine Global Stock, on such terms and conditions as the parties may agree. As additional security for any payments due to Lender, CTGL Citrine Global Israel Ltd., a wholly owned subsidiary of Citrine Global, (ii) Beezzhome Technologies Ltd. an entity wholly owned by Ora Elharar Soffer, the Chief Executive Officer of Citrine Global and (iii) Netto Holdings Ltd., [provide full corporate/company name], an unaffiliated entity under the partial control of Ilan Ben Ishay, a director on the board of Cannovation, as well as each of Ms. Elharar Soffer and Mr. Ben Ishay in their personal capacities, are providing guarantees for the repayment of any amounts that may be owing to Lender under the Credit Facility. Cannovation has agreed to indemnify Ms. Elharar Soffer and Mr. Ben Ishay for any losses they incur as a result of the guarantee | ||
| On March 7, 2023, the Company issued to the Lender shares of the Company’s common stock a commitment fee in respect of the provision of the Credit Facility. | ||
| g. | On March 15, 20232, the Company issued to a consultant in consideration of services provided to the Company. |
| h. | On
March 16, 2023, the consulting agreement originally entered into as of July 2020 with Ms Elharar Soffer, the Company’s
Chairperson, CEO and President, was amended. | |
| i. | On
March 16, 2023, the consulting agreement originally entered into as of July 2020 with Ilanit Halperin, the Company’s CFO, was amended. |
| F-36 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed in the Company’s reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the U.S. Securities and Exchange Commission (the “SEC”) rules and forms, and that such information is accumulated and communicated to the Company’s management, including the Company’s principal executive officer and chairperson of the Board, and the Company’s principal financial officer, to allow for timely decisions regarding required disclosure. In designing and evaluating the Company’s disclosure controls and procedures, the Company’s management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and the Company’s management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Based on the Company’s evaluation of the effectiveness of its disclosure controls and procedures as of December 31, 2022, the Company’s principal executive officer and principal financial officer concluded that the Company’s disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by the Company in reports that it files or submits under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including its principal executive officer and principal financial officer , to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over the Company’s financial reporting. In order to evaluate the effectiveness of internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act of 2002, the Company’s management, with the participation of the Company’s principal executive officer and principal financial officer conducted an assessment, using the criteria in Internal Control - Integrated Framework, issued by the Committee of Sponsoring Organizations of the Tredway Commission (“COSO”) (2013). The Company’s system of internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. This assessment included review of the documentation of controls, evaluation of the design effectiveness of controls, and a conclusion on this evaluation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Based on this evaluation, the Company’s management concluded that its internal control over financial reporting was effective as of December 31, 2022 as it identified no control deficiencies that constituted material weaknesses in the Company’s internal control over financial reporting, such that there is not a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Changes in Internal Control over Financial Reporting
There were no changes in the Company’s internal control over financial reporting during the quarter ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
On March 16, 2023, the consulting agreement originally entered into as of July 2020 with Soffer, the Company’s Chairperson, CEO and President, was amended. The amendment provides for the following: (i) the monthly consulting to which Ms. Elharar Soffer is entitled will increase from $20,000 to $25,000 (in invoice plus VAT if applicable) upon a listing of the Company’s stock on the Nasdaq Stock Market, retroactive to January 1, 2023, (ii) the terms contained in her original agreement and all other terms and awards previously approved by the Company’s board relating to her, including payment of her monthly fee and reimbursement of social benefits payments made by Mr Elharar Soffer, shall continue in full force and effect so long as Ms. Elharar Soffer serves as either director and /or executive officer (iii) all previous awards and bonuses previously made to her were affirmed and (iv) Ms. Elharar Soffer has agreed to defer compensation due to her until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities, at which time all of the outstanding consulting fees from March 2020 and all reimbursement for related social benefits would be paid to her. In addition, The amendment also provides that the committee of the Board that will be responsible for setting the compensation terms of senior management shall prepare and present for approval a compensation program for the Consultant that takes into consideration Ms. Elharar Soffer’s role in founding and leading the Company and that such compensation package shall be competitive with compensation programs for top senior executives/founders generally available in the market and which will include, among other things, appropriate bonuses, severance payments and other amenities generally made available in the market to senior executive and that Ms. Elharar Soffer shall receive the most extensive of such compensation terms amongst senior management.
On March 16, 2023, the consulting agreement originally entered into as of July 2020 with Ms Ilanit Halperin, the Company’s director and CFO, was amended. The amendment provides for the following: (i) the monthly consulting to which Ms. Elharar Soffer is entitled will increase from $7,500 to $10,000 ( in invoice plus VAT if applicable) upon a listing of the Company’s stock on the Nasdaq Stock Market, retroactive to January 1, 2023, (ii) the terms contained in her original agreement and all other terms and awards previously approved by the Company’s board relating to her, including payment of her monthly fee and reimbursement of social benefits payments made by Mr Elharar Soffer ,shall continue in full force and effect so long as Ms. Halperin serves as either director and /or executive officer, (iii) all previous awards and bonuses previously made to her were affirmed and (iv) s. has agreed o defer compensation due to her until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities, at which time all of the outstanding consulting fees from March 2020 and all reimbursement for related social benefits would be paid to her. In addition, The Company undertakes that the committee of the Board that will be responsible for setting the compensation terms of senior management shall prepare and present for approval a compensation program for Ms. Halperin that shall be competitive with compensation programs for senior executives generally available in the market and which will include, among other things, appropriate bonuses, severance payments and other amenities generally made available in the market to senior executives.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not Applicable.
| 46 |
PART III
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT AND CORPORATE GOVERNANCE
The Company’s directors hold office until the next annual general meeting of the stockholders or until their successors are elected and qualified. The Company’s officers are appointed by its board of directors and hold office until the earlier of their death, retirement, resignation, or removal.
The following table sets forth the names and ages of the members of the board of directors and the executive officers and the positions held by each as of March 21, 2023.
| Name | Age | Positions | ||
| Ora Elharar Soffer | 56 | Chairperson of the Board of Directors and Chief Executive Officer and President | ||
| Ilanit Halperin | 50 | Chief Financial Officer, Director, Treasurer and Secretary | ||
| Doron Birger | 70 | Director | ||
| David Kretzmer | 69 | Director |
Family Relationships
There are no family relationships between any members of the Company’s executive management and its directors.
Business Experience
The following is a brief account of the education and business experience of our current directors and executive officers:
Ora Elharar Soffer has been serving as our Director and Chair of the Board since February 2020, Chief Executive Officer since May 2020 and President since January 17, 2023 and currently serves as director in our wholly owned subsidiary CTGL Citrine Global Israel Ltd. and our majority owned subsidiary Cannovation Center Israel Ltd. Elharar Soffer is the entrepreneur behind the company and also the head of strategic business development. Additionally, Ms. Elharar Soffer is the founder, director?? and chairperson of Cannovation Center Israel Ltd.
Ms. Elharar Soffer serves as director or advisory board member in various companies. Ms. Elharar Soffer is the co-founder and CEO of Citrine SAL Investment & Holdings an Israeli company, that invests in various fields of companies and technologies and include Citrine S A L Biotech Funds, which specializes in healthcare, wellness solutions, digital health, medical devices, food tech, and botanical nutraceuticals, and Citrine S A L High-Tech Funds, which specializes in high-tech, cyber, IoT, technologies and public companies. Citrine S A L invested in various companies such as Nicast Ltd., Nanomedic Ltd., WellBe Digital Ltd., Biocep Ltd., Improdia Ltd., Intelicanna Ltd., iBOT Israel Botanicals Ltd., Cannbit Pharmaceuticals Ltd. Novomic Ltd., Dario Health, and BSP Medical, ICB - Israel China Biotechnology.
Ms. Elharar Soffer serves as director of Nicast Ltd., Nanomedic Ltd., Biocep Ltd., iBOT Israel Botanicals Ltd., Beezhome Technologies Ltd, Beyond Blade Ltd, Citrine SAL investment & holdings Ltd, Citrine SAL high tech Ltd and Citrine S A L Biotech Ltd.
Ora was Co-Founder and CEO of Chip PC Technologies, managing and leading the company from Startup stage to going public and becoming an international technology company. Ora was also co-founder of Xseed Ltd. that was sold to Elbit Systems and OR1 Investment Ltd. She is also an investor, shareholder and founder in Beezzhome Technologies Ltd., Beyond Blade Ltd, Citrine S A L investment & holdings Ltd, Citrine SAL high tech Ltd and Citrine S A L Biotech Ltd , CTGL Citrine Global Israel Ltd., Cannovation Center Israel Ltd. and more
| 47 |
Ms. Elharar Soffer is a member of the Peres Center for Peace and Innovation and of Springboard Enterprises, a global organization accelerating women’s leadership in technology and biotech companies.
Ms. Elharar Soffer completed Management Studies in the Technion - Israel Institute of Technology, and is based in Israel.
The Board believes that Ms. Elharar Soffer’s extensive history, association with and knowledge of the Company, and years of experience ideally situate her to serve on our Board.
Ilanit Halperin has been serving as a director since February 2020 and our Chief Financial Officer since May 2020 and treasurer and secretary since January 2023 and currently serves as director in our wholly owned subsidiary CTGL Citrine Global Israel Ltd. and our majority owned subsidiary Cannovation Center Israel Ltd. Ms. Halperin worked for over 21 years in one of the six largest accounting firms in Israel, for the last 11 years as a partner. She then set up her own office providing CPA and financial consulting and management services. For many years Ms. Halperin has accompanied public and private companies in Israel and abroad in diverse sectors, including industrial companies, real estate companies, technology companies, and tourism companies. Ms. Halperin has extensive experience in auditing and preparing financial statements according to Israeli, international (IFRS) and US GAAP standards. Ms. Halperin specializes in accompanying early and mature stage companies, providing, inter alia, tax advice, general financial consulting, assistance in preparing business plans, and assistance and accompaniment with investors, private placements and IPOs in Israel and the USA. Ms. Halperin has many years of experience accompanying NASDAQ and OTC-traded companies. Ms. Halperin holds a B.A. in accounting from the College of Management Academic Studies, Rishon Lezion, Israel.
The Board believes that Ms. Halperin’s extensive knowledge of the Company, her knowledge of financial matters, and years of experience ideally situate her to serve on our Board.
Doron Birger has been serving as a director since September 2020 and currently serves as director in our wholly owned subsidiary CTGL Citrine Global Israel Ltd. Mr. Birger currently serves as the chairman of the board of directors of Matricelf (TASE: MTLF) and as a director of Icecure Medical Ltd. (NASDAQ and TASE:ICCM), Kadimastem Ltd. (TASE:KDST), Pluristem (NASDAQ and TASE: PSTI) and Hera Med Ltd (ASX:HMD). Mr. Birger also serves as chairman and director of several private companies in Israel in the hi-tech sector mainly in the medical device field. From 2002 to 2007, Mr. Birger served as the chairman of the board of directors of Given Imaging Ltd. (NASDAQ and TASE:GIVN) and later on as board member until February 2014. Mr. Birger served as chief executive officer of Elron Electronic Industries, Ltd., or Elron (NASDAQ and TASE:RLRNF), from August 2002 to April 2009. Prior to that, he held other executive positions at Elron, including President since 2001, Chief Financial Officer from 1994 to August 2002, and Corporate Secretary from 1994 to 2001. Mr. Birger is a chairman and director of variety of non-profit organizations in Israel. Mr. Birger holds a B.A. and an M.A. in economics from the Hebrew University Jerusalem. Mr. Birger is based in Israel.
The Board believes that Mr. Birger’s extensive years of public company management experience ideally situate him to serve on our Board.
David Kretzmer was appointed as director in April 2021 and currently serves as director in our wholly owned subsidiary CTGL Citrine Global Israel Ltd. and our majority owned subsidiary Cannovation Center Israel Ltd. and Mr. Kretzmer is an experienced international commercial lawyer and litigator with more than 35 years of experience in international litigation and transactions concentrated on commercial law, property development and syndication, real estate law, corporate law, contracts, international trade, securities brokerage, investment banking, corporate restricting, and corporate development. In addition to his position as a director in our Company, Mr. Kretzmer is a senior partner in the law firm of Kretzmer and Associates PLLC in New York as well as the law firm Kretzmer and Associates in Tel Aviv. Mr. Kretzmer holds a Bachelor of Law from the University of the Witwatersrand, Johannesburg, South Africa and has been admitted as an Attorney in South Africa, New York and in Israel. Mr. Kretzmer is based in Israel.
The board believes that Mr. Kretzmer’s international business and legal experience ideally situate him to serve on our Board.
| 48 |
Committees of the Board of Directors
The Company does not presently have a separately constituted audit committee, compensation committee, nominating committee, executive committee or any other committees of its board of directors. As such, its entire Board acts as its audit committee.
Code of Ethics.
We have adopted a Code of Business Conduct and Ethics, or the Code of Conduct, applicable to all of our employees, executive officers and directors. The Code of Conduct is available on our website at www.citrine-global.com. Our Board must approve any waivers of the Code of Conduct for employees, executive officers and directors. In addition, we intend to post on our website all disclosures that are required by law concerning any amendments to, or waivers from, any provision of the Code of Conduct. All of our directors, executive officers and employees are required to certify in writing their understanding of and intent to comply with the Code.
Involvement in Certain Legal Proceedings.
The Company is not aware of any material legal proceedings that have occurred within the past ten years concerning any Director or control person which involved a criminal conviction, a pending criminal proceeding, a pending or concluded administrative or civil proceeding limiting one’s participation in the securities or banking industries, or a finding of securities or commodities law violations.
Section 16(a) Compliance
Section 16(a) of the Securities and Exchange Act of 1934 requires the Company’s directors and executive officers, and persons who own beneficially more than ten percent (10%) of the Company’s Common Stock, to file reports of ownership and changes of ownership with the Securities and Exchange Commission. Copies of all filed reports are required to be furnished to the Company pursuant to Section 16(a). No delinquent reports were filed during 2022 by the Company’s officers and directors and ten percent (10%) stockholders.
ITEM 11. EXECUTIVE COMPENSATION
Executive Compensation
The following table sets forth the total compensation received for services rendered in all capacities to our Company for the last two fiscal years, which was awarded to, earned by, or paid to our Chief Executive Officer and Chief Financial Officer, who are our only serving officers, whose total compensation exceeded $100,000 during 2021which we refer to collectively as our “Named Executive Officers.”
| Name and Principal Position | Year | Salary ($)(1) | Bonus ($) | Option Awards ($) (2) | All other compensation ($) | Total ($) | ||||||||||||||||
| Ora Elharar Soffer, Chairperson of the Board and Chief Executive Officer | 2022 | 360,000 | (3) | - | - | (4) | - | 360,000(3) | ||||||||||||||
| 2021 | 300,000 | (3) | - | - | (4) | - | 300,000(3) | |||||||||||||||
| Ilanit Halperin, Director and Chief Financial Officer | 2022 | 90,000 | (5) | - | (4) | - | 90,000(5) | |||||||||||||||
| 2021 | 66,000 | (5) | - | - | (4) | - | 66,000(5) | |||||||||||||||
(1) Represents annual retainer payments
| 49 |
(2) In accordance with SEC rules, the amounts in this column reflect the fair value on the grant date of the option awards granted to the named executive, calculated in accordance with ASC Topic 718. Stock options were valued using the Black-Scholes model. The grant-date fair value does not necessarily reflect the value of shares which may be received in the future with respect to these awards. The grant-date fair value of the stock options in this column is a non-cash expense for us that reflects the fair value of the stock options on the grant date and therefore does not affect our cash balance. The fair value of the stock options will likely vary from the actual value the holder receives because the actual value depends on the number of options exercised and the market price of our Common Stock on the date of exercise. For a discussion of the assumptions made in the valuation of the stock options, see Note 7 to the financial statements contained in this Annual Report on Form 10-K for the year ended December 31, 2022.
(3) These amounts represent compensation earned by Ms. Elharar Soffer during the years ended December 31, 2022 and 2021 but that by agreement is deferred until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities. See below “Consulting Agreement with Ora Elharar Soffer”.
(4) See Director compensation table for the options for shares that were awarded in August 2022.
(5) These amounts represent compensation earned by Ms. Halperin during the years ended December 31, 2022 and 2021 but is deferred until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities.
Consulting Agreements with Management and Directors
We have entered into consulting agreements with each of Ms. Elharar Soffer, our Chairperson of the Board and Chief Executive Officer, and Ms. Halperin our Chief Financial Officer and director and our directors. The following are descriptions of the material terms of our executive officers’ services and employment agreements.
Consulting Agreement with Ora Elharar Soffer
On March 16, 2023, the consulting agreement originally entered into as of July 2020 with Ms Ora Elharar Soffer, the Company’s Chairperson, CEO and President, was amended. The amendment provides for the following: (i) the monthly consulting to which Ms. Elharar Soffer is entitled will increase from $20,000 to $25,000 (in invoice plus VAT if applicable) upon a listing of the Company’s stock on the Nasdaq Stock Market, retroactive to January 1, 2023, (ii) the terms contained in her original agreement and all other terms and awards previously approved by the Company’s board relating to her, including payment of her monthly fee and reimbursement of social benefits payments made by Mr Elharar Soffer, shall continue in full force and effect so long as Ms. Elharar Soffer serves as either director and /or executive officer,(iii) all previous awards and bonuses previously made to her were affirmed and (iv) Ms. Elharar Soffer has agreed to defer compensation due to her until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities, at which time outstanding amounts due to her under the agreement would be paid to her. In addition, the amendment also provides that the committee of the Board that will be responsible for setting the compensation terms of senior management shall prepare and present for approval a compensation program for the Consultant that takes into consideration Ms. Elharar Soffer’s role in founding and leading the Company and that such compensation package shall be competitive with compensation programs for top senior executives/founders generally available in the market and which will include, among other things, appropriate bonuses, severance payments and other amenities generally made available in the market to senior executive and that Ms. Elharar Soffer shall receive the most extensive of such compensation terms amongst senior management.
In addition, on August 15, 2021, the board of directors of Cannovation Center Israel determined to adjust the compensation of the Chairperson (and interim Chief Executive Officer), Ora Elharar Soffer, to $10,000 per month, in each case retroactive to July 1, 2021. These amounts would be paid at such time as Cannovation Center shall become due and payable from, and such time as Cannovation Center Israel shall have, available funds therefor and as part of the operating budget for a minimum period of 18 months.
Further, on August 15, 2021, the board determined to award a bonus to the Company’s Chairperson of the Board, CEO, CFO, officers, directors and senior management equal to two percent (2%) of any capital raise, subject to prior repayment of the outstanding convertible loans and so long as the payment thereof would be from available funds and part of the Company’s operating budget for a minimum period of 18 months. In addition, the Board agreed to a bonus Company’s Chairperson of the Board, CEO, CFO, officers, directors and senior management of 2% from operating profits which will become payable upon the fulfillment of certain specified targets that the Board will establish, subject to prior repayment of the outstanding convertible loans and so long as the payment thereof would be from available funds and as part of the Company’s operating budget for a minimum period of 18 months. On March 30, 2022, it was agreed that Ms. Soffer would receive 65% of the allotted amount.
| 50 |
On August 9, 2022, the Company’s board also determined to grant to award to Ms. Elharar Soffer options under the 2018 Plan to purchase up to 47,128,400 shares of common stock, at a per share exercise price of $0.022. The options are scheduled to vest over a three year period, in twelve (12) equal installments, with the first instalment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. In the event of a change in control, the vesting schedule is accelerated and all unvested options vest. The stock option agreement with Ms. Elharar Soffer provides that the exercise price of the options that were awarded shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options and all other relevant terms of such options (other than the exercise price) shall continue in full force and effect following the implementation of such reverse stock split. In addition, the agreements further provide that upon a listing of the Company’s stock on the Nasdaq Stock Market, one half of the unvested options would vest.
Consulting Agreement with Ilanit Halperin
On March 16, 2023, the consulting agreement originally entered into as of July 2020 with Ms Ilanit Halperin, the Company’s director and CFO, was amended. The amendment provides for the following: (i) the monthly consulting to which Ms Ilanit Halperin is entitled will increase from $7,500 to $10,000 ( in invoice plus VAT if applicable) upon a listing of the Company’s stock on the Nasdaq Stock Market, retroactive to January 1, 2023, (ii) the terms contained in her original agreement and all other terms and awards previously approved by the Company’s board relating to her, including payment of her monthly fee and reimbursement of social benefits payments made by, Ms Ilanit Halperin shall continue in full force and effect so long as Ms. Halperin serves as either director and /or executive officer,(iii) all previous awards and bonuses previously made to her were affirmed and (iv) Ms. Halperin has agreed to defer compensation due to her until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities, at which time outstanding amounts due her under the agreement would be paid to her In addition, The Company undertakes that the committee of the Board that will be responsible for setting the compensation terms of senior management shall prepare and present for approval a compensation program for Ms. Halperin that shall be competitive with compensation programs for senior executives generally available in the market and which will include, among other things, appropriate bonuses, severance payments and other amenities generally made available in the market to senior executives.
On August 15, 2021, the Company’s board determined to award to Ms. Halperin options under the 2018 Plan to purchase up to 9,425,680 shares of common stock, at a per share exercise price of $0.05. The options vest over a two year period, in eight (8) equal installments, with the first instalment vesting on the third month anniversary of Ms. Halperin’s start date of February 27, 2020. As of the date of this report, the entirety of the options have vested. In addition, on August 15, 2021, the board of directors of Cannovation Center Israel determined to adjust the compensation of the chief financial officer, Ilanit Halperin, to $4,000 per month, in each case retroactive to July 1, 2021. These amounts would be paid at such time as Cannovation Center shall become due and payable from, and such time as Cannovation Center Israel shall have, available funds therefor and as part of the operating budget for a minimum period of 18 months. On March 30, .2022, it was agreed that Ms. Halperin would receive 25% of the allotted amount of the above referenced bonus.
On August 9, 2022, the Company’s board also determined to grant to award to Ms. Halperin options under the 2018 Plan to purchase up to 18,851,3 60shares of common stock, at a per share exercise price of $0.020. The options are scheduled to vest over a three year period, in twelve (12) equal installments, with the first instalment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. In the event of a change in control, the vesting schedule is accelerated and all unvested options vest.
The stock option agreements with Ms. Halperin provide that the exercise price of the options that were awarded shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options and all other relevant terms of such options (other than the exercise price) shall continue in full force and effect following the implementation of such reverse stock split. In addition, the agreements further provide that upon a listing of the Company’s stock on the Nasdaq Stock Market, one half of the unvested options would vest.
Consulting Agreement with Ilan Ben-Ishay
In July 2020, we entered into a consulting agreement with Mr. Ben-Ishay, a director at the Company, effective as of February 1, 2020 and as long as the Mr. Ben-Ishay serves as a director of the Company, unless earlier terminated with or without cause by any party hereto by 30 days advance written notice. Pursuant to the consulting agreement, effective as of February 2020, Mr. Ben-Ishay receives a monthly retainer of $3,500 plus VAT. In addition, Mr. Ben-Ishay is entitled to reimbursement for certain expenses, which include car, travel, lodging and meals. Mr. Ben-Ishay has agreed to defer compensation due to him until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities.
| 51 |
In addition, on August 15, 2021, the board of directors of Cannovation Center Israel determined to adjust the compensation of Ilan Ben-Ishay, director, to $2,000 per month, in each case retroactive to July 1, 2021. These amounts would be paid at such time as Cannovation Center shall become due and payable from, and such time as Cannovation Center Israel shall have, available funds therefor and as part of the operating budget for a minimum period of 18 months.
On March 30, .2022, it was agreed that Mr. Ben-Ishay would receive 5% of the allotted amount of the above referenced bonus.
On August 9, 2022, the Company’s board also determined to grant to award to Mr. Ben Ishay options under the 2018 Plan to purchase up to 18,851,360 shares of common stock, at a per share exercise price of $0.020. The options are scheduled to vest over a three year period, in twelve (12) equal installments, with the first instalment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. In the event of a change in control, the vesting schedule is accelerated and all unvested options vest. The stock option agreement with Mr. Ben -Ishay provides that the exercise price of the options that were awarded shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options and all other relevant terms of such options (other than the exercise price) shall continue in full force and effect following the implementation of such reverse stock split. In addition, the agreements further provide that upon a listing of the Company’s stock on the Nasdaq Stock Market, one half of the unvested options would vest.
On January 18, 2023, Mr. Ben Ishay resigned from his position as a director on the Board of Citrine Global Corp. but remains a director in our wholly owned subsidiary CTGL Citrine Global Israel Ltd. and Cannovation Center Israel Ltd.
Consulting Arrangement with Doron Birger
Commencing September 2020, Adv. Doron Birger, a director, is entitled to a monthly fee of $1,500 per month and certain reimbursements for traveling lodging and vehicle expenses on behalf of the Company. Beginning August, 2022, Mr. Birger has agreed to defer compensation due to him until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities.
On August 15, 2021, the Company’s board determined to award to Mr. Birger options under the 2018 Plan to purchase up to 2,365,420 shares of common stock, at a per share exercise price of $0.05. The options vest over a two year period, in eight (8) equal installments, with the first instalment vesting on the third month anniversary of Mr. Birger start date of September 20, 2020. As of the date of this report, the entirety of the options have vested.
On August 9, 2022, the Company’s board also determined to grant to award to Mr. Birger options under the 2018 Plan to purchase up to 2,356,420 shares of common stock, at a per share exercise price of $0.020. The options are scheduled to vest over a three year period, in twelve (12) equal installments, with the first instalment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. In the event of a change in control, the vesting schedule is accelerated and all unvested options vest.
The stock option agreements with Mr. Birger provide that the exercise price of the options that were awarded shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options and all other relevant terms of such options (other than the exercise price) shall continue in full force and effect following the implementation of such reverse stock split. In addition, the agreements further provide that upon a listing of the Company’s stock on the Nasdaq Stock Market, one half of the unvested options would vest.
Mr. Birger also serves as a director on the Board of Cannovation.
Consulting Arrangement with David Kretzmer
Commencing in March 2021, Adv. David Kretzmer, a director, is entitled to a monthly fee of $7,000 and certain reimbursements for traveling lodging and vehicle expenses on behalf of the Company. On August 9, 2022, Mr. David Kretzmer’s fee in respect of services provided to the Company was reduced to $1,500 per month. Mr. Kretzmer has agreed to defer compensation due to him until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities.
On August 15, 2021, the Company’s board determined to award to Mr. Kretzmer options under the 2018 Plan to purchase up to 9,425,680 shares of common stock, at a per share exercise price of $0.05. The options vest over a two year period, in eight (8) equal installments, with the first instalment vesting on the third month anniversary of Mr. Kretzmer start date of March 1, 2021. As of the date of this report, the entirety of the options have vested.
| 52 |
In addition, on August 15, 2021, the board of directors of Cannovation Center Israel determined to adjust the compensation of David Kretzmer, director, to $2,000 per month, in each case retroactive to July 1, 2021. These amounts would be paid at such time as Cannovation Center shall have, available funds therefor and as part of the operating budget for a minimum period of 18 months.
On August 9, 2022, the Company’s board also determined to grant to award to Mr. Kretzmer options under the 2018 Plan to purchase up to 2,356,420 shares of common stock, at a per share exercise price of $0.020. The options are scheduled to vest over a three year period, in twelve (12) equal installments, with the first instalment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. In the event of a change in control, the vesting schedule is accelerated and all unvested options vest.
The stock option agreements with Mr. Kretzmer provide that the exercise price of the options that were awarded shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options and all other relevant terms of such options (other than the exercise price) shall continue in full force and effect following the implementation of such reverse stock split. In addition, the agreements further provide that upon a listing of the Company’s stock on the Nasdaq Stock Market, one half of the unvested options would vest.
Mr. Kretzmer also serves on the Board of Cannovation.
Outstanding Equity Awards at December 31, 2022
The following table sets forth information concerning equity awards held by each of our Named Executive Officers as of December 31, 2022.
| Name | Number of Securities Underlying Options (#) Exercisable | Number of Securities Underlying Options (#) Unexercisable | Option ($) | Option Expiration Date | Number of Securities Underlying RSUs (#) Unvested | |||||||||||||
| Ora Elharar Soffer Director, Chief Executive Officer, President | 7,854,733 | 39,273,667 | $ | 0.022 | 8/9/2032 | - | ||||||||||||
| Ilanit Halperin, | 9,425,680 | - | $ | 0.05 | 8/15/2031 | - | ||||||||||||
| Director, Chief Financial Officer | 3,141,893 | 15,709,467 | 0.020 | 8/9/2032 | ||||||||||||||
Director Compensation
The following table provides certain information concerning the compensation for services rendered in all capacities by each director serving on the Company’s board of directors during the year ended December 31, 2022.
| Name | Fee Earned or Paid in Cash($)(1) | Option Awards($)(2) | All Other Compensation($) | Total ($) | ||||||||||||
| Ora Elharar Soffer | - | 331,487 | - | 331,487 | (3) | |||||||||||
| Ilanit Halperin | 42,000 | 142,019 | - | 184,019 | (4) | |||||||||||
| Ilan Ben-Ishay | 66,000 | 137,857 | - | 203,857 | (5) | |||||||||||
| David Kretzmer | 80,500 | 68,188 | - | 148,688 | (6) | |||||||||||
| Doron Birger | 18,000 | 22,269 | - | 40,128 | (7) | |||||||||||
(1) Payments are pursuant to the consulting agreements.
(2) In accordance with SEC rules, the amounts in this column reflect the fair value on the grant date of the option awards granted to the named executive, calculated in accordance with ASC Topic 718. Stock options were valued using the Black-Scholes model. The grant-date fair value does not necessarily reflect the value of shares which may be received in the future with respect to these awards. The grant-date fair value of the stock options in this column is a non-cash expense for us that reflects the fair value of the stock options on the grant date and therefore does not affect our cash balance. The fair value of the stock options will likely vary from the actual value the holder receives because the actual value depends on the number of options exercised and the market price of our Common Stock on the date of exercise. For a discussion of the assumptions made in the valuation of the stock options, see Note 7 to the financial statements contained in this Annual Report on Form 10-K for the year ended December 31, 2022.
| 53 |
(3) See in the Executive Compensation table above a discussion about Ora Elharar Soffer, the Company’s Chairperson of the Board and Chief Executive Officer’s executive compensation. See also above “Consulting Agreement with Ora Elharar Soffer”
(4) See in the Executive Compensation table above a discussion about Ilanit Halperin, the Company’s Chief Financial Officer’s executive compensation.
(5) Represents director compensation earned by Ilan Ben-Ishay during the year ended December 31, 2022 but that is being deferred until such time the Company shall have consummated an investment of at least $1.8 million in the Company’s securities. Mr. Ben Ishay has been serving as a director since February 2020 until his resignation from the Board on January 18 2023. Mr. Ben Ishay remains a director in our wholly owned subsidiary CTGL Citrine Global Israel Ltd. and Cannovation Center Israel Ltd.
(6) Represents director compensation earned by David Kretzmer during the year ended December 31, 2022 but is being deferred until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities.
(7) Represents director compensation earned by Doron Birger during the year ended December 31, 2022. Of this amount $7,500 is being deferred until such time as the Company shall have consummated an investment of at least $1.8 million in the Company’s securities
Golden Parachute Compensation
The Company does not currently have any agreement or understanding, whether written or unwritten, between it and its named executive officers, concerning any type of compensation, whether present, deferred or contingent, that is based on or otherwise relates to an acquisition, merger, consolidation, sale or other disposition of all or substantially all our assets.
Equity Compensation Plan
2018 Stock Incentive Plan
In December 2018, TechCare, our predecessor company, adopted the 2018 Stock Incentive Plan, or the 2018 Plan, which became effective as of December 2, 2018 by the action of its board of directors. The 2018 Plan provides for the grant of stock awards, restricted stock awards and stock options to any employee, director, officer, consultant, or advisor of the Company, or such other persons who provided bona fide services to the Company as shall be determined by a committee designated by the board of directors. If no committee is designated by the board of directors, the 2018 Plan will be administered by the board of directors. As of the date of this report the board of directors has not designated a committee to administer the 2018 Plan.
The total number of shares of common stock reserved for issuance under the 2018 Plan, either directly as stock awards or underlying options is 2,000,000 shares of common stock. The total number of shares of common stock reserved for such issuance may be increased only by a resolution adopted by the board of directors and amendment of the 2018 Plan. Awards under the 2018 Plan may be granted until December 2, 2028. The terms of under which a stock award or option is granted under the 2018 Plan shall be set forth in a written agreement, which shall be determined by the committee or the board of directors.
As of February 2021, the shares reserved for issuance under the 2018 Stock Incentive Plan was increased to 90,000,000 shares of common stock. In August 2022 the shares reserved for issuance under the 2018 Stock Incentive Plan was further increased to 180,000,000 shares of common stock
As of December 31, 2022, the total number of shares of common stock issued under the 2018 Plan, either directly as stock awards or underlying options was 122,529,342 shares of common stock.
| 54 |
2017 Employee Incentive Plan
In 2017, the Company adopted the 2017 Employee Incentive Plan, or the 2017 Plan, which became effective as of January 1, 2017 by the action of the board of directors. The 2017 Plan provided for the grant of stock awards and stock options to any employee, director, officer, consultant, or advisor of the Company, or such other persons who provided bona fide services to the Company as determined by a committee designated by the board of directors followed by the approval of the board of directors; however, if the committee was composed of a majority of the persons then comprising the board of directors, the approval of the board of directors was not necessary. If no committee was designated by the board of directors, the 2017 was to be administered by the board of directors. The board of directors did not designate a committee to administer the 2017 Plan.
As of December 31, 2022, the total number of shares of common stock issued under the 2017 Plan, either directly as stock awards or underlying options was 0 shares of common stock.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth the number of shares of our common stock beneficially owned as of March 22 2023, by (i) each of our current directors and named executive officers, (ii) all executive officers and directors as a group, and (iii) each person known by us to be the beneficial owner of more than 5% of the outstanding shares of our common stock. We have determined beneficial ownership in accordance with applicable rules of the SEC, which generally provide that beneficial ownership includes voting or investment power with respect to securities. Except as indicated by the footnotes to the table below, we believe, based on the information furnished to us, that the persons named in the table have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws.
The information set forth in the table below is based on 956,479,039 shares of our common stock issued and outstanding as of March 22, 2023. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed to be outstanding all shares of common stock subject to options, warrants or other convertible securities held by that person that are currently exercisable or will be exercisable within 60 days after March 22, 2023. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. Except as otherwise noted in the footnotes below, the address for each person listed in the table below, solely for purposes of filings with the SEC, is c/o Citrine Global, Corp. 5 Golden Beach, Caesarea 3088900, Israel.
| Name of Beneficial Owner | Common Stock Beneficially Owned | Percentage of Stock Owned | ||||||
| Principal Stockholders: | ||||||||
| Ora Elharar Soffer (1) | 354,887,780 | 36.93 | % | |||||
| Yaron Pitaru (2) | 86,619,115 | 9.09 | % | |||||
| Edan Moshe Katz (3) | 81,478,344 | 8.55 | % | |||||
| Ilan Ben Ishay (4) | 67,048,046 | 7.02 | % | |||||
| Executive Officers and Directors: | ||||||||
| Ora Elharar Soffer | 354,887,780 | 36.93 | % | |||||
| Ilanit Halperin | 13,978,678 | (5) | 1.45 | % | ||||
| Doron Birger | 2,758,156 | (6) | * | |||||
| David Kretzmer | 10,068,416 | (7) | 1.05 | % | ||||
| Ilan Ben-Ishay | 67,048,046 | (4) | ||||||
| All directors and executive officers as a group (five persons) | 448,741,075 | 45.43 | % | |||||
* Less than 1%.
(1) Includes 79,925,134 shares of common stock owned directly by Ora Elharar Soffer, 65,851,526 shares of common stock owned through Beezz Home Technologies Ltd which is 100% owned by Ora Elharar Soffer, and 201,256,386 shares of common stock owned through Citrine S A L Investment & Holdings Ltd, which is 50% owned by Beezz Home Technologies Ltd. Includes an additional 7,854,733 shares of common stock issuable upon vested options and options scheduled to vest within the next 60 days.
| 55 |
(2) Includes 19,579,952 shares of common stock owned directly by Yaron Pitaru, 12,699,937 shares of common stock owned through WealthStone Private Equity Ltd, which is 100% owned by WealthStone Holdings Ltd, which is 27% owned by Yaron Pitaru, and 54,339,226 shares of common stock owned through Citrine S A L Investment & Holdings Ltd, which is 50% owned by WealthStone Private Equity Ltd.
(3) Includes 20,141,981 shares of common stock owned directly by Edan Moshe Katz, 11,619,596 shares of common stock owned through WealthStone Private Equity Ltd, which is 100% owned by WealthStone Holdings Ltd, which is 73% owned by Golden Holdings Neto Ltd, which is 33.84% owned by Edan Moshe Katz, and 49,716,767 shares of common stock owned through Citrine S A L Investment & Holdings Ltd, which is 50% owned by WealthStone Private Equity Ltd.
(4) Includes 9,342,726 shares of common stock owned directly by Ilan Ben-Ishay, 10,634,128 shares of common stock owned through WealthStone Private Equity Ltd, which is 100% owned by WealthStone Holdings Ltd, which is 73% owned by Golden Holdings Neto Ltd, which is 30.97% owned by Ilan Ben-Ishay, and 45,500,245 shares of common stock owned through Citrine S A L Investment & Holdings Ltd, which is 50% owned by WealthStone Private Equity Ltd. Includes an additional 1,570,947 shares of common stock issuable upon vested options. On January 18, 2023, Mr. ben Ishay resigned from the Board of Directors of Citrine Global Corp but continues to serve on the board of our wholly owned subsidiary CTGL Citrine Global Israel Ltd. and our majority owned subsidiary Cannovation Center Israel Ltd..
(5) Composed of 1,411,104 shares of common stock and 12,567,574 shares issuable upon exercise of vested options.
(6) Shares of common stock issuable upon exercise of vested stock options and options scheduled to vest in 60 days.
(7) Comprised of 250,000 shares of common stock and 9,818,416 shares of common stock issuable upon exercise of vested options and options scheduled to vest in the next 60 days.
Equity Compensation Plan Information
See “Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities – Securities Authorized for Issuance under Equity Compensation Plans.”
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTORS INDEPENDENCE
The following is a description of transactions since January 1, 2021 to which we have been a participant in which the amount involved exceeded or will exceed the lesser of (i) $120,000 or (ii) 1% of the average total assets of the Company at year end for the last two completed fiscal years and in which any of our directors, executive officers or holders of more than 5% of our voting securities, or any members of their immediate family, had or will have a direct or indirect material interest, other than compensation arrangements which are described under “Executive Compensation.”
| 56 |
Convertible Loan Agreements
On April 1, 2020 the Company and certain affiliates entered into the CL Agreement with t certain Israeli Partnerships where our CEO and Chairperson is a partner (collectively, the “Buyers”) entered into a convertible loan agreement (the “CL Agreement”). Under the CL Agreement, the Buyers agree to purchase and the Company agrees to issue and sell, for up to an aggregate principal amount of $1,800,000 of the Notes, for a period starting on April 1, 2020 and ending upon the earlier of (i) 6 months thereafter and (ii) the consummation of a public offering by the Company. The Notes will bear interest at a rate of six percent (6%) with respect to amounts paid that are used for working capital purposes of the Company, provided that amounts paid that are used for investment activities of the Company may be subject to different interest rates, in accordance with the Guidelines. The conversion price per share of Common Stock shall equal 85% multiplied by the market price (as defined in the Note), representing a discount of 15%. The payment for each Note must be delivered 14 business days after delivery of the respective draw down notice, and each Note will mature 18 months thereafter. The interval between one draw down and the next must be at least thirty (30) days, provided that the Buyer may waive this requirement. Each draw down notice provided to the Buyer must be for an amount between $50,000 and $350,000, set at the Company’s discretion. The Company must use the amounts paid for the Notes in accordance with the Guidelines. The Buyer shall decide upon and provide to the Company the names of the Buyer parties which will provide the funds to the Company in respect of the Note, including the respective amounts to be transferred to the Company by each such Buyer party. The Buyer shall have the right, from time to time and at its discretion, to add other entities to the list comprising the Buyer. The Buyer may participate alongside the Company in any investment the Company makes for as long as the CL Agreement is in effect. The Company may at any time prepay an outstanding Note (principal and accrued interest) in full by paying the Buyer an amount in cash equal to 115% multiplied by the then outstanding principal amount of the Note, as well as the accrued and unpaid interest on the unpaid principal amount of the Note, provided however that in the event the Company seeks to exercise this right the Buyer will first have the option to fully convert the Note, or any remaining amount outstanding under it, into Common Stock of the Company, and the conversion amount will be equal to the amount the Company would have paid to the Buyer had the Buyer not exercised this option. On April 19, 2020 and June 12, 2020, the Company provided draw down notices under the CL Agreement for amounts of $170 thousand and $1 million, respectively, which were received in cash by the Company. On June 12, 2020, CL Agreement Amendment was executed to provide that for each draw down made by the Company under the CL Agreement, the Buyer shall be entitled to receive two types of warrants: A Warrants and B Warrants, with the A Warrants exercisable at any time between 6 and 12 months after issuance for an exercise price per share equal to 1.25 times the average of the closing prices of the 3 trading days preceding the draw down, and the B Warrants exercisable at any time between 6 and 24 months after issuance for an exercise price per share equal to 1.5 times the average of the closing prices of the 3 trading days preceding the draw down, and that the number of each of the A Warrants and the B Warrants issued will be equal to the draw down amount divided by the average of the closing prices of the 3 trading days preceding the draw down, and that these amended terms will apply in respect of all draw downs, including drawdowns made prior to the date of the amendment. On April 12, 2021, the parties to the CL Agreement amended the agreement, so that (i) the annual interest on the Notes should be changed to an nine percent (9%) applicable from January 1, 2021, (ii) the Company shall repay the loans at the time it consummates an investment of at least $5 million in the Company’s securities, and (iii) the exercise prices of each of the A Warrants and B Warrants be modified to $0.10 per share and the term of the warrants be extended by one (1) year for the A Warrants and B Warrants. The conversion and exercise price of these securities as well as the maturity dates of the notes have been adjusted as provided below.
On June 24, 2021, the Company received from Citrine 8 LP, a Buyer, a convertible loan of $350,000 made under and pursuant to the CL Agreement. Citrine agreed to honor a Draw Down Notice for, and advanced to the Company, $350,000, under the terms of the CL Agreement. As provided for under the terms of the CL Agreement, Citrine 8 LP was also issued 10,500,105 A warrants and 10,500,105 B warrants for shares of common stock, where the A warrants are exercisable beginning December 24, 2021 through December 24, 2023 and the B warrants, in each case at a per share exercise price of $0.10. The conversion and exercise price of these securities, the exercise period of the warrants as well as the maturity dates of the notes have been adjusted as provided below.
On August 13, 2021, the Company and the holders of $1,520,000 in principal amount under the CL Agreement as detailed in Note 5A and 5B above, entered into an additional agreement pursuant to which, among other things, the following terms were effected:
| (i) | Extension of the maturity date on the Outstanding CL Notes to July 31, 2023, provided, that if the Company consummates prior to maturity an investment of at least $5 million of the Company’s securities, then the Company shall repay the principal amount and accrued interest of the Notes from such proceeds; | |
| (ii) | Amendment of the conversion price on the Outstanding CL Notes to a fixed conversion price of $0.10 per share; and | |
| (iii) | Confirming the agreement of the holders of the Outstanding CL Notes to honor draw down notice for balance of remainder of the $1,800 originally committed to under the CL Agreement (i.e., $280) through March 31, 2022. |
| 57 |
On January 5, 2022, Citrine 9 LP, one of the Buyer entities (hereinafter “Citrine 9”) agreed to honor a Draw Down Notice (as defined in the Convertible Note Agreement) for, and has advanced to the Company, $180,000 on the same terms and conditions as are specified in the Convertible Note Agreement. The maturity date of the loan is the earlier of July 31, 2023 or at such time as the Company shall have consummated an investment of at least $5 million in Company securities. The terms of the advances under the Convertible note agreement were previously disclosed by the Company in Current Reports on Form 8-K filed on each of April 21, April 23, June 12, 2020 and June 24, 2021. The annual interest on the loan continues to be nine percent (9%). The principal and interest payment on the Note shall be made in New Israeli Shekels (NIS) at the conversion rate which was in effect on the date on which the loan was advanced..
As provided for under the terms of the Convertible Note Agreement, Citrine 9 will be issued 6,666,667 Series A warrants and 6,666,667 Series B warrants for shares of common stock, where the Series A warrants are exercisable beginning July 5, 2022 through July 5, 2024 and the Series B warrants are exercisable beginning July 5, 2022 through July 5, 2025, in each case at an exercise price of $0.5 per share. The conversion and exercise price of these securities, the exercise period of the warrants as well as the maturity dates of the notes have been adjusted as provided below
Additionally, on January 5, 2022, the Company and the Buyers entered into the Fourth Amendment to the Convertible Note Agreement pursuant to which the following was agreed to:
| (i) | The principal and accrued interest on all outstanding loans shall be made in New Israeli Shekels (NIS) at the conversion rate which was in effect on the date on which the loan was advanced; | |
| (ii) | The conversion price on all outstanding notes under the Convertible Note Agreement has been adjusted to a conversion price of $0.05 per share | |
| (iii) | The exercise price on all outstanding warrants issued in connection with advances made under the Convertible Note Agreement has been adjusted to an exercise price of $0.05 per share. |
On July 15, 2022, Citrine 9 LP, (hereinafter “Citrine 9”), one of the related entities and a signatory lender (to the Convertible Note Purchase Agreement entered into by the Company and several related parties (hereinafter the “Buyers”) in April 2020, as subsequently amended (the “CL Agreement”) agreed to honor a Draw Down Notice for, and has advanced to the Company, $100,000 on the same terms and conditions as are specified in the CL Agreement. The annual interest on the loan continues to be nine percent (9%). The principal and interest payments on the Note are due on July 31, 2023 and are to be made in New Israeli Shekels (NIS) at the conversion rate which was in effect on the date on which the loan was advanced. Citrine 9 was be issued 8,333,333Series A warrants and 8,333,333Series B warrants for shares of common stock, where the Series A warrants are exercisable beginning January 15, 2023 through July 15, 2024 and the Series B warrants are exercisable beginning January 15, 2023 through July 15, 2025, in each case at an exercise price of $0.05 per share On August 9, 2022, the Company’s board of directors agreed to extend the maturity date on the loans to October 31, 2023, subject to approval of Citrine 9, and to extend the exercise period of the warrants through August 9, 2027. The conversion and exercise price of these securities, the exercise period of the warrants as well as the maturity dates of the notes have been adjusted as provided below
On August 9, 2022 , the board of directors of the Company agreed to the following:
(A) The maturity date on all outstanding convertible loans under the CL Agreement was extended to October 31, 2023 (from July 31, 2023), subject to agreement of the lending entities under the CL Agreement to the extension of such maturity date; and
(B) The exercise period on all of the outstanding Series A and Series B warrants issued to date in connection with the convertible loans was extended to August 9, 2027.
On August 9, 2022, the Board agreed to issue to the related entities who advanced an aggregate of $1,170,000 in convertible loans under the CL Agreement on or before June 15, 2020 warrants for a total 5,589,172 shares of common stock, exercisable through August 9, 2027 at a per share exercise price of $0.05, in replacement of the Series A warrants for an identical number of shares issued in June 2020 in connection with such loans.
| 58 |
On September 30, 2022, Citrine Global received a loan from Citrine Hi Tech 7 LP, an Israeli limited partnership and an affiliated entity (the “Lender”), in the principal amount of $80,000. The loans bears interest at 12% per annum and is scheduled to mature on December 15, 2022. The principal and interest payment on the Note are to be made in New Israeli Shekels (NIS) at the exchange rate which was in effect on the date on which the loan was advanced. The Lender has the option, upon written notice to the Company and subject to the Company’s consent, to extend the maturity date of the loan (the “Maturity Date extension Notice”). The Lender is to provide the Maturity Date extension Notice by no later than December 5, 2022. In the event that the Company agrees to such extension, the terms of this Note shall be adjusted on a pro-rata basis, to those terms applicable to the Company’s convertible notes then outstanding under the Convertible Note Agreement, date as of April 1, 2020, as subsequently amended, amongst the Company and the affiliated parties thereto (of which the Lender is a party). On January 29, 2023, holder agreed to extend to May 31, 2024 the maturity date of the note and also agreed that upon a public offering of its securities that the Company may effect in connection with an listing of the Company’s stock on a U.S. National Securities Exchange, the note is automatically convertible into the securities that are the subject matter such offering, in whole or in part, as the Citrine Global Board may determine.
On November 14, 2022, the CL Agreement was amended to provide that until the repayment in full of all outstanding principal and accrued interest on the Notes issued thereunder and the earlier to occur of the exercise in totality of the Warrants issued in connection with the Notes or their termination by the terms thereof, if the Company issues securities in any financing transaction, including debt convertible into equity, in any equity and/or debt offering or other transaction (the “Future Financing”) and said securities contain any terms that are more favorable than the terms and provisions contained in the outstanding Notes and/or Warrants under the CL Agreement, including without limitation, an effective per share price which is lower than the then effective Conversion Price applicable to the Notes or the Exercise Price of the Warrants, then the Company shall, at the request of a majority of the Buyers, enter into amendments to the Notes and/or Warrants, as applicable, to provide for the same more favorable terms and provisions.
On January 29, 2023, the CL Agreement was further amended to extend to May 31, 2024 the maturity date thereof. The amendment also provides that upon a public offering of its securities that the Company may effect in connection with an listing of the Company’s stock on a U.S. National Securities Exchange, the note is automatically convertible into the securities that are the subject matter such offering, in whole or in part, as the Citrine Global Board may determine.
Agreements with Intelicanna
Ilanit Halperin, a director and the Chief Financial Officer of the Company, is also the Chief Financial Officer of Intelicanna. Doron Birger, a director of ours, is the chairman of the board of directors of Intelicanna Ltd. (“Intelicanna”) effective April 2021. In addition, Ora Elharar Soffer, is a shareholder in Intelicanna.
On May 31, 2020, we entered into a strategic partnership with Intelicanna via a share exchange agreement and an agreement for future issuance of shares. Furthermore, on June 25, 2020, the Citrine Global Israel has entered into a services agreement with Intelicanna to provide business development and consulting services to Intelicanna, including assistance with raising financing. Also on June 25, 2020, to assist Intelicanna to raise the first NIS 1 million towards the up to NIS 15 million mentioned in the Services Agreement, the Company and the Israeli Subsidiary entered into an agreement to grant Intelicanna NIS 1 million in cash (approximately USD 290 thousand) in direct financing for working capital purposes. On July 9, 2020, we transferred to Intelicanna NIS 500 thousand (approximately $145 thousand) on account of the above loan. In March 2021, Intelicanna repaid the loan with the 12% annual interest. On September 17, 2020 we issued to Intelicanna 2,143,470 shares of common stock in exchange for 619,589 of Intelicanna’s ordinary shares. Between August 3 – 9, 2021, we sold to an unrelated third party in an off market transaction 619,589 ordinary shares of Intelicanna for aggregate gross proceeds to the Company of 1,260,611 NIS (approximately $378,562 based on the current exchange rate). Following the sale, the Company no longer holds any Intelicanna shares. As previously disclosed, the Company obtained the Intelicanna shares in a share exchange agreement entered into with Intelicanna in September 2020. The Company’s decision to sell the Intelicanna shares was taken, in part, to avoid being subject to the terms of the Investment Company Act of 1940. In addition, on May 31, 2020, we entered into an agreement with Intelicanna for future issuance of shares. The agreement for future issuance of shares provides that a fall in a share price of a party, not exceeding 20%, measured six months after issuance of shares by both parties pursuant to a separate share exchange agreement, will be offset by the issuance of additional shares to the other party to bring up to $500 thousand the total value of the shares issued to the other party. On August 15, 2021, the Company’s board of directors determined that it is required to issue to Intelicanna 535,867 shares of the Company’s common stock and has authorized the issuance of such shares to Intelicanna.
| 59 |
Share Purchase Agreement with Nanomedic
On June 22, 2020, we entered into a share purchase agreement with Nanomedic as part of an A-1 funding round open only to existing Nanomedic shareholders and their affiliates. Our CEO and Chairperson is a director and shareholder in Nanomedic. We paid $450,000 for A-1 preferred shares of Nanomedic and also received warrants to purchase A-1 preferred shares. Such investment represents a holding of approximately 3.3% in Nanomedic. The round raised approximately $2.2 million in total. Citrine S A L Group were already beneficial shareholders of Nanomedic immediately prior to the A-1 funding round. Ilan Ben-Ishay, a director of the Company was already a beneficial shareholder of Nanomedic immediately prior to the A-1 funding round. Ora Elharar Soffer, our chairperson and CEO, was already a director of both Nanomedic and its Israeli parent company, Nicast Ltd. immediately prior to the A-1 funding round, and she was also already a beneficial shareholder of Nanomedic immediately prior to the A-1 funding round.
iBOT Israel Botanicals Ltd
On August 4, 2020, the Board of the Company approved for the Company and Citrine Global Israel to proceed with preparations for investing in iBOT. Our CEO and Chairperson, Ora Elharar Soffer, and, Ilan Ben-Ishay are directors in iBOT and Citrine SAL, one of our principal shareholders, is a principal shareholder in iBOT. iBOT has a manufacturing facility for a wide range of botanical formulations, and part of its strategy is to combine this with hemp and CBD. The Board gave its approval, subject to agreement of definitive terms and receipt of all necessary corporate and other approvals, for a proposed transaction in which (1) the Company would have an option to make one or more investments during a period of 12 months in an aggregate amount of up to $1 million; (2) the investments may be through loans, direct equity purchases, or other means, and would be based on milestones. In addition, the Board approved for the Company to proceed with preparations for entering a services agreement with iBOT pursuant to which the Company would provide consulting and other services to iBOT. Ms. Elharar Soffer, our chairperson, CEO and President and Mr. Ilan Ben Ishay, a director in Cannovation, are also directors in iBOT.
On November, 2021, the Company, Cannovation Center Israel and CTGL – Citrine Global Israel Ltd., on the one hand (collectively the “Citrine Global Group”), and iBOT, on the other hand, entered into an Exclusive Strategic Collaboration and Alliance Agreement (the “Exclusive Rights Agreement”) pursuant to which iBOT granted to the Citrine Global Group, jointly and individually, exclusive world-wide rights, solely with respect to the cannabis market, to iBOT’s botanical formulas and nutritional supplements, including, the development, manufacture, distribution and sale of such products. The exclusive rights include the right of any of the Citrine Global Group to grant rights thereunder to third parties so long as such third parties shall agree to be bound by terms consistent with those contained in this Agreement. In consideration of the grant of the rights under the Exclusive Rights Agreement, Citrine Global Group granted to iBOT the exclusive right to manufacture in State of Israel (consistent with the terms of the Manufacturing Agreement) the botanical products. In addition, so long as iBOT is in compliance with the terms of this Agreement, in the event that the Citrine Global Group determines to manufacture botanical products outside of Israel, then iBOT is to be afforded the opportunity to perform such manufacturing for the Citrine Group at iBOT’s facility in Israel provided that iBOT complies with all of the terms and conditions relating to such manufacturing project, including the price per unit, delivery schedules, packaging requirements regulation and other relevant terms.
In November 2021, iBOT granted to Citrine Global Group a pre-emption right to any equity or equity linked securities that iBOT proposes to issue to an unrelated third party with aggregate gross proceeds to the Company exceeding $1 million or which will result in a change in control in iBOT following such issuance, then iBOT is to give to the Citrine Global Group written notice of such proposed issuance and the relevant terms thereof and the Citrine Global Group shall have ten (10) days thereafter to determine if it elects to purchase a minimum of 51% of the proposed issuance on the price and other terms specified in the notice sent by iBOT (the “Pre-Emption Right”). If the Citrine Global Group elects to exercise the Pre-Emption Right, such purchase is to take place at no more than 90 days following the expiration of the 10 day notice period to the Citrine Global Group. Any iBOT securities of the Pre-Emption Right that Citrine Global Group elects to not purchase are to be sold by not later than 90 days following the end of the Citrine Global Group’s notice period and if such shares are not sold to such third party within the 90 day period, the Pre-Emption right shall apply to any subsequent proposed issuance. The preemption right does not apply to certain specified exceptions.
| 60 |
In November 2022 iBOT agreed to extend to March 31, 2023 the pre-emption right previously granted to the Citrine Global Group with respect to any equity or equity linked securities that iBOT proposes to issue to an unrelated third party with aggregate gross proceeds to iBOT exceeding $1 million or which will result 51% in a change in control in iBOT following such issuance.
On March 19, 2023, iBOT agreed to further extend to December 31, 2023 the pre-emption right previously granted to the Citrine Global Group with respect to any equity or equity linked securities that iBOT proposes to issue to an unrelated third party with aggregate gross proceeds to iBOT exceeding $1 million or which will result 51% in a change in control in iBOT following such issuance
Compensation Arrangements with Officers and Directors
On August 15, 2021, the Company’s board of directors increased the number of shares reserved for issuance under the 2018 Stock Incentive Plan to 90,000,000 shares of common stock thereunder and recommended to the Company shareholders to approve the increase in the pool to. The Board also determined to grant to each of Ilanit Halperin and David Kretzmer, directors of the Company, a grant of options to purchase 9,425,680 shares of common stock, and Doron Birger, a Company director, options to purchase 2,365,420 shares, in each case at per share exercise price of $0.05, provided, that such grant is subject to approval by the shareholders of the increase in the plan pool. The options vest over a two year period, in eight (8) equal installments, with the first instalment vesting on the third month anniversary of each individual’s start date and each further instalment on each subsequent third month anniversary, where the start date is, in the case of Ilanit Halperin February 27, 2020, in the case of Doron Birger September 20, 2020 and in the case of David Kretzmer is March 1, 2021, subject to such individual’s continued service with the Company. See below for additional options awarded in August 2022
On August 15, 2021, the board awarded a bonus to the Company’s Chairperson of the Board, CEO, CFO, officers, directors and senior management equal to two percent (2%) of any capital raise, subject to prior repayment of the outstanding convertible loans and so long as the payment thereof would be part of the Company’s operating budget for a minimum period of 18 months. In addition, the Board agreed to a bonus Company’s Chairperson of the Board, CEO, CFO, officers, directors and senior management of 2% from operating profits which will become payable upon the fulfillment of certain specified targets that the Board will establish, subject to prior repayment of the outstanding convertible loans and so long as the payment thereof is from available funds and would be part of the Company’s operating budget for a minimum period of 18 months.
On August 15, 2021, the board of directors of Cannovation Center Israel adjusted the compensation of the founder and chairperson, Ora Elharar Soffer, to $10,000 per month, and that of the chief financial officer, Ilanit Halperin, to $4,000 per month, and that of Ilan Ben-Ishay and David Kretzmer, directors, to $2,000 per month, in each case retroactive to July 1, 2021. These amounts would be paid at such time as they shall become due and payable from and as Cannovation Center Israel shall have available funds therefor.
On August 9, 2022, the Company’s Board determined to increase the number of shares reserved for issuance under the 2018 Stock Incentive Plan (the “2018 Plan”) by 90 million shares to a total of 180,000,000 shares of common stock thereunder and on August 12, 2022 the Company shareholders approved the same. On August 9, 2022, the Company’s board of directors determined to increase the number of shares reserved for issuance under the 2018 Stock Incentive Plan (the “2018 Plan”) by 90 million shares to a total of 180,000,000 shares of common stock thereunder and on August 12, 2022 the Company shareholders approved the same.
On August 9, 2022, the Board also determined to grant to the directors and officers set forth below options under the 2018 Plan. The options vest over a three year period, in twelve (12) equal installments, with the first instalment vesting on the third month anniversary of the date of grant and each further instalment on each subsequent third month anniversary, subject to such individual’s continued service with the Company. In the event of a change in control, the vesting schedule is accelerated and all unvested options vest.
| Director/Officer | Number of Options | |||
| Ora Elharar Soffer (Chairperson, CEO) | 47,128,400 | |||
| Ilanit Halperin (Director, CFO) | 18,851,360 | |||
| Ben Ishay (Director) | 18,851,360 | |||
| Doron Birger (Director) | 2,356,420 | |||
| David Kretzmer | 2,356,420 | |||
| 61 |
The options have an exercise price of $0.020 per share except for those awarded to Ms. Elharar Soffer which have an exercise price per share of $0.022. The other terms relating to the options grants are included in stock option agreements under the 2018 Plan. Amongst other things, the stock option agreements for selected service providers of Citrine Global, including our directors and officers, provide that the exercise price of the options that were awarded to date, shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options. All other relevant terms of such shall continue in full force and effect and are such reverse stock split. Any and all tax implications rest solely with the optionee and not the Company.
On November 13, 2022, the board of directors ratified the Stock Option Agreements for the previously disclosed stock options grants that were awarded in each of August 2021 and in August 2022 to service providers, including our officers and directors. With respect to selected optionee service providers to Citrine Global, including our directors and officers, the relevant stock option agreements provide that the exercise price of the options that were awarded to date , which include our officers and directors, shall remain unaffected by the implementation of a reverse stock split that the Company may implement; to avoid any doubt, such reverse stock split shall apply to the number of options shares issuable under such options and all other relevant terms of such options (other than the exercise price) shall continue in full force and effect following the implementation of such reverse stock split. Any and all tax implication of this decision shall rest solely with the optionee. In addition, on March 5, 2023, the board of directors provided that in the event that the Company’s stock is listed on the Nasdaq Stock Exchange, then one half of the awarded option will immediately vest.
Director Independence
The Company’s board of directors has determined that each of Doron Birger, David Kretzmer, Dror Shaked and David Freidenberg is an “independent director” as such term is defined by Nasdaq Marketplace Rule 5605(a)(2).
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Principal Accounting Fees and Services
The following table presents the fees for professional services rendered by our accountant, Somekh Chaikin, a member firm of KPMG International, independent registered public accounting firm, located in Tel Aviv, Israel, PCAOB ID 1057, for the two years ended December 31, 2022.
| 2022 | 2021 | |||||||
| ($ in thousands) | ||||||||
| Audit fees (1) | $ | 95 | $ | 85 | ||||
| Audit-related fees (2) | - | |||||||
| Tax fees (3) | $ | 5 | $ | 5 | ||||
| All other fees | - | |||||||
| Total: | $ | 100 | $ | 90 | ||||
| (1) | Audit fees consist of audit and review services, consents and review of documents filed with the SEC. The fee for 2022 also includes services rendered in connection with the re-audit of the financial statements for the year ended December 31, 2021. |
| (2) | Audit-related fees consist of assistance and discussion concerning financial accounting and reporting standards and other accounting issues. |
| (3) | Tax fees consist of preparation of federal and state tax returns, review of quarterly estimated tax payments, and consultation concerning tax compliance issues. |
| 62 |
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Documents filed as part of this report
(1) Financial Statements
The Consolidated Financial Statements filed as part of this annual report are identified in the Index to Consolidated Financial Statements on page F-1 hereto.
(2) Financial Statements Schedules
Financial Statement Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
(3) Exhibits
The following documents are filed as exhibits to this report on Form 10-K or incorporated by reference herein. Any document incorporated by reference is identified by a parenthetical reference to the SEC filing that included such document.
| 63 |
| 64 |
| + | Management contract or compensatory plan or arrangement |
| * | Filed herewith |
| ** | Furnished herewith |
ITEM 16. SUMMARY
Not Applicable.
| 65 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned.
| Citrine Global, Corp. | ||
| By: | /s/ Ora Elharar Soffer | |
| Ora Elharar Soffer | ||
| Chair of the Board and Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| Date: | March 22, 2023 | |
| By: | /s/ Ilanit Halperin | |
| Ilanit Halperin | ||
| Chief Financial Officer | ||
| (Principal Financial and Principal Accounting Officer) | ||
| Date: | March 22, 2023 | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Ora Elharar Soffer | Chair of the Board of Directors and Chief Executive Officer | March 22, 2023 | ||
| Ora Elharar Soffer | (Principal Executive Officer) | |||
| /s/ Ilanit Halperin | Director and Chief Financial Officer | March 22, 2023 | ||
| Ilanit Halperin | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Doron Birger | Director | March 22, 2023 | ||
| Doron Birger | ||||
| /s/ David Kretzmer | Director | March 22, 2023 | ||
| David Kretzmer |
| 66 |