Exhibit 99.3
Overview
We are a commercial biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat orphan diseases. Our products, RAVICTI® (glycerol phenylbutyrate) Oral liquid, BUPHENYL® and AMMONAPS® (sodium phenylbutyrate) Tablets and Powder, are designed to lower ammonia in the blood. Ammonia is produced in the intestine after a person eats protein and is normally detoxified in the liver by conversion to urea. Elevated levels of ammonia are potentially toxic and can lead to severe medical complications which may include death. We have developed RAVICTI, which we launched during the first quarter of 2013, to treat most urea cycle disorders (“UCD”) including 7 of the 8 and the most prevalent UCD subtypes, and are developing glycerol phenylbutyrate (“GPB”), the active pharmaceutical ingredient in RAVICTI, to treat hepatic encephalopathy (“HE”). UCD and HE are diseases in which blood ammonia is elevated. UCD are inherited rare genetic diseases caused by a deficiency of one or more enzymes or transporters that constitute the urea cycle, which in a healthy individual removes ammonia through its conversion to urea. We estimate there are approximately 2,100 cases of UCD in the United States of which approximately 1,100 have been diagnosed. However, we estimate that only about 675 patients are currently treated with medication approved by the U.S. Food and Drug Administration (“FDA”). HE may develop in some patients with liver scarring, known as cirrhosis, or acute liver failure and is a chronic complication of cirrhosis which fluctuates in severity and may lead to serious neurological damage.
On February 1, 2013, the FDA granted approval of RAVICTI for chronic management of UCD in adult and pediatric patients greater than two years of age who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. Limitations of use include treatment of patients with acute hyperammonemia (“HA”) crises for whom urgent intervention is typically necessary, patients with N-acetylglutamate synthetase (“NAGS”) deficiency for whom the safety and efficacy of RAVICTI has not been established, and UCD patients under two months of age for whom RAVICTI is contraindicated due to uncertainty as to whether newborns, who may have immature pancreatic function, can effectively digest RAVICTI.
In May 2013, we acquired BUPHENYL, an FDA-approved therapy for treatment of 3 of the most prevalent UCD subtypes, from Ucyclyd Pharma Inc. (“Ucyclyd”), a subsidiary of Valeant Pharmaceuticals International, Inc. (“Valeant”). In Europe and the Middle East, BUPHENYL is sold under the brand name AMMONAPS®. The active pharmaceutical ingredient in BUPHENYL and AMMONAPS is sodium phenylbutyrate (“NaPBA”). References to BUPHENYL in this Form 10-K include AMMONAPS when referring to the product in the Middle East and Europe. Subsequent to the acquisition, we began selling BUPHENYL within the United States to patients who have not transitioned to RAVICTI. In addition, we sell BUPHENYL in Canada based on Special Access Requests from Health Canada and through our distributors in other select regions outside the United States. In 2011, an Abbreviated New Drug Application (“ANDA”) for a generic tablet form of NaPBA was approved in the U.S., and in 2013, an ANDA for generic powder was approved. In January 2015, Health Canada approved a taste-masked granule form of NaPBA. These forms of NaPBA are owned by companies other than Hyperion. References to NaPBA in this Form 10-K include the generically available tablet and powdered forms of the drug, as well as our branded products in both powdered and tablet forms.
Although the price of BUPHENYL per gram is approximately one fifth that of RAVICTI and the prices for both therapies vary among patients because doses are individualized based on a patient’s weight and disease severity, most patients cannot afford to pay for either medication themselves. We have engaged a dedicated team at a third party call center, which serves as an integrated resource for prescription intake and distribution, reimbursement adjudication, patient financial support, and ongoing compliance support for our UCD patients. Together with distribution via two specialty pharmacies, we believe these services provide important support to UCD patients and their physicians, and help them achieve more favorable outcomes in managing their disease.
1
As part of our ongoing commitment to the patient community, we provide our UCD products at no cost to patients as we help them establish insurance coverage for our UCD products and donate to an independent foundation with an established track record of enabling patients to access medications affordably.
RAVICTI was granted orphan drug exclusivity in the United States for the maintenance treatment of patients with UCD shortly after its FDA approval in 2013. This exclusivity extends through February 1, 2020. RAVICTI has also received orphan drug designation in the European Union (“EU”), although the right to marketing exclusivity cannot be determined until we are authorized to market it in the EU. In March 2013, U.S. Patent No. 8,404,215 entitled “Methods of Therapeutic Monitoring of Nitrogen Scavenging Drugs” issued from U.S. Patent Appl. No. 13/417,137 with claims directed to methods of optimizing the dosage of nitrogen scavenging drugs based on target fasting ammonia levels. This patent will expire in March 2032, and is currently listed in the FDA publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” known as the Orange Book. In February 2014, U.S. Patent 8,642,012 entitled “Methods of Treatment Using Ammonia Scavenging Drugs” issued from U.S. Patent Appl. No. 12/350,111 with claims directed to methods of treating patients with UCD using phenylacetic acid (“PAA”) prodrugs based in part on target urinary phenylacetylglutamine (“PAGN”) levels. This patent will expire in September 2030 with Patent Term Extension (“PTE”) and is listed in the Orange Book.
In March 2012, we entered into an amended and restated collaboration agreement (the “restated collaboration agreement”) with Ucyclyd pursuant to which we obtained an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL, subject to Ucyclyd’s right to retain AMMONUL for an upfront payment of $32.0 million, plus subsequent milestone and royalty payments. We exercised this option on April 29, 2013 and Ucyclyd elected to retain AMMONUL, resulting in a net payment from Ucyclyd to us of $11.0 million upon close of the transaction. This net payment reflected the $32.0 million purchase price to retain AMMONUL due to us and the $19.0 million purchase price for BUPHENYL due to Ucyclyd, less costs of approximately $2.0 million for inventory we purchased from Ucyclyd.
We believe GPB has potential in other indications; and therefore in 2012 we completed a Phase II trial assessing the safety and efficacy of GPB in the treatment of episodic overt HE. Episodic overt HE can be diagnosed clinically through a set of signs and symptoms. The Phase II trial met its primary endpoint, which was to demonstrate that the proportion of patients experiencing an HE event was significantly lower on GPB versus placebo, both administered in addition to standard of care, which included lactulose and/or rifaximin. The FDA has granted orphan drug designation to GPB for this indication, but whether a compound receives exclusivity for an indication is not determined by the FDA until the drug has been approved for marketing. HE is a serious but potentially reversible neurological disorder that can occur in patients with cirrhosis or acute liver failure. It comprises a spectrum of neuropsychiatric abnormalities and motor disturbances that are associated with varying degrees of disability, ranging from subtle to lethal. HE is believed to occur when the brain is exposed to ammonia that is normally removed from the blood by a healthy liver. We believe that ammonia plays a central role in this disease, and the most commonly utilized therapies for the treatment of HE are believed to act by reducing ammonia. Published epidemiological data suggest that there are approximately 140,000 patients in the United States who have episodic HE. We believe GPB, if approved for HE, would treat HE through a systemic reduction of ammonia.
On June 12, 2014, the Company acquired Andromeda Biotech Ltd, (“Andromeda”) an Israeli company developing DiaPep277 for the treatment of recent onset Type 1 diabetes, from Clal Biotechnology Industries Ltd., (“CBI”). On September 8, 2014, we announced the termination of further development of DiaPep277® beyond completion of the ongoing clinical trial as a result of evidence we uncovered that certain employees of Andromeda engaged in serious misconduct that compromised clinical trial results. We subsequently terminated the Andromeda employees involved in the misconduct. We had been involved in a legal dispute with CBI related to Andromeda since our announcement of the discovery of the Andromeda employee misconduct in September 2014. On February 16, 2015 we reached an agreement with CBI and Yeda Research and Development Company Ltd (“Yeda”), the company from which Andromeda licenses the underlying DiaPep277 technology, to resolve
2
DiaPep277 related claims against one another, and we granted CBI an option to acquire all of the outstanding stock of Andromeda. In connection with the agreement, CBI will transfer to us $2.5 million in shares of our common stock it holds, valued at the average closing price of the Company’s common stock for the fifteen trading days ending on February 11, 2015. The parties have appointed a steering committee to oversee the completion of the clinical trial with representatives of CBI and Yeda and a non-voting member appointed by us. The Company’s estimated budget from October 1, 2014 through the completion of the trial remains unchanged at $10.5 million. Any increase to this budget beyond $2.25 million, if incurred as a result of the direction of the steering committee, shall require CBI to reimburse those expenses in shares of the Company’s common stock. Under the agreement, CBI’s option is exercisable through September 30, 2015 for $3.5 million payable in shares of the Company’s common stock. In addition, if the option is exercised, then Andromeda will be obligated to pay Hyperion future contingent payments if and to the extent it or its shareholders receive revenues or certain other proceeds, which are capped at $36.5 million. This amount, together with the option exercise price that Hyperion may receive, approximates the total amount Hyperion will have invested in Andromeda by the option exercise date. Under the agreement, in the event that CBI does not exercise its option, the underlying DiaPep277 technology will revert to Yeda under the license agreement between Andromeda and Yeda. Also on February 16, 2015, the Company entered into a release with Evotec International GmbH (“Evotec”) pursuant to which Evotec released its previously asserted claims that it was entitled to a milestone payment from us in connection with our acquisition of Andromeda and that it had suffered harm from recent incidents in relation to DiaPep277 in exchange for a payment of $500,000 from us.
Business Strategy
Our strategy is to commercialize a product portfolio, including RAVICTI, for the treatment of UCD and to develop GPB for the treatment of HE. The key elements of our strategy are to:
| • | Commercialize RAVICTI and enhance patient care in UCD. A cornerstone of our commercial strategy is to facilitate the transition of patients from NaPBA to RAVICTI and to grow the market through use in patients who are newly diagnosed or who have not previously been on a therapy approved by their relevant regulatory authority and whose UCD is not adequately controlled by dietary measures alone. In the U.S., we directly employ a small, scientifically-focused field team of six sales representatives and four payor account managers. We have also contracted a third-party call center focused on reimbursement adjudication and compliance support and two specialty pharmacies and one specialty distributor which distribute RAVICTI directly to patients and hospitals. If we receive approval for RAVICTI and launch in Canada, we will employ a small Canadian-based team which will work with our U.S. personnel to sell and support RAVICTI in Canada. Should we receive approval and decide to launch RAVICTI in Europe, we anticipate partnering with an EU-based company for sales and marketing support. We are currently assessing the commercial attractiveness of other ex-U.S. geographies. |
| • | Sell BUPHENYL for appropriate patients. We are marketing BUPHENYL for use by UCD patients within the United States who have not transitioned to RAVICTI. We are also marketing BUPHENYL through our distributors outside the United States. We sell BUPHENYL in Canada based on approved Special Access Program Requests that we receive from Health Canada. |
| • | Develop GPB for the treatment of HE. Based on the positive results of our Phase II trial assessing the safety and efficacy of GPB in the treatment of episodic HE, an end of Phase II meeting to discuss those trial results, and subsequent dialogue with the FDA, we are currently planning a Phase III clinical trial that we estimate will begin enrolling in the second half of 2015. |
| • | Acquire additional products and product candidates. We intend to continue to identify and may license or acquire products or product candidates that fit with our development and commercial capabilities. |
3
UCD and HE: Diseases Related to Elevated Ammonia Levels
UCD and HE are generally characterized by elevated levels of ammonia in the bloodstream. Ammonia is a potent neurotoxin, primarily produced in the intestine as a byproduct of protein metabolism. Individuals with a healthy liver detoxify ammonia by converting it to urea, which is excreted in urine. In both UCD and HE, the liver’s ability to convert ammonia to urea is diminished. UCD patients have a genetic disability, and individuals with HE have an acquired disability related to a decline in liver function and/or shunting of blood around the liver that occurs in patients with more severe cases of cirrhosis.
In both UCD and HE patients, ammonia can build up to toxic levels which can lead to severe medical complications, including death. Both UCD and HE fluctuate in severity, and patients may experience crises, called HA crises for UCD patients or HE events for HE patients, which often require hospitalization and may result in irreversible neurological damage.
UCD Background
UCD are inherited genetic diseases caused by a deficiency of one of the enzymes or transporters that are necessary for the normal function of the urea cycle. The urea cycle involves a series of biochemical steps in which ammonia, a potent neurotoxin, is converted to urea, which is excreted in the urine. UCD patients may experience HA crises which may result in irreversible brain damage, coma or death. UCD symptoms may first occur at any age depending on the severity of the disorder, with more severe defects presenting earlier in life.
Diagnosis and Prevalence
UCD are diagnosed either through newborn screening or when symptoms occur and are recognized as related to a UCD. Current newborn screening typically only detects three of the UCD subtypes and does not detect the most prevalent subtype. Thus, screening is believed to identify only approximately one-third of newborns with UCD. Initial UCD symptoms range from catastrophic illness with coma occurring within a few days of birth to milder and non-specific symptoms such as difficulty sleeping, headache, nausea, vomiting, disorientation and seizures, particularly in patients who present later in life. Because these symptoms are common to a number of ailments, physicians often do not consider the possibility of UCD and therefore may not measure levels of blood ammonia. As a result, the most mildly affected patients can go undiagnosed for decades despite having symptoms. Because many cases of UCD remain undiagnosed and because infants born with severe UCD often die without a definitive diagnosis, the exact incidence and prevalence of UCD are unknown and, we believe, likely underestimated.
We believe UCD occur in approximately 1 in 35,000 births in the United States. We estimate that there are approximately 2,100 individuals in the United States that suffer from UCD. We estimate that only half, or approximately 1,100 patients with UCD in the United States, have been diagnosed. Based on demographic data for those patients enrolled in the National Institutes of Health sponsored UCD consortium (“UCDC”) longitudinal study, we estimate that of the diagnosed UCD patient population in the United States, 28% are under 6 years of age (somewhat more than half of whom are under 2 years of age), 32% are aged 6 through 17 years and 40% are 18 years of age or older. We estimate that the UCD population with NAGS deficiency is exceedingly small and as such is financially immaterial.
Current Treatment Options for UCD and Their Limitations
On February 1, 2013, the FDA granted approval of RAVICTI for use as a nitrogen-binding agent for the chronic management of 7 of the 8 subtypes of UCD in adult and pediatric patients greater than two years of age who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. Limitations of use include treatment of patients with HA crises for whom urgent intervention is typically necessary, patients
4
with NAGS deficiency for whom the safety and efficacy of RAVICTI has not been established and UCD patients under two months of age for whom RAVICTI is contraindicated due to uncertainty as to whether newborns, who may have immature pancreatic function, can effectively digest RAVICTI.
Management of UCD involves decreasing ammonia production through reduction of protein in the diet, supplementation with essential and/or branched chain amino acids, the use of dietary supplements such as arginine and citrulline, and, when dietary measure are inadequate, the use of ammonia lowering agents, including sodium benzoate and NaPBA or Carbaglu® for NAGS deficiency. We believe that patients with mild to moderate UCD are typically treated with dietary management and that patients with more severe UCD are generally treated with FDA-approved medications, including RAVICTI or NaPBA. Liver transplantation is an option reserved for the most severely affected patients, typically those who present very early in life. Because liver transplantation is technically difficult in newborns, a company called Cytonet GmbH & Co. (“Cytonet”) is developing a therapy for severely affected newborns and recently filed a Marketing Authorization Application with the European Medicines Agency seeking approval for its liver cell therapy for treatment of UCD in children. This treatment involves the infusion of human liver cells with the aim of prolonging crisis-free survival until the patients are old enough to undergo a liver transplantation. In addition Promethera Biosciences S.A. (“Promethera”), a Belgian company has completed a 20 patient Phase I/II trial in Europe of its stem cell based therapy for treatment of UCD in the pediatric population and plans to conduct a phase IIb/III trial in UCD. Other potential therapies in early stage pre-clinical or clinical testing include gene therapy and mitochondrial enzyme replacement. For example, Aeglea Biotherapeutics has a degrading enzyme treatment in preclinical development for arginase 1 deficiency.
BUPHENYL, approved by the FDA in 1996, was the only branded FDA-approved therapy for the chronic management of 3 of the 8 subtypes of UCD, prior to RAVICTI’s approval for the same 3 subtypes, plus 4 more. BUPHENYL is available in powder and tablet forms. A generic of the tablet form of BUPHENYL was approved by the FDA in November 2011, and a generic powder form was approved by FDA in March 2013 and launched in April 2013. Similar to RAVICTI, NaPBA removes ammonia from the bloodstream and patients take the drug for the balance of their life to help maintain control of their blood ammonia. BUPHENYL is also available for the treatment of UCD in select countries throughout Europe, the Middle East, and the Asia-Pacific Region. In Europe and the Middle East the product is sold under the brand name AMMONAPS. Lucane Pharma SA obtained approval in Europe in July 2013 for its taste-masked granule formulation of NaPBA called Pheburane and in January 2015, obtained approval for Pheburane in Canada. When UCD are not well controlled or even in well-controlled patients who experience concurrent illness such as infection, or physiological stress such as pregnancy or surgery, HA crises may occur. In these acute situations, AMMONUL is often administered intravenously, and dialysis is sometimes used. AMMONUL is currently the only FDA-approved adjunctive therapy for the treatment of HA crises in patients with the most prevalent UCD. Currently, AMMONUL is not approved for use outside the United States, but is being prescribed by physicians in parts of Europe.
Limitations of Treatment Options for UCD
We believe that approximately 675 of the estimated 1,100 patients in the U.S. currently diagnosed with UCD are treated with an FDA-approved medication, including RAVICTI and NaPBA, and the remainder of UCD patients go untreated or elect to manage their disease through protein restriction and/or the use of dietary supplements. Although NaPBA is an effective treatment and in many cases is lifesaving, it has important limitations including a high pill burden or large quantity of powder that must be taken, unpleasant taste and smell, and frequent dosing (3-6 times per day), which make compliance for many UCD patients difficult. The amount of NaPBA prescribed is based on a patient’s weight or body surface area. The maximum daily dose of 20 grams requires patients to consume either 40 vitamin-sized uncoated tablets or 6.9 teaspoons of powder mixed with liquid or food. The powder form of NaPBA is often mixed with food, which can result in food aversion. Due to palatability issues with most formulations of NaPBA, gastrointestinal side effects associated with NaPBA and symptoms of their disease, we believe that up to 45% of pediatric patients have or have had a feeding tube. In addition, the sodium content of the maximum daily dose of NaPBA exceeds the FDA’s recommended daily allowance, which may lead to high blood pressure.
5
Despite the life-threatening nature of UCD and the irreversible brain damage that can occur if a patient’s disease becomes uncontrolled and an acute HA crisis occurs, non-compliance with NaPBA is common. For example, a peer reviewed journal article discussing data from a 21-year, open-label, uncontrolled, multi-center study in 206 UCD patients, which was used to support FDA approval of AMMONUL, reports that approximately 10% of HA crises were attributed to non-compliance with NaPBA. This study was initiated originally under Brusilow Enterprises, LLC’s (“Brusilow”) Investigator New Drug (“IND”) application in 1982 and later was used to support the filing of Ucyclyd’s New Drug Application (“NDA”) that resulted in FDA approval in 2005. In addition, approximately 22% of HA crises reported by patients on BUPHENYL in the year before their enrollment in our pivotal study were attributed to non-compliance.
Many patients with mild to moderate disease manage their condition through protein restriction alone and risk long-term complications of mildly elevated ammonia if the underlying disease is not well controlled. Common neurological manifestations of patients with poorly controlled mild to moderate disease include hyperactive behavior, self-injurious behavior, stroke-like episodes, behavioral problems, cognitive dysfunction, and psychiatric symptoms. Recent clinical research suggests that even mildly symptomatic patients demonstrate cognitive deficits. Even mild to moderately affected patients risk an HA crisis if their disease is poorly controlled. According to data gathered by the UCDC, approximately 40% of patients not taking NaPBA who enrolled in the UCDC sponsored longitudinal study had reported at least one acute crisis prior to enrollment.
Key Advantages of RAVICTI
Our analysis of data from our Phase II and Phase III trials evaluated the non-inferiority of RAVICTI as compared to NaPBA in controlling blood ammonia levels in adult and pediatric UCD patients. A non-inferiority trial is undertaken when a placebo-controlled trial in a life threatening condition is unethical and compares a test drug to an established treatment with the goal of showing that the test drug is at least as effective, and therefore not inferior to the established treatment, and that the test drug is, therefore, also effective. We successfully demonstrated non-inferiority in each of our Phase II and Phase III trials. We believe RAVICTI provides incremental benefits in part due to its slow release profile, which results in slower intestinal absorption of phenylbutyrate (“PBA”), which appears to provide better late afternoon and nighttime control of ammonia levels. Although not included in the FDA-approved label for RAVICTI, the following summarizes what we believe to be the key advantages of RAVICTI as compared to NaPBA based on the totality of the RAVICTI dataset:
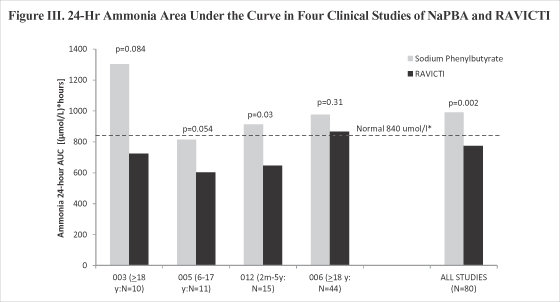
| • | Ammonia control: Four clinical trials have shown that blood ammonia is lower on RAVICTI and statistically as good as, or non-inferior, to NaPBA. A pooled analysis of the data from all patients in the Phase II and Phase III trials was performed in which all ammonia values obtained over 24 hours on RAVICTI were combined and compared with similar values obtained on NaPBA. This analysis was included in the NDA and included patients down to 2 months of age. Ammonia values as measured by 24-hour area-under-the-curve, which is a measure of the total systemic ammonia exposure experienced by patients over 24 hours, demonstrated that blood ammonia was approximately 21.9% lower after treatment with RAVICTI as compared to NaPBA (ųmol . h/L = 774.11 on RAVICTI and ųmol . h/L = 991.19 on NaPBA; p = 0.004). |
| • | Improved palatability to drive compliance: RAVICTI is a nearly odorless and tasteless liquid and requires a smaller daily drug volume (e.g., approximately 1 tablespoon contains the same amount of PBA as 40 tablets of NaPBA), all of which we believe makes RAVICTI easier and more palatable to swallow than most formulations of NaPBA. |
| • | Outcomes: In the 12-month safety extension to our pivotal Phase III trial and our studies in pediatric patients aged 6 through 17 years and 2 months through 5 years, patients on RAVICTI experienced approximately 50% fewer total HA crises than they reported having experienced in the prior year while on NaPBA. In addition, unlike RAVICTI, which contains no sodium, the sodium content of the maximum |
6
| daily dose of NaPBA exceeds the FDA’s recommended daily allowance, which may lead to high blood pressure. |
| • | Patient preference and tolerability: In our Phase II clinical trials studying daily ammonia control, of 36 patients who completed the trials, 34 expressed a preference for RAVICTI over NaPBA. Forty of the forty-four patients in our pivotal Phase III trial agreed to continue 12 months of treatment and monthly monitoring with RAVICTI beyond the initial four-week treatment period. Sixty-seven of sixty-nine patients who completed 12 months of treatment with RAVICTI elected to enroll in an expanded access protocol to continue receiving RAVICTI until it was commercially available. Moreover, of 100 pediatric and adult patients who participated in the 12 month treatment studies and completed a questionnaire pertaining to treatment and disease-related signs and symptoms, 80% reported a decrease in symptoms, 15% reported no change and 5% reported an increase in symptoms while treated with RAVICTI as compared with their prior experience on NaPBA. |
| • | Improved executive function: In the 12-month safety extension studies, 22 pediatric patients aged 6 through 17 years who underwent testing both at the beginning and end of the 12-month open label study showed a clinically and statistically significant improvement in executive function as assessed by the Behavior Rating Inventory of Executive Function (“BRIEF”). However, these pediatric patients did not show improvements in other neurocognitive areas, including intelligence. Adult patients did not improve on tests of neurocognitive function. |
RAVICTI Clinical Development in UCD Patients
The clinical efficacy of RAVICTI was evaluated in both adult and pediatric UCD patients across four short-term controlled- and three long-term open-label clinical trials (Table X).
The pivotal efficacy study, HPN-100-006, was a Phase 3, randomized, double-blind, active-controlled, crossover study to establish the non-inferiority of RAVICTI to NaPBA by comparing blood ammonia assessed as 24-hour (24-h) area under the concentration versus time curve (AUC0-24) in adult UCD patients. Scientific evidence confirming the efficacy demonstrated in the single Phase 3 study has been established in 3 open-label, controlled, fixed-sequence switchover studies (UP 1204-003, HPN-100-005, and HPN-100-012), each of which also compared ammonia control during steady-state treatment with NaPBA and RAVICTI.
In addition to these 4 comparatively short-term switch-over studies, persistent efficacy of RAVCTI in controlling blood ammonia levels and on clinical outcomes including neuropsychological function and hyperammonemic crises for up to 1 year, as well as safety, have been evaluated in both adults and children across three long-term open label studies (HPN-100-007, HPN-100-005SE, and HPN-100-012SE).
7
TableX: Summary of Hyperion-Sponsored UCD Clinical Studies
| Study Number | Phase | Study Design | Study Objective |
Subject Status/Patient Diagnosis |
Number Exposed to HPN-100/ Control(s)a |
|||||||||
| PIVOTAL |
| |||||||||||||
| HPN-100-006 |
3 | Randomized, double-blind, crossover | Efficacy and Safety | Adult UCD ³18 years |
44/45 | b | ||||||||
| SHORT-TERM SUPPORTIVE STUDIES |
||||||||||||||
| UP 1204-003 |
2 | Non-randomized, open-label, fixed-sequence, switch-over | Safety and efficacy | Adult UCD ³18 years |
10/14 | c | ||||||||
|
HPN-100-005 |
2 | Non-randomized, open-label, fixed-sequence, switch-over followed by a long-term open-label phase | Safety and efficacy | Pediatric UCD ³6 years — <18 years |
11/11 | |||||||||
|
HPN-100-012f |
2 | Non-randomized, open-label, fixed-sequence, switch-over followed by a long-term open-label phase | Safety and efficacy | Pediatric UCD ³29 days — <6 years |
15/15 | |||||||||
| LONG-TERM EFFICACY AND SAFETY STUDIES |
||||||||||||||
| HPN-100-007d |
3 | Uncontrolled | Safety | Adult and pediatric UCD | 60/- | |||||||||
| HPN-100-005 safety-extensione | 2 | Non-randomized, open-label, fixed-sequence, switch-over followed by a long-term open-label phase | Safety and efficacy | Pediatric UCD ³6 years — <18 years |
17/- | |||||||||
| HPN-100-012 safety-extensionf,g | 2 | Non-randomized, open-label, fixed-sequence, switch-over followed by a long-term open-label phase | Safety and efficacy | Pediatric UCD ³29 days — <6 years |
23/- | |||||||||
HPN-100 = glycerol phenylbutyrate (RAVICTI); UCD = urea cycle disorder.
| a | Safety population. Control for all UCD efficacy studies was NaPBA. |
| b | In the phase 3 study, HPN-100-006, active HPN-100 and control NaPBA were blinded with a matching placebo in a double-dummy design. |
| c | Study UP 1204-003 enrolled 13 unique patients; however, two patients were enrolled, assigned a subject identification number (ID), discontinued from the study, and then later re-enrolled under a different ID. Therefore, the study accounts for 15 patient IDs, 14 IDs who received at least one dose of study treatment |
8
| (NaPBA only) and are accounted for in the Safety Population. Ten of the 13 unique patients completed the study (received both NaPBA and HPN-100). |
| d | Study HPN-100-007 includes 40 patients who participated in HPN-100-006 and 20 new patients. These 60 patients consisted of 51 adult and 9 pediatric patients. |
| e | HPN-100-005SE is the 12-month extension phase of HPN-100-005 and includes 11 patients who participated in the switch-over phase of HPN-100-005 and 6 new patients who enrolled directly into the extension phase. |
| f | HPN-100-012 allowed patients ³29 days to <5 years of age to be enrolled; however, the youngest age of patients at the time of enrollment was 2 months of age. |
| g | HPN-100-012SE is the 12-month extension phase of HPN-100-012 and includes 15 patients who participated in the switch-over phase of HPN-100-012 and 7 new patients who enrolled directly into the extension phase. |
Summary of Clinical Results to Date. The following is a bulleted summary of the short and long-term clinical trial findings to date:
Short term studies (Protocols UP-1204-003, HPN-100-005, HPN-100-006 and HPN-100-012)
| • | Non-inferiority of daily ammonia control assessed as 24-hour area under the curve during dosing with RAVICTI relative to NaPBA was achieved in each of these studies. Non-inferiority was pre-defined for protocols HPN-100-005, HPN-100-006 & HPN-100-012 and the same criteria were applied retrospectively to the results of protocol UP-1204-003. |
| • | Ammonia exposure was directionally lower in each of the four switchover studies (Figure III), and by pooled analyses, 24-hour ammonia exposure was significantly lower during dosing with RAVICTI as compared with NaPBA among all patients (Figure I) as well as among all pediatric patients (Figure II). |
| • | Presumably because RAVICTI, unlike NaPBA, requires digestion by pancreatic lipases to which it is not exposed until it leaves the stomach, PBA delivered orally as RAVICTI is absorbed about 70-75% more slowly as compared to oral delivery with NaPBA. |
| • | Pharmacokinetic Differences in PAA Production Between Adult and Pediatric UCD Patients. Data from our clinical studies as well as population pharmacokinetic modeling and dosing simulations to predict PAA exposure indicate that the rate of conversion of PAA to PAGN for both RAVICTI and NaPBA varies directly with body surface area. As a result, exposure to PAA during dosing with both drugs tends to be higher among pediatric patients versus adults. High levels of PAA have been associated with reversible toxicity in previously published studies involving cancer patients who received intravenously infused PAA. No relationship has been observed so far between adverse events and PAA levels in our clinical studies of RAVICTI in UCD patients, and PAA exposure among UCD patients administered RAVICTI has been below the range associated with toxicity in these previously published studies. As compared with NaPBA, systemic exposure to PAA during equivalent steady state dosing of RAVICTI among UCD patients is modestly, but significantly, lower among adults and similar among pediatric patients. |
Long-term studies (Protocols HPN-100-005 SE, HPN-100-007 & HPN-100-012SE)
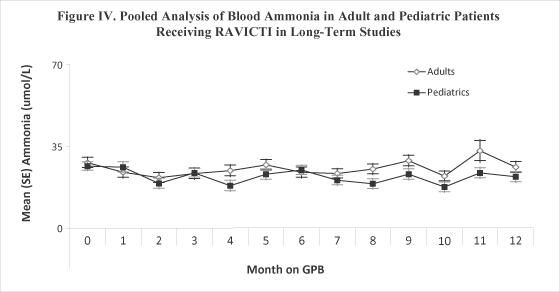
| • | Ammonia control was well maintained in both adult and pediatric patients over 12 months of dosing with average values within normal limits (Figure IV below). |
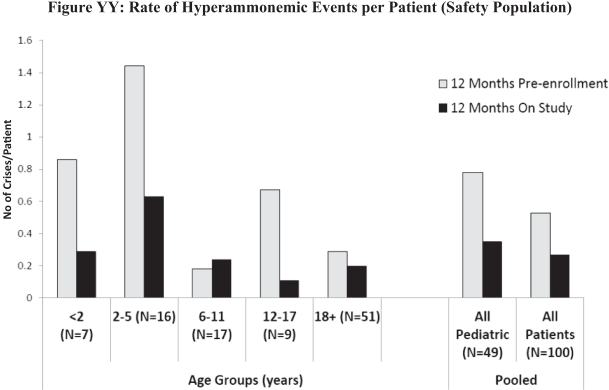
| • | There was an approximately 50% decrease in the number of HA crises experienced by patients on RAVICTI as compared with the pre enrollment period of up to 12 months (shorter for the youngest children), during which most patients had been treated with NaPBA (Figure YY). |
9
| • | Children ages 6-17 show evidence of statistically and clinically significant improvements in executive function, including behavioral regulation (e.g., flexibility, inhibitory control) and metacognitive skills (e.g., goal setting, planning, self-monitoring). |
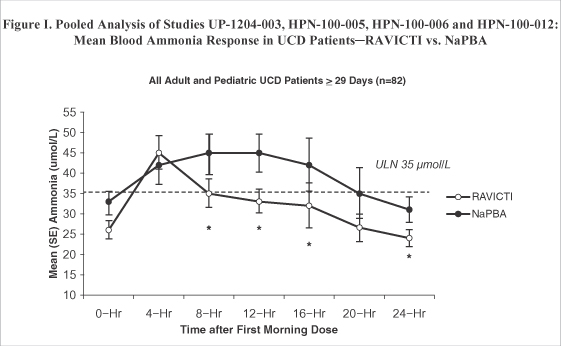
SE = standard error; ULN = upper limit of normal, *p < 0.05
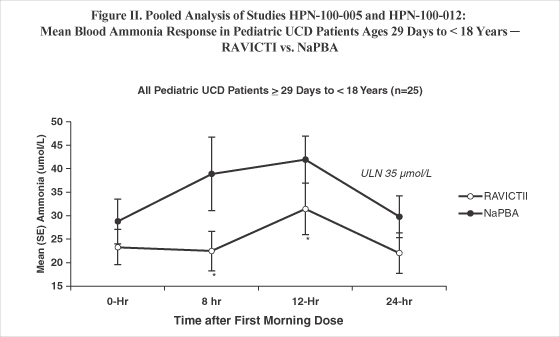
SE = standard error; ULN = upper limit of normal, *p < 0.05
10
11
HAC were recorded retrospectively for 12 months prior to enrollment. 30 patients had 53 HAC over the previous year on NaPBA and 19 patients had 27 HAC over the year on GPB
RAVICTI Nonclinical Development
As part of the development program for RAVICTI for the treatment of UCD, we conducted nonclinical genotoxicity and carcinogenicity studies to assess the risk that RAVICTI causes tumors in animals and to assess the relevant risk in humans. In a 24-month carcinogenicity study in male and female rats, six different tumor types occurred at an incidence suggestive of a relationship to RAVICTI administration. The carcinogenicity study in transgenic mice was negative, as were in vitro studies including Ames testing of all metabolites. The FDA approved label includes a summary of the carcinogenicity findings under Nonclinical Toxicology. There is no boxed warning.
Thrive Registry
As part of its ongoing commitment to the UCD community, Hyperion is sponsoring a registry comprised of UCD patients, who are eligible to participate in the registry regardless of whether they are receiving treatment for their disorder. The registry is designed to assess the impact of RAVICTI treatment on long term outcomes including hyperammonemic crises and neurocognitive development.
12
FDA Required Post-Marketing Requirements
As part of the FDA approval of RAVICTI to treat UCD, we agreed to the following post-marketing requirements:
| • | Clinical studies in pediatric patients with urea cycle disorders who are less than two months of age and also pediatric patients age 2 two months to two years. These requirements will be undertaken in the context of a single protocol, the design of which has been finalized in consultation with FDA as well as EMA in the context of a Pediatric Investigational Plan. This study began enrolling in February 2015 and will include patients under two years of age who are newly diagnosed through newborn screening or as a result of a hyperammonemic crisis as well as already diagnosed patients taking NaPBA or on dietary management alone. Ammonia levels and blood and urine metabolite (phenylacetic acid (“PAA”) and phenylacetylglutamine (“PAGN”)) levels will be checked on a fixed schedule and at the time of adverse events; |
| • | A randomized controlled clinical trial to assess the safety and efficacy of RAVICTI in treatment naïve patients with UCD; We are finalizing this protocol in consultation with FDA and anticipate that clinically stable UCD patients ages 2 months and above will be first randomized to RAVICTI vs. NaPBA after which they will undergo extended dosing with RAVICTI. Data to be collected is expected to include patient demographics and relevant history, blood ammonia, safety and tolerability, standard clinical laboratory tests, dietary protein, clinical status, neurocognitive and psychomotor status, growth and development milestones, and adverse events including HA crises. This trial is expected to start enrolling in the second half of 2015. |
| • | A drug interaction study in healthy volunteers to evaluate the effect of RAVICTI on the pharmacokinetics of a drug that is a substrate of cytochrome 3A4 has been completed. The results of the study suggest that RAVICTI administration is associated with modest induction of CYP3A4 using midazolam as a model substrate. This information is anticipated to be included in an updated RAVCTI label to be submitted this year for consideration by FDA. |
HE
Background
HE is a serious but potentially reversible neurological disorder that can occur in patients with advanced cirrhosis or acute liver failure. HE is believed to occur when the brain is exposed to gut-derived toxins that are normally removed from the blood by a healthy liver. While a variety of gut-derived toxins may contribute to HE, we believe that ammonia plays a central role in this disease. The spectrum of symptoms which constitute HE is very similar to that for UCD, including neuropsychiatric abnormalities and motor disturbances that are associated with varying degrees of disability ranging from subtle to lethal. The manifestations of HE vary over time. Similar to UCD patients who may experience HA crises, patients with episodic HE often experience periods where their symptoms worsen (“HE events”). HE events are manifested by symptoms ranging from disorientation to coma, and frequently require hospitalization. Our HE development program is targeting patients with episodic HE who have experienced past HE events and is designed to determine whether treatment with GPB will decrease the number of HE events.
We believe that the importance of ammonia is supported by the results of our Phase II study in patients with cirrhosis complicated by episodic HE. This study demonstrated not only a reduction in HE events among patients treated with GPB and a significant reduction in ammonia among patients on GPB (45.7 vs. 58.2 umol/L, p = 0.036) as compared to placebo, but also demonstrated a strong correlation between the likelihood of HE events and ammonia measured at baseline or during the study (p < 0.01).
13
Diagnosis and Prevalence
Symptoms in patients with episodic HE can range from subtle changes in personality to overt disorientation and impaired consciousness that can progress to coma or death, if untreated. Published epidemiological data suggest that there are approximately one million patients in the United States with cirrhosis of whom an estimated 140,000 have clinically recognizable episodic HE. HE is diagnosed based on the presence of compatible signs and symptoms in a patient with cirrhosis in whom other causes of brain dysfunction have been excluded. In contrast to patients with episodic HE in whom the manifestations are recognizable clinically, patients with minimal HE exhibit normal mental and neurological status upon clinical examination and need standardized neurological testing to establish a diagnosis.
The West Haven criteria, a widely used approach, grade the severity of episodic HE based upon a clinical assessment of a patient’s mental status, behavior, short-term memory, alteration of consciousness and neuromuscular function. The West Haven scale for HE ranges from Grade 1 to 4. Stable patients with Grade 1 or 2 HE are typically ambulatory and can usually be managed as outpatients. By contrast, Grade 3 and 4 patients are typically hospitalized and patients with Grade 4 HE or coma often require intensive support. Prevention of HE events is therefore important both from the standpoint of patient well-being and health care costs.
Current Therapies and Limitations
The most commonly utilized agents for the treatment of HE are poorly or non-absorbable sugars, such as lactulose or lactitol, and rifaximin, a poorly absorbed non-systemic oral antibiotic manufactured by Salix Pharmaceuticals, Ltd (“Salix”). These agents are believed to limit the local production of ammonia in the intestine. Other products currently in development include Ocera Therapeutics, Inc.’s (“Ocera”) OCR 002, or ornithine phenylacetate, which is believed to lower ammonia and is currently being studied in intravenous form in patients with acute HE events. NaPBA is not an appropriate treatment for most HE patients given the FDA warning regarding the use of the drug in patients with sodium retention and edema which is common for patients with HE.
Abdominal cramping, diarrhea and flatulence are common side effects with lactulose, making the drug difficult for many patients to tolerate. Moreover, a published review of clinical trials involving lactulose and lactitol in the treatment of HE concluded that those agents failed to demonstrate a statistically significant benefit.
Rifaximin 550 mg tablets were FDA approved in March 2010 for the reduction in risk of overt HE recurrence in patients 18 years of age or older. Although rifaximin represents the current standard of care, approximately 20% of patients experienced breakthrough HE events while taking rifaximin over a period of six months in a pivotal study. We believe the treatment and prevention of HE remains a major unmet medical need.
GPB for the Prevention of HE
Rationale for Use of GPB to Prevent HE
HE is widely assumed by clinicians to involve the systemic accumulation of ammonia resulting from impaired liver function. Therefore, we believe GPB, which lowers ammonia systemically and was shown to decrease the likelihood of HE events, can be beneficial in managing this disease. Moreover, given its mechanism of action of removing systemic ammonia from the body, GPB could be used as first line therapy. Additionally, GPB could be complementary to currently approved agents that limit the local production of ammonia.
14
GPB Clinical Development in HE Patients
Our HE clinical program to date has been comprised of two trials which have enrolled patients with cirrhosis. Our Phase II clinical trial design was similar to that used to evaluate rifaximin, the only therapy approved by the FDA for episodic HE within the last 30 years. The rifaximin trial, conducted by Salix, was a single pivotal Phase III trial which enrolled 299 patients. The primary endpoint was time to the first HE event with the key secondary endpoint of time to hospitalization.
Phase I Trial. Ucyclyd conducted a Phase I safety and pharmacokinetic study in healthy adults and adults with cirrhosis. The trial was an open-label, single and multiple dose study of GPB in 24 cirrhotic and 8 healthy subjects GPB was generally well tolerated. There were no serious adverse events or withdrawals due to adverse events. The most common individual adverse events were increased body temperature and decreased platelet count. While patients with the most severely impaired liver function tended to metabolize GPB somewhat more slowly than healthy adults, even these patients were able to effectively metabolize GPB and thereby utilize the drug for waste removal.
Phase II Trial. In 2012, we completed a Phase II trial of GPB in patients with episodic HE. Part A of this study involved an open-label dose escalation to assess the safety and pharmacokinetics of GPB in 15 patients with cirrhosis and HE. We assessed doses of 6mL and 9mL taken twice per day. The 6mL dose lowered mean fasting ammonia levels to below the average upper limit of normal and exhibited superior tolerability compared to the 9mL dose, which showed little incremental ammonia effect. The 6mL dose was therefore selected as the dose for Part B of the study.
Part B was a multi-center, randomized, double-blind trial of GPB versus placebo in patients with episodic HE, the results of which were published in the peer reviewed scientific publication, Hepatology in March 2014. This study shared many of the essential features of Salix’s pivotal trial for rifaximin in HE. In the Salix pivotal study rifaximin reduced the risk of occurrence of an HE event and risk of hospitalization due to an HE event. We followed the general design of the Salix study, recruited similar patients and applied similar definitions of HE events as the primary outcome measure. However the duration of our study was four months in contrast to Salix’s study which was six months. Also, and in contrast to the Salix trial design whereby patients exited the study after experiencing an HE event, our trial allowed patients to remain on study after their first HE event such that total events were measured. In order to be included in our study, patients must have had at least two HE events in the previous six months. The primary efficacy measure was similar to the rifaximin trial and was defined as the proportion of patients that exhibited at least one HE event while on the study. Secondary and exploratory analyses included total HE events during the study, pharmacokinetics, hospitalizations due to HE, subjects with one or more symptomatic days, and impact on minimal HE, which involves mild neurological impairment as detected by standardized testing.
Patients were allowed to continue standard of care therapy (including lactulose and/or rifaximin) while enrolled in the trial. Unlike the rifaximin pivotal trial which required that patients exit after experiencing their first HE event, our trial allowed patients to remain in the trial at the physician’s discretion after experiencing an HE event. Patients’ standard of care therapy also could be modified by their physician during the trial after experiencing their first HE event. For example, a physician could introduce rifaximin to patients not taking rifaximin at study entry.
The trial enrolled 178 patients: 88 patients from 28 sites in the U.S., 50 patients from 9 sites in Russia, and 40 patients from 7 sites in the Ukraine. The ex-U.S. sites were included both to facilitate enrollment and diversify the background standard of care. All sites were pre-qualified and continuously monitored by us throughout the study to ensure adherence to the protocol and good clinical practices (“GCP”).
The trial met its primary endpoint: the proportion of patients experiencing at least one HE event was significantly lower on GPB versus placebo (21.1% vs. 36.4%, p = 0.0214). Patients receiving GPB also
15
experienced fewer total HE events in the course of the study versus placebo (35 vs. 57; p = 0.0354 for total event rate). In addition, fewer patients on GPB experienced one or more symptomatic days (defined by a CHESS (clinical hepatic encephalopathy staging scale) score ³ 3) versus placebo (13 vs. 27; p = 0.0148).
While not statistically significant, there were trends favoring GPB in numbers of patients hospitalized for HE events, total HE-related hospitalizations and total hospital days for HE-related admissions, suggesting a potentially important pharmacoeconomic benefit to the treatment of HE with GPB. Among patients who received rifaximin at any time during the study (n=69), those receiving GPB as well experienced fewer total HE events, fewer patients hospitalized for HE events and fewer total HE hospitalizations. While the differences were not statistically significant, these trends may suggest a possible benefit of GPB in patients who have experienced HE events while taking rifaximin. However, the proportion of patients taking rifaximin at study entry that experienced at least one HE event while on study was similar for GPB and placebo (43.3% vs. 44.8%; non-significant). A summary of the results of patients on rifaximin at baseline and/or after their first HE event during the study is as follows:
Table 1: Hepatic Encephalopathy (HE) Events, Hepatic Encephalopathy-Related Hospitalizations and Blood Ammonia*
| GPB (%) | Placebo (%) | p value | ||||||||||
| All patients (n= 178) |
n=90 | n=88 | ||||||||||
| Percent (#) of patients with an HE event (1° analysis) |
21 | (19) | 36 | (32) | 0.02 | |||||||
| Percent (#) of patients with an event (WH ³ 2) |
18 | (16) | 31 | (27) | 0.04 | |||||||
| Hazard ratio (± 95% CI) based on time-to-event analysis, GPB relative to placebo |
0.56 | (0.32, 0.99) | 0.047 | |||||||||
| Total HE events |
35 | 57 | 0.04 | ** | ||||||||
| Patients reporting HE hospitalization |
10 | 16 | 0.23 | |||||||||
| HE Hospitalizations |
13 | 25 | 0.06 | ** | ||||||||
| HE Hospital days |
66 | 134 | NS | |||||||||
| Patients with CHESS score ³ 3 |
13 | 27 | 0.015 | |||||||||
| Ammonia (TNAUC; µmol/L x week) |
46 | 58 | 0.04 | |||||||||
| Not on Rifaximin at Study Entry (N= 119) |
60 | 59 | ||||||||||
| Percent (#) of patients with an HE event |
10 | (6) | 32 | (19) | 0.003 | |||||||
| Patients with an HE event, WH ³ 2 |
5 | (3) | 25 | (15) | 0.002 | |||||||
| Total HE events |
7 | 31 | <0.001 | ** | ||||||||
| HE Hospitalizations |
2 | 5 | 0.3 | ** | ||||||||
| HE Hospital days |
9 | 44 | NS | |||||||||
| Patients with CHESS score ³ 3 |
4 | (7) | 12 | (20) | 0.02 | |||||||
| Ammonia (TNAUC; µmol/L x week) |
36 | 43 | 0.08 | |||||||||
| Taking Rifaximin at Study Entry (N= 59) |
30 | 29 | ||||||||||
| Percent (#) of patients with an HE event |
43 | (13) | 45 | (13) | 0.9 | |||||||
| Percent (#) of patients with an event (WH ³ 2) |
43 | (13) | 41 | (12) | 0.9 | |||||||
| Total HE events |
28 | 26 | 0.8 | ** | ||||||||
| HE Hospitalizations |
11 | 20 | 0.1 | ** | ||||||||
| HE Hospital days |
57 | 90 | NS | |||||||||
| Patients with CHESS score ³ 3 |
9 | (30) | 15 | (52) | 0.2 | |||||||
| Ammonia (TNAUC; µmol/L x week) |
67 | 91 | 0.1 | |||||||||
| Rifaximin at Study Entry or after 1st Event (N= 69) |
31 | 38 | ||||||||||
| Percent (#) of patients with an HE event |
42 | (13) | 58 | (22) | 0.2 | |||||||
| Total HE Events |
28 | 42 | 0.6 | ** | ||||||||
| * | All analyses based on Intention to Treat Population |
| ** | Statistical analysis pertains to event rate |
16
Abbreviations: WH = West Haven, NS = not statistically significant, CHESS = Clinical Hepatic Encephalopathy Staging Scale, TNAUC = time-normalized area under the curve.
The population most similar to that enrolled in the rifaximin pivotal trial was the subgroup of patients on no rifaximin at study entry. Among these patients, there was a significant reduction in total HE events, which corresponds to an 82% reduction in the risk of experiencing a grade 2 HE event on GPB as compared with placebo.
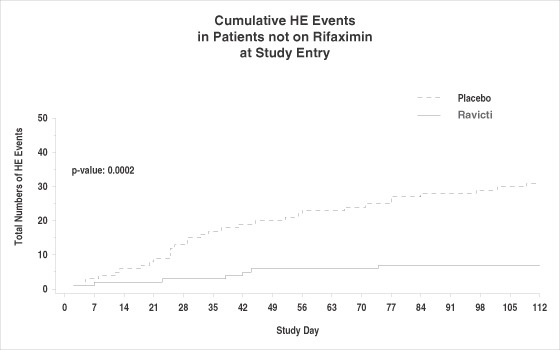
The rate of adverse events was similar on GPB versus placebo. There were three deaths in the study, two on GPB and one on placebo, all of which were judged by the clinical investigators to be unrelated to the study drug. There were 20 subjects with serious adverse events (“SAEs”) on GPB, of which one was deemed possibly related to GPB by blinded assessment at the time of the study, and 12 subjects with SAEs on placebo, of which four were deemed possibly related to the placebo by blinded assessment at the time of the study. We believe the higher number of SAEs in the GPB group reflects the greater number of Child-Pugh C patients (i.e., the most severely ill patients) randomized to GPB versus placebo (21 vs. 8).
GPB Phase III Design Planning
We had an end of Phase II meeting with the FDA in the fourth quarter of 2012 to discuss these findings and gain the FDA’s input on our plans for a Phase III trial in HE. We have initiated an SPA process with the FDA. Based on our discussion to date with the FDA, we envision a single pivotal study, similar in design to the Phase II study with respect to study population and overall design that would enroll approximately 500 patients from both the United States and other geographical areas.
During the end of Phase II meeting, the FDA expressed concern regarding the use of the West Haven grading scale, similar to the concern the FDA raised at the time of the 2010 FDA Advisory Committee meeting for rifaximin. The West Haven is a grading scale used to determine the severity of HE. It has been used by clinicians for over three decades and was used by Salix in the pivotal study of rifaximin. However, it was not developed as an outcomes tool, has never been formally validated and includes criteria such as ‘subtle change in
17
personality’ which are imprecise. At our End of Phase II meeting, the Division of Gastroenterology and Inborn Errors Products (“the review division”) indicated that we should seek consultation from the Study Endpoints and Label Development (“SEALD”), group prior to the initiation of the SPA process. The SEALD group was established by the FDA to facilitate decisions related to the approval of drugs, labels and promotional claims that are based on patient reported outcomes. Taking into account recommendations from the review division in consultation with SEALD, we developed an HE Grading Instrument, a clinician reported outcomes tool, which was received favorably by FDA. Because most HE events are anticipated to occur when patients are not at the study site, we have also developed a screening tool to be used by caregivers of the study patients which would prompt them to contact the study site in the event that the study subject exhibits manifestations which might represent an HE event. However, there is more work to be done, and we anticipate that continued discussions with the FDA regarding protocol design will result in the pivotal study commencing in the second half of 2015. Even if no formal agreement is reached with the FDA in the context of the SPA, we believe that the discussions with the FDA will be helpful and increase the probability of a successful Phase III study in HE and, ultimately, approval.
As currently designed, Part A of our Phase III pivotal study will consist of a 24 week randomized, double-blind, placebo controlled study in up to 500 adult patients who have experienced at least two documented overt HE events in the past six months, with at least one of the qualifying HE events having occurred while the subject was on stable management for HE (e.g. lactulose or rifaximin therapy). Part B of the study is a 28 week open label safety extension study. Our phase II trial indicated that 6 mL twice daily is the optimal dose. The primary endpoint is anticipated to be time to first overt HE episode of Grade 2 or above. Secondary endpoints include number of hospitalizations due to HE in all patients as well as those patients with ammonia levels above the upper limits of normal at screening, total number of HE episodes in all patients and the subgroup with ammonia levels above the upper limit of normal at screening, time to first hospitalization due to HE and time to all-cause hospitalizations.
The pivotal study is preceded by an observational study aimed at determining current standards of documentation of HE episodes at the sites targeted for the global Phase III study. Additional objectives of the observational study are to assess standard of care and health care burdens, as measured by rifaximin use and hospitalizations due to HE.
Sales, Marketing and Distribution
On February 1, 2013, the FDA granted approval of RAVICTI for use as a nitrogen-binding agent for chronic management of UCD in adult and pediatric patients greater than two years of age who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. RAVICTI is not approved for use in treating patients with HA crises for whom urgent intervention is typically necessary, patients with NAGS deficiency for whom the safety and efficacy of RAVICTI has not been established, and UCD patients under two months of age for whom RAVICTI is contraindicated due to uncertainty as to whether newborns, who may have immature pancreatic function, can effectively digest RAVICTI. In February 2013, we began commercializing RAVICTI in the U.S. and in June 2013, we began commercializing BUPHENYL inside and outside the U.S. We directly employ a small, scientifically-focused field team of six sales representatives who call on approximately 90 clinics in the US that treat UCD patients and four payor account managers who work with our five medical science liaisons to educate payors and facilitate coverage for RAVICTI.
We distribute RAVICTI through two specialty pharmacies and coordinate commercial operations through a single third party call center responsible for interfacing with patients, physicians and payors. This strategy supports a case managed approach to getting patients on treatment quickly and supporting long-term compliance. As part of our ongoing commitment to the patient community, we have implemented a comprehensive set of patient financial support services. Our call center case managers assist un-insured or under-insured patients with identifying and securing insurance. In order to assist with public and private insurance and high out-of-pocket costs, we have also made a donation to an independent foundation with a strong track record of enabling patients
18
to get affordable access to medications they seek. For uninsured patients who qualify and to assist new patients without coverage to convert to RAVICTI, we offer RAVICTI at no cost until we can help them establish coverage. As of the end of December 2014, commercial insurers responsible for approximately 95% of the covered lives in the U.S. and state Medicaid plans covering 95% of the Medicaid lives in the U.S. were reimbursing RAVICTI prescriptions.
A cornerstone of our U.S. strategy is to facilitate the transition of patients approved under FDA-labeling from NaPBA to RAVICTI. We began the transition in March 2013 concurrent with the commercial launch of RAVICTI. Based on our market research with physicians and patient preference data from our clinical trials, we anticipate that most NaPBA patients will transition to RAVICTI over time. We continue to sell BUPHENYL for any patients who are not included in the FDA-approved RAVICTI label or who have not transitioned from BUPHENYL.
As the transition of patients from NaPBA to RAVICTI is underway, we are devoting increasing efforts to expanding the number of diagnosed and treated patients through ongoing market education. As of December 31, 2014, approximately 23% of our new prescriptions (excluding clinical trial patients) were written for patients who had not previously been on NaPBA.
Outside the United States, we assumed and maintain the distribution agreements established by Ucyclyd for BUPHENYL. Swedish Orphan Biovitrum AB (“SOBI”), a related party, is the distributor of record in Europe and the Middle East and Orphan Pacific distributes BUPHENYL in Japan. We have an agreement with SOBI to support named patient sales of RAVICTI in the Middle East. If we receive approval for RAVICTI and launch in Canada, we will employ a small Canadian-based team that will work with our U.S. personnel to sell and support RAVICTI in Canada. Should we receive approval and decide to launch RAVICTI in Europe, we anticipate partnering with an EU-based company for sales and marketing support.
In the BUPHENYL market in the United States, we compete with a generic powder form of NaPBA. As of December 31, 2014, the generic powder has approximately 80% of the NaPBA powder volume. We believe there has been no impact of the generic powder sales on sales of RAVICTI or BUPHENYL tablets.
Third-Party Reimbursement in the United States
Sales of pharmaceutical products depend in significant part on the availability of coverage and adequate reimbursement by third-party payors, such as state and federal governments, including Medicare and Medicaid, managed care providers, and private insurance plans. Decisions regarding the extent of coverage and amount of reimbursement to be provided for RAVICTI and BUPHENYL are made on a plan by plan, and in some cases, patient by patient, basis. Particularly given the rarity of UCD, securing coverage and appropriate reimbursement from third-party payors requires targeted education. To that end, we employ a dedicated group of field-based payor account managers and engaging call-center based reimbursement experts focused on ensuring that clinically qualified patients have affordable access to therapy.
Within the Medicare program, as self-administered drugs, RAVICTI and BUPHENYL are reimbursed under the expanded prescription drug benefit, known as Medicare Part D. This program is a voluntary Medicare benefit administered by private plans that operate under contracts with the federal government. These Part D plans negotiate discounts with drug manufacturers, which are passed on to each of the plan’s enrollees. Historically, Part D beneficiaries have been exposed to significant out-of-pocket costs after they surpass an annual coverage limit and until they reach a catastrophic coverage threshold. However, changes in legislation will reduce this patient coverage gap, known as the donut hole, by transitioning patient responsibility in that coverage range from 100% in 2010 to only 25% in 2020. To help achieve this reduction, since 2011, pharmaceutical manufacturers have been required to pay quarterly discounts of 50% off the negotiated price of branded drugs issued to Medicare Part D patients in the donut hole.
19
An ongoing trend has been for third-party payors, including the United States government, to apply downward pressure on the reimbursement of pharmaceutical products. Also, the trend towards managed health care in the United States and the concurrent growth of organizations such as health maintenance organizations may result in lower reimbursement for pharmaceutical products. We expect that these trends will continue as these payors implement various proposals or regulatory policies, including various provisions of the recent health reform legislation that affects reimbursement of these products. There are currently, and we expect that there will continue to be, a number of federal and state proposals to implement controls on reimbursement and pricing, directly and indirectly.
Research and Development
We are conducting development activities to expand the commercial potential of RAVICTI. We sponsor and conduct clinical research activities with investigators and institutions to measure key clinical outcomes that can influence market adoption of RAVICTI.
In the years ended December 31, 2014, 2013 and 2012, we incurred $20.7 million, $10.0 million and $17.0 million, respectively, of research and development expense.
Ucyclyd Asset Purchase Agreement and Amended and Restated Collaboration Agreement
On March 22, 2012, we entered into a purchase agreement with Ucyclyd under which we purchased the worldwide rights to RAVICTI and a restated collaboration agreement under which Ucyclyd granted us an option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL at a fixed price at a future defined date, plus subsequent milestone and royalty payments, subject to Ucyclyd’s right to retain AMMONUL for a predefined price. The restated collaboration agreement superseded the collaboration agreement with Ucyclyd, dated August 23, 2007, as amended.
Asset Purchase Agreement. Under the purchase agreement, we purchased all of the worldwide rights to RAVICTI for an initial upfront payment of $6.0 million. We will also pay tiered mid to high single digit royalties on global net sales of RAVICTI and may owe regulatory milestones of up to $15.8 million related to approval of GPB in HE, regulatory milestones of up to $7.3 million per indication for approval of GPB in indications other than UCD or HE, and net sales milestones of up to $38.8 million if GPB is approved for use in indications other than UCD (such as HE) and all annual sales targets are reached. In addition, the intellectual property license agreement executed between Ucyclyd and Brusilow and the research agreement executed between Ucyclyd and Dr. Marshall L. Summar (“Summar”) were assigned to us, and we have assumed the royalty obligation under the Brusilow agreement for sales of GPB in any indication, and the royalty obligations under the Summar agreement on sales of GPB to treat HE. The Brusilow and Summar agreements provide that royalty obligations will continue, without adjustment, even if generic versions of the licensed products are introduced and sold in the relevant country.
Subject to Ucyclyd’s right to commercialize AMMONUL for UCD for as long as it owns the product, the purchase agreement prohibits Ucyclyd from developing or commercializing any product for the treatment of UCD or HE that comprises, incorporates or contains glycerol phenylbutyrate, sodium phenylbutyrate or any other active pharmaceutical ingredient that converts to PAA. This restriction is in force until the later of (a) the expiration of the last patent covering GPB in the United States, or (b) the expiry of any other market exclusivity granted by the FDA for GPB. Thereafter, the restriction will remain in force on a country-by-country basis until the later of (a) the expiration of the last patent covering GPB in the applicable country, or (b) the expiration of any other market exclusivity granted for GPB by the governing regulatory agency in the applicable country. This restriction does not prevent Ucyclyd from developing or commercializing BUPHENYL or AMMONUL for indications other than UCD or HE, and Ucyclyd retains the right to develop or commercialize the active ingredient(s) of BUPHENYL or AMMONUL for indications other than UCD or HE.
20
As part of our purchase of the worldwide rights to GPB, and in return for the payment of the royalties described above, we received a license to Ucyclyd’s manufacturing technology for use with respect to GPB. In addition, concurrent with our purchase of GPB, Ucyclyd granted us a royalty-bearing license to any developed Ucyclyd formulation technology that may be useful to our efforts with respect to GPB in UCD and HE, and we received a right to use and reference certain Ucyclyd-owned data relating to GPB.
The purchase agreement cannot be terminated by either party. However, we will have a license to certain Ucyclyd manufacturing technology, and Ucyclyd may have a license to certain of our technology, and the party granting a license will be permitted to terminate the license if the other party fails to comply with any payment obligations relating to the license and does not cure such failure within a defined time period. The license with Brusilow that was assigned to us may be terminated for any uncured breach, including of payment obligations and if we do not meet certain diligence obligations in our development and commercialization of GPB.
Amended and Restated Collaboration Agreement. On April 29, 2013, we exercised our option under the terms of the restated collaboration agreement to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and Ucyclyd elected to retain AMMONUL for a purchase price of $32.0 million. Ucyclyd paid us a net payment of $11.0 million on closing of the purchase transaction, which reflected the purchase price for BUPHENYL being set-off against Ucyclyd’s retention payment for AMMONUL and our purchase of $2.0 million in inventory. We retained a right of first negotiation should Ucyclyd later decide to sell, exclusively license, or otherwise transfer the AMMONUL assets to a third party.
We pay Ucyclyd royalties on net sales in the United States of BUPHENYL to UCD patients outside of the FDA-approved labeled age range for RAVICTI. The royalties on BUPHENYL net sales are payable at the RAVICTI royalty rate. We assumed Ucyclyd’s rights in BUPHENYL subject to its obligations to certain third-party distributors and licensees. The restated collaboration agreement provides that royalty obligations continue, without adjustment, even if generic versions of BUPHENYL are introduced and sold in the relevant country.
Following our purchase of these rights, the restated collaboration agreement cannot be terminated by either party. However, we will have a license to specified Ucyclyd manufacturing technology, and Ucyclyd will be permitted to terminate this license if we fail to comply with any payment obligations relating to the license and we fail to cure this failure within a defined time period.
Manufacturing
We currently have no manufacturing facilities and rely on third-party manufacturers to produce the active pharmaceutical ingredient (“API”) and drug products required for commercial use and for our clinical trials.
We have clinical and commercial supplies of RAVICTI API manufactured for us by two alternate suppliers, Helsinn Advanced Synthesis SA (Switzerland) (“Helsinn”) and DSM Fine Chemicals Austria (now known as DPx Fine Chemicals GmbH & Co KG (“DPx”)) on a purchase order basis. We believe our commercial requirements of API can be satisfied by Helsinn and DSM without significant delay or material additional costs. We have finished RAVICTI drug product manufactured by Lyne Laboratories, Inc. under a commercial supply agreement. We have an agreement in place for a secondary fill/finish supplier, Halo Pharmaceuticals, Inc. (“Halo”) and have obtained FDA approval of Halo as a supplier of finished drug product.
Prior to our acquisition of the worldwide rights to RAVICTI from Ucyclyd, Ucyclyd owned all manufacturing technology related to GPB, the API in RAVICTI, developed by Helsinn, other than generally applicable confidential know-how. Pursuant to the purchase agreement with Ucyclyd, Ucyclyd continues to own all GPB manufacturing technology developed as of August 23, 2007, and we own all GPB manufacturing technology developed after that date. We have a license to the GPB manufacturing technology owned by Ucyclyd.
21
When we purchased BUPHENYL under the restated collaboration agreement with Ucyclyd, we assumed all of Ucyclyd’s rights and obligations under its manufacturing agreements for the product. We obtain API for BUPHENYL on a purchase order basis from Chemie Uetikon and final manufacturing, testing and packaging of the product is provided by Pharmaceutics International Inc.
Our third-party manufacturers, their facilities and all lots of API and finished drug products are required to be in compliance with current Good Manufacturing Practices (“cGMP”). The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and finished drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports, and returned or salvaged products. The manufacturing facilities for our API’s and finished drug products must meet cGMP requirements and FDA satisfaction before any product is approved and we can manufacture commercial products. Our third-party manufacturers and their facilities, procedures and operations used in the testing and manufacture of our API and finished drug product are subject to periodic inspections by the FDA and other regulatory authorities to assess compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties. These actions could have a material impact on the availability of API and finished drug products. Contract manufacturers often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. In addition, contract manufacturers may choose to discontinue providing contract manufacturing services which could also have a material impact on the availability of our API’s and finished drug product.
Competition
We face competition from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions, among others, which may in the future develop products to treat UCD or HE. Our commercial opportunity may be reduced significantly if our competitors develop and commercialize products that are safer, more effective, more convenient, have fewer side effects or are less expensive than RAVICTI or BUPHENYL. Public announcements regarding the development of competing drugs could adversely affect the commercial potential of RAVICTI or BUPHENYL. Management of UCD involves decreasing ammonia levels through reduction of protein in the diet, amino acid supplementation and the use of ammonia lowering agents, including sodium benzoate, RAVICTI and NaPBA. Liver transplantation is an option reserved for the most severely affected patients, typically those who present very early in life. If a curative treatment for UCD is developed, RAVICTI and BUPHENYL may become obsolete for that indication.
BUPHENYL is the only branded therapy other than RAVICTI currently FDA-approved for the chronic management of patients with the most prevalent UCD. We are aware of one generic sodium phenylbutyrate tablet product manufactured by Ampolgen Pharmaceuticals, LLC, which received FDA approval in November 2011 under an ANDA and a powder form manufactured by SigmaPharm Laboratories, LLC which was approved by FDA in March 2013. Lucane Pharma SA (“Lucane”) received market authorization from the European Medicines Agency in July 2013. Lucane has announced a distribution partnership in Canada, and in January 2015 announced it had received approval from Health Canada to market its taste-masked NaPBA granules in Canada. It is our understanding that only the first phenylbutyrate-containing product approved for an indication in Canada is eligible for “data protection” which is similar to “orphan drug exclusivity” in the United States. We have been notified by Health Canada that RAVICTI is not eligible for data protection. If we cannot successfully appeal this decision to obtain data protection, we may withdraw our application for marketing approval in Canada.
We believe Lucane is also seeking approval via an Abbreviated New Drug Application (“ANDA”) in the United States. We are aware that other companies, including ForTe BV and Navinta LLC, have made efforts to develop taste masking technologies for NaPBA. We do not know whether these technologies will be introduced
22
to the market and if so, the timing or success of such introduction. In addition, Orphan Europe is conducting a clinical trial of carglumic acid to treat some of the UCD enzyme deficiencies for which RAVICTI is approved. Carglumic acid is approved to treat HA crises resulting from a rare UCD subtype and is sold under the name Carbaglu. If the results of this trial are successful and Orphan Europe is able to complete development and obtain approval of Carbaglu to treat additional UCD enzyme deficiencies, we would face competition from this compound. AMMONUL is the only FDA-approved adjunctive therapy for HA crises in patients with the most prevalent UCD. In addition, a company called Cytonet is developing a therapy for severely affected newborns which involves the infusion of human liver cells with the aim of prolonging crisis-free survival until the patients are old enough to undergo a liver transplantation. Cytonet recently filed a Marketing Authorization Application with the European Medicines Agency seeking approval for its liver cell therapy for treatment of UCD in children. In addition, Promethera has successfully completed a 20 patient Phase I/II trial in Europe of its stem cell based therapy for treatment of UCD in the pediatric population and is planning to conduct a Phase IIb/III trial. We are also aware that Aeglea Biotherapeutics has a degrading enzyme treatment in preclinical development for arginase 1 deficiency.
Currently, there is no cure for HE other than liver transplantation, which is limited by donor availability and patient eligibility. Although lactulose has been commonly used, rifaximin is the only FDA-approved therapy for reduction in risk of episodic HE recurrence. In addition to currently marketed treatments for HE, Ocera is conducting a Phase II trial of OCR 002 in patients with cirrhosis who are experiencing an HE event. To be commercially viable in HE, we must demonstrate GPB is at least as safe and effective as competitive products or can be used safely in combination. If a curative treatment for HE is developed other than liver transplantation, GPB may become obsolete for that indication.
Intellectual Property
We continue to seek patent protection in the United States and internationally for our products and product candidates. Our policy is to pursue, maintain and defend patent rights developed internally and to protect the technology, inventions and improvements that are commercially important to the development of our business. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of the existing patents upon which our product candidates rely or any patents granted to us in the future will be commercially useful in protecting our technology. We also rely on trade secrets to protect our product candidates. Our commercial success also depends in part on our non-infringement of the patents or proprietary rights of third parties. For a more comprehensive discussion of the risks related to our intellectual property, please see “Risk Factors — Risks Related to Our Intellectual Property.”
Our success depends in part on our ability to:
| • | obtain and maintain proprietary and marketing exclusivity rights for RAVICTI and BUPHENYL; |
| • | preserve trade secrets; |
| • | prevent third parties from infringing upon the proprietary rights; and |
| • | operate our business without infringing the patents and proprietary rights of third parties, both in the United States and internationally. |
RAVICTI was granted orphan drug exclusivity for the maintenance treatment of UCD, which will provide for seven years of market exclusivity from the date of the FDA approval, February 1, 2013. Additionally, we were granted orphan designation for GPB for the intermittent or chronic treatment of patients with cirrhosis and any grade HE. If we are awarded orphan drug exclusivity for HE, we would also receive seven years of orphan
23
exclusivity from the date of the FDA approval in this indication. We have licensed the rights to the GPB composition of matter patents from Brusilow, in the United States, Great Britain, Ireland, Germany, Spain and France. We will continue to have payment obligations to Brusilow under the license through 2025. The United States composition of matter patent would have expired February 7, 2015 without any term extension. However, we have applied for an extension of approximately four years under the Drug Price Competition and Patent Term Restoration Act of 1984, known as Hatch-Waxman Amendments, which if granted in full, will extend the term of this patent to 2019. We recently received notice that we have been granted an interim extension of this patent’s term through February 7, 2016 while the United States Patent and Trademark Office makes a final determination as to the length of the extension. We cannot be certain that we will be granted the full four year term of extension for which we have applied, or that we will receive any extension beyond the one year interim extension.
We also received three-year Hatch-Waxman regulatory exclusivity based on the submission of new and essential clinical trials conducted or sponsored by us in support of RAVICTI. This exclusivity prevents the FDA from giving final approval to any generic forms of RAVICTI for a period of three years from the date of the approval of RAVICTI. It also prohibits the agency from approving any 505(b)(2) NDAs that seek to reference the FDA’s approval of RAVICTI for a period of three years. This three year period runs in parallel with RAVICTI’s award of orphan drug exclusivity.
We own a variety of pending patent applications in the United States and abroad pertaining to different aspects of treatment with both RAVICTI and BUPHENYL. These patent applications, which are summarized below, provide the potential for additional patent protection extending to 2029 and beyond. However, there is a significant risk that these pending applications will not issue, or that they may issue with substantially narrower claims than those that are currently sought. There is also a risk that issued claims will not directly relate to labeled usages of RAVICTI and BUPHENYL, and therefore may be ineffective in keeping competitors out of the market.
We own issued patents in the United States and Japan and pending patent applications in Europe, Japan and Canada directed to methods of using, administering, and adjusting the dosage of drugs that operate via the active chemical entity PAA, including RAVICTI and BUPHENYL, based in part on target urinary PAGN levels. In February 2014, U.S. Patent 8,642,012 entitled “Methods of Treatment Using Ammonia Scavenging Drugs” issued from U.S. Patent Appl. No. 12/350,111 with claims directed to methods of treating UCD using PAA prodrugs based in part on analysis of target urinary PAGN levels. This patent will expire in September 2030 with PTE and is listed in the Orange Book. Any additional patents issuing from this family of applications will expire in 2029.
We own pending patent applications in the United States and internationally pursuant to the Patent Cooperation Treaty (“PCT”) directed to methods of treating nitrogen retention disorders and determining dosage of nitrogen scavenging drugs, including RAVICTI and BUPHENYL, based in part on fasting ammonia level measurements. In March 2013, U.S. Patent No. 8,404,215 entitled “Methods of Therapeutic Monitoring of Nitrogen Scavenging Drugs,” issued from U.S. Patent Appl. No. 13/417,137 with claims directed to methods of adjusting and optimizing the dosage of nitrogen scavenging drugs based on analysis of target fasting ammonia levels. This patent will expire in March 2032, and is currently listed in the Orange Book. Any additional patents issuing from this family of applications will also expire in 2032.
We own an issued patent in the United States and pending patent applications in the United States and internationally pursuant to the PCT directed to methods of treating nitrogen retention disorders and determining dosage of nitrogen scavenging drugs, including RAVICTI and BUPHENYL, based in part on PAA metabolite levels. Any patents issuing from this family of applications will expire in 2032.
We own pending patent applications in the United States and internationally pursuant to the PCT that pertain to methods of treating patients with HE and optimizing dosage of nitrogen scavenging drugs, including RAVICTI and BUPHENYL, for treating HE based in part on fasting ammonia level measurements. Any patents issuing from this family of applications will expire in 2033.
24
We own pending patent applications in the United States and internationally pursuant to the PCT that pertain to methods and systems for diagnosing, grading and treating HE episodes. Any patents issuing from this family of applications will expire in 2034.
We own pending patent applications in the United States and internationally pursuant to the PCT that pertain to methods for treating UCD and determining dosage of nitrogen scavenging drugs, including BUPHENYL and RAVICTI, based on body surface area, and methods of evaluating compliance with nitrogen scavenging drug treatment regimens based in part on analysis of urinary PAGN levels. Any patents issuing from this family of applications will expire in 2034.
We own a provisional patent application in the United States pertaining to methods of preventing hyperammonemic crises (HACs) and optimizing the dosage of nitrogen scavenging drugs, including BUPHENYL and RAVICTI, for preventings HACs, based in part on analysis of fasting ammonia levels. If nonprovisional applications are filed claiming priority to this provisional application, any patents issuing therefrom would expire in 2035. However, it is possible that no such nonprovisional applications will be filed.
We also protect our proprietary technology and processes, in part, by confidentiality and invention assignment agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Regulatory Matters
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act and related regulations. Drugs are also subject to other federal, state and local statutes and regulations. Failure to comply with the applicable United States regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an Institutional Review Board (“IRB”) of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising and promotion of our products.
The FDA’s policies may change and additional laws or regulations may be enacted that could affect our regulatory approval of RAVICTI or prevent or delay any future product candidates’ regulatory approval or approval of our current products in new disease indications or labeling changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad
25
Marketing Approval
The process required by the FDA before drugs may be marketed in the United States generally involves the following:
| • | nonclinical laboratory and animal tests; |
| • | submission of an IND application, which must become effective before clinical trials may begin; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use or uses; |
| • | pre-approval inspection of manufacturing facilities and clinical trial sites; and |
| • | FDA approval of an NDA, which must occur before a drug can be marketed or sold. |
The testing and approval process requires substantial time and financial resources, and we cannot be certain that any new approvals for our product candidates will be granted on a timely basis if at all.
Our planned clinical trials for our product candidates may not begin or be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays in:
| • | obtaining regulatory approval to commence a study; |
| • | reaching agreement with third-party clinical trial sites and their subsequent performance in conducting accurate and reliable studies on a timely basis; |
| • | obtaining institutional review board approval to conduct a study at a prospective site; |
| • | recruiting patients to participate in a study; and |
| • | supply of the drug. |
Prior to commencing the first clinical trial, an initial IND application must be submitted to the FDA. The IND application automatically becomes effective 30 days after receipt by the FDA unless the FDA within the 30-day time period raises concerns or questions about the conduct of the clinical trial and places it on hold. In such case, the IND application sponsor must resolve any outstanding concerns with the FDA before the clinical trial may begin. A separate submission to the existing IND application must be made for each successive clinical trial to be conducted during product development. Further, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. Informed consent must also be obtained from each study subject. Regulatory authorities, an IRB, a data safety monitoring board or the sponsor, may suspend or terminate a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable health risk.
For purposes of NDA approval, human clinical trials are typically conducted in phases, which may overlap:
| • | Phase I — the drug is initially given to healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. These studies may also gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational drug’s pharmacokinetics and pharmacologic effects may be obtained to permit the design of well-controlled and scientifically valid Phase II clinical trials. |
26
| • | Phase II — studies are conducted in a limited number of patients in the target population to identify possible adverse effects and safety risks, determine the efficacy of the product for specific targeted diseases and determine dosage tolerance and optimal dosage. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase III clinical trials. |
| • | Phase III — when Phase II evaluations demonstrate that a dosage range of the product appears effective and has an acceptable safety profile, and provide sufficient information for the design of Phase III studies, Phase III trials are undertaken to provide statistically significant evidence of clinical efficacy and to further test for safety in an expanded patient population at multiple clinical study sites. They are performed after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to further evaluate dosage, effectiveness and safety, to establish the overall benefit-risk relationship of the investigational drug, and to provide an adequate basis for product approval by the FDA. |
| • | Phase IV — post-marketing studies, or Phase IV clinical trials, may be conducted after initial marketing approval. These studies may be required by the FDA as a condition of approval and are used to gain additional experience from the treatment of patients in the intended therapeutic indication. The FDA also now has express statutory authority to require post-market clinical studies to address safety issues. |
All of these trials must be conducted in accordance with GCP, in order for the data to be considered reliable for regulatory purposes.
Government regulation may delay or prevent marketing of product candidates or new drugs for a considerable period of time and impose costly procedures upon our activities. We cannot be certain that the FDA or any other regulatory agency will grant approvals for any future product candidates on a timely basis, if at all. Success in early stage clinical trials does not ensure success in later stage clinical trials. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval.
The NDA Approval Process
In order to obtain approval to market a new drug in the United States, a marketing application must be submitted to the FDA that provides data establishing to the FDA’s satisfaction the safety and effectiveness of the investigational drug for the proposed indication. Each NDA submission requires a substantial user fee payment unless a waiver or exemption applies. The application includes all relevant data available from pertinent nonclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators.
The FDA will initially review the NDA for completeness before it accepts it for filing. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use or uses, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an advisory committee, typically a panel of outside experts that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
27
Based on the clinical trial results submitted in an NDA, upon the request of an applicant, the FDA may grant a priority review designation to a product, which sets the target date for FDA action on the application at six months, rather than the standard ten months, in each case with an additional two months in the case of a new chemical entity. Priority review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a significant improvement compared to marketed products or offers a therapy where no satisfactory alternative therapy exists. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
After the FDA completes its initial review of an NDA, it will communicate to the sponsor that the drug will either be approved, or it will issue a complete response letter to communicate that the NDA will not be approved in its current form and inform the sponsor of changes that must be made or additional clinical, nonclinical or manufacturing data that must be received before the application can be approved, with no implication regarding the ultimate approvability of the application.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical sites to assure compliance with GCP. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the NDA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process for a drug requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase IV studies may be made a condition to be satisfied for continuing drug approval. The results of Phase IV studies can confirm the effectiveness of a product candidate and can provide important safety information. In addition, the FDA has express statutory authority to require sponsors to conduct post-market studies to specifically address safety issues identified by the agency.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of onerous risk management plans, restrictions on distribution, or post-marketing study requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. In addition, we cannot predict what adverse governmental requirements may arise from future U.S. or foreign governmental action.
FDA Post-Approval Requirements
Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping and reporting of adverse experiences with the drug. Drug manufacturers are required to register their facilities with the FDA and certain state agencies, and are
28
subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain quality processes, manufacturing controls and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality and purity characteristics that it purports to have. Certain states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, fail to approve any NDA or other application, require us to recall a drug from distribution, shut down manufacturing operations or withdraw approval of the NDA for that drug. Noncompliance with cGMP or other requirements can result in issuance of warning letters, civil and criminal penalties, seizures and injunctive action.
Labeling, Marketing and Promotion
The FDA closely regulates the labeling, marketing and promotion of drugs. While doctors are free to prescribe any drug approved by the FDA for any use, a company can only make claims relating to safety and efficacy of a drug that are consistent with the FDA approval, and the company is allowed to market a drug only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising, injunctions, costly government investigations, and potential civil and criminal penalties.
Orphan Designation and Exclusivity
RAVICTI received orphan designation for maintenance treatment of patients with deficiencies in enzymes of the urea cycle and subsequently received exclusivity in the United States upon approval by the FDA for the maintenance treatment of patients with UCD, and GPB has a separate orphan designation for intermittent or chronic management of patients with cirrhosis and episodic HE of any grade. However, GPB cannot be considered for exclusivity until it has been approved by FDA for the treatment of HE. Under the Orphan Drug Act, the FDA may grant orphan designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States. Orphan designation must be requested before submitting an NDA.
Orphan designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. If a product that has orphan designation is the first such product to receive FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that subsequent to approval the FDA may not approve any other applications to market a drug with the same active moiety for the same disease, except in limited circumstances, for seven years. During orphan exclusivity, the FDA may only permit additional companies to market a drug with the same active chemical entity for the designated condition if such companies can demonstrate clinical superiority for the new drug, or if the company with the orphan drug exclusivity is not able to meet market demand. More than one product may also be approved by the FDA for the same orphan indication or disease as long as the products contain different active ingredients. As a result, even though RAVICTI has received orphan exclusivity, the FDA can still approve other drugs that have a different active chemical entity for use in treating the same indications or diseases covered by RAVICTI, which could create a more competitive market for us.
29
Anti-Kickback and False Claims Laws
In the United States, the research, manufacturing, distribution, sale and promotion of drug products are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General, and other state and local government agencies. For example, sales, marketing and scientific/educational grant programs must comply with the Medicare-Medicaid Anti-Fraud and Abuse Act, (the “Anti-Kickback Statute”), the False Claims Act, the privacy regulations promulgated under the Health Insurance Portability and Accountability Act (“HIPAA”), and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, and the Veterans Health Care Act of 1992. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by prison sentences, criminal fines, administrative civil money penalties, and exclusion from participation in federal healthcare programs. In addition, many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to the referral of patients for healthcare services reimbursed by any insurer, not just federal healthcare programs such as Medicare and Medicaid. Due to the breadth of these federal and state anti-kickback laws, the absence of guidance in the form of regulations or court decisions, and the potential for additional legal or regulatory change in this area, it is possible that our future sales and marketing practices and/or our future relationships with physicians might be challenged under anti-kickback laws, which could harm us.
The federal False Claims Act prohibits anyone from knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including drugs, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Although we would not submit claims directly to federal programs, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state, and third-party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, pharmaceutical companies have been prosecuted under the federal False Claims Act in connection with their off-label promotion of drugs. Penalties for a False Claims Act violation include three times the actual damages sustained by the government, plus mandatory civil penalties for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the federal False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes. If the government were to allege that we were, or if we were convicted of, violating these false claims laws, we could be subject to a substantial fine and may suffer a decline in our stock price. In addition, private individuals have the ability to bring actions under the federal False Claims Act as “whistleblowers” acting in the name of the federal government and may share in any recovery in the event the action is successful. Certain states have also enacted laws modeled after the federal False Claims Act.
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information or impose other special requirements on companies that sell and market drug products. In addition, as discussed below, as of 2013, a federal requirement requires manufacturers to track and report to the federal government certain payments made to physicians and teaching hospitals made in the previous
30
calendar year. Many of these laws contain ambiguities as to what is required to comply with the laws. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state and federal authorities.
Patient Protection and Affordable Care Act
The 2010 federal health care reform law, commonly known as the “Affordable Care Act,” includes measures that have or will significantly change the way health care is financed by both governmental and private insurers. In addition to the federal transparency law discussed above, the provisions of the Affordable Care Act of greatest importance to the pharmaceutical industry are the following:
| • | The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The Affordable Care Act made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs and biologic agents from 15.1% of average manufacturer price (“AMP”) to 23.1% of AMP, modifying the statutory definition of AMP, and adding a new rebate calculation for “line extensions” (i.e., certain new formulations, such as extended release formulations) of solid oral dosage forms of branded products. The Affordable Care Act also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization and by expanding the population potentially eligible for Medicaid drug benefits. The Centers for Medicare and Medicaid Services (“CMS”) have proposed to expand Medicaid rebate liability to the territories of the United States as well. In addition, the Affordable Care Act provides for the public availability of retail survey prices and certain weighted average AMPs. The implementation of this requirement by the CMS may also provide for the public availability of pharmacy data on cost of acquisition of cost data, which could negatively impact our sales. |
| • | In order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer The Affordable Care Act expanded the types of entities eligible to receive discounted 340B pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs when used for the orphan indication. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase. |
| • | The Affordable Care Act imposes a requirement on manufacturers of branded drugs and biologic agents to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., “donut hole”). |
| • | The Affordable Care Act imposes an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications. We qualify for exemption from payment of this fee as our products are approved only for orphan indications. |
| • | The Affordable Care Act and implementing regulations require pharmaceutical manufacturers to track and report annually certain financial arrangements with physicians and teaching hospitals, including any |
31
| “transfer of value” made or distributed to such entities, as well as any ownership or investment interests held by physicians and their immediate family members. Reports submitted under these requirements are placed in a public database. |
| • | A Patient-Centered Outcomes Research Institute was established pursuant to the Affordable Care Act to oversee, identify priorities in, and fund comparative clinical effectiveness research. The research supported by the Patient-Centered Outcomes Research Institute may affect the market for certain pharmaceutical products. |
| • | The Affordable Care Act created the Independent Payment Advisory Board which, as of 2014, has authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription drugs. Under certain circumstances, these recommendations will become law unless Congress enacts legislation that will achieve the same or greater Medicare cost savings. |
| • | The Affordable Care Act established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. Funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation from 2011 to 2019. |
Many of the provisions implementing the Affordable Care Act have only recently become effective, and at this time, it remains unclear the full effect that the Act will have on our business.
Authorization Procedures in the European Union
There are two types of marketing authorization procedures for medicinal products in the EU; the centralized authorization procedure and national authorization procedures as described below. In June 2014, we submitted a Marketing Authorisation Application for RAVICTI under the centralized procedure.
| • | Centralized procedure. The centralized procedure gives rise to marketing authorizations that are valid throughout the European Union and, by extension, in three European Economic Area, or EEA member states, Norway, Iceland and Liechtenstein. Applicants file marketing authorizations with the EMA, where they are reviewed by a relevant scientific committee, which is most likely the Committee for Medicinal Products for Human Use, or CHMP. The EMA forwards CHMP positive opinions to the European Commission, which uses them as the basis for a decision granting a marketing authorization. The centralized procedure is compulsory for human medicines that are: derived from biotechnology processes, such as recombinant DNA technology, controlled expression of genes in prokaryotes and eukaryotes and hybridoma and monoclonal antibody methods. It is also mandatory for products containing a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders, viral diseases or autoimmune diseases and other immune dysfunctions, and officially designated orphan medicines. For medicines that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA, as long as the CHMP accepts that the medicine concerned is a significant therapeutic, scientific or technical innovation, or if its authorization would be in the interest of public health. |
| • | National authorization procedures. There are also two other possible routes to authorize medicinal products in more than one EU or EEA country, which are available for investigational drug products that fall outside the scope of the centralized procedure: |
| • | Decentralized procedure. Using the decentralized procedure, an applicant may apply for simultaneous authorization in more than one EEA country of medicinal products that have not yet |
32
| been authorized in any EEA country and that do not fall within the mandatory scope of the centralized procedure. The applicant selects a so-called reference member state, or RMS, to take the lead in the review of the application. Other member states are expected to recognize the RMS decision, unless they identify a serious risk to public health. If the member states cannot resolve any such concerns between themselves, the matter is referred to the CHMP for an opinion and ultimately a binding European Commission decision. |
| • | Mutual recognition procedure. In the mutual recognition procedure, a medicine is first authorized in one EEA RMS, in accordance with the national procedures of that country. Following this, further marketing authorizations can be sought from other EEA countries in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization. As in the decentralized procedure, these concerned member states must recognize the RMS approval unless they identify a serious risk to the public health. If the member states cannot reach a consensus between themselves, the matter can be referred to the CHMP. |
Accelerated Review (European Union)
Under the centralized procedure in the EU, the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, defined by three cumulative criteria: the seriousness of the disease (e.g. heavy disabling or life-threatening diseases) to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days. Our application is being evaluated on the standard 210 day review basis, and we are in the midst of a “clock stop”.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We also offer our products for sale to federal government purchasers, which requires compliance with certain Federal Acquisition Regulation requirements and pricing requirements under the Veterans Health Care Act. We may incur significant costs to comply with such laws and regulations now or in the future.
Corporate Information and Employees
Our principal corporate offices are located at 2000 Sierra Point Parkway, Suite 400, Brisbane, California 94005 and our telephone number is (650) 745-7802. We were incorporated in Delaware on November 1, 2006 and completed our initial public offering in July 2012. We had 80 full-time employees as of December 31, 2014. None of our employees are represented by any collective bargaining unit. We believe that we maintain good relations with our employees.
Available Information
Our website address is www.hyperiontx.com. We make available on our website, free of charge, our Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as soon as reasonably practicable after we electronically file such
33
material with, or furnish it to, the Securities and Exchange Commission (the “SEC”). Our SEC reports can be accessed through the Investors section of our website. Further, a copy of this Annual Report on Form 10-K is located at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D. C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements and other information regarding our filings at http://www.sec.gov. The information found on our website is not incorporated by reference into this Annual Report on Form 10-K or any other report we file with or furnish to the SEC.
34
Our headquarters is currently located in Brisbane, California, and consists of approximately 20,468 square feet of leased office space under a lease that expires on November 18, 2019. We may require additional space and facilities as our business expands.
On March 17, 2014, we received notification of the Paragraph IV certification from Par Pharmaceutical, Inc. (“Par”) advising us that it had filed an Abbreviated New Drug Application (an “ANDA”) with the FDA for a generic version of 1.1 gm/ml RAVICTI oral liquid. The Paragraph IV certification, filed on November 19, 2013, alleges that our U.S. Patent No. 8,404,215, titled “Methods of therapeutic monitoring of nitrogen scavenging drugs,” which expires in March 2032, and U.S. Patent No. 8,642,012, titled “Methods of treatment using ammonia scavenging drugs,” which expires in September 2030, are invalid and/or will not be infringed by Par’s manufacture, use or sale of the product for which the ANDA was submitted. Par did not challenge the validity, enforceablility, or infringement of U.S. Patent No. 5,968,979, our primary composition of matter patent for RAVICTI titled “Triglycerides and ethyl esters of phenylalkanoic acid and phenylalkenoic acid useful in treatment of various disorders,” which patent has received an interim term extension through February 7, 2016 as the United States Patent and Trademark Office determines the ultimate length of the patent’s term extension. We filed suit against Par on April 23, 2014 in Federal district court in the Eastern District of Texas in order to protect our patents. On July 22, 2014, Par filed a motion to dismiss for lack of personal jurisdiction, or in the alternative, a motion to transfer the case to the Southern District of New York. We will oppose the motion.
On September 22, 2014, Clal Biotechnology Industries, Ltd., (“CBI”) filed suit against us in Superior Court of the state of Delaware for New Castle County (Case No. N14C-09-198 PRW) alleging breach of contract and breach of the implied covenant of good faith and fair dealing under our Share Purchase Agreement with CBI relating to our acquisition of Andromeda Biotech Ltd. (“Andromeda”), alleging $200.0 million in damages arising from our announcement on September 8, 2014 of our decision to terminate further development of DiaPep277 beyond completion of the ongoing clinical trial as a result of evidence we uncovered that certain employees of Andromeda engaged in serious misconduct that compromised clinical trial results. On January 16, 2015, CBI dismissed its claim against us without prejudice. On February 16, 2015 we reached an agreement with CBI and Yeda, the company from which Andromeda licenses the underlying DiaPep277 technology, to resolve DiaPep277 related claims against one another, and we granted CBI an option to acquire all of the outstanding stock of Andromeda. In connection with the agreement, CBI will transfer to us $2.5 million in shares of our common stock it holds, valued at the average closing price of the Company’s common stock for the fifteen trading days ending on February 11, 2015. The parties have appointed a steering committee to oversee the completion of the clinical trial with representatives of CBI and Yeda and a non-voting member appointed by us. The Company’s estimated budget from October 1, 2014 through the completion of the trial remains unchanged at $10.5 million. Any increase to this budget beyond $2.25 million, if incurred as a result of the direction of the steering committee, shall require CBI to reimburse those expenses in shares of the Company’s common stock. Under the agreement, CBI’s option is exercisable through September 30, 2015 for $3.5 million payable in shares of the Company’s common stock. In addition, if the option is exercised, then Andromeda will be obligated to pay Hyperion future contingent payments if and to the extent it or its shareholders receive revenues or certain other proceeds, which are capped at $36.5 million. This amount, together with the option exercise price that Hyperion may receive, approximates the total amount Hyperion will have invested in Andromeda by the option exercise date. Under the agreement, in the event that CBI does not exercise its option, the underlying DiaPep277 technology will revert to Yeda under the license agreement between Andromeda and Yeda. Also on February 16, 2015, we entered into a release with Evotec pursuant to which Evotec released its previously asserted claims to a milestone payment from us in connection with our acquisition of Andromeda and that it had suffered harm from recent incidents in relation to Diapep277in exchange for a payment of $500,000 from us.
35
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following management’s discussion and analysis should be read in conjunction with our audited consolidated financial statements and related notes that appear elsewhere in this Annual Report on Form 10-K. This Management’s Discussion and Analysis contains forward-looking statements that involve risks and uncertainties. Please see “Special Note Regarding Forward-Looking Statements” for additional factors relating to such statements, and see “Risk Factors” in Item 1 of this report for a discussion of certain risk factors applicable to our business, financial condition, and results of operations. Operating results are not necessarily indicative of results that may occur in future periods.
Overview
We are a commercial biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat orphan diseases. Our products, RAVICTI ® (glycerol phenylbutyrate) Oral Liquid, BUPHENYL® and AMMONAPS® (sodium phenylbutyrate) Tablets and Powder, are designed to lower ammonia in the blood. Ammonia is produced in the intestine after a person eats protein and is normally detoxified in the liver by conversion to urea. Elevated levels of ammonia are potentially toxic and can lead to severe medical complications which may include death. We have developed RAVICTI, which we launched during the first quarter of 2013, to treat most urea cycle disorders (“UCD”) including 7 of the 8 and the most prevalent UCD subtypes, and are developing glycerol phenylbutyrate (“GPB”), the active pharmaceutical ingredient in RAVICTI, to treat hepatic encephalopathy (“HE”). UCD and HE are diseases in which blood ammonia is elevated. UCD are inherited rare genetic diseases caused by a deficiency of one or more enzymes or transporters that constitute the urea cycle, which in a healthy individual removes ammonia through its conversion to urea. We estimate there are approximately 2,100 cases of UCD in the United States of which approximately 1,100 have been diagnosed. However, we estimate that only about 675 patients are currently treated with an FDA approved medication. HE may develop in some patients with liver scarring, known as cirrhosis, or acute liver failure and is a chronic complication of cirrhosis which fluctuates in severity and may lead to serious neurological damage.
On February 1, 2013, the U.S. Food and Drug Administration (“FDA”) granted approval of RAVICTI for chronic management of UCD in adult and pediatric patients greater than two years of age who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. Limitations of use include treatment of patients with acute hyperammonemia (“HA”) crises for whom urgent intervention is typically necessary, patients with N-acetylglutamate synthetase (“NAGS”) deficiency for whom the safety and efficacy of RAVICTI has not been established, and UCD patients under two months of age for whom RAVICTI is contraindicated due to uncertainty as to whether newborns, who may have immature pancreatic function, can effectively digest RAVICTI.
36
We originally obtained rights to develop RAVICTI in 2007 pursuant to a collaboration agreement with Ucyclyd Pharma, Inc. (“Ucyclyd”), a subsidiary of Valeant Pharmaceuticals International, Inc. In March 2012, we purchased the worldwide rights to RAVICTI for an upfront payment of $6.0 million, future payments based upon the achievement of regulatory milestones in indications other than UCD, sales milestones, and mid to high single digit royalties on global net sales of RAVICTI. Pursuant to an amended and restated collaboration agreement (the “restated collaboration agreement”), with Ucyclyd entered into in March 2012, we had an option to purchase all of Ucyclyd’s worldwide rights to BUPHENYL ® (sodium phenylbutyrate) Tablets and Powder, an FDA approved therapy for treatment of the most prevalent UCD and AMMONUL ® (sodium phenylacetate and sodium benzoate) injection 10%/10%, the only adjunctive therapy currently FDA-approved for the treatment of HA crises in UCD patients, for an upfront payment of $22.0 million, plus subsequent milestone and royalty payments. On April 29, 2013, we exercised our option to acquire BUPHENYL and AMMONUL from Ucyclyd and subsequently, Ucyclyd exercised its option to retain AMMONUL. On May 31, 2013, we completed the acquisition of BUPHENYL and we received a net payment of $11.0 million which reflected the $32.0 million contractual purchase price for AMMONUL due to us less the $19.0 million contractual purchase price for BUPHENYL due to Ucyclyd and $2.0 million payment due to Ucyclyd for inventory we purchased from Ucyclyd.
Although the price of BUPHENYL per gram is approximately one fifth that of RAVICTI and prices for both therapies vary among patients because doses are individualized based on a patient’s weight and disease severity, most patients cannot afford to pay for either medication themselves. We have engaged a dedicated team at a third party call center, which serves as an integrated resource for prescription intake and distribution, reimbursement adjudication, patient financial support, and ongoing compliance support for our UCD patients. Together with distribution via two specialty pharmacies, we believe these services provide important support to UCD patients and their physicians, and help them achieve more favorable outcomes in managing their disease. As part of our ongoing commitment to the patient community, we provide our UCD products at no cost to patients as we help them establish insurance coverage for our UCD products and donate to an independent foundation with an established track record of enabling patients to access medications affordably.
RAVICTI was granted orphan drug exclusivity in the United States for the maintenance treatment of patients with UCD shortly after its FDA approval in 2013. This exclusivity extends through February 1, 2020. RAVICTI has also received orphan drug designation in the European Union (“EU”), although the right to marketing exclusivity cannot be determined until we are authorized to market it in the EU. In March 2013, U.S. Patent No. 8,404,215 entitled “Methods of Therapeutic Monitoring of Nitrogen Scavenging Drugs” issued from U.S. Patent Appl. No. 13/417,137 with claims directed to methods of optimizing the dosage of nitrogen scavenging drugs based on target fasting ammonia levels. This patent will expire in March 2032, and is currently listed in the FDA publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” known as the Orange Book. In February 2014, U.S. Patent 8,642,012 entitled “Methods of Treatment Using Ammonia Scavenging Drugs” issued from U.S. Patent Appl. No. 12/350,111 with claims directed to methods of treating patients with UCD using phenylacetic acid (“PAA”) prodrugs based in part on target urinary phenylacetylglutamine (“PAGN”) levels. This patent will expire in September 2030 with Patent Term Extension (“PTE”) and is listed in the Orange Book.
On July 31, 2012, we completed our initial public offering (“IPO”) and issued 5,000,000 shares of our common stock at an initial offering price of $10.00 per share. We sold an additional 750,000 shares of common stock directly to our underwriters when they exercised their over-allotment option in full at the initial offering price of $10.00 per share. Our shares began trading on the NASDAQ Global Market on July 26, 2012. We received net proceeds from the IPO of $51.3 million, after deducting underwriting discounts and commissions of $4.0 million and expenses of $2.2 million.
As of December 31, 2014, we had an accumulated deficit of $128.6 million. We had net loss of $6.3 million for the year ended December 31, 2014. We had a net income of $16.6 million and a net loss $32.3 million for the years ended December 31, 2013 and 2012, respectively. We anticipate that a substantial portion of our capital
37
resources and efforts in the foreseeable future will be focused on completing the development and obtaining regulatory approval of GPB in HE and the identification and potential development of additional new product candidate.
On March 13, 2013, we completed a follow-on offering and issued 2,875,000 shares of our common stock at an offering price of $20.75 per share. In addition, we sold an additional 431,250 shares of common stock directly to our underwriters when they exercised their over-allotment option in full at an offering price of $20.75 per share. We received net proceeds from the offering of $63.7 million, after deducting underwriting discounts and commissions of $4.1 million and expenses of $0.8 million.
On August 14, 2013, we filed a shelf registration statement on Form S-3, which was declared effective by the SEC on September 13, 2013. The shelf registration statement permits: (a) the offering, issuance and sale of up to a maximum aggregate offering price of $150.0 million of our common stock, preferred stock, debt securities, warrants and/or units; (b) the sale of up to 8,727,000 shares of common stock by certain selling stockholders; and (c) the offering, issuance and sale of up to a maximum aggregate offering price of $50.0 million of our common stock that may be issued and sold under a sales agreement with Cantor Fitzgerald & Co. As of December 31, 2014, there were no sales of any securities registered pursuant to the shelf registration statement.
Financial Overview
Revenue
Our product revenues consist of the following:
| • | Revenues from the sale of RAVICTI which was approved by the FDA on February 1, 2013 and was commercially launched in the U.S. during the period ended March 31, 2013; and |
| • | Revenues from the sale of BUPHENYL which we acquired from Ucyclyd on May 31, 2013, pursuant to the restated collaboration agreement. We currently distribute BUPHENYL in the U.S. and through third party distributors in certain countries outside the U.S. |
See “Results of Operations” below for a more detailed discussion on revenues.
Cost of Sales
Our cost of sales includes third-party manufacturing costs, royalty fees payable under our restated collaboration agreement with Ucyclyd and other indirect costs including compensation cost of personnel, shipping and supplies.
We recorded costs incurred prior to the FDA approval of RAVICTI as research and development expenses in our consolidated statements of operations. We expect that cost of sales relating to RAVICTI as a percentage of revenue will increase in future periods as product manufactured prior to FDA approval, and therefore fully expensed, has been utilized. The cost of BUPHENYL sales as a percentage of revenue was higher prior to the second quarter of 2014, due to the recording of the step-value on BUPHENYL inventories acquired from Ucyclyd which was expensed to cost of sales as the inventory was sold.
38
Research and Development Expenses
We recognize research and development expenses as they are incurred. Our research and development expenses consist primarily of:
| • | salaries and related expenses for personnel, including expenses related to stock options or other stock-based compensation granted to personnel in development functions; |
| • | fees paid to clinical consultants, clinical trial sites and vendors, including clinical research organizations (“CROs”), in conjunction with implementing and monitoring our clinical trials and acquiring and evaluating clinical trial data, including all related fees, such as for investigator grants, patient screening fees, laboratory work and statistical compilation and analysis; |
| • | other consulting fees paid to third parties; |
| • | expenses related to production of clinical supplies, including fees paid to contract manufacturers; |
| • | expenses related to license fees and milestone payments under in-licensing agreements; |
| • | expenses related to compliance with drug development regulatory requirements in the United States, the European Union and other foreign jurisdictions; |
| • | depreciation and other allocated expenses; and |
| • | expenses incurred to manufacture RAVICTI prior to FDA approval. |
We expense both internal and external research and development expenses as they are incurred. We developed RAVICTI in UCD and GPB for HE in parallel, and we typically use our employees, consultants and infrastructure resources across our three programs. Thus, some of our research and development expenses are not attributable to an individual program, but rather are allocated across our three clinical stage programs and these costs were included in unallocated costs as detailed below. In 2012, unallocated costs included $5.7 million incurred in connection with the purchase of RAVICTI from Ucyclyd. Allocated expenses include salaries, stock-based compensation and related benefit expenses for our employees, consulting fees and fees paid to clinical suppliers. The following table shows our research and development expenses for the years ended December 31, 2014, 2013 and 2012 (in thousands):
| Year Ended December 31, | ||||||||||||
| (in thousands) | 2014 | 2013 | 2012 | |||||||||
| UCD Program |
$ | 10,588 | $ | 8,127 | $ | 3,463 | ||||||
| HE Program |
3,786 | 1,228 | 2,233 | |||||||||
| DiaPep 277 Program |
5,411 | — | — | |||||||||
| Unallocated |
930 | 629 | 11,350 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 20,715 | $ | 9,984 | $ | 17,046 | ||||||
|
|
|
|
|
|
|
|||||||
We expect our research and development expenses to increase for the initiation of our Phase 3 trial of GPB for the treatment of patients with episodic HE and the completion of the DIA AID 2 clinical trial of DiaPep 277 and costs associated with the termination of this clinical program. Due to the inherently unpredictable nature of product development, we are currently unable to exactly predict the expenses we will incur or the duration of the HE trial. Based on our current discussions with FDA, our estimate of the cost of our Phase 3 trial of GPB in HE will be between $50.0 million to $55.0 million from initiation of the trial until submission of data to FDA. Our estimated cost for the completion of the DIA AID 2 Phase 3 clinical trial from January 2015 forward is between $4.0 million and $6.0 million.
39
Our research and development expenditures are subject to numerous uncertainties in timing and cost to completion. Development timelines, the probability of success and development expenses can differ materially from expectations. Clinical trials in orphan diseases, such as UCD and HE, may be difficult to enroll given the small number of patients with these diseases. Completion of clinical trials may take several years or more, but the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
| • | the number of trials required for approval; |
| • | the number of sites included in the trials; |
| • | the length of time required to enroll suitable patients; |
| • | the number of patients that participate in the trials; |
| • | the drop-out or discontinuation rates of patients; |
| • | the duration of patient follow-up; |
| • | the number and complexity of analyses and tests performed during the trial; |
| • | the phase of development of the product candidate; and |
| • | the efficacy and safety profile of the product candidate. |
Our expenses related to clinical trials are based on estimates of patient enrollment and related expenses at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and contract research organizations that conduct and manage clinical trials on our behalf. We generally accrue expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity according to the protocol. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, we modify our estimates of accrued expenses accordingly on a prospective basis.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of salaries, benefits and stock-based compensation for employees in administration, finance and business development, legal, investor relations, marketing, commercial and sales functions, including fees to third party vendors providing customer support services. Other significant expenses include consulting fees, allocated facilities expenses and professional fees for accounting and legal services, including legal services associated with obtaining and maintaining patents. We expect that our selling, general and administrative expenses will increase due to the continued expansion of the commercialization of RAVICTI and marketing of BUPHENYL. We expect these increases will likely include increased expenses for insurance, expenses related to the hiring of additional personnel and payments to outside consultants, lawyers and accountants.
On March 17, 2014, we received notification of a Paragraph IV certification from the generic drug manufacturer Par Pharmaceutical, Inc. (“Par”) that it had filed an Abbreviated New Drug Application with the FDA seeking approval for a generic version of RAVICTI Oral Liquid. The Paragraph IV certification alleges that certain our patents are invalid and/or will not be infringed by Par’s manufacture, use or sale of the product for
40
which the ANDA was submitted. We filed suit against Par on April 23, 2014 to protect our patents and to obtain a stay of the FDA’s approval of Par’s ANDA. On July 22, 2014, Par filed a motion to dismiss for lack of personal jurisdiction, or in the alternative, a motion to transfer the case to the Southern District of New York. We will oppose this motion.
In 2014 we were also involved in a legal dispute with CBI, the former owner of Andromeda. On September 22, 2014, CBI filed a complaint against us in Superior Court of the state of Delaware in New Castle County alleging breach of the share purchase agreement related to the Andromeda acquisition. In the complaint, CBI raised breach of contract and breach of the implied covenant of good faith and fair dealing claims and sought damages of $200.0 million. On January 16, 2015, CBI dismissed its claim against us without prejudice. On February 16, 2015 we reached an agreement with CBI and Yeda, the company from which Andromeda licenses the underlying DiaPep277 technology, to settle DiaPep277 related claims against one another, and we granted CBI an option to acquire all of the outstanding stock of Andromeda. In connection with the agreement, CBI will transfer to us $2.5 million in shares of our common stock it holds, valued at the average closing price of the Company’s common stock for the fifteen trading days ending on February 11, 2015. The parties have appointed a steering committee to oversee the completion of the clinical trial with representatives of CBI and Yeda and a non-voting member appointed by us. The Company’s estimated budget from October 1, 2014 through the completion of the trial remains unchanged at $10.5 million. Any increase to this budget beyond $2.25 million, if incurred as a result of the direction of the steering committee, shall require CBI to reimburse those expenses in shares of the Company’s common stock. Under the agreement, CBI’s option is exercisable through September 30, 2015 for $3.5 million payable in shares of the Company’s common stock. In addition, if the option is exercised, then Andromeda will be obligated to pay Hyperion future contingent payments if and to the extent it or its shareholders receive revenues or certain other proceeds, which are capped at $36.5 million. This amount, together with the option exercise price that Hyperion may receive, approximates the total amount Hyperion will have invested in Andromeda by the option exercise date. Under the agreement, in the event that CBI does not exercise its option, the underlying DiaPep277 technology will revert to Yeda under the license agreement between Andromeda and Yeda. Also on February16, 2015, we entered into a release with Evotec pursuant to which Evotec released its previously asserted claims to a milestone payment from us in connection with our acquisition of Andromeda and that it had suffered harm from recent incidents in relation to Diapep277 in exchange for a payment of $500,000 from us.
Amortization of Intangible Asset
Amortization of intangible asset pertains to the amortization expense of BUPHENYL product rights acquired on May 31, 2013. For additional information, see Note 8 to our consolidated financial statements appearing elsewhere in this report.
Impairment of goodwill
In 2014, we recognized an impairment loss of $30.2 million relating to our acquisition of Andromeda. For additional information, see Notes 4 and 8 to our consolidated financial statements appearing elsewhere in this report.
Interest Income
Interest income consists of interest earned on our cash and cash equivalents and investments.
Interest Expense
Interest expense consists primarily of non-cash and cash interest costs related to our borrowings.
41
Gain from Settlement of Retention Option
The amount of gain from the settlement of the retention option relates to our acquisition of BUPHENYL in 2013 and is comprised of (i) fair value of BUPHENYL of $20.4 million and (ii) net cash received from Ucyclyd of $11.0 million off-set by (iii) the $0.3 million carrying value of the option to purchase the rights to BUPHENYL and AMMONUL. Accordingly, we recorded the resulting net settlement of $31.1 million as gain from our settlement of the retention option on our consolidated statements of operations in the second quarter of 2013. For additional information, see Note 4 to our consolidated financial statements appearing elsewhere in this report.
Other Income (Expense), net
For the year ended December 31, 2014, other income (expense), net was $0.7 million expense and primarily includes amortization of the discount on available-for-sale investments partially offset by the gain on foreign currency transactions. For the year ended December 31, 2013, other income (expense), net primarily includes a $0.5 million payment received from Ucyclyd in accordance with the restated collaboration agreement. For the years ended December 31, 2012, other income (expense), net consisted primarily of the changes in the fair value of the common and preferred stock warrants liability and call option liability associated with the issuance of approximately $32.5 million of convertible notes. We account for the common stock warrants issued in 2011 and preferred stock warrants issued in 2012 and 2011 at fair value and recorded as liabilities on the date of each issuance. The fair value was determined and subsequently re-measured using the Black-Scholes option-pricing model on each reporting date. On July 31, 2012, upon closing of the IPO, we performed a final re-measurement of the common stock warrants issued in 2011 and preferred stock warrants issued in 2012 and 2011, and recorded the impact of the re-measurement to other income (expense), net. On July 31, 2012, immediately prior to the closing of the IPO, the common stock warrants and the preferred stock warrants automatically net exercised into 340,361 shares of common stock.
Income Taxes
We have been granted orphan drug designation by the FDA for our products currently under development. The orphan drug designation allowed us to claim increased federal tax credits for its research and development activities. We have $22.6 million of federal credit carryforwards of which $21.1 million relates to Orphan Drug Credit claims for 2009 through 2014.
We remeasured the preliminary determination of the fair values of assets acquired and liabilities assumed in the Andromeda acquisition and subsequently impaired the goodwill. The corresponding deferred tax liabilities associated with the acquisition were written off. Given the history of operating losses in Israel, we are recording a full valuation allowance on our net deferred tax assets in Israel. This also results in the reversal of a tax benefit recorded during the second quarter of 2014 amounting to $2.9 million.
We intend to continue maintaining a valuation allowance on our deferred tax assets until there is sufficient evidence to support the reversal of all or some portion of these allowances. However, considering our current assessment of income from potential future sales, there is a reasonable possibility that, within the next year, sufficient positive evidence may become available to reach a conclusion that a significant portion of the U.S. valuation allowance will no longer be needed. As such, we may release a significant portion of our U.S. valuation allowance against our U.S. deferred tax assets within the next 12 months. This release would result in the recognition of certain deferred tax assets and a decrease to income tax expense for the period such release is recorded.
For the year ended December 31, 2014 and 2013 our income tax expense was $0.3 million and $49,000 respectively. There was no income tax expense recorded for the year ended
42
December 31, 2012. Our expected taxable income in 2015 will primarily be offset by federal and state net operating losses and credits for which a full valuation allowance has been provided.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with United States generally accepted accounting principles. Application of these principles requires management to make estimates, assumptions, and judgments that affect the amounts reported in the financial statements and accompanying notes. These estimates, assumptions, and judgments are based on information available as of the date of the financial statements; accordingly, as this information changes, the financial statements could reflect different estimates, assumptions, and judgments. Actual results could differ from those estimates.
Critical accounting estimates are necessary in the application of certain accounting policies and procedures, and are particularly susceptible to significant change. Critical accounting policies are defined as those that are reflective of significant judgments and uncertainties, and could potentially result in materially different results under different assumptions and conditions. For additional information on our critical accounting policies, please refer to the information contained in Note 2 of the consolidated financial statements appearing elsewhere in the report.
Business Combinations
We allocate the purchase price of an acquired business to the tangible and intangible assets acquired and liabilities assumed based upon their estimated fair values on the acquisition date. Any excess of the purchase price over the fair value of the net assets acquired is recorded as goodwill. The purchase price allocation process requires us to make significant estimates and assumptions, especially at the acquisition date with respect to intangible assets. Direct transaction costs associated with the business combination are expensed as incurred.
Valuation of Acquisition-Related Contingencies Resulting from the Andromeda Business Combination
In conjunction with the acquisition of Andromeda, we have recorded acquisition-related contingencies upon the achievement of specified development, regulatory approval or sales-based milestone events. The acquisition-related contingencies are measured at their respective fair values as of the acquisition date. The models used in valuing these acquisition-related contingencies require the use of significant estimates and assumptions including but not limited to:
| • | estimates of revenues and operating profits related to the product candidate; |
| • | the probability of success for unapproved product candidate considering their stage of development; |
| • | the time and resources needed to complete the development and approval of product candidate; |
| • | the life of the potential commercialized products and associated risks, including the inherent difficulties and uncertainties in developing a product candidate such as obtaining FDA and other regulatory approvals; and |
| • | risks related to the viability of and potential alternative treatments in any future target markets. |
We revalue contingent consideration obligations each quarter following the acquisition and record increases or decreases in their fair value in our condensed consolidated statements of operations.
Increases or decreases in the fair value of our contingent consideration liabilities are recorded in our consolidated statements of operations and can result from updates to assumptions such as the expected timing or
43
probability of achieving the specified milestones, changes in projected revenues or changes in discount rates. Significant judgment is employed in determining these assumptions as of the acquisition date and for each subsequent period. Updates to assumptions could have a significant impact on our results of operations in any given period. Actual results may differ from estimates.
As discussed in Note 4, to our consolidated financial statements appearing elsewhere in this report we re-measured the preliminary determination of the fair values of assets acquired and liabilities assumed as of the acquisition date and we had determined that the contingent consideration milestones and contingent liability milestones will not be achieved.
Valuation of Acquisition-Related Contingent Liability
Acquisition-related contingent liability, which consists primarily of potential milestone payments and royalty obligations, and potential payments to certain employees of Andromeda is recorded in the consolidated balance sheets at its acquisition date estimated fair value. We reassess the probability and estimates associated with the contingent liability at each reporting period. For liability payable to third parties, any increase in the liability is recorded in the consolidated statements of operations. For liabilities payable to employees, we account for the liability in accordance with ASC 450 Contingencies.
As discussed in Note 4, to our consolidated financial statements appearing elsewhere in this report, we re-measured the preliminary determination of the fair values of assets acquired and liabilities assumed as of the acquisition date and we determined that the contingent consideration milestones and contingent liability milestones will not be achieved.
Accounts Receivable
Our trade accounts receivable are recorded net of product sales allowances for prompt-payment discounts, chargebacks and doubtful accounts. We estimate chargebacks and prompt-payment discounts based on contractual terms, historical trends and our expectations regarding the utilization rates for these programs.
Inventories
Our inventories are valued at the lower of cost or market, with cost computed at standard cost (which approximates actual cost) on a first-in-first-out (FIFO) cost method and consists of raw materials, work in process and finished goods. Subsequent to the FDA approval of RAVICTI on February 1, 2013, we began capitalizing inventories as the related costs were expected to be recoverable through the commercialization of the product. Prior to the FDA approval of RAVICTI, we recorded the costs incurred as research and development expenses in the consolidated statements of operations. If information becomes available that suggest that our inventories may not be realizable, we may be required to expense a portion or all of the previously capitalized inventories.
The costs of our inventories consists mainly of third party manufacturing costs, associated compensation related costs of personnel indirectly involved in the manufacturing process and other overhead costs attributable to the manufacture of inventories.
Products that have been approved by the FDA or other regulatory authorities, such as RAVICTI are also used in clinical programs, to assess the safety and efficacy of the products for usage in diseases that have not been approved by the FDA or other regulatory authorities. The form of RAVICTI utilized for both commercial and clinical programs is identical and, as a result, the inventory has an “alternative future use”. Raw materials and purchased drug product associated with clinical development programs are included in inventory and charged to research and development expense when the product enters the research and development process and no longer can be used for commercial purposes and, therefore, does not have an “alternative future use”.
44
On May 31, 2013, we acquired BUPHENYL including inventory from Ucyclyd (see Note 4 to our consolidated financial statements appearing elsewhere in this report). We recorded these inventories at fair value in the amount of $3.9 million on the acquisition date. As of March 31, 2014, we sold the entire inventory acquired from the acquisition of BUPHENYL.
We evaluate for potential excess inventory by analyzing current and future product demand relative to the remaining product shelf life. We develop demand forecasts by considering factors such as, but not limited to, overall market potential, market share, market acceptance and patient usage. During the year ended December 31, 2014, we recorded a provision for inventory obsolescence of $0.3 million which was recorded into cost of sales in our consolidated statements of operations.
Goodwill and Intangible Assets
We record intangible assets at acquisition cost less accumulated amortization and impairment. Intangible asset with finite lives are amortized over their estimated useful life using the economic use method, which reflects the pattern that the economic benefits of the intangible asset are consumed as revenue is generated. The pattern of consumption of the economic benefits is estimated using the future projected cash flows of the intangible asset.
Goodwill and indefinite-lived intangible assets, including acquired IPR&D, are tested for impairment annually or more frequently if events or changes in circumstances between annual tests indicate that the asset may be impaired. Impairment losses on goodwill and indefinite-lived intangible assets are recognized based on a comparison of the fair value of the asset to its carrying value, without consideration of any recoverability.
The impairment test for goodwill is a two-step process. Step one consists of a comparison of the fair value of a reporting unit against its carrying amount, including the goodwill allocated to each reporting unit. We determine the fair value of the reporting unit based on the present value of estimated future cash flows as well as various price or market multiples applied to the reporting unit’s operating results, which are classified as level 3 within the fair value hierarchy (as described in Note 6 to our consolidated financial statements appearing elsewhere in this report). If the carrying amount of the reporting unit is in excess of its fair value, step two requires the comparison of the implied fair value of the reporting unit’s goodwill against the carrying amount of the reporting unit’s goodwill. Any excess of the carrying value of the reporting unit’s goodwill over the implied fair value of the reporting unit’s goodwill is recorded as an impairment loss.
Impairment of Long-lived Assets
We review our property and equipment and long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset class may not be recoverable. Recoverability is measured by comparing the carrying amount to the future net undiscounted cash flows that the assets are expected to generate. If such assets are considered impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value determined using projected discounted future net cash flows arising from the assets.
Fair Value of Financial Instruments
We measure our financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid for to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. The carrying amounts of our financial instruments, including cash equivalents, short-term investments, accounts payable, and accrued liabilities, approximate fair value due to their short maturities. The carrying amounts of long-term investments and the acquisition-related contingent consideration represent their estimated fair values.
45
Income Taxes
We are subject to income taxes in the U.S. and we use estimates in determining our provision for income taxes. We use the liability method of accounting for income taxes, whereby deferred income tax assets or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income.
Recognition of deferred income tax assets is appropriate when based on the weight of positive and negative evidence realization of such assets is more likely than not. We recognize a valuation allowance against our net deferred income tax assets if it is more likely than not that some portion of the deferred income tax assets will not be fully realizable. This assessment requires judgment as to the likelihood and amounts of future taxable income by tax jurisdiction.
We apply the provisions of FASB’s guidance on accounting for uncertainty in income taxes. We assess all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position’s sustainability and the tax benefit to be recognized is measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and we will determine whether (i) the factors underlying the sustainability assertion have changed and (ii) the amount of the recognized tax benefit is still appropriate. The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
Revenue Recognition
We recognize revenue in accordance with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 605, Revenue Recognition, when the following criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred and risk of loss has passed; and the seller’s price to the buyer is fixed or determinable and collectability is reasonably assured. We determine that persuasive evidence of an arrangement exists based on written contracts that define the terms of our arrangements. In addition, we determine that services have been delivered in accordance with the arrangement. We assesses whether the fee is fixed or determinable based on the payment terms associated with the transaction and whether the sales price is subject to refund or adjustment. We assess collectability based primarily on the customer’s payment history and on the creditworthiness of the customer.
Product Revenue
Our product revenues represent sales of RAVICTI and BUPHENYL in the U. S. and outside the U.S. We recognize revenue once all four revenue recognition criteria described above are met.
In 2013, we began distributing RAVICTI to two specialty pharmacies through a specialty distributor. The specialty pharmacies then in turn dispense RAVICTI to patients in fulfillment of prescriptions. As RAVICTI was our first commercial product, we could not reasonably assess potential product sales allowances at the time of sale to the specialty distributor. As a result, the price of RAVICTI was not deemed fixed or determinable. We deferred the recognition of revenues on product shipments of RAVICTI to the specialty distributor until the product was shipped to patients by the specialty pharmacies at which time our related product sales allowances could be reasonably estimated. Starting June 2014, we could reasonably estimate and determine sales allowances, therefore we began recognizing RAVICTI revenue at the point of sale to the specialty distributor, which resulted in the one-time non- recurring recognition of an additional $8.6 million in net revenues. The impact of this change resulted in a decrease in net loss of $7.9 million for year ended December 31, 2014, or $0.39 per basic and diluted share.
46
We sell BUPHENYL in the United States to a specialty distributor, who in turn sells this product to retail pharmacies, hospitals and other dispensing organizations. We recognize revenue from BUPHENYL sales upon receipt by the specialty distributor as we can reasonably assess potential product sales allowances at the time of sale. For product sales of BUPHENYL outside the United States, revenue is recognized once the product is accepted by the customer or once their acceptance period has expired whichever comes first.
We recognize revenue net of product sales allowances, including estimated rebates, chargebacks, prompt-payment discounts, returns, distribution service fees and Medicare Part D coverage gap reimbursements. Product shipping and handling costs are included in cost of sales.
Product Sales Allowances
We establish reserves for prompt-payment discounts, government and commercial rebates, product returns and chargebacks. Allowances relating to prompt-payment discounts and chargebacks are recorded at the time of revenue recognition, resulting in a reduction in product sales revenue and a decrease in trade accounts receivables. Allowances related to government rebates, product returns and other applicable allowances such as distributor fees are recognized at the time of revenue recognition, resulting in a reduction in product sales and an increase in accrued expenses or a reduction in the related accounts receivable depending on the nature of the sales deduction.
Co-payment assistance
We provide cash donation to a non-profit third party organization which supports UCD patients, who have commercial insurance and meet certain financial eligibility requirements, with co-payment assistance and travel costs. We account for the amount of co-payment assistance as a reduction of product revenues.
The following table summarizes the provisions, and credits/payments, for the gross to net sales deductions:
| (in thousands) | Rebates & Chargebacks |
Prompt pay discounts |
Other Sales- Related Deductions |
Total | ||||||||||||
| Balance as of December 31, 2013 |
$ | 5,051 | $ | — | $ | 184 | $ | 5,235 | ||||||||
| Provision related to current period sales |
17,846 | 4,786 | 1,966 | 24,598 | ||||||||||||
| Credits/payments |
(13,285 | ) | (4,786 | ) | (1,620 | ) | (19,691 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2014 |
$ | 9,612 | $ | — | $ | 530 | $ | 10,142 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Stock-Based Compensation
We recognize as compensation expense the fair value of stock options and other stock-based compensation issued to employees over the requisite service periods, which are typically the vesting periods. We determine the fair value of restricted stock units using the closing price of our common stock on the date of grant. We estimate the fair value of stock options using the Black-Scholes option valuation model. The fair value of employee stock options and restricted stock units is amortized on a straight-line basis over the requisite service period of the awards.
For the year ended December 31, 2014 and 2013, stock-based compensation of $0.1 million each was capitalized into inventories. Capitalized stock-based compensation is recognized as cost of sales when the related product is sold. Allocations to research and development, general and administrative and selling and marketing expense are based upon the department to which the associated employee reported.
47
Total stock-based compensation expense related to options and awards granted was allocated as follows:
| Years Ended December 31, | ||||||||||||
| (in thousands) | 2014 | 2013 | 2012 | |||||||||
| Cost of sales |
$ | 70 | $ | 27 | $ | — | ||||||
| Research and development |
1,384 | 585 | 374 | |||||||||
| Selling, general and administrative |
6,184 | 3,709 | 619 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 7,638 | $ | 4,321 | $ | 993 | ||||||
|
|
|
|
|
|
|
|||||||
Results of Operations
Comparison of the Years Ended December 31, 2014, 2013 and 2012
| Year Ended December 31, |
Increase/ (Decrease) |
% Increase/ (Decrease) |
Year Ended December 31, |
Increase/ (Decrease) |
% Increase/ (Decrease) |
|||||||||||||||||||||||||||
| (in thousands, except for percentages) | 2014 | 2013 | 2013 | 2012 | ||||||||||||||||||||||||||||
| Product revenue, net |
$ | 113,584 | $ | 42,204 | $ | 71,380 | 169 | % | $ | 42,204 | $ | — | $ | 42,204 | 100 | % | ||||||||||||||||
| Cost of sales |
13,727 | 6,740 | 6,987 | 104 | 6,740 | — | 6,740 | 100 | ||||||||||||||||||||||||
| Research and development |
20,715 | 9,984 | 10,731 | 107 | 9,984 | 17,046 | (7,062 | ) | (41 | ) | ||||||||||||||||||||||
| Selling, general and administrative |
48,522 | 35,839 | 12,683 | 35 | 35,839 | 11,487 | 24,352 | 212 | ||||||||||||||||||||||||
| Impairment of goodwill |
30,201 | — | 30,201 | 100 | — | — | — | — | ||||||||||||||||||||||||
| Amortization of intangible asset |
4,626 | 3,058 | 1,568 | 51 | 3,058 | — | 3,058 | 100 | ||||||||||||||||||||||||
| Interest income |
555 | 27 | 528 | 1,956 | 27 | 12 | 15 | 125 | ||||||||||||||||||||||||
| Interest expense |
1,601 | 1,539 | 62 | 4 | 1,539 | 3,703 | (2,164 | ) | (58 | ) | ||||||||||||||||||||||
| Gain from settlement of retention option |
— | 31,079 | (31,079 | ) | (100 | ) | 31,079 | — | 31,079 | 100 | ||||||||||||||||||||||
| Other income (expense), net |
(699 | ) | 526 | (1,225 | ) | (233 | ) | 526 | (39 | ) | 565 | 1,449 | ||||||||||||||||||||
| Income tax expense |
303 | 49 | 254 | 518 | 49 | — | 49 | 100 | ||||||||||||||||||||||||
Revenues
During the years ended December 31, 2014 and 2013, we generated $113.6 million and $42.2 million, respectively, of net product revenues. Product revenues from the sale of RAVICTI and BUPHENYL are recorded net of sales returns, co-pay assistance and estimated product sales allowances including government rebates, chargebacks, prompt -payment discounts and distributor fees.
For the year ended December 31, 2014 and 2013, net product revenues consisted of the following:
| • | net sales from RAVICTI in the amount of $95.4 million (of which $95.3 million relates to sales in the U.S. and $0.1 million relates to sales outside the U.S.) and $31.2 million, respectively; and |
| • | net sales from BUPHENYL in the amount of $18.6 million and $11.5 million, respectively (of which $10.2 million and $5.1 million, respectively, relates to sales outside the U.S. and $8.4 million and $6.4 million, respectively, of sales in the U.S.) |
The above amounts were partially offset by $0.4 million and $0.5 million, respectively, of co-payment assistance. We expect in future periods our sales from BUPHENYL to decrease in part due to increase in the usage of RAVICTI and competition from generic product sales.
48
For the year ended December 31, 2012 we did not generate any revenues from the sale of any products.
Cost of Sales
Our cost of sales includes third-party manufacturing costs, royalty fees payable under our restated collaboration agreement with Ucyclyd, and other indirect costs including compensation cost of personnel, shipping and supplies. For the year ended December 31, 2014 and 2013 total costs of sales were $13.7 million and $6.7 million, respectively. For the year ended December 31, 2014 and 2013, cost of sales consisted of BUPHENYL product costs of $5.1 million and $3.6 million, respectively and RAVICTI product costs of $8.6 million and $3.1 million, respectively. For the year ended December 31, 2014 and 2013, RAVICTI product costs included $7.6 million and $2.5 million, respectively, for royalty expenses payable under our restated collaboration agreement with Ucyclyd.
We began capitalizing inventory costs after FDA approval of RAVICTI as the related costs were expected to be recoverable through the commercialization of the product. We recorded costs incurred prior to FDA approval of RAVICTI as research and development expenses in our 2012 consolidated statement of operations. As a result, cost of sales for RAVICTI for the previous several quarters reflected a lower average per unit cost of materials as we sold product inventory that was previously expensed as research and development.
For BUPHENYL, cost of sales per unit sale was higher in 2013 due to the recording of the step-up fair value of the BUPHENYL inventories acquired from Ucyclyd which was expensed to cost of sales as of March 31, 2014 as the inventory had sold through. Accordingly, cost of sales is not indicative of expected cost of sales in future periods. Since the inventories were purchased as part of a business combination, they were recorded at fair value on the acquisition date. For additional information, see Note 4 to our consolidated financial statements appearing elsewhere in this report.
Research and Development Expenses
Research and development expenses increased by $10.7 million, or 107%, to $20.7 million for the year ended December 31, 2014, from $10.0 million for the year ended December 31, 2013. This increase was primarily due to increases of $2.3 million in compensation related expenses, $0.6 million increase in consulting costs, $1.0 million increase in regulatory related expenses, $1.4 million increase due to initiation of HE observational study costs and the initiation of the study in pediatric subjects under two years of age with UCD, and $5.4 million due to expenses incurred for the DiaPep277 Program.
Research and development expenses decreased by $7.1 million, or 41%, to $10.0 million for the year ended December 31, 2013, from $17.1 million for the year ended December 31, 2012. This decrease in research and development expenses in 2013 as compared to 2012 was primarily due to decreases of $1.4 million in clinical development costs due to completion of our HE Phase II trial in 2012 and a $5.7 million expense recognized pertaining to purchase of RAVICTI which occurred in the first quarter of 2012.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased by $12.7 million, or 35%, to $48.5 million for the year ended December 31, 2014, from $35.8 million for the year ended December 31, 2013. This increase was primarily due to a $5.6 million in compensation expense including stock-based compensation expense as a result of hiring additional employees, $3.5 million related to legal costs and professional and consulting costs, $1.7 million related to office, administrative and information technology infrastructure expenses and $1.0 million increased commercial operations expenses, and $0.7 million due to expenses incurred by Andromeda from the date of acquisition to December 31, 2014.
49
Selling, general and administrative expenses increased by $24.4 million, or 212%, to $35.8 million for the year ended December 31, 2013, from $11.5 million for the year ended December 31, 2012 primarily as a result of our commercial launch of RAVICTI and the acquisition of BUPHENYL as well as the costs associated with operating as a public company for a full year. This increase was primarily due to increases of $13.0 million in compensation expense, including stock-based compensation expense, as a result of hiring additional employees to support our commercial launch, $2.2 million in insurance and other office and administrative related expenses, $5.0 million in selling and marketing expenses pertaining to the product launch and commercialization of RAVICTI, $0.9 million in travel and related costs and $3.3 million increase in consulting costs.
Impairment of goodwill
The impairment of goodwill of $30.2 million relates to the impairment loss recognized with regards to our acquisition of Andromeda. For additional information, see Notes 4 and 8 to our consolidated financial statements appearing elsewhere in this report.
Amortization of intangible asset
The amortization of intangible asset expense pertains to the amortization expense for the BUPHENYL product rights acquired as part of the BUPHENYL acquisition on May 31, 2013. For the year ended December 31, 2014 and 2013, we recorded $4.6 million and $3.1 million, respectively in amortization expenses.
Interest Income
Interest income increased by $0.5 million for the year ended December 31, 2014 primarily due to the interest income earned from our investments portfolio. Interest income primarily included interest and dividend income from our cash and cash equivalents and investments. For the year ended December 31, 2013, the interest income was $27,000.
The changes in interest income were not significant for the years ended December 31, 2013 and 2012.
Interest Expense
Interest expense increased by $0.1 million, or 4%, to $1.6 million for the year ended December 31, 2014, from $1.5 million for the year ended December 31, 2013. Interest expense increased primarily due to the loss on extinguishment of our notes payable partially offset by reduction in interest expense due to lower rate of interest of our new loan obtained in 2014.
Interest expense decreased by $2.2 million, or 58%, to $1.5 million for the year ended December 31, 2013, from $3.7 million for the year ended December 31, 2012. Interest expense decreased primarily due to the conversion of our April 2011 Notes and October 2011 Notes to common stock upon the closing of our IPO in July 2012.
Gain from settlement of retention option
The amount of gain from the settlement of the retention option relates to our acquisition of BUPHENYL in 2013 and is comprised of (i) the fair value of BUPHENYL of $20.4 million and (ii) net cash received from Ucyclyd of $11.0 million off-set by (iii) the $0.3 million carrying value of the option to purchase the rights to BUPHENYL and AMMONUL. Accordingly, we recorded the resulting net settlement of $31.1 million as gain from our settlement of the retention option in our consolidated statements of operations. For additional information, see Note 4 to our consolidated financial statements appearing elsewhere in this report.
50
Other Income (Expense), net
For the year ended December 31, 2014, other income (expense), net was $0.7 million expense and primarily includes amortization of discount on available-for-sale investments partially offset by the gain on foreign currency transactions.
For the year ended December 31, 2013, we recorded $0.5 million in income related to Ucyclyd’s payment to us in relation to our restated collaboration agreement. For the year ended December 31, 2012, other income (expenses), net, was $39,000 of expense for the year ended December 31, 2012 primarily recording a change in fair value of approximately $43,000 of expense related to the common stock warrants and the preferred stock warrants and call option liability.
Income tax expense
For the year ended December 31, 2014 and 2013, income tax expense was $0.3 million and $49,000, respectively. There was no income tax expense recorded for the year ended December 31, 2012.
The 2014 effective tax rate differed from the U.S. federal statutory rate of 35% primarily due to the utilization of net operating loss carryforwards and tax credits for which a valuation allowance is recorded on our federal and California deferred tax assets and the tax impact of our acquisition of Andromeda. The remaining tax expense is related to other state taxes.
Our expected taxable income in 2015 will primarily be offset by federal and state net operating losses and credits for which a full valuation allowance has been provided.
Liquidity and Capital Resources
During the year ended December 31, 2014, we generated net revenues of $113.6 million. As of December 31, 2014 and December 31, 2013, our principal sources of liquidity were our cash provided by operating activities and our cash and cash equivalents and investments.
Prior to our commercialization of RAVICTI our principal source of liquidity was debt and sales of our equity securities.
In April 2012, we borrowed $10.0 million pursuant to a loan and security agreement with Silicon Valley Bank and Leader Lending, LLC — Series B (the “Lenders”). The loan carried an interest rate of 8.88%, with interest only payments for the period of 9 months from May 1, 2012. The loan was then payable in equal monthly principal payments plus interest over a period of 27 months from February 1, 2013. We issued warrants to the Lenders to purchase a total of 75,974 shares of common stock at an exercise price of $4.08 per share. These warrants have been fully exercised.
On July 31, 2012, we completed our IPO and issued 5,000,000 shares of our common stock at an initial offering price of $10.00 per share. We sold an additional 750,000 shares of common stock directly to our underwriters when they exercised their over-allotment option in full at the initial offering price of $10.00 per share. We received net proceeds from the IPO of $51.3 million, after deducting underwriting discounts and commissions of $4.0 million and expenses of $2.2 million.
In September 2012, we borrowed an additional $2.5 million from Silicon Valley Bank pursuant to the loan and security agreement. We issued an additional warrant to Silicon Valley Bank to purchase a total of 8,408 shares of common stock at an exercise price of $5.05 per share. These warrants have been fully exercised.
On March 13, 2013, we completed our follow-on offering and issued 2,875,000 shares of our common stock at an offering price of $20.75 per share. In addition, we sold an additional 431,250 shares of common stock
51
directly to underwriters when they exercised their over-allotment option in full at the offering price of $20.75 per share. We received net proceeds from the offering of $63.7 million, after deducting underwriting discounts and commissions of $4.1 million and expenses of $0.8 million.
On August 14, 2013, we filed a shelf registration statement on Form S-3, which was declared effective by the SEC on September 13, 2013. The shelf registration statement permits: (a) the offering, issuance and sale of up to a maximum aggregate offering price of $150.0 million of our common stock, preferred stock, debt securities, warrants and/or units; (b) the sale of up to 8,727,000 shares of common stock by certain selling stockholders; and (c) the offering, issuance and sale of up to a maximum aggregate offering price of $50.0 million of our common stock that may be issued and sold under a sales agreement with Cantor Fitzgerald & Co. As of December 31, 2014, there were no sales of any securities registered pursuant to the shelf registration statement.
On July 18, 2014, we entered into a new Loan and Security Agreement with Silicon Valley Bank, providing for two tranches of term loans of up to $16.0 million and a revolving credit line of up to $5.0 million. In July 2014, we drew down both tranches of the Term Loans, of which approximately $5.8 million was used to pre-pay the Company’s existing April and September 2012 notes payable. In addition, we drew down $2.0 million from the revolving line of credit facility. The actual amount of the Revolving Loans that are available from time to time under the Loan Agreement is limited to a borrowing base amount that is determined according to, among other things, a percentage of the value of eligible accounts.
At December 31, 2014, we had an accumulated deficit of $128.6 million. We expect to incur increased research and development expenses from the Phase 3 trial of GPB for the treatment of patients with episodic HE. We expect to continue to incur research and development expenses relating to the completion of the DiaPep277 Phase 3 trial and the termination of the DiaPep277 development program through mid to late 2015. In addition, we expect to continue to incur significant commercial, sales and marketing, and outsourced manufacturing expenses in connection with commercialization of RAVICTI and marketing of BUPHENYL in UCD. These increased expenses as compared to prior years include compensation expenses due to hiring additional employees, costs related to operation of our distribution network and marketing costs and general infrastructure expenses as we expand our organization. Our plans with respect to these matters include utilizing a substantial portion of our capital resources and efforts in completing the development and obtaining regulatory approval of GPB in HE and the identification and potential development of additional new product candidates. Further, as a result of our legal investigation of the serious misconduct at Andromeda, the legal disputes in which we are involved and the significant legal costs associated with the expansion of our business, our legal expenses increased significantly, and they may continue to increase in the future.
We believe that our existing cash and cash equivalents and investments as of December 31, 2014, and our future forecasted product revenues will be sufficient to fund our operations for at least the next 12 months.
Cash Flows
The following table sets forth the major sources and uses of cash for the periods set forth below (in thousands):
| Year Ended December 31, | ||||||||||||
| (in thousands) | 2014 | 2013 | 2012 | |||||||||
| Net cash (used in) provided by: |
||||||||||||
| Operating activities |
$ | 30,998 | $ | (1,212 | ) | $ | (28,531 | ) | ||||
| Investing activities |
(14,973 | ) | (34,054 | ) | 2 | |||||||
| Financing activities |
12,539 | 59,645 | 71,364 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase in cash and cash equivalents |
$ | 28,564 | $ | 24,379 | $ | 42,835 | ||||||
|
|
|
|
|
|
|
|||||||
52
Net cash provided by operating activities of $31.0 million for the year ended December 31, 2014, primarily included our net loss of $6.3 million, adjusted for non-cash items such as $30.2 million of impairment of goodwill, $0.6 million of amortization of debt discount and debt issuance cost, $4.6 million of amortization of intangible asset, $7.6 million of stock-based compensation expense, $0.4 million of amortization of discount on available-for-sale investments, $0.5 million of depreciation expense, $0.3 million of provision for inventory obsolescence, a net cash inflow of $6.6 million related to changes in operating liabilities partially offset by a net cash outflow of $13.5 million related to changes in operating assets. Cash used in operating activities of $1.2 million for the year ended December 31, 2013, primarily related to our net income of $16.6 million, adjusted for non-cash items such as $31.1 million of gain from the settlement of retention option, $0.6 million of amortization of debt discount and debt issuance costs, $3.1 million of amortization of intangible asset, $4.3 million of stock-based compensation expense, $0.3 million of other non –cash items, a net cash inflow of $9.8 million related to changes in operating liabilities partially offset by a net cash inflow of $4.8 million related to changes in operating assets. The primary use of cash in operating activities for the year ended December 31, 2012 was to fund our operations related to the development of RAVICTI in UCD and GPB in HE. Cash used in operating activities of $28.5 million for the twelve months ended December 31, 2012, primarily related to our net loss of $32.3 million, adjusted for non-cash items such as $1.3 million of amortization of debt discount, $0.8 million of re-measurement of warrants liability, $0.7 million re-measurement of call option liability and $1.0 million of stock-based compensation expense. For the twelve months ended December 31, 2012, cash used in operating activities included the $5.7 million incurred for the purchase of RAVICTI.
Net cash used in investing activities for the year ended December 31, 2014 was $15.0 million and primarily consisted of $14.0 million paid to acquire Andromeda, additions made to property, plant and equipment of $0.7 million, purchase of available-for-sale investments of $29.0 million partially offset by maturities of available-for-sale investments of $28.7 million. Net cash used in investing activities of $34.1 million for the year ended December 31, 2013 includes $43.9 million for purchases of investments and $1.1 million for additions made to property and equipment partially offset by a net payment of $11.0 million received from Ucyclyd. For the year ended December 31, 2012, net cash provided by investing activities consisted of a decrease in restricted cash of $0.3 million, partially offset by the purchase of the option to purchase rights to BUPHENYL and AMMONUL of $0.3 million and property and equipment purchases of $45,000.
Net cash provided by financing activities amounted to $12.5 million, $59.6 million and $71.4 million for the years ended December 31, 2014, 2013, and 2012, respectively. Net cash provided by financing activities for year ended December 31, 2014 primarily consisted of proceeds from the term loan and revolving credit line of $18.0 million, proceeds from issuance of common stock due to exercise of stock options of $2.6 million partially offset by payments of notes payable of $8.2 million. Net cash provided by financing activities for year ended December 31, 2013, related primarily to the proceeds from our follow-on offering $64.5 million (net of underwriting discounts and commissions), proceeds from the issuance of the common stock from stock option exercises of $0.6 million partially offset by our payments of notes payable of $4.3 million and payments of offering costs of $ 1.1 million. Net cash provided by financing activities for year ended December 31, 2012, related primarily to the proceeds from our IPO of $51.3 million (net of underwriting discounts and commissions and offering costs), proceeds from the issuance of the February 2012 Notes in the amount of $7.5 million and net proceeds from the issuance of our April 2012 Notes and September 2012 Notes totaling $12.4 million.
53
Contractual Obligations and Commitments
The following table summarizes our contractual obligations and commitments as of December 31, 2014 (in thousands):
| Payments Due By Period | ||||||||||||||||||||
| Contractual Obligations |
Total | Less than 1 Year |
1-3 Years |
3-5 Years |
More than 5 Years |
|||||||||||||||
| Principal obligations on the loan and security agreement(1) |
$ | 18,000 | $ | — | $ | 14,669 | $ | 3,331 | $ | — | ||||||||||
| Interest obligations on the loan and security agreement(1) |
2,788 | 730 | 939 | 1,119 | — | |||||||||||||||
| Operating leases(2) |
4,049 | 797 | 1,641 | 1,611 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Total(3)(4) |
$ | 24,837 | $ | 1,527 | $ | 17,249 | $ | 6,061 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | On July 18, 2014, we entered into a new Loan and Security Agreement (the “Loan Agreement”) with Silicon Valley Bank, providing for two tranches of term loans of up to $16.0 million (the “Term Loans”) and a revolving credit line of up to $5.0 million (the “Revolving Loans”). The Loan Agreement provides for an interest rate of 4.0% per year on the Term Loans and the prime rate plus 0.75% per year on the Revolving Loans, with a minimum interest requirement on the Revolving Loans. Upon the maturity date of the Term Loans, a final payment fee of 6.75% of the original principal amount of such Term Loans (the “Final Payment”) will be due. In addition, the Company drew down $2.0 million from the revolving line of credit facility. |
| (2) | Operating lease obligations consist primarily of lease payments for our Brisbane facility and office equipment. |
| (3) | This table does not include (a) any milestone payments, which may become payable to third parties under license agreements, as the timing and likelihood of such payments are not known, and (b) any royalty payments to third parties as the amounts, timing and likelihood of such payments are not known. |
| (4) | This table does not include a liability for unrecognized tax benefits related to various federal and state income tax matters of $4.2 million at December 31, 2014. The timing of the settlement of these amounts was not reasonably estimable at December 31, 2014. We do not expect payment of amounts related to the unrecognized tax benefits within the next twelve months. |
Future Funding Requirements
We may need to obtain additional financing to fund our future operations, including supporting sales and marketing activities related to RAVICTI and BUPHENYL, funding a Phase 3 trial in HE, as well as the costs related to termination of the clinical development of DiaPep277 and the development and commercialization of any additional product candidates we might acquire or develop on our own. Our future funding requirements will depend on many factors, including, but not limited to:
| • | our revenues from RAVICTI and BUPHENYL; |
| • | costs relating to the discontinuation of the DiaPep277 program, including potential liabilities from related litigation; |
| • | the amount of sales and other revenues from other products and product candidates that we may commercialize, if any, including the selling prices for such products and the availability of adequate third-party reimbursement; |
54
| • | selling and marketing costs associated with our UCD products, including the cost and timing of expanding our marketing and sales capabilities; |
| • | the progress, timing, scope and costs of our nonclinical studies and clinical trials, including the ability to timely enroll patients in our planned and potential future clinical trials; |
| • | the time and cost necessary to obtain regulatory approvals and the costs of post-marketing studies that may be required by regulatory authorities; |
| • | the costs of obtaining clinical and commercial supplies of RAVICTI, BUPHENYL and GPB; |
| • | payments of milestones and royalties to third parties; |
| • | cash requirements of any future acquisitions of product candidates; |
| • | the time and cost necessary to respond to technological and market developments; |
| • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| • | any new collaborative, licensing and other commercial relationships that we may establish. |
We believe that our current cash and cash equivalents will be sufficient to fund our operations for at least the next 12 months.
We have based these estimates on a number of assumptions that may prove to be wrong, and changing circumstances beyond our control may cause us to consume capital more rapidly than we currently anticipate. For example, we may not achieve or sustain the revenues we anticipated and our HE Phase 3 clinical trial may cost more than we expect. Because of the numerous risks and uncertainties associated with the development and commercialization of GPB or any other product candidates, we are unable to estimate with any certainty the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials.
Additional financing may not be available when we need it or may not be available on terms that are favorable to us. We may seek to raise additional capital through a combination of private and public equity offerings and debt financings. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of existing stockholders. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends.
If adequate funds are not available to us on a timely basis, or at all, we may be required to terminate or delay clinical trials or other development activities for RAVICTI and GPB, or delay our establishment of sales and marketing capabilities or other activities that may be necessary to successfully market BUPHENYL. We may elect to raise additional funds even before we need them if the conditions for raising capital are favorable.
Off-Balance Sheet Arrangements
We do not currently have, nor have we ever had, any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. In addition, we do not engage in trading activities involving non-exchange traded contracts.
55
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risks in the ordinary course of our business. These risks primarily include risk related to interest rate sensitivities and foreign currency exchange rate movements.
Interest Rate Market Risk
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio and our outstanding debt obligations.
By policy, we place our investments with highly rated credit issuers and limit the amount of credit exposure to any one issuer. As stated in our investment policy, we seek to improve the safety and likelihood of preservation of our invested funds by limiting default risk and market risk.
We mitigate default risk by investing in high credit quality securities and by positioning our portfolio to respond appropriately to a significant reduction in a credit rating of any investment issuer or guarantor. The portfolio includes only marketable securities with active secondary or resale markets to ensure portfolio liquidity.
Our cash and cash equivalents as of December 31, 2014, totaled $102.8 million and consisted primarily of cash and money market funds with original maturities of three months or less from the date of purchase. Our short-term and long-term investments totaled $43.7 million and consisted primarily of certificates of deposit, commercial paper, corporate notes and U.S. government agency securities. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of the interest rates in the United States. However, because of the short-term nature of the instruments in our portfolio, a sudden change in market interest rates would not be expected to have a material impact on our financial condition or our results of operations.
As of December 31, 2014, we had debt obligations outstanding relating to our outstanding obligations under a loan and security agreement with Silicon Valley Bank and Leader Lending, LLC totaling $16.0 million of term loans and $2.0 million of revolving credit line. Our obligations under the loan and security agreement carry an interest rate that is fixed and is not subject to fluctuations. However, to the extent in the future we enter into other long-term debt arrangements, we could be subject to fluctuations in interest rates, which could have a material impact on our future financial condition and results of operations.
We do not have any foreign currency or other derivative financial instruments.
Foreign Currency Exchange Rate Risk
We transact business in Canadian Dollars. Accordingly, we are subject to exposure from movements in foreign currency exchange rates of Canadian dollars from sales of our products and some operating expenses incurred in Canada.
At December 31, 2014, we had accounts receivable of $0.5 million denominated in foreign currencies which represented approximately 3% of our total accounts receivable.
At December 31, 2014, we had cash of $0.5 million denominated in foreign currencies which represented approximately 1% of our total cash and cash equivalents.
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this item are set forth beginning at page F-1 of this report and are incorporated herein by reference.
56
Hyperion Therapeutics, Inc.
Years Ended December 31, 2014, 2013 and 2012
| Page | ||||
| F-2 | ||||
| Consolidated Financial Statements |
||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Hyperion Therapeutics, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of comprehensive income (loss), of stockholders’ equity (deficit) and of cash flows present fairly, in all material respects, the financial position of Hyperion Therapeutics, Inc. and its subsidiaries at December 31, 2014 and December 31, 2013 and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2014 in conformity with accounting principles generally accepted in the United States of America. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
San Jose, California
March 4, 2015
F-2
Hyperion Therapeutics, Inc.
(In thousands, except share and per share amounts)
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Assets |
||||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 102,796 | $ | 74,232 | ||||
| Short-term investments |
34,487 | 28,045 | ||||||
| Accounts receivable, net |
14,864 | 4,419 | ||||||
| Inventories, net |
4,621 | 3,513 | ||||||
| Prepaid expenses and other current assets |
2,632 | 1,403 | ||||||
|
|
|
|
|
|||||
| Total current assets |
159,400 | 111,612 | ||||||
| Long-term investments |
9,226 | 15,780 | ||||||
| Property and equipment, net |
1,116 | 936 | ||||||
| Intangible asset, net |
8,816 | 13,442 | ||||||
| Other non-current assets |
1,858 | 749 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 180,416 | $ | 142,519 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities |
||||||||
| Accounts payable |
$ | 4,669 | $ | 2,292 | ||||
| Accrued liabilities |
25,509 | 12,187 | ||||||
| Deferred revenue |
224 | — | ||||||
| Notes payable, current portion |
— | 5,652 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
30,402 | 20,131 | ||||||
| Notes payable, net of current portion |
18,124 | 2,621 | ||||||
| Deferred rent |
338 | 81 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
48,864 | 22,833 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 13) |
||||||||
| Stockholders’ equity |
||||||||
| Preferred stock, par value $0.0001 — 10,000,000 shares authorized at December 31, 2014 and 2013; none issued and outstanding |
— | — | ||||||
| Common stock, par value $0.0001 — 100,000,000 shares authorized at December 31, 2014 and 2013; 20,747,013 and 20,137,145 shares issued and outstanding at December 31, 2014 and 2013, respectively |
2 | 2 | ||||||
| Additional paid-in capital |
260,225 | 242,109 | ||||||
| Accumulated other comprehensive loss |
(50 | ) | (55 | ) | ||||
| Accumulated deficit |
(128,625 | ) | (122,370 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
131,552 | 119,686 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 180,416 | $ | 142,519 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Hyperion Therapeutics, Inc.
Consolidated Statements of Operations
(In thousands, except share and per share amounts)
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Net product revenue |
$ | 113,584 | $ | 42,204 | $ | — | ||||||
| Costs and expenses: |
||||||||||||
| Cost of sales |
13,727 | 6,740 | — | |||||||||
| Research and development |
20,715 | 9,984 | 17,046 | |||||||||
| Selling, general and administrative |
48,522 | 35,839 | 11,487 | |||||||||
| Impairment of goodwill (Notes 4 and 8) |
30,201 | — | — | |||||||||
| Amortization of intangible asset |
4,626 | 3,058 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total costs and expenses |
117,791 | 55,621 | 28,533 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(4,207 | ) | (13,417 | ) | (28,533 | ) | ||||||
| Interest income |
555 | 27 | 12 | |||||||||
| Interest expense |
(1,601 | ) | (1,539 | ) | (3,703 | ) | ||||||
| Gain from settlement of retention option (Note 4) |
— | 31,079 | — | |||||||||
| Other income (expense), net |
(699 | ) | 526 | (39 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Income (loss) before income taxes |
(5,952 | ) | 16,676 | (32,263 | ) | |||||||
| Income tax expense |
303 | 49 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) |
(6,255 | ) | 16,627 | (32,263 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) per share: |
||||||||||||
| Basic |
$ | (0.31 | ) | $ | 0.86 | $ | (4.45 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Diluted |
$ | (0.31 | ) | $ | 0.80 | $ | (4.45 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Weighted average number of shares used to compute net income (loss) per share of common stock: |
||||||||||||
| Basic |
20,470,025 | 19,415,822 | 7,256,537 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted |
20,470,025 | 20,730,913 | 7,256,537 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Hyperion Therapeutics, Inc.
Consolidated Statements of Comprehensive Income (Loss)
(In thousands)
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Net income (loss) |
$ | (6,255 | ) | $ | 16,627 | $ | (32,263 | ) | ||||
| Other comprehensive income (loss): |
||||||||||||
| Unrealized gain (loss) on investments arising during the year |
5 | (55 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive income (loss) |
5 | (55 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive income (loss) |
$ | (6,250 | ) | $ | 16,572 | $ | (32,263 | ) | ||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Hyperion Therapeutics, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
(In thousands, except share and per share amounts)
| Convertible Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||
| Balances as of December 31, 2011 |
6,575,637 | 58,326 | 469,319 | — | 24,630 | — | (106,734 | ) | (82,104 | ) | ||||||||||||||||||||||||
| Conversion of Series C-1 and C-2 convertible preferred stock to common stock |
(6,575,637 | ) | (58,326 | ) | 6,575,637 | 1 | 58,325 | — | — | 58,326 | ||||||||||||||||||||||||
| Conversion of convertible notes payable and accrued interest to common stock |
— | — | 3,444,870 | — | 33,322 | — | — | 33,322 | ||||||||||||||||||||||||||
| Issuance of common stock upon automatic net exercise of warrants |
— | — | 340,361 | — | 3,901 | — | — | 3,901 | ||||||||||||||||||||||||||
| Proceeds from issuance of common stock in initial public offering, net of underwriting discounts and commissions of $4,025 and offering costs of $2,163 |
— | — | 5,750,000 | 1 | 51,311 | — | — | 51,312 | ||||||||||||||||||||||||||
| Issuance of common stock warrants in connection with notes payable |
— | — | — | — | 755 | — | — | 755 | ||||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
— | — | 66,082 | — | 147 | — | — | 147 | ||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 993 | — | — | 993 | ||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (32,263 | ) | (32,263 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Balances as of December 31, 2012 |
— | — | 16,646,269 | 2 | 173,384 | — | (138,997 | ) | 34,389 | |||||||||||||||||||||||||
| Proceeds from issuance of common stock in follow-on offering, net of underwriting discounts and commissions of $4,116 and offering costs of $776 |
— | — | 3,306,250 | — | 63,712 | — | — | 63,712 | ||||||||||||||||||||||||||
| Exercise of common stock warrants in connection with notes payable |
— | — | 67,503 | — | — | — | — | — | ||||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
— | — | 117,123 | — | 637 | — | — | 637 | ||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 4,376 | — | — | 4,376 | ||||||||||||||||||||||||||
| Other comprehensive income (loss) |
— | — | — | — | — | (55 | ) | — | (55 | ) | ||||||||||||||||||||||||
| Net income |
— | — | — | — | — | — | 16,627 | 16,627 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Balances as of December 31, 2013 |
— | — | 20,137,145 | 2 | 242,109 | (55 | ) | (122,370 | ) | 119,686 | ||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
— | — | 291,999 | — | 2,583 | — | — | 2,583 | ||||||||||||||||||||||||||
| Issuance of common stock upon release of restricted stock units |
— | — | 5,000 | — | — | — | — | — | ||||||||||||||||||||||||||
| Issuance of common stock upon acquisition of Andromeda Biotech Ltd (Note 4) |
— | — | 312,869 | — | 7,778 | — | — | 7,778 | ||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 7,647 | — | — | 7,647 | ||||||||||||||||||||||||||
| Excess tax benefit from stock-based compensation |
— | — | — | — | 108 | — | — | 108 | ||||||||||||||||||||||||||
| Other comprehensive income (loss) |
— | — | — | — | — | 5 | — | 5 | ||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (6,255 | ) | (6,255 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Balances as of December 31, 2014 |
— | $ | — | 20,747,013 | $ | 2 | $ | 260,225 | $ | (50 | ) | $ | (128,625 | ) | $ | 131,552 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-6
Hyperion Therapeutics, Inc.
Consolidated Statements of Cash Flows (In thousands)
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net income (loss) |
$ | (6,255 | ) | $ | 16,627 | $ | (32,263 | ) | ||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities |
||||||||||||
| Depreciation |
496 | 198 | 15 | |||||||||
| Amortization of debt discount and debt issuance costs |
627 | 556 | 1,381 | |||||||||
| Re-measurement of warrants liability |
— | — | 780 | |||||||||
| Re-measurement of call option liability and preferred stock liability |
— | — | (737 | ) | ||||||||
| Stock-based compensation expense |
7,568 | 4,294 | 993 | |||||||||
| Amortization of intangible asset |
4,626 | 3,058 | — | |||||||||
| Impairment of goodwill (Notes 4 and 8) |
30,201 | — | — | |||||||||
| Gain from settlement of retention option (Note 4) |
— | (31,079 | ) | — | ||||||||
| Unrealized loss on foreign currency translation adjustments |
7 | 14 | — | |||||||||
| Provision of inventory obsolescence |
346 | — | — | |||||||||
| Amortization of discount on available-for-sale investments |
397 | 51 | — | |||||||||
| Excess tax benefit from stock-based compensation |
(108 | ) | — | — | ||||||||
| Changes in assets and liabilities, net of impact of acquisition of Andromeda Biotech Ltd. (“Andromeda”) |
||||||||||||
| Accounts receivable |
(10,452 | ) | (4,433 | ) | — | |||||||
| Inventories |
(1,376 | ) | 468 | — | ||||||||
| Prepaid expenses and other assets |
(1,705 | ) | (809 | ) | (249 | ) | ||||||
| Accounts payable |
(1,857 | ) | 115 | 290 | ||||||||
| Accrued liabilities and other |
7,978 | 9,647 | 1,259 | |||||||||
| Deferred revenue |
224 | — | — | |||||||||
| Deferred rent |
281 | 81 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) operating activities |
30,998 | (1,212 | ) | (28,531 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities |
||||||||||||
| Acquisition of property and equipment |
(654 | ) | (1,085 | ) | (45 | ) | ||||||
| Purchase of available-for-sale investments |
(29,023 | ) | (43,931 | ) | — | |||||||
| Maturity of available-for-sale investments |
28,743 | — | — | |||||||||
| Acquisition of Andromeda, net of cash acquired |
(14,039 | ) | — | — | ||||||||
| Option to purchase BUPHENYL and AMMONUL (Note 4) |
— | — | (283 | ) | ||||||||
| Acquisition of rights to BUPHENYL, net of AMMONUL option (Note 4) |
— | 10,962 | — | |||||||||
| Change in restricted cash |
— | — | 330 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
(14,973 | ) | (34,054 | ) | 2 | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from issuance of common stock in initial public offering, net of underwriting discounts |
— | — | 53,475 | |||||||||
| Proceeds from issuance of common stock in follow-on offering, net of underwriting discounts |
— | 64,488 | — | |||||||||
| Proceeds from issuance of common stock from stock option exercises |
2,584 | 637 | 147 | |||||||||
| Proceeds from term loans |
16,000 | — | — | |||||||||
| Proceeds from revolving credit line |
2,000 | — | — | |||||||||
| Excess tax benefit from stock-based compensation |
108 | — | — | |||||||||
| Proceeds from issuance of convertible notes payable |
— | — | 7,504 | |||||||||
| Proceeds from issuance of notes payable, net of issuance costs |
— | — | 12,401 | |||||||||
| Payments of offering costs |
— | (1,132 | ) | (2,163 | ) | |||||||
| Principal payments under notes payable |
(8,153 | ) | (4,348 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
12,539 | 59,645 | 71,364 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase in cash and cash equivalents |
28,564 | 24,379 | 42,835 | |||||||||
| Cash and cash equivalents, beginning of period |
74,232 | 49,853 | 7,018 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 102,796 | $ | 74,232 | $ | 49,853 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental cash flow information |
||||||||||||
| Cash paid for interest |
$ | 1,526 | $ | 966 | $ | 593 | ||||||
| Cash paid for income tax |
1,099 | — | — | |||||||||
| Stock-based compensation capitalized into inventories |
79 | 82 | — | |||||||||
| Supplemental disclosure of noncash investing and financing activities |
||||||||||||
| Warrants issued in connection with notes payable |
— | — | 1,302 | |||||||||
| Option to purchase rights to BUPHENYL and AMMONUL (Note 4) |
— | 283 | — | |||||||||
| Unrealized gain (loss) on available-for-sale investments |
5 | (55 | ) | — | ||||||||
| Issuance of common stock upon automatic net exercise of warrants |
— | — | 3,901 | |||||||||
| Conversion of convertible notes payable and accrued interest to common stock |
— | — | 33,322 | |||||||||
| Conversion of Series C-1 and C-2 preferred stock to common stock |
— | — | 58,326 | |||||||||
| Acquisition of Andromeda (Note 4) |
||||||||||||
| Cash paid for acquisition |
14,345 | — | — | |||||||||
| Cash acquired in acquisition |
(306 | ) | — | — | ||||||||
| Net cash paid for acquisition |
14,039 | — | — | |||||||||
| Fair value of common stock issued in connection with the acquisition |
7,778 | — | — | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
| 1. | Formation and Business of the Company |
Hyperion Therapeutics, Inc. (the “Company”) was incorporated in the state of Delaware on November 1, 2006. The Company was in the development stage from inception through March 31, 2013. During this period, the Company’s activities consisted primarily of raising capital, negotiating a promotion and drug development collaboration agreement, establishing a management team and performing drug development activities. The Company launched RAVICTI® (glycerol phenylbutyrate) Oral Liquid during the quarter ended March 31, 2013.
On May 31, 2013, the Company acquired BUPHENYL, an FDA-approved therapy for treatment of three of the most prevalent UCD subtypes, from Ucyclyd Pharma Inc. (“Ucyclyd”), a subsidiary of Valeant Pharmaceuticals International, Inc. (“Valeant”). Subsequent to the acquisition on May 31, 2013, the Company started selling BUPHENYL Tablets and Powder within and outside the United States. The Company pays Ucyclyd royalties on net sales in the United States of BUPHENYL to UCD patients outside of the FDA-approved labeled age range for RAVICTI.
As discussed in Note 4, on June 12, 2014, the Company completed the acquisition of Andromeda Biotech Ltd. (“Andromeda”), an Israeli company. On September 8, 2014 the Company announced that it was terminating development of DiaPep277® for newly diagnosed Type 1 diabetes as a result of evidence that the Company uncovered that certain employees of Andromeda engaged in serious misconduct that compromised clinical trial results. The Company subsequently terminated the Andromeda employees involved in the misconduct. On September 22, 2014, Clal Biotechnology Industries Ltd., (“CBI”), the former owner of Andromeda filed suit against the Company in Delaware related to the Company’s announcement. On October 6, 2014 the Company entered into an interim agreement with CBI, to attempt to resolve their disputes. As discussed in Note 22, on January 16, 2015, CBI dismissed its claim against the Company without prejudice and the Company, CBI and Yeda Research and Development Company Ltd (“Yeda”) announced that they had reached an agreement to settle their disputes on February 16, 2015.
The Company is a commercial biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat disorders in the areas of orphan diseases. The Company’s products RAVICTI® (glycerol phenylbutyrate) Oral liquid, BUPHENYL® and AMMONAPS® (sodium phenylbutyrate) Tablets and Powder, are designed to lower ammonia in the blood. Ammonia is produced in the intestine after a person eats protein and is normally detoxified in the liver by conversion to urea. Elevated levels of ammonia are potentially toxic and can lead to severe medical complications which may include death. The Company developed RAVICTI, which was launched during the first quarter of 2013, to treat most urea cycle disorders (“UCD”) and is developing glycerol phenylbutyrate (“GPB”), the active pharmaceutical ingredient in RAVICTI, to treat hepatic encephalopathy (“HE”). UCD and HE are diseases in which blood ammonia is elevated. UCD are inherited rare genetic diseases caused by a deficiency of one or more enzymes or transporters that constitute the urea cycle, which in a healthy individual removes ammonia through its conversion to urea. HE may develop in some patients with liver scarring, known as cirrhosis, or acute liver failure and is a chronic complication of cirrhosis which fluctuates in severity and may lead to serious neurological damage.
On February 1, 2013, the U.S. Food and Drug Administration (“FDA”), granted approval of RAVICTI for the use as a nitrogen-binding agent for chronic management of adult and pediatric UCD patients greater than two years of age who cannot be managed by dietary protein restriction and/or amino acid supplementation alone.
On July 31, 2012, the Company completed its initial public offering (“IPO”) and issued 5,000,000 shares of its common stock at an initial offering price of $10.00 per share. In addition, the Company sold an additional 750,000 shares of common stock directly to its underwriters when they exercised their over-allotment option in
F-8
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
full at the initial offering price of $10.00 per share. The shares began trading on the NASDAQ Global Market on July 26, 2012. The Company received net proceeds from the IPO of $51.3 million, after deducting underwriting discounts and commissions of $4.0 million and expenses of $2.2 million. Upon the closing of the IPO, the Company’s Series C-1 and Series C-2 preferred stock converted into 1,912,598 and 4,663,039 shares of common stock, respectively. In addition, the principal and accrued interest under the Company’s April 2011 Notes and October 2011 Notes amounting to $18.9 million and $15.6 million, respectively, converted into 1,888,054 and 1,556,816 shares of common stock. As of July 31, 2012, the carrying value of the April 2011 Notes including the impact of the amendment to the April 2011 Notes and accrued interest was $18.4 million. As of July 31, 2012, the carrying value (net of discount) and accrued interest under the October 2011 Notes was $14.9 million. Additionally, the April 2011 common stock warrants and October 2011 preferred stock warrants were converted into 322,599 and 17,762 shares of common stock, respectively, immediately prior to the closing of the IPO.
On March 13, 2013, the Company completed its follow-on offering and issued 2,875,000 shares of its common stock at an offering price of $20.75 per share. In addition, the Company sold an additional 431,250 shares of common stock directly to its underwriters when they exercised their over-allotment option in full at the offering price of $20.75 per share. The Company received net proceeds from the offering of $63.7 million, after deducting underwriting discounts and commissions of $4.1 million and expenses of $0.8 million.
On August 14, 2013, the Company filed a shelf registration statement on Form S-3, which was declared effective by the Securities and Exchange Commission (“SEC”) on September 13, 2013. The shelf registration statement permits: (a) the offering, issuance and sale by the Company of up to a maximum aggregate offering price of $150.0 million of its common stock, preferred stock, debt securities, warrants and/or units; (b) the sale of up to 8,727,000 shares of common stock by certain selling stockholders; and (c) the offering, issuance and sale by the Company of up to a maximum aggregate offering price of $50.0 million of its common stock that may be issued and sold under a sales agreement with Cantor Fitzgerald & Co. As of December 31, 2014, there were no sales of any securities registered pursuant to the shelf registration statement.
Since inception, the Company has incurred recurring annual net operating losses from operations. During the year ended December 31, 2014, the Company incurred a loss from operations of $4.2 million. At December 31, 2014, the Company had an accumulated deficit of $128.6 million. The Company expects to incur increased research and development expenses when it initiates a Phase 3 trial of GPB for the treatment of patients with episodic HE and completes the ongoing DIA AID 2 clinical trial of DiaPep277program. In addition, the Company expects to incur increased sales and marketing expenses with the continued commercialization of RAVICTI and marketing of BUPHENYL in UCD as it expands outside of the U.S. Management’s plans with respect to these matters include utilizing a substantial portion of the Company’s capital resources and efforts in completing the development and obtaining regulatory approval for GPB in HE, expanding the Company’s organization, commercialization of RAVICTI and marketing of BUPHENYL.
| 2. | Summary of Significant Accounting Policies |
Basis of Presentation and the Use of Estimates
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of the accompanying consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, the Company evaluates its estimates, including those related to the
F-9
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
fair value of assets and liabilities, intangible asset valuation, acquisition-related contingent consideration, acquisition related contingent liability, impairment of goodwill and intangible assets, stock-based compensation expense, income taxes, revenue recognition and product sales allowances. Management bases its estimates on historical experience or on various other assumptions, including information received from its service providers, which it believes to be reasonable under the circumstances. Actual results could differ from those estimates.
Basis of Consolidation
The consolidated financial statements include the Company’s accounts and those of its wholly-owned subsidiaries: Hyperion Therapeutics Ltd., Hyperion Therapeutics Ireland Holding Ltd., Hyperion Therapeutics Ireland Operating Ltd., Hyperion Therapeutics Israel Holding Corp. Ltd., Penville Limited and Andromeda Biotech Ltd. All intercompany accounts and transactions have been eliminated.
Concentration of Credit Risk and Significant Customers
The Company’s cash and cash equivalents and investments are maintained with financial institutions located in and outside the United States. Deposits in these institutions may exceed the amount of insurance provided on such deposits. The Company has not recognized any losses from credit risks during the periods presented and management does not believe that the Company is exposed to significant credit risk from its cash and cash equivalents or investments.
The Company is also subject to credit risk from its accounts receivables related to its product sales. The Company monitors its exposure within accounts receivable and records a reserve against uncollectible accounts receivable as necessary. The Company extends credit to a specialty distributor in the United States and to international distributors, pharmacies and hospitals outside the United States. Customer creditworthiness is monitored and collateral is not required. As of December 31, 2014, there were no credit losses on the Company’s accounts receivable. As of December 31, 2014 and 2013, the specialty distributor in the United States accounted for 87% and 64% of accounts receivable balance, respectively. One international distributor accounted for 10% and 17% of the accounts receivable balance for the year ended December 31, 2014 and 2013, respectively.
The specialty distributor accounted for 91% and 88% of the net product revenue for the year ended December 31, 2014 and 2013, respectively.
Fair Value of Financial Instruments
The Company measures certain financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid for to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. The carrying amounts of the Company’s financial instruments, including cash equivalents, short-term investments, accounts payable, and accrued liabilities, approximate fair value due to their short maturities. The carrying amounts of long-term investments, acquisition-related contingent consideration and acquisition related contingent liability represent their estimated fair values. The fair value of the notes payable are based on the present value of expected future cash flows and assumptions about current interest rates and the creditworthiness of the Company.
Business Combinations
The Company allocates the purchase price of an acquired business to the tangible and intangible assets acquired and liabilities assumed based upon their estimated fair values on the acquisition date. Any excess of the
F-10
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
purchase price over the fair value of the net assets acquired is recorded as goodwill. Acquired in-process research and development (“IPR&D”) is recognized at fair value and initially characterized as an indefinite-lived intangible asset, irrespective of whether the acquired IPR&D has an alternative future use. The purchase price allocation process requires management to make significant estimates and assumptions, especially at the acquisition date with respect to intangible assets. Direct transaction costs associated with the business combination are expensed as incurred.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents include money market funds and various deposit accounts.
Investments
The Company determines the appropriate classification of its investments in debt and equity securities at the time of purchase. All of the Company’s securities are classified as available-for-sale and reported in short-term investments or long-term investments based on maturity dates and whether such assets are reasonably expected to be realized in cash or sold or consumed during the normal cycle of business. Available-for-sale investments are recorded at fair value, with unrealized gains or losses included in Accumulated Other Comprehensive Loss on the Company’s Consolidated Balance Sheets, exclusive of other-than-temporary impairment losses, if any. Short-term and long-term investments are comprised of corporate notes, commercial paper, U.S. federal government agency securities and certificates of deposit.
Accounts Receivable
Trade accounts receivable are recorded net of product sales allowances for prompt-payment discounts, chargebacks, and doubtful accounts. Estimates for chargebacks and prompt-payment discounts are based on contractual terms, historical trends and expectations regarding the utilization rates for these programs. At December 31, 2014, the Company had no allowances for doubtful accounts.
Inventories
Inventories are valued at the lower of cost or market, with cost computed at standard cost (which approximates actual cost) on a first-in-first-out (FIFO) cost method and consists of raw materials, work-in-progress and finished goods. Costs capitalized as inventories include third party manufacturing costs, associated compensation related costs of personnel indirectly involved in the manufacturing process and other overhead costs such as ancillary supplies. Subsequent to FDA approval of RAVICTI on February 1, 2013, the Company began capitalizing RAVICTI inventories as the related costs were expected to be recoverable through the commercialization of the product. Costs incurred prior to the FDA approval of RAVICTI have been recorded as research and development expense in the consolidated statements of operations. If information becomes available that suggest that inventories may not be realizable, the Company may be required to expense a portion or all of the previously capitalized inventories.
Products that have been approved by the FDA or other regulatory authorities, such as RAVICTI, are also used in clinical programs, to assess the safety and efficacy of the products for usage in diseases that have not
F-11
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
been approved by the FDA or other regulatory authorities. The form of RAVICTI utilized for both commercial and clinical programs is identical and, as a result, the inventory has an “alternative future use” as defined in authoritative guidance. Raw materials and purchased drug product associated with clinical development programs are included in inventory and charged to research and development expense when the product enters the research and development process and no longer can be used for commercial purposes and, therefore, does not have an “alternative future use”.
Intangible Assets
Intangible assets are recorded at acquisition cost less accumulated amortization and impairment. Intangible asset with finite lives are amortized over their estimated useful life using the economic use method, which reflects the pattern that the economic benefits of the intangible asset are consumed as revenue is generated. The pattern of consumption of the economic benefits is estimated using the future projected cash flows of the intangible asset.
IPR&D
The fair value of IPR&D acquired through a business combination is capitalized as an indefinite-lived intangible asset until the completion or abandonment of the related research and development activities. When the related research and development is completed, the asset is assigned a useful life and amortized.
The fair value of an IPR&D intangible asset is determined using an income approach. This approach starts with a forecast of the net cash flows expected to be generated by the asset over its estimated useful life. The net cash flows reflect the asset’s stage of completion, the probability of technical success, the projected costs to complete, expected market competition, and an assessment of the asset’s life-cycle. The net cash flows are then adjusted to present value by applying an appropriate discount rate that reflects the risk factors associated with the cash flow streams.
Goodwill
Goodwill represents the excess of the purchase price of acquired businesses over the estimated fair value of the identifiable net assets acquired. Goodwill is not amortized but is tested for impairment at least annually at the reporting unit level or more frequently if events or changes in circumstances indicate that the asset might be impaired.
Goodwill is tested for impairment annually or more frequently if events or changes in circumstances between annual tests indicate that the asset may be impaired. Impairment loss is recognized based on a comparison of the fair value of the asset to its carrying value, without consideration of any recoverability.
Impairment of Long-lived Assets
The Company reviews its property and equipment, intangible assets subject to amortization and other long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset class may not be recoverable. Indicators of potential impairment include: an adverse change in legal factors or in the business climate that could affect the value of the asset; an adverse change in the extent or manner in which the asset is used or is expected to be used, or in its physical condition; and current or forecasted operating
F-12
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
or cash flow losses that demonstrate continuing losses associated with the use of the asset. If indicators of impairment are present, the asset is tested for recoverability by comparing the carrying value of the asset to the related estimated undiscounted future cash flows expected to be derived from the asset. If the expected cash flows are less than the carrying value of the asset, then the asset is considered to be impaired and its carrying value is written down to fair value, based on the related estimated discounted future cash flows.
Indefinite-lived intangible assets, including acquired IPR&D, are tested for impairment annually or more frequently if events or changes in circumstances between annual tests indicate that the asset may be impaired. Impairment losses on indefinite-lived intangible assets are recognized based on a comparison of the fair value of the asset to its carrying value, without consideration of any recoverability.
Acquisition-Related Contingent Consideration
Acquisition-related contingent consideration, which consists primarily of potential milestone payments and royalty obligations, is recorded in the consolidated balance sheets at its acquisition date estimated fair value, in accordance with the acquisition method of accounting. The fair value of the acquisition-related contingent consideration is remeasured each reporting period, with changes in fair value recorded in the consolidated statements of operations. Changes in the fair value of the acquisition-related contingent consideration obligations result from several factors including changes in discount periods and rates, changes in the timing and amount of revenue estimates and changes in probability assumptions with respect to the likelihood of achieving specified milestone criteria. The fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined in the fair value hierarchy.
Acquisition-Related Contingent Liability
Acquisition-related contingent liability, which consists primarily of potential milestone payments and royalty obligations, and potential payments to certain employees of Andromeda is recorded in the consolidated balance sheets at its acquisition date estimated fair value. The Company reassesses the probability and estimates associated with the contingent liability at each reporting period. For liability payable to third parties, any increase in the liability is recorded in the consolidated statements of operations. For liabilities payable to employees, the Company accounts for the liability in accordance with Financial Accounting Stands Board (“FASB”) Accounting Stand Codification (“ASC”) 450 Contingencies.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation and amortization. All property and equipment is depreciated on a straight-line basis over the following estimated useful lives:
| Computer and office equipment |
3 – 5 years | |||
| Software |
3 years | |||
| Furniture and fixtures |
3 years |
Leasehold improvements are amortized over the lesser of their useful life or the term of the applicable lease. Upon sale or retirement of assets, the cost and related accumulated depreciation and amortization are removed from the consolidated balance sheet and the resulting gain or loss is reflected in operations. Maintenance and repairs are charged to operations as incurred.
F-13
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
2012 Reverse Stock Split
The Company effected a 1-for-6.09 reverse stock split of its common stock and preferred stock on July 12, 2012. Accordingly, all share and per share amounts for all periods presented in these consolidated financial statements and notes thereto, have been adjusted retroactively to reflect this reverse stock split.
Preclinical and Clinical Trial Accruals
The Company’s clinical trial accruals are based on estimates of patient enrollment and related costs at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and clinical research organizations that conduct and manage preclinical and clinical trials on the Company’s behalf. The Company accrues expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity according to the protocol. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, the Company modifies the estimates of accrued expenses accordingly. To date, the Company has had no significant adjustments to accrued preclinical and clinical trial expenses.
Revenue Recognition
The Company recognizes revenue in accordance with ASC 605, Revenue Recognition, when the following criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred and risk of loss has passed; the seller’s price to the buyer is fixed or determinable and collectability is reasonably assured. The Company determines that persuasive evidence of an arrangement exists based on written contracts that defined the terms of the arrangements. In addition, the Company determines that services have been delivered in accordance with the arrangement. The Company assesses whether the fee is fixed or determinable based on the payment terms associated with the transaction and whether the sales price is subject to refund or adjustment. The Company assesses collectability based primarily on the customer’s payment history and on the creditworthiness of the customer.
Product Revenue, net: The Company’s product revenue represents sales of RAVICTI and BUPHENYL which are recognized once all four revenue recognition criteria described above are met. The Company recognizes revenue net of product sales allowances. Product shipping and handling costs are included in cost of sales. Prior to June 2014, revenue from the sale of RAVICTI was recognized based on the amount of product sold through to the end user consumer. Starting June 2014, the Company could reasonably estimate and determine sales allowances, therefore the Company began recognizing RAVICTI revenue at the point of sale to the specialty distributor, which resulted in the one-time non-recurring recognition of an additional $8.6 million in net revenues during the year ended December 31, 2014. The impact of this change resulted in a decrease in net loss of $7.9 million for year ended December 31, 2014, or $0.39 per basic and diluted share.
Product Sales Allowances: The Company establishes reserves for prompt-payment discounts, government and commercial rebates, product returns and chargebacks. Allowances relate to prompt-payment discounts and are recorded at the time of revenue recognition, resulting in a reduction in product sales revenue and a decrease in trade accounts receivables. Accruals related to government rebates, product returns and other applicable allowances such as distributor fees are recognized at the time of revenue recognition, resulting in a reduction in product sales and an increase in accrued expenses or a reduction in the related accounts receivable.
| • | Prompt-payment discounts: The specialty distributor and specialty pharmacies are offered prompt payment discounts. The Company expects the specialty distributor and specialty pharmacies will earn |
F-14
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
| prompt payment discounts and, therefore deduct the full amount of these discounts from total product sales when revenues are recognized. The Company records prompt-payment discounts as allowances against accounts receivable on the consolidated balance sheets. |
| • | Rebates: Allowances for rebates include mandated discounts under the Medicaid Drug Rebate Program. Rebate amounts are based upon contractual agreements or legal requirements with public sector (e.g. Medicaid) benefit providers. Rebates are amounts owed after the final dispensing of the product to a benefit plan participant and are based upon contractual agreements or legal requirements with public sector benefit providers. The allowance for rebates is based on statutory discount rates and expected utilization. The Company estimates for expected utilization of rebates based on historical data and data received from the specialty pharmacies. Rebates are generally invoiced and paid in arrears so that the accrual balance consists of an estimate of the amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known prior quarter’s unpaid rebates. If actual future rebates vary from estimates, the Company may need to adjust prior period accruals, which would affect revenue in the period of adjustment. Allowance for rebates are recorded in accrued liabilities on the consolidated balance sheets. |
| • | Chargebacks: Chargebacks are discounts that occur when contracted customers purchase directly from a specialty distributor. Contracted customers, which primarily consist of Public Health Service institutions, non-profit clinics, and Federal government entities purchasing via the Federal Supply Schedule, generally purchase the product at a discounted price. The specialty distributor, in turn, charges back to the Company the difference between the price initially paid by the specialty distributor and the discounted price paid to the specialty distributor by the customer. The allowance for chargebacks is based on historical sales data and known sales to contracted customers. |
| • | Medicare Part D Coverage Gap: Medicare Part D prescription drug benefit mandates manufacturers to fund 50% of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients. The Company estimates for the expected Medicare Part D coverage gap are based on historical invoices received and in part from data received from the specialty pharmacies. Funding of the coverage gap is generally invoiced and paid in arrears so that the accrual balance consists of an estimate of the amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known prior quarters. If actual future funding varies from estimates, the Company may need to adjust prior period accruals, which would affect revenue in the period of adjustment. Estimates of the Medicare Part D coverage gap are recorded in accrued liabilities on the consolidated balance sheets. |
| • | Distribution Service Fees: The Company has a written contract with the specialty distributor that includes terms for distribution-related fees. Distributor fees are calculated at percentage of gross sales based upon agreed contracted rate. The Company accrues distributor fees at the time of the revenue recognition, resulting in reduction of product sales revenue and the recording of accrued liabilities on the consolidated balance sheets. The Company records distribution and other fees paid to its customers as a reduction of revenue, unless it receives an identifiable and separate benefit for the consideration and it can reasonably estimate the fair value of the benefit received. If both conditions are met, the Company records the consideration paid to the customer as an operating expense. These costs are typically known at the time of sale. |
| • | Product Returns: Consistent with industry practice, the Company generally offers customers a limited right to return. The Company accepts returns of products from patients resulting from breakage as |
F-15
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
| defined within the Company’s returns policy. Additionally, the Company considers several other factors in the estimation process including the expiration dates of product shipped, third party data in monitoring channel inventory levels, shelf life of the product, prescription trends and other relevant factors. Provisions for estimated product returns are recorded as accrued liabilities on the consolidated balance sheets. |
| • | Co-payment assistance: The Company provides a cash donation to a non-profit third party organization which supports patients, who have commercial insurance and meet certain financial eligibility requirements, with co-payment assistance and travel costs. The amount of co-payment assistance is accounted for by the Company as a reduction of revenues. |
Cost of sales
Cost of sales includes third-party manufacturing cost of products sold, royalty fees, and other indirect costs related to personnel compensation, shipping and supplies. Costs incurred prior to FDA approval of RAVICTI have been recorded as research and development expense in the Company’s consolidated statement of operations. The Company expects that cost of RAVICTI sales as a percentage of revenue will increase in future periods as product manufactured prior to FDA approval, and therefore fully expensed, has been utilized. Cost of BUPHENYL sales for 2013 as a percentage of revenue was higher and not indicative of cost of sales in future periods due to the recording of the step-up value on BUPHENYL inventories acquired from Ucyclyd which is expensed to cost of sales as that inventory is sold. As of December 31, 2014, the entire fair value of acquired BUPHENYL inventory has been charged to expense, primarily cost of sales.
Foreign Currency Translations
Operations in non-U.S. entities are recorded in the functional currency of each entity. For financial reporting purposes, the functional currency of an entity is determined by a review of the source of an entity’s most predominant cash flows. The reporting and functional currency of the Company and its subsidiaries is the U.S. dollar.
Gains and losses resulting from foreign currency transactions are translated at the weighted average rate of exchange prevailing during the period. Any monetary asset or liability denominated in foreign currency and is translated at the rate of exchange in effect on the balance sheet date. Any gains and losses resulting from foreign currency translations are included in other income (expense)-net in the consolidated statements of operations. The Company does not currently utilize and has not in the past utilized any foreign currency hedging strategies to mitigate the effect of its foreign currency exposure.
Research and Development Expenses
Costs related to research and development of products are charged to expense as incurred. Research and development costs include, but are not limited to, payroll and personnel expenses, clinical trial supplies, fees for clinical trial services, consulting costs and allocated overhead, including rent, equipment, depreciation and utilities.
Stock-Based Compensation
The Company accounts for stock-based employee compensation arrangements in accordance with provisions of ASC 718, Compensation — Stock Compensation. ASC 718 requires the recognition of
F-16
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
compensation expense, using a fair-value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. The Company calculates the fair value of stock options using the Black-Scholes method and expenses using the straight-line attribution approach.
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company accounts for uncertain tax positions in accordance with ASC 740-10, Accounting for Uncertainty in Income Taxes. The Company assesses all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position’s sustainability and is measured at the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and the Company will determine whether (i) the factors underlying the sustainability assertion have changed and (ii) the amount of the recognized tax benefit is still appropriate. The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
Comprehensive Income (Loss)
Comprehensive income (loss) is comprised of net income (loss) and other comprehensive loss. Other comprehensive loss includes changes in stockholders’ equity that are excluded from net income (loss), specifically changes in unrealized gains and losses on the Company’s available-for-sale securities.
Net Income (Loss) per Share of Common Stock
Basic earnings per share is computed by dividing the net income (loss) by the weighted average number of shares of common stock outstanding during the periods presented. The computation of diluted earnings per share is similar to the computation of basic earnings per share, except that the denominator is increased for the assumed exercise of dilutive options and other potentially dilutive securities using the treasury stock method, unless the effect is antidilutive.
Recent Accounting Pronouncements
In July 2013, FASB issued ASU No. 2013-11, Income Taxes (Topic 740): Presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. Under the amendments of this update an entity is required to present an unrecognized tax benefit, or a portion of an unrecognized tax benefit in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward, except as follows. To the extent a net operating loss carryforward, a similar tax loss, or a tax credit carryforward is not available at the reporting date under the tax
F-17
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
law of the applicable jurisdiction to settle any additional income taxes that would result from the disallowance of a tax position or the tax law of the applicable jurisdiction does not require the entity to use, and the entity does not intend to use, the deferred tax asset for such purpose, the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. The assessment of whether a deferred tax asset is available is based on the unrecognized tax benefit and deferred tax asset that exist at the reporting date and should be made presuming disallowance of the tax position at the reporting date. For example, an entity should not evaluate whether the deferred tax asset expires before the statute of limitations on the tax position or whether the deferred tax asset may be used prior to the unrecognized tax benefits being settled. The provisions of this update was effective prospectively for the Company in fiscal years beginning after December 15, 2013 with early adoption and retrospective application permitted. The Company adopted this guidance on January 1, 2014. The implementation did not have an impact on the Company’s consolidated financial statements.
In May 2014, FASB issued Accounting Standards Update 2014-09 (ASU 2014-09), Revenue from Contracts with Customers. ASU 2014-09 will supersede the revenue recognition requirements in Revenue Recognition (Topic 605) and requires entities to recognize revenue in a way that depicts the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. ASU 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period, which for the Company is January 1, 2017. Early adoption is not permitted. The Company is currently evaluating the potential impact the adoption of ASU 2014-09 will have on the Company’s consolidated financial statements.
| 3. | Collaboration Agreement with Ucyclyd Pharma, Inc. |
In March 2012, the Company entered into the purchase agreement with Ucyclyd under which the Company purchased the worldwide rights to RAVICTI and the amended and restated collaboration agreement (the “restated collaboration agreement”) under which Ucyclyd granted the Company an option to purchase all of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL at a fixed price at a future defined date, plus subsequent milestone and royalty payments, subject to Ucyclyd’s right to retain AMMONUL for a predefined price. The restated collaboration agreement superseded the collaboration agreement with Ucyclyd, dated August 23, 2007, as amended. The entry into the purchase agreement and the restated collaboration agreement resolved a dispute that the two parties had with respect to their rights under the prior collaboration agreement.
Under the purchase agreement, the Company made a payment of $6.0 million of which (i) $5.7 million was allocated to the worldwide rights to RAVICTI and (ii) $0.3 million was allocated to the option to purchase rights to BUPHENYL and AMMONUL, based on their relative fair values. The allocated amount to the rights to RAVICTI of $5.7 million was recorded to research and development expense in the consolidated statements of operations for the period ended March 31, 2012 due to the uncertainty of an alternative future use. The allocated amount for the option to purchase rights to BUPHENYL and AMMONUL in the amount of $0.3 million was included within other current assets and was subsequently offset against the gain recognized from the settlement of retention option (see Note 4).
The Company will also pay tiered mid to high single digit royalties on global net sales of RAVICTI and may owe regulatory milestones of up to $15.8 million related to approval of GPB in HE, regulatory milestones of up to $7.3 million per indication for approval of GPB in indications other than UCD or HE, and net sales milestones of up to $38.8 million if GPB is approved for use in indications other than UCD (such as HE) and all annual sales targets are reached.
F-18
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
In addition, the intellectual property license agreements executed between Ucyclyd and Dr. Marshall L. Summar, (“Summar”) and Ucyclyd and Brusilow Enterprises, LLC, (“Brusilow”) were assigned to the Company, and the Company has assumed the royalty and milestone obligations under the Brusilow agreement for sales of RAVICTI in any indication and the royalty obligations under the Summar agreement on sales of GPB to treat HE. The Brusilow and Summar agreements provide that royalty obligations will continue, without adjustment, even if generic versions of the licensed products are introduced and sold in the relevant country.
Under the terms of the restated collaboration agreement, the Company had an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL, subject to Ucyclyd’s option to retain rights to AMMONUL. The Company was permitted to exercise this option for 90 days beginning on the earlier of the date of the approval of RAVICTI for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. The upfront purchase price for AMMONUL and BUPHENYL was $22.0 million. If the RAVICTI New Drug Application (“NDA”) for UCD was not approved by January 1, 2013, then Ucyclyd was obligated to make monthly payments of $0.5 million to the Company until the earliest of (1) FDA approval of the RAVICTI NDA for UCD, (2) June 30, 2013 and (3) the Company’s written notification of the decision not to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL.
On February 1, 2013, the FDA approved RAVICTI for the treatment of UCD in adult and pediatric patients two years of age and older. In accordance with the restated collaboration agreement, Ucyclyd made a payment of $0.5 million during the quarter ended March 31, 2013.
On April 29, 2013, the Company exercised its option to purchase BUPHENYL and AMMONUL. Ucyclyd subsequently exercised it’s time-limited right to elect to retain all rights to AMMONUL for a contractual purchase price of $32.0 million (“retention amount”). Upon closing of the transaction, Ucyclyd paid the Company a net payment of $11.0 million, which reflects the Company’s contractual purchase price for Ucyclyd’s worldwide rights to BUPHENYL in the amount of $19.0 million being off-set against Ucyclyd’s retention amount for AMMONUL and a $2.0 million payment due to Ucyclyd for inventory that the Company purchased from Ucyclyd. The Company has retained a right of first negotiation should Ucyclyd later decide to sell, exclusively license, or otherwise transfer the AMMONUL assets to a third party.
On May 31, 2013, the Company completed the acquisition of BUPHENYL. Upon Ucyclyd’s exercise of its retention option, the Company recorded a gain of approximately $31.1 million in its consolidated statement of operations. The amount of gain is comprised of (i) fair value of BUPHENYL of $20.4 million and (ii) net cash received from Ucyclyd of $11.0 million off-set by (iii) the $0.3 million carrying value of the option to purchase the rights to BUPHENYL and AMMONUL.
| 4. | Acquisitions |
Acquisition of Andromeda
Description of the Transaction
On April 23, 2014, Hyperion Therapeutics Israel Holding Corp. Ltd. (“Hyperion Israel”), a wholly-owned subsidiary of the Company entered into a share purchase agreement (“SPA”) with Clal Biotechnology Industries Ltd. (“Clal”) to purchase all outstanding shares of Andromeda. Andromeda was incorporated in Israel in 2007 and was primarily focused on the development of DiaPep277.
F-19
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
On June 12, 2014 (the “Acquisition Date”), Hyperion Israel completed the purchase of all of the outstanding ordinary shares of Andromeda. The Andromeda acquisition is accounted for as a business combination. The results of operations of Andromeda have been included in the Company’s consolidated statements of operations starting from the Acquisition Date, the date on which the Company obtained effective control of Andromeda.
Fair Value of Consideration Transferred as of the Acquisition Date
The Company accounted for the acquisition of Andromeda as a business combination under the acquisition method of accounting. The Acquisition Date fair value of the total consideration transferred to acquire Andromeda was as follows:
| Amounts Recognized as of the acquisition date (in thousands) |
||||
| Cash paid |
$ | 14,345 | ||
| Fair value of Company’s common stock issued (312,869 shares) |
7,778 | * | ||
| Acquisition-related contingent consideration |
68,961 | |||
|
|
|
|||
| Total fair value of consideration transferred |
$ | 91,084 | ||
|
|
|
|||
| * | The fair value of the common stock was measured using the Company’s closing stock price on June 12, 2014. |
The Company estimated the fair value of the acquisition-related contingent consideration on the acquisition date using a probability-weighted income approach, which reflects the probability and timing of future payments. This fair value measurement on the acquisition date is based on key assumptions such as the anticipated timelines and probability of achieving development, regulatory approval or sales-based milestone events and projected revenues. The resulting probability-weighted cash flows are discounted using estimated discount rate that commensurate with the risks of the expected cash flows attributable to the various milestones. The material factors that may impact the fair value are the probabilities of achieving the related milestones and the discount rate. Significant increases or decreases in any of the probabilities of success would result in a significantly higher or lower fair value, respectively, and commensurate changes to this liability. The fair value relating to the acquisition-related contingent consideration at each reporting date will be updated with the changes in fair value reflected in the company’s consolidated statements of operations.
F-20
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Assets Acquired and Liabilities Assumed as of the Acquisition Date
The following table summarizes the provisional allocation of purchase price to the fair values of the assets acquired and liabilities assumed as of the acquisition date:
| Amounts Recognized as of the acquisition date (in thousands) |
||||
| Assets acquired |
||||
| IPR&D |
$ | 135,712 | ||
| Cash and cash equivalents |
306 | |||
| Prepaid expenses |
95 | |||
| Property and equipment, net |
22 | |||
| Other assets |
578 | |||
| Liabilities assumed |
||||
| Current liabilities |
(9,079 | ) | ||
| Deferred tax liability |
(6,051 | ) | ||
| Acquisition related contingent liability |
(34,288 | ) | ||
|
|
|
|||
| Total identifiable net assets |
87,295 | |||
| Goodwill |
3,789 | |||
|
|
|
|||
| Total consideration transferred |
$ | 91,084 | ||
|
|
|
|||
The amounts were considered preliminary and were subject to change once the Company finalizes the determination of the fair value of assets acquired and liabilities assumed. Thus, these amounts were subject to refinement and final determination of the fair values of net assets acquired and may result in certain adjustments to the amounts presented above.
The contingent liabilities amounting to $34.3 million relate to potential royalties and milestone payments and potential payments. The Company reassesses the probability and estimates associated with the contingent liability at each reporting period. For liability payable to third parties, any increase in the liability is recorded in the consolidated statements of operations. For liabilities payable to employees in exchange for services, the Company accounts for this liability arrangement as employee compensation over the applicable service period and in accordance with ASC 450 Contingencies.
The goodwill primarily represents the tax impact of the IPR&D and acquisition related contingencies as well as acquiring Andromeda’s assembled workforce.
Measurement period adjustments to provisional allocation of purchase price
On September 8, 2014 the Company announced the termination of further development of DiaPep277 beyond completion of the ongoing clinical trial as a result of evidence that the Company uncovered that certain employees of Andromeda engaged in serious misconduct that compromised clinical trial results. Additional evidence indicates that the biostatistics firm and certain Andromeda employees continued the improper practice of sharing and examining un-blinded data from the ongoing DIA-AID 2 trial. The Company intends to complete the ongoing Phase 3 clinical trial of DiaPep277 but does not expect to develop the product further.
The Company determined that the facts and circumstances described above existed as of the acquisition date and were not the result of intervening events subsequent to the acquisition date. In accordance with ASC 805,
F-21
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
during the measurement period, the Company recognizes adjustments to the provisional amounts as if the accounting for the business combination had been completed at the acquisition date. Accordingly, the Company re-measured the preliminary determination of the fair values of assets acquired and liabilities assumed as of the acquisition date. The measurement period adjustments primarily relate to the: (i) decrease in the estimated fair value of intangible assets, (ii) reductions in the acquisition related contingent liability, and (iii) the tax impact of pre-tax measurement period adjustments.
Adjusted Assets Acquired and Liabilities Assumed — Measurement Period Adjustments
The following table summarizes the adjusted provisional allocation of purchase price to the fair values of the assets acquired and liabilities assumed as of the Acquisition Date:
| Amounts Recognized as of September 30, 2014 (as adjusted) (in thousands) |
||||
| Assets acquired |
||||
| IPR&D |
$ | — | * | |
| Cash and cash equivalents |
306 | |||
| Prepaid expenses |
95 | |||
| Property and equipment, net |
22 | |||
| Other assets |
578 | |||
| Liabilities assumed |
||||
| Current liabilities |
(9,079 | ) | ||
| Deferred tax liability |
— | ** | ||
| Acquisition related contingent liability |
— | * | ||
|
|
|
|||
| Total identifiable net assets (liabilities) |
(8,078 | ) | ||
| Adjusted Goodwill |
30,201 | |||
|
|
|
|||
| Total consideration transferred |
$ | 22,123 | ||
|
|
|
|||
Adjusted Fair Value of Consideration Transferred
| Amounts Recognized as of September 30, 2014 (as adjusted) (in thousands) |
||||
| Cash paid |
$ | 14,345 | ||
| Fair value of Company’s common stock issued (312,869 shares) |
7,778 | |||
| Acquisition-related contingent consideration |
— | * | ||
|
|
|
|||
| Total fair value of consideration transferred |
$ | 22,123 | ||
|
|
|
|||
| * | The Company has assigned no value to the IPR&D as a result of the evidence obtained related to the improper practices regarding the clinical trials. The Company had determined that the contingent consideration milestones and contingent liability milestones will not be achieved. |
| ** | The reduction in deferred tax liability relates to the tax impact of the IPR&D and acquisition- related contingencies. |
F-22
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The Company’s allocation of purchase price to the fair values of the assets acquired and liabilities assumed as of acquisition date was determined to be final as the Company obtained the information necessary to complete the measurement process.
Acquisition of BUPHENYL from Ucyclyd Pharma, Inc.
Description of the Transaction
As discussed in Note 3, under the terms of the restated collaboration agreement, on April 29, 2013, the Company exercised its option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL. On May 17, 2013 Ucyclyd exercised its time-limited right to elect to retain all rights to AMMONUL. On May 31, 2013 (the “Acquisition Date”), the Company completed the acquisition of BUPHENYL. Accordingly, BUPHENYL results are included in Hyperion’s consolidated financial statements from the date of the acquisition. For the period from June 1, 2013 to December 31, 2013, BUPHENYL net revenue was $11.5 million.
The Company acquired BUPHENYL to enhance its commercial product portfolio and to allow the Company an opportunity to serve the entire UCD patient population, including those less than two years of age or for those patients who may prefer BUPHENYL.
Purchase Consideration and Assets Acquired
The Company accounted for the acquisition of BUPHENYL as a business combination under the acquisition method of accounting. On the Acquisition Date, the Company received a net payment of $11.0 million, which reflected the $32.0 million retention amount for AMMONUL due to the Company less the $19.0 million contractual purchase price for BUPHENYL due to Ucyclyd and a $2.0 million payment due to Ucyclyd for inventory that the Company purchased from Ucyclyd.
The fair value of purchase consideration was estimated based upon the fair value of assets acquired. The estimated fair value attributed to the BUPHENYL product rights was determined on a discounted forecast of the estimated net future cash flows to be generated from the product rights and has an expected useful life of 10 years. The following table summarizes the allocation of purchase price to the fair values of the assets acquired as of the acquisition date:
| (in thousands) | ||||
| Inventories |
$ | 3,900 | ||
| Intangible Asset — BUPHENYL Product rights |
16,500 | |||
|
|
|
|||
| Total |
$ | 20,400 | ||
|
|
|
|||
F-23
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Gain from Settlement of Retention Option
In connection with the allocation between the BUPHENYL acquisition described above and Ucyclyd’s exercise of its retention option, the Company recorded a gain of $31.1 million. The amount of gain is comprised of (i) fair value of BUPHENYL of $20.4 million and (ii) net cash received from Ucyclyd of $11.0 million off-set by (iii) the $0.3 million carrying value of the option to purchase the rights to BUPHENYL and AMMONUL. The following table summarizes the results of the Company’s allocation:
| (in thousands) | ||||
| Ucyclyd’s retention option amount |
$ | 32,000 | ||
| Amount due to Ucyclyd for BUPHENYL product rights |
(19,000 | ) | ||
| Amount due to Ucyclyd for inventory |
(2,038 | ) | ||
|
|
|
|||
| Net payment received from Ucyclyd |
10,962 | |||
| Option to purchase the rights to BUPHENYL and AMMONUL |
(283 | ) | ||
| Fair value of BUPHENYL |
20,400 | |||
|
|
|
|||
| Gain from settlement of retention option |
$ | 31,079 | ||
|
|
|
|||
Pro forma Impact of Business Combination
The following unaudited pro forma information is based on the assumption that the Andromeda acquisition occurred on January 1, 2013 and BUPHENYL acquisition occurred on January 1, 2012. The unaudited pro forma information is presented for comparative purposes only and is not necessarily indicative of the financial position or results of operations which would have been reported had the Company completed the acquisitions during these periods or which might be reported in the future. The unaudited pro forma information reflects primarily the application of the following adjustments:
| • | the elimination of the historical research and development expenses and general and administration expenses related to options granted to employees which were cancelled as part of the Andromeda acquisition; |
| • | the elimination of the historical interest expenses related to loans provided by former shareholders which were cancelled as part of the Andromeda acquisition; |
| • | the exclusion of acquisition-related costs incurred for the acquisitions; |
| • | the exclusion of impairment of goodwill recognized in relation to the Andromeda acquisition; |
| • | the inclusion of amortization expense related to the fair value of the intangible asset acquired relating to BUPHENYL; |
| • | the inclusion of the step-up value related to inventory sold that was acquired as part of the BUPHENYL acquisition. Such amounts are included in the applicable comparative period for purposes of pro forma financial information; and |
| • | as noted above, the Company issued 312,869 shares of its common stock as part of the Andromeda acquisition. The common shares were included in the pro-forma earnings per share calculation. |
F-24
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The unaudited pro forma information is not necessarily indicative of what the Company’s consolidated results of operations actually would have been had the acquisition of Andromeda been completed on January 1, 2013 and acquisition of BUPHENYL been completed on January 1, 2012. In addition, the unaudited pro forma information does not purport to project the future results of operations of the Company.
| Year Ended December 31, |
||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| ( in thousands) | ||||||||||||
| Net revenues |
$ | 113,584 | $ | 52,900 | $ | 21,501 | ||||||
| Net income (loss) |
20,390 | (12,199 | ) | (29,201 | ) | |||||||
| Net income (loss) per common share |
||||||||||||
| Basic |
0.99 | (0.62 | ) | (4.02 | ) | |||||||
| Diluted |
0.93 | (0.62 | ) | (4.02 | ) | |||||||
Acquisition-related Costs
Acquisition-related expenses consist of transaction costs which represent external costs directly related to the acquisition of Andromeda and primarily include expenditures for professional fees such as legal, accounting and other directly related incremental costs incurred to close the acquisition. Acquisition-related expenses for the year ended December 31, 2014 was $1.3 million. Acquisition-related expenses for the year ended December 31, 2013 were $0.6 million. These expenses were recorded to selling and general administrative expense in the consolidated statements of operations.
| 5. | Investments |
All investments were classified as available-for-sale at December 31, 2014 and December 31, 2013. The principal amounts of investments by contractual maturity at December 31, 2014 and December 31, 2013 are summarized in the tables below:
| Contractual Maturity Date for the Years Ending December 31, |
Total Book Value at December 31, 2014 |
Unrealized Loss |
Aggregate Fair Value at December 31, 2014 |
|||||||||||||||||
| 2015 | 2016 | |||||||||||||||||||
| Certificates of deposit |
$ | 18,405 | $ | 6,240 | $ | 24,645 | $ | (35 | ) | $ | 24,610 | |||||||||
| Corporate notes |
12,613 | 3,011 | 15,624 | (12 | ) | 15,612 | ||||||||||||||
| Commercial paper |
3,494 | — | 3,494 | (3 | ) | 3,491 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 34,512 | $ | 9,251 | $ | 43,763 | $ | (50 | ) | $ | 43,713 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Contractual Maturity Date for the Years Ending December 31, |
Total Book Value at December 31, 2013 |
Unrealized Loss |
Aggregate Fair Value at December 31, 2013 |
|||||||||||||||||
| 2014 | 2015 | |||||||||||||||||||
| Certificates of deposit |
$ | 10,525 | $ | 2,845 | $ | 13,370 | $ | (27 | ) | $ | 13,343 | |||||||||
| Corporate notes |
6,047 | 12,960 | 19,007 | (24 | ) | 18,983 | ||||||||||||||
| U.S. Government agency securities |
8,011 | — | 8,011 | (3 | ) | 8,008 | ||||||||||||||
| Commercial paper |
3,492 | — | 3,492 | (1 | ) | 3,491 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 28,075 | $ | 15,805 | $ | 43,880 | $ | (55 | ) | $ | 43,825 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-25
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The Company evaluated its investments and determined that there were no other-than-temporary impairments as of December 31, 2014.
The aggregate amounts of unrealized losses and related fair value of investments with unrealized losses as of December 31, 2014 and December 31, 2013 was as follows:
| Less Than 12 Months to Maturity |
12 Months or More to Maturity |
Total at December 31, 2014 | ||||||||||||||||||||||
| Aggregate Fair Value |
Unrealized Losses |
Aggregate Fair Value |
Unrealized Losses |
Aggregate Fair Value |
Unrealized Losses |
|||||||||||||||||||
| Certificates of deposit |
$ | 18,386 | $ | (19 | ) | $ | 6,224 | $ | (16 | ) | $ | 24,610 | $ | (35 | ) | |||||||||
| Corporate notes |
12,610 | (2 | ) | 3,002 | (10 | ) | 15,612 | (12 | ) | |||||||||||||||
| Commercial paper |
3,491 | (3 | ) | — | — | 3,491 | (3 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 34,487 | $ | (24 | ) | $ | 9,226 | $ | (26 | ) | $ | 43,713 | $ | (50 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Less Than 12 Months to Maturity |
12 Months or More to Maturity |
Total at December 31, 2013 | ||||||||||||||||||||||
| Aggregate Fair Value |
Unrealized Losses |
Aggregate Fair Value |
Unrealized Losses |
Aggregate Fair Value |
Unrealized Losses |
|||||||||||||||||||
| Certificates of deposit |
$ | 10,507 | $ | (18 | ) | $ | 2,836 | $ | (9 | ) | $ | 13,343 | $ | (27 | ) | |||||||||
| Corporate notes |
6,039 | (8 | ) | 12,944 | (16 | ) | 18,983 | (24 | ) | |||||||||||||||
| U.S. Government agency securities |
8,008 | (3 | ) | — | — | 8,008 | (3 | ) | ||||||||||||||||
| Commercial paper |
3,491 | (1 | ) | — | — | 3,491 | (1 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 28,045 | $ | (30 | ) | $ | 15,780 | $ | (25 | ) | $ | 43,825 | $ | (55 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| 6. | Fair Value Measurements |
The Company follows ASC 820-10, “Fair Value Measurements and Disclosures,” which among other things, defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, a three-tier fair value hierarchy has been established, which prioritizes the inputs used in measuring fair value as follows:
| • | Level 1 — Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date. |
| • | Level 2 — Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life. |
| • | Level 3 — Inputs reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model. |
F-26
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The following table presents the Company’s fair value hierarchy for assets and liabilities measured at fair value on a recurring basis as of December 31, 2014 and December 31, 2013 (in thousands):
| Fair Value Measurements at December 31, 2014 | ||||||||||||||||
| Quoted Price in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
||||||||||||||||
| Cash equivalents: |
||||||||||||||||
| Money market funds |
$ | 38,532 | $ | — | $ | — | $ | 38,532 | ||||||||
| Certificates of deposit |
— | 720 | — | 720 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cash equivalents |
$ | 38,532 | $ | 720 | $ | — | $ | 39,252 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Available-for-sale securities: |
||||||||||||||||
| Short-term: |
||||||||||||||||
| Certificates of deposit |
$ | — | $ | 18,386 | $ | — | $ | 18,386 | ||||||||
| Corporate notes |
— | 12,610 | — | 12,610 | ||||||||||||
| Commercial paper |
— | 3,491 | — | 3,491 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total short-term investments |
— | 34,487 | — | 34,487 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Long-term: |
||||||||||||||||
| Certificates of deposit |
— | 6,224 | — | 6,224 | ||||||||||||
| Corporate notes |
— | 3,002 | — | 3,002 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total long-term investments |
— | 9,226 | — | 9,226 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total available-for-sale securities |
$ | — | $ | 43,713 | $ | — | $ | 43,713 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair Value Measurements at December 31, 2013 | ||||||||||||||||
| Quoted Price in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
||||||||||||||||
| Cash equivalents: |
||||||||||||||||
| Money market funds |
$ | 38,506 | $ | — | $ | — | $ | 38,506 | ||||||||
| Certificates of deposit |
— | 480 | — | 480 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cash equivalents |
$ | 38,506 | $ | 480 | $ | — | $ | 38,986 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Available-for-sale securities: |
||||||||||||||||
| Short-term: |
||||||||||||||||
| Certificates of deposit |
$ | — | $ | 10,507 | $ | — | $ | 10,507 | ||||||||
| Commercial paper |
— | 3,491 | — | 3,491 | ||||||||||||
| Corporate notes |
— | 6,039 | — | 6,039 | ||||||||||||
| U.S. Government agency securities |
— | 8,008 | — | 8,008 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total short-term investments |
— | 28,045 | — | 28,045 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Long-term: |
||||||||||||||||
| Certificates of deposit |
— | 2,836 | — | 2,836 | ||||||||||||
| Corporate notes |
— | 12,944 | — | 12,944 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total long-term investments |
— | 15,780 | — | 15,780 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total available-for-sale securities |
$ | — | $ | 43,825 | $ | — | $ | 43,825 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-27
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The following table presents the carrying value and estimated fair value of the Company’s notes payable as of December 31, 2014 and December 31, 2013 (in thousands):
| December 31, 2014 | ||||||||
| Carrying Value |
Estimated Fair Value |
|||||||
| Term Loans (see Note 11) |
$ | 16,124 | $ | 16,746 | ||||
| December 31, 2013 | ||||||||
| Carrying Value |
Estimated Fair Value |
|||||||
| April and September 2012 Notes (see Note 11) |
$ | 8,273 | $ | 8,860 | ||||
Valuation Techniques
Level 1 Inputs
The Company classifies money market funds, which are valued based on quoted market prices in active markets with no valuation adjustment, as Level 1 assets within the fair value hierarchy.
Level 2 Inputs
Items classified as Level 2 within the valuation hierarchy consist of commercial paper, corporate notes, U.S. government agency securities and certificates of deposit. The Company estimates the fair values of these marketable securities by taking into consideration valuations obtained from third-party pricing sources. These pricing sources utilize industry standard valuation models, including both income and market-based approaches, for which all significant inputs are observable, either directly or indirectly, to estimate fair value. These inputs include market pricing based on real-time trade data for the same or similar securities, issuer credit spreads, benchmark yields, and other observable inputs. The Company validates the prices provided by our third-party pricing sources by obtaining market values from other pricing sources and analyzing pricing data in certain instances.
Level 3 Inputs
Notes Payable
The Company has determined that its notes payable would be classified as a Level 3 item in the fair value hierarchy. The fair value of these notes is based on the present value of expected future cash flows and assumptions about current interest rates and the credit worthiness of the Company.
Acquisition-related Contingent Consideration and Liabilities
In connection with the acquisition of Andromeda, the Company may be required to pay future consideration that is contingent upon the achievement of specified development, regulatory approval or sales-based milestone events. The Company estimates the fair value of the contingent consideration liabilities on the acquisition date and each reporting period thereafter using a probability-weighted income approach, which reflects the probability and timing of future payments. This fair value measurement is based on significant Level 3 inputs such as the anticipated timelines and probability of achieving development, regulatory approval or sales-based milestone
F-28
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
events and projected revenues. The resulting probability-weighted cash flows are discounted using estimated discount rate that commensurate with the risks of the expected cash flows attributable to the various milestones.
Each reporting period thereafter, the Company revalues these obligations by performing a review of the assumptions listed above and record increases or decreases in the fair value of these contingent consideration obligations in changes in fair value of contingent consideration expenses within the consolidated statements of operations until such time that the related product candidate receives marketing approval. In the absence of any significant changes in key assumptions, the quarterly determination of fair values of these contingent consideration obligations would primarily reflect the passage of time.
Updates to assumptions could have a significant impact on our results of operations in any given period and actual results may differ from estimates. For example, significant increases in the probability of achieving a milestone or projected revenues would result in a significantly higher fair value measurement while significant decreases in the estimated probability of achieving a milestone or projected revenues would result in a significantly lower fair value measurement. Significant increases in the discount rate or in the anticipated timelines would result in a significantly lower fair value measurement while significant decreases in the discount rate or anticipated timelines would result in a significantly higher fair value measurement.
As discussed in Note 4, the Company re-measured the preliminary determination of the fair values of assets acquired and liabilities assumed as of the acquisition date. The Company had determined that the contingent consideration milestones and contingent liability milestones will not be achieved.
| 7. | Inventories |
The following table represents the components of inventories, net of reserves (in thousands):
| December 31, 2014 |
December 31, 2013 |
|||||||
| Raw Materials |
$ | 1,787 | $ | 697 | ||||
| Work-in-process |
543 | — | ||||||
| Finished goods |
2,291 | 2,816 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 4,621 | $ | 3,513 | ||||
|
|
|
|
|
|||||
Reserves on the inventory balance was $0.3 million for the year ended December 31, 2014. There were no reserves for the year ended December 31, 2013.
| 8. | Intangible Assets and Goodwill |
Finite lived intangible asset
In May 2013, the Company acquired BUPHENYL and as part of this transaction, the Company recognized $16.5 million of an intangible asset relating to BUPHENYL product rights. The intangible asset is amortized over the estimated useful life using the economic use method, which reflects the pattern that the economic benefit of the intangible asset is consumed as revenue is generated. The pattern of consumption of the economic benefit is estimated using the future projected cash flow of the intangible asset which is reviewed on a regular basis. The Company estimated the useful life of the BUPHENYL product rights to be 10 years. The weighted average life remaining as of December 31, 2014 is 8.0 years.
F-29
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Intangible asset amortization expense was $4.6 million and $3.1 million for the years ended December 31, 2014 and 2013, respectively.
Estimated aggregate amortization expense for each of the five succeeding years ending December 31 is as follows (in thousands):
| 2015 | 2016 | 2017 | 2018 | 2019 | ||||||||||||||||
| Amortization expense |
$ | 6,495 | $ | 1,655 | $ | 1,515 | $ | 1,477 | $ | 1,441 | ||||||||||
Goodwill
As discussed in Note 4, in June 2014, the Company acquired Andromeda and as part of this transaction, the Company recognized goodwill in the amount of $30.2 million. The Company does not amortize goodwill but tests goodwill for impairment on an annual basis and in between annual tests if the Company becomes aware of any events or changes in circumstances that would more likely than not indicate a reduction in the fair value of a reporting unit below its carrying amount. Due to the Company’s decision to terminate the development of Diapep277, the Company determined that sufficient indicators of potential impairment existed to require an interim goodwill impairment analysis.
The impairment test for goodwill is a two-step process. Step one consists of a comparison of the fair value of a reporting unit against its carrying amount, including the goodwill allocated to each reporting unit. The Company determines the fair value of the reporting unit based on the present value of estimated future cash flows as well as various price or market multiples applied to the reporting unit’s operating results, which are classified as level 3 within the fair value hierarchy (as described in Note 6). If the carrying amount of the reporting unit is in excess of its fair value, step two requires the comparison of the implied fair value of the reporting unit’s goodwill against the carrying amount of the reporting unit’s goodwill. Any excess of the carrying value of the reporting unit’s goodwill over the implied fair value of the reporting unit’s goodwill is recorded as an impairment loss.
For the evaluation of the goodwill, management considered Andromeda to be a reporting unit and assigned no value to the Andromeda reporting unit. The Company also determined that the implied fair value of goodwill is zero. Thus, the excess of the carrying amount of goodwill over the implied fair value of the Andromeda reporting unit of $30.2 million was recorded as an impairment loss in the Company’s consolidated statements of operations for the year ended December 31, 2014.
| 9. | Property and Equipment |
The following table represents the components of property and equipment (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Computers and Software |
$ | 1,202 | $ | 563 | ||||
| Furniture and Fixtures |
320 | 320 | ||||||
| Office Equipment |
53 | 53 | ||||||
| Leasehold Improvements |
— | — | ||||||
| Capital work in progress |
181 | 264 | ||||||
|
|
|
|
|
|||||
| 1,756 | 1,200 | |||||||
| Less: Accumulated depreciation |
(640 | ) | (264 | ) | ||||
|
|
|
|
|
|||||
| Total property and equipment, net |
$ | 1,116 | $ | 936 | ||||
|
|
|
|
|
|||||
F-30
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Depreciation expense for the years ended December 31, 2014, 2013 and 2012 was $0.5 million, $0.2 million and $15,000, respectively.
| 10. | Accrued Liabilities |
The following table represents the components of accrued liabilities (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Clinical trial expenses |
$ | 2,399 | $ | 246 | ||||
| Payroll and related expenses |
4,134 | 4,278 | ||||||
| Gross to net sales accruals |
10,142 | 5,235 | ||||||
| Royalty payable |
2,080 | 1,154 | ||||||
| Taxes payable |
563 | 326 | ||||||
| Legal expenses payable |
410 | 45 | ||||||
| Acquisition related accrual (Note 22) |
4,615 | — | ||||||
| Other |
1,166 | 903 | ||||||
|
|
|
|
|
|||||
| $ | 25,509 | $ | 12,187 | |||||
|
|
|
|
|
|||||
| 11. | Notes Payable |
April 2011 Convertible Notes Payable
In April 2011, the Company entered into a convertible notes financing (the “April 2011 convertible notes financing”), in which it issued an aggregate principal amount of $17.5 million of convertible notes in an initial closing in April and an aggregate principal amount of $8,285 of convertible notes in subsequent closings in May 2011 (collectively, the “April 2011 Notes”) pursuant to the Convertible Note and Warrant Purchase Agreement dated April 1, 2011 (the “April 2011 Purchase Agreement”). The April 2011 Purchase Agreement permits the Company to issue up to an aggregate principal amount of $35.0 million.
The April 2011 Notes accrue interest at a rate of 6% per annum and have a maturity date of the earliest of (i) demand by the holders of 66% of the principal amount of the then-outstanding April 2011 Notes under certain circumstances, which demand may not be made earlier than December 31, 2012, as amended or (ii) an event of default. The April 2011 Notes cannot be prepaid, except on demand by the holders of the April 2011 Notes, as described above. The principal and the interest under the April 2011 Notes are automatically convertible (i) into securities that are sold in an issuance of preferred stock generating gross proceeds of at least $30.0 million, referred to herein as a qualified financing, at the lowest price at which such securities are sold to certain new investors in the qualified financing, (ii) into Series C-2 convertible preferred stock upon the occurrence of certain change of control events, unless the holders of 66% of the principal amount of the then-outstanding April 2011 Notes notify the Company of their election to accelerate the unpaid principal and interest of the April 2011 Notes in connection with the change of control event, or (iii) into common stock immediately prior to the consummation of an initial public offering, at a conversion price equal to the initial public offering price. In addition, holders of 66% of the principal amount of the then-outstanding April 2011 Notes have the option to convert the April 2011 Notes (i) in the event that the Company consummates an equity financing that is not a “qualified financing,” as described above, prior to the maturity of the April 2011 Notes, into the equity securities issued in the equity financing, or (ii) upon maturity of the April 2011 Notes, if the April 2011 Notes have not been previously converted, into shares of the Company’s Series C-2 convertible preferred stock.
F-31
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
On July 31, 2012, upon closing of the IPO, the principal and accrued interest under the Company’s April 2011 Notes amounting to $18.9 million converted into 1,888,054 shares of common stock. The carrying value, including the impact of the amendment to the April 2011 Notes as discussed below and accrued interest under the Company’s April 2011 Notes was $18.4 million as of July 31, 2012.
October 2011 Convertible Notes Payable
In October 2011, the Company entered into a convertible notes financing (the “October 2011 convertible notes financing”), in which it issued an aggregate principal amount of $7.5 million of convertible notes in an initial closing in October and an aggregate principal amount of $3,551 of convertible notes in a subsequent initial closing in November 2011 and aggregate amount of $7.5 million of convertible notes in the second closing in February 2012 (collectively, the “October 2011 Notes”) pursuant to the Convertible Note and Warrant Purchase Agreement dated October 26, 2011 (the “October 2011 Purchase Agreement”). The October 2011 Purchase Agreement permitted the Company to issue up to an aggregate principal amount of $15.0 million.
The October 2011 Notes accrue interest at a rate of 6% per annum and have a maturity date of the earliest of (i) demand by the holders of 66% of the principal amount of the then-outstanding October 2011 Notes under certain circumstances, which demand may not be made earlier than December 31, 2012, or (ii) an event of default. The October 2011 Notes cannot be prepaid, except on demand by the holders of the October 2011 Notes, as described above. The principal and the interest under the October 2011 Notes are automatically convertible (a) into securities that are sold in an issuance of preferred stock generating gross proceeds of at least $40.0 million, referred to herein as a qualified financing, equal to the quotient of (i) the outstanding principal amount plus unpaid accrued interest divided by (ii) the price per share paid by the investors purchasing new preferred stock in the qualified financing; (b) upon the occurrence of certain change in control events, into new series of preferred stock equal to the quotient of (i) the outstanding principal amount plus accrued interest divided by (ii) the Series C-2 original issue price, or (c) into common stock immediately prior to the consummation of an initial public offering, at a conversion price equal to the initial public offering price. In addition, holders of 66% of the principal amount of the then-outstanding October 2011 Notes have the option to convert the October 2011 Notes (i) in the event that the Company consummates an equity financing that is not a “qualified financing,” as described above, prior to the maturity of the October 2011 Notes, into the equity securities issued in the equity financing, or (ii) upon maturity of the October 2011 Notes, if the October 2011 Notes have not been previously converted, into shares of the Company’s Series C-2 convertible preferred stock.
On July 31, 2012, upon closing of the IPO, the principal and accrued interest under the Company’s October 2011 Notes amounting to $15.6 million converted into 1,556,816 shares of common stock. The carrying value, (net of discount) and accrued interest under the Company’s October 2011 Notes was $14.9 million as of July 31, 2012.
October 2011 Call Option Liability
The October 2011 Purchase Agreement also provides that so long as there has not been a qualified financing, change of control or initial public offering, on or before June 30, 2012, or in the event that the October 2011 Notes issued in the initial closing or subsequent initial closings have not been previously converted into common or preferred stock as set forth in the agreement, upon the election and approval of the holders of 66% of the principal amount of the then-outstanding October 2011 Notes, the Company will issue (i) notes with an aggregate principal amount of $7.5 million or (ii) up to $7.5 million of notes in the event all or a portion of the subsequent initial closing notes have been issued. The additional note amount was determined to be a call option (“October 2011 call option”) that was recorded at its fair value of $0.8 million as a debt discount that has been amortized to interest expense over the term of the October 2011 Notes.
F-32
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The Company recorded $0.7 million to other income (expense), net in its consolidated statements of operations upon issuance of the second closing of the October 2011 Notes in February 2012 for $7.5 million. As of December 31, 2012, the April 2011 Notes and October 2011 Notes were no longer outstanding as they had converted into shares of common stock upon the closing of the Company’s IPO on July 31, 2012. During the year ended December 31, 2012, the Company recorded amortization for debt discount of $0.9 million related to the October 2011 Notes.
In addition, the Company determined that the April 2011 Notes and the October 2011 Notes have contingent beneficial conversion features related to the conversion options described above. Upon the occurrence of the contingent event underlying those conversion options, the Company may recognize a charge based on the difference, if any, between the adjusted conversion price and the fair market value of common stock at the original issuance date. This charge, if any, will impact net income (loss) attributable to common stockholders and basic and diluted net income (loss) per share attributable to common stockholders.
The Company determined that no beneficial conversion features exist at the dates of issuance of the April 2011 Notes and October 2011 Notes and upon closing of the IPO on July 31, 2012, at which time these notes were converted to common stock.
April 2012 and September 2012 Notes Payable
In April 2012, the Company borrowed $10.0 million (the “April 2012 Notes”) pursuant to a loan and security agreement (the “Original Loan Agreement”) with Silicon Valley Bank and Leader Lending, LLC—Series B (the “Lenders”). The loan carried an interest rate of 8.88%, with interest only payments for the period of 9 months from May 1, 2012. The loan is then payable in equal monthly principal payments plus interest over a period of 27 months from February 1, 2013. In connection with the Loan Agreement, the Company granted a security interest in all of its assets, except intellectual property. The Company’s obligations to the Lenders include restrictions on borrowing, asset transfers, placing liens or security interest on its assets including the Company’s intellectual property, mergers and acquisitions and distributions to stockholders. The Loan Agreement also requires the Company to provide the Lenders monthly financials and compliance certificate within 30 days of each month end, annual audited financials within 180 days of each fiscal year-end and annual approved financial projections. The Company issued warrants to the Lenders to purchase a total of 75,974 shares of common stock with an exercise price of $4.08 per share. The Loan Agreement requires immediate repayment of amounts outstanding upon an event of default, as defined in the Loan Agreement, which includes events such as a payment default, a covenant default or the occurrence of a material adverse change, as defined in the Loan Agreement. In addition, a final payment equal to 6.5% of the principal loan amount is due on the earlier of (i) maturity date, (ii) prepayment of the loan or (iii) an event of default.
Pursuant to the terms of the Original Loan Agreement, once the Company raises at least $30.0 million from the sale of equity securities or subordinated debt, the Lenders also agreed to lend the Company a one-time single loan in the amount of $2.5 million (the “Bank Term Loan”). In September 2012, the Company borrowed an additional $2.5 million (the “September 2012 Note”) from Silicon Valley Bank pursuant to the terms of the Bank Term Loan. In addition, the Company issued warrants to Silicon Valley Bank to purchase a total of 8,408 shares of common stock with an exercise price of $5.05 per share. A final payment equal to 6.5% of the principal loan amount is due on the earlier of (i) maturity date, (ii) prepayment of the loan or (iii) an event of default. The principal amount outstanding under the Bank Term Loan accrues interest at a per annum rate equal to the greater of (i) 8.88% and (ii) the Treasury Rate, as defined in the Loan Agreement, on the date the loan is funded plus 8.50%, with interest only payments for the period of 9 months from the date the loan is funded. The loan is then payable in equal monthly principal payments plus interest over a period of 27 months from the date the loan is funded. These loans were extinguished during the year ended December 31, 2014.
F-33
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Term Loans
On July 18, 2014, the Company entered into a new Loan and Security Agreement (the “Loan Agreement”) with Silicon Valley Bank (“SVB”), providing for two tranches of term loans of up to $16.0 million (the “Term Loans”) and a revolving credit line of up to $5.0 million (the “Revolving Loans”). In July 2014, the Company drew down both tranches of the Term Loans, of which approximately $5.8 million was used to pre-pay the Company’s existing April and September 2012 notes payable. In addition, the Company drew down $2.0 million from the revolving line of credit facility. The actual amount of the Revolving Loans that are available from time to time under the Loan Agreement is limited to a borrowing base amount that is determined according to, among other things, a percentage of the value of eligible accounts.
In accordance with ASC Topic No. 470, “Debt – Modifications and Extinguishments” (Topic No. 470), the new loan agreement was determined to be an extinguishment of the existing debt and an issuance of new debt. As a result of the extinguishment, the Company recorded a $0.6 million loss on extinguishment of debt which includes the remaining unamortized portion of the debt discount and debt issue costs associated with the April and September 2012 loans. The loss on extinguishment of debt was included within interest expense in the Company’s consolidated statements of operations.
The Company’s obligations under the Loan Agreement are collateralized by a first priority security interest in substantially all of the Company’s assets, excluding its intellectual property. The Company has also agreed not to pledge or otherwise encumber its intellectual property assets, except that the Company may grant licenses of its intellectual property as set forth in the Loan Agreement.
The Company is required to pay interest only for the first 18 months of the Term Loans, followed by 30 equal monthly payments of interest and principal. The Term Loans will mature on June 30, 2018. The Revolving Loans will mature on July 18, 2017. The Loan Agreement provides for an interest rate of 4.0% per year on the Term Loans and the prime rate plus 0.75% per year on the Revolving Loans, with a minimum interest requirement on the Revolving Loans. Upon the maturity date of the Term Loans, a final payment fee of 6.75% of the original principal amount of such Term Loans (the “Final Payment”) will be due.
The Loan Agreement contains customary representations, warranties and covenants (including the requirement to meet one of two financial covenants) by the Company, as well as customary events of default and indemnification obligations of the Company. The financial covenants include maintaining liquidity ratio on a monthly basis and achieving revenue projections on a quarterly basis as defined in the agreement. The Loan Agreement also requires the Company to provide SVB reports and compliance certificate within 30 days of each month end, quarterly financial statements within 45 days of the end of the quarter and annual audited financials within 180 days of each fiscal year-end and annual approved financial projections. The Loan Agreement requires immediate repayment of amounts outstanding upon an event of default, as defined in the Loan Agreement, which includes events such as a payment default, a covenant default or the occurrence of a material adverse change, as defined in the Loan Agreement. Upon an event of default, after any applicable grace or cure period, all amounts owed under the Loan Agreement may be declared due and payable, including the original principal amount of the Loans, the accrued but unpaid interest thereon, the Final Payment and the prepayment fee. In addition, the Loan Agreement also requires the Company to maintain its depository accounts, operating accounts, securities accounts and all foreign exchange transactions with SVB and SVB bank’s affiliates which accounts shall represent at least the lesser of (i) seventy five percent (75%) of the dollar value of the Company and its Subsidiaries’ accounts at all financial institutions or (ii) $40.0 million; provided, however, that the Company’s obligation relating to foreign exchange transactions is subject to SVB providing commercially competitive exchange rates.
Amortization of debt discount related to the April and September 2012 notes and term loans was $0.8 million, $0.5 million and $0.4 million for the years ended December 31, 2014, 2013 and 2012, respectively.
F-34
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Future minimum payments under the Loan Agreement as of December 31, 2014 are as follows (in thousands):
| Years Ending December 31, |
||||
| 2015 |
$ | 730 | ||
| 2016 |
6,822 | |||
| 2017 |
8,786 | |||
| 2018 |
4,450 | |||
|
|
|
|||
| Total future minimum payments |
$ | 20,788 | ||
| Less: amount representing unamortized interest |
(1,709 | ) | ||
| Less: amount representing debt discount |
(955 | ) | ||
|
|
|
|||
| Total minimum payments |
$ | 18,124 | ||
| Less: current portion |
— | |||
|
|
|
|||
| Non-current portion |
$ | 18,124 | ||
|
|
|
| 12. | Warrants |
In connection with a Loan and Security Agreement entered into in October 2007, the Company issued warrants to purchase 274 shares of Series B convertible preferred stock. In June 2009, as part of the recapitalization, these warrants were converted into warrants to purchase shares of common stock. The warrants were exercisable at $1,913.05 per share and expire in October 2017 (the “October 2007 common stock warrants”). The October 2007 common stock warrants were outstanding as of December 31, 2014 and 2013.
| 13. | Commitments and Contingencies |
Operating Leases
On October 14, 2013, the Company entered into a lease agreement with 2000 Sierra Point Parkway LLC (“Landlord”) under which the Company will lease approximately 20,116 rentable square feet of office space in Brisbane, California. The initial term of the lease is six years and the lease commencement date is November 19, 2013. On December 12, 2013, the Company entered into the First Amendment to the Lease (“First Amendment”) under which the Company will lease approximately 352 rentable square feet of storage space. In addition to operating expenses and certain other additional expenses set forth in the Leases, the Company will pay total base rent of approximately $4.5 million during the initial term of the Leases.
The Company recognizes rent expense on a straight-line basis over the lease period with monthly base rent of approximately $0.1 million. In addition, the Company also leases certain office equipment under an operating lease, which expires in December 2016.
F-35
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Aggregate total future minimum lease payments under operating facility and equipment leases as of December 31, 2014 were as follows (in thousands):
| Years Ending December 31, |
||||
| 2015 |
$ | 797 | ||
| 2016 |
820 | |||
| 2017 |
821 | |||
| 2018 |
845 | |||
| 2019 |
766 | |||
|
|
|
|||
| Total |
$ | 4,049 | ||
|
|
|
Rent expense including maintenance fees for the year ended December 31, 2014, 2013 and 2012 was $0.8 million, $0.4 million and $0.3 million, respectively.
Contingencies
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. Further, the Company may be subject to certain contingent liabilities that arise in the ordinary course of its business activities. The Company accrues a liability for such matters when it is probable that future expenditures will be made and such expenditures can be reasonably estimated.
In accordance with the Company’s amended and restated certificate of incorporation and amended and restated bylaws, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. There have been no claims to date and the Company has a director and officer insurance policy that may enable it to recover a portion of any amounts paid for future claims.
The Company is contingently committed for development milestone payments as well as sales-related milestone payments and royalties relating to potential future product sales under the restated collaboration agreement and purchase agreement with Ucyclyd (Note 3). The amount, timing and likelihood of these payments are unknown as they are dependent on the occurrence of future events that may or may not occur, including approval by the FDA of GPB for HE.
Other Matters
From time to time, the Company is a party to or otherwise involved in legal proceedings, claims and government inspections and other legal matters arising in the ordinary course of business or otherwise.
On March 17, 2014, the Company received notification of a Paragraph IV certification from the generic drug manufacturer Par Pharmaceutical, Inc. (“Par”) that it had filed an Abbreviated New Drug Application with the FDA seeking approval for a generic version of RAVICTI Oral Liquid. The Paragraph IV certification alleges that certain of the Company’s patents are invalid and/or will not be infringed by Par’s manufacture, use or sale of the product for which the ANDA was submitted. The Company filed suit against Par on April 23, 2014 to protect its patents and to obtain a stay of the FDA’s approval of Par’s ANDA. On July 22, 2014, Par filed a motion to
F-36
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
dismiss for lack of personal jurisdiction, or in the alternative, a motion to transfer the case to the Southern District of New York. The Company will oppose the motion.
On September 22, 2014, CBI filed suit against the Company in Superior Court of the state of Delaware for New Castle County alleging breach of contract and breach of the implied covenant of good faith and fair dealing under the SPA with CBI relating related to the acquisition of Andromeda. CBI’s suit alleges $200.0 million in damages arising from the announcement on September 8, 2014 of the Company’s decision to terminate development of DiaPep277 after uncovering evidence of serious misconduct by certain Andromeda employees that compromised clinical trial results. The Company disputes CBI’s allegations. CBI has not served the complaint on the Company. On January 16, 2015, CBI dismissed its claim against the Company without prejudice. On February 16, 2015 the Company reached an agreement to settle with CBI and Yeda, the company from which Andromeda licenses the underlying DiaPep277 technology, DiaPep277 related claims against one another, and the Company granted CBI an option to acquire all of the outstanding stock of Andromeda. In connection with the agreement, CBI will transfer to the Company $2.5 million in shares of the common stock it holds, valued at the average closing price of the Company’s common stock for the fifteen trading days ending on February 11, 2015. The parties have appointed a steering committee to oversee the completion of the clinical trial with representatives of CBI and Yeda and a non-voting member appointed by the Company. The Company’s estimated budget from October 1, 2014 through the completion of the trial remains unchanged at $10.5 million. Any increase to this budget beyond $2.25 million, if incurred as a result of the direction of the steering committee, shall require CBI to reimburse those expenses in shares of the Company’s common stock. Under the agreement, CBI’s option is exercisable through September 30, 2015 for $3.5 million payable in shares of the Company’s common stock. In addition, if the option is exercised, then Andromeda will be obligated to pay Hyperion future contingent payments if and to the extent it or its shareholders receive revenues or certain other proceeds, which are capped at $36.5 million. This amount, together with the option exercise price that Hyperion may receive, approximates the total amount Hyperion will have invested in Andromeda by the option exercise date. Under the agreement, in the event that CBI does not exercise its option, the underlying DiaPep277 technology will revert to Yeda under the license agreement between Andromeda and Yeda. Also on February 16, 2015, the Company entered into a release with Evotec pursuant to which Evotec released its previously asserted claims to a milestone payment from the Company in connection with the acquisition of Andromeda and that it had suffered harm from recent incidents in relation to Diapep277in exchange for a payment of $500,000 from the Company.
| 14. | Preferred Stock |
On July 31, 2012, upon the closing of the IPO, the Company’s Series C-1 and Series C-2 preferred stock amounting to $58.3 million converted into 1,912,598 and 4,663,039 shares of common stock, respectively. On July 31, 2012, the Company amended its Certificate of Incorporation to decrease the number of authorized shares of preferred stock to 10,000,000 with a par value of $0.0001 per share. Pursuant to the Company’s amended and restated certificate of incorporation, the board of directors has the authority, without action by its stockholders, to designate and issue shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof.
As of December 31, 2014 and 2013, there was no preferred stock outstanding.
| 15. | Common Stock |
On July 31, 2012, the Company completed its IPO and issued 5,000,000 shares of its common stock at an initial offering price of $10.00 per share. In addition, the Company sold an additional 750,000 shares of common
F-37
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
stock directly to its underwriters when they exercised their over-allotment option in full at the initial offering price of $10.00 per share. The shares began trading on the NASDAQ Global Market on July 26, 2012. The Company received net proceeds from the IPO of $51.3 million, after deducting underwriting discounts and commissions of $4.0 million and expenses of $2.2 million.
On July 31, 2012, upon the closing of the IPO, the Company’s Series C-1 and Series C-2 preferred stock amounting to $58.3 million converted into 1,912,598 and 4,663,039 shares of common stock, respectively. In addition, the principal and accrued interest under the Company’s April 2011 Notes and October 2011 Notes amounting to $18.9 million and $15.6 million, respectively, converted into 1,888,054 and 1,556,816 shares of common stock. As of July 31, 2012, the carrying values and accrued interest under the April 2011 Notes and October 2011 Notes amounted to $18.4 million and $14.9 million, respectively. Additionally, the April 2011 common stock warrants and October 2011 preferred stock warrants were converted into 322,599 and 17,762 shares of common stock, respectively, immediately prior to the closing of the IPO. The fair value of the April 2011 common stock warrants and October 2011 preferred stock warrants as of July 31, 2012 of $3.9 million was reclassified to additional paid-in capital.
In addition, on July 31, 2012, the Company amended its Certificate of Incorporation to increase the number of authorized shares of common stock to 100,000,000 with a par value of $0.0001 per share.
Common stockholders are entitled to dividends when and if declared by the Board of Directors subject to prior rights of the preferred stockholders. The holder of each share of common stock is entitled to one vote.
On March 13, 2013, the Company completed a follow-on offering and issued 2,875,000 shares of its common stock at an offering price of $20.75 per share. In addition, the Company sold an additional 431,250 shares of common stock directly to its underwriters when they exercised their over-allotment option in full at an offering price of $20.75 per share. The Company received net proceeds from the offering of $63.7 million, after deducting underwriting discounts and commissions of $4.1 million and expenses of $0.8 million.
On August 14, 2013, the Company filed a shelf registration statement on Form S-3, which was declared effective by the SEC on September 13, 2013. The shelf registration statement permits: (a) the offering, issuance and sale by the Company of up to a maximum aggregate offering price of $150.0 million of its common stock, preferred stock, debt securities, warrants and/or units; (b) the sale of up to 8,727,000 shares of common stock by certain selling stockholders; and (c) the offering, issuance and sale by the Company of up to a maximum aggregate offering price of $50.0 million of its common stock that may be issued and sold under a sales agreement with Cantor Fitzgerald & Co. As of December 31, 2014, there were no sales of any securities registered pursuant to the shelf registration statement.
At December 31, 2014, the Company had reserved common stock for future issuances as follows:
| Issuance of options under 2012 stock plan |
688,842 | |||
| Issuance upon exercise of options under the 2006 and 2012 stock plan |
3,068,741 | |||
| Issuance upon vesting of restricted and performance stock units under the 2012 stock plan |
136,293 | |||
| Issuance upon exercise of October 2007 common stock warrants |
274 | |||
|
|
|
|||
| Total |
3,894,150 | |||
|
|
|
F-38
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
| 16. | Stock Option Plan |
2006 Plan
In December 2006, the Company adopted the 2006 Equity Incentive Plan (the “2006 Plan”), under which 457 shares of the Company’s common stock had been originally reserved for issuance to employees, directors and consultants. The 2006 Plan provides for the grant of the following stock awards: incentive stock options, non-statutory stock options, restricted stock awards, restricted stock unit awards and stock appreciation rights. Incentive stock options may be granted only to Company employees, which include officers and directors of the Company. Non-statutory stock options and stock purchase rights may be granted to employees and consultants. Stock awards other than incentive stock options may be granted to employees, directors and consultants.
The Board of Directors has the authority to determine to whom options will be granted, the number of options, the term and the exercise price. The exercise price of the stock option shall not be less than 100% of the fair market value of the common stock subject to the option on the date the option is granted. For individuals holding more than 10% of the voting rights of all classes of stock, the exercise price of an option will not be less than 110% of fair market value and the option is not exercisable after the expiration of five years from the date of the grant. Options granted to an employee who is not an officer, director or consultant shall provide for vesting of the total number of shares of common stock at a rate of at least 20% per year over five years from the date the option was granted, subject to reasonable conditions such as continued employment, at any time or during any period established by the Company. The contractual term of each option is ten years.
On July 25, 2012, the effective date of the 2012 Plan, the 2006 Plan was frozen and no additional awards will be made under the 2006 Plan. Any shares remaining available for future grant were allocated to the 2012 Plan and any shares underlying outstanding options that terminate by expiration, forfeiture, cancellation, or otherwise without issuance of such shares, will be allocated to the 2012 Plan.
Activity under the 2006 Plan is as follows:
| Outstanding Options | ||||||||||||
| Number of Shares Available for Grant |
Number of Shares Underlying Outstanding Options |
Weighted-Average Exercise Price Per Share |
||||||||||
| Balances at December 31, 2013 |
— | 1,538,197 | 3.64 | |||||||||
| Options exercised |
— | (190,263 | ) | 3.73 | ||||||||
| Options cancelled |
14,413 | (14,413 | ) | 6.78 | ||||||||
| Options re-allocated to 2012 Plan |
(14,413 | ) | — | — | ||||||||
|
|
|
|
|
|||||||||
| Balances at December 31, 2014 |
— | 1,333,521 | $ | 3.60 | ||||||||
|
|
|
|
|
|||||||||
The aggregate intrinsic value of options exercised for the year ended December 31, 2014 was $4.1 million. Intrinsic value is calculated as the difference between the exercise price of the underlying options and the fair value of the common stock for the options that had exercise prices that were lower than the fair value per share of the common stock on the date of exercise.
F-39
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Aggregate options outstanding and vested and exercisable by exercise price at December 31, 2014 for the 2006 plan are as follows (dollars in thousands except per share values):
| Options Outstanding |
Options Vested and Exercisable | |||||||||||||||||||||||
| Exercise Price |
Number Outstanding |
Aggregate Intrinsic Value |
Weighted Average Remaining Life (in Years) |
Number Outstanding |
Aggregate Intrinsic Value |
Exercise Price |
||||||||||||||||||
| $ 1.28 |
735,773 | $ | 16,717 | 4.5 | 735,773 | $ | 16,717 | $ | 1.28 | |||||||||||||||
| $ 4.08 |
252,403 | 5,028 | 6.3 | 228,490 | 4,552 | $ | 4.08 | |||||||||||||||||
| $ 7.31 |
344,405 | 5,748 | 7.3 | 217,060 | 3,623 | $ | 7.31 | |||||||||||||||||
| $ 327.95 |
940 | — | 2.5 | 940 | — | $ | 327.95 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| 1,333,521 | $ | 27,493 | 5.5 | 1,182,263 | $ | 24,892 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The intrinsic values of outstanding, vested and exercisable options were determined by multiplying the number of shares by the difference in exercise price of the options and the fair value of the common stock as of December 31, 2014 of $24.00 per share.
For the year ended December 31, 2014, options vested and expected to vest for the 2006 plan aggregated to 1,328,822 options with an intrinsic value of $27.4 million, weighted average exercise price of $3.59 per share and the weighted average remaining life of 5.5 years.
2012 Plan
In April 2012, the board of directors of the Company adopted (approved by Company’s stockholders in July 2012) the 2012 Omnibus Incentive Plan (the “2012 Plan”). The 2012 Plan provides for the grant of stock options, stock appreciation rights, restricted stock, unrestricted stock, stock units, dividend equivalent rights, other equity-based awards and cash bonus awards. The 2012 Plan became effective on July 25, 2012.
As of December 31, 2014 and 2013, the Company had reserved 688,842 shares and 576,760 shares of common stock, respectively, for issuance under the 2012 Plan.
F-40
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Activity under the 2012 Plan is as follows:
| Outstanding Options | Restricted Stock Units Outstanding |
Performance Stock Units Outstanding |
||||||||||||||||||||||||||
| Number of Shares Available for Grant |
Number of Shares Underlying Outstanding Options |
Weighted-Average Exercise Price Per Share |
Number of Shares Underlying Outstanding RSU’s |
Grant Date Fair Value |
Number of Shares Underlying Outstanding RSU’s |
Grant Date Fair Value |
||||||||||||||||||||||
| Balances at December 31, 2013 |
576,760 | 1,252,433 | 20.75 | 18,000 | 19.39 | — | — | |||||||||||||||||||||
| Additional shares authorized |
805,485 | — | — | — | — | — | — | |||||||||||||||||||||
| Options and awards granted |
(938,062 | ) | 806,919 | 25.99 | 122,155 | 26.68 | 8,988 | 25.08 | ||||||||||||||||||||
| Options exercised |
— | (101,736 | ) | 18.42 | — | — | — | — | ||||||||||||||||||||
| Awards released |
— | — | — | (5,000 | ) | 20.13 | — | — | ||||||||||||||||||||
| Options re-allocated to 2012 Plan |
14,413 | — | — | — | — | — | — | |||||||||||||||||||||
| Options and awards cancelled |
230,246 | (222,396 | ) | 22.66 | (7,850 | ) | 26.74 | — | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2014 |
688,842 | 1,735,220 | $ | 23.08 | 127,305 | $ | 25.90 | 8,988 | $ | 25.08 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The aggregate intrinsic value of options exercised for the year ended December 31, 2014 was $0.8 million.
Aggregate options outstanding and vested and exercisable by exercise price at December 31, 2014 for the 2012 plan are as follows (dollars in thousands except per share values):
| Options Outstanding | Options Vested and Exercisable | |||||||||||||||||||||||
| Exercise Price |
Number Outstanding |
Aggregate Intrinsic Value |
Weighted Average Remaining Life (in Years) |
Number Outstanding |
Aggregate Intrinsic Value |
Exercise Price |
||||||||||||||||||
| $10.32- 18.24 |
242,962 | $ | 2,799 | 7.9 | 118,837 | $ | 1,415 | $ | 10.32 - 18.24 | |||||||||||||||
| $19.39- 21.05 |
200,170 | 746 | 9.0 | 36,504 | 162 | 19.39 - 21.05 | ||||||||||||||||||
| $21.45- 24.38 |
128,450 | 220 | 8.7 | 41,514 | 83 | 21.45 - 24.38 | ||||||||||||||||||
| $24.46- 26.74 |
1,095,725 | — | 8.8 | 253,207 | — | 24.46 - 26.74 | ||||||||||||||||||
| $26.97- 28.08 |
42,313 | — | 9.3 | — | — | 26.97 - 28.08 | ||||||||||||||||||
| $29.27- 31.00 |
25,600 | — | 9.3 | — | — | 29.27 - 31.00 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| 1,735,220 | $ | 3,765 | 8.7 | 450,062 | $ | 1,660 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The intrinsic values of options vested and exercisable options as of December 31, 2014 were determined by multiplying the number of shares by the difference in exercise price of the options and the fair value of the common stock as of December 31, 2014 of $24.00 per share.
For the year ended December 31, 2014, options vested and expected to vest for the 2012 plan aggregated to 1,646,570 options with an aggregate intrinsic value of $3.6 million, weighted average exercise price of $23.02 per share and the weighted average remaining life of 8.7 years.
F-41
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Stock-Based Compensation
During the years ended December 31, 2014, 2013 and 2012 , the Company granted stock options to employees to purchase 806,919, 1,067,315 and 720,580 shares of common stock, respectively, with a weighted-average grant date fair value of $25.99, $22.90 and $6.76 per share, respectively. During the year ended December 31, 2014 and 2013, the Company granted 122,155 and 21,000 restricted stock units, respectively, to employees. There were no restricted stock units granted for the year ended December 31, 2012. During the year ended December 31, 2014, the Company granted 8,998 performance stock units to employees. There were no performance stock units granted for the years ended December 31, 2013 and 2012.
Stock-based compensation expense recognized during the years ended December 31, 2014, 2013 and 2012 includes compensation expense for stock-based awards granted to employees based on the grant date fair value estimated in accordance with the provisions of ASC 718 of $7.6 million, $4.3 million and $1.0 million, respectively. As of December 31, 2014, there was a total unrecognized compensation cost of $17.0 million related to unvested stock-based awards granted. These amounts are expected to be recognized over a period of approximately 2.5 years.
The Company determines the fair value of restricted stock units and performance stock units using the closing price of the Company’s common stock on the date of grant. The Company estimates the fair value of stock options using the Black-Scholes option valuation model. The fair value of employee stock options and restricted stock units are being amortized on a straight-line basis over the requisite service period of the awards. The fair value of the performance stock awards is being amortized based on the achievement of performance goals and over the requisite service period of the awards. The fair value of the employee stock options was estimated using the following weighted-average assumptions:
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Expected volatility |
62.76 | % | 58 | % | 65 | % | ||||||
| Risk-free interest rate |
1.90 | % | 1.14 | % | 1.05 | % | ||||||
| Dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
| Expected term (in years) |
6.00 | 5.96 | 6.00 | |||||||||
Determining Fair Value of Stock Options
The fair value of each grant of stock options was determined by the Company using the methods and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
Expected Term — The expected term of stock options represents the weighted average period the stock options are expected to be outstanding. For option grants that are considered to be “plain vanilla”, the Company has opted to use the simplified method for estimating the expected term as provided by the Securities and Exchange Commission. The simplified method calculates the expected term as the average time-to-vesting and the contractual life of the options. For other option grants, the expected term is derived from the Company’s historical data on employee exercises and post-vesting employment termination behavior taking into account the contractual life of the award.
Expected Volatility — The expected stock price volatility assumption was determined by examining the historical volatilities of a group of industry peers and the Company’s own historical volatility since the Company began trading subsequent to its IPO in July 2012.
F-42
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Risk-Free Interest Rate — The risk free rate assumption is based on U.S. Treasury instruments for which the terms were consistent with the expected term of the Company’s stock options.
Expected Dividend — The expected dividend assumption is based on the Company’s history and expectation of dividend payouts.
Forfeiture Rate — ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience.
Fair Value of Common Stock — Prior to the IPO, the fair value of the shares of common stock underlying the stock options has historically been the responsibility of and determined by the Company’s board of directors. Because there was no public market for the Company’s common stock, the board of directors determined fair value of common stock at the time of grant of the option by considering a number of objective and subjective factors including independent third-party valuations of the Company’s common stock, sales of convertible preferred stock to unrelated third parties, operating and financial performance, the lack of liquidity of capital stock and general and industry specific economic outlook, amongst other factors. Subsequent to the IPO, the fair value of the shares of the common stock underlying the stock options is the closing price on the option grant date.
Total stock-based compensation expense related to options and awards granted was allocated as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Cost of sales |
$ | 70 | $ | 27 | $ | — | ||||||
| Research and development |
1,384 | 585 | 374 | |||||||||
| Selling general and administrative |
6,184 | 3,709 | 619 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 7,638 | $ | 4,321 | $ | 993 | ||||||
|
|
|
|
|
|
|
|||||||
For the years ended December 31, 2014 and 2013, stock-based compensation of $0.1 million each was capitalized into inventories. Capitalized stock-based compensation is recognized as cost of sales when the related product is sold. Allocations to research and development, general and administrative and selling and marketing expense are based upon the department to which the associated employee reported.
| 17. | Income Taxes |
For the year ended December 31, 2014, the Company generated a pretax income of $30.0 million in the U.S. and a pretax loss of $36.0 million outside the U.S., for a net consolidated pretax loss of $6.0 million. For the years ended December 31, 2013 and 2012, the Company generated a pretax income of $16.7 million and a pretax loss of $32.3 million, respectively, in the U.S. In years prior to 2014, the Company has not generated any pretax income or loss outside the U.S.
F-43
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The components of the income tax expense are as follows (in thousands):
| Years ended December 31, |
||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Current income tax expense: |
||||||||||||
| Federal |
$ | 736 | $ | — | $ | — | ||||||
| State |
703 | 326 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| 1,439 | 326 | — | ||||||||||
|
|
|
|
|
|
|
|||||||
| Deferred income tax benefit: |
||||||||||||
| State |
(1,136 | ) | (277 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| (1,136 | ) | (277 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total income tax expense |
$ | 303 | $ | 49 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The reconciliation of income tax expense computed at the statutory federal income tax rate of 35% to amounts included in the consolidated statements of operations is as follows:
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Statutory rate |
35.0 | % | 35.0 | % | 34.0 | % | ||||||
| State tax |
0.6 | % | 5.5 | % | 5.4 | % | ||||||
| Tax credits |
57.5 | % | (5.0 | )% | 0.7 | % | ||||||
| Stock options |
(28.0 | )% | 0.2 | % | (0.7 | )% | ||||||
| Valuation allowance |
(81.8 | )% | (29.6 | )% | (37.1 | )% | ||||||
| Impact of Andromeda acquisition |
12.6 | % | — | — | ||||||||
| Change in federal tax rate |
— | (6.0 | )% | — | ||||||||
| Other |
(1.0 | )% | 0.2 | % | (2.3 | )% | ||||||
|
|
|
|
|
|
|
|||||||
| (5.1 | )% | 0.3 | % | 0.0 | % | |||||||
|
|
|
|
|
|
|
|||||||
Deferred income tax assets and liabilities reflect the net tax effects of net operating loss and tax credit carryovers and the temporary differences between the carrying amounts of assets and liabilities for financial
F-44
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
reporting and the amounts used for income tax purposes. Significant components of the Company’s deferred income tax assets are as follows (in thousands):
| Year Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Deferred income tax assets |
||||||||
| Fixed assets, capitalized intangibles and other assets |
$ | 6,030 | $ | 5,294 | ||||
| Net operating loss |
35,164 | 31,842 | ||||||
| Tax credits |
23,024 | 18,480 | ||||||
| Stock-based compensation expense |
1,660 | 1,133 | ||||||
| Accrued expenses and reserves |
3,405 | 1,502 | ||||||
| Capitalized research and development expenses |
3,237 | — | ||||||
| Tax basis in investments |
12,624 | — | ||||||
| Other |
381 | 25 | ||||||
|
|
|
|
|
|||||
| Total |
85,525 | 58,276 | ||||||
| Valuation allowance |
(84,113 | ) | (58,000 | ) | ||||
|
|
|
|
|
|||||
| Net deferred income tax assets |
$ | 1,412 | $ | 276 | ||||
|
|
|
|
|
|||||
At December 31, 2014, the non-current deferred income tax asset of $1.4 million is included in “Other non-current assets” in the consolidated balance sheets.
At December 31, 2013, current deferred income tax asset of $0.1 million is included in “Prepaid expenses and other current assets” and the non-current deferred income tax asset of $0.2 million is included in “Other non-current assets” in the consolidated balance sheets.
The amounts recorded as deferred income tax assets as of December 31, 2014 and 2013 represent the amount of tax benefits of existing deductible temporary differences and carryforwards that are more likely than not to be realized through the generation of sufficient future taxable income within the carryforward period. For the year ended December 31, 2014, the valuation allowance on deferred income tax asset increased by $26.1 million. The amount of net operating losses associated with excess tax deductions from stock-based compensation arrangements that is allocated to contributed capital if the future tax benefits are subsequently recognized is $4.0 million.
At December 31, 2014, the Company had net operating loss carryforwards of approximately $36.4 million and $107.8 million available to reduce future taxable income, if any, for both federal and California state income tax purposes, respectively. In addition, the Company had $67.3 million of Israeli net operating loss carryforwards which do not expire. The net operating loss carryforwards will begin to expire in 2031 and 2028 for federal and state income tax purposes, respectively. If the Company experiences an “ownership change” for purposes of Section 382 of the Internal Revenue Code of 1986, as amended, it may be subject to annual limits on its ability to utilize net operating loss carryforwards and credits. An ownership change is, as a general matter, triggered by sales or acquisitions of its stock in excess of 50% on a cumulative basis during a three-year period by persons owning 5% or more of our total equity value. The Company determined that an ownership change occurred in 2014 resulting in an annual limitation of approximately $50 million per year. The Company also had federal and state tax credit carryforwards of approximately $22.6 million and $0.7 million respectively, at December 31, 2014. The federal credits will expire starting in 2027, if not utilized. The California credits have no expiration date.
F-45
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
The Company was granted orphan drug designation in 2009 by the FDA for its products currently under development. The orphan drug designation allows the Company to claim increased federal tax credits for its research and development activities. Included in the $22.6 million of federal credit carryforwards is $21.1 million of Orphan Drug Credit claims for 2009 through 2014.
A reconciliation of the beginning and ending balances of the unrecognized income tax benefits during the years ended December 31, 2014, 2013 and 2012 is as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Balance at the beginning of the year |
$ | 6,269 | $ | 5,723 | $ | 5,357 | ||||||
| Changes based on prior period tax positions |
(2,325 | ) | — | (228 | ) | |||||||
| Changes based on current period tax positions |
223 | 546 | 594 | |||||||||
|
|
|
|
|
|
|
|||||||
| Balance at the end of the year |
$ | 4,167 | $ | 6,269 | $ | 5,723 | ||||||
|
|
|
|
|
|
|
|||||||
The entire amount of the unrecognized income tax benefits would not impact the Company’s effective tax rate if recognized.
There was no interest or penalties accrued through December 31, 2014. The Company’s policy is to recognize any related interest or penalties in income tax expense. The material jurisdictions in which the Company is subject to potential examination by tax authorities for tax years ended 2006 through the current period include the U.S. and California. The Company is not currently under income tax examinations by any tax authorities in federal, state or other jurisdictions.
| 18. | Defined Contribution Plan |
The Company sponsors a defined contribution plan under Section 401(k) of the Internal Revenue Code (“Plan”) covering substantially all full-time U.S. employees. Employee contributions are voluntary and are determined on an individual basis subject to the maximum allowable under federal tax regulations. Participants are always fully vested in their contributions. For the year ended December 31, 2014 and 2013, the Company made a contribution of $0.3 million and $0.2 million to the Plan. For the year ended December 31, 2012, the Company did not make any contributions to the Plan.
F-46
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
19. Net Income (Loss) per Share of Common Stock
The following table sets forth the computation of basic and diluted net income (loss) per share of common stock for the periods indicated (in thousands, except share and per share amounts):
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Net income (loss) per share |
||||||||||||
| Numerator: |
||||||||||||
| Net income (loss) attributable to common stockholders |
$ | (6,255 | ) | $ | 16,627 | $ | (32,263 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Denominator: |
||||||||||||
| Weighted-average number of common shares outstanding — basic |
20,470,025 | 19,415,822 | 7,256,537 | |||||||||
| Dilutive effect of stock-options and awards |
— | 1,315,091 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares outstanding — dilutive |
20,470,025 | 20,730,913 | 7,256,537 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) per share: |
||||||||||||
| Basic |
$ | (0.31 | ) | $ | 0.86 | $ | (4.45 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Diluted |
$ | (0.31 | ) | $ | 0.80 | $ | (4.45 | ) | ||||
|
|
|
|
|
|
|
|||||||
The following outstanding potentially dilutive securities were excluded from the computation of diluted net income (loss) per share, as the effect of including them would have been antidilutive:
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Stock options |
3,068,741 | 1,027,605 | 1,928,754 | |||||||||
| Common stock warrants |
274 | 274 | 84,656 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
3,069,015 | 1,027,879 | 2,013,410 | |||||||||
|
|
|
|
|
|
|
|||||||
| 20. | Related Party Transaction |
As part of the Company’s acquisition of BUPHENYL (see Note 4), the Company assumed the existing BUPHENYL distributors agreements, including the distribution agreement with Swedish Orphan Biovitrum AB (“SOBI”). Additionally, in the third quarter of 2013, SOBI was granted exclusive rights by the Company to distribute RAVICTI on a named patient basis for the chronic treatment of UCD in the Middle East. SOBI’s chairman, Bo Jesper Hansen, is a member of the Company’s Board of Directors. During the years ended December 31, 2014 and 2013, the Company recognized $5.1 million and $2.2 million, respectively, from sales to SOBI. As of December 31, 2014 and 2013, the accounts receivable from SOBI amounted to $1.5 million and $0.7 million, respectively.
| 21. | Segment Reporting |
The Company operates as one operating segment and uses one measurement of profitability to manage its business.
F-47
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
Net product revenue for RAVICTI and BUPHENYL for the years ended December 31, 2014 and 2013 are as follows (in thousands):
| Year Ended December 31, |
||||||||
| 2014 | 2013 | |||||||
| RAVICTI |
$ | 95,400 | $ | 31,236 | ||||
| BUPHENYL |
18,584 | 11,468 | ||||||
| Co-payment assistance |
(400 | ) | (500 | ) | ||||
|
|
|
|
|
|||||
| Total |
$ | 113,584 | $ | 42,204 | ||||
|
|
|
|
|
|||||
Net product revenue for RAVICTI and BUPHENYL by geographic region are as follows (in thousands):
| Year Ended December 31, |
||||||||
| 2014 | 2013 | |||||||
| United States |
$ | 103,303 | $ | 37,102 | ||||
| Canada |
3,723 | 2,208 | ||||||
| Rest of the world |
6,558 | 2,894 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 113,584 | $ | 42,204 | ||||
|
|
|
|
|
|||||
Long lived assets consist of property and equipment which at December 31, 2014 and 2013 are primarily in the U.S.
| 22. | Subsequent Events |
On February 16, 2015, the Company announced that it has amicably settled its disputes, and entered into a Completion of Phase 3 Clinical Trial, Option and Mutual Release Agreement (the “Release Agreement”) by and among the Company, Hyperion Therapeutics Israel Holding Corp Ltd (“Hyperion Israel”), CBI, Yeda and Andromeda regarding DiaPep277, a product candidate developed by Andromeda.
The parties to the Release Agreement appointed a steering committee with representatives of CBI and Yeda and a non-voting member from the Company to oversee completion of the Clinical Trial, the analysis of its results, and interactions with regulatory authorities. In addition, under the Release Agreement, CBI has an option to acquire Andromeda, which option expires September 30, 2015. In consideration for the option, CBI will pay to Hyperion Israel an option payment of $2.5 million in shares of the Company’s common stock held by CBI. The shares are valued based on the average closing price of the Company’s common stock for the fifteen trading days ending on February 11, 2015. If CBI exercises its option to acquire Andromeda, the option purchase price will be $3.5 million payable in shares of the Company’s common stock currently held by CBI to Hyperion Israel. In addition, if CBI exercises its option to acquire Andromeda, Andromeda will be obligated to pay Hyperion Israel future contingent payments if and to the extent it receive revenues or its shareholders receive certain other proceeds it is acquired, liquidated or makes other distributions of assets. The aggregate amount of all future contingent payments by Andromeda to Hyperion Israel is capped at $36.5 million which, together with the option exercise price that Hyperion Israel may receive, approximates the total amount the Company will have invested in Andromeda by the option exercise date. Under the Release Agreement, the Company’s estimated budget from October 2014 through completion of the Clinical Trial shall remain unchanged at $10.5 million. Any increase to the budget beyond $2.25 million, if incurred as a result of the direction of the steering committee, shall require
F-48
Hyperion Therapeutics, Inc.
Notes to Consolidated Financial Statements
CBI to reimburse expenses to Hyperion Israel. In the event that CBI does not exercise the option, the DiaPep277 intellectual property will revert to Yeda under the license agreement between Andromeda and Yeda.
Andromeda has also entered into a release agreement with Evotec International GmbH (“Evotec”) pursuant to which Andromeda has settled its disputes with Evotec regarding Evotec’s assertions that the Company’s acquisition of Andromeda triggered a €3.38 million milestone payment to it and that it suffered harm from recent incidents in relation to DiaPep277. Under the terms of the release, Evotec is releasing its claims against Andromeda in exchange for a $500,000 payment from the Company, and the Company confirms that based on its investigation of the Employee Conduct, as defined in the Release Agreement, it believes that neither Evotec nor its legal predecessor (i) had any involvement in any aspect or decision-making regarding the development of DiaPep277 and likewise, (ii) DeveloGen AG was also not involved in any way in the further development of DiaPep277 after selling it to Andromeda in June 2007, and had no role in the data generation and analyses. For the year ended December 31, 2014, the Company had $4.6 million included in “Accrued liabilities” in the Consolidated Balance Sheets. The Company considers this as a (Type 2) Subsequent event and will recognize $4.1 million as “Other Income” in the Consolidated Statements of Operations in the Quarterly Report on Form 10-Q for the period ended March 31, 2015.
| 23. | Selected Quarterly Financial Data (Unaudited) |
The following table presents selected unaudited quarterly financial information for the years ended December 31, 2014, 2013 and 2012. The results for any quarter are not necessarily indicative of future quarterly results and, accordingly, period to period comparisons should not be relied upon as an indication of future performance.
| For The Quarters Ended | ||||||||||||||||
| (in thousands, except share and per share amounts) | December 31, | September 30,(1) | June 30, | March 31, | ||||||||||||
| Year Ended December 31, 2014 |
||||||||||||||||
| Product revenue, net |
$ | 30,805 | $ | 26,201 | $ | 37,095 | $ | 19,483 | ||||||||
| Costs and expenses |
$ | 24,078 | $ | 55,031 | $ | 20,898 | $ | 17,784 | ||||||||
| Net income (loss) attributable to common stockholders |
$ | 7,096 | $ | (32,793 | ) | $ | 18,183 | $ | 1,259 | |||||||
| Net income (loss) per share attributable to common stockholders — basic |
$ | 0.34 | $ | (1.59 | ) | $ | 0.89 | $ | 0.06 | |||||||
| Net income (loss) per share attributable to common stockholders — diluted |
$ | 0.32 | $ | (1.59 | ) | $ | 0.84 | $ | 0.06 | |||||||
| For The Quarters Ended | ||||||||||||||||
| (in thousands, except share and per share amounts) | December 31, | September 30, | June 30,(2) | March 31, | ||||||||||||
| Year Ended December 31, 2013 |
||||||||||||||||
| Product revenue, net |
$ | 18,627 | $ | 15,489 | $ | 7,305 | $ | 783 | ||||||||
| Costs and expenses |
$ | 17,747 | $ | 15,037 | $ | 12,986 | $ | 9,851 | ||||||||
| Net income (loss) attributable to common stockholders |
$ | 468 | $ | 112 | $ | 25,022 | $ | (8,975 | ) | |||||||
| Net income (loss) per share attributable to common stockholders — basic |
$ | 0.02 | $ | 0.01 | $ | 1.25 | $ | (0.52 | ) | |||||||
| Net income (loss) per share attributable to common stockholders — diluted |
$ | 0.02 | $ | 0.01 | $ | 1.17 | $ | (0.52 | ) | |||||||
| (1) | For the quarter ended September 30, 2014, the Company recorded $30.2 million as an impairment charge of goodwill on the Company’s consolidated statements of operations. Also refer to the information contained in Note 4 of the consolidated financial statements appearing elsewhere in the report. |
| (2) | For the quarter ended June 30, 2013, the Company recorded a gain of $31.1 million related to the BUPHENYL acquisition on the consolidated statements of operations. Also refer to the information contained in Note 4 of the consolidated financial statements appearing elsewhere in the report. |
F-49