(1) Planned programs; not yet initiated. (2) External collaboration.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
For the fiscal year ended
or
For the transition period from to
Commission File Number
(Exact name of Registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
|
|
|
Not Applicable |
|
(Address of principal executive offices) |
(Zip Code) |
|
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
Trading Symbol |
Name of Each Exchange on Which Registered |
value $0.0001 per share |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
☒ |
Accelerated filer |
|
☐ |
|
|
|
|
|
|
|
Non-accelerated filer |
|
☐ |
Smaller reporting company |
|
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Exchange Act). Yes
The aggregate market value of the registrant’s voting ordinary shares held by non-affiliates of the registrant, based upon the $79.76 per share closing sale price of the registrant’s ordinary shares on June 30, 2022 (the last business day of the registrant’s most recently completed second quarter), was approximately $
As of February 23, 2023, the registrant had outstanding
HORIZON THERAPEUTICS PLC
FORM 10-K — ANNUAL REPORT
For the Fiscal Year Ended December 31, 2022
TABLE OF CONTENTS
PART I
Special Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K contains “forward-looking statements” — that is, statements related to future, not past, events — as defined in Section 21E of the Securities Exchange Act of 1934, as amended, that reflect our current expectations regarding the pending transaction with Amgen Inc. announced on December 12, 2022 and the anticipated closing thereof, our future growth, results of operations, business strategy and plans, financial condition, cash flows, performance, development plans and timelines, business prospects, and opportunities, as well as assumptions made by, and information currently available to, our management. Forward-looking statements include any statement that does not directly relate to a current or historical fact. Forward-looking statements generally can be identified by words such as “believe,” “may,” “could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “expect,” “should,” “would”, or similar expressions. These statements are based on current expectations and assumptions that are subject to risks and uncertainties inherent in our business, which could cause our actual results to differ materially from those indicated in the forward-looking statements. Factors that could cause actual results to differ materially from those indicated in the forward-looking statements include, without limitation: our and Amgen’s ability to consummate our pending transaction with Amgen in a timely manner, or at all, and the satisfaction (or waiver) of conditions to the consummation of our pending transaction with Amgen, including with respect to required regulatory clearances; potential delays in consummating our pending transaction with Amgen; the occurrence of any event, change or other circumstance or condition that could give rise to the termination of our transaction agreement with Amgen; the effect of the pendency of our transaction with Amgen on our business relationships, operating results and business generally; costs related to our pending transaction with Amgen; the outcome of any government investigations or legal proceedings that may be instituted against us or Amgen or any of our respective directors or officers related to the pending transaction with Amgen; our ability to successfully execute our sales and marketing strategy, including continuing to successfully recruit and retain sales and marketing personnel and to successfully build the market for our medicines; our ability to build a sustainable pipeline of new medicine candidates; whether we will be able to realize the expected benefits of strategic transactions, including whether and when such transactions will be accretive to our net income; the rate and degree of market acceptance of, and our ability and our distribution and marketing partners’ ability to obtain coverage and adequate reimbursement and pricing for, our medicines from government and third-party payers; the scope and duration of impacts of the COVID-19 pandemic on our ability to consummate our pending transaction with Amgen and on our business, our industry and the economy, including impacts to supply chains and clinical trials; our ability to maintain regulatory approvals for our medicines; our ability to conduct clinical development and obtain regulatory approvals for our medicine candidates, including potential delays in initiating and completing studies and filing for and obtaining regulatory approvals and whether data from clinical studies will support regulatory approval; our need for and ability to obtain additional financing; the accuracy of our estimates regarding future financial results; our ability to successfully execute our strategy to develop or acquire additional medicines or companies, including disruption from any future acquisition or whether any acquired development programs will be successful; our ability to manage our anticipated future growth; the ability of our medicines to compete with alternative therapies, including generic medicines and new medicines that may be developed by our competitors; our ability and our distribution and marketing partners’ ability to comply with regulatory requirements regarding the sales, marketing and manufacturing of our medicines and medicine candidates; the performance of our third-party distribution partners, licensees and manufacturers over which we have limited control; our ability to obtain and maintain intellectual property protection for our medicines; our ability to defend our intellectual property rights with respect to our medicines; our ability to operate our business without infringing the intellectual property rights of others; the loss of key commercial or management personnel; regulatory developments in the United States and other countries, including potential changes in healthcare laws and regulations; and other risks detailed below in Part I — Item 1A. “Risk Factors”. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee future results, events, levels of activity, performance or achievement. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law.
1
Risk Factors Summary
Our business faces significant risks and uncertainties. If any of the following risks are realized, our business, financial condition and results of operations could be materially and adversely affected. You should carefully review and consider the full discussion of our risk factors in the section titled “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K. Set forth below is a summary list of the principal risk factors as of the date of the filing this Annual Report on Form 10-K:
2
Item 1. Business
Unless otherwise indicated or the context otherwise requires, references to the “Company”, “we”, “us” and “our” refer to Horizon Therapeutics plc and its consolidated subsidiaries.
Overview
We are a global biotechnology company focused on the discovery, development and commercialization of medicines that address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases. Our pipeline is purposeful: we apply scientific expertise and courage to bring clinically meaningful therapies to patients. We believe science and compassion must work together to transform lives.
We substantially completed the previously announced wind down of our former inflammation business in the fourth quarter of 2022. As a result, effective in the fourth quarter of 2022, management realigned our reportable segments to reflect changes in the manner in which the chief operating decision maker assesses financial information for decision-making purposes. We transitioned our two reportable segments, the inflammation segment and the orphan segment, to one reportable segment for the year ended December 31, 2022. All prior year amounts have been reclassified to conform to our current reporting structure. Refer to Note 11, Segment and Other Information, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details.
On December 12, 2022, we announced that we had entered into a transaction agreement with Amgen Inc., or Amgen, and Pillartree Limited, or Pillartree, a wholly owned subsidiary of Amgen. The transaction agreement provides for the acquisition of Horizon by Pillartree subject to the terms and conditions of the transaction agreement. We have agreed to various covenants and agreements, including, among others, agreements to conduct our business in the ordinary course during the period between the execution of the transaction agreement and the effective time of the transaction. Outside of certain limited exceptions, we may not take, authorize, commit, resolve, or agree to do certain actions without Amgen’s consent, including: (i) acquiring businesses and disposing of significant assets; (ii) incurring capital expenditures above specified thresholds; (iii) issuing equity; (iv) incurring indebtedness; and (v) repurchasing outstanding ordinary shares. We do not believe these restrictions will prevent us from being able to fund our operations, working capital needs or capital expenditure requirements. The following discussions assume that the transaction is not consummated and we continue to operate as an independent entity. For further information, refer to Management’s Discussion and Analysis of Financial Condition and Results of Operations, included in Item 7 of this Annual Report on Form 10-K.
Our Strategy
Horizon is a leading high-growth, innovation-driven, profitable global biotechnology company. We are focused on the discovery, development and commercialization of medicines that address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases. Our three strategic goals are to: (i) maximize the value of our on-market rare disease medicines through commercial execution and clinical investment; (ii) expand our research and development, or R&D, pipeline through significant internal investment and external business development; and (iii) build a global presence in targeted international markets. Our vision is to build healthier communities, urgently and responsibly, supported by our philosophy to make a meaningful difference for patients and communities in need. We believe this generates value for our multiple stakeholders, including our shareholders.
We have made tremendous progress since our beginnings as a public company in 2011, when we had two on-market medicines and total net sales of $6.9 million. With 2022 total net sales of $3.6 billion, we now have a portfolio of 12 on-market medicines with three key growth drivers, a growing pipeline of more than 20 programs and a strong financial position that gives us the capacity to support future pipeline expansion opportunities.
We have achieved our transformation into an innovation-driven global biotechnology company through our strong strategic execution and by leveraging the three elements we believe set Horizon apart: (i) our excellence in commercial execution; (ii) our proven and disciplined business development strategy; and (iii) our strong clinical development capability. Our excellence in commercial execution has accelerated our growth trajectory and allowed us to pursue maximizing the potential of our medicines. Through our strong in-house business development capability, we acquire and license medicines focused on opportunities for which we believe we are uniquely positioned to drive value. We leverage our deep collective drug development experience and agile approach to continually explore new ways for patients to benefit from our existing medicines and develop new medicines.
3
Our Company
We are a public limited company formed under the laws of Ireland. We operate through a number of U.S. and other international subsidiaries with principal business purposes of performing R&D or manufacturing operations, serving as distributors of our medicines, holding intellectual property assets or providing us with services and financial support.
Our principal executive offices are located at 70 St. Stephen’s Green, Dublin 2, D02 E2X4, Ireland and our telephone number is 011 353 1 772 2100. Our website address is www.horizontherapeutics.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this Annual Report on Form 10-K.
Acquisitions and Divestitures
Since January 1, 2020, we completed the following acquisitions and divestitures:
The consolidated financial statements presented herein include the results of operations of the acquired businesses from the applicable dates of acquisition. Refer to Note 4, Acquisitions, Divestitures and other Arrangements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details of our acquisitions and divestitures.
Impact of COVID-19
The COVID-19 pandemic had a negative impact on our operations and net sales during 2022, including due to the emergence of new variants of the virus and resulting disruptions in healthcare operations and employee absences among our commercial team. During the first half of 2022, the omicron variant resulted in significant employee absences in our commercial organization due to illness and also impacted operations at sites of care that infuse our medicines and patient access to and willingness to visit healthcare providers. These events resulted in lower new patient enrollment forms, delays in new patients starting infusions and disruptions in therapy. In addition, our clinical trials have been and may in the future be affected by the COVID-19 pandemic.
Economic and health conditions in the United States and across most of the world are continuing to change because of the COVID-19 pandemic. Although COVID-19 is a global issue that has altered business and consumer activity, the biopharmaceutical industry is considered a critical and essential industry in the United States and many other countries and, therefore, we do not currently expect any government-imposed extended shutdowns of suppliers or distribution channels, although our suppliers and other third parties on which we rely could be impacted by employee absences due to COVID-19 illnesses. While certain of our contract manufacturers are involved in manufacturing vaccines for COVID-19, we do not currently expect these activities to impact the future supply of our medicines. In respect of our medicines, we believe we have sufficient inventory of raw materials and finished goods and we expect patients to be able to continue to receive their medicines at a site of care, for our infused medicines, and from their current pharmacies, alternative pharmacies or, if necessary, by direct shipment from our third-party providers that have such capability, for our other medicines.
4
We are continuing to actively monitor the possible impacts from the COVID-19 pandemic, including the emergence of new variants of the virus such as omicron subvariants, and may take further actions to alter our business operations as may be required by federal, state, local or foreign authorities or that we determine are in the best interests of patients, healthcare providers and our employees. There is significant uncertainty about the duration and potential impact of the COVID-19 pandemic. This means that our results could change at any time and the contemplated impact of the COVID-19 pandemic on our business results and outlook represents our estimate based on the information available as of the date of this Annual Report on Form 10-K.
For further information on the impact of COVID-19 pandemic, refer to “Risk Factors”, included in Item 1A of this Annual Report on Form 10-K.
Our Medicines
We believe our medicines address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases and provide significant advantages over existing therapies.
As of December 31, 2022, our commercial portfolio consisted of the following medicines:
Medicine |
|
Indication |
|
2022 Net Sales |
|
|
Marketing Rights |
|
|
|
|
|
|
|
|
|
|
TEPEZZA® |
|
Thyroid eye disease |
|
$ |
1,965.7 |
|
|
Worldwide |
KRYSTEXXA® |
|
Chronic refractory gout (“uncontrolled gout”) |
|
$ |
716.2 |
|
|
Worldwide |
RAVICTI |
|
Urea cycle disorders |
|
$ |
325.6 |
|
|
North America (1) |
PROCYSBI® |
|
Nephropathic cystinosis |
|
$ |
210.0 |
|
|
United States and certain other countries (2) |
UPLIZNA® |
|
Neuromyelitis optica spectrum disorder |
|
$ |
154.6 |
|
|
Worldwide, except certain countries in Asia (3) |
ACTIMMUNE® |
|
Chronic granulomatous disease and severe, malignant osteopetrosis |
|
$ |
126.1 |
|
|
United States, Canada and Japan (4) |
PENNSAID 2%® |
|
Pain of osteoarthritis of the knee(s) |
|
$ |
73.8 |
|
|
United States |
RAYOS® |
|
Rheumatoid arthritis, polymyalgia rheumatic, systemic lupus erythematosus and multiple other indications |
|
$ |
41.9 |
|
|
North America (5) |
BUPHENYL |
|
Urea cycle disorders |
|
$ |
7.3 |
|
|
North America (6) |
DUEXIS® |
|
Signs and symptoms of osteoarthritis and rheumatoid arthritis |
|
$ |
4.9 |
|
|
Worldwide |
VIMOVO® |
|
Signs and symptoms of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis |
|
$ |
1.8 |
|
|
United States |
QUINSAIR™ |
|
Treatment of chronic pulmonary infections due to Pseudomonas aeruginosa in cystic fibrosis patients |
|
$ |
1.1 |
|
|
Canada and certain other countries (7) |
5
For further information on our total revenues by medicine in each of the years ended December 31, 2022, 2021 and 2020, refer to Management's Discussion and Analysis of Financial Condition and Results of Operations, included in Item 7 of this Annual Report on Form 10-K.
TEPEZZA
TEPEZZA (teprotumumab-trbw) is a fully human monoclonal antibody and a targeted inhibitor of the insulin-like growth factor-1 receptor, or IGF-1R, that is the first and only FDA-approved medicine for the treatment of thyroid eye disease, or TED. TED is a serious, progressive and vision-threatening rare autoimmune condition. While TED often occurs in people living with hyperthyroidism or Graves’ disease, it is a distinct disease that is caused by autoantibodies activating an IGF-1R-mediated signaling complex on cells within the retro-orbital space. This leads to a cascade of negative effects, which may cause long-term, irreversible eye damage. As TED progresses, it causes serious damage – including proptosis (eye bulging), strabismus (misalignment of the eyes) and diplopia (double vision) – and in some cases can lead to blindness. Historically, patients have had to live with TED until the inflammation subsides, after which they are often left with permanent and vision-impairing consequences and may require multiple surgeries that do not completely return the patient to their pre-disease state.
Our comprehensive commercial strategy for TEPEZZA aims to enable more TED patients to benefit from TEPEZZA. We are doing this by: (i) facilitating continued TEPEZZA uptake in the treatment of TED through continued promotion of TEPEZZA to treating physicians; (ii) continuing to develop the TED market by increasing physician awareness of the disease severity and the urgency to diagnose and treat it, as well as the benefits of treatment with TEPEZZA, including through the expansion of our TEPEZZA commercial team and targeting ophthalmologists and endocrinologists; (iii) driving accelerated disease identification and time to treatment through our digital and broadcast marketing campaigns; (iv) enhancing the patient journey with our high-touch, patient-centric model as well as support for the patient and site-of-care referral processes; (v) pursuing more timely access to TEPEZZA for TED patients; (vi) conducting our Phase 4 clinical trial to inform the use TEPEZZA in chronic/low clinical activity score, or CAS, TED; and (vii) pursuing a global expansion strategy to build a global presence in targeted international markets to support the applications for potential approvals and full-scale commercial launches of TEPEZZA in Japan, Brazil, Europe and other international markets over the next several years.
To advance the continued strong growth and adoption of TEPEZZA, we are continuing to invest in significant expansion efforts in multiple areas: our commercial and field-based organization for TEPEZZA; our marketing initiatives; our long-term supply capacity; and our efforts to expand outside the United States. Due to the wind down of our former inflammation business during 2022, we redeployed a portion of our inflammation commercial team to support our TEPEZZA expansion.
With the U.S. launch of TEPEZZA in 2020 and the demonstrated benefit to U.S. patients with TED, we are pursuing a global expansion strategy to bring TEPEZZA to patients with TED in other parts of the world. Japan is one of the countries in which we are expanding and, in November 2022, we completed enrollment of a Phase 3 randomized, placebo-controlled clinical trial for the treatment of moderate-to-severe active TED patients in Japan. We initiated our build-out of infrastructure in Brazil to support potential approvals and commercial launches of TEPEZZA for TED. In October 2022, we announced that we submitted a regulatory filing to the Brazil National Health Surveillance Agency (ANVISA) for teprotumumab. In addition, since 2020, we have initiated commercial sales of teprotumumab via the Named Patient Program in other countries within Europe and Asia.
6
As the only FDA-approved medication for the treatment of TED, TEPEZZA has no direct approved competition. We believe that the results of the TEPEZZA Phase 3 and Phase 2 clinical trials present a significantly high hurdle for potential competitors, given that potentially competitive medicines would be expected to demonstrate similar or greater efficacy and safety in the treatment of TED. In addition, we have biologic reference product exclusivity in the United States covering TEPEZZA that will expire in 2032. Further, the complexity of manufacturing TEPEZZA could pose a barrier to potential biosimilar competition.
Although TEPEZZA does not face direct competition, other therapies, such as corticosteroids, have been used on an off-label basis to alleviate some of the symptoms of TED. While these therapies have not proved effective in treating the underlying disease, and carry with them potential significant side effects, their off-label use could reduce or delay treatment with TEPEZZA among the addressable patient population. Viridian Therapeutics, Inc., or Viridian, is pursuing development of three anti-IGF-1R monoclonal antibodies for TED (VRDN-001, VRDN-002 and VRDN-003). In August 2022, Viridian announced positive initial clinical data from the first cohort, 10mg/kg, of the ongoing Phase 1/2 clinical trial for an IGF-1R monoclonal antibody VRDN-001, in patients with TED. On November 14, 2022 and January 8, 2023, Viridian announced positive data for their second and third cohorts, 20mg/kg and 3mg/kg, respectively, of the ongoing Phase 1/2 trial showing improvements in signs and symptoms of TED after two infusions. In addition, in the fourth quarter of 2022, Viridian enrolled the first patient in its THRIVE Phase 3 trial of VRDN-001 with active TED. Viridian has also initiated a Phase 2 trial with a subcutaneous version of VRDN-002 in the fourth quarter of 2022. Viridian announced plans to file an investigational new drug, or IND, application for VRDN-003 in the second quarter of 2023 and subsequently begin a Phase 1 trial in healthy volunteers. In addition, Sling Therapeutics, Inc. is conducting a Phase 2b study of an oral IGF-1R for the treatment of moderate-to-severe TED and Novartis AG is conducting a Phase 3 trial of Cosentyx®(secukinumab, interleukin-17A inhibitor) in moderate-to-severe TED. Immunovant Inc., or Immunovant, initiated two Phase 3 clinical trials of a fully human anti-FcRn monoclonal antibody candidate for the treatment of active TED, also referred to as Graves’ ophthalmopathy, in the fourth quarter of 2022. Immunovant also began patient recruitment in the Phase 3 trial of batoclimab (FcRni). On January 5, 2023, Acelyrin, Inc., or Acelyrin, announced the acquisition of ValenzaBio Inc, or ValenzaBio. Previously, ValenzaBio received IND clearance and subsequently had begun a Phase 1 trial in the first half of 2022 with VB421, an anti-IGF-1R monoclonal antibody designed for subcutaneous use. In January 2023, Acelyrin also initiated a Phase 1/2 trial in active TED. Argenx SE announced a registrational trial of efgartigimod for the treatment of TED.
KRYSTEXXA
A PEGylated uric acid specific enzyme (uricase), KRYSTEXXA (pegloticase injection) is the first and only FDA-approved medicine for the treatment of uncontrolled gout. Uncontrolled gout occurs in patients who have failed to normalize serum uric acid, or sUA, and whose signs and symptoms are inadequately controlled with conventional therapies, such as xanthine oxidase inhibitors, or XOIs, at the maximum medically appropriate dose, or for whom these drugs are contraindicated.
KRYSTEXXA has a unique mechanism of action that can rapidly reverse disease progression. Unlike conventional XOI therapies, which address the over-production or under-excretion of uric acid, KRYSTEXXA converts uric acid into allantoin, a water-soluble molecule, which the body can easily eliminate through the urine. Renal excretion of allantoin is ten times more efficient than uric acid excretion. Additionally, many chronic kidney disease, or CKD, patients have gout, and the disease tends to be more prevalent as CKD advances. While conventional XOI gout therapies can place additional burden on the kidneys and have dosing limitations, KRYSTEXXA has been proven effective and safe for uncontrolled gout patients with CKD without the need to adjust dosing.
Gout is one of the most common forms of inflammatory arthritis and can be assessed by a simple blood test for the amounts of uric acid in the blood (sUA levels). Typically in gout, when uric acid levels are greater than 6.8 milligrams per deciliter, urate will crystallize and deposit. These hard deposits are known as tophi and may occur anywhere in the body, including joints, as well as organs, such as the kidney and heart. When under-treated medically, tophi often lead to bone erosions and loss of functional ability. Gout flares, a common characteristic of uncontrolled gout, are intensely painful. They may or may not be accompanied by tophi. A systemic disease, uncontrolled gout frequently causes crippling disabilities and significant joint damage. Of the 9.5 million gout sufferers in the United States, we estimate that greater than 100,000 patients have uncontrolled gout.
7
We are focused on optimizing and maximizing the benefit the medicine offers for patients as well as driving toward its peak U.S. net sales potential. Our growth strategy for KRYSTEXXA includes: (i) supporting the use of KRYSTEXXA with methotrexate following the approval of our supplemental biologics license application, or sBLA, in July 2022, which expanded KRYSTEXXA’s labeling to include co-administration with methotrexate; (ii) increasing uptake by rheumatologists; and (iii) accelerating uptake of the medicine by nephrologists.
In 2019, we added a separate group of sales representatives to call exclusively on nephrologists. Due to the wind down of our former inflammation business during 2022, we redeployed a portion of our inflammation commercial team to support our KRYSTEXXA expansion. We believe KRYSTEXXA offers a solution to a clinical need experienced by many nephrologists in dealing with uncontrolled gout patients with CKD.
As the only FDA-approved medication for the treatment of uncontrolled gout, KRYSTEXXA faces limited direct competition. We believe that the complexity of manufacturing KRYSTEXXA provides a barrier to potential biosimilar competition. However, a number of competitors have medicines in clinical trials, including Selecta Biosciences Inc., or Selecta, which has initiated a Phase 3 clinical program of a candidate for the treatment of chronic refractory gout. In September 2020, Selecta announced topline clinical data that did not meet the primary endpoint or demonstrate statistical superiority for its Phase 2 trial that compared its candidate, which includes an immunomodulator, to KRYSTEXXA alone. In July 2020, Selecta and Swedish Orphan Biovitrum AB, or Sobi, entered into a strategic licensing agreement under which Sobi will assume responsibility for certain development, regulatory, and commercial activities for this candidate. In August 2022, Selecta announced the completion of enrollment for both DISSOLVE trials, the two clinical studies of the Phase 3 DISSOLVE development program of SEL-212 for chronic refractory gout. Selecta has announced that the topline Phase 3 DISSOLVE results will be available in the first quarter of 2023. SEL-212 is a combination of Selecta’s ImmTOR immune tolerance platform and a therapeutic uricase enzyme (pegadricase).
RAVICTI
RAVICTI (glycerol phenylbutyrate) is indicated for use as a nitrogen-binding agent for chronic management of adult and pediatric patients (beginning at birth) with urea cycle disorders, or UCDs, that cannot be managed by dietary protein restriction and/or amino acid supplementation alone. UCDs are rare, life-threatening genetic disorders. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (for example, essential amino acids, arginine, citrulline or protein-free calorie supplements).
UCDs are inherited metabolic diseases caused by a deficiency of one of the enzymes or transporters that constitute the urea cycle. The urea cycle involves a series of biochemical steps in which ammonia, a potent neurotoxin, is converted to urea, which is excreted in the urine. UCD patients may experience episodes during which the ammonia levels in their blood become excessively high, called hyperammonemic crises, which may result in irreversible brain damage, coma or death. We estimate that there are approximately 2,600 patients with UCDs living in the United States, including approximately 1,000 diagnosed patients. RAVICTI is not indicated for treatment of acute hyperammonemia or for N-acetylglutamate synthase (NAGS) deficiency.
UCD symptoms may first occur at any age depending on the severity of the disorder, with more severe defects presenting earlier in life. However, a prompt diagnosis and careful management of the disease can lead to good clinical outcomes.
RAVICTI competes with older-generation nitrogen scavenger medicines. In the United States, RAVICTI competes with all forms of sodium phenylbutyrate, including BUPHENYL. RAVICTI has advantages over older-generation medicines leading to better patient adherence and compliance rates, such as its better tolerability for patients. It is ingested by mouth, requires little preparation and has little taste and lower sodium content than other nitrogen scavenger medications. RAVICTI could face competition from a few alternative medicine and treatment options that have been recently approved or are in development, including Pheburane®, a taste-masked formulation of sodium phenylbutyrate for which Medunik USA received approval from the FDA in June 2022, a gene-therapy candidate by Ultragenyx Pharmaceutical Inc., OlpruvaTM, a taste-masked formulation of sodium phenylbutyrate for which ACER Therapeutics Inc. received approval from the FDA in December 2022, an enzyme replacement for a specific UCD subtype (ARG) by Aeglea Bio Therapeutics Inc. and a mRNA-based therapeutic for a specific UCD subtype (OTC) by Arcturus Therapeutics Holdings Inc.
8
Our strategy for RAVICTI is to drive growth through increased awareness and diagnosis of UCDs; to drive conversion to RAVICTI from older-generation nitrogen scavengers, such as generic forms of sodium phenylbutyrate, based on the medicine’s differentiated benefits; to position RAVICTI as the first line of therapy; and to increase compliance rates.
In December 2018 and October 2020, we sold our rights to develop and commercialize RAVICTI outside of North America to Immedica. We previously distributed RAVICTI through a commercial partner in Europe and other non-U.S. markets. We have retained rights to RAVICTI in North America.
PROCYSBI
PROCYSBI (cysteamine bitartrate) is indicated for nephropathic cystinosis, or NC, a rare lysosomal storage disorder that results in the amino acid cystine accumulating inside the lysosomes of nearly every cell. Cystine accumulation results in the formation of crystals that lead to cell damage and death in tissues and organs throughout the body. PROCYSBI delayed-release capsules and delayed-release oral granules is the first and only FDA-approved treatment for NC with 12-hour dosing. PROCYSBI uses proprietary technology that releases cysteamine gradually, providing 12-hour continuous cystine control in adults and children 1 year of age and older. PROCYSBI granules, also called “microbeads,” are composed of cysteamine bitartrate surrounded by an acid-resistant enteric coating. To work properly, PROCYSBI microbeads must dissolve and release cysteamine bitartrate in the small intestine. The coating on the microbeads helps to control where and how medicine is released by allowing the cysteamine bitartrate to pass through the acidic stomach to the alkaline environment of the small intestine. Once in the small intestine, the coating begins to dissolve and the microbeads release cysteamine bitartrate gradually. This allows PROCYSBI to control cystine levels continuously over the dosing interval. Randomized controlled clinical trials and extended treatment with PROCYSBI therapy demonstrated consistent cystine depletion as monitored by levels of the biomarker (and surrogate marker), white blood cell cystine concentration.
In NC patients, elevated cystine can lead to cellular dysfunction and death; without treatment, the disease is usually fatal by the end of the first decade of life. Cystinosis is progressive, eventually causing irreversible tissue damage and multi-organ failure, including kidney failure, blindness, muscle wasting and premature death. NC is usually diagnosed in infancy after children display symptoms to physicians, including markedly increased urination, thirst, dehydration, gastrointestinal distress, failure to thrive, rickets, photophobia and kidney symptoms specific to Fanconi syndrome. Management of cystinosis requires lifelong therapy.
In February 2020, the FDA approved PROCYSBI Delayed-Release Oral Granules in Packets for adults and children one year of age and older living with nephropathic cystinosis. The PROCYSBI Delayed-Release Oral Granules in Packets product is the same as the PROCYSBI capsules product except in respect of the packaging format. This granules in packets dosage form provides another administration option for patients, in addition to the PROCYSBI capsules. PROCYSBI Delayed-Release Oral Granules in Packets were made commercially available in April 2020.
PROCYSBI is differentiated by its ability to control cystine concentration continuously over twelve hours. Older therapies require administration of medicine every six hours. By taking PROCYSBI, patients have to dose only twice a day, providing them greater control over their medication schedule and lifestyle. Additionally, because PROCYSBI can be administered through a feeding tube or mixed with approved foods and liquids, the patient can choose a more flexible dosing regimen. PROCYSBI may also have fewer known side effects, such as less severe bad breath (halitosis) and body odor, than older-generation therapies.
We estimate that there are approximately 500-550 patients diagnosed with NC living in the United States. In addition to patients who have already been identified, we believe that a number of patients with atypical phenotypic presentation and end-stage renal disease have their condition as a result of undiagnosed late-onset NC and would benefit from treatment with PROCYSBI.
PROCYSBI faces competition from Cystagon® for the treatment of cystinosis. Cystagon, an immediate-release cysteamine bitartrate capsule, is an older-generation systemic cystine-depleting therapy for cystinosis in the United States marketed by Mylan N.V., and by Orphan Europe SARL in markets outside of the United States. Additionally, we are also aware that AVROBIO, Inc. has a gene therapy candidate in development for the treatment of cystinosis. We believe that PROCYSBI will continue to be well received in the market and continue to expect Cystagon to be the primary competitor for PROCYSBI for the foreseeable future.
Our strategy for PROCYSBI is to drive conversion of patients from older-generation immediate-release capsules of cysteamine bitartrate; to increase the uptake of the medicine by diagnosed but untreated patients; to position PROCYSBI as a first line of therapy; and to increase compliance rates.
9
UPLIZNA
UPLIZNA (inebilizumab-cdon) is an anti-CD19 humanized monoclonal antibody that depletes B cells, including the pathogenic cells that produce autoantibodies. In some autoimmune diseases, autoantibodies secreted by plasmablasts and plasma cells attack native tissues as opposed to foreign pathogens. UPLIZNA depletes these plasmablasts that may produce pathogenic autoantibodies. UPLIZNA was approved for the treatment of adults with AQP4-IgG+ NMOSD by the FDA in June 2020, by the Japanese Ministry of Health, Labour and Welfare in March 2021, by China’s National Medical Products Administration in March 2022, by the European Commission in April 2022 and by the Brazil National Health Surveillance Agency (ANVISA) in December 2022.
NMOSD is a rare, severe autoimmune disease in which autoantibodies produced by B cells attack the optic nerve, spinal cord and brain/brainstem, often causing permanent blindness, weakness, and/or paralysis. NMOSD is characterized by unpredictable attacks and severe disability that often occurs following the first attack, accumulating with each subsequent relapse. Thus, preventing these attacks is the primary goal for disease management. NMOSD is often misdiagnosed as multiple sclerosis, or MS, which can be problematic since some MS treatments may exacerbate NMOSD. UPLIZNA is an infused medicine that works by depleting B-cells in a targeted manner and is proven to reduce NMOSD attacks.
In Japan, our strategic partner, MTPC has the rights to develop and commercialize UPLIZNA. In March 2021, MTPC received manufacturing and marketing approval for UPLIZNA in Japan. UPLIZNA was launched in Japan during the second quarter of 2021. Furthermore, in April 2022, the European Commission issued a legally binding decision based on the favorable recommendation of the Committee for Medicinal Products for Human Use of the European Medicines Agency, or EMA, to grant a Marketing Authorization for UPLIZNA for the treatment of adult patients with NMOSD in the European Union, or EU. We are continuing to invest in our European infrastructure to support the European launch of UPLIZNA for NMOSD, which began in Germany and Austria; and in France under an early access program, in the third quarter of 2022. We are also preparing for the planned commercial launch of UPLIZNA in Italy and Spain in 2023. Hansoh has rights to develop and commercialize UPLIZNA for NMOSD as well as other potential future indications in China. In March 2022, Hansoh received notice that UPLIZNA was approved by China’s National Medical Products Administration for the treatment of adults with NMOSD. The launch of UPLIZNA in China began in the first quarter of 2023. In addition, we continue to build-out our infrastructure in Brazil to support the planned commercial launch of UPLIZNA for NMOSD in 2023.
UPLIZNA is the only approved NMOSD therapy in the United States that has demonstrated a clinically relevant and durable effect on delaying worsening of disability, with a significant reduction in hospitalization. Long-term UPLIZNA treatment has been shown to be well tolerated and provide a sustained reduction in NMOSD attack risk for four or more years. UPLIZNA faces competition from eculizumab, marketed as Soliris® by AstraZeneca plc, and satralizumab, marketed as EnspryngTM by Genentech/Chugai Pharmaceuticals Co., Ltd., a subsidiary of F. Hoffmann-La Roche Ltd., each for the treatment of patients with NMOSD. In addition, AstraZeneca announced positive primary endpoint results from its Phase 3 trial with Ultomiris® (ravulizumab) in NMOSD and, if approved for this indication, UPLIZNA could face additional competition. Ultomiris is currently under regulatory review in both the United States and EU with potential approval in the first half of 2023. UPLIZNA also faces competition from rituximab, an off-label treatment that has been used for years to treat NMOSD given the lack of an approved medicine for this disease prior to 2019. Other novel treatments are under development for NMOSD, including Phase 3 candidates being developed by Beijing Mabworks Biotech Co. Ltd. and RemeGen Co. Ltd., and Phase 2 candidates, including a candidate being developed by Chord Therapeutics SA/Merck KGaA.
With respect to our strategy for UPLIZNA, which leverages the successful strategies we have employed with TEPEZZA and KRYSTEXXA, our aim is to (i) increase physician awareness of the benefits of UPLIZNA for the treatment of NMOSD, and what differentiates UPLIZNA from other medicines by generating additional trial data analyses and clinical evidence; (ii) drive patient initiation and adherence, and cultivate a positive patient experience; and (iii) maximize the potential of UPLIZNA through additional indications and global expansion, including potential approvals and commercial launches of UPLIZNA in additional markets in the coming years.
10
ACTIMMUNE
ACTIMMUNE (interferon gamma-1b) is indicated for chronic granulomatous disease, or CGD, and severe, malignant osteopetrosis, or SMO. It is a biologically manufactured protein called interferon gamma-1b that is similar to a protein the human body makes naturally. Interferon gamma helps prevent infection in CGD patients and enhances osteoclast function in SMO patients. ACTIMMUNE is the only medicine approved by the FDA to reduce the frequency and severity of serious infections associated with CGD and for delaying disease progression in patients with SMO. ACTIMMUNE is believed to work by modifying the cellular function of various cells, including those in the immune system and those that help form bones.
CGD is a genetic disorder of the immune system. It is described as a primary immunodeficiency disorder, which means it is not caused by another disease or disorder. In people who have CGD, a type of white blood cell called a phagocyte is defective. These defective phagocytes cannot generate superoxide, leading to an inability to kill harmful microorganisms such as bacteria and fungi. As a result, the immune system is weakened. People with CGD are more likely to have certain problems, such as recurrent severe and potentially life-threatening bacterial and fungal infections and chronic inflammatory conditions. These patients are prone to developing masses called granulomas, which can occur repeatedly in organs throughout the body and cause a variety of problems. We estimate that there are approximately 1,200 patients with CGD in the United States, based on an estimated incidence of 1:200,000 live births.
SMO is a form of osteopetrosis and is sometimes referred to as marble bone disease or malignant infantile osteopetrosis because it occurs in very young children. While exact numbers are not known, it has been estimated that one out of 250,000 children are born with SMO.
ACTIMMUNE currently faces limited competition. There are additional or alternative approaches used to treat patients with CGD and SMO, including the increasing trend towards the potentially curative treatment of bone marrow transplants in patients with CGD, however, there are currently no FDA approved medicines indicated for CGD and SMO on the market that compete directly with ACTIMMUNE.
Our strategy for ACTIMMUNE is to increase awareness and diagnosis of CGD; to drive utilization of ACTIMMUNE prophylaxis in newly diagnosed CGD patients as recommended in current treatment guidelines; encourage use of ACTIMMUNE in CGD patients prior to bone marrow transplant and in symptomatic carriers of x-linked CGD; and increase compliance rates overall.
BUPHENYL
BUPHENYL (sodium phenylbutyrate) tablets and BUPHENYL powder are made from granules that contain sodium phenylbutyrate as the active (chemically synthesized) ingredient and microcrystalline cellulose as a diluent.
BUPHENYL tablets for oral administration and BUPHENYL powder for oral, nasogastric, or gastrostomy tube administration are indicated as adjunctive therapy in the chronic management of patients with UCDs involving deficiencies of carbamoyl phosphate synthetase, ornithine transcarbamylase or argininosuccinic acid synthetase.
BUPHENYL is indicated for treatment of all patients with neonatal-onset deficiency (complete enzymatic deficiency, presenting within the first twenty-eight days of life). It is also indicated for treatment of patients with late-onset disease (partial enzymatic deficiency, presenting after the first month of life) who have a history of hyperammonemic encephalopathy. It is important that the diagnosis be made early and treatment initiated immediately to improve clinical outcomes. BUPHENYL must be combined with dietary protein restriction and, in some cases, essential amino acid supplementation. We distribute BUPHENYL in the United States.
QUINSAIR
QUINSAIR (levofloxacin) is a formulation of the antibiotic drug levofloxacin, suitable for inhalation via a nebulizer and indicated for the management of chronic pulmonary infections due to Pseudomonas aeruginosa in adult patients with cystic fibrosis, or CF. CF is a rare, life-threatening genetic disease affecting approximately 70,000 people worldwide, and results in build-up of abnormally thick secretions that can cause chronic lung infections and progressive lung damage in many patients that eventually leads to death.
11
INFLAMMATION MEDICINES
Our portfolio also includes medicines that treat inflammatory diseases. PENNSAID 2% (diclofenac sodium topical solution) is indicated for the treatment of pain of osteoarthritis, or OA, of the knee(s). OA is a type of arthritis that is caused by the breakdown and eventual loss of the cartilage of one or more joints. RAYOS (prednisone) is a corticosteroid indicated for the treatment of multiple conditions: rheumatoid arthritis, or RA; ankylosing spondylitis, or AS; polymyalgia rheumatica; systemic lupus erythematosus; and a number of other conditions. DUEXIS (ibuprofen/famotidine) is indicated for the relief of signs and symptoms of RA and OA and to decrease the risk of developing upper-gastrointestinal ulcers in patients who are taking ibuprofen for these indications. RA is a chronic disease that causes pain, stiffness and swelling, primarily in the joints. VIMOVO (naproxen/esomeprazole magnesium) is indicated for the relief of signs and symptoms of OA, RA and AS, and to decrease the risk of developing gastric ulcers in patients at risk of developing nonsteroidal anti-inflammatory drug-associated gastric ulcers. At present, PENNSAID 2%, DUEXIS and VIMOVO face generic competition in the United States and we are expecting a generic version of RAYOS to enter the market in 2023.
In the fourth quarter of 2022, we substantially completed our planned wind down of our former inflammation business, including active promotion efforts and associated HorizonCares support. As a result, we expect sales of our inflammation medicines to be immaterial on a go-forward basis.
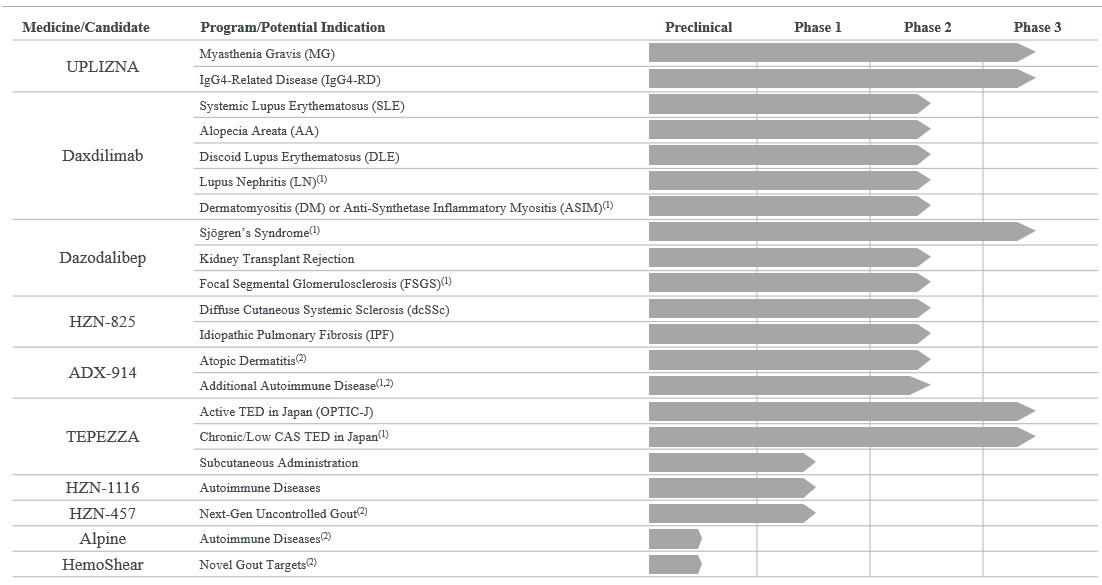
Research and Development
Our R&D programs include preclinical and clinical development of new medicine candidates, as well as development programs intended to maximize the benefit and value of our existing medicines. We devote significant resources to R&D activities that address critical unmet medical needs for people impacted by rare, autoimmune and severe inflammatory diseases. Our pipeline includes more than 20 programs. We announced the initiation of five clinical trials in 2022 and expect to initiate several more in 2023, including a planned Phase 3 program for dazodalibep in Sjögren’s syndrome. In January 2023, we announced the initiation of our daxdilimab discoid lupus erythematosus, or DLE, Phase 2 trial. The graphic below summarizes our R&D programs ranging from preclinical to Phase 3 as of March 1, 2023.
|
|
(1) Planned programs; not yet initiated. (2) External collaboration. |
|
We also have three Phase 4 programs: TEPEZZA chronic/low CAS TED, KRYSTEXXA shorter infusion duration and KRYSTEXXA monthly dosing.
12
UPLIZNA Clinical Programs
UPLIZNA is an anti-CD19 humanized monoclonal antibody that depletes B cells, including the pathogenic cells that produce autoantibodies. UPLIZNA is approved by the FDA and the European Commission for the treatment of NMOSD. We are currently evaluating UPLIZNA in two additional indications: myasthenia gravis (a Phase 3 randomized, placebo-controlled clinical trial) and IgG4-related disease (a Phase 3 randomized, placebo-controlled clinical trial).
Daxdilimab Clinical Programs
Daxdilimab is an anti-ILT7 human monoclonal antibody that depletes certain dendritic cells. Depleting these cells may interrupt the cycle of inflammation that causes tissue damage in diseases such as lupus and a variety of other autoimmune conditions. We are currently evaluating daxdilimab in two Phase 2 randomized, placebo-controlled clinical trials in systemic lupus erythematosus and DLE. We also have a Phase 2 open-label trial underway to evaluate daxdilimab in patients with alopecia areata. We expect to initiate two Phase 2 clinical trials in additional potential indications, lupus nephritis and dermatomyositis/anti-synthetase inflammatory myositis, in 2023.
Dazodalibep Clinical Programs
Dazodalibep is a CD40 ligand antagonist that blocks T-cell interaction with CD40-expressing B cells, disrupting the overactivation of the CD40 ligand co-stimulatory pathway. We are evaluating dazodalibep in several autoimmune diseases associated with the overactivation of this pathway. In September 2022 and January 2023, we announced positive top-line results of our Phase 2 randomized, placebo-controlled clinical trial in Sjögren’s syndrome: both study populations met the primary endpoints and dazodalibep was well-tolerated. Based on the positive outcome of this trial, we plan to initiate a Phase 3 program in 2023. We also completed a Phase 2 randomized, placebo-controlled clinical trial that established proof of concept in rheumatoid arthritis in May 2022. We expect to initiate a Phase 2 clinical trial evaluating dazodalibep in focal segmental glomerulosclerosis in 2023.
HZN-825 Clinical Programs
HZN-825 is an oral selective LPAR1 antagonist that prevents gene activation and has demonstrated antifibrotic activity. HZN-825 is in Phase 2 development for diffuse cutaneous systemic sclerosis and idiopathic pulmonary fibrosis.
ADX-914 Clinical Programs (collaboration with Q32 Bio Inc.)
ADX-914 is a fully human anti-IL-7Rα antibody that re-regulates adaptive immune function by blocking signaling mediated by both IL-7 and TSLP. ADX-914 is part of our global collaboration and option agreement with Q32 Bio Inc. for the treatment of autoimmune diseases, which we entered into in August 2022. We announced the initiation of a Phase 2 trial to evaluate ADX-914 in atopic dermatitis in October 2022, and we expect an additional Phase 2 trial in a second autoimmune disease to be initiated in 2023. We have an option to acquire ADX-914 exercisable through a period following completion of the Phase 2 trials; until then, Q32 is operationally responsible for the conduct of all program-related activities.
TEPEZZA Clinical Programs
TEPEZZA is an IGF-1R antagonist monoclonal antibody. It is the first and only medicine approved by the FDA for the treatment of TED. Two TEPEZZA clinical programs are underway: OPTIC-J, a Phase 3 randomized, placebo-controlled clinical trial for the treatment of moderate-to-severe active TED patients in Japan and a Phase 1 pharmacokinetic clinical trial for subcutaneous administration of TEPEZZA. Enrollment in OPTIC-J was completed in November 2022. We expect to initiate a Phase 3 clinical trial in chronic/low CAS TED in Japan in 2023.
13
HZN-1116 Autoimmune Disease Program
HZN-1116 is a human monoclonal antibody designed to neutralize the FLT3-ligand, thereby reducing both conventional and plasmacytoid dendritic cells that play a key role in driving inflammatory processes. We are currently evaluating HZN-1116 in a Phase 1 clinical trial for autoimmune diseases.
HZN-457 Gout Program (collaboration with Arrowhead Pharmaceuticals, Inc.)
HZN-457 is a small interfering RNA (siRNA) candidate designed to treat gout by silencing liver xanthine dehydrogenase. The development of HZN-457 is part of our global collaboration and license agreement with Arrowhead Pharmaceuticals, Inc. HZN-457 is aimed at addressing the unmet need for the more than 500,000 gout patients who do not respond to the current standard of conventional care and are not good candidates for KRYSTEXXA. We initiated a Phase 1 randomized, placebo-controlled trial in December 2022 to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of HZN-457.
Preclinical Programs
Our preclinical program with HemoShear Therapeutics, LLC is exploring the potential for a novel therapeutic to address the unmet need of gout patients unresponsive to conventional treatments or KRYSTEXXA. Our preclinical program with Alpine Immune Sciences, Inc. is focused on developing novel protein-based therapies for autoimmune and inflammatory diseases. We are leveraging external collaborations for these programs, using the specialized technologies of our collaboration partners in combination with our internal expertise.
Phase 4 TEPEZZA and KRYSTEXXA Programs
Our ongoing TEPEZZA Phase 4 randomized, placebo-controlled clinical trial in chronic/low CAS TED is designed to better inform physicians and payers on the safety and efficacy of TEPEZZA in TED patients with a low CAS. We completed enrollment in this trial in September 2022 and expect topline results to be available in the second quarter of 2023.
We are exploring the potential to improve the patient experience with two Phase 4 KRYSTEXXA open-label clinical trials currently underway. Both involve the co-administration of KRYSTEXXA plus the immunomodulator methotrexate. One trial is evaluating a shorter-infusion duration, and the other, our monthly dosing trial, is evaluating less frequent infusions.
An important development in 2022 was the approval by the FDA of our sBLA to expand the KRYSTEXXA label to include co-administration with methotrexate. The approval was based on six- and twelve-month results from our MIRROR randomized controlled trial completed in 2021, which demonstrated significant improvements in response rate and sustained patient response of KRYSTEXXA with methotrexate compared to KRYSTEXXA with placebo, as well as a significant reduction in infusion reactions.
14
Distribution
We use a regulatory compliant third-party logistics organization for storage and distribution of our medicines into the supply chain. Our third-party logistics provider specializes in the healthcare industry with operations that include warehousing and transportation services that can be scaled and customized to our needs based on market conditions and the demands and delivery service requirements for our medicines and materials. Their services eliminate the need to build dedicated internal infrastructures that would be difficult to scale without significant capital investment. Our third-party logistics provider warehouses all medicines in controlled regulatory compliant facilities. Incoming orders are prepared and shipped through an order entry system to ensure adequate supply and delivery of our medicines.
Sales and Marketing
As of December 31, 2022, our sales force was composed of approximately 380 sales representatives. Due to the impact of the at-risk launch of generic PENNSAID 2% and the wind down of our former inflammation business during 2022, we redeployed a portion of our inflammation commercial team to support our TEPEZZA and KRYSTEXXA expansions.
Our sales representatives focus on marketing our rare disease medicines to a limited number of healthcare practitioners who specialize in fields such as pediatric immunology, allergy, infectious diseases, metabolic disorders, rheumatology, nephrology, ophthalmology and endocrinology, to help them understand the potential benefits of our medicines. We have entered into, and may continue to enter into, agreements with third parties to commercialize our medicines outside the United States.
We have a comprehensive compliance program in place to address adherence with various laws and regulations relating to our sales, marketing, and manufacturing of our medicines, as well as certain third-party relationships, including pharmacies. Specifically with respect to pharmacies, the compliance program utilizes a variety of methods and tools to monitor and audit pharmacies, including those that participate in our patient assistance programs, to confirm their activities, adjudication and practices are consistent with our compliance policies and guidance.
15
Manufacturing, Commercial, Supply and License Agreements
We have agreements with third parties for active pharmaceutical ingredients, or APIs, biological drug substance and drug product, manufacture of our medicines, formulation and development services. We also have agreements for fill, finish and packaging services, transportation, and distribution and logistics services for certain medicines. In all cases, we retain certain levels of safety stock or maintain alternate supply relationships that we can utilize without undue disruption of our manufacturing processes if a third party fails to perform its contractual obligations.
In July 2021, we purchased a drug product biologics manufacturing facility in Waterford, Ireland, which is intended to be an additional source of manufacturing to supplement the capabilities of our third-party drug product manufacturers. We are in the process of completing the build-out and validation of this facility and assuming timely receipt of regulatory approvals, we expect the first medicine manufactured for commercial use at the facility to be approved for release in the second half of 2023. In August 2022, we submitted a planning application to build a drug substance biologics manufacturing facility adjacent to our existing drug product biologics manufacturing facility in Waterford, Ireland. Based on our current operating plan, we do not anticipate making significant investments in building a drug substance biologics manufacturing facility during 2023.
TEPEZZA
TEPEZZA is produced by culture of a genetically engineered mammalian cell line containing the DNA which encodes for teprotumumab-trbw, a fully human IgG1 monoclonal antibody. Cell culture broth is harvested and purified through filtration processes and chromatography processes prior to being formulated, frozen and shipped to the site of drug product manufacture. In support of its manufacturing process, we store multiple vials of teprotumumab-trbw master cell bank and working cell bank at multiple locations in order to ensure adequate backup should any cell bank be lost in a catastrophic event.
AGC Biologics Supply Agreement
In February 2018, we entered into a commercial supply agreement with AGC Biologics A/S (formerly known as CMC Biologics A/S), or AGC, which was amended in May 2019, December 2019 and July 2020, for the supply of TEPEZZA drug substance from AGC’s facilities in Copenhagen, Denmark and Boulder, Colorado. Pursuant to the agreement, we have agreed to purchase certain minimum annual order quantities of TEPEZZA drug substance. In addition, we must provide AGC with rolling forecasts of TEPEZZA drug substance requirements, with a portion of the forecast being a firm and binding order. The agreement has a term that runs indefinitely. Either party may terminate the agreement by giving notice at least three years in advance. Either party may also terminate the agreement for the other party’s failure to pay any undisputed sum payable under the agreement within a specified period of time, for a material breach by the other party if not cured within a specified period of time, upon the other party’s insolvency, or in the event that any material permit or regulatory license is permanently revoked preventing the performance of specified services by the other party.
AGC Development and Manufacturing Services Agreement
We have a development and manufacturing services agreement with AGC, dated June 10, 2015, which was amended in February 2018, for development and manufacturing services relating to TEPEZZA drug substance. The agreement has a term that runs until the later of the date that all work under the agreement is completed and June 2025, unless earlier terminated by us upon 30 days’ written notice. AGC can terminate the agreement after AGC has completed its services by giving 180 days’ written notice, or sooner if certain conditions are met, or upon 60 business days’ notice if AGC reasonably concludes it cannot deliver the services under the agreement despite applying commercially reasonable efforts. Either party may also terminate the agreement for the other party’s failure to pay any undisputed sum payable under the agreement within a specified period of time, for a material breach by the other party if not cured within a specified period of time, or upon the other party’s insolvency.
Catalent Indiana Supply Agreement
In December 2018, we entered into a commercial supply agreement with Catalent Indiana, LLC, or Catalent, for the supply of TEPEZZA drug product. Pursuant to the agreement, we must provide Catalent with rolling forecasts of TEPEZZA drug product requirements, with a portion of the forecast being a firm and binding order. The agreement has a term that runs until December 18, 2025, and automatically renews for two successive two-year terms unless terminated by either party at least two years in advance. The agreement may be terminated earlier by either party for a material breach by the other party, if not cured within a specified period of time, or upon the other party’s insolvency.
16
Patheon Italy Agreement
In October 2018, we entered into a master manufacturing services agreement with Patheon Pharmaceuticals, Inc., or Patheon. Pursuant to the agreement, in June 2020, we entered into a product agreement, which was amended in October 2021, for the manufacture and supply of TEPEZZA drug product in Italy. Pursuant to the master manufacturing services agreement and the amended product agreement, or, collectively, the Patheon manufacturing agreement, we must provide Patheon a monthly rolling forecast of TEPEZZA drug product requirements, with a portion of the forecast being a firm and binding order. The Patheon manufacturing agreement has a term that runs until October 2026, and automatically renews for successive three-year terms unless terminated by either party at least two years in advance. The agreement may be terminated earlier by either party for a material breach by the other party, if not cured within a specified period of time, or upon the other party’s insolvency.
Roche License Agreement
We have a license of intellectual property rights to TEPEZZA under a license agreement with Roche, effective as of June 15, 2011, as amended. Pursuant to the agreement, we have paid development and regulatory milestones totaling CHF60.0 million relating to the United States. We may be obligated to pay Roche additional development and regulatory milestones for activities outside the United States or for additional indications. We are also obligated to pay tiered royalties between 9 and 12 percent on annual worldwide net sales. The royalty terminates upon the later of (a) the expiration date of the longest-lived patent rights on a country-by-country basis; and (b) ten years after first commercial sale of TEPEZZA. Either party may terminate the agreement upon the other party’s breach of the agreement, if not cured within a specified period of time, or in the event of the other party’s bankruptcy or insolvency. Roche may also terminate the agreement if we challenge the validity of Roche’s patents. We may also terminate the agreement with nine months written notice to Roche.
Boehringer Ingelheim Biopharmaceuticals License Agreement
We have a license of certain manufacturing technology for TEPEZZA under a license agreement with Boehringer Ingelheim Biopharmaceuticals, effective as of December 21, 2016. Either party may terminate the agreement upon the other party’s material breach of the agreement if not cured within a specified period of time. Boehringer Ingelheim Biopharmaceuticals may also terminate the agreement if we challenge the validity of certain of its patent rights.
Other Agreements
In addition to the above supply and license agreements, under the agreement for the acquisition of River Vision in May 2017, we were required to pay up to $325.0 million upon the attainment of various milestones, composed of $100.0 million related to FDA approval and $225.0 million related to net sales thresholds for TEPEZZA. We made a $100.0 million milestone payment related to FDA approval during the first quarter of 2020. The agreement also included a royalty payment of 3 percent of the portion of annual worldwide net sales exceeding $300.0 million.
In April 2020, we entered into an agreement with S.R. One, Limited, or S.R. One, and an agreement with Lundbeckfond Invest A/S, or Lundbeckfond, pursuant to which we acquired all of S.R. One’s and Lundbeckfond’s beneficial rights to proceeds from certain contingent future TEPEZZA milestone and royalty payments in exchange for a one-time payment of $55.0 million to each of the respective parties. As a result of our agreements with S.R. One and Lundbeckfond in April 2020, our remaining net obligations to make TEPEZZA payments to the former stockholders of River Vision was reduced by approximately 70.25%, after including payments to a third party.
This resulted in milestone payments of $67.0 million to the other former River Vision stockholders during the year ended December 31, 2021. There are no further TEPEZZA net sales milestone obligations remaining to the former River Vision stockholders. In addition, as a result of the S.R. One and Lundbeckfond agreements, annual earnout payments of 0.893 percent are due on the portion of annual worldwide net sales exceeding $300.0 million.
17
KRYSTEXXA
KRYSTEXXA is produced by fermentation of a genetically engineered Escherichia coli bacterium containing the DNA which encodes for uricase. The complementary DNA coding for the uricase is based on mammalian sequences. Uricase is purified and is then PEGylated with a PEGylation agent to produce the bulk medicine, pegloticase. PEGylation and purification of the active drug substance is achieved by conventional column chromatography. The resulting highly purified sterile solution is filled in a single-use vial for intravenous infusion following dilution. In support of its manufacturing process, we store multiple vials of the Escherichia coli bacterium master cell bank and working cell bank at multiple locations in order to ensure adequate backup should any cell bank be lost in a catastrophic event.
NOF Supply Agreement
Under the terms of our exclusive supply agreement with NOF Corporation, or NOF, as amended, for the PEGylation agent used in the manufacture of KRYSTEXXA, we are required to issue NOF forecasts of our requirements for the PEGylation agent, a portion of which are binding. Under the agreement, we are obligated to purchase a certain minimum quantity of the PEGylation agent over specified periods of time and we are required to use NOF as our exclusive supplier for the PEGylation agent, subject to certain exceptions if NOF is unable to supply the PEGylation agent. The agreement expires in October 2024. Either we or NOF may also terminate the agreement upon a material breach, if not cured within a specified period of time, or in the event of the other party’s insolvency.
Bio-Technology General (Israel) Supply Agreement
We have a commercial supply agreement, as amended, with Bio-Technology General (Israel) Ltd, or BTG Israel, for the production of the bulk KRYSTEXXA medicine, or bulk product. Under this agreement, we have agreed to purchase certain minimum annual order quantities and are obligated to purchase at least 80 percent of our annual world-wide bulk product requirements from BTG Israel. The term of the agreement runs until December 31, 2030, and will automatically renew for successive three-year periods unless earlier terminated by either party upon three years’ prior written notice. The agreement may be terminated earlier by either party in the event of a force majeure, liquidation, dissolution, bankruptcy or insolvency of the other party, uncured material breach by the other party or after January 1, 2024, upon three years’ prior written notice. Under the agreement, if the manufacture of the bulk product is moved out of Israel, we may be required to obtain the approval of the Israel Innovation Authority (formerly known as Israeli Office of the Chief Scientist), or IIA, because certain KRYSTEXXA intellectual property was initially developed with a grant funded by the IIA. We must provide BTG with rolling forecasts of the volume of KRYSTEXXA that we expect to order, with a portion of the forecast being a firm and binding order.
Exelead PharmaSource Supply Agreement
In October 2008, Savient Pharmaceuticals, Inc. (as predecessor in interest to Crealta Pharmaceuticals LLC) and Exelead, Inc. (formerly known as Sigma Tau PharmaSource, Inc. (as successor in interest to Enzon Pharmaceuticals, Inc.)), or Exelead, entered into a commercial supply agreement, which was subsequently amended, for the packaging and supply of the final KRYSTEXXA drug product. This agreement remains in effect until terminated, and either we or Exelead may terminate the agreement with three years notice, given thirty days prior to the agreement anniversary date. Either we or Exelead may also terminate the agreement upon a material default, if not cured within a specified period of time, or in the event of the other party’s insolvency or bankruptcy.
Duke University and Mountain View Pharmaceutical License Agreement
We have a worldwide license agreement with Duke University, or Duke, and Mountain View Pharmaceuticals Inc., or MVP, which was subsequently amended. Duke developed the recombinant uricase enzyme used in KRYSTEXXA and MVP developed the PEGylation technology used in the manufacture of KRYSTEXXA. Duke and MVP may terminate the agreement if we commit fraud or for our willful misconduct or illegal conduct; upon our material breach of the agreement, if not cured within a specified period of time; upon written notice if we have committed two or more material breaches under the agreement; or in the event of our bankruptcy or insolvency. Under the terms of the agreement, we are obligated to pay Duke a mid-single digit percentage royalty on our global net sales of KRYSTEXXA and a royalty of between 5 percent and 15 percent on any global sublicense revenue. We are also obligated to pay MVP a mid-single digit percentage royalty on our net sales of KRYSTEXXA outside of the United States and royalty of between 5 percent and 15 percent on any sublicense revenue outside of the United States.
18
RAVICTI
RAVICTI is formed by the catalyzed esterification of glycerol with 4-phenylbutyric acid and the subsequent purification of the glycerol phenylbutyrate formed. The purified glycerol phenylbutyrate drug substance is filled into glass bottles for use as an oral dosage liquid.
We have a supply agreement with Seqens (Germany) for supply of 4-Phenylbutyric acid (4-PBA) needed to produce glycerol phenylbutyrate API. We have supplies of glycerol phenylbutyrate API manufactured for us by two alternate suppliers, Helsinn Advanced Synthesis SA (Switzerland) and Patheon Austria GmbH & Co KG (formerly DSM Fine Chemicals Austria) on a purchase-order basis until 2025. We have manufacturing agreements for finished RAVICTI drug product with Lyne Laboratories, Inc. and PCI Pharma Services.
Bausch Health Asset Purchase Agreement
We have an asset purchase agreement with Bausch Health Companies, Inc. (formerly Ucyclyd Pharma, Inc.), or Bausch, pursuant to which we are obligated to pay to Bausch mid-single-digit royalties on our global net sales of RAVICTI. The asset purchase agreement cannot be terminated for convenience by either party. We have a license to certain Bausch manufacturing technology related to RAVICTI; however Bausch is permitted to terminate the license if we fail to comply with any payment obligations relating to the license and do not cure such failure within a defined time period.
Brusilow License Agreement
We have a license agreement, as amended, with Saul W. Brusilow, M.D. and Brusilow Enterprises, Inc., or Brusilow, pursuant to which we license patented technology related to RAVICTI from Brusilow. Under such agreement, we are obligated to pay low-single digit royalties to Brusilow on net sales of RAVICTI that are, or were, covered by a valid claim of a licensed patent. The license agreement may be terminated for any uncured breach as well as bankruptcy. We may also terminate the agreement at any time by giving Brusilow prior written notice, in which case all rights granted to us would revert to Brusilow.
PROCYSBI
PROCYSBI drug product is composed of enteric-coated beads of cysteamine bitartrate encapsulated in gelatin capsules or packaged directly into packets. PROCYSBI drug product and API, cysteamine bitartrate, are manufactured and packaged on a contract basis by third parties.
We have an API supply agreement, as amended, with Cambrex Profarmaco Milano, or Cambrex, related to PROCYSBI API. The Cambrex supply agreement has a term that runs until November 30, 2024, and which renews for successive two-year terms if not terminated at least one year in advance. We have a manufacturing services agreement with Patheon for the manufacture and supply of PROCYSBI capsules and granules. The agreement has a term that runs until December 31, 2025, and which automatically renews for successive two-year terms if not terminated at least eighteen months in advance. In addition, we have separate agreement with another third-party contract manufacturer for the packaging of PROCYSBI granules.
UCSD License Agreement
In May 2017, we entered into an amended and restated license agreement with The Regents of the University of California, San Diego, or UCSD, which was amended in September 2018. We must pay UCSD a royalty in the mid-single digits on net sales of PROCYSBI in countries where PROCYSBI is covered by a patent right, and a royalty in the low-single digits on net sales of PROCYSBI in countries where PROCYSBI is not covered by a patent right.
19
UPLIZNA
UPLIZNA is produced by culture of a genetically engineered mammalian cell line containing the DNA which encodes for inebilizumab-cdon, a humanized IgG1 monoclonal antibody. Cell culture broth is harvested and purified through filtration processes and chromatography processes prior to being formulated, frozen, thawed, and shipped to the site of drug product manufacture. In support of its manufacturing process, we store multiple vials of inebilizumab-cdon master cell bank and working cell bank at multiple locations in order to ensure adequate backup should any cell bank be lost in a catastrophic event.
We have a commercial supply agreement with AstraZeneca Pharmaceuticals LP for the manufacture and supply of UPLIZNA drug substance and drug product. The initial period of this agreement is 10 years from April 2019 and will automatically renew for separate but successive three-year terms unless 24 months advance written notice is given by either party that it does not intend to renew. We must provide AstraZeneca Pharmaceuticals LP with rolling forecasts for drug substance and drug product, with a portion of the forecasts being a firm and binding order.
ACTIMMUNE
ACTIMMUNE is a recombinant protein that is produced by fermentation of a genetically engineered Escherichia coli bacterium containing the DNA which encodes for the human protein. Purification of the active drug substance is achieved by conventional column chromatography. The resulting active drug substance is then formulated as a highly purified sterile solution and filled in a single-use vial for subcutaneous injection, which is the ACTIMMUNE finished drug product. In support of its manufacturing process, we and Boehringer Ingelheim RCV GmbH & Co KG, or Boehringer Ingelheim, store multiple vials of the Escherichia coli bacterium master cell bank and working cell bank in order to ensure adequate backup should any cell bank be lost in a catastrophic event.
OTHER MEDICINES
PENNSAID 2% is manufactured on a contract basis by a third party. We purchase API for RAYOS from a contract manufacturer. In addition, we have contracted with two separate third-party manufacturers for the production of RAYOS tablets and for the packaging and assembling of RAYOS. BUPHENYL API is manufactured on a contract basis by a third party and final manufacturing, testing and packaging of the medicine is provided by another third party. The two APIs for DUEXIS are manufactured on a contract basis by two separate third parties. The final packaged form of DUEXIS is provided on a contract basis from an additional third party. The two APIs for VIMOVO are manufactured on a contract basis by two separate third parties. The final packaged form of VIMOVO is provided on a contract basis from an additional third party. QUINSAIR drug product, its API, levofloxacin hemihydrate, and the Zirela nebulizer device are all manufactured on a contract basis by three separate third parties.
20
Intellectual Property
Our objective is to aggressively patent the technology, inventions and improvements that we consider important to the development of our business. We have a portfolio of patents and applications based on our R&D, clinical and pharmacokinetic/pharmacodynamic modeling discoveries, and our novel formulations. We intend to continue filing patent applications seeking intellectual property protection as we generate discoveries from R&D, anticipated formulation refinements, new methods of manufacturing and clinical trial results. The intellectual property with respect to certain of our medicines is set forth below. At present, PENNSAID 2%, DUEXIS and VIMOVO face generic competition in the United States and we are expecting a generic version of RAYOS to enter the market in 2023.
We will only be able to protect our technologies and medicines from unauthorized use by third parties to the extent that valid and enforceable patents or trade secrets cover them. As such, our commercial success will depend in part on receiving and maintaining patent protection and trade secret protection of our technologies and medicines as well as successfully defending these patents against third-party challenges.
The patent positions of life sciences companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. The patent situation outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
TEPEZZA
We are either a licensee or owner of U.S. and foreign patents and applications covering TEPEZZA. If not otherwise invalidated, those patents expire between July 2024 and April 2039. We continue to prosecute and pursue patent protection to obtain additional patent coverage on TEPEZZA and its uses. Additionally, we have a biologic exclusivity in the United States covering TEPEZZA that will expire in 2032.
KRYSTEXXA
We have licenses to U.S. and foreign patents and applications covering KRYSTEXXA. If not otherwise invalidated, those patents expire between July 2023 and June 2030. We continue to prosecute and pursue patent protection to obtain additional patent coverage on KRYSTEXXA and its uses.
21
RAVICTI
We have ownership of or licenses to U.S. and foreign patents and patent applications covering RAVICTI. If not otherwise invalidated, those patents expire between 2030 and 2036. We license our rights to patents and patent applications outside of North America to Immedica.
In the United States, RAVICTI received two separate orphan drug exclusivities for two patient populations. The first of those orphan drug exclusivities expired on February 1, 2020, and the second will expire on April 28, 2024. Under our settlement and license agreement with Par Pharmaceutical, Inc., Par Pharmaceutical, Inc. may enter the market on July 1, 2025, or earlier in certain circumstances. We also have settlement and license agreements with Lupin Limited and Lupin Pharmaceuticals, Inc., or collectively Lupin; and Annora Pharma Private Limited and Hetero USA, Inc., or collectively Annora, pursuant to which Lupin and Annora may enter the market on July 1, 2026, or earlier under certain circumstances.
PROCYSBI
We have U.S. and foreign patents and patent applications covering PROCYSBI, as well as licenses from the University of California, San Diego to U.S. and foreign patents and patent applications covering PROCYSBI. If not otherwise invalidated, those patents expire between 2027 and 2036.
PROCYSBI received marketing authorization in September 2013 from the European Commission for marketing in the EU. PROCYSBI received ten years of market exclusivity, through 2023, as an orphan drug in the EU, European Economic Area, or EEA, and the United Kingdom, or UK.
In the United States, PROCYSBI received seven years of market exclusivity, until December 22, 2024, for patients one year of age to less than two years of age as an orphan drug. During December 2017, the FDA awarded pediatric exclusivity to PROCYSBI in the United States, which adds an additional six-month exclusivity period to the end of certain orphan exclusivity periods and patent terms covering PROCYSBI. Under our settlement and license agreement with Lupin, Lupin may enter the market on March 31, 2030, or earlier in certain circumstances.
UPLIZNA
We have U.S. and foreign patents and applications and licenses to U.S. and foreign patents and applications covering UPLIZNA. If not otherwise invalidated, those patents expire between 2026 and 2030. We continue to prosecute and pursue patent protection to obtain additional patent coverage on UPLIZNA and its uses. Additionally, we have biologic exclusivity in the United States covering UPLIZNA that will expire in 2032 and a patent term extension petition pending that if granted, will result in a later patent expiration in 2034.
QUINSAIR
We have U.S. and foreign patents and patent applications covering QUINSAIR, as well as licenses from PARI Pharma GmbH and Tripex Pharmaceuticals, LLC to U.S. and foreign patents and patent applications covering QUINSAIR. If not otherwise invalidated, those patents expire between 2026 and 2032.
QUINSAIR received ten years of market exclusivity in the EU, beginning with its March 2015 marketing authorization and expiring in March 2025.
Refer to Note 17, Legal Proceedings, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details on our legal proceedings relating to intellectual property matters.
22
Third-Party Coverage and Reimbursement
In both U.S. and foreign markets, our ability to commercialize our medicines successfully depends in significant part on the availability of coverage and adequate reimbursement to healthcare providers from third-party payers, including, in the United States, government payers such as the Medicare and Medicaid programs, managed care organizations and private health insurers. Third-party payers are increasingly challenging the prices charged for medicines and examining their cost effectiveness, in addition to their safety and efficacy. This is especially true in markets where over-the-counter and generic options exist. Even if coverage is made available by a third-party payer, the reimbursement rates paid for covered medicines might not be adequate. For example, third-party payers may use tiered coverage and may adversely affect demand for our medicines by not covering our medicines or by placing them in a more expensive formulary tier relative to competitive medicines (where patients have to pay relatively more out of pocket than for medicines in a lower tier). In addition, we are continuing to see a shift away from co-pay accumulators and an increase in co-pay maximizers putting higher out of pocket costs on the patient. We cannot be certain that our medicines will be covered by third-party payers or that such coverage, where available, will be adequate, or that our medicines will successfully be placed on the list of drugs covered by particular health plan formularies. Many states in the United States have also created preferred drug lists for use in their Medicaid programs and include drugs on those lists only when the manufacturers agree to pay a supplemental rebate. The industry competition to be included on such formularies and preferred drug lists often leads to downward pricing pressures on pharmaceutical companies. Also, third-party payers may refuse to include a particular branded drug on their formularies or otherwise restrict patient assistance to a branded drug when a less costly generic equivalent or other therapeutic alternative is available. In addition, because each third-party payer individually approves coverage and reimbursement levels, obtaining coverage and adequate reimbursement is a time-consuming and costly process. We may be required to provide scientific and clinical support for the use of any medicine to each third-party payer separately with no assurance that approval would be obtained, and we may need to conduct pharmacoeconomic studies to demonstrate the cost effectiveness of our medicines for formulary coverage and reimbursement. Even with studies, our medicines may be considered less safe, less effective or less cost-effective than competitive medicines, and third-party payers may not provide coverage and adequate reimbursement for our medicines or our medicine candidates. These pricing and reimbursement pressures may create negative perceptions to any medicine price increases, or limit the amount we may be able to increase our medicine prices, which may adversely affect our medicine sales and results of operations. Where coverage and reimbursement are not adequate, physicians may limit how much or under what circumstances they will prescribe or administer such medicines, and patients may decline to purchase them. This, in turn, could affect our ability to successfully commercialize our medicines and impact our profitability, results of operations, financial condition, and future success.
The U.S. market has seen a trend in which retail pharmacies have become increasingly involved in determining which prescriptions will be filled with the requested medicine or a substitute medicine, based on a number of factors, including potentially perceived medicine costs and benefits, as well as payer medicine substitution policies. Many states have in place requirements for prescribers to indicate “dispense as written” on their prescriptions if they do not want pharmacies to make medicine substitutions; these requirements are varied and not consistent across states. We may need to increasingly spend time and resources to ensure the prescriptions written for our medicines are filled as written, where appropriate.
Coverage policies, third-party reimbursement rates and medicine pricing regulation have been subject to significant change, and may change further at any time, particularly given recent political focus on the pharmaceutical industry. Even if favorable coverage and adequate reimbursement status is attained for one or more medicines, less favorable coverage policies and reimbursement rates may be implemented in the future.
23
Government Regulation
The FDA and comparable regulatory authorities in state and local jurisdictions and in foreign countries impose extensive requirements upon the non-clinical and clinical development, pre-market approval, manufacture, labeling, marketing, promotion, pricing, import, export, storage and distribution of medicines. These agencies and other regulatory authorities regulate R&D activities and the testing, approval, manufacture, quality control, safety, effectiveness, labeling, packaging, storage, recordkeeping, distribution, advertising and promotion of drugs and biologics. Failure to comply with applicable FDA or comparable foreign regulatory authority requirements may result in warning letters, fines, civil or criminal penalties, additional reporting obligations and/or authority oversight, suspension or delays in clinical development, recall or seizure of medicines, partial or total suspension of production, suspension, variation or withdrawal of marketing approval or withdrawal of a medicine from the market.
In the United States, the FDA regulates drug products under the Federal Food, Drug, and Cosmetic Act and its implementing regulations and biologics additionally under the Public Health Service Act. The process required by the FDA before medicine candidates may be marketed in the United States generally involves the following:
The results of pre-clinical tests (which include laboratory evaluation as well as GLP studies to evaluate toxicity in animals) for a particular medicine candidate, together with related manufacturing information and analytical data, are submitted as part of an IND to the FDA. The IND automatically becomes effective thirty days after receipt by the FDA, unless the FDA, within the thirty-day time period, raises concerns or questions about the conduct of the proposed clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. IND submissions may not result in FDA authorization to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during medicine development. Further, an independent institutional review board, or IRB, for each medical center proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive good clinical practice regulations and regulations for informed consent and privacy of individually identifiable information.
In the EU, approval must be obtained from the competent authorities of EU Member States before clinical trials are commenced. In addition, medicinal products may only be marketed if a marketing authorization has been obtained from the competent regulatory authorities. The related process is described below.
24
Clinical Trials in the United States. For purposes of NDA or BLA submission and approval, clinical trials are typically conducted in the following sequential phases, which may overlap:
The results of drug development, pre-clinical studies and clinical trials are submitted to the FDA as part of an NDA or BLA, as appropriate. Applications also must contain extensive chemistry, manufacturing and control information. Applications must be accompanied by a significant user fee. Once the submission has been accepted for filing, the FDA’s goal is to review applications within twelve months of submission or, if the application relates to an unmet medical need in a serious or life-threatening indication, eight months from submission. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA will typically conduct a pre-approval inspection of the manufacturer to ensure that the medicine can be reliably produced in compliance with cGMPs and will typically inspect certain clinical trial sites for compliance with good clinical practice, or GCP. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it typically follows such recommendations. The FDA may deny approval of an application by issuing a Complete Response Letter if the applicable regulatory criteria are not satisfied. A Complete Response Letter may require additional clinical data and/or trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, pre-clinical studies or manufacturing. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we or our collaborators interpret data. Approval may occur with boxed warnings on medicine labeling or Risk Evaluation and Mitigation Strategies, or REMS, which limit the labeling, distribution or promotion of a medicine. Once issued, the FDA may withdraw medicine approval if ongoing regulatory requirements are not met or if safety problems occur after the medicine reaches the market. In addition, the FDA may require testing, including Phase 4 clinical trials, and surveillance programs to monitor the safety effects of approved medicines which have been commercialized and the FDA has the power to prevent or limit further marketing of a medicine based on the results of these post-marketing programs or other information.
Clinical Trials in the EU. Similar to the United States, the various phases of preclinical and clinical research in the EU are subject to significant regulatory controls. Certain preclinical (also termed “non-clinical”) data is required in order to enable clinical trials to be used for a marketing authorization application. The requisite amount of preclinical data enables the design of a clinical trial, from Phase 1 (first-in-human clinical trials) through to Phases 2 and 3, which are quality, safety and efficacy studies. During all phases of clinical development, national competent authorities of EU Member States and other comparable regulatory authorities require extensive monitoring and auditing of all clinical activities, clinical data and clinical trial investigators.
In the EU, clinical trials are governed by the Clinical Trials Regulation (EU) No 536/2014, or CTR, which entered into application on January 31, 2022. Clinical trials of medicinal products in the EU must be conducted in accordance with EU and national regulations governing clinical trials, including the Good Clinical Practice Directive 2005/28. It is recommended that studies also be conducted in accordance with all applicable EMA, European Commission and national guidelines. Specific GCP guidelines from the European Commission apply to clinical trials of advanced therapy medicinal products, or ATMPs. The sponsor must take out a clinical trial insurance policy, and in most EU countries, the sponsor is liable to provide “no fault” compensation to any study subject injured in the clinical trial.
25
Prior to commencing a clinical trial, the sponsor must obtain a clinical trial authorization from competent authorities of EU Member States in which the sponsor intends on carrying out clinical trials, and a positive opinion from an independent Ethics Committee. The CTR, which is directly applicable in all EU Member States, introduces an application procedure through a single-entry point, the “EU portal”, the Clinical Trials Information System, or CTIS. Since January 31, 2023, the use of CTIS has become mandatory for all clinical trial sponsors submitting initial applications for the approval of their clinical trials in the EU. The CTR also establishes a single set of documents to be prepared and submitted for the application including, among other things, a copy of the trial protocol and an investigational medicinal product dossier containing information about the manufacture and quality of the medicinal product under investigation; as well as new reporting procedures for clinical trial sponsors.
A harmonized procedure for the assessment of applications for clinical trials has been introduced and is divided into two parts. Assessment of Part I is led by the competent authorities of a reference Member State selected by the trial sponsor and relates to clinical trial aspects that are considered to be scientifically harmonized across EU Member States. This assessment is then submitted to the competent authorities of all the concerned Member States in which the trial is to be conducted for their review. Part II is assessed separately by the competent authorities and Ethics Committees in each concerned EU Member State. Each concerned Member State will issue a single decision on the authorization of the clinical trial including input from the national competent authority and Ethics Committee. Individual EU Member States, therefore, retain the power to authorize the conduct of clinical trials in their territory.
The CTR establishes a general principle according to which information contained in CTIS shall be made publicly accessible unless confidentiality is justified on grounds of protecting personal data, or commercially confidential information, necessary to protect confidential communications between EU Member States in relation to the preparation of an assessment report, or necessary to ensure effective supervision of the conduct of a clinical trial by EU Member States. This confidentiality exception may be overruled if there is an overriding public interest in disclosure.
The extent to which on-going clinical trials, for which an application for approval was submitted in accordance with the Clinical Trials Directive 2001/20 before January 31, 2023, will be governed by the CTR will depend on the duration of the individual clinical trial. If a clinical trial continues for more than three years after January 31, 2022, the CTR will begin to apply to the clinical trial after expiry of this three-year period. The Regulation will apply to clinical trials from an earlier date if the clinical trial has already transitioned to the CTR framework.
In all cases, clinical trials must be conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki. Medicines used in clinical trials must be manufactured in accordance with the guidelines on cGMP and in a GMP licensed facility, which can be subject to GMP inspections.
During the development of a medicinal product, the EMA and national competent authorities of EU Member States provide the opportunity for dialogue and guidance on the development program. At the EMA level, this is usually done in the form of scientific advice, which is given by the Scientific Advice Working Party of the Committee for Medicinal Products for Human Use. A fee is incurred with each scientific advice procedure. Advice from the EMA is typically provided based on questions concerning, for example, quality (chemistry, manufacturing and controls testing), nonclinical testing and clinical studies, and pharmacovigilance plans and risk-management programs.
EU Review and Approval Process. To obtain a Marketing Authorization, or MA, for a product in the EU, an applicant must submit a Marketing Authorization Application, or MAA, either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in the EU Member States (decentralized procedure, national procedure or mutual recognition procedure). An MA may be granted only to an applicant established in the EU.
The centralized procedure provides for the grant of a single MA by the European Commission that is valid for all EU Member States. Pursuant to Regulation (EC) No 726/2004, the centralized procedure is compulsory for specific products, including for (i) medicinal products derived from biotechnological processes, (ii) products designated as orphan medicinal products, (iii) advanced therapy medicinal products, or ATMPs, and (iv) products with a new active substance indicated for the treatment of HIV/AIDS, cancer, neurodegenerative diseases, diabetes, auto immune and other immune dysfunctions and viral diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, authorization through the centralized procedure is optional on related approval.
26
Under the centralized procedure, the EMA’s Committee for Medicinal Products for Human Use, or CHMP, conducts the initial assessment of a product. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing MA.
Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated assessment may be granted by the CHMP in exceptional cases, when a medicinal product targeting an unmet medical need is expected to be of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation. If the CHMP accepts a request for accelerated assessment, the time limit of 210 days will be reduced to 150 days (excluding clock stops). The CHMP can, however, revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment.
Unlike the centralized authorization procedure, the decentralized MA procedure requires a separate application to, and leads to separate approval by, the competent authorities of each EU Member State in which the product is to be marketed. This application is identical to the application that would be submitted to the EMA for authorization through the centralized procedure. The reference EU Member State prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU Member States who, within 90 days of receipt, must decide whether to approve the assessment report and related materials. If a concerned EU Member State cannot approve the assessment report and related materials due to concerns relating to a potential serious risk to public health, disputed elements may be referred to the Heads of Medicines Agencies’ Coordination Group for Mutual Recognition and Decentralized Procedures – Human, or CMDh, for review. The subsequent decision of the European Commission is binding on all EU Member States.
The mutual recognition procedure allows companies that have a medicinal product already authorized in one EU Member State to apply for this authorization to be recognized by the competent authorities in other EU Member States. Like the decentralized procedure, the mutual recognition procedure is based on the acceptance by the competent authorities of the EU Member States of the MA of a medicinal product by the competent authorities of other EU Member States. The holder of a national MA may submit an application to the competent authority of an EU Member State requesting that this authority recognize the MA delivered by the competent authority of another EU Member State.
An MA has, in principle, an initial validity of five years. The MA may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EU Member State in which the original MA was granted. To support the application, the MA holder must provide the EMA or the competent authority with a consolidated version of the eCTD (Common Technical Document) providing up-to-date data concerning the quality, safety and efficacy of the product, including all variations introduced since the MA was granted, at least nine months before the MA ceases to be valid. The European Commission or the competent authorities of the EU Member States may decide on justified grounds relating to pharmacovigilance, to proceed with one further five year renewal period for the MA. Once subsequently definitively renewed, the MA is valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU market (for a centralized MA) or on the market of the authorizing EU Member State within three years after authorization ceases to be valid (the so-called sunset clause).
Innovative products that target an unmet medical need and are expected to be of major public health interest may be eligible for a number of expedited development and review programs, such as the Priority Medicines, or PRIME, scheme, which provides incentives similar to the breakthrough therapy designation in the United States. PRIME is a voluntary scheme aimed at enhancing the EMA’s support for the development of medicinal products that target unmet medical needs. Eligible products must target conditions for which there is an unmet medical need (there is no satisfactory method of diagnosis, prevention or treatment in the EU or, if there is, the new medicinal product will bring a major therapeutic advantage) and they must demonstrate the potential to address the unmet medical need by introducing new methods of therapy or improving existing ones. Benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and potentially accelerated MAA assessment once a dossier has been submitted.
27
In the EU, a “conditional” MA may be granted in cases where all the required safety and efficacy data are not yet available. The European Commission may grant a conditional MA for a medicinal product if it is demonstrated that all of the following criteria are met: (i) the benefit-risk balance of the medicinal product is positive; (ii) it is likely that the applicant will be able to provide comprehensive data post-authorization; (iii) the medicinal product fulfills an unmet medical need; and (iv) the benefit of the immediate availability to patients of the medicinal product is greater than the risk inherent in the fact that additional data are still required. The conditional MA is subject to conditions to be fulfilled for generating the missing data or ensuring increased safety measures. It is valid for one year and must be renewed annually until all related conditions have been fulfilled. Once any pending studies are provided, the conditional MA can be converted into a traditional MA. However, if the conditions are not fulfilled within the timeframe set by the EMA and approved by the European Commission, the MA will cease to be renewed.
An MA may also be granted “under exceptional circumstances” where the applicant can show that it is unable to provide comprehensive data on efficacy and safety under normal conditions of use even after the product has been authorized and subject to specific procedures being introduced. These circumstances may arise in particular when the intended indications are very rare and, in the state of scientific knowledge at that time, it is not possible to provide comprehensive information, or when generating data may be contrary to generally accepted ethical principles. Like a conditional MA, an MA granted in exceptional circumstances is reserved to medicinal products intended to be authorized for treatment of rare diseases or unmet medical needs for which the applicant does not hold a complete data set that is required for the grant of a standard MA. However, unlike the conditional MA, an applicant for authorization in exceptional circumstances is not subsequently required to provide the missing data. Although the MA “under exceptional circumstances” is granted definitively, the risk-benefit balance of the medicinal product is reviewed annually, and the MA will be withdrawn if the risk-benefit ratio is no longer favorable.
EU Data and Marketing Exclusivity. The EU also provides opportunities for market exclusivity. Upon receiving an MA in the EU, innovative medicinal products generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents generic or biosimilar applicants from referencing the innovator's pre-clinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization during a period of eight years from the date on which the reference product was first authorized in the EU. During the additional two-year period of market exclusivity, a generic or biosimilar marketing authorization can be submitted, and the innovator's data may be referenced, but no generic or biosimilar product can be marketed until the expiration of the market exclusivity period. The overall ten-year period can be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to authorization, is held to bring a significant clinical benefit in comparison with existing therapies.
In the EU, there is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product. For such products, the results of appropriate preclinical or clinical trials must be provided in support of an application for marketing authorization. Guidelines from the EMA detail the type of quantity of supplementary data to be provided for different types of biological products.
EU Pediatric Development. In the EU, Regulation (EC) No 1901/2006 provides that all marketing authorization applications for new medicinal products must include the results of trials conducted in the pediatric population, in compliance with a pediatric investigation plan, or PIP, agreed with the EMA’s Pediatric Committee, or PDCO. The PIP sets out the timing and measures proposed to generate data to support a pediatric indication of the medicinal product for which marketing authorization is being sought. The PDCO may grant a deferral of the obligation to implement some or all of the measures provided in the PIP until there are sufficient data to demonstrate the efficacy and safety of the product in adults. Furthermore, the obligation to provide pediatric clinical trial data can be waived by the PDCO when these data are not needed or appropriate because the product is likely to be ineffective or unsafe in children, the disease or condition for which the product is intended occurs only in adult populations, or when the product does not represent a significant therapeutic benefit over existing treatments for pediatric patients. Once the marketing authorization is obtained in all EU Member States and study results are included in the product information, even when negative, the product is eligible for a six month extension to the Supplementary Protection Certificate, or SPC, if any is in effect at the time of authorization or, in the case of orphan medicinal products, a two-year extension of orphan market exclusivity.
For other countries outside of the EU, such as certain countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product approval, pricing and reimbursement vary from country to country. In all cases, the clinical trials are to be conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
28
Orphan Medicines. Under the Orphan Drug Act, the FDA may designate a medicine as an “orphan drug” if it is intended to treat a rare disease or condition, meaning that it affects fewer than 200,000 individuals in the United States, or more in cases in which there is no reasonable expectation that the cost of developing and making a medicine available in the United States for treatment of the disease or condition will be recovered from sales of the medicine. A company must request orphan drug designation before submitting an NDA for the drug and rare disease or condition. If the request is granted, the FDA will disclose the identity of the therapeutic agent and its potential use. Orphan drug designation does not shorten the Prescription Drug User Fee Act, or PDUFA, goal dates for the regulatory review and approval process, although it does convey certain advantages such as tax benefits and exemption from the PDUFA application fee.
If a medicine with orphan designation receives the first FDA approval for the disease or condition for which it has such designation or for a select indication or use within the rare disease or condition for which it was designated, the medicine generally will receive orphan drug exclusivity. Orphan drug exclusivity means that the FDA may not approve another sponsor’s marketing application for the same drug for the same indication for seven years, except in certain limited circumstances. Orphan exclusivity does not block the approval of a different drug for the same rare disease or condition, nor does it block the approval of the same drug for different indications. If a drug designated as an orphan drug ultimately receives marketing approval for an indication broader than what was designated in its orphan drug application, it may not be entitled to exclusivity. Orphan exclusivity will not bar approval of another medicine under certain circumstances, including if a subsequent medicine with the same drug for the same indication is shown to be clinically superior to the approved medicine on the basis of greater efficacy or safety, or providing a major contribution to patient care, or if the company with orphan drug exclusivity is not able to meet market demand.
In the EU, Regulation (EC) No 141/2000, as implemented by Regulation (EC) No. 847/2000, provides that a medicine can be designated as an orphan medicinal product by the European Commission if its sponsor can establish that: (i) the product is intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions; (ii) either (a) such conditions affect not more than 5 in 10,000 persons in the EU when the application is made, or (b) the product without the benefits derived from orphan status, would not generate sufficient return in the EU to justify the necessary investment in developing the medicinal product; and (iii) there exists no satisfactory authorized method of diagnosis, prevention, or treatment of the condition that has been authorized in the EU, or even if such method exists, the product will be of significant benefit to those affected by that condition. Regulation (EC) No 847/2000 sets out further provisions for implementation of the criteria for designation of a medicinal product as an orphan medicinal product. An application for the designation of a medicinal product as an orphan medicinal product must be submitted at any stage of development of the medicinal product but before filing of an MAA. An MA for an orphan medicinal product may only include indications designated as orphan. For non-orphan indications treated with the same active pharmaceutical ingredient, a separate marketing authorization has to be sought.
Orphan medicinal product designation entitles an applicant to incentives such fee reductions or fee waivers, protocol assistance, and access to the centralized marketing authorization procedure. Upon grant of a marketing authorization, orphan medicinal products are entitled to ten years of market exclusivity for the approved therapeutic indication in all EU Member States, which means that the EMA cannot accept another marketing authorization application, or grant a marketing authorization, or accept an application to extend for a similar product for the same indication for a period of ten years. The period of market exclusivity is extendable to twelve years for medicinal products that have complied with an agreed pediatric investigation plan pursuant to Regulation 1901/2006. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications. However, marketing authorization may be granted to a similar medicinal product with the same orphan indication during the regulatory exclusivity period with the consent of the marketing authorization holder for the original orphan medicinal product or if the manufacturer of the original orphan medicinal product is unable to supply sufficient quantities. Marketing authorization may also be granted to a similar medicinal product with the same orphan indication if this medicine is safer, more effective or otherwise clinically superior to the original orphan medicinal product. The period of market exclusivity may, in addition, be reduced to six years if, at the end of the fifth year, it can be demonstrated on the basis of available evidence that the criteria for its designation as an orphan medicine are no longer satisfied, for example if the original orphan medicinal product has become sufficiently profitable not to justify maintenance of market exclusivity. A company may voluntarily remove a product from the register of orphan products. Orphan medicinal product designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
29
Following Brexit there is no pre-market orphan designation for products in the UK. Orphan designation in the UK is based on the prevalence of the condition in Great Britain. The UK Medicines and Healthcare Products Regulatory Agency, or MHRA, is responsible for reviewing applications for orphan designation at the time of the marketing authorization application (for further details on the impact the UK leaving the EU has and will have, see the section entitled ‘The Impact of Brexit’ below).
Other Regulatory Requirements. Medicines manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including recordkeeping, annual medicine quality review, payment of program fees and reporting requirements. Adverse event experience with the medicine must be reported to the FDA in a timely fashion and pharmacovigilance programs to proactively look for these adverse events are mandated by the FDA. Our medicines may be subject to REMS requirements that affect labeling, distribution or post market reporting. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Following such inspections, the FDA may issue notices on Form 483 and untitled letters or warning letters that could cause us or our third-party manufacturers to modify certain activities. A Form 483 notice, if issued at the conclusion of an FDA inspection, can list conditions the FDA investigators believe may have violated cGMP or other FDA regulations or guidelines. In addition to Form 483 notices and untitled letters, failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of medicine, injunctive action, additional reporting requirements and/or oversight by the agency, import alert or possible civil penalties. The FDA may also require us to recall a drug from distribution or withdraw approval for that medicine.
The FDA closely regulates the post-approval marketing and promotion of pharmaceuticals, including standards and regulations for direct-to-consumer advertising, dissemination of off-label information, industry-sponsored scientific and educational activities and promotional activities involving the Internet, including certain social media activities. Medicines may be marketed only for the approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to the medicine, including changes in indications, labeling, or manufacturing processes or facilities, we may be required to submit and obtain FDA approval of a new or supplemental application, which may require us to develop additional data or conduct additional pre-clinical studies and clinical trials. Failure to comply with these requirements can result in adverse publicity, untitled letters, corrective advertising and potential administrative, civil and criminal penalties, as well as damages, fines, withdrawal of regulatory approval, the curtailment or restructuring of our operations, the exclusion from participation in federal and state healthcare programs, additional reporting requirements and/or oversight by the agency, and imprisonment, any of which could adversely affect our ability to sell our medicines or operate our business and also adversely affect our financial results.
30
Physicians may, in their independent medical judgment, prescribe legally available pharmaceuticals for uses that are not described in the medicine’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across certain medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use. Additionally, a significant number of pharmaceutical companies have been the target of inquiries and investigations by various U.S. federal and state regulatory, investigative, prosecutorial and administrative entities in connection with the promotion of medicines for off-label uses and other sales practices. These investigations have alleged violations of various U.S. federal and state laws and regulations, including claims asserting antitrust violations, violations of the Food, Drug and Cosmetic Act, false claims laws, the Prescription Drug Marketing Act, or PDMA, anti-kickback laws, and other alleged violations in connection with the promotion of medicines for unapproved uses, pricing and Medicare and/or Medicaid reimbursement. If our promotional activities, including any promotional activities that a contracted sales force may perform on our behalf, fail to comply with these regulations or guidelines, we may be subject to warnings from, or enforcement action by, these authorities. In addition, our failure to follow FDA rules and guidelines relating to promotion and advertising may cause the FDA to issue warning letters or untitled letters, suspend or withdraw an approved medicine from the market, require corrective advertising or a recall or result in the imposition of fines or civil fines, additional reporting requirements and/or oversight or could result in disgorgement of money, operating restrictions, injunctions or criminal prosecution by the FDA or other U.S. regulatory agencies, any of which could harm our business. In addition, the distribution of prescription medicines is subject to the PDMA, which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription medicine samples and impose requirements to ensure accountability in distribution, including a drug pedigree which tracks the distribution of prescription drugs. Further, under the Drug Quality and Security Act, drug manufacturers are subject to a number of requirements, including, medicine identification, tracing and verification, among others, that are designed to detect and remove counterfeit, stolen, contaminated or otherwise potentially harmful drugs from the U.S. drug supply chain.
Where an MA is granted in relation to a medicinal product in the EU, the holder of the MA is required to comply with a range of regulatory requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products. Similar to the United States, both MA holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA, the European Commission and/or the competent regulatory authorities of the individual EU Member States. The holder of an MA must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of periodic safety update reports, or PSURs.
All new MAAs must include a risk management plan, or RMP, describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the MA. Such risk-minimization measures or post-authorization obligations may include additional safety monitoring, more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorization safety studies.
In the EU, the advertising and promotion of medicinal products are subject to both EU and EU Member States’ laws governing promotion of medicinal products, interactions with physicians and other healthcare professionals, misleading and comparative advertising and unfair commercial practices. Although general requirements for advertising and promotion of medicinal products are established under EU legislation, the details are governed by regulations in individual EU Member States and can differ from one country to another. For example, applicable laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics, or SmPC, as approved by the competent authorities in connection with an MA. The SmPC is the document that provides information to physicians concerning the safe and effective use of the product. Promotional activity that does not comply with the SmPC is considered off-label and is prohibited in the EU. Direct-to-consumer advertising of prescription medicinal products is also prohibited in the EU. Enforcement of advertising and promotional requirements relating to medicinal products is managed by the competent authorities of EU Member States.
31
The Impact of Brexit. The withdrawal of the UK from the EU (commonly referred to as “Brexit”) took effect on January 31, 2020. Pursuant to the formal withdrawal arrangements agreed between the UK and the EU, the UK was subject to a transition period that ended December 31, 2020, or the Transition Period, during which EU rules continued to apply. A Trade and Cooperation Agreement, or the TCA, that outlines the trading relationship between the UK and the EU was agreed in December 2020, entered into force provisionally on January 1, 2021, and has been permanently applicable since May 1, 2021. The TCA primarily focuses on ensuring free trade between the EU and the UK in relation to goods, including medicinal products. Although the body of the TCA includes general terms which apply to medicinal products, greater detail on sector-specific issues is provided in an Annex to the TCA. Since a significant portion of the regulatory framework in the UK applicable to our business and our products is derived from EU directives and regulations, Brexit has materially impacted the regulatory regime in the UK with respect to the development, manufacture, importation, approval and commercialization of our products. The regulatory changes that are a result of Brexit may also materially impact upon the development, manufacture, importation, approval and commercialization of our products in the EU, should any development or manufacture of these products take place in the UK. Among the changes that will now occur are that Great Britain (England, Scotland and Wales) will be treated as a third country. Northern Ireland will, with regard to EU regulations, continue to follow the EU regulatory rules. As part of the TCA, the EU and the UK will recognize GMP inspections carried out by the other party and the acceptance of official GMP documents issued by the other party. The TCA also encourages, although it does not oblige, the parties to consult one another on proposals to introduce significant changes to technical regulations or inspection procedures. Among the areas of absence of mutual recognition are batch testing and batch release. The UK has unilaterally agreed to accept EU batch testing and batch release. However, the EU continues to apply EU laws that require batch testing and batch release to take place in the EU territory. This means that medicinal products that are tested and released in the UK must be retested and re-released when entering the EU market for commercial use.
With respect to marketing authorizations, Great Britain has a separate regulatory submission process, approval process and a national marketing authorization. Northern Ireland will, however, continue to be covered by the marketing authorizations granted by the European Commission. Since January 1, 2021, an applicant for a centralized procedure marketing authorization can no longer be established in the UK. Since this date, companies established in the UK cannot use the centralized procedure and instead must follow one of the UK national authorization procedures to obtain an MA to market products in the UK. Until December 31, 2023, MHRA may rely on a decision taken by the European Commission on the approval of a new centralized procedure marketing authorization when determining an application for a Great Britain marketing authorization. From January 1, 2024, a new international recognition process, which will have regard to decisions made by the EMA and certain other regulatory bodies, is anticipated to be in place. The MHRA has also established its own decentralized or mutual recognition procedures which enable marketing authorizations approved in EU Member States through decentralized and mutual recognition procedures to be recognized in the UK or Great Britain. Since Brexit, the MHRA has been updating various aspects of the regulatory regime for medicinal products in the UK. These include: introducing the Innovative Licensing and Access Procedure to accelerate the time to market and facilitate patient access for innovative medicinal products; updates to the UK national approval procedure, introducing a 150-day objective for assessing applications for marketing authorizations in the UK, Great Britain and Northern Ireland and a rolling review process for marketing authorization applications (rather than a consolidated full dossier submission). It is currently unclear to what extent the UK will seek to align its regulations with the EU in the future. The UK regulatory framework in relation to clinical trials and marketing authorization is derived from existing EU legislation (as implemented into UK law, through secondary legislation). However, the Retained EU Law (Revocation and Reform) Bill published in late 2022 which is intended to remove all EU-derived legislation from the UK statute book by the end of 2023, may result in a divergence of approach between the EU and the UK.
The data exclusivity periods in the UK are currently in line with those in the EU, but the TCA provides that the periods for both data and market exclusivity are to be determined by domestic law, and so there could be divergence in the future.
Unlike in the EU, orphan designation in Great Britain following Brexit is no longer available prior to grant of marketing authorization. Applications for orphan designation are made at the same time as an application for a marketing authorization. The criteria to be granted an orphan medicinal product designation or essentially identical to those in the EU but based on the prevalence of the condition in Great Britain rather than the EU. It is therefore possible that conditions that were or would have been designated as orphan conditions in Great Britain prior to the end of the Transition Period are or would no longer be and that conditions that were not or would not have been designated as orphan conditions in the EU will be designated as such in Great Britain.
32
Healthcare Fraud and Abuse Laws. As a pharmaceutical company, certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. We may be subject to various federal and state laws targeting fraud and abuse in the healthcare industry. For example, in the United States, there are federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services or reward past purchases or recommendations. Violations of these laws can lead to significant administrative, civil and criminal penalties, including fines, imprisonment, additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement, and exclusion from participation in federal healthcare programs. These laws are applicable to manufacturers of products regulated by the FDA, such as us, and pharmacies, hospitals, physicians and other potential purchasers of such products.
The federal Anti-Kickback Statute prohibits persons and entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” is defined as any remuneration, direct or indirect, overt or covert, in cash or in kind, and has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payment, ownership interests and providing anything at less than its fair market value. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute may have been violated, and enforcement will depend on the relevant facts and circumstances. The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA, among other things, amended the intent requirement of the federal Anti-Kickback Statute to state that a person or entity need not have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the ACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act (discussed below) or the civil monetary penalties statute, which imposes penalties against any person who is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent, or to have offered improper inducements to federal health care program beneficiaries to select a particular provider or supplier. The federal Anti-Kickback Statute is broad, and despite a series of narrow safe harbors, prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs, and do not contain identical safe harbors. In addition, where such activities involve foreign government officials, they may also potentially be subject to the Foreign Corrupt Practices Act. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities, including our activities with physician customers, pharmacies, and patients, as well as our activities pursuant to partnerships with other companies and pursuant to contracts with contract research organizations, could be subject to challenge under one or more of such laws.
The federal False Claims Act prohibits any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. In addition, the ACA specified that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act. The False Claims Act has been the basis for numerous enforcement actions and settlements by pharmaceutical and other healthcare companies in connection with various alleged financial relationships with customers. In addition, a number of pharmaceutical manufacturers have reached substantial financial settlements in connection with allegedly causing false claims to be submitted because of the companies’ marketing of products for unapproved, and thus non-reimbursable, uses. Certain marketing practices, including off-label promotion, may also violate false claims laws, as well as physician self-referral laws, such as the Stark Law, which prohibit a physician from making a referral to certain designated health services with which the physician or the physician’s family member has a financial interest and prohibit submission of a claim for reimbursement pursuant to the prohibited referral. The “qui tam” provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In addition, various states have enacted similar fraud and abuse statutes or regulations, including, without limitation, false claims laws analogous to the False Claims Act, and laws analogous to the federal Anti-Kickback Statute, that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer, and there are also federal criminal false claims laws.
33
Separately, there are a number of other fraud and abuse laws that pharmaceutical manufacturers must be mindful of, particularly after a medicine candidate has been approved for marketing in the United States. For example, a federal criminal law enacted as part of, the Health Insurance Portability and Accountability Act of 1996, or HIPAA, prohibits, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payers. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. There are also federal civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent, as well as federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers.
We are also subject to analogous foreign laws of each of the above federal healthcare laws and foreign jurisdictions may require the implementation of compliance programs, disclosure of any gifts, compensation, or other remuneration provided to health professionals.
Privacy and Security Laws. We may be subject to, or our marketing activities may be limited by, HIPAA, as amended by the Health Information Technology and Clinical Health Act (HITECH) and their respective implementing regulations, which established uniform standards for certain “covered entities” (covered healthcare providers, health plans and healthcare clearinghouses) governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of protected health information. Among other things, HIPAA’s privacy and security standards are directly applicable to “business associates” — independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity as well as their covered subcontractors. In addition to possible civil and criminal penalties for violations, state attorneys general are authorized to file civil actions for damages or injunctions in federal courts to enforce HIPAA and seek attorney’s fees and costs associated with pursuing federal civil actions. Accordingly, state attorneys general (along with private plaintiffs) have brought civil actions seeking injunctions and damages resulting from alleged violations of HIPAA’s privacy and security rules. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Outside the United States, an increasing number of laws, regulations, and industry standards govern data privacy and security. For example, the EU’s General Data Protection Regulation, or EU GDPR, and the UK’s GDPR, or UK GDPR, impose strict requirements for processing personal data. Under the EU GDPR, companies may face temporary or definitive bans on data processing and other corrective actions; fines of up to €20 million or up to 4% of the annual global turnover of the infringer, whichever is greater, could be imposed; or private litigation related to processing of personal data brought by classes of data subjects or consumer protection organizations authorized at law to represent their interests.
Additionally, the California Consumer Privacy Act, or CCPA, became effective on January 1, 2020. The CCPA has been dubbed the first “EU GDPR-like” law in the United States since it creates new individual privacy rights for consumers (as that word is broadly defined in the law) and places increased privacy and security obligations on entities handling personal data of consumers or households (including health information). The CCPA requires covered companies to provide new disclosures to California consumers, provide such consumers new ways to opt-out of certain sales of personal information, and allows for a new cause of action for data breaches. Further, California voters approved a new privacy law, the California Privacy Rights Act, or CPRA, in the November 3, 2020 election. Effective starting on January 1, 2023, the CPRA significantly modified the CCPA, including by expanding consumers’ rights with respect to certain sensitive personal information. The CPRA also created a new state agency that is vested with authority to implement and enforce the CCPA and the CPRA. New legislation proposed or enacted in various other states will continue to shape the data privacy environment nationally. For example, Virginia enacted the Virginia Consumer Data Protection Act, Colorado enacted the Colorado Privacy Act, Connecticut enacted the Connecticut Data Privacy Act, and Utah enacted the Utah Consumer Privacy Act, all of which take effect in 2023.
34
“Sunshine” and Marketing Disclosure Laws. There are an increasing number of federal and state “sunshine” laws that require pharmaceutical manufacturers to make reports to states on pricing and marketing information. Several states have enacted legislation requiring pharmaceutical companies to, among other things, establish marketing compliance programs, file periodic reports with the state, and make periodic public disclosures on sales and marketing activities, and prohibiting certain other sales and marketing practices. In addition, a similar federal requirement requires certain manufacturers, including pharmaceutical manufacturers, to track and report to the federal government the following: certain payments and other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other healthcare providers (including, for example, physician assistants and nurse practitioners), and teaching hospitals and ownership or investment interests held by physicians and their immediate family members. Certain states, such as Massachusetts, also make the reported information publicly available. In addition, there are state and local laws that require pharmaceutical representatives to be licensed and comply with codes of conduct, transparency reporting, and other obligations. These laws may adversely affect our sales, marketing, and other activities with respect to our medicines in the United States by imposing administrative and compliance burdens on us. If we fail to track and report as required by these laws or otherwise comply with these laws, we could be subject to the penalty provisions of the pertinent state and federal authorities.
Outside the United States, interactions between pharmaceutical companies and health care professionals are also governed by strict laws, such as national anti-bribery laws of EU Member States, national sunshine rules and regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct. Failure to comply with these requirements could result in administrative penalties, fines or imprisonment, reputational risk and public reprimands.
Government Price Reporting. For those marketed medicines which are covered in the United States by the Medicaid programs, we have various obligations, including government price reporting and rebate requirements, which generally require medicines be offered at substantial rebates/discounts to Medicaid and certain purchasers (including “covered entities” purchasing under the 340B Drug Discount Program). We are also required to discount such medicines to authorized users of the Federal Supply Schedule of the General Services Administration, under which additional laws and requirements apply. These programs require submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations, and the guidance governing such calculations is not always clear. Compliance with such requirements can require significant investment in personnel, systems and resources, but failure to properly calculate our prices, or offer required discounts or rebates could subject us to substantial penalties. One component of the rebate and discount calculations under the Medicaid and 340B programs, respectively, is the “additional rebate”, a complex calculation which is based, in part, on the extent that a branded drug’s price increases over time more than the rate of inflation (based on the Consumer Price Index for All Urban Consumers). This comparison is based on the baseline pricing data for the first full quarter of sales associated with a branded drug’s NDA, and baseline data cannot generally be reset, even on transfer of the NDA to another manufacturer. This “additional rebate” calculation can, in some cases where price increases have been relatively high versus the first quarter of sales of the NDA, result in Medicaid rebates up to 100 percent of a drug’s “average manufacturer price” and 340B prices of one penny.
In the EU, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies (so called health technology assessments) in order to obtain reimbursement or pricing approval. For example, some EU Member States may approve a specific price for a product, or they may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other EU Member States allow companies to fix their own prices for products but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many EU Member States have increased the amount of discounts that pharmaceutical companies are required to offer. These efforts could continue as countries attempt to manage healthcare expenditures. The downward pressure on healthcare costs in general, particularly prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products onto national markets. Political, economic, and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU Member States, and parallel trade (arbitrage between low-priced and high-priced member states), can further reduce prices.
35
Penalties. Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities in the United States or foreign countries could be subject to challenge under one or more of such laws. Moreover, state governmental agencies may propose or enact laws and regulations that extend or contradict federal requirements. If we or our operations are found to be in violation of any of the state, federal, national or supranational laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including significant administrative, civil and criminal penalties, damages, fines, imprisonment, exclusion from participation in U.S. federal or state healthcare programs, or comparable foreign programs, additional reporting requirements and/or oversight and the curtailment or restructuring of our operations. To the extent that any medicine we make is sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals. Any penalties, damages, fines, curtailment or restructuring of our operations could materially adversely affect our ability to operate our business and our financial results. We maintain a comprehensive healthcare corporate compliance program. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks and risks of regulatory non-compliance cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal, state and foreign privacy, security, sunshine, government price reporting, and fraud laws may prove costly.
Impact of Healthcare Reform and Recent Public Scrutiny of Drug Pricing on Coverage, Reimbursement, and Pricing. In the United States and other potentially significant markets for our medicines, federal and state lawmakers and regulatory authorities as well as third-party payers are increasingly attempting to regulate the price of medical products and services, particularly for new and innovative medicines and therapies, which has resulted in delays of coverage decisions, barriers for product access including higher patient copays and in certain cases, leads to lower average net selling prices. Further, there is increased scrutiny of prescription drug pricing practices by federal and state lawmakers and enforcement authorities. In addition, there is an emphasis on managed healthcare in the United States and on country-specific and regional pricing and reimbursement controls in the EU, both of which will put additional pressure on medicine pricing, reimbursement and usage, which may adversely affect our future medicine sales and results of operations. These pressures can arise from rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, pharmaceutical reimbursement policies and pricing in general.
The U.S. and some foreign jurisdictions are considering or have enacted a number of additional legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our medicines profitably. Among policy makers and payers in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs (including a number of proposals pertaining to prescription drugs, specifically), improving quality and/or expanding access.
In the United States, the pharmaceutical industry has already been significantly affected by major legislative initiatives, including, for example, the ACA. The ACA, among other things, imposes a significant annual fee on companies that manufacture or import branded prescription drug products. It also contains substantial provisions intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, and impose additional health policy reforms, any or all of which may affect our business. The current administration has made efforts to bolster enrollment in the ACA. For example, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. Further, the Bipartisan Budget Act of 2018, among other things, amended the ACA, increasing in 2019 the percentage that a drug manufacturer must discount the cost of prescription drugs from 50 percent to 70 percent. Additionally, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, or IRA, into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost through a newly established manufacturer discount program. It is possible that the ACA and the IRA will be subject to judicial or Congressional challenges in the future. It is unclear how any additional healthcare reform measures of the Biden administration will impact the ACA’s many different provisions affecting the health system, the IRA, the pharmaceutical sector and our business. We continue to evaluate the effect that such challenges and measures would have on our business.
36
Other legislative changes have also been proposed and adopted since the ACA was enacted. For example, the Budget Control Act of 2011 resulted in aggregate reductions in Medicare payments to providers of up to 2 percent per fiscal year, starting in 2013, and due to subsequent legislative amendments to the statute will remain in effect through 2031, unless additional Congressional action is taken. However, COVID-19 relief legislation suspended the 2% Medicare sequester from May 1, 2020 through March 31, 2022. Following the resumption of the sequester, under current legislation the actual reduction in Medicare payments will vary from 1% in 2022 to up to 4% in the final fiscal year of this sequester. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Such laws, and others that may affect our business that have been enacted or may in the future be enacted, may result in additional reductions in Medicare and other healthcare funding. Further, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. The FDA also finalized guidance for manufacturers to obtain an additional National Drug Code for an FDA-approved drug as part of a process to provide a manufacturer a means to import its drugs that were originally intended to be marketed in and authorized for sale in a foreign country.
Also at the federal level, recent administrations have used several means to propose or implement drug pricing reform, including through federal budget proposals, executive orders and policy initiatives. For example, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In the order, the President directed the Federal Trade Commission to ban “pay for delay” patent settlement agreements, and to identify and address any efforts that impede generic or biosimilar competition. The executive order also directed the FDA to continue to work with states and Indian Tribes to develop importation programs in accordance with Section 804 of the FDCA and FDA regulations. The order directed the U.S. Department of Health and Human Services, or HHS, to increase support for generic and biosimilar drugs by improving standards for the interchangeability of biologic products, supporting biosimilar adoption by increasing education, and facilitating the approval of biosimilars by updating and clarifying existing requirements and procedures related to biologics licensing. Finally, the executive order directs HHS to submit a report detailing a comprehensive plan within 45 days to fight high prescription drug prices and reduce the amount that the federal government pays for drugs. In response to President Biden’s executive order, on September 9, 2021, HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform. These principles are government drug price negotiations, promoting increased competition including changes to supply chains and promoting biosimilars and generics and supporting public and private research. The plan sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. In addition, the IRA directs the HHS Secretary to establish a Drug Price Negotiation Program, or Program, to lower prices for certain high-expenditure, single-source prescription drugs and biologics covered under Medicare Part B and Part D that have been approved by the FDA for at least seven years for prescription drugs and at least 11 years for biologics. Under the Program, the HHS Secretary will publish a list of “selected drugs,” and will then negotiate maximum fair prices with their manufacturers. The Program will be implemented in stages. Beginning in 2026, 10 Medicare Part D “selected drugs” will be subject to price negotiations. By 2029, and in subsequent years thereafter, the number will increase to 20 drugs and biologics covered under Medicare Part B and Part D. Agreements between HHS and manufacturers will remain in place until a drug or biologic is no longer considered a “selected drug” for negotiation purposes. It is currently unclear how the IRA will be effectuated but is likely to have a significant impact on the pharmaceutical industry. Further, the Biden administration released an additional executive order on October 14, 2022, directing HHS to submit a report within 90 days on how the Center for Medicare and Medicaid Innovation can be further leveraged to test new models for lowering drug costs for Medicare and Medicaid beneficiaries. It is unclear whether this executive order or similar policy initiatives will be implemented in the future.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs, biological products and suppliers will be included in their healthcare programs.
37
Furthermore, there has been increased interest by third-party payers and governmental authorities in reference pricing systems and publication of discounts and list prices. There also has been particular and increasing legislative and enforcement interest in the United States with respect to relatively large price increases over relatively short time periods. There have been several recent state and federal lawmaker inquiries, proposed legislation and enacted legislation as was the case in California designed to, among other things, bring more transparency to drug pricing, by requiring drug companies to notify insurers and government regulators of price increases and provide an explanation of the reasons for the increase. There have also been actions to review the relationship between pricing and manufacturer patient assistance programs, and reform government program reimbursement methodologies for drugs. Further, a growing number of states have implemented, or are contemplating implementing, drug affordability boards to establish “allowable rates” for certain high-cost drugs identified by such boards.
Moreover, in the EEA some countries require the completion of additional studies that compare the cost-effectiveness of a particular medicinal product candidate to currently available therapies. This Health Technology Assessment, or HTA process, which is currently governed by the national laws of the individual EU Member States, is the procedure according to which the assessment of the public health impact, therapeutic impact and the economic and societal impact of use of a given medicinal product in the national healthcare systems of the individual country is conducted. The outcome of HTA regarding specific medicinal products will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. On January 31, 2018, the European Commission adopted a proposal for a regulation on health technologies assessment. The proposed regulation is intended to boost cooperation among EU Member States in assessing health technologies, including new medicinal products, and providing the basis for cooperation at EU level for joint clinical assessments in these areas. In December 2021 the HTA Regulation was adopted and entered into force on January 11, 2022. It will apply from 2025.
In the future, there will likely continue to be additional proposals relating to the reform of the U.S. healthcare system, some of which could further limit coverage and reimbursement of medicines, including our medicine candidates. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our medicines.
38
Irish Law Matters
As we are an Irish-incorporated company, the following matters of Irish law are relevant to the holders of our ordinary shares.
Irish Restrictions on Import and Export of Capital
Except as indicated below, there are no restrictions imposed specifically on non-residents of Ireland dealing in Irish domestic securities, which includes ordinary shares of Irish companies. Dividends and redemption proceeds also continue to be freely transferable to non-resident holders of such securities. The Financial Transfers Act 1992 gives power to the Minister for Finance of Ireland to restrict financial transfers between Ireland and other countries and persons. Financial transfers are broadly defined and include all transfers that would be movements of capital or payments within the meaning of the treaties governing the member states of the EU. The acquisition or disposal of interests in shares issued by an Irish incorporated company and associated payments falls within this definition. In addition, dividends or payments on redemption or purchase of shares and payments on a liquidation of an Irish incorporated company would fall within this definition. The Criminal Justice (Terrorist Offences) Act 2005 (as amended) also gives the Minister of Finance of Ireland the power to take various measures, including the freezing or seizure of assets, in order to combat terrorism. At present the Financial Transfers Act 1992, certain EU regulations (as implemented into Irish law) and the Criminal Justice (Terrorist Offences) Act 2005 (as amended) prohibit financial transfers involving certain persons and entities associated with the ISIL (Da’esh) and Al-Qaida organizations, the late Slobodan Milosevic and associated persons, Republic of Guinea-Bissau, Myanmar/Burma, Belarus, certain persons indicted by the International Criminal Tribunal for the former Yugoslavia, the late Osama bin Laden, Al-Qaida, the Taliban of Afghanistan, Democratic Republic of Congo, Democratic People’s Republic of Korea (North Korea), Iran, Iraq, Côte d’Ivoire, Lebanon, Liberia, Zimbabwe, South Sudan, Sudan, Somalia, Republic of Guinea, Afghanistan, Egypt, Eritrea, Libya, Syria, Tunisia, Burundi, the Central African Republic, Ukraine, Yemen, Bosnia and Herzegovina, certain known terrorists and terrorist groups, and countries that harbor certain terrorist groups, without the prior permission of the Central Bank of Ireland or the Minister of Finance (as applicable).
Any transfer of, or payment in respect of, a share or interest in a share involving the government of any country that is currently the subject of United Nations or EU sanctions, any person or body controlled by any of the foregoing, or by any person acting on behalf of the foregoing, may be subject to restrictions pursuant to such sanctions as implemented into Irish law.
Irish Taxes Applicable to U.S. Holders
Withholding Tax on Dividends. While we have no current plans to pay dividends, dividends on our ordinary shares would generally be subject to Irish Dividend Withholding Tax, or DWT, at the rate of 25 percent, unless an exemption applies.
Dividends on our ordinary shares that are owned by residents of the United States and held beneficially through the Depositary Trust Company, or DTC, will not be subject to DWT provided that the address of the beneficial owner of the ordinary shares in the records of the broker is in the United States.
Dividends on our ordinary shares that are owned by residents of the United States and held directly (outside of DTC) will not be subject to DWT provided that the shareholder has completed the appropriate Irish DWT form and this form remains valid or provides a Certification of U.S. Tax Residency, or Form IRS 6166. Such shareholders must provide the appropriate Irish DWT form or Form IRS 6166 to our transfer agent at least seven business days before the record date for the first dividend payment to which they are entitled.
If any shareholder who is resident in the United States receives a dividend subject to DWT, he or she should generally be able to make an application for a refund from the Irish Revenue Commissioners on the prescribed form (DWT Claim Form 1).
While the U.S./Ireland Double Tax Treaty contains provisions regarding withholding tax, due to the wide scope of the exemptions from DWT available under Irish domestic law, it would generally be unnecessary for a U.S. resident shareholder to rely on the treaty provisions.
Income Tax on Dividends. A shareholder who is neither resident nor ordinarily resident in Ireland and who is entitled to an exemption from DWT generally has no additional liability to Irish income tax or to the universal social charge on a dividend from us.
39
A shareholder who is neither resident nor ordinarily resident in Ireland and who is not entitled to an exemption from DWT generally has no additional liability to Irish income tax or to the universal social charge on a dividend from us. The DWT deducted by us discharges the liability to Irish income tax and to the universal social charge.
Irish Tax on Capital Gains. A shareholder who is neither resident nor ordinarily resident in Ireland and does not hold our ordinary shares in connection with a trade or business carried on by such shareholder in Ireland through a branch or agency should not be subject to Irish tax on capital gains on a disposal of our ordinary shares.
Capital Acquisitions Tax. Irish capital acquisitions tax, or CAT, is comprised principally of gift tax and inheritance tax. CAT could apply to a gift or inheritance of our ordinary shares irrespective of the place of residence, ordinary residence or domicile of the parties. This is because our ordinary shares are regarded as property situated in Ireland as our share register must be held in Ireland. The person who receives the gift or inheritance has primary liability for CAT.
CAT is levied at a rate of 33 percent above certain tax-free thresholds. The appropriate tax-free threshold is dependent upon (i) the relationship between the donor and the donee and (ii) the aggregation of the values of previous gifts and inheritances received by the donee from persons within the same category of relationship for CAT purposes. Gifts and inheritances passing between spouses are exempt from CAT. Our shareholders should consult their own tax advisers as to whether CAT is creditable or deductible in computing any domestic tax liabilities.
Stamp Duty. Irish stamp duty (if any) may become payable in respect of ordinary share transfers. However, a transfer of our ordinary shares from a seller who holds shares through DTC to a buyer who holds the acquired shares through DTC will not be subject to Irish stamp duty. A transfer of our ordinary shares (i) by a seller who holds ordinary shares outside of DTC to any buyer, or (ii) by a seller who holds the ordinary shares through DTC to a buyer who holds the acquired ordinary shares outside of DTC, may be subject to Irish stamp duty (currently at the rate of 1 percent of the price paid or the market value of the ordinary shares acquired, if greater). The person accountable for payment of stamp duty is the buyer or, in the case of a transfer by way of a gift or for less than market value, all parties to the transfer.
A shareholder who holds ordinary shares outside of DTC may transfer those ordinary shares into DTC without giving rise to Irish stamp duty provided that the shareholder would be the beneficial owner of the related book-entry interest in those ordinary shares recorded in the systems of DTC (and in exactly the same proportions) as a result of the transfer and at the time of the transfer into DTC there is no sale of those book-entry interests to a third party being contemplated by the shareholder. Similarly, a shareholder who holds ordinary shares through DTC may transfer those ordinary shares out of DTC without giving rise to Irish stamp duty provided that the shareholder would be the beneficial owner of the ordinary shares (and in exactly the same proportions) as a result of the transfer, and at the time of the transfer out of DTC there is no sale of those ordinary shares to a third party being contemplated by the shareholder. In order for the share registrar to be satisfied as to the application of this Irish stamp duty treatment where relevant, the shareholder must confirm to us that the shareholder would be the beneficial owner of the related book-entry interest in those ordinary shares recorded in the systems of DTC (and in exactly the same proportions) (or vice-versa) as a result of the transfer and there is no agreement for the sale of the related book-entry interest or the ordinary shares or an interest in the ordinary shares, as the case may be, by the shareholder to a third party being contemplated.
40
Employees and Human Capital
As of December 31, 2022, we had approximately 2,115 full-time employees. Of our employees as of December 31, 2022, approximately 660 were engaged in development, regulatory and manufacturing activities, approximately 1,005 were engaged in sales and marketing and approximately 450 were engaged in administration, including business development, finance, legal, information systems, facilities and human resources. We consider our employee relations to be good. We are committed to strict policies and procedures to maintain a safe work environment. The health and safety of our employees, customers and communities are of primary concern.
Horizon is a perennially award-winning company that has been recognized more than 65 times since 2015 for its workplace and culture. These awards are based on a combination of independent employee feedback and self-reported company data.
Our human capital management objectives include identifying, recruiting, retaining, incentivizing and integrating our existing and future employees. In addition to competitive base salaries, we offer competitive benefits to attract, retain and reward employees and also, through the granting of share-based and cash-based compensation awards, to secure and retain the services of our employees and provide long-term incentives that align the interests of employees with the interests of our shareholders. See “Focus on Employee Benefits” for additional information.
To help us measure and enhance our employees’ overall engagement and satisfaction with working at Horizon and to determine areas for improvement, we continuously seek their feedback and suggestions through periodic “pulse” surveys. In 2022, we conducted multiple pulse surveys to gain feedback on key topics such as well-being, growth and development, manager effectiveness and employee engagement. Participation in the surveys is high, with rates typically averaging above 90% for our annual surveys – and the results, based on sentiment indication, generally exceed top-quartile industry benchmarks. The favorable results indicate to us that our employees are highly motivated to go above and beyond, that they are highly engaged and that they intend to remain at Horizon, an important consideration given the highly competitive nature of our industry. We attribute some of the successful results of our surveys to the fact that we act on much of the feedback we receive from our employees.
Our Core Values
Our culture is reflected in our three core values: growth, accountability and transparency. Through these core values, our teams of highly engaged employees work to better the lives of patients and the community. This engagement is fostered by our strong emphasis on creating a diverse and inclusive culture that drives how we treat employees and expect employees to treat one another.
Growth: We are a high-growth organization that values innovation, development and evolution. We are fiercely innovating to better our communities, our patients and our employees and place a strong emphasis on personal and professional growth. Employees have access to resources to develop their teams and themselves.
Accountability: We strive to do what’s right for patients and employees through quality decisions and owning successes and failures. Employees hold each other accountable to make quality decisions that keep our company moving forward to meet the needs of patients.
Transparency: We value the collaboration that is made possible by employees trusting each other to tackle tough challenges and difficult conversations. We are courageous in our decision making, knowing it's necessary to drive our business forward.
We continuously strive to maintain an engaged workforce that is ready to serve patients and healthcare providers. To that end, employee development is central to how we improve every day. All our employees are encouraged to participate in “Growing Your Career at Horizon,” a series of learning events focused on providing guidance around leadership development. Recently – and to support continuous improvement on key culture initiatives – these events have focused on such topics as “inclusive conversations,” “strategic mindset” and “managing ambiguity.” Additionally, all our employees have unlimited and seamless access to myriad online learning resources. These resources are valuable tools that empower employees to further their professional development while contributing to the growth of our company.
As employees progress in their careers, additional opportunities become available to them, including robust programs intended to grow early career professionals, people managers and future enterprise leaders.
41
Focus on Employee Benefits
At the center of our employee experience is how we reward our employees for the impact they create. Our competitive benefits include annual equity and cash incentive plans, retirement benefits and an employee share purchase plan. In addition, we absorb most of the costs for employee medical insurance plans. We also offer a wide variety of benefits that support working families. This includes our parental and caregiver programs. As part of these programs, all caregivers have flexible paid options to care for the needs of their families. These benefits are paid at 100 percent salary. For employees pursuing adoption or surrogacy as a path to parenthood, we offer competitive reimbursement for costs associated with the legal adoption of a child or expenses incurred when using a surrogate.
We offer all full-time employees a “Make it Personal” account, which provides $500 annually for certain employee personal expenses including student loan repayment, contributions to college savings plans, donations to charitable organizations, health club memberships or purchases of personal health equipment or home office equipment. In addition, all employees have access to an annual “Make it Personal” day. This is an additional 8 hours of paid time off that employees can use to participate in something meaningful or personal to them – from volunteering at a local charity to spending time caring for a loved one.
We also offer competitive educational benefits for our employees and families. We value and encourage continued growth and development of our employees and their families. To support educational goals, we offer several programs to help offset the financial burden of college expenses, including tuition reimbursement, an executive scholarship award for graduate school and scholarships for dependents of our employees.
Our Commitment to Inclusion and Diversity
We are committed to maintaining a workplace free of discrimination, harassment, intimidation or inappropriate conduct based on sex/gender, race, color, religion, national origin, age, disability, veteran status, sexual orientation and/or any other category protected by law. We also provide equal opportunity in employment to all employees and applicants. Equal opportunity rights are applicable to recruitment, hiring, employment and employment-related decisions. In 2020, we introduced RiSE, a strategic program to further embed inclusion, diversity, equity and allyship into the organization. Through RiSE, over 20 volunteer employee leaders work together, leading nine diverse working groups, to enhance and promote our approach to inclusive recruitment, professional development, community involvement, culturally responsive benefits, patient equity and building the overall organizational inclusive culture.
Our commitment to inclusion, diversity, equity and allyship is evidenced from the top down. Our chief executive officer, or CEO, Timothy P. Walbert, was one of the first signatories to the CEO Action for Diversity & Inclusion pledge. Our top leaders have gone through in-depth assessments to determine their inclusive leadership capabilities, with coaching available for leaders who want to enhance their skillsets in these areas.
We continued to demonstrate gender and ethnicity pay equity, according to a second study conducted in 2021 by a leading third-party compensation consulting firm. The study was a follow-on study to a 2019 study that found no pay discrepancy among men, women and those of different ethnic backgrounds. Both studies analyzed employee demographic and pay data and showed that we provide equal pay for equal work, regardless of gender or ethnicity. We maintained our gender and ethnicity pay equity after significant growth in the three years since the first study. In addition, our percentage of female employees is over 50 percent and above industry standards.
42
We continue to receive multiple workplace recognitions, which we believe is evidence of our commitment to employee engagement. In 2022, we received multiple well-known published workplace rankings, including several diversity-related workplace awards:
FORTUNE Best Workplace Awards:
Diversity, Equity and Inclusion Awards:
Other Workplace-Related Awards:
Available Information
We make available free of charge on or through our internet website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission. We also regularly post copies of our press releases as well as copies of presentations and other updates about our business on our website. Our website address is www.horizontherapeutics.com. The information contained in or that can be accessed through our website is not part of this Annual Report on Form 10-K. Information is also available through the Securities and Exchange Commission’s website at www.sec.gov.
43
Item 1A. Risk Factors
Certain factors may have a material adverse effect on our business, financial condition and results of operations, and you should carefully consider them. Accordingly, in evaluating our business, we encourage you to consider the following discussion of risk factors in its entirety, in addition to other information contained in this report as well as our other public filings with the Securities and Exchange Commission, or SEC.
Risks Relating to Our Pending Transaction with Amgen
Failure to complete, or delays in completing, the pending transaction with Amgen announced on December 12, 2022 could materially and adversely affect our results of operations and our share price.
On December 12, 2022, we announced that we entered into a transaction agreement with Amgen Inc., or Amgen, and Pillartree Limited, or Pillartree, a wholly owned subsidiary of Amgen. Subject to the terms of the transaction agreement, Pillartree will acquire our company, or the Transaction, pursuant to a scheme of arrangement under Chapter 1 of Part 9 of the Companies Act 2014 of Ireland, or the Scheme, or under certain circumstances, subject to the terms of the transaction agreement, a takeover offer (as such term is defined in the Irish Takeover Rules) rather than the Scheme. As a result of the Scheme, we would become a wholly owned subsidiary of Amgen. Consummation of the Transaction is subject to certain closing conditions, some of which are not within our control, and may prevent, delay, or otherwise materially adversely affect the completion of the Transaction. On January 30, 2023, we and Amgen received a request for additional information and documentary materials, or a second request, from the Federal Trade Commission, or FTC, in connection with the FTC’s review of the Transaction. The effect of the second request is to extend the waiting period imposed by the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, until 30 days after we and Amgen have substantially complied with the second request, unless the period is extended voluntarily by the parties or terminated sooner by the FTC. In connection with the Transaction, we and Amgen have received clearances or confirmation of non-applicability related to foreign direct investment in Denmark, Italy, Germany and France and clearances related to antitrust in Germany and Austria. We cannot predict with certainty whether and when any of the required closing conditions, including receiving required regulatory clearances, will be satisfied or if additional uncertainties may arise and cannot guarantee that we will be able to successfully consummate the pending Transaction as currently contemplated under the transaction agreement or at all. Under certain limited circumstances in which the Transaction is not consummated, we may be required to reimburse Amgen for up to $278.4 million in third party costs and expenses incurred in connection with the Transaction. Risks related to the failure of the pending Transaction to be consummated include, but are not limited to, the following:
Additionally, the retention of our employees, hiring of new qualified employees and the performance of our employees may be negatively affected during the pendency of the Transaction as employees or prospective employees may experience uncertainty about their future roles following completion of the Transaction.
Further, under the transaction agreement, we are generally required to conduct our business in the ordinary course, consistent with past practice and are restricted from taking certain specified actions absent Amgen’s prior written consent, which restrictions could adversely affect our ability to conduct our business.
The occurrence of any of these events individually or in combination could materially and adversely affect our business, results of operations, financial condition, and our share price.
44
The ability to complete the Transaction is subject to the receipt of consents and clearances from government entities, which may impose conditions that could have an adverse effect or could cause either party to abandon the Transaction.
The Transaction remains subject to customary closing conditions, including, among other things, (a) the sanction by the Irish High Court of the Scheme and delivery of the court order to the Irish Registrar of Companies, (b) the receipt of required antitrust clearance in the United States, and the absence of an order or law that prevents consummation of the Transaction or imposes a burdensome condition (as defined in the transaction agreement), (c) absence of any Material Adverse Effect (as defined in the transaction agreement) from December 12, 2022 to the Sanction Date (as defined in the transaction agreement) that is continuing as of the Sanction Date, (d) the accuracy of each party’s representations and warranties subject to certain materiality and material adverse effect exceptions and (e) the performance by each party of all of its covenants and agreements under the transaction agreement in all material respects. On January 30, 2023, we and Amgen received a second request from the FTC in connection with the FTC’s review of the Transaction. The second request, and any further inquiries or actions from the FTC, could have the effect of substantially delaying, imposing restrictions on, or impeding or precluding the completion of the Transaction. In deciding whether to grant antitrust clearance, the FTC will consider the effect of the Transaction on competition and take such action under the antitrust laws as it deems necessary or desirable in the public interest. The FTC may take steps to prevent the Transaction, or condition its clearance of the Transaction on Amgen’s or our agreement to various requirements, limitations, or costs, or require divestitures or place restrictions on the conduct of Amgen’s business following the Transaction. These requirements, limitations, costs, divestitures, or restrictions may result in the delay or abandonment of the Transaction.
The transaction agreement limits our ability to pursue alternative transactions which could deter a third party from proposing an alternative transaction.
The transaction agreement contains provisions that, subject to certain exceptions, limit our ability to solicit or knowingly encourage discussions or negotiations with any third party regarding alternative acquisition proposals. It is possible that these or other provisions in the transaction agreement might discourage a potential competing acquirer that might have an interest in acquiring all or a significant part of our outstanding ordinary shares from considering or proposing an acquisition or that the price at which Amgen has proposed to acquire Horizon might result in a potential competing acquirer proposing to pay a lower per share price to acquire our ordinary shares than it might otherwise have proposed to pay.
45
Risks Related to Our Business and Industry
Our ability to generate revenues from our medicines is subject to attaining significant market acceptance among physicians, patients and healthcare payers.
Our current medicines, and other medicines or medicine candidates that we may develop or acquire, may not attain market acceptance among physicians, patients, healthcare payers or the medical community. Some of our medicines, in particular TEPEZZA and UPLIZNA, have not been on the market for an extended period of time, which subjects us to numerous risks as we attempt to increase our market share. We believe that the degree of market acceptance and our ability to generate revenues from our medicines will depend on a number of factors, including:
46
With respect to TEPEZZA, sales will depend on market acceptance and adoption by physicians, healthcare payers and patients, as well as the ability and willingness of physicians who do not have in-house infusion capability to refer patients to infusion sites of care and of certain physicians to refer patients to ocular specialists familiar with thyroid eye disease, or TED, and the use of TEPEZZA. In addition, results from our Phase 4 clinical trial evaluating the use of TEPEZZA in chronic/low clinical activity score, or CAS, TED patients will also be important for TEPEZZA’s adoption by certain physicians and payers, and we cannot assure that the results from this clinical trial will be supportive or will otherwise drive adoption. With respect to KRYSTEXXA, our ability to grow sales will be affected by the success of our sales, marketing and clinical strategies, which are intended to expand the patient population and usage of KRYSTEXXA. This includes our marketing efforts in nephrology and our studies designed to further evaluate uses and ways of enhancing the patient experience of KRYSTEXXA. With respect to RAVICTI, which is approved to treat a very limited patient population, our ability to grow sales will depend in large part on our ability to transition urea cycle disorder, or UCD, patients from BUPHENYL or generic equivalents, which are comparatively much less expensive, to RAVICTI and to educate patients and physicians on the benefits of continuing RAVICTI therapy once initiated. With respect to PROCYSBI, which is also approved to treat a very limited patient population, our ability to grow sales will depend in large part on our ability to transition patients from the first-generation immediate-release cysteamine therapy to PROCYSBI, to identify additional patients with nephropathic cystinosis and to educate patients and physicians on the benefits of continuing therapy once initiated. With respect to UPLIZNA, sales will depend on market acceptance and adoption by physicians and healthcare payers, as well as the ability and willingness of physicians who do not have in-house infusion capability to refer patients to infusion sites of care. With respect to ACTIMMUNE, while it is the only FDA-approved treatment for chronic granulomatous disease, or CGD, and severe, malignant osteopetrosis, or SMO, which are very rare conditions and, as a result, our ability to grow ACTIMMUNE sales will depend on our ability to identify additional patients with such conditions and educate patients and physicians on the benefits of continuing treatment once initiated. If our current medicines or any other medicine that we may seek approval for, or acquire, fail to attain market acceptance, we may not be able to generate significant revenue to sustain profitability, which would have a material adverse effect on our business, results of operations, financial condition and prospects (including, possibly, the value of our ordinary shares).
Our future prospects are highly dependent on our ability to successfully develop and execute commercialization strategies for each of our medicines. Failure to do so would adversely impact our financial condition and prospects.
A substantial majority of our resources are focused on the commercialization of our current medicines. Our ability to generate significant medicine revenues and to achieve commercial success in the near-term will initially depend almost entirely on our ability to successfully commercialize these medicines in the United States. Our commercialization strategy includes efforts to increase awareness of the rare conditions that each medicine is designed to treat, enhancing efforts to identify target patients and in certain cases pursue opportunities for more effective use through clinical trials, as well as opportunities for commercialization outside of the United States. Our comprehensive commercial strategy for TEPEZZA aims to enable more TED patients to benefit from TEPEZZA. We are doing this by: (i) facilitating continued TEPEZZA uptake in the treatment of TED through continued promotion of TEPEZZA to treating physicians; (ii) continuing to develop the TED market by increasing physician awareness of the disease severity and the urgency to diagnose and treat it, as well as the benefits of treatment with TEPEZZA, including through the expansion of our TEPEZZA commercial team and targeting ophthalmologists and endocrinologists; (iii) driving accelerated disease identification and time to treatment through our digital and broadcast marketing campaigns; (iv) enhancing the patient journey with our high-touch, patient-centric model as well as support for the patient and site-of-care referral processes; (v) pursuing more timely access to TEPEZZA for TED patients; (vi) conducting our Phase 4 clinical trial to inform the use TEPEZZA in chronic/low CAS TED; and (vii) pursuing a global expansion strategy, which includes bringing TEPEZZA to patients with TED outside of the United States. Our strategy with respect to KRYSTEXXA includes existing rheumatology account growth, new rheumatology account growth and accelerating nephrology growth, the use of KRYSTEXXA with methotrexate following the approval of our supplemental biologics license application, in July 2022, as well as development efforts to further evaluate uses and ways of enhancing the patient experience of KRYSTEXXA.
47
With respect to RAVICTI and PROCYSBI, our strategy includes accelerating the transition of patients from first-generation therapies, increasing the diagnosis of the associated rare conditions through patient and physician outreach; and increasing compliance rates. With respect to our strategy for UPLIZNA, which leverages the successful strategies we have employed with TEPEZZA and KRYSTEXXA, our aim is to (i) increase physician awareness of the benefits of UPLIZNA for the treatment of neuromyelitis optica spectrum disorder, or NMOSD, and what differentiates UPLIZNA from other medicines by generating additional trial data analyses and clinical evidence; (ii) drive patient initiation and adherence, and cultivate a positive patient experience; and (iii) maximize the potential of UPLIZNA through additional indications and global expansion. Our strategy with respect to ACTIMMUNE, includes increasing awareness and diagnosis of CGD, driving utilization of ACTIMMUNE prophylaxis in newly diagnosed CGD patients as recommended in current treatment guidelines, encouraging use of ACTIMMUNE in CGD patients prior to bone marrow transplant and in symptomatic carriers of x-linked CGD and increasing compliance rates overall.
We are focusing a significant portion of our commercial activities and resources on TEPEZZA, and we believe our ability to grow our long-term revenues, and a significant portion of the value of our company, relates to our ability to successfully commercialize TEPEZZA in the United States. As a medicine launched for a disease that had no previously approved treatments, successful commercialization of TEPEZZA is subject to many risks. While we believe the launch of TEPEZZA was one of the most successful launches to date for a rare disease treatment, there are numerous examples of failures to meet high expectations of market potential, including by biopharmaceutical companies with more experience and resources than us. We will need to continue training and developing our U.S. commercial team in order to continue successfully commercializing TEPEZZA. There are many factors that could cause commercialization of TEPEZZA to be unsuccessful, including a number of factors that are outside our control. Because no medicine has previously been approved by the FDA for the treatment of TED, it is especially difficult to estimate TEPEZZA’s market potential or the time it will take to increase patient and physician awareness of TED and change current treatment paradigms. In addition, some physicians that are potential prescribers of TEPEZZA do not have the necessary infusion capabilities to administer the medicine or may not have significant experience managing patients on medications like TEPEZZA and may not otherwise be able or willing to refer their patients to third-party infusion centers or other healthcare providers, which may discourage them from treating their patients with TEPEZZA. We identified certain challenges, including the often-burdensome reimbursement process, that we believe contributed to slower-than-expected growth of TEPEZZA in the first half of 2022. These challenges, as well as challenges related to a lower rate of adherence to the full course of TEPEZZA therapy, have continued to moderate TEPEZZA net sales growth. In the second half of 2022, we began executing on several opportunities to address these challenges and accelerate growth, including significantly expanding the size of our TEPEZZA sales force to allow our representatives more time with core TEPEZZA prescribers while educating other key physicians, including ophthalmologists and endocrinologists, about TED and TEPEZZA. We are also spending more time and focus on the reimbursement process to more effectively support the patient access journey. We also continue to invest significantly in direct-to-consumer advertising based on the returns we have seen to date. However, it will continue to take some time for these strategies to contribute meaningfully to TEPEZZA net sales growth and we cannot otherwise be certain that our strategies will be successful in overcoming these challenges or that new challenges to TEPEZZA adoption will not arise. In addition, if the patient population suffering from TED or that is appropriate for treatment with TEPEZZA is smaller than we estimate, if it proves difficult to identify TED patients or educate physicians as to the availability and potential benefits of TEPEZZA, or if physicians are unwilling to prescribe or patients are unwilling to take TEPEZZA for the full course of therapy, the commercial potential of TEPEZZA will be limited. Our ability to continue TEPEZZA supply could be impacted by additional government-mandated COVID-19 vaccine production orders and other risks associated with our reliance on our third-party manufacturers discussed below. We also have limited information regarding how physicians, patients and payers will respond to the pricing of TEPEZZA. Physicians may not prescribe TEPEZZA and patients may be unwilling to use TEPEZZA if coverage is not provided or reimbursement is inadequate to cover a significant portion of the cost. Thus, significant uncertainty remains regarding the commercial potential of TEPEZZA. If the continued commercialization of TEPEZZA becomes unsuccessful or perceived as disappointing, the price of our ordinary shares could decline significantly and long-term success of the medicine and our company could be harmed.
If any of our commercial strategies are unsuccessful or we fail to successfully modify our strategies over time due to changing market conditions, our ability to increase market share for our medicines, grow revenues and to sustain profitability will be harmed.
48
We are dependent on wholesale distributors for distribution of our medicines in the United States and, accordingly, our results of operations could be adversely affected if they encounter financial difficulties.
During the year ended December 31, 2022, four wholesale distributors accounted for substantially all of our sales in the United States. If one of our significant wholesale distributors encounters financial or other difficulties, such distributor may decrease the amount of business that it does with us, and we may be unable to collect all the amounts that the distributor owes on a timely basis or at all, which could negatively impact our business and results of operations. In addition, net sales of our medicines may be affected by end customer buying patterns and fluctuations in wholesaler inventory levels.
In order to increase adoption and sales of our medicines, we will need to continue developing our commercial organization as well as recruit and retain qualified sales representatives.
Part of our strategy is to continue to build a global biotechnology company to successfully execute the commercialization of our medicines in the U.S. market, and in selected markets outside the United States where we have commercial rights. We may not be able to successfully commercialize our medicines in the United States or in any other territories where we have commercial rights. In order to commercialize any approved medicines, we must continue to build our sales, marketing, distribution, managerial and other non-technical capabilities. As of December 31, 2022, we had approximately 380 sales representatives and sales management in the field. Due to the impact of the at-risk launch of generic PENNSAID 2% and wind down of our former inflammation business during 2022, we redeployed a portion of our inflammation commercial team to support our TEPEZZA and KRYSTEXXA expansions. We are further expanding our commercial team, and we may encounter difficulties transitioning sales representatives from promoting our inflammation medicines to promoting TEPEZZA and KRYSTEXXA, or in hiring additional qualified sales representatives. The pending Transaction with Amgen has also made it more difficult to attract and retain qualified employees due to the uncertainty about whether or when the Transaction will close and impact of the Transaction on our employees. We currently have limited resources compared to some of our competitors, and the continued development of our own commercial organization to market our medicines and any additional medicines we may acquire will be expensive and time-consuming. We also cannot be certain that we will be able to continue to successfully expand this capability.
As we continue to add medicines through development efforts and acquisition transactions and execute on our international expansion initiatives, the members of our sales force may have limited experience promoting certain of our medicines. To the extent we employ an acquired entity’s sales forces to promote acquired medicines, we may not be successful in continuing to retain these employees and we otherwise will have limited experience marketing these medicines under our commercial organization. In addition, prior to completing the acquisition of Viela Bio, Inc., or Viela, in March 2021, we had no experience as an organization commercializing UPLIZNA. We are required to expend significant time and resources to train our sales force to be credible and able to educate physicians on the benefits of prescribing and pharmacists dispensing our medicines. In addition, we must train our sales force to ensure that a consistent and appropriate message about our medicines is being delivered to our potential customers. Our sales representatives may also experience challenges promoting multiple medicines when we call on physicians and their office staff. We have experienced, and may continue to experience, turnover of the sales representatives that we hired or will hire, requiring us to train new sales representatives. If we are unable to recruit and retain qualified personnel outside of the United States, we may not be able to execute our global expansion strategy successfully. If we are unable to effectively train our sales force and equip them with effective materials, including medical and sales literature to help them inform and educate physicians about the benefits of our medicines and their proper administration and label indication, as well as our patient assistance programs, our efforts to successfully commercialize our medicines could be put in jeopardy, which could have a material adverse effect on our financial condition, share price and operations.
We will also have to compete with other biopharmaceutical companies to recruit, hire, train and retain commercial personnel. To the extent we rely on additional third parties to commercialize any approved medicines, we may receive less revenue than if we commercialized these medicines ourselves. In addition, we may have little or no control over the sales efforts of any third parties involved in our commercialization efforts. In the event we are unable to successfully develop and maintain our own commercial organization or collaborate with a third-party sales and marketing organization, we may not be able to commercialize our medicines and medicine candidates and execute on our business plan.
49
Coverage and reimbursement may not be available, or reimbursement may be available at only limited levels, for our medicines, which could make it difficult for us to sell our medicines profitably.
Market acceptance and sales of our medicines will depend in large part on global coverage and reimbursement policies and may be affected by future healthcare reform measures, both in the United States and other key international foreign markets. Successful commercialization of our medicines will depend in part on the availability of governmental and third-party payer reimbursement for the cost of our medicines. Government health administration authorities, private health insurers and other organizations generally provide reimbursement for healthcare. In particular, in the United States, private health insurers and other third-party payers often provide reimbursement for medicines and services based on the level at which the government (through the Medicare or Medicaid programs) provides reimbursement for such treatments. In the United States, the European Union, or EU, and other significant or potentially significant markets for our medicines and medicine candidates, government authorities and third-party payers are increasingly attempting to limit or regulate the price of medicines and services, particularly for new and innovative medicines and therapies, which has resulted in lower average selling prices. Further, the increased scrutiny of prescription drug pricing practices and emphasis on managed healthcare in the United States and on country and regional pricing and reimbursement controls in the EU and other significant or potentially significant markets will put additional pressure on medicine pricing, reimbursement and usage, which may adversely affect our medicine sales and results of operations. These pressures can arise from rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, biopharmaceutical reimbursement policies and pricing in general. These pressures may create negative reactions to any medicine price increases, or limit the amount by which we may be able to increase our medicine prices, which may adversely affect our medicine sales and results of operations.
We expect to experience pricing pressures in connection with the sale of our medicines due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative proposals relating to outcomes and quality. For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, increased the mandated Medicaid rebate from 15.1% to 23.1%, expanded the rebate to Medicaid managed care utilization and increased the types of entities eligible for the federal 340B drug discount program. As concerns continue to grow over the need for tighter oversight, there remains the possibility that the Health Resources and Services Administration or another agency under the U.S. Department of Health and Human Services, or HHS, will propose a similar regulation or that Congress will explore changes to the 340B program through legislation. For example, a bill was introduced in 2018 that would require hospitals to report their low-income utilization of the program. Further, the Centers for Medicare & Medicaid Services, or CMS, issued a final rule in 2018 that implemented civil monetary penalties for manufacturers who exceeded the ceiling price methodology for a covered outpatient drug when selling to a 340B covered entity. Pursuant to the final rule, after January 1, 2019, manufacturers must calculate 340B program ceiling prices on a quarterly basis. Moreover, manufacturers could be subject to a $5,000 penalty for each instance where they knowingly and intentionally overcharge a covered entity under the 340B program. With respect to KRYSTEXXA, the “additional rebate” methodology of the 340B pricing rules, as applied to the historical pricing of KRYSTEXXA both before and after we acquired the medicine, have resulted in a 340B ceiling price of one penny. A material portion of KRYSTEXXA prescriptions (normally in the range of low to mid-teens percent) are written by healthcare providers that are eligible for 340B drug pricing and therefore the reduction in 340B pricing to a penny has negatively impacted our net sales of KRYSTEXXA. CMS previously revised the Medicare hospital outpatient prospective payment system by creating a new, significantly reduced reimbursement methodology for drugs purchased under the 340B program for Medicare patients at hospital and other settings. However, on June 15, 2022, the Supreme Court ruled that CMS was not authorized to set the rates in the previous final rules because it did not conduct the requisite survey of acquisition data. Therefore, it was required to utilize the average price for the drug in the given year and not vary rates by hospital group. It is unclear whether CMS will attempt to implement the same rate changes by other means.
50
Patients are unlikely to use our medicines unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our medicines. Third-party payers may limit coverage to specific medicines on an approved list, also known as a formulary, which might not include all of the FDA-approved medicines for a particular indication. Moreover, a third-party payer’s decision to provide coverage for a medicine does not imply that an adequate reimbursement rate will be approved. Additionally, one third-party payer’s decision to cover a particular medicine does not ensure that other payers will also provide coverage for the medicine, or will provide coverage at an adequate reimbursement rate. Even though we have contracts with some pharmacy benefit managers, or PBMs, in the United States, that does not guarantee that they will perform in accordance with the contracts, nor does that preclude them from taking adverse actions against us, which could materially adversely affect our operating results. In addition, the existence of such PBM contracts does not guarantee coverage by such PBM’s contracted health plans or adequate reimbursement to their respective providers for our medicines. For example, some PBMs have placed some of our medicines on their exclusion lists from time to time, which has resulted in a loss of coverage for patients whose healthcare plans have adopted these PBM lists. Additional healthcare plan formularies may also exclude our medicines from coverage due to the actions of certain PBMs, future price increases we may implement, our use of our patient assistance programs or other programs whereby we assist qualified patients with certain out-of-pocket expenditures for our medicines, including donations to patient assistance programs offered by charitable foundations, or any other co-pay programs, or other reasons. If our strategies to mitigate formulary exclusions are not effective, these events may reduce the likelihood that physicians prescribe our medicines and increase the likelihood that prescriptions for our medicines are not filled.
Many EU Member States periodically review their reimbursement procedures for medicinal products, which could have an adverse impact on reimbursement status. We expect that legislators, policymakers and healthcare insurance funds in the EU Member States will continue to propose and implement cost-containing measures, such as lower maximum prices, lower or lack of reimbursement coverage and incentives to use cheaper, usually generic, products as an alternative to branded products, and/or branded products available through parallel import to keep healthcare costs down. Moreover, in order to obtain reimbursement for our products in some European countries, including some EU Member States, we may be required to compile additional data comparing the cost-effectiveness of our products to other available therapies. Health Technology Assessment, or HTA, of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU Member States, including those representing the larger markets. The HTA process is the procedure to assess therapeutic, economic and societal impact of a given medicinal product in the national healthcare systems of the individual country. The outcome of an HTA will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. The extent to which pricing and reimbursement decisions are influenced by the HTA of the specific medicinal product currently varies between EU Member States.
In December 2021, Regulation No 2021/2282 on HTA, amending Directive 2011/24/EU, was adopted in the EU. This Regulation, which entered into force in January 2022 and will apply as of January 2025, is intended to boost cooperation among EU Member States in assessing health technologies, including new medicinal products, and providing the basis for cooperation at EU level for joint clinical assessments in these areas. The Regulation foresees a three-year transitional period and will permit EU Member States to use common HTA tools, methodologies, and procedures across the EU, working together in four main areas, including joint clinical assessment of the innovative health technologies with the most potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EU Member States will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technologies, and making decisions on pricing and reimbursement. If we are unable to maintain favorable pricing and reimbursement status in EU Member States for medicine candidates that we may successfully develop and for which we may obtain regulatory approval, any anticipated revenue from and growth prospects for those products in the EU could be negatively affected.
Legislators, policymakers and healthcare insurance funds in the EU may continue to propose and implement cost-containing measures to keep healthcare costs down; particularly due to the financial strain that the COVID-19 pandemic has placed on national healthcare systems of the EU Member States. These measures could include limitations on the prices we would be able to charge for medicine candidates that we may successfully develop and for which we may obtain regulatory approval or the level of reimbursement available for these products from governmental authorities or third-party payors. Further, an increasing number of EU and other foreign countries use prices for medicinal products established in other countries as “reference prices” to help determine the price of the product in their own territory. Consequently, a downward trend in prices of medicinal products in some countries could contribute to similar downward trends elsewhere.
51
In light of such policies and the uncertainty surrounding proposed regulations and changes in the coverage and reimbursement policies of governments and third-party payers, we cannot be sure that coverage and reimbursement will be available for any of our medicines in any additional markets or for any other medicine candidates that we may develop. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our medicines. If coverage and reimbursement are not available or are available only at limited levels, we may not be able to successfully commercialize our medicines.
There may be additional pressure by payers, healthcare providers, state or foreign governments, federal regulators and Congress, to use generic drugs that contain the active ingredients found in our medicines or any other medicine candidates that we may develop or acquire. If we fail to successfully secure and maintain coverage and adequate reimbursement for our medicines or are significantly delayed in doing so, we will have difficulty achieving market acceptance of our medicines and expected revenue and profitability which would have a material adverse effect on our business, results of operations, financial condition and prospects.
We may also experience pressure from payers as well as state and federal government authorities concerning certain promotional approaches that we may implement such as our patient assistance programs or any other co-pay programs. Certain state and federal enforcement authorities and members of Congress have initiated inquiries about co-pay assistance programs. Some state legislatures have implemented or have been considering implementing laws to restrict or ban co-pay coupons for branded drugs. For example, legislation was signed into law in California that limits the use of co-pay coupons in cases where a lower cost generic drug is available and if individual ingredients in combination therapies are available over the counter at a lower cost. It is possible that similar legislation could be proposed and enacted in additional states. Additionally, numerous organizations, including biopharmaceutical manufacturers, have been subject to ongoing litigation, enforcement actions and settlements related to their patient assistance programs and support.
Our medicines are subject to extensive regulation, and we may not obtain additional regulatory approvals for our medicines.
The clinical development, manufacturing, labeling, packaging, storage, recordkeeping, advertising, promotion, export, marketing and distribution and other possible activities relating to our medicines and our medicine candidates are, and will be, subject to extensive regulation by the FDA and other regulatory authorities. Failure to comply with FDA and other applicable regulatory requirements may, either before or after medicine approval, subject us to administrative or judicially imposed sanctions. In addition, the label inclusion criteria may differ by geography.
We are pursuing a global expansion strategy, which includes bringing TEPEZZA to patients with TED and UPLIZNA to adult patients with NMOSD outside of the United States. To market any drugs or biologics outside of the United States, we and current or future collaborators must comply with numerous and varying regulatory and compliance related requirements of other countries. Approval procedures vary among countries and can involve additional medicine testing and additional administrative review periods, including obtaining reimbursement and pricing approval in select markets. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks associated with FDA approval as well as additional, presently unanticipated, risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
Applications for regulatory approval, including a marketing authorization application, or MAA, for marketing new drugs in the European Economic Area (which consists of the 27 Member States of the EU, Iceland, Liechtenstein and Norway), or EEA, must be supported by extensive clinical and pre-clinical data, as well as extensive information regarding chemistry, manufacturing and controls, or CMC, to demonstrate the safety and effectiveness of the applicable medicine candidate. The number and types of pre-clinical studies and clinical trials that will be required for regulatory approval varies depending on the medicine candidate, the disease or the condition that the medicine candidate is designed to target and the regulations applicable to any particular medicine candidate. Despite the time and expense associated with pre-clinical and clinical studies, failure can occur at any stage, and we could encounter problems that cause us to repeat or perform additional pre-clinical studies, CMC studies or clinical trials. Regulatory authorities could delay, limit or deny approval of a medicine candidate for many reasons, including because they:
52
Even if we believe that data collected from our pre-clinical studies, CMC studies and clinical trials of our medicine candidates are promising and that our information and procedures regarding CMC are sufficient, our data may not be sufficient to support marketing approval by regulatory authorities, or regulatory interpretation of these data and procedures may be unfavorable. Even if approved, medicine candidates may not be approved for all indications requested and such approval may be subject to limitations on the indicated uses for which the medicine may be marketed, restricted distribution methods or other limitations. Our business and reputation may be harmed by any failure or significant delay in obtaining regulatory approval for the sale of any of our medicine candidates. We cannot predict when or whether regulatory approval will be obtained for any medicine candidate we develop.
The ultimate approval and commercial marketing of any of our medicines in additional indications or geographies is subject to substantial uncertainty. Failure to gain additional regulatory approvals would limit the potential revenues and value of our medicines and could cause our share price to decline.
The United Kingdom’s withdrawal from the EU may have a negative effect on global economic conditions, financial markets and our business, which could reduce the price of our common shares.
Following the result of a referendum in 2016, the United Kingdom, or UK, left the EU on January 31, 2020, commonly referred to as Brexit. Pursuant to the formal withdrawal arrangements agreed between the UK and the EU, the UK was subject to a transition period until December 31, 2020 during which EU rules continued to apply. The UK and the EU have signed a EU-UK Trade and Cooperation Agreement, or TCA, which became provisionally applicable on January 1, 2021 and entered into force on May 1, 2021. This agreement provides details on how some aspects of the UK and EU’s relationship will operate going forwards however there are still many uncertainties. The TCA primarily focuses on ensuring free trade between the EU and the UK in relation to goods, including medicinal products. Although the body of the TCA includes general terms which apply to medicinal products, greater detail on sector-specific issues is provided in an Annex to the TCA. The Annex provides a framework for the recognition of Good Manufacturing Practice, or GMP, inspections and for the exchange and acceptance of official GMP documents. The regime does not, however, extended to procedures such as batch release certification. Among the changes that have occurred are that Great Britain (England, Scotland and Wales) is treated as a “third country”, a country that is not a member of the EU and whose citizens do not enjoy the EU right to free movement. Northern Ireland continues to follow many aspects of the EU regulatory rules, particularly in relation to trade in goods. As part of the TCA, the EU and the UK recognize GMP inspections carried out by the other party and the acceptance of official GMP documents issued by the other party. The TCA also encourages, although it does not oblige, the parties to consult one another on proposals to introduce significant changes to technical regulations or inspection procedures. Among the areas of absence of mutual recognition are batch testing and batch release. The UK has unilaterally agreed to accept EU batch testing and batch release. However, the EU continues to apply EU laws that require batch testing and batch release to take place in the EU territory. This means that medicinal products that are tested and released in the UK must be retested and re-released when entering the EU market for commercial use. As it relates to marketing authorizations, Great Britain has a separate regulatory submission process, approval process and a separate national marketing authorization. Northern Ireland continues, however, to be covered by the marketing authorizations granted by the European Commission. For example, the scope of a marketing authorization for a medicinal product granted by the European Commission or by the competent authorities of EU Member States will no longer encompass Great Britain (England, Scotland and Wales). In these circumstances, a separate marketing authorization granted by the UK competent authorities is required to place medicinal products on the market in Great Britain. Northern Ireland continues, however, to be covered by the marketing authorizations granted by the European Commission.
53
Since a significant proportion of the regulatory framework in the UK applicable to our business and our medicines is derived from EU directives and regulations, Brexit has materially impacted the regulatory regime with respect to the development, manufacture, importation, approval and commercialization of our medicines and medicine candidates in the UK and the EU, now that UK legislation has the potential to diverge from EU legislation. All of these changes could increase our costs and otherwise adversely affect our business. In addition, we may be required to pay taxes or duties or be subjected to other hurdles in connection with the importation of our medicine candidates into the EU. If any of these outcomes occur, we may be forced to restrict or delay efforts to seek regulatory approval in the UK or the EU for our medicine candidates, or incur significant additional expenses to operate our business, which could significantly and materially harm or delay our ability to generate revenues or achieve profitability of our business. Any further changes in international trade, tariff and import/export regulations as a result of Brexit or otherwise may impose unexpected duty costs or other non-tariff barriers on us. These developments, or the perception that any of them could occur, may significantly reduce global trade and, in particular, trade between the impacted nations and the UK. It is also possible that Brexit may negatively affect our ability to attract and retain employees, particularly those from the EU. The regulatory changes that are a result of Brexit may also materially impact upon the development, manufacture, importation, approval and commercialization of our medicines in the EEA, should any development or manufacture of these medicines take place in the UK.
Since Great Britain is no longer covered by the EU’s procedures for the grant of marketing authorizations, our medicine candidates require a separate marketing authorization for Great Britain, which involves additional administrative burden. Any delay in obtaining, or an inability to obtain, any marketing approvals, as a result of Brexit or otherwise, could prevent us from or delay us commercializing our medicine candidates in the UK and/or the EEA and restrict our ability to generate revenue and achieve and sustain profitability. If any of these outcomes occur, we may be forced to restrict or delay efforts to seek regulatory approval in the UK and/or EEA for our medicine candidates, which could significantly and materially harm our business.
In the short term there is a risk of disrupted import and export processes due to a lack of administrative processing capacity by the respective UK and EU customs agencies that may delay time-sensitive shipments and may negatively impact our product supply chain.
We are subject to ongoing obligations and continued regulatory review by the FDA and comparable foreign regulatory authorities, and we may be subject to penalties and litigation and large incremental expenses if we fail to comply with regulatory requirements or experience problems with our medicines.
Our current approved medicines (and our medicine candidates, if approved) are subject to extensive ongoing obligations and continued regulatory review with respect to many operational aspects including our manufacturing processes, labeling, packaging, distribution, storage, import, export, safety surveillance, adverse event monitoring and reporting, dispensation, advertising, promotion and recordkeeping. These requirements include submissions of safety and other post-marketing information and reports, ongoing maintenance of medicine registration and continued compliance with current good manufacturing practices, or cGMPs, good clinical practices, or GCPs, International Council for Harmonisation, or ICH, guidelines, good pharmacovigilance practice, good distribution practices and good laboratory practices, or GLPs, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for all of our medicines in clinical development and for any clinical trials that we conduct post-approval.
54
Later discovery of previously unknown problems with a medicine or medicine candidate, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
Moreover, existing regulatory approvals and any future regulatory approvals that we obtain will be subject to limitations on the approved indicated uses and patient populations for which our medicines may be marketed, the conditions of approval, requirements for potentially costly, post-market testing, including Phase 4 clinical trials, and requirements for surveillance to monitor the safety and efficacy of the medicines. Physicians nevertheless may prescribe our medicines to their patients in a manner that is inconsistent with the approved label or that is off-label. Positive clinical trial results in any of our medicine development programs increase the risk that approved biopharmaceutical forms of the same active biopharmaceutical ingredients, or APIs, may be used off-label in those indications. A significant number of biopharmaceutical companies have been the target of inquiries and investigations by various U.S. federal and state regulatory, investigative, prosecutorial and administrative entities or comparable foreign regulatory authorities in connection with the promotion of medicines for off-label uses and other sales practices. These investigations have alleged violations of various U.S. federal and state laws and regulations, including claims asserting antitrust violations, violations of the Food, Drug and Cosmetic Act, or FDCA, anti-kickback laws, and other alleged violations in connection with the promotion of medicines for unapproved uses, pricing and Medicare and/or Medicaid reimbursement and comparable foreign regulatory requirements. If we are found to have improperly promoted off-label uses of approved medicines, we may be subject to significant sanctions, civil and criminal fines and injunctions prohibiting us from engaging in specified promotional conduct.
In addition, engaging in improper promotion of our medicines for off-label uses in the United States can subject us to false claims litigation under federal and state statutes. These false claims statutes in the United States include the federal False Claims Act, which allows any individual to bring a lawsuit against a biopharmaceutical company on behalf of the federal government alleging submission of false or fraudulent claims or causing to present such false or fraudulent claims for payment by a federal program such as Medicare or Medicaid. Growth in false claims litigation has increased the risk that a biopharmaceutical company will have to defend a false claim action, pay civil money penalties, settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations and be excluded from Medicare, Medicaid and other federal and state healthcare programs.
The regulations, policies or guidance of regulatory authorities may change and new or additional statutes or government regulations may be enacted that could prevent or delay regulatory approval of our medicine candidates or further restrict or regulate post-approval activities. For example, there remains a substantial amount of uncertainty regarding internet and social media promotion of regulated medical products. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from pending or future legislation or administrative action, either in the United States or abroad. If we are unable to achieve and maintain regulatory compliance, we will not be permitted to market our drugs, which would materially adversely affect our business, results of operations and financial condition.
55
We have rights to medicines in certain jurisdictions but have little or no control over third parties that have rights to commercialize those medicines in other jurisdictions, which could adversely affect our commercialization of these medicines.
Following our sale of the rights to RAVICTI outside of North America to Medical Need Europe AB, part of the Immedica Group, or Immedica, Immedica has marketing and distribution rights to RAVICTI in those regions. Following our sale of the rights to PROCYSBI in Europe, the Middle East and Africa, or EMEA, regions to Chiesi Farmaceutici S.p.A., or Chiesi, in June 2017, or the Chiesi divestiture, Chiesi has marketing and distribution rights to PROCYSBI in the EMEA regions. MTPC has rights to develop and commercialize UPLIZNA for NMOSD as well as other potential future indications in Japan and certain other countries in Asia. Hansoh has rights to develop and commercialize UPLIZNA for NMOSD as well as other potential future indications in China. We have little or no control over Immedica’s activities with respect to RAVICTI outside of North America, over Chiesi’s activities with respect to PROCYSBI in the EMEA, or over MTPC’s or Hansoh’s activities with respect to UPLIZNA in the certain countries in Asia, even though those activities could impact our ability to successfully commercialize these medicines. For example, Immedica or its assignees, Chiesi or its assignees, MTPC or Hansoh or their respective assignees can make statements or use promotional materials with respect to RAVICTI, PROCYSBI or UPLIZNA, respectively, outside of the United States that are inconsistent with our positioning of the medicines in the United States, and could sell RAVICTI, PROCYSBI or UPLIZNA, respectively, in foreign countries at prices that are dramatically lower than the prices we charge in the United States. These activities and decisions, while occurring outside of the United States, could harm our commercialization strategy in the United States. In addition, medicine recalls or safety issues with these medicines outside the United States, even if not related to the commercial medicine we sell in the United States, could result in serious damage to the brand in the United States and impair our ability to successfully market them. We also rely on Immedica, Chiesi, MTPC and Hansoh, or their assignees to provide us with timely and accurate safety information regarding the use of these medicines outside of the United States, as we have or will have limited access to this information ourselves.
We rely on third parties to manufacture commercial supplies of all of our medicines, and we currently intend to rely, in whole or in part, on third parties to manufacture commercial supplies of any other approved medicines. The commercialization of any of our medicines could be stopped, delayed or made less profitable if those third parties fail to provide us with sufficient quantities of medicine or fail to do so at acceptable quality levels or prices or fail to maintain or achieve satisfactory regulatory compliance.
The facilities used by our third-party manufacturers to manufacture our medicines and medicine candidates must be approved by the applicable regulatory authorities. We do not control the manufacturing processes of third-party manufacturers and are currently completely dependent on our third-party manufacturing partners.
We rely on AGC Biologics A/S (formerly known as CMC Biologics A/S), or AGC Biologics, as our exclusive manufacturer of the TEPEZZA drug substance and Catalent Indiana, LLC, or Catalent, and Patheon Pharmaceuticals Inc., or Patheon (the contract development and manufacturing services organization of Thermo Fisher Scientific), as our manufacturers for TEPEZZA drug product. In December 2020, pursuant to the Defense Production Act of 1950, or DPA, Catalent was ordered to prioritize certain COVID-19 vaccine manufacturing, resulting in the cancellation of previously guaranteed and contracted TEPEZZA drug product manufacturing slots, which were required to maintain TEPEZZA supply. To offset the reduced slots allowed by the DPA and Catalent, we accelerated plans to increase the production scale of TEPEZZA drug product. In March 2021, the FDA approved a prior approval supplement to the TEPEZZA biologics license application, or BLA (which was previously approved in January 2020), giving us authorization to manufacture more TEPEZZA drug product in a batch resulting in an increased number of vials with each manufacturing slot. We commenced resupply of TEPEZZA to the market in April 2021, but we cannot guarantee that our future contracted TEPEZZA manufacturing slots at Catalent will not be rescheduled or canceled as a result of additional U.S. government-mandated COVID-19 vaccine production orders. In December 2021, we received FDA approval for our second drug product filling site at Patheon, on lines where COVID-19 products are not filled to ensure more reliable and consistent supply of TEPEZZA. While we do not currently expect COVID-19 vaccine activities by our contract manufacturers to impact the future supply of our medicines, similar circumstances to those at Catalent in December 2020 could arise in the future and could result in supply disruption to our medicines. Further, if AGC Biologics fails to supply TEPEZZA drug substance or if Catalent and Patheon fail to supply TEPEZZA drug product for a period beyond our current expectation or any manufacturer is otherwise unable to meet our volume requirements due to unexpected market demand for TEPEZZA, it may lead to further TEPEZZA supply constraints.
56
We rely on NOF Corporation, or NOF, as our exclusive supplier of the PEGylation agent that is a critical raw material in the manufacture of KRYSTEXXA. If NOF fails to supply such PEGylation agent, it may lead to KRYSTEXXA supply constraints. We rely on AstraZeneca UK Limited for the manufacture of clinical and commercial supplies of UPLIZNA, and for clinical and nonclinical supplies of the other medicine candidates acquired in the Viela acquisition. In addition, we rely on an exclusive supply agreement with Boehringer Ingelheim Biopharmaceuticals GmbH, or Boehringer Ingelheim Biopharmaceuticals, for manufacturing and supply of ACTIMMUNE. ACTIMMUNE is manufactured by starting with cells from working cell bank samples which are derived from a master cell bank. We and Boehringer Ingelheim Biopharmaceuticals separately store multiple vials of the master cell bank. In the event of catastrophic loss at our or Boehringer Ingelheim Biopharmaceuticals’ storage facility, it is possible that we could lose multiple cell banks and have the manufacturing capacity of ACTIMMUNE severely impacted by the need to substitute or replace the cell banks.
In July 2021, we purchased a drug product biologics manufacturing facility in Waterford, Ireland, which is intended to be an additional source of manufacturing to supplement the capabilities of our third-party drug product manufacturers. We are in the process of completing the build-out and validation of this facility and assuming timely receipt of regulatory approvals, we expect the first medicine manufactured for commercial use at the facility to be approved for release in the second half of 2023. In August 2022, we submitted a planning application to build a drug substance biologics manufacturing facility adjacent to our existing drug product biologics manufacturing facility in Waterford, Ireland. Based on our current operating plan, we do not anticipate making significant investments in building a drug substance biologics manufacturing facility during 2023. To the extent that we proceed with building a drug substance biologics manufacturing facility in the future, we have minimal experience in building, developing, validating, obtaining regulatory approval for or running manufacturing facilities and we therefore may not be successful in these activities. In particular, we may experience delays and unforeseen expenses in connection with building a drug substance biologics manufacturing facility or hiring qualified personnel to operate such a facility. Even if we are successful in producing medicines at the Waterford facilities for commercial sale once we receive the required regulatory approvals, we expect to remain dependent on our third-party drug product filling and drug substance manufacturing partners in the near-term and to a lesser extent in the medium/longer term, but we plan to always dual source our strategic medicines.
If we or any of our third-party manufacturers cannot successfully manufacture material that conforms to our specifications and the applicable regulatory authorities’ strict regulatory requirements, or pass regulatory inspection, we or our third-party manufacturers will not be able to secure or maintain regulatory approval for the manufacturing facilities. In addition, we have no direct control over the ability of third-party manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or any other applicable regulatory authorities do not approve these facilities for the manufacture of our medicines or if they withdraw any such approval in the future, or if our suppliers or third-party manufacturers decide they no longer want to supply our primary active ingredients or manufacture our medicines, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our medicines. To the extent our manufacturing facility or that of any third-party manufacturers that we engage with respect to our medicines are different from those currently being used for commercial supply in the United States, the FDA will need to approve such facilities prior to our sale of any medicine using these facilities.
Although we have entered into supply agreements for the manufacture and packaging of our medicines, our manufacturers may not perform as agreed or may terminate their agreements with us. We currently rely on single source suppliers for certain of our medicines. If our manufacturers terminate their agreements with us, we may have to qualify new back-up manufacturers. We rely on safety stock to mitigate the risk of our current suppliers electing to cease producing bulk drug product or ceasing to do so at acceptable prices and quality. However, we can provide no assurance that such safety stocks would be sufficient to avoid supply shortfalls in the event we have to identify and qualify new contract manufacturers.
The manufacture of medicines requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of medicines often encounter difficulties in production, particularly in scaling up and validating initial production. These problems include difficulties with production costs and yields, quality control, including stability of the medicine, quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if microbial, viral or other contaminations are discovered in the medicines or in the manufacturing facilities in which our medicines are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. We cannot assure that issues relating to the manufacture of any of our medicines will not occur in the future. Additionally, we or our third-party manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If we or our third-party manufacturers were to encounter any of these difficulties, or our third-party manufacturers otherwise fail to comply with their contractual obligations, our ability to commercialize our medicines or provide any medicine candidates to patients in clinical trials would be jeopardized.
57
Any delay or interruption in our ability to meet commercial demand for our medicines will result in the loss of potential revenues and could adversely affect our ability to gain market acceptance for these medicines. In addition, any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely.
Failures or difficulties faced at any level of our supply chain, including any further potential disruption caused by the COVID-19 pandemic, could materially adversely affect our business and delay or impede the development and commercialization of any of our medicines or medicine candidates and could have a material adverse effect on our business, results of operations, financial condition and prospects.
We face significant competition from other biopharmaceutical companies, including those marketing generic medicines and our operating results will suffer if we fail to compete effectively.
The biopharmaceutical industries are intensely competitive. We have competitors both in the United States and international markets, including major multinational biopharmaceutical companies, biotechnology companies and universities and other research institutions. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development, or R&D, staff, experienced marketing and manufacturing organizations and well-established sales forces. Additional consolidations in the biopharmaceutical industries may result in even more resources being concentrated in our competitors and we will have to find new ways to compete and may have to potentially merge with or acquire other businesses to stay competitive. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or in-licensing on an exclusive basis, medicines that are more effective and/or less costly than our medicines.
Although TEPEZZA does not face direct competition, other therapies, such as corticosteroids, have been used on an off-label basis to alleviate some of the symptoms of TED. While these therapies have not proved effective in treating the underlying disease, and carry with them potential significant side effects, their off-label use could reduce or delay treatment with TEPEZZA among the addressable patient population. Viridian Therapeutics, Inc., or Viridian, is pursuing development of three anti-IGF-1R monoclonal antibodies for TED (VRDN-001, VRDN-002 and VRDN-003). In August 2022, Viridian announced positive initial clinical data from the first cohort, 10mg/kg, of the ongoing Phase 1/2 clinical trial for an IGF-1R monoclonal antibody VRDN-001, in patients with TED. On November 14, 2022 and January 8, 2023, Viridian announced positive data for their second and third cohorts, 20mg/kg and 3mg/kg, respectively, of the ongoing Phase 1/2 trial showing improvements in signs and symptoms of TED after two infusions. In addition, in the fourth quarter of 2022, Viridian enrolled the first patient in its THRIVE Phase 3 trial of VRDN-001 with active TED. Viridian has initiated a Phase 2 trial with a subcutaneous version of VRDN-002 in the fourth quarter of 2022. Viridian also announced plans to file an investigational new drug, or IND, application for VRDN-003 in the second quarter of 2023 and subsequently begin a Phase 1 trial in healthy volunteers. In addition, Sling Therapeutics, Inc. is conducting a Phase 2b study of an oral IGF-1R for the treatment of moderate-to-severe TED and Novartis AG is conducting a Phase 3 trial of Cosentyx® (secukinumab, interleukin-17A inhibitor) in moderate-to-severe TED. Immunovant Inc., or Immunovant, initiated two Phase 3 clinical trials of a fully human anti-FcRn monoclonal antibody candidate for the treatment of active TED, also referred to as Graves’ ophthalmopathy, in the fourth quarter of 2022. Immunovant also began patient recruitment in the Phase 3 trial of batoclimab (FcRni). On January 5, 2023, Acelyrin, Inc., or Acelyrin, announced the acquisition of ValenzaBio Inc, or ValenzaBio. Previously, ValenzaBio received IND clearance and subsequently had begun a Phase 1 trial in the first half of 2022 with VB421, an anti-IGF-1R monoclonal antibody designed for subcutaneous use. In January 2023, Acelyrin also initiated a Phase 1/2 trial in active TED. Argenx SE announced a registrational trial of efgartigimod for the treatment of TED.
While KRYSTEXXA faces limited direct competition, a number of competitors have medicines in clinical trials, including Selecta Biosciences Inc., or Selecta, which has initiated a Phase 3 clinical program of a candidate for the treatment of chronic refractory gout. In September 2020, Selecta announced topline clinical data that did not meet the primary endpoint or demonstrate statistical superiority for its Phase 2 trial that compared its candidate, which includes an immunomodulator, to KRYSTEXXA alone. In July 2020, Selecta and Swedish Orphan Biovitrum AB, or Sobi, entered into a strategic licensing agreement under which Sobi will assume responsibility for certain development, regulatory, and commercial activities for this candidate. In August 2022, Selecta announced the completion of enrollment for both DISSOLVE trials, the two clinical studies of the Phase 3 DISSOLVE development program of SEL-212 for chronic refractory gout. SEL-212 is a combination of Selecta’s ImmTOR immune tolerance platform and a therapeutic uricase enzyme (pegadricase).
58
RAVICTI could face competition from a few alternative medicine and treatment options that have been recently approved or are in development, including Pheburane®, a taste-masked formulation of sodium phenylbutyrate for which Medunik USA received approval from the FDA in June 2022, a gene-therapy candidate by Ultragenyx Pharmaceutical Inc., OlpruvaTM, a taste-masked formulation of sodium phenylbutyrate for which ACER Therapeutics Inc. received approval from the FDA in December 2022, an enzyme replacement for a specific UCD subtype (ARG) by Aeglea Bio Therapeutics Inc. and a mRNA-based therapeutic for a specific UCD subtype (OTC) by Arcturus Therapeutics Holdings Inc. PROCYSBI faces competition from Cystagon® (immediate-release cysteamine bitartrate capsules) for the treatment of cystinosis. Additionally, we are also aware that AVROBIO, Inc. has a gene therapy candidate in development for the treatment of cystinosis.
UPLIZNA faces competition from eculizumab, marketed as Soliris® by AstraZeneca plc, or AstraZeneca, and satralizumab, marketed as EnspryngTM by Genentech/Chugai Pharmaceuticals Co., Ltd., a subsidiary of F. Hoffmann-La Roche Ltd., each for the treatment of patients with NMOSD. AstraZeneca announced positive primary endpoint results from its Phase 3 trial with Ultomiris® (ravulizumab) in NMOSD and, if approved for this indication, UPLIZNA could face additional competition. Ultomiris is currently under regulatory review in both the United States and EU with potential approval in the first half of 2023. UPLIZNA also faces competition from rituximab, an off-label treatment that has been used for years to treat NMOSD given the lack of an approved medicine for this disease prior to 2019. Other novel treatments are under development for NMOSD, including Phase 3 candidates being developed by Beijing Mabworks Biotech Co. Ltd. and RemeGen Co. Ltd., and Phase 2 candidates, including a candidate being developed by Chord Therapeutics SA/Merck KGaA.
We have also entered into settlement and license agreements that may allow certain of our competitors to sell generic versions of certain of our medicines in the United States, subject to the terms of such agreements. We granted (i) non-exclusive licenses to manufacture and commercialize generic versions of RAVICTI in the United States after July 1, 2025 and (ii) non-exclusive license to manufacture and commercialize a generic version of PROCYSBI in the United States after March 31, 2030. Under certain circumstances, each of these licenses could become effective on an earlier date.
ACTIMMUNE is the only medicine currently approved by the FDA specifically for the treatment of CGD and SMO. While there are additional or alternative approaches used to treat patients with CGD and SMO, there are currently no medicines on the market that compete directly with ACTIMMUNE. A widely accepted protocol to treat CGD in the United States is the use of concomitant “triple prophylactic therapy” comprising ACTIMMUNE, an oral antibiotic agent and an oral antifungal agent. However, the FDA-approved labeling for ACTIMMUNE does not discuss this “triple prophylactic therapy,” and physicians may choose to prescribe one or both of the other modalities in the absence of ACTIMMUNE. Because of the immediate and life-threatening nature of SMO, the preferred treatment option for SMO is often to have the patient undergo a bone marrow transplant which, if successful, will likely obviate the need for further use of ACTIMMUNE in that patient. Likewise, the potentially curative treatment of bone marrow transplants for patients with CGD is becoming more prevalent, which could have a material adverse effect on sales of ACTIMMUNE and its profitability. We are aware of a number of research programs investigating the potential of gene therapy as a possible cure for CGD. Additionally, other companies may be pursuing the development of medicines and treatments that target the same diseases and conditions which ACTIMMUNE is currently approved to treat. As a result, it is possible that our competitors may develop new medicines that manage CGD or SMO more effectively, cost less or possibly even cure CGD or SMO. In addition, the U.S. patents covering ACTIMMUNE expired on August 30, 2022, and although we are not currently aware of any biosimilar to ACTIMMUNE under development, the development and commercialization of any competing medicines or the discovery of any new alternative treatment for CGD or SMO could have a material adverse effect on sales of ACTIMMUNE and its profitability.
59
BUPHENYL’s composition of matter patent protection and orphan drug exclusivity have expired. Because BUPHENYL has no regulatory exclusivity or listed patents, there is nothing to prevent a competitor from submitting an ANDA for a generic version of BUPHENYL and receiving FDA approval. Generic versions of BUPHENYL to date have been priced at a discount relative to RAVICTI, and physicians, patients, or payers may decide that this less expensive alternative is preferable to RAVICTI. If this occurs, sales of RAVICTI could be materially reduced, but we would nevertheless be required to make royalty payments to Bausch Health Companies Inc. (formerly Ucyclyd Pharma, Inc.), or Bausch, and another external party, at the same royalty rates. While Bausch and its affiliates are generally contractually prohibited from developing or commercializing new medicines, anywhere in the world, for the treatment of UCD or hepatic encephalopathy, or HE, which are chemically similar to RAVICTI, they may still develop and commercialize medicines that compete with RAVICTI. For example, medicines approved for indications other than UCD and HE may still compete with RAVICTI if physicians prescribe such medicines off-label for UCD or HE. We are also aware that Recordati S.p.A (formerly known as Orphan Europe SARL), or Recordati, received FDA approval in January 2021 for carglumic acid for the treatment of acute hyperammonemia due to propionic acidemia or methylmalonic acidemia. Carglumic acid is also approved for chronic and acute hyperammonemia due to N-acetylglutamate synthase deficiency, a rare UCD subtype. RAVICTI may face additional competition from this compound.
The availability and price of our competitors’ medicines could limit the demand, and the price we are able to charge, for our medicines. We will not successfully execute on our business objectives if the market acceptance of our medicines is inhibited by price competition, if physicians are reluctant to switch from existing medicines to our medicines, or if physicians switch to other new medicines or choose to reserve our medicines for use in limited patient populations.
In addition, established biopharmaceutical companies may invest heavily to accelerate discovery and development of novel compounds or to acquire novel compounds that could make our medicines obsolete. Our ability to compete successfully with these companies and other potential competitors will depend largely on our ability to leverage our experience in clinical, regulatory and commercial development to:
Our biologic medicines and candidates may face biosimilar competition sooner than anticipated.
Even if we are successful in achieving regulatory approval to commercialize a biologic medicine candidate ahead of our competitors, our biologic medicines and candidates may face competition from biosimilar products. In the United States, the Biologics Price Competition and Innovation Act of 2009, or BPCIA, created an abbreviated pathway for FDA approval of biosimilar and interchangeable biological products based on a previously licensed reference product. Under the BPCIA, an application for a biosimilar biological product cannot be approved by the FDA until 12 years after the original reference biological product was approved under a BLA. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty and any such processes could have a material adverse effect on the future commercial prospects for our biologic medicines and candidates.
60
We believe that any of our candidates approved as a biological product under a BLA should qualify for the 12-year period of exclusivity available to reference biological products. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our candidates to be reference biological products pursuant to its interpretation of the exclusivity provisions of the BPCIA for competing products, potentially creating the opportunity for biosimilar competition sooner than anticipated. Moreover, the extent to which a biosimilar product, once approved, will be substituted for any one of our reference medicines in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing including whether a future competitor seeks an interchangeability designation for a biosimilar of one of our medicines. Under the BPCIA as well as state pharmacy laws, only interchangeable biosimilar products are considered substitutable for the reference biological product without the intervention of the health care provider who prescribed the original biological product. However, as with all prescribing decisions made in the context of a patient-provider relationship and a patient’s specific medical needs, healthcare providers are not restricted from prescribing biosimilar products in an off-label manner. In addition, a competitor could decide to forego the abbreviated approval pathway available for biosimilar products and to submit a full BLA for product licensure after completing its own preclinical studies and clinical trials. In such a situation, any exclusivity to which we may be eligible under the BPCIA would not prevent the competitor from marketing its biological product as soon as it is approved.
Data and market exclusivity is available in relation to grant of certain types of marketing authorization for medicinal products in the EU. Upon grant of a marketing authorization, innovative medicinal products are generally entitled to benefit from eight years of data exclusivity and 10 years of market exclusivity in the EU. Data exclusivity, if granted, prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product. After this period a generic or biosimilar marketing authorization application can be submitted, and the innovator’s data may be referenced. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EU until 10 years have elapsed from grant of the initial marketing authorization of the reference product in the EU. The overall ten year period may, occasionally, be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical/biological entity, and products may not qualify for data exclusivity. In the EU, the European Commission has granted marketing authorizations for several biosimilar products pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued over the past few years. In addition, companies may be developing biosimilar products in other countries that could compete with our medicines, if approved.
If competitors are able to obtain marketing approval for biosimilars referencing our medicine candidates, if approved, our future medicines may become subject to competition from such biosimilars, whether or not they are designated as interchangeable, with the attendant competitive pressure and potential adverse consequences. Such competitive products may be able to immediately compete with us in each indication for which our medicine candidates may have received approval.
61
If we are unable to maintain or realize the benefits of orphan drug exclusivity, we may face increased competition with respect to certain of our medicines.
Under the Orphan Drug Act of 1983, the FDA may designate a medicine as an orphan drug if it is a drug intended to treat a rare disease or condition affecting fewer than 200,000 people in the United States. A company that first obtains FDA approval for a designated orphan drug for the specified rare disease or condition receives orphan drug marketing exclusivity for that drug for a period of seven years from the date of its approval. PROCYSBI received ten years of market exclusivity for the treatment of nephropathic cystinosis, through 2023, as an orphan drug in the EU, EEA and UK. PROCYSBI received seven years of market exclusivity, until December 22, 2024, for patients one year of age to less than two years of age as an orphan drug in the United States. TEPEZZA has been granted orphan drug exclusivity for treatment of active (dynamic) phase Graves’ ophthalmopathy, which we expect will provide orphan drug marketing exclusivity in the United States until January 2027. In addition, UPLIZNA was granted orphan drug exclusivity for the treatment of NMOSD, which we expect will provide orphan drug marketing exclusivity in the United States until June 2027. However, despite orphan drug exclusivity, the FDA can still approve another drug containing the same active ingredient and used for the same orphan indication if it determines that a subsequent drug is safer, more effective or makes a major contribution to patient care, and orphan exclusivity can be lost if the orphan drug manufacturer is unable to ensure that a sufficient quantity of the orphan drug is available to meet the needs of patients with the rare disease or condition. Orphan drug exclusivity may also be lost if the FDA later determines that the initial request for designation was materially defective. In addition, orphan drug exclusivity does not prevent the FDA from approving competing drugs for the same or similar indication containing a different active ingredient. Outside the United States, similar limitations regarding orphan drugs also exist. If orphan drug exclusivity is lost and we were unable to successfully enforce any remaining patents covering the applicable medicine, we could be subject to generic competition and revenues from the medicine could decrease materially.
In addition, if a subsequent drug is approved for marketing for the same or a similar indication as our medicines despite orphan drug exclusivity, we may face increased competition and lose market share with respect to these medicines.
Our business operations may subject us to numerous commercial disputes, claims and/or lawsuits and such litigation may be costly and time-consuming and could materially and adversely impact our financial position and results of operations.
Operating in the biopharmaceutical industry, particularly the commercialization of medicines, involves numerous commercial relationships, complex contractual arrangements, uncertain intellectual property rights, potential product liability and other aspects that create heightened risks of disputes, claims and lawsuits. In particular, we may face claims related to the safety of our medicines, intellectual property matters, employment matters, tax matters, commercial disputes, competition, sales and marketing practices, environmental matters, personal injury, insurance coverage and acquisition or divestiture-related matters. From time to time we are involved in disputes with distributors, PBMs and licensing partners regarding our rights and performance of obligations under contractual arrangements. Any commercial dispute, claim or lawsuit may divert management’s attention away from our business, we may incur significant expenses in addressing or defending any commercial dispute, claim or lawsuit, and we may be required to pay damage awards or settlements or become subject to equitable remedies that could adversely affect our operations and financial results.
Litigation related to these disputes may be costly and time-consuming and could materially and adversely impact our financial position and results of operations if resolved against us.
A variety of risks associated with operating our business internationally could adversely affect our business.
We have operations in the United States, Ireland and in multiple other jurisdictions, and are pursuing a global expansion strategy, which includes bringing TEPEZZA to patients with TED and UPLIZNA to adult patients with NMOSD outside of the United States.
We face risks associated with our international operations, including possible unfavorable political, tax and labor conditions, which could harm our business.
62
We are subject to numerous risks associated with international business activities, including:
Our business activities outside of the United States are subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. The FCPA and similar anti-corruption laws generally prohibit offering, promising, giving, or authorizing others to give anything of value, either directly or indirectly, to non-U.S. government officials in order to improperly influence any act or decision, secure any other improper advantage, or obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the company and to devise and maintain an adequate system of internal accounting controls. As described above, our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other countries, the health care providers who prescribe biopharmaceuticals are employed by their government, and the purchasers of biopharmaceuticals are government entities; therefore, any dealings with these prescribers and purchasers may be subject to regulation under the FCPA. Recently the SEC and the U.S. Department of Justice, or DOJ, have increased their FCPA enforcement activities with respect to biopharmaceutical companies. In addition, under the Dodd–Frank Wall Street Reform and Consumer Protection Act, private individuals who report to the SEC original information that leads to successful enforcement actions may be eligible for a monetary award. We are engaged in ongoing efforts that are designed to ensure our compliance with these laws, including due diligence, training, policies, procedures and internal controls. However, there is no certainty that all employees and third-party business partners (including our distributors, wholesalers, agents, contractors, and other partners) will comply with anti-bribery laws. In particular, we do not control the actions of manufacturers and other third-party agents, although we may be liable for their actions. Violation of these laws may result in civil or criminal sanctions, which could include monetary fines, criminal penalties, and disgorgement of past profits, which could have a material adverse impact on our business and financial condition.
We are subject to tax audits around the world, and such jurisdictions may assess additional income tax against us. Although we believe our tax positions are reasonable, the final determination of tax audits could be materially different from our recorded income tax provisions and accruals. The ultimate results of an audit could have a material adverse effect on our operating results or cash flows in the period or periods for which that determination is made and could result in increases to our overall tax expense in subsequent periods.
63
These and other risks associated with our international operations may materially adversely affect our business, financial condition and results of operations.
If we fail to develop or acquire other medicine candidates or medicines, our business and prospects would be limited.
A key element of our strategy is to develop or acquire and commercialize a portfolio of other medicines or medicine candidates in addition to our current medicines, through business or medicine acquisitions. Because we do not engage in proprietary drug discovery, the success of this strategy depends in large part upon the combination of our regulatory, development and commercial capabilities and expertise and our ability to identify, select and acquire approved or clinically enabled medicine candidates for therapeutic indications that complement or augment our current medicines, or that otherwise fit into our development or strategic plans on terms that are acceptable to us. Identifying, selecting and acquiring promising medicines or medicine candidates requires substantial technical, financial and human resources expertise. Efforts to do so may not result in the actual acquisition or license of a particular medicine or medicine candidate, potentially resulting in a diversion of our management’s time and the expenditure of our resources with no resulting benefit. In addition, under the transaction agreement with Amgen, we are generally required to conduct our business in the ordinary course, consistent with past practice and are restricted from taking certain specified actions absent Amgen’s prior written consent. If the pending Transaction with Amgen is not consummated and we are unable to identify, select and acquire suitable medicines or medicine candidates from third parties or acquire businesses at valuations and on other terms acceptable to us, or if we are unable to raise capital required to acquire businesses or new medicines, our business and prospects will be limited.
Moreover, any medicine candidate we acquire may require additional, time-consuming development or regulatory efforts prior to commercial sale or prior to expansion into other indications, including pre-clinical studies if applicable, and extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All medicine candidates are prone to the risk of failure that is inherent in biopharmaceutical medicine development, including the possibility that the medicine candidate will not be shown to be sufficiently safe and/or effective for approval by regulatory authorities. In addition, we cannot assure that any such medicines that are approved will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace or be more effective or desired than other commercially available alternatives.
In addition, if we fail to successfully commercialize and further develop our medicines, there is a greater likelihood that we will fail to successfully develop a pipeline of other medicine candidates to follow our existing medicines or be able to acquire other medicines to expand our existing portfolio, and our business and prospects would be harmed.
We have experienced growth and expanded the size of our organization substantially, including in connection with our acquisition transactions, and we may experience difficulties in managing this growth as well as potential additional growth in connection with future medicine, development program or company acquisitions.
As of December 31, 2013, we employed approximately 300 full-time employees as a consolidated entity. As of December 31, 2022, we employed approximately 2,115 full-time employees, including approximately 380 sales representatives and sales management, representing a substantial change to the size of our organization. We have also experienced, and may continue to experience, turnover of the sales representatives that we hired or will hire in connection with the commercialization of our medicines, requiring us to hire and train new sales representatives. We have experienced additional turnover and difficulties in hiring new employees as a result of the pending Transaction with Amgen. Our management, personnel, systems and facilities currently in place may not be adequate to support anticipated growth, and we may not be able to retain or recruit qualified personnel in the future due to competition for personnel among biopharmaceutical businesses.
64
As our commercialization plans and strategies continue to develop, and particularly as we execute on our strategy to expand our commercial team in the United States and establish commercial capabilities outside the United States, we will need to continue to recruit and train sales and marketing personnel. In addition, as we build our R&D and manufacturing capabilities, we will need to continue to recruit and train qualified individuals in these areas. Our ability to manage any future growth effectively may require us to, among other things:
Our acquisitions have resulted in many changes, including significant changes in the corporate business and legal entity structure, the integration of other companies and their personnel with us, and changes in systems. We may encounter unexpected difficulties or incur unexpected costs, including:
If any of these factors impair our ability to continue to integrate our operations with those of any companies or businesses we acquire, we may not be able to realize the business opportunities, growth prospects and anticipated tax synergies from combining the businesses. In addition, we may be required to spend additional time or money on integration that otherwise would be spent on the development and expansion of our business.
We may not be successful in growing our commercial operations outside the United States and could encounter other challenges in growing our commercial presence, including due to risks associated with political and economic instability, operating under different legal requirements and tax complexities. If we are unable to manage our commercial growth outside of the United States, our opportunities to expand sales in other countries will be limited or we may experience greater costs with respect to our ex-U.S. commercial operations.
65
We have also broadened our acquisition strategy to include development-stage assets or programs, which entails additional risk to us. For example, if we are unable to identify programs that ultimately result in approved medicines, we may spend material amounts of our capital and other resources evaluating, acquiring and developing medicines that ultimately do not provide a return on our investment. We have less experience evaluating development-stage assets and may be at a disadvantage compared to other entities pursuing similar opportunities. Regardless, development-stage programs generally have a high rate of failure and we cannot guarantee that any such programs will ultimately be successful. While we have significantly enhanced our R&D function in recent years, we may need to enhance our clinical development and regulatory functions to properly evaluate and develop earlier-stage opportunities, which may include recruiting personnel that are knowledgeable in therapeutic areas we have not yet pursued. If we are unable to acquire promising development-stage assets or eventually obtain marketing approval for them, we may not be able to create a meaningful pipeline of new medicines and eventually realize a return on our investments. For example, a core strategic rationale for the Viela acquisition was Viela’s pipeline of medicine candidates and R&D capabilities, but if we experience clinical failures with respect to Viela’s medicine candidates and research programs or such candidates and programs do not otherwise result in marketed medicines, we will not realize the expected benefits from our substantial investment in the acquisition and subsequent development of the Viela pipeline. As our R&D plans and strategies continue to develop, including as a result of our acquisition of Viela, we will need to continue to recruit and train R&D personnel.
Our management may also have to divert a disproportionate amount of its attention away from day-to-day activities and toward managing these growth and integration activities. Our future financial performance and our ability to execute on our business plan will depend, in part, on our ability to effectively manage any future growth and our failure to effectively manage growth could have a material adverse effect on our business, results of operations, financial condition and prospects.
Our prior medicine and company acquisitions and any other strategic transactions that we may pursue in the future could have a variety of negative consequences, and we may not realize the benefits of such transactions or attempts to engage in such transactions.
We have completed multiple medicine and company acquisitions, and our strategy is to engage in additional strategic transactions with third parties, such as acquisitions of companies or divisions of companies and asset purchases of medicines, medicine candidates or technologies that we believe will complement or augment our existing business. We may also consider a variety of other business arrangements, including spin-offs, strategic partnerships, joint ventures, restructurings, divestitures, business combinations and other investments. Any such transaction may require us to incur non-recurring and other charges, increase our near and long-term expenditures, pose significant integration challenges, create additional tax, legal, accounting and operational complexities in our business, require additional expertise, result in dilution to our existing shareholders and disrupt our management and business, which could harm our operations and financial results.
We face significant competition in seeking appropriate strategic transaction opportunities and the negotiation process for any strategic transaction can be time-consuming and complex. In addition, we may not be successful in our efforts to engage in certain strategic transactions due to the pending Transaction and the restrictions under the transaction agreement with Amgen, or because our financial resources may be insufficient and/or third parties may not view our commercial and development capabilities as being adequate. Further, increasing regulatory scrutiny of acquisitions may limit our ability to pursue certain acquisitions where we have potentially competing products or clinical programs. We may not be able to expand our business or realize our strategic goals if we do not have sufficient funding or cannot borrow or raise additional capital. There is no assurance that following any of our recent acquisition transactions or any other strategic transaction, we will achieve the anticipated revenues, net income or other benefits that we believe justify such transactions. In addition, any failures or delays in entering into strategic transactions anticipated by analysts or the investment community could seriously harm our consolidated business, financial condition, results of operations or cash flow.
66
The COVID-19 global pandemic, or other actual or threatened public health epidemics or outbreaks, may continue to adversely impact our industry, including the commercialization of our medicines, our supply chain, our clinical trials, our liquidity and access to capital markets and our business development activities.
The commercialization of our medicines has been and may continue to be adversely impacted by COVID-19 and actions taken to slow its spread. For example, patients have postponed visits to healthcare provider facilities, certain healthcare providers have temporarily closed their offices or are restricting patient visits, healthcare provider employees may become generally unavailable and there could be disruptions in the operations of payers, distributors, logistics providers and other third parties that are necessary for our medicines to be prescribed, reimbursed and administered to patients. In addition, due to reduced willingness of patients to visit physician offices and infusion centers, sales of TEPEZZA, KRYSTEXXA and UPLIZNA have been negatively impacted, and this impact may continue in future quarters while COVID-19 continues to impact healthcare activities and patient visits. In the first half of 2022, demand for TEPEZZA, KRYSTEXXA and UPLIZNA was negatively impacted by the omicron variant of COVID-19. The omicron variant resulted in significant employee absences in our commercial organization due to illness and also impacted operations at sites of care that infuse TEPEZZA, KRYSTEXXA and UPLIZNA and patient access to and willingness to visit healthcare providers. These events resulted in lower new patient enrollment forms for TEPEZZA, KRYSTEXXA and UPLIZNA, delays in new patients starting infusions and disruptions in therapy. COVID-19-related restrictions have also limited our sales force activities, including access to healthcare provider offices, which has negatively impacted the effectiveness of our sales and marketing efforts. While the impact from the omicron variant has largely subsided, new COVID-19 variants, including new omicron subvariants, continue to emerge and we cannot predict how long the COVID-19 pandemic will continue to negatively impact sales of our medicines or if or when other similar disease outbreaks will emerge that cause similar disruptions.
Quarantines, shelter-in-place and similar government orders, or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could occur, related to COVID-19 or other infectious diseases could impact personnel at third-party manufacturing facilities upon which we rely, or the availability or cost of materials, which could disrupt the supply chain for our medicines. In particular, some of our suppliers of certain materials used in the production of our medicines are located in regions that have been subject to COVID-19-related actions and policies that limit the conduct of normal business operations. To the extent our suppliers and service providers are unable to comply with their obligations under our agreements with them or they are otherwise unable to deliver or are delayed in delivering goods and services to us due to COVID-19, our ability to continue meeting commercial demand for our medicines in the United States or advancing development of our medicine candidates may become impaired. At this time, we consider our medicine inventories on hand to be sufficient to meet our commercial requirements.
In addition, our clinical trials have been and may in the future be affected by the COVID-19 pandemic. Clinical site initiation and patient enrollment have experienced, and may continue to experience, delays due to staffing shortages or prioritization of hospital and healthcare resources toward COVID-19. Current or potential patients in our ongoing or planned clinical trials may also choose to not enroll, not participate in follow-up clinical visits or drop out of the trial as a precaution against or as a result of contracting COVID-19. Further, some patients may not be able or willing to comply with clinical trial protocols if healthcare services are interrupted due to COVID-19. Some clinical sites in the United States and other countries have slowed or stopped further enrollment of new patients in clinical trials, denied access to site monitors or otherwise curtailed certain operations. Similarly, our ability to recruit and retain principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19, may be adversely impacted. These events could delay our clinical trials, increase the cost of completing our clinical trials and negatively impact the integrity, reliability or robustness of the data from our clinical trials.
The spread of COVID-19 and actions taken to reduce its spread may also materially affect us economically. As a result of the COVID-19 pandemic and actions taken to slow its spread, as well as actual or anticipated changes in interest rates and economic inflation, the global credit and financial markets have experienced extreme volatility and disruptions, including diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, increases in interest rates and uncertainty about economic stability. If the equity and credit markets deteriorate, it may make any additional debt or equity financing more difficult, more costly or more dilutive. While the potential economic impact caused by, and the duration of, COVID-19 may be difficult to assess or predict, there could be a significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity and financial position or our business development activities.
67
COVID-19 continues to evolve. The extent to which COVID-19 may impact the commercialization of our medicines, our supply chain, our clinical trials, our access to capital and our business development activities, will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the duration of the pandemic, the transmissibility and severity of illness caused by new variants, the efforts by governments and businesses to contain the spread of COVID-19, business closures or business disruptions and the impact on the economy and capital markets.
We may not be able to successfully maintain our current advantageous tax status and resulting tax rates, which could adversely affect our business and financial condition, results of operations and growth prospects.
Our parent company is incorporated in Ireland and has subsidiaries maintained in Ireland and in multiple other jurisdictions. We are able to achieve a favorable tax rate through the performance of certain functions and ownership of certain assets in tax-efficient jurisdictions, including Ireland and Bermuda, together with the use of intercompany service and transfer pricing agreements, each on an arm’s length basis. Our effective tax rate may be different than experienced in the past due to numerous factors including, changes to the tax laws of jurisdictions that we operate in, the enactment of new tax treaties or changes to existing tax treaties, changes in the mix of our profitability from jurisdiction to jurisdiction, the implementation of the EU Anti-Tax Avoidance Directive (see further discussion below), the implementation of the Bermuda Economic Substance Act 2018 (effective December 31, 2018) and our inability to secure or sustain acceptable agreements with tax authorities (if applicable). Any of these factors could cause us to experience an effective tax rate significantly different from previous periods or our current expectations. Taxing authorities, such as the U.S. Internal Revenue Service, or IRS, actively audit and otherwise challenge these types of arrangements, and have done so in the biopharmaceutical industry. We expect that these challenges will continue as a result of the recent increase in scrutiny and political attention on corporate tax structures. The IRS and/or the Irish tax authorities may challenge our structure and transfer pricing arrangements through an audit or lawsuit. Responding to or defending such a challenge could be expensive and consume time and other resources, and divert management’s time and focus from operating our business. We cannot predict whether taxing authorities will conduct an audit or file a lawsuit challenging this structure, the cost involved in responding to any such audit or lawsuit, or the outcome. If we are unsuccessful in defending such a challenge, we may be required to pay taxes for prior periods, as well as interest, fines or penalties, and may be obligated to pay increased taxes in the future, any of which could require us to reduce our operating expenses, decrease efforts in support of our medicines or seek to raise additional funds, all of which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
The IRS may not agree with our conclusion that our parent company should be treated as a foreign corporation for U.S. federal income tax purposes following the combination of the businesses of Horizon Pharma, Inc., or HPI, our predecessor, and Vidara Therapeutics International Public Limited Company, or Vidara.
Although our parent company is incorporated in Ireland, the IRS may assert that it should be treated as a U.S. corporation (and, therefore, a U.S. tax resident) for U.S. federal income tax purposes pursuant to Section 7874 of the Internal Revenue Code of 1986, as amended, or the Code. A corporation is generally considered a tax resident in the jurisdiction of its organization or incorporation for U.S. federal income tax purposes. Because our parent company is an Irish incorporated entity, it would generally be classified as a foreign corporation (and, therefore, a non-U.S. tax resident) under these general rules. Section 7874 of the Code provides an exception pursuant to which a foreign incorporated entity may, in certain circumstances, be treated as a U.S. corporation for U.S. federal income tax purposes. We do not believe that our classification as a foreign corporation for U.S. federal income tax purposes is affected by Section 7874, though the IRS may disagree.
Recent and future changes to U.S. and non-U.S. tax laws could materially adversely affect our company.
Under current law, we expect our parent company to be treated as a foreign corporation for U.S. federal income tax purposes. However, changes to the rules in Section 7874 of the Code or regulations promulgated thereunder or other guidance issued by the U.S. Department of the Treasury, or the U.S. Treasury, or the IRS could adversely affect our parent company’s status as a foreign corporation for U.S. federal income tax purposes or the taxation of transactions between members of our group, and any such changes could have prospective or retroactive application. If our parent company is treated as a domestic corporation, more of our income will be taxed by the United States which may substantially increase our effective tax rate.
68
In addition, the Organization for Economic Cooperation and Development, or the OECD, released its Base Erosion and Profit Shifting project final report on October 5, 2015. This report provides the basis for international standards for corporate taxation that are designed to prevent, among other things, the artificial shifting of income to tax havens and low-tax jurisdictions, the erosion of the tax base through interest deductions on intercompany debt and the artificial avoidance of permanent establishments (i.e., tax nexus with a jurisdiction). Legislation to adopt these standards has been enacted or is currently under consideration in a number of jurisdictions. On June 7, 2017, several countries, including many countries that we operate and have subsidiaries in, participated in the signing ceremony adopting the OECD’s Multilateral Convention to Implement Tax Treaty Related Measures to Prevent Base Erosion and Profit Shifting, commonly referred to as the MLI. The MLI came into effect on July 1, 2018. In January 2019, Ireland deposited the instrument of ratification of Ireland’s MLI choices with the OECD. Ireland’s MLI came into force on May 1, 2019, however the provisions in respect of withholding taxes and other taxes levied by Ireland did not come into effect for us until January 1, 2020 (with application also depending on whether the MLI has been ratified in other jurisdictions whose tax treaties with Ireland are affected). The MLI may modify affected tax treaties making it more difficult for us to obtain advantageous tax-treaty benefits. The number of affected tax treaties could eventually be in the thousands. As a result, our income may be taxed in jurisdictions where it is not currently taxed and at higher rates of tax than it is currently taxed, which may increase our effective tax rate.
On July 12, 2016, the Anti-Tax Avoidance Directive, or ATAD, was formally adopted by the Economic and Financial Affairs Council of the EU. The stated objective of the ATAD is to provide for the effective and swift coordinated implementation of anti-base erosion and profit shifting measures at EU level. Like all directives, the ATAD is binding as to the results it aims to achieve though EU Member States are free to choose the form and method of achieving those results. In addition, the ATAD contains a number of optional provisions that present an element of choice as to how it will be implemented into law. On December 25, 2018, the Finance Act 2018 was signed into Irish law, which introduced certain elements of the ATAD, such as the Controlled Foreign Company, or CFC, regime, into Irish law. The CFC regime became effective as of January 1, 2019. The ATAD also set out a high-level framework for the introduction of Anti-hybrid provisions. Finance Act 2019 introduced Anti-hybrid legislation in Ireland with effect from January 1, 2020. Finance Act 2021 introduced further ATAD measures, such as the interest limitation rules and anti-hybrid rules to neutralize reverse-hybrid mismatches into Irish law with effect from January 1, 2022. We do not expect a material impact on our effective tax rate as a result of the introduction of these provisions.
On October 8, 2021, 136 of the 140 members of the OECD/G20 Inclusive Framework on Base Erosion and Profit Shifting, or Inclusive Framework, approved a statement providing a framework for reform of the international tax rules, or Inclusive Framework Statement. The Inclusive Framework Statement sets out the key terms for an agreement on a two-pillar solution to address the tax challenges arising from the digitalization of the economy. Pillar One focuses on nexus and profit allocation and Pillar Two provides for a global minimum effective corporate tax rate of 15%. The Inclusive Framework Statement provides that Pillar One would apply to multinational enterprises with annual global revenue above 20 billion euros and profitability above 10%, with the revenue threshold potentially reduced to 10 billion euros in the future. Based on these thresholds, we would currently be outside the scope of the Pillar One proposals. On December 20, 2021, the Inclusive Framework published detailed rules which define the scope of, and set out the mechanism for introducing, the Pillar Two global minimum effective tax rate proposal. The rules provide for the imposition of the global minimum effective tax rate on certain multinational enterprises that have consolidated revenues of at least 750 million euros in at least two out of the last four years. Based on these thresholds, we currently expect that we could fall within the scope of the Pillar Two proposals. A number of countries are currently proposing to implement core elements of the Pillar Two proposals by the start of 2024 and, on December 15, 2022, the EU adopted a Council Directive requiring aspects of the Pillar Two proposals to be transposed into the national laws of its Member States (including Ireland) by December 31, 2023. Although it is difficult at this stage to determine with precision the impact the Pillar Two proposals would have, their implementation could materially increase our effective tax rate.
69
On August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022, or IRA, which includes a minimum tax equal to 15 percent of the adjusted financial statement income of certain corporations, as well as a one percent excise tax on share buybacks. Pending further guidance, it is possible that the IRA could increase our tax liability, which could in turn adversely impact our business and future profitability. The IRA or future changes in U.S. tax laws could have a material adverse impact on the value of our deferred tax assets and liabilities, could result in significant one-time charges, and could increase our future U.S. tax expense.
Effective January 1, 2022, the Tax Cuts and Jobs Act eliminated the option to deduct research and development expenses for tax purposes in the year incurred and requires taxpayers to capitalize and subsequently amortize such expenses over five years for research activities conducted in the United States and over fifteen years for research activities conducted outside the United States. Unless U.S. Treasury issues regulations that narrow the application of this provision to a smaller subset of our research and development expenses or the provision is deferred, modified, or repealed by Congress, this provision could materially decrease our cash flows from operations with an offsetting similarly sized increase in our net deferred tax assets over these amortization periods. The actual impact of this provision will depend on multiple factors, including the amount of research and development expenses we will incur and whether we conduct our research and development activities inside or outside the United States.
We are unable to predict what tax laws may be proposed or enacted in the future or what effect such changes would have on our business. To the extent new tax laws are enacted, or new guidance released, this could have an adverse effect on our future effective tax rate. It could also lead to an increase in the complexity and cost of tax compliance. We urge our shareholders to consult with their legal and tax advisors with respect to the potential tax consequences of investing in or holding our ordinary shares.
If a United States person is treated as owning at least 10% of our ordinary shares, such holder may be subject to adverse U.S. federal income tax consequences.
If a United States person is treated as owning (directly, indirectly, or constructively) at least 10% of the value or voting power of our ordinary shares, such person may be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group (if any). Because our group includes one or more U.S. subsidiaries, certain of our non-U.S. subsidiaries could be treated as controlled foreign corporations (regardless of whether or not we are treated as a controlled foreign corporation). A United States shareholder of a controlled foreign corporation may be required to report annually and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income,” and investments in U.S. property by controlled foreign corporations, regardless of whether we make any distributions. An individual that is a United States shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a U.S. corporation that is a United States shareholder with respect to a controlled foreign corporation. Failure to comply with these reporting and tax paying obligations may subject a United States shareholder to significant monetary penalties and may prevent the statute of limitations from starting with respect to such shareholder’s U.S. federal income tax return for the year for which reporting was due. We cannot provide any assurances that we will assist investors in determining whether any of our non-U.S. subsidiaries is treated as a controlled foreign corporation or whether any investor is treated as a United States shareholder with respect to any such controlled foreign corporation or furnish to any United States shareholders information that may be necessary to comply with the aforementioned reporting and tax paying obligations. A United States investor should consult its advisors regarding the potential application of these rules to an investment in our ordinary shares.
70
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive biopharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial, manufacturing, scientific and medical personnel. We are highly dependent on our management, sales and marketing and scientific and medical personnel, including our executive officers. In order to retain valuable employees at our company, in addition to salary and annual cash incentives, we provide a mix of performance stock units, or PSUs, that vest subject to attainment of specified corporate performance goals and continued services, stock options and restricted stock units, or RSUs, that vest over time subject to continued services. The value to employees of PSUs, stock options and RSUs will be significantly affected by movements in our share price that are beyond our control, and may at any time be insufficient to counteract more lucrative offers from other companies.
Despite our efforts to retain valuable employees, members of our management, sales and marketing, regulatory affairs, clinical development, medical affairs, development and manufacturing teams may terminate their employment with us on short notice. Although we have written employment arrangements with all of our employees, these employment arrangements in the United States generally provide for at-will employment, which means that our employees can leave our employment at any time, with or without notice. In addition, the pending Transaction with Amgen has made it more difficult to attract and retain qualified employees due to the uncertainty about whether or when the transaction will close and impact of the Transaction on our employees. The loss of the services of any of our executive officers or other key employees and our inability to find suitable replacements could potentially harm our business, financial condition and prospects. We do not maintain “key man” insurance policies on the lives of these individuals or the lives of any of our other employees. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level, and senior managers as well as junior, mid-level, and senior sales and marketing, manufacturing, scientific and medical personnel.
Many of the other biopharmaceutical companies with whom we compete for qualified personnel have greater financial and other resources, different risk profiles and longer histories in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than that which we have to offer. If we are unable to continue to attract and retain high quality personnel, the rate and success at which we can develop and commercialize medicines and medicine candidates will be limited.
We are subject to federal, state and foreign healthcare laws and regulations and implementation or changes to such healthcare laws and regulations could adversely affect our business and results of operations.
The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals that change the healthcare system in ways that could impact profitability. In the United States and other countries there is significant interest in implementing regulations and legislation with the stated goals of containing healthcare costs, improving quality, and/or expanding access. The biopharmaceutical industry has been a focus of these efforts and has been significantly affected by major legislative initiatives, particularly in the United States.
The healthcare system is highly regulated in the United States and, as a biotechnology company that participates in government-regulated healthcare programs, we are subject to complex laws and regulations. Violation of these laws, or any other federal or state regulations, may subject us to significant administrative, civil and/or criminal penalties, damages, disgorgement, fines, exclusion, imprisonment, additional reporting requirements, and/or oversight from federal health care programs that could require the restructuring of our operations. Any of these could have a material adverse effect on our business and financial results. Any action against us for violation of these laws, even if we ultimately are successful in our defense, will cause us to incur significant legal expenses and divert our management’s attention away from the operation of our business.
There have been executive, Congressional and judicial challenges to the ACA. For example, on June 17, 2021 the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Thus, the ACA remains in effect in its current form. Further, prior to the U.S. Supreme Court ruling on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA.
71
In addition, the ACA has been subject to various health reform measures. For example, on August 16, 2022, President Biden signed the IRA into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket costs. Medicare will cap beneficiary costs at $2,000 per year, indexed in future years to the rate of increase in Medicare costs. Further, the IRA restructures liability under Medicare Part D beginning in 2025, through a newly established Manufacturer Discount Program, which in part requires manufacturers to provide a 10% discount in the initial phase and 20% discount in the catastrophic phase for brand drugs. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and the healthcare reform measures of the Biden administration will impact the many different provisions of the ACA and IRA affecting the health system, the biopharmaceutical sector and our business. It is also possible that the IRA could put pressure on commercial insurers to reduce coverage or reimbursement of branded medicines.
Other legislative changes have also been proposed and adopted since the ACA was enacted. For example, the Budget Control Act of 2011 resulted in aggregate reductions in Medicare payments to providers of up to 2 percent per fiscal year, starting in 2013, and due to subsequent legislative amendments to the statute will remain in effect through 2031, unless additional Congressional action is taken. However, COVID-19 relief legislation suspended the 2 percent Medicare sequester from May 1, 2020 through March 31, 2022. Following the resumption of the sequester, under current legislation the actual reduction in Medicare payments will vary from 1 percent in 2022 to up to 4 percent in the final fiscal year of this sequester. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Such laws, and others that may affect our business that have been enacted or may in the future be enacted, may result in additional reductions in Medicare and other healthcare funding.
In addition, drug pricing by biopharmaceutical companies in the United States has come under increased scrutiny. Specifically, there have been several recent state and U.S. congressional inquiries into pricing practices by biopharmaceutical companies.
In July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In the order, the President directed the Federal Trade Commission to ban “pay for delay” patent settlement agreements, and to identify and address any efforts that impede generic or biosimilar competition. The executive order also directed the FDA to continue to work with states and Indian Tribes to develop importation programs in accordance with Section 804 of the FDCA and FDA regulations. The order directed HHS to increase support for generic and biosimilar drugs by improving standards for the interchangeability of biologic products, supporting biosimilar adoption by increasing education, and facilitating the approval of biosimilars by updating and clarifying existing requirements and procedures related to biologics licensing. Finally, the executive order directs HHS to submit a report detailing a comprehensive plan within 45 days to fight high prescription drug prices and reduce the amount that the federal government pays for drugs. In response to President Biden’s executive order, on September 9, 2021, HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform. These principles are government drug price negotiations, promoting increased competition including changes to supply chains and promoting biosimilars and generics and supporting public and private research. The plan sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. The Biden administration released an additional executive order on October 14, 2022, directing HHS to submit a report within 90 days on how the Center for Medicare and Medicaid Innovation can be further leveraged to test new models for lowering drug costs for Medicare and Medicaid beneficiaries.
72
Congress continued to seek new legislative and/or administrative measures to control drug costs. Further, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. In addition, the IRA directs the HHS Secretary to establish a Drug Price Negotiation Program, or Program, to lower prices for certain high-expenditure, single-source prescription drugs and biologics covered under Medicare Part B and Part D that have been approved by the FDA for at least 7 years for prescription drugs and at least 11 years for biologics. Under the Program, the HHS Secretary will publish a list of “selected drugs,” and will then negotiate maximum fair prices with their manufacturers. The Program will be implemented in stages. Beginning in 2026, 10 Medicare Part D “selected drugs” will be subject to price negotiations. By 2029, and in subsequent years thereafter, the number will increase to 20 drugs and biologics covered under Medicare Part B and Part D. Agreements between HHS and manufacturers will remain in place until a drug or biologic is no longer considered a “selected drug” for negotiation purposes. Manufacturers who do not comply with the negotiated prices set under the Program will be subject to an excise tax based on a percentage of total sales of a “selected drug” up to 95%, or they have the option to withdraw from the Medicare and Medicaid markets. Orphan drugs approved for one indication are exempt from negotiation. Further, as early as the first quarter of 2023 for Part B and by July 2024 for Part D, the IRA will require manufacturers that increase prices of certain Medicare Part B and Part D drugs or biologics at a rate greater than inflation to pay rebates to CMS or be subject to civil monetary penalties. The IRA permits HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. It is currently unclear how the IRA will be effectuated but it is likely to have a significant negative impact on the pharmaceutical industry.
In countries in the EU, legislators, policymakers, and healthcare insurance funds continue to propose and implement cost-containing measures to keep healthcare costs down, due in part to the attention being paid to healthcare cost containment in the EU. Certain of these changes could impose limitations on the prices we will be able to charge for our medicines and any approved medicine candidates or the amounts of reimbursement available for these medicines from governmental agencies or third-party payers, may increase the tax obligations on biopharmaceutical companies such as ours, or may facilitate the introduction of generic competition with respect to our medicines.
The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our current medicines and/or those for which we may receive regulatory approval in the future.
We are subject, directly or indirectly, to federal and state healthcare fraud and abuse, transparency laws and false claims laws. Prosecutions under such laws have increased in recent years and we may become subject to such litigation. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
In the United States, we are subject directly, or indirectly through our customers and other third parties, to various state and federal fraud and abuse and transparency laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, the Civil Monetary Penalties Law prohibiting, among other things, beneficiary inducements, and similar state and local laws, federal and state privacy and security laws, such as the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, sunshine laws, government price reporting laws, and other fraud laws. Some states, such as Massachusetts, make certain reported information public. In addition, there are state and local laws that require biopharmaceutical representatives to be licensed and comply with codes of conduct, transparency reporting, and other obligations. Collectively, these laws may affect, among other things, our current and proposed research, sales, marketing and educational programs, as well as other possible relationships with customers, pharmacies, physicians, payers, and patients. We are subject to similar laws in the EEA. Outside the United States, an increasing number of laws, regulations, and industry standards may govern data privacy and security. For example, in the EEA the EU’s General Data Protection Regulation, or the EU GDPR, and in the UK the UK’s GDPR, or UK GDPR, impose strict requirements for processing personal data. For example, under the EU GDPR fines of up to €20 million or up to 4% of the annual global revenue of the infringer, whichever is greater, could be imposed; companies may face temporary or definitive bans on data processing and other corrective actions; or private litigation related to processing of personal data brought by classes of data subjects or consumer protection organizations authorized at law to represent their interests.
73
Compliance with these laws, including the development of a comprehensive compliance program, is difficult, costly and time consuming. Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Moreover, state governmental agencies may propose or enact laws and regulations that extend or contradict federal requirements. These risks may be increased where there are evolving interpretations of applicable regulatory requirements, such as those applicable to manufacturer co-pay programs. Biopharmaceutical manufacturer co-pay programs, including biopharmaceutical manufacturer donations to patient assistance programs offered by charitable foundations, are the subject of ongoing litigation, enforcement actions and settlements (involving other manufacturers and to which we are not a party) and evolving interpretations of applicable regulatory requirements and certain state laws, and any change in the regulatory or enforcement environment regarding such programs could impact our ability to offer such programs. Other recent legislation and regulatory policies contain provisions that disincentivize the use of co-pay coupons by requiring their value to be included in average sales price or best price calculations, potentially lowering reimbursement for drugs with a high use of copay coupons in Medicare Part B and Medicaid. If we are unsuccessful with our co-pay programs, we would be at a competitive disadvantage in terms of pricing versus preferred branded and generic competitors, or be subject to significant penalties. We are engaged in various business arrangements with current and potential customers, and we can give no assurance that such arrangements would not be subject to scrutiny under such laws, despite our efforts to properly structure such arrangements. Even if we structure our programs with the intent of compliance with such laws, there can be no certainty that we would not need to defend our business activities against enforcement or litigation. Further, we cannot give any assurances that prior business activities or arrangements of other companies that we acquire will not be scrutinized or subject to enforcement or litigation. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have an impact on our business, including the imposition of significant civil, criminal and administrative sanctions, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs or comparable foreign programs, imprisonment, integrity oversight and reporting obligations, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
There has also been a trend of increased federal and state regulation of payments made to physicians and other healthcare providers. The ACA, among other things, imposed reporting requirements on drug manufacturers for payments made by them to physicians (defined to include doctors, dentists, podiatrists, optometrists and licensed chiropractors), certain other healthcare providers (including, for example, physician assistants and nurse practitioners), and teaching hospitals, as well as ownership and investment interests held by physicians, and their immediate family members. Failure to submit required information may result in significant civil monetary penalties.
On August 3, 2022, we received a civil investigative demand from the United States Department of Justice, or DOJ, pursuant to the Federal False Claims Act regarding an investigation concerning potentially false information in prior authorization forms. A prior authorization form is a managed care practice whereby the payer (either a commercial insurer or a government health program) requires that the prescribing physician provide additional justification or information supporting the physician’s decision to prescribe a particular medicine. The civil investigative demand requests certain documents and information related to DUEXIS, PENNSAID 2%, VIMOVO and RAYOS. We are cooperating with the investigation and the DOJ has not indicated to us whether it believes we engaged in any wrongdoing or if we are the subject of the investigation. While we are not aware of any fraudulent scheme to provide false information in prior authorization forms for our medicines that resulted in improper payments from government healthcare programs, no assurance can be given as to the timing or outcome of the DOJ’s investigation, or that it will not result in a material adverse effect on our business.
We are unable to predict whether we could be subject to other actions under any of these or other healthcare laws, or the impact of such actions. If we are found to be in violation of, or to have encouraged or assisted the violation by third parties of any of the laws described above or other applicable state and federal fraud and abuse laws, we may be subject to penalties, including significant administrative, civil and criminal penalties, damages, fines, withdrawal of regulatory approval, imprisonment, exclusion from government healthcare reimbursement programs, contractual damages, reputational harm, diminished profits and future earnings, injunctions and other associated remedies, or private “qui tam” actions brought by individual whistleblowers in the name of the government, and the curtailment or restructuring of our operations, all of which could have a material adverse effect on our business and results of operations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
74
Our medicines or any other medicine candidate that we develop may cause undesirable side effects or have other properties that could delay or prevent regulatory approval or commercialization, result in medicine re-labeling or withdrawal from the market or have a significant impact on customer demand.
Undesirable side effects caused by any medicine candidate that we develop could result in the denial of regulatory approval by the FDA or other comparable foreign regulatory authorities for any or all targeted indications, or cause us to evaluate the future of our development programs. In our Phase 3 clinical trial evaluating TEPEZZA for the treatment of active TED, the most commonly reported treatment-emergent adverse events were muscle spasms, nausea, alopecia, diarrhea, fatigue, hyperglycemia, hearing impairment, dysgeusia, headache, dry skin and menstrual disorders. While our post-marketing studies and pharmacovigilance reporting data have shown similar rates of hearing impairment as compared to the TEPEZZA pivotal clinical trials, which is reflected in the FDA-approved label, there have been third party reports that have purported to show higher rates of hearing impairment. In addition, a recent analysis of safety data as part of our ongoing pharmacovigilance program indicated a signal of hearing impairment events of greater severity, in limited cases, than those observed in the TEPEZZA pivotal clinical trials. Based on this analysis, we are discussing with the FDA potential updates to the TEPEZZA label to further characterize the range of events reported. To the extent healthcare providers or patients become concerned with adverse events associated with TEPEZZA, including the potential for hearing impairment, it could negatively impact our ability to increase adoption of the medicine. With respect to KRYSTEXXA, the most commonly reported adverse reactions in the pivotal trial were gout flares, infusion reactions, nausea, contusion or ecchymosis, nasopharyngitis, constipation, chest pain, anaphylaxis and vomiting. When administering KRYSTEXXA with methotrexate, the most commonly reported adverse events in the MIRROR randomized control trial were gout flares, arthralgia, COVID-19, nausea and fatigue. With respect to RAVICTI, the most common side effects are diarrhea, nausea, decreased appetite, gas, vomiting, high blood levels of ammonia, headache, tiredness and dizziness. With respect to PROCYSBI, the most common side effects include vomiting, nausea, abdominal pain, breath odor, diarrhea, skin odor, fatigue, rash and headache. With respect to UPLIZNA, the most common adverse reactions across both the randomized and open-label treatment in our N-MOmentum trial for UPLIZNA were urinary tract infection, nasopharyngitis, arthralgia, upper respiratory tract infection, headache, back pain, and infusion related reaction. The most common infections reported by treated patients in the randomized and open-label periods included urinary tract infection, nasopharyngitis, upper respiratory tract infection and influenza. In addition, three deaths were reported in the ongoing open-label period. One death occurred in a patient experiencing a myelitis attack and was considered unrelated to UPLIZNA by the investigator. The second death was due to complications from mechanical ventilator-associated pneumonia in a patient who developed new neurological symptoms and seizures, the cause of which could not be definitively established. The possibility that the death was treatment-related could not be ruled out, and as a result, under the terms of the protocol for the trial, the death was assessed as treatment-related. The third death was due to COVID-19 pneumonia and was considered unrelated to UPLIZNA by the investigator. There can be no assurance a foreign regulatory authority will agree with the classifications of the deaths made by the investigators or that we will not be required to conduct additional clinical trials of UPLIZNA in order to establish an adequate safety database. The most common side effects observed in pivotal trials for ACTIMMUNE were “flu-like” or constitutional symptoms such as fever, headache, chills, myalgia and fatigue.
The FDA or other comparable foreign regulatory authorities may also require, or we may undertake, additional clinical trials to support the safety profile of our medicines or medicine candidates.
In addition, we or others may identify undesirable side effects caused by our medicines or any other medicine candidate that we may develop that receives marketing approval, or there could be perceptions that the medicine is associated with undesirable side effects. For example, product liability suits have been filed against us alleging that TEPEZZA is defectively designed and/or fails to include proper warnings regarding potential adverse events associated with hearing impairment. As a result of any such events it is possible that:
If any of these events occurred with respect to our medicines, our ability to generate significant revenues from the sale of these medicines would be significantly harmed.
75
We rely on third parties to conduct our pre-clinical and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines or if they experience regulatory compliance issues, we may not be able to obtain regulatory approval for or commercialize our medicine candidates and our business could be substantially harmed.
We have agreements with third-party contract research organizations, or CROs, to conduct our clinical programs, including those required for post-marketing commitments, and we expect to continue to rely on CROs for the completion of on-going and planned clinical trials. We may also have the need to enter into other such agreements in the future if we were to develop other medicine candidates or conduct clinical trials in additional indications for our existing medicines. We also rely heavily on these parties for the execution of our clinical studies and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol. We, our CROs and our academic research organizations are required to comply with current GCP or ICH regulations. The FDA, and comparable foreign regulatory authorities in other jurisdictions, enforce these GCP or ICH regulations through periodic inspections of trial sponsors, principal investigators and trial sites. If we or our CROs or collaborators fail to comply with applicable GCP or ICH regulations, the data generated in our clinical trials may be deemed unreliable and our submission of marketing applications may be delayed or the FDA, or such other comparable foreign regulatory authorities, may require us to perform additional clinical trials before approving our marketing applications. We cannot assure that, upon inspection, the FDA, or such other comparable foreign regulatory authorities, will determine that any of our clinical trials comply or complied with GCP or ICH regulations. In addition, our clinical trials must be conducted with medicine produced under cGMP regulations, and may require a large number of test subjects. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be implicated if any of our CROs or collaborators violates federal or state fraud and abuse or false claims laws and regulations or privacy and security laws. We must also obtain certain third-party institutional review board, or IRB, approvals and positive Ethics Committee opinions as part of the decision on the authorization of the clinical trial issued by EU Member States including input from the national competent authorities and Ethics Committee, in order to conduct our clinical trials. Delays by IRBs and Ethics Committees in providing such approvals or opinions may delay our clinical trials.
If any of our relationships with these third-party CROs or collaborators terminate, we may not be able to enter into similar arrangements on commercially reasonable terms, or at all. If CROs or collaborators do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our medicines and medicine candidates. As a result, our results of operations and the commercial prospects for our medicines and medicine candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Switching or adding additional CROs or collaborators can involve substantial cost and require extensive management time and focus. In addition, there is a natural transition period when a new CRO or collaborator commences work. As a result, delays may occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our CROs and collaborators, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition or prospects. In particular, the ability of our CROs to conduct certain of their operations, including monitoring of clinical sites, has been limited by the COVID-19 pandemic, and to the extent that our CROs are unable to fulfill their contractual obligations as a result of the COVID-19 pandemic or government orders in response to the pandemic, we may have limited or no recourse under the terms of our contractual agreements with our CROs.
Clinical development of drugs and biologics involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Clinical testing is expensive and can take many years to complete, and its outcome is uncertain. Failure can occur at any time during the clinical trial process. The results of pre-clinical studies and early clinical trials of potential medicine candidates may not be predictive of the results of later-stage clinical trials. Medicine candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through pre-clinical studies and initial clinical testing.
76
In some instances, there can be significant variability in safety and efficacy results between different clinical trials of the same medicine candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, differences in and adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. For example, while we have announced that dazodalibep met the primary endpoint in a Phase 2 clinical trial in Sjögren’s syndrome in both populations and that we plan on initiating a Phase 3 development program in this indication, there is no assurance that dazodalibep will demonstrate similar results in the planned Phase 3 trials, or demonstrate successful results in an on-going Phase 2b clinical trial in kidney transplant rejection or in our planned trial in focal segmental glomerulosclerosis.
We may experience delays in clinical trials or investigator-initiated studies. We do not know whether any additional clinical trials will be initiated in the future, begin on time, need to be redesigned, enroll patients on time or be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays related to:
Our clinical trials have been and may also in the future be affected by COVID-19 or other public health epidemics or outbreaks. For example, while it has since completed enrollment, we experienced enrollment delays in our TEPEZZA clinical trial in chronic/low CAS TED due to the impacts of the omicron variant of COVID-19. We also experienced enrollment delays in our UPLIZNA clinical trial in myasthenia gravis due to government ordered COVID-19 lockdowns in China, combined with other negative impacts related to the conflict in Ukraine. As a result, we expect data from our TEPEZZA clinical trial in chronic/low CAS TED in the second quarter of 2023 and topline data for our clinical trial of UPLIZNA in myasthenia gravis in 2024. In addition, clinical site initiation and patient enrollment may be delayed due to staffing shortages or prioritization of hospital and healthcare resources toward COVID-19. Current or potential patients in our ongoing or planned clinical trials may also choose to not enroll, not participate in follow-up clinical visits or drop out of the trial as a precaution against or as a result of contracting COVID-19. Further, some patients may not be able or willing to comply with clinical trial protocols if healthcare services are interrupted due to COVID-19. Some clinical sites in the United States and other countries have slowed or stopped further enrollment of new patients in clinical trials, denied access to site monitors or otherwise curtailed certain operations. Similarly, our ability to recruit and retain principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19, may be adversely impacted. The availability of supplies needed for the conduct of preclinical studies and clinical trials may be impacted by COVID-19 supply disruptions. For example, we depend on the availability of non-human primates to conduct certain preclinical studies that we are required to complete prior to submitting an IND and initiating clinical development. There is currently a global shortage of non-human primates available for drug development, due in part to an increase in demand from companies and other institutions developing vaccines and treatments for COVID-19. If the shortage continues, this could substantially increase the cost of conducting our preclinical development and could also result in delays to our development timelines. These events could delay our clinical trials, increase the cost of completing our clinical trials and negatively impact the integrity, reliability or robustness of the data from our clinical trials.
77
Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the medicine candidate being studied in relation to other available therapies, including any new drugs or biologics that may be approved for the indications we are investigating. In addition, if patients drop out of our trials, miss scheduled doses or follow-up visits or otherwise fail to follow trial protocols, are impacted by local disruptions, or if our trials are otherwise disrupted due to COVID-19 or actions taken to slow its spread, the integrity of data from our trials may be compromised or not accepted by the FDA or other comparable foreign regulatory authorities, which would represent a significant setback for the applicable program.
We could encounter delays if prescribing physicians encounter unresolved ethical issues associated with enrolling patients in clinical trials of our medicine candidates in lieu of prescribing existing treatments that have established safety and efficacy profiles. Further, a clinical trial may be suspended or terminated by us, our collaborators, the FDA or other comparable foreign regulatory authorities due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other comparable foreign regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a medicine candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience delays in the completion of, or if we terminate, any clinical trial of our medicine candidates, the commercial prospects of our medicine candidates will be harmed, and our ability to generate medicine revenues from any of these medicine candidates will be delayed or reduced. In addition, any delays in completing our clinical trials will increase our costs, slow down our medicine development and approval process and jeopardize our ability to commence medicine sales and generate revenues.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or comparable foreign regulatory authorities. The FDA or comparable foreign regulatory authorities may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or comparable foreign regulatory authorities may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or comparable foreign regulatory authorities and may ultimately lead to the denial of marketing approval of one or more of our medicine candidates.
Any of these occurrences may harm our business, financial condition, results of operations and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our medicine candidates.
The sizes of the patient populations suffering from some of the diseases we are targeting are small and based on estimates that may not be accurate.
Because certain of our clinical trials are focused on indications with small patient populations, our ability to enroll eligible patients may be limited or may result in slower enrollment than we anticipate. In addition, our projections of both the number of people who have some of the diseases we are targeting, as well as the subset of people with these diseases who have the potential to benefit from treatment with our medicines and any of our future medicine candidates, are estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, physician interviews, patient foundations and market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our medicines and any future medicine candidates may be limited or may not be amenable to treatment with our medicines and any of our medicine candidates, if and when approved. Even if we obtain significant market share for our medicines and any of our medicine candidates (if and when they are approved), small potential target populations for certain indications means we may never achieve profitability without obtaining market approval for additional indications.
78
Business interruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics or health pandemics, such as the current COVID-19 pandemic, and other natural or man-made disasters or business interruptions. While we carry insurance for certain of these events and have implemented disaster management plans and contingencies, the occurrence of any of these business interruptions could seriously harm our business and financial condition and increase our costs and expenses. We conduct significant management operations at both our global headquarters located in Dublin, Ireland and our U.S. office located in Deerfield, Illinois. If our Dublin or Deerfield offices were affected by a natural or man-made disaster or other business interruption, our ability to manage our domestic and foreign operations could be impaired, which could materially and adversely affect our results of operations and financial condition. We currently rely, and intend to rely in the future, on third-party manufacturers and suppliers to produce our medicines and third-party logistics partners to ship our medicines. Our ability to obtain commercial supplies of our medicines could be disrupted and our results of operations and financial condition could be materially and adversely affected if the operations of these third-party suppliers or logistics partners were affected by a man-made or natural disaster or other business interruption. The ultimate impact of such events on us, our significant suppliers and our general infrastructure is unknown.
If our information technology systems or data, or those of third parties upon which we rely, are or were compromised or threatened to be compromised, we could experience adverse consequences resulting from such compromise, including but not limited to regulatory investigations or actions; litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; loss of sales; and other adverse consequences.
In the ordinary course of our business, we may collect, receive, store, generate, use, protect, secure, dispose of, transmit, disclose, or otherwise make accessible (collectively “process”) proprietary, confidential, and sensitive data, including personal data (such as health-related data), intellectual property, and trade secrets. We may rely upon third parties (such as service providers) for our data processing-related activities. We may share or receive sensitive data with or from third parties.
Cyberattacks, malicious internet-based activity, and online and offline fraud are prevalent and continue to increase. These threats are becoming increasingly difficult to detect. These threats come from a variety of sources. In addition to traditional computer “hackers,” threat actors, personnel misconduct, or error (such as theft or misuse), sophisticated nation-state and nation-state supported actors now engage in attacks. Some actors now engage and are expected to continue to engage in cyber-attacks, including without limitation nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During times of war and other major conflicts, we, the third parties upon which we rely, may be vulnerable to a heightened risk of these attacks, including retaliatory cyber-attacks, that could materially disrupt our systems and operations, and ability to produce, sell and distribute our medicines. We may be subject to a variety of evolving threats, including but not limited to social engineering attacks, malware, denial-of-service attacks, ransomware, supply-chain attacks, software bugs, server malfunction, software or hardware failures, loss of data or other information technology assets, adware, telecommunications failures, earthquakes, fire, flood, and other similar threats. Ransomware attacks, including those perpetrated by organized criminal threat actors, nation-states, and nation-state supported actors, are becoming increasingly prevalent and severe and can lead to significant interruptions in our operations, loss or misuse of data and income, reputational harm, and diversion of funds. Extortion payments may alleviate the negative impact of a ransomware attack, but we may be unwilling or unable to make such payments due to, for example, applicable laws or regulations prohibiting payments. Similarly, supply-chain attacks have increased in frequency and severity, and we cannot guarantee that third parties and infrastructure in our supply chain have not been compromised or that they do not contain exploitable defects or bugs that could result in a breach of or disruption to our information technology systems or the third-party information technology systems that support us and our services. Our remote workforce poses increased risks to our information technology systems and data, as more of our employees work from home, utilizing network connections outside our premises. Future business transactions (such as acquisitions or integrations) could expose us to additional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies.
Any of the previously identified or similar threats could cause a security incident. A security incident could result in unauthorized, unlawful or accidental acquisition, modification, destruction, loss, alteration, encryption, disclosure of or access to data. A security incident could disrupt our (and third parties upon whom we rely) ability to provide our products and services. We may expend significant resources or modify our business activities (including our clinical trial activities) in an effort to protect against security incidents. Certain data privacy and security obligations may require us to implement and maintain specific security measures, industry-standard or reasonable security measures to protect our information technology systems and data.
79
While we have implemented security measures designed to protect against a security incident, there can be no assurance that these measures will be effective. We have not always been able in the past and may be unable in the future to detect vulnerabilities in our information technology systems because such threats and techniques change frequently, are often sophisticated in nature, and may not be detected until after a security incident has occurred. Despite our efforts to identify and remediate vulnerabilities, if any, in our information technology systems, our efforts may not be successful. Further, we may experience, delays in developing and deploying remedial measures designed to address any such identified vulnerabilities.
Applicable data privacy and security obligations may require us to notify relevant stakeholders of security incidents. Such disclosures are costly, and the disclosures or the failure to comply with such requirements, could lead to adverse impacts. If we (or a third party upon whom we rely) experience a security incident or are perceived to have experienced a security incident, we may experience adverse consequences. These consequences may include: government enforcement actions; additional reporting requirements and/or oversight; restrictions on processing data; litigation; indemnification obligations; negative publicity; reputational harm; monetary fund diversions; interruptions in our operations; financial loss; and other similar harms. Security incidents and attendant consequences may cause customers to stop using our products and services, deter new customers for using our products and services, and negatively impact our ability to grow and operate our business.
Our contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in our contracts are sufficient to protect us from liabilities, damages, or claims related to our data privacy and security obligations. We cannot be certain that (a) our liability insurance will be sufficient in type or amount to cover us against claims related to security incidents; (b) such coverage will cover any indemnification claims against us relating to any security incident, will continue to be available to us on economically reasonable terms, or at all; or (c) any insurer will not deny coverage as to any future claim. The successful assertion of one or more claims against us that exceed available insurance coverage, or the occurrence of changes in our insurance policies, including premium increases or the imposition of large deductible or co-insurance requirements, could adversely affect our reputation, business, financial condition and results of operations.
We are subject to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, policies, standards and other obligations related to data privacy and security. Our actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions; litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; loss of customers or sales; and other adverse business consequences.
In the ordinary course of business, we may process personal data and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, data we collect about trial participants in connection with clinical trials, and sensitive third-party data. Our data processing activities may subject us to numerous data privacy and security obligations, such as various laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements, and other obligations relating to data privacy and security.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, and consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act). For example, the California Consumer Privacy Act of 2018, or CCPA, requires businesses to provide specific disclosures in privacy notices and honor requests of California residents to exercise certain privacy rights. The CCPA provides for civil penalties of up to $7,500 per violation and allows private litigants affected by certain data breaches to recover significant statutory damages. Although the CCPA exempts some data processed in the context of clinical trials, the CCPA may increase compliance costs and potential liability with respect to other personal data we may maintain about California residents. In addition, the California Privacy Rights Act of 2020, effective January 1, 2023, expanded the CCPA’s requirements and establish a new regulatory agency to implement and enforce the law. Further, Virginia enacted the Virginia Consumer Data Protection Act, effective January 1, 2023, Colorado passed the Colorado Privacy Rights Act, effective July 1, 2023, Connecticut passed the Connecticut Data Privacy Act, effective July 1, 2023, and Utah passed the Utah Consumer Privacy Act, effective December 31, 2023. A number of other proposals exist for new federal and state privacy legislation that could increase our potential liability, increase our compliance costs, and affect our ability to collect and use personal information. These developments may further complicate compliance efforts and may increase legal risk and compliance costs for us, the third parties upon whom we rely.
80
Outside the United States, an increasing number of laws, regulations, and industry standards apply to data privacy and security. For example, the EU GDPR and the UK GDPR, impose strict requirements for processing personal data, and violators of these laws face significant penalties. Under the EU GDPR, companies may face temporary or definitive bans on data processing and other corrective actions; fines of up to 20 million Euros or 4% of annual global revenue, whichever is greater; or private litigation related to processing of personal data brought by classes of data subjects or consumer protection organizations authorized at law to represent their interests.
Certain jurisdictions have enacted data localization laws and cross-border personal data transfer laws, which could make it more difficult to transfer information across jurisdictions (such as transferring or receiving personal data that originates in the EU or in other foreign jurisdictions). In particular, the EEA and the UK have significantly restricted the transfer of personal data to the United States and other countries whose privacy laws it believes are inadequate. Other jurisdictions may adopt similarly stringent interpretations of their data localization and cross-border data transfer laws. Although there are currently various mechanisms that may be used to transfer personal data from the EEA and UK to the United States in compliance with law, such as the EEA and UK’s standard contractual clauses, these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States. If there is no lawful manner for us to transfer personal data from the EEA, the UK or other jurisdictions to the United States, or if the requirements for a legally compliant transfer are too onerous, we may face increased exposure to regulatory actions, the interruption or degradation of our operations, substantial fines and penalties, and injunctions against processing or transferring personal data from Europe or other foreign jurisdictions. The inability to import personal data to the United States could significantly and negatively impact our business operations, including by limiting our ability to conduct clinical trial activities in the EEA, the UK and elsewhere; limiting our ability to collaborate with third parties; or requiring us to increase our personal data processing capabilities and infrastructure in foreign jurisdictions at significant expense. Additionally, companies that transfer personal data out of the EEA and UK to other jurisdictions, particularly to the United States, are subject to increased scrutiny from regulators, individual litigants, and activist groups. Some European regulators have ordered certain companies to suspend or permanently cease certain transfers out of the EEA for allegedly violating the GDPR’s cross-border data transfer limitations.
In addition to data privacy and security laws, we may be contractually subject to industry standards adopted by industry groups and may become subject to such obligations in the future. We may also be bound by contractual obligations related to data privacy and security, and our efforts to comply with such obligations may not be successful. For example, certain privacy laws, such as the GDPR and the CCPA, require companies to impose specific contractual restrictions on their service providers. We publish privacy policies, marketing materials and other statements regarding data privacy and security. If these policies, materials, or statements are found to be deficient, lacking in transparency, deceptive, unfair, or misrepresentative of our practices, we may be subject to investigation, enforcement actions by regulators or other adverse consequences.
Obligations related to data privacy and security are quickly changing, becoming increasingly stringent, and creating regulatory uncertainty. Additionally, these obligations may be subject to differing applications and interpretations, which may be inconsistent or conflict among jurisdictions. Preparing for and complying with these obligations requires us to devote significant resources. These obligations may necessitate changes to our services, information technologies, systems, and practices and to those of any third parties that process personal data on our behalf. We may at times fail (or be perceived to have failed) in our efforts to comply with our data privacy and security obligations. Moreover, despite our efforts, our personnel or third parties on whom we rely may fail to comply with such obligations, which could negatively impact our business operations. If we or the third parties on which we rely fail, or are perceived to have failed, to address or comply with applicable data privacy and security obligations, we could face significant consequences, including but not limited to: government enforcement actions; litigation; additional reporting requirements and/or oversight; bans on processing personal data; orders to destroy or not use personal data; and imprisonment of company officials.
Any of these events could have a material adverse effect on our reputation, business, or financial condition, including but not limited to: loss of customers; interruptions or stoppages in our business operations, including clinical trials; inability to process personal data or to operate in certain jurisdictions; limited ability to develop or commercialize our medicines; expenditure of time and resources to defend any claim or inquiry; adverse publicity; or substantial changes to our business model or operations.
81
We are subject to various laws and regulations pertaining to export controls and trade and economic sanctions, which can impact our business activities and subject us to liability for noncompliance.
Our activities are subject to various U.S. and foreign export control and sanctions laws and regulations, including the U.S. Department of Commerce’s Export Administration Regulations and the U.S. Department of the Treasury’s Office of Foreign Assets Control economic and trade sanctions programs. Export control laws may restrict our ability to export, reexport, or transfer our medicines outside of the United States without authorization. Sanctions laws may prohibit or restrict our ability to provide medicines and services to certain countries, territories, entities, or individuals.
These laws and regulations are subject to frequent change which may impact the global economy and supply chains in ways that impact our business. Notably, in response to Russia’s invasion of Ukraine in February 2022, the United States and its allies significantly expanded export control and sanctions prohibitions and restrictions aimed at Russia, Belarus, and certain regions in Ukraine. We have a limited number of ongoing clinical research studies in Russia, Belarus, and non-restricted regions in Ukraine, and are monitoring the potential impact of the conflict on our clinical trial activities. We are no longer initiating new clinical trials or opening new investigator sites in these countries. With respect to our clinical trial of UPLIZNA in myasthenia gravis, disruptions in payment systems and other logistical challenges related to the conflict in Ukraine have negatively impacted enrollment and operations of clinical sites in the region which, combined with other negative impacts related to COVID-19, has delayed our expected timeline for topline data to 2024. Although we continue to work diligently with patients and sites across the impacted regions and are putting the appropriate measures in place to meet enrollment targets, further escalation of the conflict and any additional export controls and sanctions or adverse regulatory developments could restrict, prohibit, or otherwise impair our studies.
Compliance with these laws and regulations can be time- and resource-intensive. Although we are committed to complying with all applicable export control and sanctions laws and regulations, we cannot guarantee full compliance. Violations of these regimes can result in significant financial penalties, loss of licensing privileges, other administrative penalties, reputational harm, and adverse business impact.
Product liability lawsuits may cause us to incur substantial liabilities or could require us to limit commercialization of our medicines.
We face an inherent risk of product liability claims as a result of the commercial sales of our medicines and the clinical testing of our medicine candidates. In particular, we have been and may continue to be sued if any of our medicines or medicine candidates allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the medicine, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. For example, several product liability suits have been filed against us alleging that TEPEZZA is defectively designed and/or fails to include proper warnings regarding potential adverse events associated with hearing impairment. While we intend to vigorously defend ourselves in the lawsuits, no assurance can be given as to the outcome of the litigation, whether additional similar lawsuits will be initiated or whether our insurance coverage will be adequate to cover the costs of the litigation or any resulting settlements or judgments. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our medicines and medicine candidates. Even a successful defense will require significant financial and management resources.
82
Regardless of the merits or eventual outcome, liability claims may result in:
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of medicines we develop. We currently carry product liability insurance covering our clinical studies and commercial medicine sales in the amount of $125.0 million in the aggregate. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. If we determine that it is prudent to increase our product liability coverage due to the on-going commercialization of our current medicines in the United States, and/or the potential commercial launches of any of our medicines in additional markets or for additional indications, we may be unable to obtain such increased coverage on acceptable terms or at all. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Our business involves the use of hazardous materials, and we and our third-party manufacturers must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our third-party manufacturers’ activities involve the controlled storage, use and disposal of hazardous materials owned by us, including the components of our medicine candidates and other hazardous compounds. We and our manufacturers are subject to federal, state and local as well as foreign laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, state, federal or foreign authorities may curtail the use of these materials and interrupt our business operations. We currently only maintain Environmental Pollution Liability insurance coverage related to our South San Francisco facility and our Rockville, Maryland facility. If we are subject to any liability as a result of our third-party manufacturers’ activities involving hazardous materials, our business and financial condition may be adversely affected. In the future we may seek to establish longer-term third-party manufacturing arrangements, pursuant to which we would seek to obtain contractual indemnification protection from such third-party manufacturers potentially limiting this liability exposure.
83
Risks Related to our Financial Position and Capital Requirements
We may not remain profitable in future periods.
Although we recorded operating income and net income for the last several years, we may incur operating losses in the future. Losses in prior periods resulted principally from costs incurred in our development activities for our medicines and medicine candidates, commercialization activities related to our medicines and costs associated with our acquisition transactions. Our prior losses, combined with possible future losses, have had and may continue to have an adverse effect on our shareholders’ equity and working capital. Our ability to maintain profitability will depend on the revenues we generate from the sale of our medicines being sufficient to cover our operating expenses. We also expect our operating expenses to increase substantially as a result of continuing to develop our pipeline of medicine candidates, which will negatively impact our future profitability until such time, if ever, that these potential medicine candidates are approved and successfully commercialized, as well as developing our manufacturing and international sales and marketing capabilities.
We have limited sources of revenues and significant expenses. We cannot be certain that we will sustain profitability, which would depress the market price of our ordinary shares and could cause our investors to lose all or a part of their investment.
Our ability to sustain profitability depends upon our ability to generate sales of our medicines. The commercialization of our medicines has been primarily in the United States. We may never be able to successfully commercialize our medicines or develop or commercialize other medicines in the United States, which we believe represents our most significant commercial opportunity. Our ability to generate future revenues depends heavily on our success in:
Even if we do generate additional medicine sales, we may not be able to sustain profitability on a quarterly or annual basis. Our failure to remain profitable would depress the market price of our ordinary shares and could impair our ability to raise capital, expand our business, diversify our medicine offerings or continue our operations.
We may need to obtain additional financing to fund additional acquisitions.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to:
84
While we believe that our existing cash and cash equivalents, along with future cash flows based on our current expectations of continued revenue growth, will be sufficient to fund our operations, we may need to raise additional funds if we choose to expand our commercialization or development efforts more rapidly than presently anticipated, if we develop or acquire additional medicines or acquire companies, or if our revenue does not meet expectations.
We cannot be certain that additional funding will be available on acceptable terms, or at all. As a result of the COVID-19 pandemic and actions taken to slow its spread, as well as actual or anticipated changes in interest rates and economic inflation, the global credit and financial markets have at times experienced extreme volatility and disruptions, including diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. If the equity and credit markets deteriorate, it may make any additional debt or equity financing more difficult, more costly and more dilutive. In particular, rising interest rates have made debt financing more expensive which may limit our ability to finance future acquisitions through additional borrowing. In addition, the transaction agreement with Amgen contains restrictions on our ability to incur additional indebtedness or issue new equity in connection with financing activities or otherwise. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our medicines or medicine candidates or one or more of our other R&D initiatives, or delay, cut back or abandon our plans to grow the business through acquisitions. We also could be required to:
In addition, if we are unable to secure financing to support future acquisitions, our ability to execute on a key aspect of our overall growth strategy would be impaired.
Any of the above events could significantly harm our business, financial condition and prospects.
We have incurred a substantial amount of debt, which could adversely affect our business, including by restricting our ability to engage in additional transactions or incur additional indebtedness, and prevent us from meeting our debt obligations.
As of December 31, 2022, we had $2.6 billion book value, or $2.6 billion aggregate principal amount of indebtedness, including $2.0 billion in secured indebtedness.
This substantial level of debt could have important consequences to our business, including, but not limited to:
85
Our credit agreement and the indenture governing our 5.5% Senior Notes due 2027, or 2027 Senior Notes, impose, and the terms of any future indebtedness may impose, various covenants that limit our ability and/or the ability of our restricted subsidiaries’ (as designated under such agreements) to, among other things, pay dividends or distributions, repurchase equity, prepay junior debt and make certain investments, incur additional debt and issue certain preferred stock, incur liens on assets, engage in certain asset sales, consolidate with or merge or sell all or substantially all of our assets, enter into transactions with affiliates, designate subsidiaries as unrestricted subsidiaries, and allow to exist certain restrictions on the ability of restricted subsidiaries to pay dividends or make other payments to us.
Our ability to obtain future financing and engage in other transactions may be restricted by these covenants. In addition, any credit ratings will impact the cost and availability of future borrowings and our cost of capital. Our ratings at any time will reflect each rating organization’s then opinion of our financial strength, operating performance and ability to meet our debt obligations. There can be no assurance that we will achieve a particular rating or maintain a particular rating in the future. A reduction in our credit ratings may limit our ability to borrow at acceptable interest rates. If our credit ratings were downgraded or put on watch for a potential downgrade, we may not be able to sell additional debt securities or borrow money in the amounts, at the times or interest rates or upon the more favorable terms and conditions that might otherwise be available. Any impairment of our ability to obtain future financing on favorable terms could have an adverse effect on our ability to refinance any of our then-existing debt and may severely restrict our ability to execute on our business strategy, which includes the continued acquisition of additional medicines or businesses.
As a result of the COVID-19 pandemic and actions taken to slow its spread, as well as actual or anticipated changes in interest rates and economic inflation, the global credit and financial markets have experienced extreme volatility and disruptions, including diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. In addition, government efforts to stimulate economic activity in the face of the COVID-19 pandemic have caused interest rates to fluctuate and created uncertainty as to future fluctuations. If the equity and credit markets deteriorate, it may make any additional debt or equity financing more difficult, more costly or more dilutive.
We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments under or to refinance our debt obligations depends on our financial condition and operating performance, which is subject to prevailing economic, industry and competitive conditions and to certain financial, business and other factors beyond our control. For example, we expect that the COVID-19 pandemic and actions taken to slow its spread will continue to have a negative impact on net sales of our medicines, which will in turn negatively impact our cash flows. Our ability to generate cash flow to meet our payment obligations under our debt may also depend on the successful implementation of our operating and growth strategies. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. We cannot assure that we will maintain a level of cash flows from operating activities sufficient to pay the principal, premium, if any, and interest on our indebtedness.
86
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures, sell assets or business operations, seek additional capital or restructure or refinance our indebtedness. We cannot ensure that we would be able to take any of these actions, that these actions would be successful and permit us to meet our scheduled debt service obligations or that these actions would be permitted under the terms of existing or future debt agreements, including the indenture that governs the 2027 Senior Notes and our credit agreement. In addition, any failure to make payments of interest and principal on our outstanding indebtedness on a timely basis would likely result in a reduction of our credit rating, which could harm our ability to incur additional indebtedness.
If we cannot make scheduled payments on our debt, we will be in default and, as a result:
We generally have broad discretion in the use of our cash and may not use it effectively.
Our management has broad discretion in the application of our cash, and investors will be relying on the judgment of our management regarding the use of our cash. Our management may not apply our cash in ways that ultimately increase the value of any investment in our securities. We expect to use our existing cash to fund commercialization activities for our medicines, to potentially fund additional medicine, medicine candidate or business acquisitions, to potentially fund additional regulatory approvals of certain of our medicines, to potentially fund development, life cycle management or manufacturing activities of our medicines and medicine candidates, to potentially fund share repurchases, and for working capital, milestone payments, capital expenditures and general corporate purposes. We may also invest our cash in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our shareholders. If we do not invest or apply our cash in ways that enhance shareholder value, we may fail to achieve expected financial results, which could cause the price of our ordinary shares to decline.
Our ability to use net operating loss carryforwards and certain other tax attributes to offset U.S. income taxes may be limited.
Under Sections 382 and 383 of the Code, if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period), the corporation’s ability to use pre-change net operating loss carryforwards and other pre-change tax attributes to offset post-change income may be limited. Certain net operating losses generated before an August 2, 2012 ownership change and federal net operating losses and federal tax credits acquired through the Viela acquisition are subject to an annual limitation. The net operating loss carryforward and tax credit carryforward limitations are cumulative such that any use of the carryforwards below the limitations in one year will result in a corresponding increase in the limitations for the subsequent tax year.
Following certain acquisitions of a U.S. corporation by a foreign corporation, Section 7874 of the Code limits the ability of the acquired U.S. corporation and its U.S. affiliates to utilize U.S. tax attributes such as net operating losses to offset U.S. taxable income resulting from certain transactions. Based on the limited guidance available, we expect this limitation is applicable for approximately ten years following our merger transaction with Vidara with respect to certain intercompany transactions. As a result, we or our other U.S. affiliates may not be able to utilize U.S. tax attributes to offset U.S. taxable income or U.S. tax liability respectively, if any, resulting from certain intercompany taxable transactions during such period. Notwithstanding this limitation, we expect that we will be able to fully use our U.S. net operating losses and tax credits prior to their expiration. As a result of this limitation, however, it may take Horizon Therapeutics USA, Inc. (formerly known as Horizon Pharma USA, Inc. and as the successor to HPI) longer to use its net operating losses and tax credits. Moreover, contrary to these expectations, it is possible that the limitation under Section 7874 of the Code on the utilization of U.S. tax attributes could prevent us from fully utilizing our U.S. tax attributes prior to their expiration if we do not generate sufficient taxable income or tax obligations.
Any limitation on our ability to use our net operating loss and tax credit carryforwards, including the carryforwards of companies that we acquire, will likely increase the taxes we would otherwise pay in future years if we were not subject to such limitations.
87
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
From time to time, including recently as a result of the COVID-19 pandemic and actions taken to slow its spread, as well as actual or anticipated changes in interest rates and economic inflation, global credit and financial markets have experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, and uncertainty about economic stability. Our general business strategy may be adversely affected by any such economic downturn, volatile business environment and continued unpredictable and unstable market conditions. If the equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult to complete, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and share price and could require us to delay or abandon commercialization or development plans. There is a risk that one or more of our current service providers, manufacturers and other partners may not survive an economic down-turn, which could directly affect our ability to attain our operating goals on schedule and on budget.
At December 31, 2022, we had $2.4 billion of cash and cash equivalents consisting of cash, money market funds, time deposits and U.S. federal government securities. While we are not aware of any downgrades, material losses, or other significant deterioration in the fair value of our cash equivalents since December 31, 2022, no assurance can be given that deterioration in conditions of the global credit and financial markets would not negatively impact our current portfolio of cash equivalents or our ability to meet our financing objectives. Dislocations in the credit market may adversely impact the value and/or liquidity of marketable securities owned by us.
The UK’s referendum to leave the EU and the UK’s exit from the EU on January 31, 2020, or “Brexit,” has caused and may continue to cause disruptions to capital and currency markets worldwide. The full impact of Brexit, however, remains uncertain. A Trade and Cooperation Agreement, or the TCA, which outlines the trading relationship between the UK and the EU was agreed in December 2020, entered into force provisionally on January 1, 2021, and has been permanently applicable since May 1, 2021.
There remains uncertainty as to the practical impacts of Brexit and, especially in the early stages of the UK and the EU operating under different legislation, our results of operations and access to capital may be negatively affected by interest rate, exchange rate and other market and economic volatility, as well as political uncertainty. Brexit may also have a detrimental effect on our customers, distributors and suppliers, which would, in turn, adversely affect our revenues and financial condition.
While the TCA provides for the tariff-free trade of medicinal products between the UK and the EU there may be additional non-tariff costs to such trade which did not exist prior to the TCA coming into force. Further, should the UK diverge from the EU from a regulatory perspective in relation to medicinal products, tariffs could be put into place in the future. Any further changes in international trade, tariff and import/export regulations as a result of Brexit or otherwise may impose unexpected duty costs or other non-tariff barriers on us.
We could therefore, both now and in the future, face additional expenses (when compared to the position prior to the TCA coming into force) to operate our business, which could harm or delay our business. These developments, or the perception that any of them could occur, may significantly reduce global trade and, in particular, trade between the impacted nations and the UK.
88
If the London Inter-Bank Offered Rate, or LIBOR, is discontinued, interest payments under our credit agreement may be calculated using another reference rate.
In July 2017, the Chief Executive of the UK Financial Conduct Authority, or FCA, which regulates LIBOR, announced that the FCA intends to phase out the use of LIBOR by the end of 2021. However, the cessation date has been deferred to June 30, 2023 for the most commonly used tenors in U.S. dollar LIBOR (i.e., overnight and one, three and six months). In addition, the U.S. Federal Reserve, in conjunction with the Alternative Reference Rates Committee, a steering committee composed of large U.S. financial institutions, is considering replacing U.S. dollar LIBOR with the Secured Overnight Financing Rate, or SOFR, a new index calculated by short-term repurchase agreements, backed by Treasury securities. Although there have been certain issuances utilizing SOFR, it is unknown whether this or any other alternative reference rate will attain market acceptance as a replacement for LIBOR. LIBOR is used as a benchmark rate throughout our credit agreement, and our credit agreement does not address all circumstances in which LIBOR ceases to be published. There remains uncertainty regarding the future utilization of LIBOR and the nature of any replacement rate, and any potential effects of the transition away from LIBOR on us are not known. The transition process may involve, among other things, increased volatility and illiquidity in markets for instruments that currently rely on LIBOR and may result in increased borrowing costs, the effectiveness of related transactions such as hedges, uncertainty under applicable documentation, including the credit agreement, or difficult and costly processes to amend such documentation. As a result, our ability to refinance our credit agreement or other indebtedness or to hedge our exposure to floating rate instruments may be impaired, which would adversely affect the operations of our business. We do not expect the planned discontinuation of LIBOR to have a material impact on interest payments incurred under our credit agreement.
Changes in accounting rules or policies may affect our financial position and results of operations.
Accounting principles generally accepted in the United States, or GAAP, and related implementation guidelines and interpretations can be highly complex and involve subjective judgments. Changes in these rules or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations. In addition, our operation as an Irish company with multiple subsidiaries in different jurisdictions adds additional complexity to the application of GAAP and this complexity will be exacerbated further if we complete additional strategic transactions. Changes in the application of existing rules or guidance applicable to us or our wholly-owned subsidiaries could significantly affect our consolidated financial position and results of operations.
Covenants under the indenture governing our 2027 Senior Notes and our credit agreement may restrict our business and operations in many ways, and if we do not effectively manage our covenants, our financial conditions and results of operations could be adversely affected.
The indenture governing the 2027 Senior Notes and the credit agreement impose various covenants that limit our ability and/or our restricted subsidiaries’ ability to, among other things:
89
These covenants may:
If we are unable to successfully manage the limitations and decreased flexibility on our business due to our significant debt obligations, we may not be able to capitalize on strategic opportunities or grow our business to the extent we would be able to without these limitations.
Our failure to comply with any of the covenants could result in a default under the credit agreement or the indenture governing the 2027 Senior Notes, which could permit the administrative agent or the trustee, as applicable, or permit the lenders or the holders of the 2027 Senior Notes to cause the administrative agent or the trustee, as applicable, to declare all or part of any outstanding senior secured term loans or revolving loans, or the 2027 Senior Notes to be immediately due and payable or to exercise any remedies provided to the administrative agent or the trustee, including, in the case of the credit agreement proceeding against the collateral granted to secure our obligations under the credit agreement. An event of default under the credit agreement or the indenture governing the 2027 Senior Notes could also lead to an event of default under the terms of the other agreement. Any such event of default or any exercise of rights and remedies by our creditors could seriously harm our business.
If intangible assets that we have recorded in connection with our acquisition transactions become impaired, we could have to take significant charges against earnings.
In connection with the accounting for our various acquisition transactions, we have recorded significant amounts of intangible assets. Under GAAP, we must assess, at least annually and potentially more frequently, whether the value of goodwill has been impaired. Amortizing intangible assets will be assessed for impairment in the event of an impairment indicator. For example, during the year ended December 31, 2018, we recorded an impairment of $33.6 million to fully write off the book value of developed technology related to PROCYSBI in Canada and Latin America. More recently, our interim goodwill impairment test in the second quarter of 2022 indicated an impairment which represented the difference between the estimated fair value of the former inflammation reporting unit and its carrying value. As a result, we recognized an impairment charge of $56.2 million in June 2022 representing the full amount of goodwill for the former inflammation reporting unit. Refer to Note 8, Goodwill and Intangible Assets, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K. Such impairment and any reduction or other impairment of the value of goodwill or other intangible assets will result in a charge against earnings, which could materially adversely affect our results of operations and shareholders’ equity in future periods.
90
Risks Related to Our Intellectual Property
If we are unable to obtain or protect intellectual property rights related to our medicines and medicine candidates, we may not be able to compete effectively in our markets.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our medicines and medicine candidates. The strength of patents in the biopharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own may fail to result in issued patents with claims that cover our medicines in the United States or in other foreign countries. If this were to occur, early generic competition could be expected against our current medicines and other medicine candidates in development. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which prior art can invalidate a patent or prevent a patent from issuing based on a pending patent application.
Even if patents do successfully issue, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated.
Any adverse outcome in these matters or any new generic challenges that may arise could result in one or more generic versions of our medicines being launched before the expiration of the listed patents, which could adversely affect our ability to successfully execute our business strategy to increase sales of our medicines, and would negatively impact our financial condition and results of operations, including causing a significant decrease in our revenues and cash flows.
Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. If the patent applications we hold with respect to our medicines fail to issue or if their breadth or strength of protection is threatened, it could dissuade companies from collaborating with us to develop them and threaten our ability to commercialize our medicines. We cannot offer any assurances about which, if any, patents will issue or whether any issued patents will be found not invalid and not unenforceable or will go unthreatened by third parties. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to our medicines or any other medicine candidates. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States can be provoked by a third-party or instituted by us to determine which party was the first to invent any of the subject matter covered by the patent claims of our applications.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our drug discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. Although we expect all of our employees to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques.
Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and other countries. For example, if the issuance, in a given country, of a patent to us, covering an invention, is not followed by the issuance, in other countries, of patents covering the same invention, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, our ability to protect our intellectual property in those countries may be limited. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
91
If we fail to comply with our obligations in the agreements under which we license rights to technology from third parties, we could lose license rights that are important to our business.
We are party to a number of technology licenses that are important to our business and expect to enter into additional licenses in the future. For example, we hold an exclusive, worldwide license from Roche to patents and know-how for TEPEZZA. We also have exclusive sub-licenses for rights licensed to Roche for TEPEZZA by certain third-party licensors. Roche may have the right to terminate the license upon our breach, if not cured within a specified period of time. Roche may also terminate the license in the event of our bankruptcy or insolvency, or if we challenge the validity of Roche’s patents. If the license is terminated for our breach or based on our challenging the validity of Roche’s patents, then all rights and licenses granted to us by Roche would also terminate, and we may be required to assign and transfer to Roche certain filings and approvals, trademarks, and data in our possession necessary for the development and commercialization of TEPEZZA, and assign clinical trial agreements to the extent permitted. We may also be required to grant Roche an exclusive license under our patents and know-how for TEPEZZA, and to manufacture and supply TEPEZZA to Roche for a transitional period. If one or more of these licenses is terminated, it may be impossible for us to continue to commercialize TEPEZZA, which would have a material adverse effect on our business, financial condition and results of operations.
We also hold an exclusive license to patents and technology from Duke University, or Duke, and Mountain View Pharmaceuticals, Inc., or MVP, covering KRYSTEXXA. Duke and MVP may terminate the license if we commit fraud or for our willful misconduct or illegal conduct. Duke and MVP may also terminate the license upon our material breach of the agreement, if not cured within a specified period of time, or upon written notice if we have committed two or more material breaches under the agreement. Duke and MVP may also terminate the license in the event of our bankruptcy or insolvency. If the license is terminated, it may be impossible for us to continue to commercialize KRYSTEXXA, which would have a material adverse effect on our business, financial condition and results of operations.
In addition, we rely on a license from Bausch with respect to technology developed by Bausch in connection with the manufacturing of RAVICTI. The purchase agreement under which Hyperion Therapeutics, Inc., or Hyperion, purchased the rights to RAVICTI contains obligations to pay Bausch regulatory and sales milestone payments relating to RAVICTI, as well as royalties on the net sales of RAVICTI. On May 31, 2013, when Hyperion acquired BUPHENYL under a restated collaboration agreement with Bausch, Hyperion received a license to use some of the manufacturing technology developed by Bausch in connection with the manufacturing of BUPHENYL. The restated collaboration agreement also contains obligations to pay Bausch regulatory and sales milestone payments, as well as royalties on net sales of BUPHENYL. If we fail to make a required payment to Bausch and do not cure the failure within the required time period, Bausch may be able to terminate the license to use its manufacturing technology for RAVICTI and BUPHENYL. If we lose access to the Bausch manufacturing technology, we cannot guarantee that an acceptable alternative method of manufacture could be developed or acquired. Even if alternative technology could be developed or acquired, the loss of the Bausch technology could still result in substantial costs and potential periods where we would not be able to market and sell RAVICTI and/or BUPHENYL. We also license intellectual property necessary for commercialization of RAVICTI from an external party. This party may be entitled to terminate the license if we breach the agreement, including failure to pay required royalties on net sales of RAVICTI, or we do not meet specified diligence obligations in our development and commercialization of RAVICTI, and we do not cure the failure within the required time period. If the license is terminated, it may be difficult or impossible for us to continue to commercialize RAVICTI, which would have a material adverse effect on our business, financial condition and results of operations.
We are subject to contractual obligations under our amended and restated license agreement with The Regents of the University of California, San Diego, as amended, with respect to PROCYSBI. If one or more of these licenses was terminated, we would have no further right to use or exploit the related intellectual property, which would limit our ability to develop PROCYSBI in other indications, and could impact our ability to continue commercializing PROCYSBI in its approved indications.
Following our acquisition of Viela on March 15, 2021, we are a party to a number of intellectual property license agreements including (i) our licenses with Duke University and Dana-Farber Cancer Institute related to UPLIZNA, (ii) our license with SBI Biotech Co. Ltd related to daxdilimab, (iii) our license with MedImmune, LLC, or MedImmune, related to dazodalibep, (iv) our sublicense with MedImmune for its license with Lonza Sales AG, or Lonza, related to UPLIZNA and daxdilimab, (v) our sublicense with MedImmune for its license with BioWa, Inc., or BioWa, related to UPLIZNA, and (vi) our sublicense with MedImmune for its license with BioWa and Lonza related to daxdilimab. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, we may be required to make certain payments to the licensor, we may lose the exclusivity of our license, or the licensor may have the right to terminate the license, in which event we would not be able to develop or market medicines covered by the license.
92
We also license rights to know-how and trademarks for ACTIMMUNE from Genentech Inc., or Genentech. Genentech may terminate the agreement upon our material default, if not cured within a specified period of time. Genentech may also terminate the agreement in the event of our bankruptcy or insolvency. Upon such a termination of the agreement, all intellectual property rights conveyed to us under the agreement, including the rights to the ACTIMMUNE trademark, revert to Genentech. If we fail to comply with our obligations under this agreement, we could lose the ability to market and distribute ACTIMMUNE, which would have a material adverse effect on our business, financial condition and results of operations.
Some intellectual property has been discovered through government-funded programs and thus may be subject to federal regulations such as “march-in” rights, certain reporting requirements and a preference for U.S.-based companies. Compliance with such regulations may limit our exclusive rights, and limit our ability to contract with non-U.S. manufacturers.
Some of our intellectual property rights, specifically, intellectual property rights related to UPLIZNA that are in-licensed from Duke University, were generated through the use of U.S. government funding and are therefore subject to certain federal regulations. As a result, the U.S. government may have certain rights to intellectual property embodied in certain of our current or future medicine candidates pursuant to the Bayh-Dole Act of 1980, or the Bayh-Dole Act. These U.S. government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right, under certain limited circumstances, to require us to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred to as “march-in rights”). To our knowledge, however, the U.S. government has, to date, not exercised any march-in rights on any patented technology that was generated using U.S. government funds. The U.S. government also has the right to take title to these inventions if we or the applicable grantee fail to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require us to expend substantial resources. In addition, the U.S. government requires that any products embodying the subject invention or produced through the use of the subject invention be manufactured substantially in the United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. manufacturers may limit our ability to contract with non-U.S. product manufacturers for medicines covered by such intellectual property. To the extent any of our current or future intellectual property is generated through the use of U.S. government funding, the provisions of the Bayh-Dole Act may similarly apply.
The patent protection and patent prosecution for some of our medicine candidates is dependent on third parties.
While we normally seek and gain the right to fully prosecute the patents relating to our medicine candidates, there may be times when patents relating to our medicine candidates are controlled by our licensors. This is the case with current patents and patent applications licensed from MedImmune related to dazodalibep, and those licensed from Duke University related to inebilizumab. If we, or any of our future licensing partners fail to appropriately file, prosecute and maintain patent protection for patents covering any of our medicine candidates, our ability to develop and commercialize those medicine candidates may be adversely affected and we may not be able to prevent competitors from making, using, and selling competing products. In addition, even where we now have the right to control patent prosecution of patents and patent applications we have licensed from third parties, we may still be adversely affected or prejudiced by actions or inactions of our licensors.
93
Risks Related to Ownership of Our Ordinary Shares
The market price of our ordinary shares historically has been volatile and is likely to continue to be volatile, and you could lose all or part of any investment in our ordinary shares.
The trading price of our ordinary shares has been volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this report, these factors include:
94
In addition, the stock market in general, and The Nasdaq Global Select Market and the stock of biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may adversely affect the market price of our ordinary shares, regardless of our actual operating performance.
We have never declared or paid dividends on our share capital and we do not anticipate paying dividends in the foreseeable future.
We have never declared or paid any cash dividends on our ordinary shares. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future, including due to limitations that are currently imposed by our credit agreement, the indenture governing the 2027 Senior Notes and the transaction agreement with Amgen. Any return to shareholders will therefore be limited to the increase, if any, of our ordinary share price.
Future sales and issuances of our ordinary shares, securities convertible into our ordinary shares or rights to purchase ordinary shares or convertible securities could result in additional dilution of the percentage ownership of our shareholders and could cause our share price to decline.
Additional capital may be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities or securities convertible into or exchangeable for ordinary shares, our shareholders may experience substantial dilution. We may sell ordinary shares, and we may sell convertible or exchangeable securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell such ordinary shares, convertible or exchangeable securities or other equity securities in subsequent transactions, existing shareholders may be materially diluted. New investors in such subsequent transactions could gain rights, preferences and privileges senior to those of holders of ordinary shares. We also maintain equity incentive plans, including our Amended and Restated 2020 Equity Incentive Plan, as amended, Amended and Restated 2018 Equity Incentive Plan, as amended, 2014 Non-Employee Equity Plan, as amended, and 2020 Employee Share Purchase Plan, and intend to grant additional ordinary share awards under these and future plans, which will result in additional dilution to our existing shareholders.
95
Irish law differs from the laws in effect in the United States and may afford less protection to holders of our securities.
It may not be possible to enforce court judgments obtained in the United States against us in Ireland based on the civil liability provisions of the U.S. federal or state securities laws. In addition, there is some uncertainty as to whether the courts of Ireland would recognize or enforce judgments of U.S. courts obtained against us or our directors or officers based on the civil liabilities provisions of the U.S. federal or state securities laws or hear actions against us or those persons based on those laws. We have been advised that the United States currently does not have a treaty with Ireland providing for the reciprocal recognition and enforcement of judgments in civil and commercial matters. Therefore, a final judgment for the payment of money rendered by any U.S. federal or state court based on civil liability, whether or not based solely on U.S. federal or state securities laws, would not automatically or necessarily be enforceable in Ireland.
As an Irish company, we are governed by the Irish Companies Act 2014 (as amended), which differs in some material respects from laws generally applicable to U.S. corporations and shareholders, including, among others, differences relating to interested director and officer transactions and shareholder lawsuits. Likewise, the duties of directors and officers of an Irish company generally are owed to the company only. Shareholders of Irish companies generally do not have a personal right of action against directors or officers of the company and may exercise such rights of action on behalf of the company only in limited circumstances. Accordingly, holders of our securities may have more difficulty protecting their interests than would holders of securities of a corporation incorporated in a jurisdiction of the United States.
Provisions of our articles of association, and Irish law could delay or prevent a takeover of us by a third party.
Our articles of association contain provisions which could delay, defer or prevent a third-party from acquiring us, despite the possible benefit to our shareholders, or otherwise adversely affect the price of our ordinary shares. For example, our articles of association:
In addition, several mandatory provisions of Irish law could prevent or delay an acquisition of us. For example, Irish law does not permit shareholders of an Irish public limited company to take action by written consent with less than unanimous consent. We are also subject to various provisions of Irish law relating to mandatory bids, voluntary bids, requirements to make a cash offer and minimum price requirements, as well as substantial acquisition rules and rules requiring the disclosure of interests in our ordinary shares in certain circumstances.
These provisions may discourage potential takeover attempts, discourage bids for our ordinary shares at a premium over the market price or adversely affect the market price of, and the voting and other rights of the holders of, our ordinary shares. These provisions could also discourage proxy contests and make it more difficult for you and our other shareholders to elect directors other than the candidates nominated by our board of directors, and could depress the market price of our ordinary shares.
Any attempts to take us over will be subject to Irish Takeover Rules and subject to review by the Irish Takeover Panel.
We are subject to the Irish Takeover Rules, under which our board of directors will not be permitted to take any action which might frustrate Amgen’s offer for our ordinary shares or another third party offer for our ordinary shares.
96
A transfer of our ordinary shares may be subject to Irish stamp duty.
In certain circumstances, the transfer of shares in an Irish incorporated company will be subject to Irish stamp duty, which is a legal obligation of the buyer. This duty is currently charged at the rate of 1.0% of the price paid or the market value of the shares acquired, if higher. Because our ordinary shares are traded on a recognized stock exchange in the United States, an exemption from this stamp duty is available to transfers by shareholders who hold ordinary shares beneficially through brokers, which in turn hold those shares through the Depositary Trust Company, or DTC, to holders who also hold through DTC. However, a transfer by or to a record holder who holds ordinary shares directly in his, her or its own name could be subject to this stamp duty. We, in our absolute discretion and insofar as the Irish Companies Act 2014 (as amended) or any other applicable law permit, may, or may provide that one of our subsidiaries will pay Irish stamp duty arising on a transfer of our ordinary shares on behalf of the transferee of such ordinary shares. If stamp duty resulting from the transfer of ordinary shares which would otherwise be payable by the transferee is paid by us or any of our subsidiaries on behalf of the transferee, then in those circumstances, we will, on our behalf or on behalf of such subsidiary (as the case may be), be entitled to (i) seek reimbursement of the stamp duty from the transferee, (ii) set-off the stamp duty against any dividends payable to the transferee of those ordinary shares and (iii) claim a first and permanent lien on the ordinary shares on which stamp duty has been paid by us or such subsidiary for the amount of stamp duty paid. Our lien shall extend to all dividends paid on those ordinary shares.
Dividends paid by us may be subject to Irish dividend withholding tax.
In certain circumstances, as an Irish tax resident company, we will be required to deduct Irish dividend withholding tax (currently at the rate of 25%) from dividends paid to our shareholders. Shareholders that are resident in the United States, EU countries (other than Ireland) or other countries with which Ireland has signed a tax treaty (whether the treaty has been ratified or not) generally should not be subject to Irish withholding tax so long as the shareholder has provided its broker, for onward transmission to our qualifying intermediary or other designated agent (in the case of shares held beneficially), or our or its transfer agent (in the case of shares held directly), with all the necessary documentation by the appropriate due date prior to payment of the dividend. However, some shareholders may be subject to withholding tax, which could adversely affect the price of our ordinary shares.
97
General Risk Factors
Securities class action litigation could divert our management’s attention and harm our business and could subject us to significant liabilities.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the equity securities of biopharmaceutical companies. These broad market fluctuations may cause the market price of our ordinary shares to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biopharma companies have experienced significant stock price volatility in recent years. For example, following declines in our stock price, two federal securities class action lawsuits were filed in March 2016 against us and certain of our current and former officers alleging violations of the Securities Exchange Act of 1934, as amended, which lawsuits were dismissed by the plaintiffs in June 2018. Even if we are successful in defending any similar claims that may be brought in the future, such litigation could result in substantial costs and may be a distraction to our management and may lead to an unfavorable outcome that could adversely impact our financial condition and prospects.
Our employees, independent contractors, principal investigators, consultants, vendors, distributors and CROs may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, principal investigators, consultants, vendors, distributors and CROs may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or unauthorized activities that violate FDA regulations, including those laws that require the reporting of true, complete and accurate information to the FDA, manufacturing standards, federal and state healthcare fraud and abuse laws and regulations, and laws that require the true, complete and accurate reporting of financial information or data, and comparable foreign regulations. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by our employees and other third parties may also include the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a Code of Conduct and Ethics, but it is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, the exclusion from participation in federal and state healthcare programs and imprisonment.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on us avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biopharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and inter party reexamination proceedings before the United States Patent and Trademark Office, or the U.S. PTO. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which our collaborators are developing medicine candidates. As the biopharmaceutical industries expand and more patents are issued, the risk increases that our medicine candidates may be subject to claims of infringement of the patent rights of third parties.
98
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our medicines and/or any other medicine candidates. Because patent applications can take many years to issue, there may be currently pending patent applications, which may later result in issued patents that our medicine candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our medicine candidates, any molecules formed during the manufacturing process or any final medicine itself, the holders of any such patents may be able to block our ability to commercialize such medicine candidate unless we obtained a license under the applicable patents, or until such patents expire. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy, the holders of any such patent may be able to block our ability to develop and commercialize the applicable medicine candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our medicine candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing medicines, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our medicine candidates, and we have done so from time to time. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize one or more of our medicine candidates, which could harm our business significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our medicines, resulting in either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that one of our patents, or a patent of one of our licensors, is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
There are numerous post grant review proceedings available at the U.S. PTO (including inter partes review, post-grant review and ex-parte reexamination) and similar proceedings in other countries of the world that could be initiated by a third-party that could potentially negatively impact our issued patents.
Interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our collaborators or licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our ordinary shares.
99
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the U.S. PTO and foreign patent agencies in several stages over the lifetime of the patent. The U.S. PTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or licensors that control the prosecution and maintenance of our licensed patents fail to maintain the patents and patent applications covering our medicine candidates, our competitors might be able to enter the market, which would have a material adverse effect on our business.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We employ individuals who were previously employed at other biopharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of our employees’ former employers or other third parties. We may also be subject to claims that former employers or other third parties have an ownership interest in our patents. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and even if we are successful, litigation could result in substantial cost and be a distraction to our management and other employees.
Sales of a substantial number of our ordinary shares in the public market could cause our share price to decline.
If our existing shareholders sell, or indicate an intention to sell, substantial amounts of our ordinary shares in the public market, the trading price of such ordinary shares could decline. In addition, our ordinary shares that are either subject to outstanding options and restricted stock units or reserved for future issuance under our employee benefit plans are or may become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules and the Securities Act of 1933, as amended. If these additional ordinary shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our ordinary shares could decline.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our ordinary shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. If one or more of the analysts who cover us downgrade our rating or publish inaccurate or unfavorable research about our business, our share price could decline. If one or more of these analysts cease coverage of our company or fail to publish reports on our company regularly, demand for our ordinary shares could decrease, which might cause our share price and trading volume to decline.
100
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
As of December 31, 2022, we had the following office space lease agreements in place for real properties:
Location |
|
Approximate Square Feet |
|
|
Lease Expiry Date |
|
Dublin, Ireland |
|
|
80,000 |
|
|
July 1, 2032 to May 4, 2041 |
South San Francisco, California |
|
|
40,000 |
|
|
December 31, 2031 |
Rockville, Maryland |
|
|
42,000 |
|
|
August 31, 2024 to May 31, 2026 |
Chicago, Illinois |
|
|
9,200 |
|
|
December 31, 2028 |
Washington, D.C. |
|
|
6,000 |
|
|
September 30, 2024 |
Mannheim, Germany |
|
|
4,800 |
|
|
December 31, 2023 |
The above table does not include details of an agreement to lease entered into in November 2021 relating to approximately 192,000 square feet of office and laboratory space under construction in Rockville, Maryland. Lease commencement will begin when construction of the building is completed by the lessor and we have access to begin the construction of leasehold improvements. We expect to receive access to the office and laboratory space and commence the related lease in the second half of 2023 and incur leasehold improvement costs through 2024 and the first half of 2025 in order to prepare the building for occupancy.
In addition to the leases above, we purchased a three-building campus in Deerfield, Illinois. The Deerfield campus totals 70 acres and consists of approximately 650,000 square feet of office space.
Item 3. Legal Proceedings
For a description of our legal proceedings, see Note 17 of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K.
Item 4. Mine Safety Disclosures
None.
101
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our ordinary shares trade on The Nasdaq Global Select Market under the trading symbol “HZNP”.
Holders of Record
The closing price of our ordinary shares on February 23, 2023 was $110.70. As of February 23, 2023, there were approximately 25 holders of record of our ordinary shares. Because almost all of our ordinary shares are held by brokers, nominees and other institutions on behalf of shareholders, we are unable to estimate the total number of shareholders represented by these record holders.
Performance Graph
The following graph shows a comparison from December 31, 2017, through December 31, 2022, of the cumulative total return for (i) our ordinary shares, (ii) the Nasdaq Biotechnology Index and (iii) the Nasdaq U.S. Benchmark Total Return Index.
Information set forth in the graph below represents the performance of our ordinary shares from December 31, 2017, through December 31, 2022. The graph assumes an initial investment of $100.00 on December 31, 2017. The comparisons in the graph are required by the Securities and Exchange Commission and are not intended to forecast or be indicative of possible future performance of our ordinary shares.

|
|
12/31/2017 |
|
|
12/31/2018 |
|
|
12/31/2019 |
|
|
12/31/2020 |
|
|
12/31/2021 |
|
|
12/31/2022 |
|
||||||
Cumulative Returns |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Horizon Therapeutics plc |
|
$ |
100.00 |
|
|
$ |
133.84 |
|
|
$ |
247.95 |
|
|
$ |
501.03 |
|
|
$ |
738.08 |
|
|
$ |
779.45 |
|
Nasdaq Biotechnology Index |
|
|
100.00 |
|
|
|
91.14 |
|
|
|
114.02 |
|
|
|
144.15 |
|
|
|
144.18 |
|
|
|
129.59 |
|
Nasdaq U.S. Benchmark Total Return Index |
|
|
100.00 |
|
|
|
97.16 |
|
|
|
132.81 |
|
|
|
192.47 |
|
|
|
235.15 |
|
|
|
158.65 |
|
The foregoing graph and table are furnished solely with this report, and are not filed with this report, and shall not be deemed incorporated by reference into any other filing under the Securities Act of 1933, as amended, or the Securities Act, or the Securities Exchange Act of 1934, as amended, whether made by us before or after the date hereof, regardless of any general incorporation language in any such filing, except to the extent we specifically incorporate this material by reference into any such filing.
102
Dividend Policy
We have never declared or paid cash dividends on our common equity. We currently intend to retain all available funds and any future earnings to support operations and finance the growth and development of our business and do not intend to pay cash dividends on our ordinary shares for the foreseeable future. Under Irish law, dividends may only be paid, and share repurchases and redemptions must generally be funded only out of, “distributable reserves”. In addition, we are currently prohibited from paying cash dividends by the terms of our credit agreement with Citibank, N.A., as administrative and collateral agent, our $600.0 million aggregate principal amount of 5.5% Senior Notes due 2027, subject to customary exceptions, and as well as by the terms of the transaction agreement with Amgen. Any future determination as to the payment of dividends will, subject to Irish legal requirements, be at the sole discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors our board of directors deems relevant.
Securities Authorized for Issuance under Equity Compensation Plans
See Item 12 of Part III of this Annual Report on Form 10-K regarding information about securities authorized for issuance under our equity compensation plans.
Issuer Purchases of Equity Securities
The following table summarizes purchases of our ordinary shares made by or on behalf of us or any of our “affiliated purchasers” as defined in Rule 10b-18(a)(3) under the Securities Exchange Act of 1934, as amended, during each fiscal month during the three month period ended December 31, 2022:
|
|
Total Number of Shares Purchased (1) |
|
|
Average Price Paid per Share (2) |
|
|
Total Number of Shares Purchased as Part of Publicly Announced Programs (3) |
|
|
Approximate Dollar Value of Shares that May Yet Be Purchased Under the Program (4) |
|
||||
October 1 to 31, 2022 |
|
|
2,117,483 |
|
|
$ |
64.53 |
|
|
|
2,117,483 |
|
|
$ |
250,000 |
|
November 1 to 30, 2022 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
December 1 to 31, 2022 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
2,117,483 |
|
|
$ |
64.53 |
|
|
|
2,117,483 |
|
|
$ |
250,000 |
|
Irish Law Matters
See Irish Law Matters included in Item 1 of Part I of this Annual Report on Form 10-K.
Item 6. Reserved
103
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing at the end of this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should read the “Risk Factors” section of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
This section of this Annual Report on Form 10-K generally discusses 2022 and 2021 items and year-to-year comparisons between 2022 and 2021. Discussions of 2020 items and year-to-year comparisons between 2021 and 2020 that are not included in this Annual Report on Form 10-K can be found in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7 of our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, filed with the Securities and Exchange Commission, or SEC, on March 1, 2022.
Unless otherwise indicated or the context otherwise requires, references to “Horizon”, “we”, “us” and “our” refer to Horizon Therapeutics plc and its consolidated subsidiaries.
OUR BUSINESS
We are a global biotechnology company focused on the discovery, development and commercialization of medicines that address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases. Our pipeline is purposeful: we apply scientific expertise and courage to bring clinically meaningful therapies to patients. We believe science and compassion must work together to transform lives.
Effective in the fourth quarter of 2022, management realigned our reportable segments to reflect changes in the manner in which the chief operating decision maker, or CODM, assesses financial information for decision-making purposes. We transitioned our two reportable segments, the inflammation segment and the orphan segment, to one reportable segment for the year ended December 31, 2022. All prior year amounts have been reclassified to conform to our current reporting structure. Our commercial portfolio is currently composed of 12 medicines in the areas of rare diseases, gout, ophthalmology and inflammation.
On December 12, 2022, we announced we had entered into a transaction agreement with Amgen Inc., or Amgen, and Pillartree Limited, or Pillartree, a wholly owned subsidiary of Amgen. Subject to the terms of the transaction agreement, Pillartree will acquire our company, or the Transaction, pursuant to a scheme of arrangement under Chapter 1 of Part 9 of the Companies Act 2014 of Ireland, or the Scheme. As a result of the Scheme, we would become a wholly owned subsidiary of Amgen.
At the effective time of the Scheme, or the Effective Time, holders of our ordinary shares will be entitled to receive $116.50 in cash per ordinary share, or the Consideration. Our equity awards will be treated as set forth in the transaction agreement, such that:
104
On February 24, 2023, our shareholders approved the Scheme and certain scheme approval resolutions and amendments to the memorandum and articles of association of Horizon to enable the Scheme to be effected. The closing of the Transaction remains subject to customary closing conditions, including, among other things, (a) the sanction by the Irish High Court of the Scheme and delivery of the court order to the Irish Registrar of Companies, (b) the receipt of required antitrust clearance in the United States, and the absence of an order or law that prevents consummation of the Transaction or imposes a burdensome condition (as defined in the transaction agreement), (c) absence of any Material Adverse Effect (as defined in the transaction agreement) from December 12, 2022 to the Sanction Date (as defined in the transaction agreement) that is continuing as of the Sanction Date, (d) the accuracy of the other party’s representations and warranties subject to certain materiality and material adverse effect exceptions and (e) the performance by each party of all of its covenants and agreements under the transaction agreement in all material respects. On January 30, 2023, we and Amgen received a request for additional information and documentary materials, or a second request, from the Federal Trade Commission, or FTC, in connection with the FTC’s review of the Transaction. The effect of the second request is to extend the waiting period imposed by the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, until 30 days after we and Amgen have substantially complied with the second request, unless the period is extended voluntarily by the parties or terminated sooner by the FTC. In connection with the Transaction, we and Amgen have received clearances or confirmation of non-applicability related to foreign direct investment in Denmark, Italy, Germany and France and clearances related to antitrust in Germany and Austria.
We expect the Transaction to close during the first half of 2023, subject to the regulatory clearances and other customary closing conditions described above and in the transaction agreement. Additional information about the transaction agreement and the Transaction is set forth in our filings with the SEC.
105
As of December 31, 2022, our commercial portfolio consisted of the following medicines:
TEPEZZA® (teprotumumab-trbw), for intravenous infusion |
KRYSTEXXA® (pegloticase injection), for intravenous infusion |
RAVICTI® (glycerol phenylbutyrate) oral liquid |
PROCYSBI® (cysteamine bitartrate) delayed-release capsules and granules, for oral use |
UPLIZNA® (inebilizumab-cdon) injection, for intravenous use |
ACTIMMUNE® (interferon gamma-1b) injection, for subcutaneous use |
PENNSAID® (diclofenac sodium topical solution) 2% w/w, or PENNSAID 2%, for topical use |
RAYOS® (prednisone) delayed-release tablets, for oral use |
BUPHENYL® (sodium phenylbutyrate) tablets and powder, for oral use |
DUEXIS® (ibuprofen/famotidine) tablets, for oral use |
VIMOVO® (naproxen/esomeprazole magnesium) delayed-release tablets, for oral use |
QUINSAIR™ (levofloxacin) solution for inhalation |
Acquisitions and Divestitures
Since January 1, 2020, we completed the following acquisitions and divestitures:
The consolidated financial statements presented herein include the results of operations of the acquired businesses from the applicable dates of acquisition. Refer to Note 4, Acquisitions, Divestitures and other Arrangements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details of our acquisitions and divestitures.
106
Strategy
Horizon is a leading high-growth, innovation-driven, profitable global biotechnology company. We are focused on the discovery, development and commercialization of medicines that address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases. Our three strategic goals are to: (i) maximize the value of our on-market rare disease medicines through commercial execution and clinical investment; (ii) expand our research and development, or R&D, pipeline through significant internal investment and external business development; and (iii) build a global presence in targeted international markets. Our vision is to build healthier communities, urgently and responsibly, supported by our philosophy to make a meaningful difference for patients and communities in need. We believe this generates value for our multiple stakeholders, including our shareholders.
Our commercialization strategy for our on-market rare disease medicines, including our key growth drivers TEPEZZA, KRYSTEXXA and UPLIZNA, includes initiatives to increase awareness of the conditions each medicine is designed to treat, enhancing efforts to identify target patients and in certain cases pursue opportunities for international commercialization and more effective uses through clinical trials. For TEPEZZA and KRYSTEXXA, initiatives include promoting earlier treatment by driving awareness of the benefits of the medicines, and for UPLIZNA, initiatives include increasing awareness of what differentiates our medicines from other available therapies. Additional strategies for our on-market rare disease medicines include optimizing timely access for patients to the medicines and maximizing the value of the medicines through investment in clinical trials. Specifically, with respect to TEPEZZA, we expanded our commercial team, continued to invest in our direct-to-consumer marketing activities, refined our marketing and physician education strategies, and conducted extensive market analysis to identify further opportunities to accelerate growth, which we are implementing. These growth opportunities involve increasing adoption by ocular specialists and driving an urgency among ophthalmologists and endocrinologists to diagnose and refer thyroid eye disease, or TED, patients. With respect to KRYSTEXXA, after receiving U.S. Food and Drug Administration approval of our supplemental biologics license application, or sBLA, in July 2022 to expand the label to include co-treatment of KRYSTEXXA with the immunomodulator methotrexate, we launched a successful commercial campaign, expanded our commercial team and are focused on promoting the expanded label with physicians and patients.
Our R&D strategy is to expand our pipeline of preclinical and clinical development programs to drive sustainable growth, as well as maximizing the benefit and value of our existing medicines through development programs. We are (i) acquiring, licensing and developing medicines for indications that address unmet needs in rare, autoimmune and severe inflammatory diseases, particularly those in our therapeutic areas of focus; (ii) maximizing our pipeline candidates through internal R&D; (iii) expanding our early-stage pipeline through partnerships and collaborations; and (iv) continuing to build out our research capabilities to generate discovery-stage candidates internally. Our R&D pipeline includes more than 20 programs, and we announced the initiation of five clinical trials in 2022 and expect to initiate several more in 2023, including a planned Phase 3 program for dazodalibep in Sjögren’s syndrome. In January 2023, we announced the initiation of our daxdilimab discoid lupus erythematosus Phase 2 trial.
The aim of our global expansion strategy is to build a global presence in targeted international markets to support the (i) continued launch of UPLIZNA in certain European markets and Brazil this year; (ii) potential approvals and commercial launches of UPLIZNA in additional markets in the coming years; and (iii) potential approvals and full-scale commercial launches of TEPEZZA in Japan, Brazil, Europe and other international markets over the next several years. We plan to use a combination of direct marketing and partnerships for our global expansion efforts and are establishing the infrastructure needed to support these activities.
107
RESULTS OF OPERATIONS
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
Consolidated Results
|
For the Years |
|
|
|
|
|
|
|
|||||||
|
Ended December 31, |
|
|
Change |
|
|
Change |
|
|||||||
|
2022 |
|
|
2021 |
|
|
$ |
|
|
% |
|
||||
|
(in thousands, except percentages) |
|
|||||||||||||
Net sales |
$ |
3,629,044 |
|
|
$ |
3,226,410 |
|
|
$ |
402,634 |
|
|
|
12 |
% |
Cost of goods sold |
|
920,197 |
|
|
|
794,512 |
|
|
|
125,685 |
|
|
|
16 |
% |
Gross profit |
|
2,708,847 |
|
|
|
2,431,898 |
|
|
|
276,949 |
|
|
|
11 |
% |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
437,962 |
|
|
|
345,318 |
|
|
|
92,644 |
|
|
|
27 |
% |
Acquired in-process research and development and milestones |
|
56,250 |
|
|
|
86,672 |
|
|
|
(30,422 |
) |
|
|
(35 |
)% |
Selling, general and administrative |
|
1,541,052 |
|
|
|
1,446,410 |
|
|
|
94,642 |
|
|
|
7 |
% |
Impairment of goodwill |
|
56,171 |
|
|
|
— |
|
|
|
56,171 |
|
|
|
100 |
% |
Impairment of long-lived asset |
|
— |
|
|
|
12,371 |
|
|
|
(12,371 |
) |
|
|
(100 |
)% |
Gain on sale of asset |
|
— |
|
|
|
(2,000 |
) |
|
|
2,000 |
|
|
|
100 |
% |
Total operating expenses |
|
2,091,435 |
|
|
|
1,888,771 |
|
|
|
202,664 |
|
|
|
11 |
% |
Operating income |
|
617,412 |
|
|
|
543,127 |
|
|
|
74,285 |
|
|
|
14 |
% |
Other expense, net: |
|
|
|
|
|
|
|
|
|
|
|
||||
Interest expense, net |
|
(83,707 |
) |
|
|
(81,063 |
) |
|
|
(2,644 |
) |
|
|
(3 |
)% |
Foreign exchange loss |
|
(1,202 |
) |
|
|
(1,028 |
) |
|
|
(174 |
) |
|
|
(17 |
)% |
Other (expense) income, net |
|
(5,567 |
) |
|
|
1,791 |
|
|
|
(7,358 |
) |
|
|
(411 |
)% |
Total other expense, net |
|
(90,476 |
) |
|
|
(80,300 |
) |
|
|
(10,176 |
) |
|
|
(13 |
)% |
Income before expense (benefit) for income taxes |
|
526,936 |
|
|
|
462,827 |
|
|
|
64,109 |
|
|
|
14 |
% |
Expense (benefit) for income taxes |
|
5,454 |
|
|
|
(71,664 |
) |
|
|
77,118 |
|
|
|
108 |
% |
Net income |
$ |
521,482 |
|
|
$ |
534,491 |
|
|
$ |
(13,009 |
) |
|
|
(2 |
)% |
Beginning with the third quarter of 2022, we separately present upfront, milestone, and similar payments pursuant to collaborations, licenses of third-party technologies, and asset acquisitions as “Acquired in-process research and development and milestones” expenses in the consolidated statement of comprehensive income. Amounts recorded in this line item for the year ended December 31, 2022, would have historically been recorded to R&D expenses. We believe the new classification assists users of the financial statements in better understanding the payments incurred to acquire in-process research and development, or IPR&D. Prior period consolidated statements of comprehensive income have been reclassified to conform with the new classification.
Net sales. Net sales increased $402.6 million, or 12%, to $3,629.0 million during the year ended December 31, 2022, from $3,226.4 million during the year ended December 31, 2021. The increase during the year ended December 31, 2022 was primarily due to an increase in TEPEZZA net sales of $304.4 million, an increase in KRYSTEXXA net sales of $150.7 million and an increase in UPLIZNA net sales of $93.8 million, partially offset by a decrease in PENNSAID 2% net sales of $117.8 million and a decrease in DUEXIS net sales of $69.1 million when compared to the year ended December 31, 2021, each due to the impact of generic competition.
The following table presents a summary of total net sales attributed to geographic sources for the years ended December 31, 2022 and 2021 (in thousands, except percentages):
|
|
Year Ended December 31, 2022 |
|
Year Ended December 31, 2021 |
||||||||
|
|
Amount |
|
|
% of Total |
|
Amount |
|
|
% of Total |
||
United States |
|
$ |
3,589,510 |
|
|
99% |
|
$ |
3,210,020 |
|
|
100% |
Rest of world |
|
|
39,534 |
|
|
1% |
|
|
16,390 |
|
|
* |
Total net sales |
|
$ |
3,629,044 |
|
|
|
|
$ |
3,226,410 |
|
|
|
*Less than 1% |
|
|
|
|
|
|
|
|
|
|
||
108
The following table reflects the components of net sales for the years ended December 31, 2022 and 2021 (in thousands, except percentages):
|
|
Year ended December 31, |
|
|
Change |
|
|
Change |
|
|||||||
|
|
2022 |
|
|
2021 |
|
|
$ |
|
|
% |
|
||||
TEPEZZA |
|
$ |
1,965,711 |
|
|
$ |
1,661,299 |
|
|
$ |
304,412 |
|
|
|
18 |
% |
KRYSTEXXA |
|
|
716,167 |
|
|
|
565,452 |
|
|
|
150,715 |
|
|
|
27 |
% |
RAVICTI |
|
|
325,652 |
|
|
|
291,945 |
|
|
|
33,707 |
|
|
|
12 |
% |
PROCYSBI |
|
|
209,990 |
|
|
|
189,965 |
|
|
|
20,025 |
|
|
|
11 |
% |
UPLIZNA |
|
|
154,622 |
|
|
|
60,805 |
|
|
|
93,817 |
|
|
|
154 |
% |
ACTIMMUNE |
|
|
126,080 |
|
|
|
117,164 |
|
|
|
8,916 |
|
|
|
8 |
% |
PENNSAID 2% |
|
|
73,774 |
|
|
|
191,621 |
|
|
|
(117,847 |
) |
|
|
(62 |
)% |
RAYOS |
|
|
41,882 |
|
|
|
56,851 |
|
|
|
(14,969 |
) |
|
|
(26 |
)% |
BUPHENYL |
|
|
7,332 |
|
|
|
7,860 |
|
|
|
(528 |
) |
|
|
(7 |
)% |
DUEXIS |
|
|
4,901 |
|
|
|
74,023 |
|
|
|
(69,122 |
) |
|
|
(93 |
)% |
VIMOVO |
|
|
1,851 |
|
|
|
8,397 |
|
|
|
(6,546 |
) |
|
|
(78 |
)% |
QUINSAIR |
|
|
1,082 |
|
|
|
1,028 |
|
|
|
54 |
|
|
|
5 |
% |
Total net sales |
|
$ |
3,629,044 |
|
|
$ |
3,226,410 |
|
|
$ |
402,634 |
|
|
|
12 |
% |
TEPEZZA. Net sales increased $304.4 million, or 18%, to $1,965.7 million during the year ended December 31, 2022, from $1,661.3 million during the year ended December 31, 2021. Net sales increased by approximately $244.9 million due to volume growth and $59.5 million due to higher net pricing. Net sales growth for TEPEZZA was negatively impacted by the omicron variant of COVID-19 in the first half of 2022. In addition, we identified certain challenges, including the often-burdensome reimbursement process, that we believe contributed to slower-than-expected growth of TEPEZZA in the first half of 2022. These challenges, as well as challenges related to a lower rate of adherence to the full course of TEPEZZA therapy, have continued to moderate TEPEZZA net sales growth. In the second half of 2022, we began executing on several opportunities to address these challenges and accelerate growth, including significantly expanding the size of our TEPEZZA sales force to allow our representatives more time with core TEPEZZA prescribers while educating other key physicians, including ophthalmologists and endocrinologists, about TED and TEPEZZA. We are also spending more time and focus on the reimbursement process to more effectively support the patient access journey. We also continue to invest significantly in direct-to-consumer advertising based on the returns we have seen to date. However, it will continue to take some time for these strategies to contribute meaningfully to TEPEZZA net sales growth.
KRYSTEXXA. Net sales increased $150.7 million, or 27%, to $716.2 million during the year ended December 31, 2022, from $565.5 million during the year ended December 31, 2021. Net sales increased by approximately $113.4 million due to volume growth and $37.3 million due to higher net pricing. We expect net sales for KRYSTEXXA to continue to increase in future periods primarily due to the use of KRYSTEXXA with methotrexate following the approval of our sBLA in July 2022, which expanded KRYSTEXXA’s labeling to include co-administration with methotrexate.
RAVICTI. Net sales increased $33.7 million, or 12%, to $325.6 million during the year ended December 31, 2022, from $291.9 million during the year ended December 31, 2021. Net sales increased by approximately $23.8 million due to volume growth and $9.9 million due to higher net pricing.
PROCYSBI. Net sales increased $20.0 million, or 11%, to $210.0 million during the year ended December 31, 2022, from $190.0 million during the year ended December 31, 2021. Net sales increased by approximately $11.2 million due to volume growth and $8.8 million due to higher net pricing. The $8.8 million impact from higher net pricing was primarily driven by a benefit from a $7.5 million partial release of the pricing review liability recorded in the third quarter of 2022 as a result of a decision made by the Patented Medicine Prices Review Board, or PMPRB, in September 2022 relating to PROCYSBI pricing in Canada.
UPLIZNA. Net sales increased $93.8 million, or 154%, to $154.6 million during the year ended December 31, 2022, from $60.8 million during the year ended December 31, 2021. Net sales in the United States increased by $79.7 million, which was composed of an increase of $74.7 million due to higher sales volume and $5.0 million due to higher net pricing. The remaining $14.1 million increase in net sales related primarily to revenue and milestone payments from our international partners recognized during the year ended December 31, 2022.
109
PENNSAID 2%. Net sales decreased $117.8 million, or 62%, to $73.8 million during the year ended December 31, 2022, from $191.6 million during the year ended December 31, 2021. Net sales decreased by approximately $81.8 million resulting from lower net pricing and by approximately $36.0 million due to lower sales volume.
In May 2022, Apotex Corp. and its affiliate, Apotex Inc., or collectively Apotex, initiated an at-risk launch of a generic version of PENNSAID 2% in the United States. The generic competition reduced our PENNSAID 2% sales, resulting in increased utilization of co-pay and other patient assistance programs for PENNSAID 2%, which negatively impacted net pricing. We expect our net sales for PENNSAID 2% to continue declining in future periods primarily due to generic competition and the wind down of our former inflammation business, which we substantially completed in the fourth quarter of 2022.
RAYOS. Net sales decreased $15.0 million, or 26%, to $41.9 million during the year ended December 31, 2022, from $56.9 million during the year ended December 31, 2021. Net sales decreased by approximately $11.4 million resulting from lower net pricing and $3.6 million due to lower sales volume.
Under our settlement agreement with Teva Pharmaceuticals Industries Limited (formerly known as Actavis Laboratories FL, Inc., which itself was formerly known as Watson Laboratories, Inc. – Florida), or Teva, we expect Teva to enter the market with a generic version of RAYOS in 2023. As a result, we expect our net sales for RAYOS to decline in future periods.
DUEXIS. Net sales decreased $69.1 million, or 93%, to $4.9 million during the year ended December 31, 2022, from $74.0 million during the year ended December 31, 2021. Net sales decreased by approximately $61.8 million resulting from lower sales volume, primarily due to the impact of generic competition, and by approximately $7.3 million due to lower net pricing.
Due to the impact of the at-risk launch of generic PENNSAID 2%, we redeployed a portion of our inflammation commercial team to support our TEPEZZA and KRYSTEXXA expansions. In the fourth quarter of 2022, we substantially completed a wind down of our former inflammation business, including active promotion efforts and associated HorizonCares support, for our inflammation medicines. As a result, sales volumes for PENNSAID 2%, RAYOS and DUEXIS declined significantly since the second quarter of 2022 and we expect net sales of our inflammation medicines to be immaterial going forward.
The table below reconciles our gross to net sales for the years ended December 31, 2022 and 2021 (in millions, except percentages):
|
|
Year Ended December 31, 2022 |
|
|
Year Ended December 31, 2021 |
|
||||||||||
|
|
Amount |
|
|
% of Gross |
|
|
Amount |
|
|
% of Gross |
|
||||
Gross sales |
|
$ |
5,022.3 |
|
|
|
100 |
% |
|
$ |
4,903.6 |
|
|
|
100 |
% |
Adjustments to gross sales: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Prompt pay and other discounts |
|
|
(28.8 |
) |
|
|
(0.6 |
)% |
|
|
(46.0 |
) |
|
|
(0.9 |
)% |
Medicine returns |
|
|
(31.6 |
) |
|
|
(0.6 |
)% |
|
|
(17.6 |
) |
|
|
(0.4 |
)% |
Co-pay and other patient assistance |
|
|
(341.9 |
) |
|
|
(6.8 |
)% |
|
|
(599.9 |
) |
|
|
(12.2 |
)% |
Commercial rebates and wholesaler fees |
|
|
(198.0 |
) |
|
|
(3.9 |
)% |
|
|
(278.8 |
) |
|
|
(5.7 |
)% |
Government rebates and chargebacks |
|
|
(793.0 |
) |
|
|
(15.8 |
)% |
|
|
(734.9 |
) |
|
|
(15.0 |
)% |
Total adjustments |
|
|
(1,393.3 |
) |
|
|
(27.7 |
)% |
|
|
(1,677.2 |
) |
|
|
(34.2 |
)% |
Net sales |
|
$ |
3,629.0 |
|
|
|
72.3 |
% |
|
$ |
3,226.4 |
|
|
|
65.8 |
% |
During the year ended December 31, 2022, co-pay and other patient assistance costs, as a percentage of gross sales, decreased to 6.8% from 12.2% during the year ended December 31, 2021, primarily due to a decreased proportion of PENNSAID 2% and DUEXIS sold. We expect co-pay and other patient assistance costs to continue to decrease as a percentage of total gross sales.
110
On a quarter-to-quarter basis, our net sales can be impacted due to end customer buying patterns, fluctuations in wholesaler inventory levels, the impact of benefit plan changes, insurance reverification and increased co-pay expenses as patients work through deductibles. For example, our net sales in the first quarter of a year historically represents the lowest net sales quarter as a result of some of these impacts, and we expect this trend to continue in 2023. This is primarily due to annual managed care plan changes and the re-setting of patients’ medical insurance deductibles at the beginning of each year, resulting in higher co-pay and other patient assistance costs as patients meet their annual medical insurance deductibles during the first and second quarters, and higher net sales in the second half of the year after patients meet their deductibles and healthcare plans reimburse a greater portion of the total cost of our medicines.
Cost of Goods Sold. Cost of goods sold increased $125.7 million, or 16%, to $920.2 million during the year ended December 31, 2022, from $794.5 million during the year ended December 31, 2021. The increase in cost of goods sold was primarily due to an increase in inventory step-up expense, an increase in royalty and earnout expense and an increase in amortization expense. Inventory step-up expense increased by $64.1 million related to acquired units of UPLIZNA inventory sold, which increased during the year ended December 31, 2022 compared to the year ended December 31, 2021. Royalty and earnout expense increased by $48.8 million primarily due to royalties and earnouts payable on net sales of TEPEZZA, which increased during the year ended December 31, 2022 compared to the year ended December 31, 2021 due to higher net sales. Amortization expense increased $28.1 million primarily due to the acquisition of the UPLIZNA developed technology intangible asset in March 2021. In addition, we recorded a $6.5 million PENNSAID 2% inventory reserve due to the impact of generic competition on PENNSAID 2% sales. As a percentage of net sales, cost of goods sold (excluding amortization expense of $362.9 million during 2022 and $334.8 million during 2021) was 15% during the year ended December 31, 2022, compared to 14% during the year ended December 31, 2021. The increase in cost of goods sold as a percentage of net sales was primarily due to an increase in inventory step-up expense related to UPLIZNA as noted above.
Research and Development Expenses. R&D expenses increased $92.7 million, or 27%, to $438.0 million during the year ended December 31, 2022, from $345.3 million during the year ended December 31, 2021. The increase was primarily due to a $56.2 million increase in clinical trial costs reflecting increased activity in our R&D pipeline as well as the addition of certain medicine candidates and development programs following the acquisition of Viela in March 2021, and an increase of $21.4 million in consultant costs.
We expect our R&D expenses to increase significantly in future periods as a result of our on-going and planned clinical trials for our pipeline, including the medicine candidates and development programs we acquired in 2021.
Acquired In-Process Research and Development and Milestones Expenses. Acquired IPR&D and milestones expenses decreased $30.4 million, or 35%, to $56.3 million during the year ended December 31, 2022, from $86.7 million during the year ended December 31, 2021. The $56.3 million of acquired IPR&D and milestones expenses during the year ended December 31, 2022, was primarily related to an upfront payment of $15.0 million recognized in relation to the collaboration and option agreement entered into with Q32 Bio Inc., or Q32, in the third quarter of 2022 and $17.5 million recognized in the fourth quarter of 2022 relating to milestone-based development funding paid to Q32. In addition, we recognized a $15.0 million development milestone in relation to the global agreement with Arrowhead Pharmaceuticals, Inc., or Arrowhead, in the fourth quarter of 2022. During the year ended December 31, 2021, we recognized a $40.0 million upfront payment in relation to the global agreement with Arrowhead and $28.1 million relating to a stock purchase agreement with Alpine Immune Sciences, Inc., or Alpine. Refer to Note 4, Acquisitions, Divestitures and other Arrangements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
Selling, General and Administrative Expenses. Selling, general and administrative expenses increased $94.7 million, or 7%, to $1,541.1 million during the year ended December 31, 2022, from $1,446.4 million during the year ended December 31, 2021. The increase was primarily due to costs associated with the commercialization of our medicines and global expansion initiatives. These include an increase of $55.9 million in marketing program costs and an increase of $24.1 million in employee-related costs, partially offset by a decrease of $28.6 million in transaction costs, which were incurred during the year ended December 31, 2021 relating to the Viela acquisition. In addition, we incurred severance and consulting costs of $15.8 million during the year ended December 31, 2022, related to the wind down of our former inflammation business.
We expect our selling, general and administrative expenses to increase significantly in future periods primarily due to continued support for our U.S. commercial and field-based organization and global expansion activities.
111
Impairment of goodwill. During the year ended December 31, 2022, we recorded an impairment charge of $56.2 million in relation to our former inflammation reporting unit. Refer to Note 8, Goodwill and Intangible Assets, of the Notes to the Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details.
Impairment of long-lived asset. During the year ended December 31, 2021, we recorded an impairment charge of $12.4 million as a result of vacating the Lake Forest office. Refer to Note 15, Lease Obligations, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details.
Interest Expense, Net. Interest expense, net, increased $2.6 million to $83.7 million during the year ended December 31, 2022, from $81.1 million during the year ended December 31, 2021. The increase was primarily due to an increase in interest expense of $33.9 million, primarily related to increases in interest rates on the portion of our variable interest debt, partially offset by an increase in interest income of $31.2 million. Refer to Note 13, Debt Agreements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
Expense (benefit) for income taxes. During the year ended December 31, 2022, we recorded an expense for income taxes of $5.5 million and we recorded a benefit for income taxes of $71.7 million during the year ended December 31, 2021. The expense for income taxes recorded during the year ended December 31, 2022 resulted primarily from tax expense on pre-tax income and losses at the Irish statutory tax rate of $65.9 million, tax expense of $31.4 million attributable to disallowed officers’ compensation under Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, tax expense of $27.5 million recognized in respect of changes in the state tax rate expected to apply to the reversal of temporary differences between the book values and tax bases of certain assets and tax expense of $11.8 million attributable to a goodwill impairment which is non-deductible for tax purposes. These tax expenses were partially offset by tax benefit of $54.7 million recognized on the pre-tax income and losses generated in jurisdictions with statutory tax rates different than the Irish statutory tax rate, tax benefits recognized on share-based compensation of $53.1 million, tax benefit of $18.9 million recognized due to the release of valuation allowances on certain state net operating losses and $12.5 million of U.S. federal and state tax credits generated during the year.
The benefit for income taxes recorded during the year ended December 31, 2021 resulted primarily from tax expense on pre-tax income and losses at the Irish statutory tax rate of $57.9 million as offset by tax benefits recognized on share-based compensation of $71.2 million and a tax benefit of $49.4 million recognized due to a reduction in the state tax rate expected to apply to the reversal of temporary differences between the book values and tax bases of certain assets acquired through the Viela acquisition. A tax benefit of $58.5 million was recognized on the pre-tax income and losses generated in jurisdictions with statutory tax rates different than the Irish statutory tax rate and $11.6 million of U.S. federal and state tax credits were generated during the year. These tax benefits were partially offset by a tax expense of $47.1 million attributable to disallowed officers’ compensation under Section 162(m) of the Code and a tax expense of $18.7 million generated from an intercompany transfer and license of intellectual property from a U.S. subsidiary to an Irish subsidiary.
112
Non-GAAP Financial Measures
We provide certain non-GAAP financial measures, including EBITDA, or earnings before interest, taxes, depreciation and amortization, adjusted EBITDA, non-GAAP net income and non-GAAP earnings per share. These non-GAAP financial measures are intended to provide additional information on our performance, operations and profitability. Adjustments to our GAAP figures as well as EBITDA exclude acquisition/divestiture-related costs, transaction-related costs, manufacturing facility start-up costs, litigation settlements and restructuring and realignment costs, as well as non-cash items such as share-based compensation, inventory step-up expense, depreciation and amortization, non-cash interest expense, long-lived assets impairment charges, loss (gain) on equity security investments and other non-cash adjustments. Certain other special items or substantive events may also be included in the non-GAAP adjustments periodically when their magnitude is significant within the periods incurred. We maintain an established non-GAAP cost policy that guides the determination of what costs will be excluded in non-GAAP measures. We believe that these non-GAAP financial measures, when considered together with the GAAP figures, can enhance an overall understanding of our financial and operating performance. The non-GAAP financial measures are included with the intent of providing investors with a more complete understanding of our historical financial results and trends and to facilitate comparisons between periods. In addition, these non-GAAP financial measures are among the indicators our management uses for planning and forecasting purposes and measuring our performance. These non-GAAP financial measures should be considered in addition to, and not as a substitute for, or superior to, financial measures calculated in accordance with GAAP. The non-GAAP financial measures used by us may be calculated differently from, and therefore may not be comparable to, non-GAAP financial measures used by other companies.
Reconciliations of reported GAAP net income to EBITDA, adjusted EBITDA and non-GAAP net income, and the related per share amounts, were as follows (in thousands, except share and per share amounts):
|
|
|
|||||||
|
|
For the Years Ended December 31, |
|||||||
|
|
2022 |
|
|
2021 |
|
|
||
GAAP net income |
|
$ |
521,482 |
|
|
$ |
534,491 |
|
|
Depreciation (1) |
|
|
23,931 |
|
|
|
17,475 |
|
|
Amortization and step-up: |
|
|
|
|
|
|
|
||
Intangible amortization expense (2) |
|
|
366,462 |
|
|
|
336,277 |
|
|
Inventory step-up expense (3) |
|
|
91,709 |
|
|
|
27,572 |
|
|
Interest expense, net (including amortization of debt discount and deferred financing costs) |
|
|
83,707 |
|
|
|
81,063 |
|
|
Expense (benefit) for income taxes |
|
|
5,454 |
|
|
|
(71,664 |
) |
|
EBITDA |
|
|
1,092,745 |
|
|
|
925,214 |
|
|
Other non-GAAP adjustments: |
|
|
|
|
|
|
|
||
Share-based compensation (4) |
|
|
182,100 |
|
|
|
219,086 |
|
|
Impairment of goodwill (5) |
|
|
56,171 |
|
|
|
— |
|
|
Restructuring and realignment costs (6) |
|
|
16,977 |
|
|
|
26,309 |
|
|
Transaction-related costs (7) |
|
|
11,086 |
|
|
|
— |
|
|
Loss (gain) on equity security investments (8) |
|
|
6,188 |
|
|
|
(1,257 |
) |
|
Manufacturing facility start-up costs (9) |
|
|
5,552 |
|
|
|
3,622 |
|
|
Impairment of long-lived assets (10) |
|
|
— |
|
|
|
12,371 |
|
|
Litigation settlements (11) |
|
|
— |
|
|
|
5,000 |
|
|
Gain on sale of asset (12) |
|
|
— |
|
|
|
(2,000 |
) |
|
Acquisition/divestiture-related costs (13) |
|
|
(239 |
) |
|
|
95,929 |
|
|
Total of other non-GAAP adjustments |
|
|
277,835 |
|
|
|
359,060 |
|
|
Adjusted EBITDA |
|
$ |
1,370,580 |
|
|
$ |
1,284,274 |
|
|
113
|
|
For the Years Ended December 31, |
|||||||
|
|
2022 |
|
|
2021 |
|
|
||
GAAP net income |
|
$ |
521,482 |
|
|
$ |
534,491 |
|
|
Non-GAAP adjustments: |
|
|
|
|
|
|
|
||
Depreciation (1) |
|
|
23,931 |
|
|
|
17,475 |
|
|
Amortization and step-up: |
|
|
|
|
|
|
|
||
Intangible amortization expense (2) |
|
|
366,462 |
|
|
|
336,277 |
|
|
Inventory step-up expense (3) |
|
|
91,709 |
|
|
|
27,572 |
|
|
Amortization of debt discount and deferred financing costs (14) |
|
|
7,912 |
|
|
|
5,189 |
|
|
Share-based compensation (4) |
|
|
182,100 |
|
|
|
219,086 |
|
|
Impairment of goodwill (5) |
|
|
56,171 |
|
|
|
— |
|
|
Restructuring and realignment costs (6) |
|
|
16,977 |
|
|
|
26,309 |
|
|
Transaction-related costs (7) |
|
|
11,086 |
|
|
|
— |
|
|
Loss (gain) on equity security investments (8) |
|
|
6,188 |
|
|
|
(1,257 |
) |
|
Manufacturing facility start-up costs (9) |
|
|
5,552 |
|
|
|
3,622 |
|
|
Impairment of long-lived assets (10) |
|
|
— |
|
|
|
12,371 |
|
|
Litigation settlements (11) |
|
|
— |
|
|
|
5,000 |
|
|
Gain on sale of asset (12) |
|
|
— |
|
|
|
(2,000 |
) |
|
Acquisition/divestiture-related costs (13) |
|
|
(239 |
) |
|
|
95,929 |
|
|
Total pre-tax non-GAAP adjustments |
|
|
767,849 |
|
|
|
745,573 |
|
|
Income tax effect of pre-tax non-GAAP adjustments (15) |
|
|
(148,373 |
) |
|
|
(169,554 |
) |
|
Other non-GAAP income tax adjustments (16) |
|
|
3,387 |
|
|
|
(20,800 |
) |
|
Total non-GAAP adjustments |
|
|
622,863 |
|
|
|
555,219 |
|
|
Non-GAAP net income |
|
$ |
1,144,345 |
|
|
$ |
1,089,710 |
|
|
|
|
|
|
|
|
|
|
||
Non-GAAP Earnings Per Share: |
|
|
|
|
|
|
|
||
Weighted average ordinary shares – Basic |
|
|
229,108,881 |
|
|
|
225,551,410 |
|
|
|
|
|
|
|
|
|
|
||
Non-GAAP Earnings Per Share – Basic |
|
|
|
|
|
|
|
||
GAAP earnings per share - Basic |
|
$ |
2.28 |
|
|
$ |
2.37 |
|
|
Non-GAAP adjustments |
|
|
2.71 |
|
|
|
2.46 |
|
|
Non-GAAP earnings per share – Basic |
|
$ |
4.99 |
|
|
$ |
4.83 |
|
|
|
|
|
|
|
|
|
|
||
Weighted average ordinary shares – Diluted |
|
|
|
|
|
|
|
||
Weighted average ordinary shares – Basic |
|
|
229,108,881 |
|
|
|
225,551,410 |
|
|
Ordinary share equivalents |
|
|
6,130,771 |
|
|
|
10,129,073 |
|
|
Weighted average ordinary shares – Diluted |
|
|
235,239,652 |
|
|
|
235,680,483 |
|
|
|
|
|
|
|
|
|
|
||
Non-GAAP Earnings Per Share – Diluted |
|
|
|
|
|
|
|
||
GAAP earnings per share – Diluted |
|
$ |
2.22 |
|
|
$ |
2.27 |
|
|
Non-GAAP adjustments |
|
|
2.64 |
|
|
|
2.35 |
|
|
Non-GAAP earnings per share – Diluted |
|
$ |
4.86 |
|
|
$ |
4.62 |
|
|
114
non-GAAP adjustment based on the statutory income tax rate of the applicable jurisdictions for each non-GAAP adjustment.
During the year ended December 31, 2021, we recognized a U.S. federal and state tax liability on U.S. taxable income generated from an intercompany transfer and license of intellectual property from a U.S. subsidiary to an Irish subsidiary which was partially offset by the recognition of a deferred tax asset in the Irish subsidiary, resulting in a non-GAAP tax adjustment of $28.3 million. We also recognized a reduction in the state tax rate expected to apply to the reversal of temporary differences between the book values and tax bases of certain assets acquired through the Viela acquisition. The reduction in state tax rate resulted in a reduction in the deferred tax liability relating to these assets and a non-GAAP tax adjustment of $49.1 million.
115
Liquidity, Financial Position and Capital Resources
On December 12, 2022, we announced that we had entered into a transaction agreement with Amgen and Pillartree. We have agreed to various covenants and agreements, including, among others, agreements to conduct our business in the ordinary course during the period between the execution of the transaction agreement and the Effective Time. Outside of certain limited exceptions, we may not take, authorize, commit, resolve, or agree to do certain actions without Amgen’s consent, including: (i) acquiring businesses and disposing of significant assets; (ii) incurring capital expenditures above specified thresholds; (iii) issuing equity; (iv) incurring indebtedness; and (v) repurchasing outstanding ordinary shares. We do not believe these restrictions will prevent us from being able to fund our operations, working capital needs or capital expenditure requirements. The following discussion assumes that the Transaction is not consummated and we continue to operate as an independent entity.
As of December 31, 2022, we had retained earnings of $590.0 million. We expect that our sales and marketing expenses will continue to increase as a result of the commercialization of our medicines and global expansion initiatives, but we believe these cost increases will be offset by higher net sales and gross profits in future periods. Additionally, we expect that our R&D and acquired IPR&D and milestones expenses will continue to increase as we acquire or develop more development-stage medicine candidates and advance our candidates through the clinical development and regulatory approval processes. In particular, we expect to incur substantial costs in connection with advancing our pipeline of medicine candidates and development programs in on-going and planned clinical trials.
We are in the process of expanding our production capacity to meet anticipated future demand for TEPEZZA, primarily for 2023 and beyond. As of December 31, 2022, we had total purchase commitments, including the minimum annual order quantities and binding firm orders, with AGC Biologics A/S (formerly known as CMC Biologics A/S) for TEPEZZA drug substance of €72.8 million ($77.6 million converted at a Euro-to-Dollar exchange rate as of December 31, 2022 of 1.0660), to be delivered through December 2024.
We also expect to incur additional costs and to enter into additional purchase commitments in connection with our efforts to expand TEPEZZA production capacity in order to meet anticipated increases in demand.
Under our license agreement with F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc., or together referred to as Roche, our remaining obligation to Roche relating to the attainment of various TEPEZZA development and regulatory milestones is CHF43.0 million ($46.5 million when converted using a CHF-to-Dollar exchange rate at December 31, 2022 of 1.0823).
In July 2021, we completed the purchase of a drug product biologics manufacturing facility from EirGen for $67.9 million. Refer to Note 4, Acquisitions, Divestitures and other Arrangements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details. We expect to incur approximately $30.0 million in capital expenditures during 2023 in order to prepare the drug product facility to manufacture the first medicine for commercial use in the second half of 2023. In August 2022, we submitted a planning application to build a drug substance biologics manufacturing facility adjacent to our existing drug product biologics manufacturing facility in Waterford, Ireland. Based on our current operating plan, we do not anticipate making significant investments in building a drug substance biologics manufacturing facility during 2023.
On August 12, 2022, we entered into a collaboration and option agreement with Q32 related to its pipeline candidate ADX-914, a monoclonal antibody antagonist of the interleukin-7 receptor for the treatment of autoimmune and inflammatory diseases. An upfront payment of $15.0 million and milestone-based development funding of $17.5 million were paid during the year ended December 31, 2022 and recorded as acquired IPR&D and milestones expenses in the consolidated statement of comprehensive income. We may also be obligated to pay up to $22.5 million in the form of additional milestone-based development funding. If we exercise the option, we may be obligated to make up to an additional $645.0 million in closing and milestone payments, as well as tiered royalties on net sales from a high single-digit to a low double-digit percentage, inclusive of certain amounts payable to a third party under a pre-existing license agreement. Refer to Note 4, Acquisitions, Divestitures and other Arrangements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
116
On June 18, 2021, we entered into a global agreement with Arrowhead for HZN-457, a discovery-stage investigational RNA interference therapeutic being developed by Arrowhead as a potential treatment for uncontrolled gout. Under the terms of the agreement, we paid Arrowhead an upfront cash payment of $40.0 million in July 2021 and agreed to pay additional potential future milestone payments of up to $660.0 million contingent on the achievement of certain development, regulatory and commercial milestones, and low to mid-teens royalties on worldwide calendar year net sales of licensed medicines. In addition, we recognized a $15.0 million development milestone in the fourth quarter of 2022.
We are committed to invest as a strategic limited partner in four venture capital funds: Forbion Growth Opportunities Fund I C.V., Forbion Capital Fund V C.V., Aisling Capital V, L.P. and RiverVest Venture Fund V, L.P. As of December 31, 2022, the total carrying amount of our investments in these funds was $27.0 million, which is included in other long-term assets in the consolidated balance sheet, and our total future commitments to these funds are $36.2 million.
We have financed our operations to date through equity financings, debt financings and the issuance of convertible notes, along with cash flows from operations during the last several years. As of December 31, 2022, we had $2.4 billion in cash and cash equivalents and total debt with a book value of $2.6 billion and face value of $2.6 billion. We believe our existing cash and cash equivalents and our expected cash flows from our operations will be sufficient to fund our business needs for at least the next 12 months from the issuance of the financial statements in this Annual Report on Form 10-K. We do not have any financial covenants or non-financial covenants that we expect to be affected by the economic disruptions and negative effects of the COVID-19 pandemic, global macro-economic issues or inflationary pressures.
We have a significant amount of debt outstanding on a consolidated basis. For a description of our debt agreements, refer to Note 13, Debt Agreements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K. This substantial level of debt could have important consequences to our business, including, but not limited to: making it more difficult for us to satisfy our obligations; requiring a substantial portion of our cash flows from operations to be dedicated to the payment of principal and interest on our indebtedness, therefore reducing our ability to use our cash flows to fund acquisitions, capital expenditures, R&D and future business opportunities; limiting our ability to obtain additional financing, including borrowing additional funds; increasing our vulnerability to, and reducing our flexibility to respond to, general adverse economic and industry conditions, including rising interest rates; limiting our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; and placing us at a disadvantage as compared to our competitors, to the extent that they are not as highly leveraged. We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness.
In addition, the indenture governing our 5.5% Senior Notes due 2027 and our Credit Agreement impose various covenants that limit our ability and/or our restricted subsidiaries’ ability to, among other things, pay dividends or distributions, repurchase equity, prepay junior debt and make certain investments, incur additional debt and issue certain preferred stock, incur liens on assets, engage in certain asset sales or merger transactions, enter into transactions with affiliates, designate subsidiaries as unrestricted subsidiaries; and allow to exist certain restrictions on the ability of restricted subsidiaries to pay dividends or make other payments to us.
On April 25, 2022, we entered into two interest rate swap agreements with notional amounts totaling $800.0 million, effective June 24, 2022, to hedge or otherwise protect against interest rate fluctuations on a portion of our variable rate debt. The agreements effectively fix LIBOR at approximately 2.8% through December 24, 2026. These agreements were designated as cash flow hedges of the variability of future cash flows subject to the variable monthly interest rates on $800.0 million of our senior secured term loans borrowed under our Credit Agreement in December 2019 and March 2021. Refer to Note 14, Derivative Instruments and Hedging Activities, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
During the year ended December 31, 2022, we issued an aggregate of 3.8 million of our ordinary shares in connection with stock option exercises, the vesting of restricted stock units and performance stock units, and employee share purchase plan purchases. We received a total of $55.4 million in net proceeds in connection with such issuances. During the year ended December 31, 2022, we made payments of $137.2 million for employee withholding taxes relating to vesting of share-based awards.
117
In September 2022, our board of directors authorized a share repurchase program pursuant to which we may repurchase up to $500.0 million of our ordinary shares. Under the program, we may repurchase ordinary shares from time to time on the open market or through privately negotiated transactions or structured repurchase transactions. During the year ended December 31, 2022, we executed open market share repurchases of 3.9 million ordinary shares under this repurchase program for total consideration of $250.0 million. All ordinary shares repurchased were subsequently retired. The timing and amount of future repurchases, if any, will depend on a variety of factors, including the price of our ordinary shares, alternative investment opportunities, our cash resources, restrictions under our debt agreements and the transaction agreement with Amgen, corporate and regulatory requirements and market conditions. We expect that any future repurchases of our ordinary shares under the program would be funded with existing cash and cash equivalents. Refer to Note 18, Shareholders’ Equity, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
Since our inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities, other than the indemnification agreements discussed in Note 16, Commitments and Contingencies, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K.
Sources and Uses of Cash
The following table provides a summary of our cash position and cash flows for the years ended December 31, 2022 and 2021 (in thousands):
|
|
For the Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Cash, cash equivalents and restricted cash |
|
$ |
2,357,588 |
|
|
$ |
1,584,156 |
|
Cash provided by (used in): |
|
|
|
|
|
|
||
Operating activities |
|
|
1,257,842 |
|
|
|
1,035,271 |
|
Investing activities |
|
|
(134,001 |
) |
|
|
(2,994,111 |
) |
Financing activities |
|
|
(347,958 |
) |
|
|
1,470,123 |
|
Operating Cash Flows
Net cash provided by operating activities during the year ended December 31, 2022 of $1,257.8 million was primarily attributable to cash collections from gross sales, partially offset by payments made related to government rebates and patient assistance costs for our medicines, payments for inventory, payments related to selling, general and administrative expenses and payments related to R&D expenses.
Net cash provided by operating activities during the year ended December 31, 2021 of $1,035.3 million was primarily attributable to cash collections from gross sales, partially offset by payments made related to patient assistance costs and government rebates for our medicines, payments related to selling, general and administrative expenses, including transaction costs related to the Viela acquisition, and payments related to R&D expenses.
Investing Cash Flows
Net cash used in investing activities during the year ended December 31, 2022 of $134.0 million was primarily attributable to an upfront payment of $25.0 million paid to Alpine in the first quarter of 2022 relating to an exclusive license agreement entered into in December 2021, an upfront payment of $15.0 million relating to a collaboration and option agreement entered into with Q32 in the third quarter of 2022, milestone-based development funding of $17.5 million paid to Q32 in the fourth quarter of 2022 and payments related to purchases of property, plant and equipment of $64.0 million. Refer to Note 4, Acquisitions, Divestitures and other Arrangements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details on the Alpine license agreement and collaboration and option agreement with Q32.
118
Net cash used in investing activities of $2,994.1 million during the year ended December 31, 2021 was primarily attributable to payments for acquisitions, net of $2,845.3 million which was primarily attributable to $2.6 billion paid in relation to the Viela acquisition, net of acquired cash. In addition, we made a milestone payment of CHF50.0 million ($56.1 million when converted using a CHF-to-Dollar exchange rate at the date of payment of 1.1228) under our license agreement with Roche and we made a milestone payment of $67.0 million to the former River Vision stockholders during the year ended December 31, 2021. In the third quarter of 2021, we completed the purchase of a drug product biologics manufacturing facility from EirGen for $67.9 million, which included an upfront cash payment of $64.8 million and $3.1 million of additional transaction costs, legal fees and liabilities assumed and we paid an upfront cash payment of $40.0 million in relation to the global agreement with Arrowhead in July 2021.
Financing Cash Flows
Net cash used in financing activities during the year ended December 31, 2022 of $348.0 million was primarily attributable to $137.2 million in payments of employee withholding taxes relating to share-based awards, partially offset by $55.4 million in proceeds from the issuance of ordinary shares in connection with stock option exercises and employee share purchase plan purchases. In addition, we executed open market share repurchases of 3.9 million of our ordinary shares for total consideration of $250.0 million. Refer to Note 18, Shareholders’ Equity, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K, for further details on our share repurchase program.
Net cash provided by financing activities during the year ended December 31, 2021 of $1,470.1 million was primarily attributable to an additional $1.6 billion aggregate principal amount of term loans borrowed pursuant to an amendment to our Credit Agreement, the proceeds of which, in addition to a portion of our existing cash on hand, was used to pay the consideration for the Viela acquisition, partially offset by the payment of $166.0 million of employee withholding taxes relating to share-based awards.
Financial Condition as of December 31, 2022 Compared to December 31, 2021
Inventories, net. Inventories, net decreased $56.2 million, from $225.7 million during the year ended December 31, 2021 to $169.5 million during the year ended December 31, 2022. The decrease was primarily due inventory step-up expense recorded relating to UPLIZNA of $91.7 million based on the acquired units sold during the period, partially offset by an increase in finished goods of $40.1 million primarily related to finished goods on hand of TEPEZZA during the year ended December 31, 2022. Refer to Note 5, Inventories, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
Prepaid expenses and other current assets. Prepaid expenses and other current assets increased $92.2 million, from $357.1 million during the year ended December 31, 2021 to $449.3 million during the year ended December 31, 2022. The increase was primarily due to a tax benefit of $98.6 million related to deferred charges for taxes on higher intercompany inventory transfers.
Developed technology and other intangible assets, net. Developed technology and other intangible assets, net decreased $295.3 million, from $2,960.1 million during the year ended December 31, 2021 to $2,664.8 million during the year ended December 31, 2022. The decrease was primarily due to a decrease of $366.5 million related to amortization of developed technology during the year ended December 31, 2022. This was partially offset by $70.0 million of IPR&D reclassified to developed technology in the second quarter of 2022 due to the European Commission issuing a legally binding decision to grant a Marketing Authorization for UPLIZNA for the treatment of adult patients with neuromyelitis optica spectrum disorder in the European Union in April 2022. Refer to Note 8, Goodwill and Intangible Assets, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
In-process research and development. IPR&D decreased $70.0 million, from $880.0 million as of December 31, 2021 to $810.0 million as of December 31, 2022, primarily related to the reclassification of $70.0 million of IPR&D relating to UPLIZNA to developed technology in the second quarter of 2022. Refer to Note 8, Goodwill and Intangible Assets, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
Goodwill. Goodwill decreased $56.2 million, from $1,066.7 million as of December 31, 2021 to $1,010.5 million as of December 31, 2022. Our interim goodwill impairment test in the second quarter of 2022 indicated an impairment which represented the difference between the estimated fair value of our former inflammation reporting unit and its carrying value. As a result, we recognized an impairment charge of $56.2 million in June 2022 representing the full amount of goodwill for the former inflammation reporting unit. Refer to Note 8, Goodwill and Intangible Assets, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
119
Other long-term assets. Other long-term assets increased $63.4 million, from $140.7 million during the year ended December 31, 2021 to $204.1 million during the year ended December 31, 2022. The increase was primarily due to a $23.8 million increase in right-of-use assets following new leases entered into in San Francisco and Dublin, an increase of $22.5 million relating to advance payments for long-term clinical studies and $14.8 million relating to the interest rate swap asset that was recorded in connection with the interest rate swap contracts entered into during the year ended December 31, 2022. Refer to Note 14, Derivative Instruments and Hedging Activities, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
Accounts Payable. Accounts payable increased $125.7 million, from $30.1 million during the year ended December 31, 2021 to $155.8 million during the year ended December 31, 2022. The increase was primarily due to the timing of invoices received, including an increase of $77.4 million in accounts payable related to government rebates, co-pay and patient assistance costs and commercial rebates and wholesaler fees.
Accrued Expenses and other current liabilities. Accrued expenses and other current liabilities decreased $65.4 million, from $523.0 million during the year ended December 31, 2021 to $457.6 million during the year ended December 31, 2022. The decrease was primarily due to a decrease of $26.4 million in accrued payroll-related expenses, a decrease of $21.1 million in pricing review liability and a decrease of $20.1 million in accrued upfront and milestone payments.
Contractual Obligations
Our primary contractual obligations relate to our debt agreements, non-cancellable obligations under lease agreements and commitments with third parties. For information relating to our scheduled maturities with respect to our long-term debt and our lease liabilities, refer to Note 13, Debt Agreements, and Note 15, Lease Obligations, respectively, included in the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K. For information relating to our purchase commitments with our third-party manufacturers, non-cancellable advertising commitments due within one year and venture capital fund future commitments, refer to Note 16, Commitments and Contingencies, included in the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K.
In addition, for information relating to our contingent liability for uncertain tax positions, refer to Note 20, Income Taxes, included in the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K. We do not expect a significant tax payment related to these obligations within the next year. We are committed to an aggregate $2.7 billion of potential contingent future milestone payments to third parties relating to asset acquisitions and license and collaboration agreements, including those acquired through business combinations. Milestone payments generally are due and payable only upon achievement of certain developmental, regulatory and commercial milestones for which the specific timing cannot be predicted.
In July 2021, we completed the purchase of a drug product biologics manufacturing facility from EirGen for $67.9 million. We expect to incur approximately $30.0 million in capital expenditures during 2023 in order to prepare the drug product facility to manufacture the first medicine for commercial use in the second half of 2023. Refer to Note 4, Acquisitions, Divestitures and other Arrangements, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K for further details.
120
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The methods, estimates and judgments that we use in applying our critical accounting policies have a significant impact on the results that we report in our financial statements. Some of our accounting policies require us to make difficult and subjective judgments, often as a result of the need to make estimates regarding matters that are inherently uncertain.
We have identified the accounting policies and estimates listed below as those that we believe require management’s most subjective and complex judgments in estimating the effect of inherent uncertainties. This section should also be read in conjunction with Note 2, Summary of Significant Accounting Policies, of the Notes to our Consolidated Financial Statements included in Item 15 of this Annual Report on Form 10-K, which includes a discussion of these and other significant accounting policies.
Revenue Recognition
In the United States, we sell our medicines primarily to wholesale distributors, specialty distributors and specialty pharmacy providers. In other countries, we sell our medicines primarily to wholesale distributors and other third-party distribution partners. These customers subsequently resell our medicines to health care providers and patients. In addition, we enter into arrangements with health care providers and payers that provide for government-mandated or privately negotiated discounts and allowances related to our medicines. Revenue is recognized when performance obligations under the terms of a contract with a customer are satisfied. The majority of our contracts have a single performance obligation to transfer medicines. Accordingly, revenues from medicine sales are recognized when the customer obtains control of our medicines, which occurs at a point in time, typically upon delivery to the customer. Revenue is measured as the amount of consideration we expect to receive in exchange for transferring medicines and is generally based upon a list or fixed price less allowances for medicine returns, rebates and discounts. We sell our medicines to wholesale pharmaceutical distributors and pharmacies under agreements with payment terms typically less than 90 days. Discounts, rebates, returns and certain other adjustments are accounted for as variable consideration.
Medicine Sales Discounts and Allowances
The nature of our contracts gives rise to variable consideration because of allowances for medicine returns, rebates and discounts. Allowances for medicine returns, rebates and discounts are recorded at the time of sale to wholesale pharmaceutical distributors and pharmacies. We apply significant judgments and estimates in determining some of these allowances. If actual results differ from our estimates, we will be required to make adjustments to these allowances in the future. Our adjustments to gross sales are discussed further below.
Commercial Rebates
We participate in certain commercial rebate programs. Under these rebate programs, we pay a rebate to the commercial entity or third-party administrator of the program. We calculate accrued commercial rebate estimates using the expected value method. We accrue estimated rebates based on contract prices, estimated percentages of medicine that will be prescribed to qualified patients and estimated levels of inventory in the distribution channel and record the rebate as a reduction of revenue. Accrued commercial rebates are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
Co-pay and Other Patient Assistance Programs
We offer discount card and other programs to patients under which the patient receives a discount on his or her prescription. In certain circumstances when a patient’s prescription is rejected by a managed care vendor, we will pay for the full cost of the prescription. We reimburse pharmacies for this discount through third-party vendors. We reduce gross sales by the amount of actual co-pay and other patient assistance in the period based on invoices received. We also record an accrual to reduce gross sales for estimated co-pay and other patient assistance on units sold to distributors that have not yet been prescribed/dispensed to a patient. We calculate accrued co-pay and other patient assistance costs using the expected value method. The estimate is based on contract prices, estimated percentages of medicine that will be prescribed to qualified patients, average assistance paid based on reporting from the third-party vendors and estimated levels of inventory in the distribution channel. Accrued co-pay and other patient assistance costs are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
121
Sales Returns
Consistent with industry practice, we maintain a return policy that allows customers to return certain medicines within a specified period prior to and subsequent to the medicine expiration date. Generally, medicines may be returned for a period beginning six months prior to its expiration date and up to one year after its expiration date. The right of return expires on the earlier of one year after the medicine expiration date or the time that the medicine is dispensed to the patient. The majority of medicine returns result from medicine dating, which falls within the range set by our policy, and are settled through the issuance of a credit to the customer. We calculate sales returns using the expected value method. The estimate of the provision for returns is based upon our historical experience with actual returns. The return period is known to us based on the shelf life of medicines at the time of shipment. We record sales returns in “accrued expenses and other current liabilities” and as a reduction of revenue.
Government Rebates
We participate in certain government rebate programs such as Medicare Coverage Gap and Medicaid. We calculate accrued government rebate estimates using the expected value method. A significant portion of these accruals relates to our Medicaid rebates. We accrue estimated rebates based on estimated percentages of medicine prescribed to qualified patients, estimated rebate percentages and estimated levels of inventory in the distribution channel that will be prescribed to qualified patients and record the rebates as a reduction of revenue. Accrued government rebates are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
Chargebacks
We provide discounts to government qualified entities with whom we have contracted. These entities purchase medicines from the wholesale pharmaceutical distributors at a discounted price and the wholesale pharmaceutical distributors then charge back to us the difference between the current retail price and the contracted price that the entities paid for the medicines. We calculate accrued chargeback estimates using the expected value method. We accrue estimated chargebacks based on contract prices, sell-through sales data obtained from third-party information and estimated levels of inventory in the distribution channel and record the chargeback as a reduction of revenue. Accrued chargebacks are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
Refer to Note 10, Accrued Trade Discounts and Rebates, of the Notes to our Consolidated Financial Statements included in Item 15 of this Annual Report on Form 10-K, which includes a table that summarizes changes in our customer-related accruals and allowances from December 31, 2020 to December 31, 2022.
Intangible Assets
Definite-lived intangible assets are amortized over their estimated useful lives. We review our intangible assets when events or circumstances may indicate that the carrying value of these assets is not recoverable and exceeds their fair value. We measure fair value based on the estimated future discounted cash flows associated with our assets in addition to other assumptions and projections that we deem to be reasonable and supportable. The estimated useful lives, from the date of acquisition, for all identified intangible assets that are subject to amortization are between five and thirteen years.
Indefinite-lived intangible assets consist of capitalized IPR&D. IPR&D assets represent capitalized incomplete research and development projects that we acquired through business combinations. Such assets are initially measured at their acquisition date fair values and are tested for impairment, until completion or abandonment of R&D efforts associated with the projects. An IPR&D asset is considered abandoned when R&D efforts associated with the asset have ceased, and there are no plans to sell or license the asset or derive value from the asset. At that point, the asset is considered to be impaired and is written off. Upon successful completion of each project, we will make a determination about the then remaining useful life of the intangible asset and begin amortization. We test indefinite-lived intangibles, including IPR&D assets, for impairment annually during the fourth quarter and more frequently if events or changes in circumstances indicate that it is more likely than not that the asset is impaired.
122
Business Combinations
We account for business combinations in accordance with the guidance in Accounting Standards Codification Topic 805, Business Combinations, under which acquired assets and liabilities are measured at their respective estimated fair values as of the acquisition date. We may be required, as in the case of intangible assets to determine the fair value associated with these amounts by estimating the fair value using an income approach under the discounted cash flow method, which may include revenue projections and other assumptions made by us to determine the fair value.
Goodwill
Goodwill represents the excess of the purchase price of acquired businesses over the estimated fair value of the identifiable net assets acquired. Goodwill is not amortized but is tested for impairment at least annually at the reporting unit level or more frequently if events or changes in circumstances indicate that the asset might be impaired. Impairment loss, if any, is recognized based on a comparison of the fair value of the asset to its carrying value, without consideration of any recoverability. We test goodwill for impairment annually during the fourth quarter and whenever indicators of impairment exist by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If we conclude it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative impairment test is performed. If we conclude that goodwill is impaired, we will record an impairment charge in our consolidated statement of comprehensive income.
Provision for Income Taxes
We account for income taxes based upon an asset and liability approach. Deferred tax assets and liabilities represent the future tax consequences of the differences between the financial statement carrying amounts of assets and liabilities versus the tax basis of assets and liabilities. Under this method, deferred tax assets are recognized for deductible temporary differences, and operating loss and tax credit carryforwards. Deferred tax liabilities are recognized for taxable temporary differences. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Significant judgment is required in determining whether it is probable that sufficient future taxable income will be available against which a deferred tax asset can be utilized. In determining future taxable income, we are required to make assumptions including the amount of taxable income in the various jurisdictions in which we operate. These assumptions require significant judgment about forecasts of future taxable income. Actual operating results in future years could render our current assumption of recoverability of deferred tax assets inaccurate. The impact of tax rate changes on deferred tax assets and liabilities is recognized in the period that the change is enacted. From time to time, we execute intercompany transactions in response to changes in operations, regulations, tax laws, funding needs and other circumstances. These transactions require the interpretation and application of tax laws in the applicable jurisdiction to support the tax treatment taken. The valuations which support the tax treatment of the transactions require significant estimates and assumptions within discounted cash flow models. We also account for the uncertainty in income taxes by utilizing a comprehensive model for the recognition, measurement, presentation and disclosure in financial statements of any uncertain tax positions that have been taken or are expected to be taken on an income tax return. Deferred tax assets and deferred tax liabilities are netted by each tax-paying entity within each jurisdiction in our consolidated balance sheets.
New Accounting Pronouncements Impacting Critical Accounting Policies
Refer to Note 2, Summary of Significant Accounting Policies, of the Notes to our Consolidated Financial Statements included in Item 15 of this Annual Report on Form 10-K, which includes a discussion of the new accounting pronouncements impacting critical accounting policies.
123
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
We are exposed to various market risks, which include potential losses arising from adverse changes in market rates and prices, such as interest rates and foreign exchange fluctuations. We do not enter into derivatives or other financial instruments for trading or speculative purposes.
Interest Rate Risk. We are subject to interest rate fluctuation exposure through our borrowings under our Credit Agreement and our investment in money market accounts which bear a variable interest rate. Our approximately $418.0 million aggregate principal amount of senior secured term loans borrowed under our Credit Agreement in December 2019, or the 2026 Term Loans, and loans under our incremental revolving credit facility, or Revolving Credit Facility, bear interest, at our option, at a rate equal to the London Inter-Bank Offered Rate, or LIBOR, plus 2.25% per annum (subject to a 0.00% LIBOR floor), or the adjusted base rate plus 1.25% per annum with a step-down to LIBOR plus 2.00% per annum or the adjusted base rate plus 1.00% per annum at the time our leverage ratio is less than or equal to 2.00 to 1.00. The adjusted base rate is defined as the greatest of (a) LIBOR (using one-month interest period) plus 1.00%, (b) the prime rate, (c) the federal funds rate plus 0.50%, and (d) 1.00%. Our 2026 Term Loans are borrowed in LIBOR. The one-month LIBOR rate as of February 24, 2023, which was the most recent date the interest rate on the 2026 Term Loans was fixed, was 4.63%, and as a result, the interest rate on our 2026 Term Loans is currently 6.63% per annum. Our $1.6 billion aggregate principal amount of senior secured term loans borrowed under our Credit Agreement in March 2021, or the 2028 Term Loans, bear interest, at our option, at a rate equal to LIBOR, plus 2.00% per annum (subject to a 0.50% LIBOR floor), or the adjusted base rate plus 1.00% per annum with a step-down to LIBOR plus 1.75% per annum or the adjusted base rate plus 0.75% per annum at the time our leverage ratio is less than or equal to 2.00 to 1.00. Our 2028 Term Loans are based on LIBOR. The one-month LIBOR rate as of February 24, 2023, which was the most recent date the interest rate on the 2028 Term Loans was fixed, was 4.63%, and as a result, the interest rate on our 2028 Term Loans is currently 6.38% per annum. As of December 31, 2022, the Revolving Credit Facility was undrawn. Because the UK FCA, which regulates LIBOR, intended to phase out the use of LIBOR by the end of 2021, future borrowings under our Credit Agreement could be subject to reference rates other than LIBOR. However, the cessation date has been deferred to June 30, 2023, for the most commonly used tenors in U.S. dollar LIBOR (i.e., overnight and one, three and six months). We do not expect the planned discontinuation of LIBOR to have a material impact on interest payments incurred under our Credit Agreement.
As of December 31, 2022, an increase in the LIBOR of 100 basis points above the current LIBOR rate, which includes the effect of the two interest rate swaps entered into in the second quarter of 2022, would increase our interest expense related to the Credit Agreement by $12.1 million per year.
The goals of our investment policy are to preserve capital, fulfill liquidity needs and maintain fiduciary control of cash. To achieve our goal of maximizing income without assuming significant market risk, we maintain our excess cash and cash equivalents in money market funds, time deposits and U.S. federal government securities. Because of the short-term maturities of our cash equivalents, we do not believe that a decrease in interest rates would have any material negative impact on the fair value of our cash equivalents.
Foreign Currency Risk. Our purchase costs of TEPEZZA drug substance, TEPEZZA drug product with our second drug product manufacturer, Patheon Pharmaceuticals Inc. (the contract development and manufacturing services organization of Thermo Fisher Scientific), and ACTIMMUNE inventory are principally denominated in Euros and are subject to foreign currency risk. In addition, we are obligated to pay certain milestones and a royalty on sales of TEPEZZA to Roche in Swiss Francs, which obligations are subject to foreign currency risk. We also incur certain operating expenses and earn certain revenues in currencies other than the U.S. dollar in relation to our Irish operations and foreign subsidiaries. Therefore, we are subject to volatility in cash flows due to fluctuations in foreign currency exchange rates, particularly changes in the Euro and the Swiss Franc. In addition, we enter into forward currency contracts to hedge our foreign currency risk exposure.
Inflation Risk. We do not believe that inflation has had a material impact on our business or results of operations during the periods for which the financial statements are presented in this report.
Credit Risk. Historically, our accounts receivable balances have been highly concentrated with a select number of customers, consisting primarily of large wholesale biopharmaceutical distributors who, in turn, sell the medicines to pharmacies, hospitals and other customers. As of each of December 31, 2022 and 2021, our top four customers accounted for approximately 94% of our total outstanding accounts receivable balances. Given the size and creditworthiness of the customers, we have not experienced and do not expect to experience material credit related losses with such customers.
124
Item 8. Financial Statements and Supplementary Data
The financial information required by Item 8 is contained in Part IV, Item 15 of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our Chief Executive Officer and Chief Financial Officer, after evaluating the effectiveness of our “disclosure controls and procedures” (as defined in Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended, or the Exchange Act), have concluded that, as of December 31, 2022, our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive officer or officers and principal financial officer or officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management, under the supervision of our Chief Executive Officer and our Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined under Rule 13a-15(f) of the Exchange Act. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2022. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control – Integrated Framework (2013). Management’s assessment included an evaluation of the design of our internal control over financial reporting and testing of the operational effectiveness of our internal control over financial reporting. Based on management’s assessment, management has concluded that, as of December 31, 2022, our internal control over financial reporting was effective based on those criteria.
The effectiveness of our internal control over financial reporting as of December 31, 2022, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in Internal Control Over Financial Reporting
There have been no material changes to our internal control over financial reporting, as defined in Exchange Act Rules 13a-15(f) and 15d-15(f), during the three months ended December 31, 2022, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable
125
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The following table sets forth information regarding our directors and executive officers as of March 1, 2023:
Name |
|
Age |
|
|
Position with the Company |
|
Directors |
|
|
|
|
|
|
Timothy P. Walbert |
|
|
55 |
|
|
Chairman, President and Chief Executive Officer |
William F. Daniel (1,2) |
|
|
70 |
|
|
Director |
Michael Grey (1,3,4) |
|
|
70 |
|
|
Lead Independent Director |
Jeff Himawan, Ph.D. (2,4,5) |
|
|
57 |
|
|
Director |
Susan Mahony, Ph.D. (2,4,5) |
|
|
58 |
|
|
Director |
Gino Santini (2,5) |
|
|
66 |
|
|
Director |
James Shannon, M.D. (3,4,5) |
|
|
66 |
|
|
Director |
H. Thomas Watkins (1,3) |
|
|
70 |
|
|
Director |
Pascale Witz (1,3) |
|
|
56 |
|
|
Director |
Name |
|
Age |
|
|
Position with the Company |
|
Executive Officers (other than Mr. Walbert) |
|
|
|
|
|
|
Sean M. Clayton (1) |
|
|
44 |
|
|
Executive Vice President, General Counsel |
Aaron L. Cox (2) |
|
|
40 |
|
|
Executive Vice President, Chief Financial Officer |
Michael A. DesJardin |
|
|
65 |
|
|
Executive Vice President, Technical Operations and Corporate Quality |
Andy Pasternak |
|
|
52 |
|
|
Executive Vice President, Chief Strategy Officer |
Jeffrey W. Sherman, M.D., FACP |
|
|
68 |
|
|
Executive Vice President, Chief Medical Officer |
Elizabeth H.Z. Thompson, Ph.D. |
|
|
48 |
|
|
Executive Vice President, Research and Development |
126
Directors
Timothy P. Walbert. Mr. Walbert has served as our president, chief executive officer and director of Horizon since June 2008 and has served as our chairman since March 2010. From May 2007 to June 2009, Mr. Walbert served as president, chief executive officer and director of IDM Pharma, Inc., a public biotechnology company that was acquired by Takeda America Holdings, Inc. in June 2009. Prior to that, he served as executive vice president, commercial operations of NeoPharm, Inc., a public biotechnology company. From June 2001 to August 2005, Mr. Walbert served as divisional vice president and general manager of immunology, where he built and led the global development and launch of the multi-indication biologic HUMIRA, and divisional vice president, global cardiovascular strategy at Abbott, now AbbVie. From 1998 to 2001, he served as director, CELEBREX North America and arthritis team leader, Asia Pacific, Latin America and Canada at G.D. Searle & Company. Mr. Walbert has served on the board of directors of Century Therapeutics, Inc., a public biotechnology company, since October 2022, and he sits on the board of directors of the Illinois Biotechnology Innovation Organization (iBIO). Mr. Walbert is also a member of the National Organization for Rare Disorders (NORD) Advisory Board and serves on the Board of Trustees of Muhlenberg College. He previously served (i) as chairman of the board of directors of Exicure, Inc., a public clinical-stage biotechnology company, from July 2019 to February 2022; and (ii) on the board of directors of (a) Aurinia Pharmaceuticals, Inc., a public biotechnology company, from April 2020 to September 2022; (b) Assertio Holdings, Inc., a public specialty pharmaceutical company, from May 2020 to December 2020 (and before that at Zyla Life Sciences, a public pharmaceutical company, from April 2014 until May 2020, when it was acquired by Assertio); and (c) Sucampo Pharmaceuticals, Inc., a public biopharma company, from 2016 to 2018, when it was acquired by Mallinckrodt. Mr. Walbert received his bachelor of arts degree in business from Muhlenberg College, in Allentown, Pennsylvania. Our Nominating and Corporate Governance Committee and our board of directors believe that Mr. Walbert is qualified to serve as a director on the basis of his valuable biopharma industry experience, which brings important strategic insight to our board of directors as it plans our future growth.
William F. Daniel. Mr. Daniel, a chartered director and chartered accountant, is currently chair of the board of directors of Malin Corporation plc, an Ireland-based public global life sciences company. Mr. Daniel was president of the Institute of Directors of Ireland from May 2013 to May 2015, and he was originally elected to the board of the Institute of Directors in Ireland in June 2010. Prior to that, Mr. Daniel was executive vice president and company secretary of Elan Corporation plc, a public biotechnology company, and served in that role from December 2001 to December 2013, until the merger of Elan with Perrigo Company plc. He was previously an executive director of Elan between 2003 and 2007, having joined the organization as financial controller in 1994. Mr. Daniel graduated with a degree in commerce from University College Dublin. Our Nominating and Corporate Governance Committee and our board of directors believe that Mr. Daniel is qualified to serve as a director on the basis of his valuable financial and corporate governance expertise, which brings important strategic insight to our board of directors as it plans our future growth.
Michael Grey. Mr. Grey has served as chairman of the board of directors of Mirum Pharmaceuticals, Inc., a public biotechnology company, since January 2020, and as executive chairman from March 2019 to December 2019. Before that he served as chief executive officer of Mirum from the company’s inception in March 2018. He has served as executive chairman of Spruce Biosciences, Inc., a public biotechnology company, since April 2017; as executive chairman of Reneo Pharmaceuticals, Inc., a public pharmaceutical company, since December 2017; as executive chairman of Plexium, Inc., a private biotechnology company, since August 2020; and as chairman of Sorriso Pharmaceuticals, Inc., a private biotechnology company, since June 2021. He has also served as a venture partner at Pappas Ventures since January 2010. Mr. Grey served from October 2015 to January 2017 as the president and chief executive officer of Amplyx Pharmaceuticals, Inc., a private pharmaceutical company, from September 2014 to December 2017 and then as executive chairman from January 2018 until April 2021; as chairman and chief executive officer of Reneo from May 2019 until April 2020 and as executive chairman of Curzion Pharmaceuticals, Inc., a private pharmaceutical company, from May 2019 to April 2020. From February 2011 to June 2014, Mr. Grey served as president and chief executive officer of Lumena Pharmaceuticals, Inc., a biotechnology company, which was acquired by Shire plc in June 2014. He has more than 45 years of experience in the pharmaceutical and biotechnology industries and has held senior positions at a number of companies, including president and chief executive officer of SGX Pharmaceuticals, Inc. (sold to Eli Lilly and Company in 2008); president and chief executive officer of Trega Biosciences, Inc. (sold to LION Bioscience, Inc. in 2001) and president of BioChem Therapeutic Inc. Prior to these, Mr. Grey served in various roles with Glaxo, Inc. and Glaxo Holdings PLC, culminating in his position as vice president, corporate development and director of international licensing. Mr. Grey served on the boards of directors of BioMarin Pharmaceutical Inc. from December 2005 until May 2021, and Mirati Therapeutics, Inc. from November 2014 to June 2021, both public biotechnology companies. Mr. Grey received a bachelor of science degree in chemistry from the University of Nottingham in the United Kingdom. Our Nominating and Corporate Governance Committee and our board of directors believe that Mr. Grey is qualified to serve as a director on the basis of his extensive experience managing pharmaceutical and biopharma companies, which brings important strategic insight to our board of directors as it plans our future growth.
127
Jeff Himawan, Ph.D. Dr. Himawan has been a managing director of Essex Woodlands Health Ventures, a venture capital firm, since 2004. Prior to that, he was an adjunct partner at Essex Woodlands from 1999 to 2001, and he was a venture partner from 2001 to 2004. Dr. Himawan co-founded Seed-One Ventures, an early-stage venture capital firm, where he served as a managing director from 1996 to 2001. Dr. Himawan also currently serves on the board of directors of MediciNova, Inc., a public biopharma company, NexEos Bio, Inc., a private biotechnology company and Light Sciences Oncology, Inc., a private biotechnology company. Previously, Dr. Himawan served on the board of directors of Catalyst Biosciences, Inc., a public biopharma company, from 2010 to 2020. He received a bachelor of science degree in biology from the Massachusetts Institute of Technology and a doctorate in biological chemistry and molecular pharmacology from Harvard University. Our Nominating and Corporate Governance Committee and our board of directors believe that, with his doctorate in biological chemistry and molecular pharmacology and as a successful venture capitalist, Dr. Himawan brings important scientific and strategic insight to our board of directors as well as experience working with the investment community.
Susan Mahony, Ph.D. Dr. Mahony serves on the board of directors of Zymeworks Inc., a public biopharma company and Assembly Biosciences, Inc., a public biotechnology company. She served on the board of directors of Vifor Pharma AG, a public pharmaceutical company, from May 2019 until its acquisition by CSL Limited in August 2022. Previously, Dr. Mahony served as senior vice president and president of Lilly Oncology and was a member of the executive committee at Eli Lilly and Company from 2009 until her retirement in August 2018. Prior to that, Dr. Mahony served in a variety of leadership roles at Eli Lilly and Company, including senior vice president, human resources and diversity; president and general manager, Lilly Canada; and executive director, global brand development. Dr. Mahony worked in sales and marketing at Bristol-Myers Squibb Company from 1995 to 2000, at Amgen Limited from 1991 to 1995, and at Schering Plough from 1989 to 1991. Dr. Mahony also serves on the board of Chordoma Foundation, a nonprofit organization dedicated to improving the lives of those affected by chordoma. She earned bachelor of science and doctor of philosophy degrees in pharmacy from Aston University, as well as a master of business administration degree from London Business School. She was awarded an honorary doctorate from Aston University. Our Nominating and Corporate Governance Committee and our board of directors believe that Dr. Mahony is qualified to serve as a director on the basis of her industry and leadership expertise, which brings important strategic insight to our board of directors as it plans our future growth.
Gino Santini. Mr. Santini currently serves on the boards of directors of Intercept Pharmaceuticals, Inc. and Collegium Pharmaceutical, Inc., both of which are public biopharma companies. Mr. Santini also serves on the boards of directors of Artax Biopharma Inc. and Enalare Therapeutics, Inc., each a private biopharma company, and is retired from a distinguished career with Eli Lilly and Company. He served as chairman of the board of directors of AMAG Pharmaceuticals, Inc., previously a public biopharma company, from February 2012 until November 2020, when AMAG was acquired by Covis Group S. à r.l. During his tenure at Eli Lilly and Company from June 1983 to December 2010, Mr. Santini held various leadership positions. He previously served on the board of directors of Allena Pharmaceuticals, Inc., a public biopharma company, from February 2012 until September 2022. Mr. Santini, fluent in four languages, holds an undergraduate degree in mechanical engineering from the University of Bologna and a master of business administration degree from the University of Rochester. Our Nominating and Corporate Governance Committee and our board of directors believe that Mr. Santini’s extensive international and domestic commercial and business development experience brings important insight to our board of directors as it plans our future growth.
James Shannon, M.D. Dr. Shannon currently serves as chairman of the board of directors of MannKind Corporation, a public biopharma company focused on treatments for diabetes, and on the board of directors of ProQR Therapeutics NV, a public biotechnology company. From May 2012 to March 2015, Dr. Shannon served as the chief medical officer of GlaxoSmithKline, or GSK, a public biopharma company, where he was responsible for matters of patient safety, general medical governance, medical ethics and integrity, medical information as well as investigations involving human subjects relating to any GSK medicine in development or on the market. Prior to that, Dr. Shannon spent more than a decade with Novartis, a public pharmaceutical company. In his last role with the company, as global head of pharma development, he was responsible for all of Novartis’s development activities, from pre-clinical through Phase 4, and oversaw an annual development budget of approximately $4 billion. Dr. Shannon received his science and medical degrees from Queen’s University in Belfast, Northern Ireland. He also serves as chairman of the board of directors of Kyowa Kirin (NA), a private biopharma company and subsidiary of Kyowa Kirin, and on the boards of directors of Leyden Labs, a private biopharma company, and MyTomorrows, a private health-based platform that collaborates with drug developers to provide early access to treatments for patients who have exhausted all other options. Our Nominating and Corporate Governance Committee and our board of directors believe that Dr. Shannon is qualified to serve as a director on the basis of his extensive clinical development experience, which brings important insight to our board of directors as it plans our future growth.
128
H. Thomas Watkins. Mr. Watkins is a member of the board of directors of HemoShear Therapeutics LLC, a private biotechnology company. He was director, president and chief executive officer of Human Genome Sciences, Inc., or HGS, a public biopharma company, from 2004 until HGS was acquired by GlaxoSmithKline in 2012. Before leading HGS, Mr. Watkins spent over twenty years in senior roles at Abbott and its affiliates in the United States and Asia, most recently serving as the president of TAP Pharmaceutical Products, Inc., or TAP, which was jointly owned by Abbott and Takeda Pharmaceutical Company, Inc. During his tenure, he led the growth of TAP from approximately $2 billion to over $4 billion in annual revenue. Mr. Watkins began his career in 1974 with Arthur Andersen & Co. From 1979 to 1985, he was a management consultant with McKinsey and Company, Inc., working with multinational companies in the United States, Europe and Japan. Mr. Watkins previously served on the board of directors of Vanda Pharmaceuticals Inc., a public biopharma company, from September 2006 until June 2022. He holds a bachelor’s degree from William and Mary, and a master of business administration degree from the University of Chicago Graduate School of Business. Our Nominating and Corporate Governance Committee and our board of directors believe that Mr. Watkins is qualified to serve as a director on the basis of his valuable industry experience, which brings important strategic insight to our board of directors as it plans our future growth.
Pascale Witz. Ms. Witz founded PWH Advisors, a strategic consultancy firm advising healthcare and investment companies, in November 2016 and has served as its president since that time. From September 2015 to May 2016, Ms. Witz served as executive vice president, global diabetes and cardiovascular at Sanofi, a pharmaceutical company, which she joined in July 2013 as executive vice president, pharma and CHC divisions. During her tenure at Sanofi, she launched multiple medicines across three continents and strengthened the pipeline through licensing and partnerships. Prior to Sanofi, Ms. Witz served more than 17 years at GE Healthcare where, in her final role as president and chief executive officer of its pharmaceutical diagnostics business, she ran a $2 billion integrated pharmaceutical organization that encompassed research and development through commercialization. Ms. Witz also serves on the boards of directors of Fresenius Medical Care AG & Co. KGaA, a public medical supply company; Regulus Therapeutics Inc., a public biotechnology company; PerkinElmer, Inc., a public company focused on diagnostics and life science tools; PWH Advisors; Arkuda Therapeutics, Inc., a private biopharma company; CellCarta Biosciences, a private biopharma services company; WGC Clinical Services, a private biopharma services company; and Lumanity Inc., a private technology company. She also serves as chair of RTI Surgical, Inc., a private biologics implants company. Ms. Witz previously served on the board of directors of Savencia SA, a public food and dairy company, from 2016 to 2018, and of Tesaro, Inc., then a public biopharma company, from 2018 to January 2019. Ms. Witz received her master of business administration degree in economics and marketing from INSEAD and her Master of Science in biochemistry from INSA Lyon. Our Nominating and Corporate Governance Committee and our board of directors believe that Ms. Witz is qualified to serve as a director on the basis of her valuable industry experience, which brings important strategic insight to our board of directors as it plans our future growth.
Executive Officers (other than Mr. Walbert)
Sean M. Clayton. Mr. Clayton has served as our executive vice president, general counsel since February 2022. Prior to joining Horizon, Mr. Clayton was a partner at Cooley LLP, a global law firm, where he practiced from October 2004 until February 2022, most recently as the head of the firm’s San Diego corporate practice. Before that, Mr. Clayton served as a law clerk to Judge Irma E. Gonzalez (Ret.) of the U.S. District Court for the Southern District of California. Mr. Clayton received a bachelor of arts degree in political science and economics from the University of California, San Diego and a juris doctorate degree from Stanford Law School.
Aaron L. Cox. Mr. Cox has served as our executive vice president, chief financial officer since May 2022. Mr. Cox previously served as executive vice president, finance from November 2021 to May 2022; as senior vice president, corporate development, in addition to serving as chief of staff for Timothy P. Walbert, from July 2017 to November 2021; as senior director, business development, from May 2017 to July 2017 and as director, business development from April 2016 to May 2017. Prior to joining Horizon in April 2016, Mr. Cox served as vice president, capital markets at BMO Capital Markets from July 2012 to April 2016, and before that, he held investment banking roles at Stout from 2007 to 2010 and JMP Securities from 2005 to 2006. Mr. Cox received a master of business administration from the University of Chicago and a bachelor of business administration in finance from the University of Notre Dame.
129
Michael A. DesJardin. Mr. DesJardin has served as our executive vice president, technical operations and corporate quality since February 2017. Mr. DesJardin previously served as our senior vice president, technical operations from October 2016 to November 2016 and as our senior vice president, life cycle management from December 2016 to January 2017. Mr. DesJardin joined Horizon from Raptor Pharmaceutical Corp., or Raptor, a public biopharma company, in October 2016 as part of the Raptor acquisition. While at Raptor, Mr. DesJardin was the senior vice president of technical operations from April 2015 to October 2016. Prior to that, Mr. DesJardin served as senior vice president of product development at Jazz Pharmaceuticals plc (formerly Jazz Pharmaceuticals, Inc.) from July 2004 to March 2015. Mr. DesJardin spent nine years as an executive director and engineering fellow at ALZA Corporation and spent 15 years at the Dow Chemical Company working in pharmaceutical and agricultural chemical development for Marion Merrill Dow. Mr. DesJardin has over 40 years of experience in pharmaceutical development. Mr. DesJardin received a bachelor of science degree in chemical engineering from the University of California, Berkeley, and is a registered professional engineer in the State of California.
Andy Pasternak. Mr. Pasternak has served as our executive vice president, chief strategy officer since March 2020 and previously served as our executive vice president, chief business officer from November 2019 until March 2020. Prior to joining Horizon, Mr. Pasternak served as a partner of Bain & Company, Inc., a global management consulting firm, from 2008 until October 2019, where he most recently led Bain & Company’s healthcare practice in the Americas and was a member of the mergers and acquisition practice. Mr. Pasternak earned a master of business administration degree from the University of Chicago and a bachelor of arts degree in economics from Northwestern University.
Jeffrey W. Sherman, M.D., FACP. Dr. Sherman has served as our executive vice president, chief medical officer since January 2018. From September 2014 to January 2018, Dr. Sherman served as our executive vice president, research and development and chief medical officer. He joined Horizon in 2009 as our executive vice president, development, manufacturing, regulatory affairs and chief medical officer. Prior to joining Horizon, Dr. Sherman served as president and board member of the Drug Information Association (DIA), a nonprofit professional association of members who work in government regulatory, academia, patient advocacy and the pharmaceutical and medical device industry. Before that he held other management roles at IDM Pharma, Inc., Takeda Global Research & Development, NeoPharm, Inc. and G.D. Searle, LLC/Pharmacia. Dr. Sherman serves on the board of directors of Xeris BioPharma Holdings, Inc., a public biopharmaceutical company, or Xeris BioPharma, and previously served on the board of directors of Strongbridge Biopharma plc from October 2016 until October 2021 and Xeris Pharmaceuticals, Inc., or Xeris, from April 2018 until October 2021. Xeris acquired Strongbridge in October 2021 and in connection therewith both Xeris and Strongbridge became wholly owned subsidiaries of Xeris BioPharma. He also serves on the board of directors of Sorriso Pharmaceuticals, Inc., a private biotechnology company, and the Biotechnology Innovation Organization (BIO) Health Section Governing Board, and the board of advisors of the Center for Information and Study on Clinical Research Participation (CISCRP). He is an adjunct assistant professor of Medicine at the Northwestern University Feinberg School of Medicine and a diplomat of the National Board of Medical Examiners and the American Board of Internal Medicine. Dr. Sherman received his medical degree from the Rosalind Franklin University/ Chicago Medical School.
Elizabeth H.Z. Thompson, Ph.D. Dr. Thompson has served as our executive vice president, research and development since March 2021. Previously she served as our group vice president, development and external search from January 2020 to March 2021, and as our vice president, clinical development (rare disease) from June 2018 to January 2020. Prior to joining Horizon, she was with AbbVie as group scientific director, clinical development from April 2017 to May 2018 and senior scientific director, clinical development, from September 2015 to April 2017. While at AbbVie, she was the clinical lead for risankizumab (SKYRIZI), supporting global submissions and approvals. Before AbbVie, Dr. Thompson held roles at Raptor, InterMune and Amgen in a career spanning clinical development, business development and medical communications. Dr. Thompson received a bachelor of science degree in chemistry from Harvey Mudd College and a doctorate in macromolecular and cellular structure and chemistry from The Scripps Research Institute.
Board Composition
Our board of directors currently consists of nine members. We have divided our board of directors into three classes, as follows:
130
At each annual general meeting of shareholders, the successors to directors whose terms then expire will serve until the third annual general meeting following their election and until their successors are duly elected and qualified. The authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed between the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in our control or management. Our directors may be removed by ordinary resolution with majority vote of our shareholders at a general meeting provided that notice of such resolution has been given in accordance with Section 146 of the Irish Companies Act 2014. Vacancies on our board of directors may be filled only by persons elected by a majority of the directors then in office, provided that a quorum is present. A director elected by our board of directors to fill a vacancy in a class, including vacancies created by an increase in the number of directors, shall serve for the remainder of the full term of that class and until the director’s successor is duly elected and qualified.
Certain Corporate Governance Matters
Audit Committee
We have a standing Audit Committee that consists of Mr. Daniel, Mr. Grey, Mr. Watkins and Ms. Witz, with Mr. Daniel serving as the chair of the Audit Committee. Our board of directors has determined that each of Mr. Daniel, Mr. Grey, Mr. Watkins and Ms. Witz meets the independence requirements of Rule 10A-3 of the Exchange Act and the Nasdaq listing standards with respect to Audit Committee members. Our board of directors has also determined that each of Mr. Daniel, Mr. Grey, Mr. Watkins and Ms. Witz qualify as an “audit committee financial expert” within the meaning of applicable SEC rules. In making this determination, our board of directors has considered their formal education, the nature and scope of their previous experience and their financial and corporate governance expertise. Each of our independent registered public accounting firm, internal audit and management periodically meet privately with our Audit Committee.
Code of Conduct and Ethics
We have adopted a Code of Conduct and Ethics, or Code of Conduct, that applies to all officers, directors and employees, including our principal executive officer, principal financial officer, principal accounting officer and persons performing similar functions. We introduced a revised, principles-based Code of Conduct in 2021 that provides an instructive set of principles, guidelines and tools to operate our business in an ethical and compliant way. The Code of Conduct is available in the investor relations section of our website at www.horizontherapeutics.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this Annual Report on Form 10-K. If we make any substantive amendments to the Code of Conduct or grant any waiver from a provision of the Code of Conduct to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website or in a current report on Form 8-K.
Director Nominations
No material changes have been made to the procedures by which shareholders may recommend nominees to our board of directors.
131
Item 11. Executive Compensation
Compensation Discussion and Analysis
Overview
This Compensation Discussion and Analysis discusses the compensation philosophy, policies and principles underlying our executive compensation decisions for the 2022 fiscal year. It provides qualitative information on the factors relevant to these decisions and the manner in which compensation is awarded to our executive officers who have been named in the Summary Compensation Table included in this Item 11 and whom we refer to as our named executive officers, or NEOs.
Our board of directors has delegated responsibility for creating, reviewing and making recommendations regarding the compensation of our executive officers to the compensation committee of our board of directors, or Compensation Committee, which is composed of independent directors under SEC regulations and the Nasdaq Listing Rules. The role of the Compensation Committee is to oversee our compensation and benefit plans and policies, to administer our equity incentive plans and to annually review and make recommendations to our board of directors who approve all compensation decisions relating to our executive officers.
Consideration of Stockholder Advisory Votes
Our say-on-pay vote held at our 2022 annual meeting of stockholders was supported by 91 percent of the votes affirmatively cast, excluding abstentions and broker non-votes. While this vote was only advisory, our Compensation Committee interpreted it to be a very positive affirmation from our stockholders that they strongly endorse our historical compensation philosophy, policies and decisions. Accordingly, the Compensation Committee determined to not make any significant changes in how it went about reviewing and setting compensation levels for our executives as a result of the 2022 say-on-pay vote. When determining how often to hold an advisory vote on executive compensation, the board recommended and our stockholders agreed upon, an annual vote. In addition to holding an annual advisory vote on executive compensation, we conduct engagement with our stockholders on executive compensation and corporate governance issues.
132
2022 Performance Highlights
We delivered strong corporate performance during 2022. Highlights include:
133
Compensation Program and Governance
In 2022, our executive compensation program continued to emphasize three major pay considerations: long-term performance against our business strategies and goals, alignment of the interests of our shareholders, employees and patients, and risk mitigation. Our Compensation Committee is responsible for oversight of our executive compensation program. Highlights of 2022 policies and practices include:
Compensation Objectives
We believe in providing a competitive total compensation package to our executive management team through a combination of base salary, short-term performance-based cash incentives, long-term performance-based equity incentives, and severance and change-in-control benefits. Our executive compensation programs are designed to achieve the following objectives:
Our Compensation Committee believes that our executive compensation program should include short- and long-term performance incentive components, including cash and equity-based compensation, and should reward consistent performance that meets or exceeds expectations. Our Compensation Committee evaluates both performance and compensation to make sure that the total compensation provided to our executive officers remains competitive relative to compensation paid by companies of similar size and stage of development that operate in the biotech industry and appropriately reflects our relative performance and our own strategic objectives.
134
Setting Executive Compensation
The Compensation Committee seeks to ensure that our executive compensation program is properly rewarding and motivating our executive officers while aligning their goals with our business strategy and the interests of our shareholders. To do this, our Compensation Committee conducts an annual review of the aggregate level of our executive compensation, the mix of elements used to compensate our executive officers and historic compensation levels, including prior equity awards.
When setting executive compensation opportunities, the Compensation Committee considers several factors, including:
Role of Chief Executive Officer in Compensation Decisions
Our chief executive officer, or CEO, typically evaluates the performance of other executive officers and other employees, along with the performance of our company as a whole, against pre-established goals, on an annual basis and makes recommendations to the Compensation Committee with respect to annual base salary adjustments, short-term performance-based cash incentives and annual equity grants for the other executives. The Compensation Committee exercises its own independent discretion in approving compensation for all executive officers and assessing corporate performance against the pre-established goals. The CEO is not present during deliberations or voting with respect to his own compensation.
Risk Analysis
The Compensation Committee has reviewed our compensation policies applicable to our executive officers and other employees and believes that our policies do not encourage excessive and unnecessary risk-taking, and that the level of risk that they do encourage is not reasonably likely to have a material adverse effect on us. The design of our compensation policies and programs encourages our executive officers and other employees to remain focused on both our short- and long-term goals. For example, while our short-term incentive plan (our annual bonus plan) measures performance on an annual basis, our long-term incentive equity grants, which consist of time-based equity awards (RSUs) and performance-based equity awards (PSUs) vest over a number of years. Furthermore, our PSUs require that we achieve a specified level of performance over multi-year periods, which we believe encourages our executives and employees to focus on executing our long-term strategy, thus limiting the potential value of excessive risk-taking.
135
Role of Independent Consultant
Our Compensation Committee retains the services of third-party, independent executive compensation consultants from time to time, as it sees fit, in connection with the establishment of compensation programs and related policies. The Compensation Committee has engaged Aon’s Human Capital Solutions practice, a division of Aon plc, or Aon, as its independent consultant since 2016. Total fees paid to Aon in 2022 were approximately $659,000. Aon was engaged to assist and advise on multiple aspects of compensation program design and pay setting, including, but not limited to, the following services:
The Compensation Committee has assessed the independence of Aon under the applicable SEC and Nasdaq standards and concluded that the continued engagement of Aon did not raise any conflict of interest and did not adversely affect Aon’s independence.
136
Peer Group
Although our Compensation Committee has historically used survey data from Aon as a tool in determining executive compensation, it typically has not used a formula or “benchmark” to set our executives’ compensation in relation to this data. Instead, the Compensation Committee generally references the 50th percentile of comparable peer companies in combination with multiple other factors, such as the executives’ respective levels of experience, tenure and responsibility in determining the total target compensation for all executives. The peer group used for making 2022 compensation decisions and comparative performance analysis is shown below and was updated by our Compensation Committee in April 2021, with a focus on publicly traded commercial biotech and pharmaceutical companies.
Alexion was acquired in July 2021 by AstraZeneca plc and therefore is no longer a publicly traded company. However, because Alexion’s most recent fiscal year executive compensation was used in making 2022 compensation decisions and comparative performance analysis, Alexion remained in the 2022 proxy peer group.
The selection criteria used at the time of the peer group review process were:
Criteria Used to Select Peer Group in April 2021 |
||
Selection Criteria |
|
Horizon Percentile Rank |
Headcount |
between 500 and 5,500 employees |
38th percentile |
Revenue |
between $1 billion and $12 billion |
51st percentile |
Market Capitalization |
between $6.5 billion |
60th percentile |
|
and $60 billion |
(based on a 30-day average) |
137
Elements of Executive Compensation
Our executive compensation program primarily consists of base salary, annual cash incentives and long-term incentives delivered through equity and cash awards. Employees in more senior roles have an increasing proportion of their total pay package at risk and tied to performance given that their position has greater influence on our operating results.
Element |
|
Form |
|
Performance Period |
|
Objective |
Base Salary |
|
Cash (fixed) |
|
N/A |
|
• Recognition of an individual’s role, responsibilities and experience |
|
|
|
|
|
|
• Provides competitive pay for retention purposes |
Short-Term Incentive (Annual Bonus Plan) |
|
Cash (variable) |
|
Annual |
|
• Variable pay designed to reward achievement of annual financial and strategic goals |
Long-Term Incentives |
|
PSU awards (variable) |
|
Multi-year |
|
• Promotes an ownership culture |
|
|
RSU awards (variable) |
|
N/A |
|
• Aligns the interests of executives with those of shareholders |
|
|
|
|
|
|
• Provides meaningful incentives for management to execute on longer-term financial and strategic goals that drive shareholder value creation and support our retention strategy |
2022 Pay-for-Performance Overview
A significant portion of total target compensation for our CEO and other NEOs is structured in the form of variable compensation, consisting of annual performance-based incentives and PSUs.
In line with our compensation objectives, including linking executive pay with performance, short-term performance-based incentives and PSUs are dependent on Horizon’s performance, aligning our executives’ interests with those of our shareholders for near- and long-term performance. In addition, the RSU portion of the total target compensation has a time-based vesting component so that the total potential value realized from the RSU portion is dependent on our long-term share price performance.
Total target compensation for 2022 consists of annual base salary, target annual bonus and the grant date fair value of PSU and RSU grants. The grant date fair values of PSU and RSU grants were calculated in accordance with the provisions of ASC Topic 718, as reported in the Summary Compensation Table. Approximately 57 percent of our CEO’s total target compensation for 2022 was tied to the achievement of pre-established performance goals, with time-based equity awards making up approximately 37 percent of the total. Fixed compensation (annual base salary) made up the remaining 7 percent of our CEO’s total target compensation.
Base Salary
Base salaries for our executive officers are established based on the individual’s scope of responsibilities, experience and market factors. Base salaries are generally reviewed annually, typically in connection with our annual executive compensation review process. The Compensation Committee references survey and peer group data to understand the marketplace for individuals in similar positions at the peer group companies. Based on the survey and peer group data, the Compensation Committee determined that a 4 percent increase to the base salaries of our NEOs, with the exceptions of Mr. Walbert, Mr. Hoelscher (who did not receive an increase to his base salary in light of his announced retirement) and Mr. Clayton (who joined us in February 2022), was appropriate and was consistent with industry trends. In considering the 10 percent increase to Mr. Walbert’s base salary, the Compensation Committee focused on the following items: his tenure and experience as our CEO, his impact and contributions during 2021 and recent market data on CEO compensation at our highest performing peer companies.
138
The annual base salaries of our NEOs as of March 1, 2022, and the increase from their prior base salary levels, were as follows:
Executive |
|
2022 Base Salary |
|
|
% Increase |
|
||
Timothy P. Walbert |
|
$ |
1,312,615 |
|
|
|
10 |
% |
Aaron L. Cox |
|
|
644,800 |
|
|
|
4 |
% |
Paul W. Hoelscher (1) |
|
|
660,000 |
|
|
|
0 |
% |
Sean M. Clayton (2) |
|
|
650,000 |
|
|
|
— |
|
Elizabeth H.Z. Thompson, Ph.D. |
|
|
598,000 |
|
|
|
4 |
% |
Andy Pasternak |
|
|
724,148 |
|
|
|
4 |
% |
Short-Term Incentive Program: Annual Bonus
We provide performance-based cash annual bonuses as an incentive for our executives to achieve pre-established financial and strategic goals. These bonuses may range in payout from 0 percent for performance below the threshold level of achievement to 200 percent of targeted payout levels for performance at or above the maximum level of achievement.
The 2022 target bonus opportunities (expressed as a percentage of base salary paid during the full fiscal year) for our NEOs, which increased by 15 percentage points year-over-year for our CEO and by 10 percentage points year-over-year for our other NEOs (other than Mr. Clayton, who joined us in February 2022), in each case to align their respective target bonus opportunities more closely with market data, were as follows:
Executive |
|
Threshold |
|
|
Target |
|
|
Maximum |
|
|||
Timothy P. Walbert |
|
|
98 |
% |
|
|
130 |
% |
|
|
260 |
% |
Aaron L. Cox |
|
|
53 |
% |
|
|
70 |
% |
|
|
140 |
% |
Paul W. Hoelscher(1) |
|
|
53 |
% |
|
|
70 |
% |
|
|
140 |
% |
Sean M. Clayton(2) |
|
|
53 |
% |
|
|
70 |
% |
|
|
140 |
% |
Elizabeth H.Z. Thompson, Ph.D. |
|
|
53 |
% |
|
|
70 |
% |
|
|
140 |
% |
Andy Pasternak |
|
|
53 |
% |
|
|
70 |
% |
|
|
140 |
% |
In early 2022, the Compensation Committee determined that our annual bonus plan would provide our executives the opportunity to earn performance-based cash awards based on the achievement of a combination of financial goals (60 percent weighting) and strategic goals (40 percent weighting).
Financial Goals
The 60 percent weighting of the financial goals was allocated between a total net sales goal, weighted at 30 percent, and an internal adjusted earnings before interest, tax, depreciation and amortization, or internal adjusted EBITDA, goal, weighted at 30 percent. We increased the weighting of our internal adjusted EBITDA goal by 5 percent year-over-year to reflect our heightened focus on margin expansion for 2022.
Total Net Sales. The performance targets for the total net sales goal are set forth below.
|
|
Performance Levels |
||||||||||||||||
Total Net Sales ($ millions) |
|
Percentage of Target Bonus |
|
|
Threshold 75% |
|
Target 100% |
|
|
125 |
% |
|
|
150 |
% |
|
Maximum 200% |
|
|
|
|
30 |
% |
|
$3,860 |
|
$3,965 |
|
$4,060 |
|
|
$4,160 |
|
|
$4,260 |
||
139
Internal Adjusted EBITDA: The performance targets for the internal adjusted EBITDA goal are set forth below.
|
|
Performance Levels |
||||||||||||||||
Internal Adjusted EBITDA ($ millions) (1) |
|
Percentage of Target Bonus |
|
|
Threshold 75% |
|
Target 100% |
|
|
125 |
% |
|
|
150 |
% |
|
Maximum 200% |
|
|
|
|
30 |
% |
|
$1,550 |
|
$1,670 |
|
$1,774 |
|
|
$1,850 |
|
|
$1,950 |
||
Strategic Goals
The Compensation Committee established three strategic goals (with a total weighting of 40 percent) for 2022:
Research and Development, or R&D, and Technical Operations Milestones (15%)
Human Capital Management, Compliance and ESG (10%)
Business Development Initiatives (15%)
The Compensation Committee chose these three strategic goals because it believed these were the best indicators of the achievement of our operating plan, and they represented the factors most critical to increasing total shareholder value in 2022.
140
Determination of 2022 Cash Annual Bonus Amounts
In connection with its review of various compensation matters related to the pending Transaction in December 2022, the Compensation Committee discussed the annual bonus program and considered whether it remained appropriate for payouts to be based solely on achievement of the financial and strategic goals that were established earlier in the year. Specifically, the Compensation Committee considered existing progress towards achieving the corporate financial and strategic goals described above, as well as the significant shareholder value represented by the pending Transaction, the fact that management had devoted substantial attention to the process leading to the pending Transaction, the Compensation Committee’s desire for management to focus on the successful closing of the Transaction, and other intervening events that impacted the ability to achieve certain of the previously established corporate goals, including the at-risk launch of a generic version of PENNSAID 2% and our decision to wind down our former inflammation business.
Following this discussion, the Compensation Committee determined that basing annual bonus plan payouts on achievement of the financial and strategic goals established earlier in the year no longer served as the best performance indicator and on-going incentive for our executives and other employees. As a result, and in light of the significant shareholder value represented by the pending Transaction, the Compensation Committee determined that a payout at the 100 percent level was appropriate to effectively compensate executives for 2022 performance.
Based on that determination, the Compensation Committee approved cash bonus awards for our NEOs as follows:
Executive |
|
2022 Target Bonus Opportunity |
|
|
Total % of Target Bonus Earned |
|
|
2022 Earned Annual Bonus |
|
|||
Timothy P. Walbert |
|
$ |
1,681,324 |
|
|
|
100 |
% |
|
$ |
1,681,324 |
|
Aaron L. Cox |
|
|
448,554 |
|
|
|
100 |
% |
|
|
448,554 |
|
Paul W. Hoelscher |
|
172,142 |
|
|
|
100 |
% |
|
172,142 |
|
||
Sean M. Clayton(1) |
|
|
382,699 |
|
|
|
100 |
% |
|
|
382,699 |
|
Elizabeth H.Z. Thompson, Ph.D. |
|
|
415,998 |
|
|
|
100 |
% |
|
|
415,998 |
|
Andy Pasternak |
|
|
503,752 |
|
|
|
100 |
% |
|
|
503,752 |
|
In December 2022, in order to eliminate or mitigate the potential tax impact of Section 280G of the Internal Revenue Code of 1986, as amended, or the Code, or Section 280G, and Section 4999 of the Code, or Section 4999, on us and our executive officers with respect to past or future payments to certain executive officers in connection with the pending Transaction, our Compensation Committee determined that the approved cash bonus awards to Mr. Cox, Mr. Clayton, Dr. Thompson and Mr. Pasternak would be paid on December 30, 2022. The approved cash bonus awards to Mr. Walbert and Mr. Hoelscher will be paid on March 15, 2023.
141
Long-Term Incentive Program
2022 Long-term Incentive Grants. We have adopted a regular, annual long-term incentive grant schedule of awarding equity awards in the form of RSUs and PSUs to our executive officers. In addition, our performance-based equity compensation is aligned with all of our stated compensation objectives, including the linking of executive pay with performance, and aligning the interests of our executive officers and shareholders with a large portion of the equity grants vesting contingent on performance and requiring continued service. Further, we believe that annual grant cycles allow us to more easily manage shareholder dilution and burn rate, while still providing market-competitive incentive opportunities.
As part of our annual long-term incentive plan, on January 4, 2022, we awarded an equal mix of PSUs and time-vested RSUs to our NEOs (other than Mr. Hoelscher, who only received a pro-rated number of time-vested RSUs in light of his announced retirement, and Mr. Clayton, who joined us after the 2022 long-term incentive grants were made). The components of the long-term incentive plan were as follows:
|
|
2022 Long-Term Incentive Components |
||
|
|
PSUs |
|
RSUs |
Performance Criteria/Period |
|
•3-year Relative TSR |
|
N/A |
|
|
(50%) (2022-2024) |
|
|
|
|
•R&D and Technical Operations Goals |
|
|
|
|
over a 3-year period (30%) (2022-2024) |
|
|
|
|
•Financial Goals |
|
|
|
|
over a 2-year period (20%) (2022-2023) |
|
|
Maximum Award |
|
Relative TSR PSUs: |
|
N/A |
|
|
•200% of target award(1) |
|
|
|
|
R&D and Technical Operations PSUs: |
|
|
|
|
•200% of target award |
|
|
|
|
Financial PSUs: |
|
|
|
|
•200% of target award |
|
|
Service Vesting Period |
|
Relative TSR PSUs: |
|
Vest one-third annually over 3 years |
|
|
•At the end of the 2022-2024 performance period |
|
|
|
|
R&D and Technical Operations PSUs: |
|
|
|
|
•At the end of the 2022-2024 performance period |
|
|
|
|
Financial PSUs: |
|
|
|
|
•2/3 vest on January 5, 2024 |
|
|
|
|
•1/3 vest on January 5, 2025 |
|
|
Post-Issuance Holding Period |
|
1 year |
|
1 year |
142
Our NEOs received RSU and PSU grants in January 2022 for the following share amounts:
Executive |
|
Time-Vested RSUs |
|
|
Performance-Based PSUs |
|
||
Timothy P. Walbert |
|
|
73,796 |
|
|
|
73,797 |
|
Aaron L. Cox |
|
|
23,805 |
|
|
|
23,805 |
|
Paul W. Hoelscher(1) |
|
|
9,522 |
|
|
— |
|
|
Sean M. Clayton(2) |
|
— |
|
|
|
— |
|
|
Elizabeth H.Z. Thompson, Ph.D. |
|
|
21,424 |
|
|
|
21,425 |
|
Andy Pasternak |
|
|
23,805 |
|
|
|
23,805 |
|
Time-Vested RSUs
The time-vested RSUs are generally subject to three-year annual vesting over the service period beginning on January 5, 2022 and ending on January 5, 2025.
Pursuant to the agreement we entered into with Mr. Hoelscher in connection with his retirement in May 2022, which is described in the section entitled “Retirement and Transition Agreement With Paul W. Hoelscher” below, Mr. Hoelscher’s time-vested RSUs will continue to vest on their original vesting schedules subject to his continued service as a part-time advisor to us.
Performance-Based PSUs
The performance-based PSUs use three long-term performance metrics: the first component is tied to relative TSR over a three-year period, or Relative TSR PSUs; the second component is tied to the number of pre-established R&D and technical operations goals achieved over a three-year period, or R&D and Technical Operations PSUs; and the third component is tied to the achievement of two financial goals over a two-year period, or Financial PSUs. The Compensation Committee’s decision to include all longer-term (two- or three-year) metrics in the PSU award design was made in line with shareholder feedback.
The performance-based PSUs are determined by the following criteria:
Long-Term Incentive Performance
2022 PSUs. Determination of the attainment level of the Relative TSR PSUs and the attainment level of the R&D and Technical Operations PSUs will be made following the three-year performance period ending December 31, 2024. Determination of the attainment level of the Financial PSUs will be made following the two-year performance period ending December 31, 2023.
143
2021 R&D and Business Development PSUs. In January 2021, we granted performance-based PSUs tied to the number of pre-established R&D and business development goals achieved over the two-year period ending December 31, 2022, or R&D/BD PSUs. The number of R&D/BD PSUs that an executive was eligible to earn was based on the number of approved programs (i.e., active clinical-stage development programs (including acquired or in-licensed programs) targeting new indications, new product approvals, or geographical expansions with current or acquired assets) initiated, completed or acquired during the two-year performance period.
|
|
R&D/BD PSU Performance Levels |
||||||||
Multiplier |
|
0% |
|
70% |
|
100% |
|
150% |
|
200% |
Number of Approved Programs |
|
<3 |
|
3 or 4 |
|
5 or 6 |
|
7 or 8 |
|
>8 |
In December 2022, the Compensation Committee determined that we exceeded the maximum goal of more than eight approved programs during the two-year performance period. Among the key target programs that the Compensation Committee determined were achieved during the two-year performance period were:
The eligible executives therefore each vested in the number of R&D/BD PSUs set forth in the table below, which represents 200 percent (the maximum payout percentage) of their target number of R&D/BD PSUs:
Executive |
|
Target R&D/BD PSUs |
|
|
Determined R&D/BD PSUs |
|
||
Timothy P. Walbert(1) |
|
|
26,196 |
|
|
|
52,392 |
|
Aaron L. Cox(2) |
|
|
6,986 |
|
|
|
13,972 |
|
Paul W. Hoelscher(1) |
|
|
8,732 |
|
|
|
17,464 |
|
Sean M. Clayton(3) |
|
|
— |
|
|
|
— |
|
Elizabeth H.Z. Thompson, Ph.D.(3) |
|
|
— |
|
|
|
— |
|
Andy Pasternak(2) |
|
|
8,732 |
|
|
|
17,464 |
|
144
2020 Relative TSR PSUs. In January 2020, we granted performance-based PSUs tied to our TSR performance relative to the TSR of the components of the NBI over the three-year performance period ending December 31, 2022. The number of 2020 Relative TSR PSUs that an executive was eligible to earn was determined as follows, with linear interpolation between performance levels:
|
|
2020 3-Year TSR PSU Payout as a Percent of the Target (50th Percentile) |
|
|||||||||||||||||||||
Percentile Rank |
|
<25th |
|
|
25th |
|
|
50th |
|
|
60th |
|
|
75th |
|
|
≥90th |
|
||||||
Percentage Payout |
|
|
0 |
% |
|
|
50 |
% |
|
|
100 |
% |
|
|
125 |
% |
|
|
150 |
% |
|
|
200 |
% |
In December 2022, the Compensation Committee determined that, at the end of the three-year performance period, Horizon’s absolute TSR was 217 percent, which was determined to be at the 97th percentile of the NBI components for the performance period. The eligible executives therefore each vested in the number of 2020 Relative TSR PSUs set forth in the table below, which represents 200 percent (the maximum payout percentage) of their target number of 2020 Relative TSR PSUs:
Executive |
|
Target Relative TSR PSUs |
|
|
Vested Relative TSR PSUs |
|
||
Timothy P. Walbert |
|
|
52,249 |
|
|
|
104,498 |
|
Aaron L. Cox |
|
|
8,708 |
|
|
|
17,416 |
|
Paul W. Hoelscher |
|
|
13,062 |
|
|
|
26,124 |
|
Sean M. Clayton(1) |
|
|
— |
|
|
|
— |
|
Elizabeth H.Z. Thompson, Ph.D.(1) |
|
|
— |
|
|
|
— |
|
Andy Pasternak |
|
|
13,062 |
|
|
|
26,124 |
|
Additional Compensation Policies and Practices
Executive Share Ownership Guidelines. We have share ownership guidelines that establish the following minimum ownership levels within five years of the adoption of the guidelines (or within five years of the date an executive officer first becomes subject to them): five times base salary for the chief executive officer and two times base salary for executive officers. Individual ownership interest versus the guidelines is reviewed annually using an average share price for a calendar quarter prior to the review date. Shares that count toward satisfaction of these guidelines include: shares owned outright by the individual (including stock units that have vested but not yet settled); shares retained after an option exercise or issuance under another type of equity award granted under our equity incentive plans; shares retained after purchase under our Employee Share Purchase Plan; shares subject to RSUs that have not vested and shares held in trust for the benefit of the individual or his/her spouse. Any unvested PSUs and unexercised stock options, whether vested or unvested, are not counted toward satisfaction of these ownership guidelines.
Holding Period Policy. RSU and PSU awards granted to executive officers on or after January 5, 2018 provide that shares issued in settlement of such equity awards are subject to a minimum holding period of one year before the shares may be sold or transferred. RSU and PSU awards granted to Dr. Sherman prior to his becoming an executive officer in February 2020 and prior to Mr. Cox becoming an officer in May 2022 are not subject to the one-year holding period requirement.
Hedging and Pledging Policies. Our Insider Trading Policy prohibits our executive officers, other employees, non-employee directors and consultants from engaging in short sales, transactions in put or call options, hedging transactions or other inherently speculative transactions with respect to our ordinary shares at any time. In addition, none of our officers, directors, other employees or consultants may margin or pledge, or make any offer to margin or pledge, any of our ordinary shares, including without limitation, borrowing against the value of such ordinary shares, at any time.
145
Clawback Policy. As a public company, if we are required to restate our financial results due to our material noncompliance with any financial reporting requirements under the federal securities laws as a result of misconduct, our chief executive officer and chief financial officer may be legally required to reimburse us for any bonus or other incentive-based or equity-based compensation they receive in accordance with the provisions of section 304 of the Sarbanes-Oxley Act of 2002. Additionally, in January 2018, the Compensation Committee approved our Incentive Compensation Recoupment Policy, which provides for recoupment of certain compensation paid to our executive officers under certain circumstances involving material financial restatements. Any cash and equity incentive compensation that is paid, awarded or vested based on the achievement of reported financial results and that is approved, granted or awarded on or after January 5, 2018 is subject to potential recoupment in accordance with the terms of the Incentive Compensation Recoupment Policy, including but not limited to any compensation approved, granted or awarded under our annual cash bonus plan and PSUs under our long-term incentive plan.
Timing of Equity Awards. Grants of equity awards to our executive officers are generally determined and approved at our pre-scheduled quarterly Compensation Committee meetings whenever practicable. However, the Compensation Committee may otherwise approve the grant of equity awards in advance of its next scheduled meeting in connection with a new hire, promotion, and other circumstances where the Compensation Committee deems it appropriate to make such grants. Starting in 2018, our equity program has included performance vesting metrics for a performance period that begins in January. Given that, since 2018 our process has been to approve the final grants for our equity program at a special meeting of the Compensation Committee in January. We expect to continue having a significant portion of our executive officer equity compensation to include performance vesting metrics and believe it is likely that future-year grants will be approved at special Compensation Committee meetings in January.
To the extent we grant stock options, the exercise price would not be less than the closing price of our ordinary shares on Nasdaq on the grant date. It is our policy not to purposely accelerate or delay the public release of material information in consideration of a pending equity grant to allow the grantee to benefit from a more favorable exercise price. We recognize that a release of information by us in close proximity to an equity grant may appear to be an effort to time the announcement to a grantee’s benefit (even if no such benefit was intended). Accordingly, it is our policy that our management team makes a good faith effort to advise the Compensation Committee whenever it is aware that material non-public information is planned to be released to the public in close proximity to the grant of equity awards.
Accounting and Tax Considerations. We account for share-based awards exchanged for employee services in accordance with the provisions of Financial Accounting Standards Board Accounting Standards Codification Topic 718 Compensation — Stock Compensation (ASC Topic 718). Assumptions used in the calculation of these awards are included in Note 19, Share-Based and Long-Term Incentive Plans, of the Notes to Consolidated Financial Statements, included in Item 15 of this Annual Report on Form 10-K. These amounts do not necessarily correspond to the actual value recognized or that may be recognized by the NEOs.
Under Section 162(m) of the Code, compensation paid to any publicly held corporation’s “covered employees” that exceeds $1 million per taxable year for any covered employee is generally non-deductible unless it qualifies as “performance-based compensation” under Section 162(m) and is paid pursuant to a written binding contract which was in effect on November 2, 2017 and which is not modified in any material respect on or after such date.
Compensation paid to each of our “covered employees” in excess of $1 million per taxable year generally will not be deductible unless it qualifies for the performance-based compensation exception under Section 162(m) pursuant to the transition relief described above. Because of certain ambiguities and uncertainties as to the application and interpretation of Section 162(m), as well as other factors beyond the control of the Compensation Committee, no assurance can be given that any compensation paid by us will be eligible for such transition relief and be deductible by us in the future. Although the Compensation Committee will continue to consider tax implications as one factor in determining executive compensation, the Compensation Committee also looks at other factors in making its decisions and retains the flexibility to provide compensation for our NEOs in a manner consistent with the goals of our executive compensation program and the best interests of our company and our shareholders, which may include providing for compensation that is not deductible due to the deduction limit under Section 162(m). The Compensation Committee also retains the flexibility to modify compensation that was initially intended to be exempt from the deduction limit under Section 162(m) if it determines that such modifications are consistent with our business needs.
146
Severance and Change in Control Benefits. Our NEOs are provided with certain severance benefits in order to assist us in recruiting and retaining talented individuals and align the executives’ interests with the best interests of the shareholders. We believe these severance benefits are consistent with those provided by our peer group are an essential element of our overall executive compensation package due to the competitive market for executive talent in our industry. The Compensation Committee believes that the severance benefits are an important element of the NEOs’ retention and motivation and that the benefits of such severance rights agreements, including generally requiring a release of claims against us and entering into a non-competition agreement as a condition to receiving any severance benefits are in our best interests. Enhanced severance benefits are provided for a qualifying termination that occurs in connection with a change in control because the severance benefits are also intended to eliminate, or at least reduce, the reluctance of our executive officers to diligently consider and pursue potential change-in-control transactions that may be in the best interests of our shareholders.
In addition, in March 2023, we entered into an Excise Tax Gross-Up Agreement with Dr. Thompson. Entering into such agreement was consistent with our prior disclosure that in connection with the Transaction, we intended to enter into tax reimbursement agreements with certain employees, pursuant to which we would agree to make tax reimbursement payments to such employees to the extent such employees are subject, in connection with the Transaction, to an excise tax imposed by Section 4999 of the Code in an amount that generally would place them in the same after-tax position that they would have been in if no excise tax had applied and no tax reimbursement payment had been made. Under the terms of the transaction agreement with Amgen, the maximum potential tax reimbursement payments to all affected employees shall not exceed $30 million in the aggregate.
A description of the severance benefits provided under our executive officer employment agreements is provided below under the heading “Potential Payments Upon Termination or Change in Control.”
Retirement and Transition Agreement With Paul W. Hoelscher. Paul W. Hoelscher, our former executive vice president, chief financial officer, retired effective May 16, 2022. In connection with Mr. Hoelscher’s retirement, we entered into an executive employment and transition agreement with Mr. Hoelscher, or the Hoelscher Transition Agreement, which provides that, in exchange for (1) a release of claims in favor of us and (2) Mr. Hoelscher’s compliance with his contractual and legal obligations to us, including certain non-competition, non-solicitation and confidentiality provisions, we would provide the following to Mr. Hoelscher: (i) continued eligibility to receive a pro-rated 2022 bonus award (which was paid in the amount of $172,142 as further described above under “Short-Term Incentive Program: Annual Bonus—Determination of 2022 Cash Annual Bonus Amounts”; (ii) continued health benefits through December 31, 2022 (the actual amount of which was $19,322); and (iii) continued use of personal financial advisory services paid by us through December 31, 2023 (the actual amount of which for the period following Mr. Hoelscher’s retirement was $17,953, which consisted of $10,000 in financial advisory services and $7,953 in the related tax gross up).
In addition, pursuant to the terms of the Hoelscher Transition Agreement, Mr. Hoelscher agreed to serve as a part-time advisor to us from May 17, 2022 until such advisor relationship is terminated by either party, or such period, the Part-Time Advisor Period. During the Part-Time Advisor Period, we agreed to (1) pay Mr. Hoelscher a fee of (x) $27,500 per month through May 16, 2023 and (y) $5,000 per year after May 16, 2023, (2) reimburse Mr. Hoelscher for reasonable and documented out-of-pocket costs and expenses actually incurred in connection with providing the services we request, and (3) consider Mr. Hoelscher’s change of status from an employee to an advisor, and Mr. Hoelscher’s service to us during the Part-Time Advisor Period, as service as a “Consultant” and to constitute “Continuous Service” for purposes of our equity incentive plans, so, therefore, each of Mr. Hoelscher’s equity awards that were granted under any of our equity incentive plans and were outstanding as of May 16, 2022 will continue to vest in accordance with their terms during the Part-Time Advisor Period. Moreover, if we terminate the Part-Time Advisor Period after May 16, 2023 or if the Part-Time Advisor Period is terminated due to Mr. Hoelscher’s death, then, with respect to each of Mr. Hoelscher’s equity awards that are outstanding as of the date of such termination: (i) such equity award will continue to vest in accordance with its terms on and following the date of such termination as if his employment had continued through the applicable performance and vesting periods of such awards; (ii) any such equity award that is a stock option (a) may be exercised (to the extent vested on the date of exercise) until the expiration of its original full term and (b) will remain outstanding until the earlier of the date it is fully exercised or the expiration of its original full term; (iii) any such equity award that is an RSU award will remain outstanding until it is fully settled; and (iv) any such equity award that is a PSU award will remain outstanding until it is fully settled based upon the achievement of the applicable performance-based vesting conditions.
147
Deferred Compensation Plan. All of our executive officers are eligible to participate in our non-qualified Deferred Compensation Plan, which allows the participants to defer receipt of their compensation and recognition of associated income taxes without being subject to the deferral contribution limits of our 401(k) Plan, which provides additional tax and financial planning flexibility. Our policy is to match Deferred Compensation Plan deferrals under the same matching contribution formula that we apply to our 401(k) Plan. Accordingly, the matching contribution formula for our Deferred Compensation Plan is 100 percent of the first 6 percent of salary deferrals, which is the same “safe harbor” matching contribution formula that applies to our 401(k) Plan. Matching contributions to our Deferred Compensation Plan are immediately fully vested contingent upon completion of one year of employment in order to closely align to the vesting schedule of our safe harbor matching contributions to our 401(k) Plan, which are immediately fully vested when made. A description of our Deferred Compensation Plan is provided below under the heading “Nonqualified Deferred Compensation.”
Retention Bonuses. In December 2022, we granted retention bonus awards to certain key employees determined to be critical to the completion of the Transaction and the post-completion efforts, including Dr. Thompson and Mr. Clayton. Dr. Thompson’s retention bonus award, which has a value of $2,500,000, and Mr. Clayton’s retention bonus award, which has a value of $5,000,000, were each paid in a lump sum cash payment in December 2022 and are subject to repayment to us in the event of the executive’s termination for “cause” or resignation without “good reason” (each as defined in the executive’s employment agreement), as follows: (i) 100 percent of the retention bonus award is subject to repayment if such termination or resignation occurs prior to the consummation of the Transaction (referred to herein as the Completion Date); and (ii) 50 percent of the retention bonus award is subject to repayment if such termination or resignation occurs after the Completion Date and prior to the six-month anniversary of the Completion Date (such repayment requirements collectively referred to herein as the Clawback Right). In the event of the executive’s (i) termination without cause, (ii) termination due to death or disability, or (iii) resignation for good reason, in each case, at any time while a portion of the Clawback Right is outstanding, the remaining Clawback Right will lapse in connection with such termination or resignation.
Sean Clayton New-Hire Compensation. Mr. Clayton commenced serving as our executive vice president, general counsel, in February 2022. In connection with his hire, Mr. Clayton received a one-time cash sign-on bonus of $1,000,000, with the first half paid in March 2022 and the second half paid in December 2022. In the event Mr. Clayton was terminated for “cause” or he resigned without “good reason” (each as defined in Mr. Clayton’s employment agreement) during 2022, he would have been required to repay 100 percent of his total sign-on bonus. In the event Mr. Clayton is terminated for cause or he resigns without good reason during 2023, he will be required to repay 50 percent of his total sign-on bonus. In connection with his hire, we also granted a new hire RSU award to Mr. Clayton with a grant date fair value of $3,839,414 on March 1, 2022. The new hire RSU award vests in three equal annual installments, subject to Mr. Clayton’s continued service through each vesting date. For further information regarding the terms governing Mr. Clayton’s new hire RSU award, see “Potential Payments Upon Termination or Change in Control” below.
Other Benefits. All of our executive officers are eligible to receive our standard employee benefits, such as participation in our 401(k) Plan, medical, dental, vision coverage, short-term disability insurance, long-term disability insurance, group life insurance, retiree medical benefits, paid time off, holiday, and the Employee Share Purchase Plan, in each case on the same basis as our other employees. Our Paid-Time-Off Policy for employees generally allows no more than 40 paid-time-off hours to be carried over to the following year, except in cases where a carry-over limit is not legally permitted.
148
We reimburse our executive officers for any travel expenses and related tax gross ups they incur in connection with any business-related travel which does not meet the strict eligibility requirements to be treated as a non-taxable business expense reimbursement in accordance with applicable tax guidelines. We believe that the cost of providing these benefits is reasonable in light of the benefit to our business of having our executive officers more focused on attaining our business objectives in connection with any business-related travel. We also reimburse our executive officers for certain personal financial planning services incurred annually and related tax gross ups. We believe that financial planning by experts reduces the time our executives spend on that topic and assists our executives in making the most of the financial rewards provided by us. We also cover costs related to comprehensive annual executive physical health examinations for our executives. We believe the cost of providing these health examinations is reasonable in light of the benefit to our business of facilitating the health of our executives. Additionally, we have sponsored various teams, events and venues and have received tickets to various sporting events, concerts and other events in connection with such sponsorships. We have provided our executive officers with such tickets for personal entertainment, including related tax gross ups, from time to time.
Our security team regularly evaluates the level of security appropriate for our senior executives, specifically our CEO and chief financial officer, or CFO, taking into account their public profile and the critical role they play in our organization. As a result of these assessments, and based on our security team’s recommendation, our board of directors requires that our CEO use the aircraft we fractionally own through a third-party operator for all business and personal travel. Personal use of such aircraft by other executives is permitted only in limited circumstances and requires CEO approval. Additionally, based upon the recommendation of our security team and approval by our board of directors, beginning in March 2022, our CEO is also provided with a car and driver to ensure his individual safety and security. Based on ongoing analyses of security, use of a car and driver may also be provided to a select number of other executives from time to time. Lastly, beginning in 2022, reimbursement for the installation and maintenance of residential security systems is provided to our CEO, CFO and potentially other senior executives when deemed appropriate by our security team. We consider these security measures to be reasonable and appropriate expenses for the benefit to us and not a personal benefit to our executives. However, in accordance with SEC disclosure rules, the aggregate incremental cost of these services is reported in the “Summary Compensation Table.”
149
Summary Compensation Table
Name and Principal Position |
|
Year |
|
|
Salary |
|
|
Bonus (1) |
|
|
|
Stock Awards (2)(3) |
|
|
Option Awards |
|
|
Non-Equity Incentive Plan Compensation (4) |
|
|
All Other |
|
|
Total |
|
||||||||
Timothy P. Walbert |
|
|
2022 |
|
|
$ |
1,292,727 |
|
|
$ |
1,681,324 |
|
|
|
$ |
16,601,305 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
528,513 |
|
|
$ |
20,103,869 |
|
Chairman, President and |
|
|
2021 |
|
|
|
1,186,561 |
|
|
|
— |
|
|
|
|
15,818,363 |
|
|
|
— |
|
|
|
4,174,963 |
|
|
|
173,571 |
|
|
|
21,353,458 |
|
Chief Executive Officer |
|
|
2020 |
|
|
|
1,146,542 |
|
|
|
— |
|
|
|
|
16,149,668 |
|
|
|
— |
|
|
|
4,137,046 |
|
|
|
199,548 |
|
|
|
21,632,803 |
|
Aaron L. Cox |
|
|
2022 |
|
|
|
640,667 |
|
|
|
448,554 |
|
|
|
|
5,817,498 |
|
|
|
— |
|
|
|
— |
|
|
|
267,824 |
|
|
|
7,174,543 |
|
Executive Vice President, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Paul W. Hoelscher (6) |
|
|
2022 |
|
|
|
249,792 |
|
|
|
172,142 |
|
|
|
|
928,871 |
|
|
|
— |
|
|
|
— |
|
|
|
471,776 |
|
|
|
1,822,581 |
|
Former Executive Vice President, |
|
|
2021 |
|
|
|
650,653 |
|
|
|
- |
|
|
|
|
5,272,731 |
|
|
|
— |
|
|
|
1,215,500 |
|
|
|
204,773 |
|
|
|
7,343,656 |
|
Chief Financial Officer |
|
|
2020 |
|
|
|
600,569 |
|
|
|
- |
|
|
|
|
4,037,409 |
|
|
|
— |
|
|
|
1,170,683 |
|
|
|
106,485 |
|
|
|
5,915,146 |
|
Sean M. Clayton (7) |
|
|
2022 |
|
|
|
547,685 |
|
|
|
6,382,699 |
|
(8)(9) |
|
|
3,839,414 |
|
|
|
— |
|
|
|
— |
|
|
|
102,409 |
|
|
|
10,872,207 |
|
Executive Vice President, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
General Counsel |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Elizabeth H.Z. Thompson, Ph.D. |
|
|
2022 |
|
|
|
594,167 |
|
|
|
2,915,998 |
|
(10) |
|
|
4,819,700 |
|
|
|
— |
|
|
|
— |
|
|
|
151,155 |
|
|
|
8,481,020 |
|
Executive Vice President, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Research and Development |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Andy Pasternak |
|
|
2022 |
|
|
|
719,506 |
|
|
|
503,752 |
|
|
|
|
5,355,203 |
|
|
|
— |
|
|
|
— |
|
|
|
174,333 |
|
|
|
6,752,794 |
|
Executive Vice President, |
|
|
2021 |
|
|
|
692,372 |
|
|
|
— |
|
|
|
|
5,272,731 |
|
|
|
— |
|
|
|
814,368 |
|
|
|
167,855 |
|
|
|
6,947,326 |
|
Chief Strategy Officer |
|
|
2020 |
|
|
|
669,021 |
|
|
|
250,000 |
|
(11) |
|
|
4,037,409 |
|
|
|
— |
|
|
|
802,825 |
|
|
|
66,872 |
|
|
|
5,826,126 |
|
150
Name and Principal Position |
|
Year |
|
|
RSU Awards |
|
|
PSU Awards at Maximum Level |
|
|
Total Stock Awards at Maximum Level |
|
||||
Timothy P. Walbert |
|
|
2022 |
|
|
$ |
7,198,800 |
|
|
$ |
18,805,011 |
|
|
$ |
26,003,811 |
|
Chairman, President and |
|
|
2021 |
|
|
|
6,901,140 |
|
|
|
12,367,760 |
|
|
|
19,268,900 |
|
Chief Executive Officer |
|
|
2020 |
|
|
|
5,712,612 |
|
|
|
12,573,289 |
|
|
|
18,285,901 |
|
Aaron L. Cox |
|
|
2022 |
|
|
|
2,522,616 |
|
|
|
6,589,765 |
|
|
|
9,112,381 |
|
Executive Vice President, |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Paul W. Hoelscher |
|
|
2022 |
|
|
|
928,871 |
|
|
|
— |
|
|
|
928,871 |
|
Executive Vice President, |
|
|
2021 |
|
|
|
2,300,358 |
|
|
|
4,122,552 |
|
|
|
6,422,910 |
|
Chief Financial Officer |
|
|
2020 |
|
|
|
1,428,145 |
|
|
|
3,143,293 |
|
|
|
4,571,438 |
|
Sean M. Clayton |
|
|
2022 |
|
|
|
3,839,414 |
|
|
|
— |
|
|
|
3,839,414 |
|
Executive Vice President, |
|
|
|
|
|
|
|
|
|
|
|
|
||||
General Counsel |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Elizabeth H.Z. Thompson, Ph.D. |
|
|
2022 |
|
|
|
2,089,911 |
|
|
|
5,459,578 |
|
|
|
7,549,489 |
|
Executive Vice President, |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and Development |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Andy Pasternak |
|
|
2022 |
|
|
|
2,322,178 |
|
|
|
6,066,050 |
|
|
|
8,388,228 |
|
Executive Vice President, |
|
|
2021 |
|
|
|
2,300,358 |
|
|
|
4,122,552 |
|
|
|
6,422,910 |
|
Chief Strategy Officer |
|
|
2020 |
|
|
|
1,428,145 |
|
|
|
3,143,293 |
|
|
|
4,571,438 |
|
151
Name |
|
Year |
|
|
Life Insurance Benefits Imputed Income and Additional Exec Coverage (a) |
|
|
401(k) Matching Contributions |
|
|
Deferred Compensation Plan Matching Contributions |
|
|
Financial Planning/ Legal Fee Reimbursements (including tax gross up) (b) |
|
|
Other Expenses (c) |
|
|
Post Retirement Payments (d) |
|
|
Total |
|
||||||||
Timothy P. Walbert |
|
|
2022 |
|
|
$ |
19,273 |
|
|
$ |
18,300 |
|
|
$ |
238,062 |
|
|
$ |
26,930 |
|
|
$ |
225,948 |
|
|
$ |
— |
|
|
$ |
528,513 |
|
|
|
|
2021 |
|
|
|
13,091 |
|
|
|
17,400 |
|
|
|
71,194 |
|
|
|
26,930 |
|
|
|
44,957 |
|
|
|
— |
|
|
|
173,571 |
|
|
|
|
2020 |
|
|
|
16,533 |
|
|
|
11,400 |
|
|
|
121,797 |
|
|
|
26,930 |
|
|
|
22,888 |
|
|
|
— |
|
|
|
199,548 |
|
Aaron L. Cox |
|
|
2022 |
|
|
|
5,439 |
|
|
|
18,300 |
|
|
|
98,177 |
|
|
|
28,725 |
|
|
|
117,183 |
|
|
|
— |
|
|
|
267,824 |
|
Paul W. Hoelscher |
|
|
2022 |
|
|
|
11,405 |
|
|
|
18,300 |
|
|
|
60,917 |
|
|
|
31,418 |
|
|
|
68,105 |
|
|
|
281,630 |
|
|
|
471,776 |
|
|
|
|
2021 |
|
|
|
18,092 |
|
|
|
17,400 |
|
|
|
82,280 |
|
|
|
26,930 |
|
|
|
60,070 |
|
|
|
— |
|
|
|
204,773 |
|
|
|
|
2020 |
|
|
|
17,094 |
|
|
|
11,400 |
|
|
|
44,774 |
|
|
|
26,930 |
|
|
|
6,287 |
|
|
|
— |
|
|
|
106,485 |
|
Sean M. Clayton |
|
|
2022 |
|
|
|
5,306 |
|
|
|
17,995 |
|
|
|
54,018 |
|
|
|
10,862 |
|
|
|
14,228 |
|
|
|
— |
|
|
|
102,409 |
|
Elizabeth H.Z. Thompson, Ph.D. |
|
|
2022 |
|
|
|
10,458 |
|
|
|
18,300 |
|
|
|
97,551 |
|
|
|
18,501 |
|
|
|
6,345 |
|
|
|
— |
|
|
|
151,155 |
|
Andy Pasternak |
|
|
2022 |
|
|
|
9,671 |
|
|
|
18,300 |
|
|
|
122,258 |
|
|
|
18,650 |
|
|
|
5,455 |
|
|
|
— |
|
|
|
174,333 |
|
|
|
|
2021 |
|
|
|
14,573 |
|
|
|
17,400 |
|
|
|
89,712 |
|
|
|
17,953 |
|
|
|
28,217 |
|
|
|
— |
|
|
|
167,855 |
|
|
|
|
2020 |
|
|
|
438 |
|
|
|
11,400 |
|
|
|
26,758 |
|
|
|
27,554 |
|
|
|
721 |
|
|
|
— |
|
|
|
66,872 |
|
152
(6) Mr. Hoelscher retired on May 16, 2022. As discussed above in the section entitled “Retirement and Transition Agreement With Paul W. Hoelscher” in our “Compensation Discussion and Analysis” above, Mr. Hoelscher is currently serving as a part-time advisor to us.
(7) Mr. Clayton joined us on February 28, 2022.
(8) Includes a retention bonus of $5,000,000 paid to Mr. Clayton on December 30, 2022 subject to the Clawback Right. In the event of the executive’s (i) termination without cause, (ii) termination due to death or disability, or (iii) resignation for good reason, in each case, at any time while a portion of the Clawback Right is outstanding, the remaining Clawback Right will lapse in connection with such termination or resignation. For further information, see the section entitled “Additional Compensation Policies and Practices—Retention Bonuses” in our “Compensation Discussion and Analysis” above.
(9) Includes a sign-on bonus of $1,000,000 paid to Mr. Clayton, 50 percent of which was paid in March 2022 in connection with his commencement of employment with us in February 2022, and the remaining 50 percent was paid in December 2022.
(10) Includes a retention bonus of $2,500,000 paid to Dr. Thompson on December 30, 2022 subject to the Clawback Right. In the event of the executive’s (i) termination without cause, (ii) termination due to death or disability, or (iii) resignation for good reason, in each case, at any time while a portion of the Clawback Right is outstanding, the remaining Clawback Right will lapse in connection with such termination or resignation. For further information, see the section entitled “Additional Compensation Policies and Practices—Retention Bonuses” in our “Compensation Discussion and Analysis” above.
(11) Represents a sign-on bonus of $250,000 paid to Mr. Pasternak in November 2020 in connection with his commencement of employment with us in November 2019. Pursuant to the terms of his employment agreement, Mr. Pasternak received a sign-on bonus of $500,000 in November 2019 as well as the additional sign-on bonus of $250,000 in November 2020.
153
Grants of Plan-Based Awards
The following table sets forth certain information regarding grants of non-equity incentive plan and equity incentive plan-based awards to our NEOs for 2022:
|
|
|
|
|
|
Estimated Future Payouts Under |
|
|
All Other Stock Awards: Number of Shares of Stock or Units |
|
|
|
Grant Date Fair Value of Stock and Option Awards ($) (1) |
|
||||||||||||
Name |
|
Award Type |
|
Grant Date |
|
Threshold |
|
|
Target |
|
|
|
Maximum |
|
|
|
|
|
|
|
|
|||||
Timothy P. Walbert |
|
Annual Cash |
|
N/A |
|
$ |
1,450,142 |
|
|
$ |
1,681,324 |
|
(2) |
|
$ |
3,362,648 |
|
|
|
|
|
|
|
|
||
|
|
RSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
73,796 |
|
(3) |
|
$ |
7,198,800 |
|
|||
|
|
PSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
73,797 |
|
(4) |
|
|
9,402,505 |
|
|||
Aaron L. Cox |
|
Annual Cash |
|
N/A |
|
|
336,415 |
|
|
|
448,554 |
|
(5) |
|
|
897,108 |
|
|
|
|
|
|
|
|
||
|
|
RSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
23,805 |
|
(3) |
|
|
2,522,616 |
|
|||
|
|
PSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
23,805 |
|
(4) |
|
|
3,294,882 |
|
|||
Paul W. Hoelscher |
|
Annual Cash |
|
N/A |
|
|
129,107 |
|
|
|
172,142 |
|
(6) |
|
|
344,285 |
|
|
|
|
|
|
|
|
||
|
|
RSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
9,522 |
|
(3) |
|
|
928,871 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Sean M. Clayton |
|
Annual Cash |
|
N/A |
|
|
287,024 |
|
|
|
382,699 |
|
(7) |
|
|
765,397 |
|
|
|
|
|
|
|
|
||
|
|
RSU |
|
3/1/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
42,387 |
|
(3) |
|
|
3,839,414 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Elizabeth H.Z. Thompson, Ph.D. |
|
Annual Cash |
|
N/A |
|
|
311,998 |
|
|
|
415,998 |
|
(8) |
|
|
831,995 |
|
|
|
|
|
|
|
|
||
|
|
RSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
21,424 |
|
(3) |
|
|
2,089,911 |
|
|||
|
|
PSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
21,425 |
|
(4) |
|
|
2,729,789 |
|
|||
Andy Pasternak |
|
Annual Cash |
|
N/A |
|
|
377,814 |
|
|
|
503,752 |
|
(9) |
|
|
1,007,504 |
|
|
|
|
|
|
|
|
||
|
|
RSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
23,805 |
|
(3) |
|
|
2,322,178 |
|
|||
|
|
PSU |
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
23,805 |
|
(4) |
|
|
3,033,025 |
|
|||
154
Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table
Employment Agreements. Each of our NEOs has entered into a written employment agreement with us that provides for payment of base salary, target annual cash incentive compensation, eligibility for employee benefit programs and potential severance benefits. Following the retirement of Paul Hoelscher on May 16, 2022, Mr. Hoelscher ceased to be eligible for benefits under his employment agreement, except for the benefits described in the section entitled “Retirement and Transition Agreement With Paul W. Hoelscher” in our “Compensation Discussion and Analysis” above. For further information regarding the base salaries, bonuses and incentive compensation payable to our NEOs and their eligibility for our employee benefit programs, see our “Compensation Discussion and Analysis” above. For further information regarding the severance benefits provided under the employment agreements, see “Potential Payments Upon Termination or Change in Control” below.
Equity Awards. We have granted equity awards to our NEOs under our current and previous equity incentive plans. For further information regarding such equity awards, including the vesting schedules, see the “Grants of Plan-Based Awards” table and related footnotes above and “2022 Long-Term Incentive Grants” in our “Compensation Discussion and Analysis” above.
Option Repricings. Under the terms of our equity incentive plans, option repricing is not permitted without prior shareholder approval, and we did not reprice any outstanding equity awards during the year ended December 31, 2022 for our NEOs or other equity award holders.
155
Pay Ratio
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act, or Dodd-Frank Act, and Item 402(u) of Regulation S-K, we are providing the following information about the ratio of the annual total compensation of our principal executive officer to the total annual compensation of our median employee. The pay ratio included in this information is a reasonable estimate calculated in a manner consistent with Item 402(u) of Regulation S-K.
The purpose of this disclosure is to provide a measure of the equitability of pay within our company. We believe our compensation philosophy and process yield an equitable result for all of our employees. During fiscal 2022, our principal executive officer was our chairman, president and chief executive officer, Mr. Walbert.
For 2022:
Consistent with our prior year pay ratio disclosure, to identify our median compensated employee for 2022 we estimated all employees’ compensation as of October 31, 2022 (the median employee determination date). For each employee, we aggregated: (a) base salary as of October 31, 2022, (b) the target bonus for 2022 and (c) the estimated accounting value of any equity awards granted during 2022, and we then ranked this compensation measure for our employees from lowest to highest. After applying our methodology, we identified four median employees. Due to anomalous compensation characteristics of our median employees as new hires, we substituted an employee near the median whose compensation was viewed as more representative of our median employee. Amounts paid in foreign currencies were converted to U.S. Dollars based on the average annual exchange rate as of the median employee determination date. This calculation was performed for all employees, except as identified below and excluding Mr. Walbert, whether employed on a full-time, part-time or seasonal basis.
For purposes of this disclosure, all Canadian employees, totaling five individuals, were excluded from the employee population pursuant to the de minimis exemption, which permits us to exclude foreign employees, up to 5 percent of our total employee population, on a whole-country basis. As of October 31, 2022, we had 1,794 U.S. employees (excluding our CEO) and 290 non-U.S. employees, irrespective of the de minimis exemption. Applying the de minimis exemption, the total number of U.S. employees totaled 1,794, and the number of non-U.S. employees totaled 285.
The pay ratio reported above is a reasonable estimate calculated in a manner consistent with the SEC rules and based on our internal records and the methodology described above. Because the SEC rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices, the pay ratio reported by other companies may not be comparable to the pay ratio for us reported above, as other companies have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
156
Outstanding Equity Awards at December 31, 2022
The following table sets forth certain information regarding outstanding stock options, RSUs and PSUs held by our NEOs on December 31, 2022.
|
|
|
|
Option Awards |
|
Stock Awards |
||||||||||||||
Name |
|
Award Grant Date |
|
Number of Securities Underlying Unexercised Options Exercisable (#) |
|
Number of Securities Underlying Unexercised Options Unexercisable (#) |
|
Option Exercise Price ($) |
|
Option Expiration Date |
|
Number of Shares or Units of Stock that |
|
|
Market Value of Stock that Has Not Vested ($)(1) |
|
Number of Units of Stock that are Unearned and Have Not Vested (#) |
|
|
Market Value of Unearned Stock that Has Not Vested ($) (1) |
Timothy P. Walbert |
|
3/23/2015 |
|
742,565 |
|
— |
|
$22.14 |
|
3/22/2025 |
|
|
|
|
|
|
|
|
|
|
|
|
5/6/2015 |
|
1,650,000 |
|
— |
|
28.53 |
|
5/5/2025 |
|
|
|
|
|
|
|
|
|
|
|
|
1/3/2020 |
|
|
|
|
|
|
|
|
|
58,055 |
(2) |
|
$6,606,659 |
|
|
|
|
|
|
|
1/3/2020 |
|
|
|
|
|
|
|
|
|
171,968 |
(3) |
|
19,569,958 |
|
|
|
|
|
|
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
69,857 |
(2) |
|
7,949,727 |
|
|
|
|
|
|
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
52,392 |
(4) |
|
5,962,210 |
|
78,589 |
(4) |
|
$8,943,428 |
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
73,796 |
(2) |
|
8,397,985 |
|
|
|
|
|
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
73,797 |
(5) |
|
8,398,099 |
|
|
|
|
2,392,565 |
|
— |
|
|
|
|
|
426,068 |
|
|
$48,486,538 |
|
152,386 |
|
|
$17,341,527 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aaron L. Cox |
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
9,314 |
(2) |
|
$1,059,933 |
|
|
|
|
|
|
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
4,654 |
(4) |
|
|
|
20,957 |
(4) |
|
$2,384,907 |
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
15,870 |
(2) |
|
1,806,006 |
|
|
|
|
|
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23,805 |
(5) |
|
2,709,009 |
|
|
|
|
— |
|
— |
|
|
|
|
|
29,838 |
|
|
$2,865,939 |
|
44,762 |
|
|
$5,093,916 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Paul W. Hoelscher |
|
1/3/2020 |
|
|
|
|
|
|
|
|
|
9,522 |
(2) |
|
$1,083,604 |
|
|
|
|
|
|
|
1/3/2020 |
|
|
|
|
|
|
|
|
|
42,993 |
(3) |
|
4,892,603 |
|
|
|
|
|
|
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
23,286 |
(2) |
|
2,649,947 |
|
|
|
|
|
|
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
17,464 |
(4) |
|
1,987,403 |
|
26,196 |
(4) |
|
$2,981,105 |
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
9,522 |
(2) |
|
1,083,604 |
|
|
|
|
|
|
|
|
|
— |
|
— |
|
|
|
|
|
102,787 |
|
|
$11,697,161 |
|
26,196 |
|
|
$2,981,105 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sean M. Clayton |
|
3/1/2022 |
|
— |
|
— |
|
|
|
|
|
42,387 |
(2) |
|
$4,823,641 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Elizabeth H.Z. Thompson, Ph.D. |
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
4,657 |
(2) |
|
$529,967 |
|
|
|
|
|
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
14,283 |
(2) |
|
1,625,405 |
|
|
|
|
|
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21,425 |
(5) |
|
$2,438,165 |
|
|
|
|
— |
|
— |
|
|
|
|
|
18,940 |
|
|
$2,155,372 |
|
21,425 |
|
|
$2,438,165 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Andy Pasternak |
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
11,643 |
(2) |
|
$1,324,973 |
|
|
|
|
|
|
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
5,816 |
(4) |
|
661,861 |
|
26,196 |
(4) |
|
$2,981,105 |
|
|
1/4/2021 |
|
|
|
|
|
|
|
|
|
15,870 |
(2) |
|
1,806,006 |
|
|
|
|
|
|
|
1/4/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23,805 |
(5) |
|
2,709,009 |
|
|
|
|
— |
|
— |
|
|
|
|
|
33,329 |
|
|
$3,792,840 |
|
50,001 |
|
|
$5,690,114 |
157
158
Option Exercises and Stock Vested
The following table sets forth certain information regarding options exercised and stock vested for our NEOs for the fiscal year ended December 31, 2022.
|
|
Option Awards |
|
Stock Awards |
|||||
Name |
|
Number of Shares Acquired on Exercise (#) |
|
Value Realized on Exercise ($) |
|
Number of Shares Acquired on Vesting (#) |
|
|
Value Realized on Vesting ($)(1) |
Timothy P. Walbert |
|
|
|
|
|
394,646 |
(2) |
|
$40,190,749 |
|
|
|
|
|
|
20,336 |
(3) |
|
1,757,437 |
|
|
25,000 |
|
$1,696,913 |
|
|
|
|
|
|
|
25,000 |
|
1,696,500 |
|
|
|
|
|
|
|
25,000 |
|
1,881,770 |
|
|
|
|
|
|
|
25,000 |
|
1,759,163 |
|
|
|
|
|
|
|
25,000 |
|
1,969,733 |
|
|
|
|
|
|
|
25,000 |
|
2,180,840 |
|
|
|
|
|
|
|
25,000 |
|
2,256,098 |
|
|
|
|
|
|
|
25,000 |
|
2,111,130 |
|
|
|
|
|
|
|
7,400 |
|
508,508 |
|
|
|
|
|
|
|
17,600 |
|
1,194,336 |
|
|
|
|
|
|
|
25,000 |
|
1,696,500 |
|
|
|
|
|
|
|
25,000 |
|
1,754,920 |
|
|
|
|
|
Aaron L. Cox |
|
|
|
|
|
78,671 |
(4) |
|
8,011,855 |
|
|
|
|
|
|
2,526 |
(3) |
|
218,297 |
|
|
|
|
|
|
37,981 |
(5) |
|
4,322,238 |
|
|
|
|
|
|
26,925 |
(6) |
|
3,064,065 |
Paul W. Hoelscher |
|
|
|
|
|
114,197 |
(2) |
|
11,629,822 |
|
|
|
|
|
|
5,558 |
(3) |
|
480,322 |
|
|
26,090 |
|
2,453,605 |
|
|
|
|
|
|
|
215,335 |
|
19,108,371 |
|
|
|
|
|
|
|
50,000 |
|
4,628,645 |
|
|
|
|
|
|
|
36,424 |
|
3,254,849 |
|
|
|
|
|
Sean M. Clayton |
|
|
|
|
|
— |
|
|
— |
Elizabeth H.Z. Thompson, Ph.D. |
|
|
|
|
|
13,999 |
(4) |
|
1,425,658 |
|
|
|
|
|
|
19,055 |
(6) |
|
2,168,459 |
Andy Pasternak |
|
|
|
|
|
48,839 |
(4) |
|
4,973,764 |
|
|
|
|
|
|
12,421 |
(7) |
|
784,759 |
|
|
23,251 |
|
1,819,677 |
|
|
|
|
|
|
|
|
|
|
|
54,641 |
(5) |
|
6,218,146 |
|
|
|
|
|
|
34,092 |
(6) |
|
3,879,670 |
159
Pension Benefits
None of our NEOs participate in or have account balances in qualified or nonqualified defined benefit plans sponsored by us. Our Compensation Committee may elect to adopt qualified or nonqualified defined benefit plans in the future if it determines that doing so is in our best interests.
160
Nonqualified Deferred Compensation
Pursuant to our Deferred Compensation Plan, each year participants may elect to defer receipt and taxation of up to 50 percent of their salary and up to 100 percent of their incentive cash compensation. Beginning in 2021, we made matching contributions with respect to 100 percent of the first 6 percent of deferrals, which is the same general “safe harbor” matching contribution formula that we use for our 401(k) Plan, but not restricted by the compensation limits applicable to our 401(k) Plan. Prior to December 1, 2018, matching contributions generally vested in equal annual installments over a five-year period measured from the participant’s original hire date. Beginning December 1, 2018, matching contributions vest immediately, provided that the participant has provided one year of service from the participant’s original date of hire. Participants may select among phantom investment alternatives for the deemed investment of their plan accounts, which generally mirror the investment options available for our 401(k) Plan. Payments under the Deferred Compensation Plan will be distributed in the form of a lump sum payment or in up to 10 annual installments upon the participant’s termination of service or up to 10 annual installments upon a selected specified distribution date or dates made by the participant at the time of deferral. However, if a participant’s service with us terminates prior to the selected distribution date or dates, payments will commence in connection with the termination of service. Payments triggered upon a termination of service will generally commence in January or July of the next calendar year following a 6-month delay that follows the termination of service. In the event of a change in control, all plan balances will generally become immediately payable within 90 days thereafter. In addition, participants may be entitled to receive earlier payments through certain unforeseeable emergency withdrawals. Payments scheduled to be made under the Deferred Compensation Plan may be otherwise delayed or accelerated only upon the occurrence of certain specified events that comply with the requirements of Section 409A of the Code.
We fund the expenses for administering the Deferred Compensation Plan. We established a “rabbi trust” that holds Deferred Compensation Plan contributions and any credited earnings. The Deferred Compensation Plan is unfunded for tax purposes and for purposes of Title I of the Employee Retirement Income Security Act of 1974. Accordingly, amounts held in the rabbi trust are unsecured and remain subject to claims of our general creditors in the event of our insolvency in order to avoid current income taxation to the participants.
The following table sets forth certain information regarding the participation of our NEOs in the Deferred Compensation Plan for the fiscal year ended December 31, 2022.
Executive |
|
Executive Contributions (1) |
|
|
Company Contributions (2) |
|
|
Aggregate Earnings (3) |
|
|
Aggregate Distributions |
|
|
Aggregate Balance at December 31, 2022 |
|
|||||
Timothy P. Walbert |
|
$ |
259,541 |
|
|
$ |
234,780 |
|
|
$ |
(432,924 |
) |
|
$ |
52,663 |
|
|
$ |
2,510,294 |
|
Aaron L. Cox |
|
|
225,500 |
|
|
|
69,652 |
|
|
|
(246,096 |
) |
|
|
— |
|
|
|
1,043,196 |
|
Paul W. Hoelscher (4) |
|
|
60,917 |
|
|
|
60,917 |
|
|
|
(171,029 |
) |
|
|
— |
|
|
|
787,611 |
|
Sean M. Clayton (5) |
|
|
48,750 |
|
|
|
29,250 |
|
|
|
(1,332 |
) |
|
|
— |
|
|
|
76,668 |
|
Elizabeth H.Z. Thompson, Ph.D. |
|
|
90,222 |
|
|
|
90,222 |
|
|
|
(112,906 |
) |
|
|
— |
|
|
|
734,152 |
|
Andy Pasternak |
|
|
71,096 |
|
|
|
71,096 |
|
|
|
(146,590 |
) |
|
|
— |
|
|
|
800,136 |
|
161
Potential Payments Upon Termination or Change in Control
Involuntary Termination Severance Benefits
As provided under his amended employment agreement, Mr. Walbert’s severance benefit protection provides for up to 24 months’ base salary and COBRA health insurance premiums, 200 percent of target annual cash bonus, plus 18 months of time-based equity vesting acceleration in the event of a qualifying termination. As provided under their amended employment agreements, each of our NEOs other than Mr. Walbert has severance benefit protection which provide for up to 12 months’ base salary and COBRA health insurance premiums, plus 12 months of time-based equity vesting acceleration in the event of a qualifying termination.
Additionally, in the event of a qualifying termination within three months prior to or within 18 months following a change in control, Mr. Walbert has severance benefit protection of 36 months’ base salary and COBRA health insurance premiums, plus 300 percent of target annual cash bonus; each of our other NEOs has severance benefit protection of 18 months’ base salary and COBRA health insurance premiums, plus 150 percent of target annual cash bonus. In addition, time-based vesting equity awards are subject to full acceleration in a change in control related qualifying termination.
Severance benefits to our NEOs described above are payable only if there is a qualifying termination without cause or resignation for good reason. Any base salary and COBRA premium severance benefits are payable in installments over the applicable severance benefit period, target bonus severance benefits are payable in a single lump sum and equity vesting acceleration benefits are immediately effective.
The following key terms are defined in the amended employment agreements as follows:
162
Death and Disability. As provided under their amended employment agreements, in the event of a termination of employment due to death or disability, each NEO is entitled to receive a pro-rata bonus for the performance period in which the termination occurs, based on actual performance through the date of termination, and payable in a single lump sum within 30 days after termination.
Eligible Bonus Severance. The NEO employment agreements provide for eligibility to receive any earned but unpaid bonus if there is a qualifying termination without cause, resignation for good reason or termination due to death or disability. However, because our NEO bonus program currently provides that the NEO must also be employed on the scheduled bonus payment date in order to earn the bonus for a prior completed performance period, our NEOs are not currently eligible to receive any earned but unpaid bonus severance. Our NEOs are eligible to receive any target bonus severance to the extent provided in their amended employment agreements as described above.
Releases and Non-Competition. All severance benefits (other than due to death or complete disability) provided to our NEOs pursuant to their employment agreements are contingent upon (1) the executive’s execution of a standard release of claims in our favor and (2) the executive’s entering into a non-competition agreement to be effective during the period during which the executive receives severance benefits.
Sections 280G and 4999. Any payment or benefit provided under our NEOs’ employment agreements or otherwise in connection with a change in control may be subject to an excise tax under Section 4999. These payments also may not be eligible for a company tax deduction pursuant to Section 280G. Other than for Dr. Thompson, if any of these payments or benefits are subject to the excise tax, they may be reduced to provide the individual with the best after-tax result; specifically, the individual will receive either a reduced amount so that the excise tax is not triggered, or the individual will receive the full amount of the payments and benefits and then be liable for any excise tax.
For Dr. Thompson, pursuant to an Excise Tax Gross-Up Agreement we entered into with her in March 2023, we agreed to make tax reimbursement payments to her to the extent she is subject, in connection with the Transaction, to an excise tax imposed by Section 4999 of the Code in an amount that generally would place her in the same after-tax position that she would have been in if no excise tax had applied and no tax reimbursement payment had been made. The following table sets forth potential payments payable to our NEOs (other than Mr. Hoelscher, who was not serving as one of our executive officers at the end of fiscal year 2022) upon a (i) termination of employment without cause or resignation for good reason, assuming their employment was terminated on December 31, 2022, or (ii) termination of employment without cause or resignation for good reason in connection with a change in control, assuming their employment was terminated on December 31, 2022, and a change in control also occurred on such date. Pursuant to the agreement we entered into with Mr. Hoelscher in connection with his retirement in May 2022, Mr. Hoelscher received certain benefits upon his retirement, which are described in the section entitled “Retirement and Transition Agreement With Paul W. Hoelscher” in our “Compensation Discussion and Analysis” above.
163
|
|
Upon Termination Without Cause or Resignation |
|
Upon Termination Without Cause or Resignation |
||||||||||||||||
Name |
|
Cash Severance |
|
Continuation of Medical Benefits |
|
Bonus (2) |
|
Value of Accelerated Vesting (3)(4) |
|
Total |
|
Cash Severance |
|
Continuation of Medical Benefits |
|
Bonus (5) |
|
Value of Accelerated Vesting (3)(4) |
|
Total |
Timothy P. Walbert |
|
$2,625,230 |
|
$52,551 |
|
$3,412,799 |
|
$26,293,548 |
|
$32,384,128 |
|
$3,937,845 |
|
$78,827 |
|
$5,119,199 |
|
$65,828,065 |
|
$74,963,936 |
Aaron L. Cox |
|
644,800 |
|
26,276 |
|
— |
|
— |
|
671,076 |
|
967,200 |
|
39,414 |
|
677,040 |
|
8,489,480 |
|
10,173,134 |
Sean M. Clayton |
|
650,000 |
|
26,276 |
|
— |
|
1,607,880 |
|
2,284,156 |
|
975,000 |
|
39,414 |
|
682,500 |
|
4,823,641 |
|
6,520,555 |
Elizabeth H.Z. Thompson, Ph.D. (6) |
|
598,000 |
|
26,276 |
|
— |
|
— |
|
624,276 |
|
897,000 |
|
39,414 |
|
627,900 |
|
4,593,537 |
|
6,157,851 |
Andy Pasternak |
|
724,148 |
|
26,276 |
|
— |
|
— |
|
750,424 |
|
1,086,222 |
|
39,414 |
|
760,355 |
|
9,482,954 |
|
11,368,945 |
164
Non-Employee Director Compensation
Our directors perform a critical role in guiding Horizon’s strategic direction and overseeing management. Being a director entails many responsibilities and a substantial time commitment. Our compensation program for our non-employee directors reflects the critical function they perform and enables us to attract and retain highly qualified directors.
All current non-employee members of our board of directors are independent. Non-employee directors receive a combination of annual cash retainers and RSU grants in amounts that correlate to their responsibilities and levels of participation on our board of directors, including service on committees of our board of directors. Our only employee director, Mr. Walbert, receives no separate compensation for his service as a director or chairman of the board of directors.
Compensation Program
Our Compensation Committee reviews the compensation for our non-employee directors annually. To assist with the review, Aon, an independent compensation consultant, prepares a comprehensive assessment of our non-employee director compensation program, which includes:
As part of the most recent review, conducted in October 2022, our Compensation Committee determined that:
As a result of this review, in October 2022, the Compensation Committee amended our compensation policy for non-employee directors to provide that the annual cash compensation paid to the members of our Compensation Committee and to the chair of our Compensation Committee would be $15,000 and $25,000, respectively.
Peer Groups
As an Irish headquartered company traded in the United States, we consider our non-employee director compensation, both in amount and structure, against two peer groups:
Jazz Pharmaceuticals plc is in both peer groups.
165
Cash Compensation
Our compensation policy for non-employee directors who are not affiliated with any holder of more than 5 percent of our ordinary shares provides for annual cash compensation as set forth in the following table. The cash compensation is generally payable in equal quarterly installments at the end of each quarter in which the services are provided. For any independent director who joins after the beginning of the quarter, the cash compensation is pro-rated based on days served in that first partial quarter.
Director Position |
|
Annual Cash Compensation |
|
|
Non-executive chairman or lead independent director(1) |
|
$ |
115,000 |
|
All other non-employee directors(2) |
|
|
75,000 |
|
Committee chair fees |
|
|
|
|
Audit |
|
|
30,000 |
|
Compensation(3) |
|
|
25,000 |
|
Nominating and Corporate Governance |
|
|
20,000 |
|
Scientific |
|
|
20,000 |
|
Transaction |
|
|
20,000 |
|
Non-chair committee member fees |
|
|
|
|
Audit |
|
|
15,000 |
|
Compensation(4) |
|
|
15,000 |
|
Nominating and Corporate Governance |
|
|
10,000 |
|
Scientific |
|
|
12,500 |
|
Transaction |
|
|
12,500 |
|
Under our compensation policy, we reimburse our directors for their travel-related expenses, including lodging and other reasonable expenses incurred in attending meetings of the board of directors and committees of the board of directors.
Equity Compensation
On the date of each annual general meeting of shareholders that coincides with or follows the non-employee director’s initial appointment or election to our board of directors, eligible non-employee directors will automatically be granted RSUs with an aggregate value of $400,000, which will vest in full upon the earlier of the (i) first anniversary of the date of grant and (ii) date of the next annual general meeting of shareholders.
Any eligible non-employee director who is first elected or appointed to our board of directors on any date other than an annual general meeting of shareholders will automatically be granted RSUs on the date that they are first elected or appointed to our board of directors with a value equal to the annual RSU grant, prorated based on the number of days between such non-employee director’s start date and the one-year anniversary of the date of the annual general meeting of shareholders that most recently preceded such start date, which will vest in full upon the earlier of (i) the first anniversary of the date of the annual general meeting of shareholders that most recently preceded such director’s start date and (ii) the date of the next annual general meeting of shareholders.
166
Director Compensation Table
The following table sets forth compensation information for our non-employee directors who earned or received compensation under our compensation policy for non-employee directors or otherwise in 2022:
Name |
|
Fees Earned or Paid in Cash |
|
Stock Awards (1)(2) |
|
All Other Compensation |
|
|
Total |
William F. Daniel |
|
$112,500 |
|
$399,987 |
|
$10,000 |
(3) |
|
$522,487 |
Michael Grey |
|
151,250 |
|
399,987 |
|
— |
|
|
551,237 |
Jeff Himawan, Ph.D. |
|
104,375 |
|
399,987 |
|
— |
|
|
504,362 |
Susan Mahony, Ph.D. |
|
114,375 |
|
399,987 |
|
— |
|
|
514,362 |
Gino Santini |
|
95,000 |
|
399,987 |
|
— |
|
|
494,987 |
James Shannon, M.D. |
|
110,625 |
|
399,987 |
|
— |
|
|
510,612 |
Thomas H. Watkins |
|
106,250 |
|
399,987 |
|
— |
|
|
506,237 |
Pascale Witz |
|
96,250 |
|
399,987 |
|
— |
|
|
496,237 |
Non-Employee Director Share Ownership Guidelines
We have share ownership guidelines for our non-employee directors. The share ownership guidelines were amended in October 2021 and require that each director accumulate an ownership interest in our ordinary shares with a value equal to at least 5x the annual cash board service retainer within five years of the date the non-employee director first becomes subject to the guidelines.
Individual ownership interest versus the guidelines is reviewed annually using an average share price for a calendar quarter prior to the review date. Shares that count toward satisfaction of these guidelines include: shares owned outright by the individual (including stock units that have vested but not yet settled); shares subject to RSUs that have not vested; and shares held in trust for the benefit of the individual or his/her spouse.
All of our non-employee directors subject to the share ownership guidelines met the guidelines as of December 31, 2022.
167
Compensation Committee Interlocks and Insider Participation
No member of our Compensation Committee has ever been an executive officer or employee of Horizon. None of our executive officers currently serves, or has served during the last completed year, on the Compensation Committee or board of directors of any other entity that has one or more officers serving as a member of our board of directors or our Compensation Committee.
Compensation Committee Report
The Compensation Committee of our board of directors has submitted the following report for inclusion in this Annual Report on Form 10-K:
The Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis set forth above. Based on this review and discussion, the Compensation Committee has recommended to the board of directors that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K, filed by us with the SEC.
This report of the Compensation Committee is not “soliciting material,” shall not be deemed “filed” with the SEC and shall not be incorporated by reference by any general statement incorporating by reference this Annual Report on Form 10-K into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing, except to the extent that we specifically incorporate this information by reference, and shall not otherwise be deemed filed under such acts.
This report has been furnished by the Compensation Committee:
Respectively submitted,
The Compensation Committee of the Board of Directors
Susan Mahony, Ph.D., Chair
William F. Daniel
Jeff Himawan, Ph.D.
Gino Santini
168
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides information as of December 31, 2022, with respect to our ordinary shares that may be issued under our equity compensation plans:
|
|
(a) |
|
(b) |
|
(c) |
|
Plan Category |
|
Number of securities to be issued upon exercise of outstanding options, warrants, and rights (1) |
|
Weighted-average exercise price of outstanding options, warrants, and rights (2) |
|
Number of securities remaining available for future issuances under equity compensation plans (excluding securities reflected in Column (a)) |
|
Equity compensation plans approved by shareholders: |
|
|
|
|
|
|
|
2011 Equity Incentive Plan |
|
123,424 |
|
$13.41 |
|
— |
|
Amended and Restated 2014 Equity Incentive Plan |
|
4,800,693 |
|
$24.24 |
|
— |
|
2014 Non-Employee Equity Plan |
|
655,874 |
|
$15.16 |
|
483,069 |
|
Amended and Restated 2020 Equity Incentive Plan |
|
2,903,315 |
|
$— |
|
18,974,953 |
|
2020 Employee Share Purchase Plan |
|
— |
|
$— |
|
2,046,575 |
(3) |
Equity compensation plans not approved by shareholders: |
|
|
|
|
|
|
|
Amended and Restated 2018 Equity Incentive Plan |
|
1,046,782 |
|
$44.82 |
|
1,650,775 |
|
Total of all equity compensation plans: |
|
9,530,088 |
|
|
|
23,155,372 |
|
2011 Equity Incentive Plan. In July 2010, the board of directors of Horizon Pharma, Inc., or HPI, our predecessor, adopted the 2011 Equity Incentive Plan, or 2011 EIP. In June 2011, HPI’s stockholders approved the 2011 EIP and it became effective upon the signing of the underwriting agreement related to HPI’s initial public offering on July 28, 2011. Upon consummation of our merger transaction in September 2014 with Vidara Therapeutics International Public Limited Company, or Vidara Merger, we assumed the 2011 EIP, and upon the effectiveness of the 2014 EIP, no additional stock awards were or will be made under the 2011 EIP, although all outstanding stock awards granted under the 2011 EIP continue to be governed by the terms of the 2011 EIP.
Amended and Restated 2014 Equity Incentive Plan. On May 17, 2014, HPI’s board of directors adopted the 2014 EIP. On September 18, 2014, at a special meeting of the stockholders of HPI, or Special Meeting, HPI’s stockholders approved the 2014 EIP. Upon consummation of the Vidara Merger, we assumed the 2014 EIP, which served as a successor to the 2011 EIP for employee equity awards. Upon the effectiveness of the 2020 EIP, which serves as a successor to the 2014 EIP, no additional stock awards were or will be made under the 2014 EIP, although all outstanding stock awards granted under the 2014 EIP continue to be governed by the terms of the 2014 EIP.
169
2014 Non-Employee Equity Plan. On May 17, 2014, HPI’s board of directors adopted the 2014 Non-Employee Equity Plan. On September 18, 2014, at the Special Meeting, HPI’s stockholders approved the 2014 Non-Employee Equity Plan. Upon consummation of the Vidara Merger, we assumed the 2014 Non-Employee Equity Plan, which serves as a successor to the 2011 EIP for non-employee equity awards.
Amended and Restated 2020 Equity Incentive Plan. On February 19, 2020, the Compensation Committee adopted the 2020 EIP. On April 30, 2020, our shareholders approved the 2020 EIP, which serves as a successor to the 2014 EIP. On February 17, 2021, the Compensation Committee approved amending the 2020 EIP and on April 29, 2021, our shareholders approved the amendment. On February 23, 2022, the Compensation Committee approved further amending the 2020 EIP and on April 28, 2022, our shareholders approved the further amendment.
2020 Employee Share Purchase Plan. On February 19, 2020, the Compensation Committee adopted the 2020 Employee Share Purchase Plan, or 2020 ESPP. On April 30, 2020, our shareholders approved the 2020 ESPP, which serves as a successor to our 2014 Employee Share Purchase Plan.
Amended and Restated 2018 Equity Incentive Plan. On March 15, 2021, upon our completion of our acquisition of Viela Bio, Inc., or Viela, we assumed the Viela Bio 2018 Equity Incentive Plan (which was subsequently renamed the Horizon Therapeutics Public Limited Company Amended and Restated 2018 Equity Incentive Plan). Under the terms of the merger agreement for Viela, all outstanding Viela stock options assumed by us with vesting dates after June 1, 2021 (covering an aggregate of 2,180,159 shares of Viela’s common stock) were converted at a rate of 0.60 to 1 into stock options to purchase our ordinary shares (covering an aggregate of 1,318,053 of our ordinary shares), reducing the initial reserve of 3,677,603 shares available for grant, and leaving 2,359,550 remaining shares available for grant post-acquisition. The 2018 EIP provides for the grant of stock options, stock appreciation rights, restricted stock, restricted stock units and unrestricted stock. Awards under the 2018 EIP may be granted to our employees, directors and consultants or of any subsidiary; provided, however, that (i) incentive stock options may be granted only to employees and (ii) from and following March 15, 2021, the individuals eligible to receive awards under the 2018 EIP do not include any individuals who were providing services to us in any capacity prior to March 15, 2021. The 2018 EIP is administered by the Compensation Committee, provided that the Compensation Committee may delegate certain administrative powers to a subcommittee or one or more of our officers or of any subsidiary (including the power to grant awards under the 2018 EIP to employees), subject to the terms of the 2018 EIP. Unless sooner terminated by the board of directors, the 2018 EIP will terminate on January 29, 2028.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding the beneficial ownership of our ordinary shares as of January 31, 2023 for:
The table is based upon information supplied by our officers, directors and principal shareholders and/or a review of Schedules 13D and 13G documents filed with the SEC, if any, and other sources. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting and investment power with respect to the securities. Except as indicated by footnote, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all ordinary shares shown as beneficially owned by them. The number of ordinary shares used to calculate the percentage ownership of each listed person includes the ordinary shares, underlying options, warrants or other rights held by such persons that are exercisable as of April 1, 2023, which is 60 days after January 31, 2023.
Percentage of beneficial ownership is based on 228,397,129 ordinary shares outstanding as of January 31, 2023. Unless otherwise indicated, the address for the following shareholders is c/o Horizon Therapeutics plc, 70 St. Stephen’s Green, Dublin 2, D02 E2X4, Ireland.
170
|
|
Number and Percentage of Ordinary Shares |
|
|||||
Name and Address of Beneficial Owner or Identity of Group |
|
Ordinary Shares |
|
|
Percentage |
|
||
5% or Greater Shareholders: |
|
|
|
|
|
|
||
The Vanguard Group, Inc.(1) |
|
|
21,236,103 |
|
|
|
9.3 |
% |
100 Vanguard Blvd. |
|
|
|
|
|
|
||
Malvern, PA 19355 |
|
|
|
|
|
|
||
BlackRock, Inc.(2) |
|
|
14,794,254 |
|
|
|
6.5 |
% |
55 East 52nd Street |
|
|
|
|
|
|
||
New York, NY 10055 |
|
|
|
|
|
|
||
Directors (Other than Timothy P. Walbert): |
|
|
|
|
|
|
||
William F. Daniel(3) |
|
|
185,791 |
|
|
* |
|
|
Michael Grey (4) |
|
|
188,433 |
|
|
* |
|
|
Jeff Himawan, Ph.D.(5) |
|
|
141,239 |
|
|
* |
|
|
Susan Mahony, Ph.D.(6) |
|
|
16,617 |
|
|
* |
|
|
Gino Santini(7) |
|
|
187,563 |
|
|
* |
|
|
James Shannon, M.D.(8) |
|
|
95,071 |
|
|
* |
|
|
H. Thomas Watkins(9) |
|
|
246,118 |
|
|
* |
|
|
Pascale Witz(10) |
|
|
128,310 |
|
|
* |
|
|
Named Executive Officers: |
|
|
|
|
|
|
||
Timothy P. Walbert(11) |
|
|
3,266,753 |
|
|
|
1.4 |
% |
Aaron L. Cox(12) |
|
|
277 |
|
|
* |
|
|
Paul W. Hoelscher(13) |
|
|
186,158 |
|
|
* |
|
|
Sean M. Clayton(14) |
|
|
15,153 |
|
|
|
|
|
Elizabeth H.Z. Thompson, Ph.D.(15) |
|
|
16,575 |
|
|
* |
|
|
Andy Pasternak(16) |
|
|
56,338 |
|
|
|
|
|
All current executive officers and directors as a group (15 persons)(17) |
|
|
5,071,910 |
|
|
|
2.2 |
% |
* Represents beneficial ownership of less than one percent.
171
172
Policies and Procedures for Transactions with Related Persons
We maintain a written Related-Person Transactions Policy that sets forth our policies and procedures regarding the identification, review, consideration, approval and oversight of “related-person transactions.” For purposes of our policy only, a “related-person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are participants and a related person has a direct or indirect material interest. Transactions involving compensation for services provided to us as an employee, director, consultant or similar capacity by a related person are not covered by this policy. A “related person” is any executive officer, director or nominee to become director, a holder of more than 5 percent of our ordinary shares, including any immediate family members of such persons, any entity owned or controlled by such persons or the trustees of any trust of which the principal beneficiaries are any of such persons. Any related-person transaction may only be consummated if our Audit Committee has approved or ratified the transaction in accordance with the policy guidelines set forth below.
The policy imposes an affirmative duty upon each director and executive officer to identify, and we will request that significant shareholders identify, any transaction involving them, their affiliates or family members that may be considered a related-person transaction before such person engages in the transaction. Under the policy, where a transaction has been identified as a related-person transaction, management must present information regarding the proposed related-person transaction to our Audit Committee (or, where the transaction relates to compensation of related parties, to our Compensation Committee, or, where review by our Audit Committee would be inappropriate, to another independent body of our board of directors) for review. The presentation must include a description of, among other things, the material facts, the direct and indirect interests of the related persons, the benefits of the transaction to us and whether any alternative transactions are available. In considering related-person transactions, our Audit Committee (or other applicable independent body of our board of directors) takes into account the relevant available facts and circumstances including, but not limited to:
In the event a director has an interest in the proposed transaction, the director is expected to recuse himself or herself from the deliberations and approval process.
Certain Related-Person Transactions
We describe below transactions and any series of similar transactions, since the beginning of fiscal year 2022, with respect to which we were a party, will be a party, or otherwise benefited, in which:
We also describe below certain other transactions with our directors, executive officers and shareholders. We believe that the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
173
Employment Agreements
We have entered into employment agreements with our NEOs. Each of these agreements is described in the “Grants of Plan-Based Awards — Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table” and “Potential Payments Upon Termination or Change in Control” sections in Part III — Item 11, “Executive Compensation” of this Annual Report on Form 10-K.
Each of our executive officers has entered into a written employment agreement with us that provides for payment of base salary, target annual cash incentive compensation, eligibility for employee benefit programs and potential severance benefits.
Other Arrangements
Certain of our NEOs and directors have family members also employed by us. Mr. Walbert’s wife serves as our executive vice president, U.S. operations, and in 2022, she received total compensation of approximately $5,836,000. Mr. Walbert has a sister employed by us who received approximately $259,000 in total compensation in 2022. Mr. Watkins has a son employed by us who received approximately $230,000 in total compensation in 2022 and a daughter-in-law employed by us that received approximately $269,000 in total compensation in 2022.
On November 22, 2022, we entered into a research collaboration and option agreement with Xeris under which Xeris is obligated to use its proprietary formulation technology platform, XeriJect, to conduct a research program to develop an ultra-concentrated, ready-to-use, subcutaneous injection of TEPEZZA. We received an option to obtain a commercial license for any reformulated product developed under the research program. An upfront payment of $2.75 million was paid during the year ended December 31, 2022. In addition, Xeris is entitled to receive a milestone payment of $6.0 million upon the earlier of either (i) the exercise of our option or (ii) the achievement of the minimally acceptable target product profile by a reformulated product generated through the research program. If we exercise the option to continue development of and commercialize the reformulated product, Xeris may also be entitled to receive additional development and regulatory milestones and royalties on future sales. Jeffrey W. Sherman, M.D., FACP, our executive vice president, chief medical officer, is also a member of the board of directors of, and holds a beneficial interest in, Xeris BioPharma, which is the parent company of Xeris. This related party transaction was conducted in the normal course of business on an arm’s length basis.
Stock Awards Granted to Executive Officers and Directors
We have granted stock options, RSUs and PSUs to our executive officers and stock options and RSUs to our directors. Certain grants to our NEOs and directors are described in “Grants of Plan-Based Awards” and “Non-Employee Director Compensation” in Part III — Item 11, “Executive Compensation” of this Annual Report on Form 10-K, respectively.
174
Indemnification of Officers and Directors
We have entered into indemnification agreements with our directors and executive officers. The indemnification agreements require us, under the circumstances and to the extent provided for therein, to indemnify such persons to the fullest extent permitted by applicable law against certain expenses and other amounts incurred by any such person as a result of such person being made a party to certain actions, suits, proceedings and other actions by reason of the fact that such person is or was a director, officer, employee, consultant, agent or fiduciary of our company or any of our subsidiaries or other affiliated enterprises. The rights of each person who is a party to an indemnification agreement are in addition to any other rights such person may have under our Memorandum and Articles of Association, the Irish Companies Act 2014, any other agreement, a vote of the shareholders of our company, a resolution of directors of our company or otherwise. We believe that these agreements are necessary to attract and retain qualified persons as our officers and directors. We also maintain directors’ and officers’ liability insurance.
Director Independence
As required under the Nasdaq Listing Rules, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the board of directors. Our board of directors consults with our counsel to ensure that the determinations of our board of directors are consistent with relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent listing standards of Nasdaq, as in effect from time to time. Consistent with these considerations, after review of all relevant identified transactions or relationships between each director, or any of his or her family members, and us, our senior management and our independent auditors, our board of directors has affirmatively determined that, with the exception of Mr. Walbert, all of the directors are “independent directors” as defined by Rule 5605(a)(2) of the Nasdaq Listing Rules. In making this determination, our board of directors found that none of these directors (other than Mr. Walbert) had a material or other disqualifying relationship with us. Mr. Walbert is not an independent director by virtue of his current employment with us.
175
Item 14. Principal Accountant Fees and Services
Audit and All Other Fees
The following table presents fees for services rendered by PricewaterhouseCoopers LLP (United States), or PricewaterhouseCoopers, our independent registered public accounting firm, for 2022 and 2021 in the following categories:
|
|
For the Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Audit fees(1) |
|
$ |
3,075 |
|
|
$ |
3,666 |
|
All other fees(2) |
|
|
20 |
|
|
|
7 |
|
Total |
|
$ |
3,095 |
|
|
$ |
3,673 |
|
The Audit Committee has considered whether provision of the above audit-related and tax services is compatible with maintaining the registered public accounting firm’s independence and has determined that such services are compatible with maintaining the registered public accounting firm’s independence.
Pre-Approval Policies and Procedures
Pursuant to its charter, the Audit Committee is responsible for pre-approving all audit and permitted non-audit services to be performed for us by our independent registered public accounting firm or any other auditing or accounting firm. The Audit Committee pre-approved all such services in 2022 and 2021.
176
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a) Documents filed as part of this report.
The financial statements listed on the Index to Consolidated Financial Statements F-1 to F-60 are filed as part of this Annual Report on Form 10-K.
Schedule II – Valuation and Qualifying Accounts and Reserves for each of the three fiscal years ended December 31, 2022, 2021 and 2020 appearing on page F-60. Other financial statement schedules have been omitted because the required information is included in the consolidated financial statements or notes thereto or because they are not applicable or not required.
177
INDEX TO EXHIBITS
Exhibit |
|
|
Number |
|
Description of Document |
|
|
|
2.1# |
|
|
|
|
|
2.2# |
|
|
|
|
|
2.3 |
|
|
|
|
|
3.1 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3 |
|
|
4.4 |
|
|
4.5 |
|
|
4.6 |
|
|
10.1+ |
|
|
|
|
|
10.2+ |
|
|
|
|
|
10.3+ |
|
Horizon Therapeutics Public Limited Company Non-Employee Director Compensation Policy, as amended. |
|
|
|
178
10.4+ |
|
10.5+ |
|
|
|
|
|
10.6+ |
|
|
|
|
|
10.7+ |
|
|
|
|
|
10.8+ |
|
|
|
|
|
10.9+ |
|
|
|
|
|
10.10+ |
|
|
|
|
|
10.11 |
|
|
|
|
|
10.12+ |
|
|
|
|
|
10.13* |
|
|
|
|
|
10.14* |
|
|
|
|
|
10.15* |
|
179
|
|
|
10.16* |
|
|
|
|
|
10.17* |
|
|
|
|
|
10.18 |
|
|
|
|
|
10.19+ |
|
|
|
|
|
10.20+ |
|
|
|
|
|
10.21 |
|
|
|
|
|
10.22 |
|
|
|
|
|
10.23+ |
|
|
|
|
|
10.24+ |
|
|
|
|
|
10.25+ |
|
|
|
|
|
10.26** |
|
180
|
|
|
10.27** |
|
|
|
|
|
10.28 |
|
|
|
|
|
10.29 |
|
|
|
|
|
10.30* |
|
|
|
|
|
10.31* |
|
|
|
|
|
10.32* |
|
|
|
|
|
10.33 |
|
|
|
|
|
10.34* |
|
|
|
|
|
10.35* |
|
|
|
|
|
10.36+ |
|
181
|
|
|
10.37 |
|
|
|
|
|
10.38* |
|
|
|
|
|
10.39* |
|
|
|
|
|
10.40+ |
|
|
|
|
|
10.41+ |
|
|
|
|
|
10.42* |
|
|
|
|
|
10.43* |
|
|
|
|
|
10.44 |
|
|
|
|
|
10.45+ |
|
|
|
|
|
10.46* |
|
|
|
|
|
10.47 |
|
182
|
|
|
10.48 |
|
|
|
|
|
10.49+ |
|
|
|
|
|
10.50+ |
|
|
|
|
|
10.51* |
|
|
|
|
|
10.52+ |
|
|
|
|
|
10.53+ |
|
|
|
|
|
10.54+ |
|
|
|
|
|
10.55* |
|
|
|
|
|
10.56+ |
|
|
|
|
|
10.57+ |
|
|
|
|
|
21.1 |
|
Subsidiaries of Horizon Therapeutics Public Limited Company. |
|
|
|
23.1 |
|
Consent of PricewaterhouseCoopers LLP, independent registered public accounting firm. |
|
|
|
24.1 |
|
Power of Attorney. Reference is made to the signature page hereto. |
|
|
|
31.1 |
|
|
|
|
|
31.2 |
|
183
|
|
|
32.1 |
|
|
|
|
|
32.2 |
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
# Schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant hereby undertakes to furnish supplemental copies of any of the omitted schedules upon request by the U.S. Securities and Exchange Commission.
+ Indicates management contract or compensatory plan.
* Certain information in this exhibit has been omitted pursuant to Item 601 of Regulation S-K.
** Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
Item 16. Form 10-K Summary
None.
184
HORIZON THERAPEUTICS PLC
Index to Consolidated Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Horizon Therapeutics plc
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Horizon Therapeutics plc and its subsidiaries (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of comprehensive income, of shareholders' equity and of cash flows for each of the three years in the period ended December 31, 2022, including the related notes and financial statement schedule listed in the index appearing under Item 15(a)(2) (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2022 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
F-1
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Accrued Medicaid Rebates
As described in Note 2 and 10 to the consolidated financial statements, the Company has accrued government rebates and chargebacks of $235.2 million as of December 31, 2022. A significant portion of these accruals relates to the Company’s Medicaid rebates. Management calculates the Medicaid rebate allowance using the expected value method. Management accrues estimated rebates based on estimated percentages of medicine prescribed to qualified patients, estimated rebate percentages, and estimated levels of inventory in the distribution channel that will be prescribed to qualified patients and records the rebates as a reduction of revenue.
The principal considerations for our determination that performing procedures relating to accrued Medicaid rebates is a critical audit matter are (i) the significant judgment by management when developing the allowance, and (ii) a high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating the assumptions related to estimated percentages of medicine prescribed to qualified patients, estimated rebate percentages, and estimated levels of inventory in the distribution channel that will be prescribed to qualified patients.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to accrued Medicaid rebates, including controls over the assumptions used to develop the allowance. These procedures also included, among others, (i) developing an independent estimate of the accrued Medicaid rebates by utilizing third-party prescription data, the terms of the specific rebate programs, and the historical trend of actual rebate claims paid, (ii) comparing the independent estimate to management’s estimate, and (iii) testing, on a sample basis, rebate claims processed by the Company, including evaluating those claims for consistency with the terms of the specific rebate programs.
/s/
March 1, 2023
We have served as the Company’s auditor since 2009.
F-2
HORIZON THERAPEUTICS PLC
CONSOLIDATED BALANCE SHEETS
(In thousands, except nominal value and share data)
|
|
As of |
|
|
As of |
|
||
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2022 |
|
|
2021 |
|
||
ASSETS |
|
|
|
|
|
|
||
CURRENT ASSETS: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Restricted cash |
|
|
|
|
|
|
||
Accounts receivable, net |
|
|
|
|
|
|
||
Inventories, net |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
Property, plant and equipment, net |
|
|
|
|
|
|
||
Developed technology and other intangible assets, net |
|
|
|
|
|
|
||
In-process research and development |
|
|
|
|
|
|
||
Goodwill |
|
|
|
|
|
|
||
Deferred tax assets, net |
|
|
|
|
|
|
||
Other long-term assets |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
||
CURRENT LIABILITIES: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Accrued expenses and other current liabilities |
|
|
|
|
|
|
||
Accrued trade discounts and rebates |
|
|
|
|
|
|
||
Long-term debt—current portion |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
LONG-TERM LIABILITIES: |
|
|
|
|
|
|
||
Long-term debt, net |
|
|
|
|
|
|
||
Deferred tax liabilities, net |
|
|
|
|
|
|
||
Other long-term liabilities |
|
|
|
|
|
|
||
Total long-term liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
SHAREHOLDERS’ EQUITY: |
|
|
|
|
|
|
||
Ordinary shares, $ |
|
|
|
|
|
|
||
Treasury stock, |
|
|
( |
) |
|
|
( |
) |
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated other comprehensive income (loss) |
|
|
|
|
|
( |
) |
|
Retained earnings |
|
|
|
|
|
|
||
Total shareholders’ equity |
|
|
|
|
|
|
||
Total liabilities and shareholders’ equity |
|
$ |
|
|
$ |
|
||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
HORIZON THERAPEUTICS PLC
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(In thousands, except share and per share data)
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Net sales |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Cost of goods sold |
|
|
|
|
|
|
|
|
|
|||
Gross profit |
|
|
|
|
|
|
|
|
|
|||
OPERATING EXPENSES: |
|
|
|
|
|
|
|
|
|
|||
Research and development |
|
|
|
|
|
|
|
|
|
|||
Acquired in-process research and development and milestones |
|
|
|
|
|
|
|
|
|
|||
Selling, general and administrative |
|
|
|
|
|
|
|
|
|
|||
Impairment of goodwill |
|
|
|
|
|
— |
|
|
|
— |
|
|
Impairment of long-lived asset |
|
|
— |
|
|
|
|
|
|
— |
|
|
Gain on sale of assets |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Total operating expenses |
|
|
|
|
|
|
|
|
|
|||
Operating income |
|
|
|
|
|
|
|
|
|
|||
OTHER EXPENSE, NET: |
|
|
|
|
|
|
|
|
|
|||
Interest expense, net |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Foreign exchange loss |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Loss on debt extinguishment |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Other (expense) income, net |
|
|
( |
) |
|
|
|
|
|
|
||
Total other expense, net |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Income before expense (benefit) for income taxes |
|
|
|
|
|
|
|
|
|
|||
Expense (benefit) for income taxes |
|
|
|
|
|
( |
) |
|
|
|
||
Net income |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Net income per ordinary share—basic |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Weighted average ordinary shares outstanding—basic |
|
|
|
|
|
|
|
|
|
|||
Net income per ordinary share—diluted |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Weighted average ordinary shares outstanding—diluted |
|
|
|
|
|
|
|
|
|
|||
OTHER COMPREHENSIVE INCOME (LOSS), NET OF TAX |
|
|
|
|
|
|
|
|
|
|||
Interest rate swap contracts designated as cash flow hedges |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
Pension and other post-employment benefit plan remeasurements |
|
|
|
|
|
( |
) |
|
|
— |
|
|
Foreign currency translation adjustments |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Other comprehensive income (loss) |
|
|
|
|
|
( |
) |
|
|
|
||
Comprehensive income |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
HORIZON THERAPEUTICS PLC
(In thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Accumulated Other |
|
|
(Accumulated |
|
|
Total |
|
||||||||
|
|
Ordinary Shares |
|
|
Treasury Stock |
|
|
Paid-in |
|
|
Comprehensive |
|
|
Deficit) |
|
|
Shareholders’ |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
(Loss) Income |
|
|
Retained Earnings |
|
|
Equity |
|
||||||||
Balances at December 31, 2019 |
|
|
|
|
$ |
|
|
|
|
|
$ |
( |
) |
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|||||
Issuance of ordinary shares in conjunction with 2.5% Exchangeable Senior Notes due 2022 |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of ordinary shares - public offering |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of ordinary shares in conjunction with the exercise of stock options and the vesting of restricted stock units and performance stock units |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Ordinary shares withheld for payment of employees’ withholding tax liability |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Issuance of ordinary shares in conjunction with Employee Share Purchase Plan |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Share-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Foreign currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
||
Balances at December 31, 2020 |
|
|
|
|
$ |
|
|
|
|
|
$ |
( |
) |
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|||||
Issuance of ordinary shares in conjunction with the exercise of stock options |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of ordinary shares in conjunction with Employee Share Purchase Plan |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Ordinary shares withheld for payment of employees’ withholding tax liability |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Share-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Pension and other post-employment benefit plan remeasurements |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Foreign currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
||
Balances at December 31, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
( |
) |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
||||||
Issuance of ordinary shares in conjunction with the exercise of stock options |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Issuance of ordinary shares in conjunction with Employee Share Purchase Plan |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Repurchase of ordinary shares |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Ordinary shares withheld for payment of employees’ withholding tax liability |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Share-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Pension and other post-employment benefit plan remeasurements |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Foreign currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Interest rate swap contracts designated as cash flow hedges |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
||
Balances at December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
HORIZON THERAPEUTICS PLC
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|||
Net income |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|||
Depreciation and amortization expense |
|
|
|
|
|
|
|
|
|
|||
Equity-settled share-based compensation |
|
|
|
|
|
|
|
|
|
|||
Impairment of goodwill |
|
|
|
|
|
— |
|
|
|
— |
|
|
Acquired IPR&D and milestones |
|
|
|
|
|
|
|
|
|
|||
Deferred income taxes |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Amortization of debt discount and deferred financing costs |
|
|
|
|
|
|
|
|
|
|||
Impairment of long-lived asset |
|
|
— |
|
|
|
|
|
|
— |
|
|
Loss on debt extinguishment |
|
|
— |
|
|
|
— |
|
|
|
|
|
Gain on sale of assets |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Foreign exchange and other adjustments |
|
|
|
|
|
|
|
|
|
|||
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|||
Accounts receivable |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
Inventories |
|
|
|
|
|
|
|
|
( |
) |
||
Prepaid expenses and other current assets |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Accounts payable |
|
|
|
|
|
( |
) |
|
|
|
||
Accrued trade discounts and rebates |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Accrued expenses and other current liabilities |
|
|
( |
) |
|
|
|
|
|
|
||
Other non-current assets and liabilities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Net cash provided by operating activities |
|
|
|
|
|
|
|
|
|
|||
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|||
Purchases of property, plant and equipment |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payments related to license and collaboration agreements |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payments for long-term investments |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payments for acquisitions, net of cash acquired |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Proceeds from sale of assets |
|
|
— |
|
|
|
|
|
|
|
||
Change in escrow deposit for property purchase |
|
|
— |
|
|
|
— |
|
|
|
|
|
Receipts from long-term investments |
|
|
|
|
|
|
|
|
|
|||
Net cash used in investing activities |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|||
Repurchase of ordinary shares |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
Payment of employee withholding taxes relating to share-based awards |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Repayment of term loans |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
Net proceeds from term loans |
|
|
— |
|
|
|
|
|
|
— |
|
|
Repayment of senior notes |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Net proceeds from the issuance of ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
|
Proceeds from the issuance of ordinary shares in conjunction with Employee Share Purchase Plan |
|
|
|
|
|
|
|
|
|
|||
Proceeds from the issuance of ordinary shares in connection with stock option exercises |
|
|
|
|
|
|
|
|
|
|||
Net cash (used in) provided by financing activities |
|
|
( |
) |
|
|
|
|
|
|
||
Effect of foreign exchange rate changes on cash, cash equivalents and restricted cash |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
|
|
|
|
( |
) |
|
|
|
||
Cash, cash equivalents and restricted cash, beginning of the year |
|
|
|
|
|
|
|
|
|
|||
Cash, cash equivalents and restricted cash, end of the year |
|
$ |
|
|
$ |
|
|
$ |
|
|||
F-6
HORIZON THERAPEUTICS PLC
CONSOLIDATED STATEMENTS OF CASH FLOWS (CONTINUED)
(In thousands)
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
|
|||
Cash paid for interest, net of interest swap payments |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Cash paid for income taxes, net of refunds received |
|
|
|
|
|
|
|
|
|
|||
Cash paid for amounts included in the measurement of operating lease liabilities |
|
|
|
|
|
|
|
|
|
|||
SUPPLEMENTAL NON-CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
|
|||
Lease liabilities arising from obtaining right-of-use assets |
|
$ |
|
|
$ |
|
|
$ |
— |
|
||
Purchases of property, plant and equipment included in accounts payable and accrued expenses and other current liabilities |
|
|
|
|
|
|
|
|
|
|||
Purchases of acquired in-process research and development and milestones included in accrued expenses and other current liabilities |
|
|
|
|
|
|
|
|
— |
|
||
Principal amount of 2.5% Exchangeable Senior Notes due 2022 converted into ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
|
Milestone payments for TEPEZZA intangible asset included in accrued expenses and other current liabilities |
|
|
— |
|
|
|
— |
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-7
HORIZON THERAPEUTICS PLC
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022, 2021 and 2020
NOTE 1 – BASIS OF PRESENTATION AND BUSINESS OVERVIEW
Basis of Presentation
Unless otherwise indicated or the context otherwise requires, references to “Horizon”, the “Company”, “we”, “us” and “our” refer to Horizon Therapeutics plc and its consolidated subsidiaries.
Transaction agreement with Amgen Inc.
On December 12, 2022, the Company announced that it had entered into a transaction agreement with Amgen Inc. (“Amgen”) and Pillartree Limited (“Pillartree”), a wholly owned subsidiary of Amgen. Subject to the terms of the transaction agreement, Pillartree will acquire the Company (the “Transaction”), pursuant to a scheme of arrangement under Chapter 1 of Part 9 of the Companies Act 2014 of Ireland (the “Scheme”), or under certain circumstances, subject to the terms of the transaction agreement, a takeover offer (as such term is defined under the Irish Takeover Rules). As a result of the Scheme, the Company would become a wholly owned subsidiary of Amgen.
At the effective time of the Scheme (the “Effective Time”), holders of the Company’s ordinary shares will be entitled to receive $
F-8
On February 24, 2023, the Company’s shareholders approved the Scheme and certain scheme approval resolutions and amendments to the memorandum and articles of association of Horizon to enable the Scheme to be effected. The closing of the Transaction remains subject to customary closing conditions, including, among other things, (a) the sanction by the Irish High Court of the Scheme and delivery of the court order to the Irish Registrar of Companies, (b) the receipt of required antitrust clearance in the United States, and the absence of an order or law that prevents consummation of the Transaction or imposes a burdensome condition (as defined in the transaction agreement), (c) absence of any Material Adverse Effect (as defined in the transaction agreement) from December 12, 2022 to the Sanction Date (as defined in the transaction agreement) that is continuing as of the Sanction Date, (d) the accuracy of the other party’s representations and warranties subject to certain materiality and material adverse effect exceptions and (e) the performance by each party of all of its covenants and agreements under the transaction agreement in all material respects. On January 30, 2023, the Company and Amgen received a request for additional information and documentary materials, or a second request, from the Federal Trade Commission (“FTC”) in connection with the FTC’s review of the Transaction. The effect of the second request is to extend the waiting period imposed by the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, until 30 days after the Company and Amgen have substantially complied with the second request, unless the period is extended voluntarily by the parties or terminated sooner by the FTC. In connection with the Transaction, the Company and Amgen have received clearances or confirmation of non-applicability related to foreign direct investment in Denmark, Italy, Germany and France and clearances related to antitrust in Germany and Austria.
The Company expects the Transaction to close during the first half of 2023, subject to the regulatory clearances and other customary closing conditions described above and in the transaction agreement.
Acquired in-process research and development and milestones
Beginning with the third quarter of 2022, the Company separately presents upfront, milestone, and similar payments pursuant to collaborations, licenses of third-party technologies, and asset acquisitions as “Acquired in-process research and development and milestones” expenses in the consolidated statement of comprehensive income. Amounts recorded in this line item for the year ended December 31, 2022, would have historically been recorded to research and development (“R&D”) expenses. The Company believes the new classification assists users of the financial statements in better understanding the payments incurred to acquire in-process research and development (“IPR&D”). Prior period consolidated statements of comprehensive income have been reclassified to conform with the new classification. Refer to Note 2 for details on the Company’s R&D expenses and acquired IPR&D and milestones expenses accounting policies.
Business Overview
Horizon is a global biotechnology company focused on the discovery, development and commercialization of medicines that address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases. The Company’s pipeline is purposeful: it applies scientific expertise and courage to bring clinically meaningful therapies to patients. Horizon believes science and compassion must work together to transform lives. The Company’s commercial portfolio is currently composed of 12 medicines in the areas of rare diseases, gout, ophthalmology and inflammation.
As of December 31, 2022, the Company’s commercial portfolio consisted of the following medicines:
TEPEZZA® (teprotumumab-trbw), for intravenous infusion |
KRYSTEXXA® (pegloticase injection), for intravenous infusion |
RAVICTI® (glycerol phenylbutyrate) oral liquid |
PROCYSBI® (cysteamine bitartrate) delayed-release capsules and granules, for oral use |
UPLIZNA® (inebilizumab-cdon) injection, for intravenous use |
ACTIMMUNE® (interferon gamma-1b) injection, for subcutaneous use |
PENNSAID® (diclofenac sodium topical solution) 2% w/w (“PENNSAID 2%”), for topical use |
RAYOS® (prednisone) delayed-release tablets, for oral use |
BUPHENYL® (sodium phenylbutyrate) tablets and powder, for oral use |
DUEXIS® (ibuprofen/famotidine) tablets, for oral use |
VIMOVO® (naproxen/esomeprazole magnesium) delayed-release tablets, for oral use |
QUINSAIR™ (levofloxacin) solution for inhalation |
F-9
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with the accounting principles generally accepted in the United States of America (“GAAP”).
Principles of Consolidation
The consolidated financial statements include the Company’s accounts and those of its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Segment Information
Effective in the fourth quarter of 2022, management realigned the Company’s reportable segments to reflect changes in the manner in which the chief operating decision maker (“CODM”) assesses financial information for decision-making purposes. The Company transitioned its
Use of Estimates
The preparation of the accompanying consolidated financial statements in conformity with GAAP requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Foreign Currency Translation and Transactions
The reporting currency of the Company and its subsidiaries is the U.S. dollar.
The U.S. dollar is the functional currency for the Company’s Ireland and United States-based businesses and the majority of its subsidiaries. The Company has foreign subsidiaries that have the Euro and the Canadian Dollar as their functional currency. Foreign currency-denominated assets and liabilities of these subsidiaries are translated into U.S. dollars based on exchange rates prevailing at the end of the period, revenues and expenses are translated at average exchange rates prevailing during the corresponding period, and shareholders’ equity accounts are translated at historical exchange rates as of the date of any equity transaction. The effects of foreign exchange gains and losses arising from the translation of assets and liabilities of those entities where the functional currency is not the U.S. dollar are included as a component of accumulated other comprehensive income (loss) (“AOCI/L”).
Gains and losses resulting from foreign currency transactions are reflected within the Company’s results of operations.
F-10
Revenue Recognition
In the United States, the Company sells its medicines primarily to wholesale distributors and specialty pharmacy providers. In other countries, the Company sells its medicines primarily to wholesale distributors and other third-party distribution partners. These customers subsequently resell the Company’s medicines to health care providers and patients. In addition, the Company enters into arrangements with health care providers and payers that provide for government-mandated or privately negotiated discounts and allowances related to the Company’s medicines. Revenue is recognized when performance obligations under the terms of a contract with a customer are satisfied. The majority of the Company’s contracts have a single performance obligation to transfer medicines. Accordingly, revenues from medicine sales are recognized when the customer obtains control of the Company’s medicines, which occurs at a point in time, typically upon delivery to the customer. Revenue is measured as the amount of consideration the Company expects to receive in exchange for transferring medicines and is generally based upon a list or fixed price less allowances for medicine returns, rebates and discounts. The Company sells its medicines to wholesale pharmaceutical distributors and pharmacies under agreements with payment terms typically less than 90 days. Discounts, rebates, returns and certain other adjustments are accounted for as variable consideration.
Medicine Sales Discounts and Allowances
The nature of the Company’s contracts gives rise to variable consideration because of allowances for medicine returns, rebates and discounts. Allowances for medicine returns, rebates and discounts are recorded at the time of sale to wholesale pharmaceutical distributors and pharmacies. The Company applies significant judgments and estimates in determining some of these allowances. If actual results differ from its estimates, the Company will be required to make adjustments to these allowances in the future. The Company’s adjustments to gross sales are discussed further below.
Commercial Rebates
The Company participates in certain commercial rebate programs. Under these rebate programs, the Company pays a rebate to the commercial entity or third-party administrator of the program. The Company calculates accrued commercial rebate estimates using the expected value method. The Company accrues estimated rebates based on contract prices, estimated percentages of medicine that will be prescribed to qualified patients and estimated levels of inventory in the distribution channel and records the rebate as a reduction of revenue. Accrued commercial rebates are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
Distribution Service Fees
The Company includes distribution service fees paid to its wholesalers for distribution and inventory management services as a reduction to revenue. The Company calculates accrued distribution service fee estimates using the most likely amount method. The Company accrues estimated distribution fees based on contractually determined amounts, typically as a percentage of revenue. Accrued distribution service fees are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
Co-pay and Other Patient Assistance Programs
The Company offers discount card and other programs to patients under which the patient receives a discount on his or her prescription. In certain circumstances when a patient’s prescription is rejected by a managed care vendor, the Company will pay for the full cost of the prescription. The Company reimburses pharmacies for this discount through third-party vendors. The Company reduces gross sales by the amount of actual co-pay and other patient assistance in the period based on invoices received. The Company also records an accrual to reduce gross sales for estimated co-pay and other patient assistance on units sold to distributors that have not yet been prescribed/dispensed to a patient. The Company calculates accrued co-pay and other patient assistance costs using the expected value method. The estimate is based on contract prices, estimated percentages of medicine that will be prescribed to qualified patients, average assistance paid based on reporting from the third-party vendors and estimated levels of inventory in the distribution channel. Accrued co-pay and other patient assistance costs are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
F-11
Sales Returns
Consistent with industry practice, the Company maintains a return policy that allows customers to return certain medicines within a specified period prior to and subsequent to the medicine expiration date. Generally, medicines may be returned for a period beginning six months prior to its expiration date and up to one year after its expiration date. The right of return expires on the earlier of one year after the medicine expiration date or the time that the medicine is dispensed to the patient. The majority of medicine returns result from medicine dating, which falls within the range set by the Company’s policy, and are settled through the issuance of a credit to the customer. The Company calculates sales returns using the expected value method. The estimate of the provision for returns is based upon the Company’s historical experience with actual returns. The return period is known to the Company based on the shelf life of medicines at the time of shipment. The Company records sales returns in “accrued expenses and other current liabilities” and as a reduction of revenue.
Prompt Pay Discounts
As an incentive for prompt payment, the Company offers a
Government Rebates
The Company participates in certain government rebate programs such as Medicare Coverage Gap and Medicaid. The Company calculates accrued government rebate estimates using the expected value method. A significant portion of these accruals relates to the Company’s Medicaid rebates. The Company accrues estimated rebates based on estimated percentages of medicine prescribed to qualified patients, estimated rebate percentages and estimated levels of inventory in the distribution channel that will be prescribed to qualified patients and records the rebates as a reduction of revenue. Accrued government rebates are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
Chargebacks
The Company provides discounts to government qualified entities with whom the Company has contracted. These entities purchase medicines from the wholesale pharmaceutical distributors at a discounted price and the wholesale pharmaceutical distributors then charge back to the Company the difference between the current retail price and the contracted price that the entities paid for the medicines. The Company calculates accrued chargeback estimates using the expected value method. The Company accrues estimated chargebacks based on contract prices, sell-through sales data obtained from third-party information and estimated levels of inventory in the distribution channel and records the chargeback as a reduction of revenue. Accrued chargebacks are included in “accrued trade discounts and rebates” on the consolidated balance sheet.
Allowance for Credit Losses
The Company’s medicines are sold to wholesale pharmaceutical distributors and pharmacies. The Company monitors its accounts receivable balances to determine the impact, if any, of such factors as changes in customer concentration, credit risk and the realizability of its accounts receivable, and records an allowance for credit losses when applicable.
Inventories
Inventories are stated at the lower of cost or net realizable value, using the first-in, first-out convention. Inventories consist of raw materials, work-in-process and finished goods. The Company has entered into manufacturing and supply agreements for the manufacture or purchase of raw materials and production supplies. The Company’s inventories include the direct purchase cost of materials and supplies and manufacturing overhead costs. The Company reviews its inventory balance and purchase obligations to assess if it has obsolete or excess inventory and records a charge to “cost of goods sold” when applicable.
Inventories acquired in business combinations are recorded at their estimated fair values. “Step-up” represents the write-up of inventory from the lower of cost or net realizable value (the historical book value as previously recorded on the acquired company’s balance sheet) to fair market value at the acquisition date. Inventory step-up expense is recorded in the consolidated statement of comprehensive income based on actual sales, or usage, using the first-in, first-out convention.
Inventories exclude medicine sample inventory, which is included in other current assets and is expensed as a component of “selling, general and administrative” expense when shipped to sales representatives.
F-12
Cost of Goods Sold
The Company recognizes cost of goods sold in connection with its sales of each of its distributed medicines. Cost of goods sold includes all costs directly related to the acquisition of the Company’s medicines from its third-party manufacturers, including freight charges and other direct expenses such as insurance and supply chain costs. Cost of goods sold also includes amortization of intellectual property as described in the intangible assets accounting policy below, inventory step-up expense, share-based compensation, royalty payments to third parties and loss on inventory purchase commitments.
Pre-clinical Studies and Clinical Trial Accruals
The Company’s pre-clinical studies and clinical trials have historically been conducted by third-party contract research organizations and other vendors. Pre-clinical study and clinical trial expenses are based on the services received from these contract research organizations and vendors and are charged to R&D expense as incurred. Payments depend on factors such as the milestones accomplished, successful enrollment of certain numbers of patients and site initiation. In accruing service fees, the Company estimates the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, the Company adjusts the accrual accordingly.
Net Income Per Share
Basic net income per share is computed by dividing net income by the weighted-average number of ordinary shares outstanding during the period. Diluted net income per share reflects the potential dilution beyond shares for basic net income per share that could occur if securities or other contracts to issue ordinary shares were exercised, converted into ordinary shares, or resulted in the issuance of ordinary shares that would have shared in the Company’s earnings.
Cash and Cash Equivalents
The Company considers all highly liquid investments, readily convertible to cash, that mature within
Restricted Cash
Restricted cash consists primarily of balances in interest-bearing money market accounts required by a vendor for the Company’s sponsored employee business credit card program and collateral for a letter of credit.
Fair Value of Financial Instruments
The carrying amounts of the Company’s financial instruments, including cash and cash equivalents, restricted cash, accounts receivable, accounts payable and accrued expenses and other current liabilities, approximate their fair values due to their short maturities.
F-13
Derivative Instruments and Hedging Activities
All derivative instruments are recognized as either assets or liabilities at fair value on the consolidated balance sheets and are classified as current or non-current based on the scheduled maturity of the instrument. For derivatives designated as hedges, the Company assesses at inception and quarterly thereafter whether the hedging derivatives are highly effective in offsetting changes in the fair value or cash flows of the hedged item. The effective portions of changes in the fair value of a derivative designated as a cash flow hedge are reported in AOCI/L and are subsequently recognized in net income consistent with the underlying hedged item. If it is determined that a derivative is no longer highly effective as a hedge, the Company discontinues hedge accounting prospectively. If a hedged forecasted transaction becomes probable of not occurring, any gains or losses are reclassified from AOCI/L to net income. Derivatives that are not designated as hedges are adjusted to fair value through current net income.
Investments
Investments consist primarily of equity securities, bank time deposits, money market funds and U.S. federal government securities. Investments in publicly traded equity securities are reported at fair value determined using quoted market prices in active markets. Changes in the fair value of these investments are included in other (expense) income, net in the consolidated statement of comprehensive income.
Equity Method Investments
Investments in companies over which the Company has significant influence but not a controlling interest are accounted for using the equity method, with the share of earnings or losses reported in other (expense) income, net.
Concentration of Credit Risk and Other Risks and Uncertainties
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash, cash equivalents and investments. The Company’s investment policy permits investments in time deposits, U.S. federal government and federal agency securities, corporate bonds or commercial paper, money market instruments, certain qualifying money market mutual funds, certain repurchase agreements, and tax-exempt obligations of municipalities and places restrictions on credit ratings, maturities, and concentration by type and issuer. The Company is exposed to credit risk in the event of a default by the financial institutions holding the Company’s cash, cash equivalents and investments to the extent recorded on the balance sheet.
The purchase cost of TEPEZZA drug substance, TEPEZZA drug product with the Company’s second drug product manufacturer, Patheon Pharmaceuticals Inc. (“Patheon”) (the contract development and manufacturing services organization of Thermo Fisher Scientific), and ACTIMMUNE inventory are principally denominated in Euros and are subject to foreign currency risk. In addition, the Company is obligated to pay certain milestones and a royalty on sales of TEPEZZA to F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc. (together referred to as “Roche”) in Swiss Francs, which obligations are subject to foreign currency risk. The Company also incurs certain operating expenses in currencies other than the U.S. dollar in relation to its Irish operations and foreign subsidiaries. Therefore, the Company is subject to volatility in cash flows due to fluctuations in foreign currency exchange rates, particularly changes in the Euro and the Swiss Franc. In addition, the Company enters into forward currency contracts to hedge its foreign currency risk exposure.
Historically, the Company’s accounts receivable balances have been highly concentrated with a select number of customers consisting primarily of large wholesale pharmaceutical distributors who, in turn, sell the medicines to pharmacies, hospitals and other customers. As of each of December 31, 2022 and 2021, the Company’s top
The Company depends on single-source suppliers and manufacturers for certain of its medicines, medicine candidates and their active pharmaceutical ingredients.
F-14
Business Combinations
The Company accounts for business combinations in accordance with the guidance in Accounting Standards Codification Topic 805, Business Combinations (“ASC 805”) under which acquired assets and liabilities are measured at their respective estimated fair values as of the acquisition date. The Company may be required, as in the case of intangible assets, to determine the fair value associated with these amounts by estimating the fair value using an income approach under the discounted cash flow method, which may include revenue projections and other assumptions made by the Company to determine the fair value.
Provision for Income Taxes
The Company accounts for income taxes based upon an asset and liability approach. Deferred tax assets and liabilities represent the future tax consequences of the differences between the financial statement carrying amounts of assets and liabilities versus the tax basis of assets and liabilities. Under this method, deferred tax assets are recognized for deductible temporary differences, and operating loss and tax credit carryforwards. Deferred tax liabilities are recognized for taxable temporary differences. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Significant judgment is required in determining whether it is probable that sufficient future taxable income will be available against which a deferred tax asset can be utilized. In determining future taxable income, the Company is required to make assumptions including the amount of taxable income in the various jurisdictions in which the Company operates. These assumptions require significant judgment about forecasts of future taxable income. Actual operating results in future years could render the Company’s current assumption of recoverability of deferred tax assets inaccurate. The impact of tax rate changes on deferred tax assets and liabilities is recognized in the period that the change is enacted. From time to time, the Company executes intercompany transactions in response to changes in operations, regulations, tax laws, funding needs and other circumstances. These transactions require the interpretation and application of tax laws in the applicable jurisdiction to support the tax treatment taken. The valuations which support the tax treatment of the transactions require significant estimates and assumptions within discounted cash flow models. The Company also accounts for the uncertainty in income taxes by utilizing a comprehensive model for the recognition, measurement, presentation and disclosure in financial statements of any uncertain tax positions that have been taken or are expected to be taken on an income tax return. Deferred tax assets and deferred tax liabilities are netted by each tax-paying entity within each jurisdiction on the Company’s consolidated balance sheets.
Property, Plant and Equipment
Land is stated at cost. Property, plant and equipment, other than land, are stated at cost less accumulated depreciation. Depreciation is recognized using the straight-line method over the estimated useful lives of the related assets for financial reporting purposes and an accelerated method for income tax reporting purposes. Upon retirement or sale of an asset, the cost and related accumulated depreciation and amortization are removed from the balance sheet and the resulting gain or loss is reflected in operations. Repair and maintenance costs are charged to expenses as incurred and improvements are capitalized.
Leasehold improvements are amortized on a straight-line basis over the term of the applicable lease, or the useful life of the assets, whichever is shorter.
Depreciation and amortization periods for the Company’s property, plant and equipment are as follows:
Buildings |
|
|
Land improvements |
|
|
Machinery and equipment |
|
|
Furniture and fixtures |
|
|
Computer equipment |
|
|
Software |
|
|
Trade show equipment |
|
The Company capitalizes software development costs associated with internal use software, including external direct costs of materials and services and payroll costs for employees devoting time to a software project. Costs incurred during the preliminary project stage, as well as costs for maintenance and training, are expensed as incurred.
Software includes internal-use software acquired and modified to meet the Company’s internal requirements. Amortization commences when the software is ready for its intended use.
F-15
Intangible Assets
Definite-lived intangible assets are amortized over their estimated useful lives. The Company reviews its intangible assets when events or circumstances may indicate that the carrying value of these assets is not recoverable and exceeds their fair value. The Company measures fair value based on the estimated future discounted cash flows associated with these assets in addition to other assumptions and projections that the Company deems to be reasonable and supportable. The estimated useful lives, from the date of acquisition, for all identified intangible assets that are subject to amortization are between and
Indefinite-lived intangible assets consist of capitalized IPR&D. IPR&D assets represent capitalized incomplete research and development projects that the Company acquired through business combinations. Such assets are initially measured at their acquisition date fair values and are tested for impairment, until completion or abandonment of R&D efforts associated with the projects. An IPR&D asset is considered abandoned when R&D efforts associated with the asset have ceased, and there are no plans to sell or license the asset or derive value from the asset. At that point, the asset is considered to be impaired and is written off. Upon successful completion of each project, the Company will make a determination about the then remaining useful life of the intangible asset and begin amortization. The Company tests its indefinite-lived intangibles, including IPR&D assets, for impairment annually during the fourth quarter and more frequently if events or changes in circumstances indicate that it is more likely than not that the asset is impaired.
Goodwill
Goodwill represents the excess of the purchase price of acquired businesses over the estimated fair value of the identifiable net assets acquired. Goodwill is not amortized but is tested for impairment at least annually at the reporting unit level or more frequently if events or changes in circumstances indicate that the asset might be impaired. Impairment loss, if any, is recognized based on a comparison of the fair value of the asset to its carrying value, without consideration of any recoverability. The Company tests goodwill for impairment annually during the fourth quarter and whenever indicators of impairment exist by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If the Company concludes it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative impairment test is performed. If the Company concludes that goodwill is impaired, it will record an impairment charge in its consolidated statement of comprehensive income.
R&D Expenses
R&D expenses include, but are not limited to, payroll and other personnel expenses, consultant expenses, expenses incurred under agreements with contract research organizations to conduct clinical trials and expenses incurred to manufacture clinical trial materials. When milestone payments are due to third parties under R&D agreements assumed as part of business acquisitions, prior to regulatory approval, the payment obligations are recorded as R&D expenses when the milestone results are achieved. R&D expenses were $
Acquired IPR&D and Milestones Expenses
The initial costs of rights to IPR&D projects acquired in an asset acquisition are expensed as acquired IPR&D unless the project has an alternative future use. The Company also enters into collaborative agreements with third parties to develop and commercialize medicine candidates. Collaborative activities may include joint R&D and commercialization of new medicines. The Company generally receives certain licensing rights under these arrangements. Upfront payments associated with collaborative arrangements during the development stage are recorded as acquired IPR&D and milestones expenses in the consolidated statement of comprehensive income. Subsequent payments made to the partner in connection with milestones during the development stage are recorded as acquired IPR&D and milestones expenses in the consolidated statement of comprehensive income when the milestone is achieved. Acquired IPR&D and milestones expenses were $
F-16
Advertising Expenses
The Company expenses the costs of advertising as incurred. Advertising expenses were $
Deferred Financing Costs
Costs incurred in connection with debt financings have been capitalized to “Long-term debt, net” in the Company’s consolidated balance sheets as deferred financing costs, and are charged to interest expense using the effective interest method over the terms of the related debt agreements. These costs include document preparation costs, commissions, fees and expenses of investment bankers and underwriters, and accounting and legal fees.
Comprehensive Income
Comprehensive income is composed of net income and other comprehensive income (loss) (“OCI/L”). OCI/L includes certain changes in shareholders’ equity that are excluded from net income, which consist of interest rate swap contracts designated as cash flow hedges, foreign currency translation adjustments and pension and other post-employment benefit plan remeasurements. The Company reports the effect of significant reclassifications out of accumulated OCI/L on the respective line items in net income if the amount being reclassified is required under GAAP to be reclassified in its entirety to net income.
Share-Based Compensation
The Company accounts for employee share-based compensation by measuring and recognizing compensation expense for all share-based payments based on estimated grant date fair values. The Company uses the straight-line method to allocate compensation cost to reporting periods over each awardee’s requisite service period, which is generally the vesting period. Forfeitures are estimated based on historical experience at the time of grant and revised in subsequent periods if actual forfeitures differ from those estimates.
Post-employment Benefits
The Company records annual expenses relating to its defined benefit U.S. retiree medical plan based on calculations which utilize various actuarial assumptions, including discount rates, health care cost trend rates, turnover rates, and retirement rates. The Company reviews its actuarial assumptions on an annual basis and makes modifications to the assumptions based on current rates and trends. Prior service costs and credits from plan amendments, including initiation of a plan, are deferred in AOCI/L, net of tax and amortized at an equal amount in each remaining year of service until the full eligibility date of employees active as of the amendment date. Actuarial gains and losses are deferred in AOCI/L, net of tax and amortized over the remaining service attribution periods of the employees under the corridor method.
Royalties
The Company records royalty expense based on each periods’ net sales as part of cost of goods sold.
Leases
For leases with terms greater than 12 months, the Company records the related asset and obligation at the present value of lease payments over the term. The right-of-use lease asset represents the right to use the leased asset for the lease term. The lease liability represents the present value of the lease payments under the lease.
F-17
The right-of-use lease asset is initially measured at cost, which primarily comprises the initial amount of the lease liability, plus any initial direct costs incurred. All right-of-use lease assets are reviewed for impairment. The lease liability is initially measured at the present value of the lease payments, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, the Company’s secured incremental borrowing rate for the same term as the underlying lease.
The Company identified and assessed the following significant assumptions in recognizing the right-of-use lease assets and corresponding liabilities.
Expected lease term – The expected lease term includes both contractual lease periods and, when applicable, cancelable option periods. When determining the lease term, the Company includes options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option.
Incremental borrowing rate – As the Company’s leases do not provide an implicit rate, the Company obtained the incremental borrowing rate (“IBR”) based on the remaining term of each lease. The IBR is the rate of interest that a lessee would have to pay to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment.
The Company has elected not to recognize right-of-use lease assets and lease liabilities for short-term leases that have a term of 12 months or less.
The Company reports right-of-use lease assets within non-current “Other long-term assets” in its consolidated balance sheet. The Company reports the current portion of lease liabilities within “Accrued expenses and other current liabilities” and long-term lease liabilities within “Other long-term liabilities” in its consolidated balance sheet.
Contingencies
From time to time, the Company may become involved in claims and other legal matters arising in the ordinary course of business. The Company records accruals for loss contingencies to the extent that it concludes that it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. Legal fees and other expenses related to litigation are expensed as incurred and included in “selling, general and administrative” expenses.
Recent Accounting Pronouncements
From time to time, the Company adopts new accounting pronouncements issued by the Financial Accounting Standards Board (“FASB”) or other standard-setting bodies.
In March 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-04, Reference Rate Reform (Topic 848), which provides expedients and exceptions for accounting treatment of contracts which are affected by the anticipated discontinuation of the London Inter-Bank Offered Rate (“LIBOR”) and other rates resulting from rate reform that are entered into on or before December 31, 2022. In December 2022, the FASB issued ASU 2022-06 which defers the sunset date of the guidance included in Topic 848 from December 31, 2022 to December 31, 2024. The Company does not expect the planned discontinuation of LIBOR to have a material impact on interest payments incurred under the Credit Agreement (as defined below). Refer to Note 13 for further details. The discontinuation of LIBOR is expected to occur as of June 30, 2023. In the second quarter of 2022, the Company elected to apply the optional expedients for the assessment of hedge effectiveness for cash flow hedges affected by reference rate reform.
Recent authoritative guidance issued by the FASB (including technical corrections to the Accounting Standards Codification (“ASC”)), the American Institute of Certified Public Accountants and the Securities and Exchange Commission did not, or are not expected to, have a material impact on the Company’s consolidated financial statements and related disclosures.
F-18
NOTE 3 – NET INCOME PER SHARE
The following table presents basic and diluted net income per share for the years ended December 31, 2022, 2021 and 2020 (in thousands, except share and per share data):
|
|
For the Years Ended December 31, |
|
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|
|||
Basic net income per share calculation: |
|
|
|
|
|
|
|
|
|
|
|||
Numerator - net income |
|
$ |
|
|
$ |
|
|
$ |
|
|
|||
Denominator - weighted average of ordinary shares outstanding |
|
|
|
|
|
|
|
|
|
|
|||
Basic net income per share |
|
$ |
|
|
$ |
|
|
$ |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
For the Years Ended December 31, |
|
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|
|||
Diluted net income per share calculation: |
|
|
|
|
|
|
|
|
|
|
|||
Numerator - net income |
|
$ |
|
|
$ |
|
|
$ |
|
|
|||
Denominator - weighted average of ordinary shares outstanding |
|
|
|
|
|
|
|
|
|
|
|||
Diluted net income per share |
|
$ |
|
|
$ |
|
|
$ |
|
|
|||
Basic net income per share is computed by dividing net income by the weighted-average number of ordinary shares outstanding during the period. Diluted net income per share reflects the potential dilution that could occur if securities or other contracts to issue ordinary shares were exercised, converted into ordinary shares, or resulted in the issuance of ordinary shares that would have shared in the Company’s earnings.
During the years ended December 31, 2022, 2021 and 2020, the difference between the basic and diluted weighted average ordinary shares outstanding primarily represents the effect of incremental shares from the Company’s share-based compensation programs.
The outstanding securities listed in the table below were excluded from the computation of diluted net income per ordinary share for the years ended December 31, 2022, 2021 and 2020 due to being anti-dilutive:
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Stock options |
|
|
|
|
|
|
|
|
|
|||
Restricted stock units |
|
|
|
|
|
|
|
|
|
|||
Performance stock units |
|
|
|
|
|
|
|
|
|
|||
Employee share purchase plan shares |
|
|
|
|
|
|
|
|
|
|||
|
|
— |
|
|
|
— |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|||
Beginning in the fourth quarter of 2019, with the Company’s ordinary share price significantly above the $
F-19
NOTE 4 –ACQUISITIONS, DIVESTITURES AND OTHER ARRANGEMENTS
Acquisition of drug product biologics manufacturing facility
In July 2021, the Company completed the purchase of a drug product biologics manufacturing facility from EirGen Pharma Limited (“EirGen”), a subsidiary of OPKO Health, Inc. in Waterford, Ireland for $
The following table summarizes fair values of assets acquired as of the acquisition date (in thousands):
Construction in process |
|
$ |
|
|
Buildings |
|
|
|
|
Furniture and fixtures |
|
|
|
|
Definite-lived intangible assets |
|
|
|
|
Other |
|
|
|
|
Total consideration |
|
$ |
|
Acquisition of Viela Bio, Inc.
On
The total consideration for the acquisition was approximately $
Equity value ( |
|
$ |
|
|
Net settlements on the exercise of stock options |
|
|
|
|
Consideration for exchange of Viela stock options |
|
|
|
|
Total consideration |
|
$ |
|
During the year ended December 31, 2021, the Company incurred $
Pursuant to ASC 805, the Company accounted for the Viela acquisition as a business combination using the acquisition method of accounting. Identifiable assets and liabilities of Viela, including identifiable intangible assets, were recorded based on their estimated fair values as of the date of the closing of the acquisition. The excess of the purchase price over the fair value of the net assets acquired was recorded as goodwill. While all amounts were subject to adjustments, the areas subject to the most significant potential adjustments were inventory, intangible assets, IPR&D assets and deferred income taxes. As a result, the Company recorded preliminary estimates for the fair value of assets acquired and liabilities assumed as of the acquisition date. Such preliminary valuation required estimates and assumptions including, but not limited to, estimating future cash flows and direct costs in addition to developing the appropriate discount rates and current market profit margins. The Company’s management believes the fair values recognized for the assets acquired and the liabilities assumed were based on reasonable estimates and assumptions.
F-20
During the year ended December 31, 2021, the Company recorded measurement period adjustments related to deferred tax liabilities, accrued expenses and other current liabilities, accrued trade discounts and rebates, accounts receivable, prepaid expenses and other current assets and inventory, which resulted in a net reduction in goodwill of $
The following table summarizes the final values assigned to the assets acquired and the liabilities assumed by the Company along with the resulting goodwill before and after the measurement period adjustments (in thousands):
|
|
Before |
|
Adjustments |
|
After |
|
|
|||
Deferred tax liabilities, net |
|
$ |
( |
) |
$ |
|
$ |
( |
) |
|
|
Accrued expenses and other current liabilities |
|
|
( |
) |
|
( |
) |
|
( |
) |
|
Other long-term liabilities |
|
|
( |
) |
|
— |
|
|
( |
) |
|
Accounts payable |
|
|
( |
) |
|
— |
|
|
( |
) |
|
Accrued trade discounts and rebates |
|
|
( |
) |
|
( |
) |
|
( |
) |
|
Marketable securities |
|
|
|
|
— |
|
|
|
|
||
Property, plant and equipment |
|
|
|
|
— |
|
|
|
|
||
Other long-term assets |
|
|
|
|
|
|
|
|
|||
Accounts receivable |
|
|
|
|
( |
) |
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
|
|
|||
Inventories |
|
|
|
|
|
|
|
|
|||
Cash and cash equivalents |
|
|
|
|
— |
|
|
|
|
||
In-process research and development |
|
|
|
|
— |
|
|
|
|
||
Developed technology |
|
|
|
|
— |
|
|
|
|
||
(Liabilities assumed) and assets acquired |
|
|
|
|
|
|
|
|
|||
Goodwill |
|
|
|
|
( |
) |
|
|
|
||
Fair value of consideration paid |
|
$ |
|
$ |
— |
|
$ |
|
|
||
Inventories acquired included raw materials, work in process and finished goods for UPLIZNA. Inventories were recorded at their estimated fair values. The fair value of finished goods was determined based on the estimated selling price, net of selling costs and a margin on the selling activities. The fair value of work in process was determined based on estimated selling price, net of selling costs and costs to complete the manufacturing, and a margin on the selling and manufacturing activities. The fair value of raw materials was estimated to equal the replacement cost. A step-up in the value of inventory of $
Other tangible assets and liabilities were valued at their respective carrying amounts as management believes that these amounts approximated their acquisition-date fair values.
Developed technology as of the acquisition date was an intangible asset that reflected the estimated fair value of the rights to UPLIZNA in the United States. The estimated fair values of the developed technology represent valuations performed with the assistance of an independent appraisal firm based on management’s estimates, forecasted financial information and reasonable and supportable assumptions. The fair value of developed technology was determined using an income approach. The income approach explicitly recognizes that the fair value of an asset is premised upon the expected receipt of future economic benefits such as earnings and cash inflows based on current sales projections and estimated direct costs for UPLIZNA. Indications of value were developed by discounting these benefits to their acquisition-date fair value at a discount rate of
Some of the most significant assumptions inherent in the development of the asset valuation include the estimated net cash flows for each year (including net sales, cost of goods sold, sales and marketing costs and R&D costs) and the discount rate. The fair value of the UPLIZNA developed technology was capitalized as of the Viela acquisition date and is subsequently being amortized over approximately
F-21
IPR&D was related to R&D projects including:
Each IPR&D asset is considered separable from the business as each project could be sold to a third party. The fair value of each IPR&D asset was determined using an income approach. The income approach explicitly recognizes that the fair value of an asset is premised upon the expected receipt of future economic benefits such as earnings and cash inflows based on sales projections and estimated direct costs. Indications of value are developed by discounting these benefits to their present value at a discount rate of
Deferred tax assets and liabilities arise from acquisition accounting adjustments where book values of certain assets and liabilities differ from their tax bases. Deferred tax assets and liabilities are recorded at the currently enacted rates which will be in effect at the time when the temporary differences are expected to reverse in the country where the underlying assets and liabilities are located. The developed technology, IPR&D assets and inventory acquired through the Viela acquisition were located in the United States as of the acquisition date, where a U.S. tax rate of
Goodwill represents the excess of the total consideration over the estimated fair value of net assets acquired and was recorded in the consolidated balance sheet as of the acquisition date. The goodwill was primarily attributable to the establishment of a deferred tax liability for the developed technology intangible asset and the IPR&D intangible assets. Viela’s mid-stage biologics pipeline, R&D team and on-market medicine UPLIZNA, made it a complementary strategic fit with the Company’s pipeline, commercial portfolio and therapeutic areas of focus. The Company does not expect any portion of this goodwill to be deductible for tax purposes.
The following table presents certain pro forma combined results of the Company and Viela for the years ended December 31, 2021 and 2020 as if the acquisition of Viela had occurred on January 1, 2020 (in thousands):
|
|
For the Year Ended December 31, |
|
|||||||||||||||||||||
|
|
2021 |
|
|
2020 |
|
||||||||||||||||||
|
|
As reported |
|
|
Pro forma adjustments |
|
|
Pro forma |
|
|
As reported |
|
|
Pro forma adjustments |
|
|
Pro forma |
|
||||||
Net sales |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||
Net income |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
F-22
The pro forma combined financial information was prepared using the acquisition method of accounting and was based on the historical financial information of the Company and Viela. In order to reflect the pro forma information as if the acquisition occurred on January 1, 2020, the pro forma financial information includes adjustments to reflect incremental amortization expense to be incurred based on the current fair values of the identifiable intangible assets acquired; the incremental cost of medicines sold related to the fair value adjustments associated with acquisition date inventory; the additional interest expense associated with the issuance of debt to finance the acquisition; and the reclassification of transaction costs incurred during the year ended December 31, 2021 to the year ended December 31, 2020. Significant non-recurring pro forma adjustments include transaction costs of $
Acquisition of Curzion Pharmaceuticals, Inc.
On April 1, 2020, the Company acquired Curzion Pharmaceuticals, Inc. (“Curzion”), a privately held development-stage biopharma company, and its development-stage oral selective lysophosphatidic acid 1 receptor (LPAR1) antagonist, CZN001 (renamed HZN-825).
Under the terms of the acquisition agreement, the Company acquired Curzion for a $
Sale of RAVICTI and BUPHENYL Rights in Japan
On October 27, 2020, the Company sold its rights to develop and commercialize RAVICTI and BUPHENYL in Japan to Medical Need Europe AB, part of the Immedica Group, for $
Acquisition of River Vision
On
Under the acquisition agreement for River Vision, the Company agreed to pay up to $
F-23
Additionally, under the Company’s license agreement with Roche, the Company made a milestone payment of CHF
In April 2020, a subsidiary of the Company entered into an agreement with S.R. One, Limited (“S.R. One”) and an agreement with Lundbeckfond Invest A/S (“Lundbeckfond”) pursuant to which the Company acquired all of S.R. One’s and Lundbeckfond’s beneficial rights to proceeds from certain contingent future TEPEZZA milestone and royalty payments in exchange for a one-time payment of $
In addition, during the year ended December 31, 2020, the Company recorded $
Refer to Note 16 for further detail on TEPEZZA milestone payments.
Other Arrangements
Xeris Pharmaceuticals, Inc.
On November 22, 2022, the Company entered into a research collaboration and option agreement with Xeris Pharmaceuticals, Inc. (“Xeris”) under which Xeris is obligated to use its proprietary formulation technology platform, XeriJect, to conduct a research program to develop an ultra-concentrated, ready-to-use, subcutaneous injection of TEPEZZA. The Company received an option to obtain a commercial license for any reformulated product developed under the research program. An upfront payment of $
Q32 Bio Inc.
On August 12, 2022, the Company entered into a collaboration and option agreement with Q32 Bio Inc. (“Q32”) related to its pipeline candidate ADX-914, a monoclonal antibody antagonist of the interleukin-7 receptor for the treatment of autoimmune and inflammatory diseases. Under the terms of the agreement, the Company received an option to acquire the ADX-914 program, exercisable through a period of time following completion of certain planned Phase 2a trials. An upfront payment of $
F-24
Alpine Immune Sciences, Inc.
On December 15, 2021, the Company entered into an exclusive license agreement with Alpine Immune Sciences, Inc. (“Alpine”) for the development and commercialization of up to four preclinical candidates generated from Alpine’s unique discovery platform. The agreement includes licensing of a lead, potential first-in-class preclinical candidate, as well as a research partnership to jointly generate additional novel candidates. These candidates include multi-specific fusion protein-based therapeutic candidates for autoimmune and inflammatory diseases.
In connection with the execution of the license agreement, the Company entered into a stock purchase agreement with Alpine to purchase a minority stake of
In addition, Alpine is eligible to receive up to $
Arrowhead Pharmaceuticals, Inc.
On June 18, 2021, the Company entered into a global agreement with Arrowhead Pharmaceuticals, Inc. (“Arrowhead”) for HZN-457, a discovery-stage investigational RNA interference (“RNAi”) therapeutic being developed by Arrowhead as a potential treatment for uncontrolled gout. Arrowhead granted the Company a worldwide exclusive license to develop, manufacture and commercialize medicines based on the RNAi therapeutic. Arrowhead is required to use commercially reasonable efforts to conduct research and preclinical development activities for the RNAi therapeutic products. The Company must use commercially reasonable efforts in, and will be responsible for, clinical development and commercialization of the RNAi therapeutic products. Under the terms of the agreement, the Company paid Arrowhead an upfront cash payment of $
Halozyme Therapeutics, Inc.
On November 21, 2020, the Company entered into a global agreement with Halozyme Therapeutics, Inc. (“Halozyme”) that gives the Company exclusive access to Halozyme’s ENHANZE® drug delivery technology for subcutaneous (“SC”) formulation of medicines targeting IGF-1R. The Company is exploring ENHANZE to develop a SC formulation of TEPEZZA, indicated for the treatment of thyroid eye disease, a serious, progressive and vision-threatening rare autoimmune disease, potentially shortening drug administration time, reducing healthcare practitioner time and offering additional flexibility and convenience for patients. Under the terms of the agreement, the Company paid Halozyme an upfront cash payment of $
F-25
NOTE 5 – INVENTORIES
The components of inventories as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Raw materials |
|
$ |
|
|
$ |
|
||
Work-in-process |
|
|
|
|
|
|
||
Finished goods |
|
|
|
|
|
|
||
Inventories, net |
|
$ |
|
|
$ |
|
||
During the year ended December 31, 2021, as part of the Viela acquisition, a step-up in the value of inventory of $
Because inventory step-up expense is related to an acquisition, will not continue indefinitely and has a significant effect on the Company’s gross profit, gross margin percentage and net income for all affected periods, the Company discloses balance sheet and income statement amounts related to inventory step-up within the Notes to Consolidated Financial Statements.
NOTE 6 – PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Deferred charge for taxes on intercompany profit |
|
$ |
|
|
$ |
|
||
Advance payments for inventory |
|
|
|
|
|
|
||
Rabbi trust assets |
|
|
|
|
|
|
||
Prepaid income taxes and income tax receivable |
|
|
|
|
|
|
||
Other prepaid expenses and other current assets |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
$ |
|
|
$ |
|
||
Deferred charge for taxes on intercompany profit increased $
Advance payments for inventory as of December 31, 2022 and 2021, primarily represented payments made to the contract manufacturer of TEPEZZA drug substance.
F-26
NOTE 7 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Buildings |
|
$ |
|
|
$ |
|
||
Construction in process |
|
|
|
|
|
|
||
Land and land improvements |
|
|
|
|
|
|
||
Leasehold improvements |
|
|
|
|
|
|
||
Machinery and equipment |
|
|
|
|
|
|
||
Furniture and fixtures |
|
|
|
|
|
|
||
Software |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Less accumulated depreciation |
|
|
( |
) |
|
|
( |
) |
Property, plant and equipment, net |
|
$ |
|
|
$ |
|
||
Depreciation expense for the years ended December 31, 2022, 2021 and 2020 was $
NOTE 8 – GOODWILL AND INTANGIBLE ASSETS
Goodwill
The table below presents goodwill for the Company as of December 31, 2022 (in thousands):
|
|
Total |
|
|
Balance at December 31, 2021 |
|
$ |
|
|
Goodwill impairment during the year |
|
|
( |
) |
Balance at December 31, 2022 |
|
$ |
|
|
In May 2022, Apotex Corp. and its affiliate, Apotex Inc. (collectively, “Apotex”), initiated an at-risk launch of a generic version of PENNSAID 2% in the United States. The at-risk launch was expected to have an on-going negative impact on PENNSAID 2% net sales. As a result, the Company determined the generic product launch and the expected impact on PENNSAID 2% to be a triggering event to conduct an interim impairment analysis and an indicator it was more likely than not that the carrying amount of its former inflammation reporting unit exceeded its fair value as of June 30, 2022.
The Company determined the fair value of the former inflammation reporting unit as of June 30, 2022 using the income approach. The cash flow projections were based on a financial forecast developed by management that included net sales projections, which are updated annually, or more frequently based on events that may significantly impact forecasts.
The Company’s interim goodwill impairment test in the second quarter of 2022 indicated an impairment, which represented the difference between the estimated fair value of the former inflammation reporting unit and its carrying value. As a result, the Company recognized an impairment charge of $
The Company’s annual goodwill impairment test in the fourth quarter of 2022 did not indicate that it was more likely than not that the fair value of the reporting unit was less than the carrying value.
During the fourth quarter of 2022, management realigned the Company’s reportable segments to reflect changes in the manner in which the CODM assesses financial information for decision-making purposes. The Company transitioned its two reportable segments, the inflammation segment and the orphan segment, to one reportable segment for the year ended December 31, 2022. Refer to Note 11 for further details.
F-27
Intangible Assets
As of December 31, 2022, the Company’s finite-lived intangible assets consisted of developed technology related to ACTIMMUNE, KRYSTEXXA, PROCYSBI, RAVICTI, TEPEZZA and UPLIZNA. The intangible assets related to RAYOS developed technology and ACTIMMUNE customer relationships were fully amortized as of December 31, 2022.
In July 2021, in connection with the purchase of a drug product biologics manufacturing facility from EirGen, the Company capitalized $
On March 15, 2021, in connection with the acquisition of Viela, the Company capitalized $
Intangible assets as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
As of December 31, |
|
|||||||||||||||||||||
|
|
2022 |
|
|
2021 |
|
||||||||||||||||||
|
|
Cost Basis |
|
|
Accumulated |
|
|
Net Book |
|
|
Cost Basis |
|
|
Accumulated |
|
|
Net Book |
|
||||||
Developed technology (1) |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||||
In-process research and development (1) |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
||||
Other intangibles |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Total intangible assets |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||||
Amortization expense for the years ended December 31, 2022, 2021 and 2020 was $
2023 |
|
$ |
|
|
2024 |
|
|
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
Thereafter |
|
|
|
|
Total |
|
$ |
|
F-28
NOTE 9 – ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accrued expenses and other current liabilities as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Payroll-related expenses |
|
$ |
|
|
$ |
|
||
Accrued royalties |
|
|
|
|
|
|
||
R&D and manufacturing programs |
|
|
|
|
|
|
||
Consulting and professional services |
|
|
|
|
|
|
||
Allowances for returns |
|
|
|
|
|
|
||
Accrued interest |
|
|
|
|
|
|
||
Accrued upfront and milestone payments |
|
|
|
|
|
|
||
Refund liability (1) |
|
|
|
|
|
|
||
Advertising and marketing |
|
|
|
|
|
|
||
Pricing review liability |
|
|
— |
|
|
|
|
|
Accrued other |
|
|
|
|
|
|
||
Accrued expenses and other current liabilities |
|
$ |
|
|
$ |
|
||
Refund liability at December 31, 2021 |
$ |
|
|
Shipments during the year ended December 31, 2022 |
|
|
|
Remeasurement of refund liability recognized as revenue |
|
( |
) |
Refund liability at December 31, 2022 |
$ |
|
|
Less: current portion |
|
|
|
Refund liability, net of current portion |
$ |
|
F-29
NOTE 10 – ACCRUED TRADE DISCOUNTS AND REBATES
Accrued trade discounts and rebates as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Accrued government rebates and chargebacks |
|
$ |
|
|
$ |
|
||
Accrued commercial rebates and wholesaler fees |
|
|
|
|
|
|
||
Accrued co-pay and other patient assistance |
|
|
|
|
|
|
||
Accrued trade discounts and rebates |
|
$ |
|
|
$ |
|
||
Invoiced commercial rebates and wholesaler fees, |
|
|
|
|
|
— |
|
|
Total customer-related accruals and allowances |
|
$ |
|
|
$ |
|
||
The following table summarizes changes in the Company’s customer-related accruals and allowances during the years ended December 31, 2022 and 2021 (in thousands):
|
|
Government Rebates and Chargebacks |
|
|
Commercial Rebates and Wholesaler Fees |
|
|
Co-Pay and Other Patient Assistance |
|
|
Total |
|
||||
Balance at December 31, 2020 |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Current provisions relating to sales during the year ended December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Adjustments relating to prior-year sales |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payments relating to sales during the year ended |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payments relating to prior-year sales |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Viela acquisition on March 15, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Balance at December 31, 2021 |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Current provisions relating to sales during the year ended December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Adjustments relating to prior-year sales |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Payments relating to sales during the year ended |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payments relating to prior-year sales |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Balance at December 31, 2022 |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
F-30
NOTE 11 – SEGMENT AND OTHER INFORMATION
The Company substantially completed the wind down of its former inflammation business in the fourth quarter of 2022. Effective in the fourth quarter of 2022, management realigned the Company’s reportable segments to reflect changes in the manner in which the CODM assesses financial information for decision-making purposes. The Company transitioned the Company’s
The Company determined that it operates in one reportable segment, which focuses on the discovery, development and commercialization of medicines that address critical needs for people impacted by rare, autoimmune and severe inflammatory diseases. The Company’s operating segment is reported in a manner consistent with the internal reporting provided to the CODM. The Company’s chief executive officer has been identified as its CODM.
On March 15, 2021, the Company completed its acquisition of Viela. The acquisition expanded the Company’s commercial medicine portfolio by adding an additional rare disease medicine, UPLIZNA, to its commercial medicine portfolio.
The following table reflects net sales by medicine for the years ended December 31, 2022, 2021 and 2020 (in thousands):
|
|
Year Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
TEPEZZA |
|
$ |
|
|
$ |
|
|
$ |
|
|||
KRYSTEXXA |
|
|
|
|
|
|
|
|
|
|||
RAVICTI |
|
|
|
|
|
|
|
|
|
|||
PROCYSBI |
|
|
|
|
|
|
|
|
|
|||
UPLIZNA (1) |
|
|
|
|
|
|
|
— |
|
|||
ACTIMMUNE |
|
|
|
|
|
|
|
|
|
|||
PENNSAID 2% |
|
|
|
|
|
|
|
|
|
|||
RAYOS |
|
|
|
|
|
|
|
|
|
|||
BUPHENYL |
|
|
|
|
|
|
|
|
|
|||
DUEXIS |
|
|
|
|
|
|
|
|
|
|||
VIMOVO |
|
|
|
|
|
|
|
|
|
|||
QUINSAIR |
|
|
|
|
|
|
|
|
|
|||
Total net sales |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The following table presents the amount and percentage of gross sales to customers that represented more than
|
|
Year Ended December 31, |
||||||||||||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
||||||||||||
|
|
Amount |
|
|
% of Gross Sales |
|
|
Amount |
|
|
% of Gross Sales |
|
|
Amount |
|
|
% of Gross Sales |
|||
Customer A |
|
$ |
|
|
|
|
$ |
|
|
|
|
$ |
|
|
||||||
Customer B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Customer C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Customer D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Other customers |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Gross sales |
|
$ |
|
|
|
|
$ |
|
|
|
|
$ |
|
|
||||||
F-31
Geographic revenues are determined based on the country in which the Company’s customers are located. The following table presents a summary of net sales attributed to geographic sources for the years ended December 31, 2022, 2021 and 2020 (in thousands, except percentages):
|
|
Year Ended December 31, 2022 |
|
Year Ended December 31, 2021 |
|
Year Ended December 31, 2020 |
||||||||||||
|
|
Amount |
|
|
% of Total |
|
Amount |
|
|
% of Total |
|
Amount |
|
|
% of Total |
|||
United States |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
||||||
Rest of world |
|
|
|
|
|
|
|
|
* |
|
|
|
|
* |
||||
|
|
$ |
|
|
|
|
$ |
|
|
|
|
$ |
|
|
|
|||
*Less than 1% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
The following table presents total tangible long-lived assets by location as of the years ended December 31, 2022, 2021 and 2020 (in thousands):
|
|
As of December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
United States |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Ireland |
|
|
|
|
|
|
|
|
|
|||
Other |
|
|
|
|
|
|
|
|
|
|||
Total long-lived assets (1) |
|
$ |
|
|
$ |
|
|
$ |
|
|||
F-32
NOTE 12 – FAIR VALUE MEASUREMENTS
The following tables and paragraphs set forth the Company’s financial instruments that are measured at fair value on a recurring basis within the fair value hierarchy. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability. The following describes three levels of inputs that may be used to measure fair value:
Level 1—Observable inputs such as quoted prices in active markets for identical assets or liabilities;
Level 2—Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Assets and liabilities measured at fair value on a recurring basis
The following tables set forth the Company’s financial assets and liabilities at fair value on a recurring basis as of December 31, 2022 and 2021 (in thousands):
|
|
December 31, 2022 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Interest rate swap contracts |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Equity securities (1) |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Foreign currency contracts |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Other current assets |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Total assets at fair value |
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
|
|||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other long-term liabilities |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Total liabilities at fair value |
|
$ |
( |
) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
( |
) |
|
|
December 31, 2021 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Equity securities (1) |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Bank time deposits |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Other current assets |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Total assets at fair value |
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
|
|||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other long-term liabilities |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Total liabilities at fair value |
|
$ |
( |
) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
( |
) |
F-33
The Company utilizes the market approach to measure fair value for its money market funds. The market approach uses prices and other relevant information generated by market transactions involving identical or comparable assets or liabilities.
The Company’s derivative assets and liabilities include interest rate swaps, which are carried at fair value. Interest rate swaps entered into by the Company are typically executed over-the-counter and are valued using discounted cash flows along with fair value models that primarily use observable market inputs. These models take into account a variety of factors including, where applicable, maturity, interest rate yield curves, and counterparty credit risks. Refer to Note 14 for further details.
As of December 31, 2021, the Company’s cash and cash equivalents included bank time deposits which were measured at fair value using Level 2 inputs and their carrying values were approximately equal to their fair values. Level 2 inputs, obtained from various third-party data providers, represent quoted prices for similar assets in active markets, or these inputs were derived from observable market data, or if not directly observable, were derived from or corroborated by other observable market data.
The Company’s derivative assets and liabilities also include foreign currency forward contracts, which all have maturities of one month or less. The Company estimates the fair values of these contracts by using observable market inputs including the forward and spot prices for foreign currencies. Refer to Note 14 for further details.
Other current assets and other long-term liabilities recorded at fair value on a recurring basis are composed of investments held in a rabbi trust and the related deferred liability for deferred compensation arrangements. Quoted prices for this investment, primarily in mutual funds, are available in active markets. Thus, the Company’s investments related to deferred compensation arrangements and the related long-term liability are classified as Level 1 measurements in the fair value hierarchy.
N
The Company’s outstanding debt balances as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Term Loan Facility due 2028 |
|
$ |
|
|
$ |
|
||
Term Loan Facility due 2026 |
|
|
|
|
|
|
||
Senior Notes due 2027 |
|
|
|
|
|
|
||
Total face value |
|
|
|
|
|
|
||
Debt discount |
|
|
( |
) |
|
|
( |
) |
Deferred financing fees |
|
|
( |
) |
|
|
( |
) |
Total long-term debt |
|
|
|
|
|
|
||
Less: current maturities |
|
|
|
|
|
|
||
Long-term debt, net of current maturities |
|
$ |
|
|
$ |
|
||
Scheduled maturities with respect to the Company’s long-term debt are as follows (in thousands):
2023 |
|
$ |
( |
) |
2024 |
|
|
( |
) |
2025 |
|
|
( |
) |
2026 |
|
|
( |
) |
2027 |
|
|
( |
) |
Thereafter |
|
|
( |
) |
Total |
|
$ |
( |
) |
F-34
Term Loan Facility and Revolving Credit Facility
On March 15, 2021, Horizon Therapeutics USA, Inc. (the “Borrower” or “HTUSA”), a wholly-owned subsidiary of the Company, borrowed approximately $
T
The obligations under the Credit Agreement (including obligations in respect of the 2028 Term Loans, 2026 Term Loans and the Revolving Credit Facility) and any swap obligations and cash management obligations owing to a lender (or an affiliate of a lender) are guaranteed by the Company and each of the Company’s existing and subsequently acquired or formed direct and indirect subsidiaries (other than certain immaterial subsidiaries, subsidiaries whose guarantee would result in material adverse tax consequences and subsidiaries whose guarantee is prohibited by applicable law). The obligations under the Credit Agreement (including obligations in respect of the 2028 Term Loans, 2026 Term Loans and the Revolving Credit Facility) and any related swap and cash management obligations are secured, subject to customary permitted liens and other agreed upon exceptions, by a perfected security interest in (i) all tangible and intangible assets of the Borrower and the guarantors, except for certain customary excluded assets, and (ii) all of the capital stock owned by the Borrower and guarantors thereunder (limited, in the case of the stock of certain non-U.S. subsidiaries of the Borrower, to
F-35
The Credit Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among other things, restrictions on indebtedness, liens, investments, mergers, dispositions, prepayment of other indebtedness and dividends and other distributions. The Credit Agreement also contains a springing financial maintenance covenant, which requires that the Company maintain a specified leverage ratio at the end of each fiscal quarter. The covenant is tested if both the outstanding loans and letters of credit under the Revolving Credit Facility, subject to certain exceptions, exceed
Other events of default under the Credit Agreement include: (i) the failure by the Borrower to timely make payments due under the Credit Agreement; (ii) material misrepresentations or misstatements in any representation or warranty by any Loan Party when made; (iii) failure by any Loan Party to comply with the covenants under the Credit Agreement and other related agreements; (iv) certain defaults under a specified amount of other indebtedness of the Company or its subsidiaries; (v) insolvency or bankruptcy-related events with respect to the Company or any of its material subsidiaries; (vi) certain undischarged judgments against the Company or any of its restricted subsidiaries; (vii) certain ERISA-related events reasonably expected to have a material adverse effect on the Company and its restricted subsidiaries taken as a whole; (viii) certain security interests or liens under the loan documents ceasing to be, or being asserted by the Company or its restricted subsidiaries not to be, in full force and effect; (ix) any loan document or material provision thereof ceasing to be, or any challenge or assertion by any Loan Party that such loan document or material provision is not, in full force and effect; and (x) the occurrence of a change of control. If one or more events of default occurs and continues beyond any applicable cure period, the administrative agent may, with the consent of the lenders holding a majority of the loans and commitments under the facilities, or will, at the request of such lenders, terminate the commitments of the lenders to make further loans and declare all of the obligations of the Loan Parties under the Credit Agreement to be immediately due and payable.
The interest on the 2028 Term Loans is variable and as of December 31, 2022, the interest rate on the 2028 Term Loans was
The interest on the 2026 Term Loans is variable and as of December 31, 2022, the interest rate on the 2026 Term Loans was
As of December 31, 2022, the fair value of the amounts outstanding under the 2028 Term Loans and the 2026 Term Loans was approximately $
On April 25, 2022, the Company entered into two interest rate swap agreements with notional amounts totaling $
F-36
2027 Senior Notes
On July 16, 2019, HTUSA completed a private placement of $
The Company used the net proceeds from the offering of the 2027 Senior Notes, together with approximately $
The 2027 Senior Notes are HTUSA’s general unsecured senior obligations, rank equally in right of payment with all existing and future senior debt of HTUSA and rank senior in right of payment to any existing and future subordinated debt of HTUSA. The 2027 Senior Notes are effectively subordinate to all of the existing and future secured debt of HTUSA to the extent of the value of the collateral securing such debt.
The 2027 Senior Notes are unconditionally guaranteed on a senior basis by the Company and all of the Company’s restricted subsidiaries, other than HTUSA and certain immaterial subsidiaries, that guarantee the Credit Agreement. The guarantees are each guarantor’s senior unsecured obligations and rank equally in right of payment with such guarantor’s existing and future senior debt and senior in right of payment to any existing and future subordinated debt of such guarantor. The guarantees are effectively subordinated to all of the existing and future secured debt of each guarantor, including such guarantor’s guarantee under the Credit Agreement, to the extent of the value of the collateral securing such debt. The guarantees of a guarantor may be released under certain circumstances. The 2027 Senior Notes are structurally subordinated to all of the liabilities of the Company’s subsidiaries that do not guarantee the 2027 Senior Notes.
The 2027 Senior Notes accrue interest at an annual rate of
The indenture governing the 2027 Senior Notes contains covenants that limit the ability of the Company and its restricted subsidiaries to, among other things, pay dividends or distributions, repurchase equity, prepay junior debt and make certain investments, incur additional debt and issue certain preferred stock, incur liens on assets, engage in certain asset sales, merge, consolidate with or merge or sell all or substantially all of their assets, enter into transactions with affiliates, designate subsidiaries as unrestricted subsidiaries, and allow to exist certain restrictions on the ability of restricted subsidiaries to pay dividends or make other payments to the Company. Certain of the covenants will be suspended during any period in which the 2027 Senior Notes receive investment grade ratings. The indenture governing the 2027 Senior Notes also includes customary events of default.
As of December 31, 2022, the interest rate on the 2027 Senior Notes was
F-37
NOTE 14 – DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
Interest rate risk
The Company is a party to interest rate swap agreements designated as cash flow hedges with notional amounts totaling $
Foreign currency risk
The Company also enters into foreign currency forward contracts with durations of one month or less to mitigate the foreign currency risk related to certain balance sheet positions. The Company has not elected hedge accounting for these transactions and they are recorded at fair value. As of December 31, 2022, the Company had outstanding foreign currency forward contracts to sell $
The Company did not enter into any material derivative instruments during the years ended December 31, 2021 and 2020.
The following table summarizes the amounts and locations of the Company’s derivative instruments on the consolidated balance sheet as of December 31, 2022 (in thousands):
|
|
Fair value - |
|
|
Fair value - |
|
||||||
|
|
Balance sheet location |
|
December 31, 2022 |
|
|
Balance sheet location |
|
December 31, 2022 |
|
||
Interest rate swap contracts |
|
|
|
|
|
|
|
|
|
|
||
Designated as cash flow hedges |
|
Prepaid expenses and other current assets |
|
$ |
|
|
Accrued expenses and other current liabilities |
|
$ |
— |
|
|
Designated as cash flow hedges |
|
Other long-term assets |
|
|
|
|
Other long-term liabilities |
|
|
— |
|
|
Foreign currency forward contracts |
|
|
|
|
|
|
|
|
|
|
||
Not designated as hedges |
|
Prepaid expenses and other current assets |
|
|
|
|
Accrued expenses and other current liabilities |
|
|
— |
|
|
Total derivatives |
|
|
|
$ |
|
|
|
|
$ |
— |
|
|
While foreign currency forward contracts are subject to a master netting arrangement, the Company does
F-38
The following table summarizes the pre-tax amount and locations of derivative instrument net gains (losses) recognized in the consolidated statement of comprehensive income for the year ended December 31, 2022 (in thousands):
|
|
Location |
|
For the year ended |
|
|
Interest rate swap contracts designated as cash flow hedges |
|
Interest expense, net |
|
$ |
|
|
Foreign currency forward contracts not designated as cash flow hedges |
|
Foreign exchange loss |
|
|
( |
) |
The following table presents the pre-tax amounts of gains from derivative instruments recognized in other comprehensive income (loss) for the year ended December 31, 2022 (in thousands):
|
|
For the year ended |
|
|
Interest rate swap contracts designated as cash flow hedges |
|
$ |
|
|
Assuming market rates remain constant through contract maturities, the Company expects to reclassify pre-tax net gains of $
The cash flow effects of the Company’s derivative contracts in the consolidated statement of cash flows are included in operating activities.
F-39
NOTE 15 – LEASE OBLIGATIONS
As of December 31, 2022, the Company had the following office space lease agreements in place for real properties:
Location |
|
Approximate Square Feet |
|
|
Lease Expiry Date |
|
Dublin, Ireland |
|
|
|
|
||
Lake Forest, Illinois |
|
|
|
|
||
South San Francisco, California |
|
|
|
|
||
Rockville, Maryland |
|
|
|
|
||
Chicago, Illinois |
|
|
|
|
||
Washington, D.C. |
|
|
|
|
||
Mannheim, Germany |
|
|
|
|
||
The above table does not include details of an agreement to lease entered into in November 2021 relating to approximately
As of December 31, 2022 and 2021, the Company had right-of-use lease assets included in of $
In February 2021, the Company vacated the Lake Forest leased office building. As a result of the Company vacating the Lake Forest office, the Company recorded an impairment charge of $
The Company recognizes rent expense on a monthly basis over the lease term based on a straight-line method. Rent expense was $
The table below reconciles the undiscounted cash flows for each of the first five years and total of the remaining years to the lease liabilities recorded on the Company’s consolidated balance sheet as of December 31, 2022 (in thousands):
2023 |
$ |
|
|
2024 |
|
|
|
2025 |
|
|
|
2026 |
|
|
|
2027 |
|
|
|
Thereafter |
|
|
|
Total lease payments |
|
|
|
Imputed interest |
|
( |
) |
Total lease liabilities |
$ |
|
The weighted-average discount rate and remaining lease term for leases as of December 31, 2022 was
F-40
NOTE 16 – COMMITMENTS AND CONTINGENCIES
Purchase Commitments
Under the Company’s supply agreement with AGC Biologics A/S (formerly known as CMC Biologics A/S) (“AGC Biologics”), the Company has agreed to purchase certain minimum annual order quantities of TEPEZZA drug substance. In addition, the Company must provide AGC Biologics with rolling forecasts of TEPEZZA drug substance requirements, with a portion of the forecast being a firm and binding order. At December 31, 2022, the Company had binding purchase commitments with AGC Biologics for TEPEZZA drug substance of €
Under the Company’s agreement with Bio-Technology General (Israel) Ltd (“BTG Israel”), the Company has agreed to purchase certain minimum annual order quantities and is obligated to purchase at least
Under an agreement with Boehringer Ingelheim Biopharmaceuticals GmbH (“Boehringer Ingelheim Biopharmaceuticals”), Boehringer Ingelheim Biopharmaceuticals is required to manufacture and supply ACTIMMUNE to the Company. The Company is required to purchase minimum quantities of finished medicine during the term of the agreement, which term extends to at least September 30, 2024. As of December 31, 2022, the minimum purchase commitment to Boehringer Ingelheim Biopharmaceuticals was €
Excluding the above, additional purchase orders and other commitments relating to the manufacture of PROCYSBI, RAVICTI, UPLIZNA, BUPHENYL, QUINSAIR, RAYOS and VIMOVO of $
Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company’s management does not believe that any such matters, individually or in the aggregate, will have a material adverse effect on the Company’s business, financial condition, results of operations or cash flows. In addition, the Company from time to time has billing disputes with vendors in which amounts invoiced are not in accordance with the terms of their contracts.
Disclosure of ongoing matters is considered at the time of each filing and matters may be removed if the statute of limitations has lapsed or circumstances have changed that reduce the risk of exposure.
F-41
On August 3, 2022, the Company received a civil investigative demand from the United States Department of Justice (“DOJ”) pursuant to the Federal False Claims Act regarding an investigation concerning potentially false information in prior authorization forms. A prior authorization form is a managed care practice whereby the payer (either a commercial insurer or a government health program) requires that the prescribing physician provide additional justification or information supporting the physician’s decision to prescribe a particular medicine. The civil investigative demand requests certain documents and information related to DUEXIS, PENNSAID 2%, VIMOVO and RAYOS. The Company is cooperating with the investigation and the DOJ has not indicated to the Company whether it believes the Company engaged in any wrongdoing or if the Company is the subject of the investigation. While the Company is not aware of any fraudulent scheme to provide false information in prior authorization forms for its medicines that resulted in improper payments from government healthcare programs, no assurance can be given as to the timing or outcome of the DOJ’s investigation, or that it will not result in a material adverse effect on the Company’s business.
In the third and fourth quarter of 2022, the Company was served with several complaints from plaintiffs alleging to have taken TEPEZZA and suffered hearing impairments. Although hearing impairment, including deafness, was identified as a potential adverse event in the pivotal clinical trials for TEPEZZA, addressed at the FDA advisory committee meeting that considered the safety and efficacy of TEPEZZA, and listed as a potential adverse event in the FDA-approved TEPEZZA product label, the plaintiffs allege that the Company failed to adequately inform them about the risk of hearing impairment before taking the medicine. The Company intends to vigorously defend itself in the lawsuits and maintains insurance coverage for product liability claims. Nevertheless, no assurance can be given as to the outcome of the litigation, whether additional similar lawsuits will be initiated or whether the Company’s insurance coverage will be adequate to cover the costs of the litigation or any resulting settlements or judgments.
Royalty and Milestone Agreements
TEPEZZA
River Vision Acquisition Agreement and S.R. One/Lundbeckfond Agreements
Under the acquisition agreement for River Vision in May 2017, the Company agreed to pay up to $
The agreement also included a royalty payment of
S.R. One and Lundbeckfond, as two of the former River Vision stockholders, both held rights to receive approximately
Roche License Agreement
Under the Company’s license agreement with Roche, the Company is required to pay Roche up to CHF
The Company’s remaining obligation to Roche relating to the attainment of various TEPEZZA development and regulatory milestones is CHF
F-42
KRYSTEXXA
Under the terms of a license agreement with Duke University (“Duke”) and Mountain View Pharmaceuticals, Inc. (“MVP”), the Company is obligated to pay Duke a mid-single-digit royalty on its global net sales of KRYSTEXXA and a royalty of between
RAVICTI
Under the terms of an asset purchase agreement with Bausch Health Companies Inc. (formerly Ucyclyd Pharma, Inc.) (“Bausch”), the Company is obligated to pay to Bausch mid-single-digit royalties on its global net sales of RAVICTI. Under the terms of a license agreement with Saul W. Brusilow, M.D. and Brusilow Enterprises, Inc. (“Brusilow”), the Company is obligated to pay low single-digit royalties to Brusilow on net sales of RAVICTI that are covered by a valid claim of a licensed patent.
PROCYSBI
Under the terms of an amended and restated license agreement with The Regents of the University of California, San Diego (“UCSD”), as amended, the Company is obligated to pay to UCSD tiered low to mid-single-digit royalties on its net sales of PROCYSBI, including a minimum annual royalty in an amount less than $
UPLIZNA
Following the acquisition of Viela on March 15, 2021, the Company is party to a number of third-party license agreements. Under these license agreements, the Company is obligated to pay up to a total of $
ACTIMMUNE
Under an amended license agreement, with the original developer of ACTIMMUNE, the Company is obligated to pay a low single digit royalty on its annual net sales of ACTIMMUNE. In addition, under the terms of an assignment and option agreement with a separate third-party, the Company is obligated to pay low single-digit royalties on the Company’s net sales of ACTIMMUNE in the United States.
RAYOS and LODOTRA
Effective January 1, 2019, the Company was obligated to pay Vectura a mid-teens percentage royalty on its RAYOS net sales in North America, subject to a minimum royalty of $
For all of the royalty agreements entered into by the Company, a total royalty and earnout expense of $
F-43
Other Agreements
Xeris Pharmaceuticals, Inc.
On November 22, 2022, the Company entered into a research collaboration and option agreement with Xeris under which Xeris is obligated to use its proprietary formulation technology platform, XeriJect, to conduct a research program to develop an ultra-concentrated, ready-to-use, subcutaneous injection of TEPEZZA. The Company received an option to obtain a commercial license for any reformulated product developed under the research program. An upfront payment of $
Q32 Bio Inc.
On August 12, 2022, the Company entered into a collaboration and option agreement with Q32 related to its pipeline candidate ADX-914, a monoclonal antibody antagonist of the interleukin-7 receptor for the treatment of autoimmune and inflammatory diseases. An upfront payment of $
Alpine Immune Science, Inc.
On December 15, 2021, the Company entered into an exclusive license agreement with Alpine for the development and commercialization of up to four preclinical candidates generated from Alpine’s unique discovery platform. In connection with the execution of the license agreement, the Company entered into a stock purchase agreement with Alpine to purchase a minority stake of
Under the terms of the agreements, the Company paid Alpine $
Arrowhead Pharmaceuticals, Inc.
On June 18, 2021, the Company entered into a global agreement with Arrowhead for HZN-457, a discovery-stage investigational RNAi therapeutic being developed by Arrowhead as a potential treatment for uncontrolled gout. Arrowhead granted the Company a worldwide exclusive license to develop, manufacture and commercialize medicines based on the RNAi therapeutic. Arrowhead is required to use commercially reasonable efforts to conduct research and preclinical development activities for the RNAi therapeutic products. The Company must use commercially reasonable efforts in, and will be responsible for, clinical development and commercialization of the RNAi therapeutic products. Under the terms of the agreement, the Company paid Arrowhead an upfront cash payment of $
F-44
Halozyme Therapeutics, Inc.
On November 21, 2020, the Company entered into a global agreement with Halozyme that gives the Company exclusive access to Halozyme’s ENHANZE drug delivery technology for SC formulation of medicines targeting IGF-1R. The Company is exploring ENHANZE to develop a SC formulation of TEPEZZA. Under the terms of the agreement, the Company paid Halozyme an upfront cash payment of $
Curzion Pharmaceuticals, Inc.
On April 1, 2020, the Company acquired Curzion for an upfront cash payment of $
Venture capital funds
The Company is committed to invest as a strategic limited partner in four venture capital funds: Forbion Growth Opportunities Fund I C.V., Forbion Capital Fund V C.V., Aisling Capital V, L.P. and RiverVest Venture Fund V, L.P. As of December 31, 2022, the total carrying amount of the Company’s investments in these funds was $
Non-cancellable advertising commitments
As of December 31, 2022, the Company had $
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. The Company may record charges in the future as a result of these indemnification obligations.
In accordance with its memorandum and articles of association, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. Additionally, the Company has entered into, and intends to continue to enter into, separate indemnification agreements with its directors and executive officers. These agreements, among other things, require the Company to indemnify its directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of the Company’s directors or executive officers, or any of the Company’s subsidiaries or any other company or enterprise to which the person provides services at the Company’s request. The Company also has a director and officer insurance policy that enables it to recover a portion of any amounts paid for current and future potential claims.
The Company also has indemnification obligations to the former officers and directors of Viela for certain events or occurrences related to their former roles at Viela, subject to certain limits. Several individual directors and officers of Viela were named as defendants in a lawsuit, Sciannella v. Astrazeneca UK Limited et.al., Case No. 2023-0125, filed in the Court of Chancery of the State of Delaware, which alleges various breaches of fiduciary duties in connection with Viela’s decision to be acquired by the Company. The Company has a director and officer insurance policy that enables it to recover a portion of certain amounts that may be paid by (and for which the Company may be obligated to indemnity) the former Viela officers and directors as a result of the lawsuit.
F-45
NOTE 17 - LEGAL PROCEEDINGS
PENNSAID 2%
On May 6, 2022, Apotex received FDA approval to market a generic diclofenac sodium topical solution 2% (“Apotex ANDA Product”), following the apparent forfeiture by Actavis Laboratories UT, Inc. (“Actavis”) of its first-filer exclusivity. On May 13, 2022, the Company filed a complaint against Apotex asserting that the manufacture, use, offer for sale, or sale of the Apotex ANDA Product would infringe U.S. Patent No. 9,066,913 (the “‘913 patent”) in the United States District Court for the District of Delaware. The Company previously successfully enforced the ‘913 patent against Actavis in the District of New Jersey and the Federal Circuit subsequently affirmed the District Court’s ruling.
The Company purchased PENNSAID 2% from Nuvo Pharmaceuticals Inc. (“Nuvo”) in 2014. Apotex alleges that a settlement agreement entered into in January 2013 with Nuvo (“Nuvo Settlement”) provides it with a license to the ‘913 patent. The Company disputes the scope of Apotex’s settlement and license with Nuvo, contending that it does not provide Apotex with a license to the ‘913 patent, which was issued to the Company after the Company’s purchase of PENNSAID 2% from Nuvo.
On May 17, 2022, the Company moved for a preliminary injunction enjoining Apotex from engaging in the commercial manufacture, use, offer to sell, or sale of the Apotex ANDA Product. On May 27, 2022, the parties filed a stipulated preliminary injunction and a proposed expedited briefing schedule to present the underlying license dispute to the District Court by way of a motion for summary judgment, which the District Court entered on May 31, 2022. On August 23, 2022, the District Court held a hearing on the motion for summary judgment and the parties subsequently provided supplemental briefing on certain legal matters requested by the District Court. On November 7, 2022, the District Court ruled in Apotex’s favor, finding that the Nuvo Settlement provided Apotex a license to the ‘913 patent and that Apotex’s ANDA Product consequently does not infringe the ‘913 patent. Apotex subsequently alleged that the preliminary injunction was wrongfully entered and caused Apotex to suffer monetary losses. Apotex is also seeking an award of its attorney fees. The Company disputes Apotex’s allegations that it incurred monetary losses or that it is entitled to an award of attorney fees. The parties are currently briefing these issues to the District Court.
PROCYSBI
On February 2, 2022 and February 16, 2022, the Company received notice from Teva Pharmaceuticals, Inc. (“Teva”) that it had filed Abbreviated New Drug Applications (“ANDA”) with the FDA seeking approval of generic versions of PROCYSBI granules and capsules, respectively. The ANDAs contained Paragraph IV Patent Certifications alleging that the patents covering PROCYSBI granules and capsules are invalid and/or will not be infringed by Teva’s manufacture, use or sale of the medicines for which the ANDAs were submitted. On March 15, 2022, the Company filed suit against Teva in the United States District Court for the District of New Jersey for patent infringement, seeking to prevent Teva from selling its generic versions of PROCYSBI granules and capsules.
RAVICTI
On June 7, 2022, the Company received notice from Taro Pharmaceuticals Industries, Ltd. (“Taro”) that it had filed an ANDA with the FDA seeking approval of a generic version of RAVICTI. The ANDA contained Paragraph IV Patent Certification alleging that the patents covering RAVICTI are invalid and/or will not be infringed by Taro’s manufacture, use or sale of the medicine for which the ANDA was submitted. On July 20, 2022, the Company filed suit against Taro in the United States District Court for the District of New Jersey for patent infringement, seeking to prevent Taro from selling its generic version of RAVICTI.
F-46
NOTE 18 – SHAREHOLDERS’ EQUITY
In September 2022, the Company’s board of directors authorized a share repurchase program pursuant to which the Company may repurchase up to $
During the year ended December 31, 2022, the Company issued an aggregate of
During the year ended December 31, 2022, the Company made payments of $
During the year ended December 31, 2021, the Company issued an aggregate of
During the year ended December 31, 2021, the Company made payments of $
F-47
NOTE 19 – SHARE-BASED AND LONG-TERM INCENTIVE PLANS
Employee Share Purchase Plan
2020 Employee Share Purchase Plan. On February 19, 2020, the Compensation Committee adopted, subject to shareholder approval, the 2020 Employee Share Purchase Plan (“2020 ESPP”), as successor to and continuation of the 2014 Employee Share Purchase Plan (the “2014 ESPP”), including increasing the number of ordinary shares available for issuance to the Company’s employees pursuant to the exercise of purchase rights under the Company’s purchase plans by an additional
As of December 31, 2022, an aggregate of
Share-Based Compensation Plans
2011 Equity Incentive Plan. Upon the effectiveness of the Horizon Therapeutics Public Limited Company Amended and Restated 2014 Equity Incentive Plan (the “2014 EIP”), no additional stock awards were or will be made under the 2011 Equity Incentive Plan (the “2011 EIP”), although all outstanding stock awards granted under the 2011 EIP continue to be governed by the terms of the 2011 EIP.
Amended and Restated 2014 Equity Incentive Plan and 2014 Non-Employee Equity Plan. On May 17, 2014, HPI’s board of directors adopted the 2014 EIP and the Horizon Therapeutics Public Limited Company 2014 Non-Employee Equity Plan (the “2014 Non-Employee Equity Plan”). At the Special Meeting, HPI’s stockholders approved the 2014 EIP and 2014 Non-Employee Equity Plan, which serve as successors to the 2011 EIP.
Upon the effectiveness of the Horizon Therapeutics Public Limited Company Amended and Restated 2020 Equity Incentive Plan (the “2020 EIP”), no additional stock awards were or will be made under the 2014 EIP, although all outstanding stock awards granted under the 2014 EIP continue to be governed by the terms of the 2014 EIP.
The 2014 Non-Employee Equity Plan provides for the grant of non-statutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards and other forms of stock awards that may be settled in cash, shares or other property to the non-employee directors and consultants of the Company (or a subsidiary company). The Company’s board of directors has authority to suspend or terminate the 2014 Non-Employee Equity Plan at any time.
On February 20, 2019, the Compensation Committee approved, subject to shareholder approval, an amendment to the 2014 Non-Employee Equity Plan, increasing the number of ordinary shares that may be issued under the 2014 Non-Employee Equity Plan by
Amended and Restated 2020 Equity Incentive Plan. On February 19, 2020, the Compensation Committee adopted, subject to shareholder approval, the 2020 EIP, as successor to and continuation of the 2014 EIP, including increasing the number of ordinary shares available for the grant of equity awards to the Company’s employees by an additional
On February 17, 2021, the Compensation Committee approved amending the 2020 EIP, subject to shareholder approval, including increasing the number of ordinary shares available for the grant of equity awards to the Company’s employees by an additional
On February 23, 2022, the Compensation Committee approved further amending the 2020 EIP, subject to shareholder approval, to increase the aggregate number of ordinary shares available for the grant of equity awards to the Company’s employees by an additional
F-48
Amended and Restated 2018 Equity Incentive Plan. In connection with the Viela acquisition on March 15, 2021, the Company assumed the Viela Bio Amended and Restated 2018 Equity Incentive Plan (“Viela 2018 EIP”). The Viela 2018 EIP was subsequently renamed the Horizon Therapeutics Public Limited Company Amended and Restated 2018 Equity Incentive Plan (“2018 EIP”) on April 28, 2021. The maximum aggregate number of ordinary shares that may be issued under the 2018 EIP following March 15, 2021 (the “Plan Assumption Date”) is
As of December 31, 2022, an aggregate of
Stock Options
There were
Stock price (closing stock price on March 14, 2021) |
|
$ |
|
|
Weighted average fair value of converted stock options |
|
$ |
|
|
Risk-free interest rate |
|
|
||
Expected stock price volatility |
|
|
||
Dividend yield |
|
|
— |
|
Term to expiration |
|
|
||
The following table summarizes stock option activity during the year ended December 31, 2022:
|
|
|
|
|
|
|
|
Weighted Average |
|
|
|
|
||||
|
|
|
|
|
Weighted |
|
|
Contractual Term |
|
|
Aggregate |
|
||||
|
|
|
|
|
Average |
|
|
Remaining |
|
|
Intrinsic Value |
|
||||
|
|
Options |
|
|
Exercise Price |
|
|
(in years) |
|
|
(in thousands) |
|
||||
Outstanding as of December 31, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
— |
|
|
|
— |
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
— |
|
|
|
— |
|
|
Expired |
|
|
( |
) |
|
|
|
|
|
— |
|
|
|
— |
|
|
Outstanding as of December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
$ |
|
||||
Exercisable as of December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Stock options typically have a contractual term of
F-49
The following table summarizes the Company’s outstanding stock options at December 31, 2022:
|
|
Options Outstanding |
|
|
Options Exercisable |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
Weighted Average |
|
|
|
|
|
Weighted |
|
|
Weighted Average |
|
||||||
|
|
|
|
|
Weighted |
|
|
Remaining |
|
|
|
|
|
Average |
|
|
Remaining |
|
||||||
|
|
Number of options |
|
|
Average |
|
|
Contractual |
|
|
Number |
|
|
Exercise |
|
|
Contractual |
|
||||||
Exercise Price Ranges |
|
outstanding |
|
|
Exercise Price |
|
|
Term (in years) |
|
|
Exercisable |
|
|
Price |
|
|
Term (in years) |
|
||||||
$ |
|
|
|
|
$ |
|
|
|
|
|
|
|
|
$ |
|
|
|
|
||||||
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
$ |
|
|
|
|
|
|
|
|
$ |
|
|
|
|
||||||
The total intrinsic value of the options exercised during the years ended December 31, 2022, 2021 and 2020 was $
Restricted Stock Units
The following table summarizes restricted stock unit activity for the year ended December 31, 2022:
|
|
|
|
|
Weighted Average |
|
||
|
|
Number of |
|
|
Grant-Date Fair |
|
||
|
|
Units |
|
|
Value Per Unit |
|
||
Outstanding as of December 31, 2021 |
|
|
|
|
$ |
|
||
Granted |
|
|
|
|
|
|
||
Vested |
|
|
( |
) |
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
Outstanding as of December 31, 2022 |
|
|
|
|
$ |
|
||
The grant-date fair value of restricted stock units is the closing price of the Company’s ordinary shares on the date of grant.
During the years ended December 31, 2022, 2021 and 2020, the Company granted
Performance Stock Unit Awards
The following table summarizes performance stock unit awards (“PSUs”) activity for the year ended December 31, 2022:
|
|
|
|
|
Weighted |
|
|
|
|
|
Recorded |
|
||||
|
|
|
|
|
Average |
|
|
|
|
|
Weighted |
|
||||
|
|
|
|
|
Grant-Date |
|
|
Average |
|
|
Average |
|
||||
|
|
Number |
|
|
Fair Value |
|
|
Illiquidity |
|
|
Fair Value |
|
||||
|
|
of Units |
|
|
Per Unit |
|
|
Discount |
|
|
Per Unit |
|
||||
Outstanding as of December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Granted |
|
|
|
|
$ |
|
|
|
% |
|
$ |
|
||||
Forfeited |
|
|
( |
) |
|
|
|
|
|
% |
|
|
|
|||
Vested |
|
|
( |
) |
|
|
|
|
|
% |
|
|
|
|||
Performance Based Adjustment (1) |
|
|
|
|
|
|
|
|
% |
|
|
|
||||
Outstanding as of December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
F-50
On January 4, 2022, the Company awarded PSUs to key executive participants (“2022 PSUs”). The 2022 PSUs are subject to both performance-based and service based vesting provisions. The 2022 PSUs utilize three long-term performance metrics, as follows:
If a change in control occurs prior to the completion of the defined performance period, a portion of the 2022 PSUs will vest as measured through the date of the change in control which will be determined by the Compensation Committee.
On January 4, 2021, the Company awarded PSUs to key executive participants (“2021 PSUs”). The 2021 PSUs utilize three long-term performance metrics: a component tied to relative TSR over a three-year period, a component tied to technical operations and manufacturing milestones for the Company over a three-year period, and a component tied to R&D and business development milestones for the Company over a two-year period, as follows:
F-51
During 2021, the Company’s board of directors approved modifications of certain outstanding awards of two senior executives, one of whom retired in January 2022 and the other whose employment was terminated in January 2022. The modifications provided for continued vesting of performance awards post termination of services that would have otherwise been forfeited. The modifications resulted in an incremental expense of $
On January 3, 2020, the Company awarded PSUs to key executive participants (“2020 PSUs”). The 2020 PSUs utilize two performance metrics: a component tied to relative TSR over a three-year period and a component tied to certain business performance milestones for the Company over one-year and two-year periods, as follows:
F-52
As a result of the impact of the COVID-19 pandemic on certain aspects of the Company’s business in 2020, the performance goals associated with certain of the Company’s performance-based equity awards no longer reflected the Company’s expectations, causing the awards to lose their incentive to employees. Accordingly, on July 28, 2020, the Compensation Committee approved a modification to the 2020 Net Sales PSUs awarded on January 3, 2020 that were to vest based on KRYSTEXXA 2020 net sales. Following the modification, those 2020 Net Sales PSUs related to KRYSTEXXA were earned based on net sales of KRYSTEXXA achieved by the end of a modified 18-month performance period that ended on June 30, 2021 instead of a 12-month performance period that ended on December 31, 2020. As a result, with respect to the 2020 Net Sales PSUs that were earned based on net sales of KRYSTEXXA, the first one-third vested on July 1, 2021, the second one-third vested on January 5, 2022 and the vesting of the remaining one-third is unchanged and vested on January 5, 2023. There were 12 participants impacted by the modification. The total compensation cost resulting from the modification was approximately $
All PSUs outstanding on December 31, 2022 may vest in a range of between
Valuation date stock price |
|
$ |
|
|
Expected volatility |
|
|
% |
|
Risk free rate |
|
|
% |
The value of the 2022 Strategic PSUs and 2022 Financial PSUs is calculated at the end of each quarter based on the expected payout percentage based on estimated full-period performance against targets, and the Company adjusts the expense quarterly.
On January 4, 2019, the Company awarded a company-wide grant of PSUs (the “TEPEZZA PSUs”). Vesting of the TEPEZZA PSUs was contingent upon receiving shareholder approval of amendments to the 2014 EIP, which approval was received on May 2, 2019. The TEPEZZA PSUs were generally eligible to vest contingent upon receiving approval of the TEPEZZA biologics license application from the FDA no later than September 30, 2020 and the employee’s continued service with the Company. In January 2020, the Company received TEPEZZA approval from the FDA and the Company started recognizing the expense related to the TEPEZZA PSUs on that date. For all non-executive committee participants, one half of the TEPEZZA PSUs vested on the FDA approval date and one-half vested on the one-year anniversary of the FDA approval date, subject to the employee’s continued service through the applicable vesting date. The remaining
Share-Based Compensation Expense
The following table summarizes share-based compensation expense included in the Company’s consolidated statements of operations for the years ended December 31, 2022, 2021 and 2020 (in thousands):
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Cost of goods sold |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Research and development |
|
|
|
|
|
|
|
|
|
|||
Selling, general and administrative |
|
|
|
|
|
|
|
|
|
|||
Total share-based compensation expense |
|
$ |
|
|
$ |
|
|
$ |
|
|||
During the years ended December 31, 2022 and 2021, the Company recognized $
F-53
NOTE 20 – INCOME TAXES
The Company’s income before expense (benefit) for income taxes by jurisdiction for the years ended December 31, 2022, 2021 and 2020 is as follows (in thousands):
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Ireland |
|
$ |
|
|
$ |
|
|
$ |
|
|||
United States |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
Other foreign |
|
|
( |
) |
|
|
|
|
|
|
||
Income before expense (benefit) for income taxes |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The components of the expense (benefit) for income taxes were as follows for the years ended December 31, 2022, 2021 and 2020 (in thousands):
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Current expense (benefit) provision |
|
|
|
|
|
|
|
|
|
|||
Ireland |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
U.S. – Federal and State |
|
|
|
|
|
|
|
|
|
|||
Other foreign |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Total current expense (benefit) provision |
|
|
( |
) |
|
|
|
|
|
|
||
Deferred benefit provision |
|
|
|
|
|
|
|
|
|
|||
Ireland |
|
|
|
|
|
|
|
|
( |
) |
||
U.S. – Federal and State |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Other foreign |
|
|
( |
) |
|
|
|
|
|
|
||
Total deferred expense (benefit) provision |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Total expense (benefit) for income taxes |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
Total expense for income taxes was $
F-54
A reconciliation between the Irish statutory income tax rate to the Company’s effective tax rate for 2022, 2021 and 2020 is as follows (in thousands):
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Irish income tax at statutory rate (12.5%) |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Foreign tax rate differential |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Share-based compensation |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Change in valuation allowances |
|
|
( |
) |
|
|
|
|
|
|
||
U.S. federal and state tax credits |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Non-deductible in-process research and development costs |
|
|
— |
|
|
|
— |
|
|
|
|
|
Write-off of U.S. deferred tax asset related to interest expense due to Anti-Hybrid Rules |
|
|
— |
|
|
|
— |
|
|
|
|
|
Intercompany transfer and license of IP assets |
|
|
— |
|
|
|
|
|
|
|
||
U.S. state income taxes |
|
|
|
|
|
( |
) |
|
|
|
||
Uncertain tax positions |
|
|
|
|
|
( |
) |
|
|
|
||
Other non-deductible expenses |
|
|
|
|
|
|
|
|
|
|||
Goodwill impairment |
|
|
|
|
|
— |
|
|
|
— |
|
|
Change in U.S. state effective tax rate |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Disqualified compensation expense |
|
|
|
|
|
|
|
|
|
|||
Other, net |
|
|
|
|
|
|
|
|
|
|||
Expense (benefit) for income taxes |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
Effective income tax rate |
|
|
% |
|
( |
|
|
|
% |
|||
The overall effective income tax rate for 2022 of
The overall effective income tax rate for 2021 of (
The overall effective income tax rate for 2020 of
The change in the effective income tax rate in 2022 compared to that in 2021 was primarily due to changes in the state tax rate expected to apply to the reversal of temporary differences between the book values and tax bases of certain assets.
F-55
The change in the effective income tax rate in 2021 compared to that in 2020 was primarily due to an increase in excess tax benefits recognized on share-based compensation and a tax benefit of $
Significant components of the Company’s net deferred tax assets and liabilities, are as follows (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Net operating loss carryforwards |
|
$ |
|
|
$ |
|
||
Accruals and reserves |
|
|
|
|
|
|
||
Accrued compensation |
|
|
|
|
|
|
||
Intercompany interest |
|
|
|
|
|
|
||
U.S. federal and state credits |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Total deferred tax assets |
|
|
|
|
|
|
||
Valuation allowance |
|
|
( |
) |
|
|
( |
) |
Deferred tax assets, net of valuation allowance |
|
$ |
|
|
$ |
|
||
Deferred tax liabilities: |
|
|
|
|
|
|
||
Intangible assets |
|
$ |
|
|
$ |
|
||
Property, plant and equipment |
|
|
|
|
|
|
||
Interest rate swap |
|
|
|
|
|
— |
|
|
Debt discount |
|
|
|
|
|
|
||
Total deferred tax liabilities |
|
|
|
|
|
|
||
Net deferred income tax asset |
|
$ |
( |
) |
|
$ |
( |
) |
As of December 31, 2022, the Company had net operating loss carryforwards of approximately $
Utilization of certain net operating loss and tax credit carryforwards in the United States is subject to an annual limitation due to ownership change limitations provided by Sections 382 and 383 of the Internal Revenue Code of 1986, as amended. Certain net operating losses generated before an August 2, 2012 ownership change and federal net operating losses and federal tax credits acquired through the Viela acquisition are subject to an annual limitation. The U.S. federal net operating loss carryforward and U.S. federal tax credit carryforward limitation is cumulative such that any use of the carryforwards below the limitation in a particular tax year will result in a corresponding increase in the limitation for the subsequent tax year.
At December 31, 2022, the Company had $
F-56
A reconciliation of the beginning and ending amounts of valuation allowances for the years ended December 31, 2022, 2021 and 2020 is as follows (in thousands):
Valuation allowances at December 31, 2019 |
|
$ |
( |
) |
Increase for 2020 activity |
|
|
( |
) |
Release of valuation allowances |
|
|
|
|
Valuation allowances at December 31, 2020 |
|
$ |
( |
) |
Increase for 2021 activity |
|
|
( |
) |
Release of valuation allowances |
|
|
|
|
Valuation allowances at December 31, 2021 |
|
$ |
( |
) |
Increase for 2022 activity |
|
|
( |
) |
Release of valuation allowances |
|
|
|
|
Valuation allowances at December 31, 2022 |
|
$ |
( |
) |
Deferred tax valuation allowances decreased by $
The changes in the Company’s uncertain income tax positions for the years ended December 31, 2022, 2021 and 2020, excluding interest and penalties, consisted of the following (in thousands):
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Beginning balance – uncertain tax positions |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Tax positions in the year: |
|
|
|
|
|
|
|
|
|
|||
Additions |
|
|
|
|
|
|
|
|
|
|||
Acquired uncertain tax positions |
|
|
— |
|
|
|
|
|
|
— |
|
|
Tax positions related to prior years: |
|
|
|
|
|
|
|
|
|
|||
Additions |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Settlements and lapses |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Ending balance – uncertain tax positions |
|
$ |
|
|
$ |
|
|
$ |
|
|||
For the year ended December 31, 2022, the net increase in uncertain tax positions was primarily attributable to additional uncertain tax positions recognized on U.S. federal R&D credits generated during the year partially offset by lapses in statute for a portion of uncertain tax positions relating to U.S. federal orphan drug credits. In the Company’s consolidated balance sheet, uncertain tax positions (including interest and penalties) of $
At December 31, 2022, penalties of $
The Company files income tax returns in Ireland, in the United States for federal and various states, as well as in certain other jurisdictions. At December 31, 2022, open tax years in U.S. federal and certain state jurisdictions date back to 2007 due to the taxing authorities’ ability to adjust operating loss carryforwards. In Ireland, the statute of limitations expires five years from the end of the tax year or four years from the time a tax return is filed, whichever is later. Therefore, the earliest year open to examination is 2018 with the lapse of statute occurring in 2023. No changes in settled tax years have occurred to date.
F-57
NOTE 21 – EMPLOYEE BENEFIT PLANS
U.S. Retiree Medical Plan
The Company implemented its retiree medical plan effective October 1, 2021, which provides certain medical benefits to eligible retirees in the United States.
The following table summarizes the changes in benefit obligation for the years ended December 31, 2022 and 2021 for this plan (in thousands):
|
|
For the Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Benefit obligation at beginning of year |
|
$ |
|
|
$ |
— |
|
|
Prior service cost at initiation of plan |
|
|
— |
|
|
|
|
|
Service cost |
|
|
|
|
|
|
||
Interest cost |
|
|
|
|
|
|
||
|
|
( |
) |
|
|
|
||
Benefits paid net of participant contributions |
|
|
( |
) |
|
|
( |
) |
Benefit obligation at end of year |
|
$ |
|
|
$ |
|
||
As of December 31, 2022 and 2021, the unfunded status for the retiree medical plan was $
The following table summarizes the amounts recognized in the consolidated balance sheets as of December 31, 2022 and 2021 for this plan (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Accrued expenses and other current liabilities |
|
$ |
( |
) |
|
$ |
( |
) |
Other long-term liabilities |
|
|
( |
) |
|
|
( |
) |
Amounts recognized on the consolidated balance sheet |
|
$ |
( |
) |
|
$ |
( |
) |
The following table summarizes the amounts recognized in accumulated other comprehensive loss for the years ended December 31, 2022 and 2021 for this plan (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Prior service cost |
|
$ |
|
|
$ |
|
||
Actuarial (gain) loss |
|
|
( |
) |
|
|
|
|
Accumulated other comprehensive loss before income taxes |
|
$ |
|
|
$ |
|
||
Expected Benefit Payments
The following table summarizes total benefit payments, which reflect expected future service, expected to be paid to plan participants (in thousands):
2023 |
|
$ |
|
|
2024 |
|
|
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
2028 to 2031 |
|
|
|
F-58
Components of Net Periodic Benefit Cost and Amounts Recognized in Other Comprehensive (Income) Loss
The following table summarizes net period benefit cost recognized and the amounts recognized in other comprehensive (income) loss for the years ended December 31, 2022 and 2021 (in thousands):
|
|
For the Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Service cost |
|
$ |
|
|
$ |
|
||
Interest cost |
|
|
|
|
|
|
||
Amortization of prior service cost |
|
|
|
|
|
|
||
Net periodic benefit cost |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Prior service cost |
|
|
— |
|
|
|
|
|
Amortization of prior service cost |
|
|
( |
) |
|
|
( |
) |
Actuarial (gain) loss |
|
|
( |
) |
|
|
|
|
Total recognized in other comprehensive (income) loss |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
||
Total recognized in net periodic benefit cost and other comprehensive (income) loss |
|
$ |
|
|
$ |
|
||
The components of net periodic benefit cost other than service cost are included in other (expense) income, net in the consolidated statements of comprehensive income.
Weighted-Average Actuarial Assumptions and Health Care Cost Trend Rates
The following table summarizes weighted-average assumptions and health care cost trend rates used to determine the benefit obligation as of December 31, 2022 and 2021:
|
|
As of December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Discount rate |
|
|
% |
|
|
% |
||
Health care cost trend rate assumed for next year |
|
|
% |
|
|
% |
||
Rate to which the cost trend rate is assumed to decline (the “Ultimate trend rate”) |
|
|
% |
|
|
% |
||
Year that the rate reaches the ultimate trend rate |
|
|
|
|
||||
The assumptions used in calculating the December 31, 2022 measurement date benefit obligation will be used in the calculation of net periodic benefit cost in 2023.
The following table summarizes weighted-average assumptions and health care cost trend rates used to determine the net periodic benefit costs for the years ended December 31, 2022 and 2021:
|
|
For the Years Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Discount rate |
|
|
% |
|
|
% |
||
Health care cost trend rate assumed for next year |
|
|
% |
|
|
% |
||
Ultimate trend rate |
|
|
% |
|
|
% |
||
Year that the rate reaches the ultimate trend rate |
|
|
|
|
||||
F-59
Other Employee Benefit Plans
The Company sponsors a defined contribution 401(k) retirement savings plan covering all of its U.S. employees, whereby an eligible employee may elect to contribute a portion of his or her salary on a pre-tax basis, subject to applicable federal limitations. The Company is not required to make any discretionary matching of employee contributions. The Company makes a matching contribution equal to
The Company’s wholly owned Irish subsidiary sponsors a defined contribution plan covering all of its employees in Ireland. For the years ended December 31, 2022, 2021 and 2020, the Company recognized expenses of $
The Company has a non-qualified deferred compensation plan for executives. The deferred compensation plan obligations are payable in cash upon retirement, termination of employment and/or certain other times in a lump-sum distribution or in installments, as elected by the participant in accordance with the plan. As of December 31, 2022 and 2021, the deferred compensation plan liabilities totaled $
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS
For Each of the Three Fiscal Years Ended December 31, 2022, 2021 and 2020:
|
|
Balance at |
|
|
|
|
|
Additions charged to |
|
|
Deductions |
|
|
Balance at |
|
|||||
Valuation and Qualifying Accounts |
|
beginning |
|
|
|
|
|
costs and |
|
|
from |
|
|
end of |
|
|||||
(in thousands) |
|
of period |
|
|
Acquisitions |
|
|
expenses |
|
|
reserves |
|
|
period |
|
|||||
Year ended December 31, 2022: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Allowance for returns |
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|||
Allowance for prompt pay discounts |
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|||
Year ended December 31, 2021: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Allowance for returns |
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|||
Allowance for prompt pay discounts |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Year ended December 31, 2020: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Allowance for returns |
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|||
Allowance for prompt pay discounts |
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|||
F-60
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
HORIZON THERAPEUTICS PLC |
||
|
|
|
|
|
Dated: March 1, 2023 |
|
By: |
|
/s/ Timothy P. Walbert |
|
|
|
|
Timothy P. Walbert |
|
|
|
|
|
|
|
|
|
President, Chief Executive Officer and Chairman of the Board |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Timothy P. Walbert and Aaron L. Cox, and each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his or her substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
SIGNATURE |
|
TITLE |
|
DATE |
|
|
|
|
|
/s/ TIMOTHY P. WALBERT |
|
President, Chief Executive Officer and Chairman of the Board (Principal Executive Officer) |
|
March 1, 2023 |
Timothy P. Walbert |
||||
|
|
|
|
|
/s/ AARON L. COX |
|
Executive Vice President and Chief Financial Officer (Principal Financial Officer) |
|
March 1, 2023 |
Aaron L. Cox |
||||
|
|
|
|
|
/s/ PATRICK McILVENNY Patrick McIlvenny |
|
Senior Vice President and Chief Accounting Officer (Principal Accounting Officer) |
|
March 1, 2023 |
|
|
|
|
|
/s/ MICHAEL GREY |
|
Director |
|
March 1, 2023 |
Michael Grey |
||||
|
|
|
|
|
/s/ WILLIAM F. DANIEL |
|
Director |
|
March 1, 2023 |
William F. Daniel |
||||
|
|
|
|
|
/s/ JEFF HIMAWAN |
|
Director |
|
March 1, 2023 |
Jeff Himawan, Ph.D. |
||||
|
|
|
|
|
/s/ SUSAN MAHONY |
||||
Susan Mahony, Ph.D. |
|
Director |
|
March 1, 2023 |
|
|
|
|
|
/s/ GINO SANTINI |
||||
Gino Santini |
|
Director |
|
March 1, 2023 |
|
|
|
|
|
/s/ JAMES SHANNON |
||||
James Shannon, M.D. |
|
Director |
|
March 1, 2023 |
|
|
|
|
|
/s/ H. THOMAS WATKINS |
||||
H. Thomas Watkins |
|
Director |
|
March 1, 2023 |
|
|
Director |
|
March 1, 2023 |
/s/ PASCALE WITZ |
||||
Pascale Witz |